User login
Sentinel Hospitalization
As hospitalists now care for expanding numbers of America's aging patients, many of whom have chronic, debilitating illnesses or are near the end of life, there is a burgeoning need for innovative approaches to optimize quality of care and control costs, especially in the last year of life.[1, 2] In the inaugural issue of the Journal of Hospital Medicine, an overview of how hospitalists and palliative care specialists can work hand‐in‐hand to care for these seriously ill, hospitalized patients was presented.[3, 4] This perspective highlighted a symbiotic and mutually beneficial relationship between the 2 specialties based on their shared values, missions, and complementary strengths.[3, 4] Since then, a number of collaborative ventures offering palliative care for seriously ill, hospitalized patients have been developed and examined in a variety of settings.[4, 5, 6]
A key collaborative undertaking for hospitalists and palliative care specialists is the appreciation of the unique trajectory of each chronic illness toward the end of life. For example, patients with cancer or neurodegenerative disease tend to have relatively stable functional status until the final months of rapid deterioration. On the other hand, the courses of patients with chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), end‐stage renal disease, and human immunodeficiency virus/acquired immunodeficiency syndrome tend to be punctuated by episodes of acute exacerbation with often nearly complete return to previous status. Finally, dementia usually follows a slow course of gradual decline leading to death.[7] Ideally, active management of symptoms and discussion of prognosis and goals of care should happen in the early stages of these chronic illnesses, yet most often they are left until an acute hospitalization late in the disease course. The following case illustrates the point.
CASE 1
Mrs. M is an 89‐year‐old woman with Alzheimer's dementia diagnosed 7 years ago who has been cared for at home by family members. She is admitted to the hospital for urinary tract infection and volume depletion. She is bedbound, cachectic, and has a stage III decubitus ulcer. Her daughter describes a 6‐month history of feeding problems, 20‐lb weight loss, and 2 recent hospitalizations for aspiration pneumonia. She improves somewhat with hydration and intravenous antibiotics, and the physical therapist recommends rehabilitation. Mrs. M does not have decision‐making capacity, and her long‐time family physician has not inquired about care preferences or goals. The hospitalist team meets with family members to discuss the trajectory and prognosis of advanced dementia, and recommends against artificial nutrition and hydration, and for initiation of palliative care service at a skilled nursing facility.
In this example, the hospitalist team recognizes the advent of frequent infections and diminished oral intake in advanced dementia as signals of increased morbidity and mortality warranting palliative care intervention.[8] This, we suggest, represents a sentinel hospitalization, a hospitalization in the disease course that heralds a need to reassess prognosis, treatment options and intensity, and goals of care. Hospitalists are well positioned to recognize such transition points in the disease course by considering the patient's recent history of illness, to offer an impartial overview of illness progression, and to optimize patient care using principles of palliative care. Additionally, hospitalists have advantages of geographic convenience, readily available consultants, systemic support, and a detachment from the longitudinal patient‐physician relationship, which may enable more accurate medical prognostication.[9]
There are many ways to identify a sentinel hospitalization. For example, hospitalists can use the surprise question, Would you be surprised if the patient died within 12 months? on admission for the majority of cancer and dialysis patients. The answer No predicts a 3.5‐ to 7‐fold increase in 1‐year mortality.[10, 11] In a powerful predictive model for 1‐year mortality using readily available clinical, laboratory, and functional characteristics, medical inpatients in the highest quartiles have 1‐year mortality exceeding 60%.[12] Recently, several more complicated prognostic models have been derived and validated in large cohorts of medical inpatients, which predict short‐term (30‐day) and long‐term (0.5‐1 year) mortality with great accuracy.[13, 14] There are also many disease‐specific prognostic features (eg, diagnosis of metastatic disease with poor performance status or high symptom burden, progression of chronic kidney disease with consideration of hemodialysis, additional stroke in multi‐infarct dementia, and frequent exacerbation of severe COPD or severe CHF).[15, 16, 17, 18, 19, 20, 21] Finally, frequent readmissions and prolonged hospital or intensive care unit stay can also be used.[17, 19] These criteria are summarized in Table 1 with time frames.
| Common Criteria | Time Frame | References |
|---|---|---|
| ||
| No to the surprise question: Would you be surprised if the patient died in 12 months? | 1 year | [10, 11] |
| Newly diagnosed metastatic solid cancer | Various | [17] |
| Metastatic solid cancer admitted for uncontrolled symptoms | Various | [17] |
| Progressive CKD with consideration for hemodialysis | 1 year | [17, 18] |
| GOLD stage IV COPD with frequent exacerbation | Various | [20] |
| NYHAstage IV CHF with frequent exacerbation | 12 years | [21] |
| Advanced dementia with frequent UTI, aspiration PNA, and feeding problem | 12 years | [8] |
| Overall prognosis of high mortality using available indices | 30 days1 year | [12, 13, 14] |
| More than 3 admissions in last 6 months | 6 months | [17, 19] |
| Prolonged ICU stay (>7 days) | Weeks | [17, 19] |
Once a sentinel hospitalization is identified, hospitalists, with input from the patient's primary care physician and subspecialists, can then develop a comprehensive strategy to evaluate current disease management, to educate patient and family accordingly, and to actively integrate palliative care services as appropriate. The next challenge facing the care team is how to deliver the necessary palliative care since it is unnecessary and improper to ask for palliative care specialist consultation for every sentinel hospitalization. We believe that the best approach is for hospitalists to be the primary deliverers of basic palliative care in the hospital while consulting palliative care specialists for refractory symptoms and complex scenarios.[22] According to this generalist‐specialist palliative care model, physicians of all specialties should define and master a basic palliative care skill set for their patients. For hospitalists, the relevant skill set includes assessing and treating pain and other symptoms such as dyspnea, nausea and vomiting, and constipation, estimating prognosis, and initiating goals of care discussions.[22] The following case illustrates this point.
CASE 2
Ms S, a 21‐year‐old Hispanic woman with advanced, recurrent head and neck cancer, status post multiple surgeries, chemotherapy, and radiation therapy, is admitted to the hospitalist service for aspiration pneumonia, which responds to antibiotics rapidly. However, her cancer‐related somatic and neuropathic pain soon becomes refractory to opioids prescribed by the hospitalist team. She also develops significant dyspnea, xerostomia, depression, anxiety, and existential suffering. With the help of the interdisciplinary palliative care team, her pain is relieved by a patient‐controlled analgesia pump and methadone. A palliative care social worker and chaplain visit her and her family daily to address their distress. Eventually, the care team is able to provide a stable medical regimen for symptom control and to use it across the entire care continuum.
In this example, the hospitalist team, with the support of palliative care specialists, provided basic palliative care and longitudinal integration of palliative practices into the patient's overall treatment scheme. Hospitalists, given their scope of practice and sheer volume of patients, are well positioned to rapidly gain competencies in symptom management, empathic communication, and interdisciplinary teamwork.[23, 24] Hospitalists may benefit from innovative and collaborative palliative care education using interactive online modules, case simulation, communication workshops, and observed evaluation and feedback.[25] Several modes of collaboration between hospital medicine and palliative care have been developed including implementation of palliative care consult triggers on admission, palliative care participation in hospitalist interdisciplinary rounds, and disease specific, integrated management programs.[17, 26] These collaborations are particularly important, as the quality of inpatient care at the end of life is still suboptimal and more appropriate use of palliative care will be beneficial.[27] Recently, some hospitals have developed specialized inpatient palliative care units, combining intensive palliation with inpatient medical surgical level of care, as well as providing hospice care. Staffed by palliative care specialists or hospitalists, they provide efficient, cost‐saving care to patients with advanced chronic illness or terminal disease in need of intensive symptom management.[28] Finally, there is mounting evidence supporting the clinical effectiveness of palliative care in diverse specialties such as oncology, pulmonary and critical care, and nephrology.[29] For example, in the setting of metastatic non‐small cell lung cancer, early initiation of palliative care has been shown to improve symptom control and quality of life, reduce chemotherapy use at the end of life, and interestingly, prolong median survival by almost 3 months.[30] This has led to a position statement from American Society of Clinical Oncology encouraging early integration of palliative care into standard oncologic care for advanced disease.[31]
Recognizing a sentinel hospitalization allows palliative care to be integrated at transitions of care and carried forward. For patients with chronic debilitating illnesses who are approaching the end of life, appropriate care transitions will ensure that their short‐ and long‐term care matches their goals of care, assure timely clinical follow‐ups, and help reduce hospital readmission and healthcare resource utilization.[32] Importantly, timely and compassionate communication is a key to the success of both hospital medicine and palliative care. Many patients with life‐limiting diseases prefer to receive prognostic information and to discuss goals of care.[33] How this information is integrated and communicated through the care continuum is crucial, especially in the era of duty hour limits and frequent handoffs. The information exchange needs to facilitate active participation of primary care physicians who may not be involved in hospital care. Some of the innovative strategies for communication and transfer of palliative care information, such as prognosis, goals of care, family meeting consensus, and symptom control interventions, include a palliative care checklist in the electronic health record, incorporation of prognostic and family meeting information in the discharge summary, and links to the national Physician Orders for Life‐Sustaining Treatment advanced care planning program.[34] Of note, a pilot program in the United Kingdom adopting an electronic palliative care summary has reduced after‐hour emergency room visits and hospital readmissions.[35] The following case illustrates this point.
CASE 3
Mrs. K, an 82‐year‐old Russian‐speaking woman with newly diagnosed metastatic pancreatic cancer, is admitted for worsening obstructive jaundice and a second opinion about treatment. A biliary stent is placed and her jaundice slowly improves. The patient and family have requested chemotherapy. However, the oncologist determines that she would only qualify for a phase I trial given her poor performance status. The hospitalist team requests the help of the palliative care consult team to manage her severe pain, depression, and to provide support to the family. After several family meetings, the patient and family choose not to pursue chemotherapy. Given the lack of adequate support at home, she is discharged to a skilled nursing facility for short‐term rehabilitation with plans to transition to the in‐house hospice program. The hospitalist, palliative care attending physician, and the medical director of the rehabilitation facility have a 3‐way phone conference to confirm the plans of care and to ensure a smooth care transition.
In this case, the hospitalist team recognizes that this is a sentinel hospitalization for Mrs. K that requires extensive palliative care intervention. Often, transitioning to skilled nursing facilities (SNF) is the default pathway for patients needing hospice/palliative care, especially when patients and families are not yet ready to discuss prognosis realistically or to accept hospice, or there is not enough support available at home. A recent large cohort study showed that 30% of patients in their last 6‐month of life had used, and nearly 10% of such patients had died, under Medicare's posthospitalization SNF benefit.[36] Although the worsening disease trajectory may not be apparent at hospital discharge, it is more likely that the financial and practical limitation of the Medicare Hospice Benefit accounts for this observation, which includes limited home health aid hours, lack of coverage for room and board, and lower payments to SNFs.[36] Hospitalists can help address the issue of discharge location for patients needing palliative care. Sometimes this requires extensive communication before and after discharge to help enhance the transition from a rehabilitation facility to hospice/palliative care. Appropriately integrated palliative care at the time of care transitions, in the form of hospice or longitudinal home‐based palliative care rather than just routine clinic follow‐up, has the potential to reduce 30‐day readmission for chronically ill, elderly patients and for patients near the end of life.[37, 38] It is critical that national policy, suitable reimbursement, and financial incentives support this practice. A demonstration project, Better Outcomes by Optimizing Safe Transitions (BOOST), organized by the Society of Hospital Medicine, integrates palliative care evaluation into a comprehensive discharge assessment tool. This intervention has been shown to reduce readmissions to acute care hospitals.[39]
In this article, we define a sentinel hospitalization and suggest that its recognition provides an important opportunity for hospitalists to actively integrate palliative care into patients' chronic disease management programs, with inputs from patients, their families, their primary physicians and subspecialists, as well as palliative care specialists. We also recognize that within nonsentinel hospitalizations, there are important opportunities to discuss prognosis, goals of care, and advanced care planning. This approach allows the fresh eyes of hospitalists to assess the patient's current health status and prognosis, to communicate these relevant clinical issues with the patient and family, and to encourage discussions about goals of care and advanced care planning during the sentinel hospitalization. It also provides a structured vehicle for soliciting the patient's (and family's) perspectives and documenting them in the medical record. A compilation of sample items to guide discussion can be found in Table 2. Hospitalists, equipped with basic palliative care skills and supported by hospital‐ and community‐based palliative care teams, can thrive in this unique position of optimizing the quality of care for these patients.[40] Almost 20 years ago, the field of palliative care rose to national prominence on the findings of the SUPPORT (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) study, which investigated the suboptimal end‐of‐life experiences of hospitalized adult patients.[41] Since then, the fields of both hospital medicine and palliative care have grown, yet the best is still to come for their collaborative excellence, mutual education, and shared care innovation at the forefront of medicine.
| Patient/family understanding of disease process and treatment outcomes |
| Patient/family understanding of disease prognosis |
| Availability of alternative treatment options including palliative/hospice care |
| Patient/family wishes/goals of care |
| Advanced‐care planning including limitations of care |
| Inventory of symptoms (frequency, severity, modifying factors, timing, and treatments) |
| Social and financial stress |
| Emotional and existential stress |
| Social support system and caregivers |
| Living arrangements |
Disclosure: Nothing to report.
- , , , , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7:649–654.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Affairs. 2001;20:188–195.
- . Palliative care in hospitals. J Hosp Med. 2006;1:21–28.
- . Palliative care and hospitalists: a partnership for hope. J Hosp Med. 2006;1:5–6.
- , . Palliative care and the hospitalist: an opportunity for cross‐fertilization. Am J Med. 2001;111:10s–14s.
- , . Palliative care. Ann Intern Med. 2012;156:ITC2‐1, TC2‐2–15; quiz TC2‐16.
- , , , , . Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392.
- , , , et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538.
- , . Extent and determinants of error in doctor's prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–472.
- , , , et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13:837–840.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379–1384.
- , , , et al. Development and validation of a prognostic index for 1‐year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013,8:229–235.
- , , , , . Caring about prognosis: a validation study of the CARING criteria to identify hospitalized patients at high risk for death at 1 year. J Hosp Med. 2013,8:696–701.
- , , , , , , et al. Meta‐analysis of survival prediction with palliative performance scale. J Palliat Care. 2007;23:245–254.
- , , , , . Prognostic indices for older adults: a systemic review. JAMA. 2012;307:182–192.
- , . Identifying patients in need of a palliative care assessment in the hospital setting. J Palliat Med. 2011;14:17–23.
- , , , , , . Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547.
- , , . Top 10 things palliative care clinicians wished everyone knew about palliative care. Mayo Clin Proc. 2013;88:859–865.
- . Palliative and end‐of‐life care for patients with severe COPD. Eur Respir J. 2008;32:796–803.
- . Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396.
- , . Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368:1173–1175.
- , , , , , . Common myths about caring for patients with terminal illness: opportunities to improve care in the hospital setting. J Hosp Med. 2007;2:357–365.
- , , , . Opportunity lost: end‐of‐life discussions in cancer patients who die in the hospital. J Hosp Med. 2013;8:334–340.
- , , . Palliative medicine physician education in the United States: a historical review. J Palliat Med. 2013;16:230–236.
- , . Hospitalization as an opportunity to integrate palliative care in heart failure management. Curr Opin Support Palliat Care. 2009;3:247–251.
- , , , et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170:1057–1063.
- , , , , , . Outcomes of the acute palliative care unit in an academic medical center [published online ahead of print May 10, 2013]. Am J Hosp Palliat Care. doi: 10.1177/1049909113489164.
- , , , , , . Update in hospital palliative care. J Hosp Med. 2013;12:715–720.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , . Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin. 2013;63:349–363.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187.
- , , , , , . “Knowing is better”: preferences of diverse older adults for discussion prognosis. J Gen Intern Med. 2012;27:568–575.
- , , . POLST, an improvement over traditional advanced directives. Cleve Clin J Med. 2012;79:457–464.
- , , , . Use of a structured palliative care summary in patients with established cancer is associated with reduced hospital admissions by out‐of‐hours general practitioners in Grampian [published online ahead of print January 3, 2013]. BMJ Support Palliat Care. doi:10.1136/bmjspcare‐2012‐000371.
- , , , , , . Use of the Medicare posthospitalization skilled nursing benefit in the last 6 months of life. Arch Intern Med. 2012;172:1573–1579.
- , , , et al. Increased satisfaction with care and lower costs: results of a randomized trial of in‐home palliative care. J Am Geriatr Soc. 2007;55:993–1000.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- . Ensuring safe, quality care for hospitalized people with advanced illness, a core obligation for hospitalists. J Hosp Med. 2007;2:355–356.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients: the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT). JAMA. 1995;274:1591–1598.
As hospitalists now care for expanding numbers of America's aging patients, many of whom have chronic, debilitating illnesses or are near the end of life, there is a burgeoning need for innovative approaches to optimize quality of care and control costs, especially in the last year of life.[1, 2] In the inaugural issue of the Journal of Hospital Medicine, an overview of how hospitalists and palliative care specialists can work hand‐in‐hand to care for these seriously ill, hospitalized patients was presented.[3, 4] This perspective highlighted a symbiotic and mutually beneficial relationship between the 2 specialties based on their shared values, missions, and complementary strengths.[3, 4] Since then, a number of collaborative ventures offering palliative care for seriously ill, hospitalized patients have been developed and examined in a variety of settings.[4, 5, 6]
A key collaborative undertaking for hospitalists and palliative care specialists is the appreciation of the unique trajectory of each chronic illness toward the end of life. For example, patients with cancer or neurodegenerative disease tend to have relatively stable functional status until the final months of rapid deterioration. On the other hand, the courses of patients with chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), end‐stage renal disease, and human immunodeficiency virus/acquired immunodeficiency syndrome tend to be punctuated by episodes of acute exacerbation with often nearly complete return to previous status. Finally, dementia usually follows a slow course of gradual decline leading to death.[7] Ideally, active management of symptoms and discussion of prognosis and goals of care should happen in the early stages of these chronic illnesses, yet most often they are left until an acute hospitalization late in the disease course. The following case illustrates the point.
CASE 1
Mrs. M is an 89‐year‐old woman with Alzheimer's dementia diagnosed 7 years ago who has been cared for at home by family members. She is admitted to the hospital for urinary tract infection and volume depletion. She is bedbound, cachectic, and has a stage III decubitus ulcer. Her daughter describes a 6‐month history of feeding problems, 20‐lb weight loss, and 2 recent hospitalizations for aspiration pneumonia. She improves somewhat with hydration and intravenous antibiotics, and the physical therapist recommends rehabilitation. Mrs. M does not have decision‐making capacity, and her long‐time family physician has not inquired about care preferences or goals. The hospitalist team meets with family members to discuss the trajectory and prognosis of advanced dementia, and recommends against artificial nutrition and hydration, and for initiation of palliative care service at a skilled nursing facility.
In this example, the hospitalist team recognizes the advent of frequent infections and diminished oral intake in advanced dementia as signals of increased morbidity and mortality warranting palliative care intervention.[8] This, we suggest, represents a sentinel hospitalization, a hospitalization in the disease course that heralds a need to reassess prognosis, treatment options and intensity, and goals of care. Hospitalists are well positioned to recognize such transition points in the disease course by considering the patient's recent history of illness, to offer an impartial overview of illness progression, and to optimize patient care using principles of palliative care. Additionally, hospitalists have advantages of geographic convenience, readily available consultants, systemic support, and a detachment from the longitudinal patient‐physician relationship, which may enable more accurate medical prognostication.[9]
There are many ways to identify a sentinel hospitalization. For example, hospitalists can use the surprise question, Would you be surprised if the patient died within 12 months? on admission for the majority of cancer and dialysis patients. The answer No predicts a 3.5‐ to 7‐fold increase in 1‐year mortality.[10, 11] In a powerful predictive model for 1‐year mortality using readily available clinical, laboratory, and functional characteristics, medical inpatients in the highest quartiles have 1‐year mortality exceeding 60%.[12] Recently, several more complicated prognostic models have been derived and validated in large cohorts of medical inpatients, which predict short‐term (30‐day) and long‐term (0.5‐1 year) mortality with great accuracy.[13, 14] There are also many disease‐specific prognostic features (eg, diagnosis of metastatic disease with poor performance status or high symptom burden, progression of chronic kidney disease with consideration of hemodialysis, additional stroke in multi‐infarct dementia, and frequent exacerbation of severe COPD or severe CHF).[15, 16, 17, 18, 19, 20, 21] Finally, frequent readmissions and prolonged hospital or intensive care unit stay can also be used.[17, 19] These criteria are summarized in Table 1 with time frames.
| Common Criteria | Time Frame | References |
|---|---|---|
| ||
| No to the surprise question: Would you be surprised if the patient died in 12 months? | 1 year | [10, 11] |
| Newly diagnosed metastatic solid cancer | Various | [17] |
| Metastatic solid cancer admitted for uncontrolled symptoms | Various | [17] |
| Progressive CKD with consideration for hemodialysis | 1 year | [17, 18] |
| GOLD stage IV COPD with frequent exacerbation | Various | [20] |
| NYHAstage IV CHF with frequent exacerbation | 12 years | [21] |
| Advanced dementia with frequent UTI, aspiration PNA, and feeding problem | 12 years | [8] |
| Overall prognosis of high mortality using available indices | 30 days1 year | [12, 13, 14] |
| More than 3 admissions in last 6 months | 6 months | [17, 19] |
| Prolonged ICU stay (>7 days) | Weeks | [17, 19] |
Once a sentinel hospitalization is identified, hospitalists, with input from the patient's primary care physician and subspecialists, can then develop a comprehensive strategy to evaluate current disease management, to educate patient and family accordingly, and to actively integrate palliative care services as appropriate. The next challenge facing the care team is how to deliver the necessary palliative care since it is unnecessary and improper to ask for palliative care specialist consultation for every sentinel hospitalization. We believe that the best approach is for hospitalists to be the primary deliverers of basic palliative care in the hospital while consulting palliative care specialists for refractory symptoms and complex scenarios.[22] According to this generalist‐specialist palliative care model, physicians of all specialties should define and master a basic palliative care skill set for their patients. For hospitalists, the relevant skill set includes assessing and treating pain and other symptoms such as dyspnea, nausea and vomiting, and constipation, estimating prognosis, and initiating goals of care discussions.[22] The following case illustrates this point.
CASE 2
Ms S, a 21‐year‐old Hispanic woman with advanced, recurrent head and neck cancer, status post multiple surgeries, chemotherapy, and radiation therapy, is admitted to the hospitalist service for aspiration pneumonia, which responds to antibiotics rapidly. However, her cancer‐related somatic and neuropathic pain soon becomes refractory to opioids prescribed by the hospitalist team. She also develops significant dyspnea, xerostomia, depression, anxiety, and existential suffering. With the help of the interdisciplinary palliative care team, her pain is relieved by a patient‐controlled analgesia pump and methadone. A palliative care social worker and chaplain visit her and her family daily to address their distress. Eventually, the care team is able to provide a stable medical regimen for symptom control and to use it across the entire care continuum.
In this example, the hospitalist team, with the support of palliative care specialists, provided basic palliative care and longitudinal integration of palliative practices into the patient's overall treatment scheme. Hospitalists, given their scope of practice and sheer volume of patients, are well positioned to rapidly gain competencies in symptom management, empathic communication, and interdisciplinary teamwork.[23, 24] Hospitalists may benefit from innovative and collaborative palliative care education using interactive online modules, case simulation, communication workshops, and observed evaluation and feedback.[25] Several modes of collaboration between hospital medicine and palliative care have been developed including implementation of palliative care consult triggers on admission, palliative care participation in hospitalist interdisciplinary rounds, and disease specific, integrated management programs.[17, 26] These collaborations are particularly important, as the quality of inpatient care at the end of life is still suboptimal and more appropriate use of palliative care will be beneficial.[27] Recently, some hospitals have developed specialized inpatient palliative care units, combining intensive palliation with inpatient medical surgical level of care, as well as providing hospice care. Staffed by palliative care specialists or hospitalists, they provide efficient, cost‐saving care to patients with advanced chronic illness or terminal disease in need of intensive symptom management.[28] Finally, there is mounting evidence supporting the clinical effectiveness of palliative care in diverse specialties such as oncology, pulmonary and critical care, and nephrology.[29] For example, in the setting of metastatic non‐small cell lung cancer, early initiation of palliative care has been shown to improve symptom control and quality of life, reduce chemotherapy use at the end of life, and interestingly, prolong median survival by almost 3 months.[30] This has led to a position statement from American Society of Clinical Oncology encouraging early integration of palliative care into standard oncologic care for advanced disease.[31]
Recognizing a sentinel hospitalization allows palliative care to be integrated at transitions of care and carried forward. For patients with chronic debilitating illnesses who are approaching the end of life, appropriate care transitions will ensure that their short‐ and long‐term care matches their goals of care, assure timely clinical follow‐ups, and help reduce hospital readmission and healthcare resource utilization.[32] Importantly, timely and compassionate communication is a key to the success of both hospital medicine and palliative care. Many patients with life‐limiting diseases prefer to receive prognostic information and to discuss goals of care.[33] How this information is integrated and communicated through the care continuum is crucial, especially in the era of duty hour limits and frequent handoffs. The information exchange needs to facilitate active participation of primary care physicians who may not be involved in hospital care. Some of the innovative strategies for communication and transfer of palliative care information, such as prognosis, goals of care, family meeting consensus, and symptom control interventions, include a palliative care checklist in the electronic health record, incorporation of prognostic and family meeting information in the discharge summary, and links to the national Physician Orders for Life‐Sustaining Treatment advanced care planning program.[34] Of note, a pilot program in the United Kingdom adopting an electronic palliative care summary has reduced after‐hour emergency room visits and hospital readmissions.[35] The following case illustrates this point.
CASE 3
Mrs. K, an 82‐year‐old Russian‐speaking woman with newly diagnosed metastatic pancreatic cancer, is admitted for worsening obstructive jaundice and a second opinion about treatment. A biliary stent is placed and her jaundice slowly improves. The patient and family have requested chemotherapy. However, the oncologist determines that she would only qualify for a phase I trial given her poor performance status. The hospitalist team requests the help of the palliative care consult team to manage her severe pain, depression, and to provide support to the family. After several family meetings, the patient and family choose not to pursue chemotherapy. Given the lack of adequate support at home, she is discharged to a skilled nursing facility for short‐term rehabilitation with plans to transition to the in‐house hospice program. The hospitalist, palliative care attending physician, and the medical director of the rehabilitation facility have a 3‐way phone conference to confirm the plans of care and to ensure a smooth care transition.
In this case, the hospitalist team recognizes that this is a sentinel hospitalization for Mrs. K that requires extensive palliative care intervention. Often, transitioning to skilled nursing facilities (SNF) is the default pathway for patients needing hospice/palliative care, especially when patients and families are not yet ready to discuss prognosis realistically or to accept hospice, or there is not enough support available at home. A recent large cohort study showed that 30% of patients in their last 6‐month of life had used, and nearly 10% of such patients had died, under Medicare's posthospitalization SNF benefit.[36] Although the worsening disease trajectory may not be apparent at hospital discharge, it is more likely that the financial and practical limitation of the Medicare Hospice Benefit accounts for this observation, which includes limited home health aid hours, lack of coverage for room and board, and lower payments to SNFs.[36] Hospitalists can help address the issue of discharge location for patients needing palliative care. Sometimes this requires extensive communication before and after discharge to help enhance the transition from a rehabilitation facility to hospice/palliative care. Appropriately integrated palliative care at the time of care transitions, in the form of hospice or longitudinal home‐based palliative care rather than just routine clinic follow‐up, has the potential to reduce 30‐day readmission for chronically ill, elderly patients and for patients near the end of life.[37, 38] It is critical that national policy, suitable reimbursement, and financial incentives support this practice. A demonstration project, Better Outcomes by Optimizing Safe Transitions (BOOST), organized by the Society of Hospital Medicine, integrates palliative care evaluation into a comprehensive discharge assessment tool. This intervention has been shown to reduce readmissions to acute care hospitals.[39]
In this article, we define a sentinel hospitalization and suggest that its recognition provides an important opportunity for hospitalists to actively integrate palliative care into patients' chronic disease management programs, with inputs from patients, their families, their primary physicians and subspecialists, as well as palliative care specialists. We also recognize that within nonsentinel hospitalizations, there are important opportunities to discuss prognosis, goals of care, and advanced care planning. This approach allows the fresh eyes of hospitalists to assess the patient's current health status and prognosis, to communicate these relevant clinical issues with the patient and family, and to encourage discussions about goals of care and advanced care planning during the sentinel hospitalization. It also provides a structured vehicle for soliciting the patient's (and family's) perspectives and documenting them in the medical record. A compilation of sample items to guide discussion can be found in Table 2. Hospitalists, equipped with basic palliative care skills and supported by hospital‐ and community‐based palliative care teams, can thrive in this unique position of optimizing the quality of care for these patients.[40] Almost 20 years ago, the field of palliative care rose to national prominence on the findings of the SUPPORT (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) study, which investigated the suboptimal end‐of‐life experiences of hospitalized adult patients.[41] Since then, the fields of both hospital medicine and palliative care have grown, yet the best is still to come for their collaborative excellence, mutual education, and shared care innovation at the forefront of medicine.
| Patient/family understanding of disease process and treatment outcomes |
| Patient/family understanding of disease prognosis |
| Availability of alternative treatment options including palliative/hospice care |
| Patient/family wishes/goals of care |
| Advanced‐care planning including limitations of care |
| Inventory of symptoms (frequency, severity, modifying factors, timing, and treatments) |
| Social and financial stress |
| Emotional and existential stress |
| Social support system and caregivers |
| Living arrangements |
Disclosure: Nothing to report.
As hospitalists now care for expanding numbers of America's aging patients, many of whom have chronic, debilitating illnesses or are near the end of life, there is a burgeoning need for innovative approaches to optimize quality of care and control costs, especially in the last year of life.[1, 2] In the inaugural issue of the Journal of Hospital Medicine, an overview of how hospitalists and palliative care specialists can work hand‐in‐hand to care for these seriously ill, hospitalized patients was presented.[3, 4] This perspective highlighted a symbiotic and mutually beneficial relationship between the 2 specialties based on their shared values, missions, and complementary strengths.[3, 4] Since then, a number of collaborative ventures offering palliative care for seriously ill, hospitalized patients have been developed and examined in a variety of settings.[4, 5, 6]
A key collaborative undertaking for hospitalists and palliative care specialists is the appreciation of the unique trajectory of each chronic illness toward the end of life. For example, patients with cancer or neurodegenerative disease tend to have relatively stable functional status until the final months of rapid deterioration. On the other hand, the courses of patients with chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), end‐stage renal disease, and human immunodeficiency virus/acquired immunodeficiency syndrome tend to be punctuated by episodes of acute exacerbation with often nearly complete return to previous status. Finally, dementia usually follows a slow course of gradual decline leading to death.[7] Ideally, active management of symptoms and discussion of prognosis and goals of care should happen in the early stages of these chronic illnesses, yet most often they are left until an acute hospitalization late in the disease course. The following case illustrates the point.
CASE 1
Mrs. M is an 89‐year‐old woman with Alzheimer's dementia diagnosed 7 years ago who has been cared for at home by family members. She is admitted to the hospital for urinary tract infection and volume depletion. She is bedbound, cachectic, and has a stage III decubitus ulcer. Her daughter describes a 6‐month history of feeding problems, 20‐lb weight loss, and 2 recent hospitalizations for aspiration pneumonia. She improves somewhat with hydration and intravenous antibiotics, and the physical therapist recommends rehabilitation. Mrs. M does not have decision‐making capacity, and her long‐time family physician has not inquired about care preferences or goals. The hospitalist team meets with family members to discuss the trajectory and prognosis of advanced dementia, and recommends against artificial nutrition and hydration, and for initiation of palliative care service at a skilled nursing facility.
In this example, the hospitalist team recognizes the advent of frequent infections and diminished oral intake in advanced dementia as signals of increased morbidity and mortality warranting palliative care intervention.[8] This, we suggest, represents a sentinel hospitalization, a hospitalization in the disease course that heralds a need to reassess prognosis, treatment options and intensity, and goals of care. Hospitalists are well positioned to recognize such transition points in the disease course by considering the patient's recent history of illness, to offer an impartial overview of illness progression, and to optimize patient care using principles of palliative care. Additionally, hospitalists have advantages of geographic convenience, readily available consultants, systemic support, and a detachment from the longitudinal patient‐physician relationship, which may enable more accurate medical prognostication.[9]
There are many ways to identify a sentinel hospitalization. For example, hospitalists can use the surprise question, Would you be surprised if the patient died within 12 months? on admission for the majority of cancer and dialysis patients. The answer No predicts a 3.5‐ to 7‐fold increase in 1‐year mortality.[10, 11] In a powerful predictive model for 1‐year mortality using readily available clinical, laboratory, and functional characteristics, medical inpatients in the highest quartiles have 1‐year mortality exceeding 60%.[12] Recently, several more complicated prognostic models have been derived and validated in large cohorts of medical inpatients, which predict short‐term (30‐day) and long‐term (0.5‐1 year) mortality with great accuracy.[13, 14] There are also many disease‐specific prognostic features (eg, diagnosis of metastatic disease with poor performance status or high symptom burden, progression of chronic kidney disease with consideration of hemodialysis, additional stroke in multi‐infarct dementia, and frequent exacerbation of severe COPD or severe CHF).[15, 16, 17, 18, 19, 20, 21] Finally, frequent readmissions and prolonged hospital or intensive care unit stay can also be used.[17, 19] These criteria are summarized in Table 1 with time frames.
| Common Criteria | Time Frame | References |
|---|---|---|
| ||
| No to the surprise question: Would you be surprised if the patient died in 12 months? | 1 year | [10, 11] |
| Newly diagnosed metastatic solid cancer | Various | [17] |
| Metastatic solid cancer admitted for uncontrolled symptoms | Various | [17] |
| Progressive CKD with consideration for hemodialysis | 1 year | [17, 18] |
| GOLD stage IV COPD with frequent exacerbation | Various | [20] |
| NYHAstage IV CHF with frequent exacerbation | 12 years | [21] |
| Advanced dementia with frequent UTI, aspiration PNA, and feeding problem | 12 years | [8] |
| Overall prognosis of high mortality using available indices | 30 days1 year | [12, 13, 14] |
| More than 3 admissions in last 6 months | 6 months | [17, 19] |
| Prolonged ICU stay (>7 days) | Weeks | [17, 19] |
Once a sentinel hospitalization is identified, hospitalists, with input from the patient's primary care physician and subspecialists, can then develop a comprehensive strategy to evaluate current disease management, to educate patient and family accordingly, and to actively integrate palliative care services as appropriate. The next challenge facing the care team is how to deliver the necessary palliative care since it is unnecessary and improper to ask for palliative care specialist consultation for every sentinel hospitalization. We believe that the best approach is for hospitalists to be the primary deliverers of basic palliative care in the hospital while consulting palliative care specialists for refractory symptoms and complex scenarios.[22] According to this generalist‐specialist palliative care model, physicians of all specialties should define and master a basic palliative care skill set for their patients. For hospitalists, the relevant skill set includes assessing and treating pain and other symptoms such as dyspnea, nausea and vomiting, and constipation, estimating prognosis, and initiating goals of care discussions.[22] The following case illustrates this point.
CASE 2
Ms S, a 21‐year‐old Hispanic woman with advanced, recurrent head and neck cancer, status post multiple surgeries, chemotherapy, and radiation therapy, is admitted to the hospitalist service for aspiration pneumonia, which responds to antibiotics rapidly. However, her cancer‐related somatic and neuropathic pain soon becomes refractory to opioids prescribed by the hospitalist team. She also develops significant dyspnea, xerostomia, depression, anxiety, and existential suffering. With the help of the interdisciplinary palliative care team, her pain is relieved by a patient‐controlled analgesia pump and methadone. A palliative care social worker and chaplain visit her and her family daily to address their distress. Eventually, the care team is able to provide a stable medical regimen for symptom control and to use it across the entire care continuum.
In this example, the hospitalist team, with the support of palliative care specialists, provided basic palliative care and longitudinal integration of palliative practices into the patient's overall treatment scheme. Hospitalists, given their scope of practice and sheer volume of patients, are well positioned to rapidly gain competencies in symptom management, empathic communication, and interdisciplinary teamwork.[23, 24] Hospitalists may benefit from innovative and collaborative palliative care education using interactive online modules, case simulation, communication workshops, and observed evaluation and feedback.[25] Several modes of collaboration between hospital medicine and palliative care have been developed including implementation of palliative care consult triggers on admission, palliative care participation in hospitalist interdisciplinary rounds, and disease specific, integrated management programs.[17, 26] These collaborations are particularly important, as the quality of inpatient care at the end of life is still suboptimal and more appropriate use of palliative care will be beneficial.[27] Recently, some hospitals have developed specialized inpatient palliative care units, combining intensive palliation with inpatient medical surgical level of care, as well as providing hospice care. Staffed by palliative care specialists or hospitalists, they provide efficient, cost‐saving care to patients with advanced chronic illness or terminal disease in need of intensive symptom management.[28] Finally, there is mounting evidence supporting the clinical effectiveness of palliative care in diverse specialties such as oncology, pulmonary and critical care, and nephrology.[29] For example, in the setting of metastatic non‐small cell lung cancer, early initiation of palliative care has been shown to improve symptom control and quality of life, reduce chemotherapy use at the end of life, and interestingly, prolong median survival by almost 3 months.[30] This has led to a position statement from American Society of Clinical Oncology encouraging early integration of palliative care into standard oncologic care for advanced disease.[31]
Recognizing a sentinel hospitalization allows palliative care to be integrated at transitions of care and carried forward. For patients with chronic debilitating illnesses who are approaching the end of life, appropriate care transitions will ensure that their short‐ and long‐term care matches their goals of care, assure timely clinical follow‐ups, and help reduce hospital readmission and healthcare resource utilization.[32] Importantly, timely and compassionate communication is a key to the success of both hospital medicine and palliative care. Many patients with life‐limiting diseases prefer to receive prognostic information and to discuss goals of care.[33] How this information is integrated and communicated through the care continuum is crucial, especially in the era of duty hour limits and frequent handoffs. The information exchange needs to facilitate active participation of primary care physicians who may not be involved in hospital care. Some of the innovative strategies for communication and transfer of palliative care information, such as prognosis, goals of care, family meeting consensus, and symptom control interventions, include a palliative care checklist in the electronic health record, incorporation of prognostic and family meeting information in the discharge summary, and links to the national Physician Orders for Life‐Sustaining Treatment advanced care planning program.[34] Of note, a pilot program in the United Kingdom adopting an electronic palliative care summary has reduced after‐hour emergency room visits and hospital readmissions.[35] The following case illustrates this point.
CASE 3
Mrs. K, an 82‐year‐old Russian‐speaking woman with newly diagnosed metastatic pancreatic cancer, is admitted for worsening obstructive jaundice and a second opinion about treatment. A biliary stent is placed and her jaundice slowly improves. The patient and family have requested chemotherapy. However, the oncologist determines that she would only qualify for a phase I trial given her poor performance status. The hospitalist team requests the help of the palliative care consult team to manage her severe pain, depression, and to provide support to the family. After several family meetings, the patient and family choose not to pursue chemotherapy. Given the lack of adequate support at home, she is discharged to a skilled nursing facility for short‐term rehabilitation with plans to transition to the in‐house hospice program. The hospitalist, palliative care attending physician, and the medical director of the rehabilitation facility have a 3‐way phone conference to confirm the plans of care and to ensure a smooth care transition.
In this case, the hospitalist team recognizes that this is a sentinel hospitalization for Mrs. K that requires extensive palliative care intervention. Often, transitioning to skilled nursing facilities (SNF) is the default pathway for patients needing hospice/palliative care, especially when patients and families are not yet ready to discuss prognosis realistically or to accept hospice, or there is not enough support available at home. A recent large cohort study showed that 30% of patients in their last 6‐month of life had used, and nearly 10% of such patients had died, under Medicare's posthospitalization SNF benefit.[36] Although the worsening disease trajectory may not be apparent at hospital discharge, it is more likely that the financial and practical limitation of the Medicare Hospice Benefit accounts for this observation, which includes limited home health aid hours, lack of coverage for room and board, and lower payments to SNFs.[36] Hospitalists can help address the issue of discharge location for patients needing palliative care. Sometimes this requires extensive communication before and after discharge to help enhance the transition from a rehabilitation facility to hospice/palliative care. Appropriately integrated palliative care at the time of care transitions, in the form of hospice or longitudinal home‐based palliative care rather than just routine clinic follow‐up, has the potential to reduce 30‐day readmission for chronically ill, elderly patients and for patients near the end of life.[37, 38] It is critical that national policy, suitable reimbursement, and financial incentives support this practice. A demonstration project, Better Outcomes by Optimizing Safe Transitions (BOOST), organized by the Society of Hospital Medicine, integrates palliative care evaluation into a comprehensive discharge assessment tool. This intervention has been shown to reduce readmissions to acute care hospitals.[39]
In this article, we define a sentinel hospitalization and suggest that its recognition provides an important opportunity for hospitalists to actively integrate palliative care into patients' chronic disease management programs, with inputs from patients, their families, their primary physicians and subspecialists, as well as palliative care specialists. We also recognize that within nonsentinel hospitalizations, there are important opportunities to discuss prognosis, goals of care, and advanced care planning. This approach allows the fresh eyes of hospitalists to assess the patient's current health status and prognosis, to communicate these relevant clinical issues with the patient and family, and to encourage discussions about goals of care and advanced care planning during the sentinel hospitalization. It also provides a structured vehicle for soliciting the patient's (and family's) perspectives and documenting them in the medical record. A compilation of sample items to guide discussion can be found in Table 2. Hospitalists, equipped with basic palliative care skills and supported by hospital‐ and community‐based palliative care teams, can thrive in this unique position of optimizing the quality of care for these patients.[40] Almost 20 years ago, the field of palliative care rose to national prominence on the findings of the SUPPORT (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) study, which investigated the suboptimal end‐of‐life experiences of hospitalized adult patients.[41] Since then, the fields of both hospital medicine and palliative care have grown, yet the best is still to come for their collaborative excellence, mutual education, and shared care innovation at the forefront of medicine.
| Patient/family understanding of disease process and treatment outcomes |
| Patient/family understanding of disease prognosis |
| Availability of alternative treatment options including palliative/hospice care |
| Patient/family wishes/goals of care |
| Advanced‐care planning including limitations of care |
| Inventory of symptoms (frequency, severity, modifying factors, timing, and treatments) |
| Social and financial stress |
| Emotional and existential stress |
| Social support system and caregivers |
| Living arrangements |
Disclosure: Nothing to report.
- , , , , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7:649–654.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Affairs. 2001;20:188–195.
- . Palliative care in hospitals. J Hosp Med. 2006;1:21–28.
- . Palliative care and hospitalists: a partnership for hope. J Hosp Med. 2006;1:5–6.
- , . Palliative care and the hospitalist: an opportunity for cross‐fertilization. Am J Med. 2001;111:10s–14s.
- , . Palliative care. Ann Intern Med. 2012;156:ITC2‐1, TC2‐2–15; quiz TC2‐16.
- , , , , . Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392.
- , , , et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538.
- , . Extent and determinants of error in doctor's prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–472.
- , , , et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13:837–840.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379–1384.
- , , , et al. Development and validation of a prognostic index for 1‐year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013,8:229–235.
- , , , , . Caring about prognosis: a validation study of the CARING criteria to identify hospitalized patients at high risk for death at 1 year. J Hosp Med. 2013,8:696–701.
- , , , , , , et al. Meta‐analysis of survival prediction with palliative performance scale. J Palliat Care. 2007;23:245–254.
- , , , , . Prognostic indices for older adults: a systemic review. JAMA. 2012;307:182–192.
- , . Identifying patients in need of a palliative care assessment in the hospital setting. J Palliat Med. 2011;14:17–23.
- , , , , , . Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547.
- , , . Top 10 things palliative care clinicians wished everyone knew about palliative care. Mayo Clin Proc. 2013;88:859–865.
- . Palliative and end‐of‐life care for patients with severe COPD. Eur Respir J. 2008;32:796–803.
- . Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396.
- , . Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368:1173–1175.
- , , , , , . Common myths about caring for patients with terminal illness: opportunities to improve care in the hospital setting. J Hosp Med. 2007;2:357–365.
- , , , . Opportunity lost: end‐of‐life discussions in cancer patients who die in the hospital. J Hosp Med. 2013;8:334–340.
- , , . Palliative medicine physician education in the United States: a historical review. J Palliat Med. 2013;16:230–236.
- , . Hospitalization as an opportunity to integrate palliative care in heart failure management. Curr Opin Support Palliat Care. 2009;3:247–251.
- , , , et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170:1057–1063.
- , , , , , . Outcomes of the acute palliative care unit in an academic medical center [published online ahead of print May 10, 2013]. Am J Hosp Palliat Care. doi: 10.1177/1049909113489164.
- , , , , , . Update in hospital palliative care. J Hosp Med. 2013;12:715–720.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , . Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin. 2013;63:349–363.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187.
- , , , , , . “Knowing is better”: preferences of diverse older adults for discussion prognosis. J Gen Intern Med. 2012;27:568–575.
- , , . POLST, an improvement over traditional advanced directives. Cleve Clin J Med. 2012;79:457–464.
- , , , . Use of a structured palliative care summary in patients with established cancer is associated with reduced hospital admissions by out‐of‐hours general practitioners in Grampian [published online ahead of print January 3, 2013]. BMJ Support Palliat Care. doi:10.1136/bmjspcare‐2012‐000371.
- , , , , , . Use of the Medicare posthospitalization skilled nursing benefit in the last 6 months of life. Arch Intern Med. 2012;172:1573–1579.
- , , , et al. Increased satisfaction with care and lower costs: results of a randomized trial of in‐home palliative care. J Am Geriatr Soc. 2007;55:993–1000.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- . Ensuring safe, quality care for hospitalized people with advanced illness, a core obligation for hospitalists. J Hosp Med. 2007;2:355–356.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients: the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT). JAMA. 1995;274:1591–1598.
- , , , , , , et al. Evolving practice of hospital medicine and its impact on hospital throughput and efficiencies. J Hosp Med. 2012;7:649–654.
- , , , . Medicare beneficiaries' costs of care in the last year of life. Health Affairs. 2001;20:188–195.
- . Palliative care in hospitals. J Hosp Med. 2006;1:21–28.
- . Palliative care and hospitalists: a partnership for hope. J Hosp Med. 2006;1:5–6.
- , . Palliative care and the hospitalist: an opportunity for cross‐fertilization. Am J Med. 2001;111:10s–14s.
- , . Palliative care. Ann Intern Med. 2012;156:ITC2‐1, TC2‐2–15; quiz TC2‐16.
- , , , , . Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392.
- , , , et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538.
- , . Extent and determinants of error in doctor's prognoses in terminally ill patients: prospective cohort study. BMJ. 2000;320:469–472.
- , , , et al. Prognostic significance of the “surprise” question in cancer patients. J Palliat Med. 2010;13:837–840.
- , , , et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379–1384.
- , , , et al. Development and validation of a prognostic index for 1‐year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994.
- , , , , . Mortality predictions on admission as a context for organizing care activities. J Hosp Med. 2013,8:229–235.
- , , , , . Caring about prognosis: a validation study of the CARING criteria to identify hospitalized patients at high risk for death at 1 year. J Hosp Med. 2013,8:696–701.
- , , , , , , et al. Meta‐analysis of survival prediction with palliative performance scale. J Palliat Care. 2007;23:245–254.
- , , , , . Prognostic indices for older adults: a systemic review. JAMA. 2012;307:182–192.
- , . Identifying patients in need of a palliative care assessment in the hospital setting. J Palliat Med. 2011;14:17–23.
- , , , , , . Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547.
- , , . Top 10 things palliative care clinicians wished everyone knew about palliative care. Mayo Clin Proc. 2013;88:859–865.
- . Palliative and end‐of‐life care for patients with severe COPD. Eur Respir J. 2008;32:796–803.
- . Palliative care in congestive heart failure. J Am Coll Cardiol. 2009;54:386–396.
- , . Generalist plus specialist palliative care—creating a more sustainable model. N Engl J Med. 2013;368:1173–1175.
- , , , , , . Common myths about caring for patients with terminal illness: opportunities to improve care in the hospital setting. J Hosp Med. 2007;2:357–365.
- , , , . Opportunity lost: end‐of‐life discussions in cancer patients who die in the hospital. J Hosp Med. 2013;8:334–340.
- , , . Palliative medicine physician education in the United States: a historical review. J Palliat Med. 2013;16:230–236.
- , . Hospitalization as an opportunity to integrate palliative care in heart failure management. Curr Opin Support Palliat Care. 2009;3:247–251.
- , , , et al. The quality of care provided to hospitalized patients at the end of life. Arch Intern Med. 2010;170:1057–1063.
- , , , , , . Outcomes of the acute palliative care unit in an academic medical center [published online ahead of print May 10, 2013]. Am J Hosp Palliat Care. doi: 10.1177/1049909113489164.
- , , , , , . Update in hospital palliative care. J Hosp Med. 2013;12:715–720.
- , , , et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363:733–742.
- , , , . Early integration of palliative care services with standard oncology care for patients with advanced cancer. CA Cancer J Clin. 2013;63:349–363.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150:178–187.
- , , , , , . “Knowing is better”: preferences of diverse older adults for discussion prognosis. J Gen Intern Med. 2012;27:568–575.
- , , . POLST, an improvement over traditional advanced directives. Cleve Clin J Med. 2012;79:457–464.
- , , , . Use of a structured palliative care summary in patients with established cancer is associated with reduced hospital admissions by out‐of‐hours general practitioners in Grampian [published online ahead of print January 3, 2013]. BMJ Support Palliat Care. doi:10.1136/bmjspcare‐2012‐000371.
- , , , , , . Use of the Medicare posthospitalization skilled nursing benefit in the last 6 months of life. Arch Intern Med. 2012;172:1573–1579.
- , , , et al. Increased satisfaction with care and lower costs: results of a randomized trial of in‐home palliative care. J Am Geriatr Soc. 2007;55:993–1000.
- , , . 30‐day readmissions among seriously ill older adults. J Palliat Med. 2012;15:1–6.
- , , , et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8:421–427.
- . Ensuring safe, quality care for hospitalized people with advanced illness, a core obligation for hospitalists. J Hosp Med. 2007;2:355–356.
- The SUPPORT Principal Investigators. A controlled trial to improve care for seriously ill hospitalized patients: the Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments (SUPPORT). JAMA. 1995;274:1591–1598.
Patient Care Circle and Care Transitions
The focus on care transitions and readmissions is expanding beyond the development of risk scores based on objective clinical data to quality improvement interventions involving the key stakeholders in the process, namely the patients and their multidisciplinary providers.[1, 2] The Institute for Healthcare Improvement's State Action on Avoidable Rehospitalizations initiative promotes formulating a specific transition plan and developing multidisciplinary management strategies for all patients.[3] The Transition of Care Consensus Policy Statement developed by a coalition including the American College of Physicians and Society of Hospital Medicine emphasizes accountability, communication, and involvement of the patient and family members in plans of care.[4] Yet, interventions to reduce readmissions and improve the quality and safety of care transitions remain only modestly and inconsistently effective.
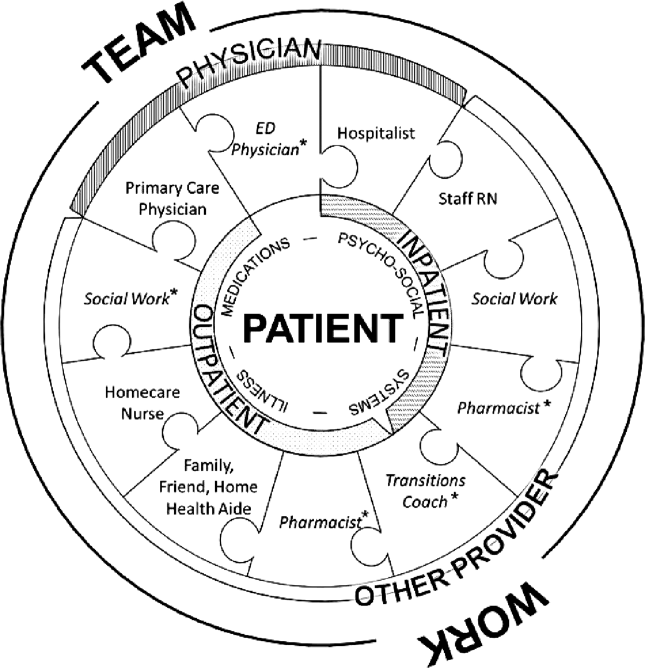
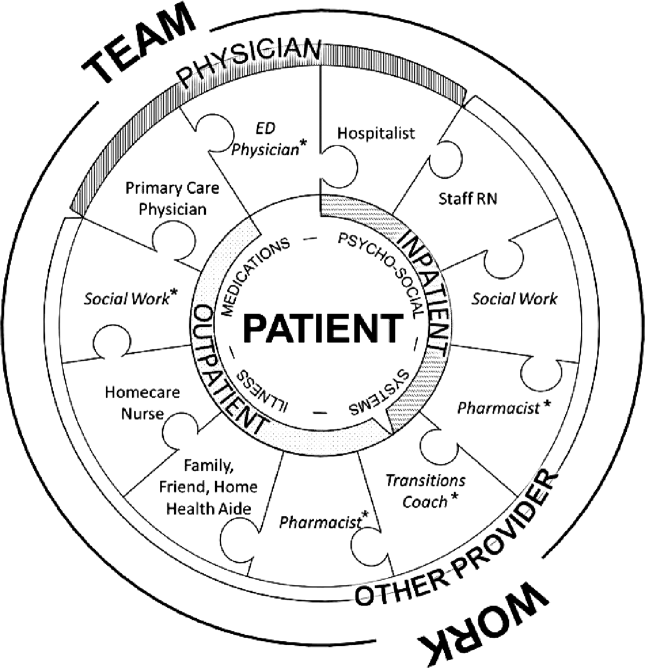
Successful interventions are those that are combined and coordinated, and shared across the hospital and community settings.[5] In this study, we sought to understand the issues leading to readmissions and barriers as perceived by patients, family members, physicians, nurses, and social workers. We compared and contrasted the perspectives by discipline and used this information to design a descriptive framework of a multidisciplinary, collaborative, and coordinated support network integral to effective care transitions, which we term a Patient Care Circle (PCC) (Figure 1).
METHODS
Study Design
We recruited a purposive sample of general medicine patients with same‐site 30‐day readmissions, and those directly involved in their care, to participate in interviews and focus groups to investigate explanations for unplanned readmissions (Table 1). We sought subjects' perspectives based on extrapolations from previous research that identified multiple stakeholders involved in the care transitions process,[1, 2, 5, 6, 7, 8] and our own professional experience with patient readmissions.
| Role | No. (%) | No. Interviewed | No. in Focus Group | |
|---|---|---|---|---|
| ||||
| Patient | 12 | NA | ||
| Male | 10 (90.9) | |||
| Average age, y, range | 3172 | |||
| Insurance | ||||
| Medicare | 5 (41.7) | |||
| Medicaid | 1 (8.3) | |||
| Medicare/Medicaid | 2 (16.7) | |||
| Private | 4 (33.3) | |||
| Race | ||||
| White | 8 (66.7) | |||
| Black | 2 (16.7) | |||
| Other | 2 (16.7) | |||
| Has PMD | 9 (75.0) | |||
| Has home caregiver (family or aide) | 10 (90.9) | |||
| Physician | ||||
| Hospitalista | 9b | |||
| Index | 10 | |||
| Readmit | 9 | |||
| Primary care physician | 5 | NA | ||
| Other provider | ||||
| RN | ||||
| Inpatient staff | 5 | 7 | ||
| Visiting home | NA | 6 | ||
| Social work | NA | 6 | ||
| Other caregivers | ||||
| Family | 2 | NA | ||
| Home aides | 0 | NA | ||
| Total | 43 | 28 | ||
Site Selection
All interviews and focus groups were conducted at New YorkPresbyterian/Weill Cornell Medical Center (NYP/WC), a large urban academic medical center in New York City serving a racially and socioeconomically diverse population. The institutional review boards at Weill Cornell Medical College and Hunter College approved this study.
Data Collection Tools
We developed semistructured interview and focus group guides (see Supporting Information, Appendixes 17, in the online version of this article) by reviewing published literature[8, 9, 10, 11, 12] and readmission pilot data that identified challenges associated with hospital discharges. Interviews were patient specific, and providers involved directly in their care were asked to consider reasons for the patients' readmissions and whether they could have been prevented. Provider interview guides were modified from the patient interview script and tailored toward their role in the patient's care.
One focus group guide was used for all sessions, allowing us to compare and contrast emerging themes across disciplines. Participants were asked to discuss perceived causes for readmissions and barriers to improvement.
All questions were open‐ended to gain insight into participants' beliefs regarding the causes of readmissions and to limit researcher bias. We iteratively reviewed and modified the guides to ensure the questions were effectively worded.
Recruiting
Using a centralized clinical database, we identified patients aged 18 years and older for interviews, who were readmitted within 30 days to NYP/WC between May 2011 and May 2012, and had an attending hospitalist during the initial and readmission visits. We confirmed patients' English fluency and cognitive ability by contacting their attending physician. Patients provided written consent prior to interview.
For interviews, we asked patients to identify their outpatient physicians and providers; inpatient hospitalists and providers were identified from the patients' charts. For focus groups, we recruited volunteers among all division hospitalists and solicited volunteer inpatient nursing, social work, and homecare nursing participants through organizational liaisons (Table 1).
Data Collection
We interviewed patients in person at their bedside. We interviewed physicians and other caregivers in person or by telephone during the course of the patient's readmission. We conducted 4 discipline‐specific 90‐minute focus groups for hospitalists, inpatient staff nurses, homecare nurses, and hospital social workers. Patient interviews and focus groups were audio‐recorded and transcribed using a professional service.
Data Analysis
We analyzed 47 transcripts (43 interviews, 4 focus groups) during research group meetings using grounded theory[13] to generate overarching themes felt to influence readmissions through iterative reviewing of transcripts. We attributed codes to salient text and documented recurring topics that emerged. Two researchers independently assessed responses from the patient‐specific interviews for variability among the various disciplines. We ended our data collection after we ceased to find new topics from participants (thematic saturation).[14]
Three researchers, in consultation with the larger team, coded the 4 focus group transcripts to generate a codebook with definitions and examples of recurring concepts. They then coded the 43 interview transcripts using the codebook. The entire team met regularly to address questions and potential discrepancies.
We achieved greater trustworthiness of the analysis by using multiple modes of triangulation, a qualitative method that relies on points of comparison and contrast.[15] We achieved methodological triangulation by using both interviews and focus groups, and achieved internal triangulation by having researchers in the clinical, social, and behavioral sciences routinely critique the evolving codebook.
RESULTS
We recruited 43 interview and 28 focus group participants (Table 1). From our transcript analysis, we generated 22 codes and categorized them into 5 themes embodying the issues pertinent to readmissions from the perspective of the stakeholders: (1) teamwork, (2) health systems navigation and management, (3) illness severity and health needs, (4) psychosocial stability; and (5) medications (Table 2).
We applied these codes and themes to build a descriptive framework depicting what we believed is the essential foundation for successful care transitions, a collaborative unified patient‐centered network to address complex healthcare‐related issues across disciplines and across settings (Figure 1). Our model illustrates the interplay between the various physician and care‐provider roles as well as the relationship of the structure of the care circle to each theme.
Care Circle Theme
Teamwork
Comprehensive, effective collaboration and communication among members of the PCC were required for the circle to function successfully and establish safe ongoing patient care across settings. Teamwork required a shared purpose and aligned incentives among all stakeholders to work as a unified patient‐centered network.
Dysfunctional teamwork led to fragmented care. Hospitalists and patients cited difficulties coordinating in‐hospital management plans with multiple consulting subspecialists. Social workers ascribed 1 potential cause for unplanned readmissions to insufficient feedback from homecare agencies regarding patients following hospital discharge:
I wouldn't mind hearing [from the home agencies][the patient] won't let me in the door' patient's doing well' or patient's still not compliant.' If we don't knowthen we can't address it [until] they come back in [to the hospital].
Meanwhile, accurate handoff of information affected the care provided by homecare nurses:
We go into assess [the patient at home] and we see something totally different than what wason a piece of paper.
Patient‐Centered Themes
Four patient‐centered themes were identified that posed challenges in the transitions process and required the support and teamwork of the PCC to deal with effectually.
Health Systems Navigation and Management
The complexities of the healthcare system in the hospital and in the community presented challenges for patients with greater needs. Meeting higher levels of patient care needs was difficult in a system where prioritizing competing responsibilities was a recurrent issue. Inpatient nurses shared:
Educat[ing] people and empower[ing] them about their health. [I]t's kind of lostwhen we have so many [tasks] that we're responsible for, the patient gets lost in all of these things. For patients requiring ongoing sub‐acute care, limited weekend and holiday hospital and skilled nursing facility personnel added to the difficulty of arranging discharges and executing care plans.
Social workers noted:
[S]ometimes people are ready for discharge and there's noprimary care physician [willing to follow them].
Obtaining additional support following discharge was another concern for patients with homecare needs:
With the Medicaid changeshomecare is going to be less [than] what's provided [now]. So they're going into a lesssafe environment. [Social worker]
Illness Severity and Health Needs
The ability to cope with disease and related stressors depended on complexity of illness, level of health literacy, and underlying psychiatric issues overlapping with the theme of psychosocial stability. Early identification and mitigation of potential postdischarge complications required PCC collaboration.
All groups agreed that patients with chronic complex comorbidities often warranted frequent access to the inpatient setting regardless of outpatient medical care:
I'm not surprised [my patient was readmitted] becausealmost anything that goes wrong leads her to the hospital. Her readmission is not avoidable because of the severity of her illness. [Primary care physician]
With patients living longer with terminal illness, several groups voiced concern regarding the frequency of hospitalizations:
People [go] into hospice in the last week of their life as opposed to in the last six months of their life.The doctor has to bring this up [I] can't do it. [Homecare nurse]
Another prevalent issue was the emotional stress that accompanies acute or exacerbations of illness. One patient shared,
I also have a four‐year‐old son. Obviously, I'm not able to care for him as much as I was. My wifehas been diagnosed with leukemia.
Psychosocial Stability
Discharge from the hospital often requires psychosocial adjustment, which may be overlooked, underestimated, or dismissed by patients and providers.
[One patient] was very visually impaired. Lives by himself. But he's youngso he wanted to go home [not] a nursing home. He got home. He got up in the middle of the night. [P]ut the wound vac[uum] on the counter [and it] fell. It broke. It started beeping. He panicked, couldn't get in touch with any of the visiting nurses because it was 2:00 a.m. And he [was readmitted], and now is saying he wants to go to sub‐acute, because he can't handle it at home. [Social worker]
Engaging patients who seemed capable of participating in their own care was often frustrating for providers:
It's depressing because you're trying to help somebody [but] they don't want to help themselves and you know you'll see them right back [in the hospital] again. [Inpatient nurse]
Social support and socioeconomic factors also impacted patients' and families' ability to cope and adjust to the community after discharge. One family member commented that he and his wife have always cared for the patient together but now he cares for her alone and must hire a private duty aide to assist.
Medications
The degree to which obtaining, understanding, and taking medications exists as an impediment to safe transitions was patient specific and dependent on all of the patient‐centered themes above. Recognition and effective intervention required a multitiered, multidisciplinary approach. Homecare nurses reflected:
Discharge planning doesn't ensure that there is someone that can go to the pharmacy to get [medications] until the [visiting] nurse comes in and sets something up.
Methods used for medication education were not always effective in reaching the patient:
I shouldn't really say that they didn't [discuss medication side effects] because I was in a lot of pain. I really don't recall somebody giving me specific [information on] side effects on the medication. [Patient]
DISCUSSION
We categorized our findings into5 principle themes that influence care transitions: teamwork, systems navigation and management, illness severity, and health needs, psychosocial stability, and medications. Many of these themes have been targeted in the literature for interventions to reduce readmissions and improve care transitions. An overarching theme of our study was the importance of the Patient Care Circle, a support system required to implement and execute comprehensive patient‐centered plans for safe and effective transitions across all settings.
Collectively, our themes emphasized that communication and comprehensive planning between all members of the PCC were instrumental to the circle's ability to address issues pertaining to the patient‐centered themes: systems navigation and management, illness severity and health needs, psychosocial stability, and medications. The strength of the bonds and collaboration within the PCC were directly dependent on the success of teamwork.
The interplay between the 4 patient‐centered themes and the degree to which they affect readmissions were variable and patient dependent. Complexities of the healthcare system and issues surrounding medications became more apparent with worsening disease severity and psychosocial instability. Complicated patients requiring more multidisciplinary interaction highlighted limitations of dispersed teams and staffing ratios. Patients faced with insurance restrictions, difficulties attending appointments, and obtaining medications required pooling the efforts of multiple PCC members to help them. Thus, these themes emphasized not only the importance of teamwork required for care coordination, but also guided the membership of the PCC to meet the patient's specific needs across the inpatient and outpatient settings.
When participants were asked to identify modifiable reasons for readmissions, the overwhelming collective response was inadequate communication and collaboration among PCC members. Clear role assignments and delegation of responsibility were also necessary to avoid gaps in care. Significant barriers to improvement included limited resources and inability to maintain the integrity of the support network needed for safe transitions.
Finally, we compared and contrasted the perceptions of the different disciplines on the factors contributing to each patient's readmission. Over all, there was substantial overlap. However, each perspective added additional layers of information allowing for a more comprehensive understanding of the problem. This demonstrated the utility of multidisciplinary patient‐centered interviews to examine readmissions and elucidate areas for intervention.
Several disciplines were not included in interviews or focus groups but were identified by our study participants as integral to a comprehensive Patient Care Circle. These include emergency medicine physicians, inpatient and outpatient pharmacists, and outpatient social workers. Some disciplines were not included due to challenges identifying discrete providers and with arranging interviews or focus groups. As their roles were mentioned several times in multiple forums, we have included them in our descriptive framework.
We designed this study with the hope of completing a full complement of patient‐specific interviews that included all stakeholders for 4 male and 4 female patients. For several reasons, we were unable to do so including challenges contacting providers and family members, and coordinating the timing of interviews with patient visits. Further, our focus on English‐speaking patients admitted to general medicine teams may limit generalizability to other vulnerable patient groups. Nevertheless, we believe we succeeded in interviewing a representative sample and obtained thematic saturation with the information obtained from our interviews and focus groups.
Last, the focus of this project was to obtain the perspectives of a full spectrum of stakeholders in the care transitions process to gain a better understanding of the reasons for readmissions. Although we did ask study participants to identify areas that may have been modifiable, we did not expand the discussion to include potential interventions, which will be the next step in our study.
CONCLUSION
Our article describes 5 main themes derived from the perspectives of multiple stakeholders involved in the care transitions process. An overarching theme was the importance of a multidisciplinary, coordinated collaborative care circle to ensure safe patient‐centered care in all settings.
The results of this study can be used by researchers and applied by care providers to improve the care transitions process. Researchers can build on our model by studying methods and interventions to improve the function of the care circle and design guidelines to create a more effective and integrated network. Institutions can adapt our methodology and tools to identify the needs of their own patient population and optimize membership in the PCC accordingly.
We feel that improving the structure and function of the care circle is necessary prior to designing interventions targeting the patient‐centered themes. Strengthening the teamwork of the PCC is fundamental to improving the quality of care transitions and reducing preventable readmissions.
Acknowledgments
The authors thank the patients, family members, social workers, nurses, and physicians who participated in their study. The authors are grateful to their research assistants for their assistance with conducting interviews, focus groups, and data collection.
Disclosures: This study was supported by the Weill Cornell Clinical and Translational Science Center: UL1 RR024996. Dr. Press is supported in part through funds provided to him as a Nanette Laitman Clinical Scholar in Public Health at the Weill Cornell Medical College. An earlier version of the article was presented as a poster at the Society of Hospital Medicine annual conference in San Diego, California in 2012.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50:599–605.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- Institute for Healthcare Improvement.State Action on Avoidable Rehospitalizations (STAAR) initiative. Available at: http://www.ihi.org/offerings/Initiatives/STAAR/Pages/Improvement.aspx. Accessed January 28, 2013.
- , , , et al. Transition of Care Consensus Policy Statement, American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatric Society–American College of Emergency Physicians–Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2008;2:314–323.
- , , , , . How‐to guide: improving transitions from the hospital to community settings to reduce avoidable rehospitalizations. Cambridge, MA: Institute for Healthcare Improvement; June 2012. Available at: www.IHI.org. Accessed December 31, 2012.
- BOOSTing care transitions. Philadelphia, PA: Society of Hospital Medicine; 2008. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. Accessed October 20, 2012.
- , , . Predictive validity of a questionnaire that identifies older persons at risk for hospital admission. J Am Geriatr Soc. 1995;43(4):374–377.
- . The care transitions program: healthcare services for improving quality and safety during care hand‐offs. Denver, CO: Care Transitions Program; 2007. Available at: http://www.caretransitions.org. Accessed October 22, 2012.
- , , , , . Did I do as best as the system would let me? Healthcare professional views on hospital to home care transitions. J Gen Intern Med. 2012;27(12):1649–1656.
- , . The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967.
- . The significance of saturation. Qual Health Res. 1995;5(2):147–149.
- . Understanding reliability and validity in qualitative research. Qual Rep. 2003;8(4):597–607.
The focus on care transitions and readmissions is expanding beyond the development of risk scores based on objective clinical data to quality improvement interventions involving the key stakeholders in the process, namely the patients and their multidisciplinary providers.[1, 2] The Institute for Healthcare Improvement's State Action on Avoidable Rehospitalizations initiative promotes formulating a specific transition plan and developing multidisciplinary management strategies for all patients.[3] The Transition of Care Consensus Policy Statement developed by a coalition including the American College of Physicians and Society of Hospital Medicine emphasizes accountability, communication, and involvement of the patient and family members in plans of care.[4] Yet, interventions to reduce readmissions and improve the quality and safety of care transitions remain only modestly and inconsistently effective.
Successful interventions are those that are combined and coordinated, and shared across the hospital and community settings.[5] In this study, we sought to understand the issues leading to readmissions and barriers as perceived by patients, family members, physicians, nurses, and social workers. We compared and contrasted the perspectives by discipline and used this information to design a descriptive framework of a multidisciplinary, collaborative, and coordinated support network integral to effective care transitions, which we term a Patient Care Circle (PCC) (Figure 1).

METHODS
Study Design
We recruited a purposive sample of general medicine patients with same‐site 30‐day readmissions, and those directly involved in their care, to participate in interviews and focus groups to investigate explanations for unplanned readmissions (Table 1). We sought subjects' perspectives based on extrapolations from previous research that identified multiple stakeholders involved in the care transitions process,[1, 2, 5, 6, 7, 8] and our own professional experience with patient readmissions.
| Role | No. (%) | No. Interviewed | No. in Focus Group | |
|---|---|---|---|---|
| ||||
| Patient | 12 | NA | ||
| Male | 10 (90.9) | |||
| Average age, y, range | 3172 | |||
| Insurance | ||||
| Medicare | 5 (41.7) | |||
| Medicaid | 1 (8.3) | |||
| Medicare/Medicaid | 2 (16.7) | |||
| Private | 4 (33.3) | |||
| Race | ||||
| White | 8 (66.7) | |||
| Black | 2 (16.7) | |||
| Other | 2 (16.7) | |||
| Has PMD | 9 (75.0) | |||
| Has home caregiver (family or aide) | 10 (90.9) | |||
| Physician | ||||
| Hospitalista | 9b | |||
| Index | 10 | |||
| Readmit | 9 | |||
| Primary care physician | 5 | NA | ||
| Other provider | ||||
| RN | ||||
| Inpatient staff | 5 | 7 | ||
| Visiting home | NA | 6 | ||
| Social work | NA | 6 | ||
| Other caregivers | ||||
| Family | 2 | NA | ||
| Home aides | 0 | NA | ||
| Total | 43 | 28 | ||
Site Selection
All interviews and focus groups were conducted at New YorkPresbyterian/Weill Cornell Medical Center (NYP/WC), a large urban academic medical center in New York City serving a racially and socioeconomically diverse population. The institutional review boards at Weill Cornell Medical College and Hunter College approved this study.
Data Collection Tools
We developed semistructured interview and focus group guides (see Supporting Information, Appendixes 17, in the online version of this article) by reviewing published literature[8, 9, 10, 11, 12] and readmission pilot data that identified challenges associated with hospital discharges. Interviews were patient specific, and providers involved directly in their care were asked to consider reasons for the patients' readmissions and whether they could have been prevented. Provider interview guides were modified from the patient interview script and tailored toward their role in the patient's care.
One focus group guide was used for all sessions, allowing us to compare and contrast emerging themes across disciplines. Participants were asked to discuss perceived causes for readmissions and barriers to improvement.
All questions were open‐ended to gain insight into participants' beliefs regarding the causes of readmissions and to limit researcher bias. We iteratively reviewed and modified the guides to ensure the questions were effectively worded.
Recruiting
Using a centralized clinical database, we identified patients aged 18 years and older for interviews, who were readmitted within 30 days to NYP/WC between May 2011 and May 2012, and had an attending hospitalist during the initial and readmission visits. We confirmed patients' English fluency and cognitive ability by contacting their attending physician. Patients provided written consent prior to interview.
For interviews, we asked patients to identify their outpatient physicians and providers; inpatient hospitalists and providers were identified from the patients' charts. For focus groups, we recruited volunteers among all division hospitalists and solicited volunteer inpatient nursing, social work, and homecare nursing participants through organizational liaisons (Table 1).
Data Collection
We interviewed patients in person at their bedside. We interviewed physicians and other caregivers in person or by telephone during the course of the patient's readmission. We conducted 4 discipline‐specific 90‐minute focus groups for hospitalists, inpatient staff nurses, homecare nurses, and hospital social workers. Patient interviews and focus groups were audio‐recorded and transcribed using a professional service.
Data Analysis
We analyzed 47 transcripts (43 interviews, 4 focus groups) during research group meetings using grounded theory[13] to generate overarching themes felt to influence readmissions through iterative reviewing of transcripts. We attributed codes to salient text and documented recurring topics that emerged. Two researchers independently assessed responses from the patient‐specific interviews for variability among the various disciplines. We ended our data collection after we ceased to find new topics from participants (thematic saturation).[14]
Three researchers, in consultation with the larger team, coded the 4 focus group transcripts to generate a codebook with definitions and examples of recurring concepts. They then coded the 43 interview transcripts using the codebook. The entire team met regularly to address questions and potential discrepancies.
We achieved greater trustworthiness of the analysis by using multiple modes of triangulation, a qualitative method that relies on points of comparison and contrast.[15] We achieved methodological triangulation by using both interviews and focus groups, and achieved internal triangulation by having researchers in the clinical, social, and behavioral sciences routinely critique the evolving codebook.
RESULTS
We recruited 43 interview and 28 focus group participants (Table 1). From our transcript analysis, we generated 22 codes and categorized them into 5 themes embodying the issues pertinent to readmissions from the perspective of the stakeholders: (1) teamwork, (2) health systems navigation and management, (3) illness severity and health needs, (4) psychosocial stability; and (5) medications (Table 2).
We applied these codes and themes to build a descriptive framework depicting what we believed is the essential foundation for successful care transitions, a collaborative unified patient‐centered network to address complex healthcare‐related issues across disciplines and across settings (Figure 1). Our model illustrates the interplay between the various physician and care‐provider roles as well as the relationship of the structure of the care circle to each theme.
Care Circle Theme
Teamwork
Comprehensive, effective collaboration and communication among members of the PCC were required for the circle to function successfully and establish safe ongoing patient care across settings. Teamwork required a shared purpose and aligned incentives among all stakeholders to work as a unified patient‐centered network.
Dysfunctional teamwork led to fragmented care. Hospitalists and patients cited difficulties coordinating in‐hospital management plans with multiple consulting subspecialists. Social workers ascribed 1 potential cause for unplanned readmissions to insufficient feedback from homecare agencies regarding patients following hospital discharge:
I wouldn't mind hearing [from the home agencies][the patient] won't let me in the door' patient's doing well' or patient's still not compliant.' If we don't knowthen we can't address it [until] they come back in [to the hospital].
Meanwhile, accurate handoff of information affected the care provided by homecare nurses:
We go into assess [the patient at home] and we see something totally different than what wason a piece of paper.
Patient‐Centered Themes
Four patient‐centered themes were identified that posed challenges in the transitions process and required the support and teamwork of the PCC to deal with effectually.
Health Systems Navigation and Management
The complexities of the healthcare system in the hospital and in the community presented challenges for patients with greater needs. Meeting higher levels of patient care needs was difficult in a system where prioritizing competing responsibilities was a recurrent issue. Inpatient nurses shared:
Educat[ing] people and empower[ing] them about their health. [I]t's kind of lostwhen we have so many [tasks] that we're responsible for, the patient gets lost in all of these things. For patients requiring ongoing sub‐acute care, limited weekend and holiday hospital and skilled nursing facility personnel added to the difficulty of arranging discharges and executing care plans.
Social workers noted:
[S]ometimes people are ready for discharge and there's noprimary care physician [willing to follow them].
Obtaining additional support following discharge was another concern for patients with homecare needs:
With the Medicaid changeshomecare is going to be less [than] what's provided [now]. So they're going into a lesssafe environment. [Social worker]
Illness Severity and Health Needs
The ability to cope with disease and related stressors depended on complexity of illness, level of health literacy, and underlying psychiatric issues overlapping with the theme of psychosocial stability. Early identification and mitigation of potential postdischarge complications required PCC collaboration.
All groups agreed that patients with chronic complex comorbidities often warranted frequent access to the inpatient setting regardless of outpatient medical care:
I'm not surprised [my patient was readmitted] becausealmost anything that goes wrong leads her to the hospital. Her readmission is not avoidable because of the severity of her illness. [Primary care physician]
With patients living longer with terminal illness, several groups voiced concern regarding the frequency of hospitalizations:
People [go] into hospice in the last week of their life as opposed to in the last six months of their life.The doctor has to bring this up [I] can't do it. [Homecare nurse]
Another prevalent issue was the emotional stress that accompanies acute or exacerbations of illness. One patient shared,
I also have a four‐year‐old son. Obviously, I'm not able to care for him as much as I was. My wifehas been diagnosed with leukemia.
Psychosocial Stability
Discharge from the hospital often requires psychosocial adjustment, which may be overlooked, underestimated, or dismissed by patients and providers.
[One patient] was very visually impaired. Lives by himself. But he's youngso he wanted to go home [not] a nursing home. He got home. He got up in the middle of the night. [P]ut the wound vac[uum] on the counter [and it] fell. It broke. It started beeping. He panicked, couldn't get in touch with any of the visiting nurses because it was 2:00 a.m. And he [was readmitted], and now is saying he wants to go to sub‐acute, because he can't handle it at home. [Social worker]
Engaging patients who seemed capable of participating in their own care was often frustrating for providers:
It's depressing because you're trying to help somebody [but] they don't want to help themselves and you know you'll see them right back [in the hospital] again. [Inpatient nurse]
Social support and socioeconomic factors also impacted patients' and families' ability to cope and adjust to the community after discharge. One family member commented that he and his wife have always cared for the patient together but now he cares for her alone and must hire a private duty aide to assist.
Medications
The degree to which obtaining, understanding, and taking medications exists as an impediment to safe transitions was patient specific and dependent on all of the patient‐centered themes above. Recognition and effective intervention required a multitiered, multidisciplinary approach. Homecare nurses reflected:
Discharge planning doesn't ensure that there is someone that can go to the pharmacy to get [medications] until the [visiting] nurse comes in and sets something up.
Methods used for medication education were not always effective in reaching the patient:
I shouldn't really say that they didn't [discuss medication side effects] because I was in a lot of pain. I really don't recall somebody giving me specific [information on] side effects on the medication. [Patient]
DISCUSSION
We categorized our findings into5 principle themes that influence care transitions: teamwork, systems navigation and management, illness severity, and health needs, psychosocial stability, and medications. Many of these themes have been targeted in the literature for interventions to reduce readmissions and improve care transitions. An overarching theme of our study was the importance of the Patient Care Circle, a support system required to implement and execute comprehensive patient‐centered plans for safe and effective transitions across all settings.
Collectively, our themes emphasized that communication and comprehensive planning between all members of the PCC were instrumental to the circle's ability to address issues pertaining to the patient‐centered themes: systems navigation and management, illness severity and health needs, psychosocial stability, and medications. The strength of the bonds and collaboration within the PCC were directly dependent on the success of teamwork.
The interplay between the 4 patient‐centered themes and the degree to which they affect readmissions were variable and patient dependent. Complexities of the healthcare system and issues surrounding medications became more apparent with worsening disease severity and psychosocial instability. Complicated patients requiring more multidisciplinary interaction highlighted limitations of dispersed teams and staffing ratios. Patients faced with insurance restrictions, difficulties attending appointments, and obtaining medications required pooling the efforts of multiple PCC members to help them. Thus, these themes emphasized not only the importance of teamwork required for care coordination, but also guided the membership of the PCC to meet the patient's specific needs across the inpatient and outpatient settings.
When participants were asked to identify modifiable reasons for readmissions, the overwhelming collective response was inadequate communication and collaboration among PCC members. Clear role assignments and delegation of responsibility were also necessary to avoid gaps in care. Significant barriers to improvement included limited resources and inability to maintain the integrity of the support network needed for safe transitions.
Finally, we compared and contrasted the perceptions of the different disciplines on the factors contributing to each patient's readmission. Over all, there was substantial overlap. However, each perspective added additional layers of information allowing for a more comprehensive understanding of the problem. This demonstrated the utility of multidisciplinary patient‐centered interviews to examine readmissions and elucidate areas for intervention.
Several disciplines were not included in interviews or focus groups but were identified by our study participants as integral to a comprehensive Patient Care Circle. These include emergency medicine physicians, inpatient and outpatient pharmacists, and outpatient social workers. Some disciplines were not included due to challenges identifying discrete providers and with arranging interviews or focus groups. As their roles were mentioned several times in multiple forums, we have included them in our descriptive framework.
We designed this study with the hope of completing a full complement of patient‐specific interviews that included all stakeholders for 4 male and 4 female patients. For several reasons, we were unable to do so including challenges contacting providers and family members, and coordinating the timing of interviews with patient visits. Further, our focus on English‐speaking patients admitted to general medicine teams may limit generalizability to other vulnerable patient groups. Nevertheless, we believe we succeeded in interviewing a representative sample and obtained thematic saturation with the information obtained from our interviews and focus groups.
Last, the focus of this project was to obtain the perspectives of a full spectrum of stakeholders in the care transitions process to gain a better understanding of the reasons for readmissions. Although we did ask study participants to identify areas that may have been modifiable, we did not expand the discussion to include potential interventions, which will be the next step in our study.
CONCLUSION
Our article describes 5 main themes derived from the perspectives of multiple stakeholders involved in the care transitions process. An overarching theme was the importance of a multidisciplinary, coordinated collaborative care circle to ensure safe patient‐centered care in all settings.
The results of this study can be used by researchers and applied by care providers to improve the care transitions process. Researchers can build on our model by studying methods and interventions to improve the function of the care circle and design guidelines to create a more effective and integrated network. Institutions can adapt our methodology and tools to identify the needs of their own patient population and optimize membership in the PCC accordingly.
We feel that improving the structure and function of the care circle is necessary prior to designing interventions targeting the patient‐centered themes. Strengthening the teamwork of the PCC is fundamental to improving the quality of care transitions and reducing preventable readmissions.
Acknowledgments
The authors thank the patients, family members, social workers, nurses, and physicians who participated in their study. The authors are grateful to their research assistants for their assistance with conducting interviews, focus groups, and data collection.
Disclosures: This study was supported by the Weill Cornell Clinical and Translational Science Center: UL1 RR024996. Dr. Press is supported in part through funds provided to him as a Nanette Laitman Clinical Scholar in Public Health at the Weill Cornell Medical College. An earlier version of the article was presented as a poster at the Society of Hospital Medicine annual conference in San Diego, California in 2012.
The focus on care transitions and readmissions is expanding beyond the development of risk scores based on objective clinical data to quality improvement interventions involving the key stakeholders in the process, namely the patients and their multidisciplinary providers.[1, 2] The Institute for Healthcare Improvement's State Action on Avoidable Rehospitalizations initiative promotes formulating a specific transition plan and developing multidisciplinary management strategies for all patients.[3] The Transition of Care Consensus Policy Statement developed by a coalition including the American College of Physicians and Society of Hospital Medicine emphasizes accountability, communication, and involvement of the patient and family members in plans of care.[4] Yet, interventions to reduce readmissions and improve the quality and safety of care transitions remain only modestly and inconsistently effective.
Successful interventions are those that are combined and coordinated, and shared across the hospital and community settings.[5] In this study, we sought to understand the issues leading to readmissions and barriers as perceived by patients, family members, physicians, nurses, and social workers. We compared and contrasted the perspectives by discipline and used this information to design a descriptive framework of a multidisciplinary, collaborative, and coordinated support network integral to effective care transitions, which we term a Patient Care Circle (PCC) (Figure 1).

METHODS
Study Design
We recruited a purposive sample of general medicine patients with same‐site 30‐day readmissions, and those directly involved in their care, to participate in interviews and focus groups to investigate explanations for unplanned readmissions (Table 1). We sought subjects' perspectives based on extrapolations from previous research that identified multiple stakeholders involved in the care transitions process,[1, 2, 5, 6, 7, 8] and our own professional experience with patient readmissions.
| Role | No. (%) | No. Interviewed | No. in Focus Group | |
|---|---|---|---|---|
| ||||
| Patient | 12 | NA | ||
| Male | 10 (90.9) | |||
| Average age, y, range | 3172 | |||
| Insurance | ||||
| Medicare | 5 (41.7) | |||
| Medicaid | 1 (8.3) | |||
| Medicare/Medicaid | 2 (16.7) | |||
| Private | 4 (33.3) | |||
| Race | ||||
| White | 8 (66.7) | |||
| Black | 2 (16.7) | |||
| Other | 2 (16.7) | |||
| Has PMD | 9 (75.0) | |||
| Has home caregiver (family or aide) | 10 (90.9) | |||
| Physician | ||||
| Hospitalista | 9b | |||
| Index | 10 | |||
| Readmit | 9 | |||
| Primary care physician | 5 | NA | ||
| Other provider | ||||
| RN | ||||
| Inpatient staff | 5 | 7 | ||
| Visiting home | NA | 6 | ||
| Social work | NA | 6 | ||
| Other caregivers | ||||
| Family | 2 | NA | ||
| Home aides | 0 | NA | ||
| Total | 43 | 28 | ||
Site Selection
All interviews and focus groups were conducted at New YorkPresbyterian/Weill Cornell Medical Center (NYP/WC), a large urban academic medical center in New York City serving a racially and socioeconomically diverse population. The institutional review boards at Weill Cornell Medical College and Hunter College approved this study.
Data Collection Tools
We developed semistructured interview and focus group guides (see Supporting Information, Appendixes 17, in the online version of this article) by reviewing published literature[8, 9, 10, 11, 12] and readmission pilot data that identified challenges associated with hospital discharges. Interviews were patient specific, and providers involved directly in their care were asked to consider reasons for the patients' readmissions and whether they could have been prevented. Provider interview guides were modified from the patient interview script and tailored toward their role in the patient's care.
One focus group guide was used for all sessions, allowing us to compare and contrast emerging themes across disciplines. Participants were asked to discuss perceived causes for readmissions and barriers to improvement.
All questions were open‐ended to gain insight into participants' beliefs regarding the causes of readmissions and to limit researcher bias. We iteratively reviewed and modified the guides to ensure the questions were effectively worded.
Recruiting
Using a centralized clinical database, we identified patients aged 18 years and older for interviews, who were readmitted within 30 days to NYP/WC between May 2011 and May 2012, and had an attending hospitalist during the initial and readmission visits. We confirmed patients' English fluency and cognitive ability by contacting their attending physician. Patients provided written consent prior to interview.
For interviews, we asked patients to identify their outpatient physicians and providers; inpatient hospitalists and providers were identified from the patients' charts. For focus groups, we recruited volunteers among all division hospitalists and solicited volunteer inpatient nursing, social work, and homecare nursing participants through organizational liaisons (Table 1).
Data Collection
We interviewed patients in person at their bedside. We interviewed physicians and other caregivers in person or by telephone during the course of the patient's readmission. We conducted 4 discipline‐specific 90‐minute focus groups for hospitalists, inpatient staff nurses, homecare nurses, and hospital social workers. Patient interviews and focus groups were audio‐recorded and transcribed using a professional service.
Data Analysis
We analyzed 47 transcripts (43 interviews, 4 focus groups) during research group meetings using grounded theory[13] to generate overarching themes felt to influence readmissions through iterative reviewing of transcripts. We attributed codes to salient text and documented recurring topics that emerged. Two researchers independently assessed responses from the patient‐specific interviews for variability among the various disciplines. We ended our data collection after we ceased to find new topics from participants (thematic saturation).[14]
Three researchers, in consultation with the larger team, coded the 4 focus group transcripts to generate a codebook with definitions and examples of recurring concepts. They then coded the 43 interview transcripts using the codebook. The entire team met regularly to address questions and potential discrepancies.
We achieved greater trustworthiness of the analysis by using multiple modes of triangulation, a qualitative method that relies on points of comparison and contrast.[15] We achieved methodological triangulation by using both interviews and focus groups, and achieved internal triangulation by having researchers in the clinical, social, and behavioral sciences routinely critique the evolving codebook.
RESULTS
We recruited 43 interview and 28 focus group participants (Table 1). From our transcript analysis, we generated 22 codes and categorized them into 5 themes embodying the issues pertinent to readmissions from the perspective of the stakeholders: (1) teamwork, (2) health systems navigation and management, (3) illness severity and health needs, (4) psychosocial stability; and (5) medications (Table 2).
We applied these codes and themes to build a descriptive framework depicting what we believed is the essential foundation for successful care transitions, a collaborative unified patient‐centered network to address complex healthcare‐related issues across disciplines and across settings (Figure 1). Our model illustrates the interplay between the various physician and care‐provider roles as well as the relationship of the structure of the care circle to each theme.
Care Circle Theme
Teamwork
Comprehensive, effective collaboration and communication among members of the PCC were required for the circle to function successfully and establish safe ongoing patient care across settings. Teamwork required a shared purpose and aligned incentives among all stakeholders to work as a unified patient‐centered network.
Dysfunctional teamwork led to fragmented care. Hospitalists and patients cited difficulties coordinating in‐hospital management plans with multiple consulting subspecialists. Social workers ascribed 1 potential cause for unplanned readmissions to insufficient feedback from homecare agencies regarding patients following hospital discharge:
I wouldn't mind hearing [from the home agencies][the patient] won't let me in the door' patient's doing well' or patient's still not compliant.' If we don't knowthen we can't address it [until] they come back in [to the hospital].
Meanwhile, accurate handoff of information affected the care provided by homecare nurses:
We go into assess [the patient at home] and we see something totally different than what wason a piece of paper.
Patient‐Centered Themes
Four patient‐centered themes were identified that posed challenges in the transitions process and required the support and teamwork of the PCC to deal with effectually.
Health Systems Navigation and Management
The complexities of the healthcare system in the hospital and in the community presented challenges for patients with greater needs. Meeting higher levels of patient care needs was difficult in a system where prioritizing competing responsibilities was a recurrent issue. Inpatient nurses shared:
Educat[ing] people and empower[ing] them about their health. [I]t's kind of lostwhen we have so many [tasks] that we're responsible for, the patient gets lost in all of these things. For patients requiring ongoing sub‐acute care, limited weekend and holiday hospital and skilled nursing facility personnel added to the difficulty of arranging discharges and executing care plans.
Social workers noted:
[S]ometimes people are ready for discharge and there's noprimary care physician [willing to follow them].
Obtaining additional support following discharge was another concern for patients with homecare needs:
With the Medicaid changeshomecare is going to be less [than] what's provided [now]. So they're going into a lesssafe environment. [Social worker]
Illness Severity and Health Needs
The ability to cope with disease and related stressors depended on complexity of illness, level of health literacy, and underlying psychiatric issues overlapping with the theme of psychosocial stability. Early identification and mitigation of potential postdischarge complications required PCC collaboration.
All groups agreed that patients with chronic complex comorbidities often warranted frequent access to the inpatient setting regardless of outpatient medical care:
I'm not surprised [my patient was readmitted] becausealmost anything that goes wrong leads her to the hospital. Her readmission is not avoidable because of the severity of her illness. [Primary care physician]
With patients living longer with terminal illness, several groups voiced concern regarding the frequency of hospitalizations:
People [go] into hospice in the last week of their life as opposed to in the last six months of their life.The doctor has to bring this up [I] can't do it. [Homecare nurse]
Another prevalent issue was the emotional stress that accompanies acute or exacerbations of illness. One patient shared,
I also have a four‐year‐old son. Obviously, I'm not able to care for him as much as I was. My wifehas been diagnosed with leukemia.
Psychosocial Stability
Discharge from the hospital often requires psychosocial adjustment, which may be overlooked, underestimated, or dismissed by patients and providers.
[One patient] was very visually impaired. Lives by himself. But he's youngso he wanted to go home [not] a nursing home. He got home. He got up in the middle of the night. [P]ut the wound vac[uum] on the counter [and it] fell. It broke. It started beeping. He panicked, couldn't get in touch with any of the visiting nurses because it was 2:00 a.m. And he [was readmitted], and now is saying he wants to go to sub‐acute, because he can't handle it at home. [Social worker]
Engaging patients who seemed capable of participating in their own care was often frustrating for providers:
It's depressing because you're trying to help somebody [but] they don't want to help themselves and you know you'll see them right back [in the hospital] again. [Inpatient nurse]
Social support and socioeconomic factors also impacted patients' and families' ability to cope and adjust to the community after discharge. One family member commented that he and his wife have always cared for the patient together but now he cares for her alone and must hire a private duty aide to assist.
Medications
The degree to which obtaining, understanding, and taking medications exists as an impediment to safe transitions was patient specific and dependent on all of the patient‐centered themes above. Recognition and effective intervention required a multitiered, multidisciplinary approach. Homecare nurses reflected:
Discharge planning doesn't ensure that there is someone that can go to the pharmacy to get [medications] until the [visiting] nurse comes in and sets something up.
Methods used for medication education were not always effective in reaching the patient:
I shouldn't really say that they didn't [discuss medication side effects] because I was in a lot of pain. I really don't recall somebody giving me specific [information on] side effects on the medication. [Patient]
DISCUSSION
We categorized our findings into5 principle themes that influence care transitions: teamwork, systems navigation and management, illness severity, and health needs, psychosocial stability, and medications. Many of these themes have been targeted in the literature for interventions to reduce readmissions and improve care transitions. An overarching theme of our study was the importance of the Patient Care Circle, a support system required to implement and execute comprehensive patient‐centered plans for safe and effective transitions across all settings.
Collectively, our themes emphasized that communication and comprehensive planning between all members of the PCC were instrumental to the circle's ability to address issues pertaining to the patient‐centered themes: systems navigation and management, illness severity and health needs, psychosocial stability, and medications. The strength of the bonds and collaboration within the PCC were directly dependent on the success of teamwork.
The interplay between the 4 patient‐centered themes and the degree to which they affect readmissions were variable and patient dependent. Complexities of the healthcare system and issues surrounding medications became more apparent with worsening disease severity and psychosocial instability. Complicated patients requiring more multidisciplinary interaction highlighted limitations of dispersed teams and staffing ratios. Patients faced with insurance restrictions, difficulties attending appointments, and obtaining medications required pooling the efforts of multiple PCC members to help them. Thus, these themes emphasized not only the importance of teamwork required for care coordination, but also guided the membership of the PCC to meet the patient's specific needs across the inpatient and outpatient settings.
When participants were asked to identify modifiable reasons for readmissions, the overwhelming collective response was inadequate communication and collaboration among PCC members. Clear role assignments and delegation of responsibility were also necessary to avoid gaps in care. Significant barriers to improvement included limited resources and inability to maintain the integrity of the support network needed for safe transitions.
Finally, we compared and contrasted the perceptions of the different disciplines on the factors contributing to each patient's readmission. Over all, there was substantial overlap. However, each perspective added additional layers of information allowing for a more comprehensive understanding of the problem. This demonstrated the utility of multidisciplinary patient‐centered interviews to examine readmissions and elucidate areas for intervention.
Several disciplines were not included in interviews or focus groups but were identified by our study participants as integral to a comprehensive Patient Care Circle. These include emergency medicine physicians, inpatient and outpatient pharmacists, and outpatient social workers. Some disciplines were not included due to challenges identifying discrete providers and with arranging interviews or focus groups. As their roles were mentioned several times in multiple forums, we have included them in our descriptive framework.
We designed this study with the hope of completing a full complement of patient‐specific interviews that included all stakeholders for 4 male and 4 female patients. For several reasons, we were unable to do so including challenges contacting providers and family members, and coordinating the timing of interviews with patient visits. Further, our focus on English‐speaking patients admitted to general medicine teams may limit generalizability to other vulnerable patient groups. Nevertheless, we believe we succeeded in interviewing a representative sample and obtained thematic saturation with the information obtained from our interviews and focus groups.
Last, the focus of this project was to obtain the perspectives of a full spectrum of stakeholders in the care transitions process to gain a better understanding of the reasons for readmissions. Although we did ask study participants to identify areas that may have been modifiable, we did not expand the discussion to include potential interventions, which will be the next step in our study.
CONCLUSION
Our article describes 5 main themes derived from the perspectives of multiple stakeholders involved in the care transitions process. An overarching theme was the importance of a multidisciplinary, coordinated collaborative care circle to ensure safe patient‐centered care in all settings.
The results of this study can be used by researchers and applied by care providers to improve the care transitions process. Researchers can build on our model by studying methods and interventions to improve the function of the care circle and design guidelines to create a more effective and integrated network. Institutions can adapt our methodology and tools to identify the needs of their own patient population and optimize membership in the PCC accordingly.
We feel that improving the structure and function of the care circle is necessary prior to designing interventions targeting the patient‐centered themes. Strengthening the teamwork of the PCC is fundamental to improving the quality of care transitions and reducing preventable readmissions.
Acknowledgments
The authors thank the patients, family members, social workers, nurses, and physicians who participated in their study. The authors are grateful to their research assistants for their assistance with conducting interviews, focus groups, and data collection.
Disclosures: This study was supported by the Weill Cornell Clinical and Translational Science Center: UL1 RR024996. Dr. Press is supported in part through funds provided to him as a Nanette Laitman Clinical Scholar in Public Health at the Weill Cornell Medical College. An earlier version of the article was presented as a poster at the Society of Hospital Medicine annual conference in San Diego, California in 2012.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50:599–605.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- Institute for Healthcare Improvement.State Action on Avoidable Rehospitalizations (STAAR) initiative. Available at: http://www.ihi.org/offerings/Initiatives/STAAR/Pages/Improvement.aspx. Accessed January 28, 2013.
- , , , et al. Transition of Care Consensus Policy Statement, American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatric Society–American College of Emergency Physicians–Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2008;2:314–323.
- , , , , . How‐to guide: improving transitions from the hospital to community settings to reduce avoidable rehospitalizations. Cambridge, MA: Institute for Healthcare Improvement; June 2012. Available at: www.IHI.org. Accessed December 31, 2012.
- BOOSTing care transitions. Philadelphia, PA: Society of Hospital Medicine; 2008. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. Accessed October 20, 2012.
- , , . Predictive validity of a questionnaire that identifies older persons at risk for hospital admission. J Am Geriatr Soc. 1995;43(4):374–377.
- . The care transitions program: healthcare services for improving quality and safety during care hand‐offs. Denver, CO: Care Transitions Program; 2007. Available at: http://www.caretransitions.org. Accessed October 22, 2012.
- , , , , . Did I do as best as the system would let me? Healthcare professional views on hospital to home care transitions. J Gen Intern Med. 2012;27(12):1649–1656.
- , . The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967.
- . The significance of saturation. Qual Health Res. 1995;5(2):147–149.
- . Understanding reliability and validity in qualitative research. Qual Rep. 2003;8(4):597–607.
- , , , et al. Factors contributing to all‐cause 30‐day readmissions: a structured case series across 18 hospitals. Med Care. 2012;50:599–605.
- , , , , , . Perceptions of readmitted patients on the transition from hospital to home. J Hosp Med. 2012;7(9):709–712.
- Institute for Healthcare Improvement.State Action on Avoidable Rehospitalizations (STAAR) initiative. Available at: http://www.ihi.org/offerings/Initiatives/STAAR/Pages/Improvement.aspx. Accessed January 28, 2013.
- , , , et al. Transition of Care Consensus Policy Statement, American College of Physicians–Society of General Internal Medicine–Society of Hospital Medicine–American Geriatric Society–American College of Emergency Physicians–Society of Academic Emergency Medicine. J Gen Intern Med. 2009;24(8):971–976.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155:520–528.
- , , , . Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2008;2:314–323.
- , , , , . How‐to guide: improving transitions from the hospital to community settings to reduce avoidable rehospitalizations. Cambridge, MA: Institute for Healthcare Improvement; June 2012. Available at: www.IHI.org. Accessed December 31, 2012.
- BOOSTing care transitions. Philadelphia, PA: Society of Hospital Medicine; 2008. Available at: http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. Accessed October 20, 2012.
- , , . Predictive validity of a questionnaire that identifies older persons at risk for hospital admission. J Am Geriatr Soc. 1995;43(4):374–377.
- . The care transitions program: healthcare services for improving quality and safety during care hand‐offs. Denver, CO: Care Transitions Program; 2007. Available at: http://www.caretransitions.org. Accessed October 22, 2012.
- , , , , . Did I do as best as the system would let me? Healthcare professional views on hospital to home care transitions. J Gen Intern Med. 2012;27(12):1649–1656.
- , . The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine; 1967.
- . The significance of saturation. Qual Health Res. 1995;5(2):147–149.
- . Understanding reliability and validity in qualitative research. Qual Rep. 2003;8(4):597–607.
© 2013 Society of Hospital Medicine
Accuracy of Hospitalist‐Performed HCUE
Hand‐carried ultrasound echocardiography (HCUE) can help noncardiologists answer well‐defined questions at patients' bedsides in less than 10 minutes.1, 2 Indeed, intensivists3 and emergency department physicians4 already use HCUE to make rapid, point‐of‐care assessments. Since cardiovascular diagnoses are common among general medicine inpatients, HCUE may become an important skill for hospitalists to learn.5
However, uncertainty exists about the duration of HCUE training for hospitalists. In 2002, experts from the American Society of Echocardiography (ASE) published recommendations on training requirements for HCUE.6 With limited data on the safety or performance of HCUE training programs, which had just begun to emerge, the ASE borrowed from the proven training recommendations for standard echocardiography (SE). They recommended that all HCUE trainees, cardiologist and noncardiologist alike, complete level 1 SE training: 75 personally‐performed and 150 personally‐interpreted echocardiographic examinations. Since then, however, several HCUE training programs designed for noncardiologists have emerged.2, 5, 710 These alternative programs suggest that the ASE's recommended duration of training may be too long, particularly for focused HCUE that is limited to a few relatively simple assessments. It is important not to overshoot the requirements of HCUE training, because doing so may discourage groups of noncardiologists, like hospitalists, who may derive great benefits from HCUE.11
To address this uncertainty for hospitalists, we first developed a brief HCUE training program to assess 6 important cardiac abnormalities. We then studied the diagnostic accuracy of HCUE by hospitalists as a test of these 6 cardiac abnormalities assessed by SE.
Patients and Methods
Setting and Subjects
This prospective cohort study was performed at Stroger Hospital of Cook County, a 500‐bed public teaching hospital in Chicago, IL, from March through May of 2007. The cohort was adult inpatients who were referred for SE on weekdays from 3 distinct patient care units (Figure 1). We used 2 sampling modes to balance practical constraints (short‐stay unit [SSU] patients were more localized and, therefore, easier to study) with clinical diversity. We consecutively sampled patients from our SSU, where adults with provisional cardiovascular diagnoses are admitted if they might be eligible for discharge with in 3 days.12 But we used random number tables with a daily unique starting point to randomly sample patients from the general medical wards and the coronary care unit (CCU). Patients were excluded if repositioning them for HCUE was potentially harmful. The study was approved by our hospital's institutional review board, and we obtained written informed consent from all enrolled patients.
SE Protocol
As part of enrolled patients' routine clinical care, SE images were acquired and interpreted in the usual fashion in our hospital's echocardiography laboratory, which performs SE on over 7,000 patients per year. Echocardiographic technicians acquired images with a General Electric Vivid 7 cardiac ultrasound machine (General Electric, Milwaukee, WI) equipped with a GE M4S 1.8 to 3.4 MHz cardiac transducer (General Electric). Technicians followed the standard adult transthoracic echocardiography scanning protocol to acquire 40 to 100 images on every patient using all available echocardiographic modalities: 2‐dimensional, M‐mode, color Doppler, continuous‐wave Doppler, pulse‐wave Doppler, and tissue Doppler.13 Blinded to HCUE results, attending physician cardiologist echocardiographers then interpreted archived images using computer software (Centricity System; General Electric) to generate final reports that were entered into patients' medical records. This software ensured that final reports were standardized, because echocardiographers' final qualitative assessments were limited to short lists of standard options; for example, in reporting left atrium (LA) size, echocardiographers chose from only 5 standard options: normal, mildly dilated, moderately dilated, severely dilated, and not interpretable. Investigators, who were also blinded to HCUE results, later abstracted SE results from these standardized report forms in patients' medical records. All echocardiographers fulfilled ASE training guidelines to independently interpret SE: a minimum of 150 personally‐performed and 300 personally‐interpreted echocardiographic examinations (training level 2).14
HCUE Training
Based on the recommendations of our cardiologist investigator (B.M.), we developed a training program for 1 hospitalist to become an HCUE instructor. Our instructor trainee (C.C.) was board‐eligible in internal medicine but had no previous formal training in cardiology or echocardiography. We a priori established that her training would continue until our cardiologist investigator determined that she was ready to train other hospitalists; this determination occurred after 5 weeks. She learned image acquisition by performing focused SE on 30 patients under the direct supervision of an echocardiographic technician. She also performed focused HCUE on 65 inpatients without direct supervision but with ongoing access to consult the technician to review archived images and troubleshoot difficulties with acquisition. She learned image interpretation by reading relevant chapters from a SE textbook15 and by participating in daily didactic sessions in which attending cardiologist echocardiographers train cardiology fellows in SE interpretation.
This hospitalist then served as the HCUE instructor for 8 other attending physician hospitalists who were board‐certified internists with no previous formal training in cardiology or echocardiography. The training program was limited to acquisition and interpretation of 2‐dimensional grayscale and color Doppler images for the 6 cardiac assessments under study (Table 1). The instructor marshaled pairs of hospitalists through the 3 components of the training program, which lasted a total of 27 hours.
|
| Six cardiac assessments learned using 2‐dimensional gray scale and color Doppler imaging |
| Left ventricular systolic dysfunction |
| Mitral valve regurgitation |
| Left atrium enlargement |
| Left ventricular hypertrophy |
| Pericardial effusion |
| Inferior vena cava diameter |
| Lecture (2 hours)* |
| Basic principles of echocardiography |
| HCUE scanning protocol and helpful techniques to optimize image quality |
| Hands‐on training with instructor |
| Orientation to machine and demonstration of scanning protocol (1 hour) |
| Sessions 1 through 3: HCUE performed on 1 patient per hour (6 patients in 6 hours) |
| Sessions 4 through 10: HCUE performed on 2 patients per hour (28 patients in 14 hours) |
| Feedback sessions on image quality and interpretation with cardiologist |
| After hands‐on training session 3 (2 hours) |
| After hands‐on training session 10 (2 hours) |
First, hospitalists attended a 2‐hour lecture on the basic principles of HCUE. Slides from this lecture and additional images of normal and abnormal findings were provided to each hospitalist on a digital video disc. Second, each hospitalist underwent 20 hours of hands‐on training in 2‐hour sessions scheduled over 2 weeks. Willing inpatients from our hospital's emergency department were used as volunteers for these hand‐on training sessions. During these sessions the instructor provided practical suggestions to optimize image quality, such as transducer location and patient positioning. In the first 3 sessions, the minimum pace was 1 patient per hour; thereafter, the pace was increased to 1 patient per half‐hour. We chose 20 hours of hands‐on training and these minimum paces because they allowed each hospitalist to attain a cumulative experience of no less than 30 patientsan amount that heralds a flattening of the HCUE learning curve among medical trainees.9 Third, each pair of hospitalists received feedback from a cardiologist investigator (B.M.) who critiqued the quality and interpretation of images acquired by hospitalists during hands‐on training sessions. Since image quality varies by patient,16 hospitalists' images were compared side‐by‐side to images recorded by the instructor on the same patients. The cardiologist also critiqued hospitalists' interpretations of both their own images and additional sets of archived images from patients with abnormal findings.
HCUE Protocol
After completing the training program and blinded to the results of SE, the 8 hospitalists performed HCUE on enrolled patients within hours of SE. We limited the time interval between tests to minimize the effect that changes in physiologic variables, such as blood pressure and intravascular volume, have on the reliability of serial echocardiographic measurements.16 Hospitalists performed HCUE with a MicroMaxx 3.4 hand‐carried ultrasound machine equipped with a cardiology software package and a 1 to 5 MHz P17 cardiac transducer (Sonosite, Inc., Bothell, WA); simultaneous electrocardiographic recording, though available, was not used. While patients laid on their own standard hospital beds or on a standard hospital gurney in a room adjacent to the SE waiting room, hospitalists positioned them without assistance from nursing staff and recorded 7 best‐quality images per patient. Patients were first positioned in a partial (3045 degrees) left lateral decubitus position to record 4 grayscale images of the short‐axis and long‐axis parasternal and 2‐chamber and 4‐chamber apical views; 2 color Doppler images of the mitral inflow were also recorded from the long‐axis parasternal and the 4‐chamber apical views. Patients were then positioned supine to record 1 grayscale image of the inferior vena cava (IVC) from the transhepatic view. Hospitalists did not perform a history or physical exam on enrolled patients, nor did they review patients' medical records.
Immediately following the HCUE, hospitalists replayed the recorded images as often as needed and entered final interpretations on data collection forms. Linear measurements were made manually with a caliper held directly to the hand‐carried ultrasound monitor. These measurements were then translated into qualitative assessments based on standard values used by our hospital's echocardiographers (Table 2).17 When a hospitalist could not confidently assess a cardiac abnormality, the final HCUE assessment was recorded as indeterminate. Hospitalists also recorded the time to perform each HCUE, which included the time to record 7 best‐quality images, to interpret the findings, and to fill out the data collection form.
| Hand‐Carried Ultrasound Echocardiography Results | |||||
|---|---|---|---|---|---|
| Cardiac Abnormality by Standard Echocardiography | Hand‐Carried Ultrasound Echocardiography Operator's Method of Assessment | Positive | Negative | ||
| |||||
| Left ventricle systolic dysfunction, mild or greater | Grade degree of abnormal wall movement and thickening during systole | Severe | Mild or moderate | Normal | Vigorous |
| Mitral valve regurgitation, severe | Classify regurgitant jet as central or eccentric, then measure as percentage of left atrium area | ||||
| Central jet | 20% | <20% | |||
| Eccentric jet | 20% | indeterminate 20% | |||
| Left atrium enlargement, moderate or severe | Measure left atrium in 3 dimensions at end diastole, then use the most abnormal dimension | Extreme | Borderline | ||
| Anteroposterior or mediolateral (cm) | 5.1 | 4.55.0 | 4.4 | ||
| Superior‐inferior (cm) | 7.1 | 6.17.0 | 6.0 | ||
| Left ventricle hypertrophy, moderate or severe | Measure thickest dimension of posterior or septal wall at end diastole | Extreme: 1.4 cm | Borderline: 1.21.3 cm | 1.1 cm | |
| Pericardial effusion, medium or large | Measure largest dimension in any view at end diastole | 1 cm | <1 cm | ||
| Inferior vena cava dilatation | Measure largest respirophasic diameter within 2 cm of right atrium | 2.1 cm | Normal: 1 to 2 cm | Contracted: 0.9 cm | |
Data Analysis
We based our sample size calculations on earlier reports of HCUE by noncardiologist trainees for assessment of left ventricular (LV) systolic function.7, 10 From these reports, we estimated a negative likelihood ratio of 0.3. In addition, we expected about a quarter of our patients to have LV systolic dysfunction (B.M., personal communication). Therefore, to achieve 95% confidence intervals (CIs) around the point estimate of a negative likelihood ratio that excluded 0.50, our upper bound for a clinically meaningful result, we needed a sample size of approximately 300 patients.18
We defined threshold levels of ordinal severity for the 6 cardiac abnormalities under study based on their clinical pertinence to hospitalists (Table 2). Here, we reasoned that abnormalities at or above these levels would likely lead to important changes in hospitalists' management of inpatients; abnormalities below these levels rarely represent cardiac disease that is worthy of an immediate change in management. Since even mild degrees of LV dysfunction have important diagnostic and therapeutic implications for most general medicine inpatients, particularly those presenting with heart failure,19 we set our threshold for LV dysfunction at mild or greater. In contrast, since neither mild nor moderate mitral regurgitation (MR) has immediate implications for medical or surgical therapy even if symptoms or LV dysfunction are present,20 we set our threshold for MR at severe. Similarly, though mild LA enlargement21 and mild LV hypertrophy22 have clear prognostic implications for patients' chronic medical conditions, we reasoned that only moderate or severe versions likely reflect underlying abnormalities that affect hospitalists' point‐of‐care decision‐making. Since cardiac tamponade is rarely both subclinical23 and due to a small pericardial effusion,24 we set our threshold for pericardial effusion size at moderate or large. Finally, we set our threshold IVC diameter, a marker of central venous volume status,25 at dilated, because volume overload is an important consideration in hospitalized cardiac patients.
Using these thresholds, investigators dichotomized echocardiographers' SE readings as normal or abnormal for each of the 6 cardiac abnormalities under study to serve as the reference standards. Hospitalists' HCUE results were then compared to the reference standards in 2 different ways. We first analyzed HCUE results as dichotomous values to calculate conventional sensitivity, specificity, and positive and negative likelihood ratios. Here we considered indeterminate HCUE results positive in a clinically conservative tradeoff that neither ignores indeterminate results nor risks falsely classifying them as negative.26 We then analyzed hospitalists' HCUE results as ordinal values for receiver operating characteristic (ROC) curve analysis. Here we considered an indeterminate result as 1 possible test result.27
To examine interobserver variability of HCUE, we first chose from the 6 possible assessments only those with a mean number of abnormal patients per hospitalist greater than 5. We reasoned that variability among assessments with lower prevalence would be predictably wide and inconclusive. We then expressed variability as standard deviations (SDs) around mean sensitivity and specificity for the 8 hospitalists.
The CIs for likelihood ratios were constructed using the likelihood‐based approach to binomial proportions of Koopman.28 The areas under ROC curves were computed using the trapezoidal rule, and the CIs for these areas were constructed using the algorithm described by DeLong et al.29 All analyses were conducted with Stata Statistical Software, Release 10 (StataCorp, College Station, TX).
Results
During the 3 month study period, 654 patients were referred for SE from the 3 participating patient care units (Figure 1). Among these, 65 patients were ineligible because their SE was performed on the weekend and 178 other patients were not randomized from the general medical wards and CCU. From the remaining eligible patients, 322 underwent HCUE and 314 (98% of 322) underwent both SE and HCUE. Individual SE assessments were not interpretable (and therefore excluded) due to poor image quality for LA enlargement in 1 patient and IVC dilatation in 30 patients. Eighty‐three percent of patients who underwent SE (260/314) were referred to assess LV function (Table 3). The prevalence of the 6 clinically pertinent cardiac abnormalities under study ranged from 1% for moderate or large pericardial effusion to 25% for LV systolic dysfunction. Overall, 40% of patients had at least 1 out of 6 cardiac abnormalities.
| Characteristic | |
|---|---|
| |
| Age, year SD (25th to 75th percentiles) | 56 13 (48 to 64) |
| Women | 146 (47) |
| Chronic obstructive pulmonary disease | 47 (15) |
| Body mass index | |
| 24.9 or less: underweight or normal | 74 (24) |
| 25 to 29.9: overweight | 94 (30) |
| 30 to 34.9: mild obesity | 75 (24) |
| 35 or greater: moderate or severe obesity | 71 (23) |
| Patient care unit | |
| Short‐stay unit | 175 (56) |
| General medical wards | 89 (28) |
| Cardiac care unit | 50 (16) |
| Indication for standard echocardiography* | |
| Left ventricular function | 260 (83) |
| Valvular function | 56 (18) |
| Wall motion abnormality | 29 (9) |
| Valvular vegetations | 22 (7) |
| Any structural heart disease | 20 (6) |
| Right ventricular function | 18 (6) |
| Other | 38 (12) |
| Standard echocardiography findings | |
| Left ventricular systolic dysfunction mild | 80 (25) |
| Inferior vena cava dilated | 45 (14) |
| Left ventricular wall thickness moderate | 33 (11) |
| Left atrium enlargement moderate | 19 (6) |
| Mitral valve regurgitation severe | 11 (4) |
| Pericardial effusion moderate | 3 (1) |
| At least 1 of the above findings | 127 (40) |
| Time difference between HCUE and standard echocardiogram, median hours (25th to 75th percentiles) | 2.8 (1.4 to 5.1) |
| Time to complete HCUE, median minutes (25th to 75th percentiles) | 28 (20 to 35) |
Each hospitalist performed a similar total number of HCUE examinations (range, 3447). The median time difference between performance of SE and HCUE was 2.8 hours (25th75th percentiles, 1.45.1). Despite the high prevalence of chronic obstructive pulmonary disease and obesity, hospitalists considered HCUE assessments indeterminate in only 2% to 6% of the 6 assessments made for each patient (Table 4). Among the 38 patients (12% of 322) with any indeterminate HCUE assessment, 24 patients had only 1 out of 6 possible. Hospitalists completed HCUE in a median time of 28 minutes (25th‐75th percentiles, 2035), which included the time to record 7 best‐quality moving images and to fill out the research data collection form.
| n (%)* | |
|---|---|
| |
| Number of indeterminate findings per patient | |
| 0 | 284 (88) |
| 1 | 24 (7) |
| 2 | 4 (1) |
| 3 or more | 10 (3) |
| Indeterminate findings by cardiac assessment | |
| Mitral valve regurgitation | 18 (6) |
| Inferior vena cava diameter | 16 (5) |
| Left ventricular hypertrophy | 15 (5) |
| Pericardial effusion | 9 (3) |
| Left atrium size | 5 (2) |
| Left ventricle systolic function | 5 (2) |
When HCUE results were analyzed as dichotomous values, positive likelihood ratios ranged from 2.5 to 21, and negative likelihood ratios ranged from 0 to 0.4 (Table 5). Positive and negative likelihood ratios were both sufficiency high and low to respectively increase and decrease by 5‐fold the prior odds of 3 out of 6 cardiac abnormalities: LV systolic dysfunction, moderate or severe MR regurgitation, and moderate or large pericardial effusion. Considering HCUE results as ordinal values for ROC analysis yielded additional diagnostic information (Figure 2). For example, the likelihood ratio of 1.0 (95% CI, 0.42.0) for borderline positive moderate or severe LA enlargement increased to 29 (range, 1362) for extreme positive results. Areas under the ROC curves were 0.9 for 4 out of 6 cardiac abnormalities.
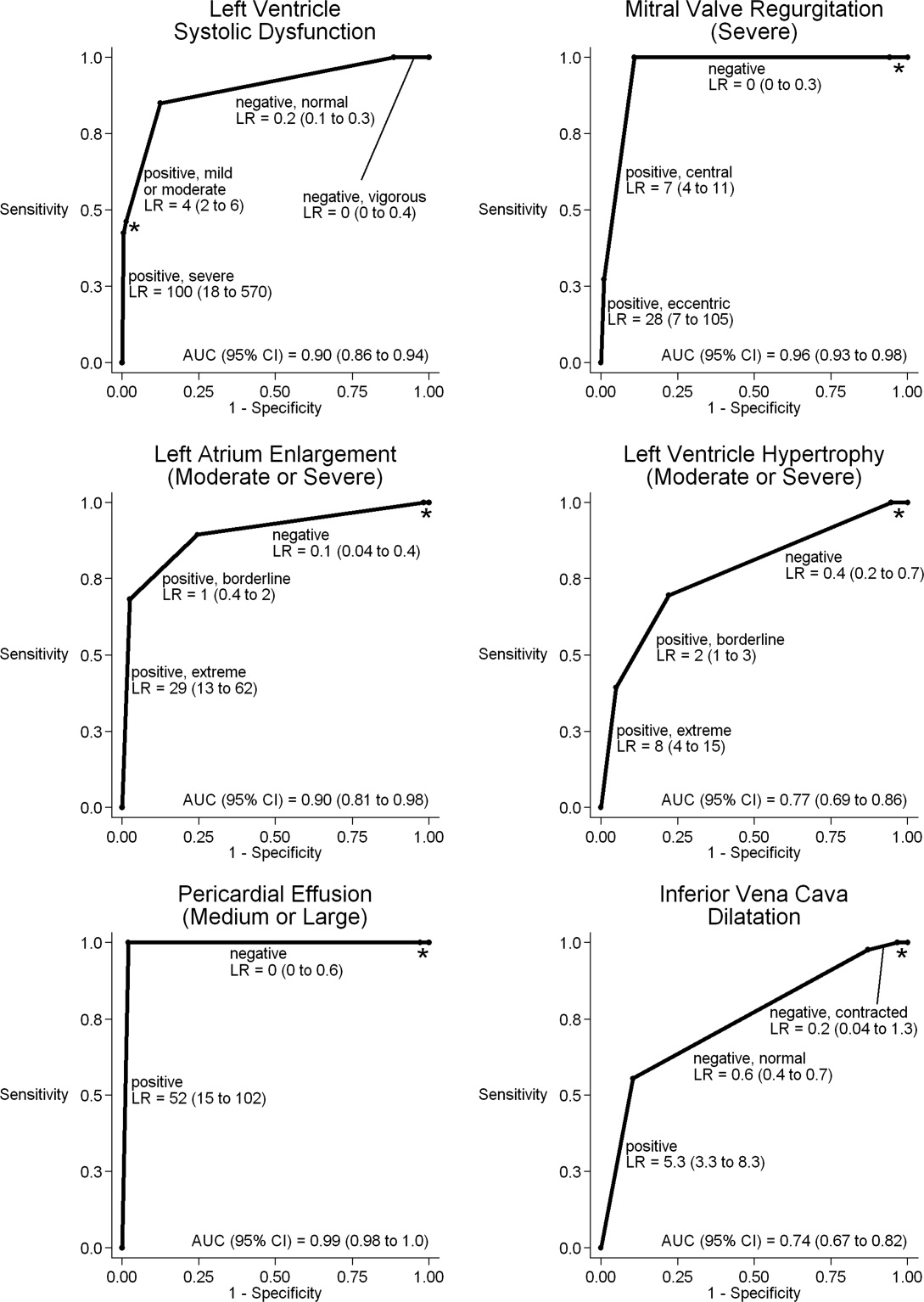
| Clinically Pertinent Cardiac Abnormality by Standard Echocardiography | Prevalence n/total n | Sensitivity* % (95% CI) | Specificity* % (95% CI) | LRpositive*, (95% CI) | LRnegative*, (95% CI) |
|---|---|---|---|---|---|
| |||||
| Left ventricular systolic dysfunction | 80/314 | 85 (7592) | 88 (8392) | 6.9 (4.99.8) | 0.2 (0.10.3) |
| Mitral valve regurgitation, severe | 11/314 | 100 (72100) | 83 (7987) | 5.9 (3.97.4) | 0 (00.3) |
| Left atrium enlargement, moderate or severe | 19/313 | 90 (6799) | 74 (6879) | 3.4 (2.54.3) | 0.1 (0.040.4) |
| Left ventricular hypertrophy, moderate or severe | 33/314 | 70 (5184) | 73 (6778) | 2.5 (1.83.3) | 0.4 (0.20.7) |
| Pericardial effusion, moderate or large | 3/314 | 100 (29100) | 95 (9297) | 21 (6.731) | 0 (00.6) |
| Inferior vena cava, dilated | 45/284 | 56 (4070) | 86 (8190) | 4.0 (2.66.0) | 0.5 (0.40.7) |
LV systolic dysfunction and IVC dilatation were both prevalent enough to meet our criterion to examine interobserver variability; the mean number of abnormal patients per hospitalist was 10 patients for LV systolic dysfunction and 6 patients for IVC dilatation. For LV systolic dysfunction, SDs around mean sensitivity (84%) and specificity (87%) were 12% and 6%, respectively. For IVC dilatation, SDs around mean sensitivity (58%) and specificity (86%) were 24% and 7%, respectively.
Discussion
We found that, after a 27‐hour training program, hospitalists performed HCUE with moderate to excellent diagnostic accuracy for 6 important cardiac abnormalities. For example, hospitalists' assessments of LV systolic function yielded positive and negative likelihood ratios of 6.9 (95% CI, 4.99.8) and 0.2 (95% CI, 0.10.3), respectively. At the bedsides of patients with acute heart failure, therefore, hospitalists could use HCUE to lower or raise the 50:50 chance of LV systolic dysfunction30 to 15% or 85%, respectively. Whether or not these posttest likelihoods are extreme enough to cross important thresholds will depend on the clinical context. Yet these findings demonstrate how HCUE has the potential to provide hospitalists with valuable point‐of‐care data that are otherwise unavailableeither because routine clinical assessments are unreliable31 or because echocardiographic services are not immediately accessible.1
In fact, recent data from the Joint Commission on Accreditation of Healthcare Organizations shows how inaccessible SE may be. Approximately one‐quarter of hospitals in the United States send home about 10% of patients with acute heart failure without echocardiographic assessment of LV systolic function before, during, or immediately after hospitalization.32 In doing so, these hospitals leave unmet the 2002 National Quality Improvement Goal of universal assessment of LV systolic function for all heart failure patients. Hospitalists could close this quality gap with routine, 10‐minute HCUE assessments in all patients admitted with acute heart failure. (Our research HCUE protocol required a median time of 28 minutes, but this included time to assess 5 other cardiac abnormalities and collect data for research purposes). Until the clinical consequences of introducing hospitalist‐performed HCUE are studied, potential benefits like this are tentative. But our findings suggest that training hospitalists to accurately perform HCUE can be successfully accomplished in just 27 hours.
Other studies of HCUE training programs for noncardiologists have also challenged the opinion that learning to perform HCUE requires more than 100 hours of training.2, 711 Yet only 1 prior study has examined an HCUE training program for hospitalists.5 In this study by Martin et al.,5 hospitalists completed 5 supervised HCUE examinations and 6 hours of interpretation training before investigators scored their image acquisition and interpretation skills from 30 unsupervised HCUE examinations. To estimate their final skill levels at the completion of all 35 examinations by accounting for an initially steep learning curve, investigators then adjusted these scores with regression models. Despite these upward adjustments, hospitalists' image acquisition and interpretation scores were low in comparison to echocardiographic technicians and cardiology fellows. Besides these adjusted measurements of hospitalists' skills, however, Martin et al.5 unfortunately did not also report standard measures of diagnostic accuracy, like those proposed by the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.33 Therefore, direct comparisons to the present study are difficult. Nevertheless, their findings suggest that a training program limited to 5 supervised HCUE examinations may be inadequate for hospitalists. In fact, the same group's earlier study of medical trainees suggested a minimum of 30 supervised HCUE examinations.9 We chose to design our hospitalist training program based on this minimum, though they surprisingly did not.5 As others continue to refine the components of hospitalist HCUE training programs, such as the optimal number of supervised examinations, our program could serve as a reasonable comparative example: more rigorous than the program designed by Martin et al.5 but more feasible than ASE level 1 training.
The number and complexity of assessments taught in HCUE training programs will determine their duration. With ongoing advancements in HCUE technology, there is a growing list of potential assessments to choose from. Although HCUE training programs ought to include assessments with proven clinical applications, there are no trials of HCUE‐directed care to inform such decisions. In their absence, therefore, we chose 6 assessments based on the following 3 criteria. First, our assessments were otherwise not reliably available from routine clinical data, such as the physical examination. Second, our assessments were straightforward: easy to learn and simple to perform. Here, we based our reasoning on an expectation that the value of HCUE lies not in highly complex, state‐of‐the‐art assessmentswhich are best left to echocardiographers equipped with SEbut in simple, routine assessments made with highly portable machines that grant noncardiologists newfound access to point‐of‐care data.34 Third, our assessments were clinically pertinent and, where appropriate, defined by cut‐points at levels of severity that often lead to changes in management. We suspect that setting high cut‐points has the salutary effects of making assessments easier to learn and more accurate, because distinguishing mild abnormalities is likely the most challenging aspect of echocardiographic interpretation.35 Whether or not our choices of assessments, and their cut‐points, are optimal has yet to be determined by future research designed to study how they affect patient outcomes. Given our hospitalists' performance in the present study, these assessments seem worthy of such future research.
Our study had several limitations. We studied physicians and patients from only 1 hospital; similar studies performed in different settings, particularly among patients with different proportions and manifestations of disease, may find different results. Nevertheless, our sampling method of prospectively enrolling consecutive patients strengthens our findings. Some echocardiographic measurement methods used by our hospitalists differed in subtle ways from echocardiography guideline recommendations.35 We chose our methods (Table 2) for 2 reasons. First, whenever possible, we chose methods of interpretation that coincided with our local cardiologists'. Second, we chose simplicity over precision. For example, the biplane method of disks, or modified Simpson's rule, is the preferred volumetric method of calculating LA size.35 This method requires tracing the contours of the LA in 2 planes and then dividing the LA volume into stacked oval disks for calculation. We chose instead to train our hospitalists in a simpler method based on 2 linear measurements. Any loss of precision, however, was balanced by a large gain in simplicity. Regardless, minor variations in LA size are not likely to affect hospitalists' bedside evaluations. Finally, we did not validate the results of our reference standard (SE) by documenting interobserver reliability. Yet, because SE is generally accurate for the 6 cardiac abnormalities under study, the effect of this bias should be small.
These limitations can be addressed best by controlled trials of HCUE‐directed care. These trials will determine the clinical impact of hospitalist‐performed HCUE and, in turn, inform our design of HCUE training programs. As the current study shows, training hospitalists to participate in such trials is feasible: like other groups of noncardiologists, hospitalists can accurately perform HCUE after a brief training program. Whether or not hospitalists should perform HCUE requires further study.
Acknowledgements
The authors thank Sonosite, Inc., Bothell, WA, for loaning us 2 MicroMaxx machines throughout the study period. They also thank the staff of the Internal Medicine Research Mentoring Program at Rush Medical College for their technical support and the staff of the Division of Neurology at Stroger Hospital for granting them access to a procedure room.
- .The physical examination of the future: echocardiography as part of the assessment.ACC Curr J Rev.1998;7:79–81.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20:477–485.
- ,.Bedside ultrasonography in the ICU: Part 2.Chest.2005;128:1766–1781.
- ,.Practical Guide to Emergency Ultrasound.1st ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120:1000–1004.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the echocardiography task force on new technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc Echocardiogr.2002;15:369–373.
- ,,,,,.The use of small personal ultrasound devices with internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,, et al.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147:476–481.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118:1010–1018.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96:1002–1006.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20:471–476.
- ,,, et al.A hospitalist‐run short stay unit: features that predict patients' length‐of‐stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- .Adult echocardiography scanning protocol. In: Templin BB, ed.Ultrasound Scanning: Principles and Protocols.2nd ed.Philadelphia, PA:Saunders;1999:426.
- ,,, et al.ACCF 2008 Recommendations for training in adult cardiovascular medicine core cardiology training (COCATS 3) (revision of the 2002 COCATS training statement).J Am Coll Cardiol.2008;51:333–414.
- ,,.The Echo Manual.2nd ed.Philadelphia, PA:Lippincott Williams 1999.
- ,,, et al.Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications.J Am Soc Echocardiogr.1991;4:203–214.
- .Textbook of Clinical Echocardiography.3rd ed.Philadelphia, PA:Elsevier Saunders;2004.
- ,,.Likelihood ratios with confidence: sample size estimation for diagnostic test studies.J Clin Epidemiol.1991;44:763–770.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2005;112;154–235.
- ,,, et al.ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2006;114:e84–e231.
- ,,, et al.Left atrial size: physiologic determinants and clinical applications.J Am Coll Cardiol.2006;47:2357–2363.
- ,,,,.Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study.N Engl J Med.1990;322:1561–1566.
- ,,,.Does this patient with a pericardial effusion have cardiac tamponade?JAMA.2007;297:1810–1818.
- .Acute cardiac tamponade.N Engl J Med.2003;349:685–690.
- ,,,,,.Evaluation of size and dynamics of the inferior vena cava as an index of right‐sided cardiac function.Am J Cardiol.1984;53:579–585.
- ,,.The influence of uninterpretability on the assessment of diagnostic tests.J Chronic Dis.1986;39:575–584.
- ,,.Relations between effectiveness of a diagnostic test, prevalence of the disease, and percentages of uninterpretable results. An example in the diagnosis of jaundice.Med Decis Making.1982;2:285–297.
- .Confidence intervals for the ratio of two binomial proportions.Biometrics.1984;40:513–517.
- ,,.Comparing the areas under two or more correlated receiver operating curves: a nonparametric approach.Biometrics.1988;44:837–845.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296:2217–2226.
- ,,, et al.Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure.Am J Med.2002;112:437–445.
- Joint Commission on Accreditation of Healthcare Organizations. Health Care Quality Data Download Website. Available at: http://www.healthcarequalitydata.org. Accessed December2008.
- ,,, et al.Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative.Clin Chem.2003;49:1–6.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78:102–112.
- ,,, et al.Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology.J Am Soc Echocardiogr.2005;18:1440–1463.
Hand‐carried ultrasound echocardiography (HCUE) can help noncardiologists answer well‐defined questions at patients' bedsides in less than 10 minutes.1, 2 Indeed, intensivists3 and emergency department physicians4 already use HCUE to make rapid, point‐of‐care assessments. Since cardiovascular diagnoses are common among general medicine inpatients, HCUE may become an important skill for hospitalists to learn.5
However, uncertainty exists about the duration of HCUE training for hospitalists. In 2002, experts from the American Society of Echocardiography (ASE) published recommendations on training requirements for HCUE.6 With limited data on the safety or performance of HCUE training programs, which had just begun to emerge, the ASE borrowed from the proven training recommendations for standard echocardiography (SE). They recommended that all HCUE trainees, cardiologist and noncardiologist alike, complete level 1 SE training: 75 personally‐performed and 150 personally‐interpreted echocardiographic examinations. Since then, however, several HCUE training programs designed for noncardiologists have emerged.2, 5, 710 These alternative programs suggest that the ASE's recommended duration of training may be too long, particularly for focused HCUE that is limited to a few relatively simple assessments. It is important not to overshoot the requirements of HCUE training, because doing so may discourage groups of noncardiologists, like hospitalists, who may derive great benefits from HCUE.11
To address this uncertainty for hospitalists, we first developed a brief HCUE training program to assess 6 important cardiac abnormalities. We then studied the diagnostic accuracy of HCUE by hospitalists as a test of these 6 cardiac abnormalities assessed by SE.
Patients and Methods
Setting and Subjects
This prospective cohort study was performed at Stroger Hospital of Cook County, a 500‐bed public teaching hospital in Chicago, IL, from March through May of 2007. The cohort was adult inpatients who were referred for SE on weekdays from 3 distinct patient care units (Figure 1). We used 2 sampling modes to balance practical constraints (short‐stay unit [SSU] patients were more localized and, therefore, easier to study) with clinical diversity. We consecutively sampled patients from our SSU, where adults with provisional cardiovascular diagnoses are admitted if they might be eligible for discharge with in 3 days.12 But we used random number tables with a daily unique starting point to randomly sample patients from the general medical wards and the coronary care unit (CCU). Patients were excluded if repositioning them for HCUE was potentially harmful. The study was approved by our hospital's institutional review board, and we obtained written informed consent from all enrolled patients.

SE Protocol
As part of enrolled patients' routine clinical care, SE images were acquired and interpreted in the usual fashion in our hospital's echocardiography laboratory, which performs SE on over 7,000 patients per year. Echocardiographic technicians acquired images with a General Electric Vivid 7 cardiac ultrasound machine (General Electric, Milwaukee, WI) equipped with a GE M4S 1.8 to 3.4 MHz cardiac transducer (General Electric). Technicians followed the standard adult transthoracic echocardiography scanning protocol to acquire 40 to 100 images on every patient using all available echocardiographic modalities: 2‐dimensional, M‐mode, color Doppler, continuous‐wave Doppler, pulse‐wave Doppler, and tissue Doppler.13 Blinded to HCUE results, attending physician cardiologist echocardiographers then interpreted archived images using computer software (Centricity System; General Electric) to generate final reports that were entered into patients' medical records. This software ensured that final reports were standardized, because echocardiographers' final qualitative assessments were limited to short lists of standard options; for example, in reporting left atrium (LA) size, echocardiographers chose from only 5 standard options: normal, mildly dilated, moderately dilated, severely dilated, and not interpretable. Investigators, who were also blinded to HCUE results, later abstracted SE results from these standardized report forms in patients' medical records. All echocardiographers fulfilled ASE training guidelines to independently interpret SE: a minimum of 150 personally‐performed and 300 personally‐interpreted echocardiographic examinations (training level 2).14
HCUE Training
Based on the recommendations of our cardiologist investigator (B.M.), we developed a training program for 1 hospitalist to become an HCUE instructor. Our instructor trainee (C.C.) was board‐eligible in internal medicine but had no previous formal training in cardiology or echocardiography. We a priori established that her training would continue until our cardiologist investigator determined that she was ready to train other hospitalists; this determination occurred after 5 weeks. She learned image acquisition by performing focused SE on 30 patients under the direct supervision of an echocardiographic technician. She also performed focused HCUE on 65 inpatients without direct supervision but with ongoing access to consult the technician to review archived images and troubleshoot difficulties with acquisition. She learned image interpretation by reading relevant chapters from a SE textbook15 and by participating in daily didactic sessions in which attending cardiologist echocardiographers train cardiology fellows in SE interpretation.
This hospitalist then served as the HCUE instructor for 8 other attending physician hospitalists who were board‐certified internists with no previous formal training in cardiology or echocardiography. The training program was limited to acquisition and interpretation of 2‐dimensional grayscale and color Doppler images for the 6 cardiac assessments under study (Table 1). The instructor marshaled pairs of hospitalists through the 3 components of the training program, which lasted a total of 27 hours.
|
| Six cardiac assessments learned using 2‐dimensional gray scale and color Doppler imaging |
| Left ventricular systolic dysfunction |
| Mitral valve regurgitation |
| Left atrium enlargement |
| Left ventricular hypertrophy |
| Pericardial effusion |
| Inferior vena cava diameter |
| Lecture (2 hours)* |
| Basic principles of echocardiography |
| HCUE scanning protocol and helpful techniques to optimize image quality |
| Hands‐on training with instructor |
| Orientation to machine and demonstration of scanning protocol (1 hour) |
| Sessions 1 through 3: HCUE performed on 1 patient per hour (6 patients in 6 hours) |
| Sessions 4 through 10: HCUE performed on 2 patients per hour (28 patients in 14 hours) |
| Feedback sessions on image quality and interpretation with cardiologist |
| After hands‐on training session 3 (2 hours) |
| After hands‐on training session 10 (2 hours) |
First, hospitalists attended a 2‐hour lecture on the basic principles of HCUE. Slides from this lecture and additional images of normal and abnormal findings were provided to each hospitalist on a digital video disc. Second, each hospitalist underwent 20 hours of hands‐on training in 2‐hour sessions scheduled over 2 weeks. Willing inpatients from our hospital's emergency department were used as volunteers for these hand‐on training sessions. During these sessions the instructor provided practical suggestions to optimize image quality, such as transducer location and patient positioning. In the first 3 sessions, the minimum pace was 1 patient per hour; thereafter, the pace was increased to 1 patient per half‐hour. We chose 20 hours of hands‐on training and these minimum paces because they allowed each hospitalist to attain a cumulative experience of no less than 30 patientsan amount that heralds a flattening of the HCUE learning curve among medical trainees.9 Third, each pair of hospitalists received feedback from a cardiologist investigator (B.M.) who critiqued the quality and interpretation of images acquired by hospitalists during hands‐on training sessions. Since image quality varies by patient,16 hospitalists' images were compared side‐by‐side to images recorded by the instructor on the same patients. The cardiologist also critiqued hospitalists' interpretations of both their own images and additional sets of archived images from patients with abnormal findings.
HCUE Protocol
After completing the training program and blinded to the results of SE, the 8 hospitalists performed HCUE on enrolled patients within hours of SE. We limited the time interval between tests to minimize the effect that changes in physiologic variables, such as blood pressure and intravascular volume, have on the reliability of serial echocardiographic measurements.16 Hospitalists performed HCUE with a MicroMaxx 3.4 hand‐carried ultrasound machine equipped with a cardiology software package and a 1 to 5 MHz P17 cardiac transducer (Sonosite, Inc., Bothell, WA); simultaneous electrocardiographic recording, though available, was not used. While patients laid on their own standard hospital beds or on a standard hospital gurney in a room adjacent to the SE waiting room, hospitalists positioned them without assistance from nursing staff and recorded 7 best‐quality images per patient. Patients were first positioned in a partial (3045 degrees) left lateral decubitus position to record 4 grayscale images of the short‐axis and long‐axis parasternal and 2‐chamber and 4‐chamber apical views; 2 color Doppler images of the mitral inflow were also recorded from the long‐axis parasternal and the 4‐chamber apical views. Patients were then positioned supine to record 1 grayscale image of the inferior vena cava (IVC) from the transhepatic view. Hospitalists did not perform a history or physical exam on enrolled patients, nor did they review patients' medical records.
Immediately following the HCUE, hospitalists replayed the recorded images as often as needed and entered final interpretations on data collection forms. Linear measurements were made manually with a caliper held directly to the hand‐carried ultrasound monitor. These measurements were then translated into qualitative assessments based on standard values used by our hospital's echocardiographers (Table 2).17 When a hospitalist could not confidently assess a cardiac abnormality, the final HCUE assessment was recorded as indeterminate. Hospitalists also recorded the time to perform each HCUE, which included the time to record 7 best‐quality images, to interpret the findings, and to fill out the data collection form.
| Hand‐Carried Ultrasound Echocardiography Results | |||||
|---|---|---|---|---|---|
| Cardiac Abnormality by Standard Echocardiography | Hand‐Carried Ultrasound Echocardiography Operator's Method of Assessment | Positive | Negative | ||
| |||||
| Left ventricle systolic dysfunction, mild or greater | Grade degree of abnormal wall movement and thickening during systole | Severe | Mild or moderate | Normal | Vigorous |
| Mitral valve regurgitation, severe | Classify regurgitant jet as central or eccentric, then measure as percentage of left atrium area | ||||
| Central jet | 20% | <20% | |||
| Eccentric jet | 20% | indeterminate 20% | |||
| Left atrium enlargement, moderate or severe | Measure left atrium in 3 dimensions at end diastole, then use the most abnormal dimension | Extreme | Borderline | ||
| Anteroposterior or mediolateral (cm) | 5.1 | 4.55.0 | 4.4 | ||
| Superior‐inferior (cm) | 7.1 | 6.17.0 | 6.0 | ||
| Left ventricle hypertrophy, moderate or severe | Measure thickest dimension of posterior or septal wall at end diastole | Extreme: 1.4 cm | Borderline: 1.21.3 cm | 1.1 cm | |
| Pericardial effusion, medium or large | Measure largest dimension in any view at end diastole | 1 cm | <1 cm | ||
| Inferior vena cava dilatation | Measure largest respirophasic diameter within 2 cm of right atrium | 2.1 cm | Normal: 1 to 2 cm | Contracted: 0.9 cm | |
Data Analysis
We based our sample size calculations on earlier reports of HCUE by noncardiologist trainees for assessment of left ventricular (LV) systolic function.7, 10 From these reports, we estimated a negative likelihood ratio of 0.3. In addition, we expected about a quarter of our patients to have LV systolic dysfunction (B.M., personal communication). Therefore, to achieve 95% confidence intervals (CIs) around the point estimate of a negative likelihood ratio that excluded 0.50, our upper bound for a clinically meaningful result, we needed a sample size of approximately 300 patients.18
We defined threshold levels of ordinal severity for the 6 cardiac abnormalities under study based on their clinical pertinence to hospitalists (Table 2). Here, we reasoned that abnormalities at or above these levels would likely lead to important changes in hospitalists' management of inpatients; abnormalities below these levels rarely represent cardiac disease that is worthy of an immediate change in management. Since even mild degrees of LV dysfunction have important diagnostic and therapeutic implications for most general medicine inpatients, particularly those presenting with heart failure,19 we set our threshold for LV dysfunction at mild or greater. In contrast, since neither mild nor moderate mitral regurgitation (MR) has immediate implications for medical or surgical therapy even if symptoms or LV dysfunction are present,20 we set our threshold for MR at severe. Similarly, though mild LA enlargement21 and mild LV hypertrophy22 have clear prognostic implications for patients' chronic medical conditions, we reasoned that only moderate or severe versions likely reflect underlying abnormalities that affect hospitalists' point‐of‐care decision‐making. Since cardiac tamponade is rarely both subclinical23 and due to a small pericardial effusion,24 we set our threshold for pericardial effusion size at moderate or large. Finally, we set our threshold IVC diameter, a marker of central venous volume status,25 at dilated, because volume overload is an important consideration in hospitalized cardiac patients.
Using these thresholds, investigators dichotomized echocardiographers' SE readings as normal or abnormal for each of the 6 cardiac abnormalities under study to serve as the reference standards. Hospitalists' HCUE results were then compared to the reference standards in 2 different ways. We first analyzed HCUE results as dichotomous values to calculate conventional sensitivity, specificity, and positive and negative likelihood ratios. Here we considered indeterminate HCUE results positive in a clinically conservative tradeoff that neither ignores indeterminate results nor risks falsely classifying them as negative.26 We then analyzed hospitalists' HCUE results as ordinal values for receiver operating characteristic (ROC) curve analysis. Here we considered an indeterminate result as 1 possible test result.27
To examine interobserver variability of HCUE, we first chose from the 6 possible assessments only those with a mean number of abnormal patients per hospitalist greater than 5. We reasoned that variability among assessments with lower prevalence would be predictably wide and inconclusive. We then expressed variability as standard deviations (SDs) around mean sensitivity and specificity for the 8 hospitalists.
The CIs for likelihood ratios were constructed using the likelihood‐based approach to binomial proportions of Koopman.28 The areas under ROC curves were computed using the trapezoidal rule, and the CIs for these areas were constructed using the algorithm described by DeLong et al.29 All analyses were conducted with Stata Statistical Software, Release 10 (StataCorp, College Station, TX).
Results
During the 3 month study period, 654 patients were referred for SE from the 3 participating patient care units (Figure 1). Among these, 65 patients were ineligible because their SE was performed on the weekend and 178 other patients were not randomized from the general medical wards and CCU. From the remaining eligible patients, 322 underwent HCUE and 314 (98% of 322) underwent both SE and HCUE. Individual SE assessments were not interpretable (and therefore excluded) due to poor image quality for LA enlargement in 1 patient and IVC dilatation in 30 patients. Eighty‐three percent of patients who underwent SE (260/314) were referred to assess LV function (Table 3). The prevalence of the 6 clinically pertinent cardiac abnormalities under study ranged from 1% for moderate or large pericardial effusion to 25% for LV systolic dysfunction. Overall, 40% of patients had at least 1 out of 6 cardiac abnormalities.
| Characteristic | |
|---|---|
| |
| Age, year SD (25th to 75th percentiles) | 56 13 (48 to 64) |
| Women | 146 (47) |
| Chronic obstructive pulmonary disease | 47 (15) |
| Body mass index | |
| 24.9 or less: underweight or normal | 74 (24) |
| 25 to 29.9: overweight | 94 (30) |
| 30 to 34.9: mild obesity | 75 (24) |
| 35 or greater: moderate or severe obesity | 71 (23) |
| Patient care unit | |
| Short‐stay unit | 175 (56) |
| General medical wards | 89 (28) |
| Cardiac care unit | 50 (16) |
| Indication for standard echocardiography* | |
| Left ventricular function | 260 (83) |
| Valvular function | 56 (18) |
| Wall motion abnormality | 29 (9) |
| Valvular vegetations | 22 (7) |
| Any structural heart disease | 20 (6) |
| Right ventricular function | 18 (6) |
| Other | 38 (12) |
| Standard echocardiography findings | |
| Left ventricular systolic dysfunction mild | 80 (25) |
| Inferior vena cava dilated | 45 (14) |
| Left ventricular wall thickness moderate | 33 (11) |
| Left atrium enlargement moderate | 19 (6) |
| Mitral valve regurgitation severe | 11 (4) |
| Pericardial effusion moderate | 3 (1) |
| At least 1 of the above findings | 127 (40) |
| Time difference between HCUE and standard echocardiogram, median hours (25th to 75th percentiles) | 2.8 (1.4 to 5.1) |
| Time to complete HCUE, median minutes (25th to 75th percentiles) | 28 (20 to 35) |
Each hospitalist performed a similar total number of HCUE examinations (range, 3447). The median time difference between performance of SE and HCUE was 2.8 hours (25th75th percentiles, 1.45.1). Despite the high prevalence of chronic obstructive pulmonary disease and obesity, hospitalists considered HCUE assessments indeterminate in only 2% to 6% of the 6 assessments made for each patient (Table 4). Among the 38 patients (12% of 322) with any indeterminate HCUE assessment, 24 patients had only 1 out of 6 possible. Hospitalists completed HCUE in a median time of 28 minutes (25th‐75th percentiles, 2035), which included the time to record 7 best‐quality moving images and to fill out the research data collection form.
| n (%)* | |
|---|---|
| |
| Number of indeterminate findings per patient | |
| 0 | 284 (88) |
| 1 | 24 (7) |
| 2 | 4 (1) |
| 3 or more | 10 (3) |
| Indeterminate findings by cardiac assessment | |
| Mitral valve regurgitation | 18 (6) |
| Inferior vena cava diameter | 16 (5) |
| Left ventricular hypertrophy | 15 (5) |
| Pericardial effusion | 9 (3) |
| Left atrium size | 5 (2) |
| Left ventricle systolic function | 5 (2) |
When HCUE results were analyzed as dichotomous values, positive likelihood ratios ranged from 2.5 to 21, and negative likelihood ratios ranged from 0 to 0.4 (Table 5). Positive and negative likelihood ratios were both sufficiency high and low to respectively increase and decrease by 5‐fold the prior odds of 3 out of 6 cardiac abnormalities: LV systolic dysfunction, moderate or severe MR regurgitation, and moderate or large pericardial effusion. Considering HCUE results as ordinal values for ROC analysis yielded additional diagnostic information (Figure 2). For example, the likelihood ratio of 1.0 (95% CI, 0.42.0) for borderline positive moderate or severe LA enlargement increased to 29 (range, 1362) for extreme positive results. Areas under the ROC curves were 0.9 for 4 out of 6 cardiac abnormalities.

| Clinically Pertinent Cardiac Abnormality by Standard Echocardiography | Prevalence n/total n | Sensitivity* % (95% CI) | Specificity* % (95% CI) | LRpositive*, (95% CI) | LRnegative*, (95% CI) |
|---|---|---|---|---|---|
| |||||
| Left ventricular systolic dysfunction | 80/314 | 85 (7592) | 88 (8392) | 6.9 (4.99.8) | 0.2 (0.10.3) |
| Mitral valve regurgitation, severe | 11/314 | 100 (72100) | 83 (7987) | 5.9 (3.97.4) | 0 (00.3) |
| Left atrium enlargement, moderate or severe | 19/313 | 90 (6799) | 74 (6879) | 3.4 (2.54.3) | 0.1 (0.040.4) |
| Left ventricular hypertrophy, moderate or severe | 33/314 | 70 (5184) | 73 (6778) | 2.5 (1.83.3) | 0.4 (0.20.7) |
| Pericardial effusion, moderate or large | 3/314 | 100 (29100) | 95 (9297) | 21 (6.731) | 0 (00.6) |
| Inferior vena cava, dilated | 45/284 | 56 (4070) | 86 (8190) | 4.0 (2.66.0) | 0.5 (0.40.7) |
LV systolic dysfunction and IVC dilatation were both prevalent enough to meet our criterion to examine interobserver variability; the mean number of abnormal patients per hospitalist was 10 patients for LV systolic dysfunction and 6 patients for IVC dilatation. For LV systolic dysfunction, SDs around mean sensitivity (84%) and specificity (87%) were 12% and 6%, respectively. For IVC dilatation, SDs around mean sensitivity (58%) and specificity (86%) were 24% and 7%, respectively.
Discussion
We found that, after a 27‐hour training program, hospitalists performed HCUE with moderate to excellent diagnostic accuracy for 6 important cardiac abnormalities. For example, hospitalists' assessments of LV systolic function yielded positive and negative likelihood ratios of 6.9 (95% CI, 4.99.8) and 0.2 (95% CI, 0.10.3), respectively. At the bedsides of patients with acute heart failure, therefore, hospitalists could use HCUE to lower or raise the 50:50 chance of LV systolic dysfunction30 to 15% or 85%, respectively. Whether or not these posttest likelihoods are extreme enough to cross important thresholds will depend on the clinical context. Yet these findings demonstrate how HCUE has the potential to provide hospitalists with valuable point‐of‐care data that are otherwise unavailableeither because routine clinical assessments are unreliable31 or because echocardiographic services are not immediately accessible.1
In fact, recent data from the Joint Commission on Accreditation of Healthcare Organizations shows how inaccessible SE may be. Approximately one‐quarter of hospitals in the United States send home about 10% of patients with acute heart failure without echocardiographic assessment of LV systolic function before, during, or immediately after hospitalization.32 In doing so, these hospitals leave unmet the 2002 National Quality Improvement Goal of universal assessment of LV systolic function for all heart failure patients. Hospitalists could close this quality gap with routine, 10‐minute HCUE assessments in all patients admitted with acute heart failure. (Our research HCUE protocol required a median time of 28 minutes, but this included time to assess 5 other cardiac abnormalities and collect data for research purposes). Until the clinical consequences of introducing hospitalist‐performed HCUE are studied, potential benefits like this are tentative. But our findings suggest that training hospitalists to accurately perform HCUE can be successfully accomplished in just 27 hours.
Other studies of HCUE training programs for noncardiologists have also challenged the opinion that learning to perform HCUE requires more than 100 hours of training.2, 711 Yet only 1 prior study has examined an HCUE training program for hospitalists.5 In this study by Martin et al.,5 hospitalists completed 5 supervised HCUE examinations and 6 hours of interpretation training before investigators scored their image acquisition and interpretation skills from 30 unsupervised HCUE examinations. To estimate their final skill levels at the completion of all 35 examinations by accounting for an initially steep learning curve, investigators then adjusted these scores with regression models. Despite these upward adjustments, hospitalists' image acquisition and interpretation scores were low in comparison to echocardiographic technicians and cardiology fellows. Besides these adjusted measurements of hospitalists' skills, however, Martin et al.5 unfortunately did not also report standard measures of diagnostic accuracy, like those proposed by the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.33 Therefore, direct comparisons to the present study are difficult. Nevertheless, their findings suggest that a training program limited to 5 supervised HCUE examinations may be inadequate for hospitalists. In fact, the same group's earlier study of medical trainees suggested a minimum of 30 supervised HCUE examinations.9 We chose to design our hospitalist training program based on this minimum, though they surprisingly did not.5 As others continue to refine the components of hospitalist HCUE training programs, such as the optimal number of supervised examinations, our program could serve as a reasonable comparative example: more rigorous than the program designed by Martin et al.5 but more feasible than ASE level 1 training.
The number and complexity of assessments taught in HCUE training programs will determine their duration. With ongoing advancements in HCUE technology, there is a growing list of potential assessments to choose from. Although HCUE training programs ought to include assessments with proven clinical applications, there are no trials of HCUE‐directed care to inform such decisions. In their absence, therefore, we chose 6 assessments based on the following 3 criteria. First, our assessments were otherwise not reliably available from routine clinical data, such as the physical examination. Second, our assessments were straightforward: easy to learn and simple to perform. Here, we based our reasoning on an expectation that the value of HCUE lies not in highly complex, state‐of‐the‐art assessmentswhich are best left to echocardiographers equipped with SEbut in simple, routine assessments made with highly portable machines that grant noncardiologists newfound access to point‐of‐care data.34 Third, our assessments were clinically pertinent and, where appropriate, defined by cut‐points at levels of severity that often lead to changes in management. We suspect that setting high cut‐points has the salutary effects of making assessments easier to learn and more accurate, because distinguishing mild abnormalities is likely the most challenging aspect of echocardiographic interpretation.35 Whether or not our choices of assessments, and their cut‐points, are optimal has yet to be determined by future research designed to study how they affect patient outcomes. Given our hospitalists' performance in the present study, these assessments seem worthy of such future research.
Our study had several limitations. We studied physicians and patients from only 1 hospital; similar studies performed in different settings, particularly among patients with different proportions and manifestations of disease, may find different results. Nevertheless, our sampling method of prospectively enrolling consecutive patients strengthens our findings. Some echocardiographic measurement methods used by our hospitalists differed in subtle ways from echocardiography guideline recommendations.35 We chose our methods (Table 2) for 2 reasons. First, whenever possible, we chose methods of interpretation that coincided with our local cardiologists'. Second, we chose simplicity over precision. For example, the biplane method of disks, or modified Simpson's rule, is the preferred volumetric method of calculating LA size.35 This method requires tracing the contours of the LA in 2 planes and then dividing the LA volume into stacked oval disks for calculation. We chose instead to train our hospitalists in a simpler method based on 2 linear measurements. Any loss of precision, however, was balanced by a large gain in simplicity. Regardless, minor variations in LA size are not likely to affect hospitalists' bedside evaluations. Finally, we did not validate the results of our reference standard (SE) by documenting interobserver reliability. Yet, because SE is generally accurate for the 6 cardiac abnormalities under study, the effect of this bias should be small.
These limitations can be addressed best by controlled trials of HCUE‐directed care. These trials will determine the clinical impact of hospitalist‐performed HCUE and, in turn, inform our design of HCUE training programs. As the current study shows, training hospitalists to participate in such trials is feasible: like other groups of noncardiologists, hospitalists can accurately perform HCUE after a brief training program. Whether or not hospitalists should perform HCUE requires further study.
Acknowledgements
The authors thank Sonosite, Inc., Bothell, WA, for loaning us 2 MicroMaxx machines throughout the study period. They also thank the staff of the Internal Medicine Research Mentoring Program at Rush Medical College for their technical support and the staff of the Division of Neurology at Stroger Hospital for granting them access to a procedure room.
Hand‐carried ultrasound echocardiography (HCUE) can help noncardiologists answer well‐defined questions at patients' bedsides in less than 10 minutes.1, 2 Indeed, intensivists3 and emergency department physicians4 already use HCUE to make rapid, point‐of‐care assessments. Since cardiovascular diagnoses are common among general medicine inpatients, HCUE may become an important skill for hospitalists to learn.5
However, uncertainty exists about the duration of HCUE training for hospitalists. In 2002, experts from the American Society of Echocardiography (ASE) published recommendations on training requirements for HCUE.6 With limited data on the safety or performance of HCUE training programs, which had just begun to emerge, the ASE borrowed from the proven training recommendations for standard echocardiography (SE). They recommended that all HCUE trainees, cardiologist and noncardiologist alike, complete level 1 SE training: 75 personally‐performed and 150 personally‐interpreted echocardiographic examinations. Since then, however, several HCUE training programs designed for noncardiologists have emerged.2, 5, 710 These alternative programs suggest that the ASE's recommended duration of training may be too long, particularly for focused HCUE that is limited to a few relatively simple assessments. It is important not to overshoot the requirements of HCUE training, because doing so may discourage groups of noncardiologists, like hospitalists, who may derive great benefits from HCUE.11
To address this uncertainty for hospitalists, we first developed a brief HCUE training program to assess 6 important cardiac abnormalities. We then studied the diagnostic accuracy of HCUE by hospitalists as a test of these 6 cardiac abnormalities assessed by SE.
Patients and Methods
Setting and Subjects
This prospective cohort study was performed at Stroger Hospital of Cook County, a 500‐bed public teaching hospital in Chicago, IL, from March through May of 2007. The cohort was adult inpatients who were referred for SE on weekdays from 3 distinct patient care units (Figure 1). We used 2 sampling modes to balance practical constraints (short‐stay unit [SSU] patients were more localized and, therefore, easier to study) with clinical diversity. We consecutively sampled patients from our SSU, where adults with provisional cardiovascular diagnoses are admitted if they might be eligible for discharge with in 3 days.12 But we used random number tables with a daily unique starting point to randomly sample patients from the general medical wards and the coronary care unit (CCU). Patients were excluded if repositioning them for HCUE was potentially harmful. The study was approved by our hospital's institutional review board, and we obtained written informed consent from all enrolled patients.

SE Protocol
As part of enrolled patients' routine clinical care, SE images were acquired and interpreted in the usual fashion in our hospital's echocardiography laboratory, which performs SE on over 7,000 patients per year. Echocardiographic technicians acquired images with a General Electric Vivid 7 cardiac ultrasound machine (General Electric, Milwaukee, WI) equipped with a GE M4S 1.8 to 3.4 MHz cardiac transducer (General Electric). Technicians followed the standard adult transthoracic echocardiography scanning protocol to acquire 40 to 100 images on every patient using all available echocardiographic modalities: 2‐dimensional, M‐mode, color Doppler, continuous‐wave Doppler, pulse‐wave Doppler, and tissue Doppler.13 Blinded to HCUE results, attending physician cardiologist echocardiographers then interpreted archived images using computer software (Centricity System; General Electric) to generate final reports that were entered into patients' medical records. This software ensured that final reports were standardized, because echocardiographers' final qualitative assessments were limited to short lists of standard options; for example, in reporting left atrium (LA) size, echocardiographers chose from only 5 standard options: normal, mildly dilated, moderately dilated, severely dilated, and not interpretable. Investigators, who were also blinded to HCUE results, later abstracted SE results from these standardized report forms in patients' medical records. All echocardiographers fulfilled ASE training guidelines to independently interpret SE: a minimum of 150 personally‐performed and 300 personally‐interpreted echocardiographic examinations (training level 2).14
HCUE Training
Based on the recommendations of our cardiologist investigator (B.M.), we developed a training program for 1 hospitalist to become an HCUE instructor. Our instructor trainee (C.C.) was board‐eligible in internal medicine but had no previous formal training in cardiology or echocardiography. We a priori established that her training would continue until our cardiologist investigator determined that she was ready to train other hospitalists; this determination occurred after 5 weeks. She learned image acquisition by performing focused SE on 30 patients under the direct supervision of an echocardiographic technician. She also performed focused HCUE on 65 inpatients without direct supervision but with ongoing access to consult the technician to review archived images and troubleshoot difficulties with acquisition. She learned image interpretation by reading relevant chapters from a SE textbook15 and by participating in daily didactic sessions in which attending cardiologist echocardiographers train cardiology fellows in SE interpretation.
This hospitalist then served as the HCUE instructor for 8 other attending physician hospitalists who were board‐certified internists with no previous formal training in cardiology or echocardiography. The training program was limited to acquisition and interpretation of 2‐dimensional grayscale and color Doppler images for the 6 cardiac assessments under study (Table 1). The instructor marshaled pairs of hospitalists through the 3 components of the training program, which lasted a total of 27 hours.
|
| Six cardiac assessments learned using 2‐dimensional gray scale and color Doppler imaging |
| Left ventricular systolic dysfunction |
| Mitral valve regurgitation |
| Left atrium enlargement |
| Left ventricular hypertrophy |
| Pericardial effusion |
| Inferior vena cava diameter |
| Lecture (2 hours)* |
| Basic principles of echocardiography |
| HCUE scanning protocol and helpful techniques to optimize image quality |
| Hands‐on training with instructor |
| Orientation to machine and demonstration of scanning protocol (1 hour) |
| Sessions 1 through 3: HCUE performed on 1 patient per hour (6 patients in 6 hours) |
| Sessions 4 through 10: HCUE performed on 2 patients per hour (28 patients in 14 hours) |
| Feedback sessions on image quality and interpretation with cardiologist |
| After hands‐on training session 3 (2 hours) |
| After hands‐on training session 10 (2 hours) |
First, hospitalists attended a 2‐hour lecture on the basic principles of HCUE. Slides from this lecture and additional images of normal and abnormal findings were provided to each hospitalist on a digital video disc. Second, each hospitalist underwent 20 hours of hands‐on training in 2‐hour sessions scheduled over 2 weeks. Willing inpatients from our hospital's emergency department were used as volunteers for these hand‐on training sessions. During these sessions the instructor provided practical suggestions to optimize image quality, such as transducer location and patient positioning. In the first 3 sessions, the minimum pace was 1 patient per hour; thereafter, the pace was increased to 1 patient per half‐hour. We chose 20 hours of hands‐on training and these minimum paces because they allowed each hospitalist to attain a cumulative experience of no less than 30 patientsan amount that heralds a flattening of the HCUE learning curve among medical trainees.9 Third, each pair of hospitalists received feedback from a cardiologist investigator (B.M.) who critiqued the quality and interpretation of images acquired by hospitalists during hands‐on training sessions. Since image quality varies by patient,16 hospitalists' images were compared side‐by‐side to images recorded by the instructor on the same patients. The cardiologist also critiqued hospitalists' interpretations of both their own images and additional sets of archived images from patients with abnormal findings.
HCUE Protocol
After completing the training program and blinded to the results of SE, the 8 hospitalists performed HCUE on enrolled patients within hours of SE. We limited the time interval between tests to minimize the effect that changes in physiologic variables, such as blood pressure and intravascular volume, have on the reliability of serial echocardiographic measurements.16 Hospitalists performed HCUE with a MicroMaxx 3.4 hand‐carried ultrasound machine equipped with a cardiology software package and a 1 to 5 MHz P17 cardiac transducer (Sonosite, Inc., Bothell, WA); simultaneous electrocardiographic recording, though available, was not used. While patients laid on their own standard hospital beds or on a standard hospital gurney in a room adjacent to the SE waiting room, hospitalists positioned them without assistance from nursing staff and recorded 7 best‐quality images per patient. Patients were first positioned in a partial (3045 degrees) left lateral decubitus position to record 4 grayscale images of the short‐axis and long‐axis parasternal and 2‐chamber and 4‐chamber apical views; 2 color Doppler images of the mitral inflow were also recorded from the long‐axis parasternal and the 4‐chamber apical views. Patients were then positioned supine to record 1 grayscale image of the inferior vena cava (IVC) from the transhepatic view. Hospitalists did not perform a history or physical exam on enrolled patients, nor did they review patients' medical records.
Immediately following the HCUE, hospitalists replayed the recorded images as often as needed and entered final interpretations on data collection forms. Linear measurements were made manually with a caliper held directly to the hand‐carried ultrasound monitor. These measurements were then translated into qualitative assessments based on standard values used by our hospital's echocardiographers (Table 2).17 When a hospitalist could not confidently assess a cardiac abnormality, the final HCUE assessment was recorded as indeterminate. Hospitalists also recorded the time to perform each HCUE, which included the time to record 7 best‐quality images, to interpret the findings, and to fill out the data collection form.
| Hand‐Carried Ultrasound Echocardiography Results | |||||
|---|---|---|---|---|---|
| Cardiac Abnormality by Standard Echocardiography | Hand‐Carried Ultrasound Echocardiography Operator's Method of Assessment | Positive | Negative | ||
| |||||
| Left ventricle systolic dysfunction, mild or greater | Grade degree of abnormal wall movement and thickening during systole | Severe | Mild or moderate | Normal | Vigorous |
| Mitral valve regurgitation, severe | Classify regurgitant jet as central or eccentric, then measure as percentage of left atrium area | ||||
| Central jet | 20% | <20% | |||
| Eccentric jet | 20% | indeterminate 20% | |||
| Left atrium enlargement, moderate or severe | Measure left atrium in 3 dimensions at end diastole, then use the most abnormal dimension | Extreme | Borderline | ||
| Anteroposterior or mediolateral (cm) | 5.1 | 4.55.0 | 4.4 | ||
| Superior‐inferior (cm) | 7.1 | 6.17.0 | 6.0 | ||
| Left ventricle hypertrophy, moderate or severe | Measure thickest dimension of posterior or septal wall at end diastole | Extreme: 1.4 cm | Borderline: 1.21.3 cm | 1.1 cm | |
| Pericardial effusion, medium or large | Measure largest dimension in any view at end diastole | 1 cm | <1 cm | ||
| Inferior vena cava dilatation | Measure largest respirophasic diameter within 2 cm of right atrium | 2.1 cm | Normal: 1 to 2 cm | Contracted: 0.9 cm | |
Data Analysis
We based our sample size calculations on earlier reports of HCUE by noncardiologist trainees for assessment of left ventricular (LV) systolic function.7, 10 From these reports, we estimated a negative likelihood ratio of 0.3. In addition, we expected about a quarter of our patients to have LV systolic dysfunction (B.M., personal communication). Therefore, to achieve 95% confidence intervals (CIs) around the point estimate of a negative likelihood ratio that excluded 0.50, our upper bound for a clinically meaningful result, we needed a sample size of approximately 300 patients.18
We defined threshold levels of ordinal severity for the 6 cardiac abnormalities under study based on their clinical pertinence to hospitalists (Table 2). Here, we reasoned that abnormalities at or above these levels would likely lead to important changes in hospitalists' management of inpatients; abnormalities below these levels rarely represent cardiac disease that is worthy of an immediate change in management. Since even mild degrees of LV dysfunction have important diagnostic and therapeutic implications for most general medicine inpatients, particularly those presenting with heart failure,19 we set our threshold for LV dysfunction at mild or greater. In contrast, since neither mild nor moderate mitral regurgitation (MR) has immediate implications for medical or surgical therapy even if symptoms or LV dysfunction are present,20 we set our threshold for MR at severe. Similarly, though mild LA enlargement21 and mild LV hypertrophy22 have clear prognostic implications for patients' chronic medical conditions, we reasoned that only moderate or severe versions likely reflect underlying abnormalities that affect hospitalists' point‐of‐care decision‐making. Since cardiac tamponade is rarely both subclinical23 and due to a small pericardial effusion,24 we set our threshold for pericardial effusion size at moderate or large. Finally, we set our threshold IVC diameter, a marker of central venous volume status,25 at dilated, because volume overload is an important consideration in hospitalized cardiac patients.
Using these thresholds, investigators dichotomized echocardiographers' SE readings as normal or abnormal for each of the 6 cardiac abnormalities under study to serve as the reference standards. Hospitalists' HCUE results were then compared to the reference standards in 2 different ways. We first analyzed HCUE results as dichotomous values to calculate conventional sensitivity, specificity, and positive and negative likelihood ratios. Here we considered indeterminate HCUE results positive in a clinically conservative tradeoff that neither ignores indeterminate results nor risks falsely classifying them as negative.26 We then analyzed hospitalists' HCUE results as ordinal values for receiver operating characteristic (ROC) curve analysis. Here we considered an indeterminate result as 1 possible test result.27
To examine interobserver variability of HCUE, we first chose from the 6 possible assessments only those with a mean number of abnormal patients per hospitalist greater than 5. We reasoned that variability among assessments with lower prevalence would be predictably wide and inconclusive. We then expressed variability as standard deviations (SDs) around mean sensitivity and specificity for the 8 hospitalists.
The CIs for likelihood ratios were constructed using the likelihood‐based approach to binomial proportions of Koopman.28 The areas under ROC curves were computed using the trapezoidal rule, and the CIs for these areas were constructed using the algorithm described by DeLong et al.29 All analyses were conducted with Stata Statistical Software, Release 10 (StataCorp, College Station, TX).
Results
During the 3 month study period, 654 patients were referred for SE from the 3 participating patient care units (Figure 1). Among these, 65 patients were ineligible because their SE was performed on the weekend and 178 other patients were not randomized from the general medical wards and CCU. From the remaining eligible patients, 322 underwent HCUE and 314 (98% of 322) underwent both SE and HCUE. Individual SE assessments were not interpretable (and therefore excluded) due to poor image quality for LA enlargement in 1 patient and IVC dilatation in 30 patients. Eighty‐three percent of patients who underwent SE (260/314) were referred to assess LV function (Table 3). The prevalence of the 6 clinically pertinent cardiac abnormalities under study ranged from 1% for moderate or large pericardial effusion to 25% for LV systolic dysfunction. Overall, 40% of patients had at least 1 out of 6 cardiac abnormalities.
| Characteristic | |
|---|---|
| |
| Age, year SD (25th to 75th percentiles) | 56 13 (48 to 64) |
| Women | 146 (47) |
| Chronic obstructive pulmonary disease | 47 (15) |
| Body mass index | |
| 24.9 or less: underweight or normal | 74 (24) |
| 25 to 29.9: overweight | 94 (30) |
| 30 to 34.9: mild obesity | 75 (24) |
| 35 or greater: moderate or severe obesity | 71 (23) |
| Patient care unit | |
| Short‐stay unit | 175 (56) |
| General medical wards | 89 (28) |
| Cardiac care unit | 50 (16) |
| Indication for standard echocardiography* | |
| Left ventricular function | 260 (83) |
| Valvular function | 56 (18) |
| Wall motion abnormality | 29 (9) |
| Valvular vegetations | 22 (7) |
| Any structural heart disease | 20 (6) |
| Right ventricular function | 18 (6) |
| Other | 38 (12) |
| Standard echocardiography findings | |
| Left ventricular systolic dysfunction mild | 80 (25) |
| Inferior vena cava dilated | 45 (14) |
| Left ventricular wall thickness moderate | 33 (11) |
| Left atrium enlargement moderate | 19 (6) |
| Mitral valve regurgitation severe | 11 (4) |
| Pericardial effusion moderate | 3 (1) |
| At least 1 of the above findings | 127 (40) |
| Time difference between HCUE and standard echocardiogram, median hours (25th to 75th percentiles) | 2.8 (1.4 to 5.1) |
| Time to complete HCUE, median minutes (25th to 75th percentiles) | 28 (20 to 35) |
Each hospitalist performed a similar total number of HCUE examinations (range, 3447). The median time difference between performance of SE and HCUE was 2.8 hours (25th75th percentiles, 1.45.1). Despite the high prevalence of chronic obstructive pulmonary disease and obesity, hospitalists considered HCUE assessments indeterminate in only 2% to 6% of the 6 assessments made for each patient (Table 4). Among the 38 patients (12% of 322) with any indeterminate HCUE assessment, 24 patients had only 1 out of 6 possible. Hospitalists completed HCUE in a median time of 28 minutes (25th‐75th percentiles, 2035), which included the time to record 7 best‐quality moving images and to fill out the research data collection form.
| n (%)* | |
|---|---|
| |
| Number of indeterminate findings per patient | |
| 0 | 284 (88) |
| 1 | 24 (7) |
| 2 | 4 (1) |
| 3 or more | 10 (3) |
| Indeterminate findings by cardiac assessment | |
| Mitral valve regurgitation | 18 (6) |
| Inferior vena cava diameter | 16 (5) |
| Left ventricular hypertrophy | 15 (5) |
| Pericardial effusion | 9 (3) |
| Left atrium size | 5 (2) |
| Left ventricle systolic function | 5 (2) |
When HCUE results were analyzed as dichotomous values, positive likelihood ratios ranged from 2.5 to 21, and negative likelihood ratios ranged from 0 to 0.4 (Table 5). Positive and negative likelihood ratios were both sufficiency high and low to respectively increase and decrease by 5‐fold the prior odds of 3 out of 6 cardiac abnormalities: LV systolic dysfunction, moderate or severe MR regurgitation, and moderate or large pericardial effusion. Considering HCUE results as ordinal values for ROC analysis yielded additional diagnostic information (Figure 2). For example, the likelihood ratio of 1.0 (95% CI, 0.42.0) for borderline positive moderate or severe LA enlargement increased to 29 (range, 1362) for extreme positive results. Areas under the ROC curves were 0.9 for 4 out of 6 cardiac abnormalities.

| Clinically Pertinent Cardiac Abnormality by Standard Echocardiography | Prevalence n/total n | Sensitivity* % (95% CI) | Specificity* % (95% CI) | LRpositive*, (95% CI) | LRnegative*, (95% CI) |
|---|---|---|---|---|---|
| |||||
| Left ventricular systolic dysfunction | 80/314 | 85 (7592) | 88 (8392) | 6.9 (4.99.8) | 0.2 (0.10.3) |
| Mitral valve regurgitation, severe | 11/314 | 100 (72100) | 83 (7987) | 5.9 (3.97.4) | 0 (00.3) |
| Left atrium enlargement, moderate or severe | 19/313 | 90 (6799) | 74 (6879) | 3.4 (2.54.3) | 0.1 (0.040.4) |
| Left ventricular hypertrophy, moderate or severe | 33/314 | 70 (5184) | 73 (6778) | 2.5 (1.83.3) | 0.4 (0.20.7) |
| Pericardial effusion, moderate or large | 3/314 | 100 (29100) | 95 (9297) | 21 (6.731) | 0 (00.6) |
| Inferior vena cava, dilated | 45/284 | 56 (4070) | 86 (8190) | 4.0 (2.66.0) | 0.5 (0.40.7) |
LV systolic dysfunction and IVC dilatation were both prevalent enough to meet our criterion to examine interobserver variability; the mean number of abnormal patients per hospitalist was 10 patients for LV systolic dysfunction and 6 patients for IVC dilatation. For LV systolic dysfunction, SDs around mean sensitivity (84%) and specificity (87%) were 12% and 6%, respectively. For IVC dilatation, SDs around mean sensitivity (58%) and specificity (86%) were 24% and 7%, respectively.
Discussion
We found that, after a 27‐hour training program, hospitalists performed HCUE with moderate to excellent diagnostic accuracy for 6 important cardiac abnormalities. For example, hospitalists' assessments of LV systolic function yielded positive and negative likelihood ratios of 6.9 (95% CI, 4.99.8) and 0.2 (95% CI, 0.10.3), respectively. At the bedsides of patients with acute heart failure, therefore, hospitalists could use HCUE to lower or raise the 50:50 chance of LV systolic dysfunction30 to 15% or 85%, respectively. Whether or not these posttest likelihoods are extreme enough to cross important thresholds will depend on the clinical context. Yet these findings demonstrate how HCUE has the potential to provide hospitalists with valuable point‐of‐care data that are otherwise unavailableeither because routine clinical assessments are unreliable31 or because echocardiographic services are not immediately accessible.1
In fact, recent data from the Joint Commission on Accreditation of Healthcare Organizations shows how inaccessible SE may be. Approximately one‐quarter of hospitals in the United States send home about 10% of patients with acute heart failure without echocardiographic assessment of LV systolic function before, during, or immediately after hospitalization.32 In doing so, these hospitals leave unmet the 2002 National Quality Improvement Goal of universal assessment of LV systolic function for all heart failure patients. Hospitalists could close this quality gap with routine, 10‐minute HCUE assessments in all patients admitted with acute heart failure. (Our research HCUE protocol required a median time of 28 minutes, but this included time to assess 5 other cardiac abnormalities and collect data for research purposes). Until the clinical consequences of introducing hospitalist‐performed HCUE are studied, potential benefits like this are tentative. But our findings suggest that training hospitalists to accurately perform HCUE can be successfully accomplished in just 27 hours.
Other studies of HCUE training programs for noncardiologists have also challenged the opinion that learning to perform HCUE requires more than 100 hours of training.2, 711 Yet only 1 prior study has examined an HCUE training program for hospitalists.5 In this study by Martin et al.,5 hospitalists completed 5 supervised HCUE examinations and 6 hours of interpretation training before investigators scored their image acquisition and interpretation skills from 30 unsupervised HCUE examinations. To estimate their final skill levels at the completion of all 35 examinations by accounting for an initially steep learning curve, investigators then adjusted these scores with regression models. Despite these upward adjustments, hospitalists' image acquisition and interpretation scores were low in comparison to echocardiographic technicians and cardiology fellows. Besides these adjusted measurements of hospitalists' skills, however, Martin et al.5 unfortunately did not also report standard measures of diagnostic accuracy, like those proposed by the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.33 Therefore, direct comparisons to the present study are difficult. Nevertheless, their findings suggest that a training program limited to 5 supervised HCUE examinations may be inadequate for hospitalists. In fact, the same group's earlier study of medical trainees suggested a minimum of 30 supervised HCUE examinations.9 We chose to design our hospitalist training program based on this minimum, though they surprisingly did not.5 As others continue to refine the components of hospitalist HCUE training programs, such as the optimal number of supervised examinations, our program could serve as a reasonable comparative example: more rigorous than the program designed by Martin et al.5 but more feasible than ASE level 1 training.
The number and complexity of assessments taught in HCUE training programs will determine their duration. With ongoing advancements in HCUE technology, there is a growing list of potential assessments to choose from. Although HCUE training programs ought to include assessments with proven clinical applications, there are no trials of HCUE‐directed care to inform such decisions. In their absence, therefore, we chose 6 assessments based on the following 3 criteria. First, our assessments were otherwise not reliably available from routine clinical data, such as the physical examination. Second, our assessments were straightforward: easy to learn and simple to perform. Here, we based our reasoning on an expectation that the value of HCUE lies not in highly complex, state‐of‐the‐art assessmentswhich are best left to echocardiographers equipped with SEbut in simple, routine assessments made with highly portable machines that grant noncardiologists newfound access to point‐of‐care data.34 Third, our assessments were clinically pertinent and, where appropriate, defined by cut‐points at levels of severity that often lead to changes in management. We suspect that setting high cut‐points has the salutary effects of making assessments easier to learn and more accurate, because distinguishing mild abnormalities is likely the most challenging aspect of echocardiographic interpretation.35 Whether or not our choices of assessments, and their cut‐points, are optimal has yet to be determined by future research designed to study how they affect patient outcomes. Given our hospitalists' performance in the present study, these assessments seem worthy of such future research.
Our study had several limitations. We studied physicians and patients from only 1 hospital; similar studies performed in different settings, particularly among patients with different proportions and manifestations of disease, may find different results. Nevertheless, our sampling method of prospectively enrolling consecutive patients strengthens our findings. Some echocardiographic measurement methods used by our hospitalists differed in subtle ways from echocardiography guideline recommendations.35 We chose our methods (Table 2) for 2 reasons. First, whenever possible, we chose methods of interpretation that coincided with our local cardiologists'. Second, we chose simplicity over precision. For example, the biplane method of disks, or modified Simpson's rule, is the preferred volumetric method of calculating LA size.35 This method requires tracing the contours of the LA in 2 planes and then dividing the LA volume into stacked oval disks for calculation. We chose instead to train our hospitalists in a simpler method based on 2 linear measurements. Any loss of precision, however, was balanced by a large gain in simplicity. Regardless, minor variations in LA size are not likely to affect hospitalists' bedside evaluations. Finally, we did not validate the results of our reference standard (SE) by documenting interobserver reliability. Yet, because SE is generally accurate for the 6 cardiac abnormalities under study, the effect of this bias should be small.
These limitations can be addressed best by controlled trials of HCUE‐directed care. These trials will determine the clinical impact of hospitalist‐performed HCUE and, in turn, inform our design of HCUE training programs. As the current study shows, training hospitalists to participate in such trials is feasible: like other groups of noncardiologists, hospitalists can accurately perform HCUE after a brief training program. Whether or not hospitalists should perform HCUE requires further study.
Acknowledgements
The authors thank Sonosite, Inc., Bothell, WA, for loaning us 2 MicroMaxx machines throughout the study period. They also thank the staff of the Internal Medicine Research Mentoring Program at Rush Medical College for their technical support and the staff of the Division of Neurology at Stroger Hospital for granting them access to a procedure room.
- .The physical examination of the future: echocardiography as part of the assessment.ACC Curr J Rev.1998;7:79–81.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20:477–485.
- ,.Bedside ultrasonography in the ICU: Part 2.Chest.2005;128:1766–1781.
- ,.Practical Guide to Emergency Ultrasound.1st ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120:1000–1004.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the echocardiography task force on new technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc Echocardiogr.2002;15:369–373.
- ,,,,,.The use of small personal ultrasound devices with internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,, et al.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147:476–481.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118:1010–1018.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96:1002–1006.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20:471–476.
- ,,, et al.A hospitalist‐run short stay unit: features that predict patients' length‐of‐stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- .Adult echocardiography scanning protocol. In: Templin BB, ed.Ultrasound Scanning: Principles and Protocols.2nd ed.Philadelphia, PA:Saunders;1999:426.
- ,,, et al.ACCF 2008 Recommendations for training in adult cardiovascular medicine core cardiology training (COCATS 3) (revision of the 2002 COCATS training statement).J Am Coll Cardiol.2008;51:333–414.
- ,,.The Echo Manual.2nd ed.Philadelphia, PA:Lippincott Williams 1999.
- ,,, et al.Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications.J Am Soc Echocardiogr.1991;4:203–214.
- .Textbook of Clinical Echocardiography.3rd ed.Philadelphia, PA:Elsevier Saunders;2004.
- ,,.Likelihood ratios with confidence: sample size estimation for diagnostic test studies.J Clin Epidemiol.1991;44:763–770.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2005;112;154–235.
- ,,, et al.ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2006;114:e84–e231.
- ,,, et al.Left atrial size: physiologic determinants and clinical applications.J Am Coll Cardiol.2006;47:2357–2363.
- ,,,,.Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study.N Engl J Med.1990;322:1561–1566.
- ,,,.Does this patient with a pericardial effusion have cardiac tamponade?JAMA.2007;297:1810–1818.
- .Acute cardiac tamponade.N Engl J Med.2003;349:685–690.
- ,,,,,.Evaluation of size and dynamics of the inferior vena cava as an index of right‐sided cardiac function.Am J Cardiol.1984;53:579–585.
- ,,.The influence of uninterpretability on the assessment of diagnostic tests.J Chronic Dis.1986;39:575–584.
- ,,.Relations between effectiveness of a diagnostic test, prevalence of the disease, and percentages of uninterpretable results. An example in the diagnosis of jaundice.Med Decis Making.1982;2:285–297.
- .Confidence intervals for the ratio of two binomial proportions.Biometrics.1984;40:513–517.
- ,,.Comparing the areas under two or more correlated receiver operating curves: a nonparametric approach.Biometrics.1988;44:837–845.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296:2217–2226.
- ,,, et al.Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure.Am J Med.2002;112:437–445.
- Joint Commission on Accreditation of Healthcare Organizations. Health Care Quality Data Download Website. Available at: http://www.healthcarequalitydata.org. Accessed December2008.
- ,,, et al.Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative.Clin Chem.2003;49:1–6.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78:102–112.
- ,,, et al.Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology.J Am Soc Echocardiogr.2005;18:1440–1463.
- .The physical examination of the future: echocardiography as part of the assessment.ACC Curr J Rev.1998;7:79–81.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20:477–485.
- ,.Bedside ultrasonography in the ICU: Part 2.Chest.2005;128:1766–1781.
- ,.Practical Guide to Emergency Ultrasound.1st ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120:1000–1004.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the echocardiography task force on new technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc Echocardiogr.2002;15:369–373.
- ,,,,,.The use of small personal ultrasound devices with internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,, et al.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147:476–481.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118:1010–1018.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96:1002–1006.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20:471–476.
- ,,, et al.A hospitalist‐run short stay unit: features that predict patients' length‐of‐stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- .Adult echocardiography scanning protocol. In: Templin BB, ed.Ultrasound Scanning: Principles and Protocols.2nd ed.Philadelphia, PA:Saunders;1999:426.
- ,,, et al.ACCF 2008 Recommendations for training in adult cardiovascular medicine core cardiology training (COCATS 3) (revision of the 2002 COCATS training statement).J Am Coll Cardiol.2008;51:333–414.
- ,,.The Echo Manual.2nd ed.Philadelphia, PA:Lippincott Williams 1999.
- ,,, et al.Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications.J Am Soc Echocardiogr.1991;4:203–214.
- .Textbook of Clinical Echocardiography.3rd ed.Philadelphia, PA:Elsevier Saunders;2004.
- ,,.Likelihood ratios with confidence: sample size estimation for diagnostic test studies.J Clin Epidemiol.1991;44:763–770.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2005;112;154–235.
- ,,, et al.ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2006;114:e84–e231.
- ,,, et al.Left atrial size: physiologic determinants and clinical applications.J Am Coll Cardiol.2006;47:2357–2363.
- ,,,,.Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study.N Engl J Med.1990;322:1561–1566.
- ,,,.Does this patient with a pericardial effusion have cardiac tamponade?JAMA.2007;297:1810–1818.
- .Acute cardiac tamponade.N Engl J Med.2003;349:685–690.
- ,,,,,.Evaluation of size and dynamics of the inferior vena cava as an index of right‐sided cardiac function.Am J Cardiol.1984;53:579–585.
- ,,.The influence of uninterpretability on the assessment of diagnostic tests.J Chronic Dis.1986;39:575–584.
- ,,.Relations between effectiveness of a diagnostic test, prevalence of the disease, and percentages of uninterpretable results. An example in the diagnosis of jaundice.Med Decis Making.1982;2:285–297.
- .Confidence intervals for the ratio of two binomial proportions.Biometrics.1984;40:513–517.
- ,,.Comparing the areas under two or more correlated receiver operating curves: a nonparametric approach.Biometrics.1988;44:837–845.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296:2217–2226.
- ,,, et al.Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure.Am J Med.2002;112:437–445.
- Joint Commission on Accreditation of Healthcare Organizations. Health Care Quality Data Download Website. Available at: http://www.healthcarequalitydata.org. Accessed December2008.
- ,,, et al.Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative.Clin Chem.2003;49:1–6.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78:102–112.
- ,,, et al.Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology.J Am Soc Echocardiogr.2005;18:1440–1463.
Copyright © 2009 Society of Hospital Medicine
Improving Central Venous Catheterization
At times central venous catheters are essential to the delivery of appropriate medical care. Because catheter‐related complications are associated with limited operator experience,1 insertion technique,2 and venous site of insertion (eg, femoral, internal jugular, or subclavian vein),3 house staff training programs strive to provide their residents with appropriate training and oversight for this skill. Most quality improvement initiatives directed at reducing complications associated with central venous catheters have focused on patients in the intensive care unit (ICU).4, 5 However, in some hospitals more central venous catheters are inserted in patients not in the ICU,6 and practices that increase the risk of complications may be more common on wards.7
In our hospital, most catheters are placed in the femoral vein. Because femoral venous placement likely increases a patient's risk of thrombosis, hematoma, and bloodstream infection,8 we developed a program to change residents' choice of venous insertion site and improve their infection‐control practices during their general medicine ward rotation. The program provided simulated hands‐on experience in a simulation laboratory. We evaluated our intervention through a firm‐based clinical trial that compared the usual practice to our intervention. We compared infection‐control practices and resident choice of venous insertion site between the intervention and control groups; we also assessed residents' knowledge about catheter‐related complications, and we monitored patients for catheter‐related complications.
METHODS
Setting and Study Design
We conducted a prospective, firm‐based clinical trial approved by the institutional review board at Cook County Hospital, a 464‐bed public teaching hospital. We evaluated all central venous catheters inserted by residents on the general medicine service from November 15, 2004, to March 31, 2005. The internal medicine residency program assigns residents to 1 of 3 firms for their entire 3 years of training. We designated 1 firm as the intervention group; the other 2 firms constituted the control group.
Educational Intervention
At the beginning of each 4‐week general medicine ward rotation, intervention‐firm residents attended an educational and simulation laboratory session. Control‐firm residents received the usual ward orientation. We conducted 6 sessions, with total attendance of 40 intervention‐firm residents, or approximately 7 residents per session. A chief medical resident experienced in catheter placement and an attending internist led and supervised each 2‐hour training session. The sessions were conducted at the Simulation Laboratory of Rush University and included a presentation about indications for central venous catheter insertion, insertion techniques, common complications, and practice placing catheters in mannequins. During the hands‐on session, each participant observed the expert insert a triple‐lumen catheter in the mannequin's internal jugular and subclavian veins. Then, with supervision, each participant practiced catheter insertion using recommended infection‐control practices (eg, use of gloves, mask, and large drape, and chlorhexidine skin preparation).
Resident Survey
Before each session, we administered a survey that assessed residents' knowledge of insertion techniques and their confidence in placing catheters at each venous insertion site. To measure change in the confidence level of residents, we distributed an abbreviated survey 2 additional times, immediately after the session and at the end of the study period. We measured confidence with answers to survey questions, which were rated on a 5‐point Likert scale, from strongly disagree to strongly agree. In addition to measuring the change in residents' confidence, the final survey repeated knowledge assessment questions, evaluated residents' attitudes regarding venous insertion sites, and asked about potential strategies to improve insertion practices.
Central Venous Catheter Detection and Monitoring
At the end of each day, residents reported catheter insertions to chief residents during routine sign‐out rounds. If a catheter had been inserted, the chief resident interviewed the resident about type of catheter, venous insertion site, duration of attempt, patient location, immediate complications, number of inserters, inserter attendance at an educational session, inserter specialty, and professional designation (eg, resident, fellow, attending), indication for insertion, and adherence to infection‐control practices. For all insertion attempts, the research team reviewed the medical record and recorded patient characteristics that might influence venous insertion site (eg, thrombocytopenia, coagulopathy, and body mass index) and evaluated patients for insertion‐related complications.
We prospectively monitored patients for mechanical (ie, pneumothorax or hematoma), thromboembolic, or infectious complications. To evaluate for pneumothorax, postinsertion chest radiographs were reviewed by a physician‐investigator, and radiologists' interpretations and progress notes were reviewed. To evaluate for infectious or other mechanical complications, progress notes also were reviewed. We required radiographic confirmation of venous thromboembolism. To categorize potential bloodstream infections, we used Centers for Disease Control and Prevention definitions.9 All medical record and radiograph reviews were performed by investigators who were masked to patient firm assignment. We monitored patients until catheter removal or hospital discharge. After patient discharge, we reviewed the electronic record, including emergency room visits and repeat hospitalizations, for 30 days after the earlier of hospital discharge or catheter removal.
Statistics
Because we were aware that temporary dialysis catheters are sometimes placed in femoral veins to preserve the subclavian or internal jugular venous sites for more permanent tunneled intravascular catheters, our prespecified plans were to compare venous insertion sites between intervention and control groups after excluding temporary dialysis catheters. To more completely describe catheter use, we also collected data on temporary dialysis catheters, and we present the results both with and without inclusion of data on temporary dialysis catheters. If multiple residents attempted to insert a catheter, we would have used the group that the final inserter was in to determine intervention versus control group assignment; however, this never occurred.
To determine resident confidence in inserting catheters, we collapsed the responses of agree and strongly agree and of disagree and strongly disagree into single categories; thus, frequency of agreement was evaluated as a dichotomous outcome. To test whether residents' confidence changed between the 3 surveys, we analyzed responses using the matched‐pair signed rank test, with the initial survey used as the referent.
We dichotomized certain continuous variables using the following cut points: body mass index 30 kg/m2; coagulopathy, international normalized ratio (INR) > 1.5; thrombocytopenia, platelets < 100 109/L. Data were entered into a relational database (Microsoft Access, Microsoft Inc., Redmond, WA) and merged analyzed using Stata software, version 8.2 (Stata Corporation, College Station, TX).
RESULTS
Patient and Catheter Characteristics
Fifty‐four catheters were inserted in 48 patients during the study period, 16 (30%) in the intervention group and 38 (70%) in the control group. Mean number of catheters inserted per resident for each 4‐week rotation was 0.24; therefore, on average, a resident would insert 1 catheter every 4 general‐medicine rotations. Most catheters were inserted between 7:00 AM and 5:00 PM; the most common reason for insertion was to administer intravenous medications to a patient without intravenous access, followed by the need for a temporary dialysis catheter. Most catheters were inserted by the medicine team rather than radiology or a subspecialty service (Table 1). Most patient characteristics and reasons for insertion were similar between groups; however, more patients in the control group had thrombocytopenia (Table 1).
| Characteristic | Central venous catheters inserted | ||
|---|---|---|---|
| Intervention (n = 16), n (%) | Control (n = 38), n (%) | P | |
| |||
| Patient | |||
| Body mass index 30 kg/m2 | 5 (31) | 11 (29) | 1.0 |
| INR > 1.5 | 3 (19) | 3 (7.9) | 0.37 |
| Platelet count < 100k | 0 (0) | 9 (24) | 0.05 |
| Charlson index, mean (interquartile range) | 2 (24) | 2 (14) | 0.58 |
| Physician inserting catheter | |||
| Resident on general medicine servicea | 15 (100) | 34/37 (92) | 1.0 |
| Subspecialty fellow | 0 (0) | 2/37 (5.3) | 1.0 |
| Radiology fellow or attending | 0 (0) | 1/37 (2.6) | 1.0 |
| Reason for insertion | |||
| No intravenous access | 7 (44) | 19 (50) | 0.67 |
| Temporary dialysis catheterb | 7 (44) | 14 (37) | 0.63 |
| Total parenteral nutrition | 1 (6.2) | 3 (7.9) | 1.0 |
| Otherc | 1 (6.2) | 2 (5.3) | 1.0 |
| Time of day of insertion | |||
| Between 7 AM and 5 PM | 12/14 (86) | 25/37 (68) | 0.30 |
Insertion Practices
Femoral venous insertion was the most common type of catheter insertion (67%), followed by internal jugular (26%) and subclavian (7%); there were no differences in insertion site between the intervention and control groups (Table 2). When we excluded temporary dialysis catheters, 39% of central venous catheters were inserted in the internal jugular vein. Although a smaller proportion of catheters inserted by the intervention group were placed in a femoral vein, the difference was not significant (Table 2).
| Intervention (n = 16), n (%) | Control (n = 38), n (%) | Risk ratio (95% CI) | P | |
|---|---|---|---|---|
| ||||
| Self‐reported practices during CVC insertion | ||||
| Mask worn | 12 (75) | 13 (34) | 2.2 (1.33.7) | 0.008 |
| Large drape used | 15 (94) | 28 (74) | 1.3 (1.01.6) | 0.14 |
| Cap worn | 3 (19) | 5 (13) | 1.4 (0.45.3) | 0.6 |
| Gown worn | 8 (50) | 18 (47) | 1.1 (0.61.9) | 0.9 |
| Sterile gloves worn | 15 (94) | 36 (95) | 1.0 (0.81.2) | 1.0 |
| Venous insertion sitea | Difference (95% CI) | |||
| Femoral | 10 (62) | 26 (68) | 6% (34%22%) | 0.67 |
| Internal jugular | 5 (31) | 9 (24) | ||
| Subclavian | 1 (6.2) | 3 (7.9) | ||
| Excluding dialysis cathetersa | n = 9 | n = 24 | ||
| Femoral | 4 (44) | 14 (58) | 14% (52%24%) | 0.7 |
| Internal jugular | 5 (56) | 8 (33) | ||
| Subclavian | 0 (0) | 2 (8) | ||
For most insertions, residents reported using sterile gloves (94%) and a large drape (80%); however, most did not report use of a sterile gown (48%), mask (46%), or cap (15%). Residents in the intervention group were more likely to report use of a mask, and there was a trend toward increased use of large drapes (Table 2). No patient characteristics predicted femoral venous insertion (data not shown).
Complications
The most frequent complication was arterial puncture (n = 4); all four occurred during femoral venous insertion attempts. Compared to subclavian or internal jugular venous placement, there was a trend toward more mechanical complications among femoral catheters (Table 3). One episode of clinical sepsis occurred, in an intervention‐group patient who had femoral and internal jugular catheters, and no pneumothoraxes or episodes of venous thromboembolism occurred (Table 3). The overall incidence of bloodstream infection was 2.7 per 1000 central‐line days; there was no difference between the intervention and control groups (9.2 versus 0 per 1000 central‐line days; P = .29).
| Complication | Femoral (n = 36), n (%) | Subclavian or IJ (n = 18), n (%) | Difference (95% CI) |
|---|---|---|---|
| |||
| Mechanical (arterial puncture, hematoma, or pneumothorax)a | 4 (11) | 0 | 11% (1%21%) |
| Venous thromboembolismb | 0 (0) | 0 (0) | 0% |
| Infection rate (per 1000 central‐line days)c | 4.3 | 7.0 | 2.7 (1913) |
Survey Responses
Before the educational session, many residents did not recognize that femoral venous catheter insertions had a higher risk of arterial puncture or venous thrombosis (Table 4); by the final survey, residents were more likely to recognize the higher risk of these complications during femoral venous insertions. Most residents recognized the higher risk of infectious complications at the femoral site (Table 4).
| Respondents in Agreement, n (%) | |||
|---|---|---|---|
| Presession n = 35 | Postsession n = 34 | Follow‐up n = 35 | |
| |||
| Knowledge | |||
| Complications are most frequent at the femoral site | 27 (77%) | 30 (86%) | |
| Arterial puncture risk is lowest at the femoral sitea | 16 (46%) | 7 (21%)b | |
| Thrombosis risk is lowest at the femoral sitea | 11 (31%) | 6 (18%) | |
| Infection risk is lowest at the femoral site | 1/33 (3%) | 0 (0%) | |
| Attitudes | |||
| I feel confident:c | |||
| Inserting a femoral CVC | 53 | 59b | 89d |
| Inserting an internal jugular CVC | 41 | 71d | 40 |
| Inserting a subclavian CVC | 24 | 65d | 34d |
| Options to increase placement in jugular or subclavian veins | |||
| Availability of ultrasound machine | 31 (89) | ||
| Expert supervisor available to assist with placement | 30 (86) | ||
| Insert CVC within 2 weeks of educational session | 30 (86) | ||
| Rotation through a service that often places CVCsa | 26 (76) | ||
| I do not plan to use this skill after my residency | 4 (11) | ||
| Barriers to inserting a subclavian or internal jugular CVC | |||
| Preexisting internal jugular or subclavian CVC | 11 (31) | ||
| For temporary dialysis, desire to preserve site | 26 (74) | ||
| Practices | |||
| More likely to remove unnecessary catheter | 29 (83) | ||
| Improved infection‐control practices | 28 (80) | ||
| Increased motivation for internal jugular or subclavian venous insertion | 27 (78) | ||
| Less likely to place a CVC | 9 (26) | ||
| Internal jugular or subclavian CVC inserted for the first time after training | 7/30 (23) | ||
Residents overwhelmingly responded that the lecture was useful (95%), that mannequins provided a valuable skill‐building exercise (90%), and that the session should be incorporated into the training program (95%). Immediately after the session, residents had increased confidence about inserting a central venous catheter at any venous site, especially for internal jugular or subclavian insertions. By the final survey, the confidence of residents about inserting catheters in the internal jugular or subclavian veins had returned to baseline but had increased for femoral‐site insertions (Table 4).
Most residents in the intervention group agreed that the educational session motivated them to remove unnecessary catheters, improve insertion‐related infection‐control practices, and place the catheter in an internal jugular or subclavian vein; some agreed because of the educational session, they were less likely to place a central venous catheter. Some reported successfully inserting a central venous catheter in the subclavian or internal jugular vein for the first time (Table 4).
DISCUSSION
An educational session designed to teach residents appropriate central venous catheter insertion practices that included simulated hands‐on training increased knowledge about insertion‐related complications and improved certain infection‐control practices. Although residents' confidence in inserting subclavian or internal jugular catheters initially improved, our training session did not change the choice of venous insertion site from femoral to subclavian or internal jugular veins, possibly because there were few opportunities for residents to insert a catheter during the 4‐week general medical ward rotations. Thus, although an active educational intervention improved the knowledge and confidence of residents, it had a minimal effect on behavior (only improved certain infection‐control practices). Catheter‐associated complications were infrequent and similar in the intervention and control groups.
Central venous catheter insertion is a skill that many general internists do not perform10; however, until recently the American Board of Internal Medicine considered it a requisite skill for internal medicine residents, and most residents at our hospital reported a desire to learn this skill. Although in our study complications were infrequent, suggesting that a change in venous insertion site is unlikely to dramatically improve patient safety, we believe that residents should become skilled at inserting catheters in internal jugular or subclavian veins, the currently recommended optimal venous insertion.8
There is evidence that single educational interventions are unlikely to result in substantial, sustained behavioral change, especially passive educational programs.11 However, a previous study documented a change in provider behavior and possibly a reduction in bloodstream infections after a single hands‐on training session.12 Our hands‐on educational format was very popular and likely improved some infection‐control practices but did not change provider behavior about choice of venous insertion site. In other institutions, mentoring residents on appropriate catheter insertion technique has been accomplished by establishing a procedure service13 or by resident rotation in a high‐volume location (eg, cardiac catheterization laboratory).14 Another option to facilitate behavioral change would be to provide a portable ultrasound machine, as requested by our residents, which may reduce complication rates.15, 16 At our hospital, we decided to supplement hands‐on training with expert bedside supervision during catheter insertion; the expert is provided through a procedure service that is led by hospitalists. The procedure service has a dedicated portable ultrasound machine to assist with internal jugular vein cannulation.
By the end of our study period, residents' confidence in subclavian or internal jugular catheter insertions had returned to presession levels; however, they reported increased confidence in femoral venous catheter insertions. These findings suggest that the session increased residents' confidence with catheter insertions in general, but not specifically for venous sites for which they had no previous experience. For subclavian or internal jugular catheter insertions, their confidence decayed to the presession baseline, likely because of few opportunities to insert catheters in patients; on average, each resident inserts 1 central venous catheter on the general medicine wards approximately every 4 months.
Our survey found that our intervention changed residents' attitudes about infection‐control practices. In particular, intervention‐group residents reported that they were more likely to remove unnecessary catheters and that they had used a mask and large drape during catheter insertion. Use of full‐barrier precautions (ie, sterile gloves and gown, large sterile drape, cap, and mask) has been shown to reduce the risk of bloodstream infection2 and is included in national guidelines.17 Adherence to these guidelines has been included in successful quality improvement initiatives.4, 5, 18 Compared to internists' adherence to recommendations for infection control reported in another survey,10 residents who attended our educational session reported more use of large sterile drapes (94% vs. 35%) or masks (75% vs. 66%); however, they were less likely to use a sterile gown (50% vs. 72%). Use of a large sterile drape is common in our hospital, likely because the drape is included in the central venous catheter package. We suspect that at our hospital, poor adherence to certain recommendations (eg, using a sterile gown) was due in part to difficulty accessing supplies. Another possibility is that use of a cap, compared to use of large drapes, is perceived as not giving the patient much additional protection. In fact, there is no evidence that using a cap provides benefit beyond that of other, more intuitively beneficial recommended infection‐control practices, such as using sterile gloves and a large sterile drape. The procedure service has addressed the supply problem by stocking hard‐to‐find items on a procedure cart.
Only 2 clinically evident complications associated with catheter insertion occurred (one patient with clinical sepsis and one with a hematoma). Although it is possible that we missed minor complications, our rates were similar to those reported by other investigators: clinically diagnosed venous thromboembolism, 0%2.2%3, 19, 20; pneumothorax, 1.4%21; catheter‐associated primary bloodstream infection, 1‐6/1000 catheter‐days.22, 23 Comparing complication rates was hindered by variability in definitions, methods of ascertainment, and populations evaluated. For example, the rate of venous thromboembolism was dramatically higher when routine diagnostic imaging was used, and detection of catheter‐associated infections likely increased when catheter‐tip cultures were routinely performed. We required clinical evidence of complications, and our study differs from others in that we evaluated general medicine ward patients.
This study had several limitations. Placement of central venous catheters on general medicine wards was less frequent than we anticipated based on a brief period of pilot data collection; therefore, our study was not powered to detect relatively small changes in venous insertion sites or differences in complications. Also, because direct observation was not possible, we relied on self‐reported adherence to infection‐control practices. However, intervention residents' self‐reported poor adherence to gown, glove, and cap use suggests that their responses were unbiased.
An educational session focused on central venous catheter insertion practices was well received by residents, increased their knowledge about complications, and improved infection‐control practices, but had no effect on increasing use of subclavian or internal jugular veins for catheter insertion. Despite continued frequent use of femoral venous catheters, clinically apparent complications were infrequent. However, we believe it is important to teach residents optimal catheter insertion techniques, including preferential placement of catheters in subclavian or internal jugular veins. Therefore, the section of hospital medicine at our hospital initiated a procedure service that provides expert bedside supervision, including use of a portable ultrasound machine, for catheter insertions.
Acknowledgements
The authors acknowledge Kathleen Murray for data collection and form development; Donald Blom for assistance with determining bloodstream infection; Laura Sadowski for developing and leading the focus group session; Yannis Guerra for assistance with the educational sessions; Oksana Barilyak, Anand Despande, and Saurabh Sharma for assistance with data collection; and chief residents Rony Ghaoui, Sean Halleran, Priya Kansal, Parag Sampat, and Sunita Nathan for interviewing residents about catheter insertions.
- ,,,,.Central vein catheterization. Failure and complication rates by three percutaneous approaches.Arch Intern Med.1986;146:259–261.
- ,,, et al.Prevention of central venous catheter‐related infections by using maximal sterile barrier precautions during insertion.Infect Control Hosp Epidemiol.1994;15:231–238.
- ,,, et al.Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial.JAMA.2001;286:700–707.
- ,,, et al.Eliminating catheter‐related bloodstream infections in the intensive care unit.Crit Care Med.2004;32:2014–2020.
- ,,, et al.The effect of an education program on the incidence of central venous catheter‐associated bloodstream infection in a medical ICU.Chest.2004;126:1612–1618.
- ,,, et al.Prevalence of the use of central venous access devices within and outside of the intensive care unit: results of a survey among hospitals in the prevention epicenter program of the Centers for Disease Control and Prevention.Infect Control Hosp Epidemiol.2003;24:942–945.
- ,,,,,.Unnecessary use of central venous catheters: the need to look outside the intensive care unit.Infect Control Hosp Epidemiol.2004;25:266–268.
- ,.Preventing complications of central venous catheterization.N Engl J Med.2003;348:1123–1133.
- ,,,,.CDC definitions for nosocomial infections, 1988.Am J Infect Control.1988;16:128–140.
- ,,,.Why is it that internists do not follow guidelines for preventing intravascular catheter infections?Infect Control Hosp Epidemiol.2005;26:525–533.
- ,,, et al.Changing provider behavior: an overview of systematic reviews of interventions.Med Care.2001;39:II2–II45.
- ,,, et al.Education of physicians‐in‐training can decrease the risk for vascular catheter infection.Ann Intern Med.2000;132:641–648.
- ,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,,.A curricular initiative for internal medicine residents to enhance proficiency in internal jugular central venous line placement.Mayo Clin Proc.2005;80:212–218.
- ,,,,,.Improvement of internal jugular vein cannulation using an ultrasound‐guided technique.Intensive Care Med.1997;23:916–919.
- ,,.Facilitation of internal jugular venous cannulation using an audio‐guided Doppler ultrasound vascular access device: results from a prospective, dual‐center, randomized, crossover clinical study.Crit Care Med.1995;23:60–65.
- ,,, et al.Guidelines for the prevention of intravascular catheter‐related infections.MMWR Morb Mortal Wkly Rep.2002;1(RR10):1–26.
- ,,, et al.The impact of bedside behavior on catheter‐related bacteremia in the intensive care unit.Arch Surg.2004;139:131–136.
- ,,,,,.A prospective evaluation of the use of femoral venous catheters in critically ill adults.Crit Care Med.1997;25:1986–1989.
- ,,,,.Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients.Chest.2000;117:178–183.
- ,,.Complications of central venous catheters: internal jugular versus subclavian access—a systematic review.Crit Care Med.2002;30:454–460.
- ,,,,,.Prospective evaluation of risk factors for bloodstream infection in patients receiving home infusion therapy.Ann Intern Med.1999;131:340–347.
- ,,,.Nosocomial infections in combined medical‐surgical intensive care units in the United States.Infect Control Hosp Epidemiol.2000;21:510–515.
At times central venous catheters are essential to the delivery of appropriate medical care. Because catheter‐related complications are associated with limited operator experience,1 insertion technique,2 and venous site of insertion (eg, femoral, internal jugular, or subclavian vein),3 house staff training programs strive to provide their residents with appropriate training and oversight for this skill. Most quality improvement initiatives directed at reducing complications associated with central venous catheters have focused on patients in the intensive care unit (ICU).4, 5 However, in some hospitals more central venous catheters are inserted in patients not in the ICU,6 and practices that increase the risk of complications may be more common on wards.7
In our hospital, most catheters are placed in the femoral vein. Because femoral venous placement likely increases a patient's risk of thrombosis, hematoma, and bloodstream infection,8 we developed a program to change residents' choice of venous insertion site and improve their infection‐control practices during their general medicine ward rotation. The program provided simulated hands‐on experience in a simulation laboratory. We evaluated our intervention through a firm‐based clinical trial that compared the usual practice to our intervention. We compared infection‐control practices and resident choice of venous insertion site between the intervention and control groups; we also assessed residents' knowledge about catheter‐related complications, and we monitored patients for catheter‐related complications.
METHODS
Setting and Study Design
We conducted a prospective, firm‐based clinical trial approved by the institutional review board at Cook County Hospital, a 464‐bed public teaching hospital. We evaluated all central venous catheters inserted by residents on the general medicine service from November 15, 2004, to March 31, 2005. The internal medicine residency program assigns residents to 1 of 3 firms for their entire 3 years of training. We designated 1 firm as the intervention group; the other 2 firms constituted the control group.
Educational Intervention
At the beginning of each 4‐week general medicine ward rotation, intervention‐firm residents attended an educational and simulation laboratory session. Control‐firm residents received the usual ward orientation. We conducted 6 sessions, with total attendance of 40 intervention‐firm residents, or approximately 7 residents per session. A chief medical resident experienced in catheter placement and an attending internist led and supervised each 2‐hour training session. The sessions were conducted at the Simulation Laboratory of Rush University and included a presentation about indications for central venous catheter insertion, insertion techniques, common complications, and practice placing catheters in mannequins. During the hands‐on session, each participant observed the expert insert a triple‐lumen catheter in the mannequin's internal jugular and subclavian veins. Then, with supervision, each participant practiced catheter insertion using recommended infection‐control practices (eg, use of gloves, mask, and large drape, and chlorhexidine skin preparation).
Resident Survey
Before each session, we administered a survey that assessed residents' knowledge of insertion techniques and their confidence in placing catheters at each venous insertion site. To measure change in the confidence level of residents, we distributed an abbreviated survey 2 additional times, immediately after the session and at the end of the study period. We measured confidence with answers to survey questions, which were rated on a 5‐point Likert scale, from strongly disagree to strongly agree. In addition to measuring the change in residents' confidence, the final survey repeated knowledge assessment questions, evaluated residents' attitudes regarding venous insertion sites, and asked about potential strategies to improve insertion practices.
Central Venous Catheter Detection and Monitoring
At the end of each day, residents reported catheter insertions to chief residents during routine sign‐out rounds. If a catheter had been inserted, the chief resident interviewed the resident about type of catheter, venous insertion site, duration of attempt, patient location, immediate complications, number of inserters, inserter attendance at an educational session, inserter specialty, and professional designation (eg, resident, fellow, attending), indication for insertion, and adherence to infection‐control practices. For all insertion attempts, the research team reviewed the medical record and recorded patient characteristics that might influence venous insertion site (eg, thrombocytopenia, coagulopathy, and body mass index) and evaluated patients for insertion‐related complications.
We prospectively monitored patients for mechanical (ie, pneumothorax or hematoma), thromboembolic, or infectious complications. To evaluate for pneumothorax, postinsertion chest radiographs were reviewed by a physician‐investigator, and radiologists' interpretations and progress notes were reviewed. To evaluate for infectious or other mechanical complications, progress notes also were reviewed. We required radiographic confirmation of venous thromboembolism. To categorize potential bloodstream infections, we used Centers for Disease Control and Prevention definitions.9 All medical record and radiograph reviews were performed by investigators who were masked to patient firm assignment. We monitored patients until catheter removal or hospital discharge. After patient discharge, we reviewed the electronic record, including emergency room visits and repeat hospitalizations, for 30 days after the earlier of hospital discharge or catheter removal.
Statistics
Because we were aware that temporary dialysis catheters are sometimes placed in femoral veins to preserve the subclavian or internal jugular venous sites for more permanent tunneled intravascular catheters, our prespecified plans were to compare venous insertion sites between intervention and control groups after excluding temporary dialysis catheters. To more completely describe catheter use, we also collected data on temporary dialysis catheters, and we present the results both with and without inclusion of data on temporary dialysis catheters. If multiple residents attempted to insert a catheter, we would have used the group that the final inserter was in to determine intervention versus control group assignment; however, this never occurred.
To determine resident confidence in inserting catheters, we collapsed the responses of agree and strongly agree and of disagree and strongly disagree into single categories; thus, frequency of agreement was evaluated as a dichotomous outcome. To test whether residents' confidence changed between the 3 surveys, we analyzed responses using the matched‐pair signed rank test, with the initial survey used as the referent.
We dichotomized certain continuous variables using the following cut points: body mass index 30 kg/m2; coagulopathy, international normalized ratio (INR) > 1.5; thrombocytopenia, platelets < 100 109/L. Data were entered into a relational database (Microsoft Access, Microsoft Inc., Redmond, WA) and merged analyzed using Stata software, version 8.2 (Stata Corporation, College Station, TX).
RESULTS
Patient and Catheter Characteristics
Fifty‐four catheters were inserted in 48 patients during the study period, 16 (30%) in the intervention group and 38 (70%) in the control group. Mean number of catheters inserted per resident for each 4‐week rotation was 0.24; therefore, on average, a resident would insert 1 catheter every 4 general‐medicine rotations. Most catheters were inserted between 7:00 AM and 5:00 PM; the most common reason for insertion was to administer intravenous medications to a patient without intravenous access, followed by the need for a temporary dialysis catheter. Most catheters were inserted by the medicine team rather than radiology or a subspecialty service (Table 1). Most patient characteristics and reasons for insertion were similar between groups; however, more patients in the control group had thrombocytopenia (Table 1).
| Characteristic | Central venous catheters inserted | ||
|---|---|---|---|
| Intervention (n = 16), n (%) | Control (n = 38), n (%) | P | |
| |||
| Patient | |||
| Body mass index 30 kg/m2 | 5 (31) | 11 (29) | 1.0 |
| INR > 1.5 | 3 (19) | 3 (7.9) | 0.37 |
| Platelet count < 100k | 0 (0) | 9 (24) | 0.05 |
| Charlson index, mean (interquartile range) | 2 (24) | 2 (14) | 0.58 |
| Physician inserting catheter | |||
| Resident on general medicine servicea | 15 (100) | 34/37 (92) | 1.0 |
| Subspecialty fellow | 0 (0) | 2/37 (5.3) | 1.0 |
| Radiology fellow or attending | 0 (0) | 1/37 (2.6) | 1.0 |
| Reason for insertion | |||
| No intravenous access | 7 (44) | 19 (50) | 0.67 |
| Temporary dialysis catheterb | 7 (44) | 14 (37) | 0.63 |
| Total parenteral nutrition | 1 (6.2) | 3 (7.9) | 1.0 |
| Otherc | 1 (6.2) | 2 (5.3) | 1.0 |
| Time of day of insertion | |||
| Between 7 AM and 5 PM | 12/14 (86) | 25/37 (68) | 0.30 |
Insertion Practices
Femoral venous insertion was the most common type of catheter insertion (67%), followed by internal jugular (26%) and subclavian (7%); there were no differences in insertion site between the intervention and control groups (Table 2). When we excluded temporary dialysis catheters, 39% of central venous catheters were inserted in the internal jugular vein. Although a smaller proportion of catheters inserted by the intervention group were placed in a femoral vein, the difference was not significant (Table 2).
| Intervention (n = 16), n (%) | Control (n = 38), n (%) | Risk ratio (95% CI) | P | |
|---|---|---|---|---|
| ||||
| Self‐reported practices during CVC insertion | ||||
| Mask worn | 12 (75) | 13 (34) | 2.2 (1.33.7) | 0.008 |
| Large drape used | 15 (94) | 28 (74) | 1.3 (1.01.6) | 0.14 |
| Cap worn | 3 (19) | 5 (13) | 1.4 (0.45.3) | 0.6 |
| Gown worn | 8 (50) | 18 (47) | 1.1 (0.61.9) | 0.9 |
| Sterile gloves worn | 15 (94) | 36 (95) | 1.0 (0.81.2) | 1.0 |
| Venous insertion sitea | Difference (95% CI) | |||
| Femoral | 10 (62) | 26 (68) | 6% (34%22%) | 0.67 |
| Internal jugular | 5 (31) | 9 (24) | ||
| Subclavian | 1 (6.2) | 3 (7.9) | ||
| Excluding dialysis cathetersa | n = 9 | n = 24 | ||
| Femoral | 4 (44) | 14 (58) | 14% (52%24%) | 0.7 |
| Internal jugular | 5 (56) | 8 (33) | ||
| Subclavian | 0 (0) | 2 (8) | ||
For most insertions, residents reported using sterile gloves (94%) and a large drape (80%); however, most did not report use of a sterile gown (48%), mask (46%), or cap (15%). Residents in the intervention group were more likely to report use of a mask, and there was a trend toward increased use of large drapes (Table 2). No patient characteristics predicted femoral venous insertion (data not shown).
Complications
The most frequent complication was arterial puncture (n = 4); all four occurred during femoral venous insertion attempts. Compared to subclavian or internal jugular venous placement, there was a trend toward more mechanical complications among femoral catheters (Table 3). One episode of clinical sepsis occurred, in an intervention‐group patient who had femoral and internal jugular catheters, and no pneumothoraxes or episodes of venous thromboembolism occurred (Table 3). The overall incidence of bloodstream infection was 2.7 per 1000 central‐line days; there was no difference between the intervention and control groups (9.2 versus 0 per 1000 central‐line days; P = .29).
| Complication | Femoral (n = 36), n (%) | Subclavian or IJ (n = 18), n (%) | Difference (95% CI) |
|---|---|---|---|
| |||
| Mechanical (arterial puncture, hematoma, or pneumothorax)a | 4 (11) | 0 | 11% (1%21%) |
| Venous thromboembolismb | 0 (0) | 0 (0) | 0% |
| Infection rate (per 1000 central‐line days)c | 4.3 | 7.0 | 2.7 (1913) |
Survey Responses
Before the educational session, many residents did not recognize that femoral venous catheter insertions had a higher risk of arterial puncture or venous thrombosis (Table 4); by the final survey, residents were more likely to recognize the higher risk of these complications during femoral venous insertions. Most residents recognized the higher risk of infectious complications at the femoral site (Table 4).
| Respondents in Agreement, n (%) | |||
|---|---|---|---|
| Presession n = 35 | Postsession n = 34 | Follow‐up n = 35 | |
| |||
| Knowledge | |||
| Complications are most frequent at the femoral site | 27 (77%) | 30 (86%) | |
| Arterial puncture risk is lowest at the femoral sitea | 16 (46%) | 7 (21%)b | |
| Thrombosis risk is lowest at the femoral sitea | 11 (31%) | 6 (18%) | |
| Infection risk is lowest at the femoral site | 1/33 (3%) | 0 (0%) | |
| Attitudes | |||
| I feel confident:c | |||
| Inserting a femoral CVC | 53 | 59b | 89d |
| Inserting an internal jugular CVC | 41 | 71d | 40 |
| Inserting a subclavian CVC | 24 | 65d | 34d |
| Options to increase placement in jugular or subclavian veins | |||
| Availability of ultrasound machine | 31 (89) | ||
| Expert supervisor available to assist with placement | 30 (86) | ||
| Insert CVC within 2 weeks of educational session | 30 (86) | ||
| Rotation through a service that often places CVCsa | 26 (76) | ||
| I do not plan to use this skill after my residency | 4 (11) | ||
| Barriers to inserting a subclavian or internal jugular CVC | |||
| Preexisting internal jugular or subclavian CVC | 11 (31) | ||
| For temporary dialysis, desire to preserve site | 26 (74) | ||
| Practices | |||
| More likely to remove unnecessary catheter | 29 (83) | ||
| Improved infection‐control practices | 28 (80) | ||
| Increased motivation for internal jugular or subclavian venous insertion | 27 (78) | ||
| Less likely to place a CVC | 9 (26) | ||
| Internal jugular or subclavian CVC inserted for the first time after training | 7/30 (23) | ||
Residents overwhelmingly responded that the lecture was useful (95%), that mannequins provided a valuable skill‐building exercise (90%), and that the session should be incorporated into the training program (95%). Immediately after the session, residents had increased confidence about inserting a central venous catheter at any venous site, especially for internal jugular or subclavian insertions. By the final survey, the confidence of residents about inserting catheters in the internal jugular or subclavian veins had returned to baseline but had increased for femoral‐site insertions (Table 4).
Most residents in the intervention group agreed that the educational session motivated them to remove unnecessary catheters, improve insertion‐related infection‐control practices, and place the catheter in an internal jugular or subclavian vein; some agreed because of the educational session, they were less likely to place a central venous catheter. Some reported successfully inserting a central venous catheter in the subclavian or internal jugular vein for the first time (Table 4).
DISCUSSION
An educational session designed to teach residents appropriate central venous catheter insertion practices that included simulated hands‐on training increased knowledge about insertion‐related complications and improved certain infection‐control practices. Although residents' confidence in inserting subclavian or internal jugular catheters initially improved, our training session did not change the choice of venous insertion site from femoral to subclavian or internal jugular veins, possibly because there were few opportunities for residents to insert a catheter during the 4‐week general medical ward rotations. Thus, although an active educational intervention improved the knowledge and confidence of residents, it had a minimal effect on behavior (only improved certain infection‐control practices). Catheter‐associated complications were infrequent and similar in the intervention and control groups.
Central venous catheter insertion is a skill that many general internists do not perform10; however, until recently the American Board of Internal Medicine considered it a requisite skill for internal medicine residents, and most residents at our hospital reported a desire to learn this skill. Although in our study complications were infrequent, suggesting that a change in venous insertion site is unlikely to dramatically improve patient safety, we believe that residents should become skilled at inserting catheters in internal jugular or subclavian veins, the currently recommended optimal venous insertion.8
There is evidence that single educational interventions are unlikely to result in substantial, sustained behavioral change, especially passive educational programs.11 However, a previous study documented a change in provider behavior and possibly a reduction in bloodstream infections after a single hands‐on training session.12 Our hands‐on educational format was very popular and likely improved some infection‐control practices but did not change provider behavior about choice of venous insertion site. In other institutions, mentoring residents on appropriate catheter insertion technique has been accomplished by establishing a procedure service13 or by resident rotation in a high‐volume location (eg, cardiac catheterization laboratory).14 Another option to facilitate behavioral change would be to provide a portable ultrasound machine, as requested by our residents, which may reduce complication rates.15, 16 At our hospital, we decided to supplement hands‐on training with expert bedside supervision during catheter insertion; the expert is provided through a procedure service that is led by hospitalists. The procedure service has a dedicated portable ultrasound machine to assist with internal jugular vein cannulation.
By the end of our study period, residents' confidence in subclavian or internal jugular catheter insertions had returned to presession levels; however, they reported increased confidence in femoral venous catheter insertions. These findings suggest that the session increased residents' confidence with catheter insertions in general, but not specifically for venous sites for which they had no previous experience. For subclavian or internal jugular catheter insertions, their confidence decayed to the presession baseline, likely because of few opportunities to insert catheters in patients; on average, each resident inserts 1 central venous catheter on the general medicine wards approximately every 4 months.
Our survey found that our intervention changed residents' attitudes about infection‐control practices. In particular, intervention‐group residents reported that they were more likely to remove unnecessary catheters and that they had used a mask and large drape during catheter insertion. Use of full‐barrier precautions (ie, sterile gloves and gown, large sterile drape, cap, and mask) has been shown to reduce the risk of bloodstream infection2 and is included in national guidelines.17 Adherence to these guidelines has been included in successful quality improvement initiatives.4, 5, 18 Compared to internists' adherence to recommendations for infection control reported in another survey,10 residents who attended our educational session reported more use of large sterile drapes (94% vs. 35%) or masks (75% vs. 66%); however, they were less likely to use a sterile gown (50% vs. 72%). Use of a large sterile drape is common in our hospital, likely because the drape is included in the central venous catheter package. We suspect that at our hospital, poor adherence to certain recommendations (eg, using a sterile gown) was due in part to difficulty accessing supplies. Another possibility is that use of a cap, compared to use of large drapes, is perceived as not giving the patient much additional protection. In fact, there is no evidence that using a cap provides benefit beyond that of other, more intuitively beneficial recommended infection‐control practices, such as using sterile gloves and a large sterile drape. The procedure service has addressed the supply problem by stocking hard‐to‐find items on a procedure cart.
Only 2 clinically evident complications associated with catheter insertion occurred (one patient with clinical sepsis and one with a hematoma). Although it is possible that we missed minor complications, our rates were similar to those reported by other investigators: clinically diagnosed venous thromboembolism, 0%2.2%3, 19, 20; pneumothorax, 1.4%21; catheter‐associated primary bloodstream infection, 1‐6/1000 catheter‐days.22, 23 Comparing complication rates was hindered by variability in definitions, methods of ascertainment, and populations evaluated. For example, the rate of venous thromboembolism was dramatically higher when routine diagnostic imaging was used, and detection of catheter‐associated infections likely increased when catheter‐tip cultures were routinely performed. We required clinical evidence of complications, and our study differs from others in that we evaluated general medicine ward patients.
This study had several limitations. Placement of central venous catheters on general medicine wards was less frequent than we anticipated based on a brief period of pilot data collection; therefore, our study was not powered to detect relatively small changes in venous insertion sites or differences in complications. Also, because direct observation was not possible, we relied on self‐reported adherence to infection‐control practices. However, intervention residents' self‐reported poor adherence to gown, glove, and cap use suggests that their responses were unbiased.
An educational session focused on central venous catheter insertion practices was well received by residents, increased their knowledge about complications, and improved infection‐control practices, but had no effect on increasing use of subclavian or internal jugular veins for catheter insertion. Despite continued frequent use of femoral venous catheters, clinically apparent complications were infrequent. However, we believe it is important to teach residents optimal catheter insertion techniques, including preferential placement of catheters in subclavian or internal jugular veins. Therefore, the section of hospital medicine at our hospital initiated a procedure service that provides expert bedside supervision, including use of a portable ultrasound machine, for catheter insertions.
Acknowledgements
The authors acknowledge Kathleen Murray for data collection and form development; Donald Blom for assistance with determining bloodstream infection; Laura Sadowski for developing and leading the focus group session; Yannis Guerra for assistance with the educational sessions; Oksana Barilyak, Anand Despande, and Saurabh Sharma for assistance with data collection; and chief residents Rony Ghaoui, Sean Halleran, Priya Kansal, Parag Sampat, and Sunita Nathan for interviewing residents about catheter insertions.
At times central venous catheters are essential to the delivery of appropriate medical care. Because catheter‐related complications are associated with limited operator experience,1 insertion technique,2 and venous site of insertion (eg, femoral, internal jugular, or subclavian vein),3 house staff training programs strive to provide their residents with appropriate training and oversight for this skill. Most quality improvement initiatives directed at reducing complications associated with central venous catheters have focused on patients in the intensive care unit (ICU).4, 5 However, in some hospitals more central venous catheters are inserted in patients not in the ICU,6 and practices that increase the risk of complications may be more common on wards.7
In our hospital, most catheters are placed in the femoral vein. Because femoral venous placement likely increases a patient's risk of thrombosis, hematoma, and bloodstream infection,8 we developed a program to change residents' choice of venous insertion site and improve their infection‐control practices during their general medicine ward rotation. The program provided simulated hands‐on experience in a simulation laboratory. We evaluated our intervention through a firm‐based clinical trial that compared the usual practice to our intervention. We compared infection‐control practices and resident choice of venous insertion site between the intervention and control groups; we also assessed residents' knowledge about catheter‐related complications, and we monitored patients for catheter‐related complications.
METHODS
Setting and Study Design
We conducted a prospective, firm‐based clinical trial approved by the institutional review board at Cook County Hospital, a 464‐bed public teaching hospital. We evaluated all central venous catheters inserted by residents on the general medicine service from November 15, 2004, to March 31, 2005. The internal medicine residency program assigns residents to 1 of 3 firms for their entire 3 years of training. We designated 1 firm as the intervention group; the other 2 firms constituted the control group.
Educational Intervention
At the beginning of each 4‐week general medicine ward rotation, intervention‐firm residents attended an educational and simulation laboratory session. Control‐firm residents received the usual ward orientation. We conducted 6 sessions, with total attendance of 40 intervention‐firm residents, or approximately 7 residents per session. A chief medical resident experienced in catheter placement and an attending internist led and supervised each 2‐hour training session. The sessions were conducted at the Simulation Laboratory of Rush University and included a presentation about indications for central venous catheter insertion, insertion techniques, common complications, and practice placing catheters in mannequins. During the hands‐on session, each participant observed the expert insert a triple‐lumen catheter in the mannequin's internal jugular and subclavian veins. Then, with supervision, each participant practiced catheter insertion using recommended infection‐control practices (eg, use of gloves, mask, and large drape, and chlorhexidine skin preparation).
Resident Survey
Before each session, we administered a survey that assessed residents' knowledge of insertion techniques and their confidence in placing catheters at each venous insertion site. To measure change in the confidence level of residents, we distributed an abbreviated survey 2 additional times, immediately after the session and at the end of the study period. We measured confidence with answers to survey questions, which were rated on a 5‐point Likert scale, from strongly disagree to strongly agree. In addition to measuring the change in residents' confidence, the final survey repeated knowledge assessment questions, evaluated residents' attitudes regarding venous insertion sites, and asked about potential strategies to improve insertion practices.
Central Venous Catheter Detection and Monitoring
At the end of each day, residents reported catheter insertions to chief residents during routine sign‐out rounds. If a catheter had been inserted, the chief resident interviewed the resident about type of catheter, venous insertion site, duration of attempt, patient location, immediate complications, number of inserters, inserter attendance at an educational session, inserter specialty, and professional designation (eg, resident, fellow, attending), indication for insertion, and adherence to infection‐control practices. For all insertion attempts, the research team reviewed the medical record and recorded patient characteristics that might influence venous insertion site (eg, thrombocytopenia, coagulopathy, and body mass index) and evaluated patients for insertion‐related complications.
We prospectively monitored patients for mechanical (ie, pneumothorax or hematoma), thromboembolic, or infectious complications. To evaluate for pneumothorax, postinsertion chest radiographs were reviewed by a physician‐investigator, and radiologists' interpretations and progress notes were reviewed. To evaluate for infectious or other mechanical complications, progress notes also were reviewed. We required radiographic confirmation of venous thromboembolism. To categorize potential bloodstream infections, we used Centers for Disease Control and Prevention definitions.9 All medical record and radiograph reviews were performed by investigators who were masked to patient firm assignment. We monitored patients until catheter removal or hospital discharge. After patient discharge, we reviewed the electronic record, including emergency room visits and repeat hospitalizations, for 30 days after the earlier of hospital discharge or catheter removal.
Statistics
Because we were aware that temporary dialysis catheters are sometimes placed in femoral veins to preserve the subclavian or internal jugular venous sites for more permanent tunneled intravascular catheters, our prespecified plans were to compare venous insertion sites between intervention and control groups after excluding temporary dialysis catheters. To more completely describe catheter use, we also collected data on temporary dialysis catheters, and we present the results both with and without inclusion of data on temporary dialysis catheters. If multiple residents attempted to insert a catheter, we would have used the group that the final inserter was in to determine intervention versus control group assignment; however, this never occurred.
To determine resident confidence in inserting catheters, we collapsed the responses of agree and strongly agree and of disagree and strongly disagree into single categories; thus, frequency of agreement was evaluated as a dichotomous outcome. To test whether residents' confidence changed between the 3 surveys, we analyzed responses using the matched‐pair signed rank test, with the initial survey used as the referent.
We dichotomized certain continuous variables using the following cut points: body mass index 30 kg/m2; coagulopathy, international normalized ratio (INR) > 1.5; thrombocytopenia, platelets < 100 109/L. Data were entered into a relational database (Microsoft Access, Microsoft Inc., Redmond, WA) and merged analyzed using Stata software, version 8.2 (Stata Corporation, College Station, TX).
RESULTS
Patient and Catheter Characteristics
Fifty‐four catheters were inserted in 48 patients during the study period, 16 (30%) in the intervention group and 38 (70%) in the control group. Mean number of catheters inserted per resident for each 4‐week rotation was 0.24; therefore, on average, a resident would insert 1 catheter every 4 general‐medicine rotations. Most catheters were inserted between 7:00 AM and 5:00 PM; the most common reason for insertion was to administer intravenous medications to a patient without intravenous access, followed by the need for a temporary dialysis catheter. Most catheters were inserted by the medicine team rather than radiology or a subspecialty service (Table 1). Most patient characteristics and reasons for insertion were similar between groups; however, more patients in the control group had thrombocytopenia (Table 1).
| Characteristic | Central venous catheters inserted | ||
|---|---|---|---|
| Intervention (n = 16), n (%) | Control (n = 38), n (%) | P | |
| |||
| Patient | |||
| Body mass index 30 kg/m2 | 5 (31) | 11 (29) | 1.0 |
| INR > 1.5 | 3 (19) | 3 (7.9) | 0.37 |
| Platelet count < 100k | 0 (0) | 9 (24) | 0.05 |
| Charlson index, mean (interquartile range) | 2 (24) | 2 (14) | 0.58 |
| Physician inserting catheter | |||
| Resident on general medicine servicea | 15 (100) | 34/37 (92) | 1.0 |
| Subspecialty fellow | 0 (0) | 2/37 (5.3) | 1.0 |
| Radiology fellow or attending | 0 (0) | 1/37 (2.6) | 1.0 |
| Reason for insertion | |||
| No intravenous access | 7 (44) | 19 (50) | 0.67 |
| Temporary dialysis catheterb | 7 (44) | 14 (37) | 0.63 |
| Total parenteral nutrition | 1 (6.2) | 3 (7.9) | 1.0 |
| Otherc | 1 (6.2) | 2 (5.3) | 1.0 |
| Time of day of insertion | |||
| Between 7 AM and 5 PM | 12/14 (86) | 25/37 (68) | 0.30 |
Insertion Practices
Femoral venous insertion was the most common type of catheter insertion (67%), followed by internal jugular (26%) and subclavian (7%); there were no differences in insertion site between the intervention and control groups (Table 2). When we excluded temporary dialysis catheters, 39% of central venous catheters were inserted in the internal jugular vein. Although a smaller proportion of catheters inserted by the intervention group were placed in a femoral vein, the difference was not significant (Table 2).
| Intervention (n = 16), n (%) | Control (n = 38), n (%) | Risk ratio (95% CI) | P | |
|---|---|---|---|---|
| ||||
| Self‐reported practices during CVC insertion | ||||
| Mask worn | 12 (75) | 13 (34) | 2.2 (1.33.7) | 0.008 |
| Large drape used | 15 (94) | 28 (74) | 1.3 (1.01.6) | 0.14 |
| Cap worn | 3 (19) | 5 (13) | 1.4 (0.45.3) | 0.6 |
| Gown worn | 8 (50) | 18 (47) | 1.1 (0.61.9) | 0.9 |
| Sterile gloves worn | 15 (94) | 36 (95) | 1.0 (0.81.2) | 1.0 |
| Venous insertion sitea | Difference (95% CI) | |||
| Femoral | 10 (62) | 26 (68) | 6% (34%22%) | 0.67 |
| Internal jugular | 5 (31) | 9 (24) | ||
| Subclavian | 1 (6.2) | 3 (7.9) | ||
| Excluding dialysis cathetersa | n = 9 | n = 24 | ||
| Femoral | 4 (44) | 14 (58) | 14% (52%24%) | 0.7 |
| Internal jugular | 5 (56) | 8 (33) | ||
| Subclavian | 0 (0) | 2 (8) | ||
For most insertions, residents reported using sterile gloves (94%) and a large drape (80%); however, most did not report use of a sterile gown (48%), mask (46%), or cap (15%). Residents in the intervention group were more likely to report use of a mask, and there was a trend toward increased use of large drapes (Table 2). No patient characteristics predicted femoral venous insertion (data not shown).
Complications
The most frequent complication was arterial puncture (n = 4); all four occurred during femoral venous insertion attempts. Compared to subclavian or internal jugular venous placement, there was a trend toward more mechanical complications among femoral catheters (Table 3). One episode of clinical sepsis occurred, in an intervention‐group patient who had femoral and internal jugular catheters, and no pneumothoraxes or episodes of venous thromboembolism occurred (Table 3). The overall incidence of bloodstream infection was 2.7 per 1000 central‐line days; there was no difference between the intervention and control groups (9.2 versus 0 per 1000 central‐line days; P = .29).
| Complication | Femoral (n = 36), n (%) | Subclavian or IJ (n = 18), n (%) | Difference (95% CI) |
|---|---|---|---|
| |||
| Mechanical (arterial puncture, hematoma, or pneumothorax)a | 4 (11) | 0 | 11% (1%21%) |
| Venous thromboembolismb | 0 (0) | 0 (0) | 0% |
| Infection rate (per 1000 central‐line days)c | 4.3 | 7.0 | 2.7 (1913) |
Survey Responses
Before the educational session, many residents did not recognize that femoral venous catheter insertions had a higher risk of arterial puncture or venous thrombosis (Table 4); by the final survey, residents were more likely to recognize the higher risk of these complications during femoral venous insertions. Most residents recognized the higher risk of infectious complications at the femoral site (Table 4).
| Respondents in Agreement, n (%) | |||
|---|---|---|---|
| Presession n = 35 | Postsession n = 34 | Follow‐up n = 35 | |
| |||
| Knowledge | |||
| Complications are most frequent at the femoral site | 27 (77%) | 30 (86%) | |
| Arterial puncture risk is lowest at the femoral sitea | 16 (46%) | 7 (21%)b | |
| Thrombosis risk is lowest at the femoral sitea | 11 (31%) | 6 (18%) | |
| Infection risk is lowest at the femoral site | 1/33 (3%) | 0 (0%) | |
| Attitudes | |||
| I feel confident:c | |||
| Inserting a femoral CVC | 53 | 59b | 89d |
| Inserting an internal jugular CVC | 41 | 71d | 40 |
| Inserting a subclavian CVC | 24 | 65d | 34d |
| Options to increase placement in jugular or subclavian veins | |||
| Availability of ultrasound machine | 31 (89) | ||
| Expert supervisor available to assist with placement | 30 (86) | ||
| Insert CVC within 2 weeks of educational session | 30 (86) | ||
| Rotation through a service that often places CVCsa | 26 (76) | ||
| I do not plan to use this skill after my residency | 4 (11) | ||
| Barriers to inserting a subclavian or internal jugular CVC | |||
| Preexisting internal jugular or subclavian CVC | 11 (31) | ||
| For temporary dialysis, desire to preserve site | 26 (74) | ||
| Practices | |||
| More likely to remove unnecessary catheter | 29 (83) | ||
| Improved infection‐control practices | 28 (80) | ||
| Increased motivation for internal jugular or subclavian venous insertion | 27 (78) | ||
| Less likely to place a CVC | 9 (26) | ||
| Internal jugular or subclavian CVC inserted for the first time after training | 7/30 (23) | ||
Residents overwhelmingly responded that the lecture was useful (95%), that mannequins provided a valuable skill‐building exercise (90%), and that the session should be incorporated into the training program (95%). Immediately after the session, residents had increased confidence about inserting a central venous catheter at any venous site, especially for internal jugular or subclavian insertions. By the final survey, the confidence of residents about inserting catheters in the internal jugular or subclavian veins had returned to baseline but had increased for femoral‐site insertions (Table 4).
Most residents in the intervention group agreed that the educational session motivated them to remove unnecessary catheters, improve insertion‐related infection‐control practices, and place the catheter in an internal jugular or subclavian vein; some agreed because of the educational session, they were less likely to place a central venous catheter. Some reported successfully inserting a central venous catheter in the subclavian or internal jugular vein for the first time (Table 4).
DISCUSSION
An educational session designed to teach residents appropriate central venous catheter insertion practices that included simulated hands‐on training increased knowledge about insertion‐related complications and improved certain infection‐control practices. Although residents' confidence in inserting subclavian or internal jugular catheters initially improved, our training session did not change the choice of venous insertion site from femoral to subclavian or internal jugular veins, possibly because there were few opportunities for residents to insert a catheter during the 4‐week general medical ward rotations. Thus, although an active educational intervention improved the knowledge and confidence of residents, it had a minimal effect on behavior (only improved certain infection‐control practices). Catheter‐associated complications were infrequent and similar in the intervention and control groups.
Central venous catheter insertion is a skill that many general internists do not perform10; however, until recently the American Board of Internal Medicine considered it a requisite skill for internal medicine residents, and most residents at our hospital reported a desire to learn this skill. Although in our study complications were infrequent, suggesting that a change in venous insertion site is unlikely to dramatically improve patient safety, we believe that residents should become skilled at inserting catheters in internal jugular or subclavian veins, the currently recommended optimal venous insertion.8
There is evidence that single educational interventions are unlikely to result in substantial, sustained behavioral change, especially passive educational programs.11 However, a previous study documented a change in provider behavior and possibly a reduction in bloodstream infections after a single hands‐on training session.12 Our hands‐on educational format was very popular and likely improved some infection‐control practices but did not change provider behavior about choice of venous insertion site. In other institutions, mentoring residents on appropriate catheter insertion technique has been accomplished by establishing a procedure service13 or by resident rotation in a high‐volume location (eg, cardiac catheterization laboratory).14 Another option to facilitate behavioral change would be to provide a portable ultrasound machine, as requested by our residents, which may reduce complication rates.15, 16 At our hospital, we decided to supplement hands‐on training with expert bedside supervision during catheter insertion; the expert is provided through a procedure service that is led by hospitalists. The procedure service has a dedicated portable ultrasound machine to assist with internal jugular vein cannulation.
By the end of our study period, residents' confidence in subclavian or internal jugular catheter insertions had returned to presession levels; however, they reported increased confidence in femoral venous catheter insertions. These findings suggest that the session increased residents' confidence with catheter insertions in general, but not specifically for venous sites for which they had no previous experience. For subclavian or internal jugular catheter insertions, their confidence decayed to the presession baseline, likely because of few opportunities to insert catheters in patients; on average, each resident inserts 1 central venous catheter on the general medicine wards approximately every 4 months.
Our survey found that our intervention changed residents' attitudes about infection‐control practices. In particular, intervention‐group residents reported that they were more likely to remove unnecessary catheters and that they had used a mask and large drape during catheter insertion. Use of full‐barrier precautions (ie, sterile gloves and gown, large sterile drape, cap, and mask) has been shown to reduce the risk of bloodstream infection2 and is included in national guidelines.17 Adherence to these guidelines has been included in successful quality improvement initiatives.4, 5, 18 Compared to internists' adherence to recommendations for infection control reported in another survey,10 residents who attended our educational session reported more use of large sterile drapes (94% vs. 35%) or masks (75% vs. 66%); however, they were less likely to use a sterile gown (50% vs. 72%). Use of a large sterile drape is common in our hospital, likely because the drape is included in the central venous catheter package. We suspect that at our hospital, poor adherence to certain recommendations (eg, using a sterile gown) was due in part to difficulty accessing supplies. Another possibility is that use of a cap, compared to use of large drapes, is perceived as not giving the patient much additional protection. In fact, there is no evidence that using a cap provides benefit beyond that of other, more intuitively beneficial recommended infection‐control practices, such as using sterile gloves and a large sterile drape. The procedure service has addressed the supply problem by stocking hard‐to‐find items on a procedure cart.
Only 2 clinically evident complications associated with catheter insertion occurred (one patient with clinical sepsis and one with a hematoma). Although it is possible that we missed minor complications, our rates were similar to those reported by other investigators: clinically diagnosed venous thromboembolism, 0%2.2%3, 19, 20; pneumothorax, 1.4%21; catheter‐associated primary bloodstream infection, 1‐6/1000 catheter‐days.22, 23 Comparing complication rates was hindered by variability in definitions, methods of ascertainment, and populations evaluated. For example, the rate of venous thromboembolism was dramatically higher when routine diagnostic imaging was used, and detection of catheter‐associated infections likely increased when catheter‐tip cultures were routinely performed. We required clinical evidence of complications, and our study differs from others in that we evaluated general medicine ward patients.
This study had several limitations. Placement of central venous catheters on general medicine wards was less frequent than we anticipated based on a brief period of pilot data collection; therefore, our study was not powered to detect relatively small changes in venous insertion sites or differences in complications. Also, because direct observation was not possible, we relied on self‐reported adherence to infection‐control practices. However, intervention residents' self‐reported poor adherence to gown, glove, and cap use suggests that their responses were unbiased.
An educational session focused on central venous catheter insertion practices was well received by residents, increased their knowledge about complications, and improved infection‐control practices, but had no effect on increasing use of subclavian or internal jugular veins for catheter insertion. Despite continued frequent use of femoral venous catheters, clinically apparent complications were infrequent. However, we believe it is important to teach residents optimal catheter insertion techniques, including preferential placement of catheters in subclavian or internal jugular veins. Therefore, the section of hospital medicine at our hospital initiated a procedure service that provides expert bedside supervision, including use of a portable ultrasound machine, for catheter insertions.
Acknowledgements
The authors acknowledge Kathleen Murray for data collection and form development; Donald Blom for assistance with determining bloodstream infection; Laura Sadowski for developing and leading the focus group session; Yannis Guerra for assistance with the educational sessions; Oksana Barilyak, Anand Despande, and Saurabh Sharma for assistance with data collection; and chief residents Rony Ghaoui, Sean Halleran, Priya Kansal, Parag Sampat, and Sunita Nathan for interviewing residents about catheter insertions.
- ,,,,.Central vein catheterization. Failure and complication rates by three percutaneous approaches.Arch Intern Med.1986;146:259–261.
- ,,, et al.Prevention of central venous catheter‐related infections by using maximal sterile barrier precautions during insertion.Infect Control Hosp Epidemiol.1994;15:231–238.
- ,,, et al.Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial.JAMA.2001;286:700–707.
- ,,, et al.Eliminating catheter‐related bloodstream infections in the intensive care unit.Crit Care Med.2004;32:2014–2020.
- ,,, et al.The effect of an education program on the incidence of central venous catheter‐associated bloodstream infection in a medical ICU.Chest.2004;126:1612–1618.
- ,,, et al.Prevalence of the use of central venous access devices within and outside of the intensive care unit: results of a survey among hospitals in the prevention epicenter program of the Centers for Disease Control and Prevention.Infect Control Hosp Epidemiol.2003;24:942–945.
- ,,,,,.Unnecessary use of central venous catheters: the need to look outside the intensive care unit.Infect Control Hosp Epidemiol.2004;25:266–268.
- ,.Preventing complications of central venous catheterization.N Engl J Med.2003;348:1123–1133.
- ,,,,.CDC definitions for nosocomial infections, 1988.Am J Infect Control.1988;16:128–140.
- ,,,.Why is it that internists do not follow guidelines for preventing intravascular catheter infections?Infect Control Hosp Epidemiol.2005;26:525–533.
- ,,, et al.Changing provider behavior: an overview of systematic reviews of interventions.Med Care.2001;39:II2–II45.
- ,,, et al.Education of physicians‐in‐training can decrease the risk for vascular catheter infection.Ann Intern Med.2000;132:641–648.
- ,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,,.A curricular initiative for internal medicine residents to enhance proficiency in internal jugular central venous line placement.Mayo Clin Proc.2005;80:212–218.
- ,,,,,.Improvement of internal jugular vein cannulation using an ultrasound‐guided technique.Intensive Care Med.1997;23:916–919.
- ,,.Facilitation of internal jugular venous cannulation using an audio‐guided Doppler ultrasound vascular access device: results from a prospective, dual‐center, randomized, crossover clinical study.Crit Care Med.1995;23:60–65.
- ,,, et al.Guidelines for the prevention of intravascular catheter‐related infections.MMWR Morb Mortal Wkly Rep.2002;1(RR10):1–26.
- ,,, et al.The impact of bedside behavior on catheter‐related bacteremia in the intensive care unit.Arch Surg.2004;139:131–136.
- ,,,,,.A prospective evaluation of the use of femoral venous catheters in critically ill adults.Crit Care Med.1997;25:1986–1989.
- ,,,,.Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients.Chest.2000;117:178–183.
- ,,.Complications of central venous catheters: internal jugular versus subclavian access—a systematic review.Crit Care Med.2002;30:454–460.
- ,,,,,.Prospective evaluation of risk factors for bloodstream infection in patients receiving home infusion therapy.Ann Intern Med.1999;131:340–347.
- ,,,.Nosocomial infections in combined medical‐surgical intensive care units in the United States.Infect Control Hosp Epidemiol.2000;21:510–515.
- ,,,,.Central vein catheterization. Failure and complication rates by three percutaneous approaches.Arch Intern Med.1986;146:259–261.
- ,,, et al.Prevention of central venous catheter‐related infections by using maximal sterile barrier precautions during insertion.Infect Control Hosp Epidemiol.1994;15:231–238.
- ,,, et al.Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial.JAMA.2001;286:700–707.
- ,,, et al.Eliminating catheter‐related bloodstream infections in the intensive care unit.Crit Care Med.2004;32:2014–2020.
- ,,, et al.The effect of an education program on the incidence of central venous catheter‐associated bloodstream infection in a medical ICU.Chest.2004;126:1612–1618.
- ,,, et al.Prevalence of the use of central venous access devices within and outside of the intensive care unit: results of a survey among hospitals in the prevention epicenter program of the Centers for Disease Control and Prevention.Infect Control Hosp Epidemiol.2003;24:942–945.
- ,,,,,.Unnecessary use of central venous catheters: the need to look outside the intensive care unit.Infect Control Hosp Epidemiol.2004;25:266–268.
- ,.Preventing complications of central venous catheterization.N Engl J Med.2003;348:1123–1133.
- ,,,,.CDC definitions for nosocomial infections, 1988.Am J Infect Control.1988;16:128–140.
- ,,,.Why is it that internists do not follow guidelines for preventing intravascular catheter infections?Infect Control Hosp Epidemiol.2005;26:525–533.
- ,,, et al.Changing provider behavior: an overview of systematic reviews of interventions.Med Care.2001;39:II2–II45.
- ,,, et al.Education of physicians‐in‐training can decrease the risk for vascular catheter infection.Ann Intern Med.2000;132:641–648.
- ,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,,.A curricular initiative for internal medicine residents to enhance proficiency in internal jugular central venous line placement.Mayo Clin Proc.2005;80:212–218.
- ,,,,,.Improvement of internal jugular vein cannulation using an ultrasound‐guided technique.Intensive Care Med.1997;23:916–919.
- ,,.Facilitation of internal jugular venous cannulation using an audio‐guided Doppler ultrasound vascular access device: results from a prospective, dual‐center, randomized, crossover clinical study.Crit Care Med.1995;23:60–65.
- ,,, et al.Guidelines for the prevention of intravascular catheter‐related infections.MMWR Morb Mortal Wkly Rep.2002;1(RR10):1–26.
- ,,, et al.The impact of bedside behavior on catheter‐related bacteremia in the intensive care unit.Arch Surg.2004;139:131–136.
- ,,,,,.A prospective evaluation of the use of femoral venous catheters in critically ill adults.Crit Care Med.1997;25:1986–1989.
- ,,,,.Deep venous thrombosis caused by femoral venous catheters in critically ill adult patients.Chest.2000;117:178–183.
- ,,.Complications of central venous catheters: internal jugular versus subclavian access—a systematic review.Crit Care Med.2002;30:454–460.
- ,,,,,.Prospective evaluation of risk factors for bloodstream infection in patients receiving home infusion therapy.Ann Intern Med.1999;131:340–347.
- ,,,.Nosocomial infections in combined medical‐surgical intensive care units in the United States.Infect Control Hosp Epidemiol.2000;21:510–515.
Copyright © 2007 Society of Hospital Medicine