User login
Comparison of immunosuppressants for early diffuse systemic sclerosis yields mixed results
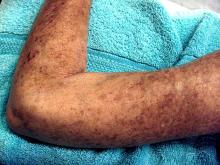
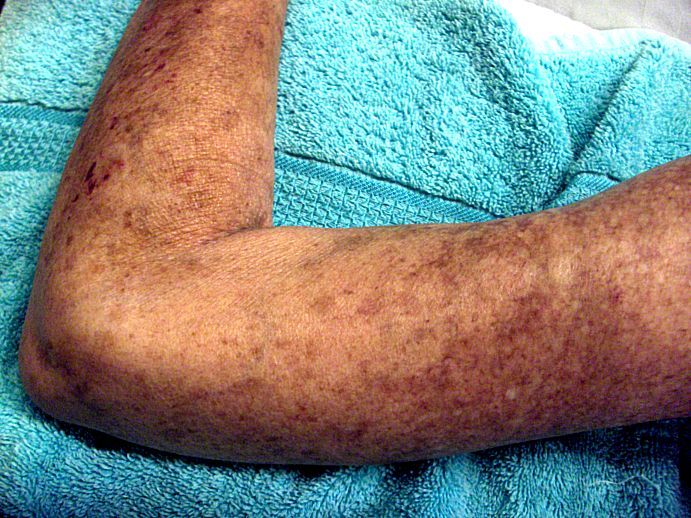
Placing patients who have early diffuse cutaneous systemic sclerosis (dcSSc ) on an immunosuppressant regimen can have a beneficial, but not necessarily sustainable, impact, according to findings from the European Scleroderma Observational Study.
“At present, there is no drug known to favorably influence disease course [because] randomized controlled trials have historically been confounded by disease rarity [and] strict entry criteria,” wrote the study investigators – led by Ariane L. Herrick, MD, of the University of Manchester (England). They sought to get around the lack of randomized, controlled trial data by comparing observational data on “the effectiveness of standard treatment approaches [in] the early management of patients with dcSSc.”
The patients receiving methotrexate had a target dose of 20-25 mg/week, either orally or subcutaneously. Patients on MMF were given two 500-mg doses a day for 2 weeks, then two 1-g doses daily. Cyclophosphamide regimens varied based on the centers, with some patients receiving one IV 500-mg/m2 dose monthly for 6-12 months and others receiving a daily dose of 1-2 mg/kg/day orally for 12 months, with most being transferred later to maintenance with methotrexate, MMF, or azathioprine. All patients underwent assessment at baseline and then every 3 months for the trial duration of 24 months; however, because the study occurred during 2010-2014 and some patients were recruited in 2013, those who joined after September 2013 were followed for only 12-24 months. Of the 326 subjects enrolled, 276 completed 12 months of follow-up and 234 completed 24 months (Ann Rheum Dis. 2017 Feb 10. doi: 10.1136/annrheumdis-2016-210503).
After weighting the 12-month outcomes between the groups by equalizing the distribution of confounding variables, all groups experienced a significant reduction in the study’s primary outcome measure, the modified Rodnan skin score (mRSS), which can range from 0 to 51. From a median baseline mRSS of 21 (interquartile range of 16-27), the mRSS for the methotrexate group fell 4.0 (IQR −5.2 to −2.7), for those on MMF it dropped 4.1 (IQR −5.3 to −2.9), for the cyclophosphamide group it decreased 3.3 (IQR −4.9 to −1.7), and for those on no immunosuppressants it dropped 2.2 (IQR −4.0 to −0.3). There were no significant differences between the groups.
Although none of the treatments had a significant effect on improving forced vital capacity (FVC) or carbon monoxide–diffusing capacity in the groups overall, the subgroup of patients with confirmed or suspected pulmonary fibrosis showed a significant difference in the rate of change over time for FVC in patients who were initially prescribed cyclophosphamide (7.4% absolute increase), but not for MMF (3.2% increase), methotrexate (2.0% decrease), or no immunosuppressant (4.0% increase). The investigators noted that this finding “confirms the relative effectiveness of cyclophosphamide in patients with pulmonary fibrosis.”
At 24 months, there were no significant differences in mortality between the four groups. After weighting, the predicted survival rates were 94% for methotrexate, 89% for MMF, 90% for cyclophosphamide, and 84% for those with no immunosuppressants. All three immunosuppressants also showed no significant difference in terms of tolerability. At this point in time, the rate of adherence to the initial protocol was comparable between the groups: 76% for methotrexate, 80% for MMF, 79% for cyclophosphamide, and 73% for those not taking an immunosuppressant, although 10 who started without an immunosuppressant later started one.
“An important point when interpreting our findings (and therefore a note of caution) is that the ‘no immunosuppressant’ group was not a control group,” Dr. Herrick and her coauthors wrote. “Patients in this group had a longer disease duration than the other three groups and were more likely to have renal involvement.”
Nevertheless, the authors contend that these findings carry a strong take-home message for clinicians: “There is a weak signal to support using immunosuppressants for early dcSSc (and in particular cyclophosphamide for patients with pulmonary fibrosis). However, it is clear that there remains a pressing need for the development of more effective and targeted treatments.”
The study was funded by a grant from the European League Against Rheumatism’s Orphan Disease Program, and additional support from Scleroderma and Raynaud’s UK. Dr. Herrick disclosed relationships with Actelion, Apricus, and GlaxoSmithKline; her coauthors disclosed numerous financial relationships of their own.
Placing patients who have early diffuse cutaneous systemic sclerosis (dcSSc ) on an immunosuppressant regimen can have a beneficial, but not necessarily sustainable, impact, according to findings from the European Scleroderma Observational Study.
“At present, there is no drug known to favorably influence disease course [because] randomized controlled trials have historically been confounded by disease rarity [and] strict entry criteria,” wrote the study investigators – led by Ariane L. Herrick, MD, of the University of Manchester (England). They sought to get around the lack of randomized, controlled trial data by comparing observational data on “the effectiveness of standard treatment approaches [in] the early management of patients with dcSSc.”
The patients receiving methotrexate had a target dose of 20-25 mg/week, either orally or subcutaneously. Patients on MMF were given two 500-mg doses a day for 2 weeks, then two 1-g doses daily. Cyclophosphamide regimens varied based on the centers, with some patients receiving one IV 500-mg/m2 dose monthly for 6-12 months and others receiving a daily dose of 1-2 mg/kg/day orally for 12 months, with most being transferred later to maintenance with methotrexate, MMF, or azathioprine. All patients underwent assessment at baseline and then every 3 months for the trial duration of 24 months; however, because the study occurred during 2010-2014 and some patients were recruited in 2013, those who joined after September 2013 were followed for only 12-24 months. Of the 326 subjects enrolled, 276 completed 12 months of follow-up and 234 completed 24 months (Ann Rheum Dis. 2017 Feb 10. doi: 10.1136/annrheumdis-2016-210503).
After weighting the 12-month outcomes between the groups by equalizing the distribution of confounding variables, all groups experienced a significant reduction in the study’s primary outcome measure, the modified Rodnan skin score (mRSS), which can range from 0 to 51. From a median baseline mRSS of 21 (interquartile range of 16-27), the mRSS for the methotrexate group fell 4.0 (IQR −5.2 to −2.7), for those on MMF it dropped 4.1 (IQR −5.3 to −2.9), for the cyclophosphamide group it decreased 3.3 (IQR −4.9 to −1.7), and for those on no immunosuppressants it dropped 2.2 (IQR −4.0 to −0.3). There were no significant differences between the groups.
Although none of the treatments had a significant effect on improving forced vital capacity (FVC) or carbon monoxide–diffusing capacity in the groups overall, the subgroup of patients with confirmed or suspected pulmonary fibrosis showed a significant difference in the rate of change over time for FVC in patients who were initially prescribed cyclophosphamide (7.4% absolute increase), but not for MMF (3.2% increase), methotrexate (2.0% decrease), or no immunosuppressant (4.0% increase). The investigators noted that this finding “confirms the relative effectiveness of cyclophosphamide in patients with pulmonary fibrosis.”
At 24 months, there were no significant differences in mortality between the four groups. After weighting, the predicted survival rates were 94% for methotrexate, 89% for MMF, 90% for cyclophosphamide, and 84% for those with no immunosuppressants. All three immunosuppressants also showed no significant difference in terms of tolerability. At this point in time, the rate of adherence to the initial protocol was comparable between the groups: 76% for methotrexate, 80% for MMF, 79% for cyclophosphamide, and 73% for those not taking an immunosuppressant, although 10 who started without an immunosuppressant later started one.
“An important point when interpreting our findings (and therefore a note of caution) is that the ‘no immunosuppressant’ group was not a control group,” Dr. Herrick and her coauthors wrote. “Patients in this group had a longer disease duration than the other three groups and were more likely to have renal involvement.”
Nevertheless, the authors contend that these findings carry a strong take-home message for clinicians: “There is a weak signal to support using immunosuppressants for early dcSSc (and in particular cyclophosphamide for patients with pulmonary fibrosis). However, it is clear that there remains a pressing need for the development of more effective and targeted treatments.”
The study was funded by a grant from the European League Against Rheumatism’s Orphan Disease Program, and additional support from Scleroderma and Raynaud’s UK. Dr. Herrick disclosed relationships with Actelion, Apricus, and GlaxoSmithKline; her coauthors disclosed numerous financial relationships of their own.
Placing patients who have early diffuse cutaneous systemic sclerosis (dcSSc ) on an immunosuppressant regimen can have a beneficial, but not necessarily sustainable, impact, according to findings from the European Scleroderma Observational Study.
“At present, there is no drug known to favorably influence disease course [because] randomized controlled trials have historically been confounded by disease rarity [and] strict entry criteria,” wrote the study investigators – led by Ariane L. Herrick, MD, of the University of Manchester (England). They sought to get around the lack of randomized, controlled trial data by comparing observational data on “the effectiveness of standard treatment approaches [in] the early management of patients with dcSSc.”
The patients receiving methotrexate had a target dose of 20-25 mg/week, either orally or subcutaneously. Patients on MMF were given two 500-mg doses a day for 2 weeks, then two 1-g doses daily. Cyclophosphamide regimens varied based on the centers, with some patients receiving one IV 500-mg/m2 dose monthly for 6-12 months and others receiving a daily dose of 1-2 mg/kg/day orally for 12 months, with most being transferred later to maintenance with methotrexate, MMF, or azathioprine. All patients underwent assessment at baseline and then every 3 months for the trial duration of 24 months; however, because the study occurred during 2010-2014 and some patients were recruited in 2013, those who joined after September 2013 were followed for only 12-24 months. Of the 326 subjects enrolled, 276 completed 12 months of follow-up and 234 completed 24 months (Ann Rheum Dis. 2017 Feb 10. doi: 10.1136/annrheumdis-2016-210503).
After weighting the 12-month outcomes between the groups by equalizing the distribution of confounding variables, all groups experienced a significant reduction in the study’s primary outcome measure, the modified Rodnan skin score (mRSS), which can range from 0 to 51. From a median baseline mRSS of 21 (interquartile range of 16-27), the mRSS for the methotrexate group fell 4.0 (IQR −5.2 to −2.7), for those on MMF it dropped 4.1 (IQR −5.3 to −2.9), for the cyclophosphamide group it decreased 3.3 (IQR −4.9 to −1.7), and for those on no immunosuppressants it dropped 2.2 (IQR −4.0 to −0.3). There were no significant differences between the groups.
Although none of the treatments had a significant effect on improving forced vital capacity (FVC) or carbon monoxide–diffusing capacity in the groups overall, the subgroup of patients with confirmed or suspected pulmonary fibrosis showed a significant difference in the rate of change over time for FVC in patients who were initially prescribed cyclophosphamide (7.4% absolute increase), but not for MMF (3.2% increase), methotrexate (2.0% decrease), or no immunosuppressant (4.0% increase). The investigators noted that this finding “confirms the relative effectiveness of cyclophosphamide in patients with pulmonary fibrosis.”
At 24 months, there were no significant differences in mortality between the four groups. After weighting, the predicted survival rates were 94% for methotrexate, 89% for MMF, 90% for cyclophosphamide, and 84% for those with no immunosuppressants. All three immunosuppressants also showed no significant difference in terms of tolerability. At this point in time, the rate of adherence to the initial protocol was comparable between the groups: 76% for methotrexate, 80% for MMF, 79% for cyclophosphamide, and 73% for those not taking an immunosuppressant, although 10 who started without an immunosuppressant later started one.
“An important point when interpreting our findings (and therefore a note of caution) is that the ‘no immunosuppressant’ group was not a control group,” Dr. Herrick and her coauthors wrote. “Patients in this group had a longer disease duration than the other three groups and were more likely to have renal involvement.”
Nevertheless, the authors contend that these findings carry a strong take-home message for clinicians: “There is a weak signal to support using immunosuppressants for early dcSSc (and in particular cyclophosphamide for patients with pulmonary fibrosis). However, it is clear that there remains a pressing need for the development of more effective and targeted treatments.”
The study was funded by a grant from the European League Against Rheumatism’s Orphan Disease Program, and additional support from Scleroderma and Raynaud’s UK. Dr. Herrick disclosed relationships with Actelion, Apricus, and GlaxoSmithKline; her coauthors disclosed numerous financial relationships of their own.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point:
Major finding: Subjects taking no immunosuppressants showed significant reduction in mRSS over 12 months (2.2; IQR, −4.0 to −0.3), but no significant difference in survival rates over 24 months.
Data source: A prospective, observational cohort study of 326 dcSSc patients recruited in 2010-2014.
Disclosures: Funded by EULAR and Scleroderma and Raynaud’s UK. Authors reported numerous financial relationships.
Gluten-free diets related to high levels of arsenic, mercury
Individuals who adopt a gluten-free diet are putting themselves at risk for uncommonly high levels of arsenic and mercury, according to the findings of a recent study published in Epidemiology.
“Despite [less than] 1% of Americans having diagnosed celiac disease, an estimated 25% of American consumers reported consuming gluten-free food in 2015, a 67% increase from 2013,” wrote the authors of the study, led by Maria Argos, PhD, of the University of Illinois at Chicago. “Despite such a dramatic shift in the diet of many Americans, little is known about how gluten-free diets might affect exposure to toxic metals found in certain foods,” they noted.
Dr. Argos and her colleagues analyzed data collected from the National Health and Nutrition Examination Survey (NHANES), which included self-reported questionnaires in which subjects indicated what type of diet they were on, if any. Data on those who indicated that they followed a gluten-free diet were analyzed to determine if their urinary and blood biomarkers indicated any exposure to toxic metals. A total of 7,471 subjects from the NHANES were included in the analysis.
“We accounted for the complex sampling design of NHANES [by] using Taylor series linearization and sampling weights, per the NHANES analytic guidelines, to ensure unbiased and nationally representative estimates,” the authors explained.
A total of 73 subjects identified themselves as following a gluten-free diet. Within this group, the mean total arsenic level in urine was found to be 12.1 mcg/L, compared to 7.8 mcg/L for the other 7,398 subjects. Levels of dimethylarsinic acid averaged 5.3 mcg/L for those who were gluten-free, but only 3.7 for everyone else, while cadmium and lead levels were also slightly higher for gluten-free individuals: 0.18 mcg/L vs. 0.16 mcg/L, and 0.40 mcg/L vs. 0.37 mcg/L, respectively.
Blood analyses showed that total mercury levels were also substantially higher in the gluten-free group, at a mean of 1.3 mcg/L compared to 0.8 mcg/L. While cadmium levels were the same between the two – both showed a mean level of 0.29 mcg/L – lead measured 1.1 mcg/dL and inorganic mercury measured 0.30 mcg/L, compared to 0.96 mcg/L and 0.28 mcg/L in everyone else, respectively.
Geometric mean ratios showed that total arsenic, total arsenic 1, and total mercury levels had the largest disparity between the two groups. Total arsenic registered a 1.5 (95% CI, 1.2-2.0), total mercury a 1.7 (95% CI, 1.1-2.4), and total arsenic 1 a 1.9 (95% CI 1.3-2.6), meaning the gluten-free group had nearly double the risk for higher levels than those on other diets.
“These findings may have important health implications since the health effects of low-level arsenic and mercury exposure from food sources are uncertain but may increase the risk for cancer and other chronic diseases,” Dr. Argos and her coauthors concluded, adding that “future studies are needed to more fully examine exposure to toxic metals from consuming gluten-free foods.”
The study was funded by a grant from the National Institutes of Health. Dr. Argos and her coauthors did not report any relevant financial disclosures.
Individuals who adopt a gluten-free diet are putting themselves at risk for uncommonly high levels of arsenic and mercury, according to the findings of a recent study published in Epidemiology.
“Despite [less than] 1% of Americans having diagnosed celiac disease, an estimated 25% of American consumers reported consuming gluten-free food in 2015, a 67% increase from 2013,” wrote the authors of the study, led by Maria Argos, PhD, of the University of Illinois at Chicago. “Despite such a dramatic shift in the diet of many Americans, little is known about how gluten-free diets might affect exposure to toxic metals found in certain foods,” they noted.
Dr. Argos and her colleagues analyzed data collected from the National Health and Nutrition Examination Survey (NHANES), which included self-reported questionnaires in which subjects indicated what type of diet they were on, if any. Data on those who indicated that they followed a gluten-free diet were analyzed to determine if their urinary and blood biomarkers indicated any exposure to toxic metals. A total of 7,471 subjects from the NHANES were included in the analysis.
“We accounted for the complex sampling design of NHANES [by] using Taylor series linearization and sampling weights, per the NHANES analytic guidelines, to ensure unbiased and nationally representative estimates,” the authors explained.
A total of 73 subjects identified themselves as following a gluten-free diet. Within this group, the mean total arsenic level in urine was found to be 12.1 mcg/L, compared to 7.8 mcg/L for the other 7,398 subjects. Levels of dimethylarsinic acid averaged 5.3 mcg/L for those who were gluten-free, but only 3.7 for everyone else, while cadmium and lead levels were also slightly higher for gluten-free individuals: 0.18 mcg/L vs. 0.16 mcg/L, and 0.40 mcg/L vs. 0.37 mcg/L, respectively.
Blood analyses showed that total mercury levels were also substantially higher in the gluten-free group, at a mean of 1.3 mcg/L compared to 0.8 mcg/L. While cadmium levels were the same between the two – both showed a mean level of 0.29 mcg/L – lead measured 1.1 mcg/dL and inorganic mercury measured 0.30 mcg/L, compared to 0.96 mcg/L and 0.28 mcg/L in everyone else, respectively.
Geometric mean ratios showed that total arsenic, total arsenic 1, and total mercury levels had the largest disparity between the two groups. Total arsenic registered a 1.5 (95% CI, 1.2-2.0), total mercury a 1.7 (95% CI, 1.1-2.4), and total arsenic 1 a 1.9 (95% CI 1.3-2.6), meaning the gluten-free group had nearly double the risk for higher levels than those on other diets.
“These findings may have important health implications since the health effects of low-level arsenic and mercury exposure from food sources are uncertain but may increase the risk for cancer and other chronic diseases,” Dr. Argos and her coauthors concluded, adding that “future studies are needed to more fully examine exposure to toxic metals from consuming gluten-free foods.”
The study was funded by a grant from the National Institutes of Health. Dr. Argos and her coauthors did not report any relevant financial disclosures.
Individuals who adopt a gluten-free diet are putting themselves at risk for uncommonly high levels of arsenic and mercury, according to the findings of a recent study published in Epidemiology.
“Despite [less than] 1% of Americans having diagnosed celiac disease, an estimated 25% of American consumers reported consuming gluten-free food in 2015, a 67% increase from 2013,” wrote the authors of the study, led by Maria Argos, PhD, of the University of Illinois at Chicago. “Despite such a dramatic shift in the diet of many Americans, little is known about how gluten-free diets might affect exposure to toxic metals found in certain foods,” they noted.
Dr. Argos and her colleagues analyzed data collected from the National Health and Nutrition Examination Survey (NHANES), which included self-reported questionnaires in which subjects indicated what type of diet they were on, if any. Data on those who indicated that they followed a gluten-free diet were analyzed to determine if their urinary and blood biomarkers indicated any exposure to toxic metals. A total of 7,471 subjects from the NHANES were included in the analysis.
“We accounted for the complex sampling design of NHANES [by] using Taylor series linearization and sampling weights, per the NHANES analytic guidelines, to ensure unbiased and nationally representative estimates,” the authors explained.
A total of 73 subjects identified themselves as following a gluten-free diet. Within this group, the mean total arsenic level in urine was found to be 12.1 mcg/L, compared to 7.8 mcg/L for the other 7,398 subjects. Levels of dimethylarsinic acid averaged 5.3 mcg/L for those who were gluten-free, but only 3.7 for everyone else, while cadmium and lead levels were also slightly higher for gluten-free individuals: 0.18 mcg/L vs. 0.16 mcg/L, and 0.40 mcg/L vs. 0.37 mcg/L, respectively.
Blood analyses showed that total mercury levels were also substantially higher in the gluten-free group, at a mean of 1.3 mcg/L compared to 0.8 mcg/L. While cadmium levels were the same between the two – both showed a mean level of 0.29 mcg/L – lead measured 1.1 mcg/dL and inorganic mercury measured 0.30 mcg/L, compared to 0.96 mcg/L and 0.28 mcg/L in everyone else, respectively.
Geometric mean ratios showed that total arsenic, total arsenic 1, and total mercury levels had the largest disparity between the two groups. Total arsenic registered a 1.5 (95% CI, 1.2-2.0), total mercury a 1.7 (95% CI, 1.1-2.4), and total arsenic 1 a 1.9 (95% CI 1.3-2.6), meaning the gluten-free group had nearly double the risk for higher levels than those on other diets.
“These findings may have important health implications since the health effects of low-level arsenic and mercury exposure from food sources are uncertain but may increase the risk for cancer and other chronic diseases,” Dr. Argos and her coauthors concluded, adding that “future studies are needed to more fully examine exposure to toxic metals from consuming gluten-free foods.”
The study was funded by a grant from the National Institutes of Health. Dr. Argos and her coauthors did not report any relevant financial disclosures.
FROM EPIDEMIOLOGY
Key clinical point:
Major finding: Total arsenic and mercury levels were higher in those that self-reported being on gluten-free diet than in those who weren’t: 12.1 mcg/L vs. 7.8 mcg/L for arsenic and 1.3 mcg/L vs. 0.8 mcg/L for mercury.
Data source: Retrospective analysis of data from the National Health and Nutrition Examination Survey during 2009-2014.
Disclosures: The National Institutes of Health funded the study. The authors had no relevant financial disclosures.
Vitamin D reduces respiratory infection risk
Administering doses of a vitamin D supplement to patients can significantly mitigate their risk of developing acute respiratory tract infections, according to a recent study published by the BMJ.
“[Existing] epidemiological and in vitro data have prompted numerous randomized controlled trials to determine whether vitamin D supplementation can decrease the risk of acute respiratory tract infection,” wrote the authors of the study, led by Adrian R. Martineau, PhD, of Queen Mary University of London. “A total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date [but] all but one of these aggregate data meta-analyses reported statistically significant heterogeneity of effect between primary trials.”
A total of 532 studies were reviewed by a panel, of which 25 studies were ultimately selected for inclusion in this analysis. The studies included were of varying lengths in terms of trial periods and involved a total of 11,321 subjects ranging from 0 to 95 years of age. Of these, 10,933 (96.6%) subjects experienced at least one acute respiratory tract infection.
No significant benefit was found in subjects who had already experienced an infection, yielding an odds ratio of 0.98 (95% CI, 0.80-1.20; P = .83). Analysis performed to quantify the risk of infection with or without Vitamin D showed that taking Vitamin D supplements significantly decreased infection risk, with an OR of 0.88 (95% CI, 0.81-0.96; P less than .001) after adjusting for age, sex, and the duration of the trial.
Results also demonstrated that bolus doses of Vitamin D did not offer any beneficial value to subjects. Those who received daily or weekly doses without bolus had a better OR, compared with those who did receive at least one bolus dose: 0.81 (95% CI, 0.72-0.91) versus 0.97 (95% CI, 0.86-1.10), respectively (P = .05). Individuals whose baseline 25-hydroxyvitamin D levels were lower than 25 nanomols per liter experienced a greater benefit than those whose levels were above 25: OR = 0.30 (95% CI, 0.17-0.53) and OR = 0.75 (95% CI, 0.60-0.95), respectively (P = .006).
“Our study reports a major new indication for vitamin D supplementation: the prevention of acute respiratory tract infection,” Dr. Martineau and his coauthors concluded, adding that a potential application for these findings would be “the introduction of public health measures such as food fortification to improve vitamin D status, particularly in settings where profound vitamin D deficiency is common.”
The study was funded by a grant from the National Institute of Health Research. Dr. Martineau and his coauthors did not report any relevant financial disclosures.
While the work undertaken by Dr. Martineau et al. is commendable, the results themselves are ultimately underwhelming. The study’s results are too heterogeneous and offer too slight a reduction in overall risk to justify a complete overhaul of clinical procedure and prescribing protocols. These findings should not change clinical practice in any significant way, and there are other groups of individuals, such as those with low serum concentrations of vitamin D, that were omitted from this analysis altogether.
Mark J. Bolland is an associate professor of medicine at the University of Auckland (New Zealand). Alison Avenell is a professor at the University of Aberdeen (Scotland).
While the work undertaken by Dr. Martineau et al. is commendable, the results themselves are ultimately underwhelming. The study’s results are too heterogeneous and offer too slight a reduction in overall risk to justify a complete overhaul of clinical procedure and prescribing protocols. These findings should not change clinical practice in any significant way, and there are other groups of individuals, such as those with low serum concentrations of vitamin D, that were omitted from this analysis altogether.
Mark J. Bolland is an associate professor of medicine at the University of Auckland (New Zealand). Alison Avenell is a professor at the University of Aberdeen (Scotland).
While the work undertaken by Dr. Martineau et al. is commendable, the results themselves are ultimately underwhelming. The study’s results are too heterogeneous and offer too slight a reduction in overall risk to justify a complete overhaul of clinical procedure and prescribing protocols. These findings should not change clinical practice in any significant way, and there are other groups of individuals, such as those with low serum concentrations of vitamin D, that were omitted from this analysis altogether.
Mark J. Bolland is an associate professor of medicine at the University of Auckland (New Zealand). Alison Avenell is a professor at the University of Aberdeen (Scotland).
Administering doses of a vitamin D supplement to patients can significantly mitigate their risk of developing acute respiratory tract infections, according to a recent study published by the BMJ.
“[Existing] epidemiological and in vitro data have prompted numerous randomized controlled trials to determine whether vitamin D supplementation can decrease the risk of acute respiratory tract infection,” wrote the authors of the study, led by Adrian R. Martineau, PhD, of Queen Mary University of London. “A total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date [but] all but one of these aggregate data meta-analyses reported statistically significant heterogeneity of effect between primary trials.”
A total of 532 studies were reviewed by a panel, of which 25 studies were ultimately selected for inclusion in this analysis. The studies included were of varying lengths in terms of trial periods and involved a total of 11,321 subjects ranging from 0 to 95 years of age. Of these, 10,933 (96.6%) subjects experienced at least one acute respiratory tract infection.
No significant benefit was found in subjects who had already experienced an infection, yielding an odds ratio of 0.98 (95% CI, 0.80-1.20; P = .83). Analysis performed to quantify the risk of infection with or without Vitamin D showed that taking Vitamin D supplements significantly decreased infection risk, with an OR of 0.88 (95% CI, 0.81-0.96; P less than .001) after adjusting for age, sex, and the duration of the trial.
Results also demonstrated that bolus doses of Vitamin D did not offer any beneficial value to subjects. Those who received daily or weekly doses without bolus had a better OR, compared with those who did receive at least one bolus dose: 0.81 (95% CI, 0.72-0.91) versus 0.97 (95% CI, 0.86-1.10), respectively (P = .05). Individuals whose baseline 25-hydroxyvitamin D levels were lower than 25 nanomols per liter experienced a greater benefit than those whose levels were above 25: OR = 0.30 (95% CI, 0.17-0.53) and OR = 0.75 (95% CI, 0.60-0.95), respectively (P = .006).
“Our study reports a major new indication for vitamin D supplementation: the prevention of acute respiratory tract infection,” Dr. Martineau and his coauthors concluded, adding that a potential application for these findings would be “the introduction of public health measures such as food fortification to improve vitamin D status, particularly in settings where profound vitamin D deficiency is common.”
The study was funded by a grant from the National Institute of Health Research. Dr. Martineau and his coauthors did not report any relevant financial disclosures.
Administering doses of a vitamin D supplement to patients can significantly mitigate their risk of developing acute respiratory tract infections, according to a recent study published by the BMJ.
“[Existing] epidemiological and in vitro data have prompted numerous randomized controlled trials to determine whether vitamin D supplementation can decrease the risk of acute respiratory tract infection,” wrote the authors of the study, led by Adrian R. Martineau, PhD, of Queen Mary University of London. “A total of five aggregate data meta-analyses incorporating data from up to 15 primary trials have been conducted to date [but] all but one of these aggregate data meta-analyses reported statistically significant heterogeneity of effect between primary trials.”
A total of 532 studies were reviewed by a panel, of which 25 studies were ultimately selected for inclusion in this analysis. The studies included were of varying lengths in terms of trial periods and involved a total of 11,321 subjects ranging from 0 to 95 years of age. Of these, 10,933 (96.6%) subjects experienced at least one acute respiratory tract infection.
No significant benefit was found in subjects who had already experienced an infection, yielding an odds ratio of 0.98 (95% CI, 0.80-1.20; P = .83). Analysis performed to quantify the risk of infection with or without Vitamin D showed that taking Vitamin D supplements significantly decreased infection risk, with an OR of 0.88 (95% CI, 0.81-0.96; P less than .001) after adjusting for age, sex, and the duration of the trial.
Results also demonstrated that bolus doses of Vitamin D did not offer any beneficial value to subjects. Those who received daily or weekly doses without bolus had a better OR, compared with those who did receive at least one bolus dose: 0.81 (95% CI, 0.72-0.91) versus 0.97 (95% CI, 0.86-1.10), respectively (P = .05). Individuals whose baseline 25-hydroxyvitamin D levels were lower than 25 nanomols per liter experienced a greater benefit than those whose levels were above 25: OR = 0.30 (95% CI, 0.17-0.53) and OR = 0.75 (95% CI, 0.60-0.95), respectively (P = .006).
“Our study reports a major new indication for vitamin D supplementation: the prevention of acute respiratory tract infection,” Dr. Martineau and his coauthors concluded, adding that a potential application for these findings would be “the introduction of public health measures such as food fortification to improve vitamin D status, particularly in settings where profound vitamin D deficiency is common.”
The study was funded by a grant from the National Institute of Health Research. Dr. Martineau and his coauthors did not report any relevant financial disclosures.
Key clinical point:
Major finding: For those receiving vitamin D, the odds ratio of reducing respiratory infection risk was 0.88 (P less than .001).
Data source: Systematic review and meta-analysis of data on 11,321 subjects taken from 25 different trials.
Disclosures: Funded by the National Institute for Health Research; authors reported no relevant financial disclosures.
Cost equilibrium in Canada: Inpatient and outpatient treatment of herpes zoster
Rates of herpes zoster infections have increased substantially since the late 1990s in Canada. This, combined with rising per-episode medical costs and constant per-episode drug costs has led to increased total outpatient costs. But this has been offset by a dramatic drop in hospitalization rates and costs, according to a study published by BMC Infectious Diseases.
“A number of recent studies have looked at the burden of HZ-PHN [herpes zoster–postherpetic neuralgia] in a variety of health care systems, including Belgium, France, Germany, Greece, Italy, Spain, the United Kingdom, and Israel. However, little recent Canadian data have been published,” wrote Kevin J. Friesen, MD, and his associates at the University of Manitoba, Winnipeg, explaining that the goal of this study was to “establish the current burden of HZ and PHN in the setting of a universal health care system, and to look at long-term trends in their treatment costs.”
From 1997-1998 through 2013-2014, there were a total of 73,886 HZ incidents. In the final year alone, incidents numbered 5,746, which represents a 49.5% increase over 1997-1998. From the first year through 2009-2010, age-adjusted rates increased at a rate of 4.7 episodes per 1,000 person-years, but after that point, that rate increased to 5.7 episodes per 1,000 person-years, and continued that way through 2013-2014 (BMC Infect Dis. 2017. doi: 10.1186/s12879-017-2185-3).
Over the same 15-year time period, costs for drugs increased from an average of $89.77 to $127.34 per incident (P less than .03). Total medical costs increased from $57.98 to $78.84 per incident (P less than .00001).
However, the total annual cost of treating HZ and PHN dipped, particularly in 2011-2012, when it was $1,997,183, compared with $2,095,633 in the first year. Hospitalization rates also declined, going from 3.1% in 1997-1998 to 1.36% in 2011-2012 and bringing mean per-episode cost for the hospital down from $397 to $195.
“The combination of these trends in per-episode costs arising from medical care and drug treatment were multiplied by the increase in the annual incidence of HZ, causing total outpatient costs to increase,” the authors explained. “However, this increase was offset by the dramatic drop in rates of hospitalization and the resulting decrease in hospital costs.”
The study was funded by a grant from Merck Canada. Dr. Friesen and his coauthors did not report any relevant financial disclosures.
Rates of herpes zoster infections have increased substantially since the late 1990s in Canada. This, combined with rising per-episode medical costs and constant per-episode drug costs has led to increased total outpatient costs. But this has been offset by a dramatic drop in hospitalization rates and costs, according to a study published by BMC Infectious Diseases.
“A number of recent studies have looked at the burden of HZ-PHN [herpes zoster–postherpetic neuralgia] in a variety of health care systems, including Belgium, France, Germany, Greece, Italy, Spain, the United Kingdom, and Israel. However, little recent Canadian data have been published,” wrote Kevin J. Friesen, MD, and his associates at the University of Manitoba, Winnipeg, explaining that the goal of this study was to “establish the current burden of HZ and PHN in the setting of a universal health care system, and to look at long-term trends in their treatment costs.”
From 1997-1998 through 2013-2014, there were a total of 73,886 HZ incidents. In the final year alone, incidents numbered 5,746, which represents a 49.5% increase over 1997-1998. From the first year through 2009-2010, age-adjusted rates increased at a rate of 4.7 episodes per 1,000 person-years, but after that point, that rate increased to 5.7 episodes per 1,000 person-years, and continued that way through 2013-2014 (BMC Infect Dis. 2017. doi: 10.1186/s12879-017-2185-3).
Over the same 15-year time period, costs for drugs increased from an average of $89.77 to $127.34 per incident (P less than .03). Total medical costs increased from $57.98 to $78.84 per incident (P less than .00001).
However, the total annual cost of treating HZ and PHN dipped, particularly in 2011-2012, when it was $1,997,183, compared with $2,095,633 in the first year. Hospitalization rates also declined, going from 3.1% in 1997-1998 to 1.36% in 2011-2012 and bringing mean per-episode cost for the hospital down from $397 to $195.
“The combination of these trends in per-episode costs arising from medical care and drug treatment were multiplied by the increase in the annual incidence of HZ, causing total outpatient costs to increase,” the authors explained. “However, this increase was offset by the dramatic drop in rates of hospitalization and the resulting decrease in hospital costs.”
The study was funded by a grant from Merck Canada. Dr. Friesen and his coauthors did not report any relevant financial disclosures.
Rates of herpes zoster infections have increased substantially since the late 1990s in Canada. This, combined with rising per-episode medical costs and constant per-episode drug costs has led to increased total outpatient costs. But this has been offset by a dramatic drop in hospitalization rates and costs, according to a study published by BMC Infectious Diseases.
“A number of recent studies have looked at the burden of HZ-PHN [herpes zoster–postherpetic neuralgia] in a variety of health care systems, including Belgium, France, Germany, Greece, Italy, Spain, the United Kingdom, and Israel. However, little recent Canadian data have been published,” wrote Kevin J. Friesen, MD, and his associates at the University of Manitoba, Winnipeg, explaining that the goal of this study was to “establish the current burden of HZ and PHN in the setting of a universal health care system, and to look at long-term trends in their treatment costs.”
From 1997-1998 through 2013-2014, there were a total of 73,886 HZ incidents. In the final year alone, incidents numbered 5,746, which represents a 49.5% increase over 1997-1998. From the first year through 2009-2010, age-adjusted rates increased at a rate of 4.7 episodes per 1,000 person-years, but after that point, that rate increased to 5.7 episodes per 1,000 person-years, and continued that way through 2013-2014 (BMC Infect Dis. 2017. doi: 10.1186/s12879-017-2185-3).
Over the same 15-year time period, costs for drugs increased from an average of $89.77 to $127.34 per incident (P less than .03). Total medical costs increased from $57.98 to $78.84 per incident (P less than .00001).
However, the total annual cost of treating HZ and PHN dipped, particularly in 2011-2012, when it was $1,997,183, compared with $2,095,633 in the first year. Hospitalization rates also declined, going from 3.1% in 1997-1998 to 1.36% in 2011-2012 and bringing mean per-episode cost for the hospital down from $397 to $195.
“The combination of these trends in per-episode costs arising from medical care and drug treatment were multiplied by the increase in the annual incidence of HZ, causing total outpatient costs to increase,” the authors explained. “However, this increase was offset by the dramatic drop in rates of hospitalization and the resulting decrease in hospital costs.”
The study was funded by a grant from Merck Canada. Dr. Friesen and his coauthors did not report any relevant financial disclosures.
FROM BMC INFECTIOUS DISEASES
Key clinical point: Increasing herpes zoster rates combined with rising per-episode medical costs and constant per-episode drug costs has led to increased total outpatient costs. But this has been offset by a dramatic drop in hospitalization rates and costs.
Major finding: Incidence of herpes zoster between 1997-1998 and 2013-2014 increased by 49.5% in Canada.
Data source: Observational cohort study of data from the Manitoba Centre for Health Policy between April 1, 1997 and March 31, 2014.
Disclosures: Funded via grant from Merck Canada. Authors reported no relevant financial disclosures.
Osteoarthritis in hip or knee can increase diabetes risk
Individuals who have osteoarthritis in the hip or knee are significantly more likely to develop diabetes than are people without the condition, according to findings from a population-based cohort study.
The relationship between osteoarthritis (OA) and new-onset diabetes was largely explained by the walking limitations brought on by OA, which was unsurprising to lead author Tetyana Kendzerska, MD, PhD, of the University of Toronto, who presented the study at the annual meeting of the Canadian Rheumatology Association.
But Dr. Kendzerska noted that even though “the World Health Organization has determined that osteoarthritis is the fastest growing chronic disease and the single most common cause of disability in older adults [and] the majority of people with OA have at least one other chronic condition – usually diabetes, high blood pressure, [or] heart disease ... few studies have examined the impact of OA on these other conditions, including on the development of diabetes.”
Dr. Kendzerska and her coauthors used existing data to study a population of 16,362 adults aged 55 or older who did not have diabetes at baseline enrollment during 1996-1998 and were then followed until 2014 for a median period of 13 years. The adults’ median age was 68 years and median body mass index was 25.3 kg/m2; 61% were female. Cox regression modeling was used to quantify any association found between osteoarthritis and diabetes.
A total of 1,637 (10%) had hip osteoarthritis, 2,431 (15%) had knee osteoarthritis, and 3,908 (24%) had some type of walking limitation. Over the course of the follow-up period, 3,539 (22%) developed diabetes. The risk for diabetes was significantly elevated in particular for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees, after adjustment for baseline age, sex, income, body mass index, preexisting hypertension and cardiovascular disease, and prior primary care exposure. However, further adjustment to the comparisons for walking limitation attenuated the relationships so that they were no longer statistically significant.
The next steps for research may be to assess the impact of evidence-based osteoarthritis care on mobility and metabolic derangements, she said.
Previous “studies have been limited by a number of methodological limitations, [such as] cross-sectional design or failure to control for other factors that may explain a relationship. Our study has addressed these limitations and thus provides important evidence to suggest that osteoarthritis-related difficulty walking contributes causally to the development of diabetes [but] now we need studies to show if effective treatment of hip and knee osteoarthritis can reduce diabetes risk.”
The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
Individuals who have osteoarthritis in the hip or knee are significantly more likely to develop diabetes than are people without the condition, according to findings from a population-based cohort study.
The relationship between osteoarthritis (OA) and new-onset diabetes was largely explained by the walking limitations brought on by OA, which was unsurprising to lead author Tetyana Kendzerska, MD, PhD, of the University of Toronto, who presented the study at the annual meeting of the Canadian Rheumatology Association.
But Dr. Kendzerska noted that even though “the World Health Organization has determined that osteoarthritis is the fastest growing chronic disease and the single most common cause of disability in older adults [and] the majority of people with OA have at least one other chronic condition – usually diabetes, high blood pressure, [or] heart disease ... few studies have examined the impact of OA on these other conditions, including on the development of diabetes.”
Dr. Kendzerska and her coauthors used existing data to study a population of 16,362 adults aged 55 or older who did not have diabetes at baseline enrollment during 1996-1998 and were then followed until 2014 for a median period of 13 years. The adults’ median age was 68 years and median body mass index was 25.3 kg/m2; 61% were female. Cox regression modeling was used to quantify any association found between osteoarthritis and diabetes.
A total of 1,637 (10%) had hip osteoarthritis, 2,431 (15%) had knee osteoarthritis, and 3,908 (24%) had some type of walking limitation. Over the course of the follow-up period, 3,539 (22%) developed diabetes. The risk for diabetes was significantly elevated in particular for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees, after adjustment for baseline age, sex, income, body mass index, preexisting hypertension and cardiovascular disease, and prior primary care exposure. However, further adjustment to the comparisons for walking limitation attenuated the relationships so that they were no longer statistically significant.
The next steps for research may be to assess the impact of evidence-based osteoarthritis care on mobility and metabolic derangements, she said.
Previous “studies have been limited by a number of methodological limitations, [such as] cross-sectional design or failure to control for other factors that may explain a relationship. Our study has addressed these limitations and thus provides important evidence to suggest that osteoarthritis-related difficulty walking contributes causally to the development of diabetes [but] now we need studies to show if effective treatment of hip and knee osteoarthritis can reduce diabetes risk.”
The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
Individuals who have osteoarthritis in the hip or knee are significantly more likely to develop diabetes than are people without the condition, according to findings from a population-based cohort study.
The relationship between osteoarthritis (OA) and new-onset diabetes was largely explained by the walking limitations brought on by OA, which was unsurprising to lead author Tetyana Kendzerska, MD, PhD, of the University of Toronto, who presented the study at the annual meeting of the Canadian Rheumatology Association.
But Dr. Kendzerska noted that even though “the World Health Organization has determined that osteoarthritis is the fastest growing chronic disease and the single most common cause of disability in older adults [and] the majority of people with OA have at least one other chronic condition – usually diabetes, high blood pressure, [or] heart disease ... few studies have examined the impact of OA on these other conditions, including on the development of diabetes.”
Dr. Kendzerska and her coauthors used existing data to study a population of 16,362 adults aged 55 or older who did not have diabetes at baseline enrollment during 1996-1998 and were then followed until 2014 for a median period of 13 years. The adults’ median age was 68 years and median body mass index was 25.3 kg/m2; 61% were female. Cox regression modeling was used to quantify any association found between osteoarthritis and diabetes.
A total of 1,637 (10%) had hip osteoarthritis, 2,431 (15%) had knee osteoarthritis, and 3,908 (24%) had some type of walking limitation. Over the course of the follow-up period, 3,539 (22%) developed diabetes. The risk for diabetes was significantly elevated in particular for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees, after adjustment for baseline age, sex, income, body mass index, preexisting hypertension and cardiovascular disease, and prior primary care exposure. However, further adjustment to the comparisons for walking limitation attenuated the relationships so that they were no longer statistically significant.
The next steps for research may be to assess the impact of evidence-based osteoarthritis care on mobility and metabolic derangements, she said.
Previous “studies have been limited by a number of methodological limitations, [such as] cross-sectional design or failure to control for other factors that may explain a relationship. Our study has addressed these limitations and thus provides important evidence to suggest that osteoarthritis-related difficulty walking contributes causally to the development of diabetes [but] now we need studies to show if effective treatment of hip and knee osteoarthritis can reduce diabetes risk.”
The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
FROM THE CRA SCIENTIFIC CONFERENCE
Key clinical point:
Major finding: The risk for diabetes was significantly elevated for individuals with two hip or knee joints with OA, where the hazard ratio was 1.25 (95% CI, 1.08-1.44; P = .003) for hips and 1.16 (95% CI, 1.04-1.29; P = .008) for knees.
Data source: Population-based cohort study of 16,362 individuals without diabetes at baseline.
Disclosures: The Canadian Institutes of Health Research funded the study. Dr. Kendzerska did not report any relevant financial disclosures.
Familial and sporadic ankylosing spondylitis differ in small ways
While differences do exist between familial and sporadic ankylosing spondylitis, key similarities suggest that the two conditions can be treated the same way, according to a new study presented at the annual meeting of the Canadian Rheumatology Association.
“AS [ankylosing spondylitis] patients with a family history of AS are not very different from patients without any family history,” Nigil Haroon, MD, of the University Health Network in Toronto, explained in an interview. “They have similar disease activity as measured by markers of inflammation [and] similar disease severity as assessed by radiographic scoring for spinal damage.”
Dr. Haroon, along with his coinvestigators – including Bruce Sheng, MD,of the same institution, who presented the study at the meeting – prospectively followed AS patients satisfying the New York criteria for a period of 15 years, collecting data on 888 eligible subjects who were eventually included in the study. Of the subjects included, 74% were male, the average age was 45.6 years (standard deviation, 13.7 years), and average disease duration was 15 years (SD, 11.5 years).
The investigators found some similarities between the 177 (20%) patients with familial AS who had at least one first- or second-generation relative with the disease and the 711 with sporadic AS. Anti–tumor necrosis factor (anti-TNF) treatment failed in 23.1% of familial AS patients and 23.6% of sporadic disease patients based on the lack of a “sustained clinical effect” for more than 1 year. There were also no differences found between the groups in clinical and radiographic severity of disease.
However, patients with familial AS did record earlier onset of disease (22.5 years vs. 24.3 years; P = .016), longer disease duration (17.4 years vs. 14.3 years; P = .003), and higher HLA-B27 positivity (90% vs. 65%; P less than .001), along with higher rates of uveitis, psoriatic arthritis, and inflammatory bowel disease.
“Some of the findings are expected, including the higher prevalence of HLA-B27 due to gene sharing in the family. ... The higher B27 sharing may also affect the uveitis prevalence as well in familial AS,” Dr. Haroon explained. “The similar radiographic progression rates and treatment responses are interesting findings.”
In terms of the ramifications of these findings, Dr. Haroon stated that clinicians should reevaluate how they prescribe drugs to their AS patients.
“The high likelihood of uveitis in familial AS patients – 43% versus 29% – may affect the choice of treatment as all drugs are not equally effective in uveitis,” he said. “As the family history of extra-articular manifestations is high in familial AS, it remains to be seen if a lower threshold for investigating symptoms suggestive of IBD/uveitis will decrease delays in diagnosis of these conditions in individuals with a family history of AS.”
Moving forward from here, Dr. Haroon called for family studies, especially those including families with multiple individuals affected with AS, as these can help identify genetic risk factors that may be contribute to the development of AS.
“There is paucity of data on familial AS,” Dr. Haroon said. “The strength of this study is the large dataset.”
The study was funded by the Canadian Rheumatology Association’s Summer Research Program, which supported Dr. Sheng. Dr. Sheng and Dr. Haroon did not report any other relevant financial disclosures.
While differences do exist between familial and sporadic ankylosing spondylitis, key similarities suggest that the two conditions can be treated the same way, according to a new study presented at the annual meeting of the Canadian Rheumatology Association.
“AS [ankylosing spondylitis] patients with a family history of AS are not very different from patients without any family history,” Nigil Haroon, MD, of the University Health Network in Toronto, explained in an interview. “They have similar disease activity as measured by markers of inflammation [and] similar disease severity as assessed by radiographic scoring for spinal damage.”
Dr. Haroon, along with his coinvestigators – including Bruce Sheng, MD,of the same institution, who presented the study at the meeting – prospectively followed AS patients satisfying the New York criteria for a period of 15 years, collecting data on 888 eligible subjects who were eventually included in the study. Of the subjects included, 74% were male, the average age was 45.6 years (standard deviation, 13.7 years), and average disease duration was 15 years (SD, 11.5 years).
The investigators found some similarities between the 177 (20%) patients with familial AS who had at least one first- or second-generation relative with the disease and the 711 with sporadic AS. Anti–tumor necrosis factor (anti-TNF) treatment failed in 23.1% of familial AS patients and 23.6% of sporadic disease patients based on the lack of a “sustained clinical effect” for more than 1 year. There were also no differences found between the groups in clinical and radiographic severity of disease.
However, patients with familial AS did record earlier onset of disease (22.5 years vs. 24.3 years; P = .016), longer disease duration (17.4 years vs. 14.3 years; P = .003), and higher HLA-B27 positivity (90% vs. 65%; P less than .001), along with higher rates of uveitis, psoriatic arthritis, and inflammatory bowel disease.
“Some of the findings are expected, including the higher prevalence of HLA-B27 due to gene sharing in the family. ... The higher B27 sharing may also affect the uveitis prevalence as well in familial AS,” Dr. Haroon explained. “The similar radiographic progression rates and treatment responses are interesting findings.”
In terms of the ramifications of these findings, Dr. Haroon stated that clinicians should reevaluate how they prescribe drugs to their AS patients.
“The high likelihood of uveitis in familial AS patients – 43% versus 29% – may affect the choice of treatment as all drugs are not equally effective in uveitis,” he said. “As the family history of extra-articular manifestations is high in familial AS, it remains to be seen if a lower threshold for investigating symptoms suggestive of IBD/uveitis will decrease delays in diagnosis of these conditions in individuals with a family history of AS.”
Moving forward from here, Dr. Haroon called for family studies, especially those including families with multiple individuals affected with AS, as these can help identify genetic risk factors that may be contribute to the development of AS.
“There is paucity of data on familial AS,” Dr. Haroon said. “The strength of this study is the large dataset.”
The study was funded by the Canadian Rheumatology Association’s Summer Research Program, which supported Dr. Sheng. Dr. Sheng and Dr. Haroon did not report any other relevant financial disclosures.
While differences do exist between familial and sporadic ankylosing spondylitis, key similarities suggest that the two conditions can be treated the same way, according to a new study presented at the annual meeting of the Canadian Rheumatology Association.
“AS [ankylosing spondylitis] patients with a family history of AS are not very different from patients without any family history,” Nigil Haroon, MD, of the University Health Network in Toronto, explained in an interview. “They have similar disease activity as measured by markers of inflammation [and] similar disease severity as assessed by radiographic scoring for spinal damage.”
Dr. Haroon, along with his coinvestigators – including Bruce Sheng, MD,of the same institution, who presented the study at the meeting – prospectively followed AS patients satisfying the New York criteria for a period of 15 years, collecting data on 888 eligible subjects who were eventually included in the study. Of the subjects included, 74% were male, the average age was 45.6 years (standard deviation, 13.7 years), and average disease duration was 15 years (SD, 11.5 years).
The investigators found some similarities between the 177 (20%) patients with familial AS who had at least one first- or second-generation relative with the disease and the 711 with sporadic AS. Anti–tumor necrosis factor (anti-TNF) treatment failed in 23.1% of familial AS patients and 23.6% of sporadic disease patients based on the lack of a “sustained clinical effect” for more than 1 year. There were also no differences found between the groups in clinical and radiographic severity of disease.
However, patients with familial AS did record earlier onset of disease (22.5 years vs. 24.3 years; P = .016), longer disease duration (17.4 years vs. 14.3 years; P = .003), and higher HLA-B27 positivity (90% vs. 65%; P less than .001), along with higher rates of uveitis, psoriatic arthritis, and inflammatory bowel disease.
“Some of the findings are expected, including the higher prevalence of HLA-B27 due to gene sharing in the family. ... The higher B27 sharing may also affect the uveitis prevalence as well in familial AS,” Dr. Haroon explained. “The similar radiographic progression rates and treatment responses are interesting findings.”
In terms of the ramifications of these findings, Dr. Haroon stated that clinicians should reevaluate how they prescribe drugs to their AS patients.
“The high likelihood of uveitis in familial AS patients – 43% versus 29% – may affect the choice of treatment as all drugs are not equally effective in uveitis,” he said. “As the family history of extra-articular manifestations is high in familial AS, it remains to be seen if a lower threshold for investigating symptoms suggestive of IBD/uveitis will decrease delays in diagnosis of these conditions in individuals with a family history of AS.”
Moving forward from here, Dr. Haroon called for family studies, especially those including families with multiple individuals affected with AS, as these can help identify genetic risk factors that may be contribute to the development of AS.
“There is paucity of data on familial AS,” Dr. Haroon said. “The strength of this study is the large dataset.”
The study was funded by the Canadian Rheumatology Association’s Summer Research Program, which supported Dr. Sheng. Dr. Sheng and Dr. Haroon did not report any other relevant financial disclosures.
FROM THE CRA SCIENTIFIC CONFERENCE
Key clinical point:
Major finding: Anti-TNF treatment failed in 23.1% of familial AS patients and 23.6% of sporadic disease patients based on the lack of a “sustained clinical effect” for more than 1 year.
Data source: Prospective cohort study of 888 patients with AS over 15 years.
Disclosures: Funded by the Canadian Rheumatology Association’s Summer Research Program. No other relevant disclosures were reported.
Targeting Paget’s bone symptoms supported in long-term trial
Long-term intensive bisphosphonate treatment of patients with Paget’s disease of bone continued to show no benefit over symptomatic treatment in a 3-year extension trial of the PRISM study, leading investigators in the trial to recommend that treatment guidelines focus more on managing the condition’s symptoms rather than normalizing bone turnover biomarkers in hope of reducing disease complications.
“The Paget’s Disease Randomized Trial of Intensive versus Symptomatic Management (PRISM) study showed that intensive bisphosphonate therapy and symptomatic treatment had similar effects on clinical outcome of PDB [Paget’s disease of bone] with respect to the occurrence of fractures, orthopedic procedures, hearing loss, bone pain, quality of life, and adverse events,” wrote the principal investigator of the PRISM-EZ (extension with zoledronic acid) phase of the trial, Stuart H. Ralston, MD, of the University of Edinburgh (Scotland), and his colleagues.
Enrollment in the extension trial, which occurred during January 2007-January 2012, meant that patients kept to the same treatment strategies except for those in the intensive arm, who switched to zoledronic acid as the treatment of first choice. Both groups received analgesics and nonsteroidal anti-inflammatory drugs as needed. Patients in the symptomatic arm received bisphosphonate treatment only if they had bone pain thought to be caused by the increased metabolic activity of PDB, whereas those in the intensive arm were prescribed “bisphosphonates as required to suppress and maintain ALP [alkaline phosphatase] concentrations within the normal range,” according to the investigators. Clinical fracture rate was the primary outcome, but Dr. Ralston and his colleagues also compared orthopedic procedures, serum total ALP concentrations, bone pain, and quality of life in both cohorts.
A higher proportion of patients in the intensive group received zoledronic acid than in the symptomatic group (28.1% vs. 10.3%; P less than .001), whereas fewer patients in the intensive group received pamidronate (4.8% vs. 15.5%; P less than .001).
Of 270 in the intensive cohort, 22 (8.1%) experienced fractures, versus 12 (5.2%) of the 232 in the symptomatic cohort, yielding a hazard ratio of 1.90 (95% confidence interval, 0.91-3.98; P = .087). Of those, fractures in the pagetic bone numbered five and two, respectively. A total of 15 (5.6%) intensive treatment subjects and 7 (3.0%) symptomatic treatment subjects required orthopedic surgery, with a hazard ratio of 1.81 (95% CI, 0.71-4.61; P = .214).
There were no differences in bodily pain, bone pain, or quality of life at year 3 as reported by patients in both cohorts. While there was a statistically significant difference between intensive and symptomatic treatment in bodily pain, physical component summary score, and arthritis-specific health index scores at year 1 of the trial, the investigators noted that the findings were below the clinically significant five-point threshold and did not persist beyond 1 year.
“The results reported here do not support the recommendations made by the Endocrine Society guideline group, which suggested that most patients with PDB should be treated with potent bisphosphonates with the aim of restoring ALP values to within the lower part of the reference range,” the authors concluded. “On the contrary, the PRISM-EZ study demonstrates that this strategy is not associated with clinical benefit and might be harmful, [so] a more appropriate indication for bisphosphonate treatment in PDB is to control bone pain thought to be due to disease activity.”
The study was funded by grants from Arthritis Research UK and the Paget’s Association. Dr. Ralston reported receiving consultancy fees on behalf of his institution from Novartis and Merck and grant support on behalf of his institution from Amgen, Eli Lilly, and UCB. One coauthor reported receiving consultancy fees from Siemens, Becton Dickinson, and Roche, and another disclosed receiving such fees from Internis.
The PRISM-EZ study is the largest and longest study of PDB and provides important information on its long-term management. The results need to be kept in perspective. All patients were treated; hence, there is no untreated group and the results apply to follow-up therapy only. In this group, repeat therapy for active PDB as measured from serum total alkaline phosphatase (ALP) versus therapy for symptoms did not make a difference in a 3-year follow-up, and the authors recommend treating only when symptoms warrant it. Longer follow-ups would be needed to definitively show a difference in a condition that is present for many years.
Roy D. Altman, MD , is professor emeritus of medicine in the division of rheumatology and immunology at the University of California, Los Angeles. He has no relevant disclosures.
The PRISM-EZ study is the largest and longest study of PDB and provides important information on its long-term management. The results need to be kept in perspective. All patients were treated; hence, there is no untreated group and the results apply to follow-up therapy only. In this group, repeat therapy for active PDB as measured from serum total alkaline phosphatase (ALP) versus therapy for symptoms did not make a difference in a 3-year follow-up, and the authors recommend treating only when symptoms warrant it. Longer follow-ups would be needed to definitively show a difference in a condition that is present for many years.
Roy D. Altman, MD , is professor emeritus of medicine in the division of rheumatology and immunology at the University of California, Los Angeles. He has no relevant disclosures.
The PRISM-EZ study is the largest and longest study of PDB and provides important information on its long-term management. The results need to be kept in perspective. All patients were treated; hence, there is no untreated group and the results apply to follow-up therapy only. In this group, repeat therapy for active PDB as measured from serum total alkaline phosphatase (ALP) versus therapy for symptoms did not make a difference in a 3-year follow-up, and the authors recommend treating only when symptoms warrant it. Longer follow-ups would be needed to definitively show a difference in a condition that is present for many years.
Roy D. Altman, MD , is professor emeritus of medicine in the division of rheumatology and immunology at the University of California, Los Angeles. He has no relevant disclosures.
Long-term intensive bisphosphonate treatment of patients with Paget’s disease of bone continued to show no benefit over symptomatic treatment in a 3-year extension trial of the PRISM study, leading investigators in the trial to recommend that treatment guidelines focus more on managing the condition’s symptoms rather than normalizing bone turnover biomarkers in hope of reducing disease complications.
“The Paget’s Disease Randomized Trial of Intensive versus Symptomatic Management (PRISM) study showed that intensive bisphosphonate therapy and symptomatic treatment had similar effects on clinical outcome of PDB [Paget’s disease of bone] with respect to the occurrence of fractures, orthopedic procedures, hearing loss, bone pain, quality of life, and adverse events,” wrote the principal investigator of the PRISM-EZ (extension with zoledronic acid) phase of the trial, Stuart H. Ralston, MD, of the University of Edinburgh (Scotland), and his colleagues.
Enrollment in the extension trial, which occurred during January 2007-January 2012, meant that patients kept to the same treatment strategies except for those in the intensive arm, who switched to zoledronic acid as the treatment of first choice. Both groups received analgesics and nonsteroidal anti-inflammatory drugs as needed. Patients in the symptomatic arm received bisphosphonate treatment only if they had bone pain thought to be caused by the increased metabolic activity of PDB, whereas those in the intensive arm were prescribed “bisphosphonates as required to suppress and maintain ALP [alkaline phosphatase] concentrations within the normal range,” according to the investigators. Clinical fracture rate was the primary outcome, but Dr. Ralston and his colleagues also compared orthopedic procedures, serum total ALP concentrations, bone pain, and quality of life in both cohorts.
A higher proportion of patients in the intensive group received zoledronic acid than in the symptomatic group (28.1% vs. 10.3%; P less than .001), whereas fewer patients in the intensive group received pamidronate (4.8% vs. 15.5%; P less than .001).
Of 270 in the intensive cohort, 22 (8.1%) experienced fractures, versus 12 (5.2%) of the 232 in the symptomatic cohort, yielding a hazard ratio of 1.90 (95% confidence interval, 0.91-3.98; P = .087). Of those, fractures in the pagetic bone numbered five and two, respectively. A total of 15 (5.6%) intensive treatment subjects and 7 (3.0%) symptomatic treatment subjects required orthopedic surgery, with a hazard ratio of 1.81 (95% CI, 0.71-4.61; P = .214).
There were no differences in bodily pain, bone pain, or quality of life at year 3 as reported by patients in both cohorts. While there was a statistically significant difference between intensive and symptomatic treatment in bodily pain, physical component summary score, and arthritis-specific health index scores at year 1 of the trial, the investigators noted that the findings were below the clinically significant five-point threshold and did not persist beyond 1 year.
“The results reported here do not support the recommendations made by the Endocrine Society guideline group, which suggested that most patients with PDB should be treated with potent bisphosphonates with the aim of restoring ALP values to within the lower part of the reference range,” the authors concluded. “On the contrary, the PRISM-EZ study demonstrates that this strategy is not associated with clinical benefit and might be harmful, [so] a more appropriate indication for bisphosphonate treatment in PDB is to control bone pain thought to be due to disease activity.”
The study was funded by grants from Arthritis Research UK and the Paget’s Association. Dr. Ralston reported receiving consultancy fees on behalf of his institution from Novartis and Merck and grant support on behalf of his institution from Amgen, Eli Lilly, and UCB. One coauthor reported receiving consultancy fees from Siemens, Becton Dickinson, and Roche, and another disclosed receiving such fees from Internis.
Long-term intensive bisphosphonate treatment of patients with Paget’s disease of bone continued to show no benefit over symptomatic treatment in a 3-year extension trial of the PRISM study, leading investigators in the trial to recommend that treatment guidelines focus more on managing the condition’s symptoms rather than normalizing bone turnover biomarkers in hope of reducing disease complications.
“The Paget’s Disease Randomized Trial of Intensive versus Symptomatic Management (PRISM) study showed that intensive bisphosphonate therapy and symptomatic treatment had similar effects on clinical outcome of PDB [Paget’s disease of bone] with respect to the occurrence of fractures, orthopedic procedures, hearing loss, bone pain, quality of life, and adverse events,” wrote the principal investigator of the PRISM-EZ (extension with zoledronic acid) phase of the trial, Stuart H. Ralston, MD, of the University of Edinburgh (Scotland), and his colleagues.
Enrollment in the extension trial, which occurred during January 2007-January 2012, meant that patients kept to the same treatment strategies except for those in the intensive arm, who switched to zoledronic acid as the treatment of first choice. Both groups received analgesics and nonsteroidal anti-inflammatory drugs as needed. Patients in the symptomatic arm received bisphosphonate treatment only if they had bone pain thought to be caused by the increased metabolic activity of PDB, whereas those in the intensive arm were prescribed “bisphosphonates as required to suppress and maintain ALP [alkaline phosphatase] concentrations within the normal range,” according to the investigators. Clinical fracture rate was the primary outcome, but Dr. Ralston and his colleagues also compared orthopedic procedures, serum total ALP concentrations, bone pain, and quality of life in both cohorts.
A higher proportion of patients in the intensive group received zoledronic acid than in the symptomatic group (28.1% vs. 10.3%; P less than .001), whereas fewer patients in the intensive group received pamidronate (4.8% vs. 15.5%; P less than .001).
Of 270 in the intensive cohort, 22 (8.1%) experienced fractures, versus 12 (5.2%) of the 232 in the symptomatic cohort, yielding a hazard ratio of 1.90 (95% confidence interval, 0.91-3.98; P = .087). Of those, fractures in the pagetic bone numbered five and two, respectively. A total of 15 (5.6%) intensive treatment subjects and 7 (3.0%) symptomatic treatment subjects required orthopedic surgery, with a hazard ratio of 1.81 (95% CI, 0.71-4.61; P = .214).
There were no differences in bodily pain, bone pain, or quality of life at year 3 as reported by patients in both cohorts. While there was a statistically significant difference between intensive and symptomatic treatment in bodily pain, physical component summary score, and arthritis-specific health index scores at year 1 of the trial, the investigators noted that the findings were below the clinically significant five-point threshold and did not persist beyond 1 year.
“The results reported here do not support the recommendations made by the Endocrine Society guideline group, which suggested that most patients with PDB should be treated with potent bisphosphonates with the aim of restoring ALP values to within the lower part of the reference range,” the authors concluded. “On the contrary, the PRISM-EZ study demonstrates that this strategy is not associated with clinical benefit and might be harmful, [so] a more appropriate indication for bisphosphonate treatment in PDB is to control bone pain thought to be due to disease activity.”
The study was funded by grants from Arthritis Research UK and the Paget’s Association. Dr. Ralston reported receiving consultancy fees on behalf of his institution from Novartis and Merck and grant support on behalf of his institution from Amgen, Eli Lilly, and UCB. One coauthor reported receiving consultancy fees from Siemens, Becton Dickinson, and Roche, and another disclosed receiving such fees from Internis.
FROM JOURNAL OF BONE AND MINERAL RESEARCH
Key clinical point:
Major finding: Intensive bisphosphonate therapy did not yield significant differences in rates of fractures (P = .087), orthopedic procedures (P = .214), and serious adverse events (RR = 1.28).
Data source: An extension study of a randomized trial involving 502 PDB patients during January 2007-January 2012.
Disclosures: The study was funded by grants from Arthritis Research UK and the Paget’s Association. Dr. Ralston reported receiving consultancy fees on behalf of his institution from Novartis and Merck and grant support on behalf of his institution from Amgen, Eli Lilly, and UCB. One coauthor reported receiving consultancy fees from Siemens, Becton Dickinson, and Roche, and another disclosed receiving such fees from Internis.
ACIP releases updated guidance for adult vaccinations
, according to the newly issued 2017 adult Recommended Immunization Schedule released by the CDC’s Advisory Committee on Immunization Practices (ACIP).
“Changes are related to concerns regarding low effectiveness of [LAIV] (FluMist, MedImmune) against influenza A(H1N1)pdm09 in the United States during the 2013-2014 and 2015-2016 influenza seasons,” wrote the authors of the report, published in Annals of Internal Medicine and led by David K. Kim, MD, of the CDC’s Immunization Services Division in Atlanta.
Another major change involves vaccination of adults with mild or severe egg allergy. The new guidance states that those with a mild egg allergy should receive either inactivated influenza vaccine or recombinant influenza vaccine, while those with severe allergies should be given one of the same vaccinations but only in a health care setting, so a clinician can monitor any signs of reaction and treat the patient accordingly.
Vaccine doses should be administered based on the patient’s age; a patient with “severe” egg allergy is one who exhibits angioedema, respiratory distress, lightheadedness, or recurrent emesis; requires epinephrine; or requires emergency medical care of any kind after consuming egg products.
Human papillomavirus (HPV) schedules have also been noticeably altered, with the CDC now considering all men and women through the ages of 21 and 26 years, respectively, who received a two-dose series of HPV vaccinations before the age of 15 to be adequately protected. Those who took only one of those two doses still need to take another dose, while men and women who have not been vaccinated should received a three-dose series at 0, 1-2, and 6 months.
Hepatitis B recommendations were also updated, with the CDC now advising: “Adults with chronic liver disease, including, but not limited to, hepatitis C virus infection, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level greater than twice the upper limit of normal, should receive a HepB series.”
Meningococcal vaccination guidelines also underwent a number of small changes pertaining to adults with anatomical or functional asplenia and human immunodeficiency virus, among other risk factors.
A number of small changes to the schedule chart were implemented to help make the immunization schedule more “clean and streamlined,” according to the CDC.
“Physicians should pay careful attention to the details found in the footnotes,” the CDC said in a statement. “The footnotes clarify who needs what vaccine, when, and at what dose.”
, according to the newly issued 2017 adult Recommended Immunization Schedule released by the CDC’s Advisory Committee on Immunization Practices (ACIP).
“Changes are related to concerns regarding low effectiveness of [LAIV] (FluMist, MedImmune) against influenza A(H1N1)pdm09 in the United States during the 2013-2014 and 2015-2016 influenza seasons,” wrote the authors of the report, published in Annals of Internal Medicine and led by David K. Kim, MD, of the CDC’s Immunization Services Division in Atlanta.
Another major change involves vaccination of adults with mild or severe egg allergy. The new guidance states that those with a mild egg allergy should receive either inactivated influenza vaccine or recombinant influenza vaccine, while those with severe allergies should be given one of the same vaccinations but only in a health care setting, so a clinician can monitor any signs of reaction and treat the patient accordingly.
Vaccine doses should be administered based on the patient’s age; a patient with “severe” egg allergy is one who exhibits angioedema, respiratory distress, lightheadedness, or recurrent emesis; requires epinephrine; or requires emergency medical care of any kind after consuming egg products.
Human papillomavirus (HPV) schedules have also been noticeably altered, with the CDC now considering all men and women through the ages of 21 and 26 years, respectively, who received a two-dose series of HPV vaccinations before the age of 15 to be adequately protected. Those who took only one of those two doses still need to take another dose, while men and women who have not been vaccinated should received a three-dose series at 0, 1-2, and 6 months.
Hepatitis B recommendations were also updated, with the CDC now advising: “Adults with chronic liver disease, including, but not limited to, hepatitis C virus infection, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level greater than twice the upper limit of normal, should receive a HepB series.”
Meningococcal vaccination guidelines also underwent a number of small changes pertaining to adults with anatomical or functional asplenia and human immunodeficiency virus, among other risk factors.
A number of small changes to the schedule chart were implemented to help make the immunization schedule more “clean and streamlined,” according to the CDC.
“Physicians should pay careful attention to the details found in the footnotes,” the CDC said in a statement. “The footnotes clarify who needs what vaccine, when, and at what dose.”
, according to the newly issued 2017 adult Recommended Immunization Schedule released by the CDC’s Advisory Committee on Immunization Practices (ACIP).
“Changes are related to concerns regarding low effectiveness of [LAIV] (FluMist, MedImmune) against influenza A(H1N1)pdm09 in the United States during the 2013-2014 and 2015-2016 influenza seasons,” wrote the authors of the report, published in Annals of Internal Medicine and led by David K. Kim, MD, of the CDC’s Immunization Services Division in Atlanta.
Another major change involves vaccination of adults with mild or severe egg allergy. The new guidance states that those with a mild egg allergy should receive either inactivated influenza vaccine or recombinant influenza vaccine, while those with severe allergies should be given one of the same vaccinations but only in a health care setting, so a clinician can monitor any signs of reaction and treat the patient accordingly.
Vaccine doses should be administered based on the patient’s age; a patient with “severe” egg allergy is one who exhibits angioedema, respiratory distress, lightheadedness, or recurrent emesis; requires epinephrine; or requires emergency medical care of any kind after consuming egg products.
Human papillomavirus (HPV) schedules have also been noticeably altered, with the CDC now considering all men and women through the ages of 21 and 26 years, respectively, who received a two-dose series of HPV vaccinations before the age of 15 to be adequately protected. Those who took only one of those two doses still need to take another dose, while men and women who have not been vaccinated should received a three-dose series at 0, 1-2, and 6 months.
Hepatitis B recommendations were also updated, with the CDC now advising: “Adults with chronic liver disease, including, but not limited to, hepatitis C virus infection, cirrhosis, fatty liver disease, alcoholic liver disease, autoimmune hepatitis, and an alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level greater than twice the upper limit of normal, should receive a HepB series.”
Meningococcal vaccination guidelines also underwent a number of small changes pertaining to adults with anatomical or functional asplenia and human immunodeficiency virus, among other risk factors.
A number of small changes to the schedule chart were implemented to help make the immunization schedule more “clean and streamlined,” according to the CDC.
“Physicians should pay careful attention to the details found in the footnotes,” the CDC said in a statement. “The footnotes clarify who needs what vaccine, when, and at what dose.”
FROM ANNALS OF INTERNAL MEDICINE
Perioperative infliximab does not increase serious infection risk
Administration of infliximab within 4 weeks of elective knee or hip arthroplasty did not have any significant effect on patients’ risk of serious infection after surgery, whereas the use of glucocorticoids increased that risk, in an analysis of a Medicare claims database.
“This increased risk with glucocorticoids has been suggested by previous studies [and] although this risk may be related in part to increased disease severity among glucocorticoid treated patients, a direct medication effect is likely. [These data suggest] that prolonged interruptions in infliximab therapy prior to surgery may be counterproductive if higher dose glucocorticoid therapy is used in substitution,” wrote the authors of the new study, led by Michael D. George, MD, of the University of Pennsylvania in Philadelphia.
Dr. George and his colleagues examined data from the U.S. Medicare claims system on 4,288 elective knee or hip arthroplasties in individuals with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis who received infliximab within 6 months prior to the operation during 2007-2013 (Arthritis Care Res. 2017 Jan 27. doi: 10.1002/acr.23209).
The patients had to have received infliximab at least three times within a year of their procedure to establish that they were receiving stable therapy over a long-term period. The investigators also looked at oral prednisone, prednisolone, and methylprednisolone prescriptions and used data on average dosing to determine how much was administered to each subject.
“Although previous studies have treated TNF stopping vs. not stopping as a dichotomous exposure based on an arbitrary (and variable) stopping definition, in this study the primary analysis evaluated stop timing as a more general categorical exposure using 4-week intervals (half the standard rheumatoid arthritis dosing interval) to allow better assessment of the optimal stop timing,” the authors explained.
Stopping infliximab within 4 weeks of the operation did not significantly influence the rate of serious infection within 30 days (adjusted odds ratio, 0.90; 95% CI, 0.60-1.34) and neither did stopping within 4-8 weeks (OR, 0.95; 95% CI, 0.62-1.36) when compared against stopping 8-12 weeks before surgery. Of the 4,288 arthroplasties, 270 serious infections (6.3%) occurred within 30 days of the operation.
There also was no significant difference between stopping within 4 weeks and 8-12 weeks in the rate of prosthetic joint infection within 1 year of the operation (hazard ratio, 0.98; 95% CI, 0.52-1.87). Overall, prosthetic joint infection occurred 2.9 times per 100 person-years.
However, glucocorticoid doses of more than 10 mg per day were risky. The odds for a serious infection within 30 days after surgery more than doubled with that level of use (OR, 2.11; 95% CI, 1.30-3.40), while the risk for a prosthetic joint infection within 1 year of the surgery also rose significantly (HR, 2.70; 95% CI, 1.30-5.60).
“This is a very well done paper that adds important observational data to our understanding of perioperative medication risk,” Dr. Goodman said.
But the study results will not, at least initially, bring about any changes to the proposed guidelines for perioperative management of patients taking antirheumatic drugs that were described at the 2016 annual meeting of the American College of Rheumatology, she said.
“We were aware of the abstract, which was also presented at the ACR last fall at the time the current perioperative medication management guidelines were presented, and it won’t change guidelines at this point,” said Dr. Goodman, who is one of the lead authors of the proposed guidelines. “[But] I think [the study] could provide important background information to use in a randomized clinical trial to compare infection on [and] not on TNF inhibitors.”
The proposed guidelines conditionally recommend that all biologics should be withheld prior to surgery in patients with inflammatory arthritis, that surgery should be planned for the end of the dosing cycle, and that current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with rheumatoid arthritis, lupus, or inflammatory arthritis.
The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
Administration of infliximab within 4 weeks of elective knee or hip arthroplasty did not have any significant effect on patients’ risk of serious infection after surgery, whereas the use of glucocorticoids increased that risk, in an analysis of a Medicare claims database.
“This increased risk with glucocorticoids has been suggested by previous studies [and] although this risk may be related in part to increased disease severity among glucocorticoid treated patients, a direct medication effect is likely. [These data suggest] that prolonged interruptions in infliximab therapy prior to surgery may be counterproductive if higher dose glucocorticoid therapy is used in substitution,” wrote the authors of the new study, led by Michael D. George, MD, of the University of Pennsylvania in Philadelphia.
Dr. George and his colleagues examined data from the U.S. Medicare claims system on 4,288 elective knee or hip arthroplasties in individuals with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis who received infliximab within 6 months prior to the operation during 2007-2013 (Arthritis Care Res. 2017 Jan 27. doi: 10.1002/acr.23209).
The patients had to have received infliximab at least three times within a year of their procedure to establish that they were receiving stable therapy over a long-term period. The investigators also looked at oral prednisone, prednisolone, and methylprednisolone prescriptions and used data on average dosing to determine how much was administered to each subject.
“Although previous studies have treated TNF stopping vs. not stopping as a dichotomous exposure based on an arbitrary (and variable) stopping definition, in this study the primary analysis evaluated stop timing as a more general categorical exposure using 4-week intervals (half the standard rheumatoid arthritis dosing interval) to allow better assessment of the optimal stop timing,” the authors explained.
Stopping infliximab within 4 weeks of the operation did not significantly influence the rate of serious infection within 30 days (adjusted odds ratio, 0.90; 95% CI, 0.60-1.34) and neither did stopping within 4-8 weeks (OR, 0.95; 95% CI, 0.62-1.36) when compared against stopping 8-12 weeks before surgery. Of the 4,288 arthroplasties, 270 serious infections (6.3%) occurred within 30 days of the operation.
There also was no significant difference between stopping within 4 weeks and 8-12 weeks in the rate of prosthetic joint infection within 1 year of the operation (hazard ratio, 0.98; 95% CI, 0.52-1.87). Overall, prosthetic joint infection occurred 2.9 times per 100 person-years.
However, glucocorticoid doses of more than 10 mg per day were risky. The odds for a serious infection within 30 days after surgery more than doubled with that level of use (OR, 2.11; 95% CI, 1.30-3.40), while the risk for a prosthetic joint infection within 1 year of the surgery also rose significantly (HR, 2.70; 95% CI, 1.30-5.60).
“This is a very well done paper that adds important observational data to our understanding of perioperative medication risk,” Dr. Goodman said.
But the study results will not, at least initially, bring about any changes to the proposed guidelines for perioperative management of patients taking antirheumatic drugs that were described at the 2016 annual meeting of the American College of Rheumatology, she said.
“We were aware of the abstract, which was also presented at the ACR last fall at the time the current perioperative medication management guidelines were presented, and it won’t change guidelines at this point,” said Dr. Goodman, who is one of the lead authors of the proposed guidelines. “[But] I think [the study] could provide important background information to use in a randomized clinical trial to compare infection on [and] not on TNF inhibitors.”
The proposed guidelines conditionally recommend that all biologics should be withheld prior to surgery in patients with inflammatory arthritis, that surgery should be planned for the end of the dosing cycle, and that current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with rheumatoid arthritis, lupus, or inflammatory arthritis.
The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
Administration of infliximab within 4 weeks of elective knee or hip arthroplasty did not have any significant effect on patients’ risk of serious infection after surgery, whereas the use of glucocorticoids increased that risk, in an analysis of a Medicare claims database.
“This increased risk with glucocorticoids has been suggested by previous studies [and] although this risk may be related in part to increased disease severity among glucocorticoid treated patients, a direct medication effect is likely. [These data suggest] that prolonged interruptions in infliximab therapy prior to surgery may be counterproductive if higher dose glucocorticoid therapy is used in substitution,” wrote the authors of the new study, led by Michael D. George, MD, of the University of Pennsylvania in Philadelphia.
Dr. George and his colleagues examined data from the U.S. Medicare claims system on 4,288 elective knee or hip arthroplasties in individuals with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis who received infliximab within 6 months prior to the operation during 2007-2013 (Arthritis Care Res. 2017 Jan 27. doi: 10.1002/acr.23209).
The patients had to have received infliximab at least three times within a year of their procedure to establish that they were receiving stable therapy over a long-term period. The investigators also looked at oral prednisone, prednisolone, and methylprednisolone prescriptions and used data on average dosing to determine how much was administered to each subject.
“Although previous studies have treated TNF stopping vs. not stopping as a dichotomous exposure based on an arbitrary (and variable) stopping definition, in this study the primary analysis evaluated stop timing as a more general categorical exposure using 4-week intervals (half the standard rheumatoid arthritis dosing interval) to allow better assessment of the optimal stop timing,” the authors explained.
Stopping infliximab within 4 weeks of the operation did not significantly influence the rate of serious infection within 30 days (adjusted odds ratio, 0.90; 95% CI, 0.60-1.34) and neither did stopping within 4-8 weeks (OR, 0.95; 95% CI, 0.62-1.36) when compared against stopping 8-12 weeks before surgery. Of the 4,288 arthroplasties, 270 serious infections (6.3%) occurred within 30 days of the operation.
There also was no significant difference between stopping within 4 weeks and 8-12 weeks in the rate of prosthetic joint infection within 1 year of the operation (hazard ratio, 0.98; 95% CI, 0.52-1.87). Overall, prosthetic joint infection occurred 2.9 times per 100 person-years.
However, glucocorticoid doses of more than 10 mg per day were risky. The odds for a serious infection within 30 days after surgery more than doubled with that level of use (OR, 2.11; 95% CI, 1.30-3.40), while the risk for a prosthetic joint infection within 1 year of the surgery also rose significantly (HR, 2.70; 95% CI, 1.30-5.60).
“This is a very well done paper that adds important observational data to our understanding of perioperative medication risk,” Dr. Goodman said.
But the study results will not, at least initially, bring about any changes to the proposed guidelines for perioperative management of patients taking antirheumatic drugs that were described at the 2016 annual meeting of the American College of Rheumatology, she said.
“We were aware of the abstract, which was also presented at the ACR last fall at the time the current perioperative medication management guidelines were presented, and it won’t change guidelines at this point,” said Dr. Goodman, who is one of the lead authors of the proposed guidelines. “[But] I think [the study] could provide important background information to use in a randomized clinical trial to compare infection on [and] not on TNF inhibitors.”
The proposed guidelines conditionally recommend that all biologics should be withheld prior to surgery in patients with inflammatory arthritis, that surgery should be planned for the end of the dosing cycle, and that current daily doses of glucocorticoids, rather than supraphysiologic doses, should be continued in adults with rheumatoid arthritis, lupus, or inflammatory arthritis.
The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Subjects on glucocorticoids had an OR of 2.11 (95% CI 1.30-3.40) for serious infection within 30 days and an HR of 2.70 (95% CI 1.30-5.60) for prosthetic joint infection within 1 year.
Data source: Retrospective cohort study of 4,288 elective knee and hip arthroplasties in Medicare patients with rheumatoid arthritis, inflammatory bowel disease, psoriasis, psoriatic arthritis, or ankylosing spondylitis during 2007-2013.
Disclosures: The National Institutes of Health, the Rheumatology Research Foundation, and the Department of Veterans Affairs funded the study. Dr. George did not report any relevant financial disclosures. Two coauthors disclosed receiving research grants or consulting fees from pharmaceutical companies for unrelated work.
Second dose rates of meningococcal vaccines low among HIV-positive men who have sex with men
ATLANTA – HIV-positive men who have sex with men should be getting vaccinated against invasive meningococcal disease twice, but an alarming majority are only getting vaccinated once, according to a new study presented at a conference on STD prevention sponsored the Centers for Disease Control and Prevention.
“This analysis underscores the need for active patient recall in order to maximize return for second dose among HIV-infected [men who have sex with men], although [that] may be resource-intensive,” said Kelly Jamison, MPH, of New York City’s department of health and mental hygiene.
Ms. Jamison and her coinvestigators examined medical record data of HIV-infected men who have sex with men who visited New York City STD clinics between Oct. 5, 2012 and Dec. 31, 2014, looking for individuals who received their first meningococcal vaccinations during that time period. The primary endpoint was to find the rate at which individuals who received the first vaccination came back within 1 year (Dec. 31, 2015) to receive a second vaccination.
The study was prompted by the invasive meningococcal disease (IMD) outbreak that New York City experienced from 2010-2013, in which 22 cases were identified in men who have sex with men, of which 55% involved men who were HIV-infected. All IMD cases involved serotype C, with a case fatality rate that was three times what public health officials anticipated at the time.
Because of this, the city launched a meningitis vaccination campaign. Vaccination was recommended for all men who have sex with men who were residents of New York City and had high-risk sexual exposure after Sept. 1, 2012. In early October, STD clinics around the city began offering free MCV4 vaccines. By late November, the recommendations were updated to include men who have sex with men who lived in specific parts of Brooklyn and had experienced high-risk sexual exposure after Sept. 1. In March 2013, the recommendations were further updated to state that all HIV-infected men who have sex with men and all such men with high-risk sexual exposure should be vaccinated. In August 2013, after the outbreak was over, the recommendations were updated one last time to state that they were recommendations for “ongoing vaccination.”
“A single dose of MCV4 is not sufficient for HIV-infected persons, so a second dose is recommended to occur 8 weeks after the first dose, and in order to increase two-dose coverage among HIV-infected MSM, STD clinicians provided the date for person to return for their second dose on a vaccine card given to patients at time of their first dose,” Ms. Jamison explained.
In total, 1,212 individuals were included in study. Over the course of the study period, only 322 (26.6%) returned within 1 year for a second vaccination. In terms of individual years, 2012 experienced the highest rate of second vaccination returns, at 38.6% (P less than .001). Of the 322 who received the second vaccination, 144 (44.7%) came to the STD clinic specifically for the second dose, 69 (21.4%) asked for the second vaccination along with other STD services, and 109 (33.9%) were “opportunistically vaccinated while presenting for other services.”
Older men who have sex with men were more likely to return for their second vaccination, as only 63 (18%) of those who did were under the age of 30. Those aged between 30 and 39 years numbered 80 (23%), those between 40 and 49 years numbered 102 (33%), and those aged 50 years or older numbered 77 (40%), meaning that older men were two to three times more likely to get that second dose (P less than .001).
“We did see suboptimal return for second doses, but this may be an underestimate, [because] we were unable to capture second doses received at non-STD clinic providers,” Ms. Jamison noted.
Ms. Jamison did not report any financial disclosures for this study.
ATLANTA – HIV-positive men who have sex with men should be getting vaccinated against invasive meningococcal disease twice, but an alarming majority are only getting vaccinated once, according to a new study presented at a conference on STD prevention sponsored the Centers for Disease Control and Prevention.
“This analysis underscores the need for active patient recall in order to maximize return for second dose among HIV-infected [men who have sex with men], although [that] may be resource-intensive,” said Kelly Jamison, MPH, of New York City’s department of health and mental hygiene.
Ms. Jamison and her coinvestigators examined medical record data of HIV-infected men who have sex with men who visited New York City STD clinics between Oct. 5, 2012 and Dec. 31, 2014, looking for individuals who received their first meningococcal vaccinations during that time period. The primary endpoint was to find the rate at which individuals who received the first vaccination came back within 1 year (Dec. 31, 2015) to receive a second vaccination.
The study was prompted by the invasive meningococcal disease (IMD) outbreak that New York City experienced from 2010-2013, in which 22 cases were identified in men who have sex with men, of which 55% involved men who were HIV-infected. All IMD cases involved serotype C, with a case fatality rate that was three times what public health officials anticipated at the time.
Because of this, the city launched a meningitis vaccination campaign. Vaccination was recommended for all men who have sex with men who were residents of New York City and had high-risk sexual exposure after Sept. 1, 2012. In early October, STD clinics around the city began offering free MCV4 vaccines. By late November, the recommendations were updated to include men who have sex with men who lived in specific parts of Brooklyn and had experienced high-risk sexual exposure after Sept. 1. In March 2013, the recommendations were further updated to state that all HIV-infected men who have sex with men and all such men with high-risk sexual exposure should be vaccinated. In August 2013, after the outbreak was over, the recommendations were updated one last time to state that they were recommendations for “ongoing vaccination.”
“A single dose of MCV4 is not sufficient for HIV-infected persons, so a second dose is recommended to occur 8 weeks after the first dose, and in order to increase two-dose coverage among HIV-infected MSM, STD clinicians provided the date for person to return for their second dose on a vaccine card given to patients at time of their first dose,” Ms. Jamison explained.
In total, 1,212 individuals were included in study. Over the course of the study period, only 322 (26.6%) returned within 1 year for a second vaccination. In terms of individual years, 2012 experienced the highest rate of second vaccination returns, at 38.6% (P less than .001). Of the 322 who received the second vaccination, 144 (44.7%) came to the STD clinic specifically for the second dose, 69 (21.4%) asked for the second vaccination along with other STD services, and 109 (33.9%) were “opportunistically vaccinated while presenting for other services.”
Older men who have sex with men were more likely to return for their second vaccination, as only 63 (18%) of those who did were under the age of 30. Those aged between 30 and 39 years numbered 80 (23%), those between 40 and 49 years numbered 102 (33%), and those aged 50 years or older numbered 77 (40%), meaning that older men were two to three times more likely to get that second dose (P less than .001).
“We did see suboptimal return for second doses, but this may be an underestimate, [because] we were unable to capture second doses received at non-STD clinic providers,” Ms. Jamison noted.
Ms. Jamison did not report any financial disclosures for this study.
ATLANTA – HIV-positive men who have sex with men should be getting vaccinated against invasive meningococcal disease twice, but an alarming majority are only getting vaccinated once, according to a new study presented at a conference on STD prevention sponsored the Centers for Disease Control and Prevention.
“This analysis underscores the need for active patient recall in order to maximize return for second dose among HIV-infected [men who have sex with men], although [that] may be resource-intensive,” said Kelly Jamison, MPH, of New York City’s department of health and mental hygiene.
Ms. Jamison and her coinvestigators examined medical record data of HIV-infected men who have sex with men who visited New York City STD clinics between Oct. 5, 2012 and Dec. 31, 2014, looking for individuals who received their first meningococcal vaccinations during that time period. The primary endpoint was to find the rate at which individuals who received the first vaccination came back within 1 year (Dec. 31, 2015) to receive a second vaccination.
The study was prompted by the invasive meningococcal disease (IMD) outbreak that New York City experienced from 2010-2013, in which 22 cases were identified in men who have sex with men, of which 55% involved men who were HIV-infected. All IMD cases involved serotype C, with a case fatality rate that was three times what public health officials anticipated at the time.
Because of this, the city launched a meningitis vaccination campaign. Vaccination was recommended for all men who have sex with men who were residents of New York City and had high-risk sexual exposure after Sept. 1, 2012. In early October, STD clinics around the city began offering free MCV4 vaccines. By late November, the recommendations were updated to include men who have sex with men who lived in specific parts of Brooklyn and had experienced high-risk sexual exposure after Sept. 1. In March 2013, the recommendations were further updated to state that all HIV-infected men who have sex with men and all such men with high-risk sexual exposure should be vaccinated. In August 2013, after the outbreak was over, the recommendations were updated one last time to state that they were recommendations for “ongoing vaccination.”
“A single dose of MCV4 is not sufficient for HIV-infected persons, so a second dose is recommended to occur 8 weeks after the first dose, and in order to increase two-dose coverage among HIV-infected MSM, STD clinicians provided the date for person to return for their second dose on a vaccine card given to patients at time of their first dose,” Ms. Jamison explained.
In total, 1,212 individuals were included in study. Over the course of the study period, only 322 (26.6%) returned within 1 year for a second vaccination. In terms of individual years, 2012 experienced the highest rate of second vaccination returns, at 38.6% (P less than .001). Of the 322 who received the second vaccination, 144 (44.7%) came to the STD clinic specifically for the second dose, 69 (21.4%) asked for the second vaccination along with other STD services, and 109 (33.9%) were “opportunistically vaccinated while presenting for other services.”
Older men who have sex with men were more likely to return for their second vaccination, as only 63 (18%) of those who did were under the age of 30. Those aged between 30 and 39 years numbered 80 (23%), those between 40 and 49 years numbered 102 (33%), and those aged 50 years or older numbered 77 (40%), meaning that older men were two to three times more likely to get that second dose (P less than .001).
“We did see suboptimal return for second doses, but this may be an underestimate, [because] we were unable to capture second doses received at non-STD clinic providers,” Ms. Jamison noted.
Ms. Jamison did not report any financial disclosures for this study.
AT THE 2016 STD PREVENTION CONFERENCE
Key clinical point:
Major finding: Only 26.6% (322 of 1,212) of men who have sex with men received a second dose within a year of receiving their first, with older men who have sex with men 2-3 times more likely to get the second dose than younger men who have sex with men.
Data source: Retrospective analysis of 1,212 men who have sex with men who visited New York City STD clinics from 2012-2015.
Disclosures: Ms. Jamison did not report any financial disclosures.