User login
Orf Virus in Humans: Case Series and Clinical Review
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
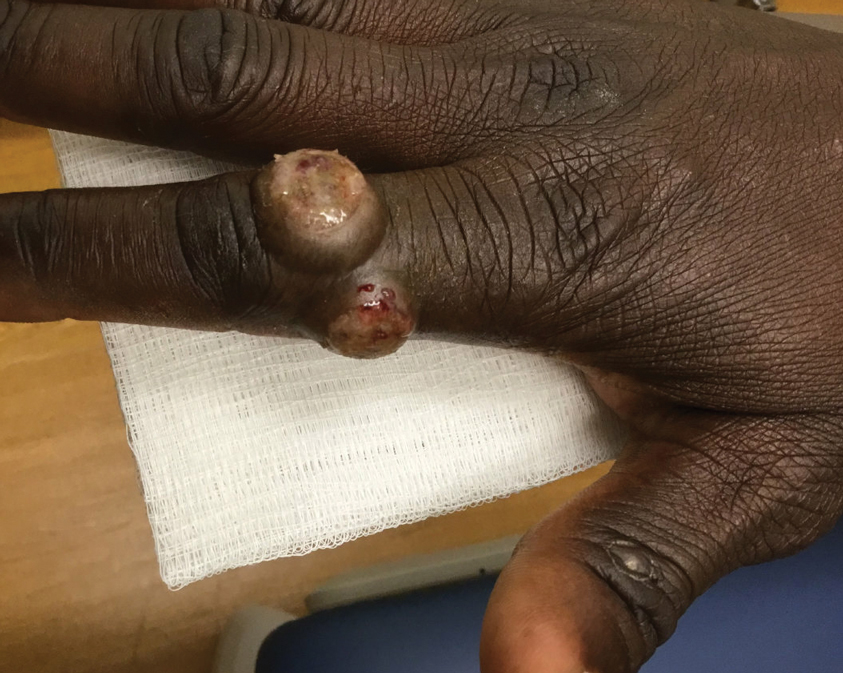
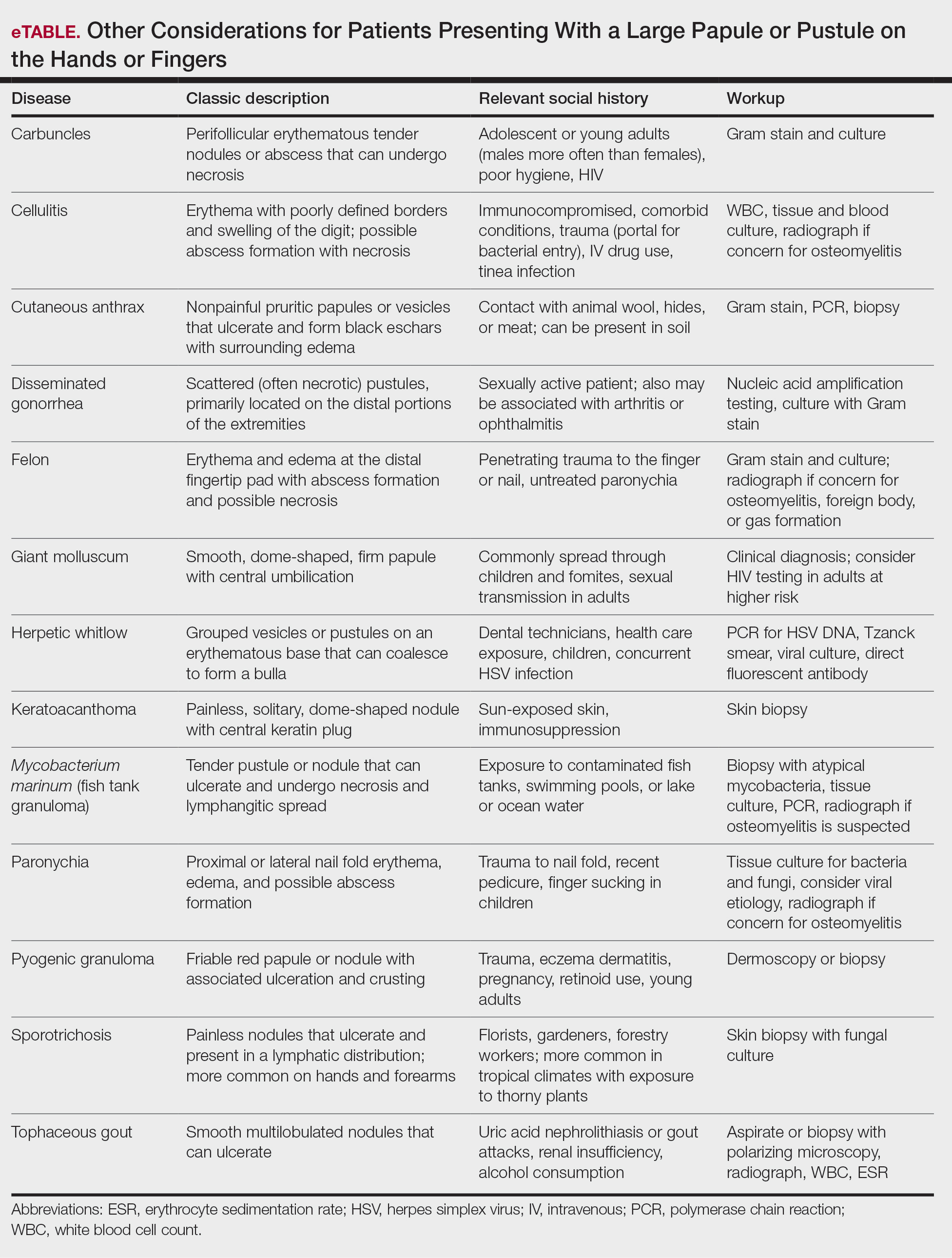
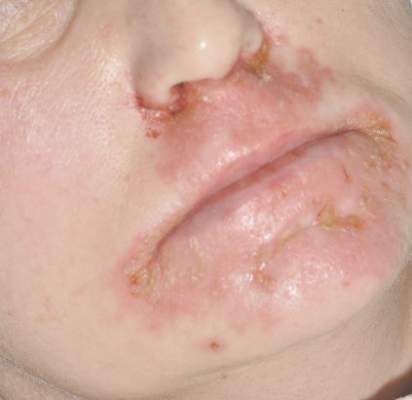
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
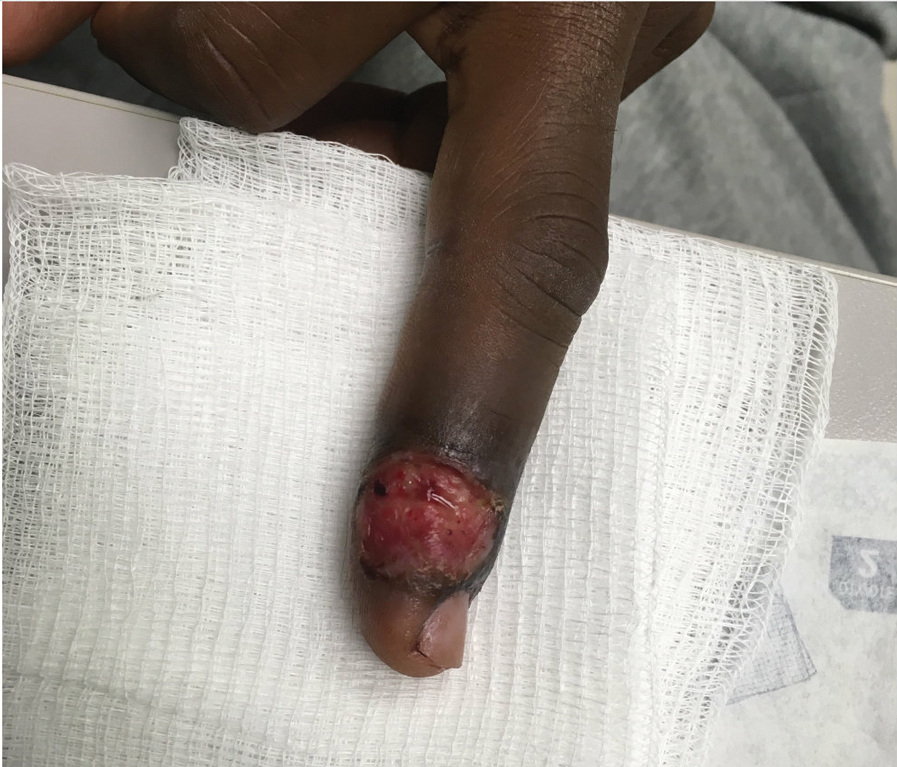
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
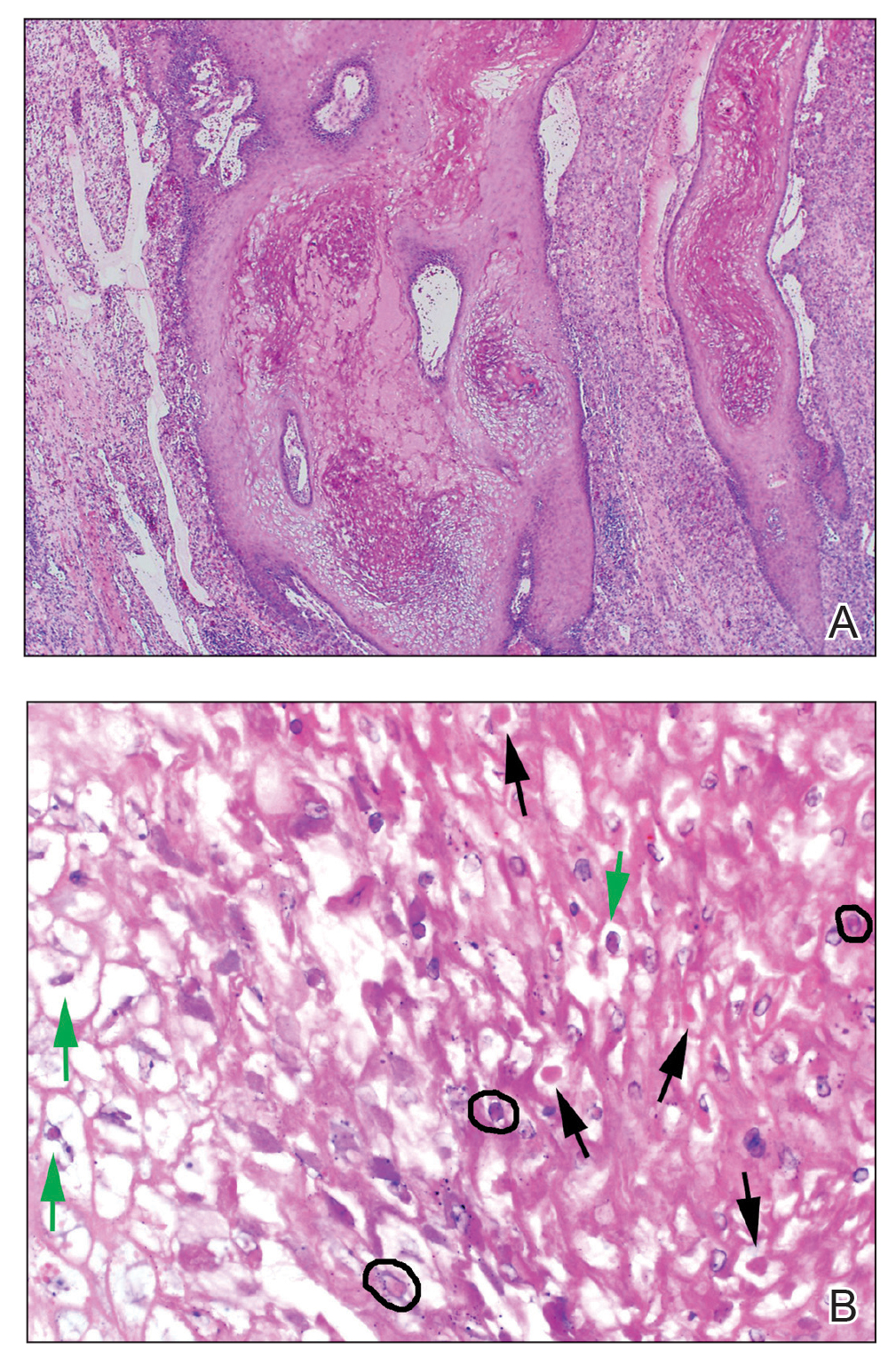
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
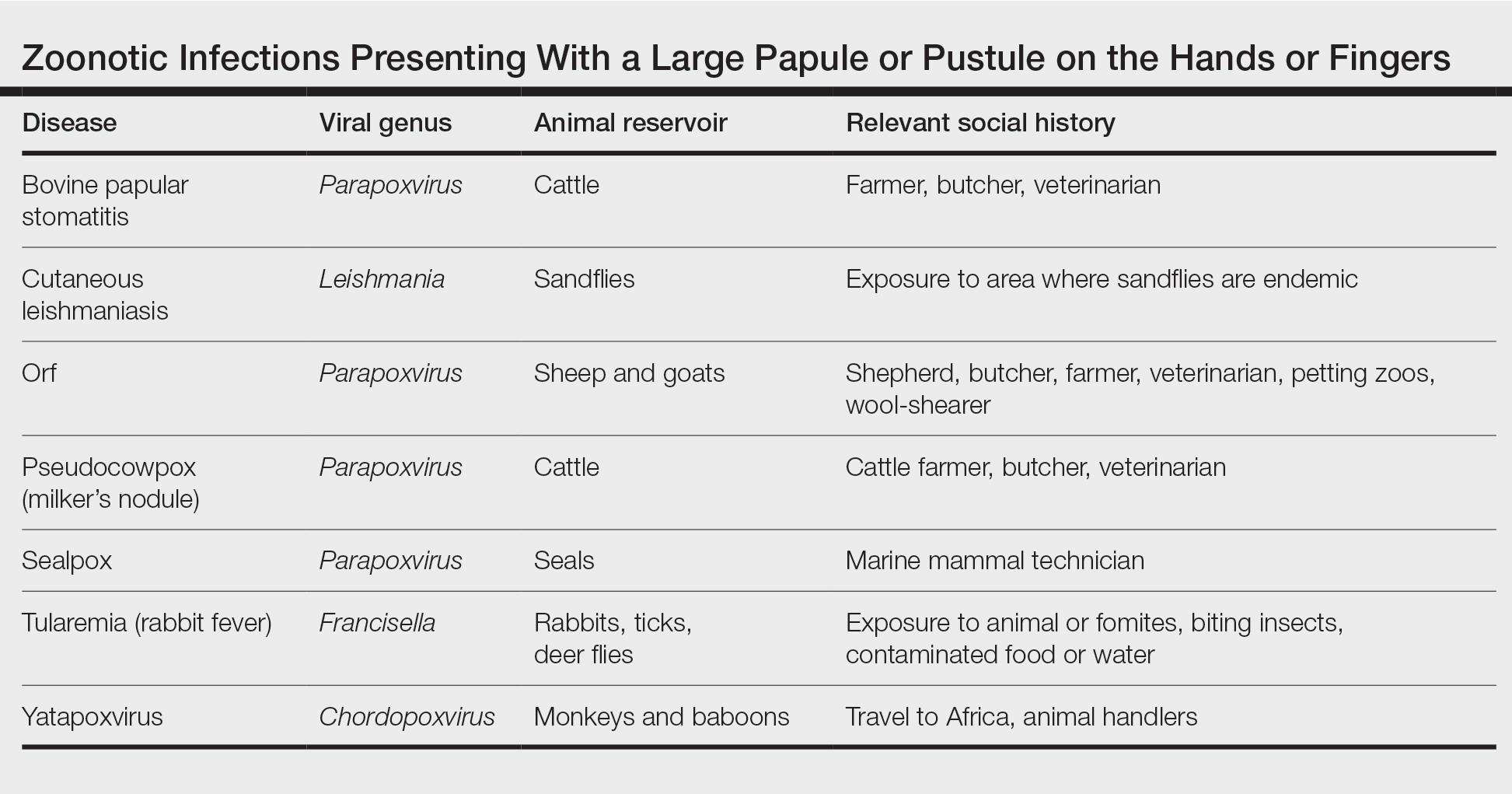
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
Practice Points
- Ecthyma contagiosum is a discrete clinical entity that occurs worldwide and demands careful attention to clinical course and social history.
- Ecthyma contagiosum is caused by orf virus, an epitheliotropic zoonotic infection that spreads from ruminants to humans.
- Early and rapid diagnosis of this classic condition is critical to prevent unnecessary biopsies or extensive testing, and determination of etiology can be important in preventing reinfection or spread to other humans by the same infected animal.
Drug-induced Linear IgA Bullous Dermatosis in a Patient With a Vancomycin-impregnated Cement Spacer
Case Report
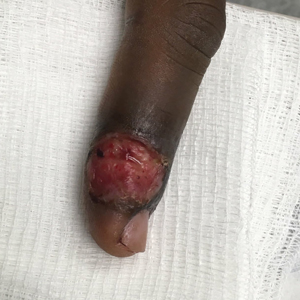
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
Case Report
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
Case Report
A 77-year-old man was admitted to the general medicine service at our institution for treatment of a diffuse macular eruption and hemorrhagic bullae 12 days after undergoing left-knee revision arthroplasty during which a cement spacer impregnated with vancomycin and tobramycin was placed. At the time of the surgery, the patient also received intravenous (IV) vancomycin and oral ciprofloxacin, which were continued postoperatively until his hospital presentation. The patient was recovering well until postoperative day 7, when he developed painful swelling and erythema surrounding the surgical wound on the left knee. Concerned that his symptoms indicated a flare of gout, he restarted a former allopurinol prescription from an outside physician after 2 years of nonuse. The skin changes progressed distally on the left leg over the next 48 hours. By postoperative day 10, he had developed serosanguinous blisters on the left knee (Figure 1A) and oral mucosa (Figure 1B), as well as erythematous nodules on the bilateral palms. He presented to our institution for emergent care on postoperative day 12 following progression of the eruption to the inguinal region (Figure 2A), buttocks (Figure 2B), and abdominal region.


Due to concerns about a potential drug reaction, the IV vancomycin, oral ciprofloxacin, and oral allopurinol were discontinued on hospital admission.

Oral prednisone 60 mg once daily and oral dapsone 25 mg once daily were initiated on hospital days 4 and 6 (postoperative days 15 and 17), respectively. A 6-week course of oral ciprofloxacin 750 mg twice daily and daptomycin 8 mg/kg once daily was initiated for bacterial coverage on hospital day 5 (postoperative day 16). Topical triamcinolone and an anesthetic mouthwash also were used to treat the mucosal involvement. The lesions stabilized on the third day of steroid therapy, and the patient was discharged 7 days after hospital admission (postoperative day 18). Dapsone was rapidly increased to 100 mg once daily over the next week for Pneumocystis jirovecii pneumonia prophylaxis. An increase in prednisone to 80 mg once daily was required 3 days after the patient was discharged due to worsening oral lesions. Five days after discharge, the patient was readmitted to the hospital for 3 days due to acute kidney injury (AKI) in which his baseline creatinine level tripled. The cause of renal impairment was unknown, resulting in empiric discontinuation of dapsone on postoperative day 27. Prophylaxis for P jirovecii pneumonia was replaced with once-monthly inhaled pentamidine. Prednisone was tapered 20 days after the original presentation (postoperative day 32) following gradual improvement of both the skin and oral lesions. At dermatology follow-up 2 weeks later, doxycycline 100 mg twice daily was added for residual inflammation of the left leg. A deep vein thrombosis was discovered in the left leg 10 days later, and 3 months of anticoagulation therapy was initiated with discontinuation of the doxycycline. The patient continued to have renal insufficiency several weeks after dapsone discontinuation and developed prominent peripheral motor neuropathy with bilateral thenar atrophy. He did not experience any skin eruptions or relapses in the weeks following prednisone cessation and underwent successful removal of the cement spacer with full left-knee reconstruction 4 months after his initial presentation to our institution. At 9-month dermatology follow-up, the LABD remained in remission.
Comment
Linear IgA bullous dermatosis is a well-documented autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction. The development of autoantibodies to antigens within the basement membrane zone leads to both cellular and humoral immune responses that facilitate the subepidermal blistering rash in LABD.2,3 Linear IgA bullous dermatosis affects all ages and races with a bimodal epidemiology. The adult form typically appears after 60 years of age, whereas the childhood form (chronic bullous disease of childhood) appears between 6 months and 6 years of age.3 Medications—particularly vancomycin—are responsible for a substantial portion of cases.1-4 In one review, vancomycin was implicated in almost half (22/52 [42.3%]) of drug-related cases of LABD.4 Other associated medications include captopril, trimethoprim-sulfamethoxazole, phenytoin, and diclo-fenac.3,4 Vancomycin-associated LABD has a substantially shorter time to onset of symptoms, with a mean of 8.6 days compared to 63.8 days for other causative agents.4
The initial treatment of drug-induced LABD is immediate discontinuation of the suspected agent(s) and supportive care.9 Although future avoidance of vancomycin is recommended in patients with a history of LABD, there are reported cases of successful rechallenges.4,10 The early removal of our patient’s cement spacer was discouraged by both the orthopedics and infectious disease consultation services due to potential complications as well as the patient’s gradual improvement during his hospital course.
Dapsone is considered the standard systemic treatment for LABD. Sulfapyridine is an alternative to dapsone, or a combination of these 2 drugs may be used. Corticosteroids can be added to each of these regimens to achieve remission, as in our case.2 Although dapsone was discontinued in the setting of the patient’s AKI, the vancomycin in the dual-eluting spacer was more likely the culprit. A review of 544 postoperative outcomes following the use of an antibiotic-impregnated cement spacer (AICS) during 2-stage arthroplasty displayed an 8- to 10-fold increase in the development of AKIs compared to the rate of AKIs following primary joint arthroplasty.10 While our patient’s AKI was not attributed to dapsone, his prominent peripheral motor neuropathy with resultant bilateral thenar atrophy was a rare complication of dapsone use. While dapsone-associated neuropathy has been reported in daily dosages of as low as 75 mg, it typically is seen in doses of at least 300 mg per day and in larger cumulative dosages.11
Despite having a well-characterized vancomycin-induced LABD in the setting of known vancomycin exposure, our patient’s case was particularly challenging given the continued presence of the vancomycin-impregnated cement spacer (VICS) in the left knee, resulting in vancomycin levels at admission and during subsequent measurements over 2 weeks that were all several-fold higher than the renal clearance predicted.
Vancomycin-associated LABD does not appear to be dose dependent and has been reported at both subtherapeutic1-3 and supratherapeutic levels,5-9 whereas toxicity reactions are more common at supratherapeutic levels.9 The literature on AICS use suggests that drug elution occurs at relatively unpredictable rates based on a variety of factors, including the type of cement used and the initial antibiotic concentration.12,13 Furthermore, the addition of tobramycin to VICSs has been found to increase the rate of vancomycin delivery through a phenomenon known as passive opportunism.14
As AICS devices allow for the delivery of higher concentrations of antibiotics to a localized area, systemic complications are considered rare but have been reported.13 Our report describes a rare case of LABD in the setting of a VICS. One clinical aspect of our case that supports the implication of VICS as the cause of the patient’s LABD is the concentration of bullae overlying the incision site on the left knee. A case of a desquamating rash in a patient with an implanted VICS has been documented in which the early lesions were localized to the surgical leg, as in our case.15 Unlike our case, there was a history of Stevens-Johnson syndrome following previous vancomycin exposure. A case of a gentamicin-impregnated cement spacer causing allergic dermatitis that was most prominent in the surgical leg also has been reported.16 An isomorphic phenomenon (Köbner phenomenon) has been suggested in the setting of
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
- Plunkett RW, Chiarello SE, Beutner EH. Linear IgA bullous dermatosis in one of two piroxicam-induced eruptions: a distinct direct immunofluorescence trend revealed by the literature. J Am Acad Dermatol. 2001;45:691-696.
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30:38-50.
- Fortuna G, Salas-Alanis JC, Guidetti E, et al. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66:988-994.
- Kuechle MK, Stegemeir E, Maynard B, et al. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30(2, pt 1):187-192.
- Neughebauer BI, Negron G, Pelton S, et al. Bullous skin disease: an unusual allergic reaction to vancomycin. Am J Med Sci. 2002;323:273-278.
- Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245.
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196-199.
- Dang LV, Byrom L, Muir J, et al. Vancomycin-induced linear IgA with mucosal and ocular involvement: a case report. Infect Dis Clin Pract. 2014;22:e119-e121.
- Luu A, Syed F, Raman G, et al. Two-stage arthroplasty for prosthetic joint infection: a systematic review of acute kidney injury, systemic toxicity and infection control [published online April 8, 2013]. J Arthroplasty. 2013;28:1490.e1-1498.e1.
- Daneshmend TK. The neurotoxicity of dapsone. Adverse Drug React Acute Poisoning Rev. 1984;3:43-58.
- Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356-368.
- Springer BD, Lee GC, Osmon D, et al. Systemic safety of high-dose antibiotic-loaded cement spacers after resection of an infected total knee arthroplasty. Clin Orthop Relat Res. 2004;427:47-51.
- Penner MJ, Masri BA, Duncan CP. Elution characteristics of vancomycin and tobramycin combined in acrylic bone-cement. J Arthroplasty. 1996;11:939-944.
- Williams B, Hanson A, Sha B. Diffuse desquamating rash following exposure to vancomycin-impregnated bone cement. Ann Pharmacother. 2014;48:1061-1065.
- Haeberle M, Wittner B. Is gentamicin-loaded bone cement a risk for developing systemic allergic dermatitis? Contact Dermatitis. 2009;60:176-177.
- McDonald HC, York NR, Pandya AG. Drug-induced linear IgA bullous dermatosis demonstrating the isomorphic phenomenon. J Am Acad Dermatol. 2010;62:897-898.
Practice Points
- Linear IgA bullous dermatosis (LABD) is an autoimmune mucocutaneous disorder characterized by linear IgA deposits at the dermoepidermal junction.
- A substantial number of cases of LABD are drug related, with vancomycin most commonly implicated.
- While antibiotic-impregnated cement spacers deliver high concentrations of local medications, systemic reactions are still possible.
- Dapsone is the first-line treatment for LABD.
An Eruption While on Total Parenteral Nutrition
The Diagnosis: Acquired Acrodermatitis Enteropathica
Acquired acrodermatitis enteropathica (AAE) is a rare disorder caused by severe zinc deficiency. Although acrodermatitis enteropathica is an autosomal-recessive disorder that typically manifests in infancy, AAE also can result from poor zinc intake, impaired absorption, or accelerated losses. There are reports of AAE in patients with zinc-deficient diets,1 eating disorders,2 bariatric and other gastrointestinal surgeries,3 malabsorptive diseases,4 and nephrotic syndrome.5
Zinc plays an important role in DNA and RNA synthesis, reactive oxygen species attenuation, and energy metabolism, allowing for proper wound healing, skin differentiation, and proliferation.6 Zinc is found in most foods, but animal protein contains higher concentrations (Table).7 Approximately 85% of zinc is stored in muscles and bones, with only a small amount of accessible zinc available in the liver. Liver stores can be depleted as quickly as 1 week.8 Total parenteral nutrition without trace element supplementation can quickly predispose patients to AAE.
 |
 |
Diagnosis of this condition requires triangulation of clinical presentation, histopathology examination, and laboratory findings. Acrodermatitis enteropathica typically is characterized by dermatitis, diarrhea, and epidermal appendage findings. In its early stages, the dermatitis often manifests with angular cheilitis and paronychia.9 Patients then develop erythema, erosions, and occasionally vesicles or psoriasiform plaques in periorificial, perineal, and acral sites (Figure 1). Epidermal appendage effects include generalized alopecia and thinning nails with white transverse ridges. Although dermatologic and gastrointestinal manifestations are the most obvious, severe AAE may cause other symptoms, including mental slowing, hypogonadism, and impaired immune function.9
Histopathology of AAE skin lesions is similar to other nutritional deficiencies. Early changes are more specific to deficiency dermatitis and include cytoplasmic pallor and ballooning degeneration of keratinocytes in the stratum spinosum and granulosum.9 Necrolysis results in confluent keratinocyte necrosis developing into subcorneal bulla. Later in the disease course, the presentation becomes psoriasiform with keratinocyte dyskeratosis and confluent parakeratosis10 (Figure 2). Dermal edema with dilated tortuous vessels and a neutrophilic infiltrate may be present throughout disease progression.
Common laboratory abnormalities used to confirm zinc deficiency are decreased plasma zinc and alkaline phosphatase levels. Plasma zinc levels should be drawn after fasting because zinc levels decrease after food intake.9 Concurrent albumin levels should be drawn to correct for low levels caused by hypoalbuminemia. Acquired acrodermatitis enteropathica has been seen in patients with only mildly decreased plasma zinc levels or even zinc levels within reference range.11 Alkaline phosphatase metalloenzyme synthesis requires zinc and a decreased level suggests zinc deficiency even with a plasma zinc level within reference range. Alkaline phosphatase levels usually can be ascertained in a matter of hours, while the zinc levels take much longer to result.
Acquired acrodermatitis enteropathica is treated with oral elemental zinc supplementation at 1 to 2 mg/kg daily.12 Diarrhea typically resolves within 24 hours, but skin lesions heal in 1 to 2 weeks or longer. Although there is no consensus on when to discontinue zinc replacement therapy, therapy generally is not lifelong. Once the patient is zinc replete and the inciting factor has resolved, patients can discontinue supplementation without risk for recurrence.
Trace elements had not been added to our patient’s total parenteral nutrition prior to admission. Basic nutrition laboratory results and zinc levels returned markedly low: 14 μg/dL (reference range, 60–120 μg/dL). Alkaline phosphatase, a zinc-dependent protein, also was low at 12 U/L (reference range, 40–150 U/L). We added trace elements and vitamins and began empiric zinc replacement with 440 mg oral zinc sulfate daily (100 mg elemental zinc). Cephalexin was prescribed for impetiginized skin lesions. The patient noted skin improvement after 3 days on zinc replacement therapy.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol. 2010;37:726-729.
- Bae-Harboe YS, Solky A, Masterpol KS. A case of acquired zinc deficiency. Dermatol Online J. 2012;18:1.
- Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn’s disease. Dermatol Basel Switz. 1996;193:361-363.
- Reichel M, Mauro TM, Ziboh VA, et al. Acrodermatitis enteropathica in a patient with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:415-417.
- Perafan-Riveros C, Franca LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- National Nutrient Database for Standard Reference, Release 28. United States Department of Agriculture, Agricultural Research Service website. http://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=309&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m. Accessed December 14, 2015.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Kumar P, Lal NR, Mondal A, et al. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
The Diagnosis: Acquired Acrodermatitis Enteropathica
Acquired acrodermatitis enteropathica (AAE) is a rare disorder caused by severe zinc deficiency. Although acrodermatitis enteropathica is an autosomal-recessive disorder that typically manifests in infancy, AAE also can result from poor zinc intake, impaired absorption, or accelerated losses. There are reports of AAE in patients with zinc-deficient diets,1 eating disorders,2 bariatric and other gastrointestinal surgeries,3 malabsorptive diseases,4 and nephrotic syndrome.5
Zinc plays an important role in DNA and RNA synthesis, reactive oxygen species attenuation, and energy metabolism, allowing for proper wound healing, skin differentiation, and proliferation.6 Zinc is found in most foods, but animal protein contains higher concentrations (Table).7 Approximately 85% of zinc is stored in muscles and bones, with only a small amount of accessible zinc available in the liver. Liver stores can be depleted as quickly as 1 week.8 Total parenteral nutrition without trace element supplementation can quickly predispose patients to AAE.
 |
 |
Diagnosis of this condition requires triangulation of clinical presentation, histopathology examination, and laboratory findings. Acrodermatitis enteropathica typically is characterized by dermatitis, diarrhea, and epidermal appendage findings. In its early stages, the dermatitis often manifests with angular cheilitis and paronychia.9 Patients then develop erythema, erosions, and occasionally vesicles or psoriasiform plaques in periorificial, perineal, and acral sites (Figure 1). Epidermal appendage effects include generalized alopecia and thinning nails with white transverse ridges. Although dermatologic and gastrointestinal manifestations are the most obvious, severe AAE may cause other symptoms, including mental slowing, hypogonadism, and impaired immune function.9
Histopathology of AAE skin lesions is similar to other nutritional deficiencies. Early changes are more specific to deficiency dermatitis and include cytoplasmic pallor and ballooning degeneration of keratinocytes in the stratum spinosum and granulosum.9 Necrolysis results in confluent keratinocyte necrosis developing into subcorneal bulla. Later in the disease course, the presentation becomes psoriasiform with keratinocyte dyskeratosis and confluent parakeratosis10 (Figure 2). Dermal edema with dilated tortuous vessels and a neutrophilic infiltrate may be present throughout disease progression.
Common laboratory abnormalities used to confirm zinc deficiency are decreased plasma zinc and alkaline phosphatase levels. Plasma zinc levels should be drawn after fasting because zinc levels decrease after food intake.9 Concurrent albumin levels should be drawn to correct for low levels caused by hypoalbuminemia. Acquired acrodermatitis enteropathica has been seen in patients with only mildly decreased plasma zinc levels or even zinc levels within reference range.11 Alkaline phosphatase metalloenzyme synthesis requires zinc and a decreased level suggests zinc deficiency even with a plasma zinc level within reference range. Alkaline phosphatase levels usually can be ascertained in a matter of hours, while the zinc levels take much longer to result.
Acquired acrodermatitis enteropathica is treated with oral elemental zinc supplementation at 1 to 2 mg/kg daily.12 Diarrhea typically resolves within 24 hours, but skin lesions heal in 1 to 2 weeks or longer. Although there is no consensus on when to discontinue zinc replacement therapy, therapy generally is not lifelong. Once the patient is zinc replete and the inciting factor has resolved, patients can discontinue supplementation without risk for recurrence.
Trace elements had not been added to our patient’s total parenteral nutrition prior to admission. Basic nutrition laboratory results and zinc levels returned markedly low: 14 μg/dL (reference range, 60–120 μg/dL). Alkaline phosphatase, a zinc-dependent protein, also was low at 12 U/L (reference range, 40–150 U/L). We added trace elements and vitamins and began empiric zinc replacement with 440 mg oral zinc sulfate daily (100 mg elemental zinc). Cephalexin was prescribed for impetiginized skin lesions. The patient noted skin improvement after 3 days on zinc replacement therapy.
The Diagnosis: Acquired Acrodermatitis Enteropathica
Acquired acrodermatitis enteropathica (AAE) is a rare disorder caused by severe zinc deficiency. Although acrodermatitis enteropathica is an autosomal-recessive disorder that typically manifests in infancy, AAE also can result from poor zinc intake, impaired absorption, or accelerated losses. There are reports of AAE in patients with zinc-deficient diets,1 eating disorders,2 bariatric and other gastrointestinal surgeries,3 malabsorptive diseases,4 and nephrotic syndrome.5
Zinc plays an important role in DNA and RNA synthesis, reactive oxygen species attenuation, and energy metabolism, allowing for proper wound healing, skin differentiation, and proliferation.6 Zinc is found in most foods, but animal protein contains higher concentrations (Table).7 Approximately 85% of zinc is stored in muscles and bones, with only a small amount of accessible zinc available in the liver. Liver stores can be depleted as quickly as 1 week.8 Total parenteral nutrition without trace element supplementation can quickly predispose patients to AAE.
 |
 |
Diagnosis of this condition requires triangulation of clinical presentation, histopathology examination, and laboratory findings. Acrodermatitis enteropathica typically is characterized by dermatitis, diarrhea, and epidermal appendage findings. In its early stages, the dermatitis often manifests with angular cheilitis and paronychia.9 Patients then develop erythema, erosions, and occasionally vesicles or psoriasiform plaques in periorificial, perineal, and acral sites (Figure 1). Epidermal appendage effects include generalized alopecia and thinning nails with white transverse ridges. Although dermatologic and gastrointestinal manifestations are the most obvious, severe AAE may cause other symptoms, including mental slowing, hypogonadism, and impaired immune function.9
Histopathology of AAE skin lesions is similar to other nutritional deficiencies. Early changes are more specific to deficiency dermatitis and include cytoplasmic pallor and ballooning degeneration of keratinocytes in the stratum spinosum and granulosum.9 Necrolysis results in confluent keratinocyte necrosis developing into subcorneal bulla. Later in the disease course, the presentation becomes psoriasiform with keratinocyte dyskeratosis and confluent parakeratosis10 (Figure 2). Dermal edema with dilated tortuous vessels and a neutrophilic infiltrate may be present throughout disease progression.
Common laboratory abnormalities used to confirm zinc deficiency are decreased plasma zinc and alkaline phosphatase levels. Plasma zinc levels should be drawn after fasting because zinc levels decrease after food intake.9 Concurrent albumin levels should be drawn to correct for low levels caused by hypoalbuminemia. Acquired acrodermatitis enteropathica has been seen in patients with only mildly decreased plasma zinc levels or even zinc levels within reference range.11 Alkaline phosphatase metalloenzyme synthesis requires zinc and a decreased level suggests zinc deficiency even with a plasma zinc level within reference range. Alkaline phosphatase levels usually can be ascertained in a matter of hours, while the zinc levels take much longer to result.
Acquired acrodermatitis enteropathica is treated with oral elemental zinc supplementation at 1 to 2 mg/kg daily.12 Diarrhea typically resolves within 24 hours, but skin lesions heal in 1 to 2 weeks or longer. Although there is no consensus on when to discontinue zinc replacement therapy, therapy generally is not lifelong. Once the patient is zinc replete and the inciting factor has resolved, patients can discontinue supplementation without risk for recurrence.
Trace elements had not been added to our patient’s total parenteral nutrition prior to admission. Basic nutrition laboratory results and zinc levels returned markedly low: 14 μg/dL (reference range, 60–120 μg/dL). Alkaline phosphatase, a zinc-dependent protein, also was low at 12 U/L (reference range, 40–150 U/L). We added trace elements and vitamins and began empiric zinc replacement with 440 mg oral zinc sulfate daily (100 mg elemental zinc). Cephalexin was prescribed for impetiginized skin lesions. The patient noted skin improvement after 3 days on zinc replacement therapy.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol. 2010;37:726-729.
- Bae-Harboe YS, Solky A, Masterpol KS. A case of acquired zinc deficiency. Dermatol Online J. 2012;18:1.
- Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn’s disease. Dermatol Basel Switz. 1996;193:361-363.
- Reichel M, Mauro TM, Ziboh VA, et al. Acrodermatitis enteropathica in a patient with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:415-417.
- Perafan-Riveros C, Franca LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- National Nutrient Database for Standard Reference, Release 28. United States Department of Agriculture, Agricultural Research Service website. http://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=309&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m. Accessed December 14, 2015.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Kumar P, Lal NR, Mondal A, et al. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol. 2010;37:726-729.
- Bae-Harboe YS, Solky A, Masterpol KS. A case of acquired zinc deficiency. Dermatol Online J. 2012;18:1.
- Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn’s disease. Dermatol Basel Switz. 1996;193:361-363.
- Reichel M, Mauro TM, Ziboh VA, et al. Acrodermatitis enteropathica in a patient with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:415-417.
- Perafan-Riveros C, Franca LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- National Nutrient Database for Standard Reference, Release 28. United States Department of Agriculture, Agricultural Research Service website. http://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=309&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m. Accessed December 14, 2015.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Kumar P, Lal NR, Mondal A, et al. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
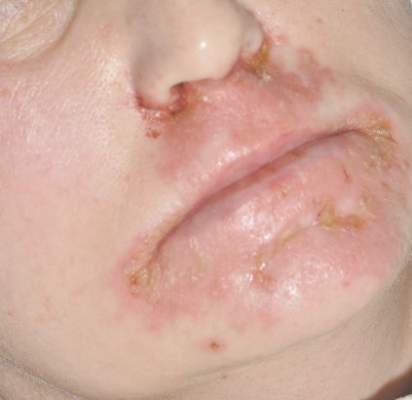
A 47-year-old woman with a history of bulimia and gastroparesis who had been on total parenteral nutrition for 8 weeks presented with a painful, perioral, perineal, and acral eruption of 7 weeks’ duration. Additionally, she had experienced diarrhea, vomiting, and a 13.5-kg weight loss in the last 4 months. Physical examination revealed perioral and perineal, well-demarcated, erythematous, scaly plaques with yellow crusting. She had edematous crusted erosions on the bilateral palms and soles and psoriasiform plaques along the right arm and flank. Punch biopsies (4 mm) from the right inguinal fold and right elbow were obtained.