User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Treatment of Grade III Acromioclavicular Separations in Professional Baseball Pitchers: A Survey of Major League Baseball Team Physicians
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Despite advancements in surgical technique and understanding of throwing mechanics, controversy persists regarding the treatment of grade III acromioclavicular (AC) joint separations, particularly in throwing athletes. Twenty-eight major league baseball (MLB) orthopedic team physicians were surveyed to determine their definitive management of a grade III AC separation in the dominant arm of a professional baseball pitcher and their experience treating AC joint separations in starting pitchers and position players. Return-to-play outcomes were also evaluated. Twenty (71.4%) team physicians recommended nonoperative intervention compared to 8 (28.6%) who would have operated acutely. Eighteen (64.3%) team physicians had treated at least 1 professional pitcher with a grade III AC separation; 51 (77.3%) pitchers had been treated nonoperatively compared to 15 (22.7%) operatively. No difference was observed in the proportion of pitchers who returned to the same level of play (P = .54), had full, unrestricted range of motion (P = .23), or had full pain relief (P = .19) between the operatively and nonoperatively treated MLB pitchers. The majority (53.6%) of physicians would not include an injection if the injury was treated nonoperatively. Open coracoclavicular reconstruction (65.2%) was preferred for operative cases; 66.7% of surgeons would also include distal clavicle excision as an adjunct procedure. About 90% of physicians would return pitchers to throwing >12 weeks after surgery compared to after 4 to 6 weeks in nonoperatively treated cases. In conclusion, MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in professional pitchers. If operative intervention is required, ligament reconstruction with adjunct distal clavicle excision were the most commonly performed procedures.
Continue to: Despite advancements in surgucal technique...
Despite advancements in surgical technique and improved understanding of the physiology of throwing mechanics, controversy persists regarding the preferred treatment for grade III acromioclavicular (AC) joint separations.1-6 Nonsurgical management has demonstrated return to prior function with fewer complications.7 However, there is a growing body of evidence demonstrating that surgical intervention is associated with more favorable outcomes8 and should be considered in patients who place high functional demands on their shoulders.9
The reported results on professional athletes in the literature remain ambivalent. Multiple small case reports/series have reported successful nonoperative treatment of elite athletes.10-12 Not surprisingly, McFarland and colleagues13 reported in 1997 that 69% of major league baseball (MLB) team physicians preferred nonoperative treatment for a theoretical starting pitcher sustaining a grade III AC separation 1 week prior to the start of the season. In contrast, reports of an inability to throw at a pre-injury level are equally commonplace.14,15 Nevertheless, all of these studies were limited to small cohorts, as the incidence of grade III AC separations in elite throwing athletes is relatively uncommon.13,16
In this study, we re-evaluated the study performed by McFarland and colleagues13 in 1997 by surveying all active MLB team orthopedic surgeons. We asked them how they would treat a grade III AC separation in a starting professional baseball pitcher. The physicians were also asked about their personal experience evaluating outcomes in these elite athletes. Given our improved understanding of the anatomy, pathophysiology, and surgical techniques for treating grade III AC separations, we hypothesize that more MLB team physicians would favor operative intervention treatment in professional baseball pitchers, as their vocation places higher demands on their shoulders.
MATERIALS AND METHODS
A questionnaire (Appendix A) was distributed to the team physicians of all 30 MLB teams. In addition to surgeon demographics, including age, years in practice, and years of taking care of an MLB team, the initial section of the questionnaire asked orthopedic surgeons how they would treat a theoretical starting pitcher who sustained a grade III AC joint separation of the dominant throwing arm 1 week prior to the start of the season. Physicians who preferred nonoperative treatment were asked whether they would use an injection (and what type), as well as when they would allow the pitcher to start a progressive interval throwing program. Physicians who preferred operative treatment were asked to rank their indications for operating, what procedure they would use (eg, open vs arthroscopic or coracoclavicular ligament repair vs reconstruction), and whether the surgical intervention would include distal clavicle excision. Both groups of physicians were also asked if their preferred treatment would change if the injury were to occur at the end of the season.
The second portion of the questionnaire asked surgeons about their experience treating AC joint separations in both starting pitchers and position players, as well as to describe the long-term outcomes of their preferred treatment, including time to return to full clearance for pitching, whether their patients returned to their prior level of play, and whether these patients had full pain relief. Finally, physicians were asked if any of the nonoperatively treated players ultimately crossed over and required operative intervention.
Continue to: Statistics...
STATISTICS
Descriptive statistics were used for continuous variables, and frequencies were used for categorical variables. Linear regression was performed to determine the correlation between the physician’s training or experience in treating AC joint separations and their recommended treatment. Fischer’s exact test/chi-square analysis was used to compare categorical variables. All tests were conducted using 2-sided hypothesis testing with statistical significance set at P < .05. All statistical analyses were conducted with SPSS 21.0 software (IBM Corporation).
RESULTS
A total of 28 MLB team physicians completed the questionnaires from 18 of the 30 MLB teams. The average age of the responders was 50.5 years (range, 34-60 years), with an average of 18.2 years in practice (range, 2-30 years) and 10.8 years (range, 1-24 years) taking care of their current professional baseball team. About 82% of the team physicians completed a sports medicine fellowship. On average, physicians saw 16.6 (range, 5-50) grade III or higher AC joint separations per year, and operated on 4.6 (range, 0-10) per year.
Nonoperative treatment was the preferred treatment for a grade III AC joint separation in a starting professional baseball pitcher for the majority of team physicians (20/28). No correlation was observed between the physician’s age (P = .881), years in practice (P = .915), years taking care of their professional team (P = .989), percentage of practice focused on shoulders (P = .986), number of AC joint injuries seen (P = .325), or number of surgeries performed per year (P = .807) with the team physician’s preferred treatment. Compared to the proportion reported originally by McFarland and colleagues13 in 1997 (69%), there was no difference in the proportion of team physicians that recommended nonoperative treatment (P = 1).
If treating this injury nonoperatively, 46.4% of physicians would also use an injection, with orthobiologics (eg, platelet-rich plasma) as the most popular choice (Table 1). No consensus was provided on the timeframe to return pitchers back to a progressive interval throwing program; however, 46.67% of physicians would return pitchers 4 to 6 weeks after a nonoperatively treated injury, while 35.7% would return pitchers 7 to 12 weeks after the initial injury.
Table 1. Treatment Preferences of Grade III AC Separation by MLB Team Physicians
Nonoperativea | |
Yes injection | 13 (46.4%) |
Cortisone | 3 (23.1%) |
Orthobiologic | 10 (76.9%) |
Local anesthetic (eg, lidocaine) | 1 (7.7%) |
Intramuscular toradol | 3 (23.1%) |
No injection | 15 (53.6%) |
Operativea | |
Open coracoclavicular ligament repair | 3 (13.0%) |
Open coracoclavicular ligament reconstruction | 15 (65.2%) |
Arthroscopic reconstruction with graft | 6 (26.1%) |
Arthroscopic repair with implant (ie, tight-rope) | 2 (8.7%) |
Distal clavicle excisionb | 16 (66.7%) |
Would not intervene operatively | 5 (17.9%) |
|
|
aRespondents were allowed to choose more than 1 treatment in each category. bChosen as an adjunct treatment.
Abbreviations: AC, acromioclavicular; MLB, major league baseball.
Most physicians (64.3%) cited functional limitations as the most important reason for indicating operative treatment, followed by pain (21.4%), and a deformity (14.3%). About 65% preferred open coracoclavicular ligament reconstruction. No physician recommended the Weaver-Dunn procedure or use of hardware (eg, hook plate). Of those who preferred an operative intervention, 66.7% would also include a distal clavicle excision, which is significantly higher than the proportion reported by McFarland and colleagues13 (23%, P = .0170). About 90% of physicians would return pitchers to play >12 weeks after operative treatment.
Continue to: If the injury occurred at the end ...
If the injury occurred at the end of the season, 7 of the 20 orthopedists (35%) who recommended nonoperative treatment said they would change to an operative intervention. Eighteen of 28 responders would have the same algorithm for MLB position players. Team physicians were less likely to recommend operative intervention in position players due to less demand on the arm and increased ability to accommodate the injury by altering their throwing mechanics.
Eighteen (64%) of the team physicians had treated at least 1 professional pitcher with a grade III AC separation in his dominant arm, and 11 (39.3%) had treated >1. Collectively, team physicians had treated 15 professional pitchers operatively, and 51 nonoperatively; only 3 patients converted to operative intervention after a failed nonoperative treatment.
Of the pitchers treated operatively, 93.3% (14) of pitchers returned to their prior level of pitching. The 1 patient who failed to return to the same level of pitching retired instead of returning to play. About 80% (12) of the pitchers had full pain relief, and 93.3% (14) had full range of motion (ROM). The pitcher who failed to regain full ROM also had a concomitant rotator cuff repair. The only complication reported from an operative intervention was a pitcher who sustained a coracoid fracture 10 months postoperatively while throwing 100 mph. Of the pitchers treated nonoperatively, 96% returned to their prior level of pitching, 92.2% (47) had full complete pain relief when throwing, and 100% had full ROM. No differences were observed between the proportion of pitchers who returned to their prior level of pitching, regained full ROM, or had full pain relief in the operative and nonoperative groups (Table 2).
Table 2. Outcomes of Treatment of Grade III AC Separation in 58 Professional Baseball Players
| Operative | Nonoperative | P-value |
Return to same level of play | 14/15 (93.3%) | 49/51 (96%) | 0.54 |
Full pain relief | 12/15 (80%) | 47/51 (92.2%) | 0.19 |
Full ROM | 14/15 (93.3%) | 51/51 (100%) | 0.23 |
Abbreviations: AC, acromioclavicular; ROM, range of motion.
DISCUSSION
Controversy persists regarding the optimal management of acute grade III AC separations, with the current available evidence potentially suggesting better cosmetic and radiological results but no definite differences in clinical results.1-6,17,18 In the absence of formal clinical practice guidelines, surgeons rely on their own experience or defer to the anecdotal experience of experts in the field. Our initial hypothesis was false in this survey of MLB team physicians taking care of overhead throwing athletes at the highest level. Our results demonstrate that despite improved techniques and an increased understanding of the pathophysiology of AC joint separations, conservative management is still the preferred treatment for acute grade III AC joint separations in professional baseball pitchers. The proportion of team physicians recommending nonoperative treatment in our series was essentially equivalent to the results reported by McFarland and colleagues13 in 1997, suggesting that the pendulum continues to favor conservative management initially. This status quo likely reflects both the dearth of literature suggesting a substantial benefit of acute operative repair, as well as the ability to accommodate with conservative measures after most grade III AC injuries, even at the highest level of athletic competition.
These results are also consistent with trends from the last few decades. In the 1970s, the overwhelming preference for treating an acute complete AC joint separation was surgical repair, with Powers and Bach10 reporting in a 1974 survey of 163 chairmen of orthopedic programs around the country that 91.5% advocated surgical treatment. However, surgical preference had reversed by the 1990s. Of the 187 chairmen and 59 team physicians surveyed by Cox19 in 1992, 72% and 86% respectively preferred nonoperative treatment in a theoretical 21-year-old athlete with a grade III AC separation. Nissen and Chatterjee20 reported in 2007 on a survey of all American Orthopaedic Society for Sports Medicine surgeons (N = 577) and Accreditation Council for Graduate Medical Education orthopedic program residency directors (N = 87) that >80% of responders preferred conservative measures for this acute injury. The reversal of trends has also been corroborated by recent multicenter trials demonstrating no difference in clinical outcomes between operative and nonoperative treatment of high grade AC joint dislocations, albeit these patients were not all high level overhead throwing athletes.17,18
Continue to: The trends in surgical interventions are notable...
The trends in surgical interventions are notable within the smaller subset of patients who are indicated for operative repair. Use of hardware and primary ligament repair, while popular in the surveys conducted in the 1970s10 and 1990s13 and even present in Nissen and Chatterjee’s20 2007 survey, were noticeably absent from our survey results, with the majority of respondents preferring open coracoclavicular ligament reconstruction. The role of distal clavicle excision has also expanded, from 23% of team physicians recommending it in 199713 to 57% to 59% in Nissen and Chatterjee’s20 2007 survey, to 66.7% in our series. This trend is not surprising as several recent cadaveric biomechanical studies have demonstrated that not only do peak graft forces not increase significantly,21 the anterior-posterior and superior-inferior motion at the AC joint following ligament reconstruction is maintained despite resection of the lateral clavicle.22 Additionally, primary distal clavicle excision may prevent the development of post-traumatic arthritis at the AC joint and osteolysis of the distal clavicle as a possible pain generator in the future.23 However, some respondents cautioned against performing a concomitant distal clavicle excision, as some biomechanical data demonstrate that resecting the distal clavicle may lead to increased horizontal translation at the AC joint despite intact superior and posterior AC capsules.24 Professional baseball pitchers may also be more lax and thus prone to more instability. Primary repair or reconstruction may not always lead to complete pre-injury stability in these individuals. This subtle unrecognized instability is hard to diagnosis and may be a persistent source of pain; thus, adding a distal clavicle excision may actually exacerbate the instability.
The nuanced indications for operative intervention, such as the presence of associated lesions were not captured by our survey.25 While most team physicians cited functional limitations as their most common reason for offering surgery, several MLB orthopedic surgeons also commented on evaluating the stability of the AC joint after a grade III injury, akin to the consensus statement from the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) Upper Extremity Committee26 in 2014 that diversified the Rockwood Grade III AC joint separation into its IIIA and IIIB classifications. The ISAKOS recommendations include initial conservative management and a second evaluation (both clinical and radiographic) for grade III lesions 3 to 6 weeks after the injury. However, as professional baseball is an incredibly profitable sport with an annual revenue approaching $9.5 billion27 and pitching salaries up to $32.5 million in 2015, serious financial considerations must be given to players who wish to avoid undergoing delayed surgery.
This study has shortcomings typical of expert opinion papers. The retrospective nature of this study places the data at risk of recall bias. Objective data (eg, terminal ROM, pain relief, and return to play) were obtained from a retrospective chart review; however, no standard documentation or collection method was used given the number of surgeons involved and, thus, conclusions based on treatment outcomes are imperfect. Another major weakness of this survey is the relatively small number of patients and respondents. An a priori power analysis was not available, as this was a retrospective review. A comparative trial will be necessary to definitively support one treatment over another. Assuming a 95% return to play in the nonoperatively treated group, approximately 300 patients would be needed in a prospective 2-armed study with 80% power to detect a 10% reduction in the incidence of return to play using an alpha level of 0.05 and assuming no loss to follow-up. This sample size would be difficult to achieve in this patient population.
However, compared to past series,13 the number of professional baseball players treated by the collective experience of these MLB team physicians is the largest reported to date. As suggested above, the rarity of this condition in elite athletes precludes the ability to have matched controls to definitively determine the optimal treatment, which may explain the lack of difference in the return to play, ROM, and pain relief outcomes. Instead, we can only extrapolate based on the collective anecdotal experience of the MLB team physicians.
CONCLUSION
Despite advances in surgical technique and understanding of throwing mechanics, the majority of MLB team physicians preferred nonoperative management for an acute grade III AC joint separation in a professional baseball pitcher. Open coracoclavicular ligament reconstruction was preferred for those who preferred operative intervention. An increasing number of orthopedic surgeons now consider a distal clavicle excision as an adjunct procedure.
This paper will be judged for the Resident Writer’s Award.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
- Spencer EE Jr. Treatment of grade III acromioclavicular joint injuries: a systematic review. Clin Orthop Relat Res. 2007;455:38-44. doi:10.1097/BLO.0b013e318030df83.
- Ceccarelli E, Bondì R, Alviti F, Garofalo R, Miulli F, Padua R. Treatment of acute grade III acromioclavicular dislocation: A lack of evidence. J Orthop Traumatol. 2008;9(2):105-108. doi:10.1007/s10195-008-0013-7.
- Smith TO, Chester R, Pearse EO, Hing CB. Operative versus non-operative management following rockwood grade III acromioclavicular separation: a meta-analysis of the current evidence base. J Orthop Traumatol. 2011;12(1):19-27. doi:10.1007/s10195-011-0127-1.
- Beitzel K, Cote MP, Apostolakos J, et al. Current concepts in the treatment of acromioclavicular joint dislocations. Arthroscopy. 2013;29(2):387-397. doi:10.1016/j.arthro.2012.11.023.
- Korsten K, Gunning AC, Leenen LP. Operative or conservative treatment in patients with rockwood type III acromioclavicular dislocation: a systematic review and update of current literature. Int Orthop. 2014;38(4):831-838. doi:10.1007/s00264-013-2143-7.
- Modi CS, Beazley J, Zywiel MG, Lawrence TM, Veillette CJ. Controversies relating to the management of acromioclavicular joint dislocations. Bone Joint J. 2013;95-B(12):1595-1602. doi:10.1302/0301-620X.95B12.31802.
- Reid D, Polson K, Johnson L. Acromioclavicular joint separations grades I-III: a review of the literature and development of best practice guidelines. Sports Med. 2012;42(8):681-696. doi:10.2165/11633460-000000000-00000.
- Farber AJ, Cascio BM, Wilckens JH. Type III acromioclavicular separation: rationale for anatomical reconstruction. Am J Orthop. 2008;37(7):349-355.
- Li X, Ma R, Bedi A, Dines DM, Altchek DW, Dines JS. Management of acromioclavicular joint injuries. J Bone Joint Surg Am. 2014;96(1):73-84. doi:10.2106/JBJS.L.00734.
- Powers JA, Bach PJ. Acromioclavicular separations. Closed or open treatment? Clin Orthop Relat Res. 1974;104(104):213-223. doi:10.1097/00003086-197410000-00024.
- Glick JM, Milburn LJ, Haggerty JF, Nishimoto D. Dislocated acromioclavicular joint: follow-up study of 35 unreduced acromioclavicular dislocations. Am J Sports Med. 1977;5(6):264-270. doi:10.1177/036354657700500614.
- Watson ST, Wyland DJ. Return to play after nonoperative management for a severe type III acromioclavicular separation in the throwing shoulder of a collegiate pitcher. Phys Sportsmed. 2015;43(1):99-103. doi:10.1080/00913847.2015.1001937.
- McFarland EG, Blivin SJ, Doehring CB, Curl LA, Silberstein C. Treatment of grade III acromioclavicular separations in professional throwing athletes: results of a survey. Am J Orthop. 1997;26(11):771-774.
- Wojtys EM, Nelson G. Conservative treatment of grade III acromioclavicular dislocations. Clin Orthop Relat Res. 1991;268(268):112-119.
- Galpin RD, Hawkins RJ, Grainger RW. A comparative analysis of operative versus nonoperative treatment of grade III acromioclavicular separations. Clin Orthop Relat Res. 1985;193(193):150-155. doi:10.1097/00003086-198503000-00020.
- Pallis M, Cameron KL, Svoboda SJ, Owens BD. Epidemiology of acromioclavicular joint injury in young athletes. Am J Sports Med. 2012;40(9):2072-2077. doi:10.1177/0363546512450162.
- Canadian Orthopaedic Trauma Society. Multicenter randomized clinical trial of nonoperative versus operative treatment of acute acromio-clavicular joint dislocation. J Orthop Trauma. 2015;29(11):479-487. doi:10.1097/BOT.0000000000000437.
- Joukainen A, Kröger H, Niemitukia L, Mäkelä EA, Väätäinen U. Results of operative and nonoperative treatment of rockwood types III and V acromioclavicular joint dislocation: a prospective, randomized trial with an 18- to 20-year follow-up. Orthop J Sports Med. 2014;2(12):2325967114560130. doi:10.1177/2325967114560130.
- Cox JS. Current method of treatment of acromioclavicular joint dislocations. Orthopedics. 1992;15(9):1041-1044.
- Nissen CW, Chatterjee A. Type III acromioclavicular separation: results of a recent survey on its management. Am J Orthop. 2007;36(2):89-93.
- Kowalsky MS, Kremenic IJ, Orishimo KF, McHugh MP, Nicholas SJ, Lee SJ. The effect of distal clavicle excision on in situ graft forces in coracoclavicular ligament reconstruction. Am J Sports Med. 2010;38(11):2313-2319. doi:10.1177/0363546510374447.
- Beaver AB, Parks BG, Hinton RY. Biomechanical analysis of distal clavicle excision with acromioclavicular joint reconstruction. Am J Sports Med. 2013;41(7):1684-1688. doi:10.1177/0363546513488750.
- Mumford EB. Acromioclavicular dislocation. J Bone Joint Surg Am. 1941;23:799-802.
- Beitzel K, Sablan N, Chowaniec DM, et al. Sequential resection of the distal clavicle and its effects on horizontal acromioclavicular joint translation. Am J Sports Med. 2012;40(3):681-685. doi:10.1177/0363546511428880.
- Arrigoni P, Brady PC, Zottarelli L, et al. Associated lesions requiring additional surgical treatment in grade 3 acromioclavicular joint dislocations. Arthroscopy. 2014;30(1):6-10. doi:10.1016/j.arthro.2013.10.006.
- Beitzel K, Mazzocca AD, Bak K, et al. ISAKOS upper extremity committee consensus statement on the need for diversification of the rockwood classification for acromioclavicular joint injuries. Arthroscopy. 2014;30(2):271-278. doi:10.1016/j.arthro.2013.11.005.
- Brown M. MLB sees record revenues for 2015, up $500 million and approaching $9.5 billion. Forbes Web site. http://www.forbes.com/sites/maurybrown/2015/12/04/mlb-sees-record-revenu.... Published December 4, 2015. Accessed February 4, 2016.
TAKE-HOME POINTS
- There was no difference in return to previous level of play between professional pitchers treated nonoperatively and operatively for grade III AC separation.
- MLB team physicians prefer nonoperative management for acute grade III AC joint separation in professional pitchers.
- The majority of MLB physicians do not use injections for nonoperative treatment of grade III AC separations; however, use of orthobiologics (eg, PRP) is becoming more commonplace.
- Persistent functional limitations and pain are the most common surgical indications for treatment of grade III AC separation in high level throwing athletes.
- If operative intervention is indicated for grade III AC separation, open coracoclavicular reconstruction and adjunct distal clavicle excision are preferred by most MLB team physicians.
Biomechanical Analysis of a Novel Buried Fixation Technique Using Headless Compression Screws for the Treatment of Patella Fractures
ABSTRACT
The traditional technique for patella fracture fixation utilizes prominent hardware. Prominent hardware use, however, results in a high rate of reoperation for symptomatic implant removal. This biomechanical study evaluates the effectiveness of a novel patella fixation technique that minimizes implant prominence.
Patellar transverse osteotomies were created in 13 pairs of cadaveric knees. Paired knees were assigned to either standard fixation (SF) using cannulated partially threaded screws and stainless steel wire tension band, or buried fixation (BF) using headless compression screws with a No. 2 FiberWire tension band and a No. 5 FiberWire cerclage suture. Quadriceps tendons were cyclically loaded to full extension followed by load to failure. The gap across the fracture site, stiffness, and load to failure were measured.
The differences in stiffness and load to failure between the 2 groups were not statistically significant. During cyclic loading, significantly greater gapping was observed across the fracture site in the BF group compared with SF group (P < .05).
Both constructs failed under loads that exceeded typical loads experienced during the postoperative rehabilitation period. Nevertheless, the BF technique demonstrated larger gap formation and a reduced load to failure than the SF technique. Further clinical studies are therefore underway to determine whether the use of constructs with decreased stability but increased patient comfort could improve clinical outcomes and reduce reoperation rates.
Continue to: Patella fractures are common...
Patella fractures are common injuries that can cause considerable disability to the knee extensor apparatus.1-3 Transverse patella fractures are the most common fracture pattern associated with patella fractures.{Harrell, 2003 #3}2 Given that the patella plays a crucial role in knee extensor biomechanics, its proper integrity is vital for physiological knee motion and ambulation.4 Traditionally, patella fractures with >2 mm of displacement have been managed with cannulated screws or Kirschner wires (K-wires) and a stainless-steel wire tension band.5-9 The goal in the treatment of patellar fractures is to reduce fracture fragments accurately and to minimize additional insults to the articular cartilage.10
Despite advances in surgical protocols and acceptable radiographic outcomes, functional impairment remains common after the treatment of patella fractures. Functional impairment includes knee pain, screw head pain, implant removal, wire breakage, and patella baja.1 The need for implant removal is one of the most common complications following the open reduction internal fixation of patella fractures.2,11 The subcutaneous and exposed nature of the patella in conjunction with soft tissue irritation resulting from standard fixation (SF) predisposes the patient toward prominence and discomfort with the retained implant. Although nonunion rates are low, the rate of implant removal can reach as high as 52%.2,10-12 To overcome some of these complications, we designed a novel buried fixation (BF) method for the treatment of transverse fractures. Our method minimizes the amount of exposed implant to improve patient comfort and potentially reduce the need for future implant removal. These effects are achieved by using headless compression screws and nonabsorbable sutures to attenuate the soft tissue irritation associated with traditional fixation.13 While our novel technique has demonstrated improved clinical results, it has not been tested biomechanically against a traditional fixation technique. Therefore, this study aims to evaluate and compare the structural integrity of our novel BF technique with that of the standard technique that uses cannulated screws and wire tension band. We hypothesized that the stability provided by our technique would be similar to that provided by SF for transverse patella fractures.
MATERIALS AND METHODS
SPECIMEN PREPARATION
Thirteen matched pairs of fresh-frozen human cadaveric knees were obtained from a Cedars-Sinai approved tissue bank. Specimens were cut midfemur and were intact to the foot. Legs with major structural bony or ligamentous abnormalities, extensor mechanism disruption, or septic knees were excluded from testing. To assess the bone quality of each specimen prior to testing, dual-energy X-ray absorptiometry was performed using a GE Lunar iDXA scanner (GE Healthcare). Specimens were stored at −30°C and thawed at room temperature for 24 hours prior to biomechanical testing.
A midline anterior approach to the patella was performed, and the extensor retinaculum, quadriceps tendon, and patellar tendon were exposed. A digital caliper was used to measure the craniocaudal and mediolateral dimensions of the patella, and a transverse osteotomy (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association [AO/OTA] type 34-C1) was created at the midway point between superior and inferior poles by using an oscillating saw. The retinaculum was then incised to the level of the midaxial line of the femur. One leg from each matched pair was allocated to the SF group, and the other was allocated to the BF group. Left and right legs were alternately assigned to each group to ensure that laterality was balanced between the 2 groups.
SURGICAL TECHNIQUE
The repair of the specimens in the SF group involved the use of 2 parallel 4.0-mm partially threaded cannulated screws (Acumed) and an 18-gauge monofilament steel wire (Ethicon) in a figure-eight tension band (Figure 1A). The repair of the specimens in the BF group involved the use of 2 parallel standard Acutrak headless compression screws (Acumed), a No. 2 FiberWire (Arthrex) in a figure-eight tension band, and a No. 5 FiberWire (Arthrex) was applied as cerclage around the patella (Figure 1B).
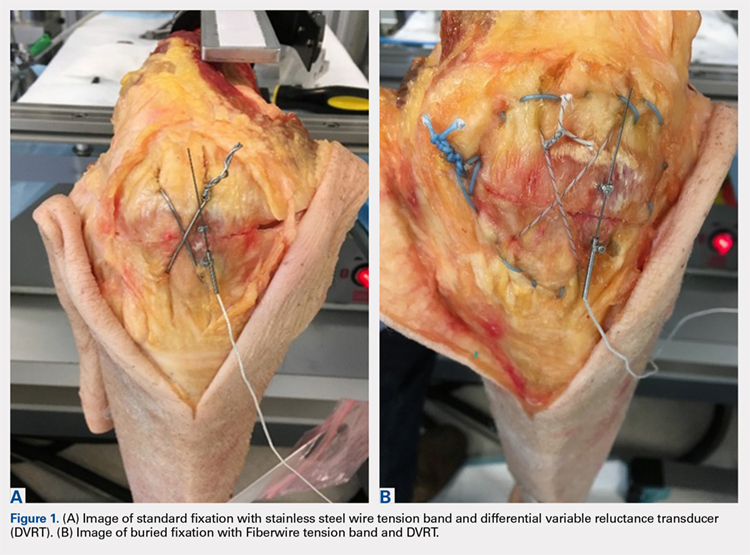
Continue to: Mechanical testing...
MECHANICAL TESTING
Mechanical testing was performed on a biaxial 370.02 Bionix Testing System (MTS Systems Corp.). The femur was rigidly and horizontally secured to a custom-built test frame, and the lower leg was left free to move. The quadriceps tendon was secured in a freeze clamp and was attached to the MTS actuator for loading via a pulley system such that raising the actuator was translated into a simulated quadriceps extensor force.
A differential variable reluctance transducer (DVRT) (Lord MicroStrain) was placed across the osteotomy site to measure the distraction, or gap, across the fracture line. The minimum load to full extension for each specimen was then determined under a slow, controlled increase in load until the leg was in a fully extended position. Any distraction across the fracture line during the initial loading phase was determined by using digital calipers. The specimen was then subjected to a preconditioning phase with 10 cycles from 0 N to full extension under the previously determined load, which was applied at the rate of 5 N/s. Meanwhile, displacement across the fracture site was recorded via the DVRT. Following the preconditioning phase, each specimen was then tested to failure in displacement control at the rate of 1.5 mm/s. Failure was defined as implant failure (screw pullout) or DVRT gapping across the osteotomy site >3 mm.10,14
Outcome measures included stiffness (N/mm), which was calculated as the slope of the linear change in load from full extension to failure vs DVRT displacement during the final loading phase; failure load (N); gapping (mm) across the osteotomy site at each cycle during the preconditioning phase; and failure mode (pullout vs >3.0 mm gap).
STATISTICAL ANALYSIS
An a priori power analysis revealed that 13 knees per group would be required to obtain an α of 0.05 and a power of 0.80. This calculation was based on a 20% difference in fracture displacement calculated by using the standard deviation and mean previously reported for cannulated screws with nonabsorbable sutures.14
Means and standard deviations for all dependent outcome measures were computed and compared across the independent measure of fixation type (BF vs SF) through repeated measures Analysis of variance (ANOVA-GLM, SAS 9.3, SAS Institute, Inc.) after controlling for bone mineral density (BMD), gender, and age. Multivariate repeated-measures ANOVA with Tukey's studentized range was applied to cyclic gap data. The mode of failure was compared across fixation type (BF vs SF) for matched data using McNemar’s test. Intracorrelations were computed and examined over all data and separately on the basis of screw fixation type (BF vs SF). All tests were considered statistically significant when P < .05.
Continue to: Results...
RESULTS
Specimen donors were 46% (6/13) male with an average age of 78.5 years (±13.77; range, 56-91 years) and 54% (7/13) female with an average age of 76.57 years (±14.37; range, 59-102 years). Average BMD was significantly lower in female (0.71 ± 0.18) than in male specimens (1.15 ± 0.33) (P < .05).
The average load to full extension across all specimens was 272 N (±54; range, 160-360 N) and was well balanced across matched pairs (270 ± 56 N for BF and 273 ± 54 N for SF). Of the 13 BF specimens, 4 experienced distraction across the fracture line during the determination of the minimum load to full extension. This initial pretest gap was measured with digital calipers (average, 1.5 mm; range, 0.90-1.85 mm) and added as an offset to the respective DVRT displacement data recorded during testing.
The total number of specimens included in the displacement data calculations decreased from 13 to 11 per group because DVRT data were not recorded during cyclic loading for 1 specimen and were considered unreliable in another. The maximum displacement measured across the fracture site during cyclic loading was significantly higher in the BF (0.94 ± 1.21) group than in the SF group (0.19 ± 0.26) as shown in the Table. The average slope of the gap per cycle for each specimen was calculated and compared between the BF and SF groups. The BF group demonstrated a significantly greater increase in gap per cycle than the SF group (Figure 2). Stiffness during load to failure was calculated for all but 1 specimen that did not display any measurable displacement during the final loading cycle. The average final stiffness and failure load between the BF and SF groups were not significantly different (Table). An equal number of specimens in both groups failed through gapping (6/13) and pullout (7/13).
Table. Means and Standard Deviations of the Main Outcome Measures
| Standard Fixation | Buried Fixation | N | P-value |
Load at Failure (N) | 1112.78 ± 457.25 | 973.20 ± 321.38 | 13 | 0.265 |
Final Stiffness (N/mm) | 358.42 ± 165.45 | 445.33 ± 310.09 | 11 | 0.175 |
Max Cyclic Gap (mm) | 0.19 ± 0.26 | 0.94 ± 1.21 | 11 | 0.026a |
Pullout: Gap Failure (ratio) | 7:6 | 7:6 | 13 | NS |
aIndicates statistical significance (P < .05).
Abbreviation: NS, not significant.
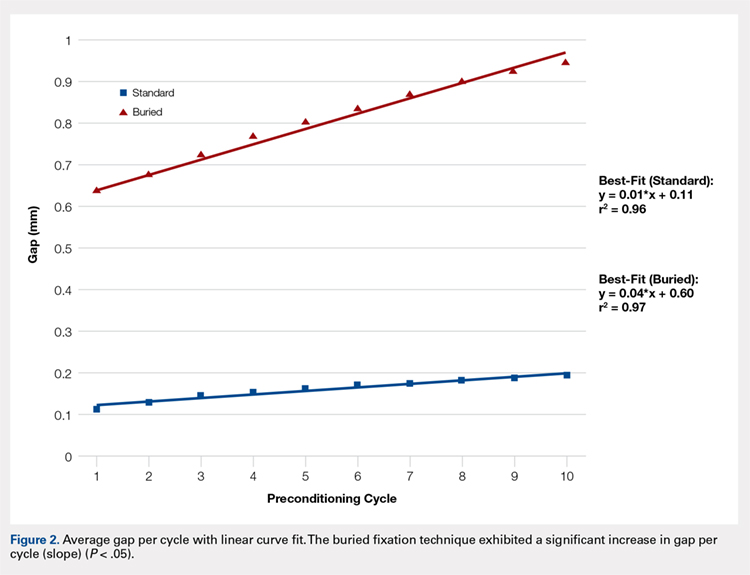
Failure load was significantly positively correlated with BMD (R = 0.62, P < .001) when all specimens were grouped together. When analyzed separately, the SF group was significantly correlated with BMD (P < .01), whereas the BF group had a marginally significant correlation (P = .06) with BMD (Figure 3). In both groups, BMD was positively correlated with stiffness and negatively correlated with gapping. Neither of these trends, however, was significant.
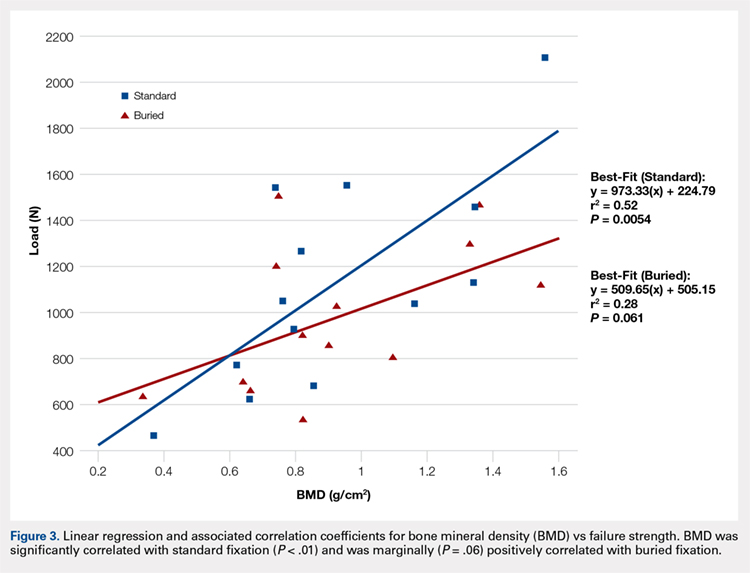
Continue to: Discussion...
DISCUSSION
We proposed a novel BF technique for the treatment of noncomminuted transverse patella fractures. Our technique utilizes headless cannulated compression screws and nonabsorbable suture tension bands. We then biomechanically compared our proposed technique with an established fixation technique that uses partially threaded cannulated screws and stainless steel wire tension bands. We hypothesized that the mechanical response of the BF technique to cyclic and failure loading would be similar to that of the SF technique. Our results demonstrate a significant increase in gap formation across the fracture site among knees and an overall reduced load to failure in the BF group (Figure 2). Whether these inferior results manifest clinically is not yet established. Both constructs could withstand forces that are typically experienced during the postoperative period. Given the high rate of symptomatic implant removal associated with the traditional technique, the low-profile buried technique might be an attractive alternative that provides increased patient comfort but may require an extended period of postoperative protection against bony ingrowths.
Patellar fixation constructs that use a combination of cannulated screws and a wire tension band provide the best resistance to patella fracture displacement when compared with screws or wires alone.4,15 Although this combination is biomechanically favorable, the steel wire often causes the painful irritation of the surrounding soft tissues and can break or migrate, thus increasing the rates of implant removal surgery to as high as 52%.4,10,12,15 We developed our novel BF technique, which uses headless compression screws and a No. 2 FiberWire tension band, to address the high rates of reoperation and patient dissatisfaction associated with the SF technique.
Headless compression screws have been successfully used in the reduction and fixation of scaphoid fractures and sesamoid fractures.16,17 The pull-out strengths of these screws are comparable with those of other commonly used screws, such as Twinfix and Herbert-Whipple screws.16 Similarly, the strength of a No. 5 FiberWire is comparable with that of an 18-gauge stainless-steel wire.14,18 Several studies have also obtained good outcomes with nonmetallic constructs that use nonabsorbable sutures alone.19,20 In this study, we utilized a No. 2 FiberWire as the tension band. The use of the No. 2 FiberWire facilitated threading through headless cannulated screws and created a low-profile knot. However, the use of thin FiberWire, despite a No. 5 FiberWire cerclage, likely contributed to the increase in distraction across the fracture.
The highest patellofemoral joint reaction force during level walking is approximately 35 kg (half body weight), which is equivalent to 350 N.15,21,22 This force is similar to the average cyclic load used in this experiment (272 ± 54 N). Gapping increased in the BF group but did not reach the defined failure value of 3 mm, and the ultimate load to failure was relatively high across both groups (SF, 1123 N; BF, 973 N). These results suggest that both fixation methods can withstand the typical patellofemoral joint forces that are experienced during the postoperative period.4 In addition, in a clinical setting, patients are placed in hinged knee braces for at least 2 weeks to limit their flexion angle and to allow for healing and bony ingrowth. Postoperative knee-brace protection presumably increases the overall strength of the fixation.
The number of specimens (n = 26) evaluated in this study was greater than that used in other biomechanical patella fracture studies.14 Furthermore, none of our specimens were reused. Our study design was further strengthened given that fellowship-trained trauma surgeons performed all surgical procedures. Finally, the data collection and analysis of numerous clinically relevant factors, such as BMD, age, and cyclical loading, contributed to the comprehensive description of each technique with respect to patient-specific criteria.
Similar to all cadaveric studies, our data only represent the immediate postoperative condition and does not represent any healing that would occur during postoperative rehabilitation. Postoperative knee-brace protection and bone healing across the fracture site would likely strengthen both constructs in a clinical setting. In addition, the average age of our specimens is 77.5 years, and therefore does not best represent the age range (20-50 years) of the typical adult population affected by patella fractures.3,23,24 Finally, postsurgical reduction was confirmed through visual inspection and not through fluoroscopy as in a clinical setting. Radiographic images were obtained after each experiment only to confirm screw placement post facto (Figures 4A, 4B).
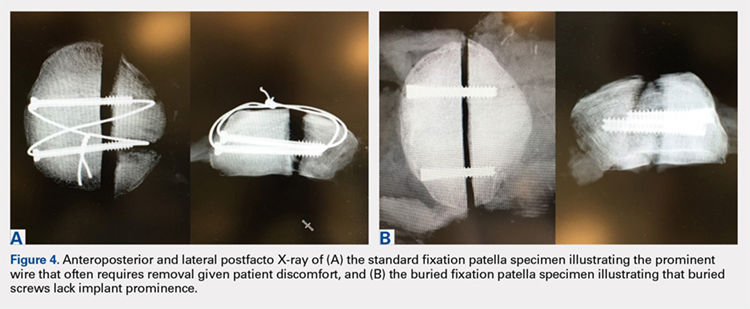
CONCLUSION
This study demonstrates the utility of a novel BF technique. Nevertheless, the proposed technique exhibited increased gapping and a lower load to failure than the current gold standard. The significance of these inferior results in clinical and functional settings has not been established. The proposed BF technique may be an appealing alternative to the SF technique given its low profile and potential to reduce the rates of future implant removal. Further studies on the long-term outcomes of patients treated through the BF technique are currently under way and will ultimately determine the utility of the proposed construct.
This paper will be judged for the Resident Writer’s Award.
- Lazaro LE, Wellman DS, Sauro G, et al. Outcomes after operative fixation of complete articular patellar fractures: assessment of functional impairment. J Bone Joint Surg Am. 2013;95(14):e96 1-8. doi:10.2106/JBJS.L.00012.
- Bostman O, Kiviluoto O, Santavirta S, Nirhamo J, Wilppula E. Fractures of the patella treated by operation. Arch Orthop Trauma Surg. 1983;102(2):78-81.
- Gwinner C, Märdian S, Schwabe P, Schaser KD, Krapohl BD, Jung TM. Current concepts review: fractures of the patella. GMS Interdiscip Plast Reconstr Surg DGPW. 2016;5:Doc01. doi:10.3205/iprs000080.
- Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
- Patel VR, Parks BG, Wang Y, Ebert FR, Jinnah RH. Fixation of patella fractures with braided polyester suture: a biomechanical study. Injury. 2000;31(1):1-6.
- Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
- Banks KE, Ambrose CG, Wheeless JS, Tissue CM, Sen M. An alternative patellar fracture fixation: a biomechanical study. J Orthop Trauma. 2013;27(6):345-351. doi:10.1097/BOT.0b013e31826623eb.
- Thelen S, Schneppendahl J, Baumgartner R, et al. Cyclic long-term loading of a bilateral fixed-angle plate in comparison with tension band wiring with K-wires or cannulated screws in transverse patella fractures. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):311-317. doi:10.1007/s00167-012-1999-1.
- Thelen S, Schneppendahl J, Jopen E, et al. Biomechanical cadaver testing of a fixed-angle plate in comparison to tension wiring and screw fixation in transverse patella fractures. Injury. 2012;43(8):1290-1295. doi:10.1016/j.injury.2012.04.020.
- LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426. doi:10.1097/BOT.0b013e318228c1a1.
- Dy CJ, Little MT, Berkes MB, et al. Meta-analysis of re-operation, nonunion, and infection after open reduction and internal fixation of patella fractures. J Trauma Acute Care Surg. 2012;73(4):928-932. doi:10.1097/TA.0b013e31825168b6.
- Smith ST, Cramer KE, Karges DE, Watson JT, Moed BR. Early complications in the operative treatment of patella fractures. J Orthop Trauma. 1997;11(3):183-187.
- Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
- Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: A pilot study. J Orthop. 2015;12(2):92-96. doi:10.1016/j.jor.2014.04.011.
- Burvant JG, Thomas KA, Alexander R, Harris MB. Evaluation of methods of internal fixation of transverse patella fractures: a biomechanical study. J Orthop Trauma. 1994;8(2):147-153.
- Crawford LA, Powell ES, Trail IA. The fixation strength of scaphoid bone screws: an in vitro investigation using polyurethane foam. J Hand Surg Am. 2012;37(2):255-260. doi:10.1016/j.jhsa.2011.10.021.
- Eddy AL, Galuppo LD, Stover SM, Taylor KT, Jensen DG. A biomechanical comparison of headless tapered variable pitch compression and ao cortical bone screws for fixation of a simulated midbody transverse fracture of the proximal sesamoid bone in horses. Vet Surg. 2004;33(3):253-262. doi:10.1111/j.1532-950X.2004.04037.x.
- Camarda L, La Gattuta A, Butera M, Siragusa F, D'Arienzo M. FiberWire tension band for patellar fractures. J Orthop Traumatol. 2016;17(1):75-80. doi:10.1007/s10195-015-0359-6.
- Camarda L, Morello S, Balistreri F, D'Arienzo A, D'Arienzo M. Non-metallic implant for patellar fracture fixation: A systematic review. Injury. 2016;47(8):1613-1617. doi:10.1016/j.injury.2016.05.039.
- Han F, Pearce CJ, Ng DQ, et al. A double button adjustable loop device is biomechanically equivalent to tension band wire in the fixation of transverse patellar fractures-A cadaveric study. Injury. 2017;48(2):270-276. doi:10.1016/j.injury.2016.11.013.
- Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126-137. doi:10.1016/j.injury.2016.11.013.
- Buff HU, Jones LC, Hungerford DS. Experimental determination of forces transmitted through the patello-femoral joint. J Biomech. 1988;21(1):17-23.
- Bostrom A. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37(8):691-697. doi:10.1111/iwj.12675.
ABSTRACT
The traditional technique for patella fracture fixation utilizes prominent hardware. Prominent hardware use, however, results in a high rate of reoperation for symptomatic implant removal. This biomechanical study evaluates the effectiveness of a novel patella fixation technique that minimizes implant prominence.
Patellar transverse osteotomies were created in 13 pairs of cadaveric knees. Paired knees were assigned to either standard fixation (SF) using cannulated partially threaded screws and stainless steel wire tension band, or buried fixation (BF) using headless compression screws with a No. 2 FiberWire tension band and a No. 5 FiberWire cerclage suture. Quadriceps tendons were cyclically loaded to full extension followed by load to failure. The gap across the fracture site, stiffness, and load to failure were measured.
The differences in stiffness and load to failure between the 2 groups were not statistically significant. During cyclic loading, significantly greater gapping was observed across the fracture site in the BF group compared with SF group (P < .05).
Both constructs failed under loads that exceeded typical loads experienced during the postoperative rehabilitation period. Nevertheless, the BF technique demonstrated larger gap formation and a reduced load to failure than the SF technique. Further clinical studies are therefore underway to determine whether the use of constructs with decreased stability but increased patient comfort could improve clinical outcomes and reduce reoperation rates.
Continue to: Patella fractures are common...
Patella fractures are common injuries that can cause considerable disability to the knee extensor apparatus.1-3 Transverse patella fractures are the most common fracture pattern associated with patella fractures.{Harrell, 2003 #3}2 Given that the patella plays a crucial role in knee extensor biomechanics, its proper integrity is vital for physiological knee motion and ambulation.4 Traditionally, patella fractures with >2 mm of displacement have been managed with cannulated screws or Kirschner wires (K-wires) and a stainless-steel wire tension band.5-9 The goal in the treatment of patellar fractures is to reduce fracture fragments accurately and to minimize additional insults to the articular cartilage.10
Despite advances in surgical protocols and acceptable radiographic outcomes, functional impairment remains common after the treatment of patella fractures. Functional impairment includes knee pain, screw head pain, implant removal, wire breakage, and patella baja.1 The need for implant removal is one of the most common complications following the open reduction internal fixation of patella fractures.2,11 The subcutaneous and exposed nature of the patella in conjunction with soft tissue irritation resulting from standard fixation (SF) predisposes the patient toward prominence and discomfort with the retained implant. Although nonunion rates are low, the rate of implant removal can reach as high as 52%.2,10-12 To overcome some of these complications, we designed a novel buried fixation (BF) method for the treatment of transverse fractures. Our method minimizes the amount of exposed implant to improve patient comfort and potentially reduce the need for future implant removal. These effects are achieved by using headless compression screws and nonabsorbable sutures to attenuate the soft tissue irritation associated with traditional fixation.13 While our novel technique has demonstrated improved clinical results, it has not been tested biomechanically against a traditional fixation technique. Therefore, this study aims to evaluate and compare the structural integrity of our novel BF technique with that of the standard technique that uses cannulated screws and wire tension band. We hypothesized that the stability provided by our technique would be similar to that provided by SF for transverse patella fractures.
MATERIALS AND METHODS
SPECIMEN PREPARATION
Thirteen matched pairs of fresh-frozen human cadaveric knees were obtained from a Cedars-Sinai approved tissue bank. Specimens were cut midfemur and were intact to the foot. Legs with major structural bony or ligamentous abnormalities, extensor mechanism disruption, or septic knees were excluded from testing. To assess the bone quality of each specimen prior to testing, dual-energy X-ray absorptiometry was performed using a GE Lunar iDXA scanner (GE Healthcare). Specimens were stored at −30°C and thawed at room temperature for 24 hours prior to biomechanical testing.
A midline anterior approach to the patella was performed, and the extensor retinaculum, quadriceps tendon, and patellar tendon were exposed. A digital caliper was used to measure the craniocaudal and mediolateral dimensions of the patella, and a transverse osteotomy (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association [AO/OTA] type 34-C1) was created at the midway point between superior and inferior poles by using an oscillating saw. The retinaculum was then incised to the level of the midaxial line of the femur. One leg from each matched pair was allocated to the SF group, and the other was allocated to the BF group. Left and right legs were alternately assigned to each group to ensure that laterality was balanced between the 2 groups.
SURGICAL TECHNIQUE
The repair of the specimens in the SF group involved the use of 2 parallel 4.0-mm partially threaded cannulated screws (Acumed) and an 18-gauge monofilament steel wire (Ethicon) in a figure-eight tension band (Figure 1A). The repair of the specimens in the BF group involved the use of 2 parallel standard Acutrak headless compression screws (Acumed), a No. 2 FiberWire (Arthrex) in a figure-eight tension band, and a No. 5 FiberWire (Arthrex) was applied as cerclage around the patella (Figure 1B).

Continue to: Mechanical testing...
MECHANICAL TESTING
Mechanical testing was performed on a biaxial 370.02 Bionix Testing System (MTS Systems Corp.). The femur was rigidly and horizontally secured to a custom-built test frame, and the lower leg was left free to move. The quadriceps tendon was secured in a freeze clamp and was attached to the MTS actuator for loading via a pulley system such that raising the actuator was translated into a simulated quadriceps extensor force.
A differential variable reluctance transducer (DVRT) (Lord MicroStrain) was placed across the osteotomy site to measure the distraction, or gap, across the fracture line. The minimum load to full extension for each specimen was then determined under a slow, controlled increase in load until the leg was in a fully extended position. Any distraction across the fracture line during the initial loading phase was determined by using digital calipers. The specimen was then subjected to a preconditioning phase with 10 cycles from 0 N to full extension under the previously determined load, which was applied at the rate of 5 N/s. Meanwhile, displacement across the fracture site was recorded via the DVRT. Following the preconditioning phase, each specimen was then tested to failure in displacement control at the rate of 1.5 mm/s. Failure was defined as implant failure (screw pullout) or DVRT gapping across the osteotomy site >3 mm.10,14
Outcome measures included stiffness (N/mm), which was calculated as the slope of the linear change in load from full extension to failure vs DVRT displacement during the final loading phase; failure load (N); gapping (mm) across the osteotomy site at each cycle during the preconditioning phase; and failure mode (pullout vs >3.0 mm gap).
STATISTICAL ANALYSIS
An a priori power analysis revealed that 13 knees per group would be required to obtain an α of 0.05 and a power of 0.80. This calculation was based on a 20% difference in fracture displacement calculated by using the standard deviation and mean previously reported for cannulated screws with nonabsorbable sutures.14
Means and standard deviations for all dependent outcome measures were computed and compared across the independent measure of fixation type (BF vs SF) through repeated measures Analysis of variance (ANOVA-GLM, SAS 9.3, SAS Institute, Inc.) after controlling for bone mineral density (BMD), gender, and age. Multivariate repeated-measures ANOVA with Tukey's studentized range was applied to cyclic gap data. The mode of failure was compared across fixation type (BF vs SF) for matched data using McNemar’s test. Intracorrelations were computed and examined over all data and separately on the basis of screw fixation type (BF vs SF). All tests were considered statistically significant when P < .05.
Continue to: Results...
RESULTS
Specimen donors were 46% (6/13) male with an average age of 78.5 years (±13.77; range, 56-91 years) and 54% (7/13) female with an average age of 76.57 years (±14.37; range, 59-102 years). Average BMD was significantly lower in female (0.71 ± 0.18) than in male specimens (1.15 ± 0.33) (P < .05).
The average load to full extension across all specimens was 272 N (±54; range, 160-360 N) and was well balanced across matched pairs (270 ± 56 N for BF and 273 ± 54 N for SF). Of the 13 BF specimens, 4 experienced distraction across the fracture line during the determination of the minimum load to full extension. This initial pretest gap was measured with digital calipers (average, 1.5 mm; range, 0.90-1.85 mm) and added as an offset to the respective DVRT displacement data recorded during testing.
The total number of specimens included in the displacement data calculations decreased from 13 to 11 per group because DVRT data were not recorded during cyclic loading for 1 specimen and were considered unreliable in another. The maximum displacement measured across the fracture site during cyclic loading was significantly higher in the BF (0.94 ± 1.21) group than in the SF group (0.19 ± 0.26) as shown in the Table. The average slope of the gap per cycle for each specimen was calculated and compared between the BF and SF groups. The BF group demonstrated a significantly greater increase in gap per cycle than the SF group (Figure 2). Stiffness during load to failure was calculated for all but 1 specimen that did not display any measurable displacement during the final loading cycle. The average final stiffness and failure load between the BF and SF groups were not significantly different (Table). An equal number of specimens in both groups failed through gapping (6/13) and pullout (7/13).
Table. Means and Standard Deviations of the Main Outcome Measures
| Standard Fixation | Buried Fixation | N | P-value |
Load at Failure (N) | 1112.78 ± 457.25 | 973.20 ± 321.38 | 13 | 0.265 |
Final Stiffness (N/mm) | 358.42 ± 165.45 | 445.33 ± 310.09 | 11 | 0.175 |
Max Cyclic Gap (mm) | 0.19 ± 0.26 | 0.94 ± 1.21 | 11 | 0.026a |
Pullout: Gap Failure (ratio) | 7:6 | 7:6 | 13 | NS |
aIndicates statistical significance (P < .05).
Abbreviation: NS, not significant.

Failure load was significantly positively correlated with BMD (R = 0.62, P < .001) when all specimens were grouped together. When analyzed separately, the SF group was significantly correlated with BMD (P < .01), whereas the BF group had a marginally significant correlation (P = .06) with BMD (Figure 3). In both groups, BMD was positively correlated with stiffness and negatively correlated with gapping. Neither of these trends, however, was significant.

Continue to: Discussion...
DISCUSSION
We proposed a novel BF technique for the treatment of noncomminuted transverse patella fractures. Our technique utilizes headless cannulated compression screws and nonabsorbable suture tension bands. We then biomechanically compared our proposed technique with an established fixation technique that uses partially threaded cannulated screws and stainless steel wire tension bands. We hypothesized that the mechanical response of the BF technique to cyclic and failure loading would be similar to that of the SF technique. Our results demonstrate a significant increase in gap formation across the fracture site among knees and an overall reduced load to failure in the BF group (Figure 2). Whether these inferior results manifest clinically is not yet established. Both constructs could withstand forces that are typically experienced during the postoperative period. Given the high rate of symptomatic implant removal associated with the traditional technique, the low-profile buried technique might be an attractive alternative that provides increased patient comfort but may require an extended period of postoperative protection against bony ingrowths.
Patellar fixation constructs that use a combination of cannulated screws and a wire tension band provide the best resistance to patella fracture displacement when compared with screws or wires alone.4,15 Although this combination is biomechanically favorable, the steel wire often causes the painful irritation of the surrounding soft tissues and can break or migrate, thus increasing the rates of implant removal surgery to as high as 52%.4,10,12,15 We developed our novel BF technique, which uses headless compression screws and a No. 2 FiberWire tension band, to address the high rates of reoperation and patient dissatisfaction associated with the SF technique.
Headless compression screws have been successfully used in the reduction and fixation of scaphoid fractures and sesamoid fractures.16,17 The pull-out strengths of these screws are comparable with those of other commonly used screws, such as Twinfix and Herbert-Whipple screws.16 Similarly, the strength of a No. 5 FiberWire is comparable with that of an 18-gauge stainless-steel wire.14,18 Several studies have also obtained good outcomes with nonmetallic constructs that use nonabsorbable sutures alone.19,20 In this study, we utilized a No. 2 FiberWire as the tension band. The use of the No. 2 FiberWire facilitated threading through headless cannulated screws and created a low-profile knot. However, the use of thin FiberWire, despite a No. 5 FiberWire cerclage, likely contributed to the increase in distraction across the fracture.
The highest patellofemoral joint reaction force during level walking is approximately 35 kg (half body weight), which is equivalent to 350 N.15,21,22 This force is similar to the average cyclic load used in this experiment (272 ± 54 N). Gapping increased in the BF group but did not reach the defined failure value of 3 mm, and the ultimate load to failure was relatively high across both groups (SF, 1123 N; BF, 973 N). These results suggest that both fixation methods can withstand the typical patellofemoral joint forces that are experienced during the postoperative period.4 In addition, in a clinical setting, patients are placed in hinged knee braces for at least 2 weeks to limit their flexion angle and to allow for healing and bony ingrowth. Postoperative knee-brace protection presumably increases the overall strength of the fixation.
The number of specimens (n = 26) evaluated in this study was greater than that used in other biomechanical patella fracture studies.14 Furthermore, none of our specimens were reused. Our study design was further strengthened given that fellowship-trained trauma surgeons performed all surgical procedures. Finally, the data collection and analysis of numerous clinically relevant factors, such as BMD, age, and cyclical loading, contributed to the comprehensive description of each technique with respect to patient-specific criteria.
Similar to all cadaveric studies, our data only represent the immediate postoperative condition and does not represent any healing that would occur during postoperative rehabilitation. Postoperative knee-brace protection and bone healing across the fracture site would likely strengthen both constructs in a clinical setting. In addition, the average age of our specimens is 77.5 years, and therefore does not best represent the age range (20-50 years) of the typical adult population affected by patella fractures.3,23,24 Finally, postsurgical reduction was confirmed through visual inspection and not through fluoroscopy as in a clinical setting. Radiographic images were obtained after each experiment only to confirm screw placement post facto (Figures 4A, 4B).

CONCLUSION
This study demonstrates the utility of a novel BF technique. Nevertheless, the proposed technique exhibited increased gapping and a lower load to failure than the current gold standard. The significance of these inferior results in clinical and functional settings has not been established. The proposed BF technique may be an appealing alternative to the SF technique given its low profile and potential to reduce the rates of future implant removal. Further studies on the long-term outcomes of patients treated through the BF technique are currently under way and will ultimately determine the utility of the proposed construct.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The traditional technique for patella fracture fixation utilizes prominent hardware. Prominent hardware use, however, results in a high rate of reoperation for symptomatic implant removal. This biomechanical study evaluates the effectiveness of a novel patella fixation technique that minimizes implant prominence.
Patellar transverse osteotomies were created in 13 pairs of cadaveric knees. Paired knees were assigned to either standard fixation (SF) using cannulated partially threaded screws and stainless steel wire tension band, or buried fixation (BF) using headless compression screws with a No. 2 FiberWire tension band and a No. 5 FiberWire cerclage suture. Quadriceps tendons were cyclically loaded to full extension followed by load to failure. The gap across the fracture site, stiffness, and load to failure were measured.
The differences in stiffness and load to failure between the 2 groups were not statistically significant. During cyclic loading, significantly greater gapping was observed across the fracture site in the BF group compared with SF group (P < .05).
Both constructs failed under loads that exceeded typical loads experienced during the postoperative rehabilitation period. Nevertheless, the BF technique demonstrated larger gap formation and a reduced load to failure than the SF technique. Further clinical studies are therefore underway to determine whether the use of constructs with decreased stability but increased patient comfort could improve clinical outcomes and reduce reoperation rates.
Continue to: Patella fractures are common...
Patella fractures are common injuries that can cause considerable disability to the knee extensor apparatus.1-3 Transverse patella fractures are the most common fracture pattern associated with patella fractures.{Harrell, 2003 #3}2 Given that the patella plays a crucial role in knee extensor biomechanics, its proper integrity is vital for physiological knee motion and ambulation.4 Traditionally, patella fractures with >2 mm of displacement have been managed with cannulated screws or Kirschner wires (K-wires) and a stainless-steel wire tension band.5-9 The goal in the treatment of patellar fractures is to reduce fracture fragments accurately and to minimize additional insults to the articular cartilage.10
Despite advances in surgical protocols and acceptable radiographic outcomes, functional impairment remains common after the treatment of patella fractures. Functional impairment includes knee pain, screw head pain, implant removal, wire breakage, and patella baja.1 The need for implant removal is one of the most common complications following the open reduction internal fixation of patella fractures.2,11 The subcutaneous and exposed nature of the patella in conjunction with soft tissue irritation resulting from standard fixation (SF) predisposes the patient toward prominence and discomfort with the retained implant. Although nonunion rates are low, the rate of implant removal can reach as high as 52%.2,10-12 To overcome some of these complications, we designed a novel buried fixation (BF) method for the treatment of transverse fractures. Our method minimizes the amount of exposed implant to improve patient comfort and potentially reduce the need for future implant removal. These effects are achieved by using headless compression screws and nonabsorbable sutures to attenuate the soft tissue irritation associated with traditional fixation.13 While our novel technique has demonstrated improved clinical results, it has not been tested biomechanically against a traditional fixation technique. Therefore, this study aims to evaluate and compare the structural integrity of our novel BF technique with that of the standard technique that uses cannulated screws and wire tension band. We hypothesized that the stability provided by our technique would be similar to that provided by SF for transverse patella fractures.
MATERIALS AND METHODS
SPECIMEN PREPARATION
Thirteen matched pairs of fresh-frozen human cadaveric knees were obtained from a Cedars-Sinai approved tissue bank. Specimens were cut midfemur and were intact to the foot. Legs with major structural bony or ligamentous abnormalities, extensor mechanism disruption, or septic knees were excluded from testing. To assess the bone quality of each specimen prior to testing, dual-energy X-ray absorptiometry was performed using a GE Lunar iDXA scanner (GE Healthcare). Specimens were stored at −30°C and thawed at room temperature for 24 hours prior to biomechanical testing.
A midline anterior approach to the patella was performed, and the extensor retinaculum, quadriceps tendon, and patellar tendon were exposed. A digital caliper was used to measure the craniocaudal and mediolateral dimensions of the patella, and a transverse osteotomy (Arbeitsgemeinschaft für Osteosynthesefragen/Orthopaedic Trauma Association [AO/OTA] type 34-C1) was created at the midway point between superior and inferior poles by using an oscillating saw. The retinaculum was then incised to the level of the midaxial line of the femur. One leg from each matched pair was allocated to the SF group, and the other was allocated to the BF group. Left and right legs were alternately assigned to each group to ensure that laterality was balanced between the 2 groups.
SURGICAL TECHNIQUE
The repair of the specimens in the SF group involved the use of 2 parallel 4.0-mm partially threaded cannulated screws (Acumed) and an 18-gauge monofilament steel wire (Ethicon) in a figure-eight tension band (Figure 1A). The repair of the specimens in the BF group involved the use of 2 parallel standard Acutrak headless compression screws (Acumed), a No. 2 FiberWire (Arthrex) in a figure-eight tension band, and a No. 5 FiberWire (Arthrex) was applied as cerclage around the patella (Figure 1B).

Continue to: Mechanical testing...
MECHANICAL TESTING
Mechanical testing was performed on a biaxial 370.02 Bionix Testing System (MTS Systems Corp.). The femur was rigidly and horizontally secured to a custom-built test frame, and the lower leg was left free to move. The quadriceps tendon was secured in a freeze clamp and was attached to the MTS actuator for loading via a pulley system such that raising the actuator was translated into a simulated quadriceps extensor force.
A differential variable reluctance transducer (DVRT) (Lord MicroStrain) was placed across the osteotomy site to measure the distraction, or gap, across the fracture line. The minimum load to full extension for each specimen was then determined under a slow, controlled increase in load until the leg was in a fully extended position. Any distraction across the fracture line during the initial loading phase was determined by using digital calipers. The specimen was then subjected to a preconditioning phase with 10 cycles from 0 N to full extension under the previously determined load, which was applied at the rate of 5 N/s. Meanwhile, displacement across the fracture site was recorded via the DVRT. Following the preconditioning phase, each specimen was then tested to failure in displacement control at the rate of 1.5 mm/s. Failure was defined as implant failure (screw pullout) or DVRT gapping across the osteotomy site >3 mm.10,14
Outcome measures included stiffness (N/mm), which was calculated as the slope of the linear change in load from full extension to failure vs DVRT displacement during the final loading phase; failure load (N); gapping (mm) across the osteotomy site at each cycle during the preconditioning phase; and failure mode (pullout vs >3.0 mm gap).
STATISTICAL ANALYSIS
An a priori power analysis revealed that 13 knees per group would be required to obtain an α of 0.05 and a power of 0.80. This calculation was based on a 20% difference in fracture displacement calculated by using the standard deviation and mean previously reported for cannulated screws with nonabsorbable sutures.14
Means and standard deviations for all dependent outcome measures were computed and compared across the independent measure of fixation type (BF vs SF) through repeated measures Analysis of variance (ANOVA-GLM, SAS 9.3, SAS Institute, Inc.) after controlling for bone mineral density (BMD), gender, and age. Multivariate repeated-measures ANOVA with Tukey's studentized range was applied to cyclic gap data. The mode of failure was compared across fixation type (BF vs SF) for matched data using McNemar’s test. Intracorrelations were computed and examined over all data and separately on the basis of screw fixation type (BF vs SF). All tests were considered statistically significant when P < .05.
Continue to: Results...
RESULTS
Specimen donors were 46% (6/13) male with an average age of 78.5 years (±13.77; range, 56-91 years) and 54% (7/13) female with an average age of 76.57 years (±14.37; range, 59-102 years). Average BMD was significantly lower in female (0.71 ± 0.18) than in male specimens (1.15 ± 0.33) (P < .05).
The average load to full extension across all specimens was 272 N (±54; range, 160-360 N) and was well balanced across matched pairs (270 ± 56 N for BF and 273 ± 54 N for SF). Of the 13 BF specimens, 4 experienced distraction across the fracture line during the determination of the minimum load to full extension. This initial pretest gap was measured with digital calipers (average, 1.5 mm; range, 0.90-1.85 mm) and added as an offset to the respective DVRT displacement data recorded during testing.
The total number of specimens included in the displacement data calculations decreased from 13 to 11 per group because DVRT data were not recorded during cyclic loading for 1 specimen and were considered unreliable in another. The maximum displacement measured across the fracture site during cyclic loading was significantly higher in the BF (0.94 ± 1.21) group than in the SF group (0.19 ± 0.26) as shown in the Table. The average slope of the gap per cycle for each specimen was calculated and compared between the BF and SF groups. The BF group demonstrated a significantly greater increase in gap per cycle than the SF group (Figure 2). Stiffness during load to failure was calculated for all but 1 specimen that did not display any measurable displacement during the final loading cycle. The average final stiffness and failure load between the BF and SF groups were not significantly different (Table). An equal number of specimens in both groups failed through gapping (6/13) and pullout (7/13).
Table. Means and Standard Deviations of the Main Outcome Measures
| Standard Fixation | Buried Fixation | N | P-value |
Load at Failure (N) | 1112.78 ± 457.25 | 973.20 ± 321.38 | 13 | 0.265 |
Final Stiffness (N/mm) | 358.42 ± 165.45 | 445.33 ± 310.09 | 11 | 0.175 |
Max Cyclic Gap (mm) | 0.19 ± 0.26 | 0.94 ± 1.21 | 11 | 0.026a |
Pullout: Gap Failure (ratio) | 7:6 | 7:6 | 13 | NS |
aIndicates statistical significance (P < .05).
Abbreviation: NS, not significant.

Failure load was significantly positively correlated with BMD (R = 0.62, P < .001) when all specimens were grouped together. When analyzed separately, the SF group was significantly correlated with BMD (P < .01), whereas the BF group had a marginally significant correlation (P = .06) with BMD (Figure 3). In both groups, BMD was positively correlated with stiffness and negatively correlated with gapping. Neither of these trends, however, was significant.

Continue to: Discussion...
DISCUSSION
We proposed a novel BF technique for the treatment of noncomminuted transverse patella fractures. Our technique utilizes headless cannulated compression screws and nonabsorbable suture tension bands. We then biomechanically compared our proposed technique with an established fixation technique that uses partially threaded cannulated screws and stainless steel wire tension bands. We hypothesized that the mechanical response of the BF technique to cyclic and failure loading would be similar to that of the SF technique. Our results demonstrate a significant increase in gap formation across the fracture site among knees and an overall reduced load to failure in the BF group (Figure 2). Whether these inferior results manifest clinically is not yet established. Both constructs could withstand forces that are typically experienced during the postoperative period. Given the high rate of symptomatic implant removal associated with the traditional technique, the low-profile buried technique might be an attractive alternative that provides increased patient comfort but may require an extended period of postoperative protection against bony ingrowths.
Patellar fixation constructs that use a combination of cannulated screws and a wire tension band provide the best resistance to patella fracture displacement when compared with screws or wires alone.4,15 Although this combination is biomechanically favorable, the steel wire often causes the painful irritation of the surrounding soft tissues and can break or migrate, thus increasing the rates of implant removal surgery to as high as 52%.4,10,12,15 We developed our novel BF technique, which uses headless compression screws and a No. 2 FiberWire tension band, to address the high rates of reoperation and patient dissatisfaction associated with the SF technique.
Headless compression screws have been successfully used in the reduction and fixation of scaphoid fractures and sesamoid fractures.16,17 The pull-out strengths of these screws are comparable with those of other commonly used screws, such as Twinfix and Herbert-Whipple screws.16 Similarly, the strength of a No. 5 FiberWire is comparable with that of an 18-gauge stainless-steel wire.14,18 Several studies have also obtained good outcomes with nonmetallic constructs that use nonabsorbable sutures alone.19,20 In this study, we utilized a No. 2 FiberWire as the tension band. The use of the No. 2 FiberWire facilitated threading through headless cannulated screws and created a low-profile knot. However, the use of thin FiberWire, despite a No. 5 FiberWire cerclage, likely contributed to the increase in distraction across the fracture.
The highest patellofemoral joint reaction force during level walking is approximately 35 kg (half body weight), which is equivalent to 350 N.15,21,22 This force is similar to the average cyclic load used in this experiment (272 ± 54 N). Gapping increased in the BF group but did not reach the defined failure value of 3 mm, and the ultimate load to failure was relatively high across both groups (SF, 1123 N; BF, 973 N). These results suggest that both fixation methods can withstand the typical patellofemoral joint forces that are experienced during the postoperative period.4 In addition, in a clinical setting, patients are placed in hinged knee braces for at least 2 weeks to limit their flexion angle and to allow for healing and bony ingrowth. Postoperative knee-brace protection presumably increases the overall strength of the fixation.
The number of specimens (n = 26) evaluated in this study was greater than that used in other biomechanical patella fracture studies.14 Furthermore, none of our specimens were reused. Our study design was further strengthened given that fellowship-trained trauma surgeons performed all surgical procedures. Finally, the data collection and analysis of numerous clinically relevant factors, such as BMD, age, and cyclical loading, contributed to the comprehensive description of each technique with respect to patient-specific criteria.
Similar to all cadaveric studies, our data only represent the immediate postoperative condition and does not represent any healing that would occur during postoperative rehabilitation. Postoperative knee-brace protection and bone healing across the fracture site would likely strengthen both constructs in a clinical setting. In addition, the average age of our specimens is 77.5 years, and therefore does not best represent the age range (20-50 years) of the typical adult population affected by patella fractures.3,23,24 Finally, postsurgical reduction was confirmed through visual inspection and not through fluoroscopy as in a clinical setting. Radiographic images were obtained after each experiment only to confirm screw placement post facto (Figures 4A, 4B).

CONCLUSION
This study demonstrates the utility of a novel BF technique. Nevertheless, the proposed technique exhibited increased gapping and a lower load to failure than the current gold standard. The significance of these inferior results in clinical and functional settings has not been established. The proposed BF technique may be an appealing alternative to the SF technique given its low profile and potential to reduce the rates of future implant removal. Further studies on the long-term outcomes of patients treated through the BF technique are currently under way and will ultimately determine the utility of the proposed construct.
This paper will be judged for the Resident Writer’s Award.
- Lazaro LE, Wellman DS, Sauro G, et al. Outcomes after operative fixation of complete articular patellar fractures: assessment of functional impairment. J Bone Joint Surg Am. 2013;95(14):e96 1-8. doi:10.2106/JBJS.L.00012.
- Bostman O, Kiviluoto O, Santavirta S, Nirhamo J, Wilppula E. Fractures of the patella treated by operation. Arch Orthop Trauma Surg. 1983;102(2):78-81.
- Gwinner C, Märdian S, Schwabe P, Schaser KD, Krapohl BD, Jung TM. Current concepts review: fractures of the patella. GMS Interdiscip Plast Reconstr Surg DGPW. 2016;5:Doc01. doi:10.3205/iprs000080.
- Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
- Patel VR, Parks BG, Wang Y, Ebert FR, Jinnah RH. Fixation of patella fractures with braided polyester suture: a biomechanical study. Injury. 2000;31(1):1-6.
- Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
- Banks KE, Ambrose CG, Wheeless JS, Tissue CM, Sen M. An alternative patellar fracture fixation: a biomechanical study. J Orthop Trauma. 2013;27(6):345-351. doi:10.1097/BOT.0b013e31826623eb.
- Thelen S, Schneppendahl J, Baumgartner R, et al. Cyclic long-term loading of a bilateral fixed-angle plate in comparison with tension band wiring with K-wires or cannulated screws in transverse patella fractures. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):311-317. doi:10.1007/s00167-012-1999-1.
- Thelen S, Schneppendahl J, Jopen E, et al. Biomechanical cadaver testing of a fixed-angle plate in comparison to tension wiring and screw fixation in transverse patella fractures. Injury. 2012;43(8):1290-1295. doi:10.1016/j.injury.2012.04.020.
- LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426. doi:10.1097/BOT.0b013e318228c1a1.
- Dy CJ, Little MT, Berkes MB, et al. Meta-analysis of re-operation, nonunion, and infection after open reduction and internal fixation of patella fractures. J Trauma Acute Care Surg. 2012;73(4):928-932. doi:10.1097/TA.0b013e31825168b6.
- Smith ST, Cramer KE, Karges DE, Watson JT, Moed BR. Early complications in the operative treatment of patella fractures. J Orthop Trauma. 1997;11(3):183-187.
- Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
- Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: A pilot study. J Orthop. 2015;12(2):92-96. doi:10.1016/j.jor.2014.04.011.
- Burvant JG, Thomas KA, Alexander R, Harris MB. Evaluation of methods of internal fixation of transverse patella fractures: a biomechanical study. J Orthop Trauma. 1994;8(2):147-153.
- Crawford LA, Powell ES, Trail IA. The fixation strength of scaphoid bone screws: an in vitro investigation using polyurethane foam. J Hand Surg Am. 2012;37(2):255-260. doi:10.1016/j.jhsa.2011.10.021.
- Eddy AL, Galuppo LD, Stover SM, Taylor KT, Jensen DG. A biomechanical comparison of headless tapered variable pitch compression and ao cortical bone screws for fixation of a simulated midbody transverse fracture of the proximal sesamoid bone in horses. Vet Surg. 2004;33(3):253-262. doi:10.1111/j.1532-950X.2004.04037.x.
- Camarda L, La Gattuta A, Butera M, Siragusa F, D'Arienzo M. FiberWire tension band for patellar fractures. J Orthop Traumatol. 2016;17(1):75-80. doi:10.1007/s10195-015-0359-6.
- Camarda L, Morello S, Balistreri F, D'Arienzo A, D'Arienzo M. Non-metallic implant for patellar fracture fixation: A systematic review. Injury. 2016;47(8):1613-1617. doi:10.1016/j.injury.2016.05.039.
- Han F, Pearce CJ, Ng DQ, et al. A double button adjustable loop device is biomechanically equivalent to tension band wire in the fixation of transverse patellar fractures-A cadaveric study. Injury. 2017;48(2):270-276. doi:10.1016/j.injury.2016.11.013.
- Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126-137. doi:10.1016/j.injury.2016.11.013.
- Buff HU, Jones LC, Hungerford DS. Experimental determination of forces transmitted through the patello-femoral joint. J Biomech. 1988;21(1):17-23.
- Bostrom A. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37(8):691-697. doi:10.1111/iwj.12675.
- Lazaro LE, Wellman DS, Sauro G, et al. Outcomes after operative fixation of complete articular patellar fractures: assessment of functional impairment. J Bone Joint Surg Am. 2013;95(14):e96 1-8. doi:10.2106/JBJS.L.00012.
- Bostman O, Kiviluoto O, Santavirta S, Nirhamo J, Wilppula E. Fractures of the patella treated by operation. Arch Orthop Trauma Surg. 1983;102(2):78-81.
- Gwinner C, Märdian S, Schwabe P, Schaser KD, Krapohl BD, Jung TM. Current concepts review: fractures of the patella. GMS Interdiscip Plast Reconstr Surg DGPW. 2016;5:Doc01. doi:10.3205/iprs000080.
- Carpenter JE, Kasman RA, Patel N, Lee ML, Goldstein SA. Biomechanical evaluation of current patella fracture fixation techniques. J Orthop Trauma. 1997;11(5):351-356.
- Patel VR, Parks BG, Wang Y, Ebert FR, Jinnah RH. Fixation of patella fractures with braided polyester suture: a biomechanical study. Injury. 2000;31(1):1-6.
- Harrell RM, Tong J, Weinhold PS, Dahners LE. Comparison of the mechanical properties of different tension band materials and suture techniques. J Orthop Trauma. 2003;17(2):119-122.
- Banks KE, Ambrose CG, Wheeless JS, Tissue CM, Sen M. An alternative patellar fracture fixation: a biomechanical study. J Orthop Trauma. 2013;27(6):345-351. doi:10.1097/BOT.0b013e31826623eb.
- Thelen S, Schneppendahl J, Baumgartner R, et al. Cyclic long-term loading of a bilateral fixed-angle plate in comparison with tension band wiring with K-wires or cannulated screws in transverse patella fractures. Knee Surg Sports Traumatol Arthrosc. 2013;21(2):311-317. doi:10.1007/s00167-012-1999-1.
- Thelen S, Schneppendahl J, Jopen E, et al. Biomechanical cadaver testing of a fixed-angle plate in comparison to tension wiring and screw fixation in transverse patella fractures. Injury. 2012;43(8):1290-1295. doi:10.1016/j.injury.2012.04.020.
- LeBrun CT, Langford JR, Sagi HC. Functional outcomes after operatively treated patella fractures. J Orthop Trauma. 2012;26(7):422-426. doi:10.1097/BOT.0b013e318228c1a1.
- Dy CJ, Little MT, Berkes MB, et al. Meta-analysis of re-operation, nonunion, and infection after open reduction and internal fixation of patella fractures. J Trauma Acute Care Surg. 2012;73(4):928-932. doi:10.1097/TA.0b013e31825168b6.
- Smith ST, Cramer KE, Karges DE, Watson JT, Moed BR. Early complications in the operative treatment of patella fractures. J Orthop Trauma. 1997;11(3):183-187.
- Berg EE. Open reduction internal fixation of displaced transverse patella fractures with figure-eight wiring through parallel cannulated compression screws. J Orthop Trauma. 1997;11(8):573-576.
- Bryant TL, Anderson CL, Stevens CG, Conrad BP, Vincent HK, Sadasivan KK. Comparison of cannulated screws with FiberWire or stainless steel wire for patella fracture fixation: A pilot study. J Orthop. 2015;12(2):92-96. doi:10.1016/j.jor.2014.04.011.
- Burvant JG, Thomas KA, Alexander R, Harris MB. Evaluation of methods of internal fixation of transverse patella fractures: a biomechanical study. J Orthop Trauma. 1994;8(2):147-153.
- Crawford LA, Powell ES, Trail IA. The fixation strength of scaphoid bone screws: an in vitro investigation using polyurethane foam. J Hand Surg Am. 2012;37(2):255-260. doi:10.1016/j.jhsa.2011.10.021.
- Eddy AL, Galuppo LD, Stover SM, Taylor KT, Jensen DG. A biomechanical comparison of headless tapered variable pitch compression and ao cortical bone screws for fixation of a simulated midbody transverse fracture of the proximal sesamoid bone in horses. Vet Surg. 2004;33(3):253-262. doi:10.1111/j.1532-950X.2004.04037.x.
- Camarda L, La Gattuta A, Butera M, Siragusa F, D'Arienzo M. FiberWire tension band for patellar fractures. J Orthop Traumatol. 2016;17(1):75-80. doi:10.1007/s10195-015-0359-6.
- Camarda L, Morello S, Balistreri F, D'Arienzo A, D'Arienzo M. Non-metallic implant for patellar fracture fixation: A systematic review. Injury. 2016;47(8):1613-1617. doi:10.1016/j.injury.2016.05.039.
- Han F, Pearce CJ, Ng DQ, et al. A double button adjustable loop device is biomechanically equivalent to tension band wire in the fixation of transverse patellar fractures-A cadaveric study. Injury. 2017;48(2):270-276. doi:10.1016/j.injury.2016.11.013.
- Reilly DT, Martens M. Experimental analysis of the quadriceps muscle force and patello-femoral joint reaction force for various activities. Acta Orthop Scand. 1972;43(2):126-137. doi:10.1016/j.injury.2016.11.013.
- Buff HU, Jones LC, Hungerford DS. Experimental determination of forces transmitted through the patello-femoral joint. J Biomech. 1988;21(1):17-23.
- Bostrom A. Fracture of the patella. A study of 422 patellar fractures. Acta Orthop Scand Suppl. 1972;143:1-80.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: A review. Injury. 2006;37(8):691-697. doi:10.1111/iwj.12675.
TAKE-HOME POINTS
- Symptomatic implant removal rates are high after patella fixation with standard techniques.
- Novel buried technique may address the issue of symptomatic implants and is an attractive alternative.
- Both techniques withstand physiologic loads, but the buried technique had overall increased gapping and lower load to failure.
- The significance of these inferior results in clinical and functional settings has not been established.
- Long-term functional outcome studies will delineate the utility of the proposed new construct.
Reasons for Readmission Following Primary Total Shoulder Arthroplasty
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
An increasing interest focuses on the rates and risk factors for hospital readmission. However, little is known regarding the readmission following total shoulder arthroplasty (TSA). This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA. Patients undergoing TSA (anatomic or reverse) as part of the American College of Surgeons National Surgical Quality Improvement Program in 2011 to 2013 were identified. The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. Using multivariate regression, demographic and comorbidity factors were tested for independent association with readmission. Finally, the reasons for readmission were characterized. A total of 3627 patients were identified. Among the admitted patients, 93 (2.56%) were readmitted within 30 days of surgery. The independent risk factors for readmission included old age (for age 60-69 years, relative risk [RR] = 1.6; for age 70-79 years, RR = 2.3; for age ≥80 years, RR = 23.1; P = .042), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission comprised pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%). Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at higher risk for readmission and should be counseled and monitored accordingly.
Continue to: Total shoulder arthroplasty...
Total shoulder arthroplasty (TSA) is performed with increasing frequency in the United States and is considered to be cost-effective.1-4 Following the procedure, patients generally achieve shoulder function and pain relief.5-8 Despite the success of the procedure, the growing literature on TSA has also reported rates of complications between 3.6% and 25% of the treated patients.9-16
In recent years, an increasing interest has focused on the rates and risk factors for unplanned hospital readmissions; these variables may not only reflect the quality of patient care but also result in considerable costs to the healthcare system. For instance, among Medicare patients, readmissions within 30 days of discharge occur in almost 20% of cases, costing $17.4 billion per year.17 Readmission rates increasingly factor into hospital performance metrics and reimbursement, including the Hospital Readmissions Reduction Program of the Patient Protection and Affordable Care Act that reduces Centers for Medicare and Medicaid Services payments to hospitals with high 30-day readmission rates.18
To date, only a few studies have evaluated readmission following TSA, with 30- to 90-day readmission rates ranging from 4.5% to 7.3%.19-23 These studies comprised single institution series20,22 and analyses of administrative databases.19,21,23 Most studies have shown that readmission occurs more often for medical than surgical reasons, with surgical reasons most commonly including infection and dislocation.19-23 However, only limited analyses have been conducted regarding risk factors for readmission.21,23 To date and to our knowledge, no study has investigated reasons for readmission following TSA using nationwide data.
This study aims to determine the rates, risk factors, and reasons for hospital readmission following primary TSA in the United States using the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database.
METHODS
DATA SOURCE
The NSQIP database was utilized to address the study purpose. NSQIP is a nationwide prospective surgical registry established by the American College of Surgeons and reports data from academic and community hospitals across the United States.24 Patients undertaking surgery at these centers are followed by the surgical clinical reviewers at the participating NSQIP sites prospectively for 30 days following the procedure to record complications including readmission. Preoperative and surgical data, such as demographics, medical comorbid diseases, and operative time, are also included. Previous studies have analyzed the complications of various orthopedic surgeries using the NSQIP data.14,16,25-30
DATA COLLECTION
We retrospectively identified from NSQIP the patients who underwent primary TSA (anatomic or reverse) in 2013 to 2014. The timeframe 2013 to 2014 was used because NSQIP only began recording reasons for readmission in 2013. The inclusion criteria were as follows: Current Procedural Terminology (CPT) code for TSA (23472); preoperative diagnosis according to the International Classification of Diseases, Ninth Revision (ICD-9) codes 714.0, 715.11, 715.31, 715.91, 715.21, 715.89, 716.xx 718.xx, 719.xx, 726.x, 727.xx, and 733.41 (where x is a wild card digit); and no missing demographic, comorbidity, or outcome data. Anatomic and reverse TSA were analyzed together because they share the same CPT code, and the NSQIP database prevents searching by the ICD-9 procedure code.
The rate of unplanned readmission to the hospital within 30 postoperative days was characterized. The reasons for readmission in this 30-day period were only available in 2013 and were determined using the ICD-9 diagnosis codes. Patient demographics were recorded for use in identifying potential risk factors for readmission; the demographic data included sex, age, smoking status, body mass index (BMI), and comorbidities, including end-stage renal disease, dyspnea on exertion, congestive heart failure, diabetes mellitus, hypertension, and chronic obstructive pulmonary disease (COPD).
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were performed using Stata version 13.1 (StataCorp). First, using bivariate and multivariate regression, demographic and comorbidity factors were tested for independent association with readmission to the hospital within 30 days of surgery. Second, among the readmitted patients, the reasons for readmission were tabulated. Of note, the reasons for readmission were only documented for the procedures performed in 2013. All tests were 2-tailed and conducted at an α level of 0.05.
RESTULTS
A total of 3627 TSA patients were identified. The mean age (± standard deviation) was 69.4 ± 9.5 years, 55.8% of patients were female, and mean BMI was 30.1 ± 7.0 years. Table 1 provides the additional demographic data. Of the 3627 included patients, 93 (2.56%) were readmitted within 30 days of surgery. The 95% confidence interval for the estimated rate of readmission reached 2.05% to 3.08%.
Table 1. Patient Population
| Number | Percent |
Total | 3627 | 100.0% |
Age |
|
|
18-59 | 539 | 14.9% |
60-69 | 1235 | 34.1% |
70-79 | 1317 | 36.3% |
≥80 | 536 | 14.8% |
Sex |
|
|
Male | 1603 | 44.2% |
Female | 2024 | 55.8% |
Body mass index |
|
|
Normal (<25 kg/m2) | 650 | 17.9% |
Overweight (25-30 kg/m2) | 1147 | 31.6% |
Obese (≥30 kg/m2) | 1830 | 50.5% |
Functional status |
|
|
Independent | 3544 | 97.7% |
Dependent | 83 | 2.3% |
Diabetes mellitus |
|
|
No | 3022 | 83.3% |
Yes | 605 | 16.7% |
Dyspnea on exertion |
|
|
No | 3393 | 93.6% |
Yes | 234 | 6.5% |
Hypertension |
|
|
No | 1192 | 32.9% |
Yes | 2435 | 67.1% |
COPD |
|
|
No | 3384 | 93.3% |
Yes | 243 | 6.7% |
Current smoker |
|
|
No | 3249 | 89.6% |
Yes | 378 | 10.4% |
Anemia |
|
|
No | 3051 | 84.1% |
Yes | 576 | 15.9% |
Abbreviation: COPD, chronic obstructive pulmonary disease.
In the bivariate analyses (Table 2), the following factors were positively associated readmission: older age (60-69 years, relative risk [RR] = 1.6; 70-79 years, RR = 2.2; ≥80 years, RR = 3.3; P = .011), dependent functional status (RR = 2.9, P = .008), and anemia (RR = 2.2, P < .001).
Table 2. Bivariate Analysis of Risk Factors for Readmission
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.011 |
18-59 | 1.30% | Ref. | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.2 | 1.0-4.9 |
|
≥80 | 4.29% | 3.3 | 1.4-7.6 |
|
Sex |
|
|
| 0.099 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.4 | 0.9-2.1 |
|
Body mass index |
|
|
| 0.764 |
Normal (<25 kg/m2) | 2.92% | Ref. | - |
|
Overweight (25-30 kg/m2) | 2.35% | 0.8 | 0.5-1.4 |
|
Obese (≥30 kg/m2) | 2.57% | 0.9 | 0.5-1.5 |
|
Functional status |
|
|
| 0.008 |
Independent | 2.45% | Ref. | - |
|
Dependent | 7.23% | 2.9 | 1.3-6.5 |
|
Diabetes mellitus |
|
|
| 0.483 |
No | 2.48% | Ref. | - |
|
Yes | 2.98% | 1.2 | 0.7-2.0 |
|
Dyspnea on exertion |
|
|
| 0.393 |
No | 2.51% | Ref. | - |
|
Yes | 3.42% | 1.4 | 0.7-2.8 |
|
Hypertension |
|
|
| 0.145 |
No | 2.01% | Ref. | - |
|
Yes | 2.83% | 1.4 | 0.9-2.2 |
|
COPD |
|
|
| 0.457 |
No | 2.51% | Ref. | - |
|
Yes | 3.29% | 1.3 | 0.6-2.7 |
|
Current smoker |
|
|
| 0.116 |
No | 2.71% | Ref. | - |
|
Yes | 1.32% | 0.5 | 0.2-1.2 |
|
Anemia |
|
|
| <0.001 |
No | 2.16% | Ref. | - |
|
Yes | 4.69% | 2.2 | 1.4-3.4 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
In the multivariate analyses (Table 3), the following factors were independent risk factors for readmission: older age (60-69 years, RR = 1.6; 70-79 years, RR = 2.3; ≥80 years, RR = 3.1; P =.027), male sex (RR = 1.6, P = .025), anemia (RR = 1.9, P = .005), and dependent functional status (RR = 2.8, P = .012). Interestingly, readmission showed no independent association with diabetes, dyspnea on exertion, BMI, COPD, hypertension, or current smoking status (P > .05 for each).
Table 3. Independent Risk Factors for Readmission on Multivariate Analysis
| Rate | RR | 95% CI | P-value |
Age |
|
|
| 0.027 |
18-59 | 1.30% | Ref | - |
|
60-69 | 2.02% | 1.6 | 0.7-3.6 |
|
70-79 | 2.89% | 2.3 | 1.0-5.1 |
|
≥80 | 4.29% | 3.1 | 1.3-7.4 |
|
Sex |
|
|
| 0.025 |
Female | 2.17% | Ref. | - |
|
Male | 3.06% | 1.6 | 1.1-2.4 |
|
Anemia |
|
|
| 0.005 |
No | 2.16% | Ref | - |
|
Yes | 4.69% | 1.9 | 1.2-3.0 |
|
Functional status |
|
|
| 0.012 |
Independent | 2.45% | Ref | - |
|
Dependent | 7.23% | 2.8 | 1.3-6.2 |
|
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; RR, relative risk.
Continue to: Table 4...
The reasons for readmission were available for 84 of the 93 readmitted patients. The most common reasons for readmission included pneumonia (14 cases, 16.7%), dislocation (7 cases, 8.3%), pulmonary embolism (7 cases, 8.3%), and surgical site infection (6 cases, 7.1%) (Table 4).
Table 4. Reasons for Readmission
| Number | Percent |
Pneumonia | 14 | 16.7% |
Dislocation | 7 | 8.3% |
Pulmonary embolism | 7 | 8.3% |
Surgical site infection | 6 | 7.1% |
Atrial fibrillation | 4 | 4.8% |
Hematoma | 4 | 4.8% |
Altered mental status | 3 | 3.6% |
Chest pain | 3 | 3.6% |
Renal insufficiency/kidney failure | 3 | 3.6% |
Urinary tract infection | 3 | 3.6% |
Acute gastric or duodenal ulcer | 2 | 2.4% |
Dermatitis/other allergic reaction | 2 | 2.4% |
Orthostatic hypotension/syncope | 2 | 2.4% |
Pain | 2 | 2.4% |
Respiratory distress | 2 | 2.4% |
Sepsis | 2 | 2.4% |
Urinary retention | 2 | 2.4% |
Acute cholecystitis | 1 | 1.2% |
Cerebrovascular accident | 1 | 1.2% |
Constipation | 1 | 1.2% |
Contusion of shoulder | 1 | 1.2% |
Deep venous thrombosis requiring therapy | 1 | 1.2% |
Gastrointestinal hemorrhage | 1 | 1.2% |
Gout | 1 | 1.2% |
Hepatic encephalopathy | 1 | 1.2% |
Intestinal infection | 1 | 1.2% |
Narcotic overdose | 1 | 1.2% |
Nausea/vomiting | 1 | 1.2% |
Proximal humerus fracture | 1 | 1.2% |
Rotator cuff tear | 1 | 1.2% |
Seroma | 1 | 1.2% |
Unspecified disease of pericardium | 1 | 1.2% |
Weakness | 1 | 1.2% |
DISCUSSION
Our analysis of 3042 TSAs from the NSQIP database suggests that unplanned readmission to the hospital occurs following about 1 in 40 cases of TSA. The study also suggests that the most common reasons for readmission encompass pneumonia, dislocation, pulmonary embolism, and surgical site infection. Old age, male sex, anemia, and dependent functional status serve as risk factors for readmission, and patients with such factors should be counseled and monitored accordingly.
In recent years, an increasing emphasis has centered on reducing rates of hospital readmission, with programs such as the Hospital Readmissions Reduction Program of the Affordable Care Act cutting reimbursements for hospitals with high 30-day readmission rates.17,18 To date, only a few studies have evaluated the reasons for readmission and readmission rates for TSA.19-23 Initial reports consisted of single-institution TSA registry reviews. For example, Mahoney and colleagues20 retrospectively evaluated shoulder arthroplasty procedures at their institution to document the readmission rates, finding a 5.9% readmission rate at 30 days. Readmission occurred more frequently in the first 30 days following discharge than in the 30- to 90-day period, with the most common reasons for readmission including medical complications, infection, and dislocation. Streubel and colleagues22 evaluated reoperation rates from their institution’s TSA registry, finding a 0.6% reoperation rate for primary TSA at 30 days and 1.5% for revision TSA. Instability and infection were the most common indications for reoperation. Our findings confirm these single-institution results and demonstrate their application to a nationwide sample of TSA, not just to high-volume academic centers. We similarly observed that dislocation, surgical site infection, and medical complications (mostly pneumonia and pulmonary embolism) were common causes of readmission, and that the 30-day readmission rate was about 1 in 40.
Several authors have since used statewide databases to analyze and determine risk factors for readmission following TSA. Lyman and colleagues19 used the New York State Database to show that higher hospital TSA surgical volume was associated with a lower rate of readmission when age and comorbidities were controlled for in a multivariate model. Old age was also associated with an increased readmission rate in their multivariate analysis, but comorbidities (as measured by the Charlson comorbidity index) presented a nonsignificant associative trend. These authors opted not to determine specific causes of readmission. Schairer and colleagues21 used State Inpatient Databases from 7 states, finding a 90-day readmission rate of 7.3%, 82% of which were due to medical complications and 18% of which were due to surgical complications (mostly infection and dislocation). Their multivariate regression revealed that male sex, reverse TSA, Medicaid insurance, patients discharged to inpatient rehabilitation or nursing facilities, medical comorbidities, and low-volume TSA hospitals were associated with readmission. Zhang and colleagues23 used the same source to show that the 90-day readmission rate reached 14% for surgically treated proximal humerus fractures and higher for patients who underwent open reduction internal fixation, were female, were African American, were discharged to a nursing facility, possessed Medicaid insurance, or experienced medical comorbidities. Most recently, Basques and colleagues31 analyzed 1505 TSA cases from 2011 and 2012 in the NSQIP database, finding a 3.3% rate of readmission, with heart disease and hypertension as risk factors for readmission. Although the limitations of the NSQIP database prevented us from analyzing surgeon and hospital TSA volume or reverse vs anatomic TSA, our results confirm that the findings from statewide database studies apply to the United States nationwide NSQIP database. Old patient age, male sex, and medical comorbidities (anemia and dependent functional status) are independent risk factors for TSA readmission. We identified pneumonia, dislocation, pulmonary embolism, and surgical site infection as the most common reasons for readmission.
This study features several limitations that should be considered when interpreting the results. Anatomic and reverse TSA share a CPT code and were not separated using NSQIP data. A number of studies have reported that reverse TSA may place patients at higher risk for readmission;20,21 however, confounding by other patient factors could play a role in this finding. The 30-day timeframe for readmission is another potential limitation; however, this timeframe is frequently used in other studies and is the relevant timeframe for the reduced reimbursement penalties from the Hospital Readmissions Reduction Program of the Affordable Care Act.18 Furthermore, the NSQIP database contains no information on surgeon or hospital TSA volume, which is a result of safeguards for patient and provider privacy. Additionally, readmission data were only available for 2011 to 2013, with causes of readmission only present in 2013. Although provided with such current information, we cannot analyze readmission trends over time, such as in response to the Affordable Care Act of 2010. Finally, although NSQIP surgical clinical reviewers strive to identify readmissions to other hospitals during their reviews of outpatient medical records, proportions of these readmissions are possibly missed. Therefore, our 30-day readmission rate may slightly underestimate the true rate.
Despite these limitations, the NSQIP database offers a unique opportunity to examine risk factors and reasons for readmission following TSA. The prior literature on readmission following TSA stemmed either from limited samples or administrative data, which feature known limitations.32 By utilizing a large, prospective, non-administrative, nationwide sample, our findings are probably both more reliable and generalizable to the country as a whole.
CONCLUSION
Unplanned readmission occurs following about 1 in 40 cases of TSA. The most common causes of readmission include pneumonia, dislocation, pulmonary embolism, and surgical site infection. Patients with old age, male sex, anemia, and dependent functional status are at a higher risk for readmission and should be counseled and monitored accordingly.
This paper will be judged for the Resident Writer’s Award.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
- Adams JE, Sperling JW, Hoskin TL, Melton LJ, Cofield RH. Shoulder arthroplasty in Olmsted County, Minnesota, 1976-2000: a population-based study. J Shoulder Elbow Surg.2006;15(1):50-55. doi:10.1016/j.jse.2005.04.009.
- Jain NB, Higgins LD, Guller U, Pietrobon R, Katz JN. Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990-2000. Arthritis Rheum.2006;55(4):591-597. doi:10.1002/art.22102.
- Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/JBJS.J.01994. doi:10.2106/JBJS.J.01994.
- Mather RC, Watters TS, Orlando LA, Bolognesi MP, Moorman CT. Cost effectiveness analysis of hemiarthroplasty and total shoulder arthroplasty. J Shoulder Elbow Surg.2010;19(3):325-334. doi:10.1016/j.jse.2009.11.057.
- Carter MJ, Mikuls TR, Nayak S, Fehringer EV, Michaud K. Impact of total shoulder arthroplasty on generic and shoulder-specific health-related quality-of-life measures: a systematic literature review and meta-analysis. J Bone Joint Surg Am. 2012;94(17):e127. doi:10.2106/JBJS.K.00204.
- Deshmukh AV, Koris M, Zurakowski D, Thornhill TS. Total shoulder arthroplasty: long-term survivorship, functional outcome, and quality of life. J Shoulder Elbow Surg. 2005;14(5):471-479. doi:10.1016/j.jse.2005.02.009.
- Montoya F, Magosch P, Scheiderer B, Lichtenberg S, Melean P, Habermeyer P. Midterm results of a total shoulder prosthesis fixed with a cementless glenoid component. J Shoulder Elbow Surg. 2013;22(5):628-635. doi:10.1016/j.jse.2012.07.005.
- Raiss P, Bruckner T, Rickert M, Walch G. Longitudinal observational study of total shoulder replacements with cement: fifteen to twenty-year follow-up. J Bone Joint Surg Am.2014;96(3):198-205. doi:10.2106/JBJS.M.00079.
- Bohsali KI, Wirth MA, Rockwood CA. Complications of total shoulder arthroplasty. J Bone Joint Surg Am. 2006;88(10):2279-2292. doi:10.2106/JBJS.F.00125.
- Chalmers PN, Gupta AK, Rahman Z, Bruce B, Romeo AA, Nicholson GP. Predictors of early complications of total shoulder arthroplasty. J Arthroplasty. 2014;29(4):856-860. doi:10.1016/j.arth.2013.07.002.
- Cheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2011;19(7):439-449.
- Papadonikolakis A, Neradilek MB, Matsen FA. Failure of the glenoid component in anatomic total shoulder arthroplasty: a systematic review of the English-language literature between 2006 and 2012. J Bone Joint Surg Am. 2013;95(24):2205-2212. doi:10.2106/JBJS.L.00552.
- Saltzman BM, Chalmers PN, Gupta AK, Romeo AA, Nicholson GP. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J Shoulder Elbow Surg.2014;23(11):1647-1654. doi:10.1016/j.jse.2014.04.015.
- Shields E, Iannuzzi JC, Thorsness R, Noyes K, Voloshin I. Perioperative complications after hemiarthroplasty and total shoulder arthroplasty are equivalent. J Shoulder Elbow Surg. 2014;23(10):1449-1453. doi:10.1016/j.jse.2014.01.052.
- Sperling JW, Hawkins RJ, Walch G, Mahoney AP, Zuckerman JD. Complications in total shoulder arthroplasty. Instr Course Lect. 2013;62:135-141.
- Shields E, Thirukumaran C, Thorsness R, Noyes K, Voloshin I. An analysis of adult patient risk factors and complications within 30 days after arthroscopic shoulder surgery. Arthroscopy. 2015;31(5):807-815. doi:10.1016/j.arthro.2014.12.011.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428. doi:10.1056/NEJMsa0803563.
- Centers for Medicare & Medicaid Services. Readmissions reduction program (HRRP). . Updated April 27, 2018. Accessed June 29, 2018.
- Lyman S, Jones EC, Bach PB, Peterson MG, Marx RG. The association between hospital volume and total shoulder arthroplasty outcomes. Clin Orthop Relat Res. 2005;432:132-137. doi:10.1097/01.blo.0000150571.51381.9a.
- Mahoney A, Bosco JA, Zuckerman JD. Readmission after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(3):377-381. doi:10.1016/j.jse.2013.08.007.
- Schairer WW, Zhang AL, Feeley BT. Hospital readmissions after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(9):1349-1355. doi:10.1016/j.jse.2013.12.004.
- Streubel PN, Simone JP, Sperling JW, Cofield R. Thirty and ninety-day reoperation rates after shoulder arthroplasty. J Bone Joint Surg Am. 2014;96(3):e17. doi:10.2106/JBJS.M.00127.
- Zhang AL, Schairer WW, Feeley BT. Hospital readmissions after surgical treatment of proximal humerus fractures: is arthroplasty safer than open reduction internal fixation? Clin Orthop Relat Res. 2014;472(8):2317-2324. doi:10.1007/s11999-014-3613-y.
- American College of Surgeons. ACS National Surgical Quality Improvement Program. http://www.acsnsqip.org. Accessed July 15, 2015.
- Basques BA, Gardner EC, Varthi AG, et al. Risk factors for short-term adverse events and readmission after arthroscopic meniscectomy: does age matter? Am J Sports Med.2015;43(1):169-175. doi:10.1177/0363546514551923.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Does resident involvement impact post-operative complications following primary total knee arthroplasty? An analysis of 24,529 cases. J Arthroplasty. 2014;29(7):1468-1472.e2. doi:10.1016/j.arth.2014.02.036.
- Haughom BD, Schairer WW, Hellman MD, Yi PH, Levine BR. Resident involvement does not influence complication after total hip arthroplasty: an analysis of 13,109 cases. J Arthroplasty. 2014;29(10):1919-1924. doi:10.1016/j.arth.2014.06.003.
- Martin CT, Gao Y, Pugely AJ, Wolf BR. 30-day morbidity and mortality after elective shoulder arthroscopy: a review of 9410 cases. J Shoulder Elbow Surg. 2013;22(12):1667-1675.e1. doi:10.1016/j.jse.2013.06.022.
- Martin CT, Pugely AJ, Gao Y, Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the national surgical quality improvement program database. J Bone Joint Surg Am. 2013;95(14):e98 1-10. doi:10.2106/JBJS.L.01440.
- Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015;24(1):24-30. doi:10.1016/j.jse.2014.05.016.
- Basques BA, Gardner EC, Toy JO, Golinvaux NS, Bohl DD, Grauer JN. Length of stay and readmission after total shoulder arthroplasty: an analysis of 1505 cases. Am J Orthop.2015;44(8):E268-E271.
- Bohl DD, Russo GS, Basques BA, et al. Variations in data collection methods between national databases affect study results: a comparison of the nationwide inpatient sample and national surgical quality improvement program databases for lumbar spine fusion procedures. J Bone Joint Surg Am. 2014;96(23):e193. doi:10.2106/JBJS.M.01490.
TAKE-HOME POINTS
- Shoulder arthroplasty is an increasingly commonly performed procedure for shoulder arthritis and other conditions.
- Unplanned readmission in the 30 days after shoulder arthroplasty occurred in about 1 of 40 cases.
- Increasing age was associated with readmission, particularly age >80 years.
- Other risk factors for readmission were male sex, anemia, and dependent functional status.
- The most common reasons for readmission were pneumonia, dislocation, pulmonary embolism, and surgical site infection.
Rheumatoid Arthritis vs Osteoarthritis: Comparison of Demographics and Trends of Joint Replacement Data from the Nationwide Inpatient Sample
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).
Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).
Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).

Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).


Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Current literature regarding complications following total joint arthroplasty have primarily focused on patients with osteoarthritis (OA), with less emphasis on the trends and in-hospital outcomes of rheumatoid arthritis (RA) patients undergoing these procedures. The purpose of this study is to analyze the outcomes and trends of RA patients undergoing total knee arthroplasty (TKA) or total hip arthroplasty (THA) compared to OA patients.
Data from the Nationwide Inpatient Sample from 2006 to 2011 was extracted using the International Classification of Diseases, Ninth Revision codes for patients that received a TKA or THA. Outcome measures included cardiovascular complications, cerebrovascular complications, pulmonary complications, wound dehiscence, and infection. Inpatient and hospital demographics including primary diagnosis, age, gender, primary payer, hospital teaching status, Charlson Comorbidity Index score, hospital bed size, location, and median household income were analyzed.
Logistic regression analysis of OA vs RA patients with patient outcomes revealed that osteoarthritic THA candidates had lower risk for cardiovascular complications, pulmonary complications, wound dehiscence, infections, and systemic complications, compared to rheumatoid patients. There was a significantly elevated risk of cerebrovascular complication in osteoarthritic THA compared to RA THA. OA patients undergoing TKA had significantly higher risk for cardiovascular and cerebrovascular complications. There were significant decreases in mechanical wounds, infection, and systemic complications in the OA TKA patients.
RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients. These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Continue to: RA is a chronic systemic inflammatory disease...
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that causes joint deterioration, leading to pain, disability, systemic complications, short lifespan, and decline in quality of life.1-3 The deterioration primarily affects the synovial membranes of joints, causing arthritis and resulting in extra-articular sequelae such as cardiovascular disease,4 pulmonary disease,5 and increased infection rates.3,6 RA is the most prevalent inflammatory arthritis worldwide and affects up to 50 cases per 100,000 in both the US and northern Europe.2,7-9 Although the gold standard of care for these patients is medical management with immunosuppressant drugs such as disease-modifying anti-rheumatic drugs (DMARDs), total joint arthroplasty (TJA) remains an important tool in the management of joint deterioration in such patients.
Total knee arthroplasty (TKA) and total hip arthroplasty (THA) are common procedures utilized to treat disorders that cause joint pain and hindered joint mobility, including osteoarthritis (OA) and RA. Given the aging population, the amount of TKAs and THAs performed in the US has consistently increased each year, with the vast majority of this increase composed of patients with OA.10 As a result, previous studies investigated the trends and outcomes of these procedures in patients with OA, but relatively less is known about the outcomes and trends of patients with RA undergoing the same surgeries.
Given that RA is a fundamentally different condition with its own pathological characteristics, an understanding of how these differences may impact postoperative outcomes in patients with RA is important. This study aims to present a comparative analysis of the trends and postoperative outcomes between patients with RA and OA undergoing TKA and THA (Figure 1, Tables 1 and 2).

Table 1. Demographics of Total Knee Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 295,637 | 42.42 | 11,325 | 48.90 | 306,962 | 42.63 |
|
65 to 79 years | 329,034 | 47.22 | 10,055 | 43.42 | 339,089 | 47.09 |
|
≥80 years | 72,197 | 10.36 | 1780 | 7.69 | 73,977 | 10.27 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 259,192 | 37.19 | 4887 | 21.12 | 264,079 | 36.68 |
|
Female | 435,855 | 62.54 | 18,248 | 78.88 | 454,103 | 63.07 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 468,632 | 67.25 | 14,532 | 77.18 | 483,164 | 67.10 |
|
Black | 39,691 | 5.7 | 2119 | 11.25 | 41,810 | 5.81 |
|
Hispanic | 28,573 | 4.1 | 1395 | 7.41 | 29,968 | 4.16 |
|
Other | 21,306 | 3.06 | 783 | 4.16 | 22,089 | 3.07 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 112,031 | 16.08 | 3417 | 14.75 | 115,448 | 16.03 |
|
Midwest | 192,595 | 27.64 | 5975 | 25.80 | 198,570 | 27.58 |
|
South | 257,855 | 37 | 9422 | 40.68 | 267,277 | 37.12 |
|
West | 134,387 | 19.28 | 4346 | 18.77 | 138,733 | 19.27 |
|
Location/teaching status of hospital |
|
|
|
|
|
| <.0001 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban non-teaching | 333,043 | 47.79 | 10,905 | 47.46 | 343,948 | 47.77 |
|
Urban teaching | 273,326 | 39.22 | 9363 | 40.75 | 282,689 | 39.26 |
|
Hospital location |
|
|
|
|
|
| .0024 |
Rural | 86,321 | 12.39 | 2709 | 11.79 | 89,030 | 12.36 |
|
Urban | 606,369 | 87.01 | 20,268 | 88.21 | 626,637 | 87.03 |
|
Hospital teaching status |
|
|
|
|
|
| <.0001 |
Teaching | 409,465 | 58.76 | 13,275 | 57.78 | 422,740 | 58.71 |
|
Non-teaching | 283,225 | 40.64 | 9702 | 42.22 | 292,927 | 40.68 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 65,342 | 9.38 | 1946 | 8.40 | 67,288 | 9.35 | <.0001 |
Diabetes | 147,292 | 21.14 | 4289 | 18.52 | 151,581 | 21.05 | <.0001 |
Obesity | 129,277 | 18.55 | 3730 | 16.11 | 133,007 | 18.47 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis.
Table 2. Demographics of Total Hip Arthroplasty Patients Based on Primary Diagnosis of Osteoarthritis or Rheumatoid Arthritis
| OA | RA | Total | P Value | |||
| No. | Percent | No. | Percent | No. | Percent | (RA vs OA) |
Age group |
|
|
|
|
|
| <.0001 |
<64 years | 133,645 | 45.18 | 4679 | 48.02 | 138,324 | 45.27 |
|
65 to 79 years | 123,628 | 41.8 | 3992 | 40.97 | 127,620 | 41.77 |
|
≥80 years | 38,513 | 13.02 | 1073 | 11.01 | 39,586 | 12.96 |
|
Gender |
|
|
|
|
|
| <.0001 |
Male | 129,708 | 43.85 | 2457 | 25.24 | 132,165 | 43.26 |
|
Female | 165,010 | 55.79 | 7278 | 74.76 | 172,288 | 56.39 |
|
Race |
|
|
|
|
|
| <.0001 |
White | 207,005 | 69.98 | 6322 | 80.08 | 213,327 | 69.82 |
|
Black | 15,505 | 5.24 | 771 | 9.77 | 16,276 | 5.33 |
|
Hispanic | 6784 | 2.29 | 522 | 6.61 | 7306 | 2.39 |
|
Other | 7209 | 2.44 | 280 | 3.55 | 7489 | 2.45 |
|
Region of hospital |
|
|
|
|
|
| <.0001 |
Northeast | 58,525 | 19.79 | 1683 | 17.27 | 60,208 | 19.71 |
|
Midwest | 79,040 | 26.72 | 2446 | 25.10 | 81,486 | 26.67 |
|
South | 95,337 | 32.23 | 3716 | 38.14 | 99,053 | 32.42 |
|
West | 62,884 | 21.26 | 1899 | 19.49 | 64,783 | 21.20 |
|
Location/teaching status of hospital |
|
|
|
|
|
| .0065 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban non-teaching | 133,061 | 44.99 | 4245 | 43.87 | 137,306 | 44.94 |
|
Urban teaching | 130,150 | 44 | 4439 | 45.87 | 134,589 | 44.05 |
|
Hospital location |
|
|
|
|
|
| .4098 |
Rural | 30,954 | 10.46 | 993 | 10.26 | 31,947 | 10.46 |
|
Urban | 263,211 | 88.99 | 8684 | 89.74 | 271,895 | 88.99 |
|
Hospital teaching status |
|
|
|
|
|
| .0077 |
Teaching | 159,313 | 53.86 | 5108 | 52.78 | 164,421 | 53.82 |
|
Non-teaching | 134,852 | 45.59 | 4569 | 47.22 | 139,421 | 45.63 |
|
Comorbidities |
|
|
|
|
|
|
|
Obstructive sleep apnea | 19,760 | 6.68 | 573 | 5.88 | 20,333 | 6.65 | .0028 |
Diabetes | 41,929 | 14.18 | 1325 | 13.60 | 43,254 | 14.16 | .1077 |
Obesity | 38,808 | 13.12 | 1100 | 11.29 | 39,908 | 13.06 | <.0001 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis
Continue to: Methods...
METHODS
Exemptions were obtained from the Institutional Review Board. Data from the Nationwide Inpatient Sample (NIS) from 2006 to 2011 were extracted using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for patients that received primary TKA or THA, as well as their comorbid conditions. No patients or populations were excluded from the sampling process. A list of all independent variables collected for analysis and provision of relevant ICD-9 codes is included in Figure 1. The NIS is the largest all-payer stratified survey of inpatient care in the US healthcare system. As of 2011, each year provides information on approximately 8 million inpatient stays from about 1000 hospitals in 46 states. All discharges from sampled hospitals are also represented in the database. All patient information is protected, and all methods were conducted in accordance with the highest ethical standards of Human and Animal Rights Research.
STATISTICAL ANALYSIS
SAS 9.2 and PROC FREQ statistics software were used to generate P values (chi square result) and analyze the trends (Cochran-Armitage). Results were weighted utilizing standard discharge weights from the NIS to ensure accurate comparison of data from different time points. P < .05 was considered statistically significant. Multivariable logistic regression analyses were performed to generate odds ratio and 95% confidence limits to assess outcomes across different demographic variables.
RESULTS
Data on 337,082 and 1,362,241 patients undergoing THA or TKA, respectively, between 2006 and 2011 were analyzed. Patients in both groups were further differentiated by a diagnosis of either OA or RA. OA was the most common diagnosis, constituting 96.8% of all arthritic THA and TKA patients. From 2006 to 2011, a 36% and 34% increase in total number of THAs and TKAs, respectively, were reported. The number of patients with OA undergoing THA and TKA steadily increased from 2006 to 2011 (Figure 2). The number of THA and TKA procedures in patients with RA followed a similar trend but at a comparatively slower rate (Figure 3). The TKA geographical trends mirrored those observed with THA. The majority of operations were performed at urban hospitals (89% THA, 87% TKA; P < .0001). Among patients with RA and OA, the majority of TKAs (47.77%; P < .0001) took place in urban non-teaching hospitals than in urban teaching hospitals (39.26%). This pattern was not the same for THA, with 44.94% being performed at urban teaching hospitals and 44.05% at urban non-teaching institutions (P < .0001). Rural hospitals accounted for a low percentage of operations for both procedures: 10.46% of THA and 12.36% of TKA (P < .0001). Large institutions (based on the number of beds) claimed the majority of cases (59% of THA and TKA).


Logistic regression analysis and odds ratios of patients with OA vs those with RA with patient outcomes adjusted for age, Charlson Comorbidity Index (CCI) score, and gender revealed that patients with OA undergoing THA had lower risk for cardiovascular (0.674; confidence interval (CI) 0.587-0.774) and pulmonary complications (0.416; CI 0.384-0.450), wound dehiscence (0.647; CI 0.561-0.747), infections (0.258; CI 0.221-0.301), and systemic complications (0.625; CI 0.562-0.695) than patients with RA. Patients with OA exhibited statistically significantly higher odds of experiencing cerebrovascular complications after THA than those with RA (1.946; CI 1.673-2.236) (Table 3). In a similar logistic regression analysis of OA vs RA in TKA, which was adjusted for age, CCI score, and gender, patients with OA had significantly higher risk for cardiovascular (1.329; CI 1.069-1.651) and cerebrovascular complications (1.635; CI 1.375-1.943) than patients with RA. Significant decreases in wound dehiscence (0.757; CI 0.639-0.896), infection (0.331; CI 0.286-0.383), and systemic complication (0.641; CI 0.565-0.729) were noted in the patients with OA and TKA (Table 4).
Table 3. Odds Ratio for In-Hospital Complications Following THA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | .674 | .587-.744 |
Cerebrovascular complication | 1.946 | 1.673-2.236 |
Pulmonary complication | .416 | .384-.450 |
Wound dehiscence | .647 | .561-.747 |
Infection | .258 | .221-.301 |
Systemic complication | .625 | .562-.695 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; THA, total hip arthroplasty.
Table 4. Odds Ratio for In-Hospital Complications Following TKA for OA Patients vs RA Patients
| Odds Ratio | Confidence Limits |
Cardiovascular complication | 1.329 | 1.069-1.651 |
Cerebrovascular complication | 1.635 | 1.375-1.943 |
Pulmonary complication | 1.03 | .995-1.223 |
Wound dehiscence | .757 | .639-.896 |
Infection | .331 | .286-.383 |
Systemic complication | .641 | .565-.729 |
Abbreviations: OA, osteoarthritis; RA, rheumatoid arthritis; TKA, total knee arthroplasty.
Continue to: Discussion...
DISCUSSION
Our results showed a continuous yearly increase from 2006 to 2011 in THA and TKA procedures at a rate of 36% and 34%, respectively; this result was consistent with existing literature.11 Despite a substantial increase in the amount of total THA and TKA procedures, the ratio of patients with RA undergoing these operations has decreased or remained nearly the same. Similar effects were found in Japan and the US when examining patients with RA undergoing TJA procedures between 2001 and 2007 and between 1992 and 2005, respectively.12-14 This observation may be explained by the advances and early initiation of pharmacologic treatment and the widespread use of DMARDs such as methotrexate (MTX), azathioprine, leflunomide, hydroxychloroquine, and biological response modifiers TNF-α and interleukin-1.15 These medications have drastically improved survival rates of patients with RA with impressive capabilities in symptom relief.15 With the increasing use of DMARDs and aggressive treatment early on in the disease process, patients with RA are showing markedly slow progression of joint deterioration, leading to a decreased need for orthopedic intervention compared with the general population.13,15
When analyzing the complication rates for patients undergoing TKA and THA, we observed that patients with RA exhibited a significant increase in the rates of infections, wound dehiscence, and systemic complications prior to discharge from the hospital compared with the OA population. The increased risk of infections was reported in previous studies assessing postoperative complication rates in TJA.16,17 A study utilizing the Norwegian Arthroplasty Registry noted an increased risk of late infection in patients with RA, leading to increased rates of revision TJA in comparison with patients with OA.16 Another study, which was based on the Canadian Institute for Health Information Discharge Abstract Database, showed that patients with RA are at an increased risk of infection only after THA and interestingly not after TKA.17 Although our study did not identify the causes of the increased infection rate, the inherent nature of the disease and the immunomodulatory drugs used to treat it may contribute to this increased infectious risk in patients with RA.6,18 Immunosuppressive DMARDs are some of the widely used medications employed to treat RA and are prime suspects of causing increased infection rates.15 The perioperative use of MTX has not been shown to cause short-term increases in infection for patients undergoing orthopedic intervention, but leflunomide and TNF-α inhibitors have been shown to cause a significant several-fold increase in risk for surgical wound infections.19,20
All patients with RA presented with significant increases for infection, wound dehiscence, and systemic complications, whereas only patients with RA undergoing THA showed increased risk of pulmonary and cardiovascular complications when compared with patients with OA. Surprisingly, in TKA, patients with RA were at a significantly decreased risk of cardiovascular complications. This observation was interesting due to cardiovascular disease being one of RA's most notable extra-articular features.4,21
Patients with RA undergoing TJA also showed significantly lower cerebrovascular complications than patients with OA. The significant reduction in risk for these complications has not been previously reported in the current literature, and it was an unexpected finding as past studies have found an increased risk in cerebrovascular disease in patients with RA. RA is an inflammatory disease exhibiting the upregulation of procoagulation factors,22 so we expected patients with RA to be at an increased risk for cerebrovascular and cardiovascular complications over patients with OA. Although we are unsure why these results were observed, we postulate that pharmaceutical interventions may confer some protection to patients with RA. For example, aspirin is commonly utilized in RA for its protective anti-platelet effect23 and may be a contributing factor to why we found low postoperative complication rates in cerebrovascular disease. However, the reason why aspirin may be protective against cerebrovascular and not cardiovascular complications remains unclear. Moreover, most guidelines suggest that aspirin be stopped prior to surgery.24 Although patients with RA were younger than those with OA, age was accounted for when analyzing the data.
A major strength of the study was the large sample size and the adjustment of potential confounding variables when examining the difference in complications between RA and OA. It is also a national US study that utilizes a validated database. Given that the patient samples in the NIS are reported in a uniform and de-identified manner, the database is considered ideal and has been extensively used for retrospective large observational cohort studies.25 However, the study also had some limitations due to the retrospective and administrative nature of the NIS database. Only data concerning patient complications during their inpatient stay at the hospital were available. Patients who may develop complications following discharge were not included in the data, providing a very small window of time for analysis. Another limitation with the database was its lack of ability to identify the severity of each patient's disease process or the medical treatment they received perioperatively. Finally, no patient-reported outcomes were determined, which would provide information on whether these complications affect the patients’ postoperational satisfaction in regard to their pain and disability.
CONCLUSION
As RA patients continue to utilize joint arthroplasty to repair deteriorated joints, understanding of how the disease process and its medical management may impact patient outcomes is important. This article reports significantly higher postoperational infection rates in RA than in patients with OA, which may be due to the medical management of the disease. Although new medications have been introduced and are being used to treat patients with RA, they have not altered the complication rate following TJA in this patient population. Thus, surgeons and other members of the management team should be familiar with the common medical conditions, co-morbidities, and medical treatments/side effects that are encountered in patients with RA. Future studies should delve into possible differences in long-term outcomes of patients with RA undergoing TKA and THA, as well as whether certain perioperative strategies and therapies (medical or physical) may decrease complications and improve outcomes.
This paper will be judged for the Resident Writer’s Award.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
- Myasoedova E, Davis JM 3rd, Crowson CS, Gabriel SE. Epidemiology of rheumatoid arthritis: rheumatoid arthritis and mortality. Curr Rheumatol Rep. 2010;12(5):379-385. doi:10.1007/s11926-010-0117-y.
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356-361. doi:10.1038/nature01661.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2011;25(4):469-483. doi:10.1016/j.berh.2011.10.009.
- Masuda H, Miyazaki T, Shimada K, et al. Disease duration and severity impacts on long-term cardiovascular events in Japanese patients with rheumatoid arthritis. J Cardiol. 2014;64(5):366-370. doi:10.1016/j.jjcc.2014.02.018.
- Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum.2010;62(6):1583-1591. doi:10.1002/art.27405.
- Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46(9):2287-2293. doi:10.1002/art.10524.
- Rossini M, Rossi E, Bernardi D, et al. Prevalence and incidence of rheumatoid arthritis in Italy. Rheumatol Int. 2014;34(5):659664. doi:10.1007/s00296-014-2974-6.
- Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006;36(3):182-188. doi:10.1016/j.semarthrit.2006.08.006.
- Carbonell J, Cobo T, Balsa A, Descalzo MA, Carmona L. The incidence of rheumatoid arthritis in Spain: results from a nationwide primary care registry. Rheumatology.2008;47(7):1088-1092. doi:10.1093/rheumatology/ken205.
- Skytta ET, Honkanen PB, Eskelinen A, Huhtala H, Remes V. Fewer and older patients with rheumatoid arthritis need total knee replacement. Scand J Rheumatol. 2012;41(5):345-349. doi:10.3109/03009742.2012.681061.
- Singh JA, Vessely MB, Harmsen WS, et al. A population-based study of trends in the use of total hip and total knee arthroplasty, 1969–2008. Mayo Clin Proc. 2010;85(10):898-904. doi:10.4065/mcp.2010.0115.
- Momohara S, Inoue E, Ikari K, et al. Decrease in orthopaedic operations, including total joint replacements, in patients with rheumatoid arthritis between 2001 and 2007: data from Japanese outpatients in a single institute-based large observational cohort (IORRA). Ann Rheum Dis. 2010;69(1):312-313. doi:10.1136/ard.2009.107599.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Howe CR, Gardner GC, Kadel NJ. Perioperative medication management for the patient with rheumatoid arthritis. J Am Acad Orthop Surg. 2006;14(9):544-551. doi:10.5435/00124635-200609000-00004.
- Schrama JC, Espehaug B, Hallan G, et al. Risk of revision for infection in primary total hip and knee arthroplasty in patients with rheumatoid arthritis compared with osteoarthritis: a prospective, population-based study on 108,786 hip and knee joint arthroplasties from the Norwegian Arthroplasty Register. Arthritis Care Res. 2010;62(4):473-479. doi:10.1002/acr.20036.
- Ravi B, Croxford R, Hollands S, et al. Increased risk of complications following total joint arthroplasty in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):254-263. doi:10.1002/art.38231.
- Au K, Reed G, Curtis JR, et al. High disease activity is associated with an increased risk of infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2011;70(5):785-791. doi:10.1136/ard.2010.128637.
- Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295(19):2275-2285. doi:10.1001/jama.295.19.2275.
- Scherrer CB, Mannion AF, Kyburz D, Vogt M, Kramers-de Quervain IA. Infection risk after orthopedic surgery in patients with inflammatory rheumatic diseases treated with immunosuppressive drugs. Arthritis Care Res. 2013;65(12):2032-2040. doi:10.1002/acr.22077.
- Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum.2012;64(1):53-61. doi:10.1002/art.33322.
- Wallberg-Jonsson S, Dahlen GH, Nilsson TK, Ranby M, Rantapaa-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. 1993;12(3):318324.
- van Heereveld HA, Laan RF, van den Hoogen FH, Malefijt MC, Novakova IR, van de Putte LB. Prevention of symptomatic thrombosis with short term (low molecular weight) heparin in patients with rheumatoid arthritis after hip or knee replacement. Ann Rheum Dis.2001;60(10):974-976. doi:10.1136/ard.60.10.974.
- Mont MA, Jacobs JJ, Boggio LN, et al. Preventing venous thromboembolic disease in patients undergoing elective hip and knee arthroplasty. J Am Acad Orthop Surg.2011;19(12):768-776.
- Bozic KJ, Bashyal RK, Anthony SG, Chiu V, Shulman B, Rubash HE. Is administratively coded comorbidity and complication data in total joint arthroplasty valid? Clin Orthop Relat Res. 2013;471(1):201-205. doi:10.1007/s11999-012-2352-1.
TAKE-HOME POINTS
- Patients undergoing THA for OA, when compared to those with RA undergoing THA, had lower risk for postoperative cardiovascular, pulmonary, wound dehiscence, infections, and systemic complications.
- Patients with OA undergoing THA had statistically significant higher risk of cerebrovascular complication compared to RA patients undergoing the same procedure.
- In TKA, OA patients had significantly higher risk for cardiovascular and cerebrovascular complications, and a significant lower risk for mechanical wounds, infection, and systemic complications.
- RA patients are at higher risk for postoperative infection, wound dehiscence, and systemic complications after TKA and THA compared to OA patients.
- These findings highlight the importance of preoperative medical clearance and management to optimize RA patients and improve the postoperative outcomes.
Outcomes After Peripheral Nerve Block in Hip Arthroscopy
ABSTRACT
Pain control following hip arthroscopy presents a significant clinical challenge, with postoperative pain requiring considerable opioid use. Peripheral nerve blocks (PNBs) have emerged as one option to improve pain and limit the consequences of opioid use. The purpose of this study is to provide a comprehensive review of outcomes associated with PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications. A systematic review of PubMed, Medline, Scopus, and Embase databases was conducted through January 2015 for English-language articles reporting outcome data, with 2 reviewers independently reviewing studies for inclusion. When available, similar outcomes were combined to generate frequency-weighted means. Six studies met the inclusion criteria for this review, reporting on 710 patients undergoing hip arthroscopy. The mean ages were 37.0 and 37.7 years for the PNB and comparator groups, respectively, with a reported total of 281 (40.5%) male and 412 (59.5%) female patients. Postoperative post-anesthesia care unit (PACU) pain was consistently reduced in the PNB group, with the use of a lower morphine equivalent dose and lower rates of inpatient admission, compared with that in the control groups. Postoperative nausea and/or vomiting as well as PACU discharge time showed mixed results. High satisfaction and few complications were reported. In conclusion, PNB is associated with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions, though similar rates of nausea/vomiting and time to discharge were reported. Current PNB techniques are varied, and future research efforts should focus on examining which of these methods provides the optimal risk-benefit profile in hip arthroscopy.
Continue to: Hip arthroscopy has emerged...
Hip arthroscopy has emerged as a useful procedure in the diagnosis and treatment of hip pathology,1-8 experiencing a substantial rise in popularity in recent years, with the number of procedures growing by a factor of 18 from 1999 to 20099 and 25 from 2006 to 2013.10 Though hip arthroscopy is beneficial in many cases, marked postoperative pain has presented a substantial challenge, with patients requiring considerable doses of opiate-based medications in the post-anesthesia care unit (PACU).11,12 Increased narcotic use carries increased side effects, including postoperative nausea and vomiting,13 and poorly managed pain leads to increased unplanned admissions.14 Furthermore, patients with chronic hip pain and long-term opioid use may experience heightened and prolonged pain following the procedure, owing to medication tolerance and reduced opioid efficacy in this setting.15
Several pain control strategies have been employed in patients undergoing hip arthroscopy. General anesthesia16,17 and combined spinal epidural (CSE)18 are commonly used. However, such techniques rely heavily on opioids for postoperative pain control,11 and epidural anesthesia commonly requires adjunctive treatments (eg, neuromuscular blockade) to ensure muscle relaxation for joint distraction.19 One technique that has been employed recently is peripheral nerve block (PNB), which has been associated with a significant decrease in postoperative opioid use and nausea and vomiting.13,20 This method has proven successful in other fields of arthroscopy, including shoulder arthroscopy, in which it resulted in faster recovery, reduced opioid consumption,21 and demonstrated cost-effectiveness22 compared with general anesthesia and knee arthroscopy.23-26 As it is a relatively new field, little is known about the use of PNB in hip arthroscopy.
The goal of this systematic review was to comprehensively review the studies reporting on PNB in hip arthroscopy. We specifically focused on outcomes, including postoperative pain; analgesic use; nausea, vomiting, and antiemetic use; discharge time; inpatient admission; and patient satisfaction, as well as the complications associated with the use of PNB. Our knowledge of outcomes associated with PNB in hip arthroscopy is based on a few individual studies that have reported on small groups of patients using a variety of outcome measures and other findings. Furthermore, each of these studies commonly reflects the experience of an individual surgeon at a single institution and, when taken alone, may not be an accurate representation of the more general outcomes associated with PNB. A comprehensive review of such studies will provide surgeons, anesthesiologists, and patients with a better understanding of the anticipated outcomes of using PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications.
MATERIALS AND METHODS
A systematic review of outcomes associated with PNB in hip arthroscopy was performed using the available English-language literature in accordance with the guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and included studies retrieved from the PubMed, Medline, Scopus, and Embase computerized literature databases. Searches were executed comprising all years from database inception through January 2015. Articles were retrieved by an electronic search of medical subject headings and keyword terms and their respective combinations (Table 1). The inclusion criteria for studies in this systematic review were studies that (1) were written in the English language and (2) reported explicit outcome data. The exclusion criteria were (1) review articles, meta-analyses, case reports, abstracts/conference papers, comments/letters, or technique articles without reported patient data and (2) basic research, biomechanics, or animal/cadaveric studies without reported patient data.
Table 1. Search Terms Entered to Identify English-Language Studies Through January 2015
Database | Search terms |
PubMed, Scopus | Keyword: (hip AND arthroscopy) AND (pain control OR pain management OR pain regimen OR nerve block OR spinal anesthesia OR regional anesthesia OR general anesthesia) |
Medline | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “Anesthesia, General” OR “Anesthesia” OR “Anesthesia, Inhalation”, OR “Balanced Anesthesia” OR “Anesthesia, Local” OR “Anesthesia, Spinal” OR “Anesthesia, Conduction” OR “Nerve Block”) |
Embase | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “General Anesthesia” OR “Anesthesia” OR “Inhalation Anesthesia”, OR “Balanced Anesthesia” OR “Local Anesthesia” OR “Spinal Anesthesia” OR “Regional Anesthesia” OR “Nerve Block”) |
The literature search strategy is outlined in the Figure. The initial title search yielded a subset of possible articles that were then further included or excluded on the basis of the contents of the article’s abstract, wherein articles were again selected on the basis of the aforementioned inclusion and exclusion criteria. Articles selected in both the title and abstract phases underwent full-text review, during which the full text of each qualifying article was reviewed. In addition, the reference sections from articles undergoing full-text review were scanned to identify any additional studies that had not been identified in the original literature search. Appropriate studies for final inclusion were then selected at this stage. The title, abstract, and full-text selection process were performed by 2 of the study authors (Dr. Steinhaus and Dr. Lynch), with any discrepancies being discussed and resolved by mutual agreement.
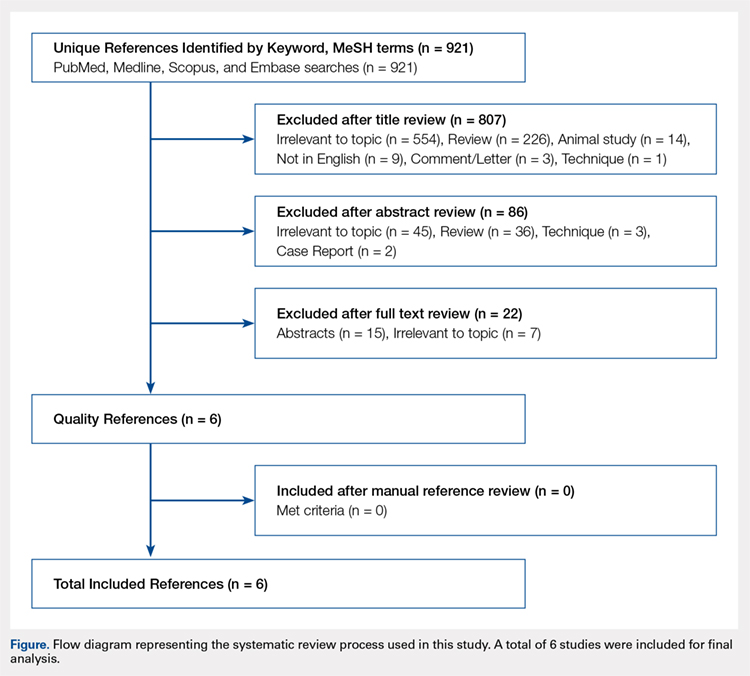
Continue to: For all 6 included studies...
For all 6 included studies,16-18,27-29 data were collected regarding the study specifics, patients included, and outcomes measured in the study. The journal of publication, type of study, level of evidence, and type of PNB, as well as the presence of a comparator group were noted (Table 2). Patient information included the number of patients at baseline and follow-up, mean age, gender, weight, height, body mass index, American Society of Anesthesiologists (ASA) status, and the specific procedures performed. In addition, data were collected on outcomes, including postoperative pain, as well as secondary outcomes and additional findings reported by the studies (Table 3). Where possible, weighted averages were calculated across all studies to obtain aggregate data.
RESULTS
STUDY INCLUSION
Six studies, all published between 2012 and 2014, were included in this systematic review (Table 2). Three studies involved lumbar plexus block, 2 studies involved femoral nerve block, and 1 study evaluated fascia iliaca block. Two studies used a control group of patients who received only general anesthesia (compared with the treatment group who received both general anesthesia and PNB); another study compared intravenous morphine with PNB; and 1 study compared CSE alone with PNB in addition to epidural.
DEMOGRAPHIC DATA
Demographic data from the included studies are presented in Table 2. In total, 710 and 549 patients were evaluated at baseline and final follow-up, respectively, which represents a follow-up rate of 77%. The frequency-weighted mean age of patients receiving PNB was 37.0 years compared with 37.7 years in the comparison groups, and the studies reported a total of 281 (40.5%) male and 412 (59.5%) female patients. The procedures performed were heterogeneously reported; therefore, totals were not tabulated, although the reported procedures included osteochondroplasty, labral débridement, labral and/or capsular repair, gluteus minimus repair, and synovectomy.
POSTOPERATIVE PAIN
Four studies reported on postoperative pain, and these data are presented in Table 3. In a retrospective study of patients receiving femoral nerve block in addition to general anesthesia, Dold and colleagues16 noted postoperative pain at 0, 15, 30, 45, and 60 minutes following arrival in the PACU, and discovered a statistically significantly lower level of pain at 60 minutes compared with inpatients receiving general anesthesia alone. YaDeau and colleagues18 found a significantly lower level of pain at rest in the PACU for those receiving CSE and lumbar plexus blockade compared with those receiving CSE alone. This significant difference did not persist at 24 hours or 6 months after the procedure, nor did it exist for pain with movement at any time point. Similarly, Schroeder and colleagues17 examined patients receiving general anesthesia and lumbar plexus block and found a significant reduction in pain immediately postoperatively in the PACU, though these effects disappeared the day following the procedure. Krych and colleagues27 also reported on postoperative pain in patients undergoing fascia iliaca blockade, although they did not include a comparator group. Outcome comparison between patients who received PNB and controls in the PACU and 1 day following the procedure are presented in Table 4.
ANALGESIC USE
Four studies reported on analgesic use after PNB, and these data are presented in Table 3. Dold and colleagues16 noted analgesic use intraoperatively, in the PACU, and in the surgical day care unit (SDCU). These authors found a significant reduction in morphine equivalent dose given in the operating room and in the PACU in the group receiving PNB, with a nonsignificant trend toward lower use of oxycodone in the SDCU. Schroeder and colleagues17 similarly reported significant reductions in morphine equivalent dose intraoperatively and in Phase I recovery for patients receiving PNB, and these differences disappeared in Phase II recovery as well as intraoperatively if the block dose was considered. In addition, these authors found a significant reduction in the use of fentanyl and hydromorphone in the operating room in the PNB group, as well as a significant reduction in the proportion of patients receiving ketorolac in the operating room or PACU. Finally, YaDeau and colleagues18 reported total analgesic usage in the PACU among PNB patients compared with those receiving CSE alone and showed a strong trend toward reduced use in the PNB group, although this difference was not significant (P = .051). PACU analgesic use is presented in Table 4.
Continue to: Postoperative nausea...
POSTOPERATIVE NAUSEA/VOMITING AND ANTIEMETIC USE
Five studies presented data on nausea, vomiting, or antiemetic use following PNB and are shown in Table 3. YaDeau and colleagues18 reported nausea among 34% of patients in the PNB group, compared with 20% in the control group, vomiting in 2% and 7%, respectively, and antiemetic use in 12% of both groups. Dold and colleagues16 identified a similar trend, with 41.1% of patients in the PNB group and 32.5% of patients in the control group experiencing postoperative nausea or vomiting, while Krych and colleagues27 noted only 10% of PNB patients with mild nausea and none requiring antiemetic use. In their study of patients receiving PNB, Schroeder and colleagues17 found a significant reduction in antiemetic use among PNB patients compared with those receiving general anesthesia alone. Similarly, Ward and colleagues29 noted a significant difference in postoperative nausea, with 10% of patients in the PNB group experiencing postoperative nausea compared with 75% of those in the comparator group who received intravenous morphine. The mean percentage of patients experiencing postoperative nausea and/or vomiting is shown in Table 4.
DISCHARGE TIME
Four studies presented data on discharge time from the PACU and are summarized in Table 3. Three of these studies included a comparator group. Both Dold and colleagues16 and YaDeau and colleagues18 reported an increase in the time to discharge for patients receiving PNB, although these differences were not significant. The study by Ward and colleagues,29 on the other hand, noted a significant reduction in the time to discharge for the PNB group. In addition to these studies, Krych and colleagues27 examined the time from skin closure to discharge for patients receiving PNB, noting a mean 199 minutes for the patients in their study. Mean times to discharge for the PNB and control groups are presented in Table 4.
INPATIENT ADMISSION
Four studies presented data on the proportion of study participants who were admitted as inpatients, and these data are shown in Table 3. Dold and colleagues16 reported no inpatient admissions in their PNB group compared with 5.0% for the control group (both cases of pain control), while YaDeau and colleagues18 found that 3 admissions occurred, with 2 in the control group (1 for oxygen desaturation and the other for intractable pain and nausea) and 1 from the PNB group (epidural spread and urinary retention). Two additional studies reported data on PNB groups alone. Krych and colleagues27 observed no overnight admissions in their study, while Nye and colleagues28 reported 1 readmission for bilateral leg numbness and weakness due to epidural spread, which resolved following discontinuation of the block. The mean proportion of inpatient admissions is presented in Table 4.
SATISFACTION
A total of 3 studies examined patient satisfaction, and these data are presented in Table 3. In their study, Ward and colleagues29 reported a significantly greater rate of satisfaction at 1 day postoperatively among the patients in the PNB group (90%) than among patients who received intravenous morphine (25%) (P < .0001). Similarly, YaDeau and colleagues18 noted greater satisfaction among the PNB group than among the control group, with PNB patients rating their satisfaction at a mean of 8.6 and control patients at a mean of 7.9 on a 10-point scale (0-10) 24 hours postoperatively, although this difference was not significant. Finally, Krych and colleagues27 found that 67% of patients were “very satisfied” and 33% were “satisfied”, based on a Likert scale.
COMPLICATIONS
Four studies presented data on complications, and these findings are summarized in Table 3. In their work, Nye and colleagues28 reported most extensively on complications associated with PNB. Overall, the authors found a rate of significant complications of 3.8%. In terms of specific complications, they noted local anesthetic systemic toxicity (0.9%), epidural spread (0.5%), sensory or motor deficits (9.4%), falls (0.5%), and catheter issues. In their study of patients receiving PNB and CSE, YaDeau and colleagues18 identified 1 patient in the PNB group with epidural spread and urinary retention, while they noted 1 case of oxygen desaturation and another case of intractable pain and nausea in the group receiving CSE alone, all 3 of which required inpatient admission. They found no permanent adverse events attributable to the PNB. In another study, Dold and colleagues16 observed no complications in patients receiving PNB compared with those in 2 admissions in the control group for inadequate pain control. Similarly, Krych and colleagues27 identified no complications in patients who received PNB in their study.
DISCUSSION
Hip arthroscopy has experienced a substantial gain in popularity in recent years, emerging as a beneficial technique for both the diagnosis and treatment of diverse hip pathologies in patients spanning a variety of demographics. Nevertheless, postoperative pain control, as well as medication side effects and unwanted patient admissions, present major challenges to the treating surgeon. As an adjuvant measure, peripheral nerve block represents one option to improve postoperative pain management, while at the same time addressing the adverse effects of considerable opioid use, which is commonly seen in these patients. Early experience with this method in hip arthroscopy was reported in a case series by Lee and colleagues.12 In an attempt to reduce postoperative pain, as well as limit the adverse effects and delay in discharge associated with considerable opioid use in the PACU, the authors used preoperative paravertebral blocks of L1 and L2 in 2 patients requiring hip arthroscopy with encouraging results. Since then, a number of studies have attempted the use of PNB in hip arthroscopy.16-18,27-29 However, we were unable to identify any prior reviews reporting on peripheral nerve blockade in hip arthroscopy, and thus this study is unique in providing a greater understanding of the outcomes associated with PNB use.
In general, we found that PNB was associated with improved outcomes. Based on the studies included in this review, there was a statistically significantly lower level of pain in the PACU for femoral nerve block (compared with general anesthesia alone)16 and lumbar plexus blockade (compared with general anesthesia17 and CSE18 alone). Nevertheless, these effects are likely short-lived, with differences disappearing the day following the procedure. In terms of analgesic use, 2 studies report significant reductions in analgesic use intraoperatively and in the PACU/Phase I recovery,16,17 with a third reporting a strong trend toward reduced analgesic use in the PACU (P = .051).18 Finally, we report fewer admissions for the PNB group, as well as high rates of satisfaction and few complications across these studies.
Continue to: Unlike these measures...
Unlike these measures, postoperative nausea, vomiting, and antiemetic use, as well as time to discharge, showed more mixed results. With regard to nausea/vomiting, 2 studies16,18 reported nonsignificantly increased rates in the PNB group, whereas others reported significant reductions in nausea/vomiting29 and in the proportion of patients receiving antiemetics.17 Similarly, mixed results were seen in terms of patient discharge time from the PACU. Two studies16,18 reported a nonsignificant increase in time to discharge for the PNB group, while another29 noted a significant reduction for the PNB group compared with those receiving intravenous morphine. These mixed results were surprising, as we expected reductions in opioid use to result in fewer instances of nausea/vomiting and a quicker time to discharge. The reasons underlying these findings are not clear, although it has been suggested that current discharge guidelines and clinical pathways limit the ability to take advantage of the accelerated timeline offered by regional anesthesia.16,30 As experience with PNB grows, our guidelines and pathways are likely to adapt to capitalize on these advantages, and future studies may show more reliable improvements in these measures.
While rare, the risk of bleeding requiring blood transfusion following hip arthroscopy is one of the most common complications of this procedure. Though the studies included in this review did not report on the need for transfusion, a recent study by Cvetanovich and colleagues10 used a national database and found that, of patients undergoing hip arthroscopy (n = 1338), 0.4% (n = 5) had bleeding requiring a transfusion, with 0.3% (n = 4) requiring return to the operating room, similar to an earlier study by Clarke and colleagues,31 who noted bleeding from the portal site in 0.4% of hip arthroscopy patients. In terms of risk factors, Cvetanovich and colleagues10 found that ASA class, older age, and prior cardiac surgery were significantly associated with minor and overall complications, whereas both regional anesthesia/monitored anesthesia care and alcohol consumption of >2 drinks a day were significantly associated with minor complications, including bleeding requiring transfusions. They noted, however, that these risk factors accounted for only 5% of the variance in complication rates, indicating that other unidentified variables better explained the variance in complication rates. These authors concluded that complications associated with hip arthroscopy are so rare that we may not be able to predict which risk factors or anesthesia types are more likely to cause them. Further characterization of bleeding following hip arthroscopy and its associated risk factors is a valuable area for future research.
LIMITATIONS
Our study contains a number of limitations. This review included studies whose level of evidence varied from I to IV; therefore, our study is limited by any bias or heterogeneity introduced in patient recruitment, selection, variability of technique, data collection, and analysis used in these studies. This heterogeneity is most apparent in the block types and comparator groups. Furthermore, several different outcome measures were reported across the 6 studies used in this review, which decreased the relevance of any one of these individual outcomes. Finally, given the limited data that currently exist for the use of PNB in hip arthroscopy, we are unable to note meaningful differences between various types of PNBs, such as differences in postoperative pain or other measures such as quadriceps weakness, which can accompany femoral nerve block.12 While it is important to read our work with these limitations in mind, this systematic review is, to our knowledge, the only comprehensive review to date of studies reporting on PNB in hip arthroscopy, providing clinicians and patients with a greater understanding of the associated outcomes across these studies.
CONCLUSION
This systematic review shows improved outcomes and few complications with PNB use in hip arthroscopy, with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions. Although opioid use was reduced in these studies, we found similar rates of postoperative nausea/vomiting as well as similar time to discharge from the PACU, which may reflect our continued reliance on outdated discharge guidelines and clinical pathways. Current attempts to provide peripheral nerve blockade are quite varied, with studies targeting femoral nerve, fascia iliaca, L1/L2 paravertebral, and lumbar plexus blockade. Future research efforts with a large prospective trial investigating these techniques should focus on which of these PNBs presents the optimal risk-benefit profile for hip arthroscopy patients and thus appropriately address the clinical questions at hand.
This paper will be judged for the Resident Writer’s Award.
- Baber YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip worthwhile? A prospective review of 328 adults investigated for hip pain. J Bone Joint Surg Br. 1999;81:600-603.
- Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379-1388.
- Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540-546.
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181-188.
- Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
- Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral debridement. Am J Sports Med. 2005;33:864-870.
- Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
- Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7:269-274.
- Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94:e23.
- Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016 Apr 8. [Epub before print].
- Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399-1402.
- Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462-465.
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105:1234-1242.
- Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206-1213.
- Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988-1993.
- Dold AP, Murnaghan L, Xing J, Abdallah FW, Brull R, Whelan DB. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med. 2014;42:144-149.
- Schroeder KM, Donnelly MJ, Anderson BM, Ford MP, Keene JS. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int. 2013;23:93-98.
- YaDeau JT, Tedore T, Goytizolo EA, et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg. 2012;115:968-972.
- Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23:1348-1353.
- Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care. 2007;35:949-952.
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol. 2015;25:255-261.
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103:428-433.
- Hadzic A, Karaca PE, Hobeika P, et al. Peripheral nerve blocks result in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesth Analg. 2005;100:976-981.
- Hsu LP, Oh S, Nuber GW, et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465-1472.
- Montes FR, Zarate E, Grueso R, et al. Comparison of spinal anesthesia with combined sciatic-femoral nerve block for outpatient knee arthroscopy. J Clin Anesth. 2008;20:415-420.
- Wulf H, Lowe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand. 2010;54:414-420.
- Krych AJ, Baran S, Kuzma SA, Smith HM, Johnson RL, Levy BA. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:843-847.
- Nye ZB, Horn JL, Crittenden W, Abrahams MS, Aziz MF. Ambulatory continuous posterior lumbar plexus blocks following hip arthroscopy: a review of 213 cases. J Clin Anesth. 2013;25:268-274.
- Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy. 2012;28:1064-1069.
- Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634-1642.
- Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-88.
ABSTRACT
Pain control following hip arthroscopy presents a significant clinical challenge, with postoperative pain requiring considerable opioid use. Peripheral nerve blocks (PNBs) have emerged as one option to improve pain and limit the consequences of opioid use. The purpose of this study is to provide a comprehensive review of outcomes associated with PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications. A systematic review of PubMed, Medline, Scopus, and Embase databases was conducted through January 2015 for English-language articles reporting outcome data, with 2 reviewers independently reviewing studies for inclusion. When available, similar outcomes were combined to generate frequency-weighted means. Six studies met the inclusion criteria for this review, reporting on 710 patients undergoing hip arthroscopy. The mean ages were 37.0 and 37.7 years for the PNB and comparator groups, respectively, with a reported total of 281 (40.5%) male and 412 (59.5%) female patients. Postoperative post-anesthesia care unit (PACU) pain was consistently reduced in the PNB group, with the use of a lower morphine equivalent dose and lower rates of inpatient admission, compared with that in the control groups. Postoperative nausea and/or vomiting as well as PACU discharge time showed mixed results. High satisfaction and few complications were reported. In conclusion, PNB is associated with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions, though similar rates of nausea/vomiting and time to discharge were reported. Current PNB techniques are varied, and future research efforts should focus on examining which of these methods provides the optimal risk-benefit profile in hip arthroscopy.
Continue to: Hip arthroscopy has emerged...
Hip arthroscopy has emerged as a useful procedure in the diagnosis and treatment of hip pathology,1-8 experiencing a substantial rise in popularity in recent years, with the number of procedures growing by a factor of 18 from 1999 to 20099 and 25 from 2006 to 2013.10 Though hip arthroscopy is beneficial in many cases, marked postoperative pain has presented a substantial challenge, with patients requiring considerable doses of opiate-based medications in the post-anesthesia care unit (PACU).11,12 Increased narcotic use carries increased side effects, including postoperative nausea and vomiting,13 and poorly managed pain leads to increased unplanned admissions.14 Furthermore, patients with chronic hip pain and long-term opioid use may experience heightened and prolonged pain following the procedure, owing to medication tolerance and reduced opioid efficacy in this setting.15
Several pain control strategies have been employed in patients undergoing hip arthroscopy. General anesthesia16,17 and combined spinal epidural (CSE)18 are commonly used. However, such techniques rely heavily on opioids for postoperative pain control,11 and epidural anesthesia commonly requires adjunctive treatments (eg, neuromuscular blockade) to ensure muscle relaxation for joint distraction.19 One technique that has been employed recently is peripheral nerve block (PNB), which has been associated with a significant decrease in postoperative opioid use and nausea and vomiting.13,20 This method has proven successful in other fields of arthroscopy, including shoulder arthroscopy, in which it resulted in faster recovery, reduced opioid consumption,21 and demonstrated cost-effectiveness22 compared with general anesthesia and knee arthroscopy.23-26 As it is a relatively new field, little is known about the use of PNB in hip arthroscopy.
The goal of this systematic review was to comprehensively review the studies reporting on PNB in hip arthroscopy. We specifically focused on outcomes, including postoperative pain; analgesic use; nausea, vomiting, and antiemetic use; discharge time; inpatient admission; and patient satisfaction, as well as the complications associated with the use of PNB. Our knowledge of outcomes associated with PNB in hip arthroscopy is based on a few individual studies that have reported on small groups of patients using a variety of outcome measures and other findings. Furthermore, each of these studies commonly reflects the experience of an individual surgeon at a single institution and, when taken alone, may not be an accurate representation of the more general outcomes associated with PNB. A comprehensive review of such studies will provide surgeons, anesthesiologists, and patients with a better understanding of the anticipated outcomes of using PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications.
MATERIALS AND METHODS
A systematic review of outcomes associated with PNB in hip arthroscopy was performed using the available English-language literature in accordance with the guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and included studies retrieved from the PubMed, Medline, Scopus, and Embase computerized literature databases. Searches were executed comprising all years from database inception through January 2015. Articles were retrieved by an electronic search of medical subject headings and keyword terms and their respective combinations (Table 1). The inclusion criteria for studies in this systematic review were studies that (1) were written in the English language and (2) reported explicit outcome data. The exclusion criteria were (1) review articles, meta-analyses, case reports, abstracts/conference papers, comments/letters, or technique articles without reported patient data and (2) basic research, biomechanics, or animal/cadaveric studies without reported patient data.
Table 1. Search Terms Entered to Identify English-Language Studies Through January 2015
Database | Search terms |
PubMed, Scopus | Keyword: (hip AND arthroscopy) AND (pain control OR pain management OR pain regimen OR nerve block OR spinal anesthesia OR regional anesthesia OR general anesthesia) |
Medline | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “Anesthesia, General” OR “Anesthesia” OR “Anesthesia, Inhalation”, OR “Balanced Anesthesia” OR “Anesthesia, Local” OR “Anesthesia, Spinal” OR “Anesthesia, Conduction” OR “Nerve Block”) |
Embase | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “General Anesthesia” OR “Anesthesia” OR “Inhalation Anesthesia”, OR “Balanced Anesthesia” OR “Local Anesthesia” OR “Spinal Anesthesia” OR “Regional Anesthesia” OR “Nerve Block”) |
The literature search strategy is outlined in the Figure. The initial title search yielded a subset of possible articles that were then further included or excluded on the basis of the contents of the article’s abstract, wherein articles were again selected on the basis of the aforementioned inclusion and exclusion criteria. Articles selected in both the title and abstract phases underwent full-text review, during which the full text of each qualifying article was reviewed. In addition, the reference sections from articles undergoing full-text review were scanned to identify any additional studies that had not been identified in the original literature search. Appropriate studies for final inclusion were then selected at this stage. The title, abstract, and full-text selection process were performed by 2 of the study authors (Dr. Steinhaus and Dr. Lynch), with any discrepancies being discussed and resolved by mutual agreement.

Continue to: For all 6 included studies...
For all 6 included studies,16-18,27-29 data were collected regarding the study specifics, patients included, and outcomes measured in the study. The journal of publication, type of study, level of evidence, and type of PNB, as well as the presence of a comparator group were noted (Table 2). Patient information included the number of patients at baseline and follow-up, mean age, gender, weight, height, body mass index, American Society of Anesthesiologists (ASA) status, and the specific procedures performed. In addition, data were collected on outcomes, including postoperative pain, as well as secondary outcomes and additional findings reported by the studies (Table 3). Where possible, weighted averages were calculated across all studies to obtain aggregate data.
RESULTS
STUDY INCLUSION
Six studies, all published between 2012 and 2014, were included in this systematic review (Table 2). Three studies involved lumbar plexus block, 2 studies involved femoral nerve block, and 1 study evaluated fascia iliaca block. Two studies used a control group of patients who received only general anesthesia (compared with the treatment group who received both general anesthesia and PNB); another study compared intravenous morphine with PNB; and 1 study compared CSE alone with PNB in addition to epidural.
DEMOGRAPHIC DATA
Demographic data from the included studies are presented in Table 2. In total, 710 and 549 patients were evaluated at baseline and final follow-up, respectively, which represents a follow-up rate of 77%. The frequency-weighted mean age of patients receiving PNB was 37.0 years compared with 37.7 years in the comparison groups, and the studies reported a total of 281 (40.5%) male and 412 (59.5%) female patients. The procedures performed were heterogeneously reported; therefore, totals were not tabulated, although the reported procedures included osteochondroplasty, labral débridement, labral and/or capsular repair, gluteus minimus repair, and synovectomy.
POSTOPERATIVE PAIN
Four studies reported on postoperative pain, and these data are presented in Table 3. In a retrospective study of patients receiving femoral nerve block in addition to general anesthesia, Dold and colleagues16 noted postoperative pain at 0, 15, 30, 45, and 60 minutes following arrival in the PACU, and discovered a statistically significantly lower level of pain at 60 minutes compared with inpatients receiving general anesthesia alone. YaDeau and colleagues18 found a significantly lower level of pain at rest in the PACU for those receiving CSE and lumbar plexus blockade compared with those receiving CSE alone. This significant difference did not persist at 24 hours or 6 months after the procedure, nor did it exist for pain with movement at any time point. Similarly, Schroeder and colleagues17 examined patients receiving general anesthesia and lumbar plexus block and found a significant reduction in pain immediately postoperatively in the PACU, though these effects disappeared the day following the procedure. Krych and colleagues27 also reported on postoperative pain in patients undergoing fascia iliaca blockade, although they did not include a comparator group. Outcome comparison between patients who received PNB and controls in the PACU and 1 day following the procedure are presented in Table 4.
ANALGESIC USE
Four studies reported on analgesic use after PNB, and these data are presented in Table 3. Dold and colleagues16 noted analgesic use intraoperatively, in the PACU, and in the surgical day care unit (SDCU). These authors found a significant reduction in morphine equivalent dose given in the operating room and in the PACU in the group receiving PNB, with a nonsignificant trend toward lower use of oxycodone in the SDCU. Schroeder and colleagues17 similarly reported significant reductions in morphine equivalent dose intraoperatively and in Phase I recovery for patients receiving PNB, and these differences disappeared in Phase II recovery as well as intraoperatively if the block dose was considered. In addition, these authors found a significant reduction in the use of fentanyl and hydromorphone in the operating room in the PNB group, as well as a significant reduction in the proportion of patients receiving ketorolac in the operating room or PACU. Finally, YaDeau and colleagues18 reported total analgesic usage in the PACU among PNB patients compared with those receiving CSE alone and showed a strong trend toward reduced use in the PNB group, although this difference was not significant (P = .051). PACU analgesic use is presented in Table 4.
Continue to: Postoperative nausea...
POSTOPERATIVE NAUSEA/VOMITING AND ANTIEMETIC USE
Five studies presented data on nausea, vomiting, or antiemetic use following PNB and are shown in Table 3. YaDeau and colleagues18 reported nausea among 34% of patients in the PNB group, compared with 20% in the control group, vomiting in 2% and 7%, respectively, and antiemetic use in 12% of both groups. Dold and colleagues16 identified a similar trend, with 41.1% of patients in the PNB group and 32.5% of patients in the control group experiencing postoperative nausea or vomiting, while Krych and colleagues27 noted only 10% of PNB patients with mild nausea and none requiring antiemetic use. In their study of patients receiving PNB, Schroeder and colleagues17 found a significant reduction in antiemetic use among PNB patients compared with those receiving general anesthesia alone. Similarly, Ward and colleagues29 noted a significant difference in postoperative nausea, with 10% of patients in the PNB group experiencing postoperative nausea compared with 75% of those in the comparator group who received intravenous morphine. The mean percentage of patients experiencing postoperative nausea and/or vomiting is shown in Table 4.
DISCHARGE TIME
Four studies presented data on discharge time from the PACU and are summarized in Table 3. Three of these studies included a comparator group. Both Dold and colleagues16 and YaDeau and colleagues18 reported an increase in the time to discharge for patients receiving PNB, although these differences were not significant. The study by Ward and colleagues,29 on the other hand, noted a significant reduction in the time to discharge for the PNB group. In addition to these studies, Krych and colleagues27 examined the time from skin closure to discharge for patients receiving PNB, noting a mean 199 minutes for the patients in their study. Mean times to discharge for the PNB and control groups are presented in Table 4.
INPATIENT ADMISSION
Four studies presented data on the proportion of study participants who were admitted as inpatients, and these data are shown in Table 3. Dold and colleagues16 reported no inpatient admissions in their PNB group compared with 5.0% for the control group (both cases of pain control), while YaDeau and colleagues18 found that 3 admissions occurred, with 2 in the control group (1 for oxygen desaturation and the other for intractable pain and nausea) and 1 from the PNB group (epidural spread and urinary retention). Two additional studies reported data on PNB groups alone. Krych and colleagues27 observed no overnight admissions in their study, while Nye and colleagues28 reported 1 readmission for bilateral leg numbness and weakness due to epidural spread, which resolved following discontinuation of the block. The mean proportion of inpatient admissions is presented in Table 4.
SATISFACTION
A total of 3 studies examined patient satisfaction, and these data are presented in Table 3. In their study, Ward and colleagues29 reported a significantly greater rate of satisfaction at 1 day postoperatively among the patients in the PNB group (90%) than among patients who received intravenous morphine (25%) (P < .0001). Similarly, YaDeau and colleagues18 noted greater satisfaction among the PNB group than among the control group, with PNB patients rating their satisfaction at a mean of 8.6 and control patients at a mean of 7.9 on a 10-point scale (0-10) 24 hours postoperatively, although this difference was not significant. Finally, Krych and colleagues27 found that 67% of patients were “very satisfied” and 33% were “satisfied”, based on a Likert scale.
COMPLICATIONS
Four studies presented data on complications, and these findings are summarized in Table 3. In their work, Nye and colleagues28 reported most extensively on complications associated with PNB. Overall, the authors found a rate of significant complications of 3.8%. In terms of specific complications, they noted local anesthetic systemic toxicity (0.9%), epidural spread (0.5%), sensory or motor deficits (9.4%), falls (0.5%), and catheter issues. In their study of patients receiving PNB and CSE, YaDeau and colleagues18 identified 1 patient in the PNB group with epidural spread and urinary retention, while they noted 1 case of oxygen desaturation and another case of intractable pain and nausea in the group receiving CSE alone, all 3 of which required inpatient admission. They found no permanent adverse events attributable to the PNB. In another study, Dold and colleagues16 observed no complications in patients receiving PNB compared with those in 2 admissions in the control group for inadequate pain control. Similarly, Krych and colleagues27 identified no complications in patients who received PNB in their study.
DISCUSSION
Hip arthroscopy has experienced a substantial gain in popularity in recent years, emerging as a beneficial technique for both the diagnosis and treatment of diverse hip pathologies in patients spanning a variety of demographics. Nevertheless, postoperative pain control, as well as medication side effects and unwanted patient admissions, present major challenges to the treating surgeon. As an adjuvant measure, peripheral nerve block represents one option to improve postoperative pain management, while at the same time addressing the adverse effects of considerable opioid use, which is commonly seen in these patients. Early experience with this method in hip arthroscopy was reported in a case series by Lee and colleagues.12 In an attempt to reduce postoperative pain, as well as limit the adverse effects and delay in discharge associated with considerable opioid use in the PACU, the authors used preoperative paravertebral blocks of L1 and L2 in 2 patients requiring hip arthroscopy with encouraging results. Since then, a number of studies have attempted the use of PNB in hip arthroscopy.16-18,27-29 However, we were unable to identify any prior reviews reporting on peripheral nerve blockade in hip arthroscopy, and thus this study is unique in providing a greater understanding of the outcomes associated with PNB use.
In general, we found that PNB was associated with improved outcomes. Based on the studies included in this review, there was a statistically significantly lower level of pain in the PACU for femoral nerve block (compared with general anesthesia alone)16 and lumbar plexus blockade (compared with general anesthesia17 and CSE18 alone). Nevertheless, these effects are likely short-lived, with differences disappearing the day following the procedure. In terms of analgesic use, 2 studies report significant reductions in analgesic use intraoperatively and in the PACU/Phase I recovery,16,17 with a third reporting a strong trend toward reduced analgesic use in the PACU (P = .051).18 Finally, we report fewer admissions for the PNB group, as well as high rates of satisfaction and few complications across these studies.
Continue to: Unlike these measures...
Unlike these measures, postoperative nausea, vomiting, and antiemetic use, as well as time to discharge, showed more mixed results. With regard to nausea/vomiting, 2 studies16,18 reported nonsignificantly increased rates in the PNB group, whereas others reported significant reductions in nausea/vomiting29 and in the proportion of patients receiving antiemetics.17 Similarly, mixed results were seen in terms of patient discharge time from the PACU. Two studies16,18 reported a nonsignificant increase in time to discharge for the PNB group, while another29 noted a significant reduction for the PNB group compared with those receiving intravenous morphine. These mixed results were surprising, as we expected reductions in opioid use to result in fewer instances of nausea/vomiting and a quicker time to discharge. The reasons underlying these findings are not clear, although it has been suggested that current discharge guidelines and clinical pathways limit the ability to take advantage of the accelerated timeline offered by regional anesthesia.16,30 As experience with PNB grows, our guidelines and pathways are likely to adapt to capitalize on these advantages, and future studies may show more reliable improvements in these measures.
While rare, the risk of bleeding requiring blood transfusion following hip arthroscopy is one of the most common complications of this procedure. Though the studies included in this review did not report on the need for transfusion, a recent study by Cvetanovich and colleagues10 used a national database and found that, of patients undergoing hip arthroscopy (n = 1338), 0.4% (n = 5) had bleeding requiring a transfusion, with 0.3% (n = 4) requiring return to the operating room, similar to an earlier study by Clarke and colleagues,31 who noted bleeding from the portal site in 0.4% of hip arthroscopy patients. In terms of risk factors, Cvetanovich and colleagues10 found that ASA class, older age, and prior cardiac surgery were significantly associated with minor and overall complications, whereas both regional anesthesia/monitored anesthesia care and alcohol consumption of >2 drinks a day were significantly associated with minor complications, including bleeding requiring transfusions. They noted, however, that these risk factors accounted for only 5% of the variance in complication rates, indicating that other unidentified variables better explained the variance in complication rates. These authors concluded that complications associated with hip arthroscopy are so rare that we may not be able to predict which risk factors or anesthesia types are more likely to cause them. Further characterization of bleeding following hip arthroscopy and its associated risk factors is a valuable area for future research.
LIMITATIONS
Our study contains a number of limitations. This review included studies whose level of evidence varied from I to IV; therefore, our study is limited by any bias or heterogeneity introduced in patient recruitment, selection, variability of technique, data collection, and analysis used in these studies. This heterogeneity is most apparent in the block types and comparator groups. Furthermore, several different outcome measures were reported across the 6 studies used in this review, which decreased the relevance of any one of these individual outcomes. Finally, given the limited data that currently exist for the use of PNB in hip arthroscopy, we are unable to note meaningful differences between various types of PNBs, such as differences in postoperative pain or other measures such as quadriceps weakness, which can accompany femoral nerve block.12 While it is important to read our work with these limitations in mind, this systematic review is, to our knowledge, the only comprehensive review to date of studies reporting on PNB in hip arthroscopy, providing clinicians and patients with a greater understanding of the associated outcomes across these studies.
CONCLUSION
This systematic review shows improved outcomes and few complications with PNB use in hip arthroscopy, with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions. Although opioid use was reduced in these studies, we found similar rates of postoperative nausea/vomiting as well as similar time to discharge from the PACU, which may reflect our continued reliance on outdated discharge guidelines and clinical pathways. Current attempts to provide peripheral nerve blockade are quite varied, with studies targeting femoral nerve, fascia iliaca, L1/L2 paravertebral, and lumbar plexus blockade. Future research efforts with a large prospective trial investigating these techniques should focus on which of these PNBs presents the optimal risk-benefit profile for hip arthroscopy patients and thus appropriately address the clinical questions at hand.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Pain control following hip arthroscopy presents a significant clinical challenge, with postoperative pain requiring considerable opioid use. Peripheral nerve blocks (PNBs) have emerged as one option to improve pain and limit the consequences of opioid use. The purpose of this study is to provide a comprehensive review of outcomes associated with PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications. A systematic review of PubMed, Medline, Scopus, and Embase databases was conducted through January 2015 for English-language articles reporting outcome data, with 2 reviewers independently reviewing studies for inclusion. When available, similar outcomes were combined to generate frequency-weighted means. Six studies met the inclusion criteria for this review, reporting on 710 patients undergoing hip arthroscopy. The mean ages were 37.0 and 37.7 years for the PNB and comparator groups, respectively, with a reported total of 281 (40.5%) male and 412 (59.5%) female patients. Postoperative post-anesthesia care unit (PACU) pain was consistently reduced in the PNB group, with the use of a lower morphine equivalent dose and lower rates of inpatient admission, compared with that in the control groups. Postoperative nausea and/or vomiting as well as PACU discharge time showed mixed results. High satisfaction and few complications were reported. In conclusion, PNB is associated with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions, though similar rates of nausea/vomiting and time to discharge were reported. Current PNB techniques are varied, and future research efforts should focus on examining which of these methods provides the optimal risk-benefit profile in hip arthroscopy.
Continue to: Hip arthroscopy has emerged...
Hip arthroscopy has emerged as a useful procedure in the diagnosis and treatment of hip pathology,1-8 experiencing a substantial rise in popularity in recent years, with the number of procedures growing by a factor of 18 from 1999 to 20099 and 25 from 2006 to 2013.10 Though hip arthroscopy is beneficial in many cases, marked postoperative pain has presented a substantial challenge, with patients requiring considerable doses of opiate-based medications in the post-anesthesia care unit (PACU).11,12 Increased narcotic use carries increased side effects, including postoperative nausea and vomiting,13 and poorly managed pain leads to increased unplanned admissions.14 Furthermore, patients with chronic hip pain and long-term opioid use may experience heightened and prolonged pain following the procedure, owing to medication tolerance and reduced opioid efficacy in this setting.15
Several pain control strategies have been employed in patients undergoing hip arthroscopy. General anesthesia16,17 and combined spinal epidural (CSE)18 are commonly used. However, such techniques rely heavily on opioids for postoperative pain control,11 and epidural anesthesia commonly requires adjunctive treatments (eg, neuromuscular blockade) to ensure muscle relaxation for joint distraction.19 One technique that has been employed recently is peripheral nerve block (PNB), which has been associated with a significant decrease in postoperative opioid use and nausea and vomiting.13,20 This method has proven successful in other fields of arthroscopy, including shoulder arthroscopy, in which it resulted in faster recovery, reduced opioid consumption,21 and demonstrated cost-effectiveness22 compared with general anesthesia and knee arthroscopy.23-26 As it is a relatively new field, little is known about the use of PNB in hip arthroscopy.
The goal of this systematic review was to comprehensively review the studies reporting on PNB in hip arthroscopy. We specifically focused on outcomes, including postoperative pain; analgesic use; nausea, vomiting, and antiemetic use; discharge time; inpatient admission; and patient satisfaction, as well as the complications associated with the use of PNB. Our knowledge of outcomes associated with PNB in hip arthroscopy is based on a few individual studies that have reported on small groups of patients using a variety of outcome measures and other findings. Furthermore, each of these studies commonly reflects the experience of an individual surgeon at a single institution and, when taken alone, may not be an accurate representation of the more general outcomes associated with PNB. A comprehensive review of such studies will provide surgeons, anesthesiologists, and patients with a better understanding of the anticipated outcomes of using PNB in hip arthroscopy. We hypothesize that the use of PNB in hip arthroscopy leads to improved outcomes and is associated with few complications.
MATERIALS AND METHODS
A systematic review of outcomes associated with PNB in hip arthroscopy was performed using the available English-language literature in accordance with the guidelines laid out by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement and included studies retrieved from the PubMed, Medline, Scopus, and Embase computerized literature databases. Searches were executed comprising all years from database inception through January 2015. Articles were retrieved by an electronic search of medical subject headings and keyword terms and their respective combinations (Table 1). The inclusion criteria for studies in this systematic review were studies that (1) were written in the English language and (2) reported explicit outcome data. The exclusion criteria were (1) review articles, meta-analyses, case reports, abstracts/conference papers, comments/letters, or technique articles without reported patient data and (2) basic research, biomechanics, or animal/cadaveric studies without reported patient data.
Table 1. Search Terms Entered to Identify English-Language Studies Through January 2015
Database | Search terms |
PubMed, Scopus | Keyword: (hip AND arthroscopy) AND (pain control OR pain management OR pain regimen OR nerve block OR spinal anesthesia OR regional anesthesia OR general anesthesia) |
Medline | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “Anesthesia, General” OR “Anesthesia” OR “Anesthesia, Inhalation”, OR “Balanced Anesthesia” OR “Anesthesia, Local” OR “Anesthesia, Spinal” OR “Anesthesia, Conduction” OR “Nerve Block”) |
Embase | MeSH (includes both MeSH terms and keywords): (Hip) AND (Arthroscopy) AND (“Pain Management” OR “General Anesthesia” OR “Anesthesia” OR “Inhalation Anesthesia”, OR “Balanced Anesthesia” OR “Local Anesthesia” OR “Spinal Anesthesia” OR “Regional Anesthesia” OR “Nerve Block”) |
The literature search strategy is outlined in the Figure. The initial title search yielded a subset of possible articles that were then further included or excluded on the basis of the contents of the article’s abstract, wherein articles were again selected on the basis of the aforementioned inclusion and exclusion criteria. Articles selected in both the title and abstract phases underwent full-text review, during which the full text of each qualifying article was reviewed. In addition, the reference sections from articles undergoing full-text review were scanned to identify any additional studies that had not been identified in the original literature search. Appropriate studies for final inclusion were then selected at this stage. The title, abstract, and full-text selection process were performed by 2 of the study authors (Dr. Steinhaus and Dr. Lynch), with any discrepancies being discussed and resolved by mutual agreement.

Continue to: For all 6 included studies...
For all 6 included studies,16-18,27-29 data were collected regarding the study specifics, patients included, and outcomes measured in the study. The journal of publication, type of study, level of evidence, and type of PNB, as well as the presence of a comparator group were noted (Table 2). Patient information included the number of patients at baseline and follow-up, mean age, gender, weight, height, body mass index, American Society of Anesthesiologists (ASA) status, and the specific procedures performed. In addition, data were collected on outcomes, including postoperative pain, as well as secondary outcomes and additional findings reported by the studies (Table 3). Where possible, weighted averages were calculated across all studies to obtain aggregate data.
RESULTS
STUDY INCLUSION
Six studies, all published between 2012 and 2014, were included in this systematic review (Table 2). Three studies involved lumbar plexus block, 2 studies involved femoral nerve block, and 1 study evaluated fascia iliaca block. Two studies used a control group of patients who received only general anesthesia (compared with the treatment group who received both general anesthesia and PNB); another study compared intravenous morphine with PNB; and 1 study compared CSE alone with PNB in addition to epidural.
DEMOGRAPHIC DATA
Demographic data from the included studies are presented in Table 2. In total, 710 and 549 patients were evaluated at baseline and final follow-up, respectively, which represents a follow-up rate of 77%. The frequency-weighted mean age of patients receiving PNB was 37.0 years compared with 37.7 years in the comparison groups, and the studies reported a total of 281 (40.5%) male and 412 (59.5%) female patients. The procedures performed were heterogeneously reported; therefore, totals were not tabulated, although the reported procedures included osteochondroplasty, labral débridement, labral and/or capsular repair, gluteus minimus repair, and synovectomy.
POSTOPERATIVE PAIN
Four studies reported on postoperative pain, and these data are presented in Table 3. In a retrospective study of patients receiving femoral nerve block in addition to general anesthesia, Dold and colleagues16 noted postoperative pain at 0, 15, 30, 45, and 60 minutes following arrival in the PACU, and discovered a statistically significantly lower level of pain at 60 minutes compared with inpatients receiving general anesthesia alone. YaDeau and colleagues18 found a significantly lower level of pain at rest in the PACU for those receiving CSE and lumbar plexus blockade compared with those receiving CSE alone. This significant difference did not persist at 24 hours or 6 months after the procedure, nor did it exist for pain with movement at any time point. Similarly, Schroeder and colleagues17 examined patients receiving general anesthesia and lumbar plexus block and found a significant reduction in pain immediately postoperatively in the PACU, though these effects disappeared the day following the procedure. Krych and colleagues27 also reported on postoperative pain in patients undergoing fascia iliaca blockade, although they did not include a comparator group. Outcome comparison between patients who received PNB and controls in the PACU and 1 day following the procedure are presented in Table 4.
ANALGESIC USE
Four studies reported on analgesic use after PNB, and these data are presented in Table 3. Dold and colleagues16 noted analgesic use intraoperatively, in the PACU, and in the surgical day care unit (SDCU). These authors found a significant reduction in morphine equivalent dose given in the operating room and in the PACU in the group receiving PNB, with a nonsignificant trend toward lower use of oxycodone in the SDCU. Schroeder and colleagues17 similarly reported significant reductions in morphine equivalent dose intraoperatively and in Phase I recovery for patients receiving PNB, and these differences disappeared in Phase II recovery as well as intraoperatively if the block dose was considered. In addition, these authors found a significant reduction in the use of fentanyl and hydromorphone in the operating room in the PNB group, as well as a significant reduction in the proportion of patients receiving ketorolac in the operating room or PACU. Finally, YaDeau and colleagues18 reported total analgesic usage in the PACU among PNB patients compared with those receiving CSE alone and showed a strong trend toward reduced use in the PNB group, although this difference was not significant (P = .051). PACU analgesic use is presented in Table 4.
Continue to: Postoperative nausea...
POSTOPERATIVE NAUSEA/VOMITING AND ANTIEMETIC USE
Five studies presented data on nausea, vomiting, or antiemetic use following PNB and are shown in Table 3. YaDeau and colleagues18 reported nausea among 34% of patients in the PNB group, compared with 20% in the control group, vomiting in 2% and 7%, respectively, and antiemetic use in 12% of both groups. Dold and colleagues16 identified a similar trend, with 41.1% of patients in the PNB group and 32.5% of patients in the control group experiencing postoperative nausea or vomiting, while Krych and colleagues27 noted only 10% of PNB patients with mild nausea and none requiring antiemetic use. In their study of patients receiving PNB, Schroeder and colleagues17 found a significant reduction in antiemetic use among PNB patients compared with those receiving general anesthesia alone. Similarly, Ward and colleagues29 noted a significant difference in postoperative nausea, with 10% of patients in the PNB group experiencing postoperative nausea compared with 75% of those in the comparator group who received intravenous morphine. The mean percentage of patients experiencing postoperative nausea and/or vomiting is shown in Table 4.
DISCHARGE TIME
Four studies presented data on discharge time from the PACU and are summarized in Table 3. Three of these studies included a comparator group. Both Dold and colleagues16 and YaDeau and colleagues18 reported an increase in the time to discharge for patients receiving PNB, although these differences were not significant. The study by Ward and colleagues,29 on the other hand, noted a significant reduction in the time to discharge for the PNB group. In addition to these studies, Krych and colleagues27 examined the time from skin closure to discharge for patients receiving PNB, noting a mean 199 minutes for the patients in their study. Mean times to discharge for the PNB and control groups are presented in Table 4.
INPATIENT ADMISSION
Four studies presented data on the proportion of study participants who were admitted as inpatients, and these data are shown in Table 3. Dold and colleagues16 reported no inpatient admissions in their PNB group compared with 5.0% for the control group (both cases of pain control), while YaDeau and colleagues18 found that 3 admissions occurred, with 2 in the control group (1 for oxygen desaturation and the other for intractable pain and nausea) and 1 from the PNB group (epidural spread and urinary retention). Two additional studies reported data on PNB groups alone. Krych and colleagues27 observed no overnight admissions in their study, while Nye and colleagues28 reported 1 readmission for bilateral leg numbness and weakness due to epidural spread, which resolved following discontinuation of the block. The mean proportion of inpatient admissions is presented in Table 4.
SATISFACTION
A total of 3 studies examined patient satisfaction, and these data are presented in Table 3. In their study, Ward and colleagues29 reported a significantly greater rate of satisfaction at 1 day postoperatively among the patients in the PNB group (90%) than among patients who received intravenous morphine (25%) (P < .0001). Similarly, YaDeau and colleagues18 noted greater satisfaction among the PNB group than among the control group, with PNB patients rating their satisfaction at a mean of 8.6 and control patients at a mean of 7.9 on a 10-point scale (0-10) 24 hours postoperatively, although this difference was not significant. Finally, Krych and colleagues27 found that 67% of patients were “very satisfied” and 33% were “satisfied”, based on a Likert scale.
COMPLICATIONS
Four studies presented data on complications, and these findings are summarized in Table 3. In their work, Nye and colleagues28 reported most extensively on complications associated with PNB. Overall, the authors found a rate of significant complications of 3.8%. In terms of specific complications, they noted local anesthetic systemic toxicity (0.9%), epidural spread (0.5%), sensory or motor deficits (9.4%), falls (0.5%), and catheter issues. In their study of patients receiving PNB and CSE, YaDeau and colleagues18 identified 1 patient in the PNB group with epidural spread and urinary retention, while they noted 1 case of oxygen desaturation and another case of intractable pain and nausea in the group receiving CSE alone, all 3 of which required inpatient admission. They found no permanent adverse events attributable to the PNB. In another study, Dold and colleagues16 observed no complications in patients receiving PNB compared with those in 2 admissions in the control group for inadequate pain control. Similarly, Krych and colleagues27 identified no complications in patients who received PNB in their study.
DISCUSSION
Hip arthroscopy has experienced a substantial gain in popularity in recent years, emerging as a beneficial technique for both the diagnosis and treatment of diverse hip pathologies in patients spanning a variety of demographics. Nevertheless, postoperative pain control, as well as medication side effects and unwanted patient admissions, present major challenges to the treating surgeon. As an adjuvant measure, peripheral nerve block represents one option to improve postoperative pain management, while at the same time addressing the adverse effects of considerable opioid use, which is commonly seen in these patients. Early experience with this method in hip arthroscopy was reported in a case series by Lee and colleagues.12 In an attempt to reduce postoperative pain, as well as limit the adverse effects and delay in discharge associated with considerable opioid use in the PACU, the authors used preoperative paravertebral blocks of L1 and L2 in 2 patients requiring hip arthroscopy with encouraging results. Since then, a number of studies have attempted the use of PNB in hip arthroscopy.16-18,27-29 However, we were unable to identify any prior reviews reporting on peripheral nerve blockade in hip arthroscopy, and thus this study is unique in providing a greater understanding of the outcomes associated with PNB use.
In general, we found that PNB was associated with improved outcomes. Based on the studies included in this review, there was a statistically significantly lower level of pain in the PACU for femoral nerve block (compared with general anesthesia alone)16 and lumbar plexus blockade (compared with general anesthesia17 and CSE18 alone). Nevertheless, these effects are likely short-lived, with differences disappearing the day following the procedure. In terms of analgesic use, 2 studies report significant reductions in analgesic use intraoperatively and in the PACU/Phase I recovery,16,17 with a third reporting a strong trend toward reduced analgesic use in the PACU (P = .051).18 Finally, we report fewer admissions for the PNB group, as well as high rates of satisfaction and few complications across these studies.
Continue to: Unlike these measures...
Unlike these measures, postoperative nausea, vomiting, and antiemetic use, as well as time to discharge, showed more mixed results. With regard to nausea/vomiting, 2 studies16,18 reported nonsignificantly increased rates in the PNB group, whereas others reported significant reductions in nausea/vomiting29 and in the proportion of patients receiving antiemetics.17 Similarly, mixed results were seen in terms of patient discharge time from the PACU. Two studies16,18 reported a nonsignificant increase in time to discharge for the PNB group, while another29 noted a significant reduction for the PNB group compared with those receiving intravenous morphine. These mixed results were surprising, as we expected reductions in opioid use to result in fewer instances of nausea/vomiting and a quicker time to discharge. The reasons underlying these findings are not clear, although it has been suggested that current discharge guidelines and clinical pathways limit the ability to take advantage of the accelerated timeline offered by regional anesthesia.16,30 As experience with PNB grows, our guidelines and pathways are likely to adapt to capitalize on these advantages, and future studies may show more reliable improvements in these measures.
While rare, the risk of bleeding requiring blood transfusion following hip arthroscopy is one of the most common complications of this procedure. Though the studies included in this review did not report on the need for transfusion, a recent study by Cvetanovich and colleagues10 used a national database and found that, of patients undergoing hip arthroscopy (n = 1338), 0.4% (n = 5) had bleeding requiring a transfusion, with 0.3% (n = 4) requiring return to the operating room, similar to an earlier study by Clarke and colleagues,31 who noted bleeding from the portal site in 0.4% of hip arthroscopy patients. In terms of risk factors, Cvetanovich and colleagues10 found that ASA class, older age, and prior cardiac surgery were significantly associated with minor and overall complications, whereas both regional anesthesia/monitored anesthesia care and alcohol consumption of >2 drinks a day were significantly associated with minor complications, including bleeding requiring transfusions. They noted, however, that these risk factors accounted for only 5% of the variance in complication rates, indicating that other unidentified variables better explained the variance in complication rates. These authors concluded that complications associated with hip arthroscopy are so rare that we may not be able to predict which risk factors or anesthesia types are more likely to cause them. Further characterization of bleeding following hip arthroscopy and its associated risk factors is a valuable area for future research.
LIMITATIONS
Our study contains a number of limitations. This review included studies whose level of evidence varied from I to IV; therefore, our study is limited by any bias or heterogeneity introduced in patient recruitment, selection, variability of technique, data collection, and analysis used in these studies. This heterogeneity is most apparent in the block types and comparator groups. Furthermore, several different outcome measures were reported across the 6 studies used in this review, which decreased the relevance of any one of these individual outcomes. Finally, given the limited data that currently exist for the use of PNB in hip arthroscopy, we are unable to note meaningful differences between various types of PNBs, such as differences in postoperative pain or other measures such as quadriceps weakness, which can accompany femoral nerve block.12 While it is important to read our work with these limitations in mind, this systematic review is, to our knowledge, the only comprehensive review to date of studies reporting on PNB in hip arthroscopy, providing clinicians and patients with a greater understanding of the associated outcomes across these studies.
CONCLUSION
This systematic review shows improved outcomes and few complications with PNB use in hip arthroscopy, with reductions in postoperative pain, analgesic use, and the rate of inpatient admissions. Although opioid use was reduced in these studies, we found similar rates of postoperative nausea/vomiting as well as similar time to discharge from the PACU, which may reflect our continued reliance on outdated discharge guidelines and clinical pathways. Current attempts to provide peripheral nerve blockade are quite varied, with studies targeting femoral nerve, fascia iliaca, L1/L2 paravertebral, and lumbar plexus blockade. Future research efforts with a large prospective trial investigating these techniques should focus on which of these PNBs presents the optimal risk-benefit profile for hip arthroscopy patients and thus appropriately address the clinical questions at hand.
This paper will be judged for the Resident Writer’s Award.
- Baber YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip worthwhile? A prospective review of 328 adults investigated for hip pain. J Bone Joint Surg Br. 1999;81:600-603.
- Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379-1388.
- Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540-546.
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181-188.
- Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
- Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral debridement. Am J Sports Med. 2005;33:864-870.
- Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
- Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7:269-274.
- Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94:e23.
- Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016 Apr 8. [Epub before print].
- Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399-1402.
- Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462-465.
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105:1234-1242.
- Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206-1213.
- Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988-1993.
- Dold AP, Murnaghan L, Xing J, Abdallah FW, Brull R, Whelan DB. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med. 2014;42:144-149.
- Schroeder KM, Donnelly MJ, Anderson BM, Ford MP, Keene JS. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int. 2013;23:93-98.
- YaDeau JT, Tedore T, Goytizolo EA, et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg. 2012;115:968-972.
- Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23:1348-1353.
- Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care. 2007;35:949-952.
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol. 2015;25:255-261.
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103:428-433.
- Hadzic A, Karaca PE, Hobeika P, et al. Peripheral nerve blocks result in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesth Analg. 2005;100:976-981.
- Hsu LP, Oh S, Nuber GW, et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465-1472.
- Montes FR, Zarate E, Grueso R, et al. Comparison of spinal anesthesia with combined sciatic-femoral nerve block for outpatient knee arthroscopy. J Clin Anesth. 2008;20:415-420.
- Wulf H, Lowe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand. 2010;54:414-420.
- Krych AJ, Baran S, Kuzma SA, Smith HM, Johnson RL, Levy BA. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:843-847.
- Nye ZB, Horn JL, Crittenden W, Abrahams MS, Aziz MF. Ambulatory continuous posterior lumbar plexus blocks following hip arthroscopy: a review of 213 cases. J Clin Anesth. 2013;25:268-274.
- Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy. 2012;28:1064-1069.
- Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634-1642.
- Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-88.
- Baber YF, Robinson AH, Villar RN. Is diagnostic arthroscopy of the hip worthwhile? A prospective review of 328 adults investigated for hip pain. J Bone Joint Surg Br. 1999;81:600-603.
- Byrd JW, Jones KS. Arthroscopic management of femoroacetabular impingement: minimum 2-year follow-up. Arthroscopy. 2011;27:1379-1388.
- Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540-546.
- O'Leary JA, Berend K, Vail TP. The relationship between diagnosis and outcome in arthroscopy of the hip. Arthroscopy. 2001;17:181-188.
- Philippon M, Schenker M, Briggs K, Kuppersmith D. Femoroacetabular impingement in 45 professional athletes: associated pathologies and return to sport following arthroscopic decompression. Knee Surg Sports Traumatol Arthrosc. 2007;15:908-914.
- Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral debridement. Am J Sports Med. 2005;33:864-870.
- Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88-92.
- Yusaf MA, Hame SL. Arthroscopy of the hip. Curr Sports Med Rep. 2008;7:269-274.
- Colvin AC, Harrast J, Harner C. Trends in hip arthroscopy. J Bone Joint Surg Am. 2012;94:e23.
- Cvetanovich GL, Chalmers PN, Levy DM, et al. Hip arthroscopy surgical volume trends and 30-day postoperative complications. Arthroscopy. 2016 Apr 8. [Epub before print].
- Baker JF, Byrne DP, Hunter K, Mulhall KJ. Post-operative opiate requirements after hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2011;19:1399-1402.
- Lee EM, Murphy KP, Ben-David B. Postoperative analgesia for hip arthroscopy: combined L1 and L2 paravertebral blocks. J Clin Anesth. 2008;20:462-465.
- Ganesh A, Rose JB, Wells L, et al. Continuous peripheral nerve blockade for inpatient and outpatient postoperative analgesia in children. Anesth Analg. 2007;105:1234-1242.
- Williams BA, Kentor ML, Vogt MT, et al. Femoral-sciatic nerve blocks for complex outpatient knee surgery are associated with less postoperative pain before same-day discharge: a review of 1,200 consecutive cases from the period 1996-1999. Anesthesiology. 2003;98:1206-1213.
- Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988-1993.
- Dold AP, Murnaghan L, Xing J, Abdallah FW, Brull R, Whelan DB. Preoperative femoral nerve block in hip arthroscopic surgery: a retrospective review of 108 consecutive cases. Am J Sports Med. 2014;42:144-149.
- Schroeder KM, Donnelly MJ, Anderson BM, Ford MP, Keene JS. The analgesic impact of preoperative lumbar plexus blocks for hip arthroscopy. A retrospective review. Hip Int. 2013;23:93-98.
- YaDeau JT, Tedore T, Goytizolo EA, et al. Lumbar plexus blockade reduces pain after hip arthroscopy: a prospective randomized controlled trial. Anesth Analg. 2012;115:968-972.
- Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23:1348-1353.
- Stevens M, Harrison G, McGrail M. A modified fascia iliaca compartment block has significant morphine-sparing effect after total hip arthroplasty. Anaesth Intensive Care. 2007;35:949-952.
- Lehmann LJ, Loosen G, Weiss C, Schmittner MD. Interscalene plexus block versus general anaesthesia for shoulder surgery: a randomized controlled study. Eur J Orthop Surg Traumatol. 2015;25:255-261.
- Gonano C, Kettner SC, Ernstbrunner M, Schebesta K, Chiari A, Marhofer P. Comparison of economical aspects of interscalene brachial plexus blockade and general anaesthesia for arthroscopic shoulder surgery. Br J Anaesth. 2009;103:428-433.
- Hadzic A, Karaca PE, Hobeika P, et al. Peripheral nerve blocks result in superior recovery profile compared with general anesthesia in outpatient knee arthroscopy. Anesth Analg. 2005;100:976-981.
- Hsu LP, Oh S, Nuber GW, et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465-1472.
- Montes FR, Zarate E, Grueso R, et al. Comparison of spinal anesthesia with combined sciatic-femoral nerve block for outpatient knee arthroscopy. J Clin Anesth. 2008;20:415-420.
- Wulf H, Lowe J, Gnutzmann KH, Steinfeldt T. Femoral nerve block with ropivacaine or bupivacaine in day case anterior crucial ligament reconstruction. Acta Anaesthesiol Scand. 2010;54:414-420.
- Krych AJ, Baran S, Kuzma SA, Smith HM, Johnson RL, Levy BA. Utility of multimodal analgesia with fascia iliaca blockade for acute pain management following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:843-847.
- Nye ZB, Horn JL, Crittenden W, Abrahams MS, Aziz MF. Ambulatory continuous posterior lumbar plexus blocks following hip arthroscopy: a review of 213 cases. J Clin Anesth. 2013;25:268-274.
- Ward JP, Albert DB, Altman R, Goldstein RY, Cuff G, Youm T. Are femoral nerve blocks effective for early postoperative pain management after hip arthroscopy? Arthroscopy. 2012;28:1064-1069.
- Liu SS, Strodtbeck WM, Richman JM, Wu CL. A comparison of regional versus general anesthesia for ambulatory anesthesia: a meta-analysis of randomized controlled trials. Anesth Analg. 2005;101:1634-1642.
- Clarke MT, Arora A, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 2003;406:84-88.
TAKE-HOME POINTS
- Postoperative PACU pain was consistently reduced in the PNB group.
- Patients with PNBs had lower postoperative pain medication requirements and lower rates of inpatient admission compared with controls.
- Similar rates of nausea/vomiting and time to discharge were reported for PNB patients and controls.
- PNBs are associated with high rates of satisfaction and few complications.
- Future research should focus on comparing across PNB techniques.
Minimum 5-Year Follow-up of Articular Surface Replacement Acetabular Components Used in Total Hip Arthroplasty
ABSTRACT
The articular surface replacement (ASR) monoblock metal-on-metal acetabular component was recalled due to a higher than expected early failure rate. We evaluated the survivorship of the device and variables that may be predictive of failure at a minimum of 5-year follow-up. A single-center, single-surgeon retrospective review was conducted in patients who received the DePuy Synthes ASR™ XL Acetabular hip system from December 2005 to November 2009. Mean values and percentages were calculated and compared using the Fisher’s exact test, simple logistic regression, and Student’s t-test. The significance level was P ≤ .05. This study included 29 patients (24 males, 5 females) with 32 ASR™ XL acetabular hip systems. Mean age and body mass index (BMI) reached 55.2 years and 28.9 kg/m2, respectively. Mean postoperative follow-up was 6.2 years. A total of 2 patients (6.9%) died of an unrelated cause and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom were available for follow-up. The 5-year revision rate was 34.4% (10 patients with 11 hip replacements). Mean time to revision was 3.1 years. Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with the increased rate for hip failure. Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months following the revision. This study demonstrates a high rate of failure of ASR acetabular components used in total hip arthroplasty at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is required.
Continue to: Metal-on-metal...
Metal-on-metal (MoM) articulations have been widely explored as an alternative to polyethylene bearings in total hip arthroplasty (THA), with proposed benefits including improved range of motion, lower dislocation rates, and enhanced durability.1 Comprising cobalt and chromium, these MoM bearings gained widespread popularity in the United States, particularly in younger and more active patients looking for longer lasting devices.
The articular surface replacement (ASR) acetabular system (DePuy Synthes) was approved for sale by the US Food and Drug Administration in 2003 and implanted in an estimated 93,000 cases.2 Since then, however, the early failure rate of the prosthesis has been well documented,3-5 leading to a formal global product recall in August 2010. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) was amongst the first to report a 6.4% rate of failure of the device at 3 years when inserted with a Corail stem.6 An acceptable upper rate of hip prosthesis failure is considered to reach 1% per year, with the majority of implants reporting well below this value. A 10.9% failure rate at 5 years was documented when the prosthesis was inserted for resurfacing. The National Joint Registry of England and Wales confirmed these findings and observed a 13% and 12% rate of failure at 5 years for the acetabular and resurfacing systems, respectively.2 With the notable failure of the ASR system, this study reports our single-center 5-year survivorship experience and evaluates any variable that might be predictive of an early failure to aid in patient counseling.
METHODS
A single-center, single-surgeon, retrospective review of a consecutive series of patients was performed from December 2005 to November 2009. This study included all patients who underwent a primary THA with a DePuy Synthes ASR™ XL Acetabular hip system. No patients were excluded. Institutional Review Board approval was obtained. Patient demographics comprising of age, gender, and body mass index (BMI) were recorded. The primary endpoint of this study was 5-year survivorship rates. Secondary endpoints included duration to revision surgery, blood cobalt and chromium levels, time interval of blood ion tests, acetabulum size, acetabular component abduction angle, and duration to follow-up.
Candidates for the ASR™ XL Acetabular hip system included young patients and/or those considered to be physically active. In a select few, ASR devices were implanted upon patient request.
All patients underwent primary total hip replacement with a DePuy Synthes ASR™ XL uncemented acetabular component and an uncemented femoral stem (DePuy Synthes, Summit, or Tri-Lock) inserted via a standard posterior approach (Figure 1). Acetabulum sizes ranged from 52 mm to 68 mm in diameter.
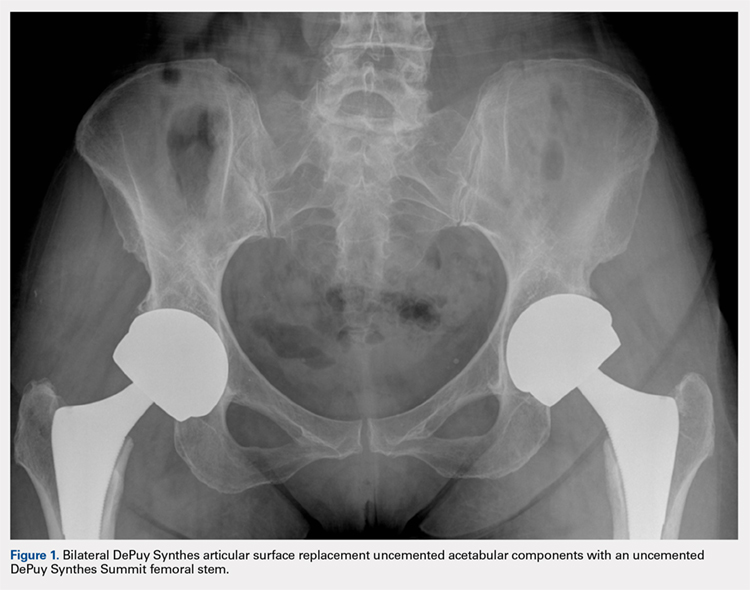
All patients were followed-up yearly in the outpatient setting. Routine (yearly) metal-ion level sampling (whole blood) was started in 2010 for all patients. Laboratory tests were conducted at a single laboratory (Lab Corp.). Abduction cup inclination angles were measured by the providing surgeon using digital radiology software (GE Centricity systems).
The Student’s t-test was used to compare mean values (such as age, BMI, and metal ion levels) between the failure and no-failure groups. The 2-sided Fisher’s exact test analyzed differences in gender. Simple logistic regression analyzed variables associated with the failure group. Significance was P ≤ .05.
Continue to: Results...
RESULTS
A total of 29 patients (24 males, 5 females) with 32 ASR hip replacements were included in this study. Indications for surgery comprised osteoarthritis (28 hips, 87.5%) and avascular necrosis of the hip (4 hips, 12.5%). Mean age and BMI were 55.2 years and 28.9 kg/m2, respectively. A total of 2 patients (6.9%) died of an unrelated cause (1 myocardial infarct, 1 suicide), and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom finished a 5-year minimum follow-up.
No implant failures were noted in the first year. The 5-year revision rate reached 34.4% (10 patients with 11 hip replacements). Mean time to revision for this subgroup was 3.1 years. Overall, an implant failure was observed in 37.5% of patients (11 patients with 12 hip replacements) at a mean postoperative follow-up of 6.2 years (Figure 2). Indications for implant revision were pain in 11 (92.7%) cases and infection in 1 (8.3%).
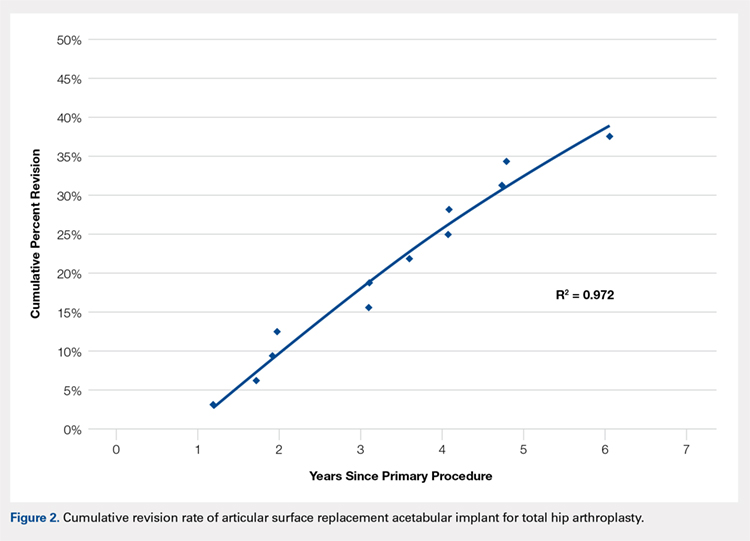
Of the 11 hips revised due to pain, 9 were performed by the original surgeon (8 were completed with primary acetabular components, 1 with a revision shell). Figure 3 shows a bilateral revision performed with primary acetabular components and retained DePuy Synthes Pinnacle femoral stems. In all these cases except 1, the ASR component was grossly loose. One case presented with pseudotumor and impingement between the femoral prosthetic neck and acetabular component after migration of a loose component. After revision, the patient returned with substantial anterior hip pain and heterotopic ossification, and failed conservative treatment, requiring another surgery with prosthesis retention, removal of heterotopic ossification, and iliopsoas lengthening. The surgery successfully relieved the symptoms. No other patients required additional surgery after their revision. In comparison to the original ASR component, the revision shell was 2 to 4 mm larger in diameter. No patient required component revision at a mean of 2.9 years after the revision surgery.
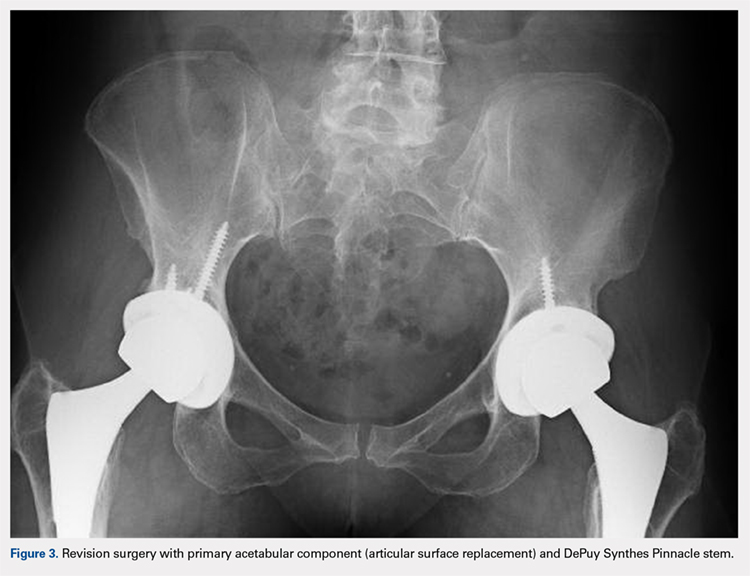
The patient with secondary revision developed a hematogenous streptococcal infection after a dental procedure performed without prophylactic antibiotics. The patient was initially lost to follow-up after the primary surgery and reported no antecedent pain prior to the revision. A substantial metal fluid collection was identified in the hip at the time of débridement and without component loosening. After débridement, the patient developed persistent metal stained wound drainage, necessitating ultimate successful treatment with a 2-stage exchange procedure.
Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with an increased rate for hip failure (Table). Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. The upper limits of blood cobalt and chromium levels reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months after the revision (Figure 4).
Table. Variables Not Associated with Early ASR Failure
|
| No Failure (n = 20) | Failure (n = 12) | P value |
Age (years) | 55.4 ± 6.4 | 54.7 ± 6.3 | .76 | |
BMI (kg/m2) | 29.7 ± 6.7 | 27.4 ± 4.0 | .29 | |
Gender | .49 | |||
Female | 3 (15%) | 3 (25%) | ||
Male | 17 (85%) | 9 (75%) | ||
Acetabulum size (mm) | 59.1 ± 3.9 | 58.3 ± 3.8 | .59 | |
Abduction angle (degrees) | 44.9 ± 4.5 | 42.3 ± 3.8 | .12 | |
Serum levels (µg/L) | ||||
Cobalt | 6.8 ± 6.0 | 7.6 ± 4.7 | .58 | |
| Chromium | 2.2 ± 1.7 | 5.0 ± 5.0 | .31 |
Continue to: Discussion...
DISCUSSION
According to the Center for Disease Control and Prevention, 310,800 total hip replacements were performed among inpatients aged 45 years and older in the US in 2010.7 Specifically, in the 55- to 64-year-old age group, the number of procedures performed tripled from 2000 through 2010. As younger and more active patients opt for hip replacements, a growing need for prosthesis with enhanced durability is observed.
Despite the early proposed advantages of large head MoM bearings, our retrospective study of the DePuy Synthes ASR™ XL Acetabular hip system yielded 15.6% and 34.4% failure rates at 3 and 5 years, respectively. These higher-than-expected rates of failure are consistent with published data. The British Hip Society reported a 21% to 35% revision rate at 4 years and 49% at 6 years for the ASR XL prosthesis.8 In comparison, other MoM prosthesis, on average, report a 12% to 15% rate of failure at 5 years.
Considerable controversy surrounds the causes of adverse wear failure in MoM bearings.9,10 The non-modular design of the ASR prostheses is frequently implicated as a cause of early failure. The lack of a central hole in the 1-piece component compromises the tactile feel of insertion, thereby reducing the surgeon’s ability to assess complete seating.11 This condition may potentially increase the abduction angle at the time of insertion. Screw fixation of the non-modular device is not possible. The ASR XL device (148° to 160°) is less than a hemisphere (180°) in size and hence features a diminished functional articular surface, further compromising implant fixation.11 The functional articular surface is defined as the optimal surface area (10 mm) needed for a MoM implant.12 Griffin and colleagues13 reported a 48 mm ASR XL component, when implanted at 45° of abduction, to function similar to an implant at 59° of abduction, leading to diminished lubrication, metallosis, and edge loading. The version of the acetabular component may similarly and adversely affect implant wear characteristics. Furthermore, the variable thickness of the implant, which is thicker at the dome and thinner at the rim, may further promote edge loading by shifting the center of rotation of the femoral head out from the center of the acetabular prosthesis.11 Studies have also shown that increased wear of the MoM articulation is associated with an acetabular component inclination angle in excess of 55°10,14 and a failure of fixation at time of implantation.15 This study, however, found no correlation between the abduction angle and risk of early implant failure for the ASR acetabular component. No correlation was also detected between the acetabulum size and revision surgery.
The AOANJRR reported loosening (44%), infection (20%), metal sensitivity (12%), fracture (9%), and dislocation of prosthesis (7%) as the indications for revision surgery for the ASR prosthesis.6 Furthermore, a single-center retrospective review of 70 consecutive MoM THAs with ultra-large diameter femoral head and monoblock acetabular components showed that 17.1% required revision within 3 years for loosening, pain, and squeaking.1 Overall, 28.6% of patients reported implant dysfunction. In this study, we observed a similar rate of failure at 3 years (15.6%) for pain (11) and infection (1). The revision surgery successfully relieved all of these symptoms. One patient presented with heterotopic ossification and anterior hip pain after the original revision and required additional surgery with prosthesis retention. No patient in this series required repeat component revisions at a mean of 2.9 years after surgery. In all but 1 case, primary acetabular components were used in the revision, and in all cases except that with infection, the femoral component was retained. Replacement shells were 2 to 4 mm larger in diameter than the original ASR component.
Recently, concerns have arisen regarding the long-term effects of serum cobalt and chromium metal ions levels. Studies have shown increased serum metal ion levels,15 groin pain,16 pseudotumor formation,17 and metallosis18 after the implantation of MoM bearings. In a case study by Mao and colleagues,19 1 patient reported headaches, anorexia, continuous metallic taste in her mouth, and weight loss. A cerebrospinal fluid analysis revealed cobalt and chromium levels at 9 and 13 nmol/L, respectively, indicating that these metal ions can cross the blood-brain barrier. Another patient reported painful muscle fatigue, night cramps, fainting spells, cognitive decline, and an inability to climb stairs. His serum cobalt level reached 258 nmol/L (reference range, 0-20 nmol/L), and chromium level totaled 88 nmol/L (reference range, 0-100 nmol/L). At 8-week follow-up after revision surgery, the symptoms of the patient had resolved, with serum cobalt levels dropping to 42 nmol/L.19 None of the patients in this study presented with any signs or symptoms of metal toxicity. The upper limits of blood cobalt and chromium levels in our study population reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. However, we noted a similar drop in post-revision blood cobalt (91% decrease) and chromium (78% decrease) levels.
In summary, our data showed a high revision rate of the DePuy Synthes ASR™ XL Acetabular hip system. Our findings are consistent with internationally published data. In the absence of reliable predictors of early failure, continued close clinical surveillance and laboratory monitoring of these patients are warranted.
CONCLUSION
This study demonstrates the high failure rate of the DePuy Synthes ASR™ XL Acetabular hip system used in THA at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is therefore required. Metal levels dropped quickly after revision, and the revision surgery can generally be performed with slightly larger primary components. Symptomatic patients with ASR hip replacements, regardless of blood metal-ion levels, were candidates for the revision surgery. Not all failed hips exhibited substantially elevated metal levels. Asymptomatic patients with high blood metal-ion levels should be closely followed-up and revision surgery should be strongly considered, consistent with recently published guidelines.20
- Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27(4):539. doi:10.1016/j.arth.2011.08.022.
- de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287. doi:10.2106/JBJS.J.01727.
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46. doi:10.1302/0301-620X.92B1.22770.
- Siebel T, Maubach S, Morlock MM. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc Inst Mech Eng H. 2006;220(2):345-353. doi:10.1243/095441105X69079.
- Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92(1):28-37. doi:10.1302/0301-620X.92B1.22769.
- Australian Orthopaedic Association National Joint Replacement Registry annual report 2010. Australian Orthopaedic Association Web site. https://aoanjrr.sahmri.com/annual-reports-2010. Accessed June 19, 2018.
- Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/databriefs/db186.pdf. Accessed July 13, 2015.
- Hodgkinson J, Skinner J, Kay P. Large diameter metal on metal bearing total hip replacements. British Hip Society Web site. https://www.britishhipsociety.com/uploaded/BHS_MOM_THR.pdf. Accessed August 6, 2015.
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93(3):315-320. doi:10.1302/0301-620X.93B3.25545.
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93(2):164-171. doi:10.1302/0301-620X.93B2.25099.
- Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6):14-18. doi:10.1016/j.arth.2011.03.027.
- De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158-1163. doi:10.1302/0301-620X.90B9.19891.
- Griffin WL, Nanson CJ, Springer BD, Davies MA, Fehring TK. Reduced articular surface of one-piece cups: a cause of runaway wear and early failure. Clin Orthop Relat Res. 2010;468(9):2328-2332. doi:10.1007/s11999-010-1383-8.
- Grammatopolous G, Pandit H, Glyn-Jones S, et al. Optimal acetablular orientation for hip resurfacing. J Bone Joint Surg Br. 2010;92(8):1072-1078. doi:10.1302/0301-620X.92B8.24194.
- MacDonalad SJ, McCalden RW, Chess DG, et al. Meta-onmetal versus polyethylene in hip arthoplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;(406):282-296.
- Bin Nasser A, Beaule PE, O'Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468(2):392-399. doi:10.1007/s11999-009-1133-y.
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80(6):653-659. doi:10.3109/17453670903473016.
- Neumann DRP, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal-on-metal total hip arthroplasty. J Arthroplasty. 2010;25(5):700-708. doi:10.1016/j.arth.2009.05.018.
- Mao X, Wong AA, Crawford RW. Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
- Information statement: current concerns with metal-on-metal hip arthroplasty. American Academy of Orthopaedic Surgeons Web site. https://aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1035%20Current%20Concerns%20with%20Metal-on-Metal%20Hip%20Arthroplasty.pdf. Accessed June 19, 2018.
ABSTRACT
The articular surface replacement (ASR) monoblock metal-on-metal acetabular component was recalled due to a higher than expected early failure rate. We evaluated the survivorship of the device and variables that may be predictive of failure at a minimum of 5-year follow-up. A single-center, single-surgeon retrospective review was conducted in patients who received the DePuy Synthes ASR™ XL Acetabular hip system from December 2005 to November 2009. Mean values and percentages were calculated and compared using the Fisher’s exact test, simple logistic regression, and Student’s t-test. The significance level was P ≤ .05. This study included 29 patients (24 males, 5 females) with 32 ASR™ XL acetabular hip systems. Mean age and body mass index (BMI) reached 55.2 years and 28.9 kg/m2, respectively. Mean postoperative follow-up was 6.2 years. A total of 2 patients (6.9%) died of an unrelated cause and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom were available for follow-up. The 5-year revision rate was 34.4% (10 patients with 11 hip replacements). Mean time to revision was 3.1 years. Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with the increased rate for hip failure. Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months following the revision. This study demonstrates a high rate of failure of ASR acetabular components used in total hip arthroplasty at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is required.
Continue to: Metal-on-metal...
Metal-on-metal (MoM) articulations have been widely explored as an alternative to polyethylene bearings in total hip arthroplasty (THA), with proposed benefits including improved range of motion, lower dislocation rates, and enhanced durability.1 Comprising cobalt and chromium, these MoM bearings gained widespread popularity in the United States, particularly in younger and more active patients looking for longer lasting devices.
The articular surface replacement (ASR) acetabular system (DePuy Synthes) was approved for sale by the US Food and Drug Administration in 2003 and implanted in an estimated 93,000 cases.2 Since then, however, the early failure rate of the prosthesis has been well documented,3-5 leading to a formal global product recall in August 2010. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) was amongst the first to report a 6.4% rate of failure of the device at 3 years when inserted with a Corail stem.6 An acceptable upper rate of hip prosthesis failure is considered to reach 1% per year, with the majority of implants reporting well below this value. A 10.9% failure rate at 5 years was documented when the prosthesis was inserted for resurfacing. The National Joint Registry of England and Wales confirmed these findings and observed a 13% and 12% rate of failure at 5 years for the acetabular and resurfacing systems, respectively.2 With the notable failure of the ASR system, this study reports our single-center 5-year survivorship experience and evaluates any variable that might be predictive of an early failure to aid in patient counseling.
METHODS
A single-center, single-surgeon, retrospective review of a consecutive series of patients was performed from December 2005 to November 2009. This study included all patients who underwent a primary THA with a DePuy Synthes ASR™ XL Acetabular hip system. No patients were excluded. Institutional Review Board approval was obtained. Patient demographics comprising of age, gender, and body mass index (BMI) were recorded. The primary endpoint of this study was 5-year survivorship rates. Secondary endpoints included duration to revision surgery, blood cobalt and chromium levels, time interval of blood ion tests, acetabulum size, acetabular component abduction angle, and duration to follow-up.
Candidates for the ASR™ XL Acetabular hip system included young patients and/or those considered to be physically active. In a select few, ASR devices were implanted upon patient request.
All patients underwent primary total hip replacement with a DePuy Synthes ASR™ XL uncemented acetabular component and an uncemented femoral stem (DePuy Synthes, Summit, or Tri-Lock) inserted via a standard posterior approach (Figure 1). Acetabulum sizes ranged from 52 mm to 68 mm in diameter.

All patients were followed-up yearly in the outpatient setting. Routine (yearly) metal-ion level sampling (whole blood) was started in 2010 for all patients. Laboratory tests were conducted at a single laboratory (Lab Corp.). Abduction cup inclination angles were measured by the providing surgeon using digital radiology software (GE Centricity systems).
The Student’s t-test was used to compare mean values (such as age, BMI, and metal ion levels) between the failure and no-failure groups. The 2-sided Fisher’s exact test analyzed differences in gender. Simple logistic regression analyzed variables associated with the failure group. Significance was P ≤ .05.
Continue to: Results...
RESULTS
A total of 29 patients (24 males, 5 females) with 32 ASR hip replacements were included in this study. Indications for surgery comprised osteoarthritis (28 hips, 87.5%) and avascular necrosis of the hip (4 hips, 12.5%). Mean age and BMI were 55.2 years and 28.9 kg/m2, respectively. A total of 2 patients (6.9%) died of an unrelated cause (1 myocardial infarct, 1 suicide), and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom finished a 5-year minimum follow-up.
No implant failures were noted in the first year. The 5-year revision rate reached 34.4% (10 patients with 11 hip replacements). Mean time to revision for this subgroup was 3.1 years. Overall, an implant failure was observed in 37.5% of patients (11 patients with 12 hip replacements) at a mean postoperative follow-up of 6.2 years (Figure 2). Indications for implant revision were pain in 11 (92.7%) cases and infection in 1 (8.3%).

Of the 11 hips revised due to pain, 9 were performed by the original surgeon (8 were completed with primary acetabular components, 1 with a revision shell). Figure 3 shows a bilateral revision performed with primary acetabular components and retained DePuy Synthes Pinnacle femoral stems. In all these cases except 1, the ASR component was grossly loose. One case presented with pseudotumor and impingement between the femoral prosthetic neck and acetabular component after migration of a loose component. After revision, the patient returned with substantial anterior hip pain and heterotopic ossification, and failed conservative treatment, requiring another surgery with prosthesis retention, removal of heterotopic ossification, and iliopsoas lengthening. The surgery successfully relieved the symptoms. No other patients required additional surgery after their revision. In comparison to the original ASR component, the revision shell was 2 to 4 mm larger in diameter. No patient required component revision at a mean of 2.9 years after the revision surgery.

The patient with secondary revision developed a hematogenous streptococcal infection after a dental procedure performed without prophylactic antibiotics. The patient was initially lost to follow-up after the primary surgery and reported no antecedent pain prior to the revision. A substantial metal fluid collection was identified in the hip at the time of débridement and without component loosening. After débridement, the patient developed persistent metal stained wound drainage, necessitating ultimate successful treatment with a 2-stage exchange procedure.
Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with an increased rate for hip failure (Table). Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. The upper limits of blood cobalt and chromium levels reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months after the revision (Figure 4).
Table. Variables Not Associated with Early ASR Failure
|
| No Failure (n = 20) | Failure (n = 12) | P value |
Age (years) | 55.4 ± 6.4 | 54.7 ± 6.3 | .76 | |
BMI (kg/m2) | 29.7 ± 6.7 | 27.4 ± 4.0 | .29 | |
Gender | .49 | |||
Female | 3 (15%) | 3 (25%) | ||
Male | 17 (85%) | 9 (75%) | ||
Acetabulum size (mm) | 59.1 ± 3.9 | 58.3 ± 3.8 | .59 | |
Abduction angle (degrees) | 44.9 ± 4.5 | 42.3 ± 3.8 | .12 | |
Serum levels (µg/L) | ||||
Cobalt | 6.8 ± 6.0 | 7.6 ± 4.7 | .58 | |
| Chromium | 2.2 ± 1.7 | 5.0 ± 5.0 | .31 |

Continue to: Discussion...
DISCUSSION
According to the Center for Disease Control and Prevention, 310,800 total hip replacements were performed among inpatients aged 45 years and older in the US in 2010.7 Specifically, in the 55- to 64-year-old age group, the number of procedures performed tripled from 2000 through 2010. As younger and more active patients opt for hip replacements, a growing need for prosthesis with enhanced durability is observed.
Despite the early proposed advantages of large head MoM bearings, our retrospective study of the DePuy Synthes ASR™ XL Acetabular hip system yielded 15.6% and 34.4% failure rates at 3 and 5 years, respectively. These higher-than-expected rates of failure are consistent with published data. The British Hip Society reported a 21% to 35% revision rate at 4 years and 49% at 6 years for the ASR XL prosthesis.8 In comparison, other MoM prosthesis, on average, report a 12% to 15% rate of failure at 5 years.
Considerable controversy surrounds the causes of adverse wear failure in MoM bearings.9,10 The non-modular design of the ASR prostheses is frequently implicated as a cause of early failure. The lack of a central hole in the 1-piece component compromises the tactile feel of insertion, thereby reducing the surgeon’s ability to assess complete seating.11 This condition may potentially increase the abduction angle at the time of insertion. Screw fixation of the non-modular device is not possible. The ASR XL device (148° to 160°) is less than a hemisphere (180°) in size and hence features a diminished functional articular surface, further compromising implant fixation.11 The functional articular surface is defined as the optimal surface area (10 mm) needed for a MoM implant.12 Griffin and colleagues13 reported a 48 mm ASR XL component, when implanted at 45° of abduction, to function similar to an implant at 59° of abduction, leading to diminished lubrication, metallosis, and edge loading. The version of the acetabular component may similarly and adversely affect implant wear characteristics. Furthermore, the variable thickness of the implant, which is thicker at the dome and thinner at the rim, may further promote edge loading by shifting the center of rotation of the femoral head out from the center of the acetabular prosthesis.11 Studies have also shown that increased wear of the MoM articulation is associated with an acetabular component inclination angle in excess of 55°10,14 and a failure of fixation at time of implantation.15 This study, however, found no correlation between the abduction angle and risk of early implant failure for the ASR acetabular component. No correlation was also detected between the acetabulum size and revision surgery.
The AOANJRR reported loosening (44%), infection (20%), metal sensitivity (12%), fracture (9%), and dislocation of prosthesis (7%) as the indications for revision surgery for the ASR prosthesis.6 Furthermore, a single-center retrospective review of 70 consecutive MoM THAs with ultra-large diameter femoral head and monoblock acetabular components showed that 17.1% required revision within 3 years for loosening, pain, and squeaking.1 Overall, 28.6% of patients reported implant dysfunction. In this study, we observed a similar rate of failure at 3 years (15.6%) for pain (11) and infection (1). The revision surgery successfully relieved all of these symptoms. One patient presented with heterotopic ossification and anterior hip pain after the original revision and required additional surgery with prosthesis retention. No patient in this series required repeat component revisions at a mean of 2.9 years after surgery. In all but 1 case, primary acetabular components were used in the revision, and in all cases except that with infection, the femoral component was retained. Replacement shells were 2 to 4 mm larger in diameter than the original ASR component.
Recently, concerns have arisen regarding the long-term effects of serum cobalt and chromium metal ions levels. Studies have shown increased serum metal ion levels,15 groin pain,16 pseudotumor formation,17 and metallosis18 after the implantation of MoM bearings. In a case study by Mao and colleagues,19 1 patient reported headaches, anorexia, continuous metallic taste in her mouth, and weight loss. A cerebrospinal fluid analysis revealed cobalt and chromium levels at 9 and 13 nmol/L, respectively, indicating that these metal ions can cross the blood-brain barrier. Another patient reported painful muscle fatigue, night cramps, fainting spells, cognitive decline, and an inability to climb stairs. His serum cobalt level reached 258 nmol/L (reference range, 0-20 nmol/L), and chromium level totaled 88 nmol/L (reference range, 0-100 nmol/L). At 8-week follow-up after revision surgery, the symptoms of the patient had resolved, with serum cobalt levels dropping to 42 nmol/L.19 None of the patients in this study presented with any signs or symptoms of metal toxicity. The upper limits of blood cobalt and chromium levels in our study population reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. However, we noted a similar drop in post-revision blood cobalt (91% decrease) and chromium (78% decrease) levels.
In summary, our data showed a high revision rate of the DePuy Synthes ASR™ XL Acetabular hip system. Our findings are consistent with internationally published data. In the absence of reliable predictors of early failure, continued close clinical surveillance and laboratory monitoring of these patients are warranted.
CONCLUSION
This study demonstrates the high failure rate of the DePuy Synthes ASR™ XL Acetabular hip system used in THA at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is therefore required. Metal levels dropped quickly after revision, and the revision surgery can generally be performed with slightly larger primary components. Symptomatic patients with ASR hip replacements, regardless of blood metal-ion levels, were candidates for the revision surgery. Not all failed hips exhibited substantially elevated metal levels. Asymptomatic patients with high blood metal-ion levels should be closely followed-up and revision surgery should be strongly considered, consistent with recently published guidelines.20
ABSTRACT
The articular surface replacement (ASR) monoblock metal-on-metal acetabular component was recalled due to a higher than expected early failure rate. We evaluated the survivorship of the device and variables that may be predictive of failure at a minimum of 5-year follow-up. A single-center, single-surgeon retrospective review was conducted in patients who received the DePuy Synthes ASR™ XL Acetabular hip system from December 2005 to November 2009. Mean values and percentages were calculated and compared using the Fisher’s exact test, simple logistic regression, and Student’s t-test. The significance level was P ≤ .05. This study included 29 patients (24 males, 5 females) with 32 ASR™ XL acetabular hip systems. Mean age and body mass index (BMI) reached 55.2 years and 28.9 kg/m2, respectively. Mean postoperative follow-up was 6.2 years. A total of 2 patients (6.9%) died of an unrelated cause and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom were available for follow-up. The 5-year revision rate was 34.4% (10 patients with 11 hip replacements). Mean time to revision was 3.1 years. Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with the increased rate for hip failure. Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months following the revision. This study demonstrates a high rate of failure of ASR acetabular components used in total hip arthroplasty at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is required.
Continue to: Metal-on-metal...
Metal-on-metal (MoM) articulations have been widely explored as an alternative to polyethylene bearings in total hip arthroplasty (THA), with proposed benefits including improved range of motion, lower dislocation rates, and enhanced durability.1 Comprising cobalt and chromium, these MoM bearings gained widespread popularity in the United States, particularly in younger and more active patients looking for longer lasting devices.
The articular surface replacement (ASR) acetabular system (DePuy Synthes) was approved for sale by the US Food and Drug Administration in 2003 and implanted in an estimated 93,000 cases.2 Since then, however, the early failure rate of the prosthesis has been well documented,3-5 leading to a formal global product recall in August 2010. The Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR) was amongst the first to report a 6.4% rate of failure of the device at 3 years when inserted with a Corail stem.6 An acceptable upper rate of hip prosthesis failure is considered to reach 1% per year, with the majority of implants reporting well below this value. A 10.9% failure rate at 5 years was documented when the prosthesis was inserted for resurfacing. The National Joint Registry of England and Wales confirmed these findings and observed a 13% and 12% rate of failure at 5 years for the acetabular and resurfacing systems, respectively.2 With the notable failure of the ASR system, this study reports our single-center 5-year survivorship experience and evaluates any variable that might be predictive of an early failure to aid in patient counseling.
METHODS
A single-center, single-surgeon, retrospective review of a consecutive series of patients was performed from December 2005 to November 2009. This study included all patients who underwent a primary THA with a DePuy Synthes ASR™ XL Acetabular hip system. No patients were excluded. Institutional Review Board approval was obtained. Patient demographics comprising of age, gender, and body mass index (BMI) were recorded. The primary endpoint of this study was 5-year survivorship rates. Secondary endpoints included duration to revision surgery, blood cobalt and chromium levels, time interval of blood ion tests, acetabulum size, acetabular component abduction angle, and duration to follow-up.
Candidates for the ASR™ XL Acetabular hip system included young patients and/or those considered to be physically active. In a select few, ASR devices were implanted upon patient request.
All patients underwent primary total hip replacement with a DePuy Synthes ASR™ XL uncemented acetabular component and an uncemented femoral stem (DePuy Synthes, Summit, or Tri-Lock) inserted via a standard posterior approach (Figure 1). Acetabulum sizes ranged from 52 mm to 68 mm in diameter.

All patients were followed-up yearly in the outpatient setting. Routine (yearly) metal-ion level sampling (whole blood) was started in 2010 for all patients. Laboratory tests were conducted at a single laboratory (Lab Corp.). Abduction cup inclination angles were measured by the providing surgeon using digital radiology software (GE Centricity systems).
The Student’s t-test was used to compare mean values (such as age, BMI, and metal ion levels) between the failure and no-failure groups. The 2-sided Fisher’s exact test analyzed differences in gender. Simple logistic regression analyzed variables associated with the failure group. Significance was P ≤ .05.
Continue to: Results...
RESULTS
A total of 29 patients (24 males, 5 females) with 32 ASR hip replacements were included in this study. Indications for surgery comprised osteoarthritis (28 hips, 87.5%) and avascular necrosis of the hip (4 hips, 12.5%). Mean age and BMI were 55.2 years and 28.9 kg/m2, respectively. A total of 2 patients (6.9%) died of an unrelated cause (1 myocardial infarct, 1 suicide), and 1 patient was lost to follow-up (3.4%), leaving 26 patients with 28 hip replacements, all of whom finished a 5-year minimum follow-up.
No implant failures were noted in the first year. The 5-year revision rate reached 34.4% (10 patients with 11 hip replacements). Mean time to revision for this subgroup was 3.1 years. Overall, an implant failure was observed in 37.5% of patients (11 patients with 12 hip replacements) at a mean postoperative follow-up of 6.2 years (Figure 2). Indications for implant revision were pain in 11 (92.7%) cases and infection in 1 (8.3%).

Of the 11 hips revised due to pain, 9 were performed by the original surgeon (8 were completed with primary acetabular components, 1 with a revision shell). Figure 3 shows a bilateral revision performed with primary acetabular components and retained DePuy Synthes Pinnacle femoral stems. In all these cases except 1, the ASR component was grossly loose. One case presented with pseudotumor and impingement between the femoral prosthetic neck and acetabular component after migration of a loose component. After revision, the patient returned with substantial anterior hip pain and heterotopic ossification, and failed conservative treatment, requiring another surgery with prosthesis retention, removal of heterotopic ossification, and iliopsoas lengthening. The surgery successfully relieved the symptoms. No other patients required additional surgery after their revision. In comparison to the original ASR component, the revision shell was 2 to 4 mm larger in diameter. No patient required component revision at a mean of 2.9 years after the revision surgery.

The patient with secondary revision developed a hematogenous streptococcal infection after a dental procedure performed without prophylactic antibiotics. The patient was initially lost to follow-up after the primary surgery and reported no antecedent pain prior to the revision. A substantial metal fluid collection was identified in the hip at the time of débridement and without component loosening. After débridement, the patient developed persistent metal stained wound drainage, necessitating ultimate successful treatment with a 2-stage exchange procedure.
Age (P = .76), gender (P = .49), BMI (P = .29), acetabular component abduction angle (P = .12), and acetabulum size (P = .59) were not associated with an increased rate for hip failure (Table). Blood cobalt (7.6 vs 6.8 µg/L, P = .58) and chromium (5.0 vs 2.2 µg/L, P = .31) levels were not significantly higher in the revised group when compared with those of the unrevised group. The upper limits of blood cobalt and chromium levels reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. In the revised group, a 91% decrease in cobalt and 78% decrease in chromium levels were observed at a mean of 6 months after the revision (Figure 4).
Table. Variables Not Associated with Early ASR Failure
|
| No Failure (n = 20) | Failure (n = 12) | P value |
Age (years) | 55.4 ± 6.4 | 54.7 ± 6.3 | .76 | |
BMI (kg/m2) | 29.7 ± 6.7 | 27.4 ± 4.0 | .29 | |
Gender | .49 | |||
Female | 3 (15%) | 3 (25%) | ||
Male | 17 (85%) | 9 (75%) | ||
Acetabulum size (mm) | 59.1 ± 3.9 | 58.3 ± 3.8 | .59 | |
Abduction angle (degrees) | 44.9 ± 4.5 | 42.3 ± 3.8 | .12 | |
Serum levels (µg/L) | ||||
Cobalt | 6.8 ± 6.0 | 7.6 ± 4.7 | .58 | |
| Chromium | 2.2 ± 1.7 | 5.0 ± 5.0 | .31 |

Continue to: Discussion...
DISCUSSION
According to the Center for Disease Control and Prevention, 310,800 total hip replacements were performed among inpatients aged 45 years and older in the US in 2010.7 Specifically, in the 55- to 64-year-old age group, the number of procedures performed tripled from 2000 through 2010. As younger and more active patients opt for hip replacements, a growing need for prosthesis with enhanced durability is observed.
Despite the early proposed advantages of large head MoM bearings, our retrospective study of the DePuy Synthes ASR™ XL Acetabular hip system yielded 15.6% and 34.4% failure rates at 3 and 5 years, respectively. These higher-than-expected rates of failure are consistent with published data. The British Hip Society reported a 21% to 35% revision rate at 4 years and 49% at 6 years for the ASR XL prosthesis.8 In comparison, other MoM prosthesis, on average, report a 12% to 15% rate of failure at 5 years.
Considerable controversy surrounds the causes of adverse wear failure in MoM bearings.9,10 The non-modular design of the ASR prostheses is frequently implicated as a cause of early failure. The lack of a central hole in the 1-piece component compromises the tactile feel of insertion, thereby reducing the surgeon’s ability to assess complete seating.11 This condition may potentially increase the abduction angle at the time of insertion. Screw fixation of the non-modular device is not possible. The ASR XL device (148° to 160°) is less than a hemisphere (180°) in size and hence features a diminished functional articular surface, further compromising implant fixation.11 The functional articular surface is defined as the optimal surface area (10 mm) needed for a MoM implant.12 Griffin and colleagues13 reported a 48 mm ASR XL component, when implanted at 45° of abduction, to function similar to an implant at 59° of abduction, leading to diminished lubrication, metallosis, and edge loading. The version of the acetabular component may similarly and adversely affect implant wear characteristics. Furthermore, the variable thickness of the implant, which is thicker at the dome and thinner at the rim, may further promote edge loading by shifting the center of rotation of the femoral head out from the center of the acetabular prosthesis.11 Studies have also shown that increased wear of the MoM articulation is associated with an acetabular component inclination angle in excess of 55°10,14 and a failure of fixation at time of implantation.15 This study, however, found no correlation between the abduction angle and risk of early implant failure for the ASR acetabular component. No correlation was also detected between the acetabulum size and revision surgery.
The AOANJRR reported loosening (44%), infection (20%), metal sensitivity (12%), fracture (9%), and dislocation of prosthesis (7%) as the indications for revision surgery for the ASR prosthesis.6 Furthermore, a single-center retrospective review of 70 consecutive MoM THAs with ultra-large diameter femoral head and monoblock acetabular components showed that 17.1% required revision within 3 years for loosening, pain, and squeaking.1 Overall, 28.6% of patients reported implant dysfunction. In this study, we observed a similar rate of failure at 3 years (15.6%) for pain (11) and infection (1). The revision surgery successfully relieved all of these symptoms. One patient presented with heterotopic ossification and anterior hip pain after the original revision and required additional surgery with prosthesis retention. No patient in this series required repeat component revisions at a mean of 2.9 years after surgery. In all but 1 case, primary acetabular components were used in the revision, and in all cases except that with infection, the femoral component was retained. Replacement shells were 2 to 4 mm larger in diameter than the original ASR component.
Recently, concerns have arisen regarding the long-term effects of serum cobalt and chromium metal ions levels. Studies have shown increased serum metal ion levels,15 groin pain,16 pseudotumor formation,17 and metallosis18 after the implantation of MoM bearings. In a case study by Mao and colleagues,19 1 patient reported headaches, anorexia, continuous metallic taste in her mouth, and weight loss. A cerebrospinal fluid analysis revealed cobalt and chromium levels at 9 and 13 nmol/L, respectively, indicating that these metal ions can cross the blood-brain barrier. Another patient reported painful muscle fatigue, night cramps, fainting spells, cognitive decline, and an inability to climb stairs. His serum cobalt level reached 258 nmol/L (reference range, 0-20 nmol/L), and chromium level totaled 88 nmol/L (reference range, 0-100 nmol/L). At 8-week follow-up after revision surgery, the symptoms of the patient had resolved, with serum cobalt levels dropping to 42 nmol/L.19 None of the patients in this study presented with any signs or symptoms of metal toxicity. The upper limits of blood cobalt and chromium levels in our study population reached 18.9 and 15.9 µg/L for the revised group and 16.8 and 5.4 µg/L for the non-revised group, respectively. However, we noted a similar drop in post-revision blood cobalt (91% decrease) and chromium (78% decrease) levels.
In summary, our data showed a high revision rate of the DePuy Synthes ASR™ XL Acetabular hip system. Our findings are consistent with internationally published data. In the absence of reliable predictors of early failure, continued close clinical surveillance and laboratory monitoring of these patients are warranted.
CONCLUSION
This study demonstrates the high failure rate of the DePuy Synthes ASR™ XL Acetabular hip system used in THA at a minimum of 5 years of follow-up. No variable that was predictive of failure could be identified in this series. Close clinical surveillance of these patients is therefore required. Metal levels dropped quickly after revision, and the revision surgery can generally be performed with slightly larger primary components. Symptomatic patients with ASR hip replacements, regardless of blood metal-ion levels, were candidates for the revision surgery. Not all failed hips exhibited substantially elevated metal levels. Asymptomatic patients with high blood metal-ion levels should be closely followed-up and revision surgery should be strongly considered, consistent with recently published guidelines.20
- Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27(4):539. doi:10.1016/j.arth.2011.08.022.
- de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287. doi:10.2106/JBJS.J.01727.
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46. doi:10.1302/0301-620X.92B1.22770.
- Siebel T, Maubach S, Morlock MM. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc Inst Mech Eng H. 2006;220(2):345-353. doi:10.1243/095441105X69079.
- Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92(1):28-37. doi:10.1302/0301-620X.92B1.22769.
- Australian Orthopaedic Association National Joint Replacement Registry annual report 2010. Australian Orthopaedic Association Web site. https://aoanjrr.sahmri.com/annual-reports-2010. Accessed June 19, 2018.
- Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/databriefs/db186.pdf. Accessed July 13, 2015.
- Hodgkinson J, Skinner J, Kay P. Large diameter metal on metal bearing total hip replacements. British Hip Society Web site. https://www.britishhipsociety.com/uploaded/BHS_MOM_THR.pdf. Accessed August 6, 2015.
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93(3):315-320. doi:10.1302/0301-620X.93B3.25545.
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93(2):164-171. doi:10.1302/0301-620X.93B2.25099.
- Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6):14-18. doi:10.1016/j.arth.2011.03.027.
- De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158-1163. doi:10.1302/0301-620X.90B9.19891.
- Griffin WL, Nanson CJ, Springer BD, Davies MA, Fehring TK. Reduced articular surface of one-piece cups: a cause of runaway wear and early failure. Clin Orthop Relat Res. 2010;468(9):2328-2332. doi:10.1007/s11999-010-1383-8.
- Grammatopolous G, Pandit H, Glyn-Jones S, et al. Optimal acetablular orientation for hip resurfacing. J Bone Joint Surg Br. 2010;92(8):1072-1078. doi:10.1302/0301-620X.92B8.24194.
- MacDonalad SJ, McCalden RW, Chess DG, et al. Meta-onmetal versus polyethylene in hip arthoplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;(406):282-296.
- Bin Nasser A, Beaule PE, O'Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468(2):392-399. doi:10.1007/s11999-009-1133-y.
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80(6):653-659. doi:10.3109/17453670903473016.
- Neumann DRP, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal-on-metal total hip arthroplasty. J Arthroplasty. 2010;25(5):700-708. doi:10.1016/j.arth.2009.05.018.
- Mao X, Wong AA, Crawford RW. Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
- Information statement: current concerns with metal-on-metal hip arthroplasty. American Academy of Orthopaedic Surgeons Web site. https://aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1035%20Current%20Concerns%20with%20Metal-on-Metal%20Hip%20Arthroplasty.pdf. Accessed June 19, 2018.
- Bernthal NM, Celestre PC, Stavrakis AI, Ludington JC, Oakes DA. Disappointing short-term results with the DePuy ASR XL metal-on-metal total hip arthroplasty. J Arthroplasty. 2012;27(4):539. doi:10.1016/j.arth.2011.08.022.
- de Steiger RN, Hang JR, Miller LN, Graves SE, Davidson DC. Five-year results of the ASR XL acetabular system and the ASR hip resurfacing system: An analysis from the Australian Orthopaedic Association National Joint Replacement Registry. J Bone Joint Surg Am. 2011;93(24):2287. doi:10.2106/JBJS.J.01727.
- Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46. doi:10.1302/0301-620X.92B1.22770.
- Siebel T, Maubach S, Morlock MM. Lessons learned from early clinical experience and results of 300 ASR hip resurfacing implantations. Proc Inst Mech Eng H. 2006;220(2):345-353. doi:10.1243/095441105X69079.
- Jameson SS, Langton DJ, Nargol AV. Articular surface replacement of the hip: a prospective single-surgeon series. J Bone Joint Surg Br. 2010;92(1):28-37. doi:10.1302/0301-620X.92B1.22769.
- Australian Orthopaedic Association National Joint Replacement Registry annual report 2010. Australian Orthopaedic Association Web site. https://aoanjrr.sahmri.com/annual-reports-2010. Accessed June 19, 2018.
- Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/nchs/data/databriefs/db186.pdf. Accessed July 13, 2015.
- Hodgkinson J, Skinner J, Kay P. Large diameter metal on metal bearing total hip replacements. British Hip Society Web site. https://www.britishhipsociety.com/uploaded/BHS_MOM_THR.pdf. Accessed August 6, 2015.
- Hart AJ, Ilo K, Underwood R, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: a prospective, CT-based study. J Bone Joint Surg Br. 2011;93(3):315-320. doi:10.1302/0301-620X.93B3.25545.
- Langton DJ, Joyce TJ, Jameson SS, et al. Adverse reaction to metal debris following hip resurfacing: the influence of component type, orientation and volumetric wear. J Bone Joint Surg Br. 2011;93(2):164-171. doi:10.1302/0301-620X.93B2.25099.
- Steele GD, Fehring TK, Odum SM, Dennos AC, Nadaud MC. Early failure of articular surface replacement XL total hip arthroplasty. J Arthroplasty. 2011;26(6):14-18. doi:10.1016/j.arth.2011.03.027.
- De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90(9):1158-1163. doi:10.1302/0301-620X.90B9.19891.
- Griffin WL, Nanson CJ, Springer BD, Davies MA, Fehring TK. Reduced articular surface of one-piece cups: a cause of runaway wear and early failure. Clin Orthop Relat Res. 2010;468(9):2328-2332. doi:10.1007/s11999-010-1383-8.
- Grammatopolous G, Pandit H, Glyn-Jones S, et al. Optimal acetablular orientation for hip resurfacing. J Bone Joint Surg Br. 2010;92(8):1072-1078. doi:10.1302/0301-620X.92B8.24194.
- MacDonalad SJ, McCalden RW, Chess DG, et al. Meta-onmetal versus polyethylene in hip arthoplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;(406):282-296.
- Bin Nasser A, Beaule PE, O'Neill M, Kim PR, Fazekas A. Incidence of groin pain after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2010;468(2):392-399. doi:10.1007/s11999-009-1133-y.
- Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009;80(6):653-659. doi:10.3109/17453670903473016.
- Neumann DRP, Thaler C, Hitzl W, Huber M, Hofstädter T, Dorn U. Long term results of a contemporary metal-on-metal total hip arthroplasty. J Arthroplasty. 2010;25(5):700-708. doi:10.1016/j.arth.2009.05.018.
- Mao X, Wong AA, Crawford RW. Cobalt toxicity--an emerging clinical problem in patients with metal-on-metal hip prostheses? Med J Aust. 2011;194(12):649-651.
- Information statement: current concerns with metal-on-metal hip arthroplasty. American Academy of Orthopaedic Surgeons Web site. https://aaos.org/uploadedFiles/PreProduction/About/Opinion_Statements/advistmt/1035%20Current%20Concerns%20with%20Metal-on-Metal%20Hip%20Arthroplasty.pdf. Accessed June 19, 2018.
TAKE-HOME POINTS
- High rate of failure of DePuy Synthes ASR™ XL Acetabular hip system used in THA, approaching 34.4% at 5 years.
- Mean time to revision was 3.1 years with pain being the most common indication for revision surgery.
- Age, gender, acetabular component abduction angle, acetabular size, and serum cobalt or chromium levels were not associated with increased rate of failure.
- Serum cobalt and chromium levels decreased significantly within 6 months of revision surgery.
- Close clinical surveillance and laboratory monitoring of patients is required.
Free Composite Serratus Anterior-Latissimus-Rib Flaps for Acute One-Stage Reconstruction of Gustilo IIIB Tibia Fractures
ABSTRACT
Gustilo IIIB injuries of the tibia with segmental bone loss continue to be a difficult reconstructive problem. The serratus anterior-latissimus-rib (SALR) composite flap consists of bone and muscle; this flap can provide soft tissue coverage and vascularized bone in a single surgical procedure. The purpose of this study is to describe the use of the SALR flap for the treatment of a large open tibia fracture with segmental bone loss, with a specific focus on postoperative complications, limb salvage, and time to union.
We reviewed the medical records of patients undergoing an SALR flap (n = 5) for the treatment of Gustilo Type IIIB tibia fractures within 1 month of injury. We compared the mechanism of injury, injury severity score, time from injury to free tissue transfer, complications, and time to radiographic and clinical union.
All patients were male, with a mean age of 25 years. On average, patients underwent free tissue transfer within 1 week of injury. The average time to radiographic union was 7 months. Two patients underwent reoperation. There were no graft failures.
Free SALR flaps can be a useful option for the treatment of high-energy tibia fractures with extensive soft tissue and bone loss. These flaps provide immediate osseous and soft tissue reconstruction with an acceptable complication profile.
Reconstruction of the lower extremity following Gustilo’s grade IIIB injuries is difficult due to loss of both combined soft tissue and segmental bone loss. Since these injuries necessitate the need for soft tissue flap coverage along with vascularized bone grafting, free fibula flaps have classically been used for reconstruction.1-3 In the setting of bilateral lower extremity injury, the contralateral fibula is often not appropriate to harvest and transfer; therefore, other sources of vascularized bone grafts must be utilized including vascularized iliac crest and rib.1-5 The vascularized iliac crest graft is insufficient to provide the bony reconstruction of bone defects >6 cm to 7 cm and does not have a reliable skin paddle.4 In contrast, free composite serratus anterior-latissimus-rib (SALR) flaps can provide both long segments of vascularized bone and abundant soft tissue coverage for large segmental defects.1-5
Continue to: Free fibula grafts have been considered...
Free fibula grafts have been considered the gold standard for the reconstruction of large (>6 cm) bone defects.6 In cases of “mangled extremities,” bone defects are associated with large soft tissue defects, which require either single-stage surgery consisting of 2 separate free flaps (ie, free fibula and free latissimus) or a 2-stage procedure where the soft tissue reconstruction precedes the bone reconstruction.2,7-9 Unlike free fibula and latissimus flaps, composite SALR flaps provide both osseous reconstruction and soft tissue in 1 flap supplied by a single vascular pedicle; unfortunately, outcomes using this flap for large Gustilo IIIB injuries are limited.1-5 The purpose of this study is to examine the use of free composite SALR flaps for soft tissue coverage in cases of Gustilo IIIB injuries with large soft tissue and bony deficits. This study specifically examines time to union, need for reoperation, and graft failure following the use of these flaps.
MATERIALS AND METHODS
Following approval from our Institutional Review Board, we retrospectively reviewed the medical records of patients undergoing a free composite SALR flap (n = 5) for the treatment of a severe open tibia fracture within 1 month of injury. All patients sustained open injuries classified as IIIB on the Gustilo-Anderson scale.10 Medical records were examined for the mechanism of injury (MOI), injury severity score (ISS), time from injury to free tissue transfer, medical comorbidities, surgical complications, and time to radiographic and clinical union. Radiographic union was determined by the presence of bridging bone on 3 of 4 of cortices on plain film radiographs.
All patients were male (n = 5), with a mean age of 25 years (range, 19-30 years) at the time of injury (Table).
Table. Demographics and Outcomes of Patients Undergoing Free Tissue Transfer
| Free Serratus Anterior-Latissimus-Rib Flaps |
Age (Mean ± SEM) | 23 ± 2 years |
Males | 5 |
Females | 0 |
Tobacco Use | 2 |
Body Mass Index (Mean ± SEM) | 26.2 ± 0.9 kg/m2 |
Injury Severity Score (Mean ± SEM) | 18 ± 5 |
Time to Tissue Transfer (Mean ± SEM) | 1 ± 0.3 weeks |
Time to Boney Union (Mean ± SEM) | 7 ± 0.7 months |
Time Non-Weight-Bearing (Mean ± SEM) | 5 ± 0.5 months |
The MOI included motorcycle collisions (n = 2), pedestrian struck by car (n = 1), motor vehicle collisions (n = 1), and direct blow to the leg (n = 1). The mean ISS of the cohort was 18 (range, 10-34) (Table). On average, patients underwent free tissue transfer within 1 week (range, 3 days to 2 weeks) from the time of injury. Patients in this cohort were followed clinically for a mean of 4 years (range, 1-6 years) after surgery. Patients were non-weight-bearing for an average of 5 months (range, 4-6 months) following their reconstructions.
RESULTS
All flaps survived. The mean time to radiographic and clinical union was 7 months (range, 6-9 months). Two patients underwent reoperation. One patient underwent a bone grafting procedure for a delayed union at 6 months postoperative, and 1 patient underwent irrigation and débridement of superficial soft tissue infection. Donor site complications occurred in 2 patients, including chronic rib pain (n = 1) and a pleural effusion requiring drainage (n = 1). At the last follow-up, all ribs had incorporated, and all patients were weight-bearing as tolerated on the limb.
CASE EXAMPLE
A 22-year-old male smoker was transferred to our facility after a motor vehicle accident with bilateral tibia fractures, 1 closed and 1 open with significant bone loss (Figures 1A, 1B).
Continue to: Surgical Technique...
SURGICAL TECHNIQUE
The patient is placed in the lateral decubitus position during the procedure. A 2-team approach is used for dissection of the flap and preparation of recipient vessels to decrease operative time. A J-shaped incision is started on the chest at the mid-axillary line and extended just over the fifth and sixth rib. The incision can be made to fall into the intermammary crease in a woman to hide the scar. The dissection begins by exposing the anterior border of the latissimus muscle (Figure 2A). Next, the latissimus is dissected to reveal the thoracodorsal vessels (Figure 2B). At this level, the thoracodorsal vessel can be traced into the axilla. The branch going into the fifth, sixth, and lower slips of the serratus are dissected. The long thoracic nerve and the thoracodorsal nerve are preserved during the dissection (Figure 2C). The fifth, sixth, and seventh slips of the serratus are preferentially included in the dissection while leaving the most superior slips of the serratus to preserve scapular stability. Dissection begins by identifying 2 adjacent rib sections of the fifth and sixth or sixth and seventh ribs. The defect in the lower extremity determines the length of rib harvested. The serratus slips are then divided anteriorly over the chest wall. The dissection is extended to the intercostal spaces of the fourth and fifth ribs. The supraperiosteal dissection is performed at the anterior margin of the rib (Figure 2D).
Continue to: Following the surgical procedure...
Following the surgical procedure, patients are made non-weight-bearing on the operative extremity until signs of healing are apparent on radiographs. In this case, at the patients’ last follow-up visit, the skin graft was healed, and there was solid fusion of the rib/tibia junction (Figures 4B, 4C).
DISCUSSION
High-energy open injuries to the lower extremities are devastating injuries, with a high rate of late amputation and poor functional outcomes.11-13 Vascularized bone grafting provides both essential osteoinductive and osteoconductive properties to segmental bone defects in areas with inadequate soft tissue coverage, particularly in the setting of >6 cm of bone loss.4,14 The results of this study show that acute reconstruction of the lower limb with a composite vascularized SALR graft is a reliable procedure with an acceptable complication profile.
The timing of soft tissue coverage should be performed as soon as the patient is medically stable enough to undergo a reconstructive procedure, ideally within 7 to 10 days; and this timetable has been shown to decrease rates of infection and free flap failure.15-19 Early coverage provides both control of the soft-tissue envelope and reduces the risk of losing bone.1 Unlike the timing of coverage, the staging of the procedure is controversial. Proponents of the 2-stage free tissue (soft tissue followed by bony flap) transfer feel that although the tissue may not be infected at the time of coverage, it is contaminated with bacteria at the time of bone reconstruction, and as such is at high risk for both infection and complications.20 Unlike 2-stage procedures, single-stage coverage provides immediate soft tissue coverage, as well as bony support. This reduces the time to bony union and negates the need for repeated surgery in a mangled extremity where secondary surgery is complicated by both scar tissue and altered anatomy.1,2 Furthermore, it has been shown that there is no difference in the rates of infection when performing a single-stage compared with a 2-stage procedure.9 In this study, SALR flaps were typically performed within 2 weeks following an injury as a single procedure. We feel this resulted in the low number of complications in the SALR group.
Unlike free fibulas, rib flaps are easily pedicled with an associated soft-tissue flap due to their blood supply, making them ideal for 1-stage reconstruction. The rib has a dual blood supply: 1 from the posterior intercostal artery, and the other, an abundant periosteal blood supply, from the serratus anterior muscle.4 The blood supply to the serratus anterior comes from the thoracodorsal artery, and usually provides 14 cm of a large-caliber pedicle, making it a reliable flap for soft tissue reconstruction.21,22 Another unique feature of the blood supply to this flap is the amount of soft tissue available for both harvest and transfer; larger portions of serratus muscle and latissimus muscle can be harvested if necessary to cover the soft tissue defect.4
Comminuted tibias with segmental bone loss are difficult to manage since they are associated with bony as well as soft tissue defects.1,12,13,23 These injuries are ideal candidates for a single-stage reconstruction using a vascularized SALR flap. In our series, the use of an SALR flap resulted ultimately in a 100% union and limb salvage rate, with no flap failures and a low complication profile. Unlike the SALR, free fibular flaps must be transferred along with a separate latissimus dorsi flap to provide enough soft tissue coverage necessary for reconstructing large Gustilo IIIB injuries, which could increase the risk of flap failure. Since ribs are composed of membranous bone and have a similar cross-sectional area to both metacarpal and metatarsals, there are concerns regarding the biomechanical properties of ribs for weight-bearing.4,22,24-26 To compensate for this relatively small cross-sectional area, 2 ribs (either consecutive or alternative) are frequently harvested.1,4,5,23 Previous studies examining the use of ribs for bony reconstruction have frequently supplemented the rib reconstruction to the tibia using screws and external fixation alone.1,4,5,23 In our series, all SALR grafts were supported with the use of an intramedullary nail (n = 3) or locked plating (n = 1). The use of this supplemental fixation of the SALR graft allowed our patients to return to full weight-bearing (mean, 6 months) much earlier than the length of time cited in previous reports (12 months) examining these injuries.1,4,5,23
Continue to: There are several limitations...
There are several limitations to this study. The small sample size and retrospective nature of the study limits the amount of data we are able to collect from the medical record and places obvious constraints on the analysis. Although all these procedures were performed at 1 institution, multiple providers were involved in the reconstruction of these injuries, and there is no standard protocol for their treatment. Similarly, although other forms of extremity reconstruction were used during this time period, there was no standard protocol that could serve as a comparator for patients who underwent an SALR compared with other reconstructive procedures.
Overall, SALR grafts provide an excellent option for 1-stage reconstruction of severe, open lower extremity injuries. In this series we noted a 100% graft success rate with an acceptable complication profile.
This paper will be judged for the Resident Writer’s Award.
1. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114(6):1457-1466. doi:10.1097/01.PRS.0000138811.88807.65.
2. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg. 1999;104(4):984-992. doi:10.1097/00006534-199909040-00013.
3. Tu YK, Yen CY, Yeh WL, Wang IC, Wang KC, Ueng SW. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthopaedica Scandinavica. 2001;72(4):359-364. doi:10.1080/000164701753542014.
4. Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99(6):1656-1665. Doi:10.1097/00006534-199705000-00028.
5. Georgescu AV, Ignatiadis I, Ileana M, Irina C, Filip A, Olariu R. Long-term results after muscle-rib flap transfer for reconstruction of composite limb defects. Microsurgery. 2011;31(3):218-222. doi:10.1002/micr.20857.
6. Moran CG, Wood MB. Vascularized bone autografts. Orthop Rev. 1993;22(2):187-197. doi:10.1097/01241398-199307000-00031.
7. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421-428. doi:10.1055/s-2007-1006751.
8. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14(6):368-374. doi:10.1002/micr.1920140603.
9. Peat BG, Liggins DF. Microvascular soft tissue reconstruction for acute tibial fractures--late complications and the role of bone grafting. Ann Plast Surg. 1990;24(6):517-520.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746. doi:10.1097/00005373-198408000-00009.
12. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. NEJM. 2002;347(24):1924-1931. doi:10.1056/NEJMoa012604.
13. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809. doi:10.2106/JBJS.E.00032.
14. Bieber EJ, Wood MB. Bone reconstruction. Clin Plast Surg. 1986;13(4):645-655.
15. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg. 2010;18(2):108-117. doi:10.5435/00124635-201002000-00005.
16. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285-292. doi:10.1055/s-2006-944324.
17. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959-966. doi:10.1302/0301-620X.82B7.0820959.
18. Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316-1322. doi:10.2106/00004623-199173090-00005.
19. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912. doi:10.1016/j.injury.2007.02.052.
20. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80(1):1-14. doi:10.1097/00006534-198707000-00002.
21. Ueng WN, Chuang CC, Shih CH. Double-rib composite free transfer to reconstruct a single-spared lower extremity defect. J Trauma. 1995;38(2):210-212.
22. Bruck JC, Bier J, Kistler D. The serratus anterior osteocutaneous free flap. J Reconstr Microsurg. 1990;6(3):209-213. doi:10.1055/s-2007-1006820.
23. Lin CH, Yazar S. Revisiting the serratus anterior rib flap for composite tibial defects. Plast Reconstr Surg. 2004;114(7):1871-1877. doi:10.1097/01.PRS.0000142767.13493.63.
24. Hui KC, Zhang F, Lineaweaver WC, Moon W, Buncke GM, Buncke HJ. Serratus anterior-rib composite flap: anatomic studies and clinical application to hand reconstruction. Ann Plast Surg. 1999;42(2):132-136. doi:10.1097/00000637-199902000-00004.
25. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59(6):799-804. doi:10.1097/00006534-197706000-00002.
26. Nusbickel FR, Dell PC, Mcandrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res. 1989;(243):65-70.
ABSTRACT
Gustilo IIIB injuries of the tibia with segmental bone loss continue to be a difficult reconstructive problem. The serratus anterior-latissimus-rib (SALR) composite flap consists of bone and muscle; this flap can provide soft tissue coverage and vascularized bone in a single surgical procedure. The purpose of this study is to describe the use of the SALR flap for the treatment of a large open tibia fracture with segmental bone loss, with a specific focus on postoperative complications, limb salvage, and time to union.
We reviewed the medical records of patients undergoing an SALR flap (n = 5) for the treatment of Gustilo Type IIIB tibia fractures within 1 month of injury. We compared the mechanism of injury, injury severity score, time from injury to free tissue transfer, complications, and time to radiographic and clinical union.
All patients were male, with a mean age of 25 years. On average, patients underwent free tissue transfer within 1 week of injury. The average time to radiographic union was 7 months. Two patients underwent reoperation. There were no graft failures.
Free SALR flaps can be a useful option for the treatment of high-energy tibia fractures with extensive soft tissue and bone loss. These flaps provide immediate osseous and soft tissue reconstruction with an acceptable complication profile.
Reconstruction of the lower extremity following Gustilo’s grade IIIB injuries is difficult due to loss of both combined soft tissue and segmental bone loss. Since these injuries necessitate the need for soft tissue flap coverage along with vascularized bone grafting, free fibula flaps have classically been used for reconstruction.1-3 In the setting of bilateral lower extremity injury, the contralateral fibula is often not appropriate to harvest and transfer; therefore, other sources of vascularized bone grafts must be utilized including vascularized iliac crest and rib.1-5 The vascularized iliac crest graft is insufficient to provide the bony reconstruction of bone defects >6 cm to 7 cm and does not have a reliable skin paddle.4 In contrast, free composite serratus anterior-latissimus-rib (SALR) flaps can provide both long segments of vascularized bone and abundant soft tissue coverage for large segmental defects.1-5
Continue to: Free fibula grafts have been considered...
Free fibula grafts have been considered the gold standard for the reconstruction of large (>6 cm) bone defects.6 In cases of “mangled extremities,” bone defects are associated with large soft tissue defects, which require either single-stage surgery consisting of 2 separate free flaps (ie, free fibula and free latissimus) or a 2-stage procedure where the soft tissue reconstruction precedes the bone reconstruction.2,7-9 Unlike free fibula and latissimus flaps, composite SALR flaps provide both osseous reconstruction and soft tissue in 1 flap supplied by a single vascular pedicle; unfortunately, outcomes using this flap for large Gustilo IIIB injuries are limited.1-5 The purpose of this study is to examine the use of free composite SALR flaps for soft tissue coverage in cases of Gustilo IIIB injuries with large soft tissue and bony deficits. This study specifically examines time to union, need for reoperation, and graft failure following the use of these flaps.
MATERIALS AND METHODS
Following approval from our Institutional Review Board, we retrospectively reviewed the medical records of patients undergoing a free composite SALR flap (n = 5) for the treatment of a severe open tibia fracture within 1 month of injury. All patients sustained open injuries classified as IIIB on the Gustilo-Anderson scale.10 Medical records were examined for the mechanism of injury (MOI), injury severity score (ISS), time from injury to free tissue transfer, medical comorbidities, surgical complications, and time to radiographic and clinical union. Radiographic union was determined by the presence of bridging bone on 3 of 4 of cortices on plain film radiographs.
All patients were male (n = 5), with a mean age of 25 years (range, 19-30 years) at the time of injury (Table).
Table. Demographics and Outcomes of Patients Undergoing Free Tissue Transfer
| Free Serratus Anterior-Latissimus-Rib Flaps |
Age (Mean ± SEM) | 23 ± 2 years |
Males | 5 |
Females | 0 |
Tobacco Use | 2 |
Body Mass Index (Mean ± SEM) | 26.2 ± 0.9 kg/m2 |
Injury Severity Score (Mean ± SEM) | 18 ± 5 |
Time to Tissue Transfer (Mean ± SEM) | 1 ± 0.3 weeks |
Time to Boney Union (Mean ± SEM) | 7 ± 0.7 months |
Time Non-Weight-Bearing (Mean ± SEM) | 5 ± 0.5 months |
The MOI included motorcycle collisions (n = 2), pedestrian struck by car (n = 1), motor vehicle collisions (n = 1), and direct blow to the leg (n = 1). The mean ISS of the cohort was 18 (range, 10-34) (Table). On average, patients underwent free tissue transfer within 1 week (range, 3 days to 2 weeks) from the time of injury. Patients in this cohort were followed clinically for a mean of 4 years (range, 1-6 years) after surgery. Patients were non-weight-bearing for an average of 5 months (range, 4-6 months) following their reconstructions.
RESULTS
All flaps survived. The mean time to radiographic and clinical union was 7 months (range, 6-9 months). Two patients underwent reoperation. One patient underwent a bone grafting procedure for a delayed union at 6 months postoperative, and 1 patient underwent irrigation and débridement of superficial soft tissue infection. Donor site complications occurred in 2 patients, including chronic rib pain (n = 1) and a pleural effusion requiring drainage (n = 1). At the last follow-up, all ribs had incorporated, and all patients were weight-bearing as tolerated on the limb.
CASE EXAMPLE
A 22-year-old male smoker was transferred to our facility after a motor vehicle accident with bilateral tibia fractures, 1 closed and 1 open with significant bone loss (Figures 1A, 1B).
Continue to: Surgical Technique...
SURGICAL TECHNIQUE
The patient is placed in the lateral decubitus position during the procedure. A 2-team approach is used for dissection of the flap and preparation of recipient vessels to decrease operative time. A J-shaped incision is started on the chest at the mid-axillary line and extended just over the fifth and sixth rib. The incision can be made to fall into the intermammary crease in a woman to hide the scar. The dissection begins by exposing the anterior border of the latissimus muscle (Figure 2A). Next, the latissimus is dissected to reveal the thoracodorsal vessels (Figure 2B). At this level, the thoracodorsal vessel can be traced into the axilla. The branch going into the fifth, sixth, and lower slips of the serratus are dissected. The long thoracic nerve and the thoracodorsal nerve are preserved during the dissection (Figure 2C). The fifth, sixth, and seventh slips of the serratus are preferentially included in the dissection while leaving the most superior slips of the serratus to preserve scapular stability. Dissection begins by identifying 2 adjacent rib sections of the fifth and sixth or sixth and seventh ribs. The defect in the lower extremity determines the length of rib harvested. The serratus slips are then divided anteriorly over the chest wall. The dissection is extended to the intercostal spaces of the fourth and fifth ribs. The supraperiosteal dissection is performed at the anterior margin of the rib (Figure 2D).
Continue to: Following the surgical procedure...
Following the surgical procedure, patients are made non-weight-bearing on the operative extremity until signs of healing are apparent on radiographs. In this case, at the patients’ last follow-up visit, the skin graft was healed, and there was solid fusion of the rib/tibia junction (Figures 4B, 4C).
DISCUSSION
High-energy open injuries to the lower extremities are devastating injuries, with a high rate of late amputation and poor functional outcomes.11-13 Vascularized bone grafting provides both essential osteoinductive and osteoconductive properties to segmental bone defects in areas with inadequate soft tissue coverage, particularly in the setting of >6 cm of bone loss.4,14 The results of this study show that acute reconstruction of the lower limb with a composite vascularized SALR graft is a reliable procedure with an acceptable complication profile.
The timing of soft tissue coverage should be performed as soon as the patient is medically stable enough to undergo a reconstructive procedure, ideally within 7 to 10 days; and this timetable has been shown to decrease rates of infection and free flap failure.15-19 Early coverage provides both control of the soft-tissue envelope and reduces the risk of losing bone.1 Unlike the timing of coverage, the staging of the procedure is controversial. Proponents of the 2-stage free tissue (soft tissue followed by bony flap) transfer feel that although the tissue may not be infected at the time of coverage, it is contaminated with bacteria at the time of bone reconstruction, and as such is at high risk for both infection and complications.20 Unlike 2-stage procedures, single-stage coverage provides immediate soft tissue coverage, as well as bony support. This reduces the time to bony union and negates the need for repeated surgery in a mangled extremity where secondary surgery is complicated by both scar tissue and altered anatomy.1,2 Furthermore, it has been shown that there is no difference in the rates of infection when performing a single-stage compared with a 2-stage procedure.9 In this study, SALR flaps were typically performed within 2 weeks following an injury as a single procedure. We feel this resulted in the low number of complications in the SALR group.
Unlike free fibulas, rib flaps are easily pedicled with an associated soft-tissue flap due to their blood supply, making them ideal for 1-stage reconstruction. The rib has a dual blood supply: 1 from the posterior intercostal artery, and the other, an abundant periosteal blood supply, from the serratus anterior muscle.4 The blood supply to the serratus anterior comes from the thoracodorsal artery, and usually provides 14 cm of a large-caliber pedicle, making it a reliable flap for soft tissue reconstruction.21,22 Another unique feature of the blood supply to this flap is the amount of soft tissue available for both harvest and transfer; larger portions of serratus muscle and latissimus muscle can be harvested if necessary to cover the soft tissue defect.4
Comminuted tibias with segmental bone loss are difficult to manage since they are associated with bony as well as soft tissue defects.1,12,13,23 These injuries are ideal candidates for a single-stage reconstruction using a vascularized SALR flap. In our series, the use of an SALR flap resulted ultimately in a 100% union and limb salvage rate, with no flap failures and a low complication profile. Unlike the SALR, free fibular flaps must be transferred along with a separate latissimus dorsi flap to provide enough soft tissue coverage necessary for reconstructing large Gustilo IIIB injuries, which could increase the risk of flap failure. Since ribs are composed of membranous bone and have a similar cross-sectional area to both metacarpal and metatarsals, there are concerns regarding the biomechanical properties of ribs for weight-bearing.4,22,24-26 To compensate for this relatively small cross-sectional area, 2 ribs (either consecutive or alternative) are frequently harvested.1,4,5,23 Previous studies examining the use of ribs for bony reconstruction have frequently supplemented the rib reconstruction to the tibia using screws and external fixation alone.1,4,5,23 In our series, all SALR grafts were supported with the use of an intramedullary nail (n = 3) or locked plating (n = 1). The use of this supplemental fixation of the SALR graft allowed our patients to return to full weight-bearing (mean, 6 months) much earlier than the length of time cited in previous reports (12 months) examining these injuries.1,4,5,23
Continue to: There are several limitations...
There are several limitations to this study. The small sample size and retrospective nature of the study limits the amount of data we are able to collect from the medical record and places obvious constraints on the analysis. Although all these procedures were performed at 1 institution, multiple providers were involved in the reconstruction of these injuries, and there is no standard protocol for their treatment. Similarly, although other forms of extremity reconstruction were used during this time period, there was no standard protocol that could serve as a comparator for patients who underwent an SALR compared with other reconstructive procedures.
Overall, SALR grafts provide an excellent option for 1-stage reconstruction of severe, open lower extremity injuries. In this series we noted a 100% graft success rate with an acceptable complication profile.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Gustilo IIIB injuries of the tibia with segmental bone loss continue to be a difficult reconstructive problem. The serratus anterior-latissimus-rib (SALR) composite flap consists of bone and muscle; this flap can provide soft tissue coverage and vascularized bone in a single surgical procedure. The purpose of this study is to describe the use of the SALR flap for the treatment of a large open tibia fracture with segmental bone loss, with a specific focus on postoperative complications, limb salvage, and time to union.
We reviewed the medical records of patients undergoing an SALR flap (n = 5) for the treatment of Gustilo Type IIIB tibia fractures within 1 month of injury. We compared the mechanism of injury, injury severity score, time from injury to free tissue transfer, complications, and time to radiographic and clinical union.
All patients were male, with a mean age of 25 years. On average, patients underwent free tissue transfer within 1 week of injury. The average time to radiographic union was 7 months. Two patients underwent reoperation. There were no graft failures.
Free SALR flaps can be a useful option for the treatment of high-energy tibia fractures with extensive soft tissue and bone loss. These flaps provide immediate osseous and soft tissue reconstruction with an acceptable complication profile.
Reconstruction of the lower extremity following Gustilo’s grade IIIB injuries is difficult due to loss of both combined soft tissue and segmental bone loss. Since these injuries necessitate the need for soft tissue flap coverage along with vascularized bone grafting, free fibula flaps have classically been used for reconstruction.1-3 In the setting of bilateral lower extremity injury, the contralateral fibula is often not appropriate to harvest and transfer; therefore, other sources of vascularized bone grafts must be utilized including vascularized iliac crest and rib.1-5 The vascularized iliac crest graft is insufficient to provide the bony reconstruction of bone defects >6 cm to 7 cm and does not have a reliable skin paddle.4 In contrast, free composite serratus anterior-latissimus-rib (SALR) flaps can provide both long segments of vascularized bone and abundant soft tissue coverage for large segmental defects.1-5
Continue to: Free fibula grafts have been considered...
Free fibula grafts have been considered the gold standard for the reconstruction of large (>6 cm) bone defects.6 In cases of “mangled extremities,” bone defects are associated with large soft tissue defects, which require either single-stage surgery consisting of 2 separate free flaps (ie, free fibula and free latissimus) or a 2-stage procedure where the soft tissue reconstruction precedes the bone reconstruction.2,7-9 Unlike free fibula and latissimus flaps, composite SALR flaps provide both osseous reconstruction and soft tissue in 1 flap supplied by a single vascular pedicle; unfortunately, outcomes using this flap for large Gustilo IIIB injuries are limited.1-5 The purpose of this study is to examine the use of free composite SALR flaps for soft tissue coverage in cases of Gustilo IIIB injuries with large soft tissue and bony deficits. This study specifically examines time to union, need for reoperation, and graft failure following the use of these flaps.
MATERIALS AND METHODS
Following approval from our Institutional Review Board, we retrospectively reviewed the medical records of patients undergoing a free composite SALR flap (n = 5) for the treatment of a severe open tibia fracture within 1 month of injury. All patients sustained open injuries classified as IIIB on the Gustilo-Anderson scale.10 Medical records were examined for the mechanism of injury (MOI), injury severity score (ISS), time from injury to free tissue transfer, medical comorbidities, surgical complications, and time to radiographic and clinical union. Radiographic union was determined by the presence of bridging bone on 3 of 4 of cortices on plain film radiographs.
All patients were male (n = 5), with a mean age of 25 years (range, 19-30 years) at the time of injury (Table).
Table. Demographics and Outcomes of Patients Undergoing Free Tissue Transfer
| Free Serratus Anterior-Latissimus-Rib Flaps |
Age (Mean ± SEM) | 23 ± 2 years |
Males | 5 |
Females | 0 |
Tobacco Use | 2 |
Body Mass Index (Mean ± SEM) | 26.2 ± 0.9 kg/m2 |
Injury Severity Score (Mean ± SEM) | 18 ± 5 |
Time to Tissue Transfer (Mean ± SEM) | 1 ± 0.3 weeks |
Time to Boney Union (Mean ± SEM) | 7 ± 0.7 months |
Time Non-Weight-Bearing (Mean ± SEM) | 5 ± 0.5 months |
The MOI included motorcycle collisions (n = 2), pedestrian struck by car (n = 1), motor vehicle collisions (n = 1), and direct blow to the leg (n = 1). The mean ISS of the cohort was 18 (range, 10-34) (Table). On average, patients underwent free tissue transfer within 1 week (range, 3 days to 2 weeks) from the time of injury. Patients in this cohort were followed clinically for a mean of 4 years (range, 1-6 years) after surgery. Patients were non-weight-bearing for an average of 5 months (range, 4-6 months) following their reconstructions.
RESULTS
All flaps survived. The mean time to radiographic and clinical union was 7 months (range, 6-9 months). Two patients underwent reoperation. One patient underwent a bone grafting procedure for a delayed union at 6 months postoperative, and 1 patient underwent irrigation and débridement of superficial soft tissue infection. Donor site complications occurred in 2 patients, including chronic rib pain (n = 1) and a pleural effusion requiring drainage (n = 1). At the last follow-up, all ribs had incorporated, and all patients were weight-bearing as tolerated on the limb.
CASE EXAMPLE
A 22-year-old male smoker was transferred to our facility after a motor vehicle accident with bilateral tibia fractures, 1 closed and 1 open with significant bone loss (Figures 1A, 1B).
Continue to: Surgical Technique...
SURGICAL TECHNIQUE
The patient is placed in the lateral decubitus position during the procedure. A 2-team approach is used for dissection of the flap and preparation of recipient vessels to decrease operative time. A J-shaped incision is started on the chest at the mid-axillary line and extended just over the fifth and sixth rib. The incision can be made to fall into the intermammary crease in a woman to hide the scar. The dissection begins by exposing the anterior border of the latissimus muscle (Figure 2A). Next, the latissimus is dissected to reveal the thoracodorsal vessels (Figure 2B). At this level, the thoracodorsal vessel can be traced into the axilla. The branch going into the fifth, sixth, and lower slips of the serratus are dissected. The long thoracic nerve and the thoracodorsal nerve are preserved during the dissection (Figure 2C). The fifth, sixth, and seventh slips of the serratus are preferentially included in the dissection while leaving the most superior slips of the serratus to preserve scapular stability. Dissection begins by identifying 2 adjacent rib sections of the fifth and sixth or sixth and seventh ribs. The defect in the lower extremity determines the length of rib harvested. The serratus slips are then divided anteriorly over the chest wall. The dissection is extended to the intercostal spaces of the fourth and fifth ribs. The supraperiosteal dissection is performed at the anterior margin of the rib (Figure 2D).
Continue to: Following the surgical procedure...
Following the surgical procedure, patients are made non-weight-bearing on the operative extremity until signs of healing are apparent on radiographs. In this case, at the patients’ last follow-up visit, the skin graft was healed, and there was solid fusion of the rib/tibia junction (Figures 4B, 4C).
DISCUSSION
High-energy open injuries to the lower extremities are devastating injuries, with a high rate of late amputation and poor functional outcomes.11-13 Vascularized bone grafting provides both essential osteoinductive and osteoconductive properties to segmental bone defects in areas with inadequate soft tissue coverage, particularly in the setting of >6 cm of bone loss.4,14 The results of this study show that acute reconstruction of the lower limb with a composite vascularized SALR graft is a reliable procedure with an acceptable complication profile.
The timing of soft tissue coverage should be performed as soon as the patient is medically stable enough to undergo a reconstructive procedure, ideally within 7 to 10 days; and this timetable has been shown to decrease rates of infection and free flap failure.15-19 Early coverage provides both control of the soft-tissue envelope and reduces the risk of losing bone.1 Unlike the timing of coverage, the staging of the procedure is controversial. Proponents of the 2-stage free tissue (soft tissue followed by bony flap) transfer feel that although the tissue may not be infected at the time of coverage, it is contaminated with bacteria at the time of bone reconstruction, and as such is at high risk for both infection and complications.20 Unlike 2-stage procedures, single-stage coverage provides immediate soft tissue coverage, as well as bony support. This reduces the time to bony union and negates the need for repeated surgery in a mangled extremity where secondary surgery is complicated by both scar tissue and altered anatomy.1,2 Furthermore, it has been shown that there is no difference in the rates of infection when performing a single-stage compared with a 2-stage procedure.9 In this study, SALR flaps were typically performed within 2 weeks following an injury as a single procedure. We feel this resulted in the low number of complications in the SALR group.
Unlike free fibulas, rib flaps are easily pedicled with an associated soft-tissue flap due to their blood supply, making them ideal for 1-stage reconstruction. The rib has a dual blood supply: 1 from the posterior intercostal artery, and the other, an abundant periosteal blood supply, from the serratus anterior muscle.4 The blood supply to the serratus anterior comes from the thoracodorsal artery, and usually provides 14 cm of a large-caliber pedicle, making it a reliable flap for soft tissue reconstruction.21,22 Another unique feature of the blood supply to this flap is the amount of soft tissue available for both harvest and transfer; larger portions of serratus muscle and latissimus muscle can be harvested if necessary to cover the soft tissue defect.4
Comminuted tibias with segmental bone loss are difficult to manage since they are associated with bony as well as soft tissue defects.1,12,13,23 These injuries are ideal candidates for a single-stage reconstruction using a vascularized SALR flap. In our series, the use of an SALR flap resulted ultimately in a 100% union and limb salvage rate, with no flap failures and a low complication profile. Unlike the SALR, free fibular flaps must be transferred along with a separate latissimus dorsi flap to provide enough soft tissue coverage necessary for reconstructing large Gustilo IIIB injuries, which could increase the risk of flap failure. Since ribs are composed of membranous bone and have a similar cross-sectional area to both metacarpal and metatarsals, there are concerns regarding the biomechanical properties of ribs for weight-bearing.4,22,24-26 To compensate for this relatively small cross-sectional area, 2 ribs (either consecutive or alternative) are frequently harvested.1,4,5,23 Previous studies examining the use of ribs for bony reconstruction have frequently supplemented the rib reconstruction to the tibia using screws and external fixation alone.1,4,5,23 In our series, all SALR grafts were supported with the use of an intramedullary nail (n = 3) or locked plating (n = 1). The use of this supplemental fixation of the SALR graft allowed our patients to return to full weight-bearing (mean, 6 months) much earlier than the length of time cited in previous reports (12 months) examining these injuries.1,4,5,23
Continue to: There are several limitations...
There are several limitations to this study. The small sample size and retrospective nature of the study limits the amount of data we are able to collect from the medical record and places obvious constraints on the analysis. Although all these procedures were performed at 1 institution, multiple providers were involved in the reconstruction of these injuries, and there is no standard protocol for their treatment. Similarly, although other forms of extremity reconstruction were used during this time period, there was no standard protocol that could serve as a comparator for patients who underwent an SALR compared with other reconstructive procedures.
Overall, SALR grafts provide an excellent option for 1-stage reconstruction of severe, open lower extremity injuries. In this series we noted a 100% graft success rate with an acceptable complication profile.
This paper will be judged for the Resident Writer’s Award.
1. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114(6):1457-1466. doi:10.1097/01.PRS.0000138811.88807.65.
2. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg. 1999;104(4):984-992. doi:10.1097/00006534-199909040-00013.
3. Tu YK, Yen CY, Yeh WL, Wang IC, Wang KC, Ueng SW. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthopaedica Scandinavica. 2001;72(4):359-364. doi:10.1080/000164701753542014.
4. Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99(6):1656-1665. Doi:10.1097/00006534-199705000-00028.
5. Georgescu AV, Ignatiadis I, Ileana M, Irina C, Filip A, Olariu R. Long-term results after muscle-rib flap transfer for reconstruction of composite limb defects. Microsurgery. 2011;31(3):218-222. doi:10.1002/micr.20857.
6. Moran CG, Wood MB. Vascularized bone autografts. Orthop Rev. 1993;22(2):187-197. doi:10.1097/01241398-199307000-00031.
7. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421-428. doi:10.1055/s-2007-1006751.
8. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14(6):368-374. doi:10.1002/micr.1920140603.
9. Peat BG, Liggins DF. Microvascular soft tissue reconstruction for acute tibial fractures--late complications and the role of bone grafting. Ann Plast Surg. 1990;24(6):517-520.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746. doi:10.1097/00005373-198408000-00009.
12. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. NEJM. 2002;347(24):1924-1931. doi:10.1056/NEJMoa012604.
13. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809. doi:10.2106/JBJS.E.00032.
14. Bieber EJ, Wood MB. Bone reconstruction. Clin Plast Surg. 1986;13(4):645-655.
15. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg. 2010;18(2):108-117. doi:10.5435/00124635-201002000-00005.
16. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285-292. doi:10.1055/s-2006-944324.
17. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959-966. doi:10.1302/0301-620X.82B7.0820959.
18. Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316-1322. doi:10.2106/00004623-199173090-00005.
19. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912. doi:10.1016/j.injury.2007.02.052.
20. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80(1):1-14. doi:10.1097/00006534-198707000-00002.
21. Ueng WN, Chuang CC, Shih CH. Double-rib composite free transfer to reconstruct a single-spared lower extremity defect. J Trauma. 1995;38(2):210-212.
22. Bruck JC, Bier J, Kistler D. The serratus anterior osteocutaneous free flap. J Reconstr Microsurg. 1990;6(3):209-213. doi:10.1055/s-2007-1006820.
23. Lin CH, Yazar S. Revisiting the serratus anterior rib flap for composite tibial defects. Plast Reconstr Surg. 2004;114(7):1871-1877. doi:10.1097/01.PRS.0000142767.13493.63.
24. Hui KC, Zhang F, Lineaweaver WC, Moon W, Buncke GM, Buncke HJ. Serratus anterior-rib composite flap: anatomic studies and clinical application to hand reconstruction. Ann Plast Surg. 1999;42(2):132-136. doi:10.1097/00000637-199902000-00004.
25. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59(6):799-804. doi:10.1097/00006534-197706000-00002.
26. Nusbickel FR, Dell PC, Mcandrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res. 1989;(243):65-70.
1. Yazar S, Lin CH, Wei FC. One-stage reconstruction of composite bone and soft-tissue defects in traumatic lower extremities. Plast Reconstr Surg. 2004;114(6):1457-1466. doi:10.1097/01.PRS.0000138811.88807.65.
2. Lin CH, Wei FC, Chen HC, Chuang DC. Outcome comparison in traumatic lower-extremity reconstruction by using various composite vascularized bone transplantation. Plast Reconstr Surg. 1999;104(4):984-992. doi:10.1097/00006534-199909040-00013.
3. Tu YK, Yen CY, Yeh WL, Wang IC, Wang KC, Ueng SW. Reconstruction of posttraumatic long bone defect with free vascularized bone graft: good outcome in 48 patients with 6 years' follow-up. Acta Orthopaedica Scandinavica. 2001;72(4):359-364. doi:10.1080/000164701753542014.
4. Lin CH, Wei FC, Levin LS, Su JI, Fan KF, Yeh WL, Hsu DT. Free composite serratus anterior and rib flaps for tibial composite bone and soft-tissue defect. Plast Reconstr Surg. 1997;99(6):1656-1665. Doi:10.1097/00006534-199705000-00028.
5. Georgescu AV, Ignatiadis I, Ileana M, Irina C, Filip A, Olariu R. Long-term results after muscle-rib flap transfer for reconstruction of composite limb defects. Microsurgery. 2011;31(3):218-222. doi:10.1002/micr.20857.
6. Moran CG, Wood MB. Vascularized bone autografts. Orthop Rev. 1993;22(2):187-197. doi:10.1097/01241398-199307000-00031.
7. Banic A, Hertel R. Double vascularized fibulas for reconstruction of large tibial defects. J Reconstr Microsurg. 1993;9(6):421-428. doi:10.1055/s-2007-1006751.
8. Malizos KN, Nunley JA, Goldner RD, Urbaniak JR, Harrelson JM. Free vascularized fibula in traumatic long bone defects and in limb salvaging following tumor resection: comparative study. Microsurgery. 1993;14(6):368-374. doi:10.1002/micr.1920140603.
9. Peat BG, Liggins DF. Microvascular soft tissue reconstruction for acute tibial fractures--late complications and the role of bone grafting. Ann Plast Surg. 1990;24(6):517-520.
10. Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58(4):453-458.
11. Gustilo RB, Mendoza RM, Williams DN. Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma. 1984;24(8):742-746. doi:10.1097/00005373-198408000-00009.
12. Bosse MJ, MacKenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. NEJM. 2002;347(24):1924-1931. doi:10.1056/NEJMoa012604.
13. MacKenzie EJ, Bosse MJ, Pollak AN, et al. Long-term persistence of disability following severe lower-limb trauma. Results of a seven-year follow-up. J Bone Joint Surg Am. 2005;87(8):1801-1809. doi:10.2106/JBJS.E.00032.
14. Bieber EJ, Wood MB. Bone reconstruction. Clin Plast Surg. 1986;13(4):645-655.
15. Melvin JS, Dombroski DG, Torbert JT, Kovach SJ, Esterhai JL, Mehta S. Open tibial shaft fractures: II. Definitive management and limb salvage. J Am Acad Orthop Surg. 2010;18(2):108-117. doi:10.5435/00124635-201002000-00005.
16. Godina M. Early microsurgical reconstruction of complex trauma of the extremities. Plast Reconstr Surg. 1986;78(3):285-292. doi:10.1055/s-2006-944324.
17. Gopal S, Majumder S, Batchelor AG, Knight SL, De Boer P, Smith RM. Fix and flap: the radical orthopaedic and plastic treatment of severe open fractures of the tibia. J Bone Joint Surg Br. 2000;82(7):959-966. doi:10.1302/0301-620X.82B7.0820959.
18. Fischer MD, Gustilo RB, Varecka TF. The timing of flap coverage, bone-grafting, and intramedullary nailing in patients who have a fracture of the tibial shaft with extensive soft-tissue injury. J Bone Joint Surg Am. 1991;73(9):1316-1322. doi:10.2106/00004623-199173090-00005.
19. Tielinen L, Lindahl JE, Tukiainen EJ. Acute unreamed intramedullary nailing and soft tissue reconstruction with muscle flaps for the treatment of severe open tibial shaft fractures. Injury. 2007;38(8):906-912. doi:10.1016/j.injury.2007.02.052.
20. Yaremchuk MJ, Brumback RJ, Manson PN, Burgess AR, Poka A, Weiland AJ. Acute and definitive management of traumatic osteocutaneous defects of the lower extremity. Plast Reconstr Surg. 1987;80(1):1-14. doi:10.1097/00006534-198707000-00002.
21. Ueng WN, Chuang CC, Shih CH. Double-rib composite free transfer to reconstruct a single-spared lower extremity defect. J Trauma. 1995;38(2):210-212.
22. Bruck JC, Bier J, Kistler D. The serratus anterior osteocutaneous free flap. J Reconstr Microsurg. 1990;6(3):209-213. doi:10.1055/s-2007-1006820.
23. Lin CH, Yazar S. Revisiting the serratus anterior rib flap for composite tibial defects. Plast Reconstr Surg. 2004;114(7):1871-1877. doi:10.1097/01.PRS.0000142767.13493.63.
24. Hui KC, Zhang F, Lineaweaver WC, Moon W, Buncke GM, Buncke HJ. Serratus anterior-rib composite flap: anatomic studies and clinical application to hand reconstruction. Ann Plast Surg. 1999;42(2):132-136. doi:10.1097/00000637-199902000-00004.
25. Buncke HJ, Furnas DW, Gordon L, Achauer BM. Free osteocutaneous flap from a rib to the tibia. Plast Reconstr Surg. 1977;59(6):799-804. doi:10.1097/00006534-197706000-00002.
26. Nusbickel FR, Dell PC, Mcandrew MP, Moore MM. Vascularized autografts for reconstruction of skeletal defects following lower extremity trauma. A review. Clin Orthop Relat Res. 1989;(243):65-70.
TAKE-HOME POINTS
- Gustilo IIIB injuries with segmental bone loss can be difficult to treat with conventional means.
- Vascularized bone grafts are beneficial for reconstructing bone defects >5 cm.
- The SALR composite flap consists of bone and muscle.
- This flap can provide soft tissue coverage and vascularized bone in a single surgical procedure.
- In this study, the use of the SALR composite flap was capable of healing large segmental bony defects at an average of 7 months.
Shoulder Arthroplasty in Patients with Rheumatoid Arthritis: A Population-Based Study Examining Utilization, Adverse Events, Length of Stay, and Cost
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).
Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.
Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
ABSTRACT
It has been suggested that the utilization of joint arthroplasty in patients with rheumatoid arthritis (RA) is decreasing; however, this observation is largely based upon evidence pertaining to lower-extremity joint arthroplasty. It remains unknown if these observed trends also hold true for shoulder arthroplasty. The purpose of this study is to utilize a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. Secondarily, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and to compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. Using a large population database in the US, we determined the annual rates of shoulder arthroplasty (overall and individual) in RA patients between 2002 and 2011. Early adverse events, length of stay, and hospitalization costs were determined and compared with those of non-RA patients undergoing shoulder arthroplasty. Overall, we identified 332,593 patients who underwent shoulder arthroplasty between 2002 and 2011, of whom 17,883 patients (5.4%) had a diagnosis of RA. Over the study period, there was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly total shoulder arthroplasty. Over the same period, there was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease. There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients. Non-RA patients had a significantly shorter length of stay; however, the difference did not appear to be clinically significant. In conclusion, the utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continue to: It has been suggested...
It has been suggested that the utilization of total joint arthroplasty (TJA) in patients with rheumatoid arthritis (RA) is decreasing over time;1 however, this observation is largely based upon evidence pertaining to lower extremity TJA.2 It remains unknown if these observed trends also hold true for shoulder arthroplasty, whereby the utilization of shoulder arthroplasty in RA patients is not limited to the management of end-stage inflammatory arthropathy. In this study, we used a nationally representative population database in the US to identify trends in the utilization of shoulder arthroplasty among patients with RA. As a secondary objective, we sought to determine the rate of early adverse events, length of stay, and hospitalization costs associated with RA patients undergoing shoulder arthroplasty and compare these outcomes to those of patients without a diagnosis of RA undergoing shoulder arthroplasty. We hypothesize that the utilization of shoulder arthroplasty in RA patients would be decreasing, but adverse events, length of stay, and hospitalization costs would not differ between patients with and without RA undergoing shoulder arthroplasty.
METHODS
We conducted a retrospective cohort study using the Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) from 2002 to 2011.3 The NIS comprises a 20% stratified sample of all hospital discharges in the US. The NIS includes information about patient characteristics (age, sex, insurance status, and medical comorbidities) and hospitalization outcomes (adverse events, costs, and length of stay). The NIS allows identification of hospitalizations according to procedures and diagnoses using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes. Given the anonymity of this study, it was exempt from Institutional Review Board ethics approval.
Hospitalizations were selected for the study based on ICD-9-CM procedural codes for hemiarthroplasty (81.81), anatomic total shoulder arthroplasty (TSA) (81.80), and reverse TSA (81.88). These patients were then stratified by an ICD-9-CM diagnosis of RA (714.X). We also utilized ICD-9-CM diagnosis codes to determine the presence of rotator cuff pathology at the time of shoulder arthroplasty (726.13, 727.61, 840.4) and to exclude patients with a history of trauma (812.X, 716.11, 733.8X). In a separate analysis, all patients in the NIS database with an ICD-9-CM diagnosis of RA were identified for each calendar year of the study, and a national estimate of RA patients was generated annually to assess overall and individual utilization rates of shoulder arthroplasty in this population (the national estimate served as the denominator).
Preoperative patient data withdrawn from the NIS included age, sex, insurance status, and medical comorbidities. An Elixhauser Comorbidity Index (ECI) was generated for each patient based on the presence of 29 comorbid conditions. The ECI was chosen because of its capacity to accurately predict mortality and represent the patient burden of comorbidities in similar administrative database studies.4-6
Early adverse events were also chosen based on ICD-9-CM diagnosis codes (Appendix A), and included the following: death, acute kidney injury, cardiac arrest, thromboembolic event, myocardial infarction, peripheral nerve injury, pneumonia, sepsis, stroke, surgical site infection, urinary tract infection, and wound dehiscence. The overall adverse event rate was defined as the occurrence of ≥1 of the above adverse events in a patient.
Appendix A. ICD-9-CM Codes Corresponding to Postoperative Adverse Events
Event | ICD-9-CM |
Acute kidney injury | 584.5-584.9 |
Cardiac arrest | 427.41, 427.5 |
Thromboembolic event | 453.2-453.4, 453.82-453.86, 415.1 |
Myocardial Infarction | 410.00-410.92 |
Peripheral nerve injury | 953.0-953.9 954.0-954.9, 955.0-955.9, 956.0-956.9 |
Pneumonia | 480.0-480.9, 481, 482.0-482.9, 483.0-483.8, 484.1-484.8, 485, 486 |
Sepsis | 038.0-038.9, 112.5, 785.52, 995.91, 995.92 |
Stroke | 430, 432, 433.01-434.91, 997.02 |
Surgical site infection | 998.51, 998.59, 996.67 |
Urinary tract infection | 599 |
Wound dehiscence | 998.30-998.33 |
Abbreviation: ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification
Length of stay and total hospital charges were available for each patient. Length of stay represents the number of calendar days a patient stayed in the hospital. All hospital charges were converted to hospitalization costs using the HCUP Cost-to-Charge Ratio Files. All hospitalization costs were adjusted for inflation using the US Bureau of Labor statistics yearly inflation calculator to represent charges in the year 2011, which was the final and most recent year in this study.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
Statistical analyses were conducted using Stata version 13.1 (StataCorp, LP). All analyses took into account the complex survey design of the NIS. Discharge weights, strata, and cluster variables were included to correctly estimate variance and to produce national estimates from the stratified sample. Pearson’s chi-squared test was used to compare age, sex, ECI, and insurance status between RA and non-RA patients undergoing shoulder arthroplasty.
Bivariate and multivariate logistic regressions were subsequently used to compare the rates of adverse events between RA and non-RA patients undergoing shoulder arthroplasty (non-RA cases were used as the reference). Multivariate linear regressions were used to compare hospital length of stay and hospitalization costs between RA and non-RA patients undergoing shoulder arthroplasty. The multivariate regressions were adjusted for baseline differences in age, sex, ECI, and insurance status. Cochran-Armitage tests for trend were used to assess trends over time. All tests were 2-tailed, and the statistical difference was established at a 2-sided α level of 0.05 (P < .05).
RESULTS
Overall, we identified 332,593 patients who underwent shoulder arthroplasty in the US between 2002 and 2011, of which 17,883 patients (5.4%) had a diagnosis of RA. In comparison with non-RA patients undergoing shoulder arthroplasty, patients with RA at the time of shoulder arthroplasty were significantly younger (65.2 ± 12.5 years vs 68.4 ± 11.0 years, P < .001), included a significantly greater proportion of female patients (76.7% vs 53.8%, P < .001), and included a significantly higher proportion of patients with Medicaid insurance (3.6% vs 2.3%, P < .001). There were no significant differences in the mean ECI between patients with and without a diagnosis of RA (Table 1). As depicted in Table 1, there were significant differences in the utilization of specific shoulder arthroplasty types between patients with and without RA, whereby a significantly greater proportion of RA patients underwent hemiarthroplasty (HA) (31.6% vs 29.3%, P = .002) and reverse TSA (7.7% vs 6.6%, P = .002), whereas a significantly greater proportion of non-RA patients underwent anatomic SA (64.0% vs 60.8%, P = .002).

Over the study period from 2002 to 2011, there was a significant increase in the overall utilization of shoulder arthroplasty in RA patients, as indicated by both the absolute number and the proportion of patients with a diagnosis of RA (P < .001) (Table 2, Figure). More specifically, 0.39% of RA patients underwent shoulder arthroplasty in 2002, as compared with 0.58% of RA patients in 2011 (P < .001) (Table 2). With respect to specific arthroplasty types, there was an exponential rise in the utilization of reverse TSA beginning in 2010 and a corresponding decrease in the rates of both HA and anatomic TSA (Table 2, Figure). In addition to changes in shoulder arthroplasty utilization over time among RA patients, we also observed a significant increase in the number of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease (9.7% in 2002 to 15.2% in 2011, P < .001).
Table 2. The Annual Utilization of Shoulder Arthroplasty Among Patients with a Diagnosis of Rheumatoid Arthritis.
Proportion of RA patients |
| ||||
Year | Overall Rate of Shoulder Arthroplastya | HA | Anatomic TSA | Reverse TSA | |
2002 | 0.39 | 0.23 | 0.16 | 0 | |
2003 | 0.37 | 0.19 | 0.18 | 0 | |
2004 | 0.46 | 0.25 | 0.21 | 0 | |
2005 | 0.46 | 0.21 | 0.25 | 0 | |
2006 | 0.47 | 0.20 | 0.27 | 0 | |
2007 | 0.55 | 0.22 | 0.33 | 0 | |
2008 | 0.47 | 0.17 | 0.30 | 0 | |
2009 | 0.50 | 0.15 | 0.35 | 0 | |
2010 | 0.58 | 0.15 | 0.37 | 0.06 | |
2011 | 0.58 | 0.12 | 0.23 | 0.23 | |
Absolute number of RA patients |
| ||||
2002 | 1295 | 768 | 527 | 0 | |
2003 | 1247 | 650 | 597 | 0 | |
2004 | 1667 | 906 | 761 | 0 | |
2005 | 1722 | 776 | 946 | 0 | |
2006 | 1847 | 794 | 1053 | 0 | |
2007 | 2249 | 910 | 1339 | 0 | |
2008 | 2194 | 799 | 1395 | 0 | |
2009 | 2407 | 724 | 1683 | 0 | |
2010 | 2869 | 722 | 1857 | 290 | |
2011 | 3193 | 649 | 1261 | 1283 | |
aRate determined as number of RA patients undergoing shoulder arthroplasty compared to the number of patients with an RA diagnosis in the stated calendar year.
Abbreviations: HA, hemiarthroplasty; RA, rheumatoid arthritis; TSA, total shoulder arthroplasty.

Continue to: Among patients with RA...
Among patients with RA undergoing shoulder arthroplasty, the overall rate of early adverse events was 3.12%, of which the most common early adverse events were urinary tract infections (1.8%), acute kidney injury (0.66%), and pneumonia (0.38%) (Table 3). As compared with patients without a diagnosis of RA undergoing shoulder arthroplasty, there were no significant differences in the overall and individual rates of early adverse events (Table 3).
Table 3. A Comparison of Early Adverse Events, Length of Stay, and Cost Between Patients With and Without Rheumatoid Arthritis (RA) Undergoing Shoulder Arthroplasty
Comparison of Early Adverse Event Rates |
| ||||
| Non-RA Patients | RA Patients | Multivariate Logistic Regression | ||
Odds Ratio | P-Value | ||||
Overall adverse event rate | 3.02% | 3.12% | 1.0 | 0.83 | |
Specific adverse event rate |
|
|
|
| |
Death | 0.08% | 0.05% | 0.9 | 0.91 | |
Acute kidney injury | 0.85% | 0.66% | 0.9 | 0.59 | |
Cardiac arrest | 0.05% | 0.05% | 1.3 | 0.70 | |
Thromboembolic event | 0.01% | 0.00% | - | - | |
Myocardial Infarction | 0.22% | 0.06% | 0.4 | 0.17 | |
Peripheral nerve injury | 0.08% | 0.11% | 1.5 | 0.45 | |
Pneumonia | 0.47% | 0.38% | 0.9 | 0.70 | |
Sepsis | 0.08% | 0.08% | 1.3 | 0.62 | |
Stroke | 0.07% | 0.05% | 0.9 | 0.93 | |
Surgical site infection | 0.09% | 0.13% | 1.4 | 0.52 | |
Urinary tract infection | 1.44% | 1.80% | 1.1 | 0.46 | |
Wound dehiscence | 0.01% | 0.05% | 3.6 | 0.09 | |
Comparison of Length of Stay and Hospital Charges | |||||
| Non-RA Patients (percent) | RA Patients (percent) | Multivariate Linear Regression | ||
Beta | P-Value | ||||
Length of staya | 2.3±2.0 | 2.4±1.6 | +0.1 | 0.002 | |
Hospitalization costb | 14,826±8,336 | 14,787±7,625 | +93 | 0.59 | |
aReported in days. bReported in 2011 US dollars, adjusted for inflation.
The mean length of stay following shoulder arthroplasty in RA patients was 2.4 ± 1.6 days, and the mean hospitalization cost was $14,787 ± $7625 (Table 3). As compared with non-RA patients undergoing shoulder arthroplasty, there were no significant differences in the mean hospitalization costs; however, non-RA patients had a significantly shorter length of stay by 0.1 days (P = .002) (Table 3).
DISCUSSION
In this study, we observed that the utilization of shoulder arthroplasty in patients with RA increased significantly in the decade from 2002 to 2011, largely related to a rise in TSA. Interestingly, we also observed a corresponding rise in the proportion of RA patients undergoing shoulder arthroplasty with a diagnosis of rotator cuff disease, and we believe that this may partly account for the recent increase in the use of the reverse TSA in this patient population. Additionally, we found shoulder arthroplasty in RA patients to be safe in the early postoperative period, with no significant increase in cost as compared with patients undergoing shoulder arthroplasty without a diagnosis of RA. Although we did observe a significant increase in length of stay among RA patients as compared with non-RA patients, the absolute difference was only 0.1 days, and given the aforementioned similarities in cost between RA and non-RA patients, we do not believe this difference to be clinically significant.
It has been theorized that the utilization of TJA in RA patients has been decreasing with improvements in medical management; however, this is largely based upon literature pertaining to lower extremity TJA.2 On the contrary, past research pertaining to the utilization of shoulder arthroplasty in RA patients has been highly variable. For instance, a Swedish study demonstrated a statistically significant decrease in admissions associated with RA-related upper limb surgery and a stable rate of shoulder arthroplasty between 1998 and 2004.7 Similarly, a Finnish study demonstrated that the annual incidence of primary joint arthroplasty in RA patients had declined from 1995 to 2010, with a greater decline for upper-limb arthroplasty as compared with lower-limb arthroplasty.8 Despite these European observations, Jain and colleagues9 reported an increasing rate of TSA among RA patients in the US between the years 1992 and 2005. In this study, we demonstrate a clear increase in the utilization of shoulder arthroplasty among RA patients between 2002 and 2011. What was most striking about our observation was that the rise in utilization appeared to be driven by an increase in TSA, whereas the utilization of HA decreased over time. This change in practice likely reflects several factors, including the multitude of studies that have demonstrated improved outcomes with anatomic TSA as compared with HA in RA patients.10-14
Perhaps the most interesting aspect of our data was the recent exponential rise in the utilization of the reverse TSA. Despite improved outcomes following TSA as compared with HA in RA patients, these outcomes all appear to be highly dependent upon the integrity of the rotator cuff.10 In fact, there is evidence that failure of the rotator cuff could be as high as 75% within 10 years of TSA in patients with RA,15 which ultimately could jeopardize the long-term durability of the TSA implant in this patient population.11 For this reason, interest in the reverse TSA for the RA patient population has increased since its introduction in the US in 2004;16 in fact, in RA patients with end-stage inflammatory arthropathy and a damaged rotator cuff, the reverse TSA has demonstrated excellent results.17-20 Based upon this evidence, it is not surprising that we found an exponential rise in the use of the reverse TSA since 2010, which corresponds to the introduction of an ICD-9 code for this implant.21 Prior to 2010, it is likely that many implanted reverse TSAs were coded as TSA, and for this reason, we believe that the observed rise in the utilization of TSA in RA patients prior to 2010 may have been partly fueled by an increase in the use of the reverse TSA. To further support this theory, there was a dramatic decrease in the use of anatomic TSA following 2010, and we believe this was related to increased awareness of the newly introduced reverse TSA code among surgeons.
Another consideration when examining the utilization of shoulder arthroplasty in RA patients is its versatility in managing different disease states, including rotator cuff disease. As has been documented in the literature, outcomes of rotator cuff repair in RA patients are discouraging.22 For this reason, it is reasonable for surgeons and patients with RA to consider alternatives to rotator cuff repair when nonoperative management has failed to provide adequate improvement in symptoms. One alternative may be shoulder arthroplasty, namely the reverse TSA. In this study, we observed a significant increase in the rate of diagnosis of rotator cuff disease among RA patients undergoing shoulder arthroplasty from 2002 to 2011 (9.7% in 2002 to 15.2% in 2011, P < .001), and it is our belief that the simultaneous increase in the diagnosis of rotator cuff disease and use of TSA is not coincidental. More specifically, there is likely an emerging trend among surgeons toward using the reverse TSA to manage rotator cuff tears in the RA population, rather than undertaking a rotator cuff repair that carries a high rate of failure. Going forward, there is a need to not only identify this trend more clearly but to also compare the outcomes between reverse TSA and rotator cuff repair in the management of rotator cuff tears in RA patients.
Continue to: In this study, we observed...
In this study, we observed that RA patients undergoing shoulder arthroplasty were significantly younger than non-RA patients undergoing shoulder arthroplasty. At first, this observation seems to counter recent literature suggesting that the age of patients with inflammatory arthropathy undergoing TJA is increasing over time;1 however, looking more closely at the data, it becomes clearer that the mean age we report is actually a relative increase as compared with past clinical studies pertaining to RA patients undergoing shoulder arthroplasty (mean ages of 47 years,23 55 years,24 60 years,10 and 62 years25). On the other hand, the continued existence of an age gap between RA and non-RA patients undergoing shoulder arthroplasty may be the result of several possible phenomena. First, this may reflect issues with patient access to and coverage of expensive biologic antirheumatic medication that would otherwise mitigate disease progression. For instance, the out-of-pocket expense for biologic medication through Medicaid and Medicare is substantial,26 which has direct implications on over two-thirds of our RA cohort. Second, it may be skewed by the proportion of RA patients who have previously been or continue to be poorly managed, enabling disease progression to end-stage arthropathy at a younger age. Ultimately, further investigation is needed to determine the reasons for this continued age disparity.
In comparing RA and non-RA patients undergoing shoulder arthroplasty, we did not find a significant difference in the overall nor the individual rates of early adverse events. This finding appears to be unique, as similar studies pertaining to total knee arthroplasty (TKA) demonstrated a significantly higher incidence of postoperative pneumonia and bleeding requiring transfusion among RA patients as compared with non-RA patients.27 In patients with RA being treated with biologic medication and undergoing shoulder arthroplasty, the frequent concern in the postoperative period is the integrity of the wound and the potential for infection.28 In this study, we did not find a significant difference in the rate of early infection, and although the difference in the rate of early wound dehiscence approached significance, it did not meet the threshold of 0.05 (P = .09). This finding is in keeping with the aforementioned NIS study pertaining to TKA, and we believe that it likely reflects the short duration of follow-up for patients in both studies. Given the nature of the database we utilized, we were only privy to complications that arose during the inpatient hospital stay, and it is likely that the clear majority of patients who develop a postoperative infection or wound dehiscence do so in the postoperative setting following discharge. A second concern regarding postoperative wound complications is the management of biologic medication in the perioperative period, which we cannot determine using this database. Despite all these limitations specific to this database, a past systematic review of reverse TSA in RA patients found a low rate of deep infection after reverse TSA in RA patients (3.3%),17 which was not higher than that after shoulder arthroplasty performed in non-RA patients.
A final demonstration from this study is that the hospital length of stay was significantly longer for RA patients than non-RA patients undergoing shoulder arthroplasty; however, given that the difference was only 0.1 days, and there was no significant difference in hospitalization cost, we are inclined to believe that statistical significance may not translate into clinical significance in this scenario. Ultimately, we do believe that length of stay is an important consideration in the current healthcare system, and given our finding that shoulder arthroplasty in the RA patient is safe in the early postoperative period, that a prolonged postoperative hospitalization is not warranted on the sole basis of a patient’s history of RA.
As with all studies using data from a search of an administrative database, such as the NIS database, this study has limitations. First, this type of research is limited by the reliability of both diagnosis and procedural coding. Although the NIS database has demonstrated high reliability,3 it is still possible that events may have been miscoded. Second, the tracking period for adverse events is limited to the inpatient hospital stay, which may be too short to detect certain postoperative complications. As such, the rates we report are likely underestimates of the true incidence of these complications, but this is true for both the RA and non-RA populations. Third, the comparisons we draw between RA and non-RA patients are limited to the scope of the NIS database and the available data; as such, we could not draw comparisons between preoperative disease stage, intraoperative findings, and postoperative course following hospital discharge. Lastly, our data are limited to a distinct period between 2002 and 2011 and may not reflect current practice. Ultimately, our findings may underestimate current trends in shoulder arthroplasty utilization among RA patients, particularly for the reverse TSA.
CONCLUSION
In this study, we found that the utilization of shoulder arthroplasty in patients with RA increased significantly from 2002 to 2011, largely related to a rise in the utilization of TSA. Similarly, we observed a rise in the proportion of RA patients undergoing shoulder arthroplasty with a corresponding diagnosis of rotator cuff disease, and we believe the increased utilization of shoulder arthroplasty among RA patients resulted from management of both end-stage inflammatory arthropathy and rotator cuff disease. Although we did not find a significant difference between RA and non-RA patients in the rates of early adverse events and overall hospitalization costs following shoulder arthroplasty, length of stay was significantly longer among RA patients; however, the absolute difference does not appear to be clinically significant.
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
- Mertelsmann-Voss C, Lyman S, Pan TJ, Goodman SM, Figgie MP, Mandl LA. US trends in rates of arthroplasty for inflammatory arthritis including rheumatoid arthritis, juvenile idiopathic arthritis, and spondyloarthritis. Arthritis Rheumatol. 2014;66(6):1432-1439. doi:10.1002/art.38384.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis. 2010;69(5):868-871. doi:10.1136/ard.2009.112474.
- HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality; 2002-2011.
- Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. doi:10.1097/00005650-199801000-00004.
- Sharabiani MT, Aylin P, Bottle A. Systematic review of comorbidity indices for administrative data. Med Care. 2012;50(12):1109-1118. doi:10.1097/MLR.0b013e31825f64d0.
- van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626-633. doi:10.1097/MLR.0b013e31819432e5.
- Weiss RJ, Ehlin A, Montgomery SM, Wick MC, Stark A, Wretenberg P. Decrease of RA-related orthopaedic surgery of the upper limbs between 1998 and 2004: data from 54,579 Swedish RA inpatients. Rheumatol Oxf. 2008 ;47(4):491-494. doi. 10.1093/rheumatology/ken009.
- Jämsen E, Virta LJ, Hakala M, Kauppi MJ, Malmivaara A, Lehto MU. The decline in joint replacement surgery in rheumatoid arthritis is associated with a concomitant increase in the intensity of anti-rheumatic therapy: a nationwide register-based study from 1995 through 2010. Acta Orthop. 2013;84(4):331-337. doi:10.3109/17453674.2013.810519.
- Jain A, Stein BE, Skolasky RL, Jones LC, Hungerford MW. Total joint arthroplasty in patients with rheumatoid arthritis: a United States experience from 1992 through 2005. J Arthroplasty. 2012;27(6):881-888. doi:10.1016/j.arth.2011.12.027.
- Barlow JD, Yuan BJ, Schleck CD, Harmsen WS, Cofield RH, Sperling JW. Shoulder arthroplasty for rheumatoid arthritis: 303 consecutive cases with minimum 5-year follow-up. J Shoulder Elbow Surg. 2014;23(6):791-799. doi:10.1016/j.jse.2013.09.016.
- Collins DN, Harryman DT, Wirth MA. Shoulder arthroplasty for the treatment of inflammatory arthritis. J Bone Joint Surg Am. 2004;86–A(11):2489-2496. doi:10.2106/00004623-200411000-00020.
- Rahme H, Mattsson P, Wikblad L, Larsson S. Cement and press-fit humeral stem fixation provides similar results in rheumatoid patients. Clin Orthop Relat Res. 2006;448:28-32. doi:10.1097/01.blo.0000224007.25636.85.
- Rozing PM, Nagels J, Rozing MP. Prognostic factors in arthroplasty in the rheumatoid shoulder. HSS J. 2011;7(1):29-36. doi:10.1007/s11420-010-9172-1.
- Sperling JW, Cofield RH, Schleck CD, Harmsen WS. Total shoulder arthroplasty versus hemiarthroplasty for rheumatoid arthritis of the shoulder: results of 303 consecutive cases. J Shoulder Elbow Surg. 2007;16(6):683-690. doi:10.1016/j.jse.2007.02.135.
- Khan A, Bunker TD, Kitson JB. Clinical and radiological follow-up of the Aequalis third-generation cemented total shoulder replacement: a minimum ten-year study. J Bone Joint Surg Br. 2009;91(12):1594-1600. doi:10.1302/0301-620X.91B12.22139.
- Guery J, Favard L, Sirveaux F, Oudet D, Mole D, Walch G. Reverse total shoulder arthroplasty: survivorship analysis of eighty replacements followed for five to ten years. J Bone Joint Surg Am. 2006;88(8):1742-1747. doi:10.2106/JBJS.E.00851.
- Gee ECA, Hanson EK, Saithna A. Reverse shoulder arthroplasty in rheumatoid arthritis: A systematic review. Open Orthop J. 2015;9:237-245. doi:10.2174/1874325001509010237.
- Holcomb JO, Hebert DJ, Mighell MA, et al. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. J Shoulder Elbow Surg. 2010;19(7):1076-1084. doi:10.1016/j.jse.2009.11.049.
- Postacchini R, Carbone S, Canero G, Ripani M, Postacchini F. Reverse shoulder prosthesis in patients with rheumatoid arthritis: a systematic review. Int Orthop. 2016;40(5):965-973. doi:10.1007/s00264-015-2916-2.
- Rittmeister M, Kerschbaumer F. Grammont reverse total shoulder arthroplasty in patients with rheumatoid arthritis and nonreconstructible rotator cuff lesions. J Shoulder Elbow Surg. 2001;10(1):17-22. doi:10.1067/mse.2001.110515.
- American Medical Association. American Medical Association Web site. www.ama-assn.org/ama. Accessed January 15, 2016.
- Smith AM, Sperling JW, Cofield RH. Rotator cuff repair in patients with rheumatoid arthritis. J Bone Joint Surg. 2005;87(8):1782-1787. doi:10.2106/JBJS.D.02452.
- Betts HM, Abu-Rajab R, Nunn T, Brooksbank AJ. Total shoulder replacement in rheumatoid disease: a 16- to 23-year follow-up. J Bone Joint Surg Br. 2009;91(9):1197-1200. doi:10.1302/0301-620X.91B9.22035.
- Geervliet PC, Somford MP, Winia P, van den Bekerom MP. Long-term results of shoulder hemiarthroplasty in patients with rheumatoid arthritis. Orthopedics. 2015;38(1):e38-e42. doi:10.3928/01477447-20150105-58.
- Hettrich CM, Weldon E III, Boorman RS, Parsons M IV, Matsen FA III. Preoperative factors associated with improvements in shoulder function after humeral hemiarthroplasty. J Bone Joint Surg. 2004;86–A(7):1446-1451.
- Yazdany J, Dudley RA, Chen R, Lin GA, Tseng CW. Coverage for high-cost specialty drugs for rheumatoid arthritis in Medicare Part D. Arthritis Rheumatol. 2015;67(6):1474-1480. doi:10.1002/art.39079.
- Jauregui JJ, Kapadia BH, Dixit A, et al. Thirty-day complications in rheumatoid patients following total knee arthroplasty. Clin Rheumatol. 2016;35(3):595-600. doi:10.1007/s10067-015-3037-4.
- Trail IA, Nuttall D. The results of shoulder arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Br. 2002;84(8):1121-1125. doi:10.1302/0301-620X.84B8.0841121
TAKE-HOME POINTS
- There was a significant increase in the utilization of shoulder arthroplasty in RA patients, particularly TSA.
- There was a significant increase in the number of RA patients who underwent shoulder arthroplasty with a diagnosis of rotator cuff disease.
- There were no significant differences in adverse events or mean hospitalization costs between RA and non-RA patients.
- Non-RA patients had a significantly shorter length of stay.
- The utilization of shoulder arthroplasty in patients with RA significantly increased from 2002 to 2011, which may partly reflect a trend toward management of rotator cuff disease with arthroplasty rather than repair.
Continuous Cryotherapy vs Ice Following Total Shoulder Arthroplasty: A Randomized Control Trial
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
ABSTRACT
Postoperative pain management is an important component of total shoulder arthroplasty (TSA). Continuous cryotherapy (CC) has been proposed as a means of improving postoperative pain control. However, CC represents an increased cost not typically covered by insurance. The purpose of this study is to compare CC to plain ice (ICE) following TSA. The hypothesis was that CC would lead to lower pain scores and decreased narcotic usage during the first 2 weeks postoperatively.
A randomized controlled trial was performed to compare CC to ICE. Forty patients were randomized to receive either CC or ICE following TSA. The rehabilitation and pain control protocols were otherwise standardized. Visual analog scales (VAS) for pain, satisfaction with cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery. Narcotic usage in morphine equivalents was also recorded.
No significant differences in preoperative pain (5.9 vs 6.8; P = .121), or postoperative pain at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593) or 14 days (2.5 vs 2.7; P = .742) were observed between the CC and ICE groups. Similarly, no differences in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval were observed between the 2 groups.
No differences in pain control, quality of sleep, patient satisfaction, or narcotic usage were detected between CC and ICE following TSA. CC may offer convenience as an advantage, but the increased cost associated with this type of treatment may not be justified.
The number of total shoulder arthroplasties (TSAs) performed annually is increasing dramatically.1 At the same time, there has been a push toward decreased length of hospital stay and earlier mobilization following joint replacement surgery. Central to these goals is adequate pain control. Multimodal pain pathways exist, and one of the safest and cheapest methods of pain control is cold therapy, which can be accomplished with continuous cryotherapy (CC) or plain ice (ICE).
Continue to: The mechanism of cryotherapy...
The mechanism of cryotherapy for controlling pain is poorly understood. Cryotherapy reduces leukocyte migration and slows down nerve signal transmission, which reduces inflammation, thereby producing a short-term analgesic effect. Stalman and colleagues2 reported on a randomized control study that evaluated the effects of postoperative cooling after knee arthroscopy. Measurements of metabolic and inflammatory markers in the synovial membrane were used to assess whether cryotherapy provides a temperature-sensitive release of prostaglandin E2. Cryotherapy lowered the temperature in the postoperative knee, and synovial prostaglandin concentrations were correlated with temperature. Because prostaglandin is a marker of inflammation and pain, the conclusion was that postoperative cooling appeared to have an anti-inflammatory effect.
The knee literature contains multiple studies that have examined the benefits of cryotherapy after both arthroscopic and arthroplasty procedures. The clinical benefits on pain have been equivocal with some studies showing improvements using cryotherapy3,4 and others showing no difference in the treatment group.5,6
Few studies have examined cryotherapy for the shoulder. Speer and colleagues7 demonstrated that postoperative use of CC was effective in reducing recovery time after shoulder surgery. However; they did not provide an ICE comparative group and did not focus specifically on TSA. In another study, Kraeutler and colleagues8 examined only arthroscopic shoulder surgery cases in a randomized prospective trial and found no significant different between CC and ICE. They concluded that there did not appear to be a significant benefit in using CC over ICE for arthroscopic shoulder procedures.
The purpose of this study is to prospectively evaluate CC and ICE following TSA. The hypothesis was that CC leads to improved pain control, less narcotic consumption, and improved quality of sleep compared to ICE in the immediate postoperative period following TSA.
MATERIALS AND METHODS
This was a prospective randomized control study of patients undergoing TSA receiving either CC or ICE postoperatively. Institutional Review Board approval was obtained before commencement of the study. Inclusion criteria included patients aged 30 to 90 years old undergoing a primary or revision shoulder arthroplasty procedure between June 2015 and January 2016. Exclusion criteria included hemiarthroplasty procedures.
Continue to: Three patients refused...
Three patients refused to participate in the study. Enrollment was performed until 40 patients were enrolled in the study (20 patients in each group). Randomization was performed with a random number generator, and patients were assigned to a treatment group following consent to participate. Complete follow-up was available for all patients. There were 13 (65%) male patients in the CC group. The average age of the CC group at the time of surgery was 68.7 years (range). There were 11 male patients in the ICE group. The average age of the ICE group at the time of surgery was 73.2 years (range). The dominant extremity was involved in 9 (45%) patients in the CC group and in 11 patients (55%) in the ICE group. Surgical case specifics are summarized in Table 1.
Table 1. Summary of Surgical Cases
| CC group (n = 20) | ICE group (n = 20) |
Primary TSA | 7 (35%) | 9 (45%) |
Primary RSA | 12 (60%) | 9 (45%) |
Revision arthroplasty | 1 (5%) | 2 (10%) |
Abbreviations: CC, continuous cryotherapy; ICE, plain ice; RSA, reverse shoulder arthroplasty; TSA, total shoulder arthroplasty.
All surgeries were performed by Dr. Denard. All patients received a single-shot interscalene nerve block prior to the procedure. A deltopectoral approach was utilized, and the subscapularis was managed with the peel technique.9 All patients were admitted to the hospital following surgery. Standard postoperative pain control consisted of as-needed intravenous morphine (1-2 mg every 2 hours, as needed) or an oral narcotic (hydrocodone/acetaminophen 5/325mg, 1-2 every 4 hours, as needed) which was also provided at discharge. However, total narcotic usage was recorded in morphine equivalents to account for substitutions. No non-steroidal anti-inflammatory drugs were allowed until 3 months postoperatively.
The CC group received treatment from a commercially available cryotherapy unit (Polar Care; Breg). All patients received instructions by a medical professional on how to use the unit. The unit was applied immediately postoperatively and set at a temperature of 45°F to 55°F. Patients were instructed to use the unit continuously during postoperative days 0 to 3. This cryotherapy was administered by a nurse while in the hospital but was left to the responsibility of the patient upon discharge. Patients were instructed to use the unit as needed for pain control during the day and continuously while asleep from days 4 to14.
The ICE group used standard ice packs postoperatively. The patients were instructed to apply an ice pack for 20 min every 2 hours while awake during days 0 to 3. This therapy was administered by a nurse while in the hospital but left to the responsibility of the patient upon discharge. Patients were instructed to use ice packs as needed for pain control during the day at a maximum of 20 minutes per hour on postoperative days 4 to 14. Compliance by both groups was monitored using a patient survey after hospital discharge. The number of hours that patients used either the CC or ICE per 24-hour period was recorded at 24 hours, 3 days, 7 days, and 14 days. The nursing staff recorded the number of hours of use of either cold modality for each patient prior to hospital discharge. The average length of stay as an inpatient was 1.2 days for the CC group and 1.3 days for the ICE group.
Visual analog scales (VAS) for pain, satisfaction with the cold therapy, and quality of sleep were recorded preoperatively and postoperatively at 24 hours, 3 days, 7 days, and 14 days following surgery.
Continue to: The Wilcoxon rank-sum test...
STATISTICAL METHOD
The Wilcoxon rank-sum test was used to assess whether scores changed significantly from the preoperative period to the different postoperative time intervals, as well as to assess the values for pain, quality of sleep, and patient satisfaction. P-values <.05 were considered significant.
RESULTS
No differences were observed in the baseline characteristics between the 2 groups. Both groups showed improvements in pain, quality of sleep, and satisfaction with the cold therapy from the preoperative period to the final follow-up.
The VAS pain scores were not different between the CC and ICE groups preoperatively (5.9 vs 6.8; P = .121) or postoperatively at 24 hours (4.2 vs 4.3; P = .989), 3 days (4.8 vs 4.7; P = .944), 7 days (2.9 vs 3.3; P = .593), or 14 days (2.5 vs 2.7; P = .742). Both cohorts demonstrated improved overall pain throughout the study period. These findings are summarized in Table 2.
Table 2. Summary of VAS Pain Scores With Cold Therapy
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
Preoperative | 5.9 ± 4.1 | 6.8 ± 5.3 | .121 | 3.3-8.3 |
24 hours | 4.2 ± 3.0 | 4.3 ± 3.1 | .989 | 2.9-5.7 |
3 days | 4.8 ± 2.7 | 4.7 ± 3.2 | .944 | 3.2-6.3 |
7 days | 2.9 ± 1.8 | 3.3 ± 2.5 | .593 | 2.1-4.4 |
14 days | 2.5 ± 2.1 | 2.7 ± 1.8 | .742 | 1.5-3.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
The number of morphine equivalents of pain medication was not different between the CC and ICE groups postoperatively at 24 hours (43 vs 38 mg; P = .579), 3 days (149 vs 116 mg; P = .201), 7 days (308 vs 228 mg; P = .181), or 14 days (431 vs 348 mg; P = .213). Both groups showed increased narcotic consumption from 24 hours postoperatively until the 2-week follow-up. Narcotic consumption is summarized in Table 3.
Table 3. Summary of Narcotic Consumption in Morphine Equivalents
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 43.0 ± 36.7 | 38.0 ± 42.9 | .579 | 17.9-60.1 |
3 days | 149.0 ± 106.5 | 116.3 ± 108.9 | .201 | 63.4-198.7 |
7 days | 308.1 ± 234.0 | 228 ± 258.3 | .181 | 107.1-348.9 |
14 days | 430.8 ± 384.2 | 347.5 ± 493.4 | .213 | 116.6-610.6 |
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice.
VAS for quality of sleep improved in both groups from 24 hours postoperatively until the final follow-up. However, no significant differences in sleep quality were observed between the CC and ICE groups postoperatively at 24 hours (5.1 vs 4.3; P = .382), 3 days (5.1 vs 5.3; P = .601), 7 days (6.0 vs 6.7; P = .319), or 14 days (6.5 vs 7.1; P = .348). The VAS scores for sleep quality are reported in Table 4.
Table 4. Summary of VAS Sleep Quality With Cold Therapya
| CC group (mean ± SD) | ICE group (mean ± SD) | P value | 95% CI |
24 hours | 5.1 ± 2.8 | 4.3 ± 2.4 | .382 | 3.2-6.4 |
3 days | 5.1 ± 1.9 | 5.3 ± 2.3 | .601 | 4.2-6.5 |
7 days | 6.0 ± 2.3 | 6.7 ± 2.1 | .319 | 4.9-7.7 |
14 days | 6.5 ± 2.3 | 7.1 ± 2.5 | .348 | 5.3-8.4 |
a0-10 rating with 10 being the highest possible score.
Abbreviations: CC, continuous cryotherapy; CI, confidence interval; ICE, plain ice; VAS, visual analog scales.
Continue to: Finally, VAS patient satisfaction...
Finally, VAS patient satisfaction scores were not different between the CC and ICE groups postoperatively at 24 hours (7.3 vs 6.1; P = .315), 3 days (6.1 vs 6.6; P = .698), 7 days (6.6 vs 6.9; P = .670), or 14 days (7.1 vs 6.3; P = .288).
While compliance within each group utilizing the randomly assigned cold modality was similar, the usage by the CC group was consistently higher at all time points recorded. No complications or reoperations were observed in either group.
DISCUSSION
The optimal method for managing postoperative pain from an arthroplasty procedure is controversial. This prospective randomized study attempted to confirm the hypothesis that CC infers better pain control, improves quality of sleep, and decreases narcotic usage compared to ICE in the first 2 weeks after a TSA procedure. The results of this study refuted our hypothesis, demonstrating no significant difference in pain control, satisfaction, narcotic usage, or sleep quality between the CC and ICE cohorts at all time points studied.
Studies on knees and lower extremities demonstrate equivocal results for the role CC plays in providing improved postoperative pain control. Thienpont10 evaluated CC in a randomized control trial comparing plain ice packs postoperatively in patients who underwent TKA. The author found no significant difference in VAS for pain or narcotic consumption in morphine equivalents. Thienpont10 recommended that CC not be used for outpatient knee arthroplasty as it is an additional cost that does not improve pain significantly. Healy and colleagues5 reported similar results that CC did not demonstrate a difference in narcotic requirement or pain control compared to plain ice packs, as well as no difference in local postoperative swelling or wound drainage. However, a recently published randomized trial by Su and colleagues11 comparing a cryopneumatic device and ICE with static compression in patients who underwent TKA demonstrated significantly lower narcotic consumption and increased ambulation distances in the treatment group. The treatment group consumed approximately 170 mg morphine equivalents less than the control group between discharge and the 2-week postoperative visit. In addition, a significant difference was observed in the satisfaction scores in the treatment group.11 Similarly, a meta-analysis by Raynor and colleagues12 on randomized clinical trials comparing cryotherapy to a placebo group after anterior cruciate ligament reconstruction showed that cryotherapy is associated with significantly lower postoperative pain (P = .02), but demonstrated no difference in postoperative drainage (P = .23) or range of motion (P = .25).
Although multiple studies have been published regarding the efficacy of cryotherapy after knee surgery, very few studies have compared CC to conventional ICE after shoulder surgery. A prospective randomized trial was performed by Singh and colleagues13 to compare CC vs no ICE in open and arthroscopic shoulder surgery patients. Both the open and arthroscopic groups receiving CC demonstrated significant reductions in pain frequency and more restful sleep at the 7-day, 14-day, and 21-day intervals compared to the control group. However, they did not compare the commercial unit to ICE. In contrast, a study by Kraeutler and colleagues8 randomized 46 patients to receive either CC or ICE in the setting of arthroscopic shoulder surgery. Although no significant difference was observed in morphine equivalent dosage between the 2 groups, the CC group used more pain medication on every postoperative day during the first week after surgery. They found no difference between the 2 groups with regards to narcotic consumption or pain scores. The results of this study mirror those by Kraeutler and colleagues,8 demonstrating no difference in pain scores, sleep quality, or narcotic consumption.
Continue to: With rising costs in the US...
With rising costs in the US healthcare system, a great deal of interest has developed in the application of value-based principles to healthcare. Value can be defined as a gain in benefits over the costs expended.14 The average cost for a commercial CC unit used in this study was $260. A pack of ICE is a nominal cost. Based on the results of this study, the cost of the commercial CC device may not be justified when compared to the cost of an ice pack.
The major strengths of this study are the randomized design and multiple data points during the early postoperative period. However, there are several limitations. First, we did not objectively measure compliance of either therapy and relied only on a patient survey. Usage of the commercial CC unit in hours decreased over half between days 3 and 14. This occurred despite training on the application and specific instructions. We believe this reflects “real-world” usage, but it is possible that compliance affected our results. Second, all patients in this study had a single-shot interscalene block. While this is standard at our institution, it is possible that either CC or ICE would have a more significant effect in the absence of an interscalene block. Finally, we did not evaluate final outcomes in this study and therefore cannot determine if the final outcome was different between the 2 groups. Our goal was simply to evaluate the first 2 weeks following surgery, as this is the most painful period following TSA.
CONCLUSION
There was no difference between CC and ICE in terms of pain control, quality of sleep, patient satisfaction, or narcotic consumption following TSA. CC may offer convenience advantages, but the increased cost associated with this type of unit may not be justified.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
1. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the United States. J Bone Joint Surg Am. 2011;93(24):2249-2254. doi:10.2106/jbjs.j.01994.
2. Stalman A, Berglund L, Dungnerc E, Arner P, Fellander-Tsai L. Temperature sensitive release of prostaglandin E2 and diminished energy requirements in synovial tissue with postoperative cryotherapy: a prospective randomized study after knee arthroscopy. J Bone Joint Surg Am. 2011;93(21):1961-1968. doi:10.2016/jbjs.j.01790.
3. Levy AS, Marmar E. The role of cold compression dressings in the postoperative treatment of total knee arthroplasty. Clin Orthop Relat Res. 1993;297:174-178. doi:10.1097/00003086-199312000-00029.
4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopaedics 1998;21(1):59-61.
5. Healy WL, Seidman J, Pfeifer BA, Brown DG. Cold compressive dressing after total knee arthroplasty. Clin Orthop Relat Res. 1994;299:143-146. doi:10.1097/00003086-199402000-00019.
6. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28-30.
7. Speer KP, Warren RF, Horowitz L. The efficacy of cryotherapy in the postoperative shoulder. J Shoulder Elbow Surg. 1996;5(1):62-68. doi:10.16/s1058-2746(96)80032-2.
8. Kraeutler MJ, Reynolds KA, Long C, McCarthy EC. Compressive cryotherapy versus ice- a prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24(6):854-859. doi:10.1016/j.jse.2015.02.004.
9. DeFranco MJ, Higgins LD, Warner JP. Subscapularis management in open shoulder surgery. J Am Acad Orthop Surg. 2010;18(12):707-717. doi:10.5435/00124635-201012000-00001.
10. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty. Clin Orthop Relat Res. 2014;472(11):3417-3423. doi:10.1007/s11999-014-3810-8.
11. Su EP, Perna M, Boettner F, Mayman DJ, et al. A prospective, multicenter, randomized trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94(11 Suppl A):153-156. doi:10.1302/0301-620x.94B11.30832.
12. Raynor MC, Pietrobon R, Guller U, Higgins LD. Cryotherapy after ACL reconstruction- a meta analysis. J Knee Surg. 2005;18(2):123-129. doi:10.1055/s-0030-1248169.
13. Singh H, Osbahr DC, Holovacs TF, Cawley PW, Speer KP. The efficacy of continuous cryotherapy on the postoperative shoulder: a prospective randomized investigation. J Shoulder Elbow Surg. 2001;10(6):522-525. doi:10.1067/mse.2001.118415.
14. Black EM, Higgins LD, Warner JP. Value based shoulder surgery: outcomes driven, cost-conscious care. J Shoulder Elbow Surg. 2013;22(7):1-10. doi:10.1016/j.se.2013.02.008.
TAKE-HOME POINTS
- CC has been proposed as a means of improving postoperative pain control.
- CC represents a cost typically not covered by insurances.
- No difference was noted between the 2 groups in quality of sleep, satisfaction with the cold therapy, or narcotic usage at any time interval.
- While CC may offer convenience advantages, the increased cost associated with this type of unit may not be justified.
- The mechanism for CC for pain control is poorly understood.
Accuracy of Distal Femoral Valgus Deformity Correction: Fixator-Assisted Nailing vs Fixator-Assisted Locked Plating
ABSTRACT
Fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP) are 2 techniques that can be used to correct distal femoral valgus deformities. The fixator aids in achieving an accurate adjustable initial reduction, which is then made permanent with either nail or plate insertion. FALP can be performed with the knee held in a neutral extended position, whereas FAN requires 30° to 90° of knee flexion to insert the nail, which may cause some alignment loss. We hypothesized that FAN may yield less accurate correction than FALP. Prospectively collected data of a consecutive cohort of patients who underwent valgus deformity femoral correction with FAN or FALP at a single institution over an 8-year period were retrospectively evaluated. Twenty extremities (18 patients) were treated using FAN (median follow-up, 5 years; range, 1-10 years), and 7 extremities (6 patients) were treated with FALP (median follow-up, 5 years; range, 1-8 years). In the FAN cohort, the mean preoperative and postoperative mechanical lateral distal femoral angles (mLDFAs) were 81° (range, 67°-86°) and 89° (range, 80°-100°), respectively (P = .009). In the FALP cohort, the mean preoperative and postoperative mLDFAs were 80° (range, 71°-87°) and 88° (range, 81°-94°), respectively (P < .001). Although the average mechanical axis deviation correction for the FALP group was greater than for the FAN group (32 mm and 27 mm, respectively), the difference was not significant (P = .66). Both methods of femoral deformity correction can be considered safe and effective. On the basis of our results, FAN and FALP are comparable in accuracy for deformity correction in the distal femur.
Multiple etiologies for distal femoral valgus deformity have been described in the literature.1-3 These can be congenital, developmental, secondary to lateral compartmental arthritis, or posttraumatic.4 If not corrected, femoral deformities alter the axial alignment and orientation of the joints, and may lead to early degenerative joint disease and abnormal leg kinematics.3,5 After correcting these deformities, the goal of treatment is to obtain anatomic distal femoral angles and neutral mechanical axis deviation (MAD), but without overcorrecting into varus. Numerous techniques to fix these deformities, such as progressive correction with external fixation or acute correction open reduction with internal fixation (ORIF), have been described.6 Modern external fixation allows for a gradual, adjustable, and more accurate correction but may produce discomfort and complications for patients.7-10 In contrast, ORIF may be more tolerable for the patient, but to achieve a precise correction, considerable technical skills and expertise are required.1,11-14
Two techniques used to correct these valgus femoral deformities in adults are fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP).1 FAN and FALP combine the advantage of external fixation (accuracy, adjustability) with the benefits of internal fixation (patient comfort), because the osteotomy and correction are performed with the guidance of a temporary external fixator and then permanently fixated by an intramedullary (IM) nail or a locking plate.1,8,11-13,15-18 Both techniques have the possibility to correct varus and valgus deformities, but whenever correcting sagittal plane angulation, the FAN technique may be more challenging. The paucity of studies available involving FAN and FALP do not lead to a conclusive preference of one technique over the other relative to the accuracy and success of correction.15,19,20
Continue to: In both FAN and FALP
In both FAN and FALP, the external fixator is applied and adjusted after the osteotomy for accurate alignment. In FALP, the plate is added without moving the leg from its straight position. However, in FAN, the knee must be flexed to 30° to 90° for insertion of the retrograde knee nail, and the alignment may be lost if the external fixation is not fully stable. Therefore, we hypothesized that FAN would be less accurate than FALP. Hence, the purposes of this study is to compare the correction achieved with FAN and FALP in patients with distal femoral valgus deformities and to describe the intraoperative complications associated with both techniques.
MATERIALS AND METHODS
After proper Institutional Review Board approval was obtained, a consecutive cohort of 35 patients who underwent femoral deformity correction with either FAN or FALP during an 8-year period (January 2002 to December 2010) was retrospectively reviewed. Eleven patients had to be excluded because of inadequate follow-up (<12 months) or because additional procedures were simultaneously performed. A total of 24 patients (27 femora) who had a mean age of 26 years (range, 14-68 years) were included in the final study cohort. Specifically, 20 femora (18 patients) were corrected using the FAN technique (7 males and 11 females; mean age, 36 years; range, 14-68 years), and 7 femora (6 patients) were fixed using the FALP technique (2 males and 4 females; mean age, 16 years; range, 15-19 years). The median follow-up in the FAN cohort was 5 years (range, 1-10 years), and the median follow-up in the FALP cohort was 5 years (range, 1-8 years) (Table 1).
| Table 1. Study Details and Demographic Characteristics | |||
| Detail | Overall | FAN | FALP |
| Number of patients | 24 | 18 | 6 |
| Number of femurs | 27 | 20 | 7 |
| Age in years (range) | 26 (14 to 68) | 36 (14 to 68) | 16 (15 to 19) |
| Male:Female | 9:15 | 7:11 | 2:4 |
| Median follow-up in years (range) | 5 (1 to 10) | 5 (1 to 10) | 5 (1 to 8) |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing
The specific measurements performed in all patients were MAD, mechanical lateral distal femoral angle (mLDFA), and medial proximal tibia angle (MPTA). These were measured from standing anteroposterior radiographs of the knee that included the femur.21 All outcome data were collected from the medical charts, operative reports, and radiographic evaluations. To ensure accuracy, all measurements were performed by 2 authors blinded to each other’s measurements. If a variation of <5% was obtained, the results were averaged and used for further analysis. Whenever a difference of >5% was obtained, the measurement was repeated by both authors for confirmation.
SURGICAL FAN TECHNIQUE
After measuring the deformity (Figure 1A) with the patient under general anesthesia on a radiolucent table, the involved lower limb is prepared and draped. Two half-pins are inserted medially, 1 proximal and 1 distal to the planned osteotomy site (Figure 1B), and then connected loosely with a monolateral external fixator. Special care is taken while placing the half-pins, not to interfere with the insertion path of the IM rod. When performing the preoperative planning, the level of osteotomy is chosen to enable the placement of at least 2 interlocking screws distal to the osteotomy. Then, a percutaneous osteotomy is performed from a lateral approach, and the bone ends are manipulated (translation and then angulation) to achieve the desired deformity correction. The external fixator is then stabilized and locked in the exact position (Figure 1C). Subsequently, retrograde reaming, nail insertion, and placement of proximal and distal locking screws are performed (Figure 1D). Blocking screws may give additional stability. The removal of the external fixator is the final step (Figure 1E).20
Continue to: When using the FAN technique...
When using the FAN technique, special attention is paid to reducing the risk of fat embolism. This can be reduced but not totally eradicated with the use of reaming irrigation devices.22-24 In our technique of FAN, the bone is cut and displaced prior to reaming so that the pressure of reaming is vented out through the osteotomy, along with the reaming contents, which theoretically can then act as a “prepositioned bone graft” that may speed healing.
SURGICAL FALP TECHNIQUE
Preoperatively, a decision concerning the planned osteotomy and the correct locking plate size is made. In addition, the outline of the plate is marked on the skin. Under general anesthesia, the patients are prepared and draped. A tourniquet is elevated around the upper thigh. Then, 2 half-pins are medially inserted, 1 proximal and 1 distal to the planned osteotomy site, and are then connected loosely with a monolateral external fixator (Figure 2A). A lateral approach to the distal femur is done, preserving the periosteum, except at the level of the osteotomy. After the osteotomy is performed (through an open lateral incision), both segments are translated (Figure 2B) and then the distal segment is angulated to achieve the desired deformity correction, and the desired position is then stabilized by tightening the external fixator connectors (Figure 2C). Subsequently, a locking plate is inserted in the submuscular-extraperiostal plane. The plate does not require being in full contact (flush) with the bone. At least 3 screws are placed on both sides of the osteotomy through a long lateral incision (Figure 2D). Bone graft may be added to the osteotomy site to encourage healing. Then, the external fixator is removed, and all incisions are closed (Figure 2E).15,19
During each of the procedures, we aimed at having “perfect alignment” with a MAD of 0 mm, in which a Bovie cord is used and passed through the center of the femoral head, knee, and ankle. However, to confirm that the surgery was successful, the actual measurements were performed on standing long-leg films. These films were obtained preoperatively and at latest follow-up. They were performed with the patella aiming forward, the toes straight ahead, feet separated enough for good balance, knees fully extended, and weight equally distributed on the feet. Postoperatively, in both cohorts, partial weight-bearing was encouraged immediately with crutches; physical therapy was instituted daily for knee range of motion. Radiographs were scheduled every 4 weeks to monitor callus formation. Full weight-bearing was allowed when at least 3 cortices were consolidated.1,15,19,20,25,26
All statistical analyses were performed with the aid of the SPSS statistical software package (SPSS). Average values and standard error of the mean were assigned to each variable. A nonparametric Mann-Whitney U test was used, and a 2-tailed P < .05 was considered significant. Correlation of continuous variables was determined by Spearman’s correlation coefficient. Also, multivariate Cox regression analyses after adjustment for age, sex, and deformity correction were used to detect associations within the study population. To evaluate whether our data were normally distributed, Shapiro-Wilk tests were performed.
Continue to: Results...
RESULTS
The mLDFA significantly improved in the FAN cohort from a mean of 81° to a mean of 89° (ranges, 67°-86° and 80°-100°; respectively; P = .001) (Figures 3A, 3B).
| Table 2. Deformity Correction | ||||
| Measurement | Cohort | Preoperative | Postoperative | P Value |
| mLDFA in degrees (range) | FAN | 81 (67 to 86) | 89 (80 to 100) | 0.001 |
| FALP | 80 (71 to 87) | 90 (88 to 94) | <0.001 | |
| Mechanical axis deviation in mm (range) | FAN | 32 (6 to 64) | 10 (0 to 22) | 0.001 |
| FALP | 34 (17 to 62) | 4 (0 to 11) | 0.002 | |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing; mLDFA, mechanical lateral distal femoral angle
After evaluating the MPTA, in the FAN cohort, we found that the mean pre- and postoperative MPTAs were not modified. These patients had a mean preoperative angle of 88° (range, 62°-100°), which was kept postoperatively to a mean of 88° (range, 78°-96°). In the FALP cohort, a slight change from 90° to 88° was observed (ranges, 82°-97° and 83°-94°, respectively). None of these changes in MPTA were significant (P > .05).
When evaluating correction of the MAD, we observed that the FAN cohort changed from a preoperative MAD of 32 mm (range, 6-64 mm) to a postoperative mean of 10 mm (range, 0-22 mm), and this correction was statistically significant. (P = .001). The FALP cohort changed from a mean of 34 mm (range, 17-62 mm) preoperatively to 4 mm (range, 0-11 mm) postoperatively, and this was also statistically significant (P = .002). The mean MAD correction for the FAN group vs FALP group was 27 mm vs 32 mm, respectively (Table 2).
In patients with valgus femoral deformity, the MAD is usually lateralized; however, in the FAN cohort, we included 3 patients with medial MADs (10 mm, 13 mm, and 40 mm). This is justified in these patients because a complex deformity of the distal femur and the proximal tibia was present. In the extreme case of a 40-mm medial MAD, the presurgery mLDFA was 76°, and the presurgery MPTA was 62°. The amount of deformity correction in this patient was 16°.
During the follow-up period, 2 complications occurred in the FAN group. One patient developed gait disturbance that resolved with physical therapy. Another had an infection at the osteotomy site. This was addressed with intravenous antibiotic therapy, surgical irrigation and débridement, hardware removal, and antegrade insertion of an antibiotic-coated nail. In the FALP group, 1 patient developed a persistent incomplete peroneal nerve palsy attributed to a 17° correction from valgus to varus, despite prophylactic peroneal nerve decompression. Nonetheless, the patient was satisfied with the result, recovered partial nerve function, and returned for correction of the contralateral leg deformity. When comparing the complications between both cohorts, no significant differences were found: 2 of 18 cases (11%) in the FAN group vs 1 of 6 cases (17%) in the FALP group (P = .78).
Continue to: The goal of this study...
DISCUSSION
The goal of this study was to compare the accuracy of deformity corrections achieved with either FAN or FALP. A number of authors have described results after deformity correction with several plating and nailing techniques; however, the information derived from comparing these 2 techniques is limited. We hypothesized that FALP would be more accurate, because less mobilization during fixation is required. However, we found no significant differences between these 2 techniques.
This study has several limitations. First, the small size of our cohort had to be further reduced owing to limited data; nevertheless, this pathology and the treatment methods used are not commonly performed, which make this cohort 1 of the largest of its type described in the literature. Also, the procedures were performed by multiple surgeons in a population with a wide age range, creating multiple additional variables that complicate the comparison of the sole differences between FAN and FALP. However, owing to these variables, the generalizability of this study may be increased, and similar outcomes can potentially be obtained by other institutions/surgeons. In addition, the variability of our follow-up period is another limitation; however, these patients were all assessed until bony union after skeletal maturity was achieved. Hence, the development of additional deformity is not expected. The lack of clinical outcome with a standardized questionnaire may also be seen as a limitation. However, because the purpose of our study was to assess both surgeries in terms of their ability to achieve angular correction, the addition of patient-reported outcomes may have increased the variability of our data.
The foremost objective in valgus deformity correction is to establish joint orientation angles within anatomic range to prevent overloading of the lateral joint and thereby prevent lateral compartmental osteoarthritis.2,20,27-29 There are 2 categories of fixation: internal and external. With FAN and FALP, we strive to have the adjustability and accuracy of external fixation with the comfort (for the patient) of internal fixation. Accurate osteotomy correction requires an accurate preoperative analysis and osteotomy close to the apex of the deformity.16,21,30-33 The most commonly used osteotomy techniques are drill-hole,31 focal dome,34 rotation, and open- or closed-wedge osteotomies.35,36 After the osteotomy, the resultant correction has to be stabilized. In recent years, the popularity of plates instead of an IM nail for internal fixation has been driven by the rapid development of low contact locking plates.16,19,26,30,37-40
There are certain advantages of using FAN over FALP. In older patients who may require a subsequent total knee arthroplasty (TKA), the midline incision used for retrograde FAN technique is identical to that made for TKA. In contrast, in a younger and more active population, with a longer life expectancy, the extra-articular FALP approach has the advantage of not violating the knee joint. In addition, locking plates may achieve a more rigid fixation than IM nails; however, the stability of IM nails can be augmented with blocking screws.
Continue to: In 20 patients, including children...
In 20 patients, including children and young adults, with frontal and sagittal plane deformities, Marangoz and colleagues7 reported on correction of valgus, varus, and procurvatum deformities using a Taylor Spatial Frame (TSF). Successful correction of severe deformities was achieved gradually with the TSF, resulting in a postoperative deformity (valgus group) of mLDFA 88.9° (range, 85°-95°).7 In a more recent study, Bar-On and colleagues15 described a series of 11 patients (18 segments) with corrective lower limb osteotomies in which all were corrected to within 2° of the planned range. Similarly, Gugenheim and Brinker20 described the use of the FAN technique to correct distal varus and valgus deformities in 14 femora. The final mean mLDFA and MAD in the valgus group were 89° (range, 88°-90°) and 5 mm (range, 0-14 mm medial), respectively.
In their comparative study, Seah and colleagues11 described monolateral frame vs FALP deformity correction in a series of 34 extremities (26 patients) that required distal femoral osteotomy. No differences related to knee range of motion or the ability to correct the deformity between internal and external fixation were reported (P > .05). Similarly, Eidelman and colleagues1 evaluated the outcomes of 6 patients (7 procedures) who underwent surgery performed with the FALP technique for distal femoral valgus deformity. They concluded that this technique is minimally invasive and can provide a precise deformity correction with minimal morbidity.
Other methods of fixation while performing FAN have been described by Jasiewicz and colleagues,22 who evaluated possible differences between the classic Ilizarov device and monolateral fixators in 19 femoral lengthening procedures. The authors concluded that there is no difference between concerning complication rate and treatment time. The use of FAN has also been described in patients with metabolic disease who required deformity correction. In this regard, Kocaoglu and colleagues12 described the use of a monolateral external fixator in combination with an IM nail in a series of 17 patients with metabolic bone disease. The authors concluded that the use of the IM nail prevented recurrence of deformity and refracture.12 Kocaoglu and colleagues14 also published a series of 25 patients treated with the FAN and LON (lengthening over a nail) technique for lengthening and deformity correction. The mean MAD improved from 33.9 mm to11.3 mm (range, 0-30 mm). In contrast, Erlap and colleagues13 compared FAN with circular external fixator for bone realignment of the lower extremity for deformities in patients with rickets. Although no significant difference was found between both groups, FAN was shown to be accurate and to provide great comfort to patients, and it also shortened the total treatment time.13 Finally, the advent of newer technologies could also provide alternatives for correcting valgus deformities. For example, Saragaglia and Chedal-Bornu6 performed 29 computer-assisted valgus knees osteotomies (27 patients) and reported that the goal hip-knee angle was achieved in 86% of patients and that the goal MPTA was achieved in 100% of patients.6
CONCLUSION
Both the FALP and FAN methods of femoral deformity correction are safe and effective surgical techniques. In our opinion, the advantages of the FALP technique result from the easy lateral surgical approach under medial external fixation and stabilization of the osteotomy without bending the knee. Ultimately, the decision to use FAN may be influenced by the surgeon’s perception of the potential need for future TKA. In such cases, a midline anterior approach with nailing is very compatible with subsequent TKA. The surgeon’s experience and preference, while keeping in mind the patient’s predilection, will play an important role in the decision-making process. Larger prospective clinical trials with larger cohorts have to be conducted to confirm our findings.
1. Eidelman M, Keren Y, Norman D. Correction of distal femoral valgus deformities in adolescents and young adults using minimally invasive fixator-assisted locking plating (FALP). J Pediatr Orthop B. 2012;21(6):558-562. doi:10.1097/BPB.0b013e328358f884.
2. Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi:10.1186/ar2272.
3. Solomin LN, Paley D, Shchepkina EA, Vilensky VA, Skomoroshko PV. A comparative study of the correction of femoral deformity between the Ilizarov apparatus and ortho-SUV Frame. Int Orthop. 2014;38(4):865-872. doi:10.1007/s00264-013-2247-0.
4. Meric G, Gracitelli GC, Aram LJ, Swank ML, Bugbee WD. Variability in distal femoral anatomy in patients undergoing total knee arthroplasty: measurements on 13,546 computed tomography scans. J Arthroplasty. 2015;30(10):1835-1838. doi:10.1016/j.arth.2015.04.024.
5. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015. doi:10.1007/s11999-014-4106-8.
6. Saragaglia D, Chedal-Bornu B. Computer-assisted osteotomy for valgus knees: medium-term results of 29 cases. Orthop Traumatol Surg Res. 2014;100(5):527-530. doi:10.1016/j.otsr.2014.04.002.
7. Marangoz S, Feldman DS, Sala DA, Hyman JE, Vitale MG. Femoral deformity correction in children and young adults using Taylor Spatial Frame. Clin Orthop Relat Res. 2008;466(12):3018-3024. doi:10.1007/s11999-008-0490-2.
8. Rogers MJ, McFadyen I, Livingstone JA, Monsell F, Jackson M, Atkins RM. Computer hexapod assisted orthopaedic surgery (CHAOS) in the correction of long bone fracture and deformity. J Orthop Trauma. 2007;21(5):337-342. doi:10.1097/BOT.0b013e3180463103.
9. Feldman DS, Madan SS, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara: acute versus gradual correction. J Pediatr Orthop. 2006;26(6):794-798. doi:10.1097/01.bpo.0000242375.64854.3d.
10. Manner HM, Huebl M, Radler C, Ganger R, Petje G, Grill F. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1(1):55-61. doi:10.1007/s11832-006-0005-1.
11. Seah KT, Shafi R, Fragomen AT, Rozbruch SR. Distal femoral osteotomy: is internal fixation better than external? Clin Orthop Relat Res. 2011;469(7):2003-2011. doi:10.1007/s11999-010-1755-0.
12. Kocaoglu M, Bilen FE, Sen C, Eralp L, Balci HI. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52-56. doi:10.1302/0301-620X.93B1.24788.
13. Eralp L, Kocaoglu M, Toker B, Balcı HI, Awad A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch Orthop Trauma Surg. 2011;131(5):581-589. doi:10.1007/s00402-010-1162-8.
14. Kocaoglu M, Eralp L, Bilen FE, Balci HI. Fixator-assisted acute femoral deformity correction and consecutive lengthening over an intramedullary nail. J Bone Joint Surg Am. 2009;91(1):152-159. doi:10.2106/JBJS.H.00114.
15. Bar-On E, Becker T, Katz K, Velkes S, Salai M, Weigl DM. Corrective lower limb osteotomies in children using temporary external fixation and percutaneous locking plates. J Child Orthop. 2009;3(2):137-143. doi:10.1007/s11832-009-0165-x.
16. Herzenberg JE, Kovar FM. External fixation assisted nailing (EFAN) and external fixation assisted plating (EFAP) for deformity correction. In: Solomin LN, ed. The Basic Principles of External Fixation Using the Ilizarov and Other Devices. 2nd ed. Italy: Springer-Verlag; 2012:1363-1378.
17. Eralp L, Kocaoglu M, Cakmak M, Ozden VE. A correction of windswept deformity by fixator assisted nailing. A report of two cases. J Bone Joint Surg Br. 2004;86(7):1065-1068.
18. Eralp L, Kocaoglu M. Distal tibial reconstruction with use of a circular external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 2):181-194. doi:10.2106/JBJS.H.00467.
19. Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(Suppl 2):B63-B76. doi:10.1016/j.injury.2003.09.026.
20. Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85–A(7):1229-1237. doi:10.2106/00004623-200307000-00008.
21. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
22. Jasiewicz B, Kacki W, Tesiorowski M, Potaczek T. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73(3):177-183.
23. Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89(11):1421-1426. doi:10.1302/0301-620X.89B11.19570.
24. Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202-207. doi:10.1097/00005373-199402000-00010.
25. Gordon JE, Goldfarb CA, Luhmann SJ, Lyons D, Schoenecker PL. Femoral lengthening over a humeral intramedullary nail in preadolescent children. J Bone Joint Surg Am. 2002;84–A(6):930-937. doi:10.2106/00004623-200206000-00006.
26. Oh CW, Song HR, Kim JW, et al. Deformity correction with submuscular plating technique in children. J Pediatr Orthop B. 2010;19(1):47-54. doi:10.1097/BPB.0b013e32832f5b06.
27. Guettler J, Glisson R, Stubbs A, Jurist K, Higgins L. The triad of varus malalignment, meniscectomy, and chondral damage: a biomechanical explanation for joint degeneration. Orthopedics. 2007;30(7):558-566.
28. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. doi:10.1002/art.23462.
29. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459-467. doi:10.1002/art.24336.
30. Paley D, HJ, Bor N. Fixator-assisted nailing of femoral and tibial deformities. Tech Orthop. 1997;12(4):260-275.
31. Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124(5):298-300. doi:10.1007/s00402-004-0646-9.
32. Strecker W, Kinzl L, Keppler P. Corrective osteotomies of the distal femur with retrograde intramedullary nail. Unfallchirurg. 2001;104(10):973-983. doi:10.1007/s001130170040.
33. Watanabe K, Tsuchiya H, Sakurakichi K, Matsubara H, Tomita K. Acute correction using focal dome osteotomy for deformity about knee joint. Arch Orthop Trauma Surg. 2008;128(12):1373-1378. doi:10.1007/s00402-008-0574-1.
34. Hankemeier S, Paley D, Pape HC, Zeichen J, Gosling T, Krettek C. Knee para-articular focal dome osteotomy. Orthopade. 2004;33(2):170-177. doi:10.1007/s00132-003-0588-x.
35. Brinkman JM, Luites JW, Wymenga AB, van Heerwaarden RJ. Early full weight bearing is safe in open-wedge high tibial osteotomy. Acta Orthop. 2010;81(2):193-198. doi:10.3109/17453671003619003.
36. Hankemeier S, Mommsen P, Krettek C, et al. Accuracy of high tibial osteotomy: comparison between open- and closed-wedge technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1328-1333. doi:10.1007/s00167-009-1020-9.
37. Hedequist D, Bishop J, Hresko T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008;28(1):6-9. doi:10.1097/bpo.0b013e31815ff301.
38. Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007;27(5):504-509. doi:10.1097/01.bpb.0000279020.96375.88.
39. Uysal M, Akpinar S, Cesur N, Hersekli MA, Tandoğan RN. Plating after lengthening (PAL): technical notes and preliminary clinical experiences. Arch Orthop Trauma Surg. 2007;127(10):889-893. doi:10.1007/s00402-007-0442-4.
40. Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. Instr Course Lect. 2008;57:25-36.
ABSTRACT
Fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP) are 2 techniques that can be used to correct distal femoral valgus deformities. The fixator aids in achieving an accurate adjustable initial reduction, which is then made permanent with either nail or plate insertion. FALP can be performed with the knee held in a neutral extended position, whereas FAN requires 30° to 90° of knee flexion to insert the nail, which may cause some alignment loss. We hypothesized that FAN may yield less accurate correction than FALP. Prospectively collected data of a consecutive cohort of patients who underwent valgus deformity femoral correction with FAN or FALP at a single institution over an 8-year period were retrospectively evaluated. Twenty extremities (18 patients) were treated using FAN (median follow-up, 5 years; range, 1-10 years), and 7 extremities (6 patients) were treated with FALP (median follow-up, 5 years; range, 1-8 years). In the FAN cohort, the mean preoperative and postoperative mechanical lateral distal femoral angles (mLDFAs) were 81° (range, 67°-86°) and 89° (range, 80°-100°), respectively (P = .009). In the FALP cohort, the mean preoperative and postoperative mLDFAs were 80° (range, 71°-87°) and 88° (range, 81°-94°), respectively (P < .001). Although the average mechanical axis deviation correction for the FALP group was greater than for the FAN group (32 mm and 27 mm, respectively), the difference was not significant (P = .66). Both methods of femoral deformity correction can be considered safe and effective. On the basis of our results, FAN and FALP are comparable in accuracy for deformity correction in the distal femur.
Multiple etiologies for distal femoral valgus deformity have been described in the literature.1-3 These can be congenital, developmental, secondary to lateral compartmental arthritis, or posttraumatic.4 If not corrected, femoral deformities alter the axial alignment and orientation of the joints, and may lead to early degenerative joint disease and abnormal leg kinematics.3,5 After correcting these deformities, the goal of treatment is to obtain anatomic distal femoral angles and neutral mechanical axis deviation (MAD), but without overcorrecting into varus. Numerous techniques to fix these deformities, such as progressive correction with external fixation or acute correction open reduction with internal fixation (ORIF), have been described.6 Modern external fixation allows for a gradual, adjustable, and more accurate correction but may produce discomfort and complications for patients.7-10 In contrast, ORIF may be more tolerable for the patient, but to achieve a precise correction, considerable technical skills and expertise are required.1,11-14
Two techniques used to correct these valgus femoral deformities in adults are fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP).1 FAN and FALP combine the advantage of external fixation (accuracy, adjustability) with the benefits of internal fixation (patient comfort), because the osteotomy and correction are performed with the guidance of a temporary external fixator and then permanently fixated by an intramedullary (IM) nail or a locking plate.1,8,11-13,15-18 Both techniques have the possibility to correct varus and valgus deformities, but whenever correcting sagittal plane angulation, the FAN technique may be more challenging. The paucity of studies available involving FAN and FALP do not lead to a conclusive preference of one technique over the other relative to the accuracy and success of correction.15,19,20
Continue to: In both FAN and FALP
In both FAN and FALP, the external fixator is applied and adjusted after the osteotomy for accurate alignment. In FALP, the plate is added without moving the leg from its straight position. However, in FAN, the knee must be flexed to 30° to 90° for insertion of the retrograde knee nail, and the alignment may be lost if the external fixation is not fully stable. Therefore, we hypothesized that FAN would be less accurate than FALP. Hence, the purposes of this study is to compare the correction achieved with FAN and FALP in patients with distal femoral valgus deformities and to describe the intraoperative complications associated with both techniques.
MATERIALS AND METHODS
After proper Institutional Review Board approval was obtained, a consecutive cohort of 35 patients who underwent femoral deformity correction with either FAN or FALP during an 8-year period (January 2002 to December 2010) was retrospectively reviewed. Eleven patients had to be excluded because of inadequate follow-up (<12 months) or because additional procedures were simultaneously performed. A total of 24 patients (27 femora) who had a mean age of 26 years (range, 14-68 years) were included in the final study cohort. Specifically, 20 femora (18 patients) were corrected using the FAN technique (7 males and 11 females; mean age, 36 years; range, 14-68 years), and 7 femora (6 patients) were fixed using the FALP technique (2 males and 4 females; mean age, 16 years; range, 15-19 years). The median follow-up in the FAN cohort was 5 years (range, 1-10 years), and the median follow-up in the FALP cohort was 5 years (range, 1-8 years) (Table 1).
| Table 1. Study Details and Demographic Characteristics | |||
| Detail | Overall | FAN | FALP |
| Number of patients | 24 | 18 | 6 |
| Number of femurs | 27 | 20 | 7 |
| Age in years (range) | 26 (14 to 68) | 36 (14 to 68) | 16 (15 to 19) |
| Male:Female | 9:15 | 7:11 | 2:4 |
| Median follow-up in years (range) | 5 (1 to 10) | 5 (1 to 10) | 5 (1 to 8) |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing
The specific measurements performed in all patients were MAD, mechanical lateral distal femoral angle (mLDFA), and medial proximal tibia angle (MPTA). These were measured from standing anteroposterior radiographs of the knee that included the femur.21 All outcome data were collected from the medical charts, operative reports, and radiographic evaluations. To ensure accuracy, all measurements were performed by 2 authors blinded to each other’s measurements. If a variation of <5% was obtained, the results were averaged and used for further analysis. Whenever a difference of >5% was obtained, the measurement was repeated by both authors for confirmation.
SURGICAL FAN TECHNIQUE
After measuring the deformity (Figure 1A) with the patient under general anesthesia on a radiolucent table, the involved lower limb is prepared and draped. Two half-pins are inserted medially, 1 proximal and 1 distal to the planned osteotomy site (Figure 1B), and then connected loosely with a monolateral external fixator. Special care is taken while placing the half-pins, not to interfere with the insertion path of the IM rod. When performing the preoperative planning, the level of osteotomy is chosen to enable the placement of at least 2 interlocking screws distal to the osteotomy. Then, a percutaneous osteotomy is performed from a lateral approach, and the bone ends are manipulated (translation and then angulation) to achieve the desired deformity correction. The external fixator is then stabilized and locked in the exact position (Figure 1C). Subsequently, retrograde reaming, nail insertion, and placement of proximal and distal locking screws are performed (Figure 1D). Blocking screws may give additional stability. The removal of the external fixator is the final step (Figure 1E).20
Continue to: When using the FAN technique...
When using the FAN technique, special attention is paid to reducing the risk of fat embolism. This can be reduced but not totally eradicated with the use of reaming irrigation devices.22-24 In our technique of FAN, the bone is cut and displaced prior to reaming so that the pressure of reaming is vented out through the osteotomy, along with the reaming contents, which theoretically can then act as a “prepositioned bone graft” that may speed healing.
SURGICAL FALP TECHNIQUE
Preoperatively, a decision concerning the planned osteotomy and the correct locking plate size is made. In addition, the outline of the plate is marked on the skin. Under general anesthesia, the patients are prepared and draped. A tourniquet is elevated around the upper thigh. Then, 2 half-pins are medially inserted, 1 proximal and 1 distal to the planned osteotomy site, and are then connected loosely with a monolateral external fixator (Figure 2A). A lateral approach to the distal femur is done, preserving the periosteum, except at the level of the osteotomy. After the osteotomy is performed (through an open lateral incision), both segments are translated (Figure 2B) and then the distal segment is angulated to achieve the desired deformity correction, and the desired position is then stabilized by tightening the external fixator connectors (Figure 2C). Subsequently, a locking plate is inserted in the submuscular-extraperiostal plane. The plate does not require being in full contact (flush) with the bone. At least 3 screws are placed on both sides of the osteotomy through a long lateral incision (Figure 2D). Bone graft may be added to the osteotomy site to encourage healing. Then, the external fixator is removed, and all incisions are closed (Figure 2E).15,19
During each of the procedures, we aimed at having “perfect alignment” with a MAD of 0 mm, in which a Bovie cord is used and passed through the center of the femoral head, knee, and ankle. However, to confirm that the surgery was successful, the actual measurements were performed on standing long-leg films. These films were obtained preoperatively and at latest follow-up. They were performed with the patella aiming forward, the toes straight ahead, feet separated enough for good balance, knees fully extended, and weight equally distributed on the feet. Postoperatively, in both cohorts, partial weight-bearing was encouraged immediately with crutches; physical therapy was instituted daily for knee range of motion. Radiographs were scheduled every 4 weeks to monitor callus formation. Full weight-bearing was allowed when at least 3 cortices were consolidated.1,15,19,20,25,26
All statistical analyses were performed with the aid of the SPSS statistical software package (SPSS). Average values and standard error of the mean were assigned to each variable. A nonparametric Mann-Whitney U test was used, and a 2-tailed P < .05 was considered significant. Correlation of continuous variables was determined by Spearman’s correlation coefficient. Also, multivariate Cox regression analyses after adjustment for age, sex, and deformity correction were used to detect associations within the study population. To evaluate whether our data were normally distributed, Shapiro-Wilk tests were performed.
Continue to: Results...
RESULTS
The mLDFA significantly improved in the FAN cohort from a mean of 81° to a mean of 89° (ranges, 67°-86° and 80°-100°; respectively; P = .001) (Figures 3A, 3B).
| Table 2. Deformity Correction | ||||
| Measurement | Cohort | Preoperative | Postoperative | P Value |
| mLDFA in degrees (range) | FAN | 81 (67 to 86) | 89 (80 to 100) | 0.001 |
| FALP | 80 (71 to 87) | 90 (88 to 94) | <0.001 | |
| Mechanical axis deviation in mm (range) | FAN | 32 (6 to 64) | 10 (0 to 22) | 0.001 |
| FALP | 34 (17 to 62) | 4 (0 to 11) | 0.002 | |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing; mLDFA, mechanical lateral distal femoral angle
After evaluating the MPTA, in the FAN cohort, we found that the mean pre- and postoperative MPTAs were not modified. These patients had a mean preoperative angle of 88° (range, 62°-100°), which was kept postoperatively to a mean of 88° (range, 78°-96°). In the FALP cohort, a slight change from 90° to 88° was observed (ranges, 82°-97° and 83°-94°, respectively). None of these changes in MPTA were significant (P > .05).
When evaluating correction of the MAD, we observed that the FAN cohort changed from a preoperative MAD of 32 mm (range, 6-64 mm) to a postoperative mean of 10 mm (range, 0-22 mm), and this correction was statistically significant. (P = .001). The FALP cohort changed from a mean of 34 mm (range, 17-62 mm) preoperatively to 4 mm (range, 0-11 mm) postoperatively, and this was also statistically significant (P = .002). The mean MAD correction for the FAN group vs FALP group was 27 mm vs 32 mm, respectively (Table 2).
In patients with valgus femoral deformity, the MAD is usually lateralized; however, in the FAN cohort, we included 3 patients with medial MADs (10 mm, 13 mm, and 40 mm). This is justified in these patients because a complex deformity of the distal femur and the proximal tibia was present. In the extreme case of a 40-mm medial MAD, the presurgery mLDFA was 76°, and the presurgery MPTA was 62°. The amount of deformity correction in this patient was 16°.
During the follow-up period, 2 complications occurred in the FAN group. One patient developed gait disturbance that resolved with physical therapy. Another had an infection at the osteotomy site. This was addressed with intravenous antibiotic therapy, surgical irrigation and débridement, hardware removal, and antegrade insertion of an antibiotic-coated nail. In the FALP group, 1 patient developed a persistent incomplete peroneal nerve palsy attributed to a 17° correction from valgus to varus, despite prophylactic peroneal nerve decompression. Nonetheless, the patient was satisfied with the result, recovered partial nerve function, and returned for correction of the contralateral leg deformity. When comparing the complications between both cohorts, no significant differences were found: 2 of 18 cases (11%) in the FAN group vs 1 of 6 cases (17%) in the FALP group (P = .78).
Continue to: The goal of this study...
DISCUSSION
The goal of this study was to compare the accuracy of deformity corrections achieved with either FAN or FALP. A number of authors have described results after deformity correction with several plating and nailing techniques; however, the information derived from comparing these 2 techniques is limited. We hypothesized that FALP would be more accurate, because less mobilization during fixation is required. However, we found no significant differences between these 2 techniques.
This study has several limitations. First, the small size of our cohort had to be further reduced owing to limited data; nevertheless, this pathology and the treatment methods used are not commonly performed, which make this cohort 1 of the largest of its type described in the literature. Also, the procedures were performed by multiple surgeons in a population with a wide age range, creating multiple additional variables that complicate the comparison of the sole differences between FAN and FALP. However, owing to these variables, the generalizability of this study may be increased, and similar outcomes can potentially be obtained by other institutions/surgeons. In addition, the variability of our follow-up period is another limitation; however, these patients were all assessed until bony union after skeletal maturity was achieved. Hence, the development of additional deformity is not expected. The lack of clinical outcome with a standardized questionnaire may also be seen as a limitation. However, because the purpose of our study was to assess both surgeries in terms of their ability to achieve angular correction, the addition of patient-reported outcomes may have increased the variability of our data.
The foremost objective in valgus deformity correction is to establish joint orientation angles within anatomic range to prevent overloading of the lateral joint and thereby prevent lateral compartmental osteoarthritis.2,20,27-29 There are 2 categories of fixation: internal and external. With FAN and FALP, we strive to have the adjustability and accuracy of external fixation with the comfort (for the patient) of internal fixation. Accurate osteotomy correction requires an accurate preoperative analysis and osteotomy close to the apex of the deformity.16,21,30-33 The most commonly used osteotomy techniques are drill-hole,31 focal dome,34 rotation, and open- or closed-wedge osteotomies.35,36 After the osteotomy, the resultant correction has to be stabilized. In recent years, the popularity of plates instead of an IM nail for internal fixation has been driven by the rapid development of low contact locking plates.16,19,26,30,37-40
There are certain advantages of using FAN over FALP. In older patients who may require a subsequent total knee arthroplasty (TKA), the midline incision used for retrograde FAN technique is identical to that made for TKA. In contrast, in a younger and more active population, with a longer life expectancy, the extra-articular FALP approach has the advantage of not violating the knee joint. In addition, locking plates may achieve a more rigid fixation than IM nails; however, the stability of IM nails can be augmented with blocking screws.
Continue to: In 20 patients, including children...
In 20 patients, including children and young adults, with frontal and sagittal plane deformities, Marangoz and colleagues7 reported on correction of valgus, varus, and procurvatum deformities using a Taylor Spatial Frame (TSF). Successful correction of severe deformities was achieved gradually with the TSF, resulting in a postoperative deformity (valgus group) of mLDFA 88.9° (range, 85°-95°).7 In a more recent study, Bar-On and colleagues15 described a series of 11 patients (18 segments) with corrective lower limb osteotomies in which all were corrected to within 2° of the planned range. Similarly, Gugenheim and Brinker20 described the use of the FAN technique to correct distal varus and valgus deformities in 14 femora. The final mean mLDFA and MAD in the valgus group were 89° (range, 88°-90°) and 5 mm (range, 0-14 mm medial), respectively.
In their comparative study, Seah and colleagues11 described monolateral frame vs FALP deformity correction in a series of 34 extremities (26 patients) that required distal femoral osteotomy. No differences related to knee range of motion or the ability to correct the deformity between internal and external fixation were reported (P > .05). Similarly, Eidelman and colleagues1 evaluated the outcomes of 6 patients (7 procedures) who underwent surgery performed with the FALP technique for distal femoral valgus deformity. They concluded that this technique is minimally invasive and can provide a precise deformity correction with minimal morbidity.
Other methods of fixation while performing FAN have been described by Jasiewicz and colleagues,22 who evaluated possible differences between the classic Ilizarov device and monolateral fixators in 19 femoral lengthening procedures. The authors concluded that there is no difference between concerning complication rate and treatment time. The use of FAN has also been described in patients with metabolic disease who required deformity correction. In this regard, Kocaoglu and colleagues12 described the use of a monolateral external fixator in combination with an IM nail in a series of 17 patients with metabolic bone disease. The authors concluded that the use of the IM nail prevented recurrence of deformity and refracture.12 Kocaoglu and colleagues14 also published a series of 25 patients treated with the FAN and LON (lengthening over a nail) technique for lengthening and deformity correction. The mean MAD improved from 33.9 mm to11.3 mm (range, 0-30 mm). In contrast, Erlap and colleagues13 compared FAN with circular external fixator for bone realignment of the lower extremity for deformities in patients with rickets. Although no significant difference was found between both groups, FAN was shown to be accurate and to provide great comfort to patients, and it also shortened the total treatment time.13 Finally, the advent of newer technologies could also provide alternatives for correcting valgus deformities. For example, Saragaglia and Chedal-Bornu6 performed 29 computer-assisted valgus knees osteotomies (27 patients) and reported that the goal hip-knee angle was achieved in 86% of patients and that the goal MPTA was achieved in 100% of patients.6
CONCLUSION
Both the FALP and FAN methods of femoral deformity correction are safe and effective surgical techniques. In our opinion, the advantages of the FALP technique result from the easy lateral surgical approach under medial external fixation and stabilization of the osteotomy without bending the knee. Ultimately, the decision to use FAN may be influenced by the surgeon’s perception of the potential need for future TKA. In such cases, a midline anterior approach with nailing is very compatible with subsequent TKA. The surgeon’s experience and preference, while keeping in mind the patient’s predilection, will play an important role in the decision-making process. Larger prospective clinical trials with larger cohorts have to be conducted to confirm our findings.
ABSTRACT
Fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP) are 2 techniques that can be used to correct distal femoral valgus deformities. The fixator aids in achieving an accurate adjustable initial reduction, which is then made permanent with either nail or plate insertion. FALP can be performed with the knee held in a neutral extended position, whereas FAN requires 30° to 90° of knee flexion to insert the nail, which may cause some alignment loss. We hypothesized that FAN may yield less accurate correction than FALP. Prospectively collected data of a consecutive cohort of patients who underwent valgus deformity femoral correction with FAN or FALP at a single institution over an 8-year period were retrospectively evaluated. Twenty extremities (18 patients) were treated using FAN (median follow-up, 5 years; range, 1-10 years), and 7 extremities (6 patients) were treated with FALP (median follow-up, 5 years; range, 1-8 years). In the FAN cohort, the mean preoperative and postoperative mechanical lateral distal femoral angles (mLDFAs) were 81° (range, 67°-86°) and 89° (range, 80°-100°), respectively (P = .009). In the FALP cohort, the mean preoperative and postoperative mLDFAs were 80° (range, 71°-87°) and 88° (range, 81°-94°), respectively (P < .001). Although the average mechanical axis deviation correction for the FALP group was greater than for the FAN group (32 mm and 27 mm, respectively), the difference was not significant (P = .66). Both methods of femoral deformity correction can be considered safe and effective. On the basis of our results, FAN and FALP are comparable in accuracy for deformity correction in the distal femur.
Multiple etiologies for distal femoral valgus deformity have been described in the literature.1-3 These can be congenital, developmental, secondary to lateral compartmental arthritis, or posttraumatic.4 If not corrected, femoral deformities alter the axial alignment and orientation of the joints, and may lead to early degenerative joint disease and abnormal leg kinematics.3,5 After correcting these deformities, the goal of treatment is to obtain anatomic distal femoral angles and neutral mechanical axis deviation (MAD), but without overcorrecting into varus. Numerous techniques to fix these deformities, such as progressive correction with external fixation or acute correction open reduction with internal fixation (ORIF), have been described.6 Modern external fixation allows for a gradual, adjustable, and more accurate correction but may produce discomfort and complications for patients.7-10 In contrast, ORIF may be more tolerable for the patient, but to achieve a precise correction, considerable technical skills and expertise are required.1,11-14
Two techniques used to correct these valgus femoral deformities in adults are fixator-assisted nailing (FAN) and fixator-assisted locked plating (FALP).1 FAN and FALP combine the advantage of external fixation (accuracy, adjustability) with the benefits of internal fixation (patient comfort), because the osteotomy and correction are performed with the guidance of a temporary external fixator and then permanently fixated by an intramedullary (IM) nail or a locking plate.1,8,11-13,15-18 Both techniques have the possibility to correct varus and valgus deformities, but whenever correcting sagittal plane angulation, the FAN technique may be more challenging. The paucity of studies available involving FAN and FALP do not lead to a conclusive preference of one technique over the other relative to the accuracy and success of correction.15,19,20
Continue to: In both FAN and FALP
In both FAN and FALP, the external fixator is applied and adjusted after the osteotomy for accurate alignment. In FALP, the plate is added without moving the leg from its straight position. However, in FAN, the knee must be flexed to 30° to 90° for insertion of the retrograde knee nail, and the alignment may be lost if the external fixation is not fully stable. Therefore, we hypothesized that FAN would be less accurate than FALP. Hence, the purposes of this study is to compare the correction achieved with FAN and FALP in patients with distal femoral valgus deformities and to describe the intraoperative complications associated with both techniques.
MATERIALS AND METHODS
After proper Institutional Review Board approval was obtained, a consecutive cohort of 35 patients who underwent femoral deformity correction with either FAN or FALP during an 8-year period (January 2002 to December 2010) was retrospectively reviewed. Eleven patients had to be excluded because of inadequate follow-up (<12 months) or because additional procedures were simultaneously performed. A total of 24 patients (27 femora) who had a mean age of 26 years (range, 14-68 years) were included in the final study cohort. Specifically, 20 femora (18 patients) were corrected using the FAN technique (7 males and 11 females; mean age, 36 years; range, 14-68 years), and 7 femora (6 patients) were fixed using the FALP technique (2 males and 4 females; mean age, 16 years; range, 15-19 years). The median follow-up in the FAN cohort was 5 years (range, 1-10 years), and the median follow-up in the FALP cohort was 5 years (range, 1-8 years) (Table 1).
| Table 1. Study Details and Demographic Characteristics | |||
| Detail | Overall | FAN | FALP |
| Number of patients | 24 | 18 | 6 |
| Number of femurs | 27 | 20 | 7 |
| Age in years (range) | 26 (14 to 68) | 36 (14 to 68) | 16 (15 to 19) |
| Male:Female | 9:15 | 7:11 | 2:4 |
| Median follow-up in years (range) | 5 (1 to 10) | 5 (1 to 10) | 5 (1 to 8) |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing
The specific measurements performed in all patients were MAD, mechanical lateral distal femoral angle (mLDFA), and medial proximal tibia angle (MPTA). These were measured from standing anteroposterior radiographs of the knee that included the femur.21 All outcome data were collected from the medical charts, operative reports, and radiographic evaluations. To ensure accuracy, all measurements were performed by 2 authors blinded to each other’s measurements. If a variation of <5% was obtained, the results were averaged and used for further analysis. Whenever a difference of >5% was obtained, the measurement was repeated by both authors for confirmation.
SURGICAL FAN TECHNIQUE
After measuring the deformity (Figure 1A) with the patient under general anesthesia on a radiolucent table, the involved lower limb is prepared and draped. Two half-pins are inserted medially, 1 proximal and 1 distal to the planned osteotomy site (Figure 1B), and then connected loosely with a monolateral external fixator. Special care is taken while placing the half-pins, not to interfere with the insertion path of the IM rod. When performing the preoperative planning, the level of osteotomy is chosen to enable the placement of at least 2 interlocking screws distal to the osteotomy. Then, a percutaneous osteotomy is performed from a lateral approach, and the bone ends are manipulated (translation and then angulation) to achieve the desired deformity correction. The external fixator is then stabilized and locked in the exact position (Figure 1C). Subsequently, retrograde reaming, nail insertion, and placement of proximal and distal locking screws are performed (Figure 1D). Blocking screws may give additional stability. The removal of the external fixator is the final step (Figure 1E).20
Continue to: When using the FAN technique...
When using the FAN technique, special attention is paid to reducing the risk of fat embolism. This can be reduced but not totally eradicated with the use of reaming irrigation devices.22-24 In our technique of FAN, the bone is cut and displaced prior to reaming so that the pressure of reaming is vented out through the osteotomy, along with the reaming contents, which theoretically can then act as a “prepositioned bone graft” that may speed healing.
SURGICAL FALP TECHNIQUE
Preoperatively, a decision concerning the planned osteotomy and the correct locking plate size is made. In addition, the outline of the plate is marked on the skin. Under general anesthesia, the patients are prepared and draped. A tourniquet is elevated around the upper thigh. Then, 2 half-pins are medially inserted, 1 proximal and 1 distal to the planned osteotomy site, and are then connected loosely with a monolateral external fixator (Figure 2A). A lateral approach to the distal femur is done, preserving the periosteum, except at the level of the osteotomy. After the osteotomy is performed (through an open lateral incision), both segments are translated (Figure 2B) and then the distal segment is angulated to achieve the desired deformity correction, and the desired position is then stabilized by tightening the external fixator connectors (Figure 2C). Subsequently, a locking plate is inserted in the submuscular-extraperiostal plane. The plate does not require being in full contact (flush) with the bone. At least 3 screws are placed on both sides of the osteotomy through a long lateral incision (Figure 2D). Bone graft may be added to the osteotomy site to encourage healing. Then, the external fixator is removed, and all incisions are closed (Figure 2E).15,19
During each of the procedures, we aimed at having “perfect alignment” with a MAD of 0 mm, in which a Bovie cord is used and passed through the center of the femoral head, knee, and ankle. However, to confirm that the surgery was successful, the actual measurements were performed on standing long-leg films. These films were obtained preoperatively and at latest follow-up. They were performed with the patella aiming forward, the toes straight ahead, feet separated enough for good balance, knees fully extended, and weight equally distributed on the feet. Postoperatively, in both cohorts, partial weight-bearing was encouraged immediately with crutches; physical therapy was instituted daily for knee range of motion. Radiographs were scheduled every 4 weeks to monitor callus formation. Full weight-bearing was allowed when at least 3 cortices were consolidated.1,15,19,20,25,26
All statistical analyses were performed with the aid of the SPSS statistical software package (SPSS). Average values and standard error of the mean were assigned to each variable. A nonparametric Mann-Whitney U test was used, and a 2-tailed P < .05 was considered significant. Correlation of continuous variables was determined by Spearman’s correlation coefficient. Also, multivariate Cox regression analyses after adjustment for age, sex, and deformity correction were used to detect associations within the study population. To evaluate whether our data were normally distributed, Shapiro-Wilk tests were performed.
Continue to: Results...
RESULTS
The mLDFA significantly improved in the FAN cohort from a mean of 81° to a mean of 89° (ranges, 67°-86° and 80°-100°; respectively; P = .001) (Figures 3A, 3B).
| Table 2. Deformity Correction | ||||
| Measurement | Cohort | Preoperative | Postoperative | P Value |
| mLDFA in degrees (range) | FAN | 81 (67 to 86) | 89 (80 to 100) | 0.001 |
| FALP | 80 (71 to 87) | 90 (88 to 94) | <0.001 | |
| Mechanical axis deviation in mm (range) | FAN | 32 (6 to 64) | 10 (0 to 22) | 0.001 |
| FALP | 34 (17 to 62) | 4 (0 to 11) | 0.002 | |
Abbreviations: FALP, fixator assisted locked plating; FAN, fixator assisted nailing; mLDFA, mechanical lateral distal femoral angle
After evaluating the MPTA, in the FAN cohort, we found that the mean pre- and postoperative MPTAs were not modified. These patients had a mean preoperative angle of 88° (range, 62°-100°), which was kept postoperatively to a mean of 88° (range, 78°-96°). In the FALP cohort, a slight change from 90° to 88° was observed (ranges, 82°-97° and 83°-94°, respectively). None of these changes in MPTA were significant (P > .05).
When evaluating correction of the MAD, we observed that the FAN cohort changed from a preoperative MAD of 32 mm (range, 6-64 mm) to a postoperative mean of 10 mm (range, 0-22 mm), and this correction was statistically significant. (P = .001). The FALP cohort changed from a mean of 34 mm (range, 17-62 mm) preoperatively to 4 mm (range, 0-11 mm) postoperatively, and this was also statistically significant (P = .002). The mean MAD correction for the FAN group vs FALP group was 27 mm vs 32 mm, respectively (Table 2).
In patients with valgus femoral deformity, the MAD is usually lateralized; however, in the FAN cohort, we included 3 patients with medial MADs (10 mm, 13 mm, and 40 mm). This is justified in these patients because a complex deformity of the distal femur and the proximal tibia was present. In the extreme case of a 40-mm medial MAD, the presurgery mLDFA was 76°, and the presurgery MPTA was 62°. The amount of deformity correction in this patient was 16°.
During the follow-up period, 2 complications occurred in the FAN group. One patient developed gait disturbance that resolved with physical therapy. Another had an infection at the osteotomy site. This was addressed with intravenous antibiotic therapy, surgical irrigation and débridement, hardware removal, and antegrade insertion of an antibiotic-coated nail. In the FALP group, 1 patient developed a persistent incomplete peroneal nerve palsy attributed to a 17° correction from valgus to varus, despite prophylactic peroneal nerve decompression. Nonetheless, the patient was satisfied with the result, recovered partial nerve function, and returned for correction of the contralateral leg deformity. When comparing the complications between both cohorts, no significant differences were found: 2 of 18 cases (11%) in the FAN group vs 1 of 6 cases (17%) in the FALP group (P = .78).
Continue to: The goal of this study...
DISCUSSION
The goal of this study was to compare the accuracy of deformity corrections achieved with either FAN or FALP. A number of authors have described results after deformity correction with several plating and nailing techniques; however, the information derived from comparing these 2 techniques is limited. We hypothesized that FALP would be more accurate, because less mobilization during fixation is required. However, we found no significant differences between these 2 techniques.
This study has several limitations. First, the small size of our cohort had to be further reduced owing to limited data; nevertheless, this pathology and the treatment methods used are not commonly performed, which make this cohort 1 of the largest of its type described in the literature. Also, the procedures were performed by multiple surgeons in a population with a wide age range, creating multiple additional variables that complicate the comparison of the sole differences between FAN and FALP. However, owing to these variables, the generalizability of this study may be increased, and similar outcomes can potentially be obtained by other institutions/surgeons. In addition, the variability of our follow-up period is another limitation; however, these patients were all assessed until bony union after skeletal maturity was achieved. Hence, the development of additional deformity is not expected. The lack of clinical outcome with a standardized questionnaire may also be seen as a limitation. However, because the purpose of our study was to assess both surgeries in terms of their ability to achieve angular correction, the addition of patient-reported outcomes may have increased the variability of our data.
The foremost objective in valgus deformity correction is to establish joint orientation angles within anatomic range to prevent overloading of the lateral joint and thereby prevent lateral compartmental osteoarthritis.2,20,27-29 There are 2 categories of fixation: internal and external. With FAN and FALP, we strive to have the adjustability and accuracy of external fixation with the comfort (for the patient) of internal fixation. Accurate osteotomy correction requires an accurate preoperative analysis and osteotomy close to the apex of the deformity.16,21,30-33 The most commonly used osteotomy techniques are drill-hole,31 focal dome,34 rotation, and open- or closed-wedge osteotomies.35,36 After the osteotomy, the resultant correction has to be stabilized. In recent years, the popularity of plates instead of an IM nail for internal fixation has been driven by the rapid development of low contact locking plates.16,19,26,30,37-40
There are certain advantages of using FAN over FALP. In older patients who may require a subsequent total knee arthroplasty (TKA), the midline incision used for retrograde FAN technique is identical to that made for TKA. In contrast, in a younger and more active population, with a longer life expectancy, the extra-articular FALP approach has the advantage of not violating the knee joint. In addition, locking plates may achieve a more rigid fixation than IM nails; however, the stability of IM nails can be augmented with blocking screws.
Continue to: In 20 patients, including children...
In 20 patients, including children and young adults, with frontal and sagittal plane deformities, Marangoz and colleagues7 reported on correction of valgus, varus, and procurvatum deformities using a Taylor Spatial Frame (TSF). Successful correction of severe deformities was achieved gradually with the TSF, resulting in a postoperative deformity (valgus group) of mLDFA 88.9° (range, 85°-95°).7 In a more recent study, Bar-On and colleagues15 described a series of 11 patients (18 segments) with corrective lower limb osteotomies in which all were corrected to within 2° of the planned range. Similarly, Gugenheim and Brinker20 described the use of the FAN technique to correct distal varus and valgus deformities in 14 femora. The final mean mLDFA and MAD in the valgus group were 89° (range, 88°-90°) and 5 mm (range, 0-14 mm medial), respectively.
In their comparative study, Seah and colleagues11 described monolateral frame vs FALP deformity correction in a series of 34 extremities (26 patients) that required distal femoral osteotomy. No differences related to knee range of motion or the ability to correct the deformity between internal and external fixation were reported (P > .05). Similarly, Eidelman and colleagues1 evaluated the outcomes of 6 patients (7 procedures) who underwent surgery performed with the FALP technique for distal femoral valgus deformity. They concluded that this technique is minimally invasive and can provide a precise deformity correction with minimal morbidity.
Other methods of fixation while performing FAN have been described by Jasiewicz and colleagues,22 who evaluated possible differences between the classic Ilizarov device and monolateral fixators in 19 femoral lengthening procedures. The authors concluded that there is no difference between concerning complication rate and treatment time. The use of FAN has also been described in patients with metabolic disease who required deformity correction. In this regard, Kocaoglu and colleagues12 described the use of a monolateral external fixator in combination with an IM nail in a series of 17 patients with metabolic bone disease. The authors concluded that the use of the IM nail prevented recurrence of deformity and refracture.12 Kocaoglu and colleagues14 also published a series of 25 patients treated with the FAN and LON (lengthening over a nail) technique for lengthening and deformity correction. The mean MAD improved from 33.9 mm to11.3 mm (range, 0-30 mm). In contrast, Erlap and colleagues13 compared FAN with circular external fixator for bone realignment of the lower extremity for deformities in patients with rickets. Although no significant difference was found between both groups, FAN was shown to be accurate and to provide great comfort to patients, and it also shortened the total treatment time.13 Finally, the advent of newer technologies could also provide alternatives for correcting valgus deformities. For example, Saragaglia and Chedal-Bornu6 performed 29 computer-assisted valgus knees osteotomies (27 patients) and reported that the goal hip-knee angle was achieved in 86% of patients and that the goal MPTA was achieved in 100% of patients.6
CONCLUSION
Both the FALP and FAN methods of femoral deformity correction are safe and effective surgical techniques. In our opinion, the advantages of the FALP technique result from the easy lateral surgical approach under medial external fixation and stabilization of the osteotomy without bending the knee. Ultimately, the decision to use FAN may be influenced by the surgeon’s perception of the potential need for future TKA. In such cases, a midline anterior approach with nailing is very compatible with subsequent TKA. The surgeon’s experience and preference, while keeping in mind the patient’s predilection, will play an important role in the decision-making process. Larger prospective clinical trials with larger cohorts have to be conducted to confirm our findings.
1. Eidelman M, Keren Y, Norman D. Correction of distal femoral valgus deformities in adolescents and young adults using minimally invasive fixator-assisted locking plating (FALP). J Pediatr Orthop B. 2012;21(6):558-562. doi:10.1097/BPB.0b013e328358f884.
2. Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi:10.1186/ar2272.
3. Solomin LN, Paley D, Shchepkina EA, Vilensky VA, Skomoroshko PV. A comparative study of the correction of femoral deformity between the Ilizarov apparatus and ortho-SUV Frame. Int Orthop. 2014;38(4):865-872. doi:10.1007/s00264-013-2247-0.
4. Meric G, Gracitelli GC, Aram LJ, Swank ML, Bugbee WD. Variability in distal femoral anatomy in patients undergoing total knee arthroplasty: measurements on 13,546 computed tomography scans. J Arthroplasty. 2015;30(10):1835-1838. doi:10.1016/j.arth.2015.04.024.
5. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015. doi:10.1007/s11999-014-4106-8.
6. Saragaglia D, Chedal-Bornu B. Computer-assisted osteotomy for valgus knees: medium-term results of 29 cases. Orthop Traumatol Surg Res. 2014;100(5):527-530. doi:10.1016/j.otsr.2014.04.002.
7. Marangoz S, Feldman DS, Sala DA, Hyman JE, Vitale MG. Femoral deformity correction in children and young adults using Taylor Spatial Frame. Clin Orthop Relat Res. 2008;466(12):3018-3024. doi:10.1007/s11999-008-0490-2.
8. Rogers MJ, McFadyen I, Livingstone JA, Monsell F, Jackson M, Atkins RM. Computer hexapod assisted orthopaedic surgery (CHAOS) in the correction of long bone fracture and deformity. J Orthop Trauma. 2007;21(5):337-342. doi:10.1097/BOT.0b013e3180463103.
9. Feldman DS, Madan SS, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara: acute versus gradual correction. J Pediatr Orthop. 2006;26(6):794-798. doi:10.1097/01.bpo.0000242375.64854.3d.
10. Manner HM, Huebl M, Radler C, Ganger R, Petje G, Grill F. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1(1):55-61. doi:10.1007/s11832-006-0005-1.
11. Seah KT, Shafi R, Fragomen AT, Rozbruch SR. Distal femoral osteotomy: is internal fixation better than external? Clin Orthop Relat Res. 2011;469(7):2003-2011. doi:10.1007/s11999-010-1755-0.
12. Kocaoglu M, Bilen FE, Sen C, Eralp L, Balci HI. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52-56. doi:10.1302/0301-620X.93B1.24788.
13. Eralp L, Kocaoglu M, Toker B, Balcı HI, Awad A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch Orthop Trauma Surg. 2011;131(5):581-589. doi:10.1007/s00402-010-1162-8.
14. Kocaoglu M, Eralp L, Bilen FE, Balci HI. Fixator-assisted acute femoral deformity correction and consecutive lengthening over an intramedullary nail. J Bone Joint Surg Am. 2009;91(1):152-159. doi:10.2106/JBJS.H.00114.
15. Bar-On E, Becker T, Katz K, Velkes S, Salai M, Weigl DM. Corrective lower limb osteotomies in children using temporary external fixation and percutaneous locking plates. J Child Orthop. 2009;3(2):137-143. doi:10.1007/s11832-009-0165-x.
16. Herzenberg JE, Kovar FM. External fixation assisted nailing (EFAN) and external fixation assisted plating (EFAP) for deformity correction. In: Solomin LN, ed. The Basic Principles of External Fixation Using the Ilizarov and Other Devices. 2nd ed. Italy: Springer-Verlag; 2012:1363-1378.
17. Eralp L, Kocaoglu M, Cakmak M, Ozden VE. A correction of windswept deformity by fixator assisted nailing. A report of two cases. J Bone Joint Surg Br. 2004;86(7):1065-1068.
18. Eralp L, Kocaoglu M. Distal tibial reconstruction with use of a circular external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 2):181-194. doi:10.2106/JBJS.H.00467.
19. Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(Suppl 2):B63-B76. doi:10.1016/j.injury.2003.09.026.
20. Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85–A(7):1229-1237. doi:10.2106/00004623-200307000-00008.
21. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
22. Jasiewicz B, Kacki W, Tesiorowski M, Potaczek T. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73(3):177-183.
23. Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89(11):1421-1426. doi:10.1302/0301-620X.89B11.19570.
24. Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202-207. doi:10.1097/00005373-199402000-00010.
25. Gordon JE, Goldfarb CA, Luhmann SJ, Lyons D, Schoenecker PL. Femoral lengthening over a humeral intramedullary nail in preadolescent children. J Bone Joint Surg Am. 2002;84–A(6):930-937. doi:10.2106/00004623-200206000-00006.
26. Oh CW, Song HR, Kim JW, et al. Deformity correction with submuscular plating technique in children. J Pediatr Orthop B. 2010;19(1):47-54. doi:10.1097/BPB.0b013e32832f5b06.
27. Guettler J, Glisson R, Stubbs A, Jurist K, Higgins L. The triad of varus malalignment, meniscectomy, and chondral damage: a biomechanical explanation for joint degeneration. Orthopedics. 2007;30(7):558-566.
28. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. doi:10.1002/art.23462.
29. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459-467. doi:10.1002/art.24336.
30. Paley D, HJ, Bor N. Fixator-assisted nailing of femoral and tibial deformities. Tech Orthop. 1997;12(4):260-275.
31. Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124(5):298-300. doi:10.1007/s00402-004-0646-9.
32. Strecker W, Kinzl L, Keppler P. Corrective osteotomies of the distal femur with retrograde intramedullary nail. Unfallchirurg. 2001;104(10):973-983. doi:10.1007/s001130170040.
33. Watanabe K, Tsuchiya H, Sakurakichi K, Matsubara H, Tomita K. Acute correction using focal dome osteotomy for deformity about knee joint. Arch Orthop Trauma Surg. 2008;128(12):1373-1378. doi:10.1007/s00402-008-0574-1.
34. Hankemeier S, Paley D, Pape HC, Zeichen J, Gosling T, Krettek C. Knee para-articular focal dome osteotomy. Orthopade. 2004;33(2):170-177. doi:10.1007/s00132-003-0588-x.
35. Brinkman JM, Luites JW, Wymenga AB, van Heerwaarden RJ. Early full weight bearing is safe in open-wedge high tibial osteotomy. Acta Orthop. 2010;81(2):193-198. doi:10.3109/17453671003619003.
36. Hankemeier S, Mommsen P, Krettek C, et al. Accuracy of high tibial osteotomy: comparison between open- and closed-wedge technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1328-1333. doi:10.1007/s00167-009-1020-9.
37. Hedequist D, Bishop J, Hresko T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008;28(1):6-9. doi:10.1097/bpo.0b013e31815ff301.
38. Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007;27(5):504-509. doi:10.1097/01.bpb.0000279020.96375.88.
39. Uysal M, Akpinar S, Cesur N, Hersekli MA, Tandoğan RN. Plating after lengthening (PAL): technical notes and preliminary clinical experiences. Arch Orthop Trauma Surg. 2007;127(10):889-893. doi:10.1007/s00402-007-0442-4.
40. Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. Instr Course Lect. 2008;57:25-36.
1. Eidelman M, Keren Y, Norman D. Correction of distal femoral valgus deformities in adolescents and young adults using minimally invasive fixator-assisted locking plating (FALP). J Pediatr Orthop B. 2012;21(6):558-562. doi:10.1097/BPB.0b013e328358f884.
2. Pelletier JP, Raynauld JP, Berthiaume MJ, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi:10.1186/ar2272.
3. Solomin LN, Paley D, Shchepkina EA, Vilensky VA, Skomoroshko PV. A comparative study of the correction of femoral deformity between the Ilizarov apparatus and ortho-SUV Frame. Int Orthop. 2014;38(4):865-872. doi:10.1007/s00264-013-2247-0.
4. Meric G, Gracitelli GC, Aram LJ, Swank ML, Bugbee WD. Variability in distal femoral anatomy in patients undergoing total knee arthroplasty: measurements on 13,546 computed tomography scans. J Arthroplasty. 2015;30(10):1835-1838. doi:10.1016/j.arth.2015.04.024.
5. Cameron JI, McCauley JC, Kermanshahi AY, Bugbee WD. Lateral opening-wedge distal femoral osteotomy: pain relief, functional improvement, and survivorship at 5 years. Clin Orthop Relat Res. 2015;473(6):2009-2015. doi:10.1007/s11999-014-4106-8.
6. Saragaglia D, Chedal-Bornu B. Computer-assisted osteotomy for valgus knees: medium-term results of 29 cases. Orthop Traumatol Surg Res. 2014;100(5):527-530. doi:10.1016/j.otsr.2014.04.002.
7. Marangoz S, Feldman DS, Sala DA, Hyman JE, Vitale MG. Femoral deformity correction in children and young adults using Taylor Spatial Frame. Clin Orthop Relat Res. 2008;466(12):3018-3024. doi:10.1007/s11999-008-0490-2.
8. Rogers MJ, McFadyen I, Livingstone JA, Monsell F, Jackson M, Atkins RM. Computer hexapod assisted orthopaedic surgery (CHAOS) in the correction of long bone fracture and deformity. J Orthop Trauma. 2007;21(5):337-342. doi:10.1097/BOT.0b013e3180463103.
9. Feldman DS, Madan SS, Ruchelsman DE, Sala DA, Lehman WB. Accuracy of correction of tibia vara: acute versus gradual correction. J Pediatr Orthop. 2006;26(6):794-798. doi:10.1097/01.bpo.0000242375.64854.3d.
10. Manner HM, Huebl M, Radler C, Ganger R, Petje G, Grill F. Accuracy of complex lower-limb deformity correction with external fixation: a comparison of the Taylor Spatial Frame with the Ilizarov ring fixator. J Child Orthop. 2007;1(1):55-61. doi:10.1007/s11832-006-0005-1.
11. Seah KT, Shafi R, Fragomen AT, Rozbruch SR. Distal femoral osteotomy: is internal fixation better than external? Clin Orthop Relat Res. 2011;469(7):2003-2011. doi:10.1007/s11999-010-1755-0.
12. Kocaoglu M, Bilen FE, Sen C, Eralp L, Balci HI. Combined technique for the correction of lower-limb deformities resulting from metabolic bone disease. J Bone Joint Surg Br. 2011;93(1):52-56. doi:10.1302/0301-620X.93B1.24788.
13. Eralp L, Kocaoglu M, Toker B, Balcı HI, Awad A. Comparison of fixator-assisted nailing versus circular external fixator for bone realignment of lower extremity angular deformities in rickets disease. Arch Orthop Trauma Surg. 2011;131(5):581-589. doi:10.1007/s00402-010-1162-8.
14. Kocaoglu M, Eralp L, Bilen FE, Balci HI. Fixator-assisted acute femoral deformity correction and consecutive lengthening over an intramedullary nail. J Bone Joint Surg Am. 2009;91(1):152-159. doi:10.2106/JBJS.H.00114.
15. Bar-On E, Becker T, Katz K, Velkes S, Salai M, Weigl DM. Corrective lower limb osteotomies in children using temporary external fixation and percutaneous locking plates. J Child Orthop. 2009;3(2):137-143. doi:10.1007/s11832-009-0165-x.
16. Herzenberg JE, Kovar FM. External fixation assisted nailing (EFAN) and external fixation assisted plating (EFAP) for deformity correction. In: Solomin LN, ed. The Basic Principles of External Fixation Using the Ilizarov and Other Devices. 2nd ed. Italy: Springer-Verlag; 2012:1363-1378.
17. Eralp L, Kocaoglu M, Cakmak M, Ozden VE. A correction of windswept deformity by fixator assisted nailing. A report of two cases. J Bone Joint Surg Br. 2004;86(7):1065-1068.
18. Eralp L, Kocaoglu M. Distal tibial reconstruction with use of a circular external fixator and an intramedullary nail. Surgical technique. J Bone Joint Surg Am. 2008;90(suppl 2 Pt 2):181-194. doi:10.2106/JBJS.H.00467.
19. Gautier E, Sommer C. Guidelines for the clinical application of the LCP. Injury. 2003;34(Suppl 2):B63-B76. doi:10.1016/j.injury.2003.09.026.
20. Gugenheim JJ Jr, Brinker MR. Bone realignment with use of temporary external fixation for distal femoral valgus and varus deformities. J Bone Joint Surg Am. 2003;85–A(7):1229-1237. doi:10.2106/00004623-200307000-00008.
21. Paley D, Herzenberg JE, Tetsworth K, McKie J, Bhave A. Deformity planning for frontal and sagittal plane corrective osteotomies. Orthop Clin North Am. 1994;25(3):425-465.
22. Jasiewicz B, Kacki W, Tesiorowski M, Potaczek T. Results of femoral lengthening over an intramedullary nail and external fixator. Chir Narzadow Ruchu Ortop Pol. 2008;73(3):177-183.
23. Pape HC, Giannoudis P. The biological and physiological effects of intramedullary reaming. J Bone Joint Surg Br. 2007;89(11):1421-1426. doi:10.1302/0301-620X.89B11.19570.
24. Wozasek GE, Simon P, Redl H, Schlag G. Intramedullary pressure changes and fat intravasation during intramedullary nailing: an experimental study in sheep. J Trauma. 1994;36(2):202-207. doi:10.1097/00005373-199402000-00010.
25. Gordon JE, Goldfarb CA, Luhmann SJ, Lyons D, Schoenecker PL. Femoral lengthening over a humeral intramedullary nail in preadolescent children. J Bone Joint Surg Am. 2002;84–A(6):930-937. doi:10.2106/00004623-200206000-00006.
26. Oh CW, Song HR, Kim JW, et al. Deformity correction with submuscular plating technique in children. J Pediatr Orthop B. 2010;19(1):47-54. doi:10.1097/BPB.0b013e32832f5b06.
27. Guettler J, Glisson R, Stubbs A, Jurist K, Higgins L. The triad of varus malalignment, meniscectomy, and chondral damage: a biomechanical explanation for joint degeneration. Orthopedics. 2007;30(7):558-566.
28. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008;58(6):1716-1726. doi:10.1002/art.23462.
29. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009;61(4):459-467. doi:10.1002/art.24336.
30. Paley D, HJ, Bor N. Fixator-assisted nailing of femoral and tibial deformities. Tech Orthop. 1997;12(4):260-275.
31. Eralp L, Kocaoğlu M, Ozkan K, Türker M. A comparison of two osteotomy techniques for tibial lengthening. Arch Orthop Trauma Surg. 2004;124(5):298-300. doi:10.1007/s00402-004-0646-9.
32. Strecker W, Kinzl L, Keppler P. Corrective osteotomies of the distal femur with retrograde intramedullary nail. Unfallchirurg. 2001;104(10):973-983. doi:10.1007/s001130170040.
33. Watanabe K, Tsuchiya H, Sakurakichi K, Matsubara H, Tomita K. Acute correction using focal dome osteotomy for deformity about knee joint. Arch Orthop Trauma Surg. 2008;128(12):1373-1378. doi:10.1007/s00402-008-0574-1.
34. Hankemeier S, Paley D, Pape HC, Zeichen J, Gosling T, Krettek C. Knee para-articular focal dome osteotomy. Orthopade. 2004;33(2):170-177. doi:10.1007/s00132-003-0588-x.
35. Brinkman JM, Luites JW, Wymenga AB, van Heerwaarden RJ. Early full weight bearing is safe in open-wedge high tibial osteotomy. Acta Orthop. 2010;81(2):193-198. doi:10.3109/17453671003619003.
36. Hankemeier S, Mommsen P, Krettek C, et al. Accuracy of high tibial osteotomy: comparison between open- and closed-wedge technique. Knee Surg Sports Traumatol Arthrosc. 2010;18(10):1328-1333. doi:10.1007/s00167-009-1020-9.
37. Hedequist D, Bishop J, Hresko T. Locking plate fixation for pediatric femur fractures. J Pediatr Orthop. 2008;28(1):6-9. doi:10.1097/bpo.0b013e31815ff301.
38. Iobst CA, Dahl MT. Limb lengthening with submuscular plate stabilization: a case series and description of the technique. J Pediatr Orthop. 2007;27(5):504-509. doi:10.1097/01.bpb.0000279020.96375.88.
39. Uysal M, Akpinar S, Cesur N, Hersekli MA, Tandoğan RN. Plating after lengthening (PAL): technical notes and preliminary clinical experiences. Arch Orthop Trauma Surg. 2007;127(10):889-893. doi:10.1007/s00402-007-0442-4.
40. Smith WR, Ziran BH, Anglen JO, Stahel PF. Locking plates: tips and tricks. Instr Course Lect. 2008;57:25-36.
TAKE-HOME POINTS
- FAN and FALP are methods to improve the accuracy of long bone deformity correction.
- Both methods include temporary stabilization of the osteotomy with an external fixator.
- FALP is technically easier, since the external fixation pins do not have to be positioned out of the path of the nail, as in FAN.
- Acute corrections in the distal femur from valgus to varus can stretch the peroneal nerve.
- FAN and FALP are equivalent techniques for improving accuracy of deformity correction.