User login
Lichenoid dermatitis with mycosis fungoides features linked to checkpoint inhibitor therapy
A patient treated with immune checkpoint inhibitor therapy for thyroid carcinoma presented with lichenoid dermatitis that resembled mycosis fungoides and also showed with monoclonal T-cell receptor gene rearrangement.
A case report published in the Journal of Cutaneous Pathology describes the “unusual” case and highlights another form of lichenoid dermatitis that may occur with immune checkpoint inhibitor therapy.
The patient was a 66-year-old man with BRAFV600E-mutated anaplastic thyroid carcinoma, who was enrolled in a clinical trial of the checkpoint inhibitor atezolizumab, in combination with the BRAF inhibitor vemurafenib and MEK inhibitor cobimetinib.
Around 11 months after starting treatment, he presented to a dermatology department with a 1.5-cm x 1.2-cm crusted, erythematous plaque on his abdomen that had appeared 3 weeks earlier. He also had several follicular-based erythematous papules on his extremities and a single verrucous papule on his index finger, which the authors wrote were likely associated with the vemurafenib therapy.
The patient had previously had a squamous cell carcinoma removed but had no history of cutaneous lymphoma.
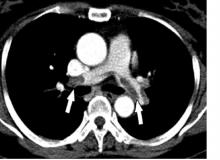
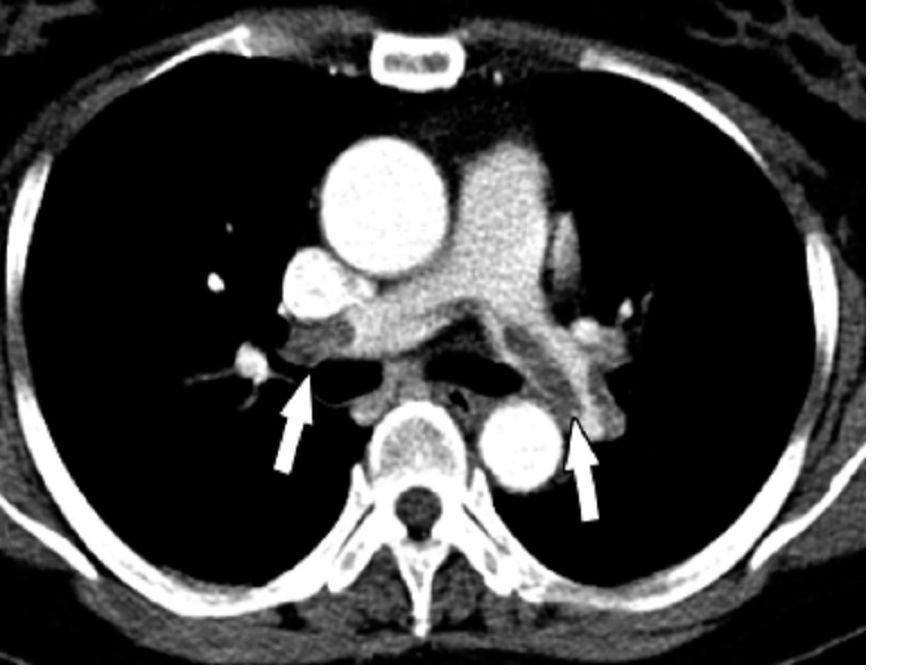
A punch biopsy of the abdominal lesion showed dense lichenoid, lymphohistiocytic infiltrate with papillary dermal fibrosis, and scattered multinucleated giant histiocytes. Immunohistochemical studies showed the lesion had an abnormal immunophenotype in which CD4+ cells were four to five times more common than CD8+ cells, and there was partial loss of CD7 expression.
The lesion was treated with 0.05% clobetasol cream and monitored without interrupting the cancer therapy. The lesion gradually reduced in size, but 4 months later, another lesion appeared on the patient’s right clavicle.
A skin biopsy revealed lichenoid lymphohistiocytic infiltrate with occasional giant cells in the superficial dermis, as well as atypical, hyperchromatic lymphocytes with clear halos. Immunohistochemical studies showed that the new lesion was similar to the earlier abdominal lesion.
T-cell receptor gene rearrangement studies on both lesions showed that the abdominal lesion had both monoclonal TCR-gamma and TCR-beta gene rearrangements. The clavicle lesion showed the same monoclonal TCR-gamma rearrangement as the abdominal lesion, but lacked the TCR-beta gene rearrangement.
The lesions continued to be treated with clobetasol cream (0.05%), and the patient remained on the anticancer treatment regimen.
Michael T. Tetzlaff, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and coauthors wrote that up to half of all patients treated with immune checkpoint inhibitors develop some kind of cutaneous immune-related adverse event, and lichenoid dermatitis is one of the most common seen in biopsies.
“Clinical and pathological recognition of monoclonal [lichenoid dermatitis associated with immune checkpoint inhibitors] in the context of [immune checkpoint inhibitor] therapy will be important for accurate diagnosis and patient care,” they wrote.
The researchers did not report financial disclosures.
SOURCE: Tetzlaff MT et al. J Cutan Pathol. 2019 Jun 29. doi: 10.1111/cup.13536.
A patient treated with immune checkpoint inhibitor therapy for thyroid carcinoma presented with lichenoid dermatitis that resembled mycosis fungoides and also showed with monoclonal T-cell receptor gene rearrangement.
A case report published in the Journal of Cutaneous Pathology describes the “unusual” case and highlights another form of lichenoid dermatitis that may occur with immune checkpoint inhibitor therapy.
The patient was a 66-year-old man with BRAFV600E-mutated anaplastic thyroid carcinoma, who was enrolled in a clinical trial of the checkpoint inhibitor atezolizumab, in combination with the BRAF inhibitor vemurafenib and MEK inhibitor cobimetinib.
Around 11 months after starting treatment, he presented to a dermatology department with a 1.5-cm x 1.2-cm crusted, erythematous plaque on his abdomen that had appeared 3 weeks earlier. He also had several follicular-based erythematous papules on his extremities and a single verrucous papule on his index finger, which the authors wrote were likely associated with the vemurafenib therapy.
The patient had previously had a squamous cell carcinoma removed but had no history of cutaneous lymphoma.
A punch biopsy of the abdominal lesion showed dense lichenoid, lymphohistiocytic infiltrate with papillary dermal fibrosis, and scattered multinucleated giant histiocytes. Immunohistochemical studies showed the lesion had an abnormal immunophenotype in which CD4+ cells were four to five times more common than CD8+ cells, and there was partial loss of CD7 expression.
The lesion was treated with 0.05% clobetasol cream and monitored without interrupting the cancer therapy. The lesion gradually reduced in size, but 4 months later, another lesion appeared on the patient’s right clavicle.
A skin biopsy revealed lichenoid lymphohistiocytic infiltrate with occasional giant cells in the superficial dermis, as well as atypical, hyperchromatic lymphocytes with clear halos. Immunohistochemical studies showed that the new lesion was similar to the earlier abdominal lesion.
T-cell receptor gene rearrangement studies on both lesions showed that the abdominal lesion had both monoclonal TCR-gamma and TCR-beta gene rearrangements. The clavicle lesion showed the same monoclonal TCR-gamma rearrangement as the abdominal lesion, but lacked the TCR-beta gene rearrangement.
The lesions continued to be treated with clobetasol cream (0.05%), and the patient remained on the anticancer treatment regimen.
Michael T. Tetzlaff, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and coauthors wrote that up to half of all patients treated with immune checkpoint inhibitors develop some kind of cutaneous immune-related adverse event, and lichenoid dermatitis is one of the most common seen in biopsies.
“Clinical and pathological recognition of monoclonal [lichenoid dermatitis associated with immune checkpoint inhibitors] in the context of [immune checkpoint inhibitor] therapy will be important for accurate diagnosis and patient care,” they wrote.
The researchers did not report financial disclosures.
SOURCE: Tetzlaff MT et al. J Cutan Pathol. 2019 Jun 29. doi: 10.1111/cup.13536.
A patient treated with immune checkpoint inhibitor therapy for thyroid carcinoma presented with lichenoid dermatitis that resembled mycosis fungoides and also showed with monoclonal T-cell receptor gene rearrangement.
A case report published in the Journal of Cutaneous Pathology describes the “unusual” case and highlights another form of lichenoid dermatitis that may occur with immune checkpoint inhibitor therapy.
The patient was a 66-year-old man with BRAFV600E-mutated anaplastic thyroid carcinoma, who was enrolled in a clinical trial of the checkpoint inhibitor atezolizumab, in combination with the BRAF inhibitor vemurafenib and MEK inhibitor cobimetinib.
Around 11 months after starting treatment, he presented to a dermatology department with a 1.5-cm x 1.2-cm crusted, erythematous plaque on his abdomen that had appeared 3 weeks earlier. He also had several follicular-based erythematous papules on his extremities and a single verrucous papule on his index finger, which the authors wrote were likely associated with the vemurafenib therapy.
The patient had previously had a squamous cell carcinoma removed but had no history of cutaneous lymphoma.
A punch biopsy of the abdominal lesion showed dense lichenoid, lymphohistiocytic infiltrate with papillary dermal fibrosis, and scattered multinucleated giant histiocytes. Immunohistochemical studies showed the lesion had an abnormal immunophenotype in which CD4+ cells were four to five times more common than CD8+ cells, and there was partial loss of CD7 expression.
The lesion was treated with 0.05% clobetasol cream and monitored without interrupting the cancer therapy. The lesion gradually reduced in size, but 4 months later, another lesion appeared on the patient’s right clavicle.
A skin biopsy revealed lichenoid lymphohistiocytic infiltrate with occasional giant cells in the superficial dermis, as well as atypical, hyperchromatic lymphocytes with clear halos. Immunohistochemical studies showed that the new lesion was similar to the earlier abdominal lesion.
T-cell receptor gene rearrangement studies on both lesions showed that the abdominal lesion had both monoclonal TCR-gamma and TCR-beta gene rearrangements. The clavicle lesion showed the same monoclonal TCR-gamma rearrangement as the abdominal lesion, but lacked the TCR-beta gene rearrangement.
The lesions continued to be treated with clobetasol cream (0.05%), and the patient remained on the anticancer treatment regimen.
Michael T. Tetzlaff, MD, PhD, of the University of Texas MD Anderson Cancer Center, Houston, and coauthors wrote that up to half of all patients treated with immune checkpoint inhibitors develop some kind of cutaneous immune-related adverse event, and lichenoid dermatitis is one of the most common seen in biopsies.
“Clinical and pathological recognition of monoclonal [lichenoid dermatitis associated with immune checkpoint inhibitors] in the context of [immune checkpoint inhibitor] therapy will be important for accurate diagnosis and patient care,” they wrote.
The researchers did not report financial disclosures.
SOURCE: Tetzlaff MT et al. J Cutan Pathol. 2019 Jun 29. doi: 10.1111/cup.13536.
FROM THE JOURNAL OF CUTANEOUS PATHOLOGY
Key clinical point:
Major finding: Checkpoint inhibitor therapy can trigger lichenoid dermatitis with mycosis fungoides–like features.
Study details: A case report involving a 66-year-old man with BRAFV600E-mutated anaplastic thyroid carcinoma.
Disclosures: The researchers did not report financial disclosures.
Source: Tetzlaff MT et al. J Cutan Pathol. 2019 Jun 29. doi: 10.1111/cup.13536.
Increased cardiovascular risk seen early on with testosterone replacement therapy
Testosterone replacement therapy is associated with an increased risk of cardiovascular and cerebrovascular events in older men, particularly during the first 2 years of use, a study in the American Journal of Medicine has found.
Simone Y. Loo of the Lady Davis Institute for Medical Research at the Jewish General Hospital in Montreal and colleagues looked at a cohort of 15,401 men aged 45 years or older with low testosterone levels, of whom 4,485 (29.1%) were prescribed testosterone replacement therapy on at least one occasion during a mean follow-up of 4.7 years. They saw that individuals who were currently using testosterone replacement therapy had a 21% increase in the risk of the composite outcome of ischemic stroke, transient ischemic attack, or myocardial infarction, compared with those who had not had hormone therapy.
In the first 6 months to 2 years after initiation of continuous treatment, the risk was even higher – at 35% – and was particularly high in individuals aged 45-59 years (at 44%).
However, the study also noted a significant 36% reduction in the risk of all-cause mortality in individuals currently using testosterone replacement therapy and a significant 28% higher risk of all-cause mortality in past users, compared with nonusers.
Concerns about the safety of testosterone replacement therapy had previously been kindled by the outcomes of the Testosterone in Older Men trial, which was stopped early because of the higher number of cardiovascular events in the treatment group. However, other randomized controlled trials have not seen that effect, the authors said.
They noted that the protective effect of current hormone replacement use on mortality was surprising, but suggested it could be the result of reverse causality, “in which physicians may discontinue TRT based on perceived deterioration of health or imminent death, because TRT is not a vital medication.”
“Moreover, TRT may be less frequently initiated among men with a higher baseline risk of mortality, particularly in the elderly, and those who received TRT may have been healthier overall, compared with their untreated counterparts,” the authors wrote.
Despite this, they suggested that larger observational studies were still needed to investigate the potential harms of testosterone replacement therapy. In the meantime, they advised that potential harms should be weighed against perceived benefits and caution be applied to prescribing.
The study was supported by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Loo S et al. Am J Med 2019 Apr 3. doi: 10.1016/j.amjmed.2019.03.022.
Testosterone replacement therapy is associated with an increased risk of cardiovascular and cerebrovascular events in older men, particularly during the first 2 years of use, a study in the American Journal of Medicine has found.
Simone Y. Loo of the Lady Davis Institute for Medical Research at the Jewish General Hospital in Montreal and colleagues looked at a cohort of 15,401 men aged 45 years or older with low testosterone levels, of whom 4,485 (29.1%) were prescribed testosterone replacement therapy on at least one occasion during a mean follow-up of 4.7 years. They saw that individuals who were currently using testosterone replacement therapy had a 21% increase in the risk of the composite outcome of ischemic stroke, transient ischemic attack, or myocardial infarction, compared with those who had not had hormone therapy.
In the first 6 months to 2 years after initiation of continuous treatment, the risk was even higher – at 35% – and was particularly high in individuals aged 45-59 years (at 44%).
However, the study also noted a significant 36% reduction in the risk of all-cause mortality in individuals currently using testosterone replacement therapy and a significant 28% higher risk of all-cause mortality in past users, compared with nonusers.
Concerns about the safety of testosterone replacement therapy had previously been kindled by the outcomes of the Testosterone in Older Men trial, which was stopped early because of the higher number of cardiovascular events in the treatment group. However, other randomized controlled trials have not seen that effect, the authors said.
They noted that the protective effect of current hormone replacement use on mortality was surprising, but suggested it could be the result of reverse causality, “in which physicians may discontinue TRT based on perceived deterioration of health or imminent death, because TRT is not a vital medication.”
“Moreover, TRT may be less frequently initiated among men with a higher baseline risk of mortality, particularly in the elderly, and those who received TRT may have been healthier overall, compared with their untreated counterparts,” the authors wrote.
Despite this, they suggested that larger observational studies were still needed to investigate the potential harms of testosterone replacement therapy. In the meantime, they advised that potential harms should be weighed against perceived benefits and caution be applied to prescribing.
The study was supported by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Loo S et al. Am J Med 2019 Apr 3. doi: 10.1016/j.amjmed.2019.03.022.
Testosterone replacement therapy is associated with an increased risk of cardiovascular and cerebrovascular events in older men, particularly during the first 2 years of use, a study in the American Journal of Medicine has found.
Simone Y. Loo of the Lady Davis Institute for Medical Research at the Jewish General Hospital in Montreal and colleagues looked at a cohort of 15,401 men aged 45 years or older with low testosterone levels, of whom 4,485 (29.1%) were prescribed testosterone replacement therapy on at least one occasion during a mean follow-up of 4.7 years. They saw that individuals who were currently using testosterone replacement therapy had a 21% increase in the risk of the composite outcome of ischemic stroke, transient ischemic attack, or myocardial infarction, compared with those who had not had hormone therapy.
In the first 6 months to 2 years after initiation of continuous treatment, the risk was even higher – at 35% – and was particularly high in individuals aged 45-59 years (at 44%).
However, the study also noted a significant 36% reduction in the risk of all-cause mortality in individuals currently using testosterone replacement therapy and a significant 28% higher risk of all-cause mortality in past users, compared with nonusers.
Concerns about the safety of testosterone replacement therapy had previously been kindled by the outcomes of the Testosterone in Older Men trial, which was stopped early because of the higher number of cardiovascular events in the treatment group. However, other randomized controlled trials have not seen that effect, the authors said.
They noted that the protective effect of current hormone replacement use on mortality was surprising, but suggested it could be the result of reverse causality, “in which physicians may discontinue TRT based on perceived deterioration of health or imminent death, because TRT is not a vital medication.”
“Moreover, TRT may be less frequently initiated among men with a higher baseline risk of mortality, particularly in the elderly, and those who received TRT may have been healthier overall, compared with their untreated counterparts,” the authors wrote.
Despite this, they suggested that larger observational studies were still needed to investigate the potential harms of testosterone replacement therapy. In the meantime, they advised that potential harms should be weighed against perceived benefits and caution be applied to prescribing.
The study was supported by the Canadian Institutes of Health Research. No conflicts of interest were reported.
SOURCE: Loo S et al. Am J Med 2019 Apr 3. doi: 10.1016/j.amjmed.2019.03.022.
FROM THE AMERICAN JOURNAL OF MEDICINE
Air pollution levels correlated with cardiorespiratory mortality, reduced life expectancy
particularly among lower-income populations, research suggests.
A study published in PLOS Medicine used vital registration and population data from across the United States for 1999-2015 to estimate the number of deaths and loss of life expectancy associated with four different models of concentrations of fine particulate matter pollution, and examine how that has changed over time.
While the current national ambient air quality standard for particle pollution is 12 mcg/m3 in almost all counties, the study found that in 1999, 59% of the 1,339 county units had concentrations above this level. At that time, the population-weighted average fine particulate matter pollution concentration for the entire country was 13.6mcg/m3. The highest level was seen in Fresno county in California, which had a fine particulate pollution concentration of 22.1 mcg/m3.
By 2015, national concentrations had declined to 8.0 mcg/m3, and the lowest observed concentration was 2.8 mcg/m3.
The investigators wrote, “Each model was applied to county-level cardiorespiratory death rates separately by sex and age group (5-year age groups from birth to 85 years and 85 years and older) because death rates vary by age group and sex, as might their associations with air pollution. From each model we estimated age-specific proportional increases in death rates (i.e. rate ratios) for each 1 mcg/m3 of PM2.5 [fine particulate matter].” The analysis revealed that fine particulate matter pollution above the lowest observed concentration of 2.8 mcg/m3 was associated with higher death rates from cardiorespiratory diseases.
Overall, researchers estimated that these higher levels contributed to 15,612 deaths from cardiorespiratory diseases in women and 14,757 deaths in men, representing 2.8% and 2.7% of all cardiorespiratory deaths, respectively. This amounted to 0.15 years of life expectancy lost in women and 0.13 years lost in men.
There was significant variation in the cost to life expectancy around the country. In the midwestern and Rocky Mountain counties in states such as New Mexico, Colorado, and Arizona, which had lower levels of air pollution, life expectancy loss was less than 0.05 years. But in southern states where the air pollution levels were highest, such as Arkansas, Oklahoma, Alabama, and around Los Angeles, the life expectancy loss was greater than 0.3 years.
“While current PM2.5 pollution is responsible for a significant mortality burden and loss of longevity, reductions in pollution since the late 1990s have benefited virtually the entire country, with the exception of 14 counties where PM2.5 increased slightly over this period,” wrote James E. Bennett, PhD, of the School of Public Health at Imperial College London and coauthors.
The primary limitation of the study is that this association between air pollution and cardiorespiratory health or life expectancy cannot be shown to be causal. Other pollutants and other environmental and behavioral factors that impact cardiorespiratory health may be significant. For example, including ozone and nitrogen dioxide levels in the models could result in different results in terms of the impact of PM2.5 on cardiorespiratory health.
The data highlighted that life expectancy loss associated with air pollution was larger in lower-income counties, those where a higher proportion of the population had a family income below the poverty line, and those where a higher proportion of the population were black or African American.
“This inequality in mortality burden occurs because lower-income counties, those with more poverty, with a greater proportion who are of black or African American race, or with a lower proportion who have graduated high school tend to have higher baseline death rates at any pollution level because of conditions associated with these covariates and hence experience a larger absolute number of deaths as a result of air pollution,” the authors wrote.
The study was funded by the U.S. Environmental Protection Agency and the Wellcome Trust. One author declared grants and personal fees from private industry, outside the submitted work.
SOURCE: Bennett JE et al. PLoS Med. 2019 Jul 23. doi: 10.1371/journal.pmed.1002856.
particularly among lower-income populations, research suggests.
A study published in PLOS Medicine used vital registration and population data from across the United States for 1999-2015 to estimate the number of deaths and loss of life expectancy associated with four different models of concentrations of fine particulate matter pollution, and examine how that has changed over time.
While the current national ambient air quality standard for particle pollution is 12 mcg/m3 in almost all counties, the study found that in 1999, 59% of the 1,339 county units had concentrations above this level. At that time, the population-weighted average fine particulate matter pollution concentration for the entire country was 13.6mcg/m3. The highest level was seen in Fresno county in California, which had a fine particulate pollution concentration of 22.1 mcg/m3.
By 2015, national concentrations had declined to 8.0 mcg/m3, and the lowest observed concentration was 2.8 mcg/m3.
The investigators wrote, “Each model was applied to county-level cardiorespiratory death rates separately by sex and age group (5-year age groups from birth to 85 years and 85 years and older) because death rates vary by age group and sex, as might their associations with air pollution. From each model we estimated age-specific proportional increases in death rates (i.e. rate ratios) for each 1 mcg/m3 of PM2.5 [fine particulate matter].” The analysis revealed that fine particulate matter pollution above the lowest observed concentration of 2.8 mcg/m3 was associated with higher death rates from cardiorespiratory diseases.
Overall, researchers estimated that these higher levels contributed to 15,612 deaths from cardiorespiratory diseases in women and 14,757 deaths in men, representing 2.8% and 2.7% of all cardiorespiratory deaths, respectively. This amounted to 0.15 years of life expectancy lost in women and 0.13 years lost in men.
There was significant variation in the cost to life expectancy around the country. In the midwestern and Rocky Mountain counties in states such as New Mexico, Colorado, and Arizona, which had lower levels of air pollution, life expectancy loss was less than 0.05 years. But in southern states where the air pollution levels were highest, such as Arkansas, Oklahoma, Alabama, and around Los Angeles, the life expectancy loss was greater than 0.3 years.
“While current PM2.5 pollution is responsible for a significant mortality burden and loss of longevity, reductions in pollution since the late 1990s have benefited virtually the entire country, with the exception of 14 counties where PM2.5 increased slightly over this period,” wrote James E. Bennett, PhD, of the School of Public Health at Imperial College London and coauthors.
The primary limitation of the study is that this association between air pollution and cardiorespiratory health or life expectancy cannot be shown to be causal. Other pollutants and other environmental and behavioral factors that impact cardiorespiratory health may be significant. For example, including ozone and nitrogen dioxide levels in the models could result in different results in terms of the impact of PM2.5 on cardiorespiratory health.
The data highlighted that life expectancy loss associated with air pollution was larger in lower-income counties, those where a higher proportion of the population had a family income below the poverty line, and those where a higher proportion of the population were black or African American.
“This inequality in mortality burden occurs because lower-income counties, those with more poverty, with a greater proportion who are of black or African American race, or with a lower proportion who have graduated high school tend to have higher baseline death rates at any pollution level because of conditions associated with these covariates and hence experience a larger absolute number of deaths as a result of air pollution,” the authors wrote.
The study was funded by the U.S. Environmental Protection Agency and the Wellcome Trust. One author declared grants and personal fees from private industry, outside the submitted work.
SOURCE: Bennett JE et al. PLoS Med. 2019 Jul 23. doi: 10.1371/journal.pmed.1002856.
particularly among lower-income populations, research suggests.
A study published in PLOS Medicine used vital registration and population data from across the United States for 1999-2015 to estimate the number of deaths and loss of life expectancy associated with four different models of concentrations of fine particulate matter pollution, and examine how that has changed over time.
While the current national ambient air quality standard for particle pollution is 12 mcg/m3 in almost all counties, the study found that in 1999, 59% of the 1,339 county units had concentrations above this level. At that time, the population-weighted average fine particulate matter pollution concentration for the entire country was 13.6mcg/m3. The highest level was seen in Fresno county in California, which had a fine particulate pollution concentration of 22.1 mcg/m3.
By 2015, national concentrations had declined to 8.0 mcg/m3, and the lowest observed concentration was 2.8 mcg/m3.
The investigators wrote, “Each model was applied to county-level cardiorespiratory death rates separately by sex and age group (5-year age groups from birth to 85 years and 85 years and older) because death rates vary by age group and sex, as might their associations with air pollution. From each model we estimated age-specific proportional increases in death rates (i.e. rate ratios) for each 1 mcg/m3 of PM2.5 [fine particulate matter].” The analysis revealed that fine particulate matter pollution above the lowest observed concentration of 2.8 mcg/m3 was associated with higher death rates from cardiorespiratory diseases.
Overall, researchers estimated that these higher levels contributed to 15,612 deaths from cardiorespiratory diseases in women and 14,757 deaths in men, representing 2.8% and 2.7% of all cardiorespiratory deaths, respectively. This amounted to 0.15 years of life expectancy lost in women and 0.13 years lost in men.
There was significant variation in the cost to life expectancy around the country. In the midwestern and Rocky Mountain counties in states such as New Mexico, Colorado, and Arizona, which had lower levels of air pollution, life expectancy loss was less than 0.05 years. But in southern states where the air pollution levels were highest, such as Arkansas, Oklahoma, Alabama, and around Los Angeles, the life expectancy loss was greater than 0.3 years.
“While current PM2.5 pollution is responsible for a significant mortality burden and loss of longevity, reductions in pollution since the late 1990s have benefited virtually the entire country, with the exception of 14 counties where PM2.5 increased slightly over this period,” wrote James E. Bennett, PhD, of the School of Public Health at Imperial College London and coauthors.
The primary limitation of the study is that this association between air pollution and cardiorespiratory health or life expectancy cannot be shown to be causal. Other pollutants and other environmental and behavioral factors that impact cardiorespiratory health may be significant. For example, including ozone and nitrogen dioxide levels in the models could result in different results in terms of the impact of PM2.5 on cardiorespiratory health.
The data highlighted that life expectancy loss associated with air pollution was larger in lower-income counties, those where a higher proportion of the population had a family income below the poverty line, and those where a higher proportion of the population were black or African American.
“This inequality in mortality burden occurs because lower-income counties, those with more poverty, with a greater proportion who are of black or African American race, or with a lower proportion who have graduated high school tend to have higher baseline death rates at any pollution level because of conditions associated with these covariates and hence experience a larger absolute number of deaths as a result of air pollution,” the authors wrote.
The study was funded by the U.S. Environmental Protection Agency and the Wellcome Trust. One author declared grants and personal fees from private industry, outside the submitted work.
SOURCE: Bennett JE et al. PLoS Med. 2019 Jul 23. doi: 10.1371/journal.pmed.1002856.
FROM PLOS MEDICINE
Key clinical point: Air pollution linked to reduced life expectancy, particularly in poorer areas.
Major finding: Fine particulate matter air pollution linked to 0.15 years of life expectancy lost in women and 0.13 years lost in men.
Study details: Population-wide data analysis for the United States.
Disclosures: The study was funded by the U.S. Environmental Protection Agency and the Wellcome Trust. One author declared grants and personal fees from private industry outside the submitted work.
Source: Bennett JE et al. PLoS Med. 2019 Jul 23. doi: 10.1371/journal.pmed.1002856.
Emicizumab follow-up shows further bleeding declines
MELBOURNE – according to data presented at the International Society on Thrombosis and Haemostasis congress.
Michael Callaghan, MD, of the Children’s Hospital of Michigan, Detroit, reported on a pooled analysis of data from 399 patients with hemophilia A who were treated with emicizumab (Hemlibra) for a median duration of 83.1 weeks, representing 650 patient-years of exposure. The studies included pediatric and adult patients, both with and without factor VIII inhibitors.
Patients enrolled in the studies had a median of eight bleeds in the 24 weeks before enrollment, but in the first 24 weeks of treatment with emicizumab, the mean annualized bleed rate dropped to 1.9. During weeks 25-48, this dropped further to 0.8, remained at that level in weeks 49-72, then declined further to 0.3 during weeks 73-96.
During the first 24 weeks of treatment, 70.8% of patients experienced zero bleeds, and 22.5% experienced 1-3 bleeds. By week 96, the number of patients experiencing zero bleeds had increased to 88.6% and nearly 100% of patients had had fewer than three bleeds during that 24-week period.
The study also reported on target joint bleeds and showed the mean annualized bleed rate in target joints decreased from 1.4 in the first 24 weeks of treatment to 0.3 in weeks 73-96, by which time 90.4% of patients reported no target joint bleeds at all. Overall, 99.2% of target joints resolved, which was defined as two or fewer spontaneous bleeding events into a target joint in a year.
“The bleed rate seemed to converge on a low number, suggesting that maybe patients that came with preexisting synovitis or inflamed joints improved over time to resemble the patients who had better joint health at the beginning of the study,” Dr. Callaghan said.
The long-term follow-up did not reveal any major safety concerns. The most common drug-related adverse event was injection site reactions, which just over one-quarter of patients reported. The main serious adverse events were bleeding related.
“With any biologic agent, we were concerned about antidrug antibodies,” Dr. Callaghan told the conference. “At this follow-up point, less than 1% of patients treated with emicizumab in this group have had neutralizing antidrug antibodies.” Most of these antibodies were detected with routine screening, but there was one patient with antidrug antibodies who developed breakthrough bleeding during the study.
In an interview, Dr. Callaghan said emicizumab was “game-changing” therapy, and that the data showed it was efficacious even long term. However, he said there were still some questions to be answered about which patients were most likely to benefit.
“How early do we start this? Do we put previously untreated patients on this, and if we do, how do we expose them to factor VIII?” he said. Other challenging questions are whether to do immune tolerance induction for patients with factor VIII inhibitors and how the drug would work for other patient groups, such as those with comorbidities or who were very active.
The study was sponsored by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Callaghan declared consultancies, grants, clinical trial involvement, speakers bureau engagements, and shares with the pharmaceutical sector.
SOURCE: Callaghan M et al. 2019 ISTH Congress, Abstract OC 60.2.
MELBOURNE – according to data presented at the International Society on Thrombosis and Haemostasis congress.
Michael Callaghan, MD, of the Children’s Hospital of Michigan, Detroit, reported on a pooled analysis of data from 399 patients with hemophilia A who were treated with emicizumab (Hemlibra) for a median duration of 83.1 weeks, representing 650 patient-years of exposure. The studies included pediatric and adult patients, both with and without factor VIII inhibitors.
Patients enrolled in the studies had a median of eight bleeds in the 24 weeks before enrollment, but in the first 24 weeks of treatment with emicizumab, the mean annualized bleed rate dropped to 1.9. During weeks 25-48, this dropped further to 0.8, remained at that level in weeks 49-72, then declined further to 0.3 during weeks 73-96.
During the first 24 weeks of treatment, 70.8% of patients experienced zero bleeds, and 22.5% experienced 1-3 bleeds. By week 96, the number of patients experiencing zero bleeds had increased to 88.6% and nearly 100% of patients had had fewer than three bleeds during that 24-week period.
The study also reported on target joint bleeds and showed the mean annualized bleed rate in target joints decreased from 1.4 in the first 24 weeks of treatment to 0.3 in weeks 73-96, by which time 90.4% of patients reported no target joint bleeds at all. Overall, 99.2% of target joints resolved, which was defined as two or fewer spontaneous bleeding events into a target joint in a year.
“The bleed rate seemed to converge on a low number, suggesting that maybe patients that came with preexisting synovitis or inflamed joints improved over time to resemble the patients who had better joint health at the beginning of the study,” Dr. Callaghan said.
The long-term follow-up did not reveal any major safety concerns. The most common drug-related adverse event was injection site reactions, which just over one-quarter of patients reported. The main serious adverse events were bleeding related.
“With any biologic agent, we were concerned about antidrug antibodies,” Dr. Callaghan told the conference. “At this follow-up point, less than 1% of patients treated with emicizumab in this group have had neutralizing antidrug antibodies.” Most of these antibodies were detected with routine screening, but there was one patient with antidrug antibodies who developed breakthrough bleeding during the study.
In an interview, Dr. Callaghan said emicizumab was “game-changing” therapy, and that the data showed it was efficacious even long term. However, he said there were still some questions to be answered about which patients were most likely to benefit.
“How early do we start this? Do we put previously untreated patients on this, and if we do, how do we expose them to factor VIII?” he said. Other challenging questions are whether to do immune tolerance induction for patients with factor VIII inhibitors and how the drug would work for other patient groups, such as those with comorbidities or who were very active.
The study was sponsored by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Callaghan declared consultancies, grants, clinical trial involvement, speakers bureau engagements, and shares with the pharmaceutical sector.
SOURCE: Callaghan M et al. 2019 ISTH Congress, Abstract OC 60.2.
MELBOURNE – according to data presented at the International Society on Thrombosis and Haemostasis congress.
Michael Callaghan, MD, of the Children’s Hospital of Michigan, Detroit, reported on a pooled analysis of data from 399 patients with hemophilia A who were treated with emicizumab (Hemlibra) for a median duration of 83.1 weeks, representing 650 patient-years of exposure. The studies included pediatric and adult patients, both with and without factor VIII inhibitors.
Patients enrolled in the studies had a median of eight bleeds in the 24 weeks before enrollment, but in the first 24 weeks of treatment with emicizumab, the mean annualized bleed rate dropped to 1.9. During weeks 25-48, this dropped further to 0.8, remained at that level in weeks 49-72, then declined further to 0.3 during weeks 73-96.
During the first 24 weeks of treatment, 70.8% of patients experienced zero bleeds, and 22.5% experienced 1-3 bleeds. By week 96, the number of patients experiencing zero bleeds had increased to 88.6% and nearly 100% of patients had had fewer than three bleeds during that 24-week period.
The study also reported on target joint bleeds and showed the mean annualized bleed rate in target joints decreased from 1.4 in the first 24 weeks of treatment to 0.3 in weeks 73-96, by which time 90.4% of patients reported no target joint bleeds at all. Overall, 99.2% of target joints resolved, which was defined as two or fewer spontaneous bleeding events into a target joint in a year.
“The bleed rate seemed to converge on a low number, suggesting that maybe patients that came with preexisting synovitis or inflamed joints improved over time to resemble the patients who had better joint health at the beginning of the study,” Dr. Callaghan said.
The long-term follow-up did not reveal any major safety concerns. The most common drug-related adverse event was injection site reactions, which just over one-quarter of patients reported. The main serious adverse events were bleeding related.
“With any biologic agent, we were concerned about antidrug antibodies,” Dr. Callaghan told the conference. “At this follow-up point, less than 1% of patients treated with emicizumab in this group have had neutralizing antidrug antibodies.” Most of these antibodies were detected with routine screening, but there was one patient with antidrug antibodies who developed breakthrough bleeding during the study.
In an interview, Dr. Callaghan said emicizumab was “game-changing” therapy, and that the data showed it was efficacious even long term. However, he said there were still some questions to be answered about which patients were most likely to benefit.
“How early do we start this? Do we put previously untreated patients on this, and if we do, how do we expose them to factor VIII?” he said. Other challenging questions are whether to do immune tolerance induction for patients with factor VIII inhibitors and how the drug would work for other patient groups, such as those with comorbidities or who were very active.
The study was sponsored by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Callaghan declared consultancies, grants, clinical trial involvement, speakers bureau engagements, and shares with the pharmaceutical sector.
SOURCE: Callaghan M et al. 2019 ISTH Congress, Abstract OC 60.2.
REPORTING FROM 2019 ISTH CONGRESS
Minor surgeries appear safe for hemophilia patients on emicizumab
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
MELBOURNE – A majority of minor surgeries can be performed in hemophilia A patients receiving emicizumab therapy without requiring prophylactic treatment with coagulation factors, according to data presented at the International Society on Thrombosis and Haemostasis congress.
Elena Santagostino, MD, PhD, from the Hemophilia and Thrombosis Center at Ospedale Maggiore Policlinico in Milan presented data from 399 patients involved in the four HAVEN trials of the humanized bispecific monoclonal antibody emicizumab (Hemlibra), which is Food and Drug Administration–approved for the prevention of bleeding episodes in individuals with hemophilia A, with or without inhibitors.
The analysis focused on the 126 patients (31.6%) who underwent at least one surgical procedure during the studies. Of the 233 surgeries, there were 215 minor procedures performed in 115 patients, and 18 major surgeries in 18 patients. All patients were receiving ongoing treatment with emicizumab, and there was no change to that treatment regimen during surgery.
“It is clear that surgery is a challenge for hemophilia,” Dr. Santagostino said. “It is a challenge for bleeding, it is a challenge for thrombosis, it is a challenge for any new drug, and this is why there is a lot of interest around this topic.”
Overall, 65.6% of minor surgeries were performed without any prophylactic coagulation factor treatment, and 90.8% of minor surgeries were conducted without postoperative bleeds requiring treatment. There were no cases of thrombosis reported.
The surgeries that did not require prophylactic coagulation factor included 42 dental procedures, 25 central venous access devices, 17 endoscopic procedures, and 12 joint procedures.
While the HAVEN studies did not allow for elective major surgery, there were still 18 unplanned major surgical situations that arose during the course of the studies. These included three hip, one knee, and one ankle arthroplasties; three synovectomies; and some dental, central venous line, and endoscopic biopsy procedures.
Of these, 15 involved prophylactic coagulant factor administration, but three procedures – including one synovectomy – were performed without prophylaxis and none resulted in a bleed.
There was one complicated bleed that occurred in a patient undergoing multiple procedures including a synovectomy, joint debridement and chondroplasty, who received prolonged treatment with recombinant Factor VIIa.
Dr. Santagostino said the findings showed surgery could be safely performed in patients who were being treated with emicizumab, both with and without inhibitors.
“A large number of minor procedures can be done without adding coagulation factors,” she said in an interview. “This is true for less invasive surgeries, such as catheter-related central venous line procedures. Even several endoscopic procedures, like a single biopsy, can be done reasonably safely.”
However she said there was still a lack of experience in dealing with hemophilia A patients who were undergoing cancer surgery, or who had significant comorbidities that might put them at higher risk of thrombosis.
“These are special patients populations that are still not investigated in the trial setting,” she said.
Commenting on the data, session cochair Liane Khoo, MD, from the Haemophilia Treatment Centre at Royal Prince Alfred Hospital in Sydney, said the results showed surgery could be performed in hemophilia A patients with and without inhibitors.
“The more we have the medication and the more experience we have, then we become more confident in using it,” she said.
The study was funded by F. Hoffman-La Roche and Chugai Pharmaceutical. Dr. Santagostino reported consultancies and speakers bureau engagements with the pharmaceutical sector.
SOURCE: Santagostino E et al. 2019 ISTH Congress, Abstract OC 60.1.
REPORTING FROM 2019 ISTH CONGRESS
Once-weekly teriparatide still achieves bone mineral density gains
Once-weekly subcutaneous injections of the osteoporosis drug teriparatide still achieve increases in bone mineral density, according to a postmarketing observational study published online in Osteoporosis and Sarcopenia.
Teriparatide is widely used as a daily, self-injection formula for osteoporosis, but in Japan, a once-weekly injectable formulation of 56.5 ug is also being used in individuals with osteoporosis who are at high risk of fracture.
In a study of 3,573 Japanese patients with osteoporosis, investigators found increases of 2.8%, 4.9%, and 6.1% in lumbar spine bone mineral density measured at 24, 48, and 72 weeks respectively. In the femoral neck, bone mineral density increased by 1.6%, 1.4%, and 2.5% at 24, 48, and 72 weeks, and total hip bone mineral density increased by 1%, 1.6%, and 2.5%.
At 24 weeks, the median percent change from baseline in the level of serum bone formation marker procollagen type I N-terminal propeptide increased 23%, and then decreased to a 4.3% median change at 48 weeks and 8.7% at 72 weeks. There were no significant changes in serum bone-type alkaline phosphatase by 48 and 72 weeks, and no changes at all in the bone turnover markers tartrate-resistant acid phosphate-5b and cross-linked N-terminal telopeptide of type I collagen.
Researchers also saw reductions in low back pain scores at all the time points, although the authors noted that the mechanism of this association was not well understood and needed further study.
“The results for efficacy parameters, including fracture incidences, in this surveillance were as expected based on the clinical studies prior to approval, indicating that the medical benefits of teriparatide were demonstrated in actual clinical practice after marketing,” wrote Dr. Emiko Ifuku and colleagues from Asahi Kasei Pharma, which manufactures the drug in Japan.
The study also looked at adherence to the once-weekly therapy, and found that 59.4% of patients were still taking the treatment at 24 weeks, and 39% were taking it at 72 weeks.
Around a quarter of patients experienced adverse reactions, with the most common being nausea (12.3%), vomiting (2.8%), headache (2.7%), and dizziness (2.2%) and most occurring within 24 weeks of starting treatment. Serious adverse reactions were reported in 26 patients (0.7%).
Asahi Kasei Pharma sponsored the study. All of the authors were employees of the company.
SOURCE: Ifuku E et al. Osteoporos Sarcopenia. 2019 Jun 26. doi: 10.1016/j.afos.2019.06.002.
Once-weekly subcutaneous injections of the osteoporosis drug teriparatide still achieve increases in bone mineral density, according to a postmarketing observational study published online in Osteoporosis and Sarcopenia.
Teriparatide is widely used as a daily, self-injection formula for osteoporosis, but in Japan, a once-weekly injectable formulation of 56.5 ug is also being used in individuals with osteoporosis who are at high risk of fracture.
In a study of 3,573 Japanese patients with osteoporosis, investigators found increases of 2.8%, 4.9%, and 6.1% in lumbar spine bone mineral density measured at 24, 48, and 72 weeks respectively. In the femoral neck, bone mineral density increased by 1.6%, 1.4%, and 2.5% at 24, 48, and 72 weeks, and total hip bone mineral density increased by 1%, 1.6%, and 2.5%.
At 24 weeks, the median percent change from baseline in the level of serum bone formation marker procollagen type I N-terminal propeptide increased 23%, and then decreased to a 4.3% median change at 48 weeks and 8.7% at 72 weeks. There were no significant changes in serum bone-type alkaline phosphatase by 48 and 72 weeks, and no changes at all in the bone turnover markers tartrate-resistant acid phosphate-5b and cross-linked N-terminal telopeptide of type I collagen.
Researchers also saw reductions in low back pain scores at all the time points, although the authors noted that the mechanism of this association was not well understood and needed further study.
“The results for efficacy parameters, including fracture incidences, in this surveillance were as expected based on the clinical studies prior to approval, indicating that the medical benefits of teriparatide were demonstrated in actual clinical practice after marketing,” wrote Dr. Emiko Ifuku and colleagues from Asahi Kasei Pharma, which manufactures the drug in Japan.
The study also looked at adherence to the once-weekly therapy, and found that 59.4% of patients were still taking the treatment at 24 weeks, and 39% were taking it at 72 weeks.
Around a quarter of patients experienced adverse reactions, with the most common being nausea (12.3%), vomiting (2.8%), headache (2.7%), and dizziness (2.2%) and most occurring within 24 weeks of starting treatment. Serious adverse reactions were reported in 26 patients (0.7%).
Asahi Kasei Pharma sponsored the study. All of the authors were employees of the company.
SOURCE: Ifuku E et al. Osteoporos Sarcopenia. 2019 Jun 26. doi: 10.1016/j.afos.2019.06.002.
Once-weekly subcutaneous injections of the osteoporosis drug teriparatide still achieve increases in bone mineral density, according to a postmarketing observational study published online in Osteoporosis and Sarcopenia.
Teriparatide is widely used as a daily, self-injection formula for osteoporosis, but in Japan, a once-weekly injectable formulation of 56.5 ug is also being used in individuals with osteoporosis who are at high risk of fracture.
In a study of 3,573 Japanese patients with osteoporosis, investigators found increases of 2.8%, 4.9%, and 6.1% in lumbar spine bone mineral density measured at 24, 48, and 72 weeks respectively. In the femoral neck, bone mineral density increased by 1.6%, 1.4%, and 2.5% at 24, 48, and 72 weeks, and total hip bone mineral density increased by 1%, 1.6%, and 2.5%.
At 24 weeks, the median percent change from baseline in the level of serum bone formation marker procollagen type I N-terminal propeptide increased 23%, and then decreased to a 4.3% median change at 48 weeks and 8.7% at 72 weeks. There were no significant changes in serum bone-type alkaline phosphatase by 48 and 72 weeks, and no changes at all in the bone turnover markers tartrate-resistant acid phosphate-5b and cross-linked N-terminal telopeptide of type I collagen.
Researchers also saw reductions in low back pain scores at all the time points, although the authors noted that the mechanism of this association was not well understood and needed further study.
“The results for efficacy parameters, including fracture incidences, in this surveillance were as expected based on the clinical studies prior to approval, indicating that the medical benefits of teriparatide were demonstrated in actual clinical practice after marketing,” wrote Dr. Emiko Ifuku and colleagues from Asahi Kasei Pharma, which manufactures the drug in Japan.
The study also looked at adherence to the once-weekly therapy, and found that 59.4% of patients were still taking the treatment at 24 weeks, and 39% were taking it at 72 weeks.
Around a quarter of patients experienced adverse reactions, with the most common being nausea (12.3%), vomiting (2.8%), headache (2.7%), and dizziness (2.2%) and most occurring within 24 weeks of starting treatment. Serious adverse reactions were reported in 26 patients (0.7%).
Asahi Kasei Pharma sponsored the study. All of the authors were employees of the company.
SOURCE: Ifuku E et al. Osteoporos Sarcopenia. 2019 Jun 26. doi: 10.1016/j.afos.2019.06.002.
FROM OSTEOPOROSIS AND SARCOPENIA
Risk of atrial fibrillation 900% higher with cancer
MELBOURNE – The overall prevalence of atrial fibrillation in people who have or have had cancer is 10 times that of individuals without cancer, according to a study presented at the International Society on Thrombosis and Haemostasis congress.
Cihan Ay, MD, of the division of hematology and hemostaseology at the Medical University of Vienna reported on a nationwide cohort study using health insurance data from more than 8.3 million people in Austria, including roughly 159,000 with a diagnosis of cancer and 113,000 with a diagnosis of atrial fibrillation.
The analysis found that, in individuals whose records showed a diagnosis of cancer, there was a 950% higher relative risk of also having a diagnosis of atrial fibrillation, compared with those with no cancer diagnosis.
The overall prevalence of atrial fibrillation among individuals with a cancer diagnosis was 9.8%, compared with 1.2% in those without cancer.
There was significant variation in relative risk according to age. Although the prevalence of atrial fibrillation increased with age, the highest relative risks were seen in the youngest age groups.
In those aged 12 years or under with a cancer diagnosis, the relative risk of atrial fibrillation was 150 times greater than in those without cancer, and in those aged 13-18 years, it was 200 times higher. At the other end of the age spectrum, individuals aged 70-79 years with a recorded cancer diagnosis, the relative risk of atrial fibrillation was still 130% higher than the noncancer population, and in those aged 80-90 years it was a significant 54% higher.
However, the analysis did not find any effect of gender on the risk of atrial fibrillation associated with cancer, regardless of the age group.
Researchers also examined the influence of different cancer types. They found the highest relative risk of atrial fibrillation was in persons with hematologic malignancies – at nine times the risk in the noncancer population – and the lowest was in the endocrine cancer patients, who had three times the risk.
Dr. Ay told the conference that the association between cancer and atrial fibrillation had been suggested in the literature, but it was still an unexplored field. “The exact magnitude of this association between cancer and atrial fibrillation is still unclear.”
There was also the question of what mechanisms might underlie the association. Dr. Ay pointed out that the health insurance database did not allow researchers to explore the temporal relationship between the two diagnoses, and therefore could not tell which came first.
One audience member queried whether the fact that cancer patients were likely to be visiting a clinician more frequently might mean that the atrial fibrillation would be more likely to be diagnosed.
To that, Dr. Ay suggested the significantly higher relative risk in children was supportive of the notion that cancer itself, or treatment effects, were influencing atrial fibrillation risk.
“There is evidence suggesting that cancer treatments are triggering atrial fibrillation,” he said in an interview. “Also, patients with cancer have situations of in which they are sick – they have neutropenia or sepsis and so on – which can also trigger atrial fibrillation.”
Given the limitations of the retrospective cohort study, Dr. Ay said he was hoping to do a prospective study that would enable baseline measurements of cancer patients to determine how much of the atrial fibrillation was preexisting.
“We have also more and more cancer survivors, and over the years they’re living longer and the likelihood of getting atrial fibrillation increases,” he added.
Commenting on the data, Gerald Soff, MD, chief of hematology at the Memorial Sloan Kettering Cancer Center in New York, said it was very important to quantify the association between cancer and atrial fibrillation.
“What’s striking to me is how many people with cancer come in with preexisting atrial fibrillation,” he said. “It could be that they have cancer and they’re already messed up, but we have, on a given day, several people coming in with newly diagnosed cancers, already on warfarin or apixaban or rivaroxaban because they have atrial fibrillation.”
Dr. Ay reported advisory board positions and speaking engagements for the pharmaceutical sector.
MELBOURNE – The overall prevalence of atrial fibrillation in people who have or have had cancer is 10 times that of individuals without cancer, according to a study presented at the International Society on Thrombosis and Haemostasis congress.
Cihan Ay, MD, of the division of hematology and hemostaseology at the Medical University of Vienna reported on a nationwide cohort study using health insurance data from more than 8.3 million people in Austria, including roughly 159,000 with a diagnosis of cancer and 113,000 with a diagnosis of atrial fibrillation.
The analysis found that, in individuals whose records showed a diagnosis of cancer, there was a 950% higher relative risk of also having a diagnosis of atrial fibrillation, compared with those with no cancer diagnosis.
The overall prevalence of atrial fibrillation among individuals with a cancer diagnosis was 9.8%, compared with 1.2% in those without cancer.
There was significant variation in relative risk according to age. Although the prevalence of atrial fibrillation increased with age, the highest relative risks were seen in the youngest age groups.
In those aged 12 years or under with a cancer diagnosis, the relative risk of atrial fibrillation was 150 times greater than in those without cancer, and in those aged 13-18 years, it was 200 times higher. At the other end of the age spectrum, individuals aged 70-79 years with a recorded cancer diagnosis, the relative risk of atrial fibrillation was still 130% higher than the noncancer population, and in those aged 80-90 years it was a significant 54% higher.
However, the analysis did not find any effect of gender on the risk of atrial fibrillation associated with cancer, regardless of the age group.
Researchers also examined the influence of different cancer types. They found the highest relative risk of atrial fibrillation was in persons with hematologic malignancies – at nine times the risk in the noncancer population – and the lowest was in the endocrine cancer patients, who had three times the risk.
Dr. Ay told the conference that the association between cancer and atrial fibrillation had been suggested in the literature, but it was still an unexplored field. “The exact magnitude of this association between cancer and atrial fibrillation is still unclear.”
There was also the question of what mechanisms might underlie the association. Dr. Ay pointed out that the health insurance database did not allow researchers to explore the temporal relationship between the two diagnoses, and therefore could not tell which came first.
One audience member queried whether the fact that cancer patients were likely to be visiting a clinician more frequently might mean that the atrial fibrillation would be more likely to be diagnosed.
To that, Dr. Ay suggested the significantly higher relative risk in children was supportive of the notion that cancer itself, or treatment effects, were influencing atrial fibrillation risk.
“There is evidence suggesting that cancer treatments are triggering atrial fibrillation,” he said in an interview. “Also, patients with cancer have situations of in which they are sick – they have neutropenia or sepsis and so on – which can also trigger atrial fibrillation.”
Given the limitations of the retrospective cohort study, Dr. Ay said he was hoping to do a prospective study that would enable baseline measurements of cancer patients to determine how much of the atrial fibrillation was preexisting.
“We have also more and more cancer survivors, and over the years they’re living longer and the likelihood of getting atrial fibrillation increases,” he added.
Commenting on the data, Gerald Soff, MD, chief of hematology at the Memorial Sloan Kettering Cancer Center in New York, said it was very important to quantify the association between cancer and atrial fibrillation.
“What’s striking to me is how many people with cancer come in with preexisting atrial fibrillation,” he said. “It could be that they have cancer and they’re already messed up, but we have, on a given day, several people coming in with newly diagnosed cancers, already on warfarin or apixaban or rivaroxaban because they have atrial fibrillation.”
Dr. Ay reported advisory board positions and speaking engagements for the pharmaceutical sector.
MELBOURNE – The overall prevalence of atrial fibrillation in people who have or have had cancer is 10 times that of individuals without cancer, according to a study presented at the International Society on Thrombosis and Haemostasis congress.
Cihan Ay, MD, of the division of hematology and hemostaseology at the Medical University of Vienna reported on a nationwide cohort study using health insurance data from more than 8.3 million people in Austria, including roughly 159,000 with a diagnosis of cancer and 113,000 with a diagnosis of atrial fibrillation.
The analysis found that, in individuals whose records showed a diagnosis of cancer, there was a 950% higher relative risk of also having a diagnosis of atrial fibrillation, compared with those with no cancer diagnosis.
The overall prevalence of atrial fibrillation among individuals with a cancer diagnosis was 9.8%, compared with 1.2% in those without cancer.
There was significant variation in relative risk according to age. Although the prevalence of atrial fibrillation increased with age, the highest relative risks were seen in the youngest age groups.
In those aged 12 years or under with a cancer diagnosis, the relative risk of atrial fibrillation was 150 times greater than in those without cancer, and in those aged 13-18 years, it was 200 times higher. At the other end of the age spectrum, individuals aged 70-79 years with a recorded cancer diagnosis, the relative risk of atrial fibrillation was still 130% higher than the noncancer population, and in those aged 80-90 years it was a significant 54% higher.
However, the analysis did not find any effect of gender on the risk of atrial fibrillation associated with cancer, regardless of the age group.
Researchers also examined the influence of different cancer types. They found the highest relative risk of atrial fibrillation was in persons with hematologic malignancies – at nine times the risk in the noncancer population – and the lowest was in the endocrine cancer patients, who had three times the risk.
Dr. Ay told the conference that the association between cancer and atrial fibrillation had been suggested in the literature, but it was still an unexplored field. “The exact magnitude of this association between cancer and atrial fibrillation is still unclear.”
There was also the question of what mechanisms might underlie the association. Dr. Ay pointed out that the health insurance database did not allow researchers to explore the temporal relationship between the two diagnoses, and therefore could not tell which came first.
One audience member queried whether the fact that cancer patients were likely to be visiting a clinician more frequently might mean that the atrial fibrillation would be more likely to be diagnosed.
To that, Dr. Ay suggested the significantly higher relative risk in children was supportive of the notion that cancer itself, or treatment effects, were influencing atrial fibrillation risk.
“There is evidence suggesting that cancer treatments are triggering atrial fibrillation,” he said in an interview. “Also, patients with cancer have situations of in which they are sick – they have neutropenia or sepsis and so on – which can also trigger atrial fibrillation.”
Given the limitations of the retrospective cohort study, Dr. Ay said he was hoping to do a prospective study that would enable baseline measurements of cancer patients to determine how much of the atrial fibrillation was preexisting.
“We have also more and more cancer survivors, and over the years they’re living longer and the likelihood of getting atrial fibrillation increases,” he added.
Commenting on the data, Gerald Soff, MD, chief of hematology at the Memorial Sloan Kettering Cancer Center in New York, said it was very important to quantify the association between cancer and atrial fibrillation.
“What’s striking to me is how many people with cancer come in with preexisting atrial fibrillation,” he said. “It could be that they have cancer and they’re already messed up, but we have, on a given day, several people coming in with newly diagnosed cancers, already on warfarin or apixaban or rivaroxaban because they have atrial fibrillation.”
Dr. Ay reported advisory board positions and speaking engagements for the pharmaceutical sector.
REPORTING FROM 2019 ISTH CONGRESS
Subcutaneous marstacimab appears safe in hemophilia A and B
MELBOURNE – Subcutaneous administration of the monoclonal antibody marstacimab (PF-06741086) provides significant reductions in bleeding rates for patients with hemophilia A and B, with reasonably safety and tolerability, according to research presented at the International Society on Thrombosis and Haemostasis congress.
Johnny Mahlangu, MBBCh, of the University of Witwatersrand in Johannesburg, South Africa, presented data from a multicenter, international phase 1B/2 open-label study involving 26 patients with severe hemophilia, who had experienced at least six acute bleeding episodes in the 6 months prior to enrollment. Twenty-three patients had hemophilia A, the remaining three patients had hemophilia B, and all were receiving on-demand treatment.
Patients were divided into one of four cohorts. Cohort 1 received a weekly 300-mg dose subcutaneously for 30 days, cohort 2 received a 300-mg loading dose followed by 150 mg weekly for 30 days, cohort 3 received 450 mg weekly for 30 days, and cohort 4 also received a 300-mg weekly dose for 30 days but consisted of patients with inhibitors.
With the primary outcome being safety, the researchers reported no treatment-related serious adverse events. There were four grade 3/4 adverse events, including two subjects who reported injection site reactions, and some generalized pruritus and erythematous rash.
Two patients discontinued treatment after reaching prespecified dose-limiting toxicity related to decreasing fibrinogen levels, compared with baseline. However, Dr. Mahlangu pointed out that, in one of these patients, the fibrinogen levels were still within normal levels but protocol required removing the patient from the study.
The study did see a significant 85%-97% reduction in annualized bleed rates across the four cohorts, including among patients with inhibitors.
“Most patients who were exposed to marstacimab actually did not bleed at all when they were receiving marstacimab, compared to when they weren’t receiving marstacimab,” Dr. Mahlangu said.
While three patients developed antidrug antibodies, this did not appear to impact the pharmacokinetics, pharmacodynamics, or safety, he said. No patients developed neutralizing antibodies, and the pharmacodynamics showed no difference between patients with hemophilia A and B.
“I would like to believe that the results of this study are fairly promising in terms of the safety, the efficacy, the pharmoacokinetics, and the pharmacodynamics,” Dr. Mahlangu said.
There is an unmet need for therapies that can be used in patients with either hemophilia A or B, and with or without inhibitors, Dr. Mahlangu said in an interview. Another unmet need that marstacimab could potentially address is for subcutaneous treatment options, he added.
“We are particularly pleased by the fact that injection-site reactions are very low, and they seem not to carry on every time the patients have injected,” Dr. Mahlangu said.
Commenting on the presentation, session cochair Julia Phillips, MD, from PathLab in Waikato, New Zealand, said subcutaneous treatments offered a huge advantage to patients with hemophilia and their families.
“Often more than one member of the family is affected. So [for] a family with children, if they have several sons with hemophilia, then doing IV injections before school on a regular basis can be quite a big burden on the family,” she said in an interview.
The study was sponsored by Pfizer. Dr. Mahlangu reported research support from and scientific advisory board and speakers bureau roles with several pharmaceutical companies, including Pfizer.
SOURCE: Mahlangu J et al. 2019. ISTH CONGRESS, Abstract OC 11.2.
MELBOURNE – Subcutaneous administration of the monoclonal antibody marstacimab (PF-06741086) provides significant reductions in bleeding rates for patients with hemophilia A and B, with reasonably safety and tolerability, according to research presented at the International Society on Thrombosis and Haemostasis congress.
Johnny Mahlangu, MBBCh, of the University of Witwatersrand in Johannesburg, South Africa, presented data from a multicenter, international phase 1B/2 open-label study involving 26 patients with severe hemophilia, who had experienced at least six acute bleeding episodes in the 6 months prior to enrollment. Twenty-three patients had hemophilia A, the remaining three patients had hemophilia B, and all were receiving on-demand treatment.
Patients were divided into one of four cohorts. Cohort 1 received a weekly 300-mg dose subcutaneously for 30 days, cohort 2 received a 300-mg loading dose followed by 150 mg weekly for 30 days, cohort 3 received 450 mg weekly for 30 days, and cohort 4 also received a 300-mg weekly dose for 30 days but consisted of patients with inhibitors.
With the primary outcome being safety, the researchers reported no treatment-related serious adverse events. There were four grade 3/4 adverse events, including two subjects who reported injection site reactions, and some generalized pruritus and erythematous rash.
Two patients discontinued treatment after reaching prespecified dose-limiting toxicity related to decreasing fibrinogen levels, compared with baseline. However, Dr. Mahlangu pointed out that, in one of these patients, the fibrinogen levels were still within normal levels but protocol required removing the patient from the study.
The study did see a significant 85%-97% reduction in annualized bleed rates across the four cohorts, including among patients with inhibitors.
“Most patients who were exposed to marstacimab actually did not bleed at all when they were receiving marstacimab, compared to when they weren’t receiving marstacimab,” Dr. Mahlangu said.
While three patients developed antidrug antibodies, this did not appear to impact the pharmacokinetics, pharmacodynamics, or safety, he said. No patients developed neutralizing antibodies, and the pharmacodynamics showed no difference between patients with hemophilia A and B.
“I would like to believe that the results of this study are fairly promising in terms of the safety, the efficacy, the pharmoacokinetics, and the pharmacodynamics,” Dr. Mahlangu said.
There is an unmet need for therapies that can be used in patients with either hemophilia A or B, and with or without inhibitors, Dr. Mahlangu said in an interview. Another unmet need that marstacimab could potentially address is for subcutaneous treatment options, he added.
“We are particularly pleased by the fact that injection-site reactions are very low, and they seem not to carry on every time the patients have injected,” Dr. Mahlangu said.
Commenting on the presentation, session cochair Julia Phillips, MD, from PathLab in Waikato, New Zealand, said subcutaneous treatments offered a huge advantage to patients with hemophilia and their families.
“Often more than one member of the family is affected. So [for] a family with children, if they have several sons with hemophilia, then doing IV injections before school on a regular basis can be quite a big burden on the family,” she said in an interview.
The study was sponsored by Pfizer. Dr. Mahlangu reported research support from and scientific advisory board and speakers bureau roles with several pharmaceutical companies, including Pfizer.
SOURCE: Mahlangu J et al. 2019. ISTH CONGRESS, Abstract OC 11.2.
MELBOURNE – Subcutaneous administration of the monoclonal antibody marstacimab (PF-06741086) provides significant reductions in bleeding rates for patients with hemophilia A and B, with reasonably safety and tolerability, according to research presented at the International Society on Thrombosis and Haemostasis congress.
Johnny Mahlangu, MBBCh, of the University of Witwatersrand in Johannesburg, South Africa, presented data from a multicenter, international phase 1B/2 open-label study involving 26 patients with severe hemophilia, who had experienced at least six acute bleeding episodes in the 6 months prior to enrollment. Twenty-three patients had hemophilia A, the remaining three patients had hemophilia B, and all were receiving on-demand treatment.
Patients were divided into one of four cohorts. Cohort 1 received a weekly 300-mg dose subcutaneously for 30 days, cohort 2 received a 300-mg loading dose followed by 150 mg weekly for 30 days, cohort 3 received 450 mg weekly for 30 days, and cohort 4 also received a 300-mg weekly dose for 30 days but consisted of patients with inhibitors.
With the primary outcome being safety, the researchers reported no treatment-related serious adverse events. There were four grade 3/4 adverse events, including two subjects who reported injection site reactions, and some generalized pruritus and erythematous rash.
Two patients discontinued treatment after reaching prespecified dose-limiting toxicity related to decreasing fibrinogen levels, compared with baseline. However, Dr. Mahlangu pointed out that, in one of these patients, the fibrinogen levels were still within normal levels but protocol required removing the patient from the study.
The study did see a significant 85%-97% reduction in annualized bleed rates across the four cohorts, including among patients with inhibitors.
“Most patients who were exposed to marstacimab actually did not bleed at all when they were receiving marstacimab, compared to when they weren’t receiving marstacimab,” Dr. Mahlangu said.
While three patients developed antidrug antibodies, this did not appear to impact the pharmacokinetics, pharmacodynamics, or safety, he said. No patients developed neutralizing antibodies, and the pharmacodynamics showed no difference between patients with hemophilia A and B.
“I would like to believe that the results of this study are fairly promising in terms of the safety, the efficacy, the pharmoacokinetics, and the pharmacodynamics,” Dr. Mahlangu said.
There is an unmet need for therapies that can be used in patients with either hemophilia A or B, and with or without inhibitors, Dr. Mahlangu said in an interview. Another unmet need that marstacimab could potentially address is for subcutaneous treatment options, he added.
“We are particularly pleased by the fact that injection-site reactions are very low, and they seem not to carry on every time the patients have injected,” Dr. Mahlangu said.
Commenting on the presentation, session cochair Julia Phillips, MD, from PathLab in Waikato, New Zealand, said subcutaneous treatments offered a huge advantage to patients with hemophilia and their families.
“Often more than one member of the family is affected. So [for] a family with children, if they have several sons with hemophilia, then doing IV injections before school on a regular basis can be quite a big burden on the family,” she said in an interview.
The study was sponsored by Pfizer. Dr. Mahlangu reported research support from and scientific advisory board and speakers bureau roles with several pharmaceutical companies, including Pfizer.
SOURCE: Mahlangu J et al. 2019. ISTH CONGRESS, Abstract OC 11.2.
REPORTING FROM 2019 ISTH CONGRESS
No reduction in PE risk with vena cava filters after severe injury
MELBOURNE – Use of a prophylactic vena cava filter to trap blood clots in severely injured patients does not appear to reduce the risk of pulmonary embolism or death, according to data presented at the International Society on Thrombosis and Haemostasis congress.
The researchers reported the outcomes of a multicenter, controlled trial in which 240 severely injured patients with a contraindication to anticoagulants were randomized to receive a vena cava filter within 72 hours of admission, or no filter. The findings were published simultaneously in the New England Journal of Medicine.
The study showed no significant differences between the filter and no-filter groups in the primary outcome of a composite of symptomatic pulmonary embolism or death from any cause at 90 days after enrollment (13.9% vs. 14.4% respectively, P = .98).
In a prespecified subgroup analysis, researchers examined patients who survived 7 days after injury and did not receive prophylactic anticoagulation in those 7 days. Among this group of patients, none of those who received the vena cava filter experienced a symptomatic pulmonary embolism between day 8 and day 90, but five patients (14.7%) in the no-filter group did.
Filters were left in place for a median duration of 27 days (11-90 days). Among the 122 patients who received a filter – which included two patients in the control group – researchers found trapped thrombi in the filter in six patients.
Transfusion requirements, and the incidence of major and nonmajor bleeding and leg deep vein thrombosis, were similar between the filter and no-filter groups. Seven patients in the filter group (5.7%) required more than one attempt to remove the filter, and in one patient the filter had to be removed surgically.
Kwok M. Ho, PhD, of the department of intensive care medicine at Royal Perth Hospital, Australia, and coauthors wrote that while vena cava filters are widely used in trauma centers to prevent pulmonary embolism in patients at high risk of bleeding, there are conflicting recommendations regarding their use, and most studies so far have been observational.
“Given the cost and risks associated with a vena cava filter, our data suggest that there is no urgency to insert the filter in patients who can be treated with prophylactic anticoagulation within 7 days after injury,” they wrote. “Unnecessary insertion of a vena cava filter has the potential to cause harm.”
However, they noted that patients with multiple, large intracranial hematomas were particularly at risk from bleeding with anticoagulant therapy, and therefore may benefit from the use of a vena cava filter.
The Medical Research Foundation of Royal Perth Hospital and the Western Australian Department of Health funded the study. Dr. Ho reported funding from the Western Australian Department of Health and the Raine Medical Research Foundation to conduct the study, as well as serving as an adviser to Medtronic and Cardinal Health.
SOURCE: Ho KM et al. N Engl J Med. 2019 Jul 7. doi: 10.156/NEJMoa1806515.
MELBOURNE – Use of a prophylactic vena cava filter to trap blood clots in severely injured patients does not appear to reduce the risk of pulmonary embolism or death, according to data presented at the International Society on Thrombosis and Haemostasis congress.
The researchers reported the outcomes of a multicenter, controlled trial in which 240 severely injured patients with a contraindication to anticoagulants were randomized to receive a vena cava filter within 72 hours of admission, or no filter. The findings were published simultaneously in the New England Journal of Medicine.
The study showed no significant differences between the filter and no-filter groups in the primary outcome of a composite of symptomatic pulmonary embolism or death from any cause at 90 days after enrollment (13.9% vs. 14.4% respectively, P = .98).
In a prespecified subgroup analysis, researchers examined patients who survived 7 days after injury and did not receive prophylactic anticoagulation in those 7 days. Among this group of patients, none of those who received the vena cava filter experienced a symptomatic pulmonary embolism between day 8 and day 90, but five patients (14.7%) in the no-filter group did.
Filters were left in place for a median duration of 27 days (11-90 days). Among the 122 patients who received a filter – which included two patients in the control group – researchers found trapped thrombi in the filter in six patients.
Transfusion requirements, and the incidence of major and nonmajor bleeding and leg deep vein thrombosis, were similar between the filter and no-filter groups. Seven patients in the filter group (5.7%) required more than one attempt to remove the filter, and in one patient the filter had to be removed surgically.
Kwok M. Ho, PhD, of the department of intensive care medicine at Royal Perth Hospital, Australia, and coauthors wrote that while vena cava filters are widely used in trauma centers to prevent pulmonary embolism in patients at high risk of bleeding, there are conflicting recommendations regarding their use, and most studies so far have been observational.
“Given the cost and risks associated with a vena cava filter, our data suggest that there is no urgency to insert the filter in patients who can be treated with prophylactic anticoagulation within 7 days after injury,” they wrote. “Unnecessary insertion of a vena cava filter has the potential to cause harm.”
However, they noted that patients with multiple, large intracranial hematomas were particularly at risk from bleeding with anticoagulant therapy, and therefore may benefit from the use of a vena cava filter.
The Medical Research Foundation of Royal Perth Hospital and the Western Australian Department of Health funded the study. Dr. Ho reported funding from the Western Australian Department of Health and the Raine Medical Research Foundation to conduct the study, as well as serving as an adviser to Medtronic and Cardinal Health.
SOURCE: Ho KM et al. N Engl J Med. 2019 Jul 7. doi: 10.156/NEJMoa1806515.
MELBOURNE – Use of a prophylactic vena cava filter to trap blood clots in severely injured patients does not appear to reduce the risk of pulmonary embolism or death, according to data presented at the International Society on Thrombosis and Haemostasis congress.
The researchers reported the outcomes of a multicenter, controlled trial in which 240 severely injured patients with a contraindication to anticoagulants were randomized to receive a vena cava filter within 72 hours of admission, or no filter. The findings were published simultaneously in the New England Journal of Medicine.
The study showed no significant differences between the filter and no-filter groups in the primary outcome of a composite of symptomatic pulmonary embolism or death from any cause at 90 days after enrollment (13.9% vs. 14.4% respectively, P = .98).
In a prespecified subgroup analysis, researchers examined patients who survived 7 days after injury and did not receive prophylactic anticoagulation in those 7 days. Among this group of patients, none of those who received the vena cava filter experienced a symptomatic pulmonary embolism between day 8 and day 90, but five patients (14.7%) in the no-filter group did.
Filters were left in place for a median duration of 27 days (11-90 days). Among the 122 patients who received a filter – which included two patients in the control group – researchers found trapped thrombi in the filter in six patients.
Transfusion requirements, and the incidence of major and nonmajor bleeding and leg deep vein thrombosis, were similar between the filter and no-filter groups. Seven patients in the filter group (5.7%) required more than one attempt to remove the filter, and in one patient the filter had to be removed surgically.
Kwok M. Ho, PhD, of the department of intensive care medicine at Royal Perth Hospital, Australia, and coauthors wrote that while vena cava filters are widely used in trauma centers to prevent pulmonary embolism in patients at high risk of bleeding, there are conflicting recommendations regarding their use, and most studies so far have been observational.
“Given the cost and risks associated with a vena cava filter, our data suggest that there is no urgency to insert the filter in patients who can be treated with prophylactic anticoagulation within 7 days after injury,” they wrote. “Unnecessary insertion of a vena cava filter has the potential to cause harm.”
However, they noted that patients with multiple, large intracranial hematomas were particularly at risk from bleeding with anticoagulant therapy, and therefore may benefit from the use of a vena cava filter.
The Medical Research Foundation of Royal Perth Hospital and the Western Australian Department of Health funded the study. Dr. Ho reported funding from the Western Australian Department of Health and the Raine Medical Research Foundation to conduct the study, as well as serving as an adviser to Medtronic and Cardinal Health.
SOURCE: Ho KM et al. N Engl J Med. 2019 Jul 7. doi: 10.156/NEJMoa1806515.
REPORTING FROM 2019 ISTH CONGRESS
Consider bleeding risk with oral anticoagulants in patients with GI cancer
MELBOURNE – The treatment of cancer-associated thrombosis may be complicated by increased bleeding risk in patients with gastrointestinal cancer, in whom direct oral anticoagulants may not be the ideal first choice, one expert reported at the International Society on Thrombosis and Haemostasis congress.
Agnes Y.Y. Lee, MD, medical director of the Thrombosis Program at Vancouver General Hospital and the University of British Columbia, spoke about the challenges and necessity of treating cancer-associated thrombosis, pointing out that about 20% of all cases of venous thromboembolism (VTE) are associated with cancer.
“In those with cancer, thrombosis can also interfere with cancer treatment, increases health care costs, and is extraordinarily burdensome to patients and their families,” she said. “Fortunately the most effective way to reduce this burden is to use anticoagulant therapy for prevention and treatment.”
While direct oral anticoagulants have been shown in several studies to be comparable to warfarin in treating most patients with thrombosis, Dr. Lee said there has been a question of how they compare in safety and efficacy to low-molecular-weight heparin in individuals with cancer.
Data from the Hokusai VTE Cancer trial, which compared oral edoxaban with subcutaneous dalteparin in patients with cancer, showed that the two treatments were comparable in time to first occurrence of thrombosis. However, the study did show a fourfold higher risk of bleeding with edoxaban, compared with that of dalteparin, among individuals with gastrointestinal cancers, a difference in bleeding rate that was not seen in patients with nongastrointestinal cancers, Dr. Lee said.
Dr. Lee pointed out that this study also showed a higher bleeding risk in patients with other bleeding risk factors, including those with primary or metastatic brain cancer.
“This study also showed that, when patients developed major bleeding, 60%-80% of them required hospitalization or an ICU stay, so major bleeding is a serious complication and certainly will increase the cost of therapy for these patients,” she said.
In the SELECT-D pilot study, which compared rivaroxaban with dalteparin in patients with cancer, there was a higher risk of bleeding for patients with esophageal or gastroesophageal cancers.
Bleeding risk is generally not well addressed in current guidelines on managing hemostasis in patients with malignancies, partly because it is difficult to quantify bleeding in these patients whose hemoglobin levels would be affected by their disease and their chemotherapy, Dr. Lee said in an interview.
“The bleeding events in cancer patients do get more complicated because there’s all this other noise in the background,” she said.
Commenting on her personal approach to treatment, Dr. Lee said she favors starting patients on low-molecular-weight heparin because it gives her time to understand patients, their disease, and their needs.
“A lot of patients arrive, and they can’t really tell me what their cancer is doing, they can’t really tell me what cancer therapy they’re going through,” she says. “And if they’re on a long list of drugs, then I have to talk to my pharmacist about whether there are drug-drug interactions.”
If patients were well managed on low-molecular-weight heparin without any bleeding, then Dr. Lee said she would consider switching them to direct oral anticoagulants.
Cochair of the session, Ingrid Pabinger, MD, from the Medical University of Vienna commented that vitamin K antagonists should not be forgotten because some patients are unable to afford low-molecular-weight heparin.
However Dr. Lee said these were last on the list for her because of the risk of drug-drug interactions, drug-food interactions, and the issues faced by patients experiencing vomiting or diarrhea with their chemotherapy.
Dr. Lee reported research funding, consultancies, and honoraria from the pharmaceutical sector.
MELBOURNE – The treatment of cancer-associated thrombosis may be complicated by increased bleeding risk in patients with gastrointestinal cancer, in whom direct oral anticoagulants may not be the ideal first choice, one expert reported at the International Society on Thrombosis and Haemostasis congress.
Agnes Y.Y. Lee, MD, medical director of the Thrombosis Program at Vancouver General Hospital and the University of British Columbia, spoke about the challenges and necessity of treating cancer-associated thrombosis, pointing out that about 20% of all cases of venous thromboembolism (VTE) are associated with cancer.
“In those with cancer, thrombosis can also interfere with cancer treatment, increases health care costs, and is extraordinarily burdensome to patients and their families,” she said. “Fortunately the most effective way to reduce this burden is to use anticoagulant therapy for prevention and treatment.”
While direct oral anticoagulants have been shown in several studies to be comparable to warfarin in treating most patients with thrombosis, Dr. Lee said there has been a question of how they compare in safety and efficacy to low-molecular-weight heparin in individuals with cancer.
Data from the Hokusai VTE Cancer trial, which compared oral edoxaban with subcutaneous dalteparin in patients with cancer, showed that the two treatments were comparable in time to first occurrence of thrombosis. However, the study did show a fourfold higher risk of bleeding with edoxaban, compared with that of dalteparin, among individuals with gastrointestinal cancers, a difference in bleeding rate that was not seen in patients with nongastrointestinal cancers, Dr. Lee said.
Dr. Lee pointed out that this study also showed a higher bleeding risk in patients with other bleeding risk factors, including those with primary or metastatic brain cancer.
“This study also showed that, when patients developed major bleeding, 60%-80% of them required hospitalization or an ICU stay, so major bleeding is a serious complication and certainly will increase the cost of therapy for these patients,” she said.
In the SELECT-D pilot study, which compared rivaroxaban with dalteparin in patients with cancer, there was a higher risk of bleeding for patients with esophageal or gastroesophageal cancers.
Bleeding risk is generally not well addressed in current guidelines on managing hemostasis in patients with malignancies, partly because it is difficult to quantify bleeding in these patients whose hemoglobin levels would be affected by their disease and their chemotherapy, Dr. Lee said in an interview.
“The bleeding events in cancer patients do get more complicated because there’s all this other noise in the background,” she said.
Commenting on her personal approach to treatment, Dr. Lee said she favors starting patients on low-molecular-weight heparin because it gives her time to understand patients, their disease, and their needs.
“A lot of patients arrive, and they can’t really tell me what their cancer is doing, they can’t really tell me what cancer therapy they’re going through,” she says. “And if they’re on a long list of drugs, then I have to talk to my pharmacist about whether there are drug-drug interactions.”
If patients were well managed on low-molecular-weight heparin without any bleeding, then Dr. Lee said she would consider switching them to direct oral anticoagulants.
Cochair of the session, Ingrid Pabinger, MD, from the Medical University of Vienna commented that vitamin K antagonists should not be forgotten because some patients are unable to afford low-molecular-weight heparin.
However Dr. Lee said these were last on the list for her because of the risk of drug-drug interactions, drug-food interactions, and the issues faced by patients experiencing vomiting or diarrhea with their chemotherapy.
Dr. Lee reported research funding, consultancies, and honoraria from the pharmaceutical sector.
MELBOURNE – The treatment of cancer-associated thrombosis may be complicated by increased bleeding risk in patients with gastrointestinal cancer, in whom direct oral anticoagulants may not be the ideal first choice, one expert reported at the International Society on Thrombosis and Haemostasis congress.
Agnes Y.Y. Lee, MD, medical director of the Thrombosis Program at Vancouver General Hospital and the University of British Columbia, spoke about the challenges and necessity of treating cancer-associated thrombosis, pointing out that about 20% of all cases of venous thromboembolism (VTE) are associated with cancer.
“In those with cancer, thrombosis can also interfere with cancer treatment, increases health care costs, and is extraordinarily burdensome to patients and their families,” she said. “Fortunately the most effective way to reduce this burden is to use anticoagulant therapy for prevention and treatment.”
While direct oral anticoagulants have been shown in several studies to be comparable to warfarin in treating most patients with thrombosis, Dr. Lee said there has been a question of how they compare in safety and efficacy to low-molecular-weight heparin in individuals with cancer.
Data from the Hokusai VTE Cancer trial, which compared oral edoxaban with subcutaneous dalteparin in patients with cancer, showed that the two treatments were comparable in time to first occurrence of thrombosis. However, the study did show a fourfold higher risk of bleeding with edoxaban, compared with that of dalteparin, among individuals with gastrointestinal cancers, a difference in bleeding rate that was not seen in patients with nongastrointestinal cancers, Dr. Lee said.
Dr. Lee pointed out that this study also showed a higher bleeding risk in patients with other bleeding risk factors, including those with primary or metastatic brain cancer.
“This study also showed that, when patients developed major bleeding, 60%-80% of them required hospitalization or an ICU stay, so major bleeding is a serious complication and certainly will increase the cost of therapy for these patients,” she said.
In the SELECT-D pilot study, which compared rivaroxaban with dalteparin in patients with cancer, there was a higher risk of bleeding for patients with esophageal or gastroesophageal cancers.
Bleeding risk is generally not well addressed in current guidelines on managing hemostasis in patients with malignancies, partly because it is difficult to quantify bleeding in these patients whose hemoglobin levels would be affected by their disease and their chemotherapy, Dr. Lee said in an interview.
“The bleeding events in cancer patients do get more complicated because there’s all this other noise in the background,” she said.
Commenting on her personal approach to treatment, Dr. Lee said she favors starting patients on low-molecular-weight heparin because it gives her time to understand patients, their disease, and their needs.
“A lot of patients arrive, and they can’t really tell me what their cancer is doing, they can’t really tell me what cancer therapy they’re going through,” she says. “And if they’re on a long list of drugs, then I have to talk to my pharmacist about whether there are drug-drug interactions.”
If patients were well managed on low-molecular-weight heparin without any bleeding, then Dr. Lee said she would consider switching them to direct oral anticoagulants.
Cochair of the session, Ingrid Pabinger, MD, from the Medical University of Vienna commented that vitamin K antagonists should not be forgotten because some patients are unable to afford low-molecular-weight heparin.
However Dr. Lee said these were last on the list for her because of the risk of drug-drug interactions, drug-food interactions, and the issues faced by patients experiencing vomiting or diarrhea with their chemotherapy.
Dr. Lee reported research funding, consultancies, and honoraria from the pharmaceutical sector.
EXPERT ANALYSIS FROM 2019 ISTH CONGRESS