User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Tranexamic Acid Reduces Perioperative Blood Loss and Hemarthrosis in Total Ankle Arthroplasty
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
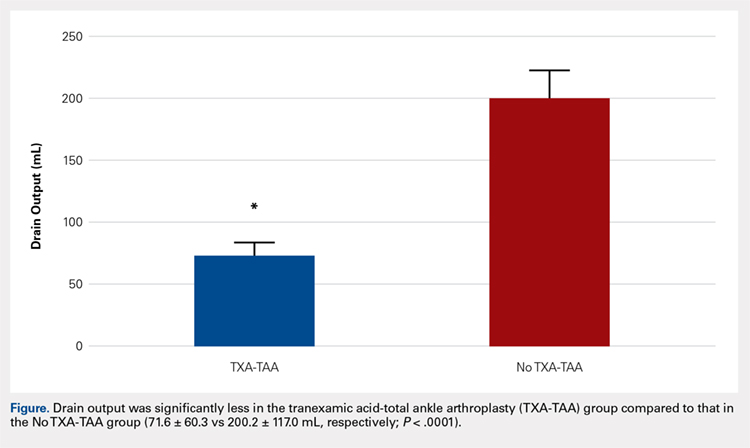
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).
RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).

RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
ABSTRACT
Tranexamic acid (TXA) is an effective agent used for reducing perioperative blood loss and decreasing the potential for postoperative hemarthrosis. We hypothesized that patients who had received intraoperative TXA during total ankle arthroplasty (TAA) would have a reduction in postoperative drain output, thereby resulting in a reduced risk of postoperative hemarthrosis and lower wound complication rates.
A retrospective review was conducted on 50 consecutive patients, 25 receiving TXA (TXA-TAA) and 25 not receiving TXA (No TXA-TAA), who underwent an uncemented TAA between September 2011 and December 2015. Demographic characteristics, drain output, preoperative and postoperative hemoglobin levels, operative and postoperative course, and minor and major wound complications of the patients were reviewed.
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P < .0001). The overall wound complication rate in the No TXA-TAA group was higher (20%, 5/25) than that in the TXA-TAA group (8%, 2/25) (P = .114). The mean change in preoperative to postoperative hemoglobin level was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively, P = .01).
TXA is an effective hemostatic agent when used during TAA. TXA reduces perioperative blood loss, hemarthrosis, and the risk of wound complications.
Continue to: End-stage ankle arthritis...
End-stage ankle arthritis is a disabling condition that may lead to poor quality of life and difficulties with activities of daily living.1 The associated mental and physical disability has been demonstrated to be as severe as in end-stage hip arthrosis.2 Operative treatment for symptomatic end-stage ankle arthritis includes arthrodesis or total ankle arthroplasty (TAA) in those refractory to nonoperative treatment.3 Newer generation implants have made TAA a more attractive option for both the surgeon and the patient.
Over the past decade, the utility of TAA has increased and attention has turned toward the management of perioperative factors that would maximize patient satisfaction and decrease the length of stay and complication rates, as well as hospital costs.4 Comprehensive literature on total knee arthroplasty (TKA) and total hip arthroplasty (THA) has demonstrated that the management of perioperative blood loss, specifically postoperative hemarthrosis, is a modifiable factor affecting patient recovery, complication rates, and hospital costs.5-8 Drain output has been used as a direct measure of intra-articular blood accumulation.9 Decreased drain output implies decreased hemarthrosis, which could potentially alleviate the pressure on the wound and decrease wound complications.
One of the major strategies that has been recognized for reducing blood loss and decreasing the potential for postoperative hemarthrosis is the use of intravenous (IV) or topical tranexamic acid (TXA).10,11 TXA is a synthetic antifibrinolytic medication that has been extensively used throughout the medical field since the 1960s to help control the bleeding cascade. This medication stabilizes clot formation without inducing a pro-coaguable state.12 Intraoperative administration of TXA has been shown to reduce drain output and decrease transfusion requirements after TKA and THA without an associated increase in patient morbidity and mortality.6,11,13-15
Currently, there is a lack of studies evaluating the utility of TXA during TAA. We hypothesize that compared with patients who had not received TXA, those who had received intraoperative TXA during TAA would have a reduction in postoperative drain output and therefore decreased hemarthrosis, lower wound complication rate, and a diminished change in preoperative to postoperative hemoglobin levels, reflecting a reduction in perioperative blood loss.
MATERIALS AND METHODS
This study was approved by the Institutional Review Board at the University at Buffalo, State University of New York. A retrospective chart review was conducted on 50 consecutive patients who underwent an uncemented TAA with the Salto Talaris total ankle prosthesis (Tornier, Inc) between September 2011 and December 2015. All surgeries were performed at 1 institution by a single fellowship surgeon trained in foot and ankle surgery through the anterior approach where a midline incision was made over the ankle. The interval between the tibialis anterior tendon and the extensor hallucis longus tendon was used. We had incorporated intraoperative TXA into the TAA surgical protocol at our institution in January 2014. We evaluated the first 25 consecutive patients who underwent TAA after TXA use began (TXA-TAA) and another 25 consecutive patients who underwent TAA before the routine use of TXA (No TXA-TAA). Inclusion criteria were patients who presented with pain, decreased function, and radiographic parameters of end-stage tibiotalar arthritis due to degenerative arthritis, rheumatoid arthritis, or posttraumatic arthritis who subsequently underwent a TAA. Exclusion criteria were patients with a contraindication for IV TXA use, a preexisting coagulopathy, or where drain output was not recorded. Contraindications for IV TXA use included patients with impaired renal clearance, recent cardiac surgery, myocardial infarction, ischemic stroke, or venous thromboembolism (VTE). Seven patients were ultimately excluded from this study based on the inclusion and exclusion criteria, 3 patients from the TXA-TAA group and 4 patients from the No TXA-TAA group.
Continue to: Charts were reviewed for demographics...
Charts were reviewed for demographics, preoperative and postoperative hemoglobin levels, indications for surgery, surgical procedures, length of surgery, postoperative drain output, length of stay, postoperative pain visual analog scale (VAS) score, minor and major wound complications, and postoperative complications. Minor wound complications were defined as the anterior surgical incision that required local wound care in office or oral antibiotics without subsequent consequences. Major wound complications were defined as requiring surgical débridement and/or any additional treatment in the operating room.16 Postoperative complications other than wound complications were defined as those requiring a subsequent surgical intervention. Patient demographics and clinical and procedural characteristics of patients in both the TXA-TAA and the No TXA-TAA groups are outlined in Table 1. There were 14 males and 11 females in the TXA-TAA group and 16 males and 9 females in the No TXA-TAA group. The mean age was 65.8 ± 10.9 years in the TXA-TAA group and 66.9 ± 8.0 years in the No TXA-TAA group (P = .69). Mean body mass index (BMI) was 31.6 ± 6.3 in the TXA-TAA group and 29.4 ± 4.9 in the No TXA-TAA group (P = .18). The primary indication for TAA was degenerative osteoarthritis in 26 patients, posttraumatic arthritis in 21 patients, and rheumatoid arthritis in 3 patients. The most common associated procedure was Achilles tendon lengthening in both groups. The mean follow-up in the TXA-TAA group was 9.3 ± 5.8 months (range, 2.0-24.0 months). Postoperative complications due to TXA administration as described in previous literature were defined as VTE, myocardial infarction, or ischemic cerebral event. The TXA-TAA group received a standard 1 g dose of IV TXA 20 minutes prior to tourniquet inflation. A tourniquet was used intraoperatively on all patients included in this study. A postoperative 400-mL surgical drain (Hemovac, Zimmer Biomet) was placed in the ankle joint in all patients and subsequently discontinued on postoperative day 1. Recent literature has reported the minor wound complication rate associated with TAA to be as high as 25% and the major wound complication rate to be 8.5%.16 To assist in reducing the risk for wound complications, our protocol traditionally uses an intra-articular surgical drain to decrease any pressure on the wound from postoperative hemarthrosis.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
aP value was calculated from t-test continuous variables and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
Total drain output was recorded in milliliters (mL) in all patients. The change between the preoperative hemoglobin level and the hemoglobin level on postoperative day 1 was calculated for each patient. The calculated blood loss was determined using Meunier’s equation, which estimates the total blood volume using Nadler’s formula and then uses preoperative hemoglobin and postoperative day 1 hemoglobin values to calculate blood loss.17,18 VAS scores (scale, 1-10) were obtained every 4 hours on postoperative day 1 according to the nursing protocol. The number 1 on the scale represents the least amount of pain, whereas 10 indicates the worst pain. The VAS scores were then averaged for each patient.
A power analysis using preliminary data determined that 15 patients were needed in each group to detect a 50% reduction in drain output at a power of 80% and a P value of 0.05. Descriptive statistics were used to analyze demographic data. We compared the demographic and clinical characteristics of patients in the TXA-TAA group with those of patients in the No TXA-TAA group using unpaired student t-tests for continuous variables and Chi-square or Fischer’s exact tests for categorical variables. Simple and adjusted linear regression analyses were used to examine the difference in drain output and blood loss between the 2 groups (TXA-TAA vs No TXA-TAA). Multivariate models were adjusted for age, BMI, and length of surgery. A P value <.05 was considered to be statistically significant. We performed all analyses using a statistical software package (SAS version 9.2, SAS Institute).

RESULTS
Drain output was significantly less in the TXA-TAA group compared to that in the No TXA-TAA group (71.6 ± 60.3 vs 200.2 ± 117.0 mL, respectively, P = .0001) (Figure). The clinical characteristics of the patients who underwent TAA with the use of TXA are outlined in Table 2. The mean change in preoperative to postoperative hemoglobin levels was significantly lower in the TXA-TAA group than in the No TXA-TAA group (1.5 ± 0.6 vs 2.0 ± 0.4 g/dL, respectively; P = .01). The calculated blood loss in patients in the TXA-TAA group was significantly lower than that in patients in the No TXA-TAA group (649.9 ± 332.7 vs 906.8 ± 287.4 mL, respectively; P = .01). No patient in either group received a blood transfusion. We did not observe a significant difference in the length of surgery between the TXA-TAA and the No TXA-TAA groups (112.8 ± 24.8 vs 108.6 ± 26.0 min, respectively; P = .57). The average American Society of Anesthesiologists’ (ASA) classification was similar between the groups (2.2 ± 0.6 and 2.2 ± 0.5, respectively; P = 1.00) as was the age-adjusted Charlson Comorbidity Index (2.8 ± 1.7 vs 2.9 ± 1.6, respectively; P = .93). Mean VAS scores on postoperative day 1 in the TXA-TAA and the No TXA-TAA group were 4.9 ± 1.7 and 5.3 ± 1.4, respectively (P = .71). The average length of stay in the TXA-TAA group was 1.6 ± 0.7 days vs 1.3 ± 0.6 days in the No TXA-TAA group (P = .23). Two patients in the TXA-TAA group had an extended hospital length of stay of 5 days due to discharge planning and social issues.
Table 2. Clinical Characteristics of Total Ankle Arthroplasty (TAA) Patients by Use of Tranexamic Acid (TXA), N = 50 | |||
|---|---|---|---|
| TXA use in TAA | P valuea | |
| Yes (n = 25 cases) | No (n = 25 controls) |
|
Clinical Characteristic |
|
|
|
Drain Output (ml), mean ± SD
| 71.6 ± 60.3 | 200.2 ± 117.0 | <0.0001 |
Preoperative to Postoperative Hgb Change (g/dL), mean ± SD
| 1.5 ± 0.6 | 2.0 ± 0.4 | 0.01 |
Blood Loss Calculated (ml), mean ± SD
| 649.9 ± 332.73 | 906.8 ± 287.4 | 0.01 |
Length of Surgery (min), mean ± SD
| 112.8 ± 24.8 | 108.6 ± 26.0 | 0.57 |
VAS scores on the POD (No.), mean ± SD
| 4.9 ± 1.7 | 5.3 ±1.4 | 0.71 |
LOS (day), mean ± SD
| 1.6 ± 0.7 | 1.3 ± 0.6 | 0.23 |
aP value was calculated from t-test for continuous variables, and Chi-square test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
Abbreviations: LOS, length of stay; VAS, visual analog scale; POD, postoperative day.
Table 3. Linear Regression Analyses of Drain Output and Blood Loss using Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), Unadjusted and Adjusted Models for Length of Surgery, N = 50 | ||||
| TXA Use in TAA (Yes vs No) | |||
Drain Output (mL)
| Regression coefficient (β) | SE | Test statistics (t) | P valuea |
Unadjusted Model | -128.6 | 26.3 | -4.89 | < 0.0001 |
Adjusted for Age | -129.6 | 26.5 | -4.89 | <0.0001 |
Adjusted for BMI | -121.8 | 26.6 | -4.57 | <0.0001 |
Adjusted for Length of Surgery | -129.6 | 26.6 | -4.86 | <0.0001 |
Multivariable Modelb | -123.4 | 27.1 | -4.55 | <0.0001 |
Blood Loss (mL)
|
|
|
|
|
Unadjusted Model | -257.0 | 87.9 | -2.92 | 0.005 |
Adjusted for Age | -263.7 | 87.4 | -3.02 | 0.004 |
Adjusted for BMI | -268.7 | 90.2 | -2.98 | 0.005 |
Adjusted for Length of Surgery | -261.3 | 88.6 | -2.94 | 0.005 |
Multivariable Modelb | -275.6 | 90.7 | -3.04 | 0.004 |
aLinear regression was used to calculate the P value. bAdjusted for age, BMI and length of surgery.
Abbreviation: BMI, body mass index.
Table 4. Patient Wound Complication Categories by Use of Tranexamic Acid (TXA) in Total Ankle Arthroplasty (TAA), N = 50 | |||
|---|---|---|---|
| TXA Use in TAA | P valuea | |
Wound Complication | Yes (n = 25 cases) | No (n = 25 controls) | 0.114 |
None, n = 46 (86%) | 23 (40%) | 20 (46%) |
|
Minor, n = 6 (12%) | 2 (4%) | 4 (8%) |
|
Major, n = 1 (2%) | 0 (0%) | 1 (4%) |
|
aP value was calculated from Fisher’s Exact test (67% cells had count <5) test for categorical variables (TXA-TAA vs No TXA-TAA comparison).
The crude linear regression model revealed a significant difference in drain output between the TXA-TAA and the No TXA-TAA groups (β = −128.6 ± 26.3, P < .0001) (Table 3). Further adjustment for age and length of surgery slightly strengthened the association (β = −129.6 ± 26.6, P < .0001). The nature of regression coefficient β showed that the mean estimate of drain output was 129.6 mL lower in the TXA-TAA group than that in the No TXA-TAA group. There was a significant difference in blood loss between the TXA-TAA and the No TXA-TAA groups in the crude linear regression model (β = −257.0 ± 87.9, P = .005). Additional adjustment for age, BMI, and length of surgery slightly strengthened the association (β = −275.6 ± 90.7, P = .004). The nature of regression coefficient β showed that the mean estimate of blood loss was 275.6 mL lower in the TXA-TAA group than in the No TXA-TAA group (Table 3).
Continue to: There was no statistically significant difference...
There was no statistically significant difference in wound complications between the TXA-TAA and the No TXA-TAA groups in this study population (P = .114). However, our results showed a higher overall wound complication rate in the No TXA-TAA group than in the TXA-TAA group (20% (5/25) vs 8% (2/25), respectively) (Table 4). In the No TXA-TAA group, there were 4 minor and 1 major wound complications. All 5 patients experiencing a postoperative wound complication required oral antibiotics for a minimum of 4 weeks and local wound care. One patient underwent a surgical débridement meeting the criteria for major wound complications. In the TXA-TAA group, there were 2 minor wound complications and no major wound complications. One patient was administered prophylactic oral antibiotics for 7 days with local wound care for blister formation without evidence of infection. The second patient experiencing a minor wound complication required 3 weeks of oral antibiotics and local wound care. No patients in either group had a deep infection requiring implant removal, IV antibiotics, or subsequent hospital admission. The surgical incisions in all patients healed after the aforementioned treatments with no persistent drainage or development of chronic wounds.
In the TXA-TAA group, there was 1 patient who sustained an intraoperative medial malleolus fracture. One patient developed an extensor hallucis longus contracture 5 months postoperatively that subsequently underwent release and lengthening. There was 1 patient in this group who sustained a distal tibia fracture 5 cm proximal to the prosthesis 3 months postoperatively after a mechanical fall. In the No TXA-TAA group, there were 2 patients who sustained intraoperative medial malleolus fractures. One patient underwent a revision of the tibial component 24 months postoperatively due to aseptic loosening. In addition, another patient in this group who sustained an Achilles tendon rupture 5 months postoperatively after a fall subsequently underwent repair with tibialis anterior tendon allograft.
There were no patients in either group who experienced any hospital readmissions in the acute follow-up period as defined by a 90-day period after discharge. There were no complications associated with TXA administration in either group.
DISCUSSION
Recent advances in total ankle prosthetic design coupled with increased survival and improved short- to midterm follow-up results make TAA an effective treatment option for end-stage ankle arthritis. Management of perioperative blood loss and reducing the potential for significant hemarthrosis and subsequent wound complications are important factors to consider for patients undergoing TAA. TXA administration is used in several centers as part of an intraoperative strategy to reduce blood loss and decrease intra-articular blood accumulation. To our knowledge, this is the first study to evaluate the management of blood loss and hemarthrosis using TXA during TAA.
IV and topical administrations of TXA have been demonstrated to be highly effective hemostatic agents in the perioperative period for TKA and THA.11 Recent literature has demonstrated a significant reduction in drain output and mean change in preoperative to postoperative hemoglobin levels in patients who received TXA compared to that in patients who did not receive TXA. The patients who did not receive TXA had more than twice as much drain output.5,10,14,19-21
Continue to: The ankle has a thin...
The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of the bone during TAA are not as extensive when compared with TKA and THA, but the intra-articular volume is smaller and the surrounding soft tissues may be less yielding when blood accumulation occurs.22 The vascular supply can be rich surrounding the ankle in the absence of arterial disease and is not as apt to tolerate dislocation and subluxation as in the case of THA or TKA.23 Shear forces can easily tear the branches of the anterior tibial artery that lie within the fascia that is continuous with the periosteum on the distal tibia.24 Reduction of hemarthrosis within the ankle joint may lead to a decrease in postoperative swelling, decreased pain, and increased range of motion due to the diminished potential for fibrosis. We also believe that there could be a reduced risk for wound complications. The current literature reports the rate of wound complications to be anywhere from 2% to 25%, with diabetes, inflammatory conditions, coronary artery disease, peripheral vascular disease, and smoking history >12-pack-years as risk factors.16,25,26 In this study, we observed a significant reduction in drain output and an overall reduced percentage of postoperative wound complications in patients who received TXA. These results demonstrate that TXA use decreases postoperative hemarthrosis.
TXA use in TKA and THA has been shown to decrease direct hospital costs and hospital length of stay.7,14,27 A recent study by Moskal and colleagues7 showed that topical TXA use has the potential to significantly decrease hospital man-hours for those patients undergoing TKA and achieve larger cost savings. Although there was no significant difference in the length of stay between the 2 groups, the average length of stay after TAA was shorter in both groups compared to the reported national average (1.49 vs 2.2 days, respectively).4 The administration of TXA in the appropriate patient has the potential to decrease hospital costs by controlling postoperative pain and swelling, allowing for earlier discharge. Long-term cost benefits could also include decreased infection rates and wound complications, and improved clinical outcomes because of improved range of motion and function scores.
The limitations of this study include the retrospective nature of its design and the relatively small sample size. The results showed nonstatistically significant differences in wound complications between the TXA-TAA and the No TXA-TAA groups, consistent with an insufficient sample size and thus inadequate power to detect the significant difference. However, this study clearly showed that the wound complication rates were higher in the No TXA-TAA group than in the TXA-TAA group, suggesting the importance of further similar studies using a larger sample size.
CONCLUSION
Current TAA offers a viable alternative to arthrodesis for end-stage ankle arthritis. TXA is an inexpensive and effective hemostatic agent used during TAA. If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management, decrease postoperative hemarthrosis, and help to reduce the risk of postoperative wound complications.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
1. Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44-46.
2. Glazebrook M, Daniels T, Younger A, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499-505. doi:10.2106/JBJS.F.01299.
3. Thomas RH, Daniels TR. Ankle arthritis. J Bone Joint Surg Am. 2003;85-A(5):923-936.
4. Zhou H, Yakavonis M, Shaw JJ, Patel A, Li X. In-patient trends and complications after total ankle arthroplasty in the United States. Orthopedics. 2016:1-6. doi:10.3928/01477447-20151228-05.
5. Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg Br. 1996;78(3):434-440.
6. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005-1015. doi:10.1302/0301-620X.96B8.33745.
7. Moskal JT, Harris RN, Capps SG. Transfusion cost savings with tranexamic acid in primary total knee arthroplasty from 2009 to 2012. J Arthroplasty. 2015;30(3):365-368. doi:10.1016/j.arth.2014.10.008.
8. Friedman R, Homering M, Holberg G, Berkowitz SD. Allogeneic blood transfusions and postoperative infections after total hip or knee arthroplasty. J Bone Joint Surg Am. 2014;96(4):272-278. doi:10.2106/JBJS.L.01268.
9. Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty. 2016;31(7):1442-1448. doi:10.1016/j.arth.2015.12.033.
10. Su EP, Su S. Strategies for reducing peri-operative blood loss in total knee arthroplasty. Bone Joint J. 2016;98-B(1 Suppl A):98-100. doi:10.1302/0301-620X.98B.36430.
11. Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96(23):1937-1944. doi:10.2106/JBJS.N.00060.
12. Cap AP, Baer DG, Orman JA, Aden J, Ryan K, Blackbourne LH. Tranexamic acid for trauma patients: a critical review of the literature. J Trauma. 2011;71(1 Suppl):S9-14. doi:10.1097/TA.0b013e31822114af.
13. Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30(2):272-276. doi:10.1016/j.arth.2014.08.022.
14. Alshryda S, Mason J, Vaghela M, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total knee replacement: a randomized controlled trial (TRANX-K). J Bone Joint Surg Am. 2013;95(21):1961-1968. doi:10.2106/JBJS.L.00907.
15. Ng W, Jerath A, Wasowicz M. Tranexamic acid: a clinical review. Anaesthesiol Intensive Ther. 2015;47(4):339-350. doi:10.5603/AIT.a2015.0011.
16. Raikin SM, Kane J, Ciminiello ME. Risk factors for incision-healing complications following total ankle arthroplasty. J Bone Joint Surg Am. 2010;92(12):2150-2155. doi:10.2106/JBJS.I.00870.
17. Meunier A, Petersson A, Good L, Berlin G. Validation of a haemoglobin dilution method for estimation of blood loss. Vox Sang. 2008;95(2):120-124. doi:10.1111/j.1423-0410.2008.01071.x.
18. Gibon E, Courpied JP, Hamadouche M. Total joint replacement and blood loss: what is the best equation? Int Orthop. 2013;37(4):735-739. doi:10.1007/s00264-013-1801-0
19. Chareancholvanich K, Siriwattanasakul P, Narkbunnam R, Pornrattanamaneewong C. Temporary clamping of drain combined with tranexamic acid reduce blood loss after total knee arthroplasty: a prospective randomized controlled trial. BMC Musculoskelet Disord. 2012;13:124.
20. Orpen NM, Little C, Walker G, Crawfurd EJ. Tranexamic acid reduces early post-operative blood loss after total knee arthroplasty: a prospective randomised controlled trial of 29 patients. Knee. 2006;13(2):106-110. doi:10.1016/j.knee.2005.11.001.
21. Veien M, Sorensen JV, Madsen F, Juelsgaard P. Tranexamic acid given intraoperatively reduces blood loss after total knee replacement: a randomized, controlled study. Acta Anaesthesiol Scand. 2002;46(10):1206-1211.
22. Draeger RW, Singh B, Parekh SG. Quantifying normal ankle joint volume: An anatomic study. Indian J Orthop. 2009;43(1):72-75. doi:10.4103/0019-5413.45326.
23. Gill LH. Challenges in total ankle arthroplasty. Foot Ankle Int. 2004;25(4):195-207. doi:10.1177/107110070402500402.
24. Taylor GI, Pan WR. Angiosomes of the leg: anatomic study and clinical implications. Plast Reconstr Surg. 1998;102(3):599-616; discussion 617-598. doi:10.1097/00006534-199809030-00001.
25. Gougoulias N, Khanna A, Maffulli N. How successful are current ankle replacements?: a systematic review of the literature. Clin Orthop Relat Res. 2010;468(1):199-208. doi:10.1007/s11999-009-0987-3.
26. Noelle S, Egidy CC, Cross MB, Gebauer M, Klauser W. Complication rates after total ankle arthroplasty in one hundred consecutive prostheses. Int Orthop. 2013;37(9):1789-1794. doi:10.1007/s00264-013-1971-9.
27. Chimento GF, Huff T, Ochsner JL Jr, Meyer M, Brandner L, Babin S. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty. 2013;28(8 Suppl):74-77. doi:10.1016/j.arth.2013.06.037.
TAKE-HOME POINTS
- TXA is an inexpensive and effective hemostatic agent used during TAA.
- The ankle has a thin soft tissue envelope that does not have elaborate elastic properties. The soft tissue release and bleeding surfaces of bone during TAA are not as extensive when compared to TKA and THA, but the intra-articular volume is smaller and surrounding soft tissues may be less yielding when blood accumulation occurs.
- If no major contraindication is present, routine use of TXA is recommended to assist in blood loss management during TAA.
- TXA decreases postoperative hemarthrosis and helps to reduce the risk of postoperative wound complications.
- The administration of TXA in the appropriate patient has the potential to decrease hospital cost by controlling postoperative pain and swelling allowing for earlier discharge.
Osteochondritis Dissecans Lesion of the Radial Head
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).
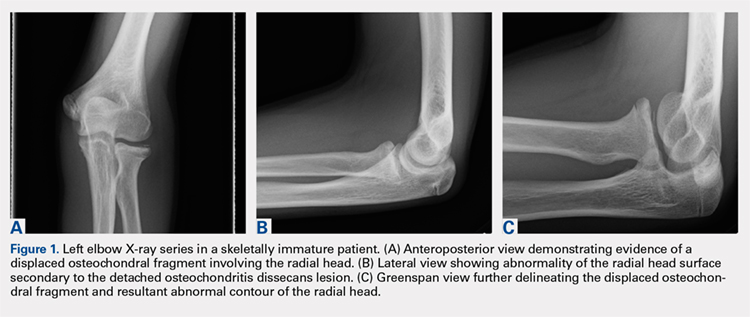
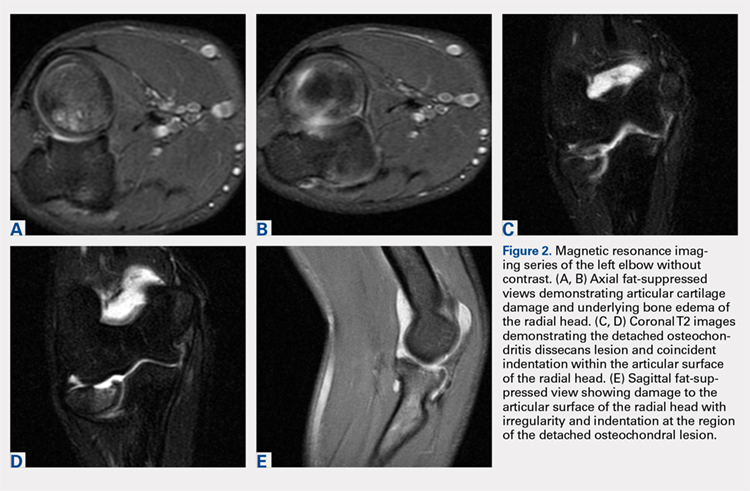
Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).
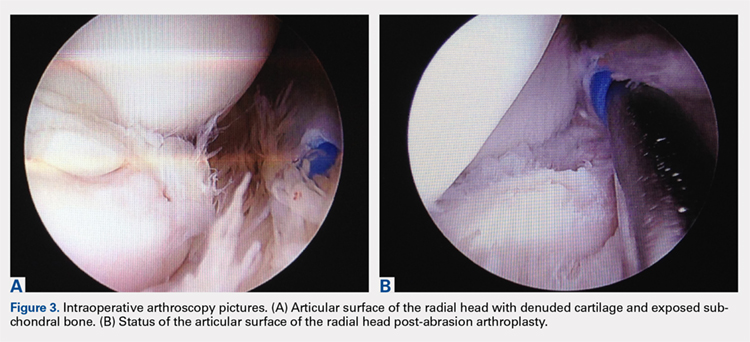
The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
ABSTRACT
This case shows an atypical presentation of an osteochondritis dissecans (OCD) lesion of the radial head with detachment diagnosed on plain radiographs and magnetic resonance imaging (MRI). OCD lesions are rather uncommon in the elbow joint; however, when present, these lesions are typically seen in throwing athletes or gymnasts who engage in activities involving repetitive trauma to the elbow. Involvement of the radial head is extremely rare, accounting for <5% of all elbow OCD lesions. Conventional radiographs have low sensitivity for detecting OCD lesions and may frequently miss these lesions in the early stages. MRI, the imaging modality of choice, can detect these lesions at the earliest stage and provide a clear picture of the involved articular cartilage and underlying bone. Treatment options can vary between nonoperative and operative management depending on several factors, including age and activity level of the patient, size and type of lesion, and clinical presentation. This case represents a radial head OCD lesion managed by arthroscopic débridement alone, resulting in a positive outcome.
Continue to: Case Report...
CASE REPORT
A healthy, 14-year-old, left-hand-dominant adolescent boy presented to the office with a chief complaint of pain localized to the posterolateral aspect of his elbow. He described an injury where he felt a “pop” in his elbow followed by immediate pain in the posterolateral elbow after throwing a pitch during a baseball game. Since the injury, the patient had experienced difficulty extending his elbow and a sharp, throbbing pain during forearm rotation. The patient also reported an intermittent clicking feeling in the elbow. Prior to this injury, he had no elbow pain. He presented in an otherwise normal state of health with no reported past medical or surgical history and no previous trauma to the left upper extremity.
Physical examination demonstrated a mild effusion of the left elbow in the region of the posterolateral corner or “soft spot” with tenderness to palpation over the radial head. The patient had restricted elbow motion with 30° to 135° of flexion. He had 90° of pronation and supination. Ligamentous examination revealed stability of the elbow to both varus and valgus stress at 30° of flexion. No deficits were observed upon upper-extremity neurovascular examination.
Plain radiographs of the left elbow were initially taken. Anteroposterior, lateral, and Greenspan views revealed evidence of a displaced osteochondral fragment of the radial head in this skeletally immature patient. No involvement of the capitellum was apparent (Figures 1A-1C). Non-contrast magnetic resonance imaging (MRI) of the left elbow was subsequently obtained to evaluate the lesion further, and the images confirmed an unstable osteochondritis dissecans (OCD) lesion of the radial head with a detached fragment entrapped within the elbow joint (Figures 2A-2E).


Elbow arthroscopy was performed to evaluate the extent of the OCD lesion to enable determination of the integrity of the cartilaginous surface and remove the loose body entrapped within the elbow joint. Multiple loose bodies (all <5 mm in size) were removed from the elbow joint. Visualization of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. The main chondral defect measured approximately 4 mm in size. Probing of the lesion confirmed no stable edge; thus, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth (Figures 3A, 3B).

The patient was started on physical therapy consisting of active and active-assisted elbow ranges of motion on postoperative day 10. At the 6-week follow up, the patient presented to the office with pain-free motion of the left elbow ranging from −5° to 135° of flexion. He maintained full pronation and supination. At this point, the patient was advised to begin a throwing program. Three months after treatment, the patient resumed baseball activities, including throwing, with pain-free, full range of motion of the elbow. The patient and the patient’s parents provided written informed consent for print and electronic publication of this case report.
Continue to: Discussion...
DISCUSSION
Elbow pain is a common complaint among young baseball players. OCD lesions, however, are an uncommon entity associated with elbow pathology.1 The overall incidence of OCD lesions is between 15 to 30 per 100,000 people.2-3 Specifically in patients aged 2 to 19 years, the incidence of elbow OCD lesions is 2.2 per 100,000 patients and 3.8 and 0.6 per 100,000 for males and females, respectively.4 Radial head OCD lesions are extremely rare, occurring in <5% of all elbow OCD cases.1 The majority of these lesions are asymptomatic and typically seen in patients who engage in repetitive overhead and upper-extremity weight-bearing activities. Reports indicate that the incidence of these lesions is on the rise and the age of presentation is decreasing, likely because of increased awareness of the disease and increasing involvement of young athletes in competitive athletics.4-5 Most patients with elbow OCD have a history of repetitive overuse of the elbow, as seen in baseball players, leading to excessive compressive and shear forces across the radiocapitellar joint and progression of the dissecans lesion.6
Patients with OCD lesions of the elbow typically present with inflammatory type symptoms and lateral elbow pain. The pain tends to be mild at rest and becomes more pronounced with activity. Patients often wait until mechanical symptoms ensue (eg, clicking, catching, or locking) before presenting to the office. On physical examination, pain in the region of the OCD lesion is usually accompanied by a mild effusion. Stiffness, particularly a loss of terminal extension, may accompany the mechanical symptoms on range of motion testing.7
Workup of elbow OCD lesions begins with obtaining plain radiographs of the elbow. Plain films are of limited use in evaluating these lesions but can help determine separation and the approximate size of the fragment.8 Further work-up must include MRI sequences, which allow for the best evaluation of the articular cartilage, underlying bone, and, specifically, the size and degree of separation of the OCD lesion.9
Nonoperative treatment of OCD lesions is usually successful if diagnosed early. Such treatment consists of activity modification, rest, anti-inflammatory medications, and a gradual return to athletic activities over the next 3 to 6 months provided the symptoms abate.10-11 During this interval, physical therapy may be employed to preserve or regain range of motion in the elbow. Clinical evidence has demonstrated improved outcomes in younger athletes with open physes.12 Returning to athletic activities is advised only when complete resolution of symptoms has been achieved and full motion about the elbow and shoulder girdle has been regained.6
If symptoms persist despite nonoperative management, or if evidence of an unstable lesion (ie, detached fragment) is obtained, operative intervention is appropriate. Operative management includes diagnostic arthroscopy of the entire elbow, removal of any small, loose bodies, and synovectomy as needed. Thereafter, the OCD lesion must be addressed. In cases of capitellar OCD lesions, if the articular cartilage surface is intact, antegrade or retrograde drilling of the subchondral bone is appropriate and will likely result in a good-to-excellent functional outcome.13-14 If disruption to the articular cartilage fissures is found or the lesion appears to be separating from the native bone, fixation of the fragment can be attempted, provided an adequate portion of the subchondral bone remains attached to the OCD lesion.6,14 Oftentimes, the bony bed must be prepared prior to fixation by removal of any fibrous tissue overlying the subchondral bone and ensuring adequate bleeding across the entire bed. Care should be taken to remove any fibrous tissue underlying the OCD lesion. If the OCD lesion is completely loose and/or the bone stock is insufficient or fragmented, arthroscopic removal of the OCD lesion followed by débridement and abrasion arthroplasty of subchondral bone is recommended.15 Improved functional outcomes from this procedure can be expected in contained lesions.15 If the patient continues to be symptomatic, osteochondral autograft or allograft procedures can be attempted depending on the size of the remaining defect.16-18
Other cases of radial head OCD lesions have been reported in the literature.19-20 In 2009, Dotzis and colleagues19 reported a case of an OCD lesion that was managed nonsurgically with observation alone as the lesion was stable and non-detached. Tatebe and colleagues20 reported 4 cases in which OCD involved the radial head and was accompanied by radial head subluxation. All lesions were located at the posteromedial aspect of the radial head with anterior subluxation of the radial head.20 Three of the cases were managed surgically via ulnar osteotomy (2 cases) and fragment removal (1 case).20 All except the 1 case treated by fragment excision revealed a good outcome.20 The patient in this case presented with a detached lesion, confirmed on MRI, with pain, mechanical symptoms, and of loss of terminal extension. Given the chronicity of the injury and the presence of mechanical symptoms, the decision was made to proceed with operative intervention. During elbow arthroscopy, multiple loose bodies were removed from the elbow joint, and inspection of the radiocapitellar joint revealed extensive cartilage damage to the radial head with multiple areas of denuded cartilage and exposed bone. Since the OCD lesion was completely loose and the bone stock was insufficient and too fragmented to attempt fixation, abrasion arthroplasty was performed to stabilize the lesion and stimulate future fibrous cartilage growth. At the 6-week follow up, the patient regained full range of motion of this elbow with no complaints of pain. At the 3-month follow up, the patient reported no pain after returning to throwing and all baseball-related activities.
CONCLUSION
This report presents an extremely rare case of an OCD lesion involving the radial head. Diagnosis and treatment of this lesion followed a protocol similar to that used for the management of capitellar OCD lesions. When dealing with elbow OCD lesions, especially in the skeletally immature patient population, nonsurgical management and a gradual return to activities should be attempted. If symptoms persist despite nonoperative management or evidence of an unstable lesion (as presented in this case) is obtained, operative intervention is appropriate.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
- Jans LB, Ditchfield M, Anna G, Jaremko JL, Verstraete KL. MR imaging findings and MR criteria for instability in osteochondritis dissecans of the elbow in children. Eur J Radiol. 2012;81(6):1306-1310. doi:10.1016/j.ejrad.2011.01.007.
- Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg. 1984;66(9):1340-1348. doi:10.2106/00004623-198466090-00003.
- Lindén B. The incidence of osteochondritis dissecans in the condyles of the femur. Acta Orthop Scand. 1976;47(6):664-667. doi:10.3109/17453677608988756.
- Kessler JI, Nikizad H, Shea KG, Jacobs JC, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320-326. doi:10.1177/0363546513510390.
- Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: current Concepts Review. Am J Sports Med. 2006;34(7):1181-1191. doi:10.1177/0363546506290127.
- Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89(6):1205-1214. doi:10.2106/JBJS.F.00622.
- Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteo Chondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216(1):207-212. doi:10.1148/radiology.216.1.r00jl29207.
- Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34(5):266-271. doi:10.1007/s00256-005-0899-6.
- Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453-1459. doi:10.2214/AJR.04.1570.
- Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27(6):728-732. doi:10.1177/03635465990270060701.
- Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;363(363):108-115. doi:10.1097/00003086-199906000-00014.
- Pill SG, Ganley TJ, Milam RA, Lou JE, Meyer JS, Flynn JM. Role of magnetic resonance imaging and clinical criteria in predicting successful nonoperative treatment of osteochondritis dissecans in children. J Pediatr Orthop. 2003;23(1):102-108. doi:10.1097/01241398-200301000-00021.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Byrd JWT, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30(4):474-478. doi:10.1177/03635465020300040401.
- Krijnen MR, Lim L, Willems WJ. Arthroscopic treatment of osteochondritis dissecans of the capitellum: report of 5 female athletes. Arthroscopy. 2003;19(2):210-214. doi:10.1053/jars.2003.50052.
- Mihara K, Suzuki K, Makiuchi D, Nishinaka N, Yamaguchi K, Tsutsui H. Surgical treatment for osteochondritis dissecans of the humeral capitellum. J Shoulder Elbow Surg. 2010;19(1):31-37. doi:10.1016/j.jse.2009.04.007.
- Yamamoto Y, Ishibashi Y, Tsuda E, Sato H, Toh S. Osteochondral autograft transplantation for osteochondritis dissecans of the elbow in juvenile baseball players: minimum 2-year follow-up. Am J Sports Med. 2006;34(5):714-720. doi:10.1177/0363546505282620.
- Ahmad CS, ElAttrache NS. Mosaicplasty for capitellar osteochondritis dissecans. In: Yamaguchi K, O'Driscoll S, King G, McKee M, eds. [In press] Advanced Reconstruction Elbow. Rosemont, IL: American Academy of Orthopaedic Surgeons.
- Dotzis A, Galissier B, Peyrou P, Longis B, Moulies D. Osteochondritis dissecans of the radial head: a case report. J Shoulder Elbow Surg. 2009;18(1):e18-e21. doi:10.1016/j.jse.2008.04.009.
- Tatebe M, Hirata H, Shinohara T, Yamamoto M, Morita A, Horii E. Pathomechanical significance of radial head subluxation in the onset of osteochondritis dissecans of the radial head. J Orthop Trauma. 2012;26(1):e4-e6. doi:10.1097/BOT.0b013e318214d678.
TAKE-HOME POINTS
- Radial Head OCD lesions are uncommon.
- Typically present in athletes that engage in repetitive trauma to elbow (throwers, gymnasts).
- MRI is the best modality for making diagnosis.
- Attempt nonsurgical treatment initially, especially in skeletally immature patients.
- If nonsurgical fails or there is an unstable lesion, consider operative intervention.
Volumetric Considerations for Valving Long-Arm Casts: The Utility of the Cast Spacer
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.
Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).
We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |
Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |
Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Fiberglass casts are frequently valved to accommodate swelling following injury or surgery. The use of cast spacers has been recommended to bridge this gap between pressure reduction and cast strength, but no studies have assessed their effect on cast pressure.
We applied 30 long-arm fiberglass casts to adult volunteers, divided between a univalve group and a bivalve group. A pediatric blood pressure bladder was applied under the cast to simulate soft tissue swelling. Valved casts were secured using an elastic wrap, 10-mm cast spacer, or 15-mm cast spacer. Measurements of cast pressure and circumference were performed at each stage and compared on the basis of type of valve and securement.
Our results indicated that cast univalving resulted in an approximately 60% reduction in cast pressures, with a 75% reduction seen in the bivalve group. The addition of cast spacers resulted in significant pressure reductions for both valving groups. The univalve group secured with a 10-mm cast spacer produced reductions in cast pressure similar to those of the elastic-wrapped bivalve cast, both with the cast padding intact and with it released.
The use of cast spacers results in significant cast pressure reductions, regardless of valving technique. A univalved cast secured with a cast spacer can produce decreases in cast pressures similar to those seen with an elastic-wrapped bivalved cast, and it is a viable option for reducing cast pressure without compromising cast structural integrity with a bivalve technique.
Continue to: Complications following closed reduction...
Complications following closed reduction and casting of pediatric forearm fractures are rare, but they do occur. Arguably the most devastating of these complications is the risk of developing compartment syndrome or Volkmann contracture secondary to injury-associated swelling under a circumferential cast.1-4 The peak in swelling can develop from 4 to 24 hours following the initial cast application,5 and as such, medical providers may not be able to identify it early because most children are discharged following closed reductions. For this reason, many providers implement prophylactic measures to minimize pressure-related complications.
A popular method for reducing pressure accumulation within a cast is to valve, or cut, the cast. Previous investigations have shown that cast valving results in significant reductions in cast pressure.2,6-9 Bivalving a circumferential cast results in significantly greater reductions in cast pressure when compared with univalve techniques;6,7,9 however, bivalving has also been shown to result in significant impairment in the structural integrity of the cast.10 An additional method to facilitate cast pressure reduction without impairing the structural integrity of the cast that accompanies a bivalve is to incorporate a cast spacer with a univalve technique to hold the split cast open.11 Although this method is commonly used in clinical practice, its ability to mitigate cast pressures has not previously been investigated.
The goal of this study is to investigate the influence of incorporating cast spacers with valved long-arm casts. We hypothesized that cast spacers would provide a greater pressure reduction for both univalved and bivalved casts when compared with the use of an elastic wrap. Additionally, we proposed that by incorporating a cast spacer with a univalved cast, we could attain pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap.
MATERIALS AND METHODS
Upon receiving approval from the Institutional Review Board, experimental testing began with the application of 30 total casts performed on uninjured adult human volunteers. Pressure readings were provided with the use of a bladder from a pediatric blood pressure cuff (Welch Allyn Inc), as previously described.6 The bladder was placed on the volar aspect of the volunteer’s forearm, held in place with a 3-in diameter cotton stockinet (3M). Cotton cast padding (Webril-Kendall) was applied, 3 in wide and 2 layers thick, and a long-arm cast was applied, 2 layers thick with 3-in wide fiberglass casting material (Scotchcast Plus Casting Tape; 3M).
Once the cast was applied and allowed to set, the blood pressure bladder was inflated to 100 mm Hg. After inflation, forearm cast circumference was measured at 2 set points, assessed at points 2 cm distal to the elbow flexor crease and 10 cm distal to the previous point (Figure 1). Using these data, we calculated estimated cast volume using the volumetric equation for a frustum. Following this point, casts were split into 2 experimental groups, univalve or bivalve, with 15 casts comprising each group. The univalve group consisted of a single cut along the dorsum of the extremity, and the bivalve group incorporated a second cut to the volar extremity. Cast valving was performed using an oscillating cast saw (Cast Vac; Stryker Instruments), with care taken to ensure the continuity of the underlying cast padding.

Continue to: Following valving, casts were secured via...
Following valving, casts were secured via 3 separate techniques: overwrap with a 3-in elastic wrap (Econo Wrap; Vitality Medical), application of two 10-mm and 15-mm cast spacers (CastWedge; DM Systems) (Figure 2). After securement, cast pressures were recorded, and circumference measurements were performed at the 2 previously identified points. The cast padding was then cut at the valve site and secured via the 3 listed techniques. Cast pressure and circumference measurements were performed at set time points (Figure 3). Changes in cast pressure were recorded in terms of the amount of change from the initial cast placement to account for differences in the size of volunteers’ forearms. Volumetric calculations were performed only for the spacer subgroups owing to the added material in the elastic wrap group. Estimated cast volume was calculated using the equation for volume of a frustum (Figure 4).



We used a 2-cast type (univalve and bivalve) by 4 securement subgroups (initial, elastic wrap, 10-mm spacer, and 15-mm spacer) design, with cast type serving as a between-subject measure and securement serving as a within-subject variable. An a priori power analysis showed that a minimum sample size of 15 subjects per condition should provide sufficient power of .80 and alpha set at .05, for a total of 30 casts. Statistical analyses were performed using IBM SPSS Statistics software version 21 (IBM). Experimental groups were analyzed using mixed-design analysis of variance (ANOVA). Post hoc comparisons between valving groups and cast securement were performed using Scheffe’s test to control for type II errors. Change in cast volume between the initial cast and cast spacers groups was compared using paired Student’s t tests. Statistical significance was predetermined as P < .05.
RESULTS
A summary of collected data for cast pressure and volume is detailed in Table 1, subdividing the variables on the basis of cast type and type of securement. Recorded pressures of the different subgroups are depicted in Figures 5 and 6 according to type of securement (initial, elastic wrap, 10-mm spacer, or 15-mm spacer). Results of the mixed-design ANOVA demonstrated significant differences between the initial cast pressure and univalve and bivalve groups (P < .05). There was a main effect for bivalve having lower pressure overall (F [1, 1)] = 3321.51, P < .001). There was also a main effect indicating that pressure was different for each type of securement (elastic wrap, 10-mm spacer, 15-mm spacer) (F [1, 28] = 538.54, P <. 01). Post hoc testing confirmed pressure decreased significantly, in descending order from elastic wrap, to 10-mm spacers, to 15-mm spacers (P < .05).
Table 1. Cumulative Data for Two Casting groups at Each Timepoint
Cast | Pressure | Standard Deviation | Volume |
Univalve |
|
|
|
Initial | 100 | --- | 2654.3 |
Elastic Wrap | 39.47 | 3.33 | --- |
10-mm Spacer | 23.93 | 2.73 | 2708.23 |
15-mm Spacer | 18.87 | 2.94 | 2734.86 |
Padding and Elastic Wrap | 20.93 | 2.91 | --- |
Padding and 10-mm Spacer | 15.46 | 2.19 | 2733.24 |
Padding and 15-mm Spacer | 0 | --- | 2819.27 |
Bivalve |
|
|
|
Initial | 100 | --- | 2839.3 |
Elastic Wrap | 25.9 | 3.17 | --- |
10-mm Spacer | 16.53 | 2.32 | 3203.13 |
15-mm Spacer | 13.6 | 2.74 | 3380.32 |
Padding and Elastic Wrap | 12.67 | 1.95 | --- |
Padding and 10-mm Spacer | 0 | --- | 3296.55 |
Padding and 15- mm Spacer | 0 | --- | 3438.67 |


Continue to: Table 2...
The summary of volumetric changes is listed in Table 2. The decrease in pressure correlated with an associated increase in cast volume, as demonstrated in Figure 7. The degree of increase in cast volume was more pronounced in the bivalve group (P < .001). The volume increased in the 15-mm group compared with the 10-mm group for both groups (P < .001) and increased for each spacer group with the release of the underlying padding (P < .05).
Table 2. Volumetric Data
Cast | Average Volumetric change (cm3) | Standard Deviation |
Univalve |
|
|
10-mm Spacer | 175.6 | 65.4 |
15-mm Spacer | 269.4 | 73.3 |
Padding and 10-mm Spacer | 202.3 | 62.5 |
Padding and 15-mm Spacer | 294.1 | 66.9 |
Bivalve |
|
|
10-mm Spacer | 363.7 | 67.2 |
15-mm Spacer | 540.9 | 85.7 |
Padding and 10-mm Spacer | 457.2 | 97.9 |
Padding and 15-mm Spacer | 599.3 | 84.2 |

Analysis of the planned comparisons demonstrated no significant difference between the bivalve with elastic wrap and univalve with 10-mm spacer subgroups (t [28] = 1.85, P = .075, d = .68). In comparing the bivalve with elastic wrap group with the univalve and 15-mm spacer subgroup, the univalve group showed significantly lower pressures [t [28] = 6.32, P < .001, d = .2.31).
DISCUSSION
Valving of circumferential casting is a well-established technique to minimize potential pressure-related complications. Previous studies have demonstrated that univalving techniques produce a 65% reduction in cast pressure, whereas bivalving produces an 80% decrease.6,7,9 Our results showed comparable pressure reductions of 75% with bivalving and 60% with univalving. The type of cast padding has been shown to have a significant effect on the cast pressure, favoring lower pressures with cotton padding over synthetic and waterproof padding, which, when released, can provide an additional 10% pressure reduction.6,7
Although bivalving techniques are superior in pressure reduction, the reduction comes at the cost of the cast’s structural integrity. Crickard and colleagues10 performed a biomechanical assessment of the structural integrity by 3-point bending of casts following univalve and bivalve compared with an intact cast. The authors found that valving resulted in a significant decrease in the casts’ bending stiffness and load to failure, with bivalved casts demonstrating a significantly lower load to failure than univalved casts. One technique that has been used to enhance the pressure reduction in univalved casting techniques is the application of a cast spacer. Rang and colleagues11 recommended this technique as part of a graded cast-splitting approach for the treatment of children’s fractures. This technique was applied to fractures with only modest anticipated swelling, which accounted for approximately 95% of casts applied in their children’s hospital. Our results support the use of cast spacers, demonstrating significant reduction in cast pressure in both univalve and bivalve techniques. Additionally, we found that a univalved cast with a 10-mm cast spacer provided pressure reduction similar to that of a bivalved cast.
The theory behind the application of cast spacers is that a split fiberglass cast will not remain open unless held in position.11 Holding the cast open is less of a restraint to pressure reduction in bivalving techniques, because the split cast no longer has the contralateral intact hinge point to resist cast opening, demonstrated in the compromise in structural integrity seen with this technique.10 By maintaining the split cast in an opened position, the effective volume of the cast is increased, which allows for the reduction in cast pressure. This is demonstrated in our results indicating an increase in estimated cast volume with an associated incremental reduction in cast pressure with the application of incrementally sized cast spacers. Although this technique does have the potential for skin irritation caused by cast expansion, as well as local swelling at the cast window location, it is a cost-effective treatment method compared with overwrapping a bivalved cast, $1.55 for 1 cast spacer vs an estimated $200 for a forearm cast application.
This study is not without its limitations. Our model does not account for the soft tissue injury associated with forearm fractures. However, by using human volunteers, we were able to include the viscoelastic properties that are omitted with nonliving models, and our results do align with those of previous investigations regarding pressure change following valving. We did not incorporate a 3-point molding technique commonly used with reduction and casting of acute forearm fractures, owing to the lack of a standardized method for applying the mold to healthy volunteers. Although molding is necessary for most fractures in which valving is considered, we believe our data still provide valuable information. Additionally, valving of circumferential casts has not been shown, prospectively, to result in a reduction of cast-related compartment syndrome, maintenance of reduction, or need for surgery.12,13 However, these results are reflective of reliable patients who completed the requisite follow-up care necessary for inclusion in a randomized controlled trial and may be applicable to unreliable patients or patient situations, a setting in which the compromise in cast structural integrity may be unacceptable.
CONCLUSION
We demonstrated that incorporating cast spacers into valved long-arm casts provides pressure reduction comparable to that achieved with the use of an elastic wrap. The addition of a 10-mm cast spacer to a univalved long-arm cast provides pressure reduction equivalent to that of a bivalved cast secured with an elastic wrap. A univalved cast secured with a cast spacer is a viable option for treatment of displaced pediatric forearm fractures, without compromising the cast’s structural integrity as required with bivalved techniques.
This paper will be judged for the Resident Writer’s Award.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
- Halanski M, Noonan KJ. Cast and splint immobilization: complications. J Am Acad Orthop Surg. 2008;16(1):30-40.
- Zaino CJ, Patel MR, Arief MS, Pivec R. The effectiveness of bivalving, cast spreading, and webril cutting to reduce cast pressure in a fiberglass short arm cast. J Bone Joint Surg Am. 2015;97(5):374-380. doi:10.2106/JBJS.N.00579.
- Rodriguez-Merchan EC. Pediatric fractures of the forearm. Clin Orthop Relat Res. 2005;(432):65-72.
- von Volkmann R. Ischaemic muscle paralyses and contractures. Clin Orthop Relat Res. 1967;50:5-56. doi:10.1097/BLO.0b013e318032561f.
- Patrick JH, Levack B. A study of pressures beneath forearm plasters. Injury. 1981;13(1):37-41.
- Roberts A, Shaw KA, Boomsma SE, Cameron CD. Effect of casting material on the cast pressure after sequential cast splitting. J Pediatr Orthop. 2017;37(1):74-77. doi:10.1097/BPO.0000000000000574.
- Garfin SR, Mubarak SJ, Evans KL, Hargens AR, Akeson WH. Quantification of intracompartmental pressure and volume under plaster casts. J Bone Joint Surg Am. 1981;63(3):449-453.
- Capo JT, Renard RL, Moulton MJ, et al. How is forearm compliance affected by various circumferential dressings? Clin Orthop Relat Res. 2014 472(10):3228-3234. doi:10.1007/s11999-014-3747-y.
- Bingold AC. On splitting plasters. A useful analogy. J Bone Joint Surg Br. 1979;61-b(3):294-295.
- Crickard CV, Riccio AI, Carney JR, Anderson TD. Analysis and comparison of the biomechanical properties of univalved and bivalved cast models. J Pediatr Orthop.2011;31(1):39-43. doi:10.1097/BPO.0b013e318202c446.
- Rang M, Wenger DR, Pring ME. Rang's Children's Fractures. 3rd ed. Wenger DR, Rang M, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Schulte D, Habernig S, Zuzak T, et al. Forearm fractures in children: split opinions about splitting the cast. Eur J Pediatr Surg. 2014;24(2):163-167. doi:10.1055/s-0033-1341412.
- Bae DS, Valim C, Connell P, Brustowicz KA, Waters PM. Bivalved versus circumferential cast immobilization for displaced forearm fractures: a randomized clinical trial to assess efficacy and safety. J Pediatr Orthop. 2017;37(4):239-246 doi:10.1097/BPO.0000000000000655.
TAKE-HOME POINTS
- Valving a long-arm cast results in decreased cast pressures.
- Univalving can produce a 60% reduction in cast pressure.
- Bivalving produces a 75% reduction in cast pressure.
- Release of the underlying cast padding produces an additional pressure reduction.
- Adding a cast spacer to a univalved cast obtains similar pressure reduction to a bivalved cast.
Preventative Care in Orthopedics: Treating Injuries Before They Happen
By 2025, it is estimated that the annual cost of treating osteoporosis-related fractures in the United States will be 25 billion dollars, which is 10 billion dollars more than was spent in 2010.1 As healthcare costs in the United States continue to skyrocket, it is imperative that orthopedic surgeons take an active role in avoiding preventable injury and disease. For orthopedic surgeons, preventative medicine will include promoting bone health and educating patients on injury prevention. By incorporating these principles into residency and fellowship education, and by leveraging the electronic medical record to support preventive care through systematic reminders, orthopedic surgeons have a critical opportunity to take a leading role in promoting prevention to our patients.
In 2009, the American Orthopaedic Association (AOA) launched a “Own the Bone” campaign, a national quality improvement program designed to optimize the treatment of osteoporosis.2 This program came about following the Surgeon General’s call, in 2004, for orthopedic surgeons to take a more active role in treating osteoporosis. The program primarily aims to improve treatment of osteoporosis after a fragility fracture in an inpatient setting. Early results from a 2010 follow-up study showed that the new emphasis on prevention inspired by this program is effective. As compared with patients who had osteoporosis work-up and treatment initiated during their hospital admission, the group of patients who were referred for osteoporosis treatment after discharge were found to have a significantly lower rate of diagnosis and treatment.3 The loss of aftercare for patients who do not obtain immediate diagnosis and treatment for osteoporosis can and should be avoided. Many hospitals now have hip fracture services with multidisciplinary input. The successful outcomes of these programs include shorter times to the operating room, shorter hospital stays, decreased readmission, and decreased 30-day mortality.4-6 These services provide an excellent opportunity to ensure that each patient has initiated management of osteoporosis before discharge. Ideally, patients would be scheduled for bone mineral density testing prior to leaving the hospital, when applicable, and would begin calcium and vitamin D supplementation or bisphosphonate treatment in the hospital, when appropriate. As part of these hip fracture services, a goal of clearly initiating or managing treatment for osteoporosis should be routinely addressed.
While patients presenting with hip fractures are an easily identifiable high-risk population, other patients present in an outpatient setting following fragility fractures, such as distal radius or vertebral compression fractures. These patients should be considered for osteoporosis work-up and counseled accordingly. A recent study compared the efficacy of the orthopedic surgeon initiating bone mineral density testing after a distal radius fracture, compared with referring the patient back to their primary care physician for testing. The study found a significantly higher rate of patients going on to bone mineral density testing when the surgeon initiated this process.7 In the era of improved digital communication, the outpatient setting offers an opportunity for clinicians to communicate with patients’ primary care physicians and initiate a multidisciplinary approach to bone health and prevention. In the outpatient setting, the orthopedist can address nutritional issues and screening on a repeated basis. Studies have demonstrated that physician counseling can be very effective in changing behavior and helping patients to stop using tobacco.8 In this vein, efforts by the physician to encourage calcium and vitamin D intake and weight-bearing exercise have the potential to be very effective.
Programs such as “Own the Bone” are crucial to orthopedists’ treatment of osteoporosis, but prevention of bone disease and fragility fracture must extend even further. Individual practitioners must be cognizant that many patients may benefit from outpatient diagnosis of osteoporosis and initiation of appropriate treatment, before fragility fractures occur. Moreover, although patients at high risk include post-menopausal women, orthopedists need to be consistently aware of osteoporosis as a disease of both genders. An estimated 2.8 million men in the United States have osteoporosis.9 A 2012 study published out of Washington, DC found a significant disparity in the rate of osteoporosis screening between men and women. Among the elderly men and women in their patient population, 60% of women underwent screening compared with only 18.4% of men.10 This gender disparity potentially represents significant physician bias regarding the risk of osteoporosis and offers an important opportunity for orthopedic surgeons to improve preventative care for this population.
Preventative care in terms of advocating for bone health should not be limited to patients presenting with fragility fractures. Education regarding smoking cessation, resistance exercise, and calcium intake are relevant to many orthopedic patients. With the advent of the electronic medical record system, a simple intervention could easily ensure that patients report on their calcium intake. A trial published in 2006 found that a simple reminder from the electronic medical record improved osteoporosis management following a fragility fracture.11 This type of intervention could certainly be expanded to include counseling on calcium and vitamin D for any orthopedic patient.
Another area in which orthopedic surgeons have an opportunity to practice good preventative care is injury prevention. Several studies examining fall prevention among the elderly have shown that physical therapy or exercise may decrease the rate of falls.12 Promotion of activity and therapy among high-risk patients by orthopedic surgeons may help to reduce fracture incidence. Injury prevention is also relevant to young, healthy patients. It is well established that neuromuscular training helps to prevent anterior cruciate ligament injuries.13 Orthopedic surgeons have an opportunity during sports physicals or as team physicians to help promote injury prevention strategies. Discussion of training regimens may prevent overuse injuries among athletes. Moreover, faced with many patients who present with significant musculoskeletal trauma, orthopedic surgeons have the opportunity to offer education regarding motorcycle helmets, seatbelt use, and avoidance of drunk driving.
New orthopedic residency educational goals were recently published to include core competencies in resident education. Among these goals is to educate residents on care of a patient with hip fracture, including counseling and management of osteoporosis.14 These milestones could be expanded to include a thorough understanding of bone health. Residents should be able to make nutritional recommendations for any patient seen as an inpatient or outpatient, identify when a referral to an endocrinologist is needed, and educate patients regarding injury and fall prevention.
As healthcare expenditures rise, so does the impetus for physicians to work to improve efficiency in the healthcare system. Furthermore, the best possible care for our patients is to prevent injury and disability before it arises, rather than to depend on our ability to intervene after the fact. Residencies and training programs should work to incorporate preventative strategies into trainee education. Hospitals and outpatient settings should include a basic bone health questionnaire in the electronic medical record. The identification and management of risk factors for injury has the potential to help our patients and to help our healthcare system, but such intervention needs to start with the clinician.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi:10.1359/jbmr.061113.
- Bunta AD. It is time for everyone to own the bone. Osteoporos Int. 2011;22 Suppl 3:477-482. doi:10.1007/s00198-011-1704-0.
- Edwards BJ, Koval K, Bunta AD, et al. Addressing secondary prevention of osteoporosis in fracture care: follow-up to “own the bone.” J Bone Joint Surg Am. 2011;93(15):e87. doi:10.2106/JBJS.I.00540.
- Sivakumar BS, McDermott LM, Bell JJ, Pulle CR, Jayamaha S, Ottley MC. Dedicated hip fracture service: implementing a novel model of care. ANZ J Surg. 2013;83(7-8):559-563. doi:10.1111/j.1445-2197.2012.06201.x.
- Khasraghi FA, Christmas C, Lee EJ, Mears SC, Wenz JF Sr. Effectiveness of a multidisciplinary team approach to hip fracture management. J Surg Orthop Adv. 2005;14(1):27-31.
- Vidan M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. doi:10.1111/j.1532-5415.2005.53466.x.
- Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953-961. doi:10.2106/JBJS.G.01121.
- Gorin SS, Heck JE. Meta-analysis of the efficacy of tobacco counseling by health care providers. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2012-2022.
- Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469(7):1900-1905. doi:10.1007/s11999-011-1780-7.
- Alswat K, Adler SM. Gender differences in osteoporosis screening: retrospective analysis. Arch Osteoporos. 2012;7:311-313. doi:10.1007/s11657-012-0113-0.
- Feldstein A, Elmer PJ, Smith DH, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006;54(3):450-457. doi:10.1111/j.1532-5415.2005.00618.x.
- Suzuki T, Kim H, Yoshida H, Ishizaki T. Randomized controlled trial of exercise intervention for the prevention of falls in community-dwelling elderly Japanese women. J Bone Miner Metab. 2004;22(6):602-611. doi:10.1007/s00774-004-0530-2.
- Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490-498. doi:10.1177/0363546505282619.
- Stern PJ, Albanese S, Bostrom M, et al. Orthopaedic surgery milestones. J Grad Med Educ. 2013;5(1 Suppl 1):36-58. doi:10.4300/JGME-05-01s1-05.
By 2025, it is estimated that the annual cost of treating osteoporosis-related fractures in the United States will be 25 billion dollars, which is 10 billion dollars more than was spent in 2010.1 As healthcare costs in the United States continue to skyrocket, it is imperative that orthopedic surgeons take an active role in avoiding preventable injury and disease. For orthopedic surgeons, preventative medicine will include promoting bone health and educating patients on injury prevention. By incorporating these principles into residency and fellowship education, and by leveraging the electronic medical record to support preventive care through systematic reminders, orthopedic surgeons have a critical opportunity to take a leading role in promoting prevention to our patients.
In 2009, the American Orthopaedic Association (AOA) launched a “Own the Bone” campaign, a national quality improvement program designed to optimize the treatment of osteoporosis.2 This program came about following the Surgeon General’s call, in 2004, for orthopedic surgeons to take a more active role in treating osteoporosis. The program primarily aims to improve treatment of osteoporosis after a fragility fracture in an inpatient setting. Early results from a 2010 follow-up study showed that the new emphasis on prevention inspired by this program is effective. As compared with patients who had osteoporosis work-up and treatment initiated during their hospital admission, the group of patients who were referred for osteoporosis treatment after discharge were found to have a significantly lower rate of diagnosis and treatment.3 The loss of aftercare for patients who do not obtain immediate diagnosis and treatment for osteoporosis can and should be avoided. Many hospitals now have hip fracture services with multidisciplinary input. The successful outcomes of these programs include shorter times to the operating room, shorter hospital stays, decreased readmission, and decreased 30-day mortality.4-6 These services provide an excellent opportunity to ensure that each patient has initiated management of osteoporosis before discharge. Ideally, patients would be scheduled for bone mineral density testing prior to leaving the hospital, when applicable, and would begin calcium and vitamin D supplementation or bisphosphonate treatment in the hospital, when appropriate. As part of these hip fracture services, a goal of clearly initiating or managing treatment for osteoporosis should be routinely addressed.
While patients presenting with hip fractures are an easily identifiable high-risk population, other patients present in an outpatient setting following fragility fractures, such as distal radius or vertebral compression fractures. These patients should be considered for osteoporosis work-up and counseled accordingly. A recent study compared the efficacy of the orthopedic surgeon initiating bone mineral density testing after a distal radius fracture, compared with referring the patient back to their primary care physician for testing. The study found a significantly higher rate of patients going on to bone mineral density testing when the surgeon initiated this process.7 In the era of improved digital communication, the outpatient setting offers an opportunity for clinicians to communicate with patients’ primary care physicians and initiate a multidisciplinary approach to bone health and prevention. In the outpatient setting, the orthopedist can address nutritional issues and screening on a repeated basis. Studies have demonstrated that physician counseling can be very effective in changing behavior and helping patients to stop using tobacco.8 In this vein, efforts by the physician to encourage calcium and vitamin D intake and weight-bearing exercise have the potential to be very effective.
Programs such as “Own the Bone” are crucial to orthopedists’ treatment of osteoporosis, but prevention of bone disease and fragility fracture must extend even further. Individual practitioners must be cognizant that many patients may benefit from outpatient diagnosis of osteoporosis and initiation of appropriate treatment, before fragility fractures occur. Moreover, although patients at high risk include post-menopausal women, orthopedists need to be consistently aware of osteoporosis as a disease of both genders. An estimated 2.8 million men in the United States have osteoporosis.9 A 2012 study published out of Washington, DC found a significant disparity in the rate of osteoporosis screening between men and women. Among the elderly men and women in their patient population, 60% of women underwent screening compared with only 18.4% of men.10 This gender disparity potentially represents significant physician bias regarding the risk of osteoporosis and offers an important opportunity for orthopedic surgeons to improve preventative care for this population.
Preventative care in terms of advocating for bone health should not be limited to patients presenting with fragility fractures. Education regarding smoking cessation, resistance exercise, and calcium intake are relevant to many orthopedic patients. With the advent of the electronic medical record system, a simple intervention could easily ensure that patients report on their calcium intake. A trial published in 2006 found that a simple reminder from the electronic medical record improved osteoporosis management following a fragility fracture.11 This type of intervention could certainly be expanded to include counseling on calcium and vitamin D for any orthopedic patient.
Another area in which orthopedic surgeons have an opportunity to practice good preventative care is injury prevention. Several studies examining fall prevention among the elderly have shown that physical therapy or exercise may decrease the rate of falls.12 Promotion of activity and therapy among high-risk patients by orthopedic surgeons may help to reduce fracture incidence. Injury prevention is also relevant to young, healthy patients. It is well established that neuromuscular training helps to prevent anterior cruciate ligament injuries.13 Orthopedic surgeons have an opportunity during sports physicals or as team physicians to help promote injury prevention strategies. Discussion of training regimens may prevent overuse injuries among athletes. Moreover, faced with many patients who present with significant musculoskeletal trauma, orthopedic surgeons have the opportunity to offer education regarding motorcycle helmets, seatbelt use, and avoidance of drunk driving.
New orthopedic residency educational goals were recently published to include core competencies in resident education. Among these goals is to educate residents on care of a patient with hip fracture, including counseling and management of osteoporosis.14 These milestones could be expanded to include a thorough understanding of bone health. Residents should be able to make nutritional recommendations for any patient seen as an inpatient or outpatient, identify when a referral to an endocrinologist is needed, and educate patients regarding injury and fall prevention.
As healthcare expenditures rise, so does the impetus for physicians to work to improve efficiency in the healthcare system. Furthermore, the best possible care for our patients is to prevent injury and disability before it arises, rather than to depend on our ability to intervene after the fact. Residencies and training programs should work to incorporate preventative strategies into trainee education. Hospitals and outpatient settings should include a basic bone health questionnaire in the electronic medical record. The identification and management of risk factors for injury has the potential to help our patients and to help our healthcare system, but such intervention needs to start with the clinician.
By 2025, it is estimated that the annual cost of treating osteoporosis-related fractures in the United States will be 25 billion dollars, which is 10 billion dollars more than was spent in 2010.1 As healthcare costs in the United States continue to skyrocket, it is imperative that orthopedic surgeons take an active role in avoiding preventable injury and disease. For orthopedic surgeons, preventative medicine will include promoting bone health and educating patients on injury prevention. By incorporating these principles into residency and fellowship education, and by leveraging the electronic medical record to support preventive care through systematic reminders, orthopedic surgeons have a critical opportunity to take a leading role in promoting prevention to our patients.
In 2009, the American Orthopaedic Association (AOA) launched a “Own the Bone” campaign, a national quality improvement program designed to optimize the treatment of osteoporosis.2 This program came about following the Surgeon General’s call, in 2004, for orthopedic surgeons to take a more active role in treating osteoporosis. The program primarily aims to improve treatment of osteoporosis after a fragility fracture in an inpatient setting. Early results from a 2010 follow-up study showed that the new emphasis on prevention inspired by this program is effective. As compared with patients who had osteoporosis work-up and treatment initiated during their hospital admission, the group of patients who were referred for osteoporosis treatment after discharge were found to have a significantly lower rate of diagnosis and treatment.3 The loss of aftercare for patients who do not obtain immediate diagnosis and treatment for osteoporosis can and should be avoided. Many hospitals now have hip fracture services with multidisciplinary input. The successful outcomes of these programs include shorter times to the operating room, shorter hospital stays, decreased readmission, and decreased 30-day mortality.4-6 These services provide an excellent opportunity to ensure that each patient has initiated management of osteoporosis before discharge. Ideally, patients would be scheduled for bone mineral density testing prior to leaving the hospital, when applicable, and would begin calcium and vitamin D supplementation or bisphosphonate treatment in the hospital, when appropriate. As part of these hip fracture services, a goal of clearly initiating or managing treatment for osteoporosis should be routinely addressed.
While patients presenting with hip fractures are an easily identifiable high-risk population, other patients present in an outpatient setting following fragility fractures, such as distal radius or vertebral compression fractures. These patients should be considered for osteoporosis work-up and counseled accordingly. A recent study compared the efficacy of the orthopedic surgeon initiating bone mineral density testing after a distal radius fracture, compared with referring the patient back to their primary care physician for testing. The study found a significantly higher rate of patients going on to bone mineral density testing when the surgeon initiated this process.7 In the era of improved digital communication, the outpatient setting offers an opportunity for clinicians to communicate with patients’ primary care physicians and initiate a multidisciplinary approach to bone health and prevention. In the outpatient setting, the orthopedist can address nutritional issues and screening on a repeated basis. Studies have demonstrated that physician counseling can be very effective in changing behavior and helping patients to stop using tobacco.8 In this vein, efforts by the physician to encourage calcium and vitamin D intake and weight-bearing exercise have the potential to be very effective.
Programs such as “Own the Bone” are crucial to orthopedists’ treatment of osteoporosis, but prevention of bone disease and fragility fracture must extend even further. Individual practitioners must be cognizant that many patients may benefit from outpatient diagnosis of osteoporosis and initiation of appropriate treatment, before fragility fractures occur. Moreover, although patients at high risk include post-menopausal women, orthopedists need to be consistently aware of osteoporosis as a disease of both genders. An estimated 2.8 million men in the United States have osteoporosis.9 A 2012 study published out of Washington, DC found a significant disparity in the rate of osteoporosis screening between men and women. Among the elderly men and women in their patient population, 60% of women underwent screening compared with only 18.4% of men.10 This gender disparity potentially represents significant physician bias regarding the risk of osteoporosis and offers an important opportunity for orthopedic surgeons to improve preventative care for this population.
Preventative care in terms of advocating for bone health should not be limited to patients presenting with fragility fractures. Education regarding smoking cessation, resistance exercise, and calcium intake are relevant to many orthopedic patients. With the advent of the electronic medical record system, a simple intervention could easily ensure that patients report on their calcium intake. A trial published in 2006 found that a simple reminder from the electronic medical record improved osteoporosis management following a fragility fracture.11 This type of intervention could certainly be expanded to include counseling on calcium and vitamin D for any orthopedic patient.
Another area in which orthopedic surgeons have an opportunity to practice good preventative care is injury prevention. Several studies examining fall prevention among the elderly have shown that physical therapy or exercise may decrease the rate of falls.12 Promotion of activity and therapy among high-risk patients by orthopedic surgeons may help to reduce fracture incidence. Injury prevention is also relevant to young, healthy patients. It is well established that neuromuscular training helps to prevent anterior cruciate ligament injuries.13 Orthopedic surgeons have an opportunity during sports physicals or as team physicians to help promote injury prevention strategies. Discussion of training regimens may prevent overuse injuries among athletes. Moreover, faced with many patients who present with significant musculoskeletal trauma, orthopedic surgeons have the opportunity to offer education regarding motorcycle helmets, seatbelt use, and avoidance of drunk driving.
New orthopedic residency educational goals were recently published to include core competencies in resident education. Among these goals is to educate residents on care of a patient with hip fracture, including counseling and management of osteoporosis.14 These milestones could be expanded to include a thorough understanding of bone health. Residents should be able to make nutritional recommendations for any patient seen as an inpatient or outpatient, identify when a referral to an endocrinologist is needed, and educate patients regarding injury and fall prevention.
As healthcare expenditures rise, so does the impetus for physicians to work to improve efficiency in the healthcare system. Furthermore, the best possible care for our patients is to prevent injury and disability before it arises, rather than to depend on our ability to intervene after the fact. Residencies and training programs should work to incorporate preventative strategies into trainee education. Hospitals and outpatient settings should include a basic bone health questionnaire in the electronic medical record. The identification and management of risk factors for injury has the potential to help our patients and to help our healthcare system, but such intervention needs to start with the clinician.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi:10.1359/jbmr.061113.
- Bunta AD. It is time for everyone to own the bone. Osteoporos Int. 2011;22 Suppl 3:477-482. doi:10.1007/s00198-011-1704-0.
- Edwards BJ, Koval K, Bunta AD, et al. Addressing secondary prevention of osteoporosis in fracture care: follow-up to “own the bone.” J Bone Joint Surg Am. 2011;93(15):e87. doi:10.2106/JBJS.I.00540.
- Sivakumar BS, McDermott LM, Bell JJ, Pulle CR, Jayamaha S, Ottley MC. Dedicated hip fracture service: implementing a novel model of care. ANZ J Surg. 2013;83(7-8):559-563. doi:10.1111/j.1445-2197.2012.06201.x.
- Khasraghi FA, Christmas C, Lee EJ, Mears SC, Wenz JF Sr. Effectiveness of a multidisciplinary team approach to hip fracture management. J Surg Orthop Adv. 2005;14(1):27-31.
- Vidan M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. doi:10.1111/j.1532-5415.2005.53466.x.
- Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953-961. doi:10.2106/JBJS.G.01121.
- Gorin SS, Heck JE. Meta-analysis of the efficacy of tobacco counseling by health care providers. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2012-2022.
- Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469(7):1900-1905. doi:10.1007/s11999-011-1780-7.
- Alswat K, Adler SM. Gender differences in osteoporosis screening: retrospective analysis. Arch Osteoporos. 2012;7:311-313. doi:10.1007/s11657-012-0113-0.
- Feldstein A, Elmer PJ, Smith DH, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006;54(3):450-457. doi:10.1111/j.1532-5415.2005.00618.x.
- Suzuki T, Kim H, Yoshida H, Ishizaki T. Randomized controlled trial of exercise intervention for the prevention of falls in community-dwelling elderly Japanese women. J Bone Miner Metab. 2004;22(6):602-611. doi:10.1007/s00774-004-0530-2.
- Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490-498. doi:10.1177/0363546505282619.
- Stern PJ, Albanese S, Bostrom M, et al. Orthopaedic surgery milestones. J Grad Med Educ. 2013;5(1 Suppl 1):36-58. doi:10.4300/JGME-05-01s1-05.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465-475. doi:10.1359/jbmr.061113.
- Bunta AD. It is time for everyone to own the bone. Osteoporos Int. 2011;22 Suppl 3:477-482. doi:10.1007/s00198-011-1704-0.
- Edwards BJ, Koval K, Bunta AD, et al. Addressing secondary prevention of osteoporosis in fracture care: follow-up to “own the bone.” J Bone Joint Surg Am. 2011;93(15):e87. doi:10.2106/JBJS.I.00540.
- Sivakumar BS, McDermott LM, Bell JJ, Pulle CR, Jayamaha S, Ottley MC. Dedicated hip fracture service: implementing a novel model of care. ANZ J Surg. 2013;83(7-8):559-563. doi:10.1111/j.1445-2197.2012.06201.x.
- Khasraghi FA, Christmas C, Lee EJ, Mears SC, Wenz JF Sr. Effectiveness of a multidisciplinary team approach to hip fracture management. J Surg Orthop Adv. 2005;14(1):27-31.
- Vidan M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. doi:10.1111/j.1532-5415.2005.53466.x.
- Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953-961. doi:10.2106/JBJS.G.01121.
- Gorin SS, Heck JE. Meta-analysis of the efficacy of tobacco counseling by health care providers. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2012-2022.
- Cawthon PM. Gender differences in osteoporosis and fractures. Clin Orthop Relat Res. 2011;469(7):1900-1905. doi:10.1007/s11999-011-1780-7.
- Alswat K, Adler SM. Gender differences in osteoporosis screening: retrospective analysis. Arch Osteoporos. 2012;7:311-313. doi:10.1007/s11657-012-0113-0.
- Feldstein A, Elmer PJ, Smith DH, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc. 2006;54(3):450-457. doi:10.1111/j.1532-5415.2005.00618.x.
- Suzuki T, Kim H, Yoshida H, Ishizaki T. Randomized controlled trial of exercise intervention for the prevention of falls in community-dwelling elderly Japanese women. J Bone Miner Metab. 2004;22(6):602-611. doi:10.1007/s00774-004-0530-2.
- Hewett TE, Ford KR, Myer GD. Anterior cruciate ligament injuries in female athletes: Part 2, a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490-498. doi:10.1177/0363546505282619.
- Stern PJ, Albanese S, Bostrom M, et al. Orthopaedic surgery milestones. J Grad Med Educ. 2013;5(1 Suppl 1):36-58. doi:10.4300/JGME-05-01s1-05.
Total Joint Arthroplasty Quality Ratings: How Are They Similar and How Are They Different?
ABSTRACT
A patient’s perception of hospital or provider quality can have far-reaching effects, as it can impact reimbursement, patient selection of a surgeon, and healthcare competition. A variety of organizations offer quality designations for orthopedic surgery and its subspecialties. Our goal is to compare total joint arthroplasty (TJA) quality designation methodology across key quality rating organizations. One researcher conducted an initial Google search to determine organizations providing quality designations for hospitals and surgeons providing orthopedic procedures with a focus on TJA. Organizations that offer quality designation specific to TJA were determined. Organizations that provided general orthopedic surgery or only surgeon-specific quality designation were excluded from the analysis. The senior author confirmed the inclusion of the final organizations. Seven organizations fit our inclusion criteria. Only the private payers and The Joint Commission required hospital accreditation to meet quality designation criteria. Total arthroplasty volume was considered in 86% of the organizations’ methodologies, and 57% of organizations utilized process measurements such as antibiotic prophylaxis and care pathways. In addition, 57% of organizations included patient experience in their methodologies. Only 29% of organizations included a cost element in their methodology. All organizations utilized outcome data and publicly reported all hospitals receiving their quality designation. Hospital quality designation methodologies are inconsistent in the context of TJA. All stakeholders (ie, providers, payers, and patients) should be involved in deciding the definition of quality.
Continue to: Healthcare in the United States...
Healthcare in the United States has begun to move toward a system focused on value for patients, defined as health outcome per dollar expended.1 Indeed, an estimated 30% of Medicare payments are now made using the so-called alternative payment models (eg, bundled payments),2 and there is an expectation that consumerism in medicine will continue to expand.3 In addition, although there is a continuing debate regarding the benefits and pitfalls of hospital mergers, there is no question whether provider consolidation has increased dramatically in recent years.4 At the core of many of these changes is the push to improve healthcare quality and reduce costs.
Quality has the ability to affect payment, patient selection of providers, and hospital competition. Patients (ie, healthcare consumers) are increasingly using the Internet to find a variety of health information.5 Accessible provider quality information online would allow patients to make more informed decisions about where to seek care. In addition, the development of transparent quality ratings could assist payers in driving beneficiaries to higher quality and better value providers, which could mean more business for the highest quality physicians and better patient outcomes with fewer complications. Some payers such as the Centers for Medicare and Medicaid Services (CMS) have already started using quality measures as part of their reimbursement strategy.6 Because CMS is the largest payer in the United States, private insurers tend to follow their lead; thus, quality measurements will become even more common as a factor in reimbursement over the coming years.
To make quality ratings useful, “quality” must be clearly defined. Clarity around which factors are considered in a quality designation will create transparency for patients and allow providers to understand how their performance is being measured so that they focus on improving outcomes for their patients. Numerous organizations, including private payers, public payers, and both not-for-profit and for-profit entities, have created quality designation programs to rate providers. However, within orthopedics and several other medical specialties, there has been an ongoing debate about what measures best reflect quality.7 Although inconsistencies in quality ratings in arthroplasty care have been noted,8 it remains unknown how each quality designation program compares with the others in terms of the factors considered in deciding quality designations.
The purpose of this study is to evaluate publicly available information from key quality designation programs for total joint arthroplasty (TJA) providers to determine what factors are considered by each organization in awarding quality designations; what similarities and differences in quality designations exist across the different organizations; and how many of the organizations publish their quality designation methodologies and final rating results.
MATERIALS AND METHODS
A directed Google search was conducted to determine organizations (ie, payers, independent firms, and government entities) that rate hospitals and/or surgeons in orthopedic surgery. The identified organizations were then examined to determine whether they provided hospital ratings for total hip and/or knee arthroplasty. Entities were included if they provided quality designations for hospitals specifically addressing TJA. Organizations that provided only general hospital, other surgical procedures, orthopedic surgery, or orthopedic surgeon-specific quality designations were excluded. A list of all organizations determined to fit the inclusion criteria was then reviewed for completeness and approved by the senior author.
Continue to: One investigator reviewed the website of each organization...
One investigator reviewed the website of each organization fitting the inclusion criteria to determine the full rating methodology in 1 sitting on July 2, 2016. Detailed notes were taken on each program using publicly available information. For organizations that used proprietary criteria for quality designation (eg, The Joint Commission [TJC]), only publicly available information was used in the analysis. Therefore, the information reported is solely based on data available online to the public.
Detailed quality designation criteria were condensed into broader categories (accreditation, volume, structural, process, outcomes, patient experience, and cost/efficiency) to capture differences between each organization reviewed. In addition, we recorded whether each organization published a list of providers that received its quality designation.
RESULTS
A total of 7 organizations fit our inclusion criteria9-15 (Table). Of these 7 organizations, 3 were private payers (Aetna, UnitedHealth, and Blue Cross Blue Shield [BCBS]), 2 were nongovernmental not-for-profit organizations (TJC and Consumer Reports), and 2 were consumer-based and/or for-profit organizations (HealthGrades and US News & World Report [USNWR]). There were no government agencies that fit our inclusion criteria. BCBS had the following 2 separate quality designations: BCBS Blue Distinction and BCBS Blue Distinction+. The only difference between the 2 BCBS ratings is that BCBS Blue Distinction+ includes cost efficiency ratings, whereas BCBS Blue Distinction does not.
Only the 3 private payers and TJC, the primary hospital accreditation body in the United States, required accreditation as part of its quality designation criteria. TJC requires its own accreditation for quality designation consideration, whereas the 3 private payers allow accreditation from one of a variety of sources. Aetna Institutes of Quality for Orthopedic Surgery requires accreditation by TJC, Healthcare Facilities Accreditation Program, American Osteopathic Association, National Integrated Accreditation for Healthcare Organizations, or Det Norske Veritas Healthcare. UnitedHealth Premium Total Joint Replacement (TJR) Specialty Center requires accreditation by TJC and/or equivalent of TJC accreditation. However, TJC accreditation equivalents are not noted in the UnitedHealth handbook. BCBS Blue Distinction and Distinction+ require accreditation by TJC, Healthcare Facilities Accreditation Program, National Integrated Accreditation for Healthcare Organizations, or Center for Improvement in Healthcare Quality. In addition, BCBS is willing to consider alternative accreditations that are at least as stringent as the national alternatives noted. However, no detailed criteria that must be met to be equivalent to the national standards are noted in the relevant quality designation handbook.
The volume of completed total hip and knee arthroplasty procedures was considered in 6 of the organizations’ quality ratings methodologies. Of those 6, all private payers, TJC (not-for-profit), and 2 for-profit rating agencies were included. Surgeon specialization in TJA was only explicitly noted as a factor considered in UnitedHealth Premium TJR Specialty Center criteria; however, the requirements for surgeon specialization were not clearly defined. In addition, the presence of a multidisciplinary clinical pathway was only explicitly considered for Aetna Institutes of Quality for Orthopedic Surgery.
Structural requirements (eg, use of electronic health records [EHR], staffing levels, etc.) were taken into account in private payer and USNWR quality methodologies. Process measures (eg, antibiotic prophylaxis and other care pathways) were considered for the private payers and TJC but not for USNWR quality designation. Cost and/or efficiency measures were factors in the quality formula for Aetna Institutes of Quality for Orthopedic Surgery and BCBS Distinction+. Aetna utilizes its own cost data and risk-adjusts using a product known as Symmetry Episode Risk Groups to determine cost-effectiveness, while BCBS uses its own Composite Facility Cost Index. Patient experience (eg, Hospital Consumer Assessment of Healthcare Providers and Systems [HCAHPS]) was incorporated into the quality formulas for 4 of the 7 quality designation programs examined.
Continue to: All of the 7 quality designation programs included...
All of the 7 quality designation programs included outcomes (ie, readmission rates and/or mortality rates) and publicly reported the hospitals receiving their quality designation. In contrast, only Aetna explicitly included the presence of multidisciplinary clinical care pathways as part of their quality designation criteria. In addition, only UnitedHealth included surgeon specialization in joint arthroplasty as a factor for quality consideration for its quality designation program. BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery were the only 2 quality designations that included at least 1 variable that fit into each of the 7 characteristics considered (accreditation, volume, structural, process, outcomes, patient experience, and cost/efficiency).
DISCUSSION
As healthcare continues to shift toward value-based delivery and payment models, quality becomes a critical factor in reimbursement and provider rankings. However, quality is a vague term. Several providers probably do not know what is required to be designated as high quality by a particular rating agency. Moreover, there are multiple quality designation programs, all using distinct criteria to determine “quality,” which further complicates the matter. Our objective was to determine the key stakeholders that provide quality designations in TJA and what criteria each organization uses in assessing quality.
Our idea of comprehensive quality is based on Avedis Donabedian’s enduring framework for healthcare quality focused on structure, process, and outcome.16 We expanded on these 3 areas and analyzed quality designations based on variables fitting into the following categories: accreditation, volume, structural, process, outcomes, patient experience, and cost/efficiency. We believe that these categories encompass a comprehensive rating system that addresses key elements of patient care. However, our results suggest that only 2 major quality designations (BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery) take all such variables into account.
All quality designation programs that we analyzed required outcome data (ie, readmission and/or mortality rates within 30 days); however, only 2 programs utilized cost in their quality designation criteria (BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery). Aetna Institutes of Quality for Orthopedic Surgery risk-adjusted for its cost-effectiveness calculations based on age, sex, and other unspecified conditions using a product known as Symmetry Episode Risk Groups. However, the organization also noted that although it did risk-adjust for inpatient mortality, it did not do so for pulmonary embolism or deep vein thrombosis. BCBS Distinction+ also utilized risk adjustment for its cost efficiency measure, and its step-by-step methodology is available online. Further, Consumer Reports does risk-adjust using logistic regression models in their quality analysis, but the description provided is minimal; it is noted that such risk adjustments are already completed by CMS prior to Consumer Reports acquiring the data. The CMS Compare model information is available on the CMS website. The data utilized by several organizations and presented on CMS Compare are already risk-adjusted using CMS’ approach. In contrast, UnitedHealth Premium TJR Specialty Center gathers its own data from providers and does not describe a risk adjustment methodology. Risk adjustment is important because the lack of risk adjustment may lead to physicians “cherry-picking” easy cases to boost positive outcomes, leading to increased financial benefits and higher quality ratings. Having a consistent risk adjustment formula will ensure accurate comparisons across outcomes and cost-effectiveness measures used by quality designation programs.
Factors considered for quality designation varied greatly from one organization to the other. The range of categories of factors considered varied from 1 (Consumer Reports only considered outcome data) to all 7 categories (BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery). Our findings are consistent with the work by Keswani and colleagues,8 which showed that there is likely variation in factors considered when rating hospital quality more broadly. Our work suggests that quality designation formulas do not appear to get more consistent when focused on TJA.
We found that all organizations in our analysis published the providers earning their quality designation. However, TJC does not provide publicly a detailed methodology on how to qualify for its quality designation. The price to purchase the necessary manual for this information is $146.00 for accredited organizations and $186.00 for all others.17 For large healthcare providers, this is not a large sum of money. Nonetheless, this provides an additional hurdle for stakeholders to gain a full understanding of the requirements to receive a TJC Gold Seal for Orthopedics.
Previous work has evaluated the consistency of and the variety of means of gauging healthcare quality. Previous work by Rothberg and colleagues18 comparing hospital rankings across 5 common consumer-oriented websites found disagreement on hospital rankings within any diagnosis and even among metrics such as mortality. Another study by Halasyamani and Davis19 found that CMS Compare and USNWR rankings were dissimilar and the authors attributed the discrepancy to different methodologies. In addition, a study by Krumholz and colleagues20 focused on Internet report cards, which measured the appropriate use of select medications and mortality rates for acute myocardial infarction as the quality metrics. The authors found that, in aggregate, there was a clear difference in quality of care and outcomes but that comparisons between 2 hospitals provided poor discrimination.20 Other work has analyzed the increasing trend of online ratings of orthopedic surgeons by patients.21 However, there remains no agreed-upon definition of quality. Thus, the use of the term “quality” in several studies may be misleading.
Our results must be interpreted keeping the limitations of our work in mind. First, we used expert knowledge and a public search engine to develop our list of organizations that provide TJA quality designations. However, there is a possibility that we did not include all relevant organizations. Second, although all authors reviewed the final data, it is possible that there was human error in the analysis of each organization’s quality designation criteria.
CONCLUSION
As healthcare progresses further toward a system that rewards providers for delivering value to patients, accurately defining and measuring quality becomes critical because it can be suggestive of value to patients, payers, and providers. Furthermore, it gives providers a goal to focus on as they strive to improve the value of care they deliver to patients. Measuring healthcare quality is currently a novel, imperfect science,22 and there continues to be a debate about what factors should be included in a quality designation formula. Nonetheless, more and more quality designations and performance measurements are being created for orthopedic care, including total hip and total knee arthroplasty. In fact, in 2016, The Leapfrog Group added readmission for patients undergoing TJA to its survey.23 Consensus on a quality definition may facilitate the movement toward a value-based healthcare system. Future research should evaluate strategies for gaining consensus among stakeholders for a universal quality metric in TJA. Surgeons, hospitals, payers, and most importantly patients should play critical roles in defining quality.
- Porter ME. A strategy for health care reform--toward a value-based system. N Engl J Med. 2009;361(2):109-112. doi:10.1056/NEJMp0904131.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316(5):525-532. doi:10.1001/jama.2016.9797.
- Mulvany C. The march to consumerism the evolution from patient to active shopper continues. Healthc Financ Manage. 2014;68(2):36-38.
- Tsai TC, Jha AK. Hospital consolidation, competition, and quality: is bigger necessarily better? JAMA. 2014;312(1):29-30. doi:10.1001/jama.2014.4692.
- Cline RJ, Haynes KM. Consumer health information seeking on the Internet: the state of the art. Health Educ Res. 2001;16(6):671-692. doi:10.1093/her/16.6.671.
- Werner RM, Kolstad JT, Stuart EA, Polsky D. The effect of pay-for-performance in hospitals: lessons for quality improvement. Health Aff (Millwood). 2011;30(4):690-698. doi:10.1377/hlthaff.2010.1277.
- Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? J Am Coll Surg. 2004;198(4):626-632. doi:10.1016/j.jamcollsurg.2003.11.017.
- Keswani A, Uhler LM, Bozic KJ. What quality metrics is my hospital being evaluated on and what are the consequences? J Arthroplast. 2016;31(6):1139-1143. doi:10.1016/j.arth.2016.01.075.
- Aetna Inc. Aetna Institutes of Quality® facilities fact book. A comprehensive reference guide for Aetna members, doctors and health care professionals. http://www.aetna.com/individuals-families-health-insurance/document-libr.... Accessed July 2, 2016.
- United HealthCare. UnitedHealth Premium® Program. https://www.uhcprovider.com/en/reports-quality-programs/premium-designation.html. Accessed July 2, 2016.
- 11. Blue Cross Blue Shield. Association. Blue Distinction Specialty Care. Selection criteria and program documentation: knee and hip replacement and spine surgery. https://www.bcbs.com/sites/default/files/fileattachments/page/KneeHip.SelectionCriteria_0.pdf. Published October 2015. Accessed July 2, 2016.
- The Joint Commission. Advanced certification for total hip and total knee replacement eligibility. https://www.jointcommission.org/advanced_certification_for_total_hip_and.... Published December 10, 2015. Accessed July 2, 2016.
- Healthgrades Operating Company. Healthgrades methodology: anatomy of a rating. https://www.healthgrades.com/quality/ratings-awards/methodology. Accessed July 2, 2016.
- Comarow A, Harder B; Dr. Foster Project Team. Methodology: U.S. News & World Report best hospitals for common care. U.S. News & World Report Web site. http://www.usnews.com/pubfiles/BHCC_MethReport_2015.pdf. Published May 20, 2015. Accessed July 2, 2016.
- Consumer Reports. How we rate hospitals. http://static3.consumerreportscdn.org/content/dam/cro/news_articles/heal.... Accessed July 2, 2016.
- Ayanian JZ, Markel H. Donabedian’s lasting framework for health care quality. N Engl J Med. 2016;375(3):205-207. doi:10.1056/NEJMp1605101.
- The Joint Commission. 2016 Certification Manuals. 2016; http://www.jcrinc.com/2016-certification-manuals/. Accessed July 2, 2016.
- Rothberg MB, Morsi E, Benjamin EM, Pekow PS, Lindenauer PK. Choosing the best hospital: the limitations of public quality reporting. Health Aff (Millwood). 2008;27(6):1680-1687. doi:10.1377/hlthaff.27.6.1680.
- Halasyamani LK, Davis MM. Conflicting measures of hospital quality: ratings from "Hospital Compare" versus "Best Hospitals". J Hosp Med. 2007;2(3):128-134. doi:10.1002/jhm.176.
- Krumholz HM, Rathore SS, Chen J, Wang Y, Radford MJ. Evaluation of a consumer-oriented internet health care report card: the risk of quality ratings based on mortality data. JAMA. 2002;287(10):1277-1287.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38(4):e257-e262. doi:10.3928/01477447-20150402-52.
- Harder B, Comarow A. Hospital Quality reporting by US News & World Report: why, how, and what's ahead. JAMA. 2015;313(19):1903-1904. doi:10.1001/jama.2015.4566.
- The Leapfrog Group. New in 2016. http://www.leapfroggroup.org/ratings-reports/new-2016. Accessed July 2, 2016.
ABSTRACT
A patient’s perception of hospital or provider quality can have far-reaching effects, as it can impact reimbursement, patient selection of a surgeon, and healthcare competition. A variety of organizations offer quality designations for orthopedic surgery and its subspecialties. Our goal is to compare total joint arthroplasty (TJA) quality designation methodology across key quality rating organizations. One researcher conducted an initial Google search to determine organizations providing quality designations for hospitals and surgeons providing orthopedic procedures with a focus on TJA. Organizations that offer quality designation specific to TJA were determined. Organizations that provided general orthopedic surgery or only surgeon-specific quality designation were excluded from the analysis. The senior author confirmed the inclusion of the final organizations. Seven organizations fit our inclusion criteria. Only the private payers and The Joint Commission required hospital accreditation to meet quality designation criteria. Total arthroplasty volume was considered in 86% of the organizations’ methodologies, and 57% of organizations utilized process measurements such as antibiotic prophylaxis and care pathways. In addition, 57% of organizations included patient experience in their methodologies. Only 29% of organizations included a cost element in their methodology. All organizations utilized outcome data and publicly reported all hospitals receiving their quality designation. Hospital quality designation methodologies are inconsistent in the context of TJA. All stakeholders (ie, providers, payers, and patients) should be involved in deciding the definition of quality.
Continue to: Healthcare in the United States...
Healthcare in the United States has begun to move toward a system focused on value for patients, defined as health outcome per dollar expended.1 Indeed, an estimated 30% of Medicare payments are now made using the so-called alternative payment models (eg, bundled payments),2 and there is an expectation that consumerism in medicine will continue to expand.3 In addition, although there is a continuing debate regarding the benefits and pitfalls of hospital mergers, there is no question whether provider consolidation has increased dramatically in recent years.4 At the core of many of these changes is the push to improve healthcare quality and reduce costs.
Quality has the ability to affect payment, patient selection of providers, and hospital competition. Patients (ie, healthcare consumers) are increasingly using the Internet to find a variety of health information.5 Accessible provider quality information online would allow patients to make more informed decisions about where to seek care. In addition, the development of transparent quality ratings could assist payers in driving beneficiaries to higher quality and better value providers, which could mean more business for the highest quality physicians and better patient outcomes with fewer complications. Some payers such as the Centers for Medicare and Medicaid Services (CMS) have already started using quality measures as part of their reimbursement strategy.6 Because CMS is the largest payer in the United States, private insurers tend to follow their lead; thus, quality measurements will become even more common as a factor in reimbursement over the coming years.
To make quality ratings useful, “quality” must be clearly defined. Clarity around which factors are considered in a quality designation will create transparency for patients and allow providers to understand how their performance is being measured so that they focus on improving outcomes for their patients. Numerous organizations, including private payers, public payers, and both not-for-profit and for-profit entities, have created quality designation programs to rate providers. However, within orthopedics and several other medical specialties, there has been an ongoing debate about what measures best reflect quality.7 Although inconsistencies in quality ratings in arthroplasty care have been noted,8 it remains unknown how each quality designation program compares with the others in terms of the factors considered in deciding quality designations.
The purpose of this study is to evaluate publicly available information from key quality designation programs for total joint arthroplasty (TJA) providers to determine what factors are considered by each organization in awarding quality designations; what similarities and differences in quality designations exist across the different organizations; and how many of the organizations publish their quality designation methodologies and final rating results.
MATERIALS AND METHODS
A directed Google search was conducted to determine organizations (ie, payers, independent firms, and government entities) that rate hospitals and/or surgeons in orthopedic surgery. The identified organizations were then examined to determine whether they provided hospital ratings for total hip and/or knee arthroplasty. Entities were included if they provided quality designations for hospitals specifically addressing TJA. Organizations that provided only general hospital, other surgical procedures, orthopedic surgery, or orthopedic surgeon-specific quality designations were excluded. A list of all organizations determined to fit the inclusion criteria was then reviewed for completeness and approved by the senior author.
Continue to: One investigator reviewed the website of each organization...
One investigator reviewed the website of each organization fitting the inclusion criteria to determine the full rating methodology in 1 sitting on July 2, 2016. Detailed notes were taken on each program using publicly available information. For organizations that used proprietary criteria for quality designation (eg, The Joint Commission [TJC]), only publicly available information was used in the analysis. Therefore, the information reported is solely based on data available online to the public.
Detailed quality designation criteria were condensed into broader categories (accreditation, volume, structural, process, outcomes, patient experience, and cost/efficiency) to capture differences between each organization reviewed. In addition, we recorded whether each organization published a list of providers that received its quality designation.
RESULTS
A total of 7 organizations fit our inclusion criteria9-15 (Table). Of these 7 organizations, 3 were private payers (Aetna, UnitedHealth, and Blue Cross Blue Shield [BCBS]), 2 were nongovernmental not-for-profit organizations (TJC and Consumer Reports), and 2 were consumer-based and/or for-profit organizations (HealthGrades and US News & World Report [USNWR]). There were no government agencies that fit our inclusion criteria. BCBS had the following 2 separate quality designations: BCBS Blue Distinction and BCBS Blue Distinction+. The only difference between the 2 BCBS ratings is that BCBS Blue Distinction+ includes cost efficiency ratings, whereas BCBS Blue Distinction does not.

Only the 3 private payers and TJC, the primary hospital accreditation body in the United States, required accreditation as part of its quality designation criteria. TJC requires its own accreditation for quality designation consideration, whereas the 3 private payers allow accreditation from one of a variety of sources. Aetna Institutes of Quality for Orthopedic Surgery requires accreditation by TJC, Healthcare Facilities Accreditation Program, American Osteopathic Association, National Integrated Accreditation for Healthcare Organizations, or Det Norske Veritas Healthcare. UnitedHealth Premium Total Joint Replacement (TJR) Specialty Center requires accreditation by TJC and/or equivalent of TJC accreditation. However, TJC accreditation equivalents are not noted in the UnitedHealth handbook. BCBS Blue Distinction and Distinction+ require accreditation by TJC, Healthcare Facilities Accreditation Program, National Integrated Accreditation for Healthcare Organizations, or Center for Improvement in Healthcare Quality. In addition, BCBS is willing to consider alternative accreditations that are at least as stringent as the national alternatives noted. However, no detailed criteria that must be met to be equivalent to the national standards are noted in the relevant quality designation handbook.
The volume of completed total hip and knee arthroplasty procedures was considered in 6 of the organizations’ quality ratings methodologies. Of those 6, all private payers, TJC (not-for-profit), and 2 for-profit rating agencies were included. Surgeon specialization in TJA was only explicitly noted as a factor considered in UnitedHealth Premium TJR Specialty Center criteria; however, the requirements for surgeon specialization were not clearly defined. In addition, the presence of a multidisciplinary clinical pathway was only explicitly considered for Aetna Institutes of Quality for Orthopedic Surgery.
Structural requirements (eg, use of electronic health records [EHR], staffing levels, etc.) were taken into account in private payer and USNWR quality methodologies. Process measures (eg, antibiotic prophylaxis and other care pathways) were considered for the private payers and TJC but not for USNWR quality designation. Cost and/or efficiency measures were factors in the quality formula for Aetna Institutes of Quality for Orthopedic Surgery and BCBS Distinction+. Aetna utilizes its own cost data and risk-adjusts using a product known as Symmetry Episode Risk Groups to determine cost-effectiveness, while BCBS uses its own Composite Facility Cost Index. Patient experience (eg, Hospital Consumer Assessment of Healthcare Providers and Systems [HCAHPS]) was incorporated into the quality formulas for 4 of the 7 quality designation programs examined.
Continue to: All of the 7 quality designation programs included...
All of the 7 quality designation programs included outcomes (ie, readmission rates and/or mortality rates) and publicly reported the hospitals receiving their quality designation. In contrast, only Aetna explicitly included the presence of multidisciplinary clinical care pathways as part of their quality designation criteria. In addition, only UnitedHealth included surgeon specialization in joint arthroplasty as a factor for quality consideration for its quality designation program. BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery were the only 2 quality designations that included at least 1 variable that fit into each of the 7 characteristics considered (accreditation, volume, structural, process, outcomes, patient experience, and cost/efficiency).
DISCUSSION
As healthcare continues to shift toward value-based delivery and payment models, quality becomes a critical factor in reimbursement and provider rankings. However, quality is a vague term. Several providers probably do not know what is required to be designated as high quality by a particular rating agency. Moreover, there are multiple quality designation programs, all using distinct criteria to determine “quality,” which further complicates the matter. Our objective was to determine the key stakeholders that provide quality designations in TJA and what criteria each organization uses in assessing quality.
Our idea of comprehensive quality is based on Avedis Donabedian’s enduring framework for healthcare quality focused on structure, process, and outcome.16 We expanded on these 3 areas and analyzed quality designations based on variables fitting into the following categories: accreditation, volume, structural, process, outcomes, patient experience, and cost/efficiency. We believe that these categories encompass a comprehensive rating system that addresses key elements of patient care. However, our results suggest that only 2 major quality designations (BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery) take all such variables into account.
All quality designation programs that we analyzed required outcome data (ie, readmission and/or mortality rates within 30 days); however, only 2 programs utilized cost in their quality designation criteria (BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery). Aetna Institutes of Quality for Orthopedic Surgery risk-adjusted for its cost-effectiveness calculations based on age, sex, and other unspecified conditions using a product known as Symmetry Episode Risk Groups. However, the organization also noted that although it did risk-adjust for inpatient mortality, it did not do so for pulmonary embolism or deep vein thrombosis. BCBS Distinction+ also utilized risk adjustment for its cost efficiency measure, and its step-by-step methodology is available online. Further, Consumer Reports does risk-adjust using logistic regression models in their quality analysis, but the description provided is minimal; it is noted that such risk adjustments are already completed by CMS prior to Consumer Reports acquiring the data. The CMS Compare model information is available on the CMS website. The data utilized by several organizations and presented on CMS Compare are already risk-adjusted using CMS’ approach. In contrast, UnitedHealth Premium TJR Specialty Center gathers its own data from providers and does not describe a risk adjustment methodology. Risk adjustment is important because the lack of risk adjustment may lead to physicians “cherry-picking” easy cases to boost positive outcomes, leading to increased financial benefits and higher quality ratings. Having a consistent risk adjustment formula will ensure accurate comparisons across outcomes and cost-effectiveness measures used by quality designation programs.
Factors considered for quality designation varied greatly from one organization to the other. The range of categories of factors considered varied from 1 (Consumer Reports only considered outcome data) to all 7 categories (BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery). Our findings are consistent with the work by Keswani and colleagues,8 which showed that there is likely variation in factors considered when rating hospital quality more broadly. Our work suggests that quality designation formulas do not appear to get more consistent when focused on TJA.
We found that all organizations in our analysis published the providers earning their quality designation. However, TJC does not provide publicly a detailed methodology on how to qualify for its quality designation. The price to purchase the necessary manual for this information is $146.00 for accredited organizations and $186.00 for all others.17 For large healthcare providers, this is not a large sum of money. Nonetheless, this provides an additional hurdle for stakeholders to gain a full understanding of the requirements to receive a TJC Gold Seal for Orthopedics.
Previous work has evaluated the consistency of and the variety of means of gauging healthcare quality. Previous work by Rothberg and colleagues18 comparing hospital rankings across 5 common consumer-oriented websites found disagreement on hospital rankings within any diagnosis and even among metrics such as mortality. Another study by Halasyamani and Davis19 found that CMS Compare and USNWR rankings were dissimilar and the authors attributed the discrepancy to different methodologies. In addition, a study by Krumholz and colleagues20 focused on Internet report cards, which measured the appropriate use of select medications and mortality rates for acute myocardial infarction as the quality metrics. The authors found that, in aggregate, there was a clear difference in quality of care and outcomes but that comparisons between 2 hospitals provided poor discrimination.20 Other work has analyzed the increasing trend of online ratings of orthopedic surgeons by patients.21 However, there remains no agreed-upon definition of quality. Thus, the use of the term “quality” in several studies may be misleading.
Our results must be interpreted keeping the limitations of our work in mind. First, we used expert knowledge and a public search engine to develop our list of organizations that provide TJA quality designations. However, there is a possibility that we did not include all relevant organizations. Second, although all authors reviewed the final data, it is possible that there was human error in the analysis of each organization’s quality designation criteria.
CONCLUSION
As healthcare progresses further toward a system that rewards providers for delivering value to patients, accurately defining and measuring quality becomes critical because it can be suggestive of value to patients, payers, and providers. Furthermore, it gives providers a goal to focus on as they strive to improve the value of care they deliver to patients. Measuring healthcare quality is currently a novel, imperfect science,22 and there continues to be a debate about what factors should be included in a quality designation formula. Nonetheless, more and more quality designations and performance measurements are being created for orthopedic care, including total hip and total knee arthroplasty. In fact, in 2016, The Leapfrog Group added readmission for patients undergoing TJA to its survey.23 Consensus on a quality definition may facilitate the movement toward a value-based healthcare system. Future research should evaluate strategies for gaining consensus among stakeholders for a universal quality metric in TJA. Surgeons, hospitals, payers, and most importantly patients should play critical roles in defining quality.
ABSTRACT
A patient’s perception of hospital or provider quality can have far-reaching effects, as it can impact reimbursement, patient selection of a surgeon, and healthcare competition. A variety of organizations offer quality designations for orthopedic surgery and its subspecialties. Our goal is to compare total joint arthroplasty (TJA) quality designation methodology across key quality rating organizations. One researcher conducted an initial Google search to determine organizations providing quality designations for hospitals and surgeons providing orthopedic procedures with a focus on TJA. Organizations that offer quality designation specific to TJA were determined. Organizations that provided general orthopedic surgery or only surgeon-specific quality designation were excluded from the analysis. The senior author confirmed the inclusion of the final organizations. Seven organizations fit our inclusion criteria. Only the private payers and The Joint Commission required hospital accreditation to meet quality designation criteria. Total arthroplasty volume was considered in 86% of the organizations’ methodologies, and 57% of organizations utilized process measurements such as antibiotic prophylaxis and care pathways. In addition, 57% of organizations included patient experience in their methodologies. Only 29% of organizations included a cost element in their methodology. All organizations utilized outcome data and publicly reported all hospitals receiving their quality designation. Hospital quality designation methodologies are inconsistent in the context of TJA. All stakeholders (ie, providers, payers, and patients) should be involved in deciding the definition of quality.
Continue to: Healthcare in the United States...
Healthcare in the United States has begun to move toward a system focused on value for patients, defined as health outcome per dollar expended.1 Indeed, an estimated 30% of Medicare payments are now made using the so-called alternative payment models (eg, bundled payments),2 and there is an expectation that consumerism in medicine will continue to expand.3 In addition, although there is a continuing debate regarding the benefits and pitfalls of hospital mergers, there is no question whether provider consolidation has increased dramatically in recent years.4 At the core of many of these changes is the push to improve healthcare quality and reduce costs.
Quality has the ability to affect payment, patient selection of providers, and hospital competition. Patients (ie, healthcare consumers) are increasingly using the Internet to find a variety of health information.5 Accessible provider quality information online would allow patients to make more informed decisions about where to seek care. In addition, the development of transparent quality ratings could assist payers in driving beneficiaries to higher quality and better value providers, which could mean more business for the highest quality physicians and better patient outcomes with fewer complications. Some payers such as the Centers for Medicare and Medicaid Services (CMS) have already started using quality measures as part of their reimbursement strategy.6 Because CMS is the largest payer in the United States, private insurers tend to follow their lead; thus, quality measurements will become even more common as a factor in reimbursement over the coming years.
To make quality ratings useful, “quality” must be clearly defined. Clarity around which factors are considered in a quality designation will create transparency for patients and allow providers to understand how their performance is being measured so that they focus on improving outcomes for their patients. Numerous organizations, including private payers, public payers, and both not-for-profit and for-profit entities, have created quality designation programs to rate providers. However, within orthopedics and several other medical specialties, there has been an ongoing debate about what measures best reflect quality.7 Although inconsistencies in quality ratings in arthroplasty care have been noted,8 it remains unknown how each quality designation program compares with the others in terms of the factors considered in deciding quality designations.
The purpose of this study is to evaluate publicly available information from key quality designation programs for total joint arthroplasty (TJA) providers to determine what factors are considered by each organization in awarding quality designations; what similarities and differences in quality designations exist across the different organizations; and how many of the organizations publish their quality designation methodologies and final rating results.
MATERIALS AND METHODS
A directed Google search was conducted to determine organizations (ie, payers, independent firms, and government entities) that rate hospitals and/or surgeons in orthopedic surgery. The identified organizations were then examined to determine whether they provided hospital ratings for total hip and/or knee arthroplasty. Entities were included if they provided quality designations for hospitals specifically addressing TJA. Organizations that provided only general hospital, other surgical procedures, orthopedic surgery, or orthopedic surgeon-specific quality designations were excluded. A list of all organizations determined to fit the inclusion criteria was then reviewed for completeness and approved by the senior author.
Continue to: One investigator reviewed the website of each organization...
One investigator reviewed the website of each organization fitting the inclusion criteria to determine the full rating methodology in 1 sitting on July 2, 2016. Detailed notes were taken on each program using publicly available information. For organizations that used proprietary criteria for quality designation (eg, The Joint Commission [TJC]), only publicly available information was used in the analysis. Therefore, the information reported is solely based on data available online to the public.
Detailed quality designation criteria were condensed into broader categories (accreditation, volume, structural, process, outcomes, patient experience, and cost/efficiency) to capture differences between each organization reviewed. In addition, we recorded whether each organization published a list of providers that received its quality designation.
RESULTS
A total of 7 organizations fit our inclusion criteria9-15 (Table). Of these 7 organizations, 3 were private payers (Aetna, UnitedHealth, and Blue Cross Blue Shield [BCBS]), 2 were nongovernmental not-for-profit organizations (TJC and Consumer Reports), and 2 were consumer-based and/or for-profit organizations (HealthGrades and US News & World Report [USNWR]). There were no government agencies that fit our inclusion criteria. BCBS had the following 2 separate quality designations: BCBS Blue Distinction and BCBS Blue Distinction+. The only difference between the 2 BCBS ratings is that BCBS Blue Distinction+ includes cost efficiency ratings, whereas BCBS Blue Distinction does not.

Only the 3 private payers and TJC, the primary hospital accreditation body in the United States, required accreditation as part of its quality designation criteria. TJC requires its own accreditation for quality designation consideration, whereas the 3 private payers allow accreditation from one of a variety of sources. Aetna Institutes of Quality for Orthopedic Surgery requires accreditation by TJC, Healthcare Facilities Accreditation Program, American Osteopathic Association, National Integrated Accreditation for Healthcare Organizations, or Det Norske Veritas Healthcare. UnitedHealth Premium Total Joint Replacement (TJR) Specialty Center requires accreditation by TJC and/or equivalent of TJC accreditation. However, TJC accreditation equivalents are not noted in the UnitedHealth handbook. BCBS Blue Distinction and Distinction+ require accreditation by TJC, Healthcare Facilities Accreditation Program, National Integrated Accreditation for Healthcare Organizations, or Center for Improvement in Healthcare Quality. In addition, BCBS is willing to consider alternative accreditations that are at least as stringent as the national alternatives noted. However, no detailed criteria that must be met to be equivalent to the national standards are noted in the relevant quality designation handbook.
The volume of completed total hip and knee arthroplasty procedures was considered in 6 of the organizations’ quality ratings methodologies. Of those 6, all private payers, TJC (not-for-profit), and 2 for-profit rating agencies were included. Surgeon specialization in TJA was only explicitly noted as a factor considered in UnitedHealth Premium TJR Specialty Center criteria; however, the requirements for surgeon specialization were not clearly defined. In addition, the presence of a multidisciplinary clinical pathway was only explicitly considered for Aetna Institutes of Quality for Orthopedic Surgery.
Structural requirements (eg, use of electronic health records [EHR], staffing levels, etc.) were taken into account in private payer and USNWR quality methodologies. Process measures (eg, antibiotic prophylaxis and other care pathways) were considered for the private payers and TJC but not for USNWR quality designation. Cost and/or efficiency measures were factors in the quality formula for Aetna Institutes of Quality for Orthopedic Surgery and BCBS Distinction+. Aetna utilizes its own cost data and risk-adjusts using a product known as Symmetry Episode Risk Groups to determine cost-effectiveness, while BCBS uses its own Composite Facility Cost Index. Patient experience (eg, Hospital Consumer Assessment of Healthcare Providers and Systems [HCAHPS]) was incorporated into the quality formulas for 4 of the 7 quality designation programs examined.
Continue to: All of the 7 quality designation programs included...
All of the 7 quality designation programs included outcomes (ie, readmission rates and/or mortality rates) and publicly reported the hospitals receiving their quality designation. In contrast, only Aetna explicitly included the presence of multidisciplinary clinical care pathways as part of their quality designation criteria. In addition, only UnitedHealth included surgeon specialization in joint arthroplasty as a factor for quality consideration for its quality designation program. BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery were the only 2 quality designations that included at least 1 variable that fit into each of the 7 characteristics considered (accreditation, volume, structural, process, outcomes, patient experience, and cost/efficiency).
DISCUSSION
As healthcare continues to shift toward value-based delivery and payment models, quality becomes a critical factor in reimbursement and provider rankings. However, quality is a vague term. Several providers probably do not know what is required to be designated as high quality by a particular rating agency. Moreover, there are multiple quality designation programs, all using distinct criteria to determine “quality,” which further complicates the matter. Our objective was to determine the key stakeholders that provide quality designations in TJA and what criteria each organization uses in assessing quality.
Our idea of comprehensive quality is based on Avedis Donabedian’s enduring framework for healthcare quality focused on structure, process, and outcome.16 We expanded on these 3 areas and analyzed quality designations based on variables fitting into the following categories: accreditation, volume, structural, process, outcomes, patient experience, and cost/efficiency. We believe that these categories encompass a comprehensive rating system that addresses key elements of patient care. However, our results suggest that only 2 major quality designations (BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery) take all such variables into account.
All quality designation programs that we analyzed required outcome data (ie, readmission and/or mortality rates within 30 days); however, only 2 programs utilized cost in their quality designation criteria (BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery). Aetna Institutes of Quality for Orthopedic Surgery risk-adjusted for its cost-effectiveness calculations based on age, sex, and other unspecified conditions using a product known as Symmetry Episode Risk Groups. However, the organization also noted that although it did risk-adjust for inpatient mortality, it did not do so for pulmonary embolism or deep vein thrombosis. BCBS Distinction+ also utilized risk adjustment for its cost efficiency measure, and its step-by-step methodology is available online. Further, Consumer Reports does risk-adjust using logistic regression models in their quality analysis, but the description provided is minimal; it is noted that such risk adjustments are already completed by CMS prior to Consumer Reports acquiring the data. The CMS Compare model information is available on the CMS website. The data utilized by several organizations and presented on CMS Compare are already risk-adjusted using CMS’ approach. In contrast, UnitedHealth Premium TJR Specialty Center gathers its own data from providers and does not describe a risk adjustment methodology. Risk adjustment is important because the lack of risk adjustment may lead to physicians “cherry-picking” easy cases to boost positive outcomes, leading to increased financial benefits and higher quality ratings. Having a consistent risk adjustment formula will ensure accurate comparisons across outcomes and cost-effectiveness measures used by quality designation programs.
Factors considered for quality designation varied greatly from one organization to the other. The range of categories of factors considered varied from 1 (Consumer Reports only considered outcome data) to all 7 categories (BCBS Distinction+ and Aetna Institutes of Quality for Orthopedic Surgery). Our findings are consistent with the work by Keswani and colleagues,8 which showed that there is likely variation in factors considered when rating hospital quality more broadly. Our work suggests that quality designation formulas do not appear to get more consistent when focused on TJA.
We found that all organizations in our analysis published the providers earning their quality designation. However, TJC does not provide publicly a detailed methodology on how to qualify for its quality designation. The price to purchase the necessary manual for this information is $146.00 for accredited organizations and $186.00 for all others.17 For large healthcare providers, this is not a large sum of money. Nonetheless, this provides an additional hurdle for stakeholders to gain a full understanding of the requirements to receive a TJC Gold Seal for Orthopedics.
Previous work has evaluated the consistency of and the variety of means of gauging healthcare quality. Previous work by Rothberg and colleagues18 comparing hospital rankings across 5 common consumer-oriented websites found disagreement on hospital rankings within any diagnosis and even among metrics such as mortality. Another study by Halasyamani and Davis19 found that CMS Compare and USNWR rankings were dissimilar and the authors attributed the discrepancy to different methodologies. In addition, a study by Krumholz and colleagues20 focused on Internet report cards, which measured the appropriate use of select medications and mortality rates for acute myocardial infarction as the quality metrics. The authors found that, in aggregate, there was a clear difference in quality of care and outcomes but that comparisons between 2 hospitals provided poor discrimination.20 Other work has analyzed the increasing trend of online ratings of orthopedic surgeons by patients.21 However, there remains no agreed-upon definition of quality. Thus, the use of the term “quality” in several studies may be misleading.
Our results must be interpreted keeping the limitations of our work in mind. First, we used expert knowledge and a public search engine to develop our list of organizations that provide TJA quality designations. However, there is a possibility that we did not include all relevant organizations. Second, although all authors reviewed the final data, it is possible that there was human error in the analysis of each organization’s quality designation criteria.
CONCLUSION
As healthcare progresses further toward a system that rewards providers for delivering value to patients, accurately defining and measuring quality becomes critical because it can be suggestive of value to patients, payers, and providers. Furthermore, it gives providers a goal to focus on as they strive to improve the value of care they deliver to patients. Measuring healthcare quality is currently a novel, imperfect science,22 and there continues to be a debate about what factors should be included in a quality designation formula. Nonetheless, more and more quality designations and performance measurements are being created for orthopedic care, including total hip and total knee arthroplasty. In fact, in 2016, The Leapfrog Group added readmission for patients undergoing TJA to its survey.23 Consensus on a quality definition may facilitate the movement toward a value-based healthcare system. Future research should evaluate strategies for gaining consensus among stakeholders for a universal quality metric in TJA. Surgeons, hospitals, payers, and most importantly patients should play critical roles in defining quality.
- Porter ME. A strategy for health care reform--toward a value-based system. N Engl J Med. 2009;361(2):109-112. doi:10.1056/NEJMp0904131.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316(5):525-532. doi:10.1001/jama.2016.9797.
- Mulvany C. The march to consumerism the evolution from patient to active shopper continues. Healthc Financ Manage. 2014;68(2):36-38.
- Tsai TC, Jha AK. Hospital consolidation, competition, and quality: is bigger necessarily better? JAMA. 2014;312(1):29-30. doi:10.1001/jama.2014.4692.
- Cline RJ, Haynes KM. Consumer health information seeking on the Internet: the state of the art. Health Educ Res. 2001;16(6):671-692. doi:10.1093/her/16.6.671.
- Werner RM, Kolstad JT, Stuart EA, Polsky D. The effect of pay-for-performance in hospitals: lessons for quality improvement. Health Aff (Millwood). 2011;30(4):690-698. doi:10.1377/hlthaff.2010.1277.
- Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? J Am Coll Surg. 2004;198(4):626-632. doi:10.1016/j.jamcollsurg.2003.11.017.
- Keswani A, Uhler LM, Bozic KJ. What quality metrics is my hospital being evaluated on and what are the consequences? J Arthroplast. 2016;31(6):1139-1143. doi:10.1016/j.arth.2016.01.075.
- Aetna Inc. Aetna Institutes of Quality® facilities fact book. A comprehensive reference guide for Aetna members, doctors and health care professionals. http://www.aetna.com/individuals-families-health-insurance/document-libr.... Accessed July 2, 2016.
- United HealthCare. UnitedHealth Premium® Program. https://www.uhcprovider.com/en/reports-quality-programs/premium-designation.html. Accessed July 2, 2016.
- 11. Blue Cross Blue Shield. Association. Blue Distinction Specialty Care. Selection criteria and program documentation: knee and hip replacement and spine surgery. https://www.bcbs.com/sites/default/files/fileattachments/page/KneeHip.SelectionCriteria_0.pdf. Published October 2015. Accessed July 2, 2016.
- The Joint Commission. Advanced certification for total hip and total knee replacement eligibility. https://www.jointcommission.org/advanced_certification_for_total_hip_and.... Published December 10, 2015. Accessed July 2, 2016.
- Healthgrades Operating Company. Healthgrades methodology: anatomy of a rating. https://www.healthgrades.com/quality/ratings-awards/methodology. Accessed July 2, 2016.
- Comarow A, Harder B; Dr. Foster Project Team. Methodology: U.S. News & World Report best hospitals for common care. U.S. News & World Report Web site. http://www.usnews.com/pubfiles/BHCC_MethReport_2015.pdf. Published May 20, 2015. Accessed July 2, 2016.
- Consumer Reports. How we rate hospitals. http://static3.consumerreportscdn.org/content/dam/cro/news_articles/heal.... Accessed July 2, 2016.
- Ayanian JZ, Markel H. Donabedian’s lasting framework for health care quality. N Engl J Med. 2016;375(3):205-207. doi:10.1056/NEJMp1605101.
- The Joint Commission. 2016 Certification Manuals. 2016; http://www.jcrinc.com/2016-certification-manuals/. Accessed July 2, 2016.
- Rothberg MB, Morsi E, Benjamin EM, Pekow PS, Lindenauer PK. Choosing the best hospital: the limitations of public quality reporting. Health Aff (Millwood). 2008;27(6):1680-1687. doi:10.1377/hlthaff.27.6.1680.
- Halasyamani LK, Davis MM. Conflicting measures of hospital quality: ratings from "Hospital Compare" versus "Best Hospitals". J Hosp Med. 2007;2(3):128-134. doi:10.1002/jhm.176.
- Krumholz HM, Rathore SS, Chen J, Wang Y, Radford MJ. Evaluation of a consumer-oriented internet health care report card: the risk of quality ratings based on mortality data. JAMA. 2002;287(10):1277-1287.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38(4):e257-e262. doi:10.3928/01477447-20150402-52.
- Harder B, Comarow A. Hospital Quality reporting by US News & World Report: why, how, and what's ahead. JAMA. 2015;313(19):1903-1904. doi:10.1001/jama.2015.4566.
- The Leapfrog Group. New in 2016. http://www.leapfroggroup.org/ratings-reports/new-2016. Accessed July 2, 2016.
- Porter ME. A strategy for health care reform--toward a value-based system. N Engl J Med. 2009;361(2):109-112. doi:10.1056/NEJMp0904131.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316(5):525-532. doi:10.1001/jama.2016.9797.
- Mulvany C. The march to consumerism the evolution from patient to active shopper continues. Healthc Financ Manage. 2014;68(2):36-38.
- Tsai TC, Jha AK. Hospital consolidation, competition, and quality: is bigger necessarily better? JAMA. 2014;312(1):29-30. doi:10.1001/jama.2014.4692.
- Cline RJ, Haynes KM. Consumer health information seeking on the Internet: the state of the art. Health Educ Res. 2001;16(6):671-692. doi:10.1093/her/16.6.671.
- Werner RM, Kolstad JT, Stuart EA, Polsky D. The effect of pay-for-performance in hospitals: lessons for quality improvement. Health Aff (Millwood). 2011;30(4):690-698. doi:10.1377/hlthaff.2010.1277.
- Birkmeyer JD, Dimick JB, Birkmeyer NJ. Measuring the quality of surgical care: structure, process, or outcomes? J Am Coll Surg. 2004;198(4):626-632. doi:10.1016/j.jamcollsurg.2003.11.017.
- Keswani A, Uhler LM, Bozic KJ. What quality metrics is my hospital being evaluated on and what are the consequences? J Arthroplast. 2016;31(6):1139-1143. doi:10.1016/j.arth.2016.01.075.
- Aetna Inc. Aetna Institutes of Quality® facilities fact book. A comprehensive reference guide for Aetna members, doctors and health care professionals. http://www.aetna.com/individuals-families-health-insurance/document-libr.... Accessed July 2, 2016.
- United HealthCare. UnitedHealth Premium® Program. https://www.uhcprovider.com/en/reports-quality-programs/premium-designation.html. Accessed July 2, 2016.
- 11. Blue Cross Blue Shield. Association. Blue Distinction Specialty Care. Selection criteria and program documentation: knee and hip replacement and spine surgery. https://www.bcbs.com/sites/default/files/fileattachments/page/KneeHip.SelectionCriteria_0.pdf. Published October 2015. Accessed July 2, 2016.
- The Joint Commission. Advanced certification for total hip and total knee replacement eligibility. https://www.jointcommission.org/advanced_certification_for_total_hip_and.... Published December 10, 2015. Accessed July 2, 2016.
- Healthgrades Operating Company. Healthgrades methodology: anatomy of a rating. https://www.healthgrades.com/quality/ratings-awards/methodology. Accessed July 2, 2016.
- Comarow A, Harder B; Dr. Foster Project Team. Methodology: U.S. News & World Report best hospitals for common care. U.S. News & World Report Web site. http://www.usnews.com/pubfiles/BHCC_MethReport_2015.pdf. Published May 20, 2015. Accessed July 2, 2016.
- Consumer Reports. How we rate hospitals. http://static3.consumerreportscdn.org/content/dam/cro/news_articles/heal.... Accessed July 2, 2016.
- Ayanian JZ, Markel H. Donabedian’s lasting framework for health care quality. N Engl J Med. 2016;375(3):205-207. doi:10.1056/NEJMp1605101.
- The Joint Commission. 2016 Certification Manuals. 2016; http://www.jcrinc.com/2016-certification-manuals/. Accessed July 2, 2016.
- Rothberg MB, Morsi E, Benjamin EM, Pekow PS, Lindenauer PK. Choosing the best hospital: the limitations of public quality reporting. Health Aff (Millwood). 2008;27(6):1680-1687. doi:10.1377/hlthaff.27.6.1680.
- Halasyamani LK, Davis MM. Conflicting measures of hospital quality: ratings from "Hospital Compare" versus "Best Hospitals". J Hosp Med. 2007;2(3):128-134. doi:10.1002/jhm.176.
- Krumholz HM, Rathore SS, Chen J, Wang Y, Radford MJ. Evaluation of a consumer-oriented internet health care report card: the risk of quality ratings based on mortality data. JAMA. 2002;287(10):1277-1287.
- Frost C, Mesfin A. Online reviews of orthopedic surgeons: an emerging trend. Orthopedics. 2015;38(4):e257-e262. doi:10.3928/01477447-20150402-52.
- Harder B, Comarow A. Hospital Quality reporting by US News & World Report: why, how, and what's ahead. JAMA. 2015;313(19):1903-1904. doi:10.1001/jama.2015.4566.
- The Leapfrog Group. New in 2016. http://www.leapfroggroup.org/ratings-reports/new-2016. Accessed July 2, 2016.
TAKE-HOME POINTS
- TJA quality designation methodologies differ substantially across rating organizations.
- Only 29% of TJA quality rating methodologies evaluated include a cost element.
- Only 57% of TJA quality rating methodologies evaluated include patient experience.
- Only 57% of TJA quality rating methodologies evaluated include process measurements, including antibiotic prophylaxis and standardized care pathways.
- There is a need for consistent definitions of quality as healthcare stakeholders continue to shift focus from volume to value.
Real-World Evidence for Safety and Effectiveness of Repeated Courses of Hyaluronic Acid Injections on the Time to Knee Replacement Surgery
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.
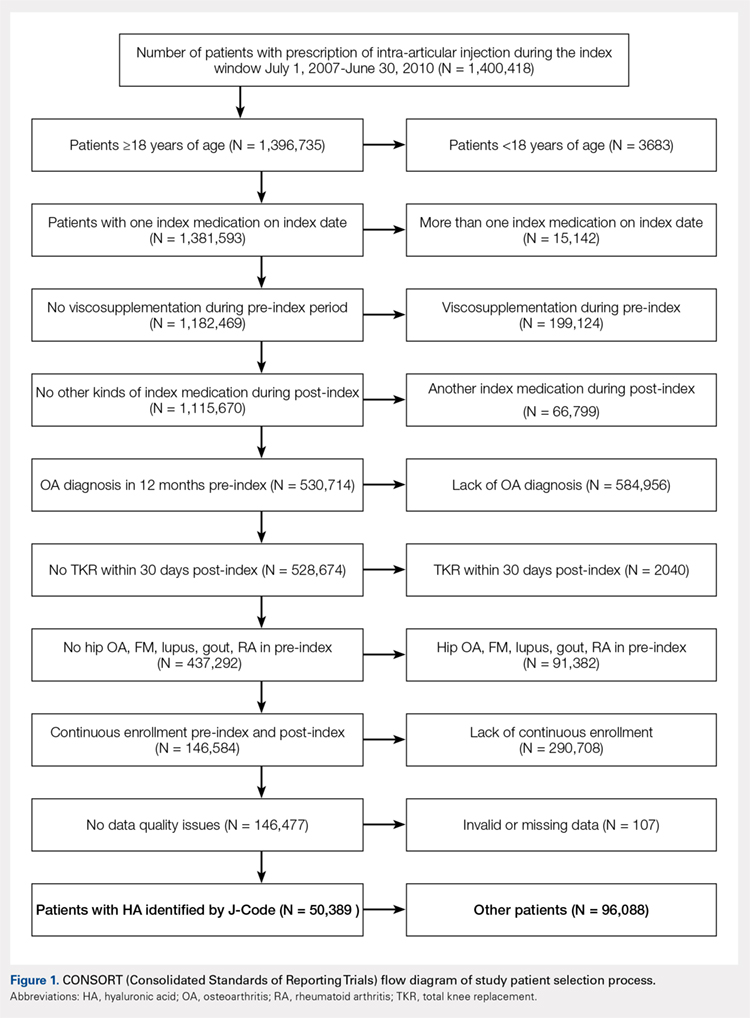
Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.
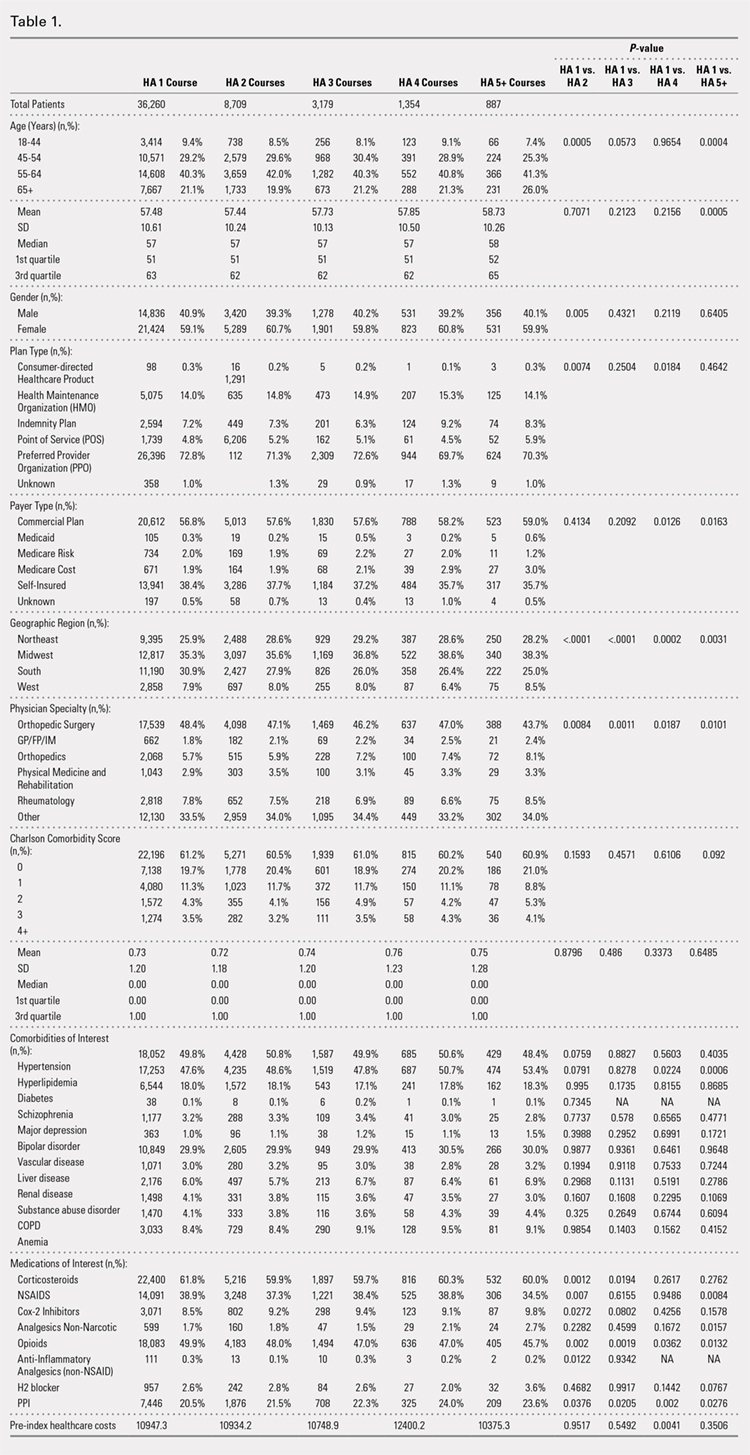
PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).
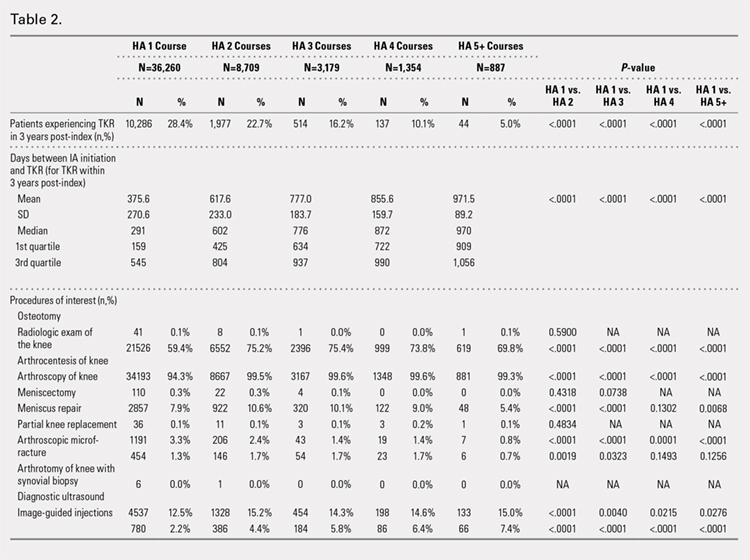
ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.
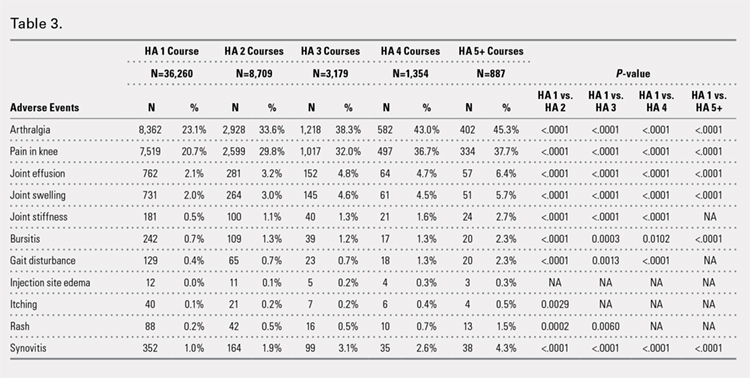
TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).
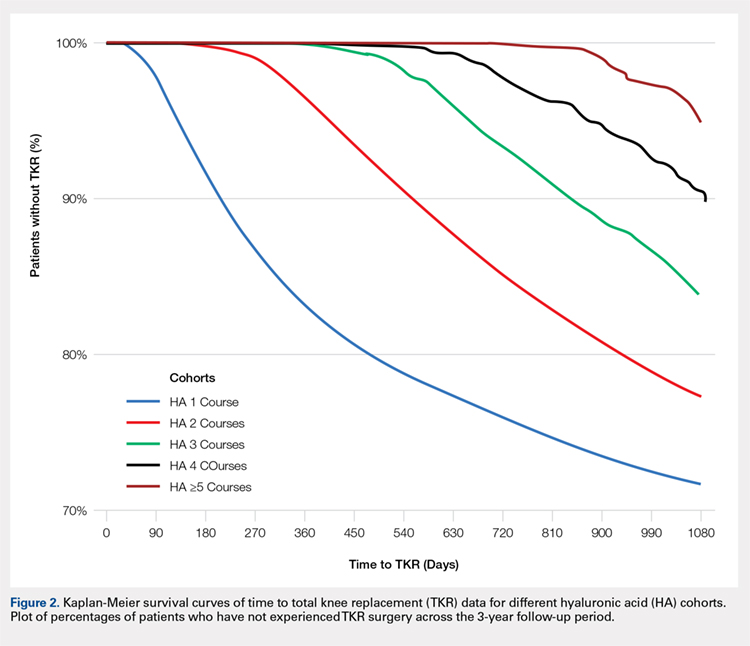
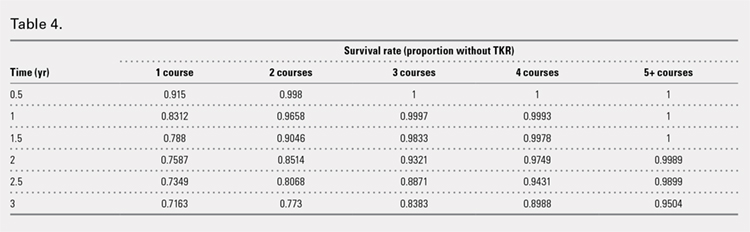
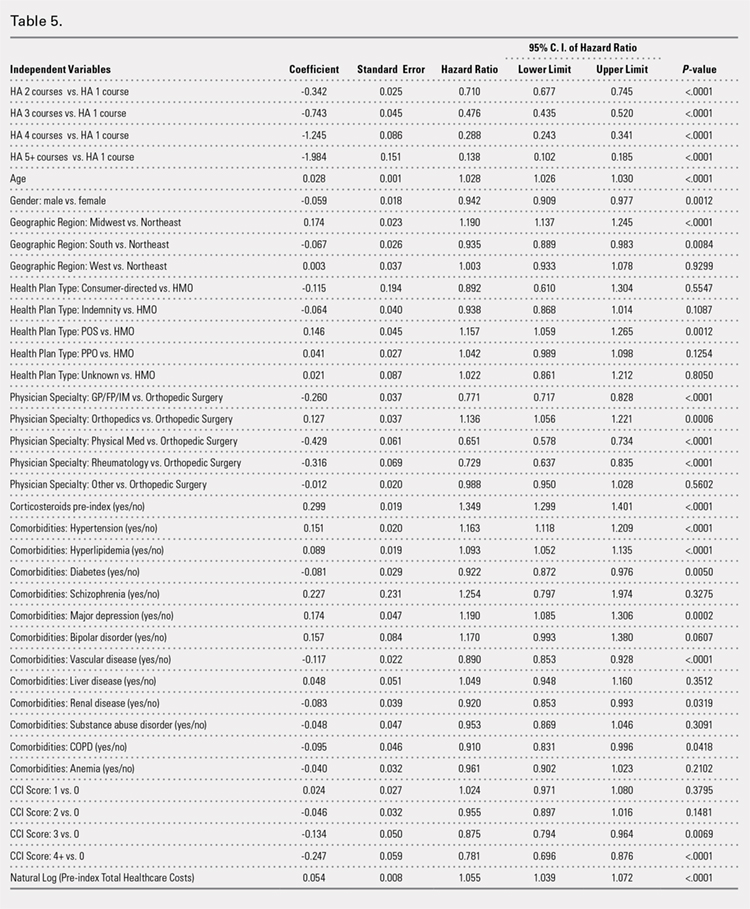
Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.

Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.

PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).

ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.

TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).



Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.

Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.

PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).

ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.

TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).



Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
TAKE-HOME POINTS
- Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years.
- HA treatment should be considered an important clinical treatment option for patients with knee OA.
- Repeated courses of treatment with HA are safe.
- Repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
- Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
The Aberrant Anterior Tibial Artery and its Surgical Risk
ABSTRACT
Vascular injury to the popliteal artery during knee surgery is uncommon, but it has significant consequences not only for the patient but also to the surgeon since it poses the threat of malpractice litigation. The vascular anatomy of the lower extremity is variable especially when it involves both the popliteal artery and its branches. An aberrant vascular course may increase the risk of iatrogenic vascular injury during surgery. Careful preoperative planning with advanced imaging can decrease the risk of a devastating vascular injury.
Continue to: Most non-traumatic injuries...
Most non-traumatic injuries to the popliteal artery are iatrogenic and may occur during total knee replacement,1-8 high tibial osteotomy,2,3,5-7 anterior cruciate ligament reconstruction,2,6 posterior cruciate ligament reconstruction,2,6,9,10 and arthroscopic meniscectomy.2,6,9 Despite the rare occurrence of complications involving the popliteal artery during such procedures, results of vessel injuries can be devastating and may also lead to malpractice litigation. Anatomic variations of the distal popliteal artery and its significance in surgery have been well documented in the literature.2-6,8,11 However, due to lack of awareness, this issue is often unintentionally disregarded. We present the case of an aberrant anterior tibial artery that was found during the review of a magnetic resonance imaging study. The patient was provided written informed consent for print and electronic publication of this case report.
CASE
A 61-year-old woman presented with a history of right knee pain from osteoarthritis that had rapidly progressed over 1 week secondary to a fall. The patient had no history of previous knee surgery. After careful evaluation of her right knee pain, treatment options were discussed. The patient agreed to proceed with total knee arthroplasty (TKA). During preoperative planning, the patient’s previous magnetic resonance imaging (MRI) was reviewed. The MRI study revealed an aberrant anterior tibial artery. The popliteal artery bifurcated at the level of the knee joint (Figures 1A-1C). After the bifurcation, the anterior tibial artery coursed anteriorly to the tibioperoneal trunk. The anterior tibial artery is seen just anterior to the popliteus muscle and just posterior to the tibial plateau cortex (Figure 2). Intraoperatively, an oscillating saw was utilized for the tibial cut. Care was taken not to penetrate the posterior cortex. An osteotome was used to elevate the tibial cut and hinge it open, and with a small mallet, finish the tibial cut. The patient had a successful TKA without complication.
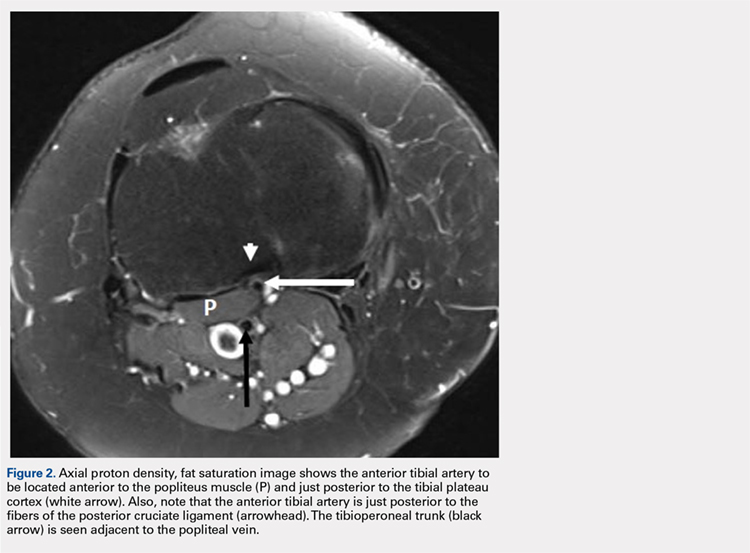
DISCUSSION
Emerging from the adductor hiatus (Hunter’s canal), the normal course of the popliteal artery is a position slightly lateral in the intercondylar fossa. It courses obliquely and posteriorly to the popliteus then bifurcates into the anterior tibial artery and the tibioperoneal trunk at the inferior border of the popliteus. The tibioperoneal trunk bifurcates into both the posterior tibial artery and the peroneal artery at the proximal tibia well below the knee joint.
There are many reported cases of popliteal artery variations.2,3,6,7,9,11-13 Variations in the popliteal artery are consequences of persistent embryonic vessels from primitive segments of the artery or abnormal fusions among them.14 According to Kim and colleagues,11 variations can be classified by the modified Lippert’s system. This system has 3 categories with 3 subtypes (Table). Variations are not uncommon and occur in 7.4% to 12% of the population.2,4,5,7,13
Table. Modified Lippert’s System11
Category (Subtype) |
|
I | Normal level of popliteal arterial branching |
IA | Usual pattern |
IB | Trifurcation- No true tibioperoneal trunk |
IC | Anterior tibioperoneal trunk- Posterior tibial artery is first branch |
II | High division of popliteal artery |
IIA | Anterior tibial artery arises at or above the knee joint |
IIB | Posterior tibial artery arises at or above the knee joint |
IIC | Peroneal artery arises at or above the knee joint |
III | Hypoplastic or aplastic branching with altered distal supply |
IIIA | Hypoplastic-aplastic posterior tibial artery |
IIIB | Hypoplastic-aplastic anterior tibial artery |
IIIC | Hypoplastic-aplastic posterior and anterior tibial artery |
Of these variations, type IIA, a high bifurcation of the anterior tibial artery, arising at or above the knee joint from the popliteal artery is the most significant. Forty-two percent of these vessels course anterior to the popliteus and make direct contact with the cortex of the posterior tibia.4 It is also the most frequent variant type reported in 1.2% to 6% of the population.3,7,11-13
Continue to: Injury to the popliteal artery...
Injury to the popliteal artery during an orthopedic procedure is believed to be under reported6 but is considered a rare complication. The incidence of popliteal artery injury in TKA is thought to be 0.03% to 0.2%.1,2,5,7,8 Vessel injury in both high tibial osteotomy and arthroscopic surgeries (lateral meniscal repair) have also been reported.5,6,8,10 Despite the rare occurrence of this complication, it may have devastating outcomes. The injury can be repaired with vascular grafting depending on its severity; however, it could also lead to compartment syndrome, loss of function, chronic ulcers, and necrosis of the affected limb resulting in below the knee amputation. The current consensus is that the popliteal artery moves posteriorly away from the tibia when the knee is in 90° of flexion,5 which is the standard position for many orthopedic knee surgeries. This position limits the risk of injuring the vessel. However, Metzdorf and colleagues,4 Smith and colleagues,6 and Zaidi and colleagues8 suggested that the vessel not be displaced posteriorly with flexion. These studies reported that the behavior of the popliteal artery varied among individuals since in some cases it had moved closer to the tibia in flexion when compared with extension.
Regardless of the behavior of the artery, it is protected by the popliteus muscle in most orthopedic knee surgeries since the majority course posterior to the muscle. However, in cases of Lippert’s type IIA variation, it not only loses protection as it courses beneath the popliteus but also is extremely vulnerable from the close relationship to the posterior tibial cortex. Klecker and colleagues2 described the aberrant artery locations related to common orthopedic procedures, which demonstrated its close proximity to various surgical plane levels. The position of the aberrant artery is approximately 1 to 1.5 cm distal to the posterior tibial joint line, just posterior to the posterior capsule, and close to the posterior cruciate ligament insertion site where the transverse tibial cut is made during TKA. This location also corresponds to the position for an inlay block and the tibial tunnel for posterior cruciate ligament reconstruction. A transverse cut for a high tibial osteotomy is approximately 1.5 to 2.5 cm distal to the posterior tibial joint line; the aberrant artery appeared directly posterior to the tibial cortex. These relationships were equivalent findings in this case. Such relationships of the aberrant anterior tibial artery to both the posterior tibial cortex and the posterior capsule increase the risk of vessel (anterior tibial artery) injury intraoperatively. The risk further increases in a revision of total knee replacement. This is secondary to limited flexibility of the vessel from scar formation which requires a more distal incision.1,4
CONCLUSION
Vascular injuries in knee surgeries are rare and often overlooked. Despite their low occurrence rate, outcomes of these injuries have grave consequences not only regarding medical but also legal matters. Variations in the popliteal artery are not uncommon and could potentially contribute to risks of vessel injury. Of these variations, the high originating anterior tibial artery poses a special risk. However, due to the low occurrence rate of these injuries, screening the general population may not be cost-effective. Since many patients already have obtained necessary imaging (preferably MRI), a careful review of these images along with preoperative planning and special care during surgery is recommended to identify popliteal artery variants and avoid iatrogenic vascular injury.
This paper will be judged for the Resident Writer’s Award.
- Abdel Karim MM, Anbar A, Keenan J. Position of the popliteal artery in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132(6):861-865. doi:10.1007/s00402-012-1479-6.
- Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery, magnetic resonance appearance, prevalence, and surgical implication. Am J Sports Medicine. 2008;36:720-727.
- Kropman RHJ, Kiela G, Moll FL, Vries JPM. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011;45:536-540.
- Metzdorf A, Jakob RP, Petropoulos P, Middleton R. Arterial injury during revision total knee replacement. A case report. Knee Surg Sports Traumatol Arthrosc. 1999;7:246-248.
- Shetty AA, Tindall AJ, Qureshi F, Divekar M, Fernando KWK. The Effect of knee flexion on the popliteal artery and its surgical significance. J Bone Joint Surg Br. 2003;85:218-222.
- Smith PN, Gelinas J, Kennedy K, Thain L, Rorabeck CH, Bourne B. Popliteal vessels in knee surgery; a magnetic resonance imaging study. Clin Orthop Rel Res. 1999;367:158-164
- Tindall AJ, Shetty AA, James KD, Middleton A, Fernando KWK. Prevalence and surgical significance of a high-origin anterior tibial artery. J Orthop Surg. 2006;14:13-16.
- Zaidi SHA, Cobb AG, Bentley G. Danger to the popliteal artery in high tibial osteotomy. J Bone Joint Surg Br. 1995;77:384-386.
- Keser S, Savranlar A, Bayar A, Ulukent SC, Ozer T, Tuncay I. Anatomic localization of the popliteal artery at the level of the knee joint: a magnetic resonance imaging study. Arthroscopy. 2006;22:656-659.
- Makino A, Costa-Paz M, Aponte-Tinao L, Ayerza MA, Muscolo L. Popliteal artery laceration during arthroscopic posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1396.
- Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants, a unified angiographic classification. Ann Surg. 1989;210:776-781.
- Day CP, Orme R. Popliteal artery branching patterns-an angiographic study. Clin Radiol. 2006;61:696-699.
- Kil SW, Jung GS. Anatomical variations of the popliteal artery and its tibial branches: Analysis in 1242 extremities. Cardiovasc Intervent Radiol. 2009;32:233-240.
- Senior HD. The development of the arteries of the human lower extremity. Am J Anat. 1919;25:55-94.
ABSTRACT
Vascular injury to the popliteal artery during knee surgery is uncommon, but it has significant consequences not only for the patient but also to the surgeon since it poses the threat of malpractice litigation. The vascular anatomy of the lower extremity is variable especially when it involves both the popliteal artery and its branches. An aberrant vascular course may increase the risk of iatrogenic vascular injury during surgery. Careful preoperative planning with advanced imaging can decrease the risk of a devastating vascular injury.
Continue to: Most non-traumatic injuries...
Most non-traumatic injuries to the popliteal artery are iatrogenic and may occur during total knee replacement,1-8 high tibial osteotomy,2,3,5-7 anterior cruciate ligament reconstruction,2,6 posterior cruciate ligament reconstruction,2,6,9,10 and arthroscopic meniscectomy.2,6,9 Despite the rare occurrence of complications involving the popliteal artery during such procedures, results of vessel injuries can be devastating and may also lead to malpractice litigation. Anatomic variations of the distal popliteal artery and its significance in surgery have been well documented in the literature.2-6,8,11 However, due to lack of awareness, this issue is often unintentionally disregarded. We present the case of an aberrant anterior tibial artery that was found during the review of a magnetic resonance imaging study. The patient was provided written informed consent for print and electronic publication of this case report.
CASE
A 61-year-old woman presented with a history of right knee pain from osteoarthritis that had rapidly progressed over 1 week secondary to a fall. The patient had no history of previous knee surgery. After careful evaluation of her right knee pain, treatment options were discussed. The patient agreed to proceed with total knee arthroplasty (TKA). During preoperative planning, the patient’s previous magnetic resonance imaging (MRI) was reviewed. The MRI study revealed an aberrant anterior tibial artery. The popliteal artery bifurcated at the level of the knee joint (Figures 1A-1C). After the bifurcation, the anterior tibial artery coursed anteriorly to the tibioperoneal trunk. The anterior tibial artery is seen just anterior to the popliteus muscle and just posterior to the tibial plateau cortex (Figure 2). Intraoperatively, an oscillating saw was utilized for the tibial cut. Care was taken not to penetrate the posterior cortex. An osteotome was used to elevate the tibial cut and hinge it open, and with a small mallet, finish the tibial cut. The patient had a successful TKA without complication.


DISCUSSION
Emerging from the adductor hiatus (Hunter’s canal), the normal course of the popliteal artery is a position slightly lateral in the intercondylar fossa. It courses obliquely and posteriorly to the popliteus then bifurcates into the anterior tibial artery and the tibioperoneal trunk at the inferior border of the popliteus. The tibioperoneal trunk bifurcates into both the posterior tibial artery and the peroneal artery at the proximal tibia well below the knee joint.
There are many reported cases of popliteal artery variations.2,3,6,7,9,11-13 Variations in the popliteal artery are consequences of persistent embryonic vessels from primitive segments of the artery or abnormal fusions among them.14 According to Kim and colleagues,11 variations can be classified by the modified Lippert’s system. This system has 3 categories with 3 subtypes (Table). Variations are not uncommon and occur in 7.4% to 12% of the population.2,4,5,7,13
Table. Modified Lippert’s System11
Category (Subtype) |
|
I | Normal level of popliteal arterial branching |
IA | Usual pattern |
IB | Trifurcation- No true tibioperoneal trunk |
IC | Anterior tibioperoneal trunk- Posterior tibial artery is first branch |
II | High division of popliteal artery |
IIA | Anterior tibial artery arises at or above the knee joint |
IIB | Posterior tibial artery arises at or above the knee joint |
IIC | Peroneal artery arises at or above the knee joint |
III | Hypoplastic or aplastic branching with altered distal supply |
IIIA | Hypoplastic-aplastic posterior tibial artery |
IIIB | Hypoplastic-aplastic anterior tibial artery |
IIIC | Hypoplastic-aplastic posterior and anterior tibial artery |
Of these variations, type IIA, a high bifurcation of the anterior tibial artery, arising at or above the knee joint from the popliteal artery is the most significant. Forty-two percent of these vessels course anterior to the popliteus and make direct contact with the cortex of the posterior tibia.4 It is also the most frequent variant type reported in 1.2% to 6% of the population.3,7,11-13
Continue to: Injury to the popliteal artery...
Injury to the popliteal artery during an orthopedic procedure is believed to be under reported6 but is considered a rare complication. The incidence of popliteal artery injury in TKA is thought to be 0.03% to 0.2%.1,2,5,7,8 Vessel injury in both high tibial osteotomy and arthroscopic surgeries (lateral meniscal repair) have also been reported.5,6,8,10 Despite the rare occurrence of this complication, it may have devastating outcomes. The injury can be repaired with vascular grafting depending on its severity; however, it could also lead to compartment syndrome, loss of function, chronic ulcers, and necrosis of the affected limb resulting in below the knee amputation. The current consensus is that the popliteal artery moves posteriorly away from the tibia when the knee is in 90° of flexion,5 which is the standard position for many orthopedic knee surgeries. This position limits the risk of injuring the vessel. However, Metzdorf and colleagues,4 Smith and colleagues,6 and Zaidi and colleagues8 suggested that the vessel not be displaced posteriorly with flexion. These studies reported that the behavior of the popliteal artery varied among individuals since in some cases it had moved closer to the tibia in flexion when compared with extension.
Regardless of the behavior of the artery, it is protected by the popliteus muscle in most orthopedic knee surgeries since the majority course posterior to the muscle. However, in cases of Lippert’s type IIA variation, it not only loses protection as it courses beneath the popliteus but also is extremely vulnerable from the close relationship to the posterior tibial cortex. Klecker and colleagues2 described the aberrant artery locations related to common orthopedic procedures, which demonstrated its close proximity to various surgical plane levels. The position of the aberrant artery is approximately 1 to 1.5 cm distal to the posterior tibial joint line, just posterior to the posterior capsule, and close to the posterior cruciate ligament insertion site where the transverse tibial cut is made during TKA. This location also corresponds to the position for an inlay block and the tibial tunnel for posterior cruciate ligament reconstruction. A transverse cut for a high tibial osteotomy is approximately 1.5 to 2.5 cm distal to the posterior tibial joint line; the aberrant artery appeared directly posterior to the tibial cortex. These relationships were equivalent findings in this case. Such relationships of the aberrant anterior tibial artery to both the posterior tibial cortex and the posterior capsule increase the risk of vessel (anterior tibial artery) injury intraoperatively. The risk further increases in a revision of total knee replacement. This is secondary to limited flexibility of the vessel from scar formation which requires a more distal incision.1,4
CONCLUSION
Vascular injuries in knee surgeries are rare and often overlooked. Despite their low occurrence rate, outcomes of these injuries have grave consequences not only regarding medical but also legal matters. Variations in the popliteal artery are not uncommon and could potentially contribute to risks of vessel injury. Of these variations, the high originating anterior tibial artery poses a special risk. However, due to the low occurrence rate of these injuries, screening the general population may not be cost-effective. Since many patients already have obtained necessary imaging (preferably MRI), a careful review of these images along with preoperative planning and special care during surgery is recommended to identify popliteal artery variants and avoid iatrogenic vascular injury.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Vascular injury to the popliteal artery during knee surgery is uncommon, but it has significant consequences not only for the patient but also to the surgeon since it poses the threat of malpractice litigation. The vascular anatomy of the lower extremity is variable especially when it involves both the popliteal artery and its branches. An aberrant vascular course may increase the risk of iatrogenic vascular injury during surgery. Careful preoperative planning with advanced imaging can decrease the risk of a devastating vascular injury.
Continue to: Most non-traumatic injuries...
Most non-traumatic injuries to the popliteal artery are iatrogenic and may occur during total knee replacement,1-8 high tibial osteotomy,2,3,5-7 anterior cruciate ligament reconstruction,2,6 posterior cruciate ligament reconstruction,2,6,9,10 and arthroscopic meniscectomy.2,6,9 Despite the rare occurrence of complications involving the popliteal artery during such procedures, results of vessel injuries can be devastating and may also lead to malpractice litigation. Anatomic variations of the distal popliteal artery and its significance in surgery have been well documented in the literature.2-6,8,11 However, due to lack of awareness, this issue is often unintentionally disregarded. We present the case of an aberrant anterior tibial artery that was found during the review of a magnetic resonance imaging study. The patient was provided written informed consent for print and electronic publication of this case report.
CASE
A 61-year-old woman presented with a history of right knee pain from osteoarthritis that had rapidly progressed over 1 week secondary to a fall. The patient had no history of previous knee surgery. After careful evaluation of her right knee pain, treatment options were discussed. The patient agreed to proceed with total knee arthroplasty (TKA). During preoperative planning, the patient’s previous magnetic resonance imaging (MRI) was reviewed. The MRI study revealed an aberrant anterior tibial artery. The popliteal artery bifurcated at the level of the knee joint (Figures 1A-1C). After the bifurcation, the anterior tibial artery coursed anteriorly to the tibioperoneal trunk. The anterior tibial artery is seen just anterior to the popliteus muscle and just posterior to the tibial plateau cortex (Figure 2). Intraoperatively, an oscillating saw was utilized for the tibial cut. Care was taken not to penetrate the posterior cortex. An osteotome was used to elevate the tibial cut and hinge it open, and with a small mallet, finish the tibial cut. The patient had a successful TKA without complication.


DISCUSSION
Emerging from the adductor hiatus (Hunter’s canal), the normal course of the popliteal artery is a position slightly lateral in the intercondylar fossa. It courses obliquely and posteriorly to the popliteus then bifurcates into the anterior tibial artery and the tibioperoneal trunk at the inferior border of the popliteus. The tibioperoneal trunk bifurcates into both the posterior tibial artery and the peroneal artery at the proximal tibia well below the knee joint.
There are many reported cases of popliteal artery variations.2,3,6,7,9,11-13 Variations in the popliteal artery are consequences of persistent embryonic vessels from primitive segments of the artery or abnormal fusions among them.14 According to Kim and colleagues,11 variations can be classified by the modified Lippert’s system. This system has 3 categories with 3 subtypes (Table). Variations are not uncommon and occur in 7.4% to 12% of the population.2,4,5,7,13
Table. Modified Lippert’s System11
Category (Subtype) |
|
I | Normal level of popliteal arterial branching |
IA | Usual pattern |
IB | Trifurcation- No true tibioperoneal trunk |
IC | Anterior tibioperoneal trunk- Posterior tibial artery is first branch |
II | High division of popliteal artery |
IIA | Anterior tibial artery arises at or above the knee joint |
IIB | Posterior tibial artery arises at or above the knee joint |
IIC | Peroneal artery arises at or above the knee joint |
III | Hypoplastic or aplastic branching with altered distal supply |
IIIA | Hypoplastic-aplastic posterior tibial artery |
IIIB | Hypoplastic-aplastic anterior tibial artery |
IIIC | Hypoplastic-aplastic posterior and anterior tibial artery |
Of these variations, type IIA, a high bifurcation of the anterior tibial artery, arising at or above the knee joint from the popliteal artery is the most significant. Forty-two percent of these vessels course anterior to the popliteus and make direct contact with the cortex of the posterior tibia.4 It is also the most frequent variant type reported in 1.2% to 6% of the population.3,7,11-13
Continue to: Injury to the popliteal artery...
Injury to the popliteal artery during an orthopedic procedure is believed to be under reported6 but is considered a rare complication. The incidence of popliteal artery injury in TKA is thought to be 0.03% to 0.2%.1,2,5,7,8 Vessel injury in both high tibial osteotomy and arthroscopic surgeries (lateral meniscal repair) have also been reported.5,6,8,10 Despite the rare occurrence of this complication, it may have devastating outcomes. The injury can be repaired with vascular grafting depending on its severity; however, it could also lead to compartment syndrome, loss of function, chronic ulcers, and necrosis of the affected limb resulting in below the knee amputation. The current consensus is that the popliteal artery moves posteriorly away from the tibia when the knee is in 90° of flexion,5 which is the standard position for many orthopedic knee surgeries. This position limits the risk of injuring the vessel. However, Metzdorf and colleagues,4 Smith and colleagues,6 and Zaidi and colleagues8 suggested that the vessel not be displaced posteriorly with flexion. These studies reported that the behavior of the popliteal artery varied among individuals since in some cases it had moved closer to the tibia in flexion when compared with extension.
Regardless of the behavior of the artery, it is protected by the popliteus muscle in most orthopedic knee surgeries since the majority course posterior to the muscle. However, in cases of Lippert’s type IIA variation, it not only loses protection as it courses beneath the popliteus but also is extremely vulnerable from the close relationship to the posterior tibial cortex. Klecker and colleagues2 described the aberrant artery locations related to common orthopedic procedures, which demonstrated its close proximity to various surgical plane levels. The position of the aberrant artery is approximately 1 to 1.5 cm distal to the posterior tibial joint line, just posterior to the posterior capsule, and close to the posterior cruciate ligament insertion site where the transverse tibial cut is made during TKA. This location also corresponds to the position for an inlay block and the tibial tunnel for posterior cruciate ligament reconstruction. A transverse cut for a high tibial osteotomy is approximately 1.5 to 2.5 cm distal to the posterior tibial joint line; the aberrant artery appeared directly posterior to the tibial cortex. These relationships were equivalent findings in this case. Such relationships of the aberrant anterior tibial artery to both the posterior tibial cortex and the posterior capsule increase the risk of vessel (anterior tibial artery) injury intraoperatively. The risk further increases in a revision of total knee replacement. This is secondary to limited flexibility of the vessel from scar formation which requires a more distal incision.1,4
CONCLUSION
Vascular injuries in knee surgeries are rare and often overlooked. Despite their low occurrence rate, outcomes of these injuries have grave consequences not only regarding medical but also legal matters. Variations in the popliteal artery are not uncommon and could potentially contribute to risks of vessel injury. Of these variations, the high originating anterior tibial artery poses a special risk. However, due to the low occurrence rate of these injuries, screening the general population may not be cost-effective. Since many patients already have obtained necessary imaging (preferably MRI), a careful review of these images along with preoperative planning and special care during surgery is recommended to identify popliteal artery variants and avoid iatrogenic vascular injury.
This paper will be judged for the Resident Writer’s Award.
- Abdel Karim MM, Anbar A, Keenan J. Position of the popliteal artery in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132(6):861-865. doi:10.1007/s00402-012-1479-6.
- Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery, magnetic resonance appearance, prevalence, and surgical implication. Am J Sports Medicine. 2008;36:720-727.
- Kropman RHJ, Kiela G, Moll FL, Vries JPM. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011;45:536-540.
- Metzdorf A, Jakob RP, Petropoulos P, Middleton R. Arterial injury during revision total knee replacement. A case report. Knee Surg Sports Traumatol Arthrosc. 1999;7:246-248.
- Shetty AA, Tindall AJ, Qureshi F, Divekar M, Fernando KWK. The Effect of knee flexion on the popliteal artery and its surgical significance. J Bone Joint Surg Br. 2003;85:218-222.
- Smith PN, Gelinas J, Kennedy K, Thain L, Rorabeck CH, Bourne B. Popliteal vessels in knee surgery; a magnetic resonance imaging study. Clin Orthop Rel Res. 1999;367:158-164
- Tindall AJ, Shetty AA, James KD, Middleton A, Fernando KWK. Prevalence and surgical significance of a high-origin anterior tibial artery. J Orthop Surg. 2006;14:13-16.
- Zaidi SHA, Cobb AG, Bentley G. Danger to the popliteal artery in high tibial osteotomy. J Bone Joint Surg Br. 1995;77:384-386.
- Keser S, Savranlar A, Bayar A, Ulukent SC, Ozer T, Tuncay I. Anatomic localization of the popliteal artery at the level of the knee joint: a magnetic resonance imaging study. Arthroscopy. 2006;22:656-659.
- Makino A, Costa-Paz M, Aponte-Tinao L, Ayerza MA, Muscolo L. Popliteal artery laceration during arthroscopic posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1396.
- Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants, a unified angiographic classification. Ann Surg. 1989;210:776-781.
- Day CP, Orme R. Popliteal artery branching patterns-an angiographic study. Clin Radiol. 2006;61:696-699.
- Kil SW, Jung GS. Anatomical variations of the popliteal artery and its tibial branches: Analysis in 1242 extremities. Cardiovasc Intervent Radiol. 2009;32:233-240.
- Senior HD. The development of the arteries of the human lower extremity. Am J Anat. 1919;25:55-94.
- Abdel Karim MM, Anbar A, Keenan J. Position of the popliteal artery in revision total knee arthroplasty. Arch Orthop Trauma Surg. 2012;132(6):861-865. doi:10.1007/s00402-012-1479-6.
- Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery, magnetic resonance appearance, prevalence, and surgical implication. Am J Sports Medicine. 2008;36:720-727.
- Kropman RHJ, Kiela G, Moll FL, Vries JPM. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011;45:536-540.
- Metzdorf A, Jakob RP, Petropoulos P, Middleton R. Arterial injury during revision total knee replacement. A case report. Knee Surg Sports Traumatol Arthrosc. 1999;7:246-248.
- Shetty AA, Tindall AJ, Qureshi F, Divekar M, Fernando KWK. The Effect of knee flexion on the popliteal artery and its surgical significance. J Bone Joint Surg Br. 2003;85:218-222.
- Smith PN, Gelinas J, Kennedy K, Thain L, Rorabeck CH, Bourne B. Popliteal vessels in knee surgery; a magnetic resonance imaging study. Clin Orthop Rel Res. 1999;367:158-164
- Tindall AJ, Shetty AA, James KD, Middleton A, Fernando KWK. Prevalence and surgical significance of a high-origin anterior tibial artery. J Orthop Surg. 2006;14:13-16.
- Zaidi SHA, Cobb AG, Bentley G. Danger to the popliteal artery in high tibial osteotomy. J Bone Joint Surg Br. 1995;77:384-386.
- Keser S, Savranlar A, Bayar A, Ulukent SC, Ozer T, Tuncay I. Anatomic localization of the popliteal artery at the level of the knee joint: a magnetic resonance imaging study. Arthroscopy. 2006;22:656-659.
- Makino A, Costa-Paz M, Aponte-Tinao L, Ayerza MA, Muscolo L. Popliteal artery laceration during arthroscopic posterior cruciate ligament reconstruction. Arthroscopy. 2005;21(11):1396.
- Kim D, Orron DE, Skillman JJ. Surgical significance of popliteal arterial variants, a unified angiographic classification. Ann Surg. 1989;210:776-781.
- Day CP, Orme R. Popliteal artery branching patterns-an angiographic study. Clin Radiol. 2006;61:696-699.
- Kil SW, Jung GS. Anatomical variations of the popliteal artery and its tibial branches: Analysis in 1242 extremities. Cardiovasc Intervent Radiol. 2009;32:233-240.
- Senior HD. The development of the arteries of the human lower extremity. Am J Anat. 1919;25:55-94.
TAKE-HOME POINTS
- Surgeon must understand and be aware of aberrant vascular anatomy around the knee.
- Careful evaluation of advance imaging for aberrant vascular anatomy is required in surgeries around the knee.
- When aberrant vascular anatomy is recognized, appropriate preoperative planning is required.
Snapping Biceps Femoris Tendon
ABSTRACT
A 23-year-old male active duty soldier presented with a biceps femoris tendon snapping over the fibular head with flexion of the knee beyond 90°. Surgical release of anomalous anterolateral tibial and lateral fibular insertions provided relief of snapping with no other repair or reconstruction required. The soldier quickly returned to full running and active duty.
Snapping biceps femoris tendon is a rare but potential cause of pain and dysfunction in the lateral knee. The possible anatomical variations and the cause of snapping must be considered when determining the operative approaches to this condition.
Continue to: Snapping in the knee...
Snapping in the knee is not as common as in other joints, such as the hip or ankle. The snapping sensation can occur from several pathologies, including the following: lateral meniscal tears, iliotibial band syndrome, proximal tibiofibular instability, snapping popliteus, peroneal nerve compression/neuritis, lateral discoid meniscus, rheumatoid nodules, plicae, congenital snapping knee, exostoses, or previous trauma.1,2 A detailed history must be provided, and physical examination and appropriate imaging must be performed to narrow down the differential diagnosis and prescribe the appropriate course of treatment for snapping.
Snapping biceps femoris syndrome is a rare cause of knee snapping. This condition has been described in various case reports.2-13 The reasons for a snapping biceps femoris can vary, and the treating provider must be ready to accommodate and treat these causes. The symptoms typically include an audible, and usually visual, lateral snapping distal to the knee joint and over the fibular head. Imaging may reveal bony abnormalities such as fibular exostoses. Magnetic resonance imaging (MRI) can aid in determining any anomalous or abnormal insertions of the biceps femoris tendon. The snapping can be debilitating, particularly in athletes or patients with high-demand occupations, and surgical intervention is often warranted.
We present a case of an active-duty military service member with symptomatic unilateral snapping biceps femoris and review the literature for treatment of this condition. Surgical release allowed the patient a quick and unrestricted return to full mission capabilities.
The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
A 23-year-old active-duty soldier presented to the orthopedic clinic with several months of noticeable snapping and pain over the lateral knee with attempted running and deep squatting activities, resulting in difficulty to perform his army duties. The patient reported no history of antecedent trauma. No locking of the knee or paresthesia distally into the leg or foot was observed.
The physical examination revealed a palpable and observable snapping of the long head of the biceps tendon over the fibular head with squatting beyond 90° in the left knee. The patient presented with full strength and no instability or joint line pain throughout the knee. Application of a posterior-to-anterior directed force over the biceps femoris proximal to the insertion allowed the patient to perform a deep squat without snapping. The radiographs demonstrated no abnormal fibular morphology (Figures 1A, 1B). Axial MRI images demonstrated an anomalous slip of the tendon inserting on the anterolateral aspect of the proximal tibia in addition to the normal insertion on the posterolateral and lateral edge of the fibular head (Figure 2) as described by Terry and LaPrade.14
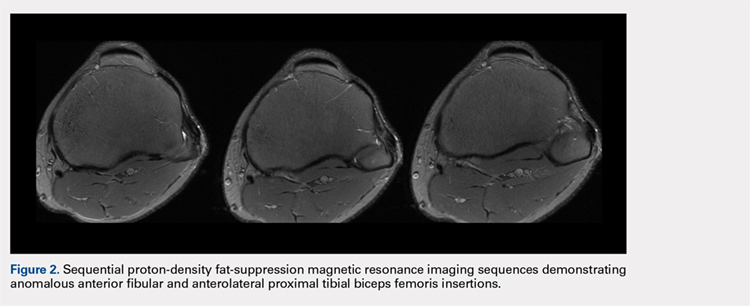
Continue to: A conservative treatment with physical therapy...
A conservative treatment with physical therapy, activity modification, and a Cho-Pat knee strap (to provide a posterior-to-anterior buttress and to prevent snapping) was attempted for 4 weeks. However, the patient could not tolerate the strap, and the activity restraints prevented him from performing his job as an active-duty soldier. Given the failure of conservative treatment, operative intervention was elected.
Upon exploration of the biceps femoris insertion, the accessory anterolateral tibial insertion was readily identified (Figure 3). Notably, the expected normal lateral edge insertion was thickened and extended beyond the lateral edge, distal, and anterior on to the fibular head (Figure 4). The anterolateral tibial band was released first. However, the snapping remained evident. The thickened anterior fibular accessory band was then released back to its normal, lateral edge, and at this point, no further snapping was observed with deep flexion of the knee. Inspection of the remaining posterolateral and lateral edge insertion demonstrated a healthy, 1-cm thick tendinous insertion. The accessory slips were completely excised, and the incision was closed without any additional repair or re-insertion (Figure 5). The patient presented no complications postoperatively. He was allowed to bear weight as tolerated and was limited to stretching and gravity resistance training for 4 weeks. At 1 month, the patient was released to progress back to full activity. By 8 weeks postoperative, he remained free of snapping and resumed his regular running routine and military duties without restriction or pain.
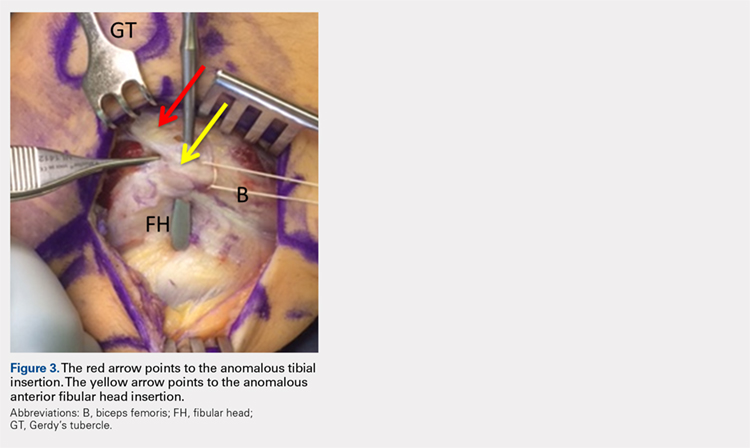
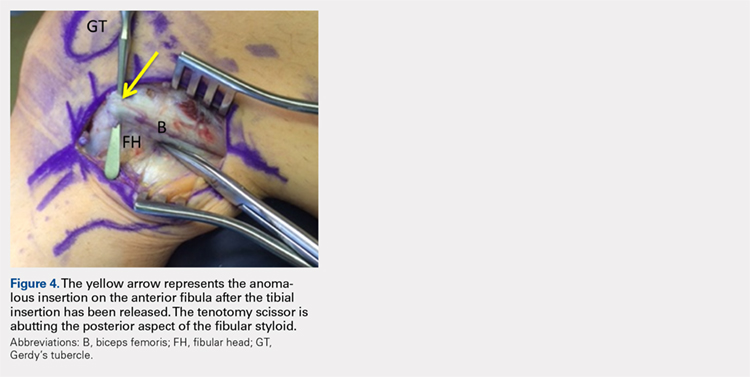

DISCUSSION
Release of the anomalous bands with no further repair or re-insertion of the biceps femoris allowed this active-duty soldier to resume full running and duty-related activities in <2 months. In this particular patient, given his anatomy, the treatment was successful. The literature indicates that optimal results and surgical approach depend upon the pathological anatomy encountered.
Date and colleagues4 described a similar anatomical anomaly as with our patient, whom after the release of tibial insertion, snapping was still observed, thus requiring the release of anterior fibular insertion. They noted the necessity of suturing the accessory limbs onto the periosteum of the fibular head to achieve a stable biceps femoris.
In other cases, abnormal bony anatomy of the fibula has been shown to cause snapping. Vavalle and Capozzi5 described a case of snapping biceps in a marathon runner, who needed partial resection of the fibular head to eliminate snapping. The runner made a full return to the sport. Fung and colleagues2 described a similar approach to a 17-year-old cyclist; however, this patient presented exostoses of the bilateral fibular heads. The exostoses were bilaterally excised, and the snapping ceased. Kristensen and colleagues13 described a patient with an anomalous tibial insertion. Rather than releasing the tibial insertion, a partial resection of the fibular head allowed for cessation of snapping.
Other authors advocate the detachment and anatomic re-insertion of the biceps femoris into the fibular head. Bernhardson and LaPrade6 reported a series of 3 patients requiring this approach with excellent results. Bansal and colleagues8 were the first to describe a soccer player with an isolated injury to the knee as a traumatic cause for a snapping biceps femoris. After failure of conservative treatment attempts, exploration and re-insertion through a bone tunnel allowed for return to the sport. Hernandez and colleagues11 and Lokiec and colleagues12 both described the reproduction of the normal biceps femoris anatomy through re-insertion procedures after identifying patients with abnormal anatomical insertions as causes for snapping.
CONCLUSION
We presented a case of an active military service member with a unilateral snapping biceps femoris tendon due to an anomalous distal insertion on both the proximal tibia and anterior fibular head. The release of abnormal insertions and maintenance of his normal anatomical insertion allowed for a quick and effective return to running and duty at full capacity. Although other surgical approaches have been described to include partial fibular head resection or anatomical re-insertion, we believe that the approach to this rare condition should be anatomy-based as the causes of snapping can significantly vary. We believe that if the normal posterolateral and lateral edge insertions of the biceps femoris are intact, removal of the abnormal anatomy without any repair or reconstruction can safely lead to successful surgical outcomes.
- Barker JU, Strauss EJ, Lodha S, Bach BR Jr. Extra-articular mimickers of lateral meniscal tears. Sports Health. 2011;3(1):82-88.
- Fung DA, Frey S, Markbreiter L. Bilateral symptomatic snapping biceps femoris tendon due to fibular exostosis. J Knee Surg. 2008;21(1):55-57.
- Mirchandani M, Gandhi P, Cai P. Poster 175 bilateral symptomatic snapping knee from biceps femoris tendon subluxation–an atypical cause of bilateral knee pain: a case report. PM R. 2016;8(9S):S218-S219.
- Date H, Hayakawa K, Yamada H. Snapping knee due to the biceps femoris tendon treated with repositioning of the anomalous tibial insertion. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1581-1583.
- Vavalle G, Capozzi M. Symptomatic snapping knee from biceps femoris tendon subluxation: an unusual case of lateral pain in a marathon runner. J Orthop Traumatol. 2010;11(4):263-266.
- Bernhardson AS, LaPrade RF. Snapping biceps femoris tendon treated with an anatomic repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1110-1112.
- Guillin R, Mendoza-Ruiz JJ, Moser T, Ropars M, Duvauferrier R, Cardinal E. Snapping biceps femoris tendon: a dynamic real-time sonographic evaluation. J Clin Ultrasound. 2010;38(8):435-437.
- Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005;12(6):458-460.
- Bagchi K, Grelsamer RP. Partial fibular head resection for bilateral snapping biceps femoris tendon. Orthopedics. 2003;26(11):1147-1149.
- Kissenberth MJ, Wilckens JH. The snapping biceps femoris tendon. Am J Knee Surg. 2000;13(1):25-28.
- Hernandez JA, Rius M. Noonan KJ. Snapping knee from anomalous biceps femoris tendon insertion: a case report. Iowa Orthop J. 1996;16:161-163.
- Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop Relat Res. 1992;(283):205-206.
- Kristensen G, Nielsen K, Blyme PJ. Snapping knee from biceps femoris tendon. A case report. Acta Orthop Scand. 1989;60(5):621.
- Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotator instability. Am J Sports Med. 1996;24:2-8.
ABSTRACT
A 23-year-old male active duty soldier presented with a biceps femoris tendon snapping over the fibular head with flexion of the knee beyond 90°. Surgical release of anomalous anterolateral tibial and lateral fibular insertions provided relief of snapping with no other repair or reconstruction required. The soldier quickly returned to full running and active duty.
Snapping biceps femoris tendon is a rare but potential cause of pain and dysfunction in the lateral knee. The possible anatomical variations and the cause of snapping must be considered when determining the operative approaches to this condition.
Continue to: Snapping in the knee...
Snapping in the knee is not as common as in other joints, such as the hip or ankle. The snapping sensation can occur from several pathologies, including the following: lateral meniscal tears, iliotibial band syndrome, proximal tibiofibular instability, snapping popliteus, peroneal nerve compression/neuritis, lateral discoid meniscus, rheumatoid nodules, plicae, congenital snapping knee, exostoses, or previous trauma.1,2 A detailed history must be provided, and physical examination and appropriate imaging must be performed to narrow down the differential diagnosis and prescribe the appropriate course of treatment for snapping.
Snapping biceps femoris syndrome is a rare cause of knee snapping. This condition has been described in various case reports.2-13 The reasons for a snapping biceps femoris can vary, and the treating provider must be ready to accommodate and treat these causes. The symptoms typically include an audible, and usually visual, lateral snapping distal to the knee joint and over the fibular head. Imaging may reveal bony abnormalities such as fibular exostoses. Magnetic resonance imaging (MRI) can aid in determining any anomalous or abnormal insertions of the biceps femoris tendon. The snapping can be debilitating, particularly in athletes or patients with high-demand occupations, and surgical intervention is often warranted.
We present a case of an active-duty military service member with symptomatic unilateral snapping biceps femoris and review the literature for treatment of this condition. Surgical release allowed the patient a quick and unrestricted return to full mission capabilities.
The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
A 23-year-old active-duty soldier presented to the orthopedic clinic with several months of noticeable snapping and pain over the lateral knee with attempted running and deep squatting activities, resulting in difficulty to perform his army duties. The patient reported no history of antecedent trauma. No locking of the knee or paresthesia distally into the leg or foot was observed.
The physical examination revealed a palpable and observable snapping of the long head of the biceps tendon over the fibular head with squatting beyond 90° in the left knee. The patient presented with full strength and no instability or joint line pain throughout the knee. Application of a posterior-to-anterior directed force over the biceps femoris proximal to the insertion allowed the patient to perform a deep squat without snapping. The radiographs demonstrated no abnormal fibular morphology (Figures 1A, 1B). Axial MRI images demonstrated an anomalous slip of the tendon inserting on the anterolateral aspect of the proximal tibia in addition to the normal insertion on the posterolateral and lateral edge of the fibular head (Figure 2) as described by Terry and LaPrade.14


Continue to: A conservative treatment with physical therapy...
A conservative treatment with physical therapy, activity modification, and a Cho-Pat knee strap (to provide a posterior-to-anterior buttress and to prevent snapping) was attempted for 4 weeks. However, the patient could not tolerate the strap, and the activity restraints prevented him from performing his job as an active-duty soldier. Given the failure of conservative treatment, operative intervention was elected.
Upon exploration of the biceps femoris insertion, the accessory anterolateral tibial insertion was readily identified (Figure 3). Notably, the expected normal lateral edge insertion was thickened and extended beyond the lateral edge, distal, and anterior on to the fibular head (Figure 4). The anterolateral tibial band was released first. However, the snapping remained evident. The thickened anterior fibular accessory band was then released back to its normal, lateral edge, and at this point, no further snapping was observed with deep flexion of the knee. Inspection of the remaining posterolateral and lateral edge insertion demonstrated a healthy, 1-cm thick tendinous insertion. The accessory slips were completely excised, and the incision was closed without any additional repair or re-insertion (Figure 5). The patient presented no complications postoperatively. He was allowed to bear weight as tolerated and was limited to stretching and gravity resistance training for 4 weeks. At 1 month, the patient was released to progress back to full activity. By 8 weeks postoperative, he remained free of snapping and resumed his regular running routine and military duties without restriction or pain.



DISCUSSION
Release of the anomalous bands with no further repair or re-insertion of the biceps femoris allowed this active-duty soldier to resume full running and duty-related activities in <2 months. In this particular patient, given his anatomy, the treatment was successful. The literature indicates that optimal results and surgical approach depend upon the pathological anatomy encountered.
Date and colleagues4 described a similar anatomical anomaly as with our patient, whom after the release of tibial insertion, snapping was still observed, thus requiring the release of anterior fibular insertion. They noted the necessity of suturing the accessory limbs onto the periosteum of the fibular head to achieve a stable biceps femoris.
In other cases, abnormal bony anatomy of the fibula has been shown to cause snapping. Vavalle and Capozzi5 described a case of snapping biceps in a marathon runner, who needed partial resection of the fibular head to eliminate snapping. The runner made a full return to the sport. Fung and colleagues2 described a similar approach to a 17-year-old cyclist; however, this patient presented exostoses of the bilateral fibular heads. The exostoses were bilaterally excised, and the snapping ceased. Kristensen and colleagues13 described a patient with an anomalous tibial insertion. Rather than releasing the tibial insertion, a partial resection of the fibular head allowed for cessation of snapping.
Other authors advocate the detachment and anatomic re-insertion of the biceps femoris into the fibular head. Bernhardson and LaPrade6 reported a series of 3 patients requiring this approach with excellent results. Bansal and colleagues8 were the first to describe a soccer player with an isolated injury to the knee as a traumatic cause for a snapping biceps femoris. After failure of conservative treatment attempts, exploration and re-insertion through a bone tunnel allowed for return to the sport. Hernandez and colleagues11 and Lokiec and colleagues12 both described the reproduction of the normal biceps femoris anatomy through re-insertion procedures after identifying patients with abnormal anatomical insertions as causes for snapping.
CONCLUSION
We presented a case of an active military service member with a unilateral snapping biceps femoris tendon due to an anomalous distal insertion on both the proximal tibia and anterior fibular head. The release of abnormal insertions and maintenance of his normal anatomical insertion allowed for a quick and effective return to running and duty at full capacity. Although other surgical approaches have been described to include partial fibular head resection or anatomical re-insertion, we believe that the approach to this rare condition should be anatomy-based as the causes of snapping can significantly vary. We believe that if the normal posterolateral and lateral edge insertions of the biceps femoris are intact, removal of the abnormal anatomy without any repair or reconstruction can safely lead to successful surgical outcomes.
ABSTRACT
A 23-year-old male active duty soldier presented with a biceps femoris tendon snapping over the fibular head with flexion of the knee beyond 90°. Surgical release of anomalous anterolateral tibial and lateral fibular insertions provided relief of snapping with no other repair or reconstruction required. The soldier quickly returned to full running and active duty.
Snapping biceps femoris tendon is a rare but potential cause of pain and dysfunction in the lateral knee. The possible anatomical variations and the cause of snapping must be considered when determining the operative approaches to this condition.
Continue to: Snapping in the knee...
Snapping in the knee is not as common as in other joints, such as the hip or ankle. The snapping sensation can occur from several pathologies, including the following: lateral meniscal tears, iliotibial band syndrome, proximal tibiofibular instability, snapping popliteus, peroneal nerve compression/neuritis, lateral discoid meniscus, rheumatoid nodules, plicae, congenital snapping knee, exostoses, or previous trauma.1,2 A detailed history must be provided, and physical examination and appropriate imaging must be performed to narrow down the differential diagnosis and prescribe the appropriate course of treatment for snapping.
Snapping biceps femoris syndrome is a rare cause of knee snapping. This condition has been described in various case reports.2-13 The reasons for a snapping biceps femoris can vary, and the treating provider must be ready to accommodate and treat these causes. The symptoms typically include an audible, and usually visual, lateral snapping distal to the knee joint and over the fibular head. Imaging may reveal bony abnormalities such as fibular exostoses. Magnetic resonance imaging (MRI) can aid in determining any anomalous or abnormal insertions of the biceps femoris tendon. The snapping can be debilitating, particularly in athletes or patients with high-demand occupations, and surgical intervention is often warranted.
We present a case of an active-duty military service member with symptomatic unilateral snapping biceps femoris and review the literature for treatment of this condition. Surgical release allowed the patient a quick and unrestricted return to full mission capabilities.
The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
A 23-year-old active-duty soldier presented to the orthopedic clinic with several months of noticeable snapping and pain over the lateral knee with attempted running and deep squatting activities, resulting in difficulty to perform his army duties. The patient reported no history of antecedent trauma. No locking of the knee or paresthesia distally into the leg or foot was observed.
The physical examination revealed a palpable and observable snapping of the long head of the biceps tendon over the fibular head with squatting beyond 90° in the left knee. The patient presented with full strength and no instability or joint line pain throughout the knee. Application of a posterior-to-anterior directed force over the biceps femoris proximal to the insertion allowed the patient to perform a deep squat without snapping. The radiographs demonstrated no abnormal fibular morphology (Figures 1A, 1B). Axial MRI images demonstrated an anomalous slip of the tendon inserting on the anterolateral aspect of the proximal tibia in addition to the normal insertion on the posterolateral and lateral edge of the fibular head (Figure 2) as described by Terry and LaPrade.14


Continue to: A conservative treatment with physical therapy...
A conservative treatment with physical therapy, activity modification, and a Cho-Pat knee strap (to provide a posterior-to-anterior buttress and to prevent snapping) was attempted for 4 weeks. However, the patient could not tolerate the strap, and the activity restraints prevented him from performing his job as an active-duty soldier. Given the failure of conservative treatment, operative intervention was elected.
Upon exploration of the biceps femoris insertion, the accessory anterolateral tibial insertion was readily identified (Figure 3). Notably, the expected normal lateral edge insertion was thickened and extended beyond the lateral edge, distal, and anterior on to the fibular head (Figure 4). The anterolateral tibial band was released first. However, the snapping remained evident. The thickened anterior fibular accessory band was then released back to its normal, lateral edge, and at this point, no further snapping was observed with deep flexion of the knee. Inspection of the remaining posterolateral and lateral edge insertion demonstrated a healthy, 1-cm thick tendinous insertion. The accessory slips were completely excised, and the incision was closed without any additional repair or re-insertion (Figure 5). The patient presented no complications postoperatively. He was allowed to bear weight as tolerated and was limited to stretching and gravity resistance training for 4 weeks. At 1 month, the patient was released to progress back to full activity. By 8 weeks postoperative, he remained free of snapping and resumed his regular running routine and military duties without restriction or pain.



DISCUSSION
Release of the anomalous bands with no further repair or re-insertion of the biceps femoris allowed this active-duty soldier to resume full running and duty-related activities in <2 months. In this particular patient, given his anatomy, the treatment was successful. The literature indicates that optimal results and surgical approach depend upon the pathological anatomy encountered.
Date and colleagues4 described a similar anatomical anomaly as with our patient, whom after the release of tibial insertion, snapping was still observed, thus requiring the release of anterior fibular insertion. They noted the necessity of suturing the accessory limbs onto the periosteum of the fibular head to achieve a stable biceps femoris.
In other cases, abnormal bony anatomy of the fibula has been shown to cause snapping. Vavalle and Capozzi5 described a case of snapping biceps in a marathon runner, who needed partial resection of the fibular head to eliminate snapping. The runner made a full return to the sport. Fung and colleagues2 described a similar approach to a 17-year-old cyclist; however, this patient presented exostoses of the bilateral fibular heads. The exostoses were bilaterally excised, and the snapping ceased. Kristensen and colleagues13 described a patient with an anomalous tibial insertion. Rather than releasing the tibial insertion, a partial resection of the fibular head allowed for cessation of snapping.
Other authors advocate the detachment and anatomic re-insertion of the biceps femoris into the fibular head. Bernhardson and LaPrade6 reported a series of 3 patients requiring this approach with excellent results. Bansal and colleagues8 were the first to describe a soccer player with an isolated injury to the knee as a traumatic cause for a snapping biceps femoris. After failure of conservative treatment attempts, exploration and re-insertion through a bone tunnel allowed for return to the sport. Hernandez and colleagues11 and Lokiec and colleagues12 both described the reproduction of the normal biceps femoris anatomy through re-insertion procedures after identifying patients with abnormal anatomical insertions as causes for snapping.
CONCLUSION
We presented a case of an active military service member with a unilateral snapping biceps femoris tendon due to an anomalous distal insertion on both the proximal tibia and anterior fibular head. The release of abnormal insertions and maintenance of his normal anatomical insertion allowed for a quick and effective return to running and duty at full capacity. Although other surgical approaches have been described to include partial fibular head resection or anatomical re-insertion, we believe that the approach to this rare condition should be anatomy-based as the causes of snapping can significantly vary. We believe that if the normal posterolateral and lateral edge insertions of the biceps femoris are intact, removal of the abnormal anatomy without any repair or reconstruction can safely lead to successful surgical outcomes.
- Barker JU, Strauss EJ, Lodha S, Bach BR Jr. Extra-articular mimickers of lateral meniscal tears. Sports Health. 2011;3(1):82-88.
- Fung DA, Frey S, Markbreiter L. Bilateral symptomatic snapping biceps femoris tendon due to fibular exostosis. J Knee Surg. 2008;21(1):55-57.
- Mirchandani M, Gandhi P, Cai P. Poster 175 bilateral symptomatic snapping knee from biceps femoris tendon subluxation–an atypical cause of bilateral knee pain: a case report. PM R. 2016;8(9S):S218-S219.
- Date H, Hayakawa K, Yamada H. Snapping knee due to the biceps femoris tendon treated with repositioning of the anomalous tibial insertion. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1581-1583.
- Vavalle G, Capozzi M. Symptomatic snapping knee from biceps femoris tendon subluxation: an unusual case of lateral pain in a marathon runner. J Orthop Traumatol. 2010;11(4):263-266.
- Bernhardson AS, LaPrade RF. Snapping biceps femoris tendon treated with an anatomic repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1110-1112.
- Guillin R, Mendoza-Ruiz JJ, Moser T, Ropars M, Duvauferrier R, Cardinal E. Snapping biceps femoris tendon: a dynamic real-time sonographic evaluation. J Clin Ultrasound. 2010;38(8):435-437.
- Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005;12(6):458-460.
- Bagchi K, Grelsamer RP. Partial fibular head resection for bilateral snapping biceps femoris tendon. Orthopedics. 2003;26(11):1147-1149.
- Kissenberth MJ, Wilckens JH. The snapping biceps femoris tendon. Am J Knee Surg. 2000;13(1):25-28.
- Hernandez JA, Rius M. Noonan KJ. Snapping knee from anomalous biceps femoris tendon insertion: a case report. Iowa Orthop J. 1996;16:161-163.
- Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop Relat Res. 1992;(283):205-206.
- Kristensen G, Nielsen K, Blyme PJ. Snapping knee from biceps femoris tendon. A case report. Acta Orthop Scand. 1989;60(5):621.
- Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotator instability. Am J Sports Med. 1996;24:2-8.
- Barker JU, Strauss EJ, Lodha S, Bach BR Jr. Extra-articular mimickers of lateral meniscal tears. Sports Health. 2011;3(1):82-88.
- Fung DA, Frey S, Markbreiter L. Bilateral symptomatic snapping biceps femoris tendon due to fibular exostosis. J Knee Surg. 2008;21(1):55-57.
- Mirchandani M, Gandhi P, Cai P. Poster 175 bilateral symptomatic snapping knee from biceps femoris tendon subluxation–an atypical cause of bilateral knee pain: a case report. PM R. 2016;8(9S):S218-S219.
- Date H, Hayakawa K, Yamada H. Snapping knee due to the biceps femoris tendon treated with repositioning of the anomalous tibial insertion. Knee Surg Sports Traumatol Arthrosc. 2012;20(8):1581-1583.
- Vavalle G, Capozzi M. Symptomatic snapping knee from biceps femoris tendon subluxation: an unusual case of lateral pain in a marathon runner. J Orthop Traumatol. 2010;11(4):263-266.
- Bernhardson AS, LaPrade RF. Snapping biceps femoris tendon treated with an anatomic repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(8):1110-1112.
- Guillin R, Mendoza-Ruiz JJ, Moser T, Ropars M, Duvauferrier R, Cardinal E. Snapping biceps femoris tendon: a dynamic real-time sonographic evaluation. J Clin Ultrasound. 2010;38(8):435-437.
- Bansal R, Taylor C, Pimpalnerkar AL. Snapping knee: an unusual biceps femoris tendon injury. Knee. 2005;12(6):458-460.
- Bagchi K, Grelsamer RP. Partial fibular head resection for bilateral snapping biceps femoris tendon. Orthopedics. 2003;26(11):1147-1149.
- Kissenberth MJ, Wilckens JH. The snapping biceps femoris tendon. Am J Knee Surg. 2000;13(1):25-28.
- Hernandez JA, Rius M. Noonan KJ. Snapping knee from anomalous biceps femoris tendon insertion: a case report. Iowa Orthop J. 1996;16:161-163.
- Lokiec F, Velkes S, Schindler A, Pritsch M. The snapping biceps femoris syndrome. Clin Orthop Relat Res. 1992;(283):205-206.
- Kristensen G, Nielsen K, Blyme PJ. Snapping knee from biceps femoris tendon. A case report. Acta Orthop Scand. 1989;60(5):621.
- Terry GC, LaPrade RF. The biceps femoris muscle complex at the knee. Its anatomy and injury patterns associated with acute anterolateral-anteromedial rotator instability. Am J Sports Med. 1996;24:2-8.
TAKE-HOME POINTS
- Snapping biceps femoris is a rare, but debilitating condition.
- Understanding the pathology from an anatomical perspective is key.
- For bone abnormalities, correct the bony pathology to relieve the snapping.
- For soft tissue abnormalities, both excisional and reconstructive approaches can be utilized.
- Preservation of normal anatomy, when possible, can help expedite recovery.
Inadvertent Perioperative Hypothermia During Orthopedic Surgery
ABSTRACT
Inadvertent perioperative hypothermia is a significant problem in patients undergoing either emergency or elective orthopedic surgery, and is associated with increased morbidity and mortality. Though in general the incidence of inadvertent perioperative hypothermia in postoperative recovery rooms has been decreasing over the last 2 decades, it still remains a significant risk in certain specialty practices, such as orthopedic surgery. This review article summarizes the currently available evidence on the incidence, risk factors, and complications of inadvertent perioperative hypothermia. Also, the effective preventive strategies in dealing with inadvertent perioperative hypothermia are reviewed and essential clinical guidelines to be followed are summarized.
Continue to: Inadvertent perioperative hypothermia...
Inadvertent perioperative hypothermia, defined as an involuntary drop in core body temperature to <35°C (95°F), is a condition associated with significant morbidity and mortality.1 This phenomenon has been reported in both emergency orthopedic admissions, such as fracture management, as well as in the elective setting such as arthroscopy, arthroplasty, and spine surgery.
In a study conducted in the United Kingdom including 781 elderly patients with a mean age of 80 years who presented with hip fractures, the 30-day mortality rate was 15.3% in patients who were admitted with a tympanic temperature of <36.5°C and only 5.1% in patients who maintained a tympanic temperature of 36.5°C to 37.5°C (odds ratio, 2.8; P > .0005).2 For an even better perspective, this analysis can be compared with the UK National Hip Fracture Database of 2013, which reported a 30-day mortality of 8.2% in patients who were admitted to the National Health Service with a diagnosis of hip fracture.3
Inadvertent perioperative hypothermia is also a common phenomenon during elective orthopedic hospital admissions. An Australian audit, which included 5050 postoperative patients, looked into the association between inadvertent perioperative hypothermia and mortality based on diagnostic criteria classifying mild hypothermia as a core temperature of <36°C and severe hypothermia as a core temperature of <35°C.4 The authors found that mild and severe hypothermia was experienced by 36% and 6% of patients, respectively. In-hospital mortality was 5.6% for normothermic patients, 8.9% for all hypothermic patients (P < .001), and 14.7% for severely hypothermic patients (P < .001). For a decrease of 1°C in core body temperature from <36°C to <35°C (but >34°C), there were higher odds of in-hospital mortality (odds ratio, 1.83; 95% confidence interval [CI], 1.20-2.60).
The physiologic response to hypothermia is to decrease heat loss by cutaneous and peripheral vasoconstriction and increase heat production by increasing the metabolic rate (eg, shivering and shifting to anaerobic metabolism). This response is blunted to a variable extent in perioperative patients for several reasons, including the effect of anesthetic drugs and old age.5
Maintenance of core body temperature >36°C is now a measured standard of perioperative care. A performance measure for perioperative temperature management was developed by the American Medical Association Physician Consortium for Performance Improvement (AMA-PCPI).6 To achieve this performance measure, mandatory documentation of use of active warming intraoperatively or a record of at least 1 body temperature ≥96.8°F (36°C) within 30 minutes immediately prior to and 15 minutes immediately after anesthesia end time is necessary. This performance measure is also endorsed by the Surgical Care Improvement Project (SCIP-Inf-10) and National Quality Forum (NQF).6
Continue to: Overall, in the last 2 decades...
Overall, in the last 2 decades, the incidence of inadvertent perioperative hypothermia has decreased, mainly due to aggressive intraoperative management.7 In spite of this, studies have shown that perioperative hypothermia remains a significant problem in patients undergoing orthopedic procedures. In a recent community hospital study conducted by the National Association for Healthcare Quality that included 4124 orthopedic patients undergoing elective surgery, it was shown that, in spite of 99% compliance to the AMA-PCPI recommendation, 7.7% of orthopedic patients were found to be hypothermic.6
Management of hypothermia has long been an integral component of “damage control surgery” and resuscitation during polytrauma, which aims to aggressively minimize hypovolemic shock and limit the development of the lethal triad of hypothermia, coagulopathy, and acidosis.8 However, critical references to prevention and management of inadvertent perioperative hypothermia are lacking in the orthopedic literature on elective surgical procedures. This review aims to bridge this knowledge gap.
Unless otherwise specified, inadvertent perioperative hypothermia in this article refers to the core body temperature. In contrast, peripheral/limb hypothermia refers primarily to the effect of tourniquet application to the involved limb and the effect after deflation of the tourniquet on core body temperature.
RISK FACTORS
There are several measurable risk factors that can contribute to inadvertent perioperative hypothermia, which can be subdivided into 3 groups: patient-related risk factors, anesthesia-related risk factors, and procedure-related risk factors (Table 1).5,9-11 It is important to note that in any given patient a combination of 2 or more risk factors predisposes them to developing inadvertent perioperative hypothermia. Conceptualizing the etiology of inadvertent perioperative hypothermia in this way helps to plan a multipronged strategy to prevent it from occurring in the first place. Some of the important risk factors for inadvertent perioperative hypothermia are discussed below.
Table 1. Risk Factors for Perioperative Hypothermia
Patient-Related Risk Factors | Anesthesia-Related Risk Factors | Procedure-Specific Risk Factors |
|
|
|
To identify patient-related risk factors, researchers from the University of Louisville conducted a study including 2138 operative patients who became hypothermic after admission, of whom 27% underwent orthopedic and spine procedures.9 The patient-related risk factors identified were a high severity of illness on admission (odds ratio, 2.81; 95% CI, 2.28-3.47), presence of a neurological disorder such as Alzheimer’s disease (odds ratio, 1.71; 95% CI,1.06-2.78), male sex (odds ratio, 1.65; 95% CI, 1.36-2.01), age >65 years (odds ratio, 1.61; 95% CI, 1.33-1.96), recent weight loss (odds ratio, 1.60; 95% CI, 1.04-2.48), anemia (odds ratio, 1.49; 95% CI, 1.12-1.98), and chronic renal failure (odds ratio, 1.43; 95% CI, 1.07-1.92). Interestingly, diabetes mellitus without end-stage organ failure was not found to be a significant risk factor (odds ratio, 0.58; 95% CI, 0.44-0.75). It is also important to note that some of these risk factors identified to contribute to perioperative hypothermia are dependent on each other and others are independent of each other. For example, chronic renal failure and anemia are dependent risk factors. In contrast, age >65 years and low body mass index as risk factors of perioperative hypothermia are independent of each other.
Continue to: The second subgroup of risk factors...
The second subgroup of risk factors for perioperative hypothermia is related to anesthesia. The effect of general and regional anesthesia on perioperative core temperature is significantly different, both in terms of intraoperative thermoregulation and postoperative recovery.12 Intraoperatively, the core body temperature during the first 2 hours of general anesthesia decreases at a rate of 1.3°C per hour due to loss of thermoregulatory cutaneous and peripheral vasoconstrictive responses resulting in heat loss exceeding metabolic heat production. However, the core temperature remains virtually constant during the subsequent 3 hours due to the return of the thermoregulatory response, which causes cutaneous and peripheral vasoconstriction and increased metabolic heat production. Postoperative recovery from the hypothermia induced by general anesthesia is significantly faster than from that induced by regional anesthesia.13
The effect of regional hypothermia on core body temperature is more complex because it must be considered in addition to the effect of an associated procedure-related variable (ie, tourniquet application). If a tourniquet is not used during a surgery with regional anesthesia, a linear decrease in core temperature follows until recovery, due to increased blood flow from the loss of sympathetic peripheral vasoconstrictive response with resultant core-to-peripheral heat redistribution to the exposed operating limb. If a tourniquet is used during surgery with regional anesthesia, there will be no significant effect of the exposed operating limb on core temperature, as there is no blood flow between them. However, once the tourniquet is deflated, the core body temperature will be affected significantly as a result of core-to-peripheral distribution of heat to the operated limb with the return of blood flow. This fall in core body temperature after tourniquet deflation can be prevented by active forced-air warming initiated from the beginning of surgery.10 The extent and rate of development of peripheral/limb hypothermia during surgery (and its subsequent effect on core body temperature) depends on several factors, including the operating room ambient temperature, duration of tourniquet application, and temperature of the irrigation fluid. Postoperative recovery from the hypothermia induced by regional anesthesia takes longer than from that induced by general anesthesia because of the prolonged period of loss of vasoconstrictive response.
The third subgroup of risk factors associated with perioperative hypothermia is procedure related. Several procedure-specific risk factors for inadvertent perioperative hypothermia during arthroscopic surgery are identified, including prolonged operating time, low blood pressure during the procedure, and low temperature of the irrigation fluid.11 It is logical to extrapolate the importance of these risk factors to other orthopedic procedures which also require prolonged operating times, are performed under hypotension, or expose the patient to irrigation fluid that is at a low temperature. Understanding the importance of each of these procedure-related risk factors is the most important from the perspective of the orthopedic surgeon when compared to the rest of the subgroups of risk factors for inadvertent perioperative hypothermia as he/she is directly responsible for them.
The ambient operating room temperature has traditionally been considered a risk factor for inadvertent hypothermia in perioperative patients, but evidence is available to the contrary. The recommended ambient room temperature as per the clinical guideline published by the American Society of PeriAnesthesia Nurses (ASPAN) is 20°C to 24°C (68°F-75°F).14 The ambient temperature can have a significant effect on peripheral/limb hypothermia when operating on a limb with a tourniquet inflated, as the limb has no blood supply to distribute heat from the core to the periphery. However, the direct effect of ambient room temperature on the patient’s core body temperature is unlikely to be clinically significant if standard active warming interventions are implemented.15
COMPLICATIONS
The increased incidence of mortality due to inadvertent hypothermia in the perioperative period has already been discussed. Several other complications of inadvertent perioperative hypothermia include increased incidence of coagulopathy, acidosis, stroke, sepsis, pneumonia, myocardial infarction, surgical site infections, altered drug metabolism, and longer hospital stays.8,9,16-18 Hypothermia, coagulopathy, and acidosis have long been recognized as a lethal triad more commonly seen in polytrauma patients than in elective orthopedic surgery, as this occurs at extremes of temperature, usually <32°C. When compared with patients who did not develop perioperative hypothermia, patients who developed hypothermia during elective operations were shown to experience an overall doubled complication rate (13.9% vs 26.3%; P < .001) of which the incidence of stroke (1.0% vs 6.5%; P < .001), pneumonia (1.3% vs 5.1%; P < .001), and sepsis (2.6% vs 7.5%; P < .001) were much more likely than myocardial infarction (1.1% vs 3.3%; P = .01) and wound infection (3.3% vs 5.0%; P = .14).9
Continue to: Prevention...
PREVENTION
Prevention of perioperative hypothermia is a core measure to improve the outcome after ambulatory and fast-track orthopedic surgery and rehabilitation. Preventive strategies for perioperative hypothermia can be grouped into passive heat retention methods and active external warming methods (Table 2). Passive methods aim to maintain body temperature by decreasing the heat loss by radiation (eg, reflective blanket), conduction (eg, layered cotton blankets and padding the operating table), or convection (eg, heat and humidity exchanger in the breathing circuits) to the surrounding environment. Active patient heating methods aim to bring in heat from the source to the patient’s body using conduction (eg, Hot Dog® [Eden Augustine Temperature Management]) or convection (eg, Bair Hugger® [Arizant Healthcare]) techniques.
Table 2. Methods to Prevent Inadvertent Perioperative Hypothermia
Passive Heat-Retention Methods | Active External Warming Methods |
| Conduction techniques:
Convection techniques:
|
Active patient warming is superior to passive heat retention methods. A recent Cochrane study assessed the effects of standard care (ie, use of layered clothing and warm blankets, etc.) and addition of extra thermal insulation by reflective blankets or active forced air warming to standard care on the perioperative core body temperature.19 They concluded that there is no clear benefit of addition of extra thermal insulation by reflective blankets compared with standard care alone. Also, forced-air warming in addition to standard care appeared to maintain core temperature better than standard care alone, by between 0.5°C and 1°C, but the clinical importance of this difference could not be inferred, as none of the included studies in this meta-analysis documented major cardiovascular outcomes.
Several clinical guidelines have been developed by not-for-profit, government, and professional organizations aimed at prevention of perioperative hypothermia as primary or secondary outcome. A clinical guideline was published by ASPAN in 2001 for assessment, prevention, and intervention in unplanned perioperative hypothermia.14 Cost and time effectiveness of the ASPAN Hypothermia Guideline was published in 2008.20 The assessment guideline includes identification of risk factors, repeated pre-/intra-/postoperative temperature measurement, and repeated clinical evaluation of the patient’s status. The preventive guideline is to maintain an ambient temperature of 20°C to 24°C (68°F-75°F) and use appropriate passive patient warming methods pre-, intra-, and postoperatively. Intervention in the form of active patient heating is advised only if the patient develops hypothermia in spite of the above-mentioned standard preventive measures. But many orthopedic ambulatory surgery centers currently use active patient warming as both a preventive and an intervention strategy.
Active patient warming by conduction devices occurs by direct physical contact with the device, which is set at a higher temperature, whereas heat transfer from the convection device to the patient occurs by a physical medium such as forced air or circulating water that moves in between the device and the patient. Any recommendation for use of a specific technique of active patient warming (ie, by the use of a conduction device or a convection device) should only be given after comparing evidence on 3 critical aspects: efficacy, safety, and cost effectiveness.
The heating efficacy and core rewarming rates of conduction and convection devices have been compared in the literature. Full-body forced-air heating with the Bair Hugger® and full-body resistive polymer heating with the Hot Dog® in healthy volunteers were found to be similar.21 Also, in a randomized study conducted on 80 orthopedic patients undergoing surgery, resistive polymer warming performed as efficiently as forced-air warming in patients undergoing orthopedic surgery.22
Continue to: Secondly, the safety of convection...
Secondly, the safety of convection devices such as the Bair Hugger® has been under intense scrutiny based on the evidence that it disrupts the laminar airflow in the operating theater.23-26 This disruption in laminar air flow has been shown to cause emission of significant levels of airborne contaminants of size >0.3 μm (germ size).27 Isolates of Staphylococcus aureus, coagulase-negative Staphylococcus species, and methicillin-resistant Staphylococcus aureus were detected in 13.5%, 3.9%, and 1.9% of forced-air blowers, respectively.28 However, the clinical effect on the rate of deep joint infection due to the disruption of laminar air flow has been examined in only 1 study. McGovern and colleagues29 reported a significant increase in deep joint infection during a period when forced air warming was used compared to a period when conductive fabric warming was used (odds ratio, 3.8; P = .024) and recommended air-free warming by a conduction device over forced-air warming for orthopedic procedures. Unfortunately, the prophylactic antibiotic regimen was not kept constant during their study period. During an overlapping time frame during which they shifted from the use of a convection device (Bair Hugger®) to a conduction device (Hot Dog®), they also changed their antibiotic regimen from gentamicin 4.5 mg/kg intravenous (IV) to gentamicin 3 mg/kg IV plus teicoplanin 400 mg IV. This change in antibiotic regimen is a major confounding factor that calls into question the validity of the conclusions drawn by the authors.
Finally, the cost effectiveness of conduction and convection devices has never been studied. Hence, based on the current evidence, it is not possible to recommend a particular type of active patient-warming device.
CONCLUSION
Orthopedic surgeons should be aware that inadvertent perioperative hypothermia is a common phenomenon in perioperative patients. It must be recognized that the maintenance of perioperative normothermia during all major orthopedic surgical procedures is desirable, as inadvertent perioperative hypothermia is shown to be associated with increased mortality and systemic morbidity, such as stroke and sepsis. Compliance with the current clinical guidelines for assessment, prevention, and treatment of inadvertent perioperative hypothermia will minimize, if not eliminate, such risk. We recommend the following essential clinical guidelines to prevent inadvertent perioperative hypothermia (Table 3). Identification of patient-, anesthesia-, and procedure-related risk factors is an integral component of assessment of the risk of inadvertent perioperative hypothermia. In order to achieve full compliance with implementation of active patient warming during surgery, it is prudent to make active warming information a part of the surgical timeout checklist. Irrespective of the presence of risk factors, passive heat retention methods should be part of perioperative management of patients undergoing elective orthopedic surgery to prevent inadvertent perioperative hypothermia. In addition, there should be a minimum threshold to utilize active patient-warming techniques, especially in patients with inherent risk factors and surgeries that take >30 minutes of operating time, either under regional or general anesthesia. As there are concerns about safety issues with the use of convection devices, we believe a multicenter randomized controlled trial is warranted.
Table 3. Recommended Essential Clinical Guidelines for Prevention of Inadvertent Perioperative Hypothermia
|
- Brown DJ, Brugger H, Boyd J, Paal P. Accidental Hypothermia. N Engl J Med. 2012;367(20):1930-1938. doi:10.1056/NEJMra1114208.
- Uzoigwe CE, Khan A, Smith RP, et al. Hypothermia and low body temperature are common and associated with high mortality in hip fracture patients. Hip Int. 2014; 24(3):237-242. doi:10.5301/hipint.5000124.
- Johansen A, Wakeman R, Boulton C, Plant F, Roberts J, Williams A. National Hip Fracture Database: National Report 2013. London, UK: National Hip Fracture Database, Royal College of Physicians; 2013.
- Karalapillai D, Story DA, Calzavacca, Licari E, Liu YL, Hart GK. Inadvertent hypothermia and mortality in postoperative intensive care patients: retrospective audit of 5050 patients. Anaesthesia. 2009;64(9):968-972. doi:10.1111/j.1365-2044.2009.05989.x.
- Horosz B, Malec-Milewska M. Inadvertent intraoperative hypothermia. Anaesthesiol Intensive Ther. 2013;45(1):38-43. doi:10.5603/AIT.2013.0009.
- Steelman VM, Perkhounkova YS, Lemke JH. The gap between compliance with the quality performance measure "perioperative temperature management” and normothermia. J Healthc Qual. 2014;37(6):333-341. doi:10.1111/jhq.12063.
- National Institute for Health and Clinical Excellence. Inadvertent Perioperative Hypothermia: The Management of Inadvertent Perioperative Hypothermia in Adults. London, UK: National Institute for Health and Clinical Excellence; 2008.
- Carlino W. Damage control resuscitation from major haemorrhage in polytrauma. Eur J Orthop Surg Traumatol. 2014;24(2):137-141. doi:10.1007/s00590-013-1172-7.
- Billeter AT, Hohmann SF, Druen D, Cannon R, Polk HC Jr. Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. Surgery. 2014;156(5):1245-1252. doi:10.1016/j.surg.2014.04.024.
- Kim YS, Jeon YS, Lee JA, et al. Intra-operative warming with a forced-air warmer in preventing hypothermia after tourniquet deflation in elderly patients. J Int Med Res. 2009;37(5):1457-1464. doi:10.1177/147323000903700521.
- Parodi D, Tobar C, Valderrama J, et al. Hip arthroscopy and hypothermia. Arthroscopy. 2012;28(7):924-928. doi:10.1016/j.arthro.2011.12.012.
- Kurz A, Sessler DI, Christensen R, Dechert M. Heat balance and distribution during the core-temperature plateau in anesthetized humans. Anesthesiology. 1995;83(3):491-499.
- Vaughan MS, Vaughan RW, Cork RC. Postoperative hypothermia in adults: relationship of age, anesthesia, and shivering to rewarming. Anesth Analg. 1981;60(10):746-751.
- American Society of PeriAnesthesia Nurses. Clinical guideline for the prevention of unplanned perioperative hypothermia. J Perianesth Nurs. 2001;16(5):305-314.
- Inaba K, Berg R, Barmparas G, et al. Prospective evaluation of ambient operating room temperature on the core temperature of injured patients undergoing emergent surgery. J Trauma Acute Care Surg. 2012;73(6):1478-1483. doi:10.1097/TA.0b013e3182781db3.
- Barie PS. Surgical site infections: epidemiology and prevention. Surg Infect (Larchmt). 2002;3 Suppl 1:S9-S21. doi:10.1089/sur.2002.3.s1-9.
- Jeran L. Patient temperature: an introduction to the clinical guideline for the prevention of unplanned perioperative hypothermia. J Perianesth Nurs. 2001;16(5):303-304.
- Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209-1215.
- Alderson P, Campbell G, Smith AF, Warttig S, Nicholson A, Lewis SR. Thermal insulation for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev. 2014;6:CD009908. doi:10.1002/14651858.CD009908.pub2.
- Berry D, Wick C, Magons P. A clinical evaluation of the cost and time effectiveness of the ASPAN Hypothermia Guideline. J Perianesth Nurs. 2008;23(1):24-35. doi:10.1016/j.jopan.2007.09.010.
- Kimberger O, Held C, Stadelmann K et al. Resistive polymer versus forced-air warming: comparable heat transfer and core rewarming rates in volunteers. Anesth Analg. 2008;107(5):1621-1626. doi:10.1213/ane.0b013e3181845502.
- Brandt S, Oguz R, Hüttner H, et al. Resistive-polymer versus forced-air warming: comparable efficacy in orthopedic patients. Anesth Analg. 2010;110(3):834-838. doi:10.1213/ANE.0b013e3181cb3f5f.
- Legg AJ, Hammer AJ. Forced-air patient warming blankets disrupt unidirectional airflow. Bone Joint J. 2013;95-B(3):407-410. doi:10.1302/0301-620X.95B3.29121.
- Dasari KB, Albrecht M, Harper M. Effect of forced-air warming on the performance of operating theatre laminar flow ventilation. Anaesthesia. 2012;67(3):244-249. doi:10.1111/j.1365-2044.2011.06983.x.
- Belani KG, Albrecht M, McGovern PD, Reed M, Nachtsheim C. Patient warming excess heat: the effects on orthopedic operating room ventilation performance. Anesth Analg. 2013;117(2):406-411. doi:10.1213/ANE.0b013e31825f81e2.
- Legg AJ, Cannon T, Hammer AJ. Do forced air patient-warming devices disrupt unidirectional downward airflow? J Bone Joint Surg Br. 2012;94(2):254-256. doi:10.1302/0301-620X.94B2.27562.
- Albrecht M, Gaithier RL, Belani K, Litchy M, Leaper D. Forced-air warming blowers: An evaluation of filtration adequacy and airborne contamination emissions in the operating room. Am J Infect Control. 2011;39(4):321-328. doi:10.1016/j.ajic.2010.06.011.
- Reed M, Kimberger O, McGovern PD, Albrecht MC. Forced-air warming design: evaluation of intake filtration, internal microbial buildup, and airborne-contamination emissions. AANA J. 2013;81(4):275-280.
- McGovern PD, Albercht M, Belani KG, et al. Forced-air warming and ultra-clean ventilation do not mix: an investigation of theatre ventilation, patient warming and joint replacement infection in orthopaedics. J Bone Joint Surg Br.2011;93(11):1537-1544. doi:10.1302/0301-620X.93B11.27124.
ABSTRACT
Inadvertent perioperative hypothermia is a significant problem in patients undergoing either emergency or elective orthopedic surgery, and is associated with increased morbidity and mortality. Though in general the incidence of inadvertent perioperative hypothermia in postoperative recovery rooms has been decreasing over the last 2 decades, it still remains a significant risk in certain specialty practices, such as orthopedic surgery. This review article summarizes the currently available evidence on the incidence, risk factors, and complications of inadvertent perioperative hypothermia. Also, the effective preventive strategies in dealing with inadvertent perioperative hypothermia are reviewed and essential clinical guidelines to be followed are summarized.
Continue to: Inadvertent perioperative hypothermia...
Inadvertent perioperative hypothermia, defined as an involuntary drop in core body temperature to <35°C (95°F), is a condition associated with significant morbidity and mortality.1 This phenomenon has been reported in both emergency orthopedic admissions, such as fracture management, as well as in the elective setting such as arthroscopy, arthroplasty, and spine surgery.
In a study conducted in the United Kingdom including 781 elderly patients with a mean age of 80 years who presented with hip fractures, the 30-day mortality rate was 15.3% in patients who were admitted with a tympanic temperature of <36.5°C and only 5.1% in patients who maintained a tympanic temperature of 36.5°C to 37.5°C (odds ratio, 2.8; P > .0005).2 For an even better perspective, this analysis can be compared with the UK National Hip Fracture Database of 2013, which reported a 30-day mortality of 8.2% in patients who were admitted to the National Health Service with a diagnosis of hip fracture.3
Inadvertent perioperative hypothermia is also a common phenomenon during elective orthopedic hospital admissions. An Australian audit, which included 5050 postoperative patients, looked into the association between inadvertent perioperative hypothermia and mortality based on diagnostic criteria classifying mild hypothermia as a core temperature of <36°C and severe hypothermia as a core temperature of <35°C.4 The authors found that mild and severe hypothermia was experienced by 36% and 6% of patients, respectively. In-hospital mortality was 5.6% for normothermic patients, 8.9% for all hypothermic patients (P < .001), and 14.7% for severely hypothermic patients (P < .001). For a decrease of 1°C in core body temperature from <36°C to <35°C (but >34°C), there were higher odds of in-hospital mortality (odds ratio, 1.83; 95% confidence interval [CI], 1.20-2.60).
The physiologic response to hypothermia is to decrease heat loss by cutaneous and peripheral vasoconstriction and increase heat production by increasing the metabolic rate (eg, shivering and shifting to anaerobic metabolism). This response is blunted to a variable extent in perioperative patients for several reasons, including the effect of anesthetic drugs and old age.5
Maintenance of core body temperature >36°C is now a measured standard of perioperative care. A performance measure for perioperative temperature management was developed by the American Medical Association Physician Consortium for Performance Improvement (AMA-PCPI).6 To achieve this performance measure, mandatory documentation of use of active warming intraoperatively or a record of at least 1 body temperature ≥96.8°F (36°C) within 30 minutes immediately prior to and 15 minutes immediately after anesthesia end time is necessary. This performance measure is also endorsed by the Surgical Care Improvement Project (SCIP-Inf-10) and National Quality Forum (NQF).6
Continue to: Overall, in the last 2 decades...
Overall, in the last 2 decades, the incidence of inadvertent perioperative hypothermia has decreased, mainly due to aggressive intraoperative management.7 In spite of this, studies have shown that perioperative hypothermia remains a significant problem in patients undergoing orthopedic procedures. In a recent community hospital study conducted by the National Association for Healthcare Quality that included 4124 orthopedic patients undergoing elective surgery, it was shown that, in spite of 99% compliance to the AMA-PCPI recommendation, 7.7% of orthopedic patients were found to be hypothermic.6
Management of hypothermia has long been an integral component of “damage control surgery” and resuscitation during polytrauma, which aims to aggressively minimize hypovolemic shock and limit the development of the lethal triad of hypothermia, coagulopathy, and acidosis.8 However, critical references to prevention and management of inadvertent perioperative hypothermia are lacking in the orthopedic literature on elective surgical procedures. This review aims to bridge this knowledge gap.
Unless otherwise specified, inadvertent perioperative hypothermia in this article refers to the core body temperature. In contrast, peripheral/limb hypothermia refers primarily to the effect of tourniquet application to the involved limb and the effect after deflation of the tourniquet on core body temperature.
RISK FACTORS
There are several measurable risk factors that can contribute to inadvertent perioperative hypothermia, which can be subdivided into 3 groups: patient-related risk factors, anesthesia-related risk factors, and procedure-related risk factors (Table 1).5,9-11 It is important to note that in any given patient a combination of 2 or more risk factors predisposes them to developing inadvertent perioperative hypothermia. Conceptualizing the etiology of inadvertent perioperative hypothermia in this way helps to plan a multipronged strategy to prevent it from occurring in the first place. Some of the important risk factors for inadvertent perioperative hypothermia are discussed below.
Table 1. Risk Factors for Perioperative Hypothermia
Patient-Related Risk Factors | Anesthesia-Related Risk Factors | Procedure-Specific Risk Factors |
|
|
|
To identify patient-related risk factors, researchers from the University of Louisville conducted a study including 2138 operative patients who became hypothermic after admission, of whom 27% underwent orthopedic and spine procedures.9 The patient-related risk factors identified were a high severity of illness on admission (odds ratio, 2.81; 95% CI, 2.28-3.47), presence of a neurological disorder such as Alzheimer’s disease (odds ratio, 1.71; 95% CI,1.06-2.78), male sex (odds ratio, 1.65; 95% CI, 1.36-2.01), age >65 years (odds ratio, 1.61; 95% CI, 1.33-1.96), recent weight loss (odds ratio, 1.60; 95% CI, 1.04-2.48), anemia (odds ratio, 1.49; 95% CI, 1.12-1.98), and chronic renal failure (odds ratio, 1.43; 95% CI, 1.07-1.92). Interestingly, diabetes mellitus without end-stage organ failure was not found to be a significant risk factor (odds ratio, 0.58; 95% CI, 0.44-0.75). It is also important to note that some of these risk factors identified to contribute to perioperative hypothermia are dependent on each other and others are independent of each other. For example, chronic renal failure and anemia are dependent risk factors. In contrast, age >65 years and low body mass index as risk factors of perioperative hypothermia are independent of each other.
Continue to: The second subgroup of risk factors...
The second subgroup of risk factors for perioperative hypothermia is related to anesthesia. The effect of general and regional anesthesia on perioperative core temperature is significantly different, both in terms of intraoperative thermoregulation and postoperative recovery.12 Intraoperatively, the core body temperature during the first 2 hours of general anesthesia decreases at a rate of 1.3°C per hour due to loss of thermoregulatory cutaneous and peripheral vasoconstrictive responses resulting in heat loss exceeding metabolic heat production. However, the core temperature remains virtually constant during the subsequent 3 hours due to the return of the thermoregulatory response, which causes cutaneous and peripheral vasoconstriction and increased metabolic heat production. Postoperative recovery from the hypothermia induced by general anesthesia is significantly faster than from that induced by regional anesthesia.13
The effect of regional hypothermia on core body temperature is more complex because it must be considered in addition to the effect of an associated procedure-related variable (ie, tourniquet application). If a tourniquet is not used during a surgery with regional anesthesia, a linear decrease in core temperature follows until recovery, due to increased blood flow from the loss of sympathetic peripheral vasoconstrictive response with resultant core-to-peripheral heat redistribution to the exposed operating limb. If a tourniquet is used during surgery with regional anesthesia, there will be no significant effect of the exposed operating limb on core temperature, as there is no blood flow between them. However, once the tourniquet is deflated, the core body temperature will be affected significantly as a result of core-to-peripheral distribution of heat to the operated limb with the return of blood flow. This fall in core body temperature after tourniquet deflation can be prevented by active forced-air warming initiated from the beginning of surgery.10 The extent and rate of development of peripheral/limb hypothermia during surgery (and its subsequent effect on core body temperature) depends on several factors, including the operating room ambient temperature, duration of tourniquet application, and temperature of the irrigation fluid. Postoperative recovery from the hypothermia induced by regional anesthesia takes longer than from that induced by general anesthesia because of the prolonged period of loss of vasoconstrictive response.
The third subgroup of risk factors associated with perioperative hypothermia is procedure related. Several procedure-specific risk factors for inadvertent perioperative hypothermia during arthroscopic surgery are identified, including prolonged operating time, low blood pressure during the procedure, and low temperature of the irrigation fluid.11 It is logical to extrapolate the importance of these risk factors to other orthopedic procedures which also require prolonged operating times, are performed under hypotension, or expose the patient to irrigation fluid that is at a low temperature. Understanding the importance of each of these procedure-related risk factors is the most important from the perspective of the orthopedic surgeon when compared to the rest of the subgroups of risk factors for inadvertent perioperative hypothermia as he/she is directly responsible for them.
The ambient operating room temperature has traditionally been considered a risk factor for inadvertent hypothermia in perioperative patients, but evidence is available to the contrary. The recommended ambient room temperature as per the clinical guideline published by the American Society of PeriAnesthesia Nurses (ASPAN) is 20°C to 24°C (68°F-75°F).14 The ambient temperature can have a significant effect on peripheral/limb hypothermia when operating on a limb with a tourniquet inflated, as the limb has no blood supply to distribute heat from the core to the periphery. However, the direct effect of ambient room temperature on the patient’s core body temperature is unlikely to be clinically significant if standard active warming interventions are implemented.15
COMPLICATIONS
The increased incidence of mortality due to inadvertent hypothermia in the perioperative period has already been discussed. Several other complications of inadvertent perioperative hypothermia include increased incidence of coagulopathy, acidosis, stroke, sepsis, pneumonia, myocardial infarction, surgical site infections, altered drug metabolism, and longer hospital stays.8,9,16-18 Hypothermia, coagulopathy, and acidosis have long been recognized as a lethal triad more commonly seen in polytrauma patients than in elective orthopedic surgery, as this occurs at extremes of temperature, usually <32°C. When compared with patients who did not develop perioperative hypothermia, patients who developed hypothermia during elective operations were shown to experience an overall doubled complication rate (13.9% vs 26.3%; P < .001) of which the incidence of stroke (1.0% vs 6.5%; P < .001), pneumonia (1.3% vs 5.1%; P < .001), and sepsis (2.6% vs 7.5%; P < .001) were much more likely than myocardial infarction (1.1% vs 3.3%; P = .01) and wound infection (3.3% vs 5.0%; P = .14).9
Continue to: Prevention...
PREVENTION
Prevention of perioperative hypothermia is a core measure to improve the outcome after ambulatory and fast-track orthopedic surgery and rehabilitation. Preventive strategies for perioperative hypothermia can be grouped into passive heat retention methods and active external warming methods (Table 2). Passive methods aim to maintain body temperature by decreasing the heat loss by radiation (eg, reflective blanket), conduction (eg, layered cotton blankets and padding the operating table), or convection (eg, heat and humidity exchanger in the breathing circuits) to the surrounding environment. Active patient heating methods aim to bring in heat from the source to the patient’s body using conduction (eg, Hot Dog® [Eden Augustine Temperature Management]) or convection (eg, Bair Hugger® [Arizant Healthcare]) techniques.
Table 2. Methods to Prevent Inadvertent Perioperative Hypothermia
Passive Heat-Retention Methods | Active External Warming Methods |
| Conduction techniques:
Convection techniques:
|
Active patient warming is superior to passive heat retention methods. A recent Cochrane study assessed the effects of standard care (ie, use of layered clothing and warm blankets, etc.) and addition of extra thermal insulation by reflective blankets or active forced air warming to standard care on the perioperative core body temperature.19 They concluded that there is no clear benefit of addition of extra thermal insulation by reflective blankets compared with standard care alone. Also, forced-air warming in addition to standard care appeared to maintain core temperature better than standard care alone, by between 0.5°C and 1°C, but the clinical importance of this difference could not be inferred, as none of the included studies in this meta-analysis documented major cardiovascular outcomes.
Several clinical guidelines have been developed by not-for-profit, government, and professional organizations aimed at prevention of perioperative hypothermia as primary or secondary outcome. A clinical guideline was published by ASPAN in 2001 for assessment, prevention, and intervention in unplanned perioperative hypothermia.14 Cost and time effectiveness of the ASPAN Hypothermia Guideline was published in 2008.20 The assessment guideline includes identification of risk factors, repeated pre-/intra-/postoperative temperature measurement, and repeated clinical evaluation of the patient’s status. The preventive guideline is to maintain an ambient temperature of 20°C to 24°C (68°F-75°F) and use appropriate passive patient warming methods pre-, intra-, and postoperatively. Intervention in the form of active patient heating is advised only if the patient develops hypothermia in spite of the above-mentioned standard preventive measures. But many orthopedic ambulatory surgery centers currently use active patient warming as both a preventive and an intervention strategy.
Active patient warming by conduction devices occurs by direct physical contact with the device, which is set at a higher temperature, whereas heat transfer from the convection device to the patient occurs by a physical medium such as forced air or circulating water that moves in between the device and the patient. Any recommendation for use of a specific technique of active patient warming (ie, by the use of a conduction device or a convection device) should only be given after comparing evidence on 3 critical aspects: efficacy, safety, and cost effectiveness.
The heating efficacy and core rewarming rates of conduction and convection devices have been compared in the literature. Full-body forced-air heating with the Bair Hugger® and full-body resistive polymer heating with the Hot Dog® in healthy volunteers were found to be similar.21 Also, in a randomized study conducted on 80 orthopedic patients undergoing surgery, resistive polymer warming performed as efficiently as forced-air warming in patients undergoing orthopedic surgery.22
Continue to: Secondly, the safety of convection...
Secondly, the safety of convection devices such as the Bair Hugger® has been under intense scrutiny based on the evidence that it disrupts the laminar airflow in the operating theater.23-26 This disruption in laminar air flow has been shown to cause emission of significant levels of airborne contaminants of size >0.3 μm (germ size).27 Isolates of Staphylococcus aureus, coagulase-negative Staphylococcus species, and methicillin-resistant Staphylococcus aureus were detected in 13.5%, 3.9%, and 1.9% of forced-air blowers, respectively.28 However, the clinical effect on the rate of deep joint infection due to the disruption of laminar air flow has been examined in only 1 study. McGovern and colleagues29 reported a significant increase in deep joint infection during a period when forced air warming was used compared to a period when conductive fabric warming was used (odds ratio, 3.8; P = .024) and recommended air-free warming by a conduction device over forced-air warming for orthopedic procedures. Unfortunately, the prophylactic antibiotic regimen was not kept constant during their study period. During an overlapping time frame during which they shifted from the use of a convection device (Bair Hugger®) to a conduction device (Hot Dog®), they also changed their antibiotic regimen from gentamicin 4.5 mg/kg intravenous (IV) to gentamicin 3 mg/kg IV plus teicoplanin 400 mg IV. This change in antibiotic regimen is a major confounding factor that calls into question the validity of the conclusions drawn by the authors.
Finally, the cost effectiveness of conduction and convection devices has never been studied. Hence, based on the current evidence, it is not possible to recommend a particular type of active patient-warming device.
CONCLUSION
Orthopedic surgeons should be aware that inadvertent perioperative hypothermia is a common phenomenon in perioperative patients. It must be recognized that the maintenance of perioperative normothermia during all major orthopedic surgical procedures is desirable, as inadvertent perioperative hypothermia is shown to be associated with increased mortality and systemic morbidity, such as stroke and sepsis. Compliance with the current clinical guidelines for assessment, prevention, and treatment of inadvertent perioperative hypothermia will minimize, if not eliminate, such risk. We recommend the following essential clinical guidelines to prevent inadvertent perioperative hypothermia (Table 3). Identification of patient-, anesthesia-, and procedure-related risk factors is an integral component of assessment of the risk of inadvertent perioperative hypothermia. In order to achieve full compliance with implementation of active patient warming during surgery, it is prudent to make active warming information a part of the surgical timeout checklist. Irrespective of the presence of risk factors, passive heat retention methods should be part of perioperative management of patients undergoing elective orthopedic surgery to prevent inadvertent perioperative hypothermia. In addition, there should be a minimum threshold to utilize active patient-warming techniques, especially in patients with inherent risk factors and surgeries that take >30 minutes of operating time, either under regional or general anesthesia. As there are concerns about safety issues with the use of convection devices, we believe a multicenter randomized controlled trial is warranted.
Table 3. Recommended Essential Clinical Guidelines for Prevention of Inadvertent Perioperative Hypothermia
|
ABSTRACT
Inadvertent perioperative hypothermia is a significant problem in patients undergoing either emergency or elective orthopedic surgery, and is associated with increased morbidity and mortality. Though in general the incidence of inadvertent perioperative hypothermia in postoperative recovery rooms has been decreasing over the last 2 decades, it still remains a significant risk in certain specialty practices, such as orthopedic surgery. This review article summarizes the currently available evidence on the incidence, risk factors, and complications of inadvertent perioperative hypothermia. Also, the effective preventive strategies in dealing with inadvertent perioperative hypothermia are reviewed and essential clinical guidelines to be followed are summarized.
Continue to: Inadvertent perioperative hypothermia...
Inadvertent perioperative hypothermia, defined as an involuntary drop in core body temperature to <35°C (95°F), is a condition associated with significant morbidity and mortality.1 This phenomenon has been reported in both emergency orthopedic admissions, such as fracture management, as well as in the elective setting such as arthroscopy, arthroplasty, and spine surgery.
In a study conducted in the United Kingdom including 781 elderly patients with a mean age of 80 years who presented with hip fractures, the 30-day mortality rate was 15.3% in patients who were admitted with a tympanic temperature of <36.5°C and only 5.1% in patients who maintained a tympanic temperature of 36.5°C to 37.5°C (odds ratio, 2.8; P > .0005).2 For an even better perspective, this analysis can be compared with the UK National Hip Fracture Database of 2013, which reported a 30-day mortality of 8.2% in patients who were admitted to the National Health Service with a diagnosis of hip fracture.3
Inadvertent perioperative hypothermia is also a common phenomenon during elective orthopedic hospital admissions. An Australian audit, which included 5050 postoperative patients, looked into the association between inadvertent perioperative hypothermia and mortality based on diagnostic criteria classifying mild hypothermia as a core temperature of <36°C and severe hypothermia as a core temperature of <35°C.4 The authors found that mild and severe hypothermia was experienced by 36% and 6% of patients, respectively. In-hospital mortality was 5.6% for normothermic patients, 8.9% for all hypothermic patients (P < .001), and 14.7% for severely hypothermic patients (P < .001). For a decrease of 1°C in core body temperature from <36°C to <35°C (but >34°C), there were higher odds of in-hospital mortality (odds ratio, 1.83; 95% confidence interval [CI], 1.20-2.60).
The physiologic response to hypothermia is to decrease heat loss by cutaneous and peripheral vasoconstriction and increase heat production by increasing the metabolic rate (eg, shivering and shifting to anaerobic metabolism). This response is blunted to a variable extent in perioperative patients for several reasons, including the effect of anesthetic drugs and old age.5
Maintenance of core body temperature >36°C is now a measured standard of perioperative care. A performance measure for perioperative temperature management was developed by the American Medical Association Physician Consortium for Performance Improvement (AMA-PCPI).6 To achieve this performance measure, mandatory documentation of use of active warming intraoperatively or a record of at least 1 body temperature ≥96.8°F (36°C) within 30 minutes immediately prior to and 15 minutes immediately after anesthesia end time is necessary. This performance measure is also endorsed by the Surgical Care Improvement Project (SCIP-Inf-10) and National Quality Forum (NQF).6
Continue to: Overall, in the last 2 decades...
Overall, in the last 2 decades, the incidence of inadvertent perioperative hypothermia has decreased, mainly due to aggressive intraoperative management.7 In spite of this, studies have shown that perioperative hypothermia remains a significant problem in patients undergoing orthopedic procedures. In a recent community hospital study conducted by the National Association for Healthcare Quality that included 4124 orthopedic patients undergoing elective surgery, it was shown that, in spite of 99% compliance to the AMA-PCPI recommendation, 7.7% of orthopedic patients were found to be hypothermic.6
Management of hypothermia has long been an integral component of “damage control surgery” and resuscitation during polytrauma, which aims to aggressively minimize hypovolemic shock and limit the development of the lethal triad of hypothermia, coagulopathy, and acidosis.8 However, critical references to prevention and management of inadvertent perioperative hypothermia are lacking in the orthopedic literature on elective surgical procedures. This review aims to bridge this knowledge gap.
Unless otherwise specified, inadvertent perioperative hypothermia in this article refers to the core body temperature. In contrast, peripheral/limb hypothermia refers primarily to the effect of tourniquet application to the involved limb and the effect after deflation of the tourniquet on core body temperature.
RISK FACTORS
There are several measurable risk factors that can contribute to inadvertent perioperative hypothermia, which can be subdivided into 3 groups: patient-related risk factors, anesthesia-related risk factors, and procedure-related risk factors (Table 1).5,9-11 It is important to note that in any given patient a combination of 2 or more risk factors predisposes them to developing inadvertent perioperative hypothermia. Conceptualizing the etiology of inadvertent perioperative hypothermia in this way helps to plan a multipronged strategy to prevent it from occurring in the first place. Some of the important risk factors for inadvertent perioperative hypothermia are discussed below.
Table 1. Risk Factors for Perioperative Hypothermia
Patient-Related Risk Factors | Anesthesia-Related Risk Factors | Procedure-Specific Risk Factors |
|
|
|
To identify patient-related risk factors, researchers from the University of Louisville conducted a study including 2138 operative patients who became hypothermic after admission, of whom 27% underwent orthopedic and spine procedures.9 The patient-related risk factors identified were a high severity of illness on admission (odds ratio, 2.81; 95% CI, 2.28-3.47), presence of a neurological disorder such as Alzheimer’s disease (odds ratio, 1.71; 95% CI,1.06-2.78), male sex (odds ratio, 1.65; 95% CI, 1.36-2.01), age >65 years (odds ratio, 1.61; 95% CI, 1.33-1.96), recent weight loss (odds ratio, 1.60; 95% CI, 1.04-2.48), anemia (odds ratio, 1.49; 95% CI, 1.12-1.98), and chronic renal failure (odds ratio, 1.43; 95% CI, 1.07-1.92). Interestingly, diabetes mellitus without end-stage organ failure was not found to be a significant risk factor (odds ratio, 0.58; 95% CI, 0.44-0.75). It is also important to note that some of these risk factors identified to contribute to perioperative hypothermia are dependent on each other and others are independent of each other. For example, chronic renal failure and anemia are dependent risk factors. In contrast, age >65 years and low body mass index as risk factors of perioperative hypothermia are independent of each other.
Continue to: The second subgroup of risk factors...
The second subgroup of risk factors for perioperative hypothermia is related to anesthesia. The effect of general and regional anesthesia on perioperative core temperature is significantly different, both in terms of intraoperative thermoregulation and postoperative recovery.12 Intraoperatively, the core body temperature during the first 2 hours of general anesthesia decreases at a rate of 1.3°C per hour due to loss of thermoregulatory cutaneous and peripheral vasoconstrictive responses resulting in heat loss exceeding metabolic heat production. However, the core temperature remains virtually constant during the subsequent 3 hours due to the return of the thermoregulatory response, which causes cutaneous and peripheral vasoconstriction and increased metabolic heat production. Postoperative recovery from the hypothermia induced by general anesthesia is significantly faster than from that induced by regional anesthesia.13
The effect of regional hypothermia on core body temperature is more complex because it must be considered in addition to the effect of an associated procedure-related variable (ie, tourniquet application). If a tourniquet is not used during a surgery with regional anesthesia, a linear decrease in core temperature follows until recovery, due to increased blood flow from the loss of sympathetic peripheral vasoconstrictive response with resultant core-to-peripheral heat redistribution to the exposed operating limb. If a tourniquet is used during surgery with regional anesthesia, there will be no significant effect of the exposed operating limb on core temperature, as there is no blood flow between them. However, once the tourniquet is deflated, the core body temperature will be affected significantly as a result of core-to-peripheral distribution of heat to the operated limb with the return of blood flow. This fall in core body temperature after tourniquet deflation can be prevented by active forced-air warming initiated from the beginning of surgery.10 The extent and rate of development of peripheral/limb hypothermia during surgery (and its subsequent effect on core body temperature) depends on several factors, including the operating room ambient temperature, duration of tourniquet application, and temperature of the irrigation fluid. Postoperative recovery from the hypothermia induced by regional anesthesia takes longer than from that induced by general anesthesia because of the prolonged period of loss of vasoconstrictive response.
The third subgroup of risk factors associated with perioperative hypothermia is procedure related. Several procedure-specific risk factors for inadvertent perioperative hypothermia during arthroscopic surgery are identified, including prolonged operating time, low blood pressure during the procedure, and low temperature of the irrigation fluid.11 It is logical to extrapolate the importance of these risk factors to other orthopedic procedures which also require prolonged operating times, are performed under hypotension, or expose the patient to irrigation fluid that is at a low temperature. Understanding the importance of each of these procedure-related risk factors is the most important from the perspective of the orthopedic surgeon when compared to the rest of the subgroups of risk factors for inadvertent perioperative hypothermia as he/she is directly responsible for them.
The ambient operating room temperature has traditionally been considered a risk factor for inadvertent hypothermia in perioperative patients, but evidence is available to the contrary. The recommended ambient room temperature as per the clinical guideline published by the American Society of PeriAnesthesia Nurses (ASPAN) is 20°C to 24°C (68°F-75°F).14 The ambient temperature can have a significant effect on peripheral/limb hypothermia when operating on a limb with a tourniquet inflated, as the limb has no blood supply to distribute heat from the core to the periphery. However, the direct effect of ambient room temperature on the patient’s core body temperature is unlikely to be clinically significant if standard active warming interventions are implemented.15
COMPLICATIONS
The increased incidence of mortality due to inadvertent hypothermia in the perioperative period has already been discussed. Several other complications of inadvertent perioperative hypothermia include increased incidence of coagulopathy, acidosis, stroke, sepsis, pneumonia, myocardial infarction, surgical site infections, altered drug metabolism, and longer hospital stays.8,9,16-18 Hypothermia, coagulopathy, and acidosis have long been recognized as a lethal triad more commonly seen in polytrauma patients than in elective orthopedic surgery, as this occurs at extremes of temperature, usually <32°C. When compared with patients who did not develop perioperative hypothermia, patients who developed hypothermia during elective operations were shown to experience an overall doubled complication rate (13.9% vs 26.3%; P < .001) of which the incidence of stroke (1.0% vs 6.5%; P < .001), pneumonia (1.3% vs 5.1%; P < .001), and sepsis (2.6% vs 7.5%; P < .001) were much more likely than myocardial infarction (1.1% vs 3.3%; P = .01) and wound infection (3.3% vs 5.0%; P = .14).9
Continue to: Prevention...
PREVENTION
Prevention of perioperative hypothermia is a core measure to improve the outcome after ambulatory and fast-track orthopedic surgery and rehabilitation. Preventive strategies for perioperative hypothermia can be grouped into passive heat retention methods and active external warming methods (Table 2). Passive methods aim to maintain body temperature by decreasing the heat loss by radiation (eg, reflective blanket), conduction (eg, layered cotton blankets and padding the operating table), or convection (eg, heat and humidity exchanger in the breathing circuits) to the surrounding environment. Active patient heating methods aim to bring in heat from the source to the patient’s body using conduction (eg, Hot Dog® [Eden Augustine Temperature Management]) or convection (eg, Bair Hugger® [Arizant Healthcare]) techniques.
Table 2. Methods to Prevent Inadvertent Perioperative Hypothermia
Passive Heat-Retention Methods | Active External Warming Methods |
| Conduction techniques:
Convection techniques:
|
Active patient warming is superior to passive heat retention methods. A recent Cochrane study assessed the effects of standard care (ie, use of layered clothing and warm blankets, etc.) and addition of extra thermal insulation by reflective blankets or active forced air warming to standard care on the perioperative core body temperature.19 They concluded that there is no clear benefit of addition of extra thermal insulation by reflective blankets compared with standard care alone. Also, forced-air warming in addition to standard care appeared to maintain core temperature better than standard care alone, by between 0.5°C and 1°C, but the clinical importance of this difference could not be inferred, as none of the included studies in this meta-analysis documented major cardiovascular outcomes.
Several clinical guidelines have been developed by not-for-profit, government, and professional organizations aimed at prevention of perioperative hypothermia as primary or secondary outcome. A clinical guideline was published by ASPAN in 2001 for assessment, prevention, and intervention in unplanned perioperative hypothermia.14 Cost and time effectiveness of the ASPAN Hypothermia Guideline was published in 2008.20 The assessment guideline includes identification of risk factors, repeated pre-/intra-/postoperative temperature measurement, and repeated clinical evaluation of the patient’s status. The preventive guideline is to maintain an ambient temperature of 20°C to 24°C (68°F-75°F) and use appropriate passive patient warming methods pre-, intra-, and postoperatively. Intervention in the form of active patient heating is advised only if the patient develops hypothermia in spite of the above-mentioned standard preventive measures. But many orthopedic ambulatory surgery centers currently use active patient warming as both a preventive and an intervention strategy.
Active patient warming by conduction devices occurs by direct physical contact with the device, which is set at a higher temperature, whereas heat transfer from the convection device to the patient occurs by a physical medium such as forced air or circulating water that moves in between the device and the patient. Any recommendation for use of a specific technique of active patient warming (ie, by the use of a conduction device or a convection device) should only be given after comparing evidence on 3 critical aspects: efficacy, safety, and cost effectiveness.
The heating efficacy and core rewarming rates of conduction and convection devices have been compared in the literature. Full-body forced-air heating with the Bair Hugger® and full-body resistive polymer heating with the Hot Dog® in healthy volunteers were found to be similar.21 Also, in a randomized study conducted on 80 orthopedic patients undergoing surgery, resistive polymer warming performed as efficiently as forced-air warming in patients undergoing orthopedic surgery.22
Continue to: Secondly, the safety of convection...
Secondly, the safety of convection devices such as the Bair Hugger® has been under intense scrutiny based on the evidence that it disrupts the laminar airflow in the operating theater.23-26 This disruption in laminar air flow has been shown to cause emission of significant levels of airborne contaminants of size >0.3 μm (germ size).27 Isolates of Staphylococcus aureus, coagulase-negative Staphylococcus species, and methicillin-resistant Staphylococcus aureus were detected in 13.5%, 3.9%, and 1.9% of forced-air blowers, respectively.28 However, the clinical effect on the rate of deep joint infection due to the disruption of laminar air flow has been examined in only 1 study. McGovern and colleagues29 reported a significant increase in deep joint infection during a period when forced air warming was used compared to a period when conductive fabric warming was used (odds ratio, 3.8; P = .024) and recommended air-free warming by a conduction device over forced-air warming for orthopedic procedures. Unfortunately, the prophylactic antibiotic regimen was not kept constant during their study period. During an overlapping time frame during which they shifted from the use of a convection device (Bair Hugger®) to a conduction device (Hot Dog®), they also changed their antibiotic regimen from gentamicin 4.5 mg/kg intravenous (IV) to gentamicin 3 mg/kg IV plus teicoplanin 400 mg IV. This change in antibiotic regimen is a major confounding factor that calls into question the validity of the conclusions drawn by the authors.
Finally, the cost effectiveness of conduction and convection devices has never been studied. Hence, based on the current evidence, it is not possible to recommend a particular type of active patient-warming device.
CONCLUSION
Orthopedic surgeons should be aware that inadvertent perioperative hypothermia is a common phenomenon in perioperative patients. It must be recognized that the maintenance of perioperative normothermia during all major orthopedic surgical procedures is desirable, as inadvertent perioperative hypothermia is shown to be associated with increased mortality and systemic morbidity, such as stroke and sepsis. Compliance with the current clinical guidelines for assessment, prevention, and treatment of inadvertent perioperative hypothermia will minimize, if not eliminate, such risk. We recommend the following essential clinical guidelines to prevent inadvertent perioperative hypothermia (Table 3). Identification of patient-, anesthesia-, and procedure-related risk factors is an integral component of assessment of the risk of inadvertent perioperative hypothermia. In order to achieve full compliance with implementation of active patient warming during surgery, it is prudent to make active warming information a part of the surgical timeout checklist. Irrespective of the presence of risk factors, passive heat retention methods should be part of perioperative management of patients undergoing elective orthopedic surgery to prevent inadvertent perioperative hypothermia. In addition, there should be a minimum threshold to utilize active patient-warming techniques, especially in patients with inherent risk factors and surgeries that take >30 minutes of operating time, either under regional or general anesthesia. As there are concerns about safety issues with the use of convection devices, we believe a multicenter randomized controlled trial is warranted.
Table 3. Recommended Essential Clinical Guidelines for Prevention of Inadvertent Perioperative Hypothermia
|
- Brown DJ, Brugger H, Boyd J, Paal P. Accidental Hypothermia. N Engl J Med. 2012;367(20):1930-1938. doi:10.1056/NEJMra1114208.
- Uzoigwe CE, Khan A, Smith RP, et al. Hypothermia and low body temperature are common and associated with high mortality in hip fracture patients. Hip Int. 2014; 24(3):237-242. doi:10.5301/hipint.5000124.
- Johansen A, Wakeman R, Boulton C, Plant F, Roberts J, Williams A. National Hip Fracture Database: National Report 2013. London, UK: National Hip Fracture Database, Royal College of Physicians; 2013.
- Karalapillai D, Story DA, Calzavacca, Licari E, Liu YL, Hart GK. Inadvertent hypothermia and mortality in postoperative intensive care patients: retrospective audit of 5050 patients. Anaesthesia. 2009;64(9):968-972. doi:10.1111/j.1365-2044.2009.05989.x.
- Horosz B, Malec-Milewska M. Inadvertent intraoperative hypothermia. Anaesthesiol Intensive Ther. 2013;45(1):38-43. doi:10.5603/AIT.2013.0009.
- Steelman VM, Perkhounkova YS, Lemke JH. The gap between compliance with the quality performance measure "perioperative temperature management” and normothermia. J Healthc Qual. 2014;37(6):333-341. doi:10.1111/jhq.12063.
- National Institute for Health and Clinical Excellence. Inadvertent Perioperative Hypothermia: The Management of Inadvertent Perioperative Hypothermia in Adults. London, UK: National Institute for Health and Clinical Excellence; 2008.
- Carlino W. Damage control resuscitation from major haemorrhage in polytrauma. Eur J Orthop Surg Traumatol. 2014;24(2):137-141. doi:10.1007/s00590-013-1172-7.
- Billeter AT, Hohmann SF, Druen D, Cannon R, Polk HC Jr. Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. Surgery. 2014;156(5):1245-1252. doi:10.1016/j.surg.2014.04.024.
- Kim YS, Jeon YS, Lee JA, et al. Intra-operative warming with a forced-air warmer in preventing hypothermia after tourniquet deflation in elderly patients. J Int Med Res. 2009;37(5):1457-1464. doi:10.1177/147323000903700521.
- Parodi D, Tobar C, Valderrama J, et al. Hip arthroscopy and hypothermia. Arthroscopy. 2012;28(7):924-928. doi:10.1016/j.arthro.2011.12.012.
- Kurz A, Sessler DI, Christensen R, Dechert M. Heat balance and distribution during the core-temperature plateau in anesthetized humans. Anesthesiology. 1995;83(3):491-499.
- Vaughan MS, Vaughan RW, Cork RC. Postoperative hypothermia in adults: relationship of age, anesthesia, and shivering to rewarming. Anesth Analg. 1981;60(10):746-751.
- American Society of PeriAnesthesia Nurses. Clinical guideline for the prevention of unplanned perioperative hypothermia. J Perianesth Nurs. 2001;16(5):305-314.
- Inaba K, Berg R, Barmparas G, et al. Prospective evaluation of ambient operating room temperature on the core temperature of injured patients undergoing emergent surgery. J Trauma Acute Care Surg. 2012;73(6):1478-1483. doi:10.1097/TA.0b013e3182781db3.
- Barie PS. Surgical site infections: epidemiology and prevention. Surg Infect (Larchmt). 2002;3 Suppl 1:S9-S21. doi:10.1089/sur.2002.3.s1-9.
- Jeran L. Patient temperature: an introduction to the clinical guideline for the prevention of unplanned perioperative hypothermia. J Perianesth Nurs. 2001;16(5):303-304.
- Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209-1215.
- Alderson P, Campbell G, Smith AF, Warttig S, Nicholson A, Lewis SR. Thermal insulation for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev. 2014;6:CD009908. doi:10.1002/14651858.CD009908.pub2.
- Berry D, Wick C, Magons P. A clinical evaluation of the cost and time effectiveness of the ASPAN Hypothermia Guideline. J Perianesth Nurs. 2008;23(1):24-35. doi:10.1016/j.jopan.2007.09.010.
- Kimberger O, Held C, Stadelmann K et al. Resistive polymer versus forced-air warming: comparable heat transfer and core rewarming rates in volunteers. Anesth Analg. 2008;107(5):1621-1626. doi:10.1213/ane.0b013e3181845502.
- Brandt S, Oguz R, Hüttner H, et al. Resistive-polymer versus forced-air warming: comparable efficacy in orthopedic patients. Anesth Analg. 2010;110(3):834-838. doi:10.1213/ANE.0b013e3181cb3f5f.
- Legg AJ, Hammer AJ. Forced-air patient warming blankets disrupt unidirectional airflow. Bone Joint J. 2013;95-B(3):407-410. doi:10.1302/0301-620X.95B3.29121.
- Dasari KB, Albrecht M, Harper M. Effect of forced-air warming on the performance of operating theatre laminar flow ventilation. Anaesthesia. 2012;67(3):244-249. doi:10.1111/j.1365-2044.2011.06983.x.
- Belani KG, Albrecht M, McGovern PD, Reed M, Nachtsheim C. Patient warming excess heat: the effects on orthopedic operating room ventilation performance. Anesth Analg. 2013;117(2):406-411. doi:10.1213/ANE.0b013e31825f81e2.
- Legg AJ, Cannon T, Hammer AJ. Do forced air patient-warming devices disrupt unidirectional downward airflow? J Bone Joint Surg Br. 2012;94(2):254-256. doi:10.1302/0301-620X.94B2.27562.
- Albrecht M, Gaithier RL, Belani K, Litchy M, Leaper D. Forced-air warming blowers: An evaluation of filtration adequacy and airborne contamination emissions in the operating room. Am J Infect Control. 2011;39(4):321-328. doi:10.1016/j.ajic.2010.06.011.
- Reed M, Kimberger O, McGovern PD, Albrecht MC. Forced-air warming design: evaluation of intake filtration, internal microbial buildup, and airborne-contamination emissions. AANA J. 2013;81(4):275-280.
- McGovern PD, Albercht M, Belani KG, et al. Forced-air warming and ultra-clean ventilation do not mix: an investigation of theatre ventilation, patient warming and joint replacement infection in orthopaedics. J Bone Joint Surg Br.2011;93(11):1537-1544. doi:10.1302/0301-620X.93B11.27124.
- Brown DJ, Brugger H, Boyd J, Paal P. Accidental Hypothermia. N Engl J Med. 2012;367(20):1930-1938. doi:10.1056/NEJMra1114208.
- Uzoigwe CE, Khan A, Smith RP, et al. Hypothermia and low body temperature are common and associated with high mortality in hip fracture patients. Hip Int. 2014; 24(3):237-242. doi:10.5301/hipint.5000124.
- Johansen A, Wakeman R, Boulton C, Plant F, Roberts J, Williams A. National Hip Fracture Database: National Report 2013. London, UK: National Hip Fracture Database, Royal College of Physicians; 2013.
- Karalapillai D, Story DA, Calzavacca, Licari E, Liu YL, Hart GK. Inadvertent hypothermia and mortality in postoperative intensive care patients: retrospective audit of 5050 patients. Anaesthesia. 2009;64(9):968-972. doi:10.1111/j.1365-2044.2009.05989.x.
- Horosz B, Malec-Milewska M. Inadvertent intraoperative hypothermia. Anaesthesiol Intensive Ther. 2013;45(1):38-43. doi:10.5603/AIT.2013.0009.
- Steelman VM, Perkhounkova YS, Lemke JH. The gap between compliance with the quality performance measure "perioperative temperature management” and normothermia. J Healthc Qual. 2014;37(6):333-341. doi:10.1111/jhq.12063.
- National Institute for Health and Clinical Excellence. Inadvertent Perioperative Hypothermia: The Management of Inadvertent Perioperative Hypothermia in Adults. London, UK: National Institute for Health and Clinical Excellence; 2008.
- Carlino W. Damage control resuscitation from major haemorrhage in polytrauma. Eur J Orthop Surg Traumatol. 2014;24(2):137-141. doi:10.1007/s00590-013-1172-7.
- Billeter AT, Hohmann SF, Druen D, Cannon R, Polk HC Jr. Unintentional perioperative hypothermia is associated with severe complications and high mortality in elective operations. Surgery. 2014;156(5):1245-1252. doi:10.1016/j.surg.2014.04.024.
- Kim YS, Jeon YS, Lee JA, et al. Intra-operative warming with a forced-air warmer in preventing hypothermia after tourniquet deflation in elderly patients. J Int Med Res. 2009;37(5):1457-1464. doi:10.1177/147323000903700521.
- Parodi D, Tobar C, Valderrama J, et al. Hip arthroscopy and hypothermia. Arthroscopy. 2012;28(7):924-928. doi:10.1016/j.arthro.2011.12.012.
- Kurz A, Sessler DI, Christensen R, Dechert M. Heat balance and distribution during the core-temperature plateau in anesthetized humans. Anesthesiology. 1995;83(3):491-499.
- Vaughan MS, Vaughan RW, Cork RC. Postoperative hypothermia in adults: relationship of age, anesthesia, and shivering to rewarming. Anesth Analg. 1981;60(10):746-751.
- American Society of PeriAnesthesia Nurses. Clinical guideline for the prevention of unplanned perioperative hypothermia. J Perianesth Nurs. 2001;16(5):305-314.
- Inaba K, Berg R, Barmparas G, et al. Prospective evaluation of ambient operating room temperature on the core temperature of injured patients undergoing emergent surgery. J Trauma Acute Care Surg. 2012;73(6):1478-1483. doi:10.1097/TA.0b013e3182781db3.
- Barie PS. Surgical site infections: epidemiology and prevention. Surg Infect (Larchmt). 2002;3 Suppl 1:S9-S21. doi:10.1089/sur.2002.3.s1-9.
- Jeran L. Patient temperature: an introduction to the clinical guideline for the prevention of unplanned perioperative hypothermia. J Perianesth Nurs. 2001;16(5):303-304.
- Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209-1215.
- Alderson P, Campbell G, Smith AF, Warttig S, Nicholson A, Lewis SR. Thermal insulation for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev. 2014;6:CD009908. doi:10.1002/14651858.CD009908.pub2.
- Berry D, Wick C, Magons P. A clinical evaluation of the cost and time effectiveness of the ASPAN Hypothermia Guideline. J Perianesth Nurs. 2008;23(1):24-35. doi:10.1016/j.jopan.2007.09.010.
- Kimberger O, Held C, Stadelmann K et al. Resistive polymer versus forced-air warming: comparable heat transfer and core rewarming rates in volunteers. Anesth Analg. 2008;107(5):1621-1626. doi:10.1213/ane.0b013e3181845502.
- Brandt S, Oguz R, Hüttner H, et al. Resistive-polymer versus forced-air warming: comparable efficacy in orthopedic patients. Anesth Analg. 2010;110(3):834-838. doi:10.1213/ANE.0b013e3181cb3f5f.
- Legg AJ, Hammer AJ. Forced-air patient warming blankets disrupt unidirectional airflow. Bone Joint J. 2013;95-B(3):407-410. doi:10.1302/0301-620X.95B3.29121.
- Dasari KB, Albrecht M, Harper M. Effect of forced-air warming on the performance of operating theatre laminar flow ventilation. Anaesthesia. 2012;67(3):244-249. doi:10.1111/j.1365-2044.2011.06983.x.
- Belani KG, Albrecht M, McGovern PD, Reed M, Nachtsheim C. Patient warming excess heat: the effects on orthopedic operating room ventilation performance. Anesth Analg. 2013;117(2):406-411. doi:10.1213/ANE.0b013e31825f81e2.
- Legg AJ, Cannon T, Hammer AJ. Do forced air patient-warming devices disrupt unidirectional downward airflow? J Bone Joint Surg Br. 2012;94(2):254-256. doi:10.1302/0301-620X.94B2.27562.
- Albrecht M, Gaithier RL, Belani K, Litchy M, Leaper D. Forced-air warming blowers: An evaluation of filtration adequacy and airborne contamination emissions in the operating room. Am J Infect Control. 2011;39(4):321-328. doi:10.1016/j.ajic.2010.06.011.
- Reed M, Kimberger O, McGovern PD, Albrecht MC. Forced-air warming design: evaluation of intake filtration, internal microbial buildup, and airborne-contamination emissions. AANA J. 2013;81(4):275-280.
- McGovern PD, Albercht M, Belani KG, et al. Forced-air warming and ultra-clean ventilation do not mix: an investigation of theatre ventilation, patient warming and joint replacement infection in orthopaedics. J Bone Joint Surg Br.2011;93(11):1537-1544. doi:10.1302/0301-620X.93B11.27124.
TAKE-HOME POINTS
- Inadvertent perioperative hypothermia, defined as an involuntary drop in core body temperature to <35°C (95°F), is a condition associated with significant morbidity and mortality.
- Maintenance of core body temperature >36°C is now a measured standard of perioperative care.
- Overall, in the last 2 decades, the incidence of inadvertent perioperative hypothermia has decreased, mainly due to aggressive intraoperative management.
- Active patient warming by conduction devices occurs by direct physical contact with the device, which is set at a higher temperature, whereas heat transfer from the convection device to the patient occurs by a physical medium such as forced air or circulating water that moves in between the device and the patient.
- Active patient warming is superior to passive heat retention methods.
Antegrade Femoral Nail Distal Interlocking Screw Causing Rupture of the Medial Patellofemoral Ligament and Patellar Instability
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.
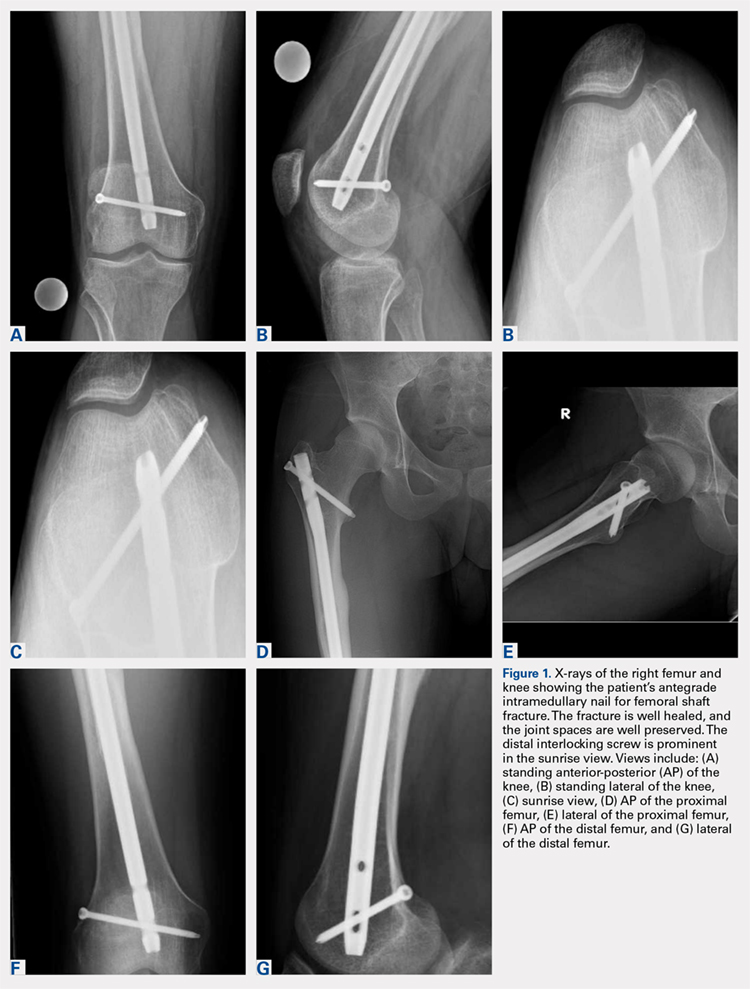
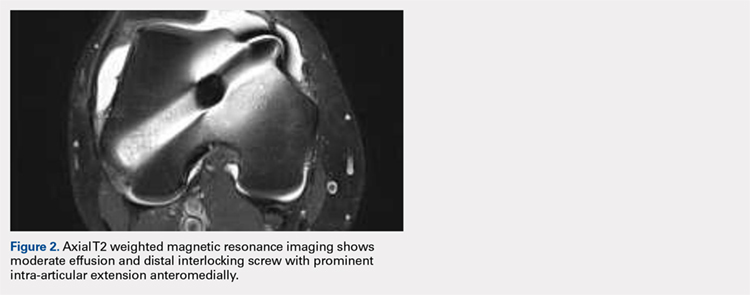
Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).
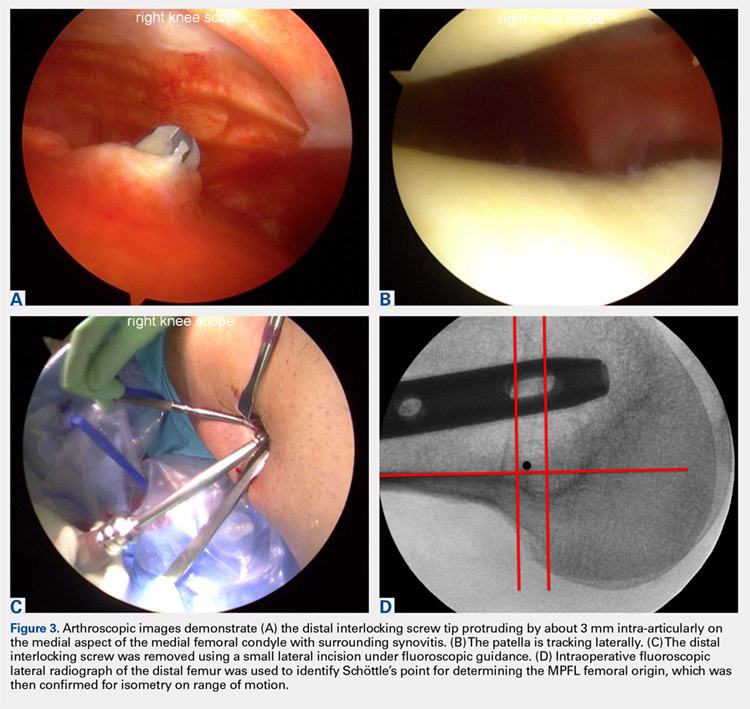
Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).
DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Antegrade reamed intramedullary nailing has the advantages of high fracture union and early weight-bearing, making it the gold standard for fixation of diaphyseal femur fractures. However, knowledge of distal femoral anatomy may mitigate the risk of secondary complications.
We present a previously unrecognized complication of antegrade femoral nailing in which a 23-year-old man sustained iatrogenic rupture of the medial patellofemoral ligament (MPFL) caused by the distal interlocking screw of the femoral nail. The patient had a history of antegrade intramedullary nailing that was revised for rotational malalignment, after which he began experiencing recurrent episodes of atraumatic bloody joint effusion and swelling of the right knee with associated patellar instability. Plain radiographs and magnetic resonance imaging revealed a large effusion with a prominent intra-articular distal interlocking screw disrupting the MPFL. The patient underwent a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability, and was able to return to his activities.
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
Continue to: Reamed intramedullary nails...
Reamed intramedullary nails are the gold standard for fixation of femoral diaphyseal fractures.1 Antegrade or retrograde nails are effective options, with the choice of technique based on factors including surgeon preference, patient factors, and concomitant injuries.2 Interlocking screws are generally placed to allow control of both rotation and length.1 Advantages of intramedullary treatment of femoral diaphyseal fractures compared with plate fixation include low rates of infection, lower nonunion rate, and faster patient mobilization and weight-bearing.3
Complications of antegrade intramedullary fixation of femoral shaft fractures include infection, nonunion, malunion, anterior cortical perforation, heterotopic ossification, abductor weakness, and soft tissue irritation from interlocking screws.2-4 Femoral intramedullary nails are not routinely removed because the hardware is rarely symptomatic and removing the nail involves additional surgical morbidity with the potential for complications.5 Interlocking screws are removed in select cases due to soft tissue irritation, generally after fracture union. Although hardware removal may help in select cases, removal of intramedullary nails is associated with low rates of symptom resolution.6-8
We present a case of iatrogenic medial patellofemoral ligament (MPFL) disruption by the distal interlocking screw leading to patellar instability, a previously unrecognized complication of antegrade femoral nailing for femoral diaphyseal fractures. The patient provided written informed consent for print and electronic publication of this case report.
CASE REPORT
We present a case of a 23-year-old man whose status was 2 years post antegrade reamed femoral intramedullary nailing at an outside institution for a right diaphyseal femur fracture. This issue was revised for external rotational malalignment, and he presented with right anterior knee pain, recurrent patellar subluxation, and recurrent effusions. The extent of external rotational malalignment and subsequent rotational correction were not evident from the available outside institution records. These symptoms began after his femoral nail revision for malalignment, and he had no subsequent trauma. The femoral fracture healed uneventfully. The patient denied any history of knee pain, swelling, or patellar instability before his femoral nail revision for malalignment. These episodes of effusion, instability, and pain occurred several times per year, generally with activities of daily living (ADL). On one occasion, he presented to a local emergency room where knee aspiration revealed no evidence of crystals or infection. The patient was referred to the senior author (Dr. Nho) for consultation.
Physical examination revealed right knee full extension with flexion to 80°. A moderate right knee effusion was present. The patient was tender over the medial femoral epicondyle and the superomedial aspect of the patella without joint line tenderness. Lateral patellar instability was present with 2 quadrants of translation (compared with 1 on the contralateral side) and patellar apprehension. The patient’s knee was ligamentously stable, and meniscal signs were absent. His lower extremity rotational profile was symmetric to the contralateral uninjured side.
Right femur and knee X-rays showed an antegrade intramedullary nail with a well-healed diaphyseal fracture and a single distal interlocking screw oriented from posterolateral to anteromedial (Figures 1A-1G). The screw tip was prominent on sunrise X-ray view anterior to the medial femoral epicondyle (Figure 1C). Magnetic resonance imaging demonstrated a large effusion and lateral patellar subluxation with a prominent intra-articular distal interlocking screw disrupting the MPFL near the femoral attachment (Figure 2). Patellar height, trochlear morphology, and tibial tubercle-trochlear groove distance were assessed and found to be normal.


Continue to: The patient elected...
The patient elected to have a right knee arthroscopic-assisted MPFL reconstruction and removal of the distal interlocking screw. Diagnostic arthroscopy revealed the distal interlocking screw to be intra-articular medially, prominent by 3 mm causing attritional disruption of the mid-substance MPFL (Figure 3A). The patella was noted to be subluxated and tracking laterally (Figure 3B). Both the anterior cruciate ligament and posterior cruciate ligament were intact, and menisci and articular cartilage were normal. The distal interlocking screw was removed under fluoroscopic guidance through a small lateral incision (Figure 3C).

Due to the nature of the longstanding attritional disruption of the MPFL in this case with associated patellar instability over a 2-year period, the decision was made to proceed with formal MPFL reconstruction as opposed to repair. A 2-cm incision was made at the medial aspect of the patella. The proximal half of the patella was decorticated. Guide pins were placed within the proximal half of the patella, ensuring at least a 1-cm bone bridge between them, and two 4.75-mm SwiveLock suture anchors (Arthrex) were inserted. A semitendinosus graft was used for MPFL reconstruction with the 2 ends of the graft secured to 2 suture anchors with a whipstitch. Lateral fluoroscopy was used to identify Schöttle’s point, denoting the femoral origin of the MPFL9 (Figure 3D). A 2-cm incision was made at this location. A guide pin was then placed at Schöttle’s point under fluoroscopic guidance, aimed proximally, and the knee was brought through a range of motion (ROM), to verify graft isometry. Once verified, the guide pin was over-reamed to 8 mm. The layer between the retinaculum and the capsule was carefully dissected, and the graft was passed extra-articularly in the plane between the retinaculum and the capsule, out through the medial incision, and docked into the bone tunnel. An 8-mm BioComposite interference screw (Arthrex) was then placed with the knee flexed to 30°. The knee was then passed through a ROM and an arthroscopic evaluation confirmed that the patella was no longer subluxated laterally. There was normal tracking of the patellofemoral joint on arthroscopic evaluation.
Postoperatively, the patient was maintained in a hinged knee brace for 6 weeks. He was weight-bearing as tolerated when locked in full extension beginning immediately postoperatively, and allowed to unlock the brace to start non-weight-bearing active flexion and extension with therapy on postoperative day 1. Radiographs confirmed removal of the distal interlocking screw (Figures 4A, 4B). Following surgery, the patient experienced resolution of his effusions, no recurrent patellar instability at 1-year postoperative, and was able to return to his ADL and recreational sporting activities (Knee Injury and Osteoarthritis Outcome Score [KOOS] ADL, 100; KOOS sporting and recreational activities, 95; quality of life, 100; Marx Activity Rating Scale, 12).

DISCUSSION
The MPFL connects the superomedial edge of the patella to the medial femur and is injured in nearly 100% of patellar dislocations.6 The femoral origin lies between the adductor tubercle and the medial epicondyle.7 The MPFL prevents lateral subluxation of the patella and acts as the major restraint during the first 20° of knee flexion. Although radiographic parameters for identifying the MPFL femoral origin have been defined by both Schöttle and colleagues9 and Stephen and colleagues10, it is important to check the isometry intraoperatively through a ROM when performing MPFL reconstruction. In this case, the patient’s history and physical examination showed patellar instability, which was determined to be iatrogenically related to the distal interlocking screw rupture of the MPFL. Following screw removal and MPFL reconstruction, the patient had no further symptoms of pain, effusion, or patellar instability and returned to his normal activities.
Femoral malrotation following intramedullary nailing of femoral shaft fractures is a common complication,4 with a 22% incidence of malrotation of at least 15° in 1 series from an academic trauma center.11 There are mixed data as to whether malrotation is more common in complex fracture patterns, in cases performed during night hours, and in cases performed by non-trauma fellowship-trained surgeons.11-13 The natural history of malrotation is not well elucidated, but there is some suggestion that it alters load bearing in the distal joints of the involved leg including the patellofemoral joint. Patients also may not tolerate malrotation due to the abnormal foot progression angle, particularly with malrotation >15°.4 In this case, the patient’s initial femoral nail was placed in an externally rotated position, requiring revision. The result of this was an unusual trajectory of the distal interlocking screw from posterolateral to anteromedial. Combined with the prominent screw tip, the trajectory of this distal interlocking screw likely contributed to the injury to the MPFL observed in this case. This trajectory would also pose potential risk to the common peroneal nerve, which is usually situated posterior to the insertion point for distal femoral interlocking screws. The prominent distal interlock screw is a well-recognized problem with femoral intramedullary nails. This issue results from the tapering of the width of the distal femur from being larger posteriorly to being smaller anteriorly. To avoid placement of a prominent distal interlocking screw, surgeons often will obtain an intraoperative anterior-posterior radiograph with the lower extremity in 30° of internal rotation to account for the angle of the medial aspect of the distal femur.
This practice represents, to our knowledge, a previously unreported cause of patellar instability as well as an unreported complication of antegrade femoral intramedullary nailing. Surgeons treating these conditions should consider this potential complication and pursue advanced imaging if patients present with these complaints after femoral intramedullary nail placement. Knowledge of both MPFL origin and insertional anatomy and avoidance of prominent distal interlocking screws in the region of the MPFL, if possible, would likely prevent this complication.
Limitations of this study include the case report design, which makes it impossible to comment on the incidence of this complication or to make comparisons regarding treatment options. There is, of course, the possibility that the patient had a concurrent MPFL injury from the injury in which he sustained the femur fracture. Nevertheless, the clinical history, examination, imaging, and arthroscopic findings all strongly suggest that the prominent distal interlocking screw was the cause of his MPFL injury and patellar instability. Finally, the point widely defined by Schöttle and colleagues12 was used for MPFL reconstruction in this case based on an intraoperative true lateral radiograph of the distal femur. It should be noted that recent literature has debated the accuracy of this method for determining the femoral origin, the anatomy of the MPFL in relation to the quadriceps, and type of fixation for MPFL reconstruction with some advocating soft tissue only fixation.14-17 For purposes of this case report, we focused on a different cause of MPFL disruption in this patient and our technique for MPFL reconstruction.
CONCLUSION
This case demonstrates that iatrogenic MPFL injury is a potential complication of antegrade femoral nailing and a previously unrecognized cause of patellar instability. Surgeons should be aware of this potential complication and strive to avoid the MPFL origin when placing their distal interlocking screw.
This paper will be judged for the Resident Writer’s Award.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
- Brumback RJ, Virkus WW. Intramedullary nailing of the femur: reamed versus nonreamed. J Am Acad Orthop Surg. 2000;8(2):83-90.
- Ricci WM, Bellabarba C, Evanoff B, Herscovici D, DiPasquale T, Sanders R. Retrograde versus antegrade nailing of femoral shaft fractures. J Orthop Trauma 2001;15(3):161-169.
- Ricci WM, Gallagher B, Haidukewych GJ. Intramedullary nailing of femoral shaft fractures: current concepts. J Am Acad Orthop Surg. 2009;17(5):296-305.
- Lindsey JD, Krieg JC. Femoral malrotation following intramedullary nail fixation. J Am Acad Orthop Surg. 2011;19(1):17-26.
- Busam ML, Esther RJ, Obremskey WT. Hardware removal: indications and expectations. J Am Acad Orthop Surg. 2006;14(2):113-120.
- Morshed S, Humphrey M, Corrales LA, Millett M, Hoffinger SA. Retention of flexible intramedullary nails following treatment of pediatric femur fractures. Arch Orthop Trauma Surg. 2007;127(7):509-514.
- Boerger TO, Patel G, Murphy JP. Is routine removal of intramedullary nails justified. Injury. 1999;30(2):79-81.
- Kellan J. Fracture healing: Does hardware removal enhance patient outcomes. Chin J Orthop Trauma (Chin). 2010;12:374-378.
- Schöttle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35(5):801-804. doi:10.1177/0363546506296415.
- Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament: location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachments. Am J Sports Med. 2012;40(8):1871-1879. doi:10.1177/0363546512449998.
- Hüfner T, Citak M, Suero EM, et al. Femoral malrotation after unreamed intramedullary nailing: an evaluation of influencing operative factors. J Orthop Trauma. 2011;25(4):224-227. doi:10.1097/BOT.0b013e3181e47e3b.
- Ayalon OB, Patel NM, Yoon RS, Donegan DJ, Koerner JD, Liporace FA. Comparing femoral version after intramedullary nailing performed by trauma-trained and non-trauma trained surgeons: is there a difference? Injury. 2014;45(7):1091-1094. doi:10.1016/j.injury.2014.01.024.
- Patel NM, Yoon RS, Cantlon MB, Koerner JD, Donegan DJ, Liporace FA. Intramedullary nailing of diaphyseal femur fractures secondary to gunshot wounds: predictors of postoperative malrotation. J Orthop Trauma. 2014;28(12):711-714. doi:10.1097/BOT.0000000000000124.
- Ziegler CG, Fulkerson JP, Edgar C. Radiographic reference points are inaccurate with and without a true lateral radiograph: the importance of anatomy in medial patellofemoral ligament reconstruction. Am J Sports Med. 2016;44(1):133-142.
- Fulkerson JP, Edgar C. Medial quadriceps tendon-femoral ligament: surgical anatomy and reconstruction technique to prevent patella instability. Arthrosc Tech. 2013;2(2):e125-e128. doi:10.1016/j.eats.2013.01.002.
- Tanaka MJ, Voss A, Fulkerson JP. The anatomic midpoint of the attachment of the medial patellofemoral complex. J Bone Joint Surg Am. 2016;98(14):1199-1205. doi:10.2106/JBJS.15.01182.
- Mochizuki T, Nimura A, Tateishi T, Yamaguchi K, Muneta T, Akita K. Anatomic study of the attachment of the medial patellofemoral ligament and its characteristic relationships to the vastus intermedius. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):305-310. doi:10.1007/s00167-012-1993-7.
TAKE-HOME POINTS
- Anterograde intramedullary nailing is the gold standard for fixation of diaphyseal femur fractures.
- Damage to the MPFL can be caused by the distal interlocking screw of an anterograde intramedullary nail.
- The trajectory of the distal interlocking screw from posterolateral to anteromedial, and a prominent screw tip, likely contributed to the injury to the MPFL observed in this case.
- Surgeons treating these conditions should pursue advanced imaging if patients present with effusion and patellar instability after femoral intramedullary nail placement.
- Distal interlocking screw removal and arthroscopic MPFL reconstruction can result in successful return of function and normal activities.