User login
In reply: Intensive therapy of type 2 diabetes (ACCORD trial)
In Reply: Dr. Najman’s concern about reasons for the slight mortality increase in the intensively treated group in ACCORD1 resonates with all of the ACCORD investigators and clinicians. However, several features of the ACCORD trial should provide reassurance about his concerns.
At the time of protocol development, it was recognized that the complexity of the protocol was such that some issues, including medication combinations, might generate safety concerns.2 The ACCORD trial—like all appropriately designed large clinical trials—has a data safety and monitoring board. The ACCORD data safety and monitoring board is composed of people with extensive experience in the conduct and analysis of clinical trials. Among its roles are ongoing evaluation of the conduct of the trial (to ensure adherence to the protocol), determination of whether the trial has achieved efficacy outcomes (based on predetermined stopping rules), and judgments as to whether there are any safety concerns. The board may request any analyses it deems necessary for the safe conduct of the trial. The board meets regularly and reports regularly to the National Heart, Lung, and Blood Institute (NHLBI) project office.
The ACCORD data safety and monitoring board has been very attentive to issues that may have been of concern during the course of the trial. Most notably, when the report by Drs. Nissen and Wolski about rosiglitazone (Avandia) was published,3 the board requested interim analyses of the ACCORD data for their review. It reported that rosiglitazone use in the ACCORD trial was not associated with a risk of increased cardiovascular events or death. The fact that they recommended to the NHLBI that the intensive glucose arm be closed early also attests to their care in ensuring the integrity of the ACCORD trial and the safety of each study participant.
Although the details of the board’s discussions are not made available to investigators (or to the public), I am quite certain that the concern about the combination of PPAR alpha and gamma agonists is on their radar screen. And in the absence of safety concerns from the ACCORD data safety and monitoring board, it would be inappropriate to report any analyses to address the question raised by Dr. Najman prior to the closure of the lipid arm in ACCORD.
Dr. Drake raises a question for which there is no easy answer. We do not know how much weight gain actually contributes to coronary heart disease risk and mortality in a group of patients whose risk factors are otherwise well treated. Weight gain is clearly associated with increasing blood pressure, more adverse lipid profiles, and probably increased nontraditional risk markers, including high-sensitivity C-reactive protein and plasminogen activator inhibitor-1. The former risk factors can be treated with additional medication, and the effects of the latter are uncertain. Thus the writer raises an excellent question, but one that does not readily lend itself to a clearly quantifiable answer for “how much weight gain is too much?”
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
In Reply: Dr. Najman’s concern about reasons for the slight mortality increase in the intensively treated group in ACCORD1 resonates with all of the ACCORD investigators and clinicians. However, several features of the ACCORD trial should provide reassurance about his concerns.
At the time of protocol development, it was recognized that the complexity of the protocol was such that some issues, including medication combinations, might generate safety concerns.2 The ACCORD trial—like all appropriately designed large clinical trials—has a data safety and monitoring board. The ACCORD data safety and monitoring board is composed of people with extensive experience in the conduct and analysis of clinical trials. Among its roles are ongoing evaluation of the conduct of the trial (to ensure adherence to the protocol), determination of whether the trial has achieved efficacy outcomes (based on predetermined stopping rules), and judgments as to whether there are any safety concerns. The board may request any analyses it deems necessary for the safe conduct of the trial. The board meets regularly and reports regularly to the National Heart, Lung, and Blood Institute (NHLBI) project office.
The ACCORD data safety and monitoring board has been very attentive to issues that may have been of concern during the course of the trial. Most notably, when the report by Drs. Nissen and Wolski about rosiglitazone (Avandia) was published,3 the board requested interim analyses of the ACCORD data for their review. It reported that rosiglitazone use in the ACCORD trial was not associated with a risk of increased cardiovascular events or death. The fact that they recommended to the NHLBI that the intensive glucose arm be closed early also attests to their care in ensuring the integrity of the ACCORD trial and the safety of each study participant.
Although the details of the board’s discussions are not made available to investigators (or to the public), I am quite certain that the concern about the combination of PPAR alpha and gamma agonists is on their radar screen. And in the absence of safety concerns from the ACCORD data safety and monitoring board, it would be inappropriate to report any analyses to address the question raised by Dr. Najman prior to the closure of the lipid arm in ACCORD.
Dr. Drake raises a question for which there is no easy answer. We do not know how much weight gain actually contributes to coronary heart disease risk and mortality in a group of patients whose risk factors are otherwise well treated. Weight gain is clearly associated with increasing blood pressure, more adverse lipid profiles, and probably increased nontraditional risk markers, including high-sensitivity C-reactive protein and plasminogen activator inhibitor-1. The former risk factors can be treated with additional medication, and the effects of the latter are uncertain. Thus the writer raises an excellent question, but one that does not readily lend itself to a clearly quantifiable answer for “how much weight gain is too much?”
In Reply: Dr. Najman’s concern about reasons for the slight mortality increase in the intensively treated group in ACCORD1 resonates with all of the ACCORD investigators and clinicians. However, several features of the ACCORD trial should provide reassurance about his concerns.
At the time of protocol development, it was recognized that the complexity of the protocol was such that some issues, including medication combinations, might generate safety concerns.2 The ACCORD trial—like all appropriately designed large clinical trials—has a data safety and monitoring board. The ACCORD data safety and monitoring board is composed of people with extensive experience in the conduct and analysis of clinical trials. Among its roles are ongoing evaluation of the conduct of the trial (to ensure adherence to the protocol), determination of whether the trial has achieved efficacy outcomes (based on predetermined stopping rules), and judgments as to whether there are any safety concerns. The board may request any analyses it deems necessary for the safe conduct of the trial. The board meets regularly and reports regularly to the National Heart, Lung, and Blood Institute (NHLBI) project office.
The ACCORD data safety and monitoring board has been very attentive to issues that may have been of concern during the course of the trial. Most notably, when the report by Drs. Nissen and Wolski about rosiglitazone (Avandia) was published,3 the board requested interim analyses of the ACCORD data for their review. It reported that rosiglitazone use in the ACCORD trial was not associated with a risk of increased cardiovascular events or death. The fact that they recommended to the NHLBI that the intensive glucose arm be closed early also attests to their care in ensuring the integrity of the ACCORD trial and the safety of each study participant.
Although the details of the board’s discussions are not made available to investigators (or to the public), I am quite certain that the concern about the combination of PPAR alpha and gamma agonists is on their radar screen. And in the absence of safety concerns from the ACCORD data safety and monitoring board, it would be inappropriate to report any analyses to address the question raised by Dr. Najman prior to the closure of the lipid arm in ACCORD.
Dr. Drake raises a question for which there is no easy answer. We do not know how much weight gain actually contributes to coronary heart disease risk and mortality in a group of patients whose risk factors are otherwise well treated. Weight gain is clearly associated with increasing blood pressure, more adverse lipid profiles, and probably increased nontraditional risk markers, including high-sensitivity C-reactive protein and plasminogen activator inhibitor-1. The former risk factors can be treated with additional medication, and the effects of the latter are uncertain. Thus the writer raises an excellent question, but one that does not readily lend itself to a clearly quantifiable answer for “how much weight gain is too much?”
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356:2457–2471.
Does intensive therapy of type 2 diabetes help or harm? Seeking accord on ACCORD
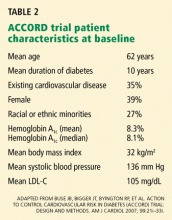
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial1–5 was designed primarily to address, in patients with type 2 diabetes at high risk of cardiovascular events, whether intensive glucose control would result in a lower risk of atherosclerotic disease events or death than would standard treatment.
It was widely expected that intensive treatment would confer either modest benefit or, at worst, no benefit. However, the glucose-lowering arm of the trial was terminated early because of a higher mortality rate in the intensively treated group. (The ACCORD trial has two other arms, which concern blood pressure and lipid-lowering, and these are continuing.)
In earlier trials in type 2 diabetes, concerns had been raised about an increased risk of cardiovascular events and possibly death associated with glucose-lowering drugs, hypoglycemia itself, or both, and these were well known when ACCORD was convened. ACCORD was very carefully designed and included careful adjudication of each cardiovascular event and death, including whether hypoglycemia might have been a proximate cause of some sudden deaths.5
Therefore, the surprising result of the higher mortality rate with intensive glycemic control in ACCORD will be fodder for discussion in many arenas over the next several years, and it poses some challenges for physicians and patients in determining treatment goals, as well as for organizations that write clinical practice guidelines (and perhaps organizations involved in pay-for-performance based on these guidelines).
Still, I believe that the ACCORD results should not substantially change our approach to treatment goals in type 2 diabetes, although hemoglobin A1c targets below 6% may not have much added value for cardiovascular risk reduction. The low overall mortality rate in all the arms of the ACCORD trial emphasizes the importance of lifestyle modification, lipid and blood pressure therapy, and encouragement of aspirin use in all patients with type 2 diabetes.
This article reflects my views as a practicing diabetologist and clinical trialist (I was an investigator in the ACCORD trial) with a long-standing interest in clinical trials and in how the results influence clinical practice. The views I express herein may not reflect the views of other ACCORD investigators, the National Heart, Lung, and Blood Institute (NHLBI), the ACCORD trial coordinating center at Wake Forest University, or its data safety and monitoring board.
RISK OF CORONARY DISEASE INCREASES WITH GLUCOSE
Many observational studies6–10 have shown that the risk of cardiovascular disease, especially coronary heart disease, is two to five times higher in people with diabetes mellitus than in people without diabetes. The risk appears to be continuous, so the higher one’s glucose or hemoglobin A1c, the higher the risk.6 This risk even extends to glucose values well below the threshold values currently used to diagnose diabetes mellitus.6 Since there is no glucose threshold for coronary heart disease, the term dysglycemia (rather than hyperglycemia) has been proposed to note the relationship between glucose and coronary heart disease. (The glucose threshold for microvascular complications of diabetes, such as retinopathy and nephropathy, appears to be between 110 and 126 mg/dL).
The clustering of multiple coronary risk factors such as obesity, dyslipidemia, and hypertension has always raised the question of whether glucose is a culprit in coronary risk or whether it simply “runs in bad company.”
EARLIER CLINICAL TRIALS SUGGEST INTENSIVE TREATMENT RAISES RISK
Even though it has been widely believed that intensive glucose-lowering would reduce cardiovascular risk in type 2 diabetes, there have been hints in previous studies that some intensive-treatment regimens might increase risk.
Two large randomized clinical trials and one small one (discussed below) addressed whether glucose control would reduce the risk of atherosclerotic vascular disease events. In each of them, an increased risk of cardiovascular events and possibly of death was seen in at least one intensively treated group.
In the following discussion, I have calculated all of the death rates as the number of deaths per 1,000 patients per year, based on published study results. In this way, we can compare the rates in the various studies (including ACCORD), regardless of the trial duration.
The university group Diabetes Program: Controvery about tolbutamide therapy
The University Group Diabetes Program (UGDP)11–16 included about 1,000 participants randomized to five treatments: tolbutamide (Orinase, a sulfonylurea), insulin in a fixed dose based on body weight, insulin in adjusted doses based on fasting glucose levels, placebo, and (later) phenformin.
In the 1970s, when the UGDP was carried out, randomized clinical trials were uncommon. Like other trials from that era, the UGDP was underpowered by today’s standards and did not have a data safety and monitoring board.
Rates of cardiovascular events and deaths (per 1,000 patient-years):
- 25 (tolbutamide group)
- 12 (placebo group).
The two insulin groups did not differ from the placebo group in their rates of cardiovascular events or death.15 The tolbutamide arm was stopped, and the ensuing controversy about how to interpret the trial results lasted for more than a decade. It also resulted in a black-box warning for tolbutamide and all subsequent sulfonylureas.
United Kingdom Prospective Diabetes Study: Method of glucose-lowering an issue
The United Kingdom Prospective Diabetes Study (UKPDS)17–27 was launched in 1977. A cohort of 5,102 patients (mean age 54 years) with newly diagnosed type 2 diabetes mellitus followed a “prudent diet” for the first 3 to 4 months. Then, if their fasting glucose levels were in the range of 6.1 to 15 mmol/L (110–270 mg/dL), they were randomized to receive various treatments.
Patients who were not obese were randomized to receive either intensive treatment or conventional treatment. The intensive-treatment group received either insulin or a sulfonylurea (chlorpropamide [Diabinese], glibenclamide, or glipizide [Glucotrol]); the conventional-treatment group received diet therapy. The sulfonylurea arm was included partly to address the UGDP results.
Patients who were obese were randomized to receive one of three treatments: intensive treatment (with the agents listed above), conventional treatment, or metformin (Fortamet, Glucophage).
The mean in-trial hemoglobin A1c level in the intensive-treatment group was 7.0%, compared with 7.9% in the conventional-treatment group.
After a mean follow-up of more than 10 years, the incidence of myocardial infarction was 16% lower in the intensive-treatment group, but the difference was not statistically significant (P = .052).
Rates of death from all causes among nonobese subjects (per 1,000 patient-years):
- 18.2–20.5 (intensive-treatment group)
- 19.9 (conventional-treatment group).
In the obese patients who received metformin, the incidence of myocardial infarction was lower than in the conventional-treatment group but not the intensive-treatment group.
Rates of death among obese patients (per 1,000 patient-years):
- 13.5 (metformin group)
- 18.9 (intensive-treatment group)
- 20.6 (conventional-treatment group).
However, a small subset (n = 587) of the original group assigned to sulfonylurea therapy whose glycemic control deteriorated during the trial were rerandomized to continue to receive a sulfonylurea alone or to have metformin added. There was a statistically significantly higher rate of cardiovascular events and a nonsignificantly higher rate of total mortality in the metformin-plus-sulfonylurea group (30.3 per 1,000 patient-years) than in the sulfonylurea-only group (19.1 per 1,000 patient-years).
These data suggested that the way glucose-lowering was achieved might be as important as the glucose levels actually achieved. However, no definite conclusions could be drawn.
In an editorial on the UKPDS, Nathan26 made a comment that may have been prescient in terms of the ACCORD trial: “Professional organizations will now scramble to decide how to translate the UKPDS results … Whether the UKPDS firmly establishes the choice of any one therapy…or any combination of therapies for the long-term treatment of type 2 diabetes is more questionable.”26
Veterans Administration feasibility study
A Veterans Administration feasibility study28,29 included 153 men (mean age 60) with type 2 diabetes (mean duration 7.8 years) who received either conventional therapy (a single daily dose of insulin) or intensive therapy (multiple doses of insulin plus a sulfonylurea). Over a mean of 27 months, the intensive-therapy group achieved a hemoglobin A1c level that was 2 percentage points lower than in the conventional-therapy group.
At 2.25 years of follow-up, cardiovascular events had occurred in 24 (24%) of the intensive-therapy group and in 16 (20%) of the standard-therapy group (P = .10).
Rates of death from all causes (per 1,000 patient-years):
- 28.9 (intensive-treatment group)
- 17.5 (conventional-treatment group).
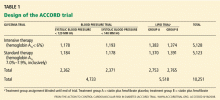
ACCORD TRIAL DESIGN
The primary outcome measured was the combined incidence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. Secondary outcomes included death from any cause. The study is also evaluating the effect of intensive treatment on microvascular disease, hypoglycemia, cognition, quality of life, and cost-effectiveness.
The ACCORD study was designed to have 89% power to detect a 15% treatment effect of intensive glycemic control compared with standard glycemic control for the primary end point.
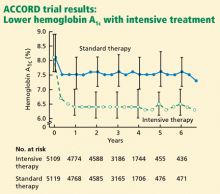
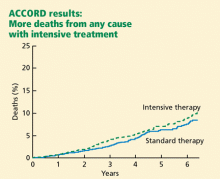
ACCORD RESULTS
Rates of death from any cause (per 1,000 patient-years):
- 14 (intensive-treatment group)
- 11 (standard-treatment group).
In the analyses available at the time that this study arm closed, the excess mortality was not attributable to any particular treatment regimen. In particular, rosiglitazone (Avandia) use did not contribute to the excess mortality. (Of note, 91.2% of the intensive-treatment group and 57.5% of the conventional-treatment group had been treated with rosiglitazone, with more than 19,000 patient-years of rosiglitazone exposure). The excess mortality was also not attributable to hypoglycemia immediately proximate to the death.
The ACCORD trial’s data safety and monitoring board recommended that this arm of the study be discontinued for safety reasons, and this recommendation was accepted by the NHLBI project office. All participants were notified by letter before the trial results were announced publicly, and all intensive-therapy group participants are now being treated by the protocol used in the standard-therapy group.1
FEWER DEATHS IN ACCORD THAN IN OTHER STUDIES IN DIABETES
The mortality rates in both arms of ACCORD were much lower than in other observational studies and clinical trials in type 2 diabetes.
The National Health and Nutrition Education Survey (NHANES),30 conducted from 1971 to 1975, included 14,374 people with diabetes between the ages of 25 and 74. Many of them were younger than the ACCORD patients, but two NHANES age-groups overlapped the ACCORD cohort. Rates of death from any cause at 22 years (per 1,000 patient-years):
- 39.7 (ages 45–64)
- 89.7 (ages 65–74).
The NHANES cohort would not have been treated as vigorously for coronary risk and other common causes of death.
UGDP, UKPDS. Death rates in the glucose-lowering trials of type 2 diabetes mellitus cited above were typically in the range of 20 deaths per 1,000 patient-years but were as high as 30 deaths per 1,000 patient-years in the UGDP tolbutamide group16 and the UK-PDS sulfonylurea-plus-metformin group.20,22,26
Steno-2.31 Half of 160 patients with type 2 diabetes were randomized to intensive strategies for controlling glucose, lipids, and blood pressure and for taking aspirin and angiotensin-converting enzyme inhibitors and following a healthy lifestyle. The other half received conventional therapy. Even in the intensive-treatment group, the mortality rate at 13 years was higher than in ACCORD. Rates of death from any cause (per 1,000 patient years):
- 22.5 (intensive-treatment group)
- 37.6 (conventional-treatment group).
After the ACCORD results were presented, two other trials addressing the question of whether lower hemoglobin A1c would reduce cardiovascular risk in type 2 diabetes have reported their outcomes:
The ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation),32,33 with 11,140 patients, had a target hemoglobin A1c of 6.5% in an intensive-treatment group and 7.3% in a usual-treatment group. The intensive-treatment group showed no difference in the rates of major macrovascular events (HR 0.94, 95% CI 0.84–1.06, P = .32) or all-cause mortality (HR 0.93, 95% CI 0.83–1.06, P = .32). The overall death rate in ADVANCE (about 18 deaths per 1,000 patient-years) was higher than in ACCORD.
The Veterans Administration Diabetes Trial included 1,791 patients.34 Like the ADVANCE trial, it also found no difference in major cardiovascular outcomes (HR 0.868, P = .11) or cardiovascular mortality rates (HR 1.258, P = .36) with intensive therapy vs conventional therapy, ie, achieved hemoglobin A1c levels of 6.9% vs 8.4% (presented at the American Diabetes Association 2008 Scientific Sessions). Hypoglycemia was associated with an increased risk of death in the standard-treatment group.
An analysis suggested that patients with a shorter duration of diabetes may have had cardiovascular benefit from intensive glucose-lowering, while those who had had it longer may have had increased risk associated with the more intensive therapy. The rate of death from all causes appears to have been higher than in ACCORD, but this could not be determined accurately from the presentations.
Comment. Thus, the ACCORD cohort as a whole has had strikingly lower death rates than in these other studies. The fact that all participants had lower glucose levels on therapy than at baseline may possibly contribute to these lower death rates. In addition, all ACCORD participants in the lipid arm received a statin; all participants in the blood pressure arm had their blood pressure lowered to levels below those commonly seen in clinical practice; participants were encouraged to exercise regularly; most participants were given diet instruction; and other healthy behaviors such as aspirin use, regular follow-up with primary care physicians, and recommendations about smoking were encouraged throughout the study. These comprehensive strategies may represent better care and thus result in lower death rates than in other studies.
POSSIBLE EXPLANATIONS FOR THE ACCORD OUTCOMES
The ACCORD trial has already stimulated fierce debate about the reasons for the higher mortality rate in the intensive-treatment group. With longer follow-up, some new risk factors for death may be identified that are not evident in the analyses of the current 460 deaths. What follows are some of my thoughts, with the caveat that they are not confirmed (supported statistically) by any currently available analyses from ACCORD.
It seems unlikely that lower glucose values as reflected by lower hemoglobin A1c values in the intensive-treatment group are an a priori explanation for the observed differences in mortality rates—especially since the mortality rates were lower than in the NHANES and clinical trial data sets cited above. If we assume that a type 1 statistical error (finding a difference where no difference actually exists) does not explain the findings, then at least four reasonable postulates exist:
Hypoglycemia may have some adverse effect, either acutely or from recurrent events that trigger a catecholamine response with associated risk for arrhythmia or increased coronary heart disease risk. However, the investigators analyzed each death to determine whether hypoglycemia was a contributing cause, and they found no statistically significant relationship between hypoglycemia and death in the intensive-treatment group.
Weight gain is common with intensive therapy. Obesity may be associated with greater cytokine production, higher concentrations of clotting factors, higher levels of free fatty acids, and other potential contributors to the risk of coronary heart disease and death. Currently, the ACCORD analyses do not suggest that weight gain explains the higher death rate.
Medications such as rosiglitazone, sulfonylureas, and the combination of a sulfonylurea plus metformin have been previously associated with increased death rates in some observational and intervention trials. These studies had some serious methodologic limitations (eg, absence of risk adjustment, events not adjudicated, small study cohorts, wide variation in study cohort characteristics) and small numbers of events.11–13,16,26,35 ACCORD analyses have not shown that any single glucose-lowering agent—including rosiglitazone—or combination of agents explains the death rates.
The stress of maintaining glycemic control has been speculated to have in some way contributed to an increased risk. To achieve intensive control, patients had to have frequent contact with their health care providers, they were often told that their hemoglobin A1c values were “too high” even when they were well below those in the American Diabetes Association guidelines, and they had to follow complex glucose-lowering regimens.
Semiquantitative measures of overall attitudes about health exist (eg, the “Feeling Thermometer” scale), but stress was not measured quantitatively in the ACCORD trial.
IMPLICATIONS OF ACCORD
In practice, most clinicians believe that the target glucose level in patients with type 2 diabetes should be as low as safely possible. This approach does not need to be modified on the basis of current information from ACCORD.
To be safe, regimens should be associated with a low risk of hypoglycemia and a low risk of weight gain. Use of combinations of medications that work by different mechanisms is still prudent. Agents should be used that may have favorable effects on other cardiovascular risk factors (eg, lipids, blood pressure, visceral fat).
Hemoglobin A1c targets below 7% are not precluded in all patients on the basis of the ACCORD results, though values lower than 6% may not have much added benefit for cardiovascular risk reduction. We should note that hemoglobin A1c was reduced in all ACCORD participants and that death rates were lower than in many other type 2 diabetic cohorts. Pending data on other outcomes in ACCORD (nephropathy, retinopathy, dementia, fracture risk), I believe it is premature for organizations to change their proposed hemoglobin A1c targets,36,37 as none have proposed values as low as the target in the ACCORD intensive-treatment group. At present, no class of glucose-lowering agents needs to be excluded from consideration on the basis of the ACCORD data.
The overall low rates of death in this population at high risk of coronary heart disease deserve comment. Not only are they lower than in other glucose-lowering trials, but they are also lower than in a number of studies of mortality in diabetes cohorts. As noted above, multiple risk factors for coronary heart disease and death were (and are) addressed in the ACCORD study participants, including repeated recommendation for lifestyle modification, intervention arms with lipid and blood pressure therapy, encouragement of aspirin use, and regular follow-up with health care providers for risk factors not managed by the ACCORD trial protocol. It is likely that multiple approaches to reducing the risk of cardiovascular disease contributed to this low mortality rate and that similar approaches will reduce the risk of coronary disease and death in regular clinical practice.
The ACCORD lipid and blood pressure arms are continuing, with results expected in 2010. The future results from ACCORD as well as from several glucose-lowering trials currently in progress (ADVANCE,32,33 Veteran’s Administration,34 Bypass Angioplasty Revascularization Investigation 2 Diabetes [BARI-2D]38) will likely help refine our understanding of the effects of glucose-lowering, glucose-lowering strategies and targets, and multiple interventions on coronary events and all-cause mortality.
For now, any strategy that lowers glucose and is associated with a low risk of hypoglycemia and does not cause excessive weight gain should be considered appropriate in patients with type 2 diabetes.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Goff DC, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:4i–20i.
- Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i.
- Gerstein HC, Riddle MC, Kendall DM, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:34i–43i.
- Bonds DE, Kurashige EM, Bergenstal R, et al. Severe hypoglycemia monitoring and risk management procedures in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:80i–89i.
- Gerstein HC. Dysglycemia, not just diabetes, is a continuous risk factor for cardiovascular disease. Evid Based Cardiovasc Med. 1997; 1:87–88.
- Gerstein HC, Pais P, Pogue J, Yusuf S. Relationship of glucose and insulin levels to the risk of myocardial infarction: a case-control study. J Am Coll Cardiol. 1999; 33:612–619.
- Gerstein HC, Capes SE. Dysglycemia: a key cardiovascular risk factor. Semin Vasc Med. 2002; 2:165–174.
- Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007; 78:305–312.
- American Diabetes Association. Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care. 1989; 12:573–579.
- Schor S. The University Group Diabetes Program. A statistician looks at the mortality results. JAMA. 1971; 217:1671–1675.
- Cornfield JThe University Group Diabetes Program. A further statistical analysis of the mortality findings. JAMA. 1971; 217:1676–1687.
- Feinstein AR. Clinical biostatistics. 8. An analytic appraisal of the University Group Diabetes Program (UGDP) study. Clin Pharmacol Ther. 1971; 12:167–191.
- The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of pheniformin therapy. Diabetes 1975; 24( suppl 1):65–184.
- Knatterud GL, Klimt CR, Levin ME, Jacobson ME, Goldner MG. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA. 1978; 240:37–42.
- Schwartz TB, Meinert CL. The UGDP controversy: thirty-four years of contentious ambiguity laid to rest. Perspect Biol Med. 2004; 47:564–574.
- Turner RC, Holman RR. Lessons from UK Prospective Diabetes Study. Diabetes Res Clin Pract 1995; 28( suppl):S151–S157.
- UKPDS Research Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:854–865.
- UKPDS Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:837–853.
- UK Prospective Diabetes Study Group. UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. Diabetes Care. 1998; 21:87–92.
- Bretzel RG, Voigt K, Schatz H. The United Kingdom Prospective Diabetes Study (UKPDS) implications for the pharmacotherapy of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 1998; 106:369–372.
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999; 281:2005–2012.
- Leslie RD. United Kingdom prospective diabetes study (UKPDS): what now or so what? Diabetes Metab Res Rev 1999; 15:65–71.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.
- Mooradian AD, Chehade J. Implications of the UK Prospective Diabetes Study: questions answered and issues remaining. Drugs Aging. 2000; 16:159–164.
- Nathan DM. Some answers, more controversy, from UKPDS. United Kingdom Prospective Diabetes Study. Lancet. 1998; 352:832–833.
- Srimanunthiphol J, Beddow R, Arakaki R. A review of the United Kingdom Prospective Diabetes Study (UKPDS) and a discussion of the implications for patient care. Hawaii Med J. 2000; 59:295–298.
- Duckworth WC, McCarren M, Abraira C. Glucose control and cardiovascular complications: the VA Diabetes Trial. Diabetes Care. 2001; 24:942–945.
- Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995; 18:1113–1123.
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998; 21:1138–1145. NHANES
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008; 358:580–591.
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572.
- Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation. Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. J Hypertens 2001; 19(suppl):S21–S28.
- Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003; 17:314–322.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):1–68.
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008; 31(suppl 1):S12–S54.
- Magee MF, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006; 97:20G–30G.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial1–5 was designed primarily to address, in patients with type 2 diabetes at high risk of cardiovascular events, whether intensive glucose control would result in a lower risk of atherosclerotic disease events or death than would standard treatment.
It was widely expected that intensive treatment would confer either modest benefit or, at worst, no benefit. However, the glucose-lowering arm of the trial was terminated early because of a higher mortality rate in the intensively treated group. (The ACCORD trial has two other arms, which concern blood pressure and lipid-lowering, and these are continuing.)
In earlier trials in type 2 diabetes, concerns had been raised about an increased risk of cardiovascular events and possibly death associated with glucose-lowering drugs, hypoglycemia itself, or both, and these were well known when ACCORD was convened. ACCORD was very carefully designed and included careful adjudication of each cardiovascular event and death, including whether hypoglycemia might have been a proximate cause of some sudden deaths.5
Therefore, the surprising result of the higher mortality rate with intensive glycemic control in ACCORD will be fodder for discussion in many arenas over the next several years, and it poses some challenges for physicians and patients in determining treatment goals, as well as for organizations that write clinical practice guidelines (and perhaps organizations involved in pay-for-performance based on these guidelines).
Still, I believe that the ACCORD results should not substantially change our approach to treatment goals in type 2 diabetes, although hemoglobin A1c targets below 6% may not have much added value for cardiovascular risk reduction. The low overall mortality rate in all the arms of the ACCORD trial emphasizes the importance of lifestyle modification, lipid and blood pressure therapy, and encouragement of aspirin use in all patients with type 2 diabetes.
This article reflects my views as a practicing diabetologist and clinical trialist (I was an investigator in the ACCORD trial) with a long-standing interest in clinical trials and in how the results influence clinical practice. The views I express herein may not reflect the views of other ACCORD investigators, the National Heart, Lung, and Blood Institute (NHLBI), the ACCORD trial coordinating center at Wake Forest University, or its data safety and monitoring board.
RISK OF CORONARY DISEASE INCREASES WITH GLUCOSE
Many observational studies6–10 have shown that the risk of cardiovascular disease, especially coronary heart disease, is two to five times higher in people with diabetes mellitus than in people without diabetes. The risk appears to be continuous, so the higher one’s glucose or hemoglobin A1c, the higher the risk.6 This risk even extends to glucose values well below the threshold values currently used to diagnose diabetes mellitus.6 Since there is no glucose threshold for coronary heart disease, the term dysglycemia (rather than hyperglycemia) has been proposed to note the relationship between glucose and coronary heart disease. (The glucose threshold for microvascular complications of diabetes, such as retinopathy and nephropathy, appears to be between 110 and 126 mg/dL).
The clustering of multiple coronary risk factors such as obesity, dyslipidemia, and hypertension has always raised the question of whether glucose is a culprit in coronary risk or whether it simply “runs in bad company.”
EARLIER CLINICAL TRIALS SUGGEST INTENSIVE TREATMENT RAISES RISK
Even though it has been widely believed that intensive glucose-lowering would reduce cardiovascular risk in type 2 diabetes, there have been hints in previous studies that some intensive-treatment regimens might increase risk.
Two large randomized clinical trials and one small one (discussed below) addressed whether glucose control would reduce the risk of atherosclerotic vascular disease events. In each of them, an increased risk of cardiovascular events and possibly of death was seen in at least one intensively treated group.
In the following discussion, I have calculated all of the death rates as the number of deaths per 1,000 patients per year, based on published study results. In this way, we can compare the rates in the various studies (including ACCORD), regardless of the trial duration.
The university group Diabetes Program: Controvery about tolbutamide therapy
The University Group Diabetes Program (UGDP)11–16 included about 1,000 participants randomized to five treatments: tolbutamide (Orinase, a sulfonylurea), insulin in a fixed dose based on body weight, insulin in adjusted doses based on fasting glucose levels, placebo, and (later) phenformin.
In the 1970s, when the UGDP was carried out, randomized clinical trials were uncommon. Like other trials from that era, the UGDP was underpowered by today’s standards and did not have a data safety and monitoring board.
Rates of cardiovascular events and deaths (per 1,000 patient-years):
- 25 (tolbutamide group)
- 12 (placebo group).
The two insulin groups did not differ from the placebo group in their rates of cardiovascular events or death.15 The tolbutamide arm was stopped, and the ensuing controversy about how to interpret the trial results lasted for more than a decade. It also resulted in a black-box warning for tolbutamide and all subsequent sulfonylureas.
United Kingdom Prospective Diabetes Study: Method of glucose-lowering an issue
The United Kingdom Prospective Diabetes Study (UKPDS)17–27 was launched in 1977. A cohort of 5,102 patients (mean age 54 years) with newly diagnosed type 2 diabetes mellitus followed a “prudent diet” for the first 3 to 4 months. Then, if their fasting glucose levels were in the range of 6.1 to 15 mmol/L (110–270 mg/dL), they were randomized to receive various treatments.
Patients who were not obese were randomized to receive either intensive treatment or conventional treatment. The intensive-treatment group received either insulin or a sulfonylurea (chlorpropamide [Diabinese], glibenclamide, or glipizide [Glucotrol]); the conventional-treatment group received diet therapy. The sulfonylurea arm was included partly to address the UGDP results.
Patients who were obese were randomized to receive one of three treatments: intensive treatment (with the agents listed above), conventional treatment, or metformin (Fortamet, Glucophage).
The mean in-trial hemoglobin A1c level in the intensive-treatment group was 7.0%, compared with 7.9% in the conventional-treatment group.
After a mean follow-up of more than 10 years, the incidence of myocardial infarction was 16% lower in the intensive-treatment group, but the difference was not statistically significant (P = .052).
Rates of death from all causes among nonobese subjects (per 1,000 patient-years):
- 18.2–20.5 (intensive-treatment group)
- 19.9 (conventional-treatment group).
In the obese patients who received metformin, the incidence of myocardial infarction was lower than in the conventional-treatment group but not the intensive-treatment group.
Rates of death among obese patients (per 1,000 patient-years):
- 13.5 (metformin group)
- 18.9 (intensive-treatment group)
- 20.6 (conventional-treatment group).
However, a small subset (n = 587) of the original group assigned to sulfonylurea therapy whose glycemic control deteriorated during the trial were rerandomized to continue to receive a sulfonylurea alone or to have metformin added. There was a statistically significantly higher rate of cardiovascular events and a nonsignificantly higher rate of total mortality in the metformin-plus-sulfonylurea group (30.3 per 1,000 patient-years) than in the sulfonylurea-only group (19.1 per 1,000 patient-years).
These data suggested that the way glucose-lowering was achieved might be as important as the glucose levels actually achieved. However, no definite conclusions could be drawn.
In an editorial on the UKPDS, Nathan26 made a comment that may have been prescient in terms of the ACCORD trial: “Professional organizations will now scramble to decide how to translate the UKPDS results … Whether the UKPDS firmly establishes the choice of any one therapy…or any combination of therapies for the long-term treatment of type 2 diabetes is more questionable.”26
Veterans Administration feasibility study
A Veterans Administration feasibility study28,29 included 153 men (mean age 60) with type 2 diabetes (mean duration 7.8 years) who received either conventional therapy (a single daily dose of insulin) or intensive therapy (multiple doses of insulin plus a sulfonylurea). Over a mean of 27 months, the intensive-therapy group achieved a hemoglobin A1c level that was 2 percentage points lower than in the conventional-therapy group.
At 2.25 years of follow-up, cardiovascular events had occurred in 24 (24%) of the intensive-therapy group and in 16 (20%) of the standard-therapy group (P = .10).
Rates of death from all causes (per 1,000 patient-years):
- 28.9 (intensive-treatment group)
- 17.5 (conventional-treatment group).
ACCORD TRIAL DESIGN
The primary outcome measured was the combined incidence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. Secondary outcomes included death from any cause. The study is also evaluating the effect of intensive treatment on microvascular disease, hypoglycemia, cognition, quality of life, and cost-effectiveness.
The ACCORD study was designed to have 89% power to detect a 15% treatment effect of intensive glycemic control compared with standard glycemic control for the primary end point.
ACCORD RESULTS
Rates of death from any cause (per 1,000 patient-years):
- 14 (intensive-treatment group)
- 11 (standard-treatment group).
In the analyses available at the time that this study arm closed, the excess mortality was not attributable to any particular treatment regimen. In particular, rosiglitazone (Avandia) use did not contribute to the excess mortality. (Of note, 91.2% of the intensive-treatment group and 57.5% of the conventional-treatment group had been treated with rosiglitazone, with more than 19,000 patient-years of rosiglitazone exposure). The excess mortality was also not attributable to hypoglycemia immediately proximate to the death.
The ACCORD trial’s data safety and monitoring board recommended that this arm of the study be discontinued for safety reasons, and this recommendation was accepted by the NHLBI project office. All participants were notified by letter before the trial results were announced publicly, and all intensive-therapy group participants are now being treated by the protocol used in the standard-therapy group.1
FEWER DEATHS IN ACCORD THAN IN OTHER STUDIES IN DIABETES
The mortality rates in both arms of ACCORD were much lower than in other observational studies and clinical trials in type 2 diabetes.
The National Health and Nutrition Education Survey (NHANES),30 conducted from 1971 to 1975, included 14,374 people with diabetes between the ages of 25 and 74. Many of them were younger than the ACCORD patients, but two NHANES age-groups overlapped the ACCORD cohort. Rates of death from any cause at 22 years (per 1,000 patient-years):
- 39.7 (ages 45–64)
- 89.7 (ages 65–74).
The NHANES cohort would not have been treated as vigorously for coronary risk and other common causes of death.
UGDP, UKPDS. Death rates in the glucose-lowering trials of type 2 diabetes mellitus cited above were typically in the range of 20 deaths per 1,000 patient-years but were as high as 30 deaths per 1,000 patient-years in the UGDP tolbutamide group16 and the UK-PDS sulfonylurea-plus-metformin group.20,22,26
Steno-2.31 Half of 160 patients with type 2 diabetes were randomized to intensive strategies for controlling glucose, lipids, and blood pressure and for taking aspirin and angiotensin-converting enzyme inhibitors and following a healthy lifestyle. The other half received conventional therapy. Even in the intensive-treatment group, the mortality rate at 13 years was higher than in ACCORD. Rates of death from any cause (per 1,000 patient years):
- 22.5 (intensive-treatment group)
- 37.6 (conventional-treatment group).
After the ACCORD results were presented, two other trials addressing the question of whether lower hemoglobin A1c would reduce cardiovascular risk in type 2 diabetes have reported their outcomes:
The ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation),32,33 with 11,140 patients, had a target hemoglobin A1c of 6.5% in an intensive-treatment group and 7.3% in a usual-treatment group. The intensive-treatment group showed no difference in the rates of major macrovascular events (HR 0.94, 95% CI 0.84–1.06, P = .32) or all-cause mortality (HR 0.93, 95% CI 0.83–1.06, P = .32). The overall death rate in ADVANCE (about 18 deaths per 1,000 patient-years) was higher than in ACCORD.
The Veterans Administration Diabetes Trial included 1,791 patients.34 Like the ADVANCE trial, it also found no difference in major cardiovascular outcomes (HR 0.868, P = .11) or cardiovascular mortality rates (HR 1.258, P = .36) with intensive therapy vs conventional therapy, ie, achieved hemoglobin A1c levels of 6.9% vs 8.4% (presented at the American Diabetes Association 2008 Scientific Sessions). Hypoglycemia was associated with an increased risk of death in the standard-treatment group.
An analysis suggested that patients with a shorter duration of diabetes may have had cardiovascular benefit from intensive glucose-lowering, while those who had had it longer may have had increased risk associated with the more intensive therapy. The rate of death from all causes appears to have been higher than in ACCORD, but this could not be determined accurately from the presentations.
Comment. Thus, the ACCORD cohort as a whole has had strikingly lower death rates than in these other studies. The fact that all participants had lower glucose levels on therapy than at baseline may possibly contribute to these lower death rates. In addition, all ACCORD participants in the lipid arm received a statin; all participants in the blood pressure arm had their blood pressure lowered to levels below those commonly seen in clinical practice; participants were encouraged to exercise regularly; most participants were given diet instruction; and other healthy behaviors such as aspirin use, regular follow-up with primary care physicians, and recommendations about smoking were encouraged throughout the study. These comprehensive strategies may represent better care and thus result in lower death rates than in other studies.
POSSIBLE EXPLANATIONS FOR THE ACCORD OUTCOMES
The ACCORD trial has already stimulated fierce debate about the reasons for the higher mortality rate in the intensive-treatment group. With longer follow-up, some new risk factors for death may be identified that are not evident in the analyses of the current 460 deaths. What follows are some of my thoughts, with the caveat that they are not confirmed (supported statistically) by any currently available analyses from ACCORD.
It seems unlikely that lower glucose values as reflected by lower hemoglobin A1c values in the intensive-treatment group are an a priori explanation for the observed differences in mortality rates—especially since the mortality rates were lower than in the NHANES and clinical trial data sets cited above. If we assume that a type 1 statistical error (finding a difference where no difference actually exists) does not explain the findings, then at least four reasonable postulates exist:
Hypoglycemia may have some adverse effect, either acutely or from recurrent events that trigger a catecholamine response with associated risk for arrhythmia or increased coronary heart disease risk. However, the investigators analyzed each death to determine whether hypoglycemia was a contributing cause, and they found no statistically significant relationship between hypoglycemia and death in the intensive-treatment group.
Weight gain is common with intensive therapy. Obesity may be associated with greater cytokine production, higher concentrations of clotting factors, higher levels of free fatty acids, and other potential contributors to the risk of coronary heart disease and death. Currently, the ACCORD analyses do not suggest that weight gain explains the higher death rate.
Medications such as rosiglitazone, sulfonylureas, and the combination of a sulfonylurea plus metformin have been previously associated with increased death rates in some observational and intervention trials. These studies had some serious methodologic limitations (eg, absence of risk adjustment, events not adjudicated, small study cohorts, wide variation in study cohort characteristics) and small numbers of events.11–13,16,26,35 ACCORD analyses have not shown that any single glucose-lowering agent—including rosiglitazone—or combination of agents explains the death rates.
The stress of maintaining glycemic control has been speculated to have in some way contributed to an increased risk. To achieve intensive control, patients had to have frequent contact with their health care providers, they were often told that their hemoglobin A1c values were “too high” even when they were well below those in the American Diabetes Association guidelines, and they had to follow complex glucose-lowering regimens.
Semiquantitative measures of overall attitudes about health exist (eg, the “Feeling Thermometer” scale), but stress was not measured quantitatively in the ACCORD trial.
IMPLICATIONS OF ACCORD
In practice, most clinicians believe that the target glucose level in patients with type 2 diabetes should be as low as safely possible. This approach does not need to be modified on the basis of current information from ACCORD.
To be safe, regimens should be associated with a low risk of hypoglycemia and a low risk of weight gain. Use of combinations of medications that work by different mechanisms is still prudent. Agents should be used that may have favorable effects on other cardiovascular risk factors (eg, lipids, blood pressure, visceral fat).
Hemoglobin A1c targets below 7% are not precluded in all patients on the basis of the ACCORD results, though values lower than 6% may not have much added benefit for cardiovascular risk reduction. We should note that hemoglobin A1c was reduced in all ACCORD participants and that death rates were lower than in many other type 2 diabetic cohorts. Pending data on other outcomes in ACCORD (nephropathy, retinopathy, dementia, fracture risk), I believe it is premature for organizations to change their proposed hemoglobin A1c targets,36,37 as none have proposed values as low as the target in the ACCORD intensive-treatment group. At present, no class of glucose-lowering agents needs to be excluded from consideration on the basis of the ACCORD data.
The overall low rates of death in this population at high risk of coronary heart disease deserve comment. Not only are they lower than in other glucose-lowering trials, but they are also lower than in a number of studies of mortality in diabetes cohorts. As noted above, multiple risk factors for coronary heart disease and death were (and are) addressed in the ACCORD study participants, including repeated recommendation for lifestyle modification, intervention arms with lipid and blood pressure therapy, encouragement of aspirin use, and regular follow-up with health care providers for risk factors not managed by the ACCORD trial protocol. It is likely that multiple approaches to reducing the risk of cardiovascular disease contributed to this low mortality rate and that similar approaches will reduce the risk of coronary disease and death in regular clinical practice.
The ACCORD lipid and blood pressure arms are continuing, with results expected in 2010. The future results from ACCORD as well as from several glucose-lowering trials currently in progress (ADVANCE,32,33 Veteran’s Administration,34 Bypass Angioplasty Revascularization Investigation 2 Diabetes [BARI-2D]38) will likely help refine our understanding of the effects of glucose-lowering, glucose-lowering strategies and targets, and multiple interventions on coronary events and all-cause mortality.
For now, any strategy that lowers glucose and is associated with a low risk of hypoglycemia and does not cause excessive weight gain should be considered appropriate in patients with type 2 diabetes.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial1–5 was designed primarily to address, in patients with type 2 diabetes at high risk of cardiovascular events, whether intensive glucose control would result in a lower risk of atherosclerotic disease events or death than would standard treatment.
It was widely expected that intensive treatment would confer either modest benefit or, at worst, no benefit. However, the glucose-lowering arm of the trial was terminated early because of a higher mortality rate in the intensively treated group. (The ACCORD trial has two other arms, which concern blood pressure and lipid-lowering, and these are continuing.)
In earlier trials in type 2 diabetes, concerns had been raised about an increased risk of cardiovascular events and possibly death associated with glucose-lowering drugs, hypoglycemia itself, or both, and these were well known when ACCORD was convened. ACCORD was very carefully designed and included careful adjudication of each cardiovascular event and death, including whether hypoglycemia might have been a proximate cause of some sudden deaths.5
Therefore, the surprising result of the higher mortality rate with intensive glycemic control in ACCORD will be fodder for discussion in many arenas over the next several years, and it poses some challenges for physicians and patients in determining treatment goals, as well as for organizations that write clinical practice guidelines (and perhaps organizations involved in pay-for-performance based on these guidelines).
Still, I believe that the ACCORD results should not substantially change our approach to treatment goals in type 2 diabetes, although hemoglobin A1c targets below 6% may not have much added value for cardiovascular risk reduction. The low overall mortality rate in all the arms of the ACCORD trial emphasizes the importance of lifestyle modification, lipid and blood pressure therapy, and encouragement of aspirin use in all patients with type 2 diabetes.
This article reflects my views as a practicing diabetologist and clinical trialist (I was an investigator in the ACCORD trial) with a long-standing interest in clinical trials and in how the results influence clinical practice. The views I express herein may not reflect the views of other ACCORD investigators, the National Heart, Lung, and Blood Institute (NHLBI), the ACCORD trial coordinating center at Wake Forest University, or its data safety and monitoring board.
RISK OF CORONARY DISEASE INCREASES WITH GLUCOSE
Many observational studies6–10 have shown that the risk of cardiovascular disease, especially coronary heart disease, is two to five times higher in people with diabetes mellitus than in people without diabetes. The risk appears to be continuous, so the higher one’s glucose or hemoglobin A1c, the higher the risk.6 This risk even extends to glucose values well below the threshold values currently used to diagnose diabetes mellitus.6 Since there is no glucose threshold for coronary heart disease, the term dysglycemia (rather than hyperglycemia) has been proposed to note the relationship between glucose and coronary heart disease. (The glucose threshold for microvascular complications of diabetes, such as retinopathy and nephropathy, appears to be between 110 and 126 mg/dL).
The clustering of multiple coronary risk factors such as obesity, dyslipidemia, and hypertension has always raised the question of whether glucose is a culprit in coronary risk or whether it simply “runs in bad company.”
EARLIER CLINICAL TRIALS SUGGEST INTENSIVE TREATMENT RAISES RISK
Even though it has been widely believed that intensive glucose-lowering would reduce cardiovascular risk in type 2 diabetes, there have been hints in previous studies that some intensive-treatment regimens might increase risk.
Two large randomized clinical trials and one small one (discussed below) addressed whether glucose control would reduce the risk of atherosclerotic vascular disease events. In each of them, an increased risk of cardiovascular events and possibly of death was seen in at least one intensively treated group.
In the following discussion, I have calculated all of the death rates as the number of deaths per 1,000 patients per year, based on published study results. In this way, we can compare the rates in the various studies (including ACCORD), regardless of the trial duration.
The university group Diabetes Program: Controvery about tolbutamide therapy
The University Group Diabetes Program (UGDP)11–16 included about 1,000 participants randomized to five treatments: tolbutamide (Orinase, a sulfonylurea), insulin in a fixed dose based on body weight, insulin in adjusted doses based on fasting glucose levels, placebo, and (later) phenformin.
In the 1970s, when the UGDP was carried out, randomized clinical trials were uncommon. Like other trials from that era, the UGDP was underpowered by today’s standards and did not have a data safety and monitoring board.
Rates of cardiovascular events and deaths (per 1,000 patient-years):
- 25 (tolbutamide group)
- 12 (placebo group).
The two insulin groups did not differ from the placebo group in their rates of cardiovascular events or death.15 The tolbutamide arm was stopped, and the ensuing controversy about how to interpret the trial results lasted for more than a decade. It also resulted in a black-box warning for tolbutamide and all subsequent sulfonylureas.
United Kingdom Prospective Diabetes Study: Method of glucose-lowering an issue
The United Kingdom Prospective Diabetes Study (UKPDS)17–27 was launched in 1977. A cohort of 5,102 patients (mean age 54 years) with newly diagnosed type 2 diabetes mellitus followed a “prudent diet” for the first 3 to 4 months. Then, if their fasting glucose levels were in the range of 6.1 to 15 mmol/L (110–270 mg/dL), they were randomized to receive various treatments.
Patients who were not obese were randomized to receive either intensive treatment or conventional treatment. The intensive-treatment group received either insulin or a sulfonylurea (chlorpropamide [Diabinese], glibenclamide, or glipizide [Glucotrol]); the conventional-treatment group received diet therapy. The sulfonylurea arm was included partly to address the UGDP results.
Patients who were obese were randomized to receive one of three treatments: intensive treatment (with the agents listed above), conventional treatment, or metformin (Fortamet, Glucophage).
The mean in-trial hemoglobin A1c level in the intensive-treatment group was 7.0%, compared with 7.9% in the conventional-treatment group.
After a mean follow-up of more than 10 years, the incidence of myocardial infarction was 16% lower in the intensive-treatment group, but the difference was not statistically significant (P = .052).
Rates of death from all causes among nonobese subjects (per 1,000 patient-years):
- 18.2–20.5 (intensive-treatment group)
- 19.9 (conventional-treatment group).
In the obese patients who received metformin, the incidence of myocardial infarction was lower than in the conventional-treatment group but not the intensive-treatment group.
Rates of death among obese patients (per 1,000 patient-years):
- 13.5 (metformin group)
- 18.9 (intensive-treatment group)
- 20.6 (conventional-treatment group).
However, a small subset (n = 587) of the original group assigned to sulfonylurea therapy whose glycemic control deteriorated during the trial were rerandomized to continue to receive a sulfonylurea alone or to have metformin added. There was a statistically significantly higher rate of cardiovascular events and a nonsignificantly higher rate of total mortality in the metformin-plus-sulfonylurea group (30.3 per 1,000 patient-years) than in the sulfonylurea-only group (19.1 per 1,000 patient-years).
These data suggested that the way glucose-lowering was achieved might be as important as the glucose levels actually achieved. However, no definite conclusions could be drawn.
In an editorial on the UKPDS, Nathan26 made a comment that may have been prescient in terms of the ACCORD trial: “Professional organizations will now scramble to decide how to translate the UKPDS results … Whether the UKPDS firmly establishes the choice of any one therapy…or any combination of therapies for the long-term treatment of type 2 diabetes is more questionable.”26
Veterans Administration feasibility study
A Veterans Administration feasibility study28,29 included 153 men (mean age 60) with type 2 diabetes (mean duration 7.8 years) who received either conventional therapy (a single daily dose of insulin) or intensive therapy (multiple doses of insulin plus a sulfonylurea). Over a mean of 27 months, the intensive-therapy group achieved a hemoglobin A1c level that was 2 percentage points lower than in the conventional-therapy group.
At 2.25 years of follow-up, cardiovascular events had occurred in 24 (24%) of the intensive-therapy group and in 16 (20%) of the standard-therapy group (P = .10).
Rates of death from all causes (per 1,000 patient-years):
- 28.9 (intensive-treatment group)
- 17.5 (conventional-treatment group).
ACCORD TRIAL DESIGN
The primary outcome measured was the combined incidence of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. Secondary outcomes included death from any cause. The study is also evaluating the effect of intensive treatment on microvascular disease, hypoglycemia, cognition, quality of life, and cost-effectiveness.
The ACCORD study was designed to have 89% power to detect a 15% treatment effect of intensive glycemic control compared with standard glycemic control for the primary end point.
ACCORD RESULTS
Rates of death from any cause (per 1,000 patient-years):
- 14 (intensive-treatment group)
- 11 (standard-treatment group).
In the analyses available at the time that this study arm closed, the excess mortality was not attributable to any particular treatment regimen. In particular, rosiglitazone (Avandia) use did not contribute to the excess mortality. (Of note, 91.2% of the intensive-treatment group and 57.5% of the conventional-treatment group had been treated with rosiglitazone, with more than 19,000 patient-years of rosiglitazone exposure). The excess mortality was also not attributable to hypoglycemia immediately proximate to the death.
The ACCORD trial’s data safety and monitoring board recommended that this arm of the study be discontinued for safety reasons, and this recommendation was accepted by the NHLBI project office. All participants were notified by letter before the trial results were announced publicly, and all intensive-therapy group participants are now being treated by the protocol used in the standard-therapy group.1
FEWER DEATHS IN ACCORD THAN IN OTHER STUDIES IN DIABETES
The mortality rates in both arms of ACCORD were much lower than in other observational studies and clinical trials in type 2 diabetes.
The National Health and Nutrition Education Survey (NHANES),30 conducted from 1971 to 1975, included 14,374 people with diabetes between the ages of 25 and 74. Many of them were younger than the ACCORD patients, but two NHANES age-groups overlapped the ACCORD cohort. Rates of death from any cause at 22 years (per 1,000 patient-years):
- 39.7 (ages 45–64)
- 89.7 (ages 65–74).
The NHANES cohort would not have been treated as vigorously for coronary risk and other common causes of death.
UGDP, UKPDS. Death rates in the glucose-lowering trials of type 2 diabetes mellitus cited above were typically in the range of 20 deaths per 1,000 patient-years but were as high as 30 deaths per 1,000 patient-years in the UGDP tolbutamide group16 and the UK-PDS sulfonylurea-plus-metformin group.20,22,26
Steno-2.31 Half of 160 patients with type 2 diabetes were randomized to intensive strategies for controlling glucose, lipids, and blood pressure and for taking aspirin and angiotensin-converting enzyme inhibitors and following a healthy lifestyle. The other half received conventional therapy. Even in the intensive-treatment group, the mortality rate at 13 years was higher than in ACCORD. Rates of death from any cause (per 1,000 patient years):
- 22.5 (intensive-treatment group)
- 37.6 (conventional-treatment group).
After the ACCORD results were presented, two other trials addressing the question of whether lower hemoglobin A1c would reduce cardiovascular risk in type 2 diabetes have reported their outcomes:
The ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation),32,33 with 11,140 patients, had a target hemoglobin A1c of 6.5% in an intensive-treatment group and 7.3% in a usual-treatment group. The intensive-treatment group showed no difference in the rates of major macrovascular events (HR 0.94, 95% CI 0.84–1.06, P = .32) or all-cause mortality (HR 0.93, 95% CI 0.83–1.06, P = .32). The overall death rate in ADVANCE (about 18 deaths per 1,000 patient-years) was higher than in ACCORD.
The Veterans Administration Diabetes Trial included 1,791 patients.34 Like the ADVANCE trial, it also found no difference in major cardiovascular outcomes (HR 0.868, P = .11) or cardiovascular mortality rates (HR 1.258, P = .36) with intensive therapy vs conventional therapy, ie, achieved hemoglobin A1c levels of 6.9% vs 8.4% (presented at the American Diabetes Association 2008 Scientific Sessions). Hypoglycemia was associated with an increased risk of death in the standard-treatment group.
An analysis suggested that patients with a shorter duration of diabetes may have had cardiovascular benefit from intensive glucose-lowering, while those who had had it longer may have had increased risk associated with the more intensive therapy. The rate of death from all causes appears to have been higher than in ACCORD, but this could not be determined accurately from the presentations.
Comment. Thus, the ACCORD cohort as a whole has had strikingly lower death rates than in these other studies. The fact that all participants had lower glucose levels on therapy than at baseline may possibly contribute to these lower death rates. In addition, all ACCORD participants in the lipid arm received a statin; all participants in the blood pressure arm had their blood pressure lowered to levels below those commonly seen in clinical practice; participants were encouraged to exercise regularly; most participants were given diet instruction; and other healthy behaviors such as aspirin use, regular follow-up with primary care physicians, and recommendations about smoking were encouraged throughout the study. These comprehensive strategies may represent better care and thus result in lower death rates than in other studies.
POSSIBLE EXPLANATIONS FOR THE ACCORD OUTCOMES
The ACCORD trial has already stimulated fierce debate about the reasons for the higher mortality rate in the intensive-treatment group. With longer follow-up, some new risk factors for death may be identified that are not evident in the analyses of the current 460 deaths. What follows are some of my thoughts, with the caveat that they are not confirmed (supported statistically) by any currently available analyses from ACCORD.
It seems unlikely that lower glucose values as reflected by lower hemoglobin A1c values in the intensive-treatment group are an a priori explanation for the observed differences in mortality rates—especially since the mortality rates were lower than in the NHANES and clinical trial data sets cited above. If we assume that a type 1 statistical error (finding a difference where no difference actually exists) does not explain the findings, then at least four reasonable postulates exist:
Hypoglycemia may have some adverse effect, either acutely or from recurrent events that trigger a catecholamine response with associated risk for arrhythmia or increased coronary heart disease risk. However, the investigators analyzed each death to determine whether hypoglycemia was a contributing cause, and they found no statistically significant relationship between hypoglycemia and death in the intensive-treatment group.
Weight gain is common with intensive therapy. Obesity may be associated with greater cytokine production, higher concentrations of clotting factors, higher levels of free fatty acids, and other potential contributors to the risk of coronary heart disease and death. Currently, the ACCORD analyses do not suggest that weight gain explains the higher death rate.
Medications such as rosiglitazone, sulfonylureas, and the combination of a sulfonylurea plus metformin have been previously associated with increased death rates in some observational and intervention trials. These studies had some serious methodologic limitations (eg, absence of risk adjustment, events not adjudicated, small study cohorts, wide variation in study cohort characteristics) and small numbers of events.11–13,16,26,35 ACCORD analyses have not shown that any single glucose-lowering agent—including rosiglitazone—or combination of agents explains the death rates.
The stress of maintaining glycemic control has been speculated to have in some way contributed to an increased risk. To achieve intensive control, patients had to have frequent contact with their health care providers, they were often told that their hemoglobin A1c values were “too high” even when they were well below those in the American Diabetes Association guidelines, and they had to follow complex glucose-lowering regimens.
Semiquantitative measures of overall attitudes about health exist (eg, the “Feeling Thermometer” scale), but stress was not measured quantitatively in the ACCORD trial.
IMPLICATIONS OF ACCORD
In practice, most clinicians believe that the target glucose level in patients with type 2 diabetes should be as low as safely possible. This approach does not need to be modified on the basis of current information from ACCORD.
To be safe, regimens should be associated with a low risk of hypoglycemia and a low risk of weight gain. Use of combinations of medications that work by different mechanisms is still prudent. Agents should be used that may have favorable effects on other cardiovascular risk factors (eg, lipids, blood pressure, visceral fat).
Hemoglobin A1c targets below 7% are not precluded in all patients on the basis of the ACCORD results, though values lower than 6% may not have much added benefit for cardiovascular risk reduction. We should note that hemoglobin A1c was reduced in all ACCORD participants and that death rates were lower than in many other type 2 diabetic cohorts. Pending data on other outcomes in ACCORD (nephropathy, retinopathy, dementia, fracture risk), I believe it is premature for organizations to change their proposed hemoglobin A1c targets,36,37 as none have proposed values as low as the target in the ACCORD intensive-treatment group. At present, no class of glucose-lowering agents needs to be excluded from consideration on the basis of the ACCORD data.
The overall low rates of death in this population at high risk of coronary heart disease deserve comment. Not only are they lower than in other glucose-lowering trials, but they are also lower than in a number of studies of mortality in diabetes cohorts. As noted above, multiple risk factors for coronary heart disease and death were (and are) addressed in the ACCORD study participants, including repeated recommendation for lifestyle modification, intervention arms with lipid and blood pressure therapy, encouragement of aspirin use, and regular follow-up with health care providers for risk factors not managed by the ACCORD trial protocol. It is likely that multiple approaches to reducing the risk of cardiovascular disease contributed to this low mortality rate and that similar approaches will reduce the risk of coronary disease and death in regular clinical practice.
The ACCORD lipid and blood pressure arms are continuing, with results expected in 2010. The future results from ACCORD as well as from several glucose-lowering trials currently in progress (ADVANCE,32,33 Veteran’s Administration,34 Bypass Angioplasty Revascularization Investigation 2 Diabetes [BARI-2D]38) will likely help refine our understanding of the effects of glucose-lowering, glucose-lowering strategies and targets, and multiple interventions on coronary events and all-cause mortality.
For now, any strategy that lowers glucose and is associated with a low risk of hypoglycemia and does not cause excessive weight gain should be considered appropriate in patients with type 2 diabetes.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Goff DC, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:4i–20i.
- Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i.
- Gerstein HC, Riddle MC, Kendall DM, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:34i–43i.
- Bonds DE, Kurashige EM, Bergenstal R, et al. Severe hypoglycemia monitoring and risk management procedures in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:80i–89i.
- Gerstein HC. Dysglycemia, not just diabetes, is a continuous risk factor for cardiovascular disease. Evid Based Cardiovasc Med. 1997; 1:87–88.
- Gerstein HC, Pais P, Pogue J, Yusuf S. Relationship of glucose and insulin levels to the risk of myocardial infarction: a case-control study. J Am Coll Cardiol. 1999; 33:612–619.
- Gerstein HC, Capes SE. Dysglycemia: a key cardiovascular risk factor. Semin Vasc Med. 2002; 2:165–174.
- Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007; 78:305–312.
- American Diabetes Association. Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care. 1989; 12:573–579.
- Schor S. The University Group Diabetes Program. A statistician looks at the mortality results. JAMA. 1971; 217:1671–1675.
- Cornfield JThe University Group Diabetes Program. A further statistical analysis of the mortality findings. JAMA. 1971; 217:1676–1687.
- Feinstein AR. Clinical biostatistics. 8. An analytic appraisal of the University Group Diabetes Program (UGDP) study. Clin Pharmacol Ther. 1971; 12:167–191.
- The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of pheniformin therapy. Diabetes 1975; 24( suppl 1):65–184.
- Knatterud GL, Klimt CR, Levin ME, Jacobson ME, Goldner MG. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA. 1978; 240:37–42.
- Schwartz TB, Meinert CL. The UGDP controversy: thirty-four years of contentious ambiguity laid to rest. Perspect Biol Med. 2004; 47:564–574.
- Turner RC, Holman RR. Lessons from UK Prospective Diabetes Study. Diabetes Res Clin Pract 1995; 28( suppl):S151–S157.
- UKPDS Research Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:854–865.
- UKPDS Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:837–853.
- UK Prospective Diabetes Study Group. UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. Diabetes Care. 1998; 21:87–92.
- Bretzel RG, Voigt K, Schatz H. The United Kingdom Prospective Diabetes Study (UKPDS) implications for the pharmacotherapy of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 1998; 106:369–372.
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999; 281:2005–2012.
- Leslie RD. United Kingdom prospective diabetes study (UKPDS): what now or so what? Diabetes Metab Res Rev 1999; 15:65–71.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.
- Mooradian AD, Chehade J. Implications of the UK Prospective Diabetes Study: questions answered and issues remaining. Drugs Aging. 2000; 16:159–164.
- Nathan DM. Some answers, more controversy, from UKPDS. United Kingdom Prospective Diabetes Study. Lancet. 1998; 352:832–833.
- Srimanunthiphol J, Beddow R, Arakaki R. A review of the United Kingdom Prospective Diabetes Study (UKPDS) and a discussion of the implications for patient care. Hawaii Med J. 2000; 59:295–298.
- Duckworth WC, McCarren M, Abraira C. Glucose control and cardiovascular complications: the VA Diabetes Trial. Diabetes Care. 2001; 24:942–945.
- Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995; 18:1113–1123.
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998; 21:1138–1145. NHANES
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008; 358:580–591.
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572.
- Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation. Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. J Hypertens 2001; 19(suppl):S21–S28.
- Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003; 17:314–322.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):1–68.
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008; 31(suppl 1):S12–S54.
- Magee MF, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006; 97:20G–30G.
- Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.
- Goff DC, Gerstein HC, Ginsberg HN, et al. Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:4i–20i.
- Buse JB, Bigger JT, Byington RP, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol 2007; 99:21i–33i.
- Gerstein HC, Riddle MC, Kendall DM, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:34i–43i.
- Bonds DE, Kurashige EM, Bergenstal R, et al. Severe hypoglycemia monitoring and risk management procedures in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007; 99:80i–89i.
- Gerstein HC. Dysglycemia, not just diabetes, is a continuous risk factor for cardiovascular disease. Evid Based Cardiovasc Med. 1997; 1:87–88.
- Gerstein HC, Pais P, Pogue J, Yusuf S. Relationship of glucose and insulin levels to the risk of myocardial infarction: a case-control study. J Am Coll Cardiol. 1999; 33:612–619.
- Gerstein HC, Capes SE. Dysglycemia: a key cardiovascular risk factor. Semin Vasc Med. 2002; 2:165–174.
- Gerstein HC, Santaguida P, Raina P, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. 2007; 78:305–312.
- American Diabetes Association. Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. Diabetes Care. 1989; 12:573–579.
- Schor S. The University Group Diabetes Program. A statistician looks at the mortality results. JAMA. 1971; 217:1671–1675.
- Cornfield JThe University Group Diabetes Program. A further statistical analysis of the mortality findings. JAMA. 1971; 217:1676–1687.
- Feinstein AR. Clinical biostatistics. 8. An analytic appraisal of the University Group Diabetes Program (UGDP) study. Clin Pharmacol Ther. 1971; 12:167–191.
- The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of pheniformin therapy. Diabetes 1975; 24( suppl 1):65–184.
- Knatterud GL, Klimt CR, Levin ME, Jacobson ME, Goldner MG. Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VII. Mortality and selected nonfatal events with insulin treatment. JAMA. 1978; 240:37–42.
- Schwartz TB, Meinert CL. The UGDP controversy: thirty-four years of contentious ambiguity laid to rest. Perspect Biol Med. 2004; 47:564–574.
- Turner RC, Holman RR. Lessons from UK Prospective Diabetes Study. Diabetes Res Clin Pract 1995; 28( suppl):S151–S157.
- UKPDS Research Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:854–865.
- UKPDS Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998; 352:837–853.
- UK Prospective Diabetes Study Group. UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. Diabetes Care. 1998; 21:87–92.
- Bretzel RG, Voigt K, Schatz H. The United Kingdom Prospective Diabetes Study (UKPDS) implications for the pharmacotherapy of type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 1998; 106:369–372.
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999; 281:2005–2012.
- Leslie RD. United Kingdom prospective diabetes study (UKPDS): what now or so what? Diabetes Metab Res Rev 1999; 15:65–71.
- Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–412.
- Mooradian AD, Chehade J. Implications of the UK Prospective Diabetes Study: questions answered and issues remaining. Drugs Aging. 2000; 16:159–164.
- Nathan DM. Some answers, more controversy, from UKPDS. United Kingdom Prospective Diabetes Study. Lancet. 1998; 352:832–833.
- Srimanunthiphol J, Beddow R, Arakaki R. A review of the United Kingdom Prospective Diabetes Study (UKPDS) and a discussion of the implications for patient care. Hawaii Med J. 2000; 59:295–298.
- Duckworth WC, McCarren M, Abraira C. Glucose control and cardiovascular complications: the VA Diabetes Trial. Diabetes Care. 2001; 24:942–945.
- Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995; 18:1113–1123.
- Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998; 21:1138–1145. NHANES
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008; 358:580–591.
- Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–2572.
- Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation. Rationale and design of the ADVANCE study: a randomised trial of blood pressure lowering and intensive glucose control in high-risk individuals with type 2 diabetes mellitus. J Hypertens 2001; 19(suppl):S21–S28.
- Abraira C, Duckworth W, McCarren M, et al. Design of the cooperative study on glycemic control and complications in diabetes mellitus type 2: Veterans Affairs Diabetes Trial. J Diabetes Complications. 2003; 17:314–322.
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007; 356:2457–2471.
- American Association of Clinical Endocrinologists. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract 2007; 13(suppl 1):1–68.
- American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care 2008; 31(suppl 1):S12–S54.
- Magee MF, Isley WL. Rationale, design, and methods for glycemic control in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Am J Cardiol 2006; 97:20G–30G.
KEY POINTS
- No obvious cause, including hypoglycemia proximate to death or the use of any particular medication, clearly explained the excess deaths, although hypoglycemia occurred more often in intensively treated participants.
- The death rates in ACCORD were lower than in population studies and in other intervention trials. It is likely that multiple approaches to reducing the risk of cardiovascular disease contributed to this low mortality rate.