User login
Levamisole-Induced Vasculopathy With Gastric Involvement in a Cocaine User
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
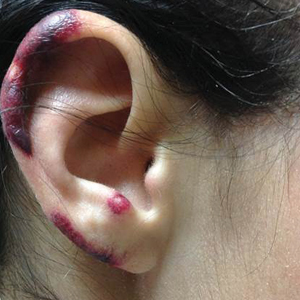
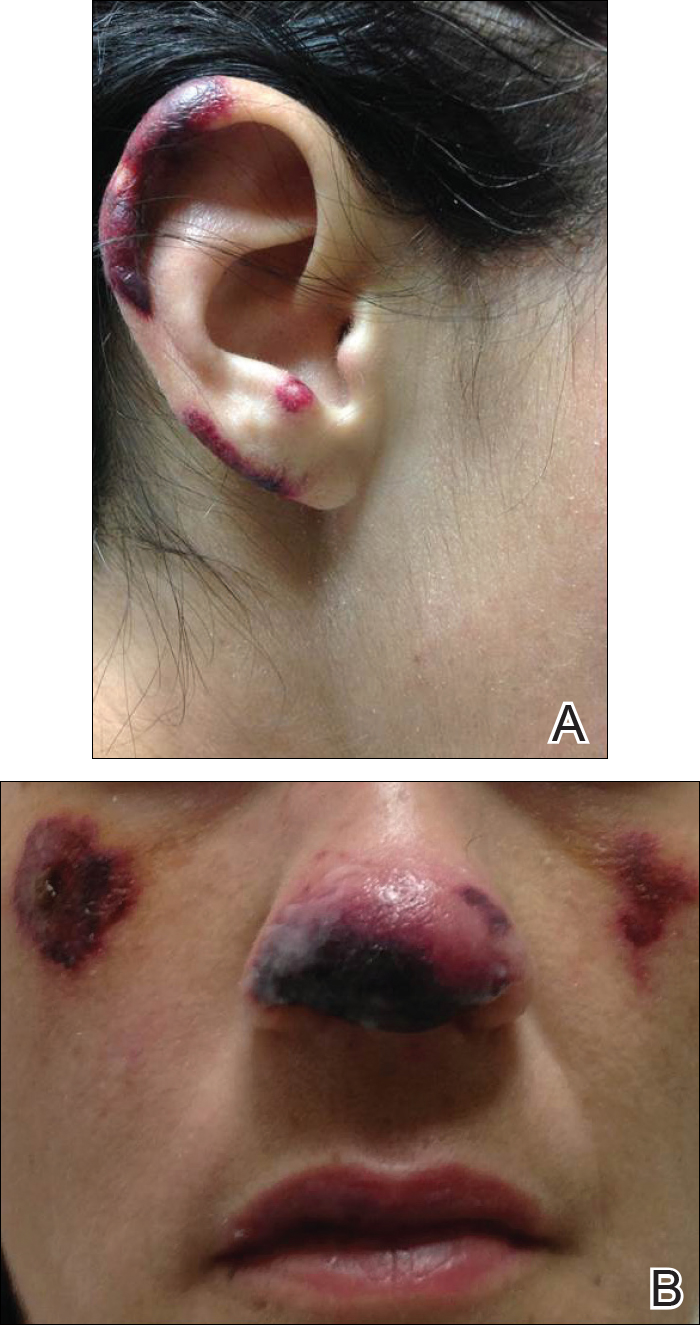
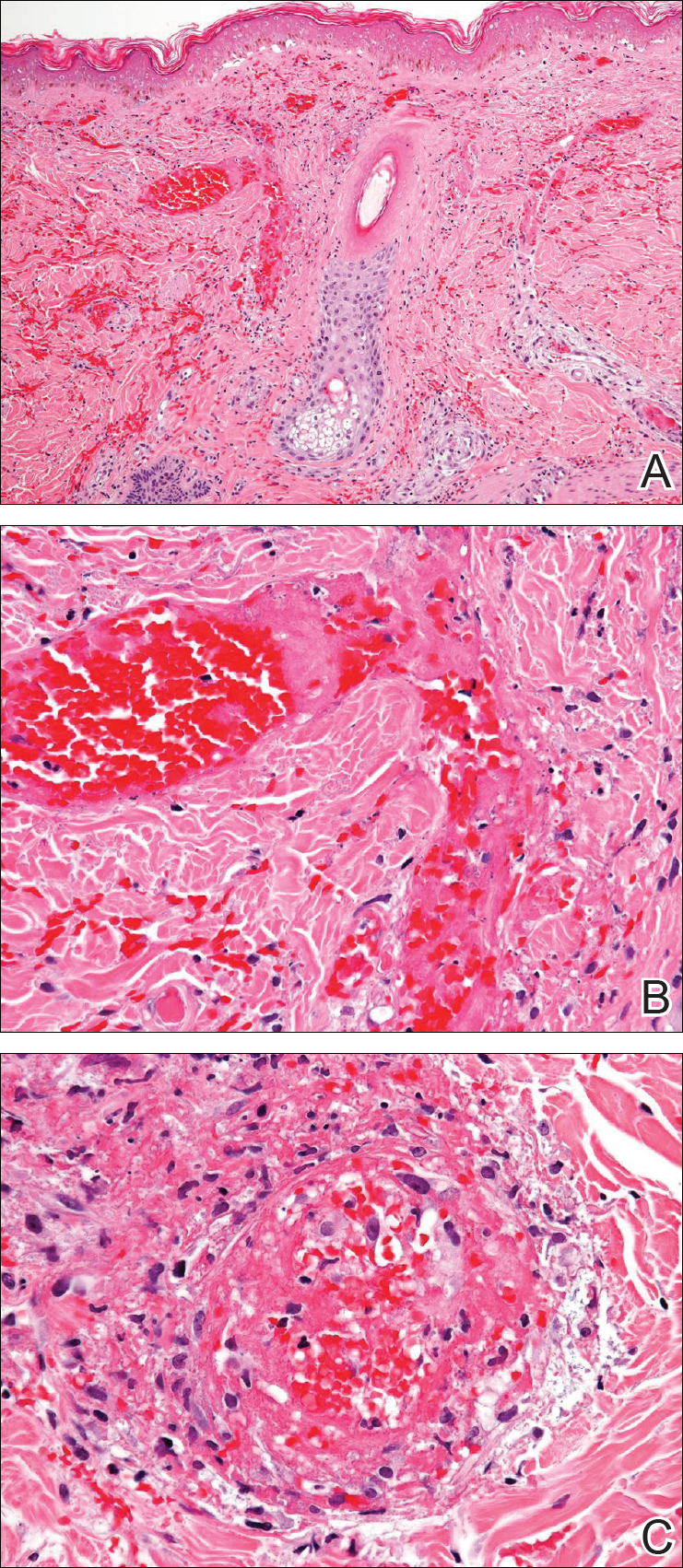
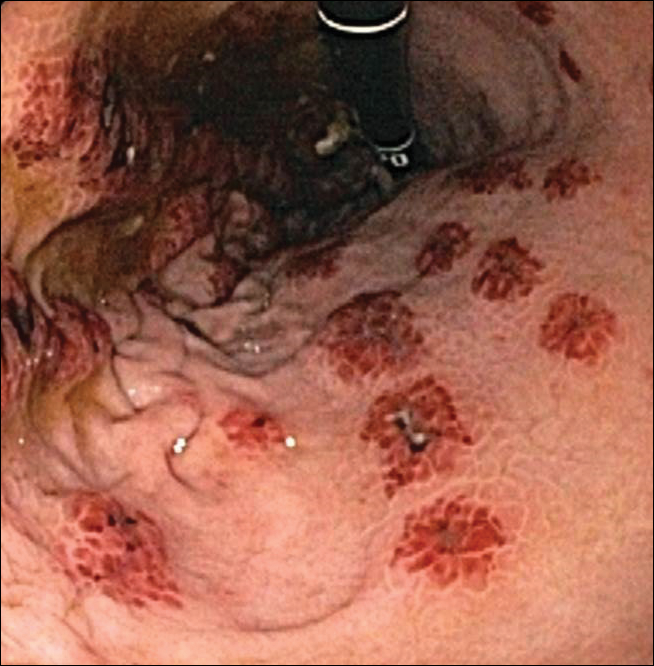
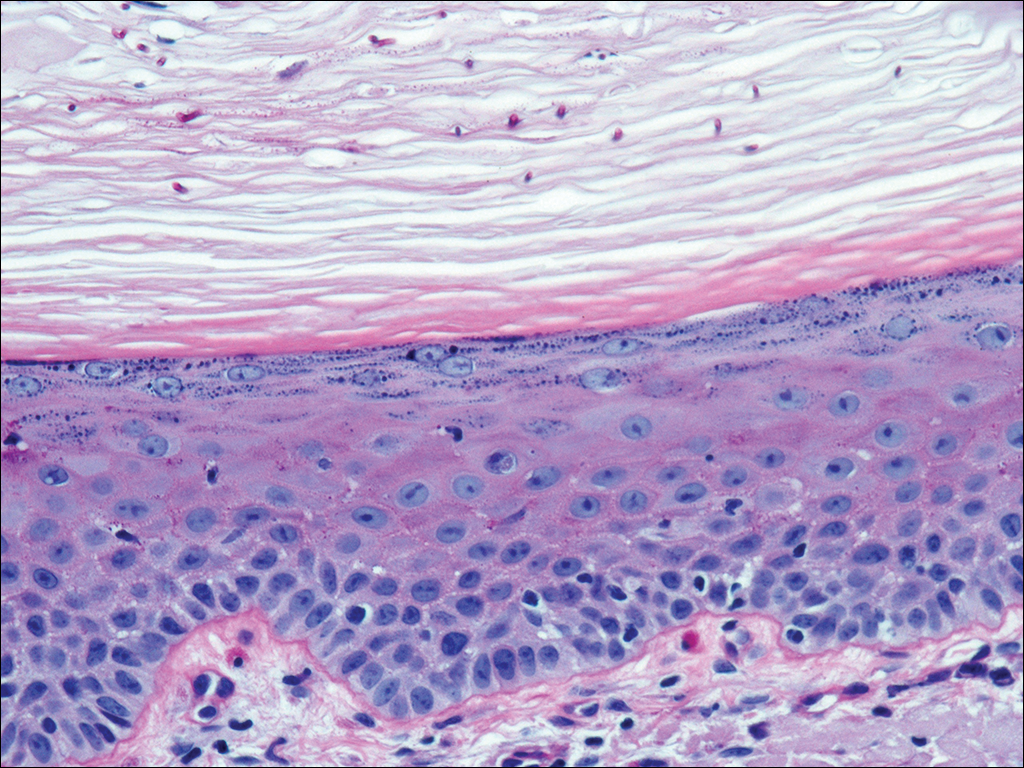
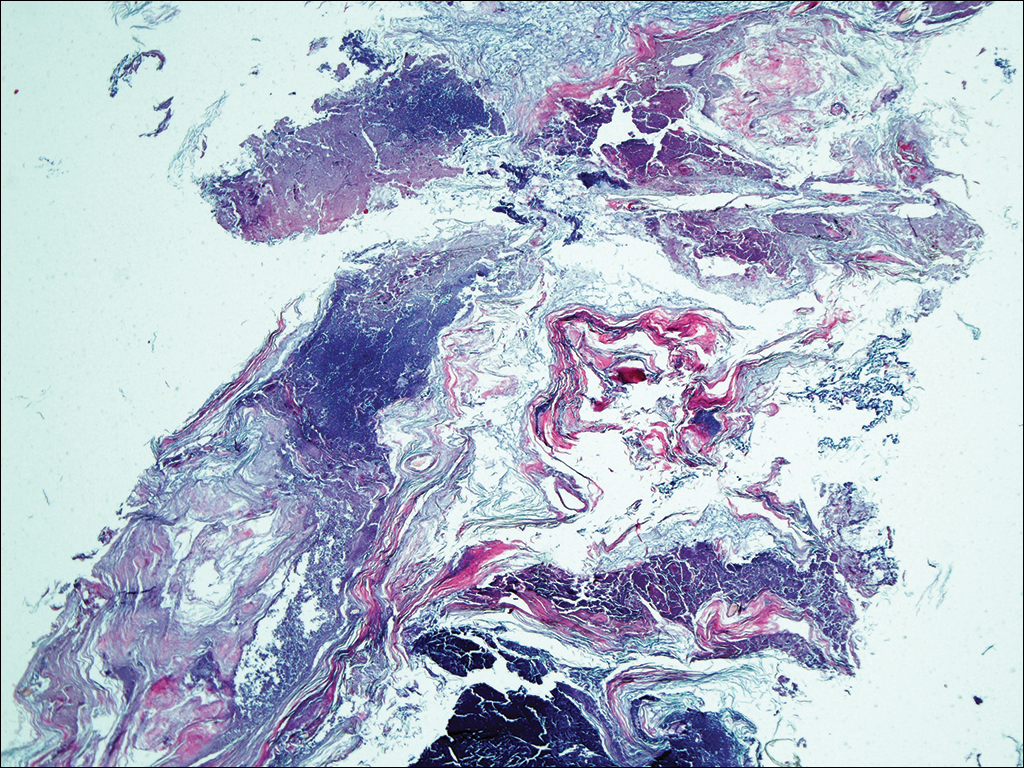
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.
Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.



Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
In 2010, two separate reports of cutaneous vasculitic/vasculopathic eruptions in patients with recent exposure to levamisole-contaminated cocaine (LCC) were published in the literature.1,2 Since then, additional reports have been published.3-6 Retiform purpura associated with cocaine use appears to be a similar condition, perhaps lying at one end of the spectrum of LCC-induced cutaneous vascular disease.7,8 Although some patients have been described as having nausea and vomiting,8,9 including one with a sudden drop in hemoglobin to 5.8 g/dL (reference range, 14.0–17.5 g/dL),10 there are no known reported cases of LCC and levamisole-induced vasculopathy in organ systems other than the skin. Herein, we report the case of a patient with levamisole-induced vasculopathy (LIV) demonstrating endoscopic evidence of gastric hemorrhage with features similar to those involving the skin.
Case Report
A 35-year-old woman with a history of hepatitis C, intravenous drug abuse, and bipolar disorder presented to the emergency department with painful necrotic lesions on the head, neck, arms, and legs of several days’ duration. Approximately 1 year prior she had been admitted to the hospital with similar lesions, with eventual partial necrosis of the left earlobe. The patient reported she had last used crack cocaine 3 days prior to the development of the lesions. A urine drug screen was positive for lorazepam, alprazolam, buprenorphine, methadone, tetrahydrocannabinol, and cocaine. She also reported abdominal pain and gastric reflux of recent onset but denied any history of gastrointestinal tract disease. During the previous admission, the patient demonstrated antinuclear antibodies at a titer of greater than 1:160 (normal, <1:40) in a smooth pattern as well as positive perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) and cytoplasmic antineutrophil cytoplasmic antibodies (c-ANCA) and positive cryoglobulins. Physical examination yielded purpuric and hemorrhagic patches and plaques on the nose, bilateral ears (Figure 1A), face (Figure 1B), arms, and legs. Older lesions exhibited evidence of evolving erosion and ulceration. A biopsy of a lesion on the right arm was obtained, demonstrating extensive epidermal necrosis, hemorrhage, fibrin thrombi within dermal blood vessels, fibrinoid mural necrosis, perivascular neutrophils, and leukocytoclasis (Figure 2). These findings were consistent with LIV caused by exposure to LCC. A complete blood cell count was unremarkable. She was started on pain management and was given prednisone to treat the cutaneous eruption. Because of continued reports of epigastric pain and discomfort on swallowing, an upper gastrointestinal endoscopy was performed. Numerous esophageal erosions and gastric submucosal hemorrhages similar to those on the skin were noted (Figure 3). Pathology taken at the time of the endoscopy demonstrated mucosal erosions, but an evaluation for vascular insult was not possible, as submucosal tissue was not obtained. As the skin lesions began to heal, the gastric symptoms gradually subsided, and the patient was released from the hospital after 7 days.



Comment
Levamisole-Contaminated Cocaine
Cocaine is a crystalline alkaloid obtained from the leaves of the coca plant.7 Fifty percent of globally produced cocaine is consumed in the United States.10 There are 2 to 5 million cocaine users in the United States; in 2009, a reported 1.6 million US adults admitted to having used cocaine in the previous month.4,11,12 Cocaine has been known to be cut with similar-appearing substances including lactose and mannitol, though caffeine, acetaminophen, methylphenidate, and other ingredients have been utilized.7
Levamisole is a synthetic imidazothiazole derivative initially developed for use as an immunomodulatory agent in patients with rheumatoid arthritis.4 It was later paired with 5-fluorouracil for administration in patients with carcinomas of the colon and breasts.4,13 In 2000, the drug was withdrawn from the US market for use in humans after an association between levamisole and agranulocytosis was noted in 2.5% to 13% of patients taking the drug for rheumatoid arthritis or as an adjuvant therapy for breast carcinoma.9,12 It still is available for veterinary use as an anthelmintic and is administered to humans in other countries. Levamisole acts as an immunomodulator by enhancing macrophage chemotaxis and upregulating T-cell functions as well as stimulating neutrophil chemotaxis and dendritic cell maturation.4 It also is known to generate autoantibodies including lupus anticoagulant, p-ANCA, c-ANCA, and antinuclear antibodies.7,14 Levamisole is known to exhibit cutaneous reactions. In 1999, Rongioletti et al14 reported 5 children with purpura of the ears who had been given levamisole for pediatric nephrotic syndrome. Involvement of other body areas was noted. Three patients developed lupus anticoagulant antibodies, 3 exhibited p-ANCA antibodies, and 1 was positive for c-ANCA antibodies. The investigators noted an exceptionally long latency period of 12 to 44 months after starting the drug. Histologically a vasculopathic/vasculitic process was noted.14 Direct immunofluorescence studies of affected skin in LIV have demonstrated IgM, IgA, IgG, C3, and fibrin staining of blood vessels.4,15 Anti–human elastase antibodies are considered both sensitive and specific for LIV and serve to differentiate it from cocaine-induced pseudovasculitis.4,7
In April 2008, the New Mexico Department of Health began evaluating several unexplained cases of agranulocytosis and noted that 11 of 21 cases were associated with cocaine use.9 Later that year, public health workers in Alberta and British Columbia, Canada, reported finding traces of levamisole in clinical specimens and drug paraphernalia of cocaine users with agranulocytosis. Officials from the New Mexico Department of Health learned of these findings and investigated the cases, finding 7 of 9 patients with idiopathic agranulocytosis had recent exposure to cocaine. None of the 21 total patients experienced any skin findings. Nausea and vomiting were common symptoms, but abdominal pain was described in only 2 patients from an additional investigation in Washington. Both of these patients used crack cocaine, and one had a positive urine test for levamisole.9
The presence of levamisole initially was detected by the US Drug Enforcement Administration in 2003. By July 2009, 69% of cocaine and 3% of heroin seized by this agency was noted to contain levamisole.16 From 2003 to 2009, the concentration of levamisole contamination rose to 10%.4 A 2011 study found levamisole in 194 of 249 cocaine-positive urine samples.16
It is unclear why cocaine producers add levamisoleto their product. Possibilities include increasing the drug’s bulk or enhancing its stimulatory effects.12 Chang et al17 posited that levamisole increases the stimulatory and euphoric effects of cocaine by increasing dopamine levels in the brain. Additionally, levamisole is metabolized to aminorex, an amphetaminelike hallucinogen that suppresses appetite, in patients with LCC.13 Vagi et al12 interviewed 10 patients who had been hospitalized for agranulocytosis secondary to use of LCC. None were aware of the presence of this additive, suggesting it was not used as a marketing tool.
Cutaneous Vasculopathy
Levamisole-induced vasculopathy (also called levamisole-induced cutaneous vasculopathy11) initially was reported by 2 separate groups in 2010.1,2 Patients typically present with tender purpuric to hemorrhagic papules, plaques, and bullae with an affinity to affect the ears, nose, and face, though other areas of the body can be affected. A pattern of retiform purpura may precede these findings in some patients. Women are disproportionately affected.11 Crack cocaine use is overrepresented in LIV compared to insufflation or snorting of the drug. Affected patients may exhibit systemic symptoms including myalgia, arthralgia, and frank arthritis.10 Additionally, 15% to 80% of patients exhibit positive antinuclear antibodies, anticardiolipin antibodies, lupus anticoagulant antibodies, p-ANCA antibodies, and c-ANCA antibodies. Magro and Wang8 hypothesized that levamisole acting in conjunction with cocaine rather than the effects of levamisole alone is responsible for some of these findings.
Histologically, the features of a vasculopathic process are noted in some patients with the presence of frank vasculitis.1 The vasculopathic component demonstrates vessel dilatation with thrombosis, eosinophilic deposits, and erythrocyte extravasation. Patients with frank vasculitis exhibit fibrinoid vessel wall necrosis and fibrin deposition, extravasated erythrocytes, endothelial cell atypia, and leukocytoclasia.3 Jacob et al3 noted interstitial and perivascular neovascularization in affected tissue, believed to represent one stage in the evolution of medium vessel vasculitis. Intercellular adhesion molecule 1 has been reported in affected vessel walls with endothelial caspase 3 expression and C5b-9 deposition.8 Magro and Wang8 believe the retiform purpura seen in the early stages of some of these patients with LIV represents a thrombotic dynamic with C5b-9 deposition and enhanced apoptosis. Overt vasculitis follows later, subsequent to the effect of ANCA antibodies and upregulated intercellular adhesion molecule 1 expression on vessel walls.
The clinical course of LIV typically is 2 to 3 weeks for lesion resolution; however, normalization of serologies may require 2 to 14 months. Observation and pain control with or without administration of systemic steroids is sufficient for most patients, but skin grafting, wound debridement, cyclosporine, mycophenolate mofetil, and plasmapheresis also have been employed.4,5 Morbidity may be substantive. One report noted LCC to be responsible for 3 cases of pulmonary hemorrhage and acute progression to chronic renal failure in another 2 patients.15 Ching and Smith18 described a patient with 52% total body surface area involvement who required skin grafting, nasal amputation, patellectomy, central upper lip excision, and amputation of the leg above the knee.
Gastrointestinal Presentation
Patients with LIV have been reported to exhibit abdominal pain, but our patient exhibited a rare presentation of visualized gastrointestinal purpura. Although support for a vasculitic/vasculopathic process requires a tissue diagnosis, the endoscopic appearance of gastric vasculitis is similar to that of cutaneous vasculitis.19 Clinicians caring for patients exposed to LCC should bear in mind that the vascular insults associated with LIV are not restricted solely to the skin.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Bradford M, Rosenberg B, Moreno J, et al. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Int Med. 2010;152:758-759.
- Jacob RS, Silva CY, Powers JG, et al. Levamisole-induced vasculopathy: a report of 2 cases and a novel histopathologic finding. Am J Dermatopathol. 2012;34:208-213.
- Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
- Pavenski K, Vandenberghe H, Jakubovic H, et al. Plasmapheresis and steroid treatment of levamisole-induced vasculopathy and associated skin necrosis in crack/cocaine users. J Cutan Med Surg. 2013;17:123-126.
- Mandrell J, Kranc CL. Prednisone and vardenafil hydrochloride refractory levamisole-induced vasculitis. Cutis. 2016;98:E15-E19.
- Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole [published online August 25, 2010]. J Cutan Pathol. 2010;37:1212-1219.
- Magro CM, Wang X. Cocaine-associated retiform purpura: a C5b-9 mediated microangiopathy syndrome associated with enhanced apoptosis and high levels of intercellular adhesion molecule-1 expression. Am J Dermatopathol 2013;35:722-730.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Espinoza LR, Alamino RP. Cocaine-induced vasculitis: clinical and immunological spectrum. Curr Rhematol Rep. 2012;14:532-538.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Vagi SJ, Sheikh S, Brackney M, et al. Passive multistate surveillance for neutropenia after of cocaine or heroin possibly contaminated with levamisole. Ann Emerg Med. 2013;61:468-474.
- Lee KC, Ladizinski, Nutan FN. Systemic complications of levamisole toxicity. J Am Acad Dermatol. 2012;67:791-792.
- Rongioletti E, Ghio L, Ginervri E, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating longer-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasmic antibody-associated diseases. Clin J Am Soc Nephrol. 2011;6:2799-2805.
- Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant. Clin Pharmacol Ther. 2010;88:408-411.
- Ching JA, Smith DJ. Levamisole-induced necrosis of skin, soft-tissue and bone: case report and review of literature. J Burn Care Res. 2012;33:E1-E5.
- Naruse G, Shimata K. Cutaneous and gastrointestinal purpura. N Engl J Med. 2013;369:1843.
Practice Points
- More than half of the cocaine illicitly consumed in the United States is contaminated with levamisole, a veterinary drug that can incite a vasculitic/vasculopathic response in the skin as well as in other organ systems.
- Because dermatologists often are the specialists to make the diagnosis of levamisole-induced vasculopathy, clinicians should be made aware that consumption of levamisole-contaminated cocaine may affect more than the skin alone.
Tinea Capitis Caused by Trichophyton rubrum Mimicking Favus
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.
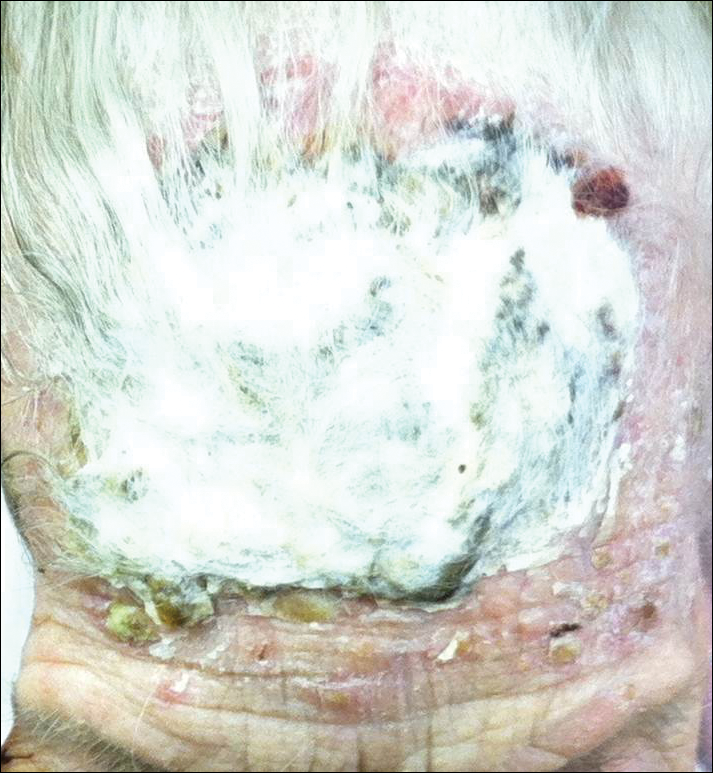
At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).

Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.


At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).


Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
In 1909, Sabouraud1 published a report delineating the clinical subsets of a chronic fungal infection of the scalp known as favus. The rarest subset was termed favus papyroide and consisted of a thin, dry, gray, parchmentlike crust up to 5 cm in diameter. Hair shafts were described as piercing the crust, with the underlying skin exhibiting erythema, moisture, and erosions. Children were reported to be affected more often than adults.1 Subsequent descriptions of patients with similar presentations have not appeared in the medical literature. In this case, an elderly woman with tinea capitis (TC) due to Trichophyton rubrum exhibited features of favus papyroide.
Case Report
An 87-year-old woman with a long history of actinic keratoses and nonmelanoma skin cancers presented to our dermatology clinic with numerous growths on the head, neck, and arms. The patient resided in a nursing home and had a history of hypertension, osteoarthritis, and mild to moderate dementia. Physical examination revealed a frail elderly woman in a wheelchair. Numerous actinic keratoses were noted on the arms and face. Examination of the scalp revealed a large, white-gray, palm-sized plaque on the crown (Figure 1) with 2 yellow, quarter-sized, hyperkeratotic nodules on the left temple and left parietal scalp. The differential diagnosis for the nodules on the temple and scalp included squamous cell carcinoma and hyperkeratotic actinic keratosis, and both lesions were biopsied. Histologically, they demonstrated pronounced hyperkeratosis and parakeratosis with numerous infiltrating neutrophils. The stratum malpighii exhibited focal atypia consistent with an actinic keratosis with areas of spongiosis and pustular folliculitis but no evidence of an invasive cutaneous malignancy. Periodic acid–Schiff stains were performed on both specimens and revealed numerous fungal hyphae within the stratum corneum (Figure 2) as well as evidence of a fungal folliculitis.


At a follow-up visit 2 weeks later, a portion of the hyperkeratotic material on the crown of the scalp was lifted free from the skin surface, removed with scissors, and submitted for histologic analysis and culture. The underlying skin exhibited substantial erythema and diffuse alopecia. The specimen consisted entirely of masses of hyperkeratotic and parakeratotic stratum corneum with numerous infiltrating neutrophils, cellular debris, and focal secondary bacterial colonization (Figure 3). Fungal hyphae and spores were readily demonstrated on Gomori methenamine-silver stain (Figure 4). A fungal culture from this material failed to demonstrate growth at 28 days. The organism was molecularly identified as T rubrum using the Sanger sequencing assay. The patient was treated with fluconazole 150 mg once daily for 3 weeks with eventual resolution of the plaque. The patient died approximately 3 months later (unrelated to her scalp infection).


Comment
Favus, or tinea favosa, is a chronic inflammatory dermatophyte infection of the scalp, less commonly involving the skin and nails.2 The classic lesion is termed a scutulum or godet consisting of concave, cup-shaped, yellow crusts typically pierced by a single hair shaft.1 With an increase in size, the scutula may become confluent. Alopecia commonly results and infected patients may exude a “cheesy” or “mousy” odor from the lesions.3 Sabouraud1 delineated 3 clinical presentations of favus: (1) favus pityroide, the most common type consisting of a seborrheic dermatitis–like picture and scutula; (2) favus impetigoide, exhibiting honey-colored crusts reminiscent of impetigo but without appreciable scutula; and (3) favus papyroide, the rarest variant, demonstrating a dry, gray, parchmentlike crust pierced by hair shafts overlying an eroded erythematous scalp.
Favus usually is acquired in childhood or adolescence and often persists into adulthood.3 It is transmitted directly by hairs, infected keratinocytes, and fomites. Child-to-child transmission is much less common than other forms of TC.4 The responsible organism is almost always Trichophyton schoenleinii, with rare cases of Trichophyton violaceum, Trichophyton verrucosum, Trichophyton mentagrophytes var quinckeanum, Microsporum canis, and Microsporum gypseum having been reported.2,5,6 This anthropophilic dermatophyte infects only humans, is capable of surviving in the same dwelling space for generations, and is believed to require prolonged exposure for transmission. Trichophyton schoenleinii was the predominant infectious cause of TC in eastern Europe in the 19th and early 20th centuries, but its incidence has dramatically declined in the last 50 years.7 A survey conducted in 1997 and published in 2001 of TC that was culture-positive for T schoenleinii in 19 European countries found only 3 cases among 3671 isolates (0.08%).8 Between 1980 and 2005, no cases were reported in the British Isles.9 Currently, favus generally is found in impoverished geographic regions with poor hygiene, malnutrition, and limited access to health care; however, endemic foci in Kentucky, Quebec, and Montreal have been reported in North America.10 Although favus rarely resolves spontaneously, T schoenleinii was eradicated in most of the world with the introduction of griseofulvin in 1958.7 Terbinafine and itraconazole are currently the drugs of choice for therapy.10
Tinea capitis is the most common fungal infection in children, with 1 in 20 US children displaying evidence of overt infection.11 Infection in adults is rare and most affected patients typically display serious illnesses with concomitant immune compromise.12 Only 3% to 5% of cases arise in patients older than 20 years.13 Adult hair appears to be relatively resistant to dermatophyte infection, probably from the fungistatic properties of long-chain fatty acids found in sebum.13 Tinea capitis in adults usually occurs in postmenopausal women, presumably from involution of sebaceous glands associated with declining estrogen levels. Patients typically exhibit erythematous scaly patches with central clearing, alopecia, varying degrees of inflammation, and few pustules, though exudative and heavily inflammatory lesions also have been described.14
In the current case, TC was not raised in the differential diagnosis. Regardless, given that scaly red patches and papules of the scalp may represent a dermatophyte infection in this patient population, clinicians are encouraged to consider this possibility. Transmission is by direct human-to-human contact and contact with objects containing fomites including brushes, combs, bedding, clothing, toys, furniture, and telephones.15 It is frequently spread among family members and classmates.16
Prior to World War II, most cases of TC in the United States were due to M canis, with Microsporum audouinii becoming more prevalent until the 1960s and 1970s when Trichophyton tonsurans began surging in incidence.12,17 Currently, the latter organism is responsible for more than 95% of TC cases in the United States.18Microsporum canis is the main causative species in Europe but varies widely by country. In the Middle East and Africa, T violaceum is responsible for many infections.
Trichophyton rubrum–associated TC appears to be a rare occurrence. A global study in 1995 noted that less than 1% of TC cases were due to T rubrum infection, most having been described in emerging nations.12 A meta-analysis of 9 studies from developed countries found only 9 of 10,145 cases of TC with a culture positive for T rubrum.14 In adults, infected patients typically exhibit either evidence of a concomitant fungal infection of the skin and/or nails or health conditions with impaired immunity, whereas in children, interfamilial spread appears more common.11
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
- Sabouraud R. Les favus atypiques, clinique. Paris. 1909;4:296-299.
- Olkit M. Favus of the scalp: an overview and update. Mycopathologia. 2010;170:143-154.
- Elewski BE. Tinea capitis: a current perspective. J Am Acad Dermatol. 2000;42:1-20.
- Aly R, Hay RJ, del Palacio A, et al. Epidemiology of tinea capitis. Med Mycol. 2000;38(suppl 1):183-188.
- Joly J, Delage G, Auger P, et al. Favus: twenty indigenous cases in the province of Quebec. Arch Dermatol. 1978;114:1647-1648.
- Garcia-Sanchez MS, Pereira M, Pereira MM, et al. Favus due to Trichophyton mentagrophytes var. quinckeanum. Dermatology. 1997;194:177-179.
- Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
- Hay RJ, Robles W, Midgley MK, et al. Tinea capitis in Europe: new perspective on an old problem. J Eur Acad Dermatol Venereol. 2001;15:229-233.
- Borman AM, Campbell CK, Fraser M, et al. Analysis of the dermatophyte species isolated in the British Isles between 1980 and 2005 and review of worldwide dermatophyte trends over the last three decades. Med Mycol. 2007;45:131-141.
- Rippon JW. Dermatophytosis and dermatomycosis. In: Rippon JW. Medical Mycology: The Pathogenic Fungi and the Pathogenic Actinomycetes. 3rd ed. Philadelphia, PA: WB Saunders; 1988:197-199.
- Abdel-Rahman SM, Penny J, Alander SW. Trichophyton rubrum tinea capitis in a young child. Ped Dermatol. 2004;21:63-65.
- Schwinn A, Ebert J, Brocker EB. Frequency of Trichophyton rubrum in tinea capitis. Mycoses. 1995;38:1-7.
- Ziemer A, Kohl K, Schroder G. Trichophyton rubrum induced inflammatory tinea capitis in a 63-year-old man. Mycoses. 2005;48:76-79.
- Anstey A, Lucke TW, Philpot C. Tinea capitis caused by Trichophyton rubrum. Br J Dermatol. 1996;135:113-115.
- Schwinn A, Ebert J, Muller I, et al. Trichophyton rubrum as the causative agent of tinea capitis in three children. Mycoses. 1995;38:9-11.
- Chang SE, Kang SK, Choi JH, et al. Tinea capitis due to Trichophyton rubrum in a neonate. Ped Dermatol. 2002;19:356-358.
- Stiller MJ, Rosenthal SA, Weinstein AS. Tinea capitis caused by Trichophyton rubrum in a 67-year-old woman with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:257-258.
- Foster KW, Ghannoum MA, Elewski BE. Epidemiologic surveillance of cutaneous fungal infection in the United States from 1999 to 2002. J Am Acad Dermatol. 2004;50:748-752.
Practice Points
- Although favus is uncommonly seen in developed countries, it still exists and can mimick other conditions, notably cutaneous malignancies.
- Favus may affect the skin and nails in addition to the hair.
- The lesions of favus may persist for many years.
An Eruption While on Total Parenteral Nutrition
The Diagnosis: Acquired Acrodermatitis Enteropathica
Acquired acrodermatitis enteropathica (AAE) is a rare disorder caused by severe zinc deficiency. Although acrodermatitis enteropathica is an autosomal-recessive disorder that typically manifests in infancy, AAE also can result from poor zinc intake, impaired absorption, or accelerated losses. There are reports of AAE in patients with zinc-deficient diets,1 eating disorders,2 bariatric and other gastrointestinal surgeries,3 malabsorptive diseases,4 and nephrotic syndrome.5
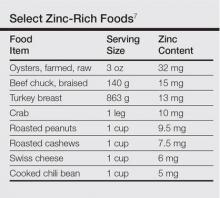
Zinc plays an important role in DNA and RNA synthesis, reactive oxygen species attenuation, and energy metabolism, allowing for proper wound healing, skin differentiation, and proliferation.6 Zinc is found in most foods, but animal protein contains higher concentrations (Table).7 Approximately 85% of zinc is stored in muscles and bones, with only a small amount of accessible zinc available in the liver. Liver stores can be depleted as quickly as 1 week.8 Total parenteral nutrition without trace element supplementation can quickly predispose patients to AAE.
 |
 |
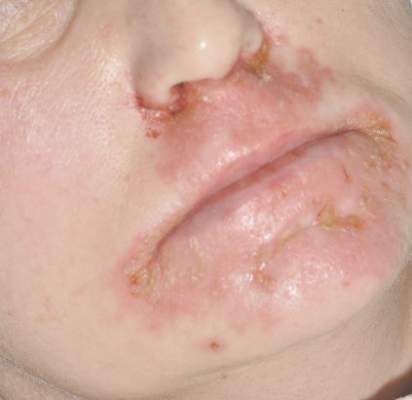
Diagnosis of this condition requires triangulation of clinical presentation, histopathology examination, and laboratory findings. Acrodermatitis enteropathica typically is characterized by dermatitis, diarrhea, and epidermal appendage findings. In its early stages, the dermatitis often manifests with angular cheilitis and paronychia.9 Patients then develop erythema, erosions, and occasionally vesicles or psoriasiform plaques in periorificial, perineal, and acral sites (Figure 1). Epidermal appendage effects include generalized alopecia and thinning nails with white transverse ridges. Although dermatologic and gastrointestinal manifestations are the most obvious, severe AAE may cause other symptoms, including mental slowing, hypogonadism, and impaired immune function.9
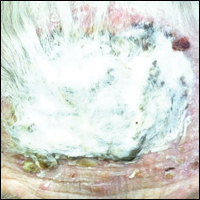
Histopathology of AAE skin lesions is similar to other nutritional deficiencies. Early changes are more specific to deficiency dermatitis and include cytoplasmic pallor and ballooning degeneration of keratinocytes in the stratum spinosum and granulosum.9 Necrolysis results in confluent keratinocyte necrosis developing into subcorneal bulla. Later in the disease course, the presentation becomes psoriasiform with keratinocyte dyskeratosis and confluent parakeratosis10 (Figure 2). Dermal edema with dilated tortuous vessels and a neutrophilic infiltrate may be present throughout disease progression.
Common laboratory abnormalities used to confirm zinc deficiency are decreased plasma zinc and alkaline phosphatase levels. Plasma zinc levels should be drawn after fasting because zinc levels decrease after food intake.9 Concurrent albumin levels should be drawn to correct for low levels caused by hypoalbuminemia. Acquired acrodermatitis enteropathica has been seen in patients with only mildly decreased plasma zinc levels or even zinc levels within reference range.11 Alkaline phosphatase metalloenzyme synthesis requires zinc and a decreased level suggests zinc deficiency even with a plasma zinc level within reference range. Alkaline phosphatase levels usually can be ascertained in a matter of hours, while the zinc levels take much longer to result.
Acquired acrodermatitis enteropathica is treated with oral elemental zinc supplementation at 1 to 2 mg/kg daily.12 Diarrhea typically resolves within 24 hours, but skin lesions heal in 1 to 2 weeks or longer. Although there is no consensus on when to discontinue zinc replacement therapy, therapy generally is not lifelong. Once the patient is zinc replete and the inciting factor has resolved, patients can discontinue supplementation without risk for recurrence.
Trace elements had not been added to our patient’s total parenteral nutrition prior to admission. Basic nutrition laboratory results and zinc levels returned markedly low: 14 μg/dL (reference range, 60–120 μg/dL). Alkaline phosphatase, a zinc-dependent protein, also was low at 12 U/L (reference range, 40–150 U/L). We added trace elements and vitamins and began empiric zinc replacement with 440 mg oral zinc sulfate daily (100 mg elemental zinc). Cephalexin was prescribed for impetiginized skin lesions. The patient noted skin improvement after 3 days on zinc replacement therapy.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol. 2010;37:726-729.
- Bae-Harboe YS, Solky A, Masterpol KS. A case of acquired zinc deficiency. Dermatol Online J. 2012;18:1.
- Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn’s disease. Dermatol Basel Switz. 1996;193:361-363.
- Reichel M, Mauro TM, Ziboh VA, et al. Acrodermatitis enteropathica in a patient with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:415-417.
- Perafan-Riveros C, Franca LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- National Nutrient Database for Standard Reference, Release 28. United States Department of Agriculture, Agricultural Research Service website. http://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=309&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m. Accessed December 14, 2015.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Kumar P, Lal NR, Mondal A, et al. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
The Diagnosis: Acquired Acrodermatitis Enteropathica
Acquired acrodermatitis enteropathica (AAE) is a rare disorder caused by severe zinc deficiency. Although acrodermatitis enteropathica is an autosomal-recessive disorder that typically manifests in infancy, AAE also can result from poor zinc intake, impaired absorption, or accelerated losses. There are reports of AAE in patients with zinc-deficient diets,1 eating disorders,2 bariatric and other gastrointestinal surgeries,3 malabsorptive diseases,4 and nephrotic syndrome.5
Zinc plays an important role in DNA and RNA synthesis, reactive oxygen species attenuation, and energy metabolism, allowing for proper wound healing, skin differentiation, and proliferation.6 Zinc is found in most foods, but animal protein contains higher concentrations (Table).7 Approximately 85% of zinc is stored in muscles and bones, with only a small amount of accessible zinc available in the liver. Liver stores can be depleted as quickly as 1 week.8 Total parenteral nutrition without trace element supplementation can quickly predispose patients to AAE.
 |
 |
Diagnosis of this condition requires triangulation of clinical presentation, histopathology examination, and laboratory findings. Acrodermatitis enteropathica typically is characterized by dermatitis, diarrhea, and epidermal appendage findings. In its early stages, the dermatitis often manifests with angular cheilitis and paronychia.9 Patients then develop erythema, erosions, and occasionally vesicles or psoriasiform plaques in periorificial, perineal, and acral sites (Figure 1). Epidermal appendage effects include generalized alopecia and thinning nails with white transverse ridges. Although dermatologic and gastrointestinal manifestations are the most obvious, severe AAE may cause other symptoms, including mental slowing, hypogonadism, and impaired immune function.9
Histopathology of AAE skin lesions is similar to other nutritional deficiencies. Early changes are more specific to deficiency dermatitis and include cytoplasmic pallor and ballooning degeneration of keratinocytes in the stratum spinosum and granulosum.9 Necrolysis results in confluent keratinocyte necrosis developing into subcorneal bulla. Later in the disease course, the presentation becomes psoriasiform with keratinocyte dyskeratosis and confluent parakeratosis10 (Figure 2). Dermal edema with dilated tortuous vessels and a neutrophilic infiltrate may be present throughout disease progression.
Common laboratory abnormalities used to confirm zinc deficiency are decreased plasma zinc and alkaline phosphatase levels. Plasma zinc levels should be drawn after fasting because zinc levels decrease after food intake.9 Concurrent albumin levels should be drawn to correct for low levels caused by hypoalbuminemia. Acquired acrodermatitis enteropathica has been seen in patients with only mildly decreased plasma zinc levels or even zinc levels within reference range.11 Alkaline phosphatase metalloenzyme synthesis requires zinc and a decreased level suggests zinc deficiency even with a plasma zinc level within reference range. Alkaline phosphatase levels usually can be ascertained in a matter of hours, while the zinc levels take much longer to result.
Acquired acrodermatitis enteropathica is treated with oral elemental zinc supplementation at 1 to 2 mg/kg daily.12 Diarrhea typically resolves within 24 hours, but skin lesions heal in 1 to 2 weeks or longer. Although there is no consensus on when to discontinue zinc replacement therapy, therapy generally is not lifelong. Once the patient is zinc replete and the inciting factor has resolved, patients can discontinue supplementation without risk for recurrence.
Trace elements had not been added to our patient’s total parenteral nutrition prior to admission. Basic nutrition laboratory results and zinc levels returned markedly low: 14 μg/dL (reference range, 60–120 μg/dL). Alkaline phosphatase, a zinc-dependent protein, also was low at 12 U/L (reference range, 40–150 U/L). We added trace elements and vitamins and began empiric zinc replacement with 440 mg oral zinc sulfate daily (100 mg elemental zinc). Cephalexin was prescribed for impetiginized skin lesions. The patient noted skin improvement after 3 days on zinc replacement therapy.
The Diagnosis: Acquired Acrodermatitis Enteropathica
Acquired acrodermatitis enteropathica (AAE) is a rare disorder caused by severe zinc deficiency. Although acrodermatitis enteropathica is an autosomal-recessive disorder that typically manifests in infancy, AAE also can result from poor zinc intake, impaired absorption, or accelerated losses. There are reports of AAE in patients with zinc-deficient diets,1 eating disorders,2 bariatric and other gastrointestinal surgeries,3 malabsorptive diseases,4 and nephrotic syndrome.5
Zinc plays an important role in DNA and RNA synthesis, reactive oxygen species attenuation, and energy metabolism, allowing for proper wound healing, skin differentiation, and proliferation.6 Zinc is found in most foods, but animal protein contains higher concentrations (Table).7 Approximately 85% of zinc is stored in muscles and bones, with only a small amount of accessible zinc available in the liver. Liver stores can be depleted as quickly as 1 week.8 Total parenteral nutrition without trace element supplementation can quickly predispose patients to AAE.
 |
 |
Diagnosis of this condition requires triangulation of clinical presentation, histopathology examination, and laboratory findings. Acrodermatitis enteropathica typically is characterized by dermatitis, diarrhea, and epidermal appendage findings. In its early stages, the dermatitis often manifests with angular cheilitis and paronychia.9 Patients then develop erythema, erosions, and occasionally vesicles or psoriasiform plaques in periorificial, perineal, and acral sites (Figure 1). Epidermal appendage effects include generalized alopecia and thinning nails with white transverse ridges. Although dermatologic and gastrointestinal manifestations are the most obvious, severe AAE may cause other symptoms, including mental slowing, hypogonadism, and impaired immune function.9
Histopathology of AAE skin lesions is similar to other nutritional deficiencies. Early changes are more specific to deficiency dermatitis and include cytoplasmic pallor and ballooning degeneration of keratinocytes in the stratum spinosum and granulosum.9 Necrolysis results in confluent keratinocyte necrosis developing into subcorneal bulla. Later in the disease course, the presentation becomes psoriasiform with keratinocyte dyskeratosis and confluent parakeratosis10 (Figure 2). Dermal edema with dilated tortuous vessels and a neutrophilic infiltrate may be present throughout disease progression.
Common laboratory abnormalities used to confirm zinc deficiency are decreased plasma zinc and alkaline phosphatase levels. Plasma zinc levels should be drawn after fasting because zinc levels decrease after food intake.9 Concurrent albumin levels should be drawn to correct for low levels caused by hypoalbuminemia. Acquired acrodermatitis enteropathica has been seen in patients with only mildly decreased plasma zinc levels or even zinc levels within reference range.11 Alkaline phosphatase metalloenzyme synthesis requires zinc and a decreased level suggests zinc deficiency even with a plasma zinc level within reference range. Alkaline phosphatase levels usually can be ascertained in a matter of hours, while the zinc levels take much longer to result.
Acquired acrodermatitis enteropathica is treated with oral elemental zinc supplementation at 1 to 2 mg/kg daily.12 Diarrhea typically resolves within 24 hours, but skin lesions heal in 1 to 2 weeks or longer. Although there is no consensus on when to discontinue zinc replacement therapy, therapy generally is not lifelong. Once the patient is zinc replete and the inciting factor has resolved, patients can discontinue supplementation without risk for recurrence.
Trace elements had not been added to our patient’s total parenteral nutrition prior to admission. Basic nutrition laboratory results and zinc levels returned markedly low: 14 μg/dL (reference range, 60–120 μg/dL). Alkaline phosphatase, a zinc-dependent protein, also was low at 12 U/L (reference range, 40–150 U/L). We added trace elements and vitamins and began empiric zinc replacement with 440 mg oral zinc sulfate daily (100 mg elemental zinc). Cephalexin was prescribed for impetiginized skin lesions. The patient noted skin improvement after 3 days on zinc replacement therapy.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol. 2010;37:726-729.
- Bae-Harboe YS, Solky A, Masterpol KS. A case of acquired zinc deficiency. Dermatol Online J. 2012;18:1.
- Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn’s disease. Dermatol Basel Switz. 1996;193:361-363.
- Reichel M, Mauro TM, Ziboh VA, et al. Acrodermatitis enteropathica in a patient with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:415-417.
- Perafan-Riveros C, Franca LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- National Nutrient Database for Standard Reference, Release 28. United States Department of Agriculture, Agricultural Research Service website. http://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=309&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m. Accessed December 14, 2015.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Kumar P, Lal NR, Mondal A, et al. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol. 2010;37:726-729.
- Bae-Harboe YS, Solky A, Masterpol KS. A case of acquired zinc deficiency. Dermatol Online J. 2012;18:1.
- Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn’s disease. Dermatol Basel Switz. 1996;193:361-363.
- Reichel M, Mauro TM, Ziboh VA, et al. Acrodermatitis enteropathica in a patient with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:415-417.
- Perafan-Riveros C, Franca LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- National Nutrient Database for Standard Reference, Release 28. United States Department of Agriculture, Agricultural Research Service website. http://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=309&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m. Accessed December 14, 2015.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Kumar P, Lal NR, Mondal A, et al. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
A 47-year-old woman with a history of bulimia and gastroparesis who had been on total parenteral nutrition for 8 weeks presented with a painful, perioral, perineal, and acral eruption of 7 weeks’ duration. Additionally, she had experienced diarrhea, vomiting, and a 13.5-kg weight loss in the last 4 months. Physical examination revealed perioral and perineal, well-demarcated, erythematous, scaly plaques with yellow crusting. She had edematous crusted erosions on the bilateral palms and soles and psoriasiform plaques along the right arm and flank. Punch biopsies (4 mm) from the right inguinal fold and right elbow were obtained.