User login
Optimizing Patient Positioning During Dermatologic Surgery
Practice Gap
Practical patient positioning is a commonly overlooked method of tension control during excision and repair that allows for easier closure.1 Although positioning is a basic step in dermatologic surgery, it often is difficult and awkward for both the patient and physician. Here, we describe basic principles in patient positioning that increase tension across the surgical site during excision and reduce tension during closure. By reducing the amount of work required for excision and closure, procedures are completed more quickly, which increases efficiency. These techniques should be considered during dermatologic surgery at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities.
Technique: Upper Back Procedures
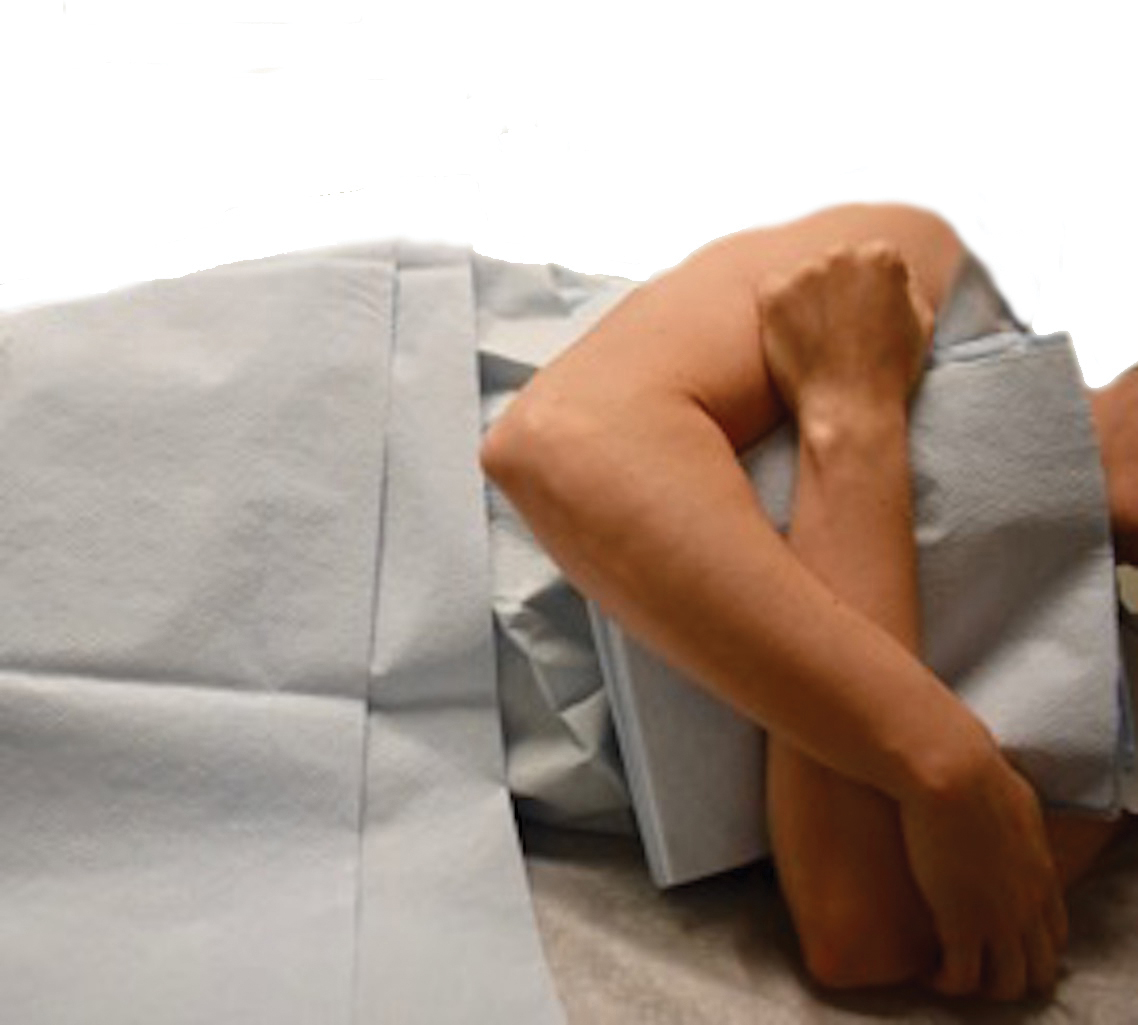
When removing lesions on the upper back, lying completely prone is uncomfortable for the patient and leaves the shoulders hyperextended.2 Instead, position the patient with the arms extended anteriorly, hugging a pillow, while lying prone or on one side (Figure 1). In this position, excision of the lesion is facilitated by increased tension across the upper back. In addition, this position is notably more comfortable for the patient. During closure, the patient should lie on the side contralateral to the surgical site, with the elbow resting at the hip and the ipsilateral arm lying parallel to the torso (Figure 2).
Following procedures on the upper back and shoulders, we typically recommend that the patient wear an arm sling on the ipsilateral side for 1 week. Doing so reliably limits mobility postoperatively and does not require the patient to constantly monitor their movement.
Technique: Lower Extremity Procedures
Anterior Lower Extremity
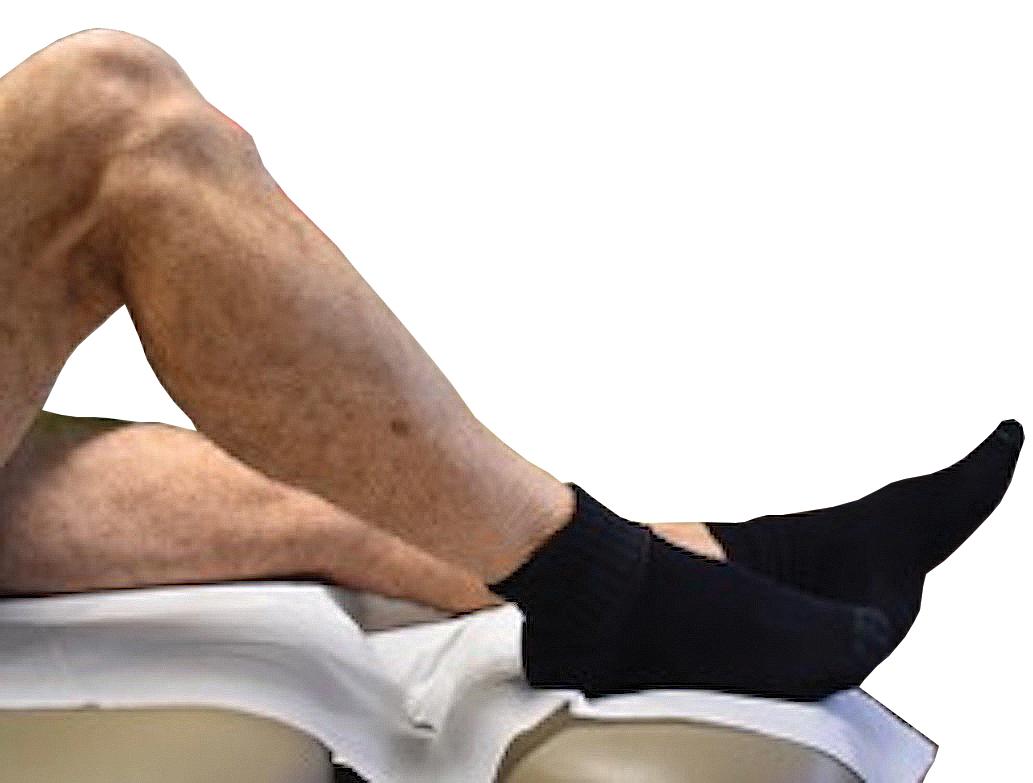
During excision of a lesion on the anterior lower extremity, we recommend that the patient be positioned with their knee bent and heel resting on the examination table. Ideally, the knee is flexed at approximately a 45° angle (Figure 3).3 In this position, excision of the lesion is facilitated by increased tension across the anterior lower extremity. During closure of these lesions, the patient should lie supine with the knee fully extended and the leg resting on the surgical bed or a pillow.
Posterior Lower Extremity
During excision of lesions on the posterior lower extremity, the patient should be positioned lying prone, with the knee fully extended, resting on the surgical bed or a pillow, which facilitates excision of the lesion by increasing tension across the site. During closure of these lesions, the patient should lie on the side contralateral to the surgical site, with the leg fully extended for support. The surgical leg should be flexed at the knee at approximately a 45° angle (Figure 4).
Practice Implications
Despite being an important step, patient positioning is an often-overlooked component of dermatologic surgery. Positioning becomes even more important in areas of high tension and repetitive motion, such as the upper back and lower extremities, where the risk of wound dehiscence and poor scar cosmesis is increased.1 Experienced dermatologic surgeons should utilize patient positioning, taking advantage of tension instead of working against it.
We have found that these 2 simple principles can aid in simplifying the excision and repair processes. Increasing tension across the surgical site during excision reduces the work required by the surgeon to reach the appropriate depth. Conversely, decreased tension across the surgical site decreases the work required for closure. These principles should be considered prior to the procedure; the patient should then be positioned in a way that maximizes tension across the surgical site during excision and minimizes tension across the surgical site during closure.
Incorporating these techniques, especially at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities, not only increases efficiency but may also reduce the risk for wound dehiscence once the patient returns home and maintains their normal level of physical activity.
- Rohrer TE, Cook JL, Kaufman AJ. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier; 2007.
- Kantor J. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. 2nd ed. McGraw-Hill Education; 2016.
- Kiwanuka E, Cruz AP. Multistep approach for improved aesthetic and functional outcomes for lower extremity wound closure after Mohs micrographic surgery. Dermatol Surg. 2017;43:704-707.
Practice Gap
Practical patient positioning is a commonly overlooked method of tension control during excision and repair that allows for easier closure.1 Although positioning is a basic step in dermatologic surgery, it often is difficult and awkward for both the patient and physician. Here, we describe basic principles in patient positioning that increase tension across the surgical site during excision and reduce tension during closure. By reducing the amount of work required for excision and closure, procedures are completed more quickly, which increases efficiency. These techniques should be considered during dermatologic surgery at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities.
Technique: Upper Back Procedures
When removing lesions on the upper back, lying completely prone is uncomfortable for the patient and leaves the shoulders hyperextended.2 Instead, position the patient with the arms extended anteriorly, hugging a pillow, while lying prone or on one side (Figure 1). In this position, excision of the lesion is facilitated by increased tension across the upper back. In addition, this position is notably more comfortable for the patient. During closure, the patient should lie on the side contralateral to the surgical site, with the elbow resting at the hip and the ipsilateral arm lying parallel to the torso (Figure 2).
Following procedures on the upper back and shoulders, we typically recommend that the patient wear an arm sling on the ipsilateral side for 1 week. Doing so reliably limits mobility postoperatively and does not require the patient to constantly monitor their movement.
Technique: Lower Extremity Procedures
Anterior Lower Extremity
During excision of a lesion on the anterior lower extremity, we recommend that the patient be positioned with their knee bent and heel resting on the examination table. Ideally, the knee is flexed at approximately a 45° angle (Figure 3).3 In this position, excision of the lesion is facilitated by increased tension across the anterior lower extremity. During closure of these lesions, the patient should lie supine with the knee fully extended and the leg resting on the surgical bed or a pillow.
Posterior Lower Extremity
During excision of lesions on the posterior lower extremity, the patient should be positioned lying prone, with the knee fully extended, resting on the surgical bed or a pillow, which facilitates excision of the lesion by increasing tension across the site. During closure of these lesions, the patient should lie on the side contralateral to the surgical site, with the leg fully extended for support. The surgical leg should be flexed at the knee at approximately a 45° angle (Figure 4).
Practice Implications
Despite being an important step, patient positioning is an often-overlooked component of dermatologic surgery. Positioning becomes even more important in areas of high tension and repetitive motion, such as the upper back and lower extremities, where the risk of wound dehiscence and poor scar cosmesis is increased.1 Experienced dermatologic surgeons should utilize patient positioning, taking advantage of tension instead of working against it.
We have found that these 2 simple principles can aid in simplifying the excision and repair processes. Increasing tension across the surgical site during excision reduces the work required by the surgeon to reach the appropriate depth. Conversely, decreased tension across the surgical site decreases the work required for closure. These principles should be considered prior to the procedure; the patient should then be positioned in a way that maximizes tension across the surgical site during excision and minimizes tension across the surgical site during closure.
Incorporating these techniques, especially at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities, not only increases efficiency but may also reduce the risk for wound dehiscence once the patient returns home and maintains their normal level of physical activity.
Practice Gap
Practical patient positioning is a commonly overlooked method of tension control during excision and repair that allows for easier closure.1 Although positioning is a basic step in dermatologic surgery, it often is difficult and awkward for both the patient and physician. Here, we describe basic principles in patient positioning that increase tension across the surgical site during excision and reduce tension during closure. By reducing the amount of work required for excision and closure, procedures are completed more quickly, which increases efficiency. These techniques should be considered during dermatologic surgery at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities.
Technique: Upper Back Procedures
When removing lesions on the upper back, lying completely prone is uncomfortable for the patient and leaves the shoulders hyperextended.2 Instead, position the patient with the arms extended anteriorly, hugging a pillow, while lying prone or on one side (Figure 1). In this position, excision of the lesion is facilitated by increased tension across the upper back. In addition, this position is notably more comfortable for the patient. During closure, the patient should lie on the side contralateral to the surgical site, with the elbow resting at the hip and the ipsilateral arm lying parallel to the torso (Figure 2).
Following procedures on the upper back and shoulders, we typically recommend that the patient wear an arm sling on the ipsilateral side for 1 week. Doing so reliably limits mobility postoperatively and does not require the patient to constantly monitor their movement.
Technique: Lower Extremity Procedures
Anterior Lower Extremity
During excision of a lesion on the anterior lower extremity, we recommend that the patient be positioned with their knee bent and heel resting on the examination table. Ideally, the knee is flexed at approximately a 45° angle (Figure 3).3 In this position, excision of the lesion is facilitated by increased tension across the anterior lower extremity. During closure of these lesions, the patient should lie supine with the knee fully extended and the leg resting on the surgical bed or a pillow.
Posterior Lower Extremity
During excision of lesions on the posterior lower extremity, the patient should be positioned lying prone, with the knee fully extended, resting on the surgical bed or a pillow, which facilitates excision of the lesion by increasing tension across the site. During closure of these lesions, the patient should lie on the side contralateral to the surgical site, with the leg fully extended for support. The surgical leg should be flexed at the knee at approximately a 45° angle (Figure 4).
Practice Implications
Despite being an important step, patient positioning is an often-overlooked component of dermatologic surgery. Positioning becomes even more important in areas of high tension and repetitive motion, such as the upper back and lower extremities, where the risk of wound dehiscence and poor scar cosmesis is increased.1 Experienced dermatologic surgeons should utilize patient positioning, taking advantage of tension instead of working against it.
We have found that these 2 simple principles can aid in simplifying the excision and repair processes. Increasing tension across the surgical site during excision reduces the work required by the surgeon to reach the appropriate depth. Conversely, decreased tension across the surgical site decreases the work required for closure. These principles should be considered prior to the procedure; the patient should then be positioned in a way that maximizes tension across the surgical site during excision and minimizes tension across the surgical site during closure.
Incorporating these techniques, especially at sites that are subject to both high tension and repetitive motion, such as the upper back and lower extremities, not only increases efficiency but may also reduce the risk for wound dehiscence once the patient returns home and maintains their normal level of physical activity.
- Rohrer TE, Cook JL, Kaufman AJ. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier; 2007.
- Kantor J. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. 2nd ed. McGraw-Hill Education; 2016.
- Kiwanuka E, Cruz AP. Multistep approach for improved aesthetic and functional outcomes for lower extremity wound closure after Mohs micrographic surgery. Dermatol Surg. 2017;43:704-707.
- Rohrer TE, Cook JL, Kaufman AJ. Flaps and Grafts in Dermatologic Surgery. 2nd ed. Elsevier; 2007.
- Kantor J. Atlas of Suturing Techniques: Approaches to Surgical Wound, Laceration, and Cosmetic Repair. 2nd ed. McGraw-Hill Education; 2016.
- Kiwanuka E, Cruz AP. Multistep approach for improved aesthetic and functional outcomes for lower extremity wound closure after Mohs micrographic surgery. Dermatol Surg. 2017;43:704-707.
Ulcerative Sarcoidosis: A Prototypical Presentation and Review
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
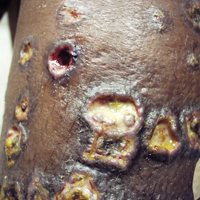
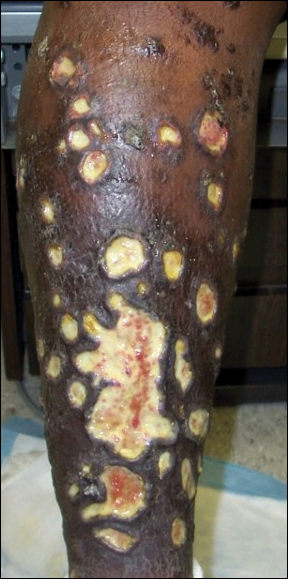
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).
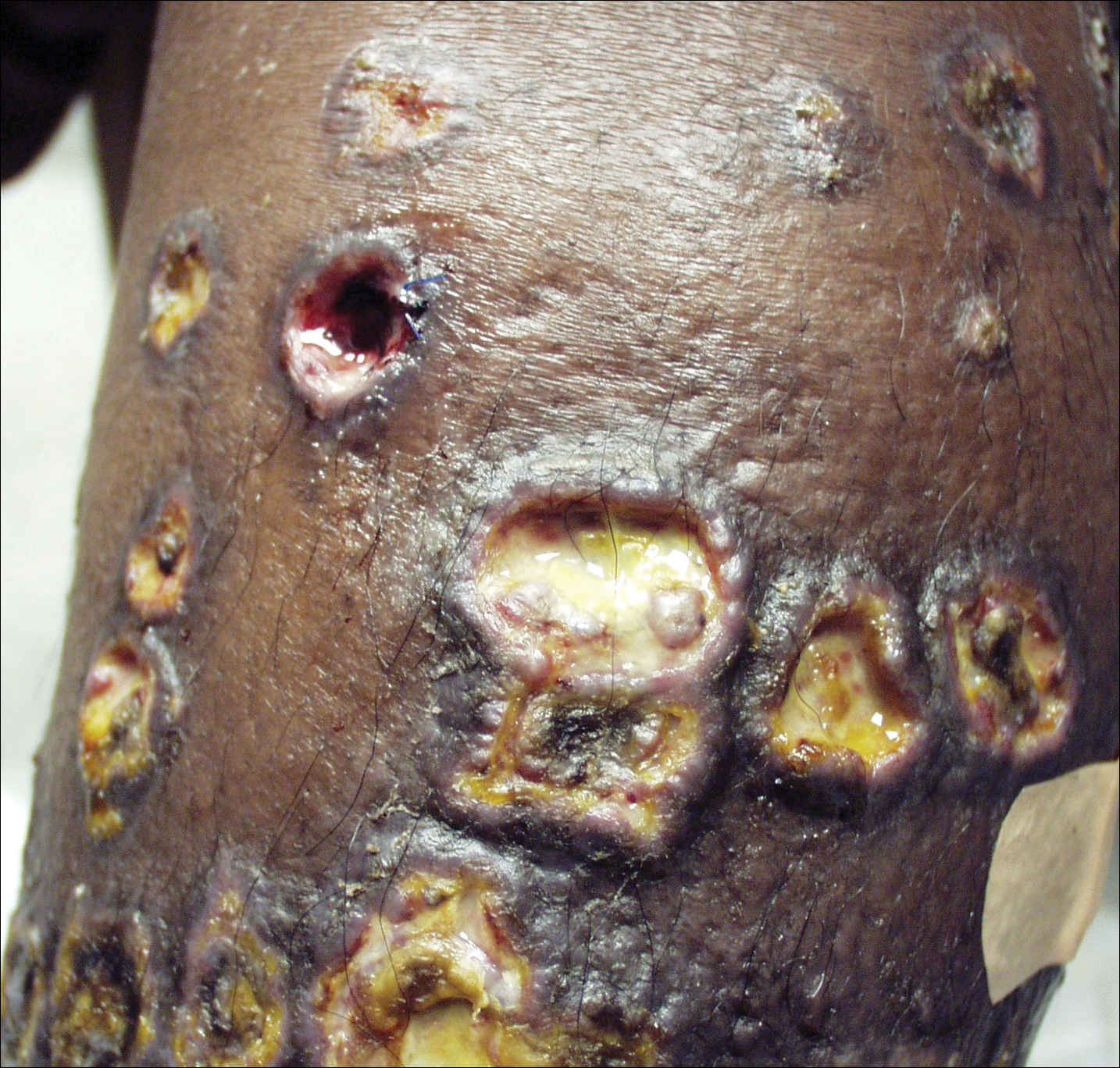
Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.
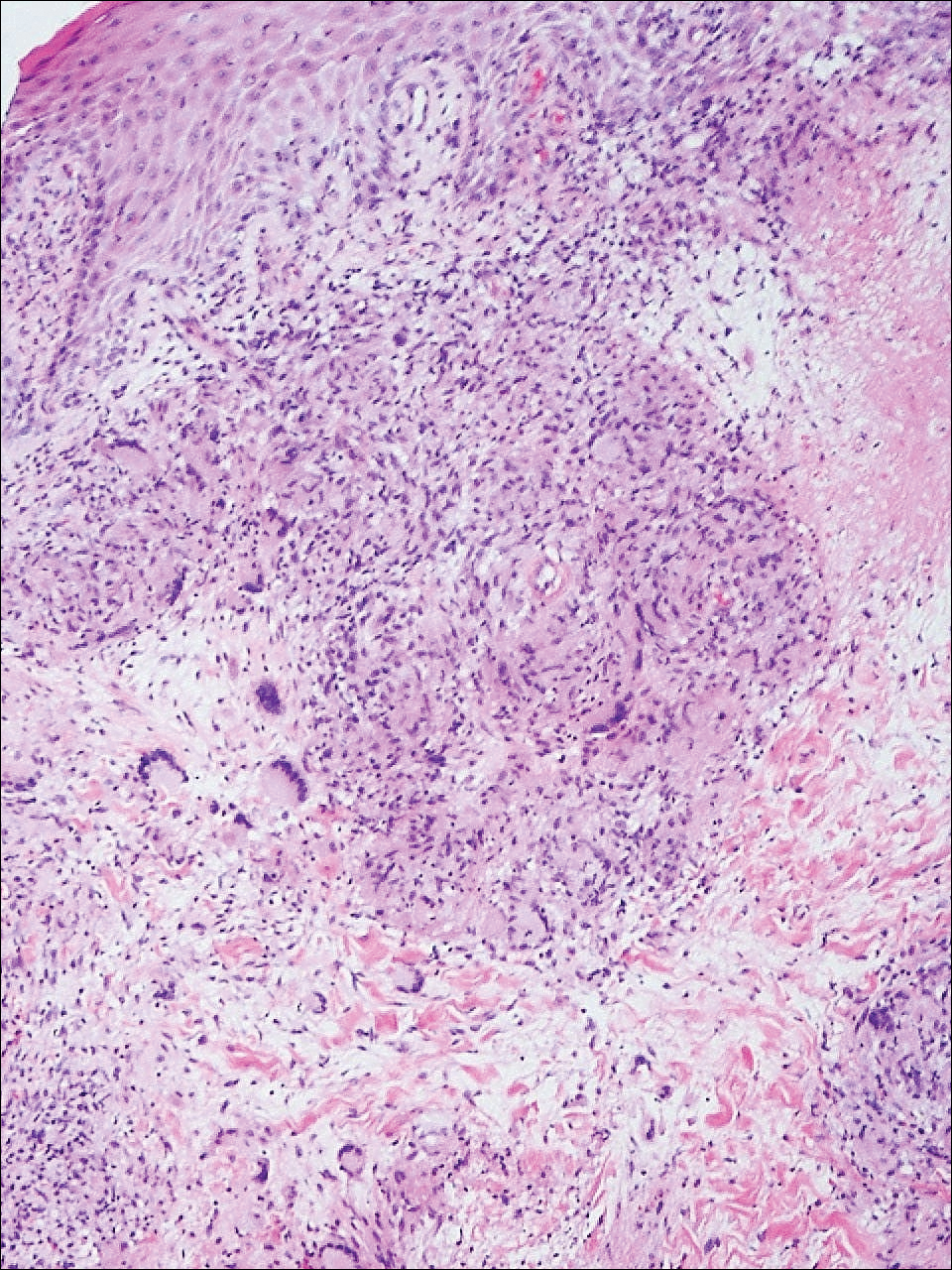
Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.
Comment
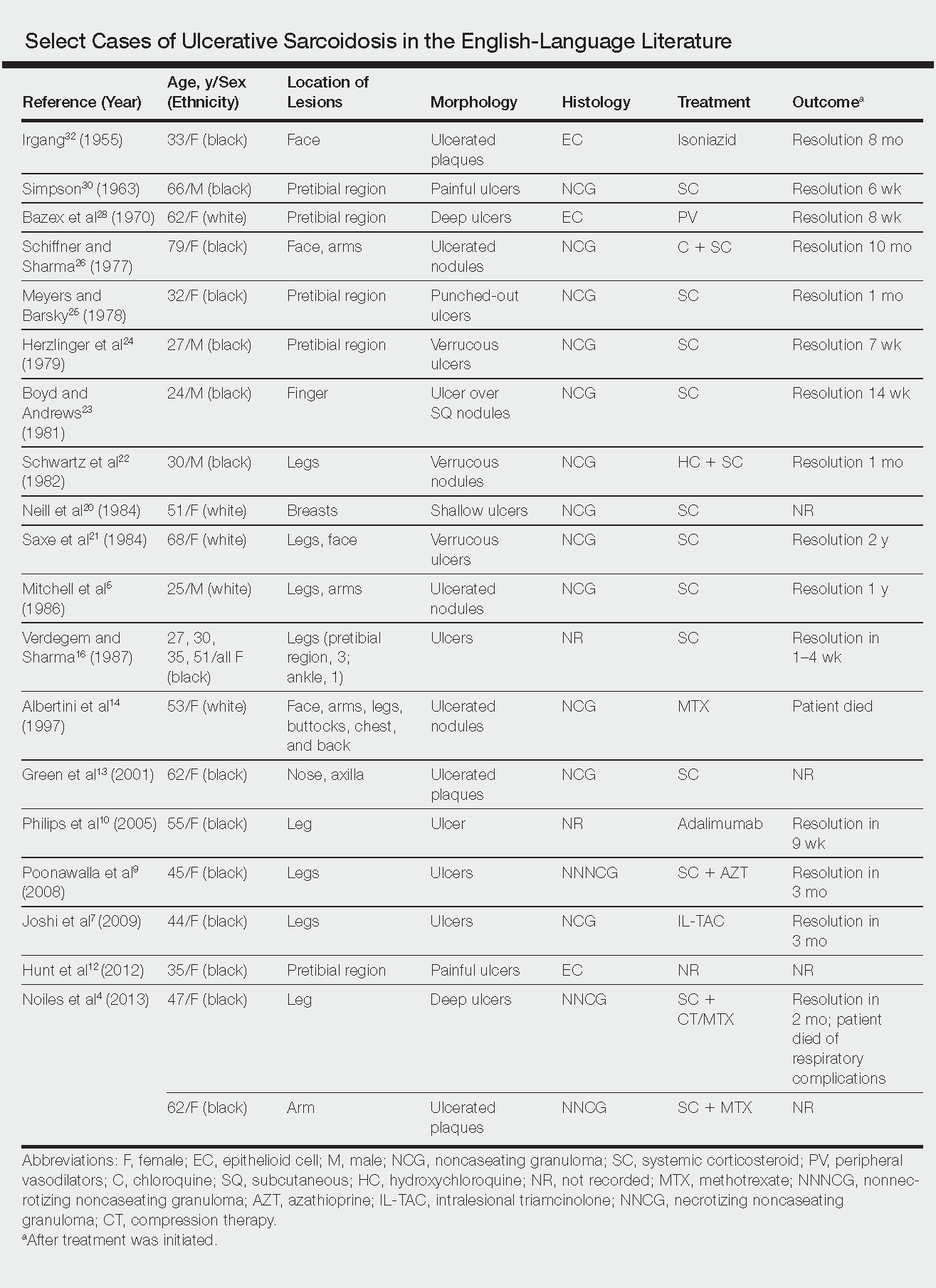
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.
The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).

Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.

Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.

Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.

The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).

Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.

Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.

Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.

The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
Practice Points
- Sarcoidosis can present as a primary ulcerative disease.
- Suspect ulcerative sarcoidosis when ulcerations are seen on the leg.
- Systemic corticosteroids may be the most effective treatment of ulcerative sarcoidosis.