User login
Hepatitis C Clinical Dashboards: Improving Liver Specialty Care Access and Quality
The VHA cares for 170,000 patients with chronic hepatitis C virus (HCV) infection, making it the largest single provider of medical care to chronic HCV patients in the U.S.1 Although HCV treatment rates within the VHA outpace those of the private sector, only half of patients with HCV infection within the VHA have accessed a liver specialist and less than a quarter have received antiviral medication.2-4
Newer HCV treatment regimens promise treatment sustained virologic response (SVR) rates—a marker of viral clearance posttreatment—of ≥ 90% in most cases but require careful patient selection and management.5 In particular, the estimated 24% of patients with HCV infection with advanced liver disease require more rapid consideration for therapy to reduce complications of cirrhosis such as liver failure, hepatocellular carcinoma, and death.6 With the advent of promising HCV therapies and rising rates of cirrhosis, there is an urgent need for population health management approach to deliver HCV care more widely and effectively.5,7
Rationale for Clinical Dashboards
Although the VHA hosts the largest integrated electronic medical record (EMR) system in the U.S., an EMR on its own does not guarantee improved patient care or access.8 EMRs can be used to document health care delivery, but they do not routinely provide information about the burden of disease in a population, nor do they identify patients most in need of care.
Clinical dashboards are tools that are geared to provide clinicians with relevant data to improve patient care. Early clinical dashboard development across the VHA was primary care focused, targeting patients with diabetes, ischemic heart disease, and hypertension. This national primary care dashboard provides clinically relevant, actionable data and enables the clinical provider to track patient progress. In addition, regional data can be aggregated for use by VISN managers.
While the impact of dashboards on quality of care is not well investigated, it remains a vital tool with the potential to transform care.9
HCV dashboards have been developed by individual VISNs and facilities across the VHA. HCV dashboards serve to identify patients most in need of antiviral therapy, expand outreach to those previously unseen by specialty care, sort patients by severity of liver disease, track treatment status, and calculate SVR.
Current HCV dashboards incorporate elements derived from the VA Corporate Data Warehouse (CDW), a national VA data repository consisting of data from all facilities’ electronic medical record systems. Updated information from the previous day is made available in VISN data warehouses and is refreshed nightly. The final result is user-friendly clinical data available in near-real time to dashboard users.
VISN 21 HCV Dashboard
Purpose and Elements
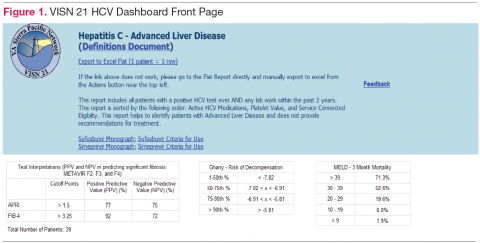
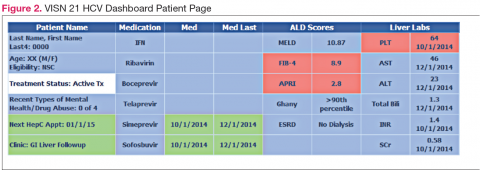
The VISN 21 HCV dashboard will be discussed as a prototype. Graphics of the VISN 21 dashboard interface are presented in Figure 1 and Figure 2. The VISN 21 HCV dashboard was developed by pharmacists with specialty training in medical informatics, health care analytics, and data management. The dashboard addresses 3 previously unmet needs in HCV care: population management, patient treatment outcome tracking, and administrative planning.
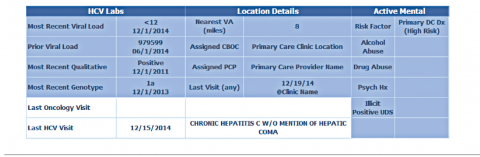
Population management. The VISN 21 HCV dashboard allows for a centralized approach to care across a large geographic area containing multiple facilities. One important function is to identify patients with advanced liver disease as well as those who have not been seen in specialty care within the previous 2 years. It also allows for pretreatment screening through identification of HCV viral characteristics (eg, genotype, viral load) and selected comorbidities (eg, renal function, mental health conditions) that may influence candidacy for specific antiviral therapies. Individual patient reports can be stratified by facility (eg, clinic or VAMC) to identify the burden of disease within a specific location.
Patient treatment outcome tracking. The HCV dashboard allows tracking of the numbers and characteristics of patients who have previously received antiviral therapy. The number of patients achieving virologic cure may be tracked at the VISN and station levels, or displayed based on user-selected parameters, such as treatment history.
Administrative planning. The high costs of HCV antiviral medication requires careful budgetary planning and close communication with local and regional leadership. The VISN 21 HCV dashboard provides information crucial to assessing future treatment needs. Specifically, it allows administrators to view the number of patients actively being treated. The dashboard also allows for comparison of treatment rates among different facilities and help allocate resources where needed.
Design Architecture
To construct the source data for the dashboards, relevant data elements are pulled into a base table using Structured Query Language (SQL) code. Subsequently, SQL Server Reporting Services (SSRS) (Microsoft, Redmond, WA) compiles the dashboard output into an interactive and user-friendly interface that can be tailored to individual end users’ needs.
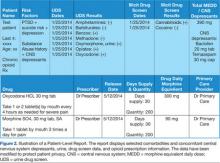
Dashboard development process. Through collaboration and survey of clinical providers, clinical factors necessary to decide patient and treatment readiness were identified. Relevant data elements include HCV genotype, selected medical and psychiatric comorbidities, prior receipt of treatment, and presence of advanced liver disease. While liver disease severity may be determined by invasive means, such as liver biopsy, the dashboard offers a noninvasive assessment using laboratory values (eg, calculated Fibrosis 4 score, Model for End Stage Liver Disease score).10,11
Once dashboard elements were selected, the variables were operationalized using data available in the CDW within the prescription, diagnostic, and laboratory data tables. As code was written, output was validated through chart review to ensure accuracy. Further validation was performed through comparison of the dashboard data with the clinical case registry, a registry of HCV viremic confirmed patients. Throughout dashboard development, the product was presented to end users to solicit requests for modifications. The code was refined over time to incorporate end user input.
Dashboard user interface. SSRS allows users to customize reports based on any variables defined within the data set including facility, severity of disease, HCV genotype, and prior antiviral treatment history among others. Results are displayed with summary information, including the total number of patients in the selected cohort, the number of patients who have been referred to a specialty liver clinic, and the number of patients who have been determined to achieve SVR. The end user has the option to export the results to excel for further use (eg, patient lists for telephone follow-up).
User recruitment. After piloting, the VISN 21 HCV dashboard was introduced during monthly pharmacy meetings and clinical telehealth encounters with providers. Feedback was solicited during the presentations and through postdevelopment surveys. In particular, providers requested spreadsheet-friendly formatting, additional informational fields consisting of mental health and substance abuse diagnoses, and identification of all patients with HCV regardless
of disease severity. A key element of dashboard refinement includes enhancing usability by solicitation of user feedback with subsequent tailoring of the user interface.12
Challenges
Many challenges exist in clinical dashboard development, expansion, and implementation including data integrity, workflow, and work culture. Data elements are often variable within a single facility, and this variation increases when identifying the same elements across facilities. For example, a laboratory test name (eg, “serum creatinine”) may exist with 2 to 3 different labels (eg, “creat,” “SCr,” “serum Cr”) within a single facility. As the variation increases, potential for inappropriate laboratory tests may be increased. Specialty clinic names also vary within and between facilities.
Local nomenclature for HCV clinic names may include “liver,” “infectious disease,” “hepatitis c,” or some variation, making it crucial for the dashboard developer to work closely with clinical staff to accurately matchspecialty clinic names being pulled from the data warehouse. Given the complexities of naming nomenclature within VA data, dashboard development requires a substantial investment of code customization and validation.
Ongoing dashboard maintenance is another important challenge due to the need for staff trained in SQL coding and familiarity with VA data warehouse architecture. Consequently, until the VHA dedicates resources to maintain such dashboards, only VISNs with existing technical knowledge and staffing will benefit from dashboards.
Usability, typically defined as “…effectiveness, efficiency and satisfaction with which the intended users can achieve their tasks in the intended context of product use,” is an additional consideration as the HCV clinical dashboard disseminates nationally.13 Standard clinic workflow is not always conducive to the use of dashboards. VHA providers use the Computerized Patient Record System (CPRS) to review and document patient notes. However, accessing the HCV dashboard involves a site hosted outside of CPRS, thereby requiring the user to take several extra steps. These and other usability factors will need to be considered as the dashboard disseminates more widely.
Finally, data describing the effectiveness of clinical dashboards is very limited. VISN 21 is tracking the number of users accessing the dashboard. However, further study is needed to determine if clinical dashboards improve patient access and quality of care as well as factors to enhance usability
Conclusion
Clinical dashboards have the ability to transform each clinical provider into a population health manager who can readily identify patients most in need of care within their facility catchment area and beyond. As HCV dashboard development and implementation grows across the VHA, there is a need to pair clinical and technological advancements with greater patient outreach and shared best practices. Understanding the factors that tie improved quality of care with usability as well as investment in dashboard development and related efforts will likely keep the VHA in the forefront of chronic care delivery.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
2. United States Department of Veterans Affairs. HCV Viremic Veterans in VHA Care in 2013 with First Fill in the Year or Ever Filled a VHA Outpatient Prescription for a HCV Antiviral Medication for the Nation, by VISN and by Station Description. http://vaww.hepatitis.va.gov/data-reports/ccr2013/RegMed-AnyFirstEverInCare-Jan14-HCVVir-HCV-2013-All.asp. Accessed October 10, 2014.
3. Rongey C, Shen H, Hamilton N, Backus LI, Asch SM, Knight S. Impact of rural residence and health system structure on quality of liver care. PloS One. 2013;8(12):e84826.
4. Beste LA, Ioannou GN. Prevalence and Treatment of Chronic Hepatitis C Virus Infection in the U.S. Department of Veterans Affairs [published online ahead of print January 19, 2015]. Epidemiologic Reviews. doi: 10.1093/epirev/mxu002.
5. Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an openlabel, randomised, phase 2 trial [published correction appears in Lancet. 2014;383(9920):870]. Lancet. 2014;383(9916):515-523.
6. United States Department of Veterans Affairs. HCV Viremic Veterans in VHA Care in 2013 who had a VHA Diagnosis of Fibrosis/Cirrhosis by FIB-4 in the year for the Nation, by VISN and by Station. http://vaww.hepatitis.va.gov/data-reports/ccr2013/Cond-FIB4CurInCare-Jan14HCVVir-2013-All.asp. Accessed October 10, 2014.
7. Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182-1188.e1.
8. Furukawa MF, King J, Patel V, Hsiao CJ, Adler-Milstein J, Jha AK. Despite substantial progress in EHR adoption, health information exchange and patient engagement remain low in office settings. Health Aff (Millwood). 2014;33(9):1672-1679.
9. Vrieze SI, Docherty A, Thuras P, et al. Best practices: The electronic medical record is an invaluable clinical tool: Let’s start using it. Psychiatric Serv. 2013;64(10):946-949.
10. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
11. Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for endstage liver disease (MELD). Hepatology. 2007;45(3):797-805.
12. Goldberg L, Lide B, Lowry S, et al. Usability and accessibility in consumer health informatics current trends and future challenges. Am J Prev Med. 2011;40(5 suppl 2):S187-S197.
13. Schumacher RM, Lowry SZ; National Institute of Standards and Technology. NIST Guide to the Processes Approach for Improving the Usability of Electronic Health Records. http://www.nist.gov/itl/hit/upload/Guide_Final_Publication_Version.pdf. Published November 29, 2010. Accessed November 24, 2014.
The VHA cares for 170,000 patients with chronic hepatitis C virus (HCV) infection, making it the largest single provider of medical care to chronic HCV patients in the U.S.1 Although HCV treatment rates within the VHA outpace those of the private sector, only half of patients with HCV infection within the VHA have accessed a liver specialist and less than a quarter have received antiviral medication.2-4
Newer HCV treatment regimens promise treatment sustained virologic response (SVR) rates—a marker of viral clearance posttreatment—of ≥ 90% in most cases but require careful patient selection and management.5 In particular, the estimated 24% of patients with HCV infection with advanced liver disease require more rapid consideration for therapy to reduce complications of cirrhosis such as liver failure, hepatocellular carcinoma, and death.6 With the advent of promising HCV therapies and rising rates of cirrhosis, there is an urgent need for population health management approach to deliver HCV care more widely and effectively.5,7
Rationale for Clinical Dashboards
Although the VHA hosts the largest integrated electronic medical record (EMR) system in the U.S., an EMR on its own does not guarantee improved patient care or access.8 EMRs can be used to document health care delivery, but they do not routinely provide information about the burden of disease in a population, nor do they identify patients most in need of care.
Clinical dashboards are tools that are geared to provide clinicians with relevant data to improve patient care. Early clinical dashboard development across the VHA was primary care focused, targeting patients with diabetes, ischemic heart disease, and hypertension. This national primary care dashboard provides clinically relevant, actionable data and enables the clinical provider to track patient progress. In addition, regional data can be aggregated for use by VISN managers.
While the impact of dashboards on quality of care is not well investigated, it remains a vital tool with the potential to transform care.9
HCV dashboards have been developed by individual VISNs and facilities across the VHA. HCV dashboards serve to identify patients most in need of antiviral therapy, expand outreach to those previously unseen by specialty care, sort patients by severity of liver disease, track treatment status, and calculate SVR.
Current HCV dashboards incorporate elements derived from the VA Corporate Data Warehouse (CDW), a national VA data repository consisting of data from all facilities’ electronic medical record systems. Updated information from the previous day is made available in VISN data warehouses and is refreshed nightly. The final result is user-friendly clinical data available in near-real time to dashboard users.
VISN 21 HCV Dashboard
Purpose and Elements
The VISN 21 HCV dashboard will be discussed as a prototype. Graphics of the VISN 21 dashboard interface are presented in Figure 1 and Figure 2. The VISN 21 HCV dashboard was developed by pharmacists with specialty training in medical informatics, health care analytics, and data management. The dashboard addresses 3 previously unmet needs in HCV care: population management, patient treatment outcome tracking, and administrative planning.
Population management. The VISN 21 HCV dashboard allows for a centralized approach to care across a large geographic area containing multiple facilities. One important function is to identify patients with advanced liver disease as well as those who have not been seen in specialty care within the previous 2 years. It also allows for pretreatment screening through identification of HCV viral characteristics (eg, genotype, viral load) and selected comorbidities (eg, renal function, mental health conditions) that may influence candidacy for specific antiviral therapies. Individual patient reports can be stratified by facility (eg, clinic or VAMC) to identify the burden of disease within a specific location.
Patient treatment outcome tracking. The HCV dashboard allows tracking of the numbers and characteristics of patients who have previously received antiviral therapy. The number of patients achieving virologic cure may be tracked at the VISN and station levels, or displayed based on user-selected parameters, such as treatment history.
Administrative planning. The high costs of HCV antiviral medication requires careful budgetary planning and close communication with local and regional leadership. The VISN 21 HCV dashboard provides information crucial to assessing future treatment needs. Specifically, it allows administrators to view the number of patients actively being treated. The dashboard also allows for comparison of treatment rates among different facilities and help allocate resources where needed.
Design Architecture
To construct the source data for the dashboards, relevant data elements are pulled into a base table using Structured Query Language (SQL) code. Subsequently, SQL Server Reporting Services (SSRS) (Microsoft, Redmond, WA) compiles the dashboard output into an interactive and user-friendly interface that can be tailored to individual end users’ needs.
Dashboard development process. Through collaboration and survey of clinical providers, clinical factors necessary to decide patient and treatment readiness were identified. Relevant data elements include HCV genotype, selected medical and psychiatric comorbidities, prior receipt of treatment, and presence of advanced liver disease. While liver disease severity may be determined by invasive means, such as liver biopsy, the dashboard offers a noninvasive assessment using laboratory values (eg, calculated Fibrosis 4 score, Model for End Stage Liver Disease score).10,11
Once dashboard elements were selected, the variables were operationalized using data available in the CDW within the prescription, diagnostic, and laboratory data tables. As code was written, output was validated through chart review to ensure accuracy. Further validation was performed through comparison of the dashboard data with the clinical case registry, a registry of HCV viremic confirmed patients. Throughout dashboard development, the product was presented to end users to solicit requests for modifications. The code was refined over time to incorporate end user input.
Dashboard user interface. SSRS allows users to customize reports based on any variables defined within the data set including facility, severity of disease, HCV genotype, and prior antiviral treatment history among others. Results are displayed with summary information, including the total number of patients in the selected cohort, the number of patients who have been referred to a specialty liver clinic, and the number of patients who have been determined to achieve SVR. The end user has the option to export the results to excel for further use (eg, patient lists for telephone follow-up).
User recruitment. After piloting, the VISN 21 HCV dashboard was introduced during monthly pharmacy meetings and clinical telehealth encounters with providers. Feedback was solicited during the presentations and through postdevelopment surveys. In particular, providers requested spreadsheet-friendly formatting, additional informational fields consisting of mental health and substance abuse diagnoses, and identification of all patients with HCV regardless
of disease severity. A key element of dashboard refinement includes enhancing usability by solicitation of user feedback with subsequent tailoring of the user interface.12
Challenges
Many challenges exist in clinical dashboard development, expansion, and implementation including data integrity, workflow, and work culture. Data elements are often variable within a single facility, and this variation increases when identifying the same elements across facilities. For example, a laboratory test name (eg, “serum creatinine”) may exist with 2 to 3 different labels (eg, “creat,” “SCr,” “serum Cr”) within a single facility. As the variation increases, potential for inappropriate laboratory tests may be increased. Specialty clinic names also vary within and between facilities.
Local nomenclature for HCV clinic names may include “liver,” “infectious disease,” “hepatitis c,” or some variation, making it crucial for the dashboard developer to work closely with clinical staff to accurately matchspecialty clinic names being pulled from the data warehouse. Given the complexities of naming nomenclature within VA data, dashboard development requires a substantial investment of code customization and validation.
Ongoing dashboard maintenance is another important challenge due to the need for staff trained in SQL coding and familiarity with VA data warehouse architecture. Consequently, until the VHA dedicates resources to maintain such dashboards, only VISNs with existing technical knowledge and staffing will benefit from dashboards.
Usability, typically defined as “…effectiveness, efficiency and satisfaction with which the intended users can achieve their tasks in the intended context of product use,” is an additional consideration as the HCV clinical dashboard disseminates nationally.13 Standard clinic workflow is not always conducive to the use of dashboards. VHA providers use the Computerized Patient Record System (CPRS) to review and document patient notes. However, accessing the HCV dashboard involves a site hosted outside of CPRS, thereby requiring the user to take several extra steps. These and other usability factors will need to be considered as the dashboard disseminates more widely.
Finally, data describing the effectiveness of clinical dashboards is very limited. VISN 21 is tracking the number of users accessing the dashboard. However, further study is needed to determine if clinical dashboards improve patient access and quality of care as well as factors to enhance usability
Conclusion
Clinical dashboards have the ability to transform each clinical provider into a population health manager who can readily identify patients most in need of care within their facility catchment area and beyond. As HCV dashboard development and implementation grows across the VHA, there is a need to pair clinical and technological advancements with greater patient outreach and shared best practices. Understanding the factors that tie improved quality of care with usability as well as investment in dashboard development and related efforts will likely keep the VHA in the forefront of chronic care delivery.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The VHA cares for 170,000 patients with chronic hepatitis C virus (HCV) infection, making it the largest single provider of medical care to chronic HCV patients in the U.S.1 Although HCV treatment rates within the VHA outpace those of the private sector, only half of patients with HCV infection within the VHA have accessed a liver specialist and less than a quarter have received antiviral medication.2-4
Newer HCV treatment regimens promise treatment sustained virologic response (SVR) rates—a marker of viral clearance posttreatment—of ≥ 90% in most cases but require careful patient selection and management.5 In particular, the estimated 24% of patients with HCV infection with advanced liver disease require more rapid consideration for therapy to reduce complications of cirrhosis such as liver failure, hepatocellular carcinoma, and death.6 With the advent of promising HCV therapies and rising rates of cirrhosis, there is an urgent need for population health management approach to deliver HCV care more widely and effectively.5,7
Rationale for Clinical Dashboards
Although the VHA hosts the largest integrated electronic medical record (EMR) system in the U.S., an EMR on its own does not guarantee improved patient care or access.8 EMRs can be used to document health care delivery, but they do not routinely provide information about the burden of disease in a population, nor do they identify patients most in need of care.
Clinical dashboards are tools that are geared to provide clinicians with relevant data to improve patient care. Early clinical dashboard development across the VHA was primary care focused, targeting patients with diabetes, ischemic heart disease, and hypertension. This national primary care dashboard provides clinically relevant, actionable data and enables the clinical provider to track patient progress. In addition, regional data can be aggregated for use by VISN managers.
While the impact of dashboards on quality of care is not well investigated, it remains a vital tool with the potential to transform care.9
HCV dashboards have been developed by individual VISNs and facilities across the VHA. HCV dashboards serve to identify patients most in need of antiviral therapy, expand outreach to those previously unseen by specialty care, sort patients by severity of liver disease, track treatment status, and calculate SVR.
Current HCV dashboards incorporate elements derived from the VA Corporate Data Warehouse (CDW), a national VA data repository consisting of data from all facilities’ electronic medical record systems. Updated information from the previous day is made available in VISN data warehouses and is refreshed nightly. The final result is user-friendly clinical data available in near-real time to dashboard users.
VISN 21 HCV Dashboard
Purpose and Elements
The VISN 21 HCV dashboard will be discussed as a prototype. Graphics of the VISN 21 dashboard interface are presented in Figure 1 and Figure 2. The VISN 21 HCV dashboard was developed by pharmacists with specialty training in medical informatics, health care analytics, and data management. The dashboard addresses 3 previously unmet needs in HCV care: population management, patient treatment outcome tracking, and administrative planning.
Population management. The VISN 21 HCV dashboard allows for a centralized approach to care across a large geographic area containing multiple facilities. One important function is to identify patients with advanced liver disease as well as those who have not been seen in specialty care within the previous 2 years. It also allows for pretreatment screening through identification of HCV viral characteristics (eg, genotype, viral load) and selected comorbidities (eg, renal function, mental health conditions) that may influence candidacy for specific antiviral therapies. Individual patient reports can be stratified by facility (eg, clinic or VAMC) to identify the burden of disease within a specific location.
Patient treatment outcome tracking. The HCV dashboard allows tracking of the numbers and characteristics of patients who have previously received antiviral therapy. The number of patients achieving virologic cure may be tracked at the VISN and station levels, or displayed based on user-selected parameters, such as treatment history.
Administrative planning. The high costs of HCV antiviral medication requires careful budgetary planning and close communication with local and regional leadership. The VISN 21 HCV dashboard provides information crucial to assessing future treatment needs. Specifically, it allows administrators to view the number of patients actively being treated. The dashboard also allows for comparison of treatment rates among different facilities and help allocate resources where needed.
Design Architecture
To construct the source data for the dashboards, relevant data elements are pulled into a base table using Structured Query Language (SQL) code. Subsequently, SQL Server Reporting Services (SSRS) (Microsoft, Redmond, WA) compiles the dashboard output into an interactive and user-friendly interface that can be tailored to individual end users’ needs.
Dashboard development process. Through collaboration and survey of clinical providers, clinical factors necessary to decide patient and treatment readiness were identified. Relevant data elements include HCV genotype, selected medical and psychiatric comorbidities, prior receipt of treatment, and presence of advanced liver disease. While liver disease severity may be determined by invasive means, such as liver biopsy, the dashboard offers a noninvasive assessment using laboratory values (eg, calculated Fibrosis 4 score, Model for End Stage Liver Disease score).10,11
Once dashboard elements were selected, the variables were operationalized using data available in the CDW within the prescription, diagnostic, and laboratory data tables. As code was written, output was validated through chart review to ensure accuracy. Further validation was performed through comparison of the dashboard data with the clinical case registry, a registry of HCV viremic confirmed patients. Throughout dashboard development, the product was presented to end users to solicit requests for modifications. The code was refined over time to incorporate end user input.
Dashboard user interface. SSRS allows users to customize reports based on any variables defined within the data set including facility, severity of disease, HCV genotype, and prior antiviral treatment history among others. Results are displayed with summary information, including the total number of patients in the selected cohort, the number of patients who have been referred to a specialty liver clinic, and the number of patients who have been determined to achieve SVR. The end user has the option to export the results to excel for further use (eg, patient lists for telephone follow-up).
User recruitment. After piloting, the VISN 21 HCV dashboard was introduced during monthly pharmacy meetings and clinical telehealth encounters with providers. Feedback was solicited during the presentations and through postdevelopment surveys. In particular, providers requested spreadsheet-friendly formatting, additional informational fields consisting of mental health and substance abuse diagnoses, and identification of all patients with HCV regardless
of disease severity. A key element of dashboard refinement includes enhancing usability by solicitation of user feedback with subsequent tailoring of the user interface.12
Challenges
Many challenges exist in clinical dashboard development, expansion, and implementation including data integrity, workflow, and work culture. Data elements are often variable within a single facility, and this variation increases when identifying the same elements across facilities. For example, a laboratory test name (eg, “serum creatinine”) may exist with 2 to 3 different labels (eg, “creat,” “SCr,” “serum Cr”) within a single facility. As the variation increases, potential for inappropriate laboratory tests may be increased. Specialty clinic names also vary within and between facilities.
Local nomenclature for HCV clinic names may include “liver,” “infectious disease,” “hepatitis c,” or some variation, making it crucial for the dashboard developer to work closely with clinical staff to accurately matchspecialty clinic names being pulled from the data warehouse. Given the complexities of naming nomenclature within VA data, dashboard development requires a substantial investment of code customization and validation.
Ongoing dashboard maintenance is another important challenge due to the need for staff trained in SQL coding and familiarity with VA data warehouse architecture. Consequently, until the VHA dedicates resources to maintain such dashboards, only VISNs with existing technical knowledge and staffing will benefit from dashboards.
Usability, typically defined as “…effectiveness, efficiency and satisfaction with which the intended users can achieve their tasks in the intended context of product use,” is an additional consideration as the HCV clinical dashboard disseminates nationally.13 Standard clinic workflow is not always conducive to the use of dashboards. VHA providers use the Computerized Patient Record System (CPRS) to review and document patient notes. However, accessing the HCV dashboard involves a site hosted outside of CPRS, thereby requiring the user to take several extra steps. These and other usability factors will need to be considered as the dashboard disseminates more widely.
Finally, data describing the effectiveness of clinical dashboards is very limited. VISN 21 is tracking the number of users accessing the dashboard. However, further study is needed to determine if clinical dashboards improve patient access and quality of care as well as factors to enhance usability
Conclusion
Clinical dashboards have the ability to transform each clinical provider into a population health manager who can readily identify patients most in need of care within their facility catchment area and beyond. As HCV dashboard development and implementation grows across the VHA, there is a need to pair clinical and technological advancements with greater patient outreach and shared best practices. Understanding the factors that tie improved quality of care with usability as well as investment in dashboard development and related efforts will likely keep the VHA in the forefront of chronic care delivery.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
2. United States Department of Veterans Affairs. HCV Viremic Veterans in VHA Care in 2013 with First Fill in the Year or Ever Filled a VHA Outpatient Prescription for a HCV Antiviral Medication for the Nation, by VISN and by Station Description. http://vaww.hepatitis.va.gov/data-reports/ccr2013/RegMed-AnyFirstEverInCare-Jan14-HCVVir-HCV-2013-All.asp. Accessed October 10, 2014.
3. Rongey C, Shen H, Hamilton N, Backus LI, Asch SM, Knight S. Impact of rural residence and health system structure on quality of liver care. PloS One. 2013;8(12):e84826.
4. Beste LA, Ioannou GN. Prevalence and Treatment of Chronic Hepatitis C Virus Infection in the U.S. Department of Veterans Affairs [published online ahead of print January 19, 2015]. Epidemiologic Reviews. doi: 10.1093/epirev/mxu002.
5. Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an openlabel, randomised, phase 2 trial [published correction appears in Lancet. 2014;383(9920):870]. Lancet. 2014;383(9916):515-523.
6. United States Department of Veterans Affairs. HCV Viremic Veterans in VHA Care in 2013 who had a VHA Diagnosis of Fibrosis/Cirrhosis by FIB-4 in the year for the Nation, by VISN and by Station. http://vaww.hepatitis.va.gov/data-reports/ccr2013/Cond-FIB4CurInCare-Jan14HCVVir-2013-All.asp. Accessed October 10, 2014.
7. Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182-1188.e1.
8. Furukawa MF, King J, Patel V, Hsiao CJ, Adler-Milstein J, Jha AK. Despite substantial progress in EHR adoption, health information exchange and patient engagement remain low in office settings. Health Aff (Millwood). 2014;33(9):1672-1679.
9. Vrieze SI, Docherty A, Thuras P, et al. Best practices: The electronic medical record is an invaluable clinical tool: Let’s start using it. Psychiatric Serv. 2013;64(10):946-949.
10. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
11. Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for endstage liver disease (MELD). Hepatology. 2007;45(3):797-805.
12. Goldberg L, Lide B, Lowry S, et al. Usability and accessibility in consumer health informatics current trends and future challenges. Am J Prev Med. 2011;40(5 suppl 2):S187-S197.
13. Schumacher RM, Lowry SZ; National Institute of Standards and Technology. NIST Guide to the Processes Approach for Improving the Usability of Electronic Health Records. http://www.nist.gov/itl/hit/upload/Guide_Final_Publication_Version.pdf. Published November 29, 2010. Accessed November 24, 2014.
1. Dominitz JA, Boyko EJ, Koepsell TD, et al. Elevated prevalence of hepatitis C infection in users of United States veterans medical centers. Hepatology. 2005;41(1):88-96.
2. United States Department of Veterans Affairs. HCV Viremic Veterans in VHA Care in 2013 with First Fill in the Year or Ever Filled a VHA Outpatient Prescription for a HCV Antiviral Medication for the Nation, by VISN and by Station Description. http://vaww.hepatitis.va.gov/data-reports/ccr2013/RegMed-AnyFirstEverInCare-Jan14-HCVVir-HCV-2013-All.asp. Accessed October 10, 2014.
3. Rongey C, Shen H, Hamilton N, Backus LI, Asch SM, Knight S. Impact of rural residence and health system structure on quality of liver care. PloS One. 2013;8(12):e84826.
4. Beste LA, Ioannou GN. Prevalence and Treatment of Chronic Hepatitis C Virus Infection in the U.S. Department of Veterans Affairs [published online ahead of print January 19, 2015]. Epidemiologic Reviews. doi: 10.1093/epirev/mxu002.
5. Lawitz E, Poordad FF, Pang PS, et al. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an openlabel, randomised, phase 2 trial [published correction appears in Lancet. 2014;383(9920):870]. Lancet. 2014;383(9916):515-523.
6. United States Department of Veterans Affairs. HCV Viremic Veterans in VHA Care in 2013 who had a VHA Diagnosis of Fibrosis/Cirrhosis by FIB-4 in the year for the Nation, by VISN and by Station. http://vaww.hepatitis.va.gov/data-reports/ccr2013/Cond-FIB4CurInCare-Jan14HCVVir-2013-All.asp. Accessed October 10, 2014.
7. Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182-1188.e1.
8. Furukawa MF, King J, Patel V, Hsiao CJ, Adler-Milstein J, Jha AK. Despite substantial progress in EHR adoption, health information exchange and patient engagement remain low in office settings. Health Aff (Millwood). 2014;33(9):1672-1679.
9. Vrieze SI, Docherty A, Thuras P, et al. Best practices: The electronic medical record is an invaluable clinical tool: Let’s start using it. Psychiatric Serv. 2013;64(10):946-949.
10. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-36.
11. Kamath PS, Kim WR; Advanced Liver Disease Study Group. The model for endstage liver disease (MELD). Hepatology. 2007;45(3):797-805.
12. Goldberg L, Lide B, Lowry S, et al. Usability and accessibility in consumer health informatics current trends and future challenges. Am J Prev Med. 2011;40(5 suppl 2):S187-S197.
13. Schumacher RM, Lowry SZ; National Institute of Standards and Technology. NIST Guide to the Processes Approach for Improving the Usability of Electronic Health Records. http://www.nist.gov/itl/hit/upload/Guide_Final_Publication_Version.pdf. Published November 29, 2010. Accessed November 24, 2014.
Using Dashboard Technology to Monitor Overdose Risk
On October 10, 2013, a Congressional hearing was held to address the issue of opioid medication prescribing within VHA facilities and clinics (House Veteran Affairs Subcommittee hearing “Between Peril and Promise: Facing the Dangers of VA’s Skyrocketing Use of Prescription Painkillers to Treat Veterans”). Several individuals testified, including the widows of 2 veterans; both their husbands had overdosed on prescribed opioid medications. One husband had been taking as many as 15 pills a day and was additionally prescribed oxycodone/acetaminophen, which led to his death.1
Alongside the widows were 2 veterans who had been treated for chronic back pain injuries sustained before and during deployment in Iraq. Both had been prescribed several pain medications, including oxycodone/acetaminophen, methadone, and morphine. One reported that as his pain increased, his doctors continued to provide him additional prescriptions; at one point he had more than 13 prescriptions and could no longer work from being so “doped up.”1
In the past 2 decades, health care professionals (HCPs) have placed greater emphasis on chronic pain management. As a result, the rate of opioid medication prescribing has increased dramatically. Since 1994, the number of opioid medication prescriptions has nearly doubled; this change has been accompanied by an increase in opioid misuse, which has resulted in accidental or intentional overdose and death.2
Based on a recent National Institute on Drug Abuse (NIDA) report, the greatest impact has been on armed forces personnel.3 Prescriptions for pain relievers quadrupled between 2001 and 2009 to almost 3.8 million within the military population. Although civilian populations are more likely to abuse illicit drugs, military personnel are at particular risk of prescription abuse, including opioid medications.3 In 2008, 11% of armed forces service members reported misusing prescription drugs, with opioid medications being the most abused. This is an approximate 5- to 6-fold increase since 2002 (2% reported misuse in 2002).3 Particularly concerning is the associated rise in suicide rates among armed forces personnel, which surpassed civilian suicide rates in 2004. In 2009, one-third of suicides among armed forces personnel involved prescription drugs.3
Certain patient characteristics or factors are related to greater overdose risk. These risk factors include prescription dosage and frequency, history of suicide attempts or self-harm behavior, history of depression or posttraumatic stress disorder (PTSD) among other mental health-related diagnoses, a history of substance and/or alcohol abuse, and within the context of opioid medication use, the concurrent use of other central nervous system (CNS) depressants.4,5 Additionally, the stresses of deployment during wartime, physical injuries sustained in combat, and the unique military culture play a particularly important role in access to substances with high abuse potential and the subsequent development of substance abuse.3
Opioid Use and Risk Factors
More than 3% of adults in the U.S. are now receiving opioid medications for chronic noncancer pain.6 Substance abuse among patients with chronic pain ranges from 14% to 40%.5 Prescription opioid medications are the fastest growing drugs of abuse and the most common cause of unintentional overdose in the U.S.4 About 17,000 deaths occur each year as a result of prescription opioid medication overdose.7 Opioid medication-related overdose deaths began to increase in the early 2000s and continue to increase. Between 1999 and 2007, the rate of unintentional overdose-related deaths in the U.S. increased by 124%, largely due to the increase of prescription opioid medications.8
High-Dose Opioid Medication Use
A study by Dunn and colleagues found that patients receiving higher doses of prescribed opioid medications were at an increased risk of overdose.6 Patients receiving 50 mg to 99 mg morphine equivalent daily dose (MEDD) had a 3.7-fold increase in overdose risk (0.7% annual overdose rate) as compared with patients who received < 50 mg MEDD (0.2% annual overdose rate). Patients receiving ≥ 100 mg MEDD had a 1.8% annual overdose rate and a 9.8-fold increase in overdose risk as compared with patients who received < 50 mg MEDD. Overall, 51 patients experienced ≥ 1 overdose event, 40 of whom experienced fatal or serious overdoses and 6 of whom attempted suicide. Patients receiving the highest doses were male, current smokers, and had a history of depression and substance abuse.6 Similarly, a study by Bohnert and colleagues found that opioid medication overdose was most likely to occur in those patients with psychiatric and substance use disorders compared with patients who had no psychiatric illness history.8
Depression
Mood disorders are common in people with chronic pain.4,5,9,10 In particular, patients with a history of depression are more likely to receive chronic opioid medication prescriptions and are at a higher risk for opioid medication abuse. A substance abuse history is the most consistent predictor of both chronic opioid medication use and abuse. However, depression without substance abuse is significantly associated with 2 forms of opioid medication abuse: self-medication for stress or sleep and overmedication (using a higher dose than prescribed). More severe cases of depression show a stronger association for potential abuse.4
PTSD
Among Iraq and Afghanistan war veterans with ≥ 1 pain-related diagnosis, veterans with PTSD and veterans with a mental health disorder other than PTSD were significantly more likely to receive opioid medications for pain than were veterans without a mental health disorder (PTSD—17.8%, adjusted relative risk [RR] 2.58; other mental health disorder—11.7%, RR 1.74; no mental health disorder—6.5%).2 Although mental health disorders in general were related to a higher risk of opioid abuse, those with PTSD in particular were more likely to receive higher prescribed dosages; to continue taking opioids for a longer period; to receive concurrent prescriptions for opioid medications, sedative hypnotics, or both; to obtain early refills; and to have comorbid alcohol and substance use disorders. Based on these results, Seal and colleagues concluded that veterans with PTSD had the highest risk of alcohol, drug, and opioid-related accidents and overdose as well as self-inflicted injuries.2
Concurrent Use of Opioids and CNS Depressants
As mentioned earlier, studies suggest that people with PTSD are at a significantly higher risk for opioid medication overdose. One factor that may contribute to this higher risk is the concurrent use of CNS depressants/sedatives, particularly benzodiazepines and alcohol.
Benzodiazepines are often prescribed for people with PTSD. One study found that the concurrent use of benzodiazepines is significantly related to opioid overdose.5 Prescribing opioids for people already abusing or dependent on alcohol or other substances increases the risk of abuse and overdose. Furthermore, the concurrent use of multiple medications is associated with aberrant behaviors, cognitive impairment, and medication abuse, potentially leading to overdose. Overall, the combined administration of these medications is responsible for higher rates of adverse events, overdose, and death related to prescription opioid medication use.5,6,11
In summary, there are various risk factors that contribute to opioid medication overdose and more generally, risk of suicide, including (1) high-dose opioid medications; (2) history of psychiatric disorders, specifically depression and PTSD; (3) history of substance use disorders; and (4) concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse.
Suicide
Suicide is the tenth leading cause of death in the U.S., with 12.4 suicide deaths per 100,000 population.12 Suicide rates are even higher among veterans. According to the VHA, the age-adjusted rate of suicide for veterans using VHA facilities and clinics was 35.9 per 100,000 person-years for fiscal year 2009.13 Several risk factors for suicide attempts include depression and other mental health disorders, substance abuse, medical disorders, and prescription medications.
Prior suicide attempts or self-harm behavior is known to increase the risk of subsequent death by suicide. About 11 attempted suicides occur per suicide death where the medical severity of prior attempts increases the risk of future suicide, as does a history of multiple self-harm episodes.14,15 One study found that the single best predictor of suicide in a veteran population was an attempt in the previous 4 months.16
Among other risk factors, previous suicide attempts and violent behavior are major behavioral flags that warrant caution and require particular consideration when prescribing opioid medications. In a national survey on drug use and health, about 18% of prescription opioid users/abusers who experienced suicidal ideations actually attempted suicide. Only 11% of individuals who never used prescription opioid medications attempted suicide after reported suicidal ideations.17
Patient Data Aggregation
The early and widespread adoption of electronic medical records (EMRs) by the VHA allowed the aggregation of patient data for quality improvement. Initially, data were aggregated, and dashboards were designed retrospectively. However, the development of regional data warehouses that update patient information daily from the EMR allowed information to be aggregated prospectively, and dashboards were designed that provided real-time information.
The purpose of the current study is to demonstrate the efficacy and future potential of dashboard technology in assessing prospectively high-risk factors for opioid overdose. Dashboards are a user-friendly application that allows providers to isolate and calculate daily morphine equivalent opioid dosages and assess patients’ risk factors for overdose on an individual basis. By using this technology, providers who prescribe opioids can get a concise summary of opioid and other medications and adjust medications to decrease overdose risk on an individual basis.
What is the Dashboard?
The VISN 22 high-risk opioid dashboard is a business intelligence tool that serves as a report card, or progress report, to provide a global view of the number of veterans who are receiving opioid prescriptions totaling >120 mg MEDD and who have characteristics (history of depression, PTSD, substance abuse, or high-risk suicide flag) and prescriptions (concomitant CNS depressants) that may increase patient risk for overdose.
The VISN 22 dashboard allows the user to navigate to an individual HCP-level and patient-level report (Figures 1 and 2). Filter settings allow report users to select only high-risk patients; it serves as a single location for pertinent details to consider for safely prescribing opioids.
To calculate daily morphine equivalents, each patient’s opioid prescriptions were evaluated. The quantity was divided by the day’s supply to calculate an average daily quantity. From there, the drug strength was used to convert to MEDD. Health care providers were informed that these conversion factors were not recommendations for clinical opioid conversions.
Implementation and Design
In 2012, the VA Pharmacy Benefits Management (PBM) in VISN 21 created a dashboard that allowed users to identify patients on high-dose opioid prescriptions. Structured query language code was used to extract data from the regional data warehouse and calculate MEDD for all patients with active opioid prescriptions. In 2013, VISN 22 expanded that dashboard to incorporate factors that could indicate a high risk for overdose or other adverse outcomes, including a history of depression, PTSD, substance abuse or high-risk suicide flag, and concomitant use of CNS depressant medications.
The high-risk opioid dashboard (Figure 3) and accompanying patient-level report were first introduced to VISN 22 HCPs in January 2013. The business intelligence tools were introduced to each facility through the VISN 22 PBM group. Training on the use of the dashboard and the report was provided, with an initial target of decreasing MEDD of > 200 mg to < 5% of all veterans prescribed opioids at each VISN 22 facility. One month later (in February 2013), a second category of veterans (those with > 120 mg but < 199 mg MEDD) was added. Also the initial MEDD > 200 mg target of < 5% was decreased to < 3% to encourage additional progress.
Eight months after the VISN 22 dashboard technology was implemented there was a 17% decrease in patients with total daily morphine equivalents > 200 mg (January 2013; 1,137 patients vs August 2013; 940 patients—a decrease of 197 patients).
From March 2013 to August 2013, VISN 22 also saw a 12% decrease in the number of patients prescribed > 120 mg MEDD but < 199 MEDD (March 2013; 2,295 vs August 2013; 2,018—a decrease of 277 patients).
Figure 4 shows opioid use as of July 2014 for VISN 22 facilities. There were further reductions in the number of patients receiving > 120 mg but < 199 mg MEDD (August 2013; 2,018 patients vs July 2014; 1,189 patients) and patients receiving > 200 mg MEDD (August 2013; 940 patients vs July 2014; 836 patients).
Case Description
In January 2013, VISN 22 implemented dashboard technology to help providers assess and monitor opioid prescription levels in relation to high-risk variables. The benefits of this dashboard technology are illustrated in the case profile that follows.
A 67-year-old male veteran had a long history of chronic pain. Pain diagnoses included osteoarthritis with spine involvement, lumbar radiculopathy, arthralgia, and peripheral neuropathy. For the past 10 years, he was prescribed opioids with modest relief of his chronic pain symptoms despite recent prescriptions totaling 300 mg MEDD. This veteran had several risk factors for overdose, including a history of depression, suicide risk, PTSD, and concomitant use of the CNS depressants alprazolam and cyclobenzaprine.
More recently, in May 2013, the veteran exhibited aberrant behavior and requested early refills for alprazolam. In response, the pharmacist discussed the case with the HCP who prescribed the opioids, noting the concomitant overdose risk factors. As a result of this interaction, the veteran was referred for mental health services, and his prescriptions for opioids were gradually decreased. He is currently stable, now receiving 120 mg MEDD, and his pain is currently described as moderately controlled on the new lower dose.
In summary, this veteran was receiving > 200 mg MEDD with several known overdose risk factors. Once the HCP was made aware of these risk factors, necessary precautions were taken, and the veteran was safely tapered to a lower dose. Dashboard technology makes the list of risk factors readily available to HCPs who are prescribing (and the pharmacists reviewing the prescriptions), thus allowing a proactive discussion of risks and benefits before continuing, renewing, or initiating opioid prescriptions.
Discussion
As reported in 2013 by NIDA, the greater availability of opioid medications and the consequent increase in prescriptions may be contributing directly to their growing misuse by both civilians and military service personnel. A direct consequence has been an increase in both accidental and intentional overdose deaths.3 Several factors are related to the risk of overdose/death, including high-dose opioid medications, a history of psychiatric disorders (specifically depression and PTSD), a history of substance use disorders, concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse, and previous attempts of suicide.
The VISN 22 high-risk opioid dashboard was a rapid information technology response to the increasing risk faced by veterans who have chronic pain and comorbid psychiatric and substance use disorders and are prescribed opioids and CNS depressants. The purpose of implementing this dashboard technology was to assist HCPs in prescribing opioids safely, using a technology that allows for the monitoring and management of concomitant suicide risk factors. Following the national Opioid Safety Initiative, this dashboard tool is being used to identify veterans who are on high-dose opioids with the goal of reducing the number of veterans on > 200 mg MEDD. The dashboard allows data to be stratified, using the concomitant risk factors for suicide to assist facilities and their providers in the identification and prioritization of highest risk patients first.
An initial review of dashboard data in VISN 22 suggests that it is a useful tool for reducing high-dose opioid prescriptions (> 200 mg MEDD and > 120 mg but < 199 mg MEDD). Across the 5 VA locations in VISN 22, in the first 8 months of implementation, 4 locations were able to lower prescription opioid medication levels to the initial target of < 5%; 2 lowered rates even further (to < 3%). The VA Greater Los Angeles Healthcare System remains at a commendable 1%. Although the number of veterans with prescriptions totaling > 200 mg MEDD has decreased as a result, a greater reduction is expected with the coordinated education and systems improvement efforts associated with the national VHA Opioid Safety Initiative. As part of the process to lower the number of patients on high-dose opioids in the future, HCP and patient education will be provided in relation to the use of dashboard technology.
Limitations
There are several limitations that affect interpretation of the usefulness of the VISN 22 high-risk opioid dashboard. Prior to the implementation of the dashboard, 2 of 5 VISN sites already had efforts in place to reduce opioid overprescribing. The VA Greater Los Angeles Healthcare System had an opioid reduction program in place before the dashboard was implemented, so it is possible reductions in opioid prescribing were a result of their previous efforts and not related to the dashboard. Similarly the VA Long Beach Healthcare System had begun a quality improvement initiative to reduce high-dose opioid prescribing prior to dashboard implementation. However, it was difficult to pinpoint the direct effect the dashboard had on patient interventions due to lack of documentation of dashboard use in the clinical notes.
A direct relationship did exist between dashboard implementation and opioid dose reduction in patients with > 200 MEDD at the remaining 3 VISN 22 facilities. Overall, this suggests that the dashboard played a significant role across all sites. Implementation of the dashboard across VISN 22 was accompanied by an education effort that resulted in an increased awareness among HCPs to evaluate certain risks in patients on high-dose opioids and to evaluate the combination of opioid and CNS depressant use. Prior to dashboard implementation, there was no standardized monitoring system that cross-referenced high-dose opioid prescribing with psychiatric illness and suicide risk factors.
Conclusions
From 2000 to 2010, opioid prescriptions nearly doubled, yet this rate was not accompanied by a change/increase in the rate of nonopioid analgesic medication prescriptions.18 Health care providers need to account for veterans’ wishes for pain treatment and be aware of options other than opioids, particularly given the risk of opioid-related accidental or intentional overdose; it is imperative that treatment become more individualized and more closely monitored.19,20 It is recommended that opioids should be the treatment of last resort in managing chronic noncancer pain. The use of opioid prescription medications should be intended as a trial, supported by clear goals and an unequivocal understanding that doses will not be indiscriminately increased.20
Health care providers who prescribe opioids are ultimately responsible for monitoring risk factors that may increase overdose and death, and dashboard technology assists them in this effort. The VISN 22 high-risk opioid dashboard is a tool that allows providers to identify and prioritize veterans who are at high risk for overdose. Initial data collected suggest that the dashboard has decreased the risk of negative consequences associated with opioid medication use today. However, the authors wish to emphasize that this technology is only part of the solution; although it can be a tool to identify actions that may need to take place and can track progress of changes in care, there must be complementary efforts in provider and patient education, improved access to mental health care, and interdisciplinary models of care that expand current chronic pain treatment options. Future considerations of this technology may include incorporating other risk factors accounting for psychosocial variables specific to military personnel that may further increase the overall risk of overdose.
Acknowledgements
The authors wish to thank the leadership of VISN 22 for their support of this initiative. Dr. Kryskalla recognizes VA OI&T for making this work possible and her family for their support. Ms. Kern would like to thank Aaron, Leslie, and Rachel Kern for their continuous support. Dr. Hauser wishes to thank Cathy, Anika, Katia, Max, and Jirina Hauser for their unwavering support.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[Published Online Ahead of Print: August 14, 2014.]
1. Brooks D. Hearing Spotlights painkiller overuse among soldiers. http://www.fayobserver.com/military/article_a6e4a2e9-827d-577c-a79a-87a6c07cf151.html. Fayobserver Website. Published October 10, 2013, Accessed June 9, 2014.
2. Seal KH, Shi Y, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
3. National Institute on Drug Abuse. DrugFacts: Substance Abuse in the Military. http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military. National Institute on Drug Abuse Website. Revised March 2013. Accessed June 9, 2014.
4. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311.
5. Pergolizzi JV Jr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443-451.
6. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85-92.
7. Substance Abuse and Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit. HHS publication No. (SMA) 13-4742. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
8. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
9. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
10. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581-624.
11. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepine, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
12. Centers for Disease Control and Prevention. FastStats: Deaths and mortality. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/deaths.htm. Updated February 13, 2014. Accessed June 9, 2014.
13. Kemp J, Bossarte R. Suicide Data Report, 2012. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed July 1, 2014.
14. National Institute of Mental Health. Suicide in the U.S. Statistics. National Institute of Mental Health Website. http://www.nimh.nih.gov/statistics/index.shtml. Accessed June 27, 2014.
15. Miller M, Hempstead K, Nguyen T, Barber C, Rosenberg-Wohl S, Azrael D. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: Implications for clinical practice. Am J Public Health. 2013;103(6):e61-e68.
16. Hartl TL, Rosen C, Drescher K, Lee TT, Gusman F. Predicting high-risk behaviors in Veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2005;193(7):464-472.
17. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
18. Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870-878.
19. Bray RM, Pemberton MR, Lane ME, Hourani LL, Mattiko MJ, Babeu LA. Substance use and mental health trends among U.S. military active duty personnel. Key findings from the 2008 DoD Health Behavior Survey. Mil Med. 2010;175(6):390-399.
20. Cuevas-Trisan RL. The unfortunate turn of events in pain management. Fed Pract. 2013;30(3):8-9.
On October 10, 2013, a Congressional hearing was held to address the issue of opioid medication prescribing within VHA facilities and clinics (House Veteran Affairs Subcommittee hearing “Between Peril and Promise: Facing the Dangers of VA’s Skyrocketing Use of Prescription Painkillers to Treat Veterans”). Several individuals testified, including the widows of 2 veterans; both their husbands had overdosed on prescribed opioid medications. One husband had been taking as many as 15 pills a day and was additionally prescribed oxycodone/acetaminophen, which led to his death.1
Alongside the widows were 2 veterans who had been treated for chronic back pain injuries sustained before and during deployment in Iraq. Both had been prescribed several pain medications, including oxycodone/acetaminophen, methadone, and morphine. One reported that as his pain increased, his doctors continued to provide him additional prescriptions; at one point he had more than 13 prescriptions and could no longer work from being so “doped up.”1
In the past 2 decades, health care professionals (HCPs) have placed greater emphasis on chronic pain management. As a result, the rate of opioid medication prescribing has increased dramatically. Since 1994, the number of opioid medication prescriptions has nearly doubled; this change has been accompanied by an increase in opioid misuse, which has resulted in accidental or intentional overdose and death.2
Based on a recent National Institute on Drug Abuse (NIDA) report, the greatest impact has been on armed forces personnel.3 Prescriptions for pain relievers quadrupled between 2001 and 2009 to almost 3.8 million within the military population. Although civilian populations are more likely to abuse illicit drugs, military personnel are at particular risk of prescription abuse, including opioid medications.3 In 2008, 11% of armed forces service members reported misusing prescription drugs, with opioid medications being the most abused. This is an approximate 5- to 6-fold increase since 2002 (2% reported misuse in 2002).3 Particularly concerning is the associated rise in suicide rates among armed forces personnel, which surpassed civilian suicide rates in 2004. In 2009, one-third of suicides among armed forces personnel involved prescription drugs.3
Certain patient characteristics or factors are related to greater overdose risk. These risk factors include prescription dosage and frequency, history of suicide attempts or self-harm behavior, history of depression or posttraumatic stress disorder (PTSD) among other mental health-related diagnoses, a history of substance and/or alcohol abuse, and within the context of opioid medication use, the concurrent use of other central nervous system (CNS) depressants.4,5 Additionally, the stresses of deployment during wartime, physical injuries sustained in combat, and the unique military culture play a particularly important role in access to substances with high abuse potential and the subsequent development of substance abuse.3
Opioid Use and Risk Factors
More than 3% of adults in the U.S. are now receiving opioid medications for chronic noncancer pain.6 Substance abuse among patients with chronic pain ranges from 14% to 40%.5 Prescription opioid medications are the fastest growing drugs of abuse and the most common cause of unintentional overdose in the U.S.4 About 17,000 deaths occur each year as a result of prescription opioid medication overdose.7 Opioid medication-related overdose deaths began to increase in the early 2000s and continue to increase. Between 1999 and 2007, the rate of unintentional overdose-related deaths in the U.S. increased by 124%, largely due to the increase of prescription opioid medications.8
High-Dose Opioid Medication Use
A study by Dunn and colleagues found that patients receiving higher doses of prescribed opioid medications were at an increased risk of overdose.6 Patients receiving 50 mg to 99 mg morphine equivalent daily dose (MEDD) had a 3.7-fold increase in overdose risk (0.7% annual overdose rate) as compared with patients who received < 50 mg MEDD (0.2% annual overdose rate). Patients receiving ≥ 100 mg MEDD had a 1.8% annual overdose rate and a 9.8-fold increase in overdose risk as compared with patients who received < 50 mg MEDD. Overall, 51 patients experienced ≥ 1 overdose event, 40 of whom experienced fatal or serious overdoses and 6 of whom attempted suicide. Patients receiving the highest doses were male, current smokers, and had a history of depression and substance abuse.6 Similarly, a study by Bohnert and colleagues found that opioid medication overdose was most likely to occur in those patients with psychiatric and substance use disorders compared with patients who had no psychiatric illness history.8
Depression
Mood disorders are common in people with chronic pain.4,5,9,10 In particular, patients with a history of depression are more likely to receive chronic opioid medication prescriptions and are at a higher risk for opioid medication abuse. A substance abuse history is the most consistent predictor of both chronic opioid medication use and abuse. However, depression without substance abuse is significantly associated with 2 forms of opioid medication abuse: self-medication for stress or sleep and overmedication (using a higher dose than prescribed). More severe cases of depression show a stronger association for potential abuse.4
PTSD
Among Iraq and Afghanistan war veterans with ≥ 1 pain-related diagnosis, veterans with PTSD and veterans with a mental health disorder other than PTSD were significantly more likely to receive opioid medications for pain than were veterans without a mental health disorder (PTSD—17.8%, adjusted relative risk [RR] 2.58; other mental health disorder—11.7%, RR 1.74; no mental health disorder—6.5%).2 Although mental health disorders in general were related to a higher risk of opioid abuse, those with PTSD in particular were more likely to receive higher prescribed dosages; to continue taking opioids for a longer period; to receive concurrent prescriptions for opioid medications, sedative hypnotics, or both; to obtain early refills; and to have comorbid alcohol and substance use disorders. Based on these results, Seal and colleagues concluded that veterans with PTSD had the highest risk of alcohol, drug, and opioid-related accidents and overdose as well as self-inflicted injuries.2
Concurrent Use of Opioids and CNS Depressants
As mentioned earlier, studies suggest that people with PTSD are at a significantly higher risk for opioid medication overdose. One factor that may contribute to this higher risk is the concurrent use of CNS depressants/sedatives, particularly benzodiazepines and alcohol.
Benzodiazepines are often prescribed for people with PTSD. One study found that the concurrent use of benzodiazepines is significantly related to opioid overdose.5 Prescribing opioids for people already abusing or dependent on alcohol or other substances increases the risk of abuse and overdose. Furthermore, the concurrent use of multiple medications is associated with aberrant behaviors, cognitive impairment, and medication abuse, potentially leading to overdose. Overall, the combined administration of these medications is responsible for higher rates of adverse events, overdose, and death related to prescription opioid medication use.5,6,11
In summary, there are various risk factors that contribute to opioid medication overdose and more generally, risk of suicide, including (1) high-dose opioid medications; (2) history of psychiatric disorders, specifically depression and PTSD; (3) history of substance use disorders; and (4) concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse.
Suicide
Suicide is the tenth leading cause of death in the U.S., with 12.4 suicide deaths per 100,000 population.12 Suicide rates are even higher among veterans. According to the VHA, the age-adjusted rate of suicide for veterans using VHA facilities and clinics was 35.9 per 100,000 person-years for fiscal year 2009.13 Several risk factors for suicide attempts include depression and other mental health disorders, substance abuse, medical disorders, and prescription medications.
Prior suicide attempts or self-harm behavior is known to increase the risk of subsequent death by suicide. About 11 attempted suicides occur per suicide death where the medical severity of prior attempts increases the risk of future suicide, as does a history of multiple self-harm episodes.14,15 One study found that the single best predictor of suicide in a veteran population was an attempt in the previous 4 months.16
Among other risk factors, previous suicide attempts and violent behavior are major behavioral flags that warrant caution and require particular consideration when prescribing opioid medications. In a national survey on drug use and health, about 18% of prescription opioid users/abusers who experienced suicidal ideations actually attempted suicide. Only 11% of individuals who never used prescription opioid medications attempted suicide after reported suicidal ideations.17
Patient Data Aggregation
The early and widespread adoption of electronic medical records (EMRs) by the VHA allowed the aggregation of patient data for quality improvement. Initially, data were aggregated, and dashboards were designed retrospectively. However, the development of regional data warehouses that update patient information daily from the EMR allowed information to be aggregated prospectively, and dashboards were designed that provided real-time information.
The purpose of the current study is to demonstrate the efficacy and future potential of dashboard technology in assessing prospectively high-risk factors for opioid overdose. Dashboards are a user-friendly application that allows providers to isolate and calculate daily morphine equivalent opioid dosages and assess patients’ risk factors for overdose on an individual basis. By using this technology, providers who prescribe opioids can get a concise summary of opioid and other medications and adjust medications to decrease overdose risk on an individual basis.
What is the Dashboard?
The VISN 22 high-risk opioid dashboard is a business intelligence tool that serves as a report card, or progress report, to provide a global view of the number of veterans who are receiving opioid prescriptions totaling >120 mg MEDD and who have characteristics (history of depression, PTSD, substance abuse, or high-risk suicide flag) and prescriptions (concomitant CNS depressants) that may increase patient risk for overdose.
The VISN 22 dashboard allows the user to navigate to an individual HCP-level and patient-level report (Figures 1 and 2). Filter settings allow report users to select only high-risk patients; it serves as a single location for pertinent details to consider for safely prescribing opioids.
To calculate daily morphine equivalents, each patient’s opioid prescriptions were evaluated. The quantity was divided by the day’s supply to calculate an average daily quantity. From there, the drug strength was used to convert to MEDD. Health care providers were informed that these conversion factors were not recommendations for clinical opioid conversions.
Implementation and Design
In 2012, the VA Pharmacy Benefits Management (PBM) in VISN 21 created a dashboard that allowed users to identify patients on high-dose opioid prescriptions. Structured query language code was used to extract data from the regional data warehouse and calculate MEDD for all patients with active opioid prescriptions. In 2013, VISN 22 expanded that dashboard to incorporate factors that could indicate a high risk for overdose or other adverse outcomes, including a history of depression, PTSD, substance abuse or high-risk suicide flag, and concomitant use of CNS depressant medications.
The high-risk opioid dashboard (Figure 3) and accompanying patient-level report were first introduced to VISN 22 HCPs in January 2013. The business intelligence tools were introduced to each facility through the VISN 22 PBM group. Training on the use of the dashboard and the report was provided, with an initial target of decreasing MEDD of > 200 mg to < 5% of all veterans prescribed opioids at each VISN 22 facility. One month later (in February 2013), a second category of veterans (those with > 120 mg but < 199 mg MEDD) was added. Also the initial MEDD > 200 mg target of < 5% was decreased to < 3% to encourage additional progress.
Eight months after the VISN 22 dashboard technology was implemented there was a 17% decrease in patients with total daily morphine equivalents > 200 mg (January 2013; 1,137 patients vs August 2013; 940 patients—a decrease of 197 patients).
From March 2013 to August 2013, VISN 22 also saw a 12% decrease in the number of patients prescribed > 120 mg MEDD but < 199 MEDD (March 2013; 2,295 vs August 2013; 2,018—a decrease of 277 patients).
Figure 4 shows opioid use as of July 2014 for VISN 22 facilities. There were further reductions in the number of patients receiving > 120 mg but < 199 mg MEDD (August 2013; 2,018 patients vs July 2014; 1,189 patients) and patients receiving > 200 mg MEDD (August 2013; 940 patients vs July 2014; 836 patients).
Case Description
In January 2013, VISN 22 implemented dashboard technology to help providers assess and monitor opioid prescription levels in relation to high-risk variables. The benefits of this dashboard technology are illustrated in the case profile that follows.
A 67-year-old male veteran had a long history of chronic pain. Pain diagnoses included osteoarthritis with spine involvement, lumbar radiculopathy, arthralgia, and peripheral neuropathy. For the past 10 years, he was prescribed opioids with modest relief of his chronic pain symptoms despite recent prescriptions totaling 300 mg MEDD. This veteran had several risk factors for overdose, including a history of depression, suicide risk, PTSD, and concomitant use of the CNS depressants alprazolam and cyclobenzaprine.
More recently, in May 2013, the veteran exhibited aberrant behavior and requested early refills for alprazolam. In response, the pharmacist discussed the case with the HCP who prescribed the opioids, noting the concomitant overdose risk factors. As a result of this interaction, the veteran was referred for mental health services, and his prescriptions for opioids were gradually decreased. He is currently stable, now receiving 120 mg MEDD, and his pain is currently described as moderately controlled on the new lower dose.
In summary, this veteran was receiving > 200 mg MEDD with several known overdose risk factors. Once the HCP was made aware of these risk factors, necessary precautions were taken, and the veteran was safely tapered to a lower dose. Dashboard technology makes the list of risk factors readily available to HCPs who are prescribing (and the pharmacists reviewing the prescriptions), thus allowing a proactive discussion of risks and benefits before continuing, renewing, or initiating opioid prescriptions.
Discussion
As reported in 2013 by NIDA, the greater availability of opioid medications and the consequent increase in prescriptions may be contributing directly to their growing misuse by both civilians and military service personnel. A direct consequence has been an increase in both accidental and intentional overdose deaths.3 Several factors are related to the risk of overdose/death, including high-dose opioid medications, a history of psychiatric disorders (specifically depression and PTSD), a history of substance use disorders, concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse, and previous attempts of suicide.
The VISN 22 high-risk opioid dashboard was a rapid information technology response to the increasing risk faced by veterans who have chronic pain and comorbid psychiatric and substance use disorders and are prescribed opioids and CNS depressants. The purpose of implementing this dashboard technology was to assist HCPs in prescribing opioids safely, using a technology that allows for the monitoring and management of concomitant suicide risk factors. Following the national Opioid Safety Initiative, this dashboard tool is being used to identify veterans who are on high-dose opioids with the goal of reducing the number of veterans on > 200 mg MEDD. The dashboard allows data to be stratified, using the concomitant risk factors for suicide to assist facilities and their providers in the identification and prioritization of highest risk patients first.
An initial review of dashboard data in VISN 22 suggests that it is a useful tool for reducing high-dose opioid prescriptions (> 200 mg MEDD and > 120 mg but < 199 mg MEDD). Across the 5 VA locations in VISN 22, in the first 8 months of implementation, 4 locations were able to lower prescription opioid medication levels to the initial target of < 5%; 2 lowered rates even further (to < 3%). The VA Greater Los Angeles Healthcare System remains at a commendable 1%. Although the number of veterans with prescriptions totaling > 200 mg MEDD has decreased as a result, a greater reduction is expected with the coordinated education and systems improvement efforts associated with the national VHA Opioid Safety Initiative. As part of the process to lower the number of patients on high-dose opioids in the future, HCP and patient education will be provided in relation to the use of dashboard technology.
Limitations
There are several limitations that affect interpretation of the usefulness of the VISN 22 high-risk opioid dashboard. Prior to the implementation of the dashboard, 2 of 5 VISN sites already had efforts in place to reduce opioid overprescribing. The VA Greater Los Angeles Healthcare System had an opioid reduction program in place before the dashboard was implemented, so it is possible reductions in opioid prescribing were a result of their previous efforts and not related to the dashboard. Similarly the VA Long Beach Healthcare System had begun a quality improvement initiative to reduce high-dose opioid prescribing prior to dashboard implementation. However, it was difficult to pinpoint the direct effect the dashboard had on patient interventions due to lack of documentation of dashboard use in the clinical notes.
A direct relationship did exist between dashboard implementation and opioid dose reduction in patients with > 200 MEDD at the remaining 3 VISN 22 facilities. Overall, this suggests that the dashboard played a significant role across all sites. Implementation of the dashboard across VISN 22 was accompanied by an education effort that resulted in an increased awareness among HCPs to evaluate certain risks in patients on high-dose opioids and to evaluate the combination of opioid and CNS depressant use. Prior to dashboard implementation, there was no standardized monitoring system that cross-referenced high-dose opioid prescribing with psychiatric illness and suicide risk factors.
Conclusions
From 2000 to 2010, opioid prescriptions nearly doubled, yet this rate was not accompanied by a change/increase in the rate of nonopioid analgesic medication prescriptions.18 Health care providers need to account for veterans’ wishes for pain treatment and be aware of options other than opioids, particularly given the risk of opioid-related accidental or intentional overdose; it is imperative that treatment become more individualized and more closely monitored.19,20 It is recommended that opioids should be the treatment of last resort in managing chronic noncancer pain. The use of opioid prescription medications should be intended as a trial, supported by clear goals and an unequivocal understanding that doses will not be indiscriminately increased.20
Health care providers who prescribe opioids are ultimately responsible for monitoring risk factors that may increase overdose and death, and dashboard technology assists them in this effort. The VISN 22 high-risk opioid dashboard is a tool that allows providers to identify and prioritize veterans who are at high risk for overdose. Initial data collected suggest that the dashboard has decreased the risk of negative consequences associated with opioid medication use today. However, the authors wish to emphasize that this technology is only part of the solution; although it can be a tool to identify actions that may need to take place and can track progress of changes in care, there must be complementary efforts in provider and patient education, improved access to mental health care, and interdisciplinary models of care that expand current chronic pain treatment options. Future considerations of this technology may include incorporating other risk factors accounting for psychosocial variables specific to military personnel that may further increase the overall risk of overdose.
Acknowledgements
The authors wish to thank the leadership of VISN 22 for their support of this initiative. Dr. Kryskalla recognizes VA OI&T for making this work possible and her family for their support. Ms. Kern would like to thank Aaron, Leslie, and Rachel Kern for their continuous support. Dr. Hauser wishes to thank Cathy, Anika, Katia, Max, and Jirina Hauser for their unwavering support.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[Published Online Ahead of Print: August 14, 2014.]
On October 10, 2013, a Congressional hearing was held to address the issue of opioid medication prescribing within VHA facilities and clinics (House Veteran Affairs Subcommittee hearing “Between Peril and Promise: Facing the Dangers of VA’s Skyrocketing Use of Prescription Painkillers to Treat Veterans”). Several individuals testified, including the widows of 2 veterans; both their husbands had overdosed on prescribed opioid medications. One husband had been taking as many as 15 pills a day and was additionally prescribed oxycodone/acetaminophen, which led to his death.1
Alongside the widows were 2 veterans who had been treated for chronic back pain injuries sustained before and during deployment in Iraq. Both had been prescribed several pain medications, including oxycodone/acetaminophen, methadone, and morphine. One reported that as his pain increased, his doctors continued to provide him additional prescriptions; at one point he had more than 13 prescriptions and could no longer work from being so “doped up.”1
In the past 2 decades, health care professionals (HCPs) have placed greater emphasis on chronic pain management. As a result, the rate of opioid medication prescribing has increased dramatically. Since 1994, the number of opioid medication prescriptions has nearly doubled; this change has been accompanied by an increase in opioid misuse, which has resulted in accidental or intentional overdose and death.2
Based on a recent National Institute on Drug Abuse (NIDA) report, the greatest impact has been on armed forces personnel.3 Prescriptions for pain relievers quadrupled between 2001 and 2009 to almost 3.8 million within the military population. Although civilian populations are more likely to abuse illicit drugs, military personnel are at particular risk of prescription abuse, including opioid medications.3 In 2008, 11% of armed forces service members reported misusing prescription drugs, with opioid medications being the most abused. This is an approximate 5- to 6-fold increase since 2002 (2% reported misuse in 2002).3 Particularly concerning is the associated rise in suicide rates among armed forces personnel, which surpassed civilian suicide rates in 2004. In 2009, one-third of suicides among armed forces personnel involved prescription drugs.3
Certain patient characteristics or factors are related to greater overdose risk. These risk factors include prescription dosage and frequency, history of suicide attempts or self-harm behavior, history of depression or posttraumatic stress disorder (PTSD) among other mental health-related diagnoses, a history of substance and/or alcohol abuse, and within the context of opioid medication use, the concurrent use of other central nervous system (CNS) depressants.4,5 Additionally, the stresses of deployment during wartime, physical injuries sustained in combat, and the unique military culture play a particularly important role in access to substances with high abuse potential and the subsequent development of substance abuse.3
Opioid Use and Risk Factors
More than 3% of adults in the U.S. are now receiving opioid medications for chronic noncancer pain.6 Substance abuse among patients with chronic pain ranges from 14% to 40%.5 Prescription opioid medications are the fastest growing drugs of abuse and the most common cause of unintentional overdose in the U.S.4 About 17,000 deaths occur each year as a result of prescription opioid medication overdose.7 Opioid medication-related overdose deaths began to increase in the early 2000s and continue to increase. Between 1999 and 2007, the rate of unintentional overdose-related deaths in the U.S. increased by 124%, largely due to the increase of prescription opioid medications.8
High-Dose Opioid Medication Use
A study by Dunn and colleagues found that patients receiving higher doses of prescribed opioid medications were at an increased risk of overdose.6 Patients receiving 50 mg to 99 mg morphine equivalent daily dose (MEDD) had a 3.7-fold increase in overdose risk (0.7% annual overdose rate) as compared with patients who received < 50 mg MEDD (0.2% annual overdose rate). Patients receiving ≥ 100 mg MEDD had a 1.8% annual overdose rate and a 9.8-fold increase in overdose risk as compared with patients who received < 50 mg MEDD. Overall, 51 patients experienced ≥ 1 overdose event, 40 of whom experienced fatal or serious overdoses and 6 of whom attempted suicide. Patients receiving the highest doses were male, current smokers, and had a history of depression and substance abuse.6 Similarly, a study by Bohnert and colleagues found that opioid medication overdose was most likely to occur in those patients with psychiatric and substance use disorders compared with patients who had no psychiatric illness history.8
Depression
Mood disorders are common in people with chronic pain.4,5,9,10 In particular, patients with a history of depression are more likely to receive chronic opioid medication prescriptions and are at a higher risk for opioid medication abuse. A substance abuse history is the most consistent predictor of both chronic opioid medication use and abuse. However, depression without substance abuse is significantly associated with 2 forms of opioid medication abuse: self-medication for stress or sleep and overmedication (using a higher dose than prescribed). More severe cases of depression show a stronger association for potential abuse.4
PTSD
Among Iraq and Afghanistan war veterans with ≥ 1 pain-related diagnosis, veterans with PTSD and veterans with a mental health disorder other than PTSD were significantly more likely to receive opioid medications for pain than were veterans without a mental health disorder (PTSD—17.8%, adjusted relative risk [RR] 2.58; other mental health disorder—11.7%, RR 1.74; no mental health disorder—6.5%).2 Although mental health disorders in general were related to a higher risk of opioid abuse, those with PTSD in particular were more likely to receive higher prescribed dosages; to continue taking opioids for a longer period; to receive concurrent prescriptions for opioid medications, sedative hypnotics, or both; to obtain early refills; and to have comorbid alcohol and substance use disorders. Based on these results, Seal and colleagues concluded that veterans with PTSD had the highest risk of alcohol, drug, and opioid-related accidents and overdose as well as self-inflicted injuries.2
Concurrent Use of Opioids and CNS Depressants
As mentioned earlier, studies suggest that people with PTSD are at a significantly higher risk for opioid medication overdose. One factor that may contribute to this higher risk is the concurrent use of CNS depressants/sedatives, particularly benzodiazepines and alcohol.
Benzodiazepines are often prescribed for people with PTSD. One study found that the concurrent use of benzodiazepines is significantly related to opioid overdose.5 Prescribing opioids for people already abusing or dependent on alcohol or other substances increases the risk of abuse and overdose. Furthermore, the concurrent use of multiple medications is associated with aberrant behaviors, cognitive impairment, and medication abuse, potentially leading to overdose. Overall, the combined administration of these medications is responsible for higher rates of adverse events, overdose, and death related to prescription opioid medication use.5,6,11
In summary, there are various risk factors that contribute to opioid medication overdose and more generally, risk of suicide, including (1) high-dose opioid medications; (2) history of psychiatric disorders, specifically depression and PTSD; (3) history of substance use disorders; and (4) concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse.
Suicide
Suicide is the tenth leading cause of death in the U.S., with 12.4 suicide deaths per 100,000 population.12 Suicide rates are even higher among veterans. According to the VHA, the age-adjusted rate of suicide for veterans using VHA facilities and clinics was 35.9 per 100,000 person-years for fiscal year 2009.13 Several risk factors for suicide attempts include depression and other mental health disorders, substance abuse, medical disorders, and prescription medications.
Prior suicide attempts or self-harm behavior is known to increase the risk of subsequent death by suicide. About 11 attempted suicides occur per suicide death where the medical severity of prior attempts increases the risk of future suicide, as does a history of multiple self-harm episodes.14,15 One study found that the single best predictor of suicide in a veteran population was an attempt in the previous 4 months.16
Among other risk factors, previous suicide attempts and violent behavior are major behavioral flags that warrant caution and require particular consideration when prescribing opioid medications. In a national survey on drug use and health, about 18% of prescription opioid users/abusers who experienced suicidal ideations actually attempted suicide. Only 11% of individuals who never used prescription opioid medications attempted suicide after reported suicidal ideations.17
Patient Data Aggregation
The early and widespread adoption of electronic medical records (EMRs) by the VHA allowed the aggregation of patient data for quality improvement. Initially, data were aggregated, and dashboards were designed retrospectively. However, the development of regional data warehouses that update patient information daily from the EMR allowed information to be aggregated prospectively, and dashboards were designed that provided real-time information.
The purpose of the current study is to demonstrate the efficacy and future potential of dashboard technology in assessing prospectively high-risk factors for opioid overdose. Dashboards are a user-friendly application that allows providers to isolate and calculate daily morphine equivalent opioid dosages and assess patients’ risk factors for overdose on an individual basis. By using this technology, providers who prescribe opioids can get a concise summary of opioid and other medications and adjust medications to decrease overdose risk on an individual basis.
What is the Dashboard?
The VISN 22 high-risk opioid dashboard is a business intelligence tool that serves as a report card, or progress report, to provide a global view of the number of veterans who are receiving opioid prescriptions totaling >120 mg MEDD and who have characteristics (history of depression, PTSD, substance abuse, or high-risk suicide flag) and prescriptions (concomitant CNS depressants) that may increase patient risk for overdose.
The VISN 22 dashboard allows the user to navigate to an individual HCP-level and patient-level report (Figures 1 and 2). Filter settings allow report users to select only high-risk patients; it serves as a single location for pertinent details to consider for safely prescribing opioids.
To calculate daily morphine equivalents, each patient’s opioid prescriptions were evaluated. The quantity was divided by the day’s supply to calculate an average daily quantity. From there, the drug strength was used to convert to MEDD. Health care providers were informed that these conversion factors were not recommendations for clinical opioid conversions.
Implementation and Design
In 2012, the VA Pharmacy Benefits Management (PBM) in VISN 21 created a dashboard that allowed users to identify patients on high-dose opioid prescriptions. Structured query language code was used to extract data from the regional data warehouse and calculate MEDD for all patients with active opioid prescriptions. In 2013, VISN 22 expanded that dashboard to incorporate factors that could indicate a high risk for overdose or other adverse outcomes, including a history of depression, PTSD, substance abuse or high-risk suicide flag, and concomitant use of CNS depressant medications.
The high-risk opioid dashboard (Figure 3) and accompanying patient-level report were first introduced to VISN 22 HCPs in January 2013. The business intelligence tools were introduced to each facility through the VISN 22 PBM group. Training on the use of the dashboard and the report was provided, with an initial target of decreasing MEDD of > 200 mg to < 5% of all veterans prescribed opioids at each VISN 22 facility. One month later (in February 2013), a second category of veterans (those with > 120 mg but < 199 mg MEDD) was added. Also the initial MEDD > 200 mg target of < 5% was decreased to < 3% to encourage additional progress.
Eight months after the VISN 22 dashboard technology was implemented there was a 17% decrease in patients with total daily morphine equivalents > 200 mg (January 2013; 1,137 patients vs August 2013; 940 patients—a decrease of 197 patients).
From March 2013 to August 2013, VISN 22 also saw a 12% decrease in the number of patients prescribed > 120 mg MEDD but < 199 MEDD (March 2013; 2,295 vs August 2013; 2,018—a decrease of 277 patients).
Figure 4 shows opioid use as of July 2014 for VISN 22 facilities. There were further reductions in the number of patients receiving > 120 mg but < 199 mg MEDD (August 2013; 2,018 patients vs July 2014; 1,189 patients) and patients receiving > 200 mg MEDD (August 2013; 940 patients vs July 2014; 836 patients).
Case Description
In January 2013, VISN 22 implemented dashboard technology to help providers assess and monitor opioid prescription levels in relation to high-risk variables. The benefits of this dashboard technology are illustrated in the case profile that follows.
A 67-year-old male veteran had a long history of chronic pain. Pain diagnoses included osteoarthritis with spine involvement, lumbar radiculopathy, arthralgia, and peripheral neuropathy. For the past 10 years, he was prescribed opioids with modest relief of his chronic pain symptoms despite recent prescriptions totaling 300 mg MEDD. This veteran had several risk factors for overdose, including a history of depression, suicide risk, PTSD, and concomitant use of the CNS depressants alprazolam and cyclobenzaprine.
More recently, in May 2013, the veteran exhibited aberrant behavior and requested early refills for alprazolam. In response, the pharmacist discussed the case with the HCP who prescribed the opioids, noting the concomitant overdose risk factors. As a result of this interaction, the veteran was referred for mental health services, and his prescriptions for opioids were gradually decreased. He is currently stable, now receiving 120 mg MEDD, and his pain is currently described as moderately controlled on the new lower dose.
In summary, this veteran was receiving > 200 mg MEDD with several known overdose risk factors. Once the HCP was made aware of these risk factors, necessary precautions were taken, and the veteran was safely tapered to a lower dose. Dashboard technology makes the list of risk factors readily available to HCPs who are prescribing (and the pharmacists reviewing the prescriptions), thus allowing a proactive discussion of risks and benefits before continuing, renewing, or initiating opioid prescriptions.
Discussion
As reported in 2013 by NIDA, the greater availability of opioid medications and the consequent increase in prescriptions may be contributing directly to their growing misuse by both civilians and military service personnel. A direct consequence has been an increase in both accidental and intentional overdose deaths.3 Several factors are related to the risk of overdose/death, including high-dose opioid medications, a history of psychiatric disorders (specifically depression and PTSD), a history of substance use disorders, concurrent use of opioid medications and prescription sedatives (specifically benzodiazepines) as well as alcohol and nonprescription drugs of abuse, and previous attempts of suicide.
The VISN 22 high-risk opioid dashboard was a rapid information technology response to the increasing risk faced by veterans who have chronic pain and comorbid psychiatric and substance use disorders and are prescribed opioids and CNS depressants. The purpose of implementing this dashboard technology was to assist HCPs in prescribing opioids safely, using a technology that allows for the monitoring and management of concomitant suicide risk factors. Following the national Opioid Safety Initiative, this dashboard tool is being used to identify veterans who are on high-dose opioids with the goal of reducing the number of veterans on > 200 mg MEDD. The dashboard allows data to be stratified, using the concomitant risk factors for suicide to assist facilities and their providers in the identification and prioritization of highest risk patients first.
An initial review of dashboard data in VISN 22 suggests that it is a useful tool for reducing high-dose opioid prescriptions (> 200 mg MEDD and > 120 mg but < 199 mg MEDD). Across the 5 VA locations in VISN 22, in the first 8 months of implementation, 4 locations were able to lower prescription opioid medication levels to the initial target of < 5%; 2 lowered rates even further (to < 3%). The VA Greater Los Angeles Healthcare System remains at a commendable 1%. Although the number of veterans with prescriptions totaling > 200 mg MEDD has decreased as a result, a greater reduction is expected with the coordinated education and systems improvement efforts associated with the national VHA Opioid Safety Initiative. As part of the process to lower the number of patients on high-dose opioids in the future, HCP and patient education will be provided in relation to the use of dashboard technology.
Limitations
There are several limitations that affect interpretation of the usefulness of the VISN 22 high-risk opioid dashboard. Prior to the implementation of the dashboard, 2 of 5 VISN sites already had efforts in place to reduce opioid overprescribing. The VA Greater Los Angeles Healthcare System had an opioid reduction program in place before the dashboard was implemented, so it is possible reductions in opioid prescribing were a result of their previous efforts and not related to the dashboard. Similarly the VA Long Beach Healthcare System had begun a quality improvement initiative to reduce high-dose opioid prescribing prior to dashboard implementation. However, it was difficult to pinpoint the direct effect the dashboard had on patient interventions due to lack of documentation of dashboard use in the clinical notes.
A direct relationship did exist between dashboard implementation and opioid dose reduction in patients with > 200 MEDD at the remaining 3 VISN 22 facilities. Overall, this suggests that the dashboard played a significant role across all sites. Implementation of the dashboard across VISN 22 was accompanied by an education effort that resulted in an increased awareness among HCPs to evaluate certain risks in patients on high-dose opioids and to evaluate the combination of opioid and CNS depressant use. Prior to dashboard implementation, there was no standardized monitoring system that cross-referenced high-dose opioid prescribing with psychiatric illness and suicide risk factors.
Conclusions
From 2000 to 2010, opioid prescriptions nearly doubled, yet this rate was not accompanied by a change/increase in the rate of nonopioid analgesic medication prescriptions.18 Health care providers need to account for veterans’ wishes for pain treatment and be aware of options other than opioids, particularly given the risk of opioid-related accidental or intentional overdose; it is imperative that treatment become more individualized and more closely monitored.19,20 It is recommended that opioids should be the treatment of last resort in managing chronic noncancer pain. The use of opioid prescription medications should be intended as a trial, supported by clear goals and an unequivocal understanding that doses will not be indiscriminately increased.20
Health care providers who prescribe opioids are ultimately responsible for monitoring risk factors that may increase overdose and death, and dashboard technology assists them in this effort. The VISN 22 high-risk opioid dashboard is a tool that allows providers to identify and prioritize veterans who are at high risk for overdose. Initial data collected suggest that the dashboard has decreased the risk of negative consequences associated with opioid medication use today. However, the authors wish to emphasize that this technology is only part of the solution; although it can be a tool to identify actions that may need to take place and can track progress of changes in care, there must be complementary efforts in provider and patient education, improved access to mental health care, and interdisciplinary models of care that expand current chronic pain treatment options. Future considerations of this technology may include incorporating other risk factors accounting for psychosocial variables specific to military personnel that may further increase the overall risk of overdose.
Acknowledgements
The authors wish to thank the leadership of VISN 22 for their support of this initiative. Dr. Kryskalla recognizes VA OI&T for making this work possible and her family for their support. Ms. Kern would like to thank Aaron, Leslie, and Rachel Kern for their continuous support. Dr. Hauser wishes to thank Cathy, Anika, Katia, Max, and Jirina Hauser for their unwavering support.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
[Published Online Ahead of Print: August 14, 2014.]
1. Brooks D. Hearing Spotlights painkiller overuse among soldiers. http://www.fayobserver.com/military/article_a6e4a2e9-827d-577c-a79a-87a6c07cf151.html. Fayobserver Website. Published October 10, 2013, Accessed June 9, 2014.
2. Seal KH, Shi Y, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
3. National Institute on Drug Abuse. DrugFacts: Substance Abuse in the Military. http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military. National Institute on Drug Abuse Website. Revised March 2013. Accessed June 9, 2014.
4. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311.
5. Pergolizzi JV Jr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443-451.
6. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85-92.
7. Substance Abuse and Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit. HHS publication No. (SMA) 13-4742. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
8. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
9. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
10. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581-624.
11. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepine, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
12. Centers for Disease Control and Prevention. FastStats: Deaths and mortality. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/deaths.htm. Updated February 13, 2014. Accessed June 9, 2014.
13. Kemp J, Bossarte R. Suicide Data Report, 2012. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed July 1, 2014.
14. National Institute of Mental Health. Suicide in the U.S. Statistics. National Institute of Mental Health Website. http://www.nimh.nih.gov/statistics/index.shtml. Accessed June 27, 2014.
15. Miller M, Hempstead K, Nguyen T, Barber C, Rosenberg-Wohl S, Azrael D. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: Implications for clinical practice. Am J Public Health. 2013;103(6):e61-e68.
16. Hartl TL, Rosen C, Drescher K, Lee TT, Gusman F. Predicting high-risk behaviors in Veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2005;193(7):464-472.
17. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
18. Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870-878.
19. Bray RM, Pemberton MR, Lane ME, Hourani LL, Mattiko MJ, Babeu LA. Substance use and mental health trends among U.S. military active duty personnel. Key findings from the 2008 DoD Health Behavior Survey. Mil Med. 2010;175(6):390-399.
20. Cuevas-Trisan RL. The unfortunate turn of events in pain management. Fed Pract. 2013;30(3):8-9.
1. Brooks D. Hearing Spotlights painkiller overuse among soldiers. http://www.fayobserver.com/military/article_a6e4a2e9-827d-577c-a79a-87a6c07cf151.html. Fayobserver Website. Published October 10, 2013, Accessed June 9, 2014.
2. Seal KH, Shi Y, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940-947.
3. National Institute on Drug Abuse. DrugFacts: Substance Abuse in the Military. http://www.drugabuse.gov/publications/drugfacts/substance-abuse-in-military. National Institute on Drug Abuse Website. Revised March 2013. Accessed June 9, 2014.
4. Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10(4):304-311.
5. Pergolizzi JV Jr, Gharibo C, Passik S, et al. Dynamic risk factors in the misuse of opioid analgesics. J Psychosom Res. 2012;72(6):443-451.
6. Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med. 2010;152(2):85-92.
7. Substance Abuse and Mental Health Services Administration. SAMHSA Opioid Overdose Prevention Toolkit. HHS publication No. (SMA) 13-4742. Rockville, MD: Substance Abuse and Mental Health Service Administration; 2013.
8. Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315-1321.
9. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: A biopsychosocial perspective. Biol Psychiatry. 2003;54(3):399-409.
10. Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull. 2007;133(4):581-624.
11. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepine, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
12. Centers for Disease Control and Prevention. FastStats: Deaths and mortality. Centers for Disease Control and Prevention Website. http://www.cdc.gov/nchs/fastats/deaths.htm. Updated February 13, 2014. Accessed June 9, 2014.
13. Kemp J, Bossarte R. Suicide Data Report, 2012. U.S. Department of Veterans Affairs Website. http://www.va.gov/opa/docs/Suicide-Data-Report-2012-final.pdf. Accessed July 1, 2014.
14. National Institute of Mental Health. Suicide in the U.S. Statistics. National Institute of Mental Health Website. http://www.nimh.nih.gov/statistics/index.shtml. Accessed June 27, 2014.
15. Miller M, Hempstead K, Nguyen T, Barber C, Rosenberg-Wohl S, Azrael D. Method choice in nonfatal self-harm as a predictor of subsequent episodes of self-harm and suicide: Implications for clinical practice. Am J Public Health. 2013;103(6):e61-e68.
16. Hartl TL, Rosen C, Drescher K, Lee TT, Gusman F. Predicting high-risk behaviors in Veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2005;193(7):464-472.
17. Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-41, HHS Publication No. (SMA) 11-4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011.
18. Daubresse M, Chang HY, Yu Y, et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51(10):870-878.
19. Bray RM, Pemberton MR, Lane ME, Hourani LL, Mattiko MJ, Babeu LA. Substance use and mental health trends among U.S. military active duty personnel. Key findings from the 2008 DoD Health Behavior Survey. Mil Med. 2010;175(6):390-399.
20. Cuevas-Trisan RL. The unfortunate turn of events in pain management. Fed Pract. 2013;30(3):8-9.