User login
Point-of-Care Ultrasound for Hospitalists: A Position Statement of the Society of Hospital Medicine
Many hospitalists incorporate point-of-care ultrasound (POCUS) into their daily practice because it adds value to their bedside evaluation of patients. However, standards for training and assessing hospitalists in POCUS have not yet been established. Other acute care specialties, including emergency medicine and critical care medicine, have already incorporated POCUS into their graduate medical education training programs, but most internal medicine residency programs are only beginning to provide POCUS training.1
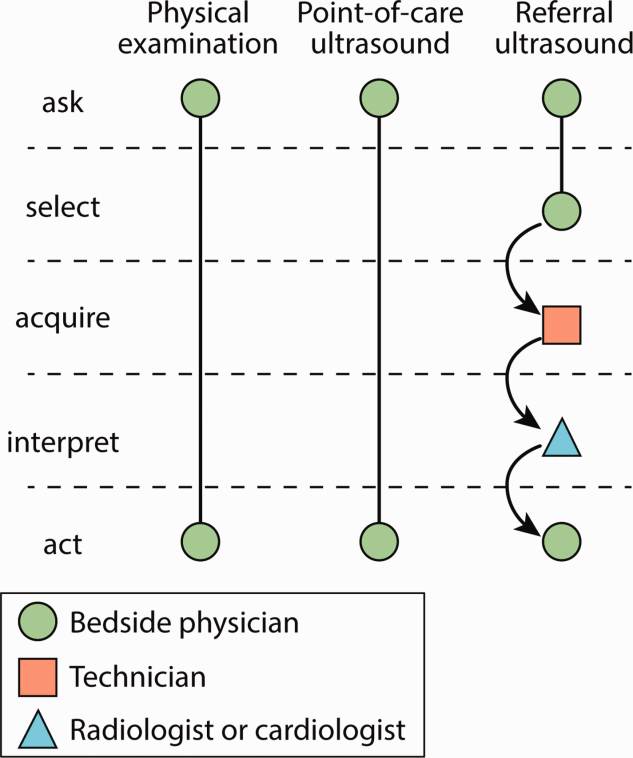
Several features distinguish POCUS from comprehensive ultrasound examinations. First, POCUS is designed to answer focused questions, whereas comprehensive ultrasound examinations evaluate all organs in an anatomical region; for example, an abdominal POCUS exam may evaluate only for presence or absence of intraperitoneal free fluid, whereas a comprehensive examination of the right upper quadrant will evaluate the liver, gallbladder, and biliary ducts. Second, POCUS examinations are generally performed by the same clinician who generates the relevant clinical question to answer with POCUS and ultimately integrates the findings into the patient’s care.2 By contrast, comprehensive ultrasound examinations involve multiple providers and steps: a clinician generates a relevant clinical question and requests an ultrasound examination that is acquired by a sonographer, interpreted by a radiologist, and reported back to the requesting clinician. Third, POCUS is often used to evaluate multiple body systems. For example, to evaluate a patient with undifferentiated hypotension, a multisystem POCUS examination of the heart, inferior vena cava, lungs, abdomen, and lower extremity veins is typically performed. Finally, POCUS examinations can be performed serially to investigate changes in clinical status or evaluate response to therapy, such as monitoring the heart, lungs, and inferior vena cava during fluid resuscitation.
The purpose of this position statement is to inform a broad audience about how hospitalists are using diagnostic and procedural applications of POCUS. This position statement does not mandate that hospitalists use POCUS. Rather, it is intended to provide guidance on the safe and effective use of POCUS by the hospitalists who use it and the administrators who oversee its use. We discuss POCUS (1) applications, (2) training, (3) assessments, and (4) program management. This position statement was reviewed and approved by the Society of Hospital Medicine (SHM) Executive Committee in March 2018.
APPLICATIONS
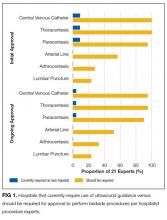
As outlined in our earlier position statements,3,4 ultrasound guidance lowers complication rates and increases success rates of invasive bedside procedures. Diagnostic POCUS can guide clinical decision making prior to bedside procedures. For instance, hospitalists may use POCUS to assess the size and character of a pleural effusion to help determine the most appropriate management strategy: observation, medical treatment, thoracentesis, chest tube placement, or surgical therapy. Furthermore, diagnostic POCUS can be used to rapidly assess for immediate postprocedural complications, such as pneumothorax, or if the patient develops new symptoms.
TRAINING
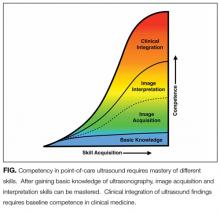
Basic Knowledge
Basic knowledge includes fundamentals of ultrasound physics; safety;4 anatomy; physiology; and device operation, including maintenance and cleaning. Basic knowledge can be taught by multiple methods, including live or recorded lectures, online modules, or directed readings.
Image Acquisition
Training should occur across multiple types of patients (eg, obese, cachectic, postsurgical) and clinical settings (eg, intensive care unit, general medicine wards, emergency department) when available. Training is largely hands-on because the relevant skills involve integration of 3D anatomy with spatial manipulation, hand-eye coordination, and fine motor movements. Virtual reality ultrasound simulators may accelerate mastery, particularly for cardiac image acquisition, and expose learners to standardized sets of pathologic findings. Real-time bedside feedback on image acquisition is ideal because understanding how ultrasound probe manipulation affects the images acquired is essential to learning.
Image Interpretation
Training in image interpretation relies on visual pattern recognition of normal and abnormal findings. Therefore, the normal to abnormal spectrum should be broad, and learners should maintain a log of what abnormalities have been identified. Giving real-time feedback at the bedside is ideal because of the connection between image acquisition and interpretation. Image interpretation can be taught through didactic sessions, image review sessions, or review of teaching files with annotated images.
Clinical Integration
Learners must interpret and integrate image findings with other clinical data considering the image quality, patient characteristics, and changing physiology. Clinical integration should be taught by instructors that share similar clinical knowledge as learners. Although sonographers are well suited to teach image acquisition, they should not be the sole instructors to teach hospitalists how to integrate ultrasound findings in clinical decision making. Likewise, emphasis should be placed on the appropriate use of POCUS within a provider’s skill set. Learners must appreciate the clinical significance of POCUS findings, including recognition of incidental findings that may require further workup. Supplemental training in clinical integration can occur through didactics that include complex patient scenarios.
Pathways
Clinical competency can be achieved with training adherent to five criteria. First, the training environment should be similar to where the trainee will practice. Second, training and feedback should occur in real time. Third, specific applications should be taught rather than broad training in “hospitalist POCUS.” Each application requires unique skills and knowledge, including image acquisition pitfalls and artifacts. Fourth, clinical competence must be achieved and demonstrated; it is not necessarily gained through experience. Fifth, once competency is achieved, continued education and feedback are necessary to ensure it is maintained.
Residency-based POCUS training pathways can best fulfill these criteria. They may eventually become commonplace, but until then alternative pathways must exist for hospitalist providers who are already in practice. There are three important attributes of such pathways. First, administrators’ expectations about learners’ clinical productivity must be realistically, but only temporarily, relaxed; otherwise, competing demands on time will likely overwhelm learners and subvert training. Second, training should begin through a local or national hands-on training program. The SHM POCUS certificate program consolidates training for common diagnostic POCUS applications for hospitalists.6 Other medical societies offer training for their respective clinical specialties.7 Third, once basic POCUS training has begun, longitudinal training should continue ideally with a local hospitalist POCUS expert.
In some settings, a subgroup of hospitalists may not desire, or be able to achieve, competency in the manual skills of POCUS image acquisition. Nevertheless, hospitalists may still find value in understanding POCUS nomenclature, image pattern recognition, and the evidence and pitfalls behind clinical integration of specific POCUS findings. This subset of POCUS skills allows hospitalists to communicate effectively with and understand the clinical decisions made by their colleagues who are competent in POCUS use.
The minimal skills a hospitalist should possess to serve as a POCUS trainer include proficiency of basic knowledge, image acquisition, image interpretation, and clinical integration of the POCUS applications being taught; effectiveness as a hands-on instructor to teach image acquisition skills; and an in-depth understanding of common POCUS pitfalls and limitations.
ASSESSMENTS
Assessment methods for POCUS can include the following: knowledge-based questions, image acquisition using task-specific checklists on human or simulation models, image interpretation using a series of videos or still images with normal and abnormal findings, clinical integration using “next best step” in a multiple choice format with POCUS images, and simulation-based clinical scenarios. Assessment methods should be aligned with local availability of resources and trainers.
Basic Knowledge
Basic knowledge can be assessed via multiple choice questions assessing knowledge of ultrasound physics, image optimization, relevant anatomy, and limitations of POCUS imaging. Basic knowledge lies primarily in the cognitive domain and does not assess manual skills.
Image Acquisition
Image acquisition can be assessed by observation and rating of image quality. Where resources allow, assessment of image acquisition is likely best done through a combination of developing an image portfolio with a minimum number of high quality images, plus direct observation of image acquisition by an expert. Various programs have utilized minimum numbers of images acquired to help define competence with image acquisition skills.6–8 Although minimums may be a necessary step to gain competence, using them as a sole means to determine competence does not account for variable learning curves.9 As with other manual skills in hospital medicine, such as ultrasound-guided bedside procedures, minimum numbers are best used as a starting point for assessments.3,10 In this regard, portfolio development with meticulous attention to the gain, depth, and proper tomographic plane of images can monitor a hospitalist’s progress toward competence by providing objective assessments and feedback. Simulation may also be used as it allows assessment of image acquisition skills and an opportunity to provide real-time feedback, similar to direct observation but without actual patients.
Image Interpretation
Image interpretation is best assessed by an expert observing the learner at bedside; however, when bedside assessment is not possible, image interpretation skills may be assessed using multiple choice or free text interpretation of archived ultrasound images with normal and abnormal findings. This is often incorporated into the portfolio development portion of a training program, as learners can submit their image interpretation along with the video clip. Both normal and abnormal images can be used to assess anatomic recognition and interpretation. Emphasis should be placed on determining when an image is suboptimal for diagnosis (eg, incomplete exam or poor-quality images). Quality assurance programs should incorporate structured feedback sessions.
Clinical Integration
Assessment of clinical integration can be completed through case scenarios that assess knowledge, interpretation of images, and integration of findings into clinical decision making, which is often delivered via a computer-based assessment. Assessments should combine specific POCUS applications to evaluate common clinical problems in hospital medicine, such as undifferentiated hypotension and dyspnea. High-fidelity simulators can be used to blend clinical case scenarios with image acquisition, image interpretation, and clinical integration. When feasible, comprehensive feedback on how providers acquire, interpret, and apply ultrasound at the bedside is likely the best mechanism to assess clinical integration. This process can be done with a hospitalist’s own patients.
General Assessment
A general assessment that includes a summative knowledge and hands-on skills assessment using task-specific checklists can be performed upon completion of training. A high-fidelity simulator with dynamic or virtual anatomy can provide reproducible standardized assessments with variation in the type and difficulty of cases. When available, we encourage the use of dynamic assessments on actual patients that have both normal and abnormal ultrasound findings because simulated patient scenarios have limitations, even with the use of high-fidelity simulators. Programs are recommended to use formative and summative assessments for evaluation. Quantitative scoring systems using checklists are likely the best framework.11,12
CERTIFICATES AND CERTIFICATION
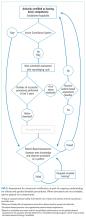
A certificate of completion is proof of a provider’s participation in an educational activity; it does not equate with competency, though it may be a step toward it. Most POCUS training workshops and short courses provide certificates of completion. Certification of competency is an attestation of a hospitalist’s basic competence within a defined scope of practice (Table 2).13 However, without longitudinal supervision and feedback, skills can decay; therefore, we recommend a longitudinal training program that provides mentored feedback and incorporates periodic competency assessments. At present, no national board certification in POCUS is available to grant external certification of competency for hospitalists.
External Certificate
Certificates of completion can be external through a national organization. An external certificate of completion designed for hospitalists includes the POCUS Certificate of Completion offered by SHM in collaboration with CHEST.6 This certificate program provides regional training options and longitudinal portfolio development. Other external certificates are also available to hospitalists.7,14,15
Most hospitalists are boarded by the American Board of Internal Medicine or the American Board of Family Medicine. These boards do not yet include certification of competency in POCUS. Other specialty boards, such as emergency medicine, include competency in POCUS. For emergency medicine, completion of an accredited residency training program and certification by the national board includes POCUS competency.
Internal Certificate
There are a few examples of successful local institutional programs that have provided internal certificates of competency.12,14 Competency assessments require significant resources including investment by both faculty and learners. Ongoing evaluation of competency should be based on quality assurance processes.
Credentialing and Privileging
The American Medical Association (AMA) House of Delegates in 1999 passed a resolution (AMA HR. 802) recommending hospitals follow specialty-specific guidelines for privileging decisions related to POCUS use.17 The resolution included a statement that, “ultrasound imaging is within the scope of practice of appropriately trained physicians.”
Some institutions have begun to rely on a combination of internal and external certificate programs to grant privileges to hospitalists.10 Although specific privileges for POCUS may not be required in some hospitals, some institutions may require certification of training and assessments prior to granting permission to use POCUS.
Hospitalist programs are encouraged to evaluate ongoing POCUS use by their providers after granting initial permission. If privileging is instituted by a hospital, hospitalists must play a significant role in determining the requirements for privileging and ongoing maintenance of skills.
Maintenance of Skills
All medical skills can decay with disuse, including those associated with POCUS.12,18 Thus, POCUS users should continue using POCUS regularly in clinical practice and participate in POCUS continuing medical education activities, ideally with ongoing assessments. Maintenance of skills may be confirmed through routine participation in a quality assurance program.
PROGRAM MANAGEMENT
Use of POCUS in hospital medicine has unique considerations, and hospitalists should be integrally involved in decision making surrounding institutional POCUS program management. Appointing a dedicated POCUS director can help a program succeed.8
Equipment and Image Archiving
Several factors are important to consider when selecting an ultrasound machine: portability, screen size, and ease of use; integration with the electronic medical record and options for image archiving; manufacturer’s service plan, including technical and clinical support; and compliance with local infection control policies. The ability to easily archive and retrieve images is essential for quality assurance, continuing education, institutional quality improvement, documentation, and reimbursement. In certain scenarios, image archiving may not be possible (such as with personal handheld devices or in emergency situations) or necessary (such as with frequent serial examinations during fluid resuscitation). An image archive is ideally linked to reports, orders, and billing software.10,19 If such linkages are not feasible, parallel external storage that complies with regulatory standards (ie, HIPAA compliance) may be suitable.20
Documentation and Billing
Components of documentation include the indication and type of ultrasound examination performed, date and time of the examination, patient identifying information, name of provider(s) acquiring and interpreting the images, specific scanning protocols used, patient position, probe used, and findings. Documentation can occur through a standalone note or as part of another note, such as a progress note. Whenever possible, documentation should be timely to facilitate communication with other providers.
Billing is supported through the AMA Current Procedural Terminology codes for “focused” or “limited” ultrasound examinations (Appendix 9). The following three criteria must be satisfied for billing. First, images must be permanently stored. Specific requirements vary by insurance policy, though current practice suggests a minimum of one image demonstrating relevant anatomy and pathology for the ultrasound examination coded. For ultrasound-guided procedures that require needle insertion, images should be captured at the point of interest, and a procedure note should reflect that the needle was guided and visualized under ultrasound.21 Second, proper documentation must be entered in the medical record. Third, local institutional privileges for POCUS must be considered. Although privileges are not required to bill, some hospitals or payers may require them.
Quality Assurance
Published guidelines on quality assurance in POCUS are available from different specialty organizations, including emergency medicine, pediatric emergency medicine, critical care, anesthesiology, obstetrics, and cardiology.8,22–28 Quality assurance is aimed at ensuring that physicians maintain basic competency in using POCUS to influence bedside decisions.
Quality assurance should be carried out by an individual or committee with expertise in POCUS. Multidisciplinary QA programs in which hospital medicine providers are working collaboratively with other POCUS providers have been demonstrated to be highly effective.10 Oversight includes ensuring that providers using POCUS are appropriately trained,10,22,28 using the equipment correctly,8,26,28 and documenting properly. Some programs have implemented mechanisms to review and provide feedback on image acquisition, interpretation, and clinical integration.8,10 Other programs have compared POCUS findings with referral studies, such as comprehensive ultrasound examinations.
CONCLUSIONS
Practicing hospitalists must continue to collaborate with their institutions to build POCUS capabilities. In particular, they must work with their local privileging body to determine what credentials are required. The distinction between certificates of completion and certificates of competency, including whether those certificates are internal or external, is important in the credentialing process.
External certificates of competency are currently unavailable for most practicing hospitalists because ABIM certification does not include POCUS-related competencies. As internal medicine residency training programs begin to adopt POCUS training and certification into their educational curricula, we foresee a need to update the ABIM Policies and Procedures for Certification. Until then, we recommend that certificates of competency be defined and granted internally by local hospitalist groups.
Given the many advantages of POCUS over traditional tools, we anticipate its increasing implementation among hospitalists in the future. As with all medical technology, its role in clinical care should be continuously reexamined and redefined through health services research. Such information will be useful in developing practice guidelines, educational curricula, and training standards.
Acknowledgments
The authors would like to thank all members that participated in the discussion and finalization of this position statement during the Point-of-care Ultrasound Faculty Retreat at the 2018 Society of Hospital Medicine Annual Conference: Saaid Abdel-Ghani, Brandon Boesch, Joel Cho, Ria Dancel, Renee Dversdal, Ricardo Franco-Sadud, Benjamin Galen, Trevor P. Jensen, Mohit Jindal, Gordon Johnson, Linda M. Kurian, Gigi Liu, Charles M. LoPresti, Brian P. Lucas, Venkat Kalidindi, Benji Matthews, Anna Maw, Gregory Mints, Kreegan Reierson, Gerard Salame, Richard Schildhouse, Daniel Schnobrich, Nilam Soni, Kirk Spencer, Hiromizu Takahashi, David M. Tierney, Tanping Wong, and Toru Yamada.
1. Schnobrich DJ, Mathews BK, Trappey BE, Muthyala BK, Olson APJ. Entrusting internal medicine residents to use point of care ultrasound: Towards improved assessment and supervision. Med Teach. 2018:1-6. doi:10.1080/0142159X.2018.1457210.
2. Soni NJ, Lucas BP. Diagnostic point-of-care ultrasound for hospitalists. J Hosp Med. 2015;10(2):120-124. doi:10.1002/jhm.2285.
3. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):117-125. doi:10.12788/jhm.2917.
4. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. doi:10.12788/jhm.2940.
5. National Council on Radiation Protection and Measurements, The Council. Implementation of the Principle of as Low as Reasonably Achievable (ALARA) for Medical and Dental Personnel.; 1990.
6. Society of Hospital Medicine. Point of Care Ultrasound course: https://www.hospitalmedicine.org/clinical-topics/ultrasonography-cert/. Accessed February 6, 2018.
7. Critical Care Ultrasonography Certificate of Completion Program. CHEST. American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography. Accessed February 6, 2018.
8. American College of Emergency Physicians Policy Statement: Emergency Ultrasound Guidelines. 2016. https://www.acep.org/Clinical---Practice-Management/ACEP-Ultrasound-Guidelines/. Accessed February 6, 2018.
9. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. doi:10.1111/acem.12653.
10. Mathews BK, Zwank M. Hospital medicine point of care ultrasound credentialing: an example protocol. J Hosp Med. 2017;12(9):767-772. doi:10.12788/jhm.2809.
11. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. doi:10.1002/jhm.468.
12. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) ultrasound program. J Hosp Med. 2018;13(8):544-550. doi:10.12788/jhm.2938.
13. Soni NJ, Tierney DM, Jensen TP, Lucas BP. Certification of point-of-care ultrasound competency. J Hosp Med. 2017;12(9):775-776. doi:10.12788/jhm.2812.
14. Ultrasound Certification for Physicians. Alliance for Physician Certification and Advancement. APCA. https://apca.org/. Accessed February 6, 2018.
15. National Board of Echocardiography, Inc. https://www.echoboards.org/EchoBoards/News/2019_Adult_Critical_Care_Echocardiography_Exam.aspx. Accessed June 18, 2018.
16. Tierney DM. Internal Medicine Bedside Ultrasound Program (IMBUS). Abbott Northwestern. http://imbus.anwresidency.com/index.html. Accessed February 6, 2018.
17. American Medical Association House of Delegates Resolution H-230.960: Privileging for Ultrasound Imaging. Resolution 802. Policy Finder Website. http://search0.ama-assn.org/search/pfonline. Published 1999. Accessed February 18, 2018.
18. Kelm D, Ratelle J, Azeem N, et al. Longitudinal ultrasound curriculum improves long-term retention among internal medicine residents. J Grad Med Educ. 2015;7(3):454-457. doi:10.4300/JGME-14-00284.1.
19. Flannigan MJ, Adhikari S. Point-of-care ultrasound work flow innovation: impact on documentation and billing. J Ultrasound Med. 2017;36(12):2467-2474. doi:10.1002/jum.14284.
20. Emergency Ultrasound: Workflow White Paper. https://www.acep.org/uploadedFiles/ACEP/memberCenter/SectionsofMembership/ultra/Workflow%20White%20Paper.pdf. Published 2013. Accessed February 18, 2018.
21. Ultrasound Coding and Reimbursement Document 2009. Emergency Ultrasound Section. American College of Emergency Physicians. http://emergencyultrasoundteaching.com/assets/2009_coding_update.pdf. Published 2009. Accessed February 18, 2018.
22. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050-1060. doi:10.1378/chest.08-2305.
23. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: general ultrasonography. Crit Care Med. 2015;43(11):2479-2502. doi:10.1097/ccm.0000000000001216.
24. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part ii: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. doi:10.1097/ccm.0000000000001847.
25. ACR–ACOG–AIUM–SRU Practice Parameter for the Performance of Obstetrical Ultrasound. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-ob.pdf. Published 2013. Accessed February 18, 2018.
26. AIUM practice guideline for documentation of an ultrasound examination. J Ultrasound Med. 2014;33(6):1098-1102. doi:10.7863/ultra.33.6.1098.
27. Marin JR, Lewiss RE. Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics. 2015;135(4):e1113-e1122. doi:10.1542/peds.2015-0343.
28. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):567-581. doi:10.1016/j.echo.2013.04.001.
Many hospitalists incorporate point-of-care ultrasound (POCUS) into their daily practice because it adds value to their bedside evaluation of patients. However, standards for training and assessing hospitalists in POCUS have not yet been established. Other acute care specialties, including emergency medicine and critical care medicine, have already incorporated POCUS into their graduate medical education training programs, but most internal medicine residency programs are only beginning to provide POCUS training.1
Several features distinguish POCUS from comprehensive ultrasound examinations. First, POCUS is designed to answer focused questions, whereas comprehensive ultrasound examinations evaluate all organs in an anatomical region; for example, an abdominal POCUS exam may evaluate only for presence or absence of intraperitoneal free fluid, whereas a comprehensive examination of the right upper quadrant will evaluate the liver, gallbladder, and biliary ducts. Second, POCUS examinations are generally performed by the same clinician who generates the relevant clinical question to answer with POCUS and ultimately integrates the findings into the patient’s care.2 By contrast, comprehensive ultrasound examinations involve multiple providers and steps: a clinician generates a relevant clinical question and requests an ultrasound examination that is acquired by a sonographer, interpreted by a radiologist, and reported back to the requesting clinician. Third, POCUS is often used to evaluate multiple body systems. For example, to evaluate a patient with undifferentiated hypotension, a multisystem POCUS examination of the heart, inferior vena cava, lungs, abdomen, and lower extremity veins is typically performed. Finally, POCUS examinations can be performed serially to investigate changes in clinical status or evaluate response to therapy, such as monitoring the heart, lungs, and inferior vena cava during fluid resuscitation.
The purpose of this position statement is to inform a broad audience about how hospitalists are using diagnostic and procedural applications of POCUS. This position statement does not mandate that hospitalists use POCUS. Rather, it is intended to provide guidance on the safe and effective use of POCUS by the hospitalists who use it and the administrators who oversee its use. We discuss POCUS (1) applications, (2) training, (3) assessments, and (4) program management. This position statement was reviewed and approved by the Society of Hospital Medicine (SHM) Executive Committee in March 2018.
APPLICATIONS
As outlined in our earlier position statements,3,4 ultrasound guidance lowers complication rates and increases success rates of invasive bedside procedures. Diagnostic POCUS can guide clinical decision making prior to bedside procedures. For instance, hospitalists may use POCUS to assess the size and character of a pleural effusion to help determine the most appropriate management strategy: observation, medical treatment, thoracentesis, chest tube placement, or surgical therapy. Furthermore, diagnostic POCUS can be used to rapidly assess for immediate postprocedural complications, such as pneumothorax, or if the patient develops new symptoms.
TRAINING
Basic Knowledge
Basic knowledge includes fundamentals of ultrasound physics; safety;4 anatomy; physiology; and device operation, including maintenance and cleaning. Basic knowledge can be taught by multiple methods, including live or recorded lectures, online modules, or directed readings.
Image Acquisition
Training should occur across multiple types of patients (eg, obese, cachectic, postsurgical) and clinical settings (eg, intensive care unit, general medicine wards, emergency department) when available. Training is largely hands-on because the relevant skills involve integration of 3D anatomy with spatial manipulation, hand-eye coordination, and fine motor movements. Virtual reality ultrasound simulators may accelerate mastery, particularly for cardiac image acquisition, and expose learners to standardized sets of pathologic findings. Real-time bedside feedback on image acquisition is ideal because understanding how ultrasound probe manipulation affects the images acquired is essential to learning.
Image Interpretation
Training in image interpretation relies on visual pattern recognition of normal and abnormal findings. Therefore, the normal to abnormal spectrum should be broad, and learners should maintain a log of what abnormalities have been identified. Giving real-time feedback at the bedside is ideal because of the connection between image acquisition and interpretation. Image interpretation can be taught through didactic sessions, image review sessions, or review of teaching files with annotated images.
Clinical Integration
Learners must interpret and integrate image findings with other clinical data considering the image quality, patient characteristics, and changing physiology. Clinical integration should be taught by instructors that share similar clinical knowledge as learners. Although sonographers are well suited to teach image acquisition, they should not be the sole instructors to teach hospitalists how to integrate ultrasound findings in clinical decision making. Likewise, emphasis should be placed on the appropriate use of POCUS within a provider’s skill set. Learners must appreciate the clinical significance of POCUS findings, including recognition of incidental findings that may require further workup. Supplemental training in clinical integration can occur through didactics that include complex patient scenarios.
Pathways
Clinical competency can be achieved with training adherent to five criteria. First, the training environment should be similar to where the trainee will practice. Second, training and feedback should occur in real time. Third, specific applications should be taught rather than broad training in “hospitalist POCUS.” Each application requires unique skills and knowledge, including image acquisition pitfalls and artifacts. Fourth, clinical competence must be achieved and demonstrated; it is not necessarily gained through experience. Fifth, once competency is achieved, continued education and feedback are necessary to ensure it is maintained.
Residency-based POCUS training pathways can best fulfill these criteria. They may eventually become commonplace, but until then alternative pathways must exist for hospitalist providers who are already in practice. There are three important attributes of such pathways. First, administrators’ expectations about learners’ clinical productivity must be realistically, but only temporarily, relaxed; otherwise, competing demands on time will likely overwhelm learners and subvert training. Second, training should begin through a local or national hands-on training program. The SHM POCUS certificate program consolidates training for common diagnostic POCUS applications for hospitalists.6 Other medical societies offer training for their respective clinical specialties.7 Third, once basic POCUS training has begun, longitudinal training should continue ideally with a local hospitalist POCUS expert.
In some settings, a subgroup of hospitalists may not desire, or be able to achieve, competency in the manual skills of POCUS image acquisition. Nevertheless, hospitalists may still find value in understanding POCUS nomenclature, image pattern recognition, and the evidence and pitfalls behind clinical integration of specific POCUS findings. This subset of POCUS skills allows hospitalists to communicate effectively with and understand the clinical decisions made by their colleagues who are competent in POCUS use.
The minimal skills a hospitalist should possess to serve as a POCUS trainer include proficiency of basic knowledge, image acquisition, image interpretation, and clinical integration of the POCUS applications being taught; effectiveness as a hands-on instructor to teach image acquisition skills; and an in-depth understanding of common POCUS pitfalls and limitations.
ASSESSMENTS
Assessment methods for POCUS can include the following: knowledge-based questions, image acquisition using task-specific checklists on human or simulation models, image interpretation using a series of videos or still images with normal and abnormal findings, clinical integration using “next best step” in a multiple choice format with POCUS images, and simulation-based clinical scenarios. Assessment methods should be aligned with local availability of resources and trainers.
Basic Knowledge
Basic knowledge can be assessed via multiple choice questions assessing knowledge of ultrasound physics, image optimization, relevant anatomy, and limitations of POCUS imaging. Basic knowledge lies primarily in the cognitive domain and does not assess manual skills.
Image Acquisition
Image acquisition can be assessed by observation and rating of image quality. Where resources allow, assessment of image acquisition is likely best done through a combination of developing an image portfolio with a minimum number of high quality images, plus direct observation of image acquisition by an expert. Various programs have utilized minimum numbers of images acquired to help define competence with image acquisition skills.6–8 Although minimums may be a necessary step to gain competence, using them as a sole means to determine competence does not account for variable learning curves.9 As with other manual skills in hospital medicine, such as ultrasound-guided bedside procedures, minimum numbers are best used as a starting point for assessments.3,10 In this regard, portfolio development with meticulous attention to the gain, depth, and proper tomographic plane of images can monitor a hospitalist’s progress toward competence by providing objective assessments and feedback. Simulation may also be used as it allows assessment of image acquisition skills and an opportunity to provide real-time feedback, similar to direct observation but without actual patients.
Image Interpretation
Image interpretation is best assessed by an expert observing the learner at bedside; however, when bedside assessment is not possible, image interpretation skills may be assessed using multiple choice or free text interpretation of archived ultrasound images with normal and abnormal findings. This is often incorporated into the portfolio development portion of a training program, as learners can submit their image interpretation along with the video clip. Both normal and abnormal images can be used to assess anatomic recognition and interpretation. Emphasis should be placed on determining when an image is suboptimal for diagnosis (eg, incomplete exam or poor-quality images). Quality assurance programs should incorporate structured feedback sessions.
Clinical Integration
Assessment of clinical integration can be completed through case scenarios that assess knowledge, interpretation of images, and integration of findings into clinical decision making, which is often delivered via a computer-based assessment. Assessments should combine specific POCUS applications to evaluate common clinical problems in hospital medicine, such as undifferentiated hypotension and dyspnea. High-fidelity simulators can be used to blend clinical case scenarios with image acquisition, image interpretation, and clinical integration. When feasible, comprehensive feedback on how providers acquire, interpret, and apply ultrasound at the bedside is likely the best mechanism to assess clinical integration. This process can be done with a hospitalist’s own patients.
General Assessment
A general assessment that includes a summative knowledge and hands-on skills assessment using task-specific checklists can be performed upon completion of training. A high-fidelity simulator with dynamic or virtual anatomy can provide reproducible standardized assessments with variation in the type and difficulty of cases. When available, we encourage the use of dynamic assessments on actual patients that have both normal and abnormal ultrasound findings because simulated patient scenarios have limitations, even with the use of high-fidelity simulators. Programs are recommended to use formative and summative assessments for evaluation. Quantitative scoring systems using checklists are likely the best framework.11,12
CERTIFICATES AND CERTIFICATION
A certificate of completion is proof of a provider’s participation in an educational activity; it does not equate with competency, though it may be a step toward it. Most POCUS training workshops and short courses provide certificates of completion. Certification of competency is an attestation of a hospitalist’s basic competence within a defined scope of practice (Table 2).13 However, without longitudinal supervision and feedback, skills can decay; therefore, we recommend a longitudinal training program that provides mentored feedback and incorporates periodic competency assessments. At present, no national board certification in POCUS is available to grant external certification of competency for hospitalists.
External Certificate
Certificates of completion can be external through a national organization. An external certificate of completion designed for hospitalists includes the POCUS Certificate of Completion offered by SHM in collaboration with CHEST.6 This certificate program provides regional training options and longitudinal portfolio development. Other external certificates are also available to hospitalists.7,14,15
Most hospitalists are boarded by the American Board of Internal Medicine or the American Board of Family Medicine. These boards do not yet include certification of competency in POCUS. Other specialty boards, such as emergency medicine, include competency in POCUS. For emergency medicine, completion of an accredited residency training program and certification by the national board includes POCUS competency.
Internal Certificate
There are a few examples of successful local institutional programs that have provided internal certificates of competency.12,14 Competency assessments require significant resources including investment by both faculty and learners. Ongoing evaluation of competency should be based on quality assurance processes.
Credentialing and Privileging
The American Medical Association (AMA) House of Delegates in 1999 passed a resolution (AMA HR. 802) recommending hospitals follow specialty-specific guidelines for privileging decisions related to POCUS use.17 The resolution included a statement that, “ultrasound imaging is within the scope of practice of appropriately trained physicians.”
Some institutions have begun to rely on a combination of internal and external certificate programs to grant privileges to hospitalists.10 Although specific privileges for POCUS may not be required in some hospitals, some institutions may require certification of training and assessments prior to granting permission to use POCUS.
Hospitalist programs are encouraged to evaluate ongoing POCUS use by their providers after granting initial permission. If privileging is instituted by a hospital, hospitalists must play a significant role in determining the requirements for privileging and ongoing maintenance of skills.
Maintenance of Skills
All medical skills can decay with disuse, including those associated with POCUS.12,18 Thus, POCUS users should continue using POCUS regularly in clinical practice and participate in POCUS continuing medical education activities, ideally with ongoing assessments. Maintenance of skills may be confirmed through routine participation in a quality assurance program.
PROGRAM MANAGEMENT
Use of POCUS in hospital medicine has unique considerations, and hospitalists should be integrally involved in decision making surrounding institutional POCUS program management. Appointing a dedicated POCUS director can help a program succeed.8
Equipment and Image Archiving
Several factors are important to consider when selecting an ultrasound machine: portability, screen size, and ease of use; integration with the electronic medical record and options for image archiving; manufacturer’s service plan, including technical and clinical support; and compliance with local infection control policies. The ability to easily archive and retrieve images is essential for quality assurance, continuing education, institutional quality improvement, documentation, and reimbursement. In certain scenarios, image archiving may not be possible (such as with personal handheld devices or in emergency situations) or necessary (such as with frequent serial examinations during fluid resuscitation). An image archive is ideally linked to reports, orders, and billing software.10,19 If such linkages are not feasible, parallel external storage that complies with regulatory standards (ie, HIPAA compliance) may be suitable.20
Documentation and Billing
Components of documentation include the indication and type of ultrasound examination performed, date and time of the examination, patient identifying information, name of provider(s) acquiring and interpreting the images, specific scanning protocols used, patient position, probe used, and findings. Documentation can occur through a standalone note or as part of another note, such as a progress note. Whenever possible, documentation should be timely to facilitate communication with other providers.
Billing is supported through the AMA Current Procedural Terminology codes for “focused” or “limited” ultrasound examinations (Appendix 9). The following three criteria must be satisfied for billing. First, images must be permanently stored. Specific requirements vary by insurance policy, though current practice suggests a minimum of one image demonstrating relevant anatomy and pathology for the ultrasound examination coded. For ultrasound-guided procedures that require needle insertion, images should be captured at the point of interest, and a procedure note should reflect that the needle was guided and visualized under ultrasound.21 Second, proper documentation must be entered in the medical record. Third, local institutional privileges for POCUS must be considered. Although privileges are not required to bill, some hospitals or payers may require them.
Quality Assurance
Published guidelines on quality assurance in POCUS are available from different specialty organizations, including emergency medicine, pediatric emergency medicine, critical care, anesthesiology, obstetrics, and cardiology.8,22–28 Quality assurance is aimed at ensuring that physicians maintain basic competency in using POCUS to influence bedside decisions.
Quality assurance should be carried out by an individual or committee with expertise in POCUS. Multidisciplinary QA programs in which hospital medicine providers are working collaboratively with other POCUS providers have been demonstrated to be highly effective.10 Oversight includes ensuring that providers using POCUS are appropriately trained,10,22,28 using the equipment correctly,8,26,28 and documenting properly. Some programs have implemented mechanisms to review and provide feedback on image acquisition, interpretation, and clinical integration.8,10 Other programs have compared POCUS findings with referral studies, such as comprehensive ultrasound examinations.
CONCLUSIONS
Practicing hospitalists must continue to collaborate with their institutions to build POCUS capabilities. In particular, they must work with their local privileging body to determine what credentials are required. The distinction between certificates of completion and certificates of competency, including whether those certificates are internal or external, is important in the credentialing process.
External certificates of competency are currently unavailable for most practicing hospitalists because ABIM certification does not include POCUS-related competencies. As internal medicine residency training programs begin to adopt POCUS training and certification into their educational curricula, we foresee a need to update the ABIM Policies and Procedures for Certification. Until then, we recommend that certificates of competency be defined and granted internally by local hospitalist groups.
Given the many advantages of POCUS over traditional tools, we anticipate its increasing implementation among hospitalists in the future. As with all medical technology, its role in clinical care should be continuously reexamined and redefined through health services research. Such information will be useful in developing practice guidelines, educational curricula, and training standards.
Acknowledgments
The authors would like to thank all members that participated in the discussion and finalization of this position statement during the Point-of-care Ultrasound Faculty Retreat at the 2018 Society of Hospital Medicine Annual Conference: Saaid Abdel-Ghani, Brandon Boesch, Joel Cho, Ria Dancel, Renee Dversdal, Ricardo Franco-Sadud, Benjamin Galen, Trevor P. Jensen, Mohit Jindal, Gordon Johnson, Linda M. Kurian, Gigi Liu, Charles M. LoPresti, Brian P. Lucas, Venkat Kalidindi, Benji Matthews, Anna Maw, Gregory Mints, Kreegan Reierson, Gerard Salame, Richard Schildhouse, Daniel Schnobrich, Nilam Soni, Kirk Spencer, Hiromizu Takahashi, David M. Tierney, Tanping Wong, and Toru Yamada.
Many hospitalists incorporate point-of-care ultrasound (POCUS) into their daily practice because it adds value to their bedside evaluation of patients. However, standards for training and assessing hospitalists in POCUS have not yet been established. Other acute care specialties, including emergency medicine and critical care medicine, have already incorporated POCUS into their graduate medical education training programs, but most internal medicine residency programs are only beginning to provide POCUS training.1
Several features distinguish POCUS from comprehensive ultrasound examinations. First, POCUS is designed to answer focused questions, whereas comprehensive ultrasound examinations evaluate all organs in an anatomical region; for example, an abdominal POCUS exam may evaluate only for presence or absence of intraperitoneal free fluid, whereas a comprehensive examination of the right upper quadrant will evaluate the liver, gallbladder, and biliary ducts. Second, POCUS examinations are generally performed by the same clinician who generates the relevant clinical question to answer with POCUS and ultimately integrates the findings into the patient’s care.2 By contrast, comprehensive ultrasound examinations involve multiple providers and steps: a clinician generates a relevant clinical question and requests an ultrasound examination that is acquired by a sonographer, interpreted by a radiologist, and reported back to the requesting clinician. Third, POCUS is often used to evaluate multiple body systems. For example, to evaluate a patient with undifferentiated hypotension, a multisystem POCUS examination of the heart, inferior vena cava, lungs, abdomen, and lower extremity veins is typically performed. Finally, POCUS examinations can be performed serially to investigate changes in clinical status or evaluate response to therapy, such as monitoring the heart, lungs, and inferior vena cava during fluid resuscitation.
The purpose of this position statement is to inform a broad audience about how hospitalists are using diagnostic and procedural applications of POCUS. This position statement does not mandate that hospitalists use POCUS. Rather, it is intended to provide guidance on the safe and effective use of POCUS by the hospitalists who use it and the administrators who oversee its use. We discuss POCUS (1) applications, (2) training, (3) assessments, and (4) program management. This position statement was reviewed and approved by the Society of Hospital Medicine (SHM) Executive Committee in March 2018.
APPLICATIONS
As outlined in our earlier position statements,3,4 ultrasound guidance lowers complication rates and increases success rates of invasive bedside procedures. Diagnostic POCUS can guide clinical decision making prior to bedside procedures. For instance, hospitalists may use POCUS to assess the size and character of a pleural effusion to help determine the most appropriate management strategy: observation, medical treatment, thoracentesis, chest tube placement, or surgical therapy. Furthermore, diagnostic POCUS can be used to rapidly assess for immediate postprocedural complications, such as pneumothorax, or if the patient develops new symptoms.
TRAINING
Basic Knowledge
Basic knowledge includes fundamentals of ultrasound physics; safety;4 anatomy; physiology; and device operation, including maintenance and cleaning. Basic knowledge can be taught by multiple methods, including live or recorded lectures, online modules, or directed readings.
Image Acquisition
Training should occur across multiple types of patients (eg, obese, cachectic, postsurgical) and clinical settings (eg, intensive care unit, general medicine wards, emergency department) when available. Training is largely hands-on because the relevant skills involve integration of 3D anatomy with spatial manipulation, hand-eye coordination, and fine motor movements. Virtual reality ultrasound simulators may accelerate mastery, particularly for cardiac image acquisition, and expose learners to standardized sets of pathologic findings. Real-time bedside feedback on image acquisition is ideal because understanding how ultrasound probe manipulation affects the images acquired is essential to learning.
Image Interpretation
Training in image interpretation relies on visual pattern recognition of normal and abnormal findings. Therefore, the normal to abnormal spectrum should be broad, and learners should maintain a log of what abnormalities have been identified. Giving real-time feedback at the bedside is ideal because of the connection between image acquisition and interpretation. Image interpretation can be taught through didactic sessions, image review sessions, or review of teaching files with annotated images.
Clinical Integration
Learners must interpret and integrate image findings with other clinical data considering the image quality, patient characteristics, and changing physiology. Clinical integration should be taught by instructors that share similar clinical knowledge as learners. Although sonographers are well suited to teach image acquisition, they should not be the sole instructors to teach hospitalists how to integrate ultrasound findings in clinical decision making. Likewise, emphasis should be placed on the appropriate use of POCUS within a provider’s skill set. Learners must appreciate the clinical significance of POCUS findings, including recognition of incidental findings that may require further workup. Supplemental training in clinical integration can occur through didactics that include complex patient scenarios.
Pathways
Clinical competency can be achieved with training adherent to five criteria. First, the training environment should be similar to where the trainee will practice. Second, training and feedback should occur in real time. Third, specific applications should be taught rather than broad training in “hospitalist POCUS.” Each application requires unique skills and knowledge, including image acquisition pitfalls and artifacts. Fourth, clinical competence must be achieved and demonstrated; it is not necessarily gained through experience. Fifth, once competency is achieved, continued education and feedback are necessary to ensure it is maintained.
Residency-based POCUS training pathways can best fulfill these criteria. They may eventually become commonplace, but until then alternative pathways must exist for hospitalist providers who are already in practice. There are three important attributes of such pathways. First, administrators’ expectations about learners’ clinical productivity must be realistically, but only temporarily, relaxed; otherwise, competing demands on time will likely overwhelm learners and subvert training. Second, training should begin through a local or national hands-on training program. The SHM POCUS certificate program consolidates training for common diagnostic POCUS applications for hospitalists.6 Other medical societies offer training for their respective clinical specialties.7 Third, once basic POCUS training has begun, longitudinal training should continue ideally with a local hospitalist POCUS expert.
In some settings, a subgroup of hospitalists may not desire, or be able to achieve, competency in the manual skills of POCUS image acquisition. Nevertheless, hospitalists may still find value in understanding POCUS nomenclature, image pattern recognition, and the evidence and pitfalls behind clinical integration of specific POCUS findings. This subset of POCUS skills allows hospitalists to communicate effectively with and understand the clinical decisions made by their colleagues who are competent in POCUS use.
The minimal skills a hospitalist should possess to serve as a POCUS trainer include proficiency of basic knowledge, image acquisition, image interpretation, and clinical integration of the POCUS applications being taught; effectiveness as a hands-on instructor to teach image acquisition skills; and an in-depth understanding of common POCUS pitfalls and limitations.
ASSESSMENTS
Assessment methods for POCUS can include the following: knowledge-based questions, image acquisition using task-specific checklists on human or simulation models, image interpretation using a series of videos or still images with normal and abnormal findings, clinical integration using “next best step” in a multiple choice format with POCUS images, and simulation-based clinical scenarios. Assessment methods should be aligned with local availability of resources and trainers.
Basic Knowledge
Basic knowledge can be assessed via multiple choice questions assessing knowledge of ultrasound physics, image optimization, relevant anatomy, and limitations of POCUS imaging. Basic knowledge lies primarily in the cognitive domain and does not assess manual skills.
Image Acquisition
Image acquisition can be assessed by observation and rating of image quality. Where resources allow, assessment of image acquisition is likely best done through a combination of developing an image portfolio with a minimum number of high quality images, plus direct observation of image acquisition by an expert. Various programs have utilized minimum numbers of images acquired to help define competence with image acquisition skills.6–8 Although minimums may be a necessary step to gain competence, using them as a sole means to determine competence does not account for variable learning curves.9 As with other manual skills in hospital medicine, such as ultrasound-guided bedside procedures, minimum numbers are best used as a starting point for assessments.3,10 In this regard, portfolio development with meticulous attention to the gain, depth, and proper tomographic plane of images can monitor a hospitalist’s progress toward competence by providing objective assessments and feedback. Simulation may also be used as it allows assessment of image acquisition skills and an opportunity to provide real-time feedback, similar to direct observation but without actual patients.
Image Interpretation
Image interpretation is best assessed by an expert observing the learner at bedside; however, when bedside assessment is not possible, image interpretation skills may be assessed using multiple choice or free text interpretation of archived ultrasound images with normal and abnormal findings. This is often incorporated into the portfolio development portion of a training program, as learners can submit their image interpretation along with the video clip. Both normal and abnormal images can be used to assess anatomic recognition and interpretation. Emphasis should be placed on determining when an image is suboptimal for diagnosis (eg, incomplete exam or poor-quality images). Quality assurance programs should incorporate structured feedback sessions.
Clinical Integration
Assessment of clinical integration can be completed through case scenarios that assess knowledge, interpretation of images, and integration of findings into clinical decision making, which is often delivered via a computer-based assessment. Assessments should combine specific POCUS applications to evaluate common clinical problems in hospital medicine, such as undifferentiated hypotension and dyspnea. High-fidelity simulators can be used to blend clinical case scenarios with image acquisition, image interpretation, and clinical integration. When feasible, comprehensive feedback on how providers acquire, interpret, and apply ultrasound at the bedside is likely the best mechanism to assess clinical integration. This process can be done with a hospitalist’s own patients.
General Assessment
A general assessment that includes a summative knowledge and hands-on skills assessment using task-specific checklists can be performed upon completion of training. A high-fidelity simulator with dynamic or virtual anatomy can provide reproducible standardized assessments with variation in the type and difficulty of cases. When available, we encourage the use of dynamic assessments on actual patients that have both normal and abnormal ultrasound findings because simulated patient scenarios have limitations, even with the use of high-fidelity simulators. Programs are recommended to use formative and summative assessments for evaluation. Quantitative scoring systems using checklists are likely the best framework.11,12
CERTIFICATES AND CERTIFICATION
A certificate of completion is proof of a provider’s participation in an educational activity; it does not equate with competency, though it may be a step toward it. Most POCUS training workshops and short courses provide certificates of completion. Certification of competency is an attestation of a hospitalist’s basic competence within a defined scope of practice (Table 2).13 However, without longitudinal supervision and feedback, skills can decay; therefore, we recommend a longitudinal training program that provides mentored feedback and incorporates periodic competency assessments. At present, no national board certification in POCUS is available to grant external certification of competency for hospitalists.
External Certificate
Certificates of completion can be external through a national organization. An external certificate of completion designed for hospitalists includes the POCUS Certificate of Completion offered by SHM in collaboration with CHEST.6 This certificate program provides regional training options and longitudinal portfolio development. Other external certificates are also available to hospitalists.7,14,15
Most hospitalists are boarded by the American Board of Internal Medicine or the American Board of Family Medicine. These boards do not yet include certification of competency in POCUS. Other specialty boards, such as emergency medicine, include competency in POCUS. For emergency medicine, completion of an accredited residency training program and certification by the national board includes POCUS competency.
Internal Certificate
There are a few examples of successful local institutional programs that have provided internal certificates of competency.12,14 Competency assessments require significant resources including investment by both faculty and learners. Ongoing evaluation of competency should be based on quality assurance processes.
Credentialing and Privileging
The American Medical Association (AMA) House of Delegates in 1999 passed a resolution (AMA HR. 802) recommending hospitals follow specialty-specific guidelines for privileging decisions related to POCUS use.17 The resolution included a statement that, “ultrasound imaging is within the scope of practice of appropriately trained physicians.”
Some institutions have begun to rely on a combination of internal and external certificate programs to grant privileges to hospitalists.10 Although specific privileges for POCUS may not be required in some hospitals, some institutions may require certification of training and assessments prior to granting permission to use POCUS.
Hospitalist programs are encouraged to evaluate ongoing POCUS use by their providers after granting initial permission. If privileging is instituted by a hospital, hospitalists must play a significant role in determining the requirements for privileging and ongoing maintenance of skills.
Maintenance of Skills
All medical skills can decay with disuse, including those associated with POCUS.12,18 Thus, POCUS users should continue using POCUS regularly in clinical practice and participate in POCUS continuing medical education activities, ideally with ongoing assessments. Maintenance of skills may be confirmed through routine participation in a quality assurance program.
PROGRAM MANAGEMENT
Use of POCUS in hospital medicine has unique considerations, and hospitalists should be integrally involved in decision making surrounding institutional POCUS program management. Appointing a dedicated POCUS director can help a program succeed.8
Equipment and Image Archiving
Several factors are important to consider when selecting an ultrasound machine: portability, screen size, and ease of use; integration with the electronic medical record and options for image archiving; manufacturer’s service plan, including technical and clinical support; and compliance with local infection control policies. The ability to easily archive and retrieve images is essential for quality assurance, continuing education, institutional quality improvement, documentation, and reimbursement. In certain scenarios, image archiving may not be possible (such as with personal handheld devices or in emergency situations) or necessary (such as with frequent serial examinations during fluid resuscitation). An image archive is ideally linked to reports, orders, and billing software.10,19 If such linkages are not feasible, parallel external storage that complies with regulatory standards (ie, HIPAA compliance) may be suitable.20
Documentation and Billing
Components of documentation include the indication and type of ultrasound examination performed, date and time of the examination, patient identifying information, name of provider(s) acquiring and interpreting the images, specific scanning protocols used, patient position, probe used, and findings. Documentation can occur through a standalone note or as part of another note, such as a progress note. Whenever possible, documentation should be timely to facilitate communication with other providers.
Billing is supported through the AMA Current Procedural Terminology codes for “focused” or “limited” ultrasound examinations (Appendix 9). The following three criteria must be satisfied for billing. First, images must be permanently stored. Specific requirements vary by insurance policy, though current practice suggests a minimum of one image demonstrating relevant anatomy and pathology for the ultrasound examination coded. For ultrasound-guided procedures that require needle insertion, images should be captured at the point of interest, and a procedure note should reflect that the needle was guided and visualized under ultrasound.21 Second, proper documentation must be entered in the medical record. Third, local institutional privileges for POCUS must be considered. Although privileges are not required to bill, some hospitals or payers may require them.
Quality Assurance
Published guidelines on quality assurance in POCUS are available from different specialty organizations, including emergency medicine, pediatric emergency medicine, critical care, anesthesiology, obstetrics, and cardiology.8,22–28 Quality assurance is aimed at ensuring that physicians maintain basic competency in using POCUS to influence bedside decisions.
Quality assurance should be carried out by an individual or committee with expertise in POCUS. Multidisciplinary QA programs in which hospital medicine providers are working collaboratively with other POCUS providers have been demonstrated to be highly effective.10 Oversight includes ensuring that providers using POCUS are appropriately trained,10,22,28 using the equipment correctly,8,26,28 and documenting properly. Some programs have implemented mechanisms to review and provide feedback on image acquisition, interpretation, and clinical integration.8,10 Other programs have compared POCUS findings with referral studies, such as comprehensive ultrasound examinations.
CONCLUSIONS
Practicing hospitalists must continue to collaborate with their institutions to build POCUS capabilities. In particular, they must work with their local privileging body to determine what credentials are required. The distinction between certificates of completion and certificates of competency, including whether those certificates are internal or external, is important in the credentialing process.
External certificates of competency are currently unavailable for most practicing hospitalists because ABIM certification does not include POCUS-related competencies. As internal medicine residency training programs begin to adopt POCUS training and certification into their educational curricula, we foresee a need to update the ABIM Policies and Procedures for Certification. Until then, we recommend that certificates of competency be defined and granted internally by local hospitalist groups.
Given the many advantages of POCUS over traditional tools, we anticipate its increasing implementation among hospitalists in the future. As with all medical technology, its role in clinical care should be continuously reexamined and redefined through health services research. Such information will be useful in developing practice guidelines, educational curricula, and training standards.
Acknowledgments
The authors would like to thank all members that participated in the discussion and finalization of this position statement during the Point-of-care Ultrasound Faculty Retreat at the 2018 Society of Hospital Medicine Annual Conference: Saaid Abdel-Ghani, Brandon Boesch, Joel Cho, Ria Dancel, Renee Dversdal, Ricardo Franco-Sadud, Benjamin Galen, Trevor P. Jensen, Mohit Jindal, Gordon Johnson, Linda M. Kurian, Gigi Liu, Charles M. LoPresti, Brian P. Lucas, Venkat Kalidindi, Benji Matthews, Anna Maw, Gregory Mints, Kreegan Reierson, Gerard Salame, Richard Schildhouse, Daniel Schnobrich, Nilam Soni, Kirk Spencer, Hiromizu Takahashi, David M. Tierney, Tanping Wong, and Toru Yamada.
1. Schnobrich DJ, Mathews BK, Trappey BE, Muthyala BK, Olson APJ. Entrusting internal medicine residents to use point of care ultrasound: Towards improved assessment and supervision. Med Teach. 2018:1-6. doi:10.1080/0142159X.2018.1457210.
2. Soni NJ, Lucas BP. Diagnostic point-of-care ultrasound for hospitalists. J Hosp Med. 2015;10(2):120-124. doi:10.1002/jhm.2285.
3. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):117-125. doi:10.12788/jhm.2917.
4. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. doi:10.12788/jhm.2940.
5. National Council on Radiation Protection and Measurements, The Council. Implementation of the Principle of as Low as Reasonably Achievable (ALARA) for Medical and Dental Personnel.; 1990.
6. Society of Hospital Medicine. Point of Care Ultrasound course: https://www.hospitalmedicine.org/clinical-topics/ultrasonography-cert/. Accessed February 6, 2018.
7. Critical Care Ultrasonography Certificate of Completion Program. CHEST. American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography. Accessed February 6, 2018.
8. American College of Emergency Physicians Policy Statement: Emergency Ultrasound Guidelines. 2016. https://www.acep.org/Clinical---Practice-Management/ACEP-Ultrasound-Guidelines/. Accessed February 6, 2018.
9. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. doi:10.1111/acem.12653.
10. Mathews BK, Zwank M. Hospital medicine point of care ultrasound credentialing: an example protocol. J Hosp Med. 2017;12(9):767-772. doi:10.12788/jhm.2809.
11. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. doi:10.1002/jhm.468.
12. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) ultrasound program. J Hosp Med. 2018;13(8):544-550. doi:10.12788/jhm.2938.
13. Soni NJ, Tierney DM, Jensen TP, Lucas BP. Certification of point-of-care ultrasound competency. J Hosp Med. 2017;12(9):775-776. doi:10.12788/jhm.2812.
14. Ultrasound Certification for Physicians. Alliance for Physician Certification and Advancement. APCA. https://apca.org/. Accessed February 6, 2018.
15. National Board of Echocardiography, Inc. https://www.echoboards.org/EchoBoards/News/2019_Adult_Critical_Care_Echocardiography_Exam.aspx. Accessed June 18, 2018.
16. Tierney DM. Internal Medicine Bedside Ultrasound Program (IMBUS). Abbott Northwestern. http://imbus.anwresidency.com/index.html. Accessed February 6, 2018.
17. American Medical Association House of Delegates Resolution H-230.960: Privileging for Ultrasound Imaging. Resolution 802. Policy Finder Website. http://search0.ama-assn.org/search/pfonline. Published 1999. Accessed February 18, 2018.
18. Kelm D, Ratelle J, Azeem N, et al. Longitudinal ultrasound curriculum improves long-term retention among internal medicine residents. J Grad Med Educ. 2015;7(3):454-457. doi:10.4300/JGME-14-00284.1.
19. Flannigan MJ, Adhikari S. Point-of-care ultrasound work flow innovation: impact on documentation and billing. J Ultrasound Med. 2017;36(12):2467-2474. doi:10.1002/jum.14284.
20. Emergency Ultrasound: Workflow White Paper. https://www.acep.org/uploadedFiles/ACEP/memberCenter/SectionsofMembership/ultra/Workflow%20White%20Paper.pdf. Published 2013. Accessed February 18, 2018.
21. Ultrasound Coding and Reimbursement Document 2009. Emergency Ultrasound Section. American College of Emergency Physicians. http://emergencyultrasoundteaching.com/assets/2009_coding_update.pdf. Published 2009. Accessed February 18, 2018.
22. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050-1060. doi:10.1378/chest.08-2305.
23. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: general ultrasonography. Crit Care Med. 2015;43(11):2479-2502. doi:10.1097/ccm.0000000000001216.
24. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part ii: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. doi:10.1097/ccm.0000000000001847.
25. ACR–ACOG–AIUM–SRU Practice Parameter for the Performance of Obstetrical Ultrasound. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-ob.pdf. Published 2013. Accessed February 18, 2018.
26. AIUM practice guideline for documentation of an ultrasound examination. J Ultrasound Med. 2014;33(6):1098-1102. doi:10.7863/ultra.33.6.1098.
27. Marin JR, Lewiss RE. Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics. 2015;135(4):e1113-e1122. doi:10.1542/peds.2015-0343.
28. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):567-581. doi:10.1016/j.echo.2013.04.001.
1. Schnobrich DJ, Mathews BK, Trappey BE, Muthyala BK, Olson APJ. Entrusting internal medicine residents to use point of care ultrasound: Towards improved assessment and supervision. Med Teach. 2018:1-6. doi:10.1080/0142159X.2018.1457210.
2. Soni NJ, Lucas BP. Diagnostic point-of-care ultrasound for hospitalists. J Hosp Med. 2015;10(2):120-124. doi:10.1002/jhm.2285.
3. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):117-125. doi:10.12788/jhm.2917.
4. Dancel R, Schnobrich D, Puri N, et al. Recommendations on the use of ultrasound guidance for adult thoracentesis: a position statement of the society of hospital medicine. J Hosp Med. 2018;13(2):126-135. doi:10.12788/jhm.2940.
5. National Council on Radiation Protection and Measurements, The Council. Implementation of the Principle of as Low as Reasonably Achievable (ALARA) for Medical and Dental Personnel.; 1990.
6. Society of Hospital Medicine. Point of Care Ultrasound course: https://www.hospitalmedicine.org/clinical-topics/ultrasonography-cert/. Accessed February 6, 2018.
7. Critical Care Ultrasonography Certificate of Completion Program. CHEST. American College of Chest Physicians. http://www.chestnet.org/Education/Advanced-Clinical-Training/Certificate-of-Completion-Program/Critical-Care-Ultrasonography. Accessed February 6, 2018.
8. American College of Emergency Physicians Policy Statement: Emergency Ultrasound Guidelines. 2016. https://www.acep.org/Clinical---Practice-Management/ACEP-Ultrasound-Guidelines/. Accessed February 6, 2018.
9. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. doi:10.1111/acem.12653.
10. Mathews BK, Zwank M. Hospital medicine point of care ultrasound credentialing: an example protocol. J Hosp Med. 2017;12(9):767-772. doi:10.12788/jhm.2809.
11. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. doi:10.1002/jhm.468.
12. Mathews BK, Reierson K, Vuong K, et al. The design and evaluation of the Comprehensive Hospitalist Assessment and Mentorship with Portfolios (CHAMP) ultrasound program. J Hosp Med. 2018;13(8):544-550. doi:10.12788/jhm.2938.
13. Soni NJ, Tierney DM, Jensen TP, Lucas BP. Certification of point-of-care ultrasound competency. J Hosp Med. 2017;12(9):775-776. doi:10.12788/jhm.2812.
14. Ultrasound Certification for Physicians. Alliance for Physician Certification and Advancement. APCA. https://apca.org/. Accessed February 6, 2018.
15. National Board of Echocardiography, Inc. https://www.echoboards.org/EchoBoards/News/2019_Adult_Critical_Care_Echocardiography_Exam.aspx. Accessed June 18, 2018.
16. Tierney DM. Internal Medicine Bedside Ultrasound Program (IMBUS). Abbott Northwestern. http://imbus.anwresidency.com/index.html. Accessed February 6, 2018.
17. American Medical Association House of Delegates Resolution H-230.960: Privileging for Ultrasound Imaging. Resolution 802. Policy Finder Website. http://search0.ama-assn.org/search/pfonline. Published 1999. Accessed February 18, 2018.
18. Kelm D, Ratelle J, Azeem N, et al. Longitudinal ultrasound curriculum improves long-term retention among internal medicine residents. J Grad Med Educ. 2015;7(3):454-457. doi:10.4300/JGME-14-00284.1.
19. Flannigan MJ, Adhikari S. Point-of-care ultrasound work flow innovation: impact on documentation and billing. J Ultrasound Med. 2017;36(12):2467-2474. doi:10.1002/jum.14284.
20. Emergency Ultrasound: Workflow White Paper. https://www.acep.org/uploadedFiles/ACEP/memberCenter/SectionsofMembership/ultra/Workflow%20White%20Paper.pdf. Published 2013. Accessed February 18, 2018.
21. Ultrasound Coding and Reimbursement Document 2009. Emergency Ultrasound Section. American College of Emergency Physicians. http://emergencyultrasoundteaching.com/assets/2009_coding_update.pdf. Published 2009. Accessed February 18, 2018.
22. Mayo PH, Beaulieu Y, Doelken P, et al. American College of Chest Physicians/La Societe de Reanimation de Langue Francaise statement on competence in critical care ultrasonography. Chest. 2009;135(4):1050-1060. doi:10.1378/chest.08-2305.
23. Frankel HL, Kirkpatrick AW, Elbarbary M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part I: general ultrasonography. Crit Care Med. 2015;43(11):2479-2502. doi:10.1097/ccm.0000000000001216.
24. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part ii: cardiac ultrasonography. Crit Care Med. 2016;44(6):1206-1227. doi:10.1097/ccm.0000000000001847.
25. ACR–ACOG–AIUM–SRU Practice Parameter for the Performance of Obstetrical Ultrasound. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/us-ob.pdf. Published 2013. Accessed February 18, 2018.
26. AIUM practice guideline for documentation of an ultrasound examination. J Ultrasound Med. 2014;33(6):1098-1102. doi:10.7863/ultra.33.6.1098.
27. Marin JR, Lewiss RE. Point-of-care ultrasonography by pediatric emergency medicine physicians. Pediatrics. 2015;135(4):e1113-e1122. doi:10.1542/peds.2015-0343.
28. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel RJ. Focused cardiac ultrasound: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):567-581. doi:10.1016/j.echo.2013.04.001.
© 2019 Society of Hospital Medicine
Recommendations on the Use of Ultrasound Guidance for Adult Thoracentesis: A Position Statement of the Society of Hospital Medicine
Approximately 1.5 million people develop a pleural effusion in the United States annually, and approximately 173,000 people (12%) undergo thoracentesis.1 A recent review of thoracenteses performed at 234 University Health System Consortium hospitals between January 2010 and September 2013 demonstrated that 16% of 132,472 thoracenteses were performed by general internists and hospitalists, 33.1% were performed by interventional radiologists, and 20.3% were performed by pulmonologists.2 The iatrogenic pneumothorax rate was not significantly different between interventional radiologists and internists (2.8% and 2.9% risk, respectively); however, the admissions associated with bedside thoracentesis were less expensive than the admissions associated with thoracentesis performed in radiology suites, even after controlling for clinical covariates.2 In addition, the use of ultrasound guidance has been associated with a reduced risk of complications and cost of thoracentesis.3,4 In most of the early published studies on ultrasound-guided thoracentesis, the procedures were performed by radiologists.5-12 However, in 2010, the British Thoracic Society published guidelines on pleural procedures and thoracic ultrasound geared toward any trained provider.13 The purpose of this guideline is to review the literature and present evidence-based recommendations on the performance of ultrasound-guided thoracentesis at the bedside.
METHODS
Detailed methods are described in Appendix 1. The Society of Hospital Medicine (SHM) Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. The expert panel members were divided into working group members, external peer reviewers, and a methodologist. All the Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the four working group members themselves. Key clinical questions were prepared prior to conducting a systematic literature search by a medical librarian. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to September 2015 initially. Updated searches were conducted in November 2016 and in August 2017 (Appendix 3). All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of the screened articles were reviewed, and the articles focusing on the use of ultrasound to guide thoracentesis were selected. Articles that discussed thoracentesis without ultrasound guidance were excluded. In addition, the following article types were excluded: non-English language, nonhuman, subjects’ age <18 years, meeting abstracts, meeting posters, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided thoracentesis were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into draft recommendations.
We used the RAND Appropriateness Method that required panel judgment and consensus.14 The 30 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: 1) Problem priority and importance, 2) Level of quality of evidence, 3) Benefit/harm balance, 4) Benefit/burden balance, and 5) Certainty/concerns about PEAF (Preferences/Equity Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (Redcap™) in December 2016 and January 2017 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale, and the degree of consensus was assessed using the RAND algorithm. Establishing a recommendation required at least 70% agreement and a strong recommendation required 80% agreement according to the RAND rules (Appendix 1, Figure 1). Disagreement was defined as >30% of panelists voting outside of the zone of the median (appropriate, uncertain, inappropriate).
RESULTS
Literature search
A total of 1,556 references were pooled from the following four different sources: a search by a certified librarian in September 2015 (1066 citations) that was updated in November 2016 (165 citations) and again in August 2017 (9 citations), working group members’ literature searches (47 citations), and a search focused on training (269 citations). The final selection included 94 articles that were abstracted into a data table and incorporated into the draft recommendations. The details of the literature search strategy are given in Appendix 3.
Recommendations
Terminology
- Thoracentesis is a procedure of aspiration of fluid from the pleural space by percutaneous insertion of a needle through the chest wall with or without the insertion of a catheter.
- In this document, ultrasound guidance refers to static guidance and site marking performed at the bedside immediately before the procedure, as opposed to real-time (dynamic) ultrasound guidance or radiology performed site marking. The static method is the most commonly used method of ultrasound guidance and is supported by current evidence.
RECOMMENDATIONS
Clinical Outcomes
1.We recommend that ultrasound should be used to guide thoracentesis to reduce the risk of complications, the most common being pneumothorax.
Rationale: Both static ultrasound guidance and dynamic ultrasound guidance have been reported to be associated with a reduced risk of pneumothorax.4-7,15-18 A meta-analysis of 24 studies that included 6,605 thoracenteses showed a significant decrease in the risk of postprocedure pneumothorax with the use of ultrasound guidance compared to the risk associated with thoracentesis performed based on landmarks alone (OR 0.3, 95% CI 0.2–0.7).3 The meta-analysis included both prospective and retrospective studies conducted using both static and dynamic ultrasound guidance.3 A large retrospective cohort study conducted by Mercaldi et al. comprising more than 61,000 patients who underwent thoracentesis also showed that ultrasound guidance was associated with reduced odds of pneumothorax (OR 0.8 [0.7–0.9]).4 When pneumothorax did occur during that hospitalization, the cost of hospitalization increased by $2800 and the length of stay increased by 1.5 days.4 A 2008 review of 19,339 thoracenteses conducted by Patel et al. also demonstrated an association between ultrasound guidance and reduced odds of pneumothorax (OR 0.8 [0.7–0.96]).18 Although these findings were significant, it is important to note that the studies of both Mercaldi et al. and Patel et al. were reviews of administrative databases conducted using the International Classification of Diseases, 9th Revision (ICD-9) codes for thoracentesis and Current Procedure Terminology–4th edition (CPT) codes for the use of ultrasound.4,18 Patel et al. identified pneumothorax using ICD-9 codes for “pneumothorax–iatrogenic” and “pneumothorax–not specified as due to the procedure.” The association between ultrasound guidance and the reduced odds of pneumothorax was driven by the latter code.18 However, as with most retrospective studies using administrative data, granular data about the patients, procedure, proceduralists, and complications were not available in these reviews and conclusions may be limited by erroneous coding or documentation.4,18 In a third retrospective cohort study, Raptopoulos et al. compared 154 landmark-based thoracenteses performed by “clinical physicians” and 188 ultrasound-guided thoracenteses performed by radiologists and found that ultrasound-guided site selection reduced the rate of pneumothorax from 18% to 3% (P < .0001).6 Finally, one single-center randomized controlled trial of 160 thoracenteses performed by pulmonologists showed that ultrasound guidance reduced the relative risk of pneumothorax by 90% (12.5% vs 1.3%; P =.009) with a number needed to treat of 9.15 It was not possible to blind the operators to the use of ultrasound guidance, but the data analysis was blinded.15 Furthermore, while there was no explicit comparison of the intervention vs. the control groups, randomization would have presumably rendered both groups similar in terms of patient characteristics and effusion characteristics.15 Ultrasound may reduce the risk of pneumothorax through several mechanisms, including identifying patients in whom thoracentesis cannot be safely performed, allowing selection of the safest needle insertion site, and revealing the optimal depth of needle insertion.
2.We recommend that ultrasound guidance should be used to increase the success rate of thoracentesis.
Rationale: Thoracentesis guided by ultrasound has lower rates of failed attempts, or “dry taps,” compared to thoracentesis guided solely by physical examination. In 1977, Ravin described a method of using ultrasound to guide successful drainage of six complex pleural effusions (empyema or loculated effusion) after multiple (5–7) failed attempts by clinicians using physical examination alone.8 In a second study by radiologists, Weingardt et al. demonstrated that 20 of 26 failed landmark-based thoracenteses were due to incorrect site selection by physical examination–15 sites were below the diaphragm and 5 sites were above the pleural effusion or in the consolidated lung–and the use of ultrasound allowed successful sampling in 14 of 16 patients who had a failed landmark-based thoracentesis.9 Diacon et al. asked 30 physicians, ranging from junior housestaff to pulmonologists, to mark 172 potential thoracentesis sites in 67 patients with pleural effusions using physical examination alone. Ultrasound was then used to evaluate the proposed puncture sites. They found that using ultrasound would have avoided puncture on “dry chests” in 2% and avoided potential laceration of a solid organ in 10% of patients compared to site selection by physical examination alone.19 Finally, Perazzo et al. randomized 160 patients to landmark-based thoracentesis and ultrasound-guided thoracentesis and demonstrated that half of the eight dry taps that occurred in the control group could be successfully drained using subsequent ultrasound guidance.15
Technique
3. We recommend that ultrasound-guided thoracentesis should be performed or closely supervised by experienced operators.
Rationale: Current evidence suggests lower complication rates when thoracentesis is performed by experienced healthcare providers. A systematic review of 6,605 thoracenteses showed a significantly lower pneumothorax rate when thoracentesis was performed by pulmonology or radiology faculty versus resident physicians (3.9% vs 8.5%; P =.04), although this finding was not significant in the four studies that directly compared this factor.3 In a quality improvement study performed by Duncan et al., pulmonology and critical care physicians combining multiple quality improvement initiatives to achieve and maintain competency decreased the rate of pneumothorax from 8.6% to 1.1% (P =.0034).20 Interventions included ultrasound training, performance of 10 thoracenteses under expert supervision, and restriction of privileges to proceduralists who perform 10 or more thoracenteses per year.20 Finally, a series of 9,320 ultrasound-guided thoracenteses performed or supervised by a single expert internist over a period of 12 years resulted in a pneumothorax rate of 0.6% and a composite complication rate of 0.98% (pneumothorax, reexpansion pulmonary edema, hemothorax, site bleeding, hematoma, splenic laceration, and vasovagal reaction).21 Notably, pneumothorax rate in resident physician hands was reported to be 8.5% in the meta-analysis performed by Gordon et al., which is similar to the initial rate in the pulmonologists who participated in the study by Duncan et al.3,20 However, after instituting formal ultrasound training and other initiatives aimed at maintaining competency, the pneumothorax rate in the study by Duncan et al. decreased to 1.1%, similar to the rate observed in the series by Ault et al.21 This suggests that training and supervision are necessary to achieve competency and reduce the rate of complications.3,20,21
4. We suggest that ultrasound guidance be used to reduce the risk of complications from thoracentesis in mechanically ventilated patients.
Rationale: The rest of this guideline refers to ultrasound-guided thoracentesis performed in spontaneously breathing patients; however, this recommendation is specific to mechanically ventilated patients. Two prospective observational studies have shown no increase in complications when ultrasound-guided thoracentesis is performed on mechanically ventilated patients compared to patients not receiving positive pressure ventilation. A feasibility study of 45 thoracenteses performed on ventilated patients reported no complications,22 whereas another study on 232 patients reported a pneumothorax rate of 1.3%.23 In a larger study conducted by Mayo et al., medicine housestaff performed thoracentesis under the supervision of intensivists who had undergone training in ultrasound prior to performing the procedure.23 In both studies, most of the patients were in a supine position, although positioning and puncture site were at the discretion of the physician, and both studies employed use of static ultrasound guidance.22,23 A large series of 9,320 ultrasound-guided thoracenteses that included 1,377 mechanically ventilated patients did not report a higher rate of pneumothorax (0.8%) compared to that in spontaneously breathing patients (0.61%).21 Finally, a meta-analysis of 19 observational studies comprising 1,124 mechanically ventilated patients who underwent pleural drainage procedures showed a low rate of pneumothorax (3.4%) and hemothorax (1.9%).24 Although the rate of complication was reported to be low in this meta-analysis, ultrasound was not employed in all studies and its use was not associated with a significant reduction in pneumothorax.24 This may be because 8 of the 19 studies used pigtail catheters or large-bore thoracostomy tubes which treat pneumothorax as they occur.24
5. We recommend that ultrasound should be used to identify the chest wall, pleura, diaphragm, lung, and subdiaphragmatic organs throughout the respiratory cycle before selecting a needle insertion site.
Rationale: The use of ultrasound improves the selection of a safe needle insertion site because sites chosen without ultrasound guidance may be below the diaphragm, over solid organs,9,19 or in locations that risk puncture of the lung.9 Visualization of the chest wall, diaphragm, and lung, which define the boundaries of a pleural effusion, allows the clinician to confirm the presence of a drainable pleural effusion and assess for other pathologies, such as ascites and tumor, that may be mistaken for a pleural effusion.22,25,26 Hypoechoic lesions can represent small loculated pleural effusions but also pleural plaques, pleural masses, peripheral lung masses, or abscesses.27,28
6. We recommend that ultrasound should be used to detect the presence or absence of an effusion and approximate the volume of pleural fluid to guide clinical decision-making.
Rationale: The presence and approximate size of pleural fluid collections are important determinants of whether thoracentesis, another procedure, or no procedure should be performed. Ultrasonography has higher sensitivity and specificity for detecting pleural effusions and better differentiates effusions from consolidations compared with chest radiography.29-42 Ultrasound allows semiquantitative estimation of pleural fluid volume to determine whether thoracentesis should be performed.41-45 When using ultrasound to choose a site for thoracentesis, the British Thoracic Society Pleural Disease guidelines recommend ≥10 mm of pleural fluid between the visceral and parietal pleura.13 Pleural effusions of <10–15 mm are considered too small to tap.22,23 In a prospective study of 45 patients, a measurement of >9.9 cm by ultrasound between the chest wall and the “V-point,” the intersection of the diaphragm and the collapsed lung, correlated with a pleural fluid volume of >1 liter.46 Another prospective study of 73 patients showed that a pleural effusion spanning >3 intercostal spaces by ultrasound also correlated with a pleural fluid volume of >1 liter.47 Anticipating the volume of fluid to be removed may aid in preplanning and procurement of larger capacity drainage containers prior to starting the procedure. Lung ultrasound can also change the management if the characteristic of the effusion suggests that an invasive procedure is unsafe or another diagnostic or therapeutic option is more appropriate.39 In a prospective cohort study of 189 mechanically ventilated patients, lung ultrasound guided the management in all patients with suspected effusion, leading to chest tube placement in 7 patients and thoracentesis in 34 patients.48
7. We recommend that ultrasound should be used to detect complex sonographic features, such as septations, to guide clinical decision-making regarding the timing and method of pleural drainage.
Rationale: Pleural effusions can be broadly categorized sonographically as simple or complex. Complex effusions are further categorized as with or without septation. Simple effusions are anechoic and are often, but not invariably, transudative.49-51 The use of sonography and computerized tomography (CT) is complementary, but features of complex pleural effusions (fibrin stranding and septations) may be better visualized by ultrasound than by CT of the thorax.52 Detection of complex features should prompt the consideration of pleural fluid sampling.53,54 Exudative effusions from tuberculosis, malignancy, or other etiologies more often include debris, septations, or other complex features.55,56 Certain features such as a swirling debris, pleural thickening, and nodularity may be more often associated with malignancy,54,56 and advanced ultrasound techniques may be used to detect a trapped lung prior to attempting drainage of a malignant pleural effusion.57 Two studies found complex septated pleural effusions to be invariably exudative50,58 and drainage was unlikely to be successful without the placement of a chest tube.50,58-60 Chest tube placement through fibrinolytic administration or video-assisted thoracoscopic surgery (VATS) may be more appropriate in the management of complex septated pleural effusions,59-61 and expert consultation with a thoracic specialist is recommended in these cases.
8. We suggest that ultrasound can be used to measure the depth from the skin surface to the parietal pleura to help select an appropriate length needle and determine the maximum needle insertion depth.
Rationale: The distance from the skin to the parietal and visceral pleura can be measured by ultrasound to determine whether thoracentesis can be safely performed and to guide selection of an adequate length needle.38 The length of needle required to penetrate the pleural space varies based on the thickness of the chest wall. Percussion of the chest wall is limited when there is more than 6 cm of subcutaneous tissue,62 making physical examination in obese patients unreliable for selecting an appropriate site or needle length for thoracentesis. Ultrasound allows visualization of deep soft tissues, well beyond the limits of percussion, and allows an accurate measurement of the chest wall.63
9. We suggest that ultrasound can be used to evaluate normal lung sliding pre- and postprocedure to rule out pneumothorax.
Rationale: Normal lung sliding indicates normal apposition and movement of visceral and parietal pleura and rules out pneumothorax with a sensitivity that exceeds that of chest radiography, according to a meta-analysis of 20 studies using computed tomography or escape of intrapleural air at the time of drainage as the gold standard.64 In this meta-analysis, the pooled sensitivity of ultrasound was reported to be 88% (85-91%) compared to 52% (49-55%) for radiography, although the analysis also suggests that the test characteristics are dependent on operator skill.64 However, although lung sliding rules out pneumothorax, absence of lung sliding is not specific for pneumothorax and other conditions, including pleural adhesions, pleurodesis, and bronchial obstruction, can cause the absence of lung sliding.64 Detection of a lung point conclusively rules in a pneumothorax.65 Provided that the preprocedure lung ultrasound examination revealed normal lung sliding, a postprocedure examination can be performed to effectively evaluate for pneumothorax. This modality does not use ionizing radiation, is less expensive than computed tomography, can be performed faster than bedside chest radiography, and is more sensitive than supine or upright chest radiography.64,66-71
10. We suggest avoiding delay or interval change in patient position between the time of marking the needle insertion site and performing the thoracentesis.
Rationale: Optimal patient positioning and ultrasound-guided site marking should be performed by the primary operator immediately before beginning an invasive procedure. Remote sonographic localization in which a radiologist marks a needle insertion site using ultrasound and the thoracentesis is performed at a later time by a different provider is an antiquated practice. Two early studies demonstrated that this practice is no safer than landmark-based thoracentesis.6,72 One prospective study of 205 patients performed in 1986 showed no significant decrease in the incidence of complications from thoracentesis performed using remote sonographic localization versus landmark-based drainage.72 Complications in that study included a total of 22 pneumothoraces and 1 hematoma. The rate of complications in the group of patients who had site marking performed by radiology faculty and subsequent thoracentesis by medicine housestaff or attending physicians was 9.7% versus a complication rate of 12.7% in the landmark-based group.72 In addition, Raptopoulos et al. observed no significant difference in the pneumothorax rate between 106 patients with landmark-based thoracenteses and 48 patients who were sonographically marked by radiology faculty and then returned to the ward for completion of the thoracentesis by medicine housestaff (19% vs. 15%, respectively).6 Both groups had significantly higher rates of pneumothorax compared to those who underwent thoracentesis performed using real-time ultrasound guidance by radiology trainees (3%).6 The authors speculated that changing the patient’s position shifted the position of the pleural effusion, ultimately leading to the reliance on physical examination for the tap site.6
11. We recommend against performing routine postprocedure chest radiographs in patients who have undergone thoracentesis successfully with ultrasound guidance and are asymptomatic with normal lung sliding postprocedure.
Rationale: Chest radiography post-thoracentesis is unlikely to add information that changes management, especially if performed routinely, but does add expense, radiation, and inconvenience.73 The most common serious complication of thoracentesis is pneumothorax, which is often accompanied by symptoms, particularly in those patients with pneumothorax large enough to warrant chest tube placement.10,74,75 Pihlajamaa et al. retrospectively studied 264 ultrasound-guided thoracenteses performed by radiologists or radiology residents and noted that of 11 pneumothoraces, only 1 necessitated chest tube placement.10 Aleman et al. prospectively studied 506 ultrasound-guided and physical examination-guided thoracenteses and found that only 1% of asymptomatic patients developed a pneumothorax.74 Eight of the 18 symptomatic patients required chest tube placement as opposed to 1 of the 488 asymptomatic patients.74 A large prospective study of 941 ultrasound-guided thoracentesis reported that only 0.3% of asymptomatic patients with no suspicion of pneumothorax required tube thoracostomy.5 Postprocedure chest radiographs may be considered when thoracentesis is performed on mechanically ventilated patients, particularly when high airway pressures exist. In a study of 434 patients undergoing thoracentesis, only 10 patients had a pneumothorax (2.3%).11 Six of these pneumothoraces occurred in 92 mechanically ventilated patients (6.5%), and 2 of these 6 patients required a chest tube.11 None of the 4 spontaneously breathing patients with pneumothorax required a chest tube.11
Training
12. We recommend that novices who use ultrasound guidance for thoracentesis should receive focused training in lung and pleural ultrasonography and hands-on practice in procedural technique.
Rationale: Healthcare providers have to gain various skills to safely perform ultrasound-guided thoracentesis independently. Trainees should learn how to use ultrasound to identify important structures (chest wall, ribs, lung, pleura, diaphragm, and subdiaphragmatic organs); detect pleural effusions with complex features, such as septations; identify consolidated lung tissue; and rule out a pneumothorax. Prospective studies done with novice learners have shown that focused training combining didactics and hands-on practice using simulation or live models improves skills to assess pleural effusions.76-84 Several additional procedural techniques such as patient positioning and needle insertion are also important but are beyond the scope of these guidelines.
13. We suggest that novices undergo simulation-based training prior to performing ultrasound-guided thoracentesis on patients.
Rationale: Simulation-based training for thoracentesis has been studied in providers with different levels of medical training, ranging from medical students and internal medicine residents to practicing pulmonologists. Studies suggest that training in a zero-risk environment with simulation task trainers leads to increased knowledge and skills without subjecting the patients to inexperienced operators.85-87 One study on simulator-based training in medical students showed skill retention at 6 months and these skills were at least partially transferred to increased competency on live patients.88 Checklists to train providers in ultrasound-guided thoracentesis have been published.89,90 An experiential training program for attending physicians that utilized task trainers, along with standardized equipment and procedural technique, resulted in a reduction in the pneumothorax rate from 8.6% to 1.1%.20
14. Training curves for novices to become competent in lung ultrasound and ultrasound-guided thoracentesis are not completely understood. We recommend that training should be tailored to the skill acquisition of the learner and the resources of the institution.
Rationale: Understanding the rates at which novices progress from performing procedures under direct supervision to performing them independently would be highly desirable to ensure patient safety, guide supervision, and maximize efficiency of training. However, there is limited research describing the rate of progression of learners through these stages, either with regard to time or number of procedures performed. Two studies have shown that with brief training programs, medical students88 and internal medicine residents87 can achieve high levels of proficiency to perform thoracentesis on simulators, which is durable over time; however, whether these findings in a simulated environment translate into clinically significant outcomes is largely unknown, and neither of these studies incorporated the use of ultrasound guidance in their training curricula.87,88 Another study of pulmonary and critical care physicians combined multiple quality improvement initiatives with a half day of ultrasound-guided thoracentesis training, a requirement to perform 10 supervised thoracenteses prior to independent practice, and an additional requirement to perform 10 thoracenteses per year to maintain privileges.20 These interventions resulted in a concentration of competency among a few proceduralists, decreasing the rate of pneumothorax from 8.6% to 1.1%.20 Degradation of skills with disuse may also occur84; thus, procedures performed infrequently should at a minimum be subjected to increased supervision and/or retesting.
KNOWLEDGE GAPS
The process of developing these guidelines revealed important gaps in the literature regarding the use of ultrasound guidance for thoracentesis. First, it is uncertain whether the use of ultrasound reduces the risk of bleeding with thoracentesis. A retrospective cohort study of 19,339 thoracenteses suggests that ultrasound guidance is associated with a 38.7% relative reduction in the odds of hemorrhage, although this reduction did not reach statistical significance (OR 0.6 [0.4–1.04]).18 Ultrasound may reduce the risk of bleeding by reducing the number of attempts and needle passes and potentially avoiding tortuous intercostal vessels, which can be found especially in elderly patients and more cephalad rib spaces.91 In an observational study of 22 patients undergoing thoracentesis, the intercostal artery (ICA) was identified by a high-frequency ultrasound transducer in 74 of 88 intercostal spaces.92 The ICA is more exposed in the intercostal space within the first 6 cm lateral to the spinous processes and can be seen as far lateral as the midaxillary line.92-95 Thus, the ICA will most likely be avoided if a procedure site is selected >6 cm lateral to the spinous processes and the needle is inserted above the rib.
Second, although all three studies conducted using real-time (dynamic) ultrasound guidance reported a pneumothorax rate of <1%, it is uncertain whether real-time ultrasound guidance confers any additional benefit compared to static guidance for site marking as direct comparisons were not made.17,96,97 It is possible that real-time ultrasound guidance may be superior to static guidance in certain situations, such as small pleural effusions of <10–15 mm that have historically been considered too small to tap.13,22,23,96
Third, although one study suggests that general internists can safely perform thoracentesis with low complication rates similar to those of interventional radiologists,2 limited data exists on how to train practicing hospitalists to use ultrasound to guide thoracentesis. The effectiveness of different training protocols to acquire competence in ultrasound-guided thoracentesis has not been compared.
Finally, the impact of ultrasound use on patient experience has yet to be explored.
CONCLUSION
The use of ultrasound guidance for thoracentesis has been associated with increased success rates and decreased complication rates. Ultrasound can be used to estimate the pleural fluid volume, characterize the effusion as simple or complex, identify an optimal needle insertion site, and reduce the need for postprocedural chest radiographs. Training and experience are essential to reap the benefits of using ultrasound for thoracentesis, although our understanding of optimal educational strategies and learning curves is limited. Once training has occurred and competence is achieved, hospitalists can perform ultrasound-guided thoracentesis as safely as radiologists, pulmonologists, and other specialists.
Acknowledgments
Collaborators from the Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Benji Mathews, Paul, Mayo, Satyen Nichani, Vicki Noble, Martin Perez, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Gerard Salame, Kirk Spencer, Vivek Tayal, David M. Tierney.
Disclosures
Ricardo Franco-Sadud reports institutional funds received from the Society of Hospital Medicine Annual Meeting for travel expenses and accommodations outside the submitted work. Nitin Puri reports Payment for lectures including service on speakers bureaus from Fujifilm Sonosite and royalties from Elsevier, both outside the submitted work. All other authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1)
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
CHAIRS: Nilam Soni, Ricardo Franco Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen. Lumbar puncture Working Group: Nilam Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen. PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Dan Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
1. Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13. 1998;139:1-119. PubMed
2. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. PubMed
3. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
5. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
6. Raptopoulos V, Davis LM, Lee G, Umali C, Lew R, Irwin RS. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917-920. PubMed
7. Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150(4):873-877. PubMed
8. Ravin CE. Thoracocentesis of loculated pleural effusions using grey scale ultrasonic guidance. Chest. 1977;71(5):666-668. PubMed
9. Weingardt JP, Guico RR, Nemcek AA, Jr., Li YP, Chiu ST. Ultrasound findings following failed, clinically directed thoracenteses. J Clin Ultrasound. 1994;22(7):419-426. PubMed
10. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
11. Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology. 1997;204(2):503-506. PubMed
12. Boland GW, Gazelle GS, Girard MJ, Mueller PR. Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR Am J Roentgenol. 1998;170(4):943-946. PubMed
13. Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(2):ii61-76. PubMed
14. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The RAND/UCLA appropriateness method user’s manual. DTIC Document; 2001.
15. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
16. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
17. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
18. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
19. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
20. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
21. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
22. Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Meziere G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25(9):955-958. PubMed
23. Mayo PH, Goltz HR, Tafreshi M, Doelken P. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125(3):1059-1062. PubMed
24. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
25. Landay M, Harless W. Ultrasonic differentiation of right pleural effusion from subphrenic fluid on longitudinal scans of the right upper quadrant: importance of recognizing the diaphragm. Radiology. 1977;123(1):155-158. PubMed
26. Mayo PH, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006;27(2):215-227. PubMed
27. Rosenberg ER. Ultrasound in the assessment of pleural densities. Chest. 1983;84(3):283-285. PubMed
28. Gorg C, Restrepo I, Schwerk WB. Sonography of malignant pleural effusion. Eur Radiol. 1997;7(8):1195-1198. PubMed
29. Gryminski J, Krakowka P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33-37. PubMed
30. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12-16. PubMed
31. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15. PubMed
32. Grimberg A, Shigueoka DC, Atallah AN, Ajzen S, Iared W. Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J.. 2010;128(2):90-95. PubMed
33. Kataoka H. Utility of thoracic sonography for follow-up examination of chronic heart failure patients with previous decompensation. Clin Cardiol. 2007;30(7):336-341. PubMed
34. Ma OJ, Mateer JR. Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312-315. PubMed
35. Rocco M, Carbone I, Morelli A, et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776-784. PubMed
36. Kocijancic I, Vidmar K, Ivanovi-Herceg Z. Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69-74. PubMed
37. Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1. PubMed
38. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
39. Medford AR, Entwisle JJ. Indications for thoracic ultrasound in chest medicine: an observational study. Postgrad Med J. 2010;86(1011):8-11. PubMed
40. Lin MS, Hwang JJ, Chong IW, et al. Ultrasonography of chest diseases: analysis of 154 cases. Gaoxiong Yi Xue Ke Xue Za Zhi . 1992;8(10):525-534. PubMed
41. Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hormann MF, Grabenwoger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191(3):681-684. PubMed
42. Vignon P, Chastagner C, Berkane V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33(8):1757-1763. PubMed
43. Usta E, Mustafi M, Ziemer G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204-207. PubMed
44. Remerand F, Dellamonica J, Mao Z, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656-664.
PubMed
45. Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318-321. PubMed
46. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracentesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
47. Lisi M, Cameli M, Mondillo S, et al. Incremental value of pocket-sized imaging device for bedside diagnosis of unilateral pleural effusions and ultrasound-guided thoracentesis. Interact Cardiovasc Thorac Surg. 2012;15(4):596-601. PubMed
48. Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57-65. PubMed
49. Chen HJ, Tu CY, Ling SJ, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol. 2008;34(3):362-369. PubMed
50. Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29-33. PubMed
51. Liang SJ, Tu CY, Chen HJ, et al. Application of ultrasound-guided pigtail catheter for drainage of pleural effusions in the ICU. Intensive Care Med. 2009;35(2):350-354. PubMed
52. McLoud TC, Flower CD. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145-1153. PubMed
53. Tu CY, Hsu WH, Hsia TC, et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274-1280. PubMed
54. Sajadieh H, Afzali F, Sajadieh V, Sajadieh A. Ultrasound as an alternative to aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci. 2004;1019:585-592. PubMed
55. Marcun R, Sustic A. Sonographic evaluation of unexplained pleural exudate: a prospective case series. Wien Klin Wochenschr. 2009;121(9-10):334-338. PubMed
56. Bugalho A, Ferreira D, Dias SS, et al. The diagnostic value of transthoracic ultrasonographic features in predicting malignancy in undiagnosed pleural effusions: a prospective observational study. Respiration. 2014;87(4):270-278. PubMed
57. Salamonsen MR, Lo AK, Ng AC, Bashirzadeh F, Wang WY, Fielding DI. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest. 2014;146(5):1286-1293. PubMed
58. Himelman RB, Callen PW. The prognostic value of loculations in parapneumonic pleural effusions. Chest. 1986;90(6):852-856. PubMed
59. Chen CH, Chen W, Chen HJ, et al. Transthoracic ultrasonography in predicting the outcome of small-bore catheter drainage in empyemas or complicated parapneumonic effusions. Ultrasound Med Biol. 2009;35(9):1468-1474. PubMed
60. Hirsch JH, Rogers JV, Mack LA. Real-time sonography of pleural opacities. AJR Am J Roentgenol. 1981;136(2):297-301. PubMed
61. Chen KY, Liaw YS, Wang HC, Luh KT, Yang PC. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837-843. PubMed
62. Diaz-Guzman E, Budev MM. Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297-303. PubMed
63. Rhyne T, Birnholz JC. Simple measurement of chest-wall thickness with ultrasound. Radiology. 1973;108(2):436-438. PubMed
64. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140(4):859-866. PubMed
65. Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434-1440. PubMed
66. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
67. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. PubMed
68. Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141(3):703-708. PubMed
69. Sartori S, Tombesi P, Trevisani L, Nielsen I, Tassinari D, Abbasciano V. Accuracy of transthoracic sonography in detection of pneumothorax after sonographically guided lung biopsy: prospective comparison with chest radiography. AJR Am J Roentgenol. 2007;188(1):37-41. PubMed
70. Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12(9):844-849. PubMed
71. Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33(6):1231-1238. PubMed
72. Kohan JM, Poe RH, Israel RH, et al. Value of chest ultrasonography versus decubitus roentgenography for thoracentesis. Am Rev Respir Dis. 1986;133(6):1124-1126. PubMed
73. Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc. 1998;73(10):948-950. PubMed
74. Aleman C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107(4):340-343. PubMed
75. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
76. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40(10):1475-1480. PubMed
77. Kotagal M, Quiroga E, Ruffatto BJ, et al. Impact of point-of-care ultrasound training on surgical residents’ confidence. J Surg Educ. 2015;72(4):e82-87. PubMed
78. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. PubMed
79. Schnobrich DJ, Olson AP, Broccard A, Duran-Nelson A. Feasibility and acceptability of a structured curriculum in teaching procedural and basic diagnostic ultrasound skills to internal medicine residents. J Grad Med Educ. 2013;5(3):493-497. PubMed
80. Chalumeau-Lemoine L, Baudel JL, Das V, et al. Results of short-term training of naive physicians in focused general ultrasonography in an intensive-care unit. Intensive Care Med. 2009;35(10):1767-1771. PubMed
81. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. PubMed
82. Ramsingh D, Alexander B, Le K, Williams W, Canales C, Cannesson M. Comparison of the didactic lecture with the simulation/model approach for the teaching of a novel perioperative ultrasound curriculum to anesthesiology residents. J Clin Anesth. 2014;26(6):443-454. PubMed
83. Sekiguchi H, Bhagra A, Gajic O, Kashani KB. A general Critical Care Ultrasonography workshop: results of a novel Web-based learning program combined with simulation-based hands-on training. J Crit Care. 2013;28(2):217.e217-212. PubMed
84. Dulohery MM, Stoven S, Kurklinsky AK, Halvorsen A, McDonald FS, Bhagra A. Ultrasound for internal medicine physicians: the future of the physical examination. J Ultrasound Med. 2014;33(6):1005-1011. PubMed
85. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
86. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
87. Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
88. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
89. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
90. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
91. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol. 2012;71(4):245-251. PubMed
92. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
93. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
94. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
95. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
96. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
97. Harnsberger HR, Lee TG, Mukuno DH. Rapid, inexpensive real-time directed thoracentesis. Radiology. 1983;146(2):545-546. PubMed
Approximately 1.5 million people develop a pleural effusion in the United States annually, and approximately 173,000 people (12%) undergo thoracentesis.1 A recent review of thoracenteses performed at 234 University Health System Consortium hospitals between January 2010 and September 2013 demonstrated that 16% of 132,472 thoracenteses were performed by general internists and hospitalists, 33.1% were performed by interventional radiologists, and 20.3% were performed by pulmonologists.2 The iatrogenic pneumothorax rate was not significantly different between interventional radiologists and internists (2.8% and 2.9% risk, respectively); however, the admissions associated with bedside thoracentesis were less expensive than the admissions associated with thoracentesis performed in radiology suites, even after controlling for clinical covariates.2 In addition, the use of ultrasound guidance has been associated with a reduced risk of complications and cost of thoracentesis.3,4 In most of the early published studies on ultrasound-guided thoracentesis, the procedures were performed by radiologists.5-12 However, in 2010, the British Thoracic Society published guidelines on pleural procedures and thoracic ultrasound geared toward any trained provider.13 The purpose of this guideline is to review the literature and present evidence-based recommendations on the performance of ultrasound-guided thoracentesis at the bedside.
METHODS
Detailed methods are described in Appendix 1. The Society of Hospital Medicine (SHM) Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. The expert panel members were divided into working group members, external peer reviewers, and a methodologist. All the Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the four working group members themselves. Key clinical questions were prepared prior to conducting a systematic literature search by a medical librarian. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to September 2015 initially. Updated searches were conducted in November 2016 and in August 2017 (Appendix 3). All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of the screened articles were reviewed, and the articles focusing on the use of ultrasound to guide thoracentesis were selected. Articles that discussed thoracentesis without ultrasound guidance were excluded. In addition, the following article types were excluded: non-English language, nonhuman, subjects’ age <18 years, meeting abstracts, meeting posters, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided thoracentesis were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into draft recommendations.
We used the RAND Appropriateness Method that required panel judgment and consensus.14 The 30 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: 1) Problem priority and importance, 2) Level of quality of evidence, 3) Benefit/harm balance, 4) Benefit/burden balance, and 5) Certainty/concerns about PEAF (Preferences/Equity Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (Redcap™) in December 2016 and January 2017 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale, and the degree of consensus was assessed using the RAND algorithm. Establishing a recommendation required at least 70% agreement and a strong recommendation required 80% agreement according to the RAND rules (Appendix 1, Figure 1). Disagreement was defined as >30% of panelists voting outside of the zone of the median (appropriate, uncertain, inappropriate).
RESULTS
Literature search
A total of 1,556 references were pooled from the following four different sources: a search by a certified librarian in September 2015 (1066 citations) that was updated in November 2016 (165 citations) and again in August 2017 (9 citations), working group members’ literature searches (47 citations), and a search focused on training (269 citations). The final selection included 94 articles that were abstracted into a data table and incorporated into the draft recommendations. The details of the literature search strategy are given in Appendix 3.
Recommendations
Terminology
- Thoracentesis is a procedure of aspiration of fluid from the pleural space by percutaneous insertion of a needle through the chest wall with or without the insertion of a catheter.
- In this document, ultrasound guidance refers to static guidance and site marking performed at the bedside immediately before the procedure, as opposed to real-time (dynamic) ultrasound guidance or radiology performed site marking. The static method is the most commonly used method of ultrasound guidance and is supported by current evidence.
RECOMMENDATIONS
Clinical Outcomes
1.We recommend that ultrasound should be used to guide thoracentesis to reduce the risk of complications, the most common being pneumothorax.
Rationale: Both static ultrasound guidance and dynamic ultrasound guidance have been reported to be associated with a reduced risk of pneumothorax.4-7,15-18 A meta-analysis of 24 studies that included 6,605 thoracenteses showed a significant decrease in the risk of postprocedure pneumothorax with the use of ultrasound guidance compared to the risk associated with thoracentesis performed based on landmarks alone (OR 0.3, 95% CI 0.2–0.7).3 The meta-analysis included both prospective and retrospective studies conducted using both static and dynamic ultrasound guidance.3 A large retrospective cohort study conducted by Mercaldi et al. comprising more than 61,000 patients who underwent thoracentesis also showed that ultrasound guidance was associated with reduced odds of pneumothorax (OR 0.8 [0.7–0.9]).4 When pneumothorax did occur during that hospitalization, the cost of hospitalization increased by $2800 and the length of stay increased by 1.5 days.4 A 2008 review of 19,339 thoracenteses conducted by Patel et al. also demonstrated an association between ultrasound guidance and reduced odds of pneumothorax (OR 0.8 [0.7–0.96]).18 Although these findings were significant, it is important to note that the studies of both Mercaldi et al. and Patel et al. were reviews of administrative databases conducted using the International Classification of Diseases, 9th Revision (ICD-9) codes for thoracentesis and Current Procedure Terminology–4th edition (CPT) codes for the use of ultrasound.4,18 Patel et al. identified pneumothorax using ICD-9 codes for “pneumothorax–iatrogenic” and “pneumothorax–not specified as due to the procedure.” The association between ultrasound guidance and the reduced odds of pneumothorax was driven by the latter code.18 However, as with most retrospective studies using administrative data, granular data about the patients, procedure, proceduralists, and complications were not available in these reviews and conclusions may be limited by erroneous coding or documentation.4,18 In a third retrospective cohort study, Raptopoulos et al. compared 154 landmark-based thoracenteses performed by “clinical physicians” and 188 ultrasound-guided thoracenteses performed by radiologists and found that ultrasound-guided site selection reduced the rate of pneumothorax from 18% to 3% (P < .0001).6 Finally, one single-center randomized controlled trial of 160 thoracenteses performed by pulmonologists showed that ultrasound guidance reduced the relative risk of pneumothorax by 90% (12.5% vs 1.3%; P =.009) with a number needed to treat of 9.15 It was not possible to blind the operators to the use of ultrasound guidance, but the data analysis was blinded.15 Furthermore, while there was no explicit comparison of the intervention vs. the control groups, randomization would have presumably rendered both groups similar in terms of patient characteristics and effusion characteristics.15 Ultrasound may reduce the risk of pneumothorax through several mechanisms, including identifying patients in whom thoracentesis cannot be safely performed, allowing selection of the safest needle insertion site, and revealing the optimal depth of needle insertion.
2.We recommend that ultrasound guidance should be used to increase the success rate of thoracentesis.
Rationale: Thoracentesis guided by ultrasound has lower rates of failed attempts, or “dry taps,” compared to thoracentesis guided solely by physical examination. In 1977, Ravin described a method of using ultrasound to guide successful drainage of six complex pleural effusions (empyema or loculated effusion) after multiple (5–7) failed attempts by clinicians using physical examination alone.8 In a second study by radiologists, Weingardt et al. demonstrated that 20 of 26 failed landmark-based thoracenteses were due to incorrect site selection by physical examination–15 sites were below the diaphragm and 5 sites were above the pleural effusion or in the consolidated lung–and the use of ultrasound allowed successful sampling in 14 of 16 patients who had a failed landmark-based thoracentesis.9 Diacon et al. asked 30 physicians, ranging from junior housestaff to pulmonologists, to mark 172 potential thoracentesis sites in 67 patients with pleural effusions using physical examination alone. Ultrasound was then used to evaluate the proposed puncture sites. They found that using ultrasound would have avoided puncture on “dry chests” in 2% and avoided potential laceration of a solid organ in 10% of patients compared to site selection by physical examination alone.19 Finally, Perazzo et al. randomized 160 patients to landmark-based thoracentesis and ultrasound-guided thoracentesis and demonstrated that half of the eight dry taps that occurred in the control group could be successfully drained using subsequent ultrasound guidance.15
Technique
3. We recommend that ultrasound-guided thoracentesis should be performed or closely supervised by experienced operators.
Rationale: Current evidence suggests lower complication rates when thoracentesis is performed by experienced healthcare providers. A systematic review of 6,605 thoracenteses showed a significantly lower pneumothorax rate when thoracentesis was performed by pulmonology or radiology faculty versus resident physicians (3.9% vs 8.5%; P =.04), although this finding was not significant in the four studies that directly compared this factor.3 In a quality improvement study performed by Duncan et al., pulmonology and critical care physicians combining multiple quality improvement initiatives to achieve and maintain competency decreased the rate of pneumothorax from 8.6% to 1.1% (P =.0034).20 Interventions included ultrasound training, performance of 10 thoracenteses under expert supervision, and restriction of privileges to proceduralists who perform 10 or more thoracenteses per year.20 Finally, a series of 9,320 ultrasound-guided thoracenteses performed or supervised by a single expert internist over a period of 12 years resulted in a pneumothorax rate of 0.6% and a composite complication rate of 0.98% (pneumothorax, reexpansion pulmonary edema, hemothorax, site bleeding, hematoma, splenic laceration, and vasovagal reaction).21 Notably, pneumothorax rate in resident physician hands was reported to be 8.5% in the meta-analysis performed by Gordon et al., which is similar to the initial rate in the pulmonologists who participated in the study by Duncan et al.3,20 However, after instituting formal ultrasound training and other initiatives aimed at maintaining competency, the pneumothorax rate in the study by Duncan et al. decreased to 1.1%, similar to the rate observed in the series by Ault et al.21 This suggests that training and supervision are necessary to achieve competency and reduce the rate of complications.3,20,21
4. We suggest that ultrasound guidance be used to reduce the risk of complications from thoracentesis in mechanically ventilated patients.
Rationale: The rest of this guideline refers to ultrasound-guided thoracentesis performed in spontaneously breathing patients; however, this recommendation is specific to mechanically ventilated patients. Two prospective observational studies have shown no increase in complications when ultrasound-guided thoracentesis is performed on mechanically ventilated patients compared to patients not receiving positive pressure ventilation. A feasibility study of 45 thoracenteses performed on ventilated patients reported no complications,22 whereas another study on 232 patients reported a pneumothorax rate of 1.3%.23 In a larger study conducted by Mayo et al., medicine housestaff performed thoracentesis under the supervision of intensivists who had undergone training in ultrasound prior to performing the procedure.23 In both studies, most of the patients were in a supine position, although positioning and puncture site were at the discretion of the physician, and both studies employed use of static ultrasound guidance.22,23 A large series of 9,320 ultrasound-guided thoracenteses that included 1,377 mechanically ventilated patients did not report a higher rate of pneumothorax (0.8%) compared to that in spontaneously breathing patients (0.61%).21 Finally, a meta-analysis of 19 observational studies comprising 1,124 mechanically ventilated patients who underwent pleural drainage procedures showed a low rate of pneumothorax (3.4%) and hemothorax (1.9%).24 Although the rate of complication was reported to be low in this meta-analysis, ultrasound was not employed in all studies and its use was not associated with a significant reduction in pneumothorax.24 This may be because 8 of the 19 studies used pigtail catheters or large-bore thoracostomy tubes which treat pneumothorax as they occur.24
5. We recommend that ultrasound should be used to identify the chest wall, pleura, diaphragm, lung, and subdiaphragmatic organs throughout the respiratory cycle before selecting a needle insertion site.
Rationale: The use of ultrasound improves the selection of a safe needle insertion site because sites chosen without ultrasound guidance may be below the diaphragm, over solid organs,9,19 or in locations that risk puncture of the lung.9 Visualization of the chest wall, diaphragm, and lung, which define the boundaries of a pleural effusion, allows the clinician to confirm the presence of a drainable pleural effusion and assess for other pathologies, such as ascites and tumor, that may be mistaken for a pleural effusion.22,25,26 Hypoechoic lesions can represent small loculated pleural effusions but also pleural plaques, pleural masses, peripheral lung masses, or abscesses.27,28
6. We recommend that ultrasound should be used to detect the presence or absence of an effusion and approximate the volume of pleural fluid to guide clinical decision-making.
Rationale: The presence and approximate size of pleural fluid collections are important determinants of whether thoracentesis, another procedure, or no procedure should be performed. Ultrasonography has higher sensitivity and specificity for detecting pleural effusions and better differentiates effusions from consolidations compared with chest radiography.29-42 Ultrasound allows semiquantitative estimation of pleural fluid volume to determine whether thoracentesis should be performed.41-45 When using ultrasound to choose a site for thoracentesis, the British Thoracic Society Pleural Disease guidelines recommend ≥10 mm of pleural fluid between the visceral and parietal pleura.13 Pleural effusions of <10–15 mm are considered too small to tap.22,23 In a prospective study of 45 patients, a measurement of >9.9 cm by ultrasound between the chest wall and the “V-point,” the intersection of the diaphragm and the collapsed lung, correlated with a pleural fluid volume of >1 liter.46 Another prospective study of 73 patients showed that a pleural effusion spanning >3 intercostal spaces by ultrasound also correlated with a pleural fluid volume of >1 liter.47 Anticipating the volume of fluid to be removed may aid in preplanning and procurement of larger capacity drainage containers prior to starting the procedure. Lung ultrasound can also change the management if the characteristic of the effusion suggests that an invasive procedure is unsafe or another diagnostic or therapeutic option is more appropriate.39 In a prospective cohort study of 189 mechanically ventilated patients, lung ultrasound guided the management in all patients with suspected effusion, leading to chest tube placement in 7 patients and thoracentesis in 34 patients.48
7. We recommend that ultrasound should be used to detect complex sonographic features, such as septations, to guide clinical decision-making regarding the timing and method of pleural drainage.
Rationale: Pleural effusions can be broadly categorized sonographically as simple or complex. Complex effusions are further categorized as with or without septation. Simple effusions are anechoic and are often, but not invariably, transudative.49-51 The use of sonography and computerized tomography (CT) is complementary, but features of complex pleural effusions (fibrin stranding and septations) may be better visualized by ultrasound than by CT of the thorax.52 Detection of complex features should prompt the consideration of pleural fluid sampling.53,54 Exudative effusions from tuberculosis, malignancy, or other etiologies more often include debris, septations, or other complex features.55,56 Certain features such as a swirling debris, pleural thickening, and nodularity may be more often associated with malignancy,54,56 and advanced ultrasound techniques may be used to detect a trapped lung prior to attempting drainage of a malignant pleural effusion.57 Two studies found complex septated pleural effusions to be invariably exudative50,58 and drainage was unlikely to be successful without the placement of a chest tube.50,58-60 Chest tube placement through fibrinolytic administration or video-assisted thoracoscopic surgery (VATS) may be more appropriate in the management of complex septated pleural effusions,59-61 and expert consultation with a thoracic specialist is recommended in these cases.
8. We suggest that ultrasound can be used to measure the depth from the skin surface to the parietal pleura to help select an appropriate length needle and determine the maximum needle insertion depth.
Rationale: The distance from the skin to the parietal and visceral pleura can be measured by ultrasound to determine whether thoracentesis can be safely performed and to guide selection of an adequate length needle.38 The length of needle required to penetrate the pleural space varies based on the thickness of the chest wall. Percussion of the chest wall is limited when there is more than 6 cm of subcutaneous tissue,62 making physical examination in obese patients unreliable for selecting an appropriate site or needle length for thoracentesis. Ultrasound allows visualization of deep soft tissues, well beyond the limits of percussion, and allows an accurate measurement of the chest wall.63
9. We suggest that ultrasound can be used to evaluate normal lung sliding pre- and postprocedure to rule out pneumothorax.
Rationale: Normal lung sliding indicates normal apposition and movement of visceral and parietal pleura and rules out pneumothorax with a sensitivity that exceeds that of chest radiography, according to a meta-analysis of 20 studies using computed tomography or escape of intrapleural air at the time of drainage as the gold standard.64 In this meta-analysis, the pooled sensitivity of ultrasound was reported to be 88% (85-91%) compared to 52% (49-55%) for radiography, although the analysis also suggests that the test characteristics are dependent on operator skill.64 However, although lung sliding rules out pneumothorax, absence of lung sliding is not specific for pneumothorax and other conditions, including pleural adhesions, pleurodesis, and bronchial obstruction, can cause the absence of lung sliding.64 Detection of a lung point conclusively rules in a pneumothorax.65 Provided that the preprocedure lung ultrasound examination revealed normal lung sliding, a postprocedure examination can be performed to effectively evaluate for pneumothorax. This modality does not use ionizing radiation, is less expensive than computed tomography, can be performed faster than bedside chest radiography, and is more sensitive than supine or upright chest radiography.64,66-71
10. We suggest avoiding delay or interval change in patient position between the time of marking the needle insertion site and performing the thoracentesis.
Rationale: Optimal patient positioning and ultrasound-guided site marking should be performed by the primary operator immediately before beginning an invasive procedure. Remote sonographic localization in which a radiologist marks a needle insertion site using ultrasound and the thoracentesis is performed at a later time by a different provider is an antiquated practice. Two early studies demonstrated that this practice is no safer than landmark-based thoracentesis.6,72 One prospective study of 205 patients performed in 1986 showed no significant decrease in the incidence of complications from thoracentesis performed using remote sonographic localization versus landmark-based drainage.72 Complications in that study included a total of 22 pneumothoraces and 1 hematoma. The rate of complications in the group of patients who had site marking performed by radiology faculty and subsequent thoracentesis by medicine housestaff or attending physicians was 9.7% versus a complication rate of 12.7% in the landmark-based group.72 In addition, Raptopoulos et al. observed no significant difference in the pneumothorax rate between 106 patients with landmark-based thoracenteses and 48 patients who were sonographically marked by radiology faculty and then returned to the ward for completion of the thoracentesis by medicine housestaff (19% vs. 15%, respectively).6 Both groups had significantly higher rates of pneumothorax compared to those who underwent thoracentesis performed using real-time ultrasound guidance by radiology trainees (3%).6 The authors speculated that changing the patient’s position shifted the position of the pleural effusion, ultimately leading to the reliance on physical examination for the tap site.6
11. We recommend against performing routine postprocedure chest radiographs in patients who have undergone thoracentesis successfully with ultrasound guidance and are asymptomatic with normal lung sliding postprocedure.
Rationale: Chest radiography post-thoracentesis is unlikely to add information that changes management, especially if performed routinely, but does add expense, radiation, and inconvenience.73 The most common serious complication of thoracentesis is pneumothorax, which is often accompanied by symptoms, particularly in those patients with pneumothorax large enough to warrant chest tube placement.10,74,75 Pihlajamaa et al. retrospectively studied 264 ultrasound-guided thoracenteses performed by radiologists or radiology residents and noted that of 11 pneumothoraces, only 1 necessitated chest tube placement.10 Aleman et al. prospectively studied 506 ultrasound-guided and physical examination-guided thoracenteses and found that only 1% of asymptomatic patients developed a pneumothorax.74 Eight of the 18 symptomatic patients required chest tube placement as opposed to 1 of the 488 asymptomatic patients.74 A large prospective study of 941 ultrasound-guided thoracentesis reported that only 0.3% of asymptomatic patients with no suspicion of pneumothorax required tube thoracostomy.5 Postprocedure chest radiographs may be considered when thoracentesis is performed on mechanically ventilated patients, particularly when high airway pressures exist. In a study of 434 patients undergoing thoracentesis, only 10 patients had a pneumothorax (2.3%).11 Six of these pneumothoraces occurred in 92 mechanically ventilated patients (6.5%), and 2 of these 6 patients required a chest tube.11 None of the 4 spontaneously breathing patients with pneumothorax required a chest tube.11
Training
12. We recommend that novices who use ultrasound guidance for thoracentesis should receive focused training in lung and pleural ultrasonography and hands-on practice in procedural technique.
Rationale: Healthcare providers have to gain various skills to safely perform ultrasound-guided thoracentesis independently. Trainees should learn how to use ultrasound to identify important structures (chest wall, ribs, lung, pleura, diaphragm, and subdiaphragmatic organs); detect pleural effusions with complex features, such as septations; identify consolidated lung tissue; and rule out a pneumothorax. Prospective studies done with novice learners have shown that focused training combining didactics and hands-on practice using simulation or live models improves skills to assess pleural effusions.76-84 Several additional procedural techniques such as patient positioning and needle insertion are also important but are beyond the scope of these guidelines.
13. We suggest that novices undergo simulation-based training prior to performing ultrasound-guided thoracentesis on patients.
Rationale: Simulation-based training for thoracentesis has been studied in providers with different levels of medical training, ranging from medical students and internal medicine residents to practicing pulmonologists. Studies suggest that training in a zero-risk environment with simulation task trainers leads to increased knowledge and skills without subjecting the patients to inexperienced operators.85-87 One study on simulator-based training in medical students showed skill retention at 6 months and these skills were at least partially transferred to increased competency on live patients.88 Checklists to train providers in ultrasound-guided thoracentesis have been published.89,90 An experiential training program for attending physicians that utilized task trainers, along with standardized equipment and procedural technique, resulted in a reduction in the pneumothorax rate from 8.6% to 1.1%.20
14. Training curves for novices to become competent in lung ultrasound and ultrasound-guided thoracentesis are not completely understood. We recommend that training should be tailored to the skill acquisition of the learner and the resources of the institution.
Rationale: Understanding the rates at which novices progress from performing procedures under direct supervision to performing them independently would be highly desirable to ensure patient safety, guide supervision, and maximize efficiency of training. However, there is limited research describing the rate of progression of learners through these stages, either with regard to time or number of procedures performed. Two studies have shown that with brief training programs, medical students88 and internal medicine residents87 can achieve high levels of proficiency to perform thoracentesis on simulators, which is durable over time; however, whether these findings in a simulated environment translate into clinically significant outcomes is largely unknown, and neither of these studies incorporated the use of ultrasound guidance in their training curricula.87,88 Another study of pulmonary and critical care physicians combined multiple quality improvement initiatives with a half day of ultrasound-guided thoracentesis training, a requirement to perform 10 supervised thoracenteses prior to independent practice, and an additional requirement to perform 10 thoracenteses per year to maintain privileges.20 These interventions resulted in a concentration of competency among a few proceduralists, decreasing the rate of pneumothorax from 8.6% to 1.1%.20 Degradation of skills with disuse may also occur84; thus, procedures performed infrequently should at a minimum be subjected to increased supervision and/or retesting.
KNOWLEDGE GAPS
The process of developing these guidelines revealed important gaps in the literature regarding the use of ultrasound guidance for thoracentesis. First, it is uncertain whether the use of ultrasound reduces the risk of bleeding with thoracentesis. A retrospective cohort study of 19,339 thoracenteses suggests that ultrasound guidance is associated with a 38.7% relative reduction in the odds of hemorrhage, although this reduction did not reach statistical significance (OR 0.6 [0.4–1.04]).18 Ultrasound may reduce the risk of bleeding by reducing the number of attempts and needle passes and potentially avoiding tortuous intercostal vessels, which can be found especially in elderly patients and more cephalad rib spaces.91 In an observational study of 22 patients undergoing thoracentesis, the intercostal artery (ICA) was identified by a high-frequency ultrasound transducer in 74 of 88 intercostal spaces.92 The ICA is more exposed in the intercostal space within the first 6 cm lateral to the spinous processes and can be seen as far lateral as the midaxillary line.92-95 Thus, the ICA will most likely be avoided if a procedure site is selected >6 cm lateral to the spinous processes and the needle is inserted above the rib.
Second, although all three studies conducted using real-time (dynamic) ultrasound guidance reported a pneumothorax rate of <1%, it is uncertain whether real-time ultrasound guidance confers any additional benefit compared to static guidance for site marking as direct comparisons were not made.17,96,97 It is possible that real-time ultrasound guidance may be superior to static guidance in certain situations, such as small pleural effusions of <10–15 mm that have historically been considered too small to tap.13,22,23,96
Third, although one study suggests that general internists can safely perform thoracentesis with low complication rates similar to those of interventional radiologists,2 limited data exists on how to train practicing hospitalists to use ultrasound to guide thoracentesis. The effectiveness of different training protocols to acquire competence in ultrasound-guided thoracentesis has not been compared.
Finally, the impact of ultrasound use on patient experience has yet to be explored.
CONCLUSION
The use of ultrasound guidance for thoracentesis has been associated with increased success rates and decreased complication rates. Ultrasound can be used to estimate the pleural fluid volume, characterize the effusion as simple or complex, identify an optimal needle insertion site, and reduce the need for postprocedural chest radiographs. Training and experience are essential to reap the benefits of using ultrasound for thoracentesis, although our understanding of optimal educational strategies and learning curves is limited. Once training has occurred and competence is achieved, hospitalists can perform ultrasound-guided thoracentesis as safely as radiologists, pulmonologists, and other specialists.
Acknowledgments
Collaborators from the Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Benji Mathews, Paul, Mayo, Satyen Nichani, Vicki Noble, Martin Perez, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Gerard Salame, Kirk Spencer, Vivek Tayal, David M. Tierney.
Disclosures
Ricardo Franco-Sadud reports institutional funds received from the Society of Hospital Medicine Annual Meeting for travel expenses and accommodations outside the submitted work. Nitin Puri reports Payment for lectures including service on speakers bureaus from Fujifilm Sonosite and royalties from Elsevier, both outside the submitted work. All other authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1)
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
CHAIRS: Nilam Soni, Ricardo Franco Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen. Lumbar puncture Working Group: Nilam Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen. PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Dan Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
Approximately 1.5 million people develop a pleural effusion in the United States annually, and approximately 173,000 people (12%) undergo thoracentesis.1 A recent review of thoracenteses performed at 234 University Health System Consortium hospitals between January 2010 and September 2013 demonstrated that 16% of 132,472 thoracenteses were performed by general internists and hospitalists, 33.1% were performed by interventional radiologists, and 20.3% were performed by pulmonologists.2 The iatrogenic pneumothorax rate was not significantly different between interventional radiologists and internists (2.8% and 2.9% risk, respectively); however, the admissions associated with bedside thoracentesis were less expensive than the admissions associated with thoracentesis performed in radiology suites, even after controlling for clinical covariates.2 In addition, the use of ultrasound guidance has been associated with a reduced risk of complications and cost of thoracentesis.3,4 In most of the early published studies on ultrasound-guided thoracentesis, the procedures were performed by radiologists.5-12 However, in 2010, the British Thoracic Society published guidelines on pleural procedures and thoracic ultrasound geared toward any trained provider.13 The purpose of this guideline is to review the literature and present evidence-based recommendations on the performance of ultrasound-guided thoracentesis at the bedside.
METHODS
Detailed methods are described in Appendix 1. The Society of Hospital Medicine (SHM) Point-of-care Ultrasound (POCUS) Task Force was assembled to carry out this guideline development project under the direction of the SHM Board of Directors, Director of Education, and Education Committee. All expert panel members were physicians or advanced practice providers with expertise in POCUS. The expert panel members were divided into working group members, external peer reviewers, and a methodologist. All the Task Force members were required to disclose any potential conflicts of interests (Appendix 2). The literature search was conducted in two independent phases. The first phase included literature searches conducted by the four working group members themselves. Key clinical questions were prepared prior to conducting a systematic literature search by a medical librarian. The Medline, Embase, CINAHL, and Cochrane medical databases were searched from 1975 to September 2015 initially. Updated searches were conducted in November 2016 and in August 2017 (Appendix 3). All article abstracts were first screened for relevance by at least two members of the working group. Full-text versions of the screened articles were reviewed, and the articles focusing on the use of ultrasound to guide thoracentesis were selected. Articles that discussed thoracentesis without ultrasound guidance were excluded. In addition, the following article types were excluded: non-English language, nonhuman, subjects’ age <18 years, meeting abstracts, meeting posters, letters, and editorials. All relevant systematic reviews, meta-analyses, randomized controlled trials, and observational studies of ultrasound-guided thoracentesis were screened and selected. Final article selection was based on working group consensus, and the selected literature was incorporated into draft recommendations.
We used the RAND Appropriateness Method that required panel judgment and consensus.14 The 30 voting members of the SHM POCUS Task Force reviewed and voted on the draft recommendations considering the following five transforming factors: 1) Problem priority and importance, 2) Level of quality of evidence, 3) Benefit/harm balance, 4) Benefit/burden balance, and 5) Certainty/concerns about PEAF (Preferences/Equity Acceptability/Feasibility). Panel members participated in two rounds of electronic voting using an internet-based electronic data collection tool (Redcap™) in December 2016 and January 2017 (Appendix 4). Voting on appropriateness was conducted using a 9-point Likert scale, and the degree of consensus was assessed using the RAND algorithm. Establishing a recommendation required at least 70% agreement and a strong recommendation required 80% agreement according to the RAND rules (Appendix 1, Figure 1). Disagreement was defined as >30% of panelists voting outside of the zone of the median (appropriate, uncertain, inappropriate).
RESULTS
Literature search
A total of 1,556 references were pooled from the following four different sources: a search by a certified librarian in September 2015 (1066 citations) that was updated in November 2016 (165 citations) and again in August 2017 (9 citations), working group members’ literature searches (47 citations), and a search focused on training (269 citations). The final selection included 94 articles that were abstracted into a data table and incorporated into the draft recommendations. The details of the literature search strategy are given in Appendix 3.
Recommendations
Terminology
- Thoracentesis is a procedure of aspiration of fluid from the pleural space by percutaneous insertion of a needle through the chest wall with or without the insertion of a catheter.
- In this document, ultrasound guidance refers to static guidance and site marking performed at the bedside immediately before the procedure, as opposed to real-time (dynamic) ultrasound guidance or radiology performed site marking. The static method is the most commonly used method of ultrasound guidance and is supported by current evidence.
RECOMMENDATIONS
Clinical Outcomes
1.We recommend that ultrasound should be used to guide thoracentesis to reduce the risk of complications, the most common being pneumothorax.
Rationale: Both static ultrasound guidance and dynamic ultrasound guidance have been reported to be associated with a reduced risk of pneumothorax.4-7,15-18 A meta-analysis of 24 studies that included 6,605 thoracenteses showed a significant decrease in the risk of postprocedure pneumothorax with the use of ultrasound guidance compared to the risk associated with thoracentesis performed based on landmarks alone (OR 0.3, 95% CI 0.2–0.7).3 The meta-analysis included both prospective and retrospective studies conducted using both static and dynamic ultrasound guidance.3 A large retrospective cohort study conducted by Mercaldi et al. comprising more than 61,000 patients who underwent thoracentesis also showed that ultrasound guidance was associated with reduced odds of pneumothorax (OR 0.8 [0.7–0.9]).4 When pneumothorax did occur during that hospitalization, the cost of hospitalization increased by $2800 and the length of stay increased by 1.5 days.4 A 2008 review of 19,339 thoracenteses conducted by Patel et al. also demonstrated an association between ultrasound guidance and reduced odds of pneumothorax (OR 0.8 [0.7–0.96]).18 Although these findings were significant, it is important to note that the studies of both Mercaldi et al. and Patel et al. were reviews of administrative databases conducted using the International Classification of Diseases, 9th Revision (ICD-9) codes for thoracentesis and Current Procedure Terminology–4th edition (CPT) codes for the use of ultrasound.4,18 Patel et al. identified pneumothorax using ICD-9 codes for “pneumothorax–iatrogenic” and “pneumothorax–not specified as due to the procedure.” The association between ultrasound guidance and the reduced odds of pneumothorax was driven by the latter code.18 However, as with most retrospective studies using administrative data, granular data about the patients, procedure, proceduralists, and complications were not available in these reviews and conclusions may be limited by erroneous coding or documentation.4,18 In a third retrospective cohort study, Raptopoulos et al. compared 154 landmark-based thoracenteses performed by “clinical physicians” and 188 ultrasound-guided thoracenteses performed by radiologists and found that ultrasound-guided site selection reduced the rate of pneumothorax from 18% to 3% (P < .0001).6 Finally, one single-center randomized controlled trial of 160 thoracenteses performed by pulmonologists showed that ultrasound guidance reduced the relative risk of pneumothorax by 90% (12.5% vs 1.3%; P =.009) with a number needed to treat of 9.15 It was not possible to blind the operators to the use of ultrasound guidance, but the data analysis was blinded.15 Furthermore, while there was no explicit comparison of the intervention vs. the control groups, randomization would have presumably rendered both groups similar in terms of patient characteristics and effusion characteristics.15 Ultrasound may reduce the risk of pneumothorax through several mechanisms, including identifying patients in whom thoracentesis cannot be safely performed, allowing selection of the safest needle insertion site, and revealing the optimal depth of needle insertion.
2.We recommend that ultrasound guidance should be used to increase the success rate of thoracentesis.
Rationale: Thoracentesis guided by ultrasound has lower rates of failed attempts, or “dry taps,” compared to thoracentesis guided solely by physical examination. In 1977, Ravin described a method of using ultrasound to guide successful drainage of six complex pleural effusions (empyema or loculated effusion) after multiple (5–7) failed attempts by clinicians using physical examination alone.8 In a second study by radiologists, Weingardt et al. demonstrated that 20 of 26 failed landmark-based thoracenteses were due to incorrect site selection by physical examination–15 sites were below the diaphragm and 5 sites were above the pleural effusion or in the consolidated lung–and the use of ultrasound allowed successful sampling in 14 of 16 patients who had a failed landmark-based thoracentesis.9 Diacon et al. asked 30 physicians, ranging from junior housestaff to pulmonologists, to mark 172 potential thoracentesis sites in 67 patients with pleural effusions using physical examination alone. Ultrasound was then used to evaluate the proposed puncture sites. They found that using ultrasound would have avoided puncture on “dry chests” in 2% and avoided potential laceration of a solid organ in 10% of patients compared to site selection by physical examination alone.19 Finally, Perazzo et al. randomized 160 patients to landmark-based thoracentesis and ultrasound-guided thoracentesis and demonstrated that half of the eight dry taps that occurred in the control group could be successfully drained using subsequent ultrasound guidance.15
Technique
3. We recommend that ultrasound-guided thoracentesis should be performed or closely supervised by experienced operators.
Rationale: Current evidence suggests lower complication rates when thoracentesis is performed by experienced healthcare providers. A systematic review of 6,605 thoracenteses showed a significantly lower pneumothorax rate when thoracentesis was performed by pulmonology or radiology faculty versus resident physicians (3.9% vs 8.5%; P =.04), although this finding was not significant in the four studies that directly compared this factor.3 In a quality improvement study performed by Duncan et al., pulmonology and critical care physicians combining multiple quality improvement initiatives to achieve and maintain competency decreased the rate of pneumothorax from 8.6% to 1.1% (P =.0034).20 Interventions included ultrasound training, performance of 10 thoracenteses under expert supervision, and restriction of privileges to proceduralists who perform 10 or more thoracenteses per year.20 Finally, a series of 9,320 ultrasound-guided thoracenteses performed or supervised by a single expert internist over a period of 12 years resulted in a pneumothorax rate of 0.6% and a composite complication rate of 0.98% (pneumothorax, reexpansion pulmonary edema, hemothorax, site bleeding, hematoma, splenic laceration, and vasovagal reaction).21 Notably, pneumothorax rate in resident physician hands was reported to be 8.5% in the meta-analysis performed by Gordon et al., which is similar to the initial rate in the pulmonologists who participated in the study by Duncan et al.3,20 However, after instituting formal ultrasound training and other initiatives aimed at maintaining competency, the pneumothorax rate in the study by Duncan et al. decreased to 1.1%, similar to the rate observed in the series by Ault et al.21 This suggests that training and supervision are necessary to achieve competency and reduce the rate of complications.3,20,21
4. We suggest that ultrasound guidance be used to reduce the risk of complications from thoracentesis in mechanically ventilated patients.
Rationale: The rest of this guideline refers to ultrasound-guided thoracentesis performed in spontaneously breathing patients; however, this recommendation is specific to mechanically ventilated patients. Two prospective observational studies have shown no increase in complications when ultrasound-guided thoracentesis is performed on mechanically ventilated patients compared to patients not receiving positive pressure ventilation. A feasibility study of 45 thoracenteses performed on ventilated patients reported no complications,22 whereas another study on 232 patients reported a pneumothorax rate of 1.3%.23 In a larger study conducted by Mayo et al., medicine housestaff performed thoracentesis under the supervision of intensivists who had undergone training in ultrasound prior to performing the procedure.23 In both studies, most of the patients were in a supine position, although positioning and puncture site were at the discretion of the physician, and both studies employed use of static ultrasound guidance.22,23 A large series of 9,320 ultrasound-guided thoracenteses that included 1,377 mechanically ventilated patients did not report a higher rate of pneumothorax (0.8%) compared to that in spontaneously breathing patients (0.61%).21 Finally, a meta-analysis of 19 observational studies comprising 1,124 mechanically ventilated patients who underwent pleural drainage procedures showed a low rate of pneumothorax (3.4%) and hemothorax (1.9%).24 Although the rate of complication was reported to be low in this meta-analysis, ultrasound was not employed in all studies and its use was not associated with a significant reduction in pneumothorax.24 This may be because 8 of the 19 studies used pigtail catheters or large-bore thoracostomy tubes which treat pneumothorax as they occur.24
5. We recommend that ultrasound should be used to identify the chest wall, pleura, diaphragm, lung, and subdiaphragmatic organs throughout the respiratory cycle before selecting a needle insertion site.
Rationale: The use of ultrasound improves the selection of a safe needle insertion site because sites chosen without ultrasound guidance may be below the diaphragm, over solid organs,9,19 or in locations that risk puncture of the lung.9 Visualization of the chest wall, diaphragm, and lung, which define the boundaries of a pleural effusion, allows the clinician to confirm the presence of a drainable pleural effusion and assess for other pathologies, such as ascites and tumor, that may be mistaken for a pleural effusion.22,25,26 Hypoechoic lesions can represent small loculated pleural effusions but also pleural plaques, pleural masses, peripheral lung masses, or abscesses.27,28
6. We recommend that ultrasound should be used to detect the presence or absence of an effusion and approximate the volume of pleural fluid to guide clinical decision-making.
Rationale: The presence and approximate size of pleural fluid collections are important determinants of whether thoracentesis, another procedure, or no procedure should be performed. Ultrasonography has higher sensitivity and specificity for detecting pleural effusions and better differentiates effusions from consolidations compared with chest radiography.29-42 Ultrasound allows semiquantitative estimation of pleural fluid volume to determine whether thoracentesis should be performed.41-45 When using ultrasound to choose a site for thoracentesis, the British Thoracic Society Pleural Disease guidelines recommend ≥10 mm of pleural fluid between the visceral and parietal pleura.13 Pleural effusions of <10–15 mm are considered too small to tap.22,23 In a prospective study of 45 patients, a measurement of >9.9 cm by ultrasound between the chest wall and the “V-point,” the intersection of the diaphragm and the collapsed lung, correlated with a pleural fluid volume of >1 liter.46 Another prospective study of 73 patients showed that a pleural effusion spanning >3 intercostal spaces by ultrasound also correlated with a pleural fluid volume of >1 liter.47 Anticipating the volume of fluid to be removed may aid in preplanning and procurement of larger capacity drainage containers prior to starting the procedure. Lung ultrasound can also change the management if the characteristic of the effusion suggests that an invasive procedure is unsafe or another diagnostic or therapeutic option is more appropriate.39 In a prospective cohort study of 189 mechanically ventilated patients, lung ultrasound guided the management in all patients with suspected effusion, leading to chest tube placement in 7 patients and thoracentesis in 34 patients.48
7. We recommend that ultrasound should be used to detect complex sonographic features, such as septations, to guide clinical decision-making regarding the timing and method of pleural drainage.
Rationale: Pleural effusions can be broadly categorized sonographically as simple or complex. Complex effusions are further categorized as with or without septation. Simple effusions are anechoic and are often, but not invariably, transudative.49-51 The use of sonography and computerized tomography (CT) is complementary, but features of complex pleural effusions (fibrin stranding and septations) may be better visualized by ultrasound than by CT of the thorax.52 Detection of complex features should prompt the consideration of pleural fluid sampling.53,54 Exudative effusions from tuberculosis, malignancy, or other etiologies more often include debris, septations, or other complex features.55,56 Certain features such as a swirling debris, pleural thickening, and nodularity may be more often associated with malignancy,54,56 and advanced ultrasound techniques may be used to detect a trapped lung prior to attempting drainage of a malignant pleural effusion.57 Two studies found complex septated pleural effusions to be invariably exudative50,58 and drainage was unlikely to be successful without the placement of a chest tube.50,58-60 Chest tube placement through fibrinolytic administration or video-assisted thoracoscopic surgery (VATS) may be more appropriate in the management of complex septated pleural effusions,59-61 and expert consultation with a thoracic specialist is recommended in these cases.
8. We suggest that ultrasound can be used to measure the depth from the skin surface to the parietal pleura to help select an appropriate length needle and determine the maximum needle insertion depth.
Rationale: The distance from the skin to the parietal and visceral pleura can be measured by ultrasound to determine whether thoracentesis can be safely performed and to guide selection of an adequate length needle.38 The length of needle required to penetrate the pleural space varies based on the thickness of the chest wall. Percussion of the chest wall is limited when there is more than 6 cm of subcutaneous tissue,62 making physical examination in obese patients unreliable for selecting an appropriate site or needle length for thoracentesis. Ultrasound allows visualization of deep soft tissues, well beyond the limits of percussion, and allows an accurate measurement of the chest wall.63
9. We suggest that ultrasound can be used to evaluate normal lung sliding pre- and postprocedure to rule out pneumothorax.
Rationale: Normal lung sliding indicates normal apposition and movement of visceral and parietal pleura and rules out pneumothorax with a sensitivity that exceeds that of chest radiography, according to a meta-analysis of 20 studies using computed tomography or escape of intrapleural air at the time of drainage as the gold standard.64 In this meta-analysis, the pooled sensitivity of ultrasound was reported to be 88% (85-91%) compared to 52% (49-55%) for radiography, although the analysis also suggests that the test characteristics are dependent on operator skill.64 However, although lung sliding rules out pneumothorax, absence of lung sliding is not specific for pneumothorax and other conditions, including pleural adhesions, pleurodesis, and bronchial obstruction, can cause the absence of lung sliding.64 Detection of a lung point conclusively rules in a pneumothorax.65 Provided that the preprocedure lung ultrasound examination revealed normal lung sliding, a postprocedure examination can be performed to effectively evaluate for pneumothorax. This modality does not use ionizing radiation, is less expensive than computed tomography, can be performed faster than bedside chest radiography, and is more sensitive than supine or upright chest radiography.64,66-71
10. We suggest avoiding delay or interval change in patient position between the time of marking the needle insertion site and performing the thoracentesis.
Rationale: Optimal patient positioning and ultrasound-guided site marking should be performed by the primary operator immediately before beginning an invasive procedure. Remote sonographic localization in which a radiologist marks a needle insertion site using ultrasound and the thoracentesis is performed at a later time by a different provider is an antiquated practice. Two early studies demonstrated that this practice is no safer than landmark-based thoracentesis.6,72 One prospective study of 205 patients performed in 1986 showed no significant decrease in the incidence of complications from thoracentesis performed using remote sonographic localization versus landmark-based drainage.72 Complications in that study included a total of 22 pneumothoraces and 1 hematoma. The rate of complications in the group of patients who had site marking performed by radiology faculty and subsequent thoracentesis by medicine housestaff or attending physicians was 9.7% versus a complication rate of 12.7% in the landmark-based group.72 In addition, Raptopoulos et al. observed no significant difference in the pneumothorax rate between 106 patients with landmark-based thoracenteses and 48 patients who were sonographically marked by radiology faculty and then returned to the ward for completion of the thoracentesis by medicine housestaff (19% vs. 15%, respectively).6 Both groups had significantly higher rates of pneumothorax compared to those who underwent thoracentesis performed using real-time ultrasound guidance by radiology trainees (3%).6 The authors speculated that changing the patient’s position shifted the position of the pleural effusion, ultimately leading to the reliance on physical examination for the tap site.6
11. We recommend against performing routine postprocedure chest radiographs in patients who have undergone thoracentesis successfully with ultrasound guidance and are asymptomatic with normal lung sliding postprocedure.
Rationale: Chest radiography post-thoracentesis is unlikely to add information that changes management, especially if performed routinely, but does add expense, radiation, and inconvenience.73 The most common serious complication of thoracentesis is pneumothorax, which is often accompanied by symptoms, particularly in those patients with pneumothorax large enough to warrant chest tube placement.10,74,75 Pihlajamaa et al. retrospectively studied 264 ultrasound-guided thoracenteses performed by radiologists or radiology residents and noted that of 11 pneumothoraces, only 1 necessitated chest tube placement.10 Aleman et al. prospectively studied 506 ultrasound-guided and physical examination-guided thoracenteses and found that only 1% of asymptomatic patients developed a pneumothorax.74 Eight of the 18 symptomatic patients required chest tube placement as opposed to 1 of the 488 asymptomatic patients.74 A large prospective study of 941 ultrasound-guided thoracentesis reported that only 0.3% of asymptomatic patients with no suspicion of pneumothorax required tube thoracostomy.5 Postprocedure chest radiographs may be considered when thoracentesis is performed on mechanically ventilated patients, particularly when high airway pressures exist. In a study of 434 patients undergoing thoracentesis, only 10 patients had a pneumothorax (2.3%).11 Six of these pneumothoraces occurred in 92 mechanically ventilated patients (6.5%), and 2 of these 6 patients required a chest tube.11 None of the 4 spontaneously breathing patients with pneumothorax required a chest tube.11
Training
12. We recommend that novices who use ultrasound guidance for thoracentesis should receive focused training in lung and pleural ultrasonography and hands-on practice in procedural technique.
Rationale: Healthcare providers have to gain various skills to safely perform ultrasound-guided thoracentesis independently. Trainees should learn how to use ultrasound to identify important structures (chest wall, ribs, lung, pleura, diaphragm, and subdiaphragmatic organs); detect pleural effusions with complex features, such as septations; identify consolidated lung tissue; and rule out a pneumothorax. Prospective studies done with novice learners have shown that focused training combining didactics and hands-on practice using simulation or live models improves skills to assess pleural effusions.76-84 Several additional procedural techniques such as patient positioning and needle insertion are also important but are beyond the scope of these guidelines.
13. We suggest that novices undergo simulation-based training prior to performing ultrasound-guided thoracentesis on patients.
Rationale: Simulation-based training for thoracentesis has been studied in providers with different levels of medical training, ranging from medical students and internal medicine residents to practicing pulmonologists. Studies suggest that training in a zero-risk environment with simulation task trainers leads to increased knowledge and skills without subjecting the patients to inexperienced operators.85-87 One study on simulator-based training in medical students showed skill retention at 6 months and these skills were at least partially transferred to increased competency on live patients.88 Checklists to train providers in ultrasound-guided thoracentesis have been published.89,90 An experiential training program for attending physicians that utilized task trainers, along with standardized equipment and procedural technique, resulted in a reduction in the pneumothorax rate from 8.6% to 1.1%.20
14. Training curves for novices to become competent in lung ultrasound and ultrasound-guided thoracentesis are not completely understood. We recommend that training should be tailored to the skill acquisition of the learner and the resources of the institution.
Rationale: Understanding the rates at which novices progress from performing procedures under direct supervision to performing them independently would be highly desirable to ensure patient safety, guide supervision, and maximize efficiency of training. However, there is limited research describing the rate of progression of learners through these stages, either with regard to time or number of procedures performed. Two studies have shown that with brief training programs, medical students88 and internal medicine residents87 can achieve high levels of proficiency to perform thoracentesis on simulators, which is durable over time; however, whether these findings in a simulated environment translate into clinically significant outcomes is largely unknown, and neither of these studies incorporated the use of ultrasound guidance in their training curricula.87,88 Another study of pulmonary and critical care physicians combined multiple quality improvement initiatives with a half day of ultrasound-guided thoracentesis training, a requirement to perform 10 supervised thoracenteses prior to independent practice, and an additional requirement to perform 10 thoracenteses per year to maintain privileges.20 These interventions resulted in a concentration of competency among a few proceduralists, decreasing the rate of pneumothorax from 8.6% to 1.1%.20 Degradation of skills with disuse may also occur84; thus, procedures performed infrequently should at a minimum be subjected to increased supervision and/or retesting.
KNOWLEDGE GAPS
The process of developing these guidelines revealed important gaps in the literature regarding the use of ultrasound guidance for thoracentesis. First, it is uncertain whether the use of ultrasound reduces the risk of bleeding with thoracentesis. A retrospective cohort study of 19,339 thoracenteses suggests that ultrasound guidance is associated with a 38.7% relative reduction in the odds of hemorrhage, although this reduction did not reach statistical significance (OR 0.6 [0.4–1.04]).18 Ultrasound may reduce the risk of bleeding by reducing the number of attempts and needle passes and potentially avoiding tortuous intercostal vessels, which can be found especially in elderly patients and more cephalad rib spaces.91 In an observational study of 22 patients undergoing thoracentesis, the intercostal artery (ICA) was identified by a high-frequency ultrasound transducer in 74 of 88 intercostal spaces.92 The ICA is more exposed in the intercostal space within the first 6 cm lateral to the spinous processes and can be seen as far lateral as the midaxillary line.92-95 Thus, the ICA will most likely be avoided if a procedure site is selected >6 cm lateral to the spinous processes and the needle is inserted above the rib.
Second, although all three studies conducted using real-time (dynamic) ultrasound guidance reported a pneumothorax rate of <1%, it is uncertain whether real-time ultrasound guidance confers any additional benefit compared to static guidance for site marking as direct comparisons were not made.17,96,97 It is possible that real-time ultrasound guidance may be superior to static guidance in certain situations, such as small pleural effusions of <10–15 mm that have historically been considered too small to tap.13,22,23,96
Third, although one study suggests that general internists can safely perform thoracentesis with low complication rates similar to those of interventional radiologists,2 limited data exists on how to train practicing hospitalists to use ultrasound to guide thoracentesis. The effectiveness of different training protocols to acquire competence in ultrasound-guided thoracentesis has not been compared.
Finally, the impact of ultrasound use on patient experience has yet to be explored.
CONCLUSION
The use of ultrasound guidance for thoracentesis has been associated with increased success rates and decreased complication rates. Ultrasound can be used to estimate the pleural fluid volume, characterize the effusion as simple or complex, identify an optimal needle insertion site, and reduce the need for postprocedural chest radiographs. Training and experience are essential to reap the benefits of using ultrasound for thoracentesis, although our understanding of optimal educational strategies and learning curves is limited. Once training has occurred and competence is achieved, hospitalists can perform ultrasound-guided thoracentesis as safely as radiologists, pulmonologists, and other specialists.
Acknowledgments
Collaborators from the Society of Hospital Medicine Point-of-care Ultrasound Task Force: Saaid Abdel-Ghani, Robert Arntfield, Jeffrey Bates, Anjali Bhagra, Michael Blaivas, Daniel Brotman, Carolina Candotti, Richard Hoppmann, Susan Hunt, Trevor P. Jensen, Venkat Kalidindi, Ketino Kobaidze, Joshua Lenchus, Benji Mathews, Paul, Mayo, Satyen Nichani, Vicki Noble, Martin Perez, Aliaksei Pustavoitau, Kreegan Reierson, Sophia Rodgers, Gerard Salame, Kirk Spencer, Vivek Tayal, David M. Tierney.
Disclosures
Ricardo Franco-Sadud reports institutional funds received from the Society of Hospital Medicine Annual Meeting for travel expenses and accommodations outside the submitted work. Nitin Puri reports Payment for lectures including service on speakers bureaus from Fujifilm Sonosite and royalties from Elsevier, both outside the submitted work. All other authors have nothing to disclose.
Funding
Brian P Lucas: Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). Nilam Soni: Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1)
Disclaimer
The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors thank all the members of the Society of Hospital Medicine Point-of-care Ultrasound Task Force and the Education Committee members for their time and dedication to develop these guidelines.
CHAIRS: Nilam Soni, Ricardo Franco Sadud, Jeff Bates. WORKING GROUPS: Thoracentesis Working Group: Ria Dancel (chair), Daniel Schnobrich, Nitin Puri. Vascular Access Working Group: Ricardo Franco (chair), Benji Matthews, Saaid Abdel-Ghani, Sophia Rodgers, Martin Perez, Daniel Schnobrich. Paracentesis Working Group: Joel Cho (chair), Benji Matthews, Kreegan Reierson, Anjali Bhagra, Trevor P. Jensen. Lumbar puncture Working Group: Nilam Soni (chair), Ricardo Franco, Gerard Salame, Josh Lenchus, Venkat Kalidindi, Ketino Kobaidze. Credentialing Working Group: Brian P Lucas (chair), David Tierney, Trevor P. Jensen. PEER REVIEWERS: Robert Arntfield, Michael Blaivas, Richard Hoppmann, Paul Mayo, Vicki Noble, Aliaksei Pustavoitau, Kirk Spencer, Vivek Tayal. METHODOLOGIST: Mahmoud El Barbary. LIBRARIAN: Loretta Grikis. SOCIETY OF HOSPITAL MEDICINE EDUCATION COMMITTEE: Dan Brotman (past chair), Satyen Nichani (current chair), Susan Hunt. SOCIETY OF HOSPITAL MEDICINE STAFF: Nick Marzano.
1. Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13. 1998;139:1-119. PubMed
2. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. PubMed
3. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
5. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
6. Raptopoulos V, Davis LM, Lee G, Umali C, Lew R, Irwin RS. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917-920. PubMed
7. Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150(4):873-877. PubMed
8. Ravin CE. Thoracocentesis of loculated pleural effusions using grey scale ultrasonic guidance. Chest. 1977;71(5):666-668. PubMed
9. Weingardt JP, Guico RR, Nemcek AA, Jr., Li YP, Chiu ST. Ultrasound findings following failed, clinically directed thoracenteses. J Clin Ultrasound. 1994;22(7):419-426. PubMed
10. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
11. Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology. 1997;204(2):503-506. PubMed
12. Boland GW, Gazelle GS, Girard MJ, Mueller PR. Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR Am J Roentgenol. 1998;170(4):943-946. PubMed
13. Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(2):ii61-76. PubMed
14. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The RAND/UCLA appropriateness method user’s manual. DTIC Document; 2001.
15. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
16. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
17. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
18. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
19. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
20. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
21. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
22. Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Meziere G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25(9):955-958. PubMed
23. Mayo PH, Goltz HR, Tafreshi M, Doelken P. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125(3):1059-1062. PubMed
24. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
25. Landay M, Harless W. Ultrasonic differentiation of right pleural effusion from subphrenic fluid on longitudinal scans of the right upper quadrant: importance of recognizing the diaphragm. Radiology. 1977;123(1):155-158. PubMed
26. Mayo PH, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006;27(2):215-227. PubMed
27. Rosenberg ER. Ultrasound in the assessment of pleural densities. Chest. 1983;84(3):283-285. PubMed
28. Gorg C, Restrepo I, Schwerk WB. Sonography of malignant pleural effusion. Eur Radiol. 1997;7(8):1195-1198. PubMed
29. Gryminski J, Krakowka P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33-37. PubMed
30. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12-16. PubMed
31. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15. PubMed
32. Grimberg A, Shigueoka DC, Atallah AN, Ajzen S, Iared W. Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J.. 2010;128(2):90-95. PubMed
33. Kataoka H. Utility of thoracic sonography for follow-up examination of chronic heart failure patients with previous decompensation. Clin Cardiol. 2007;30(7):336-341. PubMed
34. Ma OJ, Mateer JR. Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312-315. PubMed
35. Rocco M, Carbone I, Morelli A, et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776-784. PubMed
36. Kocijancic I, Vidmar K, Ivanovi-Herceg Z. Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69-74. PubMed
37. Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1. PubMed
38. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
39. Medford AR, Entwisle JJ. Indications for thoracic ultrasound in chest medicine: an observational study. Postgrad Med J. 2010;86(1011):8-11. PubMed
40. Lin MS, Hwang JJ, Chong IW, et al. Ultrasonography of chest diseases: analysis of 154 cases. Gaoxiong Yi Xue Ke Xue Za Zhi . 1992;8(10):525-534. PubMed
41. Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hormann MF, Grabenwoger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191(3):681-684. PubMed
42. Vignon P, Chastagner C, Berkane V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33(8):1757-1763. PubMed
43. Usta E, Mustafi M, Ziemer G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204-207. PubMed
44. Remerand F, Dellamonica J, Mao Z, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656-664.
PubMed
45. Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318-321. PubMed
46. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracentesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
47. Lisi M, Cameli M, Mondillo S, et al. Incremental value of pocket-sized imaging device for bedside diagnosis of unilateral pleural effusions and ultrasound-guided thoracentesis. Interact Cardiovasc Thorac Surg. 2012;15(4):596-601. PubMed
48. Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57-65. PubMed
49. Chen HJ, Tu CY, Ling SJ, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol. 2008;34(3):362-369. PubMed
50. Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29-33. PubMed
51. Liang SJ, Tu CY, Chen HJ, et al. Application of ultrasound-guided pigtail catheter for drainage of pleural effusions in the ICU. Intensive Care Med. 2009;35(2):350-354. PubMed
52. McLoud TC, Flower CD. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145-1153. PubMed
53. Tu CY, Hsu WH, Hsia TC, et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274-1280. PubMed
54. Sajadieh H, Afzali F, Sajadieh V, Sajadieh A. Ultrasound as an alternative to aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci. 2004;1019:585-592. PubMed
55. Marcun R, Sustic A. Sonographic evaluation of unexplained pleural exudate: a prospective case series. Wien Klin Wochenschr. 2009;121(9-10):334-338. PubMed
56. Bugalho A, Ferreira D, Dias SS, et al. The diagnostic value of transthoracic ultrasonographic features in predicting malignancy in undiagnosed pleural effusions: a prospective observational study. Respiration. 2014;87(4):270-278. PubMed
57. Salamonsen MR, Lo AK, Ng AC, Bashirzadeh F, Wang WY, Fielding DI. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest. 2014;146(5):1286-1293. PubMed
58. Himelman RB, Callen PW. The prognostic value of loculations in parapneumonic pleural effusions. Chest. 1986;90(6):852-856. PubMed
59. Chen CH, Chen W, Chen HJ, et al. Transthoracic ultrasonography in predicting the outcome of small-bore catheter drainage in empyemas or complicated parapneumonic effusions. Ultrasound Med Biol. 2009;35(9):1468-1474. PubMed
60. Hirsch JH, Rogers JV, Mack LA. Real-time sonography of pleural opacities. AJR Am J Roentgenol. 1981;136(2):297-301. PubMed
61. Chen KY, Liaw YS, Wang HC, Luh KT, Yang PC. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837-843. PubMed
62. Diaz-Guzman E, Budev MM. Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297-303. PubMed
63. Rhyne T, Birnholz JC. Simple measurement of chest-wall thickness with ultrasound. Radiology. 1973;108(2):436-438. PubMed
64. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140(4):859-866. PubMed
65. Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434-1440. PubMed
66. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
67. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. PubMed
68. Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141(3):703-708. PubMed
69. Sartori S, Tombesi P, Trevisani L, Nielsen I, Tassinari D, Abbasciano V. Accuracy of transthoracic sonography in detection of pneumothorax after sonographically guided lung biopsy: prospective comparison with chest radiography. AJR Am J Roentgenol. 2007;188(1):37-41. PubMed
70. Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12(9):844-849. PubMed
71. Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33(6):1231-1238. PubMed
72. Kohan JM, Poe RH, Israel RH, et al. Value of chest ultrasonography versus decubitus roentgenography for thoracentesis. Am Rev Respir Dis. 1986;133(6):1124-1126. PubMed
73. Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc. 1998;73(10):948-950. PubMed
74. Aleman C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107(4):340-343. PubMed
75. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
76. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40(10):1475-1480. PubMed
77. Kotagal M, Quiroga E, Ruffatto BJ, et al. Impact of point-of-care ultrasound training on surgical residents’ confidence. J Surg Educ. 2015;72(4):e82-87. PubMed
78. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. PubMed
79. Schnobrich DJ, Olson AP, Broccard A, Duran-Nelson A. Feasibility and acceptability of a structured curriculum in teaching procedural and basic diagnostic ultrasound skills to internal medicine residents. J Grad Med Educ. 2013;5(3):493-497. PubMed
80. Chalumeau-Lemoine L, Baudel JL, Das V, et al. Results of short-term training of naive physicians in focused general ultrasonography in an intensive-care unit. Intensive Care Med. 2009;35(10):1767-1771. PubMed
81. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. PubMed
82. Ramsingh D, Alexander B, Le K, Williams W, Canales C, Cannesson M. Comparison of the didactic lecture with the simulation/model approach for the teaching of a novel perioperative ultrasound curriculum to anesthesiology residents. J Clin Anesth. 2014;26(6):443-454. PubMed
83. Sekiguchi H, Bhagra A, Gajic O, Kashani KB. A general Critical Care Ultrasonography workshop: results of a novel Web-based learning program combined with simulation-based hands-on training. J Crit Care. 2013;28(2):217.e217-212. PubMed
84. Dulohery MM, Stoven S, Kurklinsky AK, Halvorsen A, McDonald FS, Bhagra A. Ultrasound for internal medicine physicians: the future of the physical examination. J Ultrasound Med. 2014;33(6):1005-1011. PubMed
85. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
86. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
87. Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
88. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
89. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
90. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
91. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol. 2012;71(4):245-251. PubMed
92. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
93. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
94. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
95. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
96. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
97. Harnsberger HR, Lee TG, Mukuno DH. Rapid, inexpensive real-time directed thoracentesis. Radiology. 1983;146(2):545-546. PubMed
1. Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat 13. 1998;139:1-119. PubMed
2. Kozmic SE, Wayne DB, Feinglass J, Hohmann SF, Barsuk JH. Factors associated with inpatient thoracentesis procedure quality at university hospitals. Jt Comm J Qual Patient Saf. 2016;42(1):34-40. PubMed
3. Gordon CE, Feller-Kopman D, Balk EM, Smetana GW. Pneumothorax following thoracentesis: a systematic review and meta-analysis. Arch Intern Med. 2010;170(4):332-339. PubMed
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532-538. PubMed
5. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YC, Light RW. Ultrasound-guided thoracentesis: is it a safer method? Chest. 2003;123(2):418-423. PubMed
6. Raptopoulos V, Davis LM, Lee G, Umali C, Lew R, Irwin RS. Factors affecting the development of pneumothorax associated with thoracentesis. AJR Am J Roentgenol. 1991;156(5):917-920. PubMed
7. Grogan DR, Irwin RS, Channick R, et al. Complications associated with thoracentesis. A prospective, randomized study comparing three different methods. Arch Intern Med. 1990;150(4):873-877. PubMed
8. Ravin CE. Thoracocentesis of loculated pleural effusions using grey scale ultrasonic guidance. Chest. 1977;71(5):666-668. PubMed
9. Weingardt JP, Guico RR, Nemcek AA, Jr., Li YP, Chiu ST. Ultrasound findings following failed, clinically directed thoracenteses. J Clin Ultrasound. 1994;22(7):419-426. PubMed
10. Pihlajamaa K, Bode MK, Puumalainen T, Lehtimaki A, Marjelund S, Tikkakoski T. Pneumothorax and the value of chest radiography after ultrasound-guided thoracocentesis. Acta Radiol. 2004;45(8):828-832. PubMed
11. Gervais DA, Petersein A, Lee MJ, Hahn PF, Saini S, Mueller PR. US-guided thoracentesis: requirement for postprocedure chest radiography in patients who receive mechanical ventilation versus patients who breathe spontaneously. Radiology. 1997;204(2):503-506. PubMed
12. Boland GW, Gazelle GS, Girard MJ, Mueller PR. Asymptomatic hydropneumothorax after therapeutic thoracentesis for malignant pleural effusions. AJR Am J Roentgenol. 1998;170(4):943-946. PubMed
13. Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(2):ii61-76. PubMed
14. Fitch K, Bernstein SJ, Aguilar MD, Burnand B, LaCalle JR. The RAND/UCLA appropriateness method user’s manual. DTIC Document; 2001.
15. Perazzo A, Gatto P, Barlascini C, Ferrari-Bravo M, Nicolini A. Can ultrasound guidance reduce the risk of pneumothorax following thoracentesis? J Bras Pneumol. 2014;40(1):6-12. PubMed
16. Barnes TW, Morgenthaler TI, Olson EJ, Hesley GK, Decker PA, Ryu JH. Sonographically guided thoracentesis and rate of pneumothorax. J Clin Ultrasound. 2005;33(9):442-446. PubMed
17. Cavanna L, Mordenti P, Berte R, et al. Ultrasound guidance reduces pneumothorax rate and improves safety of thoracentesis in malignant pleural effusion: report on 445 consecutive patients with advanced cancer. World J Surg Oncol. 2014;12:139. PubMed
18. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40(3):135-141. PubMed
19. Diacon AH, Brutsche MH, Soler M. Accuracy of pleural puncture sites: a prospective comparison of clinical examination with ultrasound. Chest. 2003;123(2):436-441. PubMed
20. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
21. Ault MJ, Rosen BT, Scher J, Feinglass J, Barsuk JH. Thoracentesis outcomes: a 12-year experience. Thorax. 2015;70(2):127-132. PubMed
22. Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Meziere G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25(9):955-958. PubMed
23. Mayo PH, Goltz HR, Tafreshi M, Doelken P. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125(3):1059-1062. PubMed
24. Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15(1):R46. PubMed
25. Landay M, Harless W. Ultrasonic differentiation of right pleural effusion from subphrenic fluid on longitudinal scans of the right upper quadrant: importance of recognizing the diaphragm. Radiology. 1977;123(1):155-158. PubMed
26. Mayo PH, Doelken P. Pleural ultrasonography. Clin Chest Med. 2006;27(2):215-227. PubMed
27. Rosenberg ER. Ultrasound in the assessment of pleural densities. Chest. 1983;84(3):283-285. PubMed
28. Gorg C, Restrepo I, Schwerk WB. Sonography of malignant pleural effusion. Eur Radiol. 1997;7(8):1195-1198. PubMed
29. Gryminski J, Krakowka P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. Chest. 1976;70(1):33-37. PubMed
30. Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason. 2010;12(1):12-16. PubMed
31. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9-15. PubMed
32. Grimberg A, Shigueoka DC, Atallah AN, Ajzen S, Iared W. Diagnostic accuracy of sonography for pleural effusion: systematic review. Sao Paulo Med J.. 2010;128(2):90-95. PubMed
33. Kataoka H. Utility of thoracic sonography for follow-up examination of chronic heart failure patients with previous decompensation. Clin Cardiol. 2007;30(7):336-341. PubMed
34. Ma OJ, Mateer JR. Trauma ultrasound examination versus chest radiography in the detection of hemothorax. Ann Emerg Med. 1997;29(3):312-315. PubMed
35. Rocco M, Carbone I, Morelli A, et al. Diagnostic accuracy of bedside ultrasonography in the ICU: feasibility of detecting pulmonary effusion and lung contusion in patients on respiratory support after severe blunt thoracic trauma. Acta Anaesthesiol Scand. 2008;52(6):776-784. PubMed
36. Kocijancic I, Vidmar K, Ivanovi-Herceg Z. Chest sonography versus lateral decubitus radiography in the diagnosis of small pleural effusions. J Clin Ultrasound. 2003;31(2):69-74. PubMed
37. Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):1. PubMed
38. Soni NJ, Franco R, Velez MI, et al. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10(12):811-816. PubMed
39. Medford AR, Entwisle JJ. Indications for thoracic ultrasound in chest medicine: an observational study. Postgrad Med J. 2010;86(1011):8-11. PubMed
40. Lin MS, Hwang JJ, Chong IW, et al. Ultrasonography of chest diseases: analysis of 154 cases. Gaoxiong Yi Xue Ke Xue Za Zhi . 1992;8(10):525-534. PubMed
41. Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hormann MF, Grabenwoger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191(3):681-684. PubMed
42. Vignon P, Chastagner C, Berkane V, et al. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33(8):1757-1763. PubMed
43. Usta E, Mustafi M, Ziemer G. Ultrasound estimation of volume of postoperative pleural effusion in cardiac surgery patients. Interact Cardiovasc Thorac Surg. 2010;10(2):204-207. PubMed
44. Remerand F, Dellamonica J, Mao Z, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656-664.
PubMed
45. Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32(2):318-321. PubMed
46. Zanforlin A, Gavelli G, Oboldi D, Galletti S. Ultrasound-guided thoracentesis: the V-point as a site for optimal drainage positioning. Eur Rev Med Pharmacol Sci. 2013;17(1):25-28. PubMed
47. Lisi M, Cameli M, Mondillo S, et al. Incremental value of pocket-sized imaging device for bedside diagnosis of unilateral pleural effusions and ultrasound-guided thoracentesis. Interact Cardiovasc Thorac Surg. 2012;15(4):596-601. PubMed
48. Xirouchaki N, Kondili E, Prinianakis G, Malliotakis P, Georgopoulos D. Impact of lung ultrasound on clinical decision making in critically ill patients. Intensive Care Med. 2014;40(1):57-65. PubMed
49. Chen HJ, Tu CY, Ling SJ, et al. Sonographic appearances in transudative pleural effusions: not always an anechoic pattern. Ultrasound Med Biol. 2008;34(3):362-369. PubMed
50. Yang PC, Luh KT, Chang DB, Wu HD, Yu CJ, Kuo SH. Value of sonography in determining the nature of pleural effusion: analysis of 320 cases. AJR Am J Roentgenol. 1992;159(1):29-33. PubMed
51. Liang SJ, Tu CY, Chen HJ, et al. Application of ultrasound-guided pigtail catheter for drainage of pleural effusions in the ICU. Intensive Care Med. 2009;35(2):350-354. PubMed
52. McLoud TC, Flower CD. Imaging the pleura: sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;156(6):1145-1153. PubMed
53. Tu CY, Hsu WH, Hsia TC, et al. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126(4):1274-1280. PubMed
54. Sajadieh H, Afzali F, Sajadieh V, Sajadieh A. Ultrasound as an alternative to aspiration for determining the nature of pleural effusion, especially in older people. Ann N Y Acad Sci. 2004;1019:585-592. PubMed
55. Marcun R, Sustic A. Sonographic evaluation of unexplained pleural exudate: a prospective case series. Wien Klin Wochenschr. 2009;121(9-10):334-338. PubMed
56. Bugalho A, Ferreira D, Dias SS, et al. The diagnostic value of transthoracic ultrasonographic features in predicting malignancy in undiagnosed pleural effusions: a prospective observational study. Respiration. 2014;87(4):270-278. PubMed
57. Salamonsen MR, Lo AK, Ng AC, Bashirzadeh F, Wang WY, Fielding DI. Novel use of pleural ultrasound can identify malignant entrapped lung prior to effusion drainage. Chest. 2014;146(5):1286-1293. PubMed
58. Himelman RB, Callen PW. The prognostic value of loculations in parapneumonic pleural effusions. Chest. 1986;90(6):852-856. PubMed
59. Chen CH, Chen W, Chen HJ, et al. Transthoracic ultrasonography in predicting the outcome of small-bore catheter drainage in empyemas or complicated parapneumonic effusions. Ultrasound Med Biol. 2009;35(9):1468-1474. PubMed
60. Hirsch JH, Rogers JV, Mack LA. Real-time sonography of pleural opacities. AJR Am J Roentgenol. 1981;136(2):297-301. PubMed
61. Chen KY, Liaw YS, Wang HC, Luh KT, Yang PC. Sonographic septation: a useful prognostic indicator of acute thoracic empyema. J Ultrasound Med. 2000;19(12):837-843. PubMed
62. Diaz-Guzman E, Budev MM. Accuracy of the physical examination in evaluating pleural effusion. Cleve Clin J Med. 2008;75(4):297-303. PubMed
63. Rhyne T, Birnholz JC. Simple measurement of chest-wall thickness with ultrasound. Radiology. 1973;108(2):436-438. PubMed
64. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax by radiography and ultrasonography: a meta-analysis. Chest. 2011;140(4):859-866. PubMed
65. Lichtenstein D, Meziere G, Biderman P, Gepner A. The “lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med. 2000;26(10):1434-1440. PubMed
66. Shostak E, Brylka D, Krepp J, Pua B, Sanders A. Bedside sonography for detection of postprocedure pneumothorax. J Ultrasound Med. 2013;32(6):1003-1009. PubMed
67. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta-analysis. Crit Care. 2013;17(5):R208. PubMed
68. Alrajhi K, Woo MY, Vaillancourt C. Test characteristics of ultrasonography for the detection of pneumothorax: a systematic review and meta-analysis. Chest. 2012;141(3):703-708. PubMed
69. Sartori S, Tombesi P, Trevisani L, Nielsen I, Tassinari D, Abbasciano V. Accuracy of transthoracic sonography in detection of pneumothorax after sonographically guided lung biopsy: prospective comparison with chest radiography. AJR Am J Roentgenol. 2007;188(1):37-41. PubMed
70. Blaivas M, Lyon M, Duggal S. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med. 2005;12(9):844-849. PubMed
71. Lichtenstein DA, Meziere G, Lascols N, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med. 2005;33(6):1231-1238. PubMed
72. Kohan JM, Poe RH, Israel RH, et al. Value of chest ultrasonography versus decubitus roentgenography for thoracentesis. Am Rev Respir Dis. 1986;133(6):1124-1126. PubMed
73. Capizzi SA, Prakash UB. Chest roentgenography after outpatient thoracentesis. Mayo Clin Proc. 1998;73(10):948-950. PubMed
74. Aleman C, Alegre J, Armadans L, et al. The value of chest roentgenography in the diagnosis of pneumothorax after thoracentesis. Am J Med. 1999;107(4):340-343. PubMed
75. Petersen WG, Zimmerman R. Limited utility of chest radiograph after thoracentesis. Chest. 2000;117(4):1038-1042. PubMed
76. Begot E, Grumann A, Duvoid T, et al. Ultrasonographic identification and semiquantitative assessment of unloculated pleural effusions in critically ill patients by residents after a focused training. Intensive Care Med. 2014;40(10):1475-1480. PubMed
77. Kotagal M, Quiroga E, Ruffatto BJ, et al. Impact of point-of-care ultrasound training on surgical residents’ confidence. J Surg Educ. 2015;72(4):e82-87. PubMed
78. Beaulieu Y, Laprise R, Drolet P, et al. Bedside ultrasound training using web-based e-learning and simulation early in the curriculum of residents. Crit Ultrasound J. 2015;7:1. PubMed
79. Schnobrich DJ, Olson AP, Broccard A, Duran-Nelson A. Feasibility and acceptability of a structured curriculum in teaching procedural and basic diagnostic ultrasound skills to internal medicine residents. J Grad Med Educ. 2013;5(3):493-497. PubMed
80. Chalumeau-Lemoine L, Baudel JL, Das V, et al. Results of short-term training of naive physicians in focused general ultrasonography in an intensive-care unit. Intensive Care Med. 2009;35(10):1767-1771. PubMed
81. Keddis MT, Cullen MW, Reed DA, et al. Effectiveness of an ultrasound training module for internal medicine residents. BMC Med Educ. 2011;11:75. PubMed
82. Ramsingh D, Alexander B, Le K, Williams W, Canales C, Cannesson M. Comparison of the didactic lecture with the simulation/model approach for the teaching of a novel perioperative ultrasound curriculum to anesthesiology residents. J Clin Anesth. 2014;26(6):443-454. PubMed
83. Sekiguchi H, Bhagra A, Gajic O, Kashani KB. A general Critical Care Ultrasonography workshop: results of a novel Web-based learning program combined with simulation-based hands-on training. J Crit Care. 2013;28(2):217.e217-212. PubMed
84. Dulohery MM, Stoven S, Kurklinsky AK, Halvorsen A, McDonald FS, Bhagra A. Ultrasound for internal medicine physicians: the future of the physical examination. J Ultrasound Med. 2014;33(6):1005-1011. PubMed
85. Lenchus J, Issenberg SB, Murphy D, et al. A blended approach to invasive bedside procedural instruction. Med Teach. 2011;33(2):116-123. PubMed
86. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
87. Wayne DB, Barsuk JH, O’Leary KJ, Fudala MJ, McGaghie WC. Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice. J Hosp Med. 2008;3(1):48-54. PubMed
88. Jiang G, Chen H, Wang S, et al. Learning curves and long-term outcome of simulation-based thoracentesis training for medical students. BMC Med Educ. 2011;11:39. PubMed
89. Salamonsen M, McGrath D, Steiler G, Ware R, Colt H, Fielding D. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
90. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
91. Shurtleff E, Olinger A. Posterior intercostal artery tortuosity and collateral branch points: a cadaveric study. Folia Morphol. 2012;71(4):245-251. PubMed
92. Salamonsen M, Ellis S, Paul E, Steinke K, Fielding D. Thoracic ultrasound demonstrates variable location of the intercostal artery. Respiration. 2012;83(4):323-329. PubMed
93. Salamonsen M, Dobeli K, McGrath D, et al. Physician-performed ultrasound can accurately screen for a vulnerable intercostal artery prior to chest drainage procedures. Respirology. 2013;18(6):942-947. PubMed
94. Helm EJ, Rahman NM, Talakoub O, Fox DL, Gleeson FV. Course and variation of the intercostal artery by CT scan. Chest. 2013;143(3):634-639. PubMed
95. Yoneyama H, Arahata M, Temaru R, Ishizaka S, Minami S. Evaluation of the risk of intercostal artery laceration during thoracentesis in elderly patients by using 3D-CT angiography. Intern Med. 2010;49(4):289-292. PubMed
96. Soldati G, Smargiassi A, Inchingolo R, Sher S, Valente S, Corbo GM. Ultrasound-guided pleural puncture in supine or recumbent lateral position - feasibility study. Multidiscip Respir Med. 2013;8(1):18. PubMed
97. Harnsberger HR, Lee TG, Mukuno DH. Rapid, inexpensive real-time directed thoracentesis. Radiology. 1983;146(2):545-546. PubMed
© 2018 Society of Hospital Medicine
Credentialing of Hospitalists in Ultrasound-Guided Bedside Procedures: A Position Statement of the Society of Hospital Medicine
The American Board of Internal Medicine (ABIM) changed its certification policy for bedside procedures over a decade ago.
Hospitalists increasingly perform bedside procedures with ultrasound guidance.
Therefore, the Society of Hospital Medicine (SHM) Education Committee convened a group of experts and conducted a systematic literature review in order to provide recommendations for credentialing hospitalist physicians in ultrasound-guided bedside procedures. These recommendations do not include training recommendations, aside from recommendations about remedial training for hospitalists who do not pass certification. Training is a means to competence but does not guarantee it. We believe that training recommendations ought to be considered separately.
METHODS
Working Group Formation
In January 2015, the SHM Board of Directors asked the SHM Education Committee to convene the POCUS Task Force. The purpose of the task force was to develop recommendations on ultrasound guidance for bedside procedures. The SHM Education Committee appointed 3 chairs of the task force: 1 senior member of the SHM Education Committee and 2 POCUS experts. The chairs assembled a task force of 31 members that included 5 working groups, a multispecialty peer review group, and a guideline methodologist (supplemental Appendix 1). Invitation was based on members’ past contributions to SHM POCUS-related activities, up-front commitment, and declared conflicts of interest. Working group members self-identified as “hospitalists,” whereas peer reviewers were nonhospitalists but nationally recognized POCUS physician-leaders specializing in emergency medicine, cardiology, critical care medicine, and anesthesiology. Task force membership was vetted by a chair of the SHM POCUS Task Force and the Director of Education before work began. This position statement was authored by the Credentialing Working Group together with the chairs of the other 4 working groups and a guideline methodologist.
Disclosures
Signed disclosure statements of all task force members were reviewed prior to inclusion on the task force (supplemental Appendix 2); no members received honoraria for participation. Industry representatives did not contribute to the development of the guidelines nor to any conference calls or meetings.
Literature Search Strategy
A literature search was conducted by a biomedical librarian. Records from 1979 to January of 2017 were searched in Medline, Embase, CINAHL, Cochrane, and Google Scholar (supplemental Appendix 3). Search limiters were English language and adults. Articles were manually screened to exclude nonhuman or endoscopic ultrasound applications. Final article selection was based on working group consensus.
Draft Pathways
The Credentialing Working Group drafted initial and ongoing certification pathways (Figure 1 and Figure 2). The other 4 working groups from the task force were surveyed about the elements and overall appropriateness of these draft pathways. This survey and its results have already been published.12 The Credentialing Working Group then revised the certification pathways by using these survey results and codified individual aspects of these pathways into recommendations.
Development of Position Statement
Based on the Grading of Recommendation Assessment Development and Evaluation methodology, all final article selections were initially rated as either low-quality (observational studies) or unclassifiable (expert opinion).16 These initial ratings were downgraded further because of indirectness, because none of the articles involved the intervention of interest (a credentialing pathway) in a population of interest (hospitalists) measuring the outcomes of interest (patient-level outcomes).
First, the Credentialing Working Group drafted an initial position statement composed of recommendations for credentialing pathways and other general aspects of credentialing. All final article selections were incorporated as references in a draft of the position statement and compiled in a full-text compendium. Second, feedback was provided by the other 4 task force working groups, the task force peer reviewers, and the SHM Education Committee. Feedback was incorporated by the authors of this statement who were the Credentialing Working Group, the chairs of the other 4 working groups, and a guideline methodologist. Third, final suggestions from all members of the SHM POCUS Task Force and SHM Education Committee were incorporated before final approval by the SHM Board of Directors in September 2017.
RESULTS
A total of 1438 references were identified in the original search. Manual selection led to 101 articles, which were incorporated into the following 4 domains with 16 recommendations.
General Credentialing Process
Basic Cognitive Competence Can Be Certified with Written or Oral Examinations
The ABIM defines cognitive competence as having 3 abilities: “(1) to explain indications, contraindications, patient preparation methods, sterile techniques, pain management, proper techniques for handling specimens and fluids obtained, and test results; (2) to recognize and manage complications; and, (3) to clearly explain to a patient all facets of the procedure necessary to obtain informed consent.”1 These abilities can be assessed with written or oral examinations that may be integrated into simulation- or patient-based assessments.
Minimum Thresholds of Experience to Trigger the Timing of a Patient-Based Assessment Should Be Determined by Empirical Methods
Learning curves are highly variable22-25 and even plateaus may not herald basic competence.26 Expert opinion
Hospitalists Should Formally Log All of Their Attempted Procedures, Ideally in an Electronic Medical Record
Simple self-reported numbers of procedures performed often misrepresent actual experience33,34 and do not include periprocedural complications.35,36 Thus, hospitalists should report their experience with logs of all attempted procedures, both successful and unsuccessful. Such logs must include information about supervising providers (if applicable) and patient outcomes, including periprocedural adverse events,37 but they must also remain compliant with the Health Insurance Portability and Accountability Act.
Health Information Technology Service Should Routinely Pull Collations of All Attempted Procedures from Comprehensive Electronic Medical Records
Active surveillance may reduce complications by identifying hospitalists who may benefit from further training.38 In order to facilitate active surveillance systems, documentation (such as a procedure note) should be both integrated into an electronic medical record and protocol driven,39 including procedure technique, ultrasound findings, and any safety events (both near misses and adverse events).
Multiple interacting factors, including environment, patients, baseline skills, training, experience, and skills decay, affect manual competence. Certifications that are based solely on reaching minimum thresholds of experience, even when accurate, are not valid reflections of manual competence,15,40-43 and neither are those based on self-perception.44 Patient-based assessments are, thus, necessary to ensure manual competence.45-48
Certification Assessments of Manual Competence Should Combine 2 Types of Structured Instruments: Checklists and Overall Scores
Assessments based on direct observation are more reliable when formally structured.49,50 Though checklists used in observed structured clinical examinations capture many important manual skills,51-56 they do not completely reflect a hospitalist’s manual competence;57 situations may occur in which a hospitalist meets all the individual items on a checklist but cannot perform an entire procedure with basic competence. Therefore, checklists should be paired with overall scores.58-61 Both checklists and overall scores ought to be obtained from reliable and valid instruments.
Certification Assessments Should Include Feedback
Assessments without feedback are missed learning opportunities.62 Both simulation-63 and patient-based assessments should provide feedback in real time to reinforce effective behaviors and remedy faulty ones.
If Remedial Training is Needed, Simulator-Based Training Can Supplement but Not Replace Patient-Based Training
Supervised simulator-based training allows hospitalists to master basic components of a procedure64 (including orientation to equipment, sequence of operations, dexterity, ultrasound anatomy, and real-time guidance technique) while improving both cognitive and manual skills.42,43,65-71 In addition to their role in basic training (which is outside the scope of this position statement), simulators can be useful for remedial training. To be sufficient for hospitalists who do not pass their patient-based assessments, however, remedial training that begins with simulation must also include patient-based training and assessment.72-75
Initial Credentialing Process
A Minimum Threshold of Experience Should Be Reached before Patient-Based Assessments are Conducted (Figure 1)
Initial Certification Assessments Should Ideally Begin on Simulators
Simulators allow the assurance of safe manual skills, including proper needle insertion techniques and disposal of sharp objects.3,79 If simulators are not available, however, then patient-based training and assessments can still be performed under direct observation. Safe performance of ultrasound-guided procedures during patient-based assessments (without preceding simulator-based assessments) is sufficient to certify manual competence.
Ongoing Credentialing
Certification to Perform Ultrasound-Guided Procedures Should Be Routinely Re-Evaluated During Ongoing Credentialing (Figure 2)
Observed Patient-Based Assessments Should Occur When a Periprocedural Safety Event Occurs that is Potentially Caused by “Provider Error”
Safety events include both near misses and adverse events. Information about both is ideally “flagged” and “pushed” to hospitalist group leaders by active surveillance and reporting systems. Once reviewed, if a safety event is considered to potentially have been caused by provider error (including knowledge- and skill-based errors),83 then the provider who performed the procedure should undergo an observed patient-based assessment.
Simulation-Based Practice Can Supplement Patient-Based Experience for Ongoing Credentialing
When hospitalists do not achieve a minimum threshold of patient-based experience since the antecedent certification, simulation-based training can supplement their patient-based experience.
Credentialing Infrastructure
Hospitalists Themselves Should Not Bear the Financial Costs of Developing and Maintaining Training and Certification Programs for Ultrasound-Guided Procedures
Equipment and personnel costs
Assessors Should Be Unbiased Expert Providers Who Have Demonstrated Mastery in Performance of the Procedure Being Assessed and Regularly Perform It in a Similar Practice Environment
Assessors should be expert providers who regularly perform the ultrasound-guided procedure in a similar practice environment.9,89-94 For example, providers who are not hospitalists but who are experts in an ultrasound-guided procedure and commonly perform it on the hospital wards would be acceptable assessors. However, a radiologist who only performs that procedure in a fully-staffed interventional radiology suite with fluoroscopy or computed tomography guidance would not be an acceptable assessor. More than 1 assessor may balance idiosyncratic assessments;95 but when assessments are well structured, additional assessors are generally not needed.18
If Intramural Assessors Are Not Available, Extramural Assessors May Be Considered
Intramural assessors are generally preferred because of familiarity with the local practice environment, including the available procedure kits and typical patient characteristics. Nevertheless, extramural assessors27,77,85,96 may theoretically provide even more valid assessments than intramural ones because extramural assessors are neither influenced by relationships with local hospitalists nor biased by local hospitalists’ skills.
DISCUSSION
There are no high-quality randomized trials in support of a single credentialing pathway over any other.94,102 The credentialing pathways at the center of this position statement are based on expert opinion. Our methods can be criticized straightaway, therefore, for reliance on the experience and expertise of our working group and task force. Any position statement written without high-quality supportive evidence would be appropriately subject to the same criticism. Without evidence in support of an overall pathway, we codified specific aspects of the pathways into 16 individual recommendations.
Patient-level outcomes do not back these recommendations. Consider, for example, our recommendation that certification assessments be made from structured instruments and not simply from an assessor’s gestalt. Here, the basis is not improved patient-level outcomes from a trial (such as reduced complications or increased procedural success) but improved psychometric performance from reliability studies. The body of evidence for our recommendations is similarly indirect, mostly because the outcomes studied are more proximate and, thus, less meaningful than patient-level outcomes, which are the outcomes of greatest interest but are woefully understudied for clinical competence.17,97,103
The need for high-quality evidence is most pronounced in distinguishing how recommendations should be modified for various settings. Wide variations in resources and patient-mix will make some recommendations impracticable, meaning that they could not be carried out with available resources. For example, our recommendation that credentialing decisions should ultimately rely on certifications made by assessors during patient-based assessments may not be practicable at small, rural hospitals. Such hospitals may not have access to local assessors, and they may not admit enough patients who need the types of ultrasound-guided procedures for which hospitalists seek certification (especially given the need to coordinate the schedules of patients, procedure-performing hospitalists, and assessors).
Regardless of whether some or all hospitalists at a particular hospital are expected to perform bedside procedures, technology may help to improve the practicability of our recommendations. For example, simulators may evolve to replace actual patient-level experience in achieving minimum thresholds. Certification assessments of manual skills may even someday occur entirely on simulators. Real-time high-definition video streaming enhanced with multiple cameras may allow for remote assessments. Until such advances mature, high-quality patient-level data should be sought through additional research to refine our current recommendations.
We hope that these recommendations will improve how basic competence in ultrasound-guided bedside procedures is assessed. Our ultimate goal is to improve how hospitalists perform these procedures. Patient safety is, therefore, considered paramount to cost. Nevertheless, the hospital administrative leaders and privileging committee members on our Task Force concluded that many hospitals have been seeking guidance on credentialing for bedside procedures, and the likely difficulties of implementing our recommendations (including cost) would not be prohibitive at most hospitals, especially given recognition that these recommendations can be tailored to each setting.
Acknowledgments
Collaborators from SHM POCUS Task Force are Saaid Abdel-Ghani, Michael Blaivas, Dan Brotman, Carolina Candotti, Jagriti Chadha, Joel Cho, Ria Dancel, Ricardo Franco, Richard Hoppmann, Susan Hunt, Venkat Kalidindi, Ketino Kobaidze, Josh Lenchus, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Sophia Rodgers, Gerard Salame, Daniel Schnobrich, Kirk Spencer, Vivek Tayal, Jeff Bates, Anjali Bhagra, Kreegan Reierson, Robert Arntfield, Paul Mayo, Loretta Grikis.
Disclosure
Brian P. Lucas received funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086). Nilam Soni received funding from the Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (HX002263-01A1). The contents of this publication do not represent the views of the United States Department of Veterans Affairs or the United States Government.
1. American Board of Internal Medicine. Policies and procedures for certification. Philadelphia: American Board of Internal Medicine; 2006.
2. Nichani S, Fitterman N, Lukela M, Crocker J; Society of Hospital Medicine. The Core Competencies in Hospital Medicine 2017 Revision. Section 2: Procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54 PubMed
3. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. PubMed
4. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. PubMed
5. Brown GM, Otremba M, Devine LA, Gray C, Millington SJ, Ma IW. Defining competencies for ultrasound-guided bedside procedures: consensus opinions from Canadian physicians. J Ultrasound Med. 2016;35(1):129-141. PubMed
6. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. PubMed
7. Kreisman RD. With ED ultrasound, credentialing is at issue. ED Legal Letter. 2010;21:102-103.
8. Goudie AM. Credentialing a new skill: what should the standard be for emergency department ultrasound in Australasia? Emerg Med Australas. 2010;22:263-264. PubMed
9. Maizel J, Guyomarc HL, Henon P, et al. Residents learning ultrasound-guided catheterization are not sufficiently skilled to use landmarks. Crit Care. 2014;18(1):R36. doi:10.1186/cc13741. PubMed
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. PubMed
11. Amini R, Adhikari S, Fiorello A. Ultrasound competency assessment in emergency medicine residency programs. Acad Emerg Med. 2014;21(7):799-801. PubMed
12. Jensen T, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. PubMed
13. Chang W. Is hospitalist proficiency in bedside procedures in decline? The Hospitalist. 2012. http://www.the-hospitalist.org/hospitalist/article/125236/patient-safety/hospitalist-proficiency-bedside-procedures-decline. Accessed September 30, 2017.
14. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties Performing Paracentesis Procedures at University Hospitals: Implications for Training and Certification. J Hosp Med. 2014;9(3):162-168. PubMed
15. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ Procedural Experience Does Not Ensure Competence: A Research Synthesis. J Grad Med Educ. 2017;9(2):201-208. PubMed
16. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. PubMed
17. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303-1310. PubMed
18. Miller GE. The assessment of clinical skills/competence/performance. Acad Med. 1990;65(9 Suppl):S63-S67. PubMed
19. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
20. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IWY. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. PubMed
21. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. PubMed
22. Heegeman DJ, Kieke B Jr. Learning curves, credentialing, and the need for ultrasound fellowships. Acad Emerg Med. 2003;10:404-405. PubMed
23. Jang TB, Ruggeri W, Dyne P, Kaji AH. The learning curve of resident physicians using emergency ultrasonography for cholelithaisis and cholecystitis. Acad Emerg Med. 2010;17(11):1247-1252. PubMed
24. Akhtar MI, Hamid M. Ultrasound guided central venous access; a review of literature. Anaesth Pain Intensive Care. 2015;19:317-322.
25. Bahl A, Yunker A. Assessment of the numbers–based model for evaluation of resident competency in emergency ultrasound core applications. J Emerg Med Trauma Acute Care. 2015;2015(5). doi:10.5339/jemtac.2015.5
26. Cazes N, Desmots F, Geffroy Y, Renard A, Leyral J, Chaumoitre K. Emergency ultrasound: a prospective study on sufficient adequate training for military doctors. Diagn Interv Imaging. 2013;94(11):1109-1115. PubMed
27. Arntfield RT, Millington SJ, Ainsworth CD, et al. Canadian recommendations for critical care ultrasound training and competency for the Canadian critical care society. Can Respir J. 2014;21(16):341-345.
28. Bolsin S, Colson M. The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care. 2000;12(5):433-438. PubMed
29. de Oliveira Filho GR, Helayel PE, da Conceição DB, Garzel IS, Pavei P, Ceccon MS. Learning curves and mathematical models for interventional ultrasound basic skills. Anaesth Analg. 2008;106(2):568-573. PubMed
30. Starkie T, Drake EJ. Assessment of procedural skills training and performance in anesthesia using cumulative sum analysis (cusum). Can J Anaesth. 2013;60(12):1228-1239. PubMed
31. Tierney D. Competency cut-point identification derived from a mastery learning cohort approach: A hybrid model. Ultrasound Med Biol. 2015;41:S19.
32. Rankin JH, Elkhunovich MA, Rangarajan V, Chilstrom M, Mailhot T. Learning Curves for Ultrasound Assessment of Lumbar Puncture Insertion Sites: When is Competency Established? J Emerg Med. 2016;51(1):55-62. PubMed
33. Klasko SK, Cummings RV, Glazerman LR. Resident data collection: Do the numbers add up? Am J Obstet Gynecol. 1995;172(4 Pt 1):1312-1316. PubMed
34. Tierney D. Development & analysis of a mobile POCUS tracking tool. Ultrasound Med Biol. 2015;41(suppl 4):S31.
35. Sethi MV, Zimmer J, Ure B, Lacher M. Prospective assessment of complications on a daily basis is essential to determine morbidity and mortality in routine pediatric surgery. J Pediatr Surg. 2016;51(4):630-633. PubMed
36. Fisher JC, Kuenzler KA, Tomita SS, Sinha P, Shah P, Ginsburg HB. Increased capture of pediatric surgical complications utilizing a novel case-log web application to enhance quality improvement. J Pediatr Surg. 2017;52(1):166-171. PubMed
37. Rethans JJ, Norcini JJ, Barón-Maldonado M, et al. The relationship between competence and performance: implications for assessing practice performance. Med Educ. 2002;36(10):901-909. PubMed
38. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
39. Society of Critical Care Medicine Ultrasound Certification Task Force. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. http://journals.lww.com/ccmjournal/Documents/Critical%20Care%20Ultrasound.pdf Published 2013. Accessed February 2, 2017.
40. Carraccio C, Wolfsthal SD, Englander R, Ferentz K, Martin C. Shifting paradigms: from Flexner to competencies. Acad Med. 2002;77(5):361-367. PubMed
41. Clark EG, Paparello JJ, Wayne DB, et al. Use of a national continuing medical education meeting to provide simulation-based training in temporary hemodialysis catheter insertion skills: a pre-test post-test study. Can J Kidney Health Dis. 2014;1:25-31. PubMed
42. Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. PubMed
43. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. PubMed
44. Davis DA, Mazmanian PE, Fordis M, Van Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102. PubMed
45. Shah J, Darzi A. Surgical skills assessment: an ongoing debate. BJU Int. 2001;88(7):655-660. PubMed
46. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. PubMed
47. Tolsgaard MG, Todsen T, Sorensen JL, et al. International multispecialty consensus on how to evaluate ultrasound competence: a Delphi consensus survey. PLOS One. 2013;8(2):e57687. doi:10.1371/journal.pone.0057687 PubMed
48. Moureau N, Laperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. PubMed
49. Feldman LS, Hagarty S, Ghitulescu G, Stanbridge D, Fried GM. Relationship between objective assessment of technical skills and subjective in-training evaluations in surgical residents. J Am Coll Surg. 2004;198(1):105-110. PubMed
50. Baker S, Willey B, Mitchell C. The attempt to standardize technical and analytic competence in sonography education. J Diagn Med Sonogr. 2011;27(5):203-211.
51. Tolsgaard MG, Ringsted C, Dreisler E, et al. Reliable and valid assessment of ultrasound operator competence in obstetrics and gynecology. Ultrasound Obstet Gynecol. 2014;43(4):437-443. PubMed
52. Rice J, Crichlow A, Baker M, et al. An assessment tool for the placement of ultrasound-guided peripheral intravenous access. J Grad Med Educ. 2016;8(2):202-207. PubMed
53. Hartman N, Wittler M, Askew K, Hiestand B, Manthey D. Validation of a performance checklist for ultrasound-guided internal jubular central lines for use in procedural instruction and assessment. Postgrad Med J. 2017;93(1096):67-70. PubMed
54. Primdahl SC, Todsen T, Clemmesen L, et al. Rating scale for the assessment of competence in ultrasound-guided peripheral vascular access—a Delphi Consensus Study. J Vasc Access. 2016;17(5):440-445.
55. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
56. Berg K, Riesenberg LA, Berg D, et al. The development of a validated checklist for radial arterial line placement: preliminary results. Am J Med Qual. 2014;29(3):242-246. PubMed
57. Walzak A, Bacchus M, Schaefer MP, et al. Diagnosing technical competence in six bedside procedures: comparing checklists and a global rating scale in the assessment of resident performance. Acad Med. 2015;90(8):1100-1108. PubMed
58. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for femoral venous catheterization: preliminary results. Am J Med Qual. 2014;29(5):445-450. PubMed
59. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for paracentesis: preliminary results. Am J Med Qual. 2013;28(3):227-231. PubMed
60. Huang GC, Newman LR, Schwartzstein RM, et al. Procedural competence in internal medicine residents: validity of a central venous catheter insertion assessment instrument. Acad Med. 2009;84(8):1127-1134. PubMed
61. Salamonsen M, McGrath D, Steiler G, et al. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
62. Boniface K, Yarris LM. Emergency ultrasound: Leveling the training and assessment landscape. Acad Emerg Med. 2014;21(7):803-805. PubMed
63. Boyle E, O’Keeffe D, Naughton P, Hill A, McDonnell C, Moneley D. The importance of expert feedback during endovascular simulator training. J Vasc Surg. 2011;54(1):240-248.e1. PubMed
64. Langhan TS, Rigby IJ, Walker IW, Howes D, Donnon T, Lord JA. Simulation-based training in critical resuscitation procedures improves residents’ competence. CJEM. 2009;11(6):535-539. PubMed
65. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. PubMed
66. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
67. Barsuk JH, Cohen ER, Vozenilek JA, O’Connor LM, McGaghie WC, Wayne DB. Simulation-based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23-27. PubMed
68. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706-711. PubMed
69. Ross JG. Simulation and psychomotor skill acquisition: A review of the literature. Clin Simul Nurs. 2012;8(9):e429-e435.
70. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
71. McSparron JI, Michaud GC, Gordan PL, et al. Simulation for skills-based education in pulmonary and critical care medicine. Ann Am Thorac Soc. 2015;12(4):579-586. PubMed
72. Kneebone RL, Scott W, Darzi A, Horrocks M. Simulation and clinical practice: strengthening the relationship. Med Educ. 2004;38(10):1095-1102. PubMed
73. Mema B, Harris I. The barriers and facilitators to transfer of ultrasound-guided central venous line skills from simulation to practice: exploring perceptions of learners and supervisors. Teach Learn Med. 2016;28(2):115-124. PubMed
74. Castanelli DJ. The rise of simulation in technical skills teaching and the implications for training novices in anaestheia. Anaesth Intensive Care. 2009;37(6):903-910. PubMed
75. McGaghie WC, Issenberg SB, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014;48(4):375-385. PubMed
76. Langlois SLP. Focused ultrasound training for clinicians. Crit Care Med. 2007;35(5 suppl):S138-S143.
77. Price S, Via G, Sloth E, et al. Echocardiography practice, training and accreditation in the intesive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008;6:49-83. PubMed
78. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. PubMed
79. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. PubMed
80. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. PubMed
81. Sliman Sean, Amundson S, Shaw D, Phan JN, Waalen J, Kimura B. Recently-acquired cardiac ultrasound skills are rapidly lost when not used: implications for competency in physician imaging. J Amer Coll Cardiol. 2016;67(13S):1569.
82. Kessler CS, Leone KA. The current state of core competency assessment in emergency medicine and a future research agenda: recommendations of the working group on assessment of observable learner performance. Acad Emerg Med. 2012;19(12):1354-1359. PubMed
83. Chang A, Schyve PM, Croteau RJ, O’Leary DS, Loeb JM. The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care. 2005;17(2):95-105. PubMed
84. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. PubMed
85. Das D, Kapoor M, Brown C, Ndubuisi A, Gupta S. Current status of emergency department attending physician ultrasound credentialing and quality assurance in the United States. Crit Ultrasound J. 2016;8(1):6-12. PubMed
86. Ndubuisi AK, Gupta S, Brown C, Das D. Current status and future issues in emergency department attending physician ultrasound credentialing. Ann Emerg Med. 2014;64(45):S27-S28.
87. Tandy Tk, Hoffenberg S. Emergency department ultrasound services by emergency physicians: model for gaining hospital approval. Ann Emerg Med. 1997;29(3):367-374. PubMed
88. Lewiss RE, Saul T, Del Rios M. Acquiring credentials in bedside ultrasound: a cross-sectional survey. BMJ Open. 2013;3:e003502. doi:10.1136/bmjopen-2013-003502 PubMed
89. Lanoix R. Credentialing issues in emergency ultrasonography. Emerg Med Clin North Am. 1997;15(4):913-920. PubMed
90. Scalea T, Rodriquez A, Chiu WC, et al. Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma. 1999;46(3):466-472. PubMed
91. Hertzberg BS, Kliewer MA, Bowie JD, et al. Physician training requirements in sonography: how many cases are needed for competence? AJR. 2000;174(5):1221-1227. PubMed
92. Blaivas M, Theodoro DL, Sierzenski P. Proliferation of ultrasound fellowships in emergency medicine: how do we ensure future experts are expertly trained? Acad Emerg Med. 2002;9(8):863-864. PubMed
93. Bodenham AR. Editorial II: Ultrasound imaging by anaesthetists: training and accreditation issues. Br J Anaesth. 2006;96(4):414-417. PubMed
94. Williamson JP, Twaddell SH, Lee YCG, et al. Thoracic ultrasound recognition of competence: A position paper of the Thoracic Society of Australia and New Zealand. Respirology. 2017;22(2):405-408. PubMed
95. Harrison G. Summative clinical competency assessment: a survey of ultrasound practitioners’ views. Ultrasound. 2015;23(1):11-17. PubMed
96. Evans LV, Morse JL, Hamann CJ, Osborne M, Lin Z, D'Onofrio G. The development of an independent rater system to assess residents' competence in invasive procedures. Acad Med. 2009;84(8):1135-1143. PubMed
97. Wass V, Van der Vleuten C, Shatzer J, Jones R. Assessment of clinical competence. Lancet. 2001;357(9260):945-949. PubMed
98. Arntfield RT. The utility of remote supervision with feedback as a method to deliver high-volume critical care ultrasound training. J Crit Care. 2015;30(2):441.e1-e6. PubMed
99. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound: consensus recommendations from the 2008 Council of Emergency Residency Directors Conference. Acad Emerg Med. 2009;16:S32-S36. PubMed
100. Yu E. The assessment of technical skills in a cardiology training program: is the ITER sufficient? Can J Cardiol. 2000;16(4):457-462. PubMed
101. Todsen T, Tolsgaard MG, Olsen BH, et al. Reliable and valid assessment of point-of-care ultrasonography. Ann Surg. 2015;261(2):309-315. PubMed
102. Stein JC, Nobay F. Emergency department ultrasound credentialing: a sample policy and procedure. J Emerg Med. 2009;37(2):153-159. PubMed
103. Chen FM. Burstin H, Huntington J. The importance of clinical outcomes in medical education research. Med Educ. 2005;39(4):350-351. PubMed
104. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1:48-56. PubMed
105. ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013;5(1):157-158. PubMed
106. Castillo J, Caruana CJ, Wainwright D. The changing concept of competence and categorisation of learning outcomes in Europe: Implications for the design of higher education radiography curricula at the European level. Radiography. 2011;17(3):230-234.
107. Goldstein SR. Accreditation, certification: why all the confusion? Obstet Gynecol. 2007;110(6):1396-1398. PubMed
108. Moore CL. Credentialing and reimbursement in point-of-care ultrasound. Clin Pediatr Emerg Med. 2011;12(1):73-77. PubMed
109. ten Cate O, Scheele F. Competency-based postgraduate training: can we bridge the gap between theory and clinical practice? Acad Med. 2007;82(6):542-547. PubMed
110. Abuhamad AZ, Benacerraf BR, Woletz P, Burke BL. The accreditation of ultrasound practices: impact on compliance with minimum performance guidelines. J Ultrasound Med. 2004;23(8):1023-1029. PubMed
The American Board of Internal Medicine (ABIM) changed its certification policy for bedside procedures over a decade ago.
Hospitalists increasingly perform bedside procedures with ultrasound guidance.
Therefore, the Society of Hospital Medicine (SHM) Education Committee convened a group of experts and conducted a systematic literature review in order to provide recommendations for credentialing hospitalist physicians in ultrasound-guided bedside procedures. These recommendations do not include training recommendations, aside from recommendations about remedial training for hospitalists who do not pass certification. Training is a means to competence but does not guarantee it. We believe that training recommendations ought to be considered separately.
METHODS
Working Group Formation
In January 2015, the SHM Board of Directors asked the SHM Education Committee to convene the POCUS Task Force. The purpose of the task force was to develop recommendations on ultrasound guidance for bedside procedures. The SHM Education Committee appointed 3 chairs of the task force: 1 senior member of the SHM Education Committee and 2 POCUS experts. The chairs assembled a task force of 31 members that included 5 working groups, a multispecialty peer review group, and a guideline methodologist (supplemental Appendix 1). Invitation was based on members’ past contributions to SHM POCUS-related activities, up-front commitment, and declared conflicts of interest. Working group members self-identified as “hospitalists,” whereas peer reviewers were nonhospitalists but nationally recognized POCUS physician-leaders specializing in emergency medicine, cardiology, critical care medicine, and anesthesiology. Task force membership was vetted by a chair of the SHM POCUS Task Force and the Director of Education before work began. This position statement was authored by the Credentialing Working Group together with the chairs of the other 4 working groups and a guideline methodologist.
Disclosures
Signed disclosure statements of all task force members were reviewed prior to inclusion on the task force (supplemental Appendix 2); no members received honoraria for participation. Industry representatives did not contribute to the development of the guidelines nor to any conference calls or meetings.
Literature Search Strategy
A literature search was conducted by a biomedical librarian. Records from 1979 to January of 2017 were searched in Medline, Embase, CINAHL, Cochrane, and Google Scholar (supplemental Appendix 3). Search limiters were English language and adults. Articles were manually screened to exclude nonhuman or endoscopic ultrasound applications. Final article selection was based on working group consensus.
Draft Pathways
The Credentialing Working Group drafted initial and ongoing certification pathways (Figure 1 and Figure 2). The other 4 working groups from the task force were surveyed about the elements and overall appropriateness of these draft pathways. This survey and its results have already been published.12 The Credentialing Working Group then revised the certification pathways by using these survey results and codified individual aspects of these pathways into recommendations.
Development of Position Statement
Based on the Grading of Recommendation Assessment Development and Evaluation methodology, all final article selections were initially rated as either low-quality (observational studies) or unclassifiable (expert opinion).16 These initial ratings were downgraded further because of indirectness, because none of the articles involved the intervention of interest (a credentialing pathway) in a population of interest (hospitalists) measuring the outcomes of interest (patient-level outcomes).
First, the Credentialing Working Group drafted an initial position statement composed of recommendations for credentialing pathways and other general aspects of credentialing. All final article selections were incorporated as references in a draft of the position statement and compiled in a full-text compendium. Second, feedback was provided by the other 4 task force working groups, the task force peer reviewers, and the SHM Education Committee. Feedback was incorporated by the authors of this statement who were the Credentialing Working Group, the chairs of the other 4 working groups, and a guideline methodologist. Third, final suggestions from all members of the SHM POCUS Task Force and SHM Education Committee were incorporated before final approval by the SHM Board of Directors in September 2017.
RESULTS
A total of 1438 references were identified in the original search. Manual selection led to 101 articles, which were incorporated into the following 4 domains with 16 recommendations.
General Credentialing Process
Basic Cognitive Competence Can Be Certified with Written or Oral Examinations
The ABIM defines cognitive competence as having 3 abilities: “(1) to explain indications, contraindications, patient preparation methods, sterile techniques, pain management, proper techniques for handling specimens and fluids obtained, and test results; (2) to recognize and manage complications; and, (3) to clearly explain to a patient all facets of the procedure necessary to obtain informed consent.”1 These abilities can be assessed with written or oral examinations that may be integrated into simulation- or patient-based assessments.
Minimum Thresholds of Experience to Trigger the Timing of a Patient-Based Assessment Should Be Determined by Empirical Methods
Learning curves are highly variable22-25 and even plateaus may not herald basic competence.26 Expert opinion
Hospitalists Should Formally Log All of Their Attempted Procedures, Ideally in an Electronic Medical Record
Simple self-reported numbers of procedures performed often misrepresent actual experience33,34 and do not include periprocedural complications.35,36 Thus, hospitalists should report their experience with logs of all attempted procedures, both successful and unsuccessful. Such logs must include information about supervising providers (if applicable) and patient outcomes, including periprocedural adverse events,37 but they must also remain compliant with the Health Insurance Portability and Accountability Act.
Health Information Technology Service Should Routinely Pull Collations of All Attempted Procedures from Comprehensive Electronic Medical Records
Active surveillance may reduce complications by identifying hospitalists who may benefit from further training.38 In order to facilitate active surveillance systems, documentation (such as a procedure note) should be both integrated into an electronic medical record and protocol driven,39 including procedure technique, ultrasound findings, and any safety events (both near misses and adverse events).
Multiple interacting factors, including environment, patients, baseline skills, training, experience, and skills decay, affect manual competence. Certifications that are based solely on reaching minimum thresholds of experience, even when accurate, are not valid reflections of manual competence,15,40-43 and neither are those based on self-perception.44 Patient-based assessments are, thus, necessary to ensure manual competence.45-48
Certification Assessments of Manual Competence Should Combine 2 Types of Structured Instruments: Checklists and Overall Scores
Assessments based on direct observation are more reliable when formally structured.49,50 Though checklists used in observed structured clinical examinations capture many important manual skills,51-56 they do not completely reflect a hospitalist’s manual competence;57 situations may occur in which a hospitalist meets all the individual items on a checklist but cannot perform an entire procedure with basic competence. Therefore, checklists should be paired with overall scores.58-61 Both checklists and overall scores ought to be obtained from reliable and valid instruments.
Certification Assessments Should Include Feedback
Assessments without feedback are missed learning opportunities.62 Both simulation-63 and patient-based assessments should provide feedback in real time to reinforce effective behaviors and remedy faulty ones.
If Remedial Training is Needed, Simulator-Based Training Can Supplement but Not Replace Patient-Based Training
Supervised simulator-based training allows hospitalists to master basic components of a procedure64 (including orientation to equipment, sequence of operations, dexterity, ultrasound anatomy, and real-time guidance technique) while improving both cognitive and manual skills.42,43,65-71 In addition to their role in basic training (which is outside the scope of this position statement), simulators can be useful for remedial training. To be sufficient for hospitalists who do not pass their patient-based assessments, however, remedial training that begins with simulation must also include patient-based training and assessment.72-75
Initial Credentialing Process
A Minimum Threshold of Experience Should Be Reached before Patient-Based Assessments are Conducted (Figure 1)
Initial Certification Assessments Should Ideally Begin on Simulators
Simulators allow the assurance of safe manual skills, including proper needle insertion techniques and disposal of sharp objects.3,79 If simulators are not available, however, then patient-based training and assessments can still be performed under direct observation. Safe performance of ultrasound-guided procedures during patient-based assessments (without preceding simulator-based assessments) is sufficient to certify manual competence.
Ongoing Credentialing
Certification to Perform Ultrasound-Guided Procedures Should Be Routinely Re-Evaluated During Ongoing Credentialing (Figure 2)
Observed Patient-Based Assessments Should Occur When a Periprocedural Safety Event Occurs that is Potentially Caused by “Provider Error”
Safety events include both near misses and adverse events. Information about both is ideally “flagged” and “pushed” to hospitalist group leaders by active surveillance and reporting systems. Once reviewed, if a safety event is considered to potentially have been caused by provider error (including knowledge- and skill-based errors),83 then the provider who performed the procedure should undergo an observed patient-based assessment.
Simulation-Based Practice Can Supplement Patient-Based Experience for Ongoing Credentialing
When hospitalists do not achieve a minimum threshold of patient-based experience since the antecedent certification, simulation-based training can supplement their patient-based experience.
Credentialing Infrastructure
Hospitalists Themselves Should Not Bear the Financial Costs of Developing and Maintaining Training and Certification Programs for Ultrasound-Guided Procedures
Equipment and personnel costs
Assessors Should Be Unbiased Expert Providers Who Have Demonstrated Mastery in Performance of the Procedure Being Assessed and Regularly Perform It in a Similar Practice Environment
Assessors should be expert providers who regularly perform the ultrasound-guided procedure in a similar practice environment.9,89-94 For example, providers who are not hospitalists but who are experts in an ultrasound-guided procedure and commonly perform it on the hospital wards would be acceptable assessors. However, a radiologist who only performs that procedure in a fully-staffed interventional radiology suite with fluoroscopy or computed tomography guidance would not be an acceptable assessor. More than 1 assessor may balance idiosyncratic assessments;95 but when assessments are well structured, additional assessors are generally not needed.18
If Intramural Assessors Are Not Available, Extramural Assessors May Be Considered
Intramural assessors are generally preferred because of familiarity with the local practice environment, including the available procedure kits and typical patient characteristics. Nevertheless, extramural assessors27,77,85,96 may theoretically provide even more valid assessments than intramural ones because extramural assessors are neither influenced by relationships with local hospitalists nor biased by local hospitalists’ skills.
DISCUSSION
There are no high-quality randomized trials in support of a single credentialing pathway over any other.94,102 The credentialing pathways at the center of this position statement are based on expert opinion. Our methods can be criticized straightaway, therefore, for reliance on the experience and expertise of our working group and task force. Any position statement written without high-quality supportive evidence would be appropriately subject to the same criticism. Without evidence in support of an overall pathway, we codified specific aspects of the pathways into 16 individual recommendations.
Patient-level outcomes do not back these recommendations. Consider, for example, our recommendation that certification assessments be made from structured instruments and not simply from an assessor’s gestalt. Here, the basis is not improved patient-level outcomes from a trial (such as reduced complications or increased procedural success) but improved psychometric performance from reliability studies. The body of evidence for our recommendations is similarly indirect, mostly because the outcomes studied are more proximate and, thus, less meaningful than patient-level outcomes, which are the outcomes of greatest interest but are woefully understudied for clinical competence.17,97,103
The need for high-quality evidence is most pronounced in distinguishing how recommendations should be modified for various settings. Wide variations in resources and patient-mix will make some recommendations impracticable, meaning that they could not be carried out with available resources. For example, our recommendation that credentialing decisions should ultimately rely on certifications made by assessors during patient-based assessments may not be practicable at small, rural hospitals. Such hospitals may not have access to local assessors, and they may not admit enough patients who need the types of ultrasound-guided procedures for which hospitalists seek certification (especially given the need to coordinate the schedules of patients, procedure-performing hospitalists, and assessors).
Regardless of whether some or all hospitalists at a particular hospital are expected to perform bedside procedures, technology may help to improve the practicability of our recommendations. For example, simulators may evolve to replace actual patient-level experience in achieving minimum thresholds. Certification assessments of manual skills may even someday occur entirely on simulators. Real-time high-definition video streaming enhanced with multiple cameras may allow for remote assessments. Until such advances mature, high-quality patient-level data should be sought through additional research to refine our current recommendations.
We hope that these recommendations will improve how basic competence in ultrasound-guided bedside procedures is assessed. Our ultimate goal is to improve how hospitalists perform these procedures. Patient safety is, therefore, considered paramount to cost. Nevertheless, the hospital administrative leaders and privileging committee members on our Task Force concluded that many hospitals have been seeking guidance on credentialing for bedside procedures, and the likely difficulties of implementing our recommendations (including cost) would not be prohibitive at most hospitals, especially given recognition that these recommendations can be tailored to each setting.
Acknowledgments
Collaborators from SHM POCUS Task Force are Saaid Abdel-Ghani, Michael Blaivas, Dan Brotman, Carolina Candotti, Jagriti Chadha, Joel Cho, Ria Dancel, Ricardo Franco, Richard Hoppmann, Susan Hunt, Venkat Kalidindi, Ketino Kobaidze, Josh Lenchus, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Sophia Rodgers, Gerard Salame, Daniel Schnobrich, Kirk Spencer, Vivek Tayal, Jeff Bates, Anjali Bhagra, Kreegan Reierson, Robert Arntfield, Paul Mayo, Loretta Grikis.
Disclosure
Brian P. Lucas received funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086). Nilam Soni received funding from the Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (HX002263-01A1). The contents of this publication do not represent the views of the United States Department of Veterans Affairs or the United States Government.
The American Board of Internal Medicine (ABIM) changed its certification policy for bedside procedures over a decade ago.
Hospitalists increasingly perform bedside procedures with ultrasound guidance.
Therefore, the Society of Hospital Medicine (SHM) Education Committee convened a group of experts and conducted a systematic literature review in order to provide recommendations for credentialing hospitalist physicians in ultrasound-guided bedside procedures. These recommendations do not include training recommendations, aside from recommendations about remedial training for hospitalists who do not pass certification. Training is a means to competence but does not guarantee it. We believe that training recommendations ought to be considered separately.
METHODS
Working Group Formation
In January 2015, the SHM Board of Directors asked the SHM Education Committee to convene the POCUS Task Force. The purpose of the task force was to develop recommendations on ultrasound guidance for bedside procedures. The SHM Education Committee appointed 3 chairs of the task force: 1 senior member of the SHM Education Committee and 2 POCUS experts. The chairs assembled a task force of 31 members that included 5 working groups, a multispecialty peer review group, and a guideline methodologist (supplemental Appendix 1). Invitation was based on members’ past contributions to SHM POCUS-related activities, up-front commitment, and declared conflicts of interest. Working group members self-identified as “hospitalists,” whereas peer reviewers were nonhospitalists but nationally recognized POCUS physician-leaders specializing in emergency medicine, cardiology, critical care medicine, and anesthesiology. Task force membership was vetted by a chair of the SHM POCUS Task Force and the Director of Education before work began. This position statement was authored by the Credentialing Working Group together with the chairs of the other 4 working groups and a guideline methodologist.
Disclosures
Signed disclosure statements of all task force members were reviewed prior to inclusion on the task force (supplemental Appendix 2); no members received honoraria for participation. Industry representatives did not contribute to the development of the guidelines nor to any conference calls or meetings.
Literature Search Strategy
A literature search was conducted by a biomedical librarian. Records from 1979 to January of 2017 were searched in Medline, Embase, CINAHL, Cochrane, and Google Scholar (supplemental Appendix 3). Search limiters were English language and adults. Articles were manually screened to exclude nonhuman or endoscopic ultrasound applications. Final article selection was based on working group consensus.
Draft Pathways
The Credentialing Working Group drafted initial and ongoing certification pathways (Figure 1 and Figure 2). The other 4 working groups from the task force were surveyed about the elements and overall appropriateness of these draft pathways. This survey and its results have already been published.12 The Credentialing Working Group then revised the certification pathways by using these survey results and codified individual aspects of these pathways into recommendations.
Development of Position Statement
Based on the Grading of Recommendation Assessment Development and Evaluation methodology, all final article selections were initially rated as either low-quality (observational studies) or unclassifiable (expert opinion).16 These initial ratings were downgraded further because of indirectness, because none of the articles involved the intervention of interest (a credentialing pathway) in a population of interest (hospitalists) measuring the outcomes of interest (patient-level outcomes).
First, the Credentialing Working Group drafted an initial position statement composed of recommendations for credentialing pathways and other general aspects of credentialing. All final article selections were incorporated as references in a draft of the position statement and compiled in a full-text compendium. Second, feedback was provided by the other 4 task force working groups, the task force peer reviewers, and the SHM Education Committee. Feedback was incorporated by the authors of this statement who were the Credentialing Working Group, the chairs of the other 4 working groups, and a guideline methodologist. Third, final suggestions from all members of the SHM POCUS Task Force and SHM Education Committee were incorporated before final approval by the SHM Board of Directors in September 2017.
RESULTS
A total of 1438 references were identified in the original search. Manual selection led to 101 articles, which were incorporated into the following 4 domains with 16 recommendations.
General Credentialing Process
Basic Cognitive Competence Can Be Certified with Written or Oral Examinations
The ABIM defines cognitive competence as having 3 abilities: “(1) to explain indications, contraindications, patient preparation methods, sterile techniques, pain management, proper techniques for handling specimens and fluids obtained, and test results; (2) to recognize and manage complications; and, (3) to clearly explain to a patient all facets of the procedure necessary to obtain informed consent.”1 These abilities can be assessed with written or oral examinations that may be integrated into simulation- or patient-based assessments.
Minimum Thresholds of Experience to Trigger the Timing of a Patient-Based Assessment Should Be Determined by Empirical Methods
Learning curves are highly variable22-25 and even plateaus may not herald basic competence.26 Expert opinion
Hospitalists Should Formally Log All of Their Attempted Procedures, Ideally in an Electronic Medical Record
Simple self-reported numbers of procedures performed often misrepresent actual experience33,34 and do not include periprocedural complications.35,36 Thus, hospitalists should report their experience with logs of all attempted procedures, both successful and unsuccessful. Such logs must include information about supervising providers (if applicable) and patient outcomes, including periprocedural adverse events,37 but they must also remain compliant with the Health Insurance Portability and Accountability Act.
Health Information Technology Service Should Routinely Pull Collations of All Attempted Procedures from Comprehensive Electronic Medical Records
Active surveillance may reduce complications by identifying hospitalists who may benefit from further training.38 In order to facilitate active surveillance systems, documentation (such as a procedure note) should be both integrated into an electronic medical record and protocol driven,39 including procedure technique, ultrasound findings, and any safety events (both near misses and adverse events).
Multiple interacting factors, including environment, patients, baseline skills, training, experience, and skills decay, affect manual competence. Certifications that are based solely on reaching minimum thresholds of experience, even when accurate, are not valid reflections of manual competence,15,40-43 and neither are those based on self-perception.44 Patient-based assessments are, thus, necessary to ensure manual competence.45-48
Certification Assessments of Manual Competence Should Combine 2 Types of Structured Instruments: Checklists and Overall Scores
Assessments based on direct observation are more reliable when formally structured.49,50 Though checklists used in observed structured clinical examinations capture many important manual skills,51-56 they do not completely reflect a hospitalist’s manual competence;57 situations may occur in which a hospitalist meets all the individual items on a checklist but cannot perform an entire procedure with basic competence. Therefore, checklists should be paired with overall scores.58-61 Both checklists and overall scores ought to be obtained from reliable and valid instruments.
Certification Assessments Should Include Feedback
Assessments without feedback are missed learning opportunities.62 Both simulation-63 and patient-based assessments should provide feedback in real time to reinforce effective behaviors and remedy faulty ones.
If Remedial Training is Needed, Simulator-Based Training Can Supplement but Not Replace Patient-Based Training
Supervised simulator-based training allows hospitalists to master basic components of a procedure64 (including orientation to equipment, sequence of operations, dexterity, ultrasound anatomy, and real-time guidance technique) while improving both cognitive and manual skills.42,43,65-71 In addition to their role in basic training (which is outside the scope of this position statement), simulators can be useful for remedial training. To be sufficient for hospitalists who do not pass their patient-based assessments, however, remedial training that begins with simulation must also include patient-based training and assessment.72-75
Initial Credentialing Process
A Minimum Threshold of Experience Should Be Reached before Patient-Based Assessments are Conducted (Figure 1)
Initial Certification Assessments Should Ideally Begin on Simulators
Simulators allow the assurance of safe manual skills, including proper needle insertion techniques and disposal of sharp objects.3,79 If simulators are not available, however, then patient-based training and assessments can still be performed under direct observation. Safe performance of ultrasound-guided procedures during patient-based assessments (without preceding simulator-based assessments) is sufficient to certify manual competence.
Ongoing Credentialing
Certification to Perform Ultrasound-Guided Procedures Should Be Routinely Re-Evaluated During Ongoing Credentialing (Figure 2)
Observed Patient-Based Assessments Should Occur When a Periprocedural Safety Event Occurs that is Potentially Caused by “Provider Error”
Safety events include both near misses and adverse events. Information about both is ideally “flagged” and “pushed” to hospitalist group leaders by active surveillance and reporting systems. Once reviewed, if a safety event is considered to potentially have been caused by provider error (including knowledge- and skill-based errors),83 then the provider who performed the procedure should undergo an observed patient-based assessment.
Simulation-Based Practice Can Supplement Patient-Based Experience for Ongoing Credentialing
When hospitalists do not achieve a minimum threshold of patient-based experience since the antecedent certification, simulation-based training can supplement their patient-based experience.
Credentialing Infrastructure
Hospitalists Themselves Should Not Bear the Financial Costs of Developing and Maintaining Training and Certification Programs for Ultrasound-Guided Procedures
Equipment and personnel costs
Assessors Should Be Unbiased Expert Providers Who Have Demonstrated Mastery in Performance of the Procedure Being Assessed and Regularly Perform It in a Similar Practice Environment
Assessors should be expert providers who regularly perform the ultrasound-guided procedure in a similar practice environment.9,89-94 For example, providers who are not hospitalists but who are experts in an ultrasound-guided procedure and commonly perform it on the hospital wards would be acceptable assessors. However, a radiologist who only performs that procedure in a fully-staffed interventional radiology suite with fluoroscopy or computed tomography guidance would not be an acceptable assessor. More than 1 assessor may balance idiosyncratic assessments;95 but when assessments are well structured, additional assessors are generally not needed.18
If Intramural Assessors Are Not Available, Extramural Assessors May Be Considered
Intramural assessors are generally preferred because of familiarity with the local practice environment, including the available procedure kits and typical patient characteristics. Nevertheless, extramural assessors27,77,85,96 may theoretically provide even more valid assessments than intramural ones because extramural assessors are neither influenced by relationships with local hospitalists nor biased by local hospitalists’ skills.
DISCUSSION
There are no high-quality randomized trials in support of a single credentialing pathway over any other.94,102 The credentialing pathways at the center of this position statement are based on expert opinion. Our methods can be criticized straightaway, therefore, for reliance on the experience and expertise of our working group and task force. Any position statement written without high-quality supportive evidence would be appropriately subject to the same criticism. Without evidence in support of an overall pathway, we codified specific aspects of the pathways into 16 individual recommendations.
Patient-level outcomes do not back these recommendations. Consider, for example, our recommendation that certification assessments be made from structured instruments and not simply from an assessor’s gestalt. Here, the basis is not improved patient-level outcomes from a trial (such as reduced complications or increased procedural success) but improved psychometric performance from reliability studies. The body of evidence for our recommendations is similarly indirect, mostly because the outcomes studied are more proximate and, thus, less meaningful than patient-level outcomes, which are the outcomes of greatest interest but are woefully understudied for clinical competence.17,97,103
The need for high-quality evidence is most pronounced in distinguishing how recommendations should be modified for various settings. Wide variations in resources and patient-mix will make some recommendations impracticable, meaning that they could not be carried out with available resources. For example, our recommendation that credentialing decisions should ultimately rely on certifications made by assessors during patient-based assessments may not be practicable at small, rural hospitals. Such hospitals may not have access to local assessors, and they may not admit enough patients who need the types of ultrasound-guided procedures for which hospitalists seek certification (especially given the need to coordinate the schedules of patients, procedure-performing hospitalists, and assessors).
Regardless of whether some or all hospitalists at a particular hospital are expected to perform bedside procedures, technology may help to improve the practicability of our recommendations. For example, simulators may evolve to replace actual patient-level experience in achieving minimum thresholds. Certification assessments of manual skills may even someday occur entirely on simulators. Real-time high-definition video streaming enhanced with multiple cameras may allow for remote assessments. Until such advances mature, high-quality patient-level data should be sought through additional research to refine our current recommendations.
We hope that these recommendations will improve how basic competence in ultrasound-guided bedside procedures is assessed. Our ultimate goal is to improve how hospitalists perform these procedures. Patient safety is, therefore, considered paramount to cost. Nevertheless, the hospital administrative leaders and privileging committee members on our Task Force concluded that many hospitals have been seeking guidance on credentialing for bedside procedures, and the likely difficulties of implementing our recommendations (including cost) would not be prohibitive at most hospitals, especially given recognition that these recommendations can be tailored to each setting.
Acknowledgments
Collaborators from SHM POCUS Task Force are Saaid Abdel-Ghani, Michael Blaivas, Dan Brotman, Carolina Candotti, Jagriti Chadha, Joel Cho, Ria Dancel, Ricardo Franco, Richard Hoppmann, Susan Hunt, Venkat Kalidindi, Ketino Kobaidze, Josh Lenchus, Benji Mathews, Satyen Nichani, Vicki Noble, Martin Perez, Nitin Puri, Aliaksei Pustavoitau, Sophia Rodgers, Gerard Salame, Daniel Schnobrich, Kirk Spencer, Vivek Tayal, Jeff Bates, Anjali Bhagra, Kreegan Reierson, Robert Arntfield, Paul Mayo, Loretta Grikis.
Disclosure
Brian P. Lucas received funding from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, and National Center for Translational Science (UL1TR001086). Nilam Soni received funding from the Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative (HX002263-01A1). The contents of this publication do not represent the views of the United States Department of Veterans Affairs or the United States Government.
1. American Board of Internal Medicine. Policies and procedures for certification. Philadelphia: American Board of Internal Medicine; 2006.
2. Nichani S, Fitterman N, Lukela M, Crocker J; Society of Hospital Medicine. The Core Competencies in Hospital Medicine 2017 Revision. Section 2: Procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54 PubMed
3. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. PubMed
4. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. PubMed
5. Brown GM, Otremba M, Devine LA, Gray C, Millington SJ, Ma IW. Defining competencies for ultrasound-guided bedside procedures: consensus opinions from Canadian physicians. J Ultrasound Med. 2016;35(1):129-141. PubMed
6. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. PubMed
7. Kreisman RD. With ED ultrasound, credentialing is at issue. ED Legal Letter. 2010;21:102-103.
8. Goudie AM. Credentialing a new skill: what should the standard be for emergency department ultrasound in Australasia? Emerg Med Australas. 2010;22:263-264. PubMed
9. Maizel J, Guyomarc HL, Henon P, et al. Residents learning ultrasound-guided catheterization are not sufficiently skilled to use landmarks. Crit Care. 2014;18(1):R36. doi:10.1186/cc13741. PubMed
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. PubMed
11. Amini R, Adhikari S, Fiorello A. Ultrasound competency assessment in emergency medicine residency programs. Acad Emerg Med. 2014;21(7):799-801. PubMed
12. Jensen T, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. PubMed
13. Chang W. Is hospitalist proficiency in bedside procedures in decline? The Hospitalist. 2012. http://www.the-hospitalist.org/hospitalist/article/125236/patient-safety/hospitalist-proficiency-bedside-procedures-decline. Accessed September 30, 2017.
14. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties Performing Paracentesis Procedures at University Hospitals: Implications for Training and Certification. J Hosp Med. 2014;9(3):162-168. PubMed
15. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ Procedural Experience Does Not Ensure Competence: A Research Synthesis. J Grad Med Educ. 2017;9(2):201-208. PubMed
16. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. PubMed
17. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303-1310. PubMed
18. Miller GE. The assessment of clinical skills/competence/performance. Acad Med. 1990;65(9 Suppl):S63-S67. PubMed
19. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
20. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IWY. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. PubMed
21. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. PubMed
22. Heegeman DJ, Kieke B Jr. Learning curves, credentialing, and the need for ultrasound fellowships. Acad Emerg Med. 2003;10:404-405. PubMed
23. Jang TB, Ruggeri W, Dyne P, Kaji AH. The learning curve of resident physicians using emergency ultrasonography for cholelithaisis and cholecystitis. Acad Emerg Med. 2010;17(11):1247-1252. PubMed
24. Akhtar MI, Hamid M. Ultrasound guided central venous access; a review of literature. Anaesth Pain Intensive Care. 2015;19:317-322.
25. Bahl A, Yunker A. Assessment of the numbers–based model for evaluation of resident competency in emergency ultrasound core applications. J Emerg Med Trauma Acute Care. 2015;2015(5). doi:10.5339/jemtac.2015.5
26. Cazes N, Desmots F, Geffroy Y, Renard A, Leyral J, Chaumoitre K. Emergency ultrasound: a prospective study on sufficient adequate training for military doctors. Diagn Interv Imaging. 2013;94(11):1109-1115. PubMed
27. Arntfield RT, Millington SJ, Ainsworth CD, et al. Canadian recommendations for critical care ultrasound training and competency for the Canadian critical care society. Can Respir J. 2014;21(16):341-345.
28. Bolsin S, Colson M. The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care. 2000;12(5):433-438. PubMed
29. de Oliveira Filho GR, Helayel PE, da Conceição DB, Garzel IS, Pavei P, Ceccon MS. Learning curves and mathematical models for interventional ultrasound basic skills. Anaesth Analg. 2008;106(2):568-573. PubMed
30. Starkie T, Drake EJ. Assessment of procedural skills training and performance in anesthesia using cumulative sum analysis (cusum). Can J Anaesth. 2013;60(12):1228-1239. PubMed
31. Tierney D. Competency cut-point identification derived from a mastery learning cohort approach: A hybrid model. Ultrasound Med Biol. 2015;41:S19.
32. Rankin JH, Elkhunovich MA, Rangarajan V, Chilstrom M, Mailhot T. Learning Curves for Ultrasound Assessment of Lumbar Puncture Insertion Sites: When is Competency Established? J Emerg Med. 2016;51(1):55-62. PubMed
33. Klasko SK, Cummings RV, Glazerman LR. Resident data collection: Do the numbers add up? Am J Obstet Gynecol. 1995;172(4 Pt 1):1312-1316. PubMed
34. Tierney D. Development & analysis of a mobile POCUS tracking tool. Ultrasound Med Biol. 2015;41(suppl 4):S31.
35. Sethi MV, Zimmer J, Ure B, Lacher M. Prospective assessment of complications on a daily basis is essential to determine morbidity and mortality in routine pediatric surgery. J Pediatr Surg. 2016;51(4):630-633. PubMed
36. Fisher JC, Kuenzler KA, Tomita SS, Sinha P, Shah P, Ginsburg HB. Increased capture of pediatric surgical complications utilizing a novel case-log web application to enhance quality improvement. J Pediatr Surg. 2017;52(1):166-171. PubMed
37. Rethans JJ, Norcini JJ, Barón-Maldonado M, et al. The relationship between competence and performance: implications for assessing practice performance. Med Educ. 2002;36(10):901-909. PubMed
38. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
39. Society of Critical Care Medicine Ultrasound Certification Task Force. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. http://journals.lww.com/ccmjournal/Documents/Critical%20Care%20Ultrasound.pdf Published 2013. Accessed February 2, 2017.
40. Carraccio C, Wolfsthal SD, Englander R, Ferentz K, Martin C. Shifting paradigms: from Flexner to competencies. Acad Med. 2002;77(5):361-367. PubMed
41. Clark EG, Paparello JJ, Wayne DB, et al. Use of a national continuing medical education meeting to provide simulation-based training in temporary hemodialysis catheter insertion skills: a pre-test post-test study. Can J Kidney Health Dis. 2014;1:25-31. PubMed
42. Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. PubMed
43. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. PubMed
44. Davis DA, Mazmanian PE, Fordis M, Van Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102. PubMed
45. Shah J, Darzi A. Surgical skills assessment: an ongoing debate. BJU Int. 2001;88(7):655-660. PubMed
46. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. PubMed
47. Tolsgaard MG, Todsen T, Sorensen JL, et al. International multispecialty consensus on how to evaluate ultrasound competence: a Delphi consensus survey. PLOS One. 2013;8(2):e57687. doi:10.1371/journal.pone.0057687 PubMed
48. Moureau N, Laperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. PubMed
49. Feldman LS, Hagarty S, Ghitulescu G, Stanbridge D, Fried GM. Relationship between objective assessment of technical skills and subjective in-training evaluations in surgical residents. J Am Coll Surg. 2004;198(1):105-110. PubMed
50. Baker S, Willey B, Mitchell C. The attempt to standardize technical and analytic competence in sonography education. J Diagn Med Sonogr. 2011;27(5):203-211.
51. Tolsgaard MG, Ringsted C, Dreisler E, et al. Reliable and valid assessment of ultrasound operator competence in obstetrics and gynecology. Ultrasound Obstet Gynecol. 2014;43(4):437-443. PubMed
52. Rice J, Crichlow A, Baker M, et al. An assessment tool for the placement of ultrasound-guided peripheral intravenous access. J Grad Med Educ. 2016;8(2):202-207. PubMed
53. Hartman N, Wittler M, Askew K, Hiestand B, Manthey D. Validation of a performance checklist for ultrasound-guided internal jubular central lines for use in procedural instruction and assessment. Postgrad Med J. 2017;93(1096):67-70. PubMed
54. Primdahl SC, Todsen T, Clemmesen L, et al. Rating scale for the assessment of competence in ultrasound-guided peripheral vascular access—a Delphi Consensus Study. J Vasc Access. 2016;17(5):440-445.
55. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
56. Berg K, Riesenberg LA, Berg D, et al. The development of a validated checklist for radial arterial line placement: preliminary results. Am J Med Qual. 2014;29(3):242-246. PubMed
57. Walzak A, Bacchus M, Schaefer MP, et al. Diagnosing technical competence in six bedside procedures: comparing checklists and a global rating scale in the assessment of resident performance. Acad Med. 2015;90(8):1100-1108. PubMed
58. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for femoral venous catheterization: preliminary results. Am J Med Qual. 2014;29(5):445-450. PubMed
59. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for paracentesis: preliminary results. Am J Med Qual. 2013;28(3):227-231. PubMed
60. Huang GC, Newman LR, Schwartzstein RM, et al. Procedural competence in internal medicine residents: validity of a central venous catheter insertion assessment instrument. Acad Med. 2009;84(8):1127-1134. PubMed
61. Salamonsen M, McGrath D, Steiler G, et al. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
62. Boniface K, Yarris LM. Emergency ultrasound: Leveling the training and assessment landscape. Acad Emerg Med. 2014;21(7):803-805. PubMed
63. Boyle E, O’Keeffe D, Naughton P, Hill A, McDonnell C, Moneley D. The importance of expert feedback during endovascular simulator training. J Vasc Surg. 2011;54(1):240-248.e1. PubMed
64. Langhan TS, Rigby IJ, Walker IW, Howes D, Donnon T, Lord JA. Simulation-based training in critical resuscitation procedures improves residents’ competence. CJEM. 2009;11(6):535-539. PubMed
65. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. PubMed
66. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
67. Barsuk JH, Cohen ER, Vozenilek JA, O’Connor LM, McGaghie WC, Wayne DB. Simulation-based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23-27. PubMed
68. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706-711. PubMed
69. Ross JG. Simulation and psychomotor skill acquisition: A review of the literature. Clin Simul Nurs. 2012;8(9):e429-e435.
70. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
71. McSparron JI, Michaud GC, Gordan PL, et al. Simulation for skills-based education in pulmonary and critical care medicine. Ann Am Thorac Soc. 2015;12(4):579-586. PubMed
72. Kneebone RL, Scott W, Darzi A, Horrocks M. Simulation and clinical practice: strengthening the relationship. Med Educ. 2004;38(10):1095-1102. PubMed
73. Mema B, Harris I. The barriers and facilitators to transfer of ultrasound-guided central venous line skills from simulation to practice: exploring perceptions of learners and supervisors. Teach Learn Med. 2016;28(2):115-124. PubMed
74. Castanelli DJ. The rise of simulation in technical skills teaching and the implications for training novices in anaestheia. Anaesth Intensive Care. 2009;37(6):903-910. PubMed
75. McGaghie WC, Issenberg SB, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014;48(4):375-385. PubMed
76. Langlois SLP. Focused ultrasound training for clinicians. Crit Care Med. 2007;35(5 suppl):S138-S143.
77. Price S, Via G, Sloth E, et al. Echocardiography practice, training and accreditation in the intesive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008;6:49-83. PubMed
78. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. PubMed
79. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. PubMed
80. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. PubMed
81. Sliman Sean, Amundson S, Shaw D, Phan JN, Waalen J, Kimura B. Recently-acquired cardiac ultrasound skills are rapidly lost when not used: implications for competency in physician imaging. J Amer Coll Cardiol. 2016;67(13S):1569.
82. Kessler CS, Leone KA. The current state of core competency assessment in emergency medicine and a future research agenda: recommendations of the working group on assessment of observable learner performance. Acad Emerg Med. 2012;19(12):1354-1359. PubMed
83. Chang A, Schyve PM, Croteau RJ, O’Leary DS, Loeb JM. The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care. 2005;17(2):95-105. PubMed
84. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. PubMed
85. Das D, Kapoor M, Brown C, Ndubuisi A, Gupta S. Current status of emergency department attending physician ultrasound credentialing and quality assurance in the United States. Crit Ultrasound J. 2016;8(1):6-12. PubMed
86. Ndubuisi AK, Gupta S, Brown C, Das D. Current status and future issues in emergency department attending physician ultrasound credentialing. Ann Emerg Med. 2014;64(45):S27-S28.
87. Tandy Tk, Hoffenberg S. Emergency department ultrasound services by emergency physicians: model for gaining hospital approval. Ann Emerg Med. 1997;29(3):367-374. PubMed
88. Lewiss RE, Saul T, Del Rios M. Acquiring credentials in bedside ultrasound: a cross-sectional survey. BMJ Open. 2013;3:e003502. doi:10.1136/bmjopen-2013-003502 PubMed
89. Lanoix R. Credentialing issues in emergency ultrasonography. Emerg Med Clin North Am. 1997;15(4):913-920. PubMed
90. Scalea T, Rodriquez A, Chiu WC, et al. Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma. 1999;46(3):466-472. PubMed
91. Hertzberg BS, Kliewer MA, Bowie JD, et al. Physician training requirements in sonography: how many cases are needed for competence? AJR. 2000;174(5):1221-1227. PubMed
92. Blaivas M, Theodoro DL, Sierzenski P. Proliferation of ultrasound fellowships in emergency medicine: how do we ensure future experts are expertly trained? Acad Emerg Med. 2002;9(8):863-864. PubMed
93. Bodenham AR. Editorial II: Ultrasound imaging by anaesthetists: training and accreditation issues. Br J Anaesth. 2006;96(4):414-417. PubMed
94. Williamson JP, Twaddell SH, Lee YCG, et al. Thoracic ultrasound recognition of competence: A position paper of the Thoracic Society of Australia and New Zealand. Respirology. 2017;22(2):405-408. PubMed
95. Harrison G. Summative clinical competency assessment: a survey of ultrasound practitioners’ views. Ultrasound. 2015;23(1):11-17. PubMed
96. Evans LV, Morse JL, Hamann CJ, Osborne M, Lin Z, D'Onofrio G. The development of an independent rater system to assess residents' competence in invasive procedures. Acad Med. 2009;84(8):1135-1143. PubMed
97. Wass V, Van der Vleuten C, Shatzer J, Jones R. Assessment of clinical competence. Lancet. 2001;357(9260):945-949. PubMed
98. Arntfield RT. The utility of remote supervision with feedback as a method to deliver high-volume critical care ultrasound training. J Crit Care. 2015;30(2):441.e1-e6. PubMed
99. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound: consensus recommendations from the 2008 Council of Emergency Residency Directors Conference. Acad Emerg Med. 2009;16:S32-S36. PubMed
100. Yu E. The assessment of technical skills in a cardiology training program: is the ITER sufficient? Can J Cardiol. 2000;16(4):457-462. PubMed
101. Todsen T, Tolsgaard MG, Olsen BH, et al. Reliable and valid assessment of point-of-care ultrasonography. Ann Surg. 2015;261(2):309-315. PubMed
102. Stein JC, Nobay F. Emergency department ultrasound credentialing: a sample policy and procedure. J Emerg Med. 2009;37(2):153-159. PubMed
103. Chen FM. Burstin H, Huntington J. The importance of clinical outcomes in medical education research. Med Educ. 2005;39(4):350-351. PubMed
104. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1:48-56. PubMed
105. ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013;5(1):157-158. PubMed
106. Castillo J, Caruana CJ, Wainwright D. The changing concept of competence and categorisation of learning outcomes in Europe: Implications for the design of higher education radiography curricula at the European level. Radiography. 2011;17(3):230-234.
107. Goldstein SR. Accreditation, certification: why all the confusion? Obstet Gynecol. 2007;110(6):1396-1398. PubMed
108. Moore CL. Credentialing and reimbursement in point-of-care ultrasound. Clin Pediatr Emerg Med. 2011;12(1):73-77. PubMed
109. ten Cate O, Scheele F. Competency-based postgraduate training: can we bridge the gap between theory and clinical practice? Acad Med. 2007;82(6):542-547. PubMed
110. Abuhamad AZ, Benacerraf BR, Woletz P, Burke BL. The accreditation of ultrasound practices: impact on compliance with minimum performance guidelines. J Ultrasound Med. 2004;23(8):1023-1029. PubMed
1. American Board of Internal Medicine. Policies and procedures for certification. Philadelphia: American Board of Internal Medicine; 2006.
2. Nichani S, Fitterman N, Lukela M, Crocker J; Society of Hospital Medicine. The Core Competencies in Hospital Medicine 2017 Revision. Section 2: Procedures. J Hosp Med. 2017;12(4 Suppl 1):S44-S54 PubMed
3. Lucas BP, Asbury JK, Franco-Sadud R. Training future hospitalists with simulators: a needed step toward accessible, expertly performed bedside procedures. J Hosp Med. 2009;4(7):395-396. PubMed
4. Schnobrich DJ, Gladding S, Olson APJ, Duran-Nelson A. Point-of-care ultrasound in internal medicine: a national survey of educational leadership. J Grad Med Educ. 2013;5(3):498-502. PubMed
5. Brown GM, Otremba M, Devine LA, Gray C, Millington SJ, Ma IW. Defining competencies for ultrasound-guided bedside procedures: consensus opinions from Canadian physicians. J Ultrasound Med. 2016;35(1):129-141. PubMed
6. Vaisman A, Cram P. Procedural competence among faculty in academic health centers: challenges and future directions. Acad Med. 2017;92(1):31-34. PubMed
7. Kreisman RD. With ED ultrasound, credentialing is at issue. ED Legal Letter. 2010;21:102-103.
8. Goudie AM. Credentialing a new skill: what should the standard be for emergency department ultrasound in Australasia? Emerg Med Australas. 2010;22:263-264. PubMed
9. Maizel J, Guyomarc HL, Henon P, et al. Residents learning ultrasound-guided catheterization are not sufficiently skilled to use landmarks. Crit Care. 2014;18(1):R36. doi:10.1186/cc13741. PubMed
10. American College of Emergency Physicians. Ultrasound guidelines: emergency, point-of-care, and clinical ultrasound guidelines in medicine. Ann Emerg Med. 2017;69(5):e27-e54. PubMed
11. Amini R, Adhikari S, Fiorello A. Ultrasound competency assessment in emergency medicine residency programs. Acad Emerg Med. 2014;21(7):799-801. PubMed
12. Jensen T, Soni NJ, Tierney DM, Lucas BP. Hospital privileging practices for bedside procedures: a survey of hospitalist experts. J Hosp Med. 2017;12(10):836-839. PubMed
13. Chang W. Is hospitalist proficiency in bedside procedures in decline? The Hospitalist. 2012. http://www.the-hospitalist.org/hospitalist/article/125236/patient-safety/hospitalist-proficiency-bedside-procedures-decline. Accessed September 30, 2017.
14. Barsuk JH, Feinglass J, Kozmic SE, Hohmann SF, Ganger D, Wayne DB. Specialties Performing Paracentesis Procedures at University Hospitals: Implications for Training and Certification. J Hosp Med. 2014;9(3):162-168. PubMed
15. Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Residents’ Procedural Experience Does Not Ensure Competence: A Research Synthesis. J Grad Med Educ. 2017;9(2):201-208. PubMed
16. Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401-406. PubMed
17. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303-1310. PubMed
18. Miller GE. The assessment of clinical skills/competence/performance. Acad Med. 1990;65(9 Suppl):S63-S67. PubMed
19. Grover S, Currier PF, Elinoff JM, Mouchantaf KJ, Katz JT, McMahon GT. Development of a test to evaluate residents knowledge of medical procedures. J Hosp Med. 2009;4(7):430-432. PubMed
20. Millington SJ, Wong RY, Kassen BO, Roberts JM, Ma IWY. Improving internal medicine residents’ performance, knowledge, and confidence in central venous catheterization using simulators. J Hosp Med. 2009;4(7):410-416. PubMed
21. Lenchus JD, Carvalho CM, Ferreri K, et al. Filling the void: defining invasive bedside procedural competency for internal medicine residents. J Grad Med Educ. 2013;5(4):605-612. PubMed
22. Heegeman DJ, Kieke B Jr. Learning curves, credentialing, and the need for ultrasound fellowships. Acad Emerg Med. 2003;10:404-405. PubMed
23. Jang TB, Ruggeri W, Dyne P, Kaji AH. The learning curve of resident physicians using emergency ultrasonography for cholelithaisis and cholecystitis. Acad Emerg Med. 2010;17(11):1247-1252. PubMed
24. Akhtar MI, Hamid M. Ultrasound guided central venous access; a review of literature. Anaesth Pain Intensive Care. 2015;19:317-322.
25. Bahl A, Yunker A. Assessment of the numbers–based model for evaluation of resident competency in emergency ultrasound core applications. J Emerg Med Trauma Acute Care. 2015;2015(5). doi:10.5339/jemtac.2015.5
26. Cazes N, Desmots F, Geffroy Y, Renard A, Leyral J, Chaumoitre K. Emergency ultrasound: a prospective study on sufficient adequate training for military doctors. Diagn Interv Imaging. 2013;94(11):1109-1115. PubMed
27. Arntfield RT, Millington SJ, Ainsworth CD, et al. Canadian recommendations for critical care ultrasound training and competency for the Canadian critical care society. Can Respir J. 2014;21(16):341-345.
28. Bolsin S, Colson M. The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care. 2000;12(5):433-438. PubMed
29. de Oliveira Filho GR, Helayel PE, da Conceição DB, Garzel IS, Pavei P, Ceccon MS. Learning curves and mathematical models for interventional ultrasound basic skills. Anaesth Analg. 2008;106(2):568-573. PubMed
30. Starkie T, Drake EJ. Assessment of procedural skills training and performance in anesthesia using cumulative sum analysis (cusum). Can J Anaesth. 2013;60(12):1228-1239. PubMed
31. Tierney D. Competency cut-point identification derived from a mastery learning cohort approach: A hybrid model. Ultrasound Med Biol. 2015;41:S19.
32. Rankin JH, Elkhunovich MA, Rangarajan V, Chilstrom M, Mailhot T. Learning Curves for Ultrasound Assessment of Lumbar Puncture Insertion Sites: When is Competency Established? J Emerg Med. 2016;51(1):55-62. PubMed
33. Klasko SK, Cummings RV, Glazerman LR. Resident data collection: Do the numbers add up? Am J Obstet Gynecol. 1995;172(4 Pt 1):1312-1316. PubMed
34. Tierney D. Development & analysis of a mobile POCUS tracking tool. Ultrasound Med Biol. 2015;41(suppl 4):S31.
35. Sethi MV, Zimmer J, Ure B, Lacher M. Prospective assessment of complications on a daily basis is essential to determine morbidity and mortality in routine pediatric surgery. J Pediatr Surg. 2016;51(4):630-633. PubMed
36. Fisher JC, Kuenzler KA, Tomita SS, Sinha P, Shah P, Ginsburg HB. Increased capture of pediatric surgical complications utilizing a novel case-log web application to enhance quality improvement. J Pediatr Surg. 2017;52(1):166-171. PubMed
37. Rethans JJ, Norcini JJ, Barón-Maldonado M, et al. The relationship between competence and performance: implications for assessing practice performance. Med Educ. 2002;36(10):901-909. PubMed
38. Duncan DR, Morgenthaler TI, Ryu JH, Daniels CE. Reducing iatrogenic risk in thoracentesis: establishing best practice via experiential training in a zero-risk environment. Chest. 2009;135(5):1315-1320. PubMed
39. Society of Critical Care Medicine Ultrasound Certification Task Force. Recommendations for achieving and maintaining competence and credentialing in critical care ultrasound with focused cardiac ultrasound and advanced critical care echocardiography. http://journals.lww.com/ccmjournal/Documents/Critical%20Care%20Ultrasound.pdf Published 2013. Accessed February 2, 2017.
40. Carraccio C, Wolfsthal SD, Englander R, Ferentz K, Martin C. Shifting paradigms: from Flexner to competencies. Acad Med. 2002;77(5):361-367. PubMed
41. Clark EG, Paparello JJ, Wayne DB, et al. Use of a national continuing medical education meeting to provide simulation-based training in temporary hemodialysis catheter insertion skills: a pre-test post-test study. Can J Kidney Health Dis. 2014;1:25-31. PubMed
42. Barsuk JH, Cohen ER, Caprio T, McGaghie WC, Simuni T, Wayne DB. Simulation-based education with mastery learning improves residents’ lumbar puncture skills. Neurology. 2012;79(2):132-137. PubMed
43. Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37(10):2697-2701. PubMed
44. Davis DA, Mazmanian PE, Fordis M, Van Harrison R, Thorpe KE, Perrier L. Accuracy of physician self-assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094-1102. PubMed
45. Shah J, Darzi A. Surgical skills assessment: an ongoing debate. BJU Int. 2001;88(7):655-660. PubMed
46. Lamperti M, Bodenham AR, Pittiruti M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. 2012;38(7):1105-1117. PubMed
47. Tolsgaard MG, Todsen T, Sorensen JL, et al. International multispecialty consensus on how to evaluate ultrasound competence: a Delphi consensus survey. PLOS One. 2013;8(2):e57687. doi:10.1371/journal.pone.0057687 PubMed
48. Moureau N, Laperti M, Kelly LJ, et al. Evidence-based consensus on the insertion of central venous access devices: definition of minimal requirements for training. Br J Anaesth. 2013;110(3):347-356. PubMed
49. Feldman LS, Hagarty S, Ghitulescu G, Stanbridge D, Fried GM. Relationship between objective assessment of technical skills and subjective in-training evaluations in surgical residents. J Am Coll Surg. 2004;198(1):105-110. PubMed
50. Baker S, Willey B, Mitchell C. The attempt to standardize technical and analytic competence in sonography education. J Diagn Med Sonogr. 2011;27(5):203-211.
51. Tolsgaard MG, Ringsted C, Dreisler E, et al. Reliable and valid assessment of ultrasound operator competence in obstetrics and gynecology. Ultrasound Obstet Gynecol. 2014;43(4):437-443. PubMed
52. Rice J, Crichlow A, Baker M, et al. An assessment tool for the placement of ultrasound-guided peripheral intravenous access. J Grad Med Educ. 2016;8(2):202-207. PubMed
53. Hartman N, Wittler M, Askew K, Hiestand B, Manthey D. Validation of a performance checklist for ultrasound-guided internal jubular central lines for use in procedural instruction and assessment. Postgrad Med J. 2017;93(1096):67-70. PubMed
54. Primdahl SC, Todsen T, Clemmesen L, et al. Rating scale for the assessment of competence in ultrasound-guided peripheral vascular access—a Delphi Consensus Study. J Vasc Access. 2016;17(5):440-445.
55. Berg D, Berg K, Riesenberg LA, et al. The development of a validated checklist for thoracentesis: preliminary results. Am J Med Qual. 2013;28(3):220-226. PubMed
56. Berg K, Riesenberg LA, Berg D, et al. The development of a validated checklist for radial arterial line placement: preliminary results. Am J Med Qual. 2014;29(3):242-246. PubMed
57. Walzak A, Bacchus M, Schaefer MP, et al. Diagnosing technical competence in six bedside procedures: comparing checklists and a global rating scale in the assessment of resident performance. Acad Med. 2015;90(8):1100-1108. PubMed
58. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for femoral venous catheterization: preliminary results. Am J Med Qual. 2014;29(5):445-450. PubMed
59. Riesenberg LA, Berg K, Berg D, et al. The development of a validated checklist for paracentesis: preliminary results. Am J Med Qual. 2013;28(3):227-231. PubMed
60. Huang GC, Newman LR, Schwartzstein RM, et al. Procedural competence in internal medicine residents: validity of a central venous catheter insertion assessment instrument. Acad Med. 2009;84(8):1127-1134. PubMed
61. Salamonsen M, McGrath D, Steiler G, et al. A new instrument to assess physician skill at thoracic ultrasound, including pleural effusion markup. Chest. 2013;144(3):930-934. PubMed
62. Boniface K, Yarris LM. Emergency ultrasound: Leveling the training and assessment landscape. Acad Emerg Med. 2014;21(7):803-805. PubMed
63. Boyle E, O’Keeffe D, Naughton P, Hill A, McDonnell C, Moneley D. The importance of expert feedback during endovascular simulator training. J Vasc Surg. 2011;54(1):240-248.e1. PubMed
64. Langhan TS, Rigby IJ, Walker IW, Howes D, Donnon T, Lord JA. Simulation-based training in critical resuscitation procedures improves residents’ competence. CJEM. 2009;11(6):535-539. PubMed
65. Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4(7):397-403. PubMed
66. Lenchus JD. End of the “see one, do one, teach one” era: the next generation of invasive bedside procedural instruction. J Am Osteopath Assoc. 2010;110(6):340-346. PubMed
67. Barsuk JH, Cohen ER, Vozenilek JA, O’Connor LM, McGaghie WC, Wayne DB. Simulation-based education with mastery learning improves paracentesis skills. J Grad Med Educ. 2012;4(1):23-27. PubMed
68. McGaghie WC, Issenberg SB, Cohen ER, Barsuk JH, Wayne DB. Does simulation-based medical education with deliberate practice yield better results than traditional clinical education? A meta-analytic comparative review of the evidence. Acad Med. 2011;86(6):706-711. PubMed
69. Ross JG. Simulation and psychomotor skill acquisition: A review of the literature. Clin Simul Nurs. 2012;8(9):e429-e435.
70. Barsuk JH, Cohen ER, Potts S, et al. Dissemination of a simulation-based mastery learning intervention reduces central line-associated bloodstream infections. BMJ Qual Saf. 2014;23(9):749-756. PubMed
71. McSparron JI, Michaud GC, Gordan PL, et al. Simulation for skills-based education in pulmonary and critical care medicine. Ann Am Thorac Soc. 2015;12(4):579-586. PubMed
72. Kneebone RL, Scott W, Darzi A, Horrocks M. Simulation and clinical practice: strengthening the relationship. Med Educ. 2004;38(10):1095-1102. PubMed
73. Mema B, Harris I. The barriers and facilitators to transfer of ultrasound-guided central venous line skills from simulation to practice: exploring perceptions of learners and supervisors. Teach Learn Med. 2016;28(2):115-124. PubMed
74. Castanelli DJ. The rise of simulation in technical skills teaching and the implications for training novices in anaestheia. Anaesth Intensive Care. 2009;37(6):903-910. PubMed
75. McGaghie WC, Issenberg SB, Barsuk JH, Wayne DB. A critical review of simulation-based mastery learning with translational outcomes. Med Educ. 2014;48(4):375-385. PubMed
76. Langlois SLP. Focused ultrasound training for clinicians. Crit Care Med. 2007;35(5 suppl):S138-S143.
77. Price S, Via G, Sloth E, et al. Echocardiography practice, training and accreditation in the intesive care: document for the World Interactive Network Focused on Critical Ultrasound (WINFOCUS). Cardiovasc Ultrasound. 2008;6:49-83. PubMed
78. Blehar DJ, Barton B, Gaspari RJ. Learning curves in emergency ultrasound education. Acad Emerg Med. 2015;22(5):574-582. PubMed
79. Ault MJ, Rosen BT, Ault B. The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative. J Gen Intern Med. 2006;21(5):514-517. PubMed
80. Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85(10 Suppl):S9-S12. PubMed
81. Sliman Sean, Amundson S, Shaw D, Phan JN, Waalen J, Kimura B. Recently-acquired cardiac ultrasound skills are rapidly lost when not used: implications for competency in physician imaging. J Amer Coll Cardiol. 2016;67(13S):1569.
82. Kessler CS, Leone KA. The current state of core competency assessment in emergency medicine and a future research agenda: recommendations of the working group on assessment of observable learner performance. Acad Emerg Med. 2012;19(12):1354-1359. PubMed
83. Chang A, Schyve PM, Croteau RJ, O’Leary DS, Loeb JM. The JCAHO patient safety event taxonomy: a standardized terminology and classification schema for near misses and adverse events. Int J Qual Health Care. 2005;17(2):95-105. PubMed
84. Sawyer T, White M, Zaveri P, et al. Learn, see, practice, prove, do, maintain: an evidence-based pedagogical framework for procedural skill training in medicine. Acad Med. 2015;90(8):1025-1033. PubMed
85. Das D, Kapoor M, Brown C, Ndubuisi A, Gupta S. Current status of emergency department attending physician ultrasound credentialing and quality assurance in the United States. Crit Ultrasound J. 2016;8(1):6-12. PubMed
86. Ndubuisi AK, Gupta S, Brown C, Das D. Current status and future issues in emergency department attending physician ultrasound credentialing. Ann Emerg Med. 2014;64(45):S27-S28.
87. Tandy Tk, Hoffenberg S. Emergency department ultrasound services by emergency physicians: model for gaining hospital approval. Ann Emerg Med. 1997;29(3):367-374. PubMed
88. Lewiss RE, Saul T, Del Rios M. Acquiring credentials in bedside ultrasound: a cross-sectional survey. BMJ Open. 2013;3:e003502. doi:10.1136/bmjopen-2013-003502 PubMed
89. Lanoix R. Credentialing issues in emergency ultrasonography. Emerg Med Clin North Am. 1997;15(4):913-920. PubMed
90. Scalea T, Rodriquez A, Chiu WC, et al. Focused assessment with sonography for trauma (FAST): results from an international consensus conference. J Trauma. 1999;46(3):466-472. PubMed
91. Hertzberg BS, Kliewer MA, Bowie JD, et al. Physician training requirements in sonography: how many cases are needed for competence? AJR. 2000;174(5):1221-1227. PubMed
92. Blaivas M, Theodoro DL, Sierzenski P. Proliferation of ultrasound fellowships in emergency medicine: how do we ensure future experts are expertly trained? Acad Emerg Med. 2002;9(8):863-864. PubMed
93. Bodenham AR. Editorial II: Ultrasound imaging by anaesthetists: training and accreditation issues. Br J Anaesth. 2006;96(4):414-417. PubMed
94. Williamson JP, Twaddell SH, Lee YCG, et al. Thoracic ultrasound recognition of competence: A position paper of the Thoracic Society of Australia and New Zealand. Respirology. 2017;22(2):405-408. PubMed
95. Harrison G. Summative clinical competency assessment: a survey of ultrasound practitioners’ views. Ultrasound. 2015;23(1):11-17. PubMed
96. Evans LV, Morse JL, Hamann CJ, Osborne M, Lin Z, D'Onofrio G. The development of an independent rater system to assess residents' competence in invasive procedures. Acad Med. 2009;84(8):1135-1143. PubMed
97. Wass V, Van der Vleuten C, Shatzer J, Jones R. Assessment of clinical competence. Lancet. 2001;357(9260):945-949. PubMed
98. Arntfield RT. The utility of remote supervision with feedback as a method to deliver high-volume critical care ultrasound training. J Crit Care. 2015;30(2):441.e1-e6. PubMed
99. Akhtar S, Theodoro D, Gaspari R, et al. Resident training in emergency ultrasound: consensus recommendations from the 2008 Council of Emergency Residency Directors Conference. Acad Emerg Med. 2009;16:S32-S36. PubMed
100. Yu E. The assessment of technical skills in a cardiology training program: is the ITER sufficient? Can J Cardiol. 2000;16(4):457-462. PubMed
101. Todsen T, Tolsgaard MG, Olsen BH, et al. Reliable and valid assessment of point-of-care ultrasonography. Ann Surg. 2015;261(2):309-315. PubMed
102. Stein JC, Nobay F. Emergency department ultrasound credentialing: a sample policy and procedure. J Emerg Med. 2009;37(2):153-159. PubMed
103. Chen FM. Burstin H, Huntington J. The importance of clinical outcomes in medical education research. Med Educ. 2005;39(4):350-351. PubMed
104. Dressler DD, Pistoria MJ, Budnitz TL, McKean SCW, Amin AN. Core competencies in hospital medicine: development and methodology. J Hosp Med. 2006;1:48-56. PubMed
105. ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013;5(1):157-158. PubMed
106. Castillo J, Caruana CJ, Wainwright D. The changing concept of competence and categorisation of learning outcomes in Europe: Implications for the design of higher education radiography curricula at the European level. Radiography. 2011;17(3):230-234.
107. Goldstein SR. Accreditation, certification: why all the confusion? Obstet Gynecol. 2007;110(6):1396-1398. PubMed
108. Moore CL. Credentialing and reimbursement in point-of-care ultrasound. Clin Pediatr Emerg Med. 2011;12(1):73-77. PubMed
109. ten Cate O, Scheele F. Competency-based postgraduate training: can we bridge the gap between theory and clinical practice? Acad Med. 2007;82(6):542-547. PubMed
110. Abuhamad AZ, Benacerraf BR, Woletz P, Burke BL. The accreditation of ultrasound practices: impact on compliance with minimum performance guidelines. J Ultrasound Med. 2004;23(8):1023-1029. PubMed
© 2018 Society of Hospital Medicine
Hospital Privileging Practices for Bedside Procedures: A Survey of Hospitalist Experts
Performance of 6 bedside procedures (paracentesis, thoracentesis, lumbar puncture, arthrocentesis, central venous catheter [CVC] placement, and arterial line placement) are considered core competencies for hospitalists.1 Yet, the American Board of Internal Medicine (ABIM) no longer requires demonstration of manual competency for bedside procedures, and graduates may enter the workforce with minimal or no experience performing such procedures.2 As such, the burden falls on hospital privileging committees to ensure providers have the necessary training and experience to competently perform invasive procedures before granting institutional privileges to perform them.3 Although recommendations for privileging to perform certain surgical procedures have been proposed,4,5 there are no widely accepted guidelines for initial or ongoing privileging of common invasive bedside procedures performed by hospitalists, and current privileging practices vary significantly.
In 2015, the Society of Hospital Medicine (SHM) set up a Point-of-Care Ultrasound (POCUS) Task Force to draft evidence-based guidelines on the use of ultrasound to perform bedside procedures. The recommendations for certification of competency in ultrasound-guided procedures may guide institutional privileging. The purpose of this study was to better understand current hospital privileging practices for invasive bedside procedures both with and without ultrasound guidance and how current practices are perceived by experts.
METHODS
Study Design, Setting, and Participants
After approval by the University of Texas Health Science Center at San Antonio Institutional Review Board, we conducted a survey of hospital privileging processes for bedside procedures from a convenience sample of hospitalist procedure experts on the SHM POCUS Task Force. All 21 hospitalists on the task force were invited to participate, including the authors of this article. These hospitalists represent 21 unique institutions, and all have clinical, educational, and/or research expertise in ultrasound-guided bedside procedures.
Survey Design
A 26-question, electronic survey on privileging for bedside procedures was conducted (Appendix A). Twenty questions addressed procedures in general, such as minimum numbers of procedures required and use of simulation. Six questions focused on the use of ultrasound guidance. To provide context, many questions were framed to assess a privileging process being drafted by the task force. Answers were either multiple choice or free text.
Data Collection and Analysis
All members of the task force were invited to complete the survey by e-mail during November 2016. A reminder e-mail was sent on the day after initial distribution. No compensation was offered, and participation was not required. Survey results were compiled electronically through Research Electronic Data Capture, or “REDCap”TM (Nashville, Tennessee), and data analysis was performed with Stata version 14 (College Station, Texas). Means of current and recommended minimum thresholds were calculated by excluding responses of “I don’t know,” and responses of “no minimum number threshold” were coded as 0.
RESULTS
The survey response rate was 100% (21 of 21). All experts were hospitalists, but 2 also identified themselves as intensivists. Experts practiced in a variety of hospital settings, including private university hospitals (43%), public university hospitals (19%), Veterans Affairs teaching hospitals (14%), community teaching hospitals (14%), and community nonteaching hospitals (10%). Most hospitals (90%) were teaching hospitals for internal medicine trainees. All experts have personally performed bedside procedures on a regular basis, and most (86%) had leadership roles in teaching procedures to students, residents, fellows, physician assistants, nurse practitioners, and/or physicians. Approximately half (57%) were involved in granting privileges for bedside procedures at their institutions.
Only half of the experts reported that their hospitals required a minimum number of procedures to earn initial (48%) or ongoing (52%) privileges to perform bedside procedures. Nevertheless, most experts thought there ought to be minimum numbers of procedures for initial (81%) and ongoing (81%) privileging, recommending higher minimums for both initial and ongoing privileging than are currently required at their hospitals (Figure 2).
Most hospitalist procedure experts thought that simulation training (67%) and direct observation of procedural skills (71%) should be core components of an initial privileging process. Many of the experts who did not agree with direct observation or simulation training as core components of initial privileging had concerns about feasibility with respect to manpower, availability of simulation equipment, and costs. In contrast, the majority (67%) did not think it was necessary to directly observe providers for ongoing privileging when routine monitoring was in place for periprocedural complications, which all experts (100%) agreed should be in place.
DISCUSSION
Our survey identified 3 distinct differences between hospitalist procedure experts’ recommendations and their own hospitals’ current privileging practices. First, whereas experts recommended ultrasound guidance for thoracentesis, paracentesis, and CVC placement, it is rarely a current requirement. Second, experts recommend requiring minimum numbers of procedures for both initial and ongoing privileging even though such minimums are not currently required at half of their hospitals. Third, recommended minimum numbers were generally higher than those currently in place.
The routine use of ultrasound guidance for thoracentesis, paracentesis, and CVC placement is likely a result of increased adoption based on the literature showing clinical benefits.6-9 Thus, the expert recommendations for required use of ultrasound guidance for these procedures seems both appropriate and feasible. The procedure minimums identified in our study are similar to prior ABIM guidelines when manual competency was required for board certification in internal medicine and are comparable to recent minimums proposed by the Society of Critical Care Medicine, both of which recommended a minimum of 5 to 10 per procedure.10,11 Nevertheless, no commonly agreed-upon minimum number of procedures currently exists for certification of competency, and the variability seen in the experts’ responses further supports the idea that no specific number will guarantee competence. Thus, while requiring minimum numbers of procedures was generally considered necessary by our experts, minimums alone were also considered insufficient for initial privileging because most recommended that direct observation and simulation should be part of an initial privileging process.
These findings encourage more rigorous requirements for both initial and ongoing privileging of procedures. Nevertheless, our findings were rarely unanimous. The most frequently cited reason for disagreement on our findings was feasibility and capacity for direct observation, and the absence of ultrasound equipment or simulators, particularly in resource-limited clinical environments.
Our study has several strengths and limitations. One strength is the recruitment of study experts specifically composed of hospitalist procedure experts from diverse geographic and hospital settings. Yet, we acknowledge that our findings may not be generalizable to other specialties. Another strength is we obtained 100% participation from the experts surveyed. Weaknesses of this study include the relatively small number of experts who are likely to be biased in favor of both the use of ultrasound guidance and higher standards for privileging. We also relied on self-reported data about privileging processes rather than direct observation of those practices. Finally, questions were framed in the context of only 1 possible privileging pathway, and experts may respond differently to a different framing.
CONCLUSION
Our findings may guide the development of more standardized frameworks for initial and ongoing privileging of hospitalists for invasive bedside procedures. In particular, additional privileging requirements may include the routine use of ultrasound guidance for paracentesis, thoracentesis, and CVC insertion; simulation preceding direct observation of manual skills if possible; and higher required minimums of procedures for both initial and ongoing privileging. The goal of a standardized framework for privileging should be directed at improving the quality and safety of bedside procedures but must consider feasibility in diverse clinical settings where hospitalists work.
Acknowledgments
The authors thank the hospitalists on the SHM POCUS Task Force who provided data about their institutions’ privileging processes and requirements. They are also grateful to Loretta M. Grikis, MLS, AHIP, at the White River Junction Veterans Affairs Medical Center for her assistance as a medical librarian.
Disclosure
B
1. Nichani S, Jonathan Crocker, MD, Nick Fitterman, MD, Michael Lukela, MD, Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med 2017;12(4);283-287. PubMed
2. American Board of Internal Medicine. Policies and Procedures for Certification. http://www.abim.org/certification/policies/imss/im.aspx - procedures. Published July 2016. Accessed on November 8, 2016.
3. Department of Health & Human Services. Centers for Medicare & Medicaid Services (CMS) Requirements for Hospital Medical Staff Privileging. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/downloads/SCLetter05-04.pdf. Published November 12, 2004. Accessed on November 8, 2016.
4. Blackmon SH, Cooke DT, Whyte R, et al. The Society of Thoracic Surgeons Expert Consensus Statement: A tool kit to assist thoracic surgeons seeking privileging to use new technology and perform advanced procedures in general thoracic surgery. Ann Thorac Surg. 2016;101(3):1230-1237. PubMed
5. Bhora FY, Al-Ayoubi AM, Rehmani SS, Forleiter CM, Raad WN, Belsley SG. Robotically assisted thoracic surgery: proposed guidelines for privileging and credentialing. Innovations (Phila). 2016;11(6):386-389. PubMed
6. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. PubMed
7. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40:135-141. PubMed
8. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. DOI: 10.1002/14651858.CD006962.pub2. PubMed
9. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. DOI: 10.1002/14651858.CD011447. PubMed
10. American Board of Internal Medicine. Policies and Procedures. Philadelphia, PA; July 1990.
11. Society of Critical Care Medicine Ultrasound Certification Task Force. Recommendations for Achieving and Maintaining Competence and Credentialing in Critical Care Ultrasound with Focused Cardiac Ultrasound and Advanced Critical Care Echocardiography. http://journals.lww.com/ccmjournal/Documents/Critical%20Care%20Ultrasound.pdf. Published 2013. Accessed November 8, 2016.
Performance of 6 bedside procedures (paracentesis, thoracentesis, lumbar puncture, arthrocentesis, central venous catheter [CVC] placement, and arterial line placement) are considered core competencies for hospitalists.1 Yet, the American Board of Internal Medicine (ABIM) no longer requires demonstration of manual competency for bedside procedures, and graduates may enter the workforce with minimal or no experience performing such procedures.2 As such, the burden falls on hospital privileging committees to ensure providers have the necessary training and experience to competently perform invasive procedures before granting institutional privileges to perform them.3 Although recommendations for privileging to perform certain surgical procedures have been proposed,4,5 there are no widely accepted guidelines for initial or ongoing privileging of common invasive bedside procedures performed by hospitalists, and current privileging practices vary significantly.
In 2015, the Society of Hospital Medicine (SHM) set up a Point-of-Care Ultrasound (POCUS) Task Force to draft evidence-based guidelines on the use of ultrasound to perform bedside procedures. The recommendations for certification of competency in ultrasound-guided procedures may guide institutional privileging. The purpose of this study was to better understand current hospital privileging practices for invasive bedside procedures both with and without ultrasound guidance and how current practices are perceived by experts.
METHODS
Study Design, Setting, and Participants
After approval by the University of Texas Health Science Center at San Antonio Institutional Review Board, we conducted a survey of hospital privileging processes for bedside procedures from a convenience sample of hospitalist procedure experts on the SHM POCUS Task Force. All 21 hospitalists on the task force were invited to participate, including the authors of this article. These hospitalists represent 21 unique institutions, and all have clinical, educational, and/or research expertise in ultrasound-guided bedside procedures.
Survey Design
A 26-question, electronic survey on privileging for bedside procedures was conducted (Appendix A). Twenty questions addressed procedures in general, such as minimum numbers of procedures required and use of simulation. Six questions focused on the use of ultrasound guidance. To provide context, many questions were framed to assess a privileging process being drafted by the task force. Answers were either multiple choice or free text.
Data Collection and Analysis
All members of the task force were invited to complete the survey by e-mail during November 2016. A reminder e-mail was sent on the day after initial distribution. No compensation was offered, and participation was not required. Survey results were compiled electronically through Research Electronic Data Capture, or “REDCap”TM (Nashville, Tennessee), and data analysis was performed with Stata version 14 (College Station, Texas). Means of current and recommended minimum thresholds were calculated by excluding responses of “I don’t know,” and responses of “no minimum number threshold” were coded as 0.
RESULTS
The survey response rate was 100% (21 of 21). All experts were hospitalists, but 2 also identified themselves as intensivists. Experts practiced in a variety of hospital settings, including private university hospitals (43%), public university hospitals (19%), Veterans Affairs teaching hospitals (14%), community teaching hospitals (14%), and community nonteaching hospitals (10%). Most hospitals (90%) were teaching hospitals for internal medicine trainees. All experts have personally performed bedside procedures on a regular basis, and most (86%) had leadership roles in teaching procedures to students, residents, fellows, physician assistants, nurse practitioners, and/or physicians. Approximately half (57%) were involved in granting privileges for bedside procedures at their institutions.
Only half of the experts reported that their hospitals required a minimum number of procedures to earn initial (48%) or ongoing (52%) privileges to perform bedside procedures. Nevertheless, most experts thought there ought to be minimum numbers of procedures for initial (81%) and ongoing (81%) privileging, recommending higher minimums for both initial and ongoing privileging than are currently required at their hospitals (Figure 2).
Most hospitalist procedure experts thought that simulation training (67%) and direct observation of procedural skills (71%) should be core components of an initial privileging process. Many of the experts who did not agree with direct observation or simulation training as core components of initial privileging had concerns about feasibility with respect to manpower, availability of simulation equipment, and costs. In contrast, the majority (67%) did not think it was necessary to directly observe providers for ongoing privileging when routine monitoring was in place for periprocedural complications, which all experts (100%) agreed should be in place.
DISCUSSION
Our survey identified 3 distinct differences between hospitalist procedure experts’ recommendations and their own hospitals’ current privileging practices. First, whereas experts recommended ultrasound guidance for thoracentesis, paracentesis, and CVC placement, it is rarely a current requirement. Second, experts recommend requiring minimum numbers of procedures for both initial and ongoing privileging even though such minimums are not currently required at half of their hospitals. Third, recommended minimum numbers were generally higher than those currently in place.
The routine use of ultrasound guidance for thoracentesis, paracentesis, and CVC placement is likely a result of increased adoption based on the literature showing clinical benefits.6-9 Thus, the expert recommendations for required use of ultrasound guidance for these procedures seems both appropriate and feasible. The procedure minimums identified in our study are similar to prior ABIM guidelines when manual competency was required for board certification in internal medicine and are comparable to recent minimums proposed by the Society of Critical Care Medicine, both of which recommended a minimum of 5 to 10 per procedure.10,11 Nevertheless, no commonly agreed-upon minimum number of procedures currently exists for certification of competency, and the variability seen in the experts’ responses further supports the idea that no specific number will guarantee competence. Thus, while requiring minimum numbers of procedures was generally considered necessary by our experts, minimums alone were also considered insufficient for initial privileging because most recommended that direct observation and simulation should be part of an initial privileging process.
These findings encourage more rigorous requirements for both initial and ongoing privileging of procedures. Nevertheless, our findings were rarely unanimous. The most frequently cited reason for disagreement on our findings was feasibility and capacity for direct observation, and the absence of ultrasound equipment or simulators, particularly in resource-limited clinical environments.
Our study has several strengths and limitations. One strength is the recruitment of study experts specifically composed of hospitalist procedure experts from diverse geographic and hospital settings. Yet, we acknowledge that our findings may not be generalizable to other specialties. Another strength is we obtained 100% participation from the experts surveyed. Weaknesses of this study include the relatively small number of experts who are likely to be biased in favor of both the use of ultrasound guidance and higher standards for privileging. We also relied on self-reported data about privileging processes rather than direct observation of those practices. Finally, questions were framed in the context of only 1 possible privileging pathway, and experts may respond differently to a different framing.
CONCLUSION
Our findings may guide the development of more standardized frameworks for initial and ongoing privileging of hospitalists for invasive bedside procedures. In particular, additional privileging requirements may include the routine use of ultrasound guidance for paracentesis, thoracentesis, and CVC insertion; simulation preceding direct observation of manual skills if possible; and higher required minimums of procedures for both initial and ongoing privileging. The goal of a standardized framework for privileging should be directed at improving the quality and safety of bedside procedures but must consider feasibility in diverse clinical settings where hospitalists work.
Acknowledgments
The authors thank the hospitalists on the SHM POCUS Task Force who provided data about their institutions’ privileging processes and requirements. They are also grateful to Loretta M. Grikis, MLS, AHIP, at the White River Junction Veterans Affairs Medical Center for her assistance as a medical librarian.
Disclosure
B
Performance of 6 bedside procedures (paracentesis, thoracentesis, lumbar puncture, arthrocentesis, central venous catheter [CVC] placement, and arterial line placement) are considered core competencies for hospitalists.1 Yet, the American Board of Internal Medicine (ABIM) no longer requires demonstration of manual competency for bedside procedures, and graduates may enter the workforce with minimal or no experience performing such procedures.2 As such, the burden falls on hospital privileging committees to ensure providers have the necessary training and experience to competently perform invasive procedures before granting institutional privileges to perform them.3 Although recommendations for privileging to perform certain surgical procedures have been proposed,4,5 there are no widely accepted guidelines for initial or ongoing privileging of common invasive bedside procedures performed by hospitalists, and current privileging practices vary significantly.
In 2015, the Society of Hospital Medicine (SHM) set up a Point-of-Care Ultrasound (POCUS) Task Force to draft evidence-based guidelines on the use of ultrasound to perform bedside procedures. The recommendations for certification of competency in ultrasound-guided procedures may guide institutional privileging. The purpose of this study was to better understand current hospital privileging practices for invasive bedside procedures both with and without ultrasound guidance and how current practices are perceived by experts.
METHODS
Study Design, Setting, and Participants
After approval by the University of Texas Health Science Center at San Antonio Institutional Review Board, we conducted a survey of hospital privileging processes for bedside procedures from a convenience sample of hospitalist procedure experts on the SHM POCUS Task Force. All 21 hospitalists on the task force were invited to participate, including the authors of this article. These hospitalists represent 21 unique institutions, and all have clinical, educational, and/or research expertise in ultrasound-guided bedside procedures.
Survey Design
A 26-question, electronic survey on privileging for bedside procedures was conducted (Appendix A). Twenty questions addressed procedures in general, such as minimum numbers of procedures required and use of simulation. Six questions focused on the use of ultrasound guidance. To provide context, many questions were framed to assess a privileging process being drafted by the task force. Answers were either multiple choice or free text.
Data Collection and Analysis
All members of the task force were invited to complete the survey by e-mail during November 2016. A reminder e-mail was sent on the day after initial distribution. No compensation was offered, and participation was not required. Survey results were compiled electronically through Research Electronic Data Capture, or “REDCap”TM (Nashville, Tennessee), and data analysis was performed with Stata version 14 (College Station, Texas). Means of current and recommended minimum thresholds were calculated by excluding responses of “I don’t know,” and responses of “no minimum number threshold” were coded as 0.
RESULTS
The survey response rate was 100% (21 of 21). All experts were hospitalists, but 2 also identified themselves as intensivists. Experts practiced in a variety of hospital settings, including private university hospitals (43%), public university hospitals (19%), Veterans Affairs teaching hospitals (14%), community teaching hospitals (14%), and community nonteaching hospitals (10%). Most hospitals (90%) were teaching hospitals for internal medicine trainees. All experts have personally performed bedside procedures on a regular basis, and most (86%) had leadership roles in teaching procedures to students, residents, fellows, physician assistants, nurse practitioners, and/or physicians. Approximately half (57%) were involved in granting privileges for bedside procedures at their institutions.
Only half of the experts reported that their hospitals required a minimum number of procedures to earn initial (48%) or ongoing (52%) privileges to perform bedside procedures. Nevertheless, most experts thought there ought to be minimum numbers of procedures for initial (81%) and ongoing (81%) privileging, recommending higher minimums for both initial and ongoing privileging than are currently required at their hospitals (Figure 2).
Most hospitalist procedure experts thought that simulation training (67%) and direct observation of procedural skills (71%) should be core components of an initial privileging process. Many of the experts who did not agree with direct observation or simulation training as core components of initial privileging had concerns about feasibility with respect to manpower, availability of simulation equipment, and costs. In contrast, the majority (67%) did not think it was necessary to directly observe providers for ongoing privileging when routine monitoring was in place for periprocedural complications, which all experts (100%) agreed should be in place.
DISCUSSION
Our survey identified 3 distinct differences between hospitalist procedure experts’ recommendations and their own hospitals’ current privileging practices. First, whereas experts recommended ultrasound guidance for thoracentesis, paracentesis, and CVC placement, it is rarely a current requirement. Second, experts recommend requiring minimum numbers of procedures for both initial and ongoing privileging even though such minimums are not currently required at half of their hospitals. Third, recommended minimum numbers were generally higher than those currently in place.
The routine use of ultrasound guidance for thoracentesis, paracentesis, and CVC placement is likely a result of increased adoption based on the literature showing clinical benefits.6-9 Thus, the expert recommendations for required use of ultrasound guidance for these procedures seems both appropriate and feasible. The procedure minimums identified in our study are similar to prior ABIM guidelines when manual competency was required for board certification in internal medicine and are comparable to recent minimums proposed by the Society of Critical Care Medicine, both of which recommended a minimum of 5 to 10 per procedure.10,11 Nevertheless, no commonly agreed-upon minimum number of procedures currently exists for certification of competency, and the variability seen in the experts’ responses further supports the idea that no specific number will guarantee competence. Thus, while requiring minimum numbers of procedures was generally considered necessary by our experts, minimums alone were also considered insufficient for initial privileging because most recommended that direct observation and simulation should be part of an initial privileging process.
These findings encourage more rigorous requirements for both initial and ongoing privileging of procedures. Nevertheless, our findings were rarely unanimous. The most frequently cited reason for disagreement on our findings was feasibility and capacity for direct observation, and the absence of ultrasound equipment or simulators, particularly in resource-limited clinical environments.
Our study has several strengths and limitations. One strength is the recruitment of study experts specifically composed of hospitalist procedure experts from diverse geographic and hospital settings. Yet, we acknowledge that our findings may not be generalizable to other specialties. Another strength is we obtained 100% participation from the experts surveyed. Weaknesses of this study include the relatively small number of experts who are likely to be biased in favor of both the use of ultrasound guidance and higher standards for privileging. We also relied on self-reported data about privileging processes rather than direct observation of those practices. Finally, questions were framed in the context of only 1 possible privileging pathway, and experts may respond differently to a different framing.
CONCLUSION
Our findings may guide the development of more standardized frameworks for initial and ongoing privileging of hospitalists for invasive bedside procedures. In particular, additional privileging requirements may include the routine use of ultrasound guidance for paracentesis, thoracentesis, and CVC insertion; simulation preceding direct observation of manual skills if possible; and higher required minimums of procedures for both initial and ongoing privileging. The goal of a standardized framework for privileging should be directed at improving the quality and safety of bedside procedures but must consider feasibility in diverse clinical settings where hospitalists work.
Acknowledgments
The authors thank the hospitalists on the SHM POCUS Task Force who provided data about their institutions’ privileging processes and requirements. They are also grateful to Loretta M. Grikis, MLS, AHIP, at the White River Junction Veterans Affairs Medical Center for her assistance as a medical librarian.
Disclosure
B
1. Nichani S, Jonathan Crocker, MD, Nick Fitterman, MD, Michael Lukela, MD, Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med 2017;12(4);283-287. PubMed
2. American Board of Internal Medicine. Policies and Procedures for Certification. http://www.abim.org/certification/policies/imss/im.aspx - procedures. Published July 2016. Accessed on November 8, 2016.
3. Department of Health & Human Services. Centers for Medicare & Medicaid Services (CMS) Requirements for Hospital Medical Staff Privileging. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/downloads/SCLetter05-04.pdf. Published November 12, 2004. Accessed on November 8, 2016.
4. Blackmon SH, Cooke DT, Whyte R, et al. The Society of Thoracic Surgeons Expert Consensus Statement: A tool kit to assist thoracic surgeons seeking privileging to use new technology and perform advanced procedures in general thoracic surgery. Ann Thorac Surg. 2016;101(3):1230-1237. PubMed
5. Bhora FY, Al-Ayoubi AM, Rehmani SS, Forleiter CM, Raad WN, Belsley SG. Robotically assisted thoracic surgery: proposed guidelines for privileging and credentialing. Innovations (Phila). 2016;11(6):386-389. PubMed
6. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. PubMed
7. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40:135-141. PubMed
8. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. DOI: 10.1002/14651858.CD006962.pub2. PubMed
9. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. DOI: 10.1002/14651858.CD011447. PubMed
10. American Board of Internal Medicine. Policies and Procedures. Philadelphia, PA; July 1990.
11. Society of Critical Care Medicine Ultrasound Certification Task Force. Recommendations for Achieving and Maintaining Competence and Credentialing in Critical Care Ultrasound with Focused Cardiac Ultrasound and Advanced Critical Care Echocardiography. http://journals.lww.com/ccmjournal/Documents/Critical%20Care%20Ultrasound.pdf. Published 2013. Accessed November 8, 2016.
1. Nichani S, Jonathan Crocker, MD, Nick Fitterman, MD, Michael Lukela, MD, Updating the core competencies in hospital medicine—2017 revision: Introduction and methodology. J Hosp Med 2017;12(4);283-287. PubMed
2. American Board of Internal Medicine. Policies and Procedures for Certification. http://www.abim.org/certification/policies/imss/im.aspx - procedures. Published July 2016. Accessed on November 8, 2016.
3. Department of Health & Human Services. Centers for Medicare & Medicaid Services (CMS) Requirements for Hospital Medical Staff Privileging. Centers for Medicare and Medicaid Services website. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/downloads/SCLetter05-04.pdf. Published November 12, 2004. Accessed on November 8, 2016.
4. Blackmon SH, Cooke DT, Whyte R, et al. The Society of Thoracic Surgeons Expert Consensus Statement: A tool kit to assist thoracic surgeons seeking privileging to use new technology and perform advanced procedures in general thoracic surgery. Ann Thorac Surg. 2016;101(3):1230-1237. PubMed
5. Bhora FY, Al-Ayoubi AM, Rehmani SS, Forleiter CM, Raad WN, Belsley SG. Robotically assisted thoracic surgery: proposed guidelines for privileging and credentialing. Innovations (Phila). 2016;11(6):386-389. PubMed
6. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. PubMed
7. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40:135-141. PubMed
8. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst Rev. 2015;1:CD006962. DOI: 10.1002/14651858.CD006962.pub2. PubMed
9. Brass P, Hellmich M, Kolodziej L, Schick G, Smith AF. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. DOI: 10.1002/14651858.CD011447. PubMed
10. American Board of Internal Medicine. Policies and Procedures. Philadelphia, PA; July 1990.
11. Society of Critical Care Medicine Ultrasound Certification Task Force. Recommendations for Achieving and Maintaining Competence and Credentialing in Critical Care Ultrasound with Focused Cardiac Ultrasound and Advanced Critical Care Echocardiography. http://journals.lww.com/ccmjournal/Documents/Critical%20Care%20Ultrasound.pdf. Published 2013. Accessed November 8, 2016.
© 2017 Society of Hospital Medicine
Certification of Point-of-Care Ultrasound Competency
Any conversation about point-of-care ultrasound (POCUS) inevitably brings up discussion about credentialing, privileging, and certification. While credentialing and privileging are institution-specific processes, competency certification can be extramural through a national board or intramural through an institutional process.
Some institutions have begun to develop intramural certification pathways for POCUS competency in order to grant privileges to hospitalists. In this edition of the Journal of Hospital Medicine, Mathews and Zwank2 describe a multidisciplinary collaboration to provide POCUS training, intramural certification, and quality assurance for hospitalists at one hospital in Minnesota. This model serves as a real-world example of how institutions are addressing the need to certify hospitalists in basic POCUS competency. After engaging stakeholders from radiology, critical care, emergency medicine, and cardiology, institutional standards were developed and hospitalists were assessed for basic POCUS competency. Certification included assessments of hospitalists’ knowledge, image acquisition, and image interpretation skills. The model described by Mathews did not assess competency in clinical integration but laid the groundwork for future evaluation of clinical outcomes in the cohort of certified hospitalists.
Although experts may not agree on all aspects of competency in POCUS, most will agree with the basic principles outlined by Mathews and Zwank. Initial certification should be based on training and an initial assessment of competency. Components of training should include ultrasound didactics, mentored hands-on practice, independent hands-on practice, and image interpretation practice. Ongoing certification should be based on quality assurance incorporated with an ongoing assessment of skills. Additionally, most experts will agree that competency can be recognized, and formative and summative assessments that combine a gestalt of provider skills with quantitative scoring systems using checklists are likely the best approach.
The real question is, what is the goal of certification of POCUS competency? Development of an institutional certification process demands substantive resources of the institution and time of the providers. Institutions would have to invest in equipment and staff to operate a full-time certification program, given the large number of providers that use POCUS and justify why substantive resources are being dedicated to certify POCUS skills and not others. Providers may be dissuaded from using POCUS if certification requirements are burdensome, which has potential negative consequences, such as reverting back to performing bedside procedures without ultrasound guidance or referring all patients to interventional radiology.
Conceptually, one may speculate that certification is required for providers to bill for POCUS exams, but certification is not required to bill, although institutions may require certification before granting privileges to use POCUS. However, based on the emergency medicine experience, a specialty that has been using POCUS for more than 20 years, billing may not be the main driver of POCUS use. A recent review of 2012 Medicare data revealed that <1% of emergency medicine providers received reimbursement for limited ultrasound exams.3 Despite the Accreditation Council for Graduate Medical Education (ACGME) requirement for POCUS competency of all graduating emergency medicine residents since 2001 and the increasing POCUS use reported by emergency medicine physicians,4,5 most emergency medicine physicians are not billing for POCUS exams. Maybe use of POCUS as a “quick look” or extension of the physical examination is more common than previously thought. Although billing for POCUS exams can generate some clinical revenue, the benefits for the healthcare system by expediting care,6,7 reducing ancillary testing,8,9 and reducing procedural complications10,11 likely outweigh the small gains from billing for limited ultrasound exams. As healthcare payment models evolve to reward healthcare systems that achieve good outcomes rather than services rendered, certification for the sole purpose of billing may become obsolete. Furthermore, concerns about billing increasing medical liability from using POCUS are likely overstated because few lawsuits have resulted from missed diagnoses by POCUS, and most lawsuits have been from failure to perform a POCUS exam in a timely manner.12,13
Many medical students graduating today have had some training in POCUS14 and, as this new generation of physicians enters the workforce, they will likely view POCUS as part of their routine bedside evaluation of patients. If POCUS training is integrated into medical school and residency curricula, and national board certification incorporates basic POCUS competency, then most institutions may no longer feel obligated to certify POCUS competency locally, and institutional certification programs, such as the one described by Mathews and Zwank, would become obsolete.
For now, until all providers enter the workforce with basic competency in POCUS and medical culture accepts that ultrasound is a diagnostic tool available to any trained provider, hospitalists may need to provide proof of their competence through intramural or extramural certification. The work of Mathews and Zwank provides an example of how local certification processes can be established. In a future edition of the Journal of Hospital Medicine, the Society of Hospital Medicine Point-of-Care Ultrasound Task Force will present a position statement with recommendations for certification of competency in bedside ultrasound-guided procedures.
Disclosure
Nilam Soni receives support from the U.S. Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1). Brian P. Lucas receives support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Bahner DP, Hughes D, Royall NA. I-AIM: a novel model for teaching and performing focused sonography. J Ultrasound Med. 2012;31:295-300. PubMed
2. Mathews BK, Zwank M. Hospital Medicine Point of Care Ultrasound Credentialing: An Example Protocol. J Hosp Med. 2017;12(9):767-772. PubMed
3. Hall MK, Hall J, Gross CP, et al. Use of Point-of-Care Ultrasound in the Emergency Department: Insights From the 2012 Medicare National Payment Data Set. J Ultrasound Med. 2016;35:2467-2474. PubMed
4. Amini R, Wyman MT, Hernandez NC, Guisto JA, Adhikari S. Use of Emergency Ultrasound in Arizona Community Emergency Departments. J Ultrasound Med. 2017;36(5):913-921. PubMed
5. Herbst MK, Camargo CA, Jr., Perez A, Moore CL. Use of Point-of-Care Ultrasound in Connecticut Emergency Departments. J Emerg Med. 2015;48:191-196. PubMed
6. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139:538-542. PubMed
7. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124:766-774. PubMed
8. Oks M, Cleven KL, Cardenas-Garcia J, et al. The effect of point-of-care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146:1574-1577. PubMed
9. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH, Narasimhan M. Ultrasound assessment of pulmonary embolism in patients receiving CT pulmonary angiography. Chest. 2014;145:818-823. PubMed
10. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. PubMed
11. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40:135-141. PubMed
12. Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16:1-4. PubMed
13. Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30:338-341. PubMed
14. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686. PubMed
Any conversation about point-of-care ultrasound (POCUS) inevitably brings up discussion about credentialing, privileging, and certification. While credentialing and privileging are institution-specific processes, competency certification can be extramural through a national board or intramural through an institutional process.
Some institutions have begun to develop intramural certification pathways for POCUS competency in order to grant privileges to hospitalists. In this edition of the Journal of Hospital Medicine, Mathews and Zwank2 describe a multidisciplinary collaboration to provide POCUS training, intramural certification, and quality assurance for hospitalists at one hospital in Minnesota. This model serves as a real-world example of how institutions are addressing the need to certify hospitalists in basic POCUS competency. After engaging stakeholders from radiology, critical care, emergency medicine, and cardiology, institutional standards were developed and hospitalists were assessed for basic POCUS competency. Certification included assessments of hospitalists’ knowledge, image acquisition, and image interpretation skills. The model described by Mathews did not assess competency in clinical integration but laid the groundwork for future evaluation of clinical outcomes in the cohort of certified hospitalists.
Although experts may not agree on all aspects of competency in POCUS, most will agree with the basic principles outlined by Mathews and Zwank. Initial certification should be based on training and an initial assessment of competency. Components of training should include ultrasound didactics, mentored hands-on practice, independent hands-on practice, and image interpretation practice. Ongoing certification should be based on quality assurance incorporated with an ongoing assessment of skills. Additionally, most experts will agree that competency can be recognized, and formative and summative assessments that combine a gestalt of provider skills with quantitative scoring systems using checklists are likely the best approach.
The real question is, what is the goal of certification of POCUS competency? Development of an institutional certification process demands substantive resources of the institution and time of the providers. Institutions would have to invest in equipment and staff to operate a full-time certification program, given the large number of providers that use POCUS and justify why substantive resources are being dedicated to certify POCUS skills and not others. Providers may be dissuaded from using POCUS if certification requirements are burdensome, which has potential negative consequences, such as reverting back to performing bedside procedures without ultrasound guidance or referring all patients to interventional radiology.
Conceptually, one may speculate that certification is required for providers to bill for POCUS exams, but certification is not required to bill, although institutions may require certification before granting privileges to use POCUS. However, based on the emergency medicine experience, a specialty that has been using POCUS for more than 20 years, billing may not be the main driver of POCUS use. A recent review of 2012 Medicare data revealed that <1% of emergency medicine providers received reimbursement for limited ultrasound exams.3 Despite the Accreditation Council for Graduate Medical Education (ACGME) requirement for POCUS competency of all graduating emergency medicine residents since 2001 and the increasing POCUS use reported by emergency medicine physicians,4,5 most emergency medicine physicians are not billing for POCUS exams. Maybe use of POCUS as a “quick look” or extension of the physical examination is more common than previously thought. Although billing for POCUS exams can generate some clinical revenue, the benefits for the healthcare system by expediting care,6,7 reducing ancillary testing,8,9 and reducing procedural complications10,11 likely outweigh the small gains from billing for limited ultrasound exams. As healthcare payment models evolve to reward healthcare systems that achieve good outcomes rather than services rendered, certification for the sole purpose of billing may become obsolete. Furthermore, concerns about billing increasing medical liability from using POCUS are likely overstated because few lawsuits have resulted from missed diagnoses by POCUS, and most lawsuits have been from failure to perform a POCUS exam in a timely manner.12,13
Many medical students graduating today have had some training in POCUS14 and, as this new generation of physicians enters the workforce, they will likely view POCUS as part of their routine bedside evaluation of patients. If POCUS training is integrated into medical school and residency curricula, and national board certification incorporates basic POCUS competency, then most institutions may no longer feel obligated to certify POCUS competency locally, and institutional certification programs, such as the one described by Mathews and Zwank, would become obsolete.
For now, until all providers enter the workforce with basic competency in POCUS and medical culture accepts that ultrasound is a diagnostic tool available to any trained provider, hospitalists may need to provide proof of their competence through intramural or extramural certification. The work of Mathews and Zwank provides an example of how local certification processes can be established. In a future edition of the Journal of Hospital Medicine, the Society of Hospital Medicine Point-of-Care Ultrasound Task Force will present a position statement with recommendations for certification of competency in bedside ultrasound-guided procedures.
Disclosure
Nilam Soni receives support from the U.S. Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1). Brian P. Lucas receives support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Any conversation about point-of-care ultrasound (POCUS) inevitably brings up discussion about credentialing, privileging, and certification. While credentialing and privileging are institution-specific processes, competency certification can be extramural through a national board or intramural through an institutional process.
Some institutions have begun to develop intramural certification pathways for POCUS competency in order to grant privileges to hospitalists. In this edition of the Journal of Hospital Medicine, Mathews and Zwank2 describe a multidisciplinary collaboration to provide POCUS training, intramural certification, and quality assurance for hospitalists at one hospital in Minnesota. This model serves as a real-world example of how institutions are addressing the need to certify hospitalists in basic POCUS competency. After engaging stakeholders from radiology, critical care, emergency medicine, and cardiology, institutional standards were developed and hospitalists were assessed for basic POCUS competency. Certification included assessments of hospitalists’ knowledge, image acquisition, and image interpretation skills. The model described by Mathews did not assess competency in clinical integration but laid the groundwork for future evaluation of clinical outcomes in the cohort of certified hospitalists.
Although experts may not agree on all aspects of competency in POCUS, most will agree with the basic principles outlined by Mathews and Zwank. Initial certification should be based on training and an initial assessment of competency. Components of training should include ultrasound didactics, mentored hands-on practice, independent hands-on practice, and image interpretation practice. Ongoing certification should be based on quality assurance incorporated with an ongoing assessment of skills. Additionally, most experts will agree that competency can be recognized, and formative and summative assessments that combine a gestalt of provider skills with quantitative scoring systems using checklists are likely the best approach.
The real question is, what is the goal of certification of POCUS competency? Development of an institutional certification process demands substantive resources of the institution and time of the providers. Institutions would have to invest in equipment and staff to operate a full-time certification program, given the large number of providers that use POCUS and justify why substantive resources are being dedicated to certify POCUS skills and not others. Providers may be dissuaded from using POCUS if certification requirements are burdensome, which has potential negative consequences, such as reverting back to performing bedside procedures without ultrasound guidance or referring all patients to interventional radiology.
Conceptually, one may speculate that certification is required for providers to bill for POCUS exams, but certification is not required to bill, although institutions may require certification before granting privileges to use POCUS. However, based on the emergency medicine experience, a specialty that has been using POCUS for more than 20 years, billing may not be the main driver of POCUS use. A recent review of 2012 Medicare data revealed that <1% of emergency medicine providers received reimbursement for limited ultrasound exams.3 Despite the Accreditation Council for Graduate Medical Education (ACGME) requirement for POCUS competency of all graduating emergency medicine residents since 2001 and the increasing POCUS use reported by emergency medicine physicians,4,5 most emergency medicine physicians are not billing for POCUS exams. Maybe use of POCUS as a “quick look” or extension of the physical examination is more common than previously thought. Although billing for POCUS exams can generate some clinical revenue, the benefits for the healthcare system by expediting care,6,7 reducing ancillary testing,8,9 and reducing procedural complications10,11 likely outweigh the small gains from billing for limited ultrasound exams. As healthcare payment models evolve to reward healthcare systems that achieve good outcomes rather than services rendered, certification for the sole purpose of billing may become obsolete. Furthermore, concerns about billing increasing medical liability from using POCUS are likely overstated because few lawsuits have resulted from missed diagnoses by POCUS, and most lawsuits have been from failure to perform a POCUS exam in a timely manner.12,13
Many medical students graduating today have had some training in POCUS14 and, as this new generation of physicians enters the workforce, they will likely view POCUS as part of their routine bedside evaluation of patients. If POCUS training is integrated into medical school and residency curricula, and national board certification incorporates basic POCUS competency, then most institutions may no longer feel obligated to certify POCUS competency locally, and institutional certification programs, such as the one described by Mathews and Zwank, would become obsolete.
For now, until all providers enter the workforce with basic competency in POCUS and medical culture accepts that ultrasound is a diagnostic tool available to any trained provider, hospitalists may need to provide proof of their competence through intramural or extramural certification. The work of Mathews and Zwank provides an example of how local certification processes can be established. In a future edition of the Journal of Hospital Medicine, the Society of Hospital Medicine Point-of-Care Ultrasound Task Force will present a position statement with recommendations for certification of competency in bedside ultrasound-guided procedures.
Disclosure
Nilam Soni receives support from the U.S. Department of Veterans Affairs, Quality Enhancement Research Initiative (QUERI) Partnered Evaluation Initiative Grant (HX002263-01A1). Brian P. Lucas receives support from the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Dartmouth SYNERGY, National Institutes of Health, National Center for Translational Science (UL1TR001086). The contents of this publication do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
1. Bahner DP, Hughes D, Royall NA. I-AIM: a novel model for teaching and performing focused sonography. J Ultrasound Med. 2012;31:295-300. PubMed
2. Mathews BK, Zwank M. Hospital Medicine Point of Care Ultrasound Credentialing: An Example Protocol. J Hosp Med. 2017;12(9):767-772. PubMed
3. Hall MK, Hall J, Gross CP, et al. Use of Point-of-Care Ultrasound in the Emergency Department: Insights From the 2012 Medicare National Payment Data Set. J Ultrasound Med. 2016;35:2467-2474. PubMed
4. Amini R, Wyman MT, Hernandez NC, Guisto JA, Adhikari S. Use of Emergency Ultrasound in Arizona Community Emergency Departments. J Ultrasound Med. 2017;36(5):913-921. PubMed
5. Herbst MK, Camargo CA, Jr., Perez A, Moore CL. Use of Point-of-Care Ultrasound in Connecticut Emergency Departments. J Emerg Med. 2015;48:191-196. PubMed
6. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139:538-542. PubMed
7. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124:766-774. PubMed
8. Oks M, Cleven KL, Cardenas-Garcia J, et al. The effect of point-of-care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146:1574-1577. PubMed
9. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH, Narasimhan M. Ultrasound assessment of pulmonary embolism in patients receiving CT pulmonary angiography. Chest. 2014;145:818-823. PubMed
10. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. PubMed
11. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40:135-141. PubMed
12. Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16:1-4. PubMed
13. Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30:338-341. PubMed
14. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686. PubMed
1. Bahner DP, Hughes D, Royall NA. I-AIM: a novel model for teaching and performing focused sonography. J Ultrasound Med. 2012;31:295-300. PubMed
2. Mathews BK, Zwank M. Hospital Medicine Point of Care Ultrasound Credentialing: An Example Protocol. J Hosp Med. 2017;12(9):767-772. PubMed
3. Hall MK, Hall J, Gross CP, et al. Use of Point-of-Care Ultrasound in the Emergency Department: Insights From the 2012 Medicare National Payment Data Set. J Ultrasound Med. 2016;35:2467-2474. PubMed
4. Amini R, Wyman MT, Hernandez NC, Guisto JA, Adhikari S. Use of Emergency Ultrasound in Arizona Community Emergency Departments. J Ultrasound Med. 2017;36(5):913-921. PubMed
5. Herbst MK, Camargo CA, Jr., Perez A, Moore CL. Use of Point-of-Care Ultrasound in Connecticut Emergency Departments. J Emerg Med. 2015;48:191-196. PubMed
6. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139:538-542. PubMed
7. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography by hospitalists: a randomized trial. Am J Med. 2011;124:766-774. PubMed
8. Oks M, Cleven KL, Cardenas-Garcia J, et al. The effect of point-of-care ultrasonography on imaging studies in the medical ICU: a comparative study. Chest. 2014;146:1574-1577. PubMed
9. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH, Narasimhan M. Ultrasound assessment of pulmonary embolism in patients receiving CT pulmonary angiography. Chest. 2014;145:818-823. PubMed
10. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143:532-538. PubMed
11. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance reduces complications and costs associated with thoracentesis procedures. J Clin Ultrasound. 2012;40:135-141. PubMed
12. Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16:1-4. PubMed
13. Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30:338-341. PubMed
14. Bahner DP, Goldman E, Way D, Royall NA, Liu YT. The state of ultrasound education in U.S. medical schools: results of a national survey. Acad Med. 2014;89:1681-1686. PubMed
© 2017 Society of Hospital Medicine
PoCUS for Hospitalists
Similar to the physical exam, diagnostic point‐of‐care ultrasound exams are performed at the bedside in real time by hospitalists who are seeking a diagnosis. In contrast, referral ultrasound exams involve multiple providers and several steps. Typically, an ultrasound technologist acquires images, a radiologist or cardiologist interprets the images, a report is prepared, and results are sent to the referring hospitalist (Figure 1). Another important difference is that although referral ultrasound exams are usually comprehensive evaluations of entire organs or anatomic spaces, often without specific diagnoses in mind, point‐of‐care ultrasound exams are aimed at making specific diagnoses for well‐defined clinical scenarios.[1]
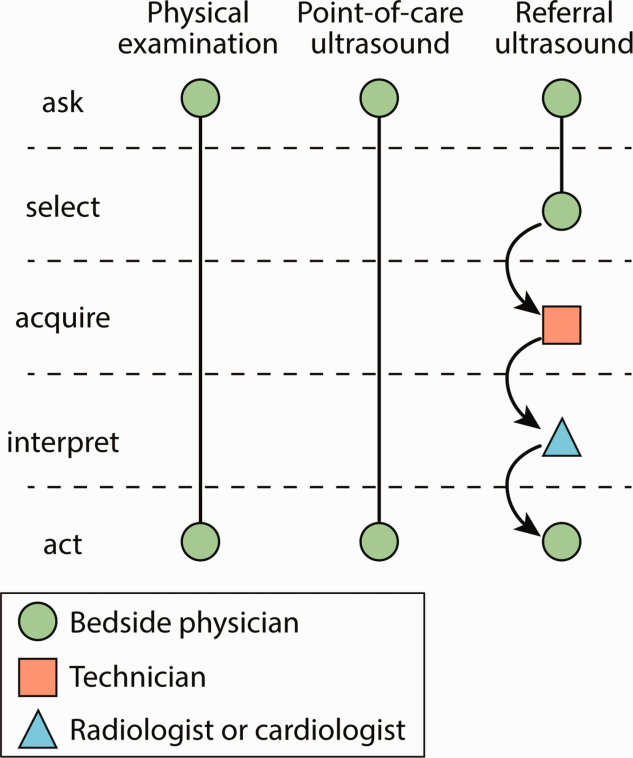
The American Medical Association has reassured providers that ultrasound imaging is within the scope of practice of appropriately trained physicians.[2] A growing body of literature demonstrates that point‐of‐care ultrasound is increasingly used by hospitalists for more than just bedside procedures. Incited by ongoing miniaturization of ultrasound devices, hospitalists are beginning to use point‐of‐care ultrasound for diagnosis, treatment, monitoring, and screening of patients (Figure 2). Our aim was to review the current literature for point‐of‐care ultrasound applications most relevant to hospitalists and highlight gaps in the current literature.
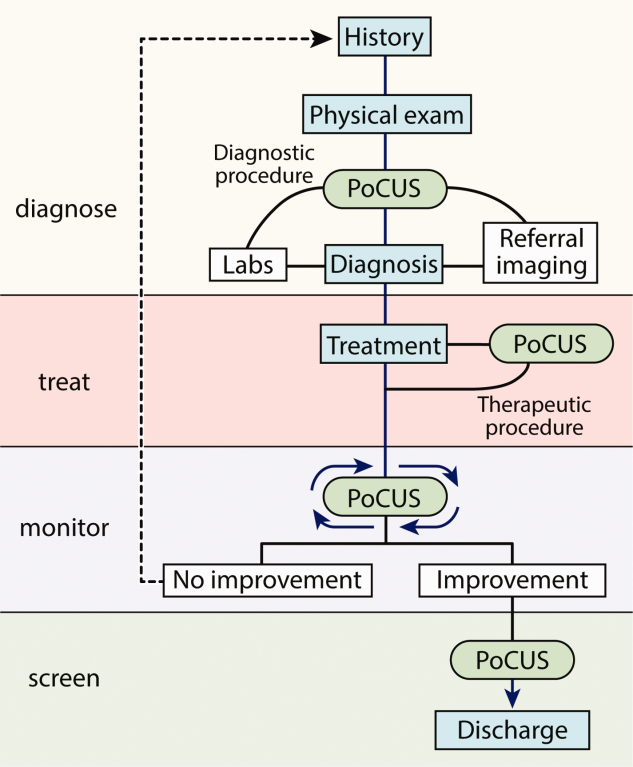
ABDOMEN
Ascites
Ultrasound is the gold standard for diagnosing ascites and can detect as little as 100 mL of ascitic fluid.[3] When ascites is not immediately evident, hospitalists can apply the principles of the FAST (Focused Assessment with Sonography in Trauma) examination to detect small amounts of ascites by evaluating the most dependent areas of the abdominopelvic cavity, the hepatorenal, left subdiaphragmatic, and rectovesicular or rectouterine spaces.[1] When ascites is identified and paracentesis is indicated, ultrasound guidance for site selection reduces bleeding complications.[4]
Aortic Aneurysm
Novice providers with limited ultrasound training can accurately screen patients for abdominal aortic aneurysm (AAA). Multiple studies from emergency departments have shown that point‐of‐care ultrasound can be used to accurately detect AAA, and a recent meta‐analysis of 7 high‐quality studies demonstrated a sensitivity of 99% (95% confidence interval [CI]: 96%‐100%) and a specificity of 98% (95% CI: 97%‐99%).[5] Hospitalists could use ultrasound to rapidly detect AAA in patients with acute abdominal pain, monitor the size in patients with known AAA, and possibly screen high‐risk patients.[6]
Hydronephrosis
Once detected, relief of postrenal obstruction usually results in rapid reversal of acute kidney injury. Although diagnostic accuracy studies of detection of hydronephrosis have yet to be conducted with hospitalists, studies of other frontline providers with limited training in renal ultrasonography have revealed sensitivities of 72% to 87% and specificities of 73% to 82% in patients with renal colic.[7, 8]
HEART
Studies of point‐of‐care cardiac ultrasound have focused most on detection of left ventricular systolic dysfunction. Yet studies among hospitalists have yielded high diagnostic accuracy for an array of abnormalities.[9, 10, 11] Lucas et al. evaluated the diagnostic accuracy of 9 hospitalists for 5 cardiac abnormalities including left ventricular systolic dysfunction after a 27‐hour, structured training program. Positive and negative likelihood ratios for point‐of‐care cardiac ultrasound increased and decreased, respectively, the prior odds by 5‐fold or more for left ventricular systolic dysfunction, severe mitral regurgitation, and moderate or large pericardial effusion. Likelihood ratios changed the prior odds by 2‐fold or more for moderate or severe left atrial enlargement, and moderate or severe left ventricle hypertrophy.[9] Martin et al. found that after a brief training program, hospitalists' image acquisition and interpretation skills were respectively below echocardiography technicians' and senior cardiology fellows' skills.[10] Yet in a follow‐up study, they found that bedside diagnosis of left ventricle systolic dysfunction, cardiomegaly, and pericardial effusion improved when point‐of‐care cardiac ultrasound supplemented hospitalists' physical examination.[11]
In 1 of the few experimental studies of the impact of point‐of‐care ultrasound on clinical care, Lucas et al. randomized general medicine patients who were referred by hospitalists for standard echocardiography to care guided by point‐of‐care cardiac ultrasound versus care guided by the referral echocardiography (usual care). Point‐of‐care cardiac ultrasound changed hospitalists' management for 37% of patients, and a post hoc subgroup analysis of heart failure patients demonstrated a statistically significant 15% reduction in length of stay.[12]
LUNGS
Pneumonia
Normally aerated lung parenchyma generates A‐lines, horizontal hyperechoic lines that are artifacts due to repeated reflections, or reverberations, between the highly reflective pleura and transducer.[1] These normal A‐lines disappear with pneumonia due to accumulation of interstitial fluid and cellular exudate in consolidated alveoli. A meta‐analysis of 9 studies of lung ultrasound to diagnose pneumonia reported pooled sensitivity of 97% (95% CI: 93%‐99%) with specificity of 94% (95% CI: 85%‐98%).[13]
Pleural Effusion
Half of patients with community‐acquired pneumonia have a pleural effusion, yet chest x‐ray often cannot differentiate pneumonia from pleural effusion, especially along the lower lung fields. Ultrasound can accurately differentiate consolidated lung from pleural effusion and is more sensitive than a chest x‐ray for detecting small pleural fluid volumes (100% vs 71%).[14] Serial monitoring of size and character of a pleural effusion can distinguish free flowing from loculated pleural effusions. Drainage of pleural effusions with ultrasound guidance is associated with a lower rate of postprocedure pneumothorax and lower total hospital costs.[15]
Pneumothorax
Lung ultrasound can accurately and rapidly detect pneumothorax after lung and pleural procedures, including thoracentesis, bronchoscopy, and transthoracic biopsy.[2] Multiple studies have demonstrated that lung ultrasound is superior to chest x‐ray. Three recent meta‐analyses reported near‐perfect specificity for both ultrasound and x‐ray. But the sensitivity of ultrasound (79%95%) was far better than that of x‐ray (40%52%) to detect pneumothorax.[16, 17]
The hallmark ultrasound findings of pneumothorax include absence of lung sliding, absence of B‐lines, and a stratified pattern using M‐mode ultrasonography (stratosphere sign). Both lung sliding and B‐lines rule out pneumothorax with a negative predictive value of 100%.[18] Absence of either finding, however, does not rule in pneumothorax with similar strength. Absent lung sliding is seen in other conditions, such as pleurodesis, mainstem intubation, and massive atelectasis; absent B‐lines are most suggestive of the normal lung (see below).[1]
Pulmonary Edema
The classic ultrasound finding of acute pulmonary edema is bilateral anterior B‐lines. In contrast to horizontal A‐lines, B‐lines are vertical, laser‐like reverberations that originate from the pleura and are due to interlobular septal edema. A linear correlation has been shown between the quantity of B‐lines and radiographic lung water score (r=0.78; P<0.01).[19] Yet B‐lines are not specific for high pulmonary capillary wedge pressure because interstitial edema can be caused by a variety of etiologies. Nonetheless, visualization of multiple B‐lines in a single intercostal space corresponds with a sensitivity of 86% to 100% and specificity of 92% to 98% for either high‐ or low‐pressure pulmonary edema.[20, 21]
VEINS
Central Venous Volume
The physiologic relationship between central venous volume and central venous pressure (CVP) is complex. Initially, there is upward stepwise progression to the stressed volume threshold, and then the relationship becomes curvilinear with the steepness of the slope dependent on the stiffness or tone of the central veins.[22]
The complexity of this relationship may explain the variable diagnostic accuracy of inferior vena cava (IVC) measurements to determine CVP, with measurements best reflecting CVP at extreme values. An IVC maximal diameter >2.0 cm predicted CVP >10 mm Hg (sensitivity 82% and specificity 84%) and pulmonary capillary wedge pressure >16 mm Hg (sensitivity 75% and specificity 83%) in 1 study.[23] Adding measurement of the collapsibility of the IVC with respiration may improve diagnostic accuracy, particularly with intermediate ranges of CVP and is recommended by current echocardiography guidelines.[24]
Nonetheless, in patients with acute dyspnea, a dilated, noncollapsing IVC may differentiate acute decompensated heart failure (ADHF) from primary pulmonary disease.[25, 26] IVC measurements may guide fluid removal in hemodialysis and heart failure patients.[27, 28] In 2 studies of patients hospitalized with ADHF, lack of improvement of IVC collapsibility index at the time of discharge was associated with higher rates of readmission.[29, 30] A follow‐up study comparing diuresis guided by IVC collapsibility to usual care in patients hospitalized with ADHF showed a reduction in hospital readmission rates (4% vs 30%, P=0.03) without an increase in hospital length of stay or renal dysfunction.[31] Patients with small, collapsed IVCs can be administered intravenous fluids safely, particularly in the setting of hypovolemic or septic shock, and the response to this fluid resuscitation can be assessed by serially measuring the change in IVC diameter.[32]
Thromboembolism
Multiple studies have shown that point‐of‐care ultrasound can accurately diagnose deep venous thrombosis (DVT) with a pooled sensitivity of 96% and specificity of 96% based on a recent meta‐analysis of 19 studies.[33] In symptomatic patients with a lung ultrasound pattern showing A‐lines, positive and negative predictive values of DVT in predicting pulmonary embolism (PE) were 94% and 98%, respectively.[34] A diagnostic accuracy study to diagnose PE using lung ultrasound to detect pleural‐ or subpleural‐based lesions yielded a sensitivity of 90%, specificity of 60%, positive predictive value of 80%, and negative predictive value of 78%.[35] In a study of 96 patients with suspected PE who underwent computed tomography pulmonary angiogram (CTPA), a focused ultrasound exam of the heart, lungs, and lower extremity veins was able to detect DVT (2.1%) or an alternative diagnosis (56.2%) in the majority of these patients, potentially obviating the need for CTPA in 58.4% of patients.[36] In addition, point‐of‐care cardiac ultrasound may reveal direct findings, such as free‐floating thrombus in the pulmonary artery, or indirect findings, such as right ventricular dilation and systolic dysfunction, septal bowing, McConnell's sign, or IVC dilation.[1] Cardiac abnormalities are more specific (88%94%) than sensitive (31%77%), and absence of cardiac abnormalities rules out massive PE, justifying withholding thrombolytic medications in most patients.[37]
RESEARCH GAPS
Most point‐of‐care ultrasound research has focused on diagnostic accuracy. Yet the training required for hospitalists to attain diagnostic competency remains controversial.[38] Evidence from cardiac point‐of‐care ultrasound training suggests that the number of supervised studies is a key determinate in competency.[39] For example, training programs based on 30 supervised studies[11, 15, 40] outperformed those based on only 5 supervised studies.[11] Nevertheless, the real value of point‐of‐care ultrasound will be in leading hospitalists to more appropriate treatment decisions that result in better outcomes for patients.[41] We believe that there are 4 important clinical areas where future research ought to focus.
First, can point‐of‐care ultrasound guide hospitalists' decision making during cardiac arrest? Current advanced cardiac life support (ACLS) guidelines recommend ruling out potentially reversible causes of cardiac arrest, including tension pneumothorax, cardiac tamponade, and massive pulmonary embolism, but traditional physical examination techniques are impractical to perform during cardiopulmonary resuscitation. Point‐of‐care ultrasound may be able to detect these conditions and facilitate emergent interventions, such as pericardiocentesis or needle decompression.[1] Identifying the absence of cardiac contractility is importantly associated with a significantly low likelihood of return of spontaneous circulation.[1, 42] Whether or not point‐of‐care ultrasound should be added to either crash carts or ACLS guideline recommendations will depend on further evidence demonstrating its value.
Second, should hospitalists seize the opportunity to screen inpatients for abdominal aortic aneurysm and asymptomatic left ventricular systolic dysfunction? Although such screening has been successfully carried out,[6, 43] widespread screening applications have been slow to develop. Ultrasound waves, themselves, impart no harm, but further research is needed to weigh the benefits of early detection against the harms of false‐positive findings.
Third, how can hospitalists best utilize bedside ultrasound to perform serial examinations of patients? Unlike referral ultrasound examinations that take single snapshots of patients at 1 point in time, point‐of‐care ultrasound allows hospitalists to iteratively monitor patients. Promising and needed applications include serial examinations of the IVC as a surrogate for central venous volume[44] during both fluid resuscitation and removal, left ventricular contraction in response to inotrope initiation, and resolution or worsening of a pneumothorax or pneumonia.
Fourth, how should hospitalists integrate point‐of‐care ultrasound into their workflow for common conditions? Recognized protocols most relevant to hospital medicine include RUSH (Rapid Ultrasound for Shock and Hypotension),[45] FALLS (Fluid Administration Limited by Lung Sonography),[46] BLUE (Bedside Lung Ultrasound in Emergency),[34] CLUE (Cardiovascular Limited Ultrasound Exam),[47] and intensive care unit‐sound.[48] Several small single‐institution studies have demonstrated that bedside ultrasound may benefit clinical decision making by differentiating cardiac versus pulmonary causes of acute dyspnea.[49, 50] However, large, validating, multicenter trials are needed. In addition, outcomes that better reflect both the patients' and payers' perspectives ought to be considered. For example, how are doctor‐patient relationships affected? Is shared decision making and patient (or physician) satisfaction improved? How are resources utilized and healthcare costs affected?
CONCLUSIONS
Hospitalists are striving to provide high‐quality, cost‐effective healthcare, and point‐of‐care ultrasound may contribute to achieving these goals by expediting diagnoses and decreasing costly ancillary testing that utilizes ionizing radiation. Hospitalists are uniquely poised to advance the field by studying how point‐of‐care ultrasound is best incorporated into patient care algorithms.
Disclosure: Nothing to report.
1. Soni NJ, Arntfield R, Kory P. Point-of-Care Ultrasound. 1st ed. Philadelphia,
PA: Saunders; 2014.
2. American Medical Association. House of Delegates. H-230.960 Privileging
for ultrasound imaging. Policy finder website. Available at:
https://ssl3.ama-assn.org/apps/ecomm/PolicyFinderForm.pl?site5www.
ama-assn.org&uri5%2fresources%2fhtml%2fPolicyFinder%2fpolicy
files%2fHnE%2fH-230.960.HTM. Accessed October 2, 2014.
3. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by
ultrasound. Radiology. 1970;96(1):15–22.
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications
and improves the cost of care among patients undergoing thoracentesis
and paracentesis. Chest. 2013;143(2):532–538.
5. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic
review: emergency department bedside ultrasonography for diagnosing
suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;
20(2):128–138.
6. Dijos M, Pucheux Y, Lafitte M, et al. Fast track echo of abdominal
aortic aneurysm using a real pocket-ultrasound device at bedside.
Echocardiography. 2012;29(3):285–290.
7. Rosen CL, Brown DF, Sagarin MJ, Chang Y, McCabe CJ, Wolfe RE.
Ultrasonography by emergency physicians in patients with suspected
ureteral colic. J Emerg Med. 1998;16(6):865–870.
8. Gaspari RJ, Horst K. Emergency ultrasound and urinalysis in the evaluation
of flank pain. Acad Emerg Med. 2005;12(12):1180–1184.
9. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of
hospitalist-performed hand-carried ultrasound echocardiography after
a brief training program. J Hosp Med. 2009;4(6):340–349.
10. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP,
Hellmann DB. Hospitalist performance of cardiac hand-carried ultrasound
after focused training. Am J Med. 2007;120(11):1000–1004.
11. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound
performed by hospitalists: does it improve the cardiac physical
examination? Am J Med. 2009;122(1):35–41.
PoCUS for Hospitalists | Soni and Lucas
An Official Publication of the Society of Hospital Medicine Journal of Hospital Medicine Vol 10 | No 2 | February 2015 123
12. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography
by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–
774.
13. Hu QJ, Shen YC, Jia LQ, et al. Diagnostic performance of lung ultrasound
in the diagnosis of pneumonia: a bivariate meta-analysis. Int J
Clin Exp Med. 2014;7(1):115–121.
14. Reissig A, Gramegna A, Aliberti S. The role of lung ultrasound in the
diagnosis and follow-up of community-acquired pneumonia. Eur J
Intern Med. 2012;23(5):391–397.
15. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance
reduces complications and costs associated with thoracentesis procedures.
J Clin Ultrasound. 2012;40(3):135–141.
16. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax
by radiography and ultrasonography: a meta-analysis. Chest.
2011;140(4):859–866.
17. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography
versus chest radiography for the diagnosis of pneumothorax: review
of the literature and meta-analysis. Crit Care. 2013;17(5):R208.
18. Lichtenstein D, Meziere G, Biderman P, Gepner A. The comet-tail
artifact: an ultrasound sign ruling out pneumothorax. Intensive Care
Med. 1999;25(4):383–388.
19. Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G.
Ultrasound lung comets: a clinically useful sign of extravascular lung
water. J Am Soc Echocardiogr. 2006;19(3):356–363.
20. Lichtenstein D, Meziere G. A lung ultrasound sign allowing bedside
distinction between pulmonary edema and COPD: the comet-tail artifact.
Intensive Care Med. 1998;24(12):1331–1334.
21. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in
the assessment of alveolar-interstitial syndrome. Am J Emerg Med.
2006;24(6):689–696.
22. Rothe CF. Reflex control of veins and vascular capacitance. Physiol
Rev. 1983;63(4):1281–1342.
23. Blair JE, Brennan JM, Goonewardena SN, Shah D, Vasaiwala S,
Spencer KT. Usefulness of hand-carried ultrasound to predict elevated
left ventricular filling pressure. Am J Cardiol. 2009;103(2):246–247.
24. Beigel R, Cercek B, Luo H, Siegel RJ. Noninvasive evaluation of right
atrial pressure. J Am Soc Echocardiogr. 2013;26(9):1033–1042.
25. Miller JB, Sen A, Strote SR, et al. Inferior vena cava assessment in the
bedside diagnosis of acute heart failure. Am J Emerg Med. 2012;
30(5):778–783.
26. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart
failure via respiratory variation of inferior vena cava diameter. Am J
Emerg Med. 2009;27(1):71–75.
27. Goonewardena SN, Spencer KT. Handcarried echocardiography to
assess hemodynamics in acute decompensated heart failure. Curr
Heart Fail Rep. 2010;7(4):219–227.
28. Guiotto G, Masarone M, Paladino F, et al. Inferior vena cava collapsibility
to guide fluid removal in slow continuous ultrafiltration: a pilot
study. Intensive Care Med. 2010;36(4):692–696.
29. Carbone F, Bovio M, Rosa GM, et al. Inferior vena cava parameters
predict readmission in ischemic heart failure. Eur J Clin Invest. 2014;
44(4):341–349.
30. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of
hand-carried ultrasound assessment of the inferior vena cava and Nterminal
pro-brain natriuretic peptide for predicting readmission after
hospitalization for acute decompensated heart failure. JACC Cardiovasc
Imaging. 2008;1(5):595–601.
31. Laffin L, Patel AR, Saha N, et al. Inferior vena cava measurement by
focused cardiac ultrasound in acute decompensated heart failure prevents
hospital readmissions. J Am Coll Cardiol. 2014;63(12 suppl):
A542.
32. Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the
respiratory variation in the inferior vena cava diameter is predictive of
fluid responsiveness in critically ill patients: systematic review and
meta-analysis. Ultrasound Med Biol. 2014;40(5):845–853.
33. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency
physician-performed ultrasonography in the diagnosis of deep-vein
thrombosis: a systematic review and meta-analysis. Thromb Haemost.
2013;109(1):137–145.
34. Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the
diagnosis of acute respiratory failure: the BLUE protocol. Chest.
2008;134(1):117–125.
35. Comert SS, Caglayan B, Akturk U, et al. The role of thoracic ultrasonography
in the diagnosis of pulmonary embolism. Ann Thorac Med.
2013;8(2):99–104.
36. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH,
Narasimhan M. Ultrasound assessment of pulmonary embolism in
patients receiving computerized tomography pulmonary angiography.
Chest. 2014;145(4):818–823.
37. Mookadam F, Jiamsripong P, Goel R, Warsame TA, Emani UR,
Khandheria BK. Critical appraisal on the utility of echocardiography
in the management of acute pulmonary embolism. Cardiol Rev. 2010;
18(1):29–37.
38. Gesensway D. Making the case for portable ultrasound. Todays Hospitalist.
2012;10:32–36.
39. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel
RJ. Focused cardiac ultrasound: recommendations from the American
Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):
567–581.
40. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire
C, Ziegelstein RC. The rate at which residents learn to use hand-held
echocardiography at the bedside. Am J Med. 2005;118(9):1010–
1018.
41. Redberg RF, Walsch J. Pay now, benefits may follow—the case of cardiac
computed tomographic angiography. N Engl J Med. 2008;359:
2309–2311.
42. Blyth L, Atkinson P, Gadd K, Lang E. Bedside focused echocardiography
as predictor of survival in cardiac arrest patients: a systematic
review. Acad Emerg Med. 2012;19(10):1119–1126.
43. Martin LD, Mathews S, Ziegelstein RC, et al. Prevalence of asymptomatic
left ventricular systolic dysfunction in at-risk medical inpatients.
Am J Med. 2013;126(1):68–73.
44. Low D, Vlasschaert M, Novak K, Chee A, Ma IWY. An argument for
using additional bedside tools, such as bedside ultrasound, for volume
status assessment in hospitalized medical patients: a needs assessment
survey. J Hosp Med. 2014;9:727–730.
45. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid
Ultrasound in SHock in the evaluation of the critically lll. Emerg Med
Clin North Am. 2010;28(1):29–56, vii.
46. Lichtenstein D. FALLS-protocol: lung ultrasound in hemodynamic
assessment of shock. Heart Lung Vessel. 2013;5(3):142–147.
47. Kimura BJ, Yogo N, O’Connell CW, Phan JN, Showalter BK,
Wolfson T. Cardiopulmonary limited ultrasound examination for
“quick-look” bedside application. Am J Cardiol. 2011;108(4):586–
590.
48. Manno E, Navarra M, Faccio L, et al. Deep impact of ultrasound in
the intensive care unit: the “ICU-sound” protocol. Anesthesiology.
2012;117(4):801–809.
49. Cibinel GA, Casoli G, Elia F, et al. Diagnostic accuracy and reproducibility
of pleural and lung ultrasound in discriminating cardiogenic
causes of acute dyspnea in the emergency department. Intern Emerg
Med. 2012;7(1):65–70.
50. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing
heart failure among acutely dyspneic patients with cardiac,
inferior vena cava, and lung ultrasonography. Am J Emerg Med.
2013;31(8):1208–1214.
Similar to the physical exam, diagnostic point‐of‐care ultrasound exams are performed at the bedside in real time by hospitalists who are seeking a diagnosis. In contrast, referral ultrasound exams involve multiple providers and several steps. Typically, an ultrasound technologist acquires images, a radiologist or cardiologist interprets the images, a report is prepared, and results are sent to the referring hospitalist (Figure 1). Another important difference is that although referral ultrasound exams are usually comprehensive evaluations of entire organs or anatomic spaces, often without specific diagnoses in mind, point‐of‐care ultrasound exams are aimed at making specific diagnoses for well‐defined clinical scenarios.[1]

The American Medical Association has reassured providers that ultrasound imaging is within the scope of practice of appropriately trained physicians.[2] A growing body of literature demonstrates that point‐of‐care ultrasound is increasingly used by hospitalists for more than just bedside procedures. Incited by ongoing miniaturization of ultrasound devices, hospitalists are beginning to use point‐of‐care ultrasound for diagnosis, treatment, monitoring, and screening of patients (Figure 2). Our aim was to review the current literature for point‐of‐care ultrasound applications most relevant to hospitalists and highlight gaps in the current literature.

ABDOMEN
Ascites
Ultrasound is the gold standard for diagnosing ascites and can detect as little as 100 mL of ascitic fluid.[3] When ascites is not immediately evident, hospitalists can apply the principles of the FAST (Focused Assessment with Sonography in Trauma) examination to detect small amounts of ascites by evaluating the most dependent areas of the abdominopelvic cavity, the hepatorenal, left subdiaphragmatic, and rectovesicular or rectouterine spaces.[1] When ascites is identified and paracentesis is indicated, ultrasound guidance for site selection reduces bleeding complications.[4]
Aortic Aneurysm
Novice providers with limited ultrasound training can accurately screen patients for abdominal aortic aneurysm (AAA). Multiple studies from emergency departments have shown that point‐of‐care ultrasound can be used to accurately detect AAA, and a recent meta‐analysis of 7 high‐quality studies demonstrated a sensitivity of 99% (95% confidence interval [CI]: 96%‐100%) and a specificity of 98% (95% CI: 97%‐99%).[5] Hospitalists could use ultrasound to rapidly detect AAA in patients with acute abdominal pain, monitor the size in patients with known AAA, and possibly screen high‐risk patients.[6]
Hydronephrosis
Once detected, relief of postrenal obstruction usually results in rapid reversal of acute kidney injury. Although diagnostic accuracy studies of detection of hydronephrosis have yet to be conducted with hospitalists, studies of other frontline providers with limited training in renal ultrasonography have revealed sensitivities of 72% to 87% and specificities of 73% to 82% in patients with renal colic.[7, 8]
HEART
Studies of point‐of‐care cardiac ultrasound have focused most on detection of left ventricular systolic dysfunction. Yet studies among hospitalists have yielded high diagnostic accuracy for an array of abnormalities.[9, 10, 11] Lucas et al. evaluated the diagnostic accuracy of 9 hospitalists for 5 cardiac abnormalities including left ventricular systolic dysfunction after a 27‐hour, structured training program. Positive and negative likelihood ratios for point‐of‐care cardiac ultrasound increased and decreased, respectively, the prior odds by 5‐fold or more for left ventricular systolic dysfunction, severe mitral regurgitation, and moderate or large pericardial effusion. Likelihood ratios changed the prior odds by 2‐fold or more for moderate or severe left atrial enlargement, and moderate or severe left ventricle hypertrophy.[9] Martin et al. found that after a brief training program, hospitalists' image acquisition and interpretation skills were respectively below echocardiography technicians' and senior cardiology fellows' skills.[10] Yet in a follow‐up study, they found that bedside diagnosis of left ventricle systolic dysfunction, cardiomegaly, and pericardial effusion improved when point‐of‐care cardiac ultrasound supplemented hospitalists' physical examination.[11]
In 1 of the few experimental studies of the impact of point‐of‐care ultrasound on clinical care, Lucas et al. randomized general medicine patients who were referred by hospitalists for standard echocardiography to care guided by point‐of‐care cardiac ultrasound versus care guided by the referral echocardiography (usual care). Point‐of‐care cardiac ultrasound changed hospitalists' management for 37% of patients, and a post hoc subgroup analysis of heart failure patients demonstrated a statistically significant 15% reduction in length of stay.[12]
LUNGS
Pneumonia
Normally aerated lung parenchyma generates A‐lines, horizontal hyperechoic lines that are artifacts due to repeated reflections, or reverberations, between the highly reflective pleura and transducer.[1] These normal A‐lines disappear with pneumonia due to accumulation of interstitial fluid and cellular exudate in consolidated alveoli. A meta‐analysis of 9 studies of lung ultrasound to diagnose pneumonia reported pooled sensitivity of 97% (95% CI: 93%‐99%) with specificity of 94% (95% CI: 85%‐98%).[13]
Pleural Effusion
Half of patients with community‐acquired pneumonia have a pleural effusion, yet chest x‐ray often cannot differentiate pneumonia from pleural effusion, especially along the lower lung fields. Ultrasound can accurately differentiate consolidated lung from pleural effusion and is more sensitive than a chest x‐ray for detecting small pleural fluid volumes (100% vs 71%).[14] Serial monitoring of size and character of a pleural effusion can distinguish free flowing from loculated pleural effusions. Drainage of pleural effusions with ultrasound guidance is associated with a lower rate of postprocedure pneumothorax and lower total hospital costs.[15]
Pneumothorax
Lung ultrasound can accurately and rapidly detect pneumothorax after lung and pleural procedures, including thoracentesis, bronchoscopy, and transthoracic biopsy.[2] Multiple studies have demonstrated that lung ultrasound is superior to chest x‐ray. Three recent meta‐analyses reported near‐perfect specificity for both ultrasound and x‐ray. But the sensitivity of ultrasound (79%95%) was far better than that of x‐ray (40%52%) to detect pneumothorax.[16, 17]
The hallmark ultrasound findings of pneumothorax include absence of lung sliding, absence of B‐lines, and a stratified pattern using M‐mode ultrasonography (stratosphere sign). Both lung sliding and B‐lines rule out pneumothorax with a negative predictive value of 100%.[18] Absence of either finding, however, does not rule in pneumothorax with similar strength. Absent lung sliding is seen in other conditions, such as pleurodesis, mainstem intubation, and massive atelectasis; absent B‐lines are most suggestive of the normal lung (see below).[1]
Pulmonary Edema
The classic ultrasound finding of acute pulmonary edema is bilateral anterior B‐lines. In contrast to horizontal A‐lines, B‐lines are vertical, laser‐like reverberations that originate from the pleura and are due to interlobular septal edema. A linear correlation has been shown between the quantity of B‐lines and radiographic lung water score (r=0.78; P<0.01).[19] Yet B‐lines are not specific for high pulmonary capillary wedge pressure because interstitial edema can be caused by a variety of etiologies. Nonetheless, visualization of multiple B‐lines in a single intercostal space corresponds with a sensitivity of 86% to 100% and specificity of 92% to 98% for either high‐ or low‐pressure pulmonary edema.[20, 21]
VEINS
Central Venous Volume
The physiologic relationship between central venous volume and central venous pressure (CVP) is complex. Initially, there is upward stepwise progression to the stressed volume threshold, and then the relationship becomes curvilinear with the steepness of the slope dependent on the stiffness or tone of the central veins.[22]
The complexity of this relationship may explain the variable diagnostic accuracy of inferior vena cava (IVC) measurements to determine CVP, with measurements best reflecting CVP at extreme values. An IVC maximal diameter >2.0 cm predicted CVP >10 mm Hg (sensitivity 82% and specificity 84%) and pulmonary capillary wedge pressure >16 mm Hg (sensitivity 75% and specificity 83%) in 1 study.[23] Adding measurement of the collapsibility of the IVC with respiration may improve diagnostic accuracy, particularly with intermediate ranges of CVP and is recommended by current echocardiography guidelines.[24]
Nonetheless, in patients with acute dyspnea, a dilated, noncollapsing IVC may differentiate acute decompensated heart failure (ADHF) from primary pulmonary disease.[25, 26] IVC measurements may guide fluid removal in hemodialysis and heart failure patients.[27, 28] In 2 studies of patients hospitalized with ADHF, lack of improvement of IVC collapsibility index at the time of discharge was associated with higher rates of readmission.[29, 30] A follow‐up study comparing diuresis guided by IVC collapsibility to usual care in patients hospitalized with ADHF showed a reduction in hospital readmission rates (4% vs 30%, P=0.03) without an increase in hospital length of stay or renal dysfunction.[31] Patients with small, collapsed IVCs can be administered intravenous fluids safely, particularly in the setting of hypovolemic or septic shock, and the response to this fluid resuscitation can be assessed by serially measuring the change in IVC diameter.[32]
Thromboembolism
Multiple studies have shown that point‐of‐care ultrasound can accurately diagnose deep venous thrombosis (DVT) with a pooled sensitivity of 96% and specificity of 96% based on a recent meta‐analysis of 19 studies.[33] In symptomatic patients with a lung ultrasound pattern showing A‐lines, positive and negative predictive values of DVT in predicting pulmonary embolism (PE) were 94% and 98%, respectively.[34] A diagnostic accuracy study to diagnose PE using lung ultrasound to detect pleural‐ or subpleural‐based lesions yielded a sensitivity of 90%, specificity of 60%, positive predictive value of 80%, and negative predictive value of 78%.[35] In a study of 96 patients with suspected PE who underwent computed tomography pulmonary angiogram (CTPA), a focused ultrasound exam of the heart, lungs, and lower extremity veins was able to detect DVT (2.1%) or an alternative diagnosis (56.2%) in the majority of these patients, potentially obviating the need for CTPA in 58.4% of patients.[36] In addition, point‐of‐care cardiac ultrasound may reveal direct findings, such as free‐floating thrombus in the pulmonary artery, or indirect findings, such as right ventricular dilation and systolic dysfunction, septal bowing, McConnell's sign, or IVC dilation.[1] Cardiac abnormalities are more specific (88%94%) than sensitive (31%77%), and absence of cardiac abnormalities rules out massive PE, justifying withholding thrombolytic medications in most patients.[37]
RESEARCH GAPS
Most point‐of‐care ultrasound research has focused on diagnostic accuracy. Yet the training required for hospitalists to attain diagnostic competency remains controversial.[38] Evidence from cardiac point‐of‐care ultrasound training suggests that the number of supervised studies is a key determinate in competency.[39] For example, training programs based on 30 supervised studies[11, 15, 40] outperformed those based on only 5 supervised studies.[11] Nevertheless, the real value of point‐of‐care ultrasound will be in leading hospitalists to more appropriate treatment decisions that result in better outcomes for patients.[41] We believe that there are 4 important clinical areas where future research ought to focus.
First, can point‐of‐care ultrasound guide hospitalists' decision making during cardiac arrest? Current advanced cardiac life support (ACLS) guidelines recommend ruling out potentially reversible causes of cardiac arrest, including tension pneumothorax, cardiac tamponade, and massive pulmonary embolism, but traditional physical examination techniques are impractical to perform during cardiopulmonary resuscitation. Point‐of‐care ultrasound may be able to detect these conditions and facilitate emergent interventions, such as pericardiocentesis or needle decompression.[1] Identifying the absence of cardiac contractility is importantly associated with a significantly low likelihood of return of spontaneous circulation.[1, 42] Whether or not point‐of‐care ultrasound should be added to either crash carts or ACLS guideline recommendations will depend on further evidence demonstrating its value.
Second, should hospitalists seize the opportunity to screen inpatients for abdominal aortic aneurysm and asymptomatic left ventricular systolic dysfunction? Although such screening has been successfully carried out,[6, 43] widespread screening applications have been slow to develop. Ultrasound waves, themselves, impart no harm, but further research is needed to weigh the benefits of early detection against the harms of false‐positive findings.
Third, how can hospitalists best utilize bedside ultrasound to perform serial examinations of patients? Unlike referral ultrasound examinations that take single snapshots of patients at 1 point in time, point‐of‐care ultrasound allows hospitalists to iteratively monitor patients. Promising and needed applications include serial examinations of the IVC as a surrogate for central venous volume[44] during both fluid resuscitation and removal, left ventricular contraction in response to inotrope initiation, and resolution or worsening of a pneumothorax or pneumonia.
Fourth, how should hospitalists integrate point‐of‐care ultrasound into their workflow for common conditions? Recognized protocols most relevant to hospital medicine include RUSH (Rapid Ultrasound for Shock and Hypotension),[45] FALLS (Fluid Administration Limited by Lung Sonography),[46] BLUE (Bedside Lung Ultrasound in Emergency),[34] CLUE (Cardiovascular Limited Ultrasound Exam),[47] and intensive care unit‐sound.[48] Several small single‐institution studies have demonstrated that bedside ultrasound may benefit clinical decision making by differentiating cardiac versus pulmonary causes of acute dyspnea.[49, 50] However, large, validating, multicenter trials are needed. In addition, outcomes that better reflect both the patients' and payers' perspectives ought to be considered. For example, how are doctor‐patient relationships affected? Is shared decision making and patient (or physician) satisfaction improved? How are resources utilized and healthcare costs affected?
CONCLUSIONS
Hospitalists are striving to provide high‐quality, cost‐effective healthcare, and point‐of‐care ultrasound may contribute to achieving these goals by expediting diagnoses and decreasing costly ancillary testing that utilizes ionizing radiation. Hospitalists are uniquely poised to advance the field by studying how point‐of‐care ultrasound is best incorporated into patient care algorithms.
Disclosure: Nothing to report.
Similar to the physical exam, diagnostic point‐of‐care ultrasound exams are performed at the bedside in real time by hospitalists who are seeking a diagnosis. In contrast, referral ultrasound exams involve multiple providers and several steps. Typically, an ultrasound technologist acquires images, a radiologist or cardiologist interprets the images, a report is prepared, and results are sent to the referring hospitalist (Figure 1). Another important difference is that although referral ultrasound exams are usually comprehensive evaluations of entire organs or anatomic spaces, often without specific diagnoses in mind, point‐of‐care ultrasound exams are aimed at making specific diagnoses for well‐defined clinical scenarios.[1]

The American Medical Association has reassured providers that ultrasound imaging is within the scope of practice of appropriately trained physicians.[2] A growing body of literature demonstrates that point‐of‐care ultrasound is increasingly used by hospitalists for more than just bedside procedures. Incited by ongoing miniaturization of ultrasound devices, hospitalists are beginning to use point‐of‐care ultrasound for diagnosis, treatment, monitoring, and screening of patients (Figure 2). Our aim was to review the current literature for point‐of‐care ultrasound applications most relevant to hospitalists and highlight gaps in the current literature.

ABDOMEN
Ascites
Ultrasound is the gold standard for diagnosing ascites and can detect as little as 100 mL of ascitic fluid.[3] When ascites is not immediately evident, hospitalists can apply the principles of the FAST (Focused Assessment with Sonography in Trauma) examination to detect small amounts of ascites by evaluating the most dependent areas of the abdominopelvic cavity, the hepatorenal, left subdiaphragmatic, and rectovesicular or rectouterine spaces.[1] When ascites is identified and paracentesis is indicated, ultrasound guidance for site selection reduces bleeding complications.[4]
Aortic Aneurysm
Novice providers with limited ultrasound training can accurately screen patients for abdominal aortic aneurysm (AAA). Multiple studies from emergency departments have shown that point‐of‐care ultrasound can be used to accurately detect AAA, and a recent meta‐analysis of 7 high‐quality studies demonstrated a sensitivity of 99% (95% confidence interval [CI]: 96%‐100%) and a specificity of 98% (95% CI: 97%‐99%).[5] Hospitalists could use ultrasound to rapidly detect AAA in patients with acute abdominal pain, monitor the size in patients with known AAA, and possibly screen high‐risk patients.[6]
Hydronephrosis
Once detected, relief of postrenal obstruction usually results in rapid reversal of acute kidney injury. Although diagnostic accuracy studies of detection of hydronephrosis have yet to be conducted with hospitalists, studies of other frontline providers with limited training in renal ultrasonography have revealed sensitivities of 72% to 87% and specificities of 73% to 82% in patients with renal colic.[7, 8]
HEART
Studies of point‐of‐care cardiac ultrasound have focused most on detection of left ventricular systolic dysfunction. Yet studies among hospitalists have yielded high diagnostic accuracy for an array of abnormalities.[9, 10, 11] Lucas et al. evaluated the diagnostic accuracy of 9 hospitalists for 5 cardiac abnormalities including left ventricular systolic dysfunction after a 27‐hour, structured training program. Positive and negative likelihood ratios for point‐of‐care cardiac ultrasound increased and decreased, respectively, the prior odds by 5‐fold or more for left ventricular systolic dysfunction, severe mitral regurgitation, and moderate or large pericardial effusion. Likelihood ratios changed the prior odds by 2‐fold or more for moderate or severe left atrial enlargement, and moderate or severe left ventricle hypertrophy.[9] Martin et al. found that after a brief training program, hospitalists' image acquisition and interpretation skills were respectively below echocardiography technicians' and senior cardiology fellows' skills.[10] Yet in a follow‐up study, they found that bedside diagnosis of left ventricle systolic dysfunction, cardiomegaly, and pericardial effusion improved when point‐of‐care cardiac ultrasound supplemented hospitalists' physical examination.[11]
In 1 of the few experimental studies of the impact of point‐of‐care ultrasound on clinical care, Lucas et al. randomized general medicine patients who were referred by hospitalists for standard echocardiography to care guided by point‐of‐care cardiac ultrasound versus care guided by the referral echocardiography (usual care). Point‐of‐care cardiac ultrasound changed hospitalists' management for 37% of patients, and a post hoc subgroup analysis of heart failure patients demonstrated a statistically significant 15% reduction in length of stay.[12]
LUNGS
Pneumonia
Normally aerated lung parenchyma generates A‐lines, horizontal hyperechoic lines that are artifacts due to repeated reflections, or reverberations, between the highly reflective pleura and transducer.[1] These normal A‐lines disappear with pneumonia due to accumulation of interstitial fluid and cellular exudate in consolidated alveoli. A meta‐analysis of 9 studies of lung ultrasound to diagnose pneumonia reported pooled sensitivity of 97% (95% CI: 93%‐99%) with specificity of 94% (95% CI: 85%‐98%).[13]
Pleural Effusion
Half of patients with community‐acquired pneumonia have a pleural effusion, yet chest x‐ray often cannot differentiate pneumonia from pleural effusion, especially along the lower lung fields. Ultrasound can accurately differentiate consolidated lung from pleural effusion and is more sensitive than a chest x‐ray for detecting small pleural fluid volumes (100% vs 71%).[14] Serial monitoring of size and character of a pleural effusion can distinguish free flowing from loculated pleural effusions. Drainage of pleural effusions with ultrasound guidance is associated with a lower rate of postprocedure pneumothorax and lower total hospital costs.[15]
Pneumothorax
Lung ultrasound can accurately and rapidly detect pneumothorax after lung and pleural procedures, including thoracentesis, bronchoscopy, and transthoracic biopsy.[2] Multiple studies have demonstrated that lung ultrasound is superior to chest x‐ray. Three recent meta‐analyses reported near‐perfect specificity for both ultrasound and x‐ray. But the sensitivity of ultrasound (79%95%) was far better than that of x‐ray (40%52%) to detect pneumothorax.[16, 17]
The hallmark ultrasound findings of pneumothorax include absence of lung sliding, absence of B‐lines, and a stratified pattern using M‐mode ultrasonography (stratosphere sign). Both lung sliding and B‐lines rule out pneumothorax with a negative predictive value of 100%.[18] Absence of either finding, however, does not rule in pneumothorax with similar strength. Absent lung sliding is seen in other conditions, such as pleurodesis, mainstem intubation, and massive atelectasis; absent B‐lines are most suggestive of the normal lung (see below).[1]
Pulmonary Edema
The classic ultrasound finding of acute pulmonary edema is bilateral anterior B‐lines. In contrast to horizontal A‐lines, B‐lines are vertical, laser‐like reverberations that originate from the pleura and are due to interlobular septal edema. A linear correlation has been shown between the quantity of B‐lines and radiographic lung water score (r=0.78; P<0.01).[19] Yet B‐lines are not specific for high pulmonary capillary wedge pressure because interstitial edema can be caused by a variety of etiologies. Nonetheless, visualization of multiple B‐lines in a single intercostal space corresponds with a sensitivity of 86% to 100% and specificity of 92% to 98% for either high‐ or low‐pressure pulmonary edema.[20, 21]
VEINS
Central Venous Volume
The physiologic relationship between central venous volume and central venous pressure (CVP) is complex. Initially, there is upward stepwise progression to the stressed volume threshold, and then the relationship becomes curvilinear with the steepness of the slope dependent on the stiffness or tone of the central veins.[22]
The complexity of this relationship may explain the variable diagnostic accuracy of inferior vena cava (IVC) measurements to determine CVP, with measurements best reflecting CVP at extreme values. An IVC maximal diameter >2.0 cm predicted CVP >10 mm Hg (sensitivity 82% and specificity 84%) and pulmonary capillary wedge pressure >16 mm Hg (sensitivity 75% and specificity 83%) in 1 study.[23] Adding measurement of the collapsibility of the IVC with respiration may improve diagnostic accuracy, particularly with intermediate ranges of CVP and is recommended by current echocardiography guidelines.[24]
Nonetheless, in patients with acute dyspnea, a dilated, noncollapsing IVC may differentiate acute decompensated heart failure (ADHF) from primary pulmonary disease.[25, 26] IVC measurements may guide fluid removal in hemodialysis and heart failure patients.[27, 28] In 2 studies of patients hospitalized with ADHF, lack of improvement of IVC collapsibility index at the time of discharge was associated with higher rates of readmission.[29, 30] A follow‐up study comparing diuresis guided by IVC collapsibility to usual care in patients hospitalized with ADHF showed a reduction in hospital readmission rates (4% vs 30%, P=0.03) without an increase in hospital length of stay or renal dysfunction.[31] Patients with small, collapsed IVCs can be administered intravenous fluids safely, particularly in the setting of hypovolemic or septic shock, and the response to this fluid resuscitation can be assessed by serially measuring the change in IVC diameter.[32]
Thromboembolism
Multiple studies have shown that point‐of‐care ultrasound can accurately diagnose deep venous thrombosis (DVT) with a pooled sensitivity of 96% and specificity of 96% based on a recent meta‐analysis of 19 studies.[33] In symptomatic patients with a lung ultrasound pattern showing A‐lines, positive and negative predictive values of DVT in predicting pulmonary embolism (PE) were 94% and 98%, respectively.[34] A diagnostic accuracy study to diagnose PE using lung ultrasound to detect pleural‐ or subpleural‐based lesions yielded a sensitivity of 90%, specificity of 60%, positive predictive value of 80%, and negative predictive value of 78%.[35] In a study of 96 patients with suspected PE who underwent computed tomography pulmonary angiogram (CTPA), a focused ultrasound exam of the heart, lungs, and lower extremity veins was able to detect DVT (2.1%) or an alternative diagnosis (56.2%) in the majority of these patients, potentially obviating the need for CTPA in 58.4% of patients.[36] In addition, point‐of‐care cardiac ultrasound may reveal direct findings, such as free‐floating thrombus in the pulmonary artery, or indirect findings, such as right ventricular dilation and systolic dysfunction, septal bowing, McConnell's sign, or IVC dilation.[1] Cardiac abnormalities are more specific (88%94%) than sensitive (31%77%), and absence of cardiac abnormalities rules out massive PE, justifying withholding thrombolytic medications in most patients.[37]
RESEARCH GAPS
Most point‐of‐care ultrasound research has focused on diagnostic accuracy. Yet the training required for hospitalists to attain diagnostic competency remains controversial.[38] Evidence from cardiac point‐of‐care ultrasound training suggests that the number of supervised studies is a key determinate in competency.[39] For example, training programs based on 30 supervised studies[11, 15, 40] outperformed those based on only 5 supervised studies.[11] Nevertheless, the real value of point‐of‐care ultrasound will be in leading hospitalists to more appropriate treatment decisions that result in better outcomes for patients.[41] We believe that there are 4 important clinical areas where future research ought to focus.
First, can point‐of‐care ultrasound guide hospitalists' decision making during cardiac arrest? Current advanced cardiac life support (ACLS) guidelines recommend ruling out potentially reversible causes of cardiac arrest, including tension pneumothorax, cardiac tamponade, and massive pulmonary embolism, but traditional physical examination techniques are impractical to perform during cardiopulmonary resuscitation. Point‐of‐care ultrasound may be able to detect these conditions and facilitate emergent interventions, such as pericardiocentesis or needle decompression.[1] Identifying the absence of cardiac contractility is importantly associated with a significantly low likelihood of return of spontaneous circulation.[1, 42] Whether or not point‐of‐care ultrasound should be added to either crash carts or ACLS guideline recommendations will depend on further evidence demonstrating its value.
Second, should hospitalists seize the opportunity to screen inpatients for abdominal aortic aneurysm and asymptomatic left ventricular systolic dysfunction? Although such screening has been successfully carried out,[6, 43] widespread screening applications have been slow to develop. Ultrasound waves, themselves, impart no harm, but further research is needed to weigh the benefits of early detection against the harms of false‐positive findings.
Third, how can hospitalists best utilize bedside ultrasound to perform serial examinations of patients? Unlike referral ultrasound examinations that take single snapshots of patients at 1 point in time, point‐of‐care ultrasound allows hospitalists to iteratively monitor patients. Promising and needed applications include serial examinations of the IVC as a surrogate for central venous volume[44] during both fluid resuscitation and removal, left ventricular contraction in response to inotrope initiation, and resolution or worsening of a pneumothorax or pneumonia.
Fourth, how should hospitalists integrate point‐of‐care ultrasound into their workflow for common conditions? Recognized protocols most relevant to hospital medicine include RUSH (Rapid Ultrasound for Shock and Hypotension),[45] FALLS (Fluid Administration Limited by Lung Sonography),[46] BLUE (Bedside Lung Ultrasound in Emergency),[34] CLUE (Cardiovascular Limited Ultrasound Exam),[47] and intensive care unit‐sound.[48] Several small single‐institution studies have demonstrated that bedside ultrasound may benefit clinical decision making by differentiating cardiac versus pulmonary causes of acute dyspnea.[49, 50] However, large, validating, multicenter trials are needed. In addition, outcomes that better reflect both the patients' and payers' perspectives ought to be considered. For example, how are doctor‐patient relationships affected? Is shared decision making and patient (or physician) satisfaction improved? How are resources utilized and healthcare costs affected?
CONCLUSIONS
Hospitalists are striving to provide high‐quality, cost‐effective healthcare, and point‐of‐care ultrasound may contribute to achieving these goals by expediting diagnoses and decreasing costly ancillary testing that utilizes ionizing radiation. Hospitalists are uniquely poised to advance the field by studying how point‐of‐care ultrasound is best incorporated into patient care algorithms.
Disclosure: Nothing to report.
1. Soni NJ, Arntfield R, Kory P. Point-of-Care Ultrasound. 1st ed. Philadelphia,
PA: Saunders; 2014.
2. American Medical Association. House of Delegates. H-230.960 Privileging
for ultrasound imaging. Policy finder website. Available at:
https://ssl3.ama-assn.org/apps/ecomm/PolicyFinderForm.pl?site5www.
ama-assn.org&uri5%2fresources%2fhtml%2fPolicyFinder%2fpolicy
files%2fHnE%2fH-230.960.HTM. Accessed October 2, 2014.
3. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by
ultrasound. Radiology. 1970;96(1):15–22.
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications
and improves the cost of care among patients undergoing thoracentesis
and paracentesis. Chest. 2013;143(2):532–538.
5. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic
review: emergency department bedside ultrasonography for diagnosing
suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;
20(2):128–138.
6. Dijos M, Pucheux Y, Lafitte M, et al. Fast track echo of abdominal
aortic aneurysm using a real pocket-ultrasound device at bedside.
Echocardiography. 2012;29(3):285–290.
7. Rosen CL, Brown DF, Sagarin MJ, Chang Y, McCabe CJ, Wolfe RE.
Ultrasonography by emergency physicians in patients with suspected
ureteral colic. J Emerg Med. 1998;16(6):865–870.
8. Gaspari RJ, Horst K. Emergency ultrasound and urinalysis in the evaluation
of flank pain. Acad Emerg Med. 2005;12(12):1180–1184.
9. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of
hospitalist-performed hand-carried ultrasound echocardiography after
a brief training program. J Hosp Med. 2009;4(6):340–349.
10. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP,
Hellmann DB. Hospitalist performance of cardiac hand-carried ultrasound
after focused training. Am J Med. 2007;120(11):1000–1004.
11. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound
performed by hospitalists: does it improve the cardiac physical
examination? Am J Med. 2009;122(1):35–41.
PoCUS for Hospitalists | Soni and Lucas
An Official Publication of the Society of Hospital Medicine Journal of Hospital Medicine Vol 10 | No 2 | February 2015 123
12. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography
by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–
774.
13. Hu QJ, Shen YC, Jia LQ, et al. Diagnostic performance of lung ultrasound
in the diagnosis of pneumonia: a bivariate meta-analysis. Int J
Clin Exp Med. 2014;7(1):115–121.
14. Reissig A, Gramegna A, Aliberti S. The role of lung ultrasound in the
diagnosis and follow-up of community-acquired pneumonia. Eur J
Intern Med. 2012;23(5):391–397.
15. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance
reduces complications and costs associated with thoracentesis procedures.
J Clin Ultrasound. 2012;40(3):135–141.
16. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax
by radiography and ultrasonography: a meta-analysis. Chest.
2011;140(4):859–866.
17. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography
versus chest radiography for the diagnosis of pneumothorax: review
of the literature and meta-analysis. Crit Care. 2013;17(5):R208.
18. Lichtenstein D, Meziere G, Biderman P, Gepner A. The comet-tail
artifact: an ultrasound sign ruling out pneumothorax. Intensive Care
Med. 1999;25(4):383–388.
19. Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G.
Ultrasound lung comets: a clinically useful sign of extravascular lung
water. J Am Soc Echocardiogr. 2006;19(3):356–363.
20. Lichtenstein D, Meziere G. A lung ultrasound sign allowing bedside
distinction between pulmonary edema and COPD: the comet-tail artifact.
Intensive Care Med. 1998;24(12):1331–1334.
21. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in
the assessment of alveolar-interstitial syndrome. Am J Emerg Med.
2006;24(6):689–696.
22. Rothe CF. Reflex control of veins and vascular capacitance. Physiol
Rev. 1983;63(4):1281–1342.
23. Blair JE, Brennan JM, Goonewardena SN, Shah D, Vasaiwala S,
Spencer KT. Usefulness of hand-carried ultrasound to predict elevated
left ventricular filling pressure. Am J Cardiol. 2009;103(2):246–247.
24. Beigel R, Cercek B, Luo H, Siegel RJ. Noninvasive evaluation of right
atrial pressure. J Am Soc Echocardiogr. 2013;26(9):1033–1042.
25. Miller JB, Sen A, Strote SR, et al. Inferior vena cava assessment in the
bedside diagnosis of acute heart failure. Am J Emerg Med. 2012;
30(5):778–783.
26. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart
failure via respiratory variation of inferior vena cava diameter. Am J
Emerg Med. 2009;27(1):71–75.
27. Goonewardena SN, Spencer KT. Handcarried echocardiography to
assess hemodynamics in acute decompensated heart failure. Curr
Heart Fail Rep. 2010;7(4):219–227.
28. Guiotto G, Masarone M, Paladino F, et al. Inferior vena cava collapsibility
to guide fluid removal in slow continuous ultrafiltration: a pilot
study. Intensive Care Med. 2010;36(4):692–696.
29. Carbone F, Bovio M, Rosa GM, et al. Inferior vena cava parameters
predict readmission in ischemic heart failure. Eur J Clin Invest. 2014;
44(4):341–349.
30. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of
hand-carried ultrasound assessment of the inferior vena cava and Nterminal
pro-brain natriuretic peptide for predicting readmission after
hospitalization for acute decompensated heart failure. JACC Cardiovasc
Imaging. 2008;1(5):595–601.
31. Laffin L, Patel AR, Saha N, et al. Inferior vena cava measurement by
focused cardiac ultrasound in acute decompensated heart failure prevents
hospital readmissions. J Am Coll Cardiol. 2014;63(12 suppl):
A542.
32. Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the
respiratory variation in the inferior vena cava diameter is predictive of
fluid responsiveness in critically ill patients: systematic review and
meta-analysis. Ultrasound Med Biol. 2014;40(5):845–853.
33. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency
physician-performed ultrasonography in the diagnosis of deep-vein
thrombosis: a systematic review and meta-analysis. Thromb Haemost.
2013;109(1):137–145.
34. Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the
diagnosis of acute respiratory failure: the BLUE protocol. Chest.
2008;134(1):117–125.
35. Comert SS, Caglayan B, Akturk U, et al. The role of thoracic ultrasonography
in the diagnosis of pulmonary embolism. Ann Thorac Med.
2013;8(2):99–104.
36. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH,
Narasimhan M. Ultrasound assessment of pulmonary embolism in
patients receiving computerized tomography pulmonary angiography.
Chest. 2014;145(4):818–823.
37. Mookadam F, Jiamsripong P, Goel R, Warsame TA, Emani UR,
Khandheria BK. Critical appraisal on the utility of echocardiography
in the management of acute pulmonary embolism. Cardiol Rev. 2010;
18(1):29–37.
38. Gesensway D. Making the case for portable ultrasound. Todays Hospitalist.
2012;10:32–36.
39. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel
RJ. Focused cardiac ultrasound: recommendations from the American
Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):
567–581.
40. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire
C, Ziegelstein RC. The rate at which residents learn to use hand-held
echocardiography at the bedside. Am J Med. 2005;118(9):1010–
1018.
41. Redberg RF, Walsch J. Pay now, benefits may follow—the case of cardiac
computed tomographic angiography. N Engl J Med. 2008;359:
2309–2311.
42. Blyth L, Atkinson P, Gadd K, Lang E. Bedside focused echocardiography
as predictor of survival in cardiac arrest patients: a systematic
review. Acad Emerg Med. 2012;19(10):1119–1126.
43. Martin LD, Mathews S, Ziegelstein RC, et al. Prevalence of asymptomatic
left ventricular systolic dysfunction in at-risk medical inpatients.
Am J Med. 2013;126(1):68–73.
44. Low D, Vlasschaert M, Novak K, Chee A, Ma IWY. An argument for
using additional bedside tools, such as bedside ultrasound, for volume
status assessment in hospitalized medical patients: a needs assessment
survey. J Hosp Med. 2014;9:727–730.
45. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid
Ultrasound in SHock in the evaluation of the critically lll. Emerg Med
Clin North Am. 2010;28(1):29–56, vii.
46. Lichtenstein D. FALLS-protocol: lung ultrasound in hemodynamic
assessment of shock. Heart Lung Vessel. 2013;5(3):142–147.
47. Kimura BJ, Yogo N, O’Connell CW, Phan JN, Showalter BK,
Wolfson T. Cardiopulmonary limited ultrasound examination for
“quick-look” bedside application. Am J Cardiol. 2011;108(4):586–
590.
48. Manno E, Navarra M, Faccio L, et al. Deep impact of ultrasound in
the intensive care unit: the “ICU-sound” protocol. Anesthesiology.
2012;117(4):801–809.
49. Cibinel GA, Casoli G, Elia F, et al. Diagnostic accuracy and reproducibility
of pleural and lung ultrasound in discriminating cardiogenic
causes of acute dyspnea in the emergency department. Intern Emerg
Med. 2012;7(1):65–70.
50. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing
heart failure among acutely dyspneic patients with cardiac,
inferior vena cava, and lung ultrasonography. Am J Emerg Med.
2013;31(8):1208–1214.
1. Soni NJ, Arntfield R, Kory P. Point-of-Care Ultrasound. 1st ed. Philadelphia,
PA: Saunders; 2014.
2. American Medical Association. House of Delegates. H-230.960 Privileging
for ultrasound imaging. Policy finder website. Available at:
https://ssl3.ama-assn.org/apps/ecomm/PolicyFinderForm.pl?site5www.
ama-assn.org&uri5%2fresources%2fhtml%2fPolicyFinder%2fpolicy
files%2fHnE%2fH-230.960.HTM. Accessed October 2, 2014.
3. Goldberg BB, Goodman GA, Clearfield HR. Evaluation of ascites by
ultrasound. Radiology. 1970;96(1):15–22.
4. Mercaldi CJ, Lanes SF. Ultrasound guidance decreases complications
and improves the cost of care among patients undergoing thoracentesis
and paracentesis. Chest. 2013;143(2):532–538.
5. Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic
review: emergency department bedside ultrasonography for diagnosing
suspected abdominal aortic aneurysm. Acad Emerg Med. 2013;
20(2):128–138.
6. Dijos M, Pucheux Y, Lafitte M, et al. Fast track echo of abdominal
aortic aneurysm using a real pocket-ultrasound device at bedside.
Echocardiography. 2012;29(3):285–290.
7. Rosen CL, Brown DF, Sagarin MJ, Chang Y, McCabe CJ, Wolfe RE.
Ultrasonography by emergency physicians in patients with suspected
ureteral colic. J Emerg Med. 1998;16(6):865–870.
8. Gaspari RJ, Horst K. Emergency ultrasound and urinalysis in the evaluation
of flank pain. Acad Emerg Med. 2005;12(12):1180–1184.
9. Lucas BP, Candotti C, Margeta B, et al. Diagnostic accuracy of
hospitalist-performed hand-carried ultrasound echocardiography after
a brief training program. J Hosp Med. 2009;4(6):340–349.
10. Martin LD, Howell EE, Ziegelstein RC, Martire C, Shapiro EP,
Hellmann DB. Hospitalist performance of cardiac hand-carried ultrasound
after focused training. Am J Med. 2007;120(11):1000–1004.
11. Martin LD, Howell EE, Ziegelstein RC, et al. Hand-carried ultrasound
performed by hospitalists: does it improve the cardiac physical
examination? Am J Med. 2009;122(1):35–41.
PoCUS for Hospitalists | Soni and Lucas
An Official Publication of the Society of Hospital Medicine Journal of Hospital Medicine Vol 10 | No 2 | February 2015 123
12. Lucas BP, Candotti C, Margeta B, et al. Hand-carried echocardiography
by hospitalists: a randomized trial. Am J Med. 2011;124(8):766–
774.
13. Hu QJ, Shen YC, Jia LQ, et al. Diagnostic performance of lung ultrasound
in the diagnosis of pneumonia: a bivariate meta-analysis. Int J
Clin Exp Med. 2014;7(1):115–121.
14. Reissig A, Gramegna A, Aliberti S. The role of lung ultrasound in the
diagnosis and follow-up of community-acquired pneumonia. Eur J
Intern Med. 2012;23(5):391–397.
15. Patel PA, Ernst FR, Gunnarsson CL. Ultrasonography guidance
reduces complications and costs associated with thoracentesis procedures.
J Clin Ultrasound. 2012;40(3):135–141.
16. Ding W, Shen Y, Yang J, He X, Zhang M. Diagnosis of pneumothorax
by radiography and ultrasonography: a meta-analysis. Chest.
2011;140(4):859–866.
17. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography
versus chest radiography for the diagnosis of pneumothorax: review
of the literature and meta-analysis. Crit Care. 2013;17(5):R208.
18. Lichtenstein D, Meziere G, Biderman P, Gepner A. The comet-tail
artifact: an ultrasound sign ruling out pneumothorax. Intensive Care
Med. 1999;25(4):383–388.
19. Picano E, Frassi F, Agricola E, Gligorova S, Gargani L, Mottola G.
Ultrasound lung comets: a clinically useful sign of extravascular lung
water. J Am Soc Echocardiogr. 2006;19(3):356–363.
20. Lichtenstein D, Meziere G. A lung ultrasound sign allowing bedside
distinction between pulmonary edema and COPD: the comet-tail artifact.
Intensive Care Med. 1998;24(12):1331–1334.
21. Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in
the assessment of alveolar-interstitial syndrome. Am J Emerg Med.
2006;24(6):689–696.
22. Rothe CF. Reflex control of veins and vascular capacitance. Physiol
Rev. 1983;63(4):1281–1342.
23. Blair JE, Brennan JM, Goonewardena SN, Shah D, Vasaiwala S,
Spencer KT. Usefulness of hand-carried ultrasound to predict elevated
left ventricular filling pressure. Am J Cardiol. 2009;103(2):246–247.
24. Beigel R, Cercek B, Luo H, Siegel RJ. Noninvasive evaluation of right
atrial pressure. J Am Soc Echocardiogr. 2013;26(9):1033–1042.
25. Miller JB, Sen A, Strote SR, et al. Inferior vena cava assessment in the
bedside diagnosis of acute heart failure. Am J Emerg Med. 2012;
30(5):778–783.
26. Blehar DJ, Dickman E, Gaspari R. Identification of congestive heart
failure via respiratory variation of inferior vena cava diameter. Am J
Emerg Med. 2009;27(1):71–75.
27. Goonewardena SN, Spencer KT. Handcarried echocardiography to
assess hemodynamics in acute decompensated heart failure. Curr
Heart Fail Rep. 2010;7(4):219–227.
28. Guiotto G, Masarone M, Paladino F, et al. Inferior vena cava collapsibility
to guide fluid removal in slow continuous ultrafiltration: a pilot
study. Intensive Care Med. 2010;36(4):692–696.
29. Carbone F, Bovio M, Rosa GM, et al. Inferior vena cava parameters
predict readmission in ischemic heart failure. Eur J Clin Invest. 2014;
44(4):341–349.
30. Goonewardena SN, Gemignani A, Ronan A, et al. Comparison of
hand-carried ultrasound assessment of the inferior vena cava and Nterminal
pro-brain natriuretic peptide for predicting readmission after
hospitalization for acute decompensated heart failure. JACC Cardiovasc
Imaging. 2008;1(5):595–601.
31. Laffin L, Patel AR, Saha N, et al. Inferior vena cava measurement by
focused cardiac ultrasound in acute decompensated heart failure prevents
hospital readmissions. J Am Coll Cardiol. 2014;63(12 suppl):
A542.
32. Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the
respiratory variation in the inferior vena cava diameter is predictive of
fluid responsiveness in critically ill patients: systematic review and
meta-analysis. Ultrasound Med Biol. 2014;40(5):845–853.
33. Pomero F, Dentali F, Borretta V, et al. Accuracy of emergency
physician-performed ultrasonography in the diagnosis of deep-vein
thrombosis: a systematic review and meta-analysis. Thromb Haemost.
2013;109(1):137–145.
34. Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the
diagnosis of acute respiratory failure: the BLUE protocol. Chest.
2008;134(1):117–125.
35. Comert SS, Caglayan B, Akturk U, et al. The role of thoracic ultrasonography
in the diagnosis of pulmonary embolism. Ann Thorac Med.
2013;8(2):99–104.
36. Koenig S, Chandra S, Alaverdian A, Dibello C, Mayo PH,
Narasimhan M. Ultrasound assessment of pulmonary embolism in
patients receiving computerized tomography pulmonary angiography.
Chest. 2014;145(4):818–823.
37. Mookadam F, Jiamsripong P, Goel R, Warsame TA, Emani UR,
Khandheria BK. Critical appraisal on the utility of echocardiography
in the management of acute pulmonary embolism. Cardiol Rev. 2010;
18(1):29–37.
38. Gesensway D. Making the case for portable ultrasound. Todays Hospitalist.
2012;10:32–36.
39. Spencer KT, Kimura BJ, Korcarz CE, Pellikka PA, Rahko PS, Siegel
RJ. Focused cardiac ultrasound: recommendations from the American
Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(6):
567–581.
40. Hellmann DB, Whiting-O’Keefe Q, Shapiro EP, Martin LD, Martire
C, Ziegelstein RC. The rate at which residents learn to use hand-held
echocardiography at the bedside. Am J Med. 2005;118(9):1010–
1018.
41. Redberg RF, Walsch J. Pay now, benefits may follow—the case of cardiac
computed tomographic angiography. N Engl J Med. 2008;359:
2309–2311.
42. Blyth L, Atkinson P, Gadd K, Lang E. Bedside focused echocardiography
as predictor of survival in cardiac arrest patients: a systematic
review. Acad Emerg Med. 2012;19(10):1119–1126.
43. Martin LD, Mathews S, Ziegelstein RC, et al. Prevalence of asymptomatic
left ventricular systolic dysfunction in at-risk medical inpatients.
Am J Med. 2013;126(1):68–73.
44. Low D, Vlasschaert M, Novak K, Chee A, Ma IWY. An argument for
using additional bedside tools, such as bedside ultrasound, for volume
status assessment in hospitalized medical patients: a needs assessment
survey. J Hosp Med. 2014;9:727–730.
45. Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid
Ultrasound in SHock in the evaluation of the critically lll. Emerg Med
Clin North Am. 2010;28(1):29–56, vii.
46. Lichtenstein D. FALLS-protocol: lung ultrasound in hemodynamic
assessment of shock. Heart Lung Vessel. 2013;5(3):142–147.
47. Kimura BJ, Yogo N, O’Connell CW, Phan JN, Showalter BK,
Wolfson T. Cardiopulmonary limited ultrasound examination for
“quick-look” bedside application. Am J Cardiol. 2011;108(4):586–
590.
48. Manno E, Navarra M, Faccio L, et al. Deep impact of ultrasound in
the intensive care unit: the “ICU-sound” protocol. Anesthesiology.
2012;117(4):801–809.
49. Cibinel GA, Casoli G, Elia F, et al. Diagnostic accuracy and reproducibility
of pleural and lung ultrasound in discriminating cardiogenic
causes of acute dyspnea in the emergency department. Intern Emerg
Med. 2012;7(1):65–70.
50. Anderson KL, Jenq KY, Fields JM, Panebianco NL, Dean AJ. Diagnosing
heart failure among acutely dyspneic patients with cardiac,
inferior vena cava, and lung ultrasonography. Am J Emerg Med.
2013;31(8):1208–1214.
© 2014 Society of Hospital Medicine
Simulator Training of Future Hospitalists
Internal medicine residency programs, the major pipeline for incoming hospitalists, often provide little hands‐on experience in bedside procedures. Some residents may only insert 1 central venous catheter every 4 months on the general medicine wards,1 and others may gain little more experience during intensive care unit rotations. As seen in the survey presented by Grover et al.2 in this issue of the Journal, after 3 years of training in all types of patient care units, residents often count their accumulated experience on their fingers and toes. Such sparse experience hardly leads to expertise. Recognizing this pervasive lack of training the American Board of Internal Medicine narrowed its certification requirements for bedside procedures in 2006.3 Residents are no longer expected to perform bedside procedures but instead to know them. This important revision acknowledges that manual skills training should neither be assumed nor expendablecontinuing to do so is too risky.4 Yet as internal medicine residency programs focus their bedside procedure training on cognitive competence, the ongoing exodus of bedside procedures to the up‐market hands of subspecialists, surgeons, anesthesiologists, and interventional radiologists5 will likely accelerate.
But why should hospitalists disrupt this trend? Bedside procedures are common and not always conveniently needed during daytime hours. Roughly one‐tenth of general medicine inpatients receive a central venous catheter (CVC) insertion, a lumbar puncture, an abdominal paracentesis, or a thoracentesis.6 Among these patients, about one‐half will urgently need procedures during off‐hours. Outside of the emergency department, hospitalists will likely remain the only group of physicians available at the bedsides of general medicine inpatients 7 days a week, 24 hours per day. Thus, in developing our particular practice system to best serve our patients,7 we believe that hospitalists ought to remain principals in ensuring that inpatients have ready access to expertly performed bedside procedures.
Yet unfortunately, given the limited training in manual skills that today's internal medicine residents receive, hospitalists are increasingly less prepared to provide this access themselves.8 State‐of‐the‐art training methods developed by medical specialties that depend largely on manual skills provide promising potential solutions for both future and practicing hospitalists.9 In particular, patient simulators can provide trainees with the essential hands‐on experience they often lack. In contrast to the ad hoc see‐one, do‐one, teach‐one method in current widespread use, training with simulators has distinct advantages. First, simulators obviate the increasingly awkward consent as patients grow savvier about safety concerns and (understandably) less tolerant of a novice's need to acquire experience.10 Second, training with simulators is controlled so that anatomic variations, comorbidities, patient discomfort, and time pressuresthough important real‐world factorscan be artificially removed in the earlier cognitive and integrative stages of training.11 Third, immediate feedback, which at the bedside of real patients is often empathetically avoided or delivered in cryptic hand signals, can be unmistakably unmuted and honest in the simulator setting. Fourth, and most important to the development of expertise, simulators can be used repeatedly, allowing trainees first to become facile in the mechanics of their performance (eg, holding an ultrasound probe for real‐time guidance or knowing how it feels to enter a vein) before attempting a procedure on a patient.
Three examples of patient simulators used to train internal medicine residents in CVC insertion are presented in this issue of the Journal.1214 Using observers who adhered to objective, a priori assessment criteria, both Rosen et al.13 and Millington et al.14 carefully demonstrate that internal medicine residents' manual skills can improve with patient simulators. Given the understood importance of hands‐on experience in manual skills training,15 these anticipated findings are important validations of simulator theory. The work by Barsuk et al.12 goes further to begin to examine whether or not simulator training actually leads to improved patient outcomesthe holy grail of such research. In this observational study, compared to residents who did not undergo simulator training, those who did undergo such training had 1 fewer needle passes during successful CVC insertions. Given the relative infrequency of periprocedural complications, this study was understandably underpowered to measure true complications, relying instead on the often‐used surrogate of needle passes. Nonetheless, this work will serve as an important initial example of why simulator training may be worth the effort.
To direct participation in simulator training, we endorse selecting trainees who will perform bedside procedures in their future practice.16 Given the trend in manual skills training among internal medicine residency training programs, hospitalist programs may need to shoulder this effort themselves. Thankfully, simulator training need not be expensive. Based on transfer‐of‐learning research,17 the fidelity of the simulator is less important than the accumulated experience it can afford. Even low‐fidelity simulators, such as the store‐bought whole chicken used by Rosen et al.,13 may preserve trainees' manual skills just as effectively as the expensive, bionic, high‐fidelity simulators used by Barsuk et al.12 and Millington et al.14
Beyond the costs of training, however, hospital administrators and hospitalist group leaders have more complex externalities and opportunity costs to weigh when evaluating which physician groups should perform bedside procedures. The intuitively lower‐cost strategy for hospitals, we believe, would be to ask hospitalists to perform bedside procedures at patients' bedsides instead of asking, say, highly‐paid interventional radiologists to perform the same procedures in fully‐staffed fluoroscopy suites. There is, however, very little research to help inform these decisions. As hospitalists, we know firsthand that modern healthcare remuneration is based more on doing than on knowing. Yet, whether or not bedside procedures afford financial incentives for hospitalists is unclearmuch will depend on local factors. Regardless of the finances, we believe that hospitalists skilled in performing common bedside procedures can improve the quality and efficiency of care delivery at patients' bedsides. So, instead of a call to arms for yet another turf battle, let's continue development of state‐of‐the‐art training methods like simulators to ensure that future hospitalists can expertly perform bedside procedures. After all, fighting for improvements in patient safety is a battle that we hospitalists know how to win.
- ,,,,,.Firm‐based trial to improve central venous catheter insertion practices.J Hosp Med.2007;2:135–142.
- ,,,,,.Development of a test to evaluate residents' knowledge of medical procedures.J Hosp Med.2009;XX:XXX–XXX.
- American Board of Internal Medicine. Policies and procedures for certification, May 2009. Available at: http://www.abim.org/default.aspx; Accessed August2009.
- .Procedural competence of internal medicine residents: time to address the gap.J Gen Intern Med.2000;15:432–433.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2006;2:143–149.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–394.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24
- ,.Teaching surgical skills—changes in the wind.N Engl J Med.2006;355:2664–2669.
- ,,,.Patients' willingness to allow residents to learn to practice medical procedures.Acad Med.2004;79:144–147.
- ,.Human performance.Belmont, CA:Brooks/Cole;1967.
- ,,,,.Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit.J Hosp Med.2009;4(7):397–403.
- ,,,,.Does personalized vascular access training on a non‐human tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI‐II).J Hosp Med.2009;4(7):423–429.
- ,,,,.Improving internal medicine residents' performance, knowledge, and confidence in central venous catheterization using simulators.J Hosp Med.2009;4(7):410–414.
- ,,,.The Cambridge handbook of expertise and expert performance.New York, NY:Cambridge University Press;2006.
- ,,.Procedural training at a crossroads: striking a balance between education, patient safety, and quality.J Hosp Med.2007;2:123–125.
- ,,, et al.The educational impact of bench model fidelity on the acquisition of technical skill: the use of clinically relevant outcome measures.Ann Surg.2004;240:374–381.
Internal medicine residency programs, the major pipeline for incoming hospitalists, often provide little hands‐on experience in bedside procedures. Some residents may only insert 1 central venous catheter every 4 months on the general medicine wards,1 and others may gain little more experience during intensive care unit rotations. As seen in the survey presented by Grover et al.2 in this issue of the Journal, after 3 years of training in all types of patient care units, residents often count their accumulated experience on their fingers and toes. Such sparse experience hardly leads to expertise. Recognizing this pervasive lack of training the American Board of Internal Medicine narrowed its certification requirements for bedside procedures in 2006.3 Residents are no longer expected to perform bedside procedures but instead to know them. This important revision acknowledges that manual skills training should neither be assumed nor expendablecontinuing to do so is too risky.4 Yet as internal medicine residency programs focus their bedside procedure training on cognitive competence, the ongoing exodus of bedside procedures to the up‐market hands of subspecialists, surgeons, anesthesiologists, and interventional radiologists5 will likely accelerate.
But why should hospitalists disrupt this trend? Bedside procedures are common and not always conveniently needed during daytime hours. Roughly one‐tenth of general medicine inpatients receive a central venous catheter (CVC) insertion, a lumbar puncture, an abdominal paracentesis, or a thoracentesis.6 Among these patients, about one‐half will urgently need procedures during off‐hours. Outside of the emergency department, hospitalists will likely remain the only group of physicians available at the bedsides of general medicine inpatients 7 days a week, 24 hours per day. Thus, in developing our particular practice system to best serve our patients,7 we believe that hospitalists ought to remain principals in ensuring that inpatients have ready access to expertly performed bedside procedures.
Yet unfortunately, given the limited training in manual skills that today's internal medicine residents receive, hospitalists are increasingly less prepared to provide this access themselves.8 State‐of‐the‐art training methods developed by medical specialties that depend largely on manual skills provide promising potential solutions for both future and practicing hospitalists.9 In particular, patient simulators can provide trainees with the essential hands‐on experience they often lack. In contrast to the ad hoc see‐one, do‐one, teach‐one method in current widespread use, training with simulators has distinct advantages. First, simulators obviate the increasingly awkward consent as patients grow savvier about safety concerns and (understandably) less tolerant of a novice's need to acquire experience.10 Second, training with simulators is controlled so that anatomic variations, comorbidities, patient discomfort, and time pressuresthough important real‐world factorscan be artificially removed in the earlier cognitive and integrative stages of training.11 Third, immediate feedback, which at the bedside of real patients is often empathetically avoided or delivered in cryptic hand signals, can be unmistakably unmuted and honest in the simulator setting. Fourth, and most important to the development of expertise, simulators can be used repeatedly, allowing trainees first to become facile in the mechanics of their performance (eg, holding an ultrasound probe for real‐time guidance or knowing how it feels to enter a vein) before attempting a procedure on a patient.
Three examples of patient simulators used to train internal medicine residents in CVC insertion are presented in this issue of the Journal.1214 Using observers who adhered to objective, a priori assessment criteria, both Rosen et al.13 and Millington et al.14 carefully demonstrate that internal medicine residents' manual skills can improve with patient simulators. Given the understood importance of hands‐on experience in manual skills training,15 these anticipated findings are important validations of simulator theory. The work by Barsuk et al.12 goes further to begin to examine whether or not simulator training actually leads to improved patient outcomesthe holy grail of such research. In this observational study, compared to residents who did not undergo simulator training, those who did undergo such training had 1 fewer needle passes during successful CVC insertions. Given the relative infrequency of periprocedural complications, this study was understandably underpowered to measure true complications, relying instead on the often‐used surrogate of needle passes. Nonetheless, this work will serve as an important initial example of why simulator training may be worth the effort.
To direct participation in simulator training, we endorse selecting trainees who will perform bedside procedures in their future practice.16 Given the trend in manual skills training among internal medicine residency training programs, hospitalist programs may need to shoulder this effort themselves. Thankfully, simulator training need not be expensive. Based on transfer‐of‐learning research,17 the fidelity of the simulator is less important than the accumulated experience it can afford. Even low‐fidelity simulators, such as the store‐bought whole chicken used by Rosen et al.,13 may preserve trainees' manual skills just as effectively as the expensive, bionic, high‐fidelity simulators used by Barsuk et al.12 and Millington et al.14
Beyond the costs of training, however, hospital administrators and hospitalist group leaders have more complex externalities and opportunity costs to weigh when evaluating which physician groups should perform bedside procedures. The intuitively lower‐cost strategy for hospitals, we believe, would be to ask hospitalists to perform bedside procedures at patients' bedsides instead of asking, say, highly‐paid interventional radiologists to perform the same procedures in fully‐staffed fluoroscopy suites. There is, however, very little research to help inform these decisions. As hospitalists, we know firsthand that modern healthcare remuneration is based more on doing than on knowing. Yet, whether or not bedside procedures afford financial incentives for hospitalists is unclearmuch will depend on local factors. Regardless of the finances, we believe that hospitalists skilled in performing common bedside procedures can improve the quality and efficiency of care delivery at patients' bedsides. So, instead of a call to arms for yet another turf battle, let's continue development of state‐of‐the‐art training methods like simulators to ensure that future hospitalists can expertly perform bedside procedures. After all, fighting for improvements in patient safety is a battle that we hospitalists know how to win.
Internal medicine residency programs, the major pipeline for incoming hospitalists, often provide little hands‐on experience in bedside procedures. Some residents may only insert 1 central venous catheter every 4 months on the general medicine wards,1 and others may gain little more experience during intensive care unit rotations. As seen in the survey presented by Grover et al.2 in this issue of the Journal, after 3 years of training in all types of patient care units, residents often count their accumulated experience on their fingers and toes. Such sparse experience hardly leads to expertise. Recognizing this pervasive lack of training the American Board of Internal Medicine narrowed its certification requirements for bedside procedures in 2006.3 Residents are no longer expected to perform bedside procedures but instead to know them. This important revision acknowledges that manual skills training should neither be assumed nor expendablecontinuing to do so is too risky.4 Yet as internal medicine residency programs focus their bedside procedure training on cognitive competence, the ongoing exodus of bedside procedures to the up‐market hands of subspecialists, surgeons, anesthesiologists, and interventional radiologists5 will likely accelerate.
But why should hospitalists disrupt this trend? Bedside procedures are common and not always conveniently needed during daytime hours. Roughly one‐tenth of general medicine inpatients receive a central venous catheter (CVC) insertion, a lumbar puncture, an abdominal paracentesis, or a thoracentesis.6 Among these patients, about one‐half will urgently need procedures during off‐hours. Outside of the emergency department, hospitalists will likely remain the only group of physicians available at the bedsides of general medicine inpatients 7 days a week, 24 hours per day. Thus, in developing our particular practice system to best serve our patients,7 we believe that hospitalists ought to remain principals in ensuring that inpatients have ready access to expertly performed bedside procedures.
Yet unfortunately, given the limited training in manual skills that today's internal medicine residents receive, hospitalists are increasingly less prepared to provide this access themselves.8 State‐of‐the‐art training methods developed by medical specialties that depend largely on manual skills provide promising potential solutions for both future and practicing hospitalists.9 In particular, patient simulators can provide trainees with the essential hands‐on experience they often lack. In contrast to the ad hoc see‐one, do‐one, teach‐one method in current widespread use, training with simulators has distinct advantages. First, simulators obviate the increasingly awkward consent as patients grow savvier about safety concerns and (understandably) less tolerant of a novice's need to acquire experience.10 Second, training with simulators is controlled so that anatomic variations, comorbidities, patient discomfort, and time pressuresthough important real‐world factorscan be artificially removed in the earlier cognitive and integrative stages of training.11 Third, immediate feedback, which at the bedside of real patients is often empathetically avoided or delivered in cryptic hand signals, can be unmistakably unmuted and honest in the simulator setting. Fourth, and most important to the development of expertise, simulators can be used repeatedly, allowing trainees first to become facile in the mechanics of their performance (eg, holding an ultrasound probe for real‐time guidance or knowing how it feels to enter a vein) before attempting a procedure on a patient.
Three examples of patient simulators used to train internal medicine residents in CVC insertion are presented in this issue of the Journal.1214 Using observers who adhered to objective, a priori assessment criteria, both Rosen et al.13 and Millington et al.14 carefully demonstrate that internal medicine residents' manual skills can improve with patient simulators. Given the understood importance of hands‐on experience in manual skills training,15 these anticipated findings are important validations of simulator theory. The work by Barsuk et al.12 goes further to begin to examine whether or not simulator training actually leads to improved patient outcomesthe holy grail of such research. In this observational study, compared to residents who did not undergo simulator training, those who did undergo such training had 1 fewer needle passes during successful CVC insertions. Given the relative infrequency of periprocedural complications, this study was understandably underpowered to measure true complications, relying instead on the often‐used surrogate of needle passes. Nonetheless, this work will serve as an important initial example of why simulator training may be worth the effort.
To direct participation in simulator training, we endorse selecting trainees who will perform bedside procedures in their future practice.16 Given the trend in manual skills training among internal medicine residency training programs, hospitalist programs may need to shoulder this effort themselves. Thankfully, simulator training need not be expensive. Based on transfer‐of‐learning research,17 the fidelity of the simulator is less important than the accumulated experience it can afford. Even low‐fidelity simulators, such as the store‐bought whole chicken used by Rosen et al.,13 may preserve trainees' manual skills just as effectively as the expensive, bionic, high‐fidelity simulators used by Barsuk et al.12 and Millington et al.14
Beyond the costs of training, however, hospital administrators and hospitalist group leaders have more complex externalities and opportunity costs to weigh when evaluating which physician groups should perform bedside procedures. The intuitively lower‐cost strategy for hospitals, we believe, would be to ask hospitalists to perform bedside procedures at patients' bedsides instead of asking, say, highly‐paid interventional radiologists to perform the same procedures in fully‐staffed fluoroscopy suites. There is, however, very little research to help inform these decisions. As hospitalists, we know firsthand that modern healthcare remuneration is based more on doing than on knowing. Yet, whether or not bedside procedures afford financial incentives for hospitalists is unclearmuch will depend on local factors. Regardless of the finances, we believe that hospitalists skilled in performing common bedside procedures can improve the quality and efficiency of care delivery at patients' bedsides. So, instead of a call to arms for yet another turf battle, let's continue development of state‐of‐the‐art training methods like simulators to ensure that future hospitalists can expertly perform bedside procedures. After all, fighting for improvements in patient safety is a battle that we hospitalists know how to win.
- ,,,,,.Firm‐based trial to improve central venous catheter insertion practices.J Hosp Med.2007;2:135–142.
- ,,,,,.Development of a test to evaluate residents' knowledge of medical procedures.J Hosp Med.2009;XX:XXX–XXX.
- American Board of Internal Medicine. Policies and procedures for certification, May 2009. Available at: http://www.abim.org/default.aspx; Accessed August2009.
- .Procedural competence of internal medicine residents: time to address the gap.J Gen Intern Med.2000;15:432–433.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2006;2:143–149.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–394.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24
- ,.Teaching surgical skills—changes in the wind.N Engl J Med.2006;355:2664–2669.
- ,,,.Patients' willingness to allow residents to learn to practice medical procedures.Acad Med.2004;79:144–147.
- ,.Human performance.Belmont, CA:Brooks/Cole;1967.
- ,,,,.Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit.J Hosp Med.2009;4(7):397–403.
- ,,,,.Does personalized vascular access training on a non‐human tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI‐II).J Hosp Med.2009;4(7):423–429.
- ,,,,.Improving internal medicine residents' performance, knowledge, and confidence in central venous catheterization using simulators.J Hosp Med.2009;4(7):410–414.
- ,,,.The Cambridge handbook of expertise and expert performance.New York, NY:Cambridge University Press;2006.
- ,,.Procedural training at a crossroads: striking a balance between education, patient safety, and quality.J Hosp Med.2007;2:123–125.
- ,,, et al.The educational impact of bench model fidelity on the acquisition of technical skill: the use of clinically relevant outcome measures.Ann Surg.2004;240:374–381.
- ,,,,,.Firm‐based trial to improve central venous catheter insertion practices.J Hosp Med.2007;2:135–142.
- ,,,,,.Development of a test to evaluate residents' knowledge of medical procedures.J Hosp Med.2009;XX:XXX–XXX.
- American Board of Internal Medicine. Policies and procedures for certification, May 2009. Available at: http://www.abim.org/default.aspx; Accessed August2009.
- .Procedural competence of internal medicine residents: time to address the gap.J Gen Intern Med.2000;15:432–433.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2006;2:143–149.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–394.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24
- ,.Teaching surgical skills—changes in the wind.N Engl J Med.2006;355:2664–2669.
- ,,,.Patients' willingness to allow residents to learn to practice medical procedures.Acad Med.2004;79:144–147.
- ,.Human performance.Belmont, CA:Brooks/Cole;1967.
- ,,,,.Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit.J Hosp Med.2009;4(7):397–403.
- ,,,,.Does personalized vascular access training on a non‐human tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI‐II).J Hosp Med.2009;4(7):423–429.
- ,,,,.Improving internal medicine residents' performance, knowledge, and confidence in central venous catheterization using simulators.J Hosp Med.2009;4(7):410–414.
- ,,,.The Cambridge handbook of expertise and expert performance.New York, NY:Cambridge University Press;2006.
- ,,.Procedural training at a crossroads: striking a balance between education, patient safety, and quality.J Hosp Med.2007;2:123–125.
- ,,, et al.The educational impact of bench model fidelity on the acquisition of technical skill: the use of clinically relevant outcome measures.Ann Surg.2004;240:374–381.
Accuracy of Hospitalist‐Performed HCUE
Hand‐carried ultrasound echocardiography (HCUE) can help noncardiologists answer well‐defined questions at patients' bedsides in less than 10 minutes.1, 2 Indeed, intensivists3 and emergency department physicians4 already use HCUE to make rapid, point‐of‐care assessments. Since cardiovascular diagnoses are common among general medicine inpatients, HCUE may become an important skill for hospitalists to learn.5
However, uncertainty exists about the duration of HCUE training for hospitalists. In 2002, experts from the American Society of Echocardiography (ASE) published recommendations on training requirements for HCUE.6 With limited data on the safety or performance of HCUE training programs, which had just begun to emerge, the ASE borrowed from the proven training recommendations for standard echocardiography (SE). They recommended that all HCUE trainees, cardiologist and noncardiologist alike, complete level 1 SE training: 75 personally‐performed and 150 personally‐interpreted echocardiographic examinations. Since then, however, several HCUE training programs designed for noncardiologists have emerged.2, 5, 710 These alternative programs suggest that the ASE's recommended duration of training may be too long, particularly for focused HCUE that is limited to a few relatively simple assessments. It is important not to overshoot the requirements of HCUE training, because doing so may discourage groups of noncardiologists, like hospitalists, who may derive great benefits from HCUE.11
To address this uncertainty for hospitalists, we first developed a brief HCUE training program to assess 6 important cardiac abnormalities. We then studied the diagnostic accuracy of HCUE by hospitalists as a test of these 6 cardiac abnormalities assessed by SE.
Patients and Methods
Setting and Subjects
This prospective cohort study was performed at Stroger Hospital of Cook County, a 500‐bed public teaching hospital in Chicago, IL, from March through May of 2007. The cohort was adult inpatients who were referred for SE on weekdays from 3 distinct patient care units (Figure 1). We used 2 sampling modes to balance practical constraints (short‐stay unit [SSU] patients were more localized and, therefore, easier to study) with clinical diversity. We consecutively sampled patients from our SSU, where adults with provisional cardiovascular diagnoses are admitted if they might be eligible for discharge with in 3 days.12 But we used random number tables with a daily unique starting point to randomly sample patients from the general medical wards and the coronary care unit (CCU). Patients were excluded if repositioning them for HCUE was potentially harmful. The study was approved by our hospital's institutional review board, and we obtained written informed consent from all enrolled patients.
SE Protocol
As part of enrolled patients' routine clinical care, SE images were acquired and interpreted in the usual fashion in our hospital's echocardiography laboratory, which performs SE on over 7,000 patients per year. Echocardiographic technicians acquired images with a General Electric Vivid 7 cardiac ultrasound machine (General Electric, Milwaukee, WI) equipped with a GE M4S 1.8 to 3.4 MHz cardiac transducer (General Electric). Technicians followed the standard adult transthoracic echocardiography scanning protocol to acquire 40 to 100 images on every patient using all available echocardiographic modalities: 2‐dimensional, M‐mode, color Doppler, continuous‐wave Doppler, pulse‐wave Doppler, and tissue Doppler.13 Blinded to HCUE results, attending physician cardiologist echocardiographers then interpreted archived images using computer software (Centricity System; General Electric) to generate final reports that were entered into patients' medical records. This software ensured that final reports were standardized, because echocardiographers' final qualitative assessments were limited to short lists of standard options; for example, in reporting left atrium (LA) size, echocardiographers chose from only 5 standard options: normal, mildly dilated, moderately dilated, severely dilated, and not interpretable. Investigators, who were also blinded to HCUE results, later abstracted SE results from these standardized report forms in patients' medical records. All echocardiographers fulfilled ASE training guidelines to independently interpret SE: a minimum of 150 personally‐performed and 300 personally‐interpreted echocardiographic examinations (training level 2).14
HCUE Training
Based on the recommendations of our cardiologist investigator (B.M.), we developed a training program for 1 hospitalist to become an HCUE instructor. Our instructor trainee (C.C.) was board‐eligible in internal medicine but had no previous formal training in cardiology or echocardiography. We a priori established that her training would continue until our cardiologist investigator determined that she was ready to train other hospitalists; this determination occurred after 5 weeks. She learned image acquisition by performing focused SE on 30 patients under the direct supervision of an echocardiographic technician. She also performed focused HCUE on 65 inpatients without direct supervision but with ongoing access to consult the technician to review archived images and troubleshoot difficulties with acquisition. She learned image interpretation by reading relevant chapters from a SE textbook15 and by participating in daily didactic sessions in which attending cardiologist echocardiographers train cardiology fellows in SE interpretation.
This hospitalist then served as the HCUE instructor for 8 other attending physician hospitalists who were board‐certified internists with no previous formal training in cardiology or echocardiography. The training program was limited to acquisition and interpretation of 2‐dimensional grayscale and color Doppler images for the 6 cardiac assessments under study (Table 1). The instructor marshaled pairs of hospitalists through the 3 components of the training program, which lasted a total of 27 hours.
|
| Six cardiac assessments learned using 2‐dimensional gray scale and color Doppler imaging |
| Left ventricular systolic dysfunction |
| Mitral valve regurgitation |
| Left atrium enlargement |
| Left ventricular hypertrophy |
| Pericardial effusion |
| Inferior vena cava diameter |
| Lecture (2 hours)* |
| Basic principles of echocardiography |
| HCUE scanning protocol and helpful techniques to optimize image quality |
| Hands‐on training with instructor |
| Orientation to machine and demonstration of scanning protocol (1 hour) |
| Sessions 1 through 3: HCUE performed on 1 patient per hour (6 patients in 6 hours) |
| Sessions 4 through 10: HCUE performed on 2 patients per hour (28 patients in 14 hours) |
| Feedback sessions on image quality and interpretation with cardiologist |
| After hands‐on training session 3 (2 hours) |
| After hands‐on training session 10 (2 hours) |
First, hospitalists attended a 2‐hour lecture on the basic principles of HCUE. Slides from this lecture and additional images of normal and abnormal findings were provided to each hospitalist on a digital video disc. Second, each hospitalist underwent 20 hours of hands‐on training in 2‐hour sessions scheduled over 2 weeks. Willing inpatients from our hospital's emergency department were used as volunteers for these hand‐on training sessions. During these sessions the instructor provided practical suggestions to optimize image quality, such as transducer location and patient positioning. In the first 3 sessions, the minimum pace was 1 patient per hour; thereafter, the pace was increased to 1 patient per half‐hour. We chose 20 hours of hands‐on training and these minimum paces because they allowed each hospitalist to attain a cumulative experience of no less than 30 patientsan amount that heralds a flattening of the HCUE learning curve among medical trainees.9 Third, each pair of hospitalists received feedback from a cardiologist investigator (B.M.) who critiqued the quality and interpretation of images acquired by hospitalists during hands‐on training sessions. Since image quality varies by patient,16 hospitalists' images were compared side‐by‐side to images recorded by the instructor on the same patients. The cardiologist also critiqued hospitalists' interpretations of both their own images and additional sets of archived images from patients with abnormal findings.
HCUE Protocol
After completing the training program and blinded to the results of SE, the 8 hospitalists performed HCUE on enrolled patients within hours of SE. We limited the time interval between tests to minimize the effect that changes in physiologic variables, such as blood pressure and intravascular volume, have on the reliability of serial echocardiographic measurements.16 Hospitalists performed HCUE with a MicroMaxx 3.4 hand‐carried ultrasound machine equipped with a cardiology software package and a 1 to 5 MHz P17 cardiac transducer (Sonosite, Inc., Bothell, WA); simultaneous electrocardiographic recording, though available, was not used. While patients laid on their own standard hospital beds or on a standard hospital gurney in a room adjacent to the SE waiting room, hospitalists positioned them without assistance from nursing staff and recorded 7 best‐quality images per patient. Patients were first positioned in a partial (3045 degrees) left lateral decubitus position to record 4 grayscale images of the short‐axis and long‐axis parasternal and 2‐chamber and 4‐chamber apical views; 2 color Doppler images of the mitral inflow were also recorded from the long‐axis parasternal and the 4‐chamber apical views. Patients were then positioned supine to record 1 grayscale image of the inferior vena cava (IVC) from the transhepatic view. Hospitalists did not perform a history or physical exam on enrolled patients, nor did they review patients' medical records.
Immediately following the HCUE, hospitalists replayed the recorded images as often as needed and entered final interpretations on data collection forms. Linear measurements were made manually with a caliper held directly to the hand‐carried ultrasound monitor. These measurements were then translated into qualitative assessments based on standard values used by our hospital's echocardiographers (Table 2).17 When a hospitalist could not confidently assess a cardiac abnormality, the final HCUE assessment was recorded as indeterminate. Hospitalists also recorded the time to perform each HCUE, which included the time to record 7 best‐quality images, to interpret the findings, and to fill out the data collection form.
| Hand‐Carried Ultrasound Echocardiography Results | |||||
|---|---|---|---|---|---|
| Cardiac Abnormality by Standard Echocardiography | Hand‐Carried Ultrasound Echocardiography Operator's Method of Assessment | Positive | Negative | ||
| |||||
| Left ventricle systolic dysfunction, mild or greater | Grade degree of abnormal wall movement and thickening during systole | Severe | Mild or moderate | Normal | Vigorous |
| Mitral valve regurgitation, severe | Classify regurgitant jet as central or eccentric, then measure as percentage of left atrium area | ||||
| Central jet | 20% | <20% | |||
| Eccentric jet | 20% | indeterminate 20% | |||
| Left atrium enlargement, moderate or severe | Measure left atrium in 3 dimensions at end diastole, then use the most abnormal dimension | Extreme | Borderline | ||
| Anteroposterior or mediolateral (cm) | 5.1 | 4.55.0 | 4.4 | ||
| Superior‐inferior (cm) | 7.1 | 6.17.0 | 6.0 | ||
| Left ventricle hypertrophy, moderate or severe | Measure thickest dimension of posterior or septal wall at end diastole | Extreme: 1.4 cm | Borderline: 1.21.3 cm | 1.1 cm | |
| Pericardial effusion, medium or large | Measure largest dimension in any view at end diastole | 1 cm | <1 cm | ||
| Inferior vena cava dilatation | Measure largest respirophasic diameter within 2 cm of right atrium | 2.1 cm | Normal: 1 to 2 cm | Contracted: 0.9 cm | |
Data Analysis
We based our sample size calculations on earlier reports of HCUE by noncardiologist trainees for assessment of left ventricular (LV) systolic function.7, 10 From these reports, we estimated a negative likelihood ratio of 0.3. In addition, we expected about a quarter of our patients to have LV systolic dysfunction (B.M., personal communication). Therefore, to achieve 95% confidence intervals (CIs) around the point estimate of a negative likelihood ratio that excluded 0.50, our upper bound for a clinically meaningful result, we needed a sample size of approximately 300 patients.18
We defined threshold levels of ordinal severity for the 6 cardiac abnormalities under study based on their clinical pertinence to hospitalists (Table 2). Here, we reasoned that abnormalities at or above these levels would likely lead to important changes in hospitalists' management of inpatients; abnormalities below these levels rarely represent cardiac disease that is worthy of an immediate change in management. Since even mild degrees of LV dysfunction have important diagnostic and therapeutic implications for most general medicine inpatients, particularly those presenting with heart failure,19 we set our threshold for LV dysfunction at mild or greater. In contrast, since neither mild nor moderate mitral regurgitation (MR) has immediate implications for medical or surgical therapy even if symptoms or LV dysfunction are present,20 we set our threshold for MR at severe. Similarly, though mild LA enlargement21 and mild LV hypertrophy22 have clear prognostic implications for patients' chronic medical conditions, we reasoned that only moderate or severe versions likely reflect underlying abnormalities that affect hospitalists' point‐of‐care decision‐making. Since cardiac tamponade is rarely both subclinical23 and due to a small pericardial effusion,24 we set our threshold for pericardial effusion size at moderate or large. Finally, we set our threshold IVC diameter, a marker of central venous volume status,25 at dilated, because volume overload is an important consideration in hospitalized cardiac patients.
Using these thresholds, investigators dichotomized echocardiographers' SE readings as normal or abnormal for each of the 6 cardiac abnormalities under study to serve as the reference standards. Hospitalists' HCUE results were then compared to the reference standards in 2 different ways. We first analyzed HCUE results as dichotomous values to calculate conventional sensitivity, specificity, and positive and negative likelihood ratios. Here we considered indeterminate HCUE results positive in a clinically conservative tradeoff that neither ignores indeterminate results nor risks falsely classifying them as negative.26 We then analyzed hospitalists' HCUE results as ordinal values for receiver operating characteristic (ROC) curve analysis. Here we considered an indeterminate result as 1 possible test result.27
To examine interobserver variability of HCUE, we first chose from the 6 possible assessments only those with a mean number of abnormal patients per hospitalist greater than 5. We reasoned that variability among assessments with lower prevalence would be predictably wide and inconclusive. We then expressed variability as standard deviations (SDs) around mean sensitivity and specificity for the 8 hospitalists.
The CIs for likelihood ratios were constructed using the likelihood‐based approach to binomial proportions of Koopman.28 The areas under ROC curves were computed using the trapezoidal rule, and the CIs for these areas were constructed using the algorithm described by DeLong et al.29 All analyses were conducted with Stata Statistical Software, Release 10 (StataCorp, College Station, TX).
Results
During the 3 month study period, 654 patients were referred for SE from the 3 participating patient care units (Figure 1). Among these, 65 patients were ineligible because their SE was performed on the weekend and 178 other patients were not randomized from the general medical wards and CCU. From the remaining eligible patients, 322 underwent HCUE and 314 (98% of 322) underwent both SE and HCUE. Individual SE assessments were not interpretable (and therefore excluded) due to poor image quality for LA enlargement in 1 patient and IVC dilatation in 30 patients. Eighty‐three percent of patients who underwent SE (260/314) were referred to assess LV function (Table 3). The prevalence of the 6 clinically pertinent cardiac abnormalities under study ranged from 1% for moderate or large pericardial effusion to 25% for LV systolic dysfunction. Overall, 40% of patients had at least 1 out of 6 cardiac abnormalities.
| Characteristic | |
|---|---|
| |
| Age, year SD (25th to 75th percentiles) | 56 13 (48 to 64) |
| Women | 146 (47) |
| Chronic obstructive pulmonary disease | 47 (15) |
| Body mass index | |
| 24.9 or less: underweight or normal | 74 (24) |
| 25 to 29.9: overweight | 94 (30) |
| 30 to 34.9: mild obesity | 75 (24) |
| 35 or greater: moderate or severe obesity | 71 (23) |
| Patient care unit | |
| Short‐stay unit | 175 (56) |
| General medical wards | 89 (28) |
| Cardiac care unit | 50 (16) |
| Indication for standard echocardiography* | |
| Left ventricular function | 260 (83) |
| Valvular function | 56 (18) |
| Wall motion abnormality | 29 (9) |
| Valvular vegetations | 22 (7) |
| Any structural heart disease | 20 (6) |
| Right ventricular function | 18 (6) |
| Other | 38 (12) |
| Standard echocardiography findings | |
| Left ventricular systolic dysfunction mild | 80 (25) |
| Inferior vena cava dilated | 45 (14) |
| Left ventricular wall thickness moderate | 33 (11) |
| Left atrium enlargement moderate | 19 (6) |
| Mitral valve regurgitation severe | 11 (4) |
| Pericardial effusion moderate | 3 (1) |
| At least 1 of the above findings | 127 (40) |
| Time difference between HCUE and standard echocardiogram, median hours (25th to 75th percentiles) | 2.8 (1.4 to 5.1) |
| Time to complete HCUE, median minutes (25th to 75th percentiles) | 28 (20 to 35) |
Each hospitalist performed a similar total number of HCUE examinations (range, 3447). The median time difference between performance of SE and HCUE was 2.8 hours (25th75th percentiles, 1.45.1). Despite the high prevalence of chronic obstructive pulmonary disease and obesity, hospitalists considered HCUE assessments indeterminate in only 2% to 6% of the 6 assessments made for each patient (Table 4). Among the 38 patients (12% of 322) with any indeterminate HCUE assessment, 24 patients had only 1 out of 6 possible. Hospitalists completed HCUE in a median time of 28 minutes (25th‐75th percentiles, 2035), which included the time to record 7 best‐quality moving images and to fill out the research data collection form.
| n (%)* | |
|---|---|
| |
| Number of indeterminate findings per patient | |
| 0 | 284 (88) |
| 1 | 24 (7) |
| 2 | 4 (1) |
| 3 or more | 10 (3) |
| Indeterminate findings by cardiac assessment | |
| Mitral valve regurgitation | 18 (6) |
| Inferior vena cava diameter | 16 (5) |
| Left ventricular hypertrophy | 15 (5) |
| Pericardial effusion | 9 (3) |
| Left atrium size | 5 (2) |
| Left ventricle systolic function | 5 (2) |
When HCUE results were analyzed as dichotomous values, positive likelihood ratios ranged from 2.5 to 21, and negative likelihood ratios ranged from 0 to 0.4 (Table 5). Positive and negative likelihood ratios were both sufficiency high and low to respectively increase and decrease by 5‐fold the prior odds of 3 out of 6 cardiac abnormalities: LV systolic dysfunction, moderate or severe MR regurgitation, and moderate or large pericardial effusion. Considering HCUE results as ordinal values for ROC analysis yielded additional diagnostic information (Figure 2). For example, the likelihood ratio of 1.0 (95% CI, 0.42.0) for borderline positive moderate or severe LA enlargement increased to 29 (range, 1362) for extreme positive results. Areas under the ROC curves were 0.9 for 4 out of 6 cardiac abnormalities.
| Clinically Pertinent Cardiac Abnormality by Standard Echocardiography | Prevalence n/total n | Sensitivity* % (95% CI) | Specificity* % (95% CI) | LRpositive*, (95% CI) | LRnegative*, (95% CI) |
|---|---|---|---|---|---|
| |||||
| Left ventricular systolic dysfunction | 80/314 | 85 (7592) | 88 (8392) | 6.9 (4.99.8) | 0.2 (0.10.3) |
| Mitral valve regurgitation, severe | 11/314 | 100 (72100) | 83 (7987) | 5.9 (3.97.4) | 0 (00.3) |
| Left atrium enlargement, moderate or severe | 19/313 | 90 (6799) | 74 (6879) | 3.4 (2.54.3) | 0.1 (0.040.4) |
| Left ventricular hypertrophy, moderate or severe | 33/314 | 70 (5184) | 73 (6778) | 2.5 (1.83.3) | 0.4 (0.20.7) |
| Pericardial effusion, moderate or large | 3/314 | 100 (29100) | 95 (9297) | 21 (6.731) | 0 (00.6) |
| Inferior vena cava, dilated | 45/284 | 56 (4070) | 86 (8190) | 4.0 (2.66.0) | 0.5 (0.40.7) |
LV systolic dysfunction and IVC dilatation were both prevalent enough to meet our criterion to examine interobserver variability; the mean number of abnormal patients per hospitalist was 10 patients for LV systolic dysfunction and 6 patients for IVC dilatation. For LV systolic dysfunction, SDs around mean sensitivity (84%) and specificity (87%) were 12% and 6%, respectively. For IVC dilatation, SDs around mean sensitivity (58%) and specificity (86%) were 24% and 7%, respectively.
Discussion
We found that, after a 27‐hour training program, hospitalists performed HCUE with moderate to excellent diagnostic accuracy for 6 important cardiac abnormalities. For example, hospitalists' assessments of LV systolic function yielded positive and negative likelihood ratios of 6.9 (95% CI, 4.99.8) and 0.2 (95% CI, 0.10.3), respectively. At the bedsides of patients with acute heart failure, therefore, hospitalists could use HCUE to lower or raise the 50:50 chance of LV systolic dysfunction30 to 15% or 85%, respectively. Whether or not these posttest likelihoods are extreme enough to cross important thresholds will depend on the clinical context. Yet these findings demonstrate how HCUE has the potential to provide hospitalists with valuable point‐of‐care data that are otherwise unavailableeither because routine clinical assessments are unreliable31 or because echocardiographic services are not immediately accessible.1
In fact, recent data from the Joint Commission on Accreditation of Healthcare Organizations shows how inaccessible SE may be. Approximately one‐quarter of hospitals in the United States send home about 10% of patients with acute heart failure without echocardiographic assessment of LV systolic function before, during, or immediately after hospitalization.32 In doing so, these hospitals leave unmet the 2002 National Quality Improvement Goal of universal assessment of LV systolic function for all heart failure patients. Hospitalists could close this quality gap with routine, 10‐minute HCUE assessments in all patients admitted with acute heart failure. (Our research HCUE protocol required a median time of 28 minutes, but this included time to assess 5 other cardiac abnormalities and collect data for research purposes). Until the clinical consequences of introducing hospitalist‐performed HCUE are studied, potential benefits like this are tentative. But our findings suggest that training hospitalists to accurately perform HCUE can be successfully accomplished in just 27 hours.
Other studies of HCUE training programs for noncardiologists have also challenged the opinion that learning to perform HCUE requires more than 100 hours of training.2, 711 Yet only 1 prior study has examined an HCUE training program for hospitalists.5 In this study by Martin et al.,5 hospitalists completed 5 supervised HCUE examinations and 6 hours of interpretation training before investigators scored their image acquisition and interpretation skills from 30 unsupervised HCUE examinations. To estimate their final skill levels at the completion of all 35 examinations by accounting for an initially steep learning curve, investigators then adjusted these scores with regression models. Despite these upward adjustments, hospitalists' image acquisition and interpretation scores were low in comparison to echocardiographic technicians and cardiology fellows. Besides these adjusted measurements of hospitalists' skills, however, Martin et al.5 unfortunately did not also report standard measures of diagnostic accuracy, like those proposed by the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.33 Therefore, direct comparisons to the present study are difficult. Nevertheless, their findings suggest that a training program limited to 5 supervised HCUE examinations may be inadequate for hospitalists. In fact, the same group's earlier study of medical trainees suggested a minimum of 30 supervised HCUE examinations.9 We chose to design our hospitalist training program based on this minimum, though they surprisingly did not.5 As others continue to refine the components of hospitalist HCUE training programs, such as the optimal number of supervised examinations, our program could serve as a reasonable comparative example: more rigorous than the program designed by Martin et al.5 but more feasible than ASE level 1 training.
The number and complexity of assessments taught in HCUE training programs will determine their duration. With ongoing advancements in HCUE technology, there is a growing list of potential assessments to choose from. Although HCUE training programs ought to include assessments with proven clinical applications, there are no trials of HCUE‐directed care to inform such decisions. In their absence, therefore, we chose 6 assessments based on the following 3 criteria. First, our assessments were otherwise not reliably available from routine clinical data, such as the physical examination. Second, our assessments were straightforward: easy to learn and simple to perform. Here, we based our reasoning on an expectation that the value of HCUE lies not in highly complex, state‐of‐the‐art assessmentswhich are best left to echocardiographers equipped with SEbut in simple, routine assessments made with highly portable machines that grant noncardiologists newfound access to point‐of‐care data.34 Third, our assessments were clinically pertinent and, where appropriate, defined by cut‐points at levels of severity that often lead to changes in management. We suspect that setting high cut‐points has the salutary effects of making assessments easier to learn and more accurate, because distinguishing mild abnormalities is likely the most challenging aspect of echocardiographic interpretation.35 Whether or not our choices of assessments, and their cut‐points, are optimal has yet to be determined by future research designed to study how they affect patient outcomes. Given our hospitalists' performance in the present study, these assessments seem worthy of such future research.
Our study had several limitations. We studied physicians and patients from only 1 hospital; similar studies performed in different settings, particularly among patients with different proportions and manifestations of disease, may find different results. Nevertheless, our sampling method of prospectively enrolling consecutive patients strengthens our findings. Some echocardiographic measurement methods used by our hospitalists differed in subtle ways from echocardiography guideline recommendations.35 We chose our methods (Table 2) for 2 reasons. First, whenever possible, we chose methods of interpretation that coincided with our local cardiologists'. Second, we chose simplicity over precision. For example, the biplane method of disks, or modified Simpson's rule, is the preferred volumetric method of calculating LA size.35 This method requires tracing the contours of the LA in 2 planes and then dividing the LA volume into stacked oval disks for calculation. We chose instead to train our hospitalists in a simpler method based on 2 linear measurements. Any loss of precision, however, was balanced by a large gain in simplicity. Regardless, minor variations in LA size are not likely to affect hospitalists' bedside evaluations. Finally, we did not validate the results of our reference standard (SE) by documenting interobserver reliability. Yet, because SE is generally accurate for the 6 cardiac abnormalities under study, the effect of this bias should be small.
These limitations can be addressed best by controlled trials of HCUE‐directed care. These trials will determine the clinical impact of hospitalist‐performed HCUE and, in turn, inform our design of HCUE training programs. As the current study shows, training hospitalists to participate in such trials is feasible: like other groups of noncardiologists, hospitalists can accurately perform HCUE after a brief training program. Whether or not hospitalists should perform HCUE requires further study.
Acknowledgements
The authors thank Sonosite, Inc., Bothell, WA, for loaning us 2 MicroMaxx machines throughout the study period. They also thank the staff of the Internal Medicine Research Mentoring Program at Rush Medical College for their technical support and the staff of the Division of Neurology at Stroger Hospital for granting them access to a procedure room.
- .The physical examination of the future: echocardiography as part of the assessment.ACC Curr J Rev.1998;7:79–81.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20:477–485.
- ,.Bedside ultrasonography in the ICU: Part 2.Chest.2005;128:1766–1781.
- ,.Practical Guide to Emergency Ultrasound.1st ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120:1000–1004.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the echocardiography task force on new technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc Echocardiogr.2002;15:369–373.
- ,,,,,.The use of small personal ultrasound devices with internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,, et al.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147:476–481.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118:1010–1018.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96:1002–1006.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20:471–476.
- ,,, et al.A hospitalist‐run short stay unit: features that predict patients' length‐of‐stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- .Adult echocardiography scanning protocol. In: Templin BB, ed.Ultrasound Scanning: Principles and Protocols.2nd ed.Philadelphia, PA:Saunders;1999:426.
- ,,, et al.ACCF 2008 Recommendations for training in adult cardiovascular medicine core cardiology training (COCATS 3) (revision of the 2002 COCATS training statement).J Am Coll Cardiol.2008;51:333–414.
- ,,.The Echo Manual.2nd ed.Philadelphia, PA:Lippincott Williams 1999.
- ,,, et al.Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications.J Am Soc Echocardiogr.1991;4:203–214.
- .Textbook of Clinical Echocardiography.3rd ed.Philadelphia, PA:Elsevier Saunders;2004.
- ,,.Likelihood ratios with confidence: sample size estimation for diagnostic test studies.J Clin Epidemiol.1991;44:763–770.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2005;112;154–235.
- ,,, et al.ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2006;114:e84–e231.
- ,,, et al.Left atrial size: physiologic determinants and clinical applications.J Am Coll Cardiol.2006;47:2357–2363.
- ,,,,.Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study.N Engl J Med.1990;322:1561–1566.
- ,,,.Does this patient with a pericardial effusion have cardiac tamponade?JAMA.2007;297:1810–1818.
- .Acute cardiac tamponade.N Engl J Med.2003;349:685–690.
- ,,,,,.Evaluation of size and dynamics of the inferior vena cava as an index of right‐sided cardiac function.Am J Cardiol.1984;53:579–585.
- ,,.The influence of uninterpretability on the assessment of diagnostic tests.J Chronic Dis.1986;39:575–584.
- ,,.Relations between effectiveness of a diagnostic test, prevalence of the disease, and percentages of uninterpretable results. An example in the diagnosis of jaundice.Med Decis Making.1982;2:285–297.
- .Confidence intervals for the ratio of two binomial proportions.Biometrics.1984;40:513–517.
- ,,.Comparing the areas under two or more correlated receiver operating curves: a nonparametric approach.Biometrics.1988;44:837–845.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296:2217–2226.
- ,,, et al.Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure.Am J Med.2002;112:437–445.
- Joint Commission on Accreditation of Healthcare Organizations. Health Care Quality Data Download Website. Available at: http://www.healthcarequalitydata.org. Accessed December2008.
- ,,, et al.Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative.Clin Chem.2003;49:1–6.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78:102–112.
- ,,, et al.Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology.J Am Soc Echocardiogr.2005;18:1440–1463.
Hand‐carried ultrasound echocardiography (HCUE) can help noncardiologists answer well‐defined questions at patients' bedsides in less than 10 minutes.1, 2 Indeed, intensivists3 and emergency department physicians4 already use HCUE to make rapid, point‐of‐care assessments. Since cardiovascular diagnoses are common among general medicine inpatients, HCUE may become an important skill for hospitalists to learn.5
However, uncertainty exists about the duration of HCUE training for hospitalists. In 2002, experts from the American Society of Echocardiography (ASE) published recommendations on training requirements for HCUE.6 With limited data on the safety or performance of HCUE training programs, which had just begun to emerge, the ASE borrowed from the proven training recommendations for standard echocardiography (SE). They recommended that all HCUE trainees, cardiologist and noncardiologist alike, complete level 1 SE training: 75 personally‐performed and 150 personally‐interpreted echocardiographic examinations. Since then, however, several HCUE training programs designed for noncardiologists have emerged.2, 5, 710 These alternative programs suggest that the ASE's recommended duration of training may be too long, particularly for focused HCUE that is limited to a few relatively simple assessments. It is important not to overshoot the requirements of HCUE training, because doing so may discourage groups of noncardiologists, like hospitalists, who may derive great benefits from HCUE.11
To address this uncertainty for hospitalists, we first developed a brief HCUE training program to assess 6 important cardiac abnormalities. We then studied the diagnostic accuracy of HCUE by hospitalists as a test of these 6 cardiac abnormalities assessed by SE.
Patients and Methods
Setting and Subjects
This prospective cohort study was performed at Stroger Hospital of Cook County, a 500‐bed public teaching hospital in Chicago, IL, from March through May of 2007. The cohort was adult inpatients who were referred for SE on weekdays from 3 distinct patient care units (Figure 1). We used 2 sampling modes to balance practical constraints (short‐stay unit [SSU] patients were more localized and, therefore, easier to study) with clinical diversity. We consecutively sampled patients from our SSU, where adults with provisional cardiovascular diagnoses are admitted if they might be eligible for discharge with in 3 days.12 But we used random number tables with a daily unique starting point to randomly sample patients from the general medical wards and the coronary care unit (CCU). Patients were excluded if repositioning them for HCUE was potentially harmful. The study was approved by our hospital's institutional review board, and we obtained written informed consent from all enrolled patients.

SE Protocol
As part of enrolled patients' routine clinical care, SE images were acquired and interpreted in the usual fashion in our hospital's echocardiography laboratory, which performs SE on over 7,000 patients per year. Echocardiographic technicians acquired images with a General Electric Vivid 7 cardiac ultrasound machine (General Electric, Milwaukee, WI) equipped with a GE M4S 1.8 to 3.4 MHz cardiac transducer (General Electric). Technicians followed the standard adult transthoracic echocardiography scanning protocol to acquire 40 to 100 images on every patient using all available echocardiographic modalities: 2‐dimensional, M‐mode, color Doppler, continuous‐wave Doppler, pulse‐wave Doppler, and tissue Doppler.13 Blinded to HCUE results, attending physician cardiologist echocardiographers then interpreted archived images using computer software (Centricity System; General Electric) to generate final reports that were entered into patients' medical records. This software ensured that final reports were standardized, because echocardiographers' final qualitative assessments were limited to short lists of standard options; for example, in reporting left atrium (LA) size, echocardiographers chose from only 5 standard options: normal, mildly dilated, moderately dilated, severely dilated, and not interpretable. Investigators, who were also blinded to HCUE results, later abstracted SE results from these standardized report forms in patients' medical records. All echocardiographers fulfilled ASE training guidelines to independently interpret SE: a minimum of 150 personally‐performed and 300 personally‐interpreted echocardiographic examinations (training level 2).14
HCUE Training
Based on the recommendations of our cardiologist investigator (B.M.), we developed a training program for 1 hospitalist to become an HCUE instructor. Our instructor trainee (C.C.) was board‐eligible in internal medicine but had no previous formal training in cardiology or echocardiography. We a priori established that her training would continue until our cardiologist investigator determined that she was ready to train other hospitalists; this determination occurred after 5 weeks. She learned image acquisition by performing focused SE on 30 patients under the direct supervision of an echocardiographic technician. She also performed focused HCUE on 65 inpatients without direct supervision but with ongoing access to consult the technician to review archived images and troubleshoot difficulties with acquisition. She learned image interpretation by reading relevant chapters from a SE textbook15 and by participating in daily didactic sessions in which attending cardiologist echocardiographers train cardiology fellows in SE interpretation.
This hospitalist then served as the HCUE instructor for 8 other attending physician hospitalists who were board‐certified internists with no previous formal training in cardiology or echocardiography. The training program was limited to acquisition and interpretation of 2‐dimensional grayscale and color Doppler images for the 6 cardiac assessments under study (Table 1). The instructor marshaled pairs of hospitalists through the 3 components of the training program, which lasted a total of 27 hours.
|
| Six cardiac assessments learned using 2‐dimensional gray scale and color Doppler imaging |
| Left ventricular systolic dysfunction |
| Mitral valve regurgitation |
| Left atrium enlargement |
| Left ventricular hypertrophy |
| Pericardial effusion |
| Inferior vena cava diameter |
| Lecture (2 hours)* |
| Basic principles of echocardiography |
| HCUE scanning protocol and helpful techniques to optimize image quality |
| Hands‐on training with instructor |
| Orientation to machine and demonstration of scanning protocol (1 hour) |
| Sessions 1 through 3: HCUE performed on 1 patient per hour (6 patients in 6 hours) |
| Sessions 4 through 10: HCUE performed on 2 patients per hour (28 patients in 14 hours) |
| Feedback sessions on image quality and interpretation with cardiologist |
| After hands‐on training session 3 (2 hours) |
| After hands‐on training session 10 (2 hours) |
First, hospitalists attended a 2‐hour lecture on the basic principles of HCUE. Slides from this lecture and additional images of normal and abnormal findings were provided to each hospitalist on a digital video disc. Second, each hospitalist underwent 20 hours of hands‐on training in 2‐hour sessions scheduled over 2 weeks. Willing inpatients from our hospital's emergency department were used as volunteers for these hand‐on training sessions. During these sessions the instructor provided practical suggestions to optimize image quality, such as transducer location and patient positioning. In the first 3 sessions, the minimum pace was 1 patient per hour; thereafter, the pace was increased to 1 patient per half‐hour. We chose 20 hours of hands‐on training and these minimum paces because they allowed each hospitalist to attain a cumulative experience of no less than 30 patientsan amount that heralds a flattening of the HCUE learning curve among medical trainees.9 Third, each pair of hospitalists received feedback from a cardiologist investigator (B.M.) who critiqued the quality and interpretation of images acquired by hospitalists during hands‐on training sessions. Since image quality varies by patient,16 hospitalists' images were compared side‐by‐side to images recorded by the instructor on the same patients. The cardiologist also critiqued hospitalists' interpretations of both their own images and additional sets of archived images from patients with abnormal findings.
HCUE Protocol
After completing the training program and blinded to the results of SE, the 8 hospitalists performed HCUE on enrolled patients within hours of SE. We limited the time interval between tests to minimize the effect that changes in physiologic variables, such as blood pressure and intravascular volume, have on the reliability of serial echocardiographic measurements.16 Hospitalists performed HCUE with a MicroMaxx 3.4 hand‐carried ultrasound machine equipped with a cardiology software package and a 1 to 5 MHz P17 cardiac transducer (Sonosite, Inc., Bothell, WA); simultaneous electrocardiographic recording, though available, was not used. While patients laid on their own standard hospital beds or on a standard hospital gurney in a room adjacent to the SE waiting room, hospitalists positioned them without assistance from nursing staff and recorded 7 best‐quality images per patient. Patients were first positioned in a partial (3045 degrees) left lateral decubitus position to record 4 grayscale images of the short‐axis and long‐axis parasternal and 2‐chamber and 4‐chamber apical views; 2 color Doppler images of the mitral inflow were also recorded from the long‐axis parasternal and the 4‐chamber apical views. Patients were then positioned supine to record 1 grayscale image of the inferior vena cava (IVC) from the transhepatic view. Hospitalists did not perform a history or physical exam on enrolled patients, nor did they review patients' medical records.
Immediately following the HCUE, hospitalists replayed the recorded images as often as needed and entered final interpretations on data collection forms. Linear measurements were made manually with a caliper held directly to the hand‐carried ultrasound monitor. These measurements were then translated into qualitative assessments based on standard values used by our hospital's echocardiographers (Table 2).17 When a hospitalist could not confidently assess a cardiac abnormality, the final HCUE assessment was recorded as indeterminate. Hospitalists also recorded the time to perform each HCUE, which included the time to record 7 best‐quality images, to interpret the findings, and to fill out the data collection form.
| Hand‐Carried Ultrasound Echocardiography Results | |||||
|---|---|---|---|---|---|
| Cardiac Abnormality by Standard Echocardiography | Hand‐Carried Ultrasound Echocardiography Operator's Method of Assessment | Positive | Negative | ||
| |||||
| Left ventricle systolic dysfunction, mild or greater | Grade degree of abnormal wall movement and thickening during systole | Severe | Mild or moderate | Normal | Vigorous |
| Mitral valve regurgitation, severe | Classify regurgitant jet as central or eccentric, then measure as percentage of left atrium area | ||||
| Central jet | 20% | <20% | |||
| Eccentric jet | 20% | indeterminate 20% | |||
| Left atrium enlargement, moderate or severe | Measure left atrium in 3 dimensions at end diastole, then use the most abnormal dimension | Extreme | Borderline | ||
| Anteroposterior or mediolateral (cm) | 5.1 | 4.55.0 | 4.4 | ||
| Superior‐inferior (cm) | 7.1 | 6.17.0 | 6.0 | ||
| Left ventricle hypertrophy, moderate or severe | Measure thickest dimension of posterior or septal wall at end diastole | Extreme: 1.4 cm | Borderline: 1.21.3 cm | 1.1 cm | |
| Pericardial effusion, medium or large | Measure largest dimension in any view at end diastole | 1 cm | <1 cm | ||
| Inferior vena cava dilatation | Measure largest respirophasic diameter within 2 cm of right atrium | 2.1 cm | Normal: 1 to 2 cm | Contracted: 0.9 cm | |
Data Analysis
We based our sample size calculations on earlier reports of HCUE by noncardiologist trainees for assessment of left ventricular (LV) systolic function.7, 10 From these reports, we estimated a negative likelihood ratio of 0.3. In addition, we expected about a quarter of our patients to have LV systolic dysfunction (B.M., personal communication). Therefore, to achieve 95% confidence intervals (CIs) around the point estimate of a negative likelihood ratio that excluded 0.50, our upper bound for a clinically meaningful result, we needed a sample size of approximately 300 patients.18
We defined threshold levels of ordinal severity for the 6 cardiac abnormalities under study based on their clinical pertinence to hospitalists (Table 2). Here, we reasoned that abnormalities at or above these levels would likely lead to important changes in hospitalists' management of inpatients; abnormalities below these levels rarely represent cardiac disease that is worthy of an immediate change in management. Since even mild degrees of LV dysfunction have important diagnostic and therapeutic implications for most general medicine inpatients, particularly those presenting with heart failure,19 we set our threshold for LV dysfunction at mild or greater. In contrast, since neither mild nor moderate mitral regurgitation (MR) has immediate implications for medical or surgical therapy even if symptoms or LV dysfunction are present,20 we set our threshold for MR at severe. Similarly, though mild LA enlargement21 and mild LV hypertrophy22 have clear prognostic implications for patients' chronic medical conditions, we reasoned that only moderate or severe versions likely reflect underlying abnormalities that affect hospitalists' point‐of‐care decision‐making. Since cardiac tamponade is rarely both subclinical23 and due to a small pericardial effusion,24 we set our threshold for pericardial effusion size at moderate or large. Finally, we set our threshold IVC diameter, a marker of central venous volume status,25 at dilated, because volume overload is an important consideration in hospitalized cardiac patients.
Using these thresholds, investigators dichotomized echocardiographers' SE readings as normal or abnormal for each of the 6 cardiac abnormalities under study to serve as the reference standards. Hospitalists' HCUE results were then compared to the reference standards in 2 different ways. We first analyzed HCUE results as dichotomous values to calculate conventional sensitivity, specificity, and positive and negative likelihood ratios. Here we considered indeterminate HCUE results positive in a clinically conservative tradeoff that neither ignores indeterminate results nor risks falsely classifying them as negative.26 We then analyzed hospitalists' HCUE results as ordinal values for receiver operating characteristic (ROC) curve analysis. Here we considered an indeterminate result as 1 possible test result.27
To examine interobserver variability of HCUE, we first chose from the 6 possible assessments only those with a mean number of abnormal patients per hospitalist greater than 5. We reasoned that variability among assessments with lower prevalence would be predictably wide and inconclusive. We then expressed variability as standard deviations (SDs) around mean sensitivity and specificity for the 8 hospitalists.
The CIs for likelihood ratios were constructed using the likelihood‐based approach to binomial proportions of Koopman.28 The areas under ROC curves were computed using the trapezoidal rule, and the CIs for these areas were constructed using the algorithm described by DeLong et al.29 All analyses were conducted with Stata Statistical Software, Release 10 (StataCorp, College Station, TX).
Results
During the 3 month study period, 654 patients were referred for SE from the 3 participating patient care units (Figure 1). Among these, 65 patients were ineligible because their SE was performed on the weekend and 178 other patients were not randomized from the general medical wards and CCU. From the remaining eligible patients, 322 underwent HCUE and 314 (98% of 322) underwent both SE and HCUE. Individual SE assessments were not interpretable (and therefore excluded) due to poor image quality for LA enlargement in 1 patient and IVC dilatation in 30 patients. Eighty‐three percent of patients who underwent SE (260/314) were referred to assess LV function (Table 3). The prevalence of the 6 clinically pertinent cardiac abnormalities under study ranged from 1% for moderate or large pericardial effusion to 25% for LV systolic dysfunction. Overall, 40% of patients had at least 1 out of 6 cardiac abnormalities.
| Characteristic | |
|---|---|
| |
| Age, year SD (25th to 75th percentiles) | 56 13 (48 to 64) |
| Women | 146 (47) |
| Chronic obstructive pulmonary disease | 47 (15) |
| Body mass index | |
| 24.9 or less: underweight or normal | 74 (24) |
| 25 to 29.9: overweight | 94 (30) |
| 30 to 34.9: mild obesity | 75 (24) |
| 35 or greater: moderate or severe obesity | 71 (23) |
| Patient care unit | |
| Short‐stay unit | 175 (56) |
| General medical wards | 89 (28) |
| Cardiac care unit | 50 (16) |
| Indication for standard echocardiography* | |
| Left ventricular function | 260 (83) |
| Valvular function | 56 (18) |
| Wall motion abnormality | 29 (9) |
| Valvular vegetations | 22 (7) |
| Any structural heart disease | 20 (6) |
| Right ventricular function | 18 (6) |
| Other | 38 (12) |
| Standard echocardiography findings | |
| Left ventricular systolic dysfunction mild | 80 (25) |
| Inferior vena cava dilated | 45 (14) |
| Left ventricular wall thickness moderate | 33 (11) |
| Left atrium enlargement moderate | 19 (6) |
| Mitral valve regurgitation severe | 11 (4) |
| Pericardial effusion moderate | 3 (1) |
| At least 1 of the above findings | 127 (40) |
| Time difference between HCUE and standard echocardiogram, median hours (25th to 75th percentiles) | 2.8 (1.4 to 5.1) |
| Time to complete HCUE, median minutes (25th to 75th percentiles) | 28 (20 to 35) |
Each hospitalist performed a similar total number of HCUE examinations (range, 3447). The median time difference between performance of SE and HCUE was 2.8 hours (25th75th percentiles, 1.45.1). Despite the high prevalence of chronic obstructive pulmonary disease and obesity, hospitalists considered HCUE assessments indeterminate in only 2% to 6% of the 6 assessments made for each patient (Table 4). Among the 38 patients (12% of 322) with any indeterminate HCUE assessment, 24 patients had only 1 out of 6 possible. Hospitalists completed HCUE in a median time of 28 minutes (25th‐75th percentiles, 2035), which included the time to record 7 best‐quality moving images and to fill out the research data collection form.
| n (%)* | |
|---|---|
| |
| Number of indeterminate findings per patient | |
| 0 | 284 (88) |
| 1 | 24 (7) |
| 2 | 4 (1) |
| 3 or more | 10 (3) |
| Indeterminate findings by cardiac assessment | |
| Mitral valve regurgitation | 18 (6) |
| Inferior vena cava diameter | 16 (5) |
| Left ventricular hypertrophy | 15 (5) |
| Pericardial effusion | 9 (3) |
| Left atrium size | 5 (2) |
| Left ventricle systolic function | 5 (2) |
When HCUE results were analyzed as dichotomous values, positive likelihood ratios ranged from 2.5 to 21, and negative likelihood ratios ranged from 0 to 0.4 (Table 5). Positive and negative likelihood ratios were both sufficiency high and low to respectively increase and decrease by 5‐fold the prior odds of 3 out of 6 cardiac abnormalities: LV systolic dysfunction, moderate or severe MR regurgitation, and moderate or large pericardial effusion. Considering HCUE results as ordinal values for ROC analysis yielded additional diagnostic information (Figure 2). For example, the likelihood ratio of 1.0 (95% CI, 0.42.0) for borderline positive moderate or severe LA enlargement increased to 29 (range, 1362) for extreme positive results. Areas under the ROC curves were 0.9 for 4 out of 6 cardiac abnormalities.

| Clinically Pertinent Cardiac Abnormality by Standard Echocardiography | Prevalence n/total n | Sensitivity* % (95% CI) | Specificity* % (95% CI) | LRpositive*, (95% CI) | LRnegative*, (95% CI) |
|---|---|---|---|---|---|
| |||||
| Left ventricular systolic dysfunction | 80/314 | 85 (7592) | 88 (8392) | 6.9 (4.99.8) | 0.2 (0.10.3) |
| Mitral valve regurgitation, severe | 11/314 | 100 (72100) | 83 (7987) | 5.9 (3.97.4) | 0 (00.3) |
| Left atrium enlargement, moderate or severe | 19/313 | 90 (6799) | 74 (6879) | 3.4 (2.54.3) | 0.1 (0.040.4) |
| Left ventricular hypertrophy, moderate or severe | 33/314 | 70 (5184) | 73 (6778) | 2.5 (1.83.3) | 0.4 (0.20.7) |
| Pericardial effusion, moderate or large | 3/314 | 100 (29100) | 95 (9297) | 21 (6.731) | 0 (00.6) |
| Inferior vena cava, dilated | 45/284 | 56 (4070) | 86 (8190) | 4.0 (2.66.0) | 0.5 (0.40.7) |
LV systolic dysfunction and IVC dilatation were both prevalent enough to meet our criterion to examine interobserver variability; the mean number of abnormal patients per hospitalist was 10 patients for LV systolic dysfunction and 6 patients for IVC dilatation. For LV systolic dysfunction, SDs around mean sensitivity (84%) and specificity (87%) were 12% and 6%, respectively. For IVC dilatation, SDs around mean sensitivity (58%) and specificity (86%) were 24% and 7%, respectively.
Discussion
We found that, after a 27‐hour training program, hospitalists performed HCUE with moderate to excellent diagnostic accuracy for 6 important cardiac abnormalities. For example, hospitalists' assessments of LV systolic function yielded positive and negative likelihood ratios of 6.9 (95% CI, 4.99.8) and 0.2 (95% CI, 0.10.3), respectively. At the bedsides of patients with acute heart failure, therefore, hospitalists could use HCUE to lower or raise the 50:50 chance of LV systolic dysfunction30 to 15% or 85%, respectively. Whether or not these posttest likelihoods are extreme enough to cross important thresholds will depend on the clinical context. Yet these findings demonstrate how HCUE has the potential to provide hospitalists with valuable point‐of‐care data that are otherwise unavailableeither because routine clinical assessments are unreliable31 or because echocardiographic services are not immediately accessible.1
In fact, recent data from the Joint Commission on Accreditation of Healthcare Organizations shows how inaccessible SE may be. Approximately one‐quarter of hospitals in the United States send home about 10% of patients with acute heart failure without echocardiographic assessment of LV systolic function before, during, or immediately after hospitalization.32 In doing so, these hospitals leave unmet the 2002 National Quality Improvement Goal of universal assessment of LV systolic function for all heart failure patients. Hospitalists could close this quality gap with routine, 10‐minute HCUE assessments in all patients admitted with acute heart failure. (Our research HCUE protocol required a median time of 28 minutes, but this included time to assess 5 other cardiac abnormalities and collect data for research purposes). Until the clinical consequences of introducing hospitalist‐performed HCUE are studied, potential benefits like this are tentative. But our findings suggest that training hospitalists to accurately perform HCUE can be successfully accomplished in just 27 hours.
Other studies of HCUE training programs for noncardiologists have also challenged the opinion that learning to perform HCUE requires more than 100 hours of training.2, 711 Yet only 1 prior study has examined an HCUE training program for hospitalists.5 In this study by Martin et al.,5 hospitalists completed 5 supervised HCUE examinations and 6 hours of interpretation training before investigators scored their image acquisition and interpretation skills from 30 unsupervised HCUE examinations. To estimate their final skill levels at the completion of all 35 examinations by accounting for an initially steep learning curve, investigators then adjusted these scores with regression models. Despite these upward adjustments, hospitalists' image acquisition and interpretation scores were low in comparison to echocardiographic technicians and cardiology fellows. Besides these adjusted measurements of hospitalists' skills, however, Martin et al.5 unfortunately did not also report standard measures of diagnostic accuracy, like those proposed by the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.33 Therefore, direct comparisons to the present study are difficult. Nevertheless, their findings suggest that a training program limited to 5 supervised HCUE examinations may be inadequate for hospitalists. In fact, the same group's earlier study of medical trainees suggested a minimum of 30 supervised HCUE examinations.9 We chose to design our hospitalist training program based on this minimum, though they surprisingly did not.5 As others continue to refine the components of hospitalist HCUE training programs, such as the optimal number of supervised examinations, our program could serve as a reasonable comparative example: more rigorous than the program designed by Martin et al.5 but more feasible than ASE level 1 training.
The number and complexity of assessments taught in HCUE training programs will determine their duration. With ongoing advancements in HCUE technology, there is a growing list of potential assessments to choose from. Although HCUE training programs ought to include assessments with proven clinical applications, there are no trials of HCUE‐directed care to inform such decisions. In their absence, therefore, we chose 6 assessments based on the following 3 criteria. First, our assessments were otherwise not reliably available from routine clinical data, such as the physical examination. Second, our assessments were straightforward: easy to learn and simple to perform. Here, we based our reasoning on an expectation that the value of HCUE lies not in highly complex, state‐of‐the‐art assessmentswhich are best left to echocardiographers equipped with SEbut in simple, routine assessments made with highly portable machines that grant noncardiologists newfound access to point‐of‐care data.34 Third, our assessments were clinically pertinent and, where appropriate, defined by cut‐points at levels of severity that often lead to changes in management. We suspect that setting high cut‐points has the salutary effects of making assessments easier to learn and more accurate, because distinguishing mild abnormalities is likely the most challenging aspect of echocardiographic interpretation.35 Whether or not our choices of assessments, and their cut‐points, are optimal has yet to be determined by future research designed to study how they affect patient outcomes. Given our hospitalists' performance in the present study, these assessments seem worthy of such future research.
Our study had several limitations. We studied physicians and patients from only 1 hospital; similar studies performed in different settings, particularly among patients with different proportions and manifestations of disease, may find different results. Nevertheless, our sampling method of prospectively enrolling consecutive patients strengthens our findings. Some echocardiographic measurement methods used by our hospitalists differed in subtle ways from echocardiography guideline recommendations.35 We chose our methods (Table 2) for 2 reasons. First, whenever possible, we chose methods of interpretation that coincided with our local cardiologists'. Second, we chose simplicity over precision. For example, the biplane method of disks, or modified Simpson's rule, is the preferred volumetric method of calculating LA size.35 This method requires tracing the contours of the LA in 2 planes and then dividing the LA volume into stacked oval disks for calculation. We chose instead to train our hospitalists in a simpler method based on 2 linear measurements. Any loss of precision, however, was balanced by a large gain in simplicity. Regardless, minor variations in LA size are not likely to affect hospitalists' bedside evaluations. Finally, we did not validate the results of our reference standard (SE) by documenting interobserver reliability. Yet, because SE is generally accurate for the 6 cardiac abnormalities under study, the effect of this bias should be small.
These limitations can be addressed best by controlled trials of HCUE‐directed care. These trials will determine the clinical impact of hospitalist‐performed HCUE and, in turn, inform our design of HCUE training programs. As the current study shows, training hospitalists to participate in such trials is feasible: like other groups of noncardiologists, hospitalists can accurately perform HCUE after a brief training program. Whether or not hospitalists should perform HCUE requires further study.
Acknowledgements
The authors thank Sonosite, Inc., Bothell, WA, for loaning us 2 MicroMaxx machines throughout the study period. They also thank the staff of the Internal Medicine Research Mentoring Program at Rush Medical College for their technical support and the staff of the Division of Neurology at Stroger Hospital for granting them access to a procedure room.
Hand‐carried ultrasound echocardiography (HCUE) can help noncardiologists answer well‐defined questions at patients' bedsides in less than 10 minutes.1, 2 Indeed, intensivists3 and emergency department physicians4 already use HCUE to make rapid, point‐of‐care assessments. Since cardiovascular diagnoses are common among general medicine inpatients, HCUE may become an important skill for hospitalists to learn.5
However, uncertainty exists about the duration of HCUE training for hospitalists. In 2002, experts from the American Society of Echocardiography (ASE) published recommendations on training requirements for HCUE.6 With limited data on the safety or performance of HCUE training programs, which had just begun to emerge, the ASE borrowed from the proven training recommendations for standard echocardiography (SE). They recommended that all HCUE trainees, cardiologist and noncardiologist alike, complete level 1 SE training: 75 personally‐performed and 150 personally‐interpreted echocardiographic examinations. Since then, however, several HCUE training programs designed for noncardiologists have emerged.2, 5, 710 These alternative programs suggest that the ASE's recommended duration of training may be too long, particularly for focused HCUE that is limited to a few relatively simple assessments. It is important not to overshoot the requirements of HCUE training, because doing so may discourage groups of noncardiologists, like hospitalists, who may derive great benefits from HCUE.11
To address this uncertainty for hospitalists, we first developed a brief HCUE training program to assess 6 important cardiac abnormalities. We then studied the diagnostic accuracy of HCUE by hospitalists as a test of these 6 cardiac abnormalities assessed by SE.
Patients and Methods
Setting and Subjects
This prospective cohort study was performed at Stroger Hospital of Cook County, a 500‐bed public teaching hospital in Chicago, IL, from March through May of 2007. The cohort was adult inpatients who were referred for SE on weekdays from 3 distinct patient care units (Figure 1). We used 2 sampling modes to balance practical constraints (short‐stay unit [SSU] patients were more localized and, therefore, easier to study) with clinical diversity. We consecutively sampled patients from our SSU, where adults with provisional cardiovascular diagnoses are admitted if they might be eligible for discharge with in 3 days.12 But we used random number tables with a daily unique starting point to randomly sample patients from the general medical wards and the coronary care unit (CCU). Patients were excluded if repositioning them for HCUE was potentially harmful. The study was approved by our hospital's institutional review board, and we obtained written informed consent from all enrolled patients.

SE Protocol
As part of enrolled patients' routine clinical care, SE images were acquired and interpreted in the usual fashion in our hospital's echocardiography laboratory, which performs SE on over 7,000 patients per year. Echocardiographic technicians acquired images with a General Electric Vivid 7 cardiac ultrasound machine (General Electric, Milwaukee, WI) equipped with a GE M4S 1.8 to 3.4 MHz cardiac transducer (General Electric). Technicians followed the standard adult transthoracic echocardiography scanning protocol to acquire 40 to 100 images on every patient using all available echocardiographic modalities: 2‐dimensional, M‐mode, color Doppler, continuous‐wave Doppler, pulse‐wave Doppler, and tissue Doppler.13 Blinded to HCUE results, attending physician cardiologist echocardiographers then interpreted archived images using computer software (Centricity System; General Electric) to generate final reports that were entered into patients' medical records. This software ensured that final reports were standardized, because echocardiographers' final qualitative assessments were limited to short lists of standard options; for example, in reporting left atrium (LA) size, echocardiographers chose from only 5 standard options: normal, mildly dilated, moderately dilated, severely dilated, and not interpretable. Investigators, who were also blinded to HCUE results, later abstracted SE results from these standardized report forms in patients' medical records. All echocardiographers fulfilled ASE training guidelines to independently interpret SE: a minimum of 150 personally‐performed and 300 personally‐interpreted echocardiographic examinations (training level 2).14
HCUE Training
Based on the recommendations of our cardiologist investigator (B.M.), we developed a training program for 1 hospitalist to become an HCUE instructor. Our instructor trainee (C.C.) was board‐eligible in internal medicine but had no previous formal training in cardiology or echocardiography. We a priori established that her training would continue until our cardiologist investigator determined that she was ready to train other hospitalists; this determination occurred after 5 weeks. She learned image acquisition by performing focused SE on 30 patients under the direct supervision of an echocardiographic technician. She also performed focused HCUE on 65 inpatients without direct supervision but with ongoing access to consult the technician to review archived images and troubleshoot difficulties with acquisition. She learned image interpretation by reading relevant chapters from a SE textbook15 and by participating in daily didactic sessions in which attending cardiologist echocardiographers train cardiology fellows in SE interpretation.
This hospitalist then served as the HCUE instructor for 8 other attending physician hospitalists who were board‐certified internists with no previous formal training in cardiology or echocardiography. The training program was limited to acquisition and interpretation of 2‐dimensional grayscale and color Doppler images for the 6 cardiac assessments under study (Table 1). The instructor marshaled pairs of hospitalists through the 3 components of the training program, which lasted a total of 27 hours.
|
| Six cardiac assessments learned using 2‐dimensional gray scale and color Doppler imaging |
| Left ventricular systolic dysfunction |
| Mitral valve regurgitation |
| Left atrium enlargement |
| Left ventricular hypertrophy |
| Pericardial effusion |
| Inferior vena cava diameter |
| Lecture (2 hours)* |
| Basic principles of echocardiography |
| HCUE scanning protocol and helpful techniques to optimize image quality |
| Hands‐on training with instructor |
| Orientation to machine and demonstration of scanning protocol (1 hour) |
| Sessions 1 through 3: HCUE performed on 1 patient per hour (6 patients in 6 hours) |
| Sessions 4 through 10: HCUE performed on 2 patients per hour (28 patients in 14 hours) |
| Feedback sessions on image quality and interpretation with cardiologist |
| After hands‐on training session 3 (2 hours) |
| After hands‐on training session 10 (2 hours) |
First, hospitalists attended a 2‐hour lecture on the basic principles of HCUE. Slides from this lecture and additional images of normal and abnormal findings were provided to each hospitalist on a digital video disc. Second, each hospitalist underwent 20 hours of hands‐on training in 2‐hour sessions scheduled over 2 weeks. Willing inpatients from our hospital's emergency department were used as volunteers for these hand‐on training sessions. During these sessions the instructor provided practical suggestions to optimize image quality, such as transducer location and patient positioning. In the first 3 sessions, the minimum pace was 1 patient per hour; thereafter, the pace was increased to 1 patient per half‐hour. We chose 20 hours of hands‐on training and these minimum paces because they allowed each hospitalist to attain a cumulative experience of no less than 30 patientsan amount that heralds a flattening of the HCUE learning curve among medical trainees.9 Third, each pair of hospitalists received feedback from a cardiologist investigator (B.M.) who critiqued the quality and interpretation of images acquired by hospitalists during hands‐on training sessions. Since image quality varies by patient,16 hospitalists' images were compared side‐by‐side to images recorded by the instructor on the same patients. The cardiologist also critiqued hospitalists' interpretations of both their own images and additional sets of archived images from patients with abnormal findings.
HCUE Protocol
After completing the training program and blinded to the results of SE, the 8 hospitalists performed HCUE on enrolled patients within hours of SE. We limited the time interval between tests to minimize the effect that changes in physiologic variables, such as blood pressure and intravascular volume, have on the reliability of serial echocardiographic measurements.16 Hospitalists performed HCUE with a MicroMaxx 3.4 hand‐carried ultrasound machine equipped with a cardiology software package and a 1 to 5 MHz P17 cardiac transducer (Sonosite, Inc., Bothell, WA); simultaneous electrocardiographic recording, though available, was not used. While patients laid on their own standard hospital beds or on a standard hospital gurney in a room adjacent to the SE waiting room, hospitalists positioned them without assistance from nursing staff and recorded 7 best‐quality images per patient. Patients were first positioned in a partial (3045 degrees) left lateral decubitus position to record 4 grayscale images of the short‐axis and long‐axis parasternal and 2‐chamber and 4‐chamber apical views; 2 color Doppler images of the mitral inflow were also recorded from the long‐axis parasternal and the 4‐chamber apical views. Patients were then positioned supine to record 1 grayscale image of the inferior vena cava (IVC) from the transhepatic view. Hospitalists did not perform a history or physical exam on enrolled patients, nor did they review patients' medical records.
Immediately following the HCUE, hospitalists replayed the recorded images as often as needed and entered final interpretations on data collection forms. Linear measurements were made manually with a caliper held directly to the hand‐carried ultrasound monitor. These measurements were then translated into qualitative assessments based on standard values used by our hospital's echocardiographers (Table 2).17 When a hospitalist could not confidently assess a cardiac abnormality, the final HCUE assessment was recorded as indeterminate. Hospitalists also recorded the time to perform each HCUE, which included the time to record 7 best‐quality images, to interpret the findings, and to fill out the data collection form.
| Hand‐Carried Ultrasound Echocardiography Results | |||||
|---|---|---|---|---|---|
| Cardiac Abnormality by Standard Echocardiography | Hand‐Carried Ultrasound Echocardiography Operator's Method of Assessment | Positive | Negative | ||
| |||||
| Left ventricle systolic dysfunction, mild or greater | Grade degree of abnormal wall movement and thickening during systole | Severe | Mild or moderate | Normal | Vigorous |
| Mitral valve regurgitation, severe | Classify regurgitant jet as central or eccentric, then measure as percentage of left atrium area | ||||
| Central jet | 20% | <20% | |||
| Eccentric jet | 20% | indeterminate 20% | |||
| Left atrium enlargement, moderate or severe | Measure left atrium in 3 dimensions at end diastole, then use the most abnormal dimension | Extreme | Borderline | ||
| Anteroposterior or mediolateral (cm) | 5.1 | 4.55.0 | 4.4 | ||
| Superior‐inferior (cm) | 7.1 | 6.17.0 | 6.0 | ||
| Left ventricle hypertrophy, moderate or severe | Measure thickest dimension of posterior or septal wall at end diastole | Extreme: 1.4 cm | Borderline: 1.21.3 cm | 1.1 cm | |
| Pericardial effusion, medium or large | Measure largest dimension in any view at end diastole | 1 cm | <1 cm | ||
| Inferior vena cava dilatation | Measure largest respirophasic diameter within 2 cm of right atrium | 2.1 cm | Normal: 1 to 2 cm | Contracted: 0.9 cm | |
Data Analysis
We based our sample size calculations on earlier reports of HCUE by noncardiologist trainees for assessment of left ventricular (LV) systolic function.7, 10 From these reports, we estimated a negative likelihood ratio of 0.3. In addition, we expected about a quarter of our patients to have LV systolic dysfunction (B.M., personal communication). Therefore, to achieve 95% confidence intervals (CIs) around the point estimate of a negative likelihood ratio that excluded 0.50, our upper bound for a clinically meaningful result, we needed a sample size of approximately 300 patients.18
We defined threshold levels of ordinal severity for the 6 cardiac abnormalities under study based on their clinical pertinence to hospitalists (Table 2). Here, we reasoned that abnormalities at or above these levels would likely lead to important changes in hospitalists' management of inpatients; abnormalities below these levels rarely represent cardiac disease that is worthy of an immediate change in management. Since even mild degrees of LV dysfunction have important diagnostic and therapeutic implications for most general medicine inpatients, particularly those presenting with heart failure,19 we set our threshold for LV dysfunction at mild or greater. In contrast, since neither mild nor moderate mitral regurgitation (MR) has immediate implications for medical or surgical therapy even if symptoms or LV dysfunction are present,20 we set our threshold for MR at severe. Similarly, though mild LA enlargement21 and mild LV hypertrophy22 have clear prognostic implications for patients' chronic medical conditions, we reasoned that only moderate or severe versions likely reflect underlying abnormalities that affect hospitalists' point‐of‐care decision‐making. Since cardiac tamponade is rarely both subclinical23 and due to a small pericardial effusion,24 we set our threshold for pericardial effusion size at moderate or large. Finally, we set our threshold IVC diameter, a marker of central venous volume status,25 at dilated, because volume overload is an important consideration in hospitalized cardiac patients.
Using these thresholds, investigators dichotomized echocardiographers' SE readings as normal or abnormal for each of the 6 cardiac abnormalities under study to serve as the reference standards. Hospitalists' HCUE results were then compared to the reference standards in 2 different ways. We first analyzed HCUE results as dichotomous values to calculate conventional sensitivity, specificity, and positive and negative likelihood ratios. Here we considered indeterminate HCUE results positive in a clinically conservative tradeoff that neither ignores indeterminate results nor risks falsely classifying them as negative.26 We then analyzed hospitalists' HCUE results as ordinal values for receiver operating characteristic (ROC) curve analysis. Here we considered an indeterminate result as 1 possible test result.27
To examine interobserver variability of HCUE, we first chose from the 6 possible assessments only those with a mean number of abnormal patients per hospitalist greater than 5. We reasoned that variability among assessments with lower prevalence would be predictably wide and inconclusive. We then expressed variability as standard deviations (SDs) around mean sensitivity and specificity for the 8 hospitalists.
The CIs for likelihood ratios were constructed using the likelihood‐based approach to binomial proportions of Koopman.28 The areas under ROC curves were computed using the trapezoidal rule, and the CIs for these areas were constructed using the algorithm described by DeLong et al.29 All analyses were conducted with Stata Statistical Software, Release 10 (StataCorp, College Station, TX).
Results
During the 3 month study period, 654 patients were referred for SE from the 3 participating patient care units (Figure 1). Among these, 65 patients were ineligible because their SE was performed on the weekend and 178 other patients were not randomized from the general medical wards and CCU. From the remaining eligible patients, 322 underwent HCUE and 314 (98% of 322) underwent both SE and HCUE. Individual SE assessments were not interpretable (and therefore excluded) due to poor image quality for LA enlargement in 1 patient and IVC dilatation in 30 patients. Eighty‐three percent of patients who underwent SE (260/314) were referred to assess LV function (Table 3). The prevalence of the 6 clinically pertinent cardiac abnormalities under study ranged from 1% for moderate or large pericardial effusion to 25% for LV systolic dysfunction. Overall, 40% of patients had at least 1 out of 6 cardiac abnormalities.
| Characteristic | |
|---|---|
| |
| Age, year SD (25th to 75th percentiles) | 56 13 (48 to 64) |
| Women | 146 (47) |
| Chronic obstructive pulmonary disease | 47 (15) |
| Body mass index | |
| 24.9 or less: underweight or normal | 74 (24) |
| 25 to 29.9: overweight | 94 (30) |
| 30 to 34.9: mild obesity | 75 (24) |
| 35 or greater: moderate or severe obesity | 71 (23) |
| Patient care unit | |
| Short‐stay unit | 175 (56) |
| General medical wards | 89 (28) |
| Cardiac care unit | 50 (16) |
| Indication for standard echocardiography* | |
| Left ventricular function | 260 (83) |
| Valvular function | 56 (18) |
| Wall motion abnormality | 29 (9) |
| Valvular vegetations | 22 (7) |
| Any structural heart disease | 20 (6) |
| Right ventricular function | 18 (6) |
| Other | 38 (12) |
| Standard echocardiography findings | |
| Left ventricular systolic dysfunction mild | 80 (25) |
| Inferior vena cava dilated | 45 (14) |
| Left ventricular wall thickness moderate | 33 (11) |
| Left atrium enlargement moderate | 19 (6) |
| Mitral valve regurgitation severe | 11 (4) |
| Pericardial effusion moderate | 3 (1) |
| At least 1 of the above findings | 127 (40) |
| Time difference between HCUE and standard echocardiogram, median hours (25th to 75th percentiles) | 2.8 (1.4 to 5.1) |
| Time to complete HCUE, median minutes (25th to 75th percentiles) | 28 (20 to 35) |
Each hospitalist performed a similar total number of HCUE examinations (range, 3447). The median time difference between performance of SE and HCUE was 2.8 hours (25th75th percentiles, 1.45.1). Despite the high prevalence of chronic obstructive pulmonary disease and obesity, hospitalists considered HCUE assessments indeterminate in only 2% to 6% of the 6 assessments made for each patient (Table 4). Among the 38 patients (12% of 322) with any indeterminate HCUE assessment, 24 patients had only 1 out of 6 possible. Hospitalists completed HCUE in a median time of 28 minutes (25th‐75th percentiles, 2035), which included the time to record 7 best‐quality moving images and to fill out the research data collection form.
| n (%)* | |
|---|---|
| |
| Number of indeterminate findings per patient | |
| 0 | 284 (88) |
| 1 | 24 (7) |
| 2 | 4 (1) |
| 3 or more | 10 (3) |
| Indeterminate findings by cardiac assessment | |
| Mitral valve regurgitation | 18 (6) |
| Inferior vena cava diameter | 16 (5) |
| Left ventricular hypertrophy | 15 (5) |
| Pericardial effusion | 9 (3) |
| Left atrium size | 5 (2) |
| Left ventricle systolic function | 5 (2) |
When HCUE results were analyzed as dichotomous values, positive likelihood ratios ranged from 2.5 to 21, and negative likelihood ratios ranged from 0 to 0.4 (Table 5). Positive and negative likelihood ratios were both sufficiency high and low to respectively increase and decrease by 5‐fold the prior odds of 3 out of 6 cardiac abnormalities: LV systolic dysfunction, moderate or severe MR regurgitation, and moderate or large pericardial effusion. Considering HCUE results as ordinal values for ROC analysis yielded additional diagnostic information (Figure 2). For example, the likelihood ratio of 1.0 (95% CI, 0.42.0) for borderline positive moderate or severe LA enlargement increased to 29 (range, 1362) for extreme positive results. Areas under the ROC curves were 0.9 for 4 out of 6 cardiac abnormalities.

| Clinically Pertinent Cardiac Abnormality by Standard Echocardiography | Prevalence n/total n | Sensitivity* % (95% CI) | Specificity* % (95% CI) | LRpositive*, (95% CI) | LRnegative*, (95% CI) |
|---|---|---|---|---|---|
| |||||
| Left ventricular systolic dysfunction | 80/314 | 85 (7592) | 88 (8392) | 6.9 (4.99.8) | 0.2 (0.10.3) |
| Mitral valve regurgitation, severe | 11/314 | 100 (72100) | 83 (7987) | 5.9 (3.97.4) | 0 (00.3) |
| Left atrium enlargement, moderate or severe | 19/313 | 90 (6799) | 74 (6879) | 3.4 (2.54.3) | 0.1 (0.040.4) |
| Left ventricular hypertrophy, moderate or severe | 33/314 | 70 (5184) | 73 (6778) | 2.5 (1.83.3) | 0.4 (0.20.7) |
| Pericardial effusion, moderate or large | 3/314 | 100 (29100) | 95 (9297) | 21 (6.731) | 0 (00.6) |
| Inferior vena cava, dilated | 45/284 | 56 (4070) | 86 (8190) | 4.0 (2.66.0) | 0.5 (0.40.7) |
LV systolic dysfunction and IVC dilatation were both prevalent enough to meet our criterion to examine interobserver variability; the mean number of abnormal patients per hospitalist was 10 patients for LV systolic dysfunction and 6 patients for IVC dilatation. For LV systolic dysfunction, SDs around mean sensitivity (84%) and specificity (87%) were 12% and 6%, respectively. For IVC dilatation, SDs around mean sensitivity (58%) and specificity (86%) were 24% and 7%, respectively.
Discussion
We found that, after a 27‐hour training program, hospitalists performed HCUE with moderate to excellent diagnostic accuracy for 6 important cardiac abnormalities. For example, hospitalists' assessments of LV systolic function yielded positive and negative likelihood ratios of 6.9 (95% CI, 4.99.8) and 0.2 (95% CI, 0.10.3), respectively. At the bedsides of patients with acute heart failure, therefore, hospitalists could use HCUE to lower or raise the 50:50 chance of LV systolic dysfunction30 to 15% or 85%, respectively. Whether or not these posttest likelihoods are extreme enough to cross important thresholds will depend on the clinical context. Yet these findings demonstrate how HCUE has the potential to provide hospitalists with valuable point‐of‐care data that are otherwise unavailableeither because routine clinical assessments are unreliable31 or because echocardiographic services are not immediately accessible.1
In fact, recent data from the Joint Commission on Accreditation of Healthcare Organizations shows how inaccessible SE may be. Approximately one‐quarter of hospitals in the United States send home about 10% of patients with acute heart failure without echocardiographic assessment of LV systolic function before, during, or immediately after hospitalization.32 In doing so, these hospitals leave unmet the 2002 National Quality Improvement Goal of universal assessment of LV systolic function for all heart failure patients. Hospitalists could close this quality gap with routine, 10‐minute HCUE assessments in all patients admitted with acute heart failure. (Our research HCUE protocol required a median time of 28 minutes, but this included time to assess 5 other cardiac abnormalities and collect data for research purposes). Until the clinical consequences of introducing hospitalist‐performed HCUE are studied, potential benefits like this are tentative. But our findings suggest that training hospitalists to accurately perform HCUE can be successfully accomplished in just 27 hours.
Other studies of HCUE training programs for noncardiologists have also challenged the opinion that learning to perform HCUE requires more than 100 hours of training.2, 711 Yet only 1 prior study has examined an HCUE training program for hospitalists.5 In this study by Martin et al.,5 hospitalists completed 5 supervised HCUE examinations and 6 hours of interpretation training before investigators scored their image acquisition and interpretation skills from 30 unsupervised HCUE examinations. To estimate their final skill levels at the completion of all 35 examinations by accounting for an initially steep learning curve, investigators then adjusted these scores with regression models. Despite these upward adjustments, hospitalists' image acquisition and interpretation scores were low in comparison to echocardiographic technicians and cardiology fellows. Besides these adjusted measurements of hospitalists' skills, however, Martin et al.5 unfortunately did not also report standard measures of diagnostic accuracy, like those proposed by the Standards for Reporting of Diagnostic Accuracy (STARD) initiative.33 Therefore, direct comparisons to the present study are difficult. Nevertheless, their findings suggest that a training program limited to 5 supervised HCUE examinations may be inadequate for hospitalists. In fact, the same group's earlier study of medical trainees suggested a minimum of 30 supervised HCUE examinations.9 We chose to design our hospitalist training program based on this minimum, though they surprisingly did not.5 As others continue to refine the components of hospitalist HCUE training programs, such as the optimal number of supervised examinations, our program could serve as a reasonable comparative example: more rigorous than the program designed by Martin et al.5 but more feasible than ASE level 1 training.
The number and complexity of assessments taught in HCUE training programs will determine their duration. With ongoing advancements in HCUE technology, there is a growing list of potential assessments to choose from. Although HCUE training programs ought to include assessments with proven clinical applications, there are no trials of HCUE‐directed care to inform such decisions. In their absence, therefore, we chose 6 assessments based on the following 3 criteria. First, our assessments were otherwise not reliably available from routine clinical data, such as the physical examination. Second, our assessments were straightforward: easy to learn and simple to perform. Here, we based our reasoning on an expectation that the value of HCUE lies not in highly complex, state‐of‐the‐art assessmentswhich are best left to echocardiographers equipped with SEbut in simple, routine assessments made with highly portable machines that grant noncardiologists newfound access to point‐of‐care data.34 Third, our assessments were clinically pertinent and, where appropriate, defined by cut‐points at levels of severity that often lead to changes in management. We suspect that setting high cut‐points has the salutary effects of making assessments easier to learn and more accurate, because distinguishing mild abnormalities is likely the most challenging aspect of echocardiographic interpretation.35 Whether or not our choices of assessments, and their cut‐points, are optimal has yet to be determined by future research designed to study how they affect patient outcomes. Given our hospitalists' performance in the present study, these assessments seem worthy of such future research.
Our study had several limitations. We studied physicians and patients from only 1 hospital; similar studies performed in different settings, particularly among patients with different proportions and manifestations of disease, may find different results. Nevertheless, our sampling method of prospectively enrolling consecutive patients strengthens our findings. Some echocardiographic measurement methods used by our hospitalists differed in subtle ways from echocardiography guideline recommendations.35 We chose our methods (Table 2) for 2 reasons. First, whenever possible, we chose methods of interpretation that coincided with our local cardiologists'. Second, we chose simplicity over precision. For example, the biplane method of disks, or modified Simpson's rule, is the preferred volumetric method of calculating LA size.35 This method requires tracing the contours of the LA in 2 planes and then dividing the LA volume into stacked oval disks for calculation. We chose instead to train our hospitalists in a simpler method based on 2 linear measurements. Any loss of precision, however, was balanced by a large gain in simplicity. Regardless, minor variations in LA size are not likely to affect hospitalists' bedside evaluations. Finally, we did not validate the results of our reference standard (SE) by documenting interobserver reliability. Yet, because SE is generally accurate for the 6 cardiac abnormalities under study, the effect of this bias should be small.
These limitations can be addressed best by controlled trials of HCUE‐directed care. These trials will determine the clinical impact of hospitalist‐performed HCUE and, in turn, inform our design of HCUE training programs. As the current study shows, training hospitalists to participate in such trials is feasible: like other groups of noncardiologists, hospitalists can accurately perform HCUE after a brief training program. Whether or not hospitalists should perform HCUE requires further study.
Acknowledgements
The authors thank Sonosite, Inc., Bothell, WA, for loaning us 2 MicroMaxx machines throughout the study period. They also thank the staff of the Internal Medicine Research Mentoring Program at Rush Medical College for their technical support and the staff of the Division of Neurology at Stroger Hospital for granting them access to a procedure room.
- .The physical examination of the future: echocardiography as part of the assessment.ACC Curr J Rev.1998;7:79–81.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20:477–485.
- ,.Bedside ultrasonography in the ICU: Part 2.Chest.2005;128:1766–1781.
- ,.Practical Guide to Emergency Ultrasound.1st ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120:1000–1004.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the echocardiography task force on new technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc Echocardiogr.2002;15:369–373.
- ,,,,,.The use of small personal ultrasound devices with internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,, et al.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147:476–481.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118:1010–1018.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96:1002–1006.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20:471–476.
- ,,, et al.A hospitalist‐run short stay unit: features that predict patients' length‐of‐stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- .Adult echocardiography scanning protocol. In: Templin BB, ed.Ultrasound Scanning: Principles and Protocols.2nd ed.Philadelphia, PA:Saunders;1999:426.
- ,,, et al.ACCF 2008 Recommendations for training in adult cardiovascular medicine core cardiology training (COCATS 3) (revision of the 2002 COCATS training statement).J Am Coll Cardiol.2008;51:333–414.
- ,,.The Echo Manual.2nd ed.Philadelphia, PA:Lippincott Williams 1999.
- ,,, et al.Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications.J Am Soc Echocardiogr.1991;4:203–214.
- .Textbook of Clinical Echocardiography.3rd ed.Philadelphia, PA:Elsevier Saunders;2004.
- ,,.Likelihood ratios with confidence: sample size estimation for diagnostic test studies.J Clin Epidemiol.1991;44:763–770.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2005;112;154–235.
- ,,, et al.ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2006;114:e84–e231.
- ,,, et al.Left atrial size: physiologic determinants and clinical applications.J Am Coll Cardiol.2006;47:2357–2363.
- ,,,,.Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study.N Engl J Med.1990;322:1561–1566.
- ,,,.Does this patient with a pericardial effusion have cardiac tamponade?JAMA.2007;297:1810–1818.
- .Acute cardiac tamponade.N Engl J Med.2003;349:685–690.
- ,,,,,.Evaluation of size and dynamics of the inferior vena cava as an index of right‐sided cardiac function.Am J Cardiol.1984;53:579–585.
- ,,.The influence of uninterpretability on the assessment of diagnostic tests.J Chronic Dis.1986;39:575–584.
- ,,.Relations between effectiveness of a diagnostic test, prevalence of the disease, and percentages of uninterpretable results. An example in the diagnosis of jaundice.Med Decis Making.1982;2:285–297.
- .Confidence intervals for the ratio of two binomial proportions.Biometrics.1984;40:513–517.
- ,,.Comparing the areas under two or more correlated receiver operating curves: a nonparametric approach.Biometrics.1988;44:837–845.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296:2217–2226.
- ,,, et al.Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure.Am J Med.2002;112:437–445.
- Joint Commission on Accreditation of Healthcare Organizations. Health Care Quality Data Download Website. Available at: http://www.healthcarequalitydata.org. Accessed December2008.
- ,,, et al.Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative.Clin Chem.2003;49:1–6.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78:102–112.
- ,,, et al.Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology.J Am Soc Echocardiogr.2005;18:1440–1463.
- .The physical examination of the future: echocardiography as part of the assessment.ACC Curr J Rev.1998;7:79–81.
- ,,.The hand‐carried echocardiographic device as an aid to the physical examination.Echocardiography.2003;20:477–485.
- ,.Bedside ultrasonography in the ICU: Part 2.Chest.2005;128:1766–1781.
- ,.Practical Guide to Emergency Ultrasound.1st ed.Philadelphia, PA:Lippincott Williams 2006.
- ,,,,,.Hospitalist performance of cardiac hand‐carried ultrasound after focused training.Am J Med.2007;120:1000–1004.
- ,,, et al.Hand‐carried cardiac ultrasound (HCU) device: recommendations regarding new technology. A report from the echocardiography task force on new technology of the Nomenclature and Standards Committee of the American Society of Echocardiography.J Am Soc Echocardiogr.2002;15:369–373.
- ,,,,,.The use of small personal ultrasound devices with internists without formal training in echocardiography.Eur J Echocardiogr.2003;4:141–147.
- ,,, et al.Feasibility of point‐of‐care echocardiography by internal medicine house staff.Am Heart J.2004;147:476–481.
- ,,,,,.The rate at which residents learn to use hand‐held echocardiography at the bedside.Am J Med.2005;118:1010–1018.
- ,,, et al.Comparison of effectiveness of hand‐carried ultrasound to bedside cardiovascular physical examination.Am J Cardiol.2005;96:1002–1006.
- ,,.Can hand‐carried ultrasound devices be extended for use by the noncardiology medical community?Echocardiography.2003;20:471–476.
- ,,, et al.A hospitalist‐run short stay unit: features that predict patients' length‐of‐stay and eventual admission to traditional inpatient services.J Hosp Med.2009;4:276–284.
- .Adult echocardiography scanning protocol. In: Templin BB, ed.Ultrasound Scanning: Principles and Protocols.2nd ed.Philadelphia, PA:Saunders;1999:426.
- ,,, et al.ACCF 2008 Recommendations for training in adult cardiovascular medicine core cardiology training (COCATS 3) (revision of the 2002 COCATS training statement).J Am Coll Cardiol.2008;51:333–414.
- ,,.The Echo Manual.2nd ed.Philadelphia, PA:Lippincott Williams 1999.
- ,,, et al.Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications.J Am Soc Echocardiogr.1991;4:203–214.
- .Textbook of Clinical Echocardiography.3rd ed.Philadelphia, PA:Elsevier Saunders;2004.
- ,,.Likelihood ratios with confidence: sample size estimation for diagnostic test studies.J Clin Epidemiol.1991;44:763–770.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2005;112;154–235.
- ,,, et al.ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2006;114:e84–e231.
- ,,, et al.Left atrial size: physiologic determinants and clinical applications.J Am Coll Cardiol.2006;47:2357–2363.
- ,,,,.Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study.N Engl J Med.1990;322:1561–1566.
- ,,,.Does this patient with a pericardial effusion have cardiac tamponade?JAMA.2007;297:1810–1818.
- .Acute cardiac tamponade.N Engl J Med.2003;349:685–690.
- ,,,,,.Evaluation of size and dynamics of the inferior vena cava as an index of right‐sided cardiac function.Am J Cardiol.1984;53:579–585.
- ,,.The influence of uninterpretability on the assessment of diagnostic tests.J Chronic Dis.1986;39:575–584.
- ,,.Relations between effectiveness of a diagnostic test, prevalence of the disease, and percentages of uninterpretable results. An example in the diagnosis of jaundice.Med Decis Making.1982;2:285–297.
- .Confidence intervals for the ratio of two binomial proportions.Biometrics.1984;40:513–517.
- ,,.Comparing the areas under two or more correlated receiver operating curves: a nonparametric approach.Biometrics.1988;44:837–845.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296:2217–2226.
- ,,, et al.Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure.Am J Med.2002;112:437–445.
- Joint Commission on Accreditation of Healthcare Organizations. Health Care Quality Data Download Website. Available at: http://www.healthcarequalitydata.org. Accessed December2008.
- ,,, et al.Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative.Clin Chem.2003;49:1–6.
- ,,.Will disruptive innovations cure health care?Harv Bus Rev.2000;78:102–112.
- ,,, et al.Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology.J Am Soc Echocardiogr.2005;18:1440–1463.
Copyright © 2009 Society of Hospital Medicine
Hospitalist‐Run Short‐Stay Unit
Short‐stay units (SSUs) are common alternatives to traditional inpatient services.1 When defined broadly to include observation units for low‐risk chest pain patients, SSUs exist in one‐third of hospitals in the United States.2 Amidst growing demands for inpatient services, SSUs have recently developed beyond observation medicine to provide more complex inpatient services in locations commonly adjacent to emergency departments (EDs).1 Hospitalists are well‐positioned to staff these emerging SSUs because of their expertise in managing complex inpatient services.3
Despite this, we found only 3 reports of hospitalist‐run SSUs designed for general medical inpatients (2 from Spain and 1 from Canada).46 Whereas these early reports introduce hospitalist‐run SSUs, they provide limited data to make firm conclusions about their usefulness or appropriate design. For example, none of these reports assessed patients' characteristics upon admission. Nor did they provide details about the services that the SSUs provided. Yet evaluation of both types of patient‐level datadescriptions of patients' needs upon admission and how these needs are met during their staysdetermine whether or not hospitalist‐run SSUs meet their potential to efficiently care for backlogs of patients who otherwise await admission to traditional inpatient services.
In order to further explore these issues, we first sought to characterize our SSU patients upon admission and record what services they received during their stays. To help interpret our results, we then investigated associations between these characteristics and measures of successfully caring for patients in our SSU.
Patients and Methods
Design and Setting
In this prospective cohort study, we included all patients admitted to the hospitalist‐run SSU of Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, from January through April of 2006. Our 14‐bed SSU opened in 2002 to reduce overcrowding on the traditional inpatient wards by admitting adult patients who require inpatient care but might be eligible for discharge within 3 days. The unit is geographically part of the ED but is staffed by resident physicians and a rotating group of hospitalist attending physicians from the Department of Medicine. At least 1 attending and resident physician are available throughout the day, including weekend days and holidays; evenings are covered by a resident who presents overnight admissions to an attending physician the following morning.
ED physicians admit general medical patients to the SSU 24 hours per day, 7 days per week. Though admissions do not require prior approval from SSU physicians, the Departments of Medicine and Emergency Medicine have collaboratively promoted 5 suggested admission‐location guidelines to admitting ED physicians (Figure 1). For candidate SSU patients, these 5 guidelines are not intended to be restrictive but to provide a framework for the complex decision‐making process that our ED physicians encounter, particularly during periods of extreme overcrowding.7 First, patients should have an anticipated stay shorter than 72 hours. Second, patients should not have an eventual need for admission to traditional inpatient services such as the general medicine wards or intensive care units; this guideline is intended to improve patient safety by reducing unnecessary handoffs between physicians.8 Third, patients with provisional cardiovascular diagnoses should be preferentially admitted to the SSU over the general medical wards; this guideline is intended to improve hospital‐wide efficiency because our SSU is equipped with continuous telemetry monitors, an exercise treadmill testing (ETT) laboratory, and other reserved cardiac tests (see Admission Characteristics and Services Received section, below). Fourth, patients' risk should be no higher than intermediate level. Admitting ED physicians are encouraged to use posted risk estimators for patients with provisional diagnoses of possible acute coronary syndrome (ACS), decompensated heart failure, asthma exacerbation, and out‐of‐control diabetes. Finally, patients should not need advanced ancillary services; these include bedside procedures (eg, central venous catheter insertions), time‐intensive nursing (eg, regular dressing changes), and complex social‐services (eg, long‐term care facility placements).
Subjects
The study subjects were all patients admitted to the SSU during the 4‐month study period. Patients were excluded from the entire study if they refused verbal consent to participate. All patients who consented were included in the description of patient admission characteristics. Thirteen patients who prematurely left the SSU against medical advice, however, were neither included in the descriptions of services received nor in the analyses of predictors of successful SSU stays. We excluded these patients because they needed services that they did not receiveincluding these patients in our analysis would tend to overestimate the efficiency of our SSU by shortening the length‐of‐stay (LOS) without adding diagnostic tests or treatments.
Data Collection
After receiving approval from the institutional review board, attending physician investigators conducted an interview, physical examination, and review of medical records for each enrolled patient within 12 hours of admission to the SSU. When ED attending physicians' provisional primary diagnoses included possible ACS or decompensated heart failure, which we knew from earlier pilot data were our 2 most common provisional diagnoses, investigators gathered patient data to be applied in validated models of risk after the study period (Figure 2).9, 10 Some of the clinical predictors required for these models are based on patients' findings on presentation to the ED. For example, Goldman's risk model for major cardiac events uses patients' initial systolic blood pressures on presentation to the ED.9 In such cases, investigators gathered needed data from electronic and paper charts generated in the ED. Upon discharge from the SSU, investigators reviewed patients' medical records a second time. All data were entered by investigators and instantly committed into an online database.
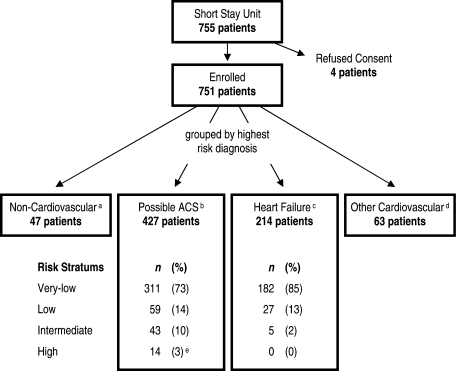
Admission Characteristics and Services Received
Patients were grouped according to the provisional diagnoses of ED attending physicians upon admission to the SSU (Figure 2). We chose to group patients by the provisional diagnoses of EDnot SSUattending physicians to better understand how ED physicians, the physicians who make the admission‐location decisions in our hospital, were using the SSU. Patients were first grouped as having possible ACS or heart failure, because patients with these provisional diagnoses were preferentially admitted to the SSU (Figure 1). When neither diagnosis was listed, patients were grouped according to ED attending physicians' first‐listed diagnoses. At the end of the study period, relevant risk models were applied to patients with possible ACS or heart failure and stratified as very low, low, intermediate, or high risk.9, 10 Patients with both possible ACS and heart failure were grouped according to the diagnosis with the highest corresponding risk assessment; if both risk assessments were the same, then the first‐listed diagnosis was used. Though developed to predict different clinical outcomes during different time periods, risk strata from the corresponding risk models were pooled across both diagnoses to develop a risk summary.
Upon discharge, investigators recorded which advanced diagnostic tests, specialty consultations, and acute care treatments patients received while in the SSU. Diagnostic tests were considered advanced if they were not routinely performed within 2 hours of being ordered. Advanced diagnostic tests were grouped into 2 types by their accessibility to ordering SSU physicians. Open access tests included echocardiograms and ETTs, which were reserved for SSU patients 6 days per week. Though the availability of open access tests was not unlimited, ordering physicians' needs for them rarely exceeded the immediate supply. On the other hand, limited access tests included both cardiac stress imaging studies, which were reserved for SSU patients on a very limited basis 4 or 5 days per week, and other tests that were not reserved for SSU patients, such as endoscopy, magnetic resonance imaging, or ultrasonography. Ordering physicians' needs for limited access tests often exceeded their immediate supply; in such cases, SSU patients were placed without priority into queues that included patients from the entire hospital.
Investigators recorded when patients received advanced diagnostic tests that were ordered by specialists. These tests, however, were not included in analyses of how services received by SSU patients affected SSU success, because SSU attending physicians were only indirectly involved in whether or not patients received these tests. Treatments were considered acute care treatments if they were commonly administered only in acute care settings, such as heparin for unstable angina or intravenous furosemide for pulmonary edema.
SSU Success
The SSU was designed to care for patients during brief stays and without eventual admission to traditional inpatient services. Therefore, we used patients' LOS and whether or not patients were admitted to traditional inpatient services as measures of SSU success. LOS was calculated from the time patients arrived in the SSU until the time they left. Therefore, neither time spent in the ED before admission to the SSU nor time spent on traditional inpatient services (if needed) contributed to our definition of LOS. Individual SSU patients were considered successfully cared for in the SSU if their LOS was less than 72 hours and they were discharged directly home from the SSU. We explored associations between these outcomes and provisional diagnoses, risk assessments, and services received.
Data Analysis
LOS data were right‐skewed; therefore, we used the Mann‐Whitney test for comparisons between 2 groups and the Kruskal‐Wallis test for comparisons among 3 or more groups. To test for trends of median LOS among ordered groupings, we used the method of Cuzick.11 We used Pearson's chi‐square test to compare proportions of patients grouped into categories and the chi‐square test for trends with equal scoring to test for trends among ordered groupings.
We performed multiple logistic regression to explore which variables were associated with SSU success. The following 5 demographic variables from Table 1 were insignificant in all single‐variable and multiple‐variable regression models that we tested and were, therefore, removed from further analyses to create more parsimonious models: gender, language, ethnicity, race, and whether or not patients had a primary care provider. Our multiple logistic regression models were fitted by maximum likelihood methods. In all of these models, odds ratios (ORs) were adjusted for patient characteristics that included age (in years), insulin‐requiring diabetes mellitus (yes or no), SSU attending physician, day of the week of SSU admission (weekday or weekend), and hospitalization during the preceding year. Confidence intervals (CIs) for predicted probabilities were computed using the delta method. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, College Station, TX).
| |
| Mean age, years (SD) (25th‐75th percentiles) | 58 (12) (49‐66) |
| Men | 389 (52) |
| Lacking a primary care provider | 256 (34) |
| Non‐English speaking | 217 (29) |
| Ethnicity is Hispanic or Latino | 105 (14) |
| Race is Black or African‐American | 480 (64) |
| Hospitalized within the preceding year | 322 (43) |
| Insulin‐requiring diabetes mellitus | 83 (11) |
| Previous coronary artery revascularization | 89 (12) |
| Provisional diagnosis* | |
| Possible acute coronary syndrome | 427 (57) |
| Heart failure | 214 (29) |
| Other cardiovascular | 62 (8) |
| Noncardiovascular | 48 (6) |
Results
Subjects
During the 4‐month study period, 755 patients were admitted to the SSU. Among these patients, 4 were excluded from our study because they refused verbal consent. In the remaining study sample of 751 patients, all were included in the descriptions of patients' admission characteristics (Table 1), but 13 patients who left prematurely were excluded in both the descriptions of services received (Table 2) and the analyses of SSU success (Tables 3 and 4).
| Service received | Possible ACS n = 418 (%) | Heart Failure n = 211 (%) | Other Cardiovascular n = 61 (%) | Noncardiovascular n = 48 (%) | Total n = 738 (%) |
|---|---|---|---|---|---|
| |||||
| Open access test* | 37 | 59 | 56 | 13 | 43 |
| Resting | |||||
| Echocardiography | 29 | 59 | 56 | 13 | 39 |
| ETT | 12 | 0 | 5 | 0 | 7 |
| Limited access test | 24 | 8 | 10 | 8 | 17 |
| Stress imaging | 21 | 5 | 7 | 2 | 14 |
| Acute care treatment | 22 | 78 | 5 | 60 | 39 |
| Specialty consultation∥ | 24 | 12 | 20 | 8 | 19 |
| Any above service | 68 | 93 | 67 | 69 | 75 |
| Provisional Diagnosis and Services Received | n | Median LOS [hours (IQR)] | Stay Longer than 72 Hours (%) | Admission to Traditional Inpatient Service (%) | Stay Longer than 72 Hours or Admission to Traditional Inpatient Service (%) |
|---|---|---|---|---|---|
| |||||
| All patients | 738 | 42 (22‐63) | 15 | 9* | 21 |
| Possible ACS | 418 | 37 (20‐57) | 13 | 10 | 20 |
| Heart failure | 211 | 47 (34‐69) | 21 | 9 | 27 |
| Other CV | 61 | 40 (21‐49) | 10 | 5 | 11 |
| Non‐CV | 48 | 40 (22‐60) | 10 | 2 | 13 |
| P value | <0.001 | 0.04 | 0.18 | 0.01 | |
| Open access test | |||||
| Yes | 320 | 46 (31‐67) | 17 | 9 | 23 |
| No | 418 | 33 (20‐52) | 13 | 9 | 20 |
| P value | <0.001 | 0.15 | 0.87 | 0.43 | |
| Limited access test | |||||
| Yes | 128 | 51 (42‐82) | 31 | 6 | 33 |
| No | 610 | 38 (21‐55) | 12 | 10 | 19 |
| P value | <0.001 | <0.001 | 0.24 | <0.001 | |
| Acute care treatment | |||||
| Yes | 287 | 46 (34‐69) | 21 | 12 | 29 |
| No | 451 | 32 (20‐50) | 11 | 7 | 16 |
| P value | <0.001 | <0.001 | 0.01 | <0.001 | |
| Specialty consultation | |||||
| Yes | 141 | 63 (40‐92) | 38 | 28 | 52 |
| No | 597 | 38 (21‐50) | 10 | 5 | 14 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Any above service | |||||
| Yes | 554 | 46 (29‐68) | 19 | 10 | 26 |
| No | 184∥ | 22 (16‐32) | 2 | 5 | 7 |
| P value | <0.001 | <0.001 | 0.03 | <0.001 | |
| Stay Longer than 72 Hours | Admission to Traditional Inpatient Service | Either Outcome | ||||
|---|---|---|---|---|---|---|
| OR* | P value | OR* | P value | OR* | P value | |
| ||||||
| Heart failure | 2.3 | 0.01 | 1.1 | 0.77 | 1.9 | 0.02 |
| Service received | ||||||
| Open access test | 1.5 | 0.10 | 1.0 | 0.89 | 1.2 | 0.32 |
| Limited access test | 5.1 | <0.001 | 0.4 | 0.03 | 2.5 | <0.001 |
| Acute care treatment | 1.7 | 0.07 | 1.4 | 0.31 | 1.6 | 0.05 |
| Specialty consultation | 6.1 | <0.001 | 13.1 | <0.001 | 8.1 | <0.001 |
Admission Characteristics and Services Received
A narrow range of provisional diagnoses were listed by ED attending physicians and 641 patients (85% of 751) were grouped as having possible ACS or heart failure (Figure 2). Patients with these diagnoses were later risk‐stratified and, when pooled across risk strata, only 14 patients (2% of 641) exceeded the suggested admission‐location criterion for the SSU of lower than high risk. Despite the array and frequency of diagnostic and treatment services that patients received, SSU physicians worked mostly independently, requesting specialty consultations for only 19% of patients (141/738; Table 2).
SSU Success
The median LOS for all patients was 42 hours (interquartile range [IRQ] 22‐63) and 156 patients (21% of 738) had unsuccessful SSU stays (Table 3). The most common reason for an unsuccessful stay was a stay longer than 72 hours (71% of 156). Among the 66 patients who required admission to traditional inpatient services, nearly one‐half (48%) were admitted expressly to receive treatments not available in the SSU after having a specialty consult.
Patients' provisional diagnoses were associated with unsuccessful stays in bivariate analyses (Table 3). In addition, when patients were grouped into 3 risk stratums (very‐low, low, and intermediate‐and‐high), unsuccessful stays increased with increasing risk. For example, in patients with possible ACS, the proportion of unsuccessful stays increased from 17% of 306 very‐low risk patients to 27% of 55 intermediate‐and‐high risk patients (P value for trend = 0.012. Similarly, in patients with heart failure, the proportion of unsuccessful stays increased from 25% of 181 very‐low risk patients to 100% of 3 intermediate‐and‐high risk patients (P value for trend = 0.004).
However, in multiple variable models that simultaneously included patients' characteristics upon admission with services received during their SSU stay, only the provisional diagnosis of heart failure was associated with unsuccessful stays (OR, 1.9; 95% CI, 1.12‐3.18); risk assessments for possible ACS (P = 0.29) and heart failure (P = 0.32) were unimportant predictors of unsuccessful stays (Table 4). On the other hand, whether or not patients received diagnostic tests, acute care treatments, or specialty consultations were important predictors. In particular, patients who received specialty consultations were much more likely to require admission to traditional inpatient services than those patients who did not (OR, 13.1; 95% CI, 6.9‐24.9) and had a 52% chance of having an unsuccessful stay (95% CI, 42‐61%; Figure 3). In addition, the accessibility of a diagnostic test was inversely proportional to the chance of having a long stay; patients who received an open access test had a 12% chance of a long stay (95% CI, 8‐16%) whereas those who received a limited access test had a 29% chance of a long stay (95% CI, 20‐39%). Receiving acute care treatments was also a significant, though less important, predictor of an unsuccessful stay (Table 4).
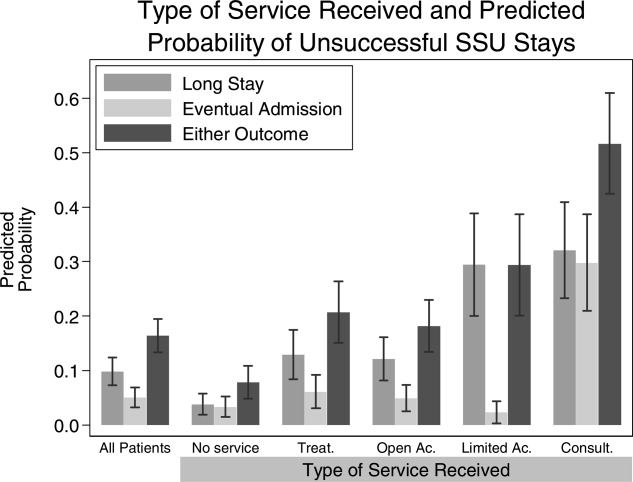
Discussion
We found that the types of services received by patients during their SSU stays were stronger predictors of long stays and eventual admissions to traditional inpatient services than patients' characteristics upon admission to the SSU. This suggests that SSUs should be focused toward matching patients' anticipated needs with readily accessible services. For example, in our SSU, which cares for over 2,250 patients annually, more than 1,200 patients will receive diagnostic tests in a given year. Among these patients, those who receive a limited access test will be more than twice as likely to have long stays than those who receive an open access test (Figure 3). Though our conclusions may not be applicable to other settings, this study is the most comprehensive description of patients admitted to a hospitalist‐run SSU. In addition, our study is the first to demonstrate that diagnostic and consultative services are the most important predictors of successful stays in SSUs. This promotes the practical strategy that hospitalists who staff SSUs should focus administratively toward gaining access to these services.
Very few of our SSU patients did not fulfill the suggested requirements of our admission location guidelines. For example, only 2% of 691 patients with either possible ACS or heart failure were high risk (Figure 2). Despite this, 21% of our patients had stays longer than 72 hours or were admitted to traditional inpatient services. The paradoxically high proportion of unsuccessful stays among mostly very‐low and low risk patients simply reflects how the clinical risk models that we used were not designed to predict unsuccessful stays. Moreover, as our multiple variable models suggest, improvements in the selection process of candidate SSU patients are more likely to come from an ability to incorporate assessments of what services patients will receive rather than from assessments of their clinical risk (Table 4). Therefore, the immediate plans of the accepting SSU physicians, the physicians who will determine what services patients eventually receive, should be incorporated in the admission‐location decision process.
Three of our findings highlight how input from accepting SSU physiciansconveyed to ED physicians before their final admission‐location decisions are mademay improve the SSU patient selection process. First, 23% of our patients were discharged home after brief stays with no advanced tests, specialty consultations, or acute care treatments (Table 3). Though some of these patients may have required inpatient services other than the ones we recorded, most were admitted with very‐low risk possible ACS; if they required overnight stays at all, many of them may have been better cared for in the ED observation unit (Figure 1). Second, 74% of the patients who required admission to traditional inpatient services were admitted for services not readily available to patients in the SSU (Table 3). Among these patients, nearly one‐third (21/66) received no advanced diagnostic tests in the SSU. This suggests that these patients should have been admitted directly to the general medical wards; doing so may have improved efficiency and quality of care by reducing unnecessary handoffs between physicians. Both types of patientsthose with minimal inpatient needs and those with more needs than the SSU can providehighlight how incorporating accepting SSU physicians' plans may improve the SSU patient selection process. After all, those best equipped to determine if the SSU will meet (or exceed) the needs of candidate patients are the SSU physicians themselves.
Third, we found that whether or not SSU physicians required assistance from specialists was the strongest predictor of unsuccessful stays: when an accepting physician determined that a patient should receive a specialty consultation, that patient's chance of having an unsuccessful stay was over 50% (Figure 3). Our study was not designed to determine how specialty consultations were associated with unsuccessful stays. We did not, for example, record whether or not hospitalists changed their diagnostic, treatment, or admission plans because of specialists' recommendations.12 Therefore, we cannot conclude that specialty consultations actually caused long stays or traditional admissions. Nevertheless, when our SSU physicians did not manage patients independent of specialty consultations, we observed a high likelihood of unsuccessful stays. Because accepting SSU physicians are the ones who will determine whether or not they need assistance from specialists, weighing their immediate plans for specialty consultations into the admission‐location decision process may improve the efficiency of SSUs. Others have recognized the importance of specialty consultations in SSUs by directly incorporating specialists as coattending physicians.13
Our study had several limitations. First, we studied mostly patients with cardiovascular diagnoses. Predictors of success in SSUs that admit patients with different diagnostic profiles may be different. In particular, SSUs that admit patients with a wide array of diagnoses may find that matching patients' needs with readily accessible services is impractical, because these needs may be too wide‐ranging. Second, our study design was observational. However, other than seasonal variations in admission patterns, there was little room for selection bias because we enrolled all consecutive admissions over the 4‐month study period, which gives us more confidence in our results. Third, our study did not record whether or not ED physicians knowingly overrode the suggested admission‐location guidelines because of limited bed availability. Yet, if shortages of beds on traditional inpatient services were driving patients who were otherwise candidates for the general medical wards in to the SSU, then we would have expected higher‐risk patients and greater needs for limited access tests. Finally, our descriptions of patients' needs were based on what diagnostic, consultative, and treatment services patients actually received; yet these needs did not include diagnostic tests that were ordered but never performed. However, any missed needs would bias our results toward no association with unsuccessful stays, because unsuccessful stays would generally increase while patients await needed services.
Future research could address these limitations through an experimental trial of traditional admissions versus admission to a hospitalist‐run SSU. And, because hospitals are complex systems of health care delivery where changes in one patient care unit often affect others in unanticipated ways,14 the impact of SSUs on other patient care units that are closely connected to SSUs, such as EDs and the general medical wards (Figure 1), should be simultaneously observed. For example, though our findings suggest that the accessibility of diagnostic tests should parallel ordering SSU physicians' needs for those tests, making all diagnostic tests open to SSU physicians may result in shortsightedly lengthening the stays of patients in other care units. Future research should also observe the decision‐making process of both the physicians who make admission‐location decisions (ED physicians) and those who determine the eventual plans for patients in the SSU (hospitalists). Accepting physicians from other patient care units have found improved outcomes of efficiency when they were involved in the complex process of deciding where to admit patients.15, 16 After an initial evaluation of a candidate SSU patient in the ED, a hospitalist who staffs both the general medical wards and the SSU would be uniquely well‐positioned to help an ED physician decide where a patient's needs would be best met. Although ED physicians will rightly be concerned that consulting SSU hospitalists may slow patient flow, hands‐on consultations of candidate SSU patients, who have a narrow range of diagnoses and low‐risk profiles, would likely be brief. In addition, because many SSUs are conveniently adjacent to EDs, the burden of communication may be minor.1 To address these questions, hospitalists who staff SSUs must continue the observed trend of working collaboratively with ED physicians.15, 17, 18
Acknowledgements
The authors thank Arthur T. Evans and Brendan M. Reilly for their insightful review of the manuscript. The authors also thank Zhaotai Cui for his assistance with statistical programming.
- ,,.A paradigm shift in the nature of care provision in emergency departments.Emerg Med J.2004;21:681–684.
- ,,.Acute coronary syndromes. In: Marx J, Hockberger R, Walls R, eds.Rosen's emergency medicine. Concepts and clinical practice.6th ed.Edinburgh:Mosby Elsevier;2006:1154–1199.
- .Taking charge of observation units for better patient flow.Todays Hospitalist Mag.2007;5(7):16–20.
- ,,, et al.Unidad de corta estancia dependiente de Medicina Interna.An Med Interna.1999;16:504–510.
- ,,,,.Factors that predict unplanned hospital readmission of patients discharged from a short stay medical unit.An Med Interna.2002;19:221–225.
- ,,, et al.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.CMAJ.2000;163(11):1477–1480.
- ,,,.Triage of patients with chest pain in the emergency department: a comparative study of physicians' decisions.Am J Med.2002;112:95–103.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,, et al.Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain.N Engl J Med.1996;334:1498–1504.
- ,,, et al.Risk stratification for in‐hospital mortality in acutely decompensated heart failure.JAMA.2005;293:572–580.
- .A Wilcoxon‐type test for trend.Stat Med.1985;4:87–90.
- ,,,,,.Diagnostic test restraint and the specialty consultation.J Gen Intern Med.1990;5:95–103.
- ,,, et al.A cooperative care model: cardiologists and hospitalists reduce length of stay in a chest pain observation unit.Crit Pathw Cardiol.2005;4:55–58.
- ,.Average length of stay, delayed discharge, and hospital congestion.BMJ.2002;325:610–611.
- .An internist in the emergency department: the IM facilitator program.HMO Pract.1991;10:42–43.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- .Hospitalists' new role in the ED: “clog busters.”Todays Hospitalist Mag.2005;3(8):15–18.
- .Kindred spirits: ED doctors, hospitalists forge a critical collaboration.Hospitalist.2007;11(7):1,16–20.
Short‐stay units (SSUs) are common alternatives to traditional inpatient services.1 When defined broadly to include observation units for low‐risk chest pain patients, SSUs exist in one‐third of hospitals in the United States.2 Amidst growing demands for inpatient services, SSUs have recently developed beyond observation medicine to provide more complex inpatient services in locations commonly adjacent to emergency departments (EDs).1 Hospitalists are well‐positioned to staff these emerging SSUs because of their expertise in managing complex inpatient services.3
Despite this, we found only 3 reports of hospitalist‐run SSUs designed for general medical inpatients (2 from Spain and 1 from Canada).46 Whereas these early reports introduce hospitalist‐run SSUs, they provide limited data to make firm conclusions about their usefulness or appropriate design. For example, none of these reports assessed patients' characteristics upon admission. Nor did they provide details about the services that the SSUs provided. Yet evaluation of both types of patient‐level datadescriptions of patients' needs upon admission and how these needs are met during their staysdetermine whether or not hospitalist‐run SSUs meet their potential to efficiently care for backlogs of patients who otherwise await admission to traditional inpatient services.
In order to further explore these issues, we first sought to characterize our SSU patients upon admission and record what services they received during their stays. To help interpret our results, we then investigated associations between these characteristics and measures of successfully caring for patients in our SSU.
Patients and Methods
Design and Setting
In this prospective cohort study, we included all patients admitted to the hospitalist‐run SSU of Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, from January through April of 2006. Our 14‐bed SSU opened in 2002 to reduce overcrowding on the traditional inpatient wards by admitting adult patients who require inpatient care but might be eligible for discharge within 3 days. The unit is geographically part of the ED but is staffed by resident physicians and a rotating group of hospitalist attending physicians from the Department of Medicine. At least 1 attending and resident physician are available throughout the day, including weekend days and holidays; evenings are covered by a resident who presents overnight admissions to an attending physician the following morning.
ED physicians admit general medical patients to the SSU 24 hours per day, 7 days per week. Though admissions do not require prior approval from SSU physicians, the Departments of Medicine and Emergency Medicine have collaboratively promoted 5 suggested admission‐location guidelines to admitting ED physicians (Figure 1). For candidate SSU patients, these 5 guidelines are not intended to be restrictive but to provide a framework for the complex decision‐making process that our ED physicians encounter, particularly during periods of extreme overcrowding.7 First, patients should have an anticipated stay shorter than 72 hours. Second, patients should not have an eventual need for admission to traditional inpatient services such as the general medicine wards or intensive care units; this guideline is intended to improve patient safety by reducing unnecessary handoffs between physicians.8 Third, patients with provisional cardiovascular diagnoses should be preferentially admitted to the SSU over the general medical wards; this guideline is intended to improve hospital‐wide efficiency because our SSU is equipped with continuous telemetry monitors, an exercise treadmill testing (ETT) laboratory, and other reserved cardiac tests (see Admission Characteristics and Services Received section, below). Fourth, patients' risk should be no higher than intermediate level. Admitting ED physicians are encouraged to use posted risk estimators for patients with provisional diagnoses of possible acute coronary syndrome (ACS), decompensated heart failure, asthma exacerbation, and out‐of‐control diabetes. Finally, patients should not need advanced ancillary services; these include bedside procedures (eg, central venous catheter insertions), time‐intensive nursing (eg, regular dressing changes), and complex social‐services (eg, long‐term care facility placements).

Subjects
The study subjects were all patients admitted to the SSU during the 4‐month study period. Patients were excluded from the entire study if they refused verbal consent to participate. All patients who consented were included in the description of patient admission characteristics. Thirteen patients who prematurely left the SSU against medical advice, however, were neither included in the descriptions of services received nor in the analyses of predictors of successful SSU stays. We excluded these patients because they needed services that they did not receiveincluding these patients in our analysis would tend to overestimate the efficiency of our SSU by shortening the length‐of‐stay (LOS) without adding diagnostic tests or treatments.
Data Collection
After receiving approval from the institutional review board, attending physician investigators conducted an interview, physical examination, and review of medical records for each enrolled patient within 12 hours of admission to the SSU. When ED attending physicians' provisional primary diagnoses included possible ACS or decompensated heart failure, which we knew from earlier pilot data were our 2 most common provisional diagnoses, investigators gathered patient data to be applied in validated models of risk after the study period (Figure 2).9, 10 Some of the clinical predictors required for these models are based on patients' findings on presentation to the ED. For example, Goldman's risk model for major cardiac events uses patients' initial systolic blood pressures on presentation to the ED.9 In such cases, investigators gathered needed data from electronic and paper charts generated in the ED. Upon discharge from the SSU, investigators reviewed patients' medical records a second time. All data were entered by investigators and instantly committed into an online database.

Admission Characteristics and Services Received
Patients were grouped according to the provisional diagnoses of ED attending physicians upon admission to the SSU (Figure 2). We chose to group patients by the provisional diagnoses of EDnot SSUattending physicians to better understand how ED physicians, the physicians who make the admission‐location decisions in our hospital, were using the SSU. Patients were first grouped as having possible ACS or heart failure, because patients with these provisional diagnoses were preferentially admitted to the SSU (Figure 1). When neither diagnosis was listed, patients were grouped according to ED attending physicians' first‐listed diagnoses. At the end of the study period, relevant risk models were applied to patients with possible ACS or heart failure and stratified as very low, low, intermediate, or high risk.9, 10 Patients with both possible ACS and heart failure were grouped according to the diagnosis with the highest corresponding risk assessment; if both risk assessments were the same, then the first‐listed diagnosis was used. Though developed to predict different clinical outcomes during different time periods, risk strata from the corresponding risk models were pooled across both diagnoses to develop a risk summary.
Upon discharge, investigators recorded which advanced diagnostic tests, specialty consultations, and acute care treatments patients received while in the SSU. Diagnostic tests were considered advanced if they were not routinely performed within 2 hours of being ordered. Advanced diagnostic tests were grouped into 2 types by their accessibility to ordering SSU physicians. Open access tests included echocardiograms and ETTs, which were reserved for SSU patients 6 days per week. Though the availability of open access tests was not unlimited, ordering physicians' needs for them rarely exceeded the immediate supply. On the other hand, limited access tests included both cardiac stress imaging studies, which were reserved for SSU patients on a very limited basis 4 or 5 days per week, and other tests that were not reserved for SSU patients, such as endoscopy, magnetic resonance imaging, or ultrasonography. Ordering physicians' needs for limited access tests often exceeded their immediate supply; in such cases, SSU patients were placed without priority into queues that included patients from the entire hospital.
Investigators recorded when patients received advanced diagnostic tests that were ordered by specialists. These tests, however, were not included in analyses of how services received by SSU patients affected SSU success, because SSU attending physicians were only indirectly involved in whether or not patients received these tests. Treatments were considered acute care treatments if they were commonly administered only in acute care settings, such as heparin for unstable angina or intravenous furosemide for pulmonary edema.
SSU Success
The SSU was designed to care for patients during brief stays and without eventual admission to traditional inpatient services. Therefore, we used patients' LOS and whether or not patients were admitted to traditional inpatient services as measures of SSU success. LOS was calculated from the time patients arrived in the SSU until the time they left. Therefore, neither time spent in the ED before admission to the SSU nor time spent on traditional inpatient services (if needed) contributed to our definition of LOS. Individual SSU patients were considered successfully cared for in the SSU if their LOS was less than 72 hours and they were discharged directly home from the SSU. We explored associations between these outcomes and provisional diagnoses, risk assessments, and services received.
Data Analysis
LOS data were right‐skewed; therefore, we used the Mann‐Whitney test for comparisons between 2 groups and the Kruskal‐Wallis test for comparisons among 3 or more groups. To test for trends of median LOS among ordered groupings, we used the method of Cuzick.11 We used Pearson's chi‐square test to compare proportions of patients grouped into categories and the chi‐square test for trends with equal scoring to test for trends among ordered groupings.
We performed multiple logistic regression to explore which variables were associated with SSU success. The following 5 demographic variables from Table 1 were insignificant in all single‐variable and multiple‐variable regression models that we tested and were, therefore, removed from further analyses to create more parsimonious models: gender, language, ethnicity, race, and whether or not patients had a primary care provider. Our multiple logistic regression models were fitted by maximum likelihood methods. In all of these models, odds ratios (ORs) were adjusted for patient characteristics that included age (in years), insulin‐requiring diabetes mellitus (yes or no), SSU attending physician, day of the week of SSU admission (weekday or weekend), and hospitalization during the preceding year. Confidence intervals (CIs) for predicted probabilities were computed using the delta method. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, College Station, TX).
| |
| Mean age, years (SD) (25th‐75th percentiles) | 58 (12) (49‐66) |
| Men | 389 (52) |
| Lacking a primary care provider | 256 (34) |
| Non‐English speaking | 217 (29) |
| Ethnicity is Hispanic or Latino | 105 (14) |
| Race is Black or African‐American | 480 (64) |
| Hospitalized within the preceding year | 322 (43) |
| Insulin‐requiring diabetes mellitus | 83 (11) |
| Previous coronary artery revascularization | 89 (12) |
| Provisional diagnosis* | |
| Possible acute coronary syndrome | 427 (57) |
| Heart failure | 214 (29) |
| Other cardiovascular | 62 (8) |
| Noncardiovascular | 48 (6) |
Results
Subjects
During the 4‐month study period, 755 patients were admitted to the SSU. Among these patients, 4 were excluded from our study because they refused verbal consent. In the remaining study sample of 751 patients, all were included in the descriptions of patients' admission characteristics (Table 1), but 13 patients who left prematurely were excluded in both the descriptions of services received (Table 2) and the analyses of SSU success (Tables 3 and 4).
| Service received | Possible ACS n = 418 (%) | Heart Failure n = 211 (%) | Other Cardiovascular n = 61 (%) | Noncardiovascular n = 48 (%) | Total n = 738 (%) |
|---|---|---|---|---|---|
| |||||
| Open access test* | 37 | 59 | 56 | 13 | 43 |
| Resting | |||||
| Echocardiography | 29 | 59 | 56 | 13 | 39 |
| ETT | 12 | 0 | 5 | 0 | 7 |
| Limited access test | 24 | 8 | 10 | 8 | 17 |
| Stress imaging | 21 | 5 | 7 | 2 | 14 |
| Acute care treatment | 22 | 78 | 5 | 60 | 39 |
| Specialty consultation∥ | 24 | 12 | 20 | 8 | 19 |
| Any above service | 68 | 93 | 67 | 69 | 75 |
| Provisional Diagnosis and Services Received | n | Median LOS [hours (IQR)] | Stay Longer than 72 Hours (%) | Admission to Traditional Inpatient Service (%) | Stay Longer than 72 Hours or Admission to Traditional Inpatient Service (%) |
|---|---|---|---|---|---|
| |||||
| All patients | 738 | 42 (22‐63) | 15 | 9* | 21 |
| Possible ACS | 418 | 37 (20‐57) | 13 | 10 | 20 |
| Heart failure | 211 | 47 (34‐69) | 21 | 9 | 27 |
| Other CV | 61 | 40 (21‐49) | 10 | 5 | 11 |
| Non‐CV | 48 | 40 (22‐60) | 10 | 2 | 13 |
| P value | <0.001 | 0.04 | 0.18 | 0.01 | |
| Open access test | |||||
| Yes | 320 | 46 (31‐67) | 17 | 9 | 23 |
| No | 418 | 33 (20‐52) | 13 | 9 | 20 |
| P value | <0.001 | 0.15 | 0.87 | 0.43 | |
| Limited access test | |||||
| Yes | 128 | 51 (42‐82) | 31 | 6 | 33 |
| No | 610 | 38 (21‐55) | 12 | 10 | 19 |
| P value | <0.001 | <0.001 | 0.24 | <0.001 | |
| Acute care treatment | |||||
| Yes | 287 | 46 (34‐69) | 21 | 12 | 29 |
| No | 451 | 32 (20‐50) | 11 | 7 | 16 |
| P value | <0.001 | <0.001 | 0.01 | <0.001 | |
| Specialty consultation | |||||
| Yes | 141 | 63 (40‐92) | 38 | 28 | 52 |
| No | 597 | 38 (21‐50) | 10 | 5 | 14 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Any above service | |||||
| Yes | 554 | 46 (29‐68) | 19 | 10 | 26 |
| No | 184∥ | 22 (16‐32) | 2 | 5 | 7 |
| P value | <0.001 | <0.001 | 0.03 | <0.001 | |
| Stay Longer than 72 Hours | Admission to Traditional Inpatient Service | Either Outcome | ||||
|---|---|---|---|---|---|---|
| OR* | P value | OR* | P value | OR* | P value | |
| ||||||
| Heart failure | 2.3 | 0.01 | 1.1 | 0.77 | 1.9 | 0.02 |
| Service received | ||||||
| Open access test | 1.5 | 0.10 | 1.0 | 0.89 | 1.2 | 0.32 |
| Limited access test | 5.1 | <0.001 | 0.4 | 0.03 | 2.5 | <0.001 |
| Acute care treatment | 1.7 | 0.07 | 1.4 | 0.31 | 1.6 | 0.05 |
| Specialty consultation | 6.1 | <0.001 | 13.1 | <0.001 | 8.1 | <0.001 |
Admission Characteristics and Services Received
A narrow range of provisional diagnoses were listed by ED attending physicians and 641 patients (85% of 751) were grouped as having possible ACS or heart failure (Figure 2). Patients with these diagnoses were later risk‐stratified and, when pooled across risk strata, only 14 patients (2% of 641) exceeded the suggested admission‐location criterion for the SSU of lower than high risk. Despite the array and frequency of diagnostic and treatment services that patients received, SSU physicians worked mostly independently, requesting specialty consultations for only 19% of patients (141/738; Table 2).
SSU Success
The median LOS for all patients was 42 hours (interquartile range [IRQ] 22‐63) and 156 patients (21% of 738) had unsuccessful SSU stays (Table 3). The most common reason for an unsuccessful stay was a stay longer than 72 hours (71% of 156). Among the 66 patients who required admission to traditional inpatient services, nearly one‐half (48%) were admitted expressly to receive treatments not available in the SSU after having a specialty consult.
Patients' provisional diagnoses were associated with unsuccessful stays in bivariate analyses (Table 3). In addition, when patients were grouped into 3 risk stratums (very‐low, low, and intermediate‐and‐high), unsuccessful stays increased with increasing risk. For example, in patients with possible ACS, the proportion of unsuccessful stays increased from 17% of 306 very‐low risk patients to 27% of 55 intermediate‐and‐high risk patients (P value for trend = 0.012. Similarly, in patients with heart failure, the proportion of unsuccessful stays increased from 25% of 181 very‐low risk patients to 100% of 3 intermediate‐and‐high risk patients (P value for trend = 0.004).
However, in multiple variable models that simultaneously included patients' characteristics upon admission with services received during their SSU stay, only the provisional diagnosis of heart failure was associated with unsuccessful stays (OR, 1.9; 95% CI, 1.12‐3.18); risk assessments for possible ACS (P = 0.29) and heart failure (P = 0.32) were unimportant predictors of unsuccessful stays (Table 4). On the other hand, whether or not patients received diagnostic tests, acute care treatments, or specialty consultations were important predictors. In particular, patients who received specialty consultations were much more likely to require admission to traditional inpatient services than those patients who did not (OR, 13.1; 95% CI, 6.9‐24.9) and had a 52% chance of having an unsuccessful stay (95% CI, 42‐61%; Figure 3). In addition, the accessibility of a diagnostic test was inversely proportional to the chance of having a long stay; patients who received an open access test had a 12% chance of a long stay (95% CI, 8‐16%) whereas those who received a limited access test had a 29% chance of a long stay (95% CI, 20‐39%). Receiving acute care treatments was also a significant, though less important, predictor of an unsuccessful stay (Table 4).

Discussion
We found that the types of services received by patients during their SSU stays were stronger predictors of long stays and eventual admissions to traditional inpatient services than patients' characteristics upon admission to the SSU. This suggests that SSUs should be focused toward matching patients' anticipated needs with readily accessible services. For example, in our SSU, which cares for over 2,250 patients annually, more than 1,200 patients will receive diagnostic tests in a given year. Among these patients, those who receive a limited access test will be more than twice as likely to have long stays than those who receive an open access test (Figure 3). Though our conclusions may not be applicable to other settings, this study is the most comprehensive description of patients admitted to a hospitalist‐run SSU. In addition, our study is the first to demonstrate that diagnostic and consultative services are the most important predictors of successful stays in SSUs. This promotes the practical strategy that hospitalists who staff SSUs should focus administratively toward gaining access to these services.
Very few of our SSU patients did not fulfill the suggested requirements of our admission location guidelines. For example, only 2% of 691 patients with either possible ACS or heart failure were high risk (Figure 2). Despite this, 21% of our patients had stays longer than 72 hours or were admitted to traditional inpatient services. The paradoxically high proportion of unsuccessful stays among mostly very‐low and low risk patients simply reflects how the clinical risk models that we used were not designed to predict unsuccessful stays. Moreover, as our multiple variable models suggest, improvements in the selection process of candidate SSU patients are more likely to come from an ability to incorporate assessments of what services patients will receive rather than from assessments of their clinical risk (Table 4). Therefore, the immediate plans of the accepting SSU physicians, the physicians who will determine what services patients eventually receive, should be incorporated in the admission‐location decision process.
Three of our findings highlight how input from accepting SSU physiciansconveyed to ED physicians before their final admission‐location decisions are mademay improve the SSU patient selection process. First, 23% of our patients were discharged home after brief stays with no advanced tests, specialty consultations, or acute care treatments (Table 3). Though some of these patients may have required inpatient services other than the ones we recorded, most were admitted with very‐low risk possible ACS; if they required overnight stays at all, many of them may have been better cared for in the ED observation unit (Figure 1). Second, 74% of the patients who required admission to traditional inpatient services were admitted for services not readily available to patients in the SSU (Table 3). Among these patients, nearly one‐third (21/66) received no advanced diagnostic tests in the SSU. This suggests that these patients should have been admitted directly to the general medical wards; doing so may have improved efficiency and quality of care by reducing unnecessary handoffs between physicians. Both types of patientsthose with minimal inpatient needs and those with more needs than the SSU can providehighlight how incorporating accepting SSU physicians' plans may improve the SSU patient selection process. After all, those best equipped to determine if the SSU will meet (or exceed) the needs of candidate patients are the SSU physicians themselves.
Third, we found that whether or not SSU physicians required assistance from specialists was the strongest predictor of unsuccessful stays: when an accepting physician determined that a patient should receive a specialty consultation, that patient's chance of having an unsuccessful stay was over 50% (Figure 3). Our study was not designed to determine how specialty consultations were associated with unsuccessful stays. We did not, for example, record whether or not hospitalists changed their diagnostic, treatment, or admission plans because of specialists' recommendations.12 Therefore, we cannot conclude that specialty consultations actually caused long stays or traditional admissions. Nevertheless, when our SSU physicians did not manage patients independent of specialty consultations, we observed a high likelihood of unsuccessful stays. Because accepting SSU physicians are the ones who will determine whether or not they need assistance from specialists, weighing their immediate plans for specialty consultations into the admission‐location decision process may improve the efficiency of SSUs. Others have recognized the importance of specialty consultations in SSUs by directly incorporating specialists as coattending physicians.13
Our study had several limitations. First, we studied mostly patients with cardiovascular diagnoses. Predictors of success in SSUs that admit patients with different diagnostic profiles may be different. In particular, SSUs that admit patients with a wide array of diagnoses may find that matching patients' needs with readily accessible services is impractical, because these needs may be too wide‐ranging. Second, our study design was observational. However, other than seasonal variations in admission patterns, there was little room for selection bias because we enrolled all consecutive admissions over the 4‐month study period, which gives us more confidence in our results. Third, our study did not record whether or not ED physicians knowingly overrode the suggested admission‐location guidelines because of limited bed availability. Yet, if shortages of beds on traditional inpatient services were driving patients who were otherwise candidates for the general medical wards in to the SSU, then we would have expected higher‐risk patients and greater needs for limited access tests. Finally, our descriptions of patients' needs were based on what diagnostic, consultative, and treatment services patients actually received; yet these needs did not include diagnostic tests that were ordered but never performed. However, any missed needs would bias our results toward no association with unsuccessful stays, because unsuccessful stays would generally increase while patients await needed services.
Future research could address these limitations through an experimental trial of traditional admissions versus admission to a hospitalist‐run SSU. And, because hospitals are complex systems of health care delivery where changes in one patient care unit often affect others in unanticipated ways,14 the impact of SSUs on other patient care units that are closely connected to SSUs, such as EDs and the general medical wards (Figure 1), should be simultaneously observed. For example, though our findings suggest that the accessibility of diagnostic tests should parallel ordering SSU physicians' needs for those tests, making all diagnostic tests open to SSU physicians may result in shortsightedly lengthening the stays of patients in other care units. Future research should also observe the decision‐making process of both the physicians who make admission‐location decisions (ED physicians) and those who determine the eventual plans for patients in the SSU (hospitalists). Accepting physicians from other patient care units have found improved outcomes of efficiency when they were involved in the complex process of deciding where to admit patients.15, 16 After an initial evaluation of a candidate SSU patient in the ED, a hospitalist who staffs both the general medical wards and the SSU would be uniquely well‐positioned to help an ED physician decide where a patient's needs would be best met. Although ED physicians will rightly be concerned that consulting SSU hospitalists may slow patient flow, hands‐on consultations of candidate SSU patients, who have a narrow range of diagnoses and low‐risk profiles, would likely be brief. In addition, because many SSUs are conveniently adjacent to EDs, the burden of communication may be minor.1 To address these questions, hospitalists who staff SSUs must continue the observed trend of working collaboratively with ED physicians.15, 17, 18
Acknowledgements
The authors thank Arthur T. Evans and Brendan M. Reilly for their insightful review of the manuscript. The authors also thank Zhaotai Cui for his assistance with statistical programming.
Short‐stay units (SSUs) are common alternatives to traditional inpatient services.1 When defined broadly to include observation units for low‐risk chest pain patients, SSUs exist in one‐third of hospitals in the United States.2 Amidst growing demands for inpatient services, SSUs have recently developed beyond observation medicine to provide more complex inpatient services in locations commonly adjacent to emergency departments (EDs).1 Hospitalists are well‐positioned to staff these emerging SSUs because of their expertise in managing complex inpatient services.3
Despite this, we found only 3 reports of hospitalist‐run SSUs designed for general medical inpatients (2 from Spain and 1 from Canada).46 Whereas these early reports introduce hospitalist‐run SSUs, they provide limited data to make firm conclusions about their usefulness or appropriate design. For example, none of these reports assessed patients' characteristics upon admission. Nor did they provide details about the services that the SSUs provided. Yet evaluation of both types of patient‐level datadescriptions of patients' needs upon admission and how these needs are met during their staysdetermine whether or not hospitalist‐run SSUs meet their potential to efficiently care for backlogs of patients who otherwise await admission to traditional inpatient services.
In order to further explore these issues, we first sought to characterize our SSU patients upon admission and record what services they received during their stays. To help interpret our results, we then investigated associations between these characteristics and measures of successfully caring for patients in our SSU.
Patients and Methods
Design and Setting
In this prospective cohort study, we included all patients admitted to the hospitalist‐run SSU of Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, from January through April of 2006. Our 14‐bed SSU opened in 2002 to reduce overcrowding on the traditional inpatient wards by admitting adult patients who require inpatient care but might be eligible for discharge within 3 days. The unit is geographically part of the ED but is staffed by resident physicians and a rotating group of hospitalist attending physicians from the Department of Medicine. At least 1 attending and resident physician are available throughout the day, including weekend days and holidays; evenings are covered by a resident who presents overnight admissions to an attending physician the following morning.
ED physicians admit general medical patients to the SSU 24 hours per day, 7 days per week. Though admissions do not require prior approval from SSU physicians, the Departments of Medicine and Emergency Medicine have collaboratively promoted 5 suggested admission‐location guidelines to admitting ED physicians (Figure 1). For candidate SSU patients, these 5 guidelines are not intended to be restrictive but to provide a framework for the complex decision‐making process that our ED physicians encounter, particularly during periods of extreme overcrowding.7 First, patients should have an anticipated stay shorter than 72 hours. Second, patients should not have an eventual need for admission to traditional inpatient services such as the general medicine wards or intensive care units; this guideline is intended to improve patient safety by reducing unnecessary handoffs between physicians.8 Third, patients with provisional cardiovascular diagnoses should be preferentially admitted to the SSU over the general medical wards; this guideline is intended to improve hospital‐wide efficiency because our SSU is equipped with continuous telemetry monitors, an exercise treadmill testing (ETT) laboratory, and other reserved cardiac tests (see Admission Characteristics and Services Received section, below). Fourth, patients' risk should be no higher than intermediate level. Admitting ED physicians are encouraged to use posted risk estimators for patients with provisional diagnoses of possible acute coronary syndrome (ACS), decompensated heart failure, asthma exacerbation, and out‐of‐control diabetes. Finally, patients should not need advanced ancillary services; these include bedside procedures (eg, central venous catheter insertions), time‐intensive nursing (eg, regular dressing changes), and complex social‐services (eg, long‐term care facility placements).

Subjects
The study subjects were all patients admitted to the SSU during the 4‐month study period. Patients were excluded from the entire study if they refused verbal consent to participate. All patients who consented were included in the description of patient admission characteristics. Thirteen patients who prematurely left the SSU against medical advice, however, were neither included in the descriptions of services received nor in the analyses of predictors of successful SSU stays. We excluded these patients because they needed services that they did not receiveincluding these patients in our analysis would tend to overestimate the efficiency of our SSU by shortening the length‐of‐stay (LOS) without adding diagnostic tests or treatments.
Data Collection
After receiving approval from the institutional review board, attending physician investigators conducted an interview, physical examination, and review of medical records for each enrolled patient within 12 hours of admission to the SSU. When ED attending physicians' provisional primary diagnoses included possible ACS or decompensated heart failure, which we knew from earlier pilot data were our 2 most common provisional diagnoses, investigators gathered patient data to be applied in validated models of risk after the study period (Figure 2).9, 10 Some of the clinical predictors required for these models are based on patients' findings on presentation to the ED. For example, Goldman's risk model for major cardiac events uses patients' initial systolic blood pressures on presentation to the ED.9 In such cases, investigators gathered needed data from electronic and paper charts generated in the ED. Upon discharge from the SSU, investigators reviewed patients' medical records a second time. All data were entered by investigators and instantly committed into an online database.

Admission Characteristics and Services Received
Patients were grouped according to the provisional diagnoses of ED attending physicians upon admission to the SSU (Figure 2). We chose to group patients by the provisional diagnoses of EDnot SSUattending physicians to better understand how ED physicians, the physicians who make the admission‐location decisions in our hospital, were using the SSU. Patients were first grouped as having possible ACS or heart failure, because patients with these provisional diagnoses were preferentially admitted to the SSU (Figure 1). When neither diagnosis was listed, patients were grouped according to ED attending physicians' first‐listed diagnoses. At the end of the study period, relevant risk models were applied to patients with possible ACS or heart failure and stratified as very low, low, intermediate, or high risk.9, 10 Patients with both possible ACS and heart failure were grouped according to the diagnosis with the highest corresponding risk assessment; if both risk assessments were the same, then the first‐listed diagnosis was used. Though developed to predict different clinical outcomes during different time periods, risk strata from the corresponding risk models were pooled across both diagnoses to develop a risk summary.
Upon discharge, investigators recorded which advanced diagnostic tests, specialty consultations, and acute care treatments patients received while in the SSU. Diagnostic tests were considered advanced if they were not routinely performed within 2 hours of being ordered. Advanced diagnostic tests were grouped into 2 types by their accessibility to ordering SSU physicians. Open access tests included echocardiograms and ETTs, which were reserved for SSU patients 6 days per week. Though the availability of open access tests was not unlimited, ordering physicians' needs for them rarely exceeded the immediate supply. On the other hand, limited access tests included both cardiac stress imaging studies, which were reserved for SSU patients on a very limited basis 4 or 5 days per week, and other tests that were not reserved for SSU patients, such as endoscopy, magnetic resonance imaging, or ultrasonography. Ordering physicians' needs for limited access tests often exceeded their immediate supply; in such cases, SSU patients were placed without priority into queues that included patients from the entire hospital.
Investigators recorded when patients received advanced diagnostic tests that were ordered by specialists. These tests, however, were not included in analyses of how services received by SSU patients affected SSU success, because SSU attending physicians were only indirectly involved in whether or not patients received these tests. Treatments were considered acute care treatments if they were commonly administered only in acute care settings, such as heparin for unstable angina or intravenous furosemide for pulmonary edema.
SSU Success
The SSU was designed to care for patients during brief stays and without eventual admission to traditional inpatient services. Therefore, we used patients' LOS and whether or not patients were admitted to traditional inpatient services as measures of SSU success. LOS was calculated from the time patients arrived in the SSU until the time they left. Therefore, neither time spent in the ED before admission to the SSU nor time spent on traditional inpatient services (if needed) contributed to our definition of LOS. Individual SSU patients were considered successfully cared for in the SSU if their LOS was less than 72 hours and they were discharged directly home from the SSU. We explored associations between these outcomes and provisional diagnoses, risk assessments, and services received.
Data Analysis
LOS data were right‐skewed; therefore, we used the Mann‐Whitney test for comparisons between 2 groups and the Kruskal‐Wallis test for comparisons among 3 or more groups. To test for trends of median LOS among ordered groupings, we used the method of Cuzick.11 We used Pearson's chi‐square test to compare proportions of patients grouped into categories and the chi‐square test for trends with equal scoring to test for trends among ordered groupings.
We performed multiple logistic regression to explore which variables were associated with SSU success. The following 5 demographic variables from Table 1 were insignificant in all single‐variable and multiple‐variable regression models that we tested and were, therefore, removed from further analyses to create more parsimonious models: gender, language, ethnicity, race, and whether or not patients had a primary care provider. Our multiple logistic regression models were fitted by maximum likelihood methods. In all of these models, odds ratios (ORs) were adjusted for patient characteristics that included age (in years), insulin‐requiring diabetes mellitus (yes or no), SSU attending physician, day of the week of SSU admission (weekday or weekend), and hospitalization during the preceding year. Confidence intervals (CIs) for predicted probabilities were computed using the delta method. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, College Station, TX).
| |
| Mean age, years (SD) (25th‐75th percentiles) | 58 (12) (49‐66) |
| Men | 389 (52) |
| Lacking a primary care provider | 256 (34) |
| Non‐English speaking | 217 (29) |
| Ethnicity is Hispanic or Latino | 105 (14) |
| Race is Black or African‐American | 480 (64) |
| Hospitalized within the preceding year | 322 (43) |
| Insulin‐requiring diabetes mellitus | 83 (11) |
| Previous coronary artery revascularization | 89 (12) |
| Provisional diagnosis* | |
| Possible acute coronary syndrome | 427 (57) |
| Heart failure | 214 (29) |
| Other cardiovascular | 62 (8) |
| Noncardiovascular | 48 (6) |
Results
Subjects
During the 4‐month study period, 755 patients were admitted to the SSU. Among these patients, 4 were excluded from our study because they refused verbal consent. In the remaining study sample of 751 patients, all were included in the descriptions of patients' admission characteristics (Table 1), but 13 patients who left prematurely were excluded in both the descriptions of services received (Table 2) and the analyses of SSU success (Tables 3 and 4).
| Service received | Possible ACS n = 418 (%) | Heart Failure n = 211 (%) | Other Cardiovascular n = 61 (%) | Noncardiovascular n = 48 (%) | Total n = 738 (%) |
|---|---|---|---|---|---|
| |||||
| Open access test* | 37 | 59 | 56 | 13 | 43 |
| Resting | |||||
| Echocardiography | 29 | 59 | 56 | 13 | 39 |
| ETT | 12 | 0 | 5 | 0 | 7 |
| Limited access test | 24 | 8 | 10 | 8 | 17 |
| Stress imaging | 21 | 5 | 7 | 2 | 14 |
| Acute care treatment | 22 | 78 | 5 | 60 | 39 |
| Specialty consultation∥ | 24 | 12 | 20 | 8 | 19 |
| Any above service | 68 | 93 | 67 | 69 | 75 |
| Provisional Diagnosis and Services Received | n | Median LOS [hours (IQR)] | Stay Longer than 72 Hours (%) | Admission to Traditional Inpatient Service (%) | Stay Longer than 72 Hours or Admission to Traditional Inpatient Service (%) |
|---|---|---|---|---|---|
| |||||
| All patients | 738 | 42 (22‐63) | 15 | 9* | 21 |
| Possible ACS | 418 | 37 (20‐57) | 13 | 10 | 20 |
| Heart failure | 211 | 47 (34‐69) | 21 | 9 | 27 |
| Other CV | 61 | 40 (21‐49) | 10 | 5 | 11 |
| Non‐CV | 48 | 40 (22‐60) | 10 | 2 | 13 |
| P value | <0.001 | 0.04 | 0.18 | 0.01 | |
| Open access test | |||||
| Yes | 320 | 46 (31‐67) | 17 | 9 | 23 |
| No | 418 | 33 (20‐52) | 13 | 9 | 20 |
| P value | <0.001 | 0.15 | 0.87 | 0.43 | |
| Limited access test | |||||
| Yes | 128 | 51 (42‐82) | 31 | 6 | 33 |
| No | 610 | 38 (21‐55) | 12 | 10 | 19 |
| P value | <0.001 | <0.001 | 0.24 | <0.001 | |
| Acute care treatment | |||||
| Yes | 287 | 46 (34‐69) | 21 | 12 | 29 |
| No | 451 | 32 (20‐50) | 11 | 7 | 16 |
| P value | <0.001 | <0.001 | 0.01 | <0.001 | |
| Specialty consultation | |||||
| Yes | 141 | 63 (40‐92) | 38 | 28 | 52 |
| No | 597 | 38 (21‐50) | 10 | 5 | 14 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | |
| Any above service | |||||
| Yes | 554 | 46 (29‐68) | 19 | 10 | 26 |
| No | 184∥ | 22 (16‐32) | 2 | 5 | 7 |
| P value | <0.001 | <0.001 | 0.03 | <0.001 | |
| Stay Longer than 72 Hours | Admission to Traditional Inpatient Service | Either Outcome | ||||
|---|---|---|---|---|---|---|
| OR* | P value | OR* | P value | OR* | P value | |
| ||||||
| Heart failure | 2.3 | 0.01 | 1.1 | 0.77 | 1.9 | 0.02 |
| Service received | ||||||
| Open access test | 1.5 | 0.10 | 1.0 | 0.89 | 1.2 | 0.32 |
| Limited access test | 5.1 | <0.001 | 0.4 | 0.03 | 2.5 | <0.001 |
| Acute care treatment | 1.7 | 0.07 | 1.4 | 0.31 | 1.6 | 0.05 |
| Specialty consultation | 6.1 | <0.001 | 13.1 | <0.001 | 8.1 | <0.001 |
Admission Characteristics and Services Received
A narrow range of provisional diagnoses were listed by ED attending physicians and 641 patients (85% of 751) were grouped as having possible ACS or heart failure (Figure 2). Patients with these diagnoses were later risk‐stratified and, when pooled across risk strata, only 14 patients (2% of 641) exceeded the suggested admission‐location criterion for the SSU of lower than high risk. Despite the array and frequency of diagnostic and treatment services that patients received, SSU physicians worked mostly independently, requesting specialty consultations for only 19% of patients (141/738; Table 2).
SSU Success
The median LOS for all patients was 42 hours (interquartile range [IRQ] 22‐63) and 156 patients (21% of 738) had unsuccessful SSU stays (Table 3). The most common reason for an unsuccessful stay was a stay longer than 72 hours (71% of 156). Among the 66 patients who required admission to traditional inpatient services, nearly one‐half (48%) were admitted expressly to receive treatments not available in the SSU after having a specialty consult.
Patients' provisional diagnoses were associated with unsuccessful stays in bivariate analyses (Table 3). In addition, when patients were grouped into 3 risk stratums (very‐low, low, and intermediate‐and‐high), unsuccessful stays increased with increasing risk. For example, in patients with possible ACS, the proportion of unsuccessful stays increased from 17% of 306 very‐low risk patients to 27% of 55 intermediate‐and‐high risk patients (P value for trend = 0.012. Similarly, in patients with heart failure, the proportion of unsuccessful stays increased from 25% of 181 very‐low risk patients to 100% of 3 intermediate‐and‐high risk patients (P value for trend = 0.004).
However, in multiple variable models that simultaneously included patients' characteristics upon admission with services received during their SSU stay, only the provisional diagnosis of heart failure was associated with unsuccessful stays (OR, 1.9; 95% CI, 1.12‐3.18); risk assessments for possible ACS (P = 0.29) and heart failure (P = 0.32) were unimportant predictors of unsuccessful stays (Table 4). On the other hand, whether or not patients received diagnostic tests, acute care treatments, or specialty consultations were important predictors. In particular, patients who received specialty consultations were much more likely to require admission to traditional inpatient services than those patients who did not (OR, 13.1; 95% CI, 6.9‐24.9) and had a 52% chance of having an unsuccessful stay (95% CI, 42‐61%; Figure 3). In addition, the accessibility of a diagnostic test was inversely proportional to the chance of having a long stay; patients who received an open access test had a 12% chance of a long stay (95% CI, 8‐16%) whereas those who received a limited access test had a 29% chance of a long stay (95% CI, 20‐39%). Receiving acute care treatments was also a significant, though less important, predictor of an unsuccessful stay (Table 4).

Discussion
We found that the types of services received by patients during their SSU stays were stronger predictors of long stays and eventual admissions to traditional inpatient services than patients' characteristics upon admission to the SSU. This suggests that SSUs should be focused toward matching patients' anticipated needs with readily accessible services. For example, in our SSU, which cares for over 2,250 patients annually, more than 1,200 patients will receive diagnostic tests in a given year. Among these patients, those who receive a limited access test will be more than twice as likely to have long stays than those who receive an open access test (Figure 3). Though our conclusions may not be applicable to other settings, this study is the most comprehensive description of patients admitted to a hospitalist‐run SSU. In addition, our study is the first to demonstrate that diagnostic and consultative services are the most important predictors of successful stays in SSUs. This promotes the practical strategy that hospitalists who staff SSUs should focus administratively toward gaining access to these services.
Very few of our SSU patients did not fulfill the suggested requirements of our admission location guidelines. For example, only 2% of 691 patients with either possible ACS or heart failure were high risk (Figure 2). Despite this, 21% of our patients had stays longer than 72 hours or were admitted to traditional inpatient services. The paradoxically high proportion of unsuccessful stays among mostly very‐low and low risk patients simply reflects how the clinical risk models that we used were not designed to predict unsuccessful stays. Moreover, as our multiple variable models suggest, improvements in the selection process of candidate SSU patients are more likely to come from an ability to incorporate assessments of what services patients will receive rather than from assessments of their clinical risk (Table 4). Therefore, the immediate plans of the accepting SSU physicians, the physicians who will determine what services patients eventually receive, should be incorporated in the admission‐location decision process.
Three of our findings highlight how input from accepting SSU physiciansconveyed to ED physicians before their final admission‐location decisions are mademay improve the SSU patient selection process. First, 23% of our patients were discharged home after brief stays with no advanced tests, specialty consultations, or acute care treatments (Table 3). Though some of these patients may have required inpatient services other than the ones we recorded, most were admitted with very‐low risk possible ACS; if they required overnight stays at all, many of them may have been better cared for in the ED observation unit (Figure 1). Second, 74% of the patients who required admission to traditional inpatient services were admitted for services not readily available to patients in the SSU (Table 3). Among these patients, nearly one‐third (21/66) received no advanced diagnostic tests in the SSU. This suggests that these patients should have been admitted directly to the general medical wards; doing so may have improved efficiency and quality of care by reducing unnecessary handoffs between physicians. Both types of patientsthose with minimal inpatient needs and those with more needs than the SSU can providehighlight how incorporating accepting SSU physicians' plans may improve the SSU patient selection process. After all, those best equipped to determine if the SSU will meet (or exceed) the needs of candidate patients are the SSU physicians themselves.
Third, we found that whether or not SSU physicians required assistance from specialists was the strongest predictor of unsuccessful stays: when an accepting physician determined that a patient should receive a specialty consultation, that patient's chance of having an unsuccessful stay was over 50% (Figure 3). Our study was not designed to determine how specialty consultations were associated with unsuccessful stays. We did not, for example, record whether or not hospitalists changed their diagnostic, treatment, or admission plans because of specialists' recommendations.12 Therefore, we cannot conclude that specialty consultations actually caused long stays or traditional admissions. Nevertheless, when our SSU physicians did not manage patients independent of specialty consultations, we observed a high likelihood of unsuccessful stays. Because accepting SSU physicians are the ones who will determine whether or not they need assistance from specialists, weighing their immediate plans for specialty consultations into the admission‐location decision process may improve the efficiency of SSUs. Others have recognized the importance of specialty consultations in SSUs by directly incorporating specialists as coattending physicians.13
Our study had several limitations. First, we studied mostly patients with cardiovascular diagnoses. Predictors of success in SSUs that admit patients with different diagnostic profiles may be different. In particular, SSUs that admit patients with a wide array of diagnoses may find that matching patients' needs with readily accessible services is impractical, because these needs may be too wide‐ranging. Second, our study design was observational. However, other than seasonal variations in admission patterns, there was little room for selection bias because we enrolled all consecutive admissions over the 4‐month study period, which gives us more confidence in our results. Third, our study did not record whether or not ED physicians knowingly overrode the suggested admission‐location guidelines because of limited bed availability. Yet, if shortages of beds on traditional inpatient services were driving patients who were otherwise candidates for the general medical wards in to the SSU, then we would have expected higher‐risk patients and greater needs for limited access tests. Finally, our descriptions of patients' needs were based on what diagnostic, consultative, and treatment services patients actually received; yet these needs did not include diagnostic tests that were ordered but never performed. However, any missed needs would bias our results toward no association with unsuccessful stays, because unsuccessful stays would generally increase while patients await needed services.
Future research could address these limitations through an experimental trial of traditional admissions versus admission to a hospitalist‐run SSU. And, because hospitals are complex systems of health care delivery where changes in one patient care unit often affect others in unanticipated ways,14 the impact of SSUs on other patient care units that are closely connected to SSUs, such as EDs and the general medical wards (Figure 1), should be simultaneously observed. For example, though our findings suggest that the accessibility of diagnostic tests should parallel ordering SSU physicians' needs for those tests, making all diagnostic tests open to SSU physicians may result in shortsightedly lengthening the stays of patients in other care units. Future research should also observe the decision‐making process of both the physicians who make admission‐location decisions (ED physicians) and those who determine the eventual plans for patients in the SSU (hospitalists). Accepting physicians from other patient care units have found improved outcomes of efficiency when they were involved in the complex process of deciding where to admit patients.15, 16 After an initial evaluation of a candidate SSU patient in the ED, a hospitalist who staffs both the general medical wards and the SSU would be uniquely well‐positioned to help an ED physician decide where a patient's needs would be best met. Although ED physicians will rightly be concerned that consulting SSU hospitalists may slow patient flow, hands‐on consultations of candidate SSU patients, who have a narrow range of diagnoses and low‐risk profiles, would likely be brief. In addition, because many SSUs are conveniently adjacent to EDs, the burden of communication may be minor.1 To address these questions, hospitalists who staff SSUs must continue the observed trend of working collaboratively with ED physicians.15, 17, 18
Acknowledgements
The authors thank Arthur T. Evans and Brendan M. Reilly for their insightful review of the manuscript. The authors also thank Zhaotai Cui for his assistance with statistical programming.
- ,,.A paradigm shift in the nature of care provision in emergency departments.Emerg Med J.2004;21:681–684.
- ,,.Acute coronary syndromes. In: Marx J, Hockberger R, Walls R, eds.Rosen's emergency medicine. Concepts and clinical practice.6th ed.Edinburgh:Mosby Elsevier;2006:1154–1199.
- .Taking charge of observation units for better patient flow.Todays Hospitalist Mag.2007;5(7):16–20.
- ,,, et al.Unidad de corta estancia dependiente de Medicina Interna.An Med Interna.1999;16:504–510.
- ,,,,.Factors that predict unplanned hospital readmission of patients discharged from a short stay medical unit.An Med Interna.2002;19:221–225.
- ,,, et al.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.CMAJ.2000;163(11):1477–1480.
- ,,,.Triage of patients with chest pain in the emergency department: a comparative study of physicians' decisions.Am J Med.2002;112:95–103.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,, et al.Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain.N Engl J Med.1996;334:1498–1504.
- ,,, et al.Risk stratification for in‐hospital mortality in acutely decompensated heart failure.JAMA.2005;293:572–580.
- .A Wilcoxon‐type test for trend.Stat Med.1985;4:87–90.
- ,,,,,.Diagnostic test restraint and the specialty consultation.J Gen Intern Med.1990;5:95–103.
- ,,, et al.A cooperative care model: cardiologists and hospitalists reduce length of stay in a chest pain observation unit.Crit Pathw Cardiol.2005;4:55–58.
- ,.Average length of stay, delayed discharge, and hospital congestion.BMJ.2002;325:610–611.
- .An internist in the emergency department: the IM facilitator program.HMO Pract.1991;10:42–43.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- .Hospitalists' new role in the ED: “clog busters.”Todays Hospitalist Mag.2005;3(8):15–18.
- .Kindred spirits: ED doctors, hospitalists forge a critical collaboration.Hospitalist.2007;11(7):1,16–20.
- ,,.A paradigm shift in the nature of care provision in emergency departments.Emerg Med J.2004;21:681–684.
- ,,.Acute coronary syndromes. In: Marx J, Hockberger R, Walls R, eds.Rosen's emergency medicine. Concepts and clinical practice.6th ed.Edinburgh:Mosby Elsevier;2006:1154–1199.
- .Taking charge of observation units for better patient flow.Todays Hospitalist Mag.2007;5(7):16–20.
- ,,, et al.Unidad de corta estancia dependiente de Medicina Interna.An Med Interna.1999;16:504–510.
- ,,,,.Factors that predict unplanned hospital readmission of patients discharged from a short stay medical unit.An Med Interna.2002;19:221–225.
- ,,, et al.Program description: a hospitalist‐run, medical short‐stay unit in a teaching hospital.CMAJ.2000;163(11):1477–1480.
- ,,,.Triage of patients with chest pain in the emergency department: a comparative study of physicians' decisions.Am J Med.2002;112:95–103.
- ,,.Gaps in the continuity of care and progress on patient safety.BMJ.2000;320:791–794.
- ,,, et al.Prediction of the need for intensive care in patients who come to emergency departments with acute chest pain.N Engl J Med.1996;334:1498–1504.
- ,,, et al.Risk stratification for in‐hospital mortality in acutely decompensated heart failure.JAMA.2005;293:572–580.
- .A Wilcoxon‐type test for trend.Stat Med.1985;4:87–90.
- ,,,,,.Diagnostic test restraint and the specialty consultation.J Gen Intern Med.1990;5:95–103.
- ,,, et al.A cooperative care model: cardiologists and hospitalists reduce length of stay in a chest pain observation unit.Crit Pathw Cardiol.2005;4:55–58.
- ,.Average length of stay, delayed discharge, and hospital congestion.BMJ.2002;325:610–611.
- .An internist in the emergency department: the IM facilitator program.HMO Pract.1991;10:42–43.
- ,,, et al.A “closed” medical intensive care unit (MICU) improves resource utilization when compared with an “open” MICU.Am J Respir Crit Care Med.1998;157:1468–1473.
- .Hospitalists' new role in the ED: “clog busters.”Todays Hospitalist Mag.2005;3(8):15–18.
- .Kindred spirits: ED doctors, hospitalists forge a critical collaboration.Hospitalist.2007;11(7):1,16–20.
Copyright © 2009 Society of Hospital Medicine
Impact of a Bedside Procedure Service
Inpatient bedside procedures are a major source of preventable adverse events in hospitals.1, 2 Unfortunately, many future inpatient physicians may lack the training3 and confidence4 to correct this problem. One proposed model for improving the teaching, performance, and evaluation of bedside procedures is a procedure service that is staffed by faculty who are experts at inpatient procedures.5 In a recent survey of internal medicine residents from our hospital, 86% (30 of 35) believed that expert supervision would improve central venous catheterization technique (Trick WE, personal communication).
Primary considerations in the development of a procedure service are the baseline demand for bedside procedures and whether a procedure service may affect this demand. Though variations in population‐based rates of some hospital procedures have been described,6, 7 there is little written on the demand for procedures performed at the bedsides of inpatients. Concomitant increases in demand and availability of other technologies810 suggest that improving the availability of bedside procedures may lead to an increase in their demand, regardless of whether such an increase benefits patients.11
Therefore, we sought to determine the impact of a bedside procedure service on the baseline number of paracenteses, thoracenteses, lumbar punctures (LPs), and central venous catheterizations (CVCs) performed on general medicine inpatients at our teaching hospital. In addition, we examined whether this service leads to more successful and safe procedure attempts.
METHODS
Design and Setting
In this prospective cohort study, the cohort was all patients admitted to the general medicine service at Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, in January and February of 2006. The general medicine inpatient service is divided into 3 firms (A, B, and C), each with 4 separate teams of physicians and students. Admissions from the emergency department or other services in the hospital, such as intensive care units (which are closed and therefore staffed by separate teams of physicians), are distributed in sequence to on‐call teams from each firm. During the study period, the availability of a bedside procedure service varied by firm. Throughout the first 4 weeks, the service was available to only 1 of 3 firms (firm A). Then, during weeks 5 through 8, the service crossed over to the other 2 firms (firms B and C) and was unavailable to the original firm. Firm assignments for residents assigned to the inpatient service for all 8 weeks did not change. Of the 16 residents assigned to firm A during the first 4 weeks, when the procedure service was available, 3 remained on the wards during the second 4 weeks, when the procedure service was not available.
We chose to collect data on 4 bedside procedures: paracentesis, thoracentesis, LP, and CVC. Similar to those at other teaching hospitals, our residents informally acquire the skills to perform these procedures while assisting and being assisted by more experienced senior residents in a see one, do one, teach one apprenticeship model of learning.4 To improve the training and performance of these bedside procedures, the Department of Medicine piloted a bedside procedure service to teach procedural skills and assist residents during these procedures. Use of the service, though voluntary, was actively encouraged at residents' monthly orientation meetings and regular conferences.
One attending inpatient physician (J.A.) staffed the bedside procedure service, which was available during normal work hours on weekdays. Requests for procedures were made by general medicine residents through an online database and, after approval by the procedure service attending physician, were performed under his direct supervision. A hand‐carried ultrasound (MicroMaxx, Sonosite, Inc., Bothell, WA) that generates a 2‐dimensional gray‐scale image was used to both confirm the presence and location of fluid prior to paracentesis and thoracentesis and provide real‐time guidance during central venous catheterization. When the bedside procedure service was unavailable, residents performed bedside procedures in the usual fashion, typically without direct attending physician supervision. But if requested, an on‐call chief medical resident with access to a hand‐carried ultrasound device used by the intensive care unit was available for assistance at any time.
Subjects
The study subjects were all patients admitted to the general medical service during the 8‐week pilot period. Patients were excluded if they had been discharged before arrival on the medical wards or if they were under the care of the general medicine service for less than 6 hours before discharge or transfer to another service. We chose 6 hours because we reasoned that such brief admissions were not potential candidates for invasive bedside procedures.
Data Collection
Each morning an investigator contacted the senior residents who had admitted patients during the previous 24‐hour shift and confirmed that newly admitted patients were under the care of the general medicine service for more than 6 hours. To examine how the number of attempts may have been affected by procedures done in the emergency room or intensive care units before admission to the general medicine service, investigators also asked admitting residents whether a bedside procedure had been attempted in the 72 hours before admission. Every general medicine service resident was asked to fill out a brief data collection form after an attempt to perform any procedure on the general medical wards. In addition, chief residents asked each member of the general medicine service at mandatory sign‐out rounds at the end of each weekday whether any procedures had been attempted, and on weekend days investigators contacted senior residents from each general medicine service team.
We report on this quality assurance study, which was conducted during a pilot phase. This report has been reviewed and judged exempt by our institutional review board.
Primary OutcomeNumber of Procedure Attempts
For all bedside procedures attempted by residents on the general medical wards, investigators determined whether the residents were members of firms that were offered the bedside procedure service and, if so, whether the procedure service attending directly supervised the procedure attempt. Multiple procedure attempts of the same type were counted for an individual patient if (1) the procedure attempts did not occur during the same admissions and (2) neither the physicians attempting the procedure nor the primary indications for it were the same. Therefore, neither attempts performed after initially unsuccessful ones nor repeated procedures, such as large‐volume therapeutic paracentesis and thoracentesis, were counted twice. We reasoned that when these criteria were met, procedure attempts could be considered independently.
Secondary Outcomes
Investigators asked residents who attempted procedures to indicate whether (1) the indication for the procedure was solely diagnostic or was, at least in part, therapeutic; (2) the procedure was successful; and (3) there were any immediate major periprocedural complications. A procedure was considered to have been successfully performed if it fulfilled 2 criteria: it had to be completed during a single continuous attempt, even if multiple sites or procedure kits were used; and it had to fulfill the indication for it being done. For example, if the indication for thoracentesis was therapeutic, this procedure would be considered successful if it yielded a large enough volume of fluid to alleviate the patient's symptoms, but if the indication was diagnostic, then thoracentesis would be considered successful if it yielded enough fluid for laboratory processing. Residents were asked to report any periprocedural complications that they considered major; 2 illustrative examples were provided: a pneumothorax and severe bleeding.
Data Analyses
On the basis of earlier pilot data, we estimated that 8%10% of all admissions to the general medicine service underwent at least 1 procedure (paracentesis, thoracentesis, lumbar puncture, or central vein catheterization). We planned for a sample size of 1900 admissions, which would have 80% power to detect a clinically meaningful 50% relative increase in the mean number of bedside procedures with a double‐sided alpha error of 0.05. We used permutation tests to compare the mean number of procedures attempted between firms and bootstrap simulation to construct 95% confidence intervals for those means and the differences between and ratios of them. Fisher's exact test was used to compare proportions of successfully performed procedures and preadmission procedure attempts. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, LP, College Station, TX).
RESULTS
Subjects
During this 8‐week pilot study, there were 2157 admissions to the general medicine service. Among these admissions, 216 were excluded from our study because the patients did not arrive on the medical wards or were not under the care of the general medicine service for at least 6 hours before discharge or before being transferred to another service. Of the remaining 1941 admissions, 935 were to firms with the bedside procedure service available, and 1006 were to firms without the service available (Fig. 1)
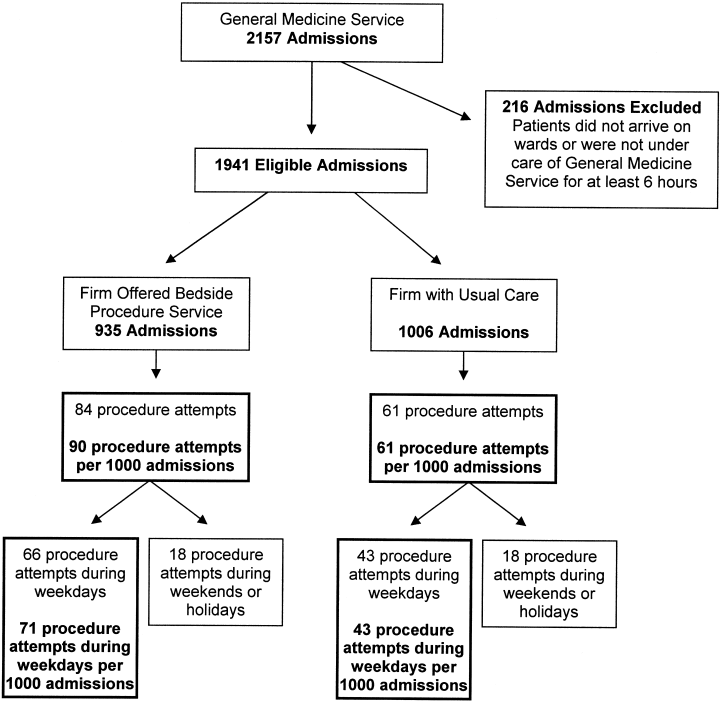
Primary OutcomeNumber of Procedure Attempts
Overall, 122 patients underwent 145 procedure attempts that met our criteria for independence. The mean number of procedure attempts in firms offered the bedside procedure service was 48% higher (90 versus 61 per 1000 admissions; RR 1.48, 95% CI 1.062.10; P = .030; Fig. 1). When procedures attempted on weekends and holidays were excluded, the relative increase in procedure attempts in firms offered the bedside procedure service was even higher (70 versus 43 per 1000 admissions; RR 1.63, 95% CI 1.092.49; P = .023; Fig. 1). When grouped according to whether procedure attempts occurred before or after crossover of the procedure service, the mean number of procedure attempts in firms was higher when the service was offered: firm A dropped from 84 to 70 per 1000 admissions (P = .58) after losing the service, whereas firms B and C increased from 57 to 94 per 1000 admissions (P = .025) on gaining the service. There were 40 procedure attempts performed on patients within 72 hours before admission, but there was no difference between firms in the proportions of these preadmission procedures (P = .43).
Secondary Outcomes
Table 1 shows how of each type of procedure contributed to the overall difference. Attempts of CVC and therapeutic paracentesis and thoracentesis accounted for 86% of the overall increase in procedure attempts for admissions to firms offered the bedside procedure service, whereas only 14% of this increase was a result of diagnostic procedures. There were no differences in the proportions of successfully performed procedures, whether grouped by firm (P = 1.0) or by direct supervision from the procedure service attending (P = .64; Table 2). There were 3 self‐reported major periprocedural complications; all were related to excessive bleeding from CVC attempts. Two occurred without direct supervision from the bedside procedure service attending, one hemomediastinum from an internal jugular CVC attempt and one groin hematoma from a femoral CVC attempt. The third, a groin hematoma from a femoral CVC attempt, occurred during direct supervision from the bedside procedure service attending.
| Bedside procedure and indication | Firms with bedside procedure service 935 admissions | Firms with usual care 1006 admissions | Absolute rate difference (proportion of overall difference)* |
|---|---|---|---|
| Total for entire study (total for weekend days and holidays) | |||
| |||
| Total | 90 (19) | 61 (18) | 29 (100%) |
| Thoracentesis | 30 (10) | 18 (7) | 12 (41%) |
| Diagnosis | 9 (5) | 6 (2) | 3 (9%) |
| Treatment | 21 (4) | 12 (5) | 9 (32%) |
| Paracentesis | 32 (5) | 25 (6) | 7 (25%) |
| Diagnosis | 9 (1) | 11 (3) | 2 (8%) |
| Treatment | 24 (4) | 14 (3) | 10 (33%) |
| Central venous catheterization | 17 (3) | 11 (4) | 6 (21%) |
| Lumbar puncture | 11 (1) | 7 (1) | 4 (13%) |
| Diagnosis | 10 (1) | 6 (1) | 4 (13%) |
| Treatment | 1 (0) | 1 (0) | 0 (0%) |
| Admission to firm with | P value of difference in proportions | ||||||
|---|---|---|---|---|---|---|---|
| Procedure service available | Usual care | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| |||||||
| Central venous catheterization | 16 | 13 | 81 | 11 | 9 | 82 | 1.00 |
| Paracentesis, thoracentesis, or lumbar puncture | 68 | 54 | 79 | 50 | 40 | 80 | 1.00 |
| Total | 84 | 67 | 80 | 61 | 49 | 80 | 1.00 |
| Procedure service attending | Pvalue of difference in proportions | ||||||
| Directly supervised | Did not directly supervise | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| Central venous catheterization | 10 | 10 | 100 | 17 | 12 | 71 | 0.28 |
| Paracentesis, thoracentesis, or lumbar puncture | 40 | 33 | 83 | 78 | 61 | 78 | 0.12 |
| Total | 50 | 43 | 86 | 95 | 73 | 77 | 0.64 |
DISCUSSION
We found that the mean number of bedside procedures increased by 48% (95% CI, 6% to 110%) from 61 to 90 per 1000 general medicine admissions when firms were offered a bedside procedure service. This suggests that a procedure service may lead to an increase in the number of procedures performed. For example, in our hospital, where 12,500 patients are admitted annually to the general medical service, 365 additional procedures per year (95% CI, 45840) may be performed if a procedure service is available. Despite this potential increase in demand, we were unable to demonstrate a parallel increase in bedside procedure success, even when the procedure service attending was directly supervising residents (Table 2). Though our conclusions may not be applicable to other settings, this study is, to our knowledge, the first to describe the demand for bedside procedures performed on general medicine inpatients at an urban teaching hospital and the first to demonstrate that this demand increases with the availability of a procedure service.
Because 86% of the observed increase in procedure attempts was due to therapeutic indications (Table 1), most of the observed difference may be due to undertreatment in the usual care cohort, overtreatment in the bedside procedure service cohort, or a combination of both. However, our study was not designed to determine if patients were undertreated because we did not review the appropriateness of physicians' decisions to not attempt procedures. And even though the bedside procedure service attending physician prospectively confirmed the appropriateness of each procedure attempt in that cohort, we did not examine what physicians' baseline treatment thresholds were or if they were lowered by the availability of the bedside procedure service.11 In other words, we cannot claim that the observed increase in procedure attempts was indicated based on patients' clinical factors. Nevertheless, the observed increase supports the important idea that discrete physician‐level decisions, in this case, whether to perform a bedside procedure, may be affected by broader system‐wide adoptions of new technologies like our bedside procedure service.12 Other nonclinical factors not observed in our study, such as fee‐for‐service compensation and variable physician‐level diagnostic and therapeutic thresholds, may also affect the rate of bedside procedures.
Our study had several limitations. We studied only one group of patients at one hospital: admissions to physicians in different settings may have different rates of bedside procedures. Our study design was observational. However, the predetermined sequential allocation of admissions and the varied assignments of the bedside procedure service during the study period should have limited selection bias. Our identification of procedure attempts, particularly in the usual care group, relied on resident physicians' self‐reports, and we cannot exclude a reporting bias. However, we believe that the daily interactions between investigators and residents from each team on the general medicine service limited the number of procedure attempts that went unrecorded. Finally, though sufficiently powered to determine our primary outcome, our study was underpowered to confirm statistical differences between firms in proportions of successfully performed procedures. For example, approximately 400 additional procedures (or more than 5000 additional admissions) would have been needed to sufficiently power the observed 9% increase in successful attempts that we observed with direct supervision by the procedure service attending (77% versus 86%; P = .64; Table 2). Our current sample size may be adequate in future research if success rates diverge as the experience of the procedure service attending increases. Though expert in performing bedside procedures, he had limited experience teaching them, particularly with the use of a hand‐carried ultrasound device. Just as there is a learning curve to gain the experience to successfully perform procedures,13 so may there be a learning curve to successfully teach procedures.14
Future research could address these limitations by more closely observing the decision‐making processes of physicians who order bedside procedures for general medicine inpatients in various settings. Our findings suggest that although patients' clinical circumstances are likely the most important consideration, nonclinical factors may also affect physicians' decisions.12 Like other multifaceted decision‐making processes of physicians,15 the complexity of this decision is important to examine because, as our pilot data suggest, a procedure service may not lead to more successful procedure attempts or reductions in the number of major complications. Although the cumulative expertise of our service or the innovative methods of training of other institutions may improve the performance of bedside procedures,5, 13 physicians' decisions about whether to order them will remain paramount, because any improvement in procedural competence will do little to reduce the relative danger of unnecessary procedures16 or the missed benefit of procedures left undone. Physicians of inpatients17, 18 should refine the indications for and anticipated benefits from these commonly performed invasive procedures.
- ,,, et al.The nature of adverse events in hospitalized patients: Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,, et al.Cost of medical injuries in Utah and Colorado.Inquiry.36;255–264.
- ,,,.Procedural Skills Training in Internal Medicine Residencies: A Survey of Program Directors.Ann Intern Med1989;111:932–38.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures.Am J Med.2006;119:71.e17–.e24.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Variation in the use of cardiac procedures after acute myocardial infarction.N Engl J Med.1995;333:573–578.
- ,,.Frequency and morbidity of inpatient procedures: report of a pilot study from two teaching hospitals.Arch Intern Med.1978;138:1809–1811.
- ,.The impact of diagnostic testing on therapeutic interventions.JAMA.1996;275:1189–1191.
- ,,, et al.Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- ,,, et al.Coronary artery bypass graft surgery in Ontario and New York State: which rate is right?Ann Intern Med.1997;126:13–19.
- ,.Avoiding the unintended consequences of growth in medical care. How might more be worse?JAMA.1999;281:446–453.
- ,,.Professional uncertainty and the problem of supplier‐induced demand.Soc Sci Med.1982;811–824.
- ,,,.A curricular initiative for internal medicine residents to enhance proficiency in internal jugular central venous line placement.Mayo Clin Proc.2005;80:212–218.
- ,,, et al.Confidence of Academic General Internists and Family Physicians to Teach Ambulatory Procedures.J Gen Intern Med.2000;15:353–360.
- ,,, et al.The impact of evidence on physicians' inpatient treatment decisions.J Gen Intern Med.2004;19:402–409.
- .Medical care—is more always better?N Engl J Med.2003;349:1665–1667.
- ,.Point/counterpoint: should hospital medicine become a distinct specialty?Hospitalist.2005;9(1):15–19.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hospital Med.2006;1:S1–S95.
Inpatient bedside procedures are a major source of preventable adverse events in hospitals.1, 2 Unfortunately, many future inpatient physicians may lack the training3 and confidence4 to correct this problem. One proposed model for improving the teaching, performance, and evaluation of bedside procedures is a procedure service that is staffed by faculty who are experts at inpatient procedures.5 In a recent survey of internal medicine residents from our hospital, 86% (30 of 35) believed that expert supervision would improve central venous catheterization technique (Trick WE, personal communication).
Primary considerations in the development of a procedure service are the baseline demand for bedside procedures and whether a procedure service may affect this demand. Though variations in population‐based rates of some hospital procedures have been described,6, 7 there is little written on the demand for procedures performed at the bedsides of inpatients. Concomitant increases in demand and availability of other technologies810 suggest that improving the availability of bedside procedures may lead to an increase in their demand, regardless of whether such an increase benefits patients.11
Therefore, we sought to determine the impact of a bedside procedure service on the baseline number of paracenteses, thoracenteses, lumbar punctures (LPs), and central venous catheterizations (CVCs) performed on general medicine inpatients at our teaching hospital. In addition, we examined whether this service leads to more successful and safe procedure attempts.
METHODS
Design and Setting
In this prospective cohort study, the cohort was all patients admitted to the general medicine service at Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, in January and February of 2006. The general medicine inpatient service is divided into 3 firms (A, B, and C), each with 4 separate teams of physicians and students. Admissions from the emergency department or other services in the hospital, such as intensive care units (which are closed and therefore staffed by separate teams of physicians), are distributed in sequence to on‐call teams from each firm. During the study period, the availability of a bedside procedure service varied by firm. Throughout the first 4 weeks, the service was available to only 1 of 3 firms (firm A). Then, during weeks 5 through 8, the service crossed over to the other 2 firms (firms B and C) and was unavailable to the original firm. Firm assignments for residents assigned to the inpatient service for all 8 weeks did not change. Of the 16 residents assigned to firm A during the first 4 weeks, when the procedure service was available, 3 remained on the wards during the second 4 weeks, when the procedure service was not available.
We chose to collect data on 4 bedside procedures: paracentesis, thoracentesis, LP, and CVC. Similar to those at other teaching hospitals, our residents informally acquire the skills to perform these procedures while assisting and being assisted by more experienced senior residents in a see one, do one, teach one apprenticeship model of learning.4 To improve the training and performance of these bedside procedures, the Department of Medicine piloted a bedside procedure service to teach procedural skills and assist residents during these procedures. Use of the service, though voluntary, was actively encouraged at residents' monthly orientation meetings and regular conferences.
One attending inpatient physician (J.A.) staffed the bedside procedure service, which was available during normal work hours on weekdays. Requests for procedures were made by general medicine residents through an online database and, after approval by the procedure service attending physician, were performed under his direct supervision. A hand‐carried ultrasound (MicroMaxx, Sonosite, Inc., Bothell, WA) that generates a 2‐dimensional gray‐scale image was used to both confirm the presence and location of fluid prior to paracentesis and thoracentesis and provide real‐time guidance during central venous catheterization. When the bedside procedure service was unavailable, residents performed bedside procedures in the usual fashion, typically without direct attending physician supervision. But if requested, an on‐call chief medical resident with access to a hand‐carried ultrasound device used by the intensive care unit was available for assistance at any time.
Subjects
The study subjects were all patients admitted to the general medical service during the 8‐week pilot period. Patients were excluded if they had been discharged before arrival on the medical wards or if they were under the care of the general medicine service for less than 6 hours before discharge or transfer to another service. We chose 6 hours because we reasoned that such brief admissions were not potential candidates for invasive bedside procedures.
Data Collection
Each morning an investigator contacted the senior residents who had admitted patients during the previous 24‐hour shift and confirmed that newly admitted patients were under the care of the general medicine service for more than 6 hours. To examine how the number of attempts may have been affected by procedures done in the emergency room or intensive care units before admission to the general medicine service, investigators also asked admitting residents whether a bedside procedure had been attempted in the 72 hours before admission. Every general medicine service resident was asked to fill out a brief data collection form after an attempt to perform any procedure on the general medical wards. In addition, chief residents asked each member of the general medicine service at mandatory sign‐out rounds at the end of each weekday whether any procedures had been attempted, and on weekend days investigators contacted senior residents from each general medicine service team.
We report on this quality assurance study, which was conducted during a pilot phase. This report has been reviewed and judged exempt by our institutional review board.
Primary OutcomeNumber of Procedure Attempts
For all bedside procedures attempted by residents on the general medical wards, investigators determined whether the residents were members of firms that were offered the bedside procedure service and, if so, whether the procedure service attending directly supervised the procedure attempt. Multiple procedure attempts of the same type were counted for an individual patient if (1) the procedure attempts did not occur during the same admissions and (2) neither the physicians attempting the procedure nor the primary indications for it were the same. Therefore, neither attempts performed after initially unsuccessful ones nor repeated procedures, such as large‐volume therapeutic paracentesis and thoracentesis, were counted twice. We reasoned that when these criteria were met, procedure attempts could be considered independently.
Secondary Outcomes
Investigators asked residents who attempted procedures to indicate whether (1) the indication for the procedure was solely diagnostic or was, at least in part, therapeutic; (2) the procedure was successful; and (3) there were any immediate major periprocedural complications. A procedure was considered to have been successfully performed if it fulfilled 2 criteria: it had to be completed during a single continuous attempt, even if multiple sites or procedure kits were used; and it had to fulfill the indication for it being done. For example, if the indication for thoracentesis was therapeutic, this procedure would be considered successful if it yielded a large enough volume of fluid to alleviate the patient's symptoms, but if the indication was diagnostic, then thoracentesis would be considered successful if it yielded enough fluid for laboratory processing. Residents were asked to report any periprocedural complications that they considered major; 2 illustrative examples were provided: a pneumothorax and severe bleeding.
Data Analyses
On the basis of earlier pilot data, we estimated that 8%10% of all admissions to the general medicine service underwent at least 1 procedure (paracentesis, thoracentesis, lumbar puncture, or central vein catheterization). We planned for a sample size of 1900 admissions, which would have 80% power to detect a clinically meaningful 50% relative increase in the mean number of bedside procedures with a double‐sided alpha error of 0.05. We used permutation tests to compare the mean number of procedures attempted between firms and bootstrap simulation to construct 95% confidence intervals for those means and the differences between and ratios of them. Fisher's exact test was used to compare proportions of successfully performed procedures and preadmission procedure attempts. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, LP, College Station, TX).
RESULTS
Subjects
During this 8‐week pilot study, there were 2157 admissions to the general medicine service. Among these admissions, 216 were excluded from our study because the patients did not arrive on the medical wards or were not under the care of the general medicine service for at least 6 hours before discharge or before being transferred to another service. Of the remaining 1941 admissions, 935 were to firms with the bedside procedure service available, and 1006 were to firms without the service available (Fig. 1)

Primary OutcomeNumber of Procedure Attempts
Overall, 122 patients underwent 145 procedure attempts that met our criteria for independence. The mean number of procedure attempts in firms offered the bedside procedure service was 48% higher (90 versus 61 per 1000 admissions; RR 1.48, 95% CI 1.062.10; P = .030; Fig. 1). When procedures attempted on weekends and holidays were excluded, the relative increase in procedure attempts in firms offered the bedside procedure service was even higher (70 versus 43 per 1000 admissions; RR 1.63, 95% CI 1.092.49; P = .023; Fig. 1). When grouped according to whether procedure attempts occurred before or after crossover of the procedure service, the mean number of procedure attempts in firms was higher when the service was offered: firm A dropped from 84 to 70 per 1000 admissions (P = .58) after losing the service, whereas firms B and C increased from 57 to 94 per 1000 admissions (P = .025) on gaining the service. There were 40 procedure attempts performed on patients within 72 hours before admission, but there was no difference between firms in the proportions of these preadmission procedures (P = .43).
Secondary Outcomes
Table 1 shows how of each type of procedure contributed to the overall difference. Attempts of CVC and therapeutic paracentesis and thoracentesis accounted for 86% of the overall increase in procedure attempts for admissions to firms offered the bedside procedure service, whereas only 14% of this increase was a result of diagnostic procedures. There were no differences in the proportions of successfully performed procedures, whether grouped by firm (P = 1.0) or by direct supervision from the procedure service attending (P = .64; Table 2). There were 3 self‐reported major periprocedural complications; all were related to excessive bleeding from CVC attempts. Two occurred without direct supervision from the bedside procedure service attending, one hemomediastinum from an internal jugular CVC attempt and one groin hematoma from a femoral CVC attempt. The third, a groin hematoma from a femoral CVC attempt, occurred during direct supervision from the bedside procedure service attending.
| Bedside procedure and indication | Firms with bedside procedure service 935 admissions | Firms with usual care 1006 admissions | Absolute rate difference (proportion of overall difference)* |
|---|---|---|---|
| Total for entire study (total for weekend days and holidays) | |||
| |||
| Total | 90 (19) | 61 (18) | 29 (100%) |
| Thoracentesis | 30 (10) | 18 (7) | 12 (41%) |
| Diagnosis | 9 (5) | 6 (2) | 3 (9%) |
| Treatment | 21 (4) | 12 (5) | 9 (32%) |
| Paracentesis | 32 (5) | 25 (6) | 7 (25%) |
| Diagnosis | 9 (1) | 11 (3) | 2 (8%) |
| Treatment | 24 (4) | 14 (3) | 10 (33%) |
| Central venous catheterization | 17 (3) | 11 (4) | 6 (21%) |
| Lumbar puncture | 11 (1) | 7 (1) | 4 (13%) |
| Diagnosis | 10 (1) | 6 (1) | 4 (13%) |
| Treatment | 1 (0) | 1 (0) | 0 (0%) |
| Admission to firm with | P value of difference in proportions | ||||||
|---|---|---|---|---|---|---|---|
| Procedure service available | Usual care | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| |||||||
| Central venous catheterization | 16 | 13 | 81 | 11 | 9 | 82 | 1.00 |
| Paracentesis, thoracentesis, or lumbar puncture | 68 | 54 | 79 | 50 | 40 | 80 | 1.00 |
| Total | 84 | 67 | 80 | 61 | 49 | 80 | 1.00 |
| Procedure service attending | Pvalue of difference in proportions | ||||||
| Directly supervised | Did not directly supervise | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| Central venous catheterization | 10 | 10 | 100 | 17 | 12 | 71 | 0.28 |
| Paracentesis, thoracentesis, or lumbar puncture | 40 | 33 | 83 | 78 | 61 | 78 | 0.12 |
| Total | 50 | 43 | 86 | 95 | 73 | 77 | 0.64 |
DISCUSSION
We found that the mean number of bedside procedures increased by 48% (95% CI, 6% to 110%) from 61 to 90 per 1000 general medicine admissions when firms were offered a bedside procedure service. This suggests that a procedure service may lead to an increase in the number of procedures performed. For example, in our hospital, where 12,500 patients are admitted annually to the general medical service, 365 additional procedures per year (95% CI, 45840) may be performed if a procedure service is available. Despite this potential increase in demand, we were unable to demonstrate a parallel increase in bedside procedure success, even when the procedure service attending was directly supervising residents (Table 2). Though our conclusions may not be applicable to other settings, this study is, to our knowledge, the first to describe the demand for bedside procedures performed on general medicine inpatients at an urban teaching hospital and the first to demonstrate that this demand increases with the availability of a procedure service.
Because 86% of the observed increase in procedure attempts was due to therapeutic indications (Table 1), most of the observed difference may be due to undertreatment in the usual care cohort, overtreatment in the bedside procedure service cohort, or a combination of both. However, our study was not designed to determine if patients were undertreated because we did not review the appropriateness of physicians' decisions to not attempt procedures. And even though the bedside procedure service attending physician prospectively confirmed the appropriateness of each procedure attempt in that cohort, we did not examine what physicians' baseline treatment thresholds were or if they were lowered by the availability of the bedside procedure service.11 In other words, we cannot claim that the observed increase in procedure attempts was indicated based on patients' clinical factors. Nevertheless, the observed increase supports the important idea that discrete physician‐level decisions, in this case, whether to perform a bedside procedure, may be affected by broader system‐wide adoptions of new technologies like our bedside procedure service.12 Other nonclinical factors not observed in our study, such as fee‐for‐service compensation and variable physician‐level diagnostic and therapeutic thresholds, may also affect the rate of bedside procedures.
Our study had several limitations. We studied only one group of patients at one hospital: admissions to physicians in different settings may have different rates of bedside procedures. Our study design was observational. However, the predetermined sequential allocation of admissions and the varied assignments of the bedside procedure service during the study period should have limited selection bias. Our identification of procedure attempts, particularly in the usual care group, relied on resident physicians' self‐reports, and we cannot exclude a reporting bias. However, we believe that the daily interactions between investigators and residents from each team on the general medicine service limited the number of procedure attempts that went unrecorded. Finally, though sufficiently powered to determine our primary outcome, our study was underpowered to confirm statistical differences between firms in proportions of successfully performed procedures. For example, approximately 400 additional procedures (or more than 5000 additional admissions) would have been needed to sufficiently power the observed 9% increase in successful attempts that we observed with direct supervision by the procedure service attending (77% versus 86%; P = .64; Table 2). Our current sample size may be adequate in future research if success rates diverge as the experience of the procedure service attending increases. Though expert in performing bedside procedures, he had limited experience teaching them, particularly with the use of a hand‐carried ultrasound device. Just as there is a learning curve to gain the experience to successfully perform procedures,13 so may there be a learning curve to successfully teach procedures.14
Future research could address these limitations by more closely observing the decision‐making processes of physicians who order bedside procedures for general medicine inpatients in various settings. Our findings suggest that although patients' clinical circumstances are likely the most important consideration, nonclinical factors may also affect physicians' decisions.12 Like other multifaceted decision‐making processes of physicians,15 the complexity of this decision is important to examine because, as our pilot data suggest, a procedure service may not lead to more successful procedure attempts or reductions in the number of major complications. Although the cumulative expertise of our service or the innovative methods of training of other institutions may improve the performance of bedside procedures,5, 13 physicians' decisions about whether to order them will remain paramount, because any improvement in procedural competence will do little to reduce the relative danger of unnecessary procedures16 or the missed benefit of procedures left undone. Physicians of inpatients17, 18 should refine the indications for and anticipated benefits from these commonly performed invasive procedures.
Inpatient bedside procedures are a major source of preventable adverse events in hospitals.1, 2 Unfortunately, many future inpatient physicians may lack the training3 and confidence4 to correct this problem. One proposed model for improving the teaching, performance, and evaluation of bedside procedures is a procedure service that is staffed by faculty who are experts at inpatient procedures.5 In a recent survey of internal medicine residents from our hospital, 86% (30 of 35) believed that expert supervision would improve central venous catheterization technique (Trick WE, personal communication).
Primary considerations in the development of a procedure service are the baseline demand for bedside procedures and whether a procedure service may affect this demand. Though variations in population‐based rates of some hospital procedures have been described,6, 7 there is little written on the demand for procedures performed at the bedsides of inpatients. Concomitant increases in demand and availability of other technologies810 suggest that improving the availability of bedside procedures may lead to an increase in their demand, regardless of whether such an increase benefits patients.11
Therefore, we sought to determine the impact of a bedside procedure service on the baseline number of paracenteses, thoracenteses, lumbar punctures (LPs), and central venous catheterizations (CVCs) performed on general medicine inpatients at our teaching hospital. In addition, we examined whether this service leads to more successful and safe procedure attempts.
METHODS
Design and Setting
In this prospective cohort study, the cohort was all patients admitted to the general medicine service at Cook County Hospital, a 500‐bed public teaching hospital in Chicago, Illinois, in January and February of 2006. The general medicine inpatient service is divided into 3 firms (A, B, and C), each with 4 separate teams of physicians and students. Admissions from the emergency department or other services in the hospital, such as intensive care units (which are closed and therefore staffed by separate teams of physicians), are distributed in sequence to on‐call teams from each firm. During the study period, the availability of a bedside procedure service varied by firm. Throughout the first 4 weeks, the service was available to only 1 of 3 firms (firm A). Then, during weeks 5 through 8, the service crossed over to the other 2 firms (firms B and C) and was unavailable to the original firm. Firm assignments for residents assigned to the inpatient service for all 8 weeks did not change. Of the 16 residents assigned to firm A during the first 4 weeks, when the procedure service was available, 3 remained on the wards during the second 4 weeks, when the procedure service was not available.
We chose to collect data on 4 bedside procedures: paracentesis, thoracentesis, LP, and CVC. Similar to those at other teaching hospitals, our residents informally acquire the skills to perform these procedures while assisting and being assisted by more experienced senior residents in a see one, do one, teach one apprenticeship model of learning.4 To improve the training and performance of these bedside procedures, the Department of Medicine piloted a bedside procedure service to teach procedural skills and assist residents during these procedures. Use of the service, though voluntary, was actively encouraged at residents' monthly orientation meetings and regular conferences.
One attending inpatient physician (J.A.) staffed the bedside procedure service, which was available during normal work hours on weekdays. Requests for procedures were made by general medicine residents through an online database and, after approval by the procedure service attending physician, were performed under his direct supervision. A hand‐carried ultrasound (MicroMaxx, Sonosite, Inc., Bothell, WA) that generates a 2‐dimensional gray‐scale image was used to both confirm the presence and location of fluid prior to paracentesis and thoracentesis and provide real‐time guidance during central venous catheterization. When the bedside procedure service was unavailable, residents performed bedside procedures in the usual fashion, typically without direct attending physician supervision. But if requested, an on‐call chief medical resident with access to a hand‐carried ultrasound device used by the intensive care unit was available for assistance at any time.
Subjects
The study subjects were all patients admitted to the general medical service during the 8‐week pilot period. Patients were excluded if they had been discharged before arrival on the medical wards or if they were under the care of the general medicine service for less than 6 hours before discharge or transfer to another service. We chose 6 hours because we reasoned that such brief admissions were not potential candidates for invasive bedside procedures.
Data Collection
Each morning an investigator contacted the senior residents who had admitted patients during the previous 24‐hour shift and confirmed that newly admitted patients were under the care of the general medicine service for more than 6 hours. To examine how the number of attempts may have been affected by procedures done in the emergency room or intensive care units before admission to the general medicine service, investigators also asked admitting residents whether a bedside procedure had been attempted in the 72 hours before admission. Every general medicine service resident was asked to fill out a brief data collection form after an attempt to perform any procedure on the general medical wards. In addition, chief residents asked each member of the general medicine service at mandatory sign‐out rounds at the end of each weekday whether any procedures had been attempted, and on weekend days investigators contacted senior residents from each general medicine service team.
We report on this quality assurance study, which was conducted during a pilot phase. This report has been reviewed and judged exempt by our institutional review board.
Primary OutcomeNumber of Procedure Attempts
For all bedside procedures attempted by residents on the general medical wards, investigators determined whether the residents were members of firms that were offered the bedside procedure service and, if so, whether the procedure service attending directly supervised the procedure attempt. Multiple procedure attempts of the same type were counted for an individual patient if (1) the procedure attempts did not occur during the same admissions and (2) neither the physicians attempting the procedure nor the primary indications for it were the same. Therefore, neither attempts performed after initially unsuccessful ones nor repeated procedures, such as large‐volume therapeutic paracentesis and thoracentesis, were counted twice. We reasoned that when these criteria were met, procedure attempts could be considered independently.
Secondary Outcomes
Investigators asked residents who attempted procedures to indicate whether (1) the indication for the procedure was solely diagnostic or was, at least in part, therapeutic; (2) the procedure was successful; and (3) there were any immediate major periprocedural complications. A procedure was considered to have been successfully performed if it fulfilled 2 criteria: it had to be completed during a single continuous attempt, even if multiple sites or procedure kits were used; and it had to fulfill the indication for it being done. For example, if the indication for thoracentesis was therapeutic, this procedure would be considered successful if it yielded a large enough volume of fluid to alleviate the patient's symptoms, but if the indication was diagnostic, then thoracentesis would be considered successful if it yielded enough fluid for laboratory processing. Residents were asked to report any periprocedural complications that they considered major; 2 illustrative examples were provided: a pneumothorax and severe bleeding.
Data Analyses
On the basis of earlier pilot data, we estimated that 8%10% of all admissions to the general medicine service underwent at least 1 procedure (paracentesis, thoracentesis, lumbar puncture, or central vein catheterization). We planned for a sample size of 1900 admissions, which would have 80% power to detect a clinically meaningful 50% relative increase in the mean number of bedside procedures with a double‐sided alpha error of 0.05. We used permutation tests to compare the mean number of procedures attempted between firms and bootstrap simulation to construct 95% confidence intervals for those means and the differences between and ratios of them. Fisher's exact test was used to compare proportions of successfully performed procedures and preadmission procedure attempts. All analyses were conducted with Stata Statistical Software, Release 9 (StataCorp, LP, College Station, TX).
RESULTS
Subjects
During this 8‐week pilot study, there were 2157 admissions to the general medicine service. Among these admissions, 216 were excluded from our study because the patients did not arrive on the medical wards or were not under the care of the general medicine service for at least 6 hours before discharge or before being transferred to another service. Of the remaining 1941 admissions, 935 were to firms with the bedside procedure service available, and 1006 were to firms without the service available (Fig. 1)

Primary OutcomeNumber of Procedure Attempts
Overall, 122 patients underwent 145 procedure attempts that met our criteria for independence. The mean number of procedure attempts in firms offered the bedside procedure service was 48% higher (90 versus 61 per 1000 admissions; RR 1.48, 95% CI 1.062.10; P = .030; Fig. 1). When procedures attempted on weekends and holidays were excluded, the relative increase in procedure attempts in firms offered the bedside procedure service was even higher (70 versus 43 per 1000 admissions; RR 1.63, 95% CI 1.092.49; P = .023; Fig. 1). When grouped according to whether procedure attempts occurred before or after crossover of the procedure service, the mean number of procedure attempts in firms was higher when the service was offered: firm A dropped from 84 to 70 per 1000 admissions (P = .58) after losing the service, whereas firms B and C increased from 57 to 94 per 1000 admissions (P = .025) on gaining the service. There were 40 procedure attempts performed on patients within 72 hours before admission, but there was no difference between firms in the proportions of these preadmission procedures (P = .43).
Secondary Outcomes
Table 1 shows how of each type of procedure contributed to the overall difference. Attempts of CVC and therapeutic paracentesis and thoracentesis accounted for 86% of the overall increase in procedure attempts for admissions to firms offered the bedside procedure service, whereas only 14% of this increase was a result of diagnostic procedures. There were no differences in the proportions of successfully performed procedures, whether grouped by firm (P = 1.0) or by direct supervision from the procedure service attending (P = .64; Table 2). There were 3 self‐reported major periprocedural complications; all were related to excessive bleeding from CVC attempts. Two occurred without direct supervision from the bedside procedure service attending, one hemomediastinum from an internal jugular CVC attempt and one groin hematoma from a femoral CVC attempt. The third, a groin hematoma from a femoral CVC attempt, occurred during direct supervision from the bedside procedure service attending.
| Bedside procedure and indication | Firms with bedside procedure service 935 admissions | Firms with usual care 1006 admissions | Absolute rate difference (proportion of overall difference)* |
|---|---|---|---|
| Total for entire study (total for weekend days and holidays) | |||
| |||
| Total | 90 (19) | 61 (18) | 29 (100%) |
| Thoracentesis | 30 (10) | 18 (7) | 12 (41%) |
| Diagnosis | 9 (5) | 6 (2) | 3 (9%) |
| Treatment | 21 (4) | 12 (5) | 9 (32%) |
| Paracentesis | 32 (5) | 25 (6) | 7 (25%) |
| Diagnosis | 9 (1) | 11 (3) | 2 (8%) |
| Treatment | 24 (4) | 14 (3) | 10 (33%) |
| Central venous catheterization | 17 (3) | 11 (4) | 6 (21%) |
| Lumbar puncture | 11 (1) | 7 (1) | 4 (13%) |
| Diagnosis | 10 (1) | 6 (1) | 4 (13%) |
| Treatment | 1 (0) | 1 (0) | 0 (0%) |
| Admission to firm with | P value of difference in proportions | ||||||
|---|---|---|---|---|---|---|---|
| Procedure service available | Usual care | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| |||||||
| Central venous catheterization | 16 | 13 | 81 | 11 | 9 | 82 | 1.00 |
| Paracentesis, thoracentesis, or lumbar puncture | 68 | 54 | 79 | 50 | 40 | 80 | 1.00 |
| Total | 84 | 67 | 80 | 61 | 49 | 80 | 1.00 |
| Procedure service attending | Pvalue of difference in proportions | ||||||
| Directly supervised | Did not directly supervise | ||||||
| Total attempts (n) | Successful | Total attempts (n) | Successful | ||||
| n | % | n | % | ||||
| Central venous catheterization | 10 | 10 | 100 | 17 | 12 | 71 | 0.28 |
| Paracentesis, thoracentesis, or lumbar puncture | 40 | 33 | 83 | 78 | 61 | 78 | 0.12 |
| Total | 50 | 43 | 86 | 95 | 73 | 77 | 0.64 |
DISCUSSION
We found that the mean number of bedside procedures increased by 48% (95% CI, 6% to 110%) from 61 to 90 per 1000 general medicine admissions when firms were offered a bedside procedure service. This suggests that a procedure service may lead to an increase in the number of procedures performed. For example, in our hospital, where 12,500 patients are admitted annually to the general medical service, 365 additional procedures per year (95% CI, 45840) may be performed if a procedure service is available. Despite this potential increase in demand, we were unable to demonstrate a parallel increase in bedside procedure success, even when the procedure service attending was directly supervising residents (Table 2). Though our conclusions may not be applicable to other settings, this study is, to our knowledge, the first to describe the demand for bedside procedures performed on general medicine inpatients at an urban teaching hospital and the first to demonstrate that this demand increases with the availability of a procedure service.
Because 86% of the observed increase in procedure attempts was due to therapeutic indications (Table 1), most of the observed difference may be due to undertreatment in the usual care cohort, overtreatment in the bedside procedure service cohort, or a combination of both. However, our study was not designed to determine if patients were undertreated because we did not review the appropriateness of physicians' decisions to not attempt procedures. And even though the bedside procedure service attending physician prospectively confirmed the appropriateness of each procedure attempt in that cohort, we did not examine what physicians' baseline treatment thresholds were or if they were lowered by the availability of the bedside procedure service.11 In other words, we cannot claim that the observed increase in procedure attempts was indicated based on patients' clinical factors. Nevertheless, the observed increase supports the important idea that discrete physician‐level decisions, in this case, whether to perform a bedside procedure, may be affected by broader system‐wide adoptions of new technologies like our bedside procedure service.12 Other nonclinical factors not observed in our study, such as fee‐for‐service compensation and variable physician‐level diagnostic and therapeutic thresholds, may also affect the rate of bedside procedures.
Our study had several limitations. We studied only one group of patients at one hospital: admissions to physicians in different settings may have different rates of bedside procedures. Our study design was observational. However, the predetermined sequential allocation of admissions and the varied assignments of the bedside procedure service during the study period should have limited selection bias. Our identification of procedure attempts, particularly in the usual care group, relied on resident physicians' self‐reports, and we cannot exclude a reporting bias. However, we believe that the daily interactions between investigators and residents from each team on the general medicine service limited the number of procedure attempts that went unrecorded. Finally, though sufficiently powered to determine our primary outcome, our study was underpowered to confirm statistical differences between firms in proportions of successfully performed procedures. For example, approximately 400 additional procedures (or more than 5000 additional admissions) would have been needed to sufficiently power the observed 9% increase in successful attempts that we observed with direct supervision by the procedure service attending (77% versus 86%; P = .64; Table 2). Our current sample size may be adequate in future research if success rates diverge as the experience of the procedure service attending increases. Though expert in performing bedside procedures, he had limited experience teaching them, particularly with the use of a hand‐carried ultrasound device. Just as there is a learning curve to gain the experience to successfully perform procedures,13 so may there be a learning curve to successfully teach procedures.14
Future research could address these limitations by more closely observing the decision‐making processes of physicians who order bedside procedures for general medicine inpatients in various settings. Our findings suggest that although patients' clinical circumstances are likely the most important consideration, nonclinical factors may also affect physicians' decisions.12 Like other multifaceted decision‐making processes of physicians,15 the complexity of this decision is important to examine because, as our pilot data suggest, a procedure service may not lead to more successful procedure attempts or reductions in the number of major complications. Although the cumulative expertise of our service or the innovative methods of training of other institutions may improve the performance of bedside procedures,5, 13 physicians' decisions about whether to order them will remain paramount, because any improvement in procedural competence will do little to reduce the relative danger of unnecessary procedures16 or the missed benefit of procedures left undone. Physicians of inpatients17, 18 should refine the indications for and anticipated benefits from these commonly performed invasive procedures.
- ,,, et al.The nature of adverse events in hospitalized patients: Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,, et al.Cost of medical injuries in Utah and Colorado.Inquiry.36;255–264.
- ,,,.Procedural Skills Training in Internal Medicine Residencies: A Survey of Program Directors.Ann Intern Med1989;111:932–38.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures.Am J Med.2006;119:71.e17–.e24.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Variation in the use of cardiac procedures after acute myocardial infarction.N Engl J Med.1995;333:573–578.
- ,,.Frequency and morbidity of inpatient procedures: report of a pilot study from two teaching hospitals.Arch Intern Med.1978;138:1809–1811.
- ,.The impact of diagnostic testing on therapeutic interventions.JAMA.1996;275:1189–1191.
- ,,, et al.Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- ,,, et al.Coronary artery bypass graft surgery in Ontario and New York State: which rate is right?Ann Intern Med.1997;126:13–19.
- ,.Avoiding the unintended consequences of growth in medical care. How might more be worse?JAMA.1999;281:446–453.
- ,,.Professional uncertainty and the problem of supplier‐induced demand.Soc Sci Med.1982;811–824.
- ,,,.A curricular initiative for internal medicine residents to enhance proficiency in internal jugular central venous line placement.Mayo Clin Proc.2005;80:212–218.
- ,,, et al.Confidence of Academic General Internists and Family Physicians to Teach Ambulatory Procedures.J Gen Intern Med.2000;15:353–360.
- ,,, et al.The impact of evidence on physicians' inpatient treatment decisions.J Gen Intern Med.2004;19:402–409.
- .Medical care—is more always better?N Engl J Med.2003;349:1665–1667.
- ,.Point/counterpoint: should hospital medicine become a distinct specialty?Hospitalist.2005;9(1):15–19.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hospital Med.2006;1:S1–S95.
- ,,, et al.The nature of adverse events in hospitalized patients: Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,, et al.Cost of medical injuries in Utah and Colorado.Inquiry.36;255–264.
- ,,,.Procedural Skills Training in Internal Medicine Residencies: A Survey of Program Directors.Ann Intern Med1989;111:932–38.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medicine procedures.Am J Med.2006;119:71.e17–.e24.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Variation in the use of cardiac procedures after acute myocardial infarction.N Engl J Med.1995;333:573–578.
- ,,.Frequency and morbidity of inpatient procedures: report of a pilot study from two teaching hospitals.Arch Intern Med.1978;138:1809–1811.
- ,.The impact of diagnostic testing on therapeutic interventions.JAMA.1996;275:1189–1191.
- ,,, et al.Does increased access to primary care reduce hospital readmissions?N Engl J Med.1996;334:1441–1447.
- ,,, et al.Coronary artery bypass graft surgery in Ontario and New York State: which rate is right?Ann Intern Med.1997;126:13–19.
- ,.Avoiding the unintended consequences of growth in medical care. How might more be worse?JAMA.1999;281:446–453.
- ,,.Professional uncertainty and the problem of supplier‐induced demand.Soc Sci Med.1982;811–824.
- ,,,.A curricular initiative for internal medicine residents to enhance proficiency in internal jugular central venous line placement.Mayo Clin Proc.2005;80:212–218.
- ,,, et al.Confidence of Academic General Internists and Family Physicians to Teach Ambulatory Procedures.J Gen Intern Med.2000;15:353–360.
- ,,, et al.The impact of evidence on physicians' inpatient treatment decisions.J Gen Intern Med.2004;19:402–409.
- .Medical care—is more always better?N Engl J Med.2003;349:1665–1667.
- ,.Point/counterpoint: should hospital medicine become a distinct specialty?Hospitalist.2005;9(1):15–19.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hospital Med.2006;1:S1–S95.
Copyright © 2007 Society of Hospital Medicine