User login
Reducing Benzodiazepine Prescribing in Older Veterans: A Direct-to-Consumer Educational Brochure
This quality improvement project used an educational brochure to help older veterans reduce their benzodiazepine use.
Benzodiazepines (BZDs) are among the most commonly prescribed medications. A recent study found that in 2008, more than 5% of Americans used a BZD, and the percentage was almost 9% among Americans aged ≥ 65 years.1,2 Among veterans, BZD use is even higher, in part because of the high prevalence of posttraumatic stress disorder (PTSD). One study found that more than 30% of veterans with PTSD received at least 1 BZD prescription.3 The risks associated with BZD treatment for PTSD are compounded by concurrent use of other sedatives and opioids prescribed for co-occurring chronic pain and insomnia.3
Older adults metabolize long-acting BZDs more slowly and generally have an increased sensitivity to the adverse effects (AEs) of all BZDs.4 In older adults, BZD use has been associated with cognitive decline, dementia, falls and consequent fractures, and adverse respiratory outcomes.5-12 The risk of most but not all of these AEs was increased with higher BZD dose or long-term BZD use, which this quality improvement project (QIP) defines as having at least a 60-day supply of BZD prescriptions dispensed within the past year.
Long-term BZD use increases with age. One study found that, among patients receiving a BZD, the rate of long-term BZD use was more than double in older adults (31.4%) than it was in adults aged between 18 and 35 years (14.7%).2 For these reasons, the 2012 Beers criteria of the American Geriatrics Society recommend avoiding all types of BZDs in the treatment of insomnia, agitation, or delirium in patients aged > 65 years.13 Despite this recommendation, the prevalence of BZD use in older adults remains high.14
Some innovative approaches have been developed to address the inappropriate use, including overuse and misuse, of BZDs in older adults.15 In one approach, direct-to-consumer (DTC) information is used to empower patients to collaborate with their physician to manage their health. Results from several studies suggest that providing older patients with information on BZD risks and benefits increases patient–physician interaction and thereby decreases inappropriate BZD use and improves health outcomes.4,16,17 One study found that perceptions of BZD risks increased 1 week after exposure to a DTC educational brochure (EB), with intention to discuss BZD discontinuation with their physician higher for patients who received the EB than it was for those who did not (83.1% vs 44.3%; P < .0001).16 The EMPOWER (Eliminating Medications Through Patient Ownership of End Results) cluster randomized controlled trial assessed the effectiveness of a DTC EB focused on BZD risks in older adults.17 In that seminal study, patients who received a DTC EB were more likely than were comparison patients to discontinue BZD within 6 months (27% vs 5%; risk difference, 23%; 95% CI, 14%-32%).
The Veterans Integrated Systems Network (VISN) 22 Academic Detailing Program is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20 With BZD use among older veterans remaining high, the VISN 22 program initiated a clinical QIP modeled on the EMPOWER trial. Veterans in VISN 22 received the DTC EB, which included information on BZD risks and encouraged them to discuss their BZD treatment with their health care provider. VISN 22 was the first VISN in the VHA to implement the EMPOWER protocol.
As this was a QIP, all eligible veterans in VISN 22 were mailed the DTC EB, thus making it difficult to estimate the impact of the EB on BZD discontinuation in this VISN. Therefore, DTC EB efficacy was estimated by comparing BZD discontinuation between VISN 22 and VISN 21, an adjacent VISN that did not mail the DTC EB.
Methods
Two QIPs were undertaken to determine the impact of DTC EB on BZD use in older veterans in the VHA.
Quality Improvement Project 1
Design. A retrospective cohort analysis was performed. The VISN 22 catchment area, which encompasses VA facilities and clinics in southern California and southern Nevada, serves about 500,000 veterans, a substantial proportion of whom are aged ≥ 65 years. Among these older veterans are active long-term BZD users, who were defined as having ≥ 60-day supply of BZD prescriptions dispensed within the past year. Each active long-term user with a BZD prescription released within 200 days before the index date (the date the user was to meet with the prescribing physician) was mailed an EB 2 to 8 weeks in advance of the visit. Excluded from analysis were veterans with a schizophrenia, spinal cord injury, or seizure disorder diagnosis recorded in both their inpatient and outpatient medical records; veterans seen by Palliative Care within the past year; and veterans who died before analysis was completed.
Education Brochure. The EB for VISN 22 (Figure 1, see
Patients. The sample consisted of all veterans identified as meeting the inclusion criteria and being enrolled in VISN 22. The EB was mailed once to veterans on a rolling basis from December 2014 to February 2016. Change in BZD use was analyzed only after 9 to 24 months had passed since the index appointment with the prescribing physician. This period included 12 weeks for BZD taper and then 6 months after taper.
Analysis. For each veteran, monthly mean lorazepam equivalent (LE) was calculated using as many as 12 fills before the index date. Average daily dose of LE was calculated by dividing the sum of LE from all included prescriptions by total number of days between the first fill and the index date. The BZD prescription fills were evaluated after the index date. Veterans who received at least 1 prescription after the index date but then had no BZD prescription activity in VA clinics for 3 consecutive months during the 9-month observation period were recorded as having tapered and then discontinued BZD. Veterans who had no BZD prescription activity in VA clinics after the index date and during the 9-month observation period were recorded as having discontinued BZD without tapering. For veterans who had BZD prescription activity in VA clinics after the index date and during the 9-month observation period, mean LE was calculated by dividing the total LE for BZD prescriptions after the index date by number of days from the first fill after the index date to the date of analysis.
Quality Improvement Project 2
Design. A retrospective cohort analysis using PSM was performed on a subgroup of the QIP-1 sample to evaluate the impact of EB on BZD prescribing in the VA during 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. Veterans in the analysis were active long-term BZD users, had at least 1 BZD prescription released within 200 days before the index date, were aged ≥ 65 years, and had an appointment scheduled with their BZD prescriber within 2 to 8 weeks (Figure 2).
Patients. VISN 22 implemented QIP-2, a real-world application of a modified EMPOWER program, by identifying eligible veterans on a rolling basis from December 2014 to August 2015. All veterans who were identified and sent an EB during this period were included in the case group. The index date was defined as the first of the month the EB was mailed. Veterans with a pending appointment were chosen because the lead time would allow them to receive the EB and prepare to discuss it with the physician during the visit.
A comparator group was drawn from the adjacent VISN 21 catchment area, which encompasses VA facilities and clinics in Hawaii, northern California, and northern Nevada. During the observation period, VISN 21 did not mail any EBs specifically addressing BZD risks. Veterans in the comparator group had an appointment scheduled with their BZD prescribing physician within 4 weeks, were aged ≥ 65 years on the index date (first of the month before the next appointment, coinciding with the date EBs were sent to VISN 22 veterans), were active long-term BZD users, and had at least 1 BZD prescription released within 200 days before the index date. All patients were followed for up to 12 months after the index date, with BZD discontinuation recorded 9 and 12 months after the index date.
Propensity Score Matching
Propensity score (PS) was estimated with logistic regression analysis with treatment as the dependent variable and baseline characteristics as the independent variables.21,22 One-to-one matching on the PS was performed using the nearest neighbor approach without replacements. Independent variables related to outcome but unrelated to EB exposure were selected for PS development.22 These variables included year of birth; male sex; Hispanic ethnicity; annual income; service connection status; region; body mass index; Charlson Comorbidity Index category; total baseline BZD dose; and diagnosis of AIDS, nonmetastatic cancer, metastatic cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), dementia, diabetes mellitus (DM), DM with complications, gastroesophageal reflux disease (GERD), general anxiety disorder (GAD), hemiparaplegia, liver disease (mild), liver disease (moderate to severe), myocardial infarction (MI), Parkinson disease, peptic ulcer disease (PUD), psychosis, renal disease, rheumatoid arthritis (RA), or substance use disorder (SUD).
The EMPOWER cluster randomized controlled trial (RCT) demonstrated the effectiveness of EB exposure in a Canadian population of elderly patients who were long-term BZD users.17 Randomized controlled trials are the gold standard for clinical trials because they can establish causal inference.23-25 Given ethical and practical concerns, however, RCTs cannot be applied to all clinical scenarios. Although EMPOWER is reported to be an effective tool in reducing BZD use in older adults, its application in a real-world, large, integrated health care system remains untested. Observational studies are often conducted as an alternative to RCTs but are subject to selection bias because of their lack of randomization.26 Therefore, robust research methods are needed to generate unbiased estimates of the impact of an intervention on an outcome. Propensity score matching simulates an RCT by balancing the covariates across treatment groups.21,22,27 Observed patient characteristics are used to estimate PS, the probability that treatment will be received. Logistic or probit regression is used to balance the potential confounding covariates between the treatment groups.Once PSs are known, mean treatment effect can be estimated without the mean model.28 In other words, PSM methods can be used to generate an unbiased estimate of the treatment.
Propensity Score Analysis
Baseline characteristics were compared using Student t test (continuous variables) and χ2 test (discrete variables). Results are presented as means and standard deviations (continuous variables) and frequency and percentage (discrete variables).
The main outcome was BZD discontinuation 9 and 12 months after the index date. A postindex lag of 6 months was used to capture any tapering (Figure 2). Discontinuation, defined as 3 consecutive months of no BZD prescription on hand, was measured for 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. An estimate was made of the difference in the proportions of BZD discontinuers who received the EB and BZD discontinuers who did not receive the EB, where mean treatment (risk difference) was presented as the absolute risk difference with a 95% CI. Standard errors and 95% CIs for the risk differences were generated with biased-corrected CIs from 1,000 bootstrap samples.
Sensitivity Analyses
Naïve multivariate logistic regression analysis was performed to evaluate the association between EB exposure and BZD discontinuation while controlling for potential confounders. Results are presented as odds ratios (ORs) and 95% CIs. Confounders identified were the same covariates used to generate the PSs.
Several analyses were performed to test the sensitivity of the methods applied using PSM by changing caliber size while maintaining the nearest neighbor approach without replacement. Linear regression analysis was performed with robust standard errors to estimate the risk difference of BZD discontinuation between EB-exposed and EB-unexposed veterans.
Statistical significance was set at P < .05. All statistical analyses were performed with Stata/SE Version 13 (College Station, TX).
Results
Quality Improvement Project 1
On a rolling basis from December 2014 to February 2016, the EB was mailed once to 3,896 VISN 22 veterans 2 to 8 weeks before a clinic appointment with their BZD prescribing physician.
Quality Improvement Project 2
Of all the VISN 22 and VISN 21 veterans, 24,420 met the inclusion and exclusion criteria. Of these 24,420 veterans, 2,020 (8.3%) were in VISN 22 and received the EB between December 2014 and August 2015 (QIP-1), and 22,400 (91.7%) were in VISN 21 and did not receive the EB.
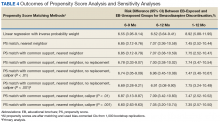
Naïve Results Before PS Matching. In the naïve analyses, a larger proportion of EB-exposed vs unexposed veterans discontinued BZD; in addition, reductions were 6.6%, 7.4%, and 9.5% larger for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (P < .0001 for all comparisons; Table 2).
After controlling for potential confounders, the naïve logistic regression analyses found EB exposure was significantly associated with 44%, 32%, and 42% increases in the odds of BZD discontinuation for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (Table 3).
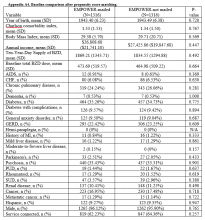
Propensity Score Matching. Before matching, there were significant differences in baseline characteristics of veterans who met the inclusion and exclusion criteria, with few exceptions (eAppendices 2 and 3, ).
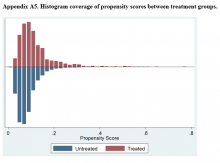
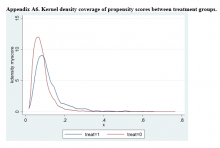
Propensity Score Matching Results. Inspection of PSs revealed good coverage across treatment groups on a histogram plot and a kernel density plot (eAppendices 5 and 6).
Discussion
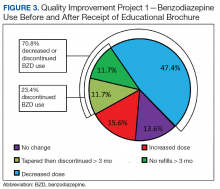
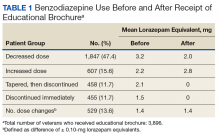
This QIP was the first to evaluate the impact of an EMPOWER-modeled DTC EB in a large, integrated health care system in the U.S. It was also the first to demonstrate potential benefits of a DTC EB designed for older veterans who are long-term BZD users. In this QIP, which mailed the EB to 3,896 veterans, 1,847 (47.4%) decreased their BZD dose, 458 (11.7%) tapered and then discontinued BZD, and 455 (11.7%) immediately discontinued BZD. The total percentage of veterans who discontinued BZD (23.4%; 913/3,896) was similar to the 27% reported in the EMPOWER trial.17 However, the risk difference between the 1,316 EB-exposed VISN 22 veterans (QIP-1) and the 1,316 EB-unexposed VISN 21 veterans in this QIP was significantly lower than the 23% risk difference in EMPOWER (though it still demonstrated a significantly larger reduction for EB-exposed veterans).17
Given this inclusion of all qualifying veterans from the catchment area studied in this QIP, and given the ethical and practical concerns, an RCT was not possible. Therefore, PSM methods were used to balance the covariates across treatment groups and thereby simulate an RCT.21,22,27 With use of the PSM approach, findings from the descriptive analysis were confirmed and potential selection bias reduced.
Study Limitations
The less robust risk difference found in this QIP has several possible explanations. The authors’ use of a DTC EB coincided with a national VA effort to reduce older veterans’ use of BZDs and other inappropriate medications. For instance, during the study period, academic detailing was being implemented to reduce use of BZDs, particularly in combination with opioids, across VHA facilities and clinics. (Academic detailing is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20) However, QIP-2 results and PS analysis of a subgroup of the original sample suggest that EB-exposed veterans were significantly more likely than were their unexposed counterparts were to discontinue BZD. To an extent, this analysis controlled for these other efforts to reduce BZD use in VHA clinics and can be considered a study strength.
Another limitation is the study design, which lacked a control group and did not consider the possibility that some facility or clinic physicians might influence others. Although the region variable was controlled for in PSM, the authors did not capture facility characteristics, including frequency of prescribing BZD and use of a protocol for enforcing the Beers criteria. Such confounders might have influenced outcomes. Unlike the EMPOWER trial,17 this QIP did not assess or exclude cognitively impaired veterans. It is reasonable to assume that these veterans might not understand some EB messages and consequently might fail to engage their physicians. Failure to initiate discussion with a physician would attenuate the impact of the EB.
Study Strengths
A strength of this QIP was its use of a DTC EB in a large, regional sample of older veterans in a real-world clinical setting. In addition, the study group (EB-exposed veterans) and the comparator group (EB-unexposed veterans) were from similar geographic areas (primarily California and Nevada).
Conclusion
Results of this study suggest that a DTC EB, designed to reduce BZD use among older veterans, was effective in helping patients lower their BZD dose and discontinue BZD. The likelihood of discontinuing BZD 9 and 12 months after the index date was significantly higher for veterans who received an EB modeled on the EMPOWER educational brochure than for a comparator group of veterans who did not receive the EB and were receiving care during the same observation period. In the future, it would be beneficial to use a design that controls for physician exposure to academic detailing focused on BZD reduction and that accounts for the cluster effects of facility practice. Despite these limitations, this QIP is the first real-world empirical example of using an EMPOWER-modeled DTC EB to decrease BZD use among older veterans. Furthermore, these results suggest that a DTC EB can be used to target other high-risk prescription drugs, such as opioids, particularly if alternative treatment options can be provided.
Acknowledgments
Dr. Hauser thanks Cathy, Anika, Katia, and Max Hauser, and Alba and Kevin Quinlan, for their support. In memory of Jirina Hauser, who died on Mother’s Day, May 14, 2017, at the age of 100.
1. Dell’osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry. 2013;28(1):7-20.
2. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
3. Bernardy NC, Lund BC, Alexander B, Friedman MJ. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
4. Roberts KJ. Patient empowerment in the United States: a critical commentary. Health Expect. 1999;2(2):82-92.
5. Paterniti S, Dufouil C, Alpérovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22(3):285-293.
6. van der Hooft CS, Schoofs MW, Ziere G, et al. Inappropriate benzodiazepine use in older adults and the risk of fracture. Br J Clin Pharmacol. 2008;66(2):276-282.
7. Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf. 2010;19(12):1248-1255.
8. Finkle WD, Der JS, Greenland S, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59(10):1883-1890.
9. de Gage SB, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231
10. Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639-658.
11. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with chronic obstructive pulmonary disease. Eur Respir J. 2014;44(2):332-340.
12. Gomm W, von Holt K, Thomé F, et al. Regular benzodiazepine and z-substance use and risk of dementia: an analysis of German claims data. J Alzheimers Dis. 2016;54(2):801-808.
13. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
14. National Institutes of Health. Despite risks, benzodiazepine use highest in older people. https://www.nih.gov/news-events/news-releases/despite-risks-benzodiaze pine-use-highest-older-people. Published December 17, 2014. Accessed July 31, 2018.
15. Airagnes G, Pelissolo A, Lavallée M, Flament M, Limosin F. Benzodiazepine misuse in the elderly: risk factors, consequences, and management. Curr Psychiatry Rep. 2016;18(10):89.
16. Martin P, Tamblyn R, Ahmed S, Tannenbaum C. A drug education tool developed for older adults changes knowledge, beliefs and risk perceptions about inappropriate benzodiazepine prescriptions in the elderly. Patient Educ Couns. 2013;92(1):81-87.
17. Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898.
18. Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263(4):549-556.
19. Fischer MA, Avorn J. Academic detailing can play a key role in assessing and implementing comparative effectiveness research findings. Health Aff (Millwood). 2012;31(10):2206-2212.
20. Wells DL, Popish S, Kay C, Torrise V, Christopher ML. VA Academic Detailing Service: implementation and lessons learned. Fed Pract. 2016;33(5):38-42.
21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424.
22. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156.
23. Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. J Ed Psych. 1974;66(5):688-701.
24. Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(4):722-729.
25. Cartwright N. What are randomized controlled trials good for? Philos Stud. 2010;147(1):59.
26. Kleinbaum DG, Morgenstern H, Kupper LL. Selection bias in epidemiologic studies. Am J Epidemiol. 1981;113(4):452-463.
27. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55.
28. Pirracchio R, Carone M, Rigon MR, Caruana E, Mebazaa A, Chevret S. Propensity score estimators for the average treatment effect and the average treatment effect on the treated may yield very different estimates. Stat Methods Med Res. 2016;25(5):1938-1954.
This quality improvement project used an educational brochure to help older veterans reduce their benzodiazepine use.
This quality improvement project used an educational brochure to help older veterans reduce their benzodiazepine use.
Benzodiazepines (BZDs) are among the most commonly prescribed medications. A recent study found that in 2008, more than 5% of Americans used a BZD, and the percentage was almost 9% among Americans aged ≥ 65 years.1,2 Among veterans, BZD use is even higher, in part because of the high prevalence of posttraumatic stress disorder (PTSD). One study found that more than 30% of veterans with PTSD received at least 1 BZD prescription.3 The risks associated with BZD treatment for PTSD are compounded by concurrent use of other sedatives and opioids prescribed for co-occurring chronic pain and insomnia.3
Older adults metabolize long-acting BZDs more slowly and generally have an increased sensitivity to the adverse effects (AEs) of all BZDs.4 In older adults, BZD use has been associated with cognitive decline, dementia, falls and consequent fractures, and adverse respiratory outcomes.5-12 The risk of most but not all of these AEs was increased with higher BZD dose or long-term BZD use, which this quality improvement project (QIP) defines as having at least a 60-day supply of BZD prescriptions dispensed within the past year.
Long-term BZD use increases with age. One study found that, among patients receiving a BZD, the rate of long-term BZD use was more than double in older adults (31.4%) than it was in adults aged between 18 and 35 years (14.7%).2 For these reasons, the 2012 Beers criteria of the American Geriatrics Society recommend avoiding all types of BZDs in the treatment of insomnia, agitation, or delirium in patients aged > 65 years.13 Despite this recommendation, the prevalence of BZD use in older adults remains high.14
Some innovative approaches have been developed to address the inappropriate use, including overuse and misuse, of BZDs in older adults.15 In one approach, direct-to-consumer (DTC) information is used to empower patients to collaborate with their physician to manage their health. Results from several studies suggest that providing older patients with information on BZD risks and benefits increases patient–physician interaction and thereby decreases inappropriate BZD use and improves health outcomes.4,16,17 One study found that perceptions of BZD risks increased 1 week after exposure to a DTC educational brochure (EB), with intention to discuss BZD discontinuation with their physician higher for patients who received the EB than it was for those who did not (83.1% vs 44.3%; P < .0001).16 The EMPOWER (Eliminating Medications Through Patient Ownership of End Results) cluster randomized controlled trial assessed the effectiveness of a DTC EB focused on BZD risks in older adults.17 In that seminal study, patients who received a DTC EB were more likely than were comparison patients to discontinue BZD within 6 months (27% vs 5%; risk difference, 23%; 95% CI, 14%-32%).
The Veterans Integrated Systems Network (VISN) 22 Academic Detailing Program is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20 With BZD use among older veterans remaining high, the VISN 22 program initiated a clinical QIP modeled on the EMPOWER trial. Veterans in VISN 22 received the DTC EB, which included information on BZD risks and encouraged them to discuss their BZD treatment with their health care provider. VISN 22 was the first VISN in the VHA to implement the EMPOWER protocol.
As this was a QIP, all eligible veterans in VISN 22 were mailed the DTC EB, thus making it difficult to estimate the impact of the EB on BZD discontinuation in this VISN. Therefore, DTC EB efficacy was estimated by comparing BZD discontinuation between VISN 22 and VISN 21, an adjacent VISN that did not mail the DTC EB.
Methods
Two QIPs were undertaken to determine the impact of DTC EB on BZD use in older veterans in the VHA.
Quality Improvement Project 1
Design. A retrospective cohort analysis was performed. The VISN 22 catchment area, which encompasses VA facilities and clinics in southern California and southern Nevada, serves about 500,000 veterans, a substantial proportion of whom are aged ≥ 65 years. Among these older veterans are active long-term BZD users, who were defined as having ≥ 60-day supply of BZD prescriptions dispensed within the past year. Each active long-term user with a BZD prescription released within 200 days before the index date (the date the user was to meet with the prescribing physician) was mailed an EB 2 to 8 weeks in advance of the visit. Excluded from analysis were veterans with a schizophrenia, spinal cord injury, or seizure disorder diagnosis recorded in both their inpatient and outpatient medical records; veterans seen by Palliative Care within the past year; and veterans who died before analysis was completed.
Education Brochure. The EB for VISN 22 (Figure 1, see
Patients. The sample consisted of all veterans identified as meeting the inclusion criteria and being enrolled in VISN 22. The EB was mailed once to veterans on a rolling basis from December 2014 to February 2016. Change in BZD use was analyzed only after 9 to 24 months had passed since the index appointment with the prescribing physician. This period included 12 weeks for BZD taper and then 6 months after taper.
Analysis. For each veteran, monthly mean lorazepam equivalent (LE) was calculated using as many as 12 fills before the index date. Average daily dose of LE was calculated by dividing the sum of LE from all included prescriptions by total number of days between the first fill and the index date. The BZD prescription fills were evaluated after the index date. Veterans who received at least 1 prescription after the index date but then had no BZD prescription activity in VA clinics for 3 consecutive months during the 9-month observation period were recorded as having tapered and then discontinued BZD. Veterans who had no BZD prescription activity in VA clinics after the index date and during the 9-month observation period were recorded as having discontinued BZD without tapering. For veterans who had BZD prescription activity in VA clinics after the index date and during the 9-month observation period, mean LE was calculated by dividing the total LE for BZD prescriptions after the index date by number of days from the first fill after the index date to the date of analysis.
Quality Improvement Project 2
Design. A retrospective cohort analysis using PSM was performed on a subgroup of the QIP-1 sample to evaluate the impact of EB on BZD prescribing in the VA during 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. Veterans in the analysis were active long-term BZD users, had at least 1 BZD prescription released within 200 days before the index date, were aged ≥ 65 years, and had an appointment scheduled with their BZD prescriber within 2 to 8 weeks (Figure 2).
Patients. VISN 22 implemented QIP-2, a real-world application of a modified EMPOWER program, by identifying eligible veterans on a rolling basis from December 2014 to August 2015. All veterans who were identified and sent an EB during this period were included in the case group. The index date was defined as the first of the month the EB was mailed. Veterans with a pending appointment were chosen because the lead time would allow them to receive the EB and prepare to discuss it with the physician during the visit.
A comparator group was drawn from the adjacent VISN 21 catchment area, which encompasses VA facilities and clinics in Hawaii, northern California, and northern Nevada. During the observation period, VISN 21 did not mail any EBs specifically addressing BZD risks. Veterans in the comparator group had an appointment scheduled with their BZD prescribing physician within 4 weeks, were aged ≥ 65 years on the index date (first of the month before the next appointment, coinciding with the date EBs were sent to VISN 22 veterans), were active long-term BZD users, and had at least 1 BZD prescription released within 200 days before the index date. All patients were followed for up to 12 months after the index date, with BZD discontinuation recorded 9 and 12 months after the index date.
Propensity Score Matching
Propensity score (PS) was estimated with logistic regression analysis with treatment as the dependent variable and baseline characteristics as the independent variables.21,22 One-to-one matching on the PS was performed using the nearest neighbor approach without replacements. Independent variables related to outcome but unrelated to EB exposure were selected for PS development.22 These variables included year of birth; male sex; Hispanic ethnicity; annual income; service connection status; region; body mass index; Charlson Comorbidity Index category; total baseline BZD dose; and diagnosis of AIDS, nonmetastatic cancer, metastatic cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), dementia, diabetes mellitus (DM), DM with complications, gastroesophageal reflux disease (GERD), general anxiety disorder (GAD), hemiparaplegia, liver disease (mild), liver disease (moderate to severe), myocardial infarction (MI), Parkinson disease, peptic ulcer disease (PUD), psychosis, renal disease, rheumatoid arthritis (RA), or substance use disorder (SUD).
The EMPOWER cluster randomized controlled trial (RCT) demonstrated the effectiveness of EB exposure in a Canadian population of elderly patients who were long-term BZD users.17 Randomized controlled trials are the gold standard for clinical trials because they can establish causal inference.23-25 Given ethical and practical concerns, however, RCTs cannot be applied to all clinical scenarios. Although EMPOWER is reported to be an effective tool in reducing BZD use in older adults, its application in a real-world, large, integrated health care system remains untested. Observational studies are often conducted as an alternative to RCTs but are subject to selection bias because of their lack of randomization.26 Therefore, robust research methods are needed to generate unbiased estimates of the impact of an intervention on an outcome. Propensity score matching simulates an RCT by balancing the covariates across treatment groups.21,22,27 Observed patient characteristics are used to estimate PS, the probability that treatment will be received. Logistic or probit regression is used to balance the potential confounding covariates between the treatment groups.Once PSs are known, mean treatment effect can be estimated without the mean model.28 In other words, PSM methods can be used to generate an unbiased estimate of the treatment.
Propensity Score Analysis
Baseline characteristics were compared using Student t test (continuous variables) and χ2 test (discrete variables). Results are presented as means and standard deviations (continuous variables) and frequency and percentage (discrete variables).
The main outcome was BZD discontinuation 9 and 12 months after the index date. A postindex lag of 6 months was used to capture any tapering (Figure 2). Discontinuation, defined as 3 consecutive months of no BZD prescription on hand, was measured for 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. An estimate was made of the difference in the proportions of BZD discontinuers who received the EB and BZD discontinuers who did not receive the EB, where mean treatment (risk difference) was presented as the absolute risk difference with a 95% CI. Standard errors and 95% CIs for the risk differences were generated with biased-corrected CIs from 1,000 bootstrap samples.
Sensitivity Analyses
Naïve multivariate logistic regression analysis was performed to evaluate the association between EB exposure and BZD discontinuation while controlling for potential confounders. Results are presented as odds ratios (ORs) and 95% CIs. Confounders identified were the same covariates used to generate the PSs.
Several analyses were performed to test the sensitivity of the methods applied using PSM by changing caliber size while maintaining the nearest neighbor approach without replacement. Linear regression analysis was performed with robust standard errors to estimate the risk difference of BZD discontinuation between EB-exposed and EB-unexposed veterans.
Statistical significance was set at P < .05. All statistical analyses were performed with Stata/SE Version 13 (College Station, TX).
Results
Quality Improvement Project 1
On a rolling basis from December 2014 to February 2016, the EB was mailed once to 3,896 VISN 22 veterans 2 to 8 weeks before a clinic appointment with their BZD prescribing physician.
Quality Improvement Project 2
Of all the VISN 22 and VISN 21 veterans, 24,420 met the inclusion and exclusion criteria. Of these 24,420 veterans, 2,020 (8.3%) were in VISN 22 and received the EB between December 2014 and August 2015 (QIP-1), and 22,400 (91.7%) were in VISN 21 and did not receive the EB.
Naïve Results Before PS Matching. In the naïve analyses, a larger proportion of EB-exposed vs unexposed veterans discontinued BZD; in addition, reductions were 6.6%, 7.4%, and 9.5% larger for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (P < .0001 for all comparisons; Table 2).
After controlling for potential confounders, the naïve logistic regression analyses found EB exposure was significantly associated with 44%, 32%, and 42% increases in the odds of BZD discontinuation for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (Table 3).
Propensity Score Matching. Before matching, there were significant differences in baseline characteristics of veterans who met the inclusion and exclusion criteria, with few exceptions (eAppendices 2 and 3, ).
Propensity Score Matching Results. Inspection of PSs revealed good coverage across treatment groups on a histogram plot and a kernel density plot (eAppendices 5 and 6).
Discussion
This QIP was the first to evaluate the impact of an EMPOWER-modeled DTC EB in a large, integrated health care system in the U.S. It was also the first to demonstrate potential benefits of a DTC EB designed for older veterans who are long-term BZD users. In this QIP, which mailed the EB to 3,896 veterans, 1,847 (47.4%) decreased their BZD dose, 458 (11.7%) tapered and then discontinued BZD, and 455 (11.7%) immediately discontinued BZD. The total percentage of veterans who discontinued BZD (23.4%; 913/3,896) was similar to the 27% reported in the EMPOWER trial.17 However, the risk difference between the 1,316 EB-exposed VISN 22 veterans (QIP-1) and the 1,316 EB-unexposed VISN 21 veterans in this QIP was significantly lower than the 23% risk difference in EMPOWER (though it still demonstrated a significantly larger reduction for EB-exposed veterans).17
Given this inclusion of all qualifying veterans from the catchment area studied in this QIP, and given the ethical and practical concerns, an RCT was not possible. Therefore, PSM methods were used to balance the covariates across treatment groups and thereby simulate an RCT.21,22,27 With use of the PSM approach, findings from the descriptive analysis were confirmed and potential selection bias reduced.
Study Limitations
The less robust risk difference found in this QIP has several possible explanations. The authors’ use of a DTC EB coincided with a national VA effort to reduce older veterans’ use of BZDs and other inappropriate medications. For instance, during the study period, academic detailing was being implemented to reduce use of BZDs, particularly in combination with opioids, across VHA facilities and clinics. (Academic detailing is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20) However, QIP-2 results and PS analysis of a subgroup of the original sample suggest that EB-exposed veterans were significantly more likely than were their unexposed counterparts were to discontinue BZD. To an extent, this analysis controlled for these other efforts to reduce BZD use in VHA clinics and can be considered a study strength.
Another limitation is the study design, which lacked a control group and did not consider the possibility that some facility or clinic physicians might influence others. Although the region variable was controlled for in PSM, the authors did not capture facility characteristics, including frequency of prescribing BZD and use of a protocol for enforcing the Beers criteria. Such confounders might have influenced outcomes. Unlike the EMPOWER trial,17 this QIP did not assess or exclude cognitively impaired veterans. It is reasonable to assume that these veterans might not understand some EB messages and consequently might fail to engage their physicians. Failure to initiate discussion with a physician would attenuate the impact of the EB.
Study Strengths
A strength of this QIP was its use of a DTC EB in a large, regional sample of older veterans in a real-world clinical setting. In addition, the study group (EB-exposed veterans) and the comparator group (EB-unexposed veterans) were from similar geographic areas (primarily California and Nevada).
Conclusion
Results of this study suggest that a DTC EB, designed to reduce BZD use among older veterans, was effective in helping patients lower their BZD dose and discontinue BZD. The likelihood of discontinuing BZD 9 and 12 months after the index date was significantly higher for veterans who received an EB modeled on the EMPOWER educational brochure than for a comparator group of veterans who did not receive the EB and were receiving care during the same observation period. In the future, it would be beneficial to use a design that controls for physician exposure to academic detailing focused on BZD reduction and that accounts for the cluster effects of facility practice. Despite these limitations, this QIP is the first real-world empirical example of using an EMPOWER-modeled DTC EB to decrease BZD use among older veterans. Furthermore, these results suggest that a DTC EB can be used to target other high-risk prescription drugs, such as opioids, particularly if alternative treatment options can be provided.
Acknowledgments
Dr. Hauser thanks Cathy, Anika, Katia, and Max Hauser, and Alba and Kevin Quinlan, for their support. In memory of Jirina Hauser, who died on Mother’s Day, May 14, 2017, at the age of 100.
Benzodiazepines (BZDs) are among the most commonly prescribed medications. A recent study found that in 2008, more than 5% of Americans used a BZD, and the percentage was almost 9% among Americans aged ≥ 65 years.1,2 Among veterans, BZD use is even higher, in part because of the high prevalence of posttraumatic stress disorder (PTSD). One study found that more than 30% of veterans with PTSD received at least 1 BZD prescription.3 The risks associated with BZD treatment for PTSD are compounded by concurrent use of other sedatives and opioids prescribed for co-occurring chronic pain and insomnia.3
Older adults metabolize long-acting BZDs more slowly and generally have an increased sensitivity to the adverse effects (AEs) of all BZDs.4 In older adults, BZD use has been associated with cognitive decline, dementia, falls and consequent fractures, and adverse respiratory outcomes.5-12 The risk of most but not all of these AEs was increased with higher BZD dose or long-term BZD use, which this quality improvement project (QIP) defines as having at least a 60-day supply of BZD prescriptions dispensed within the past year.
Long-term BZD use increases with age. One study found that, among patients receiving a BZD, the rate of long-term BZD use was more than double in older adults (31.4%) than it was in adults aged between 18 and 35 years (14.7%).2 For these reasons, the 2012 Beers criteria of the American Geriatrics Society recommend avoiding all types of BZDs in the treatment of insomnia, agitation, or delirium in patients aged > 65 years.13 Despite this recommendation, the prevalence of BZD use in older adults remains high.14
Some innovative approaches have been developed to address the inappropriate use, including overuse and misuse, of BZDs in older adults.15 In one approach, direct-to-consumer (DTC) information is used to empower patients to collaborate with their physician to manage their health. Results from several studies suggest that providing older patients with information on BZD risks and benefits increases patient–physician interaction and thereby decreases inappropriate BZD use and improves health outcomes.4,16,17 One study found that perceptions of BZD risks increased 1 week after exposure to a DTC educational brochure (EB), with intention to discuss BZD discontinuation with their physician higher for patients who received the EB than it was for those who did not (83.1% vs 44.3%; P < .0001).16 The EMPOWER (Eliminating Medications Through Patient Ownership of End Results) cluster randomized controlled trial assessed the effectiveness of a DTC EB focused on BZD risks in older adults.17 In that seminal study, patients who received a DTC EB were more likely than were comparison patients to discontinue BZD within 6 months (27% vs 5%; risk difference, 23%; 95% CI, 14%-32%).
The Veterans Integrated Systems Network (VISN) 22 Academic Detailing Program is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20 With BZD use among older veterans remaining high, the VISN 22 program initiated a clinical QIP modeled on the EMPOWER trial. Veterans in VISN 22 received the DTC EB, which included information on BZD risks and encouraged them to discuss their BZD treatment with their health care provider. VISN 22 was the first VISN in the VHA to implement the EMPOWER protocol.
As this was a QIP, all eligible veterans in VISN 22 were mailed the DTC EB, thus making it difficult to estimate the impact of the EB on BZD discontinuation in this VISN. Therefore, DTC EB efficacy was estimated by comparing BZD discontinuation between VISN 22 and VISN 21, an adjacent VISN that did not mail the DTC EB.
Methods
Two QIPs were undertaken to determine the impact of DTC EB on BZD use in older veterans in the VHA.
Quality Improvement Project 1
Design. A retrospective cohort analysis was performed. The VISN 22 catchment area, which encompasses VA facilities and clinics in southern California and southern Nevada, serves about 500,000 veterans, a substantial proportion of whom are aged ≥ 65 years. Among these older veterans are active long-term BZD users, who were defined as having ≥ 60-day supply of BZD prescriptions dispensed within the past year. Each active long-term user with a BZD prescription released within 200 days before the index date (the date the user was to meet with the prescribing physician) was mailed an EB 2 to 8 weeks in advance of the visit. Excluded from analysis were veterans with a schizophrenia, spinal cord injury, or seizure disorder diagnosis recorded in both their inpatient and outpatient medical records; veterans seen by Palliative Care within the past year; and veterans who died before analysis was completed.
Education Brochure. The EB for VISN 22 (Figure 1, see
Patients. The sample consisted of all veterans identified as meeting the inclusion criteria and being enrolled in VISN 22. The EB was mailed once to veterans on a rolling basis from December 2014 to February 2016. Change in BZD use was analyzed only after 9 to 24 months had passed since the index appointment with the prescribing physician. This period included 12 weeks for BZD taper and then 6 months after taper.
Analysis. For each veteran, monthly mean lorazepam equivalent (LE) was calculated using as many as 12 fills before the index date. Average daily dose of LE was calculated by dividing the sum of LE from all included prescriptions by total number of days between the first fill and the index date. The BZD prescription fills were evaluated after the index date. Veterans who received at least 1 prescription after the index date but then had no BZD prescription activity in VA clinics for 3 consecutive months during the 9-month observation period were recorded as having tapered and then discontinued BZD. Veterans who had no BZD prescription activity in VA clinics after the index date and during the 9-month observation period were recorded as having discontinued BZD without tapering. For veterans who had BZD prescription activity in VA clinics after the index date and during the 9-month observation period, mean LE was calculated by dividing the total LE for BZD prescriptions after the index date by number of days from the first fill after the index date to the date of analysis.
Quality Improvement Project 2
Design. A retrospective cohort analysis using PSM was performed on a subgroup of the QIP-1 sample to evaluate the impact of EB on BZD prescribing in the VA during 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. Veterans in the analysis were active long-term BZD users, had at least 1 BZD prescription released within 200 days before the index date, were aged ≥ 65 years, and had an appointment scheduled with their BZD prescriber within 2 to 8 weeks (Figure 2).
Patients. VISN 22 implemented QIP-2, a real-world application of a modified EMPOWER program, by identifying eligible veterans on a rolling basis from December 2014 to August 2015. All veterans who were identified and sent an EB during this period were included in the case group. The index date was defined as the first of the month the EB was mailed. Veterans with a pending appointment were chosen because the lead time would allow them to receive the EB and prepare to discuss it with the physician during the visit.
A comparator group was drawn from the adjacent VISN 21 catchment area, which encompasses VA facilities and clinics in Hawaii, northern California, and northern Nevada. During the observation period, VISN 21 did not mail any EBs specifically addressing BZD risks. Veterans in the comparator group had an appointment scheduled with their BZD prescribing physician within 4 weeks, were aged ≥ 65 years on the index date (first of the month before the next appointment, coinciding with the date EBs were sent to VISN 22 veterans), were active long-term BZD users, and had at least 1 BZD prescription released within 200 days before the index date. All patients were followed for up to 12 months after the index date, with BZD discontinuation recorded 9 and 12 months after the index date.
Propensity Score Matching
Propensity score (PS) was estimated with logistic regression analysis with treatment as the dependent variable and baseline characteristics as the independent variables.21,22 One-to-one matching on the PS was performed using the nearest neighbor approach without replacements. Independent variables related to outcome but unrelated to EB exposure were selected for PS development.22 These variables included year of birth; male sex; Hispanic ethnicity; annual income; service connection status; region; body mass index; Charlson Comorbidity Index category; total baseline BZD dose; and diagnosis of AIDS, nonmetastatic cancer, metastatic cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), dementia, diabetes mellitus (DM), DM with complications, gastroesophageal reflux disease (GERD), general anxiety disorder (GAD), hemiparaplegia, liver disease (mild), liver disease (moderate to severe), myocardial infarction (MI), Parkinson disease, peptic ulcer disease (PUD), psychosis, renal disease, rheumatoid arthritis (RA), or substance use disorder (SUD).
The EMPOWER cluster randomized controlled trial (RCT) demonstrated the effectiveness of EB exposure in a Canadian population of elderly patients who were long-term BZD users.17 Randomized controlled trials are the gold standard for clinical trials because they can establish causal inference.23-25 Given ethical and practical concerns, however, RCTs cannot be applied to all clinical scenarios. Although EMPOWER is reported to be an effective tool in reducing BZD use in older adults, its application in a real-world, large, integrated health care system remains untested. Observational studies are often conducted as an alternative to RCTs but are subject to selection bias because of their lack of randomization.26 Therefore, robust research methods are needed to generate unbiased estimates of the impact of an intervention on an outcome. Propensity score matching simulates an RCT by balancing the covariates across treatment groups.21,22,27 Observed patient characteristics are used to estimate PS, the probability that treatment will be received. Logistic or probit regression is used to balance the potential confounding covariates between the treatment groups.Once PSs are known, mean treatment effect can be estimated without the mean model.28 In other words, PSM methods can be used to generate an unbiased estimate of the treatment.
Propensity Score Analysis
Baseline characteristics were compared using Student t test (continuous variables) and χ2 test (discrete variables). Results are presented as means and standard deviations (continuous variables) and frequency and percentage (discrete variables).
The main outcome was BZD discontinuation 9 and 12 months after the index date. A postindex lag of 6 months was used to capture any tapering (Figure 2). Discontinuation, defined as 3 consecutive months of no BZD prescription on hand, was measured for 2 periods: 6 to 9 months and 6 to 12 months after the index date. A secondary outcome was discontinuation 1 to 12 months after the index date. An estimate was made of the difference in the proportions of BZD discontinuers who received the EB and BZD discontinuers who did not receive the EB, where mean treatment (risk difference) was presented as the absolute risk difference with a 95% CI. Standard errors and 95% CIs for the risk differences were generated with biased-corrected CIs from 1,000 bootstrap samples.
Sensitivity Analyses
Naïve multivariate logistic regression analysis was performed to evaluate the association between EB exposure and BZD discontinuation while controlling for potential confounders. Results are presented as odds ratios (ORs) and 95% CIs. Confounders identified were the same covariates used to generate the PSs.
Several analyses were performed to test the sensitivity of the methods applied using PSM by changing caliber size while maintaining the nearest neighbor approach without replacement. Linear regression analysis was performed with robust standard errors to estimate the risk difference of BZD discontinuation between EB-exposed and EB-unexposed veterans.
Statistical significance was set at P < .05. All statistical analyses were performed with Stata/SE Version 13 (College Station, TX).
Results
Quality Improvement Project 1
On a rolling basis from December 2014 to February 2016, the EB was mailed once to 3,896 VISN 22 veterans 2 to 8 weeks before a clinic appointment with their BZD prescribing physician.
Quality Improvement Project 2
Of all the VISN 22 and VISN 21 veterans, 24,420 met the inclusion and exclusion criteria. Of these 24,420 veterans, 2,020 (8.3%) were in VISN 22 and received the EB between December 2014 and August 2015 (QIP-1), and 22,400 (91.7%) were in VISN 21 and did not receive the EB.
Naïve Results Before PS Matching. In the naïve analyses, a larger proportion of EB-exposed vs unexposed veterans discontinued BZD; in addition, reductions were 6.6%, 7.4%, and 9.5% larger for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (P < .0001 for all comparisons; Table 2).
After controlling for potential confounders, the naïve logistic regression analyses found EB exposure was significantly associated with 44%, 32%, and 42% increases in the odds of BZD discontinuation for 6 to 9 months, 6 to 12 months, and 1 to 12 months after the index date, respectively (Table 3).
Propensity Score Matching. Before matching, there were significant differences in baseline characteristics of veterans who met the inclusion and exclusion criteria, with few exceptions (eAppendices 2 and 3, ).
Propensity Score Matching Results. Inspection of PSs revealed good coverage across treatment groups on a histogram plot and a kernel density plot (eAppendices 5 and 6).
Discussion
This QIP was the first to evaluate the impact of an EMPOWER-modeled DTC EB in a large, integrated health care system in the U.S. It was also the first to demonstrate potential benefits of a DTC EB designed for older veterans who are long-term BZD users. In this QIP, which mailed the EB to 3,896 veterans, 1,847 (47.4%) decreased their BZD dose, 458 (11.7%) tapered and then discontinued BZD, and 455 (11.7%) immediately discontinued BZD. The total percentage of veterans who discontinued BZD (23.4%; 913/3,896) was similar to the 27% reported in the EMPOWER trial.17 However, the risk difference between the 1,316 EB-exposed VISN 22 veterans (QIP-1) and the 1,316 EB-unexposed VISN 21 veterans in this QIP was significantly lower than the 23% risk difference in EMPOWER (though it still demonstrated a significantly larger reduction for EB-exposed veterans).17
Given this inclusion of all qualifying veterans from the catchment area studied in this QIP, and given the ethical and practical concerns, an RCT was not possible. Therefore, PSM methods were used to balance the covariates across treatment groups and thereby simulate an RCT.21,22,27 With use of the PSM approach, findings from the descriptive analysis were confirmed and potential selection bias reduced.
Study Limitations
The less robust risk difference found in this QIP has several possible explanations. The authors’ use of a DTC EB coincided with a national VA effort to reduce older veterans’ use of BZDs and other inappropriate medications. For instance, during the study period, academic detailing was being implemented to reduce use of BZDs, particularly in combination with opioids, across VHA facilities and clinics. (Academic detailing is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote physicians’ safety initiatives and align prescribing behavior with best practices.18-20) However, QIP-2 results and PS analysis of a subgroup of the original sample suggest that EB-exposed veterans were significantly more likely than were their unexposed counterparts were to discontinue BZD. To an extent, this analysis controlled for these other efforts to reduce BZD use in VHA clinics and can be considered a study strength.
Another limitation is the study design, which lacked a control group and did not consider the possibility that some facility or clinic physicians might influence others. Although the region variable was controlled for in PSM, the authors did not capture facility characteristics, including frequency of prescribing BZD and use of a protocol for enforcing the Beers criteria. Such confounders might have influenced outcomes. Unlike the EMPOWER trial,17 this QIP did not assess or exclude cognitively impaired veterans. It is reasonable to assume that these veterans might not understand some EB messages and consequently might fail to engage their physicians. Failure to initiate discussion with a physician would attenuate the impact of the EB.
Study Strengths
A strength of this QIP was its use of a DTC EB in a large, regional sample of older veterans in a real-world clinical setting. In addition, the study group (EB-exposed veterans) and the comparator group (EB-unexposed veterans) were from similar geographic areas (primarily California and Nevada).
Conclusion
Results of this study suggest that a DTC EB, designed to reduce BZD use among older veterans, was effective in helping patients lower their BZD dose and discontinue BZD. The likelihood of discontinuing BZD 9 and 12 months after the index date was significantly higher for veterans who received an EB modeled on the EMPOWER educational brochure than for a comparator group of veterans who did not receive the EB and were receiving care during the same observation period. In the future, it would be beneficial to use a design that controls for physician exposure to academic detailing focused on BZD reduction and that accounts for the cluster effects of facility practice. Despite these limitations, this QIP is the first real-world empirical example of using an EMPOWER-modeled DTC EB to decrease BZD use among older veterans. Furthermore, these results suggest that a DTC EB can be used to target other high-risk prescription drugs, such as opioids, particularly if alternative treatment options can be provided.
Acknowledgments
Dr. Hauser thanks Cathy, Anika, Katia, and Max Hauser, and Alba and Kevin Quinlan, for their support. In memory of Jirina Hauser, who died on Mother’s Day, May 14, 2017, at the age of 100.
1. Dell’osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry. 2013;28(1):7-20.
2. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
3. Bernardy NC, Lund BC, Alexander B, Friedman MJ. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
4. Roberts KJ. Patient empowerment in the United States: a critical commentary. Health Expect. 1999;2(2):82-92.
5. Paterniti S, Dufouil C, Alpérovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22(3):285-293.
6. van der Hooft CS, Schoofs MW, Ziere G, et al. Inappropriate benzodiazepine use in older adults and the risk of fracture. Br J Clin Pharmacol. 2008;66(2):276-282.
7. Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf. 2010;19(12):1248-1255.
8. Finkle WD, Der JS, Greenland S, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59(10):1883-1890.
9. de Gage SB, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231
10. Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639-658.
11. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with chronic obstructive pulmonary disease. Eur Respir J. 2014;44(2):332-340.
12. Gomm W, von Holt K, Thomé F, et al. Regular benzodiazepine and z-substance use and risk of dementia: an analysis of German claims data. J Alzheimers Dis. 2016;54(2):801-808.
13. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
14. National Institutes of Health. Despite risks, benzodiazepine use highest in older people. https://www.nih.gov/news-events/news-releases/despite-risks-benzodiaze pine-use-highest-older-people. Published December 17, 2014. Accessed July 31, 2018.
15. Airagnes G, Pelissolo A, Lavallée M, Flament M, Limosin F. Benzodiazepine misuse in the elderly: risk factors, consequences, and management. Curr Psychiatry Rep. 2016;18(10):89.
16. Martin P, Tamblyn R, Ahmed S, Tannenbaum C. A drug education tool developed for older adults changes knowledge, beliefs and risk perceptions about inappropriate benzodiazepine prescriptions in the elderly. Patient Educ Couns. 2013;92(1):81-87.
17. Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898.
18. Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263(4):549-556.
19. Fischer MA, Avorn J. Academic detailing can play a key role in assessing and implementing comparative effectiveness research findings. Health Aff (Millwood). 2012;31(10):2206-2212.
20. Wells DL, Popish S, Kay C, Torrise V, Christopher ML. VA Academic Detailing Service: implementation and lessons learned. Fed Pract. 2016;33(5):38-42.
21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424.
22. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156.
23. Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. J Ed Psych. 1974;66(5):688-701.
24. Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(4):722-729.
25. Cartwright N. What are randomized controlled trials good for? Philos Stud. 2010;147(1):59.
26. Kleinbaum DG, Morgenstern H, Kupper LL. Selection bias in epidemiologic studies. Am J Epidemiol. 1981;113(4):452-463.
27. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55.
28. Pirracchio R, Carone M, Rigon MR, Caruana E, Mebazaa A, Chevret S. Propensity score estimators for the average treatment effect and the average treatment effect on the treated may yield very different estimates. Stat Methods Med Res. 2016;25(5):1938-1954.
1. Dell’osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. Eur Psychiatry. 2013;28(1):7-20.
2. Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA Psychiatry. 2015;72(2):136-142.
3. Bernardy NC, Lund BC, Alexander B, Friedman MJ. Increased polysedative use in veterans with posttraumatic stress disorder. Pain Med. 2014;15(7):1083-1090.
4. Roberts KJ. Patient empowerment in the United States: a critical commentary. Health Expect. 1999;2(2):82-92.
5. Paterniti S, Dufouil C, Alpérovitch A. Long-term benzodiazepine use and cognitive decline in the elderly: the Epidemiology of Vascular Aging Study. J Clin Psychopharmacol. 2002;22(3):285-293.
6. van der Hooft CS, Schoofs MW, Ziere G, et al. Inappropriate benzodiazepine use in older adults and the risk of fracture. Br J Clin Pharmacol. 2008;66(2):276-282.
7. Zint K, Haefeli WE, Glynn RJ, Mogun H, Avorn J, Stürmer T. Impact of drug interactions, dosage, and duration of therapy on the risk of hip fracture associated with benzodiazepine use in older adults. Pharmacoepidemiol Drug Saf. 2010;19(12):1248-1255.
8. Finkle WD, Der JS, Greenland S, et al. Risk of fractures requiring hospitalization after an initial prescription for zolpidem, alprazolam, lorazepam, or diazepam in older adults. J Am Geriatr Soc. 2011;59(10):1883-1890.
9. de Gage SB, Bégaud B, Bazin F, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ. 2012;345:e6231
10. Tannenbaum C, Paquette A, Hilmer S, Holroyd-Leduc J, Carnahan R. A systematic review of amnestic and non-amnestic mild cognitive impairment induced by anticholinergic, antihistamine, GABAergic and opioid drugs. Drugs Aging. 2012;29(8):639-658.
11. Vozoris NT, Fischer HD, Wang X, et al. Benzodiazepine drug use and adverse respiratory outcomes among older adults with chronic obstructive pulmonary disease. Eur Respir J. 2014;44(2):332-340.
12. Gomm W, von Holt K, Thomé F, et al. Regular benzodiazepine and z-substance use and risk of dementia: an analysis of German claims data. J Alzheimers Dis. 2016;54(2):801-808.
13. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
14. National Institutes of Health. Despite risks, benzodiazepine use highest in older people. https://www.nih.gov/news-events/news-releases/despite-risks-benzodiaze pine-use-highest-older-people. Published December 17, 2014. Accessed July 31, 2018.
15. Airagnes G, Pelissolo A, Lavallée M, Flament M, Limosin F. Benzodiazepine misuse in the elderly: risk factors, consequences, and management. Curr Psychiatry Rep. 2016;18(10):89.
16. Martin P, Tamblyn R, Ahmed S, Tannenbaum C. A drug education tool developed for older adults changes knowledge, beliefs and risk perceptions about inappropriate benzodiazepine prescriptions in the elderly. Patient Educ Couns. 2013;92(1):81-87.
17. Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898.
18. Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263(4):549-556.
19. Fischer MA, Avorn J. Academic detailing can play a key role in assessing and implementing comparative effectiveness research findings. Health Aff (Millwood). 2012;31(10):2206-2212.
20. Wells DL, Popish S, Kay C, Torrise V, Christopher ML. VA Academic Detailing Service: implementation and lessons learned. Fed Pract. 2016;33(5):38-42.
21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424.
22. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156.
23. Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. J Ed Psych. 1974;66(5):688-701.
24. Greenland S. An introduction to instrumental variables for epidemiologists. Int J Epidemiol. 2000;29(4):722-729.
25. Cartwright N. What are randomized controlled trials good for? Philos Stud. 2010;147(1):59.
26. Kleinbaum DG, Morgenstern H, Kupper LL. Selection bias in epidemiologic studies. Am J Epidemiol. 1981;113(4):452-463.
27. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41-55.
28. Pirracchio R, Carone M, Rigon MR, Caruana E, Mebazaa A, Chevret S. Propensity score estimators for the average treatment effect and the average treatment effect on the treated may yield very different estimates. Stat Methods Med Res. 2016;25(5):1938-1954.
Psychotherapy Telemental Health Center and Regional Pilot
Within VHA, telemental health (TMH) refers to behavioral health services that are provided remotely, using secure communication technologies, to veterans who are separated by distance from their mental health providers.1 Telemental health sometimes involves video teleconferencing (VTC) technology, where a veteran (or group of veterans) in one location and a provider in a different location are able to communicate in real time through a computer monitor or television screen.2 In the VHA, TMH visits are typically conducted from a central location (such as a medical center hospital) to a community-based outpatient clinic (CBOC), but pilot projects have also tested VTC in homes as well.1,3,4
In addition to providing timely access to behavioral health services in rural or underserved locations, TMH eliminates travel that may be disruptive or costly and allows mental health providers to consult with or provide supervision to one another. Telemental health can be used to make diagnoses, manage care, perform checkups, and provide long-term, follow-up care. Other uses for TMH include clinical assessment, individual and group psychotherapy, psycho-educational interventions, cognitive testing, and general psychiatric care.1,5,6 More recently, TMH has been used to provide evidence-based psychotherapies (EBPs) to individuals with posttraumatic stress disorder (PTSD) and other mental health diagnoses.6,7 Such care may be particularly advantageous for veterans with PTSD, because traveling can be a burden for them or a trigger for PTSD symptoms.
Although interactive video technology is becoming widely available, its use is limited in health care systems due to lack of knowledge, education, logistical guidance, and technical training. The authors have conducted EBPs using VTC across VISN 22 in both office-to-office and office-to-home modalities and are providing EBPs using VTC to CBOCs in other VISNs across the western U.S. This article addresses these issues, outlining the necessary steps required to establish a TMH clinic and to share the successes of the EBP TMH Center and Regional Pilot used at VISN 22.
Telemental Health
Telemental health is an effective alternative to in-person treatment and is well regarded by both mental health providers and veterans. Overall, mental health providers believe it can help reduce the stigma associated with traditional mental health care and ease transportation-related issues for veterans. Telemental health allows access to care for veterans living in rural or remote areas in addition to those who are incarcerated or are otherwise unable to attend visits at primary VA facilities.2,8-10 In an assessment of TMH services in 40 CBOCs across VISN 16, most CBOC mental health providers found it to be an acceptable alternative to face-to-face care, recognize the value of TMH, and endorse a willingness to use and expand TMH programs within their clinics.11
Veterans who participated in TMH via VTC have expressed satisfaction with the decreased travel time and expenses, fewer interactions with crowds, and fewer parking problems.12 Several studies suggested that veterans preferred TMH to in-person contact due to more rapid access to care and specialists who would otherwise be unavailable at remote locations.5,10 Similarly, veterans who avoid in-person mental health care were more open to remote therapy for many of the reasons listed earlier. Studies suggest that veterans from both rural and urban locations are generally receptive to receiving mental health services via TMH.5,10
Several studies have found that TMH services may have advantages over standard in-person care. These advantages include decreasing transportation costs, travel time, and time missed at work and increasing system coverage area.13 Overall, both veterans and providers reported similar satisfaction between VTC and in-person sessions and, in some cases, prefer VTC interactions due to a sense of “easing into” intense therapies or having a “therapeutic distance” as treatment begins.12
Utility
Previous studies have shown that TMH can be used successfully to provide psychopharmacologic treatment to veterans who have major depressive disorder or schizophrenia, among other psychiatric disorders.5,8,14 Recent studies have focused on the feasibility of providing EBPs via TMH, particularly for the treatment of PTSD.12,15 Studies have shown that TMH services via VTC can be used successfully to provide cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), and prolonged exposure therapy (PE).16-21 In these studies, both PE and CPT delivered via TMH were found to be as efficacious as in-person formats. Furthermore, TMH services were successfully used in individual and group sessions.
Research has emphasized the benefits of TMH for veterans who are uncomfortable in crowds, waiting rooms, or hospital lobbies.7,12,18 For patients with PTSD who are initially limited by fears related to driving, TMH can facilitate access to care. Veterans with PTSD often avoid reminders of trauma (ie, uniforms, evidence of physical injury, artwork, photographs related to war), which can often be found at the larger VAMCs. These veterans may find mental health care services in their homes or at local CBOCs more appealing.7,12,18
Implementation
Prior to the implementation of telehealth services, many CBOC providers would refer veterans in need of specialty care to the nearest VAMC, which were sometimes many hours away.1 In response to travel and access concerns, the VA has implemented various telehealth modalities, including TMH.
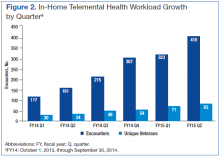
In 2008, about 230,000 veterans received mental health services via real-time clinical VTC at 300 VA CBOCs, and about 40,000 veterans enrolled in the In-Home Telehealth program.22 By 2011, > 380,000 veterans used clinic-based telehealth services and about 100,000 veterans used the in-home program.1 Between 2006 and 2008, the 98,000 veterans who used TMH modalities had fewer hospital admissions compared with those who did not; overall, the need for hospital services decreased by about 25% for those using TMH services.23
Although research suggests that TMH is an effective treatment modality, it does have limitations. A recent study noted several visual and audio difficulties that can emerge, including pixilation, “tracer” images with movement, low resolution, “frozen” or “choppy” images, delays in sound, echoes, or “mechanical sounding” voices.12 In some cases, physical details, such as crying, sniffling, or fidgeting, could not be clearly observed.12 Overall, these unforeseen issues can impact the ability to give and receive care through TMH modalities. Proper procedures need to be developed and implemented for each site.
Getting Started
Using TMH to provide mental health care at other VHA facilities requires planning and preparation. Logistics, such as preparation of the room and equipment, should be considered. Similarly, veteran and provider convenience must be considered.2,11 Before starting TMH at any VA facility, professionals working with the audiovisual technology and providing TMH care must complete necessary VA Talent Management System courses and obtain copies of certificates to assure they have met the appropriate training criteria. Providers must be credentialed to provide TMH services, including the telehealth curriculum offered by VA Employee Educational Services.2,24 An appropriate memorandum of understanding (MOU) must be created, and credentialing and privileging must also be acquired.
In addition to provider training, an information technology representative who can administer technical support as needed must be selected for both the provider and remote locations. Technologic complications can make TMH implementation much more challenging.12 As such, it is important to assure that both the veteran and the provider have the necessary TMH equipment. The selected communication device must be compatible with the technology requirements at the provider and remote facilities.12
In addition to designated technical support, the VISN TMH coordinator needs to have point-of-contact information for those who can assist with each site’s telehealth services and address the demand for EBP for PTSD or other desired services. After this information has been obtained, relationships must be developed and maintained with local leadership at each site, associated telehealth coordinators, and evidence-based therapy coordinators.
After contact has been established with remote facilities and the demand for services has been determined, there are several agreements and procedures to put in place before starting TMH services. An initial step is to develop a MOU agreement between the VISN TMH center and remote
sites that allows providers’ credentials and privileges to be shared. Also, it is important to establish a service agreement that outlines the procedures for staff at the remote site. This agreement includes checking in veterans, setting up the TMH rooms, transferring homework to VISN TMH providers, and connecting with the VISN TMH provider. In addition to service agreements, emergency procedures must be in place to ensure the safety of the veterans and the staff.24
After these agreements have been completed, the VISN TMH providers will have to complete request forms to obtain access to the Computerized Patient Record System at the remote facilities, which then must be approved by the Information Security Officer at that site. This is separate from the request at the provider’s site.12 It is essential to have points of contact for questions regarding this process. In order to facilitate referrals for TMH, electronic interfacility consult requests must be developed. Local staff need to collaborate with VISN TMH staff to ensure that the consult addresses the referral facilities need to meet the appropriate requirements.
Before the initiation of TMH services, each TMH provider has to establish clinics for scheduling appointments and obtaining workload credit. Program support assistants at the provider and remote sites must work together to ensure clinics are established correctly. This collaboration is essential for coding of visits and clinic mapping. After the clinics are “built,” appointment times will be set up based on the availability of the provider, support staff, and rooms at the remote site for the TMH session.
Once a consult is initiated, the VISN TMH EBP coordinator will review the consult and the veteran’s chart to ensure initial inclusion/exclusion criteria are met before accepting or canceling the consult. If the consult is accepted, a VISN TMH provider is assigned to the case and contacts the veteran to discuss the referral and (if the veteran is appropriate and interested) initiate services at the closest CBOC or at home. The VISN TMH regional center staff enter the appointment time for the veteran at both facility sites. The VISN TMH provider also coordinates with the CBOC staff to ensure that the veteran is checked in to the appointment and is provided with any questionnaires and necessary homework.
During the first session, the provider obtains consent from the veteran to engage in TMH services, conducts an assessment, and establishes rapport. The provider works with the veteran to develop a treatment plan for PTSD or other mental health diagnosis that will include the type of EBP. At the end of the first session, the next appointment is scheduled, and treatment materials are either mailed to the veteran or given to him or her onsite. After completing EBP, the VISN TMH center works with the referring provider to find follow-up services for the veteran.
The various steps necessary to begin an interfacility TMH clinic are summarized in Table 1.
Provider Training
Despite strong evidence of success, many providers remained skeptical about the efficacy of TMH. One study indicated that several providers in VISN 16 rarely used the established TMH programs because they were not familiar with them and applied TMH only for medication checks and consults.11 This skepticism was present in providers preparing to offer TMH as well as in providers referring veterans for TMH services. However, once providers better understood the TMH programs and had more experience using them, they were significantly more likely to use TMH for initial evaluations and ongoing psychotherapy. For these reasons, proper training and educational opportunities for practicing providers are vital to TMH implementation.9,11
To be proficient, providers need to become familiar with various TMH applications.10 Health care networks implementing TMH must ensure that their providers are well trained and prepared to give and receive proper consultation and support. Providers must also acquire several skills and familiarize themselves with available tools.9 In educating providers on the process and use of TMH, the authors suggest the following steps for TMH application:
- Learn new ways to chart in multiple systems and know how to troubleshoot during connectivity issues.
- Have an established administrative support collaborator at outpatient clinics to fax and exchange veteran homework.12
- The TMH clinic culture must be embedded where the veteran is being served in order to allow for a more realistic therapeutic feel. This type of clinic setting will allow for referrals at the veteran site and the availability to coordinate emergency procedures in the remote clinic.
Clinical Issues
Ongoing clinical issues need to be addressed continuously. Initially, referrals may be plentiful but not always appropriate. It is important to have an understanding with referring providers and remote sites about what constitutes a “good referral” as well as alternate referral options. It is imperative to outline inclusion and exclusion criteria that are clear and concise for referring providers. It is often helpful to revisit these criteria with potential referral sources after initiating services.
With the ability to provide inhome services, it is important to identify specific inclusion/exclusion criteria. Recommendations are based on research and clinical applications for exclusions, which are available on the Office of Technology Services website. These include imminent suicidality or homicidality, serious personality disorder or problematic character traits, acute substance disorders, psychotic disorders, and bipolar disorder. It is important to use sound clinical judgment, because the usual safeguards present in a remote clinic are not available for inhome services. Emergency planning is one of the most important aspects of the in-home TMH health services that are provided. The information for the emergency plan is obtained prior to initiation of services.
Emergency Plans
Each remote clinic that provides services to veterans must have an emergency plan that details procedures, phone numbers, and resources in case of medical and psychological emergencies as well as natural disasters. The VISN TMH provider will need to have a copy of the emergency plan as well as a list of contacts in case of an emergency during a TMH session.
It is recommended that TMH providers have several ways to contact key staff who can assist during an emergency. Usually the clinical coordinator and telehealth technician are the first responders to be alerted by the TMH provider during an emergency. They will then institute the remote clinic’s emergency protocol. Discussing these procedures and reviewing them with staff regularly is advisable, as key contacts may change.
In a psychological emergency, the VISN TMH provider may assist in implementing emergency procedures until a clinical counterpart at the remote site can be alerted. In the authors’ experience, VISN TMH providers have successfully de-escalated and diffused potentially emergent situations by maintaining constant realtime communication with veterans and staff by using VTC as well as interoffice communication. By offering assistance to veterans and staff during challenging situations, the VISN TMH provider will not only decrease concerns of veterans, but oftentimes integrate themselves into the treatment team of the remote clinic. The role of a VISN TMH provider can be isolative, with minimal contact with remote clinic staff, so it is important to increase visibility among staff at a remote site by communication with them even when there is not an emergency.
Treatment protocols may be determined by either administrative or clinical factors. With certain TMH interventions, the rooms used for veterans may be available for only certain periods, which may or may not fit with treatment protocols. For example, if a room is available for only an hour but a treatment protocol session is for 90 minutes, then another time slot needs to be found or a different treatment considered and offered. Although it is not ideal to have treatment protocols determined by scheduling factors, the reality of shared space at remote sites requires flexibility.
Sharing Materials and Homework Another clinical issue that is often overlooked is how to implement specific treatment protocols that entail the exchange of materials between VISN TMH providers and veterans. If materials will need to be exchanged between provider and veteran, a plan will have to be in place to facilitate this. The service agreement addresses these details, but remote staff may not always be aware of the details.
If a TMH provider opts to use faxes to send materials between a veteran and a provider, a desktop faxing program is recommended so veteran privacy is not compromised. Often, providers will wait to begin sessions until after they have received materials, but this may result in a delayed
session. One solution TMH providers can implement is mailing the materials and questionnaires to veterans before the session with clear instructions to complete them beforehand. Once the veteran arrives for the TMH session, she or he will verbally respond to the questionnaire and treatment materials. This will add time to a session but minimizes potential delays. Many of the clinical VTC units have movable cameras, so veterans can tilt the camera to show providers the forms and questionnaires.
The various steps necessary to address TMH clinical issues are summarized in Table 2.
VISIN 22 Pilot Project
The VISN 22 EBP TMH Center and Regional Pilot, based at the VA San Diego Healthcare System, was tasked with developing and providing TMH EBP services for PTSD across VISN 22 and adjacent West Coast VISNs. In addition to creating standardized procedures, troubleshooting guides were established to assist other programs with implementation. The primary focus was to increase access to EBPs for veterans with PTSD in areas where there was either no available trained providers or delays for specific services. The program established 16 clinics as well as in-home
services in VISN 22, VISN 21, and VISN 20. In fiscal year (FY) 2013, the VISN 22 EBP TMH Center and Regional Pilot provided 1,657 EBP encounters via TMH to 234 unique veterans with PTSD (Table 3).
The pilot project collected data to evaluate program effectiveness. The data were de-identified before being sent to the VA Central Office (VACO) TMH program manager. The following items were collected for the pilot: (1) clinical information; (2) consent to engage in treatment and telehealth; (3) release of information to share de-identified data to VACO for program monitoring; (4) demographic form; (5) Beck Depression Inventory-II (every other week); (6) PTSD Checklist (every other week); (7) World Health Organization Quality of Life (sessions 1, 7, final); (8) Wechsler Adult Intelligence Scale-Revised (sessions 3, 7, final); (9) satisfaction survey (final); (10) mileage not driven by veterans who receive TMH services; (11) travel pay saved by VA; (12) no-show rates; and (13) veteran, TMH provider, and referral provider satisfaction.
The growth in number of encounters and number of unique veterans has increased steadily from the first quarter of FY14 through the second quarter of FY15 (Figure 1).
In January 2013, in-home TMH services were piloted. Although occasional technical difficulties occurred, 143 EBP encounters via TMH were provided to 42 unique veterans in 2013. The service has continued to expand, and in the first half of FY14, services were provided to 64 unique veterans for a total of 278 encounters, saving veterans 3,220 travel miles and saving the VA $1,336 in travel reimbursement. In-home TMH services will continue to expand as more providers in a variety of programs are being trained by the San Diego staff on how to provide these services to veterans in their homes. In addition to decreasing mileage and travel pay, the no-show rates are lower for TMH appointments in general (averaged 8%-10% vs facility no-show rate average of 13.5%) and with the use of inhome TMH, no-show rates were kept to 2%. The growth in the number of in-home encounters and the number of unique veterans has also increased steadily from the first quarter of FY14 thru the second quarter of FY15 (Figure 2).
In-Home TMH Services
The VISN 22 EBP TMH Center and Regional Pilot often requests to have an in-person meeting with a veteran before starting TMH services in order to complete a waiver to download the software used by the VA for real-time video in-home services, a Release of Information for a Primary Support Person form, and an emergency plan.
It is also recommended that information about the veteran’s Internet connection, type of computer, type of software, presence of a camera and speakers, e-mail address, and access to secure messaging are obtained. During the initial contact with a veteran, the provider will discuss the rules and requirements to ensure HIPAA compliance. The veteran will need to have a private area for the call (not a restaurant, car, or other place where Wi-Fi is offered). Even with these discussions, some veterans will initiate services from a public place or a room in their home where family members will enter and exit frequently.
Although not required, it is recommended to have the veteran identify a primary support person and complete a release form to allow the TMH provider to contact that person in an emergency. The support person may be a person in the home (adult family member or caregiver) or someone nearby (neighbor, friend, or family member) who can contact emergency services if needed. After the necessary information is gathered and the veteran agrees to the conditions of participation, a test call will be completed. The TMH provider is often the person to conduct this call, but if available, a telehealth technician or facility telehealth coordinator may assist. The TMH provider may help the veteran download the appropriate software that is sent from the VA Scheduler software. The veteran initiates the call with the provider. Once the connection is made, the session may begin. Sites that are currently conducting in-home services have provided guides to veterans and newer TMH providers to outline the necessary steps for initiating services.
It is recommended that any provider interested in providing in-home TMH services use the Office of Technological Services help desk to assist in troubleshooting difficulties with connectivity. Challenges have included the software used for in-home TMH, periodic Internet outages, and compatibility issues.
Veteran Satisfaction
Veteran satisfaction was measured through a self-report satisfaction survey. The survey included 12 questions assessing overall experience in using TMH services. Eleven of the 12 questions included a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the last question was openended for additional comments.
A summary of the survey response of the initial 29 veterans who received TMH services suggested the following: (1) Veterans felt comfortable with using the TMH equipment and were able to see their clinician clearly; (2) Technical assistance was sufficient; (3) During the TMH session, they related to the provider as if it were a face-to-face meeting and that their needs were met; and (4) Veterans reported extremely high satisfaction with TMH and would refer TMH care to other veterans. Veterans found clinic locations very convenient and preferred the TMH modality of mental health services delivery to the alternative of travelling a long distance to see their provider (Table 4).
Written comments and recommendations from veterans supported the survey results. Most reported that they saved time and the convenience of the clinic allowed them to receive the treatment they need without interfering with their work schedule. However, some veterans still experienced trouble with travel to the remote clinic. Others felt their experience was different from the one they expected or they had a good experience via TMH but preferred face-to-face care.
Conclusion
The VISN 22 EBP TMH Center and Regional Pilot have established the infrastructure of interfacility clinics to use EBPs for the treatment of PTSD. Also, the center has provided consultation and guidance to facilities interested in developing their own TMH programs. The TMH Center now plans to expand mental health services and include medication management and EBP services for non-PTSD psychiatric diagnoses. The established infrastructure will allow providers from one facility to cover the mental health service needs of other facilities when there are absences or gaps due to leave or delays/challenges in hiring in rural locations. Finally, TMH offers the potential to offer after-hours services to veterans in other time zones during providers’ regular tours of duty.
Several other TMH programs are now expanding services into veterans’ homes. There are several sites within the VHA that have piloted this TMH modality and developed guidelines and recommendations for further expansion. Currently VACO is encouraging all VHA facilities to increase in-home telehealth services, and the Office of Telehealth Services provides details on implementation. Interested parties are encouraged to routinely visit the VACO website for updated information.
Developing and implementing a new TMH program can be an arduous task, but the program has great potential to provide veteran-centered care. As TMH sessions progress, the provider and veteran become less aware of the camera and software and more aware of the therapeutic process. Challenges and delays in implementation are to be expected—these can occur frequently during the development and implementation stages of a TMH program. Maintaining consistent communication with staff at remote sites is essential for the success of any program.
As the VHA focuses on veterancentered care, TMH services will improve access to providers with specific, needed expertise. The authors hope these experiences can facilitate the continued growth of TMH and assuage any concerns a facility or provider may have about this modality of care. Delivery of TMH care can be challenging, but the ability to provide these services to veterans at times and locations convenient to them makes these challenges worthwhile.
Acknowledgments
Dr. Hauser wishes to thank Cathy, Anika, Jirina, Katia, and Max Hauser, and Alba Pillwein for their continued support. In memory of Beverly Ostroski.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. What is telehealth? U.S. Department of Veterans Affairs Website. http://www.telehealth.va.gov. Update May 13, 2014. Accessed April 30, 2015.
2. Morland LA, Greene CJ, Rosen C, Mauldin PD, Frueh CB. Issues in the design of a randomized noninferiority clinical trial of telemental health psychotherapy for rural combat veterans with PTSD. Contemp Clin Trials. 2009;30(6):513-522.
3. Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, Acierno R. An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behav Ther. 2012;43(3):560-569.
4. Yuen EK, Gros DF, Price M, et al. Randomized controlled trial of home-based telehealth versus in-person prolonged exposure for combat-related PTSD in veterans: preliminary results. J Clin Psychol. 2015;71(6):500-512.
5. Ruskin PE, Reed S, Kumar R, et al. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatr Serv. 1998;49(8):1086-1088.
6. Gros DF, Morland LA, Greene CJ, et al. Delivery of evidence-based psychotherapy via video telehealth. J Psychopathol Behav Assess. 2013;35(4):506-521.
7. Backhaus A, Agha Z, Maglione ML, et al. Videoconferencing psychotherapy: a systematic review. Psychol Serv. 2012;9(2):111-131.
8. Egede LE, Frueh CB, Richardson LK, et al. Rationale and design: telepsychology service delivery for depressed elderly veterans. Trials. 2009;10:22.
9. Frueh BC, Deitsch SE, Santos AB, et al. Procedural and methodological issues in telepsychiatry research and program development. Psychiatr Serv. 2000;51(12):1522-1527.
10. Grubaugh AL, Cain GD, Elhai JD, Patrick SL, Frueh BC. Attitudes toward medical and mental health care delivered via telehealth applications among rural and urban primary care patients. J Nerv Ment Dis. 2008;196(2):166-170.
11. Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes towards telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425-432.
12. Thorp SR, Fidler J, Moreno L, Floto E, Agha Z. Lessons learned from studies of psychotherapy for posttraumatic stress disorder via video teleconferencing. Psychol Serv. 2012;9(2):197-199.
13. Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: predictors of treatment completion and outcome and comparison to treatment delivered in person. Behav Ther. 2011;42(2):276-283.
14. Zarate CA Jr, Weinstock L, Cukor P, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry. 1997;58(1):22-25.
15. Jones AM, Shealy KM, Reid-Quiñones K, et al. Guidelines for establishing a telemental health program to provide evidence-based therapy for trauma-exposed children and families. Psychol Serv. 2014;11(4):398-409.
16. Frueh BC, Monnier J, Grubaugh AL, Elhai JD, Yim E, Knapp R. Therapist adherence and competence with manualized cognitive-behavioral therapy for PTSD delivered via videoconferencing technology. Behav Modif. 2007;31(6):856-866.
17. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469.
18. Tuerk PW, Yoder M, Ruggiero KJ, Gros DF, Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J Trauma Stress. 2010;23(1):116-123.
19. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine- based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
20. Germain V, Marchand A, Bouchard S, Drouin MS, Guay S. Effectiveness of cognitive behavioural therapy administered by videoconference for posttraumatic stress disorder. Cogn Behav Ther. 2009;38(1):42-53.
21. Morland LA, Mackintosh M, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75(5):470-476.
22. Tuerk PW, Fortney J, Bosworth HB, et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16(1):115-117.
23. Godleski L, Darkins A, Peters J. Outcomes of 98,609 U.S. Department of Veterans Affairs patients enrolled in telemental health services, 2006-2010. Psychiatr Serv. 2012;63(4):383-385.
24. Strachan M, Gros DF, Yuen E, Ruggiero KJ, Foa EB, Acierno R. Home-based telehealth to deliver evidence-based psychotherapy in veterans with PTSD. Contemp Clin Trials. 2012;33(2):402-409.
Within VHA, telemental health (TMH) refers to behavioral health services that are provided remotely, using secure communication technologies, to veterans who are separated by distance from their mental health providers.1 Telemental health sometimes involves video teleconferencing (VTC) technology, where a veteran (or group of veterans) in one location and a provider in a different location are able to communicate in real time through a computer monitor or television screen.2 In the VHA, TMH visits are typically conducted from a central location (such as a medical center hospital) to a community-based outpatient clinic (CBOC), but pilot projects have also tested VTC in homes as well.1,3,4
In addition to providing timely access to behavioral health services in rural or underserved locations, TMH eliminates travel that may be disruptive or costly and allows mental health providers to consult with or provide supervision to one another. Telemental health can be used to make diagnoses, manage care, perform checkups, and provide long-term, follow-up care. Other uses for TMH include clinical assessment, individual and group psychotherapy, psycho-educational interventions, cognitive testing, and general psychiatric care.1,5,6 More recently, TMH has been used to provide evidence-based psychotherapies (EBPs) to individuals with posttraumatic stress disorder (PTSD) and other mental health diagnoses.6,7 Such care may be particularly advantageous for veterans with PTSD, because traveling can be a burden for them or a trigger for PTSD symptoms.
Although interactive video technology is becoming widely available, its use is limited in health care systems due to lack of knowledge, education, logistical guidance, and technical training. The authors have conducted EBPs using VTC across VISN 22 in both office-to-office and office-to-home modalities and are providing EBPs using VTC to CBOCs in other VISNs across the western U.S. This article addresses these issues, outlining the necessary steps required to establish a TMH clinic and to share the successes of the EBP TMH Center and Regional Pilot used at VISN 22.
Telemental Health
Telemental health is an effective alternative to in-person treatment and is well regarded by both mental health providers and veterans. Overall, mental health providers believe it can help reduce the stigma associated with traditional mental health care and ease transportation-related issues for veterans. Telemental health allows access to care for veterans living in rural or remote areas in addition to those who are incarcerated or are otherwise unable to attend visits at primary VA facilities.2,8-10 In an assessment of TMH services in 40 CBOCs across VISN 16, most CBOC mental health providers found it to be an acceptable alternative to face-to-face care, recognize the value of TMH, and endorse a willingness to use and expand TMH programs within their clinics.11
Veterans who participated in TMH via VTC have expressed satisfaction with the decreased travel time and expenses, fewer interactions with crowds, and fewer parking problems.12 Several studies suggested that veterans preferred TMH to in-person contact due to more rapid access to care and specialists who would otherwise be unavailable at remote locations.5,10 Similarly, veterans who avoid in-person mental health care were more open to remote therapy for many of the reasons listed earlier. Studies suggest that veterans from both rural and urban locations are generally receptive to receiving mental health services via TMH.5,10
Several studies have found that TMH services may have advantages over standard in-person care. These advantages include decreasing transportation costs, travel time, and time missed at work and increasing system coverage area.13 Overall, both veterans and providers reported similar satisfaction between VTC and in-person sessions and, in some cases, prefer VTC interactions due to a sense of “easing into” intense therapies or having a “therapeutic distance” as treatment begins.12
Utility
Previous studies have shown that TMH can be used successfully to provide psychopharmacologic treatment to veterans who have major depressive disorder or schizophrenia, among other psychiatric disorders.5,8,14 Recent studies have focused on the feasibility of providing EBPs via TMH, particularly for the treatment of PTSD.12,15 Studies have shown that TMH services via VTC can be used successfully to provide cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), and prolonged exposure therapy (PE).16-21 In these studies, both PE and CPT delivered via TMH were found to be as efficacious as in-person formats. Furthermore, TMH services were successfully used in individual and group sessions.
Research has emphasized the benefits of TMH for veterans who are uncomfortable in crowds, waiting rooms, or hospital lobbies.7,12,18 For patients with PTSD who are initially limited by fears related to driving, TMH can facilitate access to care. Veterans with PTSD often avoid reminders of trauma (ie, uniforms, evidence of physical injury, artwork, photographs related to war), which can often be found at the larger VAMCs. These veterans may find mental health care services in their homes or at local CBOCs more appealing.7,12,18
Implementation
Prior to the implementation of telehealth services, many CBOC providers would refer veterans in need of specialty care to the nearest VAMC, which were sometimes many hours away.1 In response to travel and access concerns, the VA has implemented various telehealth modalities, including TMH.
In 2008, about 230,000 veterans received mental health services via real-time clinical VTC at 300 VA CBOCs, and about 40,000 veterans enrolled in the In-Home Telehealth program.22 By 2011, > 380,000 veterans used clinic-based telehealth services and about 100,000 veterans used the in-home program.1 Between 2006 and 2008, the 98,000 veterans who used TMH modalities had fewer hospital admissions compared with those who did not; overall, the need for hospital services decreased by about 25% for those using TMH services.23
Although research suggests that TMH is an effective treatment modality, it does have limitations. A recent study noted several visual and audio difficulties that can emerge, including pixilation, “tracer” images with movement, low resolution, “frozen” or “choppy” images, delays in sound, echoes, or “mechanical sounding” voices.12 In some cases, physical details, such as crying, sniffling, or fidgeting, could not be clearly observed.12 Overall, these unforeseen issues can impact the ability to give and receive care through TMH modalities. Proper procedures need to be developed and implemented for each site.
Getting Started
Using TMH to provide mental health care at other VHA facilities requires planning and preparation. Logistics, such as preparation of the room and equipment, should be considered. Similarly, veteran and provider convenience must be considered.2,11 Before starting TMH at any VA facility, professionals working with the audiovisual technology and providing TMH care must complete necessary VA Talent Management System courses and obtain copies of certificates to assure they have met the appropriate training criteria. Providers must be credentialed to provide TMH services, including the telehealth curriculum offered by VA Employee Educational Services.2,24 An appropriate memorandum of understanding (MOU) must be created, and credentialing and privileging must also be acquired.
In addition to provider training, an information technology representative who can administer technical support as needed must be selected for both the provider and remote locations. Technologic complications can make TMH implementation much more challenging.12 As such, it is important to assure that both the veteran and the provider have the necessary TMH equipment. The selected communication device must be compatible with the technology requirements at the provider and remote facilities.12
In addition to designated technical support, the VISN TMH coordinator needs to have point-of-contact information for those who can assist with each site’s telehealth services and address the demand for EBP for PTSD or other desired services. After this information has been obtained, relationships must be developed and maintained with local leadership at each site, associated telehealth coordinators, and evidence-based therapy coordinators.
After contact has been established with remote facilities and the demand for services has been determined, there are several agreements and procedures to put in place before starting TMH services. An initial step is to develop a MOU agreement between the VISN TMH center and remote
sites that allows providers’ credentials and privileges to be shared. Also, it is important to establish a service agreement that outlines the procedures for staff at the remote site. This agreement includes checking in veterans, setting up the TMH rooms, transferring homework to VISN TMH providers, and connecting with the VISN TMH provider. In addition to service agreements, emergency procedures must be in place to ensure the safety of the veterans and the staff.24
After these agreements have been completed, the VISN TMH providers will have to complete request forms to obtain access to the Computerized Patient Record System at the remote facilities, which then must be approved by the Information Security Officer at that site. This is separate from the request at the provider’s site.12 It is essential to have points of contact for questions regarding this process. In order to facilitate referrals for TMH, electronic interfacility consult requests must be developed. Local staff need to collaborate with VISN TMH staff to ensure that the consult addresses the referral facilities need to meet the appropriate requirements.
Before the initiation of TMH services, each TMH provider has to establish clinics for scheduling appointments and obtaining workload credit. Program support assistants at the provider and remote sites must work together to ensure clinics are established correctly. This collaboration is essential for coding of visits and clinic mapping. After the clinics are “built,” appointment times will be set up based on the availability of the provider, support staff, and rooms at the remote site for the TMH session.
Once a consult is initiated, the VISN TMH EBP coordinator will review the consult and the veteran’s chart to ensure initial inclusion/exclusion criteria are met before accepting or canceling the consult. If the consult is accepted, a VISN TMH provider is assigned to the case and contacts the veteran to discuss the referral and (if the veteran is appropriate and interested) initiate services at the closest CBOC or at home. The VISN TMH regional center staff enter the appointment time for the veteran at both facility sites. The VISN TMH provider also coordinates with the CBOC staff to ensure that the veteran is checked in to the appointment and is provided with any questionnaires and necessary homework.
During the first session, the provider obtains consent from the veteran to engage in TMH services, conducts an assessment, and establishes rapport. The provider works with the veteran to develop a treatment plan for PTSD or other mental health diagnosis that will include the type of EBP. At the end of the first session, the next appointment is scheduled, and treatment materials are either mailed to the veteran or given to him or her onsite. After completing EBP, the VISN TMH center works with the referring provider to find follow-up services for the veteran.
The various steps necessary to begin an interfacility TMH clinic are summarized in Table 1.
Provider Training
Despite strong evidence of success, many providers remained skeptical about the efficacy of TMH. One study indicated that several providers in VISN 16 rarely used the established TMH programs because they were not familiar with them and applied TMH only for medication checks and consults.11 This skepticism was present in providers preparing to offer TMH as well as in providers referring veterans for TMH services. However, once providers better understood the TMH programs and had more experience using them, they were significantly more likely to use TMH for initial evaluations and ongoing psychotherapy. For these reasons, proper training and educational opportunities for practicing providers are vital to TMH implementation.9,11
To be proficient, providers need to become familiar with various TMH applications.10 Health care networks implementing TMH must ensure that their providers are well trained and prepared to give and receive proper consultation and support. Providers must also acquire several skills and familiarize themselves with available tools.9 In educating providers on the process and use of TMH, the authors suggest the following steps for TMH application:
- Learn new ways to chart in multiple systems and know how to troubleshoot during connectivity issues.
- Have an established administrative support collaborator at outpatient clinics to fax and exchange veteran homework.12
- The TMH clinic culture must be embedded where the veteran is being served in order to allow for a more realistic therapeutic feel. This type of clinic setting will allow for referrals at the veteran site and the availability to coordinate emergency procedures in the remote clinic.
Clinical Issues
Ongoing clinical issues need to be addressed continuously. Initially, referrals may be plentiful but not always appropriate. It is important to have an understanding with referring providers and remote sites about what constitutes a “good referral” as well as alternate referral options. It is imperative to outline inclusion and exclusion criteria that are clear and concise for referring providers. It is often helpful to revisit these criteria with potential referral sources after initiating services.
With the ability to provide inhome services, it is important to identify specific inclusion/exclusion criteria. Recommendations are based on research and clinical applications for exclusions, which are available on the Office of Technology Services website. These include imminent suicidality or homicidality, serious personality disorder or problematic character traits, acute substance disorders, psychotic disorders, and bipolar disorder. It is important to use sound clinical judgment, because the usual safeguards present in a remote clinic are not available for inhome services. Emergency planning is one of the most important aspects of the in-home TMH health services that are provided. The information for the emergency plan is obtained prior to initiation of services.
Emergency Plans
Each remote clinic that provides services to veterans must have an emergency plan that details procedures, phone numbers, and resources in case of medical and psychological emergencies as well as natural disasters. The VISN TMH provider will need to have a copy of the emergency plan as well as a list of contacts in case of an emergency during a TMH session.
It is recommended that TMH providers have several ways to contact key staff who can assist during an emergency. Usually the clinical coordinator and telehealth technician are the first responders to be alerted by the TMH provider during an emergency. They will then institute the remote clinic’s emergency protocol. Discussing these procedures and reviewing them with staff regularly is advisable, as key contacts may change.
In a psychological emergency, the VISN TMH provider may assist in implementing emergency procedures until a clinical counterpart at the remote site can be alerted. In the authors’ experience, VISN TMH providers have successfully de-escalated and diffused potentially emergent situations by maintaining constant realtime communication with veterans and staff by using VTC as well as interoffice communication. By offering assistance to veterans and staff during challenging situations, the VISN TMH provider will not only decrease concerns of veterans, but oftentimes integrate themselves into the treatment team of the remote clinic. The role of a VISN TMH provider can be isolative, with minimal contact with remote clinic staff, so it is important to increase visibility among staff at a remote site by communication with them even when there is not an emergency.
Treatment protocols may be determined by either administrative or clinical factors. With certain TMH interventions, the rooms used for veterans may be available for only certain periods, which may or may not fit with treatment protocols. For example, if a room is available for only an hour but a treatment protocol session is for 90 minutes, then another time slot needs to be found or a different treatment considered and offered. Although it is not ideal to have treatment protocols determined by scheduling factors, the reality of shared space at remote sites requires flexibility.
Sharing Materials and Homework Another clinical issue that is often overlooked is how to implement specific treatment protocols that entail the exchange of materials between VISN TMH providers and veterans. If materials will need to be exchanged between provider and veteran, a plan will have to be in place to facilitate this. The service agreement addresses these details, but remote staff may not always be aware of the details.
If a TMH provider opts to use faxes to send materials between a veteran and a provider, a desktop faxing program is recommended so veteran privacy is not compromised. Often, providers will wait to begin sessions until after they have received materials, but this may result in a delayed
session. One solution TMH providers can implement is mailing the materials and questionnaires to veterans before the session with clear instructions to complete them beforehand. Once the veteran arrives for the TMH session, she or he will verbally respond to the questionnaire and treatment materials. This will add time to a session but minimizes potential delays. Many of the clinical VTC units have movable cameras, so veterans can tilt the camera to show providers the forms and questionnaires.
The various steps necessary to address TMH clinical issues are summarized in Table 2.
VISIN 22 Pilot Project
The VISN 22 EBP TMH Center and Regional Pilot, based at the VA San Diego Healthcare System, was tasked with developing and providing TMH EBP services for PTSD across VISN 22 and adjacent West Coast VISNs. In addition to creating standardized procedures, troubleshooting guides were established to assist other programs with implementation. The primary focus was to increase access to EBPs for veterans with PTSD in areas where there was either no available trained providers or delays for specific services. The program established 16 clinics as well as in-home
services in VISN 22, VISN 21, and VISN 20. In fiscal year (FY) 2013, the VISN 22 EBP TMH Center and Regional Pilot provided 1,657 EBP encounters via TMH to 234 unique veterans with PTSD (Table 3).
The pilot project collected data to evaluate program effectiveness. The data were de-identified before being sent to the VA Central Office (VACO) TMH program manager. The following items were collected for the pilot: (1) clinical information; (2) consent to engage in treatment and telehealth; (3) release of information to share de-identified data to VACO for program monitoring; (4) demographic form; (5) Beck Depression Inventory-II (every other week); (6) PTSD Checklist (every other week); (7) World Health Organization Quality of Life (sessions 1, 7, final); (8) Wechsler Adult Intelligence Scale-Revised (sessions 3, 7, final); (9) satisfaction survey (final); (10) mileage not driven by veterans who receive TMH services; (11) travel pay saved by VA; (12) no-show rates; and (13) veteran, TMH provider, and referral provider satisfaction.
The growth in number of encounters and number of unique veterans has increased steadily from the first quarter of FY14 through the second quarter of FY15 (Figure 1).
In January 2013, in-home TMH services were piloted. Although occasional technical difficulties occurred, 143 EBP encounters via TMH were provided to 42 unique veterans in 2013. The service has continued to expand, and in the first half of FY14, services were provided to 64 unique veterans for a total of 278 encounters, saving veterans 3,220 travel miles and saving the VA $1,336 in travel reimbursement. In-home TMH services will continue to expand as more providers in a variety of programs are being trained by the San Diego staff on how to provide these services to veterans in their homes. In addition to decreasing mileage and travel pay, the no-show rates are lower for TMH appointments in general (averaged 8%-10% vs facility no-show rate average of 13.5%) and with the use of inhome TMH, no-show rates were kept to 2%. The growth in the number of in-home encounters and the number of unique veterans has also increased steadily from the first quarter of FY14 thru the second quarter of FY15 (Figure 2).
In-Home TMH Services
The VISN 22 EBP TMH Center and Regional Pilot often requests to have an in-person meeting with a veteran before starting TMH services in order to complete a waiver to download the software used by the VA for real-time video in-home services, a Release of Information for a Primary Support Person form, and an emergency plan.
It is also recommended that information about the veteran’s Internet connection, type of computer, type of software, presence of a camera and speakers, e-mail address, and access to secure messaging are obtained. During the initial contact with a veteran, the provider will discuss the rules and requirements to ensure HIPAA compliance. The veteran will need to have a private area for the call (not a restaurant, car, or other place where Wi-Fi is offered). Even with these discussions, some veterans will initiate services from a public place or a room in their home where family members will enter and exit frequently.
Although not required, it is recommended to have the veteran identify a primary support person and complete a release form to allow the TMH provider to contact that person in an emergency. The support person may be a person in the home (adult family member or caregiver) or someone nearby (neighbor, friend, or family member) who can contact emergency services if needed. After the necessary information is gathered and the veteran agrees to the conditions of participation, a test call will be completed. The TMH provider is often the person to conduct this call, but if available, a telehealth technician or facility telehealth coordinator may assist. The TMH provider may help the veteran download the appropriate software that is sent from the VA Scheduler software. The veteran initiates the call with the provider. Once the connection is made, the session may begin. Sites that are currently conducting in-home services have provided guides to veterans and newer TMH providers to outline the necessary steps for initiating services.
It is recommended that any provider interested in providing in-home TMH services use the Office of Technological Services help desk to assist in troubleshooting difficulties with connectivity. Challenges have included the software used for in-home TMH, periodic Internet outages, and compatibility issues.
Veteran Satisfaction
Veteran satisfaction was measured through a self-report satisfaction survey. The survey included 12 questions assessing overall experience in using TMH services. Eleven of the 12 questions included a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the last question was openended for additional comments.
A summary of the survey response of the initial 29 veterans who received TMH services suggested the following: (1) Veterans felt comfortable with using the TMH equipment and were able to see their clinician clearly; (2) Technical assistance was sufficient; (3) During the TMH session, they related to the provider as if it were a face-to-face meeting and that their needs were met; and (4) Veterans reported extremely high satisfaction with TMH and would refer TMH care to other veterans. Veterans found clinic locations very convenient and preferred the TMH modality of mental health services delivery to the alternative of travelling a long distance to see their provider (Table 4).
Written comments and recommendations from veterans supported the survey results. Most reported that they saved time and the convenience of the clinic allowed them to receive the treatment they need without interfering with their work schedule. However, some veterans still experienced trouble with travel to the remote clinic. Others felt their experience was different from the one they expected or they had a good experience via TMH but preferred face-to-face care.
Conclusion
The VISN 22 EBP TMH Center and Regional Pilot have established the infrastructure of interfacility clinics to use EBPs for the treatment of PTSD. Also, the center has provided consultation and guidance to facilities interested in developing their own TMH programs. The TMH Center now plans to expand mental health services and include medication management and EBP services for non-PTSD psychiatric diagnoses. The established infrastructure will allow providers from one facility to cover the mental health service needs of other facilities when there are absences or gaps due to leave or delays/challenges in hiring in rural locations. Finally, TMH offers the potential to offer after-hours services to veterans in other time zones during providers’ regular tours of duty.
Several other TMH programs are now expanding services into veterans’ homes. There are several sites within the VHA that have piloted this TMH modality and developed guidelines and recommendations for further expansion. Currently VACO is encouraging all VHA facilities to increase in-home telehealth services, and the Office of Telehealth Services provides details on implementation. Interested parties are encouraged to routinely visit the VACO website for updated information.
Developing and implementing a new TMH program can be an arduous task, but the program has great potential to provide veteran-centered care. As TMH sessions progress, the provider and veteran become less aware of the camera and software and more aware of the therapeutic process. Challenges and delays in implementation are to be expected—these can occur frequently during the development and implementation stages of a TMH program. Maintaining consistent communication with staff at remote sites is essential for the success of any program.
As the VHA focuses on veterancentered care, TMH services will improve access to providers with specific, needed expertise. The authors hope these experiences can facilitate the continued growth of TMH and assuage any concerns a facility or provider may have about this modality of care. Delivery of TMH care can be challenging, but the ability to provide these services to veterans at times and locations convenient to them makes these challenges worthwhile.
Acknowledgments
Dr. Hauser wishes to thank Cathy, Anika, Jirina, Katia, and Max Hauser, and Alba Pillwein for their continued support. In memory of Beverly Ostroski.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Within VHA, telemental health (TMH) refers to behavioral health services that are provided remotely, using secure communication technologies, to veterans who are separated by distance from their mental health providers.1 Telemental health sometimes involves video teleconferencing (VTC) technology, where a veteran (or group of veterans) in one location and a provider in a different location are able to communicate in real time through a computer monitor or television screen.2 In the VHA, TMH visits are typically conducted from a central location (such as a medical center hospital) to a community-based outpatient clinic (CBOC), but pilot projects have also tested VTC in homes as well.1,3,4
In addition to providing timely access to behavioral health services in rural or underserved locations, TMH eliminates travel that may be disruptive or costly and allows mental health providers to consult with or provide supervision to one another. Telemental health can be used to make diagnoses, manage care, perform checkups, and provide long-term, follow-up care. Other uses for TMH include clinical assessment, individual and group psychotherapy, psycho-educational interventions, cognitive testing, and general psychiatric care.1,5,6 More recently, TMH has been used to provide evidence-based psychotherapies (EBPs) to individuals with posttraumatic stress disorder (PTSD) and other mental health diagnoses.6,7 Such care may be particularly advantageous for veterans with PTSD, because traveling can be a burden for them or a trigger for PTSD symptoms.
Although interactive video technology is becoming widely available, its use is limited in health care systems due to lack of knowledge, education, logistical guidance, and technical training. The authors have conducted EBPs using VTC across VISN 22 in both office-to-office and office-to-home modalities and are providing EBPs using VTC to CBOCs in other VISNs across the western U.S. This article addresses these issues, outlining the necessary steps required to establish a TMH clinic and to share the successes of the EBP TMH Center and Regional Pilot used at VISN 22.
Telemental Health
Telemental health is an effective alternative to in-person treatment and is well regarded by both mental health providers and veterans. Overall, mental health providers believe it can help reduce the stigma associated with traditional mental health care and ease transportation-related issues for veterans. Telemental health allows access to care for veterans living in rural or remote areas in addition to those who are incarcerated or are otherwise unable to attend visits at primary VA facilities.2,8-10 In an assessment of TMH services in 40 CBOCs across VISN 16, most CBOC mental health providers found it to be an acceptable alternative to face-to-face care, recognize the value of TMH, and endorse a willingness to use and expand TMH programs within their clinics.11
Veterans who participated in TMH via VTC have expressed satisfaction with the decreased travel time and expenses, fewer interactions with crowds, and fewer parking problems.12 Several studies suggested that veterans preferred TMH to in-person contact due to more rapid access to care and specialists who would otherwise be unavailable at remote locations.5,10 Similarly, veterans who avoid in-person mental health care were more open to remote therapy for many of the reasons listed earlier. Studies suggest that veterans from both rural and urban locations are generally receptive to receiving mental health services via TMH.5,10
Several studies have found that TMH services may have advantages over standard in-person care. These advantages include decreasing transportation costs, travel time, and time missed at work and increasing system coverage area.13 Overall, both veterans and providers reported similar satisfaction between VTC and in-person sessions and, in some cases, prefer VTC interactions due to a sense of “easing into” intense therapies or having a “therapeutic distance” as treatment begins.12
Utility
Previous studies have shown that TMH can be used successfully to provide psychopharmacologic treatment to veterans who have major depressive disorder or schizophrenia, among other psychiatric disorders.5,8,14 Recent studies have focused on the feasibility of providing EBPs via TMH, particularly for the treatment of PTSD.12,15 Studies have shown that TMH services via VTC can be used successfully to provide cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), and prolonged exposure therapy (PE).16-21 In these studies, both PE and CPT delivered via TMH were found to be as efficacious as in-person formats. Furthermore, TMH services were successfully used in individual and group sessions.
Research has emphasized the benefits of TMH for veterans who are uncomfortable in crowds, waiting rooms, or hospital lobbies.7,12,18 For patients with PTSD who are initially limited by fears related to driving, TMH can facilitate access to care. Veterans with PTSD often avoid reminders of trauma (ie, uniforms, evidence of physical injury, artwork, photographs related to war), which can often be found at the larger VAMCs. These veterans may find mental health care services in their homes or at local CBOCs more appealing.7,12,18
Implementation
Prior to the implementation of telehealth services, many CBOC providers would refer veterans in need of specialty care to the nearest VAMC, which were sometimes many hours away.1 In response to travel and access concerns, the VA has implemented various telehealth modalities, including TMH.
In 2008, about 230,000 veterans received mental health services via real-time clinical VTC at 300 VA CBOCs, and about 40,000 veterans enrolled in the In-Home Telehealth program.22 By 2011, > 380,000 veterans used clinic-based telehealth services and about 100,000 veterans used the in-home program.1 Between 2006 and 2008, the 98,000 veterans who used TMH modalities had fewer hospital admissions compared with those who did not; overall, the need for hospital services decreased by about 25% for those using TMH services.23
Although research suggests that TMH is an effective treatment modality, it does have limitations. A recent study noted several visual and audio difficulties that can emerge, including pixilation, “tracer” images with movement, low resolution, “frozen” or “choppy” images, delays in sound, echoes, or “mechanical sounding” voices.12 In some cases, physical details, such as crying, sniffling, or fidgeting, could not be clearly observed.12 Overall, these unforeseen issues can impact the ability to give and receive care through TMH modalities. Proper procedures need to be developed and implemented for each site.
Getting Started
Using TMH to provide mental health care at other VHA facilities requires planning and preparation. Logistics, such as preparation of the room and equipment, should be considered. Similarly, veteran and provider convenience must be considered.2,11 Before starting TMH at any VA facility, professionals working with the audiovisual technology and providing TMH care must complete necessary VA Talent Management System courses and obtain copies of certificates to assure they have met the appropriate training criteria. Providers must be credentialed to provide TMH services, including the telehealth curriculum offered by VA Employee Educational Services.2,24 An appropriate memorandum of understanding (MOU) must be created, and credentialing and privileging must also be acquired.
In addition to provider training, an information technology representative who can administer technical support as needed must be selected for both the provider and remote locations. Technologic complications can make TMH implementation much more challenging.12 As such, it is important to assure that both the veteran and the provider have the necessary TMH equipment. The selected communication device must be compatible with the technology requirements at the provider and remote facilities.12
In addition to designated technical support, the VISN TMH coordinator needs to have point-of-contact information for those who can assist with each site’s telehealth services and address the demand for EBP for PTSD or other desired services. After this information has been obtained, relationships must be developed and maintained with local leadership at each site, associated telehealth coordinators, and evidence-based therapy coordinators.
After contact has been established with remote facilities and the demand for services has been determined, there are several agreements and procedures to put in place before starting TMH services. An initial step is to develop a MOU agreement between the VISN TMH center and remote
sites that allows providers’ credentials and privileges to be shared. Also, it is important to establish a service agreement that outlines the procedures for staff at the remote site. This agreement includes checking in veterans, setting up the TMH rooms, transferring homework to VISN TMH providers, and connecting with the VISN TMH provider. In addition to service agreements, emergency procedures must be in place to ensure the safety of the veterans and the staff.24
After these agreements have been completed, the VISN TMH providers will have to complete request forms to obtain access to the Computerized Patient Record System at the remote facilities, which then must be approved by the Information Security Officer at that site. This is separate from the request at the provider’s site.12 It is essential to have points of contact for questions regarding this process. In order to facilitate referrals for TMH, electronic interfacility consult requests must be developed. Local staff need to collaborate with VISN TMH staff to ensure that the consult addresses the referral facilities need to meet the appropriate requirements.
Before the initiation of TMH services, each TMH provider has to establish clinics for scheduling appointments and obtaining workload credit. Program support assistants at the provider and remote sites must work together to ensure clinics are established correctly. This collaboration is essential for coding of visits and clinic mapping. After the clinics are “built,” appointment times will be set up based on the availability of the provider, support staff, and rooms at the remote site for the TMH session.
Once a consult is initiated, the VISN TMH EBP coordinator will review the consult and the veteran’s chart to ensure initial inclusion/exclusion criteria are met before accepting or canceling the consult. If the consult is accepted, a VISN TMH provider is assigned to the case and contacts the veteran to discuss the referral and (if the veteran is appropriate and interested) initiate services at the closest CBOC or at home. The VISN TMH regional center staff enter the appointment time for the veteran at both facility sites. The VISN TMH provider also coordinates with the CBOC staff to ensure that the veteran is checked in to the appointment and is provided with any questionnaires and necessary homework.
During the first session, the provider obtains consent from the veteran to engage in TMH services, conducts an assessment, and establishes rapport. The provider works with the veteran to develop a treatment plan for PTSD or other mental health diagnosis that will include the type of EBP. At the end of the first session, the next appointment is scheduled, and treatment materials are either mailed to the veteran or given to him or her onsite. After completing EBP, the VISN TMH center works with the referring provider to find follow-up services for the veteran.
The various steps necessary to begin an interfacility TMH clinic are summarized in Table 1.
Provider Training
Despite strong evidence of success, many providers remained skeptical about the efficacy of TMH. One study indicated that several providers in VISN 16 rarely used the established TMH programs because they were not familiar with them and applied TMH only for medication checks and consults.11 This skepticism was present in providers preparing to offer TMH as well as in providers referring veterans for TMH services. However, once providers better understood the TMH programs and had more experience using them, they were significantly more likely to use TMH for initial evaluations and ongoing psychotherapy. For these reasons, proper training and educational opportunities for practicing providers are vital to TMH implementation.9,11
To be proficient, providers need to become familiar with various TMH applications.10 Health care networks implementing TMH must ensure that their providers are well trained and prepared to give and receive proper consultation and support. Providers must also acquire several skills and familiarize themselves with available tools.9 In educating providers on the process and use of TMH, the authors suggest the following steps for TMH application:
- Learn new ways to chart in multiple systems and know how to troubleshoot during connectivity issues.
- Have an established administrative support collaborator at outpatient clinics to fax and exchange veteran homework.12
- The TMH clinic culture must be embedded where the veteran is being served in order to allow for a more realistic therapeutic feel. This type of clinic setting will allow for referrals at the veteran site and the availability to coordinate emergency procedures in the remote clinic.
Clinical Issues
Ongoing clinical issues need to be addressed continuously. Initially, referrals may be plentiful but not always appropriate. It is important to have an understanding with referring providers and remote sites about what constitutes a “good referral” as well as alternate referral options. It is imperative to outline inclusion and exclusion criteria that are clear and concise for referring providers. It is often helpful to revisit these criteria with potential referral sources after initiating services.
With the ability to provide inhome services, it is important to identify specific inclusion/exclusion criteria. Recommendations are based on research and clinical applications for exclusions, which are available on the Office of Technology Services website. These include imminent suicidality or homicidality, serious personality disorder or problematic character traits, acute substance disorders, psychotic disorders, and bipolar disorder. It is important to use sound clinical judgment, because the usual safeguards present in a remote clinic are not available for inhome services. Emergency planning is one of the most important aspects of the in-home TMH health services that are provided. The information for the emergency plan is obtained prior to initiation of services.
Emergency Plans
Each remote clinic that provides services to veterans must have an emergency plan that details procedures, phone numbers, and resources in case of medical and psychological emergencies as well as natural disasters. The VISN TMH provider will need to have a copy of the emergency plan as well as a list of contacts in case of an emergency during a TMH session.
It is recommended that TMH providers have several ways to contact key staff who can assist during an emergency. Usually the clinical coordinator and telehealth technician are the first responders to be alerted by the TMH provider during an emergency. They will then institute the remote clinic’s emergency protocol. Discussing these procedures and reviewing them with staff regularly is advisable, as key contacts may change.
In a psychological emergency, the VISN TMH provider may assist in implementing emergency procedures until a clinical counterpart at the remote site can be alerted. In the authors’ experience, VISN TMH providers have successfully de-escalated and diffused potentially emergent situations by maintaining constant realtime communication with veterans and staff by using VTC as well as interoffice communication. By offering assistance to veterans and staff during challenging situations, the VISN TMH provider will not only decrease concerns of veterans, but oftentimes integrate themselves into the treatment team of the remote clinic. The role of a VISN TMH provider can be isolative, with minimal contact with remote clinic staff, so it is important to increase visibility among staff at a remote site by communication with them even when there is not an emergency.
Treatment protocols may be determined by either administrative or clinical factors. With certain TMH interventions, the rooms used for veterans may be available for only certain periods, which may or may not fit with treatment protocols. For example, if a room is available for only an hour but a treatment protocol session is for 90 minutes, then another time slot needs to be found or a different treatment considered and offered. Although it is not ideal to have treatment protocols determined by scheduling factors, the reality of shared space at remote sites requires flexibility.
Sharing Materials and Homework Another clinical issue that is often overlooked is how to implement specific treatment protocols that entail the exchange of materials between VISN TMH providers and veterans. If materials will need to be exchanged between provider and veteran, a plan will have to be in place to facilitate this. The service agreement addresses these details, but remote staff may not always be aware of the details.
If a TMH provider opts to use faxes to send materials between a veteran and a provider, a desktop faxing program is recommended so veteran privacy is not compromised. Often, providers will wait to begin sessions until after they have received materials, but this may result in a delayed
session. One solution TMH providers can implement is mailing the materials and questionnaires to veterans before the session with clear instructions to complete them beforehand. Once the veteran arrives for the TMH session, she or he will verbally respond to the questionnaire and treatment materials. This will add time to a session but minimizes potential delays. Many of the clinical VTC units have movable cameras, so veterans can tilt the camera to show providers the forms and questionnaires.
The various steps necessary to address TMH clinical issues are summarized in Table 2.
VISIN 22 Pilot Project
The VISN 22 EBP TMH Center and Regional Pilot, based at the VA San Diego Healthcare System, was tasked with developing and providing TMH EBP services for PTSD across VISN 22 and adjacent West Coast VISNs. In addition to creating standardized procedures, troubleshooting guides were established to assist other programs with implementation. The primary focus was to increase access to EBPs for veterans with PTSD in areas where there was either no available trained providers or delays for specific services. The program established 16 clinics as well as in-home
services in VISN 22, VISN 21, and VISN 20. In fiscal year (FY) 2013, the VISN 22 EBP TMH Center and Regional Pilot provided 1,657 EBP encounters via TMH to 234 unique veterans with PTSD (Table 3).
The pilot project collected data to evaluate program effectiveness. The data were de-identified before being sent to the VA Central Office (VACO) TMH program manager. The following items were collected for the pilot: (1) clinical information; (2) consent to engage in treatment and telehealth; (3) release of information to share de-identified data to VACO for program monitoring; (4) demographic form; (5) Beck Depression Inventory-II (every other week); (6) PTSD Checklist (every other week); (7) World Health Organization Quality of Life (sessions 1, 7, final); (8) Wechsler Adult Intelligence Scale-Revised (sessions 3, 7, final); (9) satisfaction survey (final); (10) mileage not driven by veterans who receive TMH services; (11) travel pay saved by VA; (12) no-show rates; and (13) veteran, TMH provider, and referral provider satisfaction.
The growth in number of encounters and number of unique veterans has increased steadily from the first quarter of FY14 through the second quarter of FY15 (Figure 1).
In January 2013, in-home TMH services were piloted. Although occasional technical difficulties occurred, 143 EBP encounters via TMH were provided to 42 unique veterans in 2013. The service has continued to expand, and in the first half of FY14, services were provided to 64 unique veterans for a total of 278 encounters, saving veterans 3,220 travel miles and saving the VA $1,336 in travel reimbursement. In-home TMH services will continue to expand as more providers in a variety of programs are being trained by the San Diego staff on how to provide these services to veterans in their homes. In addition to decreasing mileage and travel pay, the no-show rates are lower for TMH appointments in general (averaged 8%-10% vs facility no-show rate average of 13.5%) and with the use of inhome TMH, no-show rates were kept to 2%. The growth in the number of in-home encounters and the number of unique veterans has also increased steadily from the first quarter of FY14 thru the second quarter of FY15 (Figure 2).
In-Home TMH Services
The VISN 22 EBP TMH Center and Regional Pilot often requests to have an in-person meeting with a veteran before starting TMH services in order to complete a waiver to download the software used by the VA for real-time video in-home services, a Release of Information for a Primary Support Person form, and an emergency plan.
It is also recommended that information about the veteran’s Internet connection, type of computer, type of software, presence of a camera and speakers, e-mail address, and access to secure messaging are obtained. During the initial contact with a veteran, the provider will discuss the rules and requirements to ensure HIPAA compliance. The veteran will need to have a private area for the call (not a restaurant, car, or other place where Wi-Fi is offered). Even with these discussions, some veterans will initiate services from a public place or a room in their home where family members will enter and exit frequently.
Although not required, it is recommended to have the veteran identify a primary support person and complete a release form to allow the TMH provider to contact that person in an emergency. The support person may be a person in the home (adult family member or caregiver) or someone nearby (neighbor, friend, or family member) who can contact emergency services if needed. After the necessary information is gathered and the veteran agrees to the conditions of participation, a test call will be completed. The TMH provider is often the person to conduct this call, but if available, a telehealth technician or facility telehealth coordinator may assist. The TMH provider may help the veteran download the appropriate software that is sent from the VA Scheduler software. The veteran initiates the call with the provider. Once the connection is made, the session may begin. Sites that are currently conducting in-home services have provided guides to veterans and newer TMH providers to outline the necessary steps for initiating services.
It is recommended that any provider interested in providing in-home TMH services use the Office of Technological Services help desk to assist in troubleshooting difficulties with connectivity. Challenges have included the software used for in-home TMH, periodic Internet outages, and compatibility issues.
Veteran Satisfaction
Veteran satisfaction was measured through a self-report satisfaction survey. The survey included 12 questions assessing overall experience in using TMH services. Eleven of the 12 questions included a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the last question was openended for additional comments.
A summary of the survey response of the initial 29 veterans who received TMH services suggested the following: (1) Veterans felt comfortable with using the TMH equipment and were able to see their clinician clearly; (2) Technical assistance was sufficient; (3) During the TMH session, they related to the provider as if it were a face-to-face meeting and that their needs were met; and (4) Veterans reported extremely high satisfaction with TMH and would refer TMH care to other veterans. Veterans found clinic locations very convenient and preferred the TMH modality of mental health services delivery to the alternative of travelling a long distance to see their provider (Table 4).
Written comments and recommendations from veterans supported the survey results. Most reported that they saved time and the convenience of the clinic allowed them to receive the treatment they need without interfering with their work schedule. However, some veterans still experienced trouble with travel to the remote clinic. Others felt their experience was different from the one they expected or they had a good experience via TMH but preferred face-to-face care.
Conclusion
The VISN 22 EBP TMH Center and Regional Pilot have established the infrastructure of interfacility clinics to use EBPs for the treatment of PTSD. Also, the center has provided consultation and guidance to facilities interested in developing their own TMH programs. The TMH Center now plans to expand mental health services and include medication management and EBP services for non-PTSD psychiatric diagnoses. The established infrastructure will allow providers from one facility to cover the mental health service needs of other facilities when there are absences or gaps due to leave or delays/challenges in hiring in rural locations. Finally, TMH offers the potential to offer after-hours services to veterans in other time zones during providers’ regular tours of duty.
Several other TMH programs are now expanding services into veterans’ homes. There are several sites within the VHA that have piloted this TMH modality and developed guidelines and recommendations for further expansion. Currently VACO is encouraging all VHA facilities to increase in-home telehealth services, and the Office of Telehealth Services provides details on implementation. Interested parties are encouraged to routinely visit the VACO website for updated information.
Developing and implementing a new TMH program can be an arduous task, but the program has great potential to provide veteran-centered care. As TMH sessions progress, the provider and veteran become less aware of the camera and software and more aware of the therapeutic process. Challenges and delays in implementation are to be expected—these can occur frequently during the development and implementation stages of a TMH program. Maintaining consistent communication with staff at remote sites is essential for the success of any program.
As the VHA focuses on veterancentered care, TMH services will improve access to providers with specific, needed expertise. The authors hope these experiences can facilitate the continued growth of TMH and assuage any concerns a facility or provider may have about this modality of care. Delivery of TMH care can be challenging, but the ability to provide these services to veterans at times and locations convenient to them makes these challenges worthwhile.
Acknowledgments
Dr. Hauser wishes to thank Cathy, Anika, Jirina, Katia, and Max Hauser, and Alba Pillwein for their continued support. In memory of Beverly Ostroski.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. What is telehealth? U.S. Department of Veterans Affairs Website. http://www.telehealth.va.gov. Update May 13, 2014. Accessed April 30, 2015.
2. Morland LA, Greene CJ, Rosen C, Mauldin PD, Frueh CB. Issues in the design of a randomized noninferiority clinical trial of telemental health psychotherapy for rural combat veterans with PTSD. Contemp Clin Trials. 2009;30(6):513-522.
3. Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, Acierno R. An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behav Ther. 2012;43(3):560-569.
4. Yuen EK, Gros DF, Price M, et al. Randomized controlled trial of home-based telehealth versus in-person prolonged exposure for combat-related PTSD in veterans: preliminary results. J Clin Psychol. 2015;71(6):500-512.
5. Ruskin PE, Reed S, Kumar R, et al. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatr Serv. 1998;49(8):1086-1088.
6. Gros DF, Morland LA, Greene CJ, et al. Delivery of evidence-based psychotherapy via video telehealth. J Psychopathol Behav Assess. 2013;35(4):506-521.
7. Backhaus A, Agha Z, Maglione ML, et al. Videoconferencing psychotherapy: a systematic review. Psychol Serv. 2012;9(2):111-131.
8. Egede LE, Frueh CB, Richardson LK, et al. Rationale and design: telepsychology service delivery for depressed elderly veterans. Trials. 2009;10:22.
9. Frueh BC, Deitsch SE, Santos AB, et al. Procedural and methodological issues in telepsychiatry research and program development. Psychiatr Serv. 2000;51(12):1522-1527.
10. Grubaugh AL, Cain GD, Elhai JD, Patrick SL, Frueh BC. Attitudes toward medical and mental health care delivered via telehealth applications among rural and urban primary care patients. J Nerv Ment Dis. 2008;196(2):166-170.
11. Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes towards telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425-432.
12. Thorp SR, Fidler J, Moreno L, Floto E, Agha Z. Lessons learned from studies of psychotherapy for posttraumatic stress disorder via video teleconferencing. Psychol Serv. 2012;9(2):197-199.
13. Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: predictors of treatment completion and outcome and comparison to treatment delivered in person. Behav Ther. 2011;42(2):276-283.
14. Zarate CA Jr, Weinstock L, Cukor P, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry. 1997;58(1):22-25.
15. Jones AM, Shealy KM, Reid-Quiñones K, et al. Guidelines for establishing a telemental health program to provide evidence-based therapy for trauma-exposed children and families. Psychol Serv. 2014;11(4):398-409.
16. Frueh BC, Monnier J, Grubaugh AL, Elhai JD, Yim E, Knapp R. Therapist adherence and competence with manualized cognitive-behavioral therapy for PTSD delivered via videoconferencing technology. Behav Modif. 2007;31(6):856-866.
17. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469.
18. Tuerk PW, Yoder M, Ruggiero KJ, Gros DF, Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J Trauma Stress. 2010;23(1):116-123.
19. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine- based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
20. Germain V, Marchand A, Bouchard S, Drouin MS, Guay S. Effectiveness of cognitive behavioural therapy administered by videoconference for posttraumatic stress disorder. Cogn Behav Ther. 2009;38(1):42-53.
21. Morland LA, Mackintosh M, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75(5):470-476.
22. Tuerk PW, Fortney J, Bosworth HB, et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16(1):115-117.
23. Godleski L, Darkins A, Peters J. Outcomes of 98,609 U.S. Department of Veterans Affairs patients enrolled in telemental health services, 2006-2010. Psychiatr Serv. 2012;63(4):383-385.
24. Strachan M, Gros DF, Yuen E, Ruggiero KJ, Foa EB, Acierno R. Home-based telehealth to deliver evidence-based psychotherapy in veterans with PTSD. Contemp Clin Trials. 2012;33(2):402-409.
1. U.S. Department of Veterans Affairs. What is telehealth? U.S. Department of Veterans Affairs Website. http://www.telehealth.va.gov. Update May 13, 2014. Accessed April 30, 2015.
2. Morland LA, Greene CJ, Rosen C, Mauldin PD, Frueh CB. Issues in the design of a randomized noninferiority clinical trial of telemental health psychotherapy for rural combat veterans with PTSD. Contemp Clin Trials. 2009;30(6):513-522.
3. Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, Acierno R. An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behav Ther. 2012;43(3):560-569.
4. Yuen EK, Gros DF, Price M, et al. Randomized controlled trial of home-based telehealth versus in-person prolonged exposure for combat-related PTSD in veterans: preliminary results. J Clin Psychol. 2015;71(6):500-512.
5. Ruskin PE, Reed S, Kumar R, et al. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatr Serv. 1998;49(8):1086-1088.
6. Gros DF, Morland LA, Greene CJ, et al. Delivery of evidence-based psychotherapy via video telehealth. J Psychopathol Behav Assess. 2013;35(4):506-521.
7. Backhaus A, Agha Z, Maglione ML, et al. Videoconferencing psychotherapy: a systematic review. Psychol Serv. 2012;9(2):111-131.
8. Egede LE, Frueh CB, Richardson LK, et al. Rationale and design: telepsychology service delivery for depressed elderly veterans. Trials. 2009;10:22.
9. Frueh BC, Deitsch SE, Santos AB, et al. Procedural and methodological issues in telepsychiatry research and program development. Psychiatr Serv. 2000;51(12):1522-1527.
10. Grubaugh AL, Cain GD, Elhai JD, Patrick SL, Frueh BC. Attitudes toward medical and mental health care delivered via telehealth applications among rural and urban primary care patients. J Nerv Ment Dis. 2008;196(2):166-170.
11. Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes towards telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425-432.
12. Thorp SR, Fidler J, Moreno L, Floto E, Agha Z. Lessons learned from studies of psychotherapy for posttraumatic stress disorder via video teleconferencing. Psychol Serv. 2012;9(2):197-199.
13. Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: predictors of treatment completion and outcome and comparison to treatment delivered in person. Behav Ther. 2011;42(2):276-283.
14. Zarate CA Jr, Weinstock L, Cukor P, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry. 1997;58(1):22-25.
15. Jones AM, Shealy KM, Reid-Quiñones K, et al. Guidelines for establishing a telemental health program to provide evidence-based therapy for trauma-exposed children and families. Psychol Serv. 2014;11(4):398-409.
16. Frueh BC, Monnier J, Grubaugh AL, Elhai JD, Yim E, Knapp R. Therapist adherence and competence with manualized cognitive-behavioral therapy for PTSD delivered via videoconferencing technology. Behav Modif. 2007;31(6):856-866.
17. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469.
18. Tuerk PW, Yoder M, Ruggiero KJ, Gros DF, Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J Trauma Stress. 2010;23(1):116-123.
19. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine- based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
20. Germain V, Marchand A, Bouchard S, Drouin MS, Guay S. Effectiveness of cognitive behavioural therapy administered by videoconference for posttraumatic stress disorder. Cogn Behav Ther. 2009;38(1):42-53.
21. Morland LA, Mackintosh M, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75(5):470-476.
22. Tuerk PW, Fortney J, Bosworth HB, et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16(1):115-117.
23. Godleski L, Darkins A, Peters J. Outcomes of 98,609 U.S. Department of Veterans Affairs patients enrolled in telemental health services, 2006-2010. Psychiatr Serv. 2012;63(4):383-385.
24. Strachan M, Gros DF, Yuen E, Ruggiero KJ, Foa EB, Acierno R. Home-based telehealth to deliver evidence-based psychotherapy in veterans with PTSD. Contemp Clin Trials. 2012;33(2):402-409.
Using Dashboard Technology to Monitor Overdose Risk
On October 10, 2013, a Congressional hearing was held to address the issue of opioid medication prescribing within VHA facilities and clinics (House Veteran Affairs Subcommittee hearing “Between Peril and Promise: Facing the Dangers of VA’s Skyrocketing Use of Prescription Painkillers to Treat Veterans”). Several individuals testified, including the widows of 2 veterans; both their husbands had overdosed on prescribed opioid medications. One husband had been taking as many as 15 pills a day and was additionally prescribed oxycodone/acetaminophen, which led to his death.1
Alongside the widows were 2 veterans who had been treated for chronic back pain injuries sustained before and during deployment in Iraq. Both had been prescribed several pain medications, including oxycodone/acetaminophen, methadone, and morphine. One reported that as his pain increased, his doctors continued to provide him additional prescriptions; at one point he had more than 13 prescriptions and could no longer work from being so “doped up.”1
In the past 2 decades, health care professionals (HCPs) have placed greater emphasis on chronic pain management. As a result, the rate of opioid medication prescribing has increased dramatically. Since 1994, the number of opioid medication prescriptions has nearly doubled; this change has been accompanied by an increase in opioid misuse, which has resulted in accidental or intentional overdose and death.2
Based on a recent National Institute on Drug Abuse (NIDA) report, the greatest impact has been on armed forces personnel.3 Prescriptions for pain relievers quadrupled between 2001 and 2009 to almost 3.8 million within the military population. Although civilian populations are more likely to abuse illicit drugs, military personnel are at particular risk of prescription abuse, including opioid medications.3 In 2008, 11% of armed forces service members reported misusing prescription drugs, with opioid medications being the most abused. This is an approximate 5- to 6-fold increase since 2002 (2% reported misuse in 2002).3 Particularly concerning is the associated rise in suicide rates among armed forces personnel, which surpassed civilian suicide rates in 2004. In 2009, one-third of suicides among armed forces personnel involved prescription drugs.3
Certain patient characteristics or factors are related to greater overdose risk. These risk factors include prescription dosage and frequency, history of suicide attempts or self-harm behavior, history of depression or posttraumatic stress disorder (PTSD) among other mental health-related diagnoses, a history of substance and/or alcohol abuse, and within the context of opioid medication use, the concurrent use of other central nervous system (CNS) depressants.4,5 Additionally, the stresses of deployment during wartime, physical injuries sustained in combat, and the unique military culture play a particularly important role in access to substances with high abuse potential and the subsequent development of substance abuse.3
Opioid Use and Risk Factors
More than 3% of adults in the U.S. are now receiving opioid medications for chronic noncancer pain.6 Substance abuse among patients with chronic pain ranges from 14% to 40%.5 Prescription opioid medications are the fastest growing drugs of abuse and the most common cause of unintentional overdose in the U.S.4 About 17,000 deaths occur each year as a result of prescription opioid medication overdose.7 Opioid medication-related overdose deaths began to increase in the early 2000s and continue to increase. Between 1999 and 2007, the rate of unintentional overdose-related deaths in the U.S. increased by 124%, largely due to the increase of prescription opioid medications.8
High-Dose Opioid Medication Use
A study by Dunn and colleagues found that patients receiving higher doses of prescribed opioid medications were at an increased risk of overdose.6 Patients receiving 50 mg to 99 mg morphine equivalent daily dose (MEDD) had a 3.7-fold increase in overdose risk (0.7% annual overdose rate) as compared with patients who received < 50 mg MEDD (0.2% annual overdose rate). Patients receiving ≥ 100 mg MEDD had a 1.8% annual overdose rate and a 9.8-fold increase in overdose risk as compared with patients who received < 50 mg MEDD. Overall, 51 patients experienced ≥ 1 overdose event, 40 of whom experienced fatal or serious overdoses and 6 of whom attempted suicide. Patients receiving the highest doses were male, current smokers, and had a history of depression and substance abuse.6 Similarly, a study by Bohnert and colleagues found that opioid medication overdose was most likely to occur in those patients with psychiatric and substance use disorders compared with patients who had no psychiatric illness history.8
Depression
Mood disorders are common in people with chronic pain.4,5,9,10 In particular, patients with a history of depression are more likely to receive chronic opioid medication prescriptions and are at a higher risk for opioid medication abuse. A substance abuse history is the most consistent predictor of both chronic opioid medication use and abuse. However, depression without substance abuse is significantly associated with 2 forms of opioid medication abuse: self-medication for stress or sleep and overmedication (using a higher dose than prescribed). More severe cases of depression show a stronger association for potential abuse.4
PTSD
Among Iraq and Afghanistan war veterans with ≥ 1 pain-related diagnosis, veterans with PTSD and veterans with a mental health disorder other than PTSD were significantly more likely to receive opioid medications for pain than were veterans without a mental health disorder (PTSD—17.8%, adjusted relative risk [RR] 2.58; other mental health disorder—11.7%, RR 1.74; no mental health disorder—6.5%).2 Although mental health disorders in general were related to a higher risk of opioid abuse, those with PTSD in particular were more likely to receive higher prescribed dosages; to continue taking opioids for a longer period; to receive concurrent prescriptions for opioid medications, sedative hypnotics, or both; to obtain early refills; and to have comorbid alcohol and substance use disorders. Based on these results, Seal and colleagues concluded that veterans with PTSD had the highest risk of alcohol, drug, and opioid-related accidents and overdose as well as self-inflicted injuries.2
Concurrent Use of Opioids and CNS Depressants
As mentioned earlier, studies suggest that people with PTSD are at a significantly higher risk for opioid medication overdose. One factor that may contribute to this higher risk is the concurrent use of CNS depressants/sedatives, particularly benzodiazepines and alcohol.
Benzodiazepines are often prescribed for people with PTSD. One study found that the concurrent use of benzodiazepines is significantly related to opioid overdose.5 Prescribing opioids for people already abusing or dependent on alcohol or other substances increases the risk of abuse and overdose. Furthermore, the concurrent use of multiple medications is associated with aberrant behaviors, cognitive impairment, and medication abuse, potentially leading to overdose. Overall, the combined administration of these medications is responsible for higher rates of adverse events, overdose, and death related to prescription opioid medication use.5,6,11
In summary, there are various risk factors that contribute to opioid medication overdose and more generally, risk of suicide, including (1) high-dose opioid medications; (2) history of psychiatric disorders, specifically depression and PTSD; (3) history of substance use disorders; and (4) concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse.
Suicide
Suicide is the tenth leading cause of death in the U.S., with 12.4 suicide deaths per 100,000 population.12 Suicide rates are even higher among veterans. According to the VHA, the age-adjusted rate of suicide for veterans using VHA facilities and clinics was 35.9 per 100,000 person-years for fiscal year 2009.13 Several risk factors for suicide attempts include depression and other mental health disorders, substance abuse, medical disorders, and prescription medications.
Prior suicide attempts or self-harm behavior is known to increase the risk of subsequent death by suicide. About 11 attempted suicides occur per suicide death where the medical severity of prior attempts increases the risk of future suicide, as does a history of multiple self-harm episodes.14,15 One study found that the single best predictor of suicide in a veteran population was an attempt in the previous 4 months.16
Among other risk factors, previous suicide attempts and violent behavior are major behavioral flags that warrant caution and require particular consideration when prescribing opioid medications. In a national survey on drug use and health, about 18% of prescription opioid users/abusers who experienced suicidal ideations actually attempted suicide. Only 11% of individuals who never used prescription opioid medications attempted suicide after reported suicidal ideations.17
Patient Data Aggregation
The early and widespread adoption of electronic medical records (EMRs) by the VHA allowed the aggregation of patient data for quality improvement. Initially, data were aggregated, and dashboards were designed retrospectively. However, the development of regional data warehouses that update patient information daily from the EMR allowed information to be aggregated prospectively, and dashboards were designed that provided real-time information.
The purpose of the current study is to demonstrate the efficacy and future potential of dashboard technology in assessing prospectively high-risk factors for opioid overdose. Dashboards are a user-friendly application that allows providers to isolate and calculate daily morphine equivalent opioid dosages and assess patients’ risk factors for overdose on an individual basis. By using this technology, providers who prescribe opioids can get a concise summary of opioid and other medications and adjust medications to decrease overdose risk on an individual basis.
What is the Dashboard?
The VISN 22 high-risk opioid dashboard is a business intelligence tool that serves as a report card, or progress report, to provide a global view of the number of veterans who are receiving opioid prescriptions totaling >120 mg MEDD and who have characteristics (history of depression, PTSD, substance abuse, or high-risk suicide flag) and prescriptions (concomitant CNS depressants) that may increase patient risk for overdose.
The VISN 22 dashboard allows the user to navigate to an individual HCP-level and patient-level report (Figures 1 and 2). Filter settings allow report users to select only high-risk patients; it serves as a single location for pertinent details to consider for safely prescribing opioids.
To calculate daily morphine equivalents, each patient’s opioid prescriptions were evaluated. The quantity was divided by the day’s supply to calculate an average daily quantity. From there, the drug strength was used to convert to MEDD. Health care providers were informed that these conversion factors were not recommendations for clinical opioid conversions.
Implementation and Design
In 2012, the VA Pharmacy Benefits Management (PBM) in VISN 21 created a dashboard that allowed users to identify patients on high-dose opioid prescriptions. Structured query language code was used to extract data from the regional data warehouse and calculate MEDD for all patients with active opioid prescriptions. In 2013, VISN 22 expanded that dashboard to incorporate factors that could indicate a high risk for overdose or other adverse outcomes, including a history of depression, PTSD, substance abuse or high-risk suicide flag, and concomitant use of CNS depressant medications.
The high-risk opioid dashboard (Figure 3) and accompanying patient-level report were first introduced to VISN 22 HCPs in January 2013. The business intelligence tools were introduced to each facility through the VISN 22 PBM group. Training on the use of the dashboard and the report was provided, with an initial target of decreasing MEDD of > 200 mg to < 5% of all veterans prescribed opioids at each VISN 22 facility. One month later (in February 2013), a second category of veterans (those with > 120 mg but < 199 mg MEDD) was added. Also the initial MEDD > 200 mg target of < 5% was decreased to < 3% to encourage additional progress.
Eight months after the VISN 22 dashboard technology was implemented there was a 17% decrease in patients with total daily morphine equivalents > 200 mg (January 2013; 1,137 patients vs August 2013; 940 patients—a decrease of 197 patients).
From March 2013 to August 2013, VISN 22 also saw a 12% decrease in the number of patients prescribed > 120 mg MEDD but < 199 MEDD (March 2013; 2,295 vs August 2013; 2,018—a decrease of 277 patients).
Figure 4 shows opioid use as of July 2014 for VISN 22 facilities. There were further reductions in the number of patients receiving > 120 mg but < 199 mg MEDD (August 2013; 2,018 patients vs July 2014; 1,189 patients) and patients receiving > 200 mg MEDD (August 2013; 940 patients vs July 2014; 836 patients).
Case Description
In January 2013, VISN 22 implemented dashboard technology to help providers assess and monitor opioid prescription levels in relation to high-risk variables. The benefits of this dashboard technology are illustrated in the case profile that follows.
A 67-year-old male veteran had a long history of chronic pain. Pain diagnoses included osteoarthritis with spine involvement, lumbar radiculopathy, arthralgia, and peripheral neuropathy. For the past 10 years, he was prescribed opioids with modest relief of his chronic pain symptoms despite recent prescriptions totaling 300 mg MEDD. This veteran had several risk factors for overdose, including a history of depression, suicide risk, PTSD, and concomitant use of the CNS depressants alprazolam and cyclobenzaprine.
More recently, in May 2013, the veteran exhibited aberrant behavior and requested early refills for alprazolam. In response, the pharmacist discussed the case with the HCP who prescribed the opioids, noting the concomitant overdose risk factors. As a result of this interaction, the veteran was referred for mental health services, and his prescriptions for opioids were gradually decreased. He is currently stable, now receiving 120 mg MEDD, and his pain is currently described as moderately controlled on the new lower dose.
In summary, this veteran was receiving > 200 mg MEDD with several known overdose risk factors. Once the HCP was made aware of these risk factors, necessary precautions were taken, and the veteran was safely tapered to a lower dose. Dashboard technology makes the list of risk factors readily available to HCPs who are prescribing (and the pharmacists reviewing the prescriptions), thus allowing a proactive discussion of risks and benefits before continuing, renewing, or initiating opioid prescriptions.
Discussion
As reported in 2013 by NIDA, the greater availability of opioid medications and the consequent increase in prescriptions may be contributing directly to their growing misuse by both civilians and military service personnel. A direct consequence has been an increase in both accidental and intentional overdose deaths.3 Several factors are related to the risk of overdose/death, including high-dose opioid medications, a history of psychiatric disorders (specifically depression and PTSD), a history of substance use disorders, concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse, and previous attempts of suicide.
The VISN 22 high-risk opioid dashboard was a rapid information technology response to the increasing risk faced by veterans who have chronic pain and comorbid psychiatric and substance use disorders and are prescribed opioids and CNS depressants. The purpose of implementing this dashboard technology was to assist HCPs in prescribing opioids safely, using a technology that allows for the monitoring and management of concomitant suicide risk factors. Following the national Opioid Safety Initiative, this dashboard tool is being used to identify veterans who are on high-dose opioids with the goal of reducing the number of veterans on > 200 mg MEDD. The dashboard allows data to be stratified, using the concomitant risk factors for suicide to assist facilities and their providers in the identification and prioritization of highest risk patients first.
An initial review of dashboard data in VISN 22 suggests that it is a useful tool for reducing high-dose opioid prescriptions (> 200 mg MEDD and > 120 mg but < 199 mg MEDD). Across the 5 VA locations in VISN 22, in the first 8 months of implementation, 4 locations were able to lower prescription opioid medication levels to the initial target of < 5%; 2 lowered rates even further (to < 3%). The VA Greater Los Angeles Healthcare System remains at a commendable 1%. Although the number of veterans with prescriptions totaling > 200 mg MEDD has decreased as a result, a greater reduction is expected with the coordinated education and systems improvement efforts associated with the national VHA Opioid Safety Initiative. As part of the process to lower the number of patients on high-dose opioids in the future, HCP and patient education will be provided in relation to the use of dashboard technology.
Limitations
There are several limitations that affect interpretation of the usefulness of the VISN 22 high-risk opioid dashboard. Prior to the implementation of the dashboard, 2 of 5 VISN sites already had efforts in place to reduce opioid overprescribing. The VA Greater Los Angeles Healthcare System had an opioid reduction program in place before the dashboard was implemented, so it is possible reductions in opioid prescribing were a result of their previous efforts and not related to the dashboard. Similarly the VA Long Beach Healthcare System had begun a quality improvement initiative to reduce high-dose opioid prescribing prior to dashboard implementation. However, it was difficult to pinpoint the direct effect the dashboard had on patient interventions due to lack of documentation of dashboard use in the clinical notes.
A direct relationship did exist between dashboard implementation and opioid dose reduction in patients with > 200 MEDD at the remaining 3 VISN 22 facilities. Overall, this suggests that the dashboard played a significant role across all sites. Implementation of the dashboard across VISN 22 was accompanied by an education effort that resulted in an increased awareness among HCPs to evaluate certain risks in patients on high-dose opioids and to evaluate the combination of opioid and CNS depressant use. Prior to dashboard implementation, there was no standardized monitoring system that cross-referenced high-dose opioid prescribing with psychiatric illness and suicide risk factors.
Conclusions
From 2000 to 2010, opioid prescriptions nearly doubled, yet this rate was not accompanied by a change/increase in the rate of nonopioid analgesic medication prescriptions.18 Health care providers need to account for veterans’ wishes for pain treatment and be aware of options other than opioids, particularly given the risk of opioid-related accidental or intentional overdose; it is imperative that treatment become more individualized and more closely monitored.19,20 It is recommended that opioids should be the treatment of last resort in managing chronic noncancer pain. The use of opioid prescription medications should be intended as a trial, supported by clear goals and an unequivocal understanding that doses will not be indiscriminately increased.20
Health care providers who prescribe opioids are ultimately responsible for monitoring risk factors that may increase overdose and death, and dashboard technology assists them in this effort. The VISN 22 high-risk opioid dashboard is a tool that allows providers to identify and prioritize veterans who are at high risk for overdose. Initial data collected suggest that the dashboard has decreased the risk of negative consequences associated with opioid medication use today. However, the authors wish to emphasize that this technology is only part of the solution; although it can be a tool to identify actions that may need to take place and can track progress of changes in care, there must be complementary efforts in provider and patient education, improved access to mental health care, and interdisciplinary models of care that expand current chronic pain treatment options. Future considerations of this technology may include incorporating other risk factors accounting for psychosocial variables specific to military personnel that may further increase the overall risk of overdose.
Acknowledgements
The authors wish to thank the leadership of VISN 22 for their support of this initiative. Dr. Kryskalla recognizes VA OI&T for making this work possible and her family for their support. Ms. Kern would like to thank Aaron, Leslie, and Rachel Kern for their continuous support. Dr. Hauser wishes to thank Cathy, Anika, Katia, Max, and Jirina Hauser for their unwavering support.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[Published Online Ahead of Print: August 14, 2014.]
1. Brooks D. Hearing Spotlights painkiller overuse among soldiers. http://www.fayobserver.com/military/article_a6e4a2e9-827d-577c-a79a-87a6c07cf151.html. Fayobserver Website. Published October 10, 2013, Accessed June 9, 2014.
2. Seal KH, Shi Y, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
3. National Institute on Drug Abuse. DrugFacts: Substance Abuse in the Military. http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military. National Institute on Drug Abuse Website. Revised March 2013. Accessed June 9, 2014.
4. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311.
5. Pergolizzi JV Jr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443-451.
6. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85-92.
7. Substance Abuse and Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit. HHS publication No. (SMA) 13-4742. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
8. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
9. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
10. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581-624.
11. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepine, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
12. Centers for Disease Control and Prevention. FastStats: Deaths and mortality. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/deaths.htm. Updated February 13, 2014. Accessed June 9, 2014.
13. Kemp J, Bossarte R. Suicide Data Report, 2012. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed July 1, 2014.
14. National Institute of Mental Health. Suicide in the U.S. Statistics. National Institute of Mental Health Website. http://www.nimh.nih.gov/statistics/index.shtml. Accessed June 27, 2014.
15. Miller M, Hempstead K, Nguyen T, Barber C, Rosenberg-Wohl S, Azrael D. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: Implications for clinical practice. Am J Public Health. 2013;103(6):e61-e68.
16. Hartl TL, Rosen C, Drescher K, Lee TT, Gusman F. Predicting high-risk behaviors in Veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2005;193(7):464-472.
17. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
18. Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870-878.
19. Bray RM, Pemberton MR, Lane ME, Hourani LL, Mattiko MJ, Babeu LA. Substance use and mental health trends among U.S. military active duty personnel. Key findings from the 2008 DoD Health Behavior Survey. Mil Med. 2010;175(6):390-399.
20. Cuevas-Trisan RL. The unfortunate turn of events in pain management. Fed Pract. 2013;30(3):8-9.
On October 10, 2013, a Congressional hearing was held to address the issue of opioid medication prescribing within VHA facilities and clinics (House Veteran Affairs Subcommittee hearing “Between Peril and Promise: Facing the Dangers of VA’s Skyrocketing Use of Prescription Painkillers to Treat Veterans”). Several individuals testified, including the widows of 2 veterans; both their husbands had overdosed on prescribed opioid medications. One husband had been taking as many as 15 pills a day and was additionally prescribed oxycodone/acetaminophen, which led to his death.1
Alongside the widows were 2 veterans who had been treated for chronic back pain injuries sustained before and during deployment in Iraq. Both had been prescribed several pain medications, including oxycodone/acetaminophen, methadone, and morphine. One reported that as his pain increased, his doctors continued to provide him additional prescriptions; at one point he had more than 13 prescriptions and could no longer work from being so “doped up.”1
In the past 2 decades, health care professionals (HCPs) have placed greater emphasis on chronic pain management. As a result, the rate of opioid medication prescribing has increased dramatically. Since 1994, the number of opioid medication prescriptions has nearly doubled; this change has been accompanied by an increase in opioid misuse, which has resulted in accidental or intentional overdose and death.2
Based on a recent National Institute on Drug Abuse (NIDA) report, the greatest impact has been on armed forces personnel.3 Prescriptions for pain relievers quadrupled between 2001 and 2009 to almost 3.8 million within the military population. Although civilian populations are more likely to abuse illicit drugs, military personnel are at particular risk of prescription abuse, including opioid medications.3 In 2008, 11% of armed forces service members reported misusing prescription drugs, with opioid medications being the most abused. This is an approximate 5- to 6-fold increase since 2002 (2% reported misuse in 2002).3 Particularly concerning is the associated rise in suicide rates among armed forces personnel, which surpassed civilian suicide rates in 2004. In 2009, one-third of suicides among armed forces personnel involved prescription drugs.3
Certain patient characteristics or factors are related to greater overdose risk. These risk factors include prescription dosage and frequency, history of suicide attempts or self-harm behavior, history of depression or posttraumatic stress disorder (PTSD) among other mental health-related diagnoses, a history of substance and/or alcohol abuse, and within the context of opioid medication use, the concurrent use of other central nervous system (CNS) depressants.4,5 Additionally, the stresses of deployment during wartime, physical injuries sustained in combat, and the unique military culture play a particularly important role in access to substances with high abuse potential and the subsequent development of substance abuse.3
Opioid Use and Risk Factors
More than 3% of adults in the U.S. are now receiving opioid medications for chronic noncancer pain.6 Substance abuse among patients with chronic pain ranges from 14% to 40%.5 Prescription opioid medications are the fastest growing drugs of abuse and the most common cause of unintentional overdose in the U.S.4 About 17,000 deaths occur each year as a result of prescription opioid medication overdose.7 Opioid medication-related overdose deaths began to increase in the early 2000s and continue to increase. Between 1999 and 2007, the rate of unintentional overdose-related deaths in the U.S. increased by 124%, largely due to the increase of prescription opioid medications.8
High-Dose Opioid Medication Use
A study by Dunn and colleagues found that patients receiving higher doses of prescribed opioid medications were at an increased risk of overdose.6 Patients receiving 50 mg to 99 mg morphine equivalent daily dose (MEDD) had a 3.7-fold increase in overdose risk (0.7% annual overdose rate) as compared with patients who received < 50 mg MEDD (0.2% annual overdose rate). Patients receiving ≥ 100 mg MEDD had a 1.8% annual overdose rate and a 9.8-fold increase in overdose risk as compared with patients who received < 50 mg MEDD. Overall, 51 patients experienced ≥ 1 overdose event, 40 of whom experienced fatal or serious overdoses and 6 of whom attempted suicide. Patients receiving the highest doses were male, current smokers, and had a history of depression and substance abuse.6 Similarly, a study by Bohnert and colleagues found that opioid medication overdose was most likely to occur in those patients with psychiatric and substance use disorders compared with patients who had no psychiatric illness history.8
Depression
Mood disorders are common in people with chronic pain.4,5,9,10 In particular, patients with a history of depression are more likely to receive chronic opioid medication prescriptions and are at a higher risk for opioid medication abuse. A substance abuse history is the most consistent predictor of both chronic opioid medication use and abuse. However, depression without substance abuse is significantly associated with 2 forms of opioid medication abuse: self-medication for stress or sleep and overmedication (using a higher dose than prescribed). More severe cases of depression show a stronger association for potential abuse.4
PTSD
Among Iraq and Afghanistan war veterans with ≥ 1 pain-related diagnosis, veterans with PTSD and veterans with a mental health disorder other than PTSD were significantly more likely to receive opioid medications for pain than were veterans without a mental health disorder (PTSD—17.8%, adjusted relative risk [RR] 2.58; other mental health disorder—11.7%, RR 1.74; no mental health disorder—6.5%).2 Although mental health disorders in general were related to a higher risk of opioid abuse, those with PTSD in particular were more likely to receive higher prescribed dosages; to continue taking opioids for a longer period; to receive concurrent prescriptions for opioid medications, sedative hypnotics, or both; to obtain early refills; and to have comorbid alcohol and substance use disorders. Based on these results, Seal and colleagues concluded that veterans with PTSD had the highest risk of alcohol, drug, and opioid-related accidents and overdose as well as self-inflicted injuries.2
Concurrent Use of Opioids and CNS Depressants
As mentioned earlier, studies suggest that people with PTSD are at a significantly higher risk for opioid medication overdose. One factor that may contribute to this higher risk is the concurrent use of CNS depressants/sedatives, particularly benzodiazepines and alcohol.
Benzodiazepines are often prescribed for people with PTSD. One study found that the concurrent use of benzodiazepines is significantly related to opioid overdose.5 Prescribing opioids for people already abusing or dependent on alcohol or other substances increases the risk of abuse and overdose. Furthermore, the concurrent use of multiple medications is associated with aberrant behaviors, cognitive impairment, and medication abuse, potentially leading to overdose. Overall, the combined administration of these medications is responsible for higher rates of adverse events, overdose, and death related to prescription opioid medication use.5,6,11
In summary, there are various risk factors that contribute to opioid medication overdose and more generally, risk of suicide, including (1) high-dose opioid medications; (2) history of psychiatric disorders, specifically depression and PTSD; (3) history of substance use disorders; and (4) concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse.
Suicide
Suicide is the tenth leading cause of death in the U.S., with 12.4 suicide deaths per 100,000 population.12 Suicide rates are even higher among veterans. According to the VHA, the age-adjusted rate of suicide for veterans using VHA facilities and clinics was 35.9 per 100,000 person-years for fiscal year 2009.13 Several risk factors for suicide attempts include depression and other mental health disorders, substance abuse, medical disorders, and prescription medications.
Prior suicide attempts or self-harm behavior is known to increase the risk of subsequent death by suicide. About 11 attempted suicides occur per suicide death where the medical severity of prior attempts increases the risk of future suicide, as does a history of multiple self-harm episodes.14,15 One study found that the single best predictor of suicide in a veteran population was an attempt in the previous 4 months.16
Among other risk factors, previous suicide attempts and violent behavior are major behavioral flags that warrant caution and require particular consideration when prescribing opioid medications. In a national survey on drug use and health, about 18% of prescription opioid users/abusers who experienced suicidal ideations actually attempted suicide. Only 11% of individuals who never used prescription opioid medications attempted suicide after reported suicidal ideations.17
Patient Data Aggregation
The early and widespread adoption of electronic medical records (EMRs) by the VHA allowed the aggregation of patient data for quality improvement. Initially, data were aggregated, and dashboards were designed retrospectively. However, the development of regional data warehouses that update patient information daily from the EMR allowed information to be aggregated prospectively, and dashboards were designed that provided real-time information.
The purpose of the current study is to demonstrate the efficacy and future potential of dashboard technology in assessing prospectively high-risk factors for opioid overdose. Dashboards are a user-friendly application that allows providers to isolate and calculate daily morphine equivalent opioid dosages and assess patients’ risk factors for overdose on an individual basis. By using this technology, providers who prescribe opioids can get a concise summary of opioid and other medications and adjust medications to decrease overdose risk on an individual basis.
What is the Dashboard?
The VISN 22 high-risk opioid dashboard is a business intelligence tool that serves as a report card, or progress report, to provide a global view of the number of veterans who are receiving opioid prescriptions totaling >120 mg MEDD and who have characteristics (history of depression, PTSD, substance abuse, or high-risk suicide flag) and prescriptions (concomitant CNS depressants) that may increase patient risk for overdose.
The VISN 22 dashboard allows the user to navigate to an individual HCP-level and patient-level report (Figures 1 and 2). Filter settings allow report users to select only high-risk patients; it serves as a single location for pertinent details to consider for safely prescribing opioids.
To calculate daily morphine equivalents, each patient’s opioid prescriptions were evaluated. The quantity was divided by the day’s supply to calculate an average daily quantity. From there, the drug strength was used to convert to MEDD. Health care providers were informed that these conversion factors were not recommendations for clinical opioid conversions.
Implementation and Design
In 2012, the VA Pharmacy Benefits Management (PBM) in VISN 21 created a dashboard that allowed users to identify patients on high-dose opioid prescriptions. Structured query language code was used to extract data from the regional data warehouse and calculate MEDD for all patients with active opioid prescriptions. In 2013, VISN 22 expanded that dashboard to incorporate factors that could indicate a high risk for overdose or other adverse outcomes, including a history of depression, PTSD, substance abuse or high-risk suicide flag, and concomitant use of CNS depressant medications.
The high-risk opioid dashboard (Figure 3) and accompanying patient-level report were first introduced to VISN 22 HCPs in January 2013. The business intelligence tools were introduced to each facility through the VISN 22 PBM group. Training on the use of the dashboard and the report was provided, with an initial target of decreasing MEDD of > 200 mg to < 5% of all veterans prescribed opioids at each VISN 22 facility. One month later (in February 2013), a second category of veterans (those with > 120 mg but < 199 mg MEDD) was added. Also the initial MEDD > 200 mg target of < 5% was decreased to < 3% to encourage additional progress.
Eight months after the VISN 22 dashboard technology was implemented there was a 17% decrease in patients with total daily morphine equivalents > 200 mg (January 2013; 1,137 patients vs August 2013; 940 patients—a decrease of 197 patients).
From March 2013 to August 2013, VISN 22 also saw a 12% decrease in the number of patients prescribed > 120 mg MEDD but < 199 MEDD (March 2013; 2,295 vs August 2013; 2,018—a decrease of 277 patients).
Figure 4 shows opioid use as of July 2014 for VISN 22 facilities. There were further reductions in the number of patients receiving > 120 mg but < 199 mg MEDD (August 2013; 2,018 patients vs July 2014; 1,189 patients) and patients receiving > 200 mg MEDD (August 2013; 940 patients vs July 2014; 836 patients).
Case Description
In January 2013, VISN 22 implemented dashboard technology to help providers assess and monitor opioid prescription levels in relation to high-risk variables. The benefits of this dashboard technology are illustrated in the case profile that follows.
A 67-year-old male veteran had a long history of chronic pain. Pain diagnoses included osteoarthritis with spine involvement, lumbar radiculopathy, arthralgia, and peripheral neuropathy. For the past 10 years, he was prescribed opioids with modest relief of his chronic pain symptoms despite recent prescriptions totaling 300 mg MEDD. This veteran had several risk factors for overdose, including a history of depression, suicide risk, PTSD, and concomitant use of the CNS depressants alprazolam and cyclobenzaprine.
More recently, in May 2013, the veteran exhibited aberrant behavior and requested early refills for alprazolam. In response, the pharmacist discussed the case with the HCP who prescribed the opioids, noting the concomitant overdose risk factors. As a result of this interaction, the veteran was referred for mental health services, and his prescriptions for opioids were gradually decreased. He is currently stable, now receiving 120 mg MEDD, and his pain is currently described as moderately controlled on the new lower dose.
In summary, this veteran was receiving > 200 mg MEDD with several known overdose risk factors. Once the HCP was made aware of these risk factors, necessary precautions were taken, and the veteran was safely tapered to a lower dose. Dashboard technology makes the list of risk factors readily available to HCPs who are prescribing (and the pharmacists reviewing the prescriptions), thus allowing a proactive discussion of risks and benefits before continuing, renewing, or initiating opioid prescriptions.
Discussion
As reported in 2013 by NIDA, the greater availability of opioid medications and the consequent increase in prescriptions may be contributing directly to their growing misuse by both civilians and military service personnel. A direct consequence has been an increase in both accidental and intentional overdose deaths.3 Several factors are related to the risk of overdose/death, including high-dose opioid medications, a history of psychiatric disorders (specifically depression and PTSD), a history of substance use disorders, concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse, and previous attempts of suicide.
The VISN 22 high-risk opioid dashboard was a rapid information technology response to the increasing risk faced by veterans who have chronic pain and comorbid psychiatric and substance use disorders and are prescribed opioids and CNS depressants. The purpose of implementing this dashboard technology was to assist HCPs in prescribing opioids safely, using a technology that allows for the monitoring and management of concomitant suicide risk factors. Following the national Opioid Safety Initiative, this dashboard tool is being used to identify veterans who are on high-dose opioids with the goal of reducing the number of veterans on > 200 mg MEDD. The dashboard allows data to be stratified, using the concomitant risk factors for suicide to assist facilities and their providers in the identification and prioritization of highest risk patients first.
An initial review of dashboard data in VISN 22 suggests that it is a useful tool for reducing high-dose opioid prescriptions (> 200 mg MEDD and > 120 mg but < 199 mg MEDD). Across the 5 VA locations in VISN 22, in the first 8 months of implementation, 4 locations were able to lower prescription opioid medication levels to the initial target of < 5%; 2 lowered rates even further (to < 3%). The VA Greater Los Angeles Healthcare System remains at a commendable 1%. Although the number of veterans with prescriptions totaling > 200 mg MEDD has decreased as a result, a greater reduction is expected with the coordinated education and systems improvement efforts associated with the national VHA Opioid Safety Initiative. As part of the process to lower the number of patients on high-dose opioids in the future, HCP and patient education will be provided in relation to the use of dashboard technology.
Limitations
There are several limitations that affect interpretation of the usefulness of the VISN 22 high-risk opioid dashboard. Prior to the implementation of the dashboard, 2 of 5 VISN sites already had efforts in place to reduce opioid overprescribing. The VA Greater Los Angeles Healthcare System had an opioid reduction program in place before the dashboard was implemented, so it is possible reductions in opioid prescribing were a result of their previous efforts and not related to the dashboard. Similarly the VA Long Beach Healthcare System had begun a quality improvement initiative to reduce high-dose opioid prescribing prior to dashboard implementation. However, it was difficult to pinpoint the direct effect the dashboard had on patient interventions due to lack of documentation of dashboard use in the clinical notes.
A direct relationship did exist between dashboard implementation and opioid dose reduction in patients with > 200 MEDD at the remaining 3 VISN 22 facilities. Overall, this suggests that the dashboard played a significant role across all sites. Implementation of the dashboard across VISN 22 was accompanied by an education effort that resulted in an increased awareness among HCPs to evaluate certain risks in patients on high-dose opioids and to evaluate the combination of opioid and CNS depressant use. Prior to dashboard implementation, there was no standardized monitoring system that cross-referenced high-dose opioid prescribing with psychiatric illness and suicide risk factors.
Conclusions
From 2000 to 2010, opioid prescriptions nearly doubled, yet this rate was not accompanied by a change/increase in the rate of nonopioid analgesic medication prescriptions.18 Health care providers need to account for veterans’ wishes for pain treatment and be aware of options other than opioids, particularly given the risk of opioid-related accidental or intentional overdose; it is imperative that treatment become more individualized and more closely monitored.19,20 It is recommended that opioids should be the treatment of last resort in managing chronic noncancer pain. The use of opioid prescription medications should be intended as a trial, supported by clear goals and an unequivocal understanding that doses will not be indiscriminately increased.20
Health care providers who prescribe opioids are ultimately responsible for monitoring risk factors that may increase overdose and death, and dashboard technology assists them in this effort. The VISN 22 high-risk opioid dashboard is a tool that allows providers to identify and prioritize veterans who are at high risk for overdose. Initial data collected suggest that the dashboard has decreased the risk of negative consequences associated with opioid medication use today. However, the authors wish to emphasize that this technology is only part of the solution; although it can be a tool to identify actions that may need to take place and can track progress of changes in care, there must be complementary efforts in provider and patient education, improved access to mental health care, and interdisciplinary models of care that expand current chronic pain treatment options. Future considerations of this technology may include incorporating other risk factors accounting for psychosocial variables specific to military personnel that may further increase the overall risk of overdose.
Acknowledgements
The authors wish to thank the leadership of VISN 22 for their support of this initiative. Dr. Kryskalla recognizes VA OI&T for making this work possible and her family for their support. Ms. Kern would like to thank Aaron, Leslie, and Rachel Kern for their continuous support. Dr. Hauser wishes to thank Cathy, Anika, Katia, Max, and Jirina Hauser for their unwavering support.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[Published Online Ahead of Print: August 14, 2014.]
On October 10, 2013, a Congressional hearing was held to address the issue of opioid medication prescribing within VHA facilities and clinics (House Veteran Affairs Subcommittee hearing “Between Peril and Promise: Facing the Dangers of VA’s Skyrocketing Use of Prescription Painkillers to Treat Veterans”). Several individuals testified, including the widows of 2 veterans; both their husbands had overdosed on prescribed opioid medications. One husband had been taking as many as 15 pills a day and was additionally prescribed oxycodone/acetaminophen, which led to his death.1
Alongside the widows were 2 veterans who had been treated for chronic back pain injuries sustained before and during deployment in Iraq. Both had been prescribed several pain medications, including oxycodone/acetaminophen, methadone, and morphine. One reported that as his pain increased, his doctors continued to provide him additional prescriptions; at one point he had more than 13 prescriptions and could no longer work from being so “doped up.”1
In the past 2 decades, health care professionals (HCPs) have placed greater emphasis on chronic pain management. As a result, the rate of opioid medication prescribing has increased dramatically. Since 1994, the number of opioid medication prescriptions has nearly doubled; this change has been accompanied by an increase in opioid misuse, which has resulted in accidental or intentional overdose and death.2
Based on a recent National Institute on Drug Abuse (NIDA) report, the greatest impact has been on armed forces personnel.3 Prescriptions for pain relievers quadrupled between 2001 and 2009 to almost 3.8 million within the military population. Although civilian populations are more likely to abuse illicit drugs, military personnel are at particular risk of prescription abuse, including opioid medications.3 In 2008, 11% of armed forces service members reported misusing prescription drugs, with opioid medications being the most abused. This is an approximate 5- to 6-fold increase since 2002 (2% reported misuse in 2002).3 Particularly concerning is the associated rise in suicide rates among armed forces personnel, which surpassed civilian suicide rates in 2004. In 2009, one-third of suicides among armed forces personnel involved prescription drugs.3
Certain patient characteristics or factors are related to greater overdose risk. These risk factors include prescription dosage and frequency, history of suicide attempts or self-harm behavior, history of depression or posttraumatic stress disorder (PTSD) among other mental health-related diagnoses, a history of substance and/or alcohol abuse, and within the context of opioid medication use, the concurrent use of other central nervous system (CNS) depressants.4,5 Additionally, the stresses of deployment during wartime, physical injuries sustained in combat, and the unique military culture play a particularly important role in access to substances with high abuse potential and the subsequent development of substance abuse.3
Opioid Use and Risk Factors
More than 3% of adults in the U.S. are now receiving opioid medications for chronic noncancer pain.6 Substance abuse among patients with chronic pain ranges from 14% to 40%.5 Prescription opioid medications are the fastest growing drugs of abuse and the most common cause of unintentional overdose in the U.S.4 About 17,000 deaths occur each year as a result of prescription opioid medication overdose.7 Opioid medication-related overdose deaths began to increase in the early 2000s and continue to increase. Between 1999 and 2007, the rate of unintentional overdose-related deaths in the U.S. increased by 124%, largely due to the increase of prescription opioid medications.8
High-Dose Opioid Medication Use
A study by Dunn and colleagues found that patients receiving higher doses of prescribed opioid medications were at an increased risk of overdose.6 Patients receiving 50 mg to 99 mg morphine equivalent daily dose (MEDD) had a 3.7-fold increase in overdose risk (0.7% annual overdose rate) as compared with patients who received < 50 mg MEDD (0.2% annual overdose rate). Patients receiving ≥ 100 mg MEDD had a 1.8% annual overdose rate and a 9.8-fold increase in overdose risk as compared with patients who received < 50 mg MEDD. Overall, 51 patients experienced ≥ 1 overdose event, 40 of whom experienced fatal or serious overdoses and 6 of whom attempted suicide. Patients receiving the highest doses were male, current smokers, and had a history of depression and substance abuse.6 Similarly, a study by Bohnert and colleagues found that opioid medication overdose was most likely to occur in those patients with psychiatric and substance use disorders compared with patients who had no psychiatric illness history.8
Depression
Mood disorders are common in people with chronic pain.4,5,9,10 In particular, patients with a history of depression are more likely to receive chronic opioid medication prescriptions and are at a higher risk for opioid medication abuse. A substance abuse history is the most consistent predictor of both chronic opioid medication use and abuse. However, depression without substance abuse is significantly associated with 2 forms of opioid medication abuse: self-medication for stress or sleep and overmedication (using a higher dose than prescribed). More severe cases of depression show a stronger association for potential abuse.4
PTSD
Among Iraq and Afghanistan war veterans with ≥ 1 pain-related diagnosis, veterans with PTSD and veterans with a mental health disorder other than PTSD were significantly more likely to receive opioid medications for pain than were veterans without a mental health disorder (PTSD—17.8%, adjusted relative risk [RR] 2.58; other mental health disorder—11.7%, RR 1.74; no mental health disorder—6.5%).2 Although mental health disorders in general were related to a higher risk of opioid abuse, those with PTSD in particular were more likely to receive higher prescribed dosages; to continue taking opioids for a longer period; to receive concurrent prescriptions for opioid medications, sedative hypnotics, or both; to obtain early refills; and to have comorbid alcohol and substance use disorders. Based on these results, Seal and colleagues concluded that veterans with PTSD had the highest risk of alcohol, drug, and opioid-related accidents and overdose as well as self-inflicted injuries.2
Concurrent Use of Opioids and CNS Depressants
As mentioned earlier, studies suggest that people with PTSD are at a significantly higher risk for opioid medication overdose. One factor that may contribute to this higher risk is the concurrent use of CNS depressants/sedatives, particularly benzodiazepines and alcohol.
Benzodiazepines are often prescribed for people with PTSD. One study found that the concurrent use of benzodiazepines is significantly related to opioid overdose.5 Prescribing opioids for people already abusing or dependent on alcohol or other substances increases the risk of abuse and overdose. Furthermore, the concurrent use of multiple medications is associated with aberrant behaviors, cognitive impairment, and medication abuse, potentially leading to overdose. Overall, the combined administration of these medications is responsible for higher rates of adverse events, overdose, and death related to prescription opioid medication use.5,6,11
In summary, there are various risk factors that contribute to opioid medication overdose and more generally, risk of suicide, including (1) high-dose opioid medications; (2) history of psychiatric disorders, specifically depression and PTSD; (3) history of substance use disorders; and (4) concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse.
Suicide
Suicide is the tenth leading cause of death in the U.S., with 12.4 suicide deaths per 100,000 population.12 Suicide rates are even higher among veterans. According to the VHA, the age-adjusted rate of suicide for veterans using VHA facilities and clinics was 35.9 per 100,000 person-years for fiscal year 2009.13 Several risk factors for suicide attempts include depression and other mental health disorders, substance abuse, medical disorders, and prescription medications.
Prior suicide attempts or self-harm behavior is known to increase the risk of subsequent death by suicide. About 11 attempted suicides occur per suicide death where the medical severity of prior attempts increases the risk of future suicide, as does a history of multiple self-harm episodes.14,15 One study found that the single best predictor of suicide in a veteran population was an attempt in the previous 4 months.16
Among other risk factors, previous suicide attempts and violent behavior are major behavioral flags that warrant caution and require particular consideration when prescribing opioid medications. In a national survey on drug use and health, about 18% of prescription opioid users/abusers who experienced suicidal ideations actually attempted suicide. Only 11% of individuals who never used prescription opioid medications attempted suicide after reported suicidal ideations.17
Patient Data Aggregation
The early and widespread adoption of electronic medical records (EMRs) by the VHA allowed the aggregation of patient data for quality improvement. Initially, data were aggregated, and dashboards were designed retrospectively. However, the development of regional data warehouses that update patient information daily from the EMR allowed information to be aggregated prospectively, and dashboards were designed that provided real-time information.
The purpose of the current study is to demonstrate the efficacy and future potential of dashboard technology in assessing prospectively high-risk factors for opioid overdose. Dashboards are a user-friendly application that allows providers to isolate and calculate daily morphine equivalent opioid dosages and assess patients’ risk factors for overdose on an individual basis. By using this technology, providers who prescribe opioids can get a concise summary of opioid and other medications and adjust medications to decrease overdose risk on an individual basis.
What is the Dashboard?
The VISN 22 high-risk opioid dashboard is a business intelligence tool that serves as a report card, or progress report, to provide a global view of the number of veterans who are receiving opioid prescriptions totaling >120 mg MEDD and who have characteristics (history of depression, PTSD, substance abuse, or high-risk suicide flag) and prescriptions (concomitant CNS depressants) that may increase patient risk for overdose.
The VISN 22 dashboard allows the user to navigate to an individual HCP-level and patient-level report (Figures 1 and 2). Filter settings allow report users to select only high-risk patients; it serves as a single location for pertinent details to consider for safely prescribing opioids.
To calculate daily morphine equivalents, each patient’s opioid prescriptions were evaluated. The quantity was divided by the day’s supply to calculate an average daily quantity. From there, the drug strength was used to convert to MEDD. Health care providers were informed that these conversion factors were not recommendations for clinical opioid conversions.
Implementation and Design
In 2012, the VA Pharmacy Benefits Management (PBM) in VISN 21 created a dashboard that allowed users to identify patients on high-dose opioid prescriptions. Structured query language code was used to extract data from the regional data warehouse and calculate MEDD for all patients with active opioid prescriptions. In 2013, VISN 22 expanded that dashboard to incorporate factors that could indicate a high risk for overdose or other adverse outcomes, including a history of depression, PTSD, substance abuse or high-risk suicide flag, and concomitant use of CNS depressant medications.
The high-risk opioid dashboard (Figure 3) and accompanying patient-level report were first introduced to VISN 22 HCPs in January 2013. The business intelligence tools were introduced to each facility through the VISN 22 PBM group. Training on the use of the dashboard and the report was provided, with an initial target of decreasing MEDD of > 200 mg to < 5% of all veterans prescribed opioids at each VISN 22 facility. One month later (in February 2013), a second category of veterans (those with > 120 mg but < 199 mg MEDD) was added. Also the initial MEDD > 200 mg target of < 5% was decreased to < 3% to encourage additional progress.
Eight months after the VISN 22 dashboard technology was implemented there was a 17% decrease in patients with total daily morphine equivalents > 200 mg (January 2013; 1,137 patients vs August 2013; 940 patients—a decrease of 197 patients).
From March 2013 to August 2013, VISN 22 also saw a 12% decrease in the number of patients prescribed > 120 mg MEDD but < 199 MEDD (March 2013; 2,295 vs August 2013; 2,018—a decrease of 277 patients).
Figure 4 shows opioid use as of July 2014 for VISN 22 facilities. There were further reductions in the number of patients receiving > 120 mg but < 199 mg MEDD (August 2013; 2,018 patients vs July 2014; 1,189 patients) and patients receiving > 200 mg MEDD (August 2013; 940 patients vs July 2014; 836 patients).
Case Description
In January 2013, VISN 22 implemented dashboard technology to help providers assess and monitor opioid prescription levels in relation to high-risk variables. The benefits of this dashboard technology are illustrated in the case profile that follows.
A 67-year-old male veteran had a long history of chronic pain. Pain diagnoses included osteoarthritis with spine involvement, lumbar radiculopathy, arthralgia, and peripheral neuropathy. For the past 10 years, he was prescribed opioids with modest relief of his chronic pain symptoms despite recent prescriptions totaling 300 mg MEDD. This veteran had several risk factors for overdose, including a history of depression, suicide risk, PTSD, and concomitant use of the CNS depressants alprazolam and cyclobenzaprine.
More recently, in May 2013, the veteran exhibited aberrant behavior and requested early refills for alprazolam. In response, the pharmacist discussed the case with the HCP who prescribed the opioids, noting the concomitant overdose risk factors. As a result of this interaction, the veteran was referred for mental health services, and his prescriptions for opioids were gradually decreased. He is currently stable, now receiving 120 mg MEDD, and his pain is currently described as moderately controlled on the new lower dose.
In summary, this veteran was receiving > 200 mg MEDD with several known overdose risk factors. Once the HCP was made aware of these risk factors, necessary precautions were taken, and the veteran was safely tapered to a lower dose. Dashboard technology makes the list of risk factors readily available to HCPs who are prescribing (and the pharmacists reviewing the prescriptions), thus allowing a proactive discussion of risks and benefits before continuing, renewing, or initiating opioid prescriptions.
Discussion
As reported in 2013 by NIDA, the greater availability of opioid medications and the consequent increase in prescriptions may be contributing directly to their growing misuse by both civilians and military service personnel. A direct consequence has been an increase in both accidental and intentional overdose deaths.3 Several factors are related to the risk of overdose/death, including high-dose opioid medications, a history of psychiatric disorders (specifically depression and PTSD), a history of substance use disorders, concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse, and previous attempts of suicide.
The VISN 22 high-risk opioid dashboard was a rapid information technology response to the increasing risk faced by veterans who have chronic pain and comorbid psychiatric and substance use disorders and are prescribed opioids and CNS depressants. The purpose of implementing this dashboard technology was to assist HCPs in prescribing opioids safely, using a technology that allows for the monitoring and management of concomitant suicide risk factors. Following the national Opioid Safety Initiative, this dashboard tool is being used to identify veterans who are on high-dose opioids with the goal of reducing the number of veterans on > 200 mg MEDD. The dashboard allows data to be stratified, using the concomitant risk factors for suicide to assist facilities and their providers in the identification and prioritization of highest risk patients first.
An initial review of dashboard data in VISN 22 suggests that it is a useful tool for reducing high-dose opioid prescriptions (> 200 mg MEDD and > 120 mg but < 199 mg MEDD). Across the 5 VA locations in VISN 22, in the first 8 months of implementation, 4 locations were able to lower prescription opioid medication levels to the initial target of < 5%; 2 lowered rates even further (to < 3%). The VA Greater Los Angeles Healthcare System remains at a commendable 1%. Although the number of veterans with prescriptions totaling > 200 mg MEDD has decreased as a result, a greater reduction is expected with the coordinated education and systems improvement efforts associated with the national VHA Opioid Safety Initiative. As part of the process to lower the number of patients on high-dose opioids in the future, HCP and patient education will be provided in relation to the use of dashboard technology.
Limitations
There are several limitations that affect interpretation of the usefulness of the VISN 22 high-risk opioid dashboard. Prior to the implementation of the dashboard, 2 of 5 VISN sites already had efforts in place to reduce opioid overprescribing. The VA Greater Los Angeles Healthcare System had an opioid reduction program in place before the dashboard was implemented, so it is possible reductions in opioid prescribing were a result of their previous efforts and not related to the dashboard. Similarly the VA Long Beach Healthcare System had begun a quality improvement initiative to reduce high-dose opioid prescribing prior to dashboard implementation. However, it was difficult to pinpoint the direct effect the dashboard had on patient interventions due to lack of documentation of dashboard use in the clinical notes.
A direct relationship did exist between dashboard implementation and opioid dose reduction in patients with > 200 MEDD at the remaining 3 VISN 22 facilities. Overall, this suggests that the dashboard played a significant role across all sites. Implementation of the dashboard across VISN 22 was accompanied by an education effort that resulted in an increased awareness among HCPs to evaluate certain risks in patients on high-dose opioids and to evaluate the combination of opioid and CNS depressant use. Prior to dashboard implementation, there was no standardized monitoring system that cross-referenced high-dose opioid prescribing with psychiatric illness and suicide risk factors.
Conclusions
From 2000 to 2010, opioid prescriptions nearly doubled, yet this rate was not accompanied by a change/increase in the rate of nonopioid analgesic medication prescriptions.18 Health care providers need to account for veterans’ wishes for pain treatment and be aware of options other than opioids, particularly given the risk of opioid-related accidental or intentional overdose; it is imperative that treatment become more individualized and more closely monitored.19,20 It is recommended that opioids should be the treatment of last resort in managing chronic noncancer pain. The use of opioid prescription medications should be intended as a trial, supported by clear goals and an unequivocal understanding that doses will not be indiscriminately increased.20
Health care providers who prescribe opioids are ultimately responsible for monitoring risk factors that may increase overdose and death, and dashboard technology assists them in this effort. The VISN 22 high-risk opioid dashboard is a tool that allows providers to identify and prioritize veterans who are at high risk for overdose. Initial data collected suggest that the dashboard has decreased the risk of negative consequences associated with opioid medication use today. However, the authors wish to emphasize that this technology is only part of the solution; although it can be a tool to identify actions that may need to take place and can track progress of changes in care, there must be complementary efforts in provider and patient education, improved access to mental health care, and interdisciplinary models of care that expand current chronic pain treatment options. Future considerations of this technology may include incorporating other risk factors accounting for psychosocial variables specific to military personnel that may further increase the overall risk of overdose.
Acknowledgements
The authors wish to thank the leadership of VISN 22 for their support of this initiative. Dr. Kryskalla recognizes VA OI&T for making this work possible and her family for their support. Ms. Kern would like to thank Aaron, Leslie, and Rachel Kern for their continuous support. Dr. Hauser wishes to thank Cathy, Anika, Katia, Max, and Jirina Hauser for their unwavering support.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[Published Online Ahead of Print: August 14, 2014.]
1. Brooks D. Hearing Spotlights painkiller overuse among soldiers. http://www.fayobserver.com/military/article_a6e4a2e9-827d-577c-a79a-87a6c07cf151.html. Fayobserver Website. Published October 10, 2013, Accessed June 9, 2014.
2. Seal KH, Shi Y, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
3. National Institute on Drug Abuse. DrugFacts: Substance Abuse in the Military. http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military. National Institute on Drug Abuse Website. Revised March 2013. Accessed June 9, 2014.
4. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311.
5. Pergolizzi JV Jr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443-451.
6. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85-92.
7. Substance Abuse and Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit. HHS publication No. (SMA) 13-4742. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
8. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
9. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
10. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581-624.
11. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepine, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
12. Centers for Disease Control and Prevention. FastStats: Deaths and mortality. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/deaths.htm. Updated February 13, 2014. Accessed June 9, 2014.
13. Kemp J, Bossarte R. Suicide Data Report, 2012. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed July 1, 2014.
14. National Institute of Mental Health. Suicide in the U.S. Statistics. National Institute of Mental Health Website. http://www.nimh.nih.gov/statistics/index.shtml. Accessed June 27, 2014.
15. Miller M, Hempstead K, Nguyen T, Barber C, Rosenberg-Wohl S, Azrael D. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: Implications for clinical practice. Am J Public Health. 2013;103(6):e61-e68.
16. Hartl TL, Rosen C, Drescher K, Lee TT, Gusman F. Predicting high-risk behaviors in Veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2005;193(7):464-472.
17. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
18. Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870-878.
19. Bray RM, Pemberton MR, Lane ME, Hourani LL, Mattiko MJ, Babeu LA. Substance use and mental health trends among U.S. military active duty personnel. Key findings from the 2008 DoD Health Behavior Survey. Mil Med. 2010;175(6):390-399.
20. Cuevas-Trisan RL. The unfortunate turn of events in pain management. Fed Pract. 2013;30(3):8-9.
1. Brooks D. Hearing Spotlights painkiller overuse among soldiers. http://www.fayobserver.com/military/article_a6e4a2e9-827d-577c-a79a-87a6c07cf151.html. Fayobserver Website. Published October 10, 2013, Accessed June 9, 2014.
2. Seal KH, Shi Y, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
3. National Institute on Drug Abuse. DrugFacts: Substance Abuse in the Military. http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military. National Institute on Drug Abuse Website. Revised March 2013. Accessed June 9, 2014.
4. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311.
5. Pergolizzi JV Jr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443-451.
6. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85-92.
7. Substance Abuse and Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit. HHS publication No. (SMA) 13-4742. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
8. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
9. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
10. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581-624.
11. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepine, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
12. Centers for Disease Control and Prevention. FastStats: Deaths and mortality. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/deaths.htm. Updated February 13, 2014. Accessed June 9, 2014.
13. Kemp J, Bossarte R. Suicide Data Report, 2012. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed July 1, 2014.
14. National Institute of Mental Health. Suicide in the U.S. Statistics. National Institute of Mental Health Website. http://www.nimh.nih.gov/statistics/index.shtml. Accessed June 27, 2014.
15. Miller M, Hempstead K, Nguyen T, Barber C, Rosenberg-Wohl S, Azrael D. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: Implications for clinical practice. Am J Public Health. 2013;103(6):e61-e68.
16. Hartl TL, Rosen C, Drescher K, Lee TT, Gusman F. Predicting high-risk behaviors in Veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2005;193(7):464-472.
17. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
18. Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870-878.
19. Bray RM, Pemberton MR, Lane ME, Hourani LL, Mattiko MJ, Babeu LA. Substance use and mental health trends among U.S. military active duty personnel. Key findings from the 2008 DoD Health Behavior Survey. Mil Med. 2010;175(6):390-399.
20. Cuevas-Trisan RL. The unfortunate turn of events in pain management. Fed Pract. 2013;30(3):8-9.
A suicidal injection obsession
HISTORY: TIRED OF LIVING
Mr. F, age 43, presents to the emergency room with complications of type 2 diabetes mellitus: blurry vision, increased urination, fatigue, and polydipsia. Blood glucose is 676 mg/dL.
The patient flees during treatment—possibly to attempt suicide—but returns 36 hours later, noticeably disoriented. He is readmitted to the ER, where he tells staff he is considering suicide and plans to self-inject a lethal substance. The ER staff refers him to the psychiatry service.
Mr. F also complains of shortness of breath after minimal exertion, aching joints throughout his body, and intense pain in his right great toe. He has been sleeping 12 to 20 hours daily, yet has trouble sleeping at night. He persistently feels fatigued, hopeless, and helpless. He says his suicidal urges have become more intense over 2 months, but he fears he will lose his computer repair job if he is admitted. He also shows difficulties with short-term memory. We admit him for observation.
Mental status examination suggests that Mr. F is generally withdrawn. Eye contact is poor and he is quiet and evasive, possibly signaling paranoia. He spends most of his stay watching television. His thought process is linear, and he thinks constantly of suicide. During the Mini-Mental State Examination, he gives the incorrect date and county. He misses two other items on recall but gets them correct with prompts.
A mild intention tremor distorts his handwriting. He has trouble keeping his balance during the Romberg test, and his gait is mildly ataxic. Ophthalmology consult suggests that diabetic retinopathy and optic disc cupping secondary to glaucoma may be blurring his vision.
Mr. F is taking no medications but had previously used insulin twice a day, and his outpatient doctor insists he should go back on insulin. He smokes 1 pack of cigarettes per day, drinks alcohol moderately (one to two drinks/day), and does not abuse illicit drugs.
The authors’ observations
Mr. F’s depressed mood, hopelessness, concentration problems, psychomotor retardation, and suicidal thoughts suggest major depressive disorder. Depression or a delirium secondary to diabetes may account for his referential ideas.
FURTHER HISTORY: ONE SHOT AT SATISFACTION
Over the following week, Mr. F becomes more talkative as the psychiatry staff develops a therapeutic rapport. He tells his treatment team that he feels urges to self-inject liquids he finds in his hospital room, such as shower gel and beverages.
Mr. F tells us that approximately 2 years ago, he tried to kill himself by swallowing boric acid. After 6 weeks in intensive care, the poison’s physical effects resolved and he no longer appeared suicidal. The staff at that time prepared to discharge Mr. F when, while left alone in his room, he dislodged a wall-mounted sphygmomanometer, disassembled it, and broke open the mercury tube. He then injected about 3 mL of mercury into his intravenous port and swallowed another 3 mL.
A nurse who checked on Mr. F minutes after the incident did not notice the sphygmomanometer was missing. He showed the broken device to the nurse, saying, “Look what I did.” When the nurse asked why, he responded, “I was just sitting here alone and saw the thing on the wall. I thought to myself, I can do this.”
The hospital viewed the episode as another suicide attempt. Staff immediately began chelation therapy with dimercaprol, 10 mg/kg every 8 hours for 5 days, then 10 mg/kg every 12 hours for 2 weeks. Within 24 hours of ingesting mercury, Mr. F developed shortness of breath, tachycardia (104 BPM), a fever (101.8°F), and had GI complaints. Increased blood urea nitrogen, increased creatinine, and decreased urination suggested declining renal function. He developed a pruritic rash over his back and mild skin loss on his soles.
Mr. F’s mercury levels were 20.8 mg/dL (serum) and 216 mg/dL (urine) 36 hours after ingestation, and 24.8 mg/dL (serum) and 397 mg/dL (urine) after chelation. Serum mercury >5 mg/dL is usually symptomatic.
Approximately 72 hours after the incident, most pulmonary, renal, and dermal manifestations of mercury toxicity began to improve. Mr. F was discharged after 21 days. He was diagnosed with major depression and started on sertraline, 150 mg/d.
‘The best feeling.’ Two years later, Mr. F tells us he has attempted suicide at least six times. Diffuse metallic foreign bodies throughout his lung vasculature and a 9.6 mg/dL serum mercury reading confirm he has injected mercury. His painful toe is x-rayed to check for mercury deposits, but he ultimately is diagnosed with gout.
During our evaluation, Mr. F admits that “the calmest, best feeling I have ever had” was while injecting mercury, yet he fears the incident has caused permanent physical and mental damage. He describes his desire to self-inject liquids as “impulses” triggered by twice-daily subcutaneous insulin use. For this reason, he has stopped taking insulin against his doctor’s advice.
The authors’ observations
Mr. F’s mental status changes and serum mercury suggest mercury poisoning. He shows numerous heavy-metal poisoning symptoms (Box 1) as well as erethism, a malaise that can result from heavy-metal exposure.2
The patient insists that insulin shots bring on self-injection urges, but his impulsive and repetitive suicidal behavior, dysphoria, and transient paranoia suggest borderline personality disorder. His impulses may reflect a subtle, long-term personality change caused by mercury’s neurotoxic effects.1 Or they could be akin to cutting behaviors shown by some patients with personality disorders, particularly borderline personality disorder.
We ruled out substance abuse disorder, as Mr. F’s mercury ingestion was not premeditated, he has no history of illicit drug use, and intravenous elemental mercury is not psychoactive.
- Emotional lability
- Excessive shyness
- Headaches
- Hearing loss
- Insomnia
- Irritability
- Lack of ambition
- Lack of sexual desire
- Loss of confidence
- Memory loss
- Nervousness
- Neuromuscular changes (including weakness, muscle atrophy, muscle twitching)
- Performance deficits in cognitive function tests
- Polyneuropathy
- Tremor of hands
- Visual field defects
Source: Reference 1
Elemental mercury found in thermometers, lamps, and dental amalgams slowly ionizes in the blood stream before crossing the blood-brain barrier. Mercury and carbon form toxic “organic” compounds, including methylmercury (found in the environment), phenylmercury (used in some commercial products), and dimethylmercury (found in solid waste sites).
Because mercury’s half-life is 60 days, it dissipates slowly, can accumulate with chronic exposure, and stays in the blood stream long after high-dose exposure.3
Serum mercury >5 mg/dL can cause subtle, enduring neurotoxic effects, including tremor, dizziness, shortness of breath, blurry vision, decreased visual fields, depression, memory loss, and irritability.3 Serum mercury rarely exceeds 1.5 mg/dL without direct exposure.
Irritability, depressive symptoms, and renal manifestations emerge when urine mercury reaches 200 to 1,000 mg/dL. Renal, respiratory, and GI effects are seen at 1,000 to 2,000 mg/dL.
Means of exposure. Vapor inhalation is the most common means of elemental mercury exposure.3 Elemental mercury used in manufacturing vaporizes at room temperature.
Orally ingested elemental mercury is poorly absorbed from the GI tract, mostly passes unabsorbed, and is toxic only at high doses. Injected elemental mercury is poorly absorbed but can cause mechanical and immunologic effects. The psychiatric literature describes some 200 cases of mercury self-injection4-8 but offers little information on cognitive effects or long-term follow-up.
Consider heavy-metal poisoning in the differential diagnosis of patients with depressive symptoms. Ask about risk factors for environmental mercury exposure, including use of folk medicines, some cosmetics, over-the-counter nasal sprays, ophthalmic solutions, skin-lightening creams, daily fish consumption (particularly tuna or swordfish), living in a house painted with latex paint, or continuous exposure at work (Box 2).
Also ask if the patient or a household member recently ingested mercury or handled a broken thermometer. Liquid mercury on clothing and in bodily fluids may cause secondary contamination, whereas mercury vapor cannot.
Order serum mercury testing if you suspect chronic exposure. Refer patients with serum mercury ≥ 1.5 mg/dL to their primary care physicians and to a poison control center for evaluation and possible chelation. Refer patients with acute mercury exposure symptoms to the ER.
Consuming or using certain products or working in some industries increases mercury exposure risk. Mercury-containing products include:
Over-the-counter herbal remedies imported from China, Hong Kong, Haiti, and Cuba.9
Older, larger marine animals, including tuna, shark, or swordfish from mercury-contaminated waters.10,11
Vaccines and medications. Small amounts of thimerosal (ethylmercury sodium salt) were used as a preservative in some vaccines.12 Some antiseptics, eye drops, eye ointments, nasal sprays, skin-lightening creams, and gamma globulin contain mercury.
Dental amalgams are approximately 50% mercury. Each amalgam releases roughly 10 mg/d of mercury; chewing gum or grinding teeth may increase exposure.13 Some suggest removing the fillings, but this can increase mercury exposure if done incorrectly.1
Household goods, including latex paint made before 1990 and broken thermometers.3,14
Other environmental exposure, such as from burning coal, water treatment facilities, landfills, and mercury-containing fungicides.
Occupations that carry a high risk of mercury exposure include:3
Manufacturing
Batteries, cosmetics, explosives, paint/pigments, fluorescent lamps, ink, mercury vapor lamps, pharmaceuticals, switches, and rectifiers
Skilled trades
Plumbing, chlorine and caustic soda production, electroplating, felt-making, leather tanning, grinding machine operators, paper millers
Medical
Dental and medical laboratory personnel
Service industries
Hazardous-waste site personnel, painters, pesticide/fungicide production/application
Mining/processing
Cinnabar, gold, silver, copper, or zinc; metallurgy
The authors’ observations
Antidepressants generally will not reduce depression, irritability, personality changes, or apathy secondary to mercury poisoning. We have found that a psychostimulant such as methylphenidate, starting at 10 mg bid and titrating to therapeutic effect, can help treat mercury-related apathy.
We did not give Mr. F a psychostimulant, however, fearing it would worsen his impulsive behavior and disordered sleep. Also, more effectively managing Mr. F’s diabetes should improve his depression.
DISCHARGE: CHELATION CHALLENGE
Mr. F’s suicidal thoughts continued intermittently. Chelation was tried again with succimer, 1,000 mg tid for 5 days and bid for 5 more days, but the agent caused severe nausea without significantly decreasing serum mercury. He declined outpatient chelation.
After 2 weeks, Mr. F denied suicidal thoughts and said he felt physically better. He was discharged on venlafaxine, 300 mg/d, for his depressive symptoms; and metformin, 1,000 mg/d, glipizide, 10 mg bid, and rosiglitazone, 4 mg/d, to control his blood glucose. We arranged for medication management at a community mental health center. Mr. F was also told to visit the hospital’s outpatient clinic for endocrine follow-up but has not returned for 18 months.
Related resources
- Agency for Toxic Substances and Disease Registry. Information about toxic substances in the environment and diseases they may cause. www.atsdr.cdc.gov.
- Dimercaprol • BAL in Oil
- Glipizide • Glucotrol
- Metformin • Glucophage
- Methylphenidate • Ritalin, Concerta
- Rosiglitazone • Avandia
- Sertraline • Zoloft
- Succimer • Chemet
- Venlafaxine • Effexor
Dr. Matthews is an American Psychiatric Association Bristol-Myers Squibb Co. fellow in public and community psychiatry.
Dr. Hauser receives research/grant support from GlaxoSmithKline, Hoffman LaRoche, and AstraZeneca Pharmaceuticals. He is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., GlaxoSmithKline, and Janssen Pharmaceuticals.
1. Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury, March 1999. Available at: http://www.atsdr.cdc.gov/toxprofiles/tp46.html. Accessed May 4, 2005.
2. Clarkson TW, Magos L, Myers GJ. The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med 2003;349:1731-7.
3. Mercury toxicity. Agency for Toxic Substance and Disease Registry. Am Fam Physician 1992;46:1731-41.
4. Manoukian SV, Wenger NK. Mercury in the heart. Am J Cardiol 1991;67:317-8.
5. Maniatis V, Zois G, Stringaris K. IV mercury self-injection: CT imaging. AJR Am J Roentgenol 1997;169:1197-8.
6. McFee RB, Caraccio TR. Intravenous mercury injection and ingestion: clinical manifestations and management. J Toxicol Clin Toxicol 2001;39:733-8.
7. Shareeff M, Bhat YM, Adabala R, Raoof S. Shortness of breath after suicide attempt. Chest 2000;118:837-8.
8. Torres-Alanis O, Garza-Ocanas L, Pineyro-Lopez A. Intravenous self-administration of metallic mercury: report of a case with a 5-year follow-up. J Toxicol Clin Toxicol 1997;35:83-7.
9. Li AM, Chan MH, Leung TF, et al. Mercury intoxication presenting with tics. Arch Dis Child 2000;83:74-5.
10. Dewailly E, Ayotte P, Bruneau S, et al. Exposure of the Inuit population of Nunavik (Arctic Quebec) to lead and mercury. Arch Environ Health 2001;56:350-7.
11. Stephenson J. FDA warns on mercury in tuna. JAMA 2004;291:171.
12. Dantzig PI. A new cutaneous sign of mercury poisoning. Ann Intern Med 2003;139:78-80.
13. Fitzpatrick M. Heavy metal. Lancet 2003;361:1664.-
14. From the Centers for Disease Control. Acute, chronic poisoning, residential exposures to elemental mercury—Michigan, 1989-1990. JAMA 1991;266:196.-
HISTORY: TIRED OF LIVING
Mr. F, age 43, presents to the emergency room with complications of type 2 diabetes mellitus: blurry vision, increased urination, fatigue, and polydipsia. Blood glucose is 676 mg/dL.
The patient flees during treatment—possibly to attempt suicide—but returns 36 hours later, noticeably disoriented. He is readmitted to the ER, where he tells staff he is considering suicide and plans to self-inject a lethal substance. The ER staff refers him to the psychiatry service.
Mr. F also complains of shortness of breath after minimal exertion, aching joints throughout his body, and intense pain in his right great toe. He has been sleeping 12 to 20 hours daily, yet has trouble sleeping at night. He persistently feels fatigued, hopeless, and helpless. He says his suicidal urges have become more intense over 2 months, but he fears he will lose his computer repair job if he is admitted. He also shows difficulties with short-term memory. We admit him for observation.
Mental status examination suggests that Mr. F is generally withdrawn. Eye contact is poor and he is quiet and evasive, possibly signaling paranoia. He spends most of his stay watching television. His thought process is linear, and he thinks constantly of suicide. During the Mini-Mental State Examination, he gives the incorrect date and county. He misses two other items on recall but gets them correct with prompts.
A mild intention tremor distorts his handwriting. He has trouble keeping his balance during the Romberg test, and his gait is mildly ataxic. Ophthalmology consult suggests that diabetic retinopathy and optic disc cupping secondary to glaucoma may be blurring his vision.
Mr. F is taking no medications but had previously used insulin twice a day, and his outpatient doctor insists he should go back on insulin. He smokes 1 pack of cigarettes per day, drinks alcohol moderately (one to two drinks/day), and does not abuse illicit drugs.
The authors’ observations
Mr. F’s depressed mood, hopelessness, concentration problems, psychomotor retardation, and suicidal thoughts suggest major depressive disorder. Depression or a delirium secondary to diabetes may account for his referential ideas.
FURTHER HISTORY: ONE SHOT AT SATISFACTION
Over the following week, Mr. F becomes more talkative as the psychiatry staff develops a therapeutic rapport. He tells his treatment team that he feels urges to self-inject liquids he finds in his hospital room, such as shower gel and beverages.
Mr. F tells us that approximately 2 years ago, he tried to kill himself by swallowing boric acid. After 6 weeks in intensive care, the poison’s physical effects resolved and he no longer appeared suicidal. The staff at that time prepared to discharge Mr. F when, while left alone in his room, he dislodged a wall-mounted sphygmomanometer, disassembled it, and broke open the mercury tube. He then injected about 3 mL of mercury into his intravenous port and swallowed another 3 mL.
A nurse who checked on Mr. F minutes after the incident did not notice the sphygmomanometer was missing. He showed the broken device to the nurse, saying, “Look what I did.” When the nurse asked why, he responded, “I was just sitting here alone and saw the thing on the wall. I thought to myself, I can do this.”
The hospital viewed the episode as another suicide attempt. Staff immediately began chelation therapy with dimercaprol, 10 mg/kg every 8 hours for 5 days, then 10 mg/kg every 12 hours for 2 weeks. Within 24 hours of ingesting mercury, Mr. F developed shortness of breath, tachycardia (104 BPM), a fever (101.8°F), and had GI complaints. Increased blood urea nitrogen, increased creatinine, and decreased urination suggested declining renal function. He developed a pruritic rash over his back and mild skin loss on his soles.
Mr. F’s mercury levels were 20.8 mg/dL (serum) and 216 mg/dL (urine) 36 hours after ingestation, and 24.8 mg/dL (serum) and 397 mg/dL (urine) after chelation. Serum mercury >5 mg/dL is usually symptomatic.
Approximately 72 hours after the incident, most pulmonary, renal, and dermal manifestations of mercury toxicity began to improve. Mr. F was discharged after 21 days. He was diagnosed with major depression and started on sertraline, 150 mg/d.
‘The best feeling.’ Two years later, Mr. F tells us he has attempted suicide at least six times. Diffuse metallic foreign bodies throughout his lung vasculature and a 9.6 mg/dL serum mercury reading confirm he has injected mercury. His painful toe is x-rayed to check for mercury deposits, but he ultimately is diagnosed with gout.
During our evaluation, Mr. F admits that “the calmest, best feeling I have ever had” was while injecting mercury, yet he fears the incident has caused permanent physical and mental damage. He describes his desire to self-inject liquids as “impulses” triggered by twice-daily subcutaneous insulin use. For this reason, he has stopped taking insulin against his doctor’s advice.
The authors’ observations
Mr. F’s mental status changes and serum mercury suggest mercury poisoning. He shows numerous heavy-metal poisoning symptoms (Box 1) as well as erethism, a malaise that can result from heavy-metal exposure.2
The patient insists that insulin shots bring on self-injection urges, but his impulsive and repetitive suicidal behavior, dysphoria, and transient paranoia suggest borderline personality disorder. His impulses may reflect a subtle, long-term personality change caused by mercury’s neurotoxic effects.1 Or they could be akin to cutting behaviors shown by some patients with personality disorders, particularly borderline personality disorder.
We ruled out substance abuse disorder, as Mr. F’s mercury ingestion was not premeditated, he has no history of illicit drug use, and intravenous elemental mercury is not psychoactive.
- Emotional lability
- Excessive shyness
- Headaches
- Hearing loss
- Insomnia
- Irritability
- Lack of ambition
- Lack of sexual desire
- Loss of confidence
- Memory loss
- Nervousness
- Neuromuscular changes (including weakness, muscle atrophy, muscle twitching)
- Performance deficits in cognitive function tests
- Polyneuropathy
- Tremor of hands
- Visual field defects
Source: Reference 1
Elemental mercury found in thermometers, lamps, and dental amalgams slowly ionizes in the blood stream before crossing the blood-brain barrier. Mercury and carbon form toxic “organic” compounds, including methylmercury (found in the environment), phenylmercury (used in some commercial products), and dimethylmercury (found in solid waste sites).
Because mercury’s half-life is 60 days, it dissipates slowly, can accumulate with chronic exposure, and stays in the blood stream long after high-dose exposure.3
Serum mercury >5 mg/dL can cause subtle, enduring neurotoxic effects, including tremor, dizziness, shortness of breath, blurry vision, decreased visual fields, depression, memory loss, and irritability.3 Serum mercury rarely exceeds 1.5 mg/dL without direct exposure.
Irritability, depressive symptoms, and renal manifestations emerge when urine mercury reaches 200 to 1,000 mg/dL. Renal, respiratory, and GI effects are seen at 1,000 to 2,000 mg/dL.
Means of exposure. Vapor inhalation is the most common means of elemental mercury exposure.3 Elemental mercury used in manufacturing vaporizes at room temperature.
Orally ingested elemental mercury is poorly absorbed from the GI tract, mostly passes unabsorbed, and is toxic only at high doses. Injected elemental mercury is poorly absorbed but can cause mechanical and immunologic effects. The psychiatric literature describes some 200 cases of mercury self-injection4-8 but offers little information on cognitive effects or long-term follow-up.
Consider heavy-metal poisoning in the differential diagnosis of patients with depressive symptoms. Ask about risk factors for environmental mercury exposure, including use of folk medicines, some cosmetics, over-the-counter nasal sprays, ophthalmic solutions, skin-lightening creams, daily fish consumption (particularly tuna or swordfish), living in a house painted with latex paint, or continuous exposure at work (Box 2).
Also ask if the patient or a household member recently ingested mercury or handled a broken thermometer. Liquid mercury on clothing and in bodily fluids may cause secondary contamination, whereas mercury vapor cannot.
Order serum mercury testing if you suspect chronic exposure. Refer patients with serum mercury ≥ 1.5 mg/dL to their primary care physicians and to a poison control center for evaluation and possible chelation. Refer patients with acute mercury exposure symptoms to the ER.
Consuming or using certain products or working in some industries increases mercury exposure risk. Mercury-containing products include:
Over-the-counter herbal remedies imported from China, Hong Kong, Haiti, and Cuba.9
Older, larger marine animals, including tuna, shark, or swordfish from mercury-contaminated waters.10,11
Vaccines and medications. Small amounts of thimerosal (ethylmercury sodium salt) were used as a preservative in some vaccines.12 Some antiseptics, eye drops, eye ointments, nasal sprays, skin-lightening creams, and gamma globulin contain mercury.
Dental amalgams are approximately 50% mercury. Each amalgam releases roughly 10 mg/d of mercury; chewing gum or grinding teeth may increase exposure.13 Some suggest removing the fillings, but this can increase mercury exposure if done incorrectly.1
Household goods, including latex paint made before 1990 and broken thermometers.3,14
Other environmental exposure, such as from burning coal, water treatment facilities, landfills, and mercury-containing fungicides.
Occupations that carry a high risk of mercury exposure include:3
Manufacturing
Batteries, cosmetics, explosives, paint/pigments, fluorescent lamps, ink, mercury vapor lamps, pharmaceuticals, switches, and rectifiers
Skilled trades
Plumbing, chlorine and caustic soda production, electroplating, felt-making, leather tanning, grinding machine operators, paper millers
Medical
Dental and medical laboratory personnel
Service industries
Hazardous-waste site personnel, painters, pesticide/fungicide production/application
Mining/processing
Cinnabar, gold, silver, copper, or zinc; metallurgy
The authors’ observations
Antidepressants generally will not reduce depression, irritability, personality changes, or apathy secondary to mercury poisoning. We have found that a psychostimulant such as methylphenidate, starting at 10 mg bid and titrating to therapeutic effect, can help treat mercury-related apathy.
We did not give Mr. F a psychostimulant, however, fearing it would worsen his impulsive behavior and disordered sleep. Also, more effectively managing Mr. F’s diabetes should improve his depression.
DISCHARGE: CHELATION CHALLENGE
Mr. F’s suicidal thoughts continued intermittently. Chelation was tried again with succimer, 1,000 mg tid for 5 days and bid for 5 more days, but the agent caused severe nausea without significantly decreasing serum mercury. He declined outpatient chelation.
After 2 weeks, Mr. F denied suicidal thoughts and said he felt physically better. He was discharged on venlafaxine, 300 mg/d, for his depressive symptoms; and metformin, 1,000 mg/d, glipizide, 10 mg bid, and rosiglitazone, 4 mg/d, to control his blood glucose. We arranged for medication management at a community mental health center. Mr. F was also told to visit the hospital’s outpatient clinic for endocrine follow-up but has not returned for 18 months.
Related resources
- Agency for Toxic Substances and Disease Registry. Information about toxic substances in the environment and diseases they may cause. www.atsdr.cdc.gov.
- Dimercaprol • BAL in Oil
- Glipizide • Glucotrol
- Metformin • Glucophage
- Methylphenidate • Ritalin, Concerta
- Rosiglitazone • Avandia
- Sertraline • Zoloft
- Succimer • Chemet
- Venlafaxine • Effexor
Dr. Matthews is an American Psychiatric Association Bristol-Myers Squibb Co. fellow in public and community psychiatry.
Dr. Hauser receives research/grant support from GlaxoSmithKline, Hoffman LaRoche, and AstraZeneca Pharmaceuticals. He is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., GlaxoSmithKline, and Janssen Pharmaceuticals.
HISTORY: TIRED OF LIVING
Mr. F, age 43, presents to the emergency room with complications of type 2 diabetes mellitus: blurry vision, increased urination, fatigue, and polydipsia. Blood glucose is 676 mg/dL.
The patient flees during treatment—possibly to attempt suicide—but returns 36 hours later, noticeably disoriented. He is readmitted to the ER, where he tells staff he is considering suicide and plans to self-inject a lethal substance. The ER staff refers him to the psychiatry service.
Mr. F also complains of shortness of breath after minimal exertion, aching joints throughout his body, and intense pain in his right great toe. He has been sleeping 12 to 20 hours daily, yet has trouble sleeping at night. He persistently feels fatigued, hopeless, and helpless. He says his suicidal urges have become more intense over 2 months, but he fears he will lose his computer repair job if he is admitted. He also shows difficulties with short-term memory. We admit him for observation.
Mental status examination suggests that Mr. F is generally withdrawn. Eye contact is poor and he is quiet and evasive, possibly signaling paranoia. He spends most of his stay watching television. His thought process is linear, and he thinks constantly of suicide. During the Mini-Mental State Examination, he gives the incorrect date and county. He misses two other items on recall but gets them correct with prompts.
A mild intention tremor distorts his handwriting. He has trouble keeping his balance during the Romberg test, and his gait is mildly ataxic. Ophthalmology consult suggests that diabetic retinopathy and optic disc cupping secondary to glaucoma may be blurring his vision.
Mr. F is taking no medications but had previously used insulin twice a day, and his outpatient doctor insists he should go back on insulin. He smokes 1 pack of cigarettes per day, drinks alcohol moderately (one to two drinks/day), and does not abuse illicit drugs.
The authors’ observations
Mr. F’s depressed mood, hopelessness, concentration problems, psychomotor retardation, and suicidal thoughts suggest major depressive disorder. Depression or a delirium secondary to diabetes may account for his referential ideas.
FURTHER HISTORY: ONE SHOT AT SATISFACTION
Over the following week, Mr. F becomes more talkative as the psychiatry staff develops a therapeutic rapport. He tells his treatment team that he feels urges to self-inject liquids he finds in his hospital room, such as shower gel and beverages.
Mr. F tells us that approximately 2 years ago, he tried to kill himself by swallowing boric acid. After 6 weeks in intensive care, the poison’s physical effects resolved and he no longer appeared suicidal. The staff at that time prepared to discharge Mr. F when, while left alone in his room, he dislodged a wall-mounted sphygmomanometer, disassembled it, and broke open the mercury tube. He then injected about 3 mL of mercury into his intravenous port and swallowed another 3 mL.
A nurse who checked on Mr. F minutes after the incident did not notice the sphygmomanometer was missing. He showed the broken device to the nurse, saying, “Look what I did.” When the nurse asked why, he responded, “I was just sitting here alone and saw the thing on the wall. I thought to myself, I can do this.”
The hospital viewed the episode as another suicide attempt. Staff immediately began chelation therapy with dimercaprol, 10 mg/kg every 8 hours for 5 days, then 10 mg/kg every 12 hours for 2 weeks. Within 24 hours of ingesting mercury, Mr. F developed shortness of breath, tachycardia (104 BPM), a fever (101.8°F), and had GI complaints. Increased blood urea nitrogen, increased creatinine, and decreased urination suggested declining renal function. He developed a pruritic rash over his back and mild skin loss on his soles.
Mr. F’s mercury levels were 20.8 mg/dL (serum) and 216 mg/dL (urine) 36 hours after ingestation, and 24.8 mg/dL (serum) and 397 mg/dL (urine) after chelation. Serum mercury >5 mg/dL is usually symptomatic.
Approximately 72 hours after the incident, most pulmonary, renal, and dermal manifestations of mercury toxicity began to improve. Mr. F was discharged after 21 days. He was diagnosed with major depression and started on sertraline, 150 mg/d.
‘The best feeling.’ Two years later, Mr. F tells us he has attempted suicide at least six times. Diffuse metallic foreign bodies throughout his lung vasculature and a 9.6 mg/dL serum mercury reading confirm he has injected mercury. His painful toe is x-rayed to check for mercury deposits, but he ultimately is diagnosed with gout.
During our evaluation, Mr. F admits that “the calmest, best feeling I have ever had” was while injecting mercury, yet he fears the incident has caused permanent physical and mental damage. He describes his desire to self-inject liquids as “impulses” triggered by twice-daily subcutaneous insulin use. For this reason, he has stopped taking insulin against his doctor’s advice.
The authors’ observations
Mr. F’s mental status changes and serum mercury suggest mercury poisoning. He shows numerous heavy-metal poisoning symptoms (Box 1) as well as erethism, a malaise that can result from heavy-metal exposure.2
The patient insists that insulin shots bring on self-injection urges, but his impulsive and repetitive suicidal behavior, dysphoria, and transient paranoia suggest borderline personality disorder. His impulses may reflect a subtle, long-term personality change caused by mercury’s neurotoxic effects.1 Or they could be akin to cutting behaviors shown by some patients with personality disorders, particularly borderline personality disorder.
We ruled out substance abuse disorder, as Mr. F’s mercury ingestion was not premeditated, he has no history of illicit drug use, and intravenous elemental mercury is not psychoactive.
- Emotional lability
- Excessive shyness
- Headaches
- Hearing loss
- Insomnia
- Irritability
- Lack of ambition
- Lack of sexual desire
- Loss of confidence
- Memory loss
- Nervousness
- Neuromuscular changes (including weakness, muscle atrophy, muscle twitching)
- Performance deficits in cognitive function tests
- Polyneuropathy
- Tremor of hands
- Visual field defects
Source: Reference 1
Elemental mercury found in thermometers, lamps, and dental amalgams slowly ionizes in the blood stream before crossing the blood-brain barrier. Mercury and carbon form toxic “organic” compounds, including methylmercury (found in the environment), phenylmercury (used in some commercial products), and dimethylmercury (found in solid waste sites).
Because mercury’s half-life is 60 days, it dissipates slowly, can accumulate with chronic exposure, and stays in the blood stream long after high-dose exposure.3
Serum mercury >5 mg/dL can cause subtle, enduring neurotoxic effects, including tremor, dizziness, shortness of breath, blurry vision, decreased visual fields, depression, memory loss, and irritability.3 Serum mercury rarely exceeds 1.5 mg/dL without direct exposure.
Irritability, depressive symptoms, and renal manifestations emerge when urine mercury reaches 200 to 1,000 mg/dL. Renal, respiratory, and GI effects are seen at 1,000 to 2,000 mg/dL.
Means of exposure. Vapor inhalation is the most common means of elemental mercury exposure.3 Elemental mercury used in manufacturing vaporizes at room temperature.
Orally ingested elemental mercury is poorly absorbed from the GI tract, mostly passes unabsorbed, and is toxic only at high doses. Injected elemental mercury is poorly absorbed but can cause mechanical and immunologic effects. The psychiatric literature describes some 200 cases of mercury self-injection4-8 but offers little information on cognitive effects or long-term follow-up.
Consider heavy-metal poisoning in the differential diagnosis of patients with depressive symptoms. Ask about risk factors for environmental mercury exposure, including use of folk medicines, some cosmetics, over-the-counter nasal sprays, ophthalmic solutions, skin-lightening creams, daily fish consumption (particularly tuna or swordfish), living in a house painted with latex paint, or continuous exposure at work (Box 2).
Also ask if the patient or a household member recently ingested mercury or handled a broken thermometer. Liquid mercury on clothing and in bodily fluids may cause secondary contamination, whereas mercury vapor cannot.
Order serum mercury testing if you suspect chronic exposure. Refer patients with serum mercury ≥ 1.5 mg/dL to their primary care physicians and to a poison control center for evaluation and possible chelation. Refer patients with acute mercury exposure symptoms to the ER.
Consuming or using certain products or working in some industries increases mercury exposure risk. Mercury-containing products include:
Over-the-counter herbal remedies imported from China, Hong Kong, Haiti, and Cuba.9
Older, larger marine animals, including tuna, shark, or swordfish from mercury-contaminated waters.10,11
Vaccines and medications. Small amounts of thimerosal (ethylmercury sodium salt) were used as a preservative in some vaccines.12 Some antiseptics, eye drops, eye ointments, nasal sprays, skin-lightening creams, and gamma globulin contain mercury.
Dental amalgams are approximately 50% mercury. Each amalgam releases roughly 10 mg/d of mercury; chewing gum or grinding teeth may increase exposure.13 Some suggest removing the fillings, but this can increase mercury exposure if done incorrectly.1
Household goods, including latex paint made before 1990 and broken thermometers.3,14
Other environmental exposure, such as from burning coal, water treatment facilities, landfills, and mercury-containing fungicides.
Occupations that carry a high risk of mercury exposure include:3
Manufacturing
Batteries, cosmetics, explosives, paint/pigments, fluorescent lamps, ink, mercury vapor lamps, pharmaceuticals, switches, and rectifiers
Skilled trades
Plumbing, chlorine and caustic soda production, electroplating, felt-making, leather tanning, grinding machine operators, paper millers
Medical
Dental and medical laboratory personnel
Service industries
Hazardous-waste site personnel, painters, pesticide/fungicide production/application
Mining/processing
Cinnabar, gold, silver, copper, or zinc; metallurgy
The authors’ observations
Antidepressants generally will not reduce depression, irritability, personality changes, or apathy secondary to mercury poisoning. We have found that a psychostimulant such as methylphenidate, starting at 10 mg bid and titrating to therapeutic effect, can help treat mercury-related apathy.
We did not give Mr. F a psychostimulant, however, fearing it would worsen his impulsive behavior and disordered sleep. Also, more effectively managing Mr. F’s diabetes should improve his depression.
DISCHARGE: CHELATION CHALLENGE
Mr. F’s suicidal thoughts continued intermittently. Chelation was tried again with succimer, 1,000 mg tid for 5 days and bid for 5 more days, but the agent caused severe nausea without significantly decreasing serum mercury. He declined outpatient chelation.
After 2 weeks, Mr. F denied suicidal thoughts and said he felt physically better. He was discharged on venlafaxine, 300 mg/d, for his depressive symptoms; and metformin, 1,000 mg/d, glipizide, 10 mg bid, and rosiglitazone, 4 mg/d, to control his blood glucose. We arranged for medication management at a community mental health center. Mr. F was also told to visit the hospital’s outpatient clinic for endocrine follow-up but has not returned for 18 months.
Related resources
- Agency for Toxic Substances and Disease Registry. Information about toxic substances in the environment and diseases they may cause. www.atsdr.cdc.gov.
- Dimercaprol • BAL in Oil
- Glipizide • Glucotrol
- Metformin • Glucophage
- Methylphenidate • Ritalin, Concerta
- Rosiglitazone • Avandia
- Sertraline • Zoloft
- Succimer • Chemet
- Venlafaxine • Effexor
Dr. Matthews is an American Psychiatric Association Bristol-Myers Squibb Co. fellow in public and community psychiatry.
Dr. Hauser receives research/grant support from GlaxoSmithKline, Hoffman LaRoche, and AstraZeneca Pharmaceuticals. He is a speaker for Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., GlaxoSmithKline, and Janssen Pharmaceuticals.
1. Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury, March 1999. Available at: http://www.atsdr.cdc.gov/toxprofiles/tp46.html. Accessed May 4, 2005.
2. Clarkson TW, Magos L, Myers GJ. The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med 2003;349:1731-7.
3. Mercury toxicity. Agency for Toxic Substance and Disease Registry. Am Fam Physician 1992;46:1731-41.
4. Manoukian SV, Wenger NK. Mercury in the heart. Am J Cardiol 1991;67:317-8.
5. Maniatis V, Zois G, Stringaris K. IV mercury self-injection: CT imaging. AJR Am J Roentgenol 1997;169:1197-8.
6. McFee RB, Caraccio TR. Intravenous mercury injection and ingestion: clinical manifestations and management. J Toxicol Clin Toxicol 2001;39:733-8.
7. Shareeff M, Bhat YM, Adabala R, Raoof S. Shortness of breath after suicide attempt. Chest 2000;118:837-8.
8. Torres-Alanis O, Garza-Ocanas L, Pineyro-Lopez A. Intravenous self-administration of metallic mercury: report of a case with a 5-year follow-up. J Toxicol Clin Toxicol 1997;35:83-7.
9. Li AM, Chan MH, Leung TF, et al. Mercury intoxication presenting with tics. Arch Dis Child 2000;83:74-5.
10. Dewailly E, Ayotte P, Bruneau S, et al. Exposure of the Inuit population of Nunavik (Arctic Quebec) to lead and mercury. Arch Environ Health 2001;56:350-7.
11. Stephenson J. FDA warns on mercury in tuna. JAMA 2004;291:171.
12. Dantzig PI. A new cutaneous sign of mercury poisoning. Ann Intern Med 2003;139:78-80.
13. Fitzpatrick M. Heavy metal. Lancet 2003;361:1664.-
14. From the Centers for Disease Control. Acute, chronic poisoning, residential exposures to elemental mercury—Michigan, 1989-1990. JAMA 1991;266:196.-
1. Agency for Toxic Substances and Disease Registry. Toxicological profile for mercury, March 1999. Available at: http://www.atsdr.cdc.gov/toxprofiles/tp46.html. Accessed May 4, 2005.
2. Clarkson TW, Magos L, Myers GJ. The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med 2003;349:1731-7.
3. Mercury toxicity. Agency for Toxic Substance and Disease Registry. Am Fam Physician 1992;46:1731-41.
4. Manoukian SV, Wenger NK. Mercury in the heart. Am J Cardiol 1991;67:317-8.
5. Maniatis V, Zois G, Stringaris K. IV mercury self-injection: CT imaging. AJR Am J Roentgenol 1997;169:1197-8.
6. McFee RB, Caraccio TR. Intravenous mercury injection and ingestion: clinical manifestations and management. J Toxicol Clin Toxicol 2001;39:733-8.
7. Shareeff M, Bhat YM, Adabala R, Raoof S. Shortness of breath after suicide attempt. Chest 2000;118:837-8.
8. Torres-Alanis O, Garza-Ocanas L, Pineyro-Lopez A. Intravenous self-administration of metallic mercury: report of a case with a 5-year follow-up. J Toxicol Clin Toxicol 1997;35:83-7.
9. Li AM, Chan MH, Leung TF, et al. Mercury intoxication presenting with tics. Arch Dis Child 2000;83:74-5.
10. Dewailly E, Ayotte P, Bruneau S, et al. Exposure of the Inuit population of Nunavik (Arctic Quebec) to lead and mercury. Arch Environ Health 2001;56:350-7.
11. Stephenson J. FDA warns on mercury in tuna. JAMA 2004;291:171.
12. Dantzig PI. A new cutaneous sign of mercury poisoning. Ann Intern Med 2003;139:78-80.
13. Fitzpatrick M. Heavy metal. Lancet 2003;361:1664.-
14. From the Centers for Disease Control. Acute, chronic poisoning, residential exposures to elemental mercury—Michigan, 1989-1990. JAMA 1991;266:196.-
A creepy-crawly disorder
History: A mite disturbing
Mrs. K, age 60, a social worker, saw mites on her arm 3 months ago while going through a client’s old belongings. Since then, she reports, she and her house have become infested with mites.
Despite using copious amounts of lotions, baths, sprays, and prescription creams, she sees increasingly visible “creatures” all over her body and in her stool. Three doctors found no physical evidence of infestation, however, and she became indignant after one told her the problem is “in her head.”
A veterinarian treated Mrs. K’s cat for mites. Days later, Mrs. K suspected that the cat had become reinfested at home and returned it to the veterinarian. He assured her the cat was fine, but she was afraid to bring it home. The cat has remained at the veterinarian’s office—to the doctor’s displeasure—for weeks.
Two weeks after Mrs. K first spotted the mites, her husband, age 82, started believing he is infested. Mr. K, who is retired, has battled depression and drinks about a half-gallon of liquor daily.
After 2 months, Mrs. K quit her job for fear she would infest her co-workers, then locked herself and her husband in their house and allowed no visitors. Day and night for nearly 3 weeks, Mrs. K repeatedly vacuumed the house, shampooed the carpets, and sprayed the walls and furniture with a homemade insecticide. She taped the windows closed to keep bugs out and covered all furniture and surface areas with plastic. A toxic stench of insecticide and shampoo permeated every room.
A neighbor told Mrs. K’s son that his parents were locked inside their house. He came over and knocked on their door, but was refused entry. He eventually got Mrs. K out by threatening to call the police, then brought her to the emergency room.
At presentation, Mrs. K’s right leg has scratches and scabs caused by frequent scratching at mites she saw there. Her hands are reddened and dry, suggesting chemical dermatitis caused by cleaning and repeated insecticide use. Ritual cleaning and spraying has kept her from eating or sleeping; she has lost 12 lbs over 3 weeks and looks pale and tired.
A recovered alcoholic, Mrs. K has been sober for 12 years. She has no other psychiatric, medical, or dermatologic history, and has few social contacts beyond her family and workplace acquaintances.
Blood chemistry, CBC, and urine drug test results are normal. Head MRI reveal no neurologic abnormalities. Her Mini-Mental State Examination (MMSE) score (29/30) indicates no cognitive impairment.
Mrs. K is hospitalized to separate her from her allegedly bug-infested household and husband. At intake, she is panicked over leaving her husband alone and distressed that no one except she and her husband can see the bugs infesting their house and covering her skin. She asks doctors to test a small piece of toilet paper, which she says contains a sample of the bugs. She also fears that she infested her son by letting him into her house.
poll here
The authors’ observations
Mrs. K’s presentation and clinical course suggest delusional parasitosis, a fixed false belief of a parasitic infestation that can cause significant social and occupational dysfunction and medical problems. One patient calls this disorder “bugaphobia.”
The disorder may start as a self-perceived invisible infestation and evolve into visual hallucinations of bugs. Patients usually believe their skin is infested; some believe their internal organs, gums, or skin and internal organs are infested.1,2
Table 1
Medical conditions that may precede delusional parasitosis
| Anemia (severe) |
| Cancer |
| CNS infections |
| Head injury |
| Hepatitis |
| Hypertension |
| Hypovitaminosis of vitamin B12, folate, or thiamine |
| Multiple sclerosis |
| Pulmonary disease |
| Renal disease |
| Rheumatologic disease |
| Sight or hearing loss |
| Source: Reference 6 |
Some patients misinterpret scabs, abrasions, or skin irritation secondary to pesticide use as signs of infestation. Delusional parasitosis can also develop after a real, one-time infestation, as may have happened with Mrs. K.
Convinced they are infested, patients consult multiple providers—including dermatologists, gastroenterologists, and ophthalmologists—in search of the “right” treatment. They undergo numerous tests or procedures and repeatedly apply prescription creams and lotions, leading to chemical dermatitis. Patients often try to prove they are infested by bringing skin, dirt, or toilet tissue samples to doctors—this is called the “matchbox sign” because patients generally bring these samples in small boxes.4 They also may repeatedly ask veterinarians to disinfest their pets.
Described as early as 1892, delusional parasitosis has been called acrophobia, dermatophobia, parasitophobic dermatitis, parasitophobia, entomophobia, and other names.12 Researchers disagree on whether it is a primary psychiatric disorder or is secondary to a mental or physical disorder.13
Researchers have debated two neurobiologic explanations behind the disorder:
Primary sensory. Perrin in 1896 suggested that the parasitosis starts as a sensory misinterpretation, is transformed to a tactile hallucination, then becomes delusional.3
Primary delusional. Others believe delusional parasitosis starts as a hallucination, after which somatic delusional properties develop.3 Some theorists suggest that the symptoms are consistent with thalamic and parietal dysfunction or that the disorder may be a type of late-onset schizophrenia.8
Behaviors associated with “bugaphobia” may be “hardwired” into our evolutionary biology. For example, skin picking may be related to primitive grooming behavior. Its contagiousness may have its roots in animalistic pack behaviors, through which creatures adapt by copying behaviors of others in the pack.8
Delusional parasitosis is most often found in socially isolated women age >40 of average or higher intelligence. Persons in some cultures may be more susceptible than others to some types of parasitic delusions. For example, several persons in India who considered ear cleanliness crucial to attaining cultural and spiritual purity reported having ear infestation.7
Delusional parasitosis also is associated with:
- medical conditions (Table 1)6
- use of cocaine, amphetamines,8 corticosteroids,3,9 or phenelzine10
- occipital-temporal cerebral infarction11
- cognitive impairment related to dementia, depression, mental retardation, or schizophrenia/schizophreniform disorder.
Mrs. K’s delusional parasitosis may be a primary psychiatric disorder (Box). She is medically healthy and does not use drugs or alcohol. Her MMSE score is essentially normal, and she exhibited no psychotic symptoms or loss of function before her first mite sighting.
Diagnosis. Delusional parasitosis is diagnosed as delusional disorder, somatic type, if symptoms persist >1 month. Thorough laboratory and neurologic evaluation is recommended to rule out medical causes (Table 2). Eliminate schizophrenia and schizophreniform disorder with a detailed patient history and cognitive testing.
Also check for a comorbid psychiatric disorder that may be perpetuating the delusion. Delusional parasitosis often co-occurs with axis I disorders including major depressive disorder, substance abuse, dementia, and mental retardation.
poll here
The authors’ observations
Mr. K’s “bugaphobia” most likely was a form of shared secondary delusion called folie-a-deux. Between 11% and 25% of persons with primary delusional parasitosis induce secondary delusional parasitosis in another person, usually a spouse or longtime friend.2 About 50% of folie-a-deux disorders involve a married couple. Often both partners are socially isolated.4
poll here
Treatment: Between two worlds
Mrs. K was given risperidone, 2 mg/d, for delusions and anxiety, and escitalopram, 10 mg/d, preventatively for a suspected underlying depression.
As her symptoms began to clear across 2 to 3 days, Mrs. K realized most times that she was not infested, but on occasion still feared that she was. She continued to worry about her husband being alone in a mite-infested house. We reassured her that her husband would be OK and told her to let us know if the mites resurfaced on her skin.
The authors’ observations
Building rapport. When treating delusional parasitosis, be accepting and non-confrontational. These patients tend to switch doctors until they find someone who understands their problem. Developing rapport can promote treatment adherence and prevent or minimize relapse.
Table 2
5 steps to confirm ‘bugaphobia’
|
| Source: Adapted from Driscoll MS, Rothe MJ, Grant-Kels JM, Hale MS. Delusions of parasitosis: a dermatologic, psychiatric, and pharmacologic approach. J Am Acad Dermatol 1993;29:1023-33. |
4
Also communicate with other specialists to gauge medication history, confirm test findings, and rule out medical causes.
Pharmacotherapy. If symptoms do not resolve after 1 or 2 days of observation, look for a comorbid medical or mental disorder. Prescribe an atypical antipsychotic such as risperidone, 2 to 4 mg/d, or olanzapine, 2.5 mg/d, both of which have been effective against delusional parasitosis.14,16 Keep dosages low to reduce risk of sedation, extrapyramidal symptoms (EPS), and tardive dyskinesia.
Suggesting a psychotropic to patients who are convinced their problem is not psychiatric can be difficult. Try saying:
- Some people are more sensitive than others to sensations on their skin or in their body. This medication will help you tolerate the sensations.”
- or, “This drug will help reduce the anxiety your problem is causing.”
Pimozide has shown efficacy against delusional parasitosis in placebo-controlled trials,17,18 but it can alter cardiac conduction, especially at higherthan-recommended dosages. Start pimozide at 1 mg/d and increase by 1 mg/week until clinical response is achieved. Most patients respond to dosages used to treat psychotic disorders (4 to 10 mg/d).19 Order a baseline and periodic ECG to monitor for QTc prolongation, and do an abnormal involuntary movement scale examination every 3 to 6 months to test for EPS.
Other treatments that have shown benefit in case reports include naloxone, 10 mg/d;20 haloperidol, 10 mg/d; trifluoperazine, 15 mg/d; chlorpromazine, 150 to 300 mg/d; and electroconvulsive therapy.7
We have found that prognosis usually is poor after first- and second-line treatments have failed. Continue to search for a missed disorder, and add an antidepressant if an underlying depression is found or suspected.
Psychotherapy. Perform supportive and harm reduction psychotherapy immediately after diagnosis. Supportive, rapport-building approaches can get the patient to comfortably discuss the issues that led to the delusion and help him/her confront a relapse. Harm reduction can discourage patients from requesting unnecessary invasive tests, using medications and toxic insecticides, or other potentially harmful behaviors.
Cognitive-behavioral therapy may help some patients with refractory delusional parasitosis, if they have enough insight to continue treatment.
Follow-up: A bug-free future
Mrs. K was released from the hospital after 4 days, and her delusional symptoms were gone after another 3 days. We followed her for 6 months.
Upon discharge, Mrs. K and her cat moved in with her daughter’s family. Within a few weeks she was able to visit her workplace and explain what had happened. She stopped taking risperidone after 2 weeks because of excessive sedation. No depressive symptoms were present after 3 months; escitalopram was stopped.
Mrs. K’s husband continued to drink and confine himself to the house. Upon visiting him, she was horrified to find the furniture still covered with plastic and the windows taped shut. Mrs. K threatened to divorce him if he did not seek help. He eventually was treated and has been sober—and bug-free—for 15 months.
Related resources
- Bohart Museum of Entomology, University of California, Davis: Delusional parasitosis. http://delusion.ucdavis.edu.
- Chlorpromazine • Thorazine
- Escitalopram • Lexapro
- Haloperidol • Haldol
- naloxone • Narcan
- Olanzapine • Zyprexa
- Pimozide • Orap
- Phenelzine • Nardil
- Risperidone • Risperdal
- Trifluoperazine • Stelazine
Dr. Matthews is an American Psychiatric Association Bristol-Myers Squibb Co. fellow in public and community psychiatry.
Dr. Hauser receives research/grant support from and is a speaker for AstraZeneca Pharmaceuticals, Eli Lilly and Co., GlaxoSmithKline, and Hoffman LaRoche. He is also receives research/grant support from Schering-Plough Corp. and is a speaker for Abbott Laboratories and Janssen Pharmaceutica.
1. Monk BE, Rao YJ. Delusions of parasitosis with fatal outcome. South Med J 1995;88:341-2.
2. Bourgeois ML, Duhamel P, Verdoux H. Delusional parasitosis: folie a deux and attempted murder of a family doctor. Br J Psychiatry 1992;161:709-11.
3. Sherman MD, Holland GN, Holsclaw DS, et al. Delusions of ocular parasitosis. Am J Ophthalmol 1998;125:852-6.
4. Trabert W. Shared psychotic disorder in delusional parasitosis. Psychopathology 1999;32:30-4.
5. Ford EB, Calfee DP, Pearson RD. Delusions of intestinal parasitosis. South Med J 2001;94:545-7.
6. Slaughter JR, Zanol K, Rezvani H, Flax J. Psychogenic parasitosis: a case series and literature review. Psychosomatics 1998;39:491-500.
7. Srinivasan TN, Suresh TR, Jayaram V, Fernandez MP. Nature and treatment of delusional parasitosis: a different experience in India. J Dermatol 1994;33:851-5.
8. de Leon J, Antelo RE, Simpson G. Delusions of parasitosis or chronic tactile hallucinosis: hypothesis about their brain physiopathology. Compr Psychiatry 1992;33:25-33.
9. May WW, Terpenning MS. Delusional parasitosis in geriatric patients. Psychosomatics 1991;32:88-94.
10. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry 1991;159:716-7.
11. Nagaratnam N, O’Neile L. Delusional parasitosis following occipital-temporal cerebral infarction. Gen Hosp Psychiatry 2000;22:129-32.
12. Stephens MB. Delusions of parasitosis. Am Fam Physician 1999;60:2507-8.
13. Musalek M, Bach M, Passweg V, Jaeger S. The position of delusional parasitosis in psychiatric nosology and classification. Psychopathology 1990;23:115-24.
14. Gallucci G, Beard B. Risperidone and the treatment of delusions of parasitosis in an elderly patient. Psychosomatics 1995;36:578-80.
15. Elmer KB, George RM, Peterson K. Therapeutic update: use of risperidone for the treatment of monosymptomatic hypochondriacal psychosis. J Am Acad Dermatol 2000;43:683-6.
16. Fawcett RG. Olanzapine for the treatment of monosymptomatic hypochondriacal psychosis. J Clin Psychiatry 2002;63:162.-
17. Ungvari G, Vladar K. Pimozide therapy in dermatozoon delusion. Dermatol Monatsschr 1984;170:443-7.
18. Hamann K, Avnstorp C. Delusions of infestation treated by pimozide: a double-blind crossover clinical study. Acta Derm Venereol 1982;62:55-8.
19. Opler LA, Feinberg SS. The role of pimozide in clinical psychiatry: a review. J Clin Psychiatry 1991;52:221-33.
20. Botschev C, Muller N. Opiate receptor antagonists for delusions of parasitosis. Biol Psychiatry 1991;30:530-1.
History: A mite disturbing
Mrs. K, age 60, a social worker, saw mites on her arm 3 months ago while going through a client’s old belongings. Since then, she reports, she and her house have become infested with mites.
Despite using copious amounts of lotions, baths, sprays, and prescription creams, she sees increasingly visible “creatures” all over her body and in her stool. Three doctors found no physical evidence of infestation, however, and she became indignant after one told her the problem is “in her head.”
A veterinarian treated Mrs. K’s cat for mites. Days later, Mrs. K suspected that the cat had become reinfested at home and returned it to the veterinarian. He assured her the cat was fine, but she was afraid to bring it home. The cat has remained at the veterinarian’s office—to the doctor’s displeasure—for weeks.
Two weeks after Mrs. K first spotted the mites, her husband, age 82, started believing he is infested. Mr. K, who is retired, has battled depression and drinks about a half-gallon of liquor daily.
After 2 months, Mrs. K quit her job for fear she would infest her co-workers, then locked herself and her husband in their house and allowed no visitors. Day and night for nearly 3 weeks, Mrs. K repeatedly vacuumed the house, shampooed the carpets, and sprayed the walls and furniture with a homemade insecticide. She taped the windows closed to keep bugs out and covered all furniture and surface areas with plastic. A toxic stench of insecticide and shampoo permeated every room.
A neighbor told Mrs. K’s son that his parents were locked inside their house. He came over and knocked on their door, but was refused entry. He eventually got Mrs. K out by threatening to call the police, then brought her to the emergency room.
At presentation, Mrs. K’s right leg has scratches and scabs caused by frequent scratching at mites she saw there. Her hands are reddened and dry, suggesting chemical dermatitis caused by cleaning and repeated insecticide use. Ritual cleaning and spraying has kept her from eating or sleeping; she has lost 12 lbs over 3 weeks and looks pale and tired.
A recovered alcoholic, Mrs. K has been sober for 12 years. She has no other psychiatric, medical, or dermatologic history, and has few social contacts beyond her family and workplace acquaintances.
Blood chemistry, CBC, and urine drug test results are normal. Head MRI reveal no neurologic abnormalities. Her Mini-Mental State Examination (MMSE) score (29/30) indicates no cognitive impairment.
Mrs. K is hospitalized to separate her from her allegedly bug-infested household and husband. At intake, she is panicked over leaving her husband alone and distressed that no one except she and her husband can see the bugs infesting their house and covering her skin. She asks doctors to test a small piece of toilet paper, which she says contains a sample of the bugs. She also fears that she infested her son by letting him into her house.
poll here
The authors’ observations
Mrs. K’s presentation and clinical course suggest delusional parasitosis, a fixed false belief of a parasitic infestation that can cause significant social and occupational dysfunction and medical problems. One patient calls this disorder “bugaphobia.”
The disorder may start as a self-perceived invisible infestation and evolve into visual hallucinations of bugs. Patients usually believe their skin is infested; some believe their internal organs, gums, or skin and internal organs are infested.1,2
Table 1
Medical conditions that may precede delusional parasitosis
| Anemia (severe) |
| Cancer |
| CNS infections |
| Head injury |
| Hepatitis |
| Hypertension |
| Hypovitaminosis of vitamin B12, folate, or thiamine |
| Multiple sclerosis |
| Pulmonary disease |
| Renal disease |
| Rheumatologic disease |
| Sight or hearing loss |
| Source: Reference 6 |
Some patients misinterpret scabs, abrasions, or skin irritation secondary to pesticide use as signs of infestation. Delusional parasitosis can also develop after a real, one-time infestation, as may have happened with Mrs. K.
Convinced they are infested, patients consult multiple providers—including dermatologists, gastroenterologists, and ophthalmologists—in search of the “right” treatment. They undergo numerous tests or procedures and repeatedly apply prescription creams and lotions, leading to chemical dermatitis. Patients often try to prove they are infested by bringing skin, dirt, or toilet tissue samples to doctors—this is called the “matchbox sign” because patients generally bring these samples in small boxes.4 They also may repeatedly ask veterinarians to disinfest their pets.
Described as early as 1892, delusional parasitosis has been called acrophobia, dermatophobia, parasitophobic dermatitis, parasitophobia, entomophobia, and other names.12 Researchers disagree on whether it is a primary psychiatric disorder or is secondary to a mental or physical disorder.13
Researchers have debated two neurobiologic explanations behind the disorder:
Primary sensory. Perrin in 1896 suggested that the parasitosis starts as a sensory misinterpretation, is transformed to a tactile hallucination, then becomes delusional.3
Primary delusional. Others believe delusional parasitosis starts as a hallucination, after which somatic delusional properties develop.3 Some theorists suggest that the symptoms are consistent with thalamic and parietal dysfunction or that the disorder may be a type of late-onset schizophrenia.8
Behaviors associated with “bugaphobia” may be “hardwired” into our evolutionary biology. For example, skin picking may be related to primitive grooming behavior. Its contagiousness may have its roots in animalistic pack behaviors, through which creatures adapt by copying behaviors of others in the pack.8
Delusional parasitosis is most often found in socially isolated women age >40 of average or higher intelligence. Persons in some cultures may be more susceptible than others to some types of parasitic delusions. For example, several persons in India who considered ear cleanliness crucial to attaining cultural and spiritual purity reported having ear infestation.7
Delusional parasitosis also is associated with:
- medical conditions (Table 1)6
- use of cocaine, amphetamines,8 corticosteroids,3,9 or phenelzine10
- occipital-temporal cerebral infarction11
- cognitive impairment related to dementia, depression, mental retardation, or schizophrenia/schizophreniform disorder.
Mrs. K’s delusional parasitosis may be a primary psychiatric disorder (Box). She is medically healthy and does not use drugs or alcohol. Her MMSE score is essentially normal, and she exhibited no psychotic symptoms or loss of function before her first mite sighting.
Diagnosis. Delusional parasitosis is diagnosed as delusional disorder, somatic type, if symptoms persist >1 month. Thorough laboratory and neurologic evaluation is recommended to rule out medical causes (Table 2). Eliminate schizophrenia and schizophreniform disorder with a detailed patient history and cognitive testing.
Also check for a comorbid psychiatric disorder that may be perpetuating the delusion. Delusional parasitosis often co-occurs with axis I disorders including major depressive disorder, substance abuse, dementia, and mental retardation.
poll here
The authors’ observations
Mr. K’s “bugaphobia” most likely was a form of shared secondary delusion called folie-a-deux. Between 11% and 25% of persons with primary delusional parasitosis induce secondary delusional parasitosis in another person, usually a spouse or longtime friend.2 About 50% of folie-a-deux disorders involve a married couple. Often both partners are socially isolated.4
poll here
Treatment: Between two worlds
Mrs. K was given risperidone, 2 mg/d, for delusions and anxiety, and escitalopram, 10 mg/d, preventatively for a suspected underlying depression.
As her symptoms began to clear across 2 to 3 days, Mrs. K realized most times that she was not infested, but on occasion still feared that she was. She continued to worry about her husband being alone in a mite-infested house. We reassured her that her husband would be OK and told her to let us know if the mites resurfaced on her skin.
The authors’ observations
Building rapport. When treating delusional parasitosis, be accepting and non-confrontational. These patients tend to switch doctors until they find someone who understands their problem. Developing rapport can promote treatment adherence and prevent or minimize relapse.
Table 2
5 steps to confirm ‘bugaphobia’
|
| Source: Adapted from Driscoll MS, Rothe MJ, Grant-Kels JM, Hale MS. Delusions of parasitosis: a dermatologic, psychiatric, and pharmacologic approach. J Am Acad Dermatol 1993;29:1023-33. |
4
Also communicate with other specialists to gauge medication history, confirm test findings, and rule out medical causes.
Pharmacotherapy. If symptoms do not resolve after 1 or 2 days of observation, look for a comorbid medical or mental disorder. Prescribe an atypical antipsychotic such as risperidone, 2 to 4 mg/d, or olanzapine, 2.5 mg/d, both of which have been effective against delusional parasitosis.14,16 Keep dosages low to reduce risk of sedation, extrapyramidal symptoms (EPS), and tardive dyskinesia.
Suggesting a psychotropic to patients who are convinced their problem is not psychiatric can be difficult. Try saying:
- Some people are more sensitive than others to sensations on their skin or in their body. This medication will help you tolerate the sensations.”
- or, “This drug will help reduce the anxiety your problem is causing.”
Pimozide has shown efficacy against delusional parasitosis in placebo-controlled trials,17,18 but it can alter cardiac conduction, especially at higherthan-recommended dosages. Start pimozide at 1 mg/d and increase by 1 mg/week until clinical response is achieved. Most patients respond to dosages used to treat psychotic disorders (4 to 10 mg/d).19 Order a baseline and periodic ECG to monitor for QTc prolongation, and do an abnormal involuntary movement scale examination every 3 to 6 months to test for EPS.
Other treatments that have shown benefit in case reports include naloxone, 10 mg/d;20 haloperidol, 10 mg/d; trifluoperazine, 15 mg/d; chlorpromazine, 150 to 300 mg/d; and electroconvulsive therapy.7
We have found that prognosis usually is poor after first- and second-line treatments have failed. Continue to search for a missed disorder, and add an antidepressant if an underlying depression is found or suspected.
Psychotherapy. Perform supportive and harm reduction psychotherapy immediately after diagnosis. Supportive, rapport-building approaches can get the patient to comfortably discuss the issues that led to the delusion and help him/her confront a relapse. Harm reduction can discourage patients from requesting unnecessary invasive tests, using medications and toxic insecticides, or other potentially harmful behaviors.
Cognitive-behavioral therapy may help some patients with refractory delusional parasitosis, if they have enough insight to continue treatment.
Follow-up: A bug-free future
Mrs. K was released from the hospital after 4 days, and her delusional symptoms were gone after another 3 days. We followed her for 6 months.
Upon discharge, Mrs. K and her cat moved in with her daughter’s family. Within a few weeks she was able to visit her workplace and explain what had happened. She stopped taking risperidone after 2 weeks because of excessive sedation. No depressive symptoms were present after 3 months; escitalopram was stopped.
Mrs. K’s husband continued to drink and confine himself to the house. Upon visiting him, she was horrified to find the furniture still covered with plastic and the windows taped shut. Mrs. K threatened to divorce him if he did not seek help. He eventually was treated and has been sober—and bug-free—for 15 months.
Related resources
- Bohart Museum of Entomology, University of California, Davis: Delusional parasitosis. http://delusion.ucdavis.edu.
- Chlorpromazine • Thorazine
- Escitalopram • Lexapro
- Haloperidol • Haldol
- naloxone • Narcan
- Olanzapine • Zyprexa
- Pimozide • Orap
- Phenelzine • Nardil
- Risperidone • Risperdal
- Trifluoperazine • Stelazine
Dr. Matthews is an American Psychiatric Association Bristol-Myers Squibb Co. fellow in public and community psychiatry.
Dr. Hauser receives research/grant support from and is a speaker for AstraZeneca Pharmaceuticals, Eli Lilly and Co., GlaxoSmithKline, and Hoffman LaRoche. He is also receives research/grant support from Schering-Plough Corp. and is a speaker for Abbott Laboratories and Janssen Pharmaceutica.
History: A mite disturbing
Mrs. K, age 60, a social worker, saw mites on her arm 3 months ago while going through a client’s old belongings. Since then, she reports, she and her house have become infested with mites.
Despite using copious amounts of lotions, baths, sprays, and prescription creams, she sees increasingly visible “creatures” all over her body and in her stool. Three doctors found no physical evidence of infestation, however, and she became indignant after one told her the problem is “in her head.”
A veterinarian treated Mrs. K’s cat for mites. Days later, Mrs. K suspected that the cat had become reinfested at home and returned it to the veterinarian. He assured her the cat was fine, but she was afraid to bring it home. The cat has remained at the veterinarian’s office—to the doctor’s displeasure—for weeks.
Two weeks after Mrs. K first spotted the mites, her husband, age 82, started believing he is infested. Mr. K, who is retired, has battled depression and drinks about a half-gallon of liquor daily.
After 2 months, Mrs. K quit her job for fear she would infest her co-workers, then locked herself and her husband in their house and allowed no visitors. Day and night for nearly 3 weeks, Mrs. K repeatedly vacuumed the house, shampooed the carpets, and sprayed the walls and furniture with a homemade insecticide. She taped the windows closed to keep bugs out and covered all furniture and surface areas with plastic. A toxic stench of insecticide and shampoo permeated every room.
A neighbor told Mrs. K’s son that his parents were locked inside their house. He came over and knocked on their door, but was refused entry. He eventually got Mrs. K out by threatening to call the police, then brought her to the emergency room.
At presentation, Mrs. K’s right leg has scratches and scabs caused by frequent scratching at mites she saw there. Her hands are reddened and dry, suggesting chemical dermatitis caused by cleaning and repeated insecticide use. Ritual cleaning and spraying has kept her from eating or sleeping; she has lost 12 lbs over 3 weeks and looks pale and tired.
A recovered alcoholic, Mrs. K has been sober for 12 years. She has no other psychiatric, medical, or dermatologic history, and has few social contacts beyond her family and workplace acquaintances.
Blood chemistry, CBC, and urine drug test results are normal. Head MRI reveal no neurologic abnormalities. Her Mini-Mental State Examination (MMSE) score (29/30) indicates no cognitive impairment.
Mrs. K is hospitalized to separate her from her allegedly bug-infested household and husband. At intake, she is panicked over leaving her husband alone and distressed that no one except she and her husband can see the bugs infesting their house and covering her skin. She asks doctors to test a small piece of toilet paper, which she says contains a sample of the bugs. She also fears that she infested her son by letting him into her house.
poll here
The authors’ observations
Mrs. K’s presentation and clinical course suggest delusional parasitosis, a fixed false belief of a parasitic infestation that can cause significant social and occupational dysfunction and medical problems. One patient calls this disorder “bugaphobia.”
The disorder may start as a self-perceived invisible infestation and evolve into visual hallucinations of bugs. Patients usually believe their skin is infested; some believe their internal organs, gums, or skin and internal organs are infested.1,2
Table 1
Medical conditions that may precede delusional parasitosis
| Anemia (severe) |
| Cancer |
| CNS infections |
| Head injury |
| Hepatitis |
| Hypertension |
| Hypovitaminosis of vitamin B12, folate, or thiamine |
| Multiple sclerosis |
| Pulmonary disease |
| Renal disease |
| Rheumatologic disease |
| Sight or hearing loss |
| Source: Reference 6 |
Some patients misinterpret scabs, abrasions, or skin irritation secondary to pesticide use as signs of infestation. Delusional parasitosis can also develop after a real, one-time infestation, as may have happened with Mrs. K.
Convinced they are infested, patients consult multiple providers—including dermatologists, gastroenterologists, and ophthalmologists—in search of the “right” treatment. They undergo numerous tests or procedures and repeatedly apply prescription creams and lotions, leading to chemical dermatitis. Patients often try to prove they are infested by bringing skin, dirt, or toilet tissue samples to doctors—this is called the “matchbox sign” because patients generally bring these samples in small boxes.4 They also may repeatedly ask veterinarians to disinfest their pets.
Described as early as 1892, delusional parasitosis has been called acrophobia, dermatophobia, parasitophobic dermatitis, parasitophobia, entomophobia, and other names.12 Researchers disagree on whether it is a primary psychiatric disorder or is secondary to a mental or physical disorder.13
Researchers have debated two neurobiologic explanations behind the disorder:
Primary sensory. Perrin in 1896 suggested that the parasitosis starts as a sensory misinterpretation, is transformed to a tactile hallucination, then becomes delusional.3
Primary delusional. Others believe delusional parasitosis starts as a hallucination, after which somatic delusional properties develop.3 Some theorists suggest that the symptoms are consistent with thalamic and parietal dysfunction or that the disorder may be a type of late-onset schizophrenia.8
Behaviors associated with “bugaphobia” may be “hardwired” into our evolutionary biology. For example, skin picking may be related to primitive grooming behavior. Its contagiousness may have its roots in animalistic pack behaviors, through which creatures adapt by copying behaviors of others in the pack.8
Delusional parasitosis is most often found in socially isolated women age >40 of average or higher intelligence. Persons in some cultures may be more susceptible than others to some types of parasitic delusions. For example, several persons in India who considered ear cleanliness crucial to attaining cultural and spiritual purity reported having ear infestation.7
Delusional parasitosis also is associated with:
- medical conditions (Table 1)6
- use of cocaine, amphetamines,8 corticosteroids,3,9 or phenelzine10
- occipital-temporal cerebral infarction11
- cognitive impairment related to dementia, depression, mental retardation, or schizophrenia/schizophreniform disorder.
Mrs. K’s delusional parasitosis may be a primary psychiatric disorder (Box). She is medically healthy and does not use drugs or alcohol. Her MMSE score is essentially normal, and she exhibited no psychotic symptoms or loss of function before her first mite sighting.
Diagnosis. Delusional parasitosis is diagnosed as delusional disorder, somatic type, if symptoms persist >1 month. Thorough laboratory and neurologic evaluation is recommended to rule out medical causes (Table 2). Eliminate schizophrenia and schizophreniform disorder with a detailed patient history and cognitive testing.
Also check for a comorbid psychiatric disorder that may be perpetuating the delusion. Delusional parasitosis often co-occurs with axis I disorders including major depressive disorder, substance abuse, dementia, and mental retardation.
poll here
The authors’ observations
Mr. K’s “bugaphobia” most likely was a form of shared secondary delusion called folie-a-deux. Between 11% and 25% of persons with primary delusional parasitosis induce secondary delusional parasitosis in another person, usually a spouse or longtime friend.2 About 50% of folie-a-deux disorders involve a married couple. Often both partners are socially isolated.4
poll here
Treatment: Between two worlds
Mrs. K was given risperidone, 2 mg/d, for delusions and anxiety, and escitalopram, 10 mg/d, preventatively for a suspected underlying depression.
As her symptoms began to clear across 2 to 3 days, Mrs. K realized most times that she was not infested, but on occasion still feared that she was. She continued to worry about her husband being alone in a mite-infested house. We reassured her that her husband would be OK and told her to let us know if the mites resurfaced on her skin.
The authors’ observations
Building rapport. When treating delusional parasitosis, be accepting and non-confrontational. These patients tend to switch doctors until they find someone who understands their problem. Developing rapport can promote treatment adherence and prevent or minimize relapse.
Table 2
5 steps to confirm ‘bugaphobia’
|
| Source: Adapted from Driscoll MS, Rothe MJ, Grant-Kels JM, Hale MS. Delusions of parasitosis: a dermatologic, psychiatric, and pharmacologic approach. J Am Acad Dermatol 1993;29:1023-33. |
4
Also communicate with other specialists to gauge medication history, confirm test findings, and rule out medical causes.
Pharmacotherapy. If symptoms do not resolve after 1 or 2 days of observation, look for a comorbid medical or mental disorder. Prescribe an atypical antipsychotic such as risperidone, 2 to 4 mg/d, or olanzapine, 2.5 mg/d, both of which have been effective against delusional parasitosis.14,16 Keep dosages low to reduce risk of sedation, extrapyramidal symptoms (EPS), and tardive dyskinesia.
Suggesting a psychotropic to patients who are convinced their problem is not psychiatric can be difficult. Try saying:
- Some people are more sensitive than others to sensations on their skin or in their body. This medication will help you tolerate the sensations.”
- or, “This drug will help reduce the anxiety your problem is causing.”
Pimozide has shown efficacy against delusional parasitosis in placebo-controlled trials,17,18 but it can alter cardiac conduction, especially at higherthan-recommended dosages. Start pimozide at 1 mg/d and increase by 1 mg/week until clinical response is achieved. Most patients respond to dosages used to treat psychotic disorders (4 to 10 mg/d).19 Order a baseline and periodic ECG to monitor for QTc prolongation, and do an abnormal involuntary movement scale examination every 3 to 6 months to test for EPS.
Other treatments that have shown benefit in case reports include naloxone, 10 mg/d;20 haloperidol, 10 mg/d; trifluoperazine, 15 mg/d; chlorpromazine, 150 to 300 mg/d; and electroconvulsive therapy.7
We have found that prognosis usually is poor after first- and second-line treatments have failed. Continue to search for a missed disorder, and add an antidepressant if an underlying depression is found or suspected.
Psychotherapy. Perform supportive and harm reduction psychotherapy immediately after diagnosis. Supportive, rapport-building approaches can get the patient to comfortably discuss the issues that led to the delusion and help him/her confront a relapse. Harm reduction can discourage patients from requesting unnecessary invasive tests, using medications and toxic insecticides, or other potentially harmful behaviors.
Cognitive-behavioral therapy may help some patients with refractory delusional parasitosis, if they have enough insight to continue treatment.
Follow-up: A bug-free future
Mrs. K was released from the hospital after 4 days, and her delusional symptoms were gone after another 3 days. We followed her for 6 months.
Upon discharge, Mrs. K and her cat moved in with her daughter’s family. Within a few weeks she was able to visit her workplace and explain what had happened. She stopped taking risperidone after 2 weeks because of excessive sedation. No depressive symptoms were present after 3 months; escitalopram was stopped.
Mrs. K’s husband continued to drink and confine himself to the house. Upon visiting him, she was horrified to find the furniture still covered with plastic and the windows taped shut. Mrs. K threatened to divorce him if he did not seek help. He eventually was treated and has been sober—and bug-free—for 15 months.
Related resources
- Bohart Museum of Entomology, University of California, Davis: Delusional parasitosis. http://delusion.ucdavis.edu.
- Chlorpromazine • Thorazine
- Escitalopram • Lexapro
- Haloperidol • Haldol
- naloxone • Narcan
- Olanzapine • Zyprexa
- Pimozide • Orap
- Phenelzine • Nardil
- Risperidone • Risperdal
- Trifluoperazine • Stelazine
Dr. Matthews is an American Psychiatric Association Bristol-Myers Squibb Co. fellow in public and community psychiatry.
Dr. Hauser receives research/grant support from and is a speaker for AstraZeneca Pharmaceuticals, Eli Lilly and Co., GlaxoSmithKline, and Hoffman LaRoche. He is also receives research/grant support from Schering-Plough Corp. and is a speaker for Abbott Laboratories and Janssen Pharmaceutica.
1. Monk BE, Rao YJ. Delusions of parasitosis with fatal outcome. South Med J 1995;88:341-2.
2. Bourgeois ML, Duhamel P, Verdoux H. Delusional parasitosis: folie a deux and attempted murder of a family doctor. Br J Psychiatry 1992;161:709-11.
3. Sherman MD, Holland GN, Holsclaw DS, et al. Delusions of ocular parasitosis. Am J Ophthalmol 1998;125:852-6.
4. Trabert W. Shared psychotic disorder in delusional parasitosis. Psychopathology 1999;32:30-4.
5. Ford EB, Calfee DP, Pearson RD. Delusions of intestinal parasitosis. South Med J 2001;94:545-7.
6. Slaughter JR, Zanol K, Rezvani H, Flax J. Psychogenic parasitosis: a case series and literature review. Psychosomatics 1998;39:491-500.
7. Srinivasan TN, Suresh TR, Jayaram V, Fernandez MP. Nature and treatment of delusional parasitosis: a different experience in India. J Dermatol 1994;33:851-5.
8. de Leon J, Antelo RE, Simpson G. Delusions of parasitosis or chronic tactile hallucinosis: hypothesis about their brain physiopathology. Compr Psychiatry 1992;33:25-33.
9. May WW, Terpenning MS. Delusional parasitosis in geriatric patients. Psychosomatics 1991;32:88-94.
10. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry 1991;159:716-7.
11. Nagaratnam N, O’Neile L. Delusional parasitosis following occipital-temporal cerebral infarction. Gen Hosp Psychiatry 2000;22:129-32.
12. Stephens MB. Delusions of parasitosis. Am Fam Physician 1999;60:2507-8.
13. Musalek M, Bach M, Passweg V, Jaeger S. The position of delusional parasitosis in psychiatric nosology and classification. Psychopathology 1990;23:115-24.
14. Gallucci G, Beard B. Risperidone and the treatment of delusions of parasitosis in an elderly patient. Psychosomatics 1995;36:578-80.
15. Elmer KB, George RM, Peterson K. Therapeutic update: use of risperidone for the treatment of monosymptomatic hypochondriacal psychosis. J Am Acad Dermatol 2000;43:683-6.
16. Fawcett RG. Olanzapine for the treatment of monosymptomatic hypochondriacal psychosis. J Clin Psychiatry 2002;63:162.-
17. Ungvari G, Vladar K. Pimozide therapy in dermatozoon delusion. Dermatol Monatsschr 1984;170:443-7.
18. Hamann K, Avnstorp C. Delusions of infestation treated by pimozide: a double-blind crossover clinical study. Acta Derm Venereol 1982;62:55-8.
19. Opler LA, Feinberg SS. The role of pimozide in clinical psychiatry: a review. J Clin Psychiatry 1991;52:221-33.
20. Botschev C, Muller N. Opiate receptor antagonists for delusions of parasitosis. Biol Psychiatry 1991;30:530-1.
1. Monk BE, Rao YJ. Delusions of parasitosis with fatal outcome. South Med J 1995;88:341-2.
2. Bourgeois ML, Duhamel P, Verdoux H. Delusional parasitosis: folie a deux and attempted murder of a family doctor. Br J Psychiatry 1992;161:709-11.
3. Sherman MD, Holland GN, Holsclaw DS, et al. Delusions of ocular parasitosis. Am J Ophthalmol 1998;125:852-6.
4. Trabert W. Shared psychotic disorder in delusional parasitosis. Psychopathology 1999;32:30-4.
5. Ford EB, Calfee DP, Pearson RD. Delusions of intestinal parasitosis. South Med J 2001;94:545-7.
6. Slaughter JR, Zanol K, Rezvani H, Flax J. Psychogenic parasitosis: a case series and literature review. Psychosomatics 1998;39:491-500.
7. Srinivasan TN, Suresh TR, Jayaram V, Fernandez MP. Nature and treatment of delusional parasitosis: a different experience in India. J Dermatol 1994;33:851-5.
8. de Leon J, Antelo RE, Simpson G. Delusions of parasitosis or chronic tactile hallucinosis: hypothesis about their brain physiopathology. Compr Psychiatry 1992;33:25-33.
9. May WW, Terpenning MS. Delusional parasitosis in geriatric patients. Psychosomatics 1991;32:88-94.
10. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry 1991;159:716-7.
11. Nagaratnam N, O’Neile L. Delusional parasitosis following occipital-temporal cerebral infarction. Gen Hosp Psychiatry 2000;22:129-32.
12. Stephens MB. Delusions of parasitosis. Am Fam Physician 1999;60:2507-8.
13. Musalek M, Bach M, Passweg V, Jaeger S. The position of delusional parasitosis in psychiatric nosology and classification. Psychopathology 1990;23:115-24.
14. Gallucci G, Beard B. Risperidone and the treatment of delusions of parasitosis in an elderly patient. Psychosomatics 1995;36:578-80.
15. Elmer KB, George RM, Peterson K. Therapeutic update: use of risperidone for the treatment of monosymptomatic hypochondriacal psychosis. J Am Acad Dermatol 2000;43:683-6.
16. Fawcett RG. Olanzapine for the treatment of monosymptomatic hypochondriacal psychosis. J Clin Psychiatry 2002;63:162.-
17. Ungvari G, Vladar K. Pimozide therapy in dermatozoon delusion. Dermatol Monatsschr 1984;170:443-7.
18. Hamann K, Avnstorp C. Delusions of infestation treated by pimozide: a double-blind crossover clinical study. Acta Derm Venereol 1982;62:55-8.
19. Opler LA, Feinberg SS. The role of pimozide in clinical psychiatry: a review. J Clin Psychiatry 1991;52:221-33.
20. Botschev C, Muller N. Opiate receptor antagonists for delusions of parasitosis. Biol Psychiatry 1991;30:530-1.