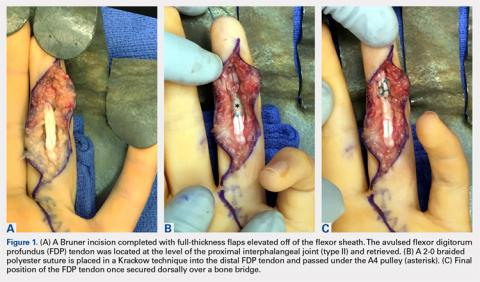
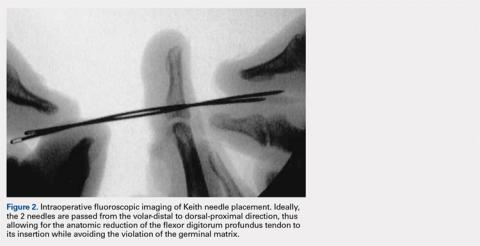
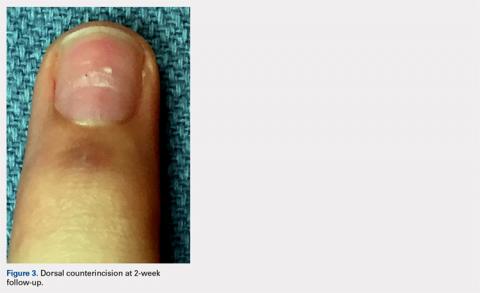
User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Looking at Ourselves
The genesis of the ongoing debate on healthcare is complex, in part because of the perceived flaws within the proposed systems. Denying the guilt that the medical profession has had is a disingenuous exercise in futility. In fact, orthopedics is possibly the most egregious among the culprits. Such a charge, supportable or not, calls for serious objective criticism.1 No one would dare question the fact that from the orthopedic point of view, the cost of healthcare delivery is an important factor because of the critical level this issue has reached. The use of expensive technology and frequent surgery are of major importance.2,3 However, I submit that it is not the high cost of the technology and surgical procedures per se but their frequent abuse.
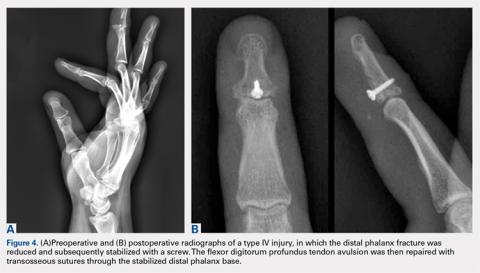
As I look at orthopedics in an objective, critical manner, I have the uncomfortable feeling that the profession is rapidly becoming a business where greater and greater profit is its primary raison d’etre. The discipline has lost much of its traditional scientific/biological foundations, and is converting itself into a technical trade heavily controlled in its educational duties and the subsequent conduct of its members by industry. This metamorphous evolution has shown ill effects as demonstrated by the loss of traditional territory to other disciplines and a borderline ridiculous fragmentation into a multitude of subspecialties that contribute to the erosion of the profession. Orthopedics is no longer a solid eclectic body of knowledge and expertise in the care of musculoskeletal conditions, but a fragmented body of techniques independent of each other. This statement is not a criticism of fragmentation per se, because fragmentation in most human endeavors is a natural evolutionary phenomenon that occurs in virtually all walks of life, and to our profession it has brought much progress. My concerns are over the apparent exaggerated degree it has reached.4
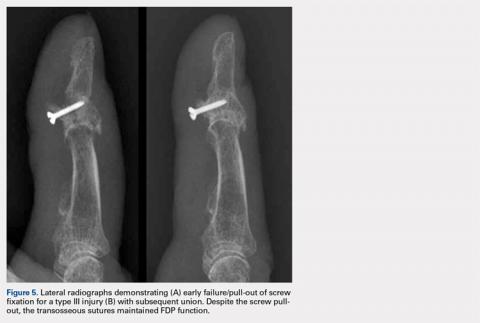
The fragmentation and erosion of orthopedics took a relatively short time to occur without any evidence of concern among the orthopedic community, which apparently assumed that the advances made by other disciplines would not compromise the security and independence it had enjoyed for generations. The spectacular advances in joint replacement began to occupy a large segment of orthopedists’ professional time. The attractive reimbursement accompanying these procedures further justified the complacency regarding the shrinking of the discipline, while the previous outsiders expanded their territory. Critical objectivity of this issue is important in the event we decide to address the consequences of further erosion and fragmentation of our profession.
There should be no question that if all, or the overwhelming majority of, orthopedists become subspecialists who take care of only a limited number of pathological conditions, the cost of care will grow exponentially. The poor, regardless of the outcome of any legislation addressing their problems, will suffer most.
In small communities there are not enough patients with conditions requiring subspecialized orthopedic services to satisfy the emotional and economic needs of the fellowship-trained orthopedists. Other physicians and allied health practitioners will fill the void and provide the needed services. However, the facts facing us today suggest that if the current trends continue unabated, orthopedics as a distinct branch of medicine may not survive. Nonetheless, people in need of musculoskeletal care will receive it from a variety of medical and paramedical people, who will gradually develop skills and knowledge in a manner comparable to that possessed today by orthopedists.
Continue to: Of major significance...
Of major significance in the overall issue of critical objectivity is the unquestionable fact that orthopedics voluntarily relegated to the surgical implant industry the control of many of its traditional educational responsibilities to the point where, at present, it is assumed that educational programs cannot be conducted without the financial support of industry. Hundreds, if not thousands, of conferences; grand rounds; local, state, and regional society meetings; the American Academy of Orthopaedic Surgeons’ (AAOS) annual meeting, and its many other educational activities take place with the financial support of industry. Such a dominance has placed the orthopedic profession in a relative subservient position, since the “generosity” provided by industry must be reciprocated. This explains the rapid and overwhelming favorable response given by the practitioners of the medical profession by seeming to accept whatever new products appear on the market. The issue has become even more complicated by the growing acceptance of kickbacks and perks for cooperation with the manufactures, simply for the use of the industrial products and assistance in advertising them.5,6.
I have previously described episodes in which I was personally involved. The one I now describe consisted of a visit by an industrial representative who approached me during my tenure as Chairman of Orthopedics at the University of Southern California.1 He offered me what he called “a very good deal” where I was to be given $200 for every one of his total joint implants I were to use, as well as from those used by any of the orthopedists working at any and all 5 hospitals affiliated with the department. I was to receive a monthly check at home, so no one else would be aware of the transactions. When I asked him what had prompted him to think I was a prostitute, all he could say was, “But Doctor Sarmiento, we do that all the time.”
I am certain that episodes of the nature of my experience occur every day of the week. Had it not been aware of their frequent occurrence, the United States Justice Department’s investigation of the “egregious unethical transgressions” and the “corruption in the relationship between the industry and orthopedics”, and the search for a solution would not have been initiated.6 What can we expect to come out of such investigations? As far as I am concerned, those who may have been accused unfairly, as well as the guilty ones, have probably stated that “all conflicts of interest have been resolved.” I insist that the orthopedic profession, and particularly its representative organizations, should stand up and, as loudly and clearly as possible, protest the despicable practice and bring about a cure for the festering ulcer.
The power of industry in controlling orthopedics has reached a previously unsuspected level. In a commentary I published in the Journal of Bone and Joint Surgery,7 I described an episode where a powerful industrial concern had prevented the release of a book I had written. The product had reached its final form in hardbound and softbound editions in English, as well as in Spanish. After much struggle to find out why at the last minute the marketing had not begun, I learned that an industrial firm had “convinced” the publishers to cancel the release of the book. Though I considered the litigation route, I realized that I did not have the financial resources for such a venture. I have experienced similar problems with other publishers in the past.7 When our representative organizations will seek involvement in this delicate issue and try to bring about a solution is as yet unknown.
A fact influencing the decline of professionalism in our ranks is the now well-known lack of credibility in an increasing number of publications.8 Some respectable journals are making a serious effort to overcome the problem, but they realize how difficult it is to see the truth, since distorting data is not that difficult, and exposing the culprits is even more so. What possible solution can be structured to resolve this embarrassing situation? It is an issue of morality that cannot be legislated; however, it can be positively influenced by the example set for the younger generations, especially by our representative officers. Unfortunately, the latter group has failed to do a very good job, since we frequently observe individuals with obvious conflicts of interest occupying positions of leadership and power.
Continue to: In the United States another move...
In the United States another move, supported by a number of well-qualified authorities in the field of medical economics, advocates the establishment of a system where all physicians would be salaried hospital employees.3 For some time I have felt inclined to support the concept, as I saw it as a logical and practical one. However, my mind has been rapidly changing, particularly after observing the growing number of hospitals employing physicians throughout some regions of the country. I ask myself, how will the number of subspecialists in each hospital be able to enroll? It cannot be open-ended, since the cost of providing a salary, malpractice insurance, secretarial support, vacation time, health insurance, and other benefits can amount to degrees the institutions cannot afford. Another discouraging find was related to the fact that the salaried doctors would receive bonuses according to the amount of work they did. This is a logical and well-intentioned move. However, it would perpetuate one of the major problems besetting the current situation: the rendering of nonessential expensive services and the performance of unnecessary surgery in order to increase the size of the bonus. Would this be a return to the current dilemma?
For the various issues I have identified and for which critical objectivity is necessary, it is essential that our representative organizations assume a leading role, concentrate on fundamental issues, and set aside time-consuming projects of questionable importance. Among those projects I identity 3 important ones: The Joint Replacement Registry, The Orthopedic Guidelines, and Evidence-Based Orthopedics. The Joint Replacement Registry can wait, since its foundations as presented today are weak and based on the illusion that the alleged success of the Scandinavian Registries can be easily duplicated in this country. I envision that 15 years from now the only thing the Registry will give us is millions of pieces of data that were already available through the traditional methods of publications and other means of dissemination of information.9
The recent infatuation with The Orthopedic Guidelines may be a temporary success that will die before they reach maturity. It is a noble effort, since it proposes a mechanism by which to provide “advice” to orthopedic surgeons regarding the degree of benefit that various treatment approaches have to offer. The problem, as I see it, is that soon the “advice” will become, in the eyes of many, “dictates” to be followed. The fear of litigation for not following the “guidelines” could result in deprivation of the independence that is acquired from experience and lessons from others. We are not children in need of instructions regarding behavior. Rather than making changes with new but probably imperfect projects, we should emphasize the credibility of publications and oral presentations. The authors of the Guidelines are appointed individuals who, like all human beings, are afflicted with the innate biases and prejudices that make them see things according to their personal perceptions, and their views are not necessarily representative of ideal situations.10
Evidence-Based Orthopedics is another noble effort to improve matters. I find in this effort the same flaws I have identified with Registries and Guidelines. There is not yet any evidence to suggest that the profession will be helped from the time and expense they require. Any publication should be based on evidence; otherwise it should not be brought to the orthopedic community. Emphasizing credibility is more likely to be beneficial to the profession and to the people we represent. To have 2 different types of articles in our journals where some are based on evidence and others lack evidence does not make sense.
In summary, orthopedics is confronting situations that require critical objectivity as we search for solutions. Some of the situations I have tried to identify may not be “problems” but examples of normal evolution or transient developments that time alone would resolve. On the other hand, some others may be of a serious nature and require our involvement. To ignore them will bring about problems for the next generation who will wonder what precluded us from seeking answers before it was too late.
1. Sarmiento A. Bare Bones: A Surgeon’s Tale. Amherst, NY: Prometheus Books; 2003.
2. Callahan D. Taming the Beloved Beast: How Medical Technology Costs are Destroying Our Health Care System. Princeton, NJ: Princeton University Press; 2009.
3. Relman AS. Doctors as the key to health care reform. N Engl J Med 2009;361(13):1225-1227. doi:10.1056/NEJMp0907925.
4. Sarmiento A. Subspecialization in orthopaedics. Has it been all for the better? J Bone Joint Surg Am. 2003;85-A(2):369-373.
5. Sarmiento A The relationship between orthopaedics and industry must be reformed. Clin Orthop Relat Res. 2003;412:38-44.
6. Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring. Newark, NJ: US Dept of Justice, US Attorney, District of New Jersey; 2007.
7. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222. doi:10.2106/JBJS.J.00888.
8. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone and Joint Surg Br. 2005;87(11):1452-1453.
9. Sarmiento A. Orthopedic registries: second thoughts. Am J Orthop. 2015;44(4):159-160.
10. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
The genesis of the ongoing debate on healthcare is complex, in part because of the perceived flaws within the proposed systems. Denying the guilt that the medical profession has had is a disingenuous exercise in futility. In fact, orthopedics is possibly the most egregious among the culprits. Such a charge, supportable or not, calls for serious objective criticism.1 No one would dare question the fact that from the orthopedic point of view, the cost of healthcare delivery is an important factor because of the critical level this issue has reached. The use of expensive technology and frequent surgery are of major importance.2,3 However, I submit that it is not the high cost of the technology and surgical procedures per se but their frequent abuse.
As I look at orthopedics in an objective, critical manner, I have the uncomfortable feeling that the profession is rapidly becoming a business where greater and greater profit is its primary raison d’etre. The discipline has lost much of its traditional scientific/biological foundations, and is converting itself into a technical trade heavily controlled in its educational duties and the subsequent conduct of its members by industry. This metamorphous evolution has shown ill effects as demonstrated by the loss of traditional territory to other disciplines and a borderline ridiculous fragmentation into a multitude of subspecialties that contribute to the erosion of the profession. Orthopedics is no longer a solid eclectic body of knowledge and expertise in the care of musculoskeletal conditions, but a fragmented body of techniques independent of each other. This statement is not a criticism of fragmentation per se, because fragmentation in most human endeavors is a natural evolutionary phenomenon that occurs in virtually all walks of life, and to our profession it has brought much progress. My concerns are over the apparent exaggerated degree it has reached.4
The fragmentation and erosion of orthopedics took a relatively short time to occur without any evidence of concern among the orthopedic community, which apparently assumed that the advances made by other disciplines would not compromise the security and independence it had enjoyed for generations. The spectacular advances in joint replacement began to occupy a large segment of orthopedists’ professional time. The attractive reimbursement accompanying these procedures further justified the complacency regarding the shrinking of the discipline, while the previous outsiders expanded their territory. Critical objectivity of this issue is important in the event we decide to address the consequences of further erosion and fragmentation of our profession.
There should be no question that if all, or the overwhelming majority of, orthopedists become subspecialists who take care of only a limited number of pathological conditions, the cost of care will grow exponentially. The poor, regardless of the outcome of any legislation addressing their problems, will suffer most.
In small communities there are not enough patients with conditions requiring subspecialized orthopedic services to satisfy the emotional and economic needs of the fellowship-trained orthopedists. Other physicians and allied health practitioners will fill the void and provide the needed services. However, the facts facing us today suggest that if the current trends continue unabated, orthopedics as a distinct branch of medicine may not survive. Nonetheless, people in need of musculoskeletal care will receive it from a variety of medical and paramedical people, who will gradually develop skills and knowledge in a manner comparable to that possessed today by orthopedists.
Continue to: Of major significance...
Of major significance in the overall issue of critical objectivity is the unquestionable fact that orthopedics voluntarily relegated to the surgical implant industry the control of many of its traditional educational responsibilities to the point where, at present, it is assumed that educational programs cannot be conducted without the financial support of industry. Hundreds, if not thousands, of conferences; grand rounds; local, state, and regional society meetings; the American Academy of Orthopaedic Surgeons’ (AAOS) annual meeting, and its many other educational activities take place with the financial support of industry. Such a dominance has placed the orthopedic profession in a relative subservient position, since the “generosity” provided by industry must be reciprocated. This explains the rapid and overwhelming favorable response given by the practitioners of the medical profession by seeming to accept whatever new products appear on the market. The issue has become even more complicated by the growing acceptance of kickbacks and perks for cooperation with the manufactures, simply for the use of the industrial products and assistance in advertising them.5,6.
I have previously described episodes in which I was personally involved. The one I now describe consisted of a visit by an industrial representative who approached me during my tenure as Chairman of Orthopedics at the University of Southern California.1 He offered me what he called “a very good deal” where I was to be given $200 for every one of his total joint implants I were to use, as well as from those used by any of the orthopedists working at any and all 5 hospitals affiliated with the department. I was to receive a monthly check at home, so no one else would be aware of the transactions. When I asked him what had prompted him to think I was a prostitute, all he could say was, “But Doctor Sarmiento, we do that all the time.”
I am certain that episodes of the nature of my experience occur every day of the week. Had it not been aware of their frequent occurrence, the United States Justice Department’s investigation of the “egregious unethical transgressions” and the “corruption in the relationship between the industry and orthopedics”, and the search for a solution would not have been initiated.6 What can we expect to come out of such investigations? As far as I am concerned, those who may have been accused unfairly, as well as the guilty ones, have probably stated that “all conflicts of interest have been resolved.” I insist that the orthopedic profession, and particularly its representative organizations, should stand up and, as loudly and clearly as possible, protest the despicable practice and bring about a cure for the festering ulcer.
The power of industry in controlling orthopedics has reached a previously unsuspected level. In a commentary I published in the Journal of Bone and Joint Surgery,7 I described an episode where a powerful industrial concern had prevented the release of a book I had written. The product had reached its final form in hardbound and softbound editions in English, as well as in Spanish. After much struggle to find out why at the last minute the marketing had not begun, I learned that an industrial firm had “convinced” the publishers to cancel the release of the book. Though I considered the litigation route, I realized that I did not have the financial resources for such a venture. I have experienced similar problems with other publishers in the past.7 When our representative organizations will seek involvement in this delicate issue and try to bring about a solution is as yet unknown.
A fact influencing the decline of professionalism in our ranks is the now well-known lack of credibility in an increasing number of publications.8 Some respectable journals are making a serious effort to overcome the problem, but they realize how difficult it is to see the truth, since distorting data is not that difficult, and exposing the culprits is even more so. What possible solution can be structured to resolve this embarrassing situation? It is an issue of morality that cannot be legislated; however, it can be positively influenced by the example set for the younger generations, especially by our representative officers. Unfortunately, the latter group has failed to do a very good job, since we frequently observe individuals with obvious conflicts of interest occupying positions of leadership and power.
Continue to: In the United States another move...
In the United States another move, supported by a number of well-qualified authorities in the field of medical economics, advocates the establishment of a system where all physicians would be salaried hospital employees.3 For some time I have felt inclined to support the concept, as I saw it as a logical and practical one. However, my mind has been rapidly changing, particularly after observing the growing number of hospitals employing physicians throughout some regions of the country. I ask myself, how will the number of subspecialists in each hospital be able to enroll? It cannot be open-ended, since the cost of providing a salary, malpractice insurance, secretarial support, vacation time, health insurance, and other benefits can amount to degrees the institutions cannot afford. Another discouraging find was related to the fact that the salaried doctors would receive bonuses according to the amount of work they did. This is a logical and well-intentioned move. However, it would perpetuate one of the major problems besetting the current situation: the rendering of nonessential expensive services and the performance of unnecessary surgery in order to increase the size of the bonus. Would this be a return to the current dilemma?
For the various issues I have identified and for which critical objectivity is necessary, it is essential that our representative organizations assume a leading role, concentrate on fundamental issues, and set aside time-consuming projects of questionable importance. Among those projects I identity 3 important ones: The Joint Replacement Registry, The Orthopedic Guidelines, and Evidence-Based Orthopedics. The Joint Replacement Registry can wait, since its foundations as presented today are weak and based on the illusion that the alleged success of the Scandinavian Registries can be easily duplicated in this country. I envision that 15 years from now the only thing the Registry will give us is millions of pieces of data that were already available through the traditional methods of publications and other means of dissemination of information.9
The recent infatuation with The Orthopedic Guidelines may be a temporary success that will die before they reach maturity. It is a noble effort, since it proposes a mechanism by which to provide “advice” to orthopedic surgeons regarding the degree of benefit that various treatment approaches have to offer. The problem, as I see it, is that soon the “advice” will become, in the eyes of many, “dictates” to be followed. The fear of litigation for not following the “guidelines” could result in deprivation of the independence that is acquired from experience and lessons from others. We are not children in need of instructions regarding behavior. Rather than making changes with new but probably imperfect projects, we should emphasize the credibility of publications and oral presentations. The authors of the Guidelines are appointed individuals who, like all human beings, are afflicted with the innate biases and prejudices that make them see things according to their personal perceptions, and their views are not necessarily representative of ideal situations.10
Evidence-Based Orthopedics is another noble effort to improve matters. I find in this effort the same flaws I have identified with Registries and Guidelines. There is not yet any evidence to suggest that the profession will be helped from the time and expense they require. Any publication should be based on evidence; otherwise it should not be brought to the orthopedic community. Emphasizing credibility is more likely to be beneficial to the profession and to the people we represent. To have 2 different types of articles in our journals where some are based on evidence and others lack evidence does not make sense.
In summary, orthopedics is confronting situations that require critical objectivity as we search for solutions. Some of the situations I have tried to identify may not be “problems” but examples of normal evolution or transient developments that time alone would resolve. On the other hand, some others may be of a serious nature and require our involvement. To ignore them will bring about problems for the next generation who will wonder what precluded us from seeking answers before it was too late.
The genesis of the ongoing debate on healthcare is complex, in part because of the perceived flaws within the proposed systems. Denying the guilt that the medical profession has had is a disingenuous exercise in futility. In fact, orthopedics is possibly the most egregious among the culprits. Such a charge, supportable or not, calls for serious objective criticism.1 No one would dare question the fact that from the orthopedic point of view, the cost of healthcare delivery is an important factor because of the critical level this issue has reached. The use of expensive technology and frequent surgery are of major importance.2,3 However, I submit that it is not the high cost of the technology and surgical procedures per se but their frequent abuse.
As I look at orthopedics in an objective, critical manner, I have the uncomfortable feeling that the profession is rapidly becoming a business where greater and greater profit is its primary raison d’etre. The discipline has lost much of its traditional scientific/biological foundations, and is converting itself into a technical trade heavily controlled in its educational duties and the subsequent conduct of its members by industry. This metamorphous evolution has shown ill effects as demonstrated by the loss of traditional territory to other disciplines and a borderline ridiculous fragmentation into a multitude of subspecialties that contribute to the erosion of the profession. Orthopedics is no longer a solid eclectic body of knowledge and expertise in the care of musculoskeletal conditions, but a fragmented body of techniques independent of each other. This statement is not a criticism of fragmentation per se, because fragmentation in most human endeavors is a natural evolutionary phenomenon that occurs in virtually all walks of life, and to our profession it has brought much progress. My concerns are over the apparent exaggerated degree it has reached.4
The fragmentation and erosion of orthopedics took a relatively short time to occur without any evidence of concern among the orthopedic community, which apparently assumed that the advances made by other disciplines would not compromise the security and independence it had enjoyed for generations. The spectacular advances in joint replacement began to occupy a large segment of orthopedists’ professional time. The attractive reimbursement accompanying these procedures further justified the complacency regarding the shrinking of the discipline, while the previous outsiders expanded their territory. Critical objectivity of this issue is important in the event we decide to address the consequences of further erosion and fragmentation of our profession.
There should be no question that if all, or the overwhelming majority of, orthopedists become subspecialists who take care of only a limited number of pathological conditions, the cost of care will grow exponentially. The poor, regardless of the outcome of any legislation addressing their problems, will suffer most.
In small communities there are not enough patients with conditions requiring subspecialized orthopedic services to satisfy the emotional and economic needs of the fellowship-trained orthopedists. Other physicians and allied health practitioners will fill the void and provide the needed services. However, the facts facing us today suggest that if the current trends continue unabated, orthopedics as a distinct branch of medicine may not survive. Nonetheless, people in need of musculoskeletal care will receive it from a variety of medical and paramedical people, who will gradually develop skills and knowledge in a manner comparable to that possessed today by orthopedists.
Continue to: Of major significance...
Of major significance in the overall issue of critical objectivity is the unquestionable fact that orthopedics voluntarily relegated to the surgical implant industry the control of many of its traditional educational responsibilities to the point where, at present, it is assumed that educational programs cannot be conducted without the financial support of industry. Hundreds, if not thousands, of conferences; grand rounds; local, state, and regional society meetings; the American Academy of Orthopaedic Surgeons’ (AAOS) annual meeting, and its many other educational activities take place with the financial support of industry. Such a dominance has placed the orthopedic profession in a relative subservient position, since the “generosity” provided by industry must be reciprocated. This explains the rapid and overwhelming favorable response given by the practitioners of the medical profession by seeming to accept whatever new products appear on the market. The issue has become even more complicated by the growing acceptance of kickbacks and perks for cooperation with the manufactures, simply for the use of the industrial products and assistance in advertising them.5,6.
I have previously described episodes in which I was personally involved. The one I now describe consisted of a visit by an industrial representative who approached me during my tenure as Chairman of Orthopedics at the University of Southern California.1 He offered me what he called “a very good deal” where I was to be given $200 for every one of his total joint implants I were to use, as well as from those used by any of the orthopedists working at any and all 5 hospitals affiliated with the department. I was to receive a monthly check at home, so no one else would be aware of the transactions. When I asked him what had prompted him to think I was a prostitute, all he could say was, “But Doctor Sarmiento, we do that all the time.”
I am certain that episodes of the nature of my experience occur every day of the week. Had it not been aware of their frequent occurrence, the United States Justice Department’s investigation of the “egregious unethical transgressions” and the “corruption in the relationship between the industry and orthopedics”, and the search for a solution would not have been initiated.6 What can we expect to come out of such investigations? As far as I am concerned, those who may have been accused unfairly, as well as the guilty ones, have probably stated that “all conflicts of interest have been resolved.” I insist that the orthopedic profession, and particularly its representative organizations, should stand up and, as loudly and clearly as possible, protest the despicable practice and bring about a cure for the festering ulcer.
The power of industry in controlling orthopedics has reached a previously unsuspected level. In a commentary I published in the Journal of Bone and Joint Surgery,7 I described an episode where a powerful industrial concern had prevented the release of a book I had written. The product had reached its final form in hardbound and softbound editions in English, as well as in Spanish. After much struggle to find out why at the last minute the marketing had not begun, I learned that an industrial firm had “convinced” the publishers to cancel the release of the book. Though I considered the litigation route, I realized that I did not have the financial resources for such a venture. I have experienced similar problems with other publishers in the past.7 When our representative organizations will seek involvement in this delicate issue and try to bring about a solution is as yet unknown.
A fact influencing the decline of professionalism in our ranks is the now well-known lack of credibility in an increasing number of publications.8 Some respectable journals are making a serious effort to overcome the problem, but they realize how difficult it is to see the truth, since distorting data is not that difficult, and exposing the culprits is even more so. What possible solution can be structured to resolve this embarrassing situation? It is an issue of morality that cannot be legislated; however, it can be positively influenced by the example set for the younger generations, especially by our representative officers. Unfortunately, the latter group has failed to do a very good job, since we frequently observe individuals with obvious conflicts of interest occupying positions of leadership and power.
Continue to: In the United States another move...
In the United States another move, supported by a number of well-qualified authorities in the field of medical economics, advocates the establishment of a system where all physicians would be salaried hospital employees.3 For some time I have felt inclined to support the concept, as I saw it as a logical and practical one. However, my mind has been rapidly changing, particularly after observing the growing number of hospitals employing physicians throughout some regions of the country. I ask myself, how will the number of subspecialists in each hospital be able to enroll? It cannot be open-ended, since the cost of providing a salary, malpractice insurance, secretarial support, vacation time, health insurance, and other benefits can amount to degrees the institutions cannot afford. Another discouraging find was related to the fact that the salaried doctors would receive bonuses according to the amount of work they did. This is a logical and well-intentioned move. However, it would perpetuate one of the major problems besetting the current situation: the rendering of nonessential expensive services and the performance of unnecessary surgery in order to increase the size of the bonus. Would this be a return to the current dilemma?
For the various issues I have identified and for which critical objectivity is necessary, it is essential that our representative organizations assume a leading role, concentrate on fundamental issues, and set aside time-consuming projects of questionable importance. Among those projects I identity 3 important ones: The Joint Replacement Registry, The Orthopedic Guidelines, and Evidence-Based Orthopedics. The Joint Replacement Registry can wait, since its foundations as presented today are weak and based on the illusion that the alleged success of the Scandinavian Registries can be easily duplicated in this country. I envision that 15 years from now the only thing the Registry will give us is millions of pieces of data that were already available through the traditional methods of publications and other means of dissemination of information.9
The recent infatuation with The Orthopedic Guidelines may be a temporary success that will die before they reach maturity. It is a noble effort, since it proposes a mechanism by which to provide “advice” to orthopedic surgeons regarding the degree of benefit that various treatment approaches have to offer. The problem, as I see it, is that soon the “advice” will become, in the eyes of many, “dictates” to be followed. The fear of litigation for not following the “guidelines” could result in deprivation of the independence that is acquired from experience and lessons from others. We are not children in need of instructions regarding behavior. Rather than making changes with new but probably imperfect projects, we should emphasize the credibility of publications and oral presentations. The authors of the Guidelines are appointed individuals who, like all human beings, are afflicted with the innate biases and prejudices that make them see things according to their personal perceptions, and their views are not necessarily representative of ideal situations.10
Evidence-Based Orthopedics is another noble effort to improve matters. I find in this effort the same flaws I have identified with Registries and Guidelines. There is not yet any evidence to suggest that the profession will be helped from the time and expense they require. Any publication should be based on evidence; otherwise it should not be brought to the orthopedic community. Emphasizing credibility is more likely to be beneficial to the profession and to the people we represent. To have 2 different types of articles in our journals where some are based on evidence and others lack evidence does not make sense.
In summary, orthopedics is confronting situations that require critical objectivity as we search for solutions. Some of the situations I have tried to identify may not be “problems” but examples of normal evolution or transient developments that time alone would resolve. On the other hand, some others may be of a serious nature and require our involvement. To ignore them will bring about problems for the next generation who will wonder what precluded us from seeking answers before it was too late.
1. Sarmiento A. Bare Bones: A Surgeon’s Tale. Amherst, NY: Prometheus Books; 2003.
2. Callahan D. Taming the Beloved Beast: How Medical Technology Costs are Destroying Our Health Care System. Princeton, NJ: Princeton University Press; 2009.
3. Relman AS. Doctors as the key to health care reform. N Engl J Med 2009;361(13):1225-1227. doi:10.1056/NEJMp0907925.
4. Sarmiento A. Subspecialization in orthopaedics. Has it been all for the better? J Bone Joint Surg Am. 2003;85-A(2):369-373.
5. Sarmiento A The relationship between orthopaedics and industry must be reformed. Clin Orthop Relat Res. 2003;412:38-44.
6. Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring. Newark, NJ: US Dept of Justice, US Attorney, District of New Jersey; 2007.
7. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222. doi:10.2106/JBJS.J.00888.
8. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone and Joint Surg Br. 2005;87(11):1452-1453.
9. Sarmiento A. Orthopedic registries: second thoughts. Am J Orthop. 2015;44(4):159-160.
10. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
1. Sarmiento A. Bare Bones: A Surgeon’s Tale. Amherst, NY: Prometheus Books; 2003.
2. Callahan D. Taming the Beloved Beast: How Medical Technology Costs are Destroying Our Health Care System. Princeton, NJ: Princeton University Press; 2009.
3. Relman AS. Doctors as the key to health care reform. N Engl J Med 2009;361(13):1225-1227. doi:10.1056/NEJMp0907925.
4. Sarmiento A. Subspecialization in orthopaedics. Has it been all for the better? J Bone Joint Surg Am. 2003;85-A(2):369-373.
5. Sarmiento A The relationship between orthopaedics and industry must be reformed. Clin Orthop Relat Res. 2003;412:38-44.
6. Five Companies in Hip and Knee Replacement Industry Avoid Prosecution by Agreeing to Compliance Rules and Monitoring. Newark, NJ: US Dept of Justice, US Attorney, District of New Jersey; 2007.
7. Sarmiento A. Infringing on freedom of speech. J Bone Joint Surg Am. 2011;93(2):222. doi:10.2106/JBJS.J.00888.
8. Carr AJ. Which research is to be believed? The ethics of industrial funding of orthopaedic research. J Bone and Joint Surg Br. 2005;87(11):1452-1453.
9. Sarmiento A. Orthopedic registries: second thoughts. Am J Orthop. 2015;44(4):159-160.
10. Sarmiento A. Thoughts on orthopedic guidelines. Am J Orthop. 2010;39(8):373-374.
The Potential Value of Dual-Energy X-Ray Absorptiometry in Orthopedics
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
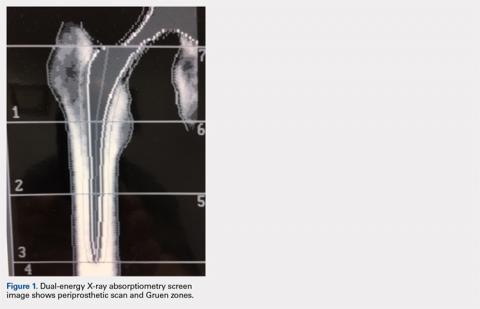
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
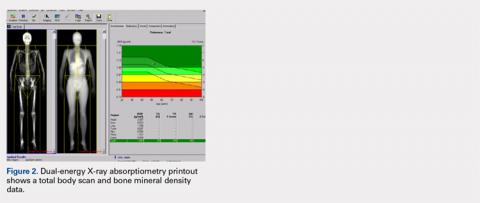
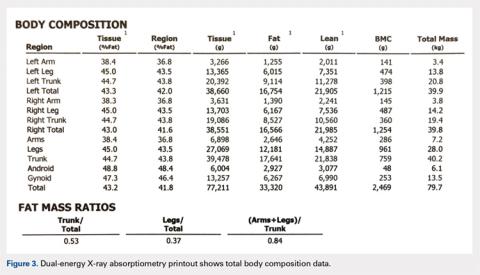
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
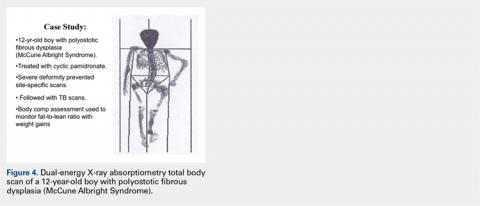
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
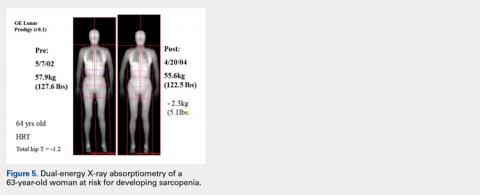
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
ABSTRACT
Dual-energy X-ray absorptiometry (DXA) is a well-established technology with an important and well-known role in measuring bone mineral density (BMD) for the purpose of determining fracture risk, diagnosing osteoporosis, and monitoring treatment efficacy. However, aside from the assessment of bone status, DXA is likely underutilized in the field of orthopedics, and most orthopedists may not be aware of the full capabilities of DXA, particularly with regard to total body scans and body composition assessment. For example, DXA would be a valuable tool for monitoring body composition after surgery where compensatory changes in the affected limb may lead to right-left asymmetry (eg, tracking lean mass change after knee surgery), rehabilitation regimens for athletes, congenital and metabolic disorders that affect the musculoskeletal system, or monitoring sarcopenia and frailty in the elderly. Furthermore, preoperative and postoperative regional scans can track BMD changes during healing or alert surgeons to impending problems such as loss of periprosthetic bone, which could lead to implant failure. This article discusses the capabilities of DXA and how this technology could be better used to the advantage of the attending orthopedist.
Dual-energy X-ray absorptiometry, abbreviated as “DXA,” (although usually abbreviated in older literature as “DEXA”) was first introduced in 1987 (Hologic QDR-1000 system, Hologic, Inc) and immediately made all previous forms of radiation-based bone mineral density (BMD) measurement systems obsolete.1 Since then, there have been many generations of the technology, with the main US manufacturers in 2017 being Hologic, Inc. and GE Lunar. There are 2 forms of DXA, peripheral systems (which usually measure BMD only in the radius, finger bones, or calcaneus) and central systems (which measure the radius, proximal femur [“hip”], lumbar spine, total body, and custom sites). The general principle of how DXA works is based on the differential attenuation of photons by bone, fat, and lean mass.2 The DXA technique uses a low- and high-energy X-ray beam produced by an X-ray tube. With the low-energy beam, attenuation by bone is greater than attenuation by soft tissue. With the high-energy beam, attenuation by bone and soft tissues are similar. The dual X-ray beams are passed through the body regions being scanned (usually posterioanteriorly), and the differential attenuation by bone and soft tissue is analyzed to produce BMD estimates. In addition, a high-quality image is produced to enable the operator of the DXA system to verify that the appropriate body region was scanned. It is important to realize that DXA is 2-dimensional (which is sometimes cited as a weakness of DXA), and the units of BMD are grams of mineral per centimeter squared (g/cm2).
Continue to: When assessing bone status...
When assessing bone status for the purpose of determining if a patient is normal, osteopenic, or osteoporotic, the skeletal sites (called regions of interest [ROI]) typically scanned are the proximal femur, lumbar spine, and radius. The BMD of the patient is then compared to a manufacturer-provided normative database of young adults (the logic being that the BMD in the young adult normative population represents maximal peak bone mass). Total body BMD and body composition can also be quantified (grams of lean and fat mass), and custom scans can be designed for other skeletal sites. Specifically, a patient’s BMD is compared to a database of sex- and age-adjusted normal values, and the deviation from normal is expressed as a T-score (the number of standard deviations the patient's BMD is above or below the average BMD of the young adult reference population) and Z-scores (the number of standard deviations a patient's BMD is above or below the average BMD of a sex- and age-matched reference population).3 The International Society for Clinical Densitometry (ISCD) has developed and published well-accepted guidelines used to assist in acquiring high-quality DXA scans and for the diagnosis of osteoporosis using BMD. The accuracy and, especially, the precision of DXA scans can be remarkable when they are performed by trained technologists, and thus, serial scans can be performed to monitor BMD and body composition changes with aging or in response to treatment.
Because of the nature of the scan mechanics and speed, the effective radiation dose with DXA is very low, expressed in microSieverts.4,5 Generally, the radiation exposure from a series of the lumbar spine, proximal femur, and distal radius is about the same as daily background radiation. Even total body scans present very low exposure due to the scan speed at which any 1 body part is exposed for only a fraction of a second.
BENEFITS OF USING DXA FOR THE ORTHEOPEDIST
At the time of this writing in 2018, the presumption could be made that most physicians in the specialties of internal medicine, rheumatology, endocrinology, radiology, and orthopedics were familiar with the capabilities of DXA to assess BMD for the purpose of diagnosing osteoporosis. However, DXA is likely underused for other purposes, as orthopedists may be unaware of the full capabilities of DXA. Printouts after a scan contain more information than simply BMD, and there are more features and applications of DXA that can potentially be useful to orthopedists.
BONE SIZE
Data from a DXA scan are expressed not only as g/cm2 (BMD) but also as total grams in the ROI (known as bone mineral content, abbreviated as BMC), and cm2 (area of the ROI). These data may appear on a separate page, being considered ancillary results. The latter 2 variables are rarely included on a report sent to a referring physician; therefore, awareness of their value is probably limited. However, there are instances where such information could be valuable when interpreting results, especially bone size.6,7 For example, on occasion, patients present with osteopenic lumbar vertebrate but larger than normal vertebral size (area). Many studies have shown that bone size is directly related to bone strength and thus fracture risk.8,9 Although an understudied phenomenon, large vertebral body size could be protective, counteracting a lower than optimal BMD. Further, because the area of the ROI is measured, it is possible to calculate the bone width (or measure directly with a ruler tool in the software if available) for the area measured. This is especially feasible for tubular bones such as the midshaft of the radius, or more specifically, the classic DXA ROI being the area approximately one third the length of the radius from the distal end, the radius 33% region (actually based on ulna length). Consequently, it is possible to use the width of the radius 33% ROI in addition to BMD and T-score when assessing fracture risk.
CASE STUDY
A 60-year-old man had a DXA series of the lumbar spine, proximal femur, and whole body. His total body T-score was 0.6 (normal), and his total proximal femur T-score was −0.8 (normal), but his lumbar spine vertebrae 2 to 4 T-score was −1.9. As the patient was osteopenic based on the lumbar spine T-score, some physicians may have initiated antiresorptive therapy, especially if other risk factors for fracture were present. Further examination of the ancillary results of the DXA scan revealed that the vertebral body height T-score was a remarkable 1.11 and 1.53 after adjustment for stature (automatic software calculation). These results suggested that the patient had vertebral bodies of above average size, which theoretically would be protective against fracture even though the BMD T-score was below normal. For this patient, this finding mitigated immediate concern about the lumbar spine T-score of −1.9. Although vertebral body size is not typically used in assessing fracture risk, it is useful information that could be factored into the decision to start treatment or watch for further change with aging.
Continue to: Case Series: Distal Radius Fractures...
CASE SERIES: DISTAL RADIUS FRACTURES
Table 1 summarizes the data comparing radius 33% ROI T-scores and ROI width in patients who fractured the contralateral radius and normal nonfractured controls.10
Table 1. Comparison of Radius Width at the 33% Region of Interest (ROI) and Bone Mineral Density T-Scores in Premenopausal Women With and Without Fractures
| 33% ROI T-score | Width of ROI, cm |
White women with distal radius fractures |
|
|
Premenopausal (<49 years), n = 36 | -0.2 + 0.9 | 1.22 + 0.11a |
Controls matched for race, age, BMIb |
|
|
Premenopausal (<49 years), n = 65 | -0.1 + 0.8 | 1.45 + 0.25 |
For premenopausal women with distal radius fractures, the width of the radius at the radius 33% ROI was significantly smaller than that in controls. However, there was no difference in T-scores between premenopausal women with distal radius fractures and controls. Thus, bone width more accurately identified women with fractures than T-scores based on BMD, and the orthopedist could use bone size in addition to BMD to predict fracture risk in a patient.
PREPARATION FOR SURGERY
For some procedures, there is potential benefit of assessing bone status prior to surgery. That is, determination of low BMD could potentially influence the type of hardware or fixation techniques used in surgery. Various studies have shown that poor bone quality and low BMD can impair purchase with various types of fixation.11-13 Low preoperative BMD has been shown to be related to high implant migration.14 Knowledge of BMD could influence the choice of screw type used or the type of implant metal (titanium vs cobalt chrome). Another example is predicting the risk of spine curvature progression in adolescent idiopathic scoliosis.15-17 It has been reported that low BMD is a risk factor for progression.15 Knowledge of BMD could potentially help with patient management strategies. For example, a patient with low BMD and vitamin D deficiency could be treated (vitamin D supplementation) prior to planning surgery in an effort to improve the low BMD.
PERIOPROSTHETIC BMD
It is possible to monitor changes in BMD around implants using the periprosthetic software application (this usually needs to be purchased separately from standard software that is installed with a system set-up). Dramatic loss of bone due to stress shielding after total hip arthroplasty (THA) can be a risk factor for implant migration or potentially outright failure of fixation or breakthrough. If bone loss occurs and is observed in the early stages, then antiresorptive treatment can be initiated to limit further loss.18,19 (Figure 1) shows the image from a periprosthetic scan.
Continue to: A 60-year-old, 215-lb man...
CASE REPORT
A 60-year-old, 215-lb man had a total hip replacement using a newly introduced cemented collared cobalt-chromium alloy femoral stem. A baseline periprosthetic DXA scan was performed 6 weeks postoperatively. Compared to baseline, the change in BMD in the Gruen zone 5 was −8.2%, +6.5%, +4.9%, and +9.46% at 3, 6, 12, and 24 months, respectively. In contrast, dramatic BMD loss was seen in Gruen zone 7 (calcar region): −33.2%, −40.8%, −37.1%, and −34.1% at 3, 6, 12, and 24 months, respectively. Similar findings in other patients led to discontinuation of use of this stem in favor of a collarless stem in which less BMD loss was seen in Gruen zone 7. Although additional technologist training is required and scans may not be reimbursable, for research purposes or for evaluating new component prototypes, the periprosthetic DXA scan capability can be useful.
Various other custom scans can be used to detect and quantify vertebral fractures (vertebral fracture assessment application), monitor healing of fractures by scanning through radiolucent cast materials, or for research purposes to assess BMD at unusual locations.21-23 Other new innovations, such as the ability to perform full-length scans of the femoral shaft and to quantify focal thickening of the lateral cortex to identify beaking, an abnormality associated with atypical femur fracture after long-term bisphosphonate use, continue to expand the utility of DXA. Using standard software, cadaver bones can be scanned prior to biomechanical testing for a variety of purposes, such as ensuring proper matching specimens in test groups. It has been reported that the common practice of using contralateral bone specimens can lead to bias, as the BMD can be significantly different in right and left bones from the same individual.9,24
TOTAL BODY BMD AND BODY COMPOSITION SCANS
Perhaps the least understood capability of DXA from our experience working with orthopedists is the ability to perform total body scans and to obtain not only total body and regional BMD but also body composition data, namely grams of lean and fat mass.25 Soft tissue (no bone pixels) is partitioned into fat and lean body mass by a calibration procedure (lean mass = total soft tissue –fat mass). DXA has become the standard for body composition assessment given the ease of data acquisition (a total body scan takes only a few minutes), accuracy, and precision of measurements. Compared with other methods (eg, skinfold thickness, bioelectrical impedance, and underwater weighing), it is the only method that gives regional values for fat mass, lean mass, and BMC (this allows the ability to compare left vs right sides).25-27 The ability to perform regional measurements cannot be overstated, as stable body weight belies potential changes with age and disease that relate to redistribution of fat and lean mass. It is not possible to identify, let alone track, such changes by measuring gross body weight on a scale or with BMI calculations. However, redistribution of fat and lean mass can be monitored in great detail using DXA. Figures 2 and 3 show the typical output from a DXA total body/body composition scan.
Total body scans with body composition analyses have many applications. For example, monitoring growth and development or treatment in patients with congenital deformity, metabolic bone disease, osteoporosis, and frailty; patients undergoing rehabilitation; and patients having surgery that could affect the use of a contralateral limb with potential hypertrophy or atrophy. Accurate assessment of percent body fat and fat distribution may help surgeons to improve risk stratification and surgical outcome.28-30 Fracture risk has been associated with muscle area.28 Simple measurements of quadriceps size underestimates atrophy, and total body composition can quantitate lean mass.30
In sports medicine, body composition assessments could be useful to monitor postoperative recovery and effectiveness of rehabilitation protocols after injury, effectiveness of conditioning and training programs, developmental changes due to sports participation, and for obtaining baseline assessment at the time of preseason physicals.27,31-34 In athletes, baseline status and morphological adaptations to training have traditionally been measured by anthropometry (eg, skinfold thickness, BMI, limb circumference, etc.), but DXA total body scanning allows for much more detailed assessments with the possibility of subregional quantitation. There is evidence for sports-specific body composition profiles and characteristic adaptations.27,31-34 Using DXA, adaptive changes as a result of training as well as changes and recovery after surgery or injury can be monitored. For example, quadriceps atrophy usually occurs to some extent after ACL repair, and bone mineral loss and muscle atrophy occur after a limb has been immobilized with a cast. DXA body composition assessment could be used to monitor leg lean mass after surgery for comparison with presurgery values or those of the contralateral noninjured side, or to track recovery of bone mineral and muscle after a cast is removed. Some technical sports, such as tennis and baseball pitching, are known to result in limb asymmetry; DXA body composition could be used to monitor development of right-left arm asymmetry in tennis players or baseball pitchers, and then measures could be taken to balance the asymmetry. Wrestlers and elite dancers are expected to maintain strict weight requirements, but diets are often poor, and as such, DXA body composition could be used to track the effects of dieting and training by comparing serial measurements to baseline to ensure that weight changes include preservation or gain of muscle mass.31
Continue to: For older patients...
For older patients being followed after orthopedic care, there is a growing concern about age-related loss of muscle mass, or sarcopenia, which can lead to functional impairment (eg, balance, gait, etc.), and physical disability leading to falling and increased risk of fracture.35-40 Even obese patients can be sarcopenic (a concept known as sarcopenic obesity), and their large body mass can mask the relative deficiency of lean mass.40 DXA total body scans can be used to monitor patients at risk for sarcopenia.
Finally, DXA total body composition scans are underused in the pediatric population. Given the low radiation burden, DXA can be used safely in children of all ages. In addition to the same uses as in adults for presurgical assessment, monitoring bone and soft-tissue changes after treatment and rehabilitation, scans can be used to monitor growth and development.41
CASE STUDY: MONITORING DEVELOPMENT AND TREATMENT
A 12-year-old boy with polyostotic fibrous dysplasia (McCune Albright Syndrome) was started on treatment with cyclic pamidronate to mitigate bone pain and reduce fracture risk. Use of DXA was planned to provide evidence of treatment efficacy by documenting increasing BMD. However, the severe skeletal deformity prevented standard site-specific DXA scans, and consequently, total body scans were effectively used to acquire the BMD data needed to monitor treatment (Figure 4).
CASE STUDY: AGE-RELATED SARCOPENIA
Figure 5 shows images of a 64-year-old woman who was followed after a distal radius fracture. A total body scan and body composition assessment was performed in 2002. At follow-up in 2004, total body weight seemed stable with only a seemingly benign 5.1-lb loss of weight, and the patient’s overall physical appearance was unchanged (Table 2).
Table 2. Age-Related Changes Potentially Leading to Sarcopenia
| Baseline, 2002 | Follow-up, 2004 | Change, % |
Body weight, kg | 57.9 (127.6 lb) | 55.6 (122.5 lb) | 4 |
BMI | 20.6 | 19.8 |
|
Total body fat, g | 13,619 | 13,390 | −1.7 |
Total body percent fat | 23.5 | 24.1 |
|
Total body lean, g | 42,038 | 39,949 | −5.0 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
However, body composition assessment revealed a disproportionate loss of lean mass, with a resultant total percent body fat increase. This imbalance between the change in fat and lean mass could lead to clinical sarcopenia unless appropriate dietary and exercise measures are taken. Such subtle developing imbalances in body composition could only be quantitated using DXA total body scans.
Continue to: It is not uncommon...
CASE STUDY: WEIGHT CHANGE IN A RECREATIONAL ATHLETE
It is not uncommon to encounter patients who have substantial weight changes as a result of lifestyle changes, such as dieting. It is also possible that body weight remains stable, but variable changes occur in the amount and distribution of fat and lean mass. Combining exercise with dieting is more likely to be associated with preservation or gain of lean mass. Such a case is presented. After a knee injury, a club tennis player reported gaining 30 lb in the subsequent 12 months. She enrolled in a DXA study, and serial body composition assessments were performed as she started a diet program and exercised on a treadmill and stationary bike. Table 3 shows body composition changes from baseline.
Table 3. Body Composition Changes After Dieting and Exercise
|
|
| Total Body | ||
| Weight, lb | Body Mass Index | Bone Mineral Density, g/cm2 | Fat, g | Lean, g |
Baseline | 160 | 27.5 | 1.245 | 29,023 | 39,610 |
12-month follow-up | 148 | 25.4 | 1.230 | 22,581 | 41,979 |
Dual-energy X-ray absorptiometry scans were performed using a GE Lunar Prodigy system.
Although gross weight using a scale clearly showed progress in losing weight, it did not provide information about redistribution of fat and lean mass. The DXA body composition assessment showed that at follow up, there was a 22% decrease in total grams of fat and a 6% increase in lean mass (changes were uniform over different body regions). Her BMI still categorized her as being overweight; however, her body composition changes demonstrated that diet and exercise were producing positive results.
CONCLUSION
There are many ways in which DXA technology could provide orthopedists with valuable baseline and postoperative and post-treatment information about their patients. This technology could be used more effectively by orthopedists in both general clinical practice and research.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
1. Miller PD. The history of bone densitometry. Bone. 2017;104:4-6 [Epub ahead of print].
2. Blake GM, Fogelman I. Technical principles of dual energy X ray absorptiometry. Semin Nucl Med. 1997;27(3):210-228.
3. Faulkner KG. The tale of the T-score: review and perspective. Osteoporo Int. 2005;16(4):347-352. doi:10.1007/s00198-004-1779-y.
4. Solomou G, Damilakis J. Radiation exposure in bone densitometry. Semin Musculoskelet Radiol. 2016;20(4):392-398. doi:10.1055/s-0036-1592430.
5. Adams J. Bone densitometry in children. Semin Musculoskelet Radiol. 2016;20(3):254-268. doi:10.1055/s-0036-1592369.
6. Duan Y, Parfitt AM, Seeman E. Vertebral bone mass, size, and volumetric density in women with spinal fractures. J Bone Miner Res. 1999;14(10):1796-1802. doi:10.1359/jbmr.1999.14.10.1796.
7. Szaulc P, Munoz F, Duboeuf F, Delmas PD. Low width of tubular bones is associated with increased risk of fragility fracture in elderly men–the MINOS study. Bone 2006;38(4):595-602. doi:10.1016/j.bone.2005.09.004.
8. Mi J, Li K, Zhao X, Zhao CQ, Li H, Zhao J. Vertebral body compressive strength evaluated by dual-energy x-ray absorptiometry and Hounsfield units in vitro. J Clin Densitom. 2018;21(1):148-153. doi:10.1016/j.jocd.2016.08.011.
9. Ambrose CG, Kiebzak GM, Sabonghy EP, et al. Biomechanical testing of cadaveric specimens: importance of bone mineral density assessment. Foot Ankle Int. 2002;23(9):850-855. doi:10.1177/107110070202300913.
10. Kiebzak G, Sassard WR. Smaller radius width in women with distal radius fractures compared to women without fractures. Cureus. 2017;9(12):e1950. doi:10.775/cureus.1950.
11. Krappinger D, Bizzotto N, Riedmann S, Kammerlander C, Hengg C, Kralinger FS. Predicting failure after surgical fixation of proximal humerus fractures. Injury 2011;42(11):1283-1288. doi:10.1016/j.injury.2011.01.017.
12. Suhm N, Hengg C, Schwyn R, Windolf M, Quarz V, Hänni M. Mechanical torque measurement predicts load to implant cut-out: a biomechanical study investigating DHS anchorage in femoral heads. Arch Orthop Trauma Surg. 2007;127(6):469-474. doi:10.1007/s00402-006-0265-8.
13. Persiani P, Ranaldi FM, Graci J, et al. Isolated olecranon fractures in children affected by osteogenesis imperfecta type I treated with single screw or tension band wiring system: outcomes and pitfalls in relation to bone mineral density. Medicine (Baltimore). 2017;96(20):e6766. doi:10.1097/MD.0000000000006766.
14. Andersen MR, Winther NS, Lind T, Schrøder HM, Flivik G, Petersen MM. Low preoperative BMD is related to high migration of tibia components in uncemented TKA–92 patients in a combined DEXA and RSA study with 2-year follow-up. J Arthroplasty. 2017;32(7):2141-2146. doi:10.1016/j.arth.2017.02.032.
15. Yip BH, Yu FW, Wang Z, et al. Prognostic value of bone mineral density on curve progression: A longitudinal cohort study of 513 girls with adolescent idiopathic scoliosis. Sci Rep. 2016;6:39220. doi:10.1038/srep39220.
16. Pourabbas Tahvildari B, Erfani MA, Nouraei H, Sadeghian M. Evaluation of bone mineral status in adolescent idiopathic scoliosis. Clin Orthop Surg. 2014;6(2):180-184. doi:10.4055/cios.2014.6.2.180.
17. Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431-1440. doi:10.1007/s00586-008-0757-z.
18. Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry--a 3-year follow-up study. J Bone Miner Res. 2001;16(6):1056-1061. doi:10.1359/jbmr.2001.16.6.1056.
19. Arabmotlagh M, Pilz M, Warzecha J, Rauschmann M. Changes of femoral periprosthetic bone mineral density 6 years after treatment with alendronate following total hip arthroplasty J Orthop Res. 2009;27(2):183-188. doi:10.1002/jor.20748.
20. Gruen TA, McNeice GM, Amstutz HC. Modes of failure of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979;(141):17-27.
21. Zeytinoglu M, Jain RK, Vokes TJ. Vertebral fracture assessment: Enhancing the diagnosis, prevention, and treatment of osteoporosis. Bone. 2017;104:54-65. doi:10.1016/j.bone.2017.03.004.
22. Kiebzak GM. Radiolucent casting tape allows for accurate measurement of forearm bone mineral density using dual-energy X-ray absorptiometry. J Clin Densitom. 1998;1(4):369-374.
23. Sung KH, Chung CY, Lee KM, et al. Correlation between central and peripheral bone mineral density around the elbow measured by dual-energy x-ray absorptiometry in healthy children and adolescents. J Clin Densitom. 2017;20(1):114-119. doi:10.1016/j.jocd.2016.04.007.
24. Hamdy R, Kiebzak GM, Seier E, Watts NB. The prevalence of significant left-right differences in hip bone mineral density. Osteoporos Int. 2006;17(12):1772-1780. doi:10.1007/s00198-006-0192-0.
25. Kelly TL, Berger N, Richardson TL. DXA body composition: Theory and practice. Appl Radiat Isot. 1998;49(5-6):511-513.
26. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3(1):35-41.
27. Bilborough JC, Greenway k, Par D, Coutts AJ. The accuracy and precision of DXA for assessing body composition in team sport athletes. J Sports Sci. 2014;32(19):1821-1828. doi:10.1080/02640414.2014.926380.
28. Malkov S, Cawthon PM, Peters KW, et al. Health ABC Study. Hip fractures risk in older men and women associated with DXA-derived measures of thigh subcutaneous fat thickness, cross-sectional muscle area, and muscle density. J Bone Miner Res. 2015;30(8):1414-1421. doi:10.1002/jbmr.2469.
29. Arangio GA, Chen C, Klady M, Reed JF. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther. 1997;26(5):238-245. doi:10.2519/jospt.1997.26.5.238.
30. Ledford CK, Millikan PD, Nickel BT, et al. Percent body fat Is more predictive of function after total joint arthroplasty than body mass index. J Bone Joint Surg. 2016;98(10):849-857. doi:10.2106/JBJS.15.00509.
31. Berlet G, Kiebzak GM, Dandar A, et al. Prospective analysis of body composition and SF36 profiles in professional dancers over a 7-month season: is there a correlation to injury? J Dance Med Sci. 2002;6(2):54-61.
32. Grant JA, Bedi A, Kurz J, Bancroft R, Gagnier JJ, Miller BS. Ability of preseason body composition and physical fitness to predict the risk of injury in male collegiate hockey players. Sports Health. 2015;7(1):45-51. doi:10.1177/1941738114540445.
33. Stewart AD, Hannan J. Subregional tissue morphometry in male athletes and controls using DXA. Int J Sport Nutr Exerc Metab. 2000;10(2):157-169. doi:10.1123/ijsnem.10.2.157.
34. Sannicandro I, Cofano G, Rosa RA, Piccinno A. Balance training exercises decrease lower-limb strength asymmetry in young tennis players. J Sports Sci Med. 2014;13(2):397-402.
35. Guglielmi G, Ponti F, Agostini M, Amadori M, Battista G, Bazzocchi A. The role of DXA in sarcopenia. Aging Clin Exp Res. 2016;28(6):1047-1060. doi:10.1007/s40520-016-0589-3.
36. Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413-421.
37. Landi F, Calvani R, Ortolani E, et al. The association between sarcopenia and functional outcomes among older patients with hip fracture undergoing in-hospital rehabilitation. Osteoporos Int. 2017;28(5):1569-1576. doi:10.1007/s00198-017-3929-z.
38. Roh YH, Noh JH, Gong HS, Baek GH. Effect of low appendicular lean mass, grip strength, and gait speed on the functional outcome after surgery for distal radius fractures. Arch Osteoporos. 2017;12(1):41. doi:10.1007/s11657-017-0335-2.
39. Miller MS, Callahan DM, Toth MJ. Skeletal muscle myofilament adaptations to aging, disease, and disuse and their effects on whole muscle performance in older adult humans. Front Physiol. 2014;5:369. doi:10.3389/fphys.2014.00369.
40. Waters DJ, Baumgartner RN. Sarcopenia and obesity. Clin Geriatr Med. 2011;27(3):401-421. doi:10.1016/j.cger.2011.03.007.
41. Bachrach LK, Gordon CM. Bone densitometry in children and adolescents. Pediatrics. 2016;138(4):e20162398. doi:10.1542/peds.2016-2398.
TAKE-HOME POINTS
- DXA is underutilized technology in orthopedics.
- More data ("ancillary data") are often available from a DXA scan then typically included in a standard report from a referral center.
- Most orthopedists are likely unaware of the detailed body composition data available with a total body scan.
- Preoperative DXA scans and knowledge of BMD may be informative when planning the type of fixation and implant metal to used.
- Serial follow-up body composition scans can be useful in monitoring the course of bone healing (mineralization) and soft tissue changes (fat and lean mass).
Blood Loss Reduction with Tranexamic Acid and a Bipolar Sealer in Direct Anterior Total Hip Arthroplasty
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
ABSTRACT
The purpose of this study is to determine the effectiveness of tranexamic acid (TXA) alone and in conjunction with a bipolar sealer in reducing postoperative transfusions during direct anterior (DA) total hip arthroplasty (THA).
In this retrospective review, we analyzed 173 consecutive patients who underwent primary unilateral DA THA performed by 2 surgeons during a 1-year period. Subjects were divided into 3 groups based on TXA use: 63 patients received TXA alone (TXA group), 49 patients received TXA in addition to a bipolar sealer (TXA + bipolar sealer group), and 61 patients received neither TXA nor a bipolar sealer (control group). Primary end points were the transfusion rate and estimated blood loss. Secondary end points were length of stay, postoperative drop in hemoglobin, and postoperative drain output.
Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). No significant difference in the rate of transfusion was found between the TXA group and the TXA + bipolar sealer group (P = .99). Estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group.
The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements. The addition of a bipolar sealer, however, does not appear to provide any additional decrease in blood loss.
Historically, patients undergoing total hip arthroplasty (THA) have significant blood loss and required blood transfusions.1-3 Blood transfusions increase not only the risk of complications but also the cost of the procedure.4-9 Although less invasive techniques in hip surgery may decrease blood loss,10-12 intraoperative blood loss remains a concern. Optimization of anemia and blood conservation techniques include preoperative autologous blood donation, perioperative hemodilution, meticulous surgical hemostasis, and the use of antifibrinolytic agents.4,5,7,13,14 Antifibrinolytics are inexpensive and have been shown to reduce blood loss during THA and total knee arthroplasty (TKA).7,15-17
Continue to: Tranexamic acid (TXA), a synthetic analog...
Tranexamic acid (TXA), a synthetic analog of the amino acid lysine, is one antifibrinolytic that has recently been adopted in total joint arthroplasty. TXA competitively inhibits the lysine binding site of plasminogen, inhibiting fibrinolysis and leading to clot stabilization.18-20 Because of its safety and low cost, TXA has been readily accepted. The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation. In contrast to standard electrocautery, a bipolar sealer uses saline to maintain tissue temperatures at <100°C, minimizing damage to surrounding tissues.21 Many applications of a bipolar sealer have been reported in the fields of surgical oncology,21 pulmonary surgery,21 liver resection,22 THA23,24 and TKA,25,26 and spine surgery.27 We recently published our reduction in transfusion rates during direct anterior (DA) THA with use of a bipolar sealer.28
Although many studies have analyzed the use of TXA and a bipolar sealer with the posterior and lateral approaches to hip arthroplasty, there is a paucity of research analyzing its use in the DA approach. This study retrospectively reviews the effectiveness of TXA alone and in conjunction with a bipolar sealer in reducing allogeneic blood transfusions in DA THA.
METHODS
This is a retrospective, comparative study evaluating the efficacy of TXA with and without a bipolar sealer in unilateral DA THA. The study included 173 patients who underwent standard DA THA performed by 2 surgeons in the period April 2013 to April 2014. Patient demographic information is summarized in Table 1.
Table 1. Demographic Data
| All (N = 173) | TXA Only (n = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Age (y)a | 64.8 ± 10.5 (28.4-87.6) | 66.9 ± 9.9 (47.2-87.6) | 62.1 ± 11.0 (28.4-86.3) | 64.7 ± 10.4 (38.3-85.8) | .31 | .24 | .03 |
Genderb |
|
|
|
| .99 | 0.95 | .94 |
Male | 82 (47.4%) | 30 (47.6%) | 23 (46.9%) | 29 (47.5%) |
|
|
|
Female | 91 (52.6%) | 33 (52.4%) | 26 (53.1%) | 32 (52.5%) |
|
|
|
BMI (kg/m2)a | 27.9 ± 4.4 (17.5-40.6) | 27.8 ± 3.3 (21.6-35.9) | 29.1 ± 5.3 (17.8-40.6) | 27.0 ± 4.5 (17.5-39.8) | .16 | .03 | .13 |
Preoperative hemoglobin levela | 13.6 ± 1.3 (10.5-17.2) | 13.9 ± 1.2 (11.5-17.1) | 13.5 ± 1.4 (10.5-16.6) | 13.5 ± 1.2 (10.5-17.2) | .10 | .98 | .10 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviations: BMI, body mass index; TXA, tranexamic acid.
Three cohorts were created based on intraoperative blood loss management practices at the surgeon’s discretion. The first group included 63 patients who underwent DA THA with TXA but not a bipolar sealer. The second group included 49 patients who underwent DA THA with TXA and a bipolar sealer. The third (control) group included 61 patients who underwent DA THA without TXA or a bipolar sealer. Data for the control group were collected prospectively as a part of a randomized trial, which demonstrated a reduction in transfusion requirements and blood loss with the use of a bipolar sealer in DA THA.28 All patients received a surgical hemovac suction drain, which was removed at 24 hours after surgery. All patients received 40 mg of enoxaparin daily for 2 weeks for venous thromboembolism prophylaxis starting the day after surgery.
All patients in the first 2 groups received 2 g of TXA administered intravenously in 2 doses: the first dose was given preoperatively, and the second dose was given immediately postoperatively in the recovery room. The bipolar sealer was utilized as needed perioperatively according to the manufacturer’s instructions to address specific bleeding targets. The common sites and steps of a DA THA, in which bleeding typically occurs, are:
- The medial femoral circumflex artery during the approach to the capsule;
- The anterior hip capsule vessels prior to capsulotomy;
- The deep branch of the medial femoral circumflex artery and the nutrient vessels to the lesser trochanter encountered while exposing the medial neck and releasing the medial capsule;
- The posterior-superior retinacular arteries encountered after femoral neck osteotomy and removal of the femoral head along the posterior capsule; and
- The branch of the obturator artery encountered during exposure of the acetabular fovea.29-31
At the time of this study, the transfusion criteria included hemoglobin <8 g/dL in the presence of clinical symptoms.
Continue to: Primary outcome measures...
OUTCOME MEASURES AND DATA ANALYSIS
Primary outcome measures were transfusion requirements and estimated blood loss. Secondary outcome measures were postoperative decrease in hemoglobin, length of stay, and postoperative drain output. Demographic and operative data were compared between groups to ensure that there were no statistically significant differences in blood loss and transfusion requirements. All data were recorded in a password encrypted file and subsequently transferred to the REDCap system (Research Electronic Data Capture, Vanderbilt University).
STATISTICAL ANALYSIS
A priori sample size calculation was performed on the basis of a prior study 28, which evaluated surgical blood loss reduction utilizing a bipolar sealer. This study suggested a sample size of 20 per group to detect the minimal clinically important difference of 1.5 (standard deviation (SD) = 1.5, α = 0.05, β = 0.20). Additionally, a general estimate for detecting a 1-unit change on an ordinal scale of 136 (SD = 1.0, α = 0.05, β = 0.20) resulted in the same number. We conservatively chose to include at least 24 patients in each study arm in the event of greater true variance. The Wilcoxon rank-sum test was used for comparison of continuous data between groups. Differences between means were analyzed using 2-sided t tests. Comparison of categorical data was performed using Pearson’s chi-square or Fisher’s exact probability test as indicated. Ordinal ranking scores were compared using the Mantel-Haenszel test.
RESULTS
There were no statistically significant differences between groups with respect to sex, age, body mass index, or preoperative hemoglobin level (Table 1). Two patients in the TXA group and 10 patients in the control group were transfused (P = .02). In the TXA + bipolar sealer group, 1 patient was transfused (P = .02). A comparison of the transfusion rate between the TXA group and the TXA + bipolar sealer group yielded no significant difference (P = .99). The estimated blood loss was 310.3 mL ± 182.5 mL in the TXA group (P = .004), 292.9 mL ± 130.8 mL in the TXA + bipolar sealer group (P = .003), and 404.9 mL ± 201.2 mL in the control group (P = .71) (Table 2).
Table 2. Patient-Related Outcomes
| TXA Only (N = 63) | TXA + Bipolar Sealer (n = 49) | Control (n = 61) | P-value (TXA vs Control) | P-value (TXA + Sealer vs Control) | P-value (TXA + Sealer vs TXA) |
Patients Transfuseda | 2 (3.2%) | 1 (2.0%) | 10 (16.4%) | .02 | .02 | .99 |
Hemoglobin Drop (g/dL)b = preoperative Hb-lowest Hb | 3.5 ± 0.8 (1.8-6.3) | 3.5 ± 1.1 (1.7-6.0) | 4.3 ± 1.2 (2.0-7.5) | <.001 | <.001 | .60 |
Total Drain Output (mL)b | 326.3 ± 197.5 (15-1050) | 309.8 ± 196.3 (20-920) | 473.6 ± 199.7 (90-960) | <.001 | <.001 | .58 |
Calculated Blood Loss (mL)b = 1000 x total Hb loss/preoperative Hb | 1217.8 ± 335.8 (573.0-2514.4) | 1289.5 ± 382.4 (536.1-2418.2) | 1514.7 ± 467.9 (789.4-3451.1) | <.001 | .005 | .43 |
Estimated Blood Loss (mL)b | 310.3 ± 182.5 (100-1400) | 292.9 ± 130.8 (75-600) | 404.9 ± 201.2 (150-1000) | .004 | .003 | .71 |
Length of Stay (d)a | 2.2 ± 0.6 (1-4) | 2.2 ± 0.9 (1-5) | 2.6 ± 0.8 (1-5) | .004 | .03 | .78 |
aResult values are expressed as mean ± standard deviation (range). bResult values are expressed as number of cases (percentage of column header population).
Abbreviation: TXA, tranexamic acid.
The total drain output was 326.3 mL ± 197.5 mL in the TXA group (P < .001 for comparison with the control group), 309.8 mL ± 196.3 mL in the TXA + bipolar sealer group (P < .001 for comparison with the control group), and 473.6 mL ± 199.7 mL in the control group (P = .58). The decrease in hemoglobin was 3.5 g/dL ± 0.8 g/dL in the TXA group (P < .001), 3.5 g/dL ± 1.1 g/dL in the TXA + bipolar sealer group (P < .001), and 4.3 g/dL ± 1.2 g/dL in the control group (Table 2). The length of stay was 2.2 ± 0.6 days for the TXA group (P = .004) and 2.2 ± 0.9 days (P = .03) for the TXA + bipolar sealer group, and 2.6 ± 0.8 days in the control group (P = .78) (Table 2).
DISCUSSION
This study shows that the use of TXA alone provides a significant decrease in transfusion rates and estimated blood loss, a benefit which was not further increased with the addition of a bipolar sealer (Table 2). Many studies have demonstrated that TXA reduces blood loss and transfusion rates in patients undergoing THA and TKA.29 However, TXA’s acceptance as a more readily used hemostatic medication has been hindered by the theoretically increased risk of thromboembolism in susceptible, high-risk patients.32-35 In a 2012 meta-analysis conducted by Yang and colleagues,36 the use of TXA led to significantly less blood loss per patient and fewer transfusions without leading to an increased risk of thromboembolic events.
Continue to: Similarly, the bipolar sealer...
Similarly, the bipolar sealer has been shown to decrease transfusion rates and stabilize perioperative hemoglobin levels.25-27 In this recent prospective clinical trial evaluating the use of a bipolar sealer during DA THA, we observed decreased intraoperative blood loss and transfusion requirements in patients managed with a bipolar sealer.28 However, in a study conducted by Barsoum and colleagues37 evaluating the use of a bipolar sealer in THA with a posterior approach, there were no significant postoperative benefits in terms of blood loss, transfusion requirements, clinical evaluations, functionality, or health-related quality of life in patients managed with a bipolar sealer.
Although the results of our research are in line with those of previous publications, it is important to address 3 limitations within this study. First, only the control group in this study was enrolled prospectively; the remaining groups were reviewed retrospectively. Second, our adoption of TXA was recent; therefore, a confounding factor is that our surgeons had more experience in the anterior approach when using TXA. Third, the established transfusion threshold of <8 g/dl for this study led to more liberal use of transfusions. Since the conclusion of this study, we have adopted stricter transfusion criteria (hemoglobin <7.0 g/dL with clinical symptoms) which has led to even lower transfusion requirements.
CONCLUSION
In the reviewed patient population, TXA decreased blood loss and transfusion requirements following DA THA. However, the addition of a bipolar sealer did not provide an advantage. The results of this study do not support the routine use of a bipolar sealer in DA THA.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
1. Sehat KR, Evans R, Newman JH. How much blood is really lost in total knee and hip arthroplasty? Knee. 2000;7(3):151-155.
2. Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC. Blood loss and replacement in total hip arthroplasty: a multicenter study. The Preoperative Autologous Blood Donation Study Group. Transfusion. 1992;32(1):63-67.
3. Pierson JL, Hannon TJ, Earles DR. A blood-conservation algorithm to reduce blood transfusions after total hip and knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(7):1512-1518.
4. Gill JB, Rosenstein A. The use of antifibrinolytic agents in total hip arthroplasty. J Arthroplasty. 2006;21(6):869-873.
5. Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93(1):39-46. doi:10.1302/0301-620X.93B1.24984.
6. Rajesparan K, Biant LC, Ahmad M, Field RE. The effect of an intravenous bolus of tranexamic acid on blood loss in total hip replacement. J Bone Joint Surg Br. 2009;91(6):776-783. doi:10.1302/0301-620X.91B6.22393.
7. Hynes MC, Calder P, Rosenfeld P, Scott G. The use of tranexamic acid to reduce blood loss during total hip arthroplasty: an observational study. Ann R Coll Surg Engl. 2005;87(2):99-101. doi:10.1308/147870805X28118.
8. Earnshaw P. Blood conservation in orthopaedic surgery: the role of epoetin alfa. Int Orthop. 2001;25(5):273-278. doi:10.1007/s002640100261.
9. Kleinman S, Chan P, Robillard P. Risks associated with transfusion of cellular blood components in Canada. Transfus Med Rev. 2003;17(2):120-162. doi:10.1053/tmrv.2003.50009.
10. Lovell TP. Single-incision direct anterior approach for total hip arthroplasty using a standard operating table. J Arthroplast. 2008;23(7 Suppl):64-68. doi:10.1016/j.arth.2008.06.027.
11. Wojciechowski P, Kusz D, Kopeć K, Borowski M. Minimally invasive approaches in total hip arthroplasty. Ortop Traumatol Rehabil. 2007;9(1):1-7.
12. Rachbauer F, Krismer M. [Minimally invasive total hip arthroplasty via direct anterior approach]. Oper Orthop Traumatol. 2008;20(3):239-251. doi:10.1007/s00064-008-1306-y.
13. Johansson T, Pettersson LG, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money: a randomized, double-blind study in 100 patients. Acta Orthop. 2005;76(3):314-319.
14. Claeys MA, Vermeersch N, Haentjens P. Reduction of blood loss with tranexamic acid in primary total hip replacement surgery. Acta Chir Belg. 2007;107(4):397-401.
15. Ido K, Neo M, Asada Y, et al. Reduction of blood loss using tranexamic acid in total knee and hip arthroplasties. Arch Orthop Trauma Surg. 2000;120(9):518-520.
16. Benoni G, Fredin H, Knebel R, Nilsson P. Blood conservation with tranexamic acid in total hip arthroplasty: a randomized, double-blind study in 40 primary operations. Acta Orthop Scand. 2001;72(5):442-448. doi:10.1080/000164701753532754.
17. Ekbäck G, Axelsson K, Ryttberg L, et al. Tranexamic acid reduces blood loss in total hip replacement surgery. Anesth Analg. 2000;91(5):1124-1130.
18. Ralley FE, Berta D, Binns V, Howard J, Naudie DDR. One intraoperative dose of tranexamic acid for patients having primary hip or knee arthroplasty. Clin Orthop Relat Res. 2010;468(7):1905-1911. doi:10.1007/s11999-009-1217-8.
19. Eubanks JD. Antifibrinolytics in major orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(3):132-138.
20. Astedt B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl. 1987;137:22-25.
21. Kirschbaum A, Kunz J, Steinfeldt T, Pehl A, Meyer C, Bartsch DK. Bipolar impedance-controlled sealing of the pulmonary artery with SealSafe G3 electric current: determination of bursting pressures in an ex vivo model. J Surg Res. 2014;192(2):611-615. doi:10.1016/j.jss.2014.07.014.
22. Romano F, Garancini M, Uggeri F, et al. Bleeding in hepatic surgery: sorting through methods to prevent it. HPB Surg. 2012;2012:169351. doi:10.1155/2012/169351.
23. Marulanda GA, Ulrich SD, Seyler TM, Delanois RE, Mont MA. Reductions in blood loss with a bipolar sealer in total hip arthroplasty. Expert Rev Med Devices. 2008;5(2):125-131. doi:10.1586/17434440.5.2.125.
24. Rosenberg AG. Reducing blood loss in total joint surgery with a saline-coupled bipolar sealing technology. J Arthroplast. 2007;22(4 Suppl 1):82-85. doi:10.1016/j.arth.2007.02.018.
25. Marulanda GA, Krebs VE, Bierbaum BE, et al. Haemostasis using a bipolar sealer in primary unilateral total knee arthroplasty. Am J Orthop. 2009;38(12):E179-E183.
26. Weeden SH, Schmidt RH, Isabell G. Haemostatic efficacy of a bipolar sealing device in minimally invasive total knee arthroplasty. J Bone Joint Surg Br Proceedings. 2009;91-B:45.
27. Gordon ZL, Son-Hing JP, Poe-Kochert C, Thompson GH. Bipolar sealer device reduces blood loss and transfusion requirements in posterior spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop. 2013;33(7):700-706. doi:10.1097/BPO.0b013e31829d5721.
28. Suarez JC, Slotkin EM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a bipolar sealer in direct anterior approach total hip arthroplasty. J Arthroplasty. 2015;30(11):1953-1958. doi:10.1016/j.arth.2015.05.023.
29. Gautier E, Ganz K, Krügel N, Gill T, Ganz R. Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg. 2000;82(5):679-683. doi:10.1302/0301-620x.82b5.10426.
30. Trueta J, Harrison MHM. The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br. 1953;35-B(3):442-461.
31. Sevitt S, Thompson RG. The distribution and anastomoses of arteries supplying the
head and neck of the femur. J Bone Joint Surg Br. 1965;47-B:560-573. doi:10.1302/0301-620X.47B3.560.
32. Saleh A, Hebeish M, Farias-Kovac M, et al. Use of hemostatic agents in hip and knee arthroplasty. JBJS. 2014;2(1):1-12. doi:10.2106/JBJS.RVW.M.00061.
33. Howes JP, Sharma V, Cohen AT. Tranexamic acid reduces blood loss after knee arthroplasty. J Bone Joint Surg Br. 1996;78(6):995-996.
34. Karkouti K. Is tranexamic acid indicated for total knee replacement surgery? Anesth Analg. 2000;91(1):244-245.
35. Graham ID, Alvarez G, Tetroe J, McAuley L, Laupacis A. Factors influencing the adoption of blood alternatives to minimize allogeneic transfusion: the perspective of eight Ontario hospitals. Can J Surg. 2002;45(2):132-140.
36. Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94(13):1153-1159. doi:10.2106/JBJS.K.00873.
37. Barsoum WK, Klika AK, Murray TG, Higuera C, Lee HH, Krebs VE. Prospective randomized evaluation of the need for blood transfusion during primary total hip arthroplasty with use of a bipolar sealer. J Bone Joint Surg Am. 2011;93(6):513-518. doi:10.2106/JBJS.J.00036.
TAKE-HOME POINTS
- TXA reduces blood loss and transfusion requirements in THA.
- The bipolar sealer enhances surgical hemostasis by sealing vessels at the surgical site through radiofrequency ablation.
- The use of TXA, with and without the concomitant use of a bipolar sealer, decreases intraoperative blood loss and postoperative transfusion requirements.
- The addition of a bipolar sealer did not offer an advantage to transfusion requirements in anterior THA.
- TXA should be used routinely in THA.
Coverage of Hand Defects with Exposed Tendons: The Use of Dermal Regeneration Template
ABSTRACT
Soft tissue defects associated with exposed tendon pose difficult reconstructive problems because of tendon adhesions, poor range of motion, poor cosmetic appearance, and donor site morbidity. Dermal regeneration template is a skin substitute widely used in reconstructive surgery, including the occasional coverage of tendons. However, postoperative functionality of the tendons has not been well documented. We report a case of using dermal regeneration template for soft tissue reconstruction overlying tendons with loss of paratenon in a patient with Dupuytren’s contracture. Dermal regeneration template may offer an alternative option for immediate tendon coverage in the hand.
Soft tissue defects overlying exposed tendon with loss of paratenon often precipitate poor clinical outcomes because of the dichotomous demands of both closing the overlying soft-tissue defect and providing a gliding surface for the underlying tendons.1 Although avoidance of adhesions and restoration of function are the primary goals of the procedure, satisfactory appearance is also desirable. Likewise, any form of coverage should ideally provide good vasculature required for complete healing and an early form of closure following débridement.2 Simple skin grafts do not adequately meet these demands because they result in a high rate of tendon adhesions,3 and also are limited in patients with limited donor skin availability or questionable underlying wound bed viability, such as in scleroderma.
In order to reduce the frequency of tendon adhesions by creating a gliding surface, the use of interpositional materials, both artificial and biologic, has been employed with varying degrees of success, including cellophane, chitosan membrane, fibrin sealant, autogenous fascial flaps, and autogenous venous grafts.4-7 Many of the autogenous flaps and grafts have been employed with good success.8 However, complications and donor site morbidity encourage alternative procedures, including the use of artificial substances.2,8-10
We present our clinical experience with a patient who underwent successful placement of Integra (Integra LifeSciences) Dermal Regeneration Template (DRT) directly over exposed tendons with a subsequent full-thickness skin graft several weeks later. The procedures were performed per the manufacturer’s specifications, resulting in 2 stages of reconstruction. In our experience, DRT can offer immediate coverage unrestricted by wound size, and provides shorter operative time and decreased donor site and surgical morbidity compared with flap coverage, while demonstrating good cosmetic results. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 74-year-old right-handed man with Dupuytren’s contracture was evaluated for recurrent symptomatic contracture causing difficulty with daily activities. He reported palpable cords and contractures in the ring and small fingers of the right hand. He had 2 prior open surgical procedures, including palmar and digital fasciectomy of both hands. On the right hand, the ring and small fingers demonstrated 90° proximal interphalangeal (PIP) and 60° metacarpophalangeal (MCP) flexion contractures. Palpable central cords were present on the flexor surfaces of both the ring and small fingers. A well-healed surgical incision, performed 22 years earlier, was present over the palmar aspect of the ring finger.
Continue to: With consideration given...
With consideration given to the patient’s recurrent contracture after a prior surgical procedure, we discussed surgical excision of the diseased cords in order to eliminate the possibility of a second recurrence and maximize the gain of motion. Following discussion with the patient, we performed palmar and digital fasciectomy of the ring and small finger contractures. Postoperatively, the patient was followed closely for wound complications and vascular status. On his return to our clinic 11 days later, the patient was noted to have dehiscence of the digital wounds in the ring and small fingers (Figure 1).
STAGE 1
During the first stage, completed 14 days following the index procedure, débridement of the wounds was performed, followed by provisional DRT coverage of the tendons, secured with 5-0 nylon sutures (Figure 2).
STAGE 2
At approximately 2 weeks after application of the DRT, a full-thickness skin graft was applied. The thickness of the graft was chosen to allow for durable coverage of the palmar skin defects. Upon successful completion of the second stage, the patient was followed and evaluated for complete wound healing. On performing an examination 14 days after surgery, the ring and small fingers demonstrated only partially healed skin graft but significantly improved range of motion (ROM), with 40° to 90° arc of motion in the PIP joint and 25° to 90° arc of motion in the MCP joint (Figure 4). Owing to their limited size, the wounds were treated with dressing changes until successful healing (Figure 5).
Hand therapy was instituted to achieve maximum mobility for covered soft tissue and tendons and to maximize tendon gliding. At 1-year follow-up, the skin was fully healed and the patient’s active PIP motion was 30° to 90°, active MCP motion was 0° to 90°, and grip strength was 90 lb on both sides. The tendons glided under a well-vascularized tissue at the DRT placement site, and no secondary tenolysis procedure was deemed necessary.
DISCUSSION
Soft tissue defects with exposed tendons may offer a number of challenges for coverage. The primary concern is the creation of a gliding surface and the restoration of a functional tendon without adhesions.2 However, surgeons must use their own clinical judgment when choosing the method of coverage so as to minimize the effects of donor site morbidity and maximize the overall functional and cosmetic outcomes. All options must be considered while selecting a material or flap that is likely to survive in the relatively avascular tendon plane.2,8,11 When considering the reconstructive ladder, skin grafts may not represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum have been damaged. That leaves the surgeon with local flaps, regional flaps, free flaps, and skin substitutes.
Continue to : Before planning closure...
Before planning closure, wound conditions should be optimized, including wound bed quality, vascularization, and bacterial loads. Experimental data suggest that the bacterial load should be brought down below a critical level of 105 bacteria per g of tissue to allow a skin graft to take. This may be problematic from a practical standpoint because quantitative bacterial cultures take about 48 hours to obtain the result, long after a decision to graft is made. As a result, the surgeon may take an aggressive approach to wound débridement, making sure that all necrotic material has been sharply débrided prior to coverage.
As Levin12 noted in 1993, decisions regarding repair of any soft tissue defect may follow a well-delineated ladder beginning with the primary choice of split-thickness skin grafts and ending with free flaps. When treating tissue defects in the hand complex, flaps are an excellent option as they replace like with like, allow minimal scarring and early rehabilitation. 13,14 Nevertheless, a few general disadvantages are inherent in flap procedure: increase in operating time, risk of flap loss, and in case of free flaps, knowledge, experience, and microsurgical ability.2 In reference to complications, the rate of flap loss found by Khouri and colleagues15 was 4.1% with a 12.1% chance of incurring some measured complication, including wound dehiscence, arterial insufficiency, and flap necrosis.
Likewise, some of the conventional local and free flaps, including cutaneous and muscular flaps, prove ineffective in preventing tendon adhesions, create unsightly postoperative contours, or increase the area of trauma on the wounded hand, encouraging the use of free fascial flaps.11 Among the wide array of potential free fascial flaps, the temporoparietal, scapular, lateral arm, radial forearm, and free serratus fascial flaps are some of the most popular for hand defects.8,9 However, these procedures require an additional surgical site, meticulous dissection, microsurgical technique at times, and increased operating cost and time.2,8-10 Furthermore, free fascial flaps have demonstrated occasional partial flap loss and a decreased survival of the overlying skin graft, leading some to advocate delayed skin graft placement.10,16,17
On the basis of these complications, Bray and colleagues11 noted that the utility of free flaps may be limited in smaller clinical settings. The primary disadvantage of using DRTs is the necessity for a second operative procedure to harvest and place the skin graft. Traditionally, this is performed 2 to 3 weeks after the initial DRT application. Nevertheless, a 1-stage procedure can be performed in an outpatient setup, minimizing the burden to the patient and the medical costs, followed by secondary intention healing.
In response to critics of the 2-stage technique, Sanger and colleagues18 described single-stage use of DRT with split-thickness skin grafts with placement of an overlying wound vacuum-assisted closure to help speed incorporation of the DRT and improve survival of the immediately grafted skin. Another viable alternative is the McCash open-palm technique.19 In the open-palm technique, a Brunner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A partial fasciectomy is performed in the palm and digit. After release, the digital incision is closed, and the palmar incision is left open. Although this well-studied and well-reported technique is known to reduce the risk of flap necrosis due to tension and hematoma,20 its main application is in the palm, as the name implies. Because in our patient the defect was palmar-digital with exposed “white structures,” we elected to use DRT.
Continue to: Although there is still...
Although there is still no perfect answer for wound coverage and closure in the hand with exposed or damaged tendons, DRT certainly performs well as a primary choice by minimizing adhesions; allowing a good ROM; and providing a durable, satisfactory cosmetic outcome. Likewise, an initial treatment with DRT does not preclude later, more elaborate reconstructive efforts, such as local or free flaps, if they continue to be indicated. DRT also does not diminish the ability to revise a tendon reconstruction if a secondary procedure is necessary. In our patient, tendon revision has not been necessary. DRT gives the surgeon a minimally invasive, efficient initial alternative to more labor-intensive, potentially morbid reconstructive procedures, without sacrificing outcome. Therefore, DRT can offer an alternative procedure in the surgeon’s armamentarium for tendon coverage in complex hand defects.
1. Flügel A. Kehrer C. Heitmann C, German G, Sauerbier M. Coverage of soft tissue defects of the hand with free fascial flaps. Microsurgery.2005;25(1):47-53.
2. Chen H, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15(4):541-554.
3. Chia J, Lim A, Peng YP. Use of an arterialized venous flap for resurfacing a circumferential soft tissue defect of a digit. Microsurgery. 2001; 21(8):374-378.
4. Wheeldon T. The use of cellophane as a permanent tendon sheath. J Bone J Surg Am; 1939;21(2):393-396.
5. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am. 1993;18(1):68-75.
6. Jones NF, Lister GD. Free skin and composite flaps. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative hand surgery. 6th ed. New York, NY: Churchill Livingstone; 2011:1721-1756.
7. Yan D, Shi X, Lui Q. Reconstruction of tendon sheath by autogenous vein graft in preventing adhesion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11(1):38-39.
8. Pederson WC. Upper extremity microsurgery. Plast Reconstr Surg. 2001;107(6):1524-1537; discussion 1538-15399, 1540-1543.
9. WintschK, Helaly P. Free flap of gliding tissue. J Reconstr Microsurg. 1986;2(3):143-151.
10. Meland NB, Weimar R. Microsurgical reconstruction: experience with free fascia flaps. Ann Plast Surg. 1991;27(1):1-8.
11. Bray PW, Boyer MI, Bowen CV. Complex injuries of the forearm. Coverage considerations. Hand Clin. 1997;13(2):263-278.
12. Levin LS. The reconstructive ladder: an orthoplastic approach. Ortho Clin North Am. 1993; 24(3):393-409.
13. Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48 (5):913-917. doi:10.1097/00005373-200005000-00016.
14. Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53(1):61-65. doi:10.1097/00005373-200207000-00013.
15. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711-721.
16. Woods JM 4th, Shack RB, Hagan KF. Free temporoparietal fascia flap in reconstruction of the lower extremity. Ann Plast Surg. 1995;34(5):501-506. doi:10.1097/00000637-199505000-00008.
17. Chung KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002;27(3):525-533.
18. Sanger C, Molnar JA, Newman CE, et al. Immediate skin grafting of an engineered dermal substitute: P37. Plast Reconstr Surg. 2005;116(3S):165.
19. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-280.
20. Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg Br. 1996;21(4):484-485.
ABSTRACT
Soft tissue defects associated with exposed tendon pose difficult reconstructive problems because of tendon adhesions, poor range of motion, poor cosmetic appearance, and donor site morbidity. Dermal regeneration template is a skin substitute widely used in reconstructive surgery, including the occasional coverage of tendons. However, postoperative functionality of the tendons has not been well documented. We report a case of using dermal regeneration template for soft tissue reconstruction overlying tendons with loss of paratenon in a patient with Dupuytren’s contracture. Dermal regeneration template may offer an alternative option for immediate tendon coverage in the hand.
Soft tissue defects overlying exposed tendon with loss of paratenon often precipitate poor clinical outcomes because of the dichotomous demands of both closing the overlying soft-tissue defect and providing a gliding surface for the underlying tendons.1 Although avoidance of adhesions and restoration of function are the primary goals of the procedure, satisfactory appearance is also desirable. Likewise, any form of coverage should ideally provide good vasculature required for complete healing and an early form of closure following débridement.2 Simple skin grafts do not adequately meet these demands because they result in a high rate of tendon adhesions,3 and also are limited in patients with limited donor skin availability or questionable underlying wound bed viability, such as in scleroderma.
In order to reduce the frequency of tendon adhesions by creating a gliding surface, the use of interpositional materials, both artificial and biologic, has been employed with varying degrees of success, including cellophane, chitosan membrane, fibrin sealant, autogenous fascial flaps, and autogenous venous grafts.4-7 Many of the autogenous flaps and grafts have been employed with good success.8 However, complications and donor site morbidity encourage alternative procedures, including the use of artificial substances.2,8-10
We present our clinical experience with a patient who underwent successful placement of Integra (Integra LifeSciences) Dermal Regeneration Template (DRT) directly over exposed tendons with a subsequent full-thickness skin graft several weeks later. The procedures were performed per the manufacturer’s specifications, resulting in 2 stages of reconstruction. In our experience, DRT can offer immediate coverage unrestricted by wound size, and provides shorter operative time and decreased donor site and surgical morbidity compared with flap coverage, while demonstrating good cosmetic results. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 74-year-old right-handed man with Dupuytren’s contracture was evaluated for recurrent symptomatic contracture causing difficulty with daily activities. He reported palpable cords and contractures in the ring and small fingers of the right hand. He had 2 prior open surgical procedures, including palmar and digital fasciectomy of both hands. On the right hand, the ring and small fingers demonstrated 90° proximal interphalangeal (PIP) and 60° metacarpophalangeal (MCP) flexion contractures. Palpable central cords were present on the flexor surfaces of both the ring and small fingers. A well-healed surgical incision, performed 22 years earlier, was present over the palmar aspect of the ring finger.
Continue to: With consideration given...
With consideration given to the patient’s recurrent contracture after a prior surgical procedure, we discussed surgical excision of the diseased cords in order to eliminate the possibility of a second recurrence and maximize the gain of motion. Following discussion with the patient, we performed palmar and digital fasciectomy of the ring and small finger contractures. Postoperatively, the patient was followed closely for wound complications and vascular status. On his return to our clinic 11 days later, the patient was noted to have dehiscence of the digital wounds in the ring and small fingers (Figure 1).
STAGE 1
During the first stage, completed 14 days following the index procedure, débridement of the wounds was performed, followed by provisional DRT coverage of the tendons, secured with 5-0 nylon sutures (Figure 2).
STAGE 2
At approximately 2 weeks after application of the DRT, a full-thickness skin graft was applied. The thickness of the graft was chosen to allow for durable coverage of the palmar skin defects. Upon successful completion of the second stage, the patient was followed and evaluated for complete wound healing. On performing an examination 14 days after surgery, the ring and small fingers demonstrated only partially healed skin graft but significantly improved range of motion (ROM), with 40° to 90° arc of motion in the PIP joint and 25° to 90° arc of motion in the MCP joint (Figure 4). Owing to their limited size, the wounds were treated with dressing changes until successful healing (Figure 5).
Hand therapy was instituted to achieve maximum mobility for covered soft tissue and tendons and to maximize tendon gliding. At 1-year follow-up, the skin was fully healed and the patient’s active PIP motion was 30° to 90°, active MCP motion was 0° to 90°, and grip strength was 90 lb on both sides. The tendons glided under a well-vascularized tissue at the DRT placement site, and no secondary tenolysis procedure was deemed necessary.
DISCUSSION
Soft tissue defects with exposed tendons may offer a number of challenges for coverage. The primary concern is the creation of a gliding surface and the restoration of a functional tendon without adhesions.2 However, surgeons must use their own clinical judgment when choosing the method of coverage so as to minimize the effects of donor site morbidity and maximize the overall functional and cosmetic outcomes. All options must be considered while selecting a material or flap that is likely to survive in the relatively avascular tendon plane.2,8,11 When considering the reconstructive ladder, skin grafts may not represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum have been damaged. That leaves the surgeon with local flaps, regional flaps, free flaps, and skin substitutes.
Continue to : Before planning closure...
Before planning closure, wound conditions should be optimized, including wound bed quality, vascularization, and bacterial loads. Experimental data suggest that the bacterial load should be brought down below a critical level of 105 bacteria per g of tissue to allow a skin graft to take. This may be problematic from a practical standpoint because quantitative bacterial cultures take about 48 hours to obtain the result, long after a decision to graft is made. As a result, the surgeon may take an aggressive approach to wound débridement, making sure that all necrotic material has been sharply débrided prior to coverage.
As Levin12 noted in 1993, decisions regarding repair of any soft tissue defect may follow a well-delineated ladder beginning with the primary choice of split-thickness skin grafts and ending with free flaps. When treating tissue defects in the hand complex, flaps are an excellent option as they replace like with like, allow minimal scarring and early rehabilitation. 13,14 Nevertheless, a few general disadvantages are inherent in flap procedure: increase in operating time, risk of flap loss, and in case of free flaps, knowledge, experience, and microsurgical ability.2 In reference to complications, the rate of flap loss found by Khouri and colleagues15 was 4.1% with a 12.1% chance of incurring some measured complication, including wound dehiscence, arterial insufficiency, and flap necrosis.
Likewise, some of the conventional local and free flaps, including cutaneous and muscular flaps, prove ineffective in preventing tendon adhesions, create unsightly postoperative contours, or increase the area of trauma on the wounded hand, encouraging the use of free fascial flaps.11 Among the wide array of potential free fascial flaps, the temporoparietal, scapular, lateral arm, radial forearm, and free serratus fascial flaps are some of the most popular for hand defects.8,9 However, these procedures require an additional surgical site, meticulous dissection, microsurgical technique at times, and increased operating cost and time.2,8-10 Furthermore, free fascial flaps have demonstrated occasional partial flap loss and a decreased survival of the overlying skin graft, leading some to advocate delayed skin graft placement.10,16,17
On the basis of these complications, Bray and colleagues11 noted that the utility of free flaps may be limited in smaller clinical settings. The primary disadvantage of using DRTs is the necessity for a second operative procedure to harvest and place the skin graft. Traditionally, this is performed 2 to 3 weeks after the initial DRT application. Nevertheless, a 1-stage procedure can be performed in an outpatient setup, minimizing the burden to the patient and the medical costs, followed by secondary intention healing.
In response to critics of the 2-stage technique, Sanger and colleagues18 described single-stage use of DRT with split-thickness skin grafts with placement of an overlying wound vacuum-assisted closure to help speed incorporation of the DRT and improve survival of the immediately grafted skin. Another viable alternative is the McCash open-palm technique.19 In the open-palm technique, a Brunner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A partial fasciectomy is performed in the palm and digit. After release, the digital incision is closed, and the palmar incision is left open. Although this well-studied and well-reported technique is known to reduce the risk of flap necrosis due to tension and hematoma,20 its main application is in the palm, as the name implies. Because in our patient the defect was palmar-digital with exposed “white structures,” we elected to use DRT.
Continue to: Although there is still...
Although there is still no perfect answer for wound coverage and closure in the hand with exposed or damaged tendons, DRT certainly performs well as a primary choice by minimizing adhesions; allowing a good ROM; and providing a durable, satisfactory cosmetic outcome. Likewise, an initial treatment with DRT does not preclude later, more elaborate reconstructive efforts, such as local or free flaps, if they continue to be indicated. DRT also does not diminish the ability to revise a tendon reconstruction if a secondary procedure is necessary. In our patient, tendon revision has not been necessary. DRT gives the surgeon a minimally invasive, efficient initial alternative to more labor-intensive, potentially morbid reconstructive procedures, without sacrificing outcome. Therefore, DRT can offer an alternative procedure in the surgeon’s armamentarium for tendon coverage in complex hand defects.
ABSTRACT
Soft tissue defects associated with exposed tendon pose difficult reconstructive problems because of tendon adhesions, poor range of motion, poor cosmetic appearance, and donor site morbidity. Dermal regeneration template is a skin substitute widely used in reconstructive surgery, including the occasional coverage of tendons. However, postoperative functionality of the tendons has not been well documented. We report a case of using dermal regeneration template for soft tissue reconstruction overlying tendons with loss of paratenon in a patient with Dupuytren’s contracture. Dermal regeneration template may offer an alternative option for immediate tendon coverage in the hand.
Soft tissue defects overlying exposed tendon with loss of paratenon often precipitate poor clinical outcomes because of the dichotomous demands of both closing the overlying soft-tissue defect and providing a gliding surface for the underlying tendons.1 Although avoidance of adhesions and restoration of function are the primary goals of the procedure, satisfactory appearance is also desirable. Likewise, any form of coverage should ideally provide good vasculature required for complete healing and an early form of closure following débridement.2 Simple skin grafts do not adequately meet these demands because they result in a high rate of tendon adhesions,3 and also are limited in patients with limited donor skin availability or questionable underlying wound bed viability, such as in scleroderma.
In order to reduce the frequency of tendon adhesions by creating a gliding surface, the use of interpositional materials, both artificial and biologic, has been employed with varying degrees of success, including cellophane, chitosan membrane, fibrin sealant, autogenous fascial flaps, and autogenous venous grafts.4-7 Many of the autogenous flaps and grafts have been employed with good success.8 However, complications and donor site morbidity encourage alternative procedures, including the use of artificial substances.2,8-10
We present our clinical experience with a patient who underwent successful placement of Integra (Integra LifeSciences) Dermal Regeneration Template (DRT) directly over exposed tendons with a subsequent full-thickness skin graft several weeks later. The procedures were performed per the manufacturer’s specifications, resulting in 2 stages of reconstruction. In our experience, DRT can offer immediate coverage unrestricted by wound size, and provides shorter operative time and decreased donor site and surgical morbidity compared with flap coverage, while demonstrating good cosmetic results. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 74-year-old right-handed man with Dupuytren’s contracture was evaluated for recurrent symptomatic contracture causing difficulty with daily activities. He reported palpable cords and contractures in the ring and small fingers of the right hand. He had 2 prior open surgical procedures, including palmar and digital fasciectomy of both hands. On the right hand, the ring and small fingers demonstrated 90° proximal interphalangeal (PIP) and 60° metacarpophalangeal (MCP) flexion contractures. Palpable central cords were present on the flexor surfaces of both the ring and small fingers. A well-healed surgical incision, performed 22 years earlier, was present over the palmar aspect of the ring finger.
Continue to: With consideration given...
With consideration given to the patient’s recurrent contracture after a prior surgical procedure, we discussed surgical excision of the diseased cords in order to eliminate the possibility of a second recurrence and maximize the gain of motion. Following discussion with the patient, we performed palmar and digital fasciectomy of the ring and small finger contractures. Postoperatively, the patient was followed closely for wound complications and vascular status. On his return to our clinic 11 days later, the patient was noted to have dehiscence of the digital wounds in the ring and small fingers (Figure 1).
STAGE 1
During the first stage, completed 14 days following the index procedure, débridement of the wounds was performed, followed by provisional DRT coverage of the tendons, secured with 5-0 nylon sutures (Figure 2).
STAGE 2
At approximately 2 weeks after application of the DRT, a full-thickness skin graft was applied. The thickness of the graft was chosen to allow for durable coverage of the palmar skin defects. Upon successful completion of the second stage, the patient was followed and evaluated for complete wound healing. On performing an examination 14 days after surgery, the ring and small fingers demonstrated only partially healed skin graft but significantly improved range of motion (ROM), with 40° to 90° arc of motion in the PIP joint and 25° to 90° arc of motion in the MCP joint (Figure 4). Owing to their limited size, the wounds were treated with dressing changes until successful healing (Figure 5).
Hand therapy was instituted to achieve maximum mobility for covered soft tissue and tendons and to maximize tendon gliding. At 1-year follow-up, the skin was fully healed and the patient’s active PIP motion was 30° to 90°, active MCP motion was 0° to 90°, and grip strength was 90 lb on both sides. The tendons glided under a well-vascularized tissue at the DRT placement site, and no secondary tenolysis procedure was deemed necessary.
DISCUSSION
Soft tissue defects with exposed tendons may offer a number of challenges for coverage. The primary concern is the creation of a gliding surface and the restoration of a functional tendon without adhesions.2 However, surgeons must use their own clinical judgment when choosing the method of coverage so as to minimize the effects of donor site morbidity and maximize the overall functional and cosmetic outcomes. All options must be considered while selecting a material or flap that is likely to survive in the relatively avascular tendon plane.2,8,11 When considering the reconstructive ladder, skin grafts may not represent a viable option in the presence of a nonvascularized wound bed, such as exposed tendon or bone, where paratenon or periosteum have been damaged. That leaves the surgeon with local flaps, regional flaps, free flaps, and skin substitutes.
Continue to : Before planning closure...
Before planning closure, wound conditions should be optimized, including wound bed quality, vascularization, and bacterial loads. Experimental data suggest that the bacterial load should be brought down below a critical level of 105 bacteria per g of tissue to allow a skin graft to take. This may be problematic from a practical standpoint because quantitative bacterial cultures take about 48 hours to obtain the result, long after a decision to graft is made. As a result, the surgeon may take an aggressive approach to wound débridement, making sure that all necrotic material has been sharply débrided prior to coverage.
As Levin12 noted in 1993, decisions regarding repair of any soft tissue defect may follow a well-delineated ladder beginning with the primary choice of split-thickness skin grafts and ending with free flaps. When treating tissue defects in the hand complex, flaps are an excellent option as they replace like with like, allow minimal scarring and early rehabilitation. 13,14 Nevertheless, a few general disadvantages are inherent in flap procedure: increase in operating time, risk of flap loss, and in case of free flaps, knowledge, experience, and microsurgical ability.2 In reference to complications, the rate of flap loss found by Khouri and colleagues15 was 4.1% with a 12.1% chance of incurring some measured complication, including wound dehiscence, arterial insufficiency, and flap necrosis.
Likewise, some of the conventional local and free flaps, including cutaneous and muscular flaps, prove ineffective in preventing tendon adhesions, create unsightly postoperative contours, or increase the area of trauma on the wounded hand, encouraging the use of free fascial flaps.11 Among the wide array of potential free fascial flaps, the temporoparietal, scapular, lateral arm, radial forearm, and free serratus fascial flaps are some of the most popular for hand defects.8,9 However, these procedures require an additional surgical site, meticulous dissection, microsurgical technique at times, and increased operating cost and time.2,8-10 Furthermore, free fascial flaps have demonstrated occasional partial flap loss and a decreased survival of the overlying skin graft, leading some to advocate delayed skin graft placement.10,16,17
On the basis of these complications, Bray and colleagues11 noted that the utility of free flaps may be limited in smaller clinical settings. The primary disadvantage of using DRTs is the necessity for a second operative procedure to harvest and place the skin graft. Traditionally, this is performed 2 to 3 weeks after the initial DRT application. Nevertheless, a 1-stage procedure can be performed in an outpatient setup, minimizing the burden to the patient and the medical costs, followed by secondary intention healing.
In response to critics of the 2-stage technique, Sanger and colleagues18 described single-stage use of DRT with split-thickness skin grafts with placement of an overlying wound vacuum-assisted closure to help speed incorporation of the DRT and improve survival of the immediately grafted skin. Another viable alternative is the McCash open-palm technique.19 In the open-palm technique, a Brunner zigzag incision is made in the affected digit. A transverse incision is made in the palm. A partial fasciectomy is performed in the palm and digit. After release, the digital incision is closed, and the palmar incision is left open. Although this well-studied and well-reported technique is known to reduce the risk of flap necrosis due to tension and hematoma,20 its main application is in the palm, as the name implies. Because in our patient the defect was palmar-digital with exposed “white structures,” we elected to use DRT.
Continue to: Although there is still...
Although there is still no perfect answer for wound coverage and closure in the hand with exposed or damaged tendons, DRT certainly performs well as a primary choice by minimizing adhesions; allowing a good ROM; and providing a durable, satisfactory cosmetic outcome. Likewise, an initial treatment with DRT does not preclude later, more elaborate reconstructive efforts, such as local or free flaps, if they continue to be indicated. DRT also does not diminish the ability to revise a tendon reconstruction if a secondary procedure is necessary. In our patient, tendon revision has not been necessary. DRT gives the surgeon a minimally invasive, efficient initial alternative to more labor-intensive, potentially morbid reconstructive procedures, without sacrificing outcome. Therefore, DRT can offer an alternative procedure in the surgeon’s armamentarium for tendon coverage in complex hand defects.
1. Flügel A. Kehrer C. Heitmann C, German G, Sauerbier M. Coverage of soft tissue defects of the hand with free fascial flaps. Microsurgery.2005;25(1):47-53.
2. Chen H, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15(4):541-554.
3. Chia J, Lim A, Peng YP. Use of an arterialized venous flap for resurfacing a circumferential soft tissue defect of a digit. Microsurgery. 2001; 21(8):374-378.
4. Wheeldon T. The use of cellophane as a permanent tendon sheath. J Bone J Surg Am; 1939;21(2):393-396.
5. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am. 1993;18(1):68-75.
6. Jones NF, Lister GD. Free skin and composite flaps. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative hand surgery. 6th ed. New York, NY: Churchill Livingstone; 2011:1721-1756.
7. Yan D, Shi X, Lui Q. Reconstruction of tendon sheath by autogenous vein graft in preventing adhesion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11(1):38-39.
8. Pederson WC. Upper extremity microsurgery. Plast Reconstr Surg. 2001;107(6):1524-1537; discussion 1538-15399, 1540-1543.
9. WintschK, Helaly P. Free flap of gliding tissue. J Reconstr Microsurg. 1986;2(3):143-151.
10. Meland NB, Weimar R. Microsurgical reconstruction: experience with free fascia flaps. Ann Plast Surg. 1991;27(1):1-8.
11. Bray PW, Boyer MI, Bowen CV. Complex injuries of the forearm. Coverage considerations. Hand Clin. 1997;13(2):263-278.
12. Levin LS. The reconstructive ladder: an orthoplastic approach. Ortho Clin North Am. 1993; 24(3):393-409.
13. Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48 (5):913-917. doi:10.1097/00005373-200005000-00016.
14. Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53(1):61-65. doi:10.1097/00005373-200207000-00013.
15. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711-721.
16. Woods JM 4th, Shack RB, Hagan KF. Free temporoparietal fascia flap in reconstruction of the lower extremity. Ann Plast Surg. 1995;34(5):501-506. doi:10.1097/00000637-199505000-00008.
17. Chung KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002;27(3):525-533.
18. Sanger C, Molnar JA, Newman CE, et al. Immediate skin grafting of an engineered dermal substitute: P37. Plast Reconstr Surg. 2005;116(3S):165.
19. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-280.
20. Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg Br. 1996;21(4):484-485.
1. Flügel A. Kehrer C. Heitmann C, German G, Sauerbier M. Coverage of soft tissue defects of the hand with free fascial flaps. Microsurgery.2005;25(1):47-53.
2. Chen H, Buchman MT, Wei FC. Free flaps for soft tissue coverage in the hand and fingers. Hand Clin. 1999;15(4):541-554.
3. Chia J, Lim A, Peng YP. Use of an arterialized venous flap for resurfacing a circumferential soft tissue defect of a digit. Microsurgery. 2001; 21(8):374-378.
4. Wheeldon T. The use of cellophane as a permanent tendon sheath. J Bone J Surg Am; 1939;21(2):393-396.
5. Frykman E, Jacobsson S, Widenfalk B. Fibrin sealant in prevention of flexor tendon adhesions: an experimental study in the rabbit. J Hand Surg Am. 1993;18(1):68-75.
6. Jones NF, Lister GD. Free skin and composite flaps. In: Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH, eds. Green’s Operative hand surgery. 6th ed. New York, NY: Churchill Livingstone; 2011:1721-1756.
7. Yan D, Shi X, Lui Q. Reconstruction of tendon sheath by autogenous vein graft in preventing adhesion. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11(1):38-39.
8. Pederson WC. Upper extremity microsurgery. Plast Reconstr Surg. 2001;107(6):1524-1537; discussion 1538-15399, 1540-1543.
9. WintschK, Helaly P. Free flap of gliding tissue. J Reconstr Microsurg. 1986;2(3):143-151.
10. Meland NB, Weimar R. Microsurgical reconstruction: experience with free fascia flaps. Ann Plast Surg. 1991;27(1):1-8.
11. Bray PW, Boyer MI, Bowen CV. Complex injuries of the forearm. Coverage considerations. Hand Clin. 1997;13(2):263-278.
12. Levin LS. The reconstructive ladder: an orthoplastic approach. Ortho Clin North Am. 1993; 24(3):393-409.
13. Hallock GG. Utility of both muscle and fascia flaps in severe lower extremity trauma. J Trauma. 2000;48 (5):913-917. doi:10.1097/00005373-200005000-00016.
14. Hallock GG. The utility of both muscle and fascia flaps in severe upper extremity trauma. J Trauma. 2002;53(1):61-65. doi:10.1097/00005373-200207000-00013.
15. Khouri RK, Cooley BC, Kunselman AR, et al. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711-721.
16. Woods JM 4th, Shack RB, Hagan KF. Free temporoparietal fascia flap in reconstruction of the lower extremity. Ann Plast Surg. 1995;34(5):501-506. doi:10.1097/00000637-199505000-00008.
17. Chung KC, Cederna PS. Endoscopic harvest of temporoparietal fascial free flaps for coverage of hand wounds. J Hand Surg Am. 2002;27(3):525-533.
18. Sanger C, Molnar JA, Newman CE, et al. Immediate skin grafting of an engineered dermal substitute: P37. Plast Reconstr Surg. 2005;116(3S):165.
19. McCash CR. The open palm technique in Dupuytren’s contracture. Br J Plast Surg. 1964;17:271-280.
20. Shaw DL, Wise DI, Holms W. Dupuytren's disease treated by palmar fasciectomy and an open palm technique. J Hand Surg Br. 1996;21(4):484-485.
TAKE-HOME POINTS
- Full thickness skin grafts are generally considered unreliable for coverage of 3-dimensional defects of the hand with tendon exposure.
- Integra (Integra LifeSciences) is a bilayer skin substitute. The “dermal” (lower) layer is a bovine collagen base with glycosaminoglycan chondroitin-6-sulfate while the upper layer is a silicone sheet that acts as a temporary epidermis.
- Despite its popularity of Integra in burn reconstruction, little has been published regarding its utility in complex hand wounds with exposed tendons.
- Small areas of exposed tendons without remaining paratenon can be successfully grafted with Integra.
- In the presence of a healthy wound bed and no necrotic tissue or infection, Integra offers a reconstructive option that allows immediate coverage of complex hand wounds.
Use of a Core Reamer for the Resection of a Central Distal Femoral Physeal Bone Bridge: A Novel Technique with 3-Year Follow-up
ABSTRACT
A central distal femoral physeal bone bridge in a boy aged 5 years and 7 months was resected with a fluoroscopically guided core reamer placed through a lateral parapatellar approach. At 3-year follow-up, the boy’s leg-length discrepancy was 3.0 cm (3.9 cm preoperatively), and the physeal bone bridge did not recur. The patient had full function and no pain or other patellofemoral complaints. This technique provided direct access to the physeal bone bridge, and complete resection was performed without injury to the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which is expected to grow normally in the absence of the bridge.
A physeal bone bridge is an osseous connection that forms across a physis. It may cause partial premature physeal arrest. Angular deformity and limb-length discrepancy are the main complications caused by physeal bone bridges.1-4 The indications for the treatment of physeal bridges are well documented.1-5 Trauma and infection are common causes of distal femoral physeal bone bridges. Arkader and colleagues6 showed that among different types of physeal bridges, the Salter-Harris type is significantly associated with complications, among which growth arrest is the most common and occurs in 27.4% of all patients.
The treatment of distal femoral physeal bone bridges is technically difficult and provides variable results. Poor results are reported in 13% to 40% of patients.7-10 Procedure failure has been attributed to incomplete resection with the persistent tethering and dislodgement of the graft.11 Methods with improved efficacy for the removal of central physeal bridges will help prevent reformation after treatment. We have used a novel technique that allows the direct resection of a central physeal bone bridge in the distal femur through the use of a fluoroscopically guided core reamer. This technique enables the complete removal of the bone bridge and the direct visual assessment of the remaining physis. The patient’s parents provided written informed consent for print and electronic publication of this case report.
CASE
A 3-year-old boy with a history of hemifacial microsomia presented for the evaluation of genu valgum and leg-length discrepancy. His intermalleolar distance at that time was 8 cm. A standing radiograph of his lower extremities demonstrated changes consistent with physiologic genu valgum. He had no history of knee trauma, infection, or pain.
At the age of 5 years and 7 months, the patient returned for a repeat evaluation and was noted to exhibit the progressive valgus deformity of the right leg and a leg-length discrepancy of 3.9 cm (Figure 1).
Continue to: With the patient supine on the operating...
OPERATIVE TECHNIQUE
With the patient supine on the operating table and after the administration of general anesthesia, 3-dimensional (3-D) fluoroscopy was used to localize the bone bridge, which confirmed the fluoroscopic location that was previously visualized through preoperative 3-D imaging. The leg was elevated, and a tourniquet was applied and inflated. A lateral parapatellar approach was used to isolate the distal femoral physis anteriorly because the bone bridge was centered just lateral to the central portion of the distal femoral physis. A Kirschner wire was placed in the center of the bridge under anteroposterior and lateral fluoroscopic imaging (Figures 3A-3E).
OUTCOME
The patient healed uneventfully, and early range-of-motion exercises were started 6 weeks postoperatively. At 6-month follow-up, his leg-length discrepancy was 2.7 cm, and the bone bridge did not recur. At 3-year follow-up, his leg-length discrepancy was 3.0 cm, and the bone bridge did not recur. Over the 3 years postoperatively, the patient exhibited 9.8 cm of growth on his operative side and 9.5 cm on his nonoperative side (Figure 5).
DISCUSSION
Given the considerable growth potential of the distal femoral physis,1,14-16 an injury to the distal femoral physis and the formation of a physeal bone bridge can have a profound effect on a young patient in terms of leg-length discrepancy and angular deformity. Fracture from trauma or infection is a common cause of physeal bone bridges.6,17-19 The etiology of our patient’s distal femoral physeal bone bridge is idiopathic, which is considerably less common than other etiologies, and the incidence of idiopathic physeal bone bridge formation is not well established in the literature. Hresko and Kasser21 identified atraumatic physeal bone bridge formations in 7 patients. Among the 13 patients with physeal bone bridges described by Broughton and colleagues,20 the cause of bridge formation is unknown in 1.
Physeal bone bridges that form centrally are particularly challenging because they are difficult to visualize through a peripheral approach. A number of methods for resecting central physeal bone bridges have been described. These methods have varying degrees of success. In 1981, Langenskiöld7 first described the creation of a metaphyseal mirror and the use of a dental mirror for visualization. This technique, however, yielded unfavorable results in 16% of patients. Williamson and Staheli9 reported poor results in 23% of patients. Loraas and Schmale4 described the use of an endoscope, termed an osteoscope, for visualization, citing advantages of superior illumination and potential for image magnification and capture. Marsh and Polzhofer8 also showed this technique to have low morbidity but poor results in 13% of patients, whereas Moreta and colleagues10 reported poor results in 2 out of 5 patients. The rate of poor results of these methods may be related to the technical difficulty of using dental mirrors and arthroscopes and can be improved by highly efficient direct methods with improved visualization, such as the method described in this article.
Continue to: Proper imaging is necessary for...
Proper imaging is necessary for the accurate quantification of bone bridges to determine resectability and to identify the best surgical approach to resection. MRI with software for the generation of 3-D physeal maps is a reproducible method with good interobserver reliability.22,23 Intraoperative computer-assisted imaging also is beneficial for determining the extent and location of the resection to ensure complete bone bridge removal.24
To our knowledge, a direct approach through parapatellar arthrotomy for the resection of a centrally located distal femoral physeal bone bridge has not been previously described. This novel technique provided direct access to the physeal bone bridge and was performed without injuring the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which may grow normally in the absence of the bridge. Instead of using a lateral or medial approach with a metaphyseal window,4 we directly approached this central bar through a parapatellar approach and were able to completely resect it under direct visualization. This obviated the need for an arthroscope or dental mirror. To remove the entire physeal bone bridge, we needed to resect completely from the anterior cortex to the posterior cortex. Although this technique potentially increased the risk of iatrogenic fracture, we believed that this risk would not differ greatly from that of disrupting the medial or lateral metaphysis and would be more stable with either axial and torsion load. At 3-year follow-up, the patient exhibited restored normal growth in his operative limb relative to that in his nonoperative limb, had not developed angular deformity, and had maintained his previously developed limb-length discrepancy that could be corrected with the epiphysiodesis of his opposite limb at a later date.
The limitations to this technique include the fact that it may be most effective with small-to moderate-sized central physeal bone bridges, although resection has shown good results with up to 70% physeal involvement.8 In this patient, the bone bridge was moderately sized (30% of the physis), centrally located, and clearly visible on fluoroscopy. These characteristics increased the technical safety and ease of the procedure. The resection of large, peripheral bridges may destabilize the distal femur. The destabilization of the distal femur, in turn, can lead to fracture. Patellofemoral mechanics may also be affected during the treatment of distal femoral physeal bone bridges. This patient has not experienced any patellofemoral dysfunction or symptoms. Given the patient’s age and significant amount of remaining growth, he will need close monitoring until he reaches skeletal maturity.
This paper will be judged for the Resident Writer’s Award.
1. Murphy GA. Disorders of tendons and fascia and adolescent and adult pes planus. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 12th edition. Philadelphia, PA: Mosby-Elsevier; 2013:3966-3972.
2. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
3. Stans AA. Excision of physeal bar. In: Wiesel SW, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:1244-1249.
4. Loraas EK, Schmale GA. Endoscopically aided physeal bar takedown and guided growth for the treatment of angular limb deformity. J Pediatr Orthop B. 2012;21(4):348-351. doi:10.1097/BPB.0b013e328346d308.
5. Inoue T, Naito M, Fuhii T, Akiyoshi Y, Yoshimura I, Takamura K. Partial physeal growth arrest treated by bridge resection and artificial dura substitute interposition. J Pediatr Orthop B. 2006;15(1):65-69. doi:10.1097/01202412-200601000-00014.
6. Arkader A, Warner WC Jr, Horn BD, Shaw RN, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop. 2007;27(6):703-708. doi:10.1097/BPO.0b013e3180dca0e5.
7. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981;1(1):3-11. doi:10.1097/01241398-198101010-00002.
8. Marsh JS, Polzhofer GK. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop. 2006;26(2):255-259. doi:10.1097/01.bpo.0000218533.43986.e1.
9. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990;10(6):769-776. doi:10.1097/01241398-199011000-00012.
10. Moreta J, Abril JC, Miranda C. Arthroscopy-assisted resection-interposition of post-traumatic central physeal bridges. Rev Esp Cir Orthop Traumatol. 2013;57(5):333-339. doi:10.1016/j.recot.2013.07.004.
11. Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;405:242-249.
12. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82(10):1432-1446. doi:10.2106/00004623-200010000-00010.
13. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
14. Rathjen KE, Kim HKW. Physeal injuries and growth disturbances. In: Flynn JM, Skaggs DL, Waters PM, eds. Rockwood and Wilkins’ Fractures in Children. 8th edition. Philadelphia, PA: Wolters-Kluwer; 2015:135-137.
15. Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma. 1972;12(4):275-281. doi:10.1097/00005373-197204000-00002.
16. Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;275:274-279.
17. Cassebaum WH, Patterson AH. Fracture of the distal femoral epiphysis. Clin Orthop Relat Res. 1965;41:79-91. doi:10.1097/00003086-196500410-00009.
18. Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pedatir Orthop. 2014;34(2):134-138. doi:10.1097/BPO.0000000000000083.
19. Roberts J. Fracture separation of the distal femoral epiphyseal growth line. J Bone Joint Surg Am. 1973;55:1324.
20. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989;71(1):13-16. doi:10.1302/0301-620X.71B1.2914983.
21. Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71(5):698-703. doi:10.2106/00004623-198971050-00009.
22. Lurie B, Koff MF, Shah P, et al. Three-dimensional magnetic resonance imaging of physeal injury: reliability and clinical utility. J Pediatr Orthop. 2014;34(3):239-245. doi:10.1097/BPO.0000000000000104.
23. Sailhan F, Chotel F, Guibal AL, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14(9):1600-1608. doi:10.1007/s00330-004-2319-z.
24. Kang HG, Yoon SJ, Kim JR. Resection of a physeal bar under computer-assisted guidance. J Bone Joint Surg Br. 2010;92(10):1452-1455. doi:10.1302/0301-620X.92B10.24587.
ABSTRACT
A central distal femoral physeal bone bridge in a boy aged 5 years and 7 months was resected with a fluoroscopically guided core reamer placed through a lateral parapatellar approach. At 3-year follow-up, the boy’s leg-length discrepancy was 3.0 cm (3.9 cm preoperatively), and the physeal bone bridge did not recur. The patient had full function and no pain or other patellofemoral complaints. This technique provided direct access to the physeal bone bridge, and complete resection was performed without injury to the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which is expected to grow normally in the absence of the bridge.
A physeal bone bridge is an osseous connection that forms across a physis. It may cause partial premature physeal arrest. Angular deformity and limb-length discrepancy are the main complications caused by physeal bone bridges.1-4 The indications for the treatment of physeal bridges are well documented.1-5 Trauma and infection are common causes of distal femoral physeal bone bridges. Arkader and colleagues6 showed that among different types of physeal bridges, the Salter-Harris type is significantly associated with complications, among which growth arrest is the most common and occurs in 27.4% of all patients.
The treatment of distal femoral physeal bone bridges is technically difficult and provides variable results. Poor results are reported in 13% to 40% of patients.7-10 Procedure failure has been attributed to incomplete resection with the persistent tethering and dislodgement of the graft.11 Methods with improved efficacy for the removal of central physeal bridges will help prevent reformation after treatment. We have used a novel technique that allows the direct resection of a central physeal bone bridge in the distal femur through the use of a fluoroscopically guided core reamer. This technique enables the complete removal of the bone bridge and the direct visual assessment of the remaining physis. The patient’s parents provided written informed consent for print and electronic publication of this case report.
CASE
A 3-year-old boy with a history of hemifacial microsomia presented for the evaluation of genu valgum and leg-length discrepancy. His intermalleolar distance at that time was 8 cm. A standing radiograph of his lower extremities demonstrated changes consistent with physiologic genu valgum. He had no history of knee trauma, infection, or pain.
At the age of 5 years and 7 months, the patient returned for a repeat evaluation and was noted to exhibit the progressive valgus deformity of the right leg and a leg-length discrepancy of 3.9 cm (Figure 1).
Continue to: With the patient supine on the operating...
OPERATIVE TECHNIQUE
With the patient supine on the operating table and after the administration of general anesthesia, 3-dimensional (3-D) fluoroscopy was used to localize the bone bridge, which confirmed the fluoroscopic location that was previously visualized through preoperative 3-D imaging. The leg was elevated, and a tourniquet was applied and inflated. A lateral parapatellar approach was used to isolate the distal femoral physis anteriorly because the bone bridge was centered just lateral to the central portion of the distal femoral physis. A Kirschner wire was placed in the center of the bridge under anteroposterior and lateral fluoroscopic imaging (Figures 3A-3E).
OUTCOME
The patient healed uneventfully, and early range-of-motion exercises were started 6 weeks postoperatively. At 6-month follow-up, his leg-length discrepancy was 2.7 cm, and the bone bridge did not recur. At 3-year follow-up, his leg-length discrepancy was 3.0 cm, and the bone bridge did not recur. Over the 3 years postoperatively, the patient exhibited 9.8 cm of growth on his operative side and 9.5 cm on his nonoperative side (Figure 5).
DISCUSSION
Given the considerable growth potential of the distal femoral physis,1,14-16 an injury to the distal femoral physis and the formation of a physeal bone bridge can have a profound effect on a young patient in terms of leg-length discrepancy and angular deformity. Fracture from trauma or infection is a common cause of physeal bone bridges.6,17-19 The etiology of our patient’s distal femoral physeal bone bridge is idiopathic, which is considerably less common than other etiologies, and the incidence of idiopathic physeal bone bridge formation is not well established in the literature. Hresko and Kasser21 identified atraumatic physeal bone bridge formations in 7 patients. Among the 13 patients with physeal bone bridges described by Broughton and colleagues,20 the cause of bridge formation is unknown in 1.
Physeal bone bridges that form centrally are particularly challenging because they are difficult to visualize through a peripheral approach. A number of methods for resecting central physeal bone bridges have been described. These methods have varying degrees of success. In 1981, Langenskiöld7 first described the creation of a metaphyseal mirror and the use of a dental mirror for visualization. This technique, however, yielded unfavorable results in 16% of patients. Williamson and Staheli9 reported poor results in 23% of patients. Loraas and Schmale4 described the use of an endoscope, termed an osteoscope, for visualization, citing advantages of superior illumination and potential for image magnification and capture. Marsh and Polzhofer8 also showed this technique to have low morbidity but poor results in 13% of patients, whereas Moreta and colleagues10 reported poor results in 2 out of 5 patients. The rate of poor results of these methods may be related to the technical difficulty of using dental mirrors and arthroscopes and can be improved by highly efficient direct methods with improved visualization, such as the method described in this article.
Continue to: Proper imaging is necessary for...
Proper imaging is necessary for the accurate quantification of bone bridges to determine resectability and to identify the best surgical approach to resection. MRI with software for the generation of 3-D physeal maps is a reproducible method with good interobserver reliability.22,23 Intraoperative computer-assisted imaging also is beneficial for determining the extent and location of the resection to ensure complete bone bridge removal.24
To our knowledge, a direct approach through parapatellar arthrotomy for the resection of a centrally located distal femoral physeal bone bridge has not been previously described. This novel technique provided direct access to the physeal bone bridge and was performed without injuring the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which may grow normally in the absence of the bridge. Instead of using a lateral or medial approach with a metaphyseal window,4 we directly approached this central bar through a parapatellar approach and were able to completely resect it under direct visualization. This obviated the need for an arthroscope or dental mirror. To remove the entire physeal bone bridge, we needed to resect completely from the anterior cortex to the posterior cortex. Although this technique potentially increased the risk of iatrogenic fracture, we believed that this risk would not differ greatly from that of disrupting the medial or lateral metaphysis and would be more stable with either axial and torsion load. At 3-year follow-up, the patient exhibited restored normal growth in his operative limb relative to that in his nonoperative limb, had not developed angular deformity, and had maintained his previously developed limb-length discrepancy that could be corrected with the epiphysiodesis of his opposite limb at a later date.
The limitations to this technique include the fact that it may be most effective with small-to moderate-sized central physeal bone bridges, although resection has shown good results with up to 70% physeal involvement.8 In this patient, the bone bridge was moderately sized (30% of the physis), centrally located, and clearly visible on fluoroscopy. These characteristics increased the technical safety and ease of the procedure. The resection of large, peripheral bridges may destabilize the distal femur. The destabilization of the distal femur, in turn, can lead to fracture. Patellofemoral mechanics may also be affected during the treatment of distal femoral physeal bone bridges. This patient has not experienced any patellofemoral dysfunction or symptoms. Given the patient’s age and significant amount of remaining growth, he will need close monitoring until he reaches skeletal maturity.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
A central distal femoral physeal bone bridge in a boy aged 5 years and 7 months was resected with a fluoroscopically guided core reamer placed through a lateral parapatellar approach. At 3-year follow-up, the boy’s leg-length discrepancy was 3.0 cm (3.9 cm preoperatively), and the physeal bone bridge did not recur. The patient had full function and no pain or other patellofemoral complaints. This technique provided direct access to the physeal bone bridge, and complete resection was performed without injury to the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which is expected to grow normally in the absence of the bridge.
A physeal bone bridge is an osseous connection that forms across a physis. It may cause partial premature physeal arrest. Angular deformity and limb-length discrepancy are the main complications caused by physeal bone bridges.1-4 The indications for the treatment of physeal bridges are well documented.1-5 Trauma and infection are common causes of distal femoral physeal bone bridges. Arkader and colleagues6 showed that among different types of physeal bridges, the Salter-Harris type is significantly associated with complications, among which growth arrest is the most common and occurs in 27.4% of all patients.
The treatment of distal femoral physeal bone bridges is technically difficult and provides variable results. Poor results are reported in 13% to 40% of patients.7-10 Procedure failure has been attributed to incomplete resection with the persistent tethering and dislodgement of the graft.11 Methods with improved efficacy for the removal of central physeal bridges will help prevent reformation after treatment. We have used a novel technique that allows the direct resection of a central physeal bone bridge in the distal femur through the use of a fluoroscopically guided core reamer. This technique enables the complete removal of the bone bridge and the direct visual assessment of the remaining physis. The patient’s parents provided written informed consent for print and electronic publication of this case report.
CASE
A 3-year-old boy with a history of hemifacial microsomia presented for the evaluation of genu valgum and leg-length discrepancy. His intermalleolar distance at that time was 8 cm. A standing radiograph of his lower extremities demonstrated changes consistent with physiologic genu valgum. He had no history of knee trauma, infection, or pain.
At the age of 5 years and 7 months, the patient returned for a repeat evaluation and was noted to exhibit the progressive valgus deformity of the right leg and a leg-length discrepancy of 3.9 cm (Figure 1).
Continue to: With the patient supine on the operating...
OPERATIVE TECHNIQUE
With the patient supine on the operating table and after the administration of general anesthesia, 3-dimensional (3-D) fluoroscopy was used to localize the bone bridge, which confirmed the fluoroscopic location that was previously visualized through preoperative 3-D imaging. The leg was elevated, and a tourniquet was applied and inflated. A lateral parapatellar approach was used to isolate the distal femoral physis anteriorly because the bone bridge was centered just lateral to the central portion of the distal femoral physis. A Kirschner wire was placed in the center of the bridge under anteroposterior and lateral fluoroscopic imaging (Figures 3A-3E).
OUTCOME
The patient healed uneventfully, and early range-of-motion exercises were started 6 weeks postoperatively. At 6-month follow-up, his leg-length discrepancy was 2.7 cm, and the bone bridge did not recur. At 3-year follow-up, his leg-length discrepancy was 3.0 cm, and the bone bridge did not recur. Over the 3 years postoperatively, the patient exhibited 9.8 cm of growth on his operative side and 9.5 cm on his nonoperative side (Figure 5).
DISCUSSION
Given the considerable growth potential of the distal femoral physis,1,14-16 an injury to the distal femoral physis and the formation of a physeal bone bridge can have a profound effect on a young patient in terms of leg-length discrepancy and angular deformity. Fracture from trauma or infection is a common cause of physeal bone bridges.6,17-19 The etiology of our patient’s distal femoral physeal bone bridge is idiopathic, which is considerably less common than other etiologies, and the incidence of idiopathic physeal bone bridge formation is not well established in the literature. Hresko and Kasser21 identified atraumatic physeal bone bridge formations in 7 patients. Among the 13 patients with physeal bone bridges described by Broughton and colleagues,20 the cause of bridge formation is unknown in 1.
Physeal bone bridges that form centrally are particularly challenging because they are difficult to visualize through a peripheral approach. A number of methods for resecting central physeal bone bridges have been described. These methods have varying degrees of success. In 1981, Langenskiöld7 first described the creation of a metaphyseal mirror and the use of a dental mirror for visualization. This technique, however, yielded unfavorable results in 16% of patients. Williamson and Staheli9 reported poor results in 23% of patients. Loraas and Schmale4 described the use of an endoscope, termed an osteoscope, for visualization, citing advantages of superior illumination and potential for image magnification and capture. Marsh and Polzhofer8 also showed this technique to have low morbidity but poor results in 13% of patients, whereas Moreta and colleagues10 reported poor results in 2 out of 5 patients. The rate of poor results of these methods may be related to the technical difficulty of using dental mirrors and arthroscopes and can be improved by highly efficient direct methods with improved visualization, such as the method described in this article.
Continue to: Proper imaging is necessary for...
Proper imaging is necessary for the accurate quantification of bone bridges to determine resectability and to identify the best surgical approach to resection. MRI with software for the generation of 3-D physeal maps is a reproducible method with good interobserver reliability.22,23 Intraoperative computer-assisted imaging also is beneficial for determining the extent and location of the resection to ensure complete bone bridge removal.24
To our knowledge, a direct approach through parapatellar arthrotomy for the resection of a centrally located distal femoral physeal bone bridge has not been previously described. This novel technique provided direct access to the physeal bone bridge and was performed without injuring the adjacent physeal cartilage in the medial and lateral columns of the distal femur, which may grow normally in the absence of the bridge. Instead of using a lateral or medial approach with a metaphyseal window,4 we directly approached this central bar through a parapatellar approach and were able to completely resect it under direct visualization. This obviated the need for an arthroscope or dental mirror. To remove the entire physeal bone bridge, we needed to resect completely from the anterior cortex to the posterior cortex. Although this technique potentially increased the risk of iatrogenic fracture, we believed that this risk would not differ greatly from that of disrupting the medial or lateral metaphysis and would be more stable with either axial and torsion load. At 3-year follow-up, the patient exhibited restored normal growth in his operative limb relative to that in his nonoperative limb, had not developed angular deformity, and had maintained his previously developed limb-length discrepancy that could be corrected with the epiphysiodesis of his opposite limb at a later date.
The limitations to this technique include the fact that it may be most effective with small-to moderate-sized central physeal bone bridges, although resection has shown good results with up to 70% physeal involvement.8 In this patient, the bone bridge was moderately sized (30% of the physis), centrally located, and clearly visible on fluoroscopy. These characteristics increased the technical safety and ease of the procedure. The resection of large, peripheral bridges may destabilize the distal femur. The destabilization of the distal femur, in turn, can lead to fracture. Patellofemoral mechanics may also be affected during the treatment of distal femoral physeal bone bridges. This patient has not experienced any patellofemoral dysfunction or symptoms. Given the patient’s age and significant amount of remaining growth, he will need close monitoring until he reaches skeletal maturity.
This paper will be judged for the Resident Writer’s Award.
1. Murphy GA. Disorders of tendons and fascia and adolescent and adult pes planus. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 12th edition. Philadelphia, PA: Mosby-Elsevier; 2013:3966-3972.
2. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
3. Stans AA. Excision of physeal bar. In: Wiesel SW, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:1244-1249.
4. Loraas EK, Schmale GA. Endoscopically aided physeal bar takedown and guided growth for the treatment of angular limb deformity. J Pediatr Orthop B. 2012;21(4):348-351. doi:10.1097/BPB.0b013e328346d308.
5. Inoue T, Naito M, Fuhii T, Akiyoshi Y, Yoshimura I, Takamura K. Partial physeal growth arrest treated by bridge resection and artificial dura substitute interposition. J Pediatr Orthop B. 2006;15(1):65-69. doi:10.1097/01202412-200601000-00014.
6. Arkader A, Warner WC Jr, Horn BD, Shaw RN, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop. 2007;27(6):703-708. doi:10.1097/BPO.0b013e3180dca0e5.
7. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981;1(1):3-11. doi:10.1097/01241398-198101010-00002.
8. Marsh JS, Polzhofer GK. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop. 2006;26(2):255-259. doi:10.1097/01.bpo.0000218533.43986.e1.
9. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990;10(6):769-776. doi:10.1097/01241398-199011000-00012.
10. Moreta J, Abril JC, Miranda C. Arthroscopy-assisted resection-interposition of post-traumatic central physeal bridges. Rev Esp Cir Orthop Traumatol. 2013;57(5):333-339. doi:10.1016/j.recot.2013.07.004.
11. Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;405:242-249.
12. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82(10):1432-1446. doi:10.2106/00004623-200010000-00010.
13. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
14. Rathjen KE, Kim HKW. Physeal injuries and growth disturbances. In: Flynn JM, Skaggs DL, Waters PM, eds. Rockwood and Wilkins’ Fractures in Children. 8th edition. Philadelphia, PA: Wolters-Kluwer; 2015:135-137.
15. Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma. 1972;12(4):275-281. doi:10.1097/00005373-197204000-00002.
16. Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;275:274-279.
17. Cassebaum WH, Patterson AH. Fracture of the distal femoral epiphysis. Clin Orthop Relat Res. 1965;41:79-91. doi:10.1097/00003086-196500410-00009.
18. Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pedatir Orthop. 2014;34(2):134-138. doi:10.1097/BPO.0000000000000083.
19. Roberts J. Fracture separation of the distal femoral epiphyseal growth line. J Bone Joint Surg Am. 1973;55:1324.
20. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989;71(1):13-16. doi:10.1302/0301-620X.71B1.2914983.
21. Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71(5):698-703. doi:10.2106/00004623-198971050-00009.
22. Lurie B, Koff MF, Shah P, et al. Three-dimensional magnetic resonance imaging of physeal injury: reliability and clinical utility. J Pediatr Orthop. 2014;34(3):239-245. doi:10.1097/BPO.0000000000000104.
23. Sailhan F, Chotel F, Guibal AL, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14(9):1600-1608. doi:10.1007/s00330-004-2319-z.
24. Kang HG, Yoon SJ, Kim JR. Resection of a physeal bar under computer-assisted guidance. J Bone Joint Surg Br. 2010;92(10):1452-1455. doi:10.1302/0301-620X.92B10.24587.
1. Murphy GA. Disorders of tendons and fascia and adolescent and adult pes planus. In: Canale ST, Beaty JH, eds. Campbell’s Operative Orthopaedics. 12th edition. Philadelphia, PA: Mosby-Elsevier; 2013:3966-3972.
2. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
3. Stans AA. Excision of physeal bar. In: Wiesel SW, ed. Operative Techniques in Orthopaedic Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2011:1244-1249.
4. Loraas EK, Schmale GA. Endoscopically aided physeal bar takedown and guided growth for the treatment of angular limb deformity. J Pediatr Orthop B. 2012;21(4):348-351. doi:10.1097/BPB.0b013e328346d308.
5. Inoue T, Naito M, Fuhii T, Akiyoshi Y, Yoshimura I, Takamura K. Partial physeal growth arrest treated by bridge resection and artificial dura substitute interposition. J Pediatr Orthop B. 2006;15(1):65-69. doi:10.1097/01202412-200601000-00014.
6. Arkader A, Warner WC Jr, Horn BD, Shaw RN, Wells L. Predicting the outcome of physeal fractures of the distal femur. J Pediatr Orthop. 2007;27(6):703-708. doi:10.1097/BPO.0b013e3180dca0e5.
7. Langenskiöld A. Surgical treatment of partial closure of the growth plate. J Pediatr Orthop. 1981;1(1):3-11. doi:10.1097/01241398-198101010-00002.
8. Marsh JS, Polzhofer GK. Arthroscopically assisted central physeal bar resection. J Pediatr Orthop. 2006;26(2):255-259. doi:10.1097/01.bpo.0000218533.43986.e1.
9. Williamson RV, Staheli LT. Partial physeal growth arrest: treatment by bridge resection and fat interposition. J Pediatr Orthop. 1990;10(6):769-776. doi:10.1097/01241398-199011000-00012.
10. Moreta J, Abril JC, Miranda C. Arthroscopy-assisted resection-interposition of post-traumatic central physeal bridges. Rev Esp Cir Orthop Traumatol. 2013;57(5):333-339. doi:10.1016/j.recot.2013.07.004.
11. Hasler CC, Foster BK. Secondary tethers after physeal bar resection: a common source of failure? Clin Orthop Relat Res. 2002;405:242-249.
12. Paley D, Bhave A, Herzenberg JE, Bowen JR. Multiplier method for predicting limb-length discrepancy. J Bone Joint Surg Am. 2000;82(10):1432-1446. doi:10.2106/00004623-200010000-00010.
13. Khoshhal KI, Kiefer GN. Physeal bridge resection. J Am Acad Orthop Surg. 2005;13(1):47-58. doi:10.5435/00124635-200501000-00007.
14. Rathjen KE, Kim HKW. Physeal injuries and growth disturbances. In: Flynn JM, Skaggs DL, Waters PM, eds. Rockwood and Wilkins’ Fractures in Children. 8th edition. Philadelphia, PA: Wolters-Kluwer; 2015:135-137.
15. Peterson CA, Peterson HA. Analysis of the incidence of injuries to the epiphyseal growth plate. J Trauma. 1972;12(4):275-281. doi:10.1097/00005373-197204000-00002.
16. Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop Relat Res. 1992;275:274-279.
17. Cassebaum WH, Patterson AH. Fracture of the distal femoral epiphysis. Clin Orthop Relat Res. 1965;41:79-91. doi:10.1097/00003086-196500410-00009.
18. Dahl WJ, Silva S, Vanderhave KL. Distal femoral physeal fixation: are smooth pins really safe? J Pedatir Orthop. 2014;34(2):134-138. doi:10.1097/BPO.0000000000000083.
19. Roberts J. Fracture separation of the distal femoral epiphyseal growth line. J Bone Joint Surg Am. 1973;55:1324.
20. Broughton NS, Dickens DR, Cole WG, Menelaus MB. Epiphyseolysis for partial growth plate arrest. Results after four years or at maturity. J Bone Joint Surg Br. 1989;71(1):13-16. doi:10.1302/0301-620X.71B1.2914983.
21. Hresko MT, Kasser JR. Physeal arrest about the knee associated with non-physeal fractures in the lower extremity. J Bone Joint Surg Am. 1989;71(5):698-703. doi:10.2106/00004623-198971050-00009.
22. Lurie B, Koff MF, Shah P, et al. Three-dimensional magnetic resonance imaging of physeal injury: reliability and clinical utility. J Pediatr Orthop. 2014;34(3):239-245. doi:10.1097/BPO.0000000000000104.
23. Sailhan F, Chotel F, Guibal AL, et al. Three-dimensional MR imaging in the assessment of physeal growth arrest. Eur Radiol. 2004;14(9):1600-1608. doi:10.1007/s00330-004-2319-z.
24. Kang HG, Yoon SJ, Kim JR. Resection of a physeal bar under computer-assisted guidance. J Bone Joint Surg Br. 2010;92(10):1452-1455. doi:10.1302/0301-620X.92B10.24587.
TAKE-HOME POINTS
- Central physeal arrest of the distal femur is challenging, but this surgical technique provides an option for treatment.
- Partial bone bridges can be resected, but advanced imaging with MRI or CT, or both, is helpful in preoperative planning.
- Regardless of the type of physeal bar resection that is chosen, it is unlikely that complete, normal bone growth will be restored and closed follow up will be needed.
A Novel Technique for the Treatment of Jersey Fingers
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
The avulsion of the flexor digitorum profundus from its insertion, or “jersey finger,” is a relatively common injury. Numerous modifications have been made to the classification and treatment of this injury since its initial description. We describe a novel variation of the surgical management of jersey finger.
The avulsion-type injury of the flexor digitorum profundus (FDP) from its insertion on the distal phalanx is relatively common. FDP avulsions are seen in athletes and nonathletes, and are the result of the sudden hyperextension of the distal interphalangeal joint during active flexion. These injuries usually occur while grasping the jersey of an opposing player and are thus commonly referred to as “jersey finger.” Initially described in 1977 by Leddy and Packer1, FDP avulsions are classified on the basis of the proximal extent of the retraction of the FDP and the presence or absence of a bony avulsion fracture fragment. Type I injuries are defined by tendon retraction to the level of the palm, where it is tethered by the lumbricals. At this level, the vinculum longus profundus (VLP) and vinculum brevis profundus (VBP) are ruptured, resulting in the substantial loss of intrinsic and extrinsic vascular supply to the tendon. In type II injuries, which are the most common type of FDP avulsions, the FDP tendon retracts to the level of the proximal interphalangeal (PIP) joint. Although the VBP is disrupted in this scenario, the VLP remains preserved because it arises at the level of the volar plate of the PIP joint. Type III lesions involve tendon avulsions with an associated bony fragment that is typically sufficiently large to not pass through the flexor sheath, thus limiting retraction to the level of the A4 pulley. Both vincula remain intact, given that the VBP originates at the distal portion of the middle phalanx. The Leddy and Packer classification was later expanded to include type IV and V injury patterns, which are less common than other injury patterns. Similar to type III injuries, type IV injuries involve a bony avulsion; however, the FDP subsequently ruptures from this fragment and the tendon subsequently retracts into the finger or palm.2,3 Type V injuries are more complex than other injury types because they involve a concomitant distal phalanx fracture with the FDP avulsion.4 Al-Qattan5 subclassified type V injuries into extra-articular (type Va) and intra-articular (type Vb) distal phalanx fractures on the basis of the distinct management of these 2 entities.
Numerous techniques have been proposed and described for the repair of FDP avulsion injuries. The pullout suture-dorsal button combination is the most widely described technique and was initially described by Bunnell.6 Unfortunately, this technique is accompanied by numerous potential postoperative complications.6 Nail plate deformity is the most commonly described complication. Other complications include local wound irritation, pain, button snagging, and repair failure. Additionally, the presence of external sutures creates a potential route of ingress for bacterial infection.
Continue to: Bone suture anchor techniques...
Bone suture anchor techniques were later utilized to repair FDP avulsions in an attempt to decrease complications associated with the external suture-button construct.7 The use of a transosseous suture without external button fixation has also been proposed. Sood and Elliot8 described a technique where the suture is passed through a hole, drilled transversely through the tuft of the distal phalanx, and affixed to the other limb. In 1999, Schultz and colleagues9 described a technique where transosseous tunnels are placed in the distal phalanx in a dorsal-to-volar direction. The suture is then passed through and tied on the dorsal surface. In this article, we propose a transosseous suture technique that may provide advantages over previously described methods.
SURGICAL TECHNIQUE
TYPES I, II, AND III
A Bruner incision is performed on the volar aspect of the affected finger, and full thickness flaps are elevated off the flexor sheath (Figures 1A-1C).
TYPES IV AND V
In cases of type IV or V injury (Figure 4A), a screw or plate construct is first used to allow for the successful reduction and fixation of the fracture (Figure 4B).
DISCUSSION
The avulsion of the FDP tendon from its insertion (zone I) on the distal phalanx is commonly called “jersey finger” and is a well-described injury that occurs most commonly in the ring finger.10 These injuries can be difficult to treat and are associated with a complication rate of as high as 60%.11,12 Bunnell’s initial description of a suture passed through the fingernail and then tied over a polypropylene button has been associated with multiple complications. Kang and colleagues13 reported abnormal nail growth, nail fold necrosis, fingertip deformity, stiffness, infection, and amputation, 43% of all complications were directly related to the button. As an alternative to the button, sutures may be tied directly over the nail plate itself via 2 separate holes.14 While this technique eliminates the complications directly associated with the button, the potential for infection remains. Additionally, increased direct pressure is placed on the nail plate and nail bed, thus potentially increasing the risk of nail deformity.
In 1994, Hallock7 initially described the use of bone anchors as an “internal fixation” alternative and cited the “expense of the apparatus” as the major drawback of this technique. McCallister and colleagues15 compared the clinical outcome of suture anchor fixation with that of the button-over-nail technique. Although they ultimately demonstrated that the clinical outcomes of the 2 techniques are not significantly different, they noted that suture anchor fixation is associated with decreased infection rate (7% vs 0%) and time to return to work. Poor bone mineral density and low cortical thickness are correlated with anchor pull-out, thus limiting its universal use.16 Furthermore, the universal use of many commonly available anchors is limited given that they are too large to be accommodated within many phalanges, particularly in women and in the small and ring fingers.17 The use of microanchors rather than mini anchors not only decreases this risk but also decreases construct strength, thus necessitating the use of 2 anchors to restore adequate fixation strength. Anchor use is associated with specific risks, including the dorsal migration of the anchor, the osteolysis of the surrounding bone, as well as the perforation of the dorsal cortex and the possible extrusion of the anchor through the phalanx and into the nail bed.18,19 Additionally, in the wake of a changing healthcare system, the cost of suture anchors, as initially noted by Hallock,7 must be considered. This consideration is particularly relevant to the use of a 2 microanchor construct, which has been advocated given its biomechanical advantage.20,21
Continue to: Transosseous tendon repair...
Transosseous tendon repair is a cost-effective option that obviates many complications commonly observed with other fixation methods. By keeping the suture within the body, the complications inherent in external sutures and buttons are eliminated, including the loss of fixation as a result of button or suture damage and facilitating hand hygiene maintenance. The rate of infection is also reduced. Moreover, the risk of nail deformities is decreased because the suture is not passed through the nail bed and nail plate in the described technique. Occasionally, some patients do note irritation from the dorsal suture knot under the thin skin proximal to the germinal matrix. This can be easily addressed in the clinic by removing the knot under local anesthesia following sufficient tendon healing. Additionally, the described technique can be used safely in pediatric patients with open physes because the needles can be placed to prevent violating the physis. This technique can be performed in conjunction with the skeletal fixation of type III, IV, and V jersey fingers. In our experience, the transosseous suture repair is more secure than the limited screw fixation, which can be accomplished in many type III jersey fingers, and in at least 1 case, has maintained flexor function when the skeletal fixation of the jersey finger has failed (Figures 5A, 5B).
All internal fixation techniques have been described previously by Sood and Elliot8 and, later, by Schultz and colleagues.9 In contrast to Sood and Elliott’s8 technique, which requires the creation of transverse tunnels, a volar-to-dorsal tunnel is technically easy to create and creates a direct repair to tendon insertion. Our technique is similar to that of Schultz and colleagues'9 but has the following differences and potential improvements:
- Keith needles are passed in a volar-to-dorsal fashion, thus allowing for the direct visualization of the transosseous tunnel origin, minimizing the size of the transosseous tunnels, and allowing for the anatomic reduction of the tendon.
- Fluoroscopy is used to confirm wire placement prior to skin incision, thus enabling precise placement and potentially allowing the needles to be placed so as to avoid physeal injury in pediatric jersey fingers.
- By using Keith needles, sutures can be passed with the same instrument that created the tunnel, thus simplifying surgical technique.
- A Krakow suture technique is used. This technique results in less gapping and higher load-to-failure than other suturing techniques.22
- A 2-0 braided suture is used, therefore strengthening repair.
This paper will be judged for the Resident Writer’s Award.
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
1. Leddy JP, Packer JW. Avulsion of the profundus tendon insertion in athletes. J Hand Surg Am. 1977;2(1):66-69. doi:https://doi.org/10.1016/S0363-5023(77)80012-9.
2. Langa V, Posner MA. Unusual rupture of a flexor profundus tendon. J Hand Surg Am. 1986;11(2):227-229. doi:https://doi.org/10.1016/S0363-5023(86)80056-9.
3. Ehlert KJ, Gould JS, Black KP. A simultaneous distal phalanx avulsion fracture with profundus tendon avulsion: A case report and review of the literature. Clin Orthop Relat Res. 1992;(283):265-269.
4. Smith JH. Avulsion of a profundus tendon with simultaneous intraarticular fracture of the distal phalanx–case report. J Hand Surg Am. 1981;6(6):600-601. doi:10.1097/00006534-198305000-00081.
5. Al-Qattan MM. Type 5 avulsion of the insertion of the flexor digitorum profundus tendon. J Hand Surg Br. 2001;26(5):427-431. doi:10.1054/jhsb.2001.0619.
6. Bunnell S. Surgery of the hand, 2nd edition. Philadelphia, PA: JB Lippincott; 1948:381-466.
7. Hallock GG. The Mitek Mini GII anchor introduced for tendon reinsertion in the hand. Ann Plast Surg. 1994;33(2):211-213.
8. Sood MK, Elliot D. A new technique of attachment of flexor tendons to the distal phalanx without a button tie-over. J Hand Surg Br. 1996;21(5):629-632. doi:https://doi.org/10.1016/S0266-7681(96)80146-X.
9. Schultz RO, Drake DB, Morgan RF. A new technique for the treatment of flexor digitorum profundus tendon avulsion. Ann Plast Surg. 1999;42(1):46-48. doi:10.1097/00000637-199901000-00008.
10. Manske PR, Lesker PA. Avulsion of the ring finger flexor digitorum profundus tendon: An experimental study. Hand 1978;10(1):52-55. doi:https://doi.org/10.1016/S0072-968X(78)80025-4.
11. Gerbino PG, Saldana MJ, Westerbeck P, Schacherer TG. Complications experienced in the rehabilitation of zone I flexor tendon injuries with dynamic traction splinting. J Hand Surg Am. 1991;16(4):680-686. doi:https://doi.org/10.1016/0363-5023(91)90194-G
12. Evans RB. Zone I flexor tendon rehabilitation with limited extension and active flexion. J Hand Ther. 2005;18(2):128-140. doi:10.1197/j.jht.2005.02.001
13. Kang N, Marsh D, Dewar D. The morbidity of the button-over-nail technique for zone 1 flexor tendon repairs. Should we still be using this technique? J Hand Surg Eur Vol. 2008;33(5):566-570. doi:10.1177/1753193408090118
14. Taras JS. Flexor tendon reconstruction: Single stage flexor tendon grafting: FDP, FDS disrupted. In: Green DP, Hotchkiss RN, Pederson WL, Wolfe SW, eds. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier Health Sciences; 2005:248-249.
15. McCallister WV, Ambrose HC, Katolik LI, Trumble TE. Comparison of pullout button versus suture anchor for zone I flexor tendon repair. J Hand Surg Am. 2006;31:246-251. doi:10.1016/j.jhsa.2005.10.020
16. Matzsuzaki H, Zaegel MA, Gelberman RH, Silva MJ. Effect of suture material and bone quality on the mechanical properties of zone 1 flexor tendon-bone reattachment with bone anchors. J Hand Surg Am. 2008;33(5):709-717. doi:10.1016/j.jhsa.2008.01.025
17. Singh R, Kakarala G, Persaud I, Roberts M, Strandring S, Compson J. The optimal length of tissue anchors for distal phalanges. A study in 395 cadaver digits. J Bone Joint Surg Br. 2006;88-B(SUPP I):37.
18. Giannikas D, Athanaselis E, Matzaroglou C, Saridis A, Tyllianakis M. An unusual complication of Mitek suture anchor use in primary treatment of flexor digitorum profundus tendon laceration: a case report. Cases J. 2009;2:9319. doi:10.1186/1757-1626-2-9319
19. Tiong WH, O'Sullivan ST. Extrusion of bone anchor suture following flexor digitorum profundus tendon avulsion injury repair. J Plast Reconstr Aesthet Surg. 2011;64(9):1242-1244. doi:10.1016/j.bjps.2011.01.016
20. Silva MJ, Hollstien SB, Brodt MD, Boyer MI, Tetro AM, Gelberman RH. Flexor digitorum profundus tendon-to-bone repair: An ex vivo biomechanical analysis of 3 pullout suture techniques. J Hand Surg Am. 1998;23(1):120-126. doi:10.1016/S0363-5023(98)80099-3
21. Latendresse K, Dona E, Scougall PJ, Schreuder FB, Puchert E, Walsh WR. Cyclic testing of pullout sutures and micro-mitek suture anchors in flexor digitorum profundus tendon distal fixation. J Hand Surg Am. 2005;30(3):471-478. doi:10.1016/j.jhsa.2004.10.014
22. Lee SK, Fajardo M, Kardashian G, Klein J, Tsai P, Christoforou D. Repair of flexor digitorum profundus to distal phalanx: a biomechanical evaluation of four techniques. J Hand Surg Am. 2011;36(10):1604-1609. doi:10.1016/j.jhsa.2011.07.017
TAKE-HOME POINTS
- Transosseous repair of FDP has been long utilized, tying the sutures over a polyethylene button at the nail plate, which is associated with significant complications.
- Avoiding use of a button decreases these complications, eliminating damage to the nailbed and eliminating external sutures, thus decreasing infection risk.
- Keith needles can be utilized to pass the sutures from volar to dorsal, and can be inserted using a wire drive; their position can be checked fluoroscopically prior to suture passage.
- This technique can be used in conjunction with skeletal fixation of associated fractures.
- This technique can be utilized in pediatric patients, placing the sutures distal to the physis.
Recurrence of Extranodal Natural Killer/T-cell Lymphoma Presenting as Tarsal Tunnel Syndrome
ABSTRACT
This case report is a rare form of lymphoma recurrence which presented as tarsal tunnel syndrome. The patient had been previously treated for the malignancy and was presumed to be in remission; however, standard radiology imaging protocols failed to include the distal extremities on these scans. The patient presented to the orthopedic clinic with tarsal tunnel symptoms and a mass in the tarsal tunnel. A complete evaluation resulted in a diagnosis of recurrence of the malignancy. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body fluorodeoxyglucose positron emission tomography computed tomography when evaluating for recurrence in patients.
Nasal-type, extranodal natural killer/T-cell lymphoma (ENKTL) is a rare form of non-Hodgkin lymphoma (NHL). Malignancies account for only 10% of NHL in Asian and South American populations. However, in Caucasians, it represents <1% of all cases. In addition, at 3:1 male to female ratio, the disease most commonly affects male patients who are 50 to 59 years old.1-3 The etiology of this malignancy is strongly related to prior infection with Epstein-Barr virus (EBV) as EBV-encoded early small ribonucleic acid on in situ hybridization of lymphoma cells is positive in 95% of cases.4-6
Typical sites of involvement include the nasal cavity, nasopharynx, and sinuses, causing patients to present with nasal obstruction, chronic sinusitis, or epistaxis. Additionally, ENKTL can occur primarily in the skin, gastrointestinal tract, spleen, and testis, whereas the bone marrow may be involved in 10% of cases. Although rare, unusual sites, including muscle, adrenals, and ovaries, have been published.7,8
Staging is best performed using the T-staging system, which accounts for the extent of local tumor involvement. Higher stages, such as T3 /T4, equate to locally advanced disease and imply a worse prognosis.9,10 Computed tomography (CT) and magnetic resonance imaging (MRI) help define local soft tissues and bony involvement. Furthermore, CT of the chest, abdomen, and pelvis as well as bone marrow biopsy are performed as part of the staging process. Lastly, fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) is often used to detect extranodal spread, define the extent of involvement, differentiate between lymphoma and inflammatory masses, and monitor for recurrence.11
Treatment for local ENKTL involves concurrent chemoradiotherapy followed by 3 cycles of etoposide, ifosfamide, cisplatin, and dexamethasone, which results in a complete response rate of 80%, and is the most favorable when comparing treatment modalities.12 Unfortunately, recurrence rates reach as high as 50%, whereas the 5-year survival rate is 59%.13,14 For recurrent or disseminated disease, high-dose chemotherapy and hematopoietic stem cell transplantation remain as alternative treatments for patients who have undergone 2 complete remissions and can be curative in some instances.13,15
Continue to: In summary, ENKTL is a rare form...
In summary, ENKTL is a rare form of NHL which classically presents in the nasal cavity; however, this type of lymphoma may present in a variety of extranodal sites.7,8 Despite the numerous published reports on ENKTL, no study has reported either primary or recurrent ENKTL in the feet or hands. To our knowledge, this is one of the first published cases of a patient who developed a rare and recurring ENKTL in the foot and ankle. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 59-year-old Caucasian woman was referred to the orthopedic foot and ankle clinic by her primary care physician for right medial ankle pain, skin ulceration, and numbness over the plantar aspect of her right foot. Upon questioning, the patient noted that the pain and numbness were present for almost 6 months. She denied trauma to the concerned area. Previously, the patient was observed and treated elsewhere for plantar fasciitis and was prescribed a brace before being immobilized in a controlled ankle motion (CAM) boot for 6 weeks. At follow-up with her outside provider, the patient had developed skin breakdown over the medial aspect of the right ankle, and this condition was presumed to be caused by the boot. After local wound care failed to improve her skin ulceration, she returned to her primary care physician, who ordered an MRI of the area and referred her to our specialty clinic.
Upon review, the patient’s past medical history included a diagnosis of nasal-type ENKTL. Her malignancy was treated with chemoradiotherapy 2 years prior to her consultation with the foot and ankle clinic.
The patient was noted by her medical oncologist and interventional radiologist to be in complete stage 4 remission since being treated. She underwent routine MRI and CT scans of the head and neck at 6-month intervals and FDG PET-CT scans at 3-month intervals, as per institutional protocol. The examinations showed no evidence of malignancy or metabolically active disease. The last imaging study occurred 2 months prior to admission to the foot and ankle clinic.
The patient consulted her medical oncologist 1 month prior to presenting to our clinic and was noted to exhibit an “excellent response to chemoradiotherapy” and “continues to remain disease free at 2 years.” She was instructed to continue routine follow-up. However, the office notes mentioned no ankle pain and non-healing wounds.
During physical examination, the patient presented an antalgic gait on the right side. Inspection demonstrated an increased circumference of the right ankle compared with the left, with a soft, palpable mass over the medial aspect of her right ankle. A 3 cm × 2 cm, grade 2 abrasion of the skin was observed over the medial mass just posterior to her medial malleolus. Range of motion was within normal limits. The patient exhibited a palpable posterior tibial artery pulse and full strength upon muscle testing of the lower extremities. She featured a positive Tinel’s sign and discomfort over the mass itself, with the pain radiating down to the plantar aspect of her foot and diffuse numbness over the plantar aspect of the foot.
Continue to: Review of her plain radiographs...
Review of her plain radiographs demonstrated no bony abnormalities, fractures, nor visible deformity (Figures 1A, 1B).
At presentation, our differential diagnosis included recurrence of the malignancy, secondary malignancy, infection, and inflammatory disease. After a lengthy discussion with the patient and consultation with our institution’s musculoskeletal oncologist, the decision was made to perform a right-ankle mass biopsy and marginal excision with wound irrigation and débridement and tarsal tunnel release.
The patient was placed in the supine position with standard prepping and draping. The medial eschar was excised in an elliptical fashion, and a curvilinear, longitudinal approach was performed within the compartment to access the mass along the posteromedial aspect of the ankle. Although no evidence of infection was observed, the tissue was thickened with areas of necrosis down to the flexor retinaculum. Once the flexor retinaculum was opened, a fibrous, plaque-like mass was observed, and it was encased with flexor tendons and neurovascular structures of the tarsal tunnel. After mass excision, a complete tarsal tunnel release was performed until the neurovascular bundle was free. Irrigation and débridement of the ulcer were performed along with complicated wound closure, and the patient was placed in a well-padded postoperative splint.
Pathology was finalized as a recurrent, EBV-positive, and nasal-type ENKTL. The patient underwent bone marrow biopsy, which yielded negative results. CT of the chest, abdomen, and pelvis were negative for the disease. FDG PET-CT, which included the extremities, was performed and demonstrated increased uptake in the right ankle, consistent with the malignancy (Figure 4).
DISCUSSION
ENKTL is an uncommon form of lymphoma and is exceedingly rare in Caucasian females.1-3 Although the patient’s primary occurrence was in the nasal cavity, recurrence in the foot and ankle must still be described.7,8 To our knowledge, this article is one of the first published cases of a patient who developed a rare-recurrence ENKTL about the foot and ankle. Occurrence in extremities is extremely rare that the staging protocol does not include FDG PET-CT of these areas. The patient’s “negative” scans led many providers to neglect the symptoms in her right ankle until the lesion had ulcerated through the skin. If one would have relied on imaging reports and outside records alone, the diagnosis would have been delayed longer or missed all together. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body FDG PET-CT when evaluating for recurrence in patients.
1. Quintanilla-Martinez L, Kremer M, Keller G, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095-2105. doi:10.1016/S0002-9440(10)63061-1.
2. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206-214. doi:10.1093/annonc/mdi037.
3. Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918.
4. Medeiros LJ, Peiper SC, Elwood L, Yano T, Raffeld M, Jaffe ES. Angiocentric immunoproliferative lesions: a molecular analysis of eight cases. Hum Pathol. 1991;22(11):1150-1157. doi:10.1016/0046-8177(91)90269-U.
5. Ho FC, Srivastava G, Loke SL, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990;8(5):271-281. doi:10.1002/hon.2900080505.
6. Gelb AB, van de Rijn M, Regula DP Jr, et al. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum Pathol. 1994;25(9):953-960. doi:10.1016/0046-8177(94)90018-3.
7. Petrella T, Delfau-Larue MH, Caillot D, et al. Nasopharyngeal lymphomas: further evidence for a natural killer cell origin. Hum Pathol. 1996;27(8):827-833. doi:10.1016/S0046-8177(96)90457-8.
8. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127(6):860-868. doi:10.1309/2F39NX1AL3L54WU8.
9. Robbins KT, Fuller LM, Vlasak M. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985;56(4):814-819. doi:10.1002/1097-0142(19850815)56.
10. Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. Am J Roentgenol. 2000;174(4):1141-1145. doi:10.2214/ajr.174.4.1741141.
11. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621. doi:10.1007/s00277-008-0494-8.
12. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027-6032. doi:10.1200/JCO.2009.23.8592.
13. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194. doi:10.1038/sj.leu.2403955.
14. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13-21. doi:10.1111/j.1365-2141.2009.07802.x.
15. Yokoyama H, Yamamoto J, Tohmiya Y, et al. Allogeneic hematopoietic stem cell transplant following chemotherapy containing l-asparaginase as a promising treatment for patients with relapsed or refractory extranodal natural killer/T cell lymphoma, nasal type. Leuk Lymphoma. 2010;51(8):1509-1512. doi:10.3109/10428194.2010.487958.
ABSTRACT
This case report is a rare form of lymphoma recurrence which presented as tarsal tunnel syndrome. The patient had been previously treated for the malignancy and was presumed to be in remission; however, standard radiology imaging protocols failed to include the distal extremities on these scans. The patient presented to the orthopedic clinic with tarsal tunnel symptoms and a mass in the tarsal tunnel. A complete evaluation resulted in a diagnosis of recurrence of the malignancy. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body fluorodeoxyglucose positron emission tomography computed tomography when evaluating for recurrence in patients.
Nasal-type, extranodal natural killer/T-cell lymphoma (ENKTL) is a rare form of non-Hodgkin lymphoma (NHL). Malignancies account for only 10% of NHL in Asian and South American populations. However, in Caucasians, it represents <1% of all cases. In addition, at 3:1 male to female ratio, the disease most commonly affects male patients who are 50 to 59 years old.1-3 The etiology of this malignancy is strongly related to prior infection with Epstein-Barr virus (EBV) as EBV-encoded early small ribonucleic acid on in situ hybridization of lymphoma cells is positive in 95% of cases.4-6
Typical sites of involvement include the nasal cavity, nasopharynx, and sinuses, causing patients to present with nasal obstruction, chronic sinusitis, or epistaxis. Additionally, ENKTL can occur primarily in the skin, gastrointestinal tract, spleen, and testis, whereas the bone marrow may be involved in 10% of cases. Although rare, unusual sites, including muscle, adrenals, and ovaries, have been published.7,8
Staging is best performed using the T-staging system, which accounts for the extent of local tumor involvement. Higher stages, such as T3 /T4, equate to locally advanced disease and imply a worse prognosis.9,10 Computed tomography (CT) and magnetic resonance imaging (MRI) help define local soft tissues and bony involvement. Furthermore, CT of the chest, abdomen, and pelvis as well as bone marrow biopsy are performed as part of the staging process. Lastly, fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) is often used to detect extranodal spread, define the extent of involvement, differentiate between lymphoma and inflammatory masses, and monitor for recurrence.11
Treatment for local ENKTL involves concurrent chemoradiotherapy followed by 3 cycles of etoposide, ifosfamide, cisplatin, and dexamethasone, which results in a complete response rate of 80%, and is the most favorable when comparing treatment modalities.12 Unfortunately, recurrence rates reach as high as 50%, whereas the 5-year survival rate is 59%.13,14 For recurrent or disseminated disease, high-dose chemotherapy and hematopoietic stem cell transplantation remain as alternative treatments for patients who have undergone 2 complete remissions and can be curative in some instances.13,15
Continue to: In summary, ENKTL is a rare form...
In summary, ENKTL is a rare form of NHL which classically presents in the nasal cavity; however, this type of lymphoma may present in a variety of extranodal sites.7,8 Despite the numerous published reports on ENKTL, no study has reported either primary or recurrent ENKTL in the feet or hands. To our knowledge, this is one of the first published cases of a patient who developed a rare and recurring ENKTL in the foot and ankle. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 59-year-old Caucasian woman was referred to the orthopedic foot and ankle clinic by her primary care physician for right medial ankle pain, skin ulceration, and numbness over the plantar aspect of her right foot. Upon questioning, the patient noted that the pain and numbness were present for almost 6 months. She denied trauma to the concerned area. Previously, the patient was observed and treated elsewhere for plantar fasciitis and was prescribed a brace before being immobilized in a controlled ankle motion (CAM) boot for 6 weeks. At follow-up with her outside provider, the patient had developed skin breakdown over the medial aspect of the right ankle, and this condition was presumed to be caused by the boot. After local wound care failed to improve her skin ulceration, she returned to her primary care physician, who ordered an MRI of the area and referred her to our specialty clinic.
Upon review, the patient’s past medical history included a diagnosis of nasal-type ENKTL. Her malignancy was treated with chemoradiotherapy 2 years prior to her consultation with the foot and ankle clinic.
The patient was noted by her medical oncologist and interventional radiologist to be in complete stage 4 remission since being treated. She underwent routine MRI and CT scans of the head and neck at 6-month intervals and FDG PET-CT scans at 3-month intervals, as per institutional protocol. The examinations showed no evidence of malignancy or metabolically active disease. The last imaging study occurred 2 months prior to admission to the foot and ankle clinic.
The patient consulted her medical oncologist 1 month prior to presenting to our clinic and was noted to exhibit an “excellent response to chemoradiotherapy” and “continues to remain disease free at 2 years.” She was instructed to continue routine follow-up. However, the office notes mentioned no ankle pain and non-healing wounds.
During physical examination, the patient presented an antalgic gait on the right side. Inspection demonstrated an increased circumference of the right ankle compared with the left, with a soft, palpable mass over the medial aspect of her right ankle. A 3 cm × 2 cm, grade 2 abrasion of the skin was observed over the medial mass just posterior to her medial malleolus. Range of motion was within normal limits. The patient exhibited a palpable posterior tibial artery pulse and full strength upon muscle testing of the lower extremities. She featured a positive Tinel’s sign and discomfort over the mass itself, with the pain radiating down to the plantar aspect of her foot and diffuse numbness over the plantar aspect of the foot.
Continue to: Review of her plain radiographs...
Review of her plain radiographs demonstrated no bony abnormalities, fractures, nor visible deformity (Figures 1A, 1B).
At presentation, our differential diagnosis included recurrence of the malignancy, secondary malignancy, infection, and inflammatory disease. After a lengthy discussion with the patient and consultation with our institution’s musculoskeletal oncologist, the decision was made to perform a right-ankle mass biopsy and marginal excision with wound irrigation and débridement and tarsal tunnel release.
The patient was placed in the supine position with standard prepping and draping. The medial eschar was excised in an elliptical fashion, and a curvilinear, longitudinal approach was performed within the compartment to access the mass along the posteromedial aspect of the ankle. Although no evidence of infection was observed, the tissue was thickened with areas of necrosis down to the flexor retinaculum. Once the flexor retinaculum was opened, a fibrous, plaque-like mass was observed, and it was encased with flexor tendons and neurovascular structures of the tarsal tunnel. After mass excision, a complete tarsal tunnel release was performed until the neurovascular bundle was free. Irrigation and débridement of the ulcer were performed along with complicated wound closure, and the patient was placed in a well-padded postoperative splint.
Pathology was finalized as a recurrent, EBV-positive, and nasal-type ENKTL. The patient underwent bone marrow biopsy, which yielded negative results. CT of the chest, abdomen, and pelvis were negative for the disease. FDG PET-CT, which included the extremities, was performed and demonstrated increased uptake in the right ankle, consistent with the malignancy (Figure 4).
DISCUSSION
ENKTL is an uncommon form of lymphoma and is exceedingly rare in Caucasian females.1-3 Although the patient’s primary occurrence was in the nasal cavity, recurrence in the foot and ankle must still be described.7,8 To our knowledge, this article is one of the first published cases of a patient who developed a rare-recurrence ENKTL about the foot and ankle. Occurrence in extremities is extremely rare that the staging protocol does not include FDG PET-CT of these areas. The patient’s “negative” scans led many providers to neglect the symptoms in her right ankle until the lesion had ulcerated through the skin. If one would have relied on imaging reports and outside records alone, the diagnosis would have been delayed longer or missed all together. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body FDG PET-CT when evaluating for recurrence in patients.
ABSTRACT
This case report is a rare form of lymphoma recurrence which presented as tarsal tunnel syndrome. The patient had been previously treated for the malignancy and was presumed to be in remission; however, standard radiology imaging protocols failed to include the distal extremities on these scans. The patient presented to the orthopedic clinic with tarsal tunnel symptoms and a mass in the tarsal tunnel. A complete evaluation resulted in a diagnosis of recurrence of the malignancy. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body fluorodeoxyglucose positron emission tomography computed tomography when evaluating for recurrence in patients.
Nasal-type, extranodal natural killer/T-cell lymphoma (ENKTL) is a rare form of non-Hodgkin lymphoma (NHL). Malignancies account for only 10% of NHL in Asian and South American populations. However, in Caucasians, it represents <1% of all cases. In addition, at 3:1 male to female ratio, the disease most commonly affects male patients who are 50 to 59 years old.1-3 The etiology of this malignancy is strongly related to prior infection with Epstein-Barr virus (EBV) as EBV-encoded early small ribonucleic acid on in situ hybridization of lymphoma cells is positive in 95% of cases.4-6
Typical sites of involvement include the nasal cavity, nasopharynx, and sinuses, causing patients to present with nasal obstruction, chronic sinusitis, or epistaxis. Additionally, ENKTL can occur primarily in the skin, gastrointestinal tract, spleen, and testis, whereas the bone marrow may be involved in 10% of cases. Although rare, unusual sites, including muscle, adrenals, and ovaries, have been published.7,8
Staging is best performed using the T-staging system, which accounts for the extent of local tumor involvement. Higher stages, such as T3 /T4, equate to locally advanced disease and imply a worse prognosis.9,10 Computed tomography (CT) and magnetic resonance imaging (MRI) help define local soft tissues and bony involvement. Furthermore, CT of the chest, abdomen, and pelvis as well as bone marrow biopsy are performed as part of the staging process. Lastly, fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) is often used to detect extranodal spread, define the extent of involvement, differentiate between lymphoma and inflammatory masses, and monitor for recurrence.11
Treatment for local ENKTL involves concurrent chemoradiotherapy followed by 3 cycles of etoposide, ifosfamide, cisplatin, and dexamethasone, which results in a complete response rate of 80%, and is the most favorable when comparing treatment modalities.12 Unfortunately, recurrence rates reach as high as 50%, whereas the 5-year survival rate is 59%.13,14 For recurrent or disseminated disease, high-dose chemotherapy and hematopoietic stem cell transplantation remain as alternative treatments for patients who have undergone 2 complete remissions and can be curative in some instances.13,15
Continue to: In summary, ENKTL is a rare form...
In summary, ENKTL is a rare form of NHL which classically presents in the nasal cavity; however, this type of lymphoma may present in a variety of extranodal sites.7,8 Despite the numerous published reports on ENKTL, no study has reported either primary or recurrent ENKTL in the feet or hands. To our knowledge, this is one of the first published cases of a patient who developed a rare and recurring ENKTL in the foot and ankle. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 59-year-old Caucasian woman was referred to the orthopedic foot and ankle clinic by her primary care physician for right medial ankle pain, skin ulceration, and numbness over the plantar aspect of her right foot. Upon questioning, the patient noted that the pain and numbness were present for almost 6 months. She denied trauma to the concerned area. Previously, the patient was observed and treated elsewhere for plantar fasciitis and was prescribed a brace before being immobilized in a controlled ankle motion (CAM) boot for 6 weeks. At follow-up with her outside provider, the patient had developed skin breakdown over the medial aspect of the right ankle, and this condition was presumed to be caused by the boot. After local wound care failed to improve her skin ulceration, she returned to her primary care physician, who ordered an MRI of the area and referred her to our specialty clinic.
Upon review, the patient’s past medical history included a diagnosis of nasal-type ENKTL. Her malignancy was treated with chemoradiotherapy 2 years prior to her consultation with the foot and ankle clinic.
The patient was noted by her medical oncologist and interventional radiologist to be in complete stage 4 remission since being treated. She underwent routine MRI and CT scans of the head and neck at 6-month intervals and FDG PET-CT scans at 3-month intervals, as per institutional protocol. The examinations showed no evidence of malignancy or metabolically active disease. The last imaging study occurred 2 months prior to admission to the foot and ankle clinic.
The patient consulted her medical oncologist 1 month prior to presenting to our clinic and was noted to exhibit an “excellent response to chemoradiotherapy” and “continues to remain disease free at 2 years.” She was instructed to continue routine follow-up. However, the office notes mentioned no ankle pain and non-healing wounds.
During physical examination, the patient presented an antalgic gait on the right side. Inspection demonstrated an increased circumference of the right ankle compared with the left, with a soft, palpable mass over the medial aspect of her right ankle. A 3 cm × 2 cm, grade 2 abrasion of the skin was observed over the medial mass just posterior to her medial malleolus. Range of motion was within normal limits. The patient exhibited a palpable posterior tibial artery pulse and full strength upon muscle testing of the lower extremities. She featured a positive Tinel’s sign and discomfort over the mass itself, with the pain radiating down to the plantar aspect of her foot and diffuse numbness over the plantar aspect of the foot.
Continue to: Review of her plain radiographs...
Review of her plain radiographs demonstrated no bony abnormalities, fractures, nor visible deformity (Figures 1A, 1B).
At presentation, our differential diagnosis included recurrence of the malignancy, secondary malignancy, infection, and inflammatory disease. After a lengthy discussion with the patient and consultation with our institution’s musculoskeletal oncologist, the decision was made to perform a right-ankle mass biopsy and marginal excision with wound irrigation and débridement and tarsal tunnel release.
The patient was placed in the supine position with standard prepping and draping. The medial eschar was excised in an elliptical fashion, and a curvilinear, longitudinal approach was performed within the compartment to access the mass along the posteromedial aspect of the ankle. Although no evidence of infection was observed, the tissue was thickened with areas of necrosis down to the flexor retinaculum. Once the flexor retinaculum was opened, a fibrous, plaque-like mass was observed, and it was encased with flexor tendons and neurovascular structures of the tarsal tunnel. After mass excision, a complete tarsal tunnel release was performed until the neurovascular bundle was free. Irrigation and débridement of the ulcer were performed along with complicated wound closure, and the patient was placed in a well-padded postoperative splint.
Pathology was finalized as a recurrent, EBV-positive, and nasal-type ENKTL. The patient underwent bone marrow biopsy, which yielded negative results. CT of the chest, abdomen, and pelvis were negative for the disease. FDG PET-CT, which included the extremities, was performed and demonstrated increased uptake in the right ankle, consistent with the malignancy (Figure 4).
DISCUSSION
ENKTL is an uncommon form of lymphoma and is exceedingly rare in Caucasian females.1-3 Although the patient’s primary occurrence was in the nasal cavity, recurrence in the foot and ankle must still be described.7,8 To our knowledge, this article is one of the first published cases of a patient who developed a rare-recurrence ENKTL about the foot and ankle. Occurrence in extremities is extremely rare that the staging protocol does not include FDG PET-CT of these areas. The patient’s “negative” scans led many providers to neglect the symptoms in her right ankle until the lesion had ulcerated through the skin. If one would have relied on imaging reports and outside records alone, the diagnosis would have been delayed longer or missed all together. This case illustrates the importance of a thorough medical history and personal review of imaging studies, and how a systematic approach can produce the correct diagnosis for any unknown lesion. Furthermore, this case may prompt oncologists to consider obtaining whole-body FDG PET-CT when evaluating for recurrence in patients.
1. Quintanilla-Martinez L, Kremer M, Keller G, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095-2105. doi:10.1016/S0002-9440(10)63061-1.
2. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206-214. doi:10.1093/annonc/mdi037.
3. Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918.
4. Medeiros LJ, Peiper SC, Elwood L, Yano T, Raffeld M, Jaffe ES. Angiocentric immunoproliferative lesions: a molecular analysis of eight cases. Hum Pathol. 1991;22(11):1150-1157. doi:10.1016/0046-8177(91)90269-U.
5. Ho FC, Srivastava G, Loke SL, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990;8(5):271-281. doi:10.1002/hon.2900080505.
6. Gelb AB, van de Rijn M, Regula DP Jr, et al. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum Pathol. 1994;25(9):953-960. doi:10.1016/0046-8177(94)90018-3.
7. Petrella T, Delfau-Larue MH, Caillot D, et al. Nasopharyngeal lymphomas: further evidence for a natural killer cell origin. Hum Pathol. 1996;27(8):827-833. doi:10.1016/S0046-8177(96)90457-8.
8. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127(6):860-868. doi:10.1309/2F39NX1AL3L54WU8.
9. Robbins KT, Fuller LM, Vlasak M. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985;56(4):814-819. doi:10.1002/1097-0142(19850815)56.
10. Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. Am J Roentgenol. 2000;174(4):1141-1145. doi:10.2214/ajr.174.4.1741141.
11. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621. doi:10.1007/s00277-008-0494-8.
12. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027-6032. doi:10.1200/JCO.2009.23.8592.
13. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194. doi:10.1038/sj.leu.2403955.
14. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13-21. doi:10.1111/j.1365-2141.2009.07802.x.
15. Yokoyama H, Yamamoto J, Tohmiya Y, et al. Allogeneic hematopoietic stem cell transplant following chemotherapy containing l-asparaginase as a promising treatment for patients with relapsed or refractory extranodal natural killer/T cell lymphoma, nasal type. Leuk Lymphoma. 2010;51(8):1509-1512. doi:10.3109/10428194.2010.487958.
1. Quintanilla-Martinez L, Kremer M, Keller G, et al. p53 mutations in nasal natural killer/T-cell lymphoma from Mexico: association with large cell morphology and advanced disease. Am J Pathol. 2001;159(6):2095-2105. doi:10.1016/S0002-9440(10)63061-1.
2. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206-214. doi:10.1093/annonc/mdi037.
3. Armitage JO. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. Blood. 1997;89(11):3909-3918.
4. Medeiros LJ, Peiper SC, Elwood L, Yano T, Raffeld M, Jaffe ES. Angiocentric immunoproliferative lesions: a molecular analysis of eight cases. Hum Pathol. 1991;22(11):1150-1157. doi:10.1016/0046-8177(91)90269-U.
5. Ho FC, Srivastava G, Loke SL, et al. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and ‘T’ cell type. Hematol Oncol. 1990;8(5):271-281. doi:10.1002/hon.2900080505.
6. Gelb AB, van de Rijn M, Regula DP Jr, et al. Epstein-Barr virus-associated natural killer-large granular lymphocyte leukemia. Hum Pathol. 1994;25(9):953-960. doi:10.1016/0046-8177(94)90018-3.
7. Petrella T, Delfau-Larue MH, Caillot D, et al. Nasopharyngeal lymphomas: further evidence for a natural killer cell origin. Hum Pathol. 1996;27(8):827-833. doi:10.1016/S0046-8177(96)90457-8.
8. Hasserjian RP, Harris NL. NK-cell lymphomas and leukemias: a spectrum of tumors with variable manifestations and immunophenotype. Am J Clin Pathol. 2007;127(6):860-868. doi:10.1309/2F39NX1AL3L54WU8.
9. Robbins KT, Fuller LM, Vlasak M. Primary lymphomas of the nasal cavity and paranasal sinuses. Cancer. 1985;56(4):814-819. doi:10.1002/1097-0142(19850815)56.
10. Ooi GC, Chim CS, Liang R, Tsang KW, Kwong YL. Nasal T-cell/natural killer cell lymphoma: CT and MR imaging features of a new clinicopathologic entity. Am J Roentgenol. 2000;174(4):1141-1145. doi:10.2214/ajr.174.4.1741141.
11. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621. doi:10.1007/s00277-008-0494-8.
12. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-cell lymphoma: consortium for improving survival of lymphoma study. J Clin Oncol. 2009;27(35):6027-6032. doi:10.1200/JCO.2009.23.8592.
13. Kwong YL. Natural killer-cell malignancies: diagnosis and treatment. Leukemia. 2005;19(12):2186-2194. doi:10.1038/sj.leu.2403955.
14. Liang R. Advances in the management and monitoring of extranodal NK/T-cell lymphoma, nasal type. Br J Haematol. 2009;147(1):13-21. doi:10.1111/j.1365-2141.2009.07802.x.
15. Yokoyama H, Yamamoto J, Tohmiya Y, et al. Allogeneic hematopoietic stem cell transplant following chemotherapy containing l-asparaginase as a promising treatment for patients with relapsed or refractory extranodal natural killer/T cell lymphoma, nasal type. Leuk Lymphoma. 2010;51(8):1509-1512. doi:10.3109/10428194.2010.487958.
TAKE-HOME POINTS
- A thorough review of systems, physical examination, and personal review of a patient’s advanced imaging is critical to avoid missed diagnosis or delays in diagnosis.
- Any mass lesion encountered in clinical practice, no matter how benign appearing, should be presumed malignant until proven otherwise.
- Fluorine-18 fluorodeoxyglucose positron emission tomography CT (18-FDG PET-CT) should include whole-body scans when evaluating patients for recurrence of malignancy.
Avulsion of the Anterior Lateral Meniscal Root Secondary to Tibial Eminence Fracture
ABSTRACT
The lateral tibial eminence shares a close relationship with the anterior root of the lateral meniscus. Limited studies have reported traumatic injury to the anterior meniscal roots in the setting of tibial eminence fractures, and reported rates of occurrence of concomitant meniscal and chondral injuries vary widely. The purpose of this article is to describe the case of a 28-year-old woman who had a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and root displacement. The anterolateral meniscal root was anatomically repaired following arthroscopic resection of the malunited fragment.
The lateral tibial eminence is intimately associated with the root attachment of the anterior horn of the lateral meniscus.1-3 Previous studies have demonstrated both the close proximity of the anterior cruciate ligament (ACL) insertion to the meniscal roots and the potential for disruption in surgical interventions, such as tibial tunnel drilling in ACL reconstruction or placement of intramedullary tibial nails.4-6 The meniscal roots play a crucial role in force distribution, and disruption of these structures has been shown to significantly increase joint contact forces. Despite the deleterious effects of this injury, limited studies have reported on traumatic injury to the meniscal roots in the setting of tibial eminence fractures.
Reported rates of occurrence of concomitant meniscal and chondral injuries occurring with tibial eminence fractures vary widely, ranging from <5% to 40%.7,8 Although fractures to the tibial eminence are more common in children, an association between these injuries and concomitant soft tissue injuries, including meniscal, chondral, and collateral ligament injuries, in the adult population has been reported.7 Monto and Cameron-Donaldson8 used magnetic resonance imaging (MRI) to evaluate tibial eminence fractures in adults and found that 23% of study subjects had associated medial meniscus tears and 18% had lateral meniscus tears. In a similar study, Ishibashi and colleagues9 found that 25% of tibial eminence fractures were associated with lateral meniscus tears and 16% with medial meniscus tears.
These studies demonstrate the potential for meniscus injuries during tibial eminence fractures. However, the authors are unaware of any reports of complete tearing of the anterior horn of the lateral meniscus in association with this injury. This is an important injury to recognize and identify intraoperatively because an injury of this nature could potentially compromise the mechanical loading patterns and health of the articular cartilage of the lateral compartment of the knee. The purpose of this article is to describe a complete avulsion of the anterolateral meniscal root due to a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomical position. The patient provided written informed consent for print and electronic publication of this case report.
Continue to: A 28-year-old active woman...
CASE
A 28-year-old active woman presented to our clinic 22 months after sustaining a right knee tibial eminence fracture that was initially treated with extension immobilization, which resulted in a fibrous malunion. She subsequently sustained a second injury resulting in displacement of the malunion fracture fragment, and was treated at another institution 10 months prior to presentation at our clinic with arthroscopic reduction and internal fixation with a cannulated screw and washer of the tibial eminence fracture. This was followed by hardware removal 6 months prior to her office visit at our clinic. At presentation, she reported worsening right knee pain, mechanical symptoms, and loss of both flexion and extension compared with her uninjured knee. Conservative management, including activity modification, extensive physical therapy, and anti-inflammatory medication following her most recent procedure, had not resulted in improvement of her symptoms.
Physical examination revealed significantly reduced knee flexion and extension (+15°-120° on the affected side compared with 5° of hyperextension to 130° flexion of the contralateral knee). Ligamentous examination demonstrated no laxity with varus or valgus stress at 0° to 30° of flexion, negative posterior drawer, and a Grade 2 Lachman and positive pivot shift. She also exhibited pain with attempted right knee terminal extension. Radiographs and computed tomography scans were obtained and reviewed. They revealed a malunited tibial eminence fracture (Figures 1A-1D).
Arthroscopic assessment of the right knee demonstrated the large osseous fragment located in the anterolateral aspect of the joint with the displaced anterior horn of the lateral meniscus attached as well as significant anterior impingement limiting knee extension. Probing of the anterolateral meniscal root in the lateral compartment showed abundant surrounding scar tissue with an abnormal attachment, representing a chronic root avulsion. A mechanical shaver was used to débride the scar tissue and expose the malunited fragment, followed by complete osseous fragment excision with a high-speed burr (Figure 3).
A soft tissue anterolateral meniscal root repair was performed by creating a 2-cm to 3-cm incision on the anterolateral tibia, just distal to the medial aspect of the Gerdy tubercle. To best restore the footprint of the repair and increase the potential for biologic healing, 2 transtibial tunnels were created at the location of the root attachment. An ACL aiming device with a cannulated sleeve was used to drill 2 bony tunnels approximately 5 mm apart, exiting at the anatomic root footprint. The drill pins were removed, leaving the 2 cannulas in place for later suture passage. A suture-passing device was used to pass 2 separate sutures through the detached meniscal root.
Continue to: Postoperatively, the patient was placed...
Postoperatively, the patient was placed on a non-weight-bearing protocol for her operative lower extremity for 6 weeks. A brace locked in extension was used for the same period of time (being removed only for physical therapy exercises). Enoxaparin was used for the first 2 weeks for deep vein thrombosis prophylaxis, followed by aspirin for an additional 4 weeks. Physical therapy was started on postoperative day 1 to begin working on early passive ROM exercises. Knee flexion was limited to 0° to 90° of flexion for the first 2 weeks and then progressed as tolerated.
DISCUSSION
This article describes a rare case of a patient with lateral meniscal anterior root avulsion in the setting of a tibial eminence fracture with subsequent malunion and root displacement. In a case such as this, delineation of the true extent of the injury is difficult because the anterior meniscal root can be torn, displaced, and nonanatomically scarred to surrounding soft tissues, making MRI interpretation challenging. Clinically, patients can present with a wide range of symptoms, including pain, mechanical symptoms, instability, and loss of knee motion.10
The anterior root of the lateral meniscus has been reported to be attached anterior to the lateral tibial eminence and adjacent to the insertion of the ACL. Fibrous connections extending from the anterior horn of the lateral meniscus attachment to the lateral tibial eminence are constant.11 Furumatsu and colleagues12 demonstrated the existence of dense fibers linking the anterior root of the lateral meniscus with the lateral aspect of the ACL tibial insertion. Acknowledging the close relationship of these structures is key to comprehending the importance of evaluating the anterior horn of the lateral meniscus in cases of tibial eminence fractures at the initial time of injury. Failure to diagnose this pathology can lead to poor clinical outcomes and early degenerative changes of the knee.
Tibial intercondylar eminence avulsion fractures are most likely to occur in children and adolescents, and are equivalent to an ACL tear in adults.13 When tibial eminence fractures occur in an older cohort, they are often combined with lesions of the menisci, capsule, or collateral ligaments.14 The initial injury in our patient demonstrated concomitant anterior root injury that progressed with time to nonanatomical healing of the root, leading to altered biomechanics. Surgical techniques available for meniscal root repair are broadly divided into transosseous suture repairs and suture anchor repairs.10 The transtibial pullout technique using 2 transtibial bone tunnels as described in this report is the senior author’s (RFL) preference because it provides a strong construct with minimal displacement of the repaired meniscus.15-17
This article describes a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomic position. Anterior meniscal root tears have been reported to result in altered biomechanics and force transmission across the knee, and therefore, anatomic repair of the anterior root is indicated.
1. James EW, LaPrade CM, Ellman MB, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic identification of the anterior and posterior root attachments of the medial and lateral menisci. Am J Sports Med. 2014;42(11):2707-2714. doi:10.1177/0363546514545863.
2. LaPrade CM, Foad A, Smith SD, et al. Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med. 2015;43(4):912-920. doi:10.1177/0363546514566191.
3. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43(2):363-369. doi:10.1177/0363546514559684.
4. Ellman MB, James EW, LaPrade CM, LaPrade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191. doi:10.1007/s00167-014-2941-5.
5. Padalecki JR, Jansson KS, Smith SD, et al. Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med. 2014;42(3):699-707. doi:10.1177/0363546513499314.
6. LaPrade CM, Jisa KA, Cram TR, LaPrade RF. Posterior lateral meniscal root tear due to a malpositioned double-bundle anterior cruciate ligament reconstruction tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3670-3673. doi:10.1007/s00167-014-3273-1.
7. Mitchell JJ, Sjostrom R, Mansour AA, et al. Incidence of meniscal injury and chondral pathology in anterior tibial spine fractures of children. J Pediatr Orthop. 2015;35(2):130-135. doi:10.1097/BPO.0000000000000249.
8. Monto RR, Cameron-Donaldson ML. Magnetic resonance imaging in the evaluation of tibial eminence fractures in adults. J Knee Surg. 2006;19(3):187-190.
9. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;(434):207-212.
10. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-3030. doi:10.1177/0363546514524162.
11. Ziegler CG, Pietrini SD, Westerhaus BD, et al. Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(4):743-752. doi:10.1177/0363546510387511.
12. Furumatsu T, Kodama Y, Maehara A, et al. The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res. 2016;57(2):91-98. doi:10.3109/03008207.2015.1081899.
13. Lubowitz JH, Grauer JD. Arthroscopic treatment of anterior cruciate ligament avulsion. Clin Orthop Rel Res. 1993;(294):242-246.
14. Falstie-Jensen S, Sondergard Petersen PE. Incarceration of the meniscus in fractures of the intercondylar eminence of the tibia in children. Injury. 1984;15(4):236-238.
15. LaPrade CM, LaPrade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43(4):899-904. doi:10.1177/0363546514563278.
16. Menge TJ, Chahla J, Dean CS, Mitchell JJ, Moatshe G, LaPrade RF. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc Tech. 2016;5(3):e679-e684. doi:10.1016/j.eats.2016.02.026.
17. Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5(5):e1111-e1116. doi:10.1016/j.eats.2016.06.005.
ABSTRACT
The lateral tibial eminence shares a close relationship with the anterior root of the lateral meniscus. Limited studies have reported traumatic injury to the anterior meniscal roots in the setting of tibial eminence fractures, and reported rates of occurrence of concomitant meniscal and chondral injuries vary widely. The purpose of this article is to describe the case of a 28-year-old woman who had a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and root displacement. The anterolateral meniscal root was anatomically repaired following arthroscopic resection of the malunited fragment.
The lateral tibial eminence is intimately associated with the root attachment of the anterior horn of the lateral meniscus.1-3 Previous studies have demonstrated both the close proximity of the anterior cruciate ligament (ACL) insertion to the meniscal roots and the potential for disruption in surgical interventions, such as tibial tunnel drilling in ACL reconstruction or placement of intramedullary tibial nails.4-6 The meniscal roots play a crucial role in force distribution, and disruption of these structures has been shown to significantly increase joint contact forces. Despite the deleterious effects of this injury, limited studies have reported on traumatic injury to the meniscal roots in the setting of tibial eminence fractures.
Reported rates of occurrence of concomitant meniscal and chondral injuries occurring with tibial eminence fractures vary widely, ranging from <5% to 40%.7,8 Although fractures to the tibial eminence are more common in children, an association between these injuries and concomitant soft tissue injuries, including meniscal, chondral, and collateral ligament injuries, in the adult population has been reported.7 Monto and Cameron-Donaldson8 used magnetic resonance imaging (MRI) to evaluate tibial eminence fractures in adults and found that 23% of study subjects had associated medial meniscus tears and 18% had lateral meniscus tears. In a similar study, Ishibashi and colleagues9 found that 25% of tibial eminence fractures were associated with lateral meniscus tears and 16% with medial meniscus tears.
These studies demonstrate the potential for meniscus injuries during tibial eminence fractures. However, the authors are unaware of any reports of complete tearing of the anterior horn of the lateral meniscus in association with this injury. This is an important injury to recognize and identify intraoperatively because an injury of this nature could potentially compromise the mechanical loading patterns and health of the articular cartilage of the lateral compartment of the knee. The purpose of this article is to describe a complete avulsion of the anterolateral meniscal root due to a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomical position. The patient provided written informed consent for print and electronic publication of this case report.
Continue to: A 28-year-old active woman...
CASE
A 28-year-old active woman presented to our clinic 22 months after sustaining a right knee tibial eminence fracture that was initially treated with extension immobilization, which resulted in a fibrous malunion. She subsequently sustained a second injury resulting in displacement of the malunion fracture fragment, and was treated at another institution 10 months prior to presentation at our clinic with arthroscopic reduction and internal fixation with a cannulated screw and washer of the tibial eminence fracture. This was followed by hardware removal 6 months prior to her office visit at our clinic. At presentation, she reported worsening right knee pain, mechanical symptoms, and loss of both flexion and extension compared with her uninjured knee. Conservative management, including activity modification, extensive physical therapy, and anti-inflammatory medication following her most recent procedure, had not resulted in improvement of her symptoms.
Physical examination revealed significantly reduced knee flexion and extension (+15°-120° on the affected side compared with 5° of hyperextension to 130° flexion of the contralateral knee). Ligamentous examination demonstrated no laxity with varus or valgus stress at 0° to 30° of flexion, negative posterior drawer, and a Grade 2 Lachman and positive pivot shift. She also exhibited pain with attempted right knee terminal extension. Radiographs and computed tomography scans were obtained and reviewed. They revealed a malunited tibial eminence fracture (Figures 1A-1D).
Arthroscopic assessment of the right knee demonstrated the large osseous fragment located in the anterolateral aspect of the joint with the displaced anterior horn of the lateral meniscus attached as well as significant anterior impingement limiting knee extension. Probing of the anterolateral meniscal root in the lateral compartment showed abundant surrounding scar tissue with an abnormal attachment, representing a chronic root avulsion. A mechanical shaver was used to débride the scar tissue and expose the malunited fragment, followed by complete osseous fragment excision with a high-speed burr (Figure 3).
A soft tissue anterolateral meniscal root repair was performed by creating a 2-cm to 3-cm incision on the anterolateral tibia, just distal to the medial aspect of the Gerdy tubercle. To best restore the footprint of the repair and increase the potential for biologic healing, 2 transtibial tunnels were created at the location of the root attachment. An ACL aiming device with a cannulated sleeve was used to drill 2 bony tunnels approximately 5 mm apart, exiting at the anatomic root footprint. The drill pins were removed, leaving the 2 cannulas in place for later suture passage. A suture-passing device was used to pass 2 separate sutures through the detached meniscal root.
Continue to: Postoperatively, the patient was placed...
Postoperatively, the patient was placed on a non-weight-bearing protocol for her operative lower extremity for 6 weeks. A brace locked in extension was used for the same period of time (being removed only for physical therapy exercises). Enoxaparin was used for the first 2 weeks for deep vein thrombosis prophylaxis, followed by aspirin for an additional 4 weeks. Physical therapy was started on postoperative day 1 to begin working on early passive ROM exercises. Knee flexion was limited to 0° to 90° of flexion for the first 2 weeks and then progressed as tolerated.
DISCUSSION
This article describes a rare case of a patient with lateral meniscal anterior root avulsion in the setting of a tibial eminence fracture with subsequent malunion and root displacement. In a case such as this, delineation of the true extent of the injury is difficult because the anterior meniscal root can be torn, displaced, and nonanatomically scarred to surrounding soft tissues, making MRI interpretation challenging. Clinically, patients can present with a wide range of symptoms, including pain, mechanical symptoms, instability, and loss of knee motion.10
The anterior root of the lateral meniscus has been reported to be attached anterior to the lateral tibial eminence and adjacent to the insertion of the ACL. Fibrous connections extending from the anterior horn of the lateral meniscus attachment to the lateral tibial eminence are constant.11 Furumatsu and colleagues12 demonstrated the existence of dense fibers linking the anterior root of the lateral meniscus with the lateral aspect of the ACL tibial insertion. Acknowledging the close relationship of these structures is key to comprehending the importance of evaluating the anterior horn of the lateral meniscus in cases of tibial eminence fractures at the initial time of injury. Failure to diagnose this pathology can lead to poor clinical outcomes and early degenerative changes of the knee.
Tibial intercondylar eminence avulsion fractures are most likely to occur in children and adolescents, and are equivalent to an ACL tear in adults.13 When tibial eminence fractures occur in an older cohort, they are often combined with lesions of the menisci, capsule, or collateral ligaments.14 The initial injury in our patient demonstrated concomitant anterior root injury that progressed with time to nonanatomical healing of the root, leading to altered biomechanics. Surgical techniques available for meniscal root repair are broadly divided into transosseous suture repairs and suture anchor repairs.10 The transtibial pullout technique using 2 transtibial bone tunnels as described in this report is the senior author’s (RFL) preference because it provides a strong construct with minimal displacement of the repaired meniscus.15-17
This article describes a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomic position. Anterior meniscal root tears have been reported to result in altered biomechanics and force transmission across the knee, and therefore, anatomic repair of the anterior root is indicated.
ABSTRACT
The lateral tibial eminence shares a close relationship with the anterior root of the lateral meniscus. Limited studies have reported traumatic injury to the anterior meniscal roots in the setting of tibial eminence fractures, and reported rates of occurrence of concomitant meniscal and chondral injuries vary widely. The purpose of this article is to describe the case of a 28-year-old woman who had a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and root displacement. The anterolateral meniscal root was anatomically repaired following arthroscopic resection of the malunited fragment.
The lateral tibial eminence is intimately associated with the root attachment of the anterior horn of the lateral meniscus.1-3 Previous studies have demonstrated both the close proximity of the anterior cruciate ligament (ACL) insertion to the meniscal roots and the potential for disruption in surgical interventions, such as tibial tunnel drilling in ACL reconstruction or placement of intramedullary tibial nails.4-6 The meniscal roots play a crucial role in force distribution, and disruption of these structures has been shown to significantly increase joint contact forces. Despite the deleterious effects of this injury, limited studies have reported on traumatic injury to the meniscal roots in the setting of tibial eminence fractures.
Reported rates of occurrence of concomitant meniscal and chondral injuries occurring with tibial eminence fractures vary widely, ranging from <5% to 40%.7,8 Although fractures to the tibial eminence are more common in children, an association between these injuries and concomitant soft tissue injuries, including meniscal, chondral, and collateral ligament injuries, in the adult population has been reported.7 Monto and Cameron-Donaldson8 used magnetic resonance imaging (MRI) to evaluate tibial eminence fractures in adults and found that 23% of study subjects had associated medial meniscus tears and 18% had lateral meniscus tears. In a similar study, Ishibashi and colleagues9 found that 25% of tibial eminence fractures were associated with lateral meniscus tears and 16% with medial meniscus tears.
These studies demonstrate the potential for meniscus injuries during tibial eminence fractures. However, the authors are unaware of any reports of complete tearing of the anterior horn of the lateral meniscus in association with this injury. This is an important injury to recognize and identify intraoperatively because an injury of this nature could potentially compromise the mechanical loading patterns and health of the articular cartilage of the lateral compartment of the knee. The purpose of this article is to describe a complete avulsion of the anterolateral meniscal root due to a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomical position. The patient provided written informed consent for print and electronic publication of this case report.
Continue to: A 28-year-old active woman...
CASE
A 28-year-old active woman presented to our clinic 22 months after sustaining a right knee tibial eminence fracture that was initially treated with extension immobilization, which resulted in a fibrous malunion. She subsequently sustained a second injury resulting in displacement of the malunion fracture fragment, and was treated at another institution 10 months prior to presentation at our clinic with arthroscopic reduction and internal fixation with a cannulated screw and washer of the tibial eminence fracture. This was followed by hardware removal 6 months prior to her office visit at our clinic. At presentation, she reported worsening right knee pain, mechanical symptoms, and loss of both flexion and extension compared with her uninjured knee. Conservative management, including activity modification, extensive physical therapy, and anti-inflammatory medication following her most recent procedure, had not resulted in improvement of her symptoms.
Physical examination revealed significantly reduced knee flexion and extension (+15°-120° on the affected side compared with 5° of hyperextension to 130° flexion of the contralateral knee). Ligamentous examination demonstrated no laxity with varus or valgus stress at 0° to 30° of flexion, negative posterior drawer, and a Grade 2 Lachman and positive pivot shift. She also exhibited pain with attempted right knee terminal extension. Radiographs and computed tomography scans were obtained and reviewed. They revealed a malunited tibial eminence fracture (Figures 1A-1D).
Arthroscopic assessment of the right knee demonstrated the large osseous fragment located in the anterolateral aspect of the joint with the displaced anterior horn of the lateral meniscus attached as well as significant anterior impingement limiting knee extension. Probing of the anterolateral meniscal root in the lateral compartment showed abundant surrounding scar tissue with an abnormal attachment, representing a chronic root avulsion. A mechanical shaver was used to débride the scar tissue and expose the malunited fragment, followed by complete osseous fragment excision with a high-speed burr (Figure 3).
A soft tissue anterolateral meniscal root repair was performed by creating a 2-cm to 3-cm incision on the anterolateral tibia, just distal to the medial aspect of the Gerdy tubercle. To best restore the footprint of the repair and increase the potential for biologic healing, 2 transtibial tunnels were created at the location of the root attachment. An ACL aiming device with a cannulated sleeve was used to drill 2 bony tunnels approximately 5 mm apart, exiting at the anatomic root footprint. The drill pins were removed, leaving the 2 cannulas in place for later suture passage. A suture-passing device was used to pass 2 separate sutures through the detached meniscal root.
Continue to: Postoperatively, the patient was placed...
Postoperatively, the patient was placed on a non-weight-bearing protocol for her operative lower extremity for 6 weeks. A brace locked in extension was used for the same period of time (being removed only for physical therapy exercises). Enoxaparin was used for the first 2 weeks for deep vein thrombosis prophylaxis, followed by aspirin for an additional 4 weeks. Physical therapy was started on postoperative day 1 to begin working on early passive ROM exercises. Knee flexion was limited to 0° to 90° of flexion for the first 2 weeks and then progressed as tolerated.
DISCUSSION
This article describes a rare case of a patient with lateral meniscal anterior root avulsion in the setting of a tibial eminence fracture with subsequent malunion and root displacement. In a case such as this, delineation of the true extent of the injury is difficult because the anterior meniscal root can be torn, displaced, and nonanatomically scarred to surrounding soft tissues, making MRI interpretation challenging. Clinically, patients can present with a wide range of symptoms, including pain, mechanical symptoms, instability, and loss of knee motion.10
The anterior root of the lateral meniscus has been reported to be attached anterior to the lateral tibial eminence and adjacent to the insertion of the ACL. Fibrous connections extending from the anterior horn of the lateral meniscus attachment to the lateral tibial eminence are constant.11 Furumatsu and colleagues12 demonstrated the existence of dense fibers linking the anterior root of the lateral meniscus with the lateral aspect of the ACL tibial insertion. Acknowledging the close relationship of these structures is key to comprehending the importance of evaluating the anterior horn of the lateral meniscus in cases of tibial eminence fractures at the initial time of injury. Failure to diagnose this pathology can lead to poor clinical outcomes and early degenerative changes of the knee.
Tibial intercondylar eminence avulsion fractures are most likely to occur in children and adolescents, and are equivalent to an ACL tear in adults.13 When tibial eminence fractures occur in an older cohort, they are often combined with lesions of the menisci, capsule, or collateral ligaments.14 The initial injury in our patient demonstrated concomitant anterior root injury that progressed with time to nonanatomical healing of the root, leading to altered biomechanics. Surgical techniques available for meniscal root repair are broadly divided into transosseous suture repairs and suture anchor repairs.10 The transtibial pullout technique using 2 transtibial bone tunnels as described in this report is the senior author’s (RFL) preference because it provides a strong construct with minimal displacement of the repaired meniscus.15-17
This article describes a complete avulsion of the anterolateral meniscal root caused by a tibial eminence fracture with resultant malunion and displacement of the root in a nonanatomic position. Anterior meniscal root tears have been reported to result in altered biomechanics and force transmission across the knee, and therefore, anatomic repair of the anterior root is indicated.
1. James EW, LaPrade CM, Ellman MB, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic identification of the anterior and posterior root attachments of the medial and lateral menisci. Am J Sports Med. 2014;42(11):2707-2714. doi:10.1177/0363546514545863.
2. LaPrade CM, Foad A, Smith SD, et al. Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med. 2015;43(4):912-920. doi:10.1177/0363546514566191.
3. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43(2):363-369. doi:10.1177/0363546514559684.
4. Ellman MB, James EW, LaPrade CM, LaPrade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191. doi:10.1007/s00167-014-2941-5.
5. Padalecki JR, Jansson KS, Smith SD, et al. Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med. 2014;42(3):699-707. doi:10.1177/0363546513499314.
6. LaPrade CM, Jisa KA, Cram TR, LaPrade RF. Posterior lateral meniscal root tear due to a malpositioned double-bundle anterior cruciate ligament reconstruction tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3670-3673. doi:10.1007/s00167-014-3273-1.
7. Mitchell JJ, Sjostrom R, Mansour AA, et al. Incidence of meniscal injury and chondral pathology in anterior tibial spine fractures of children. J Pediatr Orthop. 2015;35(2):130-135. doi:10.1097/BPO.0000000000000249.
8. Monto RR, Cameron-Donaldson ML. Magnetic resonance imaging in the evaluation of tibial eminence fractures in adults. J Knee Surg. 2006;19(3):187-190.
9. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;(434):207-212.
10. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-3030. doi:10.1177/0363546514524162.
11. Ziegler CG, Pietrini SD, Westerhaus BD, et al. Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(4):743-752. doi:10.1177/0363546510387511.
12. Furumatsu T, Kodama Y, Maehara A, et al. The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res. 2016;57(2):91-98. doi:10.3109/03008207.2015.1081899.
13. Lubowitz JH, Grauer JD. Arthroscopic treatment of anterior cruciate ligament avulsion. Clin Orthop Rel Res. 1993;(294):242-246.
14. Falstie-Jensen S, Sondergard Petersen PE. Incarceration of the meniscus in fractures of the intercondylar eminence of the tibia in children. Injury. 1984;15(4):236-238.
15. LaPrade CM, LaPrade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43(4):899-904. doi:10.1177/0363546514563278.
16. Menge TJ, Chahla J, Dean CS, Mitchell JJ, Moatshe G, LaPrade RF. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc Tech. 2016;5(3):e679-e684. doi:10.1016/j.eats.2016.02.026.
17. Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5(5):e1111-e1116. doi:10.1016/j.eats.2016.06.005.
1. James EW, LaPrade CM, Ellman MB, Wijdicks CA, Engebretsen L, LaPrade RF. Radiographic identification of the anterior and posterior root attachments of the medial and lateral menisci. Am J Sports Med. 2014;42(11):2707-2714. doi:10.1177/0363546514545863.
2. LaPrade CM, Foad A, Smith SD, et al. Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med. 2015;43(4):912-920. doi:10.1177/0363546514566191.
3. LaPrade CM, James EW, Cram TR, Feagin JA, Engebretsen L, LaPrade RF. Meniscal root tears: a classification system based on tear morphology. Am J Sports Med. 2015;43(2):363-369. doi:10.1177/0363546514559684.
4. Ellman MB, James EW, LaPrade CM, LaPrade RF. Anterior meniscus root avulsion following intramedullary nailing for a tibial shaft fracture. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1188-1191. doi:10.1007/s00167-014-2941-5.
5. Padalecki JR, Jansson KS, Smith SD, et al. Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med. 2014;42(3):699-707. doi:10.1177/0363546513499314.
6. LaPrade CM, Jisa KA, Cram TR, LaPrade RF. Posterior lateral meniscal root tear due to a malpositioned double-bundle anterior cruciate ligament reconstruction tibial tunnel. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3670-3673. doi:10.1007/s00167-014-3273-1.
7. Mitchell JJ, Sjostrom R, Mansour AA, et al. Incidence of meniscal injury and chondral pathology in anterior tibial spine fractures of children. J Pediatr Orthop. 2015;35(2):130-135. doi:10.1097/BPO.0000000000000249.
8. Monto RR, Cameron-Donaldson ML. Magnetic resonance imaging in the evaluation of tibial eminence fractures in adults. J Knee Surg. 2006;19(3):187-190.
9. Ishibashi Y, Tsuda E, Sasaki T, Toh S. Magnetic resonance imaging AIDS in detecting concomitant injuries in patients with tibial spine fractures. Clin Orthop Relat Res. 2005;(434):207-212.
10. Bhatia S, LaPrade CM, Ellman MB, LaPrade RF. Meniscal root tears significance, diagnosis, and treatment. Am J Sports Med. 2014;42(12):3016-3030. doi:10.1177/0363546514524162.
11. Ziegler CG, Pietrini SD, Westerhaus BD, et al. Arthroscopically pertinent landmarks for tunnel positioning in single-bundle and double-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39(4):743-752. doi:10.1177/0363546510387511.
12. Furumatsu T, Kodama Y, Maehara A, et al. The anterior cruciate ligament-lateral meniscus complex: a histological study. Connect Tissue Res. 2016;57(2):91-98. doi:10.3109/03008207.2015.1081899.
13. Lubowitz JH, Grauer JD. Arthroscopic treatment of anterior cruciate ligament avulsion. Clin Orthop Rel Res. 1993;(294):242-246.
14. Falstie-Jensen S, Sondergard Petersen PE. Incarceration of the meniscus in fractures of the intercondylar eminence of the tibia in children. Injury. 1984;15(4):236-238.
15. LaPrade CM, LaPrade MD, Turnbull TL, Wijdicks CA, LaPrade RF. Biomechanical evaluation of the transtibial pull-out technique for posterior medial meniscal root repairs using 1 and 2 transtibial bone tunnels. Am J Sports Med. 2015;43(4):899-904. doi:10.1177/0363546514563278.
16. Menge TJ, Chahla J, Dean CS, Mitchell JJ, Moatshe G, LaPrade RF. Anterior meniscal root repair using a transtibial double-tunnel pullout technique. Arthrosc Tech. 2016;5(3):e679-e684. doi:10.1016/j.eats.2016.02.026.
17. Menge TJ, Dean CS, Chahla J, Mitchell JJ, LaPrade RF. Anterior horn meniscal repair using an outside-in suture technique. Arthrosc Tech. 2016;5(5):e1111-e1116. doi:10.1016/j.eats.2016.06.005.
TAKE-HOME POINTS
- Root tears of all meniscal attachments have been described. A comprehensive anatomic understanding of the meniscal roots is of utmost importance to suspect root lesions.
- A detailed physical examination along with imaging methods should be performed to make the correct diagnosis. In cases of evident injuries, such as a tibial spine fracture, additional soft tissue pathology should also be assessed.
- It is important to restore all torn root attachments to restore joint loading and contact areas. An anatomical root repair is needed to yield optimal results.
- Progressive rehabilitation with early ROM starting on postoperative day 1 can help avoid loss of knee motion and arthrofibrosis.
Current Concepts in Clinical Research: Anterior Cruciate Ligament Outcome Instruments
ABSTRACT
Outcome instruments have become an essential part of the evaluation of functional recovery after anterior cruciate ligament (ACL) reconstruction. Although the clinical examination provides important objective information to assess graft integrity, stability, range of motion, and strength, these measurements do not take the patient’s perception into account. There are many knee outcome instruments, and it is challenging for surgeons to understand how to interpret clinical research and utilize these measures in a practical way. The purpose of this review is to provide an overview of the most commonly used outcome measures in patients undergoing ACL reconstruction and to examine and compare the psychometric performance (validity, reliability, responsiveness) of these measurement tools.
Anterior cruciate ligament (ACL) reconstruction is one of the most common elective orthopedic procedures.1 Despite advances in surgical techniques, ACL reconstruction is associated with a lengthy recovery time, decreased performance, and increased rate of reinjury.2 Patients undergoing ACL reconstruction are often active individuals who participate in demanding activities, and accurate assessment of their recovery helps to guide recovery counseling. In addition to objective clinical outcomes measured through physical examination, patient-reported outcome (PRO) instruments add the patient’s perspective, information critical in determining a successful outcome. A variety of outcome instruments have been used and validated for patients with ACL tears. It is important for orthopedic surgeons to know the advantages and disadvantages of each outcome tool in order to interpret clinical studies and assess postoperative patients.
Over the last 10 years, there has been an increase in the number of knee instruments and rating scales designed to measure PROs, with >54 scores designed for the ACL-deficient knee.3 No standardized instrument is currently universally accepted as superior following ACL reconstruction across the spectrum of patient populations. Clinicians and researchers must carefully consider an outcome instrument’s utility based on specific patient populations in which it has been evaluated. Appropriate selection of outcome measures is of fundamental importance for adequate demonstration of the efficacy and value of treatment interventions, especially in an era of healthcare reform with a focus on providing high-quality and cost-effective care.
The purpose of this review is to highlight current tools used to measure outcomes after ACL reconstruction. Current outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations. We have thus identified the measures most commonly used today in studies and clinical follow-up after ACL reconstruction and their various advantages and limitations. This information may enhance the orthopedic surgeon’s understanding of what outcome measures may be utilized in clinical studies.
Continue to: Patient-Reported Outcome Instruments...
PATIENT-REPORTED OUTCOME INSTRUMENTS
Recently, there has been a transition to increased use of PRO instruments rather than clinician-based postoperative assessment, largely due to the increasing emphasis on patient satisfaction in determining the value of an orthopedic intervention.4 PRO instruments are widely used to capture the patient’s perception of general health, quality of life (QOL), daily function, and pain. PRO instruments offer the benefit of allowing patients to subjectively assess their knee function during daily living and sports activities, conveying to the provider the impact of ACL reconstruction on physical, psychological, and social aspects of everyday activities. Furthermore, patient satisfaction has been shown to closely follow outcome scores related to symptoms and function.5 A multitude of specific knee-related PRO instruments have been developed and validated to measure outcomes after ACL reconstruction for both research and clinical purposes (Table).
Table. ACL Outcome Measures
|
|
|
|
| ||
Outcome Measure | Condition/Intervention | Measures | Internal Consistency (Cronbach’s a) | Test-Retest Reliability | Minimal Clinically Important Difference | Ref |
AAOS Sports Knee Scale | Many Knee | Stiffness, swelling, pain/function, locking/catching, giving way, limitation of activity, pain with activity | 0.86-0.95 | 0.68-0.96 | Unknown | 59, 60 |
ACL-QOL | Chronic ACL deficiency | Physical complaints, work, recreation and sports competition, lifestyle, social and emotional functioning | 0.93-0.98 | 6% average error | Unknown | 35, 36 |
Cincinnati Knee Rating System | ACL | Symptoms, daily and sports activities, physical examination, stability, radiographs, functional testing |
| 0.80-0.97 | 14 points (6 months), 26 points (12 months) | 39, 40, 47, 52 |
IKDC (Subjective Knee Form) | ACL | Symptoms, function, sports activity | 0.92 | 0.91-0.93 | 11.5 points; 6.3 at 6 months, 16.7 at 12 months | 48, 52, 54 |
KOOS | ACL | Pain, symptoms, activities of daily living, sport/recreation, knee-related quality of life | 0.71-0.95 | 0.75-0.93 | 8-10 points | 17 |
Lysholm | ACL | Pain, instability, locking, squatting, limp, support, swelling, stair-climbing | 0.72 | 0.94 | 8.9 | 46, 47, 55 |
Marx | Healthy patients | Activity level | 0.87 | 0.97 | Unknown | 42, 56, 57 |
Tegner | ACL | Activity level | 0.81 | 0.82 | 1 | 55, 56 |
PROMIS | Many lower extremity orthopedic conditions | Lower extremity function, central body function, activities of daily living | 0.98 | 0.96-0.99 |
| 30, 31 |
WOMAC | Hip/knee OA | Physical function, pain, stiffness | 0.81-0.95 | 0.80-0.92 | 12% baseline score or 6% max score; 9-12 points | 13, 14 |
Abbreviations: AAOS, American Academy of Orthopaedic Surgeons; ACL, anterior cruciate ligament; ACL-QOL, anterior cruciate ligament quality of life score; CAT, computer-adapting testing; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; PF, physical function; PROMIS, Patient-Reported Outcome Measurement Information System; Ref, references; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MEASUREMENT PROPERTIES
In general, clinicians and investigators should use health-related outcome measures with established reliability, validity, patient relevance, and responsiveness for assessing the specific condition.6
Reliability refers to the degree to which a measurement score is free from random error, reflecting how consistent or reproducible the instrument is when administered under the same testing conditions. Internal consistency, test-retest reliability, and measurement error are measures of reliability. Internal consistency is tested after a single administration and assesses how well items within a scale measure a single underlying dimension, represented using item-total correlation coefficients and Cronbach’s alpha. A Cronbach’s alpha of 0.70 to 0.95 is generally defined as good.7 Test-retest reliability is designed to appraise variation over time in stable patients and is represented using the intraclass correlation coefficient (ICC).8 An ICC >0.7 is considered acceptable; >0.8, good; and >0.9, excellent.9 An aspect of accuracy is whether the scoring system measures the full range of the disease or complaints. The incidence of minimum (floor) and maximum (ceiling) scores can be calculated for outcome scores. An instrument with low floor and ceiling effects, below 10% to 15%, is more inconclusive and can be more reliably used to measure patients at the high and low end of the scoring system.10
Validity is the ability of an outcome instrument to measure what it is intended to measure. Establishing validity is complex and requires evaluation of several facets, including content validity, construct validity, and criterion validity. Content validity is a relatively subjective judgment explaining the ability of an instrument to assess the critical features of the problem. Construct validity evaluates whether the questionnaire measures what it intends to measure, and is often assessed by correlating scores form one instrument to those from other proven instruments that are already accepted as valid. Finally, criterion validity assesses the correlation between the score and a previously established “gold standard” instrument.
Responsiveness is the ability of the instrument to detect a change or identify improvement or worsening of a clinical condition over time. Most frequently, the effect size (observed change/standard deviation of baseline scores) and standardized response mean (observed change/standard deviation of change) are used as measures of responsiveness. The minimal clinically important difference of an outcome measure is the smallest change in an outcome score that corresponds to a change in patient condition.
Continue to: ACL Outcome Instruments...
ACL OUTCOME INSTRUMENTS
WESTERN ONTARIO AND MCMASTER UNIVERSITIES OSTEOARTHRITIS INDEX (WOMAC LK 3.0)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.0) was developed in 1982 and is a widely used, disease-specific instrument recommended for the evaluation of treatment effects in patients with hip and knee osteoarthritis.11 Available in more than 80 languages, it is a self-administered, generic health status questionnaire developed to assess pain, function, and stiffness in daily living, taking respondents between 3 to 7.5 minutes for completion.12 Using visual analog scales, the 24 items probe the 3 subscales: pain (5 items), stiffness (2 items), and functional difficulty (17 items). Scores are calculated for each dimension, and the total score is normalized to a 100-point scale, with 0 indicating severe symptoms and 100 indicating no symptoms and higher function. The WOMAC score can also be calculated from the Knee Injury and Osteoarthritis Outcome Score (KOOS). The WOMAC questionnaire is well recognized for its good validity, reliability, and responsiveness, and is the most commonly used outcome measure for osteoarthritis.13-15 Considering its focus on older patients with osteoarthritis, it may not be appropriate for use in a young and active population.
KNEE INJURY AND OSTEOARTHRITIS OUTCOME SCORE (KOOS)
The KOOS is a knee-specific questionnaire developed as an extension of the WOMAC to evaluate the functional status and QOL of patients with any type of knee injury who are at an increased risk of developing osteoarthritis.16 The patient-based questionnaire is available in over 30 languages and covers both the short- and long-term consequences of an injury of the knee causing traumatic damage to cartilage, ligaments, and menisci. The KOOS is 42 items graded on a 5-point Likert scale, covering 5 subscales: pain (9 items), symptoms (7 items), function in activities of daily living (17 items), function in sports/recreation (5 items), and knee-related QOL (4 items). The questionnaire is self-administered and takes about 10 minutes to complete. Scores are calculated for each dimension, and the total score is transformed to a 0 to 100 scale, with 0 representing severe knee problems and 100 representing no knee problems and better outcome. An advantage of the KOOS is that it evaluates both knee injuries and osteoarthritis; therefore, it is arguably more suitable for evaluating patients over the long-term. The KOOS has been validated for several orthopedic interventions, including ACL reconstruction and rehabilitation16,17 as well as meniscectomy18 and total knee replacement.19 Population-based reference data for the adult population according to age and gender have also been established.20 The KOOS is increasingly utilized in clinical studies on ACL reconstruction.21-25 The questions of the WOMAC were retained so that a WOMAC score might be calculated separately and compared with the KOOS score.26
PATIENT-REPORTED OUTCOMES INFORMATION SYSTEM (PROMIS)
Since 2004, The National Institutes of Health (NIH) has funded the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a set of flexible tools that reliably and validly measure PROs. The PROMIS consists of a library of question banks that has been developed and operated by a network of National Institutes of Health-funded research sites and coordinating centers and covers many different health domains including pain, fatigue, anxiety, depression, social functioning, physical functioning, and sleep. PROMIS items are developed using Item Response Theory (IRT), wherein the answer to any individual item has a known mathematical probability of predicting the test taker’s overall measurement of the specific trait being tested. This is commonly administered using computer-adaptive testing (CAT), which presents to the test taker an initial item, scores the response to that item, and from the response then presents the most informative second item, and so forth until a predefined level of precision is reached. Because the items are individually validated, they can be used alone or in any combination, a feature that distinguishes the PROMIS from traditional fixed-length PRO instruments that require the completion of an instrument in its entirety to be valid.27 In recent years, orthopedic research has been published with PROMIS physical function (PF) scores as primary outcome measures.28-30 The PF item bank includes 124 items measuring upper extremity, lower extremity, central and instrumental activities of daily living. PF can be completed as a short form (SF) with a set number of questions or utilizing CAT and evaluates self-reported function and physical activity. An advantage is its ease of use and potential to minimize test burden with very few questions, often as little as 4 items, as compared to other traditional PROMs.31
Previously published work has demonstrated that, in patients undergoing meniscal surgery, the PROMIS PF CAT maintains construct validity and correlates well with currently used knee outcome instruments, including KOOS.28 Work by the same group looking at the performance of the PROMIS PF CAT in patients indicated for ACL reconstruction shows that the PROMIS PF CAT correlates well with other PRO instruments for patients with ACL injuries, (SF-36 PF [r = 0.82, P < 0.01], KOOS Sport [r = 0.70, P < 0.01], KOOS ADL [r = 0.74, P < 0.01]), does not have floor or ceiling effects in this relatively young and healthy population, and has a low test burden.32,33 Papuga and colleagues33 also compared the International Knee Documentation Committee (IKDC) and PROMIS PF CAT on 106 subjects after ACL reconstruction and found good correlation.
Continue to: Quality of Life Outcome Measure...
QUALITY OF LIFE OUTCOME MEASURE FOR ACL DEFICIENCY (ACL-QOL)
The ACL-QOL Score was developed in 1998 as a disease-specific measure for patients with chronic ACL deficiency.34 This scale consists of 32 separate items in 31 visual analog questions regarding symptoms and physical complaints, work-related concerns, recreational activities and sport participation or competition, lifestyle, and social and emotional health status relating to the knee. The raw score is transformed into a 0- to 100-point scale, with higher scores indicating a better outcome. The scale is valid, reliable, and responsive for patients with ACL insufficiency,35,36 and is not applicable to other disorders of the knee. We recommend the ACL-QOL questionnaire be used in conjunction with other currently available objective and functional outcome measures.
CINCINNATI KNEE RATING SYSTEM
The Cincinnati Knee Rating System (CKRS) was first described in 1983 and was modified to include occupational activities, athletic activities, symptoms, and functional limitations.37,38 There are 11 components, measuring symptoms and disability in sports activity, activities of daily living function, occupational rating, as well as sections that measure physical examination, laxity of the knee, and radiographic evidence of degenerative joint disease.39 The measure is scored on a 100-point scale, with higher scores indicating better outcomes. Scores have been shown to be lower as compared with other outcome measures assessing the same clinical condition.40,41 Barber-Westin and colleagues39 confirmed the reliability, validity, and responsiveness of the CKRS by testing 350 subjects with and without knee ligament injuries. In 2001, Marx42 tested the CKRS subjective form for reliability, validity, and responsiveness and found it to be acceptable for clinical research.
LYSHOLM KNEE SCORE
The Lysholm Knee Score was published in 1982 and modified in 1985, consisting of an 8-question survey that evaluates outcomes after knee ligament surgery. Items include pain, instability, locking, squatting, limping, support usage, swelling, and stair-climbing ability, with pain and instability carrying the highest weight.43 It is scored on a scale of 0 to 100, with high scores indicating higher functioning and fewer symptoms. It has been validated in patients with ACL injuries and meniscal injuries.44 Although it is widely used to measure outcomes after ACL reconstruction,45 it has received criticism in the evaluation of patients with other knee conditions.46 The main advantage of the Lysholm Knee Score is its ability to note changes in activity in the same patient across different time periods (responsiveness). A limitation of the Lysholm Knee Score is that it does not measure the domains of functioning in daily activities, sports, and recreational activities. The Lysholm scoring system’s test-retest reliability and construct validity have been evaluated,42,43,46 although there has been some concern regarding a ceiling effect and its validity, sensitivity, and reliability has been questioned.47 Therefore, it is advised that this score be used in conjunction with other PRO scores.
INTERNATIONAL KNEE DOCUMENTATION COMMITTEE (IKDC) SUBJECTIVE KNEE FORM
In 1987, members of the European Society for Knee Surgery and Arthroscopy and the American Orthopaedic Society for Sports Medicine formed the IKDC to develop a standardized method for evaluating knee injuries and treatment. The IKDC Subjective Knee Evaluation Form was initially published in 1993, and in 2001 the form was revised by the American Orthopaedic Society for Sports Medicine to become a knee-specific assessment tool rather than a disease or condition-specific tool.48 The IKDC subjective form is an 18-question, knee-specific survey designed to detect improvement or deterioration in symptoms, function, and ability to participate in sports activities experienced by patients following knee surgery or other interventions. The individual items are summed and transformed into a 0- to 100-point scale, with high scores representing higher levels of function and minimal symptoms. The IKDC is utilized to assess a variety of knee conditions including ligament, meniscus, articular cartilage, osteoarthritis, and patellofemoral pain.48,49 Thus, this form can be used to assess any condition involving the knee and allow comparison between groups with different diagnoses. The IKDC has been validated for an ACL reconstruction population,47 has been used to assess outcomes in recent clinical studies on ACL reconstruction,50,51 and is one of the most frequently used measures for patients with ACL deficiency.3 The validity, responsiveness, and reliability of the IKDC subjective form has been confirmed for both adult and adolescent populations.48,49,52-54
TEGNER ACTIVITY SCORES
The Tegner activity score was developed in 1985 and was designed to provide an objective value for a patient’s activity level.44 This scale was developed to complement the Lysholm score. It consists of 1 sport-specific activity level question on a 0 to 10 scale that evaluates an individual’s ability to compete in a sporting activity. Scores between 1 and 5 represent work or recreational sports. Scores >5 represent higher-level recreational and competitive sports. The Tegner activity score is one of the most widely used activity scoring systems for patients with knee disorders,55,56 commonly utilized with the Lysholm Knee Score.44 One disadvantage of the Tegner activity score is that it relates to specific sports rather than functional activities, which limits its generalizability. We are not aware of any studies documenting the reliability or validity of this instrument.
Continue on: Marx Activity Rating Scale...
MARX ACTIVITY RATING SCALE
The Marx activity rating scale was developed to be utilized with other knee rating scales and outcome measures as an activity assessment.57 In contrast to the Tegner activity score, the Marx activity rating scale measures function rather than sport-specific activity. The scale is a short, patient-based activity assessment that consists of a 4-question survey evaluating patients’ knee health by recording the frequency and intensity of participation in a sporting activity. Questions are scored from 0 to 4 on the basis of how often the activity is performed. The 4 sections of the Marx scale that are rated include running, cutting, decelerating, and pivoting. This scale has been validated in patients with ACL injuries, chondromalacia patellae, and meniscal lesions.42,56-58 Acceptable ceiling effects of 3% and floor effects of 8% were noted in the study of ACL-injured patients.57
AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS (AAOS) SPORTS KNEE SCALE
The American Academy of Orthopaedic Surgeons (AAOS) Sports Knee Rating Scale consists of 5 parts and 23 items, including a section addressing stiffness, swelling, pain and function (7 questions), locking/catching (4 questions), giving way (4 questions), limitations of activity (4 questions), and pain with activity (4 questions).59,60 Items may be dropped if patients select particular responses, which can lead to difficulties when using the survey. This scoring system has been found to be satisfactory when all subscales were combined and the mean was calculated.42
DISCUSSION
PRO measures play an increasingly important role in the measurement of success and impact of health care services. Specifically, for ACL reconstruction, patient satisfaction is key for demonstrating the value of operative or other interventions. Selecting a suitable outcome measurement tool can be daunting, as it can be difficult to ascertain which outcome measures are appropriate for the patient or disorder in question. As there is currently no instrument that is universally superior in the evaluation of ACL outcomes, clinicians must consider the specific patient population in which the outcome instrument has been evaluated. Investigators should also use instruments with reported minimal clinically important differences so that variation in scores can be interpreted as either clinically significant or not. When choosing which outcome instrument to use, there is rarely a single appropriate rating system that is entirely comprehensive. In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale. Activity rating scales, such as Marx or Tegner, should be included, especially when evaluating patients with low-activity lifestyles.
CONCLUSION
There are a number of reliable, valid, and responsive outcome measures that can be utilized to evaluate outcomes following ACL reconstruction in an array of patient populations. Outcome measures should be relevant to patients, easy to use, reliable, valid, and responsive to change. By increasing familiarity with these outcome measures, orthopedic surgeons and investigators can develop better studies, interpret data, and implement findings in practice with sound and informed judgment. Future research should focus on identifying the most relevant outcome metrics for assessing function following ACL reconstruction.
This paper will be judged for the Resident Writer’s Award.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370. doi:10.1177/0363546514542796.
2. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
3. Johnson DS, Smith RB. Outcome measurement in the ACL deficient knee- what’s the score? Knee. 2001;8(1):51-57. doi:10.1016/S0968-0160(01)00068-0.
4. Graham B, Green A, James M, Katz J, Swiontkowski M. Measuring patient satisfaction in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(1):80-84. doi:10.2106/JBJS.N.00811.
5. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84(9):1560-1572. doi:10.2106/00004623-200209000-00008.
6. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 1989.
7. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34-42. doi:10.1016/j.jclinepi.2006.03.012.
8. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. doi:10.2466/pr0.1966.19.1.3.
9. Scholtes VA, Terwee CB, Poolman RW. What makes a measurement instrument valid and reliable? Injury. 2011;42(3):236-240. doi:10.1016/j.injury.2010.11.042.
10. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.
12. Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67(2):216-229. doi:10.1002/acr.22415.
13. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
14. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stuckl G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12(5):331-335.
15. Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999;58(9):563-568. doi:10.1136/ard.58.9.563.
16. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi:10.2519/jospt.1998.28.2.88.
17. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406-410. doi:10.1016/j.joca.2011.01.010.
18. Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7(2):216-221. doi:10.1053/joca.1998.0153.
19. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi:10.1186/1477-7525-1-17.
20. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskeletal Disord. 2006;7(1):38. doi:10.1186/1471-2474-7-38.
21. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301-2310. doi:10.1177/0363546514549005.
22. Ventura A, Legnani C, Terzaghi C, Borgo E, Albisetti W. Revision surgery after failed ACL reconstruction with artificial ligaments: clinical, histologic and radiographic evaluation. Eur J Orthop Surg Traumatol. 2014;21(1):93-98. doi:10.1007/s00590-012-1136-3.
23. Wasserstein D, Huston LJ, Nwosu S, et al. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674-1684. doi:10.1016/j.joca.2015.05.025.
24. Zaffagnini S, Grassi A, Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
25. Duffee A, Magnussen RA, Pedroza AD, Flanigan DC; MOON Group, Kaeding CC. Transtibial ACL femoral tunnel preparation increases odds of repeat ipsilateral knee surgery. J Bone Joint Surg Am. 2013;95(22):2035-2042. doi:10.2106/JBJS.M.00187.
26. Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5(2):231-241. doi:10.1007/BF02032362.
27. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
28. Hancock KJ, Glass NA, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954-958. doi:10.2106/JBJS.16.00848.
29. Hung M, Clegg Do, Greene T, et al. Evaluation of the PROMIS physical function item bank in orthopedic patients. J Orthop Res. 2011;29(6):947-953. doi:10.1002/jor.21308.
30. Hung M, Baumhauer JF, Brodsky JW, et al; Orthopaedic Foot & Ankle Outcomes Research (OFAR) of the American Orthopaedic Foot & Ankle Society (AOFAS). Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int. 2014;35(6):592-599. doi:10.1177/1071100714528492.
31. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443. doi:10.1097/BOT.0000000000000059.
32. Hancock, et al. PROMIS: A valid and efficient outcomes instrument for patients with ACL tears. KSSTA. In press.
33. Scott, et al. Performance of PROMIS physical function compared with KOOS, SF-36, Eq5D, and Marx activity scale in patients who undergo ACL reconstruction. In press.
34. Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793-801. doi:10.1002/jor.22591.
35. Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350-359. doi:10.1177/03635465980260030201.
36. Lafave MR, Hiemstra L, Kerslake S, Heard M, Buchko G. Validity, reliability, and responsiveness of the anterior cruciate ligament quality of life measure: a continuation of its overall validation. Clin J Sport Med. 2017;27(1):57-63. doi:10.1097/JSM.0000000000000292.
37. Noyes FR, McGinniss GH, Mooar LA. Functional disability in the anterior cruciate insufficient knee syndrome: Review of knee rating systems and projected risk factors in determining treatment. Sports Med. 1984;1(4):278-302. doi:10.2165/00007256-198401040-00004.
38. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee: Part II. The results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174. doi:10.2106/00004623-198365020-00004.
39. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-416. doi:10.1177/03635465990270040201.
40. Bollen S, Seedhorn BB. A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med. 1991;19(2):189-190. doi:10.1177/036354659101900215.
41. Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ. Critical analysis of knee ligament rating systems. Am J Sports Med. 1995;23(6):660-667. doi:10.1177/036354659502300604.
42. Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83(10):1459-1469. doi:10.2106/00004623-200110000-00001.
43. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154. doi:10.1177/036354658201000306.
44. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-49. doi:10.1097/00003086-198509000-00007.
45. Lukianov AV, Gillquist J, Grana WA, DeHaven KE. An anterior cruciate ligament (ACL) evaluation format for assessment of artificial or autologous anterior cruciate reconstruction results. Clin Orthop Relat Res. 1987;218:167-180. doi:10.1097/00003086-198705000-00024.
46. Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31. doi:10.1007/BF01565994.
47. Risberg MA, Holm I, Steen J, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):152-159. doi:10.1007/s001670050140.
48. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. doi:10.1177/03635465010290051301.
49. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-1573. doi:10.1177/0363546506288855.
50. Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(2):914-923. doi:10.2519/jospt.2014.4852.
51. Lentz TA, Zeppieri G Jr, George SZ, et al. Comparison of physical impairment, functional and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345-353. doi:10.1177/0363546514559707.
52. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. doi:10.1177/0363546509354163.
53. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-234. doi:10.1007/BF01560215.
54. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38(12):2443-2447. doi:10.1177/0363546510374873.
55. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897. doi:10.1177/0363546508330143.
56. Negahban H, Mostafaee N, Sohani SM, et al. Reliability and validity of the Tegner and Marx activity rating scales in Iranian patients with anterior cruciate ligament injury. Disabil Rehabil. 2011;33(23-24):2305-2310. doi:10.3109/09638288.2011.570409.
57. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-218. doi:10.1177/03635465010290021601.
58. Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAM-ASK Trial Team. Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414-1423. doi:10.1093/rheumatology/keh362.
59. American Academy of Orthopaedic Surgeons. Scoring algorithms for the lower limb: Outcomes data collection instrument. Rosemon, IL: American Academy of Orthopaedic Surgeons; 1998.
60. Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am. 2004;86-A(5):902-909.
ABSTRACT
Outcome instruments have become an essential part of the evaluation of functional recovery after anterior cruciate ligament (ACL) reconstruction. Although the clinical examination provides important objective information to assess graft integrity, stability, range of motion, and strength, these measurements do not take the patient’s perception into account. There are many knee outcome instruments, and it is challenging for surgeons to understand how to interpret clinical research and utilize these measures in a practical way. The purpose of this review is to provide an overview of the most commonly used outcome measures in patients undergoing ACL reconstruction and to examine and compare the psychometric performance (validity, reliability, responsiveness) of these measurement tools.
Anterior cruciate ligament (ACL) reconstruction is one of the most common elective orthopedic procedures.1 Despite advances in surgical techniques, ACL reconstruction is associated with a lengthy recovery time, decreased performance, and increased rate of reinjury.2 Patients undergoing ACL reconstruction are often active individuals who participate in demanding activities, and accurate assessment of their recovery helps to guide recovery counseling. In addition to objective clinical outcomes measured through physical examination, patient-reported outcome (PRO) instruments add the patient’s perspective, information critical in determining a successful outcome. A variety of outcome instruments have been used and validated for patients with ACL tears. It is important for orthopedic surgeons to know the advantages and disadvantages of each outcome tool in order to interpret clinical studies and assess postoperative patients.
Over the last 10 years, there has been an increase in the number of knee instruments and rating scales designed to measure PROs, with >54 scores designed for the ACL-deficient knee.3 No standardized instrument is currently universally accepted as superior following ACL reconstruction across the spectrum of patient populations. Clinicians and researchers must carefully consider an outcome instrument’s utility based on specific patient populations in which it has been evaluated. Appropriate selection of outcome measures is of fundamental importance for adequate demonstration of the efficacy and value of treatment interventions, especially in an era of healthcare reform with a focus on providing high-quality and cost-effective care.
The purpose of this review is to highlight current tools used to measure outcomes after ACL reconstruction. Current outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations. We have thus identified the measures most commonly used today in studies and clinical follow-up after ACL reconstruction and their various advantages and limitations. This information may enhance the orthopedic surgeon’s understanding of what outcome measures may be utilized in clinical studies.
Continue to: Patient-Reported Outcome Instruments...
PATIENT-REPORTED OUTCOME INSTRUMENTS
Recently, there has been a transition to increased use of PRO instruments rather than clinician-based postoperative assessment, largely due to the increasing emphasis on patient satisfaction in determining the value of an orthopedic intervention.4 PRO instruments are widely used to capture the patient’s perception of general health, quality of life (QOL), daily function, and pain. PRO instruments offer the benefit of allowing patients to subjectively assess their knee function during daily living and sports activities, conveying to the provider the impact of ACL reconstruction on physical, psychological, and social aspects of everyday activities. Furthermore, patient satisfaction has been shown to closely follow outcome scores related to symptoms and function.5 A multitude of specific knee-related PRO instruments have been developed and validated to measure outcomes after ACL reconstruction for both research and clinical purposes (Table).
Table. ACL Outcome Measures
|
|
|
|
| ||
Outcome Measure | Condition/Intervention | Measures | Internal Consistency (Cronbach’s a) | Test-Retest Reliability | Minimal Clinically Important Difference | Ref |
AAOS Sports Knee Scale | Many Knee | Stiffness, swelling, pain/function, locking/catching, giving way, limitation of activity, pain with activity | 0.86-0.95 | 0.68-0.96 | Unknown | 59, 60 |
ACL-QOL | Chronic ACL deficiency | Physical complaints, work, recreation and sports competition, lifestyle, social and emotional functioning | 0.93-0.98 | 6% average error | Unknown | 35, 36 |
Cincinnati Knee Rating System | ACL | Symptoms, daily and sports activities, physical examination, stability, radiographs, functional testing |
| 0.80-0.97 | 14 points (6 months), 26 points (12 months) | 39, 40, 47, 52 |
IKDC (Subjective Knee Form) | ACL | Symptoms, function, sports activity | 0.92 | 0.91-0.93 | 11.5 points; 6.3 at 6 months, 16.7 at 12 months | 48, 52, 54 |
KOOS | ACL | Pain, symptoms, activities of daily living, sport/recreation, knee-related quality of life | 0.71-0.95 | 0.75-0.93 | 8-10 points | 17 |
Lysholm | ACL | Pain, instability, locking, squatting, limp, support, swelling, stair-climbing | 0.72 | 0.94 | 8.9 | 46, 47, 55 |
Marx | Healthy patients | Activity level | 0.87 | 0.97 | Unknown | 42, 56, 57 |
Tegner | ACL | Activity level | 0.81 | 0.82 | 1 | 55, 56 |
PROMIS | Many lower extremity orthopedic conditions | Lower extremity function, central body function, activities of daily living | 0.98 | 0.96-0.99 |
| 30, 31 |
WOMAC | Hip/knee OA | Physical function, pain, stiffness | 0.81-0.95 | 0.80-0.92 | 12% baseline score or 6% max score; 9-12 points | 13, 14 |
Abbreviations: AAOS, American Academy of Orthopaedic Surgeons; ACL, anterior cruciate ligament; ACL-QOL, anterior cruciate ligament quality of life score; CAT, computer-adapting testing; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; PF, physical function; PROMIS, Patient-Reported Outcome Measurement Information System; Ref, references; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MEASUREMENT PROPERTIES
In general, clinicians and investigators should use health-related outcome measures with established reliability, validity, patient relevance, and responsiveness for assessing the specific condition.6
Reliability refers to the degree to which a measurement score is free from random error, reflecting how consistent or reproducible the instrument is when administered under the same testing conditions. Internal consistency, test-retest reliability, and measurement error are measures of reliability. Internal consistency is tested after a single administration and assesses how well items within a scale measure a single underlying dimension, represented using item-total correlation coefficients and Cronbach’s alpha. A Cronbach’s alpha of 0.70 to 0.95 is generally defined as good.7 Test-retest reliability is designed to appraise variation over time in stable patients and is represented using the intraclass correlation coefficient (ICC).8 An ICC >0.7 is considered acceptable; >0.8, good; and >0.9, excellent.9 An aspect of accuracy is whether the scoring system measures the full range of the disease or complaints. The incidence of minimum (floor) and maximum (ceiling) scores can be calculated for outcome scores. An instrument with low floor and ceiling effects, below 10% to 15%, is more inconclusive and can be more reliably used to measure patients at the high and low end of the scoring system.10
Validity is the ability of an outcome instrument to measure what it is intended to measure. Establishing validity is complex and requires evaluation of several facets, including content validity, construct validity, and criterion validity. Content validity is a relatively subjective judgment explaining the ability of an instrument to assess the critical features of the problem. Construct validity evaluates whether the questionnaire measures what it intends to measure, and is often assessed by correlating scores form one instrument to those from other proven instruments that are already accepted as valid. Finally, criterion validity assesses the correlation between the score and a previously established “gold standard” instrument.
Responsiveness is the ability of the instrument to detect a change or identify improvement or worsening of a clinical condition over time. Most frequently, the effect size (observed change/standard deviation of baseline scores) and standardized response mean (observed change/standard deviation of change) are used as measures of responsiveness. The minimal clinically important difference of an outcome measure is the smallest change in an outcome score that corresponds to a change in patient condition.
Continue to: ACL Outcome Instruments...
ACL OUTCOME INSTRUMENTS
WESTERN ONTARIO AND MCMASTER UNIVERSITIES OSTEOARTHRITIS INDEX (WOMAC LK 3.0)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.0) was developed in 1982 and is a widely used, disease-specific instrument recommended for the evaluation of treatment effects in patients with hip and knee osteoarthritis.11 Available in more than 80 languages, it is a self-administered, generic health status questionnaire developed to assess pain, function, and stiffness in daily living, taking respondents between 3 to 7.5 minutes for completion.12 Using visual analog scales, the 24 items probe the 3 subscales: pain (5 items), stiffness (2 items), and functional difficulty (17 items). Scores are calculated for each dimension, and the total score is normalized to a 100-point scale, with 0 indicating severe symptoms and 100 indicating no symptoms and higher function. The WOMAC score can also be calculated from the Knee Injury and Osteoarthritis Outcome Score (KOOS). The WOMAC questionnaire is well recognized for its good validity, reliability, and responsiveness, and is the most commonly used outcome measure for osteoarthritis.13-15 Considering its focus on older patients with osteoarthritis, it may not be appropriate for use in a young and active population.
KNEE INJURY AND OSTEOARTHRITIS OUTCOME SCORE (KOOS)
The KOOS is a knee-specific questionnaire developed as an extension of the WOMAC to evaluate the functional status and QOL of patients with any type of knee injury who are at an increased risk of developing osteoarthritis.16 The patient-based questionnaire is available in over 30 languages and covers both the short- and long-term consequences of an injury of the knee causing traumatic damage to cartilage, ligaments, and menisci. The KOOS is 42 items graded on a 5-point Likert scale, covering 5 subscales: pain (9 items), symptoms (7 items), function in activities of daily living (17 items), function in sports/recreation (5 items), and knee-related QOL (4 items). The questionnaire is self-administered and takes about 10 minutes to complete. Scores are calculated for each dimension, and the total score is transformed to a 0 to 100 scale, with 0 representing severe knee problems and 100 representing no knee problems and better outcome. An advantage of the KOOS is that it evaluates both knee injuries and osteoarthritis; therefore, it is arguably more suitable for evaluating patients over the long-term. The KOOS has been validated for several orthopedic interventions, including ACL reconstruction and rehabilitation16,17 as well as meniscectomy18 and total knee replacement.19 Population-based reference data for the adult population according to age and gender have also been established.20 The KOOS is increasingly utilized in clinical studies on ACL reconstruction.21-25 The questions of the WOMAC were retained so that a WOMAC score might be calculated separately and compared with the KOOS score.26
PATIENT-REPORTED OUTCOMES INFORMATION SYSTEM (PROMIS)
Since 2004, The National Institutes of Health (NIH) has funded the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a set of flexible tools that reliably and validly measure PROs. The PROMIS consists of a library of question banks that has been developed and operated by a network of National Institutes of Health-funded research sites and coordinating centers and covers many different health domains including pain, fatigue, anxiety, depression, social functioning, physical functioning, and sleep. PROMIS items are developed using Item Response Theory (IRT), wherein the answer to any individual item has a known mathematical probability of predicting the test taker’s overall measurement of the specific trait being tested. This is commonly administered using computer-adaptive testing (CAT), which presents to the test taker an initial item, scores the response to that item, and from the response then presents the most informative second item, and so forth until a predefined level of precision is reached. Because the items are individually validated, they can be used alone or in any combination, a feature that distinguishes the PROMIS from traditional fixed-length PRO instruments that require the completion of an instrument in its entirety to be valid.27 In recent years, orthopedic research has been published with PROMIS physical function (PF) scores as primary outcome measures.28-30 The PF item bank includes 124 items measuring upper extremity, lower extremity, central and instrumental activities of daily living. PF can be completed as a short form (SF) with a set number of questions or utilizing CAT and evaluates self-reported function and physical activity. An advantage is its ease of use and potential to minimize test burden with very few questions, often as little as 4 items, as compared to other traditional PROMs.31
Previously published work has demonstrated that, in patients undergoing meniscal surgery, the PROMIS PF CAT maintains construct validity and correlates well with currently used knee outcome instruments, including KOOS.28 Work by the same group looking at the performance of the PROMIS PF CAT in patients indicated for ACL reconstruction shows that the PROMIS PF CAT correlates well with other PRO instruments for patients with ACL injuries, (SF-36 PF [r = 0.82, P < 0.01], KOOS Sport [r = 0.70, P < 0.01], KOOS ADL [r = 0.74, P < 0.01]), does not have floor or ceiling effects in this relatively young and healthy population, and has a low test burden.32,33 Papuga and colleagues33 also compared the International Knee Documentation Committee (IKDC) and PROMIS PF CAT on 106 subjects after ACL reconstruction and found good correlation.
Continue to: Quality of Life Outcome Measure...
QUALITY OF LIFE OUTCOME MEASURE FOR ACL DEFICIENCY (ACL-QOL)
The ACL-QOL Score was developed in 1998 as a disease-specific measure for patients with chronic ACL deficiency.34 This scale consists of 32 separate items in 31 visual analog questions regarding symptoms and physical complaints, work-related concerns, recreational activities and sport participation or competition, lifestyle, and social and emotional health status relating to the knee. The raw score is transformed into a 0- to 100-point scale, with higher scores indicating a better outcome. The scale is valid, reliable, and responsive for patients with ACL insufficiency,35,36 and is not applicable to other disorders of the knee. We recommend the ACL-QOL questionnaire be used in conjunction with other currently available objective and functional outcome measures.
CINCINNATI KNEE RATING SYSTEM
The Cincinnati Knee Rating System (CKRS) was first described in 1983 and was modified to include occupational activities, athletic activities, symptoms, and functional limitations.37,38 There are 11 components, measuring symptoms and disability in sports activity, activities of daily living function, occupational rating, as well as sections that measure physical examination, laxity of the knee, and radiographic evidence of degenerative joint disease.39 The measure is scored on a 100-point scale, with higher scores indicating better outcomes. Scores have been shown to be lower as compared with other outcome measures assessing the same clinical condition.40,41 Barber-Westin and colleagues39 confirmed the reliability, validity, and responsiveness of the CKRS by testing 350 subjects with and without knee ligament injuries. In 2001, Marx42 tested the CKRS subjective form for reliability, validity, and responsiveness and found it to be acceptable for clinical research.
LYSHOLM KNEE SCORE
The Lysholm Knee Score was published in 1982 and modified in 1985, consisting of an 8-question survey that evaluates outcomes after knee ligament surgery. Items include pain, instability, locking, squatting, limping, support usage, swelling, and stair-climbing ability, with pain and instability carrying the highest weight.43 It is scored on a scale of 0 to 100, with high scores indicating higher functioning and fewer symptoms. It has been validated in patients with ACL injuries and meniscal injuries.44 Although it is widely used to measure outcomes after ACL reconstruction,45 it has received criticism in the evaluation of patients with other knee conditions.46 The main advantage of the Lysholm Knee Score is its ability to note changes in activity in the same patient across different time periods (responsiveness). A limitation of the Lysholm Knee Score is that it does not measure the domains of functioning in daily activities, sports, and recreational activities. The Lysholm scoring system’s test-retest reliability and construct validity have been evaluated,42,43,46 although there has been some concern regarding a ceiling effect and its validity, sensitivity, and reliability has been questioned.47 Therefore, it is advised that this score be used in conjunction with other PRO scores.
INTERNATIONAL KNEE DOCUMENTATION COMMITTEE (IKDC) SUBJECTIVE KNEE FORM
In 1987, members of the European Society for Knee Surgery and Arthroscopy and the American Orthopaedic Society for Sports Medicine formed the IKDC to develop a standardized method for evaluating knee injuries and treatment. The IKDC Subjective Knee Evaluation Form was initially published in 1993, and in 2001 the form was revised by the American Orthopaedic Society for Sports Medicine to become a knee-specific assessment tool rather than a disease or condition-specific tool.48 The IKDC subjective form is an 18-question, knee-specific survey designed to detect improvement or deterioration in symptoms, function, and ability to participate in sports activities experienced by patients following knee surgery or other interventions. The individual items are summed and transformed into a 0- to 100-point scale, with high scores representing higher levels of function and minimal symptoms. The IKDC is utilized to assess a variety of knee conditions including ligament, meniscus, articular cartilage, osteoarthritis, and patellofemoral pain.48,49 Thus, this form can be used to assess any condition involving the knee and allow comparison between groups with different diagnoses. The IKDC has been validated for an ACL reconstruction population,47 has been used to assess outcomes in recent clinical studies on ACL reconstruction,50,51 and is one of the most frequently used measures for patients with ACL deficiency.3 The validity, responsiveness, and reliability of the IKDC subjective form has been confirmed for both adult and adolescent populations.48,49,52-54
TEGNER ACTIVITY SCORES
The Tegner activity score was developed in 1985 and was designed to provide an objective value for a patient’s activity level.44 This scale was developed to complement the Lysholm score. It consists of 1 sport-specific activity level question on a 0 to 10 scale that evaluates an individual’s ability to compete in a sporting activity. Scores between 1 and 5 represent work or recreational sports. Scores >5 represent higher-level recreational and competitive sports. The Tegner activity score is one of the most widely used activity scoring systems for patients with knee disorders,55,56 commonly utilized with the Lysholm Knee Score.44 One disadvantage of the Tegner activity score is that it relates to specific sports rather than functional activities, which limits its generalizability. We are not aware of any studies documenting the reliability or validity of this instrument.
Continue on: Marx Activity Rating Scale...
MARX ACTIVITY RATING SCALE
The Marx activity rating scale was developed to be utilized with other knee rating scales and outcome measures as an activity assessment.57 In contrast to the Tegner activity score, the Marx activity rating scale measures function rather than sport-specific activity. The scale is a short, patient-based activity assessment that consists of a 4-question survey evaluating patients’ knee health by recording the frequency and intensity of participation in a sporting activity. Questions are scored from 0 to 4 on the basis of how often the activity is performed. The 4 sections of the Marx scale that are rated include running, cutting, decelerating, and pivoting. This scale has been validated in patients with ACL injuries, chondromalacia patellae, and meniscal lesions.42,56-58 Acceptable ceiling effects of 3% and floor effects of 8% were noted in the study of ACL-injured patients.57
AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS (AAOS) SPORTS KNEE SCALE
The American Academy of Orthopaedic Surgeons (AAOS) Sports Knee Rating Scale consists of 5 parts and 23 items, including a section addressing stiffness, swelling, pain and function (7 questions), locking/catching (4 questions), giving way (4 questions), limitations of activity (4 questions), and pain with activity (4 questions).59,60 Items may be dropped if patients select particular responses, which can lead to difficulties when using the survey. This scoring system has been found to be satisfactory when all subscales were combined and the mean was calculated.42
DISCUSSION
PRO measures play an increasingly important role in the measurement of success and impact of health care services. Specifically, for ACL reconstruction, patient satisfaction is key for demonstrating the value of operative or other interventions. Selecting a suitable outcome measurement tool can be daunting, as it can be difficult to ascertain which outcome measures are appropriate for the patient or disorder in question. As there is currently no instrument that is universally superior in the evaluation of ACL outcomes, clinicians must consider the specific patient population in which the outcome instrument has been evaluated. Investigators should also use instruments with reported minimal clinically important differences so that variation in scores can be interpreted as either clinically significant or not. When choosing which outcome instrument to use, there is rarely a single appropriate rating system that is entirely comprehensive. In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale. Activity rating scales, such as Marx or Tegner, should be included, especially when evaluating patients with low-activity lifestyles.
CONCLUSION
There are a number of reliable, valid, and responsive outcome measures that can be utilized to evaluate outcomes following ACL reconstruction in an array of patient populations. Outcome measures should be relevant to patients, easy to use, reliable, valid, and responsive to change. By increasing familiarity with these outcome measures, orthopedic surgeons and investigators can develop better studies, interpret data, and implement findings in practice with sound and informed judgment. Future research should focus on identifying the most relevant outcome metrics for assessing function following ACL reconstruction.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Outcome instruments have become an essential part of the evaluation of functional recovery after anterior cruciate ligament (ACL) reconstruction. Although the clinical examination provides important objective information to assess graft integrity, stability, range of motion, and strength, these measurements do not take the patient’s perception into account. There are many knee outcome instruments, and it is challenging for surgeons to understand how to interpret clinical research and utilize these measures in a practical way. The purpose of this review is to provide an overview of the most commonly used outcome measures in patients undergoing ACL reconstruction and to examine and compare the psychometric performance (validity, reliability, responsiveness) of these measurement tools.
Anterior cruciate ligament (ACL) reconstruction is one of the most common elective orthopedic procedures.1 Despite advances in surgical techniques, ACL reconstruction is associated with a lengthy recovery time, decreased performance, and increased rate of reinjury.2 Patients undergoing ACL reconstruction are often active individuals who participate in demanding activities, and accurate assessment of their recovery helps to guide recovery counseling. In addition to objective clinical outcomes measured through physical examination, patient-reported outcome (PRO) instruments add the patient’s perspective, information critical in determining a successful outcome. A variety of outcome instruments have been used and validated for patients with ACL tears. It is important for orthopedic surgeons to know the advantages and disadvantages of each outcome tool in order to interpret clinical studies and assess postoperative patients.
Over the last 10 years, there has been an increase in the number of knee instruments and rating scales designed to measure PROs, with >54 scores designed for the ACL-deficient knee.3 No standardized instrument is currently universally accepted as superior following ACL reconstruction across the spectrum of patient populations. Clinicians and researchers must carefully consider an outcome instrument’s utility based on specific patient populations in which it has been evaluated. Appropriate selection of outcome measures is of fundamental importance for adequate demonstration of the efficacy and value of treatment interventions, especially in an era of healthcare reform with a focus on providing high-quality and cost-effective care.
The purpose of this review is to highlight current tools used to measure outcomes after ACL reconstruction. Current outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations. We have thus identified the measures most commonly used today in studies and clinical follow-up after ACL reconstruction and their various advantages and limitations. This information may enhance the orthopedic surgeon’s understanding of what outcome measures may be utilized in clinical studies.
Continue to: Patient-Reported Outcome Instruments...
PATIENT-REPORTED OUTCOME INSTRUMENTS
Recently, there has been a transition to increased use of PRO instruments rather than clinician-based postoperative assessment, largely due to the increasing emphasis on patient satisfaction in determining the value of an orthopedic intervention.4 PRO instruments are widely used to capture the patient’s perception of general health, quality of life (QOL), daily function, and pain. PRO instruments offer the benefit of allowing patients to subjectively assess their knee function during daily living and sports activities, conveying to the provider the impact of ACL reconstruction on physical, psychological, and social aspects of everyday activities. Furthermore, patient satisfaction has been shown to closely follow outcome scores related to symptoms and function.5 A multitude of specific knee-related PRO instruments have been developed and validated to measure outcomes after ACL reconstruction for both research and clinical purposes (Table).
Table. ACL Outcome Measures
|
|
|
|
| ||
Outcome Measure | Condition/Intervention | Measures | Internal Consistency (Cronbach’s a) | Test-Retest Reliability | Minimal Clinically Important Difference | Ref |
AAOS Sports Knee Scale | Many Knee | Stiffness, swelling, pain/function, locking/catching, giving way, limitation of activity, pain with activity | 0.86-0.95 | 0.68-0.96 | Unknown | 59, 60 |
ACL-QOL | Chronic ACL deficiency | Physical complaints, work, recreation and sports competition, lifestyle, social and emotional functioning | 0.93-0.98 | 6% average error | Unknown | 35, 36 |
Cincinnati Knee Rating System | ACL | Symptoms, daily and sports activities, physical examination, stability, radiographs, functional testing |
| 0.80-0.97 | 14 points (6 months), 26 points (12 months) | 39, 40, 47, 52 |
IKDC (Subjective Knee Form) | ACL | Symptoms, function, sports activity | 0.92 | 0.91-0.93 | 11.5 points; 6.3 at 6 months, 16.7 at 12 months | 48, 52, 54 |
KOOS | ACL | Pain, symptoms, activities of daily living, sport/recreation, knee-related quality of life | 0.71-0.95 | 0.75-0.93 | 8-10 points | 17 |
Lysholm | ACL | Pain, instability, locking, squatting, limp, support, swelling, stair-climbing | 0.72 | 0.94 | 8.9 | 46, 47, 55 |
Marx | Healthy patients | Activity level | 0.87 | 0.97 | Unknown | 42, 56, 57 |
Tegner | ACL | Activity level | 0.81 | 0.82 | 1 | 55, 56 |
PROMIS | Many lower extremity orthopedic conditions | Lower extremity function, central body function, activities of daily living | 0.98 | 0.96-0.99 |
| 30, 31 |
WOMAC | Hip/knee OA | Physical function, pain, stiffness | 0.81-0.95 | 0.80-0.92 | 12% baseline score or 6% max score; 9-12 points | 13, 14 |
Abbreviations: AAOS, American Academy of Orthopaedic Surgeons; ACL, anterior cruciate ligament; ACL-QOL, anterior cruciate ligament quality of life score; CAT, computer-adapting testing; IKDC, International Knee Documentation Committee; KOOS, Knee Injury and Osteoarthritis Outcome Score; OA, osteoarthritis; PF, physical function; PROMIS, Patient-Reported Outcome Measurement Information System; Ref, references; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
MEASUREMENT PROPERTIES
In general, clinicians and investigators should use health-related outcome measures with established reliability, validity, patient relevance, and responsiveness for assessing the specific condition.6
Reliability refers to the degree to which a measurement score is free from random error, reflecting how consistent or reproducible the instrument is when administered under the same testing conditions. Internal consistency, test-retest reliability, and measurement error are measures of reliability. Internal consistency is tested after a single administration and assesses how well items within a scale measure a single underlying dimension, represented using item-total correlation coefficients and Cronbach’s alpha. A Cronbach’s alpha of 0.70 to 0.95 is generally defined as good.7 Test-retest reliability is designed to appraise variation over time in stable patients and is represented using the intraclass correlation coefficient (ICC).8 An ICC >0.7 is considered acceptable; >0.8, good; and >0.9, excellent.9 An aspect of accuracy is whether the scoring system measures the full range of the disease or complaints. The incidence of minimum (floor) and maximum (ceiling) scores can be calculated for outcome scores. An instrument with low floor and ceiling effects, below 10% to 15%, is more inconclusive and can be more reliably used to measure patients at the high and low end of the scoring system.10
Validity is the ability of an outcome instrument to measure what it is intended to measure. Establishing validity is complex and requires evaluation of several facets, including content validity, construct validity, and criterion validity. Content validity is a relatively subjective judgment explaining the ability of an instrument to assess the critical features of the problem. Construct validity evaluates whether the questionnaire measures what it intends to measure, and is often assessed by correlating scores form one instrument to those from other proven instruments that are already accepted as valid. Finally, criterion validity assesses the correlation between the score and a previously established “gold standard” instrument.
Responsiveness is the ability of the instrument to detect a change or identify improvement or worsening of a clinical condition over time. Most frequently, the effect size (observed change/standard deviation of baseline scores) and standardized response mean (observed change/standard deviation of change) are used as measures of responsiveness. The minimal clinically important difference of an outcome measure is the smallest change in an outcome score that corresponds to a change in patient condition.
Continue to: ACL Outcome Instruments...
ACL OUTCOME INSTRUMENTS
WESTERN ONTARIO AND MCMASTER UNIVERSITIES OSTEOARTHRITIS INDEX (WOMAC LK 3.0)
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC LK 3.0) was developed in 1982 and is a widely used, disease-specific instrument recommended for the evaluation of treatment effects in patients with hip and knee osteoarthritis.11 Available in more than 80 languages, it is a self-administered, generic health status questionnaire developed to assess pain, function, and stiffness in daily living, taking respondents between 3 to 7.5 minutes for completion.12 Using visual analog scales, the 24 items probe the 3 subscales: pain (5 items), stiffness (2 items), and functional difficulty (17 items). Scores are calculated for each dimension, and the total score is normalized to a 100-point scale, with 0 indicating severe symptoms and 100 indicating no symptoms and higher function. The WOMAC score can also be calculated from the Knee Injury and Osteoarthritis Outcome Score (KOOS). The WOMAC questionnaire is well recognized for its good validity, reliability, and responsiveness, and is the most commonly used outcome measure for osteoarthritis.13-15 Considering its focus on older patients with osteoarthritis, it may not be appropriate for use in a young and active population.
KNEE INJURY AND OSTEOARTHRITIS OUTCOME SCORE (KOOS)
The KOOS is a knee-specific questionnaire developed as an extension of the WOMAC to evaluate the functional status and QOL of patients with any type of knee injury who are at an increased risk of developing osteoarthritis.16 The patient-based questionnaire is available in over 30 languages and covers both the short- and long-term consequences of an injury of the knee causing traumatic damage to cartilage, ligaments, and menisci. The KOOS is 42 items graded on a 5-point Likert scale, covering 5 subscales: pain (9 items), symptoms (7 items), function in activities of daily living (17 items), function in sports/recreation (5 items), and knee-related QOL (4 items). The questionnaire is self-administered and takes about 10 minutes to complete. Scores are calculated for each dimension, and the total score is transformed to a 0 to 100 scale, with 0 representing severe knee problems and 100 representing no knee problems and better outcome. An advantage of the KOOS is that it evaluates both knee injuries and osteoarthritis; therefore, it is arguably more suitable for evaluating patients over the long-term. The KOOS has been validated for several orthopedic interventions, including ACL reconstruction and rehabilitation16,17 as well as meniscectomy18 and total knee replacement.19 Population-based reference data for the adult population according to age and gender have also been established.20 The KOOS is increasingly utilized in clinical studies on ACL reconstruction.21-25 The questions of the WOMAC were retained so that a WOMAC score might be calculated separately and compared with the KOOS score.26
PATIENT-REPORTED OUTCOMES INFORMATION SYSTEM (PROMIS)
Since 2004, The National Institutes of Health (NIH) has funded the development of the Patient-Reported Outcome Measurement Information System (PROMIS), a set of flexible tools that reliably and validly measure PROs. The PROMIS consists of a library of question banks that has been developed and operated by a network of National Institutes of Health-funded research sites and coordinating centers and covers many different health domains including pain, fatigue, anxiety, depression, social functioning, physical functioning, and sleep. PROMIS items are developed using Item Response Theory (IRT), wherein the answer to any individual item has a known mathematical probability of predicting the test taker’s overall measurement of the specific trait being tested. This is commonly administered using computer-adaptive testing (CAT), which presents to the test taker an initial item, scores the response to that item, and from the response then presents the most informative second item, and so forth until a predefined level of precision is reached. Because the items are individually validated, they can be used alone or in any combination, a feature that distinguishes the PROMIS from traditional fixed-length PRO instruments that require the completion of an instrument in its entirety to be valid.27 In recent years, orthopedic research has been published with PROMIS physical function (PF) scores as primary outcome measures.28-30 The PF item bank includes 124 items measuring upper extremity, lower extremity, central and instrumental activities of daily living. PF can be completed as a short form (SF) with a set number of questions or utilizing CAT and evaluates self-reported function and physical activity. An advantage is its ease of use and potential to minimize test burden with very few questions, often as little as 4 items, as compared to other traditional PROMs.31
Previously published work has demonstrated that, in patients undergoing meniscal surgery, the PROMIS PF CAT maintains construct validity and correlates well with currently used knee outcome instruments, including KOOS.28 Work by the same group looking at the performance of the PROMIS PF CAT in patients indicated for ACL reconstruction shows that the PROMIS PF CAT correlates well with other PRO instruments for patients with ACL injuries, (SF-36 PF [r = 0.82, P < 0.01], KOOS Sport [r = 0.70, P < 0.01], KOOS ADL [r = 0.74, P < 0.01]), does not have floor or ceiling effects in this relatively young and healthy population, and has a low test burden.32,33 Papuga and colleagues33 also compared the International Knee Documentation Committee (IKDC) and PROMIS PF CAT on 106 subjects after ACL reconstruction and found good correlation.
Continue to: Quality of Life Outcome Measure...
QUALITY OF LIFE OUTCOME MEASURE FOR ACL DEFICIENCY (ACL-QOL)
The ACL-QOL Score was developed in 1998 as a disease-specific measure for patients with chronic ACL deficiency.34 This scale consists of 32 separate items in 31 visual analog questions regarding symptoms and physical complaints, work-related concerns, recreational activities and sport participation or competition, lifestyle, and social and emotional health status relating to the knee. The raw score is transformed into a 0- to 100-point scale, with higher scores indicating a better outcome. The scale is valid, reliable, and responsive for patients with ACL insufficiency,35,36 and is not applicable to other disorders of the knee. We recommend the ACL-QOL questionnaire be used in conjunction with other currently available objective and functional outcome measures.
CINCINNATI KNEE RATING SYSTEM
The Cincinnati Knee Rating System (CKRS) was first described in 1983 and was modified to include occupational activities, athletic activities, symptoms, and functional limitations.37,38 There are 11 components, measuring symptoms and disability in sports activity, activities of daily living function, occupational rating, as well as sections that measure physical examination, laxity of the knee, and radiographic evidence of degenerative joint disease.39 The measure is scored on a 100-point scale, with higher scores indicating better outcomes. Scores have been shown to be lower as compared with other outcome measures assessing the same clinical condition.40,41 Barber-Westin and colleagues39 confirmed the reliability, validity, and responsiveness of the CKRS by testing 350 subjects with and without knee ligament injuries. In 2001, Marx42 tested the CKRS subjective form for reliability, validity, and responsiveness and found it to be acceptable for clinical research.
LYSHOLM KNEE SCORE
The Lysholm Knee Score was published in 1982 and modified in 1985, consisting of an 8-question survey that evaluates outcomes after knee ligament surgery. Items include pain, instability, locking, squatting, limping, support usage, swelling, and stair-climbing ability, with pain and instability carrying the highest weight.43 It is scored on a scale of 0 to 100, with high scores indicating higher functioning and fewer symptoms. It has been validated in patients with ACL injuries and meniscal injuries.44 Although it is widely used to measure outcomes after ACL reconstruction,45 it has received criticism in the evaluation of patients with other knee conditions.46 The main advantage of the Lysholm Knee Score is its ability to note changes in activity in the same patient across different time periods (responsiveness). A limitation of the Lysholm Knee Score is that it does not measure the domains of functioning in daily activities, sports, and recreational activities. The Lysholm scoring system’s test-retest reliability and construct validity have been evaluated,42,43,46 although there has been some concern regarding a ceiling effect and its validity, sensitivity, and reliability has been questioned.47 Therefore, it is advised that this score be used in conjunction with other PRO scores.
INTERNATIONAL KNEE DOCUMENTATION COMMITTEE (IKDC) SUBJECTIVE KNEE FORM
In 1987, members of the European Society for Knee Surgery and Arthroscopy and the American Orthopaedic Society for Sports Medicine formed the IKDC to develop a standardized method for evaluating knee injuries and treatment. The IKDC Subjective Knee Evaluation Form was initially published in 1993, and in 2001 the form was revised by the American Orthopaedic Society for Sports Medicine to become a knee-specific assessment tool rather than a disease or condition-specific tool.48 The IKDC subjective form is an 18-question, knee-specific survey designed to detect improvement or deterioration in symptoms, function, and ability to participate in sports activities experienced by patients following knee surgery or other interventions. The individual items are summed and transformed into a 0- to 100-point scale, with high scores representing higher levels of function and minimal symptoms. The IKDC is utilized to assess a variety of knee conditions including ligament, meniscus, articular cartilage, osteoarthritis, and patellofemoral pain.48,49 Thus, this form can be used to assess any condition involving the knee and allow comparison between groups with different diagnoses. The IKDC has been validated for an ACL reconstruction population,47 has been used to assess outcomes in recent clinical studies on ACL reconstruction,50,51 and is one of the most frequently used measures for patients with ACL deficiency.3 The validity, responsiveness, and reliability of the IKDC subjective form has been confirmed for both adult and adolescent populations.48,49,52-54
TEGNER ACTIVITY SCORES
The Tegner activity score was developed in 1985 and was designed to provide an objective value for a patient’s activity level.44 This scale was developed to complement the Lysholm score. It consists of 1 sport-specific activity level question on a 0 to 10 scale that evaluates an individual’s ability to compete in a sporting activity. Scores between 1 and 5 represent work or recreational sports. Scores >5 represent higher-level recreational and competitive sports. The Tegner activity score is one of the most widely used activity scoring systems for patients with knee disorders,55,56 commonly utilized with the Lysholm Knee Score.44 One disadvantage of the Tegner activity score is that it relates to specific sports rather than functional activities, which limits its generalizability. We are not aware of any studies documenting the reliability or validity of this instrument.
Continue on: Marx Activity Rating Scale...
MARX ACTIVITY RATING SCALE
The Marx activity rating scale was developed to be utilized with other knee rating scales and outcome measures as an activity assessment.57 In contrast to the Tegner activity score, the Marx activity rating scale measures function rather than sport-specific activity. The scale is a short, patient-based activity assessment that consists of a 4-question survey evaluating patients’ knee health by recording the frequency and intensity of participation in a sporting activity. Questions are scored from 0 to 4 on the basis of how often the activity is performed. The 4 sections of the Marx scale that are rated include running, cutting, decelerating, and pivoting. This scale has been validated in patients with ACL injuries, chondromalacia patellae, and meniscal lesions.42,56-58 Acceptable ceiling effects of 3% and floor effects of 8% were noted in the study of ACL-injured patients.57
AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS (AAOS) SPORTS KNEE SCALE
The American Academy of Orthopaedic Surgeons (AAOS) Sports Knee Rating Scale consists of 5 parts and 23 items, including a section addressing stiffness, swelling, pain and function (7 questions), locking/catching (4 questions), giving way (4 questions), limitations of activity (4 questions), and pain with activity (4 questions).59,60 Items may be dropped if patients select particular responses, which can lead to difficulties when using the survey. This scoring system has been found to be satisfactory when all subscales were combined and the mean was calculated.42
DISCUSSION
PRO measures play an increasingly important role in the measurement of success and impact of health care services. Specifically, for ACL reconstruction, patient satisfaction is key for demonstrating the value of operative or other interventions. Selecting a suitable outcome measurement tool can be daunting, as it can be difficult to ascertain which outcome measures are appropriate for the patient or disorder in question. As there is currently no instrument that is universally superior in the evaluation of ACL outcomes, clinicians must consider the specific patient population in which the outcome instrument has been evaluated. Investigators should also use instruments with reported minimal clinically important differences so that variation in scores can be interpreted as either clinically significant or not. When choosing which outcome instrument to use, there is rarely a single appropriate rating system that is entirely comprehensive. In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale. Activity rating scales, such as Marx or Tegner, should be included, especially when evaluating patients with low-activity lifestyles.
CONCLUSION
There are a number of reliable, valid, and responsive outcome measures that can be utilized to evaluate outcomes following ACL reconstruction in an array of patient populations. Outcome measures should be relevant to patients, easy to use, reliable, valid, and responsive to change. By increasing familiarity with these outcome measures, orthopedic surgeons and investigators can develop better studies, interpret data, and implement findings in practice with sound and informed judgment. Future research should focus on identifying the most relevant outcome metrics for assessing function following ACL reconstruction.
This paper will be judged for the Resident Writer’s Award.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370. doi:10.1177/0363546514542796.
2. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
3. Johnson DS, Smith RB. Outcome measurement in the ACL deficient knee- what’s the score? Knee. 2001;8(1):51-57. doi:10.1016/S0968-0160(01)00068-0.
4. Graham B, Green A, James M, Katz J, Swiontkowski M. Measuring patient satisfaction in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(1):80-84. doi:10.2106/JBJS.N.00811.
5. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84(9):1560-1572. doi:10.2106/00004623-200209000-00008.
6. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 1989.
7. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34-42. doi:10.1016/j.jclinepi.2006.03.012.
8. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. doi:10.2466/pr0.1966.19.1.3.
9. Scholtes VA, Terwee CB, Poolman RW. What makes a measurement instrument valid and reliable? Injury. 2011;42(3):236-240. doi:10.1016/j.injury.2010.11.042.
10. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.
12. Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67(2):216-229. doi:10.1002/acr.22415.
13. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
14. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stuckl G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12(5):331-335.
15. Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999;58(9):563-568. doi:10.1136/ard.58.9.563.
16. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi:10.2519/jospt.1998.28.2.88.
17. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406-410. doi:10.1016/j.joca.2011.01.010.
18. Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7(2):216-221. doi:10.1053/joca.1998.0153.
19. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi:10.1186/1477-7525-1-17.
20. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskeletal Disord. 2006;7(1):38. doi:10.1186/1471-2474-7-38.
21. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301-2310. doi:10.1177/0363546514549005.
22. Ventura A, Legnani C, Terzaghi C, Borgo E, Albisetti W. Revision surgery after failed ACL reconstruction with artificial ligaments: clinical, histologic and radiographic evaluation. Eur J Orthop Surg Traumatol. 2014;21(1):93-98. doi:10.1007/s00590-012-1136-3.
23. Wasserstein D, Huston LJ, Nwosu S, et al. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674-1684. doi:10.1016/j.joca.2015.05.025.
24. Zaffagnini S, Grassi A, Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
25. Duffee A, Magnussen RA, Pedroza AD, Flanigan DC; MOON Group, Kaeding CC. Transtibial ACL femoral tunnel preparation increases odds of repeat ipsilateral knee surgery. J Bone Joint Surg Am. 2013;95(22):2035-2042. doi:10.2106/JBJS.M.00187.
26. Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5(2):231-241. doi:10.1007/BF02032362.
27. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
28. Hancock KJ, Glass NA, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954-958. doi:10.2106/JBJS.16.00848.
29. Hung M, Clegg Do, Greene T, et al. Evaluation of the PROMIS physical function item bank in orthopedic patients. J Orthop Res. 2011;29(6):947-953. doi:10.1002/jor.21308.
30. Hung M, Baumhauer JF, Brodsky JW, et al; Orthopaedic Foot & Ankle Outcomes Research (OFAR) of the American Orthopaedic Foot & Ankle Society (AOFAS). Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int. 2014;35(6):592-599. doi:10.1177/1071100714528492.
31. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443. doi:10.1097/BOT.0000000000000059.
32. Hancock, et al. PROMIS: A valid and efficient outcomes instrument for patients with ACL tears. KSSTA. In press.
33. Scott, et al. Performance of PROMIS physical function compared with KOOS, SF-36, Eq5D, and Marx activity scale in patients who undergo ACL reconstruction. In press.
34. Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793-801. doi:10.1002/jor.22591.
35. Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350-359. doi:10.1177/03635465980260030201.
36. Lafave MR, Hiemstra L, Kerslake S, Heard M, Buchko G. Validity, reliability, and responsiveness of the anterior cruciate ligament quality of life measure: a continuation of its overall validation. Clin J Sport Med. 2017;27(1):57-63. doi:10.1097/JSM.0000000000000292.
37. Noyes FR, McGinniss GH, Mooar LA. Functional disability in the anterior cruciate insufficient knee syndrome: Review of knee rating systems and projected risk factors in determining treatment. Sports Med. 1984;1(4):278-302. doi:10.2165/00007256-198401040-00004.
38. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee: Part II. The results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174. doi:10.2106/00004623-198365020-00004.
39. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-416. doi:10.1177/03635465990270040201.
40. Bollen S, Seedhorn BB. A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med. 1991;19(2):189-190. doi:10.1177/036354659101900215.
41. Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ. Critical analysis of knee ligament rating systems. Am J Sports Med. 1995;23(6):660-667. doi:10.1177/036354659502300604.
42. Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83(10):1459-1469. doi:10.2106/00004623-200110000-00001.
43. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154. doi:10.1177/036354658201000306.
44. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-49. doi:10.1097/00003086-198509000-00007.
45. Lukianov AV, Gillquist J, Grana WA, DeHaven KE. An anterior cruciate ligament (ACL) evaluation format for assessment of artificial or autologous anterior cruciate reconstruction results. Clin Orthop Relat Res. 1987;218:167-180. doi:10.1097/00003086-198705000-00024.
46. Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31. doi:10.1007/BF01565994.
47. Risberg MA, Holm I, Steen J, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):152-159. doi:10.1007/s001670050140.
48. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. doi:10.1177/03635465010290051301.
49. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-1573. doi:10.1177/0363546506288855.
50. Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(2):914-923. doi:10.2519/jospt.2014.4852.
51. Lentz TA, Zeppieri G Jr, George SZ, et al. Comparison of physical impairment, functional and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345-353. doi:10.1177/0363546514559707.
52. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. doi:10.1177/0363546509354163.
53. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-234. doi:10.1007/BF01560215.
54. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38(12):2443-2447. doi:10.1177/0363546510374873.
55. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897. doi:10.1177/0363546508330143.
56. Negahban H, Mostafaee N, Sohani SM, et al. Reliability and validity of the Tegner and Marx activity rating scales in Iranian patients with anterior cruciate ligament injury. Disabil Rehabil. 2011;33(23-24):2305-2310. doi:10.3109/09638288.2011.570409.
57. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-218. doi:10.1177/03635465010290021601.
58. Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAM-ASK Trial Team. Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414-1423. doi:10.1093/rheumatology/keh362.
59. American Academy of Orthopaedic Surgeons. Scoring algorithms for the lower limb: Outcomes data collection instrument. Rosemon, IL: American Academy of Orthopaedic Surgeons; 1998.
60. Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am. 2004;86-A(5):902-909.
1. Mall NA, Chalmers PN, Moric M, et al. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363-2370. doi:10.1177/0363546514542796.
2. Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517-2522. doi:10.1177/0363546512459476.
3. Johnson DS, Smith RB. Outcome measurement in the ACL deficient knee- what’s the score? Knee. 2001;8(1):51-57. doi:10.1016/S0968-0160(01)00068-0.
4. Graham B, Green A, James M, Katz J, Swiontkowski M. Measuring patient satisfaction in orthopaedic surgery. J Bone Joint Surg Am. 2015;97(1):80-84. doi:10.2106/JBJS.N.00811.
5. Kocher MS, Steadman JR, Briggs K, Zurakowski D, Sterett WI, Hawkins RJ. Determinants of patient satisfaction with outcome after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2002;84(9):1560-1572. doi:10.2106/00004623-200209000-00008.
6. Streiner DL, Norman GR. Health Measurement Scales: A Practical Guide to their Development and Use. Oxford: Oxford University Press; 1989.
7. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34-42. doi:10.1016/j.jclinepi.2006.03.012.
8. Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19(1):3-11. doi:10.2466/pr0.1966.19.1.3.
9. Scholtes VA, Terwee CB, Poolman RW. What makes a measurement instrument valid and reliable? Injury. 2011;42(3):236-240. doi:10.1016/j.injury.2010.11.042.
10. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
11. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt L. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833-1840.
12. Gandek B. Measurement properties of the Western Ontario and McMaster Universities Osteoarthritis Index: a systematic review. Arthritis Care Res (Hoboken). 2015;67(2):216-229. doi:10.1002/acr.22415.
13. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384-391. doi:10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
14. Ryser L, Wright BD, Aeschlimann A, Mariacher-Gehler S, Stuckl G. A new look at the Western Ontario and McMaster Universities Osteoarthritis Index using Rasch analysis. Arthritis Care Res. 1999;12(5):331-335.
15. Wolfe F, Kong SX. Rasch analysis of the Western Ontario MacMaster questionnaire (WOMAC) in 2205 patients with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Ann Rheum Dis. 1999;58(9):563-568. doi:10.1136/ard.58.9.563.
16. Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS)—development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi:10.2519/jospt.1998.28.2.88.
17. Salavati M, Akhbari B, Mohammadi F, Mazaheri M, Khorrami M. Knee injury and Osteoarthritis Outcome Score (KOOS): reliability and validity in competitive athletes after anterior cruciate ligament reconstruction. Osteoarthritis Cartilage. 2011;19(4):406-410. doi:10.1016/j.joca.2011.01.010.
18. Roos EM, Roos HP, Lohmander LS. WOMAC Osteoarthritis Index—additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee. Western Ontario and MacMaster Universities. Osteoarthritis Cartilage. 1999;7(2):216-221. doi:10.1053/joca.1998.0153.
19. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS)—validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1(1):17. doi:10.1186/1477-7525-1-17.
20. Paradowski PT, Bergman S, Sunden-Lundius A, Lohmander LS, Roos EM. Knee complaints vary with age and gender in the adult population: population-based reference data for the Knee injury and Osteoarthritis Outcome Score (KOOS). BMC Musculoskeletal Disord. 2006;7(1):38. doi:10.1186/1471-2474-7-38.
21. MARS Group. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301-2310. doi:10.1177/0363546514549005.
22. Ventura A, Legnani C, Terzaghi C, Borgo E, Albisetti W. Revision surgery after failed ACL reconstruction with artificial ligaments: clinical, histologic and radiographic evaluation. Eur J Orthop Surg Traumatol. 2014;21(1):93-98. doi:10.1007/s00590-012-1136-3.
23. Wasserstein D, Huston LJ, Nwosu S, et al. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674-1684. doi:10.1016/j.joca.2015.05.025.
24. Zaffagnini S, Grassi A, Muccioli GM, et al. Return to sport after anterior cruciate ligament reconstruction in professional soccer players. Knee. 2014;21(3):731-735. doi:10.1016/j.knee.2014.02.005.
25. Duffee A, Magnussen RA, Pedroza AD, Flanigan DC; MOON Group, Kaeding CC. Transtibial ACL femoral tunnel preparation increases odds of repeat ipsilateral knee surgery. J Bone Joint Surg Am. 2013;95(22):2035-2042. doi:10.2106/JBJS.M.00187.
26. Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5(2):231-241. doi:10.1007/BF02032362.
27. Fries J, Rose M, Krishnan E. The PROMIS of better outcome assessment: responsiveness, floor and ceiling effects, and Internet administration. J Rheumatol. 2011;38(8):1759-1764. doi:10.3899/jrheum.110402.
28. Hancock KJ, Glass NA, Anthony CA, et al. Performance of PROMIS for healthy patients undergoing meniscal surgery. J Bone Joint Surg Am. 2017;99(11):954-958. doi:10.2106/JBJS.16.00848.
29. Hung M, Clegg Do, Greene T, et al. Evaluation of the PROMIS physical function item bank in orthopedic patients. J Orthop Res. 2011;29(6):947-953. doi:10.1002/jor.21308.
30. Hung M, Baumhauer JF, Brodsky JW, et al; Orthopaedic Foot & Ankle Outcomes Research (OFAR) of the American Orthopaedic Foot & Ankle Society (AOFAS). Psychometric comparison of the PROMIS physical function CAT with the FAAM and FFI for measuring patient-reported outcomes. Foot Ankle Int. 2014;35(6):592-599. doi:10.1177/1071100714528492.
31. Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized adaptive testing using the PROMIS physical function item bank reduces test burden with less ceiling effects compared with the short musculoskeletal function assessment in orthopaedic trauma patients. J Orthop Trauma. 2014;28(8):439-443. doi:10.1097/BOT.0000000000000059.
32. Hancock, et al. PROMIS: A valid and efficient outcomes instrument for patients with ACL tears. KSSTA. In press.
33. Scott, et al. Performance of PROMIS physical function compared with KOOS, SF-36, Eq5D, and Marx activity scale in patients who undergo ACL reconstruction. In press.
34. Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793-801. doi:10.1002/jor.22591.
35. Mohtadi N. Development and validation of the quality of life outcome measure (questionnaire) for chronic anterior cruciate ligament deficiency. Am J Sports Med. 1998;26(3):350-359. doi:10.1177/03635465980260030201.
36. Lafave MR, Hiemstra L, Kerslake S, Heard M, Buchko G. Validity, reliability, and responsiveness of the anterior cruciate ligament quality of life measure: a continuation of its overall validation. Clin J Sport Med. 2017;27(1):57-63. doi:10.1097/JSM.0000000000000292.
37. Noyes FR, McGinniss GH, Mooar LA. Functional disability in the anterior cruciate insufficient knee syndrome: Review of knee rating systems and projected risk factors in determining treatment. Sports Med. 1984;1(4):278-302. doi:10.2165/00007256-198401040-00004.
38. Noyes FR, Matthews DS, Mooar PA, Grood ES. The symptomatic anterior cruciate-deficient knee: Part II. The results of rehabilitation, activity modification, and counseling on functional disability. J Bone Joint Surg Am. 1983;65(2):163-174. doi:10.2106/00004623-198365020-00004.
39. Barber-Westin SD, Noyes FR, McCloskey JW. Rigorous statistical reliability, validity, and responsiveness testing of the Cincinnati knee rating system in 350 subjects with uninjured, injured, or anterior cruciate ligament-reconstructed knees. Am J Sports Med. 1999;27(4):402-416. doi:10.1177/03635465990270040201.
40. Bollen S, Seedhorn BB. A comparison of the Lysholm and Cincinnati knee scoring questionnaires. Am J Sports Med. 1991;19(2):189-190. doi:10.1177/036354659101900215.
41. Sgaglione NA, Del Pizzo W, Fox JM, Friedman MJ. Critical analysis of knee ligament rating systems. Am J Sports Med. 1995;23(6):660-667. doi:10.1177/036354659502300604.
42. Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am. 2001;83(10):1459-1469. doi:10.2106/00004623-200110000-00001.
43. Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10(3):150-154. doi:10.1177/036354658201000306.
44. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;198:43-49. doi:10.1097/00003086-198509000-00007.
45. Lukianov AV, Gillquist J, Grana WA, DeHaven KE. An anterior cruciate ligament (ACL) evaluation format for assessment of artificial or autologous anterior cruciate reconstruction results. Clin Orthop Relat Res. 1987;218:167-180. doi:10.1097/00003086-198705000-00024.
46. Bengtsson J, Mollborg J, Werner S. A study for testing the sensitivity and reliability of the Lysholm knee scoring scale. Knee Surg Sports Traumatol Arthrosc. 1996;4(1):27-31. doi:10.1007/BF01565994.
47. Risberg MA, Holm I, Steen J, Beynnon BD. Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc. 1999;7(3):152-159. doi:10.1007/s001670050140.
48. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29(5):600-613. doi:10.1177/03635465010290051301.
49. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34(10):1567-1573. doi:10.1177/0363546506288855.
50. Logerstedt D, Di Stasi S, Grindem H, et al. Self-reported knee function can identify athletes who fail return-to-activity criteria up to 1 year after anterior cruciate ligament reconstruction: a Delaware-Oslo ACL cohort study. J Orthop Sports Phys Ther. 2014;44(2):914-923. doi:10.2519/jospt.2014.4852.
51. Lentz TA, Zeppieri G Jr, George SZ, et al. Comparison of physical impairment, functional and psychosocial measures based on fear of reinjury/lack of confidence and return-to-sport status after ACL reconstruction. Am J Sports Med. 2015;43(2):345-353. doi:10.1177/0363546514559707.
52. Greco NJ, Anderson AF, Mann BJ, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form in comparison to the Western Ontario and McMaster Universities Osteoarthritis Index, modified Cincinnati Knee Rating System, and Short Form 36 in patients with focal articular cartilage defects. Am J Sports Med. 2010;38(5):891-902. doi:10.1177/0363546509354163.
53. Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1(3-4):226-234. doi:10.1007/BF01560215.
54. Schmitt LC, Paterno MV, Huang S. Validity and internal consistency of the International Knee Documentation Committee Subjective Knee Evaluation Form in children and adolescents. Am J Sports Med. 2010;38(12):2443-2447. doi:10.1177/0363546510374873.
55. Briggs KK, Lysholm J, Tegner Y, Rodkey WG, Kocher MS, Steadman JR. The reliability, validity, and responsiveness of the Lysholm and Tegner activity scale for anterior cruciate ligament injuries of the knee: 25 years later. Am J Sports Med. 2009;37(5):890-897. doi:10.1177/0363546508330143.
56. Negahban H, Mostafaee N, Sohani SM, et al. Reliability and validity of the Tegner and Marx activity rating scales in Iranian patients with anterior cruciate ligament injury. Disabil Rehabil. 2011;33(23-24):2305-2310. doi:10.3109/09638288.2011.570409.
57. Marx RG, Stump TJ, Jones EC, Wickiewicz TL, Warren RF. Development and evaluation of an activity rating scale for disorders of the knee. Am J Sports Med. 2001;29(2):213-218. doi:10.1177/03635465010290021601.
58. Garratt AM, Brealey S, Gillespie WJ, in collaboration with the DAM-ASK Trial Team. Patient-assessed health instruments for the knee: a structured review. Rheumatology. 2004;43(11):1414-1423. doi:10.1093/rheumatology/keh362.
59. American Academy of Orthopaedic Surgeons. Scoring algorithms for the lower limb: Outcomes data collection instrument. Rosemon, IL: American Academy of Orthopaedic Surgeons; 1998.
60. Johanson NA, Liang MH, Daltroy L, Rudicel S, Richmond J. American Academy of Orthopaedic Surgeons lower limb outcomes assessment instruments. Reliability, validity, and sensitivity to change. J Bone Joint Surg Am. 2004;86-A(5):902-909.
TAKE-HOME POINTS
- PRO instruments are widely used to capture patient perception of general health, QOL, daily function, and pain, and are an essential part of evaluation after ACL reconstruction.
- ACL outcome measures vary widely in regards to their validity, reliability, minimal clinically important difference, and applicability to specific patient populations.
- There is currently no standardized instrument universally accepted as superior following ACL reconstruction.
- In most cases, a general health outcome measure should be used in combination with a condition-specific rating scale.
- Activity rating scales, such as Marx or Tegner, should be included when evaluating patients with low-activity lifestyles.
Drs. O'Neil, Meadows, and Patterson Earn Annual AJO Resident Writer's Awards

Bryan Hanypsiak, MD, Editor-in-Chief of The American Journal of Orthopedics, along with Darla Conrad, Senior Director, North America, and Mindy Edgar, Manager, Academic Alliances from the Johnson & Johnson Institute presented the 2017 Resident Writer’s Award to the three winners at the American Academy of Orthopaedic Surgeons (AAOS) annual meeting in New Orleans. All articles published in 2017 with a resident as the first-listed author and accepted through the journal’s standard blinded-review process were eligible for this award. The annual Resident Writer's Award is sponsored by Johnson & Johnson. Papers published in 2018 will be judged by The American Journal of Orthopedics Editorial Board, and honoraria will be presented to the winners at the 2019 AAOS annual meeting.
Joseph T. O’Neil, MD
Molly C. Meadows, MD
Joseph T. Patterson, MD
See more information about the 2018 Resident Writer's Award.
Supported by Johnson & Johnson

Bryan Hanypsiak, MD, Editor-in-Chief of The American Journal of Orthopedics, along with Darla Conrad, Senior Director, North America, and Mindy Edgar, Manager, Academic Alliances from the Johnson & Johnson Institute presented the 2017 Resident Writer’s Award to the three winners at the American Academy of Orthopaedic Surgeons (AAOS) annual meeting in New Orleans. All articles published in 2017 with a resident as the first-listed author and accepted through the journal’s standard blinded-review process were eligible for this award. The annual Resident Writer's Award is sponsored by Johnson & Johnson. Papers published in 2018 will be judged by The American Journal of Orthopedics Editorial Board, and honoraria will be presented to the winners at the 2019 AAOS annual meeting.
Joseph T. O’Neil, MD
Molly C. Meadows, MD
Joseph T. Patterson, MD
See more information about the 2018 Resident Writer's Award.
Supported by Johnson & Johnson

Bryan Hanypsiak, MD, Editor-in-Chief of The American Journal of Orthopedics, along with Darla Conrad, Senior Director, North America, and Mindy Edgar, Manager, Academic Alliances from the Johnson & Johnson Institute presented the 2017 Resident Writer’s Award to the three winners at the American Academy of Orthopaedic Surgeons (AAOS) annual meeting in New Orleans. All articles published in 2017 with a resident as the first-listed author and accepted through the journal’s standard blinded-review process were eligible for this award. The annual Resident Writer's Award is sponsored by Johnson & Johnson. Papers published in 2018 will be judged by The American Journal of Orthopedics Editorial Board, and honoraria will be presented to the winners at the 2019 AAOS annual meeting.
Joseph T. O’Neil, MD
Molly C. Meadows, MD
Joseph T. Patterson, MD
See more information about the 2018 Resident Writer's Award.
Supported by Johnson & Johnson