User login
Performance status, molecular testing key to metastatic cancer prognosis
according to Sam Brondfield, MD, MA, an inpatient medical oncologist at the University of California, San Francisco.
Oncologists have at their fingertips a voluminous and ever-growing body of clinical trials data to draw on for prognostication. Yet many hospitalists will be surprised to learn that this wealth of information is of little value in the inpatient settings where they work, he said at HM20 Virtual, hosted by the Society of Hospital Medicine.
“The applicability of clinical trials data to hospitalized patients is generally poor. That’s an important caveat to keep in mind,” Dr. Brondfield said.
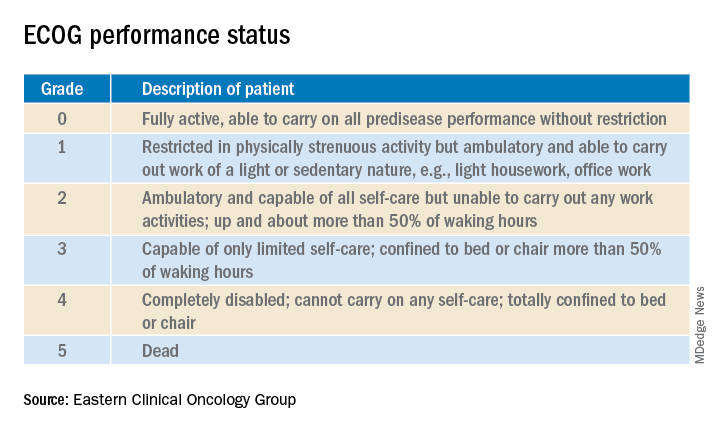
Enrollment in clinical trials is usually restricted to patients with a score of 0 or 1 on the Eastern Clinical Oncology Group Performance Status, meaning their cancer is causing minimal or no disruption to their life (see graphic). Sometimes trials will include patients with a performance status of 2 on the ECOG scale, a tool developed nearly 40 years ago, but clinical trials virtually never enroll those with an ECOG status of 3 or 4. Yet most hospitalized patients with metastatic cancer have an ECOG performance status of 3 or worse. Thus, the clinical trials outcome data are of little relevance.
“In oncology the distinction between ECOG 2 and 3 is very important,” Dr. Brondfield emphasized.
When he talks about treatment options with hospitalized patients who have metastatic cancer and poor performance status – that is, ECOG 3 or 4 – he’ll often say: “Assuming you feel better and can go home, that’s when these clinical trial data may apply better to you.”
Dr. Brondfield cautioned against quoting the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 5-year overall survival data when hospitalized patients with advanced cancer ask how long they have to live. For one thing, the national average 5-year overall survival figure is hardly an individualized assessment. Plus, oncology is a fast-moving field in which important treatment advances occur all the time, and the SEER data lag far behind. For example, when Dr. Brondfield recently looked up the current SEER 5-year survival for patients diagnosed with metastatic non–small cell lung cancer (NSCLC), the figure quoted was less than 6%, and it was drawn from data accrued in 2009-2015. That simply doesn’t reflect contemporary practice.
Indeed, it’s no longer true that the average survival of patients with metastatic NSCLC is less than a year. In the practice-changing KEYNOTE-189 randomized trial, which accrued participants in 2016-2017, the median overall survival of patients randomized to pembrolizumab (Keytruda) plus standard cytotoxic chemotherapy was 22 months, compared with 11 months with chemotherapy plus placebo (J Clin Oncol. 2020 May 10. doi: 10.1200/JCO.19.03136). As a result, immunotherapy with a programmed death–1 inhibitor such as pembrolizumab in combination with chemotherapy is now standard practice in patients with metastatic NSCLC without targetable mutations.
Performance status guides treatment decision-making
Hospitalists can help oncologists in decision-making regarding whether to offer palliative systemic therapy to patients with advanced metastatic cancer and poor performance status by determining whether that status is caused by the cancer itself or some other cause that’s not easily reversible, such as liver failure.
Take, for example, the inpatient with advanced SCLC. This is an aggressive and chemosensitive cancer. Dr. Brondfield said he is among many medical oncologists who are convinced that, if poor performance status in a patient with advanced SCLC is caused by the cancer itself, prompt initiation of inpatient chemotherapy should be recommended to elicit a response that improves quality of life and performance status in the short term. If, on the other hand, the poor performance status is caused by organ failure or some other issue that can’t easily be improved, hospice may be more appropriate.
“The contour of SCLC over time is that despite its treatment responsiveness it inevitably recurs. But with chemotherapy you can give people in this situation months of quality time, so we generally try to treat these sorts of patients,” Dr. Brondfield explained.
The National Comprehensive Cancer Network guidelines upon which oncologists rely leave lots of room for interpretation regarding the appropriateness of inpatient chemotherapy in patients with advanced cancer and poor patient performance status. Citing “knowledge that’s been passed down across oncology generations,” Dr. Brondfield said he and many of his colleagues believe early palliative supportive care rather than systemic cytotoxic cancer-directed therapy is appropriate for patients with poor performance status who have one of several specific relatively nonchemoresponsive types of metastatic cancer. These include esophageal, gastric, and head and neck cancers.
On the other hand, advanced SCLC isn’t the only type of metastatic cancer that’s so chemosensitive that he and many other oncologists believe aggressive chemotherapy should be offered even in the face of poor patient performance status attributable to the cancer itself.
Take, for example, colorectal cancer with no more than five metastases to the lung or liver, provided those metastases are treatable with resection or radiation. “Those patients are actually curable at a high rate. They have about a 30%-40% cure rate. So those patients, even if they have poor performance status, if we can get them up for surgery or radiation, we usually do try to treat them aggressively,” Dr. Brondfield said.
There are other often chemoresponsive metastatic cancers for which oncologists frequently recommend aggressive treatment to improve quality of life in patients with poor performance status. These cancers include aggressive lymphomas, which are actually often curable; multiple myeloma; testicular and germ cell cancers; NSCLC with a targetable mutation, which is often responsive to oral medications; and prostate and well-differentiated thyroid cancers, which can usually be treated with hormone- or iodine-based therapies rather than more toxic intravenous cytotoxic chemotherapy.
The impact of inpatient palliative chemotherapy in patients with poor performance status and advanced solid cancers not on the short list of highly chemosensitive cancers has not been well studied. A recent retrospective study of 228 such patients who received inpatient palliative chemotherapy at a large Brazilian academic medical center provided little reason for enthusiasm regarding the practice. Survival was short, with 30- and 60-day survival rates of 56% and 39%, respectively. Plus, 30% of patients were admitted to the ICU, where they received aggressive and costly end-of-life care. The investigators found these results suggestive of overprescribing of inpatient palliative chemotherapy (BMC Palliat Care. 2019 May 20;18[1]:42. doi: 10.1186/s12904-019-0427-4).
Of note, the investigators found in a multivariate analysis that an elevated bilirubin was associated with a 217% increased risk of 30-day mortality, and hypercalcemia was associated with a 119% increased risk.
“That’s something to take into account when these decisions are being made,” Dr. Brondfield advised.
In response to an audience comment that oncologists often seem overly optimistic about prognosis, Dr. Brondfield observed, “I think it’s very common for there to be a disagreement between the oncologist wanting to be aggressive for a sick inpatient and the hospitalist or generalist provider thinking: ‘This person looks way too sick for chemotherapy.’ ”
For this reason he is a firm believer in having multidisciplinary conversations regarding prognosis in challenging situations involving hospitalized patients with advanced cancer. An oncologist can bring to such discussions a detailed understanding of clinical trial and molecular data as well as information about the patient’s response to the first round of therapy. But lots of other factors are relevant to prognosis, including nutritional status, comorbidities, and the intuitive eyeball test of how a patient might do. The patient’s family, primary care provider, oncologist, the hospitalist, and the palliative care team will have perspectives of their own.
Molecular testing is now the norm in metastatic cancers
These days oncologists order molecular testing for most patients with metastatic carcinomas to determine eligibility for targeted therapy, suitability for participation in clinical trials, prognostication, and/or assistance in determining the site of origin if that’s unclear.
A single-pass fine needle aspiration biopsy doesn’t provide enough tissue for molecular testing. It’s therefore important to order initially a multipass fine needle aspiration to avoid the need for a repeat biopsy, which is uncomfortable for the patient and can delay diagnosis and treatment.
Dr. Brondfield advised waiting for molecular testing results to come in before trying to prognosticate in patients with a metastatic cancer for which targetable mutations might be present. Survival rates can vary substantially depending upon those test results. Take, for example, metastatic NSCLC: Just within the past year, clinical trials have been published reporting overall survival rates of 39 months in patients with treatable mutations in epidermal growth factor receptor, 42 months with anaplastic lymphoma kinase mutations, and 51 months in patients whose tumor signature features mutations in c-ros oncogene 1, as compared with 22 months with no targetable mutations in the KEYNOTE-189 trial.
“There’s a lot of heterogeneity around how metastatic tumors behave and respond to therapy. Not all metastatic cancers are the same,” the oncologist emphasized.
according to Sam Brondfield, MD, MA, an inpatient medical oncologist at the University of California, San Francisco.

Oncologists have at their fingertips a voluminous and ever-growing body of clinical trials data to draw on for prognostication. Yet many hospitalists will be surprised to learn that this wealth of information is of little value in the inpatient settings where they work, he said at HM20 Virtual, hosted by the Society of Hospital Medicine.
“The applicability of clinical trials data to hospitalized patients is generally poor. That’s an important caveat to keep in mind,” Dr. Brondfield said.
Enrollment in clinical trials is usually restricted to patients with a score of 0 or 1 on the Eastern Clinical Oncology Group Performance Status, meaning their cancer is causing minimal or no disruption to their life (see graphic). Sometimes trials will include patients with a performance status of 2 on the ECOG scale, a tool developed nearly 40 years ago, but clinical trials virtually never enroll those with an ECOG status of 3 or 4. Yet most hospitalized patients with metastatic cancer have an ECOG performance status of 3 or worse. Thus, the clinical trials outcome data are of little relevance.
“In oncology the distinction between ECOG 2 and 3 is very important,” Dr. Brondfield emphasized.
When he talks about treatment options with hospitalized patients who have metastatic cancer and poor performance status – that is, ECOG 3 or 4 – he’ll often say: “Assuming you feel better and can go home, that’s when these clinical trial data may apply better to you.”
Dr. Brondfield cautioned against quoting the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 5-year overall survival data when hospitalized patients with advanced cancer ask how long they have to live. For one thing, the national average 5-year overall survival figure is hardly an individualized assessment. Plus, oncology is a fast-moving field in which important treatment advances occur all the time, and the SEER data lag far behind. For example, when Dr. Brondfield recently looked up the current SEER 5-year survival for patients diagnosed with metastatic non–small cell lung cancer (NSCLC), the figure quoted was less than 6%, and it was drawn from data accrued in 2009-2015. That simply doesn’t reflect contemporary practice.
Indeed, it’s no longer true that the average survival of patients with metastatic NSCLC is less than a year. In the practice-changing KEYNOTE-189 randomized trial, which accrued participants in 2016-2017, the median overall survival of patients randomized to pembrolizumab (Keytruda) plus standard cytotoxic chemotherapy was 22 months, compared with 11 months with chemotherapy plus placebo (J Clin Oncol. 2020 May 10. doi: 10.1200/JCO.19.03136). As a result, immunotherapy with a programmed death–1 inhibitor such as pembrolizumab in combination with chemotherapy is now standard practice in patients with metastatic NSCLC without targetable mutations.
Performance status guides treatment decision-making
Hospitalists can help oncologists in decision-making regarding whether to offer palliative systemic therapy to patients with advanced metastatic cancer and poor performance status by determining whether that status is caused by the cancer itself or some other cause that’s not easily reversible, such as liver failure.
Take, for example, the inpatient with advanced SCLC. This is an aggressive and chemosensitive cancer. Dr. Brondfield said he is among many medical oncologists who are convinced that, if poor performance status in a patient with advanced SCLC is caused by the cancer itself, prompt initiation of inpatient chemotherapy should be recommended to elicit a response that improves quality of life and performance status in the short term. If, on the other hand, the poor performance status is caused by organ failure or some other issue that can’t easily be improved, hospice may be more appropriate.
“The contour of SCLC over time is that despite its treatment responsiveness it inevitably recurs. But with chemotherapy you can give people in this situation months of quality time, so we generally try to treat these sorts of patients,” Dr. Brondfield explained.
The National Comprehensive Cancer Network guidelines upon which oncologists rely leave lots of room for interpretation regarding the appropriateness of inpatient chemotherapy in patients with advanced cancer and poor patient performance status. Citing “knowledge that’s been passed down across oncology generations,” Dr. Brondfield said he and many of his colleagues believe early palliative supportive care rather than systemic cytotoxic cancer-directed therapy is appropriate for patients with poor performance status who have one of several specific relatively nonchemoresponsive types of metastatic cancer. These include esophageal, gastric, and head and neck cancers.
On the other hand, advanced SCLC isn’t the only type of metastatic cancer that’s so chemosensitive that he and many other oncologists believe aggressive chemotherapy should be offered even in the face of poor patient performance status attributable to the cancer itself.
Take, for example, colorectal cancer with no more than five metastases to the lung or liver, provided those metastases are treatable with resection or radiation. “Those patients are actually curable at a high rate. They have about a 30%-40% cure rate. So those patients, even if they have poor performance status, if we can get them up for surgery or radiation, we usually do try to treat them aggressively,” Dr. Brondfield said.
There are other often chemoresponsive metastatic cancers for which oncologists frequently recommend aggressive treatment to improve quality of life in patients with poor performance status. These cancers include aggressive lymphomas, which are actually often curable; multiple myeloma; testicular and germ cell cancers; NSCLC with a targetable mutation, which is often responsive to oral medications; and prostate and well-differentiated thyroid cancers, which can usually be treated with hormone- or iodine-based therapies rather than more toxic intravenous cytotoxic chemotherapy.
The impact of inpatient palliative chemotherapy in patients with poor performance status and advanced solid cancers not on the short list of highly chemosensitive cancers has not been well studied. A recent retrospective study of 228 such patients who received inpatient palliative chemotherapy at a large Brazilian academic medical center provided little reason for enthusiasm regarding the practice. Survival was short, with 30- and 60-day survival rates of 56% and 39%, respectively. Plus, 30% of patients were admitted to the ICU, where they received aggressive and costly end-of-life care. The investigators found these results suggestive of overprescribing of inpatient palliative chemotherapy (BMC Palliat Care. 2019 May 20;18[1]:42. doi: 10.1186/s12904-019-0427-4).
Of note, the investigators found in a multivariate analysis that an elevated bilirubin was associated with a 217% increased risk of 30-day mortality, and hypercalcemia was associated with a 119% increased risk.
“That’s something to take into account when these decisions are being made,” Dr. Brondfield advised.
In response to an audience comment that oncologists often seem overly optimistic about prognosis, Dr. Brondfield observed, “I think it’s very common for there to be a disagreement between the oncologist wanting to be aggressive for a sick inpatient and the hospitalist or generalist provider thinking: ‘This person looks way too sick for chemotherapy.’ ”
For this reason he is a firm believer in having multidisciplinary conversations regarding prognosis in challenging situations involving hospitalized patients with advanced cancer. An oncologist can bring to such discussions a detailed understanding of clinical trial and molecular data as well as information about the patient’s response to the first round of therapy. But lots of other factors are relevant to prognosis, including nutritional status, comorbidities, and the intuitive eyeball test of how a patient might do. The patient’s family, primary care provider, oncologist, the hospitalist, and the palliative care team will have perspectives of their own.
Molecular testing is now the norm in metastatic cancers
These days oncologists order molecular testing for most patients with metastatic carcinomas to determine eligibility for targeted therapy, suitability for participation in clinical trials, prognostication, and/or assistance in determining the site of origin if that’s unclear.
A single-pass fine needle aspiration biopsy doesn’t provide enough tissue for molecular testing. It’s therefore important to order initially a multipass fine needle aspiration to avoid the need for a repeat biopsy, which is uncomfortable for the patient and can delay diagnosis and treatment.
Dr. Brondfield advised waiting for molecular testing results to come in before trying to prognosticate in patients with a metastatic cancer for which targetable mutations might be present. Survival rates can vary substantially depending upon those test results. Take, for example, metastatic NSCLC: Just within the past year, clinical trials have been published reporting overall survival rates of 39 months in patients with treatable mutations in epidermal growth factor receptor, 42 months with anaplastic lymphoma kinase mutations, and 51 months in patients whose tumor signature features mutations in c-ros oncogene 1, as compared with 22 months with no targetable mutations in the KEYNOTE-189 trial.
“There’s a lot of heterogeneity around how metastatic tumors behave and respond to therapy. Not all metastatic cancers are the same,” the oncologist emphasized.
according to Sam Brondfield, MD, MA, an inpatient medical oncologist at the University of California, San Francisco.

Oncologists have at their fingertips a voluminous and ever-growing body of clinical trials data to draw on for prognostication. Yet many hospitalists will be surprised to learn that this wealth of information is of little value in the inpatient settings where they work, he said at HM20 Virtual, hosted by the Society of Hospital Medicine.
“The applicability of clinical trials data to hospitalized patients is generally poor. That’s an important caveat to keep in mind,” Dr. Brondfield said.
Enrollment in clinical trials is usually restricted to patients with a score of 0 or 1 on the Eastern Clinical Oncology Group Performance Status, meaning their cancer is causing minimal or no disruption to their life (see graphic). Sometimes trials will include patients with a performance status of 2 on the ECOG scale, a tool developed nearly 40 years ago, but clinical trials virtually never enroll those with an ECOG status of 3 or 4. Yet most hospitalized patients with metastatic cancer have an ECOG performance status of 3 or worse. Thus, the clinical trials outcome data are of little relevance.
“In oncology the distinction between ECOG 2 and 3 is very important,” Dr. Brondfield emphasized.
When he talks about treatment options with hospitalized patients who have metastatic cancer and poor performance status – that is, ECOG 3 or 4 – he’ll often say: “Assuming you feel better and can go home, that’s when these clinical trial data may apply better to you.”
Dr. Brondfield cautioned against quoting the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) 5-year overall survival data when hospitalized patients with advanced cancer ask how long they have to live. For one thing, the national average 5-year overall survival figure is hardly an individualized assessment. Plus, oncology is a fast-moving field in which important treatment advances occur all the time, and the SEER data lag far behind. For example, when Dr. Brondfield recently looked up the current SEER 5-year survival for patients diagnosed with metastatic non–small cell lung cancer (NSCLC), the figure quoted was less than 6%, and it was drawn from data accrued in 2009-2015. That simply doesn’t reflect contemporary practice.
Indeed, it’s no longer true that the average survival of patients with metastatic NSCLC is less than a year. In the practice-changing KEYNOTE-189 randomized trial, which accrued participants in 2016-2017, the median overall survival of patients randomized to pembrolizumab (Keytruda) plus standard cytotoxic chemotherapy was 22 months, compared with 11 months with chemotherapy plus placebo (J Clin Oncol. 2020 May 10. doi: 10.1200/JCO.19.03136). As a result, immunotherapy with a programmed death–1 inhibitor such as pembrolizumab in combination with chemotherapy is now standard practice in patients with metastatic NSCLC without targetable mutations.
Performance status guides treatment decision-making
Hospitalists can help oncologists in decision-making regarding whether to offer palliative systemic therapy to patients with advanced metastatic cancer and poor performance status by determining whether that status is caused by the cancer itself or some other cause that’s not easily reversible, such as liver failure.
Take, for example, the inpatient with advanced SCLC. This is an aggressive and chemosensitive cancer. Dr. Brondfield said he is among many medical oncologists who are convinced that, if poor performance status in a patient with advanced SCLC is caused by the cancer itself, prompt initiation of inpatient chemotherapy should be recommended to elicit a response that improves quality of life and performance status in the short term. If, on the other hand, the poor performance status is caused by organ failure or some other issue that can’t easily be improved, hospice may be more appropriate.
“The contour of SCLC over time is that despite its treatment responsiveness it inevitably recurs. But with chemotherapy you can give people in this situation months of quality time, so we generally try to treat these sorts of patients,” Dr. Brondfield explained.
The National Comprehensive Cancer Network guidelines upon which oncologists rely leave lots of room for interpretation regarding the appropriateness of inpatient chemotherapy in patients with advanced cancer and poor patient performance status. Citing “knowledge that’s been passed down across oncology generations,” Dr. Brondfield said he and many of his colleagues believe early palliative supportive care rather than systemic cytotoxic cancer-directed therapy is appropriate for patients with poor performance status who have one of several specific relatively nonchemoresponsive types of metastatic cancer. These include esophageal, gastric, and head and neck cancers.
On the other hand, advanced SCLC isn’t the only type of metastatic cancer that’s so chemosensitive that he and many other oncologists believe aggressive chemotherapy should be offered even in the face of poor patient performance status attributable to the cancer itself.
Take, for example, colorectal cancer with no more than five metastases to the lung or liver, provided those metastases are treatable with resection or radiation. “Those patients are actually curable at a high rate. They have about a 30%-40% cure rate. So those patients, even if they have poor performance status, if we can get them up for surgery or radiation, we usually do try to treat them aggressively,” Dr. Brondfield said.
There are other often chemoresponsive metastatic cancers for which oncologists frequently recommend aggressive treatment to improve quality of life in patients with poor performance status. These cancers include aggressive lymphomas, which are actually often curable; multiple myeloma; testicular and germ cell cancers; NSCLC with a targetable mutation, which is often responsive to oral medications; and prostate and well-differentiated thyroid cancers, which can usually be treated with hormone- or iodine-based therapies rather than more toxic intravenous cytotoxic chemotherapy.
The impact of inpatient palliative chemotherapy in patients with poor performance status and advanced solid cancers not on the short list of highly chemosensitive cancers has not been well studied. A recent retrospective study of 228 such patients who received inpatient palliative chemotherapy at a large Brazilian academic medical center provided little reason for enthusiasm regarding the practice. Survival was short, with 30- and 60-day survival rates of 56% and 39%, respectively. Plus, 30% of patients were admitted to the ICU, where they received aggressive and costly end-of-life care. The investigators found these results suggestive of overprescribing of inpatient palliative chemotherapy (BMC Palliat Care. 2019 May 20;18[1]:42. doi: 10.1186/s12904-019-0427-4).
Of note, the investigators found in a multivariate analysis that an elevated bilirubin was associated with a 217% increased risk of 30-day mortality, and hypercalcemia was associated with a 119% increased risk.
“That’s something to take into account when these decisions are being made,” Dr. Brondfield advised.
In response to an audience comment that oncologists often seem overly optimistic about prognosis, Dr. Brondfield observed, “I think it’s very common for there to be a disagreement between the oncologist wanting to be aggressive for a sick inpatient and the hospitalist or generalist provider thinking: ‘This person looks way too sick for chemotherapy.’ ”
For this reason he is a firm believer in having multidisciplinary conversations regarding prognosis in challenging situations involving hospitalized patients with advanced cancer. An oncologist can bring to such discussions a detailed understanding of clinical trial and molecular data as well as information about the patient’s response to the first round of therapy. But lots of other factors are relevant to prognosis, including nutritional status, comorbidities, and the intuitive eyeball test of how a patient might do. The patient’s family, primary care provider, oncologist, the hospitalist, and the palliative care team will have perspectives of their own.
Molecular testing is now the norm in metastatic cancers
These days oncologists order molecular testing for most patients with metastatic carcinomas to determine eligibility for targeted therapy, suitability for participation in clinical trials, prognostication, and/or assistance in determining the site of origin if that’s unclear.
A single-pass fine needle aspiration biopsy doesn’t provide enough tissue for molecular testing. It’s therefore important to order initially a multipass fine needle aspiration to avoid the need for a repeat biopsy, which is uncomfortable for the patient and can delay diagnosis and treatment.
Dr. Brondfield advised waiting for molecular testing results to come in before trying to prognosticate in patients with a metastatic cancer for which targetable mutations might be present. Survival rates can vary substantially depending upon those test results. Take, for example, metastatic NSCLC: Just within the past year, clinical trials have been published reporting overall survival rates of 39 months in patients with treatable mutations in epidermal growth factor receptor, 42 months with anaplastic lymphoma kinase mutations, and 51 months in patients whose tumor signature features mutations in c-ros oncogene 1, as compared with 22 months with no targetable mutations in the KEYNOTE-189 trial.
“There’s a lot of heterogeneity around how metastatic tumors behave and respond to therapy. Not all metastatic cancers are the same,” the oncologist emphasized.
FROM HM20 VIRTUAL
Novel botulinum toxin type A earns high marks for forehead lines
, Jeremy B. Green, MD, said at the virtual annual meeting of the American Academy of Dermatology.
When the study is completed, conclusions can be reached about the investigational product’s durability of benefit for treatment of dynamic forehead lines, which are notoriously challenging to treat. However, much is already known about the product’s durability for treatment of glabellar lines, as demonstrated in SAKURA 1 and SAKURA 2, two pivotal, phase 3, multicenter, randomized, double-blind, placebo-controlled trials totaling 609 patients.
In SAKURA 1 and 2, glabellar line severity didn’t return to baseline until a median of 28 and 26 weeks after injection. In contrast, as the study authors noted, the majority of patients whose glabellar lines are treated with the currently available botulinum toxin type A products are no longer responders by 3-4 months after treatment. Since surveys indicate most patients receive repeated injections every 5-6 months, that means they’re walking around with suboptimal results for the last 2-3 months before their next treatment session (Plast Reconstr Surg. 2020 Jan;145[1]:45-58).
This investigational neuromodulator, known as DaxibotulinumtoxinA for Injection, or DAXI, is composed of a highly purified 150-KDa botulinum toxin type A coupled with a proprietary stabilizing peptide. The product is formulated without human serum albumin and, once reconstituted, is stable at room temperature.
Dr. Green, a dermatologist in private practice in Coral Gables, Fla., reported on 61 participants in the phase 2 study, all with moderate or severe forehead lines and glabellar lines as assessed by both investigators and patients on structured scales. The patients’ glabellar lines were treated with 40 U of DAXI at baseline. Then 2 weeks later, their dynamic forehead lines were treated with either 12 U, 18 U, 24 U, or 30 U of DAXI. This sequential treatment recapitulates the approach widely used in clinical practice, he noted.
At baseline, two-thirds of patients had severe forehead lines at maximum eyebrow elevation as determined by Investigator Global Assessment – Forehead Wrinkle Severity and Patient Forehead Wrinkle Severity. The other third of participants had moderate forehead lines.
The primary endpoint was the presence of no or mild forehead lines by investigator assessment 4 weeks after treatment. This was achieved in 86% of patients who received 12 U of DAXI, 87% who recieved 18 U, 94% who received 24 U, and 100% of those who received 30 U.
“There appears to be a dose-dependent response, but this hasn’t yet been statistically analyzed,” Dr. Green said.
By patient assessment, there were no or only mild forehead lines at 4 weeks in 57% of those who received the lowest dose of DAXI, with rates of 80%, 100%, and 93% in those who received 18 U, 24 U, and 30 U.
At week 4, 57% of patients who got 12 U of DAXI pronounced themselves “satisfied” or “very satisfied” with DAXI therapy, as did 73%, 100%, and 93% of those who got the higher doses.
The treatment-related adverse events consisted of a smattering of cases of edema, erythema, or headache, similar to what’s described in the product labeling of all the neuromodulators.
Revance Therapeutics has applied to the Food and Drug Administration for marketing approval of DAXI for the treatment of glabellar lines. A regulatory decision is expected in late November. The company is also developing DAXI for the treatment of variety of neurologic and musculoskeletal conditions, including poststroke upper limb spasticity.
In an interview, Dr. Green said he was favorably impressed with DAXI’s durability for amelioration of forehead lines in the patients he personally treated in the ongoing phase 2 study, although there was no head-to-head comparison with other neuromodulators in the trial. He’s not aware of any planned phase 3 trial aimed at obtaining a forehead line indication.
“Of course, all four of the neuromodulators currently approved in the U.S. have glabellar line indications, but all are also used off-label in other locations, so I would imagine that DAXI will be used similarly if and when it is FDA-approved,” the dermatologist added.
He reported serving as a paid investigator for Revance.
, Jeremy B. Green, MD, said at the virtual annual meeting of the American Academy of Dermatology.
When the study is completed, conclusions can be reached about the investigational product’s durability of benefit for treatment of dynamic forehead lines, which are notoriously challenging to treat. However, much is already known about the product’s durability for treatment of glabellar lines, as demonstrated in SAKURA 1 and SAKURA 2, two pivotal, phase 3, multicenter, randomized, double-blind, placebo-controlled trials totaling 609 patients.
In SAKURA 1 and 2, glabellar line severity didn’t return to baseline until a median of 28 and 26 weeks after injection. In contrast, as the study authors noted, the majority of patients whose glabellar lines are treated with the currently available botulinum toxin type A products are no longer responders by 3-4 months after treatment. Since surveys indicate most patients receive repeated injections every 5-6 months, that means they’re walking around with suboptimal results for the last 2-3 months before their next treatment session (Plast Reconstr Surg. 2020 Jan;145[1]:45-58).
This investigational neuromodulator, known as DaxibotulinumtoxinA for Injection, or DAXI, is composed of a highly purified 150-KDa botulinum toxin type A coupled with a proprietary stabilizing peptide. The product is formulated without human serum albumin and, once reconstituted, is stable at room temperature.
Dr. Green, a dermatologist in private practice in Coral Gables, Fla., reported on 61 participants in the phase 2 study, all with moderate or severe forehead lines and glabellar lines as assessed by both investigators and patients on structured scales. The patients’ glabellar lines were treated with 40 U of DAXI at baseline. Then 2 weeks later, their dynamic forehead lines were treated with either 12 U, 18 U, 24 U, or 30 U of DAXI. This sequential treatment recapitulates the approach widely used in clinical practice, he noted.
At baseline, two-thirds of patients had severe forehead lines at maximum eyebrow elevation as determined by Investigator Global Assessment – Forehead Wrinkle Severity and Patient Forehead Wrinkle Severity. The other third of participants had moderate forehead lines.
The primary endpoint was the presence of no or mild forehead lines by investigator assessment 4 weeks after treatment. This was achieved in 86% of patients who received 12 U of DAXI, 87% who recieved 18 U, 94% who received 24 U, and 100% of those who received 30 U.
“There appears to be a dose-dependent response, but this hasn’t yet been statistically analyzed,” Dr. Green said.
By patient assessment, there were no or only mild forehead lines at 4 weeks in 57% of those who received the lowest dose of DAXI, with rates of 80%, 100%, and 93% in those who received 18 U, 24 U, and 30 U.
At week 4, 57% of patients who got 12 U of DAXI pronounced themselves “satisfied” or “very satisfied” with DAXI therapy, as did 73%, 100%, and 93% of those who got the higher doses.
The treatment-related adverse events consisted of a smattering of cases of edema, erythema, or headache, similar to what’s described in the product labeling of all the neuromodulators.
Revance Therapeutics has applied to the Food and Drug Administration for marketing approval of DAXI for the treatment of glabellar lines. A regulatory decision is expected in late November. The company is also developing DAXI for the treatment of variety of neurologic and musculoskeletal conditions, including poststroke upper limb spasticity.
In an interview, Dr. Green said he was favorably impressed with DAXI’s durability for amelioration of forehead lines in the patients he personally treated in the ongoing phase 2 study, although there was no head-to-head comparison with other neuromodulators in the trial. He’s not aware of any planned phase 3 trial aimed at obtaining a forehead line indication.
“Of course, all four of the neuromodulators currently approved in the U.S. have glabellar line indications, but all are also used off-label in other locations, so I would imagine that DAXI will be used similarly if and when it is FDA-approved,” the dermatologist added.
He reported serving as a paid investigator for Revance.
, Jeremy B. Green, MD, said at the virtual annual meeting of the American Academy of Dermatology.
When the study is completed, conclusions can be reached about the investigational product’s durability of benefit for treatment of dynamic forehead lines, which are notoriously challenging to treat. However, much is already known about the product’s durability for treatment of glabellar lines, as demonstrated in SAKURA 1 and SAKURA 2, two pivotal, phase 3, multicenter, randomized, double-blind, placebo-controlled trials totaling 609 patients.
In SAKURA 1 and 2, glabellar line severity didn’t return to baseline until a median of 28 and 26 weeks after injection. In contrast, as the study authors noted, the majority of patients whose glabellar lines are treated with the currently available botulinum toxin type A products are no longer responders by 3-4 months after treatment. Since surveys indicate most patients receive repeated injections every 5-6 months, that means they’re walking around with suboptimal results for the last 2-3 months before their next treatment session (Plast Reconstr Surg. 2020 Jan;145[1]:45-58).
This investigational neuromodulator, known as DaxibotulinumtoxinA for Injection, or DAXI, is composed of a highly purified 150-KDa botulinum toxin type A coupled with a proprietary stabilizing peptide. The product is formulated without human serum albumin and, once reconstituted, is stable at room temperature.
Dr. Green, a dermatologist in private practice in Coral Gables, Fla., reported on 61 participants in the phase 2 study, all with moderate or severe forehead lines and glabellar lines as assessed by both investigators and patients on structured scales. The patients’ glabellar lines were treated with 40 U of DAXI at baseline. Then 2 weeks later, their dynamic forehead lines were treated with either 12 U, 18 U, 24 U, or 30 U of DAXI. This sequential treatment recapitulates the approach widely used in clinical practice, he noted.
At baseline, two-thirds of patients had severe forehead lines at maximum eyebrow elevation as determined by Investigator Global Assessment – Forehead Wrinkle Severity and Patient Forehead Wrinkle Severity. The other third of participants had moderate forehead lines.
The primary endpoint was the presence of no or mild forehead lines by investigator assessment 4 weeks after treatment. This was achieved in 86% of patients who received 12 U of DAXI, 87% who recieved 18 U, 94% who received 24 U, and 100% of those who received 30 U.
“There appears to be a dose-dependent response, but this hasn’t yet been statistically analyzed,” Dr. Green said.
By patient assessment, there were no or only mild forehead lines at 4 weeks in 57% of those who received the lowest dose of DAXI, with rates of 80%, 100%, and 93% in those who received 18 U, 24 U, and 30 U.
At week 4, 57% of patients who got 12 U of DAXI pronounced themselves “satisfied” or “very satisfied” with DAXI therapy, as did 73%, 100%, and 93% of those who got the higher doses.
The treatment-related adverse events consisted of a smattering of cases of edema, erythema, or headache, similar to what’s described in the product labeling of all the neuromodulators.
Revance Therapeutics has applied to the Food and Drug Administration for marketing approval of DAXI for the treatment of glabellar lines. A regulatory decision is expected in late November. The company is also developing DAXI for the treatment of variety of neurologic and musculoskeletal conditions, including poststroke upper limb spasticity.
In an interview, Dr. Green said he was favorably impressed with DAXI’s durability for amelioration of forehead lines in the patients he personally treated in the ongoing phase 2 study, although there was no head-to-head comparison with other neuromodulators in the trial. He’s not aware of any planned phase 3 trial aimed at obtaining a forehead line indication.
“Of course, all four of the neuromodulators currently approved in the U.S. have glabellar line indications, but all are also used off-label in other locations, so I would imagine that DAXI will be used similarly if and when it is FDA-approved,” the dermatologist added.
He reported serving as a paid investigator for Revance.
FROM AAD 20
Risk stratification key in acute pulmonary embolism
All intermediate-risk pulmonary embolism is not the same, Victor F. Tapson, MD, declared at HM20 Virtual, hosted by the Society of Hospital Medicine.
This additional classification is worthwhile because it has important treatment implications.
Patients with intermediate- to low-risk PE, along with those who have truly low-risk PE, require anticoagulation only. In contrast, patients with intermediate- to high-risk PE are at increased risk of decompensation. They have a much higher in-hospital mortality than those with intermediate- to low-risk PE. So hospitalists may want to consult their hospitals’ PE response team (PERT), if there is one, or whoever on staff is involved in helping make decisions about the appropriateness of more aggressive interventions, such as catheter-directed thrombolysis or catheter-directed clot extraction, said Dr. Tapson, director of the venous thromboembolism and pulmonary vascular disease research program at Cedars-Sinai Medical Center in Los Angeles.
“We don’t have evidence of any real proven mortality difference yet in the intermediate-high risk PE group by being more aggressive. I think if the right patients were studied we could see a mortality difference. But one thing I’ve noted is that by being more aggressive – in a cautious manner, in selected patients – we clearly shorten the hospital stay by doing catheter-directed therapy in some of these folks. It saves money,” he observed.
Once the diagnosis of PE is confirmed, the first priority is to get anticoagulation started in all patients with an acceptable bleeding risk, since there is convincing evidence that anticoagulation reduces mortality in PE. The 2019 European Society of Cardiology guidelines recommend a direct-acting oral anticoagulant over warfarin on the basis of persuasive evidence of lower risk of major bleeding coupled with equal or better effectiveness in preventing recurrent PE.
Dr. Tapson said it’s worthwhile for hospitalists to take a close look at these European guidelines (Eur Respir J. 2019 Oct 9. doi: 10.1183/13993003.01647-2019).
“I think our Europeans friends did a really nice job with those guidelines. They’re great guidelines, better than many of the others out there. I think they’re very, very usable,” he said. “I took part in the ACCP [American College of Chest Physicians] guidelines for years. I think they’re very rigorous in terms of the evidence base, but because they’re so rigorous there’s just tons of 2C recommendations, which are basically suggestions. The ESC guidelines are more robust.”
Risk stratification
Once anticoagulation is on board, the next task is risk stratification to determine the need for more aggressive therapy. A high-risk PE is best defined hemodynamically as one causing a systolic blood pressure below 90 mm Hg for at least 15 minutes. The term “high risk” is increasingly replacing “massive” PE, because the size of the clot doesn’t necessarily correlate with its hemodynamic impact.
An intermediate-risk PE is marked by a simplified Pulmonary Embolism Severity Index (sPESI) score of 1 or more, right ventricular dysfunction on echocardiography or CT angiography, or an elevated cardiac troponin level.
The sPESI is a validated, user-friendly tool that grants 1 point each for age over 80, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%.
“All you really need to know about a patient’s sPESI score is: Is it more than zero?” he explained.
Indeed, patients with an sPESI score of 0 have a 30-day mortality of 1%. With a score of 1 or more, however, that risk jumps to 10.9%.
No scoring system is 100% accurate, though, and Dr. Tapson emphasized that clinician gestalt plays an important role in PE risk stratification. In terms of clinical indicators of risk, he pays special attention to heart rate.
“I think if I had to pick the one thing that drives my decision the most about whether someone needs more aggressive therapy than anticoagulation, it’s probably heart rate,” he said. “If the heart rate is 70, the patient is probably very stable. Of course, that might not hold up in a patient with conduction problems or who is on a beta blocker, but in general if I see someone who looks good, has a relatively small PE, and a low heart rate, it makes me feel much better. If the heart rate is 130 or 120, I’m much more concerned.”
Both the European guidelines and the PERT Consortium guidelines on the diagnosis, treatment, and follow-up of acute PE (Clin Appl Thromb Hemost. 2019 Jun 17. doi: 10.1177/1076029619853037), which Dr. Tapson coauthored, recommend substratifying intermediate-risk PE into intermediate to low or intermediate to high risk. It’s a straightforward matter: If a patient has either right ventricular dysfunction on imaging or an elevated cardiac troponin, that’s an intermediate- to low-risk PE warranting anticoagulation only. On the other hand, if both right ventricular dysfunction and an elevated troponin are present, the patient has an intermediate- to high-risk PE. Since this distinction translates to a difference in outcome, a consultation with PERT or an experienced PE interventionalist is in order for the intermediate- to high-risk PE, he said.
Dr. Tapson reported receiving research funding from Bayer, Bristol-Myers Squibb, Janssen, BiO2, EKOS/BTG, and Daiichi. He is also a consultant to Janssen and BiO2, and on speakers’ bureaus for EKOS/BTG and Janssen.
All intermediate-risk pulmonary embolism is not the same, Victor F. Tapson, MD, declared at HM20 Virtual, hosted by the Society of Hospital Medicine.
This additional classification is worthwhile because it has important treatment implications.
Patients with intermediate- to low-risk PE, along with those who have truly low-risk PE, require anticoagulation only. In contrast, patients with intermediate- to high-risk PE are at increased risk of decompensation. They have a much higher in-hospital mortality than those with intermediate- to low-risk PE. So hospitalists may want to consult their hospitals’ PE response team (PERT), if there is one, or whoever on staff is involved in helping make decisions about the appropriateness of more aggressive interventions, such as catheter-directed thrombolysis or catheter-directed clot extraction, said Dr. Tapson, director of the venous thromboembolism and pulmonary vascular disease research program at Cedars-Sinai Medical Center in Los Angeles.
“We don’t have evidence of any real proven mortality difference yet in the intermediate-high risk PE group by being more aggressive. I think if the right patients were studied we could see a mortality difference. But one thing I’ve noted is that by being more aggressive – in a cautious manner, in selected patients – we clearly shorten the hospital stay by doing catheter-directed therapy in some of these folks. It saves money,” he observed.
Once the diagnosis of PE is confirmed, the first priority is to get anticoagulation started in all patients with an acceptable bleeding risk, since there is convincing evidence that anticoagulation reduces mortality in PE. The 2019 European Society of Cardiology guidelines recommend a direct-acting oral anticoagulant over warfarin on the basis of persuasive evidence of lower risk of major bleeding coupled with equal or better effectiveness in preventing recurrent PE.
Dr. Tapson said it’s worthwhile for hospitalists to take a close look at these European guidelines (Eur Respir J. 2019 Oct 9. doi: 10.1183/13993003.01647-2019).
“I think our Europeans friends did a really nice job with those guidelines. They’re great guidelines, better than many of the others out there. I think they’re very, very usable,” he said. “I took part in the ACCP [American College of Chest Physicians] guidelines for years. I think they’re very rigorous in terms of the evidence base, but because they’re so rigorous there’s just tons of 2C recommendations, which are basically suggestions. The ESC guidelines are more robust.”
Risk stratification
Once anticoagulation is on board, the next task is risk stratification to determine the need for more aggressive therapy. A high-risk PE is best defined hemodynamically as one causing a systolic blood pressure below 90 mm Hg for at least 15 minutes. The term “high risk” is increasingly replacing “massive” PE, because the size of the clot doesn’t necessarily correlate with its hemodynamic impact.
An intermediate-risk PE is marked by a simplified Pulmonary Embolism Severity Index (sPESI) score of 1 or more, right ventricular dysfunction on echocardiography or CT angiography, or an elevated cardiac troponin level.
The sPESI is a validated, user-friendly tool that grants 1 point each for age over 80, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%.
“All you really need to know about a patient’s sPESI score is: Is it more than zero?” he explained.
Indeed, patients with an sPESI score of 0 have a 30-day mortality of 1%. With a score of 1 or more, however, that risk jumps to 10.9%.
No scoring system is 100% accurate, though, and Dr. Tapson emphasized that clinician gestalt plays an important role in PE risk stratification. In terms of clinical indicators of risk, he pays special attention to heart rate.
“I think if I had to pick the one thing that drives my decision the most about whether someone needs more aggressive therapy than anticoagulation, it’s probably heart rate,” he said. “If the heart rate is 70, the patient is probably very stable. Of course, that might not hold up in a patient with conduction problems or who is on a beta blocker, but in general if I see someone who looks good, has a relatively small PE, and a low heart rate, it makes me feel much better. If the heart rate is 130 or 120, I’m much more concerned.”
Both the European guidelines and the PERT Consortium guidelines on the diagnosis, treatment, and follow-up of acute PE (Clin Appl Thromb Hemost. 2019 Jun 17. doi: 10.1177/1076029619853037), which Dr. Tapson coauthored, recommend substratifying intermediate-risk PE into intermediate to low or intermediate to high risk. It’s a straightforward matter: If a patient has either right ventricular dysfunction on imaging or an elevated cardiac troponin, that’s an intermediate- to low-risk PE warranting anticoagulation only. On the other hand, if both right ventricular dysfunction and an elevated troponin are present, the patient has an intermediate- to high-risk PE. Since this distinction translates to a difference in outcome, a consultation with PERT or an experienced PE interventionalist is in order for the intermediate- to high-risk PE, he said.
Dr. Tapson reported receiving research funding from Bayer, Bristol-Myers Squibb, Janssen, BiO2, EKOS/BTG, and Daiichi. He is also a consultant to Janssen and BiO2, and on speakers’ bureaus for EKOS/BTG and Janssen.
All intermediate-risk pulmonary embolism is not the same, Victor F. Tapson, MD, declared at HM20 Virtual, hosted by the Society of Hospital Medicine.
This additional classification is worthwhile because it has important treatment implications.
Patients with intermediate- to low-risk PE, along with those who have truly low-risk PE, require anticoagulation only. In contrast, patients with intermediate- to high-risk PE are at increased risk of decompensation. They have a much higher in-hospital mortality than those with intermediate- to low-risk PE. So hospitalists may want to consult their hospitals’ PE response team (PERT), if there is one, or whoever on staff is involved in helping make decisions about the appropriateness of more aggressive interventions, such as catheter-directed thrombolysis or catheter-directed clot extraction, said Dr. Tapson, director of the venous thromboembolism and pulmonary vascular disease research program at Cedars-Sinai Medical Center in Los Angeles.
“We don’t have evidence of any real proven mortality difference yet in the intermediate-high risk PE group by being more aggressive. I think if the right patients were studied we could see a mortality difference. But one thing I’ve noted is that by being more aggressive – in a cautious manner, in selected patients – we clearly shorten the hospital stay by doing catheter-directed therapy in some of these folks. It saves money,” he observed.
Once the diagnosis of PE is confirmed, the first priority is to get anticoagulation started in all patients with an acceptable bleeding risk, since there is convincing evidence that anticoagulation reduces mortality in PE. The 2019 European Society of Cardiology guidelines recommend a direct-acting oral anticoagulant over warfarin on the basis of persuasive evidence of lower risk of major bleeding coupled with equal or better effectiveness in preventing recurrent PE.
Dr. Tapson said it’s worthwhile for hospitalists to take a close look at these European guidelines (Eur Respir J. 2019 Oct 9. doi: 10.1183/13993003.01647-2019).
“I think our Europeans friends did a really nice job with those guidelines. They’re great guidelines, better than many of the others out there. I think they’re very, very usable,” he said. “I took part in the ACCP [American College of Chest Physicians] guidelines for years. I think they’re very rigorous in terms of the evidence base, but because they’re so rigorous there’s just tons of 2C recommendations, which are basically suggestions. The ESC guidelines are more robust.”
Risk stratification
Once anticoagulation is on board, the next task is risk stratification to determine the need for more aggressive therapy. A high-risk PE is best defined hemodynamically as one causing a systolic blood pressure below 90 mm Hg for at least 15 minutes. The term “high risk” is increasingly replacing “massive” PE, because the size of the clot doesn’t necessarily correlate with its hemodynamic impact.
An intermediate-risk PE is marked by a simplified Pulmonary Embolism Severity Index (sPESI) score of 1 or more, right ventricular dysfunction on echocardiography or CT angiography, or an elevated cardiac troponin level.
The sPESI is a validated, user-friendly tool that grants 1 point each for age over 80, background cardiopulmonary disease, a systolic blood pressure below 100 mm Hg, cancer, a heart rate of 110 bpm or more, and an oxygen saturation level below 90%.
“All you really need to know about a patient’s sPESI score is: Is it more than zero?” he explained.
Indeed, patients with an sPESI score of 0 have a 30-day mortality of 1%. With a score of 1 or more, however, that risk jumps to 10.9%.
No scoring system is 100% accurate, though, and Dr. Tapson emphasized that clinician gestalt plays an important role in PE risk stratification. In terms of clinical indicators of risk, he pays special attention to heart rate.
“I think if I had to pick the one thing that drives my decision the most about whether someone needs more aggressive therapy than anticoagulation, it’s probably heart rate,” he said. “If the heart rate is 70, the patient is probably very stable. Of course, that might not hold up in a patient with conduction problems or who is on a beta blocker, but in general if I see someone who looks good, has a relatively small PE, and a low heart rate, it makes me feel much better. If the heart rate is 130 or 120, I’m much more concerned.”
Both the European guidelines and the PERT Consortium guidelines on the diagnosis, treatment, and follow-up of acute PE (Clin Appl Thromb Hemost. 2019 Jun 17. doi: 10.1177/1076029619853037), which Dr. Tapson coauthored, recommend substratifying intermediate-risk PE into intermediate to low or intermediate to high risk. It’s a straightforward matter: If a patient has either right ventricular dysfunction on imaging or an elevated cardiac troponin, that’s an intermediate- to low-risk PE warranting anticoagulation only. On the other hand, if both right ventricular dysfunction and an elevated troponin are present, the patient has an intermediate- to high-risk PE. Since this distinction translates to a difference in outcome, a consultation with PERT or an experienced PE interventionalist is in order for the intermediate- to high-risk PE, he said.
Dr. Tapson reported receiving research funding from Bayer, Bristol-Myers Squibb, Janssen, BiO2, EKOS/BTG, and Daiichi. He is also a consultant to Janssen and BiO2, and on speakers’ bureaus for EKOS/BTG and Janssen.
FROM HM20 VIRTUAL
A ‘foolproof’ way to diagnose narrow complex tachycardias on EKGs
A hospitalist looking at an EKG showing a narrow complex tachycardia needs to be able to come up with an accurate diagnosis of the rhythm pronto. And hospitalist Meghan Mary Walsh, MD, MPH, has developed a simple and efficient method for doing so within a minute or two that she’s used with great success on the wards and in teaching medical students and residents for nearly a decade.
she promised at HM20 Virtual, hosted by the Society of Hospital Medicine.
Her method involves asking three questions about the 12-lead EKG:
1) What’s the rate?
A narrow complex tachycardia by definition needs to be both narrow and fast, with a QRS complex of less than 0.12 seconds and a heart rate above 100 bpm. Knowing how far above 100 bpm the rate is will help with the differential diagnosis.
2) Is the rhythm regular or irregular?
“If I put the EKG 10 feet away from you, you should still be able to look at it and say the QRS is either systematically marching out – boom, boom, boom – or there is an irregular sea of QRS complexes where the RR intervals are variable and inconsistent,” said Dr. Walsh, a hospitalist at the University of Minnesota, Minneapolis, and chief academic officer at Hennepin Healthcare, where she oversees all medical students and residents training in the health system.
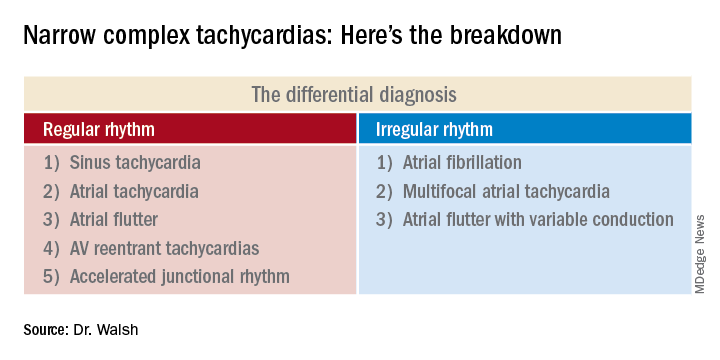
This distinction between a regular and irregular rhythm immediately narrows the differential by dividing the diagnostic possibilities into two columns (See chart). She urged her audience to commit the list to memory or keep it handy on their cell phone or in a notebook.
“If it’s irregular I’m going down the right column; if it’s regular I’m going down the left. And then I’m systematically running the drill,” she explained.
3) Are upright p waves present before each QRS complex in leads II and V1?
This information rules out some of the eight items in the differential diagnosis and rules in others.
Narrow complex tachycardias with an irregular rhythm
There are only three:
Atrial fibrillation: The heart rate is typically 110-160 bpm, although it can occasionally go higher. The rhythm is irregularly irregular: No two RR intervals on the EKG are exactly the same. And there are no p waves.
“If it’s faster than 100 bpm, irregularly irregular, and no p waves, the conclusion is very simple: It’s AFib,” Dr. Walsh said.
Multifocal atrial tachycardia (MAT): The heart rate is generally 100-150 bpm but can sometimes climb to about 180 bpm. The PP, PR, and RR intervals are varied, inconsistent, and don’t repeat. Most importantly, there are three or more different p wave morphologies in the same lead. One p wave might look like a tall mountain peak, another could be short and flat, and perhaps the next is big and broad.
MAT often occurs in patients with a structurally abnormal atrium – for example, in the setting of pulmonary hypertension leading to right atrial enlargement, with resultant depolarization occurring all over the atrium.
“Don’t confuse MAT with AFib: One has p waves, one does not. Otherwise they can look very similar,” she said.
Atrial flutter with variable conduction: A hallmark of this reentrant tachycardia is the atrial flutter waves occurring at about 300 bpm between each QRS complex.
“On board renewal exams, the question is often asked, ‘Which leads are the best identifiers of atrial flutter?’ And the answer is the inferior leads II, III, and aVF,” she said.
Another classic feature of atrial flutter with variable conduction is cluster beating attributable to a varied ventricular response. This results in a repeated pattern of irregular RR intervals: There might be a 2:1 block in AV conduction for several beats, then maybe a 4:1 block for several more, with resultant lengthening of the RR interval, then 3:1, with shortening of RR. This regularly irregular sequence is repeated throughout the EKG.
“Look for a pattern amidst the chaos,” the hospitalist advised.
The heart rate might be roughly 150 bpm with a 2:1 block, or 100 bpm with a 3:1 block. The p waves in atrial flutter with variable conduction can be either negatively or positively deflected.
Narrow complex tachycardias with a regular rhythm*
Sinus tachycardia: The heart rate is typically less than 160 bpm, the QRS complexes show a regular pattern, and upright p waves are clearly visible in leads II and V1.
The distinguishing feature of this arrhythmia is the ramping up and ramping down of the heart rate. The tachycardia is typically less than 160 bpm. But the rate doesn’t suddenly jump from, say, 70 to140 bpm in a flash while the patient is lying in the hospital bed. A trip to the telemetry room for a look at the telemetry strip will tell the tale: The heart rate will have progressively ramped up from 70, to 80, then 90, then 100, 110, 120, 130, to perhaps 140 bpm. And then it will similarly ramp back down in stages, with the up/down pattern being repeated.
Sinus tachycardia is generally a reflection of underlying significant systemic illness, such as sepsis, hypotension, or anemia.
Atrial tachycardia: The heart rate is generally 100-140 bpm, and p waves are present. But unlike in sinus tachycardia, the patient with atrial tachycardia lying in bed with a heart rate of 140 bpm is not in a state of profound neurohormonal activation and is not all that sick.
Another diagnostic clue is provided by a look at the telemonitoring strip. Unlike in sinus tachycardia, where the heart rate ramps up and then back down repeatedly, in atrial tachycardia the heart rate very quickly ramps up in stages to, say, 140 bpm, and then hangs there.
Atrial flutter: This is the only narrow complex tachycardia that appears in both the regular and irregular rhythm columns. It belongs in the irregular rhythm column when there is variable conduction and cluster beating, with a regularly irregular pattern of RR intervals. In contrast, when atrial flutter is in the regular rhythm column, it’s because the atrioventricular node is steadily conducting the atrial depolarizations at a rate of about 300 bpm. So there’s no cluster beating. As in atrial flutter with variable conduction, the flutter waves are visible most often in leads II, III, and aVF, where they can be either positively or negatively deflected.
AV reentrant tachycardias: These reentrant tachycardias can take two forms. In atrioventricular nodal reentrant tachycardia (AVnRT), the aberrant pathway is found entirely within the AV node, whereas in atrioventricular reentrant tachycardia (AVRT) the aberrant pathway is found outside the AV node. AVnRT is more common than AVRT. As in atrial flutter, there is no ramp up in heart rate. Patients will be lying in their hospital bed with a heart rate of, say, 80 bpm, and then suddenly it jumps to 180, 200, or even as high as 240 bpm “almost in a split second,” Dr. Walsh said.
No other narrow complex tachycardia reaches so high a heart rate. In both of these reentrant tachycardias the p waves are often buried in the QRS complex and can be tough to see. It’s very difficult to differentiate AVnRT from AVRT except by an electrophysiologic study.
Accelerated junctional tachycardia: This is most commonly the slowest of the narrow complex tachycardias, with a heart rate of less than 120 bpm.
“In the case of accelerated junctional tachycardia, think slow, think ‘regular,’ think of a rate often just over 100, usually with p waves after the QRS that are inverted because there’s retrograde conduction,” she advised.
She reported having no financial conflicts of interest regarding her presentation.
Correction, 8/19/20: An earlier version of this article mischaracterized the type of rhythm noted in this subhead.
A hospitalist looking at an EKG showing a narrow complex tachycardia needs to be able to come up with an accurate diagnosis of the rhythm pronto. And hospitalist Meghan Mary Walsh, MD, MPH, has developed a simple and efficient method for doing so within a minute or two that she’s used with great success on the wards and in teaching medical students and residents for nearly a decade.

she promised at HM20 Virtual, hosted by the Society of Hospital Medicine.
Her method involves asking three questions about the 12-lead EKG:
1) What’s the rate?
A narrow complex tachycardia by definition needs to be both narrow and fast, with a QRS complex of less than 0.12 seconds and a heart rate above 100 bpm. Knowing how far above 100 bpm the rate is will help with the differential diagnosis.
2) Is the rhythm regular or irregular?
“If I put the EKG 10 feet away from you, you should still be able to look at it and say the QRS is either systematically marching out – boom, boom, boom – or there is an irregular sea of QRS complexes where the RR intervals are variable and inconsistent,” said Dr. Walsh, a hospitalist at the University of Minnesota, Minneapolis, and chief academic officer at Hennepin Healthcare, where she oversees all medical students and residents training in the health system.
This distinction between a regular and irregular rhythm immediately narrows the differential by dividing the diagnostic possibilities into two columns (See chart). She urged her audience to commit the list to memory or keep it handy on their cell phone or in a notebook.
“If it’s irregular I’m going down the right column; if it’s regular I’m going down the left. And then I’m systematically running the drill,” she explained.
3) Are upright p waves present before each QRS complex in leads II and V1?
This information rules out some of the eight items in the differential diagnosis and rules in others.
Narrow complex tachycardias with an irregular rhythm
There are only three:
Atrial fibrillation: The heart rate is typically 110-160 bpm, although it can occasionally go higher. The rhythm is irregularly irregular: No two RR intervals on the EKG are exactly the same. And there are no p waves.
“If it’s faster than 100 bpm, irregularly irregular, and no p waves, the conclusion is very simple: It’s AFib,” Dr. Walsh said.
Multifocal atrial tachycardia (MAT): The heart rate is generally 100-150 bpm but can sometimes climb to about 180 bpm. The PP, PR, and RR intervals are varied, inconsistent, and don’t repeat. Most importantly, there are three or more different p wave morphologies in the same lead. One p wave might look like a tall mountain peak, another could be short and flat, and perhaps the next is big and broad.
MAT often occurs in patients with a structurally abnormal atrium – for example, in the setting of pulmonary hypertension leading to right atrial enlargement, with resultant depolarization occurring all over the atrium.
“Don’t confuse MAT with AFib: One has p waves, one does not. Otherwise they can look very similar,” she said.
Atrial flutter with variable conduction: A hallmark of this reentrant tachycardia is the atrial flutter waves occurring at about 300 bpm between each QRS complex.
“On board renewal exams, the question is often asked, ‘Which leads are the best identifiers of atrial flutter?’ And the answer is the inferior leads II, III, and aVF,” she said.
Another classic feature of atrial flutter with variable conduction is cluster beating attributable to a varied ventricular response. This results in a repeated pattern of irregular RR intervals: There might be a 2:1 block in AV conduction for several beats, then maybe a 4:1 block for several more, with resultant lengthening of the RR interval, then 3:1, with shortening of RR. This regularly irregular sequence is repeated throughout the EKG.
“Look for a pattern amidst the chaos,” the hospitalist advised.
The heart rate might be roughly 150 bpm with a 2:1 block, or 100 bpm with a 3:1 block. The p waves in atrial flutter with variable conduction can be either negatively or positively deflected.
Narrow complex tachycardias with a regular rhythm*
Sinus tachycardia: The heart rate is typically less than 160 bpm, the QRS complexes show a regular pattern, and upright p waves are clearly visible in leads II and V1.
The distinguishing feature of this arrhythmia is the ramping up and ramping down of the heart rate. The tachycardia is typically less than 160 bpm. But the rate doesn’t suddenly jump from, say, 70 to140 bpm in a flash while the patient is lying in the hospital bed. A trip to the telemetry room for a look at the telemetry strip will tell the tale: The heart rate will have progressively ramped up from 70, to 80, then 90, then 100, 110, 120, 130, to perhaps 140 bpm. And then it will similarly ramp back down in stages, with the up/down pattern being repeated.
Sinus tachycardia is generally a reflection of underlying significant systemic illness, such as sepsis, hypotension, or anemia.
Atrial tachycardia: The heart rate is generally 100-140 bpm, and p waves are present. But unlike in sinus tachycardia, the patient with atrial tachycardia lying in bed with a heart rate of 140 bpm is not in a state of profound neurohormonal activation and is not all that sick.
Another diagnostic clue is provided by a look at the telemonitoring strip. Unlike in sinus tachycardia, where the heart rate ramps up and then back down repeatedly, in atrial tachycardia the heart rate very quickly ramps up in stages to, say, 140 bpm, and then hangs there.
Atrial flutter: This is the only narrow complex tachycardia that appears in both the regular and irregular rhythm columns. It belongs in the irregular rhythm column when there is variable conduction and cluster beating, with a regularly irregular pattern of RR intervals. In contrast, when atrial flutter is in the regular rhythm column, it’s because the atrioventricular node is steadily conducting the atrial depolarizations at a rate of about 300 bpm. So there’s no cluster beating. As in atrial flutter with variable conduction, the flutter waves are visible most often in leads II, III, and aVF, where they can be either positively or negatively deflected.
AV reentrant tachycardias: These reentrant tachycardias can take two forms. In atrioventricular nodal reentrant tachycardia (AVnRT), the aberrant pathway is found entirely within the AV node, whereas in atrioventricular reentrant tachycardia (AVRT) the aberrant pathway is found outside the AV node. AVnRT is more common than AVRT. As in atrial flutter, there is no ramp up in heart rate. Patients will be lying in their hospital bed with a heart rate of, say, 80 bpm, and then suddenly it jumps to 180, 200, or even as high as 240 bpm “almost in a split second,” Dr. Walsh said.
No other narrow complex tachycardia reaches so high a heart rate. In both of these reentrant tachycardias the p waves are often buried in the QRS complex and can be tough to see. It’s very difficult to differentiate AVnRT from AVRT except by an electrophysiologic study.
Accelerated junctional tachycardia: This is most commonly the slowest of the narrow complex tachycardias, with a heart rate of less than 120 bpm.
“In the case of accelerated junctional tachycardia, think slow, think ‘regular,’ think of a rate often just over 100, usually with p waves after the QRS that are inverted because there’s retrograde conduction,” she advised.
She reported having no financial conflicts of interest regarding her presentation.
Correction, 8/19/20: An earlier version of this article mischaracterized the type of rhythm noted in this subhead.
A hospitalist looking at an EKG showing a narrow complex tachycardia needs to be able to come up with an accurate diagnosis of the rhythm pronto. And hospitalist Meghan Mary Walsh, MD, MPH, has developed a simple and efficient method for doing so within a minute or two that she’s used with great success on the wards and in teaching medical students and residents for nearly a decade.

she promised at HM20 Virtual, hosted by the Society of Hospital Medicine.
Her method involves asking three questions about the 12-lead EKG:
1) What’s the rate?
A narrow complex tachycardia by definition needs to be both narrow and fast, with a QRS complex of less than 0.12 seconds and a heart rate above 100 bpm. Knowing how far above 100 bpm the rate is will help with the differential diagnosis.
2) Is the rhythm regular or irregular?
“If I put the EKG 10 feet away from you, you should still be able to look at it and say the QRS is either systematically marching out – boom, boom, boom – or there is an irregular sea of QRS complexes where the RR intervals are variable and inconsistent,” said Dr. Walsh, a hospitalist at the University of Minnesota, Minneapolis, and chief academic officer at Hennepin Healthcare, where she oversees all medical students and residents training in the health system.
This distinction between a regular and irregular rhythm immediately narrows the differential by dividing the diagnostic possibilities into two columns (See chart). She urged her audience to commit the list to memory or keep it handy on their cell phone or in a notebook.
“If it’s irregular I’m going down the right column; if it’s regular I’m going down the left. And then I’m systematically running the drill,” she explained.
3) Are upright p waves present before each QRS complex in leads II and V1?
This information rules out some of the eight items in the differential diagnosis and rules in others.
Narrow complex tachycardias with an irregular rhythm
There are only three:
Atrial fibrillation: The heart rate is typically 110-160 bpm, although it can occasionally go higher. The rhythm is irregularly irregular: No two RR intervals on the EKG are exactly the same. And there are no p waves.
“If it’s faster than 100 bpm, irregularly irregular, and no p waves, the conclusion is very simple: It’s AFib,” Dr. Walsh said.
Multifocal atrial tachycardia (MAT): The heart rate is generally 100-150 bpm but can sometimes climb to about 180 bpm. The PP, PR, and RR intervals are varied, inconsistent, and don’t repeat. Most importantly, there are three or more different p wave morphologies in the same lead. One p wave might look like a tall mountain peak, another could be short and flat, and perhaps the next is big and broad.
MAT often occurs in patients with a structurally abnormal atrium – for example, in the setting of pulmonary hypertension leading to right atrial enlargement, with resultant depolarization occurring all over the atrium.
“Don’t confuse MAT with AFib: One has p waves, one does not. Otherwise they can look very similar,” she said.
Atrial flutter with variable conduction: A hallmark of this reentrant tachycardia is the atrial flutter waves occurring at about 300 bpm between each QRS complex.
“On board renewal exams, the question is often asked, ‘Which leads are the best identifiers of atrial flutter?’ And the answer is the inferior leads II, III, and aVF,” she said.
Another classic feature of atrial flutter with variable conduction is cluster beating attributable to a varied ventricular response. This results in a repeated pattern of irregular RR intervals: There might be a 2:1 block in AV conduction for several beats, then maybe a 4:1 block for several more, with resultant lengthening of the RR interval, then 3:1, with shortening of RR. This regularly irregular sequence is repeated throughout the EKG.
“Look for a pattern amidst the chaos,” the hospitalist advised.
The heart rate might be roughly 150 bpm with a 2:1 block, or 100 bpm with a 3:1 block. The p waves in atrial flutter with variable conduction can be either negatively or positively deflected.
Narrow complex tachycardias with a regular rhythm*
Sinus tachycardia: The heart rate is typically less than 160 bpm, the QRS complexes show a regular pattern, and upright p waves are clearly visible in leads II and V1.
The distinguishing feature of this arrhythmia is the ramping up and ramping down of the heart rate. The tachycardia is typically less than 160 bpm. But the rate doesn’t suddenly jump from, say, 70 to140 bpm in a flash while the patient is lying in the hospital bed. A trip to the telemetry room for a look at the telemetry strip will tell the tale: The heart rate will have progressively ramped up from 70, to 80, then 90, then 100, 110, 120, 130, to perhaps 140 bpm. And then it will similarly ramp back down in stages, with the up/down pattern being repeated.
Sinus tachycardia is generally a reflection of underlying significant systemic illness, such as sepsis, hypotension, or anemia.
Atrial tachycardia: The heart rate is generally 100-140 bpm, and p waves are present. But unlike in sinus tachycardia, the patient with atrial tachycardia lying in bed with a heart rate of 140 bpm is not in a state of profound neurohormonal activation and is not all that sick.
Another diagnostic clue is provided by a look at the telemonitoring strip. Unlike in sinus tachycardia, where the heart rate ramps up and then back down repeatedly, in atrial tachycardia the heart rate very quickly ramps up in stages to, say, 140 bpm, and then hangs there.
Atrial flutter: This is the only narrow complex tachycardia that appears in both the regular and irregular rhythm columns. It belongs in the irregular rhythm column when there is variable conduction and cluster beating, with a regularly irregular pattern of RR intervals. In contrast, when atrial flutter is in the regular rhythm column, it’s because the atrioventricular node is steadily conducting the atrial depolarizations at a rate of about 300 bpm. So there’s no cluster beating. As in atrial flutter with variable conduction, the flutter waves are visible most often in leads II, III, and aVF, where they can be either positively or negatively deflected.
AV reentrant tachycardias: These reentrant tachycardias can take two forms. In atrioventricular nodal reentrant tachycardia (AVnRT), the aberrant pathway is found entirely within the AV node, whereas in atrioventricular reentrant tachycardia (AVRT) the aberrant pathway is found outside the AV node. AVnRT is more common than AVRT. As in atrial flutter, there is no ramp up in heart rate. Patients will be lying in their hospital bed with a heart rate of, say, 80 bpm, and then suddenly it jumps to 180, 200, or even as high as 240 bpm “almost in a split second,” Dr. Walsh said.
No other narrow complex tachycardia reaches so high a heart rate. In both of these reentrant tachycardias the p waves are often buried in the QRS complex and can be tough to see. It’s very difficult to differentiate AVnRT from AVRT except by an electrophysiologic study.
Accelerated junctional tachycardia: This is most commonly the slowest of the narrow complex tachycardias, with a heart rate of less than 120 bpm.
“In the case of accelerated junctional tachycardia, think slow, think ‘regular,’ think of a rate often just over 100, usually with p waves after the QRS that are inverted because there’s retrograde conduction,” she advised.
She reported having no financial conflicts of interest regarding her presentation.
Correction, 8/19/20: An earlier version of this article mischaracterized the type of rhythm noted in this subhead.
FROM HM20 VIRTUAL
COVID-19/heart connection: What hospitalists need to know
The heart-related manifestations of COVID-19 are a serious matter, but no one should make the mistake of thinking of COVID-19 as primarily a cardiac disease, according to Jeffrey C. Trost, MD, a cardiologist at Johns Hopkins University, Baltimore.
he said in offering a preview of his upcoming clinical update at HM20 Virtual, hosted by the Society of Hospital Medicine.
For this reason, in his clinical update talk, titled “COVID-19 and the Heart: What Every Hospitalist Should Know,” he’ll urge hospitalists to be conservative in ordering cardiac biomarker tests such troponin and natriuretic peptide levels. The focus should appropriately be on the subset of COVID-19 patients having the same symptoms suggestive of acute coronary syndrome, heart failure, or new-onset cardiomyopathy that would trigger laboratory testing in non–COVID-19 patients.
“Be more selective. Definitely do not routinely monitor troponin or [N-terminal of the prohormone brain natriuretic peptide] in patients just because they have COVID-19. A lot of patients with COVID-19 have these labs drawn, especially in the emergency department. We see a high signal-to-noise ratio: not infrequently the values are abnormal, and yet we don’t really know what that means,” said Dr. Trost, who is also director of the cardiac catheterization laboratory at Johns Hopkins Bayview Medical Center.
COVID-19 patients with preexisting heart disease are clearly at increased risk of severe forms of the infectious illness. In his talk, Dr. Trost will review the epidemiology of this association. He’ll also discuss the varied cardiac manifestations of COVID-19, consisting of myocarditis or other forms of new-onset cardiomyopathy, acute coronary syndrome, heart failure, and arrhythmias.
Many questions regarding COVID-19 and the heart remain unanswered for now, such as the mechanism and long-term implications of the phenomenon of ST-elevation acute coronary syndrome with chest pain in the presence of unobstructed coronary arteries, which Dr. Trost and others have encountered. Or the extent to which COVID-19–associated myocarditis is directly virus mediated as opposed to an autoimmune process.
“We’re relying completely on case reports at this point,” according to the cardiologist.
But one major issue has, thankfully, been put to rest on the basis of persuasive evidence which Dr. Trost plans to highlight: Millions of patients on ACE inhibitors or angiotensin receptor blockers can now rest assured that taking those medications doesn’t place them at increased risk of becoming infected with the novel coronavirus or, if infected, developing severe complications of COVID-19. Earlier in the pandemic that had been a legitimate theoretic concern based upon a plausible mechanism.
“I think we as physicians can now confidently say that we don’t need to stop these medicines in folks,” Dr. Trost said.
COVID-19 and the Heart: What Every Hospitalist Should Know
Live Q&A: Wednesday, Aug. 19, 3:30 p.m. to 4:30 p.m. ET
The heart-related manifestations of COVID-19 are a serious matter, but no one should make the mistake of thinking of COVID-19 as primarily a cardiac disease, according to Jeffrey C. Trost, MD, a cardiologist at Johns Hopkins University, Baltimore.
he said in offering a preview of his upcoming clinical update at HM20 Virtual, hosted by the Society of Hospital Medicine.
For this reason, in his clinical update talk, titled “COVID-19 and the Heart: What Every Hospitalist Should Know,” he’ll urge hospitalists to be conservative in ordering cardiac biomarker tests such troponin and natriuretic peptide levels. The focus should appropriately be on the subset of COVID-19 patients having the same symptoms suggestive of acute coronary syndrome, heart failure, or new-onset cardiomyopathy that would trigger laboratory testing in non–COVID-19 patients.
“Be more selective. Definitely do not routinely monitor troponin or [N-terminal of the prohormone brain natriuretic peptide] in patients just because they have COVID-19. A lot of patients with COVID-19 have these labs drawn, especially in the emergency department. We see a high signal-to-noise ratio: not infrequently the values are abnormal, and yet we don’t really know what that means,” said Dr. Trost, who is also director of the cardiac catheterization laboratory at Johns Hopkins Bayview Medical Center.
COVID-19 patients with preexisting heart disease are clearly at increased risk of severe forms of the infectious illness. In his talk, Dr. Trost will review the epidemiology of this association. He’ll also discuss the varied cardiac manifestations of COVID-19, consisting of myocarditis or other forms of new-onset cardiomyopathy, acute coronary syndrome, heart failure, and arrhythmias.
Many questions regarding COVID-19 and the heart remain unanswered for now, such as the mechanism and long-term implications of the phenomenon of ST-elevation acute coronary syndrome with chest pain in the presence of unobstructed coronary arteries, which Dr. Trost and others have encountered. Or the extent to which COVID-19–associated myocarditis is directly virus mediated as opposed to an autoimmune process.
“We’re relying completely on case reports at this point,” according to the cardiologist.
But one major issue has, thankfully, been put to rest on the basis of persuasive evidence which Dr. Trost plans to highlight: Millions of patients on ACE inhibitors or angiotensin receptor blockers can now rest assured that taking those medications doesn’t place them at increased risk of becoming infected with the novel coronavirus or, if infected, developing severe complications of COVID-19. Earlier in the pandemic that had been a legitimate theoretic concern based upon a plausible mechanism.
“I think we as physicians can now confidently say that we don’t need to stop these medicines in folks,” Dr. Trost said.
COVID-19 and the Heart: What Every Hospitalist Should Know
Live Q&A: Wednesday, Aug. 19, 3:30 p.m. to 4:30 p.m. ET
The heart-related manifestations of COVID-19 are a serious matter, but no one should make the mistake of thinking of COVID-19 as primarily a cardiac disease, according to Jeffrey C. Trost, MD, a cardiologist at Johns Hopkins University, Baltimore.
he said in offering a preview of his upcoming clinical update at HM20 Virtual, hosted by the Society of Hospital Medicine.
For this reason, in his clinical update talk, titled “COVID-19 and the Heart: What Every Hospitalist Should Know,” he’ll urge hospitalists to be conservative in ordering cardiac biomarker tests such troponin and natriuretic peptide levels. The focus should appropriately be on the subset of COVID-19 patients having the same symptoms suggestive of acute coronary syndrome, heart failure, or new-onset cardiomyopathy that would trigger laboratory testing in non–COVID-19 patients.
“Be more selective. Definitely do not routinely monitor troponin or [N-terminal of the prohormone brain natriuretic peptide] in patients just because they have COVID-19. A lot of patients with COVID-19 have these labs drawn, especially in the emergency department. We see a high signal-to-noise ratio: not infrequently the values are abnormal, and yet we don’t really know what that means,” said Dr. Trost, who is also director of the cardiac catheterization laboratory at Johns Hopkins Bayview Medical Center.
COVID-19 patients with preexisting heart disease are clearly at increased risk of severe forms of the infectious illness. In his talk, Dr. Trost will review the epidemiology of this association. He’ll also discuss the varied cardiac manifestations of COVID-19, consisting of myocarditis or other forms of new-onset cardiomyopathy, acute coronary syndrome, heart failure, and arrhythmias.
Many questions regarding COVID-19 and the heart remain unanswered for now, such as the mechanism and long-term implications of the phenomenon of ST-elevation acute coronary syndrome with chest pain in the presence of unobstructed coronary arteries, which Dr. Trost and others have encountered. Or the extent to which COVID-19–associated myocarditis is directly virus mediated as opposed to an autoimmune process.
“We’re relying completely on case reports at this point,” according to the cardiologist.
But one major issue has, thankfully, been put to rest on the basis of persuasive evidence which Dr. Trost plans to highlight: Millions of patients on ACE inhibitors or angiotensin receptor blockers can now rest assured that taking those medications doesn’t place them at increased risk of becoming infected with the novel coronavirus or, if infected, developing severe complications of COVID-19. Earlier in the pandemic that had been a legitimate theoretic concern based upon a plausible mechanism.
“I think we as physicians can now confidently say that we don’t need to stop these medicines in folks,” Dr. Trost said.
COVID-19 and the Heart: What Every Hospitalist Should Know
Live Q&A: Wednesday, Aug. 19, 3:30 p.m. to 4:30 p.m. ET
CT-FFR offers a noninvasive ‘one-stop shop’ for pre-TAVR assessment
Fractional flow reserve derived noninvasively from coronary CT angiography is a safe and accurate method for assessing the significance of coronary artery disease in patients with severe aortic stenosis who are headed for transcatheter aortic valve replacement (TAVR), according to results of the CAST-FFR prospective study.
Indeed, utilization of coronary CT angiography–derived fractional flow reserve (CT-FFR) for this purpose offers the advantage of using a single noninvasive imaging method to replace two invasive procedures: coronary angiography to assess the anatomy of coronary lesions, and conventional FFR using a pressure wire to determine the functional significance of a given coronary stenosis as a cause of ischemia, Michael Michail, MBBS, explained in reporting the results at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“Because up to 50% of patients with severe aortic stenosis undergoing TAVR have coexisting coronary artery disease, it remains common practice to perform prior invasive coronary angiography. However, this is associated with inherent risks, particularly in an elderly cohort with comorbidities. Additionally, coronary angiography provides no information on the functional impact of coronary stenoses, which may be important in guiding revascularization decisions prior to TAVR,” noted Dr. Michail, a cardiologist at Monash University, Melbourne.
Simulating FFR: ‘A one-stop shop cardiac CT’
Dr. Michail presented the results of the prospective CAST-FFR study, the first evaluation of CT-FFR for assessment of coronary arteries in patients with severe symptomatic aortic stenosis. This method uses computational fluid dynamics to transform data obtained noninvasively from a standard coronary CT angiography acquisition into a simulated FFR. And it offers the potential to streamline patient care.
“In current practice we see elderly patients with a long pre-TAVR assessment period, with numerous appointments and invasive procedures. Our vision is a one-stop shop cardiac CT that will provide the cardiologist with a complete assessment of the annular measurements, peripheral vasculature, and the coronary arteries ahead of their procedure,” according to Dr. Michail.
“We believe the ability to perform the requisite coronary assessment using CT-FFR will translate to improved patient care in several ways,” he continued. “Firstly, this will shorten the number of tests and overall diagnostic journey for patients. It will reduce the risk from unnecessary invasive procedures, and this will also reduce discomfort for the patient. Based on emerging evidence on the adverse prognostic impact of functionally significant coronary disease in aortic stenosis, this data has the potential to improve procedural risk stratification. And finally, contingent on further data, this may improve lesion selection for upfront revascularization.”
The CAST-FFR study was a small, single-center, proof-of-concept study in which 42 patients with severe aortic stenosis underwent both coronary CT angiography and conventional FFR with a pressure wire. The CT data was sent to a core laboratory for conversion into CT-FFR by evaluators blinded to the conventional FFR values.
Of the 42 participants, 39 (93%) had usable CT-FFR data on 60 coronary vessels. Dr. Michail and coinvestigators found a strong correlation between the conventional pressure wire FFR and CT-FFR findings, with a receiver operating characteristic area under the curve of 0.83 per vessel. CT-FFR had a diagnostic sensitivity and specificity of 73.9% and 78.4%, respectively, with a positive predictive value of 68%, a negative predictive value of 82.9%, and a diagnostic accuracy of 76.7%.
He cited as study limitations the small size, the fact that patients with previous revascularization or significant left ventricular impairment were excluded, and the study cohort’s relative youth.
“With a mean age of 76.2 years, it’s unclear whether these results can be extrapolated to very elderly patients with more calcified arteries undergoing TAVR. Encouragingly, though, a subgroup analysis based on calcium score showed no effect on accuracy,” according to the cardiologist.
CT-FFR may ‘shorten the diagnostic journey’ for fragile patients
Discussant Daniele Andreini, MD, PhD, praised the investigators’ concept of integrating the functional assessment provided by CT-FFR into a one-stop shop examination by cardiac CT angiography for TAVR planning.
“I would like to underline one of Dr. Michail’s messages: It’s really important to shorten the diagnostic journey for these fragile, older patients with aortic stenosis in order to improve safety, use less contrast, and avoid complications,” said Dr. Andreini, a cardiologist at the University of Milan and director of the cardiovascular CT and radiology unit at Monzino Cardiology Center, also in Milan.
Both Dr. Michail and Dr. Andreini reported having no financial conflicts of interest.
Fractional flow reserve derived noninvasively from coronary CT angiography is a safe and accurate method for assessing the significance of coronary artery disease in patients with severe aortic stenosis who are headed for transcatheter aortic valve replacement (TAVR), according to results of the CAST-FFR prospective study.
Indeed, utilization of coronary CT angiography–derived fractional flow reserve (CT-FFR) for this purpose offers the advantage of using a single noninvasive imaging method to replace two invasive procedures: coronary angiography to assess the anatomy of coronary lesions, and conventional FFR using a pressure wire to determine the functional significance of a given coronary stenosis as a cause of ischemia, Michael Michail, MBBS, explained in reporting the results at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“Because up to 50% of patients with severe aortic stenosis undergoing TAVR have coexisting coronary artery disease, it remains common practice to perform prior invasive coronary angiography. However, this is associated with inherent risks, particularly in an elderly cohort with comorbidities. Additionally, coronary angiography provides no information on the functional impact of coronary stenoses, which may be important in guiding revascularization decisions prior to TAVR,” noted Dr. Michail, a cardiologist at Monash University, Melbourne.
Simulating FFR: ‘A one-stop shop cardiac CT’
Dr. Michail presented the results of the prospective CAST-FFR study, the first evaluation of CT-FFR for assessment of coronary arteries in patients with severe symptomatic aortic stenosis. This method uses computational fluid dynamics to transform data obtained noninvasively from a standard coronary CT angiography acquisition into a simulated FFR. And it offers the potential to streamline patient care.
“In current practice we see elderly patients with a long pre-TAVR assessment period, with numerous appointments and invasive procedures. Our vision is a one-stop shop cardiac CT that will provide the cardiologist with a complete assessment of the annular measurements, peripheral vasculature, and the coronary arteries ahead of their procedure,” according to Dr. Michail.
“We believe the ability to perform the requisite coronary assessment using CT-FFR will translate to improved patient care in several ways,” he continued. “Firstly, this will shorten the number of tests and overall diagnostic journey for patients. It will reduce the risk from unnecessary invasive procedures, and this will also reduce discomfort for the patient. Based on emerging evidence on the adverse prognostic impact of functionally significant coronary disease in aortic stenosis, this data has the potential to improve procedural risk stratification. And finally, contingent on further data, this may improve lesion selection for upfront revascularization.”
The CAST-FFR study was a small, single-center, proof-of-concept study in which 42 patients with severe aortic stenosis underwent both coronary CT angiography and conventional FFR with a pressure wire. The CT data was sent to a core laboratory for conversion into CT-FFR by evaluators blinded to the conventional FFR values.
Of the 42 participants, 39 (93%) had usable CT-FFR data on 60 coronary vessels. Dr. Michail and coinvestigators found a strong correlation between the conventional pressure wire FFR and CT-FFR findings, with a receiver operating characteristic area under the curve of 0.83 per vessel. CT-FFR had a diagnostic sensitivity and specificity of 73.9% and 78.4%, respectively, with a positive predictive value of 68%, a negative predictive value of 82.9%, and a diagnostic accuracy of 76.7%.
He cited as study limitations the small size, the fact that patients with previous revascularization or significant left ventricular impairment were excluded, and the study cohort’s relative youth.
“With a mean age of 76.2 years, it’s unclear whether these results can be extrapolated to very elderly patients with more calcified arteries undergoing TAVR. Encouragingly, though, a subgroup analysis based on calcium score showed no effect on accuracy,” according to the cardiologist.
CT-FFR may ‘shorten the diagnostic journey’ for fragile patients
Discussant Daniele Andreini, MD, PhD, praised the investigators’ concept of integrating the functional assessment provided by CT-FFR into a one-stop shop examination by cardiac CT angiography for TAVR planning.
“I would like to underline one of Dr. Michail’s messages: It’s really important to shorten the diagnostic journey for these fragile, older patients with aortic stenosis in order to improve safety, use less contrast, and avoid complications,” said Dr. Andreini, a cardiologist at the University of Milan and director of the cardiovascular CT and radiology unit at Monzino Cardiology Center, also in Milan.
Both Dr. Michail and Dr. Andreini reported having no financial conflicts of interest.
Fractional flow reserve derived noninvasively from coronary CT angiography is a safe and accurate method for assessing the significance of coronary artery disease in patients with severe aortic stenosis who are headed for transcatheter aortic valve replacement (TAVR), according to results of the CAST-FFR prospective study.
Indeed, utilization of coronary CT angiography–derived fractional flow reserve (CT-FFR) for this purpose offers the advantage of using a single noninvasive imaging method to replace two invasive procedures: coronary angiography to assess the anatomy of coronary lesions, and conventional FFR using a pressure wire to determine the functional significance of a given coronary stenosis as a cause of ischemia, Michael Michail, MBBS, explained in reporting the results at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“Because up to 50% of patients with severe aortic stenosis undergoing TAVR have coexisting coronary artery disease, it remains common practice to perform prior invasive coronary angiography. However, this is associated with inherent risks, particularly in an elderly cohort with comorbidities. Additionally, coronary angiography provides no information on the functional impact of coronary stenoses, which may be important in guiding revascularization decisions prior to TAVR,” noted Dr. Michail, a cardiologist at Monash University, Melbourne.
Simulating FFR: ‘A one-stop shop cardiac CT’
Dr. Michail presented the results of the prospective CAST-FFR study, the first evaluation of CT-FFR for assessment of coronary arteries in patients with severe symptomatic aortic stenosis. This method uses computational fluid dynamics to transform data obtained noninvasively from a standard coronary CT angiography acquisition into a simulated FFR. And it offers the potential to streamline patient care.
“In current practice we see elderly patients with a long pre-TAVR assessment period, with numerous appointments and invasive procedures. Our vision is a one-stop shop cardiac CT that will provide the cardiologist with a complete assessment of the annular measurements, peripheral vasculature, and the coronary arteries ahead of their procedure,” according to Dr. Michail.
“We believe the ability to perform the requisite coronary assessment using CT-FFR will translate to improved patient care in several ways,” he continued. “Firstly, this will shorten the number of tests and overall diagnostic journey for patients. It will reduce the risk from unnecessary invasive procedures, and this will also reduce discomfort for the patient. Based on emerging evidence on the adverse prognostic impact of functionally significant coronary disease in aortic stenosis, this data has the potential to improve procedural risk stratification. And finally, contingent on further data, this may improve lesion selection for upfront revascularization.”
The CAST-FFR study was a small, single-center, proof-of-concept study in which 42 patients with severe aortic stenosis underwent both coronary CT angiography and conventional FFR with a pressure wire. The CT data was sent to a core laboratory for conversion into CT-FFR by evaluators blinded to the conventional FFR values.
Of the 42 participants, 39 (93%) had usable CT-FFR data on 60 coronary vessels. Dr. Michail and coinvestigators found a strong correlation between the conventional pressure wire FFR and CT-FFR findings, with a receiver operating characteristic area under the curve of 0.83 per vessel. CT-FFR had a diagnostic sensitivity and specificity of 73.9% and 78.4%, respectively, with a positive predictive value of 68%, a negative predictive value of 82.9%, and a diagnostic accuracy of 76.7%.
He cited as study limitations the small size, the fact that patients with previous revascularization or significant left ventricular impairment were excluded, and the study cohort’s relative youth.
“With a mean age of 76.2 years, it’s unclear whether these results can be extrapolated to very elderly patients with more calcified arteries undergoing TAVR. Encouragingly, though, a subgroup analysis based on calcium score showed no effect on accuracy,” according to the cardiologist.
CT-FFR may ‘shorten the diagnostic journey’ for fragile patients
Discussant Daniele Andreini, MD, PhD, praised the investigators’ concept of integrating the functional assessment provided by CT-FFR into a one-stop shop examination by cardiac CT angiography for TAVR planning.
“I would like to underline one of Dr. Michail’s messages: It’s really important to shorten the diagnostic journey for these fragile, older patients with aortic stenosis in order to improve safety, use less contrast, and avoid complications,” said Dr. Andreini, a cardiologist at the University of Milan and director of the cardiovascular CT and radiology unit at Monzino Cardiology Center, also in Milan.
Both Dr. Michail and Dr. Andreini reported having no financial conflicts of interest.
REPORTING FROM EUROPCR 2020
Beyond PASI 100: striving for molecular clearance
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
All PASI 100 responses to psoriasis therapy are not the same, Andrew Blauvelt, MD, declared at the virtual annual meeting of the American Academy of Dermatology.
He presented a first-of-its-kind study that potentially opens the door to a new, more rigorous standard for treatment success in psoriasis: Not simply cleared lesional skin as captured by a Psoriasis Area and Severity Index (PASI) 100 response, but also clearance of residual psoriasis signs and symptoms – as well as what he termed “molecular clearance.”
“We’ve found that clearing skin with drugs utilizing different mechanisms of action may lead to differential consequences for our patients,” observed Dr. Blauvelt, a dermatologist and clinical trialist who is president of the Oregon Medical Research Center, Portland.
A PASI 100 response, traditionally considered an elusive goal for the great majority of patients with severe psoriasis, can now often be achieved using today’s top-tier, high-performance biologics. But Dr. Blauvelt and his coinvestigators are interested in pushing even beyond PASI 100 to a new frontier of therapeutic benefit.
He presented a secondary analysis of the previously reported VOYAGE 1 and 2 head-to-head randomized trials of guselkumab (Tremfya) versus adalimumab (Humira) for treatment of moderate to severe psoriasis. This new analysis, which focused exclusively on PASI 100 responders by week 24, demonstrated that patients with a PASI 100 response to guselkumab, an interleukin (IL)-23 inhibitor, had significantly fewer persistent symptoms and signs of psoriasis than those whose skin clearance was attained using adalimumab, a tumor necrosis factor (TNF) inhibitor.
Moreover,
The analysis included 16 participants in the VOYAGE trials who achieved PASI 100 at week 24 on guselkumab and 5 who did so on adalimumab. At baseline and again at week 24, these individuals completed the Psoriasis Symptoms and Signs Diary (PSSD). Also, biopsies of lesional and nonlesional skin were obtained at baseline and of cleared lesional skin at week 24 for transcriptomic microarray analysis of the expression of many thousands of genes.
Persistent psoriasis symptoms despite cleared skin
The PSSD involves patient ratings of various psoriasis symptoms and signs. Total scores can range from 0 (symptom- and sign-free) up to 100. At week 24, a significantly higher proportion of guselkumab-treated PASI 100 responders had a total PSSD score of zero: 55%, versus 43% in the adalimumab group. This was consistently true across the board for each of the individual signs and symptoms assessed. For example, 61% of the guselkumab group gave themselves a zero for itch, as did 50% of the adalimumab group. Sixty-four percent on guselkumab and 52% on adalimumab reported being free of redness. And 78% of the guselkumab group reported being pain-free, compared with 69% with adalimumab, Dr. Blauvelt reported.
Gene expression analysis
At baseline, more than 2,300 dysregulated genes were identified in lesional skin while functioning normally in nonlesional skin. The great majority of these initially dysregulated genes became normalized in cleared lesional skin in PASI 100 responders at week 24. However, 25 of the genes remained dysregulated in cleared lesional skin, meaning they displayed less than 75% of normal function. Ten of these 25 genes with dysregulated expression at follow-up showed abnormal function in patients with residual symptoms despite cleared skin, but they functioned normally in those without persistent symptoms. This raises the possibility that the residual symptoms of psoriasis were attributable to the abnormal gene functioning, according to Dr. Blauvelt.
Of note, 9 of the 10 dysregulated genes in cleared lesional skin of patients with residual symptoms were present in the adalimumab group; these included two genes localized to the epidermal differentiation complex as well as the psoriasis-specific proline-rich 9 gene known as PRR9, which is induced by IL-17A. In contrast, only four genes, none of which were localized to the epidermal differentiation complex, were insufficiently normalized in the cleared lesional skin of guselkumab-treated PASI-100 responders.
“Nothing like this analysis has ever been done before,” the dermatologist observed. “It’s a pilot study. Perhaps with more data like this, we’ll be using this type of information in clinical practice to go beyond clearing patients’ skin.”
Dr. Blauvelt reported serving as a scientific advisor to and paid clinical investigator for Janssen, which sponsored the study, as well as for roughly two dozen other pharmaceutical companies.
FROM AAD 20
Oculostenotic reflex still holds sway, survey shows
Most interventional cardiologists still rely solely upon angiography in making revascularization decisions about intermediate stenoses in the setting of stable coronary artery disease – and in doing so they end up making the wrong call nearly 40% of the time, according to the results of an international survey presented at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We saw a strong tendency to visually overestimate the percent diameter stenosis,” reported Gabor G. Toth, MD, an interventional cardiologist at the Medical University of Graz (Austria).
The same tendency has been highlighted in numerous randomized trials and observational studies. That’s why both European and U.S. guidelines now strongly recommend invasive functional assessment, such as fractional-flow reserve (FFR) testing, in evaluating the significance of intermediate stenoses in the absence of noninvasive evidence of ischemia. The new survey findings point to an important disconnect between these guideline recommendations and current clinical practice, he noted.
Dr. Toth presented the results of the second web-based, international survey on interventional decision-making strategy sponsored by the European Association of Percutaneous Cardiovascular Interventions. He contrasted the findings with those of the previously reported first international online survey, conducted 6 years earlier, for which he was first author (Circ Cardiovasc Interv. 2014 Dec;7[6]:751-9).
The two surveys were identically designed. In both, participants answered questions that enabled investigators to place them into one of four categories based upon the extent of their experience in interventional cardiology. The participants were also presented with 5 angiograms of focal intermediate stenoses and asked to determine the stenosis significance of each lesion. No information on the functional significance of the stenoses was included; however, the respondents could request additional diagnostic information by “ordering” adjunctive invasive functional assessment tests, including FFR, quantitative coronary angiography, intravascular ultrasound, or optical coherence tomography. Importantly, participating cardiologists were asked to make their decisions based upon best possible clinical practice in a hypothetical scenario where financial constraints had no role.
The second international survey was conducted during the latter half of 2019. The 334 interventional cardiologists who responded performed a total of 978 case evaluations including 2,054 coronary lesion assessments.
About 59% of all decisions were made solely on the basis of angiographic appearance without any information as to the functional significance of a given stenosis: Indeed, 13% of all stenoses were thereby declared to be “certainly” nonsignificant, and 46% were deemed “certainly” significant. In total, that figure was down significantly from the 71% rate in the first survey. In the first survey, 47% of decisions based upon angiographic appearance alone were discordant with FFR results known to the investigators, compared with a 39% discordance rate in the second survey.
Of the physician decisions made in the second survey, 10% involved a request for intravascular imaging, essentially unchanged from the 9% rate in the first survey. However, there was a significant increase over time in requests for invasive functional assessment tests: 25% in the first survey, rising to 31% in the second. This increase was entirely driven by additional requests for data on nonhyperemic pressure ratios; there was no difference in requests for FFR testing between the 2013 and 2019 surveys.
Clinician experience played an interesting role in decision-making: “Experience does not have an impact on the accuracy of angiographically based decisions, but experience does have an impact on understanding the need for adjunctive functional diagnostic testing,” Dr. Toth explained.
Indeed, 21% of decisions made by the least-experienced interventional cardiologists involved a request for adjunctive invasive functional assessment, compared with 24% of decisions by physicians in the third quartile of experience, 32% in the second, and 37% of decisions made by the most experienced clinicians.
Discussant Michael Haude, MD, PhD, said that “these results clearly show that eyeball angioguidance is still the dominant tool used in decision-making, and that this eyeball angioguidance continuously overestimates the stenosis when you compare the results to quantitative coronary angiography.
“These results, surprisingly for me, show a quite low uptake of the invasive functional assessments despite overwhelming scientific data leading to clear guideline-based recommendations. Why is this the case, even after financial constraints are ruled out? Probably because FFR is still a complex invasive procedure. Maybe, in the future, quantitative flow-ratio angiography [which requires no pressure wire] or CT-based FFR will be more popular,” said Dr. Haude, an interventional cardiologist at the Rheinland Clinic in Neuss, Germany.
He reported receiving research grants from Biotronik and serving as a paid consultant to that company as well as Cardiac Dimensions, Orbus Neich, and Philips. Dr. Toth reported having no financial conflicts regarding the international survey.
Most interventional cardiologists still rely solely upon angiography in making revascularization decisions about intermediate stenoses in the setting of stable coronary artery disease – and in doing so they end up making the wrong call nearly 40% of the time, according to the results of an international survey presented at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We saw a strong tendency to visually overestimate the percent diameter stenosis,” reported Gabor G. Toth, MD, an interventional cardiologist at the Medical University of Graz (Austria).
The same tendency has been highlighted in numerous randomized trials and observational studies. That’s why both European and U.S. guidelines now strongly recommend invasive functional assessment, such as fractional-flow reserve (FFR) testing, in evaluating the significance of intermediate stenoses in the absence of noninvasive evidence of ischemia. The new survey findings point to an important disconnect between these guideline recommendations and current clinical practice, he noted.
Dr. Toth presented the results of the second web-based, international survey on interventional decision-making strategy sponsored by the European Association of Percutaneous Cardiovascular Interventions. He contrasted the findings with those of the previously reported first international online survey, conducted 6 years earlier, for which he was first author (Circ Cardiovasc Interv. 2014 Dec;7[6]:751-9).
The two surveys were identically designed. In both, participants answered questions that enabled investigators to place them into one of four categories based upon the extent of their experience in interventional cardiology. The participants were also presented with 5 angiograms of focal intermediate stenoses and asked to determine the stenosis significance of each lesion. No information on the functional significance of the stenoses was included; however, the respondents could request additional diagnostic information by “ordering” adjunctive invasive functional assessment tests, including FFR, quantitative coronary angiography, intravascular ultrasound, or optical coherence tomography. Importantly, participating cardiologists were asked to make their decisions based upon best possible clinical practice in a hypothetical scenario where financial constraints had no role.
The second international survey was conducted during the latter half of 2019. The 334 interventional cardiologists who responded performed a total of 978 case evaluations including 2,054 coronary lesion assessments.
About 59% of all decisions were made solely on the basis of angiographic appearance without any information as to the functional significance of a given stenosis: Indeed, 13% of all stenoses were thereby declared to be “certainly” nonsignificant, and 46% were deemed “certainly” significant. In total, that figure was down significantly from the 71% rate in the first survey. In the first survey, 47% of decisions based upon angiographic appearance alone were discordant with FFR results known to the investigators, compared with a 39% discordance rate in the second survey.
Of the physician decisions made in the second survey, 10% involved a request for intravascular imaging, essentially unchanged from the 9% rate in the first survey. However, there was a significant increase over time in requests for invasive functional assessment tests: 25% in the first survey, rising to 31% in the second. This increase was entirely driven by additional requests for data on nonhyperemic pressure ratios; there was no difference in requests for FFR testing between the 2013 and 2019 surveys.
Clinician experience played an interesting role in decision-making: “Experience does not have an impact on the accuracy of angiographically based decisions, but experience does have an impact on understanding the need for adjunctive functional diagnostic testing,” Dr. Toth explained.
Indeed, 21% of decisions made by the least-experienced interventional cardiologists involved a request for adjunctive invasive functional assessment, compared with 24% of decisions by physicians in the third quartile of experience, 32% in the second, and 37% of decisions made by the most experienced clinicians.
Discussant Michael Haude, MD, PhD, said that “these results clearly show that eyeball angioguidance is still the dominant tool used in decision-making, and that this eyeball angioguidance continuously overestimates the stenosis when you compare the results to quantitative coronary angiography.
“These results, surprisingly for me, show a quite low uptake of the invasive functional assessments despite overwhelming scientific data leading to clear guideline-based recommendations. Why is this the case, even after financial constraints are ruled out? Probably because FFR is still a complex invasive procedure. Maybe, in the future, quantitative flow-ratio angiography [which requires no pressure wire] or CT-based FFR will be more popular,” said Dr. Haude, an interventional cardiologist at the Rheinland Clinic in Neuss, Germany.
He reported receiving research grants from Biotronik and serving as a paid consultant to that company as well as Cardiac Dimensions, Orbus Neich, and Philips. Dr. Toth reported having no financial conflicts regarding the international survey.
Most interventional cardiologists still rely solely upon angiography in making revascularization decisions about intermediate stenoses in the setting of stable coronary artery disease – and in doing so they end up making the wrong call nearly 40% of the time, according to the results of an international survey presented at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“We saw a strong tendency to visually overestimate the percent diameter stenosis,” reported Gabor G. Toth, MD, an interventional cardiologist at the Medical University of Graz (Austria).
The same tendency has been highlighted in numerous randomized trials and observational studies. That’s why both European and U.S. guidelines now strongly recommend invasive functional assessment, such as fractional-flow reserve (FFR) testing, in evaluating the significance of intermediate stenoses in the absence of noninvasive evidence of ischemia. The new survey findings point to an important disconnect between these guideline recommendations and current clinical practice, he noted.
Dr. Toth presented the results of the second web-based, international survey on interventional decision-making strategy sponsored by the European Association of Percutaneous Cardiovascular Interventions. He contrasted the findings with those of the previously reported first international online survey, conducted 6 years earlier, for which he was first author (Circ Cardiovasc Interv. 2014 Dec;7[6]:751-9).
The two surveys were identically designed. In both, participants answered questions that enabled investigators to place them into one of four categories based upon the extent of their experience in interventional cardiology. The participants were also presented with 5 angiograms of focal intermediate stenoses and asked to determine the stenosis significance of each lesion. No information on the functional significance of the stenoses was included; however, the respondents could request additional diagnostic information by “ordering” adjunctive invasive functional assessment tests, including FFR, quantitative coronary angiography, intravascular ultrasound, or optical coherence tomography. Importantly, participating cardiologists were asked to make their decisions based upon best possible clinical practice in a hypothetical scenario where financial constraints had no role.
The second international survey was conducted during the latter half of 2019. The 334 interventional cardiologists who responded performed a total of 978 case evaluations including 2,054 coronary lesion assessments.
About 59% of all decisions were made solely on the basis of angiographic appearance without any information as to the functional significance of a given stenosis: Indeed, 13% of all stenoses were thereby declared to be “certainly” nonsignificant, and 46% were deemed “certainly” significant. In total, that figure was down significantly from the 71% rate in the first survey. In the first survey, 47% of decisions based upon angiographic appearance alone were discordant with FFR results known to the investigators, compared with a 39% discordance rate in the second survey.
Of the physician decisions made in the second survey, 10% involved a request for intravascular imaging, essentially unchanged from the 9% rate in the first survey. However, there was a significant increase over time in requests for invasive functional assessment tests: 25% in the first survey, rising to 31% in the second. This increase was entirely driven by additional requests for data on nonhyperemic pressure ratios; there was no difference in requests for FFR testing between the 2013 and 2019 surveys.
Clinician experience played an interesting role in decision-making: “Experience does not have an impact on the accuracy of angiographically based decisions, but experience does have an impact on understanding the need for adjunctive functional diagnostic testing,” Dr. Toth explained.
Indeed, 21% of decisions made by the least-experienced interventional cardiologists involved a request for adjunctive invasive functional assessment, compared with 24% of decisions by physicians in the third quartile of experience, 32% in the second, and 37% of decisions made by the most experienced clinicians.
Discussant Michael Haude, MD, PhD, said that “these results clearly show that eyeball angioguidance is still the dominant tool used in decision-making, and that this eyeball angioguidance continuously overestimates the stenosis when you compare the results to quantitative coronary angiography.
“These results, surprisingly for me, show a quite low uptake of the invasive functional assessments despite overwhelming scientific data leading to clear guideline-based recommendations. Why is this the case, even after financial constraints are ruled out? Probably because FFR is still a complex invasive procedure. Maybe, in the future, quantitative flow-ratio angiography [which requires no pressure wire] or CT-based FFR will be more popular,” said Dr. Haude, an interventional cardiologist at the Rheinland Clinic in Neuss, Germany.
He reported receiving research grants from Biotronik and serving as a paid consultant to that company as well as Cardiac Dimensions, Orbus Neich, and Philips. Dr. Toth reported having no financial conflicts regarding the international survey.
REPORTING FROM EUROPCR 2020
New topicals for excessive sweating are in sight
Safe and effective of novel agents presented at the virtual annual meeting of the American Academy of Dermatology.
Both investigational topical anticholinergic agents – 5% sofpironium bromide (SPB) gel and 1% glycopyrronium bromide (GPB) cream – met all of the efficacy and safety endpoints required by the Food and Drug Administration.
Primary axillary hyperhidrosis, or symmetrical bilateral excessive armpit sweating, has a prevalence worldwide of 1%-16%, with 5%-6% the most frequently cited numbers. The condition has a strong adverse impact on quality of life. Primary axillary hyperhidrosis is not caused by a disorder of the sweat glands; rather, it’s actually a dysregulation of the autonomic nervous system leading to disproportionate sweating, explained Christoph Abels, MD, PhD, medical director at Dr. August Wolff in Bielefeld, Germany.
“What’s surprising is that more than 50% of patients do not receive appropriate treatment, very likely due to lack of awareness or embarrassment,” he added.
Also, many patients are put off by the systemic side effects of the oral anticholinergic agents, which are the current off-label treatment mainstay for patients with moderate or severe disease, according to Tomoko Fujimoto, MD, PhD, director of Ikebukuro Nishiguchi Fukurou Dermatology, near Tokyo.
Sofpironium bromide gel
Dr. Fujimoto presented the results of a phase 3, double-blind, multicenter, 6-week, vehicle-controlled clinical trial conducted in 281 Japanese patients with moderate to severe primary axillary hyperhidrosis as defined by a baseline score of 3 or 4 on the 4-point Hyperhidrosis Disease Severity Scale (HDSS). Participants were randomized to self-application of 5% SPB gel or its vehicle once daily before bedtime.
Sofpironium bromide blocks the cholinergic response mediated by the M3 muscarinic receptor subtype expressed on eccrine sweat glands, thereby inhibiting sweating. The drug then undergoes breakdown into an inactive metabolite after reaching the blood.
An important aspect of both SPB gel and GPB cream is that these agents are rolled onto the axillae using a dedicated applicator. Patients never touch the medications with their hands, thus avoiding accidental exposure to the mucous membranes. This largely prevents problems with mydriasis and blurred vision as anticholinergic side effects, which has been an issue with glycopyrronium tosylate topical cloth wipes (Qbrexza), the first FDA-approved treatment for primary axillary hyperhidrosis.
The primary endpoint in the Japanese study was at least a 1-point improvement on the HDSS plus at least a 50% reduction in gravimetric sweat production between baseline and week 6. This composite outcome was achieved in 53.9% of patients in the active treatment arm, compared with 36.4% of controls.
The secondary endpoint consisting of a week-6 HDSS score of 1 or 2 – that is, underarm sweating that’s either never noticeable or is tolerable – occurred in 60.3% of the sofpironium bromide group and 47.9% of controls, a between-group difference that achieved statistical significance by week 2, when the rates were 46.8% and 28.2%.
The reduction in total gravimetric weight of axillary sweat from a mean baseline of 227 mg collected over 5 minutes was also significantly greater in the SPB group: a decrease of 157.6 mg, compared with 127.6 mg in controls; a between-group difference that also was significant by week 2. The mean Dermatology Life Quality Index score dropped by 6.8 points in the active-treatment arm from a baseline of 11.3, a significant improvement over the mean 4.5-point drop in controls.
A new 5-point measure of subjective symptoms of primary axillary hyperhidrosis – the Hyperhidrosis Disease Severity Measure–Axilla (HDSM-Ax) – improved by 1.41 points in the SPB group, significantly better than the 0.93 points in vehicle-treated controls. About 48% of patients on SBP experienced at least a 1.5-point reduction on the HDSM-Ax, compared with 26% of controls.
Regarding safety, there was a 2% incidence of application-site itch or scale in the SBP group. Anticholinergic side effects consisted of a single case of mydriasis, another of constipation, and two complaints of thirst, all mild, none resulting in treatment discontinuation. There were no reports of headache or blurred vision.
“These results indicate that the safety risks of sofpironium bromide can be considered small and controllable,” Dr. Fujimoto said. “Moreover, sofpironium bromide is a topical agent that patients can use by themselves, so it is highly convenient, unlike, say, botulinum toxin type A injections.”
Glycopyrronium bromide cream
Following on the heels of a recently published dose-ranging study (Br J Dermatol. 2020 Jan;182[1]:229-231), Dr. Abels presented the 4-week outcomes of a phase 3a, double-blind, randomized, five-country trial of once-daily 1% GPB cream or placebo in 171 patients with moderate or severe primary axillary hyperhidrosis. A phase 3b, open-label, 72-week, long-term safety trial is ongoing in 516 patients.
The primary endpoint of the 4-week trial was the reduction in gravimetric sweat production from day 1 to day 29. A reduction of 50% or more was documented in 57.5% of the patients on GBP and 34.5% of controls. A 75% or greater reduction occurred in 32.2% of the active-treatment group and 16.7% of those on placebo. And a decrease of at least 90% was seen in 23% of patients on topical GBP, compared with 9.5% of controls. All these between-group differences were significant.
The FDA now requires a quality of life measurement as a coprimary endpoint in phase 3 hyperhidrosis studies, and the phase 3 GBP trial also served as the successful validation study for a new patient-reported quality of life instrument designed specifically for this purpose. The new tool, known as the Hyperhydrosis Quality of Life questionnaire (HidroQol), proved much more sensitive than the HDSS or DLQI for evaluating clinical improvement in response to treatment (Br J Dermatol. 2020 Jun 8. doi: 10.1111/bjd.19300).
Initial results from the long-term phase 3b safety study should be available this fall on the first 100 patients followed on topical GBP for 1 year and for 300 followed for 6 months, Dr. Abels said.
Dr. Fujimoto reported serving as a paid consultant to and speaker for Kaken Pharmaceutical, which is developing SBP gel with Brickell Biotech. Dr. Abels is an employee of the company that is developing GPB cream.
Safe and effective of novel agents presented at the virtual annual meeting of the American Academy of Dermatology.
Both investigational topical anticholinergic agents – 5% sofpironium bromide (SPB) gel and 1% glycopyrronium bromide (GPB) cream – met all of the efficacy and safety endpoints required by the Food and Drug Administration.
Primary axillary hyperhidrosis, or symmetrical bilateral excessive armpit sweating, has a prevalence worldwide of 1%-16%, with 5%-6% the most frequently cited numbers. The condition has a strong adverse impact on quality of life. Primary axillary hyperhidrosis is not caused by a disorder of the sweat glands; rather, it’s actually a dysregulation of the autonomic nervous system leading to disproportionate sweating, explained Christoph Abels, MD, PhD, medical director at Dr. August Wolff in Bielefeld, Germany.
“What’s surprising is that more than 50% of patients do not receive appropriate treatment, very likely due to lack of awareness or embarrassment,” he added.
Also, many patients are put off by the systemic side effects of the oral anticholinergic agents, which are the current off-label treatment mainstay for patients with moderate or severe disease, according to Tomoko Fujimoto, MD, PhD, director of Ikebukuro Nishiguchi Fukurou Dermatology, near Tokyo.
Sofpironium bromide gel
Dr. Fujimoto presented the results of a phase 3, double-blind, multicenter, 6-week, vehicle-controlled clinical trial conducted in 281 Japanese patients with moderate to severe primary axillary hyperhidrosis as defined by a baseline score of 3 or 4 on the 4-point Hyperhidrosis Disease Severity Scale (HDSS). Participants were randomized to self-application of 5% SPB gel or its vehicle once daily before bedtime.
Sofpironium bromide blocks the cholinergic response mediated by the M3 muscarinic receptor subtype expressed on eccrine sweat glands, thereby inhibiting sweating. The drug then undergoes breakdown into an inactive metabolite after reaching the blood.
An important aspect of both SPB gel and GPB cream is that these agents are rolled onto the axillae using a dedicated applicator. Patients never touch the medications with their hands, thus avoiding accidental exposure to the mucous membranes. This largely prevents problems with mydriasis and blurred vision as anticholinergic side effects, which has been an issue with glycopyrronium tosylate topical cloth wipes (Qbrexza), the first FDA-approved treatment for primary axillary hyperhidrosis.
The primary endpoint in the Japanese study was at least a 1-point improvement on the HDSS plus at least a 50% reduction in gravimetric sweat production between baseline and week 6. This composite outcome was achieved in 53.9% of patients in the active treatment arm, compared with 36.4% of controls.
The secondary endpoint consisting of a week-6 HDSS score of 1 or 2 – that is, underarm sweating that’s either never noticeable or is tolerable – occurred in 60.3% of the sofpironium bromide group and 47.9% of controls, a between-group difference that achieved statistical significance by week 2, when the rates were 46.8% and 28.2%.
The reduction in total gravimetric weight of axillary sweat from a mean baseline of 227 mg collected over 5 minutes was also significantly greater in the SPB group: a decrease of 157.6 mg, compared with 127.6 mg in controls; a between-group difference that also was significant by week 2. The mean Dermatology Life Quality Index score dropped by 6.8 points in the active-treatment arm from a baseline of 11.3, a significant improvement over the mean 4.5-point drop in controls.
A new 5-point measure of subjective symptoms of primary axillary hyperhidrosis – the Hyperhidrosis Disease Severity Measure–Axilla (HDSM-Ax) – improved by 1.41 points in the SPB group, significantly better than the 0.93 points in vehicle-treated controls. About 48% of patients on SBP experienced at least a 1.5-point reduction on the HDSM-Ax, compared with 26% of controls.
Regarding safety, there was a 2% incidence of application-site itch or scale in the SBP group. Anticholinergic side effects consisted of a single case of mydriasis, another of constipation, and two complaints of thirst, all mild, none resulting in treatment discontinuation. There were no reports of headache or blurred vision.
“These results indicate that the safety risks of sofpironium bromide can be considered small and controllable,” Dr. Fujimoto said. “Moreover, sofpironium bromide is a topical agent that patients can use by themselves, so it is highly convenient, unlike, say, botulinum toxin type A injections.”
Glycopyrronium bromide cream
Following on the heels of a recently published dose-ranging study (Br J Dermatol. 2020 Jan;182[1]:229-231), Dr. Abels presented the 4-week outcomes of a phase 3a, double-blind, randomized, five-country trial of once-daily 1% GPB cream or placebo in 171 patients with moderate or severe primary axillary hyperhidrosis. A phase 3b, open-label, 72-week, long-term safety trial is ongoing in 516 patients.
The primary endpoint of the 4-week trial was the reduction in gravimetric sweat production from day 1 to day 29. A reduction of 50% or more was documented in 57.5% of the patients on GBP and 34.5% of controls. A 75% or greater reduction occurred in 32.2% of the active-treatment group and 16.7% of those on placebo. And a decrease of at least 90% was seen in 23% of patients on topical GBP, compared with 9.5% of controls. All these between-group differences were significant.
The FDA now requires a quality of life measurement as a coprimary endpoint in phase 3 hyperhidrosis studies, and the phase 3 GBP trial also served as the successful validation study for a new patient-reported quality of life instrument designed specifically for this purpose. The new tool, known as the Hyperhydrosis Quality of Life questionnaire (HidroQol), proved much more sensitive than the HDSS or DLQI for evaluating clinical improvement in response to treatment (Br J Dermatol. 2020 Jun 8. doi: 10.1111/bjd.19300).
Initial results from the long-term phase 3b safety study should be available this fall on the first 100 patients followed on topical GBP for 1 year and for 300 followed for 6 months, Dr. Abels said.
Dr. Fujimoto reported serving as a paid consultant to and speaker for Kaken Pharmaceutical, which is developing SBP gel with Brickell Biotech. Dr. Abels is an employee of the company that is developing GPB cream.
Safe and effective of novel agents presented at the virtual annual meeting of the American Academy of Dermatology.
Both investigational topical anticholinergic agents – 5% sofpironium bromide (SPB) gel and 1% glycopyrronium bromide (GPB) cream – met all of the efficacy and safety endpoints required by the Food and Drug Administration.
Primary axillary hyperhidrosis, or symmetrical bilateral excessive armpit sweating, has a prevalence worldwide of 1%-16%, with 5%-6% the most frequently cited numbers. The condition has a strong adverse impact on quality of life. Primary axillary hyperhidrosis is not caused by a disorder of the sweat glands; rather, it’s actually a dysregulation of the autonomic nervous system leading to disproportionate sweating, explained Christoph Abels, MD, PhD, medical director at Dr. August Wolff in Bielefeld, Germany.
“What’s surprising is that more than 50% of patients do not receive appropriate treatment, very likely due to lack of awareness or embarrassment,” he added.
Also, many patients are put off by the systemic side effects of the oral anticholinergic agents, which are the current off-label treatment mainstay for patients with moderate or severe disease, according to Tomoko Fujimoto, MD, PhD, director of Ikebukuro Nishiguchi Fukurou Dermatology, near Tokyo.
Sofpironium bromide gel
Dr. Fujimoto presented the results of a phase 3, double-blind, multicenter, 6-week, vehicle-controlled clinical trial conducted in 281 Japanese patients with moderate to severe primary axillary hyperhidrosis as defined by a baseline score of 3 or 4 on the 4-point Hyperhidrosis Disease Severity Scale (HDSS). Participants were randomized to self-application of 5% SPB gel or its vehicle once daily before bedtime.
Sofpironium bromide blocks the cholinergic response mediated by the M3 muscarinic receptor subtype expressed on eccrine sweat glands, thereby inhibiting sweating. The drug then undergoes breakdown into an inactive metabolite after reaching the blood.
An important aspect of both SPB gel and GPB cream is that these agents are rolled onto the axillae using a dedicated applicator. Patients never touch the medications with their hands, thus avoiding accidental exposure to the mucous membranes. This largely prevents problems with mydriasis and blurred vision as anticholinergic side effects, which has been an issue with glycopyrronium tosylate topical cloth wipes (Qbrexza), the first FDA-approved treatment for primary axillary hyperhidrosis.
The primary endpoint in the Japanese study was at least a 1-point improvement on the HDSS plus at least a 50% reduction in gravimetric sweat production between baseline and week 6. This composite outcome was achieved in 53.9% of patients in the active treatment arm, compared with 36.4% of controls.
The secondary endpoint consisting of a week-6 HDSS score of 1 or 2 – that is, underarm sweating that’s either never noticeable or is tolerable – occurred in 60.3% of the sofpironium bromide group and 47.9% of controls, a between-group difference that achieved statistical significance by week 2, when the rates were 46.8% and 28.2%.
The reduction in total gravimetric weight of axillary sweat from a mean baseline of 227 mg collected over 5 minutes was also significantly greater in the SPB group: a decrease of 157.6 mg, compared with 127.6 mg in controls; a between-group difference that also was significant by week 2. The mean Dermatology Life Quality Index score dropped by 6.8 points in the active-treatment arm from a baseline of 11.3, a significant improvement over the mean 4.5-point drop in controls.
A new 5-point measure of subjective symptoms of primary axillary hyperhidrosis – the Hyperhidrosis Disease Severity Measure–Axilla (HDSM-Ax) – improved by 1.41 points in the SPB group, significantly better than the 0.93 points in vehicle-treated controls. About 48% of patients on SBP experienced at least a 1.5-point reduction on the HDSM-Ax, compared with 26% of controls.
Regarding safety, there was a 2% incidence of application-site itch or scale in the SBP group. Anticholinergic side effects consisted of a single case of mydriasis, another of constipation, and two complaints of thirst, all mild, none resulting in treatment discontinuation. There were no reports of headache or blurred vision.
“These results indicate that the safety risks of sofpironium bromide can be considered small and controllable,” Dr. Fujimoto said. “Moreover, sofpironium bromide is a topical agent that patients can use by themselves, so it is highly convenient, unlike, say, botulinum toxin type A injections.”
Glycopyrronium bromide cream
Following on the heels of a recently published dose-ranging study (Br J Dermatol. 2020 Jan;182[1]:229-231), Dr. Abels presented the 4-week outcomes of a phase 3a, double-blind, randomized, five-country trial of once-daily 1% GPB cream or placebo in 171 patients with moderate or severe primary axillary hyperhidrosis. A phase 3b, open-label, 72-week, long-term safety trial is ongoing in 516 patients.
The primary endpoint of the 4-week trial was the reduction in gravimetric sweat production from day 1 to day 29. A reduction of 50% or more was documented in 57.5% of the patients on GBP and 34.5% of controls. A 75% or greater reduction occurred in 32.2% of the active-treatment group and 16.7% of those on placebo. And a decrease of at least 90% was seen in 23% of patients on topical GBP, compared with 9.5% of controls. All these between-group differences were significant.
The FDA now requires a quality of life measurement as a coprimary endpoint in phase 3 hyperhidrosis studies, and the phase 3 GBP trial also served as the successful validation study for a new patient-reported quality of life instrument designed specifically for this purpose. The new tool, known as the Hyperhydrosis Quality of Life questionnaire (HidroQol), proved much more sensitive than the HDSS or DLQI for evaluating clinical improvement in response to treatment (Br J Dermatol. 2020 Jun 8. doi: 10.1111/bjd.19300).
Initial results from the long-term phase 3b safety study should be available this fall on the first 100 patients followed on topical GBP for 1 year and for 300 followed for 6 months, Dr. Abels said.
Dr. Fujimoto reported serving as a paid consultant to and speaker for Kaken Pharmaceutical, which is developing SBP gel with Brickell Biotech. Dr. Abels is an employee of the company that is developing GPB cream.
FROM AAD 20
Tendyne transcatheter mitral valve shows sustained benefits at 2 years
Two-year outcomes following transcatheter mitral valve replacement with the Tendyne prosthesis showed durable control of mitral regurgitation, sustained improvement in quality of life and functional capacity, and a marked drop-off in the rate of hospitalization for heart failure, David W.M. Muller, MBBS, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The outcomes can be considered very acceptable considering the advanced age and underlying comorbidities. The data show that, at 2 years, we still have excellent control of the MR [mitral regurgitation], with more than 93% of patients having no MR at all, and there were no patients with 1+ or greater MR,” said Dr. Muller, director of cardiac catheterization laboratories at St. Vincent’s Hospital in Sydney.
He presented for the first time the 2-year results for the first 100 patients enrolled in the long-term Tendyne Expanded Clinical Study. The 1-year outcomes were reported previously (J Am Coll Cardiol. 2019 Mar 26;73[11]:1250-60).
The transcatheter Tendyne mitral valve system received European marketing approval in early 2020 for commercial use in patients with severe symptomatic MR who aren’t candidates for open surgery or transcatheter mitral repair using the MitraClip device. The Tendyne remains investigational in the United States, where a large phase 3 randomized head-to-head trial of the Tendyne device and the FDA-approved MitraClip is ongoing.
At enrollment, the first 100 patients in the long-term prospective Tendyne study averaged 75 years of age, 66% were New York Heart Association functional class III or IV, 89% had secondary or mixed etiology MR, and 39% had been hospitalized for heart failure during the preceding 6 months. Ninety-two percent of participants had 4+ MR before implantation. The group’s average Society of Thoracic Surgeons Predicted Risk of Mortality score was 7.8%.
The all-cause mortality rate was 27% at 1 year and 39% at 2 years, with 87% of deaths being attributable to cardiovascular causes. At 2 years, 82% of survivors were NYHA class I or II. Their Kansas City Cardiomyopathy Questionnaire score improved by 19.1 points from an average of 49 at baseline. There was no evidence of structural valve dysfunction. Their heart failure hospitalization rate improved from 1.3 events per patient per year to 0.6 per patient-year at 1 year and 0.51 at 2 years.
Discussant Francesco Maisano, MD, was favorably impressed by the acute outcomes of transcatheter mitral valve replacement with the Tendyne device.
“There were very few procedural or in-hospital complications. Success was obtained in almost every patient. There was no procedural mortality. The 30-day mortality of 6.0% was reasonable in patients with a baseline STS score of 7.8%; that’s pretty good, I would say,” observed Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
However, he voiced concerns about the 35% incidence of major bleeding and 5% rate of disabling stroke at 2 years, which he deemed “pretty high.”
“This underlies the open issue of anticoagulation following these kinds of procedures. This obviously requires further work in the next years,” he said.
Dr. Muller reported receiving research grants from and serving as a consultant to Abbott, which markets the MitraClip and is developing the Tendyne system. He also serves as a consultant to Edwards Lifesciences and Medtronic. Dr. Maisano also reported serving as a consultant to several medical device companies.
Two-year outcomes following transcatheter mitral valve replacement with the Tendyne prosthesis showed durable control of mitral regurgitation, sustained improvement in quality of life and functional capacity, and a marked drop-off in the rate of hospitalization for heart failure, David W.M. Muller, MBBS, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The outcomes can be considered very acceptable considering the advanced age and underlying comorbidities. The data show that, at 2 years, we still have excellent control of the MR [mitral regurgitation], with more than 93% of patients having no MR at all, and there were no patients with 1+ or greater MR,” said Dr. Muller, director of cardiac catheterization laboratories at St. Vincent’s Hospital in Sydney.
He presented for the first time the 2-year results for the first 100 patients enrolled in the long-term Tendyne Expanded Clinical Study. The 1-year outcomes were reported previously (J Am Coll Cardiol. 2019 Mar 26;73[11]:1250-60).
The transcatheter Tendyne mitral valve system received European marketing approval in early 2020 for commercial use in patients with severe symptomatic MR who aren’t candidates for open surgery or transcatheter mitral repair using the MitraClip device. The Tendyne remains investigational in the United States, where a large phase 3 randomized head-to-head trial of the Tendyne device and the FDA-approved MitraClip is ongoing.
At enrollment, the first 100 patients in the long-term prospective Tendyne study averaged 75 years of age, 66% were New York Heart Association functional class III or IV, 89% had secondary or mixed etiology MR, and 39% had been hospitalized for heart failure during the preceding 6 months. Ninety-two percent of participants had 4+ MR before implantation. The group’s average Society of Thoracic Surgeons Predicted Risk of Mortality score was 7.8%.
The all-cause mortality rate was 27% at 1 year and 39% at 2 years, with 87% of deaths being attributable to cardiovascular causes. At 2 years, 82% of survivors were NYHA class I or II. Their Kansas City Cardiomyopathy Questionnaire score improved by 19.1 points from an average of 49 at baseline. There was no evidence of structural valve dysfunction. Their heart failure hospitalization rate improved from 1.3 events per patient per year to 0.6 per patient-year at 1 year and 0.51 at 2 years.
Discussant Francesco Maisano, MD, was favorably impressed by the acute outcomes of transcatheter mitral valve replacement with the Tendyne device.
“There were very few procedural or in-hospital complications. Success was obtained in almost every patient. There was no procedural mortality. The 30-day mortality of 6.0% was reasonable in patients with a baseline STS score of 7.8%; that’s pretty good, I would say,” observed Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
However, he voiced concerns about the 35% incidence of major bleeding and 5% rate of disabling stroke at 2 years, which he deemed “pretty high.”
“This underlies the open issue of anticoagulation following these kinds of procedures. This obviously requires further work in the next years,” he said.
Dr. Muller reported receiving research grants from and serving as a consultant to Abbott, which markets the MitraClip and is developing the Tendyne system. He also serves as a consultant to Edwards Lifesciences and Medtronic. Dr. Maisano also reported serving as a consultant to several medical device companies.
Two-year outcomes following transcatheter mitral valve replacement with the Tendyne prosthesis showed durable control of mitral regurgitation, sustained improvement in quality of life and functional capacity, and a marked drop-off in the rate of hospitalization for heart failure, David W.M. Muller, MBBS, MD, reported at the virtual annual meeting of the European Association of Percutaneous Cardiovascular Interventions.
“The outcomes can be considered very acceptable considering the advanced age and underlying comorbidities. The data show that, at 2 years, we still have excellent control of the MR [mitral regurgitation], with more than 93% of patients having no MR at all, and there were no patients with 1+ or greater MR,” said Dr. Muller, director of cardiac catheterization laboratories at St. Vincent’s Hospital in Sydney.
He presented for the first time the 2-year results for the first 100 patients enrolled in the long-term Tendyne Expanded Clinical Study. The 1-year outcomes were reported previously (J Am Coll Cardiol. 2019 Mar 26;73[11]:1250-60).
The transcatheter Tendyne mitral valve system received European marketing approval in early 2020 for commercial use in patients with severe symptomatic MR who aren’t candidates for open surgery or transcatheter mitral repair using the MitraClip device. The Tendyne remains investigational in the United States, where a large phase 3 randomized head-to-head trial of the Tendyne device and the FDA-approved MitraClip is ongoing.
At enrollment, the first 100 patients in the long-term prospective Tendyne study averaged 75 years of age, 66% were New York Heart Association functional class III or IV, 89% had secondary or mixed etiology MR, and 39% had been hospitalized for heart failure during the preceding 6 months. Ninety-two percent of participants had 4+ MR before implantation. The group’s average Society of Thoracic Surgeons Predicted Risk of Mortality score was 7.8%.
The all-cause mortality rate was 27% at 1 year and 39% at 2 years, with 87% of deaths being attributable to cardiovascular causes. At 2 years, 82% of survivors were NYHA class I or II. Their Kansas City Cardiomyopathy Questionnaire score improved by 19.1 points from an average of 49 at baseline. There was no evidence of structural valve dysfunction. Their heart failure hospitalization rate improved from 1.3 events per patient per year to 0.6 per patient-year at 1 year and 0.51 at 2 years.
Discussant Francesco Maisano, MD, was favorably impressed by the acute outcomes of transcatheter mitral valve replacement with the Tendyne device.
“There were very few procedural or in-hospital complications. Success was obtained in almost every patient. There was no procedural mortality. The 30-day mortality of 6.0% was reasonable in patients with a baseline STS score of 7.8%; that’s pretty good, I would say,” observed Dr. Maisano, professor of cardiac surgery at the University of Zürich and a pioneer of catheter-based mitral and tricuspid interventions.
However, he voiced concerns about the 35% incidence of major bleeding and 5% rate of disabling stroke at 2 years, which he deemed “pretty high.”
“This underlies the open issue of anticoagulation following these kinds of procedures. This obviously requires further work in the next years,” he said.
Dr. Muller reported receiving research grants from and serving as a consultant to Abbott, which markets the MitraClip and is developing the Tendyne system. He also serves as a consultant to Edwards Lifesciences and Medtronic. Dr. Maisano also reported serving as a consultant to several medical device companies.
FROM EUROPCR 2020