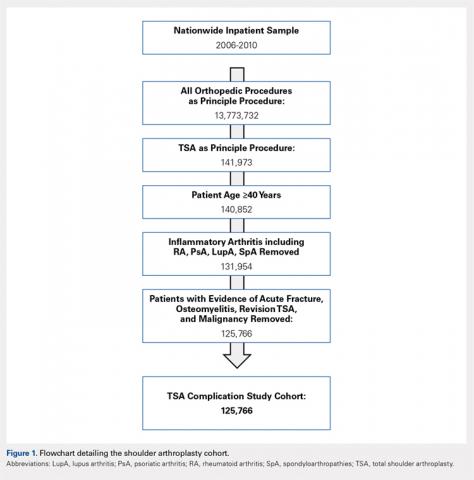
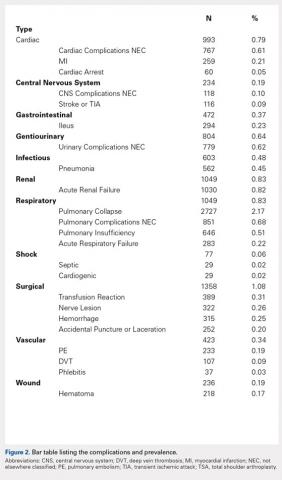
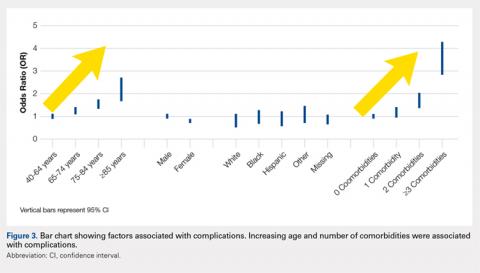
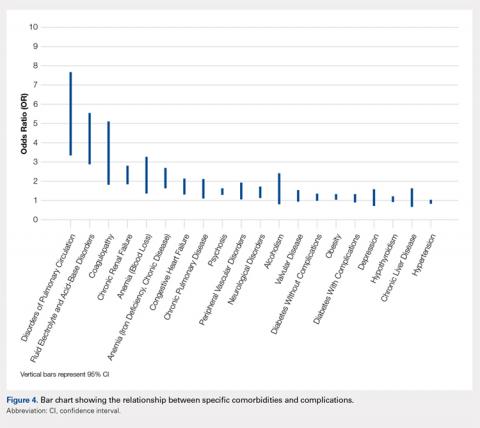
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Patient Preferences in Office-Based Orthopedic Care: A Prospective Evaluation
ABSTRACT
Patient satisfaction has become a topic of interest within orthopedics as the landscape of provider reimbursement continues to evolve to reward value of care. Online internet physician rating sites are becoming increasingly popular ways for patients to subjectively express their provider experience. Understanding what patients value during their episode of care is important in the modern healthcare environment. The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. A prospective survey was electronically administered to 212 patients in an adult reconstruction clinic. One hundred ninety-six patients (92.5%) completed the survey. Demographic questions regarding age, sex, ethnicity, and prior adult reconstruction surgical history were obtained. When patients were asked how much time they would like the doctor to spend with them on a routine visit, the most common answer was 10 to 15 minutes (41.3%), with only 10.2% patients desiring >20 minutes. The majority of patients (83.1%) believe ≥30 minutes is too long to wait to see their surgeon. Less than half of patients (41.8%) stated that they would feel as though they were receiving below average care if seen only by a nurse practitioner or physician’s assistant at a postoperative visit. Patients reported no significant age, gender, or ethnicity preferences for their physician. Recommendations from friends or other physicians was the most common (66.4%) way for patients to find their physicians, while 12.2% utilized online rating sites during their search. Optimizing patient experiences in the office may include keeping wait times to <30 minutes and educating patients on the roles of physician extenders. More work needs to be done to further elucidate variables influencing the subjective patient experience with their orthopedic care.
Continue to: Patient satisfaction...
Patient satisfaction has become an important focus in the rapidly changing healthcare environment due to the significant impact it has on healthcare delivery, healthcare economics, assessment of the quality of care, development of patient-care models, and quality improvement initiatives.1-4 Historically, the quality of care was measured by objective metrics such as complication rates, range-of-motion, and the provider’s expert opinion on the outcome. While those metrics are still impactful variables when defining a successful outcome, the medical community is now increasingly recognizing the importance of patients’ perspectives when defining successful treatments. Patient satisfaction is now highly regarded by clinicians and the government when considering outcomes and is even being incorporated into determining the value of care. Under the Affordable Care Act, patients assumed a more active role in clinical decision-making as well as in creating quality and efficiency initiatives.5,6 By 2017, 2% of the United States government’s Medicare payments will be redistributed among hospitals and physicians based on their quality and efficiency metrics, which are largely determined by patients’ evaluations of care.7 As a result, there has been significant interest in identifying variables influencing patient satisfaction and subjective outcomes.8,9
Patient satisfaction is related to both the outcomes of care and the process of care. As first described by Donabedian,10patients may be satisfied with the successful outcome of their care, but dissatisfied with how they received their care. The process of care is complex and considers many aspects of healthcare delivery, including time, cost, healthcare provider interactions, and burdens faced. While patient satisfaction with outcomes and process of care are heavily related, they should be regarded separately. It is essential that providers understand what variables are important to patients with regards to how they experience healthcare and choose their provider, especially surrounding elective procedures such as hip and knee arthroplasty.11,12
Within orthopedic surgery, patient satisfaction scores are beginning to be incorporated as part of the standard-of-care quality metrics obtained along with patient-reported outcome measures (PROMs) at defined time points postoperatively. Furthermore, PROMs and patient satisfaction data are becoming an increasingly important component of medical decision-making.13-16 Several authors have reported that increased patient satisfaction is correlated with increased compliance, improved treatment outcomes across numerous medical settings, including orthopedics, decreased risk of litigation, and higher patient ratings of the quality of care.17,18 Various factors, including meeting of expectations, staff politeness, the communication skills of the surgeon, and waiting times, have been suggested to influence eventual patient satisfaction within the surgical literature.19-21 However, within orthopedic surgery there is a paucity of investigations evaluating how patients determine preferences and satisfaction with the process of care.
The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. The authors hypothesize that the majority of patients find their physicians through online rating sites or recommendations from family and friends. The authors believe that patients expect to be seen in <30 minutes and will be unsatisfied overall with the amount of time that they spend with their physician.
Continue to: METHODS...
METHODS
The senior author (BRL) and a research team created a 15-question survey to evaluate patient preferences regarding the demographic characteristics (eg, age, gender, ethnicity) of their physician, wait times in a waiting room, time spent with the physician, care received from physician extenders (eg, nurse practitioners, physician assistants), and how they learned of their physician (Appendix). An a priori power analysis was conducted to determine that approximately 200 patients were needed for inclusion.11,22 Following Institutional Review Board approval (ORA 15051104), the survey was administered to 212 patients in a single-surgeon, adult reconstruction clinic. The survey was digitally administered on a touch-screen tablet using an electronic independent third party survey center (SurveyMonkey Inc) devoid of any identifying data. The survey was offered to all patients >21 years of age who were English-speaking and in the common area as patients waiting to be seen, from June 2015 to March 2016. A research assistant approached patients in the waiting room and asked if they would like to participate in a short survey regarding what factors influence the patient-physician relationship from the patient’s perspective.
Appendix 1
- Do you wish to partake in this 3-minute survey?
- Have you had a prior knee or hip replacement?
- What is your age?
- 30-40 years
- 40-50 years
- 50-60 years
- 60-70 years
- 70-80 years
- 80+ years
- What is your gender?
- Which of the following best represents your racial or ethnic heritage?
- African American
- How much time would you like the doctor to spend talking to you on a routine visit?
- 0-5 minutes
- 5-10 minutes
- 10-15 minutes
- 15-20 minutes
- 20-30 minutes
- >30 minutes
- How long is too long to wait to see the doctor?
- 10 minutes
- 20 minutes
- 30 minutes
- 40 minutes
- 50 minutes
- An hour or more
- If you were to only see a physician’s assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care?
- Overall I am satisfied with the time the doctor spends with me.
- If you were to need a major surgery, would you want the physician to tell you what he or she would do if they were in your shoes?
- Would you prefer your doctor to be the same race/ethnicity as you?
- No
- No Preference
- Would you feel more comfortable with a male as opposed to a female orthopedic surgeon?
- Would you feel more comfortable with a female as opposed to a male orthopedic surgeon?
- What age would you like your physician to be?
- 25-35 years old
- 35-45 years old
- 45-55 years old
- 55-65 years old
- 65 years and older
- No preference
- How do you usually find your physician?
- Friends’ recommendations
- Healthcare provider’s recommendations
- Insurance plans
- Online research/ratings
- Other
Descriptive statistics were used to analyze subject demographics and survey responses. Chi-square analyses and multinomial logistic regressions were utilized to compare responses. All statistical analyses were conducted using SPSS version 24.0 software (SPSS Inc). Statistical significance was set at P < 0.05.
RESULTS
Of the 212 patients who were invited to participate, 196 patients (92.4%) agreed and completed the survey. Demographic and surgical history information can be found in Table 1. The majority of patients were female (62%) and above the age of 50 years (92.4%). Almost half (48.5%) of patients had a prior hip or knee replacement.
Table 1. Survey Respondent Demographics
| Number | Percent |
Age Range | ||
30-40 years | 4 | 2.0% |
40-50 years | 11 | 5.6% |
50-60 years | 47 | 24.0% |
60-70 years | 84 | 42.9% |
70-80 years | 41 | 20.9% |
>80 years | 9 | 4.6% |
Gender | ||
Male | 74 | 37.8% |
Female | 122 | 62.2% |
Ethnicity | ||
African American | 39 | 19.9% |
Asian | 3 | 1.5% |
Caucasian | 140 | 71.4% |
Hispanic | 10 | 5.1% |
Other | 4 | 2.0% |
Prior knee or hip replacement | ||
Yes | 95 | 48.5% |
No | 55 | 28.1% |
No Response | 46 | 23.5% |
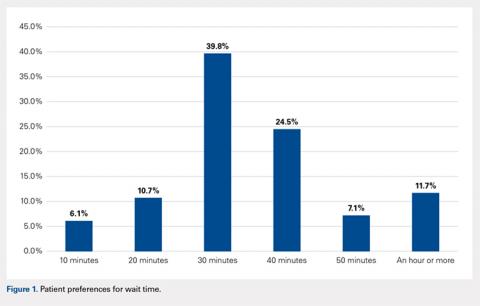
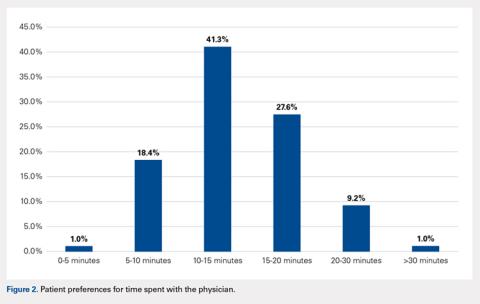
When asked how long is too long to wait to see the doctor, 30 minutes (39.8%) was most commonly selected, followed by 40 minutes (24.5%) (Figure 1). When asked how much time patients would like the doctor to spend with them during an office visit, the majority (68.9%) selected either 10 to 15 minutes (41.3%) or 15 to 20 minutes (27.6%) (Figure 2). The majority of patients (92.3%) were satisfied with the amount of time the doctor spent with them. In addition, 94.9% of respondents would want their doctor to tell them what they would do if they were in the patient’s shoes when making decisions regarding their medical care (Table 2). Less than half of respondents (41.8%) believe that seeing a physician extender (eg, nurse practitioner or physician assistant) at a postoperative visit would result in a lower quality of care (Table 2).
Table 2. Responses to Survey Questions
If you were to only see a physician's assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care? | ||
Answer choices | Number | Percent |
No | 114 | 58.2% |
Yes | 82 | 41.8% |
If you were to need a major surgery would you want the physician to tell you what he or she would do if they were in your shoes? | ||
Answer choices | Number | Percent |
No | 10 | 5.1% |
Yes | 186 | 94.9% |
Would you prefer your doctor to be the same race/ethnicity as you? | ||
Answer choices | Number | Percent |
No | 29 | 14.8% |
Yes | 3 | 1.5% |
No Preference | 164 | 83.7% |
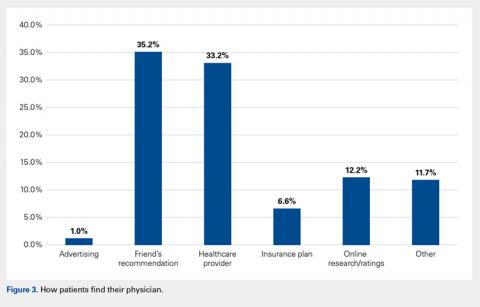
When asked if patients preferred a doctor of the same race/ethnicity, the vast majority (83.7%) had no preference (Table 2). There was no significant difference found between male and female respondents when asked if they would feel more comfortable with a male as opposed to a female orthopedic surgeon (P = .118) and vice versa (P = .604) (Table 3). Most patients preferred a physician between the ages of 45 and 55 years (39.3%), followed by 35 and 45 years (23.0%); however, this preference was not statistically significant (Table 4). Older patients were more likely to prefer younger physicians (odds ratio, 4.612 for 25-35 years of age; odds ratio, 1.328 for 35-45 years of age). Only 12.2% of patients reported online research/rating sites as the main resource utilized when selecting their physician (Figure 3). The majority (68.4%) reported that recommendations from either friends (35.2%) or healthcare providers (33.2%) were the main avenues through which they found their physicians.
Table 3. Overall Responses to Questions Regarding Male and Female Orthopedic Surgeonsa
Would you feel more comfortable with a male as opposed to a female orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 164 | 83.7% | 106 (86.9%) | 58 (78.4%) | 0.118 |
Yes | 32 | 16.3% | 16 (13.1%) | 16 (21.6%) |
|
Would you feel more comfortable with a female as opposed to a male orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 186 | 94.9% | 115 (94.3%) | 71 (95.9%) | 0.604 |
Yes | 10 | 5.1% | 7 (5.7%) | 3 (4.1%) |
|
aResponses were broken down by gender and compared utilizing a 2 x 2 chi-square analysis to test for significant differences in respondents’ gender preferences for their orthopedic surgeon.
Table 4. Patient Preferences Regarding Physician Age
What age would you like your physician to be? |
| 95% Confidence Interval | ||||
Answer Choices | Number or Responses | Percent | P value | Exp(β) | Lower Bound | Upper Bound |
25-35 years | 1 | 0.5% | 0.217 | 4.612 | 0.407 | 52.283 |
35-45 years | 45 | 23.0% | 0.161 | 1.328 | 0.893 | 1.975 |
45-55 years | 77 | 39.3% | 0.159 | 1.276 | 0.909 | 1.791 |
55-65 years | 9 | 4.6% | 0.483 | 1.302 | 0.624 | 2.717 |
≥65 years | 2 | 1.0% | 0.272 | 0.491 | 0.138 | 1.748 |
No preferencea | 62 | 31.6% | Reference | |||
aNo preference was used as the reference category for the answer choices, while the age bracket “>80 years” was used as the reference for the age of respondent variable.
Continue to: DISCUSSION...
DISCUSSION
The results of this study demonstrate that patients have several expectations and preferences with regards to the care they receive from physicians in the office. Patients prefer to wait <30 minutes before seeing their provider and desire only 10 to 20 minutes with their doctor. Patients do not have specific preferences with regards to the gender or ethnicity of their physician but would prefer a physician in the middle of their career, aged 45 to 55 years. Ultimately, patients do believe that seeing a physician at a postoperative visit is important, as just under half of patients thought that seeing a physician extender alone at a postoperative visit resulted in a lower quality of care.
While these results were obtained in a population specifically seeking the care of an orthopedic adult reconstruction surgeon, the results demonstrate that patients do not necessarily desire an unreasonable amount of time with their doctor. Patients simply want to be seen in a timely fashion and receive the full undivided attention of their doctor for approximately 20 minutes. Similarly, Patterson and colleagues22 found, in their series of 182 patients who presented to an orthopedic surgeon, that there was a significant correlation between time spent with the surgeon and overall patient satisfaction. Interestingly, the authors reported that patient satisfaction was not correlated with education level, sex, marital status, whether the patients were evaluated by a resident physician before seeing the attending surgeon, self-reported mental status, tobacco usage, the type of clinic visit, or the waiting time to see the surgeon (average, about 40 minutes for this cohort).22 Similarly, Teunis and colleagues23 reported an average 32-minute wait time in 81 patients presenting for care at an orthopedic hand clinic and demonstrated that a longer wait time was associated with decreased patient satisfaction. These results corroborate the findings of this study that a short wait time is important to patients when evaluating the process of care. Additionally, patients do not have unreasonable expectations with regards to the amount of time they would like to spend with the physician. A physician who has a clinic for 9 hours a day would thus be able to see 54 patients and still spend at least 10 minutes with each patient. The quality of the physician-patient interaction is likely more important than the actual amount of time spent; however, based on this study, patients do have certain expectations about how much time physicians should spend with them.
There were no significant sex, age, or ethnicity preferences in our specific patient cohort. However, a sizable percentage of respondents, 41.8%, believed that they were receiving inferior care if they only saw a physician extender at a routine follow-up visit. Many orthopedic surgeons rely on the care provided by physician extenders to enable them to see additional patients. Physician extenders are well trained to provide high-quality care, including at routine postoperative visits. The results of this study, that many patients believe physician extenders provide lower-quality care, may be a result of inadequate patient education regarding the extensive training and education physician extenders undergo. Physician extenders are qualified, licensed healthcare professionals who are playing increasingly important roles within orthopedics and medicine as a whole. As the demand for orthopedic surgeons to see more patients increases, so does the role of physician extenders. Future research is warranted into educating the public regarding the importance of these healthcare providers and the adequacy of their training.
While many practices now routinely obtain patient satisfaction scores, another modality through which patients can express their satisfaction and experiences with healthcare providers is through online internet physician rating sites (IPRS). These sites have exploded in number and popularity in recent years and, according to some studies, have a very real effect on provider selection.24 Interestingly, a low percentage of patients in this study utilized IPRS reviews to find their doctors. In a recent prospective survey study of 1000 consecutive patients presenting for care at the Mayo Clinic, Burkle and Keegan24 reported that 27% of patients would choose not to see a physician based on a negative IPRS review. Interestingly, only 1.0% of patients reported finding their doctor through advertising. Numerous authors have recently addressed advertising in orthopedic surgery, specifically direct-to-consumer marking, including the influence of physician self-promotion on patients.25,26 Specifically, Halawi and Barsoum26 discussed how direct-to-consumer marketing is commonly disseminated to the public through television and print advertisements, which are modalities more commonly utilized by older generations. However, many advertising agencies are moving toward internet-based advertising, especially through orthopedic group and individual surgeon websites for self-promoting advertisement, as approximately 75% of Americans use the internet for health-related information.25,27 The fact that many patients in this study did not utilize IPRS reviews or advertising (much of which is electronic) may be a result of the older, less internet-centric demographic that is often seen in an adult reconstruction clinic. Future research is warranted to determine what demographic of patients value IPRS reviews and how those reviews influence physician selection and the patient experience.
There are several limitations to this study. First, the majority of the surveyed population was Caucasian, and our results may not be equally reflective of diverse ethnic backgrounds. Second, the cohort size, while based on previous studies conducted in a similar fashion, may be underpowered to detect significant differences for 1 or more of these questions. In addition, having a question regarding the patient’s medical background or experiences may have provided further insight as to why patients selected the answers that they did. Furthermore, questions regarding the patient’s education level, religious background, and income brackets may have provided further context in which to evaluate their responses. These questions were omitted in an effort to keep the questionnaire at a length that would maximize enrollment and prevent survey fatigue. Future research is warranted to determine what patient-specific, injury/symptom-specific, and treatment-specific variables influence the subjective patient experience.
CONCLUSION
The vast majority of patients desire only 10 to 20 minutes with their doctor and are highly satisfied with the amount of time their surgeon spends with them. Patients reported no significant gender- or ethnicity-based preferences for their doctor. The majority of patients believe that a wait time exceeding 30 minutes is too long. A greater effort needs to be made to educate patients and the public about the significant and effective roles nurse practitioners and physician assistants can play within the healthcare system. While this cohort did not report notable utilization of IPRS reviews, it remains essential to understand what factors influence patients’ subjective experiences with their providers to ensure that patients achieve their desired outcomes, and report as such on these websites as they continue to gain popularity. Diminishing clinic wait times and understanding patient preferences may lead to a greater percentage of “satisfied” patients. While the majority of focus has been and will likely continue to be on improving patients’ satisfaction with their outcomes, more work needs to be done focusing specifically on the process through which outcomes are achieved.
1. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84–A(9):1560-1572.
2. Carr-Hill RA. The measurement of patient satisfaction. J Public Health Med. 1992;14(3):236-249.
3. Ross CK, Steward CA, Sinacore JM. A comparative study of seven measures of patient satisfaction. Med Care. 1995;33(4):392-406. doi:10.1097/00005650-199504000-00006.
4. Strasser S, Aharony L, Greenberger D. The patient satisfaction process: moving toward a comprehensive model. Med Care Rev. 1993;50(2):219-248. doi:10.1177/107755879305000205.
5. Bozic KJ. Orthopaedic healthcare worldwide: shared medical decision making in orthopaedics. Clin Orthop Relat Res. 2013;471(5):1412-1414. doi:10.1007/s11999-013-2838-5.
6. Youm J, Chenok KE, Belkora J, Chiu V, Bozic KJ. The emerging case for shared decision making in orthopaedics. Instr Course Lect. 2013;62:587-594. doi:10.2106/00004623-201210170-00011.
7. Blumenthal D, Abrams M, Nuzum R. The affordable CARE Act at 5 years. N Engl J Med. 2015;373(16):1580. doi:10.1056/NEJMc1510015.
8. Shirley ED, Sanders JO. Patient satisfaction: implications and predictors of success. J Bone Joint Surg Am. 2013;95(10):e69. doi:10.2106/JBJS.L.01048.
9. Morris BJ, Jahangir AA, Sethi MK. Patient satisfaction: an emerging health policy issue. AAOS Now Web site. http://www.aaos.org/AAOSNow/2013/Jun/advocacy/advocacy5/?ssopc=1. Published June 2013. Accessed November 19, 2016.
10. Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743-1748. doi:10.1001/jama.260.12.1743.
11. Bozic KJ, Kaufman D, Chan VC, Caminiti S, Lewis C. Factors that influence provider selection for elective total joint arthroplasty. Clin Orthop Relat Res. 2013;471(6):1865-1872. doi:10.1007/s11999-012-2640-9.
12. Davies AR, Ware JE Jr. Involving consumers in quality of care assessment. Health Aff (Millwood). 1988;7(1):33-48.
13. Black N, Burke L, Forrest CB, et al. Patient-reported outcomes: pathways to better health, better services, and better societies. Qual Life Res. 2016;25(5):1103-1112. doi:10.1007/s11136-015-1168-3.
14. Gilbert A, Sebag-Montefiore D, Davidson S, Velikova G. Use of patient-reported outcomes to measure symptoms and health related quality of life in the clinic. Gynecol Oncol. 2015;136(3):429-439. doi:10.1016/j.ygyno.2014.11.071.
15. Van Der Wees PJ, Nijhuis-Van Der Sanden MW, Ayanian JZ, Black N, Westert GP, Schneider EC. Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q. 2014;92(4):754-775. doi:10.1111/1468-0009.12091.
16. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. Total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(Suppl 1):104-109. doi:10.2106/JBJS.N.00328.
17. Williams B. Patient satisfaction: a valid concept? Soc Sci Med. 1994;38(4):509-516. doi:10.1016/0277-9536(94)90247-X.
18. Hickson GB, Clayton EW, Entman SS, et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583-1587. doi:10.1001/jama.1994.03520200039032.
19. Larsson BW, Larsson G, Chantereau MW, von Holstein KS. International comparisons of patients' views on quality of care. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2005;18(1):62-73. doi:10.1108/09526860510576974.
20. McLafferty RB, Williams RG, Lambert AD, Dunnington GL. Surgeon communication behaviors that lead patients to not recommend the surgeon to family members or friends: analysis and impact. Surgery. 2006;140(4):616-624. doi:https://doi.org/10.1016/j.surg.2006.06.021.
21. Mira JJ, Tomás O, Virtudes-Pérez M, Nebot C, Rodríguez-Marín J. Predictors of patient satisfaction in surgery. Surgery. 2009;145(5):536-541. doi:10.1016/j.surg.2009.01.012.
22. Patterson BM, Eskildsen SM, Clement RC, et al. Patient satisfaction is associated with time with provider but not clinic wait time among orthopedic patients. Orthopedics. 2017;40(1):43-48. doi:10.3928/01477447-20161013-05.
23. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated With patient satisfaction. Clin Orthop Relat Res. 2015;473(7):2362-2368. doi:10.1007/s11999-014-4090-z.
24. Burkle CM, Keegan MT. Popularity of internet physician rating sites and their apparent influence on patients' choices of physicians. BMC Health Serv Res. 2015;15:416. doi:10.1186/s12913-015-1099-2.
25. Mohney S, Lee DJ, Elfar JC. The effect of orthopedic advertising and self-promotion on a naive population. Am J Orthop. 2016;45(4):E227-E232.
26. Halawi MJ, Barsoum WK. Direct-to-consumer marketing: implications for patient care and orthopedic education. Am J Orthop. 2016;45(6):E335-E336.
27. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156. doi:10.1007/s11606-010-1425-7.
ABSTRACT
Patient satisfaction has become a topic of interest within orthopedics as the landscape of provider reimbursement continues to evolve to reward value of care. Online internet physician rating sites are becoming increasingly popular ways for patients to subjectively express their provider experience. Understanding what patients value during their episode of care is important in the modern healthcare environment. The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. A prospective survey was electronically administered to 212 patients in an adult reconstruction clinic. One hundred ninety-six patients (92.5%) completed the survey. Demographic questions regarding age, sex, ethnicity, and prior adult reconstruction surgical history were obtained. When patients were asked how much time they would like the doctor to spend with them on a routine visit, the most common answer was 10 to 15 minutes (41.3%), with only 10.2% patients desiring >20 minutes. The majority of patients (83.1%) believe ≥30 minutes is too long to wait to see their surgeon. Less than half of patients (41.8%) stated that they would feel as though they were receiving below average care if seen only by a nurse practitioner or physician’s assistant at a postoperative visit. Patients reported no significant age, gender, or ethnicity preferences for their physician. Recommendations from friends or other physicians was the most common (66.4%) way for patients to find their physicians, while 12.2% utilized online rating sites during their search. Optimizing patient experiences in the office may include keeping wait times to <30 minutes and educating patients on the roles of physician extenders. More work needs to be done to further elucidate variables influencing the subjective patient experience with their orthopedic care.
Continue to: Patient satisfaction...
Patient satisfaction has become an important focus in the rapidly changing healthcare environment due to the significant impact it has on healthcare delivery, healthcare economics, assessment of the quality of care, development of patient-care models, and quality improvement initiatives.1-4 Historically, the quality of care was measured by objective metrics such as complication rates, range-of-motion, and the provider’s expert opinion on the outcome. While those metrics are still impactful variables when defining a successful outcome, the medical community is now increasingly recognizing the importance of patients’ perspectives when defining successful treatments. Patient satisfaction is now highly regarded by clinicians and the government when considering outcomes and is even being incorporated into determining the value of care. Under the Affordable Care Act, patients assumed a more active role in clinical decision-making as well as in creating quality and efficiency initiatives.5,6 By 2017, 2% of the United States government’s Medicare payments will be redistributed among hospitals and physicians based on their quality and efficiency metrics, which are largely determined by patients’ evaluations of care.7 As a result, there has been significant interest in identifying variables influencing patient satisfaction and subjective outcomes.8,9
Patient satisfaction is related to both the outcomes of care and the process of care. As first described by Donabedian,10patients may be satisfied with the successful outcome of their care, but dissatisfied with how they received their care. The process of care is complex and considers many aspects of healthcare delivery, including time, cost, healthcare provider interactions, and burdens faced. While patient satisfaction with outcomes and process of care are heavily related, they should be regarded separately. It is essential that providers understand what variables are important to patients with regards to how they experience healthcare and choose their provider, especially surrounding elective procedures such as hip and knee arthroplasty.11,12
Within orthopedic surgery, patient satisfaction scores are beginning to be incorporated as part of the standard-of-care quality metrics obtained along with patient-reported outcome measures (PROMs) at defined time points postoperatively. Furthermore, PROMs and patient satisfaction data are becoming an increasingly important component of medical decision-making.13-16 Several authors have reported that increased patient satisfaction is correlated with increased compliance, improved treatment outcomes across numerous medical settings, including orthopedics, decreased risk of litigation, and higher patient ratings of the quality of care.17,18 Various factors, including meeting of expectations, staff politeness, the communication skills of the surgeon, and waiting times, have been suggested to influence eventual patient satisfaction within the surgical literature.19-21 However, within orthopedic surgery there is a paucity of investigations evaluating how patients determine preferences and satisfaction with the process of care.
The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. The authors hypothesize that the majority of patients find their physicians through online rating sites or recommendations from family and friends. The authors believe that patients expect to be seen in <30 minutes and will be unsatisfied overall with the amount of time that they spend with their physician.
Continue to: METHODS...
METHODS
The senior author (BRL) and a research team created a 15-question survey to evaluate patient preferences regarding the demographic characteristics (eg, age, gender, ethnicity) of their physician, wait times in a waiting room, time spent with the physician, care received from physician extenders (eg, nurse practitioners, physician assistants), and how they learned of their physician (Appendix). An a priori power analysis was conducted to determine that approximately 200 patients were needed for inclusion.11,22 Following Institutional Review Board approval (ORA 15051104), the survey was administered to 212 patients in a single-surgeon, adult reconstruction clinic. The survey was digitally administered on a touch-screen tablet using an electronic independent third party survey center (SurveyMonkey Inc) devoid of any identifying data. The survey was offered to all patients >21 years of age who were English-speaking and in the common area as patients waiting to be seen, from June 2015 to March 2016. A research assistant approached patients in the waiting room and asked if they would like to participate in a short survey regarding what factors influence the patient-physician relationship from the patient’s perspective.
Appendix 1
- Do you wish to partake in this 3-minute survey?
- Have you had a prior knee or hip replacement?
- What is your age?
- 30-40 years
- 40-50 years
- 50-60 years
- 60-70 years
- 70-80 years
- 80+ years
- What is your gender?
- Which of the following best represents your racial or ethnic heritage?
- African American
- How much time would you like the doctor to spend talking to you on a routine visit?
- 0-5 minutes
- 5-10 minutes
- 10-15 minutes
- 15-20 minutes
- 20-30 minutes
- >30 minutes
- How long is too long to wait to see the doctor?
- 10 minutes
- 20 minutes
- 30 minutes
- 40 minutes
- 50 minutes
- An hour or more
- If you were to only see a physician’s assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care?
- Overall I am satisfied with the time the doctor spends with me.
- If you were to need a major surgery, would you want the physician to tell you what he or she would do if they were in your shoes?
- Would you prefer your doctor to be the same race/ethnicity as you?
- No
- No Preference
- Would you feel more comfortable with a male as opposed to a female orthopedic surgeon?
- Would you feel more comfortable with a female as opposed to a male orthopedic surgeon?
- What age would you like your physician to be?
- 25-35 years old
- 35-45 years old
- 45-55 years old
- 55-65 years old
- 65 years and older
- No preference
- How do you usually find your physician?
- Friends’ recommendations
- Healthcare provider’s recommendations
- Insurance plans
- Online research/ratings
- Other
Descriptive statistics were used to analyze subject demographics and survey responses. Chi-square analyses and multinomial logistic regressions were utilized to compare responses. All statistical analyses were conducted using SPSS version 24.0 software (SPSS Inc). Statistical significance was set at P < 0.05.
RESULTS
Of the 212 patients who were invited to participate, 196 patients (92.4%) agreed and completed the survey. Demographic and surgical history information can be found in Table 1. The majority of patients were female (62%) and above the age of 50 years (92.4%). Almost half (48.5%) of patients had a prior hip or knee replacement.
Table 1. Survey Respondent Demographics
| Number | Percent |
Age Range | ||
30-40 years | 4 | 2.0% |
40-50 years | 11 | 5.6% |
50-60 years | 47 | 24.0% |
60-70 years | 84 | 42.9% |
70-80 years | 41 | 20.9% |
>80 years | 9 | 4.6% |
Gender | ||
Male | 74 | 37.8% |
Female | 122 | 62.2% |
Ethnicity | ||
African American | 39 | 19.9% |
Asian | 3 | 1.5% |
Caucasian | 140 | 71.4% |
Hispanic | 10 | 5.1% |
Other | 4 | 2.0% |
Prior knee or hip replacement | ||
Yes | 95 | 48.5% |
No | 55 | 28.1% |
No Response | 46 | 23.5% |
When asked how long is too long to wait to see the doctor, 30 minutes (39.8%) was most commonly selected, followed by 40 minutes (24.5%) (Figure 1). When asked how much time patients would like the doctor to spend with them during an office visit, the majority (68.9%) selected either 10 to 15 minutes (41.3%) or 15 to 20 minutes (27.6%) (Figure 2). The majority of patients (92.3%) were satisfied with the amount of time the doctor spent with them. In addition, 94.9% of respondents would want their doctor to tell them what they would do if they were in the patient’s shoes when making decisions regarding their medical care (Table 2). Less than half of respondents (41.8%) believe that seeing a physician extender (eg, nurse practitioner or physician assistant) at a postoperative visit would result in a lower quality of care (Table 2).
Table 2. Responses to Survey Questions
If you were to only see a physician's assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care? | ||
Answer choices | Number | Percent |
No | 114 | 58.2% |
Yes | 82 | 41.8% |
If you were to need a major surgery would you want the physician to tell you what he or she would do if they were in your shoes? | ||
Answer choices | Number | Percent |
No | 10 | 5.1% |
Yes | 186 | 94.9% |
Would you prefer your doctor to be the same race/ethnicity as you? | ||
Answer choices | Number | Percent |
No | 29 | 14.8% |
Yes | 3 | 1.5% |
No Preference | 164 | 83.7% |
When asked if patients preferred a doctor of the same race/ethnicity, the vast majority (83.7%) had no preference (Table 2). There was no significant difference found between male and female respondents when asked if they would feel more comfortable with a male as opposed to a female orthopedic surgeon (P = .118) and vice versa (P = .604) (Table 3). Most patients preferred a physician between the ages of 45 and 55 years (39.3%), followed by 35 and 45 years (23.0%); however, this preference was not statistically significant (Table 4). Older patients were more likely to prefer younger physicians (odds ratio, 4.612 for 25-35 years of age; odds ratio, 1.328 for 35-45 years of age). Only 12.2% of patients reported online research/rating sites as the main resource utilized when selecting their physician (Figure 3). The majority (68.4%) reported that recommendations from either friends (35.2%) or healthcare providers (33.2%) were the main avenues through which they found their physicians.
Table 3. Overall Responses to Questions Regarding Male and Female Orthopedic Surgeonsa
Would you feel more comfortable with a male as opposed to a female orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 164 | 83.7% | 106 (86.9%) | 58 (78.4%) | 0.118 |
Yes | 32 | 16.3% | 16 (13.1%) | 16 (21.6%) |
|
Would you feel more comfortable with a female as opposed to a male orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 186 | 94.9% | 115 (94.3%) | 71 (95.9%) | 0.604 |
Yes | 10 | 5.1% | 7 (5.7%) | 3 (4.1%) |
|
aResponses were broken down by gender and compared utilizing a 2 x 2 chi-square analysis to test for significant differences in respondents’ gender preferences for their orthopedic surgeon.
Table 4. Patient Preferences Regarding Physician Age
What age would you like your physician to be? |
| 95% Confidence Interval | ||||
Answer Choices | Number or Responses | Percent | P value | Exp(β) | Lower Bound | Upper Bound |
25-35 years | 1 | 0.5% | 0.217 | 4.612 | 0.407 | 52.283 |
35-45 years | 45 | 23.0% | 0.161 | 1.328 | 0.893 | 1.975 |
45-55 years | 77 | 39.3% | 0.159 | 1.276 | 0.909 | 1.791 |
55-65 years | 9 | 4.6% | 0.483 | 1.302 | 0.624 | 2.717 |
≥65 years | 2 | 1.0% | 0.272 | 0.491 | 0.138 | 1.748 |
No preferencea | 62 | 31.6% | Reference | |||
aNo preference was used as the reference category for the answer choices, while the age bracket “>80 years” was used as the reference for the age of respondent variable.
Continue to: DISCUSSION...
DISCUSSION
The results of this study demonstrate that patients have several expectations and preferences with regards to the care they receive from physicians in the office. Patients prefer to wait <30 minutes before seeing their provider and desire only 10 to 20 minutes with their doctor. Patients do not have specific preferences with regards to the gender or ethnicity of their physician but would prefer a physician in the middle of their career, aged 45 to 55 years. Ultimately, patients do believe that seeing a physician at a postoperative visit is important, as just under half of patients thought that seeing a physician extender alone at a postoperative visit resulted in a lower quality of care.
While these results were obtained in a population specifically seeking the care of an orthopedic adult reconstruction surgeon, the results demonstrate that patients do not necessarily desire an unreasonable amount of time with their doctor. Patients simply want to be seen in a timely fashion and receive the full undivided attention of their doctor for approximately 20 minutes. Similarly, Patterson and colleagues22 found, in their series of 182 patients who presented to an orthopedic surgeon, that there was a significant correlation between time spent with the surgeon and overall patient satisfaction. Interestingly, the authors reported that patient satisfaction was not correlated with education level, sex, marital status, whether the patients were evaluated by a resident physician before seeing the attending surgeon, self-reported mental status, tobacco usage, the type of clinic visit, or the waiting time to see the surgeon (average, about 40 minutes for this cohort).22 Similarly, Teunis and colleagues23 reported an average 32-minute wait time in 81 patients presenting for care at an orthopedic hand clinic and demonstrated that a longer wait time was associated with decreased patient satisfaction. These results corroborate the findings of this study that a short wait time is important to patients when evaluating the process of care. Additionally, patients do not have unreasonable expectations with regards to the amount of time they would like to spend with the physician. A physician who has a clinic for 9 hours a day would thus be able to see 54 patients and still spend at least 10 minutes with each patient. The quality of the physician-patient interaction is likely more important than the actual amount of time spent; however, based on this study, patients do have certain expectations about how much time physicians should spend with them.
There were no significant sex, age, or ethnicity preferences in our specific patient cohort. However, a sizable percentage of respondents, 41.8%, believed that they were receiving inferior care if they only saw a physician extender at a routine follow-up visit. Many orthopedic surgeons rely on the care provided by physician extenders to enable them to see additional patients. Physician extenders are well trained to provide high-quality care, including at routine postoperative visits. The results of this study, that many patients believe physician extenders provide lower-quality care, may be a result of inadequate patient education regarding the extensive training and education physician extenders undergo. Physician extenders are qualified, licensed healthcare professionals who are playing increasingly important roles within orthopedics and medicine as a whole. As the demand for orthopedic surgeons to see more patients increases, so does the role of physician extenders. Future research is warranted into educating the public regarding the importance of these healthcare providers and the adequacy of their training.
While many practices now routinely obtain patient satisfaction scores, another modality through which patients can express their satisfaction and experiences with healthcare providers is through online internet physician rating sites (IPRS). These sites have exploded in number and popularity in recent years and, according to some studies, have a very real effect on provider selection.24 Interestingly, a low percentage of patients in this study utilized IPRS reviews to find their doctors. In a recent prospective survey study of 1000 consecutive patients presenting for care at the Mayo Clinic, Burkle and Keegan24 reported that 27% of patients would choose not to see a physician based on a negative IPRS review. Interestingly, only 1.0% of patients reported finding their doctor through advertising. Numerous authors have recently addressed advertising in orthopedic surgery, specifically direct-to-consumer marking, including the influence of physician self-promotion on patients.25,26 Specifically, Halawi and Barsoum26 discussed how direct-to-consumer marketing is commonly disseminated to the public through television and print advertisements, which are modalities more commonly utilized by older generations. However, many advertising agencies are moving toward internet-based advertising, especially through orthopedic group and individual surgeon websites for self-promoting advertisement, as approximately 75% of Americans use the internet for health-related information.25,27 The fact that many patients in this study did not utilize IPRS reviews or advertising (much of which is electronic) may be a result of the older, less internet-centric demographic that is often seen in an adult reconstruction clinic. Future research is warranted to determine what demographic of patients value IPRS reviews and how those reviews influence physician selection and the patient experience.
There are several limitations to this study. First, the majority of the surveyed population was Caucasian, and our results may not be equally reflective of diverse ethnic backgrounds. Second, the cohort size, while based on previous studies conducted in a similar fashion, may be underpowered to detect significant differences for 1 or more of these questions. In addition, having a question regarding the patient’s medical background or experiences may have provided further insight as to why patients selected the answers that they did. Furthermore, questions regarding the patient’s education level, religious background, and income brackets may have provided further context in which to evaluate their responses. These questions were omitted in an effort to keep the questionnaire at a length that would maximize enrollment and prevent survey fatigue. Future research is warranted to determine what patient-specific, injury/symptom-specific, and treatment-specific variables influence the subjective patient experience.
CONCLUSION
The vast majority of patients desire only 10 to 20 minutes with their doctor and are highly satisfied with the amount of time their surgeon spends with them. Patients reported no significant gender- or ethnicity-based preferences for their doctor. The majority of patients believe that a wait time exceeding 30 minutes is too long. A greater effort needs to be made to educate patients and the public about the significant and effective roles nurse practitioners and physician assistants can play within the healthcare system. While this cohort did not report notable utilization of IPRS reviews, it remains essential to understand what factors influence patients’ subjective experiences with their providers to ensure that patients achieve their desired outcomes, and report as such on these websites as they continue to gain popularity. Diminishing clinic wait times and understanding patient preferences may lead to a greater percentage of “satisfied” patients. While the majority of focus has been and will likely continue to be on improving patients’ satisfaction with their outcomes, more work needs to be done focusing specifically on the process through which outcomes are achieved.
ABSTRACT
Patient satisfaction has become a topic of interest within orthopedics as the landscape of provider reimbursement continues to evolve to reward value of care. Online internet physician rating sites are becoming increasingly popular ways for patients to subjectively express their provider experience. Understanding what patients value during their episode of care is important in the modern healthcare environment. The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. A prospective survey was electronically administered to 212 patients in an adult reconstruction clinic. One hundred ninety-six patients (92.5%) completed the survey. Demographic questions regarding age, sex, ethnicity, and prior adult reconstruction surgical history were obtained. When patients were asked how much time they would like the doctor to spend with them on a routine visit, the most common answer was 10 to 15 minutes (41.3%), with only 10.2% patients desiring >20 minutes. The majority of patients (83.1%) believe ≥30 minutes is too long to wait to see their surgeon. Less than half of patients (41.8%) stated that they would feel as though they were receiving below average care if seen only by a nurse practitioner or physician’s assistant at a postoperative visit. Patients reported no significant age, gender, or ethnicity preferences for their physician. Recommendations from friends or other physicians was the most common (66.4%) way for patients to find their physicians, while 12.2% utilized online rating sites during their search. Optimizing patient experiences in the office may include keeping wait times to <30 minutes and educating patients on the roles of physician extenders. More work needs to be done to further elucidate variables influencing the subjective patient experience with their orthopedic care.
Continue to: Patient satisfaction...
Patient satisfaction has become an important focus in the rapidly changing healthcare environment due to the significant impact it has on healthcare delivery, healthcare economics, assessment of the quality of care, development of patient-care models, and quality improvement initiatives.1-4 Historically, the quality of care was measured by objective metrics such as complication rates, range-of-motion, and the provider’s expert opinion on the outcome. While those metrics are still impactful variables when defining a successful outcome, the medical community is now increasingly recognizing the importance of patients’ perspectives when defining successful treatments. Patient satisfaction is now highly regarded by clinicians and the government when considering outcomes and is even being incorporated into determining the value of care. Under the Affordable Care Act, patients assumed a more active role in clinical decision-making as well as in creating quality and efficiency initiatives.5,6 By 2017, 2% of the United States government’s Medicare payments will be redistributed among hospitals and physicians based on their quality and efficiency metrics, which are largely determined by patients’ evaluations of care.7 As a result, there has been significant interest in identifying variables influencing patient satisfaction and subjective outcomes.8,9
Patient satisfaction is related to both the outcomes of care and the process of care. As first described by Donabedian,10patients may be satisfied with the successful outcome of their care, but dissatisfied with how they received their care. The process of care is complex and considers many aspects of healthcare delivery, including time, cost, healthcare provider interactions, and burdens faced. While patient satisfaction with outcomes and process of care are heavily related, they should be regarded separately. It is essential that providers understand what variables are important to patients with regards to how they experience healthcare and choose their provider, especially surrounding elective procedures such as hip and knee arthroplasty.11,12
Within orthopedic surgery, patient satisfaction scores are beginning to be incorporated as part of the standard-of-care quality metrics obtained along with patient-reported outcome measures (PROMs) at defined time points postoperatively. Furthermore, PROMs and patient satisfaction data are becoming an increasingly important component of medical decision-making.13-16 Several authors have reported that increased patient satisfaction is correlated with increased compliance, improved treatment outcomes across numerous medical settings, including orthopedics, decreased risk of litigation, and higher patient ratings of the quality of care.17,18 Various factors, including meeting of expectations, staff politeness, the communication skills of the surgeon, and waiting times, have been suggested to influence eventual patient satisfaction within the surgical literature.19-21 However, within orthopedic surgery there is a paucity of investigations evaluating how patients determine preferences and satisfaction with the process of care.
The purpose of this study is to determine what preferences, if any, patients have when selecting their physician and how they experience care in an outpatient orthopedic setting. The authors hypothesize that the majority of patients find their physicians through online rating sites or recommendations from family and friends. The authors believe that patients expect to be seen in <30 minutes and will be unsatisfied overall with the amount of time that they spend with their physician.
Continue to: METHODS...
METHODS
The senior author (BRL) and a research team created a 15-question survey to evaluate patient preferences regarding the demographic characteristics (eg, age, gender, ethnicity) of their physician, wait times in a waiting room, time spent with the physician, care received from physician extenders (eg, nurse practitioners, physician assistants), and how they learned of their physician (Appendix). An a priori power analysis was conducted to determine that approximately 200 patients were needed for inclusion.11,22 Following Institutional Review Board approval (ORA 15051104), the survey was administered to 212 patients in a single-surgeon, adult reconstruction clinic. The survey was digitally administered on a touch-screen tablet using an electronic independent third party survey center (SurveyMonkey Inc) devoid of any identifying data. The survey was offered to all patients >21 years of age who were English-speaking and in the common area as patients waiting to be seen, from June 2015 to March 2016. A research assistant approached patients in the waiting room and asked if they would like to participate in a short survey regarding what factors influence the patient-physician relationship from the patient’s perspective.
Appendix 1
- Do you wish to partake in this 3-minute survey?
- Have you had a prior knee or hip replacement?
- What is your age?
- 30-40 years
- 40-50 years
- 50-60 years
- 60-70 years
- 70-80 years
- 80+ years
- What is your gender?
- Which of the following best represents your racial or ethnic heritage?
- African American
- How much time would you like the doctor to spend talking to you on a routine visit?
- 0-5 minutes
- 5-10 minutes
- 10-15 minutes
- 15-20 minutes
- 20-30 minutes
- >30 minutes
- How long is too long to wait to see the doctor?
- 10 minutes
- 20 minutes
- 30 minutes
- 40 minutes
- 50 minutes
- An hour or more
- If you were to only see a physician’s assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care?
- Overall I am satisfied with the time the doctor spends with me.
- If you were to need a major surgery, would you want the physician to tell you what he or she would do if they were in your shoes?
- Would you prefer your doctor to be the same race/ethnicity as you?
- No
- No Preference
- Would you feel more comfortable with a male as opposed to a female orthopedic surgeon?
- Would you feel more comfortable with a female as opposed to a male orthopedic surgeon?
- What age would you like your physician to be?
- 25-35 years old
- 35-45 years old
- 45-55 years old
- 55-65 years old
- 65 years and older
- No preference
- How do you usually find your physician?
- Friends’ recommendations
- Healthcare provider’s recommendations
- Insurance plans
- Online research/ratings
- Other
Descriptive statistics were used to analyze subject demographics and survey responses. Chi-square analyses and multinomial logistic regressions were utilized to compare responses. All statistical analyses were conducted using SPSS version 24.0 software (SPSS Inc). Statistical significance was set at P < 0.05.
RESULTS
Of the 212 patients who were invited to participate, 196 patients (92.4%) agreed and completed the survey. Demographic and surgical history information can be found in Table 1. The majority of patients were female (62%) and above the age of 50 years (92.4%). Almost half (48.5%) of patients had a prior hip or knee replacement.
Table 1. Survey Respondent Demographics
| Number | Percent |
Age Range | ||
30-40 years | 4 | 2.0% |
40-50 years | 11 | 5.6% |
50-60 years | 47 | 24.0% |
60-70 years | 84 | 42.9% |
70-80 years | 41 | 20.9% |
>80 years | 9 | 4.6% |
Gender | ||
Male | 74 | 37.8% |
Female | 122 | 62.2% |
Ethnicity | ||
African American | 39 | 19.9% |
Asian | 3 | 1.5% |
Caucasian | 140 | 71.4% |
Hispanic | 10 | 5.1% |
Other | 4 | 2.0% |
Prior knee or hip replacement | ||
Yes | 95 | 48.5% |
No | 55 | 28.1% |
No Response | 46 | 23.5% |
When asked how long is too long to wait to see the doctor, 30 minutes (39.8%) was most commonly selected, followed by 40 minutes (24.5%) (Figure 1). When asked how much time patients would like the doctor to spend with them during an office visit, the majority (68.9%) selected either 10 to 15 minutes (41.3%) or 15 to 20 minutes (27.6%) (Figure 2). The majority of patients (92.3%) were satisfied with the amount of time the doctor spent with them. In addition, 94.9% of respondents would want their doctor to tell them what they would do if they were in the patient’s shoes when making decisions regarding their medical care (Table 2). Less than half of respondents (41.8%) believe that seeing a physician extender (eg, nurse practitioner or physician assistant) at a postoperative visit would result in a lower quality of care (Table 2).
Table 2. Responses to Survey Questions
If you were to only see a physician's assistant or nurse practitioner at your follow-up visit and not the doctor, would you feel like you were getting below average care? | ||
Answer choices | Number | Percent |
No | 114 | 58.2% |
Yes | 82 | 41.8% |
If you were to need a major surgery would you want the physician to tell you what he or she would do if they were in your shoes? | ||
Answer choices | Number | Percent |
No | 10 | 5.1% |
Yes | 186 | 94.9% |
Would you prefer your doctor to be the same race/ethnicity as you? | ||
Answer choices | Number | Percent |
No | 29 | 14.8% |
Yes | 3 | 1.5% |
No Preference | 164 | 83.7% |
When asked if patients preferred a doctor of the same race/ethnicity, the vast majority (83.7%) had no preference (Table 2). There was no significant difference found between male and female respondents when asked if they would feel more comfortable with a male as opposed to a female orthopedic surgeon (P = .118) and vice versa (P = .604) (Table 3). Most patients preferred a physician between the ages of 45 and 55 years (39.3%), followed by 35 and 45 years (23.0%); however, this preference was not statistically significant (Table 4). Older patients were more likely to prefer younger physicians (odds ratio, 4.612 for 25-35 years of age; odds ratio, 1.328 for 35-45 years of age). Only 12.2% of patients reported online research/rating sites as the main resource utilized when selecting their physician (Figure 3). The majority (68.4%) reported that recommendations from either friends (35.2%) or healthcare providers (33.2%) were the main avenues through which they found their physicians.
Table 3. Overall Responses to Questions Regarding Male and Female Orthopedic Surgeonsa
Would you feel more comfortable with a male as opposed to a female orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 164 | 83.7% | 106 (86.9%) | 58 (78.4%) | 0.118 |
Yes | 32 | 16.3% | 16 (13.1%) | 16 (21.6%) |
|
Would you feel more comfortable with a female as opposed to a male orthopedic surgeon? | |||||
Answer choices | Number | Percent | Female responses | Male responses | P value |
No | 186 | 94.9% | 115 (94.3%) | 71 (95.9%) | 0.604 |
Yes | 10 | 5.1% | 7 (5.7%) | 3 (4.1%) |
|
aResponses were broken down by gender and compared utilizing a 2 x 2 chi-square analysis to test for significant differences in respondents’ gender preferences for their orthopedic surgeon.
Table 4. Patient Preferences Regarding Physician Age
What age would you like your physician to be? |
| 95% Confidence Interval | ||||
Answer Choices | Number or Responses | Percent | P value | Exp(β) | Lower Bound | Upper Bound |
25-35 years | 1 | 0.5% | 0.217 | 4.612 | 0.407 | 52.283 |
35-45 years | 45 | 23.0% | 0.161 | 1.328 | 0.893 | 1.975 |
45-55 years | 77 | 39.3% | 0.159 | 1.276 | 0.909 | 1.791 |
55-65 years | 9 | 4.6% | 0.483 | 1.302 | 0.624 | 2.717 |
≥65 years | 2 | 1.0% | 0.272 | 0.491 | 0.138 | 1.748 |
No preferencea | 62 | 31.6% | Reference | |||
aNo preference was used as the reference category for the answer choices, while the age bracket “>80 years” was used as the reference for the age of respondent variable.
Continue to: DISCUSSION...
DISCUSSION
The results of this study demonstrate that patients have several expectations and preferences with regards to the care they receive from physicians in the office. Patients prefer to wait <30 minutes before seeing their provider and desire only 10 to 20 minutes with their doctor. Patients do not have specific preferences with regards to the gender or ethnicity of their physician but would prefer a physician in the middle of their career, aged 45 to 55 years. Ultimately, patients do believe that seeing a physician at a postoperative visit is important, as just under half of patients thought that seeing a physician extender alone at a postoperative visit resulted in a lower quality of care.
While these results were obtained in a population specifically seeking the care of an orthopedic adult reconstruction surgeon, the results demonstrate that patients do not necessarily desire an unreasonable amount of time with their doctor. Patients simply want to be seen in a timely fashion and receive the full undivided attention of their doctor for approximately 20 minutes. Similarly, Patterson and colleagues22 found, in their series of 182 patients who presented to an orthopedic surgeon, that there was a significant correlation between time spent with the surgeon and overall patient satisfaction. Interestingly, the authors reported that patient satisfaction was not correlated with education level, sex, marital status, whether the patients were evaluated by a resident physician before seeing the attending surgeon, self-reported mental status, tobacco usage, the type of clinic visit, or the waiting time to see the surgeon (average, about 40 minutes for this cohort).22 Similarly, Teunis and colleagues23 reported an average 32-minute wait time in 81 patients presenting for care at an orthopedic hand clinic and demonstrated that a longer wait time was associated with decreased patient satisfaction. These results corroborate the findings of this study that a short wait time is important to patients when evaluating the process of care. Additionally, patients do not have unreasonable expectations with regards to the amount of time they would like to spend with the physician. A physician who has a clinic for 9 hours a day would thus be able to see 54 patients and still spend at least 10 minutes with each patient. The quality of the physician-patient interaction is likely more important than the actual amount of time spent; however, based on this study, patients do have certain expectations about how much time physicians should spend with them.
There were no significant sex, age, or ethnicity preferences in our specific patient cohort. However, a sizable percentage of respondents, 41.8%, believed that they were receiving inferior care if they only saw a physician extender at a routine follow-up visit. Many orthopedic surgeons rely on the care provided by physician extenders to enable them to see additional patients. Physician extenders are well trained to provide high-quality care, including at routine postoperative visits. The results of this study, that many patients believe physician extenders provide lower-quality care, may be a result of inadequate patient education regarding the extensive training and education physician extenders undergo. Physician extenders are qualified, licensed healthcare professionals who are playing increasingly important roles within orthopedics and medicine as a whole. As the demand for orthopedic surgeons to see more patients increases, so does the role of physician extenders. Future research is warranted into educating the public regarding the importance of these healthcare providers and the adequacy of their training.
While many practices now routinely obtain patient satisfaction scores, another modality through which patients can express their satisfaction and experiences with healthcare providers is through online internet physician rating sites (IPRS). These sites have exploded in number and popularity in recent years and, according to some studies, have a very real effect on provider selection.24 Interestingly, a low percentage of patients in this study utilized IPRS reviews to find their doctors. In a recent prospective survey study of 1000 consecutive patients presenting for care at the Mayo Clinic, Burkle and Keegan24 reported that 27% of patients would choose not to see a physician based on a negative IPRS review. Interestingly, only 1.0% of patients reported finding their doctor through advertising. Numerous authors have recently addressed advertising in orthopedic surgery, specifically direct-to-consumer marking, including the influence of physician self-promotion on patients.25,26 Specifically, Halawi and Barsoum26 discussed how direct-to-consumer marketing is commonly disseminated to the public through television and print advertisements, which are modalities more commonly utilized by older generations. However, many advertising agencies are moving toward internet-based advertising, especially through orthopedic group and individual surgeon websites for self-promoting advertisement, as approximately 75% of Americans use the internet for health-related information.25,27 The fact that many patients in this study did not utilize IPRS reviews or advertising (much of which is electronic) may be a result of the older, less internet-centric demographic that is often seen in an adult reconstruction clinic. Future research is warranted to determine what demographic of patients value IPRS reviews and how those reviews influence physician selection and the patient experience.
There are several limitations to this study. First, the majority of the surveyed population was Caucasian, and our results may not be equally reflective of diverse ethnic backgrounds. Second, the cohort size, while based on previous studies conducted in a similar fashion, may be underpowered to detect significant differences for 1 or more of these questions. In addition, having a question regarding the patient’s medical background or experiences may have provided further insight as to why patients selected the answers that they did. Furthermore, questions regarding the patient’s education level, religious background, and income brackets may have provided further context in which to evaluate their responses. These questions were omitted in an effort to keep the questionnaire at a length that would maximize enrollment and prevent survey fatigue. Future research is warranted to determine what patient-specific, injury/symptom-specific, and treatment-specific variables influence the subjective patient experience.
CONCLUSION
The vast majority of patients desire only 10 to 20 minutes with their doctor and are highly satisfied with the amount of time their surgeon spends with them. Patients reported no significant gender- or ethnicity-based preferences for their doctor. The majority of patients believe that a wait time exceeding 30 minutes is too long. A greater effort needs to be made to educate patients and the public about the significant and effective roles nurse practitioners and physician assistants can play within the healthcare system. While this cohort did not report notable utilization of IPRS reviews, it remains essential to understand what factors influence patients’ subjective experiences with their providers to ensure that patients achieve their desired outcomes, and report as such on these websites as they continue to gain popularity. Diminishing clinic wait times and understanding patient preferences may lead to a greater percentage of “satisfied” patients. While the majority of focus has been and will likely continue to be on improving patients’ satisfaction with their outcomes, more work needs to be done focusing specifically on the process through which outcomes are achieved.
1. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84–A(9):1560-1572.
2. Carr-Hill RA. The measurement of patient satisfaction. J Public Health Med. 1992;14(3):236-249.
3. Ross CK, Steward CA, Sinacore JM. A comparative study of seven measures of patient satisfaction. Med Care. 1995;33(4):392-406. doi:10.1097/00005650-199504000-00006.
4. Strasser S, Aharony L, Greenberger D. The patient satisfaction process: moving toward a comprehensive model. Med Care Rev. 1993;50(2):219-248. doi:10.1177/107755879305000205.
5. Bozic KJ. Orthopaedic healthcare worldwide: shared medical decision making in orthopaedics. Clin Orthop Relat Res. 2013;471(5):1412-1414. doi:10.1007/s11999-013-2838-5.
6. Youm J, Chenok KE, Belkora J, Chiu V, Bozic KJ. The emerging case for shared decision making in orthopaedics. Instr Course Lect. 2013;62:587-594. doi:10.2106/00004623-201210170-00011.
7. Blumenthal D, Abrams M, Nuzum R. The affordable CARE Act at 5 years. N Engl J Med. 2015;373(16):1580. doi:10.1056/NEJMc1510015.
8. Shirley ED, Sanders JO. Patient satisfaction: implications and predictors of success. J Bone Joint Surg Am. 2013;95(10):e69. doi:10.2106/JBJS.L.01048.
9. Morris BJ, Jahangir AA, Sethi MK. Patient satisfaction: an emerging health policy issue. AAOS Now Web site. http://www.aaos.org/AAOSNow/2013/Jun/advocacy/advocacy5/?ssopc=1. Published June 2013. Accessed November 19, 2016.
10. Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743-1748. doi:10.1001/jama.260.12.1743.
11. Bozic KJ, Kaufman D, Chan VC, Caminiti S, Lewis C. Factors that influence provider selection for elective total joint arthroplasty. Clin Orthop Relat Res. 2013;471(6):1865-1872. doi:10.1007/s11999-012-2640-9.
12. Davies AR, Ware JE Jr. Involving consumers in quality of care assessment. Health Aff (Millwood). 1988;7(1):33-48.
13. Black N, Burke L, Forrest CB, et al. Patient-reported outcomes: pathways to better health, better services, and better societies. Qual Life Res. 2016;25(5):1103-1112. doi:10.1007/s11136-015-1168-3.
14. Gilbert A, Sebag-Montefiore D, Davidson S, Velikova G. Use of patient-reported outcomes to measure symptoms and health related quality of life in the clinic. Gynecol Oncol. 2015;136(3):429-439. doi:10.1016/j.ygyno.2014.11.071.
15. Van Der Wees PJ, Nijhuis-Van Der Sanden MW, Ayanian JZ, Black N, Westert GP, Schneider EC. Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q. 2014;92(4):754-775. doi:10.1111/1468-0009.12091.
16. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. Total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(Suppl 1):104-109. doi:10.2106/JBJS.N.00328.
17. Williams B. Patient satisfaction: a valid concept? Soc Sci Med. 1994;38(4):509-516. doi:10.1016/0277-9536(94)90247-X.
18. Hickson GB, Clayton EW, Entman SS, et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583-1587. doi:10.1001/jama.1994.03520200039032.
19. Larsson BW, Larsson G, Chantereau MW, von Holstein KS. International comparisons of patients' views on quality of care. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2005;18(1):62-73. doi:10.1108/09526860510576974.
20. McLafferty RB, Williams RG, Lambert AD, Dunnington GL. Surgeon communication behaviors that lead patients to not recommend the surgeon to family members or friends: analysis and impact. Surgery. 2006;140(4):616-624. doi:https://doi.org/10.1016/j.surg.2006.06.021.
21. Mira JJ, Tomás O, Virtudes-Pérez M, Nebot C, Rodríguez-Marín J. Predictors of patient satisfaction in surgery. Surgery. 2009;145(5):536-541. doi:10.1016/j.surg.2009.01.012.
22. Patterson BM, Eskildsen SM, Clement RC, et al. Patient satisfaction is associated with time with provider but not clinic wait time among orthopedic patients. Orthopedics. 2017;40(1):43-48. doi:10.3928/01477447-20161013-05.
23. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated With patient satisfaction. Clin Orthop Relat Res. 2015;473(7):2362-2368. doi:10.1007/s11999-014-4090-z.
24. Burkle CM, Keegan MT. Popularity of internet physician rating sites and their apparent influence on patients' choices of physicians. BMC Health Serv Res. 2015;15:416. doi:10.1186/s12913-015-1099-2.
25. Mohney S, Lee DJ, Elfar JC. The effect of orthopedic advertising and self-promotion on a naive population. Am J Orthop. 2016;45(4):E227-E232.
26. Halawi MJ, Barsoum WK. Direct-to-consumer marketing: implications for patient care and orthopedic education. Am J Orthop. 2016;45(6):E335-E336.
27. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156. doi:10.1007/s11606-010-1425-7.
1. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84–A(9):1560-1572.
2. Carr-Hill RA. The measurement of patient satisfaction. J Public Health Med. 1992;14(3):236-249.
3. Ross CK, Steward CA, Sinacore JM. A comparative study of seven measures of patient satisfaction. Med Care. 1995;33(4):392-406. doi:10.1097/00005650-199504000-00006.
4. Strasser S, Aharony L, Greenberger D. The patient satisfaction process: moving toward a comprehensive model. Med Care Rev. 1993;50(2):219-248. doi:10.1177/107755879305000205.
5. Bozic KJ. Orthopaedic healthcare worldwide: shared medical decision making in orthopaedics. Clin Orthop Relat Res. 2013;471(5):1412-1414. doi:10.1007/s11999-013-2838-5.
6. Youm J, Chenok KE, Belkora J, Chiu V, Bozic KJ. The emerging case for shared decision making in orthopaedics. Instr Course Lect. 2013;62:587-594. doi:10.2106/00004623-201210170-00011.
7. Blumenthal D, Abrams M, Nuzum R. The affordable CARE Act at 5 years. N Engl J Med. 2015;373(16):1580. doi:10.1056/NEJMc1510015.
8. Shirley ED, Sanders JO. Patient satisfaction: implications and predictors of success. J Bone Joint Surg Am. 2013;95(10):e69. doi:10.2106/JBJS.L.01048.
9. Morris BJ, Jahangir AA, Sethi MK. Patient satisfaction: an emerging health policy issue. AAOS Now Web site. http://www.aaos.org/AAOSNow/2013/Jun/advocacy/advocacy5/?ssopc=1. Published June 2013. Accessed November 19, 2016.
10. Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260(12):1743-1748. doi:10.1001/jama.260.12.1743.
11. Bozic KJ, Kaufman D, Chan VC, Caminiti S, Lewis C. Factors that influence provider selection for elective total joint arthroplasty. Clin Orthop Relat Res. 2013;471(6):1865-1872. doi:10.1007/s11999-012-2640-9.
12. Davies AR, Ware JE Jr. Involving consumers in quality of care assessment. Health Aff (Millwood). 1988;7(1):33-48.
13. Black N, Burke L, Forrest CB, et al. Patient-reported outcomes: pathways to better health, better services, and better societies. Qual Life Res. 2016;25(5):1103-1112. doi:10.1007/s11136-015-1168-3.
14. Gilbert A, Sebag-Montefiore D, Davidson S, Velikova G. Use of patient-reported outcomes to measure symptoms and health related quality of life in the clinic. Gynecol Oncol. 2015;136(3):429-439. doi:10.1016/j.ygyno.2014.11.071.
15. Van Der Wees PJ, Nijhuis-Van Der Sanden MW, Ayanian JZ, Black N, Westert GP, Schneider EC. Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q. 2014;92(4):754-775. doi:10.1111/1468-0009.12091.
16. Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. Total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(Suppl 1):104-109. doi:10.2106/JBJS.N.00328.
17. Williams B. Patient satisfaction: a valid concept? Soc Sci Med. 1994;38(4):509-516. doi:10.1016/0277-9536(94)90247-X.
18. Hickson GB, Clayton EW, Entman SS, et al. Obstetricians' prior malpractice experience and patients' satisfaction with care. JAMA. 1994;272(20):1583-1587. doi:10.1001/jama.1994.03520200039032.
19. Larsson BW, Larsson G, Chantereau MW, von Holstein KS. International comparisons of patients' views on quality of care. Int J Health Care Qual Assur Inc Leadersh Health Serv. 2005;18(1):62-73. doi:10.1108/09526860510576974.
20. McLafferty RB, Williams RG, Lambert AD, Dunnington GL. Surgeon communication behaviors that lead patients to not recommend the surgeon to family members or friends: analysis and impact. Surgery. 2006;140(4):616-624. doi:https://doi.org/10.1016/j.surg.2006.06.021.
21. Mira JJ, Tomás O, Virtudes-Pérez M, Nebot C, Rodríguez-Marín J. Predictors of patient satisfaction in surgery. Surgery. 2009;145(5):536-541. doi:10.1016/j.surg.2009.01.012.
22. Patterson BM, Eskildsen SM, Clement RC, et al. Patient satisfaction is associated with time with provider but not clinic wait time among orthopedic patients. Orthopedics. 2017;40(1):43-48. doi:10.3928/01477447-20161013-05.
23. Teunis T, Thornton ER, Jayakumar P, Ring D. Time seeing a hand surgeon is not associated With patient satisfaction. Clin Orthop Relat Res. 2015;473(7):2362-2368. doi:10.1007/s11999-014-4090-z.
24. Burkle CM, Keegan MT. Popularity of internet physician rating sites and their apparent influence on patients' choices of physicians. BMC Health Serv Res. 2015;15:416. doi:10.1186/s12913-015-1099-2.
25. Mohney S, Lee DJ, Elfar JC. The effect of orthopedic advertising and self-promotion on a naive population. Am J Orthop. 2016;45(4):E227-E232.
26. Halawi MJ, Barsoum WK. Direct-to-consumer marketing: implications for patient care and orthopedic education. Am J Orthop. 2016;45(6):E335-E336.
27. Mostaghimi A, Crotty BH, Landon BE. The availability and nature of physician information on the internet. J Gen Intern Med. 2010;25(11):1152-1156. doi:10.1007/s11606-010-1425-7.
TAKE-HOME POINTS
- The vast majority of patients desire only 10 to 20 minutes with their doctor and are highly satisfied with the amount of time their surgeon spends with them.
- Patients reported no significant gender- or ethnicity-based preferences for their doctor.
- The majority of patients believe that a wait time exceeding 30 minutes is too long.
- Nearly 42% of respondents felt they would be receiving below average medical care if seen only by a nurse practitioner or physician’s assistant at a postoperative appointment.
- Recommendations from friends is the most common way patients find their physicians.
Hip and Core Muscle Injuries in Soccer
ABSTRACT
Soccer is the most popular sport in the world and has the fourth highest number of sports injuries. Hip and groin injuries account for 14% of soccer injuries and can be difficult to recognize and treat as they often require a high level of suspicion and advanced imaging. Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain. Conservative approaches are typically the first line of treatment, but operative intervention has been reported to result in higher rates of return to sport in athletes with hip-related and inguinal-related groin pain injuries. In patients with concurrent hip-related and inguinal-related groin pain, the failure to recognize the relationship and treat both conditions may result in lower rates of return to sport. Preseason screening programs can identify high-risk athletes, who may benefit from a targeted prevention program. Further study on exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
Continue to: Each year, the global audience for soccer grows...
Each year, the global audience for soccer grows. Soccer has long surpassed all other sports as the most popular sport in the world, reaching 3.2 billion viewers during the 2014 World Cup.1 In the latest Fédération Internationale de Football Association (FIFA) Big Count survey, the organization estimated that 265 million people are actively involved in soccer, accounting for approximately 4% of the world’s population.2 Moreover, the number of people playing soccer increased by 9.5% within 6 years after the previous Big Count Survey.2 In the United States, soccer accounts for the fourth most common cause of sports injuries next to basketball, exercise, and football with approximately 228,000 injuries per year.3 The total cost of treatment related to worldwide soccer injuries tops $30 billion.4 The most common body parts injured are the thigh (25%), knee (18%), and hip and/or groin (14%).5
Hip and groin injuries in soccer players can be separated into 3 main categories based on the Doha Agreement:6 (1) defined clinical entities for groin pain, (2) hip-related groin pain, and (3) other causes of groin pain in athletes. Defined clinical entities include adductor-related, iliopsoas-related, inguinal-related (sports hernia/athletic pubalgia), and pubic-related groin pain; while hip-related groin pain includes hip morphologic abnormalities, labral tears, and chondral injuries. Included in other causes of groin pain are injuries not clinically defined. The Doha Agreement has acknowledged that not all causes of groin pain fit into the classification system including injuries of the rectus femoris, but they will be included under defined clinical entities for groin pain in this review. While they are not a cause of groin pain, proximal hamstring and gluteal and piriformis injuries are important causes of posterior and lateral hip pain in soccer players and will also be covered in the first section of this review.
DEFINED CLINICAL ENTITIES FOR GROIN PAIN IN SOCCER ATHLETES
ADDUCTOR-RELATED GROIN PAIN
Acute groin pain in soccer players is most commonly caused by muscle strain.7 Of the muscle strains, 66% involve the adductor longus, 25% the iliopsoas, and 23% the rectus femoris.7 The Doha Agreement defines adductor-related groin pain as adductor tenderness and pain on resisted adduction.6 Adductor longus strains in soccer players are typically noncontact injuries (62.5%) and most commonly the result of kicking (40%).7-9 Many athletes will remember a pop at the time of the original injury.9 The combination of history and physical examination is usually sufficient for diagnosis; however, magnetic resonance imaging (MRI) may be helpful in complicated situations with a reported 86% sensitivity and 89% specificity.10 The average playing time lost is 2 weeks.5 Management includes rest, anti-inflammatory medication, physical therapy with core strengthening, and avoidance of aggressive stretching. While partial and distal avulsions can heal with conservative measures, proximal osseous and retracted avulsions of the adductor longus can be treated surgically.11
Continue to: ILIOPSOAS-RELATED GROIN PAIN...
ILIOPSOAS-RELATED GROIN PAIN
Iliopsoas strains account for 25% of acute groin strains and typically result from an impact that causes eccentric overload while kicking the ball.7,12 Iliopsoas-related groin pain is defined by the Doha Agreement as groin pain that is reproducible with resisted hip flexion or hip flexor stretch.6 Iliopsoas strains respond well to conservative treatment such as rest, anti-inflammatory medication, and physical therapy. Rarely do these athletes become surgical candidates in the acute setting. Chronic cases of iliopsoas pathology occasionally require an arthroscopic intervention.
INGUINAL-RELATED GROIN PAIN
Inguinal-related groin pain is one of the most misleading diagnoses in sports because of its poorly defined and under-researched nature. The varying nomenclature of this entity illustrates the heterogeneity and includes sports hernia,9,13-15 athletic pubalgia,16 core muscle injury,17 athletic hernia,18 Gilmore’s groin,15 osteitis pubis,19 sportsman’s hernia,20,21 sportsmen’s groin,22 symphysis syndrome,23 and inguinal disruption.24 It is important to realize that in inguinal-related groin pain, regardless of the nomenclature, there is no true hernia present. The Doha Agreement has defined inguinal-related groin pain as “pain in the location of the inguinal region with associated tenderness of the inguinal canal,” which “is more likely if the pain is aggravated with resistance testing of the abdominal muscles or on Valsalva/cough/sneeze.”6 The condition is a painful soft tissue injury in the groin or inguinal area, involving a constellation of various anatomic areas including the abdominal musculature, sacroiliac joint, neural structures, pubic symphysis, adductors, and hip joint. This may account for up to 50% of chronic groin pain.25,26
One important theory in the development of inguinal-related groin pain is its relationship with femoroacetabular impingement (FAI). Cadaver studies demonstrate that cam deformities cause a 35% increase in motion at the pubic symphysis altering the biomechanics of the adductors and abdominal musculature and, with repetitive stress, may lead to tearing or attenuation of the transversalis fascia, rectus abdominis, internal obliques, and/or external obliques.12,27,28 Another prevailing theory of this is that the increased pubic stress causes weakness in the posterior portion of the inguinal canal, which then stretches and entraps the genitofemoral, ilioinguinal, lateral femoral cutaneous, or obturator nerves, ultimately causing pain.28,29
Physical examination findings include pain over the conjoined tendon, pubic tubercle/symphysis (present in 22% of patients), adductor origin (36%), and inguinal ring.25,30 Pain with resisted sit-ups is present in 46% of patients and pain with coughing/Valsalva is present in 10%.25,30,31 Selective injections can be a critical part of the evaluation to differentiate inguinal-related groin pain from FAI, osteitis pubis, and adductor strains while helping to determine the appropriate treatment.25,32 The role of advanced imaging is unclear as the clinical entity is still uncertain and the standard imaging findings have not been definitively established.33 However, several studies have reported MRI findings suggestive of inguinal-related groin pain. One of the more common MRI findings is the “secondary cleft sign,” which requires injecting a dye into the pubic symphysis.34 Several studies have shown that the radiographic dye extravasates preferentially into the side where the groin symptoms exist and are thought to be secondary to micro-tearing at the common attachment of the musculotendinous structures to the anterior pubis.34,35 However, it should be noted that the lack of imaging findings does not exclude the possibility of inguinal-related groin pathology.
Initial treatment consists of rest, anti-inflammatory medication, injections, and physical therapy with core strengthening.25 A study by Paajanen and colleagues36 suggested that early surgical intervention may be preferred over conservative management in a randomized trial comparing physical therapy, injections, anti-inflammatory medication, and rest vs an extraperitoneal laparoscopic mesh repair behind the pubic symphysis. In the conservative group, 20% of athletes returned to sport at 1 month, 27% at 3 months, and 50% at 12 months.36 In comparison, the surgical group had 67% return to sport at 1 month, 90% at 3 months, and 97% at 12 months.36 If surgical management is chosen, there are a variety of surgical options including laparoscopy, open or mini-open repairs of the abdominal musculature/fascia or pelvic floor with and without mesh, neurolysis, and adductor release. Muschawek and Berger37described a series of 129 patients that had an open-suture repair of the posterior wall of the inguinal canal with 67% of professional athletes returning to sport within 2 weeks and 83.7% of athletes returning to sport overall. The rates of return to play are consistently 80% to 100% without demonstrated superiority of one technique over another up to this point.30
Continue to: PUBIC-RELATED GROIN PAIN...
PUBIC-RELATED GROIN PAIN
Pubic-related groin pain is defined as tenderness to palpation over the pubic symphysis and adjacent bone.6 Osteitis pubis is a chronic overuse injury characterized by localized pain to the pubic symphysis and is believed to be caused by repetitive microtrauma from a dynamic rotation of the sacroiliac joint with suggested imbalances between the rectus abdominis and the adductor musculature.12,38 In soccer players, the condition may be related to the constant torsional stresses of kicking, running, or twisting.12 If performed, radiographs often show lytic areas of the pubic symphysis, widening of the symphysis, sclerosis, and cystic changes, while bone marrow edema may be present on MRI.38Management consists of rest, anti-inflammatory medication, and corticosteroid injections with gentle stretching once asymptomatic.12,39
RECTUS FEMORIS INJURIES
The most common injury to the rectus femoris is a strain as a result of an eccentric overload while a soccer player is hit trying to extend his or her leg to kick a ball.12 In pediatric soccer athletes, an avulsion of the anterior inferior iliac spine from the direct head of the rectus femoris is the second most common avulsion injury.40 Radiographs are diagnostic and can help determine treatment. Most avulsions are minimally displaced and can be treated conservatively, but surgical intervention should be considered for an avulsion >2 cm.12
PROXIMAL HAMSTRING INJURIES
Proximal hamstring injuries are important causes of acute posterior hip pain and are caused by an eccentric overload in hip flexion and knee extension.25 In soccer players, the typical mechanism is that the planted leg slipping on the playing turf creates a sudden violent flexion of the hip with the knee in an extended position. While relatively uncommon, when a significant avulsion occurs in a professional athlete, surgical intervention is often necessary. In general, these injuries may involve partial or full avulsions off the ischial tuberosity or separation of the bony apophysis in pediatric athletes. A physical examination in the acute setting typically demonstrates massive posterior thigh ecchymosis, a palpable defect, and/or weakness with knee flexion. Imaging is helpful to confirm the diagnosis and evaluate for surgical repair. Radiographs may show a bony avulsion, which is more commonly seen in pediatric apophyseal avulsions. MRI can be used to differentiate a complete tear (involving all 3 tendons) vs a partial tear and evaluate for retraction of the tendon distally. Complete and partial tears of 2 tendons with retraction of >2 cm should be surgically repaired.25 Partial tears without tendon retraction may be treated conservatively with rest, anti-inflammatory medication, and physical therapy and then followed later by a hamstring prevention program.25 We have found that biologic augmentation with platelet-rich plasma can help accelerate healing in partial thickness injuries; however, the evidence is conflicting.
GLUTEAL INJURIES
Chronic overuse injuries of the gluteal musculature are common causes of lateral hip pain. Abductor overuse caused by weakness in the gluteus medius with a normal tensor fascia lata can cause pain with sitting and side-lying.25Overuse of the gluteal muscles with muscular imbalances along with increased tension on the iliotibial band can lead to greater trochanteric pain syndrome.25 A physical examination may demonstrate tenderness over the greater trochanter bursa and positive flexion, abduction, and external rotation testing.25 Abductor overuse syndrome and greater trochanteric pain syndrome are best treated with anti-inflammatory medication and physical therapy to balance the core/pelvic musculature.41
PIRIFORMIS INJURIES
Piriformis syndrome is a compressive neuropathy of the sciatic nerve. The mechanism of injury in the athlete is through a minor trauma to the buttock or pelvis.25,42,43 Presenting symptoms include pain with sitting and internal rotation of the hip.12 Zeren and colleagues42 published the only study that includes 2 cases of bilateral piriformis syndrome in professional soccer players. The diagnosis was confirmed with electromyography that was negative at rest and positive when measured after running.42 The athletes exhausted conservative treatment with physical therapy, anti-inflammatory medications, injections, and rest and were treated with surgical decompression.42 Both players returned to professional soccer after 6 months and played for an average of 7 years.42
Continue to: HIP-RELATED GROIN PAIN IN SOCCER ATHLETES...
HIP-RELATED GROIN PAIN IN SOCCER ATHLETES
Hip-related groin pain has garnered more attention in the last several years after being a previously underdiagnosed entity. One study found that practitioners treated groin pain in athletes for 7 months on average before recognizing that the pathology was intra-articular.44 FAI, labral tears, and chondral injuries are the major intra-articular pathologies that cause groin pain in athletes and ultimately impaired performance.45,46
FEMOROACETABULAR IMPINGEMENT
FAI is caused by pincer-type, cam-type, or combined-type deformities. Pincer lesions are defined as an increased acetabular overhang, while cam lesions are described as an increased bone at the femoral head/neck junction. These deformities in isolation or in combination cause decreased hip motion and increased contact pressures between the anterolateral acetabulum and femoral head-neck junction, which may ultimately lead to labral tears, chondral lesions, and osteoarthritis.47 During hip flexion, cam deformities impact the anterolateral acetabulum, preferentially causing articular cartilage damage, while sparing the labrum.25 Conversely, pincer deformities cause repetitive microtrauma to the labrum, crushing it between the acetabular rim and femoral neck with secondary damage to the articular cartilage.25 Over time, the damage to the labrum and articular cartilage may lead to premature osteoarthritis, which occurs at a much younger age in the athletic population.48
We know from previous studies that soccer athletes have a high prevalence of morphologic abnormalities of the hip, most commonly FAI. Gerhardt and colleagues49 documented the prevalence of hip morphologic abnormalities in elite soccer players and found abnormalities in 72% of men and over 50% of women. It should be noted that this series looked at asymptomatic athletes; however, it has been shown that hip dysmorphia is a risk factor for hip and groin injuries and may provide an opportunity for injury prevention strategies.50
Physical examination findings in FAI include decreased hip internal rotation and pain with provocative testing. Wyss and colleagues51 measured hip internal rotation in athletes with and without FAI. They found that the athletes with FAI have an average of 4° of internal rotation compared with that of the non-FAI athletes with 28°.51 A worsening internal rotation deficit has been linked to increasing severity of the deformity and when <20° was correlated with joint damage.51 Provocative testing has a high sensitivity with a recent meta-analysis demonstrating the most sensitive tests to be the anterior impingement test (flexion-adduction-internal rotation) with 94% to 99% sensitivity and the flexion-internal rotation test with 96% sensitivity.52 While provocative tests are sensitive, there is no current consensus on physical examination findings that are specific in the diagnosis of FAI.6 Diagnosis is made with both positive physical examination and radiographic morphologic findings (alpha angle >55°).33 Advanced imaging with an MRI arthrogram can be helpful in diagnosing underlying injuries such as labral tears in athletes presenting with compatible symptoms.
Symptomatic patients are typically treated surgically through either open or arthroscopic procedures, which have favorable and comparable functional results, biomechanics, and return to sport.53 In soccer players, return to sport at the professional level after arthroscopic surgery was found to be 96%.54 Players returned to sport on average 9.2 months postoperatively and played an average of 70 games after surgery.54
Continue to: LABRAL TEARS...
LABRAL TEARS
Labral tears present with groin pain, limited hip range of motion, and symptoms of catching, locking, and instability.25Causes of labral tears include trauma, FAI, hip dysplasia, capsular laxity, and degeneration.55 Labral tears rarely occur in isolation and have a high association (87%) with morphologic abnormalities of the hip, most commonly FAI and occasionally dysplasia.56,57 Physical examination findings include positive anterior impingement tests (flexion-adduction-internal rotation) in athletes with anterior labral tears and, less commonly, positive flexion, abduction, and external rotation tests for athletes with lateral and posterolateral labral tears.57 Radiographic imaging is used to evaluate for concurrent morphologic abnormalities of the hip, and MRI arthrogram is used to confirm the diagnosis of a labral tear with a sensitivity of 76% to 91%.58 Initial treatment consists of conservative treatment, which includes rest, anti-inflammatory medication, activity modification, and physical therapy. In patient refractory to conservative treatment, arthroscopic surgery is effective with high rates of return to sport.59 It is important to note that when treating labral tears surgically, any morphologic abnormality needs to be addressed to prevent recurrence of the tear.
CHONDRAL INJURIES
Focal chondral lesions in the hip are commonly found in athletes with FAI and labral tears during arthroscopic evaluation.60 Full-thickness defects and unstable flaps in weight-bearing areas are indications for surgical intervention with microfracture.60 There are no studies examining the efficacy of microfracture in isolation; however, Locks and colleagues54 have demonstrated a 96% return to professional soccer after an arthroscopic treatment for FAI and found that severe chondral damage with microfracture did not lengthen the return to sport.
RELATIONSHIP BETWEEN INGUINAL-RELATED GROIN PAIN AND FEMOROACETABULAR IMPINGEMENT
The altered biomechanics and restricted range of motion in athletes with FAI cause an increase in compensatory motion at the pelvis and lumbosacral areas, which may contribute to the development of inguinal-related groin pain, bursitis, adductor, and gluteal dysfunction.25 In athletes with concurrent intra-articular hip pathology and inguinal-related groin pain, treating 1 condition in isolation will result in poor results. Larson and colleagues61 found that when only inguinal-related groin pain or FAI were addressed, return to sport was only 25% and 50%, respectively, while concurrent surgical treatment resulted in a return to sport of 89%.
DISCUSSION AND FUTURE DIRECTIONS
Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need a surgical intervention. Efforts are underway to determine the role and efficacy of identifying high-risk athletes that may benefit from targeted prevention strategies. Wyles and colleagues48 identified adolescent athletes with hip internal rotation of <10° and found at 5-year follow-up that 95% had abnormal MRI findings compared with 54% in the age-matched control group. Wollin and colleagues62 developed an in-season screening protocol using adductor strength reductions of 15%, adductor/abductor strength ratio <0.9, and hip and groin outcome scores <75 as indicators of at-risk individuals. By employing preseason and in-season screening protocols, we can identify high-risk athletes for further workup and close follow-up throughout the season. Pelvic radiographs in these high-risk athletes may help us determine the presence of abnormalities in hip morphology, which would place an athlete into a high-risk group where prevention strategies could then be employed. There are no data available to determine the most effective prevention strategy at this time. However, levels II and III evidence exists indicating that exercise programs may reduce the incidence of groin injuries.63 Additional strategies, like limiting adolescent playing time similar to strategies employed in baseball pitches with pitch counts, could potentially reduce the potential for injury. Further studies on preseason screening and in-season monitoring protocols, targeted exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
1. Kantar Media. 2014 FIFA World Cup Brazil television audience report. https://resources.fifa.com/mm/document/affederation/tv/02/74/55/57/2014f...(draft5)(issuedate14.12.15)_neutral.pdf. Accessed March 20, 2018.
2. Fédération Internationale de Football Association. FIFA Big Count. http://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Published July 2007. Accessed March 20, 2018.
3. United States Consumer Product Safety Commission. Neiss data highlights - 2015. https://www.cpsc.gov/s3fs-public/2015 Neiss data highlights.pdf. Accessed March 20, 2018.
4. Hassabi M, Mohammad-Javad Mortazavi S, Giti MR, Hassabi M, Mansournia MA, Shapouran S. Injury profile of a professional soccer team in the premier league of Iran. Asian J Sports Med. 2010;1(4):201-208.
5. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558.
6. Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med. 2015;49(12):768-774.
7. Serner A, Tol JL, Jomaah N, et al. Diagnosis of acute groin injuries: a prospective study of 110 athletes. Am J Sports Med. 2015;43(8):1857-1864. doi:10.1177/0363546515585123.
8. Eckard TG, Padua DA, Dompier TP, Dalton SL, Thorborg K, Kerr ZY. Epidemiology of hip flexor and hip adductor strains in national collegiate athletic association athletes, 2009/2010-2014/2015. Am J Sports Med. 2017;45(12):2713-2722. doi:10.1177/0363546517716179.
9. Hopkins JN, Brown W, Lee CA. Sports hernia: definition, evaluation, and treatment. JBJS Rev. 2017;5(9):e6. doi:10.2106/JBJS.RVW.17.00022.
10. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438. doi:10.1148/rg.285075217.
11. Vogt S, Ansah P, Imhoff AB. Complete osseous avulsion of the adductor longus muscle: acute repair with three Wberwire suture anchors. Arch Orthop Trauma Surg. 2007;127:613-615. doi:10.1007/s00402-007-0328-5.
12. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533. doi:10.1177/03635465010290042501.
13. Choi HR, Elattar O, Dills VD, Busconi B. Return to play after sports hernia surgery. Clin Sports Med. 2016;35(4):621-636. doi:10.1016/j.csm.2016.05.007.
14. Garvey JF, Hazard H. Sports hernia or groin disruption injury? Chronic athletic groin pain: a retrospective study of 100 patients with long-term follow-up. Hernia. 2014;18(6):815-823. doi:10.1007/s10029-013-1161-0.
15. Gilmore J. Groin pain in the soccer athlete: fact, fiction, and treatment. Clin Sports Med. 1998;17(4):787-793, vii. doi:10.1016/S0278-5919(05)70119-8.
16. Cohen B, Kleinhenz D, Schiller J, Tabaddor R. Understanding athletic pubalgia: a review. R I Med J. 2016;99(10):31-35.
17. Ross JR, Stone RM, Larson CM. Core muscle injury/sports hernia/athletic pubalgia, and femoroacetabular impingement. Sports Med Arthrosc Rev. 2015;23(4):213-220. doi:10.1097/JSA.0000000000000083.
18. Swan KG Jr, Wolcott M. The athletic hernia: a systematic review. Clin Orthop Relat Res. 2007;455:78-87. doi:10.1097/BLO.0b013e31802eb3ea.
19. Matikainen M, Hermunen H, Paajanen H. Athletic pubalgia in females: predictive value of MRI in outcomes of endoscopic surgery. Orthop J Sports Med. 2017;5(8):2325967117720171. doi:10.1177/2325967117720171.
20. Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14(1):17-25. doi:10.1007/s10029-009-0611-1.
21. Paksoy M, Sekmen U. Sportsman hernia; the review of current diagnosis and treatment modalities. Ulusal Cerrahi Derg. 2016;32(2):122-129. doi:10.5152/UCD.2015.3132.
22. Pokorny H, Resinger C, Fischer I, et al. Fast early recovery after transabdominal preperitoneal repair in athletes with sportsman's groin: a prospective clinical cohort study. J Laparoendosc Adv Surg Tech A. 2017;27(3):272-276. doi:10.1089/lap.2016.0188.
23. Biedert RM, Warnke K, Meyer S. Symphysis syndrome in athletes: surgical treatment for chronic lower abdominal, groin, and adductor pain in athletes. Clin J Sport Med. 2003;13(5):278-284.
24. Sheen AJ, Stephenson BM, Lloyd DM, et al. 'Treatment of the sportsman's groin': British Hernia Society's 2014 position statement based on the Manchester Consensus Conference. Br J Sports Med. 2014;48(14):1079-1087.
25. Miller M, Thompson S. DeLee & Drez's Orthopaedic Sports Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
26. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. J Sci Med Sport. 1995;27:76-79.
27. Dimitrakopoulou A, Schilders E. Sportsman's hernia? An ambiguous term. J Hip Preserv Surg. 2016;3(1):16-22. doi:10.1093/jhps/hnv083.
28. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6. doi:10.3389/fsurg.2016.00006.
29. Muschaweck U, Berger LM. Sportsmen's groin-diagnostic approach and treatment with the minimal repair technique: a single-center uncontrolled clinical review. Sports Health. 2010;2(3):216-221. doi:10.1177/1941738110367623.
30. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144. doi:10.1177/1941738114523557.
31. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. Am J Sports Med. 2000; 28(1):2-8. doi:10.1177/03635465000280011501.
32. Gerhardt MB, Mandelbaum BR, Hutchinson WB. Ancillary modalities in the treatment of athletic groin Pain: Local Anesthetics, Corticosteroids, and Orthobiologics. In: Diduch DR, Brunt LM, eds. Sports Hernia and Athletic Pubalgia: Diagnosis and Treatment. Boston, MA: Springer US; 2014:183-187.
33. Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
34. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167. doi:10.1148/radiol.2351040045.
35. Byrne CA, Bowden DJ, Alkhayat A, Kavanagh EC, Eustace SJ. Sports-related groin pain secondary to symphysis pubis disorders: correlation between MRI findings and outcome after fluoroscopy-guided injection of steroid and local anesthetic. Am J Roentgenol. 2017;209(2):380-388. doi:10.2214/AJR.16.17578.
36. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: a randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman's hernia (athletic pubalgia). Surgery. 2011;150(1):99-107. doi:10.1016/j.surg.2011.02.016.
37. Muschaweck U, Berger L. Minimal repair technique of sportsmen's groin: an innovative open-suture repair to treat chronic inguinal pain. Hernia. 2010;14(1):27-33. doi:10.1007/s10029-009-0614-y.
38. Lynch TS, Bedi A, Larson CM. Athletic hip injuries. J Am Acad Orthop Surg. 2017;25(4):269-279. doi:10.5435/JAAOS-D-16-00171.
39. Holt MA, Keene JS, Graf BK, Helwig DC. Treatment of osteitis pubis in athletes. Results of corticosteroid injections. Am J Sports Med. 1995;23(5):601-606.doi:10.1177/036354659502300515.
40. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30(3):127-131. doi: 10.1007/s002560000319.
41. Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829.
42. Zeren B, Canbek U, Oztekin HH, Imerci A, Akgun U. Bilateral piriformis syndrome in two elite soccer players: report of two cases. Orthop Traumatol Surg Res. 2015;101(8):987-990. doi:10.1016/j.otsr.2015.07.022.
43. Keskula DR, Tamburello M. Conservative management of piriformis syndrome. J Athl Train. 1992;27(2):102-110.
44. Byrd JW, Jones KS. Hip arthroscopy in athletes. Clin Sports Med. 2001;20(4):749-761.
45. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral tears. Arthroscopy.2015;31(11):2106-2111.
46. Mullins K, Hanlon M, Carton P. Differences in athletic performance between sportsmen with symptomatic femoroacetabular impingement and healthy controls. Clin J Sport Med.2018;28(4):370-376. doi:10.1097/JSM.0000000000000460.
47. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-120. doi:10.1097/01.blo.0000096804.78689.c2.
48. Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036-3043. doi:10.1177/0363546517719460 .
49. Gerhardt MB, Romero AA, Silvers HJ, Harris DJ, Watanabe D, Mandelbaum BR. The prevalence of radiographic hip abnormalities in elite soccer players. Am J Sports Med. 2012;40(3):584-588. doi:10.1177/0363546511432711.
50. Larson CM, Ross JR, Kuhn AW, et al. Radiographic hip anatomy correlates with range of motion and symptoms in national hockey league players. Am J Sports Med. 2017;45(7):1633-1639. doi:10.1177/0363546517692542.
51. Wyss TF, Clark JM, Weishaupt D, Notzli HP. Correlation between internal rotation and bony anatomy in the hip. Clin Orthop Relat Res. 2007;460:152-158. doi:10.1097/BLO.0b013e3180399430.
52. Reiman MP, Goode AP, Cook CE, Holmich P, Thorborg K. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med. 2015;49:811. doi:10.1136/bjsports-2014-094302.
53. Papalia R, Del Buono A, Franceschi F, Marinozzi A, Maffulli N, Denaro V. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop. 2012;36(5):903-914. doi:10.1007/s00264-011-1443-z.
54. Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for femoroacetabular impingement in professional soccer players. Am J Sports Med. 2018;46(2):273-279. doi:10.1177/0363546517738741.
55. Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21(12):1496-1504. doi:10.1016/j.arthro.2005.08.013.
56. Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145-150. doi:10.1097/01.blo.0000136903.01368.20.
57. Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus débridement versus reconstruction. J Am Acad Orthop Surg. 2017;25(3):e53-e62. doi:10.5435/JAAOS-D-16-00144.
58. Frank JS, Gambacorta PL, Eisner EA. Hip pathology in the adolescent athlete. J Am Acad Orthop Surg. 2013;21(11):665-674. doi:10.5435/JAAOS-21-11-665.
59. Singh PJ, O'Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26(6):743-749. doi:10.1016/j.arthro.2009.10.010.
60. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-335. doi:10.1016/j.csm.2005.12.004.
61. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775. doi:10.1016/j.arthro.2011.01.018.
62. Wollin M, Thorborg K, Welvaert M, Pizzari T. In-season monitoring of hip and groin strength, health and function in elite youth soccer: implementing an early detection and management strategy over two consecutive seasons. J Sci Med Sport. 2018;21(10):988. doi:10.1016/j.jsams.2018.03.004.
63. Charlton PC, Drew MK, Mentiplay BF, Grimaldi A, Clark RA. Exercise interventions for the prevention and treatment of groin pain and injury in athletes: a critical and systematic review. Sports Med. 2017;47:2011. doi:10.1007/s40279-017-0742-y.
ABSTRACT
Soccer is the most popular sport in the world and has the fourth highest number of sports injuries. Hip and groin injuries account for 14% of soccer injuries and can be difficult to recognize and treat as they often require a high level of suspicion and advanced imaging. Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain. Conservative approaches are typically the first line of treatment, but operative intervention has been reported to result in higher rates of return to sport in athletes with hip-related and inguinal-related groin pain injuries. In patients with concurrent hip-related and inguinal-related groin pain, the failure to recognize the relationship and treat both conditions may result in lower rates of return to sport. Preseason screening programs can identify high-risk athletes, who may benefit from a targeted prevention program. Further study on exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
Continue to: Each year, the global audience for soccer grows...
Each year, the global audience for soccer grows. Soccer has long surpassed all other sports as the most popular sport in the world, reaching 3.2 billion viewers during the 2014 World Cup.1 In the latest Fédération Internationale de Football Association (FIFA) Big Count survey, the organization estimated that 265 million people are actively involved in soccer, accounting for approximately 4% of the world’s population.2 Moreover, the number of people playing soccer increased by 9.5% within 6 years after the previous Big Count Survey.2 In the United States, soccer accounts for the fourth most common cause of sports injuries next to basketball, exercise, and football with approximately 228,000 injuries per year.3 The total cost of treatment related to worldwide soccer injuries tops $30 billion.4 The most common body parts injured are the thigh (25%), knee (18%), and hip and/or groin (14%).5
Hip and groin injuries in soccer players can be separated into 3 main categories based on the Doha Agreement:6 (1) defined clinical entities for groin pain, (2) hip-related groin pain, and (3) other causes of groin pain in athletes. Defined clinical entities include adductor-related, iliopsoas-related, inguinal-related (sports hernia/athletic pubalgia), and pubic-related groin pain; while hip-related groin pain includes hip morphologic abnormalities, labral tears, and chondral injuries. Included in other causes of groin pain are injuries not clinically defined. The Doha Agreement has acknowledged that not all causes of groin pain fit into the classification system including injuries of the rectus femoris, but they will be included under defined clinical entities for groin pain in this review. While they are not a cause of groin pain, proximal hamstring and gluteal and piriformis injuries are important causes of posterior and lateral hip pain in soccer players and will also be covered in the first section of this review.
DEFINED CLINICAL ENTITIES FOR GROIN PAIN IN SOCCER ATHLETES
ADDUCTOR-RELATED GROIN PAIN
Acute groin pain in soccer players is most commonly caused by muscle strain.7 Of the muscle strains, 66% involve the adductor longus, 25% the iliopsoas, and 23% the rectus femoris.7 The Doha Agreement defines adductor-related groin pain as adductor tenderness and pain on resisted adduction.6 Adductor longus strains in soccer players are typically noncontact injuries (62.5%) and most commonly the result of kicking (40%).7-9 Many athletes will remember a pop at the time of the original injury.9 The combination of history and physical examination is usually sufficient for diagnosis; however, magnetic resonance imaging (MRI) may be helpful in complicated situations with a reported 86% sensitivity and 89% specificity.10 The average playing time lost is 2 weeks.5 Management includes rest, anti-inflammatory medication, physical therapy with core strengthening, and avoidance of aggressive stretching. While partial and distal avulsions can heal with conservative measures, proximal osseous and retracted avulsions of the adductor longus can be treated surgically.11
Continue to: ILIOPSOAS-RELATED GROIN PAIN...
ILIOPSOAS-RELATED GROIN PAIN
Iliopsoas strains account for 25% of acute groin strains and typically result from an impact that causes eccentric overload while kicking the ball.7,12 Iliopsoas-related groin pain is defined by the Doha Agreement as groin pain that is reproducible with resisted hip flexion or hip flexor stretch.6 Iliopsoas strains respond well to conservative treatment such as rest, anti-inflammatory medication, and physical therapy. Rarely do these athletes become surgical candidates in the acute setting. Chronic cases of iliopsoas pathology occasionally require an arthroscopic intervention.
INGUINAL-RELATED GROIN PAIN
Inguinal-related groin pain is one of the most misleading diagnoses in sports because of its poorly defined and under-researched nature. The varying nomenclature of this entity illustrates the heterogeneity and includes sports hernia,9,13-15 athletic pubalgia,16 core muscle injury,17 athletic hernia,18 Gilmore’s groin,15 osteitis pubis,19 sportsman’s hernia,20,21 sportsmen’s groin,22 symphysis syndrome,23 and inguinal disruption.24 It is important to realize that in inguinal-related groin pain, regardless of the nomenclature, there is no true hernia present. The Doha Agreement has defined inguinal-related groin pain as “pain in the location of the inguinal region with associated tenderness of the inguinal canal,” which “is more likely if the pain is aggravated with resistance testing of the abdominal muscles or on Valsalva/cough/sneeze.”6 The condition is a painful soft tissue injury in the groin or inguinal area, involving a constellation of various anatomic areas including the abdominal musculature, sacroiliac joint, neural structures, pubic symphysis, adductors, and hip joint. This may account for up to 50% of chronic groin pain.25,26
One important theory in the development of inguinal-related groin pain is its relationship with femoroacetabular impingement (FAI). Cadaver studies demonstrate that cam deformities cause a 35% increase in motion at the pubic symphysis altering the biomechanics of the adductors and abdominal musculature and, with repetitive stress, may lead to tearing or attenuation of the transversalis fascia, rectus abdominis, internal obliques, and/or external obliques.12,27,28 Another prevailing theory of this is that the increased pubic stress causes weakness in the posterior portion of the inguinal canal, which then stretches and entraps the genitofemoral, ilioinguinal, lateral femoral cutaneous, or obturator nerves, ultimately causing pain.28,29
Physical examination findings include pain over the conjoined tendon, pubic tubercle/symphysis (present in 22% of patients), adductor origin (36%), and inguinal ring.25,30 Pain with resisted sit-ups is present in 46% of patients and pain with coughing/Valsalva is present in 10%.25,30,31 Selective injections can be a critical part of the evaluation to differentiate inguinal-related groin pain from FAI, osteitis pubis, and adductor strains while helping to determine the appropriate treatment.25,32 The role of advanced imaging is unclear as the clinical entity is still uncertain and the standard imaging findings have not been definitively established.33 However, several studies have reported MRI findings suggestive of inguinal-related groin pain. One of the more common MRI findings is the “secondary cleft sign,” which requires injecting a dye into the pubic symphysis.34 Several studies have shown that the radiographic dye extravasates preferentially into the side where the groin symptoms exist and are thought to be secondary to micro-tearing at the common attachment of the musculotendinous structures to the anterior pubis.34,35 However, it should be noted that the lack of imaging findings does not exclude the possibility of inguinal-related groin pathology.
Initial treatment consists of rest, anti-inflammatory medication, injections, and physical therapy with core strengthening.25 A study by Paajanen and colleagues36 suggested that early surgical intervention may be preferred over conservative management in a randomized trial comparing physical therapy, injections, anti-inflammatory medication, and rest vs an extraperitoneal laparoscopic mesh repair behind the pubic symphysis. In the conservative group, 20% of athletes returned to sport at 1 month, 27% at 3 months, and 50% at 12 months.36 In comparison, the surgical group had 67% return to sport at 1 month, 90% at 3 months, and 97% at 12 months.36 If surgical management is chosen, there are a variety of surgical options including laparoscopy, open or mini-open repairs of the abdominal musculature/fascia or pelvic floor with and without mesh, neurolysis, and adductor release. Muschawek and Berger37described a series of 129 patients that had an open-suture repair of the posterior wall of the inguinal canal with 67% of professional athletes returning to sport within 2 weeks and 83.7% of athletes returning to sport overall. The rates of return to play are consistently 80% to 100% without demonstrated superiority of one technique over another up to this point.30
Continue to: PUBIC-RELATED GROIN PAIN...
PUBIC-RELATED GROIN PAIN
Pubic-related groin pain is defined as tenderness to palpation over the pubic symphysis and adjacent bone.6 Osteitis pubis is a chronic overuse injury characterized by localized pain to the pubic symphysis and is believed to be caused by repetitive microtrauma from a dynamic rotation of the sacroiliac joint with suggested imbalances between the rectus abdominis and the adductor musculature.12,38 In soccer players, the condition may be related to the constant torsional stresses of kicking, running, or twisting.12 If performed, radiographs often show lytic areas of the pubic symphysis, widening of the symphysis, sclerosis, and cystic changes, while bone marrow edema may be present on MRI.38Management consists of rest, anti-inflammatory medication, and corticosteroid injections with gentle stretching once asymptomatic.12,39
RECTUS FEMORIS INJURIES
The most common injury to the rectus femoris is a strain as a result of an eccentric overload while a soccer player is hit trying to extend his or her leg to kick a ball.12 In pediatric soccer athletes, an avulsion of the anterior inferior iliac spine from the direct head of the rectus femoris is the second most common avulsion injury.40 Radiographs are diagnostic and can help determine treatment. Most avulsions are minimally displaced and can be treated conservatively, but surgical intervention should be considered for an avulsion >2 cm.12
PROXIMAL HAMSTRING INJURIES
Proximal hamstring injuries are important causes of acute posterior hip pain and are caused by an eccentric overload in hip flexion and knee extension.25 In soccer players, the typical mechanism is that the planted leg slipping on the playing turf creates a sudden violent flexion of the hip with the knee in an extended position. While relatively uncommon, when a significant avulsion occurs in a professional athlete, surgical intervention is often necessary. In general, these injuries may involve partial or full avulsions off the ischial tuberosity or separation of the bony apophysis in pediatric athletes. A physical examination in the acute setting typically demonstrates massive posterior thigh ecchymosis, a palpable defect, and/or weakness with knee flexion. Imaging is helpful to confirm the diagnosis and evaluate for surgical repair. Radiographs may show a bony avulsion, which is more commonly seen in pediatric apophyseal avulsions. MRI can be used to differentiate a complete tear (involving all 3 tendons) vs a partial tear and evaluate for retraction of the tendon distally. Complete and partial tears of 2 tendons with retraction of >2 cm should be surgically repaired.25 Partial tears without tendon retraction may be treated conservatively with rest, anti-inflammatory medication, and physical therapy and then followed later by a hamstring prevention program.25 We have found that biologic augmentation with platelet-rich plasma can help accelerate healing in partial thickness injuries; however, the evidence is conflicting.
GLUTEAL INJURIES
Chronic overuse injuries of the gluteal musculature are common causes of lateral hip pain. Abductor overuse caused by weakness in the gluteus medius with a normal tensor fascia lata can cause pain with sitting and side-lying.25Overuse of the gluteal muscles with muscular imbalances along with increased tension on the iliotibial band can lead to greater trochanteric pain syndrome.25 A physical examination may demonstrate tenderness over the greater trochanter bursa and positive flexion, abduction, and external rotation testing.25 Abductor overuse syndrome and greater trochanteric pain syndrome are best treated with anti-inflammatory medication and physical therapy to balance the core/pelvic musculature.41
PIRIFORMIS INJURIES
Piriformis syndrome is a compressive neuropathy of the sciatic nerve. The mechanism of injury in the athlete is through a minor trauma to the buttock or pelvis.25,42,43 Presenting symptoms include pain with sitting and internal rotation of the hip.12 Zeren and colleagues42 published the only study that includes 2 cases of bilateral piriformis syndrome in professional soccer players. The diagnosis was confirmed with electromyography that was negative at rest and positive when measured after running.42 The athletes exhausted conservative treatment with physical therapy, anti-inflammatory medications, injections, and rest and were treated with surgical decompression.42 Both players returned to professional soccer after 6 months and played for an average of 7 years.42
Continue to: HIP-RELATED GROIN PAIN IN SOCCER ATHLETES...
HIP-RELATED GROIN PAIN IN SOCCER ATHLETES
Hip-related groin pain has garnered more attention in the last several years after being a previously underdiagnosed entity. One study found that practitioners treated groin pain in athletes for 7 months on average before recognizing that the pathology was intra-articular.44 FAI, labral tears, and chondral injuries are the major intra-articular pathologies that cause groin pain in athletes and ultimately impaired performance.45,46
FEMOROACETABULAR IMPINGEMENT
FAI is caused by pincer-type, cam-type, or combined-type deformities. Pincer lesions are defined as an increased acetabular overhang, while cam lesions are described as an increased bone at the femoral head/neck junction. These deformities in isolation or in combination cause decreased hip motion and increased contact pressures between the anterolateral acetabulum and femoral head-neck junction, which may ultimately lead to labral tears, chondral lesions, and osteoarthritis.47 During hip flexion, cam deformities impact the anterolateral acetabulum, preferentially causing articular cartilage damage, while sparing the labrum.25 Conversely, pincer deformities cause repetitive microtrauma to the labrum, crushing it between the acetabular rim and femoral neck with secondary damage to the articular cartilage.25 Over time, the damage to the labrum and articular cartilage may lead to premature osteoarthritis, which occurs at a much younger age in the athletic population.48
We know from previous studies that soccer athletes have a high prevalence of morphologic abnormalities of the hip, most commonly FAI. Gerhardt and colleagues49 documented the prevalence of hip morphologic abnormalities in elite soccer players and found abnormalities in 72% of men and over 50% of women. It should be noted that this series looked at asymptomatic athletes; however, it has been shown that hip dysmorphia is a risk factor for hip and groin injuries and may provide an opportunity for injury prevention strategies.50
Physical examination findings in FAI include decreased hip internal rotation and pain with provocative testing. Wyss and colleagues51 measured hip internal rotation in athletes with and without FAI. They found that the athletes with FAI have an average of 4° of internal rotation compared with that of the non-FAI athletes with 28°.51 A worsening internal rotation deficit has been linked to increasing severity of the deformity and when <20° was correlated with joint damage.51 Provocative testing has a high sensitivity with a recent meta-analysis demonstrating the most sensitive tests to be the anterior impingement test (flexion-adduction-internal rotation) with 94% to 99% sensitivity and the flexion-internal rotation test with 96% sensitivity.52 While provocative tests are sensitive, there is no current consensus on physical examination findings that are specific in the diagnosis of FAI.6 Diagnosis is made with both positive physical examination and radiographic morphologic findings (alpha angle >55°).33 Advanced imaging with an MRI arthrogram can be helpful in diagnosing underlying injuries such as labral tears in athletes presenting with compatible symptoms.
Symptomatic patients are typically treated surgically through either open or arthroscopic procedures, which have favorable and comparable functional results, biomechanics, and return to sport.53 In soccer players, return to sport at the professional level after arthroscopic surgery was found to be 96%.54 Players returned to sport on average 9.2 months postoperatively and played an average of 70 games after surgery.54
Continue to: LABRAL TEARS...
LABRAL TEARS
Labral tears present with groin pain, limited hip range of motion, and symptoms of catching, locking, and instability.25Causes of labral tears include trauma, FAI, hip dysplasia, capsular laxity, and degeneration.55 Labral tears rarely occur in isolation and have a high association (87%) with morphologic abnormalities of the hip, most commonly FAI and occasionally dysplasia.56,57 Physical examination findings include positive anterior impingement tests (flexion-adduction-internal rotation) in athletes with anterior labral tears and, less commonly, positive flexion, abduction, and external rotation tests for athletes with lateral and posterolateral labral tears.57 Radiographic imaging is used to evaluate for concurrent morphologic abnormalities of the hip, and MRI arthrogram is used to confirm the diagnosis of a labral tear with a sensitivity of 76% to 91%.58 Initial treatment consists of conservative treatment, which includes rest, anti-inflammatory medication, activity modification, and physical therapy. In patient refractory to conservative treatment, arthroscopic surgery is effective with high rates of return to sport.59 It is important to note that when treating labral tears surgically, any morphologic abnormality needs to be addressed to prevent recurrence of the tear.
CHONDRAL INJURIES
Focal chondral lesions in the hip are commonly found in athletes with FAI and labral tears during arthroscopic evaluation.60 Full-thickness defects and unstable flaps in weight-bearing areas are indications for surgical intervention with microfracture.60 There are no studies examining the efficacy of microfracture in isolation; however, Locks and colleagues54 have demonstrated a 96% return to professional soccer after an arthroscopic treatment for FAI and found that severe chondral damage with microfracture did not lengthen the return to sport.
RELATIONSHIP BETWEEN INGUINAL-RELATED GROIN PAIN AND FEMOROACETABULAR IMPINGEMENT
The altered biomechanics and restricted range of motion in athletes with FAI cause an increase in compensatory motion at the pelvis and lumbosacral areas, which may contribute to the development of inguinal-related groin pain, bursitis, adductor, and gluteal dysfunction.25 In athletes with concurrent intra-articular hip pathology and inguinal-related groin pain, treating 1 condition in isolation will result in poor results. Larson and colleagues61 found that when only inguinal-related groin pain or FAI were addressed, return to sport was only 25% and 50%, respectively, while concurrent surgical treatment resulted in a return to sport of 89%.
DISCUSSION AND FUTURE DIRECTIONS
Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need a surgical intervention. Efforts are underway to determine the role and efficacy of identifying high-risk athletes that may benefit from targeted prevention strategies. Wyles and colleagues48 identified adolescent athletes with hip internal rotation of <10° and found at 5-year follow-up that 95% had abnormal MRI findings compared with 54% in the age-matched control group. Wollin and colleagues62 developed an in-season screening protocol using adductor strength reductions of 15%, adductor/abductor strength ratio <0.9, and hip and groin outcome scores <75 as indicators of at-risk individuals. By employing preseason and in-season screening protocols, we can identify high-risk athletes for further workup and close follow-up throughout the season. Pelvic radiographs in these high-risk athletes may help us determine the presence of abnormalities in hip morphology, which would place an athlete into a high-risk group where prevention strategies could then be employed. There are no data available to determine the most effective prevention strategy at this time. However, levels II and III evidence exists indicating that exercise programs may reduce the incidence of groin injuries.63 Additional strategies, like limiting adolescent playing time similar to strategies employed in baseball pitches with pitch counts, could potentially reduce the potential for injury. Further studies on preseason screening and in-season monitoring protocols, targeted exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
ABSTRACT
Soccer is the most popular sport in the world and has the fourth highest number of sports injuries. Hip and groin injuries account for 14% of soccer injuries and can be difficult to recognize and treat as they often require a high level of suspicion and advanced imaging. Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain. Conservative approaches are typically the first line of treatment, but operative intervention has been reported to result in higher rates of return to sport in athletes with hip-related and inguinal-related groin pain injuries. In patients with concurrent hip-related and inguinal-related groin pain, the failure to recognize the relationship and treat both conditions may result in lower rates of return to sport. Preseason screening programs can identify high-risk athletes, who may benefit from a targeted prevention program. Further study on exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
Continue to: Each year, the global audience for soccer grows...
Each year, the global audience for soccer grows. Soccer has long surpassed all other sports as the most popular sport in the world, reaching 3.2 billion viewers during the 2014 World Cup.1 In the latest Fédération Internationale de Football Association (FIFA) Big Count survey, the organization estimated that 265 million people are actively involved in soccer, accounting for approximately 4% of the world’s population.2 Moreover, the number of people playing soccer increased by 9.5% within 6 years after the previous Big Count Survey.2 In the United States, soccer accounts for the fourth most common cause of sports injuries next to basketball, exercise, and football with approximately 228,000 injuries per year.3 The total cost of treatment related to worldwide soccer injuries tops $30 billion.4 The most common body parts injured are the thigh (25%), knee (18%), and hip and/or groin (14%).5
Hip and groin injuries in soccer players can be separated into 3 main categories based on the Doha Agreement:6 (1) defined clinical entities for groin pain, (2) hip-related groin pain, and (3) other causes of groin pain in athletes. Defined clinical entities include adductor-related, iliopsoas-related, inguinal-related (sports hernia/athletic pubalgia), and pubic-related groin pain; while hip-related groin pain includes hip morphologic abnormalities, labral tears, and chondral injuries. Included in other causes of groin pain are injuries not clinically defined. The Doha Agreement has acknowledged that not all causes of groin pain fit into the classification system including injuries of the rectus femoris, but they will be included under defined clinical entities for groin pain in this review. While they are not a cause of groin pain, proximal hamstring and gluteal and piriformis injuries are important causes of posterior and lateral hip pain in soccer players and will also be covered in the first section of this review.
DEFINED CLINICAL ENTITIES FOR GROIN PAIN IN SOCCER ATHLETES
ADDUCTOR-RELATED GROIN PAIN
Acute groin pain in soccer players is most commonly caused by muscle strain.7 Of the muscle strains, 66% involve the adductor longus, 25% the iliopsoas, and 23% the rectus femoris.7 The Doha Agreement defines adductor-related groin pain as adductor tenderness and pain on resisted adduction.6 Adductor longus strains in soccer players are typically noncontact injuries (62.5%) and most commonly the result of kicking (40%).7-9 Many athletes will remember a pop at the time of the original injury.9 The combination of history and physical examination is usually sufficient for diagnosis; however, magnetic resonance imaging (MRI) may be helpful in complicated situations with a reported 86% sensitivity and 89% specificity.10 The average playing time lost is 2 weeks.5 Management includes rest, anti-inflammatory medication, physical therapy with core strengthening, and avoidance of aggressive stretching. While partial and distal avulsions can heal with conservative measures, proximal osseous and retracted avulsions of the adductor longus can be treated surgically.11
Continue to: ILIOPSOAS-RELATED GROIN PAIN...
ILIOPSOAS-RELATED GROIN PAIN
Iliopsoas strains account for 25% of acute groin strains and typically result from an impact that causes eccentric overload while kicking the ball.7,12 Iliopsoas-related groin pain is defined by the Doha Agreement as groin pain that is reproducible with resisted hip flexion or hip flexor stretch.6 Iliopsoas strains respond well to conservative treatment such as rest, anti-inflammatory medication, and physical therapy. Rarely do these athletes become surgical candidates in the acute setting. Chronic cases of iliopsoas pathology occasionally require an arthroscopic intervention.
INGUINAL-RELATED GROIN PAIN
Inguinal-related groin pain is one of the most misleading diagnoses in sports because of its poorly defined and under-researched nature. The varying nomenclature of this entity illustrates the heterogeneity and includes sports hernia,9,13-15 athletic pubalgia,16 core muscle injury,17 athletic hernia,18 Gilmore’s groin,15 osteitis pubis,19 sportsman’s hernia,20,21 sportsmen’s groin,22 symphysis syndrome,23 and inguinal disruption.24 It is important to realize that in inguinal-related groin pain, regardless of the nomenclature, there is no true hernia present. The Doha Agreement has defined inguinal-related groin pain as “pain in the location of the inguinal region with associated tenderness of the inguinal canal,” which “is more likely if the pain is aggravated with resistance testing of the abdominal muscles or on Valsalva/cough/sneeze.”6 The condition is a painful soft tissue injury in the groin or inguinal area, involving a constellation of various anatomic areas including the abdominal musculature, sacroiliac joint, neural structures, pubic symphysis, adductors, and hip joint. This may account for up to 50% of chronic groin pain.25,26
One important theory in the development of inguinal-related groin pain is its relationship with femoroacetabular impingement (FAI). Cadaver studies demonstrate that cam deformities cause a 35% increase in motion at the pubic symphysis altering the biomechanics of the adductors and abdominal musculature and, with repetitive stress, may lead to tearing or attenuation of the transversalis fascia, rectus abdominis, internal obliques, and/or external obliques.12,27,28 Another prevailing theory of this is that the increased pubic stress causes weakness in the posterior portion of the inguinal canal, which then stretches and entraps the genitofemoral, ilioinguinal, lateral femoral cutaneous, or obturator nerves, ultimately causing pain.28,29
Physical examination findings include pain over the conjoined tendon, pubic tubercle/symphysis (present in 22% of patients), adductor origin (36%), and inguinal ring.25,30 Pain with resisted sit-ups is present in 46% of patients and pain with coughing/Valsalva is present in 10%.25,30,31 Selective injections can be a critical part of the evaluation to differentiate inguinal-related groin pain from FAI, osteitis pubis, and adductor strains while helping to determine the appropriate treatment.25,32 The role of advanced imaging is unclear as the clinical entity is still uncertain and the standard imaging findings have not been definitively established.33 However, several studies have reported MRI findings suggestive of inguinal-related groin pain. One of the more common MRI findings is the “secondary cleft sign,” which requires injecting a dye into the pubic symphysis.34 Several studies have shown that the radiographic dye extravasates preferentially into the side where the groin symptoms exist and are thought to be secondary to micro-tearing at the common attachment of the musculotendinous structures to the anterior pubis.34,35 However, it should be noted that the lack of imaging findings does not exclude the possibility of inguinal-related groin pathology.
Initial treatment consists of rest, anti-inflammatory medication, injections, and physical therapy with core strengthening.25 A study by Paajanen and colleagues36 suggested that early surgical intervention may be preferred over conservative management in a randomized trial comparing physical therapy, injections, anti-inflammatory medication, and rest vs an extraperitoneal laparoscopic mesh repair behind the pubic symphysis. In the conservative group, 20% of athletes returned to sport at 1 month, 27% at 3 months, and 50% at 12 months.36 In comparison, the surgical group had 67% return to sport at 1 month, 90% at 3 months, and 97% at 12 months.36 If surgical management is chosen, there are a variety of surgical options including laparoscopy, open or mini-open repairs of the abdominal musculature/fascia or pelvic floor with and without mesh, neurolysis, and adductor release. Muschawek and Berger37described a series of 129 patients that had an open-suture repair of the posterior wall of the inguinal canal with 67% of professional athletes returning to sport within 2 weeks and 83.7% of athletes returning to sport overall. The rates of return to play are consistently 80% to 100% without demonstrated superiority of one technique over another up to this point.30
Continue to: PUBIC-RELATED GROIN PAIN...
PUBIC-RELATED GROIN PAIN
Pubic-related groin pain is defined as tenderness to palpation over the pubic symphysis and adjacent bone.6 Osteitis pubis is a chronic overuse injury characterized by localized pain to the pubic symphysis and is believed to be caused by repetitive microtrauma from a dynamic rotation of the sacroiliac joint with suggested imbalances between the rectus abdominis and the adductor musculature.12,38 In soccer players, the condition may be related to the constant torsional stresses of kicking, running, or twisting.12 If performed, radiographs often show lytic areas of the pubic symphysis, widening of the symphysis, sclerosis, and cystic changes, while bone marrow edema may be present on MRI.38Management consists of rest, anti-inflammatory medication, and corticosteroid injections with gentle stretching once asymptomatic.12,39
RECTUS FEMORIS INJURIES
The most common injury to the rectus femoris is a strain as a result of an eccentric overload while a soccer player is hit trying to extend his or her leg to kick a ball.12 In pediatric soccer athletes, an avulsion of the anterior inferior iliac spine from the direct head of the rectus femoris is the second most common avulsion injury.40 Radiographs are diagnostic and can help determine treatment. Most avulsions are minimally displaced and can be treated conservatively, but surgical intervention should be considered for an avulsion >2 cm.12
PROXIMAL HAMSTRING INJURIES
Proximal hamstring injuries are important causes of acute posterior hip pain and are caused by an eccentric overload in hip flexion and knee extension.25 In soccer players, the typical mechanism is that the planted leg slipping on the playing turf creates a sudden violent flexion of the hip with the knee in an extended position. While relatively uncommon, when a significant avulsion occurs in a professional athlete, surgical intervention is often necessary. In general, these injuries may involve partial or full avulsions off the ischial tuberosity or separation of the bony apophysis in pediatric athletes. A physical examination in the acute setting typically demonstrates massive posterior thigh ecchymosis, a palpable defect, and/or weakness with knee flexion. Imaging is helpful to confirm the diagnosis and evaluate for surgical repair. Radiographs may show a bony avulsion, which is more commonly seen in pediatric apophyseal avulsions. MRI can be used to differentiate a complete tear (involving all 3 tendons) vs a partial tear and evaluate for retraction of the tendon distally. Complete and partial tears of 2 tendons with retraction of >2 cm should be surgically repaired.25 Partial tears without tendon retraction may be treated conservatively with rest, anti-inflammatory medication, and physical therapy and then followed later by a hamstring prevention program.25 We have found that biologic augmentation with platelet-rich plasma can help accelerate healing in partial thickness injuries; however, the evidence is conflicting.
GLUTEAL INJURIES
Chronic overuse injuries of the gluteal musculature are common causes of lateral hip pain. Abductor overuse caused by weakness in the gluteus medius with a normal tensor fascia lata can cause pain with sitting and side-lying.25Overuse of the gluteal muscles with muscular imbalances along with increased tension on the iliotibial band can lead to greater trochanteric pain syndrome.25 A physical examination may demonstrate tenderness over the greater trochanter bursa and positive flexion, abduction, and external rotation testing.25 Abductor overuse syndrome and greater trochanteric pain syndrome are best treated with anti-inflammatory medication and physical therapy to balance the core/pelvic musculature.41
PIRIFORMIS INJURIES
Piriformis syndrome is a compressive neuropathy of the sciatic nerve. The mechanism of injury in the athlete is through a minor trauma to the buttock or pelvis.25,42,43 Presenting symptoms include pain with sitting and internal rotation of the hip.12 Zeren and colleagues42 published the only study that includes 2 cases of bilateral piriformis syndrome in professional soccer players. The diagnosis was confirmed with electromyography that was negative at rest and positive when measured after running.42 The athletes exhausted conservative treatment with physical therapy, anti-inflammatory medications, injections, and rest and were treated with surgical decompression.42 Both players returned to professional soccer after 6 months and played for an average of 7 years.42
Continue to: HIP-RELATED GROIN PAIN IN SOCCER ATHLETES...
HIP-RELATED GROIN PAIN IN SOCCER ATHLETES
Hip-related groin pain has garnered more attention in the last several years after being a previously underdiagnosed entity. One study found that practitioners treated groin pain in athletes for 7 months on average before recognizing that the pathology was intra-articular.44 FAI, labral tears, and chondral injuries are the major intra-articular pathologies that cause groin pain in athletes and ultimately impaired performance.45,46
FEMOROACETABULAR IMPINGEMENT
FAI is caused by pincer-type, cam-type, or combined-type deformities. Pincer lesions are defined as an increased acetabular overhang, while cam lesions are described as an increased bone at the femoral head/neck junction. These deformities in isolation or in combination cause decreased hip motion and increased contact pressures between the anterolateral acetabulum and femoral head-neck junction, which may ultimately lead to labral tears, chondral lesions, and osteoarthritis.47 During hip flexion, cam deformities impact the anterolateral acetabulum, preferentially causing articular cartilage damage, while sparing the labrum.25 Conversely, pincer deformities cause repetitive microtrauma to the labrum, crushing it between the acetabular rim and femoral neck with secondary damage to the articular cartilage.25 Over time, the damage to the labrum and articular cartilage may lead to premature osteoarthritis, which occurs at a much younger age in the athletic population.48
We know from previous studies that soccer athletes have a high prevalence of morphologic abnormalities of the hip, most commonly FAI. Gerhardt and colleagues49 documented the prevalence of hip morphologic abnormalities in elite soccer players and found abnormalities in 72% of men and over 50% of women. It should be noted that this series looked at asymptomatic athletes; however, it has been shown that hip dysmorphia is a risk factor for hip and groin injuries and may provide an opportunity for injury prevention strategies.50
Physical examination findings in FAI include decreased hip internal rotation and pain with provocative testing. Wyss and colleagues51 measured hip internal rotation in athletes with and without FAI. They found that the athletes with FAI have an average of 4° of internal rotation compared with that of the non-FAI athletes with 28°.51 A worsening internal rotation deficit has been linked to increasing severity of the deformity and when <20° was correlated with joint damage.51 Provocative testing has a high sensitivity with a recent meta-analysis demonstrating the most sensitive tests to be the anterior impingement test (flexion-adduction-internal rotation) with 94% to 99% sensitivity and the flexion-internal rotation test with 96% sensitivity.52 While provocative tests are sensitive, there is no current consensus on physical examination findings that are specific in the diagnosis of FAI.6 Diagnosis is made with both positive physical examination and radiographic morphologic findings (alpha angle >55°).33 Advanced imaging with an MRI arthrogram can be helpful in diagnosing underlying injuries such as labral tears in athletes presenting with compatible symptoms.
Symptomatic patients are typically treated surgically through either open or arthroscopic procedures, which have favorable and comparable functional results, biomechanics, and return to sport.53 In soccer players, return to sport at the professional level after arthroscopic surgery was found to be 96%.54 Players returned to sport on average 9.2 months postoperatively and played an average of 70 games after surgery.54
Continue to: LABRAL TEARS...
LABRAL TEARS
Labral tears present with groin pain, limited hip range of motion, and symptoms of catching, locking, and instability.25Causes of labral tears include trauma, FAI, hip dysplasia, capsular laxity, and degeneration.55 Labral tears rarely occur in isolation and have a high association (87%) with morphologic abnormalities of the hip, most commonly FAI and occasionally dysplasia.56,57 Physical examination findings include positive anterior impingement tests (flexion-adduction-internal rotation) in athletes with anterior labral tears and, less commonly, positive flexion, abduction, and external rotation tests for athletes with lateral and posterolateral labral tears.57 Radiographic imaging is used to evaluate for concurrent morphologic abnormalities of the hip, and MRI arthrogram is used to confirm the diagnosis of a labral tear with a sensitivity of 76% to 91%.58 Initial treatment consists of conservative treatment, which includes rest, anti-inflammatory medication, activity modification, and physical therapy. In patient refractory to conservative treatment, arthroscopic surgery is effective with high rates of return to sport.59 It is important to note that when treating labral tears surgically, any morphologic abnormality needs to be addressed to prevent recurrence of the tear.
CHONDRAL INJURIES
Focal chondral lesions in the hip are commonly found in athletes with FAI and labral tears during arthroscopic evaluation.60 Full-thickness defects and unstable flaps in weight-bearing areas are indications for surgical intervention with microfracture.60 There are no studies examining the efficacy of microfracture in isolation; however, Locks and colleagues54 have demonstrated a 96% return to professional soccer after an arthroscopic treatment for FAI and found that severe chondral damage with microfracture did not lengthen the return to sport.
RELATIONSHIP BETWEEN INGUINAL-RELATED GROIN PAIN AND FEMOROACETABULAR IMPINGEMENT
The altered biomechanics and restricted range of motion in athletes with FAI cause an increase in compensatory motion at the pelvis and lumbosacral areas, which may contribute to the development of inguinal-related groin pain, bursitis, adductor, and gluteal dysfunction.25 In athletes with concurrent intra-articular hip pathology and inguinal-related groin pain, treating 1 condition in isolation will result in poor results. Larson and colleagues61 found that when only inguinal-related groin pain or FAI were addressed, return to sport was only 25% and 50%, respectively, while concurrent surgical treatment resulted in a return to sport of 89%.
DISCUSSION AND FUTURE DIRECTIONS
Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need a surgical intervention. Efforts are underway to determine the role and efficacy of identifying high-risk athletes that may benefit from targeted prevention strategies. Wyles and colleagues48 identified adolescent athletes with hip internal rotation of <10° and found at 5-year follow-up that 95% had abnormal MRI findings compared with 54% in the age-matched control group. Wollin and colleagues62 developed an in-season screening protocol using adductor strength reductions of 15%, adductor/abductor strength ratio <0.9, and hip and groin outcome scores <75 as indicators of at-risk individuals. By employing preseason and in-season screening protocols, we can identify high-risk athletes for further workup and close follow-up throughout the season. Pelvic radiographs in these high-risk athletes may help us determine the presence of abnormalities in hip morphology, which would place an athlete into a high-risk group where prevention strategies could then be employed. There are no data available to determine the most effective prevention strategy at this time. However, levels II and III evidence exists indicating that exercise programs may reduce the incidence of groin injuries.63 Additional strategies, like limiting adolescent playing time similar to strategies employed in baseball pitches with pitch counts, could potentially reduce the potential for injury. Further studies on preseason screening and in-season monitoring protocols, targeted exercise therapy, early surgical intervention, and potential biologic intervention are needed to determine the most effective methods of preventing groin injuries in athletes.
1. Kantar Media. 2014 FIFA World Cup Brazil television audience report. https://resources.fifa.com/mm/document/affederation/tv/02/74/55/57/2014f...(draft5)(issuedate14.12.15)_neutral.pdf. Accessed March 20, 2018.
2. Fédération Internationale de Football Association. FIFA Big Count. http://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Published July 2007. Accessed March 20, 2018.
3. United States Consumer Product Safety Commission. Neiss data highlights - 2015. https://www.cpsc.gov/s3fs-public/2015 Neiss data highlights.pdf. Accessed March 20, 2018.
4. Hassabi M, Mohammad-Javad Mortazavi S, Giti MR, Hassabi M, Mansournia MA, Shapouran S. Injury profile of a professional soccer team in the premier league of Iran. Asian J Sports Med. 2010;1(4):201-208.
5. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558.
6. Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med. 2015;49(12):768-774.
7. Serner A, Tol JL, Jomaah N, et al. Diagnosis of acute groin injuries: a prospective study of 110 athletes. Am J Sports Med. 2015;43(8):1857-1864. doi:10.1177/0363546515585123.
8. Eckard TG, Padua DA, Dompier TP, Dalton SL, Thorborg K, Kerr ZY. Epidemiology of hip flexor and hip adductor strains in national collegiate athletic association athletes, 2009/2010-2014/2015. Am J Sports Med. 2017;45(12):2713-2722. doi:10.1177/0363546517716179.
9. Hopkins JN, Brown W, Lee CA. Sports hernia: definition, evaluation, and treatment. JBJS Rev. 2017;5(9):e6. doi:10.2106/JBJS.RVW.17.00022.
10. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438. doi:10.1148/rg.285075217.
11. Vogt S, Ansah P, Imhoff AB. Complete osseous avulsion of the adductor longus muscle: acute repair with three Wberwire suture anchors. Arch Orthop Trauma Surg. 2007;127:613-615. doi:10.1007/s00402-007-0328-5.
12. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533. doi:10.1177/03635465010290042501.
13. Choi HR, Elattar O, Dills VD, Busconi B. Return to play after sports hernia surgery. Clin Sports Med. 2016;35(4):621-636. doi:10.1016/j.csm.2016.05.007.
14. Garvey JF, Hazard H. Sports hernia or groin disruption injury? Chronic athletic groin pain: a retrospective study of 100 patients with long-term follow-up. Hernia. 2014;18(6):815-823. doi:10.1007/s10029-013-1161-0.
15. Gilmore J. Groin pain in the soccer athlete: fact, fiction, and treatment. Clin Sports Med. 1998;17(4):787-793, vii. doi:10.1016/S0278-5919(05)70119-8.
16. Cohen B, Kleinhenz D, Schiller J, Tabaddor R. Understanding athletic pubalgia: a review. R I Med J. 2016;99(10):31-35.
17. Ross JR, Stone RM, Larson CM. Core muscle injury/sports hernia/athletic pubalgia, and femoroacetabular impingement. Sports Med Arthrosc Rev. 2015;23(4):213-220. doi:10.1097/JSA.0000000000000083.
18. Swan KG Jr, Wolcott M. The athletic hernia: a systematic review. Clin Orthop Relat Res. 2007;455:78-87. doi:10.1097/BLO.0b013e31802eb3ea.
19. Matikainen M, Hermunen H, Paajanen H. Athletic pubalgia in females: predictive value of MRI in outcomes of endoscopic surgery. Orthop J Sports Med. 2017;5(8):2325967117720171. doi:10.1177/2325967117720171.
20. Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14(1):17-25. doi:10.1007/s10029-009-0611-1.
21. Paksoy M, Sekmen U. Sportsman hernia; the review of current diagnosis and treatment modalities. Ulusal Cerrahi Derg. 2016;32(2):122-129. doi:10.5152/UCD.2015.3132.
22. Pokorny H, Resinger C, Fischer I, et al. Fast early recovery after transabdominal preperitoneal repair in athletes with sportsman's groin: a prospective clinical cohort study. J Laparoendosc Adv Surg Tech A. 2017;27(3):272-276. doi:10.1089/lap.2016.0188.
23. Biedert RM, Warnke K, Meyer S. Symphysis syndrome in athletes: surgical treatment for chronic lower abdominal, groin, and adductor pain in athletes. Clin J Sport Med. 2003;13(5):278-284.
24. Sheen AJ, Stephenson BM, Lloyd DM, et al. 'Treatment of the sportsman's groin': British Hernia Society's 2014 position statement based on the Manchester Consensus Conference. Br J Sports Med. 2014;48(14):1079-1087.
25. Miller M, Thompson S. DeLee & Drez's Orthopaedic Sports Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
26. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. J Sci Med Sport. 1995;27:76-79.
27. Dimitrakopoulou A, Schilders E. Sportsman's hernia? An ambiguous term. J Hip Preserv Surg. 2016;3(1):16-22. doi:10.1093/jhps/hnv083.
28. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6. doi:10.3389/fsurg.2016.00006.
29. Muschaweck U, Berger LM. Sportsmen's groin-diagnostic approach and treatment with the minimal repair technique: a single-center uncontrolled clinical review. Sports Health. 2010;2(3):216-221. doi:10.1177/1941738110367623.
30. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144. doi:10.1177/1941738114523557.
31. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. Am J Sports Med. 2000; 28(1):2-8. doi:10.1177/03635465000280011501.
32. Gerhardt MB, Mandelbaum BR, Hutchinson WB. Ancillary modalities in the treatment of athletic groin Pain: Local Anesthetics, Corticosteroids, and Orthobiologics. In: Diduch DR, Brunt LM, eds. Sports Hernia and Athletic Pubalgia: Diagnosis and Treatment. Boston, MA: Springer US; 2014:183-187.
33. Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
34. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167. doi:10.1148/radiol.2351040045.
35. Byrne CA, Bowden DJ, Alkhayat A, Kavanagh EC, Eustace SJ. Sports-related groin pain secondary to symphysis pubis disorders: correlation between MRI findings and outcome after fluoroscopy-guided injection of steroid and local anesthetic. Am J Roentgenol. 2017;209(2):380-388. doi:10.2214/AJR.16.17578.
36. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: a randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman's hernia (athletic pubalgia). Surgery. 2011;150(1):99-107. doi:10.1016/j.surg.2011.02.016.
37. Muschaweck U, Berger L. Minimal repair technique of sportsmen's groin: an innovative open-suture repair to treat chronic inguinal pain. Hernia. 2010;14(1):27-33. doi:10.1007/s10029-009-0614-y.
38. Lynch TS, Bedi A, Larson CM. Athletic hip injuries. J Am Acad Orthop Surg. 2017;25(4):269-279. doi:10.5435/JAAOS-D-16-00171.
39. Holt MA, Keene JS, Graf BK, Helwig DC. Treatment of osteitis pubis in athletes. Results of corticosteroid injections. Am J Sports Med. 1995;23(5):601-606.doi:10.1177/036354659502300515.
40. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30(3):127-131. doi: 10.1007/s002560000319.
41. Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829.
42. Zeren B, Canbek U, Oztekin HH, Imerci A, Akgun U. Bilateral piriformis syndrome in two elite soccer players: report of two cases. Orthop Traumatol Surg Res. 2015;101(8):987-990. doi:10.1016/j.otsr.2015.07.022.
43. Keskula DR, Tamburello M. Conservative management of piriformis syndrome. J Athl Train. 1992;27(2):102-110.
44. Byrd JW, Jones KS. Hip arthroscopy in athletes. Clin Sports Med. 2001;20(4):749-761.
45. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral tears. Arthroscopy.2015;31(11):2106-2111.
46. Mullins K, Hanlon M, Carton P. Differences in athletic performance between sportsmen with symptomatic femoroacetabular impingement and healthy controls. Clin J Sport Med.2018;28(4):370-376. doi:10.1097/JSM.0000000000000460.
47. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-120. doi:10.1097/01.blo.0000096804.78689.c2.
48. Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036-3043. doi:10.1177/0363546517719460 .
49. Gerhardt MB, Romero AA, Silvers HJ, Harris DJ, Watanabe D, Mandelbaum BR. The prevalence of radiographic hip abnormalities in elite soccer players. Am J Sports Med. 2012;40(3):584-588. doi:10.1177/0363546511432711.
50. Larson CM, Ross JR, Kuhn AW, et al. Radiographic hip anatomy correlates with range of motion and symptoms in national hockey league players. Am J Sports Med. 2017;45(7):1633-1639. doi:10.1177/0363546517692542.
51. Wyss TF, Clark JM, Weishaupt D, Notzli HP. Correlation between internal rotation and bony anatomy in the hip. Clin Orthop Relat Res. 2007;460:152-158. doi:10.1097/BLO.0b013e3180399430.
52. Reiman MP, Goode AP, Cook CE, Holmich P, Thorborg K. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med. 2015;49:811. doi:10.1136/bjsports-2014-094302.
53. Papalia R, Del Buono A, Franceschi F, Marinozzi A, Maffulli N, Denaro V. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop. 2012;36(5):903-914. doi:10.1007/s00264-011-1443-z.
54. Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for femoroacetabular impingement in professional soccer players. Am J Sports Med. 2018;46(2):273-279. doi:10.1177/0363546517738741.
55. Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21(12):1496-1504. doi:10.1016/j.arthro.2005.08.013.
56. Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145-150. doi:10.1097/01.blo.0000136903.01368.20.
57. Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus débridement versus reconstruction. J Am Acad Orthop Surg. 2017;25(3):e53-e62. doi:10.5435/JAAOS-D-16-00144.
58. Frank JS, Gambacorta PL, Eisner EA. Hip pathology in the adolescent athlete. J Am Acad Orthop Surg. 2013;21(11):665-674. doi:10.5435/JAAOS-21-11-665.
59. Singh PJ, O'Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26(6):743-749. doi:10.1016/j.arthro.2009.10.010.
60. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-335. doi:10.1016/j.csm.2005.12.004.
61. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775. doi:10.1016/j.arthro.2011.01.018.
62. Wollin M, Thorborg K, Welvaert M, Pizzari T. In-season monitoring of hip and groin strength, health and function in elite youth soccer: implementing an early detection and management strategy over two consecutive seasons. J Sci Med Sport. 2018;21(10):988. doi:10.1016/j.jsams.2018.03.004.
63. Charlton PC, Drew MK, Mentiplay BF, Grimaldi A, Clark RA. Exercise interventions for the prevention and treatment of groin pain and injury in athletes: a critical and systematic review. Sports Med. 2017;47:2011. doi:10.1007/s40279-017-0742-y.
1. Kantar Media. 2014 FIFA World Cup Brazil television audience report. https://resources.fifa.com/mm/document/affederation/tv/02/74/55/57/2014f...(draft5)(issuedate14.12.15)_neutral.pdf. Accessed March 20, 2018.
2. Fédération Internationale de Football Association. FIFA Big Count. http://www.fifa.com/mm/document/fifafacts/bcoffsurv/emaga_9384_10704.pdf. Published July 2007. Accessed March 20, 2018.
3. United States Consumer Product Safety Commission. Neiss data highlights - 2015. https://www.cpsc.gov/s3fs-public/2015 Neiss data highlights.pdf. Accessed March 20, 2018.
4. Hassabi M, Mohammad-Javad Mortazavi S, Giti MR, Hassabi M, Mansournia MA, Shapouran S. Injury profile of a professional soccer team in the premier league of Iran. Asian J Sports Med. 2010;1(4):201-208.
5. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558.
6. Weir A, Brukner P, Delahunt E, et al. Doha agreement meeting on terminology and definitions in groin pain in athletes. Br J Sports Med. 2015;49(12):768-774.
7. Serner A, Tol JL, Jomaah N, et al. Diagnosis of acute groin injuries: a prospective study of 110 athletes. Am J Sports Med. 2015;43(8):1857-1864. doi:10.1177/0363546515585123.
8. Eckard TG, Padua DA, Dompier TP, Dalton SL, Thorborg K, Kerr ZY. Epidemiology of hip flexor and hip adductor strains in national collegiate athletic association athletes, 2009/2010-2014/2015. Am J Sports Med. 2017;45(12):2713-2722. doi:10.1177/0363546517716179.
9. Hopkins JN, Brown W, Lee CA. Sports hernia: definition, evaluation, and treatment. JBJS Rev. 2017;5(9):e6. doi:10.2106/JBJS.RVW.17.00022.
10. Omar IM, Zoga AC, Kavanagh EC, et al. Athletic pubalgia and "sports hernia": optimal MR imaging technique and findings. Radiographics. 2008;28(5):1415-1438. doi:10.1148/rg.285075217.
11. Vogt S, Ansah P, Imhoff AB. Complete osseous avulsion of the adductor longus muscle: acute repair with three Wberwire suture anchors. Arch Orthop Trauma Surg. 2007;127:613-615. doi:10.1007/s00402-007-0328-5.
12. Anderson K, Strickland SM, Warren R. Hip and groin injuries in athletes. Am J Sports Med. 2001;29(4):521-533. doi:10.1177/03635465010290042501.
13. Choi HR, Elattar O, Dills VD, Busconi B. Return to play after sports hernia surgery. Clin Sports Med. 2016;35(4):621-636. doi:10.1016/j.csm.2016.05.007.
14. Garvey JF, Hazard H. Sports hernia or groin disruption injury? Chronic athletic groin pain: a retrospective study of 100 patients with long-term follow-up. Hernia. 2014;18(6):815-823. doi:10.1007/s10029-013-1161-0.
15. Gilmore J. Groin pain in the soccer athlete: fact, fiction, and treatment. Clin Sports Med. 1998;17(4):787-793, vii. doi:10.1016/S0278-5919(05)70119-8.
16. Cohen B, Kleinhenz D, Schiller J, Tabaddor R. Understanding athletic pubalgia: a review. R I Med J. 2016;99(10):31-35.
17. Ross JR, Stone RM, Larson CM. Core muscle injury/sports hernia/athletic pubalgia, and femoroacetabular impingement. Sports Med Arthrosc Rev. 2015;23(4):213-220. doi:10.1097/JSA.0000000000000083.
18. Swan KG Jr, Wolcott M. The athletic hernia: a systematic review. Clin Orthop Relat Res. 2007;455:78-87. doi:10.1097/BLO.0b013e31802eb3ea.
19. Matikainen M, Hermunen H, Paajanen H. Athletic pubalgia in females: predictive value of MRI in outcomes of endoscopic surgery. Orthop J Sports Med. 2017;5(8):2325967117720171. doi:10.1177/2325967117720171.
20. Garvey JF, Read JW, Turner A. Sportsman hernia: what can we do? Hernia. 2010;14(1):17-25. doi:10.1007/s10029-009-0611-1.
21. Paksoy M, Sekmen U. Sportsman hernia; the review of current diagnosis and treatment modalities. Ulusal Cerrahi Derg. 2016;32(2):122-129. doi:10.5152/UCD.2015.3132.
22. Pokorny H, Resinger C, Fischer I, et al. Fast early recovery after transabdominal preperitoneal repair in athletes with sportsman's groin: a prospective clinical cohort study. J Laparoendosc Adv Surg Tech A. 2017;27(3):272-276. doi:10.1089/lap.2016.0188.
23. Biedert RM, Warnke K, Meyer S. Symphysis syndrome in athletes: surgical treatment for chronic lower abdominal, groin, and adductor pain in athletes. Clin J Sport Med. 2003;13(5):278-284.
24. Sheen AJ, Stephenson BM, Lloyd DM, et al. 'Treatment of the sportsman's groin': British Hernia Society's 2014 position statement based on the Manchester Consensus Conference. Br J Sports Med. 2014;48(14):1079-1087.
25. Miller M, Thompson S. DeLee & Drez's Orthopaedic Sports Medicine. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2015.
26. Lovell G. The diagnosis of chronic groin pain in athletes: a review of 189 cases. J Sci Med Sport. 1995;27:76-79.
27. Dimitrakopoulou A, Schilders E. Sportsman's hernia? An ambiguous term. J Hip Preserv Surg. 2016;3(1):16-22. doi:10.1093/jhps/hnv083.
28. Strosberg DS, Ellis TJ, Renton DB. The role of femoroacetabular impingement in core muscle injury/athletic pubalgia: diagnosis and management. Front Surg. 2016;3:6. doi:10.3389/fsurg.2016.00006.
29. Muschaweck U, Berger LM. Sportsmen's groin-diagnostic approach and treatment with the minimal repair technique: a single-center uncontrolled clinical review. Sports Health. 2010;2(3):216-221. doi:10.1177/1941738110367623.
30. Larson CM. Sports hernia/athletic pubalgia: evaluation and management. Sports Health. 2014;6(2):139-144. doi:10.1177/1941738114523557.
31. Meyers WC, Foley DP, Garrett WE, Lohnes JH, Mandlebaum BR. Management of severe lower abdominal or inguinal pain in high-performance athletes. Am J Sports Med. 2000; 28(1):2-8. doi:10.1177/03635465000280011501.
32. Gerhardt MB, Mandelbaum BR, Hutchinson WB. Ancillary modalities in the treatment of athletic groin Pain: Local Anesthetics, Corticosteroids, and Orthobiologics. In: Diduch DR, Brunt LM, eds. Sports Hernia and Athletic Pubalgia: Diagnosis and Treatment. Boston, MA: Springer US; 2014:183-187.
33. Notzli HP, Wyss TF, Stoecklin CH, et al. The contour of the femoral head-neck junction as a predictor for the risk of anterior impingement. J Bone Joint Surg Br. 2002;84(4):556-560.
34. Brennan D, O’Connell MJ, Ryan M, et al. Secondary cleft sign as a marker of injury in athletes with groin pain: MR image appearance and interpretation. Radiology. 2005;235(1):162-167. doi:10.1148/radiol.2351040045.
35. Byrne CA, Bowden DJ, Alkhayat A, Kavanagh EC, Eustace SJ. Sports-related groin pain secondary to symphysis pubis disorders: correlation between MRI findings and outcome after fluoroscopy-guided injection of steroid and local anesthetic. Am J Roentgenol. 2017;209(2):380-388. doi:10.2214/AJR.16.17578.
36. Paajanen H, Brinck T, Hermunen H, Airo I. Laparoscopic surgery for chronic groin pain in athletes is more effective than nonoperative treatment: a randomized clinical trial with magnetic resonance imaging of 60 patients with sportsman's hernia (athletic pubalgia). Surgery. 2011;150(1):99-107. doi:10.1016/j.surg.2011.02.016.
37. Muschaweck U, Berger L. Minimal repair technique of sportsmen's groin: an innovative open-suture repair to treat chronic inguinal pain. Hernia. 2010;14(1):27-33. doi:10.1007/s10029-009-0614-y.
38. Lynch TS, Bedi A, Larson CM. Athletic hip injuries. J Am Acad Orthop Surg. 2017;25(4):269-279. doi:10.5435/JAAOS-D-16-00171.
39. Holt MA, Keene JS, Graf BK, Helwig DC. Treatment of osteitis pubis in athletes. Results of corticosteroid injections. Am J Sports Med. 1995;23(5):601-606.doi:10.1177/036354659502300515.
40. Rossi F, Dragoni S. Acute avulsion fractures of the pelvis in adolescent competitive athletes: prevalence, location and sports distribution of 203 cases collected. Skeletal Radiol. 2001;30(3):127-131. doi: 10.1007/s002560000319.
41. Grumet RC, Frank RM, Slabaugh MA, Virkus WW, Bush-Joseph CA, Nho SJ. Lateral hip pain in an athletic population: differential diagnosis and treatment options. Sports Health. 2010;2(3):191-196. doi:10.1177/1941738110366829.
42. Zeren B, Canbek U, Oztekin HH, Imerci A, Akgun U. Bilateral piriformis syndrome in two elite soccer players: report of two cases. Orthop Traumatol Surg Res. 2015;101(8):987-990. doi:10.1016/j.otsr.2015.07.022.
43. Keskula DR, Tamburello M. Conservative management of piriformis syndrome. J Athl Train. 1992;27(2):102-110.
44. Byrd JW, Jones KS. Hip arthroscopy in athletes. Clin Sports Med. 2001;20(4):749-761.
45. Nepple JJ, Goljan P, Briggs KK, Garvey SE, Ryan M, Philippon MJ. Hip strength deficits in patients with symptomatic femoroacetabular impingement and labral tears. Arthroscopy.2015;31(11):2106-2111.
46. Mullins K, Hanlon M, Carton P. Differences in athletic performance between sportsmen with symptomatic femoroacetabular impingement and healthy controls. Clin J Sport Med.2018;28(4):370-376. doi:10.1097/JSM.0000000000000460.
47. Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112-120. doi:10.1097/01.blo.0000096804.78689.c2.
48. Wyles CC, Norambuena GA, Howe BM, et al. Cam deformities and limited hip range of motion are associated with early osteoarthritic changes in adolescent athletes: a prospective matched cohort study. Am J Sports Med. 2017;45(13):3036-3043. doi:10.1177/0363546517719460 .
49. Gerhardt MB, Romero AA, Silvers HJ, Harris DJ, Watanabe D, Mandelbaum BR. The prevalence of radiographic hip abnormalities in elite soccer players. Am J Sports Med. 2012;40(3):584-588. doi:10.1177/0363546511432711.
50. Larson CM, Ross JR, Kuhn AW, et al. Radiographic hip anatomy correlates with range of motion and symptoms in national hockey league players. Am J Sports Med. 2017;45(7):1633-1639. doi:10.1177/0363546517692542.
51. Wyss TF, Clark JM, Weishaupt D, Notzli HP. Correlation between internal rotation and bony anatomy in the hip. Clin Orthop Relat Res. 2007;460:152-158. doi:10.1097/BLO.0b013e3180399430.
52. Reiman MP, Goode AP, Cook CE, Holmich P, Thorborg K. Diagnostic accuracy of clinical tests for the diagnosis of hip femoroacetabular impingement/labral tear: a systematic review with meta-analysis. Br J Sports Med. 2015;49:811. doi:10.1136/bjsports-2014-094302.
53. Papalia R, Del Buono A, Franceschi F, Marinozzi A, Maffulli N, Denaro V. Femoroacetabular impingement syndrome management: arthroscopy or open surgery? Int Orthop. 2012;36(5):903-914. doi:10.1007/s00264-011-1443-z.
54. Locks R, Utsunomiya H, Briggs KK, McNamara S, Chahla J, Philippon MJ. Return to play after hip arthroscopic surgery for femoroacetabular impingement in professional soccer players. Am J Sports Med. 2018;46(2):273-279. doi:10.1177/0363546517738741.
55. Kelly BT, Weiland DE, Schenker ML, Philippon MJ. Arthroscopic labral repair in the hip: surgical technique and review of the literature. Arthroscopy. 2005;21(12):1496-1504. doi:10.1016/j.arthro.2005.08.013.
56. Wenger DE, Kendell KR, Miner MR, Trousdale RT. Acetabular labral tears rarely occur in the absence of bony abnormalities. Clin Orthop Relat Res. 2004;426:145-150. doi:10.1097/01.blo.0000136903.01368.20.
57. Domb BG, Hartigan DE, Perets I. Decision making for labral treatment in the hip: repair versus débridement versus reconstruction. J Am Acad Orthop Surg. 2017;25(3):e53-e62. doi:10.5435/JAAOS-D-16-00144.
58. Frank JS, Gambacorta PL, Eisner EA. Hip pathology in the adolescent athlete. J Am Acad Orthop Surg. 2013;21(11):665-674. doi:10.5435/JAAOS-21-11-665.
59. Singh PJ, O'Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26(6):743-749. doi:10.1016/j.arthro.2009.10.010.
60. Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25(2):327-335. doi:10.1016/j.csm.2005.12.004.
61. Larson CM, Pierce BR, Giveans MR. Treatment of athletes with symptomatic intra-articular hip pathology and athletic pubalgia/sports hernia: a case series. Arthroscopy.2011;27(6):768-775. doi:10.1016/j.arthro.2011.01.018.
62. Wollin M, Thorborg K, Welvaert M, Pizzari T. In-season monitoring of hip and groin strength, health and function in elite youth soccer: implementing an early detection and management strategy over two consecutive seasons. J Sci Med Sport. 2018;21(10):988. doi:10.1016/j.jsams.2018.03.004.
63. Charlton PC, Drew MK, Mentiplay BF, Grimaldi A, Clark RA. Exercise interventions for the prevention and treatment of groin pain and injury in athletes: a critical and systematic review. Sports Med. 2017;47:2011. doi:10.1007/s40279-017-0742-y.
TAKE-HOME POINTS
- Groin injuries in soccer players can cause significant decreases in athletic performance, result in lost playing time, and may ultimately need surgical intervention.
- Groin pain can be separated into 3 categories: (1) defined clinical entities for groin pain (adductor-related, iliopsoas-related, inguinal-related [sports hernias/athletic pubalgia], and pubic-related groin pain), (2) hip-related groin pain (hip morphologic abnormalities, labral tears, and chondral injuries), and (3) other causes of groin pain.
- Acute groin pain in soccer players is most commonly caused by muscle strain involving the adductor longus, the iliopsoas or the rectus femoris.
- Inguinal-related groin pain is a common cause of chronic groin pain and typically is the most challenging to treat with a complex pathophysiology and a high association with femoroacetabular impingement.
- Hip-related groin pain (femoroacetabular impingement, labral tears, and chondral injuries) usually respond well to surgical intervention and has high rates of return to sport.
The Effect of Playing Position on Injury Risk in Male Soccer Players: Systematic Review of the Literature and Risk Considerations for Each Playing Position
ABSTRACT
Soccer (football) is a complex contact sport with a substantial risk of injury. As injury surveillance is the first step of the injury prevention paradigm, soccer epidemiology is well reported in the existing literature, but less is known about the actual role of player position on the general injury risk.
The goal of this study is to present the existing evidence regarding the influence of player’s position on general injury risk in male soccer.
A systematic review of the Medline database was carried out. Only English written studies on male soccer and citing playing position as a possible determinant of injury risk were included. One hundred and two full texts were evaluated for eligibility, and 11 studies were selected for the qualitative synthesis.
Of the 11 studies included in the systematic review, 5 didn’t find any significant correlation with between player’s position and general injury risk, while the remaining 6 studies found player’s position to be correlated with injury risk, with mixed findings depending on each study. The most consistent finding was a tendency for goalkeepers (GKs) to sustain less injuries compared to outfield players. When considering only the studies reporting just the match injury risk, forwards seemed to be at higher risk, even if there wasn’t a complete agreement.
Few studies have evaluated a possible effect of playing position on general injury risk in male soccer. There is no agreement if weather or not different playing positions are associated to a higher injury risk. GKs seem to be at lower risk of injury when compared to outfield players.
Continue to: Soccer, known worldwide as football, is the most...
Soccer, known worldwide as football, is the most common practiced sport worldwide. Soccer is also a complex contact sport with a substantial injury risk,1 that is well documented in the current literature. According to a recent systematic review, general injury incidence in male soccer ranged from 2.0 injuries to 19.4 injuries per 1000 hours of exposure in youth male soccer and from 2.48 injuries to 9.4 injuries per 1000 hours of exposure in elite male soccer.1 It is also well established that the injury risk is greater in matches than training.1 Soccer’s injuries are well known to be a socioeconomic burden for elite, youth, and recreational players.2 Different authors have underlined the problem that nowadays the game is faster, and players need to have a better physical performance as they are subjected to important efforts both metabolically and biomechanically during match play. In the last decades, thanks to different research groups involved with professional soccer,3 there has been an increasing interest in soccer injuries’ epidemiology and for preventative measures.4 A deep comprehension of injury epidemiology is in fact the first necessary step for successful preventative measures. Regarding a possible correlation between playing position and injury incidence, there is a lack of consensus in the literature. Player position (goalkeeper [GK], defenders, midfielder [MF], or striker) may affect injury risk, as different roles are associated to different intensity during match play5 and experience different combination of anticipated or non-anticipated movement patterns.6 Previous authors underlined that few studies have evaluated a possible influence of playing position on injury incidence and severity.7
The main goal of this systematic review is to present the existing evidence regarding the influence of player position on injury incidence in male soccer and to present practical considerations on each field position in relation to the injury’s risk.
METHODS
DATA SOURCES AND SELECTION CRITERIA
We searched the Medline database for key terms and their variations to identify appropriate studies on injury epidemiology in soccer and specific player position influence. The keywords included: injury epidemiology soccer [OR] injury epidemiology football; position specific injury epidemiology soccer [OR] football. We limited our search to originally published English-language research articles.
Relevant data were extracted for study characteristics to ensure the included studies met certain criteria. The inclusion criteria were prospective design with minimum 6-month observational period, exclusively male soccer players’ cohorts, reported injury incidence, and documented player position in correlation with a measure of injury risk.
As stated above, we only included studies on male soccer. We also did not consider studies limited to a single injury type, considering only studies analyzing and documenting all injuries. We did not exclude studies on youth soccer but we didn’t consider studies on ≥2 more sports or mixed male and female studies.
Data were extracted by an author (FDV) and qualitatively controlled by another one (BM). Controversy were solved through discussion or confrontation with another author (LL).
Results of the included studies are presented only qualitatively because of different methodologies we encountered in documenting the potential effects of player’s role. Some studies reported differences in injury incidence within groups, others reported the proportion of injuries for each subgroup.
Continue to: RESULTS...
RESULTS
STUDY SELECTION
Of the 1609 potential items we found in the existing literature, 102 full-text articles were screened for eligibility. Only 11 papers met the inclusion criteria and were included in the systematic review, including 2 studies on youth soccer and 9 studies on adult soccer (Figure 1). Five of the selected studies tracked only match injuries, while the remaining 6 studies presented data on both match and training injuries. As a matter of fact, the effect of player position was not so commonly evaluated or at least reported in the existing literature. Studies’ characteristics and main findings regarding player’s position are reported in Table.
GENERAL INJURY RISK AND PLAYING POSITION
Of the 11 studies included for qualitative synthesis, 5 studies reported no significant effect of player’s position on general risk of injury,7-11 3 studies reported a greater risk in forwards,12-14 1 study reported a greater risk in MFs,15 1 study reported a greater risk in forwards and central defenders,8 and finally 1 study reported a significant lower risk in GKs.16 Additionally, 2 more studies reported GKs to be at the lowest injury risk,12,13 another study reported GKs to have lost the lower number of matches,8 1 study didn’t consider the GK position in the analysis due to the low number of injuries,17 limiting the analysis on the outfield positions.
Out of the 5 studies reporting no significant effect of playing position on injury risk, 1 study found a tendency to more injuries in forward players,10 a second study found a tendency for higher injury risk in midfielders,18 and a third study found a tendency for higher risk in defenders.17 Considering only the 5 studies reporting data on match injuries, 3 reported a higher risk in forwards,12-14 while a fourth one reported a tendency for increased risk in forwards10 even if not statistically significant. On the other hand, evaluating the 6 studies reporting data on match and training injuries, most of the studies, 4 out of 6, reported no effect of playing position.17-20 The main findings of the studies are also expressed graphically in Figures 2A, 2B.
DISCUSSION
The main finding of this study is that there is substantially no agreement regarding the effect of player position on general injury risk in male soccer.
First, we must underline that not many studies have evaluated prospectively the influence of player’s position on injury risk. Of the 11 selected studies, 5 (5/11) reported no significant effect of playing position,7,10,17,18,20 while the remaining studies (6/11)8,12-16 reported a significant effect of player position on the risk of injury, with various results depending on the single study. It should be noted that the 2 studies with the longest observational period (15 consecutive seasons)16,19 did not report any difference in injury risk considering only the outfield playing positions.
Continue to: We will now review the findings...
We will now review the findings of our systematic review based on player position. One of the more consistent trends that we found is the possible occurrence of different injury epidemiology in GKs compared to outfield players. One study reported a significant lower incidence of match injuries for GKs, 12.9 injuries per 1000 game hours vs 22.6 injuries per 1000 game hours of outfield positions.16 This result is remarkable, even considering the very long observational period (15 seasons). Other 2 studies, not reporting position specific injury incidence (but proportion of injuries) also agreed on the topic.12,13 On the other hand, Morgan and Oberlander9 reported no differences between GKs and other positions. Anecdotally, unpublished Major League Soccer data regarding the most recent seasons seems to support these findings with GKs sustaining the lower proportion of injuries. By a physiological point of view, somatotype and body composition have been reported to differ between GKs and the other playing positions in young male soccer players.21 The uniqueness of the GK somatotype and role may reflect on a predisposition to a different pattern of injuries. Ekstrand and colleagues22 reported that GKs have a higher incidence of upper extremity fractures, the same group demonstrated a possible tendency for more head and neck injuries9 and a lower risk of medial collateral ligament injuries.23 On the other hand, GKs seems to be at lower injury risk for the playing pattern differences with outfield players. The reduced distance GKs cover during the match, as well as less direct contacts with opponents, may be factors that potentially explain this finding.
In relation to forwards, 4 studies interestingly stated that forwards were at increased risk of injury,12-14 although 1 report had similar risk of injury with forwards and defenders.8 Most of the studies only on match injuries reported some association between forward position and injury risk (Figures 2A, 2B), so attackers may be at higher risk of match injuries when compared to the other playing positions. There are different possible explanations for this finding. First, it is demonstrated that the clear majority of soccer incidents happen in the mid-defensive zone and in the score-box,24 2 typical attackers’ zones, where most of duels and tackles may happen. So, forwards may be more prone to match injuries because of the intensity of match play in their typical playing zones. Also, fast kicking and acceleration/deceleration activities of the attackers may predispose for thigh muscle injuries, accounting up to 25% of the total lay off time in professional soccer.25 However, these considerations are still yet to be proven.
When considering defenders, 1 report indicated defenders (and forwards) to be at potential greater risk of injury,8which is similar to the report from Shalaj and colleagues,17 although it did not report a statistically significant result. A direct playing style, with defenders and strikers being more involved in the game can potentially explain this finding. However, the specific epidemiology of defenders may be more complicated. Defenders may be predisposed to knee injuries, such as injury to the anterior cruciate ligament (ACL). In fact, Walden and colleagues,11 in a video-analysis study, reported that the 77% of ACL injuries happened in defending situations. In addition, Brophy and colleagues,6 in another video-analysis study, reported a 73% of ACL injuries happened while defending. A likely explanation is the nature of the defender’s role in soccer, reactive to the attacking team actions. Many times, defenders try high risk maneuvers while tackling the opponent, with minimal motor planning time and consciousness. This is well described by Walden and colleagues,11 with the pressing mechanism ACL injury, when the injured player is pressing the opponent in the attempt to get the ball but eventually falls into a high-risk position.
When considering MFs, Deehan and colleagues15 found a significant higher risk in MFs in youth soccer. This result is partially according to Morgan and Oberlander18 who reported a non-statistically significant greater injury incidence in MFs. MFs are generally the players that cover more distance during a soccer match and it is logical to think that they would be predisposed to a large volume of acceleration/deceleration activities,19 potentially relating to injury risk, especially to muscles injuries. A previous study on thigh muscle injuries in youth soccer reporting higher injury risk in MFs, followed by forwards.19 Consistent with these results, another study on a mixed male and female cohort on high school soccer revealed more injuries in MFs, followed by forwards.26
Continue to: The results of this systematic review...
The results of this systematic review reveal mixed reports on injury risk in relation to playing position, the more consistent results through studies was that GKs may be at lower injury risk compared to the outfield players, even if there wasn’t complete agreement. One should note that in modern soccer the specific role of any player at 1 position may not be entirely consistent with another player in the same position. Within the same “position group”, there may also be players with completely different qualitative playing demands (eg, wing defender and central defender). So, even with the strongest study design, it may be difficult to give a simple and clear message about playing position and injury risk due to the variability of the playing styles and players at each position.
This study has several limitations and the results must be considered and interpreted with caution. First, we limited our search to male soccer, so the results may not be applicable to female soccer. Secondly, the interpretation of study findings wasn’t easy because of the different report modalities of the different papers included in the systematic review. Finally, we included reports from a total of a 23-year time span and from different countries and continents. The game may have evolved through years and there may be differences in the style of playing within countries that potentially could interfere with injury risk.
However, this is the first paper systematically evaluating the existing literature on position specific injury risk in male soccer players. Future studies, with prospective design and a consistent method to evaluate the player position as a potential factor related to injury risk, are needed. Match and training injuries should be evaluated separately as playing position may be more related to match injury risk.
CONCLUSION
There is no agreement in the existing literature regarding weather or not player position influence the general injury risk in male soccer. The GKs may have a lower risk of injury if compared to outfield players.
1. Pfirrmann D, Herbst M, Ingelfinger P, Simon P, Tug S. Analysis of injury incidences in male professional adult and elite youth soccer players: a systematic review. J Athl Train. 2016;51(5):410-424. doi:10.4085/1062-6050-51.6.03.
2. Eirale C, Gillogly S, Singh G, Chamari K. Injury and illness epidemiology in soccer - effects of global geographical differences - a call for standardized and consistent research studies. Biol Sport. 2017;34(3):249-254. doi:10.5114/biolsport.2017.66002.
3. Ekstrand J, Hägglund M, Waldén M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
4. Silvers-Granelli HJ, Bizzini M, Arundale A, Mandelbaum BR, Snyder-Mackler L. Does the FIFA 11+ Injury Prevention Program reduce the incidence of ACL injury in male soccer players? Clin Orthop Relat Res. 2017;475(10):2447-2455. doi:10.1007/s11999-017-5342-5.
5. Di Salvo V, Gregson W, Atkinson G, Tordoff P, Drust B. Analysis of high intensity activity in premier league soccer. Int J Sports Med. 2009;30(3):205-212. doi:10.1055/s-0028-1105950.
6. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
7. Dauty M, Collon S. Incidence of injuries in French professional soccer players. Int J Sports Med. 2011;32(12):965-969. doi:10.1055/s-0031-1283188.
8. Mallo J, Dellal A. Injury risk in professional football players with special reference to the playing position and training periodization. J Sports Med Phys Fitness. 2012;52(6):631-638.
9. Nilsson M, Hägglund M, Ekstrand J, Waldén M. Head and neck injuries in professional soccer. Clin J Sport Med. 2013;23(4):255-260. doi:10.1097/JSM.0b013e31827ee6f8.
10. Timpka T, Risto O, Björmsjö M. Boys soccer league injuries: a community-based study of time-loss from sports participation and long-term sequelae. Eur J Public Health. 2008;18(1):19-24.
11. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
12. Andersen TE, Larsen Ø, Tenga A, Engebretsen L, Bahr R. Football incident analysis: a new video based method to describe injury mechanisms in professional football. Br J Sports Med. 2003;37(3):226-232.
13. Arliani GG, Lara PHS, Astur DC, Pedrinelli A, Pagura JR, Cohen M. Orthopaedics injuries in male professional football players in Brazil: a prospective comparison between two divisions. Muscles Ligaments Tendons J. 2018;7(3), 524-531. doi:10.11138/mltj/2017.7.3.524.
14. Carling C, Orhant E, LeGall F. Match injuries in professional soccer: inter-seasonal variation and effects of competition type, match congestion and positional role. Int J Sports Med. 2010;31(4):271-276. doi:10.1055/s-0029-1243646.
15. Deehan DJ, Bell K, McCaskie AW. Adolescent musculoskeletal injuries in a football academy. J Bone Joint Surg Br. 2007;89(1):5-8.
16. Aoki H, O'Hata N, Kohno T, Morikawa T, Seki J. A 15-year prospective epidemiological account of acute traumatic injuries during official professional soccer league matches in Japan. Am J Sports Med. 2012;40(5):1006-1014. doi:10.1177/0363546512438695.
17. Shalaj I, Tishukaj F, Bachl N, Tschan H, Wessner B, Csapo R. Injuries in professional male football players in Kosovo: a descriptive epidemiological study. BMC Musculoskelet Disord. 2016;17:338. doi:10.1186/s12891-016-1202-9.
18. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430.
19. Cloke D, Moore O, Shah T, Rushton S, Shirley MD, Deehan DJ. Thigh muscle injuries in youth soccer: predictors of recovery. Am J Sports Med. 2012;40(2):433-439. doi:10.1177/0363546511428800.
20. Mallo J, González P, Veiga S, Navarro E. Injury incidence in a spanish sub-elite professional football team: a prospective study during four consecutive seasons. J Sports Sci Med. 2011;10(4):731-736.
21. Cárdenas-Fernández V, Chinchilla-Minguet JL, Castillo-Rodríguez A. Somatotype and body composition in young soccer players according to the playing position and sport success. J Strength Cond Res. 2017. doi:10.1519/JSC.0000000000002125. [Epub ahead of print]
22. Ekstrand J, Hägglund M, Törnqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
23. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
24. Andersen TE, Tenga A, Engebretsen L, Bahr R. Video analysis of injuries and incidents in Norwegian professional football. Br J Sports Med. 2004;38(5):626-631.
25. Ueblacker P, Müller-Wohlfahrt HW, Ekstrand J. Epidemiological and clinical outcome comparison of indirect (‘strain’) versus direct (‘contusion’) anterior and posterior thigh muscle injuries in male elite football players: UEFA Elite League study of 2287 thigh injuries (2001-2013). Br J Sports Med. 2015;49(22):1461-1465. doi:10.1136/bjsports-2014-094285.
26. Khodaee M, Currie DW, Asif IM, Comstock RD. Nine-year study of US high school soccer injuries: data from a national sports injury surveillance programme. Br J Sports Med. 2017;51(3):185-193. doi:10.1136/bjsports-2015-095946.
ABSTRACT
Soccer (football) is a complex contact sport with a substantial risk of injury. As injury surveillance is the first step of the injury prevention paradigm, soccer epidemiology is well reported in the existing literature, but less is known about the actual role of player position on the general injury risk.
The goal of this study is to present the existing evidence regarding the influence of player’s position on general injury risk in male soccer.
A systematic review of the Medline database was carried out. Only English written studies on male soccer and citing playing position as a possible determinant of injury risk were included. One hundred and two full texts were evaluated for eligibility, and 11 studies were selected for the qualitative synthesis.
Of the 11 studies included in the systematic review, 5 didn’t find any significant correlation with between player’s position and general injury risk, while the remaining 6 studies found player’s position to be correlated with injury risk, with mixed findings depending on each study. The most consistent finding was a tendency for goalkeepers (GKs) to sustain less injuries compared to outfield players. When considering only the studies reporting just the match injury risk, forwards seemed to be at higher risk, even if there wasn’t a complete agreement.
Few studies have evaluated a possible effect of playing position on general injury risk in male soccer. There is no agreement if weather or not different playing positions are associated to a higher injury risk. GKs seem to be at lower risk of injury when compared to outfield players.
Continue to: Soccer, known worldwide as football, is the most...
Soccer, known worldwide as football, is the most common practiced sport worldwide. Soccer is also a complex contact sport with a substantial injury risk,1 that is well documented in the current literature. According to a recent systematic review, general injury incidence in male soccer ranged from 2.0 injuries to 19.4 injuries per 1000 hours of exposure in youth male soccer and from 2.48 injuries to 9.4 injuries per 1000 hours of exposure in elite male soccer.1 It is also well established that the injury risk is greater in matches than training.1 Soccer’s injuries are well known to be a socioeconomic burden for elite, youth, and recreational players.2 Different authors have underlined the problem that nowadays the game is faster, and players need to have a better physical performance as they are subjected to important efforts both metabolically and biomechanically during match play. In the last decades, thanks to different research groups involved with professional soccer,3 there has been an increasing interest in soccer injuries’ epidemiology and for preventative measures.4 A deep comprehension of injury epidemiology is in fact the first necessary step for successful preventative measures. Regarding a possible correlation between playing position and injury incidence, there is a lack of consensus in the literature. Player position (goalkeeper [GK], defenders, midfielder [MF], or striker) may affect injury risk, as different roles are associated to different intensity during match play5 and experience different combination of anticipated or non-anticipated movement patterns.6 Previous authors underlined that few studies have evaluated a possible influence of playing position on injury incidence and severity.7
The main goal of this systematic review is to present the existing evidence regarding the influence of player position on injury incidence in male soccer and to present practical considerations on each field position in relation to the injury’s risk.
METHODS
DATA SOURCES AND SELECTION CRITERIA
We searched the Medline database for key terms and their variations to identify appropriate studies on injury epidemiology in soccer and specific player position influence. The keywords included: injury epidemiology soccer [OR] injury epidemiology football; position specific injury epidemiology soccer [OR] football. We limited our search to originally published English-language research articles.
Relevant data were extracted for study characteristics to ensure the included studies met certain criteria. The inclusion criteria were prospective design with minimum 6-month observational period, exclusively male soccer players’ cohorts, reported injury incidence, and documented player position in correlation with a measure of injury risk.
As stated above, we only included studies on male soccer. We also did not consider studies limited to a single injury type, considering only studies analyzing and documenting all injuries. We did not exclude studies on youth soccer but we didn’t consider studies on ≥2 more sports or mixed male and female studies.
Data were extracted by an author (FDV) and qualitatively controlled by another one (BM). Controversy were solved through discussion or confrontation with another author (LL).
Results of the included studies are presented only qualitatively because of different methodologies we encountered in documenting the potential effects of player’s role. Some studies reported differences in injury incidence within groups, others reported the proportion of injuries for each subgroup.
Continue to: RESULTS...
RESULTS
STUDY SELECTION
Of the 1609 potential items we found in the existing literature, 102 full-text articles were screened for eligibility. Only 11 papers met the inclusion criteria and were included in the systematic review, including 2 studies on youth soccer and 9 studies on adult soccer (Figure 1). Five of the selected studies tracked only match injuries, while the remaining 6 studies presented data on both match and training injuries. As a matter of fact, the effect of player position was not so commonly evaluated or at least reported in the existing literature. Studies’ characteristics and main findings regarding player’s position are reported in Table.
GENERAL INJURY RISK AND PLAYING POSITION
Of the 11 studies included for qualitative synthesis, 5 studies reported no significant effect of player’s position on general risk of injury,7-11 3 studies reported a greater risk in forwards,12-14 1 study reported a greater risk in MFs,15 1 study reported a greater risk in forwards and central defenders,8 and finally 1 study reported a significant lower risk in GKs.16 Additionally, 2 more studies reported GKs to be at the lowest injury risk,12,13 another study reported GKs to have lost the lower number of matches,8 1 study didn’t consider the GK position in the analysis due to the low number of injuries,17 limiting the analysis on the outfield positions.
Out of the 5 studies reporting no significant effect of playing position on injury risk, 1 study found a tendency to more injuries in forward players,10 a second study found a tendency for higher injury risk in midfielders,18 and a third study found a tendency for higher risk in defenders.17 Considering only the 5 studies reporting data on match injuries, 3 reported a higher risk in forwards,12-14 while a fourth one reported a tendency for increased risk in forwards10 even if not statistically significant. On the other hand, evaluating the 6 studies reporting data on match and training injuries, most of the studies, 4 out of 6, reported no effect of playing position.17-20 The main findings of the studies are also expressed graphically in Figures 2A, 2B.
DISCUSSION
The main finding of this study is that there is substantially no agreement regarding the effect of player position on general injury risk in male soccer.
First, we must underline that not many studies have evaluated prospectively the influence of player’s position on injury risk. Of the 11 selected studies, 5 (5/11) reported no significant effect of playing position,7,10,17,18,20 while the remaining studies (6/11)8,12-16 reported a significant effect of player position on the risk of injury, with various results depending on the single study. It should be noted that the 2 studies with the longest observational period (15 consecutive seasons)16,19 did not report any difference in injury risk considering only the outfield playing positions.
Continue to: We will now review the findings...
We will now review the findings of our systematic review based on player position. One of the more consistent trends that we found is the possible occurrence of different injury epidemiology in GKs compared to outfield players. One study reported a significant lower incidence of match injuries for GKs, 12.9 injuries per 1000 game hours vs 22.6 injuries per 1000 game hours of outfield positions.16 This result is remarkable, even considering the very long observational period (15 seasons). Other 2 studies, not reporting position specific injury incidence (but proportion of injuries) also agreed on the topic.12,13 On the other hand, Morgan and Oberlander9 reported no differences between GKs and other positions. Anecdotally, unpublished Major League Soccer data regarding the most recent seasons seems to support these findings with GKs sustaining the lower proportion of injuries. By a physiological point of view, somatotype and body composition have been reported to differ between GKs and the other playing positions in young male soccer players.21 The uniqueness of the GK somatotype and role may reflect on a predisposition to a different pattern of injuries. Ekstrand and colleagues22 reported that GKs have a higher incidence of upper extremity fractures, the same group demonstrated a possible tendency for more head and neck injuries9 and a lower risk of medial collateral ligament injuries.23 On the other hand, GKs seems to be at lower injury risk for the playing pattern differences with outfield players. The reduced distance GKs cover during the match, as well as less direct contacts with opponents, may be factors that potentially explain this finding.
In relation to forwards, 4 studies interestingly stated that forwards were at increased risk of injury,12-14 although 1 report had similar risk of injury with forwards and defenders.8 Most of the studies only on match injuries reported some association between forward position and injury risk (Figures 2A, 2B), so attackers may be at higher risk of match injuries when compared to the other playing positions. There are different possible explanations for this finding. First, it is demonstrated that the clear majority of soccer incidents happen in the mid-defensive zone and in the score-box,24 2 typical attackers’ zones, where most of duels and tackles may happen. So, forwards may be more prone to match injuries because of the intensity of match play in their typical playing zones. Also, fast kicking and acceleration/deceleration activities of the attackers may predispose for thigh muscle injuries, accounting up to 25% of the total lay off time in professional soccer.25 However, these considerations are still yet to be proven.
When considering defenders, 1 report indicated defenders (and forwards) to be at potential greater risk of injury,8which is similar to the report from Shalaj and colleagues,17 although it did not report a statistically significant result. A direct playing style, with defenders and strikers being more involved in the game can potentially explain this finding. However, the specific epidemiology of defenders may be more complicated. Defenders may be predisposed to knee injuries, such as injury to the anterior cruciate ligament (ACL). In fact, Walden and colleagues,11 in a video-analysis study, reported that the 77% of ACL injuries happened in defending situations. In addition, Brophy and colleagues,6 in another video-analysis study, reported a 73% of ACL injuries happened while defending. A likely explanation is the nature of the defender’s role in soccer, reactive to the attacking team actions. Many times, defenders try high risk maneuvers while tackling the opponent, with minimal motor planning time and consciousness. This is well described by Walden and colleagues,11 with the pressing mechanism ACL injury, when the injured player is pressing the opponent in the attempt to get the ball but eventually falls into a high-risk position.
When considering MFs, Deehan and colleagues15 found a significant higher risk in MFs in youth soccer. This result is partially according to Morgan and Oberlander18 who reported a non-statistically significant greater injury incidence in MFs. MFs are generally the players that cover more distance during a soccer match and it is logical to think that they would be predisposed to a large volume of acceleration/deceleration activities,19 potentially relating to injury risk, especially to muscles injuries. A previous study on thigh muscle injuries in youth soccer reporting higher injury risk in MFs, followed by forwards.19 Consistent with these results, another study on a mixed male and female cohort on high school soccer revealed more injuries in MFs, followed by forwards.26
Continue to: The results of this systematic review...
The results of this systematic review reveal mixed reports on injury risk in relation to playing position, the more consistent results through studies was that GKs may be at lower injury risk compared to the outfield players, even if there wasn’t complete agreement. One should note that in modern soccer the specific role of any player at 1 position may not be entirely consistent with another player in the same position. Within the same “position group”, there may also be players with completely different qualitative playing demands (eg, wing defender and central defender). So, even with the strongest study design, it may be difficult to give a simple and clear message about playing position and injury risk due to the variability of the playing styles and players at each position.
This study has several limitations and the results must be considered and interpreted with caution. First, we limited our search to male soccer, so the results may not be applicable to female soccer. Secondly, the interpretation of study findings wasn’t easy because of the different report modalities of the different papers included in the systematic review. Finally, we included reports from a total of a 23-year time span and from different countries and continents. The game may have evolved through years and there may be differences in the style of playing within countries that potentially could interfere with injury risk.
However, this is the first paper systematically evaluating the existing literature on position specific injury risk in male soccer players. Future studies, with prospective design and a consistent method to evaluate the player position as a potential factor related to injury risk, are needed. Match and training injuries should be evaluated separately as playing position may be more related to match injury risk.
CONCLUSION
There is no agreement in the existing literature regarding weather or not player position influence the general injury risk in male soccer. The GKs may have a lower risk of injury if compared to outfield players.
ABSTRACT
Soccer (football) is a complex contact sport with a substantial risk of injury. As injury surveillance is the first step of the injury prevention paradigm, soccer epidemiology is well reported in the existing literature, but less is known about the actual role of player position on the general injury risk.
The goal of this study is to present the existing evidence regarding the influence of player’s position on general injury risk in male soccer.
A systematic review of the Medline database was carried out. Only English written studies on male soccer and citing playing position as a possible determinant of injury risk were included. One hundred and two full texts were evaluated for eligibility, and 11 studies were selected for the qualitative synthesis.
Of the 11 studies included in the systematic review, 5 didn’t find any significant correlation with between player’s position and general injury risk, while the remaining 6 studies found player’s position to be correlated with injury risk, with mixed findings depending on each study. The most consistent finding was a tendency for goalkeepers (GKs) to sustain less injuries compared to outfield players. When considering only the studies reporting just the match injury risk, forwards seemed to be at higher risk, even if there wasn’t a complete agreement.
Few studies have evaluated a possible effect of playing position on general injury risk in male soccer. There is no agreement if weather or not different playing positions are associated to a higher injury risk. GKs seem to be at lower risk of injury when compared to outfield players.
Continue to: Soccer, known worldwide as football, is the most...
Soccer, known worldwide as football, is the most common practiced sport worldwide. Soccer is also a complex contact sport with a substantial injury risk,1 that is well documented in the current literature. According to a recent systematic review, general injury incidence in male soccer ranged from 2.0 injuries to 19.4 injuries per 1000 hours of exposure in youth male soccer and from 2.48 injuries to 9.4 injuries per 1000 hours of exposure in elite male soccer.1 It is also well established that the injury risk is greater in matches than training.1 Soccer’s injuries are well known to be a socioeconomic burden for elite, youth, and recreational players.2 Different authors have underlined the problem that nowadays the game is faster, and players need to have a better physical performance as they are subjected to important efforts both metabolically and biomechanically during match play. In the last decades, thanks to different research groups involved with professional soccer,3 there has been an increasing interest in soccer injuries’ epidemiology and for preventative measures.4 A deep comprehension of injury epidemiology is in fact the first necessary step for successful preventative measures. Regarding a possible correlation between playing position and injury incidence, there is a lack of consensus in the literature. Player position (goalkeeper [GK], defenders, midfielder [MF], or striker) may affect injury risk, as different roles are associated to different intensity during match play5 and experience different combination of anticipated or non-anticipated movement patterns.6 Previous authors underlined that few studies have evaluated a possible influence of playing position on injury incidence and severity.7
The main goal of this systematic review is to present the existing evidence regarding the influence of player position on injury incidence in male soccer and to present practical considerations on each field position in relation to the injury’s risk.
METHODS
DATA SOURCES AND SELECTION CRITERIA
We searched the Medline database for key terms and their variations to identify appropriate studies on injury epidemiology in soccer and specific player position influence. The keywords included: injury epidemiology soccer [OR] injury epidemiology football; position specific injury epidemiology soccer [OR] football. We limited our search to originally published English-language research articles.
Relevant data were extracted for study characteristics to ensure the included studies met certain criteria. The inclusion criteria were prospective design with minimum 6-month observational period, exclusively male soccer players’ cohorts, reported injury incidence, and documented player position in correlation with a measure of injury risk.
As stated above, we only included studies on male soccer. We also did not consider studies limited to a single injury type, considering only studies analyzing and documenting all injuries. We did not exclude studies on youth soccer but we didn’t consider studies on ≥2 more sports or mixed male and female studies.
Data were extracted by an author (FDV) and qualitatively controlled by another one (BM). Controversy were solved through discussion or confrontation with another author (LL).
Results of the included studies are presented only qualitatively because of different methodologies we encountered in documenting the potential effects of player’s role. Some studies reported differences in injury incidence within groups, others reported the proportion of injuries for each subgroup.
Continue to: RESULTS...
RESULTS
STUDY SELECTION
Of the 1609 potential items we found in the existing literature, 102 full-text articles were screened for eligibility. Only 11 papers met the inclusion criteria and were included in the systematic review, including 2 studies on youth soccer and 9 studies on adult soccer (Figure 1). Five of the selected studies tracked only match injuries, while the remaining 6 studies presented data on both match and training injuries. As a matter of fact, the effect of player position was not so commonly evaluated or at least reported in the existing literature. Studies’ characteristics and main findings regarding player’s position are reported in Table.
GENERAL INJURY RISK AND PLAYING POSITION
Of the 11 studies included for qualitative synthesis, 5 studies reported no significant effect of player’s position on general risk of injury,7-11 3 studies reported a greater risk in forwards,12-14 1 study reported a greater risk in MFs,15 1 study reported a greater risk in forwards and central defenders,8 and finally 1 study reported a significant lower risk in GKs.16 Additionally, 2 more studies reported GKs to be at the lowest injury risk,12,13 another study reported GKs to have lost the lower number of matches,8 1 study didn’t consider the GK position in the analysis due to the low number of injuries,17 limiting the analysis on the outfield positions.
Out of the 5 studies reporting no significant effect of playing position on injury risk, 1 study found a tendency to more injuries in forward players,10 a second study found a tendency for higher injury risk in midfielders,18 and a third study found a tendency for higher risk in defenders.17 Considering only the 5 studies reporting data on match injuries, 3 reported a higher risk in forwards,12-14 while a fourth one reported a tendency for increased risk in forwards10 even if not statistically significant. On the other hand, evaluating the 6 studies reporting data on match and training injuries, most of the studies, 4 out of 6, reported no effect of playing position.17-20 The main findings of the studies are also expressed graphically in Figures 2A, 2B.
DISCUSSION
The main finding of this study is that there is substantially no agreement regarding the effect of player position on general injury risk in male soccer.
First, we must underline that not many studies have evaluated prospectively the influence of player’s position on injury risk. Of the 11 selected studies, 5 (5/11) reported no significant effect of playing position,7,10,17,18,20 while the remaining studies (6/11)8,12-16 reported a significant effect of player position on the risk of injury, with various results depending on the single study. It should be noted that the 2 studies with the longest observational period (15 consecutive seasons)16,19 did not report any difference in injury risk considering only the outfield playing positions.
Continue to: We will now review the findings...
We will now review the findings of our systematic review based on player position. One of the more consistent trends that we found is the possible occurrence of different injury epidemiology in GKs compared to outfield players. One study reported a significant lower incidence of match injuries for GKs, 12.9 injuries per 1000 game hours vs 22.6 injuries per 1000 game hours of outfield positions.16 This result is remarkable, even considering the very long observational period (15 seasons). Other 2 studies, not reporting position specific injury incidence (but proportion of injuries) also agreed on the topic.12,13 On the other hand, Morgan and Oberlander9 reported no differences between GKs and other positions. Anecdotally, unpublished Major League Soccer data regarding the most recent seasons seems to support these findings with GKs sustaining the lower proportion of injuries. By a physiological point of view, somatotype and body composition have been reported to differ between GKs and the other playing positions in young male soccer players.21 The uniqueness of the GK somatotype and role may reflect on a predisposition to a different pattern of injuries. Ekstrand and colleagues22 reported that GKs have a higher incidence of upper extremity fractures, the same group demonstrated a possible tendency for more head and neck injuries9 and a lower risk of medial collateral ligament injuries.23 On the other hand, GKs seems to be at lower injury risk for the playing pattern differences with outfield players. The reduced distance GKs cover during the match, as well as less direct contacts with opponents, may be factors that potentially explain this finding.
In relation to forwards, 4 studies interestingly stated that forwards were at increased risk of injury,12-14 although 1 report had similar risk of injury with forwards and defenders.8 Most of the studies only on match injuries reported some association between forward position and injury risk (Figures 2A, 2B), so attackers may be at higher risk of match injuries when compared to the other playing positions. There are different possible explanations for this finding. First, it is demonstrated that the clear majority of soccer incidents happen in the mid-defensive zone and in the score-box,24 2 typical attackers’ zones, where most of duels and tackles may happen. So, forwards may be more prone to match injuries because of the intensity of match play in their typical playing zones. Also, fast kicking and acceleration/deceleration activities of the attackers may predispose for thigh muscle injuries, accounting up to 25% of the total lay off time in professional soccer.25 However, these considerations are still yet to be proven.
When considering defenders, 1 report indicated defenders (and forwards) to be at potential greater risk of injury,8which is similar to the report from Shalaj and colleagues,17 although it did not report a statistically significant result. A direct playing style, with defenders and strikers being more involved in the game can potentially explain this finding. However, the specific epidemiology of defenders may be more complicated. Defenders may be predisposed to knee injuries, such as injury to the anterior cruciate ligament (ACL). In fact, Walden and colleagues,11 in a video-analysis study, reported that the 77% of ACL injuries happened in defending situations. In addition, Brophy and colleagues,6 in another video-analysis study, reported a 73% of ACL injuries happened while defending. A likely explanation is the nature of the defender’s role in soccer, reactive to the attacking team actions. Many times, defenders try high risk maneuvers while tackling the opponent, with minimal motor planning time and consciousness. This is well described by Walden and colleagues,11 with the pressing mechanism ACL injury, when the injured player is pressing the opponent in the attempt to get the ball but eventually falls into a high-risk position.
When considering MFs, Deehan and colleagues15 found a significant higher risk in MFs in youth soccer. This result is partially according to Morgan and Oberlander18 who reported a non-statistically significant greater injury incidence in MFs. MFs are generally the players that cover more distance during a soccer match and it is logical to think that they would be predisposed to a large volume of acceleration/deceleration activities,19 potentially relating to injury risk, especially to muscles injuries. A previous study on thigh muscle injuries in youth soccer reporting higher injury risk in MFs, followed by forwards.19 Consistent with these results, another study on a mixed male and female cohort on high school soccer revealed more injuries in MFs, followed by forwards.26
Continue to: The results of this systematic review...
The results of this systematic review reveal mixed reports on injury risk in relation to playing position, the more consistent results through studies was that GKs may be at lower injury risk compared to the outfield players, even if there wasn’t complete agreement. One should note that in modern soccer the specific role of any player at 1 position may not be entirely consistent with another player in the same position. Within the same “position group”, there may also be players with completely different qualitative playing demands (eg, wing defender and central defender). So, even with the strongest study design, it may be difficult to give a simple and clear message about playing position and injury risk due to the variability of the playing styles and players at each position.
This study has several limitations and the results must be considered and interpreted with caution. First, we limited our search to male soccer, so the results may not be applicable to female soccer. Secondly, the interpretation of study findings wasn’t easy because of the different report modalities of the different papers included in the systematic review. Finally, we included reports from a total of a 23-year time span and from different countries and continents. The game may have evolved through years and there may be differences in the style of playing within countries that potentially could interfere with injury risk.
However, this is the first paper systematically evaluating the existing literature on position specific injury risk in male soccer players. Future studies, with prospective design and a consistent method to evaluate the player position as a potential factor related to injury risk, are needed. Match and training injuries should be evaluated separately as playing position may be more related to match injury risk.
CONCLUSION
There is no agreement in the existing literature regarding weather or not player position influence the general injury risk in male soccer. The GKs may have a lower risk of injury if compared to outfield players.
1. Pfirrmann D, Herbst M, Ingelfinger P, Simon P, Tug S. Analysis of injury incidences in male professional adult and elite youth soccer players: a systematic review. J Athl Train. 2016;51(5):410-424. doi:10.4085/1062-6050-51.6.03.
2. Eirale C, Gillogly S, Singh G, Chamari K. Injury and illness epidemiology in soccer - effects of global geographical differences - a call for standardized and consistent research studies. Biol Sport. 2017;34(3):249-254. doi:10.5114/biolsport.2017.66002.
3. Ekstrand J, Hägglund M, Waldén M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
4. Silvers-Granelli HJ, Bizzini M, Arundale A, Mandelbaum BR, Snyder-Mackler L. Does the FIFA 11+ Injury Prevention Program reduce the incidence of ACL injury in male soccer players? Clin Orthop Relat Res. 2017;475(10):2447-2455. doi:10.1007/s11999-017-5342-5.
5. Di Salvo V, Gregson W, Atkinson G, Tordoff P, Drust B. Analysis of high intensity activity in premier league soccer. Int J Sports Med. 2009;30(3):205-212. doi:10.1055/s-0028-1105950.
6. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
7. Dauty M, Collon S. Incidence of injuries in French professional soccer players. Int J Sports Med. 2011;32(12):965-969. doi:10.1055/s-0031-1283188.
8. Mallo J, Dellal A. Injury risk in professional football players with special reference to the playing position and training periodization. J Sports Med Phys Fitness. 2012;52(6):631-638.
9. Nilsson M, Hägglund M, Ekstrand J, Waldén M. Head and neck injuries in professional soccer. Clin J Sport Med. 2013;23(4):255-260. doi:10.1097/JSM.0b013e31827ee6f8.
10. Timpka T, Risto O, Björmsjö M. Boys soccer league injuries: a community-based study of time-loss from sports participation and long-term sequelae. Eur J Public Health. 2008;18(1):19-24.
11. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
12. Andersen TE, Larsen Ø, Tenga A, Engebretsen L, Bahr R. Football incident analysis: a new video based method to describe injury mechanisms in professional football. Br J Sports Med. 2003;37(3):226-232.
13. Arliani GG, Lara PHS, Astur DC, Pedrinelli A, Pagura JR, Cohen M. Orthopaedics injuries in male professional football players in Brazil: a prospective comparison between two divisions. Muscles Ligaments Tendons J. 2018;7(3), 524-531. doi:10.11138/mltj/2017.7.3.524.
14. Carling C, Orhant E, LeGall F. Match injuries in professional soccer: inter-seasonal variation and effects of competition type, match congestion and positional role. Int J Sports Med. 2010;31(4):271-276. doi:10.1055/s-0029-1243646.
15. Deehan DJ, Bell K, McCaskie AW. Adolescent musculoskeletal injuries in a football academy. J Bone Joint Surg Br. 2007;89(1):5-8.
16. Aoki H, O'Hata N, Kohno T, Morikawa T, Seki J. A 15-year prospective epidemiological account of acute traumatic injuries during official professional soccer league matches in Japan. Am J Sports Med. 2012;40(5):1006-1014. doi:10.1177/0363546512438695.
17. Shalaj I, Tishukaj F, Bachl N, Tschan H, Wessner B, Csapo R. Injuries in professional male football players in Kosovo: a descriptive epidemiological study. BMC Musculoskelet Disord. 2016;17:338. doi:10.1186/s12891-016-1202-9.
18. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430.
19. Cloke D, Moore O, Shah T, Rushton S, Shirley MD, Deehan DJ. Thigh muscle injuries in youth soccer: predictors of recovery. Am J Sports Med. 2012;40(2):433-439. doi:10.1177/0363546511428800.
20. Mallo J, González P, Veiga S, Navarro E. Injury incidence in a spanish sub-elite professional football team: a prospective study during four consecutive seasons. J Sports Sci Med. 2011;10(4):731-736.
21. Cárdenas-Fernández V, Chinchilla-Minguet JL, Castillo-Rodríguez A. Somatotype and body composition in young soccer players according to the playing position and sport success. J Strength Cond Res. 2017. doi:10.1519/JSC.0000000000002125. [Epub ahead of print]
22. Ekstrand J, Hägglund M, Törnqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
23. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
24. Andersen TE, Tenga A, Engebretsen L, Bahr R. Video analysis of injuries and incidents in Norwegian professional football. Br J Sports Med. 2004;38(5):626-631.
25. Ueblacker P, Müller-Wohlfahrt HW, Ekstrand J. Epidemiological and clinical outcome comparison of indirect (‘strain’) versus direct (‘contusion’) anterior and posterior thigh muscle injuries in male elite football players: UEFA Elite League study of 2287 thigh injuries (2001-2013). Br J Sports Med. 2015;49(22):1461-1465. doi:10.1136/bjsports-2014-094285.
26. Khodaee M, Currie DW, Asif IM, Comstock RD. Nine-year study of US high school soccer injuries: data from a national sports injury surveillance programme. Br J Sports Med. 2017;51(3):185-193. doi:10.1136/bjsports-2015-095946.
1. Pfirrmann D, Herbst M, Ingelfinger P, Simon P, Tug S. Analysis of injury incidences in male professional adult and elite youth soccer players: a systematic review. J Athl Train. 2016;51(5):410-424. doi:10.4085/1062-6050-51.6.03.
2. Eirale C, Gillogly S, Singh G, Chamari K. Injury and illness epidemiology in soccer - effects of global geographical differences - a call for standardized and consistent research studies. Biol Sport. 2017;34(3):249-254. doi:10.5114/biolsport.2017.66002.
3. Ekstrand J, Hägglund M, Waldén M. Injury incidence and injury patterns in professional football: the UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
4. Silvers-Granelli HJ, Bizzini M, Arundale A, Mandelbaum BR, Snyder-Mackler L. Does the FIFA 11+ Injury Prevention Program reduce the incidence of ACL injury in male soccer players? Clin Orthop Relat Res. 2017;475(10):2447-2455. doi:10.1007/s11999-017-5342-5.
5. Di Salvo V, Gregson W, Atkinson G, Tordoff P, Drust B. Analysis of high intensity activity in premier league soccer. Int J Sports Med. 2009;30(3):205-212. doi:10.1055/s-0028-1105950.
6. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
7. Dauty M, Collon S. Incidence of injuries in French professional soccer players. Int J Sports Med. 2011;32(12):965-969. doi:10.1055/s-0031-1283188.
8. Mallo J, Dellal A. Injury risk in professional football players with special reference to the playing position and training periodization. J Sports Med Phys Fitness. 2012;52(6):631-638.
9. Nilsson M, Hägglund M, Ekstrand J, Waldén M. Head and neck injuries in professional soccer. Clin J Sport Med. 2013;23(4):255-260. doi:10.1097/JSM.0b013e31827ee6f8.
10. Timpka T, Risto O, Björmsjö M. Boys soccer league injuries: a community-based study of time-loss from sports participation and long-term sequelae. Eur J Public Health. 2008;18(1):19-24.
11. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
12. Andersen TE, Larsen Ø, Tenga A, Engebretsen L, Bahr R. Football incident analysis: a new video based method to describe injury mechanisms in professional football. Br J Sports Med. 2003;37(3):226-232.
13. Arliani GG, Lara PHS, Astur DC, Pedrinelli A, Pagura JR, Cohen M. Orthopaedics injuries in male professional football players in Brazil: a prospective comparison between two divisions. Muscles Ligaments Tendons J. 2018;7(3), 524-531. doi:10.11138/mltj/2017.7.3.524.
14. Carling C, Orhant E, LeGall F. Match injuries in professional soccer: inter-seasonal variation and effects of competition type, match congestion and positional role. Int J Sports Med. 2010;31(4):271-276. doi:10.1055/s-0029-1243646.
15. Deehan DJ, Bell K, McCaskie AW. Adolescent musculoskeletal injuries in a football academy. J Bone Joint Surg Br. 2007;89(1):5-8.
16. Aoki H, O'Hata N, Kohno T, Morikawa T, Seki J. A 15-year prospective epidemiological account of acute traumatic injuries during official professional soccer league matches in Japan. Am J Sports Med. 2012;40(5):1006-1014. doi:10.1177/0363546512438695.
17. Shalaj I, Tishukaj F, Bachl N, Tschan H, Wessner B, Csapo R. Injuries in professional male football players in Kosovo: a descriptive epidemiological study. BMC Musculoskelet Disord. 2016;17:338. doi:10.1186/s12891-016-1202-9.
18. Morgan BE, Oberlander MA. An examination of injuries in major league soccer. The inaugural season. Am J Sports Med. 2001;29(4):426-430.
19. Cloke D, Moore O, Shah T, Rushton S, Shirley MD, Deehan DJ. Thigh muscle injuries in youth soccer: predictors of recovery. Am J Sports Med. 2012;40(2):433-439. doi:10.1177/0363546511428800.
20. Mallo J, González P, Veiga S, Navarro E. Injury incidence in a spanish sub-elite professional football team: a prospective study during four consecutive seasons. J Sports Sci Med. 2011;10(4):731-736.
21. Cárdenas-Fernández V, Chinchilla-Minguet JL, Castillo-Rodríguez A. Somatotype and body composition in young soccer players according to the playing position and sport success. J Strength Cond Res. 2017. doi:10.1519/JSC.0000000000002125. [Epub ahead of print]
22. Ekstrand J, Hägglund M, Törnqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
23. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
24. Andersen TE, Tenga A, Engebretsen L, Bahr R. Video analysis of injuries and incidents in Norwegian professional football. Br J Sports Med. 2004;38(5):626-631.
25. Ueblacker P, Müller-Wohlfahrt HW, Ekstrand J. Epidemiological and clinical outcome comparison of indirect (‘strain’) versus direct (‘contusion’) anterior and posterior thigh muscle injuries in male elite football players: UEFA Elite League study of 2287 thigh injuries (2001-2013). Br J Sports Med. 2015;49(22):1461-1465. doi:10.1136/bjsports-2014-094285.
26. Khodaee M, Currie DW, Asif IM, Comstock RD. Nine-year study of US high school soccer injuries: data from a national sports injury surveillance programme. Br J Sports Med. 2017;51(3):185-193. doi:10.1136/bjsports-2015-095946.
TAKE-HOME POINTS
- Playing positions haven’t been extensively evaluated as an injury risk factor in elite, non-elite, and youth soccer (football).
- Different playing positions may have different injury rates and patterns due to different load, different movement patterns, and peculiar combination of anticipated and non-anticipated (reactive movements).
- The existing literature suggests that goalkeepers seem to be at lower general injury risk if compared to outfield players in male soccer (football).
- There is also a tendency towards strikers (forwards) to be at higher risk of match (but not training) injuries. This result is however not consistent between all the studies considered and should be interpreted cautiously.
- When studying injury risk in male soccer match and training injuries should be considered separately and playing position should be evaluated as a potential predictor of injury incidence.
Upper Extremity Injuries in Soccer
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
ABSTRACT
Upper limb injuries in soccer represent only a marginal portion of injuries, however this is mainly true for outfield players. Goalkeepers are reported to have up to 5 times more upper extremity injuries, many of them requiring substantial time-loss for treatment and rehabilitation. The most common upper extremity injury locations are the shoulder/clavicle followed by the hand/finger/thumb, elbow, wrist, forearm, and upper arm. The mechanism of injury, presentation, physical examination, and imaging features all play a significant role in reaching the correct diagnosis. Taking to consideration the position the player plays and his demands will also enable tailoring the optimal treatment plan that allows timely and safe return to play. This article discusses common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: Upper limb injuries in association with soccer...
Upper limb injuries in association with soccer have been reported to represent only 3% of all time-loss injuries in professional soccer players1. However, they are considered an increasing problem in recent years2-4 and have been reported in high proportions in children under the age of 15 years.5 Some of the reasons for the increase in upper extremity injuries may be explained by modern soccer tactics that have been characterized by high speed, pressing, and marking.2 Furthermore, upper extremity injuries may still be underestimated in soccer, mainly because outfield players are sometimes able to train and play even when they suffer from an upper extremity injury.
Unsurprisingly, upper extremity injuries are reported to be up to 5 times more common in goalkeepers than in outfield players,1,2 reaching a high rate of up to 18% of all injuries among professional goalkeepers. The usage of upper extremities to stop the ball and repeated reaching to the ball and landing on the ground with changing upper extremity positions are some of the contributors to the increased upper extremity injury risk in goalkeepers.
Following 57 male professional European soccer teams from 16 countries between the years 2001 and 2011, Ekstrand and colleagues1 showed that 90% of upper extremity injuries are traumatic, and only 10% are related to overuse. They also reported that the most common upper extremity injury location is the shoulder/clavicle (56%), followed by the hand/finger/thumb (24%), elbow (10%), wrist (5%), forearm (4%), and upper arm (1%). Specifically, the 6 most common injuries are acromioclavicular joint (ACJ) sprain (13%), shoulder dislocation (12%), hand metacarpal fracture (8%), shoulder rotator cuff tendinopathy (6%), hand phalanx fracture (6%), and shoulder ACJ dislocation (5%).
This article will discuss common upper extremity injuries observed in soccer players, focusing on proper diagnosis and optimal management.
Continue to: THE SHOULDER...
THE SHOULDER
The majority of upper extremity injuries in professional soccer players are shoulder injuries.1,2,4 Almost a third of these injuries (28%) are considered severe, preventing participation in training and matches for 28 days or more.6Ekstrand and colleagues1 reported that shoulder dislocation represents the most severe upper extremity injury with a mean of 41 days of absence from soccer. When considering the position of the player, they further demonstrated that absence from full training and matches is twice as long for goalkeepers as for outfield players, which reflects the importance of shoulder function for goalkeepers.
In terms of the mechanism of shoulder instability injuries in soccer players, more than half (56%) of these injuries occur with a high-energy mechanism in the recognized position of combined humeral abduction and external rotation against a force of external rotation and horizontal extension.3 However, almost a quarter (24%) occur with a mechanism of varied upper extremity position and low-energy trauma, and 20% of injuries are either a low energy injury with little or no contact or gradual onset. These unique characteristics of shoulder instability injuries in soccer players should be accounted for during training and may imply that current training programs are suboptimal for the prevention of upper extremity injuries and shoulder injuries. Ejnisman and colleagues2 reported on the development of a Fédération Internationale de Football Association (FIFA) 11+ shoulder injury prevention program for soccer goalkeepers as one of the ways to promote training programs that address the risk of shoulder injuries.
Reporting on the management of severe shoulder injuries requiring surgery in 25 professional soccer players in England, between 2007 and 2011, Hart and Funk3 found that the majority of subjects (88%) reported a dislocation as a feature of their presentation. Twenty-one (84%) subjects were diagnosed with labral injuries, of which 7 had an associated Hill-Sachs lesion. Two (8%) subjects were diagnosed with rotator cuff tears requiring repair, and 2 (8%) subjects had a combination of rotator cuff and labral injury repair. All patients underwent arthroscopic repair, except for 5 who had a Latarjet coracoid transfer. Post-surgery, all players were able to return to unrestricted participation in soccer at a mean of 11.4 weeks, with no significant difference between goalkeepers and outfield players and no recurrences at a mean of 91 weeks’ follow-up.
Up to one-third of shoulder instability injuries in soccer players are reported to be recurrences,1,3 which emphasizes the need to carefully assess soccer players before clearing them to return to play. These data raise the controversy over the treatment of first time shoulder dislocators and may support early surgical intervention.7-9 In terms of the preferred surgical intervention in these cases, Balg and Boileau10 suggested a simple scoring system based on factors derived from a preoperative questionnaire, physical examination, and anteroposterior radiographs to help distinguish between patients who will benefit from an arthroscopic anterior stabilization using suture anchors and those who will require a bony procedure (open or arthroscopic). Cerciello and colleagues11 reported excellent results for bony stabilization (modified Latarjet) in a population of 26 soccer players (28 shoulders) affected by chronic anterior instability. Only 1 player did not return to soccer, and 18 players (20 shoulders, 71%) returned to the same level. One re-dislocation was noted in a goalkeeper 74 months after surgery.
An injury to the ACJ has been previously reported to be the most prevalent type of shoulder injury in contact sports.12In soccer, injury to the ACJ is responsible for 18% of upper extremity injuries, and the majority (72%) are sprains.1Interestingly, but unsurprisingly, implications of such an injury differ significantly between goalkeepers and outfield players with up to 3 times longer required absence periods for goalkeepers vs outfield players sustaining the same injury.
ACJ injury is commonly the result of a direct fall on the shoulder with the arm adducted or extended. Six grades of ACJ injuries have been described and distinguished by the injured anatomical structure (acromioclavicular ligaments and coracoclavicular ligaments) and the direction and magnitude of clavicular dislocation.13,14 Presentation will usually include anterior shoulder pain, a noticeable swelling or change in morphology of the lateral end of the clavicle (mainly in dislocation types), and sharp pain provoked by palpation of the ACJ. Radiographic imaging will confirm the diagnosis and help with identifying the specific grade/type of injury.
Decision making and management of acute ACJ injury should be based on the type/grade of injury. Nonoperative treatment is recommended for types I and II, and most athletes have a successful outcome with a full return to play.12Types IV, V, and VI are treated early with operative intervention, mostly due to the morbidity associated with prolonged dislocation of the joint and subsequent soft tissue damage.12 Treatment of type III injury remains controversial. Pereira-Graterol and colleagues15 reported the effectiveness of clavicular hook plate (DePuy Synthes) in the surgical stabilization of grade III ACJ dislocation in 11 professional soccer players. At a mean follow-up of 4 years, they showed excellent functional results with full shoulder range of motion at 5 weeks and latest return to soccer at 6 months. The hook plate was removed after 16 weeks in 10 patients in whom no apparent complication was observed.
Continue to: THE ELBOW...
THE ELBOW
Ekstrand and colleagues1 reported that 10% of all upper extremity injuries in professional soccer players are elbow injuries, of which only 19% are considered severe injuries that require more than 28 days of absence from playing soccer. The most common elbow injuries in their cohort were elbow medial collateral ligament (MCL) sprain and olecranon bursitis.
Elbow MCL is the primary constraint of the elbow joint to valgus stress, and MCL sprain occurs when the elbow is subjected to a valgus, or laterally directed force, which distracts the medial side of the elbow, exceeding the tensile properties of the MCL.16 A thorough physical examination that includes valgus stress tests through the arc of elbow flexion and extension to elicit a possible subjective feeling of apprehension, instability, or localized pain is essential for optimal evaluation and treatment.16,17 Imaging studies (X-ray and stress X-rays, dynamic ultrasound, computed tomography [CT], magnetic resonance imaging [MRI], and MR arthrography) have a role in further establishing the diagnosis and identifying possible additional associated injuries.16 The treatment plan should be specifically tailored to the individual athlete, depending on his demands and the degree of MCL injury. In soccer, which is a non-throwing shoulder sport, nonoperative treatment should be the preferred initial treatment in most cases. Ekstrand and colleagues1 showed that this injury requires a mean of 4 days of absence from soccer for outfield players and a mean of 21 days of absence from soccer for goalkeepers, thereby indicating more severe sprains and cautious return to soccer in goalkeepers. Athletes who fail nonoperative treatment are candidates for MCL reconstruction.16
The olecranon bursa is a synovium-lined sac that facilitates gliding between the olecranon and overlying skin. Olecranon bursitis is characterized by accumulation of fluid in the bursa with or without inflammation. The fluid can be serous, sanguineous, or purulent depending on the etiology.18 In soccer, traumatic etiology is common, but infection secondary to cuts or scratches of the skin around the elbow or previous therapeutic injections around the elbow should always be ruled out. Local pain, swelling, warmth, and redness are usually the presenting symptoms. Aseptic olecranon bursitis may be managed non-surgically with ‘‘benign neglect’’ and avoidance of pressure to the area, non-steroidal anti-inflammatory drugs, needle aspiration, corticosteroid injection, compression dressings, and/or padded splinting; whereas acute septic bursitis requires needle aspiration for diagnosis, appropriate oral or intravenous antibiotics directed toward the offending organism, and, when clinically indicated, surgical evacuation/excision of the bursa.18 When treating this condition with cortisone injection, possible complications, such as skin atrophy, secondary infection, and chronic local pain, have been reported and should be considered.19
Severe elbow injuries in professional athletes in general,20-22 and soccer players specifically, are elbow subluxations/dislocations and elbow fracture. The mechanism of injury is usually contact injury with an opponent player or a fall on the palm with the arm extended. Posterolateral is the most common type of elbow dislocation. Elbow dislocations are further classified into simple (no associated fractures) and complex (associated with fractures) categories.22 Simple dislocations are usually treated with early mobilization after closed reduction; it is associated with a low risk for re-dislocation and with generally good results. The complex type of elbow fracture dislocation is more difficult to treat, has higher complication and re-dislocation rates, and requires operative treatment in most cases compared with simple dislocation.22 The “terrible triad” of the elbow (posterior elbow dislocation, radial head fracture, and coronoid fracture) represents a specific complex elbow dislocation scenario that is difficult to manage because of conflicting aims of ensuring elbow stability while maintaining early range of motion.22
Isolated fracture around the elbow should be treated based on known principles of fracture management: mechanism of injury, fracture patterns, fracture displacement, intra-articular involvement, soft tissue condition, and associated injuries.
Continue to: THE WRIST...
THE WRIST
Ekstrand and colleagues1 reported that 5% of all upper extremity injuries in their cohort of professional soccer players are wrist injuries, of which, only 2% are considered severe injuries that require >28 days of absence from playing soccer. The more common wrist injuries in soccer, which is considered a high-impact sport, are fractures (distal radius, scaphoid, capitate), and less reported injuries are dislocations (lunate, perilunate) and ligamentous injuries or tears (scapholunate ligament).23
Distal radius fractures in high-impact sports, like soccer, usually occur as a result of a fall on an out-stretched hand and will usually be more comminuted, displaced, and intra-articular compared with low-impact sports.23 All these aforementioned characteristics usually indicate surgical management of open reduction and internal fixation, which will allow for rapid start of rehabilitation and return to play.
Scaphoid fracture is the most common carpal bone fracture and presents unique challenges in terms of diagnosis and optimal treatment24 in professional athletes. A typical injury scenario would be a player falling on an outstretched hand and sustaining a scaphoid fracture during a match or training session. The player may acknowledge some wrist pain but will often continue to play with minimal or no limitation. As wrist pain and swelling become more evident after the match/training session, the player will seek medical evaluation.24 A complete wrist and upper extremity examination should be performed in addition to a specific assessment, which includes palpation of the distal scaphoid pole at the distal wrist flexion crease, palpation of the scaphoid waist through the wrist snuff box, and palpation dorsally just distal to the Lister tubercle at the scapholunate joint. Any wrist injury that results in decreased range of motion, snuff box swelling, and scaphoid tenderness should be further evaluated with imaging. Plain radiographs with special scaphoid views are the initial preferred imaging studies, but occult fracture will require an additional study such as a bone scan, CT, or MRI. Several studies have validated the benefit of MRI and the fact that it may outweigh the costs associated with lost productivity from unnecessary cast immobilization, especially in elite athletes.23-25Casting the patient with a nondisplaced scaphoid waist fracture has been the traditional treatment; however, stiffness, weakness, and deconditioning that can occur with long-term casting required for scaphoid fractures are significant impairments for the professional athlete and usually end the player’s season. Surgical treatment, which was traditionally indicated for displaced or proximal pole fractures, is currently also considered for non-displaced scaphoid waist fractures in professional athletes. This treatment allows for a rapid return to the rehabilitation of the extremity and possible early return to professional sport. In view of the known complications (eg, malunion, nonunion, and avascular necrosis), return to soccer can be considered when imaging confirms advanced healing, which some consider as at least 50% of bone fracture bridging on CT scan, no pain, excellent motion, and at least 80% of normal grip strength.24 Outfield players can return to play with a protective cast or brace until full healing is observed on imaging.
Continue to: THE HAND/FINGERS/THUMB...
THE HAND/FINGERS/THUMB
Almost a quarter of upper extremity injuries in professional soccer players were reported to involve the hand, fingers, and thumb. A quarter of them were classified as severe injuries requiring >28 days of absence from playing soccer.1Specifically, hand metacarpal and phalanx fractures are the most common reported injuries in sports in general,26 and in soccer,1 and account for 14% of all upper extremity injuries1 in professional soccer players. Goalkeepers require a functional hand to play, whereas an outfielder can play with protection on the injured area; thus, the period of absence from soccer in these injuries is significantly different between goalkeepers and outfielders with more than 4 times longer absence from soccer for a goalkeeper compared with an outfielder. The fifth ray has been shown to be the most frequently fractured ray in the hand in soccer with 51.7% of all hand fractures reported.26 The common mechanism is a full hit on the hand, and a direct hit from the ball is another possible mechanism in goalkeepers.
In general, the diagnosis of hand injuries requires evaluation of the mechanism of injury and injury symptoms, proper and comprehensive physical examination of the whole extremity, and prompt imaging. In most cases, plain radiographs in several projections will suffice for the diagnosis of obvious fractures, but CT scan is an additional modality that allows for improved appreciation of occult or complex and comminuted fracture patterns. MRI or ultrasound can be used additionally whenever associated soft tissue injury is suspected. Optimal management of the hand is based on the specific characteristics of the fractures, which include location, direction of the fracture line, presence of comminution, displacement, articular involvement, and associated soft tissue injury. Nondisplaced extra-articular fractures often can be treated with buddy taping or splinting, whereas intra-articular fractures often require surgical treatment. Displaced fractures of the hand have a tendency to angulate volarly because of attachments of the interosseous muscles. Marginal fractures or avulsion fractures involving the metacarpals or phalanges can be sentinels of serious associated soft tissue injuries.27
Phalangeal fractures can potentially affect the function of the entire hand; therefore, no malrotation is acceptable for phalangeal fractures because they can lead to overlap and malalignment of the digit. Displaced or malrotated fractures should be reduced either by closed or open techniques. Acceptable reduction is <6 mm of shortening, <15° of angulation, and no rotational deformity.27,28 Nondisplaced phalangeal fractures can be treated nonoperatively with buddy taping and splinting with good results.27 Interphalangeal (IP) dislocations can be reduced on the sidelines and then taped or splinted. Any injury with a force significant enough to cause joint dislocation indicates further evaluation for associated fractures and ligamentous injury or tear. The proximal interphalangeal (PIP) joint is the most common IP joint dislocation and is usually a dorsal dislocation. Reduction is often achieved by traction and flexion of the middle phalanx,27 followed by splinting of the finger with the PIP in 30° of flexion or an extension block splint.29 Successful reduction with no associated intra-articular fractures involving more than a third of the joint can be further managed nonoperatively with the splint, allowing 2 to 4 weeks for the volar plate, joint capsule, and collateral ligaments to heal. Additional 2 to 4 weeks of splinting with buddy taping to the adjacent finger is usually recommended.29
The “Mallet finger” injury can be observed in goalkeepers and is caused by a flexion force on the tip of the finger while the distal interphalangeal (DIP) joint is extended. This force results in tearing of the extensor tendon or an avulsion fracture at the tendinous attachment on the dorsal lip of the distal phalangeal base. The classic mechanism of injury is an extended finger struck on the tip by a ball. Physical examination will indicate loss of DIP joint active extension, and the joint rests in an abnormally flexed position. Treatment typically consists of splinting the DIP joint in extension for 6 to 8 weeks. Operative treatment is reserved for severe injuries or fractures involving greater than one-third of the articular surface of the DIP joint or with failed nonoperative treatment.27
Metacarpal fractures can be subdivided into distal, metacarpal neck, metacarpal shaft, and metacarpal base fractures. Metacarpal shaft fractures raise a specific concern regarding rotation, because even a small degree of rotation can create a substantial degree of deformity at the fingertip. This concern must be addressed during evaluation of the player. Fractures of the metacarpal base most commonly involve the fourth and fifth metacarpals and are often reduced easily but have a tendency to re-subluxate, which may indicate operative treatment. Most fractures of the metacarpals are low energy and result in simple fracture patterns that can be treated nonoperatively. Open reduction is reserved for high-energy trauma, fractures with excessive angulation, or multiple fractures.27
Continue to: An important subgroup of metacarpal injuries...
An important subgroup of metacarpal injuries involves the base of the thumb. These injuries result from an axial load applied to the thumb. The most common injury is the “Bennett fracture,” which is an intra-articular fracture or dislocation involving the base of the first metacarpal. Bennett fractures are unstable fractures; unless properly recognized and treated, this intra-articular fracture subluxation may result in an unstable arthritic first carpometacarpal joint. These fractures are most commonly treated with closed or open reduction combined with internal fixation.27 “Rolando fractures” are similar in location and etiology but are comminuted and usually require operative treatment.27, 29
Another common hand injury found in soccer goalkeepers and involving the base of the thumb is disruption of the ulnar collateral ligament (UCL) of the first metacarpophalangeal (MCP) joint as a result of an acute radial or valgus stress on the thumb. Known as “gamekeeper’s thumb” or “skier’s thumb,” this injury can occur in the form of an avulsion fracture, an isolated ligament tear, or combined fracture and ligament rupture. On examination, swelling and tenderness over the thumb UCL are observed. A MCP joint stress test should be performed by gently applying a radially directed force to the thumb while stabilizing the metacarpal bone at both 0° and 30° at the MCP joint. Increased laxity, a soft or nonexistent end point, and gaping of the joint, as compared with the contralateral side, will indicate this injury.29 Radiographs may show a small avulsion fracture fragment at the ulnar aspect of the base of the first metacarpal and at the attachment of the UCL. A Stener lesion is an abnormality that occurs when the thumb adductor muscle aponeurosis interposes between the 2 ends of the ruptured UCL, preventing UCL healing by immobilization alone. Ultrasound and MRI are additional imaging modalities that can assist with the diagnosis of a Stener lesion. The presence of a Stener lesion is a prime indication for surgical intervention. A nondisplaced fracture or isolated ligament injury with no evidence of a Stener lesion can be treated nonoperatively with splinting of the thumb and may lead to healing and restoration of stability. However, in professional players, surgical repair is often times preferred.27
CONCLUSION
Upper extremity injuries are less common injuries among soccer players, but their prevalence is on the rise in recent years. Modern playing tactics and the increase in participation in soccer in younger age groups may be 2 contributing factors to this rise. Given the characteristics of their unique playing role and specific demands, the risk for upper extremity injuries among goalkeepers is significantly higher than that in outfielders and will usually result in a long absence period from soccer before they return to play. A thorough understanding of the mechanism of injury, players’ complaints and presentation, osseous and soft tissue involvement based on a systematic physical examination, imaging features, and treatment options is important for the optimal care of the players. Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
1. Ekstrand J, Hagglund M, Tornqvist H, et al. Upper extremity injuries in male elite football players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1626-1632. doi:10.1007/s00167-012-2164-6.
2. Ejnisman B, Barbosa G, Andreoli CV, et al. Shoulder injuries in soccer goalkeepers: Review and development of a FIFA 11+ shoulder injury prevention program. Open Access J Sports Med. 2016;7:75-80. doi:10.2147/OAJSM.S97917.
3. Hart D, Funk L. Serious shoulder injuries in professional soccer: Return to participation after surgery. Knee Surg Sports Traumatol Arthrosc. 2015;23(7):2123-2129. doi:10.1007/s00167-013-2796-1.
4. Longo UG, Loppini M, Berton A, Martinelli N, Maffulli N, Denaro V. Shoulder injuries in soccer players. Clin Cases Miner Bone Metab. 2012;9(3):138-141.
5. Faude O, Rossler R, Junge A. Football injuries in children and adolescent players: Are there clues for prevention? Sports Med. 2013;43(9):819-837. doi:10.1007/s40279-013-0061-x.
6. Ekstrand J, Hagglund M, Walden M. Injury incidence and injury patterns in professional football: The UEFA injury study. Br J Sports Med. 2011;45(7):553-558. doi:10.1136/bjsm.2009.060582.
7. Boone JL, Arciero RA. First-time anterior shoulder dislocations: Has the standard changed? Br J Sports Med. 2010;44(5):355-360. doi:10.1136/bjsm.2009.062596.
8. Handoll HH, Almaiyah MA, Rangan A. Surgical versus non-surgical treatment for acute anterior shoulder dislocation. Cochrane Database Syst Rev. 2004;(1):CD004325.
9. Kirkley A, Werstine R, Ratjek A, Griffin S. Prospective randomized clinical trial comparing the effectiveness of immediate arthroscopic stabilization versus immobilization and rehabilitation in first traumatic anterior dislocations of the shoulder: Long-term evaluation. Arthroscopy. 2005;21(1):55-63.
10. Balg F, Boileau P. The instability severity index score. A simple pre-operative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89(11):1470-1477.
11. Cerciello S, Edwards TB, Walch G. Chronic anterior glenohumeral instability in soccer players: Results for a series of 28 shoulders treated with the latarjet procedure. J Orthop Traumatol. 2012;13(4):197-202. doi:10.1007/s10195-012-0201-3.
12. Bishop JY, Kaeding C. Treatment of the acute traumatic acromioclavicular separation. Sports Med Arthrosc Rev. 2006;14(4):237-245. doi:10.1097/01.jsa.0000212330.32969.6e.
13. de Putter CE, van Beeck EF, Burdorf A, et al. Increase in upper extremity fractures in young male soccer players in the netherlands, 1998-2009. Scand J Med Sci Sports. 2015;25(4):462-466. doi:10.1111/sms.12287.
14. Rockwood CJ, Williams G, Young D. Disorders of the acromioclavicular joint. In: Rockwood CJ, Matsen FA III, eds. The Shoulder. 2nd ed. Philadelphia: WB Saunders; 1998:483-553.
15. Pereira-Graterol E, Alvarez-Diaz P, Seijas R, Ares O, Cusco X, Cugat R. Treatment and evolution of grade III acromioclavicular dislocations in soccer players. Knee Surg Sports Traumatol Arthrosc. 2013;21(7):1633-1635. doi:10.1007/s00167-012-2186-0.
16. Rahman RK, Levine WN, Ahmad CS. Elbow medial collateral ligament injuries. Curr Rev Musculoskelet Med. 2008;1(3-4):197-204. doi:10.1007/s12178-008-9026-3.
17. Redler LH, Watling JP, Ahmad CS. Physical examination of the throwing athlete's elbow. Am J Orthop. 2015;44(1):13-18.
18. Sayegh ET, Strauch RJ. Treatment of olecranon bursitis: A systematic review. Arch Orthop Trauma Surg. 2014;134(11):1517-1536. doi:10.1007/s00402-014-2088-3.
19. Weinstein PS, Canoso JJ, Wohlgethan JR. Long-term follow-up of corticosteroid injection for traumatic olecranon bursitis. Ann Rheum Dis. 1984;43(1):44-46.
20. Carlisle JC, Goldfarb CA, Mall N, Powell JW, Matava MJ. Upper extremity injuries in the national football league: Part II: Elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36(10):1945-1952. doi:10.1177/0363546508318198.
21. Dizdarevic I, Low S, Currie DW, Comstock RD, Hammoud S, Atanda A Jr. Epidemiology of elbow dislocations in high school athletes. Am J Sports Med. 2016;44(1):202-208. doi:10.1177/0363546515610527.
22. Saati AZ, McKee MD. Fracture-dislocation of the elbow: Diagnosis, treatment, and prognosis. Hand Clin. 2004;20(4):405-414.
23. Bancroft LW. Wrist injuries: A comparison between high- and low-impact sports. Radiol Clin North Am. 2013;51(2):299-311. doi:10.1016/j.rcl.2012.09.017.
24. Belsky MR, Leibman MI, Ruchelsman DE. Scaphoid fracture in the elite athlete. Hand Clin. 2012;28(3):78, vii. doi:10.1016/j.hcl.2012.05.005.
25. Mallee W, Doornberg JN, Ring D, van Dijk CN, Maas M, Goslings JC. Comparison of CT and MRI for diagnosis of suspected scaphoid fractures. J Bone Joint Surg Am. 2011;93(1):20-28. doi:10.2106/JBJS.I.01523.
26. Aitken S, Court-Brown CM. The epidemiology of sports-related fractures of the hand. Injury. 2008;39(12):1377-1383. doi:10.1016/j.injury.2008.04.012.
27. Peterson JJ, Bancroft LW. Injuries of the fingers and thumb in the athlete. Clin Sports Med. 2006;25(3):viii.
28. Walsh JJ 4th. Fractures of the hand and carpal navicular bone in athletes. South Med J. 2004;97(8):762-765.
29. Hong E. Hand injuries in sports medicine. Prim Care. 2005;32(1):91-103.
TAKE-HOME POINTS
- Upper extremity injuries in soccer are not common, however they can reach up to 18% of all injuries in professional goalkeepers.
- Common injury locations in the upper extremity in soccer are the shoulder/clavicle, hand/finger/thumb, the elbow, and the wrist and most of these injuries are traumatic injuries.
- Mechanism of injury, players’ complaints and presentation, physical examination, and imaging features are all important for a proper evaluation and optimal management.
- Position of play is an important consideration in the management of upper extremity injuries in soccer. Outfield players may be able to return to play before a complete resolution of their injury, with protective accessories.
- Prompt and accurate diagnosis and appropriate management are essential for improved outcomes and timely return to play.
Soccer or Football Medicine? Global Sports Medicine for a Global Game
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
Any given weekend where the sun is shining in the United States, you can jump in your car and see children competing on the soccer field. Soccer, known as football in other countries, is one of the most played sports in the US with over 25 million children participating every year. Despite Americans’ mass participation in youth soccer, this level of enthusiasm hasn’t necessarily translated into soccer being one of our most watched sports. On an international level, soccer is not only a sport but a way of life, and it is often described as “the beautiful game”, as visions of Pelé, Kaká, Messi, Ronaldo, and others can invoke emotional responses in the hearts of so many people across the world.
Over the course of the past 20 years, the enthusiasm for soccer in the US has grown significantly as defined not only by the number of youth players on the field but also now by the increased number of professional teams, energetic supporters in the stands, and fans watching on their televisions at home. This exponential growth started with the success of our US Soccer National Teams in the 1990s, including the 1994 World Cup held in the US, and became cemented into the culture of American sports with the birth, development, and subsequent growth of Major League Soccer (MLS) across the country. Despite the recent disappointment of the US Men’s National Team not making the 2018 World Cup, Americans should remain excited that our US Women’s National Team is prepared to be a contender in the 2019 World Cup, our US Men’s National Team will certainly make a significant push to compete in the 2022 World Cup, and the US is again ready to re-energize Americans’ interest in soccer by hosting a collaborative bid for the Men’s 2026 World Cup!
Now that I have hopefully energized all of our readers about the current and future impact of soccer within the US, I am personally excited about being an active member of the soccer medicine community through my roles as the Chief Medical Officer of the Orlando City Soccer Club, including Orlando City Lions MLS team and Orlando Pride National Women’s Soccer League (NWSL) team, and a Team Physician for US Soccer. What most people don’t realize in the sports medicine community and beyond is that our MLS and US soccer medical teams have been working tirelessly for the last 20 years to not only provide top-notch medical care within our country but to create one of the best medical structures in the world.
Over the last several years, I have learned that our soccer medical community is fortunate to have strength in numbers. In fact, our international colleagues provide a collaborative team to help push the limits on medical innovation so that we constantly reflect upon the quality of care that we are providing for the ultimate improvement of the medical care for all of our players. I recently returned from a trip to Barcelona for the Isokinetic Medical Group Football, known as soccer in the US, Medicine Outcomes Meeting where over 3000 participants from almost 100 countries around the world attended. After previous involvement in Major League Baseball and the National Football League, and since my integration into the soccer medicine community several years ago, I have been amazed and challenged by the complexity of pathology that we see in soccer players and the attention to detail that is required to successfully transition a soccer player back to the field while also preventing a subsequent injury. In fact, soccer players require a unique combination of skill, fitness, performance, nutrition, and sustainability to be successful at the highest level of soccer. As a sports medicine community in the US, we have come so far but yet still have so much left to learn. I’m certainly excited that we will be able to build and share this knowledge base with not only my fellow Americans but also our international colleagues abroad. Who knows, after the 2026 World Cup, the further growth and solidification of soccer and soccer medicine in the US might enable me to change the title for my editorial with no resulting confusion: “Global Football Medicine for a Global Game”.
The Three H’s: Head, Heart, and Heat Considerations in Soccer
ABSTRACT
Soccer requires significant physical conditioning and endurance, as well as the physicality required for contact play. In order to keep athletes safe, it is important that coaches, medical staff, and the players themselves are educated on the most common dangers to their health that they may encounter on a soccer pitch. This article aims to review the current literature and recommendations on concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer, with a goal of educating all those who help to keep athletes healthy and competing to their full potential.
Continue to: Soccer is one of the most popular sports...
Soccer is one of the most popular sports in the modern world and requires significant physical conditioning and endurance, as well as the physicality required for contact play. This article covers the topics of concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer players. We provide a review of the prevention, recognition, and management required to keep athletes safe on the soccer pitch, both in practice and in competitive play.
HEAD
With an estimated 1.6 to 3.8 million sports-related mild traumatic brain injuries (ie, concussions) occurring annually in the United States,1 there has been an appropriate increase in the focus on prevention and treatment of these injuries. For more than a decade the spotlight has been on concussions that occur in American football, but other sports have also had to examine the prevalence of concussions in their sport. This is certainly true for soccer.
There has been a steady increase in soccer participation in the United States. From 1973 to 2014 there was a 4-fold increase in high school boys and a 35-fold increase in high school girls playing soccer.2 Currently, there are more than 3.7 million youth who play on teams under the supervision of the US Soccer Federation, the sport’s national governing body.3 With the growth of the sport, there has also been an intensified focus on injury prevention in soccer players, including concussive brain injuries.
A recent study examined injury rates in high school soccer players and noted that concussion is the second most common injury (17.9%), after ligament sprains (29.7%).4 The overall injury rate was 2.06 per 1000 athletic exposures (AEs [defined as participation in practice or game play]), but higher in games (4.42 per 1000 AEs) than during practices (1.05 per 1000 AEs). The overall concussion rate was 0.36 per 1000 AEs.4
Most concussions (54.8%) resulted in missing play between 1 to 3 weeks, but a sizeable portion of the athletes (14.9%) were out of play for more than 3 weeks. Additionally, 10.7% of all medical disqualifications were due to concussive injuries. Khodaee and colleagues4 found no statistically significant difference in concussion rates between male and female soccer players over the 9-year period of time that they examined, however previous studies have found higher rates in female athletes.5
In soccer, as in other sports, there is a concern about both the adequate recognition of concussions during practice and play and the underreporting of concussions by athletes. The US Soccer Federation has taken a proactive stance on addressing concussion in youth soccer by developing the “Recognize to Recover” program.6 Recognize to Recover is the US Soccer Federation’s “comprehensive player health and safety program aimed at promoting safe play and reducing injuries in soccer players of all ages.” The website provides an educational video geared toward players, along with links to concussion assessment tools, the US Soccer Federation Concussion Protocol, and US Soccer Federation-Centers for Disease Control fact sheets for athletes, parents, and coaches.6
Continue to: A challenge for all sports...
A challenge for all sports is allowing adequate evaluation of a suspected concussion by properly trained healthcare professionals. The 2017 Berlin Concussion in Sport Group position paper stated, “when a concussion is suspected, the athlete should be removed from the sporting environment and a multimodal assessment should be conducted in standardized fashion (eg, Sport Concussion Assessment Tool- 5th edition). Sporting bodies should allow adequate time to conduct this evaluation.”7 However, the International Federation of Football Association (FIFA) rules limit substitutions to 3 over the course of the game, which can make a thorough evaluation of players difficult as trainers and coaches are under increased pressure to quickly determine whether to use one of their valuable substitutions. Fortunately, the National Collegiate Athletic Association (NCAA) soccer has mitigated this issue by allowing unlimited substitutions during matches, and high school teams generally follow similar rules.
One of the goals of any safety education program is not only to raise the awareness of the signs and symptoms of concussion by all those involved in the sport, but also to increase the number of athletes who self-report their symptoms and decrease those who hide any possible concussions. A study found that a majority (58.6%) of middle school soccer players continued to play while experiencing concussion symptoms.5 However, in a very recent (not yet published) study, 92% of US Soccer Federation players reported that they did seek out a medical evaluation for their concussion.8 This is certainly a positive sign and further research needs to clarify what methods of education or training will maintain this level of self-reporting in soccer players.
There has also been an increased focus on understanding the mechanism of injury of concussions. In soccer, concussions can occur from player-to-player contact, contact with the player surface, contact with playing apparatus (eg, goal posts) and non-contact mechanisms. While there has been a focus on concussions from heading the ball, player-to-player contact is the most common cause of concussions. A 2017 study of 7- to 12-year-old soccer players in 4 European countries found that about 1 out of 10 concussions were caused by heading the ball.9 Comstock and colleageues10 found slightly higher numbers, roughly 25% to 30% (depending on gender), but 70% to 78% (again depending on gender) of those were caused by player-to-player contact rather than contact with the ball.
To date there has not been any meta-analytic review evaluating the cognitive and physical symptoms associated with heading in soccer. A recent review paper stated that the “current evidence seems insufficient to support a ban of heading in children’s football (soccer).”10 However, in December 2015 the US Soccer Federation included age-specific heading limitations. Players ages 10 years and under “shall not engage in heading, either in practices or in games” and players age 11 years and 12 years should have “limited heading in practice; maximum of 30 minutes of heading training per week, with no more than 15-20 headers per player, per week.”11 US Soccer Federation officials acknowledged the limitations in the current science regarding heading in young soccer players but chose to err on the side of caution until further empirical evidence regarding the risks associated with repetitive heading is available.
The US Soccer Federation is also exploring other ways to reduce the incidence rate of concussions, including ensuring that the age-appropriate sized ball is used in practice and play, possible rule changes, evaluation of different playing surfaces, and equipment usage. To date, there is no strong evidence to support the use of mouth guards or helmets to reduce concussions in soccer. Additionally, the current data about the value of head impact sensors in soccer has not supported its widespread use.
Continue to: Finally, the issue of the prevalence...
Finally, the issue of the prevalence of chronic traumatic encephalopathy (CTE) in soccer players is beyond the scope of this article. The expert opinion from the 2017 US Soccer Federation, Major League Soccer (MLS), and National Women’s Soccer League (NWSL) conference concluded, “At present, no data exist that support that soccer participation is a risk factor for the development of neurodegenerative disease. Similarly, at this time, consistent with evidence discussed in the Berlin Concussion in Sport Group (CISG) Consensus Conference, our review suggests no causal relationship has been demonstrated between soccer and CTE pathology.”12
The more we know about concussions, both in general and those sustained during soccer play, the better we are able to diagnose and manage these injuries in our athletes. An important step is creating evidence-based protocols that evolve as our knowledge of concussions does as well. In April 2017, the US Soccer Federation, MLS, and the NWSL held a joint summit entitled, “Head Injury in Soccer: From Science to the Field” to address the current evidence-based science of concussions in soccer.8 An article discussing the findings of this meeting is forthcoming and will undoubtedly guide further development of concussion protocols for soccer players of all ages.
HEART
The physiologic demands of soccer place considerable stress on the cardiovascular system. Participation in training and competition is characterized by a combination of aerobic and anaerobic physiology with the typical athlete covering approximately 10 km over the course of the 90-minute match. The primary role of the heart and blood vessels is to supply the exercising skeletal muscle with oxygen and energy substrate and to clear the byproducts of metabolism. Among healthy athletes without cardiovascular disease, these processes are typically well tolerated and may be associated with beneficial cardiovascular adaptations over time. However, competitive soccer players are not completely immune to cardiovascular disease. Athletes across the age and competition spectrum may develop symptoms suggestive of underlying cardiovascular disease during play including exertional chest pain, inappropriate shortness of breath, palpitations, and syncope. These athletes require timely clinical evaluation. In extremely rare but high visibility cases, competitive soccer players may succumb to cardiac arrest on the pitch, underscoring the need for comprehensive emergency action plans (EAPs). We provide the practicing clinician with an overview of cardiovascular issues relevant to the competitive soccer athlete.
CARDIOVASCULAR ADAPTATIONS TO SPORT
The pressure (ie, repetitive surges in systemic blood pressure) and volume (ie, sustained increases in high cardiac output) challenges inherent in soccer participation place stress on the cardiovascular system. Healthy athletes across the age spectrum typically tolerate the hemodynamic stressors of participation without issues. Athletes that engage in training and competition over months to years often develop beneficial adaptations of the cardiovascular system that enhance on-field performance and contribute to optimal long-term health. Detailed discussion of how the heart and blood vessels respond to exercise training is beyond the scope of this article, but the interested reader is referred to several prior publications.13,14 In brief, the heart of the healthy soccer athlete demonstrates the balanced mild chamber dilation and wall thickening characteristic of left ventricular eccentric remodeling. This form of exercise-induced cardiac remodeling facilitates maintenance of high stroke volume during exercise with minimal increases in cardiac work. In parallel, routine aerobic exercise training confers favorable changes in the systemic arterial system, which leads to reductions in age-associated ventricular stiffening and maintenance of healthy low blood pressure. It must be emphasized that the healthy heart muscle dilation and thickening that develop in response to sports participation, regardless of age, ethnicity, or gender, are relatively mild and should not be confused with common forms of heart muscle disease that may be seen in athletes at risk for adverse outcomes. In some situations, consultation with an extreme sports cardiologist may be required to differentiate exercise-induced remodeling from over heart muscle pathology.15
Continue to: THE SYMPTOMATIC ATHLETE...
THE SYMPTOMATIC ATHLETE
Any athlete presenting with symptoms suggestive of underlying cardiovascular disease should be withheld from training and competition until a comprehensive clinical evaluation has been completed. Common manifestations of underlying heart disease that occur in soccer players include exertional chest pain/pressure/tightness, shortness of breath that is out of proportion to workload, palpitations or the perception of irregular cardiac activity, and syncope. Chest discomfort, inappropriate shortness of breath, and palpitations that occur during training or competition should be managed with immediate removal from the playing field and prompt medical assessment. In many cases, thorough evaluation will involve collaboration between sports medicine and sports cardiology providers. Evaluations must be individualized on a case-by-case basis as tailored to the athlete’s presenting chief complaint and prior medical history. Most of these assessments will include a detailed medical history and physical examination, a 12-lead electrocardiogram (ECG), provocative exercise testing in a controlled environment, noninvasive cardiac imaging, and in some cases ambulatory rhythm monitoring. Uniformly, the athlete should be withheld from further training and competition until high-risk cardiovascular disease has been excluded. We refer the interested reader to a comprehensive discussion of symptom-based assessment of the athlete with suspected cardiovascular disease.16
Syncope (sudden and abrupt loss of consciousness with spontaneous neurologic recovery) is common among trained athletes. The vast majority of syncope is caused by “neurocardiogenic” mechanisms and carries a benign prognosis. Benign neurally-mediated syncope most often occurs outside of training and competition among athletes with heightened vagal tone and a predisposed susceptibility to triggers including pain, anxiety, emotional stimulation, and sudden postural change. Athletes who experience neurally-mediated syncope outside of training and competition routinely report a pre-event prodrome or aura that permits them to lower themselves to the ground, thereby avoiding injury. A distinct, but similarly benign and common, form of neurally-mediated fainting is post-exertional syncope. Here, fainting occurs within seconds of abrupt termination of exercise due to a rapid reduction cardiac preload and corollary cerebral blood supply. When either form of neurally-mediated syncope is suggested by a comprehensive medical history, normal physical examination, and a normal 12- lead ECG, further evaluation is unnecessary. However, the athlete and their coaching staff should be educated about avoidance tactics including hydration, dietary sodium supplementation, and avoidance of abrupt exercise termination as neutrally-mediated syncope tends to be recurrent without such measures. Fainting episodes that occur during training or competition that are not clearly post-exertional should be considered a medical emergency and should prompt comprehensive evaluation by a qualified cardiovascular specialist. Working closely with team physicians and athletic training staff, it is the responsibility of sports cardiologists to exclude potentially life-threatening forms of electrical, muscular, coronary, and valvular heart disease. Ideally, this evaluation should be conducted rapidly to avoid unnecessary delays in return to play.
CARDIAC ARREST AND SUDDEN DEATH
Numerous high-visibility cases of cardiac arrest on the soccer pitch have alerted the sporting community to the potential for these rare and potentially tragic events. Definitive incidence statistics defining the risk of cardiac arrest among soccer players are lacking. Data from the NCAA database suggest a sudden death incidence rate among collegiate male soccer athletes of approximately 1:24,000 athlete years.8 Similar data documenting incidence among female athletes and among those at lower (ie, youth level and high school) and higher (ie, professional) levels of play are unavailable. Underlying cardiovascular disease in the forms of heart muscle abnormalities (ie, genetic and acquired cardiomyopathy), coronary artery abnormalities (ie, genetic coronary anomalies and atherosclerotic coronary disease), valvular heart problems (ie, congenitally malformed aortic valves), and primary disturbances of the cardiac electrical system (ie, Wolf-Parkinson-White syndrome, long QT syndrome, etc.) explain a substantial percentage of on-pitch cardiac arrest. However, it is increasingly recognized that a significant minority of sudden cardiac deaths among athletes occur in the absence of attributable cardiovascular abnormality.17 Such cases, often referred to as “sudden unexplained death,” present unique challenges in the context of pre-participation screening, as they are undetectable and thus unpredictable.
Reduction of cardiac arrest and sudden death may best be accomplished through a combination of focused pre-participation screening and the development and implementation of a comprehensive EAP. Pre-participation involves the performance of a battery of tests prior to training and competition that are geared toward the detection of occult high-risk cardiovascular disease. Recommendations regarding pre-participation screening vary both across and within countries. Current US recommendations call for a focused medical history and physical examination prior to training and competition with consideration of the addition of a 12-lead ECG on a local level based on expertise and available resources.18 Conversely, current European guidelines including those endorsed by FIFA, suggest routine inclusion of a 12-lead ECG and in some cases, a transthoracic ECG.19 It must be emphasized that no screening approach has been confirmed to reduce the incidence of sudden death, and the decision to extend screening beyond the medical history and physical examination to include a 12-lead ECG or echocardiogram may come at the cost of increased false positive testing. In practice, decisions about how and when to screen are ideally made at a local level after consideration of medical and financial resources.20
Continue to: Even the most comprehensive approach...
Even the most comprehensive approach to screening and evaluation of symptomatic athletes will not completely eliminate on-pitch cardiac arrest. Thus, all stakeholders that engage in the oversight of organized soccer must be committed to the development and implementation of an EAP.21 Key components of an effective EAP include the training of coaching staff, athletic trainers, and players in basic cardiopulmonary resuscitation, access to and training in the use of automated external defibrillators, and a triage/transport protocol that ensures timely access to advanced cardiac life support. Much like screening, emergency action planning involving these key core components must be developed and tailored locally. In the era of contemporary organized athletics, the absence of an EAP at any level of competition, from youth to professional leagues, is unacceptable. Effective EAPs must be developed, documented, and rehearsed at regular intervals. For the health and safety of competitive soccer players, as well as coaching staff and spectators, these steps are of critical importance.
HEAT
Heat-related illnesses can be serious and, at times, even life threatening. It is important for athletic staff and athletes to be well versed in the prevention, signs and symptoms, and treatment of heat-related illnesses in order to prevent serious and lasting injury. We aim to educate physicians about the prevention, recognition, and management of heat-related injury, and stress the importance of similarly educating athletes and coaching staff.
Exertional heat illnesses most often occur at temperatures >86°F, however they can occur at any temperature with heavy exertion.22 Signs and symptoms can be nonspecific early on, including weakness, fatigue, headache, nausea, and dizziness. Later signs can include imbalance, altered mentation, confusion, and behavior that is out of character such as irritability or aggression.23 It is easy to see how the later signs can be confused for concussion in the right context. We cover the recognition and treatment of two common and serious heat-related illnesses: heat exhaustion and exertional heat stroke (EHS).
HEAT EXHAUSTION
Heat exhaustion occurs when an athlete cannot continue to exercise due to weakness and fatigue. While the exact mechanism is not well understood, it has been established that the combined effect of heat and dehydration have been proven to decrease exercise capacity and performance to a greater degree than either alone. The heat created by the body during exercise is 15 to 20 times greater than when at rest, and can increase core body temperature by 1°C every 5 minutes if no heat is lost, such as through sweating.24 Additionally, when fluid deficits reach >3% to 5% of total body water, sweat production and skin blood flow decline, blunting the ability for the body to cool itself and causing progressive elevation of core body temperature if the athlete continues exerting him or herself. When fluid deficits reach 6% to 10%, cardiac output, sweat, and muscle blood flow decrease, likely leading to the symptoms seen with heat exhaustion: weakness, profound fatigue, and occasionally confusion and disorientation. Athletes with suspected heat exhaustion should be moved to a cooler area, laid down with legs elevated, and orally rehydrated. If they do not improve with oral rehydration, they may require intravenous fluids. The diagnosis of heat exhaustion hinges on a rectal temperature of <104°F; if >104°F the athlete should be presumed to have heat stroke, which will be addressed in the following paragraphs. Players can be cleared to return to play in mild cases within 24 to 48 hours with gradual increases in exercise intensity.24
EXERTIONAL HEAT STROKE
EHS occurs when the body can no longer regulate the core body temperature and it rises to upwards of 104°F. In EHS, elevated core body temperature is associated with evidence of end organ dysfunction. The most easily identified on the playing field is likely central nervous system dysfunction, including irritability, confusion, irrational behavior, lethargy, dizziness, confusion, and even loss of consciousness. Temperature should be measured with rectal temperature only, as other methods of measurement have been shown to be consistently inaccurate.22 Heat stroke can be confused with exertional hyponatremia, heat exhaustion, or concussion, especially when core body temperature cannot be determined. However, EHS should always be the presumed cause of altered mentation when no rectal temperature is available because rapid cooling is critical to minimizing lasting effects. Morbidity and mortality are directly related to the length of time required to cool the athlete under 40°C (104°F).24 Cooling should be completed on site prior to transport to a medical facility and is best achieved with submersion in an ice bath (ie, a kiddie pool or soaking tub full of ice and water).22,25 If an ice bath is not available, ice bags should be applied to the neck axilla and groin and exchanged for fresh bags every 2 to 3 minutes.22 Ice bags have been shown to be inferior to whole body cooling, only cooling the athlete .04°C to .08°C/min compared to .15°C to .24°C/min with the ice bath.24 All other tests should be delayed until cooling is achieved, unless they can be completed while cooling the athlete. The athlete can be removed from the ice bath once rectal temperatures reach <101°F to 102°F.23 If the athlete returns to baseline after cooling, transportation to a medical facility may not be necessary. However, they should refrain from physical activity and heat exposure for at least 7 days and should be evaluated by a physician at that time. If all labs are normal and the athlete is asymptomatic, they can start progressive return to play under the direction of an athletic trainer or a sports medicine physician.23
Continue to: HEAT-RELATED ILLNESS...
HEAT-RELATED ILLNESS
It is impossible to predict exactly which athletes will be most at risk for heat-related illness, so it is important to have a high degree of suspicion when environmental conditions are right. Athletes with recent illness, fever, or lack of sleep are at higher risk. Additional intrinsic risk factors include low fitness level, obesity, and inadequate hydration. Athletes who are highly competitive or motivated can be more likely to push through the early signs of illness or be reluctant to report symptoms.23 Those with a history of exertional heat illness are more at risk for developing it again in the future.23
The extrinsic risk factors for the development of heat-related illness are much easier to identify and modify in order to keep athletes safe. High temperature and high humidity conditions, heavy sun exposure, and exposure to similar conditions the preceding day put athletes at risk for exertional heat illness. Risks are even greater when the exercise is prolonged or intense with few breaks and access to hydration is limited.23 Therefore, prevention of exertional heat illness is centered on these external risk factors.
Each team should have a heat policy as part of their EAP aimed at prevention and early recognition of heat-related illness. This policy should be shared with all athletes and coaches. The plan should be centered on acclimatization, activity modification, and early recognition and management as previously discussed. The US Soccer Federation “Recognize to Recover” Heat Guidelines suggest a 3-step process for appropriate activity modification:22
1. Find the wet bulb globe temperature, either using a wet bulb globe thermometer or the temperature and humidity (Figure 1).
2. Find your regional weather category on the map (Figure 2).
3. Find your alert level and work to rest ratio recommendations (Figure 3).
Scheduled hydration breaks should be given as listed in Figure 3. Breaks of 4 minutes should be given for each 30 minutes of continuous practice or play. In a regulation 90-minute match, a hydration break should be given at 30 and 75 minutes (with half time at 45 minutes) at minimum. Athletes should be educated about where hydration can be accessed, and given unlimited access to hydration even outside of planned breaks.22
Acclimatization to conditions is another integral part of preventing heat-related illness. It allows the body time to adapt to exercising in heat gradually, with a measured progression of exertion over the course of 10 to 14 days. The “Recognize to Recover” Heat Guidelines also provide guidance on acclimatization, and specifics can be found on the website.1 Generally speaking, the warmest part of the day, usually between 11 AM and 4 PM, should be avoided for all training sessions, and length of practice and exertion should be gradually increased over 2 weeks.22
In summary, appropriate acclimatization, hydration, activity modification, and education of athletes and staff are essential for the prevention of heat-related illness. Athletes and staff should understand the signs and symptoms of heat-related illness so that it can be recognized early and treated appropriately. If an athlete is altered in the heat and rectal temperature is >104°F or rectal temperature cannot be obtained, rapid cooling using an ice bath or ice bags is essential to prevent the morbidity and mortality associated with EHS. Above all, teams should have an explicit plan that includes protocols for acclimatization, activity modification, and all necessary equipment to prevent and treat heat-related illnesses should they occur, and ultimately keep athletes safe and healthy.
1. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain Injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
2. The National Federation of State High School Associations. 2013-14 high school athletics participation survey. http://www.nfhs.org/ParticipationStatics/PDF/2013-14_Participation_Survey_PDF.pdf. Accessed August 6, 2018.
3. Youth Council. US Soccer Federation Web site. https://www.ussoccer.com/about/affiliates/youth-council. Accessed July 31, 2018.
4. Khodaee M, Currie DW, Asif IM, Comstock RD. Nine-year study of US high school soccer injuries: data from a national sports injury surveillance programme. Br J Sports Med. 2017;51(3):185-193. doi:10.1136/bjsports-2015-095946.
5. Schallmo MS, Weiner JA, Hsu WK. Sport and sex-specific trends in the epidemiology of concussions sustained by high school athletes. J Bone Joint Surg Am. 2017;99(15):1314-1320. doi:10.2106/JBJS.16.01573.
6. US Soccer Federation. U.S. Soccer’s comprehensive player health and safety program. Recognize to Recover Web site. http://www.recognizetorecover.org/#us-soccers-comprehensive-player-health-and-safety-program. Accessed July 31, 2018.
7. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi:10.1136/bjsports-2017-097699.
8. Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123(15):1594-1600. doi:10.1161/CIRCULATIONAHA.110.004622.
9. Faude O, Rössler R, Junge A, et al. Head injuries in children’s football-results from two prospective cohort studies in four European countries. Scand J Med Sci Sports. 2017;27(12):1986-1992. doi:10.1111/sms.12839.
10. Comstock RD, Currie DW, Pierpoint LA, Grubenhoff JA, Fields SK. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169(9):830-837. doi:10.1001/jamapediatrics.2015.1062.
11. Tarnutzer AA. Should heading be forbidden in children’s football? Sci Med Football. 2018;2(1):75-79.
12. US Soccer Federation. US Soccer, NWSL and MLS to host “head injury in soccer; science to field”. https://www.ussoccer.com/stories/2017/04/18/17/35/20170418-news-us-soccer-nwsl-mls-host-head-injury-in-soccer-science-to-field. Published April 18, 2017. Accessed August 6, 2018.
13. Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. 2012;54(5):380-386. doi:10.1016/j.pcad.2012.01.006.
14. Baggish AL, Wood MJ. Athlete's heart and cardiovascular care of the athlete: scientific and clinical update. Circulation. 2011;123(23):2723-2735. doi:10.1161/CIRCULATIONAHA.110.981571.
15. Kim JH, Baggish AL. Differentiating exercise-induced cardiac adaptations from cardiac pathology: the "Grey Zone" of clinical uncertainty. Can J Cardiol. 2016;32(4):429-437. doi:10.1016/j.cjca.2015.11.025.
16. Baggish AL, Battle RW, Beckerman JG, et al; ACC’s Sports and Exercise Council Leadership Group. Sports cardiology: core curriculum for providing cardiovascular care to competitive athletes and highly active people. J Am Coll Cardiol. 2017;70(15):1902-1918. doi:10.1016/j.jacc.2017.08.055.
17. Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation. 2015;132(1):10-19. doi:10.1161/CIRCULATIONAHA.115.015431.
18. Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66(21):2356-2361. doi:10.1016/j.jacc.2015.09.034.
19. Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516-524.
20. Baggish AL, Kovacs RJ. Preparticipation cardiovascular screening: clinical partnership is the only certainty. Br J Sports Med. 2017;51(3):150-151. doi:10.1136/bjsports-2016-096954.
21. Hainline B, Drezner J, Baggish A, et al. Interassociation consensus statement on cardiovascular care of college student-athletes. J Athl Train. 2016;51(4):344-357. doi:10.4085/j.jacc.2016.03.527.
22. US Soccer Federation. Environmental conditions. Recognize to Recover Web site. http://www.recognizetorecover.org/environmental/#environmental-conditions. Accessed April 15, 2018.
23. Korey Stringer Institute. Emergency conditions: heat illnesses. University of Connecticut Web site. https://ksi.uconn.edu/. Accessed April 15, 2018.
24. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
25. Belval LN, Casa DJ, Adams WM, et al. Consensus statement- prehospital care of exertional heat stroke. Prehosp Emerg Care. 2018;22(3):392-397. doi:10.1080/10903127.2017.1392666.
ABSTRACT
Soccer requires significant physical conditioning and endurance, as well as the physicality required for contact play. In order to keep athletes safe, it is important that coaches, medical staff, and the players themselves are educated on the most common dangers to their health that they may encounter on a soccer pitch. This article aims to review the current literature and recommendations on concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer, with a goal of educating all those who help to keep athletes healthy and competing to their full potential.
Continue to: Soccer is one of the most popular sports...
Soccer is one of the most popular sports in the modern world and requires significant physical conditioning and endurance, as well as the physicality required for contact play. This article covers the topics of concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer players. We provide a review of the prevention, recognition, and management required to keep athletes safe on the soccer pitch, both in practice and in competitive play.
HEAD
With an estimated 1.6 to 3.8 million sports-related mild traumatic brain injuries (ie, concussions) occurring annually in the United States,1 there has been an appropriate increase in the focus on prevention and treatment of these injuries. For more than a decade the spotlight has been on concussions that occur in American football, but other sports have also had to examine the prevalence of concussions in their sport. This is certainly true for soccer.
There has been a steady increase in soccer participation in the United States. From 1973 to 2014 there was a 4-fold increase in high school boys and a 35-fold increase in high school girls playing soccer.2 Currently, there are more than 3.7 million youth who play on teams under the supervision of the US Soccer Federation, the sport’s national governing body.3 With the growth of the sport, there has also been an intensified focus on injury prevention in soccer players, including concussive brain injuries.
A recent study examined injury rates in high school soccer players and noted that concussion is the second most common injury (17.9%), after ligament sprains (29.7%).4 The overall injury rate was 2.06 per 1000 athletic exposures (AEs [defined as participation in practice or game play]), but higher in games (4.42 per 1000 AEs) than during practices (1.05 per 1000 AEs). The overall concussion rate was 0.36 per 1000 AEs.4
Most concussions (54.8%) resulted in missing play between 1 to 3 weeks, but a sizeable portion of the athletes (14.9%) were out of play for more than 3 weeks. Additionally, 10.7% of all medical disqualifications were due to concussive injuries. Khodaee and colleagues4 found no statistically significant difference in concussion rates between male and female soccer players over the 9-year period of time that they examined, however previous studies have found higher rates in female athletes.5
In soccer, as in other sports, there is a concern about both the adequate recognition of concussions during practice and play and the underreporting of concussions by athletes. The US Soccer Federation has taken a proactive stance on addressing concussion in youth soccer by developing the “Recognize to Recover” program.6 Recognize to Recover is the US Soccer Federation’s “comprehensive player health and safety program aimed at promoting safe play and reducing injuries in soccer players of all ages.” The website provides an educational video geared toward players, along with links to concussion assessment tools, the US Soccer Federation Concussion Protocol, and US Soccer Federation-Centers for Disease Control fact sheets for athletes, parents, and coaches.6
Continue to: A challenge for all sports...
A challenge for all sports is allowing adequate evaluation of a suspected concussion by properly trained healthcare professionals. The 2017 Berlin Concussion in Sport Group position paper stated, “when a concussion is suspected, the athlete should be removed from the sporting environment and a multimodal assessment should be conducted in standardized fashion (eg, Sport Concussion Assessment Tool- 5th edition). Sporting bodies should allow adequate time to conduct this evaluation.”7 However, the International Federation of Football Association (FIFA) rules limit substitutions to 3 over the course of the game, which can make a thorough evaluation of players difficult as trainers and coaches are under increased pressure to quickly determine whether to use one of their valuable substitutions. Fortunately, the National Collegiate Athletic Association (NCAA) soccer has mitigated this issue by allowing unlimited substitutions during matches, and high school teams generally follow similar rules.
One of the goals of any safety education program is not only to raise the awareness of the signs and symptoms of concussion by all those involved in the sport, but also to increase the number of athletes who self-report their symptoms and decrease those who hide any possible concussions. A study found that a majority (58.6%) of middle school soccer players continued to play while experiencing concussion symptoms.5 However, in a very recent (not yet published) study, 92% of US Soccer Federation players reported that they did seek out a medical evaluation for their concussion.8 This is certainly a positive sign and further research needs to clarify what methods of education or training will maintain this level of self-reporting in soccer players.
There has also been an increased focus on understanding the mechanism of injury of concussions. In soccer, concussions can occur from player-to-player contact, contact with the player surface, contact with playing apparatus (eg, goal posts) and non-contact mechanisms. While there has been a focus on concussions from heading the ball, player-to-player contact is the most common cause of concussions. A 2017 study of 7- to 12-year-old soccer players in 4 European countries found that about 1 out of 10 concussions were caused by heading the ball.9 Comstock and colleageues10 found slightly higher numbers, roughly 25% to 30% (depending on gender), but 70% to 78% (again depending on gender) of those were caused by player-to-player contact rather than contact with the ball.
To date there has not been any meta-analytic review evaluating the cognitive and physical symptoms associated with heading in soccer. A recent review paper stated that the “current evidence seems insufficient to support a ban of heading in children’s football (soccer).”10 However, in December 2015 the US Soccer Federation included age-specific heading limitations. Players ages 10 years and under “shall not engage in heading, either in practices or in games” and players age 11 years and 12 years should have “limited heading in practice; maximum of 30 minutes of heading training per week, with no more than 15-20 headers per player, per week.”11 US Soccer Federation officials acknowledged the limitations in the current science regarding heading in young soccer players but chose to err on the side of caution until further empirical evidence regarding the risks associated with repetitive heading is available.
The US Soccer Federation is also exploring other ways to reduce the incidence rate of concussions, including ensuring that the age-appropriate sized ball is used in practice and play, possible rule changes, evaluation of different playing surfaces, and equipment usage. To date, there is no strong evidence to support the use of mouth guards or helmets to reduce concussions in soccer. Additionally, the current data about the value of head impact sensors in soccer has not supported its widespread use.
Continue to: Finally, the issue of the prevalence...
Finally, the issue of the prevalence of chronic traumatic encephalopathy (CTE) in soccer players is beyond the scope of this article. The expert opinion from the 2017 US Soccer Federation, Major League Soccer (MLS), and National Women’s Soccer League (NWSL) conference concluded, “At present, no data exist that support that soccer participation is a risk factor for the development of neurodegenerative disease. Similarly, at this time, consistent with evidence discussed in the Berlin Concussion in Sport Group (CISG) Consensus Conference, our review suggests no causal relationship has been demonstrated between soccer and CTE pathology.”12
The more we know about concussions, both in general and those sustained during soccer play, the better we are able to diagnose and manage these injuries in our athletes. An important step is creating evidence-based protocols that evolve as our knowledge of concussions does as well. In April 2017, the US Soccer Federation, MLS, and the NWSL held a joint summit entitled, “Head Injury in Soccer: From Science to the Field” to address the current evidence-based science of concussions in soccer.8 An article discussing the findings of this meeting is forthcoming and will undoubtedly guide further development of concussion protocols for soccer players of all ages.
HEART
The physiologic demands of soccer place considerable stress on the cardiovascular system. Participation in training and competition is characterized by a combination of aerobic and anaerobic physiology with the typical athlete covering approximately 10 km over the course of the 90-minute match. The primary role of the heart and blood vessels is to supply the exercising skeletal muscle with oxygen and energy substrate and to clear the byproducts of metabolism. Among healthy athletes without cardiovascular disease, these processes are typically well tolerated and may be associated with beneficial cardiovascular adaptations over time. However, competitive soccer players are not completely immune to cardiovascular disease. Athletes across the age and competition spectrum may develop symptoms suggestive of underlying cardiovascular disease during play including exertional chest pain, inappropriate shortness of breath, palpitations, and syncope. These athletes require timely clinical evaluation. In extremely rare but high visibility cases, competitive soccer players may succumb to cardiac arrest on the pitch, underscoring the need for comprehensive emergency action plans (EAPs). We provide the practicing clinician with an overview of cardiovascular issues relevant to the competitive soccer athlete.
CARDIOVASCULAR ADAPTATIONS TO SPORT
The pressure (ie, repetitive surges in systemic blood pressure) and volume (ie, sustained increases in high cardiac output) challenges inherent in soccer participation place stress on the cardiovascular system. Healthy athletes across the age spectrum typically tolerate the hemodynamic stressors of participation without issues. Athletes that engage in training and competition over months to years often develop beneficial adaptations of the cardiovascular system that enhance on-field performance and contribute to optimal long-term health. Detailed discussion of how the heart and blood vessels respond to exercise training is beyond the scope of this article, but the interested reader is referred to several prior publications.13,14 In brief, the heart of the healthy soccer athlete demonstrates the balanced mild chamber dilation and wall thickening characteristic of left ventricular eccentric remodeling. This form of exercise-induced cardiac remodeling facilitates maintenance of high stroke volume during exercise with minimal increases in cardiac work. In parallel, routine aerobic exercise training confers favorable changes in the systemic arterial system, which leads to reductions in age-associated ventricular stiffening and maintenance of healthy low blood pressure. It must be emphasized that the healthy heart muscle dilation and thickening that develop in response to sports participation, regardless of age, ethnicity, or gender, are relatively mild and should not be confused with common forms of heart muscle disease that may be seen in athletes at risk for adverse outcomes. In some situations, consultation with an extreme sports cardiologist may be required to differentiate exercise-induced remodeling from over heart muscle pathology.15
Continue to: THE SYMPTOMATIC ATHLETE...
THE SYMPTOMATIC ATHLETE
Any athlete presenting with symptoms suggestive of underlying cardiovascular disease should be withheld from training and competition until a comprehensive clinical evaluation has been completed. Common manifestations of underlying heart disease that occur in soccer players include exertional chest pain/pressure/tightness, shortness of breath that is out of proportion to workload, palpitations or the perception of irregular cardiac activity, and syncope. Chest discomfort, inappropriate shortness of breath, and palpitations that occur during training or competition should be managed with immediate removal from the playing field and prompt medical assessment. In many cases, thorough evaluation will involve collaboration between sports medicine and sports cardiology providers. Evaluations must be individualized on a case-by-case basis as tailored to the athlete’s presenting chief complaint and prior medical history. Most of these assessments will include a detailed medical history and physical examination, a 12-lead electrocardiogram (ECG), provocative exercise testing in a controlled environment, noninvasive cardiac imaging, and in some cases ambulatory rhythm monitoring. Uniformly, the athlete should be withheld from further training and competition until high-risk cardiovascular disease has been excluded. We refer the interested reader to a comprehensive discussion of symptom-based assessment of the athlete with suspected cardiovascular disease.16
Syncope (sudden and abrupt loss of consciousness with spontaneous neurologic recovery) is common among trained athletes. The vast majority of syncope is caused by “neurocardiogenic” mechanisms and carries a benign prognosis. Benign neurally-mediated syncope most often occurs outside of training and competition among athletes with heightened vagal tone and a predisposed susceptibility to triggers including pain, anxiety, emotional stimulation, and sudden postural change. Athletes who experience neurally-mediated syncope outside of training and competition routinely report a pre-event prodrome or aura that permits them to lower themselves to the ground, thereby avoiding injury. A distinct, but similarly benign and common, form of neurally-mediated fainting is post-exertional syncope. Here, fainting occurs within seconds of abrupt termination of exercise due to a rapid reduction cardiac preload and corollary cerebral blood supply. When either form of neurally-mediated syncope is suggested by a comprehensive medical history, normal physical examination, and a normal 12- lead ECG, further evaluation is unnecessary. However, the athlete and their coaching staff should be educated about avoidance tactics including hydration, dietary sodium supplementation, and avoidance of abrupt exercise termination as neutrally-mediated syncope tends to be recurrent without such measures. Fainting episodes that occur during training or competition that are not clearly post-exertional should be considered a medical emergency and should prompt comprehensive evaluation by a qualified cardiovascular specialist. Working closely with team physicians and athletic training staff, it is the responsibility of sports cardiologists to exclude potentially life-threatening forms of electrical, muscular, coronary, and valvular heart disease. Ideally, this evaluation should be conducted rapidly to avoid unnecessary delays in return to play.
CARDIAC ARREST AND SUDDEN DEATH
Numerous high-visibility cases of cardiac arrest on the soccer pitch have alerted the sporting community to the potential for these rare and potentially tragic events. Definitive incidence statistics defining the risk of cardiac arrest among soccer players are lacking. Data from the NCAA database suggest a sudden death incidence rate among collegiate male soccer athletes of approximately 1:24,000 athlete years.8 Similar data documenting incidence among female athletes and among those at lower (ie, youth level and high school) and higher (ie, professional) levels of play are unavailable. Underlying cardiovascular disease in the forms of heart muscle abnormalities (ie, genetic and acquired cardiomyopathy), coronary artery abnormalities (ie, genetic coronary anomalies and atherosclerotic coronary disease), valvular heart problems (ie, congenitally malformed aortic valves), and primary disturbances of the cardiac electrical system (ie, Wolf-Parkinson-White syndrome, long QT syndrome, etc.) explain a substantial percentage of on-pitch cardiac arrest. However, it is increasingly recognized that a significant minority of sudden cardiac deaths among athletes occur in the absence of attributable cardiovascular abnormality.17 Such cases, often referred to as “sudden unexplained death,” present unique challenges in the context of pre-participation screening, as they are undetectable and thus unpredictable.
Reduction of cardiac arrest and sudden death may best be accomplished through a combination of focused pre-participation screening and the development and implementation of a comprehensive EAP. Pre-participation involves the performance of a battery of tests prior to training and competition that are geared toward the detection of occult high-risk cardiovascular disease. Recommendations regarding pre-participation screening vary both across and within countries. Current US recommendations call for a focused medical history and physical examination prior to training and competition with consideration of the addition of a 12-lead ECG on a local level based on expertise and available resources.18 Conversely, current European guidelines including those endorsed by FIFA, suggest routine inclusion of a 12-lead ECG and in some cases, a transthoracic ECG.19 It must be emphasized that no screening approach has been confirmed to reduce the incidence of sudden death, and the decision to extend screening beyond the medical history and physical examination to include a 12-lead ECG or echocardiogram may come at the cost of increased false positive testing. In practice, decisions about how and when to screen are ideally made at a local level after consideration of medical and financial resources.20
Continue to: Even the most comprehensive approach...
Even the most comprehensive approach to screening and evaluation of symptomatic athletes will not completely eliminate on-pitch cardiac arrest. Thus, all stakeholders that engage in the oversight of organized soccer must be committed to the development and implementation of an EAP.21 Key components of an effective EAP include the training of coaching staff, athletic trainers, and players in basic cardiopulmonary resuscitation, access to and training in the use of automated external defibrillators, and a triage/transport protocol that ensures timely access to advanced cardiac life support. Much like screening, emergency action planning involving these key core components must be developed and tailored locally. In the era of contemporary organized athletics, the absence of an EAP at any level of competition, from youth to professional leagues, is unacceptable. Effective EAPs must be developed, documented, and rehearsed at regular intervals. For the health and safety of competitive soccer players, as well as coaching staff and spectators, these steps are of critical importance.
HEAT
Heat-related illnesses can be serious and, at times, even life threatening. It is important for athletic staff and athletes to be well versed in the prevention, signs and symptoms, and treatment of heat-related illnesses in order to prevent serious and lasting injury. We aim to educate physicians about the prevention, recognition, and management of heat-related injury, and stress the importance of similarly educating athletes and coaching staff.
Exertional heat illnesses most often occur at temperatures >86°F, however they can occur at any temperature with heavy exertion.22 Signs and symptoms can be nonspecific early on, including weakness, fatigue, headache, nausea, and dizziness. Later signs can include imbalance, altered mentation, confusion, and behavior that is out of character such as irritability or aggression.23 It is easy to see how the later signs can be confused for concussion in the right context. We cover the recognition and treatment of two common and serious heat-related illnesses: heat exhaustion and exertional heat stroke (EHS).
HEAT EXHAUSTION
Heat exhaustion occurs when an athlete cannot continue to exercise due to weakness and fatigue. While the exact mechanism is not well understood, it has been established that the combined effect of heat and dehydration have been proven to decrease exercise capacity and performance to a greater degree than either alone. The heat created by the body during exercise is 15 to 20 times greater than when at rest, and can increase core body temperature by 1°C every 5 minutes if no heat is lost, such as through sweating.24 Additionally, when fluid deficits reach >3% to 5% of total body water, sweat production and skin blood flow decline, blunting the ability for the body to cool itself and causing progressive elevation of core body temperature if the athlete continues exerting him or herself. When fluid deficits reach 6% to 10%, cardiac output, sweat, and muscle blood flow decrease, likely leading to the symptoms seen with heat exhaustion: weakness, profound fatigue, and occasionally confusion and disorientation. Athletes with suspected heat exhaustion should be moved to a cooler area, laid down with legs elevated, and orally rehydrated. If they do not improve with oral rehydration, they may require intravenous fluids. The diagnosis of heat exhaustion hinges on a rectal temperature of <104°F; if >104°F the athlete should be presumed to have heat stroke, which will be addressed in the following paragraphs. Players can be cleared to return to play in mild cases within 24 to 48 hours with gradual increases in exercise intensity.24
EXERTIONAL HEAT STROKE
EHS occurs when the body can no longer regulate the core body temperature and it rises to upwards of 104°F. In EHS, elevated core body temperature is associated with evidence of end organ dysfunction. The most easily identified on the playing field is likely central nervous system dysfunction, including irritability, confusion, irrational behavior, lethargy, dizziness, confusion, and even loss of consciousness. Temperature should be measured with rectal temperature only, as other methods of measurement have been shown to be consistently inaccurate.22 Heat stroke can be confused with exertional hyponatremia, heat exhaustion, or concussion, especially when core body temperature cannot be determined. However, EHS should always be the presumed cause of altered mentation when no rectal temperature is available because rapid cooling is critical to minimizing lasting effects. Morbidity and mortality are directly related to the length of time required to cool the athlete under 40°C (104°F).24 Cooling should be completed on site prior to transport to a medical facility and is best achieved with submersion in an ice bath (ie, a kiddie pool or soaking tub full of ice and water).22,25 If an ice bath is not available, ice bags should be applied to the neck axilla and groin and exchanged for fresh bags every 2 to 3 minutes.22 Ice bags have been shown to be inferior to whole body cooling, only cooling the athlete .04°C to .08°C/min compared to .15°C to .24°C/min with the ice bath.24 All other tests should be delayed until cooling is achieved, unless they can be completed while cooling the athlete. The athlete can be removed from the ice bath once rectal temperatures reach <101°F to 102°F.23 If the athlete returns to baseline after cooling, transportation to a medical facility may not be necessary. However, they should refrain from physical activity and heat exposure for at least 7 days and should be evaluated by a physician at that time. If all labs are normal and the athlete is asymptomatic, they can start progressive return to play under the direction of an athletic trainer or a sports medicine physician.23
Continue to: HEAT-RELATED ILLNESS...
HEAT-RELATED ILLNESS
It is impossible to predict exactly which athletes will be most at risk for heat-related illness, so it is important to have a high degree of suspicion when environmental conditions are right. Athletes with recent illness, fever, or lack of sleep are at higher risk. Additional intrinsic risk factors include low fitness level, obesity, and inadequate hydration. Athletes who are highly competitive or motivated can be more likely to push through the early signs of illness or be reluctant to report symptoms.23 Those with a history of exertional heat illness are more at risk for developing it again in the future.23
The extrinsic risk factors for the development of heat-related illness are much easier to identify and modify in order to keep athletes safe. High temperature and high humidity conditions, heavy sun exposure, and exposure to similar conditions the preceding day put athletes at risk for exertional heat illness. Risks are even greater when the exercise is prolonged or intense with few breaks and access to hydration is limited.23 Therefore, prevention of exertional heat illness is centered on these external risk factors.
Each team should have a heat policy as part of their EAP aimed at prevention and early recognition of heat-related illness. This policy should be shared with all athletes and coaches. The plan should be centered on acclimatization, activity modification, and early recognition and management as previously discussed. The US Soccer Federation “Recognize to Recover” Heat Guidelines suggest a 3-step process for appropriate activity modification:22
1. Find the wet bulb globe temperature, either using a wet bulb globe thermometer or the temperature and humidity (Figure 1).
2. Find your regional weather category on the map (Figure 2).
3. Find your alert level and work to rest ratio recommendations (Figure 3).
Scheduled hydration breaks should be given as listed in Figure 3. Breaks of 4 minutes should be given for each 30 minutes of continuous practice or play. In a regulation 90-minute match, a hydration break should be given at 30 and 75 minutes (with half time at 45 minutes) at minimum. Athletes should be educated about where hydration can be accessed, and given unlimited access to hydration even outside of planned breaks.22
Acclimatization to conditions is another integral part of preventing heat-related illness. It allows the body time to adapt to exercising in heat gradually, with a measured progression of exertion over the course of 10 to 14 days. The “Recognize to Recover” Heat Guidelines also provide guidance on acclimatization, and specifics can be found on the website.1 Generally speaking, the warmest part of the day, usually between 11 AM and 4 PM, should be avoided for all training sessions, and length of practice and exertion should be gradually increased over 2 weeks.22
In summary, appropriate acclimatization, hydration, activity modification, and education of athletes and staff are essential for the prevention of heat-related illness. Athletes and staff should understand the signs and symptoms of heat-related illness so that it can be recognized early and treated appropriately. If an athlete is altered in the heat and rectal temperature is >104°F or rectal temperature cannot be obtained, rapid cooling using an ice bath or ice bags is essential to prevent the morbidity and mortality associated with EHS. Above all, teams should have an explicit plan that includes protocols for acclimatization, activity modification, and all necessary equipment to prevent and treat heat-related illnesses should they occur, and ultimately keep athletes safe and healthy.
ABSTRACT
Soccer requires significant physical conditioning and endurance, as well as the physicality required for contact play. In order to keep athletes safe, it is important that coaches, medical staff, and the players themselves are educated on the most common dangers to their health that they may encounter on a soccer pitch. This article aims to review the current literature and recommendations on concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer, with a goal of educating all those who help to keep athletes healthy and competing to their full potential.
Continue to: Soccer is one of the most popular sports...
Soccer is one of the most popular sports in the modern world and requires significant physical conditioning and endurance, as well as the physicality required for contact play. This article covers the topics of concussion, cardiovascular considerations, and heat-related illness as they relate to competitive soccer players. We provide a review of the prevention, recognition, and management required to keep athletes safe on the soccer pitch, both in practice and in competitive play.
HEAD
With an estimated 1.6 to 3.8 million sports-related mild traumatic brain injuries (ie, concussions) occurring annually in the United States,1 there has been an appropriate increase in the focus on prevention and treatment of these injuries. For more than a decade the spotlight has been on concussions that occur in American football, but other sports have also had to examine the prevalence of concussions in their sport. This is certainly true for soccer.
There has been a steady increase in soccer participation in the United States. From 1973 to 2014 there was a 4-fold increase in high school boys and a 35-fold increase in high school girls playing soccer.2 Currently, there are more than 3.7 million youth who play on teams under the supervision of the US Soccer Federation, the sport’s national governing body.3 With the growth of the sport, there has also been an intensified focus on injury prevention in soccer players, including concussive brain injuries.
A recent study examined injury rates in high school soccer players and noted that concussion is the second most common injury (17.9%), after ligament sprains (29.7%).4 The overall injury rate was 2.06 per 1000 athletic exposures (AEs [defined as participation in practice or game play]), but higher in games (4.42 per 1000 AEs) than during practices (1.05 per 1000 AEs). The overall concussion rate was 0.36 per 1000 AEs.4
Most concussions (54.8%) resulted in missing play between 1 to 3 weeks, but a sizeable portion of the athletes (14.9%) were out of play for more than 3 weeks. Additionally, 10.7% of all medical disqualifications were due to concussive injuries. Khodaee and colleagues4 found no statistically significant difference in concussion rates between male and female soccer players over the 9-year period of time that they examined, however previous studies have found higher rates in female athletes.5
In soccer, as in other sports, there is a concern about both the adequate recognition of concussions during practice and play and the underreporting of concussions by athletes. The US Soccer Federation has taken a proactive stance on addressing concussion in youth soccer by developing the “Recognize to Recover” program.6 Recognize to Recover is the US Soccer Federation’s “comprehensive player health and safety program aimed at promoting safe play and reducing injuries in soccer players of all ages.” The website provides an educational video geared toward players, along with links to concussion assessment tools, the US Soccer Federation Concussion Protocol, and US Soccer Federation-Centers for Disease Control fact sheets for athletes, parents, and coaches.6
Continue to: A challenge for all sports...
A challenge for all sports is allowing adequate evaluation of a suspected concussion by properly trained healthcare professionals. The 2017 Berlin Concussion in Sport Group position paper stated, “when a concussion is suspected, the athlete should be removed from the sporting environment and a multimodal assessment should be conducted in standardized fashion (eg, Sport Concussion Assessment Tool- 5th edition). Sporting bodies should allow adequate time to conduct this evaluation.”7 However, the International Federation of Football Association (FIFA) rules limit substitutions to 3 over the course of the game, which can make a thorough evaluation of players difficult as trainers and coaches are under increased pressure to quickly determine whether to use one of their valuable substitutions. Fortunately, the National Collegiate Athletic Association (NCAA) soccer has mitigated this issue by allowing unlimited substitutions during matches, and high school teams generally follow similar rules.
One of the goals of any safety education program is not only to raise the awareness of the signs and symptoms of concussion by all those involved in the sport, but also to increase the number of athletes who self-report their symptoms and decrease those who hide any possible concussions. A study found that a majority (58.6%) of middle school soccer players continued to play while experiencing concussion symptoms.5 However, in a very recent (not yet published) study, 92% of US Soccer Federation players reported that they did seek out a medical evaluation for their concussion.8 This is certainly a positive sign and further research needs to clarify what methods of education or training will maintain this level of self-reporting in soccer players.
There has also been an increased focus on understanding the mechanism of injury of concussions. In soccer, concussions can occur from player-to-player contact, contact with the player surface, contact with playing apparatus (eg, goal posts) and non-contact mechanisms. While there has been a focus on concussions from heading the ball, player-to-player contact is the most common cause of concussions. A 2017 study of 7- to 12-year-old soccer players in 4 European countries found that about 1 out of 10 concussions were caused by heading the ball.9 Comstock and colleageues10 found slightly higher numbers, roughly 25% to 30% (depending on gender), but 70% to 78% (again depending on gender) of those were caused by player-to-player contact rather than contact with the ball.
To date there has not been any meta-analytic review evaluating the cognitive and physical symptoms associated with heading in soccer. A recent review paper stated that the “current evidence seems insufficient to support a ban of heading in children’s football (soccer).”10 However, in December 2015 the US Soccer Federation included age-specific heading limitations. Players ages 10 years and under “shall not engage in heading, either in practices or in games” and players age 11 years and 12 years should have “limited heading in practice; maximum of 30 minutes of heading training per week, with no more than 15-20 headers per player, per week.”11 US Soccer Federation officials acknowledged the limitations in the current science regarding heading in young soccer players but chose to err on the side of caution until further empirical evidence regarding the risks associated with repetitive heading is available.
The US Soccer Federation is also exploring other ways to reduce the incidence rate of concussions, including ensuring that the age-appropriate sized ball is used in practice and play, possible rule changes, evaluation of different playing surfaces, and equipment usage. To date, there is no strong evidence to support the use of mouth guards or helmets to reduce concussions in soccer. Additionally, the current data about the value of head impact sensors in soccer has not supported its widespread use.
Continue to: Finally, the issue of the prevalence...
Finally, the issue of the prevalence of chronic traumatic encephalopathy (CTE) in soccer players is beyond the scope of this article. The expert opinion from the 2017 US Soccer Federation, Major League Soccer (MLS), and National Women’s Soccer League (NWSL) conference concluded, “At present, no data exist that support that soccer participation is a risk factor for the development of neurodegenerative disease. Similarly, at this time, consistent with evidence discussed in the Berlin Concussion in Sport Group (CISG) Consensus Conference, our review suggests no causal relationship has been demonstrated between soccer and CTE pathology.”12
The more we know about concussions, both in general and those sustained during soccer play, the better we are able to diagnose and manage these injuries in our athletes. An important step is creating evidence-based protocols that evolve as our knowledge of concussions does as well. In April 2017, the US Soccer Federation, MLS, and the NWSL held a joint summit entitled, “Head Injury in Soccer: From Science to the Field” to address the current evidence-based science of concussions in soccer.8 An article discussing the findings of this meeting is forthcoming and will undoubtedly guide further development of concussion protocols for soccer players of all ages.
HEART
The physiologic demands of soccer place considerable stress on the cardiovascular system. Participation in training and competition is characterized by a combination of aerobic and anaerobic physiology with the typical athlete covering approximately 10 km over the course of the 90-minute match. The primary role of the heart and blood vessels is to supply the exercising skeletal muscle with oxygen and energy substrate and to clear the byproducts of metabolism. Among healthy athletes without cardiovascular disease, these processes are typically well tolerated and may be associated with beneficial cardiovascular adaptations over time. However, competitive soccer players are not completely immune to cardiovascular disease. Athletes across the age and competition spectrum may develop symptoms suggestive of underlying cardiovascular disease during play including exertional chest pain, inappropriate shortness of breath, palpitations, and syncope. These athletes require timely clinical evaluation. In extremely rare but high visibility cases, competitive soccer players may succumb to cardiac arrest on the pitch, underscoring the need for comprehensive emergency action plans (EAPs). We provide the practicing clinician with an overview of cardiovascular issues relevant to the competitive soccer athlete.
CARDIOVASCULAR ADAPTATIONS TO SPORT
The pressure (ie, repetitive surges in systemic blood pressure) and volume (ie, sustained increases in high cardiac output) challenges inherent in soccer participation place stress on the cardiovascular system. Healthy athletes across the age spectrum typically tolerate the hemodynamic stressors of participation without issues. Athletes that engage in training and competition over months to years often develop beneficial adaptations of the cardiovascular system that enhance on-field performance and contribute to optimal long-term health. Detailed discussion of how the heart and blood vessels respond to exercise training is beyond the scope of this article, but the interested reader is referred to several prior publications.13,14 In brief, the heart of the healthy soccer athlete demonstrates the balanced mild chamber dilation and wall thickening characteristic of left ventricular eccentric remodeling. This form of exercise-induced cardiac remodeling facilitates maintenance of high stroke volume during exercise with minimal increases in cardiac work. In parallel, routine aerobic exercise training confers favorable changes in the systemic arterial system, which leads to reductions in age-associated ventricular stiffening and maintenance of healthy low blood pressure. It must be emphasized that the healthy heart muscle dilation and thickening that develop in response to sports participation, regardless of age, ethnicity, or gender, are relatively mild and should not be confused with common forms of heart muscle disease that may be seen in athletes at risk for adverse outcomes. In some situations, consultation with an extreme sports cardiologist may be required to differentiate exercise-induced remodeling from over heart muscle pathology.15
Continue to: THE SYMPTOMATIC ATHLETE...
THE SYMPTOMATIC ATHLETE
Any athlete presenting with symptoms suggestive of underlying cardiovascular disease should be withheld from training and competition until a comprehensive clinical evaluation has been completed. Common manifestations of underlying heart disease that occur in soccer players include exertional chest pain/pressure/tightness, shortness of breath that is out of proportion to workload, palpitations or the perception of irregular cardiac activity, and syncope. Chest discomfort, inappropriate shortness of breath, and palpitations that occur during training or competition should be managed with immediate removal from the playing field and prompt medical assessment. In many cases, thorough evaluation will involve collaboration between sports medicine and sports cardiology providers. Evaluations must be individualized on a case-by-case basis as tailored to the athlete’s presenting chief complaint and prior medical history. Most of these assessments will include a detailed medical history and physical examination, a 12-lead electrocardiogram (ECG), provocative exercise testing in a controlled environment, noninvasive cardiac imaging, and in some cases ambulatory rhythm monitoring. Uniformly, the athlete should be withheld from further training and competition until high-risk cardiovascular disease has been excluded. We refer the interested reader to a comprehensive discussion of symptom-based assessment of the athlete with suspected cardiovascular disease.16
Syncope (sudden and abrupt loss of consciousness with spontaneous neurologic recovery) is common among trained athletes. The vast majority of syncope is caused by “neurocardiogenic” mechanisms and carries a benign prognosis. Benign neurally-mediated syncope most often occurs outside of training and competition among athletes with heightened vagal tone and a predisposed susceptibility to triggers including pain, anxiety, emotional stimulation, and sudden postural change. Athletes who experience neurally-mediated syncope outside of training and competition routinely report a pre-event prodrome or aura that permits them to lower themselves to the ground, thereby avoiding injury. A distinct, but similarly benign and common, form of neurally-mediated fainting is post-exertional syncope. Here, fainting occurs within seconds of abrupt termination of exercise due to a rapid reduction cardiac preload and corollary cerebral blood supply. When either form of neurally-mediated syncope is suggested by a comprehensive medical history, normal physical examination, and a normal 12- lead ECG, further evaluation is unnecessary. However, the athlete and their coaching staff should be educated about avoidance tactics including hydration, dietary sodium supplementation, and avoidance of abrupt exercise termination as neutrally-mediated syncope tends to be recurrent without such measures. Fainting episodes that occur during training or competition that are not clearly post-exertional should be considered a medical emergency and should prompt comprehensive evaluation by a qualified cardiovascular specialist. Working closely with team physicians and athletic training staff, it is the responsibility of sports cardiologists to exclude potentially life-threatening forms of electrical, muscular, coronary, and valvular heart disease. Ideally, this evaluation should be conducted rapidly to avoid unnecessary delays in return to play.
CARDIAC ARREST AND SUDDEN DEATH
Numerous high-visibility cases of cardiac arrest on the soccer pitch have alerted the sporting community to the potential for these rare and potentially tragic events. Definitive incidence statistics defining the risk of cardiac arrest among soccer players are lacking. Data from the NCAA database suggest a sudden death incidence rate among collegiate male soccer athletes of approximately 1:24,000 athlete years.8 Similar data documenting incidence among female athletes and among those at lower (ie, youth level and high school) and higher (ie, professional) levels of play are unavailable. Underlying cardiovascular disease in the forms of heart muscle abnormalities (ie, genetic and acquired cardiomyopathy), coronary artery abnormalities (ie, genetic coronary anomalies and atherosclerotic coronary disease), valvular heart problems (ie, congenitally malformed aortic valves), and primary disturbances of the cardiac electrical system (ie, Wolf-Parkinson-White syndrome, long QT syndrome, etc.) explain a substantial percentage of on-pitch cardiac arrest. However, it is increasingly recognized that a significant minority of sudden cardiac deaths among athletes occur in the absence of attributable cardiovascular abnormality.17 Such cases, often referred to as “sudden unexplained death,” present unique challenges in the context of pre-participation screening, as they are undetectable and thus unpredictable.
Reduction of cardiac arrest and sudden death may best be accomplished through a combination of focused pre-participation screening and the development and implementation of a comprehensive EAP. Pre-participation involves the performance of a battery of tests prior to training and competition that are geared toward the detection of occult high-risk cardiovascular disease. Recommendations regarding pre-participation screening vary both across and within countries. Current US recommendations call for a focused medical history and physical examination prior to training and competition with consideration of the addition of a 12-lead ECG on a local level based on expertise and available resources.18 Conversely, current European guidelines including those endorsed by FIFA, suggest routine inclusion of a 12-lead ECG and in some cases, a transthoracic ECG.19 It must be emphasized that no screening approach has been confirmed to reduce the incidence of sudden death, and the decision to extend screening beyond the medical history and physical examination to include a 12-lead ECG or echocardiogram may come at the cost of increased false positive testing. In practice, decisions about how and when to screen are ideally made at a local level after consideration of medical and financial resources.20
Continue to: Even the most comprehensive approach...
Even the most comprehensive approach to screening and evaluation of symptomatic athletes will not completely eliminate on-pitch cardiac arrest. Thus, all stakeholders that engage in the oversight of organized soccer must be committed to the development and implementation of an EAP.21 Key components of an effective EAP include the training of coaching staff, athletic trainers, and players in basic cardiopulmonary resuscitation, access to and training in the use of automated external defibrillators, and a triage/transport protocol that ensures timely access to advanced cardiac life support. Much like screening, emergency action planning involving these key core components must be developed and tailored locally. In the era of contemporary organized athletics, the absence of an EAP at any level of competition, from youth to professional leagues, is unacceptable. Effective EAPs must be developed, documented, and rehearsed at regular intervals. For the health and safety of competitive soccer players, as well as coaching staff and spectators, these steps are of critical importance.
HEAT
Heat-related illnesses can be serious and, at times, even life threatening. It is important for athletic staff and athletes to be well versed in the prevention, signs and symptoms, and treatment of heat-related illnesses in order to prevent serious and lasting injury. We aim to educate physicians about the prevention, recognition, and management of heat-related injury, and stress the importance of similarly educating athletes and coaching staff.
Exertional heat illnesses most often occur at temperatures >86°F, however they can occur at any temperature with heavy exertion.22 Signs and symptoms can be nonspecific early on, including weakness, fatigue, headache, nausea, and dizziness. Later signs can include imbalance, altered mentation, confusion, and behavior that is out of character such as irritability or aggression.23 It is easy to see how the later signs can be confused for concussion in the right context. We cover the recognition and treatment of two common and serious heat-related illnesses: heat exhaustion and exertional heat stroke (EHS).
HEAT EXHAUSTION
Heat exhaustion occurs when an athlete cannot continue to exercise due to weakness and fatigue. While the exact mechanism is not well understood, it has been established that the combined effect of heat and dehydration have been proven to decrease exercise capacity and performance to a greater degree than either alone. The heat created by the body during exercise is 15 to 20 times greater than when at rest, and can increase core body temperature by 1°C every 5 minutes if no heat is lost, such as through sweating.24 Additionally, when fluid deficits reach >3% to 5% of total body water, sweat production and skin blood flow decline, blunting the ability for the body to cool itself and causing progressive elevation of core body temperature if the athlete continues exerting him or herself. When fluid deficits reach 6% to 10%, cardiac output, sweat, and muscle blood flow decrease, likely leading to the symptoms seen with heat exhaustion: weakness, profound fatigue, and occasionally confusion and disorientation. Athletes with suspected heat exhaustion should be moved to a cooler area, laid down with legs elevated, and orally rehydrated. If they do not improve with oral rehydration, they may require intravenous fluids. The diagnosis of heat exhaustion hinges on a rectal temperature of <104°F; if >104°F the athlete should be presumed to have heat stroke, which will be addressed in the following paragraphs. Players can be cleared to return to play in mild cases within 24 to 48 hours with gradual increases in exercise intensity.24
EXERTIONAL HEAT STROKE
EHS occurs when the body can no longer regulate the core body temperature and it rises to upwards of 104°F. In EHS, elevated core body temperature is associated with evidence of end organ dysfunction. The most easily identified on the playing field is likely central nervous system dysfunction, including irritability, confusion, irrational behavior, lethargy, dizziness, confusion, and even loss of consciousness. Temperature should be measured with rectal temperature only, as other methods of measurement have been shown to be consistently inaccurate.22 Heat stroke can be confused with exertional hyponatremia, heat exhaustion, or concussion, especially when core body temperature cannot be determined. However, EHS should always be the presumed cause of altered mentation when no rectal temperature is available because rapid cooling is critical to minimizing lasting effects. Morbidity and mortality are directly related to the length of time required to cool the athlete under 40°C (104°F).24 Cooling should be completed on site prior to transport to a medical facility and is best achieved with submersion in an ice bath (ie, a kiddie pool or soaking tub full of ice and water).22,25 If an ice bath is not available, ice bags should be applied to the neck axilla and groin and exchanged for fresh bags every 2 to 3 minutes.22 Ice bags have been shown to be inferior to whole body cooling, only cooling the athlete .04°C to .08°C/min compared to .15°C to .24°C/min with the ice bath.24 All other tests should be delayed until cooling is achieved, unless they can be completed while cooling the athlete. The athlete can be removed from the ice bath once rectal temperatures reach <101°F to 102°F.23 If the athlete returns to baseline after cooling, transportation to a medical facility may not be necessary. However, they should refrain from physical activity and heat exposure for at least 7 days and should be evaluated by a physician at that time. If all labs are normal and the athlete is asymptomatic, they can start progressive return to play under the direction of an athletic trainer or a sports medicine physician.23
Continue to: HEAT-RELATED ILLNESS...
HEAT-RELATED ILLNESS
It is impossible to predict exactly which athletes will be most at risk for heat-related illness, so it is important to have a high degree of suspicion when environmental conditions are right. Athletes with recent illness, fever, or lack of sleep are at higher risk. Additional intrinsic risk factors include low fitness level, obesity, and inadequate hydration. Athletes who are highly competitive or motivated can be more likely to push through the early signs of illness or be reluctant to report symptoms.23 Those with a history of exertional heat illness are more at risk for developing it again in the future.23
The extrinsic risk factors for the development of heat-related illness are much easier to identify and modify in order to keep athletes safe. High temperature and high humidity conditions, heavy sun exposure, and exposure to similar conditions the preceding day put athletes at risk for exertional heat illness. Risks are even greater when the exercise is prolonged or intense with few breaks and access to hydration is limited.23 Therefore, prevention of exertional heat illness is centered on these external risk factors.
Each team should have a heat policy as part of their EAP aimed at prevention and early recognition of heat-related illness. This policy should be shared with all athletes and coaches. The plan should be centered on acclimatization, activity modification, and early recognition and management as previously discussed. The US Soccer Federation “Recognize to Recover” Heat Guidelines suggest a 3-step process for appropriate activity modification:22
1. Find the wet bulb globe temperature, either using a wet bulb globe thermometer or the temperature and humidity (Figure 1).
2. Find your regional weather category on the map (Figure 2).
3. Find your alert level and work to rest ratio recommendations (Figure 3).
Scheduled hydration breaks should be given as listed in Figure 3. Breaks of 4 minutes should be given for each 30 minutes of continuous practice or play. In a regulation 90-minute match, a hydration break should be given at 30 and 75 minutes (with half time at 45 minutes) at minimum. Athletes should be educated about where hydration can be accessed, and given unlimited access to hydration even outside of planned breaks.22
Acclimatization to conditions is another integral part of preventing heat-related illness. It allows the body time to adapt to exercising in heat gradually, with a measured progression of exertion over the course of 10 to 14 days. The “Recognize to Recover” Heat Guidelines also provide guidance on acclimatization, and specifics can be found on the website.1 Generally speaking, the warmest part of the day, usually between 11 AM and 4 PM, should be avoided for all training sessions, and length of practice and exertion should be gradually increased over 2 weeks.22
In summary, appropriate acclimatization, hydration, activity modification, and education of athletes and staff are essential for the prevention of heat-related illness. Athletes and staff should understand the signs and symptoms of heat-related illness so that it can be recognized early and treated appropriately. If an athlete is altered in the heat and rectal temperature is >104°F or rectal temperature cannot be obtained, rapid cooling using an ice bath or ice bags is essential to prevent the morbidity and mortality associated with EHS. Above all, teams should have an explicit plan that includes protocols for acclimatization, activity modification, and all necessary equipment to prevent and treat heat-related illnesses should they occur, and ultimately keep athletes safe and healthy.
1. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain Injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
2. The National Federation of State High School Associations. 2013-14 high school athletics participation survey. http://www.nfhs.org/ParticipationStatics/PDF/2013-14_Participation_Survey_PDF.pdf. Accessed August 6, 2018.
3. Youth Council. US Soccer Federation Web site. https://www.ussoccer.com/about/affiliates/youth-council. Accessed July 31, 2018.
4. Khodaee M, Currie DW, Asif IM, Comstock RD. Nine-year study of US high school soccer injuries: data from a national sports injury surveillance programme. Br J Sports Med. 2017;51(3):185-193. doi:10.1136/bjsports-2015-095946.
5. Schallmo MS, Weiner JA, Hsu WK. Sport and sex-specific trends in the epidemiology of concussions sustained by high school athletes. J Bone Joint Surg Am. 2017;99(15):1314-1320. doi:10.2106/JBJS.16.01573.
6. US Soccer Federation. U.S. Soccer’s comprehensive player health and safety program. Recognize to Recover Web site. http://www.recognizetorecover.org/#us-soccers-comprehensive-player-health-and-safety-program. Accessed July 31, 2018.
7. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi:10.1136/bjsports-2017-097699.
8. Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123(15):1594-1600. doi:10.1161/CIRCULATIONAHA.110.004622.
9. Faude O, Rössler R, Junge A, et al. Head injuries in children’s football-results from two prospective cohort studies in four European countries. Scand J Med Sci Sports. 2017;27(12):1986-1992. doi:10.1111/sms.12839.
10. Comstock RD, Currie DW, Pierpoint LA, Grubenhoff JA, Fields SK. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169(9):830-837. doi:10.1001/jamapediatrics.2015.1062.
11. Tarnutzer AA. Should heading be forbidden in children’s football? Sci Med Football. 2018;2(1):75-79.
12. US Soccer Federation. US Soccer, NWSL and MLS to host “head injury in soccer; science to field”. https://www.ussoccer.com/stories/2017/04/18/17/35/20170418-news-us-soccer-nwsl-mls-host-head-injury-in-soccer-science-to-field. Published April 18, 2017. Accessed August 6, 2018.
13. Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. 2012;54(5):380-386. doi:10.1016/j.pcad.2012.01.006.
14. Baggish AL, Wood MJ. Athlete's heart and cardiovascular care of the athlete: scientific and clinical update. Circulation. 2011;123(23):2723-2735. doi:10.1161/CIRCULATIONAHA.110.981571.
15. Kim JH, Baggish AL. Differentiating exercise-induced cardiac adaptations from cardiac pathology: the "Grey Zone" of clinical uncertainty. Can J Cardiol. 2016;32(4):429-437. doi:10.1016/j.cjca.2015.11.025.
16. Baggish AL, Battle RW, Beckerman JG, et al; ACC’s Sports and Exercise Council Leadership Group. Sports cardiology: core curriculum for providing cardiovascular care to competitive athletes and highly active people. J Am Coll Cardiol. 2017;70(15):1902-1918. doi:10.1016/j.jacc.2017.08.055.
17. Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation. 2015;132(1):10-19. doi:10.1161/CIRCULATIONAHA.115.015431.
18. Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66(21):2356-2361. doi:10.1016/j.jacc.2015.09.034.
19. Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516-524.
20. Baggish AL, Kovacs RJ. Preparticipation cardiovascular screening: clinical partnership is the only certainty. Br J Sports Med. 2017;51(3):150-151. doi:10.1136/bjsports-2016-096954.
21. Hainline B, Drezner J, Baggish A, et al. Interassociation consensus statement on cardiovascular care of college student-athletes. J Athl Train. 2016;51(4):344-357. doi:10.4085/j.jacc.2016.03.527.
22. US Soccer Federation. Environmental conditions. Recognize to Recover Web site. http://www.recognizetorecover.org/environmental/#environmental-conditions. Accessed April 15, 2018.
23. Korey Stringer Institute. Emergency conditions: heat illnesses. University of Connecticut Web site. https://ksi.uconn.edu/. Accessed April 15, 2018.
24. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
25. Belval LN, Casa DJ, Adams WM, et al. Consensus statement- prehospital care of exertional heat stroke. Prehosp Emerg Care. 2018;22(3):392-397. doi:10.1080/10903127.2017.1392666.
1. Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain Injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375-378.
2. The National Federation of State High School Associations. 2013-14 high school athletics participation survey. http://www.nfhs.org/ParticipationStatics/PDF/2013-14_Participation_Survey_PDF.pdf. Accessed August 6, 2018.
3. Youth Council. US Soccer Federation Web site. https://www.ussoccer.com/about/affiliates/youth-council. Accessed July 31, 2018.
4. Khodaee M, Currie DW, Asif IM, Comstock RD. Nine-year study of US high school soccer injuries: data from a national sports injury surveillance programme. Br J Sports Med. 2017;51(3):185-193. doi:10.1136/bjsports-2015-095946.
5. Schallmo MS, Weiner JA, Hsu WK. Sport and sex-specific trends in the epidemiology of concussions sustained by high school athletes. J Bone Joint Surg Am. 2017;99(15):1314-1320. doi:10.2106/JBJS.16.01573.
6. US Soccer Federation. U.S. Soccer’s comprehensive player health and safety program. Recognize to Recover Web site. http://www.recognizetorecover.org/#us-soccers-comprehensive-player-health-and-safety-program. Accessed July 31, 2018.
7. McCrory P, Meeuwisse W, Dvořák J, et al. Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838-847. doi:10.1136/bjsports-2017-097699.
8. Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123(15):1594-1600. doi:10.1161/CIRCULATIONAHA.110.004622.
9. Faude O, Rössler R, Junge A, et al. Head injuries in children’s football-results from two prospective cohort studies in four European countries. Scand J Med Sci Sports. 2017;27(12):1986-1992. doi:10.1111/sms.12839.
10. Comstock RD, Currie DW, Pierpoint LA, Grubenhoff JA, Fields SK. An evidence-based discussion of heading the ball and concussions in high school soccer. JAMA Pediatr. 2015;169(9):830-837. doi:10.1001/jamapediatrics.2015.1062.
11. Tarnutzer AA. Should heading be forbidden in children’s football? Sci Med Football. 2018;2(1):75-79.
12. US Soccer Federation. US Soccer, NWSL and MLS to host “head injury in soccer; science to field”. https://www.ussoccer.com/stories/2017/04/18/17/35/20170418-news-us-soccer-nwsl-mls-host-head-injury-in-soccer-science-to-field. Published April 18, 2017. Accessed August 6, 2018.
13. Weiner RB, Baggish AL. Exercise-induced cardiac remodeling. Prog Cardiovasc Dis. 2012;54(5):380-386. doi:10.1016/j.pcad.2012.01.006.
14. Baggish AL, Wood MJ. Athlete's heart and cardiovascular care of the athlete: scientific and clinical update. Circulation. 2011;123(23):2723-2735. doi:10.1161/CIRCULATIONAHA.110.981571.
15. Kim JH, Baggish AL. Differentiating exercise-induced cardiac adaptations from cardiac pathology: the "Grey Zone" of clinical uncertainty. Can J Cardiol. 2016;32(4):429-437. doi:10.1016/j.cjca.2015.11.025.
16. Baggish AL, Battle RW, Beckerman JG, et al; ACC’s Sports and Exercise Council Leadership Group. Sports cardiology: core curriculum for providing cardiovascular care to competitive athletes and highly active people. J Am Coll Cardiol. 2017;70(15):1902-1918. doi:10.1016/j.jacc.2017.08.055.
17. Harmon KG, Asif IM, Maleszewski JJ, et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation. 2015;132(1):10-19. doi:10.1161/CIRCULATIONAHA.115.015431.
18. Maron BJ, Levine BD, Washington RL, Baggish AL, Kovacs RJ, Maron MS. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015;66(21):2356-2361. doi:10.1016/j.jacc.2015.09.034.
19. Corrado D, Pelliccia A, Bjørnstad HH, et al. Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(5):516-524.
20. Baggish AL, Kovacs RJ. Preparticipation cardiovascular screening: clinical partnership is the only certainty. Br J Sports Med. 2017;51(3):150-151. doi:10.1136/bjsports-2016-096954.
21. Hainline B, Drezner J, Baggish A, et al. Interassociation consensus statement on cardiovascular care of college student-athletes. J Athl Train. 2016;51(4):344-357. doi:10.4085/j.jacc.2016.03.527.
22. US Soccer Federation. Environmental conditions. Recognize to Recover Web site. http://www.recognizetorecover.org/environmental/#environmental-conditions. Accessed April 15, 2018.
23. Korey Stringer Institute. Emergency conditions: heat illnesses. University of Connecticut Web site. https://ksi.uconn.edu/. Accessed April 15, 2018.
24. American College of Sports Medicine, Armstrong LE, Casa DJ, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exerc. 2007;39(3):556-572.
25. Belval LN, Casa DJ, Adams WM, et al. Consensus statement- prehospital care of exertional heat stroke. Prehosp Emerg Care. 2018;22(3):392-397. doi:10.1080/10903127.2017.1392666.
TAKE-HOME POINTS
- Current concussion education programs such as “Recognize to Recover” aim to increase self-reporting of concussion symptoms by players, and recognition and appropriate evaluation by medical and coaching staff.
- Athletes who develop symptoms suggestive of underlying cardiovascular disease during play, including exertional chest pain, inappropriate shortness of breath, palpitations, and syncope should be withheld from play until they can be evaluated by a qualified medical professional.
- Key components of an effective EAP include the training of coaching staff, athletic trainers, and players in basic cardiopulmonary resuscitation, access to and training in the use of automated external defibrillators, and a triage/transport protocol that ensures timely access to advanced cardiac life support.
- Exertional heat stroke should always be the presumed cause of altered mentation when no rectal temperature is available because rapid cooling is critical to minimizing lasting effects.
- Prevention of exertional heat illness should center around appropriate acclimatization, access to adequate hydration and scheduled hydration breaks, and avoiding exertion all together when conditions are too dangerous.
Knee Injuries in Elite Level Soccer Players
ABSTRACT
As one of the most popular sports in the world, soccer injury rates involving the knee continue to rise. An alarming trend of knee injuries, including increased anterior cruciate ligament ruptures, underscores the need to review our current understanding of these injuries in soccer players. This article includes a critical review of the epidemiology of knee injuries in soccer, anterior cruciate ligament and other ligamentous injuries, cartilage and meniscal injury, post-traumatic osteoarthritis, as well as current prevention initiatives.
Continue to: EPIDEMIOLOGY...
EPIDEMIOLOGY
There are currently 28 players on each of the Major League Soccer (MLS) teams, and during the 2013 to 2014 academic year, the National Federation of State High School Associations (NFHS) reported that 417,419 boys and 374,564 girls played high school soccer and the National Collegiate Athletic Association (NCAA) reported that 23,602 males and 26,358 females played collegiate soccer.5 As such, knee injuries in this population are a major concern for those involved in sports medicine. Several injuries occurring during soccer involve the lower extremity, particularly the knee.1 In fact, multiple reports estimate that up to 17.6% of soccer-related injuries presenting to the emergency room involved the knee.1,6-9 The majority of these injuries are noncontact injuries, although contact injuries do still occur.10,11
Risk factors for injuries in soccer may be non-modifiable (such as age and gender) and modifiable (such as level of conditioning, force, balance, and flexibility). Inadequate lower motor coordination may result in injury in the adolescent population, and advanced age >28 years in males and >25 years in females is considered as a high-risk factor for injury.12,13 Importantly, gender and age have been reported to play a significant role as risk factors for ACL injury.6 In fact, female players have a 3 to 5 times higher risk of significant knee injury, including ACL injuries, than male players.4,14-16 Preventative programs such as the FIFA 11+ program have been set forth to augment conditioning as part of managing the modifiable risk factors.
Like American football, playing on artificial turf has been questioned as a contributor to injury compared to playing on natural grass.17,18 In recent years, newer generations of artificial turf have been developed to more closely replicate the characteristics of natural grass. Meyers19 compared the incidence, mechanisms, and severity of match-related collegiate men’s soccer injuries on artificial turf and those on natural grass and demonstrated no significant difference in knee injuries between the 2 surfaces. This finding was consistent with previous studies that reported no difference in the incidence of knee injuries on either surface in women’s collegiate and elite-level soccer.15,20,21
Continue to: ACL INJURIES...
ACL INJURIES
ACL injuries are life-changing events that can significantly affect the career of a soccer athlete. As a major stabilizer of the knee, the ACL primarily prevents anterior tibial translation with the anteromedial bundle and secondarily resists tibial rotation with the posterolateral bundle. The ligament takes origin from the posteromedial aspect of the lateral femoral condyle and inserts anterior to the tibial intercondylar eminence. Grading of ACL injuries is based on the Lachman test, which is performed between 20°and 30° of knee flexion and measures the amount of anterior tibial translation relative to the femur (A = firm endpoint, B = no endpoint; grade I: 3-5 mm, grade II (A/B): 5-10 mm, grade III (A/B): >10 mm).
ACL injury may occur via contact or noncontact mechanisms. Noncontact mechanisms of ACL injury in soccer athletes contribute to about 85% of injuries.6,22-25 Typical noncontact mechanism of injury involves a forceful valgus collapse with the knee near full extension and combined external or internal rotation of the tibia23,26 (Figure 1). This on-field scenario generally involves cutting and torsional movement, as well as landing after a jump, particularly in 1-legged stance. Similarly, a disturbance in balance caused by the opponent may incite a noncontact mechanism resulting in ACL rupture.6,27 Video analyses of professional soccer players have also demonstrated a higher risk of noncontact ACL injury within the first 9 minutes of the match, with the most common playing situation resulting in injury being pressing, followed by kicking and heading.24,25,28 Contact mechanisms resulting in ACL injury, however, are not an uncommon occurrence in soccer players with higher risk for certain positions. Brophy and colleagues29 reviewed ACL injuries in professional and collegiate soccer players and reported a higher risk of ACL injury during defending and tackling. Similarly, Faude and colleagues30 found the risk of injury to be higher in defenders and strikers than in goalkeepers and midfielders.

Female athletes participating in elite-level athletics, especially soccer, represent a high-risk group for ACL injury. In fact, these soccer athletes experience ACL injury at an incidence 3 times higher than that in male athletes.31-35 Female soccer athletes may also be at risk for reinjury to the ACL and contralateral ACL injury. Female gender, in combination with participation in soccer, thus represents a high-risk group for ACL tear in athletics. Allen and colleagues36 retrospectively reviewed 180 female patients who had undergone ACL reconstruction (ACLR) (90 soccer players and 90 non-soccer players) over a mean period of 68.8 months. In their series, soccer players sustained significantly more ACL injuries than non-soccer players, including graft failures (11% vs 1%) and contralateral ACL tears (17% vs 4%).
ACLR is the gold standard treatment for elite soccer athletes. A recent survey of MLS team orthopedic surgeons revealed several important details regarding decision-making in ACLR in this population. From a technical standpoint, the vast majority of surgeons used a single incision, arthroscopically assisted, single-bundle reconstruction (91%). Femoral tunnel drilling was almost equally split between transtibial (51%) and use of an accessory medial portal (46%). Bone-patella-tendon-bone (BPTB) autograft was the most preferred graft choice (68%), and quadriceps tendon autograft was the least preferred. The majority of surgeons preformed ACLR within 4 weeks and permitted return to sport (RTS) without restrictions at 6 to 8 months.37
Continue to: There is a scarcity of literature regarding...
There is a scarcity of literature regarding the use of soft tissue and BPTB allografts in soccer athletes. However, one study reported no difference in patient-reported outcomes and return to preinjury level of activity (including soccer) with the use of either autograft or allograft BPTB in ACLR.38 The authors’ preference was to avoid the use of allograft in elite-level soccer athletes as the reported rate of ACL re-tear was 4 to 8 times higher than that with autograft reconstruction, as shown in athletes and military personnel.39,40 BPTB autograft and hamstring autograft (semitendinosus and/or gracilis) are common graft choices for soccer athletes. Gifstad and colleagues41 compared BPTB autograft and hamstring autograft in 45,998 primary ACLRs performed in Scandinavia. Although the cohort included, but was not limited to, soccer players, the authors reported an overall risk of revision that was significantly lower in the BPTB autograft group than in the hamstring autograft group (hazard ratio, 0.63; 95% confidence interval, 0.53-0.74).41 Mohammadi and colleagues42 prospectively compared the functional outcomes of 42 competitive soccer players who underwent ACLR with BPTB autograft vs those who underwent ACLR with hamstring autograft at the time of RTS. Players who had undergone ACLR with hamstring autograft demonstrated greater quadriceps torque, as well as better performance with triple-hop, crossover-hop, and jump-landing tests; however, both groups demonstrated similar hamstring torque and performance in 2 other hop tests.42 In the authors’ opinion, there may be a concern regarding the use of hamstring autograft in elite soccer players considering that hamstring strains are extremely common in this athletic population; however, further research would be necessary to elucidate whether this is an actual or a theoretical risk. Although not yet studied in elite-level athletes, early clinical results of ACL repair with suture augmentation show promise for certain injury patterns. These include proximal femoral ACL avulsion injuries (Sherman type 1) of excellent tissue quality that have the ability to be reapproximated to the femoral origin43 (Figures 2A, 2B). In a recent series,43-45 early clinical outcomes were found to be excellent and maintained at midterm follow-up.
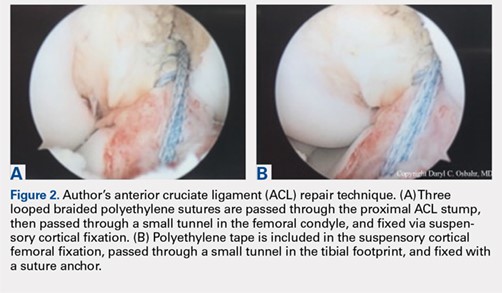
In the NCAA soccer athletes, an overall RTS rate of 85% has been reported in those undergoing ACLR, with a significantly higher rate observed in scholarship versus non-scholarship athletes.46 Howard and colleagues46 reported median time to unrestricted game play of 6.1 months, with 75% returning to the same or higher level position on the depth chart. Among their studied collegiate soccer athletes, 32% reported continued participation in soccer on some level after college (recreational, semiprofessional, or professional).46 RTS rates for MLS soccer players have also been reported to be high, ranging from 74% to 77%, most of them returning within the following season at 10 ± 2.8 months.47,48 These findings were consistent with the RTS rate of 72% reported by the Multicenter Orthopaedic Outcomes Network (MOON) group, which analyzed 100 female and male soccer players undergoing ACLR at a minimum 7-year follow-up. In this series, Brophy and colleagues29,49 reported an RTS at 12 ± 14.3 months, with 85% returning to the same or a higher level of play prior to their injury. Erickson and colleagues47 analyzed a series of 57 ACLRs performed in MLS athletes and reported no significant difference in preinjury or postoperative performance, or between cases and uninjured controls. Arundale and colleagues48 demonstrated no significantly increased risk of lower extremity injury in MLS athletes after ACLR, but the athletes had significantly shorter careers than their uninjured counterparts. Curiously, RTS rates for European professional soccer athletes have been reported to be substantially higher at 95% to 97%.50,51 Although we can only speculate the reasons for such a discrepancy, the difference in RTS rates for similar athletes highlights a need for objective criteria to determine and report RTS rates, while also providing guidelines to prevent reinjury. Such a consensus among orthopedists is not yet present in the literature.
Soccer players and adolescent age in combination have been shown to portend a 3-fold increased risk of revision surgery for ACL failure in a cohort of 16,930 patients from the Swedish National Knee Ligament Register.52 Published data regarding ACL failure and management of revision ACLR in elite-level soccer athletes are currently lacking. However, low failure rates of 3% to 10% requiring revision reconstruction have been reported.47,49 Arundale and colleagues48 reported 2 incidences of players with ACL graft failures, 1 BPTB autograft and 1 BPTB allograft, both of whom were able to return to MLS after revision ACLR. It is the authors’ preference to use ipsilateral hamstring autograft or contralateral BPTB autograft when an ACL revision reconstruction is required.
Continue to: OTHER LIGAMENTOUS INJURIES...
OTHER LIGAMENTOUS INJURIES
The majority of research efforts regarding knee injuries in this population are focused on the ACL. Correspondingly, literature regarding injury to the collateral ligaments and the posterior cruciate ligament (PCL) in soccer players is sparse. The lateral collateral ligament (LCL) and the medial collateral ligament (MCL) play important roles as primary stabilizers to varus and valgus forces, respectively. The PCL is the primary posterior stabilizer of the knee, preventing posterior translation of the tibia. Injury to these structures may result in significant time lost from soccer and risk of reinjury.53,54
The MCL is the one of the most commonly injured ligaments in sports, including soccer.53,55 The injury mechanism generally involves contact with a resulting valgus force applied to the knee.55 Grading of MCL injuries is based on the amount of medial joint gapping with applied valgus force during examination (grade I: <5 mm, grade II: 5-10 mm, grade III: >10 mm). Kramer and colleagues53 reviewed collateral ligament injuries in the adolescent population and found that MCL injuries occurred 4 times more often than LCL injuries and about 25% were grade III injuries, most commonly occurring in American football and soccer players. Soccer also touts the highest sport-specific MCL injury rate for high school and collegiate athletics, particularly for female NCAA soccer players.56 At the professional level, Lundblad and colleagues55 reported 346 MCL injuries in 27 European teams over an 11-year period, of which 70% were contact-related, and the average time-off from soccer was 28 days.
Most surgeons treat isolated MCL injuries nonoperatively, regardless of grade.57,58 This includes activity modification, use of a hinged knee brace, quadriceps strengthening, and progressive return to play. The literature currently lacks substantial data to guide MCL injury management, specifically in elite soccer athletes. In our experience, grade I injuries are managed nonoperatively and RTS is allowed at 4 to 6 weeks. Grade II injuries are also managed nonoperatively and RTS is allowed at 6 to 8 weeks. Grade III injuries are generally allowed RTS at 8 to 12 weeks and may be considered for surgery in the context of concomitant injuries (eg, posteromedial capsular injury, multiligamentous knee injuries, and meniscal injuries). In some athletes, we consider using a varus unloader brace to help maximize decreased stress on the MCL while still allowing the athlete to be fully weight-bearing. We have found it less ideal to limit weight-bearing in elite athletes, which may negatively affect overall lower extremity neuromuscular proprioception and potentially prolong a safe return to play. Some athletes may experience prolonged soreness at the MCL femoral or tibial attachment despite being able to return to play. It is important to counsel athletes about these prolonged symptoms to set expectations, as this may even occur with grade I MCL injuries. Other rare instances where surgical management may be indicated include persistent pain and instability following nonoperative treatment of grade III injuries and highly displaced tibial avulsions of the ligament resulting in poor healing.59,60
Data regarding LCL injuries in soccer are extremely sparse. In our experience, treatment and RTS rates for isolated LCL injuries are similar to those for MCL injuries. However, it is worth noting that one-quarter of LCL injuries may occur in combination with injury to other posterolateral corner structures.53
PCL injuries are more commonly associated with vehicular trauma but have also been reported to occur in sports at a rate of 33% to 40%.61,62 The mechanism of injury in athletes generally involves a fall onto the hyperflexed knee with the foot in plantarflexion or a direct blow to the anterior tibia in a flexed knee.62,63 Classification of PCL injuries is based on posterior translation of the tibia relative to the femur with the knee flexed to 90°(grade I: 1-5 mm, grade II: 6-10 mm, grade III: >10 mm). In one cohort of 62 patients with isolated PCL injuries, soccer was found to be among the top 5 causes of injury.64 A Scandinavian review of 1287 patients who underwent PCL reconstruction found soccer to be the sport with the highest number of injuries (13.1%).65 The goalkeeper was most commonly subjected to this injury.62 Krutsch and colleagues54 compared PCL injuries in new, professional soccer players to those in players at the closest amateur level of play. In their series, 90% of PCL injuries occurred during preseason in players who were at a lower level of play in the previous season. This finding suggested that a rapid increase in training and playing intensity may have been a significant risk factor for PCL injury. Substantial literature supporting nonoperative or operative management of PCL injuries in soccer athletes is currently lacking. Historically, nonoperative treatment has been the initial management for isolated PCL injuries; however, surgical intervention has become increasingly used for both isolated and combined PCL injuries.66
Continue to: CARTILAGE AND MENISCAL INJURIES...
CARTILAGE AND MENISCAL INJURIES
The prevalence of osteoarthritis (OA) in retired soccer players is high.67,68 Articular cartilage degeneration with subsequent OA occurs in up to 32% of soccer players and ultimately leads to significant disability and retirement from the sport. High physical demands and concomitant knee injuries probably predispose to the development of posttraumatic OA.69-71
Several techniques addressing cartilage débridement or restoration have been reported, with successful RTS but with variable durability.72-75 Recently, Andrade and colleagues76 performed a systematic review of 217 articular cartilage defects in soccer players that were treated using restoration techniques, including chondroplasty, microfracture, autologous chondrocyte implantation (ACI), and osteochondral autograft. Although no superior technique could be ascertained, microfracture and osteochondral autograft procedures led to the quickest return to play, and ACI techniques enhanced long-standing clinical and functional results.76 More recently, osteochondral allograft transplantation has also been described with an 84% return to some level of activity (including soccer) and 60% of athletes returning to high-level sports participation at a mean follow-up of 4.5 years77 (Figures 3A-3C). Although chondroplasty may be successful and allow for a quicker return to play in some soccer players (return to play from 6-12 weeks), the authors believe that a strong cartilage scaffold repair strategy with early weight-bearing, including osteochondral autograft and allograft procedures (return to play from 6-9 months), must also be considered in focal chondral defects to optimize both short-term and potential long-term success.
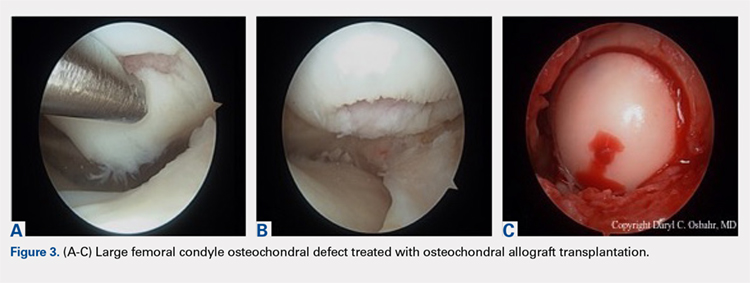
Meniscal injuries are also prevalent in the soccer population, and consistent with ACL injuries, female players are at least twice as likely to sustain a meniscal tear.78,79 Meniscal damage can occur in isolation or in association with ACL rupture. Repair techniques should be strongly considered as chondral changes in the setting of meniscal deficiency are a significant short- and long-term concern for elite athletes. However, due to intrinsically poor healing potential, partial meniscectomy is unfortunately more often performed.79,80 In either case, meniscal deficiency is recognized as a precursor to the development of OA as meniscal functionality is lost and the articular cartilage is subjected to increased biomechanical loading.81,82 Nawabi and colleagues83 analyzed RTS in 90 professional soccer players following partial meniscectomy. Median RTS was at 7 weeks for lateral meniscectomies and at 5 weeks for medial meniscectomies. RTS probability was 5.99 times greater after medial meniscectomy at all time points. Lateral meniscectomies were associated with an increased risk of postoperative adverse events, reoperation, and a significantly lower rate of return to play.83 In the case of severe meniscal deficiency, particularly post-meniscectomy, meniscal allograft transplantation (MAT) may be considered. In a series of MATs in lower division Spanish players, 12/14 (85.7%) returned to play at an average of 7.6 months.84 A more recent series of professional players reported 9/12 (75%) RTS as professionals and 2/12 (17%) as semiprofessionals at an average of 10.5 months.85 The authors’ strong preference is to perform meniscus-saving procedures whenever possible. Due to the longer recovery and return to play associated with meniscus repair than partial meniscectomy, most of the soccer players will often prefer to proceed with partial meniscectomy. Despite the ultimate treatment, it is critical that the surgeon and the soccer player have an in-depth conversation concerning the risks and benefits for each procedure and individualize treatment to the individual soccer player accordingly.
Continue to: INJURY PREVENTION...
INJURY PREVENTION
Given the breadth and the prevalence of soccer-related injuries, the FIFA11+ program was developed in 2006 as an injury prevention measure (Figure 4). The warm-up program includes 15 structured exercises emphasizing core stabilization, thigh muscle training, proprioception, dynamic stabilization, and plyometric exercises. The routine is believed to be easily executed and effective at preventing the incidence of noncontact injuries.86,87 Recently, Sadigursky and colleagues1 performed a systematic review of randomized clinical trials examining the efficacy of FIFA11+. The authors reported a reduction in injuries by 30% and a relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of the program.1 Post-training programs may also be beneficial as it has been shown that performing FIFA11+ both before and after training reduced overall injury rates in male, amateur soccer players.88 Regardless of the prevention program, it is critical that every league, team, medical team, and athlete have a thorough injury prevention strategy to help keep players healthy and not wait until they have instead sustained a significant injury.

CONCLUSION
Knee injuries are common in soccer, with an alarming number of ACL injuries, as well as other significant pathology. Understanding the unique epidemiology, risk factors, treatment, and injury prevention strategies is critically important in helping medical professionals provide care for all levels of elite soccer players.
1. Sadigursky D, Braid JA, De Lira DNL, Machado BAB, Carneiro RJF, Colavolpe PO. The FIFA 11+ injury prevention program for soccer players: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:18. doi:10.1186/s13102-017-0083-z.
2. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929-938. doi:10.2165/00007256-200434130-00004.
3. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
4. Agel J, Rockwood T, Klossner D. Collegiate ACL Injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. doi:10.1097/JSM.0000000000000290.
5. Kerr ZY, Pierpoint LA, Currie DW, Wasserman EB, Comstock RD. Epidemiologic comparisons of soccer-related injuries presenting to emergency departments and reported within high school and collegiate settings. Inj Epidemiol. 2017;4(1):19. doi:10.1186/s40621-017-0116-9.
6. Volpi P, Bisciotti GN, Chamari K, Cena E, Carimati G, Bragazzi NL. Risk factors of anterior cruciate ligament injury in football players: a systematic review of the literature. Muscles Ligaments Tendons J. 2016;6(4):480-485. doi:10.11138/mltj/2016.6.4.480.
7. Smith NA, Chounthirath T, Xiang H. Soccer-related injuries treated in emergency departments: 1990-2014. Pediatrics. 2016;138(4). doi:10.1542/peds.2016-0346.
8. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2):288-293. doi:10.1177/0363546506294060.
9. Adams AL, Schiff MA. Childhood soccer injuries treated in U.S. emergency departments. Acad Emerg Med. 2006;13(5):571-574. doi:10.1197/j.aem.2005.12.015.
10. Woods C, Hawkins R, Hulse M, Hodson A. The football association medical research programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med. 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436.
11. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28(5 Suppl):S58-68. doi:10.1177/28.suppl_5.s-58.
12. Ostenberg A, Roos H. Injury risk factors in female European football. a prospective study of 123 players during one season. Scand J Med Sci Sports. 2000;10(5):279-285. doi:10.1034/j.1600-0838.2000.010005279.x.
13. Backous DD, Friedl KE, Smith NJ, Parr TJ, Carpine WD. Soccer injuries and their relation to physical maturity. Am J Dis Child. 1988;142(8):839-842. doi:10.1001/archpedi.1988.02150080045019.
14. Grimm NL, Jacobs JC, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2049-2056. doi:10.1177/0363546514556737.
15. Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women's soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
16. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934.
17. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650. doi:10.1177/03635465000280050401.
18. Levy IM, Skovron ML, Agel J. Living with artificial grass: a knowledge update. Part 1: Basic science. Am J Sports Med. 1990;18(4):406-412. doi:10.1177/036354659001800413.
19. Meyers MC. Incidence, Mechanisms, and severity of match-related collegiate men's soccer injuries on fieldturf and natural grass surfaces: a 6-year prospective study. Am J Sports Med. 2017;45(3):708-718. doi:10.1177/0363546516671715.
20. Ekstrand J, Hägglund M, Fuller CW. Comparison of injuries sustained on artificial turf and grass by male and female elite football players. Scand J Med Sci Sports. 2011;21(6):824-832. doi:10.1111/j.1600-0838.2010.01118.x.
21. Meyers MC. Incidence, mechanisms, and severity of match-related collegiate women's soccer injuries on FieldTurf and natural grass surfaces: a 5-year prospective study. Am J Sports Med. 2013;41(10):2409-2420. doi:10.1177/0363546513498994.
22. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195. doi:10.1016/j.knee.2012.07.006.
23. Rothenberg P, Grau L, Kaplan L, Baraga MG. Knee injuries in american football: an epidemiological review. Am J Orthop. 2016;45(6):368-373.
24. Waldén M, Hägglund M, Magnusson H, Ekstrand J. Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):11-19. doi:10.1007/s00167-010-1170-9.
25. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012. doi:10.1177/0363546503261724.
27. Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med. 2005;39(4):212-216; discussion 212-216. doi:10.1136/bjsm.2004.011973.
28. Grassi A, Smiley SP, Roberti di Sarsina T, et al. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: a YouTube-based video analysis. Eur J Orthop Surg Traumatol. 2017;27(7):967-981. doi:10.1007/s00590-017-1905-0.
29. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
30. Faude O, Junge A, Kindermann W, Dvorak J. Risk factors for injuries in elite female soccer players. Br J Sports Med. 2006;40(9):785-790. doi:10.1136/bjsm.2006.027540.
31. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. doi:10.1177/0363546504269937.
32. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98-102. doi:10.1177/03635465000280012901.
33. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701. doi:10.1177/036354659502300611.
34. Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899-904. doi:10.1177/0363546505285582.
35. Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320-1325.e1326. doi:10.1016/j.arthro.2007.07.003.
36. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492-2498. doi:10.1177/0363546516648439.
37. Farber J, Harris JD, Kolstad K, McCulloch PC. Treatment of anterior cruciate ligament injuries by major league soccer team physicians. Orthop J Sports Med. 2014;2(11):2325967114559892. doi:10.1177/2325967114559892.
38. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-66. doi:10.1016/j.arthro.2010.01.004.
39. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81. doi:10.1177/1941738110386185.
40. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246. doi:10.1177/0363546512443945.
41. Gifstad T, Foss OA, Engebretsen L, et al. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42(10):2319-2328. doi:10.1177/0363546514548164.
42. Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271-1277. doi:10.2106/JBJS.L.00724.
43. van der List JP, DiFelice GS. Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1529-e1534. doi:10.1016/j.eats.2017.06.009.
44. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. doi:10.1177/2325967116672176.
45. DiFelice GS, van der List JP. Clinical outcomes of arthroscopic primary repair of proximal anterior cruciate ligament tears are maintained at mid-term follow-up. Arthroscopy. 2018;34(4):1085-1093. doi:10.1016/j.arthro.2017.10.028.
46. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in national collegiate athletic association division I soccer athletes: a study of the southeastern conference. Am J Sports Med. 2016;44(2):433-439. doi:10.1177/0363546515614315.
47. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):2325967113497189. doi:10.1177/2325967113497189.
48. Arundale AJH, Silvers-Granelli HJ, Snyder-Mackler L. Career length and injury incidence after anterior cruciate ligament reconstruction in major league soccer players. Orthop J Sports Med. 2018;6(1):2325967117750825. doi:10.1177/2325967117750825.
49. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
50. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
51. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744-750. doi:10.1136/bjsports-2015-095952.
52. Andernord D, Desai N, Björnsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43(1):121-127. doi:10.1177/0363546514552788.
53. Kramer DE, Miller PE, Berrahou IK, Yen YM, Heyworth BE. Collateral ligament knee injuries in pediatric and adolescent athletes. J Pediatr Orthop. 2017. doi:10.1097/BPO.0000000000001112.
54. Krutsch W, Zeman F, Zellner J, Pfeifer C, Nerlich M, Angele P. Increase in ACL and PCL injuries after implementation of a new professional football league. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2271-2279. doi:10.1007/s00167-014-3357-y.
55. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
56. Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 Through 2013-2014. Am J Sports Med. 2016;44(6):1565-1572. doi:10.1177/0363546516630927.
57. Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37(6):1116-1122. doi:10.1177/0363546509332498.
58. Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. doi:10.2106/JBJS.I.01229.
59. Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. doi:10.1177/0363546510385999.
60. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293. doi:10.1007/s11999-009-0992-6
61. Fanelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529. doi:10.1016/0749-8063(95)90127-2.
62. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191. doi:10.1007/s00402-002-0471-y.
63. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557. doi:10.1177/036354658701500606.
64. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146. doi:10.1007/s11420-007-9058-z.
65. Owesen C, Sandven-Thrane S, Lind M, Forssblad M, Granan LP, Årøen A. Epidemiology of surgically treated posterior cruciate ligament injuries in Scandinavia. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2384-2391. doi:10.1007/s00167-015-3786-2.
66. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092. doi:10.1177/0363546515572770.
67. Anderson CL. High rate of osteoarthritis of the knee in former soccer players. Med Sci Sports Exerc. 1986;18(1):141.
68. Arliani GG, Astur DC, Yamada RK, et al. Early osteoarthritis and reduced quality of life after retirement in former professional soccer players. Clinics (Sao Paulo). 2014;69(9):589-594. doi:10.6061/clinics/2014(09)03.
69. Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39(8):473-482. doi:10.1136/bjsm.2004.015511.
70. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329-333. doi:10.1111/j.1600-0838.2005.00497.x.
71. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. doi:10.1177/0363546507307396.
72. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-1646. doi:10.1177/0363546505275647
73. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484. doi:10.1053/jars.2003.50112.
74. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65-72.
75. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-133. doi:10.5435/JAAOS-22-02-121.
76. Andrade R, Vasta S, Papalia R, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players' knees: a systematic review. Arthroscopy. 2016;32(7):1466-1477. doi:10.1016/j.arthro.2016.01.055.
77. Görtz S, Williams RJ, Gersoff WK, Bugbee WD. Osteochondral and meniscal allograft transplantation in the football (soccer) player. Cartilage. 2012;3(1 Suppl):37S-42S. doi:10.1177/1947603511416974.
78. Junge A, Grimm K, Feddermann N, Dvorak J. Precompetition orthopedic assessment of international elite football players. Clin J Sport Med. 2009;19(4):326-328. doi:10.1097/JSM.0b013e3181b21b56.
79. Salzmann GM, Preiss S, Zenobi-Wong M, Harder LP, Maier D, Dvorák J. Osteoarthritis in Football. Cartilage. 2017;8(2):162-172. doi:10.1177/1947603516648186.
80. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. doi:10.1016/j.biomaterials.2011.06.037
81. Freutel M, Seitz AM, Ignatius A, Dürselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):74-82. doi:10.1007/s00167-014-2951-3.
82. Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89-106. doi:10.1093/bmb/ldq043.
83. Nawabi DH, Cro S, Hamid IP, Williams A. Return to play after lateral meniscectomy compared with medial meniscectomy in elite professional soccer players. Am J Sports Med. 2014;42(9):2193-2198. doi:10.1177/0363546514540271.
84. Alentorn-Geli E, Vázquez RS, Díaz PA, Cuscó X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340-343. doi:10.1097/JSM.0b013e3181f207dc.
85. Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382-388. doi:10.1177/0363546513508763.
86. Bizzini M, Dvorak J. FIFA 11+: an effective programme to prevent football injuries in various player groups worldwide-a narrative review. Br J Sports Med. 2015;49(9):577-579. doi:10.1136/bjsports-2015-094765.
87. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
88. Al Attar WSA, Soomro N, Pappas E, Sinclair PJ, Sanders RH. Adding a post-training FIFA 11+ exercise program to the pre-training FIFA 11+ injury prevention program reduces injury rates among male amateur soccer players: a cluster-randomised trial. J Physiother. 2017;63(4):235-242. doi:10.1016/j.jphys.2017.08.004.
ABSTRACT
As one of the most popular sports in the world, soccer injury rates involving the knee continue to rise. An alarming trend of knee injuries, including increased anterior cruciate ligament ruptures, underscores the need to review our current understanding of these injuries in soccer players. This article includes a critical review of the epidemiology of knee injuries in soccer, anterior cruciate ligament and other ligamentous injuries, cartilage and meniscal injury, post-traumatic osteoarthritis, as well as current prevention initiatives.
Continue to: EPIDEMIOLOGY...
EPIDEMIOLOGY
There are currently 28 players on each of the Major League Soccer (MLS) teams, and during the 2013 to 2014 academic year, the National Federation of State High School Associations (NFHS) reported that 417,419 boys and 374,564 girls played high school soccer and the National Collegiate Athletic Association (NCAA) reported that 23,602 males and 26,358 females played collegiate soccer.5 As such, knee injuries in this population are a major concern for those involved in sports medicine. Several injuries occurring during soccer involve the lower extremity, particularly the knee.1 In fact, multiple reports estimate that up to 17.6% of soccer-related injuries presenting to the emergency room involved the knee.1,6-9 The majority of these injuries are noncontact injuries, although contact injuries do still occur.10,11
Risk factors for injuries in soccer may be non-modifiable (such as age and gender) and modifiable (such as level of conditioning, force, balance, and flexibility). Inadequate lower motor coordination may result in injury in the adolescent population, and advanced age >28 years in males and >25 years in females is considered as a high-risk factor for injury.12,13 Importantly, gender and age have been reported to play a significant role as risk factors for ACL injury.6 In fact, female players have a 3 to 5 times higher risk of significant knee injury, including ACL injuries, than male players.4,14-16 Preventative programs such as the FIFA 11+ program have been set forth to augment conditioning as part of managing the modifiable risk factors.
Like American football, playing on artificial turf has been questioned as a contributor to injury compared to playing on natural grass.17,18 In recent years, newer generations of artificial turf have been developed to more closely replicate the characteristics of natural grass. Meyers19 compared the incidence, mechanisms, and severity of match-related collegiate men’s soccer injuries on artificial turf and those on natural grass and demonstrated no significant difference in knee injuries between the 2 surfaces. This finding was consistent with previous studies that reported no difference in the incidence of knee injuries on either surface in women’s collegiate and elite-level soccer.15,20,21
Continue to: ACL INJURIES...
ACL INJURIES
ACL injuries are life-changing events that can significantly affect the career of a soccer athlete. As a major stabilizer of the knee, the ACL primarily prevents anterior tibial translation with the anteromedial bundle and secondarily resists tibial rotation with the posterolateral bundle. The ligament takes origin from the posteromedial aspect of the lateral femoral condyle and inserts anterior to the tibial intercondylar eminence. Grading of ACL injuries is based on the Lachman test, which is performed between 20°and 30° of knee flexion and measures the amount of anterior tibial translation relative to the femur (A = firm endpoint, B = no endpoint; grade I: 3-5 mm, grade II (A/B): 5-10 mm, grade III (A/B): >10 mm).
ACL injury may occur via contact or noncontact mechanisms. Noncontact mechanisms of ACL injury in soccer athletes contribute to about 85% of injuries.6,22-25 Typical noncontact mechanism of injury involves a forceful valgus collapse with the knee near full extension and combined external or internal rotation of the tibia23,26 (Figure 1). This on-field scenario generally involves cutting and torsional movement, as well as landing after a jump, particularly in 1-legged stance. Similarly, a disturbance in balance caused by the opponent may incite a noncontact mechanism resulting in ACL rupture.6,27 Video analyses of professional soccer players have also demonstrated a higher risk of noncontact ACL injury within the first 9 minutes of the match, with the most common playing situation resulting in injury being pressing, followed by kicking and heading.24,25,28 Contact mechanisms resulting in ACL injury, however, are not an uncommon occurrence in soccer players with higher risk for certain positions. Brophy and colleagues29 reviewed ACL injuries in professional and collegiate soccer players and reported a higher risk of ACL injury during defending and tackling. Similarly, Faude and colleagues30 found the risk of injury to be higher in defenders and strikers than in goalkeepers and midfielders.

Female athletes participating in elite-level athletics, especially soccer, represent a high-risk group for ACL injury. In fact, these soccer athletes experience ACL injury at an incidence 3 times higher than that in male athletes.31-35 Female soccer athletes may also be at risk for reinjury to the ACL and contralateral ACL injury. Female gender, in combination with participation in soccer, thus represents a high-risk group for ACL tear in athletics. Allen and colleagues36 retrospectively reviewed 180 female patients who had undergone ACL reconstruction (ACLR) (90 soccer players and 90 non-soccer players) over a mean period of 68.8 months. In their series, soccer players sustained significantly more ACL injuries than non-soccer players, including graft failures (11% vs 1%) and contralateral ACL tears (17% vs 4%).
ACLR is the gold standard treatment for elite soccer athletes. A recent survey of MLS team orthopedic surgeons revealed several important details regarding decision-making in ACLR in this population. From a technical standpoint, the vast majority of surgeons used a single incision, arthroscopically assisted, single-bundle reconstruction (91%). Femoral tunnel drilling was almost equally split between transtibial (51%) and use of an accessory medial portal (46%). Bone-patella-tendon-bone (BPTB) autograft was the most preferred graft choice (68%), and quadriceps tendon autograft was the least preferred. The majority of surgeons preformed ACLR within 4 weeks and permitted return to sport (RTS) without restrictions at 6 to 8 months.37
Continue to: There is a scarcity of literature regarding...
There is a scarcity of literature regarding the use of soft tissue and BPTB allografts in soccer athletes. However, one study reported no difference in patient-reported outcomes and return to preinjury level of activity (including soccer) with the use of either autograft or allograft BPTB in ACLR.38 The authors’ preference was to avoid the use of allograft in elite-level soccer athletes as the reported rate of ACL re-tear was 4 to 8 times higher than that with autograft reconstruction, as shown in athletes and military personnel.39,40 BPTB autograft and hamstring autograft (semitendinosus and/or gracilis) are common graft choices for soccer athletes. Gifstad and colleagues41 compared BPTB autograft and hamstring autograft in 45,998 primary ACLRs performed in Scandinavia. Although the cohort included, but was not limited to, soccer players, the authors reported an overall risk of revision that was significantly lower in the BPTB autograft group than in the hamstring autograft group (hazard ratio, 0.63; 95% confidence interval, 0.53-0.74).41 Mohammadi and colleagues42 prospectively compared the functional outcomes of 42 competitive soccer players who underwent ACLR with BPTB autograft vs those who underwent ACLR with hamstring autograft at the time of RTS. Players who had undergone ACLR with hamstring autograft demonstrated greater quadriceps torque, as well as better performance with triple-hop, crossover-hop, and jump-landing tests; however, both groups demonstrated similar hamstring torque and performance in 2 other hop tests.42 In the authors’ opinion, there may be a concern regarding the use of hamstring autograft in elite soccer players considering that hamstring strains are extremely common in this athletic population; however, further research would be necessary to elucidate whether this is an actual or a theoretical risk. Although not yet studied in elite-level athletes, early clinical results of ACL repair with suture augmentation show promise for certain injury patterns. These include proximal femoral ACL avulsion injuries (Sherman type 1) of excellent tissue quality that have the ability to be reapproximated to the femoral origin43 (Figures 2A, 2B). In a recent series,43-45 early clinical outcomes were found to be excellent and maintained at midterm follow-up.

In the NCAA soccer athletes, an overall RTS rate of 85% has been reported in those undergoing ACLR, with a significantly higher rate observed in scholarship versus non-scholarship athletes.46 Howard and colleagues46 reported median time to unrestricted game play of 6.1 months, with 75% returning to the same or higher level position on the depth chart. Among their studied collegiate soccer athletes, 32% reported continued participation in soccer on some level after college (recreational, semiprofessional, or professional).46 RTS rates for MLS soccer players have also been reported to be high, ranging from 74% to 77%, most of them returning within the following season at 10 ± 2.8 months.47,48 These findings were consistent with the RTS rate of 72% reported by the Multicenter Orthopaedic Outcomes Network (MOON) group, which analyzed 100 female and male soccer players undergoing ACLR at a minimum 7-year follow-up. In this series, Brophy and colleagues29,49 reported an RTS at 12 ± 14.3 months, with 85% returning to the same or a higher level of play prior to their injury. Erickson and colleagues47 analyzed a series of 57 ACLRs performed in MLS athletes and reported no significant difference in preinjury or postoperative performance, or between cases and uninjured controls. Arundale and colleagues48 demonstrated no significantly increased risk of lower extremity injury in MLS athletes after ACLR, but the athletes had significantly shorter careers than their uninjured counterparts. Curiously, RTS rates for European professional soccer athletes have been reported to be substantially higher at 95% to 97%.50,51 Although we can only speculate the reasons for such a discrepancy, the difference in RTS rates for similar athletes highlights a need for objective criteria to determine and report RTS rates, while also providing guidelines to prevent reinjury. Such a consensus among orthopedists is not yet present in the literature.
Soccer players and adolescent age in combination have been shown to portend a 3-fold increased risk of revision surgery for ACL failure in a cohort of 16,930 patients from the Swedish National Knee Ligament Register.52 Published data regarding ACL failure and management of revision ACLR in elite-level soccer athletes are currently lacking. However, low failure rates of 3% to 10% requiring revision reconstruction have been reported.47,49 Arundale and colleagues48 reported 2 incidences of players with ACL graft failures, 1 BPTB autograft and 1 BPTB allograft, both of whom were able to return to MLS after revision ACLR. It is the authors’ preference to use ipsilateral hamstring autograft or contralateral BPTB autograft when an ACL revision reconstruction is required.
Continue to: OTHER LIGAMENTOUS INJURIES...
OTHER LIGAMENTOUS INJURIES
The majority of research efforts regarding knee injuries in this population are focused on the ACL. Correspondingly, literature regarding injury to the collateral ligaments and the posterior cruciate ligament (PCL) in soccer players is sparse. The lateral collateral ligament (LCL) and the medial collateral ligament (MCL) play important roles as primary stabilizers to varus and valgus forces, respectively. The PCL is the primary posterior stabilizer of the knee, preventing posterior translation of the tibia. Injury to these structures may result in significant time lost from soccer and risk of reinjury.53,54
The MCL is the one of the most commonly injured ligaments in sports, including soccer.53,55 The injury mechanism generally involves contact with a resulting valgus force applied to the knee.55 Grading of MCL injuries is based on the amount of medial joint gapping with applied valgus force during examination (grade I: <5 mm, grade II: 5-10 mm, grade III: >10 mm). Kramer and colleagues53 reviewed collateral ligament injuries in the adolescent population and found that MCL injuries occurred 4 times more often than LCL injuries and about 25% were grade III injuries, most commonly occurring in American football and soccer players. Soccer also touts the highest sport-specific MCL injury rate for high school and collegiate athletics, particularly for female NCAA soccer players.56 At the professional level, Lundblad and colleagues55 reported 346 MCL injuries in 27 European teams over an 11-year period, of which 70% were contact-related, and the average time-off from soccer was 28 days.
Most surgeons treat isolated MCL injuries nonoperatively, regardless of grade.57,58 This includes activity modification, use of a hinged knee brace, quadriceps strengthening, and progressive return to play. The literature currently lacks substantial data to guide MCL injury management, specifically in elite soccer athletes. In our experience, grade I injuries are managed nonoperatively and RTS is allowed at 4 to 6 weeks. Grade II injuries are also managed nonoperatively and RTS is allowed at 6 to 8 weeks. Grade III injuries are generally allowed RTS at 8 to 12 weeks and may be considered for surgery in the context of concomitant injuries (eg, posteromedial capsular injury, multiligamentous knee injuries, and meniscal injuries). In some athletes, we consider using a varus unloader brace to help maximize decreased stress on the MCL while still allowing the athlete to be fully weight-bearing. We have found it less ideal to limit weight-bearing in elite athletes, which may negatively affect overall lower extremity neuromuscular proprioception and potentially prolong a safe return to play. Some athletes may experience prolonged soreness at the MCL femoral or tibial attachment despite being able to return to play. It is important to counsel athletes about these prolonged symptoms to set expectations, as this may even occur with grade I MCL injuries. Other rare instances where surgical management may be indicated include persistent pain and instability following nonoperative treatment of grade III injuries and highly displaced tibial avulsions of the ligament resulting in poor healing.59,60
Data regarding LCL injuries in soccer are extremely sparse. In our experience, treatment and RTS rates for isolated LCL injuries are similar to those for MCL injuries. However, it is worth noting that one-quarter of LCL injuries may occur in combination with injury to other posterolateral corner structures.53
PCL injuries are more commonly associated with vehicular trauma but have also been reported to occur in sports at a rate of 33% to 40%.61,62 The mechanism of injury in athletes generally involves a fall onto the hyperflexed knee with the foot in plantarflexion or a direct blow to the anterior tibia in a flexed knee.62,63 Classification of PCL injuries is based on posterior translation of the tibia relative to the femur with the knee flexed to 90°(grade I: 1-5 mm, grade II: 6-10 mm, grade III: >10 mm). In one cohort of 62 patients with isolated PCL injuries, soccer was found to be among the top 5 causes of injury.64 A Scandinavian review of 1287 patients who underwent PCL reconstruction found soccer to be the sport with the highest number of injuries (13.1%).65 The goalkeeper was most commonly subjected to this injury.62 Krutsch and colleagues54 compared PCL injuries in new, professional soccer players to those in players at the closest amateur level of play. In their series, 90% of PCL injuries occurred during preseason in players who were at a lower level of play in the previous season. This finding suggested that a rapid increase in training and playing intensity may have been a significant risk factor for PCL injury. Substantial literature supporting nonoperative or operative management of PCL injuries in soccer athletes is currently lacking. Historically, nonoperative treatment has been the initial management for isolated PCL injuries; however, surgical intervention has become increasingly used for both isolated and combined PCL injuries.66
Continue to: CARTILAGE AND MENISCAL INJURIES...
CARTILAGE AND MENISCAL INJURIES
The prevalence of osteoarthritis (OA) in retired soccer players is high.67,68 Articular cartilage degeneration with subsequent OA occurs in up to 32% of soccer players and ultimately leads to significant disability and retirement from the sport. High physical demands and concomitant knee injuries probably predispose to the development of posttraumatic OA.69-71
Several techniques addressing cartilage débridement or restoration have been reported, with successful RTS but with variable durability.72-75 Recently, Andrade and colleagues76 performed a systematic review of 217 articular cartilage defects in soccer players that were treated using restoration techniques, including chondroplasty, microfracture, autologous chondrocyte implantation (ACI), and osteochondral autograft. Although no superior technique could be ascertained, microfracture and osteochondral autograft procedures led to the quickest return to play, and ACI techniques enhanced long-standing clinical and functional results.76 More recently, osteochondral allograft transplantation has also been described with an 84% return to some level of activity (including soccer) and 60% of athletes returning to high-level sports participation at a mean follow-up of 4.5 years77 (Figures 3A-3C). Although chondroplasty may be successful and allow for a quicker return to play in some soccer players (return to play from 6-12 weeks), the authors believe that a strong cartilage scaffold repair strategy with early weight-bearing, including osteochondral autograft and allograft procedures (return to play from 6-9 months), must also be considered in focal chondral defects to optimize both short-term and potential long-term success.

Meniscal injuries are also prevalent in the soccer population, and consistent with ACL injuries, female players are at least twice as likely to sustain a meniscal tear.78,79 Meniscal damage can occur in isolation or in association with ACL rupture. Repair techniques should be strongly considered as chondral changes in the setting of meniscal deficiency are a significant short- and long-term concern for elite athletes. However, due to intrinsically poor healing potential, partial meniscectomy is unfortunately more often performed.79,80 In either case, meniscal deficiency is recognized as a precursor to the development of OA as meniscal functionality is lost and the articular cartilage is subjected to increased biomechanical loading.81,82 Nawabi and colleagues83 analyzed RTS in 90 professional soccer players following partial meniscectomy. Median RTS was at 7 weeks for lateral meniscectomies and at 5 weeks for medial meniscectomies. RTS probability was 5.99 times greater after medial meniscectomy at all time points. Lateral meniscectomies were associated with an increased risk of postoperative adverse events, reoperation, and a significantly lower rate of return to play.83 In the case of severe meniscal deficiency, particularly post-meniscectomy, meniscal allograft transplantation (MAT) may be considered. In a series of MATs in lower division Spanish players, 12/14 (85.7%) returned to play at an average of 7.6 months.84 A more recent series of professional players reported 9/12 (75%) RTS as professionals and 2/12 (17%) as semiprofessionals at an average of 10.5 months.85 The authors’ strong preference is to perform meniscus-saving procedures whenever possible. Due to the longer recovery and return to play associated with meniscus repair than partial meniscectomy, most of the soccer players will often prefer to proceed with partial meniscectomy. Despite the ultimate treatment, it is critical that the surgeon and the soccer player have an in-depth conversation concerning the risks and benefits for each procedure and individualize treatment to the individual soccer player accordingly.
Continue to: INJURY PREVENTION...
INJURY PREVENTION
Given the breadth and the prevalence of soccer-related injuries, the FIFA11+ program was developed in 2006 as an injury prevention measure (Figure 4). The warm-up program includes 15 structured exercises emphasizing core stabilization, thigh muscle training, proprioception, dynamic stabilization, and plyometric exercises. The routine is believed to be easily executed and effective at preventing the incidence of noncontact injuries.86,87 Recently, Sadigursky and colleagues1 performed a systematic review of randomized clinical trials examining the efficacy of FIFA11+. The authors reported a reduction in injuries by 30% and a relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of the program.1 Post-training programs may also be beneficial as it has been shown that performing FIFA11+ both before and after training reduced overall injury rates in male, amateur soccer players.88 Regardless of the prevention program, it is critical that every league, team, medical team, and athlete have a thorough injury prevention strategy to help keep players healthy and not wait until they have instead sustained a significant injury.

CONCLUSION
Knee injuries are common in soccer, with an alarming number of ACL injuries, as well as other significant pathology. Understanding the unique epidemiology, risk factors, treatment, and injury prevention strategies is critically important in helping medical professionals provide care for all levels of elite soccer players.
ABSTRACT
As one of the most popular sports in the world, soccer injury rates involving the knee continue to rise. An alarming trend of knee injuries, including increased anterior cruciate ligament ruptures, underscores the need to review our current understanding of these injuries in soccer players. This article includes a critical review of the epidemiology of knee injuries in soccer, anterior cruciate ligament and other ligamentous injuries, cartilage and meniscal injury, post-traumatic osteoarthritis, as well as current prevention initiatives.
Continue to: EPIDEMIOLOGY...
EPIDEMIOLOGY
There are currently 28 players on each of the Major League Soccer (MLS) teams, and during the 2013 to 2014 academic year, the National Federation of State High School Associations (NFHS) reported that 417,419 boys and 374,564 girls played high school soccer and the National Collegiate Athletic Association (NCAA) reported that 23,602 males and 26,358 females played collegiate soccer.5 As such, knee injuries in this population are a major concern for those involved in sports medicine. Several injuries occurring during soccer involve the lower extremity, particularly the knee.1 In fact, multiple reports estimate that up to 17.6% of soccer-related injuries presenting to the emergency room involved the knee.1,6-9 The majority of these injuries are noncontact injuries, although contact injuries do still occur.10,11
Risk factors for injuries in soccer may be non-modifiable (such as age and gender) and modifiable (such as level of conditioning, force, balance, and flexibility). Inadequate lower motor coordination may result in injury in the adolescent population, and advanced age >28 years in males and >25 years in females is considered as a high-risk factor for injury.12,13 Importantly, gender and age have been reported to play a significant role as risk factors for ACL injury.6 In fact, female players have a 3 to 5 times higher risk of significant knee injury, including ACL injuries, than male players.4,14-16 Preventative programs such as the FIFA 11+ program have been set forth to augment conditioning as part of managing the modifiable risk factors.
Like American football, playing on artificial turf has been questioned as a contributor to injury compared to playing on natural grass.17,18 In recent years, newer generations of artificial turf have been developed to more closely replicate the characteristics of natural grass. Meyers19 compared the incidence, mechanisms, and severity of match-related collegiate men’s soccer injuries on artificial turf and those on natural grass and demonstrated no significant difference in knee injuries between the 2 surfaces. This finding was consistent with previous studies that reported no difference in the incidence of knee injuries on either surface in women’s collegiate and elite-level soccer.15,20,21
Continue to: ACL INJURIES...
ACL INJURIES
ACL injuries are life-changing events that can significantly affect the career of a soccer athlete. As a major stabilizer of the knee, the ACL primarily prevents anterior tibial translation with the anteromedial bundle and secondarily resists tibial rotation with the posterolateral bundle. The ligament takes origin from the posteromedial aspect of the lateral femoral condyle and inserts anterior to the tibial intercondylar eminence. Grading of ACL injuries is based on the Lachman test, which is performed between 20°and 30° of knee flexion and measures the amount of anterior tibial translation relative to the femur (A = firm endpoint, B = no endpoint; grade I: 3-5 mm, grade II (A/B): 5-10 mm, grade III (A/B): >10 mm).
ACL injury may occur via contact or noncontact mechanisms. Noncontact mechanisms of ACL injury in soccer athletes contribute to about 85% of injuries.6,22-25 Typical noncontact mechanism of injury involves a forceful valgus collapse with the knee near full extension and combined external or internal rotation of the tibia23,26 (Figure 1). This on-field scenario generally involves cutting and torsional movement, as well as landing after a jump, particularly in 1-legged stance. Similarly, a disturbance in balance caused by the opponent may incite a noncontact mechanism resulting in ACL rupture.6,27 Video analyses of professional soccer players have also demonstrated a higher risk of noncontact ACL injury within the first 9 minutes of the match, with the most common playing situation resulting in injury being pressing, followed by kicking and heading.24,25,28 Contact mechanisms resulting in ACL injury, however, are not an uncommon occurrence in soccer players with higher risk for certain positions. Brophy and colleagues29 reviewed ACL injuries in professional and collegiate soccer players and reported a higher risk of ACL injury during defending and tackling. Similarly, Faude and colleagues30 found the risk of injury to be higher in defenders and strikers than in goalkeepers and midfielders.

Female athletes participating in elite-level athletics, especially soccer, represent a high-risk group for ACL injury. In fact, these soccer athletes experience ACL injury at an incidence 3 times higher than that in male athletes.31-35 Female soccer athletes may also be at risk for reinjury to the ACL and contralateral ACL injury. Female gender, in combination with participation in soccer, thus represents a high-risk group for ACL tear in athletics. Allen and colleagues36 retrospectively reviewed 180 female patients who had undergone ACL reconstruction (ACLR) (90 soccer players and 90 non-soccer players) over a mean period of 68.8 months. In their series, soccer players sustained significantly more ACL injuries than non-soccer players, including graft failures (11% vs 1%) and contralateral ACL tears (17% vs 4%).
ACLR is the gold standard treatment for elite soccer athletes. A recent survey of MLS team orthopedic surgeons revealed several important details regarding decision-making in ACLR in this population. From a technical standpoint, the vast majority of surgeons used a single incision, arthroscopically assisted, single-bundle reconstruction (91%). Femoral tunnel drilling was almost equally split between transtibial (51%) and use of an accessory medial portal (46%). Bone-patella-tendon-bone (BPTB) autograft was the most preferred graft choice (68%), and quadriceps tendon autograft was the least preferred. The majority of surgeons preformed ACLR within 4 weeks and permitted return to sport (RTS) without restrictions at 6 to 8 months.37
Continue to: There is a scarcity of literature regarding...
There is a scarcity of literature regarding the use of soft tissue and BPTB allografts in soccer athletes. However, one study reported no difference in patient-reported outcomes and return to preinjury level of activity (including soccer) with the use of either autograft or allograft BPTB in ACLR.38 The authors’ preference was to avoid the use of allograft in elite-level soccer athletes as the reported rate of ACL re-tear was 4 to 8 times higher than that with autograft reconstruction, as shown in athletes and military personnel.39,40 BPTB autograft and hamstring autograft (semitendinosus and/or gracilis) are common graft choices for soccer athletes. Gifstad and colleagues41 compared BPTB autograft and hamstring autograft in 45,998 primary ACLRs performed in Scandinavia. Although the cohort included, but was not limited to, soccer players, the authors reported an overall risk of revision that was significantly lower in the BPTB autograft group than in the hamstring autograft group (hazard ratio, 0.63; 95% confidence interval, 0.53-0.74).41 Mohammadi and colleagues42 prospectively compared the functional outcomes of 42 competitive soccer players who underwent ACLR with BPTB autograft vs those who underwent ACLR with hamstring autograft at the time of RTS. Players who had undergone ACLR with hamstring autograft demonstrated greater quadriceps torque, as well as better performance with triple-hop, crossover-hop, and jump-landing tests; however, both groups demonstrated similar hamstring torque and performance in 2 other hop tests.42 In the authors’ opinion, there may be a concern regarding the use of hamstring autograft in elite soccer players considering that hamstring strains are extremely common in this athletic population; however, further research would be necessary to elucidate whether this is an actual or a theoretical risk. Although not yet studied in elite-level athletes, early clinical results of ACL repair with suture augmentation show promise for certain injury patterns. These include proximal femoral ACL avulsion injuries (Sherman type 1) of excellent tissue quality that have the ability to be reapproximated to the femoral origin43 (Figures 2A, 2B). In a recent series,43-45 early clinical outcomes were found to be excellent and maintained at midterm follow-up.

In the NCAA soccer athletes, an overall RTS rate of 85% has been reported in those undergoing ACLR, with a significantly higher rate observed in scholarship versus non-scholarship athletes.46 Howard and colleagues46 reported median time to unrestricted game play of 6.1 months, with 75% returning to the same or higher level position on the depth chart. Among their studied collegiate soccer athletes, 32% reported continued participation in soccer on some level after college (recreational, semiprofessional, or professional).46 RTS rates for MLS soccer players have also been reported to be high, ranging from 74% to 77%, most of them returning within the following season at 10 ± 2.8 months.47,48 These findings were consistent with the RTS rate of 72% reported by the Multicenter Orthopaedic Outcomes Network (MOON) group, which analyzed 100 female and male soccer players undergoing ACLR at a minimum 7-year follow-up. In this series, Brophy and colleagues29,49 reported an RTS at 12 ± 14.3 months, with 85% returning to the same or a higher level of play prior to their injury. Erickson and colleagues47 analyzed a series of 57 ACLRs performed in MLS athletes and reported no significant difference in preinjury or postoperative performance, or between cases and uninjured controls. Arundale and colleagues48 demonstrated no significantly increased risk of lower extremity injury in MLS athletes after ACLR, but the athletes had significantly shorter careers than their uninjured counterparts. Curiously, RTS rates for European professional soccer athletes have been reported to be substantially higher at 95% to 97%.50,51 Although we can only speculate the reasons for such a discrepancy, the difference in RTS rates for similar athletes highlights a need for objective criteria to determine and report RTS rates, while also providing guidelines to prevent reinjury. Such a consensus among orthopedists is not yet present in the literature.
Soccer players and adolescent age in combination have been shown to portend a 3-fold increased risk of revision surgery for ACL failure in a cohort of 16,930 patients from the Swedish National Knee Ligament Register.52 Published data regarding ACL failure and management of revision ACLR in elite-level soccer athletes are currently lacking. However, low failure rates of 3% to 10% requiring revision reconstruction have been reported.47,49 Arundale and colleagues48 reported 2 incidences of players with ACL graft failures, 1 BPTB autograft and 1 BPTB allograft, both of whom were able to return to MLS after revision ACLR. It is the authors’ preference to use ipsilateral hamstring autograft or contralateral BPTB autograft when an ACL revision reconstruction is required.
Continue to: OTHER LIGAMENTOUS INJURIES...
OTHER LIGAMENTOUS INJURIES
The majority of research efforts regarding knee injuries in this population are focused on the ACL. Correspondingly, literature regarding injury to the collateral ligaments and the posterior cruciate ligament (PCL) in soccer players is sparse. The lateral collateral ligament (LCL) and the medial collateral ligament (MCL) play important roles as primary stabilizers to varus and valgus forces, respectively. The PCL is the primary posterior stabilizer of the knee, preventing posterior translation of the tibia. Injury to these structures may result in significant time lost from soccer and risk of reinjury.53,54
The MCL is the one of the most commonly injured ligaments in sports, including soccer.53,55 The injury mechanism generally involves contact with a resulting valgus force applied to the knee.55 Grading of MCL injuries is based on the amount of medial joint gapping with applied valgus force during examination (grade I: <5 mm, grade II: 5-10 mm, grade III: >10 mm). Kramer and colleagues53 reviewed collateral ligament injuries in the adolescent population and found that MCL injuries occurred 4 times more often than LCL injuries and about 25% were grade III injuries, most commonly occurring in American football and soccer players. Soccer also touts the highest sport-specific MCL injury rate for high school and collegiate athletics, particularly for female NCAA soccer players.56 At the professional level, Lundblad and colleagues55 reported 346 MCL injuries in 27 European teams over an 11-year period, of which 70% were contact-related, and the average time-off from soccer was 28 days.
Most surgeons treat isolated MCL injuries nonoperatively, regardless of grade.57,58 This includes activity modification, use of a hinged knee brace, quadriceps strengthening, and progressive return to play. The literature currently lacks substantial data to guide MCL injury management, specifically in elite soccer athletes. In our experience, grade I injuries are managed nonoperatively and RTS is allowed at 4 to 6 weeks. Grade II injuries are also managed nonoperatively and RTS is allowed at 6 to 8 weeks. Grade III injuries are generally allowed RTS at 8 to 12 weeks and may be considered for surgery in the context of concomitant injuries (eg, posteromedial capsular injury, multiligamentous knee injuries, and meniscal injuries). In some athletes, we consider using a varus unloader brace to help maximize decreased stress on the MCL while still allowing the athlete to be fully weight-bearing. We have found it less ideal to limit weight-bearing in elite athletes, which may negatively affect overall lower extremity neuromuscular proprioception and potentially prolong a safe return to play. Some athletes may experience prolonged soreness at the MCL femoral or tibial attachment despite being able to return to play. It is important to counsel athletes about these prolonged symptoms to set expectations, as this may even occur with grade I MCL injuries. Other rare instances where surgical management may be indicated include persistent pain and instability following nonoperative treatment of grade III injuries and highly displaced tibial avulsions of the ligament resulting in poor healing.59,60
Data regarding LCL injuries in soccer are extremely sparse. In our experience, treatment and RTS rates for isolated LCL injuries are similar to those for MCL injuries. However, it is worth noting that one-quarter of LCL injuries may occur in combination with injury to other posterolateral corner structures.53
PCL injuries are more commonly associated with vehicular trauma but have also been reported to occur in sports at a rate of 33% to 40%.61,62 The mechanism of injury in athletes generally involves a fall onto the hyperflexed knee with the foot in plantarflexion or a direct blow to the anterior tibia in a flexed knee.62,63 Classification of PCL injuries is based on posterior translation of the tibia relative to the femur with the knee flexed to 90°(grade I: 1-5 mm, grade II: 6-10 mm, grade III: >10 mm). In one cohort of 62 patients with isolated PCL injuries, soccer was found to be among the top 5 causes of injury.64 A Scandinavian review of 1287 patients who underwent PCL reconstruction found soccer to be the sport with the highest number of injuries (13.1%).65 The goalkeeper was most commonly subjected to this injury.62 Krutsch and colleagues54 compared PCL injuries in new, professional soccer players to those in players at the closest amateur level of play. In their series, 90% of PCL injuries occurred during preseason in players who were at a lower level of play in the previous season. This finding suggested that a rapid increase in training and playing intensity may have been a significant risk factor for PCL injury. Substantial literature supporting nonoperative or operative management of PCL injuries in soccer athletes is currently lacking. Historically, nonoperative treatment has been the initial management for isolated PCL injuries; however, surgical intervention has become increasingly used for both isolated and combined PCL injuries.66
Continue to: CARTILAGE AND MENISCAL INJURIES...
CARTILAGE AND MENISCAL INJURIES
The prevalence of osteoarthritis (OA) in retired soccer players is high.67,68 Articular cartilage degeneration with subsequent OA occurs in up to 32% of soccer players and ultimately leads to significant disability and retirement from the sport. High physical demands and concomitant knee injuries probably predispose to the development of posttraumatic OA.69-71
Several techniques addressing cartilage débridement or restoration have been reported, with successful RTS but with variable durability.72-75 Recently, Andrade and colleagues76 performed a systematic review of 217 articular cartilage defects in soccer players that were treated using restoration techniques, including chondroplasty, microfracture, autologous chondrocyte implantation (ACI), and osteochondral autograft. Although no superior technique could be ascertained, microfracture and osteochondral autograft procedures led to the quickest return to play, and ACI techniques enhanced long-standing clinical and functional results.76 More recently, osteochondral allograft transplantation has also been described with an 84% return to some level of activity (including soccer) and 60% of athletes returning to high-level sports participation at a mean follow-up of 4.5 years77 (Figures 3A-3C). Although chondroplasty may be successful and allow for a quicker return to play in some soccer players (return to play from 6-12 weeks), the authors believe that a strong cartilage scaffold repair strategy with early weight-bearing, including osteochondral autograft and allograft procedures (return to play from 6-9 months), must also be considered in focal chondral defects to optimize both short-term and potential long-term success.

Meniscal injuries are also prevalent in the soccer population, and consistent with ACL injuries, female players are at least twice as likely to sustain a meniscal tear.78,79 Meniscal damage can occur in isolation or in association with ACL rupture. Repair techniques should be strongly considered as chondral changes in the setting of meniscal deficiency are a significant short- and long-term concern for elite athletes. However, due to intrinsically poor healing potential, partial meniscectomy is unfortunately more often performed.79,80 In either case, meniscal deficiency is recognized as a precursor to the development of OA as meniscal functionality is lost and the articular cartilage is subjected to increased biomechanical loading.81,82 Nawabi and colleagues83 analyzed RTS in 90 professional soccer players following partial meniscectomy. Median RTS was at 7 weeks for lateral meniscectomies and at 5 weeks for medial meniscectomies. RTS probability was 5.99 times greater after medial meniscectomy at all time points. Lateral meniscectomies were associated with an increased risk of postoperative adverse events, reoperation, and a significantly lower rate of return to play.83 In the case of severe meniscal deficiency, particularly post-meniscectomy, meniscal allograft transplantation (MAT) may be considered. In a series of MATs in lower division Spanish players, 12/14 (85.7%) returned to play at an average of 7.6 months.84 A more recent series of professional players reported 9/12 (75%) RTS as professionals and 2/12 (17%) as semiprofessionals at an average of 10.5 months.85 The authors’ strong preference is to perform meniscus-saving procedures whenever possible. Due to the longer recovery and return to play associated with meniscus repair than partial meniscectomy, most of the soccer players will often prefer to proceed with partial meniscectomy. Despite the ultimate treatment, it is critical that the surgeon and the soccer player have an in-depth conversation concerning the risks and benefits for each procedure and individualize treatment to the individual soccer player accordingly.
Continue to: INJURY PREVENTION...
INJURY PREVENTION
Given the breadth and the prevalence of soccer-related injuries, the FIFA11+ program was developed in 2006 as an injury prevention measure (Figure 4). The warm-up program includes 15 structured exercises emphasizing core stabilization, thigh muscle training, proprioception, dynamic stabilization, and plyometric exercises. The routine is believed to be easily executed and effective at preventing the incidence of noncontact injuries.86,87 Recently, Sadigursky and colleagues1 performed a systematic review of randomized clinical trials examining the efficacy of FIFA11+. The authors reported a reduction in injuries by 30% and a relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of the program.1 Post-training programs may also be beneficial as it has been shown that performing FIFA11+ both before and after training reduced overall injury rates in male, amateur soccer players.88 Regardless of the prevention program, it is critical that every league, team, medical team, and athlete have a thorough injury prevention strategy to help keep players healthy and not wait until they have instead sustained a significant injury.

CONCLUSION
Knee injuries are common in soccer, with an alarming number of ACL injuries, as well as other significant pathology. Understanding the unique epidemiology, risk factors, treatment, and injury prevention strategies is critically important in helping medical professionals provide care for all levels of elite soccer players.
1. Sadigursky D, Braid JA, De Lira DNL, Machado BAB, Carneiro RJF, Colavolpe PO. The FIFA 11+ injury prevention program for soccer players: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:18. doi:10.1186/s13102-017-0083-z.
2. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929-938. doi:10.2165/00007256-200434130-00004.
3. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
4. Agel J, Rockwood T, Klossner D. Collegiate ACL Injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. doi:10.1097/JSM.0000000000000290.
5. Kerr ZY, Pierpoint LA, Currie DW, Wasserman EB, Comstock RD. Epidemiologic comparisons of soccer-related injuries presenting to emergency departments and reported within high school and collegiate settings. Inj Epidemiol. 2017;4(1):19. doi:10.1186/s40621-017-0116-9.
6. Volpi P, Bisciotti GN, Chamari K, Cena E, Carimati G, Bragazzi NL. Risk factors of anterior cruciate ligament injury in football players: a systematic review of the literature. Muscles Ligaments Tendons J. 2016;6(4):480-485. doi:10.11138/mltj/2016.6.4.480.
7. Smith NA, Chounthirath T, Xiang H. Soccer-related injuries treated in emergency departments: 1990-2014. Pediatrics. 2016;138(4). doi:10.1542/peds.2016-0346.
8. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2):288-293. doi:10.1177/0363546506294060.
9. Adams AL, Schiff MA. Childhood soccer injuries treated in U.S. emergency departments. Acad Emerg Med. 2006;13(5):571-574. doi:10.1197/j.aem.2005.12.015.
10. Woods C, Hawkins R, Hulse M, Hodson A. The football association medical research programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med. 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436.
11. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28(5 Suppl):S58-68. doi:10.1177/28.suppl_5.s-58.
12. Ostenberg A, Roos H. Injury risk factors in female European football. a prospective study of 123 players during one season. Scand J Med Sci Sports. 2000;10(5):279-285. doi:10.1034/j.1600-0838.2000.010005279.x.
13. Backous DD, Friedl KE, Smith NJ, Parr TJ, Carpine WD. Soccer injuries and their relation to physical maturity. Am J Dis Child. 1988;142(8):839-842. doi:10.1001/archpedi.1988.02150080045019.
14. Grimm NL, Jacobs JC, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2049-2056. doi:10.1177/0363546514556737.
15. Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women's soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
16. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934.
17. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650. doi:10.1177/03635465000280050401.
18. Levy IM, Skovron ML, Agel J. Living with artificial grass: a knowledge update. Part 1: Basic science. Am J Sports Med. 1990;18(4):406-412. doi:10.1177/036354659001800413.
19. Meyers MC. Incidence, Mechanisms, and severity of match-related collegiate men's soccer injuries on fieldturf and natural grass surfaces: a 6-year prospective study. Am J Sports Med. 2017;45(3):708-718. doi:10.1177/0363546516671715.
20. Ekstrand J, Hägglund M, Fuller CW. Comparison of injuries sustained on artificial turf and grass by male and female elite football players. Scand J Med Sci Sports. 2011;21(6):824-832. doi:10.1111/j.1600-0838.2010.01118.x.
21. Meyers MC. Incidence, mechanisms, and severity of match-related collegiate women's soccer injuries on FieldTurf and natural grass surfaces: a 5-year prospective study. Am J Sports Med. 2013;41(10):2409-2420. doi:10.1177/0363546513498994.
22. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195. doi:10.1016/j.knee.2012.07.006.
23. Rothenberg P, Grau L, Kaplan L, Baraga MG. Knee injuries in american football: an epidemiological review. Am J Orthop. 2016;45(6):368-373.
24. Waldén M, Hägglund M, Magnusson H, Ekstrand J. Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):11-19. doi:10.1007/s00167-010-1170-9.
25. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012. doi:10.1177/0363546503261724.
27. Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med. 2005;39(4):212-216; discussion 212-216. doi:10.1136/bjsm.2004.011973.
28. Grassi A, Smiley SP, Roberti di Sarsina T, et al. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: a YouTube-based video analysis. Eur J Orthop Surg Traumatol. 2017;27(7):967-981. doi:10.1007/s00590-017-1905-0.
29. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
30. Faude O, Junge A, Kindermann W, Dvorak J. Risk factors for injuries in elite female soccer players. Br J Sports Med. 2006;40(9):785-790. doi:10.1136/bjsm.2006.027540.
31. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. doi:10.1177/0363546504269937.
32. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98-102. doi:10.1177/03635465000280012901.
33. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701. doi:10.1177/036354659502300611.
34. Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899-904. doi:10.1177/0363546505285582.
35. Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320-1325.e1326. doi:10.1016/j.arthro.2007.07.003.
36. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492-2498. doi:10.1177/0363546516648439.
37. Farber J, Harris JD, Kolstad K, McCulloch PC. Treatment of anterior cruciate ligament injuries by major league soccer team physicians. Orthop J Sports Med. 2014;2(11):2325967114559892. doi:10.1177/2325967114559892.
38. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-66. doi:10.1016/j.arthro.2010.01.004.
39. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81. doi:10.1177/1941738110386185.
40. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246. doi:10.1177/0363546512443945.
41. Gifstad T, Foss OA, Engebretsen L, et al. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42(10):2319-2328. doi:10.1177/0363546514548164.
42. Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271-1277. doi:10.2106/JBJS.L.00724.
43. van der List JP, DiFelice GS. Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1529-e1534. doi:10.1016/j.eats.2017.06.009.
44. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. doi:10.1177/2325967116672176.
45. DiFelice GS, van der List JP. Clinical outcomes of arthroscopic primary repair of proximal anterior cruciate ligament tears are maintained at mid-term follow-up. Arthroscopy. 2018;34(4):1085-1093. doi:10.1016/j.arthro.2017.10.028.
46. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in national collegiate athletic association division I soccer athletes: a study of the southeastern conference. Am J Sports Med. 2016;44(2):433-439. doi:10.1177/0363546515614315.
47. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):2325967113497189. doi:10.1177/2325967113497189.
48. Arundale AJH, Silvers-Granelli HJ, Snyder-Mackler L. Career length and injury incidence after anterior cruciate ligament reconstruction in major league soccer players. Orthop J Sports Med. 2018;6(1):2325967117750825. doi:10.1177/2325967117750825.
49. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
50. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
51. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744-750. doi:10.1136/bjsports-2015-095952.
52. Andernord D, Desai N, Björnsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43(1):121-127. doi:10.1177/0363546514552788.
53. Kramer DE, Miller PE, Berrahou IK, Yen YM, Heyworth BE. Collateral ligament knee injuries in pediatric and adolescent athletes. J Pediatr Orthop. 2017. doi:10.1097/BPO.0000000000001112.
54. Krutsch W, Zeman F, Zellner J, Pfeifer C, Nerlich M, Angele P. Increase in ACL and PCL injuries after implementation of a new professional football league. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2271-2279. doi:10.1007/s00167-014-3357-y.
55. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
56. Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 Through 2013-2014. Am J Sports Med. 2016;44(6):1565-1572. doi:10.1177/0363546516630927.
57. Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37(6):1116-1122. doi:10.1177/0363546509332498.
58. Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. doi:10.2106/JBJS.I.01229.
59. Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. doi:10.1177/0363546510385999.
60. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293. doi:10.1007/s11999-009-0992-6
61. Fanelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529. doi:10.1016/0749-8063(95)90127-2.
62. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191. doi:10.1007/s00402-002-0471-y.
63. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557. doi:10.1177/036354658701500606.
64. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146. doi:10.1007/s11420-007-9058-z.
65. Owesen C, Sandven-Thrane S, Lind M, Forssblad M, Granan LP, Årøen A. Epidemiology of surgically treated posterior cruciate ligament injuries in Scandinavia. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2384-2391. doi:10.1007/s00167-015-3786-2.
66. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092. doi:10.1177/0363546515572770.
67. Anderson CL. High rate of osteoarthritis of the knee in former soccer players. Med Sci Sports Exerc. 1986;18(1):141.
68. Arliani GG, Astur DC, Yamada RK, et al. Early osteoarthritis and reduced quality of life after retirement in former professional soccer players. Clinics (Sao Paulo). 2014;69(9):589-594. doi:10.6061/clinics/2014(09)03.
69. Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39(8):473-482. doi:10.1136/bjsm.2004.015511.
70. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329-333. doi:10.1111/j.1600-0838.2005.00497.x.
71. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. doi:10.1177/0363546507307396.
72. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-1646. doi:10.1177/0363546505275647
73. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484. doi:10.1053/jars.2003.50112.
74. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65-72.
75. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-133. doi:10.5435/JAAOS-22-02-121.
76. Andrade R, Vasta S, Papalia R, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players' knees: a systematic review. Arthroscopy. 2016;32(7):1466-1477. doi:10.1016/j.arthro.2016.01.055.
77. Görtz S, Williams RJ, Gersoff WK, Bugbee WD. Osteochondral and meniscal allograft transplantation in the football (soccer) player. Cartilage. 2012;3(1 Suppl):37S-42S. doi:10.1177/1947603511416974.
78. Junge A, Grimm K, Feddermann N, Dvorak J. Precompetition orthopedic assessment of international elite football players. Clin J Sport Med. 2009;19(4):326-328. doi:10.1097/JSM.0b013e3181b21b56.
79. Salzmann GM, Preiss S, Zenobi-Wong M, Harder LP, Maier D, Dvorák J. Osteoarthritis in Football. Cartilage. 2017;8(2):162-172. doi:10.1177/1947603516648186.
80. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. doi:10.1016/j.biomaterials.2011.06.037
81. Freutel M, Seitz AM, Ignatius A, Dürselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):74-82. doi:10.1007/s00167-014-2951-3.
82. Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89-106. doi:10.1093/bmb/ldq043.
83. Nawabi DH, Cro S, Hamid IP, Williams A. Return to play after lateral meniscectomy compared with medial meniscectomy in elite professional soccer players. Am J Sports Med. 2014;42(9):2193-2198. doi:10.1177/0363546514540271.
84. Alentorn-Geli E, Vázquez RS, Díaz PA, Cuscó X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340-343. doi:10.1097/JSM.0b013e3181f207dc.
85. Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382-388. doi:10.1177/0363546513508763.
86. Bizzini M, Dvorak J. FIFA 11+: an effective programme to prevent football injuries in various player groups worldwide-a narrative review. Br J Sports Med. 2015;49(9):577-579. doi:10.1136/bjsports-2015-094765.
87. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
88. Al Attar WSA, Soomro N, Pappas E, Sinclair PJ, Sanders RH. Adding a post-training FIFA 11+ exercise program to the pre-training FIFA 11+ injury prevention program reduces injury rates among male amateur soccer players: a cluster-randomised trial. J Physiother. 2017;63(4):235-242. doi:10.1016/j.jphys.2017.08.004.
1. Sadigursky D, Braid JA, De Lira DNL, Machado BAB, Carneiro RJF, Colavolpe PO. The FIFA 11+ injury prevention program for soccer players: a systematic review. BMC Sports Sci Med Rehabil. 2017;9:18. doi:10.1186/s13102-017-0083-z.
2. Junge A, Dvorak J. Soccer injuries: a review on incidence and prevention. Sports Med. 2004;34(13):929-938. doi:10.2165/00007256-200434130-00004.
3. Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311-319.
4. Agel J, Rockwood T, Klossner D. Collegiate ACL Injury rates across 15 sports: National collegiate athletic association injury surveillance system data update (2004-2005 Through 2012-2013). Clin J Sport Med. 2016;26(6):518-523. doi:10.1097/JSM.0000000000000290.
5. Kerr ZY, Pierpoint LA, Currie DW, Wasserman EB, Comstock RD. Epidemiologic comparisons of soccer-related injuries presenting to emergency departments and reported within high school and collegiate settings. Inj Epidemiol. 2017;4(1):19. doi:10.1186/s40621-017-0116-9.
6. Volpi P, Bisciotti GN, Chamari K, Cena E, Carimati G, Bragazzi NL. Risk factors of anterior cruciate ligament injury in football players: a systematic review of the literature. Muscles Ligaments Tendons J. 2016;6(4):480-485. doi:10.11138/mltj/2016.6.4.480.
7. Smith NA, Chounthirath T, Xiang H. Soccer-related injuries treated in emergency departments: 1990-2014. Pediatrics. 2016;138(4). doi:10.1542/peds.2016-0346.
8. Leininger RE, Knox CL, Comstock RD. Epidemiology of 1.6 million pediatric soccer-related injuries presenting to US emergency departments from 1990 to 2003. Am J Sports Med. 2007;35(2):288-293. doi:10.1177/0363546506294060.
9. Adams AL, Schiff MA. Childhood soccer injuries treated in U.S. emergency departments. Acad Emerg Med. 2006;13(5):571-574. doi:10.1197/j.aem.2005.12.015.
10. Woods C, Hawkins R, Hulse M, Hodson A. The football association medical research programme: an audit of injuries in professional football-analysis of preseason injuries. Br J Sports Med. 2002;36(6):436-441. doi:10.1136/bjsm.36.6.436.
11. Chomiak J, Junge A, Peterson L, Dvorak J. Severe injuries in football players. Influencing factors. Am J Sports Med. 2000;28(5 Suppl):S58-68. doi:10.1177/28.suppl_5.s-58.
12. Ostenberg A, Roos H. Injury risk factors in female European football. a prospective study of 123 players during one season. Scand J Med Sci Sports. 2000;10(5):279-285. doi:10.1034/j.1600-0838.2000.010005279.x.
13. Backous DD, Friedl KE, Smith NJ, Parr TJ, Carpine WD. Soccer injuries and their relation to physical maturity. Am J Dis Child. 1988;142(8):839-842. doi:10.1001/archpedi.1988.02150080045019.
14. Grimm NL, Jacobs JC, Kim J, Denney BS, Shea KG. Anterior cruciate ligament and knee injury prevention programs for soccer players: a systematic review and meta-analysis. Am J Sports Med. 2015;43(8):2049-2056. doi:10.1177/0363546514556737.
15. Dick R, Putukian M, Agel J, Evans TA, Marshall SW. Descriptive epidemiology of collegiate women's soccer injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2002-2003. J Athl Train. 2007;42(2):278-285.
16. Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394-412. doi:10.1136/bjsm.2008.048934.
17. Guskiewicz KM, Weaver NL, Padua DA, Garrett WE. Epidemiology of concussion in collegiate and high school football players. Am J Sports Med. 2000;28(5):643-650. doi:10.1177/03635465000280050401.
18. Levy IM, Skovron ML, Agel J. Living with artificial grass: a knowledge update. Part 1: Basic science. Am J Sports Med. 1990;18(4):406-412. doi:10.1177/036354659001800413.
19. Meyers MC. Incidence, Mechanisms, and severity of match-related collegiate men's soccer injuries on fieldturf and natural grass surfaces: a 6-year prospective study. Am J Sports Med. 2017;45(3):708-718. doi:10.1177/0363546516671715.
20. Ekstrand J, Hägglund M, Fuller CW. Comparison of injuries sustained on artificial turf and grass by male and female elite football players. Scand J Med Sci Sports. 2011;21(6):824-832. doi:10.1111/j.1600-0838.2010.01118.x.
21. Meyers MC. Incidence, mechanisms, and severity of match-related collegiate women's soccer injuries on FieldTurf and natural grass surfaces: a 5-year prospective study. Am J Sports Med. 2013;41(10):2409-2420. doi:10.1177/0363546513498994.
22. Dragoo JL, Braun HJ, Harris AH. The effect of playing surface on the incidence of ACL injuries in National Collegiate Athletic Association American Football. Knee. 2013;20(3):191-195. doi:10.1016/j.knee.2012.07.006.
23. Rothenberg P, Grau L, Kaplan L, Baraga MG. Knee injuries in american football: an epidemiological review. Am J Orthop. 2016;45(6):368-373.
24. Waldén M, Hägglund M, Magnusson H, Ekstrand J. Anterior cruciate ligament injury in elite football: a prospective three-cohort study. Knee Surg Sports Traumatol Arthrosc. 2011;19(1):11-19. doi:10.1007/s00167-010-1170-9.
25. Waldén M, Krosshaug T, Bjørneboe J, Andersen TE, Faul O, Hägglund M. Three distinct mechanisms predominate in non-contact anterior cruciate ligament injuries in male professional football players: a systematic video analysis of 39 cases. Br J Sports Med. 2015;49(22):1452-1460. doi:10.1136/bjsports-2014-094573.
26. Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002-1012. doi:10.1177/0363546503261724.
27. Giza E, Mithöfer K, Farrell L, Zarins B, Gill T. Injuries in women's professional soccer. Br J Sports Med. 2005;39(4):212-216; discussion 212-216. doi:10.1136/bjsm.2004.011973.
28. Grassi A, Smiley SP, Roberti di Sarsina T, et al. Mechanisms and situations of anterior cruciate ligament injuries in professional male soccer players: a YouTube-based video analysis. Eur J Orthop Surg Traumatol. 2017;27(7):967-981. doi:10.1007/s00590-017-1905-0.
29. Brophy RH, Stepan JG, Silvers HJ, Mandelbaum BR. Defending puts the anterior cruciate ligament at risk during soccer: a gender-based analysis. Sports Health. 2015;7(3):244-249. doi:10.1177/1941738114535184.
30. Faude O, Junge A, Kindermann W, Dvorak J. Risk factors for injuries in elite female soccer players. Br J Sports Med. 2006;40(9):785-790. doi:10.1136/bjsm.2006.027540.
31. Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524-530. doi:10.1177/0363546504269937.
32. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000;28(1):98-102. doi:10.1177/03635465000280012901.
33. Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694-701. doi:10.1177/036354659502300611.
34. Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899-904. doi:10.1177/0363546505285582.
35. Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320-1325.e1326. doi:10.1016/j.arthro.2007.07.003.
36. Allen MM, Pareek A, Krych AJ, et al. Are female soccer players at an increased risk of second anterior cruciate ligament injury compared with their athletic peers? Am J Sports Med. 2016;44(10):2492-2498. doi:10.1177/0363546516648439.
37. Farber J, Harris JD, Kolstad K, McCulloch PC. Treatment of anterior cruciate ligament injuries by major league soccer team physicians. Orthop J Sports Med. 2014;2(11):2325967114559892. doi:10.1177/2325967114559892.
38. Mascarenhas R, Tranovich M, Karpie JC, Irrgang JJ, Fu FH, Harner CD. Patellar tendon anterior cruciate ligament reconstruction in the high-demand patient: evaluation of autograft versus allograft reconstruction. Arthroscopy. 2010;26(9 Suppl):S58-66. doi:10.1016/j.arthro.2010.01.004.
39. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73-81. doi:10.1177/1941738110386185.
40. Pallis M, Svoboda SJ, Cameron KL, Owens BD. Survival comparison of allograft and autograft anterior cruciate ligament reconstruction at the United States Military Academy. Am J Sports Med. 2012;40(6):1242-1246. doi:10.1177/0363546512443945.
41. Gifstad T, Foss OA, Engebretsen L, et al. Lower risk of revision with patellar tendon autografts compared with hamstring autografts: a registry study based on 45,998 primary ACL reconstructions in Scandinavia. Am J Sports Med. 2014;42(10):2319-2328. doi:10.1177/0363546514548164.
42. Mohammadi F, Salavati M, Akhbari B, Mazaheri M, Mohsen Mir S, Etemadi Y. Comparison of functional outcome measures after ACL reconstruction in competitive soccer players: a randomized trial. J Bone Joint Surg Am. 2013;95(14):1271-1277. doi:10.2106/JBJS.L.00724.
43. van der List JP, DiFelice GS. Arthroscopic primary anterior cruciate ligament repair with suture augmentation. Arthrosc Tech. 2017;6(5):e1529-e1534. doi:10.1016/j.eats.2017.06.009.
44. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4(11):2325967116672176. doi:10.1177/2325967116672176.
45. DiFelice GS, van der List JP. Clinical outcomes of arthroscopic primary repair of proximal anterior cruciate ligament tears are maintained at mid-term follow-up. Arthroscopy. 2018;34(4):1085-1093. doi:10.1016/j.arthro.2017.10.028.
46. Howard JS, Lembach ML, Metzler AV, Johnson DL. Rates and determinants of return to play after anterior cruciate ligament reconstruction in national collegiate athletic association division I soccer athletes: a study of the southeastern conference. Am J Sports Med. 2016;44(2):433-439. doi:10.1177/0363546515614315.
47. Erickson BJ, Harris JD, Cvetanovich GL, et al. Performance and return to sport after anterior cruciate ligament reconstruction in male major league soccer players. Orthop J Sports Med. 2013;1(2):2325967113497189. doi:10.1177/2325967113497189.
48. Arundale AJH, Silvers-Granelli HJ, Snyder-Mackler L. Career length and injury incidence after anterior cruciate ligament reconstruction in major league soccer players. Orthop J Sports Med. 2018;6(1):2325967117750825. doi:10.1177/2325967117750825.
49. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
50. Zaffagnini S, Grassi A, Marcheggiani Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
51. Waldén M, Hägglund M, Magnusson H, Ekstrand J. ACL injuries in men's professional football: a 15-year prospective study on time trends and return-to-play rates reveals only 65% of players still play at the top level 3 years after ACL rupture. Br J Sports Med. 2016;50(12):744-750. doi:10.1136/bjsports-2015-095952.
52. Andernord D, Desai N, Björnsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43(1):121-127. doi:10.1177/0363546514552788.
53. Kramer DE, Miller PE, Berrahou IK, Yen YM, Heyworth BE. Collateral ligament knee injuries in pediatric and adolescent athletes. J Pediatr Orthop. 2017. doi:10.1097/BPO.0000000000001112.
54. Krutsch W, Zeman F, Zellner J, Pfeifer C, Nerlich M, Angele P. Increase in ACL and PCL injuries after implementation of a new professional football league. Knee Surg Sports Traumatol Arthrosc. 2016;24(7):2271-2279. doi:10.1007/s00167-014-3357-y.
55. Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759-762. doi:10.1136/bjsports-2013-092305.
56. Stanley LE, Kerr ZY, Dompier TP, Padua DA. Sex differences in the incidence of anterior cruciate ligament, medial collateral ligament, and meniscal injuries in collegiate and high school sports: 2009-2010 Through 2013-2014. Am J Sports Med. 2016;44(6):1565-1572. doi:10.1177/0363546516630927.
57. Lind M, Jakobsen BW, Lund B, Hansen MS, Abdallah O, Christiansen SE. Anatomical reconstruction of the medial collateral ligament and posteromedial corner of the knee in patients with chronic medial collateral ligament instability. Am J Sports Med. 2009;37(6):1116-1122. doi:10.1177/0363546509332498.
58. Wijdicks CA, Griffith CJ, Johansen S, Engebretsen L, LaPrade RF. Injuries to the medial collateral ligament and associated medial structures of the knee. J Bone Joint Surg Am. 2010;92(5):1266-1280. doi:10.2106/JBJS.I.01229.
59. Marchant MH, Tibor LM, Sekiya JK, Hardaker WT, Garrett WE, Taylor DC. Management of medial-sided knee injuries, part 1: medial collateral ligament. Am J Sports Med. 2011;39(5):1102-1113. doi:10.1177/0363546510385999.
60. Corten K, Hoser C, Fink C, Bellemans J. Case reports: a Stener-like lesion of the medial collateral ligament of the knee. Clin Orthop Relat Res. 2010;468(1):289-293. doi:10.1007/s11999-009-0992-6
61. Fanelli GC, Edson CJ. Posterior cruciate ligament injuries in trauma patients: Part II. Arthroscopy. 1995;11(5):526-529. doi:10.1016/0749-8063(95)90127-2.
62. Schulz MS, Russe K, Weiler A, Eichhorn HJ, Strobel MJ. Epidemiology of posterior cruciate ligament injuries. Arch Orthop Trauma Surg. 2003;123(4):186-191. doi:10.1007/s00402-002-0471-y.
63. Fowler PJ, Messieh SS. Isolated posterior cruciate ligament injuries in athletes. Am J Sports Med. 1987;15(6):553-557. doi:10.1177/036354658701500606.
64. Patel DV, Allen AA, Warren RF, Wickiewicz TL, Simonian PT. The nonoperative treatment of acute, isolated (partial or complete) posterior cruciate ligament-deficient knees: an intermediate-term follow-up study. HSS J. 2007;3(2):137-146. doi:10.1007/s11420-007-9058-z.
65. Owesen C, Sandven-Thrane S, Lind M, Forssblad M, Granan LP, Årøen A. Epidemiology of surgically treated posterior cruciate ligament injuries in Scandinavia. Knee Surg Sports Traumatol Arthrosc. 2017;25(8):2384-2391. doi:10.1007/s00167-015-3786-2.
66. LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF. Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. 2015;43(12):3077-3092. doi:10.1177/0363546515572770.
67. Anderson CL. High rate of osteoarthritis of the knee in former soccer players. Med Sci Sports Exerc. 1986;18(1):141.
68. Arliani GG, Astur DC, Yamada RK, et al. Early osteoarthritis and reduced quality of life after retirement in former professional soccer players. Clinics (Sao Paulo). 2014;69(9):589-594. doi:10.6061/clinics/2014(09)03.
69. Wong P, Hong Y. Soccer injury in the lower extremities. Br J Sports Med. 2005;39(8):473-482. doi:10.1136/bjsm.2004.015511.
70. Thelin N, Holmberg S, Thelin A. Knee injuries account for the sports-related increased risk of knee osteoarthritis. Scand J Med Sci Sports. 2006;16(5):329-333. doi:10.1111/j.1600-0838.2005.00497.x.
71. Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756-1769. doi:10.1177/0363546507307396.
72. Mithöfer K, Peterson L, Mandelbaum BR, Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33(11):1639-1646. doi:10.1177/0363546505275647
73. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477-484. doi:10.1053/jars.2003.50112.
74. Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A Suppl 1:65-72.
75. Sherman SL, Garrity J, Bauer K, Cook J, Stannard J, Bugbee W. Fresh osteochondral allograft transplantation for the knee: current concepts. J Am Acad Orthop Surg. 2014;22(2):121-133. doi:10.5435/JAAOS-22-02-121.
76. Andrade R, Vasta S, Papalia R, et al. Prevalence of articular cartilage lesions and surgical clinical outcomes in football (soccer) players' knees: a systematic review. Arthroscopy. 2016;32(7):1466-1477. doi:10.1016/j.arthro.2016.01.055.
77. Görtz S, Williams RJ, Gersoff WK, Bugbee WD. Osteochondral and meniscal allograft transplantation in the football (soccer) player. Cartilage. 2012;3(1 Suppl):37S-42S. doi:10.1177/1947603511416974.
78. Junge A, Grimm K, Feddermann N, Dvorak J. Precompetition orthopedic assessment of international elite football players. Clin J Sport Med. 2009;19(4):326-328. doi:10.1097/JSM.0b013e3181b21b56.
79. Salzmann GM, Preiss S, Zenobi-Wong M, Harder LP, Maier D, Dvorák J. Osteoarthritis in Football. Cartilage. 2017;8(2):162-172. doi:10.1177/1947603516648186.
80. Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials. 2011;32(30):7411-7431. doi:10.1016/j.biomaterials.2011.06.037
81. Freutel M, Seitz AM, Ignatius A, Dürselen L. Influence of partial meniscectomy on attachment forces, superficial strain and contact mechanics in porcine knee joints. Knee Surg Sports Traumatol Arthrosc. 2015;23(1):74-82. doi:10.1007/s00167-014-2951-3.
82. Papalia R, Del Buono A, Osti L, Denaro V, Maffulli N. Meniscectomy as a risk factor for knee osteoarthritis: a systematic review. Br Med Bull. 2011;99:89-106. doi:10.1093/bmb/ldq043.
83. Nawabi DH, Cro S, Hamid IP, Williams A. Return to play after lateral meniscectomy compared with medial meniscectomy in elite professional soccer players. Am J Sports Med. 2014;42(9):2193-2198. doi:10.1177/0363546514540271.
84. Alentorn-Geli E, Vázquez RS, Díaz PA, Cuscó X, Cugat R. Arthroscopic meniscal transplants in soccer players: outcomes at 2- to 5-year follow-up. Clin J Sport Med. 2010;20(5):340-343. doi:10.1097/JSM.0b013e3181f207dc.
85. Marcacci M, Marcheggiani Muccioli GM, Grassi A, et al. Arthroscopic meniscus allograft transplantation in male professional soccer players: a 36-month follow-up study. Am J Sports Med. 2014;42(2):382-388. doi:10.1177/0363546513508763.
86. Bizzini M, Dvorak J. FIFA 11+: an effective programme to prevent football injuries in various player groups worldwide-a narrative review. Br J Sports Med. 2015;49(9):577-579. doi:10.1136/bjsports-2015-094765.
87. Junge A, Lamprecht M, Stamm H, et al. Countrywide campaign to prevent soccer injuries in Swiss amateur players. Am J Sports Med. 2011;39(1):57-63. doi:10.1177/0363546510377424.
88. Al Attar WSA, Soomro N, Pappas E, Sinclair PJ, Sanders RH. Adding a post-training FIFA 11+ exercise program to the pre-training FIFA 11+ injury prevention program reduces injury rates among male amateur soccer players: a cluster-randomised trial. J Physiother. 2017;63(4):235-242. doi:10.1016/j.jphys.2017.08.004.
TAKE-HOME POINTS
- Soccer is one of the most popular sports in the world and has a high incidence of resultant knee injuries.
- Significant, identifiable risk factors put soccer players at risk for serious knee injuries, such as ACL ruptures; age, female sex, and position played influence injury susceptibility.
- ACL injury most commonly occurs via non-contact mechanisms, and female players are at a significantly higher risk of ACL injury than male counterparts.
- The prevalence of osteoarthritis in retired soccer players is high, underscoring the need to be familiar with meniscal and cartilage repair/restoration techniques and associated outcomes.
- The FIFA11+ program reduces injury by 30%, with reported relative risk of 0.70 for lower limb injuries, highlighting the significant preventative importance of this warm-up program.
Medical Complications and Outcomes After Total Shoulder Arthroplasty: A Nationwide Analysis
ABSTRACT
There is a paucity of evidence describing the types and rates of postoperative complications following total shoulder arthroplasty (TSA). We sought to analyze the complications following TSA and determine their effects on described outcome measures.
Using discharge data from the weighted Nationwide Inpatient Sample from 2006 to 2010, patients who underwent primary TSA were identified. The prevalence of specific complications was identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. The data from this database represent events occurring during admission, prior to discharge. The associations between patient characteristics, complications, and outcomes of TSA were evaluated. The specific outcomes analyzed in this study were mortality and length of stay (LOS).
A total of 125,766 patients were identified. The rate of complication after TSA was 6.7% (8457 patients). The most frequent complications were respiratory, renal, and cardiac, occurring in 2.9%, 0.8%, and 0.8% of cases, respectively. Increasing age and total number of preoperative comorbidities significantly increased the likelihood of having a complication. The prevalence of postoperative shock and central nervous system, cardiac, vascular, and respiratory complications was significantly higher in patients who suffered postoperative mortality (88 patients; 0.07% mortality rate) than in those who survived surgery (P < 0.0001). In terms of LOS, shock and infectious and vascular complications most significantly increased the length of hospitalization.
Postoperative complications following TSA are not uncommon and occur in >6% of patients. Older patients and certain comorbidities are associated with complications after surgery. These complications are associated with postoperative mortality and increased LOS.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) provides a predictably high level of satisfaction with survival as high as 92% at 15 years.1 As implant instrumentation and surgical technique and understanding have improved, the frequency of TSAs being performed has also increased.2 Although there are enough data on long-term surgical complications following TSA,1,3-6 there is a paucity of evidence delineating the incidence and types of postoperative complications during hospitalization. Several current issues motivate the improved understanding of TSA, including the increasing number of TSAs being performed, the desire to improve quality of care, and the desire to create financially efficient healthcare.
The purpose of this study is to detail the postoperative complications that occur following TSA using a large national database. Specifically, our goals are to determine the incidence and types of complications after shoulder arthroplasty, determine the patient factors that are associated with these complications, and evaluate the effects of these complications on postoperative in-hospital mortality and length of stay (LOS). Our hypothesis is that there would be a correlation between specific patient factors and complications and that these complications would adversely correlate to patient postoperative outcomes.
METHODS
DESIGN
We conducted a retrospective analysis of TSAs captured by the Nationwide Inpatient Sample (NIS) database between 2006 and 2010. The NIS is the largest all-payer inpatient database that is currently available to the public in the United States.7
The NIS is a part of the Healthcare Cost and Utilization Project funded by the Agency for Healthcare Research and Quality (AHRQ) and the US Department of Health and Human Services. The NIS database is designed to approximate a 20% sample of US hospitals and the patients they serve, including community, academic, general, and specialty-specific hospitals such as orthopedic hospitals.7 The 2010 update of the NIS database contains discharge data from 1051 hospitals across 45 states, with a representative sample of >39 million inpatient hospital stays.7 The NIS database and its data sources have been independently validated and assessed for quality each year since 1988.8Furthermore, comparative analysis of multiple database elements and distributions has been validated against standard norms, including the National Hospital Discharge Survey.9 The NIS database has been used in numerous published studies.2,10,11
PATIENT SELECTION
The yearly NIS databases from 2006 to 2010 were compiled. Patients aged ≥40 years who underwent a TSA were identified using the International Classification of Diseases, 9th Revision (ICD-9), procedural code 81.80. Exclusion criteria were patients with a primary or a secondary diagnosis of humeral or scapular fracture, chronic osteomyelitis, rheumatologic diseases, or evidence of concurrent malignancy (Figure 1).
Native to NIS are patient demographics, including age, sex, and race. Patient comorbidities as described by Elixhauser and colleagues12 are also included in the database.
Continue to: OUTCOMES...
OUTCOMES
The primary outcome of this study was a description of the type and frequency of postoperative complications of TSA. To conduct this analysis, we queried the TSA cohort for specific ICD-9 codes representing acute cardiac, central nervous system, infectious, gastrointestinal, genitourinary, postoperative shock, renal, respiratory, surgical, vascular, and wound complications. The ICD-9 codes used to identify complications were modeled according to previous literature on various surgical applications and were further parsed to reflect only acute postoperative diagnoses13-15(see the Appendix for the comprehensive list of ICD-9 codes).
Two additional outcomes were analyzed, including postoperative mortality and LOS. Postoperative mortality was defined as death occurring prior to discharge. We calculated the average LOS among the complication and the noncomplication cohort.
STATISTICAL ANALYSIS
Patient demographics and target outcomes of the study were analyzed by frequency distribution. Where applicable, the chi-square and the Student’s t tests were used to confirm the statistical difference for dichotomous and continuous variables, respectively. Multivariate regressions were performed after controlling for possible clustering of the data using a generalized estimating equation following a previous analytical methodology.16-20 The results are reported with odds ratios and 95% confidence intervals where applicable, all statistical tests with P ≤ 0.05 were considered to be significant, and all statistical tests were two-sided. We conducted all analyses using SAS, version 9.2 (SAS Institute).
RESULTS
From 2006 to 2010, a weighted sample of 141,973 patients was found to undergo a TSA. After applying our inclusion and exclusion criteria, our study cohort consisted of 125,766 patients (Figure 1).
Continue to: OVERALL TSA COHORT DEMOGRAPHICS...
OVERALL TSA COHORT DEMOGRAPHICS
The average age of the TSA cohort was 69.4 years (standard deviation [SD], 21.20), and 54.1% were females. The cohort had significant comorbidities, with 83.3% of them having at least 1 comorbidity at the time of surgery. Specifically, 31.3% of the patients had 1 comorbidity, 26.5% had 2 comorbidities, and 25.4% had ≥3 comorbidities. Hypertension was the most common comorbidity present in 66.2% of patients, and diabetes was the second most common comorbidity with a prevalence of 16.8%.
COMPLICATION COHORT DEMOGRAPHICS
An overall postoperative complication rate of 6.7% (weighted sample of 8457 patients) was noted in the overall TSA cohort. The TSA cohort was dichotomized into patients who suffered at least 1 complication (weighted, n = 8457) and patients undergoing routine TSAs (weighted, n = 117,308). The average age was significantly higher in the complication vs routine cohort (71.38 vs 69.27 years, P < 0.0001). Similarly, there were significantly more comorbidities (2.51 vs 1.71, P < 0.0001) in the complication cohort.
COMPLICATIONS
We noted a complication rate of 6.7% (weighted sample of 8457 patients). A single complication was noted in 5% of these patients, whereas 1.3% and 0.4% of the patients had 2 and ≥3 complications, respectively. Respiratory abnormalities (2.9%), acute renal failure (0.8%), and cardiac complications (0.8%) were the most prevalent complications after TSA. The list of complications is detailed in Figure 2. Logistic regression analysis of patient characteristics predicting complications showed that advanced age (odds ratio [OR], 2.1 in those aged ≥85 years) and increasing number of comorbidities (≥3; OR, 3.5) were most significant in predicting complications (all P < 0.0001) (Figure 3). Despite the ubiquity of hypertension in this patient population, it was not a significant predictor of complication (OR, 0.9); in contrast, pulmonary disorders (OR, 5.1) and fluid and electrolyte disorders (4.0) were most strongly associated with the development of a postoperative complication after surgery (Figure 4).
EFFECT OF COMPLICATIONS ON LOS
The average length of hospitalization was 2.3 days (95% confidence interval, 2.22-2.25) among the entire cohort. The average LOS was longer in the complication cohort (3.9 days) than in patients who did not have a complication (2.1 days, P < 0.0001). Of the specific complications noted, hemodynamic shock (11.8 days); infectious, most commonly pneumonia (7.6 days); and vascular complications (6.9 days) were associated with the longest hospitalizations. This result is summarized in Figure 5.
MORTALITY
An overall postoperative (in-house) mortality rate of 0.07% was noted (weighted, n = 88). Comparison between the patient cohort that died vs those who survived TSA resulted in significant differences in the rates of complications. Complications that were most significantly different between the cohorts included cardiac (60.47% vs 0.75%, P < 0.0001), postoperative shock (26.61% vs 0.04%, P < 0.0001), and respiratory complications (43.1% vs 2.8%, P < 0.0001). It is important to note that the overall rate of postoperative shock was exceedingly low in the TSA cohort, but it was highly prevalent in the mortality cohort, occurring in 26.61% of patients. A summary of the mortality statistics is presented in Figure 6.
Continue to: DISCUSSION...
DISCUSSION
TSA continues to be associated with high levels of satisfaction;1 as a result, its incidence is increasing.2 As our understanding and efficiency improves nationally, it is imperative that we determine the short-term and longer-term outcomes and complications. In addition, the factors that may affect prognosis must be elucidated to provide a more individualized and effective standard of care. To date, most of the outcome studies of TSA have evaluated long-term outcomes and specific implant-related complications.1,5,6,21,22 Our intent was to evaluate the complications that occur in the postoperative period and their effect on unique “patient care” outcomes. With knowledge of these complications and the predisposing factors, we can better assess patients, risk-stratify, and provide appropriate guidelines.
We noted that complications occurring after TSA are not uncommon, with >6% of patients suffering a postoperative complication. In this study, the number of complications noted was associated with worse patient outcomes. In addition, we noted that patients undergoing a TSA have a significant burden of comorbidities; however, hematologic and fluid disorders (eg, iron deficiency anemia, pulmonary circulatory disorders, and fluid imbalances) were most important in predicting postoperative complications.
Increased LOS in the hospital after TSA was associated with the occurrence of complications. Of all noted complications, shock and infectious and vascular complications led to the longest hospitalizations. Hospital-acquired pneumonia was the most common infectious etiology, while pulmonary embolism and deep vein thrombosis were the most consistent vascular complications. Although seldom studied in the TSA population, a similar finding has been noted in patients after THA. O’Malley and colleagues,23 using the American College of Surgeon’s National Surgical Quality Improvement Program database, identified independent factors that were associated with complications and average prolonged LOS. They noted that the occurrence of major complications was associated with a prolonged LOS. Some, but not all the major complications, included organ space infection, cardiac events, pneumonia, and venous thromboembolic events.23 Therefore, attempts to limit the amount of time spent in hospitals and control the associated costs must focus on managing the incidence of complications.
Postoperative mortality after TSA was uncommon, occurring in 0.07% of the patients in this study. The low incidence of mortality noted in this study is probably related to the fact that our data represent mortality, whereas in the hospital and, unlike most mortality studies, it does not account for patient demise that may occur in the months after surgery. Other reports have noted that mortality occurs in <1.5% of these patients.24-28 Singh and colleagues25 observed in their evaluation of perioperative mortality after TSA a mortality rate of 0.8% with 90 days after 4380 shoulder replacements performed at their institution. Using multivariate analysis, they were able to identify associations between mortality and increasing American Society of Anesthesiology (ASA) class and Charlson Comorbidity Index. These results in relation to ours would indicate that the majority of patients who die after shoulder arthroplasty do so after initial discharge. Although we could not determine a causal relationship between mortality and patient comorbidities, we noted that certain complications strongly correlated with mortality. In patients who died, there was a relatively high incidence of cardiac (60.5%) and respiratory (43.1%) complications. Similarly, although postoperative shock was almost nonexistent in the patients who survived surgery (0.04%), it was much more common in the patients who suffered mortality (26.6%).
This study is not without limitations. Data were extracted from a national database, therefore precluding the inclusion of specific details of surgery and functional assessment. Inherent to ICD-9 coding, we were unable to assess the exact detail and severity of complications. For instance, we cannot be certain what criteria were used to define “acute renal failure” for each patient. This study is retrospective in nature and therefore adequate randomization and standardization of patients is not possible. Similarly, the nature of the database may not allow for exacting our inclusion and exclusion criteria. However, the large sample size of the patient population lessens the chance of potential biases and type 2 errors. Prior to October 2010, reverse shoulder arthroplasty was coded under the ICD-9procedural code 81.80 as TSA. Therefore, there is some overlap between TSA and reverse shoulder arthroplasty in our data. Reverse shoulder arthroplasty is now coded under ICD-9 procedural code 81.88. It is possible that results may differ if reverse shoulder arthroplasty were excluded from our patient cohort. This can be an area of future research.
CONCLUSION
Although much is known about the long-term hardware and functional complications after TSA, in this study, we have attempted to broaden the understanding of perioperative complications and the associated sequelae. Complications are common after TSA surgery and are related to adverse outcomes. In the setting of healthcare changes, the surgeon and the patient must understand the cause, types, incidence, and outcomes of medical and surgical complications after surgery. This allows for more accurate “standard of care” metrics. Further large-volume multicenter studies are needed to gain further insight into the short- and long-term outcomes of TSA.
1. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863. doi:10.1016/j.jse.2008.11.020.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
3. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution's experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43–48. doi:10.1016/j.jse.2013.03.010.
4. Boyd AD Jr, Aliabadi P, Thornhill TS. Postoperative proximal migration in total shoulder arthroplasty. Incidence and significance. J Arthroplasty. 1991;6(1):31-37. doi:10.1016/S0883-5403(06)80154-3.
5. Choi T, Horodyski M, Struk AM, Sahajpal DT, Wright TW. Incidence of early radiolucent lines after glenoid component insertion for total shoulder arthroplasty: a radiographic study comparing pressurized and unpressurized cementing techniques. J Shoulder Elbow Surg. 2013;22(3):403-408. doi:10.1016/j.jse.2012.05.041.
6. Favard L, Katz D, Colmar M, Benkalfate T, Thomazeau H, Emily S. Total shoulder arthroplasty - arthroplasty for glenohumeral arthropathies: results and complications after a minimum follow-up of 8 years according to the type of arthroplasty and etiology. Orthop Traumatol Surg Res. 2012;98(4 Suppl):S41-S47. doi:10.1016/j.otsr.2012.04.003.
7. Agency for Healthcare Research and Quality. Introduction to the HCUP national inpatient sample (NIS) 2012. https://hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2012.pdf 2012. Accessed June 9, 2013.
8. Agency for Healthcare Research and Quality. HCUP quality control procedures. https://hcup-us.ahrq.gov/db/quality.pdf. Accessed June 15, 2013.
9. Agency for Healthcare Research and Quality. Comparative analysis of HCUP and NHDS inpatient discharge data: technical supplement 13. https://archive.ahrq.gov/research/data/hcup/nhds/niscomp.html. Accessed June 15, 2013.
10. Rajaee SS, Trofa D, Matzkin E, Smith E. National trends in primary total hip arthroplasty in extremely young patients: a focus on bearing surface usage. J Arthroplasty. 2012;27(10):1870-1878. doi:10.1016/j.arth.2012.04.006.
11. Bozic KJ, Kurtz S, Lau E, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(7):1614-1620. doi:10.2106/JBJS.H.01220.
12. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
13. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66. doi:10.1001/jama.2009.956.
14. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036. doi:10.2106/JBJS.L.00269.
15. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148. doi:10.1016/j.arth.2013.04.001.
16. Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138-1144. doi:10.1056/NEJMsa011788.
17. Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Temporal trends in radical prostatectomy complications from 1991 to 1998. J Urol. 2003;169(4):1443-1448. doi:10.1097/01.ju.0000056046.16588.e4.
18. Abdollah F, Sun M, Schmitges J, et al. Surgical caseload is an important determinant of continent urinary diversion rate at radical cystectomy: a population-based study. Ann Surg Oncol. 2011;18(9):2680-2687. doi:10.1245/s10434-011-1618-2.
19. Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139(8):658-665. doi:10.7326/0003-4819-139-8-200310210-00009.
20. Joice GA, Deibert CM, Kates M, Spencer BA, McKiernan JM. "Never events”: centers for Medicare and Medicaid Services complications after radical cystectomy. Urology. 2013;81(3):527-532. doi:10.1016/j.urology.2012.09.050.
21. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188. doi:10.2106/JBJS.G.00966.
22. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-e1710. doi:10.2106/JBJS.K.00580.
23. O'Malley NT, Fleming FJ, Gunzler DD, Messing SP, Kates SL. Factors independently associated with complications and length of stay after hip arthroplasty: analysis of the National Surgical Quality Improvement Program. J Arthroplasty. 2012;27(10):1832-1837. doi:10.1016/j.arth.2012.04.025.
24. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888. doi:10.1016/S0883-5403(03)00269-9.
25. Singh JA, Sperling JW, Cofield RH. Ninety day mortality and its predictors after primary shoulder arthroplasty: an analysis of 4,019 patients from 1976-2008. BMC Musculoskelet Disord. 2011;12:231. doi:10.1186/1471-2474-12-231.
26. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O'Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722. doi:10.1007/s11999-009-0996-2.
27. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;455:183-189. doi:10.1097/01.blo.0000238839.26423.8d.
28. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005.
ABSTRACT
There is a paucity of evidence describing the types and rates of postoperative complications following total shoulder arthroplasty (TSA). We sought to analyze the complications following TSA and determine their effects on described outcome measures.
Using discharge data from the weighted Nationwide Inpatient Sample from 2006 to 2010, patients who underwent primary TSA were identified. The prevalence of specific complications was identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. The data from this database represent events occurring during admission, prior to discharge. The associations between patient characteristics, complications, and outcomes of TSA were evaluated. The specific outcomes analyzed in this study were mortality and length of stay (LOS).
A total of 125,766 patients were identified. The rate of complication after TSA was 6.7% (8457 patients). The most frequent complications were respiratory, renal, and cardiac, occurring in 2.9%, 0.8%, and 0.8% of cases, respectively. Increasing age and total number of preoperative comorbidities significantly increased the likelihood of having a complication. The prevalence of postoperative shock and central nervous system, cardiac, vascular, and respiratory complications was significantly higher in patients who suffered postoperative mortality (88 patients; 0.07% mortality rate) than in those who survived surgery (P < 0.0001). In terms of LOS, shock and infectious and vascular complications most significantly increased the length of hospitalization.
Postoperative complications following TSA are not uncommon and occur in >6% of patients. Older patients and certain comorbidities are associated with complications after surgery. These complications are associated with postoperative mortality and increased LOS.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) provides a predictably high level of satisfaction with survival as high as 92% at 15 years.1 As implant instrumentation and surgical technique and understanding have improved, the frequency of TSAs being performed has also increased.2 Although there are enough data on long-term surgical complications following TSA,1,3-6 there is a paucity of evidence delineating the incidence and types of postoperative complications during hospitalization. Several current issues motivate the improved understanding of TSA, including the increasing number of TSAs being performed, the desire to improve quality of care, and the desire to create financially efficient healthcare.
The purpose of this study is to detail the postoperative complications that occur following TSA using a large national database. Specifically, our goals are to determine the incidence and types of complications after shoulder arthroplasty, determine the patient factors that are associated with these complications, and evaluate the effects of these complications on postoperative in-hospital mortality and length of stay (LOS). Our hypothesis is that there would be a correlation between specific patient factors and complications and that these complications would adversely correlate to patient postoperative outcomes.
METHODS
DESIGN
We conducted a retrospective analysis of TSAs captured by the Nationwide Inpatient Sample (NIS) database between 2006 and 2010. The NIS is the largest all-payer inpatient database that is currently available to the public in the United States.7
The NIS is a part of the Healthcare Cost and Utilization Project funded by the Agency for Healthcare Research and Quality (AHRQ) and the US Department of Health and Human Services. The NIS database is designed to approximate a 20% sample of US hospitals and the patients they serve, including community, academic, general, and specialty-specific hospitals such as orthopedic hospitals.7 The 2010 update of the NIS database contains discharge data from 1051 hospitals across 45 states, with a representative sample of >39 million inpatient hospital stays.7 The NIS database and its data sources have been independently validated and assessed for quality each year since 1988.8Furthermore, comparative analysis of multiple database elements and distributions has been validated against standard norms, including the National Hospital Discharge Survey.9 The NIS database has been used in numerous published studies.2,10,11
PATIENT SELECTION
The yearly NIS databases from 2006 to 2010 were compiled. Patients aged ≥40 years who underwent a TSA were identified using the International Classification of Diseases, 9th Revision (ICD-9), procedural code 81.80. Exclusion criteria were patients with a primary or a secondary diagnosis of humeral or scapular fracture, chronic osteomyelitis, rheumatologic diseases, or evidence of concurrent malignancy (Figure 1).
Native to NIS are patient demographics, including age, sex, and race. Patient comorbidities as described by Elixhauser and colleagues12 are also included in the database.
Continue to: OUTCOMES...
OUTCOMES
The primary outcome of this study was a description of the type and frequency of postoperative complications of TSA. To conduct this analysis, we queried the TSA cohort for specific ICD-9 codes representing acute cardiac, central nervous system, infectious, gastrointestinal, genitourinary, postoperative shock, renal, respiratory, surgical, vascular, and wound complications. The ICD-9 codes used to identify complications were modeled according to previous literature on various surgical applications and were further parsed to reflect only acute postoperative diagnoses13-15(see the Appendix for the comprehensive list of ICD-9 codes).
Two additional outcomes were analyzed, including postoperative mortality and LOS. Postoperative mortality was defined as death occurring prior to discharge. We calculated the average LOS among the complication and the noncomplication cohort.
STATISTICAL ANALYSIS
Patient demographics and target outcomes of the study were analyzed by frequency distribution. Where applicable, the chi-square and the Student’s t tests were used to confirm the statistical difference for dichotomous and continuous variables, respectively. Multivariate regressions were performed after controlling for possible clustering of the data using a generalized estimating equation following a previous analytical methodology.16-20 The results are reported with odds ratios and 95% confidence intervals where applicable, all statistical tests with P ≤ 0.05 were considered to be significant, and all statistical tests were two-sided. We conducted all analyses using SAS, version 9.2 (SAS Institute).
RESULTS
From 2006 to 2010, a weighted sample of 141,973 patients was found to undergo a TSA. After applying our inclusion and exclusion criteria, our study cohort consisted of 125,766 patients (Figure 1).
Continue to: OVERALL TSA COHORT DEMOGRAPHICS...
OVERALL TSA COHORT DEMOGRAPHICS
The average age of the TSA cohort was 69.4 years (standard deviation [SD], 21.20), and 54.1% were females. The cohort had significant comorbidities, with 83.3% of them having at least 1 comorbidity at the time of surgery. Specifically, 31.3% of the patients had 1 comorbidity, 26.5% had 2 comorbidities, and 25.4% had ≥3 comorbidities. Hypertension was the most common comorbidity present in 66.2% of patients, and diabetes was the second most common comorbidity with a prevalence of 16.8%.
COMPLICATION COHORT DEMOGRAPHICS
An overall postoperative complication rate of 6.7% (weighted sample of 8457 patients) was noted in the overall TSA cohort. The TSA cohort was dichotomized into patients who suffered at least 1 complication (weighted, n = 8457) and patients undergoing routine TSAs (weighted, n = 117,308). The average age was significantly higher in the complication vs routine cohort (71.38 vs 69.27 years, P < 0.0001). Similarly, there were significantly more comorbidities (2.51 vs 1.71, P < 0.0001) in the complication cohort.
COMPLICATIONS
We noted a complication rate of 6.7% (weighted sample of 8457 patients). A single complication was noted in 5% of these patients, whereas 1.3% and 0.4% of the patients had 2 and ≥3 complications, respectively. Respiratory abnormalities (2.9%), acute renal failure (0.8%), and cardiac complications (0.8%) were the most prevalent complications after TSA. The list of complications is detailed in Figure 2. Logistic regression analysis of patient characteristics predicting complications showed that advanced age (odds ratio [OR], 2.1 in those aged ≥85 years) and increasing number of comorbidities (≥3; OR, 3.5) were most significant in predicting complications (all P < 0.0001) (Figure 3). Despite the ubiquity of hypertension in this patient population, it was not a significant predictor of complication (OR, 0.9); in contrast, pulmonary disorders (OR, 5.1) and fluid and electrolyte disorders (4.0) were most strongly associated with the development of a postoperative complication after surgery (Figure 4).
EFFECT OF COMPLICATIONS ON LOS
The average length of hospitalization was 2.3 days (95% confidence interval, 2.22-2.25) among the entire cohort. The average LOS was longer in the complication cohort (3.9 days) than in patients who did not have a complication (2.1 days, P < 0.0001). Of the specific complications noted, hemodynamic shock (11.8 days); infectious, most commonly pneumonia (7.6 days); and vascular complications (6.9 days) were associated with the longest hospitalizations. This result is summarized in Figure 5.
MORTALITY
An overall postoperative (in-house) mortality rate of 0.07% was noted (weighted, n = 88). Comparison between the patient cohort that died vs those who survived TSA resulted in significant differences in the rates of complications. Complications that were most significantly different between the cohorts included cardiac (60.47% vs 0.75%, P < 0.0001), postoperative shock (26.61% vs 0.04%, P < 0.0001), and respiratory complications (43.1% vs 2.8%, P < 0.0001). It is important to note that the overall rate of postoperative shock was exceedingly low in the TSA cohort, but it was highly prevalent in the mortality cohort, occurring in 26.61% of patients. A summary of the mortality statistics is presented in Figure 6.
Continue to: DISCUSSION...
DISCUSSION
TSA continues to be associated with high levels of satisfaction;1 as a result, its incidence is increasing.2 As our understanding and efficiency improves nationally, it is imperative that we determine the short-term and longer-term outcomes and complications. In addition, the factors that may affect prognosis must be elucidated to provide a more individualized and effective standard of care. To date, most of the outcome studies of TSA have evaluated long-term outcomes and specific implant-related complications.1,5,6,21,22 Our intent was to evaluate the complications that occur in the postoperative period and their effect on unique “patient care” outcomes. With knowledge of these complications and the predisposing factors, we can better assess patients, risk-stratify, and provide appropriate guidelines.
We noted that complications occurring after TSA are not uncommon, with >6% of patients suffering a postoperative complication. In this study, the number of complications noted was associated with worse patient outcomes. In addition, we noted that patients undergoing a TSA have a significant burden of comorbidities; however, hematologic and fluid disorders (eg, iron deficiency anemia, pulmonary circulatory disorders, and fluid imbalances) were most important in predicting postoperative complications.
Increased LOS in the hospital after TSA was associated with the occurrence of complications. Of all noted complications, shock and infectious and vascular complications led to the longest hospitalizations. Hospital-acquired pneumonia was the most common infectious etiology, while pulmonary embolism and deep vein thrombosis were the most consistent vascular complications. Although seldom studied in the TSA population, a similar finding has been noted in patients after THA. O’Malley and colleagues,23 using the American College of Surgeon’s National Surgical Quality Improvement Program database, identified independent factors that were associated with complications and average prolonged LOS. They noted that the occurrence of major complications was associated with a prolonged LOS. Some, but not all the major complications, included organ space infection, cardiac events, pneumonia, and venous thromboembolic events.23 Therefore, attempts to limit the amount of time spent in hospitals and control the associated costs must focus on managing the incidence of complications.
Postoperative mortality after TSA was uncommon, occurring in 0.07% of the patients in this study. The low incidence of mortality noted in this study is probably related to the fact that our data represent mortality, whereas in the hospital and, unlike most mortality studies, it does not account for patient demise that may occur in the months after surgery. Other reports have noted that mortality occurs in <1.5% of these patients.24-28 Singh and colleagues25 observed in their evaluation of perioperative mortality after TSA a mortality rate of 0.8% with 90 days after 4380 shoulder replacements performed at their institution. Using multivariate analysis, they were able to identify associations between mortality and increasing American Society of Anesthesiology (ASA) class and Charlson Comorbidity Index. These results in relation to ours would indicate that the majority of patients who die after shoulder arthroplasty do so after initial discharge. Although we could not determine a causal relationship between mortality and patient comorbidities, we noted that certain complications strongly correlated with mortality. In patients who died, there was a relatively high incidence of cardiac (60.5%) and respiratory (43.1%) complications. Similarly, although postoperative shock was almost nonexistent in the patients who survived surgery (0.04%), it was much more common in the patients who suffered mortality (26.6%).
This study is not without limitations. Data were extracted from a national database, therefore precluding the inclusion of specific details of surgery and functional assessment. Inherent to ICD-9 coding, we were unable to assess the exact detail and severity of complications. For instance, we cannot be certain what criteria were used to define “acute renal failure” for each patient. This study is retrospective in nature and therefore adequate randomization and standardization of patients is not possible. Similarly, the nature of the database may not allow for exacting our inclusion and exclusion criteria. However, the large sample size of the patient population lessens the chance of potential biases and type 2 errors. Prior to October 2010, reverse shoulder arthroplasty was coded under the ICD-9procedural code 81.80 as TSA. Therefore, there is some overlap between TSA and reverse shoulder arthroplasty in our data. Reverse shoulder arthroplasty is now coded under ICD-9 procedural code 81.88. It is possible that results may differ if reverse shoulder arthroplasty were excluded from our patient cohort. This can be an area of future research.
CONCLUSION
Although much is known about the long-term hardware and functional complications after TSA, in this study, we have attempted to broaden the understanding of perioperative complications and the associated sequelae. Complications are common after TSA surgery and are related to adverse outcomes. In the setting of healthcare changes, the surgeon and the patient must understand the cause, types, incidence, and outcomes of medical and surgical complications after surgery. This allows for more accurate “standard of care” metrics. Further large-volume multicenter studies are needed to gain further insight into the short- and long-term outcomes of TSA.
ABSTRACT
There is a paucity of evidence describing the types and rates of postoperative complications following total shoulder arthroplasty (TSA). We sought to analyze the complications following TSA and determine their effects on described outcome measures.
Using discharge data from the weighted Nationwide Inpatient Sample from 2006 to 2010, patients who underwent primary TSA were identified. The prevalence of specific complications was identified using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes. The data from this database represent events occurring during admission, prior to discharge. The associations between patient characteristics, complications, and outcomes of TSA were evaluated. The specific outcomes analyzed in this study were mortality and length of stay (LOS).
A total of 125,766 patients were identified. The rate of complication after TSA was 6.7% (8457 patients). The most frequent complications were respiratory, renal, and cardiac, occurring in 2.9%, 0.8%, and 0.8% of cases, respectively. Increasing age and total number of preoperative comorbidities significantly increased the likelihood of having a complication. The prevalence of postoperative shock and central nervous system, cardiac, vascular, and respiratory complications was significantly higher in patients who suffered postoperative mortality (88 patients; 0.07% mortality rate) than in those who survived surgery (P < 0.0001). In terms of LOS, shock and infectious and vascular complications most significantly increased the length of hospitalization.
Postoperative complications following TSA are not uncommon and occur in >6% of patients. Older patients and certain comorbidities are associated with complications after surgery. These complications are associated with postoperative mortality and increased LOS.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) provides a predictably high level of satisfaction with survival as high as 92% at 15 years.1 As implant instrumentation and surgical technique and understanding have improved, the frequency of TSAs being performed has also increased.2 Although there are enough data on long-term surgical complications following TSA,1,3-6 there is a paucity of evidence delineating the incidence and types of postoperative complications during hospitalization. Several current issues motivate the improved understanding of TSA, including the increasing number of TSAs being performed, the desire to improve quality of care, and the desire to create financially efficient healthcare.
The purpose of this study is to detail the postoperative complications that occur following TSA using a large national database. Specifically, our goals are to determine the incidence and types of complications after shoulder arthroplasty, determine the patient factors that are associated with these complications, and evaluate the effects of these complications on postoperative in-hospital mortality and length of stay (LOS). Our hypothesis is that there would be a correlation between specific patient factors and complications and that these complications would adversely correlate to patient postoperative outcomes.
METHODS
DESIGN
We conducted a retrospective analysis of TSAs captured by the Nationwide Inpatient Sample (NIS) database between 2006 and 2010. The NIS is the largest all-payer inpatient database that is currently available to the public in the United States.7
The NIS is a part of the Healthcare Cost and Utilization Project funded by the Agency for Healthcare Research and Quality (AHRQ) and the US Department of Health and Human Services. The NIS database is designed to approximate a 20% sample of US hospitals and the patients they serve, including community, academic, general, and specialty-specific hospitals such as orthopedic hospitals.7 The 2010 update of the NIS database contains discharge data from 1051 hospitals across 45 states, with a representative sample of >39 million inpatient hospital stays.7 The NIS database and its data sources have been independently validated and assessed for quality each year since 1988.8Furthermore, comparative analysis of multiple database elements and distributions has been validated against standard norms, including the National Hospital Discharge Survey.9 The NIS database has been used in numerous published studies.2,10,11
PATIENT SELECTION
The yearly NIS databases from 2006 to 2010 were compiled. Patients aged ≥40 years who underwent a TSA were identified using the International Classification of Diseases, 9th Revision (ICD-9), procedural code 81.80. Exclusion criteria were patients with a primary or a secondary diagnosis of humeral or scapular fracture, chronic osteomyelitis, rheumatologic diseases, or evidence of concurrent malignancy (Figure 1).
Native to NIS are patient demographics, including age, sex, and race. Patient comorbidities as described by Elixhauser and colleagues12 are also included in the database.
Continue to: OUTCOMES...
OUTCOMES
The primary outcome of this study was a description of the type and frequency of postoperative complications of TSA. To conduct this analysis, we queried the TSA cohort for specific ICD-9 codes representing acute cardiac, central nervous system, infectious, gastrointestinal, genitourinary, postoperative shock, renal, respiratory, surgical, vascular, and wound complications. The ICD-9 codes used to identify complications were modeled according to previous literature on various surgical applications and were further parsed to reflect only acute postoperative diagnoses13-15(see the Appendix for the comprehensive list of ICD-9 codes).
Two additional outcomes were analyzed, including postoperative mortality and LOS. Postoperative mortality was defined as death occurring prior to discharge. We calculated the average LOS among the complication and the noncomplication cohort.
STATISTICAL ANALYSIS
Patient demographics and target outcomes of the study were analyzed by frequency distribution. Where applicable, the chi-square and the Student’s t tests were used to confirm the statistical difference for dichotomous and continuous variables, respectively. Multivariate regressions were performed after controlling for possible clustering of the data using a generalized estimating equation following a previous analytical methodology.16-20 The results are reported with odds ratios and 95% confidence intervals where applicable, all statistical tests with P ≤ 0.05 were considered to be significant, and all statistical tests were two-sided. We conducted all analyses using SAS, version 9.2 (SAS Institute).
RESULTS
From 2006 to 2010, a weighted sample of 141,973 patients was found to undergo a TSA. After applying our inclusion and exclusion criteria, our study cohort consisted of 125,766 patients (Figure 1).
Continue to: OVERALL TSA COHORT DEMOGRAPHICS...
OVERALL TSA COHORT DEMOGRAPHICS
The average age of the TSA cohort was 69.4 years (standard deviation [SD], 21.20), and 54.1% were females. The cohort had significant comorbidities, with 83.3% of them having at least 1 comorbidity at the time of surgery. Specifically, 31.3% of the patients had 1 comorbidity, 26.5% had 2 comorbidities, and 25.4% had ≥3 comorbidities. Hypertension was the most common comorbidity present in 66.2% of patients, and diabetes was the second most common comorbidity with a prevalence of 16.8%.
COMPLICATION COHORT DEMOGRAPHICS
An overall postoperative complication rate of 6.7% (weighted sample of 8457 patients) was noted in the overall TSA cohort. The TSA cohort was dichotomized into patients who suffered at least 1 complication (weighted, n = 8457) and patients undergoing routine TSAs (weighted, n = 117,308). The average age was significantly higher in the complication vs routine cohort (71.38 vs 69.27 years, P < 0.0001). Similarly, there were significantly more comorbidities (2.51 vs 1.71, P < 0.0001) in the complication cohort.
COMPLICATIONS
We noted a complication rate of 6.7% (weighted sample of 8457 patients). A single complication was noted in 5% of these patients, whereas 1.3% and 0.4% of the patients had 2 and ≥3 complications, respectively. Respiratory abnormalities (2.9%), acute renal failure (0.8%), and cardiac complications (0.8%) were the most prevalent complications after TSA. The list of complications is detailed in Figure 2. Logistic regression analysis of patient characteristics predicting complications showed that advanced age (odds ratio [OR], 2.1 in those aged ≥85 years) and increasing number of comorbidities (≥3; OR, 3.5) were most significant in predicting complications (all P < 0.0001) (Figure 3). Despite the ubiquity of hypertension in this patient population, it was not a significant predictor of complication (OR, 0.9); in contrast, pulmonary disorders (OR, 5.1) and fluid and electrolyte disorders (4.0) were most strongly associated with the development of a postoperative complication after surgery (Figure 4).
EFFECT OF COMPLICATIONS ON LOS
The average length of hospitalization was 2.3 days (95% confidence interval, 2.22-2.25) among the entire cohort. The average LOS was longer in the complication cohort (3.9 days) than in patients who did not have a complication (2.1 days, P < 0.0001). Of the specific complications noted, hemodynamic shock (11.8 days); infectious, most commonly pneumonia (7.6 days); and vascular complications (6.9 days) were associated with the longest hospitalizations. This result is summarized in Figure 5.
MORTALITY
An overall postoperative (in-house) mortality rate of 0.07% was noted (weighted, n = 88). Comparison between the patient cohort that died vs those who survived TSA resulted in significant differences in the rates of complications. Complications that were most significantly different between the cohorts included cardiac (60.47% vs 0.75%, P < 0.0001), postoperative shock (26.61% vs 0.04%, P < 0.0001), and respiratory complications (43.1% vs 2.8%, P < 0.0001). It is important to note that the overall rate of postoperative shock was exceedingly low in the TSA cohort, but it was highly prevalent in the mortality cohort, occurring in 26.61% of patients. A summary of the mortality statistics is presented in Figure 6.
Continue to: DISCUSSION...
DISCUSSION
TSA continues to be associated with high levels of satisfaction;1 as a result, its incidence is increasing.2 As our understanding and efficiency improves nationally, it is imperative that we determine the short-term and longer-term outcomes and complications. In addition, the factors that may affect prognosis must be elucidated to provide a more individualized and effective standard of care. To date, most of the outcome studies of TSA have evaluated long-term outcomes and specific implant-related complications.1,5,6,21,22 Our intent was to evaluate the complications that occur in the postoperative period and their effect on unique “patient care” outcomes. With knowledge of these complications and the predisposing factors, we can better assess patients, risk-stratify, and provide appropriate guidelines.
We noted that complications occurring after TSA are not uncommon, with >6% of patients suffering a postoperative complication. In this study, the number of complications noted was associated with worse patient outcomes. In addition, we noted that patients undergoing a TSA have a significant burden of comorbidities; however, hematologic and fluid disorders (eg, iron deficiency anemia, pulmonary circulatory disorders, and fluid imbalances) were most important in predicting postoperative complications.
Increased LOS in the hospital after TSA was associated with the occurrence of complications. Of all noted complications, shock and infectious and vascular complications led to the longest hospitalizations. Hospital-acquired pneumonia was the most common infectious etiology, while pulmonary embolism and deep vein thrombosis were the most consistent vascular complications. Although seldom studied in the TSA population, a similar finding has been noted in patients after THA. O’Malley and colleagues,23 using the American College of Surgeon’s National Surgical Quality Improvement Program database, identified independent factors that were associated with complications and average prolonged LOS. They noted that the occurrence of major complications was associated with a prolonged LOS. Some, but not all the major complications, included organ space infection, cardiac events, pneumonia, and venous thromboembolic events.23 Therefore, attempts to limit the amount of time spent in hospitals and control the associated costs must focus on managing the incidence of complications.
Postoperative mortality after TSA was uncommon, occurring in 0.07% of the patients in this study. The low incidence of mortality noted in this study is probably related to the fact that our data represent mortality, whereas in the hospital and, unlike most mortality studies, it does not account for patient demise that may occur in the months after surgery. Other reports have noted that mortality occurs in <1.5% of these patients.24-28 Singh and colleagues25 observed in their evaluation of perioperative mortality after TSA a mortality rate of 0.8% with 90 days after 4380 shoulder replacements performed at their institution. Using multivariate analysis, they were able to identify associations between mortality and increasing American Society of Anesthesiology (ASA) class and Charlson Comorbidity Index. These results in relation to ours would indicate that the majority of patients who die after shoulder arthroplasty do so after initial discharge. Although we could not determine a causal relationship between mortality and patient comorbidities, we noted that certain complications strongly correlated with mortality. In patients who died, there was a relatively high incidence of cardiac (60.5%) and respiratory (43.1%) complications. Similarly, although postoperative shock was almost nonexistent in the patients who survived surgery (0.04%), it was much more common in the patients who suffered mortality (26.6%).
This study is not without limitations. Data were extracted from a national database, therefore precluding the inclusion of specific details of surgery and functional assessment. Inherent to ICD-9 coding, we were unable to assess the exact detail and severity of complications. For instance, we cannot be certain what criteria were used to define “acute renal failure” for each patient. This study is retrospective in nature and therefore adequate randomization and standardization of patients is not possible. Similarly, the nature of the database may not allow for exacting our inclusion and exclusion criteria. However, the large sample size of the patient population lessens the chance of potential biases and type 2 errors. Prior to October 2010, reverse shoulder arthroplasty was coded under the ICD-9procedural code 81.80 as TSA. Therefore, there is some overlap between TSA and reverse shoulder arthroplasty in our data. Reverse shoulder arthroplasty is now coded under ICD-9 procedural code 81.88. It is possible that results may differ if reverse shoulder arthroplasty were excluded from our patient cohort. This can be an area of future research.
CONCLUSION
Although much is known about the long-term hardware and functional complications after TSA, in this study, we have attempted to broaden the understanding of perioperative complications and the associated sequelae. Complications are common after TSA surgery and are related to adverse outcomes. In the setting of healthcare changes, the surgeon and the patient must understand the cause, types, incidence, and outcomes of medical and surgical complications after surgery. This allows for more accurate “standard of care” metrics. Further large-volume multicenter studies are needed to gain further insight into the short- and long-term outcomes of TSA.
1. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863. doi:10.1016/j.jse.2008.11.020.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
3. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution's experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43–48. doi:10.1016/j.jse.2013.03.010.
4. Boyd AD Jr, Aliabadi P, Thornhill TS. Postoperative proximal migration in total shoulder arthroplasty. Incidence and significance. J Arthroplasty. 1991;6(1):31-37. doi:10.1016/S0883-5403(06)80154-3.
5. Choi T, Horodyski M, Struk AM, Sahajpal DT, Wright TW. Incidence of early radiolucent lines after glenoid component insertion for total shoulder arthroplasty: a radiographic study comparing pressurized and unpressurized cementing techniques. J Shoulder Elbow Surg. 2013;22(3):403-408. doi:10.1016/j.jse.2012.05.041.
6. Favard L, Katz D, Colmar M, Benkalfate T, Thomazeau H, Emily S. Total shoulder arthroplasty - arthroplasty for glenohumeral arthropathies: results and complications after a minimum follow-up of 8 years according to the type of arthroplasty and etiology. Orthop Traumatol Surg Res. 2012;98(4 Suppl):S41-S47. doi:10.1016/j.otsr.2012.04.003.
7. Agency for Healthcare Research and Quality. Introduction to the HCUP national inpatient sample (NIS) 2012. https://hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2012.pdf 2012. Accessed June 9, 2013.
8. Agency for Healthcare Research and Quality. HCUP quality control procedures. https://hcup-us.ahrq.gov/db/quality.pdf. Accessed June 15, 2013.
9. Agency for Healthcare Research and Quality. Comparative analysis of HCUP and NHDS inpatient discharge data: technical supplement 13. https://archive.ahrq.gov/research/data/hcup/nhds/niscomp.html. Accessed June 15, 2013.
10. Rajaee SS, Trofa D, Matzkin E, Smith E. National trends in primary total hip arthroplasty in extremely young patients: a focus on bearing surface usage. J Arthroplasty. 2012;27(10):1870-1878. doi:10.1016/j.arth.2012.04.006.
11. Bozic KJ, Kurtz S, Lau E, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(7):1614-1620. doi:10.2106/JBJS.H.01220.
12. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
13. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66. doi:10.1001/jama.2009.956.
14. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036. doi:10.2106/JBJS.L.00269.
15. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148. doi:10.1016/j.arth.2013.04.001.
16. Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138-1144. doi:10.1056/NEJMsa011788.
17. Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Temporal trends in radical prostatectomy complications from 1991 to 1998. J Urol. 2003;169(4):1443-1448. doi:10.1097/01.ju.0000056046.16588.e4.
18. Abdollah F, Sun M, Schmitges J, et al. Surgical caseload is an important determinant of continent urinary diversion rate at radical cystectomy: a population-based study. Ann Surg Oncol. 2011;18(9):2680-2687. doi:10.1245/s10434-011-1618-2.
19. Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139(8):658-665. doi:10.7326/0003-4819-139-8-200310210-00009.
20. Joice GA, Deibert CM, Kates M, Spencer BA, McKiernan JM. "Never events”: centers for Medicare and Medicaid Services complications after radical cystectomy. Urology. 2013;81(3):527-532. doi:10.1016/j.urology.2012.09.050.
21. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188. doi:10.2106/JBJS.G.00966.
22. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-e1710. doi:10.2106/JBJS.K.00580.
23. O'Malley NT, Fleming FJ, Gunzler DD, Messing SP, Kates SL. Factors independently associated with complications and length of stay after hip arthroplasty: analysis of the National Surgical Quality Improvement Program. J Arthroplasty. 2012;27(10):1832-1837. doi:10.1016/j.arth.2012.04.025.
24. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888. doi:10.1016/S0883-5403(03)00269-9.
25. Singh JA, Sperling JW, Cofield RH. Ninety day mortality and its predictors after primary shoulder arthroplasty: an analysis of 4,019 patients from 1976-2008. BMC Musculoskelet Disord. 2011;12:231. doi:10.1186/1471-2474-12-231.
26. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O'Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722. doi:10.1007/s11999-009-0996-2.
27. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;455:183-189. doi:10.1097/01.blo.0000238839.26423.8d.
28. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005.
1. Fox TJ, Cil A, Sperling JW, Sanchez-Sotelo J, Schleck CD, Cofield RH. Survival of the glenoid component in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(6):859-863. doi:10.1016/j.jse.2008.11.020.
2. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994.
3. Ahmadi S, Lawrence TM, Sahota S, et al. The incidence and risk factors for blood transfusion in revision shoulder arthroplasty: our institution's experience and review of the literature. J Shoulder Elbow Surg. 2014;23(1):43–48. doi:10.1016/j.jse.2013.03.010.
4. Boyd AD Jr, Aliabadi P, Thornhill TS. Postoperative proximal migration in total shoulder arthroplasty. Incidence and significance. J Arthroplasty. 1991;6(1):31-37. doi:10.1016/S0883-5403(06)80154-3.
5. Choi T, Horodyski M, Struk AM, Sahajpal DT, Wright TW. Incidence of early radiolucent lines after glenoid component insertion for total shoulder arthroplasty: a radiographic study comparing pressurized and unpressurized cementing techniques. J Shoulder Elbow Surg. 2013;22(3):403-408. doi:10.1016/j.jse.2012.05.041.
6. Favard L, Katz D, Colmar M, Benkalfate T, Thomazeau H, Emily S. Total shoulder arthroplasty - arthroplasty for glenohumeral arthropathies: results and complications after a minimum follow-up of 8 years according to the type of arthroplasty and etiology. Orthop Traumatol Surg Res. 2012;98(4 Suppl):S41-S47. doi:10.1016/j.otsr.2012.04.003.
7. Agency for Healthcare Research and Quality. Introduction to the HCUP national inpatient sample (NIS) 2012. https://hcup-us.ahrq.gov/db/nation/nis/NISIntroduction2012.pdf 2012. Accessed June 9, 2013.
8. Agency for Healthcare Research and Quality. HCUP quality control procedures. https://hcup-us.ahrq.gov/db/quality.pdf. Accessed June 15, 2013.
9. Agency for Healthcare Research and Quality. Comparative analysis of HCUP and NHDS inpatient discharge data: technical supplement 13. https://archive.ahrq.gov/research/data/hcup/nhds/niscomp.html. Accessed June 15, 2013.
10. Rajaee SS, Trofa D, Matzkin E, Smith E. National trends in primary total hip arthroplasty in extremely young patients: a focus on bearing surface usage. J Arthroplasty. 2012;27(10):1870-1878. doi:10.1016/j.arth.2012.04.006.
11. Bozic KJ, Kurtz S, Lau E, et al. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(7):1614-1620. doi:10.2106/JBJS.H.01220.
12. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
13. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA. 2009;302(1):58-66. doi:10.1001/jama.2009.956.
14. Lin CA, Kuo AC, Takemoto S. Comorbidities and perioperative complications in HIV-positive patients undergoing primary total hip and knee arthroplasty. J Bone Joint Surg Am. 2013;95(11):1028-1036. doi:10.2106/JBJS.L.00269.
15. Rasouli MR, Maltenfort MG, Ross D, Hozack WJ, Memtsoudis SG, Parvizi J. Perioperative morbidity and mortality following bilateral total hip arthroplasty. J Arthroplasty. 2014;29(1):142-148. doi:10.1016/j.arth.2013.04.001.
16. Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138-1144. doi:10.1056/NEJMsa011788.
17. Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Temporal trends in radical prostatectomy complications from 1991 to 1998. J Urol. 2003;169(4):1443-1448. doi:10.1097/01.ju.0000056046.16588.e4.
18. Abdollah F, Sun M, Schmitges J, et al. Surgical caseload is an important determinant of continent urinary diversion rate at radical cystectomy: a population-based study. Ann Surg Oncol. 2011;18(9):2680-2687. doi:10.1245/s10434-011-1618-2.
19. Panageas KS, Schrag D, Riedel E, Bach PB, Begg CB. The effect of clustering of outcomes on the association of procedure volume and surgical outcomes. Ann Intern Med. 2003;139(8):658-665. doi:10.7326/0003-4819-139-8-200310210-00009.
20. Joice GA, Deibert CM, Kates M, Spencer BA, McKiernan JM. "Never events”: centers for Medicare and Medicaid Services complications after radical cystectomy. Urology. 2013;81(3):527-532. doi:10.1016/j.urology.2012.09.050.
21. Taunton MJ, McIntosh AL, Sperling JW, Cofield RH. Total shoulder arthroplasty with a metal-backed, bone-ingrowth glenoid component. Medium to long-term results. J Bone Joint Surg Am. 2008;90(10):2180-2188. doi:10.2106/JBJS.G.00966.
22. Raiss P, Schmitt M, Bruckner T, et al. Results of cemented total shoulder replacement with a minimum follow-up of ten years. J Bone Joint Surg Am. 2012;94(23):e1711-e1710. doi:10.2106/JBJS.K.00580.
23. O'Malley NT, Fleming FJ, Gunzler DD, Messing SP, Kates SL. Factors independently associated with complications and length of stay after hip arthroplasty: analysis of the National Surgical Quality Improvement Program. J Arthroplasty. 2012;27(10):1832-1837. doi:10.1016/j.arth.2012.04.025.
24. White CB, Sperling JW, Cofield RH, Rowland CM. Ninety-day mortality after shoulder arthroplasty. J Arthroplasty. 2003;18(7):886-888. doi:10.1016/S0883-5403(03)00269-9.
25. Singh JA, Sperling JW, Cofield RH. Ninety day mortality and its predictors after primary shoulder arthroplasty: an analysis of 4,019 patients from 1976-2008. BMC Musculoskelet Disord. 2011;12:231. doi:10.1186/1471-2474-12-231.
26. Fehringer EV, Mikuls TR, Michaud KD, Henderson WG, O'Dell JR. Shoulder arthroplasties have fewer complications than hip or knee arthroplasties in US veterans. Clin Orthop Relat Res. 2010;468(3):717-722. doi:10.1007/s11999-009-0996-2.
27. Farmer KW, Hammond JW, Queale WS, Keyurapan E, McFarland EG. Shoulder arthroplasty versus hip and knee arthroplasties: a comparison of outcomes. Clin Orthop Relat Res. 2007;455:183-189. doi:10.1097/01.blo.0000238839.26423.8d.
28. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563. doi:10.1016/j.jse.2010.11.005.
TAKE HOME POINTS
- Medical complications are common (6.7%) after total shoulder arthroplasty.
- Age and preoperative medical comorbidities increased the risk of a postoperative complication.
- The most frequent medical complications are respiratory, renal, and cardiac.
- Length of stay was effected most by shock, infections, and vascular complications.
- Mortality was associated with major complications such as, shock, central nervous system, cardiac, vascular, and respiratory complications.
The In Vivo Impact of Leukocyte Injections on Normal Rat Achilles Tendons: Potential Detriment to Tendon Morphology, Cellularity, and Vascularity
ABSTRACT
In this study, we determine the in vivo effects of injecting sub-populations of leukocytes into normal rat Achilles tendons via a controlled laboratory study. Allogenic monocytes, granulocytes, or plasma were injected into 24 healthy rat Achilles tendons. Treated and contralateral un-treated control tendons then assessed for cellularity, histologic morphology, and vascularity after 7 and 14 days. Significant increases of 221% and 249% in cellularity (P = 0.014) were seen on day 14 within Achilles tendons injected with granulocytes as compared to plasma and monocytes, respectively. Also, significant improvement in morphology (P = 0.029) between days 7 and 14 was seen for the granulocyte injected Achilles tendons. Significant increases in cellularity after an injection of granulocytes, compared to monocytes and plasma, corresponds to a significant increase in inflammation within the tissue, suggesting that leukocyte-rich platelet-rich plasma (PRP) preparations are proinflammatory and potentially catabolic when injected into tendon tissue. The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Continue to: Tendinopathies are debilitating conditions...
Tendinopathies are debilitating conditions affecting patients worldwide every day. They arise most frequently from tendon overuse resulting in pathology.1 There are 2 major subtypes of tendinopathy: tendinosis and tendinitis. Tendinosis, the more common condition, is characterized by long-term, chronic degradation of tendon tissue resulting in fibrosis from infiltrating fibroblasts.2 Tendinitis, the less common condition, is characterized by an acute inflammatory response and inflammatory cell infiltrate.2 Both conditions are common, with Achilles tendinopathy affecting 11% of runners and lateral epicondylitis affecting 1% to 3% of the general population.3,4 Many sports-related overuse injuries, such as tendinopathies, go undiagnosed for extended periods of time because medical attention is avoided in order to prevent time loss from training or competing.5 These delays could be eliminated if a non-surgical option for treating tendon pathology was available.
Tendinopathies are believed to result from tendon overuse that causes micro-damage to collagen, as well as from significant changes in protein and enzyme composition within the tendon.6 The damage accumulates over time and eventually leads to chronic inflammation or fibrotic change within tendons, in both cases weakening the tendon and causing pain. Currently, accepted treatments for tendinopathies include: nonsteroidal anti-inflammatory drugs, physical therapy, ultrasound, laser-therapy, corticosteroids, glyceryl trinitrate patches, extracorporeal shock wave therapy, sclerotherapy, and surgery.7 Recently, platelet-rich plasma (PRP) therapy has emerged as a promising treatment for tendinopathies, as well as a variety of other orthopedic indications.
PRP consists of autologous blood from the patient, centrifuged to increase the amount of platelets in the sample above baseline, and subsequently injected around an affected tendon or joint.8 PRP is used to treat tendinopathy because it can supply injured tendons with blood components that aid in healing, which tendons do not receive due to poor vascularity.9 These components include growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor, and leukocytes that can stimulate an inflammatory response within the injured tissue.10 The inflammatory response from the PRP induces a more robust reconstruction and revascularization of the injured tissue, stimulating proliferation, and remodeling.11,12However, significant variability exists within the platelets, leukocytes, and growth factors that comprise PRP. This is attributed to 3 major causes. First, current commercial preparations of PRP result in differing platelet concentrations, as well as leukocyte-rich and leukocyte-poor compositions.13,14 Variability in platelet concentrations results in unreliable amounts of growth factors, including cytokines, TGF-β, PDGF, VEGF and basic fibroblast growth factor in each preparation, while leukocyte levels affect inflammation, all leading to variable effects for each preparation.15,16Second, despite sex and age of the PRP donor not being significant factors influencing variation in growth factor concentrations, the existence of an unexplained variation in concentrations of growth factors between different donors has been observed.17 Third, the selection of activating agents, bovine thrombin or calcium chloride, and their application, whether to the elbow, shoulder, or knee, produces variability.18
While the effects of platelets and growth factors in PRP have been well studied, less is known about the effects of differing cell types. Recently it was reported that the concentrations of leukocytes directly affect the outcomes of PRP injections. McCarrel and colleagues19,20 found that as the number of leukocytes increased, there was a concomitant increase in the expression of inflammatory cytokines and catabolic activity. This effect may result in inferior healing of injured tissues and is attributed to the release of pro-inflammatory cytokines such as interleukin-1β from the leukocytes.21 There is also evidence that minimizing the catabolic effect of leukocytes may be just as important to tissue healing as the maximizing anabolic effect of platelets and growth factors.22
The use of PRP has been highly disputed in recent years due to conflicting reports of its success in treating orthopedic conditions. Numerous favorable studies have shown benefit for treating chronic and acute orthopedic injuries including; rotator cuff tear repair, chronic refractory patellar tendinopathy, and chronic lateral tendinosis/epicondylitis.23-26 Concurrently, articles demonstrating no significant effects from PRP have also been published. One study claiming that PRP injections did not improve outcomes of chronic Achilles tendinopathy did not differentiate whether patients had tendinosis or tendinitis, and did not consider leukocyte concentration in their PRP preparations27 Another study that determined PRP is not beneficial to the healing of ruptured Achilles tendons after surgical repair also failed to consider the concentration of leukocytes in their PRP preparations.28 One of the difficulties in comparing these studies is their heterogeneous nature. This arises from the use of different conditions in each study that makes the studies incomparable. Variations in PRP preparations lead to different concentrations of growth factors, platelets, and leukocyte concentrations. Additionally, tendinopathy models were not specified as tendinosis and tendonitis, and models or patients were not controlled for age, sex, or comorbidities. Given that leukocyte-rich and leukocyte-poor PRP preparations are currently widely used in clinical practice, the discovery of which type of preparation is indicated in which setting is paramount to evidence-based use of this treatment modality. Due to reports suggesting that leukocytes may be detrimental to tendon healing, determining which types of leukocytes are responsible for these effects is vital. As such, the purpose of this study is to determine the in vivo effects of sub-populations of leukocytes on normal rat tendons. This study design allowed us to isolate the effects of the injections to induce a response and remove confounding effects of normal healing response to a damaged tendon and effects from the injection itself. Our hypothesis was that the injection of leukocytes would cause an inflammatory response in rat tendons, leading to catabolic outcomes.
Continue to: METHODS...
METHODS
This was a prospective, in vivo, placebo controlled, randomized animal study. The University’s Institutional Animal Care and Use Committee approved all procedures prior to initiation. Twenty-four male Sprague-Dawley rats were randomized to 3 treatment groups (n = 8): monocytes; granulocytes, and; plasma, as a negative control.
Allogenic blood from 6 additional rats was collected into K2EDTA tubes via cardiac puncture. Allogenic, as opposed to autogenic, blood is commonly used in rat models because of low immunogenic response to blood from rats of the same strain and litter.29,30 The blood was then pooled and the red cells lysed by incubation with Red Blood Cell Lysis Buffer (Roche). The samples were then sorted into fractions containing monocytes and granulocytes using fluorescence activated cell sorting (FACS) using a FACSAria (BD Biosciences). Cells were sorted using Purified PE Mouse Anti-Rat CD11b/c antibodies (BD Pharmingen) specific to monocytes, granulocytes, macrophages, dendritic cells, and microglia, APC-Cy7 Mouse Anti-Rat CD45 antibodies (BD Pharmingen) specific to all hematopoietic cells except erythrocytes, and FITC Mouse Anti-Rat CD42d antibodies (BD Pharmingen) specific to megakaryocytes and platelets. 20 μL of 0.2 mg/mL CD11b/c, 20 μL of 0.2 mg/mL CD 45, and 10 μL of 0.5 mg/mL CD42d antibodies were added to 1 mL of condensed non-red cells collected from the 6 rats and incubated at room temperature in the dark for 15 minutes. A fraction containing only platelet-poor plasma was also collected. For all treatments the injection volume was 75 μL. Rats in the monocyte group were injected with 200,000 cells in platelet-poor plasma, those in the granulocyte group were injected with 900,000 cells in platelet-poor plasma, and rats in the plasma control group received only platelet-poor plasma. The cell concentrations were based on previous studies that documented these concentrations that are found in typical leukocyte-rich PRP preparations.13
The animals were anesthetized with isoflurane gas and then injected aseptically once into their right Achilles tendon. The left Achilles tendon was used as an un-injected control, giving a total of 48 total Achilles tendons studied. At days 7 and 14 post-injection, 4 rats from each group were euthanized and the Achilles tendons were harvested.
The tendons were fixed in neutral buffered formalin for 24 hours and then embedded in paraffin and sectioned sagittally at 12 μm. The tendons were then stained with hematoxylin and eosin (H&E) using standard histological protocols and examined by 3 individuals trained to assess cellularity and morphology. All samples were assigned unrecognizable numbers and randomized prior to examination by individuals. Cell counts were based on the number of nuclei present in 3 mid-tendon high-power fields (400x) per sample. Morphology was graded on a scale of 1 to 3, with 1 being a normal tendon and 3 having severe pathology with total loss of alignment and crimping on 3 low-power fields (100x) per sample (Figures 1A-1G).
Vascularity was assessed by immunohistochemical staining using Rabbit Polyclonal Anti-CD31 antibodies (Abcam), a marker for vascular endothelial cells, using a Vectastain ABC Kit (Vector Laboratories) system and the ImmPACT AEC Peroxidase (HRP) Substrate (Vector Laboratories). Following staining, automated image analysis was performed (Bioquant). Briefly, all areas that did not contain tendon were masked. CD31 positive areas were then quantified using global thresholding. Vascularity was then calculated as ratio of CD31 positive area to total tendon area. Analyses were performed on 3 mid-tendon medium-power (200x) fields per sample.
For cellularity and morphology, the results for the injected tendons were normalized to those of their contralateral untreated controls and reported as a percentage. Results for vascularity were compared directly between treated tendons. Differences were assessed between groups at each time-point using Independent Samples Median Tests. When significant differences were identified, pairwise comparisons were performed to identify the source of the differences. All analyses were conducted using SPSS (V22, SAS Institute) with significant differences determined for values of P < 0.05.
RESULTS
No significant differences in cellularity between groups were seen at day 7 (P = 0.368) (Figures 1A-1G). However, a significant difference in cellularity between groups was seen at day 14 (P = 0.014). Pairwise tests showed there to be a significant increase in the number of cells in the tendons treated with granulocytes from 221% and 249% in cellularity (P = 0.014) on day 14, as compared to both monocytes and plasma, respectively. Morphologically, no significant differences were seen between groups at either time-point (P = 0.091 for day 7 and P = 1.000 for day 14) (Figures 2A-2G). However, a significant improvement in morphology was observed from day 7 to day 14 in the granulocyte group from 60% to 165% (P = 0.029). Finally, no differences were seen in vascularity between treatment groups at either time-point (P = 0.368 for day 7 and P = 0.535 for day 14) (Figures 3A-3G).
Continue to: DISCUSSION...
DISCUSSION
Our hypothesis that the injection of leukocytes would cause an inflammatory response in rat tendons leading to catabolic outcomes was confirmed in the granulocyte group. It should be noted that prior to the catabolic outcome, there was a transient anabolic effect in the granulocyte group during the second week. Deterioration in morphology was observed in the tendons injected with granulocytes on day 7, which subsequently recovered in the following week. We found that injecting granulocytes into normal tendons resulted in an increase in inflammatory cellularity, when compared to monocytes and plasma injections.
Limitations inherent in this study are those similar to other in vivo studies. To begin with, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 Another limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the immunohistochemistry (IHC) and morphological data are clear, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. The increased cellularity could be due to the increased number of cells injected into the tendon; however, our conclusions are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32
In terms of morphology, we hypothesized that degenerative changes would be seen in the tendons that were injected with granulocytes due to the inflammatory action of these cells. As part of the granulocyte response, neutrophils release proteases and macrophages can stimulate collagen synthesis via fibroblasts, both causing change within the extracellular matrix.33,34 Indeed, we observed a significant change in tissue morphology in the granulocyte group over the course of 14 days. As the degenerative and regenerative effects of granulocytes take time to present, this is likely what we observed to occur between day 7 and 14 after treatment. These observations are also consistent with prior observations that leukocyte-rich PRP injections can be detrimental to tendon healing, but beneficial to tissue degeneration in the setting of chronic tendonitis.20
We hypothesized that the vascularity of the tendons would be similar in all preparations. This was based on previous studies demonstrating that the lack of platelets in the platelet-poor plasma fraction is sufficient to deplete VEGF, the angiogenic agent in PRP.35 In this study, there were no observable differences in vascularity of platelet-poor plasma, monocyte, and granulocyte injections. We attribute this to the lack of VEGF in any of these preparations. The aforementioned study also showed that the lack of platelets in injection was enough to prevent the angiogenic effect of this treatment.35
Continue to: The goal of this study was...
The goal of this study was to assess the morphology, cellularity, and vascularity of normal tendons after injections of different leukocyte populations. This is clinically important because of the potential to tailor future PRP injections on a patient-by-patient basis. In patients requiring an anabolic response, leukocyte-poor PRP may be the best option. In contrast, when patient pathology requires an inflammatory response to improve healing36 or breakdown fibrotic tissue, as seen in tendinosis, leukocyte-rich PRP may be warranted. Further, properly controlled clinical studies are needed to validate these recommendations.
Limitations inherent in this study are those similar to other in vivo studies. First, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 A second limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the IHC and morphological data show clear changes, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. The last limitation of this study is the lack of functional mechanical testing since, clinically, healing of the tendon is also related to the strength of the tendon. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. Moreover, our results are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32 It is interesting to note that the increase in inflammation does not lead to an increase in vascularity as could be expected.
CONCLUSION
We found that the injection of leukocytes into healthy rat Achilles tendons increases inflammation, as evidenced by increased cellularity and disrupted morphology, which suggests that leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis. However, these preparations may be useful for a specific subset of tendinopathies, including chronic tendinosis.
1. Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225-239.
2. Bass E. Tendinopathy: why the difference between tendinitis and tendinosis matters. Int J Ther Massage Bodywork. 2012;5(1):14-17.
3. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
4. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
5. Bahr R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br J Sports Med. 2009;43(13):966-972.
6. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867.
7. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539-1554.
8. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602-608.
9. Smith JW. Blood Supply of Tendons. Am J Surg. 1965;109:272-276.
10. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825-853.
11. Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3(3):226-250.
12. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
13. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316.
14. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742-1749.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271.
16. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212-218.
17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.
18. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344-352.
19. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033-1042.
20. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(141-148).
21. Pillitteri D, Bassus S, Boller K, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18(2):119-127.
22. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42-49.
23. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
24. Maniscalco P, Gambera D, Lunati A, et al. The "Cascade" membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223-226.
25. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909-915.
26. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
27. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
28. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38-47.
29. Welsh KI, Burgos H, Batchelor JR. The immune response to allogeneic rat platelets; Ag-B antigens in matrix form lacking Ia. Eur J Immunol. 1977;7(5):267-272.
30. Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats : relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721-1727.
31. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140.
33. Palmgren MS, deShazo RD, Carter RM, Zimny ML, Shah SV. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J Allergy Clin Immunol. 1992;89(4):905-915.
34. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727-737.
35. Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
36. Su B, O'Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013;115(6):892-899.
ABSTRACT
In this study, we determine the in vivo effects of injecting sub-populations of leukocytes into normal rat Achilles tendons via a controlled laboratory study. Allogenic monocytes, granulocytes, or plasma were injected into 24 healthy rat Achilles tendons. Treated and contralateral un-treated control tendons then assessed for cellularity, histologic morphology, and vascularity after 7 and 14 days. Significant increases of 221% and 249% in cellularity (P = 0.014) were seen on day 14 within Achilles tendons injected with granulocytes as compared to plasma and monocytes, respectively. Also, significant improvement in morphology (P = 0.029) between days 7 and 14 was seen for the granulocyte injected Achilles tendons. Significant increases in cellularity after an injection of granulocytes, compared to monocytes and plasma, corresponds to a significant increase in inflammation within the tissue, suggesting that leukocyte-rich platelet-rich plasma (PRP) preparations are proinflammatory and potentially catabolic when injected into tendon tissue. The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Continue to: Tendinopathies are debilitating conditions...
Tendinopathies are debilitating conditions affecting patients worldwide every day. They arise most frequently from tendon overuse resulting in pathology.1 There are 2 major subtypes of tendinopathy: tendinosis and tendinitis. Tendinosis, the more common condition, is characterized by long-term, chronic degradation of tendon tissue resulting in fibrosis from infiltrating fibroblasts.2 Tendinitis, the less common condition, is characterized by an acute inflammatory response and inflammatory cell infiltrate.2 Both conditions are common, with Achilles tendinopathy affecting 11% of runners and lateral epicondylitis affecting 1% to 3% of the general population.3,4 Many sports-related overuse injuries, such as tendinopathies, go undiagnosed for extended periods of time because medical attention is avoided in order to prevent time loss from training or competing.5 These delays could be eliminated if a non-surgical option for treating tendon pathology was available.
Tendinopathies are believed to result from tendon overuse that causes micro-damage to collagen, as well as from significant changes in protein and enzyme composition within the tendon.6 The damage accumulates over time and eventually leads to chronic inflammation or fibrotic change within tendons, in both cases weakening the tendon and causing pain. Currently, accepted treatments for tendinopathies include: nonsteroidal anti-inflammatory drugs, physical therapy, ultrasound, laser-therapy, corticosteroids, glyceryl trinitrate patches, extracorporeal shock wave therapy, sclerotherapy, and surgery.7 Recently, platelet-rich plasma (PRP) therapy has emerged as a promising treatment for tendinopathies, as well as a variety of other orthopedic indications.
PRP consists of autologous blood from the patient, centrifuged to increase the amount of platelets in the sample above baseline, and subsequently injected around an affected tendon or joint.8 PRP is used to treat tendinopathy because it can supply injured tendons with blood components that aid in healing, which tendons do not receive due to poor vascularity.9 These components include growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor, and leukocytes that can stimulate an inflammatory response within the injured tissue.10 The inflammatory response from the PRP induces a more robust reconstruction and revascularization of the injured tissue, stimulating proliferation, and remodeling.11,12However, significant variability exists within the platelets, leukocytes, and growth factors that comprise PRP. This is attributed to 3 major causes. First, current commercial preparations of PRP result in differing platelet concentrations, as well as leukocyte-rich and leukocyte-poor compositions.13,14 Variability in platelet concentrations results in unreliable amounts of growth factors, including cytokines, TGF-β, PDGF, VEGF and basic fibroblast growth factor in each preparation, while leukocyte levels affect inflammation, all leading to variable effects for each preparation.15,16Second, despite sex and age of the PRP donor not being significant factors influencing variation in growth factor concentrations, the existence of an unexplained variation in concentrations of growth factors between different donors has been observed.17 Third, the selection of activating agents, bovine thrombin or calcium chloride, and their application, whether to the elbow, shoulder, or knee, produces variability.18
While the effects of platelets and growth factors in PRP have been well studied, less is known about the effects of differing cell types. Recently it was reported that the concentrations of leukocytes directly affect the outcomes of PRP injections. McCarrel and colleagues19,20 found that as the number of leukocytes increased, there was a concomitant increase in the expression of inflammatory cytokines and catabolic activity. This effect may result in inferior healing of injured tissues and is attributed to the release of pro-inflammatory cytokines such as interleukin-1β from the leukocytes.21 There is also evidence that minimizing the catabolic effect of leukocytes may be just as important to tissue healing as the maximizing anabolic effect of platelets and growth factors.22
The use of PRP has been highly disputed in recent years due to conflicting reports of its success in treating orthopedic conditions. Numerous favorable studies have shown benefit for treating chronic and acute orthopedic injuries including; rotator cuff tear repair, chronic refractory patellar tendinopathy, and chronic lateral tendinosis/epicondylitis.23-26 Concurrently, articles demonstrating no significant effects from PRP have also been published. One study claiming that PRP injections did not improve outcomes of chronic Achilles tendinopathy did not differentiate whether patients had tendinosis or tendinitis, and did not consider leukocyte concentration in their PRP preparations27 Another study that determined PRP is not beneficial to the healing of ruptured Achilles tendons after surgical repair also failed to consider the concentration of leukocytes in their PRP preparations.28 One of the difficulties in comparing these studies is their heterogeneous nature. This arises from the use of different conditions in each study that makes the studies incomparable. Variations in PRP preparations lead to different concentrations of growth factors, platelets, and leukocyte concentrations. Additionally, tendinopathy models were not specified as tendinosis and tendonitis, and models or patients were not controlled for age, sex, or comorbidities. Given that leukocyte-rich and leukocyte-poor PRP preparations are currently widely used in clinical practice, the discovery of which type of preparation is indicated in which setting is paramount to evidence-based use of this treatment modality. Due to reports suggesting that leukocytes may be detrimental to tendon healing, determining which types of leukocytes are responsible for these effects is vital. As such, the purpose of this study is to determine the in vivo effects of sub-populations of leukocytes on normal rat tendons. This study design allowed us to isolate the effects of the injections to induce a response and remove confounding effects of normal healing response to a damaged tendon and effects from the injection itself. Our hypothesis was that the injection of leukocytes would cause an inflammatory response in rat tendons, leading to catabolic outcomes.
Continue to: METHODS...
METHODS
This was a prospective, in vivo, placebo controlled, randomized animal study. The University’s Institutional Animal Care and Use Committee approved all procedures prior to initiation. Twenty-four male Sprague-Dawley rats were randomized to 3 treatment groups (n = 8): monocytes; granulocytes, and; plasma, as a negative control.
Allogenic blood from 6 additional rats was collected into K2EDTA tubes via cardiac puncture. Allogenic, as opposed to autogenic, blood is commonly used in rat models because of low immunogenic response to blood from rats of the same strain and litter.29,30 The blood was then pooled and the red cells lysed by incubation with Red Blood Cell Lysis Buffer (Roche). The samples were then sorted into fractions containing monocytes and granulocytes using fluorescence activated cell sorting (FACS) using a FACSAria (BD Biosciences). Cells were sorted using Purified PE Mouse Anti-Rat CD11b/c antibodies (BD Pharmingen) specific to monocytes, granulocytes, macrophages, dendritic cells, and microglia, APC-Cy7 Mouse Anti-Rat CD45 antibodies (BD Pharmingen) specific to all hematopoietic cells except erythrocytes, and FITC Mouse Anti-Rat CD42d antibodies (BD Pharmingen) specific to megakaryocytes and platelets. 20 μL of 0.2 mg/mL CD11b/c, 20 μL of 0.2 mg/mL CD 45, and 10 μL of 0.5 mg/mL CD42d antibodies were added to 1 mL of condensed non-red cells collected from the 6 rats and incubated at room temperature in the dark for 15 minutes. A fraction containing only platelet-poor plasma was also collected. For all treatments the injection volume was 75 μL. Rats in the monocyte group were injected with 200,000 cells in platelet-poor plasma, those in the granulocyte group were injected with 900,000 cells in platelet-poor plasma, and rats in the plasma control group received only platelet-poor plasma. The cell concentrations were based on previous studies that documented these concentrations that are found in typical leukocyte-rich PRP preparations.13
The animals were anesthetized with isoflurane gas and then injected aseptically once into their right Achilles tendon. The left Achilles tendon was used as an un-injected control, giving a total of 48 total Achilles tendons studied. At days 7 and 14 post-injection, 4 rats from each group were euthanized and the Achilles tendons were harvested.
The tendons were fixed in neutral buffered formalin for 24 hours and then embedded in paraffin and sectioned sagittally at 12 μm. The tendons were then stained with hematoxylin and eosin (H&E) using standard histological protocols and examined by 3 individuals trained to assess cellularity and morphology. All samples were assigned unrecognizable numbers and randomized prior to examination by individuals. Cell counts were based on the number of nuclei present in 3 mid-tendon high-power fields (400x) per sample. Morphology was graded on a scale of 1 to 3, with 1 being a normal tendon and 3 having severe pathology with total loss of alignment and crimping on 3 low-power fields (100x) per sample (Figures 1A-1G).
Vascularity was assessed by immunohistochemical staining using Rabbit Polyclonal Anti-CD31 antibodies (Abcam), a marker for vascular endothelial cells, using a Vectastain ABC Kit (Vector Laboratories) system and the ImmPACT AEC Peroxidase (HRP) Substrate (Vector Laboratories). Following staining, automated image analysis was performed (Bioquant). Briefly, all areas that did not contain tendon were masked. CD31 positive areas were then quantified using global thresholding. Vascularity was then calculated as ratio of CD31 positive area to total tendon area. Analyses were performed on 3 mid-tendon medium-power (200x) fields per sample.
For cellularity and morphology, the results for the injected tendons were normalized to those of their contralateral untreated controls and reported as a percentage. Results for vascularity were compared directly between treated tendons. Differences were assessed between groups at each time-point using Independent Samples Median Tests. When significant differences were identified, pairwise comparisons were performed to identify the source of the differences. All analyses were conducted using SPSS (V22, SAS Institute) with significant differences determined for values of P < 0.05.
RESULTS
No significant differences in cellularity between groups were seen at day 7 (P = 0.368) (Figures 1A-1G). However, a significant difference in cellularity between groups was seen at day 14 (P = 0.014). Pairwise tests showed there to be a significant increase in the number of cells in the tendons treated with granulocytes from 221% and 249% in cellularity (P = 0.014) on day 14, as compared to both monocytes and plasma, respectively. Morphologically, no significant differences were seen between groups at either time-point (P = 0.091 for day 7 and P = 1.000 for day 14) (Figures 2A-2G). However, a significant improvement in morphology was observed from day 7 to day 14 in the granulocyte group from 60% to 165% (P = 0.029). Finally, no differences were seen in vascularity between treatment groups at either time-point (P = 0.368 for day 7 and P = 0.535 for day 14) (Figures 3A-3G).
Continue to: DISCUSSION...
DISCUSSION
Our hypothesis that the injection of leukocytes would cause an inflammatory response in rat tendons leading to catabolic outcomes was confirmed in the granulocyte group. It should be noted that prior to the catabolic outcome, there was a transient anabolic effect in the granulocyte group during the second week. Deterioration in morphology was observed in the tendons injected with granulocytes on day 7, which subsequently recovered in the following week. We found that injecting granulocytes into normal tendons resulted in an increase in inflammatory cellularity, when compared to monocytes and plasma injections.
Limitations inherent in this study are those similar to other in vivo studies. To begin with, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 Another limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the immunohistochemistry (IHC) and morphological data are clear, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. The increased cellularity could be due to the increased number of cells injected into the tendon; however, our conclusions are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32
In terms of morphology, we hypothesized that degenerative changes would be seen in the tendons that were injected with granulocytes due to the inflammatory action of these cells. As part of the granulocyte response, neutrophils release proteases and macrophages can stimulate collagen synthesis via fibroblasts, both causing change within the extracellular matrix.33,34 Indeed, we observed a significant change in tissue morphology in the granulocyte group over the course of 14 days. As the degenerative and regenerative effects of granulocytes take time to present, this is likely what we observed to occur between day 7 and 14 after treatment. These observations are also consistent with prior observations that leukocyte-rich PRP injections can be detrimental to tendon healing, but beneficial to tissue degeneration in the setting of chronic tendonitis.20
We hypothesized that the vascularity of the tendons would be similar in all preparations. This was based on previous studies demonstrating that the lack of platelets in the platelet-poor plasma fraction is sufficient to deplete VEGF, the angiogenic agent in PRP.35 In this study, there were no observable differences in vascularity of platelet-poor plasma, monocyte, and granulocyte injections. We attribute this to the lack of VEGF in any of these preparations. The aforementioned study also showed that the lack of platelets in injection was enough to prevent the angiogenic effect of this treatment.35
Continue to: The goal of this study was...
The goal of this study was to assess the morphology, cellularity, and vascularity of normal tendons after injections of different leukocyte populations. This is clinically important because of the potential to tailor future PRP injections on a patient-by-patient basis. In patients requiring an anabolic response, leukocyte-poor PRP may be the best option. In contrast, when patient pathology requires an inflammatory response to improve healing36 or breakdown fibrotic tissue, as seen in tendinosis, leukocyte-rich PRP may be warranted. Further, properly controlled clinical studies are needed to validate these recommendations.
Limitations inherent in this study are those similar to other in vivo studies. First, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 A second limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the IHC and morphological data show clear changes, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. The last limitation of this study is the lack of functional mechanical testing since, clinically, healing of the tendon is also related to the strength of the tendon. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. Moreover, our results are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32 It is interesting to note that the increase in inflammation does not lead to an increase in vascularity as could be expected.
CONCLUSION
We found that the injection of leukocytes into healthy rat Achilles tendons increases inflammation, as evidenced by increased cellularity and disrupted morphology, which suggests that leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis. However, these preparations may be useful for a specific subset of tendinopathies, including chronic tendinosis.
ABSTRACT
In this study, we determine the in vivo effects of injecting sub-populations of leukocytes into normal rat Achilles tendons via a controlled laboratory study. Allogenic monocytes, granulocytes, or plasma were injected into 24 healthy rat Achilles tendons. Treated and contralateral un-treated control tendons then assessed for cellularity, histologic morphology, and vascularity after 7 and 14 days. Significant increases of 221% and 249% in cellularity (P = 0.014) were seen on day 14 within Achilles tendons injected with granulocytes as compared to plasma and monocytes, respectively. Also, significant improvement in morphology (P = 0.029) between days 7 and 14 was seen for the granulocyte injected Achilles tendons. Significant increases in cellularity after an injection of granulocytes, compared to monocytes and plasma, corresponds to a significant increase in inflammation within the tissue, suggesting that leukocyte-rich platelet-rich plasma (PRP) preparations are proinflammatory and potentially catabolic when injected into tendon tissue. The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Continue to: Tendinopathies are debilitating conditions...
Tendinopathies are debilitating conditions affecting patients worldwide every day. They arise most frequently from tendon overuse resulting in pathology.1 There are 2 major subtypes of tendinopathy: tendinosis and tendinitis. Tendinosis, the more common condition, is characterized by long-term, chronic degradation of tendon tissue resulting in fibrosis from infiltrating fibroblasts.2 Tendinitis, the less common condition, is characterized by an acute inflammatory response and inflammatory cell infiltrate.2 Both conditions are common, with Achilles tendinopathy affecting 11% of runners and lateral epicondylitis affecting 1% to 3% of the general population.3,4 Many sports-related overuse injuries, such as tendinopathies, go undiagnosed for extended periods of time because medical attention is avoided in order to prevent time loss from training or competing.5 These delays could be eliminated if a non-surgical option for treating tendon pathology was available.
Tendinopathies are believed to result from tendon overuse that causes micro-damage to collagen, as well as from significant changes in protein and enzyme composition within the tendon.6 The damage accumulates over time and eventually leads to chronic inflammation or fibrotic change within tendons, in both cases weakening the tendon and causing pain. Currently, accepted treatments for tendinopathies include: nonsteroidal anti-inflammatory drugs, physical therapy, ultrasound, laser-therapy, corticosteroids, glyceryl trinitrate patches, extracorporeal shock wave therapy, sclerotherapy, and surgery.7 Recently, platelet-rich plasma (PRP) therapy has emerged as a promising treatment for tendinopathies, as well as a variety of other orthopedic indications.
PRP consists of autologous blood from the patient, centrifuged to increase the amount of platelets in the sample above baseline, and subsequently injected around an affected tendon or joint.8 PRP is used to treat tendinopathy because it can supply injured tendons with blood components that aid in healing, which tendons do not receive due to poor vascularity.9 These components include growth factors, such as platelet derived growth factor (PDGF), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), endothelial growth factor, and leukocytes that can stimulate an inflammatory response within the injured tissue.10 The inflammatory response from the PRP induces a more robust reconstruction and revascularization of the injured tissue, stimulating proliferation, and remodeling.11,12However, significant variability exists within the platelets, leukocytes, and growth factors that comprise PRP. This is attributed to 3 major causes. First, current commercial preparations of PRP result in differing platelet concentrations, as well as leukocyte-rich and leukocyte-poor compositions.13,14 Variability in platelet concentrations results in unreliable amounts of growth factors, including cytokines, TGF-β, PDGF, VEGF and basic fibroblast growth factor in each preparation, while leukocyte levels affect inflammation, all leading to variable effects for each preparation.15,16Second, despite sex and age of the PRP donor not being significant factors influencing variation in growth factor concentrations, the existence of an unexplained variation in concentrations of growth factors between different donors has been observed.17 Third, the selection of activating agents, bovine thrombin or calcium chloride, and their application, whether to the elbow, shoulder, or knee, produces variability.18
While the effects of platelets and growth factors in PRP have been well studied, less is known about the effects of differing cell types. Recently it was reported that the concentrations of leukocytes directly affect the outcomes of PRP injections. McCarrel and colleagues19,20 found that as the number of leukocytes increased, there was a concomitant increase in the expression of inflammatory cytokines and catabolic activity. This effect may result in inferior healing of injured tissues and is attributed to the release of pro-inflammatory cytokines such as interleukin-1β from the leukocytes.21 There is also evidence that minimizing the catabolic effect of leukocytes may be just as important to tissue healing as the maximizing anabolic effect of platelets and growth factors.22
The use of PRP has been highly disputed in recent years due to conflicting reports of its success in treating orthopedic conditions. Numerous favorable studies have shown benefit for treating chronic and acute orthopedic injuries including; rotator cuff tear repair, chronic refractory patellar tendinopathy, and chronic lateral tendinosis/epicondylitis.23-26 Concurrently, articles demonstrating no significant effects from PRP have also been published. One study claiming that PRP injections did not improve outcomes of chronic Achilles tendinopathy did not differentiate whether patients had tendinosis or tendinitis, and did not consider leukocyte concentration in their PRP preparations27 Another study that determined PRP is not beneficial to the healing of ruptured Achilles tendons after surgical repair also failed to consider the concentration of leukocytes in their PRP preparations.28 One of the difficulties in comparing these studies is their heterogeneous nature. This arises from the use of different conditions in each study that makes the studies incomparable. Variations in PRP preparations lead to different concentrations of growth factors, platelets, and leukocyte concentrations. Additionally, tendinopathy models were not specified as tendinosis and tendonitis, and models or patients were not controlled for age, sex, or comorbidities. Given that leukocyte-rich and leukocyte-poor PRP preparations are currently widely used in clinical practice, the discovery of which type of preparation is indicated in which setting is paramount to evidence-based use of this treatment modality. Due to reports suggesting that leukocytes may be detrimental to tendon healing, determining which types of leukocytes are responsible for these effects is vital. As such, the purpose of this study is to determine the in vivo effects of sub-populations of leukocytes on normal rat tendons. This study design allowed us to isolate the effects of the injections to induce a response and remove confounding effects of normal healing response to a damaged tendon and effects from the injection itself. Our hypothesis was that the injection of leukocytes would cause an inflammatory response in rat tendons, leading to catabolic outcomes.
Continue to: METHODS...
METHODS
This was a prospective, in vivo, placebo controlled, randomized animal study. The University’s Institutional Animal Care and Use Committee approved all procedures prior to initiation. Twenty-four male Sprague-Dawley rats were randomized to 3 treatment groups (n = 8): monocytes; granulocytes, and; plasma, as a negative control.
Allogenic blood from 6 additional rats was collected into K2EDTA tubes via cardiac puncture. Allogenic, as opposed to autogenic, blood is commonly used in rat models because of low immunogenic response to blood from rats of the same strain and litter.29,30 The blood was then pooled and the red cells lysed by incubation with Red Blood Cell Lysis Buffer (Roche). The samples were then sorted into fractions containing monocytes and granulocytes using fluorescence activated cell sorting (FACS) using a FACSAria (BD Biosciences). Cells were sorted using Purified PE Mouse Anti-Rat CD11b/c antibodies (BD Pharmingen) specific to monocytes, granulocytes, macrophages, dendritic cells, and microglia, APC-Cy7 Mouse Anti-Rat CD45 antibodies (BD Pharmingen) specific to all hematopoietic cells except erythrocytes, and FITC Mouse Anti-Rat CD42d antibodies (BD Pharmingen) specific to megakaryocytes and platelets. 20 μL of 0.2 mg/mL CD11b/c, 20 μL of 0.2 mg/mL CD 45, and 10 μL of 0.5 mg/mL CD42d antibodies were added to 1 mL of condensed non-red cells collected from the 6 rats and incubated at room temperature in the dark for 15 minutes. A fraction containing only platelet-poor plasma was also collected. For all treatments the injection volume was 75 μL. Rats in the monocyte group were injected with 200,000 cells in platelet-poor plasma, those in the granulocyte group were injected with 900,000 cells in platelet-poor plasma, and rats in the plasma control group received only platelet-poor plasma. The cell concentrations were based on previous studies that documented these concentrations that are found in typical leukocyte-rich PRP preparations.13
The animals were anesthetized with isoflurane gas and then injected aseptically once into their right Achilles tendon. The left Achilles tendon was used as an un-injected control, giving a total of 48 total Achilles tendons studied. At days 7 and 14 post-injection, 4 rats from each group were euthanized and the Achilles tendons were harvested.
The tendons were fixed in neutral buffered formalin for 24 hours and then embedded in paraffin and sectioned sagittally at 12 μm. The tendons were then stained with hematoxylin and eosin (H&E) using standard histological protocols and examined by 3 individuals trained to assess cellularity and morphology. All samples were assigned unrecognizable numbers and randomized prior to examination by individuals. Cell counts were based on the number of nuclei present in 3 mid-tendon high-power fields (400x) per sample. Morphology was graded on a scale of 1 to 3, with 1 being a normal tendon and 3 having severe pathology with total loss of alignment and crimping on 3 low-power fields (100x) per sample (Figures 1A-1G).
Vascularity was assessed by immunohistochemical staining using Rabbit Polyclonal Anti-CD31 antibodies (Abcam), a marker for vascular endothelial cells, using a Vectastain ABC Kit (Vector Laboratories) system and the ImmPACT AEC Peroxidase (HRP) Substrate (Vector Laboratories). Following staining, automated image analysis was performed (Bioquant). Briefly, all areas that did not contain tendon were masked. CD31 positive areas were then quantified using global thresholding. Vascularity was then calculated as ratio of CD31 positive area to total tendon area. Analyses were performed on 3 mid-tendon medium-power (200x) fields per sample.
For cellularity and morphology, the results for the injected tendons were normalized to those of their contralateral untreated controls and reported as a percentage. Results for vascularity were compared directly between treated tendons. Differences were assessed between groups at each time-point using Independent Samples Median Tests. When significant differences were identified, pairwise comparisons were performed to identify the source of the differences. All analyses were conducted using SPSS (V22, SAS Institute) with significant differences determined for values of P < 0.05.
RESULTS
No significant differences in cellularity between groups were seen at day 7 (P = 0.368) (Figures 1A-1G). However, a significant difference in cellularity between groups was seen at day 14 (P = 0.014). Pairwise tests showed there to be a significant increase in the number of cells in the tendons treated with granulocytes from 221% and 249% in cellularity (P = 0.014) on day 14, as compared to both monocytes and plasma, respectively. Morphologically, no significant differences were seen between groups at either time-point (P = 0.091 for day 7 and P = 1.000 for day 14) (Figures 2A-2G). However, a significant improvement in morphology was observed from day 7 to day 14 in the granulocyte group from 60% to 165% (P = 0.029). Finally, no differences were seen in vascularity between treatment groups at either time-point (P = 0.368 for day 7 and P = 0.535 for day 14) (Figures 3A-3G).
Continue to: DISCUSSION...
DISCUSSION
Our hypothesis that the injection of leukocytes would cause an inflammatory response in rat tendons leading to catabolic outcomes was confirmed in the granulocyte group. It should be noted that prior to the catabolic outcome, there was a transient anabolic effect in the granulocyte group during the second week. Deterioration in morphology was observed in the tendons injected with granulocytes on day 7, which subsequently recovered in the following week. We found that injecting granulocytes into normal tendons resulted in an increase in inflammatory cellularity, when compared to monocytes and plasma injections.
Limitations inherent in this study are those similar to other in vivo studies. To begin with, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 Another limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the immunohistochemistry (IHC) and morphological data are clear, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. The increased cellularity could be due to the increased number of cells injected into the tendon; however, our conclusions are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32
In terms of morphology, we hypothesized that degenerative changes would be seen in the tendons that were injected with granulocytes due to the inflammatory action of these cells. As part of the granulocyte response, neutrophils release proteases and macrophages can stimulate collagen synthesis via fibroblasts, both causing change within the extracellular matrix.33,34 Indeed, we observed a significant change in tissue morphology in the granulocyte group over the course of 14 days. As the degenerative and regenerative effects of granulocytes take time to present, this is likely what we observed to occur between day 7 and 14 after treatment. These observations are also consistent with prior observations that leukocyte-rich PRP injections can be detrimental to tendon healing, but beneficial to tissue degeneration in the setting of chronic tendonitis.20
We hypothesized that the vascularity of the tendons would be similar in all preparations. This was based on previous studies demonstrating that the lack of platelets in the platelet-poor plasma fraction is sufficient to deplete VEGF, the angiogenic agent in PRP.35 In this study, there were no observable differences in vascularity of platelet-poor plasma, monocyte, and granulocyte injections. We attribute this to the lack of VEGF in any of these preparations. The aforementioned study also showed that the lack of platelets in injection was enough to prevent the angiogenic effect of this treatment.35
Continue to: The goal of this study was...
The goal of this study was to assess the morphology, cellularity, and vascularity of normal tendons after injections of different leukocyte populations. This is clinically important because of the potential to tailor future PRP injections on a patient-by-patient basis. In patients requiring an anabolic response, leukocyte-poor PRP may be the best option. In contrast, when patient pathology requires an inflammatory response to improve healing36 or breakdown fibrotic tissue, as seen in tendinosis, leukocyte-rich PRP may be warranted. Further, properly controlled clinical studies are needed to validate these recommendations.
Limitations inherent in this study are those similar to other in vivo studies. First, the results of injections into rat tendons may not be translatable to human tendons. Despite this limitation, the rat is a common model for tendon research.31 A second limitation is that this study injected healthy Achilles tendons, rather than tendons with preexisting tendinopathy. In a naturally occurring tendinopathy, there may be other factors present that interact with PRP, and this model negates the contribution of these factors. Finally, while the IHC and morphological data show clear changes, the cellularity data are not clear in identifying the type of cells that were increased by granulocyte injection. However, the cells appeared rounded, resembling inflammatory infiltrate; a common cell type seen in tendons.2 While fibroblasts are also a common infiltrate during chronic tendinopathy, they are generally flat and appear on H&E as long spindle shaped cells. The last limitation of this study is the lack of functional mechanical testing since, clinically, healing of the tendon is also related to the strength of the tendon. Thus, we believe the increased cellularity of the tendons after granulocyte injections is representative of an increase in inflammation. Moreover, our results are consistent with the increased inflammation previously reported linking leukocytes to tendon inflammation.20,22,32 It is interesting to note that the increase in inflammation does not lead to an increase in vascularity as could be expected.
CONCLUSION
We found that the injection of leukocytes into healthy rat Achilles tendons increases inflammation, as evidenced by increased cellularity and disrupted morphology, which suggests that leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis. However, these preparations may be useful for a specific subset of tendinopathies, including chronic tendinosis.
1. Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225-239.
2. Bass E. Tendinopathy: why the difference between tendinitis and tendinosis matters. Int J Ther Massage Bodywork. 2012;5(1):14-17.
3. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
4. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
5. Bahr R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br J Sports Med. 2009;43(13):966-972.
6. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867.
7. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539-1554.
8. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602-608.
9. Smith JW. Blood Supply of Tendons. Am J Surg. 1965;109:272-276.
10. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825-853.
11. Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3(3):226-250.
12. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
13. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316.
14. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742-1749.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271.
16. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212-218.
17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.
18. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344-352.
19. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033-1042.
20. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(141-148).
21. Pillitteri D, Bassus S, Boller K, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18(2):119-127.
22. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42-49.
23. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
24. Maniscalco P, Gambera D, Lunati A, et al. The "Cascade" membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223-226.
25. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909-915.
26. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
27. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
28. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38-47.
29. Welsh KI, Burgos H, Batchelor JR. The immune response to allogeneic rat platelets; Ag-B antigens in matrix form lacking Ia. Eur J Immunol. 1977;7(5):267-272.
30. Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats : relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721-1727.
31. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140.
33. Palmgren MS, deShazo RD, Carter RM, Zimny ML, Shah SV. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J Allergy Clin Immunol. 1992;89(4):905-915.
34. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727-737.
35. Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
36. Su B, O'Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013;115(6):892-899.
1. Herring SA, Nilson KL. Introduction to overuse injuries. Clin Sports Med. 1987;6(2):225-239.
2. Bass E. Tendinopathy: why the difference between tendinitis and tendinosis matters. Int J Ther Massage Bodywork. 2012;5(1):14-17.
3. James SL, Bates BT, Osternig LR. Injuries to runners. Am J Sports Med. 1978;6(2):40-50.
4. Allander E. Prevalence, incidence, and remission rates of some common rheumatic diseases or syndromes. Scand J Rheumatol. 1974;3(3):145-153.
5. Bahr R. No injuries, but plenty of pain? On the methodology for recording overuse symptoms in sports. Br J Sports Med. 2009;43(13):966-972.
6. Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med. 2009;37(9):1855-1867.
7. Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539-1554.
8. Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17(10):602-608.
9. Smith JW. Blood Supply of Tendons. Am J Surg. 1965;109:272-276.
10. Wu PI, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825-853.
11. Nguyen RT, Borg-Stein J, McInnis K. Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R. 2011;3(3):226-250.
12. Broughton G 2nd, Janis JE, Attinger CE. Wound healing: an overview. Plast Reconstr Surg. 2006;117(7 Suppl):1e-S-32e-S.
13. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316.
14. Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742-1749.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271.
16. Cho HS, Song IH, Park SY, Sung MC, Ahn MW, Song KE. Individual variation in growth factor concentrations in platelet-rich plasma and its influence on human mesenchymal stem cells. Korean J Lab Med. 2011;31(3):212-218.
17. Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97-102.
18. Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344-352.
19. McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res. 2009;27(8):1033-1042.
20. McCarrel TM, Minas T, Fortier LA. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J Bone Joint Surg Am. 2012;94(19):e143(141-148).
21. Pillitteri D, Bassus S, Boller K, et al. Thrombin-induced interleukin 1beta synthesis in platelet suspensions: impact of contaminating leukocytes. Platelets. 2007;18(2):119-127.
22. Boswell SG, Schnabel LV, Mohammed HO, Sundman EA, Minas T, Fortier LA. Increasing platelet concentrations in leukocyte-reduced platelet-rich plasma decrease collagen gene synthesis in tendons. Am J Sports Med. 2014;42(1):42-49.
23. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
24. Maniscalco P, Gambera D, Lunati A, et al. The "Cascade" membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed. 2008;79(3):223-226.
25. Filardo G, Kon E, Della Villa S, Vincentelli F, Fornasari PM, Marcacci M. Use of platelet-rich plasma for the treatment of refractory jumper's knee. Int Orthop. 2010;34(6):909-915.
26. Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
27. de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149.
28. Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human achilles tendon ruptures: a randomized single-blind study. Am J Sports Med. 2011;39(1):38-47.
29. Welsh KI, Burgos H, Batchelor JR. The immune response to allogeneic rat platelets; Ag-B antigens in matrix form lacking Ia. Eur J Immunol. 1977;7(5):267-272.
30. Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats : relationship between blood fractions and brain cell death. Stroke. 2000;31(7):1721-1727.
31. Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annu Rev Biomed Eng. 2012;14:47-71.
32. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140.
33. Palmgren MS, deShazo RD, Carter RM, Zimny ML, Shah SV. Mechanisms of neutrophil damage to human alveolar extracellular matrix: the role of serine and metalloproteases. J Allergy Clin Immunol. 1992;89(4):905-915.
34. Khalil N, Bereznay O, Sporn M, Greenberg AH. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989;170(3):727-737.
35. Zhou Y, Zhang J, Wu H, Hogan MV, Wang JH. The differential effects of leukocyte-containing and pure platelet-rich plasma (PRP) on tendon stem/progenitor cells - implications of PRP application for the clinical treatment of tendon injuries. Stem Cell Res Ther. 2015;6:173.
36. Su B, O'Connor JP. NSAID therapy effects on healing of bone, tendon, and the enthesis. J Appl Physiol (1985). 2013;115(6):892-899.
TAKE-HOME POINTS
- Injection of leukocytes into healthy rat Achilles tendons increases inflammation.
- Injection of leukocytes into healthy rat Achilles tendons does not affect vascularity.
- Leukocyte-rich PRP preparations may be contraindicated in settings of acute tendonitis.
- Leukocyte-rich PRP preparations may be useful for chronic tendinosis.
- The concentration and composition of white blood cells within PRP preparations is variable and needs to be better understood in order to optimize clinical utility of PRP injections.
Timing of Adverse Events Following Geriatric Hip Fracture Surgery: A Study of 19,873 Patients in the American College of Surgeons National Surgical Quality Improvement Program
ABSTRACT
This study uses a prospective surgical registry to characterize the timing of 10 postoperative adverse events following geriatric hip fracture surgery. There were 19,873 patients identified who were ≥70 years undergoing surgery for hip fracture as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). The median postoperative day of diagnosis (and interquartile range) for myocardial infarction was 3 (1-5), cardiac arrest requiring cardiopulmonary resuscitation 3 (0-8), stroke 3 (1-10), pneumonia 4 (2-10), pulmonary embolism 4 (2-11), urinary tract infection 7 (2-13), deep vein thrombosis 9 (4-16), sepsis 9 (4-18), mortality 11 (6-19), and surgical site infection 16 (11-22). For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30. Findings help to enable more targeted clinical surveillance, inform patient counseling, and determine the duration of follow-up required to study specific adverse events effectively. Orthopedic surgeons should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Continue to: Geriatric hip fracture surgery is associated with...
Geriatric hip fracture surgery is associated with a higher rate of occurrence of postoperative adverse events than any other commonly performed orthopedic procedure.1-4 Indeed, the 90-day mortality rate following a geriatric hip fracture surgery may be as high as 15%2 and the 30-day morbidity rate as high as 30%.3 Furthermore, more than half of postoperative mortalities following orthopedic procedures occur after surgery for hip fracture.4 Therefore, extensive research has been conducted regarding interventions to reduce the rates of adverse events following a hip fracture surgery.5-12 For example, randomized trials have been conducted involving venous thromboembolism prophylaxis,5,6nutritional supplementation,7 delirium prevention,8-10 anemia correction,11 geriatrics consultation,9 and anesthetic technique.12
Despite these extensive research efforts, there is currently little information in the literature regarding when postoperative adverse events occur. A clear depiction of the timing of adverse events could help target clinical surveillance, inform patient counseling, and determine the duration of follow-up required for studies. The reason that the timing of adverse events has not been previously characterized may be that the sample sizes available through standard single- or multi-institutional studies may be insufficient to accurately characterize the timing of rare adverse events (eg, myocardial infarction, stroke, etc.). Moreover, although administrative datasets have become common data sources for investigation of rare postoperative adverse events,13-16 such data sources often do not contain data on the timing of diagnosis.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a relatively new and growing surgical registry.1,3,13-22 The registry follows up patients undergoing surgical procedures at several hundred community and academic institutions nationwide. Unlike the administrative datasets discussed above, the ACS-NSQIP characterizes the postoperative day of diagnosis of well-defined adverse events during the first 30 postoperative days.22
In this study, data collected by the ACS-NSQIP are used to characterize the timing of 10 specific postoperative adverse events following a geriatric hip fracture surgery.
Continue to: METHODS...
METHODS
A retrospective analysis of data collected prospectively through the ACS-NSQIP was conducted. Geriatric patients who underwent hip fracture surgery during 2010 to 2013 were identified. Specific inclusion criteria were (1) International Classification of Diseases, Ninth Revision, diagnosis code 820, (2) primary Current Procedural Terminology codes 27125, 27130, 27235, 27236, 27244, or 27245, and (3) age ≥70 years.
The ACS-NSQIP captures patient demographic, comorbidity, and procedural characteristics at baseline.22 At the end of the 30-day follow-up period, the ACS-NSQIP personnel review both inpatient and outpatient charts to characterize the occurrence vs nonoccurrence of specific postoperative adverse events.22-25 When an adverse event does occur, the postoperative day of diagnosis is recorded.
For this study, the following adverse event categories were investigated: myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, pulmonary embolism, urinary tract infection, deep vein thrombosis, sepsis (either with or without shock), mortality, and surgical site infection (including superficial surgical site infection, deep surgical site infection, and organ or space surgical site infection). Detailed definitions of each adverse event are provided in ACS-NSQIP materials.22
First, the 30-day incidence (and the associated 95% confidence interval) was determined for each adverse event. Second, the median postoperative day of diagnosis (and the associated interquartile range) was determined for each adverse event. Third, the postoperative length of stay was used to estimate the proportion of diagnoses occurring prior to vs following discharge for each adverse event. Finally, multivariate Cox proportional hazards models were used to identify independent risk factors for earlier occurrence of postoperative adverse events. The final models were selected using a backward stepwise process that sequentially eliminated variables with the weakest associations until all variables had P < .05.
Because the ACS-NSQIP reports timing data in calendar days, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, it was not possible to ascertain whether the diagnosis occurred prior to or following discharge. For this study, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, the adverse event was considered to have been diagnosed following discharge. The rationale for this is that for most of the adverse events, it was thought to be unlikely that an inpatient would be discharged before the end of the same day as an inpatient diagnosis. However, there was one exception to this rule; when the postoperative day of discharge, the postoperative length of stay, and the postoperative day of death were all equivalent, the adverse event was considered to have occurred prior to discharge. This is because when a patient dies during the initial inpatient stay, the ACS-NSQIP considers the postoperative length of stay to be equivalent to the postoperative day of death. This makes it much more likely that a diagnosis on the final hospital day had occurred in a patient who had not been discharged.
The mandatory ACS-NSQIP statement is “The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”26
Continue to: RESULTS...
RESULTS
In total, 19,873 geriatric patients undergoing a hip fracture surgery were identified (Table 1). The rates of adverse events ranged from 6.7% for urinary tract infection to 0.6% for pulmonary embolism (Table 2).
Table 1. Patient Population
| Number | Percent |
Total | 19,873 | 100.0% |
Age |
|
|
70-74 years | 1852 | 9.3% |
75-79 years | 2764 | 13.9% |
80-84 years | 4328 | 21.8% |
85-89 years | 5525 | 27.8% |
≥90 years | 5404 | 27.2% |
Sex |
|
|
Male | 5359 | 27.0% |
Female | 14,514 | 73.0% |
Body mass index |
|
|
<30 kg/m2 | 17,733 | 89.2% |
≥30 kg/m2 | 2140 | 10.8% |
Functional status |
|
|
Independent | 14,348 | 72.2% |
Dependent | 5525 | 27.8% |
Diabetes | 3321 | 16.7% |
Congestive heart failure | 738 | 3.7% |
Dyspnea on exertion | 1542 | 7.8% |
Hypertension | 14,265 | 71.8% |
End-stage renal disease | 322 | 1.6% |
COPD | 2239 | 11.3% |
Current smoker | 1506 | 7.6% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2. Patients with Adverse Events Diagnosed During the First 30 postoperative days (N = 19,873)
Adverse Event | Number | Percent | 95% CI |
Urinary tract infection | 1321 | 6.7% | 6.3%-7.0% |
Mortality | 1240 | 6.2% | 5.9%-6.6% |
Pneumonia | 771 | 3.9% | 3.6%-4.2% |
Sepsis | 428 | 2.2% | 2.0%-2.4% |
Myocardial infarction | 347 | 1.8% | 1.6%-1.9% |
Surgical site infection | 247 | 1.2% | 1.1%-1.4% |
Deep vein thrombosis | 199 | 1.0% | 0.9%-1.1% |
Stroke | 144 | 0.7% | 0.6%-0.8% |
Cardiac arrest | 136 | 0.7% | 0.6%-0.8% |
Pulmonary embolism | 126 | 0.6% | 0.5%-0.7% |
Abbreviation: CI, confidence interval.
Figure 1 depicts the timing of postoperative adverse events in detail in histograms and timing curves. For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
Figure 2 provides the summary statistics for adverse events diagnosed in the first 30 postoperative days. The median postoperative day of diagnosis (and the interquartile range) was 3 (1-5) for myocardial infarction, 3 (0-8) for cardiac arrest requiring cardiopulmonary resuscitation, 3 (1-10) for stroke, 4 (2-10) for pneumonia, 4 (2-11) for pulmonary embolism, 7 (2-13) for urinary tract infection, 9 (4-16) for deep vein thrombosis, 9 (4-18) for sepsis, 11 (6-19) for mortality, and 16 (11-22) for surgical site infection.
Figure 3 depicts the timing of adverse events relative to discharge. The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
Table 3 shows the independent risk factors for earlier occurrence of adverse events. Following multivariate stepwise selection of final models, at least 1 patient characteristic was independently associated with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death. In contrast, no patient characteristics were independently associated with the timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, and surgical site infection.
Table 3. Timing of Diagnosis of Adverse Eventsa
Adverse events and associated baseline characteristic(s) | Median postoperative day of diagnosis with vs without baseline characteristic | P-valueb |
Cardiac arrest |
|
|
End-stage renal disease | 1 vs 3 | .005 |
Stroke |
|
|
Hypertension | 4 vs 2 | .025 |
Dependent functional status | 2 vs 4 | .027 |
Urinary tract infection |
|
|
Female sex | 6 vs 8 | .009 |
Deep vein thrombosis |
|
|
Body mass index ≥30 kg/m2 | 5 vs 10 | .015 |
Death |
|
|
End-stage renal disease | 10 vs 11 | .031 |
aBaseline characteristics that were independently associated with the timing of each adverse event were identified through a backwards stepwise selection process initially including all characteristics listed in Table 1, and sequentially excluding characteristics with the weakest associations until only characteristics with P < .05 remained. Independent associations with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death are shown. There were no characteristics independently associated with timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, or surgical site infection; hence, these adverse events are not listed in the table.
bFrom final Cox proportional hazards models identified through multivariate stepwise selection.
Continue to: DISCUSSION...
DISCUSSION
Adverse events are extremely common following a geriatric hip fracture surgery.1-4 Despite extensive investigation regarding methods to prevent these events,5-12 there is limited published description of the timing at which such events occur. This study used a large prospectively followed up cohort of geriatric patients undergoing a hip fracture surgery to deliver a better description of the timing of adverse events than was previously available. The findings of this study should enable more targeted clinical surveillance, inform patient counseling, and help determine the duration of follow-up required for studies on adverse events.
There was wide variability in the timing at which the different postoperative adverse events were diagnosed (Figures 1, 2). Myocardial infarction was diagnosed the earliest, with more than three-fourth of diagnoses in the first postoperative week. Other relatively early-diagnosed adverse events included cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, and pulmonary embolism.
The latest-diagnosed adverse event was surgical site infection (Figures 1, 2). Surgical site infection was actually the only adverse event with a rate of diagnosis during the first week that was lower than the rate of diagnosis later in the month (as can be seen by the inflection in the timing curve for surgical site infection in Figure 1). Mortality showed a relatively consistent rate of diagnosis throughout the entire first postoperative month. Other relatively late-diagnosed postoperative events, including sepsis, deep vein thrombosis, and urinary tract infection, showed varying degrees of decreased rate of diagnosis near the end of the first postoperative month. Of note, for the later-diagnosed adverse events, the estimated median and interquartile ranges (Figure 2) were presumably quite biased toward earlier diagnosis, as the 30-day follow-up period clearly failed to capture a large proportion of later-occurring adverse events (Figure 1).
Certain risk factors were independently associated with earlier occurrence of adverse events. Perhaps most strikingly, body mass index in the obese range was associated with substantially earlier occurrence of deep vein thrombosis (median of 5 vs 10 days). This finding suggests that clinical monitoring for deep vein thrombosis should be performed earlier in patients with greater body mass index. Also notable is the earlier occurrence of cardiac arrest and death among patients with end-stage renal disease than among those without. Patients with end-stage renal disease may have a greater risk for these adverse events immediately following the cardiac stresses of surgery.27 Similarly, such patients may be more prone to early electrolyte abnormalities and arrhythmia.
Continue to: In addition to its clinical implications, this study...
In addition to its clinical implications, this study informs about the interpretation of the many studies of adverse events following hip fracture procedures that have been conducted using retrospective data. Several such studies have relied on inpatient-only administrative databases.4,13,14,28-35 As clearly demonstrated in Figure 3, for most of the commonly studied adverse events, inpatient-only databases failed to capture a large proportion of adverse events occurring in the first postoperative month. This highlights a substantial limitation of this commonly published type of study that is often not emphasized in the literature.
There has also been an increase in the publication of studies of adverse events following a hip fracture surgery using the ACS-NSQIP data.3,13,14,17,18,21 As discussed, the ACS-NSQIP provides data on 30-days of follow-up. This relatively extended follow-up is often touted as a distinct advantage. However, this study demonstrates that even the 30-day follow-up afforded by the ACS-NSQIP is limited in its ability to enable investigation of the later-occurring adverse events (Figure 1). In particular, the rate of surgical site infection shows little sign of slowing by postoperative day 30. Similarly, the rates of mortality, sepsis, deep vein thrombosis, and urinary tract infection remain substantial.
This study does have limitations. First, as discussed, the duration of follow-up is a limitation of any ACS-NSQIP-based investigation, including this study. Second, the ACS-NSQIP does not capture relevant orthopedic-specific outcomes (eg, screw cutout). In addition, it could not be determined with certainty whether adverse events occurring on the final hospital day occurred prior to or following discharge. However, only a small proportion of most of the adverse events was diagnosed on the final hospital day. Finally, the ACS-NSQIP reports on days from the operation until diagnosis of the adverse event. Although some adverse events are probably diagnosed quickly after they have occurred (eg, myocardial infarction and cardiac arrest), other adverse events may have a delayed diagnosis (eg, surgical site infection may be identified days after its initial occurrence during a follow-up examination). Therefore, it is important to note the subtle distinction between occurrence and diagnosis throughout the article. This article reports on the timing of diagnosis, not actual occurrence.
CONCLUSION
The timing of postoperative adverse events has been understudied in the past. This may be due to an inability of standard single- or multi-institutional investigations to achieve sample sizes adequate to study the less commonly occurring adverse events. Using a relatively new prospective surgical registry, this study provides a far more detailed description of the timing of adverse events following surgery than was previously available. The authors anticipate that these data can be used to inform patient counseling, target clinical surveillance, and direct clinical research. The authors chose to study the timing of postoperative adverse events following geriatric hip fracture surgery because of the high rate of adverse events associated with the procedure. However, future ACS-NSQIP studies may involve characterization of the timing of adverse events following other orthopedic and non-orthopedic procedures.
This paper will be judged for the Resident Writer’s Award.
1. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/jbjs.i.00735.
2. Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799-806. doi:10.2106/jbjs.h.01204.
3. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma.2014;28(2):63-69. doi:10.1097/BOT.0b013e3182a22744.
4. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84-a(4):562-572.
5. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003;163(11):1337-1342. doi:10.1001/archinte.163.11.1337.
6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev.2002;(4):Cd000305. doi:10.1002/14651858.cd000305.
7. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst Rev. 2004;(1):Cd001880. doi:10.1002/14651858.CD001880.pub2.
8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi:10.1046/j.1532-5415.2001.49108.x.
9. Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. J Am Geriatr Soc. 2012;60(4):733-739. doi:10.1111/j.1532-5415.2012.03899.x.
10. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 Suppl 2:S282-S288. doi:10.1111/j.1532-5415.2011.03691.x.
11. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92(2):265-269. doi:10.2106/jbjs.i.00883.
12. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455. doi:10.1093/oxfordjournals.bja.a013468.
13. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
14. Bohl DD, Grauer JN, Leopold SS. Editor's spotlight/Take 5: nationwide inpatient sample and national surgical quality improvement program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671. doi:10.1007/s11999-014-3595-9.
15. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/jbjs.m.01490.
16. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: "Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198. doi:10.2106/jbjs.n.00890.
17. Basques BA, Bohl DD, Golinvaux NS, Leslie MP, Baumgaertner MR, Grauer JN. Postoperative length of stay and thirty-day readmission following geriatric hip fracture: an analysis of 8,434 patients. J Orthop Trauma. 2015;29(3):e115-e120. doi:10.1097/bot.0000000000000222.
18. Golinvaux NS, Bohl DD, Basques BA, Baumgaertner MR, Grauer JN. Diabetes confers little to no increased risk of postoperative complications after hip fracture surgery in geriatric patients. Clin Orthop Relat Res. 2015;473(3):1043-1051. doi:10.1007/s11999-014-3945-7.
19. Maciejewski ML, Radcliff TA, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276. doi:10.1682/jrrd.2013.02.0041.
20. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res.2015;473(5):1574-1581. doi:10.1007/s11999-014-3597-7.
21. Bohl DD, Basques BA, Golinvaux NS, Miller CP, Baumgaertner MR, Grauer JN. Extramedullary compared with intramedullary implants for intertrochanteric hip fractures: thirty-day outcomes of 4432 procedures from the ACS NSQIP database. J Bone Joint Surg Am. 2014;96(22):1871-1877. doi:10.2106/jbjs.n.00041.
22. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine (Phila Pa 1976). 2009;34(18):1956-1962. doi:10.1097/BRS.0b013e3181ab930e.
23. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA.2009;302(1):58-66. doi:10.1001/jama.2009.956.
24. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267. doi:10.1016/j.yasu.2010.05.003.
25. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
26. ACS-NSQIP. Data Use Agreement. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip/participant-use/puf-form. Accessed September 20, 2018.
27. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi:10.1161/hy1001.096358.
28. Browne JA, Cook C, Olson SA, Bolognesi MP. Resident duty-hour reform associated with increased morbidity following hip fracture. J Bone Joint Surg Am. 2009;91(9):2079-2085. doi:10.2106/jbjs.h.01240.
29. Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809-814. doi:10.1097/TA.0b013e31816166bb.
30. Menendez ME, Ring D. Failure to rescue after proximal femur fracture surgery. J Orthop Trauma. 2015;29(3):e96-e102. doi:10.1097/bot.0000000000000234.
31. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9-17. doi:10.2106/jbjs.j.01077.
32. Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a “July effect”? Am J Orthop. 2009;38(12):606-611.
33. Koval KJ, Rust CL, Spratt KF. The effect of hospital setting and teaching status on outcomes after hip fracture. Am J Orthop. 2011;40(1):19-28.
34. Bacon WE. Secular trends in hip fracture occurrence and survival: age and sex differences. J Aging Health. 1996;8(4):538-553. doi:10.1177/089826439600800404.
35. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248-2249. doi:10.1111/jgs.12567.
ABSTRACT
This study uses a prospective surgical registry to characterize the timing of 10 postoperative adverse events following geriatric hip fracture surgery. There were 19,873 patients identified who were ≥70 years undergoing surgery for hip fracture as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). The median postoperative day of diagnosis (and interquartile range) for myocardial infarction was 3 (1-5), cardiac arrest requiring cardiopulmonary resuscitation 3 (0-8), stroke 3 (1-10), pneumonia 4 (2-10), pulmonary embolism 4 (2-11), urinary tract infection 7 (2-13), deep vein thrombosis 9 (4-16), sepsis 9 (4-18), mortality 11 (6-19), and surgical site infection 16 (11-22). For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30. Findings help to enable more targeted clinical surveillance, inform patient counseling, and determine the duration of follow-up required to study specific adverse events effectively. Orthopedic surgeons should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Continue to: Geriatric hip fracture surgery is associated with...
Geriatric hip fracture surgery is associated with a higher rate of occurrence of postoperative adverse events than any other commonly performed orthopedic procedure.1-4 Indeed, the 90-day mortality rate following a geriatric hip fracture surgery may be as high as 15%2 and the 30-day morbidity rate as high as 30%.3 Furthermore, more than half of postoperative mortalities following orthopedic procedures occur after surgery for hip fracture.4 Therefore, extensive research has been conducted regarding interventions to reduce the rates of adverse events following a hip fracture surgery.5-12 For example, randomized trials have been conducted involving venous thromboembolism prophylaxis,5,6nutritional supplementation,7 delirium prevention,8-10 anemia correction,11 geriatrics consultation,9 and anesthetic technique.12
Despite these extensive research efforts, there is currently little information in the literature regarding when postoperative adverse events occur. A clear depiction of the timing of adverse events could help target clinical surveillance, inform patient counseling, and determine the duration of follow-up required for studies. The reason that the timing of adverse events has not been previously characterized may be that the sample sizes available through standard single- or multi-institutional studies may be insufficient to accurately characterize the timing of rare adverse events (eg, myocardial infarction, stroke, etc.). Moreover, although administrative datasets have become common data sources for investigation of rare postoperative adverse events,13-16 such data sources often do not contain data on the timing of diagnosis.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a relatively new and growing surgical registry.1,3,13-22 The registry follows up patients undergoing surgical procedures at several hundred community and academic institutions nationwide. Unlike the administrative datasets discussed above, the ACS-NSQIP characterizes the postoperative day of diagnosis of well-defined adverse events during the first 30 postoperative days.22
In this study, data collected by the ACS-NSQIP are used to characterize the timing of 10 specific postoperative adverse events following a geriatric hip fracture surgery.
Continue to: METHODS...
METHODS
A retrospective analysis of data collected prospectively through the ACS-NSQIP was conducted. Geriatric patients who underwent hip fracture surgery during 2010 to 2013 were identified. Specific inclusion criteria were (1) International Classification of Diseases, Ninth Revision, diagnosis code 820, (2) primary Current Procedural Terminology codes 27125, 27130, 27235, 27236, 27244, or 27245, and (3) age ≥70 years.
The ACS-NSQIP captures patient demographic, comorbidity, and procedural characteristics at baseline.22 At the end of the 30-day follow-up period, the ACS-NSQIP personnel review both inpatient and outpatient charts to characterize the occurrence vs nonoccurrence of specific postoperative adverse events.22-25 When an adverse event does occur, the postoperative day of diagnosis is recorded.
For this study, the following adverse event categories were investigated: myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, pulmonary embolism, urinary tract infection, deep vein thrombosis, sepsis (either with or without shock), mortality, and surgical site infection (including superficial surgical site infection, deep surgical site infection, and organ or space surgical site infection). Detailed definitions of each adverse event are provided in ACS-NSQIP materials.22
First, the 30-day incidence (and the associated 95% confidence interval) was determined for each adverse event. Second, the median postoperative day of diagnosis (and the associated interquartile range) was determined for each adverse event. Third, the postoperative length of stay was used to estimate the proportion of diagnoses occurring prior to vs following discharge for each adverse event. Finally, multivariate Cox proportional hazards models were used to identify independent risk factors for earlier occurrence of postoperative adverse events. The final models were selected using a backward stepwise process that sequentially eliminated variables with the weakest associations until all variables had P < .05.
Because the ACS-NSQIP reports timing data in calendar days, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, it was not possible to ascertain whether the diagnosis occurred prior to or following discharge. For this study, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, the adverse event was considered to have been diagnosed following discharge. The rationale for this is that for most of the adverse events, it was thought to be unlikely that an inpatient would be discharged before the end of the same day as an inpatient diagnosis. However, there was one exception to this rule; when the postoperative day of discharge, the postoperative length of stay, and the postoperative day of death were all equivalent, the adverse event was considered to have occurred prior to discharge. This is because when a patient dies during the initial inpatient stay, the ACS-NSQIP considers the postoperative length of stay to be equivalent to the postoperative day of death. This makes it much more likely that a diagnosis on the final hospital day had occurred in a patient who had not been discharged.
The mandatory ACS-NSQIP statement is “The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”26
Continue to: RESULTS...
RESULTS
In total, 19,873 geriatric patients undergoing a hip fracture surgery were identified (Table 1). The rates of adverse events ranged from 6.7% for urinary tract infection to 0.6% for pulmonary embolism (Table 2).
Table 1. Patient Population
| Number | Percent |
Total | 19,873 | 100.0% |
Age |
|
|
70-74 years | 1852 | 9.3% |
75-79 years | 2764 | 13.9% |
80-84 years | 4328 | 21.8% |
85-89 years | 5525 | 27.8% |
≥90 years | 5404 | 27.2% |
Sex |
|
|
Male | 5359 | 27.0% |
Female | 14,514 | 73.0% |
Body mass index |
|
|
<30 kg/m2 | 17,733 | 89.2% |
≥30 kg/m2 | 2140 | 10.8% |
Functional status |
|
|
Independent | 14,348 | 72.2% |
Dependent | 5525 | 27.8% |
Diabetes | 3321 | 16.7% |
Congestive heart failure | 738 | 3.7% |
Dyspnea on exertion | 1542 | 7.8% |
Hypertension | 14,265 | 71.8% |
End-stage renal disease | 322 | 1.6% |
COPD | 2239 | 11.3% |
Current smoker | 1506 | 7.6% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2. Patients with Adverse Events Diagnosed During the First 30 postoperative days (N = 19,873)
Adverse Event | Number | Percent | 95% CI |
Urinary tract infection | 1321 | 6.7% | 6.3%-7.0% |
Mortality | 1240 | 6.2% | 5.9%-6.6% |
Pneumonia | 771 | 3.9% | 3.6%-4.2% |
Sepsis | 428 | 2.2% | 2.0%-2.4% |
Myocardial infarction | 347 | 1.8% | 1.6%-1.9% |
Surgical site infection | 247 | 1.2% | 1.1%-1.4% |
Deep vein thrombosis | 199 | 1.0% | 0.9%-1.1% |
Stroke | 144 | 0.7% | 0.6%-0.8% |
Cardiac arrest | 136 | 0.7% | 0.6%-0.8% |
Pulmonary embolism | 126 | 0.6% | 0.5%-0.7% |
Abbreviation: CI, confidence interval.
Figure 1 depicts the timing of postoperative adverse events in detail in histograms and timing curves. For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
Figure 2 provides the summary statistics for adverse events diagnosed in the first 30 postoperative days. The median postoperative day of diagnosis (and the interquartile range) was 3 (1-5) for myocardial infarction, 3 (0-8) for cardiac arrest requiring cardiopulmonary resuscitation, 3 (1-10) for stroke, 4 (2-10) for pneumonia, 4 (2-11) for pulmonary embolism, 7 (2-13) for urinary tract infection, 9 (4-16) for deep vein thrombosis, 9 (4-18) for sepsis, 11 (6-19) for mortality, and 16 (11-22) for surgical site infection.
Figure 3 depicts the timing of adverse events relative to discharge. The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
Table 3 shows the independent risk factors for earlier occurrence of adverse events. Following multivariate stepwise selection of final models, at least 1 patient characteristic was independently associated with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death. In contrast, no patient characteristics were independently associated with the timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, and surgical site infection.
Table 3. Timing of Diagnosis of Adverse Eventsa
Adverse events and associated baseline characteristic(s) | Median postoperative day of diagnosis with vs without baseline characteristic | P-valueb |
Cardiac arrest |
|
|
End-stage renal disease | 1 vs 3 | .005 |
Stroke |
|
|
Hypertension | 4 vs 2 | .025 |
Dependent functional status | 2 vs 4 | .027 |
Urinary tract infection |
|
|
Female sex | 6 vs 8 | .009 |
Deep vein thrombosis |
|
|
Body mass index ≥30 kg/m2 | 5 vs 10 | .015 |
Death |
|
|
End-stage renal disease | 10 vs 11 | .031 |
aBaseline characteristics that were independently associated with the timing of each adverse event were identified through a backwards stepwise selection process initially including all characteristics listed in Table 1, and sequentially excluding characteristics with the weakest associations until only characteristics with P < .05 remained. Independent associations with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death are shown. There were no characteristics independently associated with timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, or surgical site infection; hence, these adverse events are not listed in the table.
bFrom final Cox proportional hazards models identified through multivariate stepwise selection.
Continue to: DISCUSSION...
DISCUSSION
Adverse events are extremely common following a geriatric hip fracture surgery.1-4 Despite extensive investigation regarding methods to prevent these events,5-12 there is limited published description of the timing at which such events occur. This study used a large prospectively followed up cohort of geriatric patients undergoing a hip fracture surgery to deliver a better description of the timing of adverse events than was previously available. The findings of this study should enable more targeted clinical surveillance, inform patient counseling, and help determine the duration of follow-up required for studies on adverse events.
There was wide variability in the timing at which the different postoperative adverse events were diagnosed (Figures 1, 2). Myocardial infarction was diagnosed the earliest, with more than three-fourth of diagnoses in the first postoperative week. Other relatively early-diagnosed adverse events included cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, and pulmonary embolism.
The latest-diagnosed adverse event was surgical site infection (Figures 1, 2). Surgical site infection was actually the only adverse event with a rate of diagnosis during the first week that was lower than the rate of diagnosis later in the month (as can be seen by the inflection in the timing curve for surgical site infection in Figure 1). Mortality showed a relatively consistent rate of diagnosis throughout the entire first postoperative month. Other relatively late-diagnosed postoperative events, including sepsis, deep vein thrombosis, and urinary tract infection, showed varying degrees of decreased rate of diagnosis near the end of the first postoperative month. Of note, for the later-diagnosed adverse events, the estimated median and interquartile ranges (Figure 2) were presumably quite biased toward earlier diagnosis, as the 30-day follow-up period clearly failed to capture a large proportion of later-occurring adverse events (Figure 1).
Certain risk factors were independently associated with earlier occurrence of adverse events. Perhaps most strikingly, body mass index in the obese range was associated with substantially earlier occurrence of deep vein thrombosis (median of 5 vs 10 days). This finding suggests that clinical monitoring for deep vein thrombosis should be performed earlier in patients with greater body mass index. Also notable is the earlier occurrence of cardiac arrest and death among patients with end-stage renal disease than among those without. Patients with end-stage renal disease may have a greater risk for these adverse events immediately following the cardiac stresses of surgery.27 Similarly, such patients may be more prone to early electrolyte abnormalities and arrhythmia.
Continue to: In addition to its clinical implications, this study...
In addition to its clinical implications, this study informs about the interpretation of the many studies of adverse events following hip fracture procedures that have been conducted using retrospective data. Several such studies have relied on inpatient-only administrative databases.4,13,14,28-35 As clearly demonstrated in Figure 3, for most of the commonly studied adverse events, inpatient-only databases failed to capture a large proportion of adverse events occurring in the first postoperative month. This highlights a substantial limitation of this commonly published type of study that is often not emphasized in the literature.
There has also been an increase in the publication of studies of adverse events following a hip fracture surgery using the ACS-NSQIP data.3,13,14,17,18,21 As discussed, the ACS-NSQIP provides data on 30-days of follow-up. This relatively extended follow-up is often touted as a distinct advantage. However, this study demonstrates that even the 30-day follow-up afforded by the ACS-NSQIP is limited in its ability to enable investigation of the later-occurring adverse events (Figure 1). In particular, the rate of surgical site infection shows little sign of slowing by postoperative day 30. Similarly, the rates of mortality, sepsis, deep vein thrombosis, and urinary tract infection remain substantial.
This study does have limitations. First, as discussed, the duration of follow-up is a limitation of any ACS-NSQIP-based investigation, including this study. Second, the ACS-NSQIP does not capture relevant orthopedic-specific outcomes (eg, screw cutout). In addition, it could not be determined with certainty whether adverse events occurring on the final hospital day occurred prior to or following discharge. However, only a small proportion of most of the adverse events was diagnosed on the final hospital day. Finally, the ACS-NSQIP reports on days from the operation until diagnosis of the adverse event. Although some adverse events are probably diagnosed quickly after they have occurred (eg, myocardial infarction and cardiac arrest), other adverse events may have a delayed diagnosis (eg, surgical site infection may be identified days after its initial occurrence during a follow-up examination). Therefore, it is important to note the subtle distinction between occurrence and diagnosis throughout the article. This article reports on the timing of diagnosis, not actual occurrence.
CONCLUSION
The timing of postoperative adverse events has been understudied in the past. This may be due to an inability of standard single- or multi-institutional investigations to achieve sample sizes adequate to study the less commonly occurring adverse events. Using a relatively new prospective surgical registry, this study provides a far more detailed description of the timing of adverse events following surgery than was previously available. The authors anticipate that these data can be used to inform patient counseling, target clinical surveillance, and direct clinical research. The authors chose to study the timing of postoperative adverse events following geriatric hip fracture surgery because of the high rate of adverse events associated with the procedure. However, future ACS-NSQIP studies may involve characterization of the timing of adverse events following other orthopedic and non-orthopedic procedures.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
This study uses a prospective surgical registry to characterize the timing of 10 postoperative adverse events following geriatric hip fracture surgery. There were 19,873 patients identified who were ≥70 years undergoing surgery for hip fracture as part of the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). The median postoperative day of diagnosis (and interquartile range) for myocardial infarction was 3 (1-5), cardiac arrest requiring cardiopulmonary resuscitation 3 (0-8), stroke 3 (1-10), pneumonia 4 (2-10), pulmonary embolism 4 (2-11), urinary tract infection 7 (2-13), deep vein thrombosis 9 (4-16), sepsis 9 (4-18), mortality 11 (6-19), and surgical site infection 16 (11-22). For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30. Findings help to enable more targeted clinical surveillance, inform patient counseling, and determine the duration of follow-up required to study specific adverse events effectively. Orthopedic surgeons should have the lowest threshold for testing for each adverse event during the time period of greatest risk.
Continue to: Geriatric hip fracture surgery is associated with...
Geriatric hip fracture surgery is associated with a higher rate of occurrence of postoperative adverse events than any other commonly performed orthopedic procedure.1-4 Indeed, the 90-day mortality rate following a geriatric hip fracture surgery may be as high as 15%2 and the 30-day morbidity rate as high as 30%.3 Furthermore, more than half of postoperative mortalities following orthopedic procedures occur after surgery for hip fracture.4 Therefore, extensive research has been conducted regarding interventions to reduce the rates of adverse events following a hip fracture surgery.5-12 For example, randomized trials have been conducted involving venous thromboembolism prophylaxis,5,6nutritional supplementation,7 delirium prevention,8-10 anemia correction,11 geriatrics consultation,9 and anesthetic technique.12
Despite these extensive research efforts, there is currently little information in the literature regarding when postoperative adverse events occur. A clear depiction of the timing of adverse events could help target clinical surveillance, inform patient counseling, and determine the duration of follow-up required for studies. The reason that the timing of adverse events has not been previously characterized may be that the sample sizes available through standard single- or multi-institutional studies may be insufficient to accurately characterize the timing of rare adverse events (eg, myocardial infarction, stroke, etc.). Moreover, although administrative datasets have become common data sources for investigation of rare postoperative adverse events,13-16 such data sources often do not contain data on the timing of diagnosis.
The American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) is a relatively new and growing surgical registry.1,3,13-22 The registry follows up patients undergoing surgical procedures at several hundred community and academic institutions nationwide. Unlike the administrative datasets discussed above, the ACS-NSQIP characterizes the postoperative day of diagnosis of well-defined adverse events during the first 30 postoperative days.22
In this study, data collected by the ACS-NSQIP are used to characterize the timing of 10 specific postoperative adverse events following a geriatric hip fracture surgery.
Continue to: METHODS...
METHODS
A retrospective analysis of data collected prospectively through the ACS-NSQIP was conducted. Geriatric patients who underwent hip fracture surgery during 2010 to 2013 were identified. Specific inclusion criteria were (1) International Classification of Diseases, Ninth Revision, diagnosis code 820, (2) primary Current Procedural Terminology codes 27125, 27130, 27235, 27236, 27244, or 27245, and (3) age ≥70 years.
The ACS-NSQIP captures patient demographic, comorbidity, and procedural characteristics at baseline.22 At the end of the 30-day follow-up period, the ACS-NSQIP personnel review both inpatient and outpatient charts to characterize the occurrence vs nonoccurrence of specific postoperative adverse events.22-25 When an adverse event does occur, the postoperative day of diagnosis is recorded.
For this study, the following adverse event categories were investigated: myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, pulmonary embolism, urinary tract infection, deep vein thrombosis, sepsis (either with or without shock), mortality, and surgical site infection (including superficial surgical site infection, deep surgical site infection, and organ or space surgical site infection). Detailed definitions of each adverse event are provided in ACS-NSQIP materials.22
First, the 30-day incidence (and the associated 95% confidence interval) was determined for each adverse event. Second, the median postoperative day of diagnosis (and the associated interquartile range) was determined for each adverse event. Third, the postoperative length of stay was used to estimate the proportion of diagnoses occurring prior to vs following discharge for each adverse event. Finally, multivariate Cox proportional hazards models were used to identify independent risk factors for earlier occurrence of postoperative adverse events. The final models were selected using a backward stepwise process that sequentially eliminated variables with the weakest associations until all variables had P < .05.
Because the ACS-NSQIP reports timing data in calendar days, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, it was not possible to ascertain whether the diagnosis occurred prior to or following discharge. For this study, when the postoperative length of stay was equivalent to the postoperative day of diagnosis, the adverse event was considered to have been diagnosed following discharge. The rationale for this is that for most of the adverse events, it was thought to be unlikely that an inpatient would be discharged before the end of the same day as an inpatient diagnosis. However, there was one exception to this rule; when the postoperative day of discharge, the postoperative length of stay, and the postoperative day of death were all equivalent, the adverse event was considered to have occurred prior to discharge. This is because when a patient dies during the initial inpatient stay, the ACS-NSQIP considers the postoperative length of stay to be equivalent to the postoperative day of death. This makes it much more likely that a diagnosis on the final hospital day had occurred in a patient who had not been discharged.
The mandatory ACS-NSQIP statement is “The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS-NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.”26
Continue to: RESULTS...
RESULTS
In total, 19,873 geriatric patients undergoing a hip fracture surgery were identified (Table 1). The rates of adverse events ranged from 6.7% for urinary tract infection to 0.6% for pulmonary embolism (Table 2).
Table 1. Patient Population
| Number | Percent |
Total | 19,873 | 100.0% |
Age |
|
|
70-74 years | 1852 | 9.3% |
75-79 years | 2764 | 13.9% |
80-84 years | 4328 | 21.8% |
85-89 years | 5525 | 27.8% |
≥90 years | 5404 | 27.2% |
Sex |
|
|
Male | 5359 | 27.0% |
Female | 14,514 | 73.0% |
Body mass index |
|
|
<30 kg/m2 | 17,733 | 89.2% |
≥30 kg/m2 | 2140 | 10.8% |
Functional status |
|
|
Independent | 14,348 | 72.2% |
Dependent | 5525 | 27.8% |
Diabetes | 3321 | 16.7% |
Congestive heart failure | 738 | 3.7% |
Dyspnea on exertion | 1542 | 7.8% |
Hypertension | 14,265 | 71.8% |
End-stage renal disease | 322 | 1.6% |
COPD | 2239 | 11.3% |
Current smoker | 1506 | 7.6% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2. Patients with Adverse Events Diagnosed During the First 30 postoperative days (N = 19,873)
Adverse Event | Number | Percent | 95% CI |
Urinary tract infection | 1321 | 6.7% | 6.3%-7.0% |
Mortality | 1240 | 6.2% | 5.9%-6.6% |
Pneumonia | 771 | 3.9% | 3.6%-4.2% |
Sepsis | 428 | 2.2% | 2.0%-2.4% |
Myocardial infarction | 347 | 1.8% | 1.6%-1.9% |
Surgical site infection | 247 | 1.2% | 1.1%-1.4% |
Deep vein thrombosis | 199 | 1.0% | 0.9%-1.1% |
Stroke | 144 | 0.7% | 0.6%-0.8% |
Cardiac arrest | 136 | 0.7% | 0.6%-0.8% |
Pulmonary embolism | 126 | 0.6% | 0.5%-0.7% |
Abbreviation: CI, confidence interval.
Figure 1 depicts the timing of postoperative adverse events in detail in histograms and timing curves. For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30. For the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
Figure 2 provides the summary statistics for adverse events diagnosed in the first 30 postoperative days. The median postoperative day of diagnosis (and the interquartile range) was 3 (1-5) for myocardial infarction, 3 (0-8) for cardiac arrest requiring cardiopulmonary resuscitation, 3 (1-10) for stroke, 4 (2-10) for pneumonia, 4 (2-11) for pulmonary embolism, 7 (2-13) for urinary tract infection, 9 (4-16) for deep vein thrombosis, 9 (4-18) for sepsis, 11 (6-19) for mortality, and 16 (11-22) for surgical site infection.
Figure 3 depicts the timing of adverse events relative to discharge. The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
Table 3 shows the independent risk factors for earlier occurrence of adverse events. Following multivariate stepwise selection of final models, at least 1 patient characteristic was independently associated with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death. In contrast, no patient characteristics were independently associated with the timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, and surgical site infection.
Table 3. Timing of Diagnosis of Adverse Eventsa
Adverse events and associated baseline characteristic(s) | Median postoperative day of diagnosis with vs without baseline characteristic | P-valueb |
Cardiac arrest |
|
|
End-stage renal disease | 1 vs 3 | .005 |
Stroke |
|
|
Hypertension | 4 vs 2 | .025 |
Dependent functional status | 2 vs 4 | .027 |
Urinary tract infection |
|
|
Female sex | 6 vs 8 | .009 |
Deep vein thrombosis |
|
|
Body mass index ≥30 kg/m2 | 5 vs 10 | .015 |
Death |
|
|
End-stage renal disease | 10 vs 11 | .031 |
aBaseline characteristics that were independently associated with the timing of each adverse event were identified through a backwards stepwise selection process initially including all characteristics listed in Table 1, and sequentially excluding characteristics with the weakest associations until only characteristics with P < .05 remained. Independent associations with the timing of cardiac arrest, stroke, urinary tract infection, deep vein thrombosis, and death are shown. There were no characteristics independently associated with timing of myocardial infarction, pneumonia, pulmonary embolism, sepsis, or surgical site infection; hence, these adverse events are not listed in the table.
bFrom final Cox proportional hazards models identified through multivariate stepwise selection.
Continue to: DISCUSSION...
DISCUSSION
Adverse events are extremely common following a geriatric hip fracture surgery.1-4 Despite extensive investigation regarding methods to prevent these events,5-12 there is limited published description of the timing at which such events occur. This study used a large prospectively followed up cohort of geriatric patients undergoing a hip fracture surgery to deliver a better description of the timing of adverse events than was previously available. The findings of this study should enable more targeted clinical surveillance, inform patient counseling, and help determine the duration of follow-up required for studies on adverse events.
There was wide variability in the timing at which the different postoperative adverse events were diagnosed (Figures 1, 2). Myocardial infarction was diagnosed the earliest, with more than three-fourth of diagnoses in the first postoperative week. Other relatively early-diagnosed adverse events included cardiac arrest requiring cardiopulmonary resuscitation, stroke, pneumonia, and pulmonary embolism.
The latest-diagnosed adverse event was surgical site infection (Figures 1, 2). Surgical site infection was actually the only adverse event with a rate of diagnosis during the first week that was lower than the rate of diagnosis later in the month (as can be seen by the inflection in the timing curve for surgical site infection in Figure 1). Mortality showed a relatively consistent rate of diagnosis throughout the entire first postoperative month. Other relatively late-diagnosed postoperative events, including sepsis, deep vein thrombosis, and urinary tract infection, showed varying degrees of decreased rate of diagnosis near the end of the first postoperative month. Of note, for the later-diagnosed adverse events, the estimated median and interquartile ranges (Figure 2) were presumably quite biased toward earlier diagnosis, as the 30-day follow-up period clearly failed to capture a large proportion of later-occurring adverse events (Figure 1).
Certain risk factors were independently associated with earlier occurrence of adverse events. Perhaps most strikingly, body mass index in the obese range was associated with substantially earlier occurrence of deep vein thrombosis (median of 5 vs 10 days). This finding suggests that clinical monitoring for deep vein thrombosis should be performed earlier in patients with greater body mass index. Also notable is the earlier occurrence of cardiac arrest and death among patients with end-stage renal disease than among those without. Patients with end-stage renal disease may have a greater risk for these adverse events immediately following the cardiac stresses of surgery.27 Similarly, such patients may be more prone to early electrolyte abnormalities and arrhythmia.
Continue to: In addition to its clinical implications, this study...
In addition to its clinical implications, this study informs about the interpretation of the many studies of adverse events following hip fracture procedures that have been conducted using retrospective data. Several such studies have relied on inpatient-only administrative databases.4,13,14,28-35 As clearly demonstrated in Figure 3, for most of the commonly studied adverse events, inpatient-only databases failed to capture a large proportion of adverse events occurring in the first postoperative month. This highlights a substantial limitation of this commonly published type of study that is often not emphasized in the literature.
There has also been an increase in the publication of studies of adverse events following a hip fracture surgery using the ACS-NSQIP data.3,13,14,17,18,21 As discussed, the ACS-NSQIP provides data on 30-days of follow-up. This relatively extended follow-up is often touted as a distinct advantage. However, this study demonstrates that even the 30-day follow-up afforded by the ACS-NSQIP is limited in its ability to enable investigation of the later-occurring adverse events (Figure 1). In particular, the rate of surgical site infection shows little sign of slowing by postoperative day 30. Similarly, the rates of mortality, sepsis, deep vein thrombosis, and urinary tract infection remain substantial.
This study does have limitations. First, as discussed, the duration of follow-up is a limitation of any ACS-NSQIP-based investigation, including this study. Second, the ACS-NSQIP does not capture relevant orthopedic-specific outcomes (eg, screw cutout). In addition, it could not be determined with certainty whether adverse events occurring on the final hospital day occurred prior to or following discharge. However, only a small proportion of most of the adverse events was diagnosed on the final hospital day. Finally, the ACS-NSQIP reports on days from the operation until diagnosis of the adverse event. Although some adverse events are probably diagnosed quickly after they have occurred (eg, myocardial infarction and cardiac arrest), other adverse events may have a delayed diagnosis (eg, surgical site infection may be identified days after its initial occurrence during a follow-up examination). Therefore, it is important to note the subtle distinction between occurrence and diagnosis throughout the article. This article reports on the timing of diagnosis, not actual occurrence.
CONCLUSION
The timing of postoperative adverse events has been understudied in the past. This may be due to an inability of standard single- or multi-institutional investigations to achieve sample sizes adequate to study the less commonly occurring adverse events. Using a relatively new prospective surgical registry, this study provides a far more detailed description of the timing of adverse events following surgery than was previously available. The authors anticipate that these data can be used to inform patient counseling, target clinical surveillance, and direct clinical research. The authors chose to study the timing of postoperative adverse events following geriatric hip fracture surgery because of the high rate of adverse events associated with the procedure. However, future ACS-NSQIP studies may involve characterization of the timing of adverse events following other orthopedic and non-orthopedic procedures.
This paper will be judged for the Resident Writer’s Award.
1. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/jbjs.i.00735.
2. Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799-806. doi:10.2106/jbjs.h.01204.
3. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma.2014;28(2):63-69. doi:10.1097/BOT.0b013e3182a22744.
4. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84-a(4):562-572.
5. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003;163(11):1337-1342. doi:10.1001/archinte.163.11.1337.
6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev.2002;(4):Cd000305. doi:10.1002/14651858.cd000305.
7. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst Rev. 2004;(1):Cd001880. doi:10.1002/14651858.CD001880.pub2.
8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi:10.1046/j.1532-5415.2001.49108.x.
9. Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. J Am Geriatr Soc. 2012;60(4):733-739. doi:10.1111/j.1532-5415.2012.03899.x.
10. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 Suppl 2:S282-S288. doi:10.1111/j.1532-5415.2011.03691.x.
11. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92(2):265-269. doi:10.2106/jbjs.i.00883.
12. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455. doi:10.1093/oxfordjournals.bja.a013468.
13. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
14. Bohl DD, Grauer JN, Leopold SS. Editor's spotlight/Take 5: nationwide inpatient sample and national surgical quality improvement program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671. doi:10.1007/s11999-014-3595-9.
15. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/jbjs.m.01490.
16. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: "Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198. doi:10.2106/jbjs.n.00890.
17. Basques BA, Bohl DD, Golinvaux NS, Leslie MP, Baumgaertner MR, Grauer JN. Postoperative length of stay and thirty-day readmission following geriatric hip fracture: an analysis of 8,434 patients. J Orthop Trauma. 2015;29(3):e115-e120. doi:10.1097/bot.0000000000000222.
18. Golinvaux NS, Bohl DD, Basques BA, Baumgaertner MR, Grauer JN. Diabetes confers little to no increased risk of postoperative complications after hip fracture surgery in geriatric patients. Clin Orthop Relat Res. 2015;473(3):1043-1051. doi:10.1007/s11999-014-3945-7.
19. Maciejewski ML, Radcliff TA, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276. doi:10.1682/jrrd.2013.02.0041.
20. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res.2015;473(5):1574-1581. doi:10.1007/s11999-014-3597-7.
21. Bohl DD, Basques BA, Golinvaux NS, Miller CP, Baumgaertner MR, Grauer JN. Extramedullary compared with intramedullary implants for intertrochanteric hip fractures: thirty-day outcomes of 4432 procedures from the ACS NSQIP database. J Bone Joint Surg Am. 2014;96(22):1871-1877. doi:10.2106/jbjs.n.00041.
22. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine (Phila Pa 1976). 2009;34(18):1956-1962. doi:10.1097/BRS.0b013e3181ab930e.
23. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA.2009;302(1):58-66. doi:10.1001/jama.2009.956.
24. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267. doi:10.1016/j.yasu.2010.05.003.
25. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
26. ACS-NSQIP. Data Use Agreement. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip/participant-use/puf-form. Accessed September 20, 2018.
27. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi:10.1161/hy1001.096358.
28. Browne JA, Cook C, Olson SA, Bolognesi MP. Resident duty-hour reform associated with increased morbidity following hip fracture. J Bone Joint Surg Am. 2009;91(9):2079-2085. doi:10.2106/jbjs.h.01240.
29. Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809-814. doi:10.1097/TA.0b013e31816166bb.
30. Menendez ME, Ring D. Failure to rescue after proximal femur fracture surgery. J Orthop Trauma. 2015;29(3):e96-e102. doi:10.1097/bot.0000000000000234.
31. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9-17. doi:10.2106/jbjs.j.01077.
32. Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a “July effect”? Am J Orthop. 2009;38(12):606-611.
33. Koval KJ, Rust CL, Spratt KF. The effect of hospital setting and teaching status on outcomes after hip fracture. Am J Orthop. 2011;40(1):19-28.
34. Bacon WE. Secular trends in hip fracture occurrence and survival: age and sex differences. J Aging Health. 1996;8(4):538-553. doi:10.1177/089826439600800404.
35. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248-2249. doi:10.1111/jgs.12567.
1. Schilling PL, Hallstrom BR, Birkmeyer JD, Carpenter JE. Prioritizing perioperative quality improvement in orthopaedic surgery. J Bone Joint Surg Am. 2010;92(9):1884-1889. doi:10.2106/jbjs.i.00735.
2. Forte ML, Virnig BA, Swiontkowski MF, et al. Ninety-day mortality after intertrochanteric hip fracture: does provider volume matter? J Bone Joint Surg Am. 2010;92(4):799-806. doi:10.2106/jbjs.h.01204.
3. Pugely AJ, Martin CT, Gao Y, Klocke NF, Callaghan JJ, Marsh JL. A risk calculator for short-term morbidity and mortality after hip fracture surgery. J Orthop Trauma.2014;28(2):63-69. doi:10.1097/BOT.0b013e3182a22744.
4. Bhattacharyya T, Iorio R, Healy WL. Rate of and risk factors for acute inpatient mortality after orthopaedic surgery. J Bone Joint Surg Am. 2002;84-a(4):562-572.
5. Eriksson BI, Lassen MR. Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2003;163(11):1337-1342. doi:10.1001/archinte.163.11.1337.
6. Handoll HH, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev.2002;(4):Cd000305. doi:10.1002/14651858.cd000305.
7. Avenell A, Handoll HH. Nutritional supplementation for hip fracture aftercare in the elderly. Cochrane Database Syst Rev. 2004;(1):Cd001880. doi:10.1002/14651858.CD001880.pub2.
8. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49(5):516-522. doi:10.1046/j.1532-5415.2001.49108.x.
9. Deschodt M, Braes T, Flamaing J, et al. Preventing delirium in older adults with recent hip fracture through multidisciplinary geriatric consultation. J Am Geriatr Soc. 2012;60(4):733-739. doi:10.1111/j.1532-5415.2012.03899.x.
10. Marcantonio ER, Palihnich K, Appleton P, Davis RB. Pilot randomized trial of donepezil hydrochloride for delirium after hip fracture. J Am Geriatr Soc. 2011;59 Suppl 2:S282-S288. doi:10.1111/j.1532-5415.2011.03691.x.
11. Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92(2):265-269. doi:10.2106/jbjs.i.00883.
12. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450-455. doi:10.1093/oxfordjournals.bja.a013468.
13. Bohl DD, Basques BA, Golinvaux NS, Baumgaertner MR, Grauer JN. Nationwide Inpatient Sample and National Surgical Quality Improvement Program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1672-1680. doi:10.1007/s11999-014-3559-0.
14. Bohl DD, Grauer JN, Leopold SS. Editor's spotlight/Take 5: nationwide inpatient sample and national surgical quality improvement program give different results in hip fracture studies. Clin Orthop Relat Res. 2014;472(6):1667-1671. doi:10.1007/s11999-014-3595-9.
15. Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/jbjs.m.01490.
16. Levin PE. Apples, oranges, and national databases: commentary on an article by Daniel D. Bohl, MPH, et al.: "Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures.” J Bone Joint Surg Am. 2014;96(23):e198. doi:10.2106/jbjs.n.00890.
17. Basques BA, Bohl DD, Golinvaux NS, Leslie MP, Baumgaertner MR, Grauer JN. Postoperative length of stay and thirty-day readmission following geriatric hip fracture: an analysis of 8,434 patients. J Orthop Trauma. 2015;29(3):e115-e120. doi:10.1097/bot.0000000000000222.
18. Golinvaux NS, Bohl DD, Basques BA, Baumgaertner MR, Grauer JN. Diabetes confers little to no increased risk of postoperative complications after hip fracture surgery in geriatric patients. Clin Orthop Relat Res. 2015;473(3):1043-1051. doi:10.1007/s11999-014-3945-7.
19. Maciejewski ML, Radcliff TA, Henderson WG, et al. Determinants of postsurgical discharge setting for male hip fracture patients. J Rehabil Res Dev. 2013;50(9):1267-1276. doi:10.1682/jrrd.2013.02.0041.
20. Molina CS, Thakore RV, Blumer A, Obremskey WT, Sethi MK. Use of the National Surgical Quality Improvement Program in orthopaedic surgery. Clin Orthop Relat Res.2015;473(5):1574-1581. doi:10.1007/s11999-014-3597-7.
21. Bohl DD, Basques BA, Golinvaux NS, Miller CP, Baumgaertner MR, Grauer JN. Extramedullary compared with intramedullary implants for intertrochanteric hip fractures: thirty-day outcomes of 4432 procedures from the ACS NSQIP database. J Bone Joint Surg Am. 2014;96(22):1871-1877. doi:10.2106/jbjs.n.00041.
22. Alosh H, Riley LH 3rd, Skolasky RL. Insurance status, geography, race, and ethnicity as predictors of anterior cervical spine surgery rates and in-hospital mortality: an examination of United States trends from 1992 to 2005. Spine (Phila Pa 1976). 2009;34(18):1956-1962. doi:10.1097/BRS.0b013e3181ab930e.
23. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA.2009;302(1):58-66. doi:10.1001/jama.2009.956.
24. Ingraham AM, Richards KE, Hall BL, Ko CY. Quality improvement in surgery: the American College of Surgeons National Surgical Quality Improvement Program approach. Adv Surg. 2010;44(1):251-267. doi:10.1016/j.yasu.2010.05.003.
25. Shiloach M, Frencher SK Jr, Steeger JE, et al. Toward robust information: data quality and inter-rater reliability in the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2010;210(1):6-16. doi:10.1016/j.jamcollsurg.2009.09.031.
26. ACS-NSQIP. Data Use Agreement. American College of Surgeons Web site. https://www.facs.org/quality-programs/acs-nsqip/participant-use/puf-form. Accessed September 20, 2018.
27. Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938-942. doi:10.1161/hy1001.096358.
28. Browne JA, Cook C, Olson SA, Bolognesi MP. Resident duty-hour reform associated with increased morbidity following hip fracture. J Bone Joint Surg Am. 2009;91(9):2079-2085. doi:10.2106/jbjs.h.01240.
29. Browne JA, Pietrobon R, Olson SA. Hip fracture outcomes: does surgeon or hospital volume really matter? J Trauma. 2009;66(3):809-814. doi:10.1097/TA.0b013e31816166bb.
30. Menendez ME, Ring D. Failure to rescue after proximal femur fracture surgery. J Orthop Trauma. 2015;29(3):e96-e102. doi:10.1097/bot.0000000000000234.
31. Nikkel LE, Fox EJ, Black KP, Davis C, Andersen L, Hollenbeak CS. Impact of comorbidities on hospitalization costs following hip fracture. J Bone Joint Surg Am. 2012;94(1):9-17. doi:10.2106/jbjs.j.01077.
32. Anderson KL, Koval KJ, Spratt KF. Hip fracture outcome: is there a “July effect”? Am J Orthop. 2009;38(12):606-611.
33. Koval KJ, Rust CL, Spratt KF. The effect of hospital setting and teaching status on outcomes after hip fracture. Am J Orthop. 2011;40(1):19-28.
34. Bacon WE. Secular trends in hip fracture occurrence and survival: age and sex differences. J Aging Health. 1996;8(4):538-553. doi:10.1177/089826439600800404.
35. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248-2249. doi:10.1111/jgs.12567.
TAKE-HOME POINTS
- The median postoperative day of diagnosis for myocardial infarction was 3, 3 for cardiac arrest requiring cardiopulmonary resuscitation, 3 for stroke, 4 for pneumonia, 4 for pulmonary embolism, 7 for urinary tract infection, 9 for deep vein thrombosis, 9 for sepsis, 11 for mortality, and 16 for surgical site infection.
- For the earliest diagnosed adverse events, the rate of adverse events had diminished by postoperative day 30; however, for the later diagnosed adverse events, the rate of adverse events remained high at postoperative day 30.
- The proportions of adverse events diagnosed prior to discharge were 81.0% for myocardial infarction, 77.8% for stroke, 76.1% for cardiac arrest requiring cardiopulmonary resuscitation, 71.9% for pulmonary embolism, 71.1% for pneumonia, 58.0% for urinary tract infection, 52.1% for sepsis, 46.9% for deep vein thrombosis, 44.3% for mortality, and 27.6% for surgical site infection.
- These results facilitate targeted clinical surveillance, guide patient counseling, and inform the duration of follow-up required in research studies.
- Clinicians should have the lowest threshold for testing for each adverse event during the time period of greatest risk.