User login
What Is the Best Approach to a Cavitary Lung Lesion?
Case
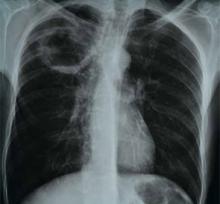
A 66-year-old homeless man with a history of smoking and cirrhosis due to alcoholism presents to the hospital with a productive cough and fever for one month. He has traveled around Arizona and New Mexico but has never left the country. His complete blood count (CBC) is notable for a white blood cell count of 13,000. His chest X-ray reveals a 1.7-cm right upper lobe cavitary lung lesion (see Figure 1). What is the best approach to this patient’s cavitary lung lesion?
Overview
Cavitary lung lesions are relatively common findings on chest imaging and often pose a diagnostic challenge to the hospitalist. Having a standard approach to the evaluation of a cavitary lung lesion can facilitate an expedited workup.
A lung cavity is defined radiographically as a lucent area contained within a consolidation, mass, or nodule.1 Cavities usually are accompanied by thick walls, greater than 4 mm. These should be differentiated from cysts, which are not surrounded by consolidation, mass, or nodule, and are accompanied by a thinner wall.2
The differential diagnosis of a cavitary lung lesion is broad and can be delineated into categories of infectious and noninfectious etiologies (see Figure 2). Infectious causes include bacterial, fungal, and, rarely, parasitic agents. Noninfectious causes encompass malignant, rheumatologic, and other less common etiologies such as infarct related to pulmonary embolism.
The clinical presentation and assessment of risk factors for a particular patient are of the utmost importance in delineating next steps for evaluation and management (see Table 1). For those patients of older age with smoking history, specific occupational or environmental exposures, and weight loss, the most common etiology is neoplasm. Common infectious causes include lung abscess and necrotizing pneumonia, as well as tuberculosis. The approach to diagnosis should be based on a composite of the clinical presentation, patient characteristics, and radiographic appearance of the cavity.
Guidelines for the approach to cavitary lung lesions are lacking, yet a thorough understanding of the initial approach is important for those practicing hospital medicine. Key components in the approach to diagnosis of a solitary cavitary lesion are outlined in this article.
Diagnosis of Infectious Causes
In the initial evaluation of a cavitary lung lesion, it is important to first determine if the cause is an infectious process. The infectious etiologies to consider include lung abscess and necrotizing pneumonia, tuberculosis, and septic emboli. Important components in the clinical presentation include presence of cough, fever, night sweats, chills, and symptoms that have lasted less than one month, as well as comorbid conditions, drug or alcohol abuse, and history of immunocompromise (e.g. HIV, immunosuppressive therapy, or organ transplant).
Given the public health considerations and impact of treatment, tuberculosis (TB) will be discussed in its own category.
Tuberculosis. Given the fact that TB patients require airborne isolation, the disease must be considered early in the evaluation of a cavitary lung lesion. Patients with TB often present with more chronic symptoms, such as fevers, night sweats, weight loss, and hemoptysis. Immunocompromised state, travel to endemic regions, and incarceration increase the likelihood of TB. Nontuberculous mycobacterium (i.e., M. kansasii) should also be considered in endemic areas.
For those patients in whom TB is suspected, airborne isolation must be initiated promptly. The provider should obtain three sputum samples for acid-fast bacillus (AFB) smear and culture when risk factors are present. Most patients with reactivation TB have abnormal chest X-rays, with approximately 20% of those patients having air-fluid levels and the majority of cases affecting the upper lobes.3 Cavities may be seen in patients with primary or reactivation TB.3
Lung abscess and necrotizing pneumonia. Lung abscesses are cavities associated with necrosis caused by a microbial infection. The term necrotizing pneumonia typically is used when there are multiple smaller (smaller than 2 cm) associated lung abscesses, although both lung abscess and necrotizing pneumonia represent a similar pathophysiologic process and are along the same continuum. Lung abscess is suspected with the presence of predisposing risk factors to aspiration (e.g. alcoholism) and poor dentition. History of cough, fever, putrid sputum, night sweats, and weight loss may indicate subacute or chronic development of a lung abscess. Physical examination might be significant for signs of pneumonia and gingivitis.
Organisms that cause lung abscesses include anaerobes (most common), TB, methicillin-resistant Staphylococcus aureus (MRSA), post-influenza illness, endemic fungi, and Nocardia, among others.4 In immunocompromised patients, more common considerations include TB, Mycobacterium avium complex, other mycobacteria, Pseudomonas aeruginosa, Nocardia, Cryptococcus, Aspergillus, endemic fungi (e.g. Coccidiodes in the Southwest and Histoplasma in the Midwest), and, less commonly, Pneumocystis jiroveci.4 The likelihood of each organism is dependent on the patient’s risk factors. Initial laboratory testing includes sputum and blood cultures, as well as serologic testing for endemic fungi, especially in immunocompromised patients.
Imaging may reveal a cavitary lesion in the dependent pulmonary segments (posterior segments of the upper lobes or superior segments of the lower lobes), at times associated with a pleural effusion or infiltrate. The most common appearance of a lung abscess is an asymmetric cavity with an air-fluid level and a wall with a ragged or smooth border. CT scan is often indicated when X-rays are equivocal and when cases are of uncertain cause or are unresponsive to antibiotic therapy. Bronchoscopy is reserved for patients with an immunocompromising condition, atypical presentation, or lack of response to treatment.
For those cavitary lesions in which there is a high degree of suspicion for lung abscess, empiric treatment should include antibiotics active against anaerobes and MRSA if the patient has risk factors. Patients often receive an empiric trial of antibiotics prior to biopsy unless there are clear indications that the cavitary lung lesion is related to cancer. Lung abscesses typically drain spontaneously, and transthoracic or endobronchial drainage is not usually recommended as initial management due to risk of pneumothorax and formation of bronchopleural fistula.
Lung abscesses should be followed to resolution with serial chest imaging. If the lung abscess does not resolve, it would be appropriate to consult thoracic surgery, interventional radiology, or pulmonary, depending on the location of the abscess and the local expertise with transthoracic or endobronchial drainage and surgical resection.
Septic emboli. Septic emboli are a less common cause of cavitary lung lesions. This entity should be considered in patients with a history of IV drug use or infected indwelling devices (central venous catheters, pacemaker wires, and right-sided prosthetic heart valves). Physical examination should include an assessment for signs of endocarditis and inspection for infected indwelling devices. In patients with IV drug use, the likely pathogen is S. aureus.
Oropharyngeal infection or indwelling catheters may predispose patients to septic thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, a rare but important cause of septic emboli.5 Laboratory testing includes culture for sputum and blood and culture of the infected device if applicable. On chest X-ray, septic emboli commonly appear as nodules located in the lung periphery. CT scan is more sensitive for detecting cavitation associated with septic emboli.
Diagnosis of Noninfectious Causes
Upon identification of a cavitary lung lesion, noninfectious etiologies must also be entertained. Noninfectious etiologies include malignancy, rheumatologic diseases, pulmonary embolism, and other causes. Important components in the clinical presentation include the presence of constitutional symptoms (fevers, weight loss, night sweats), smoking history, family history, and an otherwise complete review of systems. Physical exam should include evaluation for lymphadenopathy, cachexia, rash, clubbing, and other symptoms pertinent to the suspected etiology.
Malignancy. Perhaps most important among noninfectious causes of cavitary lung lesions is malignancy, and a high index of suspicion is warranted given that it is commonly the first diagnosis to consider overall.2 Cavities can form in primary lung cancers (e.g. bronchogenic carcinomas), lung tumors such as lymphoma or Kaposi’s sarcoma, or in metastatic disease. Cavitation has been detected in 7%-11% of primary lung cancers by plain radiography and in 22% by computed tomography.5 Cancers of squamous cell origin are the most likely to cavitate; this holds true for both primary lung tumors and metastatic tumors.6 Additionally, cavitation portends a worse prognosis.7
Clinicians should review any available prior chest imaging studies to look for a change in the quality or size of a cavitary lung lesion. Neoplasms are typically of variable size with irregular thick walls (greater than 4 mm) on CT scan, with higher specificity for neoplasm in those with a wall thickness greater than 15 mm.2
When the diagnosis is less clear, the decision to embark on more advanced diagnostic methods, such as biopsy, should rest on the provider’s clinical suspicion for a certain disease process. When a lung cancer is suspected, consultation with pulmonary and interventional radiology should be obtained to determine the best approach for biopsy.
Rheumatologic. Less common causes of cavitary lesions include those related to rheumatologic diseases (e.g. granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis). One study demonstrated that cavitary lung nodules occur in 37% of patients with granulomatosis with polyangiitis.8
Although uncommon, cavitary nodules can also be seen in rheumatoid arthritis and sarcoidosis. Given that patients with rheumatologic diseases are often treated with immunosuppressive agents, infection must remain high on the differential. Suspicion of a rheumatologic cause should prompt the clinician to obtain appropriate serologic testing and consultation as needed.
Pulmonary embolism. Although often not considered in the evaluation of cavitary lung lesions, pulmonary embolism (PE) can lead to infarction and the formation of a cavitary lesion. Pulmonary infarction has been reported to occur in as many as one third of cases of PE.9 Cavitary lesions also have been described in chronic thromboembolic disease.10
Other. Uncommon causes of cavitary lesions include bronchiolitis obliterans with organizing pneumonia, Langerhans cell histiocytosis, and amyloidosis, among others. The hospitalist should keep a broad differential and involve consultants if the diagnosis remains unclear after initial diagnostic evaluation.
Back to the Case
The patient’s fever and productive cough, in combination with recent travel and location of the cavitary lesion, increase his risk for tuberculosis and endemic fungi, such as Coccidioides. This patient was placed on respiratory isolation with AFBs obtained to rule out TB, with Coccidioides antibodies, Cyptococcal antigen titers, and sputum for fungus sent to evaluate for an endemic fungus. He had a chest CT, which revealed a 17-mm cavitary mass within the right upper lobe that contained an air-fluid level indicating lung abscess. Coccidioides, cryptococcal, fungal sputum, and TB studies were negative.
The patient was treated empirically with clindamycin given the high prevalence of anaerobes in lung abscess. He was followed as an outpatient and had a chest X-ray showing resolution of the lesion at six months. The purpose of the X-ray was two-fold: to monitor the effect of antibiotic treatment and to evaluate for persistence of the cavitation given the neoplastic risk factors of older age and smoking.
Bottom Line
The best approach to a patient with a cavitary lung lesion includes assessing the clinical presentation and risk factors, differentiating infectious from noninfectious causes, and then utilizing this information to further direct the diagnostic evaluation. Consultation with a subspecialist or further testing such as biopsy should be considered if the etiology remains undefined after the initial evaluation.
Drs. Rendon, Pizanis, Montanaro, and Kraai are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque.
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744-752.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, Overturf GD. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94(2):316-320.
- Yazbeck MF, Dahdel M, Kalra A, Browne AS, Pratter MR. Lung abscess: update on microbiology and management. Am J Ther. 2012;21(3):217-221. doi: 10.1097/MJT.0b013e3182383c9b.
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305-333.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5(6):523-530.
- Kolodziejski LS, Dyczek S, Duda K, Góralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66-73.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21(1):1-7.
- Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2(3):11-21.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269-1271.
Case
A 66-year-old homeless man with a history of smoking and cirrhosis due to alcoholism presents to the hospital with a productive cough and fever for one month. He has traveled around Arizona and New Mexico but has never left the country. His complete blood count (CBC) is notable for a white blood cell count of 13,000. His chest X-ray reveals a 1.7-cm right upper lobe cavitary lung lesion (see Figure 1). What is the best approach to this patient’s cavitary lung lesion?
Overview
Cavitary lung lesions are relatively common findings on chest imaging and often pose a diagnostic challenge to the hospitalist. Having a standard approach to the evaluation of a cavitary lung lesion can facilitate an expedited workup.
A lung cavity is defined radiographically as a lucent area contained within a consolidation, mass, or nodule.1 Cavities usually are accompanied by thick walls, greater than 4 mm. These should be differentiated from cysts, which are not surrounded by consolidation, mass, or nodule, and are accompanied by a thinner wall.2
The differential diagnosis of a cavitary lung lesion is broad and can be delineated into categories of infectious and noninfectious etiologies (see Figure 2). Infectious causes include bacterial, fungal, and, rarely, parasitic agents. Noninfectious causes encompass malignant, rheumatologic, and other less common etiologies such as infarct related to pulmonary embolism.
The clinical presentation and assessment of risk factors for a particular patient are of the utmost importance in delineating next steps for evaluation and management (see Table 1). For those patients of older age with smoking history, specific occupational or environmental exposures, and weight loss, the most common etiology is neoplasm. Common infectious causes include lung abscess and necrotizing pneumonia, as well as tuberculosis. The approach to diagnosis should be based on a composite of the clinical presentation, patient characteristics, and radiographic appearance of the cavity.
Guidelines for the approach to cavitary lung lesions are lacking, yet a thorough understanding of the initial approach is important for those practicing hospital medicine. Key components in the approach to diagnosis of a solitary cavitary lesion are outlined in this article.
Diagnosis of Infectious Causes
In the initial evaluation of a cavitary lung lesion, it is important to first determine if the cause is an infectious process. The infectious etiologies to consider include lung abscess and necrotizing pneumonia, tuberculosis, and septic emboli. Important components in the clinical presentation include presence of cough, fever, night sweats, chills, and symptoms that have lasted less than one month, as well as comorbid conditions, drug or alcohol abuse, and history of immunocompromise (e.g. HIV, immunosuppressive therapy, or organ transplant).
Given the public health considerations and impact of treatment, tuberculosis (TB) will be discussed in its own category.
Tuberculosis. Given the fact that TB patients require airborne isolation, the disease must be considered early in the evaluation of a cavitary lung lesion. Patients with TB often present with more chronic symptoms, such as fevers, night sweats, weight loss, and hemoptysis. Immunocompromised state, travel to endemic regions, and incarceration increase the likelihood of TB. Nontuberculous mycobacterium (i.e., M. kansasii) should also be considered in endemic areas.
For those patients in whom TB is suspected, airborne isolation must be initiated promptly. The provider should obtain three sputum samples for acid-fast bacillus (AFB) smear and culture when risk factors are present. Most patients with reactivation TB have abnormal chest X-rays, with approximately 20% of those patients having air-fluid levels and the majority of cases affecting the upper lobes.3 Cavities may be seen in patients with primary or reactivation TB.3
Lung abscess and necrotizing pneumonia. Lung abscesses are cavities associated with necrosis caused by a microbial infection. The term necrotizing pneumonia typically is used when there are multiple smaller (smaller than 2 cm) associated lung abscesses, although both lung abscess and necrotizing pneumonia represent a similar pathophysiologic process and are along the same continuum. Lung abscess is suspected with the presence of predisposing risk factors to aspiration (e.g. alcoholism) and poor dentition. History of cough, fever, putrid sputum, night sweats, and weight loss may indicate subacute or chronic development of a lung abscess. Physical examination might be significant for signs of pneumonia and gingivitis.
Organisms that cause lung abscesses include anaerobes (most common), TB, methicillin-resistant Staphylococcus aureus (MRSA), post-influenza illness, endemic fungi, and Nocardia, among others.4 In immunocompromised patients, more common considerations include TB, Mycobacterium avium complex, other mycobacteria, Pseudomonas aeruginosa, Nocardia, Cryptococcus, Aspergillus, endemic fungi (e.g. Coccidiodes in the Southwest and Histoplasma in the Midwest), and, less commonly, Pneumocystis jiroveci.4 The likelihood of each organism is dependent on the patient’s risk factors. Initial laboratory testing includes sputum and blood cultures, as well as serologic testing for endemic fungi, especially in immunocompromised patients.
Imaging may reveal a cavitary lesion in the dependent pulmonary segments (posterior segments of the upper lobes or superior segments of the lower lobes), at times associated with a pleural effusion or infiltrate. The most common appearance of a lung abscess is an asymmetric cavity with an air-fluid level and a wall with a ragged or smooth border. CT scan is often indicated when X-rays are equivocal and when cases are of uncertain cause or are unresponsive to antibiotic therapy. Bronchoscopy is reserved for patients with an immunocompromising condition, atypical presentation, or lack of response to treatment.
For those cavitary lesions in which there is a high degree of suspicion for lung abscess, empiric treatment should include antibiotics active against anaerobes and MRSA if the patient has risk factors. Patients often receive an empiric trial of antibiotics prior to biopsy unless there are clear indications that the cavitary lung lesion is related to cancer. Lung abscesses typically drain spontaneously, and transthoracic or endobronchial drainage is not usually recommended as initial management due to risk of pneumothorax and formation of bronchopleural fistula.
Lung abscesses should be followed to resolution with serial chest imaging. If the lung abscess does not resolve, it would be appropriate to consult thoracic surgery, interventional radiology, or pulmonary, depending on the location of the abscess and the local expertise with transthoracic or endobronchial drainage and surgical resection.
Septic emboli. Septic emboli are a less common cause of cavitary lung lesions. This entity should be considered in patients with a history of IV drug use or infected indwelling devices (central venous catheters, pacemaker wires, and right-sided prosthetic heart valves). Physical examination should include an assessment for signs of endocarditis and inspection for infected indwelling devices. In patients with IV drug use, the likely pathogen is S. aureus.
Oropharyngeal infection or indwelling catheters may predispose patients to septic thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, a rare but important cause of septic emboli.5 Laboratory testing includes culture for sputum and blood and culture of the infected device if applicable. On chest X-ray, septic emboli commonly appear as nodules located in the lung periphery. CT scan is more sensitive for detecting cavitation associated with septic emboli.
Diagnosis of Noninfectious Causes
Upon identification of a cavitary lung lesion, noninfectious etiologies must also be entertained. Noninfectious etiologies include malignancy, rheumatologic diseases, pulmonary embolism, and other causes. Important components in the clinical presentation include the presence of constitutional symptoms (fevers, weight loss, night sweats), smoking history, family history, and an otherwise complete review of systems. Physical exam should include evaluation for lymphadenopathy, cachexia, rash, clubbing, and other symptoms pertinent to the suspected etiology.
Malignancy. Perhaps most important among noninfectious causes of cavitary lung lesions is malignancy, and a high index of suspicion is warranted given that it is commonly the first diagnosis to consider overall.2 Cavities can form in primary lung cancers (e.g. bronchogenic carcinomas), lung tumors such as lymphoma or Kaposi’s sarcoma, or in metastatic disease. Cavitation has been detected in 7%-11% of primary lung cancers by plain radiography and in 22% by computed tomography.5 Cancers of squamous cell origin are the most likely to cavitate; this holds true for both primary lung tumors and metastatic tumors.6 Additionally, cavitation portends a worse prognosis.7
Clinicians should review any available prior chest imaging studies to look for a change in the quality or size of a cavitary lung lesion. Neoplasms are typically of variable size with irregular thick walls (greater than 4 mm) on CT scan, with higher specificity for neoplasm in those with a wall thickness greater than 15 mm.2
When the diagnosis is less clear, the decision to embark on more advanced diagnostic methods, such as biopsy, should rest on the provider’s clinical suspicion for a certain disease process. When a lung cancer is suspected, consultation with pulmonary and interventional radiology should be obtained to determine the best approach for biopsy.
Rheumatologic. Less common causes of cavitary lesions include those related to rheumatologic diseases (e.g. granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis). One study demonstrated that cavitary lung nodules occur in 37% of patients with granulomatosis with polyangiitis.8
Although uncommon, cavitary nodules can also be seen in rheumatoid arthritis and sarcoidosis. Given that patients with rheumatologic diseases are often treated with immunosuppressive agents, infection must remain high on the differential. Suspicion of a rheumatologic cause should prompt the clinician to obtain appropriate serologic testing and consultation as needed.
Pulmonary embolism. Although often not considered in the evaluation of cavitary lung lesions, pulmonary embolism (PE) can lead to infarction and the formation of a cavitary lesion. Pulmonary infarction has been reported to occur in as many as one third of cases of PE.9 Cavitary lesions also have been described in chronic thromboembolic disease.10
Other. Uncommon causes of cavitary lesions include bronchiolitis obliterans with organizing pneumonia, Langerhans cell histiocytosis, and amyloidosis, among others. The hospitalist should keep a broad differential and involve consultants if the diagnosis remains unclear after initial diagnostic evaluation.
Back to the Case
The patient’s fever and productive cough, in combination with recent travel and location of the cavitary lesion, increase his risk for tuberculosis and endemic fungi, such as Coccidioides. This patient was placed on respiratory isolation with AFBs obtained to rule out TB, with Coccidioides antibodies, Cyptococcal antigen titers, and sputum for fungus sent to evaluate for an endemic fungus. He had a chest CT, which revealed a 17-mm cavitary mass within the right upper lobe that contained an air-fluid level indicating lung abscess. Coccidioides, cryptococcal, fungal sputum, and TB studies were negative.
The patient was treated empirically with clindamycin given the high prevalence of anaerobes in lung abscess. He was followed as an outpatient and had a chest X-ray showing resolution of the lesion at six months. The purpose of the X-ray was two-fold: to monitor the effect of antibiotic treatment and to evaluate for persistence of the cavitation given the neoplastic risk factors of older age and smoking.
Bottom Line
The best approach to a patient with a cavitary lung lesion includes assessing the clinical presentation and risk factors, differentiating infectious from noninfectious causes, and then utilizing this information to further direct the diagnostic evaluation. Consultation with a subspecialist or further testing such as biopsy should be considered if the etiology remains undefined after the initial evaluation.
Drs. Rendon, Pizanis, Montanaro, and Kraai are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque.
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744-752.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, Overturf GD. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94(2):316-320.
- Yazbeck MF, Dahdel M, Kalra A, Browne AS, Pratter MR. Lung abscess: update on microbiology and management. Am J Ther. 2012;21(3):217-221. doi: 10.1097/MJT.0b013e3182383c9b.
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305-333.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5(6):523-530.
- Kolodziejski LS, Dyczek S, Duda K, Góralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66-73.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21(1):1-7.
- Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2(3):11-21.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269-1271.
Case
A 66-year-old homeless man with a history of smoking and cirrhosis due to alcoholism presents to the hospital with a productive cough and fever for one month. He has traveled around Arizona and New Mexico but has never left the country. His complete blood count (CBC) is notable for a white blood cell count of 13,000. His chest X-ray reveals a 1.7-cm right upper lobe cavitary lung lesion (see Figure 1). What is the best approach to this patient’s cavitary lung lesion?
Overview
Cavitary lung lesions are relatively common findings on chest imaging and often pose a diagnostic challenge to the hospitalist. Having a standard approach to the evaluation of a cavitary lung lesion can facilitate an expedited workup.
A lung cavity is defined radiographically as a lucent area contained within a consolidation, mass, or nodule.1 Cavities usually are accompanied by thick walls, greater than 4 mm. These should be differentiated from cysts, which are not surrounded by consolidation, mass, or nodule, and are accompanied by a thinner wall.2
The differential diagnosis of a cavitary lung lesion is broad and can be delineated into categories of infectious and noninfectious etiologies (see Figure 2). Infectious causes include bacterial, fungal, and, rarely, parasitic agents. Noninfectious causes encompass malignant, rheumatologic, and other less common etiologies such as infarct related to pulmonary embolism.
The clinical presentation and assessment of risk factors for a particular patient are of the utmost importance in delineating next steps for evaluation and management (see Table 1). For those patients of older age with smoking history, specific occupational or environmental exposures, and weight loss, the most common etiology is neoplasm. Common infectious causes include lung abscess and necrotizing pneumonia, as well as tuberculosis. The approach to diagnosis should be based on a composite of the clinical presentation, patient characteristics, and radiographic appearance of the cavity.
Guidelines for the approach to cavitary lung lesions are lacking, yet a thorough understanding of the initial approach is important for those practicing hospital medicine. Key components in the approach to diagnosis of a solitary cavitary lesion are outlined in this article.
Diagnosis of Infectious Causes
In the initial evaluation of a cavitary lung lesion, it is important to first determine if the cause is an infectious process. The infectious etiologies to consider include lung abscess and necrotizing pneumonia, tuberculosis, and septic emboli. Important components in the clinical presentation include presence of cough, fever, night sweats, chills, and symptoms that have lasted less than one month, as well as comorbid conditions, drug or alcohol abuse, and history of immunocompromise (e.g. HIV, immunosuppressive therapy, or organ transplant).
Given the public health considerations and impact of treatment, tuberculosis (TB) will be discussed in its own category.
Tuberculosis. Given the fact that TB patients require airborne isolation, the disease must be considered early in the evaluation of a cavitary lung lesion. Patients with TB often present with more chronic symptoms, such as fevers, night sweats, weight loss, and hemoptysis. Immunocompromised state, travel to endemic regions, and incarceration increase the likelihood of TB. Nontuberculous mycobacterium (i.e., M. kansasii) should also be considered in endemic areas.
For those patients in whom TB is suspected, airborne isolation must be initiated promptly. The provider should obtain three sputum samples for acid-fast bacillus (AFB) smear and culture when risk factors are present. Most patients with reactivation TB have abnormal chest X-rays, with approximately 20% of those patients having air-fluid levels and the majority of cases affecting the upper lobes.3 Cavities may be seen in patients with primary or reactivation TB.3
Lung abscess and necrotizing pneumonia. Lung abscesses are cavities associated with necrosis caused by a microbial infection. The term necrotizing pneumonia typically is used when there are multiple smaller (smaller than 2 cm) associated lung abscesses, although both lung abscess and necrotizing pneumonia represent a similar pathophysiologic process and are along the same continuum. Lung abscess is suspected with the presence of predisposing risk factors to aspiration (e.g. alcoholism) and poor dentition. History of cough, fever, putrid sputum, night sweats, and weight loss may indicate subacute or chronic development of a lung abscess. Physical examination might be significant for signs of pneumonia and gingivitis.
Organisms that cause lung abscesses include anaerobes (most common), TB, methicillin-resistant Staphylococcus aureus (MRSA), post-influenza illness, endemic fungi, and Nocardia, among others.4 In immunocompromised patients, more common considerations include TB, Mycobacterium avium complex, other mycobacteria, Pseudomonas aeruginosa, Nocardia, Cryptococcus, Aspergillus, endemic fungi (e.g. Coccidiodes in the Southwest and Histoplasma in the Midwest), and, less commonly, Pneumocystis jiroveci.4 The likelihood of each organism is dependent on the patient’s risk factors. Initial laboratory testing includes sputum and blood cultures, as well as serologic testing for endemic fungi, especially in immunocompromised patients.
Imaging may reveal a cavitary lesion in the dependent pulmonary segments (posterior segments of the upper lobes or superior segments of the lower lobes), at times associated with a pleural effusion or infiltrate. The most common appearance of a lung abscess is an asymmetric cavity with an air-fluid level and a wall with a ragged or smooth border. CT scan is often indicated when X-rays are equivocal and when cases are of uncertain cause or are unresponsive to antibiotic therapy. Bronchoscopy is reserved for patients with an immunocompromising condition, atypical presentation, or lack of response to treatment.
For those cavitary lesions in which there is a high degree of suspicion for lung abscess, empiric treatment should include antibiotics active against anaerobes and MRSA if the patient has risk factors. Patients often receive an empiric trial of antibiotics prior to biopsy unless there are clear indications that the cavitary lung lesion is related to cancer. Lung abscesses typically drain spontaneously, and transthoracic or endobronchial drainage is not usually recommended as initial management due to risk of pneumothorax and formation of bronchopleural fistula.
Lung abscesses should be followed to resolution with serial chest imaging. If the lung abscess does not resolve, it would be appropriate to consult thoracic surgery, interventional radiology, or pulmonary, depending on the location of the abscess and the local expertise with transthoracic or endobronchial drainage and surgical resection.
Septic emboli. Septic emboli are a less common cause of cavitary lung lesions. This entity should be considered in patients with a history of IV drug use or infected indwelling devices (central venous catheters, pacemaker wires, and right-sided prosthetic heart valves). Physical examination should include an assessment for signs of endocarditis and inspection for infected indwelling devices. In patients with IV drug use, the likely pathogen is S. aureus.
Oropharyngeal infection or indwelling catheters may predispose patients to septic thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, a rare but important cause of septic emboli.5 Laboratory testing includes culture for sputum and blood and culture of the infected device if applicable. On chest X-ray, septic emboli commonly appear as nodules located in the lung periphery. CT scan is more sensitive for detecting cavitation associated with septic emboli.
Diagnosis of Noninfectious Causes
Upon identification of a cavitary lung lesion, noninfectious etiologies must also be entertained. Noninfectious etiologies include malignancy, rheumatologic diseases, pulmonary embolism, and other causes. Important components in the clinical presentation include the presence of constitutional symptoms (fevers, weight loss, night sweats), smoking history, family history, and an otherwise complete review of systems. Physical exam should include evaluation for lymphadenopathy, cachexia, rash, clubbing, and other symptoms pertinent to the suspected etiology.
Malignancy. Perhaps most important among noninfectious causes of cavitary lung lesions is malignancy, and a high index of suspicion is warranted given that it is commonly the first diagnosis to consider overall.2 Cavities can form in primary lung cancers (e.g. bronchogenic carcinomas), lung tumors such as lymphoma or Kaposi’s sarcoma, or in metastatic disease. Cavitation has been detected in 7%-11% of primary lung cancers by plain radiography and in 22% by computed tomography.5 Cancers of squamous cell origin are the most likely to cavitate; this holds true for both primary lung tumors and metastatic tumors.6 Additionally, cavitation portends a worse prognosis.7
Clinicians should review any available prior chest imaging studies to look for a change in the quality or size of a cavitary lung lesion. Neoplasms are typically of variable size with irregular thick walls (greater than 4 mm) on CT scan, with higher specificity for neoplasm in those with a wall thickness greater than 15 mm.2
When the diagnosis is less clear, the decision to embark on more advanced diagnostic methods, such as biopsy, should rest on the provider’s clinical suspicion for a certain disease process. When a lung cancer is suspected, consultation with pulmonary and interventional radiology should be obtained to determine the best approach for biopsy.
Rheumatologic. Less common causes of cavitary lesions include those related to rheumatologic diseases (e.g. granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis). One study demonstrated that cavitary lung nodules occur in 37% of patients with granulomatosis with polyangiitis.8
Although uncommon, cavitary nodules can also be seen in rheumatoid arthritis and sarcoidosis. Given that patients with rheumatologic diseases are often treated with immunosuppressive agents, infection must remain high on the differential. Suspicion of a rheumatologic cause should prompt the clinician to obtain appropriate serologic testing and consultation as needed.
Pulmonary embolism. Although often not considered in the evaluation of cavitary lung lesions, pulmonary embolism (PE) can lead to infarction and the formation of a cavitary lesion. Pulmonary infarction has been reported to occur in as many as one third of cases of PE.9 Cavitary lesions also have been described in chronic thromboembolic disease.10
Other. Uncommon causes of cavitary lesions include bronchiolitis obliterans with organizing pneumonia, Langerhans cell histiocytosis, and amyloidosis, among others. The hospitalist should keep a broad differential and involve consultants if the diagnosis remains unclear after initial diagnostic evaluation.
Back to the Case
The patient’s fever and productive cough, in combination with recent travel and location of the cavitary lesion, increase his risk for tuberculosis and endemic fungi, such as Coccidioides. This patient was placed on respiratory isolation with AFBs obtained to rule out TB, with Coccidioides antibodies, Cyptococcal antigen titers, and sputum for fungus sent to evaluate for an endemic fungus. He had a chest CT, which revealed a 17-mm cavitary mass within the right upper lobe that contained an air-fluid level indicating lung abscess. Coccidioides, cryptococcal, fungal sputum, and TB studies were negative.
The patient was treated empirically with clindamycin given the high prevalence of anaerobes in lung abscess. He was followed as an outpatient and had a chest X-ray showing resolution of the lesion at six months. The purpose of the X-ray was two-fold: to monitor the effect of antibiotic treatment and to evaluate for persistence of the cavitation given the neoplastic risk factors of older age and smoking.
Bottom Line
The best approach to a patient with a cavitary lung lesion includes assessing the clinical presentation and risk factors, differentiating infectious from noninfectious causes, and then utilizing this information to further direct the diagnostic evaluation. Consultation with a subspecialist or further testing such as biopsy should be considered if the etiology remains undefined after the initial evaluation.
Drs. Rendon, Pizanis, Montanaro, and Kraai are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque.
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744-752.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, Overturf GD. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94(2):316-320.
- Yazbeck MF, Dahdel M, Kalra A, Browne AS, Pratter MR. Lung abscess: update on microbiology and management. Am J Ther. 2012;21(3):217-221. doi: 10.1097/MJT.0b013e3182383c9b.
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305-333.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5(6):523-530.
- Kolodziejski LS, Dyczek S, Duda K, Góralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66-73.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21(1):1-7.
- Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2(3):11-21.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269-1271.
How Should Common Symptoms at the End of Life be Managed?
Case
A 58-year-old male with colon cancer metastatic to the liver and lungs presents with vomiting, dyspnea, and abdominal pain. His disease has progressed through third-line chemotherapy and his care is now focused entirely on symptom management. He has not had a bowel movement in five days and he began vomiting two days ago.
Overview
The majority of patients in the United States die in acute-care hospitals. The Study to Understand Prognosis and Preferences for Outcomes and Risks of Treatments (SUPPORT), which evaluated the courses of close to 10,000 hospitalized patients with serious and life-limiting illnesses, illustrated that patients’ end-of-life (EOL) experiences often are characterized by poor symptom management and invasive care that is not congruent with the patients’ overall goals of care.1 Studies of factors identified as priorities in EOL care have consistently shown that excellent pain and symptom management are highly valued by patients and families. As the hospitalist movement continues to grow, hospitalists will play a large role in caring for patients at EOL and will need to know how to provide adequate pain and symptom management so that high-quality care can be achieved.
Pain: A Basic Tenet
A basic tenet of palliative medicine is to evaluate and treat all types of suffering.2 Physical pain at EOL is frequently accompanied by other types of pain, such as psychological, social, religious, or existential pain. However, this review will focus on the pharmacologic management of physical pain.
Pain management must begin with a thorough evaluation of the severity, location, and characteristics of the discomfort to assess which therapies are most likely to be beneficial (see Table 1).3 The consistent use of one scale of pain severity (such as 0-10, or mild/moderate/severe) assists in the choice of initial dose of pain medication, in determining the response to the medication, and in assessing the need for change in dose.4
Opioids are the foundation of pain management in advanced diseases because they are available in a number of formulations and, when dosed appropriately, they are effective and safe. Starting doses and equianalgesic doses of common opioids are presented in Table 2. Guidelines recommend the use of short-acting opioids for dose titration to gain control of poorly controlled pain.3 If a patient is experiencing mild pain on a specific regimen, the medication dose can be increased up to 25%; by 25% to 50%, if pain is moderate; and 50% to 100%, if severe.5 When the pain is better-controlled, the total amount of pain medication used in 24 hours (24-hour dose) can be converted to a long-acting formulation for more consistent pain management. Because there is a constant component to most advanced pain syndromes, it is recommended that pain medication is given on a standing basis, with as-needed (prn) doses available for exacerbations of pain.3 Prn doses of short-acting medication (equivalent to approximately 10% of the 24-hour dose of medication) should be available at one- or two-hour intervals prn (longer if hepatic or renal impairment is present) for IV or PO medications, respectively.
Opioids often are categorized as low potency (i.e. codeine, hydrocodone) and high-potency (i.e. oxycodone, morphine, hydromorphone, fentanyl). When given in “equianalgesic doses,” the analgesic effect and common side effects (nausea/vomiting, constipation, sedation, confusion, pruritis) of different opioids can vary in different patients. Due to differences in levels of expressed subtypes of opioid receptors, a given patient might be more sensitive to the analgesic effect or side effects of a specific medication. Therefore, if dose escalation of one opioid is inadequate to control pain and further increases in dose are limited by intolerable side effects, rotation to another opioid is recommended.4 Tables documenting equianalgesic doses of different opioids are based on only moderate evidence from equivalency trials performed in healthy volunteers.6 Due to interpatient differences in responses, it is recommended that the equianalgesic dose of the new medication be decreased by 25% to 50% for initial dosing.5
Certain treatments are indicated for specific pain syndromes. Bony metastases respond to NSAIDs, bisphosphonates, and radiation therapy in addition to opioid medications. As focal back pain is the first symptom of spinal cord compression, clinicians should have a high index of suspicion for compression in any patient with malignancy and new back pain. Steroids and radiation therapy are considered emergent treatments for pain control and to prevent paralysis in this circumstance. Pain due to bowel obstruction is usually colicky in nature and responds well to octreotide as discussed in the section on nausea and vomiting. Steroids (such as dexamethasone 4 mg PO bid-tid) might be an effective adjuvant medication in bone pain, tumor pain, or inflammation.
*Half this dose should be used in renal or liver dysfunction and in the elderly.
Preferred in renal dysfunction.
SOURCES: Adapted from Assessment and treatment of physical pain associated with life-limiting illness. Hospice and Palliative Care Training for Physicians: UNIPAC. Vol 3. 3rd ed. Glenview, IL: American Academy of Hospice and Palliative Medicine; 2008, and Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
Back to the Case
At home, the patient was taking 60 mg of extended-release morphine twice daily and six doses per day of 15-mg immediate-release morphine for breakthrough pain. This is the equivalent of 210 mg of oral morphine in 24 hours. His pain is severe on this regimen, but it is unclear how much of this medication he is absorbing due to his vomiting. Using the IV route of administration and a patient-controlled analgesia (PCA) system will enable rapid dose titration and pain control. The equivalent of the 24-hour dose of 210 mg oral morphine is 70 mg IV morphine, which is equivalent to a drip basal rate of approximately 3 mg IV morphine per hour. This basal rate with a bolus dose of 7 mg (10% of the 24-hour dose) IV morphine q1 hour prn is reasonable as a starting point.
Review of the Data: Nausea and Vomiting
Nausea and vomiting affect 40% to 70% of patients in a palliative setting.7 A thorough history and physical exam can enable one to determine the most likely causes, pathways, and receptors involved in the process of nausea and vomiting. It is important to review the timing, frequency, and triggers of vomiting. The oral, abdominal, neurologic, and rectal exams, in addition to a complete chemistry panel, offer helpful information. The most common etiologies and recommended medications are included in Table 3. It is worthwhile to note that serotonin-antagonists (i.e. ondansetron) are first-line therapies only for chemotherapy and radiation-therapy-induced emesis. If a 24-hour trial of one antiemetic therapy is ineffective, one should reassess the etiology and escalate the antiemetic dose, or add a second therapy with a different (pertinent) mechanism of action. Although most studies of antiemetic therapy are case series, there is good evidence for this mechanistic approach.8
*EPS: extrapyramidal symptoms
The various insults and pathways that can cause vomiting are quite complex. The medullary vomiting center (VC) receives vestibular, peripheral (via splanchnic and vagal nerves), and higher cortical inputs and is the final common pathway in the vomiting reflex. The chemoreceptor trigger zone (CTZ) near the fourth ventricle receives input from the vagal and splanchnic nerves, and generates output to the VC.
General dietary recommendations are to avoid sweet, fatty, and highly salted or spiced foods. Small portions of bland foods without strong odors are best tolerated.7 Constipation commonly contributes to nausea and vomiting and should be managed with disimpaction, enemas, and laxatives as tolerated. Imaging may be required to make the important distinction between partial and complete bowel obstruction, as the treatments differ. Surgical procedures, such as colostomy or placement of a venting gastrostomy tube, can relieve pain and vomiting associated with complete bowel obstruction.
Back to the Case
The patient is found to have a fecal impaction on rectal exam, but vomiting persists after disimpaction and enema use. Imaging documents a complete bowel obstruction at the site of a palpable mass in the right upper quadrant and multiple large hepatic metastases. Octreotide is initiated to decrease intestinal secretions and peristalsis. Steroids are given to decrease tumor burden and associated inflammation in the intestine and liver, as well as to relieve distension of the hepatic capsule. Haloperidol is used in low doses to control episodes of nausea.
Review of the Data: Dyspnea
Dyspnea is a common symptom faced by patients at EOL. An estimated 50% of patients who are evaluated in acute-care hospitals seek treatment for the management of this often-crippling symptom.10 Unfortunately, as disease burden progresses, the incidence of dyspnea increases towards EOL, and the presence and severity of dyspnea is strongly correlated with mortality.
It is imperative for providers to appreciate that dyspnea is a subjective symptom, similar to pain. The presence and severity of dyspnea, therefore, depends on patient report. Given its subjective nature, the degree of dyspnea experienced by a patient might not correlate with objective laboratory findings or test results. In practice, the severity of dyspnea is commonly assessed with a numeric rating scale (0-10), verbal analogue scale, or with verbal descriptors (mild, moderate, severe). It is important to determine the underlying etiology of the dyspnea and, if possible, to target interventions to relieve the underlying cause. However, at the end of life, the burdens of invasive studies to determine the exact cause of dyspnea might outweigh the benefits, and invasive testing might not correlate with patients’ and families’ goals of care. In that instance, the goal of treatment should be aggressive symptom management and providers should use clinical judgment to tailor therapies based on the patient’s underlying illness, physical examination, and perhaps on noninvasive radiological or laboratory findings. Below are nonpharmacological and pharmacological interventions that can be employed to help alleviate dyspnea in the actively dying patient.
Nonpharmacological Management
A handheld fan aimed near the patient’s face has been shown to reduce the sensation of dyspnea.11 This relatively safe and inexpensive intervention has no major side effects and can provide improvement in this distressing symptom.
Often, the first line of therapy in the hospital setting for a patient reporting dyspnea is the administration of oxygen therapy. However, recent evidence does not show superiority of oxygen over air inhalation via nasal prongs for dyspnea in patients with advanced cancer or heart failure.12,13
Pharmacological Management
Opioids are first-line therapy for alleviating dyspnea in patients at EOL. The administration of opioids has been shown in systematic reviews to provide effective management of dyspnea.14,15 Practice guidelines by leading expert groups advocate for the use of opioids in the management of dyspnea for patients with advanced malignant and noncancer diseases.10,16 Fear of causing unintended respiratory sedation with opioids limits the prescription of opioids for dyspnea. However, studies have not found a change in mortality with the use of opioids appropriately titrated to control dyspnea.17
Studies examining the role of benzodiazepines in dyspnea management are conflicting. Anecdotal clinical evidence in actively dying patients supports treating dyspnea with benzodiazepines in conjunction with opioid therapy. Benzodiazepines are most beneficial when there is an anxiety-related component to the dyspnea.
Many patients with advanced disease and evidence of airflow obstruction will benefit from nebulized bronchodilator therapy for dyspnea. Patients with dyspnea from fluid overload (i.e. end-stage congestive heart failure or renal disease) might benefit from systemic diuretics. An increasing number of trials are under way to evaluate the efficacy of nebulized furosemide in the symptomatic management of dyspnea.
Back to the Case
The patient’s clinical course decompensates, and he begins to report worsening dyspnea in addition to his underlying pain. He becomes increasingly anxious about what this new symptom means. In addition to having a discussion about disease progression and prognosis, you increase his PCA basal dose to morphine 4 mg/hour to help him with this new symptom. You also add low-dose lorazepam 0.5 mg IV q8 hours as an adjunct agent for his dyspnea. The patient reports improvement of his symptom burden.
Review of the Data: Secretions
Physiological changes occur as a patient enters the active phase of dying. Two such changes are the loss of the ability to swallow and a reduced cough reflex. These changes culminate in an inability to clear secretions, which pool in the oropharynx and the airways. As the patient breathes, air moves over the pooled secretions and produces a gurgling sound that is referred to as the “death rattle.” The onset of this clinical marker has been shown to have significant prognostic significance for predicting imminent death within a period of hours to days. Proposed treatments for the symptom are listed below.
Nonpharmacological Management
Nonpharmacological options include repositioning the patient in a manner that facilitates postural draining.18 Careful and gentle oral suctioning might help reduce secretions if they are salivary in origin. This will not help to clear deeper bronchial secretions. Suctioning of deeper secretions often causes more burden than benefit, as this can cause repeated trauma and possible bleeding.
Family and caregivers at the bedside can find the “death rattle” quite disturbing and often fear that their loved one is “drowning.” Education and counseling that this is not the case, and that the development of secretions is a natural part of the dying process, can help alleviate this concern. Explaining that pharmacological agents can be titrated to decrease secretions is also reassuring to caregivers.
Pharmacological Management
Pharmacological options for secretion management include utilizing anticholinergic medications to prevent the formation of further secretions. These medications are standard of care for managing the death rattle and have been found to be most efficacious if started earlier in the actively dying phase.19,20 Anticholinergic medications include glycopyrrolate (0.2 mg IV q8 hours), atropine sulfate ophthalmological drops (1% solution, 1-2 drops SL q6 hours), hyoscyamine (0.125 mg one to four times a day), and scopolamine (1.5 mg patch q72 hours). These medications all have possible side effects typical of anticholinergic agents, including delirium, constipation, blurred vision, and urinary retention.
Back to the Case
The patient becomes increasingly lethargic. You meet with his family and explain that he is actively dying. His family reiterates that the goals of medical care should focus on maximizing symptom management. His family is concerned about the “gurgly” sound they hear and want to know if that means he is suffering. You educate the family about expected changes that occur with the dying process and inform them that glycopyrrolate 0.2 mg IV q8 hour will be started to minimize further secretions.
Bottom Line
Pain, nausea, dyspnea, and secretions are common end-of-life symptoms that hospitalists should be competent in treating.
Dr. Litrivis is an associate director and assistant professor at the Mount Sinai School of Medicine in New York, and Dr. Neale is an assistant professor at the University of New Mexico School of Medicine in Albuquerque.
References
- The SUPPORT Principal Investigators. A controlled trial to improve the care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
- World Health Organization Definition of Palliative Care. World Health Organization website. Available at: http://www.who.int/cancer/palliative/definition/en/. Accessed April 12, 2012.
- NCCN Guidelines Version 2. 2011 Adult Cancer Pain. National Comprehensive Cancer Network website. Available at: http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed April 12, 2012.
- Whitecar PS, Jonas AP, Clasen ME. Managing pain in the dying patient. Am Fam Physician. 2000;61(3):755-764.
- Bial A, Levine S. Assessment and treatment of physical pain associated with life-limiting illness. Hospice and Palliative Care Training for Physicians: UNIPAC. Vol 3. 3rd ed. Glenview, IL: American Academy of Hospice and Palliative Medicine; 2008.
- Sydney M, et al. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
- Mannix KA. Gastrointestinal symptoms. In: Doyle D, Hanks G, Cherny N, Calman K, eds. Oxford Textbook of Palliative Medicine. 3rd ed. New York, NY: Oxford University Press; 2005.
- Tyler LS. Nausea and vomiting in palliative care. In: Lipman AG, Jackson KC, Tyler LS, eds. Evidence-Based Symptom Control in Palliative Care. New York, NY: The Hawthorn Press; 2000.
- Policzer JS, Sobel J. Management of Selected Nonpain Symptoms of Life-Limiting Illness. Hospice and Palliative Care Training for Physicians: UNIPAC. Vol 4. 3rd ed. Glenview, IL: American Academy of Hospice and Palliative Medicine; 2008.
- Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4): 435-452.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized controlled, crossover trial. J Pain Symptom Manage. 2010;39(5): 831-838.
- Philip J, Gold M, Milner A, Di Iulio J, Miller B, Spruyt O. A randomized, double-blind, crossover trial of the effect of oxygen on dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2006;32(6):541-550.
- Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnea in adults. Cochrane Database Syst Rev. 2008;(3):CD004769.
- Jennings AL, Davies AN, Higgins JP, Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev. 2001;(4):CD002066.
- Ben-Aharon I, Gafter-Gvili A, Paul M, Leibovici, L, Stemmer, SM. Interventions for alleviating cancer-related dyspnea. A systematic review. J Clin Oncol. 2008;26(14): 2396-2404.
- Qaseem A, Snow V, Shekelle P, et al. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148(2):141-146
- Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nat Clin Pract Oncol. 2008;5(2):90–100.
- Bickel K, Arnold R. EPERC Fast Facts Documents #109 Death Rattle and Oral Secretions, 2nd ed. Available at: http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_109.htm. Accessed April 15, 2012.
- Wildiers H, Dhaenekint C, Demeulenaere P, et al. Atropine, hyoscine butylbromide, or scopalamine are equally effective for the treatment of death rattle in terminal care. J Pain Symptom Manage. 2009;38(1):124-133.
- Hugel H, Ellershaw J, Gambles M. Respiratory tract secretions in the dying patient: a comparison between glycopyrronium and hyoscine hydrobromide. J Palliat Med. 2006;9(2):279-285.
Case
A 58-year-old male with colon cancer metastatic to the liver and lungs presents with vomiting, dyspnea, and abdominal pain. His disease has progressed through third-line chemotherapy and his care is now focused entirely on symptom management. He has not had a bowel movement in five days and he began vomiting two days ago.
Overview
The majority of patients in the United States die in acute-care hospitals. The Study to Understand Prognosis and Preferences for Outcomes and Risks of Treatments (SUPPORT), which evaluated the courses of close to 10,000 hospitalized patients with serious and life-limiting illnesses, illustrated that patients’ end-of-life (EOL) experiences often are characterized by poor symptom management and invasive care that is not congruent with the patients’ overall goals of care.1 Studies of factors identified as priorities in EOL care have consistently shown that excellent pain and symptom management are highly valued by patients and families. As the hospitalist movement continues to grow, hospitalists will play a large role in caring for patients at EOL and will need to know how to provide adequate pain and symptom management so that high-quality care can be achieved.
Pain: A Basic Tenet
A basic tenet of palliative medicine is to evaluate and treat all types of suffering.2 Physical pain at EOL is frequently accompanied by other types of pain, such as psychological, social, religious, or existential pain. However, this review will focus on the pharmacologic management of physical pain.
Pain management must begin with a thorough evaluation of the severity, location, and characteristics of the discomfort to assess which therapies are most likely to be beneficial (see Table 1).3 The consistent use of one scale of pain severity (such as 0-10, or mild/moderate/severe) assists in the choice of initial dose of pain medication, in determining the response to the medication, and in assessing the need for change in dose.4
Opioids are the foundation of pain management in advanced diseases because they are available in a number of formulations and, when dosed appropriately, they are effective and safe. Starting doses and equianalgesic doses of common opioids are presented in Table 2. Guidelines recommend the use of short-acting opioids for dose titration to gain control of poorly controlled pain.3 If a patient is experiencing mild pain on a specific regimen, the medication dose can be increased up to 25%; by 25% to 50%, if pain is moderate; and 50% to 100%, if severe.5 When the pain is better-controlled, the total amount of pain medication used in 24 hours (24-hour dose) can be converted to a long-acting formulation for more consistent pain management. Because there is a constant component to most advanced pain syndromes, it is recommended that pain medication is given on a standing basis, with as-needed (prn) doses available for exacerbations of pain.3 Prn doses of short-acting medication (equivalent to approximately 10% of the 24-hour dose of medication) should be available at one- or two-hour intervals prn (longer if hepatic or renal impairment is present) for IV or PO medications, respectively.
Opioids often are categorized as low potency (i.e. codeine, hydrocodone) and high-potency (i.e. oxycodone, morphine, hydromorphone, fentanyl). When given in “equianalgesic doses,” the analgesic effect and common side effects (nausea/vomiting, constipation, sedation, confusion, pruritis) of different opioids can vary in different patients. Due to differences in levels of expressed subtypes of opioid receptors, a given patient might be more sensitive to the analgesic effect or side effects of a specific medication. Therefore, if dose escalation of one opioid is inadequate to control pain and further increases in dose are limited by intolerable side effects, rotation to another opioid is recommended.4 Tables documenting equianalgesic doses of different opioids are based on only moderate evidence from equivalency trials performed in healthy volunteers.6 Due to interpatient differences in responses, it is recommended that the equianalgesic dose of the new medication be decreased by 25% to 50% for initial dosing.5
Certain treatments are indicated for specific pain syndromes. Bony metastases respond to NSAIDs, bisphosphonates, and radiation therapy in addition to opioid medications. As focal back pain is the first symptom of spinal cord compression, clinicians should have a high index of suspicion for compression in any patient with malignancy and new back pain. Steroids and radiation therapy are considered emergent treatments for pain control and to prevent paralysis in this circumstance. Pain due to bowel obstruction is usually colicky in nature and responds well to octreotide as discussed in the section on nausea and vomiting. Steroids (such as dexamethasone 4 mg PO bid-tid) might be an effective adjuvant medication in bone pain, tumor pain, or inflammation.
*Half this dose should be used in renal or liver dysfunction and in the elderly.
Preferred in renal dysfunction.
SOURCES: Adapted from Assessment and treatment of physical pain associated with life-limiting illness. Hospice and Palliative Care Training for Physicians: UNIPAC. Vol 3. 3rd ed. Glenview, IL: American Academy of Hospice and Palliative Medicine; 2008, and Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
Back to the Case
At home, the patient was taking 60 mg of extended-release morphine twice daily and six doses per day of 15-mg immediate-release morphine for breakthrough pain. This is the equivalent of 210 mg of oral morphine in 24 hours. His pain is severe on this regimen, but it is unclear how much of this medication he is absorbing due to his vomiting. Using the IV route of administration and a patient-controlled analgesia (PCA) system will enable rapid dose titration and pain control. The equivalent of the 24-hour dose of 210 mg oral morphine is 70 mg IV morphine, which is equivalent to a drip basal rate of approximately 3 mg IV morphine per hour. This basal rate with a bolus dose of 7 mg (10% of the 24-hour dose) IV morphine q1 hour prn is reasonable as a starting point.
Review of the Data: Nausea and Vomiting
Nausea and vomiting affect 40% to 70% of patients in a palliative setting.7 A thorough history and physical exam can enable one to determine the most likely causes, pathways, and receptors involved in the process of nausea and vomiting. It is important to review the timing, frequency, and triggers of vomiting. The oral, abdominal, neurologic, and rectal exams, in addition to a complete chemistry panel, offer helpful information. The most common etiologies and recommended medications are included in Table 3. It is worthwhile to note that serotonin-antagonists (i.e. ondansetron) are first-line therapies only for chemotherapy and radiation-therapy-induced emesis. If a 24-hour trial of one antiemetic therapy is ineffective, one should reassess the etiology and escalate the antiemetic dose, or add a second therapy with a different (pertinent) mechanism of action. Although most studies of antiemetic therapy are case series, there is good evidence for this mechanistic approach.8
*EPS: extrapyramidal symptoms
The various insults and pathways that can cause vomiting are quite complex. The medullary vomiting center (VC) receives vestibular, peripheral (via splanchnic and vagal nerves), and higher cortical inputs and is the final common pathway in the vomiting reflex. The chemoreceptor trigger zone (CTZ) near the fourth ventricle receives input from the vagal and splanchnic nerves, and generates output to the VC.
General dietary recommendations are to avoid sweet, fatty, and highly salted or spiced foods. Small portions of bland foods without strong odors are best tolerated.7 Constipation commonly contributes to nausea and vomiting and should be managed with disimpaction, enemas, and laxatives as tolerated. Imaging may be required to make the important distinction between partial and complete bowel obstruction, as the treatments differ. Surgical procedures, such as colostomy or placement of a venting gastrostomy tube, can relieve pain and vomiting associated with complete bowel obstruction.
Back to the Case
The patient is found to have a fecal impaction on rectal exam, but vomiting persists after disimpaction and enema use. Imaging documents a complete bowel obstruction at the site of a palpable mass in the right upper quadrant and multiple large hepatic metastases. Octreotide is initiated to decrease intestinal secretions and peristalsis. Steroids are given to decrease tumor burden and associated inflammation in the intestine and liver, as well as to relieve distension of the hepatic capsule. Haloperidol is used in low doses to control episodes of nausea.
Review of the Data: Dyspnea
Dyspnea is a common symptom faced by patients at EOL. An estimated 50% of patients who are evaluated in acute-care hospitals seek treatment for the management of this often-crippling symptom.10 Unfortunately, as disease burden progresses, the incidence of dyspnea increases towards EOL, and the presence and severity of dyspnea is strongly correlated with mortality.
It is imperative for providers to appreciate that dyspnea is a subjective symptom, similar to pain. The presence and severity of dyspnea, therefore, depends on patient report. Given its subjective nature, the degree of dyspnea experienced by a patient might not correlate with objective laboratory findings or test results. In practice, the severity of dyspnea is commonly assessed with a numeric rating scale (0-10), verbal analogue scale, or with verbal descriptors (mild, moderate, severe). It is important to determine the underlying etiology of the dyspnea and, if possible, to target interventions to relieve the underlying cause. However, at the end of life, the burdens of invasive studies to determine the exact cause of dyspnea might outweigh the benefits, and invasive testing might not correlate with patients’ and families’ goals of care. In that instance, the goal of treatment should be aggressive symptom management and providers should use clinical judgment to tailor therapies based on the patient’s underlying illness, physical examination, and perhaps on noninvasive radiological or laboratory findings. Below are nonpharmacological and pharmacological interventions that can be employed to help alleviate dyspnea in the actively dying patient.
Nonpharmacological Management
A handheld fan aimed near the patient’s face has been shown to reduce the sensation of dyspnea.11 This relatively safe and inexpensive intervention has no major side effects and can provide improvement in this distressing symptom.
Often, the first line of therapy in the hospital setting for a patient reporting dyspnea is the administration of oxygen therapy. However, recent evidence does not show superiority of oxygen over air inhalation via nasal prongs for dyspnea in patients with advanced cancer or heart failure.12,13
Pharmacological Management
Opioids are first-line therapy for alleviating dyspnea in patients at EOL. The administration of opioids has been shown in systematic reviews to provide effective management of dyspnea.14,15 Practice guidelines by leading expert groups advocate for the use of opioids in the management of dyspnea for patients with advanced malignant and noncancer diseases.10,16 Fear of causing unintended respiratory sedation with opioids limits the prescription of opioids for dyspnea. However, studies have not found a change in mortality with the use of opioids appropriately titrated to control dyspnea.17
Studies examining the role of benzodiazepines in dyspnea management are conflicting. Anecdotal clinical evidence in actively dying patients supports treating dyspnea with benzodiazepines in conjunction with opioid therapy. Benzodiazepines are most beneficial when there is an anxiety-related component to the dyspnea.
Many patients with advanced disease and evidence of airflow obstruction will benefit from nebulized bronchodilator therapy for dyspnea. Patients with dyspnea from fluid overload (i.e. end-stage congestive heart failure or renal disease) might benefit from systemic diuretics. An increasing number of trials are under way to evaluate the efficacy of nebulized furosemide in the symptomatic management of dyspnea.
Back to the Case
The patient’s clinical course decompensates, and he begins to report worsening dyspnea in addition to his underlying pain. He becomes increasingly anxious about what this new symptom means. In addition to having a discussion about disease progression and prognosis, you increase his PCA basal dose to morphine 4 mg/hour to help him with this new symptom. You also add low-dose lorazepam 0.5 mg IV q8 hours as an adjunct agent for his dyspnea. The patient reports improvement of his symptom burden.
Review of the Data: Secretions
Physiological changes occur as a patient enters the active phase of dying. Two such changes are the loss of the ability to swallow and a reduced cough reflex. These changes culminate in an inability to clear secretions, which pool in the oropharynx and the airways. As the patient breathes, air moves over the pooled secretions and produces a gurgling sound that is referred to as the “death rattle.” The onset of this clinical marker has been shown to have significant prognostic significance for predicting imminent death within a period of hours to days. Proposed treatments for the symptom are listed below.
Nonpharmacological Management
Nonpharmacological options include repositioning the patient in a manner that facilitates postural draining.18 Careful and gentle oral suctioning might help reduce secretions if they are salivary in origin. This will not help to clear deeper bronchial secretions. Suctioning of deeper secretions often causes more burden than benefit, as this can cause repeated trauma and possible bleeding.
Family and caregivers at the bedside can find the “death rattle” quite disturbing and often fear that their loved one is “drowning.” Education and counseling that this is not the case, and that the development of secretions is a natural part of the dying process, can help alleviate this concern. Explaining that pharmacological agents can be titrated to decrease secretions is also reassuring to caregivers.
Pharmacological Management
Pharmacological options for secretion management include utilizing anticholinergic medications to prevent the formation of further secretions. These medications are standard of care for managing the death rattle and have been found to be most efficacious if started earlier in the actively dying phase.19,20 Anticholinergic medications include glycopyrrolate (0.2 mg IV q8 hours), atropine sulfate ophthalmological drops (1% solution, 1-2 drops SL q6 hours), hyoscyamine (0.125 mg one to four times a day), and scopolamine (1.5 mg patch q72 hours). These medications all have possible side effects typical of anticholinergic agents, including delirium, constipation, blurred vision, and urinary retention.
Back to the Case
The patient becomes increasingly lethargic. You meet with his family and explain that he is actively dying. His family reiterates that the goals of medical care should focus on maximizing symptom management. His family is concerned about the “gurgly” sound they hear and want to know if that means he is suffering. You educate the family about expected changes that occur with the dying process and inform them that glycopyrrolate 0.2 mg IV q8 hour will be started to minimize further secretions.
Bottom Line
Pain, nausea, dyspnea, and secretions are common end-of-life symptoms that hospitalists should be competent in treating.
Dr. Litrivis is an associate director and assistant professor at the Mount Sinai School of Medicine in New York, and Dr. Neale is an assistant professor at the University of New Mexico School of Medicine in Albuquerque.
References
- The SUPPORT Principal Investigators. A controlled trial to improve the care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
- World Health Organization Definition of Palliative Care. World Health Organization website. Available at: http://www.who.int/cancer/palliative/definition/en/. Accessed April 12, 2012.
- NCCN Guidelines Version 2. 2011 Adult Cancer Pain. National Comprehensive Cancer Network website. Available at: http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed April 12, 2012.
- Whitecar PS, Jonas AP, Clasen ME. Managing pain in the dying patient. Am Fam Physician. 2000;61(3):755-764.
- Bial A, Levine S. Assessment and treatment of physical pain associated with life-limiting illness. Hospice and Palliative Care Training for Physicians: UNIPAC. Vol 3. 3rd ed. Glenview, IL: American Academy of Hospice and Palliative Medicine; 2008.
- Sydney M, et al. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
- Mannix KA. Gastrointestinal symptoms. In: Doyle D, Hanks G, Cherny N, Calman K, eds. Oxford Textbook of Palliative Medicine. 3rd ed. New York, NY: Oxford University Press; 2005.
- Tyler LS. Nausea and vomiting in palliative care. In: Lipman AG, Jackson KC, Tyler LS, eds. Evidence-Based Symptom Control in Palliative Care. New York, NY: The Hawthorn Press; 2000.
- Policzer JS, Sobel J. Management of Selected Nonpain Symptoms of Life-Limiting Illness. Hospice and Palliative Care Training for Physicians: UNIPAC. Vol 4. 3rd ed. Glenview, IL: American Academy of Hospice and Palliative Medicine; 2008.
- Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4): 435-452.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized controlled, crossover trial. J Pain Symptom Manage. 2010;39(5): 831-838.
- Philip J, Gold M, Milner A, Di Iulio J, Miller B, Spruyt O. A randomized, double-blind, crossover trial of the effect of oxygen on dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2006;32(6):541-550.
- Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnea in adults. Cochrane Database Syst Rev. 2008;(3):CD004769.
- Jennings AL, Davies AN, Higgins JP, Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev. 2001;(4):CD002066.
- Ben-Aharon I, Gafter-Gvili A, Paul M, Leibovici, L, Stemmer, SM. Interventions for alleviating cancer-related dyspnea. A systematic review. J Clin Oncol. 2008;26(14): 2396-2404.
- Qaseem A, Snow V, Shekelle P, et al. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148(2):141-146
- Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nat Clin Pract Oncol. 2008;5(2):90–100.
- Bickel K, Arnold R. EPERC Fast Facts Documents #109 Death Rattle and Oral Secretions, 2nd ed. Available at: http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_109.htm. Accessed April 15, 2012.
- Wildiers H, Dhaenekint C, Demeulenaere P, et al. Atropine, hyoscine butylbromide, or scopalamine are equally effective for the treatment of death rattle in terminal care. J Pain Symptom Manage. 2009;38(1):124-133.
- Hugel H, Ellershaw J, Gambles M. Respiratory tract secretions in the dying patient: a comparison between glycopyrronium and hyoscine hydrobromide. J Palliat Med. 2006;9(2):279-285.
Case
A 58-year-old male with colon cancer metastatic to the liver and lungs presents with vomiting, dyspnea, and abdominal pain. His disease has progressed through third-line chemotherapy and his care is now focused entirely on symptom management. He has not had a bowel movement in five days and he began vomiting two days ago.
Overview
The majority of patients in the United States die in acute-care hospitals. The Study to Understand Prognosis and Preferences for Outcomes and Risks of Treatments (SUPPORT), which evaluated the courses of close to 10,000 hospitalized patients with serious and life-limiting illnesses, illustrated that patients’ end-of-life (EOL) experiences often are characterized by poor symptom management and invasive care that is not congruent with the patients’ overall goals of care.1 Studies of factors identified as priorities in EOL care have consistently shown that excellent pain and symptom management are highly valued by patients and families. As the hospitalist movement continues to grow, hospitalists will play a large role in caring for patients at EOL and will need to know how to provide adequate pain and symptom management so that high-quality care can be achieved.
Pain: A Basic Tenet
A basic tenet of palliative medicine is to evaluate and treat all types of suffering.2 Physical pain at EOL is frequently accompanied by other types of pain, such as psychological, social, religious, or existential pain. However, this review will focus on the pharmacologic management of physical pain.
Pain management must begin with a thorough evaluation of the severity, location, and characteristics of the discomfort to assess which therapies are most likely to be beneficial (see Table 1).3 The consistent use of one scale of pain severity (such as 0-10, or mild/moderate/severe) assists in the choice of initial dose of pain medication, in determining the response to the medication, and in assessing the need for change in dose.4
Opioids are the foundation of pain management in advanced diseases because they are available in a number of formulations and, when dosed appropriately, they are effective and safe. Starting doses and equianalgesic doses of common opioids are presented in Table 2. Guidelines recommend the use of short-acting opioids for dose titration to gain control of poorly controlled pain.3 If a patient is experiencing mild pain on a specific regimen, the medication dose can be increased up to 25%; by 25% to 50%, if pain is moderate; and 50% to 100%, if severe.5 When the pain is better-controlled, the total amount of pain medication used in 24 hours (24-hour dose) can be converted to a long-acting formulation for more consistent pain management. Because there is a constant component to most advanced pain syndromes, it is recommended that pain medication is given on a standing basis, with as-needed (prn) doses available for exacerbations of pain.3 Prn doses of short-acting medication (equivalent to approximately 10% of the 24-hour dose of medication) should be available at one- or two-hour intervals prn (longer if hepatic or renal impairment is present) for IV or PO medications, respectively.
Opioids often are categorized as low potency (i.e. codeine, hydrocodone) and high-potency (i.e. oxycodone, morphine, hydromorphone, fentanyl). When given in “equianalgesic doses,” the analgesic effect and common side effects (nausea/vomiting, constipation, sedation, confusion, pruritis) of different opioids can vary in different patients. Due to differences in levels of expressed subtypes of opioid receptors, a given patient might be more sensitive to the analgesic effect or side effects of a specific medication. Therefore, if dose escalation of one opioid is inadequate to control pain and further increases in dose are limited by intolerable side effects, rotation to another opioid is recommended.4 Tables documenting equianalgesic doses of different opioids are based on only moderate evidence from equivalency trials performed in healthy volunteers.6 Due to interpatient differences in responses, it is recommended that the equianalgesic dose of the new medication be decreased by 25% to 50% for initial dosing.5
Certain treatments are indicated for specific pain syndromes. Bony metastases respond to NSAIDs, bisphosphonates, and radiation therapy in addition to opioid medications. As focal back pain is the first symptom of spinal cord compression, clinicians should have a high index of suspicion for compression in any patient with malignancy and new back pain. Steroids and radiation therapy are considered emergent treatments for pain control and to prevent paralysis in this circumstance. Pain due to bowel obstruction is usually colicky in nature and responds well to octreotide as discussed in the section on nausea and vomiting. Steroids (such as dexamethasone 4 mg PO bid-tid) might be an effective adjuvant medication in bone pain, tumor pain, or inflammation.
*Half this dose should be used in renal or liver dysfunction and in the elderly.
Preferred in renal dysfunction.
SOURCES: Adapted from Assessment and treatment of physical pain associated with life-limiting illness. Hospice and Palliative Care Training for Physicians: UNIPAC. Vol 3. 3rd ed. Glenview, IL: American Academy of Hospice and Palliative Medicine; 2008, and Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
Back to the Case
At home, the patient was taking 60 mg of extended-release morphine twice daily and six doses per day of 15-mg immediate-release morphine for breakthrough pain. This is the equivalent of 210 mg of oral morphine in 24 hours. His pain is severe on this regimen, but it is unclear how much of this medication he is absorbing due to his vomiting. Using the IV route of administration and a patient-controlled analgesia (PCA) system will enable rapid dose titration and pain control. The equivalent of the 24-hour dose of 210 mg oral morphine is 70 mg IV morphine, which is equivalent to a drip basal rate of approximately 3 mg IV morphine per hour. This basal rate with a bolus dose of 7 mg (10% of the 24-hour dose) IV morphine q1 hour prn is reasonable as a starting point.
Review of the Data: Nausea and Vomiting
Nausea and vomiting affect 40% to 70% of patients in a palliative setting.7 A thorough history and physical exam can enable one to determine the most likely causes, pathways, and receptors involved in the process of nausea and vomiting. It is important to review the timing, frequency, and triggers of vomiting. The oral, abdominal, neurologic, and rectal exams, in addition to a complete chemistry panel, offer helpful information. The most common etiologies and recommended medications are included in Table 3. It is worthwhile to note that serotonin-antagonists (i.e. ondansetron) are first-line therapies only for chemotherapy and radiation-therapy-induced emesis. If a 24-hour trial of one antiemetic therapy is ineffective, one should reassess the etiology and escalate the antiemetic dose, or add a second therapy with a different (pertinent) mechanism of action. Although most studies of antiemetic therapy are case series, there is good evidence for this mechanistic approach.8
*EPS: extrapyramidal symptoms
The various insults and pathways that can cause vomiting are quite complex. The medullary vomiting center (VC) receives vestibular, peripheral (via splanchnic and vagal nerves), and higher cortical inputs and is the final common pathway in the vomiting reflex. The chemoreceptor trigger zone (CTZ) near the fourth ventricle receives input from the vagal and splanchnic nerves, and generates output to the VC.
General dietary recommendations are to avoid sweet, fatty, and highly salted or spiced foods. Small portions of bland foods without strong odors are best tolerated.7 Constipation commonly contributes to nausea and vomiting and should be managed with disimpaction, enemas, and laxatives as tolerated. Imaging may be required to make the important distinction between partial and complete bowel obstruction, as the treatments differ. Surgical procedures, such as colostomy or placement of a venting gastrostomy tube, can relieve pain and vomiting associated with complete bowel obstruction.
Back to the Case
The patient is found to have a fecal impaction on rectal exam, but vomiting persists after disimpaction and enema use. Imaging documents a complete bowel obstruction at the site of a palpable mass in the right upper quadrant and multiple large hepatic metastases. Octreotide is initiated to decrease intestinal secretions and peristalsis. Steroids are given to decrease tumor burden and associated inflammation in the intestine and liver, as well as to relieve distension of the hepatic capsule. Haloperidol is used in low doses to control episodes of nausea.
Review of the Data: Dyspnea
Dyspnea is a common symptom faced by patients at EOL. An estimated 50% of patients who are evaluated in acute-care hospitals seek treatment for the management of this often-crippling symptom.10 Unfortunately, as disease burden progresses, the incidence of dyspnea increases towards EOL, and the presence and severity of dyspnea is strongly correlated with mortality.
It is imperative for providers to appreciate that dyspnea is a subjective symptom, similar to pain. The presence and severity of dyspnea, therefore, depends on patient report. Given its subjective nature, the degree of dyspnea experienced by a patient might not correlate with objective laboratory findings or test results. In practice, the severity of dyspnea is commonly assessed with a numeric rating scale (0-10), verbal analogue scale, or with verbal descriptors (mild, moderate, severe). It is important to determine the underlying etiology of the dyspnea and, if possible, to target interventions to relieve the underlying cause. However, at the end of life, the burdens of invasive studies to determine the exact cause of dyspnea might outweigh the benefits, and invasive testing might not correlate with patients’ and families’ goals of care. In that instance, the goal of treatment should be aggressive symptom management and providers should use clinical judgment to tailor therapies based on the patient’s underlying illness, physical examination, and perhaps on noninvasive radiological or laboratory findings. Below are nonpharmacological and pharmacological interventions that can be employed to help alleviate dyspnea in the actively dying patient.
Nonpharmacological Management
A handheld fan aimed near the patient’s face has been shown to reduce the sensation of dyspnea.11 This relatively safe and inexpensive intervention has no major side effects and can provide improvement in this distressing symptom.
Often, the first line of therapy in the hospital setting for a patient reporting dyspnea is the administration of oxygen therapy. However, recent evidence does not show superiority of oxygen over air inhalation via nasal prongs for dyspnea in patients with advanced cancer or heart failure.12,13
Pharmacological Management
Opioids are first-line therapy for alleviating dyspnea in patients at EOL. The administration of opioids has been shown in systematic reviews to provide effective management of dyspnea.14,15 Practice guidelines by leading expert groups advocate for the use of opioids in the management of dyspnea for patients with advanced malignant and noncancer diseases.10,16 Fear of causing unintended respiratory sedation with opioids limits the prescription of opioids for dyspnea. However, studies have not found a change in mortality with the use of opioids appropriately titrated to control dyspnea.17
Studies examining the role of benzodiazepines in dyspnea management are conflicting. Anecdotal clinical evidence in actively dying patients supports treating dyspnea with benzodiazepines in conjunction with opioid therapy. Benzodiazepines are most beneficial when there is an anxiety-related component to the dyspnea.
Many patients with advanced disease and evidence of airflow obstruction will benefit from nebulized bronchodilator therapy for dyspnea. Patients with dyspnea from fluid overload (i.e. end-stage congestive heart failure or renal disease) might benefit from systemic diuretics. An increasing number of trials are under way to evaluate the efficacy of nebulized furosemide in the symptomatic management of dyspnea.
Back to the Case
The patient’s clinical course decompensates, and he begins to report worsening dyspnea in addition to his underlying pain. He becomes increasingly anxious about what this new symptom means. In addition to having a discussion about disease progression and prognosis, you increase his PCA basal dose to morphine 4 mg/hour to help him with this new symptom. You also add low-dose lorazepam 0.5 mg IV q8 hours as an adjunct agent for his dyspnea. The patient reports improvement of his symptom burden.
Review of the Data: Secretions
Physiological changes occur as a patient enters the active phase of dying. Two such changes are the loss of the ability to swallow and a reduced cough reflex. These changes culminate in an inability to clear secretions, which pool in the oropharynx and the airways. As the patient breathes, air moves over the pooled secretions and produces a gurgling sound that is referred to as the “death rattle.” The onset of this clinical marker has been shown to have significant prognostic significance for predicting imminent death within a period of hours to days. Proposed treatments for the symptom are listed below.
Nonpharmacological Management
Nonpharmacological options include repositioning the patient in a manner that facilitates postural draining.18 Careful and gentle oral suctioning might help reduce secretions if they are salivary in origin. This will not help to clear deeper bronchial secretions. Suctioning of deeper secretions often causes more burden than benefit, as this can cause repeated trauma and possible bleeding.
Family and caregivers at the bedside can find the “death rattle” quite disturbing and often fear that their loved one is “drowning.” Education and counseling that this is not the case, and that the development of secretions is a natural part of the dying process, can help alleviate this concern. Explaining that pharmacological agents can be titrated to decrease secretions is also reassuring to caregivers.
Pharmacological Management
Pharmacological options for secretion management include utilizing anticholinergic medications to prevent the formation of further secretions. These medications are standard of care for managing the death rattle and have been found to be most efficacious if started earlier in the actively dying phase.19,20 Anticholinergic medications include glycopyrrolate (0.2 mg IV q8 hours), atropine sulfate ophthalmological drops (1% solution, 1-2 drops SL q6 hours), hyoscyamine (0.125 mg one to four times a day), and scopolamine (1.5 mg patch q72 hours). These medications all have possible side effects typical of anticholinergic agents, including delirium, constipation, blurred vision, and urinary retention.
Back to the Case
The patient becomes increasingly lethargic. You meet with his family and explain that he is actively dying. His family reiterates that the goals of medical care should focus on maximizing symptom management. His family is concerned about the “gurgly” sound they hear and want to know if that means he is suffering. You educate the family about expected changes that occur with the dying process and inform them that glycopyrrolate 0.2 mg IV q8 hour will be started to minimize further secretions.
Bottom Line
Pain, nausea, dyspnea, and secretions are common end-of-life symptoms that hospitalists should be competent in treating.
Dr. Litrivis is an associate director and assistant professor at the Mount Sinai School of Medicine in New York, and Dr. Neale is an assistant professor at the University of New Mexico School of Medicine in Albuquerque.
References
- The SUPPORT Principal Investigators. A controlled trial to improve the care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). JAMA. 1995;274(20):1591-1598.
- World Health Organization Definition of Palliative Care. World Health Organization website. Available at: http://www.who.int/cancer/palliative/definition/en/. Accessed April 12, 2012.
- NCCN Guidelines Version 2. 2011 Adult Cancer Pain. National Comprehensive Cancer Network website. Available at: http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf. Accessed April 12, 2012.
- Whitecar PS, Jonas AP, Clasen ME. Managing pain in the dying patient. Am Fam Physician. 2000;61(3):755-764.
- Bial A, Levine S. Assessment and treatment of physical pain associated with life-limiting illness. Hospice and Palliative Care Training for Physicians: UNIPAC. Vol 3. 3rd ed. Glenview, IL: American Academy of Hospice and Palliative Medicine; 2008.
- Sydney M, et al. Evidence-based standards for cancer pain management. J Clin Oncol. 2008;26(23):3879-3885.
- Mannix KA. Gastrointestinal symptoms. In: Doyle D, Hanks G, Cherny N, Calman K, eds. Oxford Textbook of Palliative Medicine. 3rd ed. New York, NY: Oxford University Press; 2005.
- Tyler LS. Nausea and vomiting in palliative care. In: Lipman AG, Jackson KC, Tyler LS, eds. Evidence-Based Symptom Control in Palliative Care. New York, NY: The Hawthorn Press; 2000.
- Policzer JS, Sobel J. Management of Selected Nonpain Symptoms of Life-Limiting Illness. Hospice and Palliative Care Training for Physicians: UNIPAC. Vol 4. 3rd ed. Glenview, IL: American Academy of Hospice and Palliative Medicine; 2008.
- Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4): 435-452.
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S. Does the use of a handheld fan improve chronic dyspnea? A randomized controlled, crossover trial. J Pain Symptom Manage. 2010;39(5): 831-838.
- Philip J, Gold M, Milner A, Di Iulio J, Miller B, Spruyt O. A randomized, double-blind, crossover trial of the effect of oxygen on dyspnea in patients with advanced cancer. J Pain Symptom Manage. 2006;32(6):541-550.
- Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnea in adults. Cochrane Database Syst Rev. 2008;(3):CD004769.
- Jennings AL, Davies AN, Higgins JP, Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev. 2001;(4):CD002066.
- Ben-Aharon I, Gafter-Gvili A, Paul M, Leibovici, L, Stemmer, SM. Interventions for alleviating cancer-related dyspnea. A systematic review. J Clin Oncol. 2008;26(14): 2396-2404.
- Qaseem A, Snow V, Shekelle P, et al. Evidence-based interventions to improve the palliative care of pain, dyspnea, and depression at the end of life: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148(2):141-146
- Booth S, Moosavi SH, Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: a systematic review of pharmacological therapy. Nat Clin Pract Oncol. 2008;5(2):90–100.
- Bickel K, Arnold R. EPERC Fast Facts Documents #109 Death Rattle and Oral Secretions, 2nd ed. Available at: http://www.eperc.mcw.edu/EPERC/FastFactsIndex/ff_109.htm. Accessed April 15, 2012.
- Wildiers H, Dhaenekint C, Demeulenaere P, et al. Atropine, hyoscine butylbromide, or scopalamine are equally effective for the treatment of death rattle in terminal care. J Pain Symptom Manage. 2009;38(1):124-133.
- Hugel H, Ellershaw J, Gambles M. Respiratory tract secretions in the dying patient: a comparison between glycopyrronium and hyoscine hydrobromide. J Palliat Med. 2006;9(2):279-285.