User login
Cemiplimab-Associated Eruption of Generalized Eruptive Keratoacanthoma of Grzybowski
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.
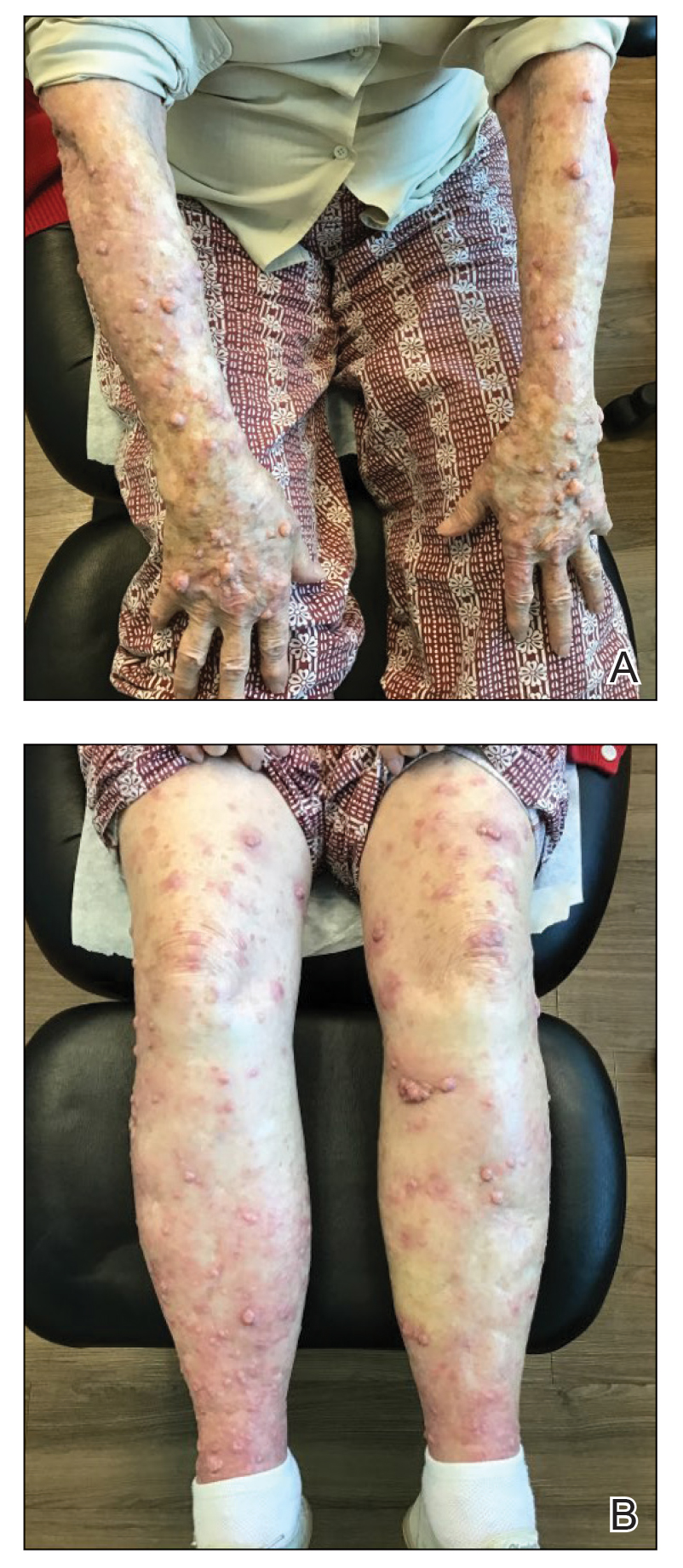
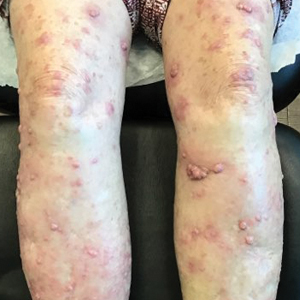
The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
To the Editor:
Treatment of cancer, including cutaneous malignancy, has been transformed by the use of immunotherapeutic agents such as immune checkpoint inhibitors (ICIs) that target cytotoxic T lymphocyte-associated antigen 4, programmed cell-death protein 1 (PD-1), or programmed cell-death ligand 1 (PD-L1). However, these drugs are associated with a distinct set of immune-related adverse events (IRAEs). We present a case of generalized eruptive keratoacanthoma of Grzybowski associated with the ICI cemiplimab.
A 94-year-old White woman presented to the dermatology clinic with acute onset of extensive, locally advanced cutaneous squamous cell carcinoma (cSCC) of the upper right posterolateral calf as well as multiple noninvasive cSCCs of the arms and legs. Her medical history was remarkable for widespread actinic keratoses and numerous cSCCs. The patient had no personal or family history of melanoma. Various cSCCs had required treatment with electrodesiccation and curettage, topical or intralesional 5-fluorouracil, and Mohs micrographic surgery. Approximately 1 year prior to presentation, oral acitretin was initiated to help control the cSCC. Given the extent of locally advanced disease, which was considered unresectable, she was referred to oncology but continued to follow up with dermatology. Positron emission tomography was remarkable for hypermetabolic cutaneous thickening in the upper right posterolateral calf with no evidence of visceral disease.

The patient was started on cemiplimab, an anti-PD-1 monoclonal antibody ICI indicated for the treatment of both metastatic and advanced cSCC. After 4 cycles of intravenous cemiplimab, the patient developed widespread nodules covering the arms and legs (Figure 1) as well as associated tenderness and pruritus. Biopsies of nodules revealed superficially invasive, well-differentiated cSCC consistent with keratoacanthoma. Although a lymphocytic infiltrate was present, no other specific reaction pattern, such as a lichenoid infiltrate, was present (Figure 2).

Positron emission tomography was repeated, demonstrating resolution of the right calf lesion; however, new diffuse cutaneous lesions and inguinal lymph node involvement were present, again without evidence of visceral disease. Given the clinical and histologic findings, a diagnosis of generalized eruptive keratoacanthoma of Grzybowski was made. Cemiplimab was discontinued after the fifth cycle. The patient declined further systemic treatment, instead choosing a regimen of topical steroids and an emollient.
Immunotherapeutics have transformed cancer therapy, which includes ICIs that target cytotoxic T lymphocyte-associated antigen 4, PD-1, or PD-L1. Increased activity of these checkpoints allows tumor cells to downregulate T-cell activation, thereby evading immune destruction. When PD-1 on T cells binds PD-L1 on tumor cells, T lymphocytes are inhibited from cytotoxic-mediated killing. Therefore, anti-PD-1 ICIs such as cemiplimab permit T-lymphocyte activation and destruction of malignant cells. However, this unique mechanism of immunotherapy is associated with an array of IRAEs, which often manifest in a delayed and prolonged fashion.1 Immune-related adverse events most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.2 Notably, patients with certain tumors who experience these adverse effects might be more likely to have superior overall survival; therefore, IRAEs are sometimes used as an indicator of favorable treatment response.2,3
Dermatologic IRAEs associated with the use of a PD-1 inhibitor include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.4,5 Eruptions of keratoacanthoma rarely have been reported following treatment with the PD-1 inhibitors nivolumab and pembrolizumab.3,6,7 In our patient, we believe the profound and generalized eruptive keratoacanthoma—a well-differentiated cSCC variant—was related to treatment of locally advanced cSCC with cemiplimab. The mechanism underlying the formation of anti-PD-1 eruptive keratoacanthoma is not well understood. In susceptible patients, it is plausible that the inflammatory environment permitted by ICIs paradoxically induces regression of tumors such as locally invasive cSCC and simultaneously promotes formation of keratoacanthoma.
The role of inflammation in the pathogenesis and progression of cSCC is complex and possibly involves contrasting roles of leukocyte subpopulations.8 The increased incidence of cSCC in the immunocompromised population,8 PD-L1 overexpression in cSCC,9,10 and successful treatment of cSCC with PD-1 inhibition10 all suggest that inhibition of specific inflammatory pathways is pivotal in tumor pathogenesis. However, increased inflammation, particularly inflammation driven by T lymphocytes and Langerhans cells, also is believed to play a key role in the formation of cSCCs, including the degeneration of actinic keratosis into cSCC. Moreover, because keratoacanthomas are believed to be a cSCC variant and also are associated with PD-L1 overexpression,9 it is perplexing that PD-1 blockade may result in eruptive keratoacanthoma in some patients while also treating locally advanced cSCC, as seen in our patient. Successful treatment of keratoacanthoma with anti-inflammatory intralesional or topical corticosteroids adds to this complicated picture.3
We hypothesize that the pathogenesis of invasive cSCC and keratoacanthoma shares certain immune-mediated mechanisms but also differs in distinct manners. To understand the relationship between systemic treatment of cSCC and eruptive keratoacanthoma, further research is required.
In addition, the RAS/BRAF/MEK oncogenic pathway may be involved in the development of cSCCs associated with anti-PD-1. It is hypothesized that BRAF and MEK inhibition increases T-cell infiltration and increases PD-L1 expression on tumor cells,11 thus increasing the susceptibility of those cells to PD-1 blockade. Further supporting a relationship between the RAS/BRAF/MEK and PD-1 pathways, BRAF inhibitors are associated with development of SCCs and verrucal keratosis by upregulation of the RAS pathway.12,13 Perhaps a common mechanism underlying these pathways results in their shared association for an increased risk for cSCC upon blockade. More research is needed to fully elucidate the underlying biochemical mechanism of immunotherapy and formation of SCCs, such as keratoacanthoma.
Treatment of solitary keratoacanthoma often involves surgical excision; however, the sheer number of lesions in eruptive keratoacanthoma presents a larger dilemma. Because oral systemic retinoids have been shown to be most effective for treating eruptive keratoacanthoma, they are considered first-line therapy as monotherapy or in combination with surgical excision.3 Other treatment options include intralesional or topical corticosteroids, cyclosporine, 5-fluorouracil, imiquimod, and cryotherapy.3,6
The development of ICIs has revolutionized the treatment of cutaneous malignancy, yet we have a great deal more to comprehend on the systemic effects of these medications. Although IRAEs may signal a better response to therapy, some of these effects regrettably can be dose limiting. In our patient, cemiplimab was successful in treating locally advanced cSCC, but treatment also resulted in devastating widespread eruptive keratoacanthoma. The mechanism of this kind of eruption has yet to be understood; we hypothesize that it likely involves T lymphocyte–driven inflammation and the interplay of molecular and immune-mediated pathways.
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
- Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. doi:10.1038/s41572-020-0160-6
- Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. doi:10.1186/s40425-019-0805-8
- Freites-Martinez A, Kwong BY, Rieger KE, et al. Eruptive keratoacanthomas associated with pembrolizumab therapy. JAMA Dermatol. 2017;153:694-697. doi:10.1001/jamadermatol.2017.0989
- Shen J, Chang J, Mendenhall M, et al. Diverse cutaneous adverse eruptions caused by anti-programmed cell death-1 (PD-1) and anti-programmed cell death ligand-1 (PD-L1) immunotherapies: clinicalfeatures and management. Ther Adv Med Oncol. 2018;10:1758834017751634. doi:10.1177/1758834017751634
- Bandino JP, Perry DM, Clarke CE, et al. Two cases of anti-programmed cell death 1-associated bullous pemphigoid-like disease and eruptive keratoacanthomas featuring combined histopathology. J Eur Acad Dermatol Venereol. 2017;31:E378-E380. doi:10.1111/jdv.14179
- Marsh RL, Kolodney JA, Iyengar S, et al. Formation of eruptive cutaneous squamous cell carcinomas after programmed cell death protein-1 blockade. JAAD Case Rep. 2020;6:390-393. doi:10.1016/j.jdcr.2020.02.024
- Antonov NK, Nair KG, Halasz CL. Transient eruptive keratoacanthomas associated with nivolumab. JAAD Case Rep. 2019;5:342-345. doi:10.1016/j.jdcr.2019.01.025
- Bottomley MJ, Thomson J, Harwood C, et al. The role of the immune system in cutaneous squamous cell carcinoma. Int J Mol Sci. 2019;20:2009. doi:10.3390/ijms20082009
- Gambichler T, Gnielka M, Rüddel I, et al. Expression of PD-L1 in keratoacanthoma and different stages of progression in cutaneous squamous cell carcinoma. Cancer Immunol Immunother. 2017;66:1199-1204. doi:10.1007/s00262-017-2015-x
- Patel R, Chang ALS. Immune checkpoint inhibitors for treating advanced cutaneous squamous cell carcinoma. Am J Clin Dermatol. 2019;20:477-482. doi:10.1007/s40257-019-00426-w
- Rozeman EA, Blank CU. Combining checkpoint inhibition and targeted therapy in melanoma. Nat Med. 2019;25:879-882. doi:10.1038/s41591-019-0482-7
- Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20-23. doi:10.3816/CGC.2009.n.003
- Chen P, Chen F, Zhou B. Systematic review and meta-analysis of prevalence of dermatological toxicities associated with vemurafenib treatment in patients with melanoma. Clin Exp Dermatol. 2019;44:243-251. doi:10.1111/ced.13751
Practice Points
- Immunotherapy, including immune checkpoint inhibitors such as programmed cell-death protein 1 (PD-1) inhibitors, is associated with an array of immune-related adverse events that often manifest in a delayed and prolonged manner. They most commonly affect the gastrointestinal tract as well as the endocrine and dermatologic systems.
- Dermatologic adverse effects associated with PD-1 inhibitors include lichenoid reactions, pruritus, morbilliform eruptions, vitiligo, and bullous pemphigoid.
- Eruptions of keratoacanthoma rarely have been reported following treatment with PD-1 inhibitors such as cemiplimab, nivolumab, and pembrolizumab.
Metastatic Adamantinoma Presenting as a Cutaneous Papule
To the Editor:
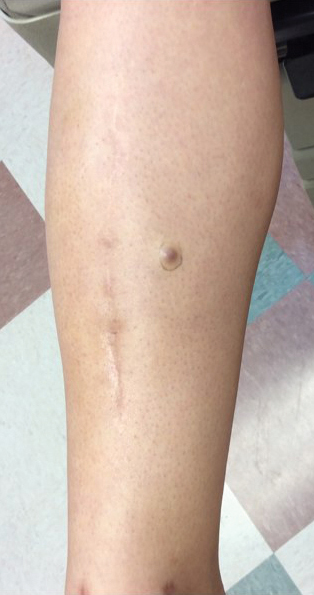
A 34-year-old woman with a history of adamantinoma of the right tibia that had been surgically resected with tibial reconstruction 5 years prior presented with a mildly tender, enlarging lesion on the right distal shin of 6 months’ duration that had started to change color. Review of systems was otherwise negative. Physical examination revealed an 8-mm, slightly tender, rubbery, pink papule adjacent to the surgical scar over the right tibia (Figure 1). Given the rapid growth of the lesion and its proximity to the surgical site, a punch biopsy was performed.
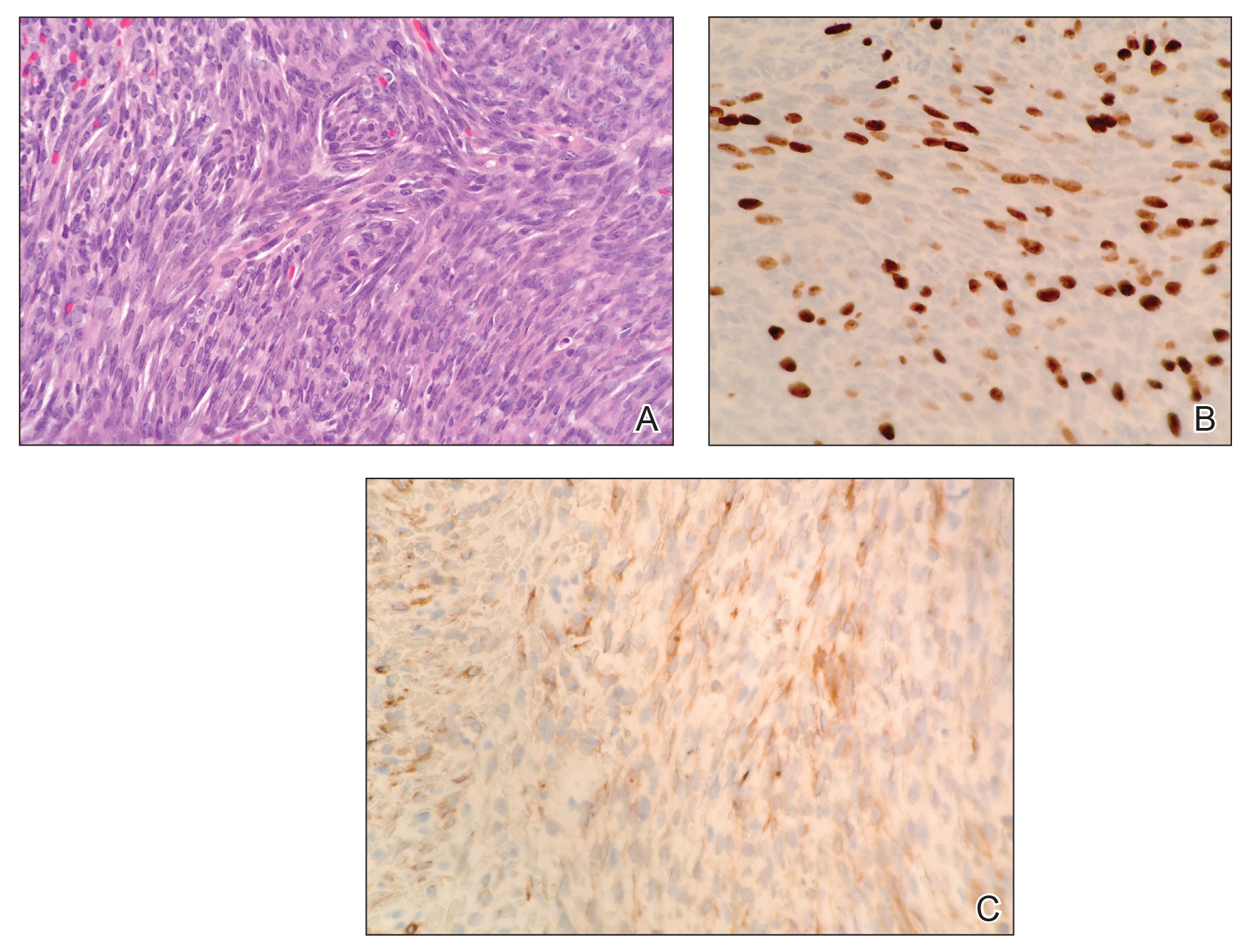
Histopathologic examination demonstrated a densely cellular dermal tumor composed of spindle cells with large hyperchromatic nuclei, numerous mitotic figures, and minimal eosinophilic cytoplasm (Figure 2A). Immunohistochemical studies revealed that approximately 40% of the tumor nuclei were immunoreactive to Ki-67 (Figure 2B), and total cytokeratin was focally positive (Figure 2C). A diagnosis of metastatic adamantinoma was made. Positron emission tomography and magnetic resonance imaging revealed new lytic lesions involving the T10 and L2 vertebrae (without frank spinal cord compression) and the right superior sacrum. Additionally, a small pulmonary nodule on the left upper lobe was noted on positron emission tomography, but it was below the size threshold for reliable detection. A computed tomography–guided biopsy of the T10 lesion demonstrated metastatic adamantinoma. The patient underwent a spinal stabilization procedure and discussed options regarding further oncologic and palliative management.
Adamantinoma is an extremely rare primary malignant bone tumor that typically involves the anterior portion of the tibial metaphysis or diaphysis in approximately 90% of cases. Young adults most commonly are affected in the third or fourth decades of life.1 Although the histogenesis is not clearly understood, experts have theorized that fetal implantation during embryogenesis or traumatic implantation of epithelial cells may be causes of this tumor and may explain the close pathologic similarity to basal cell carcinoma.2
Adamantinomas are slow growing, and as a result, patients often present with gradual onset of pain and swelling that persists for years.3,4 Metastasis occurs in 10% to 30% of patients, typically located in regional lymph nodes, the lungs, and distant bone.1,4 Our case represents a rare instance of adamantinoma metastasis to the skin. Although primary adamantinomas consist of both epithelial and stromal components, the typical metastatic lesions of adamantinomas are solely epithelial (often in a spindle-cell pattern),1 as was seen in our patient.
Operative removal via amputation or en bloc resection with limb salvage is the current treatment of choice. Adamantinomas are highly radioresistant, and chemotherapy has shown minimal efficacy.3,5
In conclusion, the presence of cutaneous metastasis from an adamantinoma is rare. Our case emphasizes this tumor’s potential for late metastasis as well as late recurrence.3,6 Most importantly, dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
- Schowinsky JT, Ormond DR, Kleinschmidt-DeMasters BK. Tibial adamantinoma: late metastasis to the brain. J Neuropathol Exp Neurol. 2015;74:95-97.
- Jain D, Jain VK, Vasishta RK, et al. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:8.
- Qureshi AA, Shott S, Mallin BA, et al. Current trends in the management of adamantinoma of long bones. an international study. J Bone Joint Surg Am. 2000;82-A:1122-1131.
- Desai SS, Jambhekar N, Agarwal M, et al. Adamantinoma of tibia: a study of 12 cases. J Surg Oncol. 2006;93:429-433.
- Weiss SW, Dorfman HD. Adamantinoma of long bone. an analysis of nine new cases with emphasis on metastasizing lesions and fibrous dysplasia-like changes. Hum Pathol. 1977;8:141-153.
- Szendroi M, Antal I, Arató G. Adamantinoma of long bones: a long-term follow-up study of 11 cases. Pathol Oncol Res. 2009;15:209-216.
To the Editor:
A 34-year-old woman with a history of adamantinoma of the right tibia that had been surgically resected with tibial reconstruction 5 years prior presented with a mildly tender, enlarging lesion on the right distal shin of 6 months’ duration that had started to change color. Review of systems was otherwise negative. Physical examination revealed an 8-mm, slightly tender, rubbery, pink papule adjacent to the surgical scar over the right tibia (Figure 1). Given the rapid growth of the lesion and its proximity to the surgical site, a punch biopsy was performed.
Histopathologic examination demonstrated a densely cellular dermal tumor composed of spindle cells with large hyperchromatic nuclei, numerous mitotic figures, and minimal eosinophilic cytoplasm (Figure 2A). Immunohistochemical studies revealed that approximately 40% of the tumor nuclei were immunoreactive to Ki-67 (Figure 2B), and total cytokeratin was focally positive (Figure 2C). A diagnosis of metastatic adamantinoma was made. Positron emission tomography and magnetic resonance imaging revealed new lytic lesions involving the T10 and L2 vertebrae (without frank spinal cord compression) and the right superior sacrum. Additionally, a small pulmonary nodule on the left upper lobe was noted on positron emission tomography, but it was below the size threshold for reliable detection. A computed tomography–guided biopsy of the T10 lesion demonstrated metastatic adamantinoma. The patient underwent a spinal stabilization procedure and discussed options regarding further oncologic and palliative management.
Adamantinoma is an extremely rare primary malignant bone tumor that typically involves the anterior portion of the tibial metaphysis or diaphysis in approximately 90% of cases. Young adults most commonly are affected in the third or fourth decades of life.1 Although the histogenesis is not clearly understood, experts have theorized that fetal implantation during embryogenesis or traumatic implantation of epithelial cells may be causes of this tumor and may explain the close pathologic similarity to basal cell carcinoma.2
Adamantinomas are slow growing, and as a result, patients often present with gradual onset of pain and swelling that persists for years.3,4 Metastasis occurs in 10% to 30% of patients, typically located in regional lymph nodes, the lungs, and distant bone.1,4 Our case represents a rare instance of adamantinoma metastasis to the skin. Although primary adamantinomas consist of both epithelial and stromal components, the typical metastatic lesions of adamantinomas are solely epithelial (often in a spindle-cell pattern),1 as was seen in our patient.
Operative removal via amputation or en bloc resection with limb salvage is the current treatment of choice. Adamantinomas are highly radioresistant, and chemotherapy has shown minimal efficacy.3,5
In conclusion, the presence of cutaneous metastasis from an adamantinoma is rare. Our case emphasizes this tumor’s potential for late metastasis as well as late recurrence.3,6 Most importantly, dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
To the Editor:
A 34-year-old woman with a history of adamantinoma of the right tibia that had been surgically resected with tibial reconstruction 5 years prior presented with a mildly tender, enlarging lesion on the right distal shin of 6 months’ duration that had started to change color. Review of systems was otherwise negative. Physical examination revealed an 8-mm, slightly tender, rubbery, pink papule adjacent to the surgical scar over the right tibia (Figure 1). Given the rapid growth of the lesion and its proximity to the surgical site, a punch biopsy was performed.
Histopathologic examination demonstrated a densely cellular dermal tumor composed of spindle cells with large hyperchromatic nuclei, numerous mitotic figures, and minimal eosinophilic cytoplasm (Figure 2A). Immunohistochemical studies revealed that approximately 40% of the tumor nuclei were immunoreactive to Ki-67 (Figure 2B), and total cytokeratin was focally positive (Figure 2C). A diagnosis of metastatic adamantinoma was made. Positron emission tomography and magnetic resonance imaging revealed new lytic lesions involving the T10 and L2 vertebrae (without frank spinal cord compression) and the right superior sacrum. Additionally, a small pulmonary nodule on the left upper lobe was noted on positron emission tomography, but it was below the size threshold for reliable detection. A computed tomography–guided biopsy of the T10 lesion demonstrated metastatic adamantinoma. The patient underwent a spinal stabilization procedure and discussed options regarding further oncologic and palliative management.
Adamantinoma is an extremely rare primary malignant bone tumor that typically involves the anterior portion of the tibial metaphysis or diaphysis in approximately 90% of cases. Young adults most commonly are affected in the third or fourth decades of life.1 Although the histogenesis is not clearly understood, experts have theorized that fetal implantation during embryogenesis or traumatic implantation of epithelial cells may be causes of this tumor and may explain the close pathologic similarity to basal cell carcinoma.2
Adamantinomas are slow growing, and as a result, patients often present with gradual onset of pain and swelling that persists for years.3,4 Metastasis occurs in 10% to 30% of patients, typically located in regional lymph nodes, the lungs, and distant bone.1,4 Our case represents a rare instance of adamantinoma metastasis to the skin. Although primary adamantinomas consist of both epithelial and stromal components, the typical metastatic lesions of adamantinomas are solely epithelial (often in a spindle-cell pattern),1 as was seen in our patient.
Operative removal via amputation or en bloc resection with limb salvage is the current treatment of choice. Adamantinomas are highly radioresistant, and chemotherapy has shown minimal efficacy.3,5
In conclusion, the presence of cutaneous metastasis from an adamantinoma is rare. Our case emphasizes this tumor’s potential for late metastasis as well as late recurrence.3,6 Most importantly, dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
- Schowinsky JT, Ormond DR, Kleinschmidt-DeMasters BK. Tibial adamantinoma: late metastasis to the brain. J Neuropathol Exp Neurol. 2015;74:95-97.
- Jain D, Jain VK, Vasishta RK, et al. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:8.
- Qureshi AA, Shott S, Mallin BA, et al. Current trends in the management of adamantinoma of long bones. an international study. J Bone Joint Surg Am. 2000;82-A:1122-1131.
- Desai SS, Jambhekar N, Agarwal M, et al. Adamantinoma of tibia: a study of 12 cases. J Surg Oncol. 2006;93:429-433.
- Weiss SW, Dorfman HD. Adamantinoma of long bone. an analysis of nine new cases with emphasis on metastasizing lesions and fibrous dysplasia-like changes. Hum Pathol. 1977;8:141-153.
- Szendroi M, Antal I, Arató G. Adamantinoma of long bones: a long-term follow-up study of 11 cases. Pathol Oncol Res. 2009;15:209-216.
- Schowinsky JT, Ormond DR, Kleinschmidt-DeMasters BK. Tibial adamantinoma: late metastasis to the brain. J Neuropathol Exp Neurol. 2015;74:95-97.
- Jain D, Jain VK, Vasishta RK, et al. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:8.
- Qureshi AA, Shott S, Mallin BA, et al. Current trends in the management of adamantinoma of long bones. an international study. J Bone Joint Surg Am. 2000;82-A:1122-1131.
- Desai SS, Jambhekar N, Agarwal M, et al. Adamantinoma of tibia: a study of 12 cases. J Surg Oncol. 2006;93:429-433.
- Weiss SW, Dorfman HD. Adamantinoma of long bone. an analysis of nine new cases with emphasis on metastasizing lesions and fibrous dysplasia-like changes. Hum Pathol. 1977;8:141-153.
- Szendroi M, Antal I, Arató G. Adamantinoma of long bones: a long-term follow-up study of 11 cases. Pathol Oncol Res. 2009;15:209-216.
Practice Points
- Metastatic adamantinoma of the skin is a rare clinical scenario.
- Dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
Multinucleate Cell Angiohistiocytoma
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
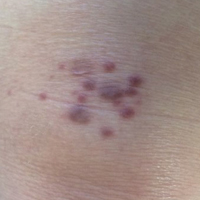
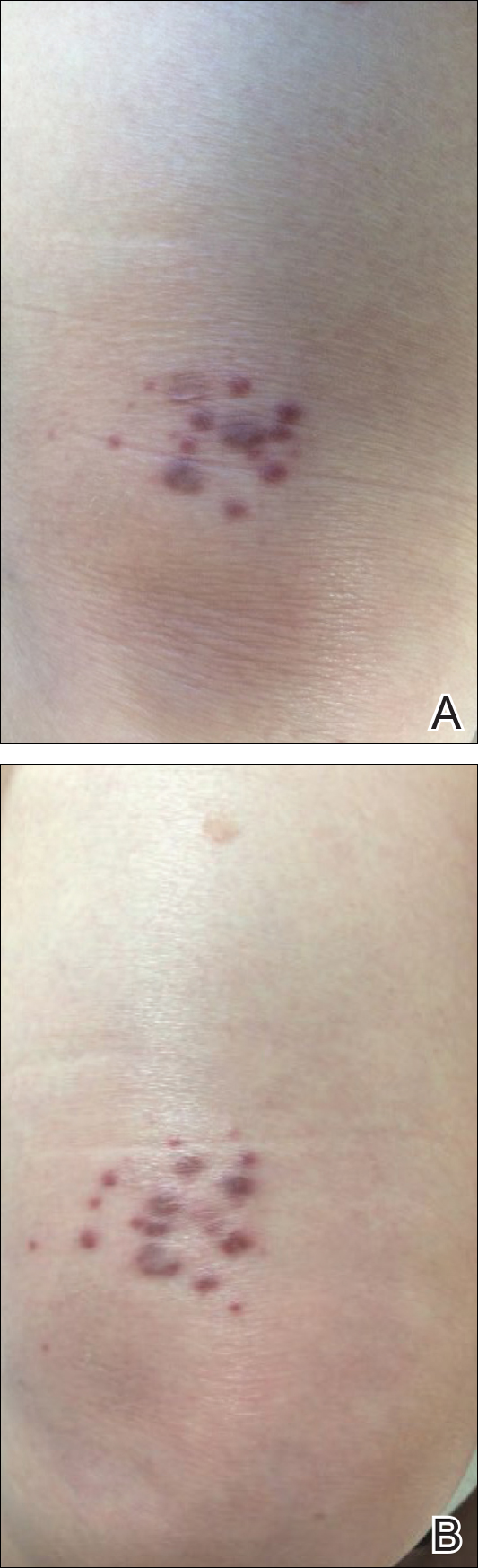
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
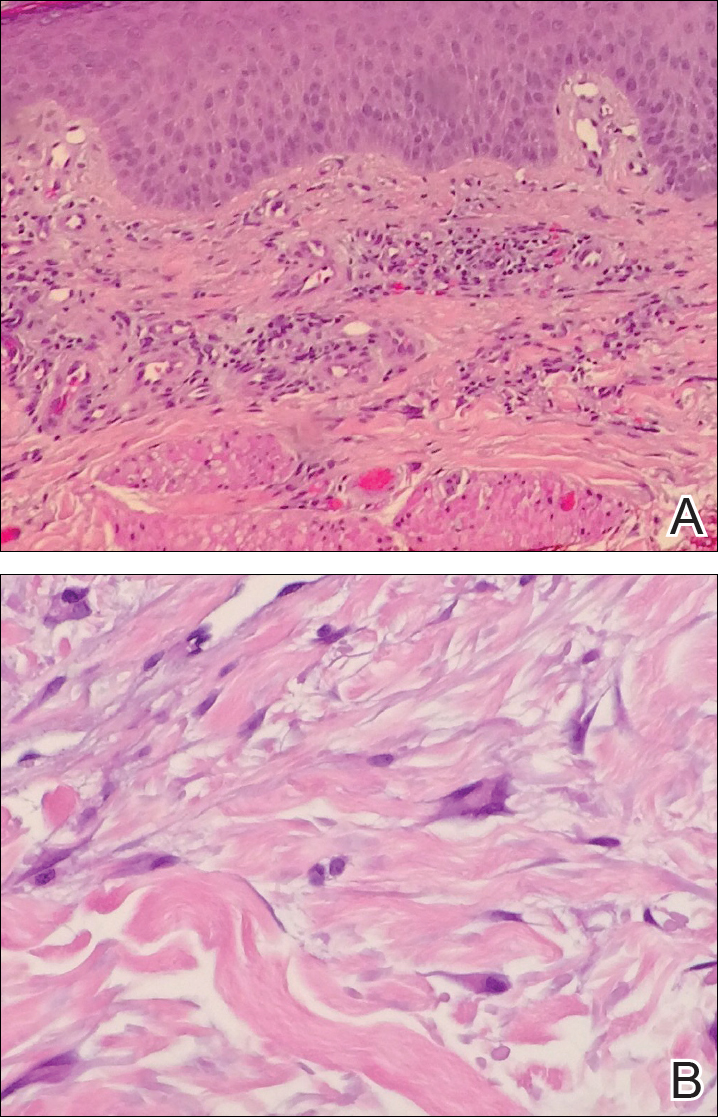
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.
At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.


At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
Multinucleate cell angiohistiocytoma (MCAH) is a rare benign, soft-tissue tumor first described in 1985 by Smith and Jones1 that presents clinically as erythematous to violaceous papules most commonly affecting females on the dorsal aspect of the hands and face.2 Multinucleate cell angiohistiocytoma is histologically characterized by vascular and histiocytic proliferations with dermal fibrosis. Few cases have been reported of lesions affecting the lower extremities. We report a case of MCAH affecting the legs.
Case Report
An 83-year-old white man with a history of basal cell carcinoma presented for evaluation of grouped, well-circumscribed, soft, red-violet, painless papules on the right anterior thigh that had been present for 8 months (Figure 1A). A review of symptoms was negative for immunologic, respiratory, and hematologic changes. The patient’s medical history also was remarkable for prostate cancer treated with radiation 18 years prior as well as right hip and left knee implants. The initial clinical impression was Kaposi sarcoma or a granulomatous disorder.
Histopathologic evaluation of a deep shave biopsy initially determined the lesion to be scar tissue without other pathologic findings. The patient returned to the clinic 12 months later for a complete skin examination given his history of skin cancer. Compared to clinical photographs taken a year prior, new violaceous papules were noted on the right thigh (Figure 1B) and left calf. Furthermore, there was no recurrence of the lesion at the prior biopsy site. Shave biopsies of the papules on the right thigh and left calf demonstrated similar histologic findings to each other. There was a mild increase in the number of small blood vessels in the superficial dermis (Figure 2A). A mild perivascular lymphocytic infiltrate surrounded some of these blood vessels. The endothelial cells had small nuclei with no evidence of nuclear pleomorphism. Careful examination of the interstitial dermis revealed scattered multinucleate cells with angulated cytoplasm (Figure 2B). Immunostaining for CD31 and human herpesvirus 8 were negative, excluding an infiltrative vascular tumor and Kaposi sarcoma, respectively. The diagnosis of MCAH was made based on the histopathologic findings.


At 1-year follow-up, the condition was stable with no gross changes in the lesions based on prior photographs. Once again, there was no recurrence of the excised lesions at both biopsy sites.
Comment
Presentation
A systematic review of published reports determined that 79% of MCAH cases occur in females, with an average age of onset of 50.1 years.2 However, MCAH likely is underreported due to the overall lack of knowledge regarding this condition by physicians and pathologists. The hands and face are the most commonly affected areas, though other sites of involvement have been reported, including the lower extremities,3,4 oral mucosa and upper lip,5,6 and trunk,7,8 as well as generalized distribution.9-12 Additionally, 1 case presented as a single plaque on the trunk rather than having papular or nodular morphology.8 Multinucleate cell angiohistiocytoma lesions generally are asymptomatic, though pruritus may be present.13 The condition is regarded as benign, though a minority of cases have exhibited spontaneous resolution.14-16
Histopathology
Multinucleate cell angiohistiocytoma histology demonstrates full-thickness dermal microvessel proliferation and fibrosis with characteristic multinucleate giant cells.2,3 Vascular endothelial cells stained positive for CD68 in 60% of cases2 as well as the normal endothelial markers (ie, factor VIII, CD31, CD34). The multinucleate giant cells exhibit immunoreactivity for macrophage/histiocytic markers factor XIIIa and CD68.
Etiology
The pathogenesis of MCAH is not yet fully understood, but it is considered to be a benign vascular or fibrohistiocytic neoplasm.17 Calderaro et al18 described a series of 8 patients who developed MCAH either within a cutaneous neoplastic process or in conjunction with various cutaneous reactive conditions, including hidradenitis suppurativa and chronic radiation dermatitis, as well as overlying a bone prosthesis placed due to degenerative arthritis. These cases suggest that MCAH, or possibly a subset of the disease, is a reactive process.
Differential Diagnosis
The differential diagnosis for MCAH includes Kaposi sarcoma clinically and dermatofibroma and fibrous papules histologically. Sass et al21 determined the in vitro behavior of cultured MCAH cells to contrast markedly with Kaposi sarcoma–derived cells. Although Kaposi sarcoma–derived cells exhibited invasive behavior, cells isolated from MCAH lesions were less elongated and were unable to traverse basement membranes.
Treatment
Surgical excision or cryotherapy appear to be definitive treatments of MCAH; however, a number of cases have reported light and laser modalities as successful alternatives to excision. One case of MCAH affecting the face was treated with pulsed dye laser monotherapy.22 This modality allowed selective coagulation of the vascular structures in MCAH. At 8-month follow-up, the initial lesion was noted to be completely cleared, though similar lesions had recently appeared elsewhere on the face.22 Another case of MCAH affecting the leg was treated with pulsed dye laser and both topical and intralesional corticosteroid combination therapy. In this case, the lesion failed to respond to treatment, which may suggest that facial localization could influence response in pulsed dye laser treatment.3
Intense pulsed light also has been reported as a definitive treatment in 2 cases.2,13 Slight erythema and transient pruritus have been reported immediately following treatment. In this case, complete resolution with only residual hyperpigmentation was reported at 2-month follow-up, with no recurrence during 12 months of follow-up.13
Argon laser therapy has been used in 2 cases. After a single session, lesions were no longer palpable, with no scarring noted at 8 weeks follow-up.23 Lastly, 2 cases of MCAH have been successfully treated with the CO2 laser, with no relapse noted at 2.5- or 5-month follow-up, respectively.24
Conclusion
Multinucleate cell angiohistiocytoma is a rare and likely underdiagnosed dermatologic condition that is believed to be a reactive process. Characteristic histology of MCAH demonstrates microvascular proliferations of the dermis with multinucleate giant cells amidst a fibrous background. Although surgical excision is curative, there are reports in which laser and light therapies were used to effectively treat MCAH.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
- Smith NP, Jones EW. Multinucleate cell angiohistiocytoma—a new entity. Br J Dermatol. 1985;113:15.
- Frew JW. Multinucleate cell angiohistiocytoma: clinicopathological correlation of 142 cases with insights into etiology and pathogenesis. Am J Dermatopathol. 2015;37:222-228.
- Applebaum DS, Shuja F, Hicks L, et al. Multinucleate cell angiohistiocytoma: a case report and review of the literature. Dermatol Online J. 2014;20:22610.
- Sagdeo A, Chu EY, Elenitsas R, et al. Multiple asymptomatic violaceous macules on the thigh. Multinucleate cell angiohistiocytoma (MCAH). JAMA Dermatol. 2013;149:357-363.
- Rawal YB, Anderson KM, Rawal SY. Multinucleate cell angiohistiocytoma: an uncommon mucosal tumour. Clin Exp Dermatol. 2009;34:333-336.
- Jones AC, Mullins D, Jimenez F. Multinucleate cell angiohistiocytoma of the upper lip. Oral Surg Oral Med Oral Pathol. 1994;78:743-747.
- Doshi-Chougule BN, Gust A, Mentzel T, et al. Multinucleate cell angiohistiocytoma with hypertrophic nerves. J Cutan Pathol. 2013;40:1048-1053.
- Issa AA, Lui H, Shapiro J, et al. Plaque-type multinucleate cell angiohistiocytoma. J Cutan Med Surg. 1998;3:112-114.
- Doane JA, Purdy K, Pasternak S. Generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2015;19:323-325.
- Marti N, Monteagudo C, Revert A, et al. Multiple papules on the trunk and extremities. generalized multinucleate cell angiohistiocytoma. Int J Dermatol. 2013;52:544-546.
- O’Blenes CA, Walsh NM, Green PJ, et al. Novel case of generalized multinucleate cell angiohistiocytoma. J Cutan Med Surg. 2010;14:178-180.
- Chang SN, Kim HS, Kim SC, et al. Generalized multinucleate cell angiohistiocytoma. J Am Acad Dermatol. 1996;35:320-322.
- Fernández-Jorge B, Del Pozo J, García-Silva J, et al. Multinucleate cell angiohistiocytoma: treatment using intense pulsed light. Dermatol Surg. 2009;35:1141-1143.
- Perez LP, Zulaica A, Rodriguez L, et al. Multinucleate cell angiohistiocytoma. report of five cases. J Cutan Pathol. 2006;33:349-352.
- Shapiro PE, Nova MP, Rosmarin LA, et al. Multinucleate cell angiohistiocytoma: a distinct entity diagnosable by clinical and histologic features. J Am Acad Dermatol. 1994;30:417-422.
- Jaconelli L, Kanitakis J, Ktiouet S, et al. Multinucleate cell angiohistiocytoma: report of three new cases and literature review. Dermatol Online J. 2009;15:4.
- Jones WE, Cerio R, Smith NP. Multinucleate cell angiohistiocytoma: an acquired vascular anomaly to be distinguished from Kaposi’s sarcoma. Br J Dermatol. 1990;122:651-663.
- Calderaro J, Rethers L, Ortonne N. Multinucleated cells angiohistiocytoma: a reactive lesion? Am J Dermatopathol. 2010;32:415-417.
- Cesinaro AM, Roncati L, Maiorana A. Estrogen receptor alpha overexpression in multinucleate cell angiohistiocytoma: new insights into the pathogenesis of a reactive process. Am J Dermatopathol. 2010;32:655-659.
- Losordo DW, Isner JM. Estrogen and angiogenesis: a review. Arterioscler Thromb Vasc Biol. 2001;21:6-12.
- Sass U, Noel JC, Andre J, et al. Multinucleate cell angiohistiocytoma: report of two cases with no evidence of human herpesvirus-8 infection. J Cutan Pathol. 2000;27:258-261.
- Richer V, Lui H. Facial multinucleate cell angiohistiocytoma: long-term remission with 585 nm pulsed dye laser. Clin Exp Dermatol. 2016;41:312-313.
- Kopera D, Smolle J, Kerl H. Multinucleate cell angiohistiocytoma: treatment with argon laser. Br J Dermatol. 1995;133:308-310.
- Väkevä L, Saksela O, Kariniemi AL. Multinucleate cell angiohistiocytoma: a report of four cases in Finland. Acta Derm Venereol. 2003;83:222-223.
Practice Points
- Multinucleate cell angiohistiocytoma (MCAH) is a rare underrecognized cutaneous tumor presenting as erythematous to violaceous papules.
- Although it clinically mimics Kaposi sarcoma, MCAH may be distinguished histopathologically by negative immunostaining for human herpesvirus 8.
- Surgical excision and laser therapies are definitive treatments for MCAH, which is a benign lesion.