User login
The Current State of Advanced Practice Provider Fellowships in Hospital Medicine: A Survey of Program Directors
Postgraduate training for physician assistants (PAs) and nurse practitioners (NPs) is a rapidly evolving field. It has been estimated that the number of these advanced practice providers (APPs) almost doubled between 2000 and 2016 (from 15.3 to 28.2 per 100 physicians) and is expected to double again by 2030.
Historically, postgraduate APP fellowships have functioned to help bridge the gap in clinical practice experience between physicians and APPs.
First described in 2010 by the Mayo Clinic,
METHODS
This was a cross-sectional study of all APP adult and pediatric fellowships in hospital medicine, in the United States, that were identifiable through May 2018. Multiple methods were used to identify all active fellowships. First, all training programs offering a Hospital Medicine Fellowship in the ARC-PA and Association of Postgraduate PA Programs databases were noted. Second, questionnaires were given out at the NP/PA forum at the national SHM conference in 2018 to gather information on existing APP fellowships. Third, similar online requests to identify known programs were posted to the SHM web forum Hospital Medicine Exchange (HMX). Fourth, Internet searches were used to discover additional programs. Once those fellowships were identified, surveys were sent to their program directors (PDs). These surveys not only asked the PDs about their fellowship but also asked them to identify additional APP fellowships beyond those that we had captured. Once additional programs were identified, a second round of surveys was sent to their PDs. This was performed in an iterative fashion until no additional fellowships were discovered.
The survey tool was developed and validated internally in the AAMC Survey Development style18 and was influenced by prior validated surveys of postgraduate medical fellowships.10,
A web-based survey format (Qualtrics) was used to distribute the questionnaire e-mail to the PDs. Follow up e-mail reminders were sent to all nonresponders to encourage full participation. Survey completion was voluntary; no financial incentives or gifts were offered. IRB approval was obtained at Johns Hopkins Bayview (IRB number 00181629). Descriptive statistics (proportions, means, and ranges as appropriate) were calculated for all variables. Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, Texas. StataCorp LP) was used for data analysis.
RESULTS
In total, 11 fellowships were identified using our multimethod approach. We found four (36%) programs by utilizing existing online databases, two (18%) through the SHM questionnaire and HMX forum, three (27%) through internet searches, and the remaining two (18%) were referred to us by the other PDs who were surveyed. Of the programs surveyed, 10 were adult programs and one was a pediatric program. Surveys were sent to the PDs of the 11 fellowships, and all but one of them (10/11, 91%) responded. Respondent programs were given alphabetical designations A through J (Table).
Fellowship and Individual Characteristics
Most programs have been in existence for five years or fewer. Eighty percent of the programs are about one year in duration; two outlier programs have fellowship lengths of six months and 18 months. The main hospital where training occurs has a mean of 496 beds (range 213 to 900). Ninety percent of the hospitals also have physician residency training programs. Sixty percent of programs enroll two to four fellows per year while 40% enroll five or more. The salary range paid by the programs is $55,000 to >$70,000, and half the programs pay more than $65,000.
The majority of fellows accepted into APP fellowships in hospital medicine are women. Eighty percent of fellows are 26-30 years old, and 90% of fellows have been out of NP or PA school for one year or less. Both NP and PA applicants are accepted in 80% of fellowships.
Program Rationales
All programs reported that training and retaining applicants is the main driver for developing their fellowship, and 50% of them offer financial incentives for retention upon successful completion of the program. Forty percent of PDs stated that there is an implicit or explicit understanding that successful completion of the fellowship would result in further employment. Over the last five years, 89% (range: 71%-100%) of graduates were asked to remain for a full-time position after program completion.
In addition to training and retention, building an interprofessional team (50%), managing patient volume (30%), and reducing overhead (20%) were also reported as rationales for program development. The majority of programs (80%) have fellows bill for clinical services, and five of those eight programs do so after their fellows become more clinically competent.
Curricula
Of the nine adult programs, 67% teach explicitly to SHM core competencies and 33% send their fellows to the SHM NP/PA Boot Camp. Thirty percent of fellowships partner formally with either a physician residency or a local PA program to develop educational content. Six of the nine programs with active physician residencies, including the pediatric fellowship, offer shared educational experiences for the residents and APPs.
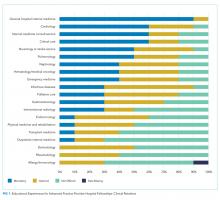
There are notable differences in clinical rotations between the programs (Figure 1). No single rotation is universally required, although general hospital internal medicine is required in all adult fellowships. The majority (80%) of programs offer at least one elective. Six programs reported mandatory rotations outside the department of medicine, most commonly neurology or the stroke service (four programs). Only one program reported only general medicine rotations, with no subspecialty electives.
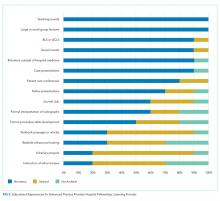
There are also differences between programs with respect to educational experiences and learning formats (Figure 2). Each fellowship takes a unique approach to clinical instruction; teaching rounds and lecture attendance are the only experiences that are mandatory across the board. Grand rounds are available, but not required, in all programs. Ninety percent of programs offer or require fellow presentations, journal clubs, reading assignments, or scholarly projects. Fellow presentations (70%) and journal club attendance (60%) are required in more than half the programs; however, reading assignments (30%) and scholarly projects (20%) are rarely required.
Methods of Fellow Assessment
Each program surveyed has a unique method of fellow assessment. Ninety percent of the programs use more than one method to assess their fellows. Faculty reviews are most commonly used and are conducted in all rotations in 80% of fellowships. Both self-assessment exercises and written examinations are used in some rotations by the majority of programs. Capstone projects are required infrequently (30%).
DISCUSSION
We found several commonalities between the fellowships surveyed. Many of the program characteristics, such as years in operation, salary, duration, and lack of accreditation, are quite similar. Most fellowships also have a similar rationale for building their programs and use resources from the SHM to inform their curricula. Fellows, on average, share several demographic characteristics, such as age, gender, and time out of schooling. Conversely, we found wide variability in clinical rotations, the general teaching structure, and methods of fellow evaluation.
There have been several publications detailing successful individual APP fellowships in medical subspecialties,
It is noteworthy that every program surveyed was created with training and retention in mind, rather than other factors like decreasing overhead or managing patient volume. Training one’s own APPs so that they can learn on the job, come to understand expectations within a group, and witness the culture is extremely valuable. From a patient safety standpoint, it has been documented that physician hospitalists straight out of residency have a higher patient mortality compared with more experienced providers.
Several limitations to this study should be considered. While we used multiple strategies to locate as many fellowships as possible, it is unlikely that we successfully captured all existing programs, and new programs are being developed annually. We also relied on self-reported data from PDs. While we would expect PDs to provide accurate data, we could not externally validate their answers. Additionally, although our survey tool was reviewed extensively and validated internally, it was developed de novo for this study.
CONCLUSION
APP fellowships in hospital medicine have experienced marked growth since the first program was described in 2010. The majority of programs are 12 months long, operate in existing teaching centers, and are intended to further enhance the training and retention of newly graduated PAs and NPs. Despite their similarities, fellowships have striking variability in their methods of teaching and assessing their learners. Best practices have yet to be identified, and further study is required to determine how to standardize curricula across the board.
Acknowledgments
Disclosures
The authors report no conflicts of interest.
Funding
This project was supported by the Johns Hopkins School of Medicine Biostatistics, Epidemiology and Data Management (BEAD) Core. Dr. Wright is the Anne Gaines and G. Thomas Miller Professor of Medicine, which is supported through the Johns Hopkins’ Center for Innovative Medicine.
1. Auerbach DI, Staiger DO, Buerhaus PI. Growing ranks of advanced practice clinicians — implications for the physician workforce. N Engl J Med. 2018;378(25):2358-2360. doi: 10.1056/nejmp1801869. PubMed
2. Darves B. Midlevels make a rocky entrance into hospital medicine. Todays Hospitalist. 2007;5(1):28-32.
3. Polansky M. A historical perspective on postgraduate physician assistant education and the association of postgraduate physician assistant programs. J Physician Assist Educ. 2007;18(3):100-108. doi: 10.1097/01367895-200718030-00014.
4. FNP & AGNP Certification Candidate Handbook. The American Academy of Nurse Practitioners National Certification Board, Inc; 2018. https://www.aanpcert.org/resource/documents/AGNP FNP Candidate Handbook.pdf. Accessed December 20, 2018
5. Become a PA: Getting Your Prerequisites and Certification. AAPA. https://www.aapa.org/career-central/become-a-pa/. Accessed December 20, 2018.
6. ACGME Common Program Requirements. ACGME; 2017. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRs_2017-07-01.pdf. Accessed December 20, 2018
7. Committee on the Learning Health Care System in America; Institute of Medicine, Smith MD, Smith M, Saunders R, Stuckhardt L, McGinnis JM. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2013. PubMed
8. The Future of Nursing LEADING CHANGE, ADVANCING HEALTH. THE NATIONAL ACADEMIES PRESS; 2014. https://www.nap.edu/read/12956/chapter/1. Accessed December 16, 2018.
9. Hussaini SS, Bushardt RL, Gonsalves WC, et al. Accreditation and implications of clinical postgraduate pa training programs. JAAPA. 2016:29:1-7. doi: 10.1097/01.jaa.0000482298.17821.fb. PubMed
10. Polansky M, Garver GJH, Hilton G. Postgraduate clinical education of physician assistants. J Physician Assist Educ. 2012;23(1):39-45. doi: 10.1097/01367895-201223010-00008.
11. Will KK, Budavari AI, Wilkens JA, Mishark K, Hartsell ZC. A hospitalist postgraduate training program for physician assistants. J Hosp Med. 2010;5(2):94-98. doi: 10.1002/jhm.619. PubMed
12. Kartha A, Restuccia JD, Burgess JF, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. doi: 10.1002/jhm.2231. PubMed
13. Singh S, Fletcher KE, Schapira MM, et al. A comparison of outcomes of general medical inpatient care provided by a hospitalist-physician assistant model vs a traditional resident-based model. J Hosp Med. 2011;6(3):122-130. doi: 10.1002/jhm.826. PubMed
14. Hussaini SS, Bushardt RL, Gonsalves WC, et al. Accreditation and implications of clinical postgraduate PA training programs. JAAPA. 2016;29(5):1-7. doi: 10.1097/01.jaa.0000482298.17821.fb. PubMed
15. Postgraduate Programs. ARC-PA. http://www.arc-pa.org/accreditation/postgraduate-programs. Accessed September 13, 2018.
16. National Nurse Practitioner Residency & Fellowship Training Consortium: Mission. https://www.nppostgradtraining.com/About-Us/Mission. Accessed September 27, 2018.
17. NP/PA Boot Camp. State of Hospital Medicine | Society of Hospital Medicine. http://www.hospitalmedicine.org/events/nppa-boot-camp. Accessed September 13, 2018.
18. Gehlbach H, Artino Jr AR, Durning SJ. AM last page: survey development guidance for medical education researchers. Acad Med. 2010;85(5):925. doi: 10.1097/ACM.0b013e3181dd3e88.” Accessed March 10, 2018. PubMed
19. Kraus C, Carlisle T, Carney D. Emergency Medicine Physician Assistant (EMPA) post-graduate training programs: program characteristics and training curricula. West J Emerg Med. 2018;19(5):803-807. doi: 10.5811/westjem.2018.6.37892.
20. Shah NH, Rhim HJH, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. doi: 10.1002/jhm.2571. PubMed
21. Thompson BM, Searle NS, Gruppen LD, Hatem CJ, Nelson E. A national survey of medical education fellowships. Med Educ Online. 2011;16(1):5642. doi: 10.3402/meo.v16i0.5642. PubMed
22. Hooker R. A physician assistant rheumatology fellowship. JAAPA. 2013;26(6):49-52. doi: 10.1097/01.jaa.0000430346.04435.e4 PubMed
23. Keizer T, Trangle M. the benefits of a physician assistant and/or nurse practitioner psychiatric postgraduate training program. Acad Psychiatry. 2015;39(6):691-694. doi: 10.1007/s40596-015-0331-z. PubMed
24. Miller A, Weiss J, Hill V, Lindaman K, Emory C. Implementation of a postgraduate orthopaedic physician assistant fellowship for improved specialty training. JBJS Journal of Orthopaedics for Physician Assistants. 2017:1. doi: 10.2106/jbjs.jopa.17.00021.
25. Sharma P, Brooks M, Roomiany P, Verma L, Criscione-Schreiber L. physician assistant student training for the inpatient setting. J Physician Assist Educ. 2017;28(4):189-195. doi: 10.1097/jpa.0000000000000174. PubMed
26. Goodwin JS, Salameh H, Zhou J, Singh S, Kuo Y-F, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized medicare population. JAMA Intern Med. 2018;178(2):196. doi: 10.1001/jamainternmed.2017.7049. PubMed
27. Barnes H. Exploring the factors that influence nurse practitioner role transition. J Nurse Pract. 2015;11(2):178-183. doi: 10.1016/j.nurpra.2014.11.004. PubMed
28. Will K, Williams J, Hilton G, Wilson L, Geyer H. Perceived efficacy and utility of postgraduate physician assistant training programs. JAAPA. 2016;29(3):46-48. doi: 10.1097/01.jaa.0000480569.39885.c8. PubMed
29. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2011;7(3):190-194. doi: 10.1002/jhm.1001. PubMed
30. Cate O. Competency-based postgraduate medical education: past, present and future. GMS J Med Educ. 2017:34(5). doi: 10.3205/zma001146. PubMed
31. Exploring the ACGME Core Competencies (Part 1 of 7). NEJM Knowledge. https://knowledgeplus.nejm.org/blog/exploring-acgme-core-competencies/. Accessed October 24, 2018.
32. Core Competencies. Core Competencies | Society of Hospital Medicine. http://www.hospitalmedicine.org/professional-development/core-competencies/. Accessed October 24, 2018.
Postgraduate training for physician assistants (PAs) and nurse practitioners (NPs) is a rapidly evolving field. It has been estimated that the number of these advanced practice providers (APPs) almost doubled between 2000 and 2016 (from 15.3 to 28.2 per 100 physicians) and is expected to double again by 2030.
Historically, postgraduate APP fellowships have functioned to help bridge the gap in clinical practice experience between physicians and APPs.
First described in 2010 by the Mayo Clinic,
METHODS
This was a cross-sectional study of all APP adult and pediatric fellowships in hospital medicine, in the United States, that were identifiable through May 2018. Multiple methods were used to identify all active fellowships. First, all training programs offering a Hospital Medicine Fellowship in the ARC-PA and Association of Postgraduate PA Programs databases were noted. Second, questionnaires were given out at the NP/PA forum at the national SHM conference in 2018 to gather information on existing APP fellowships. Third, similar online requests to identify known programs were posted to the SHM web forum Hospital Medicine Exchange (HMX). Fourth, Internet searches were used to discover additional programs. Once those fellowships were identified, surveys were sent to their program directors (PDs). These surveys not only asked the PDs about their fellowship but also asked them to identify additional APP fellowships beyond those that we had captured. Once additional programs were identified, a second round of surveys was sent to their PDs. This was performed in an iterative fashion until no additional fellowships were discovered.
The survey tool was developed and validated internally in the AAMC Survey Development style18 and was influenced by prior validated surveys of postgraduate medical fellowships.10,
A web-based survey format (Qualtrics) was used to distribute the questionnaire e-mail to the PDs. Follow up e-mail reminders were sent to all nonresponders to encourage full participation. Survey completion was voluntary; no financial incentives or gifts were offered. IRB approval was obtained at Johns Hopkins Bayview (IRB number 00181629). Descriptive statistics (proportions, means, and ranges as appropriate) were calculated for all variables. Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, Texas. StataCorp LP) was used for data analysis.
RESULTS
In total, 11 fellowships were identified using our multimethod approach. We found four (36%) programs by utilizing existing online databases, two (18%) through the SHM questionnaire and HMX forum, three (27%) through internet searches, and the remaining two (18%) were referred to us by the other PDs who were surveyed. Of the programs surveyed, 10 were adult programs and one was a pediatric program. Surveys were sent to the PDs of the 11 fellowships, and all but one of them (10/11, 91%) responded. Respondent programs were given alphabetical designations A through J (Table).
Fellowship and Individual Characteristics
Most programs have been in existence for five years or fewer. Eighty percent of the programs are about one year in duration; two outlier programs have fellowship lengths of six months and 18 months. The main hospital where training occurs has a mean of 496 beds (range 213 to 900). Ninety percent of the hospitals also have physician residency training programs. Sixty percent of programs enroll two to four fellows per year while 40% enroll five or more. The salary range paid by the programs is $55,000 to >$70,000, and half the programs pay more than $65,000.
The majority of fellows accepted into APP fellowships in hospital medicine are women. Eighty percent of fellows are 26-30 years old, and 90% of fellows have been out of NP or PA school for one year or less. Both NP and PA applicants are accepted in 80% of fellowships.
Program Rationales
All programs reported that training and retaining applicants is the main driver for developing their fellowship, and 50% of them offer financial incentives for retention upon successful completion of the program. Forty percent of PDs stated that there is an implicit or explicit understanding that successful completion of the fellowship would result in further employment. Over the last five years, 89% (range: 71%-100%) of graduates were asked to remain for a full-time position after program completion.
In addition to training and retention, building an interprofessional team (50%), managing patient volume (30%), and reducing overhead (20%) were also reported as rationales for program development. The majority of programs (80%) have fellows bill for clinical services, and five of those eight programs do so after their fellows become more clinically competent.
Curricula
Of the nine adult programs, 67% teach explicitly to SHM core competencies and 33% send their fellows to the SHM NP/PA Boot Camp. Thirty percent of fellowships partner formally with either a physician residency or a local PA program to develop educational content. Six of the nine programs with active physician residencies, including the pediatric fellowship, offer shared educational experiences for the residents and APPs.
There are notable differences in clinical rotations between the programs (Figure 1). No single rotation is universally required, although general hospital internal medicine is required in all adult fellowships. The majority (80%) of programs offer at least one elective. Six programs reported mandatory rotations outside the department of medicine, most commonly neurology or the stroke service (four programs). Only one program reported only general medicine rotations, with no subspecialty electives.
There are also differences between programs with respect to educational experiences and learning formats (Figure 2). Each fellowship takes a unique approach to clinical instruction; teaching rounds and lecture attendance are the only experiences that are mandatory across the board. Grand rounds are available, but not required, in all programs. Ninety percent of programs offer or require fellow presentations, journal clubs, reading assignments, or scholarly projects. Fellow presentations (70%) and journal club attendance (60%) are required in more than half the programs; however, reading assignments (30%) and scholarly projects (20%) are rarely required.
Methods of Fellow Assessment
Each program surveyed has a unique method of fellow assessment. Ninety percent of the programs use more than one method to assess their fellows. Faculty reviews are most commonly used and are conducted in all rotations in 80% of fellowships. Both self-assessment exercises and written examinations are used in some rotations by the majority of programs. Capstone projects are required infrequently (30%).
DISCUSSION
We found several commonalities between the fellowships surveyed. Many of the program characteristics, such as years in operation, salary, duration, and lack of accreditation, are quite similar. Most fellowships also have a similar rationale for building their programs and use resources from the SHM to inform their curricula. Fellows, on average, share several demographic characteristics, such as age, gender, and time out of schooling. Conversely, we found wide variability in clinical rotations, the general teaching structure, and methods of fellow evaluation.
There have been several publications detailing successful individual APP fellowships in medical subspecialties,
It is noteworthy that every program surveyed was created with training and retention in mind, rather than other factors like decreasing overhead or managing patient volume. Training one’s own APPs so that they can learn on the job, come to understand expectations within a group, and witness the culture is extremely valuable. From a patient safety standpoint, it has been documented that physician hospitalists straight out of residency have a higher patient mortality compared with more experienced providers.
Several limitations to this study should be considered. While we used multiple strategies to locate as many fellowships as possible, it is unlikely that we successfully captured all existing programs, and new programs are being developed annually. We also relied on self-reported data from PDs. While we would expect PDs to provide accurate data, we could not externally validate their answers. Additionally, although our survey tool was reviewed extensively and validated internally, it was developed de novo for this study.
CONCLUSION
APP fellowships in hospital medicine have experienced marked growth since the first program was described in 2010. The majority of programs are 12 months long, operate in existing teaching centers, and are intended to further enhance the training and retention of newly graduated PAs and NPs. Despite their similarities, fellowships have striking variability in their methods of teaching and assessing their learners. Best practices have yet to be identified, and further study is required to determine how to standardize curricula across the board.
Acknowledgments
Disclosures
The authors report no conflicts of interest.
Funding
This project was supported by the Johns Hopkins School of Medicine Biostatistics, Epidemiology and Data Management (BEAD) Core. Dr. Wright is the Anne Gaines and G. Thomas Miller Professor of Medicine, which is supported through the Johns Hopkins’ Center for Innovative Medicine.
Postgraduate training for physician assistants (PAs) and nurse practitioners (NPs) is a rapidly evolving field. It has been estimated that the number of these advanced practice providers (APPs) almost doubled between 2000 and 2016 (from 15.3 to 28.2 per 100 physicians) and is expected to double again by 2030.
Historically, postgraduate APP fellowships have functioned to help bridge the gap in clinical practice experience between physicians and APPs.
First described in 2010 by the Mayo Clinic,
METHODS
This was a cross-sectional study of all APP adult and pediatric fellowships in hospital medicine, in the United States, that were identifiable through May 2018. Multiple methods were used to identify all active fellowships. First, all training programs offering a Hospital Medicine Fellowship in the ARC-PA and Association of Postgraduate PA Programs databases were noted. Second, questionnaires were given out at the NP/PA forum at the national SHM conference in 2018 to gather information on existing APP fellowships. Third, similar online requests to identify known programs were posted to the SHM web forum Hospital Medicine Exchange (HMX). Fourth, Internet searches were used to discover additional programs. Once those fellowships were identified, surveys were sent to their program directors (PDs). These surveys not only asked the PDs about their fellowship but also asked them to identify additional APP fellowships beyond those that we had captured. Once additional programs were identified, a second round of surveys was sent to their PDs. This was performed in an iterative fashion until no additional fellowships were discovered.
The survey tool was developed and validated internally in the AAMC Survey Development style18 and was influenced by prior validated surveys of postgraduate medical fellowships.10,
A web-based survey format (Qualtrics) was used to distribute the questionnaire e-mail to the PDs. Follow up e-mail reminders were sent to all nonresponders to encourage full participation. Survey completion was voluntary; no financial incentives or gifts were offered. IRB approval was obtained at Johns Hopkins Bayview (IRB number 00181629). Descriptive statistics (proportions, means, and ranges as appropriate) were calculated for all variables. Stata 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, Texas. StataCorp LP) was used for data analysis.
RESULTS
In total, 11 fellowships were identified using our multimethod approach. We found four (36%) programs by utilizing existing online databases, two (18%) through the SHM questionnaire and HMX forum, three (27%) through internet searches, and the remaining two (18%) were referred to us by the other PDs who were surveyed. Of the programs surveyed, 10 were adult programs and one was a pediatric program. Surveys were sent to the PDs of the 11 fellowships, and all but one of them (10/11, 91%) responded. Respondent programs were given alphabetical designations A through J (Table).
Fellowship and Individual Characteristics
Most programs have been in existence for five years or fewer. Eighty percent of the programs are about one year in duration; two outlier programs have fellowship lengths of six months and 18 months. The main hospital where training occurs has a mean of 496 beds (range 213 to 900). Ninety percent of the hospitals also have physician residency training programs. Sixty percent of programs enroll two to four fellows per year while 40% enroll five or more. The salary range paid by the programs is $55,000 to >$70,000, and half the programs pay more than $65,000.
The majority of fellows accepted into APP fellowships in hospital medicine are women. Eighty percent of fellows are 26-30 years old, and 90% of fellows have been out of NP or PA school for one year or less. Both NP and PA applicants are accepted in 80% of fellowships.
Program Rationales
All programs reported that training and retaining applicants is the main driver for developing their fellowship, and 50% of them offer financial incentives for retention upon successful completion of the program. Forty percent of PDs stated that there is an implicit or explicit understanding that successful completion of the fellowship would result in further employment. Over the last five years, 89% (range: 71%-100%) of graduates were asked to remain for a full-time position after program completion.
In addition to training and retention, building an interprofessional team (50%), managing patient volume (30%), and reducing overhead (20%) were also reported as rationales for program development. The majority of programs (80%) have fellows bill for clinical services, and five of those eight programs do so after their fellows become more clinically competent.
Curricula
Of the nine adult programs, 67% teach explicitly to SHM core competencies and 33% send their fellows to the SHM NP/PA Boot Camp. Thirty percent of fellowships partner formally with either a physician residency or a local PA program to develop educational content. Six of the nine programs with active physician residencies, including the pediatric fellowship, offer shared educational experiences for the residents and APPs.
There are notable differences in clinical rotations between the programs (Figure 1). No single rotation is universally required, although general hospital internal medicine is required in all adult fellowships. The majority (80%) of programs offer at least one elective. Six programs reported mandatory rotations outside the department of medicine, most commonly neurology or the stroke service (four programs). Only one program reported only general medicine rotations, with no subspecialty electives.
There are also differences between programs with respect to educational experiences and learning formats (Figure 2). Each fellowship takes a unique approach to clinical instruction; teaching rounds and lecture attendance are the only experiences that are mandatory across the board. Grand rounds are available, but not required, in all programs. Ninety percent of programs offer or require fellow presentations, journal clubs, reading assignments, or scholarly projects. Fellow presentations (70%) and journal club attendance (60%) are required in more than half the programs; however, reading assignments (30%) and scholarly projects (20%) are rarely required.
Methods of Fellow Assessment
Each program surveyed has a unique method of fellow assessment. Ninety percent of the programs use more than one method to assess their fellows. Faculty reviews are most commonly used and are conducted in all rotations in 80% of fellowships. Both self-assessment exercises and written examinations are used in some rotations by the majority of programs. Capstone projects are required infrequently (30%).
DISCUSSION
We found several commonalities between the fellowships surveyed. Many of the program characteristics, such as years in operation, salary, duration, and lack of accreditation, are quite similar. Most fellowships also have a similar rationale for building their programs and use resources from the SHM to inform their curricula. Fellows, on average, share several demographic characteristics, such as age, gender, and time out of schooling. Conversely, we found wide variability in clinical rotations, the general teaching structure, and methods of fellow evaluation.
There have been several publications detailing successful individual APP fellowships in medical subspecialties,
It is noteworthy that every program surveyed was created with training and retention in mind, rather than other factors like decreasing overhead or managing patient volume. Training one’s own APPs so that they can learn on the job, come to understand expectations within a group, and witness the culture is extremely valuable. From a patient safety standpoint, it has been documented that physician hospitalists straight out of residency have a higher patient mortality compared with more experienced providers.
Several limitations to this study should be considered. While we used multiple strategies to locate as many fellowships as possible, it is unlikely that we successfully captured all existing programs, and new programs are being developed annually. We also relied on self-reported data from PDs. While we would expect PDs to provide accurate data, we could not externally validate their answers. Additionally, although our survey tool was reviewed extensively and validated internally, it was developed de novo for this study.
CONCLUSION
APP fellowships in hospital medicine have experienced marked growth since the first program was described in 2010. The majority of programs are 12 months long, operate in existing teaching centers, and are intended to further enhance the training and retention of newly graduated PAs and NPs. Despite their similarities, fellowships have striking variability in their methods of teaching and assessing their learners. Best practices have yet to be identified, and further study is required to determine how to standardize curricula across the board.
Acknowledgments
Disclosures
The authors report no conflicts of interest.
Funding
This project was supported by the Johns Hopkins School of Medicine Biostatistics, Epidemiology and Data Management (BEAD) Core. Dr. Wright is the Anne Gaines and G. Thomas Miller Professor of Medicine, which is supported through the Johns Hopkins’ Center for Innovative Medicine.
1. Auerbach DI, Staiger DO, Buerhaus PI. Growing ranks of advanced practice clinicians — implications for the physician workforce. N Engl J Med. 2018;378(25):2358-2360. doi: 10.1056/nejmp1801869. PubMed
2. Darves B. Midlevels make a rocky entrance into hospital medicine. Todays Hospitalist. 2007;5(1):28-32.
3. Polansky M. A historical perspective on postgraduate physician assistant education and the association of postgraduate physician assistant programs. J Physician Assist Educ. 2007;18(3):100-108. doi: 10.1097/01367895-200718030-00014.
4. FNP & AGNP Certification Candidate Handbook. The American Academy of Nurse Practitioners National Certification Board, Inc; 2018. https://www.aanpcert.org/resource/documents/AGNP FNP Candidate Handbook.pdf. Accessed December 20, 2018
5. Become a PA: Getting Your Prerequisites and Certification. AAPA. https://www.aapa.org/career-central/become-a-pa/. Accessed December 20, 2018.
6. ACGME Common Program Requirements. ACGME; 2017. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRs_2017-07-01.pdf. Accessed December 20, 2018
7. Committee on the Learning Health Care System in America; Institute of Medicine, Smith MD, Smith M, Saunders R, Stuckhardt L, McGinnis JM. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2013. PubMed
8. The Future of Nursing LEADING CHANGE, ADVANCING HEALTH. THE NATIONAL ACADEMIES PRESS; 2014. https://www.nap.edu/read/12956/chapter/1. Accessed December 16, 2018.
9. Hussaini SS, Bushardt RL, Gonsalves WC, et al. Accreditation and implications of clinical postgraduate pa training programs. JAAPA. 2016:29:1-7. doi: 10.1097/01.jaa.0000482298.17821.fb. PubMed
10. Polansky M, Garver GJH, Hilton G. Postgraduate clinical education of physician assistants. J Physician Assist Educ. 2012;23(1):39-45. doi: 10.1097/01367895-201223010-00008.
11. Will KK, Budavari AI, Wilkens JA, Mishark K, Hartsell ZC. A hospitalist postgraduate training program for physician assistants. J Hosp Med. 2010;5(2):94-98. doi: 10.1002/jhm.619. PubMed
12. Kartha A, Restuccia JD, Burgess JF, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. doi: 10.1002/jhm.2231. PubMed
13. Singh S, Fletcher KE, Schapira MM, et al. A comparison of outcomes of general medical inpatient care provided by a hospitalist-physician assistant model vs a traditional resident-based model. J Hosp Med. 2011;6(3):122-130. doi: 10.1002/jhm.826. PubMed
14. Hussaini SS, Bushardt RL, Gonsalves WC, et al. Accreditation and implications of clinical postgraduate PA training programs. JAAPA. 2016;29(5):1-7. doi: 10.1097/01.jaa.0000482298.17821.fb. PubMed
15. Postgraduate Programs. ARC-PA. http://www.arc-pa.org/accreditation/postgraduate-programs. Accessed September 13, 2018.
16. National Nurse Practitioner Residency & Fellowship Training Consortium: Mission. https://www.nppostgradtraining.com/About-Us/Mission. Accessed September 27, 2018.
17. NP/PA Boot Camp. State of Hospital Medicine | Society of Hospital Medicine. http://www.hospitalmedicine.org/events/nppa-boot-camp. Accessed September 13, 2018.
18. Gehlbach H, Artino Jr AR, Durning SJ. AM last page: survey development guidance for medical education researchers. Acad Med. 2010;85(5):925. doi: 10.1097/ACM.0b013e3181dd3e88.” Accessed March 10, 2018. PubMed
19. Kraus C, Carlisle T, Carney D. Emergency Medicine Physician Assistant (EMPA) post-graduate training programs: program characteristics and training curricula. West J Emerg Med. 2018;19(5):803-807. doi: 10.5811/westjem.2018.6.37892.
20. Shah NH, Rhim HJH, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. doi: 10.1002/jhm.2571. PubMed
21. Thompson BM, Searle NS, Gruppen LD, Hatem CJ, Nelson E. A national survey of medical education fellowships. Med Educ Online. 2011;16(1):5642. doi: 10.3402/meo.v16i0.5642. PubMed
22. Hooker R. A physician assistant rheumatology fellowship. JAAPA. 2013;26(6):49-52. doi: 10.1097/01.jaa.0000430346.04435.e4 PubMed
23. Keizer T, Trangle M. the benefits of a physician assistant and/or nurse practitioner psychiatric postgraduate training program. Acad Psychiatry. 2015;39(6):691-694. doi: 10.1007/s40596-015-0331-z. PubMed
24. Miller A, Weiss J, Hill V, Lindaman K, Emory C. Implementation of a postgraduate orthopaedic physician assistant fellowship for improved specialty training. JBJS Journal of Orthopaedics for Physician Assistants. 2017:1. doi: 10.2106/jbjs.jopa.17.00021.
25. Sharma P, Brooks M, Roomiany P, Verma L, Criscione-Schreiber L. physician assistant student training for the inpatient setting. J Physician Assist Educ. 2017;28(4):189-195. doi: 10.1097/jpa.0000000000000174. PubMed
26. Goodwin JS, Salameh H, Zhou J, Singh S, Kuo Y-F, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized medicare population. JAMA Intern Med. 2018;178(2):196. doi: 10.1001/jamainternmed.2017.7049. PubMed
27. Barnes H. Exploring the factors that influence nurse practitioner role transition. J Nurse Pract. 2015;11(2):178-183. doi: 10.1016/j.nurpra.2014.11.004. PubMed
28. Will K, Williams J, Hilton G, Wilson L, Geyer H. Perceived efficacy and utility of postgraduate physician assistant training programs. JAAPA. 2016;29(3):46-48. doi: 10.1097/01.jaa.0000480569.39885.c8. PubMed
29. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2011;7(3):190-194. doi: 10.1002/jhm.1001. PubMed
30. Cate O. Competency-based postgraduate medical education: past, present and future. GMS J Med Educ. 2017:34(5). doi: 10.3205/zma001146. PubMed
31. Exploring the ACGME Core Competencies (Part 1 of 7). NEJM Knowledge. https://knowledgeplus.nejm.org/blog/exploring-acgme-core-competencies/. Accessed October 24, 2018.
32. Core Competencies. Core Competencies | Society of Hospital Medicine. http://www.hospitalmedicine.org/professional-development/core-competencies/. Accessed October 24, 2018.
1. Auerbach DI, Staiger DO, Buerhaus PI. Growing ranks of advanced practice clinicians — implications for the physician workforce. N Engl J Med. 2018;378(25):2358-2360. doi: 10.1056/nejmp1801869. PubMed
2. Darves B. Midlevels make a rocky entrance into hospital medicine. Todays Hospitalist. 2007;5(1):28-32.
3. Polansky M. A historical perspective on postgraduate physician assistant education and the association of postgraduate physician assistant programs. J Physician Assist Educ. 2007;18(3):100-108. doi: 10.1097/01367895-200718030-00014.
4. FNP & AGNP Certification Candidate Handbook. The American Academy of Nurse Practitioners National Certification Board, Inc; 2018. https://www.aanpcert.org/resource/documents/AGNP FNP Candidate Handbook.pdf. Accessed December 20, 2018
5. Become a PA: Getting Your Prerequisites and Certification. AAPA. https://www.aapa.org/career-central/become-a-pa/. Accessed December 20, 2018.
6. ACGME Common Program Requirements. ACGME; 2017. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRs_2017-07-01.pdf. Accessed December 20, 2018
7. Committee on the Learning Health Care System in America; Institute of Medicine, Smith MD, Smith M, Saunders R, Stuckhardt L, McGinnis JM. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2013. PubMed
8. The Future of Nursing LEADING CHANGE, ADVANCING HEALTH. THE NATIONAL ACADEMIES PRESS; 2014. https://www.nap.edu/read/12956/chapter/1. Accessed December 16, 2018.
9. Hussaini SS, Bushardt RL, Gonsalves WC, et al. Accreditation and implications of clinical postgraduate pa training programs. JAAPA. 2016:29:1-7. doi: 10.1097/01.jaa.0000482298.17821.fb. PubMed
10. Polansky M, Garver GJH, Hilton G. Postgraduate clinical education of physician assistants. J Physician Assist Educ. 2012;23(1):39-45. doi: 10.1097/01367895-201223010-00008.
11. Will KK, Budavari AI, Wilkens JA, Mishark K, Hartsell ZC. A hospitalist postgraduate training program for physician assistants. J Hosp Med. 2010;5(2):94-98. doi: 10.1002/jhm.619. PubMed
12. Kartha A, Restuccia JD, Burgess JF, et al. Nurse practitioner and physician assistant scope of practice in 118 acute care hospitals. J Hosp Med. 2014;9(10):615-620. doi: 10.1002/jhm.2231. PubMed
13. Singh S, Fletcher KE, Schapira MM, et al. A comparison of outcomes of general medical inpatient care provided by a hospitalist-physician assistant model vs a traditional resident-based model. J Hosp Med. 2011;6(3):122-130. doi: 10.1002/jhm.826. PubMed
14. Hussaini SS, Bushardt RL, Gonsalves WC, et al. Accreditation and implications of clinical postgraduate PA training programs. JAAPA. 2016;29(5):1-7. doi: 10.1097/01.jaa.0000482298.17821.fb. PubMed
15. Postgraduate Programs. ARC-PA. http://www.arc-pa.org/accreditation/postgraduate-programs. Accessed September 13, 2018.
16. National Nurse Practitioner Residency & Fellowship Training Consortium: Mission. https://www.nppostgradtraining.com/About-Us/Mission. Accessed September 27, 2018.
17. NP/PA Boot Camp. State of Hospital Medicine | Society of Hospital Medicine. http://www.hospitalmedicine.org/events/nppa-boot-camp. Accessed September 13, 2018.
18. Gehlbach H, Artino Jr AR, Durning SJ. AM last page: survey development guidance for medical education researchers. Acad Med. 2010;85(5):925. doi: 10.1097/ACM.0b013e3181dd3e88.” Accessed March 10, 2018. PubMed
19. Kraus C, Carlisle T, Carney D. Emergency Medicine Physician Assistant (EMPA) post-graduate training programs: program characteristics and training curricula. West J Emerg Med. 2018;19(5):803-807. doi: 10.5811/westjem.2018.6.37892.
20. Shah NH, Rhim HJH, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. doi: 10.1002/jhm.2571. PubMed
21. Thompson BM, Searle NS, Gruppen LD, Hatem CJ, Nelson E. A national survey of medical education fellowships. Med Educ Online. 2011;16(1):5642. doi: 10.3402/meo.v16i0.5642. PubMed
22. Hooker R. A physician assistant rheumatology fellowship. JAAPA. 2013;26(6):49-52. doi: 10.1097/01.jaa.0000430346.04435.e4 PubMed
23. Keizer T, Trangle M. the benefits of a physician assistant and/or nurse practitioner psychiatric postgraduate training program. Acad Psychiatry. 2015;39(6):691-694. doi: 10.1007/s40596-015-0331-z. PubMed
24. Miller A, Weiss J, Hill V, Lindaman K, Emory C. Implementation of a postgraduate orthopaedic physician assistant fellowship for improved specialty training. JBJS Journal of Orthopaedics for Physician Assistants. 2017:1. doi: 10.2106/jbjs.jopa.17.00021.
25. Sharma P, Brooks M, Roomiany P, Verma L, Criscione-Schreiber L. physician assistant student training for the inpatient setting. J Physician Assist Educ. 2017;28(4):189-195. doi: 10.1097/jpa.0000000000000174. PubMed
26. Goodwin JS, Salameh H, Zhou J, Singh S, Kuo Y-F, Nattinger AB. Association of hospitalist years of experience with mortality in the hospitalized medicare population. JAMA Intern Med. 2018;178(2):196. doi: 10.1001/jamainternmed.2017.7049. PubMed
27. Barnes H. Exploring the factors that influence nurse practitioner role transition. J Nurse Pract. 2015;11(2):178-183. doi: 10.1016/j.nurpra.2014.11.004. PubMed
28. Will K, Williams J, Hilton G, Wilson L, Geyer H. Perceived efficacy and utility of postgraduate physician assistant training programs. JAAPA. 2016;29(3):46-48. doi: 10.1097/01.jaa.0000480569.39885.c8. PubMed
29. Torok H, Lackner C, Landis R, Wright S. Learning needs of physician assistants working in hospital medicine. J Hosp Med. 2011;7(3):190-194. doi: 10.1002/jhm.1001. PubMed
30. Cate O. Competency-based postgraduate medical education: past, present and future. GMS J Med Educ. 2017:34(5). doi: 10.3205/zma001146. PubMed
31. Exploring the ACGME Core Competencies (Part 1 of 7). NEJM Knowledge. https://knowledgeplus.nejm.org/blog/exploring-acgme-core-competencies/. Accessed October 24, 2018.
32. Core Competencies. Core Competencies | Society of Hospital Medicine. http://www.hospitalmedicine.org/professional-development/core-competencies/. Accessed October 24, 2018.
© 2019 Society of Hospital Medicine
When patients on target-specific oral anticoagulants need surgery
More then 2.5 million patients in the United States are on long-term anticoagulation therapy for atrial fibrillation, venous thromboembolic disease, or mechanical heart valves,1 and the number is expected to rise as the population ages. Each year, about 10% of these patients undergo an invasive procedure or surgery that requires temporary interruption of anticoagulation.2
Most physicians are familiar with the perioperative management of warfarin, a vitamin K antagonist, since for decades it has been the sole oral anticoagulant available. However, many physicians lack experience with the three target-specific oral anticoagulants (TSOACs; also known as “novel” oral anticoagulants) approved so far: the direct thrombin inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
With their rapid onset of action, predictable pharmacokinetics, relatively short half-lives, and fewer drug-drug interactions than warfarin, TSOACs overcome many of the limitations of the older oral anticoagulant warfarin. In many ways, these qualities simplify the perioperative management of anticoagulation. At the same time, these new drugs also bring new challenges: caution is needed in patients with renal impairment; the level of anticoagulation is difficult to assess; and there is no specific antidote or standardized procedure to reverse their anticoagulant effect. While various periprocedural protocols for TSOAC therapy have been proposed, evidence-based guidelines are still to come.
This article first discusses the pharmacology of dabigatran, rivaroxaban, and apixaban that is pertinent to the perioperative period. It then briefly reviews the general principles of perioperative management of anticoagulation. The final section provides specific recommendations for the perioperative management of TSOACs.
PHARMACOLOGY OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
Dabigatran, a factor IIa inhibitor
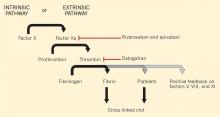
Dabigatran is an oral direct thrombin (factor IIa) inhibitor. It exerts its anticoagulant effect by blocking the generation of fibrin, inhibiting platelet aggregation, and dampening the activity of factors V, VIII, and XI (Figure 1).3,4 From its introduction in October 2010 through August 2012, nearly 3.7 million prescriptions were dispensed to 725,000 patients in the United States.5
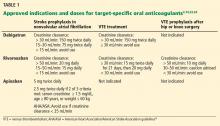
Indications for dabigatran. Dabigatran is approved in the United States and Canada for preventing stroke in nonvalvular atrial fibrillation (Table 1).6 More recently, it received US approval for treating deep vein thrombosis or pulmonary embolism after 5 to 10 days of a parenteral anticoagulant.7,8 It is also approved in Europe and Canada for preventing venous thromboembolism (VTE) after total hip replacement and knee arthroplasty.9,10
Dabigatran is contraindicated in patients with a mechanical heart valve, based on a phase 2 study in which it conferred a higher risk of thromboembolism and bleeding than warfarin.3,11
Pharmacokinetics of dabigatran. Dabigatran is formulated as a prodrug, dabigatran etexilate, in a capsule containing multiple small pellets.12 The capsules should not be crushed, as this significantly increases oral bioavailability. The prodrug is absorbed across the gastric mucosa and is then rapidly converted to the active form (Table 2).
Plasma concentrations peak within 2 hours of ingestion, which means that therapeutic anticoagulation is achieved shortly after taking the drug.
Only 35% of dabigatran is protein-bound, which allows it to be removed by hemodialysis. Nearly 85% of the drug is eliminated in the urine. It has a half-life of 13 to 15 hours in patients with normal renal function.3 However, its half-life increases to about 27 hours in patients whose creatinine clearance is less than 30 mL/min. As a result, the dose must be reduced in patients with renal impairment (Table 1).
Dabigatran is not metabolized by the cytochrome P450 enzymes, but it is a substrate for P-glycoprotein, so it still has the potential for drug-drug interactions.3 Practitioners should be familiar with these potential interactions (Table 3), as they can result in higher- or lower-than-expected plasma concentrations of dabigatran in the perioperative period.13
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is an oral direct factor Xa inhibitor. It has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular atrial fibrillation, for VTE treatment, and for VTE prophylaxis after hip replacement or knee replacement (Table 1).14–20 It has not yet been studied in patients with hip fracture.
Pharmacokinetics of rivaroxaban. Rivaroxaban is manufactured as a tablet that is best absorbed in the stomach (Table 2).14 In contrast to dabigatran, it can be crushed and, for example, mixed with applesauce for patients who have trouble swallowing. It can also be mixed with water and given via nasogastric tube; however, postpyloric administration should be avoided.
Plasma concentrations peak within a few hours after ingestion. Rivaroxaban is highly protein-bound, so it cannot be eliminated by hemodialysis.
The drug relies on renal elimination to a smaller degree than dabigatran, with one-third of the dose eliminated unchanged in the urine, one-third eliminated in the urine as inactive metabolite, and the remaining one-third eliminated in the feces. However, enough parent compound is cleared through the kidneys that the half-life of rivaroxaban increases from 8.3 hours in healthy individuals to 9.5 hours in patients whose creatinine clearance is less than 30 mL/min.21 As with dabigatran, the dose must be adjusted for renal impairment (Table 1).
Rivaroxaban has significant liver metabolism, specifically through the cytochrome P450 3A4 enzyme, and it is also a substrate of P-glycoprotein. Therefore, potential drug-drug interactions must be taken into account, as they may lead to important alterations in plasma concentrations (Table 3).
Apixaban, a factor Xa inhibitor
Apixaban is also an oral direct factor Xa inhibitor. It is the newest of the oral anticoagulants to be approved in the United States, specifically for preventing stroke in nonvalvular atrial fibrillation (Table 1).22
Pharmacokinetics of apixaban. Apixaban is produced as a tablet that is absorbed slowly through the gastrointestinal tract, mainly the distal small bowel and ascending colon (Table 2).23
Peak plasma concentrations are reached a few hours after ingestion. Like rivaroxaban, apixaban is highly protein-bound, so it cannot be removed by hemodialysis.
Apixaban is similar to rivaroxaban in that 27% of the parent compound is cleared through the kidneys, it undergoes significant hepatic metabolism through cytochrome P450 3A4, and it is a substrate for P-glycoprotein.
Drug-drug interactions must be considered as a potential source of altered drug exposure and clearance (Table 3).
Unlike dabigatran and rivaroxaban, dose reduction is not based on the calculated creatinine clearance. Instead, a reduced dose is required if the patient meets two of the following three criteria:
- Serum creatinine level ≥ 1.5 mg/dL
- Age ≥ 80
- Weight ≤ 60 kg (Table 1).
The American Heart Association/American Stroke Association guidelines further recommend against using apixaban in patients with a creatinine clearance less than 25 mL/min.24
Edoxaban, a factor Xa inhibitor in development
Edoxaban (Savaysa), another factor Xa inhibitor, is available in Japan and has been submitted for approval in the United States for treating VTE and for preventing stroke in patients with
PERIOPERATIVE CONSIDERATIONS IN ANTICOAGULATION
Before addressing the perioperative management of TSOACs, let us review the evidence guiding the perioperative management of any chronic anticoagulant.
In fact, no large prospective randomized trial has clearly defined the risks and benefits of using or withholding a bridging anticoagulation strategy around surgery and other procedures, though the PERIOP 2 and BRIDGE trials are currently ongoing.25,26 There are some data regarding continuing anticoagulation without interruption, but they have mainly been derived from specific groups (eg, patients on warfarin undergoing cardiac pacemaker or defibrillator placement) and in procedures that pose a very low risk of bleeding complications (eg, minor dental extractions, cataract surgery, dermatologic procedures).2,27 Recommendations are, therefore, necessarily based on small perioperative trials and data gleaned from cohort review and from studies that did not involve surgical patients.
Ultimately, the decisions whether to discontinue oral anticoagulants and whether to employ bridging anticoagulation are based on assumptions about the risks of bleeding and the risk of thrombotic events, with similar assumptions regarding the effects of anticoagulants on both outcomes. In addition, the relative acceptance of bleeding vs thrombotic risks implicitly guides these complex decisions.
Perioperative bleeding risk
Many risk factors specific to the patient and to the type of surgery affect the rates and severity of perioperative bleeding.28
As for patient-specific risk factors, a small retrospective cohort analysis revealed that a HAS-BLED score of 3 or higher was highly discriminating in predicting perioperative bleeding in atrial fibrillation patients receiving anticoagulation.29 (The HAS-BLED score is based on hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [age > 65] and drug therapy.30) However, there are no widely validated tools that incorporate patient-specific factors to accurately predict bleeding risk in an individual patient.
Therefore, the American College of Chest Physicians (ACCP) guidelines suggest coarsely categorizing bleeding risk as either low or high solely on the basis of the type of procedure.2 Procedures considered “high-risk” have a risk greater than 1.5% to 2% and include urologic surgery involving the prostate or kidney, colonic polyp resections, surgeries involving highly vascular organs such as the liver or spleen, joint replacements, cancer surgeries, and cardiac or neurosurgical procedures.
Perioperative thrombotic risk
The ACCP guidelines2 place patients with atrial fibrillation, VTE, or mechanical heart valves in three risk groups for perioperative thromboembolism without anticoagulation, based on their annual risk of a thrombotic event:
- High risk—annual risk of a thrombotic event > 10%
- Moderate risk—5% to 10%
- Low risk—< 5%.
Comparing the risks calculated by these methods with the real-world risk of perioperative thrombosis highlights the problem of applying nonperioperative risk calculations: the perioperative period exposes patients to a higher risk than these models would predict.31 Nonetheless, these risk categorizations likely have some validity in stratifying patients into risk groups, even if the absolute risks are inaccurate.
Perioperative bridging for patients taking warfarin
Many patients with atrial fibrillation, VTE, or a mechanical heart valve need to interrupt their warfarin therapy because of the bleeding risk of an upcoming procedure.
The perioperative management of warfarin and other vitamin K antagonists is challenging because of the pharmacokinetics and pharmacodynamics of these drugs. Because it has a long half-life, warfarin usually must be stopped 4 to 5 days before a procedure in order to allow not only adequate clearance of the drug itself, but also restoration of functional clotting factors to normal or near-normal levels.12 Warfarin can generally be resumed 12 to 24 hours after surgery, assuming adequate hemostasis has been achieved, and it will again take several days for the INR to reach the therapeutic range.
The ACCP guidelines recommend using the perioperative risk of thromboembolism to make decisions about the need for bridging anticoagulation during warfarin interruption.2 They suggest that patients at high risk of thrombosis receive bridging with an alternative anticoagulant such as low-molecular-weight heparin or unfractionated heparin, because of the prolonged duration of subtherapeutic anticoagulation.
There has been clinical interest in using a TSOAC instead of low-molecular-weight or unfractionated heparin for bridging in the perioperative setting. Although this approach may be attractive from a cost and convenience perspective, it cannot be endorsed as yet because of the lack of information on the pros and cons of such an approach.
Patients at low thrombotic risk do not require bridging. In patients at moderate risk, the decision to bridge or not to bridge is based on careful consideration of patient-specific and surgery-specific factors.
PERIOPERATIVE MANAGEMENT OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
As summarized above, the perioperative management strategy for chronic anticoagulation is based on limited evidence, even for drugs as well established as warfarin.
The most recent ACCP guidelines on the perioperative management of antithrombotic therapy do not mention TSOACs.2 For now, the management strategy must be based on the pharmacokinetics of the drugs, package inserts from the manufacturers, and expert recommendations.3,14,23,32–34 Fortunately, because TSOACs have a more favorable pharmacokinetic profile than that of warfarin, their perioperative uses should be more streamlined. As always, the goal is to minimize the risk of both periprocedural bleeding and thromboembolism.
Timing of cessation of anticoagulation
The timing of cessation of TSOACs before an elective procedure depends primarily on two factors: the bleeding risk of the procedure and the patient’s renal function. Complete clearance of the medication is not necessary in all circumstances.
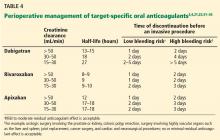
TSOACs should be stopped four to five half-lives before a procedure with a high bleeding risk, so that there is no or only minimal residual anticoagulant effect. The drug can be stopped two to three half-lives before a procedure with a low bleeding risk. Remember: the half-life increases as creatinine clearance decreases.
Specific recommendations may vary across institutions, but a suggested strategy is shown in Table 4.3,4,21,23,32–35 For the small subset of patients on P-glycoprotein or cytochrome P450 inhibitors or inducers, further adjustment in the time of discontinuation may be required.
Therapy does not need to be interrupted for procedures with a very low bleeding risk, as defined above.33,34 There is also preliminary evidence that TSOACs, similar to warfarin, may be continued during cardiac pacemaker or defibrillator placement.36
Evidence from clinical trials of perioperative TSOAC management
While the above recommendations are logical, studies are needed to prospectively evaluate perioperative management strategies.
The RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), which compared the effects of dabigatran and warfarin in preventing stroke in patients with atrial fibrillation, is one of the few clinical trials that also looked at periprocedural bleeding.37 About a quarter of the RE-LY participants required interruption of anticoagulation for a procedure.
Warfarin was managed according to local practices. For most of the study, the protocol required that dabigatran be discontinued 24 hours before a procedure, regardless of renal function or procedure type. The protocol was later amended and closely mirrored the management plan outlined in Table 4.
With either protocol, there was no statistically significant difference between dabigatran and warfarin in the rates of bleeding and thrombotic complications in the 7 days before or 30 days after the procedure.
A major limitation of the study was that most patients underwent a procedure with a low bleeding risk, so the analysis was likely underpowered to evaluate rates of bleeding in higher-risk procedures.
The ROCKET-AF trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) also shed light on periprocedural bleeding.15 About 15% of the participants required temporary interruption of anticoagulation for a surgical or invasive procedure.38
The study protocol called for discontinuing rivaroxaban 2 days before any procedure. Warfarin was to be held for 4 days to achieve a goal INR of 1.5 or less.15
Rates of major and nonmajor clinically significant bleeding at 30 days were similar with rivaroxaban and with warfarin.38 As with the RE-LY trial, the retrospective analysis was probably underpowered for assessing rates of bleeding in procedures with higher risk.
Perioperative bridging
While stopping a TSOAC in the perioperative period decreases the risk of bleeding, it naturally increases the risk of thromboembolism. However, patients on TSOACs should not routinely require perioperative bridging with an alternative anticoagulant, regardless of thrombotic risk.
Of note, dabigatran, rivaroxaban, and apixaban carry black-box warnings that discontinuation places patients at higher risk of thrombotic events.3,14,23 These warnings further state that coverage with an alternative anticoagulant should be strongly considered during interruption of therapy for reasons other than pathologic bleeding.
However, it does not necessarily follow that perioperative bridging is required. For example, the warning for rivaroxaban is based on the finding in the ROCKET-AF trial that patients in the rivaroxaban group had higher rates of stroke than those in the warfarin group after the study drugs were stopped at the end of the trial.39 While there was initial concern that this could represent a prothrombotic rebound effect, the authors subsequently showed that patients in the rivaroxaban group were more likely to have had a subtherapeutic INR when transitioning to open-label vitamin-K-antagonist therapy.39,40 There was no difference in the rate of stroke or systemic embolism between the rivaroxaban and warfarin groups when anticoagulation was temporarily interrupted for a procedure.38
The risks and benefits of perioperative bridging with TSOACs are difficult to evaluate, given the dearth of trial data. In the RE-LY trial, only 17% of patients on dabigatran and 28% of patients on warfarin underwent periprocedural bridging.37 The selection criteria and protocol for bridging were not reported. In the ROCKET-AF trial, only 9% of patients received bridging therapy despite a mean CHADS2 score of 3.4.38 (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes; 2 points for stroke or transient ischemic attack.) The decision to bridge or not was left to the individual investigator. As a result, the literature offers diverse opinions about the appropriateness of transitioning to an alternative anticoagulant.41–43
Bridging does not make sense in most instances, since anticoagulants such as low-molecular-weight heparin have pharmacokinetics similar to those of the available TSOACs and also depend on renal clearance.41 However, there may be situations in which patients must be switched to a parenteral anticoagulant such as unfractionated or low-molecular-weight heparin. For example, if a TSOAC has to be held, the patient has acute renal failure, and a needed procedure is still several days away, it would be reasonable to start a heparin drip for an inpatient at increased thrombotic risk.
In patients with normal renal function, these alternative anticoagulants should be started at the time the next TSOAC dose would have been due.3,14,23 In patients with reduced renal function, initiation of an alternative anticoagulant may need to be delayed 12 to 48 hours depending on which TSOAC is being used, as well as on the degree of renal dysfunction. This delay would help ensure that the onset of anticoagulation with the alternative anticoagulant is timed with the offset of therapeutic anticoagulation with the TSOAC.
Although limited, information from available coagulation assays may assist with the timing of initiation of an alternative anticoagulant (see the following section on laboratory monitoring). Serial testing with appropriate coagulation assays may help identify when most of a TSOAC has been cleared from a patient.
Laboratory monitoring
Inevitably, some patients on TSOACs require urgent or emergency surgery. In certain situations, such as before an orthopedic spine procedure, in which the complications of bleeding could be devastating, it may be necessary to know if any residual anticoagulant effect is present.
Monitoring dabigatran. As one might expect, direct thrombin inhibitors such as dabigatran can prolong the prothrombin time and activated partial thromboplastin time (aPTT).44–47 However, the prothrombin time is not recommended for assessing the level of anticoagulation from dabigatran. Many institutions may be using a normal aPTT to rule out therapeutic concentrations of dabigatran, based on results from early in vitro and ex vivo studies.46 While appealing from a practical standpoint, practitioners should exercise caution when relying on the aPTT to assess the risk of perioperative bleeding. A more recent investigation in patients treated with dabigatran found that up to 35% of patients with a normal aPTT still had a plasma concentration in the therapeutic range.48
The thrombin time and ecarin clotting time are more sensitive tests for dabigatran. A normal thrombin time or ecarin clotting time indicates that no or only minimal dabigatran is present.48 Unfortunately, these two tests often are either unavailable or are associated with long turnaround times, which limits their usefulness in the perioperative setting.
Monitoring rivaroxaban and apixaban. Factor Xa inhibitors such as rivaroxaban and apixaban can also influence the prothrombin time and aPTT (Figure 1).44–47,49,50 The aPTT is relatively insensitive to these drugs at low concentrations. It has been suggested that a normal prothrombin time can reasonably exclude therapeutic concentrations of rivaroxaban.45,46 However, the effects on the prothrombin time are highly variable, changing with the reagent used.49,50 In addition, apixaban appears to have less impact on the prothrombin time overall. The INR is not recommended for monitoring the effect of factor Xa inhibitors.
Anti-factor Xa assays likely represent the best option to provide true quantitative information on the level of anticoagulation with either rivaroxaban or apixaban. However, the assays must be specifically calibrated for each drug for results to be useful. (Anti-factor Xa assays cannot be used for heparin or low-molecular-weight heparin.) Further, most institutions do not yet have this capability. When appropriately calibrated, normal anti-factor Xa levels would exclude any effect of rivaroxaban or apixaban.
Reversal of anticoagulation
If patients on TSOACs require emergency surgery or present with significant bleeding in the setting of persistent anticoagulation, it may be necessary to try to reverse the anticoagulation.
Unlike warfarin or heparin, TSOACS do not have specific reversal agents, though specific antidotes are being developed. For example, researchers are evaluating antibodies capable of neutralizing dabigatran, as well as recombinant thrombin and factor Xa molecules that could antagonize dabigatran and rivaroxaban, respectively.51–53
Reversal can be attempted by neutralizing or removing the offending drug. Activated charcoal may be able to reduce absorption of TSOACs that were recently ingested,44 and dabigatran can be removed by hemodialysis.
However, certain practical considerations may limit the use of dialysis in the perioperative period. Insertion of a temporary dialysis line in an anticoagulated patient poses additional bleeding risks. A standard 4-hour hemodialysis session may remove only about 70% of dabigatran from the plasma, which may not be enough to prevent perioperative bleeding.54 Dabigatran also tends to redistribute from adipose tissue back into plasma after each dialysis session.55 Serial sessions of high-flux intermittent hemodialysis or continuous renal replacement therapy may therefore be needed to counteract rebound elevations in the dabigatran concentration.
Reversal can also be attempted through activation of the coagulation cascade via other mechanisms. Fresh-frozen plasma is unlikely to be a practical solution for reversal.44 Although it can readily replace the clotting factors depleted by vitamin K antagonists, large volumes of fresh-frozen plasma would be needed to overwhelm thrombin or factor Xa inhibition by TSOACs.
There are limited data on the use of prothrombin complex concentrates or recombinant activated factor VIIa in patients on TSOACs, though their use can be considered.56 In a trial in 12 healthy participants, a nonactivated four-factor prothrombin complex concentrate containing factors II, VII, IX, and X immediately and completely reversed the anticoagulant effect of rivaroxaban but had no effect on dabigatran.57 Before 2013, there were no nonactivated four-factor prothrombin complex concentrates available in the United States. The FDA has since approved Kcentra for the urgent reversal of vitamin K antagonists, meaning that the reversal of TSOACs in major bleeding events would still be off-label.58 Giving any of the clotting factors carries a risk of thromboembolism.
Resumption of anticoagulation
TSOACs have a rapid onset of action, and therapeutic levels are reached within a few hours of administration.
Extrapolating from the ACCP guidelines, TSOACs can generally be restarted at therapeutic doses 24 hours after low-bleeding-risk procedures.2 Therapeutic dosing should be delayed 48 to 72 hours after a procedure with a high bleeding risk, assuming adequate hemostasis has been achieved. Prophylactic unfractionated heparin or low-molecular-weight heparin therapy can be given in the interim if deemed safe. Alternatively, for orthopedic patients ultimately transitioning back to therapeutic rivaroxaban after hip or knee arthroplasty, prophylactic rivaroxaban doses can be started 6 to 10 hours after surgery.14
There are numerous reasons why the resumption of TSOACs may have to be delayed after surgery, including nothing-by-mouth status, postoperative nausea and vomiting, ileus, gastric or bowel resection, and the anticipated need for future procedures. Since dabigatran capsules cannot be crushed, they cannot be given via nasogastric tube in patients with postoperative dysphagia. Parenteral anticoagulants should be used until these issues resolve.
Unfractionated heparin is still the preferred anticoagulant in unstable or potentially unstable patients, given its ease of monitoring, quick offset of action, and reversibility. When patients have stabilized, TSOACs can be resumed when the next dose of low-molecular-weight heparin would have been due or when the unfractionated heparin drip is discontinued.3,14,23
UNTIL EVIDENCE-BASED GUIDELINES ARE DEVELOPED
The development of TSOACs has ushered in an exciting new era for anticoagulant therapy. Providers involved in perioperative medicine will increasingly encounter patients on dabigatran, rivaroxaban, and apixaban. However, until evidence-based guidelines are developed for these new anticoagulants, clinicians will have to apply their knowledge of pharmacology and critically evaluate expert recommendations in order to manage patients safely throughout the perioperative period.
- Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):299S–339S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Boehringer Ingelheim Pharmaceuticals, Inc. PRADAXA (dabigatran) package insert. http://bidocs.boehringer-ingelheim.com/BIWebAc-cess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed August 6, 2014.
- Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology 2013; 118:1466–1474.
- US Food and Drug Administration (FDA). FDA drug safety communication: update on the risk for serious bleeding events with the anticoagulant Pradaxa (dabigatran). www.fda.gov/drugs/drugsafety/ucm326580.htm. Accessed August 6, 2014.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Eriksson BI, Dahl OE, Rosencher N, Büller HR, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Janssen Pharmaceuticals, Inc. XARELTO (rivaroxaban) package insert. www.xareltohcp.com/about-xarelto/about-xarelto.html?utm_source=google&utm_medium=cpc&utm_campaign=Branded+-+Broad&utm_term=xarelto%20rivaroxaban&utm_content=Xarelto+Rivaroxaban|mkwid|sxSDxPb4m_dc|pcrid|34667840494. Accessed August 6, 2014.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–391.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008; 372:31–39.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 2010; 70:703–712.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Bristol-Myers Squibb Company. ELIQUIS (apixaban) package insert. www.eliquis.com/index.aspx. Accessed August 6, 2014.
- Furie KL, Goldstein LB, Albers GW, et al; American Heart Association Stroke Council. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:3442–3453.
- ClinicalTrials.gov, US National Institutes of Health. PERIOP 2 - A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin. http://clinicaltri-als.gov/show/NCT00432796. Accessed August 6, 2014.
- ClinicalTrials.gov, US National Institutes of Health. Effectiveness of Bridging Anticoagulation for Surgery (The BRIDGE Study). http://clinicaltrials.gov/ct2/show/NCT00786474. Accessed August 6, 2014.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J 2013; 165:427.e1–433.e1.
- Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost 2012; 108:65–73.
- Pisters R, Lane DA, Nieuwlaaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 2010; 138:1093–1100.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis 2013; 36:212–222.
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012; 120:2954–2962.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87(suppl 1):S141–S145.
- Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111:1165–1168.
- Healey JS, Eikelboom J, Douketis J, et al; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126:343–348.
- Sherwood MW, Douketis JD, Patel MR, et al; on behalf of the ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 129:1850–1859.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol 2013; 61:659–660.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation 2012; 126:1573–1576.
- Sié P, Samama CM, Godier A, et al; Working Group on Perioperative Haemostasis. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and the French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis 2011; 104:669–676.
- King CS, Holley AB, Moores LK. Moving toward a more ideal anticoagulant: the oral direct thrombin and factor Xa inhibitors. Chest 2013; 143:1106–1116.
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11:756–760.
- Baglin T, Keeling D, Kitchen S; British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 2012; 159:427–429.
- Mani H, Kasper A, Lindhoff-Last E. Measuring the anticoagulant effects of target specific oral anticoagulants—reasons, methods and current limitations. J Thromb Thrombolysis 2013; 36:187–194.
- Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013; 11:1493–1502.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013; 33:1223–1245.
- Van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol 2011; 57:E1130.
- Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold D, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. Abstract presented at the International Society of Thrombosis and Haemostasis 2013 Congress. http://onlinelibrary.wiley.com/doi/10.1111/jth.2013.11.issue-s2/issuetoc. Accessed August 6, 2014.
- Lu G, Luan P, Hollenbach SJ, et al. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors (abstract). J Thromb Haemost 2009; 7(suppl 2):abstract OC-TH-107.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis 2013; 36:195–202.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128:1234–1243.
More then 2.5 million patients in the United States are on long-term anticoagulation therapy for atrial fibrillation, venous thromboembolic disease, or mechanical heart valves,1 and the number is expected to rise as the population ages. Each year, about 10% of these patients undergo an invasive procedure or surgery that requires temporary interruption of anticoagulation.2
Most physicians are familiar with the perioperative management of warfarin, a vitamin K antagonist, since for decades it has been the sole oral anticoagulant available. However, many physicians lack experience with the three target-specific oral anticoagulants (TSOACs; also known as “novel” oral anticoagulants) approved so far: the direct thrombin inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
With their rapid onset of action, predictable pharmacokinetics, relatively short half-lives, and fewer drug-drug interactions than warfarin, TSOACs overcome many of the limitations of the older oral anticoagulant warfarin. In many ways, these qualities simplify the perioperative management of anticoagulation. At the same time, these new drugs also bring new challenges: caution is needed in patients with renal impairment; the level of anticoagulation is difficult to assess; and there is no specific antidote or standardized procedure to reverse their anticoagulant effect. While various periprocedural protocols for TSOAC therapy have been proposed, evidence-based guidelines are still to come.
This article first discusses the pharmacology of dabigatran, rivaroxaban, and apixaban that is pertinent to the perioperative period. It then briefly reviews the general principles of perioperative management of anticoagulation. The final section provides specific recommendations for the perioperative management of TSOACs.
PHARMACOLOGY OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
Dabigatran, a factor IIa inhibitor
Dabigatran is an oral direct thrombin (factor IIa) inhibitor. It exerts its anticoagulant effect by blocking the generation of fibrin, inhibiting platelet aggregation, and dampening the activity of factors V, VIII, and XI (Figure 1).3,4 From its introduction in October 2010 through August 2012, nearly 3.7 million prescriptions were dispensed to 725,000 patients in the United States.5
Indications for dabigatran. Dabigatran is approved in the United States and Canada for preventing stroke in nonvalvular atrial fibrillation (Table 1).6 More recently, it received US approval for treating deep vein thrombosis or pulmonary embolism after 5 to 10 days of a parenteral anticoagulant.7,8 It is also approved in Europe and Canada for preventing venous thromboembolism (VTE) after total hip replacement and knee arthroplasty.9,10
Dabigatran is contraindicated in patients with a mechanical heart valve, based on a phase 2 study in which it conferred a higher risk of thromboembolism and bleeding than warfarin.3,11
Pharmacokinetics of dabigatran. Dabigatran is formulated as a prodrug, dabigatran etexilate, in a capsule containing multiple small pellets.12 The capsules should not be crushed, as this significantly increases oral bioavailability. The prodrug is absorbed across the gastric mucosa and is then rapidly converted to the active form (Table 2).
Plasma concentrations peak within 2 hours of ingestion, which means that therapeutic anticoagulation is achieved shortly after taking the drug.
Only 35% of dabigatran is protein-bound, which allows it to be removed by hemodialysis. Nearly 85% of the drug is eliminated in the urine. It has a half-life of 13 to 15 hours in patients with normal renal function.3 However, its half-life increases to about 27 hours in patients whose creatinine clearance is less than 30 mL/min. As a result, the dose must be reduced in patients with renal impairment (Table 1).
Dabigatran is not metabolized by the cytochrome P450 enzymes, but it is a substrate for P-glycoprotein, so it still has the potential for drug-drug interactions.3 Practitioners should be familiar with these potential interactions (Table 3), as they can result in higher- or lower-than-expected plasma concentrations of dabigatran in the perioperative period.13
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is an oral direct factor Xa inhibitor. It has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular atrial fibrillation, for VTE treatment, and for VTE prophylaxis after hip replacement or knee replacement (Table 1).14–20 It has not yet been studied in patients with hip fracture.
Pharmacokinetics of rivaroxaban. Rivaroxaban is manufactured as a tablet that is best absorbed in the stomach (Table 2).14 In contrast to dabigatran, it can be crushed and, for example, mixed with applesauce for patients who have trouble swallowing. It can also be mixed with water and given via nasogastric tube; however, postpyloric administration should be avoided.
Plasma concentrations peak within a few hours after ingestion. Rivaroxaban is highly protein-bound, so it cannot be eliminated by hemodialysis.
The drug relies on renal elimination to a smaller degree than dabigatran, with one-third of the dose eliminated unchanged in the urine, one-third eliminated in the urine as inactive metabolite, and the remaining one-third eliminated in the feces. However, enough parent compound is cleared through the kidneys that the half-life of rivaroxaban increases from 8.3 hours in healthy individuals to 9.5 hours in patients whose creatinine clearance is less than 30 mL/min.21 As with dabigatran, the dose must be adjusted for renal impairment (Table 1).
Rivaroxaban has significant liver metabolism, specifically through the cytochrome P450 3A4 enzyme, and it is also a substrate of P-glycoprotein. Therefore, potential drug-drug interactions must be taken into account, as they may lead to important alterations in plasma concentrations (Table 3).
Apixaban, a factor Xa inhibitor
Apixaban is also an oral direct factor Xa inhibitor. It is the newest of the oral anticoagulants to be approved in the United States, specifically for preventing stroke in nonvalvular atrial fibrillation (Table 1).22
Pharmacokinetics of apixaban. Apixaban is produced as a tablet that is absorbed slowly through the gastrointestinal tract, mainly the distal small bowel and ascending colon (Table 2).23
Peak plasma concentrations are reached a few hours after ingestion. Like rivaroxaban, apixaban is highly protein-bound, so it cannot be removed by hemodialysis.
Apixaban is similar to rivaroxaban in that 27% of the parent compound is cleared through the kidneys, it undergoes significant hepatic metabolism through cytochrome P450 3A4, and it is a substrate for P-glycoprotein.
Drug-drug interactions must be considered as a potential source of altered drug exposure and clearance (Table 3).
Unlike dabigatran and rivaroxaban, dose reduction is not based on the calculated creatinine clearance. Instead, a reduced dose is required if the patient meets two of the following three criteria:
- Serum creatinine level ≥ 1.5 mg/dL
- Age ≥ 80
- Weight ≤ 60 kg (Table 1).
The American Heart Association/American Stroke Association guidelines further recommend against using apixaban in patients with a creatinine clearance less than 25 mL/min.24
Edoxaban, a factor Xa inhibitor in development
Edoxaban (Savaysa), another factor Xa inhibitor, is available in Japan and has been submitted for approval in the United States for treating VTE and for preventing stroke in patients with
PERIOPERATIVE CONSIDERATIONS IN ANTICOAGULATION
Before addressing the perioperative management of TSOACs, let us review the evidence guiding the perioperative management of any chronic anticoagulant.
In fact, no large prospective randomized trial has clearly defined the risks and benefits of using or withholding a bridging anticoagulation strategy around surgery and other procedures, though the PERIOP 2 and BRIDGE trials are currently ongoing.25,26 There are some data regarding continuing anticoagulation without interruption, but they have mainly been derived from specific groups (eg, patients on warfarin undergoing cardiac pacemaker or defibrillator placement) and in procedures that pose a very low risk of bleeding complications (eg, minor dental extractions, cataract surgery, dermatologic procedures).2,27 Recommendations are, therefore, necessarily based on small perioperative trials and data gleaned from cohort review and from studies that did not involve surgical patients.
Ultimately, the decisions whether to discontinue oral anticoagulants and whether to employ bridging anticoagulation are based on assumptions about the risks of bleeding and the risk of thrombotic events, with similar assumptions regarding the effects of anticoagulants on both outcomes. In addition, the relative acceptance of bleeding vs thrombotic risks implicitly guides these complex decisions.
Perioperative bleeding risk
Many risk factors specific to the patient and to the type of surgery affect the rates and severity of perioperative bleeding.28
As for patient-specific risk factors, a small retrospective cohort analysis revealed that a HAS-BLED score of 3 or higher was highly discriminating in predicting perioperative bleeding in atrial fibrillation patients receiving anticoagulation.29 (The HAS-BLED score is based on hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [age > 65] and drug therapy.30) However, there are no widely validated tools that incorporate patient-specific factors to accurately predict bleeding risk in an individual patient.
Therefore, the American College of Chest Physicians (ACCP) guidelines suggest coarsely categorizing bleeding risk as either low or high solely on the basis of the type of procedure.2 Procedures considered “high-risk” have a risk greater than 1.5% to 2% and include urologic surgery involving the prostate or kidney, colonic polyp resections, surgeries involving highly vascular organs such as the liver or spleen, joint replacements, cancer surgeries, and cardiac or neurosurgical procedures.
Perioperative thrombotic risk
The ACCP guidelines2 place patients with atrial fibrillation, VTE, or mechanical heart valves in three risk groups for perioperative thromboembolism without anticoagulation, based on their annual risk of a thrombotic event:
- High risk—annual risk of a thrombotic event > 10%
- Moderate risk—5% to 10%
- Low risk—< 5%.
Comparing the risks calculated by these methods with the real-world risk of perioperative thrombosis highlights the problem of applying nonperioperative risk calculations: the perioperative period exposes patients to a higher risk than these models would predict.31 Nonetheless, these risk categorizations likely have some validity in stratifying patients into risk groups, even if the absolute risks are inaccurate.
Perioperative bridging for patients taking warfarin
Many patients with atrial fibrillation, VTE, or a mechanical heart valve need to interrupt their warfarin therapy because of the bleeding risk of an upcoming procedure.
The perioperative management of warfarin and other vitamin K antagonists is challenging because of the pharmacokinetics and pharmacodynamics of these drugs. Because it has a long half-life, warfarin usually must be stopped 4 to 5 days before a procedure in order to allow not only adequate clearance of the drug itself, but also restoration of functional clotting factors to normal or near-normal levels.12 Warfarin can generally be resumed 12 to 24 hours after surgery, assuming adequate hemostasis has been achieved, and it will again take several days for the INR to reach the therapeutic range.
The ACCP guidelines recommend using the perioperative risk of thromboembolism to make decisions about the need for bridging anticoagulation during warfarin interruption.2 They suggest that patients at high risk of thrombosis receive bridging with an alternative anticoagulant such as low-molecular-weight heparin or unfractionated heparin, because of the prolonged duration of subtherapeutic anticoagulation.
There has been clinical interest in using a TSOAC instead of low-molecular-weight or unfractionated heparin for bridging in the perioperative setting. Although this approach may be attractive from a cost and convenience perspective, it cannot be endorsed as yet because of the lack of information on the pros and cons of such an approach.
Patients at low thrombotic risk do not require bridging. In patients at moderate risk, the decision to bridge or not to bridge is based on careful consideration of patient-specific and surgery-specific factors.
PERIOPERATIVE MANAGEMENT OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
As summarized above, the perioperative management strategy for chronic anticoagulation is based on limited evidence, even for drugs as well established as warfarin.
The most recent ACCP guidelines on the perioperative management of antithrombotic therapy do not mention TSOACs.2 For now, the management strategy must be based on the pharmacokinetics of the drugs, package inserts from the manufacturers, and expert recommendations.3,14,23,32–34 Fortunately, because TSOACs have a more favorable pharmacokinetic profile than that of warfarin, their perioperative uses should be more streamlined. As always, the goal is to minimize the risk of both periprocedural bleeding and thromboembolism.
Timing of cessation of anticoagulation
The timing of cessation of TSOACs before an elective procedure depends primarily on two factors: the bleeding risk of the procedure and the patient’s renal function. Complete clearance of the medication is not necessary in all circumstances.
TSOACs should be stopped four to five half-lives before a procedure with a high bleeding risk, so that there is no or only minimal residual anticoagulant effect. The drug can be stopped two to three half-lives before a procedure with a low bleeding risk. Remember: the half-life increases as creatinine clearance decreases.
Specific recommendations may vary across institutions, but a suggested strategy is shown in Table 4.3,4,21,23,32–35 For the small subset of patients on P-glycoprotein or cytochrome P450 inhibitors or inducers, further adjustment in the time of discontinuation may be required.
Therapy does not need to be interrupted for procedures with a very low bleeding risk, as defined above.33,34 There is also preliminary evidence that TSOACs, similar to warfarin, may be continued during cardiac pacemaker or defibrillator placement.36
Evidence from clinical trials of perioperative TSOAC management
While the above recommendations are logical, studies are needed to prospectively evaluate perioperative management strategies.
The RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), which compared the effects of dabigatran and warfarin in preventing stroke in patients with atrial fibrillation, is one of the few clinical trials that also looked at periprocedural bleeding.37 About a quarter of the RE-LY participants required interruption of anticoagulation for a procedure.
Warfarin was managed according to local practices. For most of the study, the protocol required that dabigatran be discontinued 24 hours before a procedure, regardless of renal function or procedure type. The protocol was later amended and closely mirrored the management plan outlined in Table 4.
With either protocol, there was no statistically significant difference between dabigatran and warfarin in the rates of bleeding and thrombotic complications in the 7 days before or 30 days after the procedure.
A major limitation of the study was that most patients underwent a procedure with a low bleeding risk, so the analysis was likely underpowered to evaluate rates of bleeding in higher-risk procedures.
The ROCKET-AF trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) also shed light on periprocedural bleeding.15 About 15% of the participants required temporary interruption of anticoagulation for a surgical or invasive procedure.38
The study protocol called for discontinuing rivaroxaban 2 days before any procedure. Warfarin was to be held for 4 days to achieve a goal INR of 1.5 or less.15
Rates of major and nonmajor clinically significant bleeding at 30 days were similar with rivaroxaban and with warfarin.38 As with the RE-LY trial, the retrospective analysis was probably underpowered for assessing rates of bleeding in procedures with higher risk.
Perioperative bridging
While stopping a TSOAC in the perioperative period decreases the risk of bleeding, it naturally increases the risk of thromboembolism. However, patients on TSOACs should not routinely require perioperative bridging with an alternative anticoagulant, regardless of thrombotic risk.
Of note, dabigatran, rivaroxaban, and apixaban carry black-box warnings that discontinuation places patients at higher risk of thrombotic events.3,14,23 These warnings further state that coverage with an alternative anticoagulant should be strongly considered during interruption of therapy for reasons other than pathologic bleeding.
However, it does not necessarily follow that perioperative bridging is required. For example, the warning for rivaroxaban is based on the finding in the ROCKET-AF trial that patients in the rivaroxaban group had higher rates of stroke than those in the warfarin group after the study drugs were stopped at the end of the trial.39 While there was initial concern that this could represent a prothrombotic rebound effect, the authors subsequently showed that patients in the rivaroxaban group were more likely to have had a subtherapeutic INR when transitioning to open-label vitamin-K-antagonist therapy.39,40 There was no difference in the rate of stroke or systemic embolism between the rivaroxaban and warfarin groups when anticoagulation was temporarily interrupted for a procedure.38
The risks and benefits of perioperative bridging with TSOACs are difficult to evaluate, given the dearth of trial data. In the RE-LY trial, only 17% of patients on dabigatran and 28% of patients on warfarin underwent periprocedural bridging.37 The selection criteria and protocol for bridging were not reported. In the ROCKET-AF trial, only 9% of patients received bridging therapy despite a mean CHADS2 score of 3.4.38 (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes; 2 points for stroke or transient ischemic attack.) The decision to bridge or not was left to the individual investigator. As a result, the literature offers diverse opinions about the appropriateness of transitioning to an alternative anticoagulant.41–43
Bridging does not make sense in most instances, since anticoagulants such as low-molecular-weight heparin have pharmacokinetics similar to those of the available TSOACs and also depend on renal clearance.41 However, there may be situations in which patients must be switched to a parenteral anticoagulant such as unfractionated or low-molecular-weight heparin. For example, if a TSOAC has to be held, the patient has acute renal failure, and a needed procedure is still several days away, it would be reasonable to start a heparin drip for an inpatient at increased thrombotic risk.
In patients with normal renal function, these alternative anticoagulants should be started at the time the next TSOAC dose would have been due.3,14,23 In patients with reduced renal function, initiation of an alternative anticoagulant may need to be delayed 12 to 48 hours depending on which TSOAC is being used, as well as on the degree of renal dysfunction. This delay would help ensure that the onset of anticoagulation with the alternative anticoagulant is timed with the offset of therapeutic anticoagulation with the TSOAC.
Although limited, information from available coagulation assays may assist with the timing of initiation of an alternative anticoagulant (see the following section on laboratory monitoring). Serial testing with appropriate coagulation assays may help identify when most of a TSOAC has been cleared from a patient.
Laboratory monitoring
Inevitably, some patients on TSOACs require urgent or emergency surgery. In certain situations, such as before an orthopedic spine procedure, in which the complications of bleeding could be devastating, it may be necessary to know if any residual anticoagulant effect is present.
Monitoring dabigatran. As one might expect, direct thrombin inhibitors such as dabigatran can prolong the prothrombin time and activated partial thromboplastin time (aPTT).44–47 However, the prothrombin time is not recommended for assessing the level of anticoagulation from dabigatran. Many institutions may be using a normal aPTT to rule out therapeutic concentrations of dabigatran, based on results from early in vitro and ex vivo studies.46 While appealing from a practical standpoint, practitioners should exercise caution when relying on the aPTT to assess the risk of perioperative bleeding. A more recent investigation in patients treated with dabigatran found that up to 35% of patients with a normal aPTT still had a plasma concentration in the therapeutic range.48
The thrombin time and ecarin clotting time are more sensitive tests for dabigatran. A normal thrombin time or ecarin clotting time indicates that no or only minimal dabigatran is present.48 Unfortunately, these two tests often are either unavailable or are associated with long turnaround times, which limits their usefulness in the perioperative setting.
Monitoring rivaroxaban and apixaban. Factor Xa inhibitors such as rivaroxaban and apixaban can also influence the prothrombin time and aPTT (Figure 1).44–47,49,50 The aPTT is relatively insensitive to these drugs at low concentrations. It has been suggested that a normal prothrombin time can reasonably exclude therapeutic concentrations of rivaroxaban.45,46 However, the effects on the prothrombin time are highly variable, changing with the reagent used.49,50 In addition, apixaban appears to have less impact on the prothrombin time overall. The INR is not recommended for monitoring the effect of factor Xa inhibitors.
Anti-factor Xa assays likely represent the best option to provide true quantitative information on the level of anticoagulation with either rivaroxaban or apixaban. However, the assays must be specifically calibrated for each drug for results to be useful. (Anti-factor Xa assays cannot be used for heparin or low-molecular-weight heparin.) Further, most institutions do not yet have this capability. When appropriately calibrated, normal anti-factor Xa levels would exclude any effect of rivaroxaban or apixaban.
Reversal of anticoagulation
If patients on TSOACs require emergency surgery or present with significant bleeding in the setting of persistent anticoagulation, it may be necessary to try to reverse the anticoagulation.
Unlike warfarin or heparin, TSOACS do not have specific reversal agents, though specific antidotes are being developed. For example, researchers are evaluating antibodies capable of neutralizing dabigatran, as well as recombinant thrombin and factor Xa molecules that could antagonize dabigatran and rivaroxaban, respectively.51–53
Reversal can be attempted by neutralizing or removing the offending drug. Activated charcoal may be able to reduce absorption of TSOACs that were recently ingested,44 and dabigatran can be removed by hemodialysis.
However, certain practical considerations may limit the use of dialysis in the perioperative period. Insertion of a temporary dialysis line in an anticoagulated patient poses additional bleeding risks. A standard 4-hour hemodialysis session may remove only about 70% of dabigatran from the plasma, which may not be enough to prevent perioperative bleeding.54 Dabigatran also tends to redistribute from adipose tissue back into plasma after each dialysis session.55 Serial sessions of high-flux intermittent hemodialysis or continuous renal replacement therapy may therefore be needed to counteract rebound elevations in the dabigatran concentration.
Reversal can also be attempted through activation of the coagulation cascade via other mechanisms. Fresh-frozen plasma is unlikely to be a practical solution for reversal.44 Although it can readily replace the clotting factors depleted by vitamin K antagonists, large volumes of fresh-frozen plasma would be needed to overwhelm thrombin or factor Xa inhibition by TSOACs.
There are limited data on the use of prothrombin complex concentrates or recombinant activated factor VIIa in patients on TSOACs, though their use can be considered.56 In a trial in 12 healthy participants, a nonactivated four-factor prothrombin complex concentrate containing factors II, VII, IX, and X immediately and completely reversed the anticoagulant effect of rivaroxaban but had no effect on dabigatran.57 Before 2013, there were no nonactivated four-factor prothrombin complex concentrates available in the United States. The FDA has since approved Kcentra for the urgent reversal of vitamin K antagonists, meaning that the reversal of TSOACs in major bleeding events would still be off-label.58 Giving any of the clotting factors carries a risk of thromboembolism.
Resumption of anticoagulation
TSOACs have a rapid onset of action, and therapeutic levels are reached within a few hours of administration.
Extrapolating from the ACCP guidelines, TSOACs can generally be restarted at therapeutic doses 24 hours after low-bleeding-risk procedures.2 Therapeutic dosing should be delayed 48 to 72 hours after a procedure with a high bleeding risk, assuming adequate hemostasis has been achieved. Prophylactic unfractionated heparin or low-molecular-weight heparin therapy can be given in the interim if deemed safe. Alternatively, for orthopedic patients ultimately transitioning back to therapeutic rivaroxaban after hip or knee arthroplasty, prophylactic rivaroxaban doses can be started 6 to 10 hours after surgery.14
There are numerous reasons why the resumption of TSOACs may have to be delayed after surgery, including nothing-by-mouth status, postoperative nausea and vomiting, ileus, gastric or bowel resection, and the anticipated need for future procedures. Since dabigatran capsules cannot be crushed, they cannot be given via nasogastric tube in patients with postoperative dysphagia. Parenteral anticoagulants should be used until these issues resolve.
Unfractionated heparin is still the preferred anticoagulant in unstable or potentially unstable patients, given its ease of monitoring, quick offset of action, and reversibility. When patients have stabilized, TSOACs can be resumed when the next dose of low-molecular-weight heparin would have been due or when the unfractionated heparin drip is discontinued.3,14,23
UNTIL EVIDENCE-BASED GUIDELINES ARE DEVELOPED
The development of TSOACs has ushered in an exciting new era for anticoagulant therapy. Providers involved in perioperative medicine will increasingly encounter patients on dabigatran, rivaroxaban, and apixaban. However, until evidence-based guidelines are developed for these new anticoagulants, clinicians will have to apply their knowledge of pharmacology and critically evaluate expert recommendations in order to manage patients safely throughout the perioperative period.
More then 2.5 million patients in the United States are on long-term anticoagulation therapy for atrial fibrillation, venous thromboembolic disease, or mechanical heart valves,1 and the number is expected to rise as the population ages. Each year, about 10% of these patients undergo an invasive procedure or surgery that requires temporary interruption of anticoagulation.2
Most physicians are familiar with the perioperative management of warfarin, a vitamin K antagonist, since for decades it has been the sole oral anticoagulant available. However, many physicians lack experience with the three target-specific oral anticoagulants (TSOACs; also known as “novel” oral anticoagulants) approved so far: the direct thrombin inhibitor dabigatran (Pradaxa) and the direct factor Xa inhibitors rivaroxaban (Xarelto) and apixaban (Eliquis).
With their rapid onset of action, predictable pharmacokinetics, relatively short half-lives, and fewer drug-drug interactions than warfarin, TSOACs overcome many of the limitations of the older oral anticoagulant warfarin. In many ways, these qualities simplify the perioperative management of anticoagulation. At the same time, these new drugs also bring new challenges: caution is needed in patients with renal impairment; the level of anticoagulation is difficult to assess; and there is no specific antidote or standardized procedure to reverse their anticoagulant effect. While various periprocedural protocols for TSOAC therapy have been proposed, evidence-based guidelines are still to come.
This article first discusses the pharmacology of dabigatran, rivaroxaban, and apixaban that is pertinent to the perioperative period. It then briefly reviews the general principles of perioperative management of anticoagulation. The final section provides specific recommendations for the perioperative management of TSOACs.
PHARMACOLOGY OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
Dabigatran, a factor IIa inhibitor
Dabigatran is an oral direct thrombin (factor IIa) inhibitor. It exerts its anticoagulant effect by blocking the generation of fibrin, inhibiting platelet aggregation, and dampening the activity of factors V, VIII, and XI (Figure 1).3,4 From its introduction in October 2010 through August 2012, nearly 3.7 million prescriptions were dispensed to 725,000 patients in the United States.5
Indications for dabigatran. Dabigatran is approved in the United States and Canada for preventing stroke in nonvalvular atrial fibrillation (Table 1).6 More recently, it received US approval for treating deep vein thrombosis or pulmonary embolism after 5 to 10 days of a parenteral anticoagulant.7,8 It is also approved in Europe and Canada for preventing venous thromboembolism (VTE) after total hip replacement and knee arthroplasty.9,10
Dabigatran is contraindicated in patients with a mechanical heart valve, based on a phase 2 study in which it conferred a higher risk of thromboembolism and bleeding than warfarin.3,11
Pharmacokinetics of dabigatran. Dabigatran is formulated as a prodrug, dabigatran etexilate, in a capsule containing multiple small pellets.12 The capsules should not be crushed, as this significantly increases oral bioavailability. The prodrug is absorbed across the gastric mucosa and is then rapidly converted to the active form (Table 2).
Plasma concentrations peak within 2 hours of ingestion, which means that therapeutic anticoagulation is achieved shortly after taking the drug.
Only 35% of dabigatran is protein-bound, which allows it to be removed by hemodialysis. Nearly 85% of the drug is eliminated in the urine. It has a half-life of 13 to 15 hours in patients with normal renal function.3 However, its half-life increases to about 27 hours in patients whose creatinine clearance is less than 30 mL/min. As a result, the dose must be reduced in patients with renal impairment (Table 1).
Dabigatran is not metabolized by the cytochrome P450 enzymes, but it is a substrate for P-glycoprotein, so it still has the potential for drug-drug interactions.3 Practitioners should be familiar with these potential interactions (Table 3), as they can result in higher- or lower-than-expected plasma concentrations of dabigatran in the perioperative period.13
Rivaroxaban, a factor Xa inhibitor
Rivaroxaban is an oral direct factor Xa inhibitor. It has been approved by the US Food and Drug Administration (FDA) for the prevention of stroke in nonvalvular atrial fibrillation, for VTE treatment, and for VTE prophylaxis after hip replacement or knee replacement (Table 1).14–20 It has not yet been studied in patients with hip fracture.
Pharmacokinetics of rivaroxaban. Rivaroxaban is manufactured as a tablet that is best absorbed in the stomach (Table 2).14 In contrast to dabigatran, it can be crushed and, for example, mixed with applesauce for patients who have trouble swallowing. It can also be mixed with water and given via nasogastric tube; however, postpyloric administration should be avoided.
Plasma concentrations peak within a few hours after ingestion. Rivaroxaban is highly protein-bound, so it cannot be eliminated by hemodialysis.
The drug relies on renal elimination to a smaller degree than dabigatran, with one-third of the dose eliminated unchanged in the urine, one-third eliminated in the urine as inactive metabolite, and the remaining one-third eliminated in the feces. However, enough parent compound is cleared through the kidneys that the half-life of rivaroxaban increases from 8.3 hours in healthy individuals to 9.5 hours in patients whose creatinine clearance is less than 30 mL/min.21 As with dabigatran, the dose must be adjusted for renal impairment (Table 1).
Rivaroxaban has significant liver metabolism, specifically through the cytochrome P450 3A4 enzyme, and it is also a substrate of P-glycoprotein. Therefore, potential drug-drug interactions must be taken into account, as they may lead to important alterations in plasma concentrations (Table 3).
Apixaban, a factor Xa inhibitor
Apixaban is also an oral direct factor Xa inhibitor. It is the newest of the oral anticoagulants to be approved in the United States, specifically for preventing stroke in nonvalvular atrial fibrillation (Table 1).22
Pharmacokinetics of apixaban. Apixaban is produced as a tablet that is absorbed slowly through the gastrointestinal tract, mainly the distal small bowel and ascending colon (Table 2).23
Peak plasma concentrations are reached a few hours after ingestion. Like rivaroxaban, apixaban is highly protein-bound, so it cannot be removed by hemodialysis.
Apixaban is similar to rivaroxaban in that 27% of the parent compound is cleared through the kidneys, it undergoes significant hepatic metabolism through cytochrome P450 3A4, and it is a substrate for P-glycoprotein.
Drug-drug interactions must be considered as a potential source of altered drug exposure and clearance (Table 3).
Unlike dabigatran and rivaroxaban, dose reduction is not based on the calculated creatinine clearance. Instead, a reduced dose is required if the patient meets two of the following three criteria:
- Serum creatinine level ≥ 1.5 mg/dL
- Age ≥ 80
- Weight ≤ 60 kg (Table 1).
The American Heart Association/American Stroke Association guidelines further recommend against using apixaban in patients with a creatinine clearance less than 25 mL/min.24
Edoxaban, a factor Xa inhibitor in development
Edoxaban (Savaysa), another factor Xa inhibitor, is available in Japan and has been submitted for approval in the United States for treating VTE and for preventing stroke in patients with
PERIOPERATIVE CONSIDERATIONS IN ANTICOAGULATION
Before addressing the perioperative management of TSOACs, let us review the evidence guiding the perioperative management of any chronic anticoagulant.
In fact, no large prospective randomized trial has clearly defined the risks and benefits of using or withholding a bridging anticoagulation strategy around surgery and other procedures, though the PERIOP 2 and BRIDGE trials are currently ongoing.25,26 There are some data regarding continuing anticoagulation without interruption, but they have mainly been derived from specific groups (eg, patients on warfarin undergoing cardiac pacemaker or defibrillator placement) and in procedures that pose a very low risk of bleeding complications (eg, minor dental extractions, cataract surgery, dermatologic procedures).2,27 Recommendations are, therefore, necessarily based on small perioperative trials and data gleaned from cohort review and from studies that did not involve surgical patients.
Ultimately, the decisions whether to discontinue oral anticoagulants and whether to employ bridging anticoagulation are based on assumptions about the risks of bleeding and the risk of thrombotic events, with similar assumptions regarding the effects of anticoagulants on both outcomes. In addition, the relative acceptance of bleeding vs thrombotic risks implicitly guides these complex decisions.
Perioperative bleeding risk
Many risk factors specific to the patient and to the type of surgery affect the rates and severity of perioperative bleeding.28
As for patient-specific risk factors, a small retrospective cohort analysis revealed that a HAS-BLED score of 3 or higher was highly discriminating in predicting perioperative bleeding in atrial fibrillation patients receiving anticoagulation.29 (The HAS-BLED score is based on hypertension, abnormal renal or liver function, stroke, bleeding, labile international normalized ratio [INR], elderly [age > 65] and drug therapy.30) However, there are no widely validated tools that incorporate patient-specific factors to accurately predict bleeding risk in an individual patient.
Therefore, the American College of Chest Physicians (ACCP) guidelines suggest coarsely categorizing bleeding risk as either low or high solely on the basis of the type of procedure.2 Procedures considered “high-risk” have a risk greater than 1.5% to 2% and include urologic surgery involving the prostate or kidney, colonic polyp resections, surgeries involving highly vascular organs such as the liver or spleen, joint replacements, cancer surgeries, and cardiac or neurosurgical procedures.
Perioperative thrombotic risk
The ACCP guidelines2 place patients with atrial fibrillation, VTE, or mechanical heart valves in three risk groups for perioperative thromboembolism without anticoagulation, based on their annual risk of a thrombotic event:
- High risk—annual risk of a thrombotic event > 10%
- Moderate risk—5% to 10%
- Low risk—< 5%.
Comparing the risks calculated by these methods with the real-world risk of perioperative thrombosis highlights the problem of applying nonperioperative risk calculations: the perioperative period exposes patients to a higher risk than these models would predict.31 Nonetheless, these risk categorizations likely have some validity in stratifying patients into risk groups, even if the absolute risks are inaccurate.
Perioperative bridging for patients taking warfarin
Many patients with atrial fibrillation, VTE, or a mechanical heart valve need to interrupt their warfarin therapy because of the bleeding risk of an upcoming procedure.
The perioperative management of warfarin and other vitamin K antagonists is challenging because of the pharmacokinetics and pharmacodynamics of these drugs. Because it has a long half-life, warfarin usually must be stopped 4 to 5 days before a procedure in order to allow not only adequate clearance of the drug itself, but also restoration of functional clotting factors to normal or near-normal levels.12 Warfarin can generally be resumed 12 to 24 hours after surgery, assuming adequate hemostasis has been achieved, and it will again take several days for the INR to reach the therapeutic range.
The ACCP guidelines recommend using the perioperative risk of thromboembolism to make decisions about the need for bridging anticoagulation during warfarin interruption.2 They suggest that patients at high risk of thrombosis receive bridging with an alternative anticoagulant such as low-molecular-weight heparin or unfractionated heparin, because of the prolonged duration of subtherapeutic anticoagulation.
There has been clinical interest in using a TSOAC instead of low-molecular-weight or unfractionated heparin for bridging in the perioperative setting. Although this approach may be attractive from a cost and convenience perspective, it cannot be endorsed as yet because of the lack of information on the pros and cons of such an approach.
Patients at low thrombotic risk do not require bridging. In patients at moderate risk, the decision to bridge or not to bridge is based on careful consideration of patient-specific and surgery-specific factors.
PERIOPERATIVE MANAGEMENT OF TARGET-SPECIFIC ORAL ANTICOAGULANTS
As summarized above, the perioperative management strategy for chronic anticoagulation is based on limited evidence, even for drugs as well established as warfarin.
The most recent ACCP guidelines on the perioperative management of antithrombotic therapy do not mention TSOACs.2 For now, the management strategy must be based on the pharmacokinetics of the drugs, package inserts from the manufacturers, and expert recommendations.3,14,23,32–34 Fortunately, because TSOACs have a more favorable pharmacokinetic profile than that of warfarin, their perioperative uses should be more streamlined. As always, the goal is to minimize the risk of both periprocedural bleeding and thromboembolism.
Timing of cessation of anticoagulation
The timing of cessation of TSOACs before an elective procedure depends primarily on two factors: the bleeding risk of the procedure and the patient’s renal function. Complete clearance of the medication is not necessary in all circumstances.
TSOACs should be stopped four to five half-lives before a procedure with a high bleeding risk, so that there is no or only minimal residual anticoagulant effect. The drug can be stopped two to three half-lives before a procedure with a low bleeding risk. Remember: the half-life increases as creatinine clearance decreases.
Specific recommendations may vary across institutions, but a suggested strategy is shown in Table 4.3,4,21,23,32–35 For the small subset of patients on P-glycoprotein or cytochrome P450 inhibitors or inducers, further adjustment in the time of discontinuation may be required.
Therapy does not need to be interrupted for procedures with a very low bleeding risk, as defined above.33,34 There is also preliminary evidence that TSOACs, similar to warfarin, may be continued during cardiac pacemaker or defibrillator placement.36
Evidence from clinical trials of perioperative TSOAC management
While the above recommendations are logical, studies are needed to prospectively evaluate perioperative management strategies.
The RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), which compared the effects of dabigatran and warfarin in preventing stroke in patients with atrial fibrillation, is one of the few clinical trials that also looked at periprocedural bleeding.37 About a quarter of the RE-LY participants required interruption of anticoagulation for a procedure.
Warfarin was managed according to local practices. For most of the study, the protocol required that dabigatran be discontinued 24 hours before a procedure, regardless of renal function or procedure type. The protocol was later amended and closely mirrored the management plan outlined in Table 4.
With either protocol, there was no statistically significant difference between dabigatran and warfarin in the rates of bleeding and thrombotic complications in the 7 days before or 30 days after the procedure.
A major limitation of the study was that most patients underwent a procedure with a low bleeding risk, so the analysis was likely underpowered to evaluate rates of bleeding in higher-risk procedures.
The ROCKET-AF trial (Rivaroxaban Once-daily Oral Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) also shed light on periprocedural bleeding.15 About 15% of the participants required temporary interruption of anticoagulation for a surgical or invasive procedure.38
The study protocol called for discontinuing rivaroxaban 2 days before any procedure. Warfarin was to be held for 4 days to achieve a goal INR of 1.5 or less.15
Rates of major and nonmajor clinically significant bleeding at 30 days were similar with rivaroxaban and with warfarin.38 As with the RE-LY trial, the retrospective analysis was probably underpowered for assessing rates of bleeding in procedures with higher risk.
Perioperative bridging
While stopping a TSOAC in the perioperative period decreases the risk of bleeding, it naturally increases the risk of thromboembolism. However, patients on TSOACs should not routinely require perioperative bridging with an alternative anticoagulant, regardless of thrombotic risk.
Of note, dabigatran, rivaroxaban, and apixaban carry black-box warnings that discontinuation places patients at higher risk of thrombotic events.3,14,23 These warnings further state that coverage with an alternative anticoagulant should be strongly considered during interruption of therapy for reasons other than pathologic bleeding.
However, it does not necessarily follow that perioperative bridging is required. For example, the warning for rivaroxaban is based on the finding in the ROCKET-AF trial that patients in the rivaroxaban group had higher rates of stroke than those in the warfarin group after the study drugs were stopped at the end of the trial.39 While there was initial concern that this could represent a prothrombotic rebound effect, the authors subsequently showed that patients in the rivaroxaban group were more likely to have had a subtherapeutic INR when transitioning to open-label vitamin-K-antagonist therapy.39,40 There was no difference in the rate of stroke or systemic embolism between the rivaroxaban and warfarin groups when anticoagulation was temporarily interrupted for a procedure.38
The risks and benefits of perioperative bridging with TSOACs are difficult to evaluate, given the dearth of trial data. In the RE-LY trial, only 17% of patients on dabigatran and 28% of patients on warfarin underwent periprocedural bridging.37 The selection criteria and protocol for bridging were not reported. In the ROCKET-AF trial, only 9% of patients received bridging therapy despite a mean CHADS2 score of 3.4.38 (The CHADS2 score is calculated as 1 point each for congestive heart failure, hypertension, age ≥ 75, and diabetes; 2 points for stroke or transient ischemic attack.) The decision to bridge or not was left to the individual investigator. As a result, the literature offers diverse opinions about the appropriateness of transitioning to an alternative anticoagulant.41–43
Bridging does not make sense in most instances, since anticoagulants such as low-molecular-weight heparin have pharmacokinetics similar to those of the available TSOACs and also depend on renal clearance.41 However, there may be situations in which patients must be switched to a parenteral anticoagulant such as unfractionated or low-molecular-weight heparin. For example, if a TSOAC has to be held, the patient has acute renal failure, and a needed procedure is still several days away, it would be reasonable to start a heparin drip for an inpatient at increased thrombotic risk.
In patients with normal renal function, these alternative anticoagulants should be started at the time the next TSOAC dose would have been due.3,14,23 In patients with reduced renal function, initiation of an alternative anticoagulant may need to be delayed 12 to 48 hours depending on which TSOAC is being used, as well as on the degree of renal dysfunction. This delay would help ensure that the onset of anticoagulation with the alternative anticoagulant is timed with the offset of therapeutic anticoagulation with the TSOAC.
Although limited, information from available coagulation assays may assist with the timing of initiation of an alternative anticoagulant (see the following section on laboratory monitoring). Serial testing with appropriate coagulation assays may help identify when most of a TSOAC has been cleared from a patient.
Laboratory monitoring
Inevitably, some patients on TSOACs require urgent or emergency surgery. In certain situations, such as before an orthopedic spine procedure, in which the complications of bleeding could be devastating, it may be necessary to know if any residual anticoagulant effect is present.
Monitoring dabigatran. As one might expect, direct thrombin inhibitors such as dabigatran can prolong the prothrombin time and activated partial thromboplastin time (aPTT).44–47 However, the prothrombin time is not recommended for assessing the level of anticoagulation from dabigatran. Many institutions may be using a normal aPTT to rule out therapeutic concentrations of dabigatran, based on results from early in vitro and ex vivo studies.46 While appealing from a practical standpoint, practitioners should exercise caution when relying on the aPTT to assess the risk of perioperative bleeding. A more recent investigation in patients treated with dabigatran found that up to 35% of patients with a normal aPTT still had a plasma concentration in the therapeutic range.48
The thrombin time and ecarin clotting time are more sensitive tests for dabigatran. A normal thrombin time or ecarin clotting time indicates that no or only minimal dabigatran is present.48 Unfortunately, these two tests often are either unavailable or are associated with long turnaround times, which limits their usefulness in the perioperative setting.
Monitoring rivaroxaban and apixaban. Factor Xa inhibitors such as rivaroxaban and apixaban can also influence the prothrombin time and aPTT (Figure 1).44–47,49,50 The aPTT is relatively insensitive to these drugs at low concentrations. It has been suggested that a normal prothrombin time can reasonably exclude therapeutic concentrations of rivaroxaban.45,46 However, the effects on the prothrombin time are highly variable, changing with the reagent used.49,50 In addition, apixaban appears to have less impact on the prothrombin time overall. The INR is not recommended for monitoring the effect of factor Xa inhibitors.
Anti-factor Xa assays likely represent the best option to provide true quantitative information on the level of anticoagulation with either rivaroxaban or apixaban. However, the assays must be specifically calibrated for each drug for results to be useful. (Anti-factor Xa assays cannot be used for heparin or low-molecular-weight heparin.) Further, most institutions do not yet have this capability. When appropriately calibrated, normal anti-factor Xa levels would exclude any effect of rivaroxaban or apixaban.
Reversal of anticoagulation
If patients on TSOACs require emergency surgery or present with significant bleeding in the setting of persistent anticoagulation, it may be necessary to try to reverse the anticoagulation.
Unlike warfarin or heparin, TSOACS do not have specific reversal agents, though specific antidotes are being developed. For example, researchers are evaluating antibodies capable of neutralizing dabigatran, as well as recombinant thrombin and factor Xa molecules that could antagonize dabigatran and rivaroxaban, respectively.51–53
Reversal can be attempted by neutralizing or removing the offending drug. Activated charcoal may be able to reduce absorption of TSOACs that were recently ingested,44 and dabigatran can be removed by hemodialysis.
However, certain practical considerations may limit the use of dialysis in the perioperative period. Insertion of a temporary dialysis line in an anticoagulated patient poses additional bleeding risks. A standard 4-hour hemodialysis session may remove only about 70% of dabigatran from the plasma, which may not be enough to prevent perioperative bleeding.54 Dabigatran also tends to redistribute from adipose tissue back into plasma after each dialysis session.55 Serial sessions of high-flux intermittent hemodialysis or continuous renal replacement therapy may therefore be needed to counteract rebound elevations in the dabigatran concentration.
Reversal can also be attempted through activation of the coagulation cascade via other mechanisms. Fresh-frozen plasma is unlikely to be a practical solution for reversal.44 Although it can readily replace the clotting factors depleted by vitamin K antagonists, large volumes of fresh-frozen plasma would be needed to overwhelm thrombin or factor Xa inhibition by TSOACs.
There are limited data on the use of prothrombin complex concentrates or recombinant activated factor VIIa in patients on TSOACs, though their use can be considered.56 In a trial in 12 healthy participants, a nonactivated four-factor prothrombin complex concentrate containing factors II, VII, IX, and X immediately and completely reversed the anticoagulant effect of rivaroxaban but had no effect on dabigatran.57 Before 2013, there were no nonactivated four-factor prothrombin complex concentrates available in the United States. The FDA has since approved Kcentra for the urgent reversal of vitamin K antagonists, meaning that the reversal of TSOACs in major bleeding events would still be off-label.58 Giving any of the clotting factors carries a risk of thromboembolism.
Resumption of anticoagulation
TSOACs have a rapid onset of action, and therapeutic levels are reached within a few hours of administration.
Extrapolating from the ACCP guidelines, TSOACs can generally be restarted at therapeutic doses 24 hours after low-bleeding-risk procedures.2 Therapeutic dosing should be delayed 48 to 72 hours after a procedure with a high bleeding risk, assuming adequate hemostasis has been achieved. Prophylactic unfractionated heparin or low-molecular-weight heparin therapy can be given in the interim if deemed safe. Alternatively, for orthopedic patients ultimately transitioning back to therapeutic rivaroxaban after hip or knee arthroplasty, prophylactic rivaroxaban doses can be started 6 to 10 hours after surgery.14
There are numerous reasons why the resumption of TSOACs may have to be delayed after surgery, including nothing-by-mouth status, postoperative nausea and vomiting, ileus, gastric or bowel resection, and the anticipated need for future procedures. Since dabigatran capsules cannot be crushed, they cannot be given via nasogastric tube in patients with postoperative dysphagia. Parenteral anticoagulants should be used until these issues resolve.
Unfractionated heparin is still the preferred anticoagulant in unstable or potentially unstable patients, given its ease of monitoring, quick offset of action, and reversibility. When patients have stabilized, TSOACs can be resumed when the next dose of low-molecular-weight heparin would have been due or when the unfractionated heparin drip is discontinued.3,14,23
UNTIL EVIDENCE-BASED GUIDELINES ARE DEVELOPED
The development of TSOACs has ushered in an exciting new era for anticoagulant therapy. Providers involved in perioperative medicine will increasingly encounter patients on dabigatran, rivaroxaban, and apixaban. However, until evidence-based guidelines are developed for these new anticoagulants, clinicians will have to apply their knowledge of pharmacology and critically evaluate expert recommendations in order to manage patients safely throughout the perioperative period.
- Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):299S–339S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Boehringer Ingelheim Pharmaceuticals, Inc. PRADAXA (dabigatran) package insert. http://bidocs.boehringer-ingelheim.com/BIWebAc-cess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed August 6, 2014.
- Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology 2013; 118:1466–1474.
- US Food and Drug Administration (FDA). FDA drug safety communication: update on the risk for serious bleeding events with the anticoagulant Pradaxa (dabigatran). www.fda.gov/drugs/drugsafety/ucm326580.htm. Accessed August 6, 2014.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Eriksson BI, Dahl OE, Rosencher N, Büller HR, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Janssen Pharmaceuticals, Inc. XARELTO (rivaroxaban) package insert. www.xareltohcp.com/about-xarelto/about-xarelto.html?utm_source=google&utm_medium=cpc&utm_campaign=Branded+-+Broad&utm_term=xarelto%20rivaroxaban&utm_content=Xarelto+Rivaroxaban|mkwid|sxSDxPb4m_dc|pcrid|34667840494. Accessed August 6, 2014.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–391.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008; 372:31–39.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 2010; 70:703–712.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Bristol-Myers Squibb Company. ELIQUIS (apixaban) package insert. www.eliquis.com/index.aspx. Accessed August 6, 2014.
- Furie KL, Goldstein LB, Albers GW, et al; American Heart Association Stroke Council. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:3442–3453.
- ClinicalTrials.gov, US National Institutes of Health. PERIOP 2 - A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin. http://clinicaltri-als.gov/show/NCT00432796. Accessed August 6, 2014.
- ClinicalTrials.gov, US National Institutes of Health. Effectiveness of Bridging Anticoagulation for Surgery (The BRIDGE Study). http://clinicaltrials.gov/ct2/show/NCT00786474. Accessed August 6, 2014.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J 2013; 165:427.e1–433.e1.
- Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost 2012; 108:65–73.
- Pisters R, Lane DA, Nieuwlaaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 2010; 138:1093–1100.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis 2013; 36:212–222.
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012; 120:2954–2962.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87(suppl 1):S141–S145.
- Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111:1165–1168.
- Healey JS, Eikelboom J, Douketis J, et al; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126:343–348.
- Sherwood MW, Douketis JD, Patel MR, et al; on behalf of the ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 129:1850–1859.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol 2013; 61:659–660.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation 2012; 126:1573–1576.
- Sié P, Samama CM, Godier A, et al; Working Group on Perioperative Haemostasis. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and the French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis 2011; 104:669–676.
- King CS, Holley AB, Moores LK. Moving toward a more ideal anticoagulant: the oral direct thrombin and factor Xa inhibitors. Chest 2013; 143:1106–1116.
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11:756–760.
- Baglin T, Keeling D, Kitchen S; British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 2012; 159:427–429.
- Mani H, Kasper A, Lindhoff-Last E. Measuring the anticoagulant effects of target specific oral anticoagulants—reasons, methods and current limitations. J Thromb Thrombolysis 2013; 36:187–194.
- Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013; 11:1493–1502.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013; 33:1223–1245.
- Van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol 2011; 57:E1130.
- Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold D, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. Abstract presented at the International Society of Thrombosis and Haemostasis 2013 Congress. http://onlinelibrary.wiley.com/doi/10.1111/jth.2013.11.issue-s2/issuetoc. Accessed August 6, 2014.
- Lu G, Luan P, Hollenbach SJ, et al. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors (abstract). J Thromb Haemost 2009; 7(suppl 2):abstract OC-TH-107.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis 2013; 36:195–202.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128:1234–1243.
- Douketis JD, Berger PB, Dunn AS, et al; American College of Chest Physicians. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133(suppl 6):299S–339S.
- Douketis JD, Spyropoulos AC, Spencer FA, et al; American College of Chest Physicians. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e326S–e350S.
- Boehringer Ingelheim Pharmaceuticals, Inc. PRADAXA (dabigatran) package insert. http://bidocs.boehringer-ingelheim.com/BIWebAc-cess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed August 6, 2014.
- Levy JH, Faraoni D, Spring JL, Douketis JD, Samama CM. Managing new oral anticoagulants in the perioperative and intensive care unit setting. Anesthesiology 2013; 118:1466–1474.
- US Food and Drug Administration (FDA). FDA drug safety communication: update on the risk for serious bleeding events with the anticoagulant Pradaxa (dabigatran). www.fda.gov/drugs/drugsafety/ucm326580.htm. Accessed August 6, 2014.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Schulman S, Kearon C, Kakkar AK, et al; RE-MEDY Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013; 368:709–718.
- Eriksson BI, Dahl OE, Rosencher N, Büller HR, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet 2007; 370:949–956.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-MODEL Study Group. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al; RE-ALIGN Investigators. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013; 369:1206–1214.
- Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G; American College of Chest Physicians. Oral anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e44S–e88S.
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos 2008; 36:386–399.
- Janssen Pharmaceuticals, Inc. XARELTO (rivaroxaban) package insert. www.xareltohcp.com/about-xarelto/about-xarelto.html?utm_source=google&utm_medium=cpc&utm_campaign=Branded+-+Broad&utm_term=xarelto%20rivaroxaban&utm_content=Xarelto+Rivaroxaban|mkwid|sxSDxPb4m_dc|pcrid|34667840494. Accessed August 6, 2014.
- Patel MR, Mahaffey KW, Garg J, et al; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365:883–391.
- EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–2510.
- EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366:1287–1297.
- Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358:2765–2775.
- Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008; 372:31–39.
- Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358:2776–2786.
- Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct factor Xa inhibitor. Br J Clin Pharmacol 2010; 70:703–712.
- Granger CB, Alexander JH, McMurray JJ, et al; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365:981–992.
- Bristol-Myers Squibb Company. ELIQUIS (apixaban) package insert. www.eliquis.com/index.aspx. Accessed August 6, 2014.
- Furie KL, Goldstein LB, Albers GW, et al; American Heart Association Stroke Council. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012; 43:3442–3453.
- ClinicalTrials.gov, US National Institutes of Health. PERIOP 2 - A Safety and Effectiveness Study of LMWH Bridging Therapy Versus Placebo Bridging Therapy for Patients on Long Term Warfarin and Require Temporary Interruption of Their Warfarin. http://clinicaltri-als.gov/show/NCT00432796. Accessed August 6, 2014.
- ClinicalTrials.gov, US National Institutes of Health. Effectiveness of Bridging Anticoagulation for Surgery (The BRIDGE Study). http://clinicaltrials.gov/ct2/show/NCT00786474. Accessed August 6, 2014.
- Birnie DH, Healey JS, Wells GA, et al; BRUISE CONTROL Investigators. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med 2013; 368:2084–2093.
- Oberweis BS, Nukala S, Rosenberg A, et al. Thrombotic and bleeding complications after orthopedic surgery. Am Heart J 2013; 165:427.e1–433.e1.
- Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS-BLED score predicts bleedings during bridging of chronic oral anticoagulation. Results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost 2012; 108:65–73.
- Pisters R, Lane DA, Nieuwlaaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation. The Euro Heart Survey. Chest 2010; 138:1093–1100.
- Kaatz S, Douketis JD, Zhou H, Gage BF, White RH. Risk of stroke after surgery in patients with and without chronic atrial fibrillation. J Thromb Haemost 2010; 8:884–890.
- van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate—a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost 2010; 103:1116–1127.
- Connolly G, Spyropoulos AC. Practical issues, limitations, and periprocedural management of the NOAC’s. J Thromb Thrombolysis 2013; 36:212–222.
- Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood 2012; 120:2954–2962.
- Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol 2012; 87(suppl 1):S141–S145.
- Rowley CP, Bernard ML, Brabham WW, et al. Safety of continuous anticoagulation with dabigatran during implantation of cardiac rhythm devices. Am J Cardiol 2013; 111:1165–1168.
- Healey JS, Eikelboom J, Douketis J, et al; RE-LY Investigators. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) randomized trial. Circulation 2012; 126:343–348.
- Sherwood MW, Douketis JD, Patel MR, et al; on behalf of the ROCKET AF Investigators. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation 2014; 129:1850–1859.
- Patel MR, Hellkamp AS, Lokhnygina Y, et al. Outcomes of discontinuing rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: analysis from the ROCKET AF trial (Rivaroxaban Once-Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation). J Am Coll Cardiol 2013; 61:651–658.
- Reynolds MR. Discontinuation of rivaroxaban: filling in the gaps. J Am Coll Cardiol 2013; 61:659–660.
- Turpie AG, Kreutz R, Llau J, Norrving B, Haas S. Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 2012; 108:876–886.
- Gallego P, Apostolakis S, Lip GY. Bridging evidence-based practice and practice-based evidence in periprocedural anticoagulation. Circulation 2012; 126:1573–1576.
- Sié P, Samama CM, Godier A, et al; Working Group on Perioperative Haemostasis. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and the French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis 2011; 104:669–676.
- King CS, Holley AB, Moores LK. Moving toward a more ideal anticoagulant: the oral direct thrombin and factor Xa inhibitors. Chest 2013; 143:1106–1116.
- Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W. Measuring oral direct inhibitors (ODIs) of thrombin and factor Xa: a recommendation from the Subcommittee on Control of Anticoagulation of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2013; 11:756–760.
- Baglin T, Keeling D, Kitchen S; British Committee for Standards in Haematology. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol 2012; 159:427–429.
- Mani H, Kasper A, Lindhoff-Last E. Measuring the anticoagulant effects of target specific oral anticoagulants—reasons, methods and current limitations. J Thromb Thrombolysis 2013; 36:187–194.
- Hawes EM, Deal AM, Funk-Adcock D, et al. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost 2013; 11:1493–1502.
- Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti-Xa assay is preferable to prothrombin time assay. Thromb Haemost 2010; 104:1263–1271.
- Smythe MA, Fanikos J, Gulseth MP, et al. Rivaroxaban: practical considerations for ensuring safety and efficacy. Pharmacotherapy 2013; 33:1223–1245.
- Van Ryn J, Litzenburger T, Waterman A, et al. Dabigatran anticoagulant activity is neutralized by an antibody selective to dabigatran in in vitro and in vivo models. J Am Coll Cardiol 2011; 57:E1130.
- Sheffield W, Lambourne M, Bhakta V, Eltringham-Smith L, Arnold D, Crowther M. Active site-mutated thrombin S195A but not active site-blocked thrombin counteracts the anticoagulant activity of dabigatran in plasma. Abstract presented at the International Society of Thrombosis and Haemostasis 2013 Congress. http://onlinelibrary.wiley.com/doi/10.1111/jth.2013.11.issue-s2/issuetoc. Accessed August 6, 2014.
- Lu G, Luan P, Hollenbach SJ, et al. Reconstructed recombinant factor Xa as an antidote to reverse anticoagulation by factor Xa inhibitors (abstract). J Thromb Haemost 2009; 7(suppl 2):abstract OC-TH-107.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 2010; 49:259–268.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol 2013; 8:1533–1539.
- Kaatz S, Crowther M. Reversal of target-specific oral anticoagulants. J Thromb Thrombolysis 2013; 36:195–202.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation 2011; 124:1573–1579.
- Sarode R, Milling TJ, Refaai MA, et al. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation 2013; 128:1234–1243.
KEY POINTS
- How long before surgery to stop a TSOAC depends on the bleeding risk of the procedure and the patient’s renal function.
- Perioperative bridging is generally unnecessary for patients on TSOACs.
- Routine coagulation assays such as the prothrombin time and activated partial thromboplastin time do not reliably reflect the degree of anticoagulation with TSOACs.
- There are no specific antidotes or standardized reversal strategies for TSOACs.
- TSOACs have a rapid onset of action and should only be restarted postoperatively once hemostasis has been confirmed.
How Should Patients with Acute Hip Fractures Be Managed Perioperatively?
Case
A 91-year-old man with Alzheimer’s dementia presents with severe right hip pain after a fall at his nursing home. His family reports that he is dependent in most of his activities of daily living (ADLs) and can normally ambulate short distances with a walker. He is alert and oriented at baseline but has been more confused since his wife died a week earlier from pneumonia. His only new medication is lorazepam as needed for anxiety. On admission, the patient is diagnosed with a displaced femoral neck fracture, delirium, and healthcare-associated pneumonia, with a new oxygen requirement of 5 L/min. The orthopedic surgery service requests a medicine consult. How should this patient be managed perioperatively?
Overview
Hip fractures are a major health burden on the United States’ geriatric population. The lifetime risk of hip fracture is approximately 17% for Caucasian women and 6% for Caucasian men.1 In 2010, an estimated 258,000 people aged 65 years and older were hospitalized with hip fractures.2 This number is expected to climb to 289,000 by 2030.
In total, hip fractures directly cost the healthcare system about $18 billion per year.1
Hip fractures, like most other geriatric syndromes, are almost invariably multifactorial in etiology. They occur at the intersection of general frailty, bone fragility, and fall risk. Hip fractures too often trigger a further downward spiral in elderly patients, as deconditioning and acute complications compound chronic comorbidities and compromise any remaining physiologic reserve. Mortality after a hip fracture approaches 25% at one year.3 An excess mortality risk persists for at least 10 years.4 Of the patients who survive six months, only 50% can perform their ADLs, and only 25% can perform their instrumental ADLs as well as they could prior to their fracture.5,6
Unsurprisingly, older adults with hip fractures are five times more likely to require nursing home placement at one year.5
Hospitalists frequently encounter patients with hip fractures in the perioperative setting. Given their close collaboration with orthopedic surgeons and emphasis on transitions of care, hospitalists can play an important role in reversing the trajectory of death and disability following hip fractures. Key aspects of inpatient management are outlined below.
Hip Fracture Repair
Hip fractures can be divided into intracapsular (femoral neck) or extracapsular (intratrochanteric or subtrochanteric) fractures. Their relative frequencies are listed in Table 1.7
Surgery types. Femoral neck fractures typically are the most difficult to heal, given a limited regional blood supply.5,7 Displaced femoral neck fractures require either a hemiarthroplasty or total hip arthroplasty. Over time, hemiarthroplasties tend to cause hip pain from acetabular erosion, so they are better suited for less active, elderly patients. Nondisplaced femoral neck, intratrochanteric, and subtrochanteric fractures are usually managed with open reduction and internal fixation.
The overall goal of surgery is to return patients to their prior level of functioning. In the short term, surgery also provides pain relief and allows for early mobilization. Nonoperative management is generally reserved for patients with very high operative risk or limited life expectancies or those who are bedridden at baseline.
Timing of surgery. In general, hip fracture repair should be performed within 24-48 hours of admission in patients who are medically stable. Though early surgery may not improve functional outcomes or mortality, it has been associated with improved pain control, decreased length of stay, and fewer major complications.8 Patients with active medical conditions (e.g. pneumonia) should be medically optimized before proceeding with surgery. A 2011 study found that most of the excess in-hospital mortality associated with surgical delays beyond five days was attributable to the active medical issues rather than to the delay itself.9
Prevention of Perioperative Complications
The principles of geriatric medicine should be applied to the care of elderly patients with hip fractures. Emphasis should be placed on early recognition of treatable conditions and avoidance of iatrogenesis. Careful assessment of medical problems, social support, and functional status within an interdisciplinary framework is recommended. Such a multi-faceted approach has been shown to reduce overall complications in hip fracture patients.10 Specific complications are discussed in more detail below.
Delirium. Delirium is the most common complication after hip fracture surgery, with a prevalence of 35%-65%.7 Proper pain control, minimization of polypharmacy, removal of tethers, and frequent reorientation are among the many preventive measures that should be implemented.
Venous thromboembolism (VTE). VTE is a leading cause of morbidity and mortality for hip fracture patients.11 Without prophylaxis, about 1.8% will develop symptomatic deep venous thromboses, and 1% will develop symptomatic pulmonary emboli in the first seven to 14 days after surgery. An estimated 4.3% will develop symptomatic VTE in the first 35 days after surgery.
The American College of Chest Physicians recommends that patients undergoing hip fracture surgery receive VTE prophylaxis for a minimum of 10-14 days postoperatively.11 Extending prophylaxis out to 35 days is reasonable. Low molecular-weight heparin is preferred over low-dose unfractionated heparin, fondaparinux, warfarin, and aspirin. Patients should receive preoperative VTE prophylaxis if surgery is delayed.
Postoperative infections. Urinary tract infections (UTIs) are the most common infectious complication after hip fracture surgery.7 If not caught early, they can result in urosepsis, prosthetic joint infections, and death. After the first 48 hours of urinary catheterization, the risk of a UTI is 5%-10% per day.12
Therefore, catheters should be removed within 24-48 hours of surgery.
Acute blood loss anemia. Anemia is common in hip fracture patients. It may be present on admission or develop as a result of intraoperative blood loss, ongoing drain output, or fluid resuscitation.
The recent FOCUS trial, which helped to clarify the optimal transfusion threshold for patients after hip fracture surgery, compared a liberal versus restrictive transfusion strategy in patients with cardiovascular disease.13 Transfusing for a hemoglobin < 10 g/dL, as opposed to transfusing for symptoms or a hemoglobin < 8 g/dL, did not improve mortality, in-hospital morbidity (including myocardial infarction), or functional status at 60 days.
Pressure ulcers. Patients with hip fractures are at risk of developing decubitus ulcers. One study found the incidence of new pressure ulcers to be 16% at seven days and 36% at 32 days after initial hospitalization.14 Multicomponent interventions have been shown to successfully reduce the rate of hospital-acquired pressure ulcers.15
Medical Management of Osteoporosis
The World Heath Organization defines osteoporosis as a bone mineral density of at least 2.5 standard deviations below that of a “normal” young adult as measured on DEXA scan, or a T-score ≤ -2.5.16 However, it is important to recognize that bone strength depends not only on the quantity of bone but also on the quality. Any patient who sustains a hip fracture with minimal trauma (e.g. a fall from standing height) should be considered to have osteoporosis, regardless of T-score.
Patients with their first hip fracture are 2.5 times more likely to have a future fragility fracture.17 Hospitalists must therefore make secondary prevention a priority. Medical management focuses on maintaining bone strength, slowing further bone loss, and preventing future falls.
Evaluation. A directed history and physical examination should be completed to screen for secondary causes of osteoporosis. A basic laboratory workup is reasonable in the inpatient setting (see Table 2).17 Other tests, such as a serum and urine protein electrophoresis, can be obtained as clinically indicated.
Patients require counseling directed at lifestyle factors, including the importance of weight-bearing exercise, smoking cessation, and avoidance of excessive alcohol intake. A comprehensive falls assessment is also warranted.
Treatment. All patients with hip fractures should be discharged from the hospital on calcium and vitamin D supplementation, unless there is a specific contraindication.18 Guidelines vary by organization, but the National Osteoporosis Foundation’s recommendations are listed in Table 3.17,19,20 Dietary calcium is usually insufficient to meet the daily requirement.
Bisphosphonates are considered first-line therapy for osteoporosis.17 The HORIZON trial was a randomized, placebo-controlled study that evaluated annual zoledronic acid infusions in hip fracture patients who were intolerant of oral bisphosphonates.21 Zoledronic acid reduced the rate of new fractures by 35% after 1.9 years, with a number needed to treat (NNT) of 19. It also improved survival by 28%, for an NNT of 27. All subjects also received calcium and vitamin D supplementation.
Both hospitalists and orthopedists might worry about bisphosphonates adversely affecting bone healing in the acute setting. Subsequent analyses from the HORIZON trial suggest that bisphosphonates can be safely started as soon as two weeks after surgery.22,23
Transitions of care. Despite well-established guidelines for the treatment of osteoporosis, patients with hip fractures often are undertreated. A retrospective study of 420 acute hip fracture patients found that only 37% received calcium, 36% received vitamin D, and 31% received a bisphosphonate on discharge.24 A prospective study of 1,075 women with new osteoporotic fractures found that only 17% had started anti-osteoporosis medications at one year.25
Hospitalists should recognize and address potential barriers to appropriate medical therapy. Patient-related obstacles may include the cost of medications, concerns about side effects, and lack of a PCP.24,25 Hospitalists should document the diagnosis of osteoporosis in the medical record so subsequent providers are attuned to the issue.26 They should also clarify the ownership of osteoporosis across the continuum of care, because medicine consultants, orthopedists, primary care or rehabilitation physicians, and subspecialists may all be involved. Hospitalists can certainly take advantage of this window of opportunity by starting patients on osteoporosis treatment and ensuring smooth transitions of care on discharge.
Back to the Case
The patient was started on intravenous antibiotics for healthcare-associated pneumonia with improvement of his oxygen requirement to 3 L/min. He underwent a right hemiarthroplasty on hospital day five and tolerated the procedure well. His delirium resolved with treatment of his infection, pain control, discontinuation of lorazepam, and other conservative measures. He was given VTE prophylaxis pre- and postoperatively. His urinary catheter was discontinued on day one after surgery. He was started on calcium supplementation and vitamin D repletion after his 25-OH vitamin D level returned low at 14 ng/mL.
The patient progressed well with physical and occupational therapy and was discharged back to his skilled nursing facility, with plans to start a bisphosphonate in two weeks.
Bottom Line
Hospitalists should be familiar with the best practices for the perioperative management of hip fracture patients.
Dr. Anderson and Dr. Wolfe are hospitalists at the University of Colorado Hospital in Aurora. Dr. Anderson directs the medicine consult service, and Dr. Wolfe serves as the associate director.
References
- The Joint Commission. Improving and measuring osteoporosis treatment 2008. Oakbrook Terrace, Il; 2008. Available at: http://www.jointcommission.org/improving_and_measuring_osteoporosis_management/. Accessed September 29, 2013.
- Stevens JA, Rudd RA. The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24(10):2725-2728.
- Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality, and costs. J Am Geriatr Soc. 2003;51(3):364-370. Haentjens P, Magaziner J, Colón-Emeric CS, et al.
- Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380-390.
- Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA. 2012;307(20):2185-2194.
- Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45(3):M101-107.
- Bateman L, Vuppala S, Porada P, et al. Medical management in the acute hip fracture patient: a comprehensive review for the internist. Ochsner J. 2012;12(2):101-110.
- Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
- Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233.
- Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482.
- Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
- Wald H, Epstein A, Kramer A. Extended use of indwelling urinary catheters in postoperative hip fracture patients. Med Care. 2005;43(10):1009-1017.
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
- Baumgarten M, Margolis DJ, Orwig DL, et al. Pressure ulcers in elderly patients with hip fracture across the continuum of care. J Am Geriatr Soc. 2009;57(5):863-870.
- Sullivan N, Schoelles KM. Preventing in-facility pressure ulcers as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):410-416.
- World Health Organization. Prevention and management of osteoporosis: Report of a WHO scientific group 2000. Geneva, Switzerland; 2000. WHO technical report series; 921. Available at: http://whqlibdoc.who.int/trs/who_trs_921.pdf. Accessed July 9, 2013.
- National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis 2013. Washington, D.C.; 2013. Available at: www.nof.org/files/nof/public/content/file/917/upload/481.pdf. Accessed September 29, 2013.
- Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657-666.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary reference intakes for calcium and vitamin D. Washington, D.C.: National Academies Press; 2011. Lyles KW, Colón-Emeric CS, Magaziner JS, et al.
- Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357: nihpa40967.
- Colón-Emeric CS, Nordsletten L, Olson S, et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int. 2011;22(8):2329-2336.
- Eriksen EF, Lyles KW, Colón-Emeric CS, et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J Bone Miner Res. 2009;24(7):1308-1313.
- Byszewski A, Lemay G, Molnar F, Azad N, McMartin SE. Closing the osteoporosis care gap in hip fracture patients: an opportunity to decrease recurrent fractures and hospital admissions. J Osteoporos. 2011;2011:404969.
- Greenspan SL, Wyman, A, Hoovan FH, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60(3):455-461.
- Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med. 2000;109(4):326-328.
Case
A 91-year-old man with Alzheimer’s dementia presents with severe right hip pain after a fall at his nursing home. His family reports that he is dependent in most of his activities of daily living (ADLs) and can normally ambulate short distances with a walker. He is alert and oriented at baseline but has been more confused since his wife died a week earlier from pneumonia. His only new medication is lorazepam as needed for anxiety. On admission, the patient is diagnosed with a displaced femoral neck fracture, delirium, and healthcare-associated pneumonia, with a new oxygen requirement of 5 L/min. The orthopedic surgery service requests a medicine consult. How should this patient be managed perioperatively?
Overview
Hip fractures are a major health burden on the United States’ geriatric population. The lifetime risk of hip fracture is approximately 17% for Caucasian women and 6% for Caucasian men.1 In 2010, an estimated 258,000 people aged 65 years and older were hospitalized with hip fractures.2 This number is expected to climb to 289,000 by 2030.
In total, hip fractures directly cost the healthcare system about $18 billion per year.1
Hip fractures, like most other geriatric syndromes, are almost invariably multifactorial in etiology. They occur at the intersection of general frailty, bone fragility, and fall risk. Hip fractures too often trigger a further downward spiral in elderly patients, as deconditioning and acute complications compound chronic comorbidities and compromise any remaining physiologic reserve. Mortality after a hip fracture approaches 25% at one year.3 An excess mortality risk persists for at least 10 years.4 Of the patients who survive six months, only 50% can perform their ADLs, and only 25% can perform their instrumental ADLs as well as they could prior to their fracture.5,6
Unsurprisingly, older adults with hip fractures are five times more likely to require nursing home placement at one year.5
Hospitalists frequently encounter patients with hip fractures in the perioperative setting. Given their close collaboration with orthopedic surgeons and emphasis on transitions of care, hospitalists can play an important role in reversing the trajectory of death and disability following hip fractures. Key aspects of inpatient management are outlined below.
Hip Fracture Repair
Hip fractures can be divided into intracapsular (femoral neck) or extracapsular (intratrochanteric or subtrochanteric) fractures. Their relative frequencies are listed in Table 1.7
Surgery types. Femoral neck fractures typically are the most difficult to heal, given a limited regional blood supply.5,7 Displaced femoral neck fractures require either a hemiarthroplasty or total hip arthroplasty. Over time, hemiarthroplasties tend to cause hip pain from acetabular erosion, so they are better suited for less active, elderly patients. Nondisplaced femoral neck, intratrochanteric, and subtrochanteric fractures are usually managed with open reduction and internal fixation.
The overall goal of surgery is to return patients to their prior level of functioning. In the short term, surgery also provides pain relief and allows for early mobilization. Nonoperative management is generally reserved for patients with very high operative risk or limited life expectancies or those who are bedridden at baseline.
Timing of surgery. In general, hip fracture repair should be performed within 24-48 hours of admission in patients who are medically stable. Though early surgery may not improve functional outcomes or mortality, it has been associated with improved pain control, decreased length of stay, and fewer major complications.8 Patients with active medical conditions (e.g. pneumonia) should be medically optimized before proceeding with surgery. A 2011 study found that most of the excess in-hospital mortality associated with surgical delays beyond five days was attributable to the active medical issues rather than to the delay itself.9
Prevention of Perioperative Complications
The principles of geriatric medicine should be applied to the care of elderly patients with hip fractures. Emphasis should be placed on early recognition of treatable conditions and avoidance of iatrogenesis. Careful assessment of medical problems, social support, and functional status within an interdisciplinary framework is recommended. Such a multi-faceted approach has been shown to reduce overall complications in hip fracture patients.10 Specific complications are discussed in more detail below.
Delirium. Delirium is the most common complication after hip fracture surgery, with a prevalence of 35%-65%.7 Proper pain control, minimization of polypharmacy, removal of tethers, and frequent reorientation are among the many preventive measures that should be implemented.
Venous thromboembolism (VTE). VTE is a leading cause of morbidity and mortality for hip fracture patients.11 Without prophylaxis, about 1.8% will develop symptomatic deep venous thromboses, and 1% will develop symptomatic pulmonary emboli in the first seven to 14 days after surgery. An estimated 4.3% will develop symptomatic VTE in the first 35 days after surgery.
The American College of Chest Physicians recommends that patients undergoing hip fracture surgery receive VTE prophylaxis for a minimum of 10-14 days postoperatively.11 Extending prophylaxis out to 35 days is reasonable. Low molecular-weight heparin is preferred over low-dose unfractionated heparin, fondaparinux, warfarin, and aspirin. Patients should receive preoperative VTE prophylaxis if surgery is delayed.
Postoperative infections. Urinary tract infections (UTIs) are the most common infectious complication after hip fracture surgery.7 If not caught early, they can result in urosepsis, prosthetic joint infections, and death. After the first 48 hours of urinary catheterization, the risk of a UTI is 5%-10% per day.12
Therefore, catheters should be removed within 24-48 hours of surgery.
Acute blood loss anemia. Anemia is common in hip fracture patients. It may be present on admission or develop as a result of intraoperative blood loss, ongoing drain output, or fluid resuscitation.
The recent FOCUS trial, which helped to clarify the optimal transfusion threshold for patients after hip fracture surgery, compared a liberal versus restrictive transfusion strategy in patients with cardiovascular disease.13 Transfusing for a hemoglobin < 10 g/dL, as opposed to transfusing for symptoms or a hemoglobin < 8 g/dL, did not improve mortality, in-hospital morbidity (including myocardial infarction), or functional status at 60 days.
Pressure ulcers. Patients with hip fractures are at risk of developing decubitus ulcers. One study found the incidence of new pressure ulcers to be 16% at seven days and 36% at 32 days after initial hospitalization.14 Multicomponent interventions have been shown to successfully reduce the rate of hospital-acquired pressure ulcers.15
Medical Management of Osteoporosis
The World Heath Organization defines osteoporosis as a bone mineral density of at least 2.5 standard deviations below that of a “normal” young adult as measured on DEXA scan, or a T-score ≤ -2.5.16 However, it is important to recognize that bone strength depends not only on the quantity of bone but also on the quality. Any patient who sustains a hip fracture with minimal trauma (e.g. a fall from standing height) should be considered to have osteoporosis, regardless of T-score.
Patients with their first hip fracture are 2.5 times more likely to have a future fragility fracture.17 Hospitalists must therefore make secondary prevention a priority. Medical management focuses on maintaining bone strength, slowing further bone loss, and preventing future falls.
Evaluation. A directed history and physical examination should be completed to screen for secondary causes of osteoporosis. A basic laboratory workup is reasonable in the inpatient setting (see Table 2).17 Other tests, such as a serum and urine protein electrophoresis, can be obtained as clinically indicated.
Patients require counseling directed at lifestyle factors, including the importance of weight-bearing exercise, smoking cessation, and avoidance of excessive alcohol intake. A comprehensive falls assessment is also warranted.
Treatment. All patients with hip fractures should be discharged from the hospital on calcium and vitamin D supplementation, unless there is a specific contraindication.18 Guidelines vary by organization, but the National Osteoporosis Foundation’s recommendations are listed in Table 3.17,19,20 Dietary calcium is usually insufficient to meet the daily requirement.
Bisphosphonates are considered first-line therapy for osteoporosis.17 The HORIZON trial was a randomized, placebo-controlled study that evaluated annual zoledronic acid infusions in hip fracture patients who were intolerant of oral bisphosphonates.21 Zoledronic acid reduced the rate of new fractures by 35% after 1.9 years, with a number needed to treat (NNT) of 19. It also improved survival by 28%, for an NNT of 27. All subjects also received calcium and vitamin D supplementation.
Both hospitalists and orthopedists might worry about bisphosphonates adversely affecting bone healing in the acute setting. Subsequent analyses from the HORIZON trial suggest that bisphosphonates can be safely started as soon as two weeks after surgery.22,23
Transitions of care. Despite well-established guidelines for the treatment of osteoporosis, patients with hip fractures often are undertreated. A retrospective study of 420 acute hip fracture patients found that only 37% received calcium, 36% received vitamin D, and 31% received a bisphosphonate on discharge.24 A prospective study of 1,075 women with new osteoporotic fractures found that only 17% had started anti-osteoporosis medications at one year.25
Hospitalists should recognize and address potential barriers to appropriate medical therapy. Patient-related obstacles may include the cost of medications, concerns about side effects, and lack of a PCP.24,25 Hospitalists should document the diagnosis of osteoporosis in the medical record so subsequent providers are attuned to the issue.26 They should also clarify the ownership of osteoporosis across the continuum of care, because medicine consultants, orthopedists, primary care or rehabilitation physicians, and subspecialists may all be involved. Hospitalists can certainly take advantage of this window of opportunity by starting patients on osteoporosis treatment and ensuring smooth transitions of care on discharge.
Back to the Case
The patient was started on intravenous antibiotics for healthcare-associated pneumonia with improvement of his oxygen requirement to 3 L/min. He underwent a right hemiarthroplasty on hospital day five and tolerated the procedure well. His delirium resolved with treatment of his infection, pain control, discontinuation of lorazepam, and other conservative measures. He was given VTE prophylaxis pre- and postoperatively. His urinary catheter was discontinued on day one after surgery. He was started on calcium supplementation and vitamin D repletion after his 25-OH vitamin D level returned low at 14 ng/mL.
The patient progressed well with physical and occupational therapy and was discharged back to his skilled nursing facility, with plans to start a bisphosphonate in two weeks.
Bottom Line
Hospitalists should be familiar with the best practices for the perioperative management of hip fracture patients.
Dr. Anderson and Dr. Wolfe are hospitalists at the University of Colorado Hospital in Aurora. Dr. Anderson directs the medicine consult service, and Dr. Wolfe serves as the associate director.
References
- The Joint Commission. Improving and measuring osteoporosis treatment 2008. Oakbrook Terrace, Il; 2008. Available at: http://www.jointcommission.org/improving_and_measuring_osteoporosis_management/. Accessed September 29, 2013.
- Stevens JA, Rudd RA. The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24(10):2725-2728.
- Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality, and costs. J Am Geriatr Soc. 2003;51(3):364-370. Haentjens P, Magaziner J, Colón-Emeric CS, et al.
- Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380-390.
- Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA. 2012;307(20):2185-2194.
- Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45(3):M101-107.
- Bateman L, Vuppala S, Porada P, et al. Medical management in the acute hip fracture patient: a comprehensive review for the internist. Ochsner J. 2012;12(2):101-110.
- Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
- Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233.
- Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482.
- Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
- Wald H, Epstein A, Kramer A. Extended use of indwelling urinary catheters in postoperative hip fracture patients. Med Care. 2005;43(10):1009-1017.
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
- Baumgarten M, Margolis DJ, Orwig DL, et al. Pressure ulcers in elderly patients with hip fracture across the continuum of care. J Am Geriatr Soc. 2009;57(5):863-870.
- Sullivan N, Schoelles KM. Preventing in-facility pressure ulcers as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):410-416.
- World Health Organization. Prevention and management of osteoporosis: Report of a WHO scientific group 2000. Geneva, Switzerland; 2000. WHO technical report series; 921. Available at: http://whqlibdoc.who.int/trs/who_trs_921.pdf. Accessed July 9, 2013.
- National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis 2013. Washington, D.C.; 2013. Available at: www.nof.org/files/nof/public/content/file/917/upload/481.pdf. Accessed September 29, 2013.
- Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657-666.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary reference intakes for calcium and vitamin D. Washington, D.C.: National Academies Press; 2011. Lyles KW, Colón-Emeric CS, Magaziner JS, et al.
- Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357: nihpa40967.
- Colón-Emeric CS, Nordsletten L, Olson S, et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int. 2011;22(8):2329-2336.
- Eriksen EF, Lyles KW, Colón-Emeric CS, et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J Bone Miner Res. 2009;24(7):1308-1313.
- Byszewski A, Lemay G, Molnar F, Azad N, McMartin SE. Closing the osteoporosis care gap in hip fracture patients: an opportunity to decrease recurrent fractures and hospital admissions. J Osteoporos. 2011;2011:404969.
- Greenspan SL, Wyman, A, Hoovan FH, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60(3):455-461.
- Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med. 2000;109(4):326-328.
Case
A 91-year-old man with Alzheimer’s dementia presents with severe right hip pain after a fall at his nursing home. His family reports that he is dependent in most of his activities of daily living (ADLs) and can normally ambulate short distances with a walker. He is alert and oriented at baseline but has been more confused since his wife died a week earlier from pneumonia. His only new medication is lorazepam as needed for anxiety. On admission, the patient is diagnosed with a displaced femoral neck fracture, delirium, and healthcare-associated pneumonia, with a new oxygen requirement of 5 L/min. The orthopedic surgery service requests a medicine consult. How should this patient be managed perioperatively?
Overview
Hip fractures are a major health burden on the United States’ geriatric population. The lifetime risk of hip fracture is approximately 17% for Caucasian women and 6% for Caucasian men.1 In 2010, an estimated 258,000 people aged 65 years and older were hospitalized with hip fractures.2 This number is expected to climb to 289,000 by 2030.
In total, hip fractures directly cost the healthcare system about $18 billion per year.1
Hip fractures, like most other geriatric syndromes, are almost invariably multifactorial in etiology. They occur at the intersection of general frailty, bone fragility, and fall risk. Hip fractures too often trigger a further downward spiral in elderly patients, as deconditioning and acute complications compound chronic comorbidities and compromise any remaining physiologic reserve. Mortality after a hip fracture approaches 25% at one year.3 An excess mortality risk persists for at least 10 years.4 Of the patients who survive six months, only 50% can perform their ADLs, and only 25% can perform their instrumental ADLs as well as they could prior to their fracture.5,6
Unsurprisingly, older adults with hip fractures are five times more likely to require nursing home placement at one year.5
Hospitalists frequently encounter patients with hip fractures in the perioperative setting. Given their close collaboration with orthopedic surgeons and emphasis on transitions of care, hospitalists can play an important role in reversing the trajectory of death and disability following hip fractures. Key aspects of inpatient management are outlined below.
Hip Fracture Repair
Hip fractures can be divided into intracapsular (femoral neck) or extracapsular (intratrochanteric or subtrochanteric) fractures. Their relative frequencies are listed in Table 1.7
Surgery types. Femoral neck fractures typically are the most difficult to heal, given a limited regional blood supply.5,7 Displaced femoral neck fractures require either a hemiarthroplasty or total hip arthroplasty. Over time, hemiarthroplasties tend to cause hip pain from acetabular erosion, so they are better suited for less active, elderly patients. Nondisplaced femoral neck, intratrochanteric, and subtrochanteric fractures are usually managed with open reduction and internal fixation.
The overall goal of surgery is to return patients to their prior level of functioning. In the short term, surgery also provides pain relief and allows for early mobilization. Nonoperative management is generally reserved for patients with very high operative risk or limited life expectancies or those who are bedridden at baseline.
Timing of surgery. In general, hip fracture repair should be performed within 24-48 hours of admission in patients who are medically stable. Though early surgery may not improve functional outcomes or mortality, it has been associated with improved pain control, decreased length of stay, and fewer major complications.8 Patients with active medical conditions (e.g. pneumonia) should be medically optimized before proceeding with surgery. A 2011 study found that most of the excess in-hospital mortality associated with surgical delays beyond five days was attributable to the active medical issues rather than to the delay itself.9
Prevention of Perioperative Complications
The principles of geriatric medicine should be applied to the care of elderly patients with hip fractures. Emphasis should be placed on early recognition of treatable conditions and avoidance of iatrogenesis. Careful assessment of medical problems, social support, and functional status within an interdisciplinary framework is recommended. Such a multi-faceted approach has been shown to reduce overall complications in hip fracture patients.10 Specific complications are discussed in more detail below.
Delirium. Delirium is the most common complication after hip fracture surgery, with a prevalence of 35%-65%.7 Proper pain control, minimization of polypharmacy, removal of tethers, and frequent reorientation are among the many preventive measures that should be implemented.
Venous thromboembolism (VTE). VTE is a leading cause of morbidity and mortality for hip fracture patients.11 Without prophylaxis, about 1.8% will develop symptomatic deep venous thromboses, and 1% will develop symptomatic pulmonary emboli in the first seven to 14 days after surgery. An estimated 4.3% will develop symptomatic VTE in the first 35 days after surgery.
The American College of Chest Physicians recommends that patients undergoing hip fracture surgery receive VTE prophylaxis for a minimum of 10-14 days postoperatively.11 Extending prophylaxis out to 35 days is reasonable. Low molecular-weight heparin is preferred over low-dose unfractionated heparin, fondaparinux, warfarin, and aspirin. Patients should receive preoperative VTE prophylaxis if surgery is delayed.
Postoperative infections. Urinary tract infections (UTIs) are the most common infectious complication after hip fracture surgery.7 If not caught early, they can result in urosepsis, prosthetic joint infections, and death. After the first 48 hours of urinary catheterization, the risk of a UTI is 5%-10% per day.12
Therefore, catheters should be removed within 24-48 hours of surgery.
Acute blood loss anemia. Anemia is common in hip fracture patients. It may be present on admission or develop as a result of intraoperative blood loss, ongoing drain output, or fluid resuscitation.
The recent FOCUS trial, which helped to clarify the optimal transfusion threshold for patients after hip fracture surgery, compared a liberal versus restrictive transfusion strategy in patients with cardiovascular disease.13 Transfusing for a hemoglobin < 10 g/dL, as opposed to transfusing for symptoms or a hemoglobin < 8 g/dL, did not improve mortality, in-hospital morbidity (including myocardial infarction), or functional status at 60 days.
Pressure ulcers. Patients with hip fractures are at risk of developing decubitus ulcers. One study found the incidence of new pressure ulcers to be 16% at seven days and 36% at 32 days after initial hospitalization.14 Multicomponent interventions have been shown to successfully reduce the rate of hospital-acquired pressure ulcers.15
Medical Management of Osteoporosis
The World Heath Organization defines osteoporosis as a bone mineral density of at least 2.5 standard deviations below that of a “normal” young adult as measured on DEXA scan, or a T-score ≤ -2.5.16 However, it is important to recognize that bone strength depends not only on the quantity of bone but also on the quality. Any patient who sustains a hip fracture with minimal trauma (e.g. a fall from standing height) should be considered to have osteoporosis, regardless of T-score.
Patients with their first hip fracture are 2.5 times more likely to have a future fragility fracture.17 Hospitalists must therefore make secondary prevention a priority. Medical management focuses on maintaining bone strength, slowing further bone loss, and preventing future falls.
Evaluation. A directed history and physical examination should be completed to screen for secondary causes of osteoporosis. A basic laboratory workup is reasonable in the inpatient setting (see Table 2).17 Other tests, such as a serum and urine protein electrophoresis, can be obtained as clinically indicated.
Patients require counseling directed at lifestyle factors, including the importance of weight-bearing exercise, smoking cessation, and avoidance of excessive alcohol intake. A comprehensive falls assessment is also warranted.
Treatment. All patients with hip fractures should be discharged from the hospital on calcium and vitamin D supplementation, unless there is a specific contraindication.18 Guidelines vary by organization, but the National Osteoporosis Foundation’s recommendations are listed in Table 3.17,19,20 Dietary calcium is usually insufficient to meet the daily requirement.
Bisphosphonates are considered first-line therapy for osteoporosis.17 The HORIZON trial was a randomized, placebo-controlled study that evaluated annual zoledronic acid infusions in hip fracture patients who were intolerant of oral bisphosphonates.21 Zoledronic acid reduced the rate of new fractures by 35% after 1.9 years, with a number needed to treat (NNT) of 19. It also improved survival by 28%, for an NNT of 27. All subjects also received calcium and vitamin D supplementation.
Both hospitalists and orthopedists might worry about bisphosphonates adversely affecting bone healing in the acute setting. Subsequent analyses from the HORIZON trial suggest that bisphosphonates can be safely started as soon as two weeks after surgery.22,23
Transitions of care. Despite well-established guidelines for the treatment of osteoporosis, patients with hip fractures often are undertreated. A retrospective study of 420 acute hip fracture patients found that only 37% received calcium, 36% received vitamin D, and 31% received a bisphosphonate on discharge.24 A prospective study of 1,075 women with new osteoporotic fractures found that only 17% had started anti-osteoporosis medications at one year.25
Hospitalists should recognize and address potential barriers to appropriate medical therapy. Patient-related obstacles may include the cost of medications, concerns about side effects, and lack of a PCP.24,25 Hospitalists should document the diagnosis of osteoporosis in the medical record so subsequent providers are attuned to the issue.26 They should also clarify the ownership of osteoporosis across the continuum of care, because medicine consultants, orthopedists, primary care or rehabilitation physicians, and subspecialists may all be involved. Hospitalists can certainly take advantage of this window of opportunity by starting patients on osteoporosis treatment and ensuring smooth transitions of care on discharge.
Back to the Case
The patient was started on intravenous antibiotics for healthcare-associated pneumonia with improvement of his oxygen requirement to 3 L/min. He underwent a right hemiarthroplasty on hospital day five and tolerated the procedure well. His delirium resolved with treatment of his infection, pain control, discontinuation of lorazepam, and other conservative measures. He was given VTE prophylaxis pre- and postoperatively. His urinary catheter was discontinued on day one after surgery. He was started on calcium supplementation and vitamin D repletion after his 25-OH vitamin D level returned low at 14 ng/mL.
The patient progressed well with physical and occupational therapy and was discharged back to his skilled nursing facility, with plans to start a bisphosphonate in two weeks.
Bottom Line
Hospitalists should be familiar with the best practices for the perioperative management of hip fracture patients.
Dr. Anderson and Dr. Wolfe are hospitalists at the University of Colorado Hospital in Aurora. Dr. Anderson directs the medicine consult service, and Dr. Wolfe serves as the associate director.
References
- The Joint Commission. Improving and measuring osteoporosis treatment 2008. Oakbrook Terrace, Il; 2008. Available at: http://www.jointcommission.org/improving_and_measuring_osteoporosis_management/. Accessed September 29, 2013.
- Stevens JA, Rudd RA. The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24(10):2725-2728.
- Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality, and costs. J Am Geriatr Soc. 2003;51(3):364-370. Haentjens P, Magaziner J, Colón-Emeric CS, et al.
- Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380-390.
- Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA. 2012;307(20):2185-2194.
- Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45(3):M101-107.
- Bateman L, Vuppala S, Porada P, et al. Medical management in the acute hip fracture patient: a comprehensive review for the internist. Ochsner J. 2012;12(2):101-110.
- Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
- Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233.
- Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482.
- Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
- Wald H, Epstein A, Kramer A. Extended use of indwelling urinary catheters in postoperative hip fracture patients. Med Care. 2005;43(10):1009-1017.
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
- Baumgarten M, Margolis DJ, Orwig DL, et al. Pressure ulcers in elderly patients with hip fracture across the continuum of care. J Am Geriatr Soc. 2009;57(5):863-870.
- Sullivan N, Schoelles KM. Preventing in-facility pressure ulcers as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):410-416.
- World Health Organization. Prevention and management of osteoporosis: Report of a WHO scientific group 2000. Geneva, Switzerland; 2000. WHO technical report series; 921. Available at: http://whqlibdoc.who.int/trs/who_trs_921.pdf. Accessed July 9, 2013.
- National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis 2013. Washington, D.C.; 2013. Available at: www.nof.org/files/nof/public/content/file/917/upload/481.pdf. Accessed September 29, 2013.
- Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657-666.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary reference intakes for calcium and vitamin D. Washington, D.C.: National Academies Press; 2011. Lyles KW, Colón-Emeric CS, Magaziner JS, et al.
- Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357: nihpa40967.
- Colón-Emeric CS, Nordsletten L, Olson S, et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int. 2011;22(8):2329-2336.
- Eriksen EF, Lyles KW, Colón-Emeric CS, et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J Bone Miner Res. 2009;24(7):1308-1313.
- Byszewski A, Lemay G, Molnar F, Azad N, McMartin SE. Closing the osteoporosis care gap in hip fracture patients: an opportunity to decrease recurrent fractures and hospital admissions. J Osteoporos. 2011;2011:404969.
- Greenspan SL, Wyman, A, Hoovan FH, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60(3):455-461.
- Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med. 2000;109(4):326-328.
What Is the Role of BNP in Diagnosis and Management of Acutely Decompensated Heart Failure?
Case
A 76-year-old woman with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and atrial fibrillation presents with shortness of breath. She is tachypneic, her pulse is 105 beats per minute, and her blood pressure is 105/60 mm/Hg. She is obese and has an immeasurable venous pressure with decreased breath sounds in both lung bases, and irregular and distant heart sounds. What is the role of brain (or B-type) natriuretic peptide (BNP) in the diagnosis and management of this patient?
Overview
Each year, more than 1 million patients are admitted to hospitals with acutely decompensated heart failure (ADHF). Although many of these patients carry a pre-admission diagnosis of CHF, their common presenting symptoms are not specific for ADHF, which leads to delays in diagnosis and therapy initiation, and increased diagnostic costs and potentially worse outcomes. Clinical risk scores from NHANES and the Framingham heart study have limited sensitivity, missing nearly 20% of patients.1,2 Moreover, these scores are underused by clinicians who depend heavily on clinical gestalt.3
Once ADHF is diagnosed, ongoing bedside assessment of volume status is a difficult and inexact science. The physiologic goal is achievement of normal left ventricular end diastolic volume; however, surrogate measures of this status, including weight change, venous pressure, and pulmonary and cardiac auscultatory findings, have significant limitations. After discharge, patients have high and heterogeneous risks of readmission, death, and other adverse events. Identifying patients with the highest risk might allow for intensive strategies to improve outcomes.
BNP is a neurohormone released from the ventricular cells in response to increased cardiac filling pressures. Plasma measurements of BNP have been shown to reflect volume status, to predict risk at admission and discharge, and to serve as a treatment guide in a variety of clinical settings.4 This simple laboratory test increasingly has been used to diagnose and manage ADHF; its utility and limitations deserve critical review.
Review of the Data
CHF diagnosis. Since introduction of the rapid BNP assay, several trials have evaluated its clinical utility in determining whether ADHF is the cause of a patient’s dyspnea. The largest of these trials, the Breathing Not Properly Multinational Study, conducted by McCullough et al, enrolled nearly 1,600 patients who presented with the primary complaint of dyspnea.5 After reviewing conventional clinical information, ED physicians were asked to determine the likelihood that ADHF was the etiology of a patient’s dyspnea. These likelihoods were classified as low (<20%), intermediate (20%-80%), or high (>80%). The admission BNP was recorded but was not available for the ED physician decisions.
The “gold standard” was the opinion of two adjudicating cardiologists who reviewed the cases retrospectively and determined whether the dyspnea resulted from ADHF. They were blinded to both the ED physician’s opinion and the BNP results. The accuracy of the ED physician’s initial assessment and the impact of the BNP results were compared with this gold standard.
For the entire cohort, the use of BNP (with a cutoff point of 100 pg/mL) would have improved the ED physician’s assessment from 74% diagnostic accuracy to 81%, which is statistically significant. Most important, in those patients initially given an intermediate likelihood of CHF, BNP results correctly classified 75% of these patients and rarely missed ADHF cases (<10%).
Atrial fibrillation. Since the original trials that established a BNP cutoff of 100 pg/mL for determining the presence of ADHF, several adjustments have been suggested. The presence of atrial fibrillation has been shown to increase BNP values independent of cardiac filling pressures. Breidthardt et al examined patients with atrial fibrillation presenting with dyspnea.4 In their analysis, using a cutoff of 100 pg/mL remained robust in identifying patients without ADHF. However, in the 100 pg/mL-500 pg/mL range, the test was not able to discriminate between atrial fibrillation and ADHF. Values greater than 500 pg/mL proved accurate in supporting the diagnosis of ADHF.
Renal failure. Renal dysfunction also elevates BNP levels independent of filling pressures. McCullough et al re-examined data from their Breathing Not Properly Multinational Study and found that the glomerular filtration rate (GFR) was inversely related to BNP levels.5 They recommend using a cutoff point of 200 pg/mL when the GFR is below 60 mg/dL. Other authors recommend not using BNP levels to diagnose ADHF when the GFR is less than 60 mg/dL due to the lack of data supporting this approach. Until clarified, clinicians should be cautious of interpreting BNP elevations in the setting of kidney disease.
Obesity. Obesity has a negative effect on BNP levels, decreasing the sensitivity of the test in these patients.6 Although no study defines how to adjust for body mass index (BMI), clinicians should be cautious about using a low BNP to rule out ADHF in a dyspneic obese patient.
Historical BNP values. If historical BNP values are available, studies of biological variation have shown that an increase to 123% from 66% from baseline is representative of a clinically meaningful increase in cardiac filling pressures. Less significant changes could merely represent biological variation and should be cautiously interpreted.7
Cost effectiveness. The cost effectiveness of using BNP measurements in dyspneic ED patients has been examined as well. Mueller et al found in a Swiss hospital that BNP testing was associated with a 25% decrease in treatment cost, length of stay (LOS), and ICU usage.8 However, LOS is significantly longer in Switzerland compared with the U.S., and given that much of the cost savings was attributed to reducing LOS, it is not possible to extrapolate these data to the U.S. health system. More evidence is needed to truly evaluate the cost effectiveness of BNP testing.
Serial BNP testing. Once a patient has been diagnosed with ADHF and admitted to the hospital, diuretics are indicated with the goal of achieving euvolemia. The bedside assessment of volume status remains a difficult and inexact science, and failure to appropriately remove fluid is associated with readmissions. Conversely, overdiuresis with a concomitant rise in creatinine has been associated with increased morbidity and mortality.
Several studies have shown that the reduction of volume associated with diuretic administration is coupled with a rapid decrease in BNP levels. Therefore, serial BNP measurement has been evaluated as a tool to guide the daily assessment of volume status in patients admitted with ADHF. Unfortunately, frequent measurements of BNP reveal that a great deal of variance, or “noise,” is present in these repeat measurements. Data do not clearly show how to incorporate serial BNP measurements into daily diuretic management.9
Mortality prediction. Nearly 3.5% of admitted heart failure patients will die during their hospitalization. For perspective, the rate of hospital mortality with acute myocardial infarction is 7%. BNP serves as a powerful and independent predictor of inpatient mortality. The ADHERE (Acute Decompensated Heart Failure National Registry) study showed that when divided into BNP quartiles of <430 pg/mL, 430 pg/mL to 839 pg/mL, 840 pg/mL to 1,729 pg/mL, and >1,730 pg/mL, patients’ risk of inpatient death was accurately predicted as 1.9%, 2.8%, 3.8%, and 6.0%, respectively.10 Even when adjusted for other risk factors, BNP remained a powerful predictor; the mortality rate more than doubled from the lowest to highest quartile.
Different strategies have been proposed to improve the outcomes in these highest-risk patients; however, to date, no evidence-based strategy offers a meaningful way to reduce inpatient mortality beyond the current standard of care.
Readmission and 30-day mortality. The 30-day readmission rate after discharge for ADHF is more than than 25%. A study of Medicare patients showed that more than $17 billion (more than 15% of all Medicare payments to hospitals) was associated with unplanned rehospitalizations.11 As bundling payment trends develop, hospitals have an enormous incentive to identify CHF patients with the highest risk of readmission and attempt to mitigate that risk.
From a patient-centered view, upon hospital discharge a patient with ADHF also realizes a 1 in 10 chance of dying within the first 30 days.
At discharge, BNP serves as a powerful and independent marker of increased risk of readmission, morbidity, and mortality. O’Connor et al developed a discharge risk model in patients with severe left ventricular dysfunction; the ESCAPE risk model and discharge score showed elevated BNP was the single most powerful predictor of six-month mortality.12 For every doubling of the BNP, the odds of death at six months increased by 1.4 times.
After combining discharge BNP with other factors, the ESCAPE discharge score was fairly successful at discriminating between patients who would and would not survive to six months. By identifying these outpatients, intensive management strategies could be focused on individuals with the highest risk. The data support the idea that readmission reductions are significant when outpatients obtain early follow-up. Many healthcare centers struggle to schedule early follow-up for all heart failure patients.
As such, the ability to target individuals with the highest discharge scores for intensive follow-up might improve outcomes. These patients could undergo early evaluation for such advanced therapies as resynchronization, left ventricular assist device implantation, or listing for transplantation. Currently, this strategy is not proven. It also is possible that these high-risk patients might have such advanced diseases that their risk cannot be modified by our current medications and advanced therapies.
Back to the Case
This patient has symptoms and signs that could be caused by ADHF or COPD. Her presentation is consistent with an intermediate probability of ADHF. A rapid BNP reveals a level of 950 pg/mL.
Even considering the higher cutoff required because of her coexistent atrial fibrillation, her BNP is consistent with ADHF. Additionally, her obesity likely has decreased the true value of her BNP. A previous BNP drawn when the patient was not in ADHF was 250 ng/mL, meaning that at least a 70% increase is present.
She was admitted and treated with intravenous diuretics with improvement in her congestion and relief of her symptoms. Daily BNPs were not drawn and her diuretics were titrated based on bedside clinical assessments. Her admission BNP elevation would predict a moderately high risk of short- and intermediate term of morbidity and mortality.
At discharge, a repeat BNP also could add to her risk stratification, though it would not be clear what do with this prognostic information beyond the standard of care.
Bottom Line
BNP measurement in specific situations can complement conventional clinical information in determining the presence of ADHF and also can enhance clinicians’ ability to risk-stratify patients during and after hospitalization. TH
Dr. Wolfe is a hospitalist and assistant professor of medicine at the University of Colorado Denver.
References
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301-306.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285(26):1441-1446.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
- Breidthardt T, Noveanu M, Cayir S, et al. The use of B-type natriuretic peptide in the management of patients with atrial fibrillation and dyspnea. Int J Cardiol. 2009;136(2):193-199.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
- Iwanaga Y, Hihara Y, Nizuma S, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13(8):663-667.
- O’Hanlon R, O’Shea P, Ledwidge M. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13(1):50-55.
- Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(1):1081-1087.
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152(5):828-834.
- Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;48 (19):1943-1950.
- Jencks SF, Williams MC, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
- O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872-878.
Case
A 76-year-old woman with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and atrial fibrillation presents with shortness of breath. She is tachypneic, her pulse is 105 beats per minute, and her blood pressure is 105/60 mm/Hg. She is obese and has an immeasurable venous pressure with decreased breath sounds in both lung bases, and irregular and distant heart sounds. What is the role of brain (or B-type) natriuretic peptide (BNP) in the diagnosis and management of this patient?
Overview
Each year, more than 1 million patients are admitted to hospitals with acutely decompensated heart failure (ADHF). Although many of these patients carry a pre-admission diagnosis of CHF, their common presenting symptoms are not specific for ADHF, which leads to delays in diagnosis and therapy initiation, and increased diagnostic costs and potentially worse outcomes. Clinical risk scores from NHANES and the Framingham heart study have limited sensitivity, missing nearly 20% of patients.1,2 Moreover, these scores are underused by clinicians who depend heavily on clinical gestalt.3
Once ADHF is diagnosed, ongoing bedside assessment of volume status is a difficult and inexact science. The physiologic goal is achievement of normal left ventricular end diastolic volume; however, surrogate measures of this status, including weight change, venous pressure, and pulmonary and cardiac auscultatory findings, have significant limitations. After discharge, patients have high and heterogeneous risks of readmission, death, and other adverse events. Identifying patients with the highest risk might allow for intensive strategies to improve outcomes.
BNP is a neurohormone released from the ventricular cells in response to increased cardiac filling pressures. Plasma measurements of BNP have been shown to reflect volume status, to predict risk at admission and discharge, and to serve as a treatment guide in a variety of clinical settings.4 This simple laboratory test increasingly has been used to diagnose and manage ADHF; its utility and limitations deserve critical review.
Review of the Data
CHF diagnosis. Since introduction of the rapid BNP assay, several trials have evaluated its clinical utility in determining whether ADHF is the cause of a patient’s dyspnea. The largest of these trials, the Breathing Not Properly Multinational Study, conducted by McCullough et al, enrolled nearly 1,600 patients who presented with the primary complaint of dyspnea.5 After reviewing conventional clinical information, ED physicians were asked to determine the likelihood that ADHF was the etiology of a patient’s dyspnea. These likelihoods were classified as low (<20%), intermediate (20%-80%), or high (>80%). The admission BNP was recorded but was not available for the ED physician decisions.
The “gold standard” was the opinion of two adjudicating cardiologists who reviewed the cases retrospectively and determined whether the dyspnea resulted from ADHF. They were blinded to both the ED physician’s opinion and the BNP results. The accuracy of the ED physician’s initial assessment and the impact of the BNP results were compared with this gold standard.
For the entire cohort, the use of BNP (with a cutoff point of 100 pg/mL) would have improved the ED physician’s assessment from 74% diagnostic accuracy to 81%, which is statistically significant. Most important, in those patients initially given an intermediate likelihood of CHF, BNP results correctly classified 75% of these patients and rarely missed ADHF cases (<10%).
Atrial fibrillation. Since the original trials that established a BNP cutoff of 100 pg/mL for determining the presence of ADHF, several adjustments have been suggested. The presence of atrial fibrillation has been shown to increase BNP values independent of cardiac filling pressures. Breidthardt et al examined patients with atrial fibrillation presenting with dyspnea.4 In their analysis, using a cutoff of 100 pg/mL remained robust in identifying patients without ADHF. However, in the 100 pg/mL-500 pg/mL range, the test was not able to discriminate between atrial fibrillation and ADHF. Values greater than 500 pg/mL proved accurate in supporting the diagnosis of ADHF.
Renal failure. Renal dysfunction also elevates BNP levels independent of filling pressures. McCullough et al re-examined data from their Breathing Not Properly Multinational Study and found that the glomerular filtration rate (GFR) was inversely related to BNP levels.5 They recommend using a cutoff point of 200 pg/mL when the GFR is below 60 mg/dL. Other authors recommend not using BNP levels to diagnose ADHF when the GFR is less than 60 mg/dL due to the lack of data supporting this approach. Until clarified, clinicians should be cautious of interpreting BNP elevations in the setting of kidney disease.
Obesity. Obesity has a negative effect on BNP levels, decreasing the sensitivity of the test in these patients.6 Although no study defines how to adjust for body mass index (BMI), clinicians should be cautious about using a low BNP to rule out ADHF in a dyspneic obese patient.
Historical BNP values. If historical BNP values are available, studies of biological variation have shown that an increase to 123% from 66% from baseline is representative of a clinically meaningful increase in cardiac filling pressures. Less significant changes could merely represent biological variation and should be cautiously interpreted.7
Cost effectiveness. The cost effectiveness of using BNP measurements in dyspneic ED patients has been examined as well. Mueller et al found in a Swiss hospital that BNP testing was associated with a 25% decrease in treatment cost, length of stay (LOS), and ICU usage.8 However, LOS is significantly longer in Switzerland compared with the U.S., and given that much of the cost savings was attributed to reducing LOS, it is not possible to extrapolate these data to the U.S. health system. More evidence is needed to truly evaluate the cost effectiveness of BNP testing.
Serial BNP testing. Once a patient has been diagnosed with ADHF and admitted to the hospital, diuretics are indicated with the goal of achieving euvolemia. The bedside assessment of volume status remains a difficult and inexact science, and failure to appropriately remove fluid is associated with readmissions. Conversely, overdiuresis with a concomitant rise in creatinine has been associated with increased morbidity and mortality.
Several studies have shown that the reduction of volume associated with diuretic administration is coupled with a rapid decrease in BNP levels. Therefore, serial BNP measurement has been evaluated as a tool to guide the daily assessment of volume status in patients admitted with ADHF. Unfortunately, frequent measurements of BNP reveal that a great deal of variance, or “noise,” is present in these repeat measurements. Data do not clearly show how to incorporate serial BNP measurements into daily diuretic management.9
Mortality prediction. Nearly 3.5% of admitted heart failure patients will die during their hospitalization. For perspective, the rate of hospital mortality with acute myocardial infarction is 7%. BNP serves as a powerful and independent predictor of inpatient mortality. The ADHERE (Acute Decompensated Heart Failure National Registry) study showed that when divided into BNP quartiles of <430 pg/mL, 430 pg/mL to 839 pg/mL, 840 pg/mL to 1,729 pg/mL, and >1,730 pg/mL, patients’ risk of inpatient death was accurately predicted as 1.9%, 2.8%, 3.8%, and 6.0%, respectively.10 Even when adjusted for other risk factors, BNP remained a powerful predictor; the mortality rate more than doubled from the lowest to highest quartile.
Different strategies have been proposed to improve the outcomes in these highest-risk patients; however, to date, no evidence-based strategy offers a meaningful way to reduce inpatient mortality beyond the current standard of care.
Readmission and 30-day mortality. The 30-day readmission rate after discharge for ADHF is more than than 25%. A study of Medicare patients showed that more than $17 billion (more than 15% of all Medicare payments to hospitals) was associated with unplanned rehospitalizations.11 As bundling payment trends develop, hospitals have an enormous incentive to identify CHF patients with the highest risk of readmission and attempt to mitigate that risk.
From a patient-centered view, upon hospital discharge a patient with ADHF also realizes a 1 in 10 chance of dying within the first 30 days.
At discharge, BNP serves as a powerful and independent marker of increased risk of readmission, morbidity, and mortality. O’Connor et al developed a discharge risk model in patients with severe left ventricular dysfunction; the ESCAPE risk model and discharge score showed elevated BNP was the single most powerful predictor of six-month mortality.12 For every doubling of the BNP, the odds of death at six months increased by 1.4 times.
After combining discharge BNP with other factors, the ESCAPE discharge score was fairly successful at discriminating between patients who would and would not survive to six months. By identifying these outpatients, intensive management strategies could be focused on individuals with the highest risk. The data support the idea that readmission reductions are significant when outpatients obtain early follow-up. Many healthcare centers struggle to schedule early follow-up for all heart failure patients.
As such, the ability to target individuals with the highest discharge scores for intensive follow-up might improve outcomes. These patients could undergo early evaluation for such advanced therapies as resynchronization, left ventricular assist device implantation, or listing for transplantation. Currently, this strategy is not proven. It also is possible that these high-risk patients might have such advanced diseases that their risk cannot be modified by our current medications and advanced therapies.
Back to the Case
This patient has symptoms and signs that could be caused by ADHF or COPD. Her presentation is consistent with an intermediate probability of ADHF. A rapid BNP reveals a level of 950 pg/mL.
Even considering the higher cutoff required because of her coexistent atrial fibrillation, her BNP is consistent with ADHF. Additionally, her obesity likely has decreased the true value of her BNP. A previous BNP drawn when the patient was not in ADHF was 250 ng/mL, meaning that at least a 70% increase is present.
She was admitted and treated with intravenous diuretics with improvement in her congestion and relief of her symptoms. Daily BNPs were not drawn and her diuretics were titrated based on bedside clinical assessments. Her admission BNP elevation would predict a moderately high risk of short- and intermediate term of morbidity and mortality.
At discharge, a repeat BNP also could add to her risk stratification, though it would not be clear what do with this prognostic information beyond the standard of care.
Bottom Line
BNP measurement in specific situations can complement conventional clinical information in determining the presence of ADHF and also can enhance clinicians’ ability to risk-stratify patients during and after hospitalization. TH
Dr. Wolfe is a hospitalist and assistant professor of medicine at the University of Colorado Denver.
References
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301-306.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285(26):1441-1446.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
- Breidthardt T, Noveanu M, Cayir S, et al. The use of B-type natriuretic peptide in the management of patients with atrial fibrillation and dyspnea. Int J Cardiol. 2009;136(2):193-199.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
- Iwanaga Y, Hihara Y, Nizuma S, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13(8):663-667.
- O’Hanlon R, O’Shea P, Ledwidge M. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13(1):50-55.
- Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(1):1081-1087.
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152(5):828-834.
- Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;48 (19):1943-1950.
- Jencks SF, Williams MC, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
- O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872-878.
Case
A 76-year-old woman with a history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), and atrial fibrillation presents with shortness of breath. She is tachypneic, her pulse is 105 beats per minute, and her blood pressure is 105/60 mm/Hg. She is obese and has an immeasurable venous pressure with decreased breath sounds in both lung bases, and irregular and distant heart sounds. What is the role of brain (or B-type) natriuretic peptide (BNP) in the diagnosis and management of this patient?
Overview
Each year, more than 1 million patients are admitted to hospitals with acutely decompensated heart failure (ADHF). Although many of these patients carry a pre-admission diagnosis of CHF, their common presenting symptoms are not specific for ADHF, which leads to delays in diagnosis and therapy initiation, and increased diagnostic costs and potentially worse outcomes. Clinical risk scores from NHANES and the Framingham heart study have limited sensitivity, missing nearly 20% of patients.1,2 Moreover, these scores are underused by clinicians who depend heavily on clinical gestalt.3
Once ADHF is diagnosed, ongoing bedside assessment of volume status is a difficult and inexact science. The physiologic goal is achievement of normal left ventricular end diastolic volume; however, surrogate measures of this status, including weight change, venous pressure, and pulmonary and cardiac auscultatory findings, have significant limitations. After discharge, patients have high and heterogeneous risks of readmission, death, and other adverse events. Identifying patients with the highest risk might allow for intensive strategies to improve outcomes.
BNP is a neurohormone released from the ventricular cells in response to increased cardiac filling pressures. Plasma measurements of BNP have been shown to reflect volume status, to predict risk at admission and discharge, and to serve as a treatment guide in a variety of clinical settings.4 This simple laboratory test increasingly has been used to diagnose and manage ADHF; its utility and limitations deserve critical review.
Review of the Data
CHF diagnosis. Since introduction of the rapid BNP assay, several trials have evaluated its clinical utility in determining whether ADHF is the cause of a patient’s dyspnea. The largest of these trials, the Breathing Not Properly Multinational Study, conducted by McCullough et al, enrolled nearly 1,600 patients who presented with the primary complaint of dyspnea.5 After reviewing conventional clinical information, ED physicians were asked to determine the likelihood that ADHF was the etiology of a patient’s dyspnea. These likelihoods were classified as low (<20%), intermediate (20%-80%), or high (>80%). The admission BNP was recorded but was not available for the ED physician decisions.
The “gold standard” was the opinion of two adjudicating cardiologists who reviewed the cases retrospectively and determined whether the dyspnea resulted from ADHF. They were blinded to both the ED physician’s opinion and the BNP results. The accuracy of the ED physician’s initial assessment and the impact of the BNP results were compared with this gold standard.
For the entire cohort, the use of BNP (with a cutoff point of 100 pg/mL) would have improved the ED physician’s assessment from 74% diagnostic accuracy to 81%, which is statistically significant. Most important, in those patients initially given an intermediate likelihood of CHF, BNP results correctly classified 75% of these patients and rarely missed ADHF cases (<10%).
Atrial fibrillation. Since the original trials that established a BNP cutoff of 100 pg/mL for determining the presence of ADHF, several adjustments have been suggested. The presence of atrial fibrillation has been shown to increase BNP values independent of cardiac filling pressures. Breidthardt et al examined patients with atrial fibrillation presenting with dyspnea.4 In their analysis, using a cutoff of 100 pg/mL remained robust in identifying patients without ADHF. However, in the 100 pg/mL-500 pg/mL range, the test was not able to discriminate between atrial fibrillation and ADHF. Values greater than 500 pg/mL proved accurate in supporting the diagnosis of ADHF.
Renal failure. Renal dysfunction also elevates BNP levels independent of filling pressures. McCullough et al re-examined data from their Breathing Not Properly Multinational Study and found that the glomerular filtration rate (GFR) was inversely related to BNP levels.5 They recommend using a cutoff point of 200 pg/mL when the GFR is below 60 mg/dL. Other authors recommend not using BNP levels to diagnose ADHF when the GFR is less than 60 mg/dL due to the lack of data supporting this approach. Until clarified, clinicians should be cautious of interpreting BNP elevations in the setting of kidney disease.
Obesity. Obesity has a negative effect on BNP levels, decreasing the sensitivity of the test in these patients.6 Although no study defines how to adjust for body mass index (BMI), clinicians should be cautious about using a low BNP to rule out ADHF in a dyspneic obese patient.
Historical BNP values. If historical BNP values are available, studies of biological variation have shown that an increase to 123% from 66% from baseline is representative of a clinically meaningful increase in cardiac filling pressures. Less significant changes could merely represent biological variation and should be cautiously interpreted.7
Cost effectiveness. The cost effectiveness of using BNP measurements in dyspneic ED patients has been examined as well. Mueller et al found in a Swiss hospital that BNP testing was associated with a 25% decrease in treatment cost, length of stay (LOS), and ICU usage.8 However, LOS is significantly longer in Switzerland compared with the U.S., and given that much of the cost savings was attributed to reducing LOS, it is not possible to extrapolate these data to the U.S. health system. More evidence is needed to truly evaluate the cost effectiveness of BNP testing.
Serial BNP testing. Once a patient has been diagnosed with ADHF and admitted to the hospital, diuretics are indicated with the goal of achieving euvolemia. The bedside assessment of volume status remains a difficult and inexact science, and failure to appropriately remove fluid is associated with readmissions. Conversely, overdiuresis with a concomitant rise in creatinine has been associated with increased morbidity and mortality.
Several studies have shown that the reduction of volume associated with diuretic administration is coupled with a rapid decrease in BNP levels. Therefore, serial BNP measurement has been evaluated as a tool to guide the daily assessment of volume status in patients admitted with ADHF. Unfortunately, frequent measurements of BNP reveal that a great deal of variance, or “noise,” is present in these repeat measurements. Data do not clearly show how to incorporate serial BNP measurements into daily diuretic management.9
Mortality prediction. Nearly 3.5% of admitted heart failure patients will die during their hospitalization. For perspective, the rate of hospital mortality with acute myocardial infarction is 7%. BNP serves as a powerful and independent predictor of inpatient mortality. The ADHERE (Acute Decompensated Heart Failure National Registry) study showed that when divided into BNP quartiles of <430 pg/mL, 430 pg/mL to 839 pg/mL, 840 pg/mL to 1,729 pg/mL, and >1,730 pg/mL, patients’ risk of inpatient death was accurately predicted as 1.9%, 2.8%, 3.8%, and 6.0%, respectively.10 Even when adjusted for other risk factors, BNP remained a powerful predictor; the mortality rate more than doubled from the lowest to highest quartile.
Different strategies have been proposed to improve the outcomes in these highest-risk patients; however, to date, no evidence-based strategy offers a meaningful way to reduce inpatient mortality beyond the current standard of care.
Readmission and 30-day mortality. The 30-day readmission rate after discharge for ADHF is more than than 25%. A study of Medicare patients showed that more than $17 billion (more than 15% of all Medicare payments to hospitals) was associated with unplanned rehospitalizations.11 As bundling payment trends develop, hospitals have an enormous incentive to identify CHF patients with the highest risk of readmission and attempt to mitigate that risk.
From a patient-centered view, upon hospital discharge a patient with ADHF also realizes a 1 in 10 chance of dying within the first 30 days.
At discharge, BNP serves as a powerful and independent marker of increased risk of readmission, morbidity, and mortality. O’Connor et al developed a discharge risk model in patients with severe left ventricular dysfunction; the ESCAPE risk model and discharge score showed elevated BNP was the single most powerful predictor of six-month mortality.12 For every doubling of the BNP, the odds of death at six months increased by 1.4 times.
After combining discharge BNP with other factors, the ESCAPE discharge score was fairly successful at discriminating between patients who would and would not survive to six months. By identifying these outpatients, intensive management strategies could be focused on individuals with the highest risk. The data support the idea that readmission reductions are significant when outpatients obtain early follow-up. Many healthcare centers struggle to schedule early follow-up for all heart failure patients.
As such, the ability to target individuals with the highest discharge scores for intensive follow-up might improve outcomes. These patients could undergo early evaluation for such advanced therapies as resynchronization, left ventricular assist device implantation, or listing for transplantation. Currently, this strategy is not proven. It also is possible that these high-risk patients might have such advanced diseases that their risk cannot be modified by our current medications and advanced therapies.
Back to the Case
This patient has symptoms and signs that could be caused by ADHF or COPD. Her presentation is consistent with an intermediate probability of ADHF. A rapid BNP reveals a level of 950 pg/mL.
Even considering the higher cutoff required because of her coexistent atrial fibrillation, her BNP is consistent with ADHF. Additionally, her obesity likely has decreased the true value of her BNP. A previous BNP drawn when the patient was not in ADHF was 250 ng/mL, meaning that at least a 70% increase is present.
She was admitted and treated with intravenous diuretics with improvement in her congestion and relief of her symptoms. Daily BNPs were not drawn and her diuretics were titrated based on bedside clinical assessments. Her admission BNP elevation would predict a moderately high risk of short- and intermediate term of morbidity and mortality.
At discharge, a repeat BNP also could add to her risk stratification, though it would not be clear what do with this prognostic information beyond the standard of care.
Bottom Line
BNP measurement in specific situations can complement conventional clinical information in determining the presence of ADHF and also can enhance clinicians’ ability to risk-stratify patients during and after hospitalization. TH
Dr. Wolfe is a hospitalist and assistant professor of medicine at the University of Colorado Denver.
References
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20(2):301-306.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Eng J Med. 1971;285(26):1441-1446.
- Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294(15):1944-1956.
- Breidthardt T, Noveanu M, Cayir S, et al. The use of B-type natriuretic peptide in the management of patients with atrial fibrillation and dyspnea. Int J Cardiol. 2009;136(2):193-199.
- McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41(3):571-579.
- Iwanaga Y, Hihara Y, Nizuma S, et al. BNP in overweight and obese patients with heart failure: an analysis based on the BNP-LV diastolic wall stress relationship. J Card Fail. 2007;13(8):663-667.
- O’Hanlon R, O’Shea P, Ledwidge M. The biologic variability of B-type natriuretic peptide and N-terminal pro-B-type natriuretic peptide in stable heart failure patients. J Card Fail. 2007;13(1):50-55.
- Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Arch Intern Med. 2006;166(1):1081-1087.
- Wu AH. Serial testing of B-type natriuretic peptide and NTpro-BNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Am Heart J. 2006;152(5):828-834.
- Fonarow GC, Peacock WF, Phillips CO, et al. ADHERE Scientific Advisory Committee and Investigators. Admission B-type natriuretic peptide levels and in-hospital mortality in acute decompensated heart failure. J Am Coll Cardiol. 2007;48 (19):1943-1950.
- Jencks SF, Williams MC, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418-1428.
- O’Connor CM, Hasselblad V, Mehta RH, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872-878.