User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
AJO Awards Molly C. Meadows, MD, Second-Place Resident Writer's Award
2017 AJO Resident Writer's Awards
Second-Place Award
An Original Study
Effects of Platelet-Rich Plasma and Indomethacin on Biomechanics of Rotator Cuff Repair
Molly C. Meadows, MD, David M. Levy, MD, Christopher M. Ferry, MS, Thomas R. Gardner, MCE, Takeshi Teratani, MD, and Christopher S. Ahmad, MD
Dr. Meadows is currently in her chief year of orthopedic surgery residency training at Rush University Medical Center. Prior to residency, she completed undergraduate education at Brown University and medical school at Columbia University. Dr. Meadows is beginning a sports medicine fellowship at Stanford University in August 2018, and she plans to pursue a pediatric orthopedic fellowship thereafter.
Her research interests include osteochondritis dissecans lesions, patellofemoral disorders, and other sports injuries in the skeletally immature population.
Read the full version of Dr. Meadows' original study.
2017 AJO Resident Writer's Awards
Second-Place Award
An Original Study
Effects of Platelet-Rich Plasma and Indomethacin on Biomechanics of Rotator Cuff Repair
Molly C. Meadows, MD, David M. Levy, MD, Christopher M. Ferry, MS, Thomas R. Gardner, MCE, Takeshi Teratani, MD, and Christopher S. Ahmad, MD
Dr. Meadows is currently in her chief year of orthopedic surgery residency training at Rush University Medical Center. Prior to residency, she completed undergraduate education at Brown University and medical school at Columbia University. Dr. Meadows is beginning a sports medicine fellowship at Stanford University in August 2018, and she plans to pursue a pediatric orthopedic fellowship thereafter.
Her research interests include osteochondritis dissecans lesions, patellofemoral disorders, and other sports injuries in the skeletally immature population.
Read the full version of Dr. Meadows' original study.
2017 AJO Resident Writer's Awards
Second-Place Award
An Original Study
Effects of Platelet-Rich Plasma and Indomethacin on Biomechanics of Rotator Cuff Repair
Molly C. Meadows, MD, David M. Levy, MD, Christopher M. Ferry, MS, Thomas R. Gardner, MCE, Takeshi Teratani, MD, and Christopher S. Ahmad, MD
Dr. Meadows is currently in her chief year of orthopedic surgery residency training at Rush University Medical Center. Prior to residency, she completed undergraduate education at Brown University and medical school at Columbia University. Dr. Meadows is beginning a sports medicine fellowship at Stanford University in August 2018, and she plans to pursue a pediatric orthopedic fellowship thereafter.
Her research interests include osteochondritis dissecans lesions, patellofemoral disorders, and other sports injuries in the skeletally immature population.
Read the full version of Dr. Meadows' original study.
AJO Awards Joseph T. Patterson, MD, Third-Place Resident Writer's Award
2017 AJO Resident Writer's Awards
Third-Place Award
An Original Study
Does Preoperative Pneumonia Affect Complications of Geriatric Hip Fracture Surgery?
Joseph T. Patterson, MD, Daniel D. Bohl, MD, MPH, Bryce A. Basques, MD, Alexander H. Arzeno, MD, and Jonathan Grauer, MD
Dr. Patterson is completing his orthopedic surgery residency at the University of California San Francisco, and will continue training with a fellowship in orthopedic trauma at Harborview Medical Center. Prior to residency, he completed undergraduate education at the University of California Los Angeles and medical school at Yale University.
His research interests include geriatric hip fracture care, interdisciplinary trauma care performance improvement, and outcome assessment in orthopedic trauma.
Read the full version of Dr. Patterson's original study.
2017 AJO Resident Writer's Awards
Third-Place Award
An Original Study
Does Preoperative Pneumonia Affect Complications of Geriatric Hip Fracture Surgery?
Joseph T. Patterson, MD, Daniel D. Bohl, MD, MPH, Bryce A. Basques, MD, Alexander H. Arzeno, MD, and Jonathan Grauer, MD
Dr. Patterson is completing his orthopedic surgery residency at the University of California San Francisco, and will continue training with a fellowship in orthopedic trauma at Harborview Medical Center. Prior to residency, he completed undergraduate education at the University of California Los Angeles and medical school at Yale University.
His research interests include geriatric hip fracture care, interdisciplinary trauma care performance improvement, and outcome assessment in orthopedic trauma.
Read the full version of Dr. Patterson's original study.
2017 AJO Resident Writer's Awards
Third-Place Award
An Original Study
Does Preoperative Pneumonia Affect Complications of Geriatric Hip Fracture Surgery?
Joseph T. Patterson, MD, Daniel D. Bohl, MD, MPH, Bryce A. Basques, MD, Alexander H. Arzeno, MD, and Jonathan Grauer, MD
Dr. Patterson is completing his orthopedic surgery residency at the University of California San Francisco, and will continue training with a fellowship in orthopedic trauma at Harborview Medical Center. Prior to residency, he completed undergraduate education at the University of California Los Angeles and medical school at Yale University.
His research interests include geriatric hip fracture care, interdisciplinary trauma care performance improvement, and outcome assessment in orthopedic trauma.
Read the full version of Dr. Patterson's original study.
AJO Awards Joseph T. O'Neil, MD, First-Place Resident Writer's Award
2017 AJO Resident Writer's Awards
First-Place Award
An Original Study
Prospective Evaluation of Opioid Consumption After Distal Radius Fracture Repair Surgery
Joseph T. O’Neil, MD, Mark L. Wang, MD, PhD, Nayoung Kim, BS, Mitchell Maltenfort, PhD, and Asif M. Ilyas, MD
Dr. O'Neil completed his orthopedic surgery residency training at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania. Prior to residency, he completed undergraduate education at the University of Notre Dame and medical school at Thomas Jefferson University. He was born and raised in the Philadelphia area.
Dr. O'Neil is currently an orthopedic foot and ankle surgery fellow at Union Memorial Hospital in Baltimore, Maryland.
His research interests include total ankle arthroplasty; the diagnosis, treatment, and prevention of periprosthetic joint infection in the ankle; as well as helping to combat the opioid epidemic in the United States by better understanding patterns of prescribing and use following common orthopedic surgical procedures.
Read the full version of Dr. O'Neil's original study.
2017 AJO Resident Writer's Awards
First-Place Award
An Original Study
Prospective Evaluation of Opioid Consumption After Distal Radius Fracture Repair Surgery
Joseph T. O’Neil, MD, Mark L. Wang, MD, PhD, Nayoung Kim, BS, Mitchell Maltenfort, PhD, and Asif M. Ilyas, MD
Dr. O'Neil completed his orthopedic surgery residency training at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania. Prior to residency, he completed undergraduate education at the University of Notre Dame and medical school at Thomas Jefferson University. He was born and raised in the Philadelphia area.
Dr. O'Neil is currently an orthopedic foot and ankle surgery fellow at Union Memorial Hospital in Baltimore, Maryland.
His research interests include total ankle arthroplasty; the diagnosis, treatment, and prevention of periprosthetic joint infection in the ankle; as well as helping to combat the opioid epidemic in the United States by better understanding patterns of prescribing and use following common orthopedic surgical procedures.
Read the full version of Dr. O'Neil's original study.
2017 AJO Resident Writer's Awards
First-Place Award
An Original Study
Prospective Evaluation of Opioid Consumption After Distal Radius Fracture Repair Surgery
Joseph T. O’Neil, MD, Mark L. Wang, MD, PhD, Nayoung Kim, BS, Mitchell Maltenfort, PhD, and Asif M. Ilyas, MD
Dr. O'Neil completed his orthopedic surgery residency training at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania. Prior to residency, he completed undergraduate education at the University of Notre Dame and medical school at Thomas Jefferson University. He was born and raised in the Philadelphia area.
Dr. O'Neil is currently an orthopedic foot and ankle surgery fellow at Union Memorial Hospital in Baltimore, Maryland.
His research interests include total ankle arthroplasty; the diagnosis, treatment, and prevention of periprosthetic joint infection in the ankle; as well as helping to combat the opioid epidemic in the United States by better understanding patterns of prescribing and use following common orthopedic surgical procedures.
Read the full version of Dr. O'Neil's original study.
2018 Resident Writer’s Award Information
The 2018 Resident Writer’s Award competition is sponsored by Johnson & Johnson. Orthopedic residents are invited to submit original studies, review papers, or case reports for publication. Papers published in 2018 will be judged by The American Journal of Orthopedics Editorial Board. Honoraria will be presented to the winners at the 2019 AAOS annual meeting.
- $1,500 for the First-Place Award
- $1,000 for the Second-Place Award
- $500 for the Third-Place Award
To quality for consideration, papers must have the resident as the first-listed author and must be accepted through the journal’s standard blinded-review process. Papers submitted in 2018 but not published until 2019 will automatically qualify for the 2019 competition. Manuscripts should be prepared according to our Information for the Authors and submitted via our online submission system, Editorial Manager®, at www.editorialmanager.com/AmJOrthop.
Read more about this year's RWA winners.
Supported by Johnson & Johnson
The 2018 Resident Writer’s Award competition is sponsored by Johnson & Johnson. Orthopedic residents are invited to submit original studies, review papers, or case reports for publication. Papers published in 2018 will be judged by The American Journal of Orthopedics Editorial Board. Honoraria will be presented to the winners at the 2019 AAOS annual meeting.
- $1,500 for the First-Place Award
- $1,000 for the Second-Place Award
- $500 for the Third-Place Award
To quality for consideration, papers must have the resident as the first-listed author and must be accepted through the journal’s standard blinded-review process. Papers submitted in 2018 but not published until 2019 will automatically qualify for the 2019 competition. Manuscripts should be prepared according to our Information for the Authors and submitted via our online submission system, Editorial Manager®, at www.editorialmanager.com/AmJOrthop.
Read more about this year's RWA winners.
Supported by Johnson & Johnson
The 2018 Resident Writer’s Award competition is sponsored by Johnson & Johnson. Orthopedic residents are invited to submit original studies, review papers, or case reports for publication. Papers published in 2018 will be judged by The American Journal of Orthopedics Editorial Board. Honoraria will be presented to the winners at the 2019 AAOS annual meeting.
- $1,500 for the First-Place Award
- $1,000 for the Second-Place Award
- $500 for the Third-Place Award
To quality for consideration, papers must have the resident as the first-listed author and must be accepted through the journal’s standard blinded-review process. Papers submitted in 2018 but not published until 2019 will automatically qualify for the 2019 competition. Manuscripts should be prepared according to our Information for the Authors and submitted via our online submission system, Editorial Manager®, at www.editorialmanager.com/AmJOrthop.
Read more about this year's RWA winners.
Supported by Johnson & Johnson
Proximal Humerus Fracture 3-D Modeling
ABSTRACT
The objective of this study is to determine the reproducibility and feasibility of using 3-dimensional (3-D) computer simulation of proximal humerus fracture computed tomography (CT) scans for fracture reduction. We hypothesized that anatomic reconstruction with 3-D models would be anatomically accurate and reproducible.
Preoperative CT scans of 28 patients with 3- and 4-part (AO classification 11-B1, 11-B2, 11-C1, 11-C2) proximal humerus fractures who were treated by hemiarthroplasty were converted into 3-D computer models. The displaced fractured fragments were anatomically reduced with computer simulation by 2 fellowship-trained shoulder surgeons, and measurements were made of the reconstructed proximal humerus.
The measurements of the reconstructed models had very good to excellent interobserver and intraobserver reliability. The reconstructions of these humerus fractures showed interclass correlation coefficients ranging from 0.71 to 0.93 between 1 observer and from 0.82 to 0.98 between 2 different observers. The fracture reduction was judged against normal proximal humerus geometry to determine reduction accuracy.
The 3-D modeling techniques used to reconstruct 3- and 4-part proximal humerus fractures were reliable and accurate. This technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of open reduction and internal fixation or hemiarthroplasty for 3- and 4-part proximal humerus fractures.
The treatment of proximal humerus fractures is influenced by multiple factors, including patient age, associated injuries, bone quality, and fracture pattern. Three- and 4-part fractures are among the more severe of these fractures, which may result in vascular compromise to the humeral head, leading to avascular necrosis. Surgical goals for the management of these fractures are to optimize functional outcomes by re-creating a stable construct with a functional rotator cuff by open reduction and internal fixation (ORIF), hemiarthroplasty with tuberosity ORIF, or reverse shoulder replacement. Achieving a good outcome following hemiarthroplasty is dependent on many factors, including anatomic tuberosity healing and component positioning.1,2,3 Repairing the greater tuberosity in a near-anatomic position has been shown to greatly affect the results of hemiarthroplasty for fracture.3,4
Continue to: Three-dimensional (3-D) modeling...
Three-dimensional (3-D) modeling is increasingly being used in preoperative planning of shoulder arthroplasty and determining proper proximal humeral fracture treatment. 5 However, no studies have examined the reconstruction of a fractured proximal humerus into native anatomy using computer simulation. The purpose of this study is to determine the accuracy and reliability of anatomically reconstructing the preinjury proximal humerus using 3-D computer models created from postinjury computed tomography (CT) scans. The results of this study could lead to useful techniques employing CT–based models for patient-specific preoperative planning of proximal humeral fracture ORIF and during tuberosity reduction and fixation during hemiarthroplasty for fracture. We hypothesize that it is feasible to reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures with high reliability based on interobserver and intraobserver review.
METHODS
After Institutional Review Board approval was obtained, we reviewed the medical records of consecutive patients with a diagnosis of proximal humeral fracture and the treatment codes for hemiarthroplasty from 2000 to 2013. Inclusion criteria included 3- and 4-part fractures (AO classifications 11-B1, 11-B2, 11-C1, 11-C2). CT scans with insufficient quality to differentiate bone from soft tissue (inadequate signal-to-noise ratio) were excluded from the study. A total of 28 patients with adequate CT scans met the criteria for inclusion in this study.
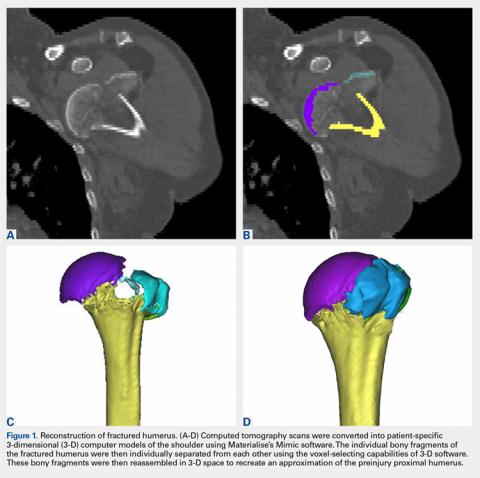
The CT scan protocol included 0.5-mm axial cuts with inclusion of the proximal humerus in the Digital Imaging and Communications in Medicine format. These CT scans were converted into patient-specific 3-D computer models of the shoulder using Mimics software (Materialise Inc.). The use of this software to produce anatomically accurate models has previously been verified in a shoulder model.6,7 The tuberosity fragments were then individually separated from each other using the voxel-selecting capabilities of 3-D software and manipulated with translation and rotation for anatomic reduction (Figures 1A-1D, Figure 2).
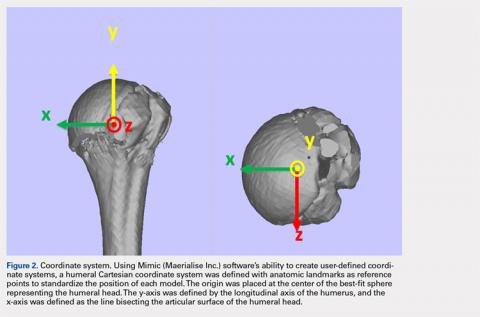
The de-identified anatomically reconstructed shoulder models were then uploaded into Materialise’s Magics rapid prototyping software, and a user-defined humeral Cartesian coordinate system was defined with anatomic landmarks as reference points to standardize the position of each model (Figure 3).8,9
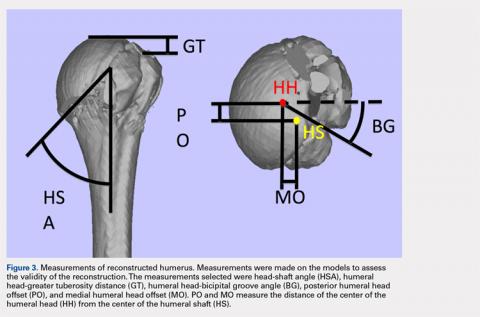
A series of measurements were made on these models to assess the validity and reliability of the reassembly. The bicipital groove at the anatomic neck was used to measure humeral head version as described by Kummer and colleagues.10 The head-shaft angle, humeral head-greater tuberosity distance, humeral head-bicipital groove angle, and posterior and medial humeral head offset were measured directly on the reconstructed humerus.
Continue to: Two fellowship-trained shoulder...
Two fellowship-trained shoulder surgeons independently reassembled these fracture fragments via computer simulation. Interobserver reliability testing was conducted on these reconstructions by measuring the geometry between the 2 different surgeons’ reconstructions. Intraobserver reliability testing was conducted by 1 surgeon repeating the reconstructions with 4-week intervals between trials and measuring the geometry between the 2 different trials. The average dimensions of the reconstructed proximal humerus fractures were compared with the geometry of normal humeri reported in previously conducted anatomic studies.11,12,13
STATISTICS
The measured dimensions of the 28 reassembled proximal humeri models were averaged across all trials between the 2 fellowship-trained surgeons and compared with the range of normal dimensions of a healthy proximal humerus using the 2 one-sided tests (TOST) method for equivalence between 2 means given a range. The interobserver and intraobserver reliabilities were quantified using the interclass correlation coefficient. An excellent correlation was defined as a correlation coefficient >0.81; very good was defined as 0.61 to 0.80; and good was defined as 0.41 to 0.60.
RESULTS
Of the patients studied, 9 (32.1%) were male, and the average age at the time of CT scanning was 72 years. Of the 28 patients with fracture, 18 (64.2%) had 3-part fractures (AO classifications 11-B1, 11-B2), and 10 (35.8%) had 4-part fractures (AO classifications 11-C1, 11-C2). When examining the location of the intertubercular fracture line, we found that 13 (46.4%) fractures went through the bicipital groove. Of the remaining fracture lines, 9 (32.1%) extended into the greater tuberosity and 6 (21.4%) extended into the lesser tuberosity.
All users were able to reconstruct all 28 fractures using this technique. The average measured dimensions fell within the range of dimensions of a normal healthy proximal humerus specified in the literature to within a 95% confidence interval using the TOST for equivalence, in which we compared measured values with ranges reported in the literature (Table).11,12,13
Table. Dimensions of Proximal Humerus Geometry
| Normal Parameters | Average Dimensions From Trials | Dimensions From Literature |
| Head shaft angle | 43.5° ± 1° | 42.5° ± 12.5° |
| Head to greater tuberosity distance | 4.9 mm ± 0.4 mm | 8 mm ± 3.2 mm |
Head to bicipital groove angle (anatomic neck) | 26.4° ± 2° | 27.3° ± 14° |
| Posterior humeral head offset | 1.6 mm ± 0.3 mm | 4 mm ± 6 mm |
| Medial humeral head offset | 4.5 mm ± 0.3 mm | 9 mm ± 5 mm |
The reconstructions of these humerus fractures showed intraclass correlation coefficients ranging from 0.71 to 0.93 in 1 observer and interclass correlation coefficients from 0.82 to 0.98 between 2 different observers (Table).
DISCUSSION
This study demonstrates that it is feasible to reliably and accurately reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures. Poor outcomes after hemiarthroplasty for proximal humerus fractures are mostly related to tuberosity malpositioning, resorption, or failure of fixation and resultant dysfunction of the rotator cuff.14,15,16 These studies highlight the importance of accurate tuberosity reduction during surgical care of these fractures.
Continue to: The 3-D computer model...
The 3-D computer model reconstruction of 3- and 4-part proximal humerus fractures were reliable and valid. The interclass correlation coefficients showed very good to excellent interobserver and intraobserver reliability for all measurements conducted. The averaged dimensions from all trials fell within the appropriate range of dimensions for a normal healthy humerus reported in the literature, as verified by the TOST method.11,12,13 The 3-D modeling capabilities demonstrated in this study allowed a greater understanding of the fracture patterns present in 3- and 4-part (AO classifications 11-B1, 11-B2, 11-C1, 11-C2) humerus fractures.
Overreduction of greater tuberosity to create cortical overlap with the lateral shaft may be used to promote bony union. As a result of this distalization, there may be extra strains placed on the rotator cuff, making the patient more prone to rotator cuff tear, as well as improperly balancing the dynamic stabilizers of the shoulder. Poor clinical outcomes in hemiarthroplasty for proximal humerus fractures have been correlated with a greater tuberosity placed distal relative to the humeral head by 1 cm in a study2 and by 2 cm in another.3
This study has several limitations. The first is the assumption that our injured patients had preinjury proximal humerus geometry within the range of normal dimensions of a healthy humerus. Unfortunately, because we were unable to obtain CT scans of the contralateral shoulder, we had to use standard proximal humerus geometry as the control. Another limitation, inherent in the technique, is that only cortical and dense trabecular bone was modeled, so that comminuted or osteoporotic bone was not well modeled. This study did not correlate the findings from these models with clinical outcomes. A prospective study is needed to evaluate the impact of this 3-D modeling on fracture reductions and clinical outcomes.
This study demonstrates that patient-specific modeling of proximal humerus fracture 3-D CT scans may help surgeons reliably and accurately reconstruct fractures. This technique may have utility in the preoperative planning of tuberosity fracture reduction and hemiarthroplasty. It gives surgeons the ability to visualize fracture fragments, and the process of reconstructing the fragments may help surgeons understand the required maneuvers for reduction at the time of surgery. This technique also provides dimensions of the patient’s native humerus, thus potentially improving the anatomic accuracy of the reduction or hemiarthroplasty reconstruction. With the new trend toward patient-specific instrumentation, this study also provides a means of planning the size of the humeral prostheses as well as the version relative to the biceps groove and intertubercular fracture line.
CONCLUSION
This study demonstrates the feasibility of using 3-D computer modeling of complex proximal humerus fractures in anatomic reconstruction. These techniques of computer-simulated 3-D models are valid and reliable. We believe that this technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of hemiarthroplasty for 3- and 4-part proximal humerus fractures by providing improved understanding of the patient’s native humeral geometry and tuberosity reduction.
1. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412. doi:10.1067/mse.2002.124527.
2. Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):569-577. doi:10.1016/S1058274603002131.
3. Greiner SH, Kaab MJ, Kroning I, Scheibel M, Perka C. Reconstruction of humeral length and centering of the prosthetic head in hemiarthroplasty for proximal humeral fractures. J Shoulder Elbow Surg. 2008;17(5):709-714. doi:10.1016/j.jse.2008.03.004.
4. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24. doi:10.1016/j.jse.2006.05.008.
5. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491. doi:10.1016/j.jse.2007.09.006.
6. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832. doi:10.1016/j.jse.2008.01.141.
7. Yongpravat C, Kim HM, Gardner TR, Bigliani LU, Levine WN, Ahmad CS. Glenoid implant orientation and cement failure in total shoulder arthroplasty: a finite element analysis. J Shoulder Elbow Surg. 2013;22(7):940-947. doi:10.1016/j.jse.2012.09.007.
8. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865. doi:10.1302/0301-620X.79B5.0790857.
9. Wu G, van der Helm FC, Veeger HE, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981-992.
10. Kummer FJ, Perkins R, Zuckerman JD. The use of the bicipital groove for alignment of the humeral stem in shoulder arthroplasty. J Shoulder Elbow Surg. 1998;7(2):144-146. doi:10.1016/S1058-2746(98)90225-7.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326. doi:10.1016/S1058-2746(96)80060-7.
13. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(1 Suppl S):99S-104S. doi:10.1016/j.jse.2004.09.025.
14. Prakash U, McGurty DW, Dent JA. Hemiarthroplasty for severe fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):428-430. doi:10.1067/mse.2002.126615.
15. Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85-A(7):1215-1223.
16. Zyto K, Wallace WA, Frostick SP, Preston BJ. Outcome after hemiarthroplasty for three- and four-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1998;7(2):85-89. doi:10.1016/S1058-2746(98)90215-4.
ABSTRACT
The objective of this study is to determine the reproducibility and feasibility of using 3-dimensional (3-D) computer simulation of proximal humerus fracture computed tomography (CT) scans for fracture reduction. We hypothesized that anatomic reconstruction with 3-D models would be anatomically accurate and reproducible.
Preoperative CT scans of 28 patients with 3- and 4-part (AO classification 11-B1, 11-B2, 11-C1, 11-C2) proximal humerus fractures who were treated by hemiarthroplasty were converted into 3-D computer models. The displaced fractured fragments were anatomically reduced with computer simulation by 2 fellowship-trained shoulder surgeons, and measurements were made of the reconstructed proximal humerus.
The measurements of the reconstructed models had very good to excellent interobserver and intraobserver reliability. The reconstructions of these humerus fractures showed interclass correlation coefficients ranging from 0.71 to 0.93 between 1 observer and from 0.82 to 0.98 between 2 different observers. The fracture reduction was judged against normal proximal humerus geometry to determine reduction accuracy.
The 3-D modeling techniques used to reconstruct 3- and 4-part proximal humerus fractures were reliable and accurate. This technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of open reduction and internal fixation or hemiarthroplasty for 3- and 4-part proximal humerus fractures.
The treatment of proximal humerus fractures is influenced by multiple factors, including patient age, associated injuries, bone quality, and fracture pattern. Three- and 4-part fractures are among the more severe of these fractures, which may result in vascular compromise to the humeral head, leading to avascular necrosis. Surgical goals for the management of these fractures are to optimize functional outcomes by re-creating a stable construct with a functional rotator cuff by open reduction and internal fixation (ORIF), hemiarthroplasty with tuberosity ORIF, or reverse shoulder replacement. Achieving a good outcome following hemiarthroplasty is dependent on many factors, including anatomic tuberosity healing and component positioning.1,2,3 Repairing the greater tuberosity in a near-anatomic position has been shown to greatly affect the results of hemiarthroplasty for fracture.3,4
Continue to: Three-dimensional (3-D) modeling...
Three-dimensional (3-D) modeling is increasingly being used in preoperative planning of shoulder arthroplasty and determining proper proximal humeral fracture treatment. 5 However, no studies have examined the reconstruction of a fractured proximal humerus into native anatomy using computer simulation. The purpose of this study is to determine the accuracy and reliability of anatomically reconstructing the preinjury proximal humerus using 3-D computer models created from postinjury computed tomography (CT) scans. The results of this study could lead to useful techniques employing CT–based models for patient-specific preoperative planning of proximal humeral fracture ORIF and during tuberosity reduction and fixation during hemiarthroplasty for fracture. We hypothesize that it is feasible to reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures with high reliability based on interobserver and intraobserver review.
METHODS
After Institutional Review Board approval was obtained, we reviewed the medical records of consecutive patients with a diagnosis of proximal humeral fracture and the treatment codes for hemiarthroplasty from 2000 to 2013. Inclusion criteria included 3- and 4-part fractures (AO classifications 11-B1, 11-B2, 11-C1, 11-C2). CT scans with insufficient quality to differentiate bone from soft tissue (inadequate signal-to-noise ratio) were excluded from the study. A total of 28 patients with adequate CT scans met the criteria for inclusion in this study.
The CT scan protocol included 0.5-mm axial cuts with inclusion of the proximal humerus in the Digital Imaging and Communications in Medicine format. These CT scans were converted into patient-specific 3-D computer models of the shoulder using Mimics software (Materialise Inc.). The use of this software to produce anatomically accurate models has previously been verified in a shoulder model.6,7 The tuberosity fragments were then individually separated from each other using the voxel-selecting capabilities of 3-D software and manipulated with translation and rotation for anatomic reduction (Figures 1A-1D, Figure 2).
The de-identified anatomically reconstructed shoulder models were then uploaded into Materialise’s Magics rapid prototyping software, and a user-defined humeral Cartesian coordinate system was defined with anatomic landmarks as reference points to standardize the position of each model (Figure 3).8,9
A series of measurements were made on these models to assess the validity and reliability of the reassembly. The bicipital groove at the anatomic neck was used to measure humeral head version as described by Kummer and colleagues.10 The head-shaft angle, humeral head-greater tuberosity distance, humeral head-bicipital groove angle, and posterior and medial humeral head offset were measured directly on the reconstructed humerus.
Continue to: Two fellowship-trained shoulder...
Two fellowship-trained shoulder surgeons independently reassembled these fracture fragments via computer simulation. Interobserver reliability testing was conducted on these reconstructions by measuring the geometry between the 2 different surgeons’ reconstructions. Intraobserver reliability testing was conducted by 1 surgeon repeating the reconstructions with 4-week intervals between trials and measuring the geometry between the 2 different trials. The average dimensions of the reconstructed proximal humerus fractures were compared with the geometry of normal humeri reported in previously conducted anatomic studies.11,12,13
STATISTICS
The measured dimensions of the 28 reassembled proximal humeri models were averaged across all trials between the 2 fellowship-trained surgeons and compared with the range of normal dimensions of a healthy proximal humerus using the 2 one-sided tests (TOST) method for equivalence between 2 means given a range. The interobserver and intraobserver reliabilities were quantified using the interclass correlation coefficient. An excellent correlation was defined as a correlation coefficient >0.81; very good was defined as 0.61 to 0.80; and good was defined as 0.41 to 0.60.
RESULTS
Of the patients studied, 9 (32.1%) were male, and the average age at the time of CT scanning was 72 years. Of the 28 patients with fracture, 18 (64.2%) had 3-part fractures (AO classifications 11-B1, 11-B2), and 10 (35.8%) had 4-part fractures (AO classifications 11-C1, 11-C2). When examining the location of the intertubercular fracture line, we found that 13 (46.4%) fractures went through the bicipital groove. Of the remaining fracture lines, 9 (32.1%) extended into the greater tuberosity and 6 (21.4%) extended into the lesser tuberosity.
All users were able to reconstruct all 28 fractures using this technique. The average measured dimensions fell within the range of dimensions of a normal healthy proximal humerus specified in the literature to within a 95% confidence interval using the TOST for equivalence, in which we compared measured values with ranges reported in the literature (Table).11,12,13
Table. Dimensions of Proximal Humerus Geometry
| Normal Parameters | Average Dimensions From Trials | Dimensions From Literature |
| Head shaft angle | 43.5° ± 1° | 42.5° ± 12.5° |
| Head to greater tuberosity distance | 4.9 mm ± 0.4 mm | 8 mm ± 3.2 mm |
Head to bicipital groove angle (anatomic neck) | 26.4° ± 2° | 27.3° ± 14° |
| Posterior humeral head offset | 1.6 mm ± 0.3 mm | 4 mm ± 6 mm |
| Medial humeral head offset | 4.5 mm ± 0.3 mm | 9 mm ± 5 mm |
The reconstructions of these humerus fractures showed intraclass correlation coefficients ranging from 0.71 to 0.93 in 1 observer and interclass correlation coefficients from 0.82 to 0.98 between 2 different observers (Table).
DISCUSSION
This study demonstrates that it is feasible to reliably and accurately reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures. Poor outcomes after hemiarthroplasty for proximal humerus fractures are mostly related to tuberosity malpositioning, resorption, or failure of fixation and resultant dysfunction of the rotator cuff.14,15,16 These studies highlight the importance of accurate tuberosity reduction during surgical care of these fractures.
Continue to: The 3-D computer model...
The 3-D computer model reconstruction of 3- and 4-part proximal humerus fractures were reliable and valid. The interclass correlation coefficients showed very good to excellent interobserver and intraobserver reliability for all measurements conducted. The averaged dimensions from all trials fell within the appropriate range of dimensions for a normal healthy humerus reported in the literature, as verified by the TOST method.11,12,13 The 3-D modeling capabilities demonstrated in this study allowed a greater understanding of the fracture patterns present in 3- and 4-part (AO classifications 11-B1, 11-B2, 11-C1, 11-C2) humerus fractures.
Overreduction of greater tuberosity to create cortical overlap with the lateral shaft may be used to promote bony union. As a result of this distalization, there may be extra strains placed on the rotator cuff, making the patient more prone to rotator cuff tear, as well as improperly balancing the dynamic stabilizers of the shoulder. Poor clinical outcomes in hemiarthroplasty for proximal humerus fractures have been correlated with a greater tuberosity placed distal relative to the humeral head by 1 cm in a study2 and by 2 cm in another.3
This study has several limitations. The first is the assumption that our injured patients had preinjury proximal humerus geometry within the range of normal dimensions of a healthy humerus. Unfortunately, because we were unable to obtain CT scans of the contralateral shoulder, we had to use standard proximal humerus geometry as the control. Another limitation, inherent in the technique, is that only cortical and dense trabecular bone was modeled, so that comminuted or osteoporotic bone was not well modeled. This study did not correlate the findings from these models with clinical outcomes. A prospective study is needed to evaluate the impact of this 3-D modeling on fracture reductions and clinical outcomes.
This study demonstrates that patient-specific modeling of proximal humerus fracture 3-D CT scans may help surgeons reliably and accurately reconstruct fractures. This technique may have utility in the preoperative planning of tuberosity fracture reduction and hemiarthroplasty. It gives surgeons the ability to visualize fracture fragments, and the process of reconstructing the fragments may help surgeons understand the required maneuvers for reduction at the time of surgery. This technique also provides dimensions of the patient’s native humerus, thus potentially improving the anatomic accuracy of the reduction or hemiarthroplasty reconstruction. With the new trend toward patient-specific instrumentation, this study also provides a means of planning the size of the humeral prostheses as well as the version relative to the biceps groove and intertubercular fracture line.
CONCLUSION
This study demonstrates the feasibility of using 3-D computer modeling of complex proximal humerus fractures in anatomic reconstruction. These techniques of computer-simulated 3-D models are valid and reliable. We believe that this technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of hemiarthroplasty for 3- and 4-part proximal humerus fractures by providing improved understanding of the patient’s native humeral geometry and tuberosity reduction.
ABSTRACT
The objective of this study is to determine the reproducibility and feasibility of using 3-dimensional (3-D) computer simulation of proximal humerus fracture computed tomography (CT) scans for fracture reduction. We hypothesized that anatomic reconstruction with 3-D models would be anatomically accurate and reproducible.
Preoperative CT scans of 28 patients with 3- and 4-part (AO classification 11-B1, 11-B2, 11-C1, 11-C2) proximal humerus fractures who were treated by hemiarthroplasty were converted into 3-D computer models. The displaced fractured fragments were anatomically reduced with computer simulation by 2 fellowship-trained shoulder surgeons, and measurements were made of the reconstructed proximal humerus.
The measurements of the reconstructed models had very good to excellent interobserver and intraobserver reliability. The reconstructions of these humerus fractures showed interclass correlation coefficients ranging from 0.71 to 0.93 between 1 observer and from 0.82 to 0.98 between 2 different observers. The fracture reduction was judged against normal proximal humerus geometry to determine reduction accuracy.
The 3-D modeling techniques used to reconstruct 3- and 4-part proximal humerus fractures were reliable and accurate. This technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of open reduction and internal fixation or hemiarthroplasty for 3- and 4-part proximal humerus fractures.
The treatment of proximal humerus fractures is influenced by multiple factors, including patient age, associated injuries, bone quality, and fracture pattern. Three- and 4-part fractures are among the more severe of these fractures, which may result in vascular compromise to the humeral head, leading to avascular necrosis. Surgical goals for the management of these fractures are to optimize functional outcomes by re-creating a stable construct with a functional rotator cuff by open reduction and internal fixation (ORIF), hemiarthroplasty with tuberosity ORIF, or reverse shoulder replacement. Achieving a good outcome following hemiarthroplasty is dependent on many factors, including anatomic tuberosity healing and component positioning.1,2,3 Repairing the greater tuberosity in a near-anatomic position has been shown to greatly affect the results of hemiarthroplasty for fracture.3,4
Continue to: Three-dimensional (3-D) modeling...
Three-dimensional (3-D) modeling is increasingly being used in preoperative planning of shoulder arthroplasty and determining proper proximal humeral fracture treatment. 5 However, no studies have examined the reconstruction of a fractured proximal humerus into native anatomy using computer simulation. The purpose of this study is to determine the accuracy and reliability of anatomically reconstructing the preinjury proximal humerus using 3-D computer models created from postinjury computed tomography (CT) scans. The results of this study could lead to useful techniques employing CT–based models for patient-specific preoperative planning of proximal humeral fracture ORIF and during tuberosity reduction and fixation during hemiarthroplasty for fracture. We hypothesize that it is feasible to reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures with high reliability based on interobserver and intraobserver review.
METHODS
After Institutional Review Board approval was obtained, we reviewed the medical records of consecutive patients with a diagnosis of proximal humeral fracture and the treatment codes for hemiarthroplasty from 2000 to 2013. Inclusion criteria included 3- and 4-part fractures (AO classifications 11-B1, 11-B2, 11-C1, 11-C2). CT scans with insufficient quality to differentiate bone from soft tissue (inadequate signal-to-noise ratio) were excluded from the study. A total of 28 patients with adequate CT scans met the criteria for inclusion in this study.
The CT scan protocol included 0.5-mm axial cuts with inclusion of the proximal humerus in the Digital Imaging and Communications in Medicine format. These CT scans were converted into patient-specific 3-D computer models of the shoulder using Mimics software (Materialise Inc.). The use of this software to produce anatomically accurate models has previously been verified in a shoulder model.6,7 The tuberosity fragments were then individually separated from each other using the voxel-selecting capabilities of 3-D software and manipulated with translation and rotation for anatomic reduction (Figures 1A-1D, Figure 2).
The de-identified anatomically reconstructed shoulder models were then uploaded into Materialise’s Magics rapid prototyping software, and a user-defined humeral Cartesian coordinate system was defined with anatomic landmarks as reference points to standardize the position of each model (Figure 3).8,9
A series of measurements were made on these models to assess the validity and reliability of the reassembly. The bicipital groove at the anatomic neck was used to measure humeral head version as described by Kummer and colleagues.10 The head-shaft angle, humeral head-greater tuberosity distance, humeral head-bicipital groove angle, and posterior and medial humeral head offset were measured directly on the reconstructed humerus.
Continue to: Two fellowship-trained shoulder...
Two fellowship-trained shoulder surgeons independently reassembled these fracture fragments via computer simulation. Interobserver reliability testing was conducted on these reconstructions by measuring the geometry between the 2 different surgeons’ reconstructions. Intraobserver reliability testing was conducted by 1 surgeon repeating the reconstructions with 4-week intervals between trials and measuring the geometry between the 2 different trials. The average dimensions of the reconstructed proximal humerus fractures were compared with the geometry of normal humeri reported in previously conducted anatomic studies.11,12,13
STATISTICS
The measured dimensions of the 28 reassembled proximal humeri models were averaged across all trials between the 2 fellowship-trained surgeons and compared with the range of normal dimensions of a healthy proximal humerus using the 2 one-sided tests (TOST) method for equivalence between 2 means given a range. The interobserver and intraobserver reliabilities were quantified using the interclass correlation coefficient. An excellent correlation was defined as a correlation coefficient >0.81; very good was defined as 0.61 to 0.80; and good was defined as 0.41 to 0.60.
RESULTS
Of the patients studied, 9 (32.1%) were male, and the average age at the time of CT scanning was 72 years. Of the 28 patients with fracture, 18 (64.2%) had 3-part fractures (AO classifications 11-B1, 11-B2), and 10 (35.8%) had 4-part fractures (AO classifications 11-C1, 11-C2). When examining the location of the intertubercular fracture line, we found that 13 (46.4%) fractures went through the bicipital groove. Of the remaining fracture lines, 9 (32.1%) extended into the greater tuberosity and 6 (21.4%) extended into the lesser tuberosity.
All users were able to reconstruct all 28 fractures using this technique. The average measured dimensions fell within the range of dimensions of a normal healthy proximal humerus specified in the literature to within a 95% confidence interval using the TOST for equivalence, in which we compared measured values with ranges reported in the literature (Table).11,12,13
Table. Dimensions of Proximal Humerus Geometry
| Normal Parameters | Average Dimensions From Trials | Dimensions From Literature |
| Head shaft angle | 43.5° ± 1° | 42.5° ± 12.5° |
| Head to greater tuberosity distance | 4.9 mm ± 0.4 mm | 8 mm ± 3.2 mm |
Head to bicipital groove angle (anatomic neck) | 26.4° ± 2° | 27.3° ± 14° |
| Posterior humeral head offset | 1.6 mm ± 0.3 mm | 4 mm ± 6 mm |
| Medial humeral head offset | 4.5 mm ± 0.3 mm | 9 mm ± 5 mm |
The reconstructions of these humerus fractures showed intraclass correlation coefficients ranging from 0.71 to 0.93 in 1 observer and interclass correlation coefficients from 0.82 to 0.98 between 2 different observers (Table).
DISCUSSION
This study demonstrates that it is feasible to reliably and accurately reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures. Poor outcomes after hemiarthroplasty for proximal humerus fractures are mostly related to tuberosity malpositioning, resorption, or failure of fixation and resultant dysfunction of the rotator cuff.14,15,16 These studies highlight the importance of accurate tuberosity reduction during surgical care of these fractures.
Continue to: The 3-D computer model...
The 3-D computer model reconstruction of 3- and 4-part proximal humerus fractures were reliable and valid. The interclass correlation coefficients showed very good to excellent interobserver and intraobserver reliability for all measurements conducted. The averaged dimensions from all trials fell within the appropriate range of dimensions for a normal healthy humerus reported in the literature, as verified by the TOST method.11,12,13 The 3-D modeling capabilities demonstrated in this study allowed a greater understanding of the fracture patterns present in 3- and 4-part (AO classifications 11-B1, 11-B2, 11-C1, 11-C2) humerus fractures.
Overreduction of greater tuberosity to create cortical overlap with the lateral shaft may be used to promote bony union. As a result of this distalization, there may be extra strains placed on the rotator cuff, making the patient more prone to rotator cuff tear, as well as improperly balancing the dynamic stabilizers of the shoulder. Poor clinical outcomes in hemiarthroplasty for proximal humerus fractures have been correlated with a greater tuberosity placed distal relative to the humeral head by 1 cm in a study2 and by 2 cm in another.3
This study has several limitations. The first is the assumption that our injured patients had preinjury proximal humerus geometry within the range of normal dimensions of a healthy humerus. Unfortunately, because we were unable to obtain CT scans of the contralateral shoulder, we had to use standard proximal humerus geometry as the control. Another limitation, inherent in the technique, is that only cortical and dense trabecular bone was modeled, so that comminuted or osteoporotic bone was not well modeled. This study did not correlate the findings from these models with clinical outcomes. A prospective study is needed to evaluate the impact of this 3-D modeling on fracture reductions and clinical outcomes.
This study demonstrates that patient-specific modeling of proximal humerus fracture 3-D CT scans may help surgeons reliably and accurately reconstruct fractures. This technique may have utility in the preoperative planning of tuberosity fracture reduction and hemiarthroplasty. It gives surgeons the ability to visualize fracture fragments, and the process of reconstructing the fragments may help surgeons understand the required maneuvers for reduction at the time of surgery. This technique also provides dimensions of the patient’s native humerus, thus potentially improving the anatomic accuracy of the reduction or hemiarthroplasty reconstruction. With the new trend toward patient-specific instrumentation, this study also provides a means of planning the size of the humeral prostheses as well as the version relative to the biceps groove and intertubercular fracture line.
CONCLUSION
This study demonstrates the feasibility of using 3-D computer modeling of complex proximal humerus fractures in anatomic reconstruction. These techniques of computer-simulated 3-D models are valid and reliable. We believe that this technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of hemiarthroplasty for 3- and 4-part proximal humerus fractures by providing improved understanding of the patient’s native humeral geometry and tuberosity reduction.
1. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412. doi:10.1067/mse.2002.124527.
2. Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):569-577. doi:10.1016/S1058274603002131.
3. Greiner SH, Kaab MJ, Kroning I, Scheibel M, Perka C. Reconstruction of humeral length and centering of the prosthetic head in hemiarthroplasty for proximal humeral fractures. J Shoulder Elbow Surg. 2008;17(5):709-714. doi:10.1016/j.jse.2008.03.004.
4. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24. doi:10.1016/j.jse.2006.05.008.
5. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491. doi:10.1016/j.jse.2007.09.006.
6. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832. doi:10.1016/j.jse.2008.01.141.
7. Yongpravat C, Kim HM, Gardner TR, Bigliani LU, Levine WN, Ahmad CS. Glenoid implant orientation and cement failure in total shoulder arthroplasty: a finite element analysis. J Shoulder Elbow Surg. 2013;22(7):940-947. doi:10.1016/j.jse.2012.09.007.
8. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865. doi:10.1302/0301-620X.79B5.0790857.
9. Wu G, van der Helm FC, Veeger HE, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981-992.
10. Kummer FJ, Perkins R, Zuckerman JD. The use of the bicipital groove for alignment of the humeral stem in shoulder arthroplasty. J Shoulder Elbow Surg. 1998;7(2):144-146. doi:10.1016/S1058-2746(98)90225-7.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326. doi:10.1016/S1058-2746(96)80060-7.
13. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(1 Suppl S):99S-104S. doi:10.1016/j.jse.2004.09.025.
14. Prakash U, McGurty DW, Dent JA. Hemiarthroplasty for severe fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):428-430. doi:10.1067/mse.2002.126615.
15. Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85-A(7):1215-1223.
16. Zyto K, Wallace WA, Frostick SP, Preston BJ. Outcome after hemiarthroplasty for three- and four-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1998;7(2):85-89. doi:10.1016/S1058-2746(98)90215-4.
1. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412. doi:10.1067/mse.2002.124527.
2. Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):569-577. doi:10.1016/S1058274603002131.
3. Greiner SH, Kaab MJ, Kroning I, Scheibel M, Perka C. Reconstruction of humeral length and centering of the prosthetic head in hemiarthroplasty for proximal humeral fractures. J Shoulder Elbow Surg. 2008;17(5):709-714. doi:10.1016/j.jse.2008.03.004.
4. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24. doi:10.1016/j.jse.2006.05.008.
5. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491. doi:10.1016/j.jse.2007.09.006.
6. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832. doi:10.1016/j.jse.2008.01.141.
7. Yongpravat C, Kim HM, Gardner TR, Bigliani LU, Levine WN, Ahmad CS. Glenoid implant orientation and cement failure in total shoulder arthroplasty: a finite element analysis. J Shoulder Elbow Surg. 2013;22(7):940-947. doi:10.1016/j.jse.2012.09.007.
8. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865. doi:10.1302/0301-620X.79B5.0790857.
9. Wu G, van der Helm FC, Veeger HE, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981-992.
10. Kummer FJ, Perkins R, Zuckerman JD. The use of the bicipital groove for alignment of the humeral stem in shoulder arthroplasty. J Shoulder Elbow Surg. 1998;7(2):144-146. doi:10.1016/S1058-2746(98)90225-7.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326. doi:10.1016/S1058-2746(96)80060-7.
13. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(1 Suppl S):99S-104S. doi:10.1016/j.jse.2004.09.025.
14. Prakash U, McGurty DW, Dent JA. Hemiarthroplasty for severe fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):428-430. doi:10.1067/mse.2002.126615.
15. Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85-A(7):1215-1223.
16. Zyto K, Wallace WA, Frostick SP, Preston BJ. Outcome after hemiarthroplasty for three- and four-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1998;7(2):85-89. doi:10.1016/S1058-2746(98)90215-4.
TAKE-HOME POINTS
- Proximal humerus fractures may be better understood with 3-D CT imaging.
- 3-D computer modeling of complex proximal humerus fractures allows an understanding of tuebroisty reduction durring ORIF or hemiarthroplasty.
- 3-D modeling enhances preoperative planning for hemiarthroplasty implant size and position relative to the repaired tuberosity fragments.
- 3-D modeling of fracture reduction can help surgeons understand the patient’s native humeral geometry and anatomy.
- Preoperative evaluation of fracture characteristics and fragment reduction help surgeons better understand surgical solutions.
Short-Term Storage of Platelet-Rich Plasma at Room Temperature Does Not Affect Growth Factor or Catabolic Cytokine Concentration
ABSTRACT
The aim of this study was to provide clinical recommendations about the use of platelet-rich plasma (PRP) that was subjected to short-term storage at room temperature. We determined bioactive growth factor and cytokine concentrations as indicators of platelet and white blood cell degranulation in blood and PRP. Additionally, this study sought to validate the use of manual, direct smear analysis as an alternative to automated methods for platelet quantification in PRP.
Blood was used to generate low-leukocyte PRP (Llo PRP) or high-leukocyte PRP (Lhi PRP). Blood was either processed immediately or kept at room temperature for 2 or 4 hours prior to generation of PRP, which was then held at room temperature for 0, 1, 2, or 4 hours. Subsequently, bioactive transforming growth factor beta-1 and matrix metalloproteinase-9 were measured by ELISA (enzyme-linked immunosorbent assay). Manual and automated platelet counts were performed on all blood and PRP samples.
There were no differences in growth factor or cytokine concentration when blood or Llo PRP or Lhi PRP was retained at room temperature for up to 4 hours. Manual, direct smear analysis for platelet quantification was not different from the use of automated machine counting for PRP samples, but in the starting blood samples, manual platelet counts were significantly higher than those generated using automated technology.
When there is a delay of up to 4 hours in the generation of PRP from blood or in the application of PRP to the patient, bioactive growth factor and cytokine concentrations remain stable in both blood and PRP. A manual direct counting method is a simple, cost-effective, and valid method to measure the contents of the PRP product being delivered to the patient.
Platelet-rich plasma (PRP) is used to promote healing in many areas of medicine, such as dental surgery,1,2 soft-tissue injury,3,4 orthopedic surgery,5,6 wound healing,7 and veterinary medicine.8,9 Despite its extensive use, there are still questions about the clinical efficacy of PRP.10-12 Due to biological heterogeneity between patients13,14 and differences between available manufacturing kits,13,15 PRP can be highly variable between patients. There are classification schemes to categorize the various types of PRP,16-18 which can be divided broadly into low-leukocyte PRP (Llo PRP) and high-leukocyte PRP (Lhi PRP). PRP can be used as a point of care therapy, prepared and used immediately, or it can be used during a surgical procedure. In some institutions, blood is drawn by a phlebotomist, processed in the hospital laboratory, and then delivered to the operating room. In other instances, PRP is generated patient-side by the primary attending physician’s team, who draws the blood and processes it for immediate use.5,19 Delays at any step in these various scenarios could lead to the blood or the resultant PRP remaining at room temperature from minutes to several hours prior to administration to the patient. This variability in PRP protocols between clinical and surgical settings adds to concerns regarding the stability and efficacy of the biologic.
Continue to: When performing clinical or research...
When performing clinical or research studies using PRP, it is important to report the contents of the PRP delivered to the patient. By documenting the cellular content of the PRP delivered to the patient, the common questions of optimal platelet dose and the importance of leukocytes in PRP can begin to be answered. There are some known factors that contribute to PRP variability, such as patient biology and operator technique, but there are many other unknown factors. In some instances, there is a failure to generate PRP, defined as a lower platelet count in the PRP preparation than in the starting blood sample.13,14 To measure the platelet and cellular contents of the starting blood and PRP, samples can be submitted to a clinical pathology laboratory for a complete blood count, which adds cost to the patient above the typically unreimbursed cost of the PRP injection itself. An alternative method for measuring platelet concentrations is the use of direct smear analysis on glass slides. The use of direct smears to measure platelet concentration is well validated for blood,20,21 but the use of direct smears of PRP for determining platelet concentrations has not been previously validated. The use of manual platelet counts would provide an alternative to automated platelet counting for clinical and preclinical research studies to characterize the type of PRP administered to the patient.
The primary aim of this study was to determine if retention of blood or PRP at room temperature for various time intervals had an effect on final growth factor or catabolic cytokine concentration. Bioactive transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) were measured as representatives of growth factors and catabolic cytokines, respectively. The secondary aim was to identify if manual platelet counts were an accurate reflection of automated counts. The outcomes of these experiments should provide immediately relevant information for the clinical application of PRP.
MATERIALS AND METHODS
Blood Collection and Generation of PRP
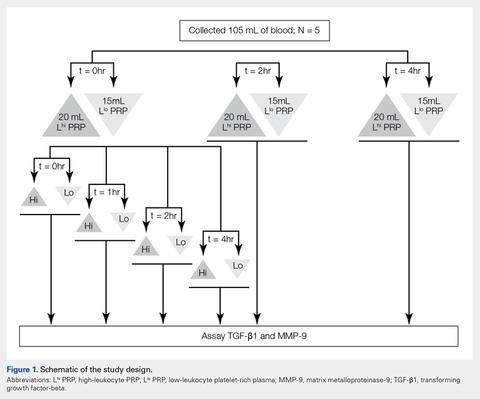
Under Institutional Review Board approval, blood (105 mL) was collected from healthy human volunteers (N = 5) into a syringe containing acid citrate dextrose anticoagulant to a final concentration of 10% acid citrate dextrose. Three 15-mL aliquots of blood were used to generate Llo PRP (Autologous Conditioned Plasma Double Syringe, Arthrex) and three 20-mL aliquots were used to generate Lhi PRP (SmartPReP 2, Harvest Technologies) (Figure 1).
Automated and Manual Counts
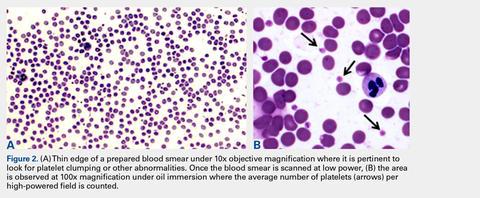
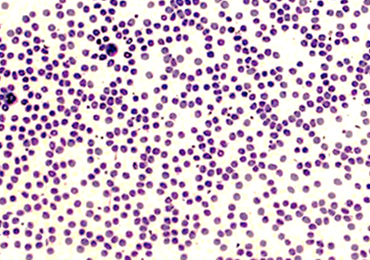
Automated complete blood counts were performed by a board certified clinical pathologist in the clinical pathology department of Cornell University on all blood, Llo PRP, and Lhi PRP samples. A manual platelet count, using a modified Giemsa stain,22 was performed on smears of all blood and PRP samples (Video). Slides were scanned at 10x magnification to identify an area where many red blood cells were present while maintaining a clear field of view (Figure 2A). The magnification was then increased to 100x using oil immersion, and the total number of platelets was counted in 10 fields of view (Figure 2B).
Growth Factor and Catabolic Cytokine Measurements
Blood and PRP samples were thawed for ELISA (enzyme-linked immunosorbent assay) analysis. TGF-β1 concentration was determined using the TGF-β1 Emax ImmunoAssay System (Promega Corporation), which measures biologically active TGF-β1. We chose TGF-β1 because it is commonly measured in PRP studies as an anabolic cytokine with multiple effects on tissue healing. The functions of TGF-β1 include stimulation of undifferentiated mesenchymal cell proliferation; regulation of endothelial, fibroblast, and osteoblast mitogenesis; coordination of collagen synthesis; promotion of endothelial chemotaxis and angiogenesis; activation of extracellular matrix synthesis in cartilage; and reduction of the catabolic activity of interluekin-1 and MMPs.23-25 In addition, TGF-β1 concentration strongly correlates with platelet concentration.26 MMP-9 concentration was determined using the MMP-9 Biotrak Activity Assay (GE Healthcare Biosciences) which measures both active and pro- forms of MMP-9. In PRP, MMP-9 was measured as an indicator of white blood cell (WBC) concentration.26 A catabolic cytokine capable of degrading collagen,13,27 MMP-9 has been linked to poor healing.28 For both assays, samples were measured in duplicate using a multiple detection plate reader (Tecan Safire).
Continue to: Statistical Analysis...
Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
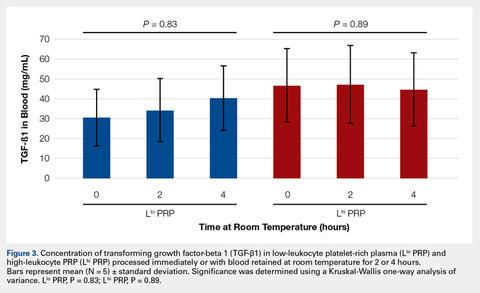
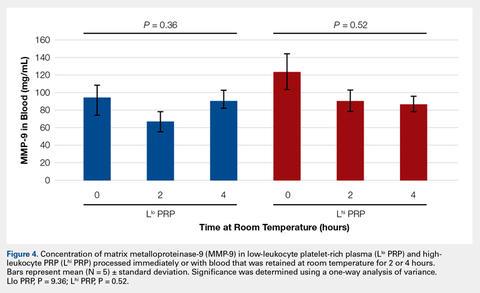
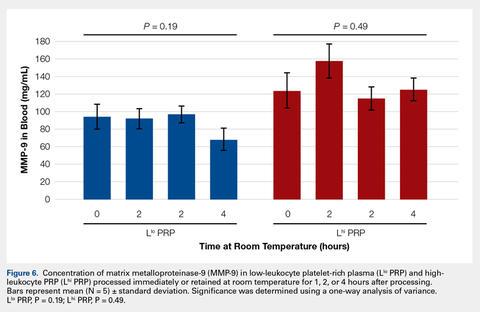
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5). 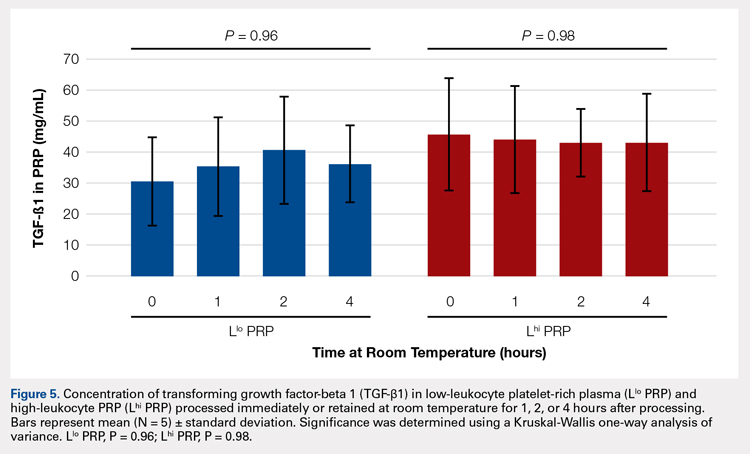
Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| |||||
| Automated Count | Manual Count | P Value | ||
| Mean ± SD | Range | Mean ± SD | Range |
|
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...
DISCUSSION
The primary aim of this study was to improve the clinical use of PRP by characterizing changes that might occur due to extended preparation times. Physicians commonly question the stability of blood or PRP if it is retained at room temperature prior to being administered to the patient. Clinical recommendations to optimize PRP preparation can be derived from a better understanding of the stability of platelets and WBCs, which contribute to the anabolic and catabolic cytokines in PRP.
The results of this study suggest that platelets and WBCs remain stable in blood and both Llo PRP and Lhi PRP for up to 4 hours. The use of bioactive ELISAs to measure TGF-β1 and MMP-9 allows for determination of stability of the PRP product retained at room temperature for up to 4 hours. This provides a time buffer to allow for delays from either institutional logistics or unanticipated clinical delays, without adverse effects on the generation of the final PRP product. As with all biologics, there are many factors that contribute to variability, but a relatively short delay of up to 4 hours in either generation of PRP from blood or in administration of PRP to the patient does not appear to contribute to that variability. Similar studies have been performed on equine PRP and suggest that growth factor concentrations remain stable for up to 6 hours after preparation of PRP29 and in human PRP, which implies that although samples degrade over time, platelet integrity might be acceptable for clinical use for up to 5 days after preparation, particularly if stored in oxygen.30 In contrast to this study, neither of the previously published reports used assays to measure biological activity in the stored PRP. Regardless of the variability between the studies with respect to the type of PRP evaluated and the outcome measures used, all of the studies support the concept that PRP can be stored at room temperature for at least a few hours before clinical use.
Centrifugation of blood does not guarantee the generation of PRP.13,14 In some cases, platelet counts in PRP are similar to or even less than that in the starting whole blood sample. To determine whether a clinical outcome is attributed to PRP, it is vital to know the platelet concentration and, arguably, the WBC concentration in the blood used to generate PRP and in the PRP sample administered to the patient. The platelet concentration in blood and PRP samples can be quantified using automated or manual methods. The use of automated methods can add significant cost to a study or procedure. Manually evaluating a blood smear is an accepted, though more time consuming, method of analyzing cellular components of a blood sample. Depending on the standard operating procedure of the laboratory, manual smears are often done in conjunction with an automated count. This identifies abnormalities in cellular shape or size, or platelet clumping, which are not consistently recognized by automated methods. Manually evaluating a blood smear does take some training, but the material cost is very low, which has added value for clinical or preclinical research studies. Interestingly, the results of this study indicate that manual platelet counts in blood may be more accurate than the count generated from an automated counter because the automated platelet counts were falsely low due to platelet clumping. Platelet clumping can occur as early as 1 hour after blood collection, regardless of the type of anticoagulant used.31
LIMITATIONS
The sample size of this study was small. However, variability in PRP has been well documented in multiple other studies using slightly larger sample sizes.13,14,16 Another potential limitation of this study could be that only one growth factor, TGF-β1, and one catabolic cytokine, MMP-9, were used as surrogate measures to represent platelet and WBC stability, respectively. We chose TGF-β1 because it is correlated with platelet concentrations14,15,26 and MMP-9 because it is an indicator of catabolic factors in PRP that have been correlated with WBC concentrations.26
CONCLUSION
This study illustrated that growth factor and cytokine concentrations in both Llo PRP and Lhi PRP are stable for up to 4 hours. The clinical implications of these results suggest that if the generation or administration of PRP is delayed by up to 4 hours, the resultant PRP retains its bioactivity and is acceptable for clinical application. However, given the known variability of PRP generated due to patient and manufacturer variability,13,14 it is still important to ensure that the product is indeed PRP, with an increase in platelet number over the starting sample of blood. This validation can be performed with a simple and cost-effective manual smear analysis of blood and PRP. The results of this study provide information that can be immediately translated into clinical, surgical, and research practices.
1. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng Part B Rev. 2008;14(3):249-258. doi:10.1089/ten.teb.2008.0062.
2. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
3. Monto RR. Platelet rich plasma treatment for chronic achilles tendinosis. Foot Ankle Int. 2012;33(5):379-385. doi:10.3113/FAI.2012.0379.
4. Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot ankle Int. 2011;32(11):1032-1039. doi:10.3113/FAI.2011.1032.
5. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245-251. doi:10.1177/0363546506294078.
6. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676-682. doi:10.1007/s00167-009-0762-8.
7. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434-439. doi:10.1177/154405910508400507.
8. Bosch G, René van Weeren P, Barneveld A, van Schie HTM. Computerised analysis of standardised ultrasonographic images to monitor the repair of surgically created core lesions in equine superficial digital flexor tendons following treatment with intratendinous platelet rich plasma or placebo. Vet J. 2011;187(1):92-98. doi:10.1016/j.tvjl.2009.10.014.
9. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569-1576. doi:10.1007/s00264-011-1237-3.
10. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165-174. doi:10.1007/s12178-008-9032-5.
11. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298-307. doi:10.2106/JBJS.K.00154.
12. Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20(1):2-9. doi:10.1016/j.fas.2013.08.001.
13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429-439. doi:10.1016/j.arthro.2011.10.018.
14. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316. doi:10.2106/JBJS.K.00430.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi:10.1177/0363546510387517.
16. Arnoczky SP, Sheibani-Rad S, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180-185. doi:10.1097/JSA.0b013e3182999712.
17. Dohan Ehrenfest DM, Bielecki T, Corso M Del, Inchingolo F, Sammartino G. Shedding light in the controversial terminology for platelet-rich products: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), platelet-leukocyte gel (PLG), preparation rich in growth factors (PRGF), classification and commercialism. J Biomed Mater Res Part A. 2010;95A(4):1280-1282. doi:10.1002/jbm.a.32894.
18. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167. doi:10.1016/j.tibtech.2008.11.009.
19. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
20. Malok M, Titchener EH, Bridgers C, Lee BY, Bamberg R. Comparison of two platelet count estimation methodologies for peripheral blood smears. Clin Lab Sci. 2007;20(3):154-160.
21. Gulati G, Uppal G, Florea AD, Gong J. Detection of platelet clumps on peripheral blood smears by CellaVision DM96 System and Microscopic Review. Lab Med. 2014;45(4):368-371. doi:10.1309/LM604RQVKVLRFXOR.
22. Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med. 2013;33(1):1-7. doi:10.3343/alm.2013.33.1.1.
23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x.
24. Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8(1):12-26.
25. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715. doi:10.1007/s11999-011-1857-3.
26. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi:10.1177/0363546511417792.
27. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411-422.
28. Watelet JB, Demetter P, Claeys C, Van Cauwenberge P, Cuvelier C, Bachert C. Neutrophil-derived metalloproteinase-9 predicts healing quality after sinus surgery. Laryngoscope. 2005;115(1):56-61. doi:10.1097/01.mlg.0000150674.30237.3f.
29. Hauschild G, Geburek F, Gosheger G, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. 2017;13(1):7. doi:10.1186/s12917-016-0920-4.
30. Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2017;74(2):71-77. doi:10.1080/09674845.2016.1233792.
31. McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12(3):277-285.
ABSTRACT
The aim of this study was to provide clinical recommendations about the use of platelet-rich plasma (PRP) that was subjected to short-term storage at room temperature. We determined bioactive growth factor and cytokine concentrations as indicators of platelet and white blood cell degranulation in blood and PRP. Additionally, this study sought to validate the use of manual, direct smear analysis as an alternative to automated methods for platelet quantification in PRP.
Blood was used to generate low-leukocyte PRP (Llo PRP) or high-leukocyte PRP (Lhi PRP). Blood was either processed immediately or kept at room temperature for 2 or 4 hours prior to generation of PRP, which was then held at room temperature for 0, 1, 2, or 4 hours. Subsequently, bioactive transforming growth factor beta-1 and matrix metalloproteinase-9 were measured by ELISA (enzyme-linked immunosorbent assay). Manual and automated platelet counts were performed on all blood and PRP samples.
There were no differences in growth factor or cytokine concentration when blood or Llo PRP or Lhi PRP was retained at room temperature for up to 4 hours. Manual, direct smear analysis for platelet quantification was not different from the use of automated machine counting for PRP samples, but in the starting blood samples, manual platelet counts were significantly higher than those generated using automated technology.
When there is a delay of up to 4 hours in the generation of PRP from blood or in the application of PRP to the patient, bioactive growth factor and cytokine concentrations remain stable in both blood and PRP. A manual direct counting method is a simple, cost-effective, and valid method to measure the contents of the PRP product being delivered to the patient.
Platelet-rich plasma (PRP) is used to promote healing in many areas of medicine, such as dental surgery,1,2 soft-tissue injury,3,4 orthopedic surgery,5,6 wound healing,7 and veterinary medicine.8,9 Despite its extensive use, there are still questions about the clinical efficacy of PRP.10-12 Due to biological heterogeneity between patients13,14 and differences between available manufacturing kits,13,15 PRP can be highly variable between patients. There are classification schemes to categorize the various types of PRP,16-18 which can be divided broadly into low-leukocyte PRP (Llo PRP) and high-leukocyte PRP (Lhi PRP). PRP can be used as a point of care therapy, prepared and used immediately, or it can be used during a surgical procedure. In some institutions, blood is drawn by a phlebotomist, processed in the hospital laboratory, and then delivered to the operating room. In other instances, PRP is generated patient-side by the primary attending physician’s team, who draws the blood and processes it for immediate use.5,19 Delays at any step in these various scenarios could lead to the blood or the resultant PRP remaining at room temperature from minutes to several hours prior to administration to the patient. This variability in PRP protocols between clinical and surgical settings adds to concerns regarding the stability and efficacy of the biologic.
Continue to: When performing clinical or research...
When performing clinical or research studies using PRP, it is important to report the contents of the PRP delivered to the patient. By documenting the cellular content of the PRP delivered to the patient, the common questions of optimal platelet dose and the importance of leukocytes in PRP can begin to be answered. There are some known factors that contribute to PRP variability, such as patient biology and operator technique, but there are many other unknown factors. In some instances, there is a failure to generate PRP, defined as a lower platelet count in the PRP preparation than in the starting blood sample.13,14 To measure the platelet and cellular contents of the starting blood and PRP, samples can be submitted to a clinical pathology laboratory for a complete blood count, which adds cost to the patient above the typically unreimbursed cost of the PRP injection itself. An alternative method for measuring platelet concentrations is the use of direct smear analysis on glass slides. The use of direct smears to measure platelet concentration is well validated for blood,20,21 but the use of direct smears of PRP for determining platelet concentrations has not been previously validated. The use of manual platelet counts would provide an alternative to automated platelet counting for clinical and preclinical research studies to characterize the type of PRP administered to the patient.
The primary aim of this study was to determine if retention of blood or PRP at room temperature for various time intervals had an effect on final growth factor or catabolic cytokine concentration. Bioactive transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) were measured as representatives of growth factors and catabolic cytokines, respectively. The secondary aim was to identify if manual platelet counts were an accurate reflection of automated counts. The outcomes of these experiments should provide immediately relevant information for the clinical application of PRP.
MATERIALS AND METHODS
Blood Collection and Generation of PRP
Under Institutional Review Board approval, blood (105 mL) was collected from healthy human volunteers (N = 5) into a syringe containing acid citrate dextrose anticoagulant to a final concentration of 10% acid citrate dextrose. Three 15-mL aliquots of blood were used to generate Llo PRP (Autologous Conditioned Plasma Double Syringe, Arthrex) and three 20-mL aliquots were used to generate Lhi PRP (SmartPReP 2, Harvest Technologies) (Figure 1).
Automated and Manual Counts
Automated complete blood counts were performed by a board certified clinical pathologist in the clinical pathology department of Cornell University on all blood, Llo PRP, and Lhi PRP samples. A manual platelet count, using a modified Giemsa stain,22 was performed on smears of all blood and PRP samples (Video). Slides were scanned at 10x magnification to identify an area where many red blood cells were present while maintaining a clear field of view (Figure 2A). The magnification was then increased to 100x using oil immersion, and the total number of platelets was counted in 10 fields of view (Figure 2B).
Growth Factor and Catabolic Cytokine Measurements
Blood and PRP samples were thawed for ELISA (enzyme-linked immunosorbent assay) analysis. TGF-β1 concentration was determined using the TGF-β1 Emax ImmunoAssay System (Promega Corporation), which measures biologically active TGF-β1. We chose TGF-β1 because it is commonly measured in PRP studies as an anabolic cytokine with multiple effects on tissue healing. The functions of TGF-β1 include stimulation of undifferentiated mesenchymal cell proliferation; regulation of endothelial, fibroblast, and osteoblast mitogenesis; coordination of collagen synthesis; promotion of endothelial chemotaxis and angiogenesis; activation of extracellular matrix synthesis in cartilage; and reduction of the catabolic activity of interluekin-1 and MMPs.23-25 In addition, TGF-β1 concentration strongly correlates with platelet concentration.26 MMP-9 concentration was determined using the MMP-9 Biotrak Activity Assay (GE Healthcare Biosciences) which measures both active and pro- forms of MMP-9. In PRP, MMP-9 was measured as an indicator of white blood cell (WBC) concentration.26 A catabolic cytokine capable of degrading collagen,13,27 MMP-9 has been linked to poor healing.28 For both assays, samples were measured in duplicate using a multiple detection plate reader (Tecan Safire).
Continue to: Statistical Analysis...
Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5). 
Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| |||||
| Automated Count | Manual Count | P Value | ||
| Mean ± SD | Range | Mean ± SD | Range |
|
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...
DISCUSSION
The primary aim of this study was to improve the clinical use of PRP by characterizing changes that might occur due to extended preparation times. Physicians commonly question the stability of blood or PRP if it is retained at room temperature prior to being administered to the patient. Clinical recommendations to optimize PRP preparation can be derived from a better understanding of the stability of platelets and WBCs, which contribute to the anabolic and catabolic cytokines in PRP.
The results of this study suggest that platelets and WBCs remain stable in blood and both Llo PRP and Lhi PRP for up to 4 hours. The use of bioactive ELISAs to measure TGF-β1 and MMP-9 allows for determination of stability of the PRP product retained at room temperature for up to 4 hours. This provides a time buffer to allow for delays from either institutional logistics or unanticipated clinical delays, without adverse effects on the generation of the final PRP product. As with all biologics, there are many factors that contribute to variability, but a relatively short delay of up to 4 hours in either generation of PRP from blood or in administration of PRP to the patient does not appear to contribute to that variability. Similar studies have been performed on equine PRP and suggest that growth factor concentrations remain stable for up to 6 hours after preparation of PRP29 and in human PRP, which implies that although samples degrade over time, platelet integrity might be acceptable for clinical use for up to 5 days after preparation, particularly if stored in oxygen.30 In contrast to this study, neither of the previously published reports used assays to measure biological activity in the stored PRP. Regardless of the variability between the studies with respect to the type of PRP evaluated and the outcome measures used, all of the studies support the concept that PRP can be stored at room temperature for at least a few hours before clinical use.
Centrifugation of blood does not guarantee the generation of PRP.13,14 In some cases, platelet counts in PRP are similar to or even less than that in the starting whole blood sample. To determine whether a clinical outcome is attributed to PRP, it is vital to know the platelet concentration and, arguably, the WBC concentration in the blood used to generate PRP and in the PRP sample administered to the patient. The platelet concentration in blood and PRP samples can be quantified using automated or manual methods. The use of automated methods can add significant cost to a study or procedure. Manually evaluating a blood smear is an accepted, though more time consuming, method of analyzing cellular components of a blood sample. Depending on the standard operating procedure of the laboratory, manual smears are often done in conjunction with an automated count. This identifies abnormalities in cellular shape or size, or platelet clumping, which are not consistently recognized by automated methods. Manually evaluating a blood smear does take some training, but the material cost is very low, which has added value for clinical or preclinical research studies. Interestingly, the results of this study indicate that manual platelet counts in blood may be more accurate than the count generated from an automated counter because the automated platelet counts were falsely low due to platelet clumping. Platelet clumping can occur as early as 1 hour after blood collection, regardless of the type of anticoagulant used.31
LIMITATIONS
The sample size of this study was small. However, variability in PRP has been well documented in multiple other studies using slightly larger sample sizes.13,14,16 Another potential limitation of this study could be that only one growth factor, TGF-β1, and one catabolic cytokine, MMP-9, were used as surrogate measures to represent platelet and WBC stability, respectively. We chose TGF-β1 because it is correlated with platelet concentrations14,15,26 and MMP-9 because it is an indicator of catabolic factors in PRP that have been correlated with WBC concentrations.26
CONCLUSION
This study illustrated that growth factor and cytokine concentrations in both Llo PRP and Lhi PRP are stable for up to 4 hours. The clinical implications of these results suggest that if the generation or administration of PRP is delayed by up to 4 hours, the resultant PRP retains its bioactivity and is acceptable for clinical application. However, given the known variability of PRP generated due to patient and manufacturer variability,13,14 it is still important to ensure that the product is indeed PRP, with an increase in platelet number over the starting sample of blood. This validation can be performed with a simple and cost-effective manual smear analysis of blood and PRP. The results of this study provide information that can be immediately translated into clinical, surgical, and research practices.
ABSTRACT
The aim of this study was to provide clinical recommendations about the use of platelet-rich plasma (PRP) that was subjected to short-term storage at room temperature. We determined bioactive growth factor and cytokine concentrations as indicators of platelet and white blood cell degranulation in blood and PRP. Additionally, this study sought to validate the use of manual, direct smear analysis as an alternative to automated methods for platelet quantification in PRP.
Blood was used to generate low-leukocyte PRP (Llo PRP) or high-leukocyte PRP (Lhi PRP). Blood was either processed immediately or kept at room temperature for 2 or 4 hours prior to generation of PRP, which was then held at room temperature for 0, 1, 2, or 4 hours. Subsequently, bioactive transforming growth factor beta-1 and matrix metalloproteinase-9 were measured by ELISA (enzyme-linked immunosorbent assay). Manual and automated platelet counts were performed on all blood and PRP samples.
There were no differences in growth factor or cytokine concentration when blood or Llo PRP or Lhi PRP was retained at room temperature for up to 4 hours. Manual, direct smear analysis for platelet quantification was not different from the use of automated machine counting for PRP samples, but in the starting blood samples, manual platelet counts were significantly higher than those generated using automated technology.
When there is a delay of up to 4 hours in the generation of PRP from blood or in the application of PRP to the patient, bioactive growth factor and cytokine concentrations remain stable in both blood and PRP. A manual direct counting method is a simple, cost-effective, and valid method to measure the contents of the PRP product being delivered to the patient.
Platelet-rich plasma (PRP) is used to promote healing in many areas of medicine, such as dental surgery,1,2 soft-tissue injury,3,4 orthopedic surgery,5,6 wound healing,7 and veterinary medicine.8,9 Despite its extensive use, there are still questions about the clinical efficacy of PRP.10-12 Due to biological heterogeneity between patients13,14 and differences between available manufacturing kits,13,15 PRP can be highly variable between patients. There are classification schemes to categorize the various types of PRP,16-18 which can be divided broadly into low-leukocyte PRP (Llo PRP) and high-leukocyte PRP (Lhi PRP). PRP can be used as a point of care therapy, prepared and used immediately, or it can be used during a surgical procedure. In some institutions, blood is drawn by a phlebotomist, processed in the hospital laboratory, and then delivered to the operating room. In other instances, PRP is generated patient-side by the primary attending physician’s team, who draws the blood and processes it for immediate use.5,19 Delays at any step in these various scenarios could lead to the blood or the resultant PRP remaining at room temperature from minutes to several hours prior to administration to the patient. This variability in PRP protocols between clinical and surgical settings adds to concerns regarding the stability and efficacy of the biologic.
Continue to: When performing clinical or research...
When performing clinical or research studies using PRP, it is important to report the contents of the PRP delivered to the patient. By documenting the cellular content of the PRP delivered to the patient, the common questions of optimal platelet dose and the importance of leukocytes in PRP can begin to be answered. There are some known factors that contribute to PRP variability, such as patient biology and operator technique, but there are many other unknown factors. In some instances, there is a failure to generate PRP, defined as a lower platelet count in the PRP preparation than in the starting blood sample.13,14 To measure the platelet and cellular contents of the starting blood and PRP, samples can be submitted to a clinical pathology laboratory for a complete blood count, which adds cost to the patient above the typically unreimbursed cost of the PRP injection itself. An alternative method for measuring platelet concentrations is the use of direct smear analysis on glass slides. The use of direct smears to measure platelet concentration is well validated for blood,20,21 but the use of direct smears of PRP for determining platelet concentrations has not been previously validated. The use of manual platelet counts would provide an alternative to automated platelet counting for clinical and preclinical research studies to characterize the type of PRP administered to the patient.
The primary aim of this study was to determine if retention of blood or PRP at room temperature for various time intervals had an effect on final growth factor or catabolic cytokine concentration. Bioactive transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) were measured as representatives of growth factors and catabolic cytokines, respectively. The secondary aim was to identify if manual platelet counts were an accurate reflection of automated counts. The outcomes of these experiments should provide immediately relevant information for the clinical application of PRP.
MATERIALS AND METHODS
Blood Collection and Generation of PRP
Under Institutional Review Board approval, blood (105 mL) was collected from healthy human volunteers (N = 5) into a syringe containing acid citrate dextrose anticoagulant to a final concentration of 10% acid citrate dextrose. Three 15-mL aliquots of blood were used to generate Llo PRP (Autologous Conditioned Plasma Double Syringe, Arthrex) and three 20-mL aliquots were used to generate Lhi PRP (SmartPReP 2, Harvest Technologies) (Figure 1).
Automated and Manual Counts
Automated complete blood counts were performed by a board certified clinical pathologist in the clinical pathology department of Cornell University on all blood, Llo PRP, and Lhi PRP samples. A manual platelet count, using a modified Giemsa stain,22 was performed on smears of all blood and PRP samples (Video). Slides were scanned at 10x magnification to identify an area where many red blood cells were present while maintaining a clear field of view (Figure 2A). The magnification was then increased to 100x using oil immersion, and the total number of platelets was counted in 10 fields of view (Figure 2B).
Growth Factor and Catabolic Cytokine Measurements
Blood and PRP samples were thawed for ELISA (enzyme-linked immunosorbent assay) analysis. TGF-β1 concentration was determined using the TGF-β1 Emax ImmunoAssay System (Promega Corporation), which measures biologically active TGF-β1. We chose TGF-β1 because it is commonly measured in PRP studies as an anabolic cytokine with multiple effects on tissue healing. The functions of TGF-β1 include stimulation of undifferentiated mesenchymal cell proliferation; regulation of endothelial, fibroblast, and osteoblast mitogenesis; coordination of collagen synthesis; promotion of endothelial chemotaxis and angiogenesis; activation of extracellular matrix synthesis in cartilage; and reduction of the catabolic activity of interluekin-1 and MMPs.23-25 In addition, TGF-β1 concentration strongly correlates with platelet concentration.26 MMP-9 concentration was determined using the MMP-9 Biotrak Activity Assay (GE Healthcare Biosciences) which measures both active and pro- forms of MMP-9. In PRP, MMP-9 was measured as an indicator of white blood cell (WBC) concentration.26 A catabolic cytokine capable of degrading collagen,13,27 MMP-9 has been linked to poor healing.28 For both assays, samples were measured in duplicate using a multiple detection plate reader (Tecan Safire).
Continue to: Statistical Analysis...
Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5). 
Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| |||||
| Automated Count | Manual Count | P Value | ||
| Mean ± SD | Range | Mean ± SD | Range |
|
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...
DISCUSSION
The primary aim of this study was to improve the clinical use of PRP by characterizing changes that might occur due to extended preparation times. Physicians commonly question the stability of blood or PRP if it is retained at room temperature prior to being administered to the patient. Clinical recommendations to optimize PRP preparation can be derived from a better understanding of the stability of platelets and WBCs, which contribute to the anabolic and catabolic cytokines in PRP.
The results of this study suggest that platelets and WBCs remain stable in blood and both Llo PRP and Lhi PRP for up to 4 hours. The use of bioactive ELISAs to measure TGF-β1 and MMP-9 allows for determination of stability of the PRP product retained at room temperature for up to 4 hours. This provides a time buffer to allow for delays from either institutional logistics or unanticipated clinical delays, without adverse effects on the generation of the final PRP product. As with all biologics, there are many factors that contribute to variability, but a relatively short delay of up to 4 hours in either generation of PRP from blood or in administration of PRP to the patient does not appear to contribute to that variability. Similar studies have been performed on equine PRP and suggest that growth factor concentrations remain stable for up to 6 hours after preparation of PRP29 and in human PRP, which implies that although samples degrade over time, platelet integrity might be acceptable for clinical use for up to 5 days after preparation, particularly if stored in oxygen.30 In contrast to this study, neither of the previously published reports used assays to measure biological activity in the stored PRP. Regardless of the variability between the studies with respect to the type of PRP evaluated and the outcome measures used, all of the studies support the concept that PRP can be stored at room temperature for at least a few hours before clinical use.
Centrifugation of blood does not guarantee the generation of PRP.13,14 In some cases, platelet counts in PRP are similar to or even less than that in the starting whole blood sample. To determine whether a clinical outcome is attributed to PRP, it is vital to know the platelet concentration and, arguably, the WBC concentration in the blood used to generate PRP and in the PRP sample administered to the patient. The platelet concentration in blood and PRP samples can be quantified using automated or manual methods. The use of automated methods can add significant cost to a study or procedure. Manually evaluating a blood smear is an accepted, though more time consuming, method of analyzing cellular components of a blood sample. Depending on the standard operating procedure of the laboratory, manual smears are often done in conjunction with an automated count. This identifies abnormalities in cellular shape or size, or platelet clumping, which are not consistently recognized by automated methods. Manually evaluating a blood smear does take some training, but the material cost is very low, which has added value for clinical or preclinical research studies. Interestingly, the results of this study indicate that manual platelet counts in blood may be more accurate than the count generated from an automated counter because the automated platelet counts were falsely low due to platelet clumping. Platelet clumping can occur as early as 1 hour after blood collection, regardless of the type of anticoagulant used.31
LIMITATIONS
The sample size of this study was small. However, variability in PRP has been well documented in multiple other studies using slightly larger sample sizes.13,14,16 Another potential limitation of this study could be that only one growth factor, TGF-β1, and one catabolic cytokine, MMP-9, were used as surrogate measures to represent platelet and WBC stability, respectively. We chose TGF-β1 because it is correlated with platelet concentrations14,15,26 and MMP-9 because it is an indicator of catabolic factors in PRP that have been correlated with WBC concentrations.26
CONCLUSION
This study illustrated that growth factor and cytokine concentrations in both Llo PRP and Lhi PRP are stable for up to 4 hours. The clinical implications of these results suggest that if the generation or administration of PRP is delayed by up to 4 hours, the resultant PRP retains its bioactivity and is acceptable for clinical application. However, given the known variability of PRP generated due to patient and manufacturer variability,13,14 it is still important to ensure that the product is indeed PRP, with an increase in platelet number over the starting sample of blood. This validation can be performed with a simple and cost-effective manual smear analysis of blood and PRP. The results of this study provide information that can be immediately translated into clinical, surgical, and research practices.
1. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng Part B Rev. 2008;14(3):249-258. doi:10.1089/ten.teb.2008.0062.
2. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
3. Monto RR. Platelet rich plasma treatment for chronic achilles tendinosis. Foot Ankle Int. 2012;33(5):379-385. doi:10.3113/FAI.2012.0379.
4. Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot ankle Int. 2011;32(11):1032-1039. doi:10.3113/FAI.2011.1032.
5. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245-251. doi:10.1177/0363546506294078.
6. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676-682. doi:10.1007/s00167-009-0762-8.
7. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434-439. doi:10.1177/154405910508400507.
8. Bosch G, René van Weeren P, Barneveld A, van Schie HTM. Computerised analysis of standardised ultrasonographic images to monitor the repair of surgically created core lesions in equine superficial digital flexor tendons following treatment with intratendinous platelet rich plasma or placebo. Vet J. 2011;187(1):92-98. doi:10.1016/j.tvjl.2009.10.014.
9. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569-1576. doi:10.1007/s00264-011-1237-3.
10. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165-174. doi:10.1007/s12178-008-9032-5.
11. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298-307. doi:10.2106/JBJS.K.00154.
12. Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20(1):2-9. doi:10.1016/j.fas.2013.08.001.
13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429-439. doi:10.1016/j.arthro.2011.10.018.
14. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316. doi:10.2106/JBJS.K.00430.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi:10.1177/0363546510387517.
16. Arnoczky SP, Sheibani-Rad S, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180-185. doi:10.1097/JSA.0b013e3182999712.
17. Dohan Ehrenfest DM, Bielecki T, Corso M Del, Inchingolo F, Sammartino G. Shedding light in the controversial terminology for platelet-rich products: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), platelet-leukocyte gel (PLG), preparation rich in growth factors (PRGF), classification and commercialism. J Biomed Mater Res Part A. 2010;95A(4):1280-1282. doi:10.1002/jbm.a.32894.
18. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167. doi:10.1016/j.tibtech.2008.11.009.
19. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
20. Malok M, Titchener EH, Bridgers C, Lee BY, Bamberg R. Comparison of two platelet count estimation methodologies for peripheral blood smears. Clin Lab Sci. 2007;20(3):154-160.
21. Gulati G, Uppal G, Florea AD, Gong J. Detection of platelet clumps on peripheral blood smears by CellaVision DM96 System and Microscopic Review. Lab Med. 2014;45(4):368-371. doi:10.1309/LM604RQVKVLRFXOR.
22. Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med. 2013;33(1):1-7. doi:10.3343/alm.2013.33.1.1.
23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x.
24. Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8(1):12-26.
25. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715. doi:10.1007/s11999-011-1857-3.
26. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi:10.1177/0363546511417792.
27. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411-422.
28. Watelet JB, Demetter P, Claeys C, Van Cauwenberge P, Cuvelier C, Bachert C. Neutrophil-derived metalloproteinase-9 predicts healing quality after sinus surgery. Laryngoscope. 2005;115(1):56-61. doi:10.1097/01.mlg.0000150674.30237.3f.
29. Hauschild G, Geburek F, Gosheger G, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. 2017;13(1):7. doi:10.1186/s12917-016-0920-4.
30. Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2017;74(2):71-77. doi:10.1080/09674845.2016.1233792.
31. McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12(3):277-285.
1. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng Part B Rev. 2008;14(3):249-258. doi:10.1089/ten.teb.2008.0062.
2. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
3. Monto RR. Platelet rich plasma treatment for chronic achilles tendinosis. Foot Ankle Int. 2012;33(5):379-385. doi:10.3113/FAI.2012.0379.
4. Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot ankle Int. 2011;32(11):1032-1039. doi:10.3113/FAI.2011.1032.
5. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245-251. doi:10.1177/0363546506294078.
6. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676-682. doi:10.1007/s00167-009-0762-8.
7. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434-439. doi:10.1177/154405910508400507.
8. Bosch G, René van Weeren P, Barneveld A, van Schie HTM. Computerised analysis of standardised ultrasonographic images to monitor the repair of surgically created core lesions in equine superficial digital flexor tendons following treatment with intratendinous platelet rich plasma or placebo. Vet J. 2011;187(1):92-98. doi:10.1016/j.tvjl.2009.10.014.
9. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569-1576. doi:10.1007/s00264-011-1237-3.
10. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165-174. doi:10.1007/s12178-008-9032-5.
11. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298-307. doi:10.2106/JBJS.K.00154.
12. Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20(1):2-9. doi:10.1016/j.fas.2013.08.001.
13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429-439. doi:10.1016/j.arthro.2011.10.018.
14. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316. doi:10.2106/JBJS.K.00430.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi:10.1177/0363546510387517.
16. Arnoczky SP, Sheibani-Rad S, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180-185. doi:10.1097/JSA.0b013e3182999712.
17. Dohan Ehrenfest DM, Bielecki T, Corso M Del, Inchingolo F, Sammartino G. Shedding light in the controversial terminology for platelet-rich products: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), platelet-leukocyte gel (PLG), preparation rich in growth factors (PRGF), classification and commercialism. J Biomed Mater Res Part A. 2010;95A(4):1280-1282. doi:10.1002/jbm.a.32894.
18. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167. doi:10.1016/j.tibtech.2008.11.009.
19. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
20. Malok M, Titchener EH, Bridgers C, Lee BY, Bamberg R. Comparison of two platelet count estimation methodologies for peripheral blood smears. Clin Lab Sci. 2007;20(3):154-160.
21. Gulati G, Uppal G, Florea AD, Gong J. Detection of platelet clumps on peripheral blood smears by CellaVision DM96 System and Microscopic Review. Lab Med. 2014;45(4):368-371. doi:10.1309/LM604RQVKVLRFXOR.
22. Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med. 2013;33(1):1-7. doi:10.3343/alm.2013.33.1.1.
23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x.
24. Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8(1):12-26.
25. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715. doi:10.1007/s11999-011-1857-3.
26. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi:10.1177/0363546511417792.
27. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411-422.
28. Watelet JB, Demetter P, Claeys C, Van Cauwenberge P, Cuvelier C, Bachert C. Neutrophil-derived metalloproteinase-9 predicts healing quality after sinus surgery. Laryngoscope. 2005;115(1):56-61. doi:10.1097/01.mlg.0000150674.30237.3f.
29. Hauschild G, Geburek F, Gosheger G, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. 2017;13(1):7. doi:10.1186/s12917-016-0920-4.
30. Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2017;74(2):71-77. doi:10.1080/09674845.2016.1233792.
31. McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12(3):277-285.
TAKE-HOME POINTS
- Blood can be stored at room temperature for up to 4 hours before making PRP without loss in activity.
- PRP can be stored at room temperature for up to 4 hours before administration to a patient without loss in activity.
- Manual, direct smear analysis is as accurate as automated counting for measuring platelet concentration in PRP.
Fat Fracture: A Rare Cause of Anterior and Medial Knee Pain in a Professional Baseball Player
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
ABSTRACT
Blunt trauma to the anterior knee typically results in a contusion or fracture of the patella. Additionally, injury to the extensor mechanism may come from a partial or full disruption of the patellar or quadriceps tendon. A professional baseball player suffered an injury to his knee after he collided with an outfield wall. Acute swelling in the suprapatellar soft tissues concealed a palpable defect, which initially was suspected to be an injury to the quadriceps tendon. Magnetic resonance imaging of the knee revealed an intact extensor mechanism; moreover, a fracture of the subcutaneous fat anterior to the quadriceps tendon was evident and diagnosed as a fat fracture.
Fat fracture is a rare diagnosis, and to the best of our knowledge, this is the first reported diagnosis in a professional athlete. Conservative management including, but not limited to, range of motion exercises, hydrotherapy, and iontophoresis effectively treated the athlete’s injury.
Blunt trauma to the anterior knee can result in a contusion or fracture of the patella, subluxation of the patella, and injury to the quadriceps or patellar tendon. Typically, a contusion or non-displaced fracture of the patella clinically presents with a direct anterior effusion and point tenderness. A displaced fracture or tendon deficit typically has an extensor lag or weakness in extension. Fat fracture or traumatic lipomata has been previously described in 1 case of anterior knee pain after blunt injury.1
In this article, we present the case of a 32-year-old professional baseball player who suffered a blunt injury to his left knee after collision with the outfield wall and experienced both anterior and medial knee pain. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 32-year-old outfielder for a professional baseball team was attempting a catch in the outfield when his left knee collided with the padded outfield wall in a semiflexed position. The player was able to walk off the field in the middle of the inning; however, he then experienced increasing pain and was unable to return to play. He had no prior history of significant knee pain or injury. He complained only of pain, with no instability or sensation of catching or locking.
Continue to: Physical examination of the patient...
Physical examination of the patient revealed a grade 1+ swelling over the anterior aspect of the superior pole of the patella in the prepatellar region, as well as medially over the medial femoral condyle. However, there was no joint effusion. Palpation of the superomedial aspect of the patella elicited pain, but no medial joint line tenderness was elicited. Percussion testing to the patella was negative. There were no gross palpable defects in the extensor mechanism, and the patient was able to perform a straight leg raise against resistance with pain.
Mild coronal laxity of the patella was noted compared with that of the contralateral knee. Hip range of motion (ROM) was intact, but knee ROM was limited to 110° of flexion, with the complaint of anterior tightness at this position. He was able to fully extend his knee without symptoms. The knee was stable to varus and valgus stress at both 0° and 30° of flexion. Lachman and anterior and posterior drawer tests were negative and symmetric to the contralateral knee. The McMurray test for meniscal pathology also was negative. Radiographs of the left knee were completed and were negative for fracture.
OUTCOMES
The initial clinical diagnosis was a patellar contusion and sprain of the medial retinaculum, and the athlete was treated with multiple modalities available in the athletic training room. Rehabilitation included activity modifications, passive and active ROM activities, quadriceps isometric exercises, and neuromuscular control activities. Adjunctive modalities included cryotherapy, hydrotherapy, topical hematoma cream, and iontophoresis.2 This aggressive treatment was continued for 3 days with decreased but persistent pain with running drills and limited knee flexion. Repeat clinical examination revealed a decreased swelling, but there was evidence of a clinically palpable defect anteriorly proximal to the patella. Although the patient could perform a straight leg raise, a partial injury to the quadriceps became plausible. Magnetic resonance imaging (MRI) of the left knee was performed, owing to the persistent pain and limited flexion despite aggressive conservative management, as well as the palpable soft-tissue defect.
MRI was performed using a 3T (Tesla) system (GE Healthcare) with a GE Healthcare Precision 8-channel knee coil. Routine knee protocol imaging was performed to include the distal quadriceps tendon due to clinical concern for a quadriceps tear. Sagittal proton density and proton-density fat-saturated (PD FS), coronal T1 and PD FS, and axial T1 and PD FS sequences were acquired.
An acutely marginated, 1.5 cm × 3 cm, longitudinal and transverse fluid defect “crevasse” was identified at the midline in the prepatellar subcutaneous fat overlying the distal quadriceps tendon and corresponded to a clinically palpable abnormality (Figures 1, 2).
Continue to: These findings explained...
These findings explained the delayed course in resolution of symptoms. Over the next 48 hours, continued conservative management, as outlined above, led to the resolution of symptoms, and the athlete returned to play. At a 2-month follow-up, the athlete described normal function in his knee without any residual symptoms. He returned to play without any symptoms. At 6 months, the athlete underwent MRI of the same knee for an unrelated reason. MRI revealed a healed fat fracture with resolution of the fluid defect in the subcutaneous fat (Figures 4A, 4B).
DISCUSSION
A fat fracture was first described in 1972 in 12 cases of buttock fat fractures after blunt trauma.3 The authors explained that fat lobules are typically arranged in layers and supported by horizontal and vertical fibrous septa. Typical loads flatten the lobules and disperse the forces throughout the layer. However, abnormal loads to a local area disrupt the fat lobules and shear the septa, resulting in decreased integrity of the interface between the epidermis and the fascia.
However, the extremities typically have less adipose tissue than in the buttocks, and the anterior knee is prone to blunt trauma. A previous description of a fat fracture in the knee noted a palpable defect in the quadriceps tendon and an inability to perform a straight leg raise. Our case initially presented with swelling, which concealed any soft-tissue defect. Furthermore, a straight leg raise was always intact despite the fat fracture defect surfacing after anterior swelling subsided. However, the disparity in these 2 cases highlights the spectrum of injury that is possible, as well as the difficulty in diagnosing a fat fracture. The previous report used ultrasound to confirm the diagnosis and assess the integrity of adjacent musculotendinous structures. An ultrasound may be readily available in athletic training rooms.1 Of note, to the best of our knowledge, this is the first case in the literature to report a fat fracture in a professional athlete and in baseball players. Furthermore, this case report describes an athlete who presented with anterior and medial knee pain. The edema from the fat fracture dispersed into the medial prepatellar bursa, which could be confused with edema from an injury to the medial-sided soft tissues.
Although these injuries do not require operative management, conservative measures may not be as effective as those in a patellar contusion or ligamentous sprain, and prolonged treatment may be necessary. Additionally, healthcare providers should be aware of this possible source of injury and counsel on an appropriate recovery time. Ideally, further recognition of such injuries can facilitate improved management and a faster return to activity.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
1. Thomas RH, Holt MD, James SH, White PG. 'Fat fracture'—a physical sign mimicking tendon rupture. J Bone Joint Surg Br. 2001;83(2):204-205.
2. Antich T, Randall CC, Westbrook RA, Morrissey MC, Brewster CE. Physical therapy treatment of knee extensor mechanism disorders: comparison of four treatment modalities*. J Orthop Sports Phys Ther. 1986;8(5):255-259.
3. Meggitt BF, Wilson JN. The battered buttock syndrome—fat fractures. A report on a group of traumatic lipomata. Br J Surg. 1972;59(3):165-169.
TAKE-HOME POINTS
- A fat fracture should be considered in the setting of a blunt injury to the anterior knee when a palpable soft-tissue defect is observed and the extensor mechanism is clinically intact.
- An ultrasound or MRI can assist in making the diagnosis, which can aid in guiding the patient with management and in determining the expected duration of symptoms.
- Injuries to the anterior knee that may present as contusions but have a prolonged course of symptoms should not be overlooked.
Dual Radial Styloid and Volar Plating for Unstable Fractures of the Distal Radius
ABSTRACT
As the operative management of displaced distal radius fractures evolves, intraoperative techniques and fixation strategies evolve as well. Achieving and maintaining an acceptable reduction is paramount but can be difficult with particular fracture patterns. In this article, we describe the use of a radial column plate as a reduction tool in the management of unstable distal radius fractures, along with clinical and radiographic clinical outcomes. This technique can be useful in situations where multiplanar instability exists, or simply when intraoperative assistance is limited. Surgeons can expect acceptable radiographic and clinical outcomes when using this technique, although effects on scar formation and wrist range of motion are currently not known.
Continue to: Distal radius fractures...
Distal radius fractures are among the most common orthopedic injuries encountered; their reported incidence is >640,000 annually and is estimated to increase.1-4 The management of these injuries has evolved from closed reduction and casting to percutaneous pinning and internal fixation, as the importance of achieving and maintaining an anatomic reduction has become more apparent.5-7 More recently, volar locking plates have emerged as a way to prevent complications associated with dorsal plating. Most authors agree that volar locked plating achieves stable fixation and allows for early postoperative wrist range of motion (ROM).5,8-11 However, a volar approach to a dorsally unstable fracture creates difficulty with regard to reduction at the time of surgery and several reports have noted mechanical failure with utilization of locked volar plating alone.12-15
Dual plating of unstable distal radius fractures with a volar locking plate and a radial column plate has been described in the past in the setting of severely comminuted fractures or in patterns with a large radial styloid fragment that was not addressed with a volar locking plate alone.16-19 The purpose of this study is to present the use of the radial column plate as a tool that allows a surgeon to achieve and maintain reduction during open reduction and internal fixation (ORIF) of an unstable distal radius fracture.
OPERATIVE TECHNIQUE
Patients for whom ORIF is indicated include those with unstable distal radius fractures, with or without intra-articular extension and involvement of both the intermediate and lateral columns.
The patient is positioned supine on the operating table with the operative hand placed palm-up on a radiolucent hand table. A volar approach to the distal radius is undertaken, utilizing the interval between the flexor carpi radialis (FCR) tendon and the radial artery. The floor of the FCR sheath is incised, and a self-retaining retractor with blunt tips can be placed to permit visualization. The pronator quadratus (PQ) is sharply reflected off the radial boarder of the distal radius and approximately 1 mm to 2 mm proximal to the radiocarpal joint with an L-shaped incision for fracture site exposure. The brachioradialis is then identified and tenotomized with a scalpel (Figure 1).
A preliminary reduction is then performed using a combination of axial traction and palmar translation of the carpus. The surgeon should not be concerned with radial height or inclination at this point; however, volar tilt should be established as best as possible. A rolled towel is placed dorsal to the metacarpals, holding the wrist in a flexed position as it is placed back onto the radiolucent hand table.
Continue to: A 7 to 8 hole...
A 7 to 8 hole 2.0-mm reconstruction plate (DePuy Synthes) is bent to the shape of the radial boarder of the distal radius. Undercontouring of the plate is necessary to allow for its use as a reduction tool. The plate is then applied to the radial column ensuring that the distal aspect of the plate engages the distal fracture fragment(s) (Figure 2). A single 2.4-mm fully threaded cortical screw in the radial to ulnar direction is then placed bicortically in the proximal fragment in the hole nearest the fracture site. As the screw is tightened, the plate will push the distal fragment(s) due to its undercontoured shape, and in doing so, will restore radial height and inclination (Figure 3). As this screw is being used as a “working screw,” it will be longer than needed after final tightening. A second screw is then placed proximally to prevent rotation of the plate, and the initial screw can be replaced if its length is of concern. If it is the intention of the surgeon to remove the plate prior to wound closure, the second screw is typically not necessary, and there is no indication for exchanging the first screw if it is long.
At this point, final changes to the reduction can still be performed, as the distal fragment(s) has no fixation except for a buttress plate on its radial border. However, the pressure applied by this plate is still typically adequate to maintain a reduction without the use of percutaneous pins or an assistant holding the reduction. Volar fixation is then applied and positioned under both direct visualization and fluoroscopic aid, and cortical and locking screws are inserted as needed (Figure 4). The radial styloid plate can then be removed; however, it is our preference to leave it in place, as we have not seen any postoperative issues that we can attribute to this technique. The PQ is then repaired over the volar locking plate directly to the radial column plate.
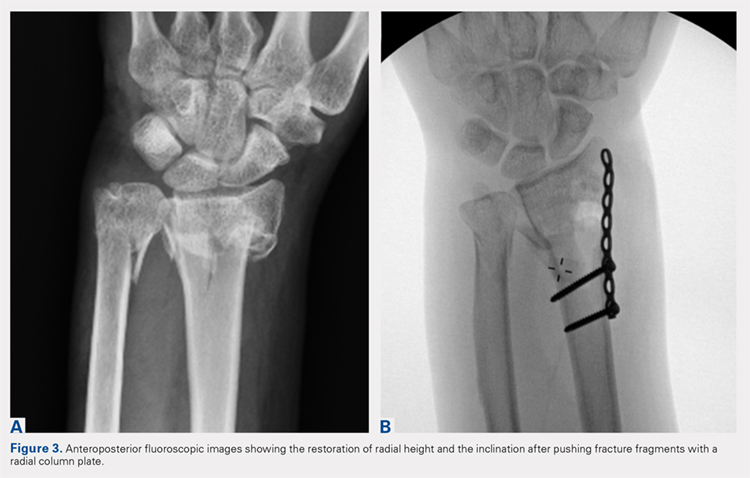
At our institution, patients are maintained in a plaster volar-based wrist splint for a period of 2 weeks postoperatively. After splint and suture removal, active and passive ROM exercises of the wrist and hand are initiated, and a custom thermoplast volar wrist splint is manufactured. This splint is to be worn at all times except during physical therapy. At the 6-week postoperative visit, all restrictions are lifted, assuming there are no complications or unexpected issues. Patients are then seen for follow-up at 3 and 6 months postoperatively. Continued follow-up is indicated if patients are following an abnormal clinical or radiographic course.
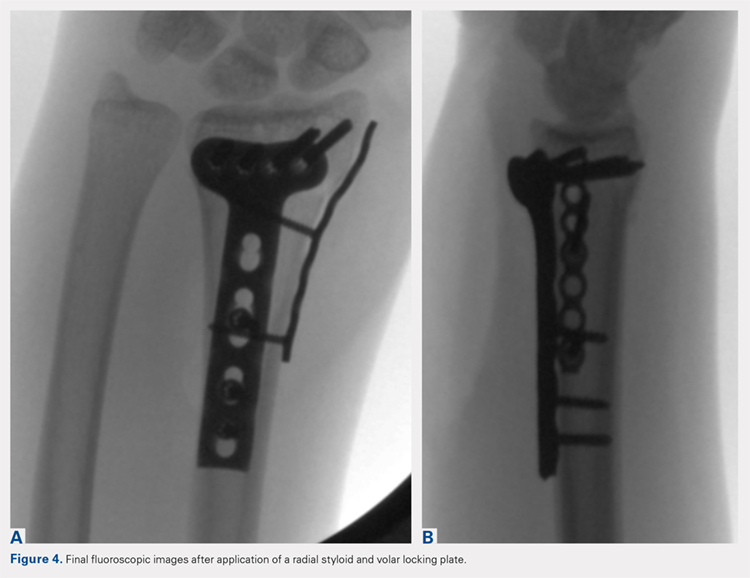
MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a clinical outcomes registry was queried to identify all patients treated operatively by the senior author (DGL) for a distal radius fracture at our Level 1 trauma center between August 2002 and December 2013. Adult (age >18 years) patients with isolated distal radius fractures treated with a radial styloid plate were included for initial review (N = 261). Patients for whom 6-month clinical or radiographic outcomes were unknown were then excluded (n = 225).
Patient demographics were recorded from the existing database along with visual analog scale, Quick Disabilities of the Arm, Shoulder and Hand (DASH), and short form 36 (SF-36) physical component scores (PCS) and mental component scores (MCS) from the final follow-up visit. Injury and intraoperative and final radiographs were assessed by a single reviewer (MRG) using calibrated radiographs on our institution’s picture archiving and communication system. Radial height, radial inclination, and volar tilt were documented for each time point except for radial height, which was not recorded for intraoperative fluoroscopy images due to lack of calibration. Intra-articular extension was noted on injury films. Wound complications, the presence of a deep or superficial infection, and removal of implants after union were recorded.
Continue to: RESULTS
RESULTS
Thirty-six patients met the inclusion criteria and were therefore included in the study. The average age at the time of surgery was 60.6 years (range, 25-87 years), 27 patients (75%) were female, and 21 (58%) had left-sided injuries. Patient comorbidities can be seen in Table 1. Twenty-six fractures (72.2%) had intra-articular extension. Average follow-up was 15.6 months (range, 6-53.9 months).
Table 1. Comorbidities of Patients Treated with Radial Column Plating
| Total No. of patients | 36 | |
| Diabetes mellitus | 2 | 5.6% |
| Hyperlipidemia | 7 | 19.4% |
| Hypertension | 11 | 30.6% |
| Current smoker | 4 | 11.1% |
| Current alcohol abuse | 1 | 2.8% |
| Peripheral vascular disease | 0 | 0.0% |
| Mean body mass index | 27.0 | Range: 19-34.5 |
Radiographic measurements at the time of injury, surgery, and final follow-up can be seen in Table 2. As previously noted, radial height could not be recorded on intraoperative films due to the use of fluoroscopy, which is not calibrated at our institution. The average changes in radial inclination and volar tilt from the time of surgery (intraoperative fluoroscopy) to final follow-up were 0.46° (range, −4.4°-4.3°) and 0.24° (range, −10.6°-9.6°), respectively. All patients had acceptable radial height, radial inclination, and volar tilt at final follow-up. Clinical outcomes were obtained at a mean of 15.6 months (range, 6-54 months) and were generally good, with a mean DASH score of 20.7 (range, 0-57.5), SF-36 PCS of 45.4 (range, 22.7-68.0), and SF-36 MCS of 50.5 (range, 22.3-64.1) (Table 3). Of the 36 patients with 6-month outcome scores, 13 (36.1%) elected for implant removal after fracture union at a mean of 7.6 months after index surgery (range, 3.2-49.8 months). No infections or wound complications were noted.
Table 2. Radiographic Measurements for Patients Treated with Radial Column Plating
| Mean Measurement | Range | |
| Injury radiographs | ||
| Radial inclination (degrees) | 7.3 | −22.9-22 |
| Radial height (mm) | 3.3 | −14.9-11.5 |
| Volar tilt (degrees) | −10.4 | −49.2-33.9 |
| Intraoperative fluoroscopy | ||
| Radial inclination (degrees) | 21.1 | 13.1-26.6 |
| Volar tilt (degrees) | 6.2 | −3.6-12.2 |
| Final radiographs | ||
| Radial inclination (degrees) | 21.5 | 14.5-29.2 |
| Radial height (mm) | 11.0 | 7.6-14.6 |
| Volar tilt (degrees) | 6.8 | −12.4-18.8 |
DISCUSSION
In this article, we described the use of a radial column plate as a tool to achieve and maintain a reduction during the surgical fixation of an unstable distal radius fracture with a volar locking plate. We have further presented a series of 36 patients treated in this manner and their clinical and radiographic outcomes. This technique permits the maintenance of coronal alignment, thereby limiting the use of percutaneous techniques or the need to manually hold fracture fragments in a reduced position, which may be valuable to the surgeon who is operating without a surgical assistant.
Table 3. Clinical Outcome Scores at Final Follow-Up for Patients Treated with Radial Column Plating
| Outcome Score | Mean Score | Range |
| VAS | 1.4 | 0-7.5 |
| DASH | 20.7 | 0-57.5 |
| PCS | 45.4 | 22.7-68 |
| MCS | 50.5 | 22.3-64.1 |
Abbreviations: DASH, Quick Disabilities of the Arm, Shoulder and Hand; MCS, mental component scores; PCS, physical component scores; VAS, visual analog scale.
In addition to its value as a reduction tool, unlike traditional temporary k-wire fixation, we believe that the utilization of a radial styloid plate allows for increased stability until fracture union is achieved. Biomechanical studies have demonstrated favorable results with the use of a radial column plate. Grindel and colleagues20 evaluated dual radial styloid and volar radius plating vs volar plating alone in their biomechanical comparison of 8 cadaveric matched hand and forearm pairs. Specimens were fixated with a volar locking plate, and a 1-cm wedge osteotomy was created dorsally approximately 2 cm from the articular margin. The distal fragment was then osteotomized longitudinally between the 2 ulnar and 2 radial distal locking screws to create a fracture pattern that mimics a dorsally unstable injury with intra-articular extension. Half of the specimens then underwent radial styloid plating with 2 screws securing the construct proximally, and load-to-failure testing was performed. The authors found that utilization of both the volar and radial styloid plates resulted in 50% increased stiffness and 76% increased force-to-failure as compared with radial styloid plating alone. Similar, although not statistically significant, results were found by Blythe and colleagues.21 In their cadaveric study, dorsal and volar plating with an additional radial column plate resulted in improved stiffness with axial loading compared to volar or dorsal plating alone 21.
Two prior studies have presented outcome data after fixation of distal radius fractures with radial column and volar radius dual plating. Tang and colleagues16 described this technique and presented postoperative outcomes in 8 patients followed for an average of 35 weeks. They reported a 100% union rate, no loss of reduction, and a mean DASH score of 19.9. Jacobi and colleagues17 also described this technique in their 2010 report. In their cohort of 10 patients treated by multiple surgeons, they found a mean of 39° of flexion, 49° of extension, 75° of pronation, and 75° of supination at 24 months postoperatively. Eight patients were rated as “excellent,” 1 as “good,” and 1 as “fair” according to the Gartland and Werley score, with all 10 cases achieving bony union. No cases demonstrated loss of volar tilt, radial length, or radial inclination. In both studies, however, the use of the radial column plate was advocated as a fragment-specific fixation tool and not as a reduction tool.
Continue to: Although 1-year DASH scores...
Although 1-year DASH scores for volar plating alone have been shown in the literature to be consistently within 6 and 13, 3-month and 6-month scores have historically been >18.22-27 Our short-term clinical results (Table 3) are comparable to these historic controls. Further, within our cohort there were no cases of nonunion, postoperative infection, or wound complications, and radiographic measures show maintenance of reduction at final follow-up (Table 2).
We do recognize that 36.1% (13/36) of our cohort had their distal radius implants removed. Although this incidence is high, it stems from the fact that patients who elect for implant removal are more likely to have had an atypical postoperative course and are therefore followed for longer than 6 months. Those who do not elect for removal are typically discharged from care after their 3-month postoperative visit, and were therefore not eligible for inclusion in this study. Overall, a total of 261 patients have been treated with this technique by the senior surgeon. Of those patients, only 28 (10.7%) underwent removal of surgical implants. If the remaining patients had been followed for the full 6 months, it is likely that outcome scores would have been skewed in a more favorable direction.
Surgeons electing to utilize radial styloid plating for displaced distal radius fractures should recognize that the required increased surgical dissection might lead to increased scar formation and postoperative stiffness. A limitation of this study is the lack of quantitative wrist ROM data. Future studies may compare final clinical outcomes and ROM for patients treated with and without radial column fixation.
CONCLUSION
We advocate for the use of a radial column plate as a tool to help achieve and maintain fracture reduction in the setting of an unstable distal radius fracture being treated with ORIF. This technique may be particularly useful when a surgical assistant is not available. Surgeons can expect clinical and radiographic results that are similar to those of volar locked plating alone.
1. Larsen CF, Lauritsen J. Epidemiology of acute wrist trauma. Int J Epidemiol. 1993;22(5):911-916.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915. doi:10.1053/jhsu.2001.26322.
3. Melton LJ 3rd, Amadio PC, Crowson CS, O'Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8(4):341-348.
4. Hagino H, Yamamoto K, Ohshiro H, Nakamura T, Kishimoto H, Nose T. Changing incidence of hip, distal radius, and proximal humerus fractures in Tottori Prefecture, Japan. Bone. 1999;24(3):265-270.
5. Diaz-Garcia RJ, Chung KC. The evolution of distal radius fracture management: A historical treatise. Hand Clin. 2012;28(2):105-111. doi:10.1016/j.hcl.2012.02.007.
6. McQueen M, Caspers J. Colles fracture: Does the anatomical result affect the final function? J Bone Joint Surg Br. 1988;70(4):649-651.
7. Stewart HD, Innes AR, Burke FD. Factors affecting the outcome of Colles' fracture: an anatomical and functional study. Injury. 1985;16(5):289-295.
8. Knight D, Hajducka C, Will E, McQueen M. Locked volar plating for unstable distal radial fractures: Clinical and radiological outcomes. Injury. 2010;41(2):184-189. doi:10.1016/j.injury.2009.08.024.
9. Anakwe R, Khan L, Cook R, McEachan J. Locked volar plating for complex distal radius fractures: patient reported outcomes and satisfaction. J Orthop Surg Res. 2010;5:51. doi:10.1186/1749799X-5-51.
10. Gruber G, Gruber K, Giessauf C, et al. Volar plate fixation of AO type C2 and C3 distal radius fractures, a single-center study of 55 patients. J Orthop Trauma. 2008;22(7):467-472. doi:10.1097/BOT.0b013e318180db09.
11. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861. doi:10.2106/JBJS.G.01569.
12. Foo TL, Gan AW, Soh T, Chew WY. Mechanical failure of the distal radius volar locking plate. J Orthop Surg (Hong Kong). 2013;21(3):332-336. doi:10.11777/230949901302100314.
13. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189. doi:10.1007/s11552-010-9313-5.
14. Min W, Kaplan K, Miyamoto R, Tejwani NC. A unique failure mechanism of a distal radius fracture fixed with volar plating--a case report. Bull NYU Hosp Jt Dis. 2010;68(4):304-306.
15. Cao J, Ozer K. Failure of volar locking plate fixation of an extraarticular distal radius fracture: A case report. Patient Saf Surg. 2010;4(1):19. doi:10.1186/1754-9493-4-19.
16. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149. doi:10.1097/BTH.0b013e3181cae14d.
17. Jacobi M, Wahl P, Kohut G. Repositioning and stabilization of the radial styloid process in comminuted fractures of the distal radius using a single approach: The radio-volar double plating technique. J Orthop Surg Res. 2010;5:55. doi:10.1186/1749-799X-5-55.
18. Rikli DA, Regazzoni P. The double plating technique for distal radius fractures. Tech Hand Up Extrem Surg. 2000;4(2):107-114.
19. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):588-592.
20. Grindel SI, Wang M, Gerlach M, McGrady LM, Brown S. Biomechanical comparison of fixed-angle volar plate versus fixed-angle volar plate plus fragment-specific fixation in a cadaveric distal radius fracture model. J Hand Surg Am. 2007;32(2):194-199. doi:10.1016/j.jhsa.2006.12.003.
21. Blythe M, Stoffel K, Jarrett P, Kuster M. Volar versus dorsal locking plates with and without radial styloid locking plates for the fixation of dorsally comminuted distal radius fractures: A biomechanical study in cadavers. J Hand Surg Am. 2006;31(10):1587-1593. doi:10.1016/j.jhsa.2006.09.011.
22. Loveridge J, Ahearn N, Gee C, Pearson D, Sivaloganathan S, Bhatia R. Treatment of distal radial fractures with the DVR-A plate--the early bristol experience. Hand Surg. 2013;18(2):159-167. doi:10.1142/S0218810413500184.
23. Karantana A, Downing ND, Forward DP, et al. Surgical treatment of distal radial fractures with a volar locking plate versus conventional percutaneous methods: a randomized controlled trial. J Bone Joint Surg Am. 2013;95(19):1737-1744. doi:10.2106/JBJS.L.00232.
24. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: A randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221. doi:10.1302/0301-620X.90B9.20521.
25. von Recum J, Matschke S, Jupiter JB, et al. Characteristics of two different locking compression plates in the volar fixation of complex articular distal radius fractures. Bone Joint Res. 2012;1(6):111-117. doi:10.1302/2046-3758.16.2000008.
26. Safi A, Hart R, Těknědžjan B, Kozák T. Treatment of extra-articular and simple articular distal radial fractures with intramedullary nail versus volar locking plate. J Hand Surg Eur Vol. 2013;38(7):774-779. doi:10.1177/1753193413478715.
27. Kim JK, Park SD. Outcomes after volar plate fixation of low-grade open and closed distal radius fractures are similar. Clin Orthop Relat Res. 2013;471(6):2030-2035. doi:10.1007/s11999-013-2798-9.
ABSTRACT
As the operative management of displaced distal radius fractures evolves, intraoperative techniques and fixation strategies evolve as well. Achieving and maintaining an acceptable reduction is paramount but can be difficult with particular fracture patterns. In this article, we describe the use of a radial column plate as a reduction tool in the management of unstable distal radius fractures, along with clinical and radiographic clinical outcomes. This technique can be useful in situations where multiplanar instability exists, or simply when intraoperative assistance is limited. Surgeons can expect acceptable radiographic and clinical outcomes when using this technique, although effects on scar formation and wrist range of motion are currently not known.
Continue to: Distal radius fractures...
Distal radius fractures are among the most common orthopedic injuries encountered; their reported incidence is >640,000 annually and is estimated to increase.1-4 The management of these injuries has evolved from closed reduction and casting to percutaneous pinning and internal fixation, as the importance of achieving and maintaining an anatomic reduction has become more apparent.5-7 More recently, volar locking plates have emerged as a way to prevent complications associated with dorsal plating. Most authors agree that volar locked plating achieves stable fixation and allows for early postoperative wrist range of motion (ROM).5,8-11 However, a volar approach to a dorsally unstable fracture creates difficulty with regard to reduction at the time of surgery and several reports have noted mechanical failure with utilization of locked volar plating alone.12-15
Dual plating of unstable distal radius fractures with a volar locking plate and a radial column plate has been described in the past in the setting of severely comminuted fractures or in patterns with a large radial styloid fragment that was not addressed with a volar locking plate alone.16-19 The purpose of this study is to present the use of the radial column plate as a tool that allows a surgeon to achieve and maintain reduction during open reduction and internal fixation (ORIF) of an unstable distal radius fracture.
OPERATIVE TECHNIQUE
Patients for whom ORIF is indicated include those with unstable distal radius fractures, with or without intra-articular extension and involvement of both the intermediate and lateral columns.
The patient is positioned supine on the operating table with the operative hand placed palm-up on a radiolucent hand table. A volar approach to the distal radius is undertaken, utilizing the interval between the flexor carpi radialis (FCR) tendon and the radial artery. The floor of the FCR sheath is incised, and a self-retaining retractor with blunt tips can be placed to permit visualization. The pronator quadratus (PQ) is sharply reflected off the radial boarder of the distal radius and approximately 1 mm to 2 mm proximal to the radiocarpal joint with an L-shaped incision for fracture site exposure. The brachioradialis is then identified and tenotomized with a scalpel (Figure 1).

A preliminary reduction is then performed using a combination of axial traction and palmar translation of the carpus. The surgeon should not be concerned with radial height or inclination at this point; however, volar tilt should be established as best as possible. A rolled towel is placed dorsal to the metacarpals, holding the wrist in a flexed position as it is placed back onto the radiolucent hand table.
Continue to: A 7 to 8 hole...
A 7 to 8 hole 2.0-mm reconstruction plate (DePuy Synthes) is bent to the shape of the radial boarder of the distal radius. Undercontouring of the plate is necessary to allow for its use as a reduction tool. The plate is then applied to the radial column ensuring that the distal aspect of the plate engages the distal fracture fragment(s) (Figure 2). A single 2.4-mm fully threaded cortical screw in the radial to ulnar direction is then placed bicortically in the proximal fragment in the hole nearest the fracture site. As the screw is tightened, the plate will push the distal fragment(s) due to its undercontoured shape, and in doing so, will restore radial height and inclination (Figure 3). As this screw is being used as a “working screw,” it will be longer than needed after final tightening. A second screw is then placed proximally to prevent rotation of the plate, and the initial screw can be replaced if its length is of concern. If it is the intention of the surgeon to remove the plate prior to wound closure, the second screw is typically not necessary, and there is no indication for exchanging the first screw if it is long.

At this point, final changes to the reduction can still be performed, as the distal fragment(s) has no fixation except for a buttress plate on its radial border. However, the pressure applied by this plate is still typically adequate to maintain a reduction without the use of percutaneous pins or an assistant holding the reduction. Volar fixation is then applied and positioned under both direct visualization and fluoroscopic aid, and cortical and locking screws are inserted as needed (Figure 4). The radial styloid plate can then be removed; however, it is our preference to leave it in place, as we have not seen any postoperative issues that we can attribute to this technique. The PQ is then repaired over the volar locking plate directly to the radial column plate.

At our institution, patients are maintained in a plaster volar-based wrist splint for a period of 2 weeks postoperatively. After splint and suture removal, active and passive ROM exercises of the wrist and hand are initiated, and a custom thermoplast volar wrist splint is manufactured. This splint is to be worn at all times except during physical therapy. At the 6-week postoperative visit, all restrictions are lifted, assuming there are no complications or unexpected issues. Patients are then seen for follow-up at 3 and 6 months postoperatively. Continued follow-up is indicated if patients are following an abnormal clinical or radiographic course.

MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a clinical outcomes registry was queried to identify all patients treated operatively by the senior author (DGL) for a distal radius fracture at our Level 1 trauma center between August 2002 and December 2013. Adult (age >18 years) patients with isolated distal radius fractures treated with a radial styloid plate were included for initial review (N = 261). Patients for whom 6-month clinical or radiographic outcomes were unknown were then excluded (n = 225).
Patient demographics were recorded from the existing database along with visual analog scale, Quick Disabilities of the Arm, Shoulder and Hand (DASH), and short form 36 (SF-36) physical component scores (PCS) and mental component scores (MCS) from the final follow-up visit. Injury and intraoperative and final radiographs were assessed by a single reviewer (MRG) using calibrated radiographs on our institution’s picture archiving and communication system. Radial height, radial inclination, and volar tilt were documented for each time point except for radial height, which was not recorded for intraoperative fluoroscopy images due to lack of calibration. Intra-articular extension was noted on injury films. Wound complications, the presence of a deep or superficial infection, and removal of implants after union were recorded.
Continue to: RESULTS
RESULTS
Thirty-six patients met the inclusion criteria and were therefore included in the study. The average age at the time of surgery was 60.6 years (range, 25-87 years), 27 patients (75%) were female, and 21 (58%) had left-sided injuries. Patient comorbidities can be seen in Table 1. Twenty-six fractures (72.2%) had intra-articular extension. Average follow-up was 15.6 months (range, 6-53.9 months).
Table 1. Comorbidities of Patients Treated with Radial Column Plating
| Total No. of patients | 36 | |
| Diabetes mellitus | 2 | 5.6% |
| Hyperlipidemia | 7 | 19.4% |
| Hypertension | 11 | 30.6% |
| Current smoker | 4 | 11.1% |
| Current alcohol abuse | 1 | 2.8% |
| Peripheral vascular disease | 0 | 0.0% |
| Mean body mass index | 27.0 | Range: 19-34.5 |
Radiographic measurements at the time of injury, surgery, and final follow-up can be seen in Table 2. As previously noted, radial height could not be recorded on intraoperative films due to the use of fluoroscopy, which is not calibrated at our institution. The average changes in radial inclination and volar tilt from the time of surgery (intraoperative fluoroscopy) to final follow-up were 0.46° (range, −4.4°-4.3°) and 0.24° (range, −10.6°-9.6°), respectively. All patients had acceptable radial height, radial inclination, and volar tilt at final follow-up. Clinical outcomes were obtained at a mean of 15.6 months (range, 6-54 months) and were generally good, with a mean DASH score of 20.7 (range, 0-57.5), SF-36 PCS of 45.4 (range, 22.7-68.0), and SF-36 MCS of 50.5 (range, 22.3-64.1) (Table 3). Of the 36 patients with 6-month outcome scores, 13 (36.1%) elected for implant removal after fracture union at a mean of 7.6 months after index surgery (range, 3.2-49.8 months). No infections or wound complications were noted.
Table 2. Radiographic Measurements for Patients Treated with Radial Column Plating
| Mean Measurement | Range | |
| Injury radiographs | ||
| Radial inclination (degrees) | 7.3 | −22.9-22 |
| Radial height (mm) | 3.3 | −14.9-11.5 |
| Volar tilt (degrees) | −10.4 | −49.2-33.9 |
| Intraoperative fluoroscopy | ||
| Radial inclination (degrees) | 21.1 | 13.1-26.6 |
| Volar tilt (degrees) | 6.2 | −3.6-12.2 |
| Final radiographs | ||
| Radial inclination (degrees) | 21.5 | 14.5-29.2 |
| Radial height (mm) | 11.0 | 7.6-14.6 |
| Volar tilt (degrees) | 6.8 | −12.4-18.8 |
DISCUSSION
In this article, we described the use of a radial column plate as a tool to achieve and maintain a reduction during the surgical fixation of an unstable distal radius fracture with a volar locking plate. We have further presented a series of 36 patients treated in this manner and their clinical and radiographic outcomes. This technique permits the maintenance of coronal alignment, thereby limiting the use of percutaneous techniques or the need to manually hold fracture fragments in a reduced position, which may be valuable to the surgeon who is operating without a surgical assistant.
Table 3. Clinical Outcome Scores at Final Follow-Up for Patients Treated with Radial Column Plating
| Outcome Score | Mean Score | Range |
| VAS | 1.4 | 0-7.5 |
| DASH | 20.7 | 0-57.5 |
| PCS | 45.4 | 22.7-68 |
| MCS | 50.5 | 22.3-64.1 |
Abbreviations: DASH, Quick Disabilities of the Arm, Shoulder and Hand; MCS, mental component scores; PCS, physical component scores; VAS, visual analog scale.
In addition to its value as a reduction tool, unlike traditional temporary k-wire fixation, we believe that the utilization of a radial styloid plate allows for increased stability until fracture union is achieved. Biomechanical studies have demonstrated favorable results with the use of a radial column plate. Grindel and colleagues20 evaluated dual radial styloid and volar radius plating vs volar plating alone in their biomechanical comparison of 8 cadaveric matched hand and forearm pairs. Specimens were fixated with a volar locking plate, and a 1-cm wedge osteotomy was created dorsally approximately 2 cm from the articular margin. The distal fragment was then osteotomized longitudinally between the 2 ulnar and 2 radial distal locking screws to create a fracture pattern that mimics a dorsally unstable injury with intra-articular extension. Half of the specimens then underwent radial styloid plating with 2 screws securing the construct proximally, and load-to-failure testing was performed. The authors found that utilization of both the volar and radial styloid plates resulted in 50% increased stiffness and 76% increased force-to-failure as compared with radial styloid plating alone. Similar, although not statistically significant, results were found by Blythe and colleagues.21 In their cadaveric study, dorsal and volar plating with an additional radial column plate resulted in improved stiffness with axial loading compared to volar or dorsal plating alone 21.
Two prior studies have presented outcome data after fixation of distal radius fractures with radial column and volar radius dual plating. Tang and colleagues16 described this technique and presented postoperative outcomes in 8 patients followed for an average of 35 weeks. They reported a 100% union rate, no loss of reduction, and a mean DASH score of 19.9. Jacobi and colleagues17 also described this technique in their 2010 report. In their cohort of 10 patients treated by multiple surgeons, they found a mean of 39° of flexion, 49° of extension, 75° of pronation, and 75° of supination at 24 months postoperatively. Eight patients were rated as “excellent,” 1 as “good,” and 1 as “fair” according to the Gartland and Werley score, with all 10 cases achieving bony union. No cases demonstrated loss of volar tilt, radial length, or radial inclination. In both studies, however, the use of the radial column plate was advocated as a fragment-specific fixation tool and not as a reduction tool.
Continue to: Although 1-year DASH scores...
Although 1-year DASH scores for volar plating alone have been shown in the literature to be consistently within 6 and 13, 3-month and 6-month scores have historically been >18.22-27 Our short-term clinical results (Table 3) are comparable to these historic controls. Further, within our cohort there were no cases of nonunion, postoperative infection, or wound complications, and radiographic measures show maintenance of reduction at final follow-up (Table 2).
We do recognize that 36.1% (13/36) of our cohort had their distal radius implants removed. Although this incidence is high, it stems from the fact that patients who elect for implant removal are more likely to have had an atypical postoperative course and are therefore followed for longer than 6 months. Those who do not elect for removal are typically discharged from care after their 3-month postoperative visit, and were therefore not eligible for inclusion in this study. Overall, a total of 261 patients have been treated with this technique by the senior surgeon. Of those patients, only 28 (10.7%) underwent removal of surgical implants. If the remaining patients had been followed for the full 6 months, it is likely that outcome scores would have been skewed in a more favorable direction.
Surgeons electing to utilize radial styloid plating for displaced distal radius fractures should recognize that the required increased surgical dissection might lead to increased scar formation and postoperative stiffness. A limitation of this study is the lack of quantitative wrist ROM data. Future studies may compare final clinical outcomes and ROM for patients treated with and without radial column fixation.
CONCLUSION
We advocate for the use of a radial column plate as a tool to help achieve and maintain fracture reduction in the setting of an unstable distal radius fracture being treated with ORIF. This technique may be particularly useful when a surgical assistant is not available. Surgeons can expect clinical and radiographic results that are similar to those of volar locked plating alone.
ABSTRACT
As the operative management of displaced distal radius fractures evolves, intraoperative techniques and fixation strategies evolve as well. Achieving and maintaining an acceptable reduction is paramount but can be difficult with particular fracture patterns. In this article, we describe the use of a radial column plate as a reduction tool in the management of unstable distal radius fractures, along with clinical and radiographic clinical outcomes. This technique can be useful in situations where multiplanar instability exists, or simply when intraoperative assistance is limited. Surgeons can expect acceptable radiographic and clinical outcomes when using this technique, although effects on scar formation and wrist range of motion are currently not known.
Continue to: Distal radius fractures...
Distal radius fractures are among the most common orthopedic injuries encountered; their reported incidence is >640,000 annually and is estimated to increase.1-4 The management of these injuries has evolved from closed reduction and casting to percutaneous pinning and internal fixation, as the importance of achieving and maintaining an anatomic reduction has become more apparent.5-7 More recently, volar locking plates have emerged as a way to prevent complications associated with dorsal plating. Most authors agree that volar locked plating achieves stable fixation and allows for early postoperative wrist range of motion (ROM).5,8-11 However, a volar approach to a dorsally unstable fracture creates difficulty with regard to reduction at the time of surgery and several reports have noted mechanical failure with utilization of locked volar plating alone.12-15
Dual plating of unstable distal radius fractures with a volar locking plate and a radial column plate has been described in the past in the setting of severely comminuted fractures or in patterns with a large radial styloid fragment that was not addressed with a volar locking plate alone.16-19 The purpose of this study is to present the use of the radial column plate as a tool that allows a surgeon to achieve and maintain reduction during open reduction and internal fixation (ORIF) of an unstable distal radius fracture.
OPERATIVE TECHNIQUE
Patients for whom ORIF is indicated include those with unstable distal radius fractures, with or without intra-articular extension and involvement of both the intermediate and lateral columns.
The patient is positioned supine on the operating table with the operative hand placed palm-up on a radiolucent hand table. A volar approach to the distal radius is undertaken, utilizing the interval between the flexor carpi radialis (FCR) tendon and the radial artery. The floor of the FCR sheath is incised, and a self-retaining retractor with blunt tips can be placed to permit visualization. The pronator quadratus (PQ) is sharply reflected off the radial boarder of the distal radius and approximately 1 mm to 2 mm proximal to the radiocarpal joint with an L-shaped incision for fracture site exposure. The brachioradialis is then identified and tenotomized with a scalpel (Figure 1).

A preliminary reduction is then performed using a combination of axial traction and palmar translation of the carpus. The surgeon should not be concerned with radial height or inclination at this point; however, volar tilt should be established as best as possible. A rolled towel is placed dorsal to the metacarpals, holding the wrist in a flexed position as it is placed back onto the radiolucent hand table.
Continue to: A 7 to 8 hole...
A 7 to 8 hole 2.0-mm reconstruction plate (DePuy Synthes) is bent to the shape of the radial boarder of the distal radius. Undercontouring of the plate is necessary to allow for its use as a reduction tool. The plate is then applied to the radial column ensuring that the distal aspect of the plate engages the distal fracture fragment(s) (Figure 2). A single 2.4-mm fully threaded cortical screw in the radial to ulnar direction is then placed bicortically in the proximal fragment in the hole nearest the fracture site. As the screw is tightened, the plate will push the distal fragment(s) due to its undercontoured shape, and in doing so, will restore radial height and inclination (Figure 3). As this screw is being used as a “working screw,” it will be longer than needed after final tightening. A second screw is then placed proximally to prevent rotation of the plate, and the initial screw can be replaced if its length is of concern. If it is the intention of the surgeon to remove the plate prior to wound closure, the second screw is typically not necessary, and there is no indication for exchanging the first screw if it is long.

At this point, final changes to the reduction can still be performed, as the distal fragment(s) has no fixation except for a buttress plate on its radial border. However, the pressure applied by this plate is still typically adequate to maintain a reduction without the use of percutaneous pins or an assistant holding the reduction. Volar fixation is then applied and positioned under both direct visualization and fluoroscopic aid, and cortical and locking screws are inserted as needed (Figure 4). The radial styloid plate can then be removed; however, it is our preference to leave it in place, as we have not seen any postoperative issues that we can attribute to this technique. The PQ is then repaired over the volar locking plate directly to the radial column plate.

At our institution, patients are maintained in a plaster volar-based wrist splint for a period of 2 weeks postoperatively. After splint and suture removal, active and passive ROM exercises of the wrist and hand are initiated, and a custom thermoplast volar wrist splint is manufactured. This splint is to be worn at all times except during physical therapy. At the 6-week postoperative visit, all restrictions are lifted, assuming there are no complications or unexpected issues. Patients are then seen for follow-up at 3 and 6 months postoperatively. Continued follow-up is indicated if patients are following an abnormal clinical or radiographic course.

MATERIALS AND METHODS
After Institutional Review Board approval was obtained, a clinical outcomes registry was queried to identify all patients treated operatively by the senior author (DGL) for a distal radius fracture at our Level 1 trauma center between August 2002 and December 2013. Adult (age >18 years) patients with isolated distal radius fractures treated with a radial styloid plate were included for initial review (N = 261). Patients for whom 6-month clinical or radiographic outcomes were unknown were then excluded (n = 225).
Patient demographics were recorded from the existing database along with visual analog scale, Quick Disabilities of the Arm, Shoulder and Hand (DASH), and short form 36 (SF-36) physical component scores (PCS) and mental component scores (MCS) from the final follow-up visit. Injury and intraoperative and final radiographs were assessed by a single reviewer (MRG) using calibrated radiographs on our institution’s picture archiving and communication system. Radial height, radial inclination, and volar tilt were documented for each time point except for radial height, which was not recorded for intraoperative fluoroscopy images due to lack of calibration. Intra-articular extension was noted on injury films. Wound complications, the presence of a deep or superficial infection, and removal of implants after union were recorded.
Continue to: RESULTS
RESULTS
Thirty-six patients met the inclusion criteria and were therefore included in the study. The average age at the time of surgery was 60.6 years (range, 25-87 years), 27 patients (75%) were female, and 21 (58%) had left-sided injuries. Patient comorbidities can be seen in Table 1. Twenty-six fractures (72.2%) had intra-articular extension. Average follow-up was 15.6 months (range, 6-53.9 months).
Table 1. Comorbidities of Patients Treated with Radial Column Plating
| Total No. of patients | 36 | |
| Diabetes mellitus | 2 | 5.6% |
| Hyperlipidemia | 7 | 19.4% |
| Hypertension | 11 | 30.6% |
| Current smoker | 4 | 11.1% |
| Current alcohol abuse | 1 | 2.8% |
| Peripheral vascular disease | 0 | 0.0% |
| Mean body mass index | 27.0 | Range: 19-34.5 |
Radiographic measurements at the time of injury, surgery, and final follow-up can be seen in Table 2. As previously noted, radial height could not be recorded on intraoperative films due to the use of fluoroscopy, which is not calibrated at our institution. The average changes in radial inclination and volar tilt from the time of surgery (intraoperative fluoroscopy) to final follow-up were 0.46° (range, −4.4°-4.3°) and 0.24° (range, −10.6°-9.6°), respectively. All patients had acceptable radial height, radial inclination, and volar tilt at final follow-up. Clinical outcomes were obtained at a mean of 15.6 months (range, 6-54 months) and were generally good, with a mean DASH score of 20.7 (range, 0-57.5), SF-36 PCS of 45.4 (range, 22.7-68.0), and SF-36 MCS of 50.5 (range, 22.3-64.1) (Table 3). Of the 36 patients with 6-month outcome scores, 13 (36.1%) elected for implant removal after fracture union at a mean of 7.6 months after index surgery (range, 3.2-49.8 months). No infections or wound complications were noted.
Table 2. Radiographic Measurements for Patients Treated with Radial Column Plating
| Mean Measurement | Range | |
| Injury radiographs | ||
| Radial inclination (degrees) | 7.3 | −22.9-22 |
| Radial height (mm) | 3.3 | −14.9-11.5 |
| Volar tilt (degrees) | −10.4 | −49.2-33.9 |
| Intraoperative fluoroscopy | ||
| Radial inclination (degrees) | 21.1 | 13.1-26.6 |
| Volar tilt (degrees) | 6.2 | −3.6-12.2 |
| Final radiographs | ||
| Radial inclination (degrees) | 21.5 | 14.5-29.2 |
| Radial height (mm) | 11.0 | 7.6-14.6 |
| Volar tilt (degrees) | 6.8 | −12.4-18.8 |
DISCUSSION
In this article, we described the use of a radial column plate as a tool to achieve and maintain a reduction during the surgical fixation of an unstable distal radius fracture with a volar locking plate. We have further presented a series of 36 patients treated in this manner and their clinical and radiographic outcomes. This technique permits the maintenance of coronal alignment, thereby limiting the use of percutaneous techniques or the need to manually hold fracture fragments in a reduced position, which may be valuable to the surgeon who is operating without a surgical assistant.
Table 3. Clinical Outcome Scores at Final Follow-Up for Patients Treated with Radial Column Plating
| Outcome Score | Mean Score | Range |
| VAS | 1.4 | 0-7.5 |
| DASH | 20.7 | 0-57.5 |
| PCS | 45.4 | 22.7-68 |
| MCS | 50.5 | 22.3-64.1 |
Abbreviations: DASH, Quick Disabilities of the Arm, Shoulder and Hand; MCS, mental component scores; PCS, physical component scores; VAS, visual analog scale.
In addition to its value as a reduction tool, unlike traditional temporary k-wire fixation, we believe that the utilization of a radial styloid plate allows for increased stability until fracture union is achieved. Biomechanical studies have demonstrated favorable results with the use of a radial column plate. Grindel and colleagues20 evaluated dual radial styloid and volar radius plating vs volar plating alone in their biomechanical comparison of 8 cadaveric matched hand and forearm pairs. Specimens were fixated with a volar locking plate, and a 1-cm wedge osteotomy was created dorsally approximately 2 cm from the articular margin. The distal fragment was then osteotomized longitudinally between the 2 ulnar and 2 radial distal locking screws to create a fracture pattern that mimics a dorsally unstable injury with intra-articular extension. Half of the specimens then underwent radial styloid plating with 2 screws securing the construct proximally, and load-to-failure testing was performed. The authors found that utilization of both the volar and radial styloid plates resulted in 50% increased stiffness and 76% increased force-to-failure as compared with radial styloid plating alone. Similar, although not statistically significant, results were found by Blythe and colleagues.21 In their cadaveric study, dorsal and volar plating with an additional radial column plate resulted in improved stiffness with axial loading compared to volar or dorsal plating alone 21.
Two prior studies have presented outcome data after fixation of distal radius fractures with radial column and volar radius dual plating. Tang and colleagues16 described this technique and presented postoperative outcomes in 8 patients followed for an average of 35 weeks. They reported a 100% union rate, no loss of reduction, and a mean DASH score of 19.9. Jacobi and colleagues17 also described this technique in their 2010 report. In their cohort of 10 patients treated by multiple surgeons, they found a mean of 39° of flexion, 49° of extension, 75° of pronation, and 75° of supination at 24 months postoperatively. Eight patients were rated as “excellent,” 1 as “good,” and 1 as “fair” according to the Gartland and Werley score, with all 10 cases achieving bony union. No cases demonstrated loss of volar tilt, radial length, or radial inclination. In both studies, however, the use of the radial column plate was advocated as a fragment-specific fixation tool and not as a reduction tool.
Continue to: Although 1-year DASH scores...
Although 1-year DASH scores for volar plating alone have been shown in the literature to be consistently within 6 and 13, 3-month and 6-month scores have historically been >18.22-27 Our short-term clinical results (Table 3) are comparable to these historic controls. Further, within our cohort there were no cases of nonunion, postoperative infection, or wound complications, and radiographic measures show maintenance of reduction at final follow-up (Table 2).
We do recognize that 36.1% (13/36) of our cohort had their distal radius implants removed. Although this incidence is high, it stems from the fact that patients who elect for implant removal are more likely to have had an atypical postoperative course and are therefore followed for longer than 6 months. Those who do not elect for removal are typically discharged from care after their 3-month postoperative visit, and were therefore not eligible for inclusion in this study. Overall, a total of 261 patients have been treated with this technique by the senior surgeon. Of those patients, only 28 (10.7%) underwent removal of surgical implants. If the remaining patients had been followed for the full 6 months, it is likely that outcome scores would have been skewed in a more favorable direction.
Surgeons electing to utilize radial styloid plating for displaced distal radius fractures should recognize that the required increased surgical dissection might lead to increased scar formation and postoperative stiffness. A limitation of this study is the lack of quantitative wrist ROM data. Future studies may compare final clinical outcomes and ROM for patients treated with and without radial column fixation.
CONCLUSION
We advocate for the use of a radial column plate as a tool to help achieve and maintain fracture reduction in the setting of an unstable distal radius fracture being treated with ORIF. This technique may be particularly useful when a surgical assistant is not available. Surgeons can expect clinical and radiographic results that are similar to those of volar locked plating alone.
1. Larsen CF, Lauritsen J. Epidemiology of acute wrist trauma. Int J Epidemiol. 1993;22(5):911-916.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915. doi:10.1053/jhsu.2001.26322.
3. Melton LJ 3rd, Amadio PC, Crowson CS, O'Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8(4):341-348.
4. Hagino H, Yamamoto K, Ohshiro H, Nakamura T, Kishimoto H, Nose T. Changing incidence of hip, distal radius, and proximal humerus fractures in Tottori Prefecture, Japan. Bone. 1999;24(3):265-270.
5. Diaz-Garcia RJ, Chung KC. The evolution of distal radius fracture management: A historical treatise. Hand Clin. 2012;28(2):105-111. doi:10.1016/j.hcl.2012.02.007.
6. McQueen M, Caspers J. Colles fracture: Does the anatomical result affect the final function? J Bone Joint Surg Br. 1988;70(4):649-651.
7. Stewart HD, Innes AR, Burke FD. Factors affecting the outcome of Colles' fracture: an anatomical and functional study. Injury. 1985;16(5):289-295.
8. Knight D, Hajducka C, Will E, McQueen M. Locked volar plating for unstable distal radial fractures: Clinical and radiological outcomes. Injury. 2010;41(2):184-189. doi:10.1016/j.injury.2009.08.024.
9. Anakwe R, Khan L, Cook R, McEachan J. Locked volar plating for complex distal radius fractures: patient reported outcomes and satisfaction. J Orthop Surg Res. 2010;5:51. doi:10.1186/1749799X-5-51.
10. Gruber G, Gruber K, Giessauf C, et al. Volar plate fixation of AO type C2 and C3 distal radius fractures, a single-center study of 55 patients. J Orthop Trauma. 2008;22(7):467-472. doi:10.1097/BOT.0b013e318180db09.
11. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861. doi:10.2106/JBJS.G.01569.
12. Foo TL, Gan AW, Soh T, Chew WY. Mechanical failure of the distal radius volar locking plate. J Orthop Surg (Hong Kong). 2013;21(3):332-336. doi:10.11777/230949901302100314.
13. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189. doi:10.1007/s11552-010-9313-5.
14. Min W, Kaplan K, Miyamoto R, Tejwani NC. A unique failure mechanism of a distal radius fracture fixed with volar plating--a case report. Bull NYU Hosp Jt Dis. 2010;68(4):304-306.
15. Cao J, Ozer K. Failure of volar locking plate fixation of an extraarticular distal radius fracture: A case report. Patient Saf Surg. 2010;4(1):19. doi:10.1186/1754-9493-4-19.
16. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149. doi:10.1097/BTH.0b013e3181cae14d.
17. Jacobi M, Wahl P, Kohut G. Repositioning and stabilization of the radial styloid process in comminuted fractures of the distal radius using a single approach: The radio-volar double plating technique. J Orthop Surg Res. 2010;5:55. doi:10.1186/1749-799X-5-55.
18. Rikli DA, Regazzoni P. The double plating technique for distal radius fractures. Tech Hand Up Extrem Surg. 2000;4(2):107-114.
19. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):588-592.
20. Grindel SI, Wang M, Gerlach M, McGrady LM, Brown S. Biomechanical comparison of fixed-angle volar plate versus fixed-angle volar plate plus fragment-specific fixation in a cadaveric distal radius fracture model. J Hand Surg Am. 2007;32(2):194-199. doi:10.1016/j.jhsa.2006.12.003.
21. Blythe M, Stoffel K, Jarrett P, Kuster M. Volar versus dorsal locking plates with and without radial styloid locking plates for the fixation of dorsally comminuted distal radius fractures: A biomechanical study in cadavers. J Hand Surg Am. 2006;31(10):1587-1593. doi:10.1016/j.jhsa.2006.09.011.
22. Loveridge J, Ahearn N, Gee C, Pearson D, Sivaloganathan S, Bhatia R. Treatment of distal radial fractures with the DVR-A plate--the early bristol experience. Hand Surg. 2013;18(2):159-167. doi:10.1142/S0218810413500184.
23. Karantana A, Downing ND, Forward DP, et al. Surgical treatment of distal radial fractures with a volar locking plate versus conventional percutaneous methods: a randomized controlled trial. J Bone Joint Surg Am. 2013;95(19):1737-1744. doi:10.2106/JBJS.L.00232.
24. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: A randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221. doi:10.1302/0301-620X.90B9.20521.
25. von Recum J, Matschke S, Jupiter JB, et al. Characteristics of two different locking compression plates in the volar fixation of complex articular distal radius fractures. Bone Joint Res. 2012;1(6):111-117. doi:10.1302/2046-3758.16.2000008.
26. Safi A, Hart R, Těknědžjan B, Kozák T. Treatment of extra-articular and simple articular distal radial fractures with intramedullary nail versus volar locking plate. J Hand Surg Eur Vol. 2013;38(7):774-779. doi:10.1177/1753193413478715.
27. Kim JK, Park SD. Outcomes after volar plate fixation of low-grade open and closed distal radius fractures are similar. Clin Orthop Relat Res. 2013;471(6):2030-2035. doi:10.1007/s11999-013-2798-9.
1. Larsen CF, Lauritsen J. Epidemiology of acute wrist trauma. Int J Epidemiol. 1993;22(5):911-916.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915. doi:10.1053/jhsu.2001.26322.
3. Melton LJ 3rd, Amadio PC, Crowson CS, O'Fallon WM. Long-term trends in the incidence of distal forearm fractures. Osteoporos Int. 1998;8(4):341-348.
4. Hagino H, Yamamoto K, Ohshiro H, Nakamura T, Kishimoto H, Nose T. Changing incidence of hip, distal radius, and proximal humerus fractures in Tottori Prefecture, Japan. Bone. 1999;24(3):265-270.
5. Diaz-Garcia RJ, Chung KC. The evolution of distal radius fracture management: A historical treatise. Hand Clin. 2012;28(2):105-111. doi:10.1016/j.hcl.2012.02.007.
6. McQueen M, Caspers J. Colles fracture: Does the anatomical result affect the final function? J Bone Joint Surg Br. 1988;70(4):649-651.
7. Stewart HD, Innes AR, Burke FD. Factors affecting the outcome of Colles' fracture: an anatomical and functional study. Injury. 1985;16(5):289-295.
8. Knight D, Hajducka C, Will E, McQueen M. Locked volar plating for unstable distal radial fractures: Clinical and radiological outcomes. Injury. 2010;41(2):184-189. doi:10.1016/j.injury.2009.08.024.
9. Anakwe R, Khan L, Cook R, McEachan J. Locked volar plating for complex distal radius fractures: patient reported outcomes and satisfaction. J Orthop Surg Res. 2010;5:51. doi:10.1186/1749799X-5-51.
10. Gruber G, Gruber K, Giessauf C, et al. Volar plate fixation of AO type C2 and C3 distal radius fractures, a single-center study of 55 patients. J Orthop Trauma. 2008;22(7):467-472. doi:10.1097/BOT.0b013e318180db09.
11. Koval KJ, Harrast JJ, Anglen JO, Weinstein JN. Fractures of the distal part of the radius. The evolution of practice over time. Where’s the evidence? J Bone Joint Surg Am. 2008;90(9):1855-1861. doi:10.2106/JBJS.G.01569.
12. Foo TL, Gan AW, Soh T, Chew WY. Mechanical failure of the distal radius volar locking plate. J Orthop Surg (Hong Kong). 2013;21(3):332-336. doi:10.11777/230949901302100314.
13. Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand (N Y). 2011;6(2):185-189. doi:10.1007/s11552-010-9313-5.
14. Min W, Kaplan K, Miyamoto R, Tejwani NC. A unique failure mechanism of a distal radius fracture fixed with volar plating--a case report. Bull NYU Hosp Jt Dis. 2010;68(4):304-306.
15. Cao J, Ozer K. Failure of volar locking plate fixation of an extraarticular distal radius fracture: A case report. Patient Saf Surg. 2010;4(1):19. doi:10.1186/1754-9493-4-19.
16. Tang P, Ding A, Uzumcugil A. Radial column and volar plating (RCVP) for distal radius fractures with a radial styloid component or severe comminution. Tech Hand Up Extrem Surg. 2010;14(3):143-149. doi:10.1097/BTH.0b013e3181cae14d.
17. Jacobi M, Wahl P, Kohut G. Repositioning and stabilization of the radial styloid process in comminuted fractures of the distal radius using a single approach: The radio-volar double plating technique. J Orthop Surg Res. 2010;5:55. doi:10.1186/1749-799X-5-55.
18. Rikli DA, Regazzoni P. The double plating technique for distal radius fractures. Tech Hand Up Extrem Surg. 2000;4(2):107-114.
19. Rikli DA, Regazzoni P. Fractures of the distal end of the radius treated by internal fixation and early function. A preliminary report of 20 cases. J Bone Joint Surg Br. 1996;78(4):588-592.
20. Grindel SI, Wang M, Gerlach M, McGrady LM, Brown S. Biomechanical comparison of fixed-angle volar plate versus fixed-angle volar plate plus fragment-specific fixation in a cadaveric distal radius fracture model. J Hand Surg Am. 2007;32(2):194-199. doi:10.1016/j.jhsa.2006.12.003.
21. Blythe M, Stoffel K, Jarrett P, Kuster M. Volar versus dorsal locking plates with and without radial styloid locking plates for the fixation of dorsally comminuted distal radius fractures: A biomechanical study in cadavers. J Hand Surg Am. 2006;31(10):1587-1593. doi:10.1016/j.jhsa.2006.09.011.
22. Loveridge J, Ahearn N, Gee C, Pearson D, Sivaloganathan S, Bhatia R. Treatment of distal radial fractures with the DVR-A plate--the early bristol experience. Hand Surg. 2013;18(2):159-167. doi:10.1142/S0218810413500184.
23. Karantana A, Downing ND, Forward DP, et al. Surgical treatment of distal radial fractures with a volar locking plate versus conventional percutaneous methods: a randomized controlled trial. J Bone Joint Surg Am. 2013;95(19):1737-1744. doi:10.2106/JBJS.L.00232.
24. Egol K, Walsh M, Tejwani N, McLaurin T, Wynn C, Paksima N. Bridging external fixation and supplementary kirschner-wire fixation versus volar locked plating for unstable fractures of the distal radius: A randomised, prospective trial. J Bone Joint Surg Br. 2008;90(9):1214-1221. doi:10.1302/0301-620X.90B9.20521.
25. von Recum J, Matschke S, Jupiter JB, et al. Characteristics of two different locking compression plates in the volar fixation of complex articular distal radius fractures. Bone Joint Res. 2012;1(6):111-117. doi:10.1302/2046-3758.16.2000008.
26. Safi A, Hart R, Těknědžjan B, Kozák T. Treatment of extra-articular and simple articular distal radial fractures with intramedullary nail versus volar locking plate. J Hand Surg Eur Vol. 2013;38(7):774-779. doi:10.1177/1753193413478715.
27. Kim JK, Park SD. Outcomes after volar plate fixation of low-grade open and closed distal radius fractures are similar. Clin Orthop Relat Res. 2013;471(6):2030-2035. doi:10.1007/s11999-013-2798-9.
TAKE-HOME POINTS
- Radial column fixation can be used as a reduction tool in unstable distal radius fractures.
- Radial column fixation can help maintain reduction until union in unstable distal radius fractures when combined with volar plating.
- When operating without an assistant, radial column plating can assist in reduction maintenance when other techniques are not successful and holding a reduction manually is not possible.
- Acceptable clinical and radiographic outcomes can be achieved with the use of dual radial styloid and volar plating for unstable distal radius fractures.
- The effects of increased dissection during radial column fixation in distal radius fractures with regard to scar formation and wrist ROM is currently not known.
Implant Survivorship and Complication Rates After Total Knee Arthroplasty With a Third-Generation Cemented System: 15-Year Follow-Up
ABSTRACT
This work is a retrospective cohort study evaluating patients who had undergone third-generation cemented total knee arthroplasty (TKA) with prostheses (NexGen, Zimmer Biomet) utilizing posterior-stabilized (PS) and cruciate-retaining (CR) designs at a single center at their 15-year follow-up.
The purpose of this study is to determine the functional knee scores, reoperations, and long-term survivorship for patients with the NexGen Zimmer Biomet Knee system at the 15-year follow-up. In total, 99 patients who had undergone primary TKA were followed for 15 years.
At the 15-year follow-up, survivorship in both study groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups also showed similar functionality: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Reoperation rates were 2% for the PS group and 0% for the CR group (P = .38). No differences in any of the outcomes analyzed were observed between patients who had CR TKA and those who had undergone PS TKA.
Our study found no significant differences in functional outcomes between PS and CR NexGen knee implants. Patients treated by both methods showed excellent longevity and survivorship at the 15-year follow-up.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) is an orthopedic procedure with increasing demand.1 Over the past 2 decades, a surge in TKA implants has been observed. Of the available prosthetic designs, only a few implants with long-term follow-up have been reported.2-9 The NexGen TKA system (Zimmer Biomet) has been shown to have excellent clinical and radiographic results at an intermediate follow-up term of 8 years.10 This system is a third-generation prosthetic design that was developed to improve problems seen with its predecessors, such as the Miller-Galante II system (Zimmer Biomet), the Insall-Burstein II system (Zimmer Biomet), and the Constrained Condylar Knee (Zimmer Biomet), which were mainly for patellar maltracking.11-17 The NexGen TKA system is a fixed-bearing system designed to include an anatomic femoral trochlea with the option of cruciate-retaining (CR), posterior-stabilized (PS), or more constrained implants. This study evaluates the long-term success of the CR and PS NexGen TKA systems. Outcomes measured include functional knee scores and reoperation rates at the 15-year follow-up. Based on the measured outcomes, potential differences between the PS and CR implants from this system are cited.
MATERIAL AND METHODS
Between July 1995 and July 1997, 334 consecutive primary TKAs were performed on 287 patients at our institution. In total, 167 patients (186 knees) underwent posterior CR TKAs with the NexGen CR prosthesis (Zimmer Biomet), and 120 patients (148 knees) underwent PS TKAs using the NexGen Legacy PS prosthesis (Zimmer Biomet). This retrospective double cohort study was reviewed and approved by our Institutional Review Board. At the 15-year postoperative follow-up, 99 patients were available (Figure 1).
The CR and PS implants were used with similar frequencies by the surgeons who performed the procedures. Patients were not randomized into either the PS- or CR-implant teams; the final decision on implant selection was left to the operating surgeon’s discretion. However, in addition to standard indications for TKA (pain and disability associated with severe arthritic change seen on radiographs and refractory to conservative measures), absolute contraindications to the CR implant included severe combined deformity (flexion contraction >30° combined with a varus or valgus deformity >20°) or posterior cruciate ligament insufficiency (often associated with inflammatory arthritis).
The surgical technique for the CR and PS designs was identical, and included a median parapatellar approach, femoral rotational alignment perpendicular to the transepicondylar axis, measured resection of the flexion and extension gaps, intramedullary femoral alignment, and extramedullary tibial alignment. All components were cemented, and the patella of each patient was resurfaced. All patients received preoperative antibiotics that were continued for 48 hours postoperatively, and 4 weeks of anticoagulation with dose-adjusted warfarin to maintain an international normalized ratio of 1.5 to 2.0.
Patients were observed postoperatively at the 5- to 8-year and 15-year time points. The 5-year data were previously published in 2005 by Bozic and colleagues.10 Patients available for follow-up at the 15-year time-point were evaluated using the 100-point Hospital for Special Surgery (HSS) knee scoring system, which assigns up to 30 points for pain, 22 points for function, 18 points for range of motion, and 10 points each for quadricep strength, deformity, and instability. In addition, common medical conditions limiting patient activity were assessed; these included joint replacement; arthritis in another joint, the back, or spine; weakness or fatigue; breathing or heart ailments; and others.
Continue to: At the 15-year follow-up...
At the 15-year follow-up, patients were contacted via telephone to obtain their HSS knee scores. If patients were unavailable/unable to answer the questions asked, knee score information was collected from a first-degree relative or caretaker. Patients that could not be contacted by phone were sent a HSS knee score survey to their last known address. The online Social Security Death Index was queried for confirmation of death. If deceased, a first-degree relative was contacted for confirmation.
Survivorship was evaluated using revision for any reason and revision for aseptic loosening as separate endpoints via the Kaplan-Meier product-limit method, and the CR and PS TKA groups were compared using the log-rank test. The power of the study for detecting differences between the TKA groups was determined to be 80%, based on a moderate hazard ratio of 1.5, using the log-rank test. Differences between PS and CR TKAs were assessed using the Pearson chi-square test for knee pain and functional outcomes, Fisher’s exact test for patient limitations, such as joint replacement, and the non-parametric Mann Whitney U-test for median pain scores (Table 1). Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were performed to assess whether self-reported knee scores were significantly correlated with physician-based knee scores. Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation at 95% confidence intervals. Statistical analysis was conducted using IBM SPSS Statistics (version 21.0, IBM). Two-tailed P < .05 was considered statistically significant.
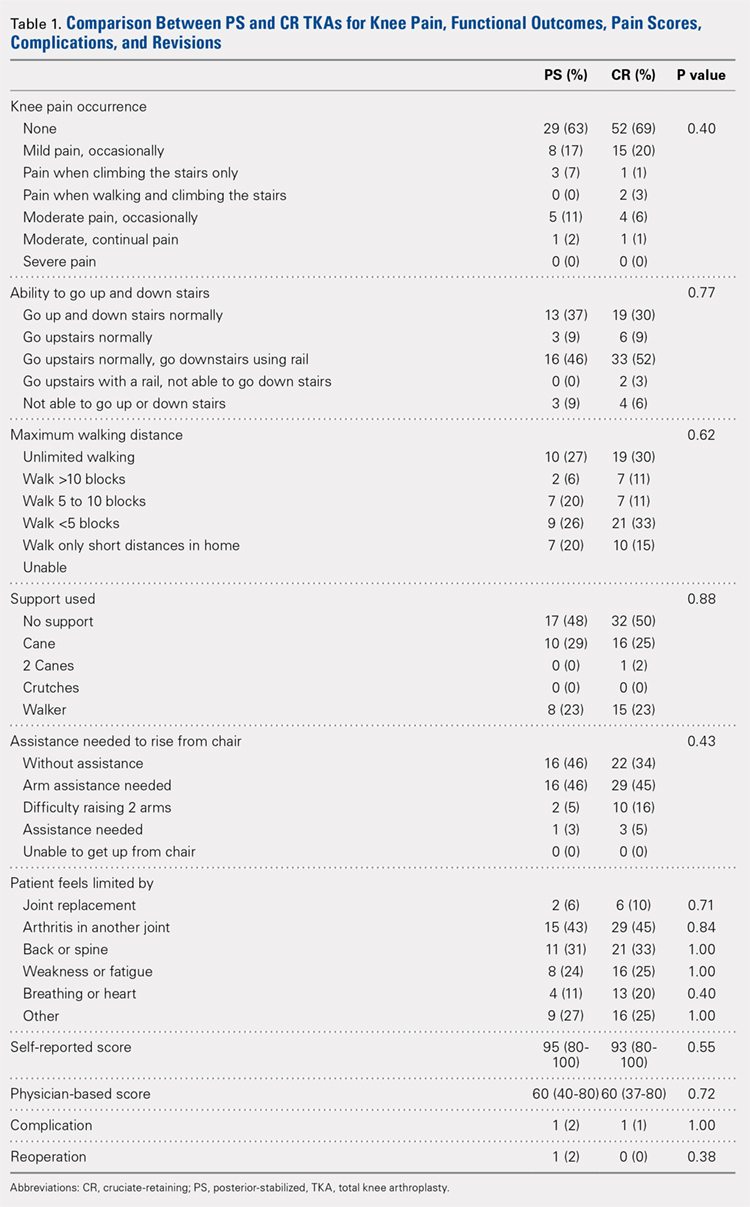
RESULTS
Of the 287 patients (334 knees) who had primary TKAs, 99 patients (121 knees; 75 CR and 46 PS) were available at the 15-year follow-up. A total of 155 patients (171 knees) died before the 15-year follow-up, and 33 (42 knees) were lost to follow-up (Figure 1). The functional status of the knees of patients who were lost to follow-up or who had died since the previous follow-up data were published is unknown.
Demographic and outcome data for the cohort of 121 TKAs (99 patients) are summarized in Table 2. The median age at surgery was 64 years, and 71% of the cohort was female.

At the 15-year follow-up, survivorship in both groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups were also similar functionally: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Approximately half of the patients in both groups (52% PS; 50% CR; P = .88) required walking support (canes or walkers) and nearly half of both groups (46% PS; 48% CR; P = .62) could walk <5 blocks or only short distances in their homes. In addition, 46% of the patients in both groups reported needing arm assistance to functionally rise from a chair (P = .43); 91% of the patients in both groups could also walk up and down stairs (P = .77). No statistical difference in the medical conditions limiting the patients in the 2 groups was found: joint replacement (2% PS; 6% CR; P = .71), arthritis in another joint (43% PS; 45% CR; P = .84), back or spine arthritis (31% PS; 33% CR; P = 1.00), weakness or fatigue (24% PS; 25% CR; P = 1.00), breathing or heart ailments (11% PS; 20% CR; P = .40), and other reasons (27% PS; 25% CR; P = 1.00). In addition, median self-reported knee scores were 95 and 93 points for the PS and CR groups, respectively (P = .55).
Continue to: Patients reported 2 complications...
Patients reported 2 complications since the previous 5- to 8-year follow-up, 1 in each group. The first case underwent a PS TKA that required open reduction internal fixation for a bilateral supracondylar peri-prosthesis femur fracture following a fall, which was subsequently complicated with infection and ultimately led to above-the-knee amputation. In the second case, a CR TKA patient experienced persistent swelling and knee instability. The patient followed up with a local orthopaedist, but to date, no reoperations on the knee have been reported.
Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were moderately correlated with physician-based knee scores (rs = 0.42; P < .001).
Reoperation rates were 2% for PS and 0% for CR (P = .38). Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation and no significance difference between the PS and CR groups was revealed (log-rank test = 1.40, P = .24, Figure 2).
DISCUSSION
The success of TKA in pain relief and restoration of function has led to increased demands for this surgery.1 Such demand has enabled the introduction of a new joint replacement prosthesis to the market.18 Considering the increased incidence of osteoarthritis in the younger population (<55 years of age), critically reviewing the longevity and durability of TKA implant designs is of great importance. Compared with other TKA implant designs, the NexGen Zimmer Biomet Knee system has shown excellent longevity at the 15-year follow-up.5,6,9,11-15 Our study began with 136 patients, and, after eliminating the deceased, those lost to follow-up, and non-responders, a total of 99 patients were available for the 15-year follow-up. At this time-point, 80% of the PS implants and 89% of the CR implants were associated with no or mild pain. Survivorship at the 15-year follow-up was similar in both groups: 98% for PS TKAs and 100% for CR TKAs. The reoperation rate was low in both groups, and no evidence of aseptic loosening was found. Based on our results, the NexGen Zimmer Biomet Knee system can be concluded to show excellent longevity and functional outcomes at the 15-year follow-up.
Our study includes several limiting factors that were taken into consideration during the analysis of the results. One of the main limitations of this work is that it required a 15-year follow-up of predominantly elderly patients; many of the participants may be expected to be deceased at this time-point. In our study, a total of 7 patients were confirmed to be deceased by a first-degree relative or the Social Security Death Index. In addition, unlike Bozic and colleagues’10 previous 5-year follow-up study, radiographic imaging data were not collected at the 15-year follow-up. However, given that this study aimed to assess the functional knee scores and reoperation rates of the PS and CR NexGen Zimmer Biomet Knee system, radiographic information did not appear to be necessary.
CONCLUSION
This study found no significant differences in functional outcomes between the PS and CR NexGen knee implants. Patients who received these implants showed excellent longevity and survivorship at their 15-year follow-up.
1. Lützner J, Hübel U, Kirschner S, Günther KP, Krummenauer F. Langzeitergebnisse in der Knieendoprothetik. Chirurg. 2011;82(7):618-624. doi:10.1007/s00104-010-2001-8.
2. Font-Rodriguez DE, Scuderi GR, Insall J. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;345:79-86.
3. Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10-17.
4. Van Loon CJM, Wisse MA, de Waal Malefijt MC, Jansen RH, Veth RPH. The kinematic total knee arthroplasty. Arch Orth Traum Surg. 2000;120(1-2):48-52. doi:10.1007/PL00021215.
5. Buechel FFS. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40-50.
6. Ito J, Koshino T, Okamoto R, Saito T. 15-year follow-up study of total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplasty. 2003;18(8):984-992. doi:10.1016/S0883-5403(03)00262-6.
7. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. J Bone Joint Surg. 2005;87(3):598-603. doi:10.2106/JBJS.C.00591.
8. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67-70. doi:10.1016/j.arth.2007.05.001.
9. Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A randomised controlled trial of cemented versus cementless press-fit condylar total knee replacement: 15-year survival analysis. J Bone Joint Surg. 2007;89-B(12):1608-1614. doi:10.1302/0301-620x.89b12.19363.
10. Bozic KJ, Kinder J, Menegini M, Zurakowski D, Rosenberg AG, Galante JO. Implant survivorship and complication rates after total knee arthroplasty with a third-generation cemented system: 5 to 8 years followup. Clin Orthop Relat Res. 2005;430:117-124. doi:10.1097/01.blo.0000146539.23869.14.
11. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kißlinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378-381. doi:10.1007/s002640100294.
12. Kirk PG, Rorabeck CH, Bourne RB. Clinical comparison of the Miller Galante I and AMK total knee systems. J Arthroplasty. 1994;9(2):131-136. doi:10.1016/0883-5403(94)90061-2.
13. Kobori M, Kamisato S, Yoshida M, Kobori K. Revision of failed metal-backed patellar component of Miller/Galante-I total knee prosthesis. J Orthop Sci. 2000;5(5):436-438. doi:10.1007/s007760070020.
14. Larson CM, Lachiewicz PF. Patellofemoral complications with the insall-burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14(3):288-292. doi:http://dx.doi.org/10.1016/S0883-5403(99)90053-0.
15. Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14(3):152-156.
16. Miyagi T, Matsuda S, Miura H, Nagamine R, Urabe K. Changes in patellar tracking after total knee arthroplasty: 10-year follow-up of Miller-Balante I knees. Orthopedics. 2002;25(8):811-813. doi:10.3928/0147-7447-20020801-10.
17. Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg. 2002;84(10):1849-1855.
18. Anand R, Graves SE, de Steiger RN, et al. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg. 2011;93(3):51-54. doi:10.2106/JBJS.K.00867.
ABSTRACT
This work is a retrospective cohort study evaluating patients who had undergone third-generation cemented total knee arthroplasty (TKA) with prostheses (NexGen, Zimmer Biomet) utilizing posterior-stabilized (PS) and cruciate-retaining (CR) designs at a single center at their 15-year follow-up.
The purpose of this study is to determine the functional knee scores, reoperations, and long-term survivorship for patients with the NexGen Zimmer Biomet Knee system at the 15-year follow-up. In total, 99 patients who had undergone primary TKA were followed for 15 years.
At the 15-year follow-up, survivorship in both study groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups also showed similar functionality: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Reoperation rates were 2% for the PS group and 0% for the CR group (P = .38). No differences in any of the outcomes analyzed were observed between patients who had CR TKA and those who had undergone PS TKA.
Our study found no significant differences in functional outcomes between PS and CR NexGen knee implants. Patients treated by both methods showed excellent longevity and survivorship at the 15-year follow-up.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) is an orthopedic procedure with increasing demand.1 Over the past 2 decades, a surge in TKA implants has been observed. Of the available prosthetic designs, only a few implants with long-term follow-up have been reported.2-9 The NexGen TKA system (Zimmer Biomet) has been shown to have excellent clinical and radiographic results at an intermediate follow-up term of 8 years.10 This system is a third-generation prosthetic design that was developed to improve problems seen with its predecessors, such as the Miller-Galante II system (Zimmer Biomet), the Insall-Burstein II system (Zimmer Biomet), and the Constrained Condylar Knee (Zimmer Biomet), which were mainly for patellar maltracking.11-17 The NexGen TKA system is a fixed-bearing system designed to include an anatomic femoral trochlea with the option of cruciate-retaining (CR), posterior-stabilized (PS), or more constrained implants. This study evaluates the long-term success of the CR and PS NexGen TKA systems. Outcomes measured include functional knee scores and reoperation rates at the 15-year follow-up. Based on the measured outcomes, potential differences between the PS and CR implants from this system are cited.
MATERIAL AND METHODS
Between July 1995 and July 1997, 334 consecutive primary TKAs were performed on 287 patients at our institution. In total, 167 patients (186 knees) underwent posterior CR TKAs with the NexGen CR prosthesis (Zimmer Biomet), and 120 patients (148 knees) underwent PS TKAs using the NexGen Legacy PS prosthesis (Zimmer Biomet). This retrospective double cohort study was reviewed and approved by our Institutional Review Board. At the 15-year postoperative follow-up, 99 patients were available (Figure 1).

The CR and PS implants were used with similar frequencies by the surgeons who performed the procedures. Patients were not randomized into either the PS- or CR-implant teams; the final decision on implant selection was left to the operating surgeon’s discretion. However, in addition to standard indications for TKA (pain and disability associated with severe arthritic change seen on radiographs and refractory to conservative measures), absolute contraindications to the CR implant included severe combined deformity (flexion contraction >30° combined with a varus or valgus deformity >20°) or posterior cruciate ligament insufficiency (often associated with inflammatory arthritis).
The surgical technique for the CR and PS designs was identical, and included a median parapatellar approach, femoral rotational alignment perpendicular to the transepicondylar axis, measured resection of the flexion and extension gaps, intramedullary femoral alignment, and extramedullary tibial alignment. All components were cemented, and the patella of each patient was resurfaced. All patients received preoperative antibiotics that were continued for 48 hours postoperatively, and 4 weeks of anticoagulation with dose-adjusted warfarin to maintain an international normalized ratio of 1.5 to 2.0.
Patients were observed postoperatively at the 5- to 8-year and 15-year time points. The 5-year data were previously published in 2005 by Bozic and colleagues.10 Patients available for follow-up at the 15-year time-point were evaluated using the 100-point Hospital for Special Surgery (HSS) knee scoring system, which assigns up to 30 points for pain, 22 points for function, 18 points for range of motion, and 10 points each for quadricep strength, deformity, and instability. In addition, common medical conditions limiting patient activity were assessed; these included joint replacement; arthritis in another joint, the back, or spine; weakness or fatigue; breathing or heart ailments; and others.
Continue to: At the 15-year follow-up...
At the 15-year follow-up, patients were contacted via telephone to obtain their HSS knee scores. If patients were unavailable/unable to answer the questions asked, knee score information was collected from a first-degree relative or caretaker. Patients that could not be contacted by phone were sent a HSS knee score survey to their last known address. The online Social Security Death Index was queried for confirmation of death. If deceased, a first-degree relative was contacted for confirmation.
Survivorship was evaluated using revision for any reason and revision for aseptic loosening as separate endpoints via the Kaplan-Meier product-limit method, and the CR and PS TKA groups were compared using the log-rank test. The power of the study for detecting differences between the TKA groups was determined to be 80%, based on a moderate hazard ratio of 1.5, using the log-rank test. Differences between PS and CR TKAs were assessed using the Pearson chi-square test for knee pain and functional outcomes, Fisher’s exact test for patient limitations, such as joint replacement, and the non-parametric Mann Whitney U-test for median pain scores (Table 1). Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were performed to assess whether self-reported knee scores were significantly correlated with physician-based knee scores. Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation at 95% confidence intervals. Statistical analysis was conducted using IBM SPSS Statistics (version 21.0, IBM). Two-tailed P < .05 was considered statistically significant.

RESULTS
Of the 287 patients (334 knees) who had primary TKAs, 99 patients (121 knees; 75 CR and 46 PS) were available at the 15-year follow-up. A total of 155 patients (171 knees) died before the 15-year follow-up, and 33 (42 knees) were lost to follow-up (Figure 1). The functional status of the knees of patients who were lost to follow-up or who had died since the previous follow-up data were published is unknown.
Demographic and outcome data for the cohort of 121 TKAs (99 patients) are summarized in Table 2. The median age at surgery was 64 years, and 71% of the cohort was female.

At the 15-year follow-up, survivorship in both groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups were also similar functionally: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Approximately half of the patients in both groups (52% PS; 50% CR; P = .88) required walking support (canes or walkers) and nearly half of both groups (46% PS; 48% CR; P = .62) could walk <5 blocks or only short distances in their homes. In addition, 46% of the patients in both groups reported needing arm assistance to functionally rise from a chair (P = .43); 91% of the patients in both groups could also walk up and down stairs (P = .77). No statistical difference in the medical conditions limiting the patients in the 2 groups was found: joint replacement (2% PS; 6% CR; P = .71), arthritis in another joint (43% PS; 45% CR; P = .84), back or spine arthritis (31% PS; 33% CR; P = 1.00), weakness or fatigue (24% PS; 25% CR; P = 1.00), breathing or heart ailments (11% PS; 20% CR; P = .40), and other reasons (27% PS; 25% CR; P = 1.00). In addition, median self-reported knee scores were 95 and 93 points for the PS and CR groups, respectively (P = .55).
Continue to: Patients reported 2 complications...
Patients reported 2 complications since the previous 5- to 8-year follow-up, 1 in each group. The first case underwent a PS TKA that required open reduction internal fixation for a bilateral supracondylar peri-prosthesis femur fracture following a fall, which was subsequently complicated with infection and ultimately led to above-the-knee amputation. In the second case, a CR TKA patient experienced persistent swelling and knee instability. The patient followed up with a local orthopaedist, but to date, no reoperations on the knee have been reported.
Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were moderately correlated with physician-based knee scores (rs = 0.42; P < .001).
Reoperation rates were 2% for PS and 0% for CR (P = .38). Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation and no significance difference between the PS and CR groups was revealed (log-rank test = 1.40, P = .24, Figure 2).

DISCUSSION
The success of TKA in pain relief and restoration of function has led to increased demands for this surgery.1 Such demand has enabled the introduction of a new joint replacement prosthesis to the market.18 Considering the increased incidence of osteoarthritis in the younger population (<55 years of age), critically reviewing the longevity and durability of TKA implant designs is of great importance. Compared with other TKA implant designs, the NexGen Zimmer Biomet Knee system has shown excellent longevity at the 15-year follow-up.5,6,9,11-15 Our study began with 136 patients, and, after eliminating the deceased, those lost to follow-up, and non-responders, a total of 99 patients were available for the 15-year follow-up. At this time-point, 80% of the PS implants and 89% of the CR implants were associated with no or mild pain. Survivorship at the 15-year follow-up was similar in both groups: 98% for PS TKAs and 100% for CR TKAs. The reoperation rate was low in both groups, and no evidence of aseptic loosening was found. Based on our results, the NexGen Zimmer Biomet Knee system can be concluded to show excellent longevity and functional outcomes at the 15-year follow-up.
Our study includes several limiting factors that were taken into consideration during the analysis of the results. One of the main limitations of this work is that it required a 15-year follow-up of predominantly elderly patients; many of the participants may be expected to be deceased at this time-point. In our study, a total of 7 patients were confirmed to be deceased by a first-degree relative or the Social Security Death Index. In addition, unlike Bozic and colleagues’10 previous 5-year follow-up study, radiographic imaging data were not collected at the 15-year follow-up. However, given that this study aimed to assess the functional knee scores and reoperation rates of the PS and CR NexGen Zimmer Biomet Knee system, radiographic information did not appear to be necessary.
CONCLUSION
This study found no significant differences in functional outcomes between the PS and CR NexGen knee implants. Patients who received these implants showed excellent longevity and survivorship at their 15-year follow-up.
ABSTRACT
This work is a retrospective cohort study evaluating patients who had undergone third-generation cemented total knee arthroplasty (TKA) with prostheses (NexGen, Zimmer Biomet) utilizing posterior-stabilized (PS) and cruciate-retaining (CR) designs at a single center at their 15-year follow-up.
The purpose of this study is to determine the functional knee scores, reoperations, and long-term survivorship for patients with the NexGen Zimmer Biomet Knee system at the 15-year follow-up. In total, 99 patients who had undergone primary TKA were followed for 15 years.
At the 15-year follow-up, survivorship in both study groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups also showed similar functionality: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Reoperation rates were 2% for the PS group and 0% for the CR group (P = .38). No differences in any of the outcomes analyzed were observed between patients who had CR TKA and those who had undergone PS TKA.
Our study found no significant differences in functional outcomes between PS and CR NexGen knee implants. Patients treated by both methods showed excellent longevity and survivorship at the 15-year follow-up.
Continue to: Total knee arthroplasty...
Total knee arthroplasty (TKA) is an orthopedic procedure with increasing demand.1 Over the past 2 decades, a surge in TKA implants has been observed. Of the available prosthetic designs, only a few implants with long-term follow-up have been reported.2-9 The NexGen TKA system (Zimmer Biomet) has been shown to have excellent clinical and radiographic results at an intermediate follow-up term of 8 years.10 This system is a third-generation prosthetic design that was developed to improve problems seen with its predecessors, such as the Miller-Galante II system (Zimmer Biomet), the Insall-Burstein II system (Zimmer Biomet), and the Constrained Condylar Knee (Zimmer Biomet), which were mainly for patellar maltracking.11-17 The NexGen TKA system is a fixed-bearing system designed to include an anatomic femoral trochlea with the option of cruciate-retaining (CR), posterior-stabilized (PS), or more constrained implants. This study evaluates the long-term success of the CR and PS NexGen TKA systems. Outcomes measured include functional knee scores and reoperation rates at the 15-year follow-up. Based on the measured outcomes, potential differences between the PS and CR implants from this system are cited.
MATERIAL AND METHODS
Between July 1995 and July 1997, 334 consecutive primary TKAs were performed on 287 patients at our institution. In total, 167 patients (186 knees) underwent posterior CR TKAs with the NexGen CR prosthesis (Zimmer Biomet), and 120 patients (148 knees) underwent PS TKAs using the NexGen Legacy PS prosthesis (Zimmer Biomet). This retrospective double cohort study was reviewed and approved by our Institutional Review Board. At the 15-year postoperative follow-up, 99 patients were available (Figure 1).

The CR and PS implants were used with similar frequencies by the surgeons who performed the procedures. Patients were not randomized into either the PS- or CR-implant teams; the final decision on implant selection was left to the operating surgeon’s discretion. However, in addition to standard indications for TKA (pain and disability associated with severe arthritic change seen on radiographs and refractory to conservative measures), absolute contraindications to the CR implant included severe combined deformity (flexion contraction >30° combined with a varus or valgus deformity >20°) or posterior cruciate ligament insufficiency (often associated with inflammatory arthritis).
The surgical technique for the CR and PS designs was identical, and included a median parapatellar approach, femoral rotational alignment perpendicular to the transepicondylar axis, measured resection of the flexion and extension gaps, intramedullary femoral alignment, and extramedullary tibial alignment. All components were cemented, and the patella of each patient was resurfaced. All patients received preoperative antibiotics that were continued for 48 hours postoperatively, and 4 weeks of anticoagulation with dose-adjusted warfarin to maintain an international normalized ratio of 1.5 to 2.0.
Patients were observed postoperatively at the 5- to 8-year and 15-year time points. The 5-year data were previously published in 2005 by Bozic and colleagues.10 Patients available for follow-up at the 15-year time-point were evaluated using the 100-point Hospital for Special Surgery (HSS) knee scoring system, which assigns up to 30 points for pain, 22 points for function, 18 points for range of motion, and 10 points each for quadricep strength, deformity, and instability. In addition, common medical conditions limiting patient activity were assessed; these included joint replacement; arthritis in another joint, the back, or spine; weakness or fatigue; breathing or heart ailments; and others.
Continue to: At the 15-year follow-up...
At the 15-year follow-up, patients were contacted via telephone to obtain their HSS knee scores. If patients were unavailable/unable to answer the questions asked, knee score information was collected from a first-degree relative or caretaker. Patients that could not be contacted by phone were sent a HSS knee score survey to their last known address. The online Social Security Death Index was queried for confirmation of death. If deceased, a first-degree relative was contacted for confirmation.
Survivorship was evaluated using revision for any reason and revision for aseptic loosening as separate endpoints via the Kaplan-Meier product-limit method, and the CR and PS TKA groups were compared using the log-rank test. The power of the study for detecting differences between the TKA groups was determined to be 80%, based on a moderate hazard ratio of 1.5, using the log-rank test. Differences between PS and CR TKAs were assessed using the Pearson chi-square test for knee pain and functional outcomes, Fisher’s exact test for patient limitations, such as joint replacement, and the non-parametric Mann Whitney U-test for median pain scores (Table 1). Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were performed to assess whether self-reported knee scores were significantly correlated with physician-based knee scores. Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation at 95% confidence intervals. Statistical analysis was conducted using IBM SPSS Statistics (version 21.0, IBM). Two-tailed P < .05 was considered statistically significant.

RESULTS
Of the 287 patients (334 knees) who had primary TKAs, 99 patients (121 knees; 75 CR and 46 PS) were available at the 15-year follow-up. A total of 155 patients (171 knees) died before the 15-year follow-up, and 33 (42 knees) were lost to follow-up (Figure 1). The functional status of the knees of patients who were lost to follow-up or who had died since the previous follow-up data were published is unknown.
Demographic and outcome data for the cohort of 121 TKAs (99 patients) are summarized in Table 2. The median age at surgery was 64 years, and 71% of the cohort was female.

At the 15-year follow-up, survivorship in both groups was similar: 98% for PS TKAs and 100% for CR TKAs. The 2 groups were also similar functionally: 80% of the PS implants and 89% of the CR implants were associated with no or mild pain (P = .40). Approximately half of the patients in both groups (52% PS; 50% CR; P = .88) required walking support (canes or walkers) and nearly half of both groups (46% PS; 48% CR; P = .62) could walk <5 blocks or only short distances in their homes. In addition, 46% of the patients in both groups reported needing arm assistance to functionally rise from a chair (P = .43); 91% of the patients in both groups could also walk up and down stairs (P = .77). No statistical difference in the medical conditions limiting the patients in the 2 groups was found: joint replacement (2% PS; 6% CR; P = .71), arthritis in another joint (43% PS; 45% CR; P = .84), back or spine arthritis (31% PS; 33% CR; P = 1.00), weakness or fatigue (24% PS; 25% CR; P = 1.00), breathing or heart ailments (11% PS; 20% CR; P = .40), and other reasons (27% PS; 25% CR; P = 1.00). In addition, median self-reported knee scores were 95 and 93 points for the PS and CR groups, respectively (P = .55).
Continue to: Patients reported 2 complications...
Patients reported 2 complications since the previous 5- to 8-year follow-up, 1 in each group. The first case underwent a PS TKA that required open reduction internal fixation for a bilateral supracondylar peri-prosthesis femur fracture following a fall, which was subsequently complicated with infection and ultimately led to above-the-knee amputation. In the second case, a CR TKA patient experienced persistent swelling and knee instability. The patient followed up with a local orthopaedist, but to date, no reoperations on the knee have been reported.
Spearman correlations between the patients’ self-reported knee scores (as a percentage of normal) and physician-based knee scores were moderately correlated with physician-based knee scores (rs = 0.42; P < .001).
Reoperation rates were 2% for PS and 0% for CR (P = .38). Kaplan-Meier analysis was performed to evaluate time-related freedom from reoperation and no significance difference between the PS and CR groups was revealed (log-rank test = 1.40, P = .24, Figure 2).

DISCUSSION
The success of TKA in pain relief and restoration of function has led to increased demands for this surgery.1 Such demand has enabled the introduction of a new joint replacement prosthesis to the market.18 Considering the increased incidence of osteoarthritis in the younger population (<55 years of age), critically reviewing the longevity and durability of TKA implant designs is of great importance. Compared with other TKA implant designs, the NexGen Zimmer Biomet Knee system has shown excellent longevity at the 15-year follow-up.5,6,9,11-15 Our study began with 136 patients, and, after eliminating the deceased, those lost to follow-up, and non-responders, a total of 99 patients were available for the 15-year follow-up. At this time-point, 80% of the PS implants and 89% of the CR implants were associated with no or mild pain. Survivorship at the 15-year follow-up was similar in both groups: 98% for PS TKAs and 100% for CR TKAs. The reoperation rate was low in both groups, and no evidence of aseptic loosening was found. Based on our results, the NexGen Zimmer Biomet Knee system can be concluded to show excellent longevity and functional outcomes at the 15-year follow-up.
Our study includes several limiting factors that were taken into consideration during the analysis of the results. One of the main limitations of this work is that it required a 15-year follow-up of predominantly elderly patients; many of the participants may be expected to be deceased at this time-point. In our study, a total of 7 patients were confirmed to be deceased by a first-degree relative or the Social Security Death Index. In addition, unlike Bozic and colleagues’10 previous 5-year follow-up study, radiographic imaging data were not collected at the 15-year follow-up. However, given that this study aimed to assess the functional knee scores and reoperation rates of the PS and CR NexGen Zimmer Biomet Knee system, radiographic information did not appear to be necessary.
CONCLUSION
This study found no significant differences in functional outcomes between the PS and CR NexGen knee implants. Patients who received these implants showed excellent longevity and survivorship at their 15-year follow-up.
1. Lützner J, Hübel U, Kirschner S, Günther KP, Krummenauer F. Langzeitergebnisse in der Knieendoprothetik. Chirurg. 2011;82(7):618-624. doi:10.1007/s00104-010-2001-8.
2. Font-Rodriguez DE, Scuderi GR, Insall J. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;345:79-86.
3. Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10-17.
4. Van Loon CJM, Wisse MA, de Waal Malefijt MC, Jansen RH, Veth RPH. The kinematic total knee arthroplasty. Arch Orth Traum Surg. 2000;120(1-2):48-52. doi:10.1007/PL00021215.
5. Buechel FFS. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40-50.
6. Ito J, Koshino T, Okamoto R, Saito T. 15-year follow-up study of total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplasty. 2003;18(8):984-992. doi:10.1016/S0883-5403(03)00262-6.
7. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. J Bone Joint Surg. 2005;87(3):598-603. doi:10.2106/JBJS.C.00591.
8. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67-70. doi:10.1016/j.arth.2007.05.001.
9. Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A randomised controlled trial of cemented versus cementless press-fit condylar total knee replacement: 15-year survival analysis. J Bone Joint Surg. 2007;89-B(12):1608-1614. doi:10.1302/0301-620x.89b12.19363.
10. Bozic KJ, Kinder J, Menegini M, Zurakowski D, Rosenberg AG, Galante JO. Implant survivorship and complication rates after total knee arthroplasty with a third-generation cemented system: 5 to 8 years followup. Clin Orthop Relat Res. 2005;430:117-124. doi:10.1097/01.blo.0000146539.23869.14.
11. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kißlinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378-381. doi:10.1007/s002640100294.
12. Kirk PG, Rorabeck CH, Bourne RB. Clinical comparison of the Miller Galante I and AMK total knee systems. J Arthroplasty. 1994;9(2):131-136. doi:10.1016/0883-5403(94)90061-2.
13. Kobori M, Kamisato S, Yoshida M, Kobori K. Revision of failed metal-backed patellar component of Miller/Galante-I total knee prosthesis. J Orthop Sci. 2000;5(5):436-438. doi:10.1007/s007760070020.
14. Larson CM, Lachiewicz PF. Patellofemoral complications with the insall-burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14(3):288-292. doi:http://dx.doi.org/10.1016/S0883-5403(99)90053-0.
15. Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14(3):152-156.
16. Miyagi T, Matsuda S, Miura H, Nagamine R, Urabe K. Changes in patellar tracking after total knee arthroplasty: 10-year follow-up of Miller-Balante I knees. Orthopedics. 2002;25(8):811-813. doi:10.3928/0147-7447-20020801-10.
17. Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg. 2002;84(10):1849-1855.
18. Anand R, Graves SE, de Steiger RN, et al. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg. 2011;93(3):51-54. doi:10.2106/JBJS.K.00867.
1. Lützner J, Hübel U, Kirschner S, Günther KP, Krummenauer F. Langzeitergebnisse in der Knieendoprothetik. Chirurg. 2011;82(7):618-624. doi:10.1007/s00104-010-2001-8.
2. Font-Rodriguez DE, Scuderi GR, Insall J. Survivorship of cemented total knee arthroplasty. Clin Orthop Relat Res. 1997;345:79-86.
3. Rodriguez JA, Bhende H, Ranawat CS. Total condylar knee replacement: a 20-year followup study. Clin Orthop Relat Res. 2001;388:10-17.
4. Van Loon CJM, Wisse MA, de Waal Malefijt MC, Jansen RH, Veth RPH. The kinematic total knee arthroplasty. Arch Orth Traum Surg. 2000;120(1-2):48-52. doi:10.1007/PL00021215.
5. Buechel FFS. Long-term followup after mobile-bearing total knee replacement. Clin Orthop Relat Res. 2002;404:40-50.
6. Ito J, Koshino T, Okamoto R, Saito T. 15-year follow-up study of total knee arthroplasty in patients with rheumatoid arthritis. J Arthroplasty. 2003;18(8):984-992. doi:10.1016/S0883-5403(03)00262-6.
7. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. J Bone Joint Surg. 2005;87(3):598-603. doi:10.2106/JBJS.C.00591.
8. Duffy GP, Crowder AR, Trousdale RR, Berry DJ. Cemented total knee arthroplasty using a modern prosthesis in young patients with osteoarthritis. J Arthroplasty. 2007;22(6 Suppl 2):67-70. doi:10.1016/j.arth.2007.05.001.
9. Baker PN, Khaw FM, Kirk LMG, Esler CNA, Gregg PJ. A randomised controlled trial of cemented versus cementless press-fit condylar total knee replacement: 15-year survival analysis. J Bone Joint Surg. 2007;89-B(12):1608-1614. doi:10.1302/0301-620x.89b12.19363.
10. Bozic KJ, Kinder J, Menegini M, Zurakowski D, Rosenberg AG, Galante JO. Implant survivorship and complication rates after total knee arthroplasty with a third-generation cemented system: 5 to 8 years followup. Clin Orthop Relat Res. 2005;430:117-124. doi:10.1097/01.blo.0000146539.23869.14.
11. Effenberger H, Berka J, Hilzensauer G, Ramsauer T, Dorn U, Kißlinger E. Miller-Galante total knee arthroplasty: the importance of material and design on the revision rate. Int Orthop. 2001;25(6):378-381. doi:10.1007/s002640100294.
12. Kirk PG, Rorabeck CH, Bourne RB. Clinical comparison of the Miller Galante I and AMK total knee systems. J Arthroplasty. 1994;9(2):131-136. doi:10.1016/0883-5403(94)90061-2.
13. Kobori M, Kamisato S, Yoshida M, Kobori K. Revision of failed metal-backed patellar component of Miller/Galante-I total knee prosthesis. J Orthop Sci. 2000;5(5):436-438. doi:10.1007/s007760070020.
14. Larson CM, Lachiewicz PF. Patellofemoral complications with the insall-burstein II posterior-stabilized total knee arthroplasty. J Arthroplasty. 1999;14(3):288-292. doi:http://dx.doi.org/10.1016/S0883-5403(99)90053-0.
15. Matsuda S, Miura H, Nagamine R, Urabe K, Hirata G, Iwamoto Y. Effect of femoral and tibial component position on patellar tracking following total knee arthroplasty: 10-year follow-up of Miller-Galante I knees. Am J Knee Surg. 2001;14(3):152-156.
16. Miyagi T, Matsuda S, Miura H, Nagamine R, Urabe K. Changes in patellar tracking after total knee arthroplasty: 10-year follow-up of Miller-Balante I knees. Orthopedics. 2002;25(8):811-813. doi:10.3928/0147-7447-20020801-10.
17. Rao AR, Engh GA, Collier MB, Lounici S. Tibial interface wear in retrieved total knee components and correlations with modular insert motion. J Bone Joint Surg. 2002;84(10):1849-1855.
18. Anand R, Graves SE, de Steiger RN, et al. What is the benefit of introducing new hip and knee prostheses? J Bone Joint Surg. 2011;93(3):51-54. doi:10.2106/JBJS.K.00867.
TAKE-HOME POINTS
- TKA has a high success rate in pain relief and restoration of function in patients with severe osteoarthritis.
- NexGen (Zimmer Biomet) knee implants showed excellent functional outcomes at 15 years.
- There are no significant differences in functional outcomes between the PS and CR knee systems.
- NexGen knee implants showed excellent longevity and survivorship at 15-year follow-up with no evidence of aseptic loosening.
- There is an increased incidence of knee osteoarthritis in the younger population (<55 years of age).
The Prevention and Treatment of Femoral Trial Head Loss in Total Hip Arthroplasty
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
ABSTRACT
This article aims to provide the information necessary to prevent femoral trial head loss and to offer information regarding retrieval of the trial head if it is lost within the surgical field. These techniques can be used to help guide practice in the future. A review of the literature was conducted using a computerized search of PubMed in regard to this issue to investigate how such an occurrence can be prevented and what steps can be taken if preventative measures fail.
Continue to: Total hip arthroplasty...
Total hip arthroplasty (THA) is becoming an increasingly common procedure. Although this procedure is frequently performed, intraoperative complications still arise; therefore, methods of preventing and ameliorating these complications must be devised. One such complication is the loss of the femoral trial head component within the patient.
Loss of the trial head has been documented in THA cases that have used a number of different surgical approaches.1 Although it is uncommon to lose the trial within the pelvis, it is not an entirely unlikely phenomenon. The possibility of such an event makes prevention important, especially given the associated morbidity that loss of the component could cause. Fortunately, there are preventative measures that can be taken to minimize the probability of losing the femoral trial head, in addition to techniques that can be utilized if prevention fails.
SURGICAL TECHNIQUE
PREVENTION
Firstly, it is important to avoid the use of worn-out femoral trial components. It is thought that the incidence of femoral trial head loss is increased when the trunnion is older and has been used repeatedly.2,3 Therefore, it is advised that the use of worn femoral trial stems and other older trial components be avoided.
When the femoral trial head disengages anteriorly, it has the potential to enter the pelvis/retroperitoneal space.2,4 The femoral trial head may move more freely in the absence of resistance offered by the anterior capsule.4 Therefore, when extensive anterior capsular dissection has taken place, such as during extensive capsulectomy, caution should be exercised when manipulating the hip. This emphasizes the necessity to closely monitor the head during any manipulation, particularly in the presence of significant anterior capsule disruption.
Modular hip arthroplasty prosthetics allow for various intraoperative changes to be made to the femoral component, providing greater specificity to the prosthesis.5 However, the modularity of the femoral component has been described as a factor contributing to loss of the femoral trial head.4 This also has been discussed with respect to the implantable prosthetic femoral head itself because of disengagement from the femoral stem during reduction and dislocation.4
Continue to: Case reports have cited...
Case reports have cited the tension of the soft tissues as a definitive factor in trial head loss.1,4,6 These reports discuss the notion that more tension within the soft tissue can increase the likelihood that the trial head will dislodge during reduction or dislocation. Surgeons should therefore consider taking special care when manipulating the trial joint when the soft tissues are particularly tight and offer significant resistance. It has been suggested that the incision be packed with gauze during reductions when the soft tissue is under significant tension in order to keep the femoral trial head from entering the pelvis.6
A simple technique that can be utilized in the prevention of femoral trial head loss is the placement of a suture through the apical hole in the trial head to aid in the retrieval of the implant if it is lost.1 Madsen and colleagues1 suggest the placement of a No.1 (or thicker) suture through this hole. Although this takes some time to perform, it could prove useful in the prevention of complicated implant loss.
Lastly, and perhaps most importantly, it is essential that there is communication and understanding between the surgeon and any assistants. This has been noted to be particularly important during posterior or lateral surgical approaches when the trial head can be lost during attempts at reduction with traction and internal rotation.2 Given the possibility of losing the trial head during this reduction maneuver, communication between the team during the reduction is instrumental.
RETRIEVAL
If the femoral trial head dissociates from the trunnion of the femoral trial manipulation, there are some techniques that can be used to aid in retrieval. It has been described that when the trial head is lost within the surgical wound, it can travel underneath the rectus femoris muscle and cross the pelvic brim, subsequently entering the pelvis along the psoas tendon, as the psoas bursa offers little resistance to the smooth femoral trial head.1 The trial head has been found to follow this path along the psoas tendon until it is located in the posterior pelvis within the retroperitoneal space.1,7 What follows is a compilation of techniques for approaching loss of the femoral trial head when it occurs.
The femoral trial head is round and smooth, which complicates its retrieval. If the surgeon tries to simply grab the component with fingers, it may slip away into the pelvis. When trialing the hip to assess for anterior stability, if the femoral trial head is lost, the leg should not be moved.7 At this point, a manual attempt to recover the trial head before it moves into the pelvis along the psoas tendon should be made.7 It is possible that the femoral trial head may spin when trying to retrieve it, however this should still be attempted before a formal additional surgical approach is employed.7 It has also been noted that one can manually simultaneously press down on the hypogastrium toward the iliac fossa in order to inhibit the movement of the disarticulated trial head from advancing proximally.3 After performing this maneuver, the femoral trial head can be retrieved through the inguinal canal.3
Continue to: Additional surgical approaches...
Additional surgical approaches can also be utilized for retrieval of the femoral trial head if other measures fail. Callaghan and colleagues7 describe a separate surgical approach that can be used to retrieve the trial component after losing the trial head during a posterolateral approach for THA. This technique is commenced by making a 6-cm to 7-cm incision along the iliac crest to the anteromedial aspect of the anterior superior iliac spine.7 The interval between the iliacus and the inner table of the iliac wing is developed, and an attempt is made to locate the femoral trial head and guide it distally along the pelvis toward the hip. Fingers or napkin forceps can be used to accomplish this advancement of the trial head distally toward the hip, and once reaching surgical site, the trial can then be retrieved.7 Further extension of the incision can be made distally if this limited approach is unsuccessful.7 In the event the femoral trial head is still unable to be retrieved, the authors suggest considering a dedicated retroperitoneal approach for trial retrieval after the arthroplasty procedure has been completed.7
Another method for retrieval of the femoral trial head has been described specifically in the setting of a direct lateral approach.8 Kalra and colleagues8 describe a case in which the trial femoral head dislocated anteriorly, and although it was unable to be visualized, the component was able to be palpated posterior to the superior pubic ramus. With the trial head still disassociated within the pelvis, the final implants were implanted. Although the trial was unable to be viewed, using the same incision for the direct lateral approach, the trial femoral head was guided posteriorly toward the sciatic notch. A posterior approach to the hip was then performed using the same initial direct lateral incision used. Subsequent exposure and release of the external rotators and posterior capsule was performed, as was release of the insertion of the gluteus maximus in order to facilitate better visualization and to prevent excessive tension on the sciatic nerve. Blunt finger dissection of the soft tissues was then performed, and the trial head was retrieved from the sciatic notch with a Kocher clamp.8
Madsen and colleagues1 highlight two different cases in which the trial head was lost into the pelvis when using an anterolateral (modified Watson-Jones) approach to the hip to perform THA. As previously alluded to, the trial heads traveled along the patients’ psoas muscle and stopped directly anterior to their sacroiliac joint. In both cases, the trial head was retrieved using a large Satinsky aortic clamp, which enabled the surgeons to drag the trial head to the pelvic brim where it could be removed with a hemostat.1
Multiple authors have discussed the decision to leave the component within the pelvis if the femoral trial head cannot be retrieved.2,4,7 Batouk and colleagues4 noted that in a case of loss of the femoral trial head, the component would be unlikely to disrupt any of the structures within the pelvis, and in the absence of compression of any vital structures, leaving the implant in the patient could be considered. Although the short-term follow-up of 3 months noted in this particular case did not yield any obvious detriment to the patient in regard to symptomatology, the authors note that the long-term effects of such a practice is unclear.4 In another case, in which the decision was made to leave the femoral trial head, the patient at postoperative week 6 began to hear clicking in the hip with an associated loss of range of motion.7 This subsequently prompted removal of the trial component.
DISCUSSION
Although not a particularly common complication, loss of the femoral trial head can occur; therefore, a plan of action should be in place to prevent its loss or to retrieve it if prevention is ineffective. Given the modularity of various arthroplasty systems in regard to the different trial components or even the final implantable prosthetic devices, component loss is a possibility. An understanding of this complication and the appropriate steps to approaching it could aid in preventing patient morbidity. Because of this, it is imperative that surgeons who perform THA be aware of the potential complications and the measures that can be taken to address them.
Continue to: CONCLUSION
CONCLUSION
The femoral trial head often can be quickly and easily recovered; however, trial component recovery can sometimes be more complicated. Loss of the trial femoral head could potentially occur during dislocation, reduction, or any of the trial positions. An example of a more complicated recovery is when the femoral trial head is lost into the retroperitoneal space, which could occur when trialing the hip in extension to assess the anterior stability of the hip. Loss of the femoral trial head is an avoidable occurrence, and it has the potential to cause a number of complications as well as the need for additional incisions/surgery to retrieve the femoral trial head. The subsequent issues that could arise after loss occurs can not only lead to extensive surgical complications, but can also foster patient dissatisfaction regarding surgical outcomes. Therefore, consistent attempts to utilize preventative techniques are essential. As discussed, simple measures such as placement of a suture through the apical hole of the trial component and adequate communication between those involved in reduction and trialing maneuvers, can serve to avert femoral trial head loss.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
1. Madsen WY, Mitchell BS, Kates SL. Successful intraoperative retrieval of dislocated femoral trial head during total hip arthroplasty. J Arthroplasty. 2012;27(5):820.e9-e11. doi:10.1016/j.arth.2011.08.006.
2. Ozkan K, Ugutmen E, Altintas F, Eren A, Mahirogullari M. Intraoperative dislocation of the prosthetic femoral head into the pelvis during total hip arthroplasty. Acta Orthop Belg. 2008;74(4):553-555.
3. Rachbauer F, Nogler M, Krismer M, Moritz M. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):881-882.
4. Batouk O, Gilbart M, Jain R. Intraoperative dislocation of the trial femoral head into the pelvis during total hip arthroplasty: a case report. J Bone Joint Surg Am. 2001;83-A(10):1549-1551.
5. Srinivasan A, Jung E, Levine BR. Modularity of the femoral component in total hip arthroplasty. J Am Acad Orthop Surg. 2012;20(4):214-222. doi:10.5435/JAAOS-20-04-214.
6. Princep A. Intraoperative migration of the trial femoral head into the pelvis during total hip arthroplasty: prevention and retrieval. J Bone Joint Surg Am. 2002;84-A(5):880-881.
7. Callaghan JJ, McAndrew C, Boese CK, Forest E. Intrapelvic migration of the trial femoral head during total hip arthroplasty: is retrieval necessary? A report of four cases. Iowa Orthop J. 2006;26:60-62.
8. Kalra K, Ries MD, Bozic KJ. Intrapelvic displacement of a trial femoral head during total hip arthroplasty and a method to retrieve it. J Arthroplasty 2011;26(2):338.e21-e23. doi:10.1016/j.arth.2009.12.005.
TAKE-HOME POINTS
- Femoral head trial loss is a complication that can occur during THA.
- This event can be a source of avoidable morbidity.
- Preventative measures can be taken to avoid this complication.
- If preventative measures fail, retrieval of the femoral trial head can be performed.
- A thorough understanding of preventative and retrieval methods is essential for surgeons that perform THA.