User login
Reducing Benzodiazepine Prescribing in Older Veterans: A Direct-to-Consumer Educational Brochure
This quality improvement project used an educational brochure to help older veterans reduce their benzodiazepine use.
Benzodiazepines (BZDs) are among the most commonly prescribed medications. A recent study found that in 2008, more than 5% of Americans used a BZD, and the percentage was almost 9% among Americans aged ≥ 65 years.1,2 Among veterans, BZD use is even higher, in part because of the high prevalence of posttraumatic stress disorder (PTSD). One study found that more than 30% of veterans with PTSD received at least 1 BZD prescription.3 The risks associated with BZD treatment for PTSD are compounded by concurrent use of other sedatives and opioids prescribed for co-occurring chronic pain and insomnia.3
Older adults metabolize long-acting BZDs more slowly and generally have an increased sensitivity to the adverse effects (AEs) of all BZDs.4 In older adults, BZD use has been associated with cognitive decline, dementia, falls and consequent fractures, and adverse respiratory outcomes.5-12 The risk of most but not all of these AEs was increased with higher BZD dose or long-term BZD use, which this quality improvement project (QIP) defines as having at least a 60-day supply of BZD prescriptions dispensed within the past year.
Long-term BZD use increases with age. One study found that, among patients receiving a BZD, the rate of long-term BZD use was more than double in older adults (31.4%) than it was in adults aged between 18 and 35 years (14.7%).2 For these reasons, the 2012 Beers criteria of the American Geriatrics Society recommend avoiding all types of BZDs in the treatment of insomnia, agitation, or delirium in patients aged > 65 years.13 Despite this recommendation, the prevalence of BZD use in older adults remains high.14
Some innovative approaches have been developed to address the inappropriate use, including overuse and misuse, of BZDs in older adults.15 In one approach, direct-to-consumer (DTC) information is used to empower patients to collaborate with their physician to manage their health. Results from several studies suggest that providing older patients with information on BZD risks and benefits increases patient–physician interaction and thereby decreases inappropriate BZD use and improves health outcomes.4,16,17 One study found that perceptions of BZD risks increased 1 week after exposure to a DTC educational brochure (EB), with intention to discuss BZD discontinuation with their physician higher for patients who received the EB than it was for those who did not (83.1% vs 44.3%; P < .0001).16 The EMPOWER (Eliminating Medications Through Patient Ownership of End Results) cluster randomized controlled trial assessed the effectiveness of a DTC EB focused on BZD risks in older adults.17 In that seminal study, patients who received a DTC EB were more likely than were comparison patients to discontinue BZD within 6 months (27% vs 5%; risk difference, 23%; 95% CI, 14%-32%).
The Veterans Integrated Systems Network (VISN) 22 Academic Detailing Program is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20 With BZD use among older veterans remaining high, the VISN 22 program initiated a clinical QIP modeled on the EMPOWER trial. Veterans in VISN 22 received the DTC EB, which included information on BZD risks and encouraged them to discuss their BZD treatment with their health care provider. VISN 22 was the first VISN in the VHA to implement the EMPOWER protocol.
As this was a QIP, all eligible veterans in VISN 22 were mailed the DTC EB, thus making it difficult to estimate the impact of the EB on BZD discontinuation in this VISN. Therefore, DTC EB efficacy was estimated by comparing BZD discontinuation between VISN 22 and VISN 21, an adjacent VISN that did not mail the DTC EB.
Methods
Two QIPs were undertaken to determine the impact of DTC EB on BZD use in older veterans in the VHA.
Quality Improvement Project 1
Design. A retrospective cohort analysis was performed. The VISN 22 catchment area, which encompasses VA facilities and clinics in southern California and southern Nevada, serves about 500,000 veterans, a substantial proportion of whom are aged ≥ 65 years. Among these older veterans are active long-term BZD users, who were defined as having ≥ 60-day supply of BZD prescriptions dispensed within the past year. Each active long-term user with a BZD prescription released within 200 days before the index date (the date the user was to meet with the prescribing physician) was mailed an EB 2 to 8 weeks in advance of the visit. Excluded from analysis were veterans with a schizophrenia, spinal cord injury, or seizure disorder diagnosis recorded in both their inpatient and outpatient medical records; veterans seen by Palliative Care within the past year; and veterans who died before analysis was completed.
Education Brochure. The EB for VISN 22 (Figure 1, see
Patients. The sample consisted of all veterans identified as meeting the inclusion criteria and being enrolled in VISN 22. The EB was mailed once to veterans on a rolling basis from December 2014 to February 2016. Change in BZD use was analyzed only after 9 to 24 months had passed since the index appointment with the prescribing physician. This period included 12 weeks for BZD taper and then 6 months after taper.
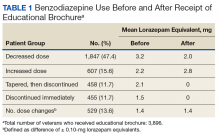
Analysis. For each veteran, monthly mean lorazepam equivalent (LE) was calculated using as many as 12 fills before the index date. Average daily dose of LE was calculated by dividing the sum of LE from all included prescriptions by total number of days between the first fill and the index date. The BZD prescription fills were evaluated after the index date. Veterans who received at least 1 prescription after the index date but then had no BZD prescription activity in VA clinics for 3 consecutive months during the 9-month observation period were recorded as having tapered and then discontinued BZD. Veterans who had no BZD prescription activity in VA clinics after the index date and during the 9-month observation period were recorded as having discontinued BZD without tapering. For veterans who had BZD prescription activity in VA clinics after the index date and during the 9-month observation period, mean LE was calculated by dividing the total LE for BZD prescriptions after the index date by number of days from the first fill after the index date to the date of analysis.
Quality Improvement Project 2
Design. A retrospective cohort analysis using PSM was performed on a subgroup of the QIP-1 sample to evaluate the impact of EB on BZD prescribing in the VA during 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. Veterans in the analysis were active long-term BZD users, had at least 1 BZD prescription released within 200 days before the index date, were aged ≥ 65 years, and had an appointment scheduled with their BZD prescriber within 2 to 8 weeks (Figure 2).
Patients. VISN 22 implemented QIP-2, a real-world application of a modified EMPOWER program, by identifying eligible veterans on a rolling basis from December 2014 to August 2015. All veterans who were identified and sent an EB during this period were included in the case group. The index date was defined as the first of the month the EB was mailed. Veterans with a pending appointment were chosen because the lead time would allow them to receive the EB and prepare to discuss it with the physician during the visit.
A comparator group was drawn from the adjacent VISN 21 catchment area, which encompasses VA facilities and clinics in Hawaii, northern California, and northern Nevada. During the observation period, VISN 21 did not mail any EBs specifically addressing BZD risks. Veterans in the comparator group had an appointment scheduled with their BZD prescribing physician within 4 weeks, were aged ≥ 65 years on the index date (first of the month before the next appointment, coinciding with the date EBs were sent to VISN 22 veterans), were active long-term BZD users, and had at least 1 BZD prescription released within 200 days before the index date. All patients were followed for up to 12 months after the index date, with BZD discontinuation recorded 9 and 12 months after the index date.
Propensity Score Matching
Propensity score (PS) was estimated with logistic regression analysis with treatment as the dependent variable and baseline characteristics as the independent variables.21,22 One-to-one matching on the PS was performed using the nearest neighbor approach without replacements. Independent variables related to outcome but unrelated to EB exposure were selected for PS development.22 These variables included year of birth; male sex; Hispanic ethnicity; annual income; service connection status; region; body mass index; Charlson Comorbidity Index category; total baseline BZD dose; and diagnosis of AIDS, nonmetastatic cancer, metastatic cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), dementia, diabetes mellitus (DM), DM with complications, gastroesophageal reflux disease (GERD), general anxiety disorder (GAD), hemiparaplegia, liver disease (mild), liver disease (moderate to severe), myocardial infarction (MI), Parkinson disease, peptic ulcer disease (PUD), psychosis, renal disease, rheumatoid arthritis (RA), or substance use disorder (SUD).
The EMPOWER cluster randomized controlled trial (RCT) demonstrated the effectiveness of EB exposure in a Canadian population of elderly patients who were long-term BZD users.17 Randomized controlled trials are the gold standard for clinical trials because they can establish causal inference.23-25 Given ethical and practical concerns, however, RCTs cannot be applied to all clinical scenarios. Although EMPOWER is reported to be an effective tool in reducing BZD use in older adults, its application in a real-world, large, integrated health care system remains untested. Observational studies are often conducted as an alternative to RCTs but are subject to selection bias because of their lack of randomization.26 Therefore, robust research methods are needed to generate unbiased estimates of the impact of an intervention on an outcome. Propensity score matching simulates an RCT by balancing the covariates across treatment groups.21,22,27 Observed patient characteristics are used to estimate PS, the probability that treatment will be received. Logistic or probit regression is used to balance the potential confounding covariates between the treatment groups.Once PSs are known, mean treatment effect can be estimated without the mean model.28 In other words, PSM methods can be used to generate an unbiased estimate of the treatment.
Propensity Score Analysis
Baseline characteristics were compared using Student t test (continuous variables) and χ2 test (discrete variables). Results are presented as means and standard deviations (continuous variables) and frequency and percentage (discrete variables).
The main outcome was BZD discontinuation 9 and 12 months after the index date. A postindex lag of 6 months was used to capture any tapering (Figure 2). Discontinuation, defined as 3 consecutive months of no BZD prescription on hand, was measured for 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. An estimate was made of the difference in the proportions of BZD discontinuers who received the EB and BZD discontinuers who did not receive the EB, where mean treatment (risk difference) was presented as the absolute risk difference with a 95% CI. Standard errors and 95% CIs for the risk differences were generated with biased-corrected CIs from 1,000 bootstrap samples.
Sensitivity Analyses
Naïve multivariate logistic regression analysis was performed to evaluate the association between EB exposure and BZD discontinuation while controlling for potential confounders. Results are presented as odds ratios (ORs) and 95% CIs. Confounders identified were the same covariates used to generate the PSs.
Several analyses were performed to test the sensitivity of the methods applied using PSM by changing caliber size while maintaining the nearest neighbor approach without replacement. Linear regression analysis was performed with robust standard errors to estimate the risk difference of BZD discontinuation between EB-exposed and EB-unexposed veterans.
Statistical significance was set at P < .05. All statistical analyses were performed with Stata/SE Version 13 (College Station, TX).
Results
Quality Improvement Project 1
On a rolling basis from December 2014 to February 2016, the EB was mailed once to 3,896 VISN 22 veterans 2 to 8 weeks before a clinic appointment with their BZD prescribing physician.
Quality Improvement Project 2
Of all the VISN 22 and VISN 21 veterans, 24,420 met the inclusion and exclusion criteria. Of these 24,420 veterans, 2,020 (8.3%) were in VISN 22 and received the EB between December 2014 and August 2015 (QIP-1), and 22,400 (91.7%) were in VISN 21 and did not receive the EB.
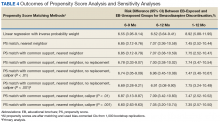
Naïve Results Before PS Matching. In the naïve analyses, a larger proportion of EB-exposed vs unexposed veterans discontinued BZD; in addition, reductions were 6.6%, 7.4%, and 9.5% larger for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (P < .0001 for all comparisons; Table 2).
After controlling for potential confounders, the naïve logistic regression analyses found EB exposure was significantly associated with 44%, 32%, and 42% increases in the odds of BZD discontinuation for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (Table 3).
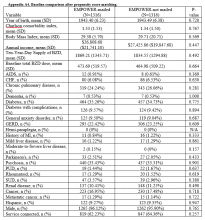
Propensity Score Matching. Before matching, there were significant differences in baseline characteristics of veterans who met the inclusion and exclusion criteria, with few exceptions (eAppendices 2 and 3, ).
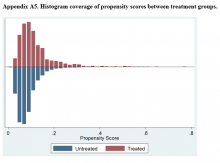
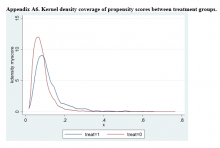
Propensity Score Matching Results. Inspection of PSs revealed good coverage across treatment groups on a histogram plot and a kernel density plot (eAppendices 5 and 6).
Discussion
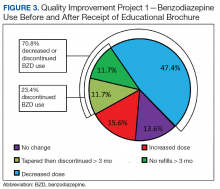
This QIP was the first to evaluate the impact of an EMPOWER-modeled DTC EB in a large, integrated health care system in the U.S. It was also the first to demonstrate potential benefits of a DTC EB designed for older veterans who are long-term BZD users. In this QIP, which mailed the EB to 3,896 veterans, 1,847 (47.4%) decreased their BZD dose, 458 (11.7%) tapered and then discontinued BZD, and 455 (11.7%) immediately discontinued BZD. The total percentage of veterans who discontinued BZD (23.4%; 913/3,896) was similar to the 27% reported in the EMPOWER trial.17 However, the risk difference between the 1,316 EB-exposed VISN 22 veterans (QIP-1) and the 1,316 EB-unexposed VISN 21 veterans in this QIP was significantly lower than the 23% risk difference in EMPOWER (though it still demonstrated a significantly larger reduction for EB-exposed veterans).17
Given this inclusion of all qualifying veterans from the catchment area studied in this QIP, and given the ethical and practical concerns, an RCT was not possible. Therefore, PSM methods were used to balance the covariates across treatment groups and thereby simulate an RCT.21,22,27 With use of the PSM approach, findings from the descriptive analysis were confirmed and potential selection bias reduced.
Study Limitations
The less robust risk difference found in this QIP has several possible explanations. The authors’ use of a DTC EB coincided with a national VA effort to reduce older veterans’ use of BZDs and other inappropriate medications. For instance, during the study period, academic detailing was being implemented to reduce use of BZDs, particularly in combination with opioids, across VHA facilities and clinics. (Academic detailing is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20) However, QIP-2 results and PS analysis of a subgroup of the original sample suggest that EB-exposed veterans were significantly more likely than were their unexposed counterparts were to discontinue BZD. To an extent, this analysis controlled for these other efforts to reduce BZD use in VHA clinics and can be considered a study strength.
Another limitation is the study design, which lacked a control group and did not consider the possibility that some facility or clinic physicians might influence others. Although the region variable was controlled for in PSM, the authors did not capture facility characteristics, including frequency of prescribing BZD and use of a protocol for enforcing the Beers criteria. Such confounders might have influenced outcomes. Unlike the EMPOWER trial,17 this QIP did not assess or exclude cognitively impaired veterans. It is reasonable to assume that these veterans might not understand some EB messages and consequently might fail to engage their physicians. Failure to initiate discussion with a physician would attenuate the impact of the EB.
Study Strengths
A strength of this QIP was its use of a DTC EB in a large, regional sample of older veterans in a real-world clinical setting. In addition, the study group (EB-exposed veterans) and the comparator group (EB-unexposed veterans) were from similar geographic areas (primarily California and Nevada).
Conclusion
Results of this study suggest that a DTC EB, designed to reduce BZD use among older veterans, was effective in helping patients lower their BZD dose and discontinue BZD. The likelihood of discontinuing BZD 9 and 12 months after the index date was significantly higher for veterans who received an EB modeled on the EMPOWER educational brochure than for a comparator group of veterans who did not receive the EB and were receiving care during the same observation period. In the future, it would be beneficial to use a design that controls for physician exposure to academic detailing focused on BZD reduction and that accounts for the cluster effects of facility practice. Despite these limitations, this QIP is the first real-world empirical example of using an EMPOWER-modeled DTC EB to decrease BZD use among older veterans. Furthermore, these results suggest that a DTC EB can be used to target other high-risk prescription drugs, such as opioids, particularly if alternative treatment options can be provided.
Acknowledgments
Dr. Hauser thanks Cathy, Anika, Katia, and Max Hauser, and Alba and Kevin Quinlan, for their support. In memory of Jirina Hauser, who died on Mother’s Day, May 14, 2017, at the age of 100.
1. Dell’osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry. 2013;28(1):7-20.
2. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
3. Bernardy NC, Lund BC, Alexander B, Friedman MJ. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
4. Roberts KJ. Patient empowerment in the United States: a critical commentary. Health Expect. 1999;2(2):82-92.
5. Paterniti S, Dufouil C, Alpérovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22(3):285-293.
6. van der Hooft CS, Schoofs MW, Ziere G, et al. Inappropriate benzodiazepine use in older adults and the risk of fracture. Br J Clin Pharmacol. 2008;66(2):276-282.
7. Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf. 2010;19(12):1248-1255.
8. Finkle WD, Der JS, Greenland S, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59(10):1883-1890.
9. de Gage SB, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231
10. Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639-658.
11. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with chronic obstructive pulmonary disease. Eur Respir J. 2014;44(2):332-340.
12. Gomm W, von Holt K, Thomé F, et al. Regular benzodiazepine and z-substance use and risk of dementia: an analysis of German claims data. J Alzheimers Dis. 2016;54(2):801-808.
13. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
14. National Institutes of Health. Despite risks, benzodiazepine use highest in older people. https://www.nih.gov/news-events/news-releases/despite-risks-benzodiaze pine-use-highest-older-people. Published December 17, 2014. Accessed July 31, 2018.
15. Airagnes G, Pelissolo A, Lavallée M, Flament M, Limosin F. Benzodiazepine misuse in the elderly: risk factors, consequences, and management. Curr Psychiatry Rep. 2016;18(10):89.
16. Martin P, Tamblyn R, Ahmed S, Tannenbaum C. A drug education tool developed for older adults changes knowledge, beliefs and risk perceptions about inappropriate benzodiazepine prescriptions in the elderly. Patient Educ Couns. 2013;92(1):81-87.
17. Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898.
18. Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263(4):549-556.
19. Fischer MA, Avorn J. Academic detailing can play a key role in assessing and implementing comparative effectiveness research findings. Health Aff (Millwood). 2012;31(10):2206-2212.
20. Wells DL, Popish S, Kay C, Torrise V, Christopher ML. VA Academic Detailing Service: implementation and lessons learned. Fed Pract. 2016;33(5):38-42.
21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424.
22. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156.
23. Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. J Ed Psych. 1974;66(5):688-701.
24. Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(4):722-729.
25. Cartwright N. What are randomized controlled trials good for? Philos Stud. 2010;147(1):59.
26. Kleinbaum DG, Morgenstern H, Kupper LL. Selection bias in epidemiologic studies. Am J Epidemiol. 1981;113(4):452-463.
27. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55.
28. Pirracchio R, Carone M, Rigon MR, Caruana E, Mebazaa A, Chevret S. Propensity score estimators for the average treatment effect and the average treatment effect on the treated may yield very different estimates. Stat Methods Med Res. 2016;25(5):1938-1954.
This quality improvement project used an educational brochure to help older veterans reduce their benzodiazepine use.
This quality improvement project used an educational brochure to help older veterans reduce their benzodiazepine use.
Benzodiazepines (BZDs) are among the most commonly prescribed medications. A recent study found that in 2008, more than 5% of Americans used a BZD, and the percentage was almost 9% among Americans aged ≥ 65 years.1,2 Among veterans, BZD use is even higher, in part because of the high prevalence of posttraumatic stress disorder (PTSD). One study found that more than 30% of veterans with PTSD received at least 1 BZD prescription.3 The risks associated with BZD treatment for PTSD are compounded by concurrent use of other sedatives and opioids prescribed for co-occurring chronic pain and insomnia.3
Older adults metabolize long-acting BZDs more slowly and generally have an increased sensitivity to the adverse effects (AEs) of all BZDs.4 In older adults, BZD use has been associated with cognitive decline, dementia, falls and consequent fractures, and adverse respiratory outcomes.5-12 The risk of most but not all of these AEs was increased with higher BZD dose or long-term BZD use, which this quality improvement project (QIP) defines as having at least a 60-day supply of BZD prescriptions dispensed within the past year.
Long-term BZD use increases with age. One study found that, among patients receiving a BZD, the rate of long-term BZD use was more than double in older adults (31.4%) than it was in adults aged between 18 and 35 years (14.7%).2 For these reasons, the 2012 Beers criteria of the American Geriatrics Society recommend avoiding all types of BZDs in the treatment of insomnia, agitation, or delirium in patients aged > 65 years.13 Despite this recommendation, the prevalence of BZD use in older adults remains high.14
Some innovative approaches have been developed to address the inappropriate use, including overuse and misuse, of BZDs in older adults.15 In one approach, direct-to-consumer (DTC) information is used to empower patients to collaborate with their physician to manage their health. Results from several studies suggest that providing older patients with information on BZD risks and benefits increases patient–physician interaction and thereby decreases inappropriate BZD use and improves health outcomes.4,16,17 One study found that perceptions of BZD risks increased 1 week after exposure to a DTC educational brochure (EB), with intention to discuss BZD discontinuation with their physician higher for patients who received the EB than it was for those who did not (83.1% vs 44.3%; P < .0001).16 The EMPOWER (Eliminating Medications Through Patient Ownership of End Results) cluster randomized controlled trial assessed the effectiveness of a DTC EB focused on BZD risks in older adults.17 In that seminal study, patients who received a DTC EB were more likely than were comparison patients to discontinue BZD within 6 months (27% vs 5%; risk difference, 23%; 95% CI, 14%-32%).
The Veterans Integrated Systems Network (VISN) 22 Academic Detailing Program is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20 With BZD use among older veterans remaining high, the VISN 22 program initiated a clinical QIP modeled on the EMPOWER trial. Veterans in VISN 22 received the DTC EB, which included information on BZD risks and encouraged them to discuss their BZD treatment with their health care provider. VISN 22 was the first VISN in the VHA to implement the EMPOWER protocol.
As this was a QIP, all eligible veterans in VISN 22 were mailed the DTC EB, thus making it difficult to estimate the impact of the EB on BZD discontinuation in this VISN. Therefore, DTC EB efficacy was estimated by comparing BZD discontinuation between VISN 22 and VISN 21, an adjacent VISN that did not mail the DTC EB.
Methods
Two QIPs were undertaken to determine the impact of DTC EB on BZD use in older veterans in the VHA.
Quality Improvement Project 1
Design. A retrospective cohort analysis was performed. The VISN 22 catchment area, which encompasses VA facilities and clinics in southern California and southern Nevada, serves about 500,000 veterans, a substantial proportion of whom are aged ≥ 65 years. Among these older veterans are active long-term BZD users, who were defined as having ≥ 60-day supply of BZD prescriptions dispensed within the past year. Each active long-term user with a BZD prescription released within 200 days before the index date (the date the user was to meet with the prescribing physician) was mailed an EB 2 to 8 weeks in advance of the visit. Excluded from analysis were veterans with a schizophrenia, spinal cord injury, or seizure disorder diagnosis recorded in both their inpatient and outpatient medical records; veterans seen by Palliative Care within the past year; and veterans who died before analysis was completed.
Education Brochure. The EB for VISN 22 (Figure 1, see
Patients. The sample consisted of all veterans identified as meeting the inclusion criteria and being enrolled in VISN 22. The EB was mailed once to veterans on a rolling basis from December 2014 to February 2016. Change in BZD use was analyzed only after 9 to 24 months had passed since the index appointment with the prescribing physician. This period included 12 weeks for BZD taper and then 6 months after taper.
Analysis. For each veteran, monthly mean lorazepam equivalent (LE) was calculated using as many as 12 fills before the index date. Average daily dose of LE was calculated by dividing the sum of LE from all included prescriptions by total number of days between the first fill and the index date. The BZD prescription fills were evaluated after the index date. Veterans who received at least 1 prescription after the index date but then had no BZD prescription activity in VA clinics for 3 consecutive months during the 9-month observation period were recorded as having tapered and then discontinued BZD. Veterans who had no BZD prescription activity in VA clinics after the index date and during the 9-month observation period were recorded as having discontinued BZD without tapering. For veterans who had BZD prescription activity in VA clinics after the index date and during the 9-month observation period, mean LE was calculated by dividing the total LE for BZD prescriptions after the index date by number of days from the first fill after the index date to the date of analysis.
Quality Improvement Project 2
Design. A retrospective cohort analysis using PSM was performed on a subgroup of the QIP-1 sample to evaluate the impact of EB on BZD prescribing in the VA during 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. Veterans in the analysis were active long-term BZD users, had at least 1 BZD prescription released within 200 days before the index date, were aged ≥ 65 years, and had an appointment scheduled with their BZD prescriber within 2 to 8 weeks (Figure 2).
Patients. VISN 22 implemented QIP-2, a real-world application of a modified EMPOWER program, by identifying eligible veterans on a rolling basis from December 2014 to August 2015. All veterans who were identified and sent an EB during this period were included in the case group. The index date was defined as the first of the month the EB was mailed. Veterans with a pending appointment were chosen because the lead time would allow them to receive the EB and prepare to discuss it with the physician during the visit.
A comparator group was drawn from the adjacent VISN 21 catchment area, which encompasses VA facilities and clinics in Hawaii, northern California, and northern Nevada. During the observation period, VISN 21 did not mail any EBs specifically addressing BZD risks. Veterans in the comparator group had an appointment scheduled with their BZD prescribing physician within 4 weeks, were aged ≥ 65 years on the index date (first of the month before the next appointment, coinciding with the date EBs were sent to VISN 22 veterans), were active long-term BZD users, and had at least 1 BZD prescription released within 200 days before the index date. All patients were followed for up to 12 months after the index date, with BZD discontinuation recorded 9 and 12 months after the index date.
Propensity Score Matching
Propensity score (PS) was estimated with logistic regression analysis with treatment as the dependent variable and baseline characteristics as the independent variables.21,22 One-to-one matching on the PS was performed using the nearest neighbor approach without replacements. Independent variables related to outcome but unrelated to EB exposure were selected for PS development.22 These variables included year of birth; male sex; Hispanic ethnicity; annual income; service connection status; region; body mass index; Charlson Comorbidity Index category; total baseline BZD dose; and diagnosis of AIDS, nonmetastatic cancer, metastatic cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), dementia, diabetes mellitus (DM), DM with complications, gastroesophageal reflux disease (GERD), general anxiety disorder (GAD), hemiparaplegia, liver disease (mild), liver disease (moderate to severe), myocardial infarction (MI), Parkinson disease, peptic ulcer disease (PUD), psychosis, renal disease, rheumatoid arthritis (RA), or substance use disorder (SUD).
The EMPOWER cluster randomized controlled trial (RCT) demonstrated the effectiveness of EB exposure in a Canadian population of elderly patients who were long-term BZD users.17 Randomized controlled trials are the gold standard for clinical trials because they can establish causal inference.23-25 Given ethical and practical concerns, however, RCTs cannot be applied to all clinical scenarios. Although EMPOWER is reported to be an effective tool in reducing BZD use in older adults, its application in a real-world, large, integrated health care system remains untested. Observational studies are often conducted as an alternative to RCTs but are subject to selection bias because of their lack of randomization.26 Therefore, robust research methods are needed to generate unbiased estimates of the impact of an intervention on an outcome. Propensity score matching simulates an RCT by balancing the covariates across treatment groups.21,22,27 Observed patient characteristics are used to estimate PS, the probability that treatment will be received. Logistic or probit regression is used to balance the potential confounding covariates between the treatment groups.Once PSs are known, mean treatment effect can be estimated without the mean model.28 In other words, PSM methods can be used to generate an unbiased estimate of the treatment.
Propensity Score Analysis
Baseline characteristics were compared using Student t test (continuous variables) and χ2 test (discrete variables). Results are presented as means and standard deviations (continuous variables) and frequency and percentage (discrete variables).
The main outcome was BZD discontinuation 9 and 12 months after the index date. A postindex lag of 6 months was used to capture any tapering (Figure 2). Discontinuation, defined as 3 consecutive months of no BZD prescription on hand, was measured for 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. An estimate was made of the difference in the proportions of BZD discontinuers who received the EB and BZD discontinuers who did not receive the EB, where mean treatment (risk difference) was presented as the absolute risk difference with a 95% CI. Standard errors and 95% CIs for the risk differences were generated with biased-corrected CIs from 1,000 bootstrap samples.
Sensitivity Analyses
Naïve multivariate logistic regression analysis was performed to evaluate the association between EB exposure and BZD discontinuation while controlling for potential confounders. Results are presented as odds ratios (ORs) and 95% CIs. Confounders identified were the same covariates used to generate the PSs.
Several analyses were performed to test the sensitivity of the methods applied using PSM by changing caliber size while maintaining the nearest neighbor approach without replacement. Linear regression analysis was performed with robust standard errors to estimate the risk difference of BZD discontinuation between EB-exposed and EB-unexposed veterans.
Statistical significance was set at P < .05. All statistical analyses were performed with Stata/SE Version 13 (College Station, TX).
Results
Quality Improvement Project 1
On a rolling basis from December 2014 to February 2016, the EB was mailed once to 3,896 VISN 22 veterans 2 to 8 weeks before a clinic appointment with their BZD prescribing physician.
Quality Improvement Project 2
Of all the VISN 22 and VISN 21 veterans, 24,420 met the inclusion and exclusion criteria. Of these 24,420 veterans, 2,020 (8.3%) were in VISN 22 and received the EB between December 2014 and August 2015 (QIP-1), and 22,400 (91.7%) were in VISN 21 and did not receive the EB.
Naïve Results Before PS Matching. In the naïve analyses, a larger proportion of EB-exposed vs unexposed veterans discontinued BZD; in addition, reductions were 6.6%, 7.4%, and 9.5% larger for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (P < .0001 for all comparisons; Table 2).
After controlling for potential confounders, the naïve logistic regression analyses found EB exposure was significantly associated with 44%, 32%, and 42% increases in the odds of BZD discontinuation for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (Table 3).
Propensity Score Matching. Before matching, there were significant differences in baseline characteristics of veterans who met the inclusion and exclusion criteria, with few exceptions (eAppendices 2 and 3, ).
Propensity Score Matching Results. Inspection of PSs revealed good coverage across treatment groups on a histogram plot and a kernel density plot (eAppendices 5 and 6).
Discussion
This QIP was the first to evaluate the impact of an EMPOWER-modeled DTC EB in a large, integrated health care system in the U.S. It was also the first to demonstrate potential benefits of a DTC EB designed for older veterans who are long-term BZD users. In this QIP, which mailed the EB to 3,896 veterans, 1,847 (47.4%) decreased their BZD dose, 458 (11.7%) tapered and then discontinued BZD, and 455 (11.7%) immediately discontinued BZD. The total percentage of veterans who discontinued BZD (23.4%; 913/3,896) was similar to the 27% reported in the EMPOWER trial.17 However, the risk difference between the 1,316 EB-exposed VISN 22 veterans (QIP-1) and the 1,316 EB-unexposed VISN 21 veterans in this QIP was significantly lower than the 23% risk difference in EMPOWER (though it still demonstrated a significantly larger reduction for EB-exposed veterans).17
Given this inclusion of all qualifying veterans from the catchment area studied in this QIP, and given the ethical and practical concerns, an RCT was not possible. Therefore, PSM methods were used to balance the covariates across treatment groups and thereby simulate an RCT.21,22,27 With use of the PSM approach, findings from the descriptive analysis were confirmed and potential selection bias reduced.
Study Limitations
The less robust risk difference found in this QIP has several possible explanations. The authors’ use of a DTC EB coincided with a national VA effort to reduce older veterans’ use of BZDs and other inappropriate medications. For instance, during the study period, academic detailing was being implemented to reduce use of BZDs, particularly in combination with opioids, across VHA facilities and clinics. (Academic detailing is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20) However, QIP-2 results and PS analysis of a subgroup of the original sample suggest that EB-exposed veterans were significantly more likely than were their unexposed counterparts were to discontinue BZD. To an extent, this analysis controlled for these other efforts to reduce BZD use in VHA clinics and can be considered a study strength.
Another limitation is the study design, which lacked a control group and did not consider the possibility that some facility or clinic physicians might influence others. Although the region variable was controlled for in PSM, the authors did not capture facility characteristics, including frequency of prescribing BZD and use of a protocol for enforcing the Beers criteria. Such confounders might have influenced outcomes. Unlike the EMPOWER trial,17 this QIP did not assess or exclude cognitively impaired veterans. It is reasonable to assume that these veterans might not understand some EB messages and consequently might fail to engage their physicians. Failure to initiate discussion with a physician would attenuate the impact of the EB.
Study Strengths
A strength of this QIP was its use of a DTC EB in a large, regional sample of older veterans in a real-world clinical setting. In addition, the study group (EB-exposed veterans) and the comparator group (EB-unexposed veterans) were from similar geographic areas (primarily California and Nevada).
Conclusion
Results of this study suggest that a DTC EB, designed to reduce BZD use among older veterans, was effective in helping patients lower their BZD dose and discontinue BZD. The likelihood of discontinuing BZD 9 and 12 months after the index date was significantly higher for veterans who received an EB modeled on the EMPOWER educational brochure than for a comparator group of veterans who did not receive the EB and were receiving care during the same observation period. In the future, it would be beneficial to use a design that controls for physician exposure to academic detailing focused on BZD reduction and that accounts for the cluster effects of facility practice. Despite these limitations, this QIP is the first real-world empirical example of using an EMPOWER-modeled DTC EB to decrease BZD use among older veterans. Furthermore, these results suggest that a DTC EB can be used to target other high-risk prescription drugs, such as opioids, particularly if alternative treatment options can be provided.
Acknowledgments
Dr. Hauser thanks Cathy, Anika, Katia, and Max Hauser, and Alba and Kevin Quinlan, for their support. In memory of Jirina Hauser, who died on Mother’s Day, May 14, 2017, at the age of 100.
Benzodiazepines (BZDs) are among the most commonly prescribed medications. A recent study found that in 2008, more than 5% of Americans used a BZD, and the percentage was almost 9% among Americans aged ≥ 65 years.1,2 Among veterans, BZD use is even higher, in part because of the high prevalence of posttraumatic stress disorder (PTSD). One study found that more than 30% of veterans with PTSD received at least 1 BZD prescription.3 The risks associated with BZD treatment for PTSD are compounded by concurrent use of other sedatives and opioids prescribed for co-occurring chronic pain and insomnia.3
Older adults metabolize long-acting BZDs more slowly and generally have an increased sensitivity to the adverse effects (AEs) of all BZDs.4 In older adults, BZD use has been associated with cognitive decline, dementia, falls and consequent fractures, and adverse respiratory outcomes.5-12 The risk of most but not all of these AEs was increased with higher BZD dose or long-term BZD use, which this quality improvement project (QIP) defines as having at least a 60-day supply of BZD prescriptions dispensed within the past year.
Long-term BZD use increases with age. One study found that, among patients receiving a BZD, the rate of long-term BZD use was more than double in older adults (31.4%) than it was in adults aged between 18 and 35 years (14.7%).2 For these reasons, the 2012 Beers criteria of the American Geriatrics Society recommend avoiding all types of BZDs in the treatment of insomnia, agitation, or delirium in patients aged > 65 years.13 Despite this recommendation, the prevalence of BZD use in older adults remains high.14
Some innovative approaches have been developed to address the inappropriate use, including overuse and misuse, of BZDs in older adults.15 In one approach, direct-to-consumer (DTC) information is used to empower patients to collaborate with their physician to manage their health. Results from several studies suggest that providing older patients with information on BZD risks and benefits increases patient–physician interaction and thereby decreases inappropriate BZD use and improves health outcomes.4,16,17 One study found that perceptions of BZD risks increased 1 week after exposure to a DTC educational brochure (EB), with intention to discuss BZD discontinuation with their physician higher for patients who received the EB than it was for those who did not (83.1% vs 44.3%; P < .0001).16 The EMPOWER (Eliminating Medications Through Patient Ownership of End Results) cluster randomized controlled trial assessed the effectiveness of a DTC EB focused on BZD risks in older adults.17 In that seminal study, patients who received a DTC EB were more likely than were comparison patients to discontinue BZD within 6 months (27% vs 5%; risk difference, 23%; 95% CI, 14%-32%).
The Veterans Integrated Systems Network (VISN) 22 Academic Detailing Program is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20 With BZD use among older veterans remaining high, the VISN 22 program initiated a clinical QIP modeled on the EMPOWER trial. Veterans in VISN 22 received the DTC EB, which included information on BZD risks and encouraged them to discuss their BZD treatment with their health care provider. VISN 22 was the first VISN in the VHA to implement the EMPOWER protocol.
As this was a QIP, all eligible veterans in VISN 22 were mailed the DTC EB, thus making it difficult to estimate the impact of the EB on BZD discontinuation in this VISN. Therefore, DTC EB efficacy was estimated by comparing BZD discontinuation between VISN 22 and VISN 21, an adjacent VISN that did not mail the DTC EB.
Methods
Two QIPs were undertaken to determine the impact of DTC EB on BZD use in older veterans in the VHA.
Quality Improvement Project 1
Design. A retrospective cohort analysis was performed. The VISN 22 catchment area, which encompasses VA facilities and clinics in southern California and southern Nevada, serves about 500,000 veterans, a substantial proportion of whom are aged ≥ 65 years. Among these older veterans are active long-term BZD users, who were defined as having ≥ 60-day supply of BZD prescriptions dispensed within the past year. Each active long-term user with a BZD prescription released within 200 days before the index date (the date the user was to meet with the prescribing physician) was mailed an EB 2 to 8 weeks in advance of the visit. Excluded from analysis were veterans with a schizophrenia, spinal cord injury, or seizure disorder diagnosis recorded in both their inpatient and outpatient medical records; veterans seen by Palliative Care within the past year; and veterans who died before analysis was completed.
Education Brochure. The EB for VISN 22 (Figure 1, see
Patients. The sample consisted of all veterans identified as meeting the inclusion criteria and being enrolled in VISN 22. The EB was mailed once to veterans on a rolling basis from December 2014 to February 2016. Change in BZD use was analyzed only after 9 to 24 months had passed since the index appointment with the prescribing physician. This period included 12 weeks for BZD taper and then 6 months after taper.
Analysis. For each veteran, monthly mean lorazepam equivalent (LE) was calculated using as many as 12 fills before the index date. Average daily dose of LE was calculated by dividing the sum of LE from all included prescriptions by total number of days between the first fill and the index date. The BZD prescription fills were evaluated after the index date. Veterans who received at least 1 prescription after the index date but then had no BZD prescription activity in VA clinics for 3 consecutive months during the 9-month observation period were recorded as having tapered and then discontinued BZD. Veterans who had no BZD prescription activity in VA clinics after the index date and during the 9-month observation period were recorded as having discontinued BZD without tapering. For veterans who had BZD prescription activity in VA clinics after the index date and during the 9-month observation period, mean LE was calculated by dividing the total LE for BZD prescriptions after the index date by number of days from the first fill after the index date to the date of analysis.
Quality Improvement Project 2
Design. A retrospective cohort analysis using PSM was performed on a subgroup of the QIP-1 sample to evaluate the impact of EB on BZD prescribing in the VA during 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. Veterans in the analysis were active long-term BZD users, had at least 1 BZD prescription released within 200 days before the index date, were aged ≥ 65 years, and had an appointment scheduled with their BZD prescriber within 2 to 8 weeks (Figure 2).
Patients. VISN 22 implemented QIP-2, a real-world application of a modified EMPOWER program, by identifying eligible veterans on a rolling basis from December 2014 to August 2015. All veterans who were identified and sent an EB during this period were included in the case group. The index date was defined as the first of the month the EB was mailed. Veterans with a pending appointment were chosen because the lead time would allow them to receive the EB and prepare to discuss it with the physician during the visit.
A comparator group was drawn from the adjacent VISN 21 catchment area, which encompasses VA facilities and clinics in Hawaii, northern California, and northern Nevada. During the observation period, VISN 21 did not mail any EBs specifically addressing BZD risks. Veterans in the comparator group had an appointment scheduled with their BZD prescribing physician within 4 weeks, were aged ≥ 65 years on the index date (first of the month before the next appointment, coinciding with the date EBs were sent to VISN 22 veterans), were active long-term BZD users, and had at least 1 BZD prescription released within 200 days before the index date. All patients were followed for up to 12 months after the index date, with BZD discontinuation recorded 9 and 12 months after the index date.
Propensity Score Matching
Propensity score (PS) was estimated with logistic regression analysis with treatment as the dependent variable and baseline characteristics as the independent variables.21,22 One-to-one matching on the PS was performed using the nearest neighbor approach without replacements. Independent variables related to outcome but unrelated to EB exposure were selected for PS development.22 These variables included year of birth; male sex; Hispanic ethnicity; annual income; service connection status; region; body mass index; Charlson Comorbidity Index category; total baseline BZD dose; and diagnosis of AIDS, nonmetastatic cancer, metastatic cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), dementia, diabetes mellitus (DM), DM with complications, gastroesophageal reflux disease (GERD), general anxiety disorder (GAD), hemiparaplegia, liver disease (mild), liver disease (moderate to severe), myocardial infarction (MI), Parkinson disease, peptic ulcer disease (PUD), psychosis, renal disease, rheumatoid arthritis (RA), or substance use disorder (SUD).
The EMPOWER cluster randomized controlled trial (RCT) demonstrated the effectiveness of EB exposure in a Canadian population of elderly patients who were long-term BZD users.17 Randomized controlled trials are the gold standard for clinical trials because they can establish causal inference.23-25 Given ethical and practical concerns, however, RCTs cannot be applied to all clinical scenarios. Although EMPOWER is reported to be an effective tool in reducing BZD use in older adults, its application in a real-world, large, integrated health care system remains untested. Observational studies are often conducted as an alternative to RCTs but are subject to selection bias because of their lack of randomization.26 Therefore, robust research methods are needed to generate unbiased estimates of the impact of an intervention on an outcome. Propensity score matching simulates an RCT by balancing the covariates across treatment groups.21,22,27 Observed patient characteristics are used to estimate PS, the probability that treatment will be received. Logistic or probit regression is used to balance the potential confounding covariates between the treatment groups.Once PSs are known, mean treatment effect can be estimated without the mean model.28 In other words, PSM methods can be used to generate an unbiased estimate of the treatment.
Propensity Score Analysis
Baseline characteristics were compared using Student t test (continuous variables) and χ2 test (discrete variables). Results are presented as means and standard deviations (continuous variables) and frequency and percentage (discrete variables).
The main outcome was BZD discontinuation 9 and 12 months after the index date. A postindex lag of 6 months was used to capture any tapering (Figure 2). Discontinuation, defined as 3 consecutive months of no BZD prescription on hand, was measured for 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. An estimate was made of the difference in the proportions of BZD discontinuers who received the EB and BZD discontinuers who did not receive the EB, where mean treatment (risk difference) was presented as the absolute risk difference with a 95% CI. Standard errors and 95% CIs for the risk differences were generated with biased-corrected CIs from 1,000 bootstrap samples.
Sensitivity Analyses
Naïve multivariate logistic regression analysis was performed to evaluate the association between EB exposure and BZD discontinuation while controlling for potential confounders. Results are presented as odds ratios (ORs) and 95% CIs. Confounders identified were the same covariates used to generate the PSs.
Several analyses were performed to test the sensitivity of the methods applied using PSM by changing caliber size while maintaining the nearest neighbor approach without replacement. Linear regression analysis was performed with robust standard errors to estimate the risk difference of BZD discontinuation between EB-exposed and EB-unexposed veterans.
Statistical significance was set at P < .05. All statistical analyses were performed with Stata/SE Version 13 (College Station, TX).
Results
Quality Improvement Project 1
On a rolling basis from December 2014 to February 2016, the EB was mailed once to 3,896 VISN 22 veterans 2 to 8 weeks before a clinic appointment with their BZD prescribing physician.
Quality Improvement Project 2
Of all the VISN 22 and VISN 21 veterans, 24,420 met the inclusion and exclusion criteria. Of these 24,420 veterans, 2,020 (8.3%) were in VISN 22 and received the EB between December 2014 and August 2015 (QIP-1), and 22,400 (91.7%) were in VISN 21 and did not receive the EB.
Naïve Results Before PS Matching. In the naïve analyses, a larger proportion of EB-exposed vs unexposed veterans discontinued BZD; in addition, reductions were 6.6%, 7.4%, and 9.5% larger for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (P < .0001 for all comparisons; Table 2).
After controlling for potential confounders, the naïve logistic regression analyses found EB exposure was significantly associated with 44%, 32%, and 42% increases in the odds of BZD discontinuation for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (Table 3).
Propensity Score Matching. Before matching, there were significant differences in baseline characteristics of veterans who met the inclusion and exclusion criteria, with few exceptions (eAppendices 2 and 3, ).
Propensity Score Matching Results. Inspection of PSs revealed good coverage across treatment groups on a histogram plot and a kernel density plot (eAppendices 5 and 6).
Discussion
This QIP was the first to evaluate the impact of an EMPOWER-modeled DTC EB in a large, integrated health care system in the U.S. It was also the first to demonstrate potential benefits of a DTC EB designed for older veterans who are long-term BZD users. In this QIP, which mailed the EB to 3,896 veterans, 1,847 (47.4%) decreased their BZD dose, 458 (11.7%) tapered and then discontinued BZD, and 455 (11.7%) immediately discontinued BZD. The total percentage of veterans who discontinued BZD (23.4%; 913/3,896) was similar to the 27% reported in the EMPOWER trial.17 However, the risk difference between the 1,316 EB-exposed VISN 22 veterans (QIP-1) and the 1,316 EB-unexposed VISN 21 veterans in this QIP was significantly lower than the 23% risk difference in EMPOWER (though it still demonstrated a significantly larger reduction for EB-exposed veterans).17
Given this inclusion of all qualifying veterans from the catchment area studied in this QIP, and given the ethical and practical concerns, an RCT was not possible. Therefore, PSM methods were used to balance the covariates across treatment groups and thereby simulate an RCT.21,22,27 With use of the PSM approach, findings from the descriptive analysis were confirmed and potential selection bias reduced.
Study Limitations
The less robust risk difference found in this QIP has several possible explanations. The authors’ use of a DTC EB coincided with a national VA effort to reduce older veterans’ use of BZDs and other inappropriate medications. For instance, during the study period, academic detailing was being implemented to reduce use of BZDs, particularly in combination with opioids, across VHA facilities and clinics. (Academic detailing is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20) However, QIP-2 results and PS analysis of a subgroup of the original sample suggest that EB-exposed veterans were significantly more likely than were their unexposed counterparts were to discontinue BZD. To an extent, this analysis controlled for these other efforts to reduce BZD use in VHA clinics and can be considered a study strength.
Another limitation is the study design, which lacked a control group and did not consider the possibility that some facility or clinic physicians might influence others. Although the region variable was controlled for in PSM, the authors did not capture facility characteristics, including frequency of prescribing BZD and use of a protocol for enforcing the Beers criteria. Such confounders might have influenced outcomes. Unlike the EMPOWER trial,17 this QIP did not assess or exclude cognitively impaired veterans. It is reasonable to assume that these veterans might not understand some EB messages and consequently might fail to engage their physicians. Failure to initiate discussion with a physician would attenuate the impact of the EB.
Study Strengths
A strength of this QIP was its use of a DTC EB in a large, regional sample of older veterans in a real-world clinical setting. In addition, the study group (EB-exposed veterans) and the comparator group (EB-unexposed veterans) were from similar geographic areas (primarily California and Nevada).
Conclusion
Results of this study suggest that a DTC EB, designed to reduce BZD use among older veterans, was effective in helping patients lower their BZD dose and discontinue BZD. The likelihood of discontinuing BZD 9 and 12 months after the index date was significantly higher for veterans who received an EB modeled on the EMPOWER educational brochure than for a comparator group of veterans who did not receive the EB and were receiving care during the same observation period. In the future, it would be beneficial to use a design that controls for physician exposure to academic detailing focused on BZD reduction and that accounts for the cluster effects of facility practice. Despite these limitations, this QIP is the first real-world empirical example of using an EMPOWER-modeled DTC EB to decrease BZD use among older veterans. Furthermore, these results suggest that a DTC EB can be used to target other high-risk prescription drugs, such as opioids, particularly if alternative treatment options can be provided.
Acknowledgments
Dr. Hauser thanks Cathy, Anika, Katia, and Max Hauser, and Alba and Kevin Quinlan, for their support. In memory of Jirina Hauser, who died on Mother’s Day, May 14, 2017, at the age of 100.
1. Dell’osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry. 2013;28(1):7-20.
2. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
3. Bernardy NC, Lund BC, Alexander B, Friedman MJ. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
4. Roberts KJ. Patient empowerment in the United States: a critical commentary. Health Expect. 1999;2(2):82-92.
5. Paterniti S, Dufouil C, Alpérovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22(3):285-293.
6. van der Hooft CS, Schoofs MW, Ziere G, et al. Inappropriate benzodiazepine use in older adults and the risk of fracture. Br J Clin Pharmacol. 2008;66(2):276-282.
7. Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf. 2010;19(12):1248-1255.
8. Finkle WD, Der JS, Greenland S, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59(10):1883-1890.
9. de Gage SB, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231
10. Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639-658.
11. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with chronic obstructive pulmonary disease. Eur Respir J. 2014;44(2):332-340.
12. Gomm W, von Holt K, Thomé F, et al. Regular benzodiazepine and z-substance use and risk of dementia: an analysis of German claims data. J Alzheimers Dis. 2016;54(2):801-808.
13. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
14. National Institutes of Health. Despite risks, benzodiazepine use highest in older people. https://www.nih.gov/news-events/news-releases/despite-risks-benzodiaze pine-use-highest-older-people. Published December 17, 2014. Accessed July 31, 2018.
15. Airagnes G, Pelissolo A, Lavallée M, Flament M, Limosin F. Benzodiazepine misuse in the elderly: risk factors, consequences, and management. Curr Psychiatry Rep. 2016;18(10):89.
16. Martin P, Tamblyn R, Ahmed S, Tannenbaum C. A drug education tool developed for older adults changes knowledge, beliefs and risk perceptions about inappropriate benzodiazepine prescriptions in the elderly. Patient Educ Couns. 2013;92(1):81-87.
17. Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898.
18. Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263(4):549-556.
19. Fischer MA, Avorn J. Academic detailing can play a key role in assessing and implementing comparative effectiveness research findings. Health Aff (Millwood). 2012;31(10):2206-2212.
20. Wells DL, Popish S, Kay C, Torrise V, Christopher ML. VA Academic Detailing Service: implementation and lessons learned. Fed Pract. 2016;33(5):38-42.
21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424.
22. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156.
23. Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. J Ed Psych. 1974;66(5):688-701.
24. Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(4):722-729.
25. Cartwright N. What are randomized controlled trials good for? Philos Stud. 2010;147(1):59.
26. Kleinbaum DG, Morgenstern H, Kupper LL. Selection bias in epidemiologic studies. Am J Epidemiol. 1981;113(4):452-463.
27. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55.
28. Pirracchio R, Carone M, Rigon MR, Caruana E, Mebazaa A, Chevret S. Propensity score estimators for the average treatment effect and the average treatment effect on the treated may yield very different estimates. Stat Methods Med Res. 2016;25(5):1938-1954.
1. Dell’osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry. 2013;28(1):7-20.
2. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
3. Bernardy NC, Lund BC, Alexander B, Friedman MJ. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
4. Roberts KJ. Patient empowerment in the United States: a critical commentary. Health Expect. 1999;2(2):82-92.
5. Paterniti S, Dufouil C, Alpérovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22(3):285-293.
6. van der Hooft CS, Schoofs MW, Ziere G, et al. Inappropriate benzodiazepine use in older adults and the risk of fracture. Br J Clin Pharmacol. 2008;66(2):276-282.
7. Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf. 2010;19(12):1248-1255.
8. Finkle WD, Der JS, Greenland S, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59(10):1883-1890.
9. de Gage SB, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231
10. Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639-658.
11. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with chronic obstructive pulmonary disease. Eur Respir J. 2014;44(2):332-340.
12. Gomm W, von Holt K, Thomé F, et al. Regular benzodiazepine and z-substance use and risk of dementia: an analysis of German claims data. J Alzheimers Dis. 2016;54(2):801-808.
13. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
14. National Institutes of Health. Despite risks, benzodiazepine use highest in older people. https://www.nih.gov/news-events/news-releases/despite-risks-benzodiaze pine-use-highest-older-people. Published December 17, 2014. Accessed July 31, 2018.
15. Airagnes G, Pelissolo A, Lavallée M, Flament M, Limosin F. Benzodiazepine misuse in the elderly: risk factors, consequences, and management. Curr Psychiatry Rep. 2016;18(10):89.
16. Martin P, Tamblyn R, Ahmed S, Tannenbaum C. A drug education tool developed for older adults changes knowledge, beliefs and risk perceptions about inappropriate benzodiazepine prescriptions in the elderly. Patient Educ Couns. 2013;92(1):81-87.
17. Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898.
18. Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263(4):549-556.
19. Fischer MA, Avorn J. Academic detailing can play a key role in assessing and implementing comparative effectiveness research findings. Health Aff (Millwood). 2012;31(10):2206-2212.
20. Wells DL, Popish S, Kay C, Torrise V, Christopher ML. VA Academic Detailing Service: implementation and lessons learned. Fed Pract. 2016;33(5):38-42.
21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424.
22. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156.
23. Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. J Ed Psych. 1974;66(5):688-701.
24. Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(4):722-729.
25. Cartwright N. What are randomized controlled trials good for? Philos Stud. 2010;147(1):59.
26. Kleinbaum DG, Morgenstern H, Kupper LL. Selection bias in epidemiologic studies. Am J Epidemiol. 1981;113(4):452-463.
27. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55.
28. Pirracchio R, Carone M, Rigon MR, Caruana E, Mebazaa A, Chevret S. Propensity score estimators for the average treatment effect and the average treatment effect on the treated may yield very different estimates. Stat Methods Med Res. 2016;25(5):1938-1954.
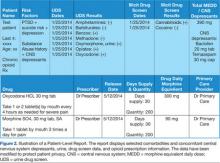
Using Dashboard Technology to Monitor Overdose Risk
On October 10, 2013, a Congressional hearing was held to address the issue of opioid medication prescribing within VHA facilities and clinics (House Veteran Affairs Subcommittee hearing “Between Peril and Promise: Facing the Dangers of VA’s Skyrocketing Use of Prescription Painkillers to Treat Veterans”). Several individuals testified, including the widows of 2 veterans; both their husbands had overdosed on prescribed opioid medications. One husband had been taking as many as 15 pills a day and was additionally prescribed oxycodone/acetaminophen, which led to his death.1
Alongside the widows were 2 veterans who had been treated for chronic back pain injuries sustained before and during deployment in Iraq. Both had been prescribed several pain medications, including oxycodone/acetaminophen, methadone, and morphine. One reported that as his pain increased, his doctors continued to provide him additional prescriptions; at one point he had more than 13 prescriptions and could no longer work from being so “doped up.”1
In the past 2 decades, health care professionals (HCPs) have placed greater emphasis on chronic pain management. As a result, the rate of opioid medication prescribing has increased dramatically. Since 1994, the number of opioid medication prescriptions has nearly doubled; this change has been accompanied by an increase in opioid misuse, which has resulted in accidental or intentional overdose and death.2
Based on a recent National Institute on Drug Abuse (NIDA) report, the greatest impact has been on armed forces personnel.3 Prescriptions for pain relievers quadrupled between 2001 and 2009 to almost 3.8 million within the military population. Although civilian populations are more likely to abuse illicit drugs, military personnel are at particular risk of prescription abuse, including opioid medications.3 In 2008, 11% of armed forces service members reported misusing prescription drugs, with opioid medications being the most abused. This is an approximate 5- to 6-fold increase since 2002 (2% reported misuse in 2002).3 Particularly concerning is the associated rise in suicide rates among armed forces personnel, which surpassed civilian suicide rates in 2004. In 2009, one-third of suicides among armed forces personnel involved prescription drugs.3
Certain patient characteristics or factors are related to greater overdose risk. These risk factors include prescription dosage and frequency, history of suicide attempts or self-harm behavior, history of depression or posttraumatic stress disorder (PTSD) among other mental health-related diagnoses, a history of substance and/or alcohol abuse, and within the context of opioid medication use, the concurrent use of other central nervous system (CNS) depressants.4,5 Additionally, the stresses of deployment during wartime, physical injuries sustained in combat, and the unique military culture play a particularly important role in access to substances with high abuse potential and the subsequent development of substance abuse.3
Opioid Use and Risk Factors
More than 3% of adults in the U.S. are now receiving opioid medications for chronic noncancer pain.6 Substance abuse among patients with chronic pain ranges from 14% to 40%.5 Prescription opioid medications are the fastest growing drugs of abuse and the most common cause of unintentional overdose in the U.S.4 About 17,000 deaths occur each year as a result of prescription opioid medication overdose.7 Opioid medication-related overdose deaths began to increase in the early 2000s and continue to increase. Between 1999 and 2007, the rate of unintentional overdose-related deaths in the U.S. increased by 124%, largely due to the increase of prescription opioid medications.8
High-Dose Opioid Medication Use
A study by Dunn and colleagues found that patients receiving higher doses of prescribed opioid medications were at an increased risk of overdose.6 Patients receiving 50 mg to 99 mg morphine equivalent daily dose (MEDD) had a 3.7-fold increase in overdose risk (0.7% annual overdose rate) as compared with patients who received < 50 mg MEDD (0.2% annual overdose rate). Patients receiving ≥ 100 mg MEDD had a 1.8% annual overdose rate and a 9.8-fold increase in overdose risk as compared with patients who received < 50 mg MEDD. Overall, 51 patients experienced ≥ 1 overdose event, 40 of whom experienced fatal or serious overdoses and 6 of whom attempted suicide. Patients receiving the highest doses were male, current smokers, and had a history of depression and substance abuse.6 Similarly, a study by Bohnert and colleagues found that opioid medication overdose was most likely to occur in those patients with psychiatric and substance use disorders compared with patients who had no psychiatric illness history.8
Depression
Mood disorders are common in people with chronic pain.4,5,9,10 In particular, patients with a history of depression are more likely to receive chronic opioid medication prescriptions and are at a higher risk for opioid medication abuse. A substance abuse history is the most consistent predictor of both chronic opioid medication use and abuse. However, depression without substance abuse is significantly associated with 2 forms of opioid medication abuse: self-medication for stress or sleep and overmedication (using a higher dose than prescribed). More severe cases of depression show a stronger association for potential abuse.4
PTSD
Among Iraq and Afghanistan war veterans with ≥ 1 pain-related diagnosis, veterans with PTSD and veterans with a mental health disorder other than PTSD were significantly more likely to receive opioid medications for pain than were veterans without a mental health disorder (PTSD—17.8%, adjusted relative risk [RR] 2.58; other mental health disorder—11.7%, RR 1.74; no mental health disorder—6.5%).2 Although mental health disorders in general were related to a higher risk of opioid abuse, those with PTSD in particular were more likely to receive higher prescribed dosages; to continue taking opioids for a longer period; to receive concurrent prescriptions for opioid medications, sedative hypnotics, or both; to obtain early refills; and to have comorbid alcohol and substance use disorders. Based on these results, Seal and colleagues concluded that veterans with PTSD had the highest risk of alcohol, drug, and opioid-related accidents and overdose as well as self-inflicted injuries.2
Concurrent Use of Opioids and CNS Depressants
As mentioned earlier, studies suggest that people with PTSD are at a significantly higher risk for opioid medication overdose. One factor that may contribute to this higher risk is the concurrent use of CNS depressants/sedatives, particularly benzodiazepines and alcohol.
Benzodiazepines are often prescribed for people with PTSD. One study found that the concurrent use of benzodiazepines is significantly related to opioid overdose.5 Prescribing opioids for people already abusing or dependent on alcohol or other substances increases the risk of abuse and overdose. Furthermore, the concurrent use of multiple medications is associated with aberrant behaviors, cognitive impairment, and medication abuse, potentially leading to overdose. Overall, the combined administration of these medications is responsible for higher rates of adverse events, overdose, and death related to prescription opioid medication use.5,6,11
In summary, there are various risk factors that contribute to opioid medication overdose and more generally, risk of suicide, including (1) high-dose opioid medications; (2) history of psychiatric disorders, specifically depression and PTSD; (3) history of substance use disorders; and (4) concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse.
Suicide
Suicide is the tenth leading cause of death in the U.S., with 12.4 suicide deaths per 100,000 population.12 Suicide rates are even higher among veterans. According to the VHA, the age-adjusted rate of suicide for veterans using VHA facilities and clinics was 35.9 per 100,000 person-years for fiscal year 2009.13 Several risk factors for suicide attempts include depression and other mental health disorders, substance abuse, medical disorders, and prescription medications.
Prior suicide attempts or self-harm behavior is known to increase the risk of subsequent death by suicide. About 11 attempted suicides occur per suicide death where the medical severity of prior attempts increases the risk of future suicide, as does a history of multiple self-harm episodes.14,15 One study found that the single best predictor of suicide in a veteran population was an attempt in the previous 4 months.16
Among other risk factors, previous suicide attempts and violent behavior are major behavioral flags that warrant caution and require particular consideration when prescribing opioid medications. In a national survey on drug use and health, about 18% of prescription opioid users/abusers who experienced suicidal ideations actually attempted suicide. Only 11% of individuals who never used prescription opioid medications attempted suicide after reported suicidal ideations.17
Patient Data Aggregation
The early and widespread adoption of electronic medical records (EMRs) by the VHA allowed the aggregation of patient data for quality improvement. Initially, data were aggregated, and dashboards were designed retrospectively. However, the development of regional data warehouses that update patient information daily from the EMR allowed information to be aggregated prospectively, and dashboards were designed that provided real-time information.
The purpose of the current study is to demonstrate the efficacy and future potential of dashboard technology in assessing prospectively high-risk factors for opioid overdose. Dashboards are a user-friendly application that allows providers to isolate and calculate daily morphine equivalent opioid dosages and assess patients’ risk factors for overdose on an individual basis. By using this technology, providers who prescribe opioids can get a concise summary of opioid and other medications and adjust medications to decrease overdose risk on an individual basis.
What is the Dashboard?
The VISN 22 high-risk opioid dashboard is a business intelligence tool that serves as a report card, or progress report, to provide a global view of the number of veterans who are receiving opioid prescriptions totaling >120 mg MEDD and who have characteristics (history of depression, PTSD, substance abuse, or high-risk suicide flag) and prescriptions (concomitant CNS depressants) that may increase patient risk for overdose.
The VISN 22 dashboard allows the user to navigate to an individual HCP-level and patient-level report (Figures 1 and 2). Filter settings allow report users to select only high-risk patients; it serves as a single location for pertinent details to consider for safely prescribing opioids.
To calculate daily morphine equivalents, each patient’s opioid prescriptions were evaluated. The quantity was divided by the day’s supply to calculate an average daily quantity. From there, the drug strength was used to convert to MEDD. Health care providers were informed that these conversion factors were not recommendations for clinical opioid conversions.
Implementation and Design
In 2012, the VA Pharmacy Benefits Management (PBM) in VISN 21 created a dashboard that allowed users to identify patients on high-dose opioid prescriptions. Structured query language code was used to extract data from the regional data warehouse and calculate MEDD for all patients with active opioid prescriptions. In 2013, VISN 22 expanded that dashboard to incorporate factors that could indicate a high risk for overdose or other adverse outcomes, including a history of depression, PTSD, substance abuse or high-risk suicide flag, and concomitant use of CNS depressant medications.
The high-risk opioid dashboard (Figure 3) and accompanying patient-level report were first introduced to VISN 22 HCPs in January 2013. The business intelligence tools were introduced to each facility through the VISN 22 PBM group. Training on the use of the dashboard and the report was provided, with an initial target of decreasing MEDD of > 200 mg to < 5% of all veterans prescribed opioids at each VISN 22 facility. One month later (in February 2013), a second category of veterans (those with > 120 mg but < 199 mg MEDD) was added. Also the initial MEDD > 200 mg target of < 5% was decreased to < 3% to encourage additional progress.
Eight months after the VISN 22 dashboard technology was implemented there was a 17% decrease in patients with total daily morphine equivalents > 200 mg (January 2013; 1,137 patients vs August 2013; 940 patients—a decrease of 197 patients).
From March 2013 to August 2013, VISN 22 also saw a 12% decrease in the number of patients prescribed > 120 mg MEDD but < 199 MEDD (March 2013; 2,295 vs August 2013; 2,018—a decrease of 277 patients).
Figure 4 shows opioid use as of July 2014 for VISN 22 facilities. There were further reductions in the number of patients receiving > 120 mg but < 199 mg MEDD (August 2013; 2,018 patients vs July 2014; 1,189 patients) and patients receiving > 200 mg MEDD (August 2013; 940 patients vs July 2014; 836 patients).
Case Description
In January 2013, VISN 22 implemented dashboard technology to help providers assess and monitor opioid prescription levels in relation to high-risk variables. The benefits of this dashboard technology are illustrated in the case profile that follows.
A 67-year-old male veteran had a long history of chronic pain. Pain diagnoses included osteoarthritis with spine involvement, lumbar radiculopathy, arthralgia, and peripheral neuropathy. For the past 10 years, he was prescribed opioids with modest relief of his chronic pain symptoms despite recent prescriptions totaling 300 mg MEDD. This veteran had several risk factors for overdose, including a history of depression, suicide risk, PTSD, and concomitant use of the CNS depressants alprazolam and cyclobenzaprine.
More recently, in May 2013, the veteran exhibited aberrant behavior and requested early refills for alprazolam. In response, the pharmacist discussed the case with the HCP who prescribed the opioids, noting the concomitant overdose risk factors. As a result of this interaction, the veteran was referred for mental health services, and his prescriptions for opioids were gradually decreased. He is currently stable, now receiving 120 mg MEDD, and his pain is currently described as moderately controlled on the new lower dose.
In summary, this veteran was receiving > 200 mg MEDD with several known overdose risk factors. Once the HCP was made aware of these risk factors, necessary precautions were taken, and the veteran was safely tapered to a lower dose. Dashboard technology makes the list of risk factors readily available to HCPs who are prescribing (and the pharmacists reviewing the prescriptions), thus allowing a proactive discussion of risks and benefits before continuing, renewing, or initiating opioid prescriptions.
Discussion
As reported in 2013 by NIDA, the greater availability of opioid medications and the consequent increase in prescriptions may be contributing directly to their growing misuse by both civilians and military service personnel. A direct consequence has been an increase in both accidental and intentional overdose deaths.3 Several factors are related to the risk of overdose/death, including high-dose opioid medications, a history of psychiatric disorders (specifically depression and PTSD), a history of substance use disorders, concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse, and previous attempts of suicide.
The VISN 22 high-risk opioid dashboard was a rapid information technology response to the increasing risk faced by veterans who have chronic pain and comorbid psychiatric and substance use disorders and are prescribed opioids and CNS depressants. The purpose of implementing this dashboard technology was to assist HCPs in prescribing opioids safely, using a technology that allows for the monitoring and management of concomitant suicide risk factors. Following the national Opioid Safety Initiative, this dashboard tool is being used to identify veterans who are on high-dose opioids with the goal of reducing the number of veterans on > 200 mg MEDD. The dashboard allows data to be stratified, using the concomitant risk factors for suicide to assist facilities and their providers in the identification and prioritization of highest risk patients first.
An initial review of dashboard data in VISN 22 suggests that it is a useful tool for reducing high-dose opioid prescriptions (> 200 mg MEDD and > 120 mg but < 199 mg MEDD). Across the 5 VA locations in VISN 22, in the first 8 months of implementation, 4 locations were able to lower prescription opioid medication levels to the initial target of < 5%; 2 lowered rates even further (to < 3%). The VA Greater Los Angeles Healthcare System remains at a commendable 1%. Although the number of veterans with prescriptions totaling > 200 mg MEDD has decreased as a result, a greater reduction is expected with the coordinated education and systems improvement efforts associated with the national VHA Opioid Safety Initiative. As part of the process to lower the number of patients on high-dose opioids in the future, HCP and patient education will be provided in relation to the use of dashboard technology.
Limitations
There are several limitations that affect interpretation of the usefulness of the VISN 22 high-risk opioid dashboard. Prior to the implementation of the dashboard, 2 of 5 VISN sites already had efforts in place to reduce opioid overprescribing. The VA Greater Los Angeles Healthcare System had an opioid reduction program in place before the dashboard was implemented, so it is possible reductions in opioid prescribing were a result of their previous efforts and not related to the dashboard. Similarly the VA Long Beach Healthcare System had begun a quality improvement initiative to reduce high-dose opioid prescribing prior to dashboard implementation. However, it was difficult to pinpoint the direct effect the dashboard had on patient interventions due to lack of documentation of dashboard use in the clinical notes.
A direct relationship did exist between dashboard implementation and opioid dose reduction in patients with > 200 MEDD at the remaining 3 VISN 22 facilities. Overall, this suggests that the dashboard played a significant role across all sites. Implementation of the dashboard across VISN 22 was accompanied by an education effort that resulted in an increased awareness among HCPs to evaluate certain risks in patients on high-dose opioids and to evaluate the combination of opioid and CNS depressant use. Prior to dashboard implementation, there was no standardized monitoring system that cross-referenced high-dose opioid prescribing with psychiatric illness and suicide risk factors.
Conclusions
From 2000 to 2010, opioid prescriptions nearly doubled, yet this rate was not accompanied by a change/increase in the rate of nonopioid analgesic medication prescriptions.18 Health care providers need to account for veterans’ wishes for pain treatment and be aware of options other than opioids, particularly given the risk of opioid-related accidental or intentional overdose; it is imperative that treatment become more individualized and more closely monitored.19,20 It is recommended that opioids should be the treatment of last resort in managing chronic noncancer pain. The use of opioid prescription medications should be intended as a trial, supported by clear goals and an unequivocal understanding that doses will not be indiscriminately increased.20
Health care providers who prescribe opioids are ultimately responsible for monitoring risk factors that may increase overdose and death, and dashboard technology assists them in this effort. The VISN 22 high-risk opioid dashboard is a tool that allows providers to identify and prioritize veterans who are at high risk for overdose. Initial data collected suggest that the dashboard has decreased the risk of negative consequences associated with opioid medication use today. However, the authors wish to emphasize that this technology is only part of the solution; although it can be a tool to identify actions that may need to take place and can track progress of changes in care, there must be complementary efforts in provider and patient education, improved access to mental health care, and interdisciplinary models of care that expand current chronic pain treatment options. Future considerations of this technology may include incorporating other risk factors accounting for psychosocial variables specific to military personnel that may further increase the overall risk of overdose.
Acknowledgements
The authors wish to thank the leadership of VISN 22 for their support of this initiative. Dr. Kryskalla recognizes VA OI&T for making this work possible and her family for their support. Ms. Kern would like to thank Aaron, Leslie, and Rachel Kern for their continuous support. Dr. Hauser wishes to thank Cathy, Anika, Katia, Max, and Jirina Hauser for their unwavering support.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[Published Online Ahead of Print: August 14, 2014.]
1. Brooks D. Hearing Spotlights painkiller overuse among soldiers. http://www.fayobserver.com/military/article_a6e4a2e9-827d-577c-a79a-87a6c07cf151.html. Fayobserver Website. Published October 10, 2013, Accessed June 9, 2014.
2. Seal KH, Shi Y, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
3. National Institute on Drug Abuse. DrugFacts: Substance Abuse in the Military. http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military. National Institute on Drug Abuse Website. Revised March 2013. Accessed June 9, 2014.
4. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311.
5. Pergolizzi JV Jr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443-451.
6. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85-92.
7. Substance Abuse and Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit. HHS publication No. (SMA) 13-4742. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
8. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
9. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
10. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581-624.
11. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepine, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
12. Centers for Disease Control and Prevention. FastStats: Deaths and mortality. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/deaths.htm. Updated February 13, 2014. Accessed June 9, 2014.
13. Kemp J, Bossarte R. Suicide Data Report, 2012. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed July 1, 2014.
14. National Institute of Mental Health. Suicide in the U.S. Statistics. National Institute of Mental Health Website. http://www.nimh.nih.gov/statistics/index.shtml. Accessed June 27, 2014.
15. Miller M, Hempstead K, Nguyen T, Barber C, Rosenberg-Wohl S, Azrael D. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: Implications for clinical practice. Am J Public Health. 2013;103(6):e61-e68.
16. Hartl TL, Rosen C, Drescher K, Lee TT, Gusman F. Predicting high-risk behaviors in Veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2005;193(7):464-472.
17. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
18. Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870-878.
19. Bray RM, Pemberton MR, Lane ME, Hourani LL, Mattiko MJ, Babeu LA. Substance use and mental health trends among U.S. military active duty personnel. Key findings from the 2008 DoD Health Behavior Survey. Mil Med. 2010;175(6):390-399.
20. Cuevas-Trisan RL. The unfortunate turn of events in pain management. Fed Pract. 2013;30(3):8-9.
On October 10, 2013, a Congressional hearing was held to address the issue of opioid medication prescribing within VHA facilities and clinics (House Veteran Affairs Subcommittee hearing “Between Peril and Promise: Facing the Dangers of VA’s Skyrocketing Use of Prescription Painkillers to Treat Veterans”). Several individuals testified, including the widows of 2 veterans; both their husbands had overdosed on prescribed opioid medications. One husband had been taking as many as 15 pills a day and was additionally prescribed oxycodone/acetaminophen, which led to his death.1
Alongside the widows were 2 veterans who had been treated for chronic back pain injuries sustained before and during deployment in Iraq. Both had been prescribed several pain medications, including oxycodone/acetaminophen, methadone, and morphine. One reported that as his pain increased, his doctors continued to provide him additional prescriptions; at one point he had more than 13 prescriptions and could no longer work from being so “doped up.”1
In the past 2 decades, health care professionals (HCPs) have placed greater emphasis on chronic pain management. As a result, the rate of opioid medication prescribing has increased dramatically. Since 1994, the number of opioid medication prescriptions has nearly doubled; this change has been accompanied by an increase in opioid misuse, which has resulted in accidental or intentional overdose and death.2
Based on a recent National Institute on Drug Abuse (NIDA) report, the greatest impact has been on armed forces personnel.3 Prescriptions for pain relievers quadrupled between 2001 and 2009 to almost 3.8 million within the military population. Although civilian populations are more likely to abuse illicit drugs, military personnel are at particular risk of prescription abuse, including opioid medications.3 In 2008, 11% of armed forces service members reported misusing prescription drugs, with opioid medications being the most abused. This is an approximate 5- to 6-fold increase since 2002 (2% reported misuse in 2002).3 Particularly concerning is the associated rise in suicide rates among armed forces personnel, which surpassed civilian suicide rates in 2004. In 2009, one-third of suicides among armed forces personnel involved prescription drugs.3
Certain patient characteristics or factors are related to greater overdose risk. These risk factors include prescription dosage and frequency, history of suicide attempts or self-harm behavior, history of depression or posttraumatic stress disorder (PTSD) among other mental health-related diagnoses, a history of substance and/or alcohol abuse, and within the context of opioid medication use, the concurrent use of other central nervous system (CNS) depressants.4,5 Additionally, the stresses of deployment during wartime, physical injuries sustained in combat, and the unique military culture play a particularly important role in access to substances with high abuse potential and the subsequent development of substance abuse.3
Opioid Use and Risk Factors
More than 3% of adults in the U.S. are now receiving opioid medications for chronic noncancer pain.6 Substance abuse among patients with chronic pain ranges from 14% to 40%.5 Prescription opioid medications are the fastest growing drugs of abuse and the most common cause of unintentional overdose in the U.S.4 About 17,000 deaths occur each year as a result of prescription opioid medication overdose.7 Opioid medication-related overdose deaths began to increase in the early 2000s and continue to increase. Between 1999 and 2007, the rate of unintentional overdose-related deaths in the U.S. increased by 124%, largely due to the increase of prescription opioid medications.8
High-Dose Opioid Medication Use
A study by Dunn and colleagues found that patients receiving higher doses of prescribed opioid medications were at an increased risk of overdose.6 Patients receiving 50 mg to 99 mg morphine equivalent daily dose (MEDD) had a 3.7-fold increase in overdose risk (0.7% annual overdose rate) as compared with patients who received < 50 mg MEDD (0.2% annual overdose rate). Patients receiving ≥ 100 mg MEDD had a 1.8% annual overdose rate and a 9.8-fold increase in overdose risk as compared with patients who received < 50 mg MEDD. Overall, 51 patients experienced ≥ 1 overdose event, 40 of whom experienced fatal or serious overdoses and 6 of whom attempted suicide. Patients receiving the highest doses were male, current smokers, and had a history of depression and substance abuse.6 Similarly, a study by Bohnert and colleagues found that opioid medication overdose was most likely to occur in those patients with psychiatric and substance use disorders compared with patients who had no psychiatric illness history.8
Depression
Mood disorders are common in people with chronic pain.4,5,9,10 In particular, patients with a history of depression are more likely to receive chronic opioid medication prescriptions and are at a higher risk for opioid medication abuse. A substance abuse history is the most consistent predictor of both chronic opioid medication use and abuse. However, depression without substance abuse is significantly associated with 2 forms of opioid medication abuse: self-medication for stress or sleep and overmedication (using a higher dose than prescribed). More severe cases of depression show a stronger association for potential abuse.4
PTSD
Among Iraq and Afghanistan war veterans with ≥ 1 pain-related diagnosis, veterans with PTSD and veterans with a mental health disorder other than PTSD were significantly more likely to receive opioid medications for pain than were veterans without a mental health disorder (PTSD—17.8%, adjusted relative risk [RR] 2.58; other mental health disorder—11.7%, RR 1.74; no mental health disorder—6.5%).2 Although mental health disorders in general were related to a higher risk of opioid abuse, those with PTSD in particular were more likely to receive higher prescribed dosages; to continue taking opioids for a longer period; to receive concurrent prescriptions for opioid medications, sedative hypnotics, or both; to obtain early refills; and to have comorbid alcohol and substance use disorders. Based on these results, Seal and colleagues concluded that veterans with PTSD had the highest risk of alcohol, drug, and opioid-related accidents and overdose as well as self-inflicted injuries.2
Concurrent Use of Opioids and CNS Depressants
As mentioned earlier, studies suggest that people with PTSD are at a significantly higher risk for opioid medication overdose. One factor that may contribute to this higher risk is the concurrent use of CNS depressants/sedatives, particularly benzodiazepines and alcohol.
Benzodiazepines are often prescribed for people with PTSD. One study found that the concurrent use of benzodiazepines is significantly related to opioid overdose.5 Prescribing opioids for people already abusing or dependent on alcohol or other substances increases the risk of abuse and overdose. Furthermore, the concurrent use of multiple medications is associated with aberrant behaviors, cognitive impairment, and medication abuse, potentially leading to overdose. Overall, the combined administration of these medications is responsible for higher rates of adverse events, overdose, and death related to prescription opioid medication use.5,6,11
In summary, there are various risk factors that contribute to opioid medication overdose and more generally, risk of suicide, including (1) high-dose opioid medications; (2) history of psychiatric disorders, specifically depression and PTSD; (3) history of substance use disorders; and (4) concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse.
Suicide
Suicide is the tenth leading cause of death in the U.S., with 12.4 suicide deaths per 100,000 population.12 Suicide rates are even higher among veterans. According to the VHA, the age-adjusted rate of suicide for veterans using VHA facilities and clinics was 35.9 per 100,000 person-years for fiscal year 2009.13 Several risk factors for suicide attempts include depression and other mental health disorders, substance abuse, medical disorders, and prescription medications.
Prior suicide attempts or self-harm behavior is known to increase the risk of subsequent death by suicide. About 11 attempted suicides occur per suicide death where the medical severity of prior attempts increases the risk of future suicide, as does a history of multiple self-harm episodes.14,15 One study found that the single best predictor of suicide in a veteran population was an attempt in the previous 4 months.16
Among other risk factors, previous suicide attempts and violent behavior are major behavioral flags that warrant caution and require particular consideration when prescribing opioid medications. In a national survey on drug use and health, about 18% of prescription opioid users/abusers who experienced suicidal ideations actually attempted suicide. Only 11% of individuals who never used prescription opioid medications attempted suicide after reported suicidal ideations.17
Patient Data Aggregation
The early and widespread adoption of electronic medical records (EMRs) by the VHA allowed the aggregation of patient data for quality improvement. Initially, data were aggregated, and dashboards were designed retrospectively. However, the development of regional data warehouses that update patient information daily from the EMR allowed information to be aggregated prospectively, and dashboards were designed that provided real-time information.
The purpose of the current study is to demonstrate the efficacy and future potential of dashboard technology in assessing prospectively high-risk factors for opioid overdose. Dashboards are a user-friendly application that allows providers to isolate and calculate daily morphine equivalent opioid dosages and assess patients’ risk factors for overdose on an individual basis. By using this technology, providers who prescribe opioids can get a concise summary of opioid and other medications and adjust medications to decrease overdose risk on an individual basis.
What is the Dashboard?
The VISN 22 high-risk opioid dashboard is a business intelligence tool that serves as a report card, or progress report, to provide a global view of the number of veterans who are receiving opioid prescriptions totaling >120 mg MEDD and who have characteristics (history of depression, PTSD, substance abuse, or high-risk suicide flag) and prescriptions (concomitant CNS depressants) that may increase patient risk for overdose.
The VISN 22 dashboard allows the user to navigate to an individual HCP-level and patient-level report (Figures 1 and 2). Filter settings allow report users to select only high-risk patients; it serves as a single location for pertinent details to consider for safely prescribing opioids.
To calculate daily morphine equivalents, each patient’s opioid prescriptions were evaluated. The quantity was divided by the day’s supply to calculate an average daily quantity. From there, the drug strength was used to convert to MEDD. Health care providers were informed that these conversion factors were not recommendations for clinical opioid conversions.
Implementation and Design
In 2012, the VA Pharmacy Benefits Management (PBM) in VISN 21 created a dashboard that allowed users to identify patients on high-dose opioid prescriptions. Structured query language code was used to extract data from the regional data warehouse and calculate MEDD for all patients with active opioid prescriptions. In 2013, VISN 22 expanded that dashboard to incorporate factors that could indicate a high risk for overdose or other adverse outcomes, including a history of depression, PTSD, substance abuse or high-risk suicide flag, and concomitant use of CNS depressant medications.
The high-risk opioid dashboard (Figure 3) and accompanying patient-level report were first introduced to VISN 22 HCPs in January 2013. The business intelligence tools were introduced to each facility through the VISN 22 PBM group. Training on the use of the dashboard and the report was provided, with an initial target of decreasing MEDD of > 200 mg to < 5% of all veterans prescribed opioids at each VISN 22 facility. One month later (in February 2013), a second category of veterans (those with > 120 mg but < 199 mg MEDD) was added. Also the initial MEDD > 200 mg target of < 5% was decreased to < 3% to encourage additional progress.
Eight months after the VISN 22 dashboard technology was implemented there was a 17% decrease in patients with total daily morphine equivalents > 200 mg (January 2013; 1,137 patients vs August 2013; 940 patients—a decrease of 197 patients).
From March 2013 to August 2013, VISN 22 also saw a 12% decrease in the number of patients prescribed > 120 mg MEDD but < 199 MEDD (March 2013; 2,295 vs August 2013; 2,018—a decrease of 277 patients).
Figure 4 shows opioid use as of July 2014 for VISN 22 facilities. There were further reductions in the number of patients receiving > 120 mg but < 199 mg MEDD (August 2013; 2,018 patients vs July 2014; 1,189 patients) and patients receiving > 200 mg MEDD (August 2013; 940 patients vs July 2014; 836 patients).
Case Description
In January 2013, VISN 22 implemented dashboard technology to help providers assess and monitor opioid prescription levels in relation to high-risk variables. The benefits of this dashboard technology are illustrated in the case profile that follows.
A 67-year-old male veteran had a long history of chronic pain. Pain diagnoses included osteoarthritis with spine involvement, lumbar radiculopathy, arthralgia, and peripheral neuropathy. For the past 10 years, he was prescribed opioids with modest relief of his chronic pain symptoms despite recent prescriptions totaling 300 mg MEDD. This veteran had several risk factors for overdose, including a history of depression, suicide risk, PTSD, and concomitant use of the CNS depressants alprazolam and cyclobenzaprine.
More recently, in May 2013, the veteran exhibited aberrant behavior and requested early refills for alprazolam. In response, the pharmacist discussed the case with the HCP who prescribed the opioids, noting the concomitant overdose risk factors. As a result of this interaction, the veteran was referred for mental health services, and his prescriptions for opioids were gradually decreased. He is currently stable, now receiving 120 mg MEDD, and his pain is currently described as moderately controlled on the new lower dose.
In summary, this veteran was receiving > 200 mg MEDD with several known overdose risk factors. Once the HCP was made aware of these risk factors, necessary precautions were taken, and the veteran was safely tapered to a lower dose. Dashboard technology makes the list of risk factors readily available to HCPs who are prescribing (and the pharmacists reviewing the prescriptions), thus allowing a proactive discussion of risks and benefits before continuing, renewing, or initiating opioid prescriptions.
Discussion
As reported in 2013 by NIDA, the greater availability of opioid medications and the consequent increase in prescriptions may be contributing directly to their growing misuse by both civilians and military service personnel. A direct consequence has been an increase in both accidental and intentional overdose deaths.3 Several factors are related to the risk of overdose/death, including high-dose opioid medications, a history of psychiatric disorders (specifically depression and PTSD), a history of substance use disorders, concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse, and previous attempts of suicide.
The VISN 22 high-risk opioid dashboard was a rapid information technology response to the increasing risk faced by veterans who have chronic pain and comorbid psychiatric and substance use disorders and are prescribed opioids and CNS depressants. The purpose of implementing this dashboard technology was to assist HCPs in prescribing opioids safely, using a technology that allows for the monitoring and management of concomitant suicide risk factors. Following the national Opioid Safety Initiative, this dashboard tool is being used to identify veterans who are on high-dose opioids with the goal of reducing the number of veterans on > 200 mg MEDD. The dashboard allows data to be stratified, using the concomitant risk factors for suicide to assist facilities and their providers in the identification and prioritization of highest risk patients first.
An initial review of dashboard data in VISN 22 suggests that it is a useful tool for reducing high-dose opioid prescriptions (> 200 mg MEDD and > 120 mg but < 199 mg MEDD). Across the 5 VA locations in VISN 22, in the first 8 months of implementation, 4 locations were able to lower prescription opioid medication levels to the initial target of < 5%; 2 lowered rates even further (to < 3%). The VA Greater Los Angeles Healthcare System remains at a commendable 1%. Although the number of veterans with prescriptions totaling > 200 mg MEDD has decreased as a result, a greater reduction is expected with the coordinated education and systems improvement efforts associated with the national VHA Opioid Safety Initiative. As part of the process to lower the number of patients on high-dose opioids in the future, HCP and patient education will be provided in relation to the use of dashboard technology.
Limitations
There are several limitations that affect interpretation of the usefulness of the VISN 22 high-risk opioid dashboard. Prior to the implementation of the dashboard, 2 of 5 VISN sites already had efforts in place to reduce opioid overprescribing. The VA Greater Los Angeles Healthcare System had an opioid reduction program in place before the dashboard was implemented, so it is possible reductions in opioid prescribing were a result of their previous efforts and not related to the dashboard. Similarly the VA Long Beach Healthcare System had begun a quality improvement initiative to reduce high-dose opioid prescribing prior to dashboard implementation. However, it was difficult to pinpoint the direct effect the dashboard had on patient interventions due to lack of documentation of dashboard use in the clinical notes.
A direct relationship did exist between dashboard implementation and opioid dose reduction in patients with > 200 MEDD at the remaining 3 VISN 22 facilities. Overall, this suggests that the dashboard played a significant role across all sites. Implementation of the dashboard across VISN 22 was accompanied by an education effort that resulted in an increased awareness among HCPs to evaluate certain risks in patients on high-dose opioids and to evaluate the combination of opioid and CNS depressant use. Prior to dashboard implementation, there was no standardized monitoring system that cross-referenced high-dose opioid prescribing with psychiatric illness and suicide risk factors.
Conclusions
From 2000 to 2010, opioid prescriptions nearly doubled, yet this rate was not accompanied by a change/increase in the rate of nonopioid analgesic medication prescriptions.18 Health care providers need to account for veterans’ wishes for pain treatment and be aware of options other than opioids, particularly given the risk of opioid-related accidental or intentional overdose; it is imperative that treatment become more individualized and more closely monitored.19,20 It is recommended that opioids should be the treatment of last resort in managing chronic noncancer pain. The use of opioid prescription medications should be intended as a trial, supported by clear goals and an unequivocal understanding that doses will not be indiscriminately increased.20
Health care providers who prescribe opioids are ultimately responsible for monitoring risk factors that may increase overdose and death, and dashboard technology assists them in this effort. The VISN 22 high-risk opioid dashboard is a tool that allows providers to identify and prioritize veterans who are at high risk for overdose. Initial data collected suggest that the dashboard has decreased the risk of negative consequences associated with opioid medication use today. However, the authors wish to emphasize that this technology is only part of the solution; although it can be a tool to identify actions that may need to take place and can track progress of changes in care, there must be complementary efforts in provider and patient education, improved access to mental health care, and interdisciplinary models of care that expand current chronic pain treatment options. Future considerations of this technology may include incorporating other risk factors accounting for psychosocial variables specific to military personnel that may further increase the overall risk of overdose.
Acknowledgements
The authors wish to thank the leadership of VISN 22 for their support of this initiative. Dr. Kryskalla recognizes VA OI&T for making this work possible and her family for their support. Ms. Kern would like to thank Aaron, Leslie, and Rachel Kern for their continuous support. Dr. Hauser wishes to thank Cathy, Anika, Katia, Max, and Jirina Hauser for their unwavering support.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[Published Online Ahead of Print: August 14, 2014.]
On October 10, 2013, a Congressional hearing was held to address the issue of opioid medication prescribing within VHA facilities and clinics (House Veteran Affairs Subcommittee hearing “Between Peril and Promise: Facing the Dangers of VA’s Skyrocketing Use of Prescription Painkillers to Treat Veterans”). Several individuals testified, including the widows of 2 veterans; both their husbands had overdosed on prescribed opioid medications. One husband had been taking as many as 15 pills a day and was additionally prescribed oxycodone/acetaminophen, which led to his death.1
Alongside the widows were 2 veterans who had been treated for chronic back pain injuries sustained before and during deployment in Iraq. Both had been prescribed several pain medications, including oxycodone/acetaminophen, methadone, and morphine. One reported that as his pain increased, his doctors continued to provide him additional prescriptions; at one point he had more than 13 prescriptions and could no longer work from being so “doped up.”1
In the past 2 decades, health care professionals (HCPs) have placed greater emphasis on chronic pain management. As a result, the rate of opioid medication prescribing has increased dramatically. Since 1994, the number of opioid medication prescriptions has nearly doubled; this change has been accompanied by an increase in opioid misuse, which has resulted in accidental or intentional overdose and death.2
Based on a recent National Institute on Drug Abuse (NIDA) report, the greatest impact has been on armed forces personnel.3 Prescriptions for pain relievers quadrupled between 2001 and 2009 to almost 3.8 million within the military population. Although civilian populations are more likely to abuse illicit drugs, military personnel are at particular risk of prescription abuse, including opioid medications.3 In 2008, 11% of armed forces service members reported misusing prescription drugs, with opioid medications being the most abused. This is an approximate 5- to 6-fold increase since 2002 (2% reported misuse in 2002).3 Particularly concerning is the associated rise in suicide rates among armed forces personnel, which surpassed civilian suicide rates in 2004. In 2009, one-third of suicides among armed forces personnel involved prescription drugs.3
Certain patient characteristics or factors are related to greater overdose risk. These risk factors include prescription dosage and frequency, history of suicide attempts or self-harm behavior, history of depression or posttraumatic stress disorder (PTSD) among other mental health-related diagnoses, a history of substance and/or alcohol abuse, and within the context of opioid medication use, the concurrent use of other central nervous system (CNS) depressants.4,5 Additionally, the stresses of deployment during wartime, physical injuries sustained in combat, and the unique military culture play a particularly important role in access to substances with high abuse potential and the subsequent development of substance abuse.3
Opioid Use and Risk Factors
More than 3% of adults in the U.S. are now receiving opioid medications for chronic noncancer pain.6 Substance abuse among patients with chronic pain ranges from 14% to 40%.5 Prescription opioid medications are the fastest growing drugs of abuse and the most common cause of unintentional overdose in the U.S.4 About 17,000 deaths occur each year as a result of prescription opioid medication overdose.7 Opioid medication-related overdose deaths began to increase in the early 2000s and continue to increase. Between 1999 and 2007, the rate of unintentional overdose-related deaths in the U.S. increased by 124%, largely due to the increase of prescription opioid medications.8
High-Dose Opioid Medication Use
A study by Dunn and colleagues found that patients receiving higher doses of prescribed opioid medications were at an increased risk of overdose.6 Patients receiving 50 mg to 99 mg morphine equivalent daily dose (MEDD) had a 3.7-fold increase in overdose risk (0.7% annual overdose rate) as compared with patients who received < 50 mg MEDD (0.2% annual overdose rate). Patients receiving ≥ 100 mg MEDD had a 1.8% annual overdose rate and a 9.8-fold increase in overdose risk as compared with patients who received < 50 mg MEDD. Overall, 51 patients experienced ≥ 1 overdose event, 40 of whom experienced fatal or serious overdoses and 6 of whom attempted suicide. Patients receiving the highest doses were male, current smokers, and had a history of depression and substance abuse.6 Similarly, a study by Bohnert and colleagues found that opioid medication overdose was most likely to occur in those patients with psychiatric and substance use disorders compared with patients who had no psychiatric illness history.8
Depression
Mood disorders are common in people with chronic pain.4,5,9,10 In particular, patients with a history of depression are more likely to receive chronic opioid medication prescriptions and are at a higher risk for opioid medication abuse. A substance abuse history is the most consistent predictor of both chronic opioid medication use and abuse. However, depression without substance abuse is significantly associated with 2 forms of opioid medication abuse: self-medication for stress or sleep and overmedication (using a higher dose than prescribed). More severe cases of depression show a stronger association for potential abuse.4
PTSD
Among Iraq and Afghanistan war veterans with ≥ 1 pain-related diagnosis, veterans with PTSD and veterans with a mental health disorder other than PTSD were significantly more likely to receive opioid medications for pain than were veterans without a mental health disorder (PTSD—17.8%, adjusted relative risk [RR] 2.58; other mental health disorder—11.7%, RR 1.74; no mental health disorder—6.5%).2 Although mental health disorders in general were related to a higher risk of opioid abuse, those with PTSD in particular were more likely to receive higher prescribed dosages; to continue taking opioids for a longer period; to receive concurrent prescriptions for opioid medications, sedative hypnotics, or both; to obtain early refills; and to have comorbid alcohol and substance use disorders. Based on these results, Seal and colleagues concluded that veterans with PTSD had the highest risk of alcohol, drug, and opioid-related accidents and overdose as well as self-inflicted injuries.2
Concurrent Use of Opioids and CNS Depressants
As mentioned earlier, studies suggest that people with PTSD are at a significantly higher risk for opioid medication overdose. One factor that may contribute to this higher risk is the concurrent use of CNS depressants/sedatives, particularly benzodiazepines and alcohol.
Benzodiazepines are often prescribed for people with PTSD. One study found that the concurrent use of benzodiazepines is significantly related to opioid overdose.5 Prescribing opioids for people already abusing or dependent on alcohol or other substances increases the risk of abuse and overdose. Furthermore, the concurrent use of multiple medications is associated with aberrant behaviors, cognitive impairment, and medication abuse, potentially leading to overdose. Overall, the combined administration of these medications is responsible for higher rates of adverse events, overdose, and death related to prescription opioid medication use.5,6,11
In summary, there are various risk factors that contribute to opioid medication overdose and more generally, risk of suicide, including (1) high-dose opioid medications; (2) history of psychiatric disorders, specifically depression and PTSD; (3) history of substance use disorders; and (4) concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse.
Suicide
Suicide is the tenth leading cause of death in the U.S., with 12.4 suicide deaths per 100,000 population.12 Suicide rates are even higher among veterans. According to the VHA, the age-adjusted rate of suicide for veterans using VHA facilities and clinics was 35.9 per 100,000 person-years for fiscal year 2009.13 Several risk factors for suicide attempts include depression and other mental health disorders, substance abuse, medical disorders, and prescription medications.
Prior suicide attempts or self-harm behavior is known to increase the risk of subsequent death by suicide. About 11 attempted suicides occur per suicide death where the medical severity of prior attempts increases the risk of future suicide, as does a history of multiple self-harm episodes.14,15 One study found that the single best predictor of suicide in a veteran population was an attempt in the previous 4 months.16
Among other risk factors, previous suicide attempts and violent behavior are major behavioral flags that warrant caution and require particular consideration when prescribing opioid medications. In a national survey on drug use and health, about 18% of prescription opioid users/abusers who experienced suicidal ideations actually attempted suicide. Only 11% of individuals who never used prescription opioid medications attempted suicide after reported suicidal ideations.17
Patient Data Aggregation
The early and widespread adoption of electronic medical records (EMRs) by the VHA allowed the aggregation of patient data for quality improvement. Initially, data were aggregated, and dashboards were designed retrospectively. However, the development of regional data warehouses that update patient information daily from the EMR allowed information to be aggregated prospectively, and dashboards were designed that provided real-time information.
The purpose of the current study is to demonstrate the efficacy and future potential of dashboard technology in assessing prospectively high-risk factors for opioid overdose. Dashboards are a user-friendly application that allows providers to isolate and calculate daily morphine equivalent opioid dosages and assess patients’ risk factors for overdose on an individual basis. By using this technology, providers who prescribe opioids can get a concise summary of opioid and other medications and adjust medications to decrease overdose risk on an individual basis.
What is the Dashboard?
The VISN 22 high-risk opioid dashboard is a business intelligence tool that serves as a report card, or progress report, to provide a global view of the number of veterans who are receiving opioid prescriptions totaling >120 mg MEDD and who have characteristics (history of depression, PTSD, substance abuse, or high-risk suicide flag) and prescriptions (concomitant CNS depressants) that may increase patient risk for overdose.
The VISN 22 dashboard allows the user to navigate to an individual HCP-level and patient-level report (Figures 1 and 2). Filter settings allow report users to select only high-risk patients; it serves as a single location for pertinent details to consider for safely prescribing opioids.
To calculate daily morphine equivalents, each patient’s opioid prescriptions were evaluated. The quantity was divided by the day’s supply to calculate an average daily quantity. From there, the drug strength was used to convert to MEDD. Health care providers were informed that these conversion factors were not recommendations for clinical opioid conversions.
Implementation and Design
In 2012, the VA Pharmacy Benefits Management (PBM) in VISN 21 created a dashboard that allowed users to identify patients on high-dose opioid prescriptions. Structured query language code was used to extract data from the regional data warehouse and calculate MEDD for all patients with active opioid prescriptions. In 2013, VISN 22 expanded that dashboard to incorporate factors that could indicate a high risk for overdose or other adverse outcomes, including a history of depression, PTSD, substance abuse or high-risk suicide flag, and concomitant use of CNS depressant medications.
The high-risk opioid dashboard (Figure 3) and accompanying patient-level report were first introduced to VISN 22 HCPs in January 2013. The business intelligence tools were introduced to each facility through the VISN 22 PBM group. Training on the use of the dashboard and the report was provided, with an initial target of decreasing MEDD of > 200 mg to < 5% of all veterans prescribed opioids at each VISN 22 facility. One month later (in February 2013), a second category of veterans (those with > 120 mg but < 199 mg MEDD) was added. Also the initial MEDD > 200 mg target of < 5% was decreased to < 3% to encourage additional progress.
Eight months after the VISN 22 dashboard technology was implemented there was a 17% decrease in patients with total daily morphine equivalents > 200 mg (January 2013; 1,137 patients vs August 2013; 940 patients—a decrease of 197 patients).
From March 2013 to August 2013, VISN 22 also saw a 12% decrease in the number of patients prescribed > 120 mg MEDD but < 199 MEDD (March 2013; 2,295 vs August 2013; 2,018—a decrease of 277 patients).
Figure 4 shows opioid use as of July 2014 for VISN 22 facilities. There were further reductions in the number of patients receiving > 120 mg but < 199 mg MEDD (August 2013; 2,018 patients vs July 2014; 1,189 patients) and patients receiving > 200 mg MEDD (August 2013; 940 patients vs July 2014; 836 patients).
Case Description
In January 2013, VISN 22 implemented dashboard technology to help providers assess and monitor opioid prescription levels in relation to high-risk variables. The benefits of this dashboard technology are illustrated in the case profile that follows.
A 67-year-old male veteran had a long history of chronic pain. Pain diagnoses included osteoarthritis with spine involvement, lumbar radiculopathy, arthralgia, and peripheral neuropathy. For the past 10 years, he was prescribed opioids with modest relief of his chronic pain symptoms despite recent prescriptions totaling 300 mg MEDD. This veteran had several risk factors for overdose, including a history of depression, suicide risk, PTSD, and concomitant use of the CNS depressants alprazolam and cyclobenzaprine.
More recently, in May 2013, the veteran exhibited aberrant behavior and requested early refills for alprazolam. In response, the pharmacist discussed the case with the HCP who prescribed the opioids, noting the concomitant overdose risk factors. As a result of this interaction, the veteran was referred for mental health services, and his prescriptions for opioids were gradually decreased. He is currently stable, now receiving 120 mg MEDD, and his pain is currently described as moderately controlled on the new lower dose.
In summary, this veteran was receiving > 200 mg MEDD with several known overdose risk factors. Once the HCP was made aware of these risk factors, necessary precautions were taken, and the veteran was safely tapered to a lower dose. Dashboard technology makes the list of risk factors readily available to HCPs who are prescribing (and the pharmacists reviewing the prescriptions), thus allowing a proactive discussion of risks and benefits before continuing, renewing, or initiating opioid prescriptions.
Discussion
As reported in 2013 by NIDA, the greater availability of opioid medications and the consequent increase in prescriptions may be contributing directly to their growing misuse by both civilians and military service personnel. A direct consequence has been an increase in both accidental and intentional overdose deaths.3 Several factors are related to the risk of overdose/death, including high-dose opioid medications, a history of psychiatric disorders (specifically depression and PTSD), a history of substance use disorders, concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse, and previous attempts of suicide.
The VISN 22 high-risk opioid dashboard was a rapid information technology response to the increasing risk faced by veterans who have chronic pain and comorbid psychiatric and substance use disorders and are prescribed opioids and CNS depressants. The purpose of implementing this dashboard technology was to assist HCPs in prescribing opioids safely, using a technology that allows for the monitoring and management of concomitant suicide risk factors. Following the national Opioid Safety Initiative, this dashboard tool is being used to identify veterans who are on high-dose opioids with the goal of reducing the number of veterans on > 200 mg MEDD. The dashboard allows data to be stratified, using the concomitant risk factors for suicide to assist facilities and their providers in the identification and prioritization of highest risk patients first.
An initial review of dashboard data in VISN 22 suggests that it is a useful tool for reducing high-dose opioid prescriptions (> 200 mg MEDD and > 120 mg but < 199 mg MEDD). Across the 5 VA locations in VISN 22, in the first 8 months of implementation, 4 locations were able to lower prescription opioid medication levels to the initial target of < 5%; 2 lowered rates even further (to < 3%). The VA Greater Los Angeles Healthcare System remains at a commendable 1%. Although the number of veterans with prescriptions totaling > 200 mg MEDD has decreased as a result, a greater reduction is expected with the coordinated education and systems improvement efforts associated with the national VHA Opioid Safety Initiative. As part of the process to lower the number of patients on high-dose opioids in the future, HCP and patient education will be provided in relation to the use of dashboard technology.
Limitations
There are several limitations that affect interpretation of the usefulness of the VISN 22 high-risk opioid dashboard. Prior to the implementation of the dashboard, 2 of 5 VISN sites already had efforts in place to reduce opioid overprescribing. The VA Greater Los Angeles Healthcare System had an opioid reduction program in place before the dashboard was implemented, so it is possible reductions in opioid prescribing were a result of their previous efforts and not related to the dashboard. Similarly the VA Long Beach Healthcare System had begun a quality improvement initiative to reduce high-dose opioid prescribing prior to dashboard implementation. However, it was difficult to pinpoint the direct effect the dashboard had on patient interventions due to lack of documentation of dashboard use in the clinical notes.
A direct relationship did exist between dashboard implementation and opioid dose reduction in patients with > 200 MEDD at the remaining 3 VISN 22 facilities. Overall, this suggests that the dashboard played a significant role across all sites. Implementation of the dashboard across VISN 22 was accompanied by an education effort that resulted in an increased awareness among HCPs to evaluate certain risks in patients on high-dose opioids and to evaluate the combination of opioid and CNS depressant use. Prior to dashboard implementation, there was no standardized monitoring system that cross-referenced high-dose opioid prescribing with psychiatric illness and suicide risk factors.
Conclusions
From 2000 to 2010, opioid prescriptions nearly doubled, yet this rate was not accompanied by a change/increase in the rate of nonopioid analgesic medication prescriptions.18 Health care providers need to account for veterans’ wishes for pain treatment and be aware of options other than opioids, particularly given the risk of opioid-related accidental or intentional overdose; it is imperative that treatment become more individualized and more closely monitored.19,20 It is recommended that opioids should be the treatment of last resort in managing chronic noncancer pain. The use of opioid prescription medications should be intended as a trial, supported by clear goals and an unequivocal understanding that doses will not be indiscriminately increased.20
Health care providers who prescribe opioids are ultimately responsible for monitoring risk factors that may increase overdose and death, and dashboard technology assists them in this effort. The VISN 22 high-risk opioid dashboard is a tool that allows providers to identify and prioritize veterans who are at high risk for overdose. Initial data collected suggest that the dashboard has decreased the risk of negative consequences associated with opioid medication use today. However, the authors wish to emphasize that this technology is only part of the solution; although it can be a tool to identify actions that may need to take place and can track progress of changes in care, there must be complementary efforts in provider and patient education, improved access to mental health care, and interdisciplinary models of care that expand current chronic pain treatment options. Future considerations of this technology may include incorporating other risk factors accounting for psychosocial variables specific to military personnel that may further increase the overall risk of overdose.
Acknowledgements
The authors wish to thank the leadership of VISN 22 for their support of this initiative. Dr. Kryskalla recognizes VA OI&T for making this work possible and her family for their support. Ms. Kern would like to thank Aaron, Leslie, and Rachel Kern for their continuous support. Dr. Hauser wishes to thank Cathy, Anika, Katia, Max, and Jirina Hauser for their unwavering support.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[Published Online Ahead of Print: August 14, 2014.]
1. Brooks D. Hearing Spotlights painkiller overuse among soldiers. http://www.fayobserver.com/military/article_a6e4a2e9-827d-577c-a79a-87a6c07cf151.html. Fayobserver Website. Published October 10, 2013, Accessed June 9, 2014.
2. Seal KH, Shi Y, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
3. National Institute on Drug Abuse. DrugFacts: Substance Abuse in the Military. http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military. National Institute on Drug Abuse Website. Revised March 2013. Accessed June 9, 2014.
4. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311.
5. Pergolizzi JV Jr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443-451.
6. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85-92.
7. Substance Abuse and Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit. HHS publication No. (SMA) 13-4742. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
8. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
9. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
10. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581-624.
11. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepine, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
12. Centers for Disease Control and Prevention. FastStats: Deaths and mortality. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/deaths.htm. Updated February 13, 2014. Accessed June 9, 2014.
13. Kemp J, Bossarte R. Suicide Data Report, 2012. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed July 1, 2014.
14. National Institute of Mental Health. Suicide in the U.S. Statistics. National Institute of Mental Health Website. http://www.nimh.nih.gov/statistics/index.shtml. Accessed June 27, 2014.
15. Miller M, Hempstead K, Nguyen T, Barber C, Rosenberg-Wohl S, Azrael D. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: Implications for clinical practice. Am J Public Health. 2013;103(6):e61-e68.
16. Hartl TL, Rosen C, Drescher K, Lee TT, Gusman F. Predicting high-risk behaviors in Veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2005;193(7):464-472.
17. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
18. Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870-878.
19. Bray RM, Pemberton MR, Lane ME, Hourani LL, Mattiko MJ, Babeu LA. Substance use and mental health trends among U.S. military active duty personnel. Key findings from the 2008 DoD Health Behavior Survey. Mil Med. 2010;175(6):390-399.
20. Cuevas-Trisan RL. The unfortunate turn of events in pain management. Fed Pract. 2013;30(3):8-9.
1. Brooks D. Hearing Spotlights painkiller overuse among soldiers. http://www.fayobserver.com/military/article_a6e4a2e9-827d-577c-a79a-87a6c07cf151.html. Fayobserver Website. Published October 10, 2013, Accessed June 9, 2014.
2. Seal KH, Shi Y, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
3. National Institute on Drug Abuse. DrugFacts: Substance Abuse in the Military. http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military. National Institute on Drug Abuse Website. Revised March 2013. Accessed June 9, 2014.
4. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311.
5. Pergolizzi JV Jr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443-451.
6. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85-92.
7. Substance Abuse and Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit. HHS publication No. (SMA) 13-4742. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
8. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
9. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
10. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581-624.
11. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepine, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
12. Centers for Disease Control and Prevention. FastStats: Deaths and mortality. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/deaths.htm. Updated February 13, 2014. Accessed June 9, 2014.
13. Kemp J, Bossarte R. Suicide Data Report, 2012. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed July 1, 2014.
14. National Institute of Mental Health. Suicide in the U.S. Statistics. National Institute of Mental Health Website. http://www.nimh.nih.gov/statistics/index.shtml. Accessed June 27, 2014.
15. Miller M, Hempstead K, Nguyen T, Barber C, Rosenberg-Wohl S, Azrael D. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: Implications for clinical practice. Am J Public Health. 2013;103(6):e61-e68.
16. Hartl TL, Rosen C, Drescher K, Lee TT, Gusman F. Predicting high-risk behaviors in Veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2005;193(7):464-472.
17. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
18. Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870-878.
19. Bray RM, Pemberton MR, Lane ME, Hourani LL, Mattiko MJ, Babeu LA. Substance use and mental health trends among U.S. military active duty personnel. Key findings from the 2008 DoD Health Behavior Survey. Mil Med. 2010;175(6):390-399.
20. Cuevas-Trisan RL. The unfortunate turn of events in pain management. Fed Pract. 2013;30(3):8-9.