User login
Psychotherapy Telemental Health Center and Regional Pilot
Within VHA, telemental health (TMH) refers to behavioral health services that are provided remotely, using secure communication technologies, to veterans who are separated by distance from their mental health providers.1 Telemental health sometimes involves video teleconferencing (VTC) technology, where a veteran (or group of veterans) in one location and a provider in a different location are able to communicate in real time through a computer monitor or television screen.2 In the VHA, TMH visits are typically conducted from a central location (such as a medical center hospital) to a community-based outpatient clinic (CBOC), but pilot projects have also tested VTC in homes as well.1,3,4
In addition to providing timely access to behavioral health services in rural or underserved locations, TMH eliminates travel that may be disruptive or costly and allows mental health providers to consult with or provide supervision to one another. Telemental health can be used to make diagnoses, manage care, perform checkups, and provide long-term, follow-up care. Other uses for TMH include clinical assessment, individual and group psychotherapy, psycho-educational interventions, cognitive testing, and general psychiatric care.1,5,6 More recently, TMH has been used to provide evidence-based psychotherapies (EBPs) to individuals with posttraumatic stress disorder (PTSD) and other mental health diagnoses.6,7 Such care may be particularly advantageous for veterans with PTSD, because traveling can be a burden for them or a trigger for PTSD symptoms.
Although interactive video technology is becoming widely available, its use is limited in health care systems due to lack of knowledge, education, logistical guidance, and technical training. The authors have conducted EBPs using VTC across VISN 22 in both office-to-office and office-to-home modalities and are providing EBPs using VTC to CBOCs in other VISNs across the western U.S. This article addresses these issues, outlining the necessary steps required to establish a TMH clinic and to share the successes of the EBP TMH Center and Regional Pilot used at VISN 22.
Telemental Health
Telemental health is an effective alternative to in-person treatment and is well regarded by both mental health providers and veterans. Overall, mental health providers believe it can help reduce the stigma associated with traditional mental health care and ease transportation-related issues for veterans. Telemental health allows access to care for veterans living in rural or remote areas in addition to those who are incarcerated or are otherwise unable to attend visits at primary VA facilities.2,8-10 In an assessment of TMH services in 40 CBOCs across VISN 16, most CBOC mental health providers found it to be an acceptable alternative to face-to-face care, recognize the value of TMH, and endorse a willingness to use and expand TMH programs within their clinics.11
Veterans who participated in TMH via VTC have expressed satisfaction with the decreased travel time and expenses, fewer interactions with crowds, and fewer parking problems.12 Several studies suggested that veterans preferred TMH to in-person contact due to more rapid access to care and specialists who would otherwise be unavailable at remote locations.5,10 Similarly, veterans who avoid in-person mental health care were more open to remote therapy for many of the reasons listed earlier. Studies suggest that veterans from both rural and urban locations are generally receptive to receiving mental health services via TMH.5,10
Several studies have found that TMH services may have advantages over standard in-person care. These advantages include decreasing transportation costs, travel time, and time missed at work and increasing system coverage area.13 Overall, both veterans and providers reported similar satisfaction between VTC and in-person sessions and, in some cases, prefer VTC interactions due to a sense of “easing into” intense therapies or having a “therapeutic distance” as treatment begins.12
Utility
Previous studies have shown that TMH can be used successfully to provide psychopharmacologic treatment to veterans who have major depressive disorder or schizophrenia, among other psychiatric disorders.5,8,14 Recent studies have focused on the feasibility of providing EBPs via TMH, particularly for the treatment of PTSD.12,15 Studies have shown that TMH services via VTC can be used successfully to provide cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), and prolonged exposure therapy (PE).16-21 In these studies, both PE and CPT delivered via TMH were found to be as efficacious as in-person formats. Furthermore, TMH services were successfully used in individual and group sessions.
Research has emphasized the benefits of TMH for veterans who are uncomfortable in crowds, waiting rooms, or hospital lobbies.7,12,18 For patients with PTSD who are initially limited by fears related to driving, TMH can facilitate access to care. Veterans with PTSD often avoid reminders of trauma (ie, uniforms, evidence of physical injury, artwork, photographs related to war), which can often be found at the larger VAMCs. These veterans may find mental health care services in their homes or at local CBOCs more appealing.7,12,18
Implementation
Prior to the implementation of telehealth services, many CBOC providers would refer veterans in need of specialty care to the nearest VAMC, which were sometimes many hours away.1 In response to travel and access concerns, the VA has implemented various telehealth modalities, including TMH.
In 2008, about 230,000 veterans received mental health services via real-time clinical VTC at 300 VA CBOCs, and about 40,000 veterans enrolled in the In-Home Telehealth program.22 By 2011, > 380,000 veterans used clinic-based telehealth services and about 100,000 veterans used the in-home program.1 Between 2006 and 2008, the 98,000 veterans who used TMH modalities had fewer hospital admissions compared with those who did not; overall, the need for hospital services decreased by about 25% for those using TMH services.23
Although research suggests that TMH is an effective treatment modality, it does have limitations. A recent study noted several visual and audio difficulties that can emerge, including pixilation, “tracer” images with movement, low resolution, “frozen” or “choppy” images, delays in sound, echoes, or “mechanical sounding” voices.12 In some cases, physical details, such as crying, sniffling, or fidgeting, could not be clearly observed.12 Overall, these unforeseen issues can impact the ability to give and receive care through TMH modalities. Proper procedures need to be developed and implemented for each site.
Getting Started
Using TMH to provide mental health care at other VHA facilities requires planning and preparation. Logistics, such as preparation of the room and equipment, should be considered. Similarly, veteran and provider convenience must be considered.2,11 Before starting TMH at any VA facility, professionals working with the audiovisual technology and providing TMH care must complete necessary VA Talent Management System courses and obtain copies of certificates to assure they have met the appropriate training criteria. Providers must be credentialed to provide TMH services, including the telehealth curriculum offered by VA Employee Educational Services.2,24 An appropriate memorandum of understanding (MOU) must be created, and credentialing and privileging must also be acquired.
In addition to provider training, an information technology representative who can administer technical support as needed must be selected for both the provider and remote locations. Technologic complications can make TMH implementation much more challenging.12 As such, it is important to assure that both the veteran and the provider have the necessary TMH equipment. The selected communication device must be compatible with the technology requirements at the provider and remote facilities.12
In addition to designated technical support, the VISN TMH coordinator needs to have point-of-contact information for those who can assist with each site’s telehealth services and address the demand for EBP for PTSD or other desired services. After this information has been obtained, relationships must be developed and maintained with local leadership at each site, associated telehealth coordinators, and evidence-based therapy coordinators.
After contact has been established with remote facilities and the demand for services has been determined, there are several agreements and procedures to put in place before starting TMH services. An initial step is to develop a MOU agreement between the VISN TMH center and remote
sites that allows providers’ credentials and privileges to be shared. Also, it is important to establish a service agreement that outlines the procedures for staff at the remote site. This agreement includes checking in veterans, setting up the TMH rooms, transferring homework to VISN TMH providers, and connecting with the VISN TMH provider. In addition to service agreements, emergency procedures must be in place to ensure the safety of the veterans and the staff.24
After these agreements have been completed, the VISN TMH providers will have to complete request forms to obtain access to the Computerized Patient Record System at the remote facilities, which then must be approved by the Information Security Officer at that site. This is separate from the request at the provider’s site.12 It is essential to have points of contact for questions regarding this process. In order to facilitate referrals for TMH, electronic interfacility consult requests must be developed. Local staff need to collaborate with VISN TMH staff to ensure that the consult addresses the referral facilities need to meet the appropriate requirements.
Before the initiation of TMH services, each TMH provider has to establish clinics for scheduling appointments and obtaining workload credit. Program support assistants at the provider and remote sites must work together to ensure clinics are established correctly. This collaboration is essential for coding of visits and clinic mapping. After the clinics are “built,” appointment times will be set up based on the availability of the provider, support staff, and rooms at the remote site for the TMH session.
Once a consult is initiated, the VISN TMH EBP coordinator will review the consult and the veteran’s chart to ensure initial inclusion/exclusion criteria are met before accepting or canceling the consult. If the consult is accepted, a VISN TMH provider is assigned to the case and contacts the veteran to discuss the referral and (if the veteran is appropriate and interested) initiate services at the closest CBOC or at home. The VISN TMH regional center staff enter the appointment time for the veteran at both facility sites. The VISN TMH provider also coordinates with the CBOC staff to ensure that the veteran is checked in to the appointment and is provided with any questionnaires and necessary homework.
During the first session, the provider obtains consent from the veteran to engage in TMH services, conducts an assessment, and establishes rapport. The provider works with the veteran to develop a treatment plan for PTSD or other mental health diagnosis that will include the type of EBP. At the end of the first session, the next appointment is scheduled, and treatment materials are either mailed to the veteran or given to him or her onsite. After completing EBP, the VISN TMH center works with the referring provider to find follow-up services for the veteran.
The various steps necessary to begin an interfacility TMH clinic are summarized in Table 1.
Provider Training
Despite strong evidence of success, many providers remained skeptical about the efficacy of TMH. One study indicated that several providers in VISN 16 rarely used the established TMH programs because they were not familiar with them and applied TMH only for medication checks and consults.11 This skepticism was present in providers preparing to offer TMH as well as in providers referring veterans for TMH services. However, once providers better understood the TMH programs and had more experience using them, they were significantly more likely to use TMH for initial evaluations and ongoing psychotherapy. For these reasons, proper training and educational opportunities for practicing providers are vital to TMH implementation.9,11
To be proficient, providers need to become familiar with various TMH applications.10 Health care networks implementing TMH must ensure that their providers are well trained and prepared to give and receive proper consultation and support. Providers must also acquire several skills and familiarize themselves with available tools.9 In educating providers on the process and use of TMH, the authors suggest the following steps for TMH application:
- Learn new ways to chart in multiple systems and know how to troubleshoot during connectivity issues.
- Have an established administrative support collaborator at outpatient clinics to fax and exchange veteran homework.12
- The TMH clinic culture must be embedded where the veteran is being served in order to allow for a more realistic therapeutic feel. This type of clinic setting will allow for referrals at the veteran site and the availability to coordinate emergency procedures in the remote clinic.
Clinical Issues
Ongoing clinical issues need to be addressed continuously. Initially, referrals may be plentiful but not always appropriate. It is important to have an understanding with referring providers and remote sites about what constitutes a “good referral” as well as alternate referral options. It is imperative to outline inclusion and exclusion criteria that are clear and concise for referring providers. It is often helpful to revisit these criteria with potential referral sources after initiating services.
With the ability to provide inhome services, it is important to identify specific inclusion/exclusion criteria. Recommendations are based on research and clinical applications for exclusions, which are available on the Office of Technology Services website. These include imminent suicidality or homicidality, serious personality disorder or problematic character traits, acute substance disorders, psychotic disorders, and bipolar disorder. It is important to use sound clinical judgment, because the usual safeguards present in a remote clinic are not available for inhome services. Emergency planning is one of the most important aspects of the in-home TMH health services that are provided. The information for the emergency plan is obtained prior to initiation of services.
Emergency Plans
Each remote clinic that provides services to veterans must have an emergency plan that details procedures, phone numbers, and resources in case of medical and psychological emergencies as well as natural disasters. The VISN TMH provider will need to have a copy of the emergency plan as well as a list of contacts in case of an emergency during a TMH session.
It is recommended that TMH providers have several ways to contact key staff who can assist during an emergency. Usually the clinical coordinator and telehealth technician are the first responders to be alerted by the TMH provider during an emergency. They will then institute the remote clinic’s emergency protocol. Discussing these procedures and reviewing them with staff regularly is advisable, as key contacts may change.
In a psychological emergency, the VISN TMH provider may assist in implementing emergency procedures until a clinical counterpart at the remote site can be alerted. In the authors’ experience, VISN TMH providers have successfully de-escalated and diffused potentially emergent situations by maintaining constant realtime communication with veterans and staff by using VTC as well as interoffice communication. By offering assistance to veterans and staff during challenging situations, the VISN TMH provider will not only decrease concerns of veterans, but oftentimes integrate themselves into the treatment team of the remote clinic. The role of a VISN TMH provider can be isolative, with minimal contact with remote clinic staff, so it is important to increase visibility among staff at a remote site by communication with them even when there is not an emergency.
Treatment protocols may be determined by either administrative or clinical factors. With certain TMH interventions, the rooms used for veterans may be available for only certain periods, which may or may not fit with treatment protocols. For example, if a room is available for only an hour but a treatment protocol session is for 90 minutes, then another time slot needs to be found or a different treatment considered and offered. Although it is not ideal to have treatment protocols determined by scheduling factors, the reality of shared space at remote sites requires flexibility.
Sharing Materials and Homework Another clinical issue that is often overlooked is how to implement specific treatment protocols that entail the exchange of materials between VISN TMH providers and veterans. If materials will need to be exchanged between provider and veteran, a plan will have to be in place to facilitate this. The service agreement addresses these details, but remote staff may not always be aware of the details.
If a TMH provider opts to use faxes to send materials between a veteran and a provider, a desktop faxing program is recommended so veteran privacy is not compromised. Often, providers will wait to begin sessions until after they have received materials, but this may result in a delayed
session. One solution TMH providers can implement is mailing the materials and questionnaires to veterans before the session with clear instructions to complete them beforehand. Once the veteran arrives for the TMH session, she or he will verbally respond to the questionnaire and treatment materials. This will add time to a session but minimizes potential delays. Many of the clinical VTC units have movable cameras, so veterans can tilt the camera to show providers the forms and questionnaires.
The various steps necessary to address TMH clinical issues are summarized in Table 2.
VISIN 22 Pilot Project
The VISN 22 EBP TMH Center and Regional Pilot, based at the VA San Diego Healthcare System, was tasked with developing and providing TMH EBP services for PTSD across VISN 22 and adjacent West Coast VISNs. In addition to creating standardized procedures, troubleshooting guides were established to assist other programs with implementation. The primary focus was to increase access to EBPs for veterans with PTSD in areas where there was either no available trained providers or delays for specific services. The program established 16 clinics as well as in-home
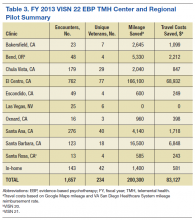
services in VISN 22, VISN 21, and VISN 20. In fiscal year (FY) 2013, the VISN 22 EBP TMH Center and Regional Pilot provided 1,657 EBP encounters via TMH to 234 unique veterans with PTSD (Table 3).
The pilot project collected data to evaluate program effectiveness. The data were de-identified before being sent to the VA Central Office (VACO) TMH program manager. The following items were collected for the pilot: (1) clinical information; (2) consent to engage in treatment and telehealth; (3) release of information to share de-identified data to VACO for program monitoring; (4) demographic form; (5) Beck Depression Inventory-II (every other week); (6) PTSD Checklist (every other week); (7) World Health Organization Quality of Life (sessions 1, 7, final); (8) Wechsler Adult Intelligence Scale-Revised (sessions 3, 7, final); (9) satisfaction survey (final); (10) mileage not driven by veterans who receive TMH services; (11) travel pay saved by VA; (12) no-show rates; and (13) veteran, TMH provider, and referral provider satisfaction.
The growth in number of encounters and number of unique veterans has increased steadily from the first quarter of FY14 through the second quarter of FY15 (Figure 1).
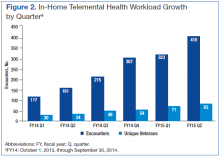
In January 2013, in-home TMH services were piloted. Although occasional technical difficulties occurred, 143 EBP encounters via TMH were provided to 42 unique veterans in 2013. The service has continued to expand, and in the first half of FY14, services were provided to 64 unique veterans for a total of 278 encounters, saving veterans 3,220 travel miles and saving the VA $1,336 in travel reimbursement. In-home TMH services will continue to expand as more providers in a variety of programs are being trained by the San Diego staff on how to provide these services to veterans in their homes. In addition to decreasing mileage and travel pay, the no-show rates are lower for TMH appointments in general (averaged 8%-10% vs facility no-show rate average of 13.5%) and with the use of inhome TMH, no-show rates were kept to 2%. The growth in the number of in-home encounters and the number of unique veterans has also increased steadily from the first quarter of FY14 thru the second quarter of FY15 (Figure 2).
In-Home TMH Services
The VISN 22 EBP TMH Center and Regional Pilot often requests to have an in-person meeting with a veteran before starting TMH services in order to complete a waiver to download the software used by the VA for real-time video in-home services, a Release of Information for a Primary Support Person form, and an emergency plan.
It is also recommended that information about the veteran’s Internet connection, type of computer, type of software, presence of a camera and speakers, e-mail address, and access to secure messaging are obtained. During the initial contact with a veteran, the provider will discuss the rules and requirements to ensure HIPAA compliance. The veteran will need to have a private area for the call (not a restaurant, car, or other place where Wi-Fi is offered). Even with these discussions, some veterans will initiate services from a public place or a room in their home where family members will enter and exit frequently.
Although not required, it is recommended to have the veteran identify a primary support person and complete a release form to allow the TMH provider to contact that person in an emergency. The support person may be a person in the home (adult family member or caregiver) or someone nearby (neighbor, friend, or family member) who can contact emergency services if needed. After the necessary information is gathered and the veteran agrees to the conditions of participation, a test call will be completed. The TMH provider is often the person to conduct this call, but if available, a telehealth technician or facility telehealth coordinator may assist. The TMH provider may help the veteran download the appropriate software that is sent from the VA Scheduler software. The veteran initiates the call with the provider. Once the connection is made, the session may begin. Sites that are currently conducting in-home services have provided guides to veterans and newer TMH providers to outline the necessary steps for initiating services.
It is recommended that any provider interested in providing in-home TMH services use the Office of Technological Services help desk to assist in troubleshooting difficulties with connectivity. Challenges have included the software used for in-home TMH, periodic Internet outages, and compatibility issues.
Veteran Satisfaction
Veteran satisfaction was measured through a self-report satisfaction survey. The survey included 12 questions assessing overall experience in using TMH services. Eleven of the 12 questions included a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the last question was openended for additional comments.
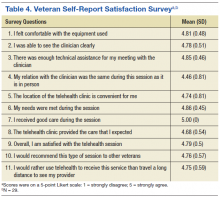
A summary of the survey response of the initial 29 veterans who received TMH services suggested the following: (1) Veterans felt comfortable with using the TMH equipment and were able to see their clinician clearly; (2) Technical assistance was sufficient; (3) During the TMH session, they related to the provider as if it were a face-to-face meeting and that their needs were met; and (4) Veterans reported extremely high satisfaction with TMH and would refer TMH care to other veterans. Veterans found clinic locations very convenient and preferred the TMH modality of mental health services delivery to the alternative of travelling a long distance to see their provider (Table 4).
Written comments and recommendations from veterans supported the survey results. Most reported that they saved time and the convenience of the clinic allowed them to receive the treatment they need without interfering with their work schedule. However, some veterans still experienced trouble with travel to the remote clinic. Others felt their experience was different from the one they expected or they had a good experience via TMH but preferred face-to-face care.
Conclusion
The VISN 22 EBP TMH Center and Regional Pilot have established the infrastructure of interfacility clinics to use EBPs for the treatment of PTSD. Also, the center has provided consultation and guidance to facilities interested in developing their own TMH programs. The TMH Center now plans to expand mental health services and include medication management and EBP services for non-PTSD psychiatric diagnoses. The established infrastructure will allow providers from one facility to cover the mental health service needs of other facilities when there are absences or gaps due to leave or delays/challenges in hiring in rural locations. Finally, TMH offers the potential to offer after-hours services to veterans in other time zones during providers’ regular tours of duty.
Several other TMH programs are now expanding services into veterans’ homes. There are several sites within the VHA that have piloted this TMH modality and developed guidelines and recommendations for further expansion. Currently VACO is encouraging all VHA facilities to increase in-home telehealth services, and the Office of Telehealth Services provides details on implementation. Interested parties are encouraged to routinely visit the VACO website for updated information.
Developing and implementing a new TMH program can be an arduous task, but the program has great potential to provide veteran-centered care. As TMH sessions progress, the provider and veteran become less aware of the camera and software and more aware of the therapeutic process. Challenges and delays in implementation are to be expected—these can occur frequently during the development and implementation stages of a TMH program. Maintaining consistent communication with staff at remote sites is essential for the success of any program.
As the VHA focuses on veterancentered care, TMH services will improve access to providers with specific, needed expertise. The authors hope these experiences can facilitate the continued growth of TMH and assuage any concerns a facility or provider may have about this modality of care. Delivery of TMH care can be challenging, but the ability to provide these services to veterans at times and locations convenient to them makes these challenges worthwhile.
Acknowledgments
Dr. Hauser wishes to thank Cathy, Anika, Jirina, Katia, and Max Hauser, and Alba Pillwein for their continued support. In memory of Beverly Ostroski.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. What is telehealth? U.S. Department of Veterans Affairs Website. http://www.telehealth.va.gov. Update May 13, 2014. Accessed April 30, 2015.
2. Morland LA, Greene CJ, Rosen C, Mauldin PD, Frueh CB. Issues in the design of a randomized noninferiority clinical trial of telemental health psychotherapy for rural combat veterans with PTSD. Contemp Clin Trials. 2009;30(6):513-522.
3. Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, Acierno R. An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behav Ther. 2012;43(3):560-569.
4. Yuen EK, Gros DF, Price M, et al. Randomized controlled trial of home-based telehealth versus in-person prolonged exposure for combat-related PTSD in veterans: preliminary results. J Clin Psychol. 2015;71(6):500-512.
5. Ruskin PE, Reed S, Kumar R, et al. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatr Serv. 1998;49(8):1086-1088.
6. Gros DF, Morland LA, Greene CJ, et al. Delivery of evidence-based psychotherapy via video telehealth. J Psychopathol Behav Assess. 2013;35(4):506-521.
7. Backhaus A, Agha Z, Maglione ML, et al. Videoconferencing psychotherapy: a systematic review. Psychol Serv. 2012;9(2):111-131.
8. Egede LE, Frueh CB, Richardson LK, et al. Rationale and design: telepsychology service delivery for depressed elderly veterans. Trials. 2009;10:22.
9. Frueh BC, Deitsch SE, Santos AB, et al. Procedural and methodological issues in telepsychiatry research and program development. Psychiatr Serv. 2000;51(12):1522-1527.
10. Grubaugh AL, Cain GD, Elhai JD, Patrick SL, Frueh BC. Attitudes toward medical and mental health care delivered via telehealth applications among rural and urban primary care patients. J Nerv Ment Dis. 2008;196(2):166-170.
11. Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes towards telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425-432.
12. Thorp SR, Fidler J, Moreno L, Floto E, Agha Z. Lessons learned from studies of psychotherapy for posttraumatic stress disorder via video teleconferencing. Psychol Serv. 2012;9(2):197-199.
13. Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: predictors of treatment completion and outcome and comparison to treatment delivered in person. Behav Ther. 2011;42(2):276-283.
14. Zarate CA Jr, Weinstock L, Cukor P, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry. 1997;58(1):22-25.
15. Jones AM, Shealy KM, Reid-Quiñones K, et al. Guidelines for establishing a telemental health program to provide evidence-based therapy for trauma-exposed children and families. Psychol Serv. 2014;11(4):398-409.
16. Frueh BC, Monnier J, Grubaugh AL, Elhai JD, Yim E, Knapp R. Therapist adherence and competence with manualized cognitive-behavioral therapy for PTSD delivered via videoconferencing technology. Behav Modif. 2007;31(6):856-866.
17. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469.
18. Tuerk PW, Yoder M, Ruggiero KJ, Gros DF, Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J Trauma Stress. 2010;23(1):116-123.
19. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine- based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
20. Germain V, Marchand A, Bouchard S, Drouin MS, Guay S. Effectiveness of cognitive behavioural therapy administered by videoconference for posttraumatic stress disorder. Cogn Behav Ther. 2009;38(1):42-53.
21. Morland LA, Mackintosh M, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75(5):470-476.
22. Tuerk PW, Fortney J, Bosworth HB, et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16(1):115-117.
23. Godleski L, Darkins A, Peters J. Outcomes of 98,609 U.S. Department of Veterans Affairs patients enrolled in telemental health services, 2006-2010. Psychiatr Serv. 2012;63(4):383-385.
24. Strachan M, Gros DF, Yuen E, Ruggiero KJ, Foa EB, Acierno R. Home-based telehealth to deliver evidence-based psychotherapy in veterans with PTSD. Contemp Clin Trials. 2012;33(2):402-409.
Within VHA, telemental health (TMH) refers to behavioral health services that are provided remotely, using secure communication technologies, to veterans who are separated by distance from their mental health providers.1 Telemental health sometimes involves video teleconferencing (VTC) technology, where a veteran (or group of veterans) in one location and a provider in a different location are able to communicate in real time through a computer monitor or television screen.2 In the VHA, TMH visits are typically conducted from a central location (such as a medical center hospital) to a community-based outpatient clinic (CBOC), but pilot projects have also tested VTC in homes as well.1,3,4
In addition to providing timely access to behavioral health services in rural or underserved locations, TMH eliminates travel that may be disruptive or costly and allows mental health providers to consult with or provide supervision to one another. Telemental health can be used to make diagnoses, manage care, perform checkups, and provide long-term, follow-up care. Other uses for TMH include clinical assessment, individual and group psychotherapy, psycho-educational interventions, cognitive testing, and general psychiatric care.1,5,6 More recently, TMH has been used to provide evidence-based psychotherapies (EBPs) to individuals with posttraumatic stress disorder (PTSD) and other mental health diagnoses.6,7 Such care may be particularly advantageous for veterans with PTSD, because traveling can be a burden for them or a trigger for PTSD symptoms.
Although interactive video technology is becoming widely available, its use is limited in health care systems due to lack of knowledge, education, logistical guidance, and technical training. The authors have conducted EBPs using VTC across VISN 22 in both office-to-office and office-to-home modalities and are providing EBPs using VTC to CBOCs in other VISNs across the western U.S. This article addresses these issues, outlining the necessary steps required to establish a TMH clinic and to share the successes of the EBP TMH Center and Regional Pilot used at VISN 22.
Telemental Health
Telemental health is an effective alternative to in-person treatment and is well regarded by both mental health providers and veterans. Overall, mental health providers believe it can help reduce the stigma associated with traditional mental health care and ease transportation-related issues for veterans. Telemental health allows access to care for veterans living in rural or remote areas in addition to those who are incarcerated or are otherwise unable to attend visits at primary VA facilities.2,8-10 In an assessment of TMH services in 40 CBOCs across VISN 16, most CBOC mental health providers found it to be an acceptable alternative to face-to-face care, recognize the value of TMH, and endorse a willingness to use and expand TMH programs within their clinics.11
Veterans who participated in TMH via VTC have expressed satisfaction with the decreased travel time and expenses, fewer interactions with crowds, and fewer parking problems.12 Several studies suggested that veterans preferred TMH to in-person contact due to more rapid access to care and specialists who would otherwise be unavailable at remote locations.5,10 Similarly, veterans who avoid in-person mental health care were more open to remote therapy for many of the reasons listed earlier. Studies suggest that veterans from both rural and urban locations are generally receptive to receiving mental health services via TMH.5,10
Several studies have found that TMH services may have advantages over standard in-person care. These advantages include decreasing transportation costs, travel time, and time missed at work and increasing system coverage area.13 Overall, both veterans and providers reported similar satisfaction between VTC and in-person sessions and, in some cases, prefer VTC interactions due to a sense of “easing into” intense therapies or having a “therapeutic distance” as treatment begins.12
Utility
Previous studies have shown that TMH can be used successfully to provide psychopharmacologic treatment to veterans who have major depressive disorder or schizophrenia, among other psychiatric disorders.5,8,14 Recent studies have focused on the feasibility of providing EBPs via TMH, particularly for the treatment of PTSD.12,15 Studies have shown that TMH services via VTC can be used successfully to provide cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), and prolonged exposure therapy (PE).16-21 In these studies, both PE and CPT delivered via TMH were found to be as efficacious as in-person formats. Furthermore, TMH services were successfully used in individual and group sessions.
Research has emphasized the benefits of TMH for veterans who are uncomfortable in crowds, waiting rooms, or hospital lobbies.7,12,18 For patients with PTSD who are initially limited by fears related to driving, TMH can facilitate access to care. Veterans with PTSD often avoid reminders of trauma (ie, uniforms, evidence of physical injury, artwork, photographs related to war), which can often be found at the larger VAMCs. These veterans may find mental health care services in their homes or at local CBOCs more appealing.7,12,18
Implementation
Prior to the implementation of telehealth services, many CBOC providers would refer veterans in need of specialty care to the nearest VAMC, which were sometimes many hours away.1 In response to travel and access concerns, the VA has implemented various telehealth modalities, including TMH.
In 2008, about 230,000 veterans received mental health services via real-time clinical VTC at 300 VA CBOCs, and about 40,000 veterans enrolled in the In-Home Telehealth program.22 By 2011, > 380,000 veterans used clinic-based telehealth services and about 100,000 veterans used the in-home program.1 Between 2006 and 2008, the 98,000 veterans who used TMH modalities had fewer hospital admissions compared with those who did not; overall, the need for hospital services decreased by about 25% for those using TMH services.23
Although research suggests that TMH is an effective treatment modality, it does have limitations. A recent study noted several visual and audio difficulties that can emerge, including pixilation, “tracer” images with movement, low resolution, “frozen” or “choppy” images, delays in sound, echoes, or “mechanical sounding” voices.12 In some cases, physical details, such as crying, sniffling, or fidgeting, could not be clearly observed.12 Overall, these unforeseen issues can impact the ability to give and receive care through TMH modalities. Proper procedures need to be developed and implemented for each site.
Getting Started
Using TMH to provide mental health care at other VHA facilities requires planning and preparation. Logistics, such as preparation of the room and equipment, should be considered. Similarly, veteran and provider convenience must be considered.2,11 Before starting TMH at any VA facility, professionals working with the audiovisual technology and providing TMH care must complete necessary VA Talent Management System courses and obtain copies of certificates to assure they have met the appropriate training criteria. Providers must be credentialed to provide TMH services, including the telehealth curriculum offered by VA Employee Educational Services.2,24 An appropriate memorandum of understanding (MOU) must be created, and credentialing and privileging must also be acquired.
In addition to provider training, an information technology representative who can administer technical support as needed must be selected for both the provider and remote locations. Technologic complications can make TMH implementation much more challenging.12 As such, it is important to assure that both the veteran and the provider have the necessary TMH equipment. The selected communication device must be compatible with the technology requirements at the provider and remote facilities.12
In addition to designated technical support, the VISN TMH coordinator needs to have point-of-contact information for those who can assist with each site’s telehealth services and address the demand for EBP for PTSD or other desired services. After this information has been obtained, relationships must be developed and maintained with local leadership at each site, associated telehealth coordinators, and evidence-based therapy coordinators.
After contact has been established with remote facilities and the demand for services has been determined, there are several agreements and procedures to put in place before starting TMH services. An initial step is to develop a MOU agreement between the VISN TMH center and remote
sites that allows providers’ credentials and privileges to be shared. Also, it is important to establish a service agreement that outlines the procedures for staff at the remote site. This agreement includes checking in veterans, setting up the TMH rooms, transferring homework to VISN TMH providers, and connecting with the VISN TMH provider. In addition to service agreements, emergency procedures must be in place to ensure the safety of the veterans and the staff.24
After these agreements have been completed, the VISN TMH providers will have to complete request forms to obtain access to the Computerized Patient Record System at the remote facilities, which then must be approved by the Information Security Officer at that site. This is separate from the request at the provider’s site.12 It is essential to have points of contact for questions regarding this process. In order to facilitate referrals for TMH, electronic interfacility consult requests must be developed. Local staff need to collaborate with VISN TMH staff to ensure that the consult addresses the referral facilities need to meet the appropriate requirements.
Before the initiation of TMH services, each TMH provider has to establish clinics for scheduling appointments and obtaining workload credit. Program support assistants at the provider and remote sites must work together to ensure clinics are established correctly. This collaboration is essential for coding of visits and clinic mapping. After the clinics are “built,” appointment times will be set up based on the availability of the provider, support staff, and rooms at the remote site for the TMH session.
Once a consult is initiated, the VISN TMH EBP coordinator will review the consult and the veteran’s chart to ensure initial inclusion/exclusion criteria are met before accepting or canceling the consult. If the consult is accepted, a VISN TMH provider is assigned to the case and contacts the veteran to discuss the referral and (if the veteran is appropriate and interested) initiate services at the closest CBOC or at home. The VISN TMH regional center staff enter the appointment time for the veteran at both facility sites. The VISN TMH provider also coordinates with the CBOC staff to ensure that the veteran is checked in to the appointment and is provided with any questionnaires and necessary homework.
During the first session, the provider obtains consent from the veteran to engage in TMH services, conducts an assessment, and establishes rapport. The provider works with the veteran to develop a treatment plan for PTSD or other mental health diagnosis that will include the type of EBP. At the end of the first session, the next appointment is scheduled, and treatment materials are either mailed to the veteran or given to him or her onsite. After completing EBP, the VISN TMH center works with the referring provider to find follow-up services for the veteran.
The various steps necessary to begin an interfacility TMH clinic are summarized in Table 1.
Provider Training
Despite strong evidence of success, many providers remained skeptical about the efficacy of TMH. One study indicated that several providers in VISN 16 rarely used the established TMH programs because they were not familiar with them and applied TMH only for medication checks and consults.11 This skepticism was present in providers preparing to offer TMH as well as in providers referring veterans for TMH services. However, once providers better understood the TMH programs and had more experience using them, they were significantly more likely to use TMH for initial evaluations and ongoing psychotherapy. For these reasons, proper training and educational opportunities for practicing providers are vital to TMH implementation.9,11
To be proficient, providers need to become familiar with various TMH applications.10 Health care networks implementing TMH must ensure that their providers are well trained and prepared to give and receive proper consultation and support. Providers must also acquire several skills and familiarize themselves with available tools.9 In educating providers on the process and use of TMH, the authors suggest the following steps for TMH application:
- Learn new ways to chart in multiple systems and know how to troubleshoot during connectivity issues.
- Have an established administrative support collaborator at outpatient clinics to fax and exchange veteran homework.12
- The TMH clinic culture must be embedded where the veteran is being served in order to allow for a more realistic therapeutic feel. This type of clinic setting will allow for referrals at the veteran site and the availability to coordinate emergency procedures in the remote clinic.
Clinical Issues
Ongoing clinical issues need to be addressed continuously. Initially, referrals may be plentiful but not always appropriate. It is important to have an understanding with referring providers and remote sites about what constitutes a “good referral” as well as alternate referral options. It is imperative to outline inclusion and exclusion criteria that are clear and concise for referring providers. It is often helpful to revisit these criteria with potential referral sources after initiating services.
With the ability to provide inhome services, it is important to identify specific inclusion/exclusion criteria. Recommendations are based on research and clinical applications for exclusions, which are available on the Office of Technology Services website. These include imminent suicidality or homicidality, serious personality disorder or problematic character traits, acute substance disorders, psychotic disorders, and bipolar disorder. It is important to use sound clinical judgment, because the usual safeguards present in a remote clinic are not available for inhome services. Emergency planning is one of the most important aspects of the in-home TMH health services that are provided. The information for the emergency plan is obtained prior to initiation of services.
Emergency Plans
Each remote clinic that provides services to veterans must have an emergency plan that details procedures, phone numbers, and resources in case of medical and psychological emergencies as well as natural disasters. The VISN TMH provider will need to have a copy of the emergency plan as well as a list of contacts in case of an emergency during a TMH session.
It is recommended that TMH providers have several ways to contact key staff who can assist during an emergency. Usually the clinical coordinator and telehealth technician are the first responders to be alerted by the TMH provider during an emergency. They will then institute the remote clinic’s emergency protocol. Discussing these procedures and reviewing them with staff regularly is advisable, as key contacts may change.
In a psychological emergency, the VISN TMH provider may assist in implementing emergency procedures until a clinical counterpart at the remote site can be alerted. In the authors’ experience, VISN TMH providers have successfully de-escalated and diffused potentially emergent situations by maintaining constant realtime communication with veterans and staff by using VTC as well as interoffice communication. By offering assistance to veterans and staff during challenging situations, the VISN TMH provider will not only decrease concerns of veterans, but oftentimes integrate themselves into the treatment team of the remote clinic. The role of a VISN TMH provider can be isolative, with minimal contact with remote clinic staff, so it is important to increase visibility among staff at a remote site by communication with them even when there is not an emergency.
Treatment protocols may be determined by either administrative or clinical factors. With certain TMH interventions, the rooms used for veterans may be available for only certain periods, which may or may not fit with treatment protocols. For example, if a room is available for only an hour but a treatment protocol session is for 90 minutes, then another time slot needs to be found or a different treatment considered and offered. Although it is not ideal to have treatment protocols determined by scheduling factors, the reality of shared space at remote sites requires flexibility.
Sharing Materials and Homework Another clinical issue that is often overlooked is how to implement specific treatment protocols that entail the exchange of materials between VISN TMH providers and veterans. If materials will need to be exchanged between provider and veteran, a plan will have to be in place to facilitate this. The service agreement addresses these details, but remote staff may not always be aware of the details.
If a TMH provider opts to use faxes to send materials between a veteran and a provider, a desktop faxing program is recommended so veteran privacy is not compromised. Often, providers will wait to begin sessions until after they have received materials, but this may result in a delayed
session. One solution TMH providers can implement is mailing the materials and questionnaires to veterans before the session with clear instructions to complete them beforehand. Once the veteran arrives for the TMH session, she or he will verbally respond to the questionnaire and treatment materials. This will add time to a session but minimizes potential delays. Many of the clinical VTC units have movable cameras, so veterans can tilt the camera to show providers the forms and questionnaires.
The various steps necessary to address TMH clinical issues are summarized in Table 2.
VISIN 22 Pilot Project
The VISN 22 EBP TMH Center and Regional Pilot, based at the VA San Diego Healthcare System, was tasked with developing and providing TMH EBP services for PTSD across VISN 22 and adjacent West Coast VISNs. In addition to creating standardized procedures, troubleshooting guides were established to assist other programs with implementation. The primary focus was to increase access to EBPs for veterans with PTSD in areas where there was either no available trained providers or delays for specific services. The program established 16 clinics as well as in-home
services in VISN 22, VISN 21, and VISN 20. In fiscal year (FY) 2013, the VISN 22 EBP TMH Center and Regional Pilot provided 1,657 EBP encounters via TMH to 234 unique veterans with PTSD (Table 3).
The pilot project collected data to evaluate program effectiveness. The data were de-identified before being sent to the VA Central Office (VACO) TMH program manager. The following items were collected for the pilot: (1) clinical information; (2) consent to engage in treatment and telehealth; (3) release of information to share de-identified data to VACO for program monitoring; (4) demographic form; (5) Beck Depression Inventory-II (every other week); (6) PTSD Checklist (every other week); (7) World Health Organization Quality of Life (sessions 1, 7, final); (8) Wechsler Adult Intelligence Scale-Revised (sessions 3, 7, final); (9) satisfaction survey (final); (10) mileage not driven by veterans who receive TMH services; (11) travel pay saved by VA; (12) no-show rates; and (13) veteran, TMH provider, and referral provider satisfaction.
The growth in number of encounters and number of unique veterans has increased steadily from the first quarter of FY14 through the second quarter of FY15 (Figure 1).
In January 2013, in-home TMH services were piloted. Although occasional technical difficulties occurred, 143 EBP encounters via TMH were provided to 42 unique veterans in 2013. The service has continued to expand, and in the first half of FY14, services were provided to 64 unique veterans for a total of 278 encounters, saving veterans 3,220 travel miles and saving the VA $1,336 in travel reimbursement. In-home TMH services will continue to expand as more providers in a variety of programs are being trained by the San Diego staff on how to provide these services to veterans in their homes. In addition to decreasing mileage and travel pay, the no-show rates are lower for TMH appointments in general (averaged 8%-10% vs facility no-show rate average of 13.5%) and with the use of inhome TMH, no-show rates were kept to 2%. The growth in the number of in-home encounters and the number of unique veterans has also increased steadily from the first quarter of FY14 thru the second quarter of FY15 (Figure 2).
In-Home TMH Services
The VISN 22 EBP TMH Center and Regional Pilot often requests to have an in-person meeting with a veteran before starting TMH services in order to complete a waiver to download the software used by the VA for real-time video in-home services, a Release of Information for a Primary Support Person form, and an emergency plan.
It is also recommended that information about the veteran’s Internet connection, type of computer, type of software, presence of a camera and speakers, e-mail address, and access to secure messaging are obtained. During the initial contact with a veteran, the provider will discuss the rules and requirements to ensure HIPAA compliance. The veteran will need to have a private area for the call (not a restaurant, car, or other place where Wi-Fi is offered). Even with these discussions, some veterans will initiate services from a public place or a room in their home where family members will enter and exit frequently.
Although not required, it is recommended to have the veteran identify a primary support person and complete a release form to allow the TMH provider to contact that person in an emergency. The support person may be a person in the home (adult family member or caregiver) or someone nearby (neighbor, friend, or family member) who can contact emergency services if needed. After the necessary information is gathered and the veteran agrees to the conditions of participation, a test call will be completed. The TMH provider is often the person to conduct this call, but if available, a telehealth technician or facility telehealth coordinator may assist. The TMH provider may help the veteran download the appropriate software that is sent from the VA Scheduler software. The veteran initiates the call with the provider. Once the connection is made, the session may begin. Sites that are currently conducting in-home services have provided guides to veterans and newer TMH providers to outline the necessary steps for initiating services.
It is recommended that any provider interested in providing in-home TMH services use the Office of Technological Services help desk to assist in troubleshooting difficulties with connectivity. Challenges have included the software used for in-home TMH, periodic Internet outages, and compatibility issues.
Veteran Satisfaction
Veteran satisfaction was measured through a self-report satisfaction survey. The survey included 12 questions assessing overall experience in using TMH services. Eleven of the 12 questions included a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the last question was openended for additional comments.
A summary of the survey response of the initial 29 veterans who received TMH services suggested the following: (1) Veterans felt comfortable with using the TMH equipment and were able to see their clinician clearly; (2) Technical assistance was sufficient; (3) During the TMH session, they related to the provider as if it were a face-to-face meeting and that their needs were met; and (4) Veterans reported extremely high satisfaction with TMH and would refer TMH care to other veterans. Veterans found clinic locations very convenient and preferred the TMH modality of mental health services delivery to the alternative of travelling a long distance to see their provider (Table 4).
Written comments and recommendations from veterans supported the survey results. Most reported that they saved time and the convenience of the clinic allowed them to receive the treatment they need without interfering with their work schedule. However, some veterans still experienced trouble with travel to the remote clinic. Others felt their experience was different from the one they expected or they had a good experience via TMH but preferred face-to-face care.
Conclusion
The VISN 22 EBP TMH Center and Regional Pilot have established the infrastructure of interfacility clinics to use EBPs for the treatment of PTSD. Also, the center has provided consultation and guidance to facilities interested in developing their own TMH programs. The TMH Center now plans to expand mental health services and include medication management and EBP services for non-PTSD psychiatric diagnoses. The established infrastructure will allow providers from one facility to cover the mental health service needs of other facilities when there are absences or gaps due to leave or delays/challenges in hiring in rural locations. Finally, TMH offers the potential to offer after-hours services to veterans in other time zones during providers’ regular tours of duty.
Several other TMH programs are now expanding services into veterans’ homes. There are several sites within the VHA that have piloted this TMH modality and developed guidelines and recommendations for further expansion. Currently VACO is encouraging all VHA facilities to increase in-home telehealth services, and the Office of Telehealth Services provides details on implementation. Interested parties are encouraged to routinely visit the VACO website for updated information.
Developing and implementing a new TMH program can be an arduous task, but the program has great potential to provide veteran-centered care. As TMH sessions progress, the provider and veteran become less aware of the camera and software and more aware of the therapeutic process. Challenges and delays in implementation are to be expected—these can occur frequently during the development and implementation stages of a TMH program. Maintaining consistent communication with staff at remote sites is essential for the success of any program.
As the VHA focuses on veterancentered care, TMH services will improve access to providers with specific, needed expertise. The authors hope these experiences can facilitate the continued growth of TMH and assuage any concerns a facility or provider may have about this modality of care. Delivery of TMH care can be challenging, but the ability to provide these services to veterans at times and locations convenient to them makes these challenges worthwhile.
Acknowledgments
Dr. Hauser wishes to thank Cathy, Anika, Jirina, Katia, and Max Hauser, and Alba Pillwein for their continued support. In memory of Beverly Ostroski.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Within VHA, telemental health (TMH) refers to behavioral health services that are provided remotely, using secure communication technologies, to veterans who are separated by distance from their mental health providers.1 Telemental health sometimes involves video teleconferencing (VTC) technology, where a veteran (or group of veterans) in one location and a provider in a different location are able to communicate in real time through a computer monitor or television screen.2 In the VHA, TMH visits are typically conducted from a central location (such as a medical center hospital) to a community-based outpatient clinic (CBOC), but pilot projects have also tested VTC in homes as well.1,3,4
In addition to providing timely access to behavioral health services in rural or underserved locations, TMH eliminates travel that may be disruptive or costly and allows mental health providers to consult with or provide supervision to one another. Telemental health can be used to make diagnoses, manage care, perform checkups, and provide long-term, follow-up care. Other uses for TMH include clinical assessment, individual and group psychotherapy, psycho-educational interventions, cognitive testing, and general psychiatric care.1,5,6 More recently, TMH has been used to provide evidence-based psychotherapies (EBPs) to individuals with posttraumatic stress disorder (PTSD) and other mental health diagnoses.6,7 Such care may be particularly advantageous for veterans with PTSD, because traveling can be a burden for them or a trigger for PTSD symptoms.
Although interactive video technology is becoming widely available, its use is limited in health care systems due to lack of knowledge, education, logistical guidance, and technical training. The authors have conducted EBPs using VTC across VISN 22 in both office-to-office and office-to-home modalities and are providing EBPs using VTC to CBOCs in other VISNs across the western U.S. This article addresses these issues, outlining the necessary steps required to establish a TMH clinic and to share the successes of the EBP TMH Center and Regional Pilot used at VISN 22.
Telemental Health
Telemental health is an effective alternative to in-person treatment and is well regarded by both mental health providers and veterans. Overall, mental health providers believe it can help reduce the stigma associated with traditional mental health care and ease transportation-related issues for veterans. Telemental health allows access to care for veterans living in rural or remote areas in addition to those who are incarcerated or are otherwise unable to attend visits at primary VA facilities.2,8-10 In an assessment of TMH services in 40 CBOCs across VISN 16, most CBOC mental health providers found it to be an acceptable alternative to face-to-face care, recognize the value of TMH, and endorse a willingness to use and expand TMH programs within their clinics.11
Veterans who participated in TMH via VTC have expressed satisfaction with the decreased travel time and expenses, fewer interactions with crowds, and fewer parking problems.12 Several studies suggested that veterans preferred TMH to in-person contact due to more rapid access to care and specialists who would otherwise be unavailable at remote locations.5,10 Similarly, veterans who avoid in-person mental health care were more open to remote therapy for many of the reasons listed earlier. Studies suggest that veterans from both rural and urban locations are generally receptive to receiving mental health services via TMH.5,10
Several studies have found that TMH services may have advantages over standard in-person care. These advantages include decreasing transportation costs, travel time, and time missed at work and increasing system coverage area.13 Overall, both veterans and providers reported similar satisfaction between VTC and in-person sessions and, in some cases, prefer VTC interactions due to a sense of “easing into” intense therapies or having a “therapeutic distance” as treatment begins.12
Utility
Previous studies have shown that TMH can be used successfully to provide psychopharmacologic treatment to veterans who have major depressive disorder or schizophrenia, among other psychiatric disorders.5,8,14 Recent studies have focused on the feasibility of providing EBPs via TMH, particularly for the treatment of PTSD.12,15 Studies have shown that TMH services via VTC can be used successfully to provide cognitive behavioral therapy (CBT), cognitive processing therapy (CPT), and prolonged exposure therapy (PE).16-21 In these studies, both PE and CPT delivered via TMH were found to be as efficacious as in-person formats. Furthermore, TMH services were successfully used in individual and group sessions.
Research has emphasized the benefits of TMH for veterans who are uncomfortable in crowds, waiting rooms, or hospital lobbies.7,12,18 For patients with PTSD who are initially limited by fears related to driving, TMH can facilitate access to care. Veterans with PTSD often avoid reminders of trauma (ie, uniforms, evidence of physical injury, artwork, photographs related to war), which can often be found at the larger VAMCs. These veterans may find mental health care services in their homes or at local CBOCs more appealing.7,12,18
Implementation
Prior to the implementation of telehealth services, many CBOC providers would refer veterans in need of specialty care to the nearest VAMC, which were sometimes many hours away.1 In response to travel and access concerns, the VA has implemented various telehealth modalities, including TMH.
In 2008, about 230,000 veterans received mental health services via real-time clinical VTC at 300 VA CBOCs, and about 40,000 veterans enrolled in the In-Home Telehealth program.22 By 2011, > 380,000 veterans used clinic-based telehealth services and about 100,000 veterans used the in-home program.1 Between 2006 and 2008, the 98,000 veterans who used TMH modalities had fewer hospital admissions compared with those who did not; overall, the need for hospital services decreased by about 25% for those using TMH services.23
Although research suggests that TMH is an effective treatment modality, it does have limitations. A recent study noted several visual and audio difficulties that can emerge, including pixilation, “tracer” images with movement, low resolution, “frozen” or “choppy” images, delays in sound, echoes, or “mechanical sounding” voices.12 In some cases, physical details, such as crying, sniffling, or fidgeting, could not be clearly observed.12 Overall, these unforeseen issues can impact the ability to give and receive care through TMH modalities. Proper procedures need to be developed and implemented for each site.
Getting Started
Using TMH to provide mental health care at other VHA facilities requires planning and preparation. Logistics, such as preparation of the room and equipment, should be considered. Similarly, veteran and provider convenience must be considered.2,11 Before starting TMH at any VA facility, professionals working with the audiovisual technology and providing TMH care must complete necessary VA Talent Management System courses and obtain copies of certificates to assure they have met the appropriate training criteria. Providers must be credentialed to provide TMH services, including the telehealth curriculum offered by VA Employee Educational Services.2,24 An appropriate memorandum of understanding (MOU) must be created, and credentialing and privileging must also be acquired.
In addition to provider training, an information technology representative who can administer technical support as needed must be selected for both the provider and remote locations. Technologic complications can make TMH implementation much more challenging.12 As such, it is important to assure that both the veteran and the provider have the necessary TMH equipment. The selected communication device must be compatible with the technology requirements at the provider and remote facilities.12
In addition to designated technical support, the VISN TMH coordinator needs to have point-of-contact information for those who can assist with each site’s telehealth services and address the demand for EBP for PTSD or other desired services. After this information has been obtained, relationships must be developed and maintained with local leadership at each site, associated telehealth coordinators, and evidence-based therapy coordinators.
After contact has been established with remote facilities and the demand for services has been determined, there are several agreements and procedures to put in place before starting TMH services. An initial step is to develop a MOU agreement between the VISN TMH center and remote
sites that allows providers’ credentials and privileges to be shared. Also, it is important to establish a service agreement that outlines the procedures for staff at the remote site. This agreement includes checking in veterans, setting up the TMH rooms, transferring homework to VISN TMH providers, and connecting with the VISN TMH provider. In addition to service agreements, emergency procedures must be in place to ensure the safety of the veterans and the staff.24
After these agreements have been completed, the VISN TMH providers will have to complete request forms to obtain access to the Computerized Patient Record System at the remote facilities, which then must be approved by the Information Security Officer at that site. This is separate from the request at the provider’s site.12 It is essential to have points of contact for questions regarding this process. In order to facilitate referrals for TMH, electronic interfacility consult requests must be developed. Local staff need to collaborate with VISN TMH staff to ensure that the consult addresses the referral facilities need to meet the appropriate requirements.
Before the initiation of TMH services, each TMH provider has to establish clinics for scheduling appointments and obtaining workload credit. Program support assistants at the provider and remote sites must work together to ensure clinics are established correctly. This collaboration is essential for coding of visits and clinic mapping. After the clinics are “built,” appointment times will be set up based on the availability of the provider, support staff, and rooms at the remote site for the TMH session.
Once a consult is initiated, the VISN TMH EBP coordinator will review the consult and the veteran’s chart to ensure initial inclusion/exclusion criteria are met before accepting or canceling the consult. If the consult is accepted, a VISN TMH provider is assigned to the case and contacts the veteran to discuss the referral and (if the veteran is appropriate and interested) initiate services at the closest CBOC or at home. The VISN TMH regional center staff enter the appointment time for the veteran at both facility sites. The VISN TMH provider also coordinates with the CBOC staff to ensure that the veteran is checked in to the appointment and is provided with any questionnaires and necessary homework.
During the first session, the provider obtains consent from the veteran to engage in TMH services, conducts an assessment, and establishes rapport. The provider works with the veteran to develop a treatment plan for PTSD or other mental health diagnosis that will include the type of EBP. At the end of the first session, the next appointment is scheduled, and treatment materials are either mailed to the veteran or given to him or her onsite. After completing EBP, the VISN TMH center works with the referring provider to find follow-up services for the veteran.
The various steps necessary to begin an interfacility TMH clinic are summarized in Table 1.
Provider Training
Despite strong evidence of success, many providers remained skeptical about the efficacy of TMH. One study indicated that several providers in VISN 16 rarely used the established TMH programs because they were not familiar with them and applied TMH only for medication checks and consults.11 This skepticism was present in providers preparing to offer TMH as well as in providers referring veterans for TMH services. However, once providers better understood the TMH programs and had more experience using them, they were significantly more likely to use TMH for initial evaluations and ongoing psychotherapy. For these reasons, proper training and educational opportunities for practicing providers are vital to TMH implementation.9,11
To be proficient, providers need to become familiar with various TMH applications.10 Health care networks implementing TMH must ensure that their providers are well trained and prepared to give and receive proper consultation and support. Providers must also acquire several skills and familiarize themselves with available tools.9 In educating providers on the process and use of TMH, the authors suggest the following steps for TMH application:
- Learn new ways to chart in multiple systems and know how to troubleshoot during connectivity issues.
- Have an established administrative support collaborator at outpatient clinics to fax and exchange veteran homework.12
- The TMH clinic culture must be embedded where the veteran is being served in order to allow for a more realistic therapeutic feel. This type of clinic setting will allow for referrals at the veteran site and the availability to coordinate emergency procedures in the remote clinic.
Clinical Issues
Ongoing clinical issues need to be addressed continuously. Initially, referrals may be plentiful but not always appropriate. It is important to have an understanding with referring providers and remote sites about what constitutes a “good referral” as well as alternate referral options. It is imperative to outline inclusion and exclusion criteria that are clear and concise for referring providers. It is often helpful to revisit these criteria with potential referral sources after initiating services.
With the ability to provide inhome services, it is important to identify specific inclusion/exclusion criteria. Recommendations are based on research and clinical applications for exclusions, which are available on the Office of Technology Services website. These include imminent suicidality or homicidality, serious personality disorder or problematic character traits, acute substance disorders, psychotic disorders, and bipolar disorder. It is important to use sound clinical judgment, because the usual safeguards present in a remote clinic are not available for inhome services. Emergency planning is one of the most important aspects of the in-home TMH health services that are provided. The information for the emergency plan is obtained prior to initiation of services.
Emergency Plans
Each remote clinic that provides services to veterans must have an emergency plan that details procedures, phone numbers, and resources in case of medical and psychological emergencies as well as natural disasters. The VISN TMH provider will need to have a copy of the emergency plan as well as a list of contacts in case of an emergency during a TMH session.
It is recommended that TMH providers have several ways to contact key staff who can assist during an emergency. Usually the clinical coordinator and telehealth technician are the first responders to be alerted by the TMH provider during an emergency. They will then institute the remote clinic’s emergency protocol. Discussing these procedures and reviewing them with staff regularly is advisable, as key contacts may change.
In a psychological emergency, the VISN TMH provider may assist in implementing emergency procedures until a clinical counterpart at the remote site can be alerted. In the authors’ experience, VISN TMH providers have successfully de-escalated and diffused potentially emergent situations by maintaining constant realtime communication with veterans and staff by using VTC as well as interoffice communication. By offering assistance to veterans and staff during challenging situations, the VISN TMH provider will not only decrease concerns of veterans, but oftentimes integrate themselves into the treatment team of the remote clinic. The role of a VISN TMH provider can be isolative, with minimal contact with remote clinic staff, so it is important to increase visibility among staff at a remote site by communication with them even when there is not an emergency.
Treatment protocols may be determined by either administrative or clinical factors. With certain TMH interventions, the rooms used for veterans may be available for only certain periods, which may or may not fit with treatment protocols. For example, if a room is available for only an hour but a treatment protocol session is for 90 minutes, then another time slot needs to be found or a different treatment considered and offered. Although it is not ideal to have treatment protocols determined by scheduling factors, the reality of shared space at remote sites requires flexibility.
Sharing Materials and Homework Another clinical issue that is often overlooked is how to implement specific treatment protocols that entail the exchange of materials between VISN TMH providers and veterans. If materials will need to be exchanged between provider and veteran, a plan will have to be in place to facilitate this. The service agreement addresses these details, but remote staff may not always be aware of the details.
If a TMH provider opts to use faxes to send materials between a veteran and a provider, a desktop faxing program is recommended so veteran privacy is not compromised. Often, providers will wait to begin sessions until after they have received materials, but this may result in a delayed
session. One solution TMH providers can implement is mailing the materials and questionnaires to veterans before the session with clear instructions to complete them beforehand. Once the veteran arrives for the TMH session, she or he will verbally respond to the questionnaire and treatment materials. This will add time to a session but minimizes potential delays. Many of the clinical VTC units have movable cameras, so veterans can tilt the camera to show providers the forms and questionnaires.
The various steps necessary to address TMH clinical issues are summarized in Table 2.
VISIN 22 Pilot Project
The VISN 22 EBP TMH Center and Regional Pilot, based at the VA San Diego Healthcare System, was tasked with developing and providing TMH EBP services for PTSD across VISN 22 and adjacent West Coast VISNs. In addition to creating standardized procedures, troubleshooting guides were established to assist other programs with implementation. The primary focus was to increase access to EBPs for veterans with PTSD in areas where there was either no available trained providers or delays for specific services. The program established 16 clinics as well as in-home
services in VISN 22, VISN 21, and VISN 20. In fiscal year (FY) 2013, the VISN 22 EBP TMH Center and Regional Pilot provided 1,657 EBP encounters via TMH to 234 unique veterans with PTSD (Table 3).
The pilot project collected data to evaluate program effectiveness. The data were de-identified before being sent to the VA Central Office (VACO) TMH program manager. The following items were collected for the pilot: (1) clinical information; (2) consent to engage in treatment and telehealth; (3) release of information to share de-identified data to VACO for program monitoring; (4) demographic form; (5) Beck Depression Inventory-II (every other week); (6) PTSD Checklist (every other week); (7) World Health Organization Quality of Life (sessions 1, 7, final); (8) Wechsler Adult Intelligence Scale-Revised (sessions 3, 7, final); (9) satisfaction survey (final); (10) mileage not driven by veterans who receive TMH services; (11) travel pay saved by VA; (12) no-show rates; and (13) veteran, TMH provider, and referral provider satisfaction.
The growth in number of encounters and number of unique veterans has increased steadily from the first quarter of FY14 through the second quarter of FY15 (Figure 1).
In January 2013, in-home TMH services were piloted. Although occasional technical difficulties occurred, 143 EBP encounters via TMH were provided to 42 unique veterans in 2013. The service has continued to expand, and in the first half of FY14, services were provided to 64 unique veterans for a total of 278 encounters, saving veterans 3,220 travel miles and saving the VA $1,336 in travel reimbursement. In-home TMH services will continue to expand as more providers in a variety of programs are being trained by the San Diego staff on how to provide these services to veterans in their homes. In addition to decreasing mileage and travel pay, the no-show rates are lower for TMH appointments in general (averaged 8%-10% vs facility no-show rate average of 13.5%) and with the use of inhome TMH, no-show rates were kept to 2%. The growth in the number of in-home encounters and the number of unique veterans has also increased steadily from the first quarter of FY14 thru the second quarter of FY15 (Figure 2).
In-Home TMH Services
The VISN 22 EBP TMH Center and Regional Pilot often requests to have an in-person meeting with a veteran before starting TMH services in order to complete a waiver to download the software used by the VA for real-time video in-home services, a Release of Information for a Primary Support Person form, and an emergency plan.
It is also recommended that information about the veteran’s Internet connection, type of computer, type of software, presence of a camera and speakers, e-mail address, and access to secure messaging are obtained. During the initial contact with a veteran, the provider will discuss the rules and requirements to ensure HIPAA compliance. The veteran will need to have a private area for the call (not a restaurant, car, or other place where Wi-Fi is offered). Even with these discussions, some veterans will initiate services from a public place or a room in their home where family members will enter and exit frequently.
Although not required, it is recommended to have the veteran identify a primary support person and complete a release form to allow the TMH provider to contact that person in an emergency. The support person may be a person in the home (adult family member or caregiver) or someone nearby (neighbor, friend, or family member) who can contact emergency services if needed. After the necessary information is gathered and the veteran agrees to the conditions of participation, a test call will be completed. The TMH provider is often the person to conduct this call, but if available, a telehealth technician or facility telehealth coordinator may assist. The TMH provider may help the veteran download the appropriate software that is sent from the VA Scheduler software. The veteran initiates the call with the provider. Once the connection is made, the session may begin. Sites that are currently conducting in-home services have provided guides to veterans and newer TMH providers to outline the necessary steps for initiating services.
It is recommended that any provider interested in providing in-home TMH services use the Office of Technological Services help desk to assist in troubleshooting difficulties with connectivity. Challenges have included the software used for in-home TMH, periodic Internet outages, and compatibility issues.
Veteran Satisfaction
Veteran satisfaction was measured through a self-report satisfaction survey. The survey included 12 questions assessing overall experience in using TMH services. Eleven of the 12 questions included a 5-point Likert scale (1 = strongly disagree, 5 = strongly agree); the last question was openended for additional comments.
A summary of the survey response of the initial 29 veterans who received TMH services suggested the following: (1) Veterans felt comfortable with using the TMH equipment and were able to see their clinician clearly; (2) Technical assistance was sufficient; (3) During the TMH session, they related to the provider as if it were a face-to-face meeting and that their needs were met; and (4) Veterans reported extremely high satisfaction with TMH and would refer TMH care to other veterans. Veterans found clinic locations very convenient and preferred the TMH modality of mental health services delivery to the alternative of travelling a long distance to see their provider (Table 4).
Written comments and recommendations from veterans supported the survey results. Most reported that they saved time and the convenience of the clinic allowed them to receive the treatment they need without interfering with their work schedule. However, some veterans still experienced trouble with travel to the remote clinic. Others felt their experience was different from the one they expected or they had a good experience via TMH but preferred face-to-face care.
Conclusion
The VISN 22 EBP TMH Center and Regional Pilot have established the infrastructure of interfacility clinics to use EBPs for the treatment of PTSD. Also, the center has provided consultation and guidance to facilities interested in developing their own TMH programs. The TMH Center now plans to expand mental health services and include medication management and EBP services for non-PTSD psychiatric diagnoses. The established infrastructure will allow providers from one facility to cover the mental health service needs of other facilities when there are absences or gaps due to leave or delays/challenges in hiring in rural locations. Finally, TMH offers the potential to offer after-hours services to veterans in other time zones during providers’ regular tours of duty.
Several other TMH programs are now expanding services into veterans’ homes. There are several sites within the VHA that have piloted this TMH modality and developed guidelines and recommendations for further expansion. Currently VACO is encouraging all VHA facilities to increase in-home telehealth services, and the Office of Telehealth Services provides details on implementation. Interested parties are encouraged to routinely visit the VACO website for updated information.
Developing and implementing a new TMH program can be an arduous task, but the program has great potential to provide veteran-centered care. As TMH sessions progress, the provider and veteran become less aware of the camera and software and more aware of the therapeutic process. Challenges and delays in implementation are to be expected—these can occur frequently during the development and implementation stages of a TMH program. Maintaining consistent communication with staff at remote sites is essential for the success of any program.
As the VHA focuses on veterancentered care, TMH services will improve access to providers with specific, needed expertise. The authors hope these experiences can facilitate the continued growth of TMH and assuage any concerns a facility or provider may have about this modality of care. Delivery of TMH care can be challenging, but the ability to provide these services to veterans at times and locations convenient to them makes these challenges worthwhile.
Acknowledgments
Dr. Hauser wishes to thank Cathy, Anika, Jirina, Katia, and Max Hauser, and Alba Pillwein for their continued support. In memory of Beverly Ostroski.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. U.S. Department of Veterans Affairs. What is telehealth? U.S. Department of Veterans Affairs Website. http://www.telehealth.va.gov. Update May 13, 2014. Accessed April 30, 2015.
2. Morland LA, Greene CJ, Rosen C, Mauldin PD, Frueh CB. Issues in the design of a randomized noninferiority clinical trial of telemental health psychotherapy for rural combat veterans with PTSD. Contemp Clin Trials. 2009;30(6):513-522.
3. Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, Acierno R. An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behav Ther. 2012;43(3):560-569.
4. Yuen EK, Gros DF, Price M, et al. Randomized controlled trial of home-based telehealth versus in-person prolonged exposure for combat-related PTSD in veterans: preliminary results. J Clin Psychol. 2015;71(6):500-512.
5. Ruskin PE, Reed S, Kumar R, et al. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatr Serv. 1998;49(8):1086-1088.
6. Gros DF, Morland LA, Greene CJ, et al. Delivery of evidence-based psychotherapy via video telehealth. J Psychopathol Behav Assess. 2013;35(4):506-521.
7. Backhaus A, Agha Z, Maglione ML, et al. Videoconferencing psychotherapy: a systematic review. Psychol Serv. 2012;9(2):111-131.
8. Egede LE, Frueh CB, Richardson LK, et al. Rationale and design: telepsychology service delivery for depressed elderly veterans. Trials. 2009;10:22.
9. Frueh BC, Deitsch SE, Santos AB, et al. Procedural and methodological issues in telepsychiatry research and program development. Psychiatr Serv. 2000;51(12):1522-1527.
10. Grubaugh AL, Cain GD, Elhai JD, Patrick SL, Frueh BC. Attitudes toward medical and mental health care delivered via telehealth applications among rural and urban primary care patients. J Nerv Ment Dis. 2008;196(2):166-170.
11. Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes towards telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425-432.
12. Thorp SR, Fidler J, Moreno L, Floto E, Agha Z. Lessons learned from studies of psychotherapy for posttraumatic stress disorder via video teleconferencing. Psychol Serv. 2012;9(2):197-199.
13. Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: predictors of treatment completion and outcome and comparison to treatment delivered in person. Behav Ther. 2011;42(2):276-283.
14. Zarate CA Jr, Weinstock L, Cukor P, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry. 1997;58(1):22-25.
15. Jones AM, Shealy KM, Reid-Quiñones K, et al. Guidelines for establishing a telemental health program to provide evidence-based therapy for trauma-exposed children and families. Psychol Serv. 2014;11(4):398-409.
16. Frueh BC, Monnier J, Grubaugh AL, Elhai JD, Yim E, Knapp R. Therapist adherence and competence with manualized cognitive-behavioral therapy for PTSD delivered via videoconferencing technology. Behav Modif. 2007;31(6):856-866.
17. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469.
18. Tuerk PW, Yoder M, Ruggiero KJ, Gros DF, Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J Trauma Stress. 2010;23(1):116-123.
19. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine- based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
20. Germain V, Marchand A, Bouchard S, Drouin MS, Guay S. Effectiveness of cognitive behavioural therapy administered by videoconference for posttraumatic stress disorder. Cogn Behav Ther. 2009;38(1):42-53.
21. Morland LA, Mackintosh M, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75(5):470-476.
22. Tuerk PW, Fortney J, Bosworth HB, et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16(1):115-117.
23. Godleski L, Darkins A, Peters J. Outcomes of 98,609 U.S. Department of Veterans Affairs patients enrolled in telemental health services, 2006-2010. Psychiatr Serv. 2012;63(4):383-385.
24. Strachan M, Gros DF, Yuen E, Ruggiero KJ, Foa EB, Acierno R. Home-based telehealth to deliver evidence-based psychotherapy in veterans with PTSD. Contemp Clin Trials. 2012;33(2):402-409.
1. U.S. Department of Veterans Affairs. What is telehealth? U.S. Department of Veterans Affairs Website. http://www.telehealth.va.gov. Update May 13, 2014. Accessed April 30, 2015.
2. Morland LA, Greene CJ, Rosen C, Mauldin PD, Frueh CB. Issues in the design of a randomized noninferiority clinical trial of telemental health psychotherapy for rural combat veterans with PTSD. Contemp Clin Trials. 2009;30(6):513-522.
3. Strachan M, Gros DF, Ruggiero KJ, Lejuez CW, Acierno R. An integrated approach to delivering exposure-based treatment for symptoms of PTSD and depression in OIF/OEF veterans: preliminary findings. Behav Ther. 2012;43(3):560-569.
4. Yuen EK, Gros DF, Price M, et al. Randomized controlled trial of home-based telehealth versus in-person prolonged exposure for combat-related PTSD in veterans: preliminary results. J Clin Psychol. 2015;71(6):500-512.
5. Ruskin PE, Reed S, Kumar R, et al. Reliability and acceptability of psychiatric diagnosis via telecommunication and audiovisual technology. Psychiatr Serv. 1998;49(8):1086-1088.
6. Gros DF, Morland LA, Greene CJ, et al. Delivery of evidence-based psychotherapy via video telehealth. J Psychopathol Behav Assess. 2013;35(4):506-521.
7. Backhaus A, Agha Z, Maglione ML, et al. Videoconferencing psychotherapy: a systematic review. Psychol Serv. 2012;9(2):111-131.
8. Egede LE, Frueh CB, Richardson LK, et al. Rationale and design: telepsychology service delivery for depressed elderly veterans. Trials. 2009;10:22.
9. Frueh BC, Deitsch SE, Santos AB, et al. Procedural and methodological issues in telepsychiatry research and program development. Psychiatr Serv. 2000;51(12):1522-1527.
10. Grubaugh AL, Cain GD, Elhai JD, Patrick SL, Frueh BC. Attitudes toward medical and mental health care delivered via telehealth applications among rural and urban primary care patients. J Nerv Ment Dis. 2008;196(2):166-170.
11. Jameson JP, Farmer MS, Head KJ, Fortney J, Teal CR. VA community mental health service providers’ utilization of and attitudes towards telemental health care: the gatekeeper’s perspective. J Rural Health. 2011;27(4):425-432.
12. Thorp SR, Fidler J, Moreno L, Floto E, Agha Z. Lessons learned from studies of psychotherapy for posttraumatic stress disorder via video teleconferencing. Psychol Serv. 2012;9(2):197-199.
13. Gros DF, Yoder M, Tuerk PW, Lozano BE, Acierno R. Exposure therapy for PTSD delivered to veterans via telehealth: predictors of treatment completion and outcome and comparison to treatment delivered in person. Behav Ther. 2011;42(2):276-283.
14. Zarate CA Jr, Weinstock L, Cukor P, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry. 1997;58(1):22-25.
15. Jones AM, Shealy KM, Reid-Quiñones K, et al. Guidelines for establishing a telemental health program to provide evidence-based therapy for trauma-exposed children and families. Psychol Serv. 2014;11(4):398-409.
16. Frueh BC, Monnier J, Grubaugh AL, Elhai JD, Yim E, Knapp R. Therapist adherence and competence with manualized cognitive-behavioral therapy for PTSD delivered via videoconferencing technology. Behav Modif. 2007;31(6):856-866.
17. Morland LA, Hynes AK, Mackintosh MA, Resick PA, Chard KM. Group cognitive processing therapy delivered to veterans via telehealth: a pilot cohort. J Trauma Stress. 2011;24(4):465-469.
18. Tuerk PW, Yoder M, Ruggiero KJ, Gros DF, Acierno R. A pilot study of prolonged exposure therapy for posttraumatic stress disorder delivered via telehealth technology. J Trauma Stress. 2010;23(1):116-123.
19. Fortney JC, Pyne JM, Kimbrell TA, et al. Telemedicine- based collaborative care for posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(1):58-67.
20. Germain V, Marchand A, Bouchard S, Drouin MS, Guay S. Effectiveness of cognitive behavioural therapy administered by videoconference for posttraumatic stress disorder. Cogn Behav Ther. 2009;38(1):42-53.
21. Morland LA, Mackintosh M, Greene CJ, et al. Cognitive processing therapy for posttraumatic stress disorder delivered to rural veterans via telemental health: a randomized noninferiority clinical trial. J Clin Psychiatry. 2014;75(5):470-476.
22. Tuerk PW, Fortney J, Bosworth HB, et al. Toward the development of national telehealth services: the role of Veterans Health Administration and future directions for research. Telemed J E Health. 2010;16(1):115-117.
23. Godleski L, Darkins A, Peters J. Outcomes of 98,609 U.S. Department of Veterans Affairs patients enrolled in telemental health services, 2006-2010. Psychiatr Serv. 2012;63(4):383-385.
24. Strachan M, Gros DF, Yuen E, Ruggiero KJ, Foa EB, Acierno R. Home-based telehealth to deliver evidence-based psychotherapy in veterans with PTSD. Contemp Clin Trials. 2012;33(2):402-409.
Skin infections in athletes: Treating the patient, protecting the team
• Do not permit athletes with wet, weeping lesions to return to play. C
• Familiarize yourself with the rules governing the team sports your patients participate in and use them to guide return-to-play decisions. C
• Explain to athletes with herpes or bacterial infections that they cannot participate while the lesions are active, even if they are covered with occlusive dressings. C
Strength of recommendation (SOR)
A: Good-quality patient-oriented evidence
B: Inconsistent or limited-quality patient-oriented evidence
C: Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Shane O, a 16-year-old on his high school wrestling team, presents with red, scaly, well-defined plaques on his forehead. The coach spotted the lesions and removed him from play. The patient reports that he’s training for a competitive event and is anxious to return to practice.
You take skin scrapings for a potassium chloride (KOH) test, which is negative, and for a dermatophyte culture. The culture will take 7 to 14 days, however. What should you do in the meantime?
Chances are you’ve cared for countless patients with cutaneous infections. But when the individual who’s infected participates in a team sport, it’s a game changer. In addition to treating the infection, it is necessary to take steps to prevent an outbreak among other players.
More than half of the infections incurred by athletes are cutaneous lesions1—not surprising considering the numerous opportunities sports play provides for common skin conditions to spread. Predisposing factors, in addition to skin-to-skin contact and abrasions, include shared close quarters (eg, locker rooms and showers), shared sports equipment, and poor hygiene.2-5
For a competitive athlete like Shane, the loss of practice may be the most worrisome aspect of a cutaneous infection. From a medical perspective, there are far more pressing concerns. Contact with a teammate’s active herpes simplex lesion (HSV-1), for example, could result in a lifelong infection, and a methicillin-resistant Staphylococcus aureus (MRSA) infection can be a source of significant morbidity for anyone who is infected. There is also the potential for a public health hazard. In addition to prompt, accurate diagnosis, a treatment approach that considers both the need to protect others and the importance of getting the player back in the game as soon as it is safe to do so is critical.
Athlete’s foot, ringworm, jock itch—fungal infections are easily spread
Fungal infections are a common cause of skin disease in athletes. Dermatophytes can spread from fomites such as mats, floors, laundry items, and shared clothing, combs, and brushes, as well as in swimming pools and from direct contact with carriers.2
National Collegiate Athletic Association (NCAA) data show that fungi account for 22% of the skin infections that athletes involved in competitive wrestling develop.6 Sports with skin-to-skin contact, such as wrestling, pose the greatest risk. Fungal infection is the No. 1 reason cited for missing wrestling practice, according to the NCAA.6
Fungal infections are not just a wrestler’s problem, however. Tinea pedis (athlete’s foot) is common among runners and swimmers, and tinea corporis (ringworm), in prepubertal athletes.5
Fungal skin infections are caused by 3 dermatophyte genera: Trichophyton, Epidermophyton, and Microsporum. In the United States, T rubrum is responsible for most cutaneous fungal infections, including tinea corporis, tinea unguium (nail), tinea pedis, and tinea cruris (commonly called jock itch).5
Tinea corporis (Figure 1) may present with areas of central hypopigmentation that give it a ring-like appearance. This type of fungal infection also includes tinea gladiatorum, so named for its high prevalence among wrestlers and characterized by well-defined, red scaling plaques on the head, neck, and upper extremities, often with an irregular border.7
Tinea pedis typically develops in the web spaces of the toes, with skin maceration often accompanied by thick scaling or desquamation. Tinea cruris presents as erythematous plaques in the pubic and inguinal areas.8
Onychomycosis can affect the nail plate, resulting in thickening, change in color, and alteration of nail texture.9
T tonsurans is the dominant pathogen for tinea capitis—fungal infection of the scalp,5 which can be inflammatory or noninflammatory. The noninflammatory form may present with gray-patch scaling, seborrheic dermatitis-like scale, hair thinning without significant scaling, or patches of “black-dot” alopecia. Inflammatory forms of tinea capitis may present as anything from localized pustules to widespread abscesses, or may remain in an asymptomatic carrier state.10
When to confirm a clinical diagnosis
Fungal infections can often be diagnosed based on clinical presentation, but confirmation is important when systemic therapy is required—for tinea capitis, in particular. The traditional method is an examination of skin scraped from the edge of a lesion and treated with 10% to 20% KOH, gently heated, and viewed under light microscopy.8
Alternatively, fungal infection can be confirmed retrospectively, by culture on selective media. Dermatophyte test medium (DTM) is convenient and easy to use, and usually reveals fungal growth within 7 days. A newer selective media, DBM (bromothymol blue is the pH indicator) is a modification of the DTM formulation that may offer earlier and more accurate identification of fungi.11
Keep in mind, however, that a negative KOH preparation or culture does not necessarily rule out a fungal infection.12 Polymerase chain reaction (PCR), an emerging technology performed on a specimen swab, has a greater sensitivity than either KOH or culture in identifying fungal pathogens. PCR can identify the presence of fungi even if the dermatophyte growth on culture is hidden by the overgrowth of Candida albicans.13
In patients with onychomycosis, dermatophytes may be exceedingly difficult to isolate on either KOH or culture medium, and the results of these tests often conflict. Onychomycosis is best diagnosed histopathologically by examination of periodic acid-Schiff-stained nail clippings.14
Topical or systemic treatment?
Treatment of dermatophyte infections is site-dependent. For simple epidermal infection, other than scalp or nail, topical therapy is first-line treatment. In a recent Cochrane review, topical allylamines, azoles, butenafine, ciclopirox olamine, tolciclate, and tolnaftate were all found to be effective. However, the allylamines had the greatest efficacy, which increased with duration of use.15
Topical terbinafine was found to be effective in as little as one to 3 days of treat-ment for tinea pedis. Mycological cure with near total symptom elimination at 28 days was reported in 61% and 78% of those receiving topical treatment for one day and 3 days, respectively; the difference was not statistically significant.16
Use this topical when bacterial infection complicates care. Although 1% naftifine gel requires a prescription and costs more than many other topicals, its advantages may offset the higher costs. Once-daily naftifine gel is as effective as other allylamines that require twice-daily application, and has both antihistamine and corticosteroid effects to offset inflammation. What’s more, naftifine is active against both gram-positive and gram-negative bacteria; therefore, it should be considered in instances in which bacterial superinfection is a possibility, as suggested by a high degree of inflammation with bright red and yellow crusts.17,18
Scalp, nail, and complicated foot infections typically require systemic therapy. Griseofulvin is the most widely used systemic treatment for tinea capitis.10 While terbinafine requires a shorter duration of treatment (4-6 weeks) and is similar in efficacy—except in cases of microsporum infection of the scalp, for which griseofulvin has been found to have higher cure rates19—it is often not used because it has a higher cost.
Tinea pedis and onychomycosis often occur concurrently, making eradication difficult and increasing the potential for reinfection. For recalcitrant cases of onychomycosis, a combination of topical and systemic therapy may be required, along with trimming, debridement, nail abrasion, and partial nail avulsion.20 A recent study found laser therapy to be a promising treatment for onychomycosis, but randomized controlled trials have yet to be done.21
NCAA and NFHS rules. Both the NCAA and the National Federation of State
High School Associations (NFHS) mandate that a wrestler with tinea corporis receive a minimum of 72 hours of topical therapy prior to participation; 14 days of systemic antifungal therapy are required for athletes with tinea capitis.22,23 The NCAA allows wrestlers to be cleared to participate on an individual basis, at the discretion of the examining physician or certified trainer.
The degree of disease involvement, the activity of disease as judged by KOH preparation, or the review of therapeutic regimen and the ability to properly cover lesions securely are taken into account.22 Proper coverage could consist of a semiocclusive or occlusive dressing such as film, foam, hydrogel, or hydrocolloid covered with stretch tape.24 Similarly, the NFHS permits a wrestler to participate once the lesion is deemed to be no longer contagious and can be covered with a bio-occlusive dressing.23
Prophylactic oral fluconazole, given in a 3-day regimen twice during the season to all team members, has been shown to be successful in reducing a high burden of tinea gladiatorum in a high school wrestling setting.25
CASE You presumptively diagnose tinea gladiatorum based on both the presentation and the patient’s history as a wrestler and prescribe topical terbinafine therapy twice daily. You schedule a follow-up appointment in 3 days, and tell Shane he must refrain from wrestling practice at least until then.
Staph and strep infections
Bacterial skin infections are also common among athletes, with S aureus reported to be responsible for 22% of infectious disease outbreaks.26 Here, too, infections occur primarily in contact sports such as football, rugby, and soccer, as well as wrestling.
There are several types of bacterial dermatoses: impetigo, folliculitis, furuncles, carbuncles, abscesses, and cellulitis. Most are caused by group A beta-hemolytic Streptococcus or S aureus and can be easily treated, but identification of the pathogen is needed to facilitate healing and a safe return to play (TABLE 1).27
When to suspect CA-MRSA
Community-acquired methicillin-resistant S aureus (CA-MRSA) was first reported in an athletic population in the 1960s, and in 1993 the first documented outbreak of CA-MRSA in a sports setting—involving 6 high school wrestlers from Vermont—was reported.28
The athlete with CA-MRSA usually presents with a painful, purulent, swollen, red abscess-like lesion, sometimes described as (or mistaken for) a spider bite. The patient may also develop fever, fatigue, and malaise. Culturing the wound for identification of the bacterium and for susceptibility to antibiotic therapy is needed for a definitive diagnosis of CA-MRSA.
Is incision and drainage sufficient treatment? The standard treatment for uncomplicated CA-MRSA lesions is incision and drainage. Several studies have found this to be adequate for simple lesions.29 Others have reported increased treatment failure with simple incision and drainage and shown that the addition of antimicrobial therapy helps decrease further tissue damage and morbidity.29
With no clear consensus as to when and whether to add oral antibiotic therapy after incision and drainage of a CA-MRSA lesion, decisions should be based on the severity of the lesion, the presence or absence of systemic symptoms, and the potential risk of bacterial spread to other team members.
Choosing an antimicrobial agent. Antimicrobial treatment should be guided by culture and sensitivity results, as well as the regional incidence of CA-MRSA. Empiric treatments for CA-MRSA are trimethoprim-sulfamethoxazole, doxycycline, and clindamycin, taken for 7 to 14 days. If you’re considering the use of clindamycin and there is known resistance to erythromycin from antimicrobial sensitivities, a double disc diffusion (D-test) should be ordered to detect inducible macrolide resistance that can occur in some strains of CA-MRSA.29,30
Fluoroquinolones and certain macrolides should not be used, due to resistance to these antibiotics.1
Topicals for superficial lesions. Topical antibiotic therapy with mupirocin or retapamulin should be reserved for superficial CA-MRSA lesions, such as impetigo.31,32 Caution must be used when mupirocin is prescribed, however, due to recent studies showing increasing resistance to mupirocin, especially when used for nasal decolonizing purposes.29
Once treatment is initiated, see the athlete every 2 to 3 days. Resolution usually occurs in 10 to 14 days. The athlete can return to play after 72 hours of treatment, however, provided there is evidence of clinical improvement, no further drainage from the infected lesion, and no new lesions have developed.1,30
Containing CA-MRSA. Mass nasal decolonization—applying topical mupirocin in the nares of infected athletes as well as their teammates—has been attempted to prevent the spread of CA-MRSA. But there is no evidence to suggest that mupirocin or any other intranasal antimicrobial is effective in preventing the spread of CA-MRSA, and nasal decolonization should not be attempted in any community setting.29 In fact, studies have found that attempts at nasal decolonization can actually lead to increased bacterial resistance and a recurrence of colonization.29,31,32
HSV-1 is highly prevalent
Herpes simplex virus-1 (HSV-1) is a common problem in athletes who play team sports that involve skin-to-skin contact. It is particularly prevalent among competitive wrestlers—earning it the name herpes gladiatorum.
In fact, HSV-1 is widespread throughout the country: Its prevalence in the general population is 58%.33 It is estimated that nearly half (47%) of cutaneous infections in collegiate athletes are caused by the herpes virus, making HSV-1 the most common pathogen of skin infections in this group.34 The clinical presentation of active HSV-1 depends on whether the infection is primary or recurrent.
So which form of HSV-1 is it?
Primary lesions may be preceded by a prodromal period with systemic symptoms, such as fever. Oral lesions and enlarged cervical and submandibular lymph nodes follow.35 The lesions, which are typically painful, can be found on the lips, buccal mucosa, or tongue—nearly anywhere in and around the oral cavity (Figure 2). They are vesicular at first, then ulcerate. Healing typically takes 10 to 14 days.36
In recurrent HSV-1, clusters of vesicles with erythematous borders typically occur. In those who play contact sports, HSV lesions can be found not only in the typical facial and oral areas, but anywhere on the head, face, torso, or extremities, as well.
Identifying herpetic whitlow. Presenting as a cluster of herpetic vesicles on the hands, fingers, or toes, herpetic whitlow is a common presentation of recurrent HSV-1. Recognition of these lesions is usually adequate for a diagnosis of recurrent infection, but confirmation can be obtained by laboratory or serologic testing.
Testing should be considered when you suspect that a patient has a primary herpes infection, as HSV-1 is a lifelong diagnosis. Viral culture is technique dependent and limited by 50% sensitivity, as cultures can take from 2 to 4 days to grow.35 The Tzanck test (direct microscopic examination of skin scrapings) has fallen out of favor, and PCR is now the gold standard.
Compared with viral culture, PCR has been shown to detect 80% of positive cases.35 However, PCR—using swab specimens taken from the lesions—is expensive and must be sent to a lab for analysis. One study suggests that while less sensitive than PCR, the Tzanck test can still be a reliably sensitive method of diagnosis when done properly, at less cost and with quicker
results.37
Serologic tests of HSV-1 IgM and IgG antibodies are also available. Serum IgG levels remain elevated in patients with previous infections. In primary infections, IgM is most useful as it can detect recent or active infection, but results may be falsely negative for several days after infection.38
Once diagnosed, management of HSV-1 in athletes is based on whether the infection is primary or recurrent. In both cases, oral antivirals are needed. Acyclovir is the gold standard, but famciclovir and valacyclovir have been proven to be equally effective.39 Both the NCAA and NFHS have published guidelines addressing the question of return to play (TABLE 2).22,23
In addition to the management of primary and recurrent infections of HSV-1, it is recommended that athletes and coaches known to be HSV-1 seropositive be treated with prophylactic suppressive therapy.40
The Centers for Disease Control and Prevention recommends acyclovir 400 mg bid or valacyclovir 500 mg/d as prophylaxis for anyone with ≤10 recurrences per year. For those with >10 recurrences annually, valacyclovir 1000 mg/d is recommended for the rest of the season.40 This has been shown to be effective in the reduction of outbreaks among wrestlers after a large outbreak occurred in 2007 in Minnesota,40,41 and NCAA and NFHS guidelines now support prophylactic therapy during competitive season.22,23 Before prescribing it, clinicians need to consider both the benefits of prophylactic antiviral therapy and the risks of promoting HSV-1 resistance.
Focus on prevention and squelching outbreaks
For cutaneous infections, as with so many medical conditions, prevention is paramount. With good preventive practices, many, if not all, of the skin infections common among athletes can be eliminated and outbreaks can be squelched.
Good hygienic practice is the cornerstone of prevention. Patients who participate in team sports should be advised to:
- shower immediately after practice
- refrain from sharing personal equipment like uniforms, towels, razors, and headgear
- launder workout clothes and towels after each use
- immediately cleanse and cover any abrasions that occur during practice.
Athletes should also be advised to ask their trainer or coach to check their skin for lesions on a regular basis. Surveillance should be instituted to prohibit athletes with cutaneous lesions from participating until they are sufficiently treated.
Although the role that environmental contamination plays in transmission of infection is uncertain, it is recommended that all sports equipment, playing surfaces, and locker rooms be disinfected daily with either a freshly made bactericidal (1/100 bleach/water solution)23,24 or an appropriate product. The Environmental Protection Agency provides a list of commercial products that have been proven to prevent the spread of MRSA on such surfaces (http://epa.gov/oppad001/chemregindex.htm) on its Web site.42
CASE When Shane returned 3 days later, the erythema had resolved, and minimal scaling remained. You tell him to continue to use the topical terbinafine twice a day for 10 days. You also show him how to apply a bio-occlusive dressing and clear him for practice, provided the lesions are fully covered.
You also talk to Shane about prevention, recommending that he immediately clean and cover any abrasion or other skin trauma that occurs during practice and suggesting that he ask his trainer to regularly check team members for skin lesions.
At Day 7, the DTM culture is positive, confirming a dermatophyte infection.
CORRESPONDENCE
Nilesh Shah, MD, 20 Olive Street, Suite 201, Akron, OH 44310;
shahn@summahealth.org
ACKNOWLEDGEMENT
The authors would like to thank Tom Bartsokas, MD, for his help with this manuscript.
1. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers Association position statement: skin diseases. J Athletic Training. 2010;45:411-428.
2. Brandi G, Sisti M, Paparini A, et al. Swimming pools and fungi: an environmental epidemiology survey in Italian indoor swimming facilities. Int J Environ Health Res. 2007;17:197-206.
3. Dienst WL Jr, Dightman L, Dworkin MS. Diagnosis, treatment and pinning down skin infections: diagnosis, treatment and prevention in wrestlers. Physician Sportsmed. 2005;25:45-56.
4. Ilkit M, GÜmral R, Saraçli MA, et al. Trichophyton tonsurans scalp carriage among wrestlers in a national competition in Turkey. Mycopathologia. 2011;172:215-222.
5. Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
6. Agel J, Ransone J, Dick R, et al. Descriptive epidemiology of collegiate men’s wrestling injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Training. 2007;42:303-310.
7. Adams BB. Tinea corporis gladiatorum. J Am Acad Dermatol. 2002;47:286-297.
8. Pecci M, Comeau D, Chawla V. Skin conditions in the athlete. Am J Sport Med. 2009;37:406-418.
9. Beaven DW, Brooks SE. Color Atlas of the Nail in Clinical Diagnosis. 2nd ed. London: Mosby-Wolfe; 1994.
10. Gupta AK, Summerbell RC. Tinea capitis. Med Mycol. 2000;38:255-287.
11. Li XF, Shen YN, Chen W, et al. A new medium for diagnosis of dermatophyte infection. Eur J Dermatol. 2009;19:34-37.
12. Akcaglar S, Ener B, Toker SC, et al. A comparative study of dermatophyte infections in Bursa Turkey. Med Mycol. 2011;49:602-607.
13. Garg J, Tilak R, Garg A, et al. Rapid detection of dermatophytes from skin and hair. BMC Res Notes. 2009;18:60.
14. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
15. Crawford F, Hollis S. Topical treatments for fungal infections of the ski and nails of the foot. Cochrane Database Syst Rev. 2007;(3):CD001434.
16. Evans EGV, Seaman RAJ, James IGV. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol. 1994;130:83-88.
17. Gupta AK, Ryder JE, Cooper EA. Naftifine: a review. J Cutan Med Surg. 2008;12:51-58.
18. Friedrich M. Inflammatory tinea pedis with bacterial superinfection effectively treated with isoconazole nitrate and diflucortolone valerate combination therapy. Mycoses. 2013;56(suppl 1):S23-S25.
19. González U, Seaton T, Bergus G, et al. Systemic antifungal therapy for tinea capitis in children. Cochrane Database Syst Rev. 2007;(4):CD004685.
20. Baran R, Hay RJ, Garduno JI. Review of antifungal therapy, part II: treatment rationale, including specific patient populations. J Dermatol Treat. 2008;19:168-175.
21. Zhang RN, Wang DK, Zhuo FL, et al. Long-pulse Nd:YAG 1064- nm laser treatment for onychomycosis. Chin Med J (Engl). 2012;125:3288-3291.
22. Guideline 2j: Skin infections in athletics. In: National Collegiate Athletic Association Sports Medicine Handbook. 22nd ed. Indianapolis, IN: National Collegiate Athletic Association; 2011:63.
23. National Federation of State High School Associations, Sports Medicine Advisory Committee. General guidelines for sports hygiene, skin infections and communicable diseases. Revised October 2012. Available at: http://www.nfhs.org/search. aspx?searchtext=skin%20infection. Accessed May 15, 2013.
24. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers’ Association position statement: skin diseases. J Athl Training. 2010;45:411-428.
25. Brickman K, Einstein E, Sinha S, et al. Fluconazole as a prophylactic measure for tinea gladiatorum in high school wrestlers. Clin J Sport Med. 2009;19:412-414.
26. Turbeville S, Cowan L, Greenfield R. Infectious disease outbreaks in competitive sports. Am J Sports Med. 2006;34:1860-1865.
27. Sedgwick P, Dexter W, Smith C. Bacterial dermatoses in sports. Clin Sports Med. 2007;26:383-396.
28. Patel A, Fischer S, Calfee R, et al. Locker room acquired methicillin-resistance Staphylococcus aureus. Orthopedics. 2007;30:532-535.
29. David M, Daum R. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616-687.
30. Benjamin H, Nikore V, Takagishi J. Practical management: community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA): the latest sports epidemic. Clin J Sport Med. 2007;17:393-397.
31. Rhin JA, Posfay-Barbe K, Harner CD, et al. Community-acquired methicillin-resistant Staphylococcus aureus outbreak in a local high school football team unsuccessful interventions. Pediatr Infect Dis J. 2005;24:841-843.
32. Loeb M, Main C, Walker-Dilks C, et al. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;(4):CD003340.
33. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964-973.
34. Yard EE, Collins CL, Dick RW, et al. An epidemiologic comparison of high school and college wrestling injuries. Am J Sports Med. 2008;36:57-64.
35. Usatine RP, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
36. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
37. Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541-555.
38. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9:86-90.
39. Ozcan A, Senol M, Saglam H, et al. Comparison of the Tzanck test and polymerase chain reaction in the diagnosis of cutaneous herpes simplex and varicella zoster virus infections. Int J Dermatol. 2007;46:1177-1179.
40. Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clin Microbiol Infect. 2006;12:463-469.
41. Anderson BJ. Managing herpes gladiatorum outbreaks in competitive wrestling: the 2007 Minnesota experience. Curr Sports Med Rep. 2008;7:323-327.
42. Environmental Protection Agency. EPA’s registered sterilizers, tuberculocides, and antimicrobial products against certain human public health bacteria and viruses. October 2012. Available at: http://epa.gov/oppad001/chemregindex.htm. Accessed November 13, 2012.
• Do not permit athletes with wet, weeping lesions to return to play. C
• Familiarize yourself with the rules governing the team sports your patients participate in and use them to guide return-to-play decisions. C
• Explain to athletes with herpes or bacterial infections that they cannot participate while the lesions are active, even if they are covered with occlusive dressings. C
Strength of recommendation (SOR)
A: Good-quality patient-oriented evidence
B: Inconsistent or limited-quality patient-oriented evidence
C: Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Shane O, a 16-year-old on his high school wrestling team, presents with red, scaly, well-defined plaques on his forehead. The coach spotted the lesions and removed him from play. The patient reports that he’s training for a competitive event and is anxious to return to practice.
You take skin scrapings for a potassium chloride (KOH) test, which is negative, and for a dermatophyte culture. The culture will take 7 to 14 days, however. What should you do in the meantime?
Chances are you’ve cared for countless patients with cutaneous infections. But when the individual who’s infected participates in a team sport, it’s a game changer. In addition to treating the infection, it is necessary to take steps to prevent an outbreak among other players.
More than half of the infections incurred by athletes are cutaneous lesions1—not surprising considering the numerous opportunities sports play provides for common skin conditions to spread. Predisposing factors, in addition to skin-to-skin contact and abrasions, include shared close quarters (eg, locker rooms and showers), shared sports equipment, and poor hygiene.2-5
For a competitive athlete like Shane, the loss of practice may be the most worrisome aspect of a cutaneous infection. From a medical perspective, there are far more pressing concerns. Contact with a teammate’s active herpes simplex lesion (HSV-1), for example, could result in a lifelong infection, and a methicillin-resistant Staphylococcus aureus (MRSA) infection can be a source of significant morbidity for anyone who is infected. There is also the potential for a public health hazard. In addition to prompt, accurate diagnosis, a treatment approach that considers both the need to protect others and the importance of getting the player back in the game as soon as it is safe to do so is critical.
Athlete’s foot, ringworm, jock itch—fungal infections are easily spread
Fungal infections are a common cause of skin disease in athletes. Dermatophytes can spread from fomites such as mats, floors, laundry items, and shared clothing, combs, and brushes, as well as in swimming pools and from direct contact with carriers.2
National Collegiate Athletic Association (NCAA) data show that fungi account for 22% of the skin infections that athletes involved in competitive wrestling develop.6 Sports with skin-to-skin contact, such as wrestling, pose the greatest risk. Fungal infection is the No. 1 reason cited for missing wrestling practice, according to the NCAA.6
Fungal infections are not just a wrestler’s problem, however. Tinea pedis (athlete’s foot) is common among runners and swimmers, and tinea corporis (ringworm), in prepubertal athletes.5
Fungal skin infections are caused by 3 dermatophyte genera: Trichophyton, Epidermophyton, and Microsporum. In the United States, T rubrum is responsible for most cutaneous fungal infections, including tinea corporis, tinea unguium (nail), tinea pedis, and tinea cruris (commonly called jock itch).5
Tinea corporis (Figure 1) may present with areas of central hypopigmentation that give it a ring-like appearance. This type of fungal infection also includes tinea gladiatorum, so named for its high prevalence among wrestlers and characterized by well-defined, red scaling plaques on the head, neck, and upper extremities, often with an irregular border.7
Tinea pedis typically develops in the web spaces of the toes, with skin maceration often accompanied by thick scaling or desquamation. Tinea cruris presents as erythematous plaques in the pubic and inguinal areas.8
Onychomycosis can affect the nail plate, resulting in thickening, change in color, and alteration of nail texture.9
T tonsurans is the dominant pathogen for tinea capitis—fungal infection of the scalp,5 which can be inflammatory or noninflammatory. The noninflammatory form may present with gray-patch scaling, seborrheic dermatitis-like scale, hair thinning without significant scaling, or patches of “black-dot” alopecia. Inflammatory forms of tinea capitis may present as anything from localized pustules to widespread abscesses, or may remain in an asymptomatic carrier state.10
When to confirm a clinical diagnosis
Fungal infections can often be diagnosed based on clinical presentation, but confirmation is important when systemic therapy is required—for tinea capitis, in particular. The traditional method is an examination of skin scraped from the edge of a lesion and treated with 10% to 20% KOH, gently heated, and viewed under light microscopy.8
Alternatively, fungal infection can be confirmed retrospectively, by culture on selective media. Dermatophyte test medium (DTM) is convenient and easy to use, and usually reveals fungal growth within 7 days. A newer selective media, DBM (bromothymol blue is the pH indicator) is a modification of the DTM formulation that may offer earlier and more accurate identification of fungi.11
Keep in mind, however, that a negative KOH preparation or culture does not necessarily rule out a fungal infection.12 Polymerase chain reaction (PCR), an emerging technology performed on a specimen swab, has a greater sensitivity than either KOH or culture in identifying fungal pathogens. PCR can identify the presence of fungi even if the dermatophyte growth on culture is hidden by the overgrowth of Candida albicans.13
In patients with onychomycosis, dermatophytes may be exceedingly difficult to isolate on either KOH or culture medium, and the results of these tests often conflict. Onychomycosis is best diagnosed histopathologically by examination of periodic acid-Schiff-stained nail clippings.14
Topical or systemic treatment?
Treatment of dermatophyte infections is site-dependent. For simple epidermal infection, other than scalp or nail, topical therapy is first-line treatment. In a recent Cochrane review, topical allylamines, azoles, butenafine, ciclopirox olamine, tolciclate, and tolnaftate were all found to be effective. However, the allylamines had the greatest efficacy, which increased with duration of use.15
Topical terbinafine was found to be effective in as little as one to 3 days of treat-ment for tinea pedis. Mycological cure with near total symptom elimination at 28 days was reported in 61% and 78% of those receiving topical treatment for one day and 3 days, respectively; the difference was not statistically significant.16
Use this topical when bacterial infection complicates care. Although 1% naftifine gel requires a prescription and costs more than many other topicals, its advantages may offset the higher costs. Once-daily naftifine gel is as effective as other allylamines that require twice-daily application, and has both antihistamine and corticosteroid effects to offset inflammation. What’s more, naftifine is active against both gram-positive and gram-negative bacteria; therefore, it should be considered in instances in which bacterial superinfection is a possibility, as suggested by a high degree of inflammation with bright red and yellow crusts.17,18
Scalp, nail, and complicated foot infections typically require systemic therapy. Griseofulvin is the most widely used systemic treatment for tinea capitis.10 While terbinafine requires a shorter duration of treatment (4-6 weeks) and is similar in efficacy—except in cases of microsporum infection of the scalp, for which griseofulvin has been found to have higher cure rates19—it is often not used because it has a higher cost.
Tinea pedis and onychomycosis often occur concurrently, making eradication difficult and increasing the potential for reinfection. For recalcitrant cases of onychomycosis, a combination of topical and systemic therapy may be required, along with trimming, debridement, nail abrasion, and partial nail avulsion.20 A recent study found laser therapy to be a promising treatment for onychomycosis, but randomized controlled trials have yet to be done.21
NCAA and NFHS rules. Both the NCAA and the National Federation of State
High School Associations (NFHS) mandate that a wrestler with tinea corporis receive a minimum of 72 hours of topical therapy prior to participation; 14 days of systemic antifungal therapy are required for athletes with tinea capitis.22,23 The NCAA allows wrestlers to be cleared to participate on an individual basis, at the discretion of the examining physician or certified trainer.
The degree of disease involvement, the activity of disease as judged by KOH preparation, or the review of therapeutic regimen and the ability to properly cover lesions securely are taken into account.22 Proper coverage could consist of a semiocclusive or occlusive dressing such as film, foam, hydrogel, or hydrocolloid covered with stretch tape.24 Similarly, the NFHS permits a wrestler to participate once the lesion is deemed to be no longer contagious and can be covered with a bio-occlusive dressing.23
Prophylactic oral fluconazole, given in a 3-day regimen twice during the season to all team members, has been shown to be successful in reducing a high burden of tinea gladiatorum in a high school wrestling setting.25
CASE You presumptively diagnose tinea gladiatorum based on both the presentation and the patient’s history as a wrestler and prescribe topical terbinafine therapy twice daily. You schedule a follow-up appointment in 3 days, and tell Shane he must refrain from wrestling practice at least until then.
Staph and strep infections
Bacterial skin infections are also common among athletes, with S aureus reported to be responsible for 22% of infectious disease outbreaks.26 Here, too, infections occur primarily in contact sports such as football, rugby, and soccer, as well as wrestling.
There are several types of bacterial dermatoses: impetigo, folliculitis, furuncles, carbuncles, abscesses, and cellulitis. Most are caused by group A beta-hemolytic Streptococcus or S aureus and can be easily treated, but identification of the pathogen is needed to facilitate healing and a safe return to play (TABLE 1).27
When to suspect CA-MRSA
Community-acquired methicillin-resistant S aureus (CA-MRSA) was first reported in an athletic population in the 1960s, and in 1993 the first documented outbreak of CA-MRSA in a sports setting—involving 6 high school wrestlers from Vermont—was reported.28
The athlete with CA-MRSA usually presents with a painful, purulent, swollen, red abscess-like lesion, sometimes described as (or mistaken for) a spider bite. The patient may also develop fever, fatigue, and malaise. Culturing the wound for identification of the bacterium and for susceptibility to antibiotic therapy is needed for a definitive diagnosis of CA-MRSA.
Is incision and drainage sufficient treatment? The standard treatment for uncomplicated CA-MRSA lesions is incision and drainage. Several studies have found this to be adequate for simple lesions.29 Others have reported increased treatment failure with simple incision and drainage and shown that the addition of antimicrobial therapy helps decrease further tissue damage and morbidity.29
With no clear consensus as to when and whether to add oral antibiotic therapy after incision and drainage of a CA-MRSA lesion, decisions should be based on the severity of the lesion, the presence or absence of systemic symptoms, and the potential risk of bacterial spread to other team members.
Choosing an antimicrobial agent. Antimicrobial treatment should be guided by culture and sensitivity results, as well as the regional incidence of CA-MRSA. Empiric treatments for CA-MRSA are trimethoprim-sulfamethoxazole, doxycycline, and clindamycin, taken for 7 to 14 days. If you’re considering the use of clindamycin and there is known resistance to erythromycin from antimicrobial sensitivities, a double disc diffusion (D-test) should be ordered to detect inducible macrolide resistance that can occur in some strains of CA-MRSA.29,30
Fluoroquinolones and certain macrolides should not be used, due to resistance to these antibiotics.1
Topicals for superficial lesions. Topical antibiotic therapy with mupirocin or retapamulin should be reserved for superficial CA-MRSA lesions, such as impetigo.31,32 Caution must be used when mupirocin is prescribed, however, due to recent studies showing increasing resistance to mupirocin, especially when used for nasal decolonizing purposes.29
Once treatment is initiated, see the athlete every 2 to 3 days. Resolution usually occurs in 10 to 14 days. The athlete can return to play after 72 hours of treatment, however, provided there is evidence of clinical improvement, no further drainage from the infected lesion, and no new lesions have developed.1,30
Containing CA-MRSA. Mass nasal decolonization—applying topical mupirocin in the nares of infected athletes as well as their teammates—has been attempted to prevent the spread of CA-MRSA. But there is no evidence to suggest that mupirocin or any other intranasal antimicrobial is effective in preventing the spread of CA-MRSA, and nasal decolonization should not be attempted in any community setting.29 In fact, studies have found that attempts at nasal decolonization can actually lead to increased bacterial resistance and a recurrence of colonization.29,31,32
HSV-1 is highly prevalent
Herpes simplex virus-1 (HSV-1) is a common problem in athletes who play team sports that involve skin-to-skin contact. It is particularly prevalent among competitive wrestlers—earning it the name herpes gladiatorum.
In fact, HSV-1 is widespread throughout the country: Its prevalence in the general population is 58%.33 It is estimated that nearly half (47%) of cutaneous infections in collegiate athletes are caused by the herpes virus, making HSV-1 the most common pathogen of skin infections in this group.34 The clinical presentation of active HSV-1 depends on whether the infection is primary or recurrent.
So which form of HSV-1 is it?
Primary lesions may be preceded by a prodromal period with systemic symptoms, such as fever. Oral lesions and enlarged cervical and submandibular lymph nodes follow.35 The lesions, which are typically painful, can be found on the lips, buccal mucosa, or tongue—nearly anywhere in and around the oral cavity (Figure 2). They are vesicular at first, then ulcerate. Healing typically takes 10 to 14 days.36
In recurrent HSV-1, clusters of vesicles with erythematous borders typically occur. In those who play contact sports, HSV lesions can be found not only in the typical facial and oral areas, but anywhere on the head, face, torso, or extremities, as well.
Identifying herpetic whitlow. Presenting as a cluster of herpetic vesicles on the hands, fingers, or toes, herpetic whitlow is a common presentation of recurrent HSV-1. Recognition of these lesions is usually adequate for a diagnosis of recurrent infection, but confirmation can be obtained by laboratory or serologic testing.
Testing should be considered when you suspect that a patient has a primary herpes infection, as HSV-1 is a lifelong diagnosis. Viral culture is technique dependent and limited by 50% sensitivity, as cultures can take from 2 to 4 days to grow.35 The Tzanck test (direct microscopic examination of skin scrapings) has fallen out of favor, and PCR is now the gold standard.
Compared with viral culture, PCR has been shown to detect 80% of positive cases.35 However, PCR—using swab specimens taken from the lesions—is expensive and must be sent to a lab for analysis. One study suggests that while less sensitive than PCR, the Tzanck test can still be a reliably sensitive method of diagnosis when done properly, at less cost and with quicker
results.37
Serologic tests of HSV-1 IgM and IgG antibodies are also available. Serum IgG levels remain elevated in patients with previous infections. In primary infections, IgM is most useful as it can detect recent or active infection, but results may be falsely negative for several days after infection.38
Once diagnosed, management of HSV-1 in athletes is based on whether the infection is primary or recurrent. In both cases, oral antivirals are needed. Acyclovir is the gold standard, but famciclovir and valacyclovir have been proven to be equally effective.39 Both the NCAA and NFHS have published guidelines addressing the question of return to play (TABLE 2).22,23
In addition to the management of primary and recurrent infections of HSV-1, it is recommended that athletes and coaches known to be HSV-1 seropositive be treated with prophylactic suppressive therapy.40
The Centers for Disease Control and Prevention recommends acyclovir 400 mg bid or valacyclovir 500 mg/d as prophylaxis for anyone with ≤10 recurrences per year. For those with >10 recurrences annually, valacyclovir 1000 mg/d is recommended for the rest of the season.40 This has been shown to be effective in the reduction of outbreaks among wrestlers after a large outbreak occurred in 2007 in Minnesota,40,41 and NCAA and NFHS guidelines now support prophylactic therapy during competitive season.22,23 Before prescribing it, clinicians need to consider both the benefits of prophylactic antiviral therapy and the risks of promoting HSV-1 resistance.
Focus on prevention and squelching outbreaks
For cutaneous infections, as with so many medical conditions, prevention is paramount. With good preventive practices, many, if not all, of the skin infections common among athletes can be eliminated and outbreaks can be squelched.
Good hygienic practice is the cornerstone of prevention. Patients who participate in team sports should be advised to:
- shower immediately after practice
- refrain from sharing personal equipment like uniforms, towels, razors, and headgear
- launder workout clothes and towels after each use
- immediately cleanse and cover any abrasions that occur during practice.
Athletes should also be advised to ask their trainer or coach to check their skin for lesions on a regular basis. Surveillance should be instituted to prohibit athletes with cutaneous lesions from participating until they are sufficiently treated.
Although the role that environmental contamination plays in transmission of infection is uncertain, it is recommended that all sports equipment, playing surfaces, and locker rooms be disinfected daily with either a freshly made bactericidal (1/100 bleach/water solution)23,24 or an appropriate product. The Environmental Protection Agency provides a list of commercial products that have been proven to prevent the spread of MRSA on such surfaces (http://epa.gov/oppad001/chemregindex.htm) on its Web site.42
CASE When Shane returned 3 days later, the erythema had resolved, and minimal scaling remained. You tell him to continue to use the topical terbinafine twice a day for 10 days. You also show him how to apply a bio-occlusive dressing and clear him for practice, provided the lesions are fully covered.
You also talk to Shane about prevention, recommending that he immediately clean and cover any abrasion or other skin trauma that occurs during practice and suggesting that he ask his trainer to regularly check team members for skin lesions.
At Day 7, the DTM culture is positive, confirming a dermatophyte infection.
CORRESPONDENCE
Nilesh Shah, MD, 20 Olive Street, Suite 201, Akron, OH 44310;
shahn@summahealth.org
ACKNOWLEDGEMENT
The authors would like to thank Tom Bartsokas, MD, for his help with this manuscript.
• Do not permit athletes with wet, weeping lesions to return to play. C
• Familiarize yourself with the rules governing the team sports your patients participate in and use them to guide return-to-play decisions. C
• Explain to athletes with herpes or bacterial infections that they cannot participate while the lesions are active, even if they are covered with occlusive dressings. C
Strength of recommendation (SOR)
A: Good-quality patient-oriented evidence
B: Inconsistent or limited-quality patient-oriented evidence
C: Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Shane O, a 16-year-old on his high school wrestling team, presents with red, scaly, well-defined plaques on his forehead. The coach spotted the lesions and removed him from play. The patient reports that he’s training for a competitive event and is anxious to return to practice.
You take skin scrapings for a potassium chloride (KOH) test, which is negative, and for a dermatophyte culture. The culture will take 7 to 14 days, however. What should you do in the meantime?
Chances are you’ve cared for countless patients with cutaneous infections. But when the individual who’s infected participates in a team sport, it’s a game changer. In addition to treating the infection, it is necessary to take steps to prevent an outbreak among other players.
More than half of the infections incurred by athletes are cutaneous lesions1—not surprising considering the numerous opportunities sports play provides for common skin conditions to spread. Predisposing factors, in addition to skin-to-skin contact and abrasions, include shared close quarters (eg, locker rooms and showers), shared sports equipment, and poor hygiene.2-5
For a competitive athlete like Shane, the loss of practice may be the most worrisome aspect of a cutaneous infection. From a medical perspective, there are far more pressing concerns. Contact with a teammate’s active herpes simplex lesion (HSV-1), for example, could result in a lifelong infection, and a methicillin-resistant Staphylococcus aureus (MRSA) infection can be a source of significant morbidity for anyone who is infected. There is also the potential for a public health hazard. In addition to prompt, accurate diagnosis, a treatment approach that considers both the need to protect others and the importance of getting the player back in the game as soon as it is safe to do so is critical.
Athlete’s foot, ringworm, jock itch—fungal infections are easily spread
Fungal infections are a common cause of skin disease in athletes. Dermatophytes can spread from fomites such as mats, floors, laundry items, and shared clothing, combs, and brushes, as well as in swimming pools and from direct contact with carriers.2
National Collegiate Athletic Association (NCAA) data show that fungi account for 22% of the skin infections that athletes involved in competitive wrestling develop.6 Sports with skin-to-skin contact, such as wrestling, pose the greatest risk. Fungal infection is the No. 1 reason cited for missing wrestling practice, according to the NCAA.6
Fungal infections are not just a wrestler’s problem, however. Tinea pedis (athlete’s foot) is common among runners and swimmers, and tinea corporis (ringworm), in prepubertal athletes.5
Fungal skin infections are caused by 3 dermatophyte genera: Trichophyton, Epidermophyton, and Microsporum. In the United States, T rubrum is responsible for most cutaneous fungal infections, including tinea corporis, tinea unguium (nail), tinea pedis, and tinea cruris (commonly called jock itch).5
Tinea corporis (Figure 1) may present with areas of central hypopigmentation that give it a ring-like appearance. This type of fungal infection also includes tinea gladiatorum, so named for its high prevalence among wrestlers and characterized by well-defined, red scaling plaques on the head, neck, and upper extremities, often with an irregular border.7
Tinea pedis typically develops in the web spaces of the toes, with skin maceration often accompanied by thick scaling or desquamation. Tinea cruris presents as erythematous plaques in the pubic and inguinal areas.8
Onychomycosis can affect the nail plate, resulting in thickening, change in color, and alteration of nail texture.9
T tonsurans is the dominant pathogen for tinea capitis—fungal infection of the scalp,5 which can be inflammatory or noninflammatory. The noninflammatory form may present with gray-patch scaling, seborrheic dermatitis-like scale, hair thinning without significant scaling, or patches of “black-dot” alopecia. Inflammatory forms of tinea capitis may present as anything from localized pustules to widespread abscesses, or may remain in an asymptomatic carrier state.10
When to confirm a clinical diagnosis
Fungal infections can often be diagnosed based on clinical presentation, but confirmation is important when systemic therapy is required—for tinea capitis, in particular. The traditional method is an examination of skin scraped from the edge of a lesion and treated with 10% to 20% KOH, gently heated, and viewed under light microscopy.8
Alternatively, fungal infection can be confirmed retrospectively, by culture on selective media. Dermatophyte test medium (DTM) is convenient and easy to use, and usually reveals fungal growth within 7 days. A newer selective media, DBM (bromothymol blue is the pH indicator) is a modification of the DTM formulation that may offer earlier and more accurate identification of fungi.11
Keep in mind, however, that a negative KOH preparation or culture does not necessarily rule out a fungal infection.12 Polymerase chain reaction (PCR), an emerging technology performed on a specimen swab, has a greater sensitivity than either KOH or culture in identifying fungal pathogens. PCR can identify the presence of fungi even if the dermatophyte growth on culture is hidden by the overgrowth of Candida albicans.13
In patients with onychomycosis, dermatophytes may be exceedingly difficult to isolate on either KOH or culture medium, and the results of these tests often conflict. Onychomycosis is best diagnosed histopathologically by examination of periodic acid-Schiff-stained nail clippings.14
Topical or systemic treatment?
Treatment of dermatophyte infections is site-dependent. For simple epidermal infection, other than scalp or nail, topical therapy is first-line treatment. In a recent Cochrane review, topical allylamines, azoles, butenafine, ciclopirox olamine, tolciclate, and tolnaftate were all found to be effective. However, the allylamines had the greatest efficacy, which increased with duration of use.15
Topical terbinafine was found to be effective in as little as one to 3 days of treat-ment for tinea pedis. Mycological cure with near total symptom elimination at 28 days was reported in 61% and 78% of those receiving topical treatment for one day and 3 days, respectively; the difference was not statistically significant.16
Use this topical when bacterial infection complicates care. Although 1% naftifine gel requires a prescription and costs more than many other topicals, its advantages may offset the higher costs. Once-daily naftifine gel is as effective as other allylamines that require twice-daily application, and has both antihistamine and corticosteroid effects to offset inflammation. What’s more, naftifine is active against both gram-positive and gram-negative bacteria; therefore, it should be considered in instances in which bacterial superinfection is a possibility, as suggested by a high degree of inflammation with bright red and yellow crusts.17,18
Scalp, nail, and complicated foot infections typically require systemic therapy. Griseofulvin is the most widely used systemic treatment for tinea capitis.10 While terbinafine requires a shorter duration of treatment (4-6 weeks) and is similar in efficacy—except in cases of microsporum infection of the scalp, for which griseofulvin has been found to have higher cure rates19—it is often not used because it has a higher cost.
Tinea pedis and onychomycosis often occur concurrently, making eradication difficult and increasing the potential for reinfection. For recalcitrant cases of onychomycosis, a combination of topical and systemic therapy may be required, along with trimming, debridement, nail abrasion, and partial nail avulsion.20 A recent study found laser therapy to be a promising treatment for onychomycosis, but randomized controlled trials have yet to be done.21
NCAA and NFHS rules. Both the NCAA and the National Federation of State
High School Associations (NFHS) mandate that a wrestler with tinea corporis receive a minimum of 72 hours of topical therapy prior to participation; 14 days of systemic antifungal therapy are required for athletes with tinea capitis.22,23 The NCAA allows wrestlers to be cleared to participate on an individual basis, at the discretion of the examining physician or certified trainer.
The degree of disease involvement, the activity of disease as judged by KOH preparation, or the review of therapeutic regimen and the ability to properly cover lesions securely are taken into account.22 Proper coverage could consist of a semiocclusive or occlusive dressing such as film, foam, hydrogel, or hydrocolloid covered with stretch tape.24 Similarly, the NFHS permits a wrestler to participate once the lesion is deemed to be no longer contagious and can be covered with a bio-occlusive dressing.23
Prophylactic oral fluconazole, given in a 3-day regimen twice during the season to all team members, has been shown to be successful in reducing a high burden of tinea gladiatorum in a high school wrestling setting.25
CASE You presumptively diagnose tinea gladiatorum based on both the presentation and the patient’s history as a wrestler and prescribe topical terbinafine therapy twice daily. You schedule a follow-up appointment in 3 days, and tell Shane he must refrain from wrestling practice at least until then.
Staph and strep infections
Bacterial skin infections are also common among athletes, with S aureus reported to be responsible for 22% of infectious disease outbreaks.26 Here, too, infections occur primarily in contact sports such as football, rugby, and soccer, as well as wrestling.
There are several types of bacterial dermatoses: impetigo, folliculitis, furuncles, carbuncles, abscesses, and cellulitis. Most are caused by group A beta-hemolytic Streptococcus or S aureus and can be easily treated, but identification of the pathogen is needed to facilitate healing and a safe return to play (TABLE 1).27
When to suspect CA-MRSA
Community-acquired methicillin-resistant S aureus (CA-MRSA) was first reported in an athletic population in the 1960s, and in 1993 the first documented outbreak of CA-MRSA in a sports setting—involving 6 high school wrestlers from Vermont—was reported.28
The athlete with CA-MRSA usually presents with a painful, purulent, swollen, red abscess-like lesion, sometimes described as (or mistaken for) a spider bite. The patient may also develop fever, fatigue, and malaise. Culturing the wound for identification of the bacterium and for susceptibility to antibiotic therapy is needed for a definitive diagnosis of CA-MRSA.
Is incision and drainage sufficient treatment? The standard treatment for uncomplicated CA-MRSA lesions is incision and drainage. Several studies have found this to be adequate for simple lesions.29 Others have reported increased treatment failure with simple incision and drainage and shown that the addition of antimicrobial therapy helps decrease further tissue damage and morbidity.29
With no clear consensus as to when and whether to add oral antibiotic therapy after incision and drainage of a CA-MRSA lesion, decisions should be based on the severity of the lesion, the presence or absence of systemic symptoms, and the potential risk of bacterial spread to other team members.
Choosing an antimicrobial agent. Antimicrobial treatment should be guided by culture and sensitivity results, as well as the regional incidence of CA-MRSA. Empiric treatments for CA-MRSA are trimethoprim-sulfamethoxazole, doxycycline, and clindamycin, taken for 7 to 14 days. If you’re considering the use of clindamycin and there is known resistance to erythromycin from antimicrobial sensitivities, a double disc diffusion (D-test) should be ordered to detect inducible macrolide resistance that can occur in some strains of CA-MRSA.29,30
Fluoroquinolones and certain macrolides should not be used, due to resistance to these antibiotics.1
Topicals for superficial lesions. Topical antibiotic therapy with mupirocin or retapamulin should be reserved for superficial CA-MRSA lesions, such as impetigo.31,32 Caution must be used when mupirocin is prescribed, however, due to recent studies showing increasing resistance to mupirocin, especially when used for nasal decolonizing purposes.29
Once treatment is initiated, see the athlete every 2 to 3 days. Resolution usually occurs in 10 to 14 days. The athlete can return to play after 72 hours of treatment, however, provided there is evidence of clinical improvement, no further drainage from the infected lesion, and no new lesions have developed.1,30
Containing CA-MRSA. Mass nasal decolonization—applying topical mupirocin in the nares of infected athletes as well as their teammates—has been attempted to prevent the spread of CA-MRSA. But there is no evidence to suggest that mupirocin or any other intranasal antimicrobial is effective in preventing the spread of CA-MRSA, and nasal decolonization should not be attempted in any community setting.29 In fact, studies have found that attempts at nasal decolonization can actually lead to increased bacterial resistance and a recurrence of colonization.29,31,32
HSV-1 is highly prevalent
Herpes simplex virus-1 (HSV-1) is a common problem in athletes who play team sports that involve skin-to-skin contact. It is particularly prevalent among competitive wrestlers—earning it the name herpes gladiatorum.
In fact, HSV-1 is widespread throughout the country: Its prevalence in the general population is 58%.33 It is estimated that nearly half (47%) of cutaneous infections in collegiate athletes are caused by the herpes virus, making HSV-1 the most common pathogen of skin infections in this group.34 The clinical presentation of active HSV-1 depends on whether the infection is primary or recurrent.
So which form of HSV-1 is it?
Primary lesions may be preceded by a prodromal period with systemic symptoms, such as fever. Oral lesions and enlarged cervical and submandibular lymph nodes follow.35 The lesions, which are typically painful, can be found on the lips, buccal mucosa, or tongue—nearly anywhere in and around the oral cavity (Figure 2). They are vesicular at first, then ulcerate. Healing typically takes 10 to 14 days.36
In recurrent HSV-1, clusters of vesicles with erythematous borders typically occur. In those who play contact sports, HSV lesions can be found not only in the typical facial and oral areas, but anywhere on the head, face, torso, or extremities, as well.
Identifying herpetic whitlow. Presenting as a cluster of herpetic vesicles on the hands, fingers, or toes, herpetic whitlow is a common presentation of recurrent HSV-1. Recognition of these lesions is usually adequate for a diagnosis of recurrent infection, but confirmation can be obtained by laboratory or serologic testing.
Testing should be considered when you suspect that a patient has a primary herpes infection, as HSV-1 is a lifelong diagnosis. Viral culture is technique dependent and limited by 50% sensitivity, as cultures can take from 2 to 4 days to grow.35 The Tzanck test (direct microscopic examination of skin scrapings) has fallen out of favor, and PCR is now the gold standard.
Compared with viral culture, PCR has been shown to detect 80% of positive cases.35 However, PCR—using swab specimens taken from the lesions—is expensive and must be sent to a lab for analysis. One study suggests that while less sensitive than PCR, the Tzanck test can still be a reliably sensitive method of diagnosis when done properly, at less cost and with quicker
results.37
Serologic tests of HSV-1 IgM and IgG antibodies are also available. Serum IgG levels remain elevated in patients with previous infections. In primary infections, IgM is most useful as it can detect recent or active infection, but results may be falsely negative for several days after infection.38
Once diagnosed, management of HSV-1 in athletes is based on whether the infection is primary or recurrent. In both cases, oral antivirals are needed. Acyclovir is the gold standard, but famciclovir and valacyclovir have been proven to be equally effective.39 Both the NCAA and NFHS have published guidelines addressing the question of return to play (TABLE 2).22,23
In addition to the management of primary and recurrent infections of HSV-1, it is recommended that athletes and coaches known to be HSV-1 seropositive be treated with prophylactic suppressive therapy.40
The Centers for Disease Control and Prevention recommends acyclovir 400 mg bid or valacyclovir 500 mg/d as prophylaxis for anyone with ≤10 recurrences per year. For those with >10 recurrences annually, valacyclovir 1000 mg/d is recommended for the rest of the season.40 This has been shown to be effective in the reduction of outbreaks among wrestlers after a large outbreak occurred in 2007 in Minnesota,40,41 and NCAA and NFHS guidelines now support prophylactic therapy during competitive season.22,23 Before prescribing it, clinicians need to consider both the benefits of prophylactic antiviral therapy and the risks of promoting HSV-1 resistance.
Focus on prevention and squelching outbreaks
For cutaneous infections, as with so many medical conditions, prevention is paramount. With good preventive practices, many, if not all, of the skin infections common among athletes can be eliminated and outbreaks can be squelched.
Good hygienic practice is the cornerstone of prevention. Patients who participate in team sports should be advised to:
- shower immediately after practice
- refrain from sharing personal equipment like uniforms, towels, razors, and headgear
- launder workout clothes and towels after each use
- immediately cleanse and cover any abrasions that occur during practice.
Athletes should also be advised to ask their trainer or coach to check their skin for lesions on a regular basis. Surveillance should be instituted to prohibit athletes with cutaneous lesions from participating until they are sufficiently treated.
Although the role that environmental contamination plays in transmission of infection is uncertain, it is recommended that all sports equipment, playing surfaces, and locker rooms be disinfected daily with either a freshly made bactericidal (1/100 bleach/water solution)23,24 or an appropriate product. The Environmental Protection Agency provides a list of commercial products that have been proven to prevent the spread of MRSA on such surfaces (http://epa.gov/oppad001/chemregindex.htm) on its Web site.42
CASE When Shane returned 3 days later, the erythema had resolved, and minimal scaling remained. You tell him to continue to use the topical terbinafine twice a day for 10 days. You also show him how to apply a bio-occlusive dressing and clear him for practice, provided the lesions are fully covered.
You also talk to Shane about prevention, recommending that he immediately clean and cover any abrasion or other skin trauma that occurs during practice and suggesting that he ask his trainer to regularly check team members for skin lesions.
At Day 7, the DTM culture is positive, confirming a dermatophyte infection.
CORRESPONDENCE
Nilesh Shah, MD, 20 Olive Street, Suite 201, Akron, OH 44310;
shahn@summahealth.org
ACKNOWLEDGEMENT
The authors would like to thank Tom Bartsokas, MD, for his help with this manuscript.
1. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers Association position statement: skin diseases. J Athletic Training. 2010;45:411-428.
2. Brandi G, Sisti M, Paparini A, et al. Swimming pools and fungi: an environmental epidemiology survey in Italian indoor swimming facilities. Int J Environ Health Res. 2007;17:197-206.
3. Dienst WL Jr, Dightman L, Dworkin MS. Diagnosis, treatment and pinning down skin infections: diagnosis, treatment and prevention in wrestlers. Physician Sportsmed. 2005;25:45-56.
4. Ilkit M, GÜmral R, Saraçli MA, et al. Trichophyton tonsurans scalp carriage among wrestlers in a national competition in Turkey. Mycopathologia. 2011;172:215-222.
5. Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
6. Agel J, Ransone J, Dick R, et al. Descriptive epidemiology of collegiate men’s wrestling injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Training. 2007;42:303-310.
7. Adams BB. Tinea corporis gladiatorum. J Am Acad Dermatol. 2002;47:286-297.
8. Pecci M, Comeau D, Chawla V. Skin conditions in the athlete. Am J Sport Med. 2009;37:406-418.
9. Beaven DW, Brooks SE. Color Atlas of the Nail in Clinical Diagnosis. 2nd ed. London: Mosby-Wolfe; 1994.
10. Gupta AK, Summerbell RC. Tinea capitis. Med Mycol. 2000;38:255-287.
11. Li XF, Shen YN, Chen W, et al. A new medium for diagnosis of dermatophyte infection. Eur J Dermatol. 2009;19:34-37.
12. Akcaglar S, Ener B, Toker SC, et al. A comparative study of dermatophyte infections in Bursa Turkey. Med Mycol. 2011;49:602-607.
13. Garg J, Tilak R, Garg A, et al. Rapid detection of dermatophytes from skin and hair. BMC Res Notes. 2009;18:60.
14. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
15. Crawford F, Hollis S. Topical treatments for fungal infections of the ski and nails of the foot. Cochrane Database Syst Rev. 2007;(3):CD001434.
16. Evans EGV, Seaman RAJ, James IGV. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol. 1994;130:83-88.
17. Gupta AK, Ryder JE, Cooper EA. Naftifine: a review. J Cutan Med Surg. 2008;12:51-58.
18. Friedrich M. Inflammatory tinea pedis with bacterial superinfection effectively treated with isoconazole nitrate and diflucortolone valerate combination therapy. Mycoses. 2013;56(suppl 1):S23-S25.
19. González U, Seaton T, Bergus G, et al. Systemic antifungal therapy for tinea capitis in children. Cochrane Database Syst Rev. 2007;(4):CD004685.
20. Baran R, Hay RJ, Garduno JI. Review of antifungal therapy, part II: treatment rationale, including specific patient populations. J Dermatol Treat. 2008;19:168-175.
21. Zhang RN, Wang DK, Zhuo FL, et al. Long-pulse Nd:YAG 1064- nm laser treatment for onychomycosis. Chin Med J (Engl). 2012;125:3288-3291.
22. Guideline 2j: Skin infections in athletics. In: National Collegiate Athletic Association Sports Medicine Handbook. 22nd ed. Indianapolis, IN: National Collegiate Athletic Association; 2011:63.
23. National Federation of State High School Associations, Sports Medicine Advisory Committee. General guidelines for sports hygiene, skin infections and communicable diseases. Revised October 2012. Available at: http://www.nfhs.org/search. aspx?searchtext=skin%20infection. Accessed May 15, 2013.
24. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers’ Association position statement: skin diseases. J Athl Training. 2010;45:411-428.
25. Brickman K, Einstein E, Sinha S, et al. Fluconazole as a prophylactic measure for tinea gladiatorum in high school wrestlers. Clin J Sport Med. 2009;19:412-414.
26. Turbeville S, Cowan L, Greenfield R. Infectious disease outbreaks in competitive sports. Am J Sports Med. 2006;34:1860-1865.
27. Sedgwick P, Dexter W, Smith C. Bacterial dermatoses in sports. Clin Sports Med. 2007;26:383-396.
28. Patel A, Fischer S, Calfee R, et al. Locker room acquired methicillin-resistance Staphylococcus aureus. Orthopedics. 2007;30:532-535.
29. David M, Daum R. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616-687.
30. Benjamin H, Nikore V, Takagishi J. Practical management: community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA): the latest sports epidemic. Clin J Sport Med. 2007;17:393-397.
31. Rhin JA, Posfay-Barbe K, Harner CD, et al. Community-acquired methicillin-resistant Staphylococcus aureus outbreak in a local high school football team unsuccessful interventions. Pediatr Infect Dis J. 2005;24:841-843.
32. Loeb M, Main C, Walker-Dilks C, et al. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;(4):CD003340.
33. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964-973.
34. Yard EE, Collins CL, Dick RW, et al. An epidemiologic comparison of high school and college wrestling injuries. Am J Sports Med. 2008;36:57-64.
35. Usatine RP, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
36. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
37. Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541-555.
38. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9:86-90.
39. Ozcan A, Senol M, Saglam H, et al. Comparison of the Tzanck test and polymerase chain reaction in the diagnosis of cutaneous herpes simplex and varicella zoster virus infections. Int J Dermatol. 2007;46:1177-1179.
40. Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clin Microbiol Infect. 2006;12:463-469.
41. Anderson BJ. Managing herpes gladiatorum outbreaks in competitive wrestling: the 2007 Minnesota experience. Curr Sports Med Rep. 2008;7:323-327.
42. Environmental Protection Agency. EPA’s registered sterilizers, tuberculocides, and antimicrobial products against certain human public health bacteria and viruses. October 2012. Available at: http://epa.gov/oppad001/chemregindex.htm. Accessed November 13, 2012.
1. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers Association position statement: skin diseases. J Athletic Training. 2010;45:411-428.
2. Brandi G, Sisti M, Paparini A, et al. Swimming pools and fungi: an environmental epidemiology survey in Italian indoor swimming facilities. Int J Environ Health Res. 2007;17:197-206.
3. Dienst WL Jr, Dightman L, Dworkin MS. Diagnosis, treatment and pinning down skin infections: diagnosis, treatment and prevention in wrestlers. Physician Sportsmed. 2005;25:45-56.
4. Ilkit M, GÜmral R, Saraçli MA, et al. Trichophyton tonsurans scalp carriage among wrestlers in a national competition in Turkey. Mycopathologia. 2011;172:215-222.
5. Seebacher C, Bouchara JP, Mignon B. Updates on the epidemiology of dermatophyte infections. Mycopathologia. 2008;166:335-352.
6. Agel J, Ransone J, Dick R, et al. Descriptive epidemiology of collegiate men’s wrestling injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athl Training. 2007;42:303-310.
7. Adams BB. Tinea corporis gladiatorum. J Am Acad Dermatol. 2002;47:286-297.
8. Pecci M, Comeau D, Chawla V. Skin conditions in the athlete. Am J Sport Med. 2009;37:406-418.
9. Beaven DW, Brooks SE. Color Atlas of the Nail in Clinical Diagnosis. 2nd ed. London: Mosby-Wolfe; 1994.
10. Gupta AK, Summerbell RC. Tinea capitis. Med Mycol. 2000;38:255-287.
11. Li XF, Shen YN, Chen W, et al. A new medium for diagnosis of dermatophyte infection. Eur J Dermatol. 2009;19:34-37.
12. Akcaglar S, Ener B, Toker SC, et al. A comparative study of dermatophyte infections in Bursa Turkey. Med Mycol. 2011;49:602-607.
13. Garg J, Tilak R, Garg A, et al. Rapid detection of dermatophytes from skin and hair. BMC Res Notes. 2009;18:60.
14. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
15. Crawford F, Hollis S. Topical treatments for fungal infections of the ski and nails of the foot. Cochrane Database Syst Rev. 2007;(3):CD001434.
16. Evans EGV, Seaman RAJ, James IGV. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol. 1994;130:83-88.
17. Gupta AK, Ryder JE, Cooper EA. Naftifine: a review. J Cutan Med Surg. 2008;12:51-58.
18. Friedrich M. Inflammatory tinea pedis with bacterial superinfection effectively treated with isoconazole nitrate and diflucortolone valerate combination therapy. Mycoses. 2013;56(suppl 1):S23-S25.
19. González U, Seaton T, Bergus G, et al. Systemic antifungal therapy for tinea capitis in children. Cochrane Database Syst Rev. 2007;(4):CD004685.
20. Baran R, Hay RJ, Garduno JI. Review of antifungal therapy, part II: treatment rationale, including specific patient populations. J Dermatol Treat. 2008;19:168-175.
21. Zhang RN, Wang DK, Zhuo FL, et al. Long-pulse Nd:YAG 1064- nm laser treatment for onychomycosis. Chin Med J (Engl). 2012;125:3288-3291.
22. Guideline 2j: Skin infections in athletics. In: National Collegiate Athletic Association Sports Medicine Handbook. 22nd ed. Indianapolis, IN: National Collegiate Athletic Association; 2011:63.
23. National Federation of State High School Associations, Sports Medicine Advisory Committee. General guidelines for sports hygiene, skin infections and communicable diseases. Revised October 2012. Available at: http://www.nfhs.org/search. aspx?searchtext=skin%20infection. Accessed May 15, 2013.
24. Zinder SM, Basler RS, Foley J, et al. National Athletic Trainers’ Association position statement: skin diseases. J Athl Training. 2010;45:411-428.
25. Brickman K, Einstein E, Sinha S, et al. Fluconazole as a prophylactic measure for tinea gladiatorum in high school wrestlers. Clin J Sport Med. 2009;19:412-414.
26. Turbeville S, Cowan L, Greenfield R. Infectious disease outbreaks in competitive sports. Am J Sports Med. 2006;34:1860-1865.
27. Sedgwick P, Dexter W, Smith C. Bacterial dermatoses in sports. Clin Sports Med. 2007;26:383-396.
28. Patel A, Fischer S, Calfee R, et al. Locker room acquired methicillin-resistance Staphylococcus aureus. Orthopedics. 2007;30:532-535.
29. David M, Daum R. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616-687.
30. Benjamin H, Nikore V, Takagishi J. Practical management: community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA): the latest sports epidemic. Clin J Sport Med. 2007;17:393-397.
31. Rhin JA, Posfay-Barbe K, Harner CD, et al. Community-acquired methicillin-resistant Staphylococcus aureus outbreak in a local high school football team unsuccessful interventions. Pediatr Infect Dis J. 2005;24:841-843.
32. Loeb M, Main C, Walker-Dilks C, et al. Antimicrobial drugs for treating methicillin-resistant Staphylococcus aureus colonization. Cochrane Database Syst Rev. 2003;(4):CD003340.
33. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964-973.
34. Yard EE, Collins CL, Dick RW, et al. An epidemiologic comparison of high school and college wrestling injuries. Am J Sports Med. 2008;36:57-64.
35. Usatine RP, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075-1082.
36. Cernik C, Gallina K, Brodell RT. The treatment of herpes simplex infections: an evidence-based review. Arch Intern Med. 2008;168:1137-1144.
37. Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;26:541-555.
38. Anderson BJ. The effectiveness of valacyclovir in preventing reactivation of herpes gladiatorum in wrestlers. Clin J Sport Med. 1999;9:86-90.
39. Ozcan A, Senol M, Saglam H, et al. Comparison of the Tzanck test and polymerase chain reaction in the diagnosis of cutaneous herpes simplex and varicella zoster virus infections. Int J Dermatol. 2007;46:1177-1179.
40. Morrow R, Friedrich D. Performance of a novel test for IgM and IgG antibodies in subjects with culture-documented genital herpes simplex virus-1 or -2 infection. Clin Microbiol Infect. 2006;12:463-469.
41. Anderson BJ. Managing herpes gladiatorum outbreaks in competitive wrestling: the 2007 Minnesota experience. Curr Sports Med Rep. 2008;7:323-327.
42. Environmental Protection Agency. EPA’s registered sterilizers, tuberculocides, and antimicrobial products against certain human public health bacteria and viruses. October 2012. Available at: http://epa.gov/oppad001/chemregindex.htm. Accessed November 13, 2012.
Shoulder pain: 3 cases to test your diagnostic skills
Shoulder pain is a common reason for visits to primary care physicians, who are most likely to diagnose it as rotator cuff tendinitis1,2—often erroneously. The complexity of the joint and the overlapping pathologies that may present as shoulder pain highlight the need to take a closer look when dealing with this diagnostic challenge.
Often, a targeted medical history—including the mechanism of injury and pain-provoking and pain-relieving factors—and a problem-based physical examination (incorporating many of the maneuvers highlighted in the text and tables that follow) will lead to an accurate diagnosis without the need for imaging studies. We recommend that imaging be reserved for patients who don’t respond to conventional treatments, cases in which the diagnosis is in doubt, and instances in which surgical intervention is being considered.
The 3 cases* that follow, and the take-away message incorporated in each, will give you an opportunity to test—and to hone—your shoulder pain diagnostic skill.
CASE 1 The history: Jesse, a 17-year-old student who’s active in football and track, came in during track season complaining of severe left shoulder pain. He denied any traumatic event or previous injury to the shoulder, but reported that any motion involving the shoulder caused pain. It hurt at night, the patient said, when he lay on his left side.
The physical: No muscle atrophy, redness, or swelling was evident, nor was there any indication of asymmetry or ecchymosis in the affected area. Jesse’s neck range of motion was normal; he had a very hard time with any active motion of the shoulder, however, because of the pain.
Evaluation of scapular motion demonstrated scapular dyskinesis3,4 without winging. Passive motion of the glenohumeral joint was much better than active motion. Strength testing appeared to be grossly intact but was limited by the pain. Shoulder impingement testing was positive. Sensation and deep tendon reflexes were intact.
Patients' names have been changed to protect their privacy
What’s the diagnosis?
Subacromial bursitis, suggested by the patient’s pain and altered scapular motion, was our working diagnosis, and we administered a subacromial injection of corticosteroid with lidocaine, for diagnostic as well as therapeutic purposes. Reexamination after the injection revealed immediate partial improvement in resting pain, range of motion, and strength. We referred Jesse to physical therapy with a focus on scapular stabilization and rotator cuff strength.
Three months later, Jesse returned to our office, complaining of weakness in his left shoulder. The pain had subsided a week after his first appointment, so he’d never gone to physical therapy. The weakness, which he had first noticed about 2 months after starting a lifting program in preparation for football season, was limited to resistance exercises, especially overhead shoulder presses and bench press. There were no other changes in his history, and he reported no reinjuries.
Physical examination revealed atrophy of the supraspinatus and infraspinatus muscles (FIGURE 1) and external rotation and shoulder abduction (in the scapular plane) resistance tests revealed weakness of these muscles. There was no scapular winging. The cervical spine exam was normal, and neurovascular status was intact in both upper extremities.
FIGURE 1
Severe shoulder pain, followed by weakness
Physical examination reveals atrophy of the patient’s supraspinatus (^) and infraspinatus (+) muscles.
New evidence points to nerve injury. Based on Jesse’s current history and physical, nerve injury was our new working diagnosis. (We considered the possibility of a rotator cuff tear, but this was not corroborated by the history.)
We ordered an electromyogram/nerve conduction velocity study to localize the lesion. The test revealed a brachial plexitis/neuritis (also known as Parsonage-Turner syndrome or brachial amyotrophy). The etiology of most atraumatic brachial plexopathies is unknown, and most are thought to be viral or autoimmune in nature.5,6
A classic case of Parsonage-Turner syndrome. The typical presentation of Parsonage-Turner syndrome (like Jesse’s) is one of acute, intense shoulder pain for no known reason. After 1 to 3 weeks, the pain resolves and the patient is left with weakness, usually of the supraspinatus and infraspinatus muscles. The weakness typically resolves with time, but full resolution may take 6 to 9 months.5,6 (In Jesse’s case, it took about 6 months.)
The take-away message: Look beyond the shoulder
As this case illustrates, not all shoulder pain originates in the shoulder. When evaluating shoulder pain, it is essential to consider other causes. The differential diagnosis for shoulder pain includes cervical spine disorders, cholecystitis (right shoulder), diaphragmatic irritation (eg, in the case of splenic rupture, usually involving the left shoulder), cardiac disease, and thoracic outlet syndrome.7
Evaluation of the cervical spine should be part of a complete shoulder examination. It is vital to follow a systematic approach that carefully assesses the cervical region for the possibility of nerve root impingement and radicular dysfunction masquerading as a primary shoulder disorder. (TABLE 18,9 details pain and sensory distribution patterns, reflex involvement, and potential motor impairments associated with various spinal nerve root levels.)
TABLE 1
Assessing the cervical spine
| Nerve root | Pain distribution | Sensory distribution | Reflex changes | Motor involvement |
|---|---|---|---|---|
| C5 | Lateral neck/upper trapezius | Lateral arm | Biceps | Deltoid, biceps |
| C6 | Base of neck/upper trapezius to superior glenohumeral joint | Radial aspect of distal forearm, thenar eminence, and index finger | Brachioradialis | Biceps, extensor carpi radialis longus and brevis (wrist extension) |
| C7 | Base of neck, almost entire upper quadrant of the back | Third finger | Triceps | Triceps, wrist flexion, finger extension |
| C8 | No shoulder pain | 4th and 5th fingers, distal half of forearm (ulna side) | None | Finger flexion (grip strength) |
| Adapted from: Miller JD, et al. Am Fam Physician. 20008; Eubanks JD. Am Fam Physician. 2010.9 | ||||
Practitioners should develop their own approach to “clearing the neck.” A logical order is to note posture of the head/neck/shoulders, observe active motion, perform palpation and provocative tests, and then assess neurologic function with sensation/reflex/strength testing. Provocative tests that can help to identify cervical involvement relating to shoulder pain include Spurling’s maneuver, axial compression test, abduction relief sign, and Lhermitte’s sign.10,11
CASE 2 The history: Mark, a 17-year-old, right-handed volleyball player, presented with right shoulder pain, which he felt whenever he spiked or served the ball. The pain started last season, Mark said, diminished during the months when he wasn’t playing, then got progressively worse as his activity level increased. The pain was in the posterior aspect of the shoulder.
The physical: Physical examination revealed a well-developed, but thin (6’4”, 170 pounds) young man who was not in distress. The general examination was benign, and a joint-specific exam showed no asymmetry or atrophy on inspection and no tenderness to palpation over the posterior and anterior soft tissues of the right shoulder. Rotator cuff testing yielded intact strength for all 4 muscles, but external rotation and supraspinatus testing elicited pain. The crank test, drawer sign, load and shift test, relocation test, and sulcus sign, detailed in TABLE 2,12-14 were all positive for shoulder instability; the Clunk and O’Brien tests were negative, and the contralateral shoulder exam was within normal limits. General joint laxity was observed, with the ability to oppose the thumb to the volar forearm and hyperextension noted in both elbows and knees. There were no outward signs of connective tissue disease.
Because of the chronicity of Mark’s pain and the progressive nature of his symptoms, we ordered radiographs, including anterior-posterior, lateral axillary, and scapular Y views. These films showed a nearly skeletally mature male without bony abnormalities; the humeral head was well located in the glenoid.
TABLE 2
Testing for shoulder instability12-14
| Test | Procedure | Positive result/implication |
|---|---|---|
| Apprehension | Patient supine, arm abducted 90º, externally rotated with anteriorly directed force applied to humeral head | Pain/apprehension with force suggests anterior instability |
| Relocation* | Patient supine, posteriorly directed force applied to humeral head | Relief with force suggests anterior instability |
| Crank | Patient sitting, arm abducted 90º, elbow flexed to 90º, humerus supported with forced external rotation | Pain/apprehension with forced external rotation suggests anterior instability |
| Load and shift | Patient supine, arm held by examiner and abducted 90º, force applied along axis of humerus to "seat" the humerus within the glenoid, followed by anterior force directed to humeral head | Pain and appreciable translation felt with anterior force suggest anterior instability |
| Drawer | Patient sitting, arm at side, proximal humeral shaft grasped by examiner, seating the humeral head within the glenoid then applying anterior translational force | Pain and appreciable translation felt with anterior force suggest anterior instability |
| Sulcus | Patient sitting, arm at side, forearm grasped by examiner with an inferior/caudally directed force applied | Sulcus or depression seen inferior to acromion as humeral head subluxes posteriorly, pathognomonic for multidirectional instability |
| Clunk | Patient supine, examiner grasps at forearm and humeral shaft, with humeral head seated within the fossa, taking the arm through passive ROM from extension through forward flexion | Clunk sound or clicking sensation suggests labral tear |
| O’Brien | Patient sitting, arm is forward flexed to 90º and fully adducted and internally rotated; patient resists downward motion. If pain is elicited, the maneuver is repeated in external rotation | Pain elicited with resisted downward motion in internal rotation but relieved with external rotation suggests labral pathology |
| *Perform only if apprehension test is positive. ROM, range of motion | ||
What’s the diagnosis?
Multidirectional instability with recurrent subluxations and probable acute rotator cuff tendinitis was our provisional diagnosis. Treatment focused on physical therapy, with a concentration on scapular stabilization and rotator cuff strengthening.
Shoulder instability is relatively common and represents a spectrum of disorders ranging from dislocation to subluxation to simple laxity.12,13 A complete loss of humeral articulation within the glenoid fossa is evidence of dislocation, whereas subluxation includes approximation of the humeral head to the limits of the glenoid rim. Dislocation typically results from trauma, whereas subluxation can be the result of microtrauma and repetitive overuse injury. Anterior instability is the most common type and is reported in as many as 95% of all dislocations.13
The take-away message: Rule out instability
The shoulder is one of the most complex joints in the body. The rotator cuff structures, the glenoid labrum, and the collective capsular ligaments provide structural stability to the glenohumeral joint.12,13 The shoulder is vulnerable to instability because the shallow glenoid fossa offers little bony support for the humeral head. Thus, instability should always be included in an assessment of shoulder pain.
Key factors to consider in identifying shoulder instability include the location of the pain, the direction of traumatic force applied, the presence of a known complete dislocation vs apprehension with specific movement, the position of the arm in which pain is elicited, a previous occurrence of instability (subluxation or dislocation), and the presence of tingling or numbness.12-14 The maneuvers detailed in TABLE 212-14 can help identify instability, as they did in this case. Patients with hypermobility are at increased risk for shoulder instability, so a targeted exam and patient history aimed at identifying signs and symptoms of hypermobility is needed, as well.
Ask the patient to attempt to:
- bend the thumb to the volar forearm
- place hands to the floor with hyperextended knees
- perform maximal hyperextension of the fifth metacarpophalangeal joint (>90° is a positive result).
Findings from the medical history that indicate a predisposition to instability include generalized joint laxity, Ehlers-Danlos syndrome, Marfan syndrome, osteogenesis imperfecta, hyperhomocysteinuria, hyperlysinemia, benign joint hypermobility syndrome, juvenile rheumatoid arthritis, and previous shoulder or patellar dislocations.
Imaging tips: Scapular Y and/or axillary lateral views should always be included when ordering imaging studies for suspected instability/dislocations, as 50% of posterior dislocations are missed on standard shoulder x-rays that do not include them.12 In reviewing the x-rays, it is important to look for signs of a compression fracture of the posterior humeral head (known as a Hill-Sachs lesion) for anterior shoulder dislocations, and fractures to the anterior glenoid rim (known as a Bankart lesion).12-14
CASE 3 The history: Robert, a right-handed, 50-year-old motorcycle instructor, came to our office because of chronic right shoulder pain. The pain, located over the anterior portion of the glenohumeral joint, developed insidiously about 3 or 4 years ago, the patient reported. He had finally decided to seek help because he’d recently experienced an acute exacerbation of pain brought on by shoveling snow, after which he also noticed associated weakness, a clicking/popping on active motion, and mild loss of motion.
The physical: Robert’s cervical spine exam was unremarkable. He demonstrated full active range of motion (ROM) without exacerbation of right shoulder symptoms, and special tests for disc pathology at the neck were negative. Active ROM testing of the right shoulder revealed full abduction, with only minimal pain; full flexion, with moderate pain noted initially at 49°; full extension, with a painful arc noted at 50°; and full horizontal adduction, with a painful arc noted at the halfway point. The testing also revealed that his right thumb was 3 inches lower than the left on reaching for the opposite scapula. At the superior aspect of the acromioclavicular (AC) joint, 2+ tenderness was noted; 3+ tenderness was noted at the greater tubercle of the humerus.
After inspecting the shoulder region for alterations in bony landmarks, muscle bulk, carrying position, and movement characteristics, palpation of the region was performed.
When assessing shoulder strength, there are a variety of tests for each functioning component of the rotator cuff structures (TABLE 3).15-17 Manual muscle tests revealed: 4-/5 on external rotation (French horn test), 3+/5 on the lift-off test, and 5-/5 on all other tests for right shoulder function. Impingement testing was slightly positive, or pain producing, on Hawkins and Neer tests.18,19 For the Hawkins test, the examiner flexes the arm to 90° of shoulder flexion with the elbow flexed at 90°, then internally rotates the shoulder. For the Neer test, the arm is fully elevated in the scapular plane and internally rotated by the examiner.
The subscapularis muscle, which functions primarily in internal rotation, is tested by the French horn and lift-off tests. The teres minor muscle, which performs external rotation, is tested by the French horn test of external rotation. And the supraspinatus muscle, which performs abduction and external rotation, is tested by the empty can (also known as the Jobe) and full can tests. Some researchers suggest that the empty can test is better for diagnosing impingement, based on evidence showing that the full can test is better at diagnosing supraspinatus tears because it causes less pain during testing.20
TABLE 3
Suspect rotator cuff involvement?7,15-17
What’s the diagnosis?
Rotator cuff tear was suspected because Robert had positive elements of the “rotator cuff triad”—supraspinatus weakness (as indicated by a positive empty can test), external rotation weakness (revealed by the French horn test), and a positive Hawkins impingement test. We ordered diagnostic studies, including plain radiographs, which revealed degenerative changes at the acromioclavicular joint, decreased acromiohumeral interval, and no significant changes at the glenohumeral joint (FIGURE 2), and magnetic resonance imaging (MRI) of the right shoulder. The MRI revealed a large, full-thickness rotator cuff tear of the supraspinatus tendon with retraction. A torn and retracted biceps tendon and AC joint osteoarthritis were also shown, likely causing a mass effect on the supraspinatus. The patient underwent surgery to repair the torn rotator cuff, with excellent results.
FIGURE 2
Chronic right shoulder pain
An AP view of the patient’s right shoulder shows acromioclavicular joint narrowing and degeneration and subtle narrowing of the acromiohumeral interval.
The take-away message: Keep the rotator-cuff triad in mind
Because none of the tests that comprise the triad is specific enough alone to diagnose a rotator cuff tear,15,20,21 Murrell and Walton16 suggested that the 3 tests be considered together for diagnostic purposes. If all 3 are positive, there is a 98% chance of a rotator cuff tear; if 2 tests are positive and the patient is older than 60 years, the findings are suggestive of a tear; and if all 3 tests (plus the drop arm test) are negative, there is less than a 5% chance of a major rotator cuff tear.16
CORRESPONDENCE Nilesh Shah, MD, Summa Center for Sports Health, 20 Olive Street, Suite 201, Akron, OH 44310; ShahN@summahealth.org
1. Van der Windt DA, Koes BW, De Jong BA, et al. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54:959-964.
2. Johansson K, Adolfsson L, Foldevi M. Attitudes toward management of patients with subacromial pain in Swedish primary care. Fam Pract. 1999;16:233-237.
3. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11:142-151.
4. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26:325-337.
5. Vanermen B, Aertgeerts M, Hoogmartens M, et al. The syndrome of Parsonage and Turner. Discussion of clinical features with a review of 8 cases. Acta Orthop Belg. 1991;57:414-419.
6. Misamore GW, Lehman DE. Parsonage-Turner syndrome (acute brachial neuritis). J Bone Joint Surg. 1996;78:1405-1408.
7. Stevenson J, Trojian T. Evaluation of shoulder pain. J Fam Pract. 2002;51:605-611.
8. Miller JD, Pruitt RN, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
9. Eubanks JD. Cervical radiculopathy: nonoperative management of neck pain and radicular symptoms. Am Fam Physician. 2010;81:33-40.
10. Malanga GA, Landes P, Nadler SF. Provocative tests in cervical spine examination: historical basis and scientific analyses. Pain Physician. 2003;6:199-205.
11. Huston M, Ellis R. eds. Textbook of Musculoskeletal Medicine. Oxford, UK: Oxford University Press; 2005.
12. Mahaffey BL, Smith PA. Shoulder instability in young athletes. Am Fam Physician. 1999;59:2773-2782, 2787.
13. Petron DJ, Khan U. The shoulder and upper extremity. In: McKeag DB, Moeller JL. ACSM’s Primary Care Sports Medicine. 2nd ed. Philadelphia, Pa: Wolters Kluwer, Lippincott Williams & Wilkins; 2007:359–373.
14. Woodward TW, Best TM. The painful shoulder: part I. clinical evaluation. Am Fam Physician. 2000;61:3079-3088.
15. Kelly BT, Kadrmas WR, Speer KP. The manual muscle examination for rotator cuff strength: an electromyographic investigation. Am J Sports Med. 1996;24:581-588.
16. Murrell GA, Walton JR. Diagnosis of rotator cuff tears. Lancet. 2001;357:769-770.
17. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347-352.
18. Neer CS, Welsh RP. The shoulder in sports. Orthop Clin North Am. 1977;8:583-591.
19. Hawkins RJ, Kennedy JC. Impingement syndrome in athletics. Am J Sports Med. 1980;8:151-163.
20. Itoi E, Kido T, Sano A, et al. Which is more useful, the “full can test” or the “empty can test,” in detecting the torn supraspinatus tendon? Am J Sports Med. 1999;27:65-68.
21. Boettcher CE, Ginn KA, Cathers I. The ‘empty can’ and ‘full can’ tests do not selectively activate supraspinatus. J Sci Med Sport. 2009;12:435-439.
Shoulder pain is a common reason for visits to primary care physicians, who are most likely to diagnose it as rotator cuff tendinitis1,2—often erroneously. The complexity of the joint and the overlapping pathologies that may present as shoulder pain highlight the need to take a closer look when dealing with this diagnostic challenge.
Often, a targeted medical history—including the mechanism of injury and pain-provoking and pain-relieving factors—and a problem-based physical examination (incorporating many of the maneuvers highlighted in the text and tables that follow) will lead to an accurate diagnosis without the need for imaging studies. We recommend that imaging be reserved for patients who don’t respond to conventional treatments, cases in which the diagnosis is in doubt, and instances in which surgical intervention is being considered.
The 3 cases* that follow, and the take-away message incorporated in each, will give you an opportunity to test—and to hone—your shoulder pain diagnostic skill.
CASE 1 The history: Jesse, a 17-year-old student who’s active in football and track, came in during track season complaining of severe left shoulder pain. He denied any traumatic event or previous injury to the shoulder, but reported that any motion involving the shoulder caused pain. It hurt at night, the patient said, when he lay on his left side.
The physical: No muscle atrophy, redness, or swelling was evident, nor was there any indication of asymmetry or ecchymosis in the affected area. Jesse’s neck range of motion was normal; he had a very hard time with any active motion of the shoulder, however, because of the pain.
Evaluation of scapular motion demonstrated scapular dyskinesis3,4 without winging. Passive motion of the glenohumeral joint was much better than active motion. Strength testing appeared to be grossly intact but was limited by the pain. Shoulder impingement testing was positive. Sensation and deep tendon reflexes were intact.
Patients' names have been changed to protect their privacy
What’s the diagnosis?
Subacromial bursitis, suggested by the patient’s pain and altered scapular motion, was our working diagnosis, and we administered a subacromial injection of corticosteroid with lidocaine, for diagnostic as well as therapeutic purposes. Reexamination after the injection revealed immediate partial improvement in resting pain, range of motion, and strength. We referred Jesse to physical therapy with a focus on scapular stabilization and rotator cuff strength.
Three months later, Jesse returned to our office, complaining of weakness in his left shoulder. The pain had subsided a week after his first appointment, so he’d never gone to physical therapy. The weakness, which he had first noticed about 2 months after starting a lifting program in preparation for football season, was limited to resistance exercises, especially overhead shoulder presses and bench press. There were no other changes in his history, and he reported no reinjuries.
Physical examination revealed atrophy of the supraspinatus and infraspinatus muscles (FIGURE 1) and external rotation and shoulder abduction (in the scapular plane) resistance tests revealed weakness of these muscles. There was no scapular winging. The cervical spine exam was normal, and neurovascular status was intact in both upper extremities.
FIGURE 1
Severe shoulder pain, followed by weakness
Physical examination reveals atrophy of the patient’s supraspinatus (^) and infraspinatus (+) muscles.
New evidence points to nerve injury. Based on Jesse’s current history and physical, nerve injury was our new working diagnosis. (We considered the possibility of a rotator cuff tear, but this was not corroborated by the history.)
We ordered an electromyogram/nerve conduction velocity study to localize the lesion. The test revealed a brachial plexitis/neuritis (also known as Parsonage-Turner syndrome or brachial amyotrophy). The etiology of most atraumatic brachial plexopathies is unknown, and most are thought to be viral or autoimmune in nature.5,6
A classic case of Parsonage-Turner syndrome. The typical presentation of Parsonage-Turner syndrome (like Jesse’s) is one of acute, intense shoulder pain for no known reason. After 1 to 3 weeks, the pain resolves and the patient is left with weakness, usually of the supraspinatus and infraspinatus muscles. The weakness typically resolves with time, but full resolution may take 6 to 9 months.5,6 (In Jesse’s case, it took about 6 months.)
The take-away message: Look beyond the shoulder
As this case illustrates, not all shoulder pain originates in the shoulder. When evaluating shoulder pain, it is essential to consider other causes. The differential diagnosis for shoulder pain includes cervical spine disorders, cholecystitis (right shoulder), diaphragmatic irritation (eg, in the case of splenic rupture, usually involving the left shoulder), cardiac disease, and thoracic outlet syndrome.7
Evaluation of the cervical spine should be part of a complete shoulder examination. It is vital to follow a systematic approach that carefully assesses the cervical region for the possibility of nerve root impingement and radicular dysfunction masquerading as a primary shoulder disorder. (TABLE 18,9 details pain and sensory distribution patterns, reflex involvement, and potential motor impairments associated with various spinal nerve root levels.)
TABLE 1
Assessing the cervical spine
| Nerve root | Pain distribution | Sensory distribution | Reflex changes | Motor involvement |
|---|---|---|---|---|
| C5 | Lateral neck/upper trapezius | Lateral arm | Biceps | Deltoid, biceps |
| C6 | Base of neck/upper trapezius to superior glenohumeral joint | Radial aspect of distal forearm, thenar eminence, and index finger | Brachioradialis | Biceps, extensor carpi radialis longus and brevis (wrist extension) |
| C7 | Base of neck, almost entire upper quadrant of the back | Third finger | Triceps | Triceps, wrist flexion, finger extension |
| C8 | No shoulder pain | 4th and 5th fingers, distal half of forearm (ulna side) | None | Finger flexion (grip strength) |
| Adapted from: Miller JD, et al. Am Fam Physician. 20008; Eubanks JD. Am Fam Physician. 2010.9 | ||||
Practitioners should develop their own approach to “clearing the neck.” A logical order is to note posture of the head/neck/shoulders, observe active motion, perform palpation and provocative tests, and then assess neurologic function with sensation/reflex/strength testing. Provocative tests that can help to identify cervical involvement relating to shoulder pain include Spurling’s maneuver, axial compression test, abduction relief sign, and Lhermitte’s sign.10,11
CASE 2 The history: Mark, a 17-year-old, right-handed volleyball player, presented with right shoulder pain, which he felt whenever he spiked or served the ball. The pain started last season, Mark said, diminished during the months when he wasn’t playing, then got progressively worse as his activity level increased. The pain was in the posterior aspect of the shoulder.
The physical: Physical examination revealed a well-developed, but thin (6’4”, 170 pounds) young man who was not in distress. The general examination was benign, and a joint-specific exam showed no asymmetry or atrophy on inspection and no tenderness to palpation over the posterior and anterior soft tissues of the right shoulder. Rotator cuff testing yielded intact strength for all 4 muscles, but external rotation and supraspinatus testing elicited pain. The crank test, drawer sign, load and shift test, relocation test, and sulcus sign, detailed in TABLE 2,12-14 were all positive for shoulder instability; the Clunk and O’Brien tests were negative, and the contralateral shoulder exam was within normal limits. General joint laxity was observed, with the ability to oppose the thumb to the volar forearm and hyperextension noted in both elbows and knees. There were no outward signs of connective tissue disease.
Because of the chronicity of Mark’s pain and the progressive nature of his symptoms, we ordered radiographs, including anterior-posterior, lateral axillary, and scapular Y views. These films showed a nearly skeletally mature male without bony abnormalities; the humeral head was well located in the glenoid.
TABLE 2
Testing for shoulder instability12-14
| Test | Procedure | Positive result/implication |
|---|---|---|
| Apprehension | Patient supine, arm abducted 90º, externally rotated with anteriorly directed force applied to humeral head | Pain/apprehension with force suggests anterior instability |
| Relocation* | Patient supine, posteriorly directed force applied to humeral head | Relief with force suggests anterior instability |
| Crank | Patient sitting, arm abducted 90º, elbow flexed to 90º, humerus supported with forced external rotation | Pain/apprehension with forced external rotation suggests anterior instability |
| Load and shift | Patient supine, arm held by examiner and abducted 90º, force applied along axis of humerus to "seat" the humerus within the glenoid, followed by anterior force directed to humeral head | Pain and appreciable translation felt with anterior force suggest anterior instability |
| Drawer | Patient sitting, arm at side, proximal humeral shaft grasped by examiner, seating the humeral head within the glenoid then applying anterior translational force | Pain and appreciable translation felt with anterior force suggest anterior instability |
| Sulcus | Patient sitting, arm at side, forearm grasped by examiner with an inferior/caudally directed force applied | Sulcus or depression seen inferior to acromion as humeral head subluxes posteriorly, pathognomonic for multidirectional instability |
| Clunk | Patient supine, examiner grasps at forearm and humeral shaft, with humeral head seated within the fossa, taking the arm through passive ROM from extension through forward flexion | Clunk sound or clicking sensation suggests labral tear |
| O’Brien | Patient sitting, arm is forward flexed to 90º and fully adducted and internally rotated; patient resists downward motion. If pain is elicited, the maneuver is repeated in external rotation | Pain elicited with resisted downward motion in internal rotation but relieved with external rotation suggests labral pathology |
| *Perform only if apprehension test is positive. ROM, range of motion | ||
What’s the diagnosis?
Multidirectional instability with recurrent subluxations and probable acute rotator cuff tendinitis was our provisional diagnosis. Treatment focused on physical therapy, with a concentration on scapular stabilization and rotator cuff strengthening.
Shoulder instability is relatively common and represents a spectrum of disorders ranging from dislocation to subluxation to simple laxity.12,13 A complete loss of humeral articulation within the glenoid fossa is evidence of dislocation, whereas subluxation includes approximation of the humeral head to the limits of the glenoid rim. Dislocation typically results from trauma, whereas subluxation can be the result of microtrauma and repetitive overuse injury. Anterior instability is the most common type and is reported in as many as 95% of all dislocations.13
The take-away message: Rule out instability
The shoulder is one of the most complex joints in the body. The rotator cuff structures, the glenoid labrum, and the collective capsular ligaments provide structural stability to the glenohumeral joint.12,13 The shoulder is vulnerable to instability because the shallow glenoid fossa offers little bony support for the humeral head. Thus, instability should always be included in an assessment of shoulder pain.
Key factors to consider in identifying shoulder instability include the location of the pain, the direction of traumatic force applied, the presence of a known complete dislocation vs apprehension with specific movement, the position of the arm in which pain is elicited, a previous occurrence of instability (subluxation or dislocation), and the presence of tingling or numbness.12-14 The maneuvers detailed in TABLE 212-14 can help identify instability, as they did in this case. Patients with hypermobility are at increased risk for shoulder instability, so a targeted exam and patient history aimed at identifying signs and symptoms of hypermobility is needed, as well.
Ask the patient to attempt to:
- bend the thumb to the volar forearm
- place hands to the floor with hyperextended knees
- perform maximal hyperextension of the fifth metacarpophalangeal joint (>90° is a positive result).
Findings from the medical history that indicate a predisposition to instability include generalized joint laxity, Ehlers-Danlos syndrome, Marfan syndrome, osteogenesis imperfecta, hyperhomocysteinuria, hyperlysinemia, benign joint hypermobility syndrome, juvenile rheumatoid arthritis, and previous shoulder or patellar dislocations.
Imaging tips: Scapular Y and/or axillary lateral views should always be included when ordering imaging studies for suspected instability/dislocations, as 50% of posterior dislocations are missed on standard shoulder x-rays that do not include them.12 In reviewing the x-rays, it is important to look for signs of a compression fracture of the posterior humeral head (known as a Hill-Sachs lesion) for anterior shoulder dislocations, and fractures to the anterior glenoid rim (known as a Bankart lesion).12-14
CASE 3 The history: Robert, a right-handed, 50-year-old motorcycle instructor, came to our office because of chronic right shoulder pain. The pain, located over the anterior portion of the glenohumeral joint, developed insidiously about 3 or 4 years ago, the patient reported. He had finally decided to seek help because he’d recently experienced an acute exacerbation of pain brought on by shoveling snow, after which he also noticed associated weakness, a clicking/popping on active motion, and mild loss of motion.
The physical: Robert’s cervical spine exam was unremarkable. He demonstrated full active range of motion (ROM) without exacerbation of right shoulder symptoms, and special tests for disc pathology at the neck were negative. Active ROM testing of the right shoulder revealed full abduction, with only minimal pain; full flexion, with moderate pain noted initially at 49°; full extension, with a painful arc noted at 50°; and full horizontal adduction, with a painful arc noted at the halfway point. The testing also revealed that his right thumb was 3 inches lower than the left on reaching for the opposite scapula. At the superior aspect of the acromioclavicular (AC) joint, 2+ tenderness was noted; 3+ tenderness was noted at the greater tubercle of the humerus.
After inspecting the shoulder region for alterations in bony landmarks, muscle bulk, carrying position, and movement characteristics, palpation of the region was performed.
When assessing shoulder strength, there are a variety of tests for each functioning component of the rotator cuff structures (TABLE 3).15-17 Manual muscle tests revealed: 4-/5 on external rotation (French horn test), 3+/5 on the lift-off test, and 5-/5 on all other tests for right shoulder function. Impingement testing was slightly positive, or pain producing, on Hawkins and Neer tests.18,19 For the Hawkins test, the examiner flexes the arm to 90° of shoulder flexion with the elbow flexed at 90°, then internally rotates the shoulder. For the Neer test, the arm is fully elevated in the scapular plane and internally rotated by the examiner.
The subscapularis muscle, which functions primarily in internal rotation, is tested by the French horn and lift-off tests. The teres minor muscle, which performs external rotation, is tested by the French horn test of external rotation. And the supraspinatus muscle, which performs abduction and external rotation, is tested by the empty can (also known as the Jobe) and full can tests. Some researchers suggest that the empty can test is better for diagnosing impingement, based on evidence showing that the full can test is better at diagnosing supraspinatus tears because it causes less pain during testing.20
TABLE 3
Suspect rotator cuff involvement?7,15-17
What’s the diagnosis?
Rotator cuff tear was suspected because Robert had positive elements of the “rotator cuff triad”—supraspinatus weakness (as indicated by a positive empty can test), external rotation weakness (revealed by the French horn test), and a positive Hawkins impingement test. We ordered diagnostic studies, including plain radiographs, which revealed degenerative changes at the acromioclavicular joint, decreased acromiohumeral interval, and no significant changes at the glenohumeral joint (FIGURE 2), and magnetic resonance imaging (MRI) of the right shoulder. The MRI revealed a large, full-thickness rotator cuff tear of the supraspinatus tendon with retraction. A torn and retracted biceps tendon and AC joint osteoarthritis were also shown, likely causing a mass effect on the supraspinatus. The patient underwent surgery to repair the torn rotator cuff, with excellent results.
FIGURE 2
Chronic right shoulder pain
An AP view of the patient’s right shoulder shows acromioclavicular joint narrowing and degeneration and subtle narrowing of the acromiohumeral interval.
The take-away message: Keep the rotator-cuff triad in mind
Because none of the tests that comprise the triad is specific enough alone to diagnose a rotator cuff tear,15,20,21 Murrell and Walton16 suggested that the 3 tests be considered together for diagnostic purposes. If all 3 are positive, there is a 98% chance of a rotator cuff tear; if 2 tests are positive and the patient is older than 60 years, the findings are suggestive of a tear; and if all 3 tests (plus the drop arm test) are negative, there is less than a 5% chance of a major rotator cuff tear.16
CORRESPONDENCE Nilesh Shah, MD, Summa Center for Sports Health, 20 Olive Street, Suite 201, Akron, OH 44310; ShahN@summahealth.org
Shoulder pain is a common reason for visits to primary care physicians, who are most likely to diagnose it as rotator cuff tendinitis1,2—often erroneously. The complexity of the joint and the overlapping pathologies that may present as shoulder pain highlight the need to take a closer look when dealing with this diagnostic challenge.
Often, a targeted medical history—including the mechanism of injury and pain-provoking and pain-relieving factors—and a problem-based physical examination (incorporating many of the maneuvers highlighted in the text and tables that follow) will lead to an accurate diagnosis without the need for imaging studies. We recommend that imaging be reserved for patients who don’t respond to conventional treatments, cases in which the diagnosis is in doubt, and instances in which surgical intervention is being considered.
The 3 cases* that follow, and the take-away message incorporated in each, will give you an opportunity to test—and to hone—your shoulder pain diagnostic skill.
CASE 1 The history: Jesse, a 17-year-old student who’s active in football and track, came in during track season complaining of severe left shoulder pain. He denied any traumatic event or previous injury to the shoulder, but reported that any motion involving the shoulder caused pain. It hurt at night, the patient said, when he lay on his left side.
The physical: No muscle atrophy, redness, or swelling was evident, nor was there any indication of asymmetry or ecchymosis in the affected area. Jesse’s neck range of motion was normal; he had a very hard time with any active motion of the shoulder, however, because of the pain.
Evaluation of scapular motion demonstrated scapular dyskinesis3,4 without winging. Passive motion of the glenohumeral joint was much better than active motion. Strength testing appeared to be grossly intact but was limited by the pain. Shoulder impingement testing was positive. Sensation and deep tendon reflexes were intact.
Patients' names have been changed to protect their privacy
What’s the diagnosis?
Subacromial bursitis, suggested by the patient’s pain and altered scapular motion, was our working diagnosis, and we administered a subacromial injection of corticosteroid with lidocaine, for diagnostic as well as therapeutic purposes. Reexamination after the injection revealed immediate partial improvement in resting pain, range of motion, and strength. We referred Jesse to physical therapy with a focus on scapular stabilization and rotator cuff strength.
Three months later, Jesse returned to our office, complaining of weakness in his left shoulder. The pain had subsided a week after his first appointment, so he’d never gone to physical therapy. The weakness, which he had first noticed about 2 months after starting a lifting program in preparation for football season, was limited to resistance exercises, especially overhead shoulder presses and bench press. There were no other changes in his history, and he reported no reinjuries.
Physical examination revealed atrophy of the supraspinatus and infraspinatus muscles (FIGURE 1) and external rotation and shoulder abduction (in the scapular plane) resistance tests revealed weakness of these muscles. There was no scapular winging. The cervical spine exam was normal, and neurovascular status was intact in both upper extremities.
FIGURE 1
Severe shoulder pain, followed by weakness
Physical examination reveals atrophy of the patient’s supraspinatus (^) and infraspinatus (+) muscles.
New evidence points to nerve injury. Based on Jesse’s current history and physical, nerve injury was our new working diagnosis. (We considered the possibility of a rotator cuff tear, but this was not corroborated by the history.)
We ordered an electromyogram/nerve conduction velocity study to localize the lesion. The test revealed a brachial plexitis/neuritis (also known as Parsonage-Turner syndrome or brachial amyotrophy). The etiology of most atraumatic brachial plexopathies is unknown, and most are thought to be viral or autoimmune in nature.5,6
A classic case of Parsonage-Turner syndrome. The typical presentation of Parsonage-Turner syndrome (like Jesse’s) is one of acute, intense shoulder pain for no known reason. After 1 to 3 weeks, the pain resolves and the patient is left with weakness, usually of the supraspinatus and infraspinatus muscles. The weakness typically resolves with time, but full resolution may take 6 to 9 months.5,6 (In Jesse’s case, it took about 6 months.)
The take-away message: Look beyond the shoulder
As this case illustrates, not all shoulder pain originates in the shoulder. When evaluating shoulder pain, it is essential to consider other causes. The differential diagnosis for shoulder pain includes cervical spine disorders, cholecystitis (right shoulder), diaphragmatic irritation (eg, in the case of splenic rupture, usually involving the left shoulder), cardiac disease, and thoracic outlet syndrome.7
Evaluation of the cervical spine should be part of a complete shoulder examination. It is vital to follow a systematic approach that carefully assesses the cervical region for the possibility of nerve root impingement and radicular dysfunction masquerading as a primary shoulder disorder. (TABLE 18,9 details pain and sensory distribution patterns, reflex involvement, and potential motor impairments associated with various spinal nerve root levels.)
TABLE 1
Assessing the cervical spine
| Nerve root | Pain distribution | Sensory distribution | Reflex changes | Motor involvement |
|---|---|---|---|---|
| C5 | Lateral neck/upper trapezius | Lateral arm | Biceps | Deltoid, biceps |
| C6 | Base of neck/upper trapezius to superior glenohumeral joint | Radial aspect of distal forearm, thenar eminence, and index finger | Brachioradialis | Biceps, extensor carpi radialis longus and brevis (wrist extension) |
| C7 | Base of neck, almost entire upper quadrant of the back | Third finger | Triceps | Triceps, wrist flexion, finger extension |
| C8 | No shoulder pain | 4th and 5th fingers, distal half of forearm (ulna side) | None | Finger flexion (grip strength) |
| Adapted from: Miller JD, et al. Am Fam Physician. 20008; Eubanks JD. Am Fam Physician. 2010.9 | ||||
Practitioners should develop their own approach to “clearing the neck.” A logical order is to note posture of the head/neck/shoulders, observe active motion, perform palpation and provocative tests, and then assess neurologic function with sensation/reflex/strength testing. Provocative tests that can help to identify cervical involvement relating to shoulder pain include Spurling’s maneuver, axial compression test, abduction relief sign, and Lhermitte’s sign.10,11
CASE 2 The history: Mark, a 17-year-old, right-handed volleyball player, presented with right shoulder pain, which he felt whenever he spiked or served the ball. The pain started last season, Mark said, diminished during the months when he wasn’t playing, then got progressively worse as his activity level increased. The pain was in the posterior aspect of the shoulder.
The physical: Physical examination revealed a well-developed, but thin (6’4”, 170 pounds) young man who was not in distress. The general examination was benign, and a joint-specific exam showed no asymmetry or atrophy on inspection and no tenderness to palpation over the posterior and anterior soft tissues of the right shoulder. Rotator cuff testing yielded intact strength for all 4 muscles, but external rotation and supraspinatus testing elicited pain. The crank test, drawer sign, load and shift test, relocation test, and sulcus sign, detailed in TABLE 2,12-14 were all positive for shoulder instability; the Clunk and O’Brien tests were negative, and the contralateral shoulder exam was within normal limits. General joint laxity was observed, with the ability to oppose the thumb to the volar forearm and hyperextension noted in both elbows and knees. There were no outward signs of connective tissue disease.
Because of the chronicity of Mark’s pain and the progressive nature of his symptoms, we ordered radiographs, including anterior-posterior, lateral axillary, and scapular Y views. These films showed a nearly skeletally mature male without bony abnormalities; the humeral head was well located in the glenoid.
TABLE 2
Testing for shoulder instability12-14
| Test | Procedure | Positive result/implication |
|---|---|---|
| Apprehension | Patient supine, arm abducted 90º, externally rotated with anteriorly directed force applied to humeral head | Pain/apprehension with force suggests anterior instability |
| Relocation* | Patient supine, posteriorly directed force applied to humeral head | Relief with force suggests anterior instability |
| Crank | Patient sitting, arm abducted 90º, elbow flexed to 90º, humerus supported with forced external rotation | Pain/apprehension with forced external rotation suggests anterior instability |
| Load and shift | Patient supine, arm held by examiner and abducted 90º, force applied along axis of humerus to "seat" the humerus within the glenoid, followed by anterior force directed to humeral head | Pain and appreciable translation felt with anterior force suggest anterior instability |
| Drawer | Patient sitting, arm at side, proximal humeral shaft grasped by examiner, seating the humeral head within the glenoid then applying anterior translational force | Pain and appreciable translation felt with anterior force suggest anterior instability |
| Sulcus | Patient sitting, arm at side, forearm grasped by examiner with an inferior/caudally directed force applied | Sulcus or depression seen inferior to acromion as humeral head subluxes posteriorly, pathognomonic for multidirectional instability |
| Clunk | Patient supine, examiner grasps at forearm and humeral shaft, with humeral head seated within the fossa, taking the arm through passive ROM from extension through forward flexion | Clunk sound or clicking sensation suggests labral tear |
| O’Brien | Patient sitting, arm is forward flexed to 90º and fully adducted and internally rotated; patient resists downward motion. If pain is elicited, the maneuver is repeated in external rotation | Pain elicited with resisted downward motion in internal rotation but relieved with external rotation suggests labral pathology |
| *Perform only if apprehension test is positive. ROM, range of motion | ||
What’s the diagnosis?
Multidirectional instability with recurrent subluxations and probable acute rotator cuff tendinitis was our provisional diagnosis. Treatment focused on physical therapy, with a concentration on scapular stabilization and rotator cuff strengthening.
Shoulder instability is relatively common and represents a spectrum of disorders ranging from dislocation to subluxation to simple laxity.12,13 A complete loss of humeral articulation within the glenoid fossa is evidence of dislocation, whereas subluxation includes approximation of the humeral head to the limits of the glenoid rim. Dislocation typically results from trauma, whereas subluxation can be the result of microtrauma and repetitive overuse injury. Anterior instability is the most common type and is reported in as many as 95% of all dislocations.13
The take-away message: Rule out instability
The shoulder is one of the most complex joints in the body. The rotator cuff structures, the glenoid labrum, and the collective capsular ligaments provide structural stability to the glenohumeral joint.12,13 The shoulder is vulnerable to instability because the shallow glenoid fossa offers little bony support for the humeral head. Thus, instability should always be included in an assessment of shoulder pain.
Key factors to consider in identifying shoulder instability include the location of the pain, the direction of traumatic force applied, the presence of a known complete dislocation vs apprehension with specific movement, the position of the arm in which pain is elicited, a previous occurrence of instability (subluxation or dislocation), and the presence of tingling or numbness.12-14 The maneuvers detailed in TABLE 212-14 can help identify instability, as they did in this case. Patients with hypermobility are at increased risk for shoulder instability, so a targeted exam and patient history aimed at identifying signs and symptoms of hypermobility is needed, as well.
Ask the patient to attempt to:
- bend the thumb to the volar forearm
- place hands to the floor with hyperextended knees
- perform maximal hyperextension of the fifth metacarpophalangeal joint (>90° is a positive result).
Findings from the medical history that indicate a predisposition to instability include generalized joint laxity, Ehlers-Danlos syndrome, Marfan syndrome, osteogenesis imperfecta, hyperhomocysteinuria, hyperlysinemia, benign joint hypermobility syndrome, juvenile rheumatoid arthritis, and previous shoulder or patellar dislocations.
Imaging tips: Scapular Y and/or axillary lateral views should always be included when ordering imaging studies for suspected instability/dislocations, as 50% of posterior dislocations are missed on standard shoulder x-rays that do not include them.12 In reviewing the x-rays, it is important to look for signs of a compression fracture of the posterior humeral head (known as a Hill-Sachs lesion) for anterior shoulder dislocations, and fractures to the anterior glenoid rim (known as a Bankart lesion).12-14
CASE 3 The history: Robert, a right-handed, 50-year-old motorcycle instructor, came to our office because of chronic right shoulder pain. The pain, located over the anterior portion of the glenohumeral joint, developed insidiously about 3 or 4 years ago, the patient reported. He had finally decided to seek help because he’d recently experienced an acute exacerbation of pain brought on by shoveling snow, after which he also noticed associated weakness, a clicking/popping on active motion, and mild loss of motion.
The physical: Robert’s cervical spine exam was unremarkable. He demonstrated full active range of motion (ROM) without exacerbation of right shoulder symptoms, and special tests for disc pathology at the neck were negative. Active ROM testing of the right shoulder revealed full abduction, with only minimal pain; full flexion, with moderate pain noted initially at 49°; full extension, with a painful arc noted at 50°; and full horizontal adduction, with a painful arc noted at the halfway point. The testing also revealed that his right thumb was 3 inches lower than the left on reaching for the opposite scapula. At the superior aspect of the acromioclavicular (AC) joint, 2+ tenderness was noted; 3+ tenderness was noted at the greater tubercle of the humerus.
After inspecting the shoulder region for alterations in bony landmarks, muscle bulk, carrying position, and movement characteristics, palpation of the region was performed.
When assessing shoulder strength, there are a variety of tests for each functioning component of the rotator cuff structures (TABLE 3).15-17 Manual muscle tests revealed: 4-/5 on external rotation (French horn test), 3+/5 on the lift-off test, and 5-/5 on all other tests for right shoulder function. Impingement testing was slightly positive, or pain producing, on Hawkins and Neer tests.18,19 For the Hawkins test, the examiner flexes the arm to 90° of shoulder flexion with the elbow flexed at 90°, then internally rotates the shoulder. For the Neer test, the arm is fully elevated in the scapular plane and internally rotated by the examiner.
The subscapularis muscle, which functions primarily in internal rotation, is tested by the French horn and lift-off tests. The teres minor muscle, which performs external rotation, is tested by the French horn test of external rotation. And the supraspinatus muscle, which performs abduction and external rotation, is tested by the empty can (also known as the Jobe) and full can tests. Some researchers suggest that the empty can test is better for diagnosing impingement, based on evidence showing that the full can test is better at diagnosing supraspinatus tears because it causes less pain during testing.20
TABLE 3
Suspect rotator cuff involvement?7,15-17
What’s the diagnosis?
Rotator cuff tear was suspected because Robert had positive elements of the “rotator cuff triad”—supraspinatus weakness (as indicated by a positive empty can test), external rotation weakness (revealed by the French horn test), and a positive Hawkins impingement test. We ordered diagnostic studies, including plain radiographs, which revealed degenerative changes at the acromioclavicular joint, decreased acromiohumeral interval, and no significant changes at the glenohumeral joint (FIGURE 2), and magnetic resonance imaging (MRI) of the right shoulder. The MRI revealed a large, full-thickness rotator cuff tear of the supraspinatus tendon with retraction. A torn and retracted biceps tendon and AC joint osteoarthritis were also shown, likely causing a mass effect on the supraspinatus. The patient underwent surgery to repair the torn rotator cuff, with excellent results.
FIGURE 2
Chronic right shoulder pain
An AP view of the patient’s right shoulder shows acromioclavicular joint narrowing and degeneration and subtle narrowing of the acromiohumeral interval.
The take-away message: Keep the rotator-cuff triad in mind
Because none of the tests that comprise the triad is specific enough alone to diagnose a rotator cuff tear,15,20,21 Murrell and Walton16 suggested that the 3 tests be considered together for diagnostic purposes. If all 3 are positive, there is a 98% chance of a rotator cuff tear; if 2 tests are positive and the patient is older than 60 years, the findings are suggestive of a tear; and if all 3 tests (plus the drop arm test) are negative, there is less than a 5% chance of a major rotator cuff tear.16
CORRESPONDENCE Nilesh Shah, MD, Summa Center for Sports Health, 20 Olive Street, Suite 201, Akron, OH 44310; ShahN@summahealth.org
1. Van der Windt DA, Koes BW, De Jong BA, et al. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54:959-964.
2. Johansson K, Adolfsson L, Foldevi M. Attitudes toward management of patients with subacromial pain in Swedish primary care. Fam Pract. 1999;16:233-237.
3. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11:142-151.
4. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26:325-337.
5. Vanermen B, Aertgeerts M, Hoogmartens M, et al. The syndrome of Parsonage and Turner. Discussion of clinical features with a review of 8 cases. Acta Orthop Belg. 1991;57:414-419.
6. Misamore GW, Lehman DE. Parsonage-Turner syndrome (acute brachial neuritis). J Bone Joint Surg. 1996;78:1405-1408.
7. Stevenson J, Trojian T. Evaluation of shoulder pain. J Fam Pract. 2002;51:605-611.
8. Miller JD, Pruitt RN, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
9. Eubanks JD. Cervical radiculopathy: nonoperative management of neck pain and radicular symptoms. Am Fam Physician. 2010;81:33-40.
10. Malanga GA, Landes P, Nadler SF. Provocative tests in cervical spine examination: historical basis and scientific analyses. Pain Physician. 2003;6:199-205.
11. Huston M, Ellis R. eds. Textbook of Musculoskeletal Medicine. Oxford, UK: Oxford University Press; 2005.
12. Mahaffey BL, Smith PA. Shoulder instability in young athletes. Am Fam Physician. 1999;59:2773-2782, 2787.
13. Petron DJ, Khan U. The shoulder and upper extremity. In: McKeag DB, Moeller JL. ACSM’s Primary Care Sports Medicine. 2nd ed. Philadelphia, Pa: Wolters Kluwer, Lippincott Williams & Wilkins; 2007:359–373.
14. Woodward TW, Best TM. The painful shoulder: part I. clinical evaluation. Am Fam Physician. 2000;61:3079-3088.
15. Kelly BT, Kadrmas WR, Speer KP. The manual muscle examination for rotator cuff strength: an electromyographic investigation. Am J Sports Med. 1996;24:581-588.
16. Murrell GA, Walton JR. Diagnosis of rotator cuff tears. Lancet. 2001;357:769-770.
17. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347-352.
18. Neer CS, Welsh RP. The shoulder in sports. Orthop Clin North Am. 1977;8:583-591.
19. Hawkins RJ, Kennedy JC. Impingement syndrome in athletics. Am J Sports Med. 1980;8:151-163.
20. Itoi E, Kido T, Sano A, et al. Which is more useful, the “full can test” or the “empty can test,” in detecting the torn supraspinatus tendon? Am J Sports Med. 1999;27:65-68.
21. Boettcher CE, Ginn KA, Cathers I. The ‘empty can’ and ‘full can’ tests do not selectively activate supraspinatus. J Sci Med Sport. 2009;12:435-439.
1. Van der Windt DA, Koes BW, De Jong BA, et al. Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 1995;54:959-964.
2. Johansson K, Adolfsson L, Foldevi M. Attitudes toward management of patients with subacromial pain in Swedish primary care. Fam Pract. 1999;16:233-237.
3. Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11:142-151.
4. Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26:325-337.
5. Vanermen B, Aertgeerts M, Hoogmartens M, et al. The syndrome of Parsonage and Turner. Discussion of clinical features with a review of 8 cases. Acta Orthop Belg. 1991;57:414-419.
6. Misamore GW, Lehman DE. Parsonage-Turner syndrome (acute brachial neuritis). J Bone Joint Surg. 1996;78:1405-1408.
7. Stevenson J, Trojian T. Evaluation of shoulder pain. J Fam Pract. 2002;51:605-611.
8. Miller JD, Pruitt RN, McDonald TJ. Acute brachial plexus neuritis: an uncommon cause of shoulder pain. Am Fam Physician. 2000;62:2067-2072.
9. Eubanks JD. Cervical radiculopathy: nonoperative management of neck pain and radicular symptoms. Am Fam Physician. 2010;81:33-40.
10. Malanga GA, Landes P, Nadler SF. Provocative tests in cervical spine examination: historical basis and scientific analyses. Pain Physician. 2003;6:199-205.
11. Huston M, Ellis R. eds. Textbook of Musculoskeletal Medicine. Oxford, UK: Oxford University Press; 2005.
12. Mahaffey BL, Smith PA. Shoulder instability in young athletes. Am Fam Physician. 1999;59:2773-2782, 2787.
13. Petron DJ, Khan U. The shoulder and upper extremity. In: McKeag DB, Moeller JL. ACSM’s Primary Care Sports Medicine. 2nd ed. Philadelphia, Pa: Wolters Kluwer, Lippincott Williams & Wilkins; 2007:359–373.
14. Woodward TW, Best TM. The painful shoulder: part I. clinical evaluation. Am Fam Physician. 2000;61:3079-3088.
15. Kelly BT, Kadrmas WR, Speer KP. The manual muscle examination for rotator cuff strength: an electromyographic investigation. Am J Sports Med. 1996;24:581-588.
16. Murrell GA, Walton JR. Diagnosis of rotator cuff tears. Lancet. 2001;357:769-770.
17. Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg. 1994;3:347-352.
18. Neer CS, Welsh RP. The shoulder in sports. Orthop Clin North Am. 1977;8:583-591.
19. Hawkins RJ, Kennedy JC. Impingement syndrome in athletics. Am J Sports Med. 1980;8:151-163.
20. Itoi E, Kido T, Sano A, et al. Which is more useful, the “full can test” or the “empty can test,” in detecting the torn supraspinatus tendon? Am J Sports Med. 1999;27:65-68.
21. Boettcher CE, Ginn KA, Cathers I. The ‘empty can’ and ‘full can’ tests do not selectively activate supraspinatus. J Sci Med Sport. 2009;12:435-439.