User login
Peristomal Pyoderma Gangrenosum at an Ileostomy Site
To the Editor:
Peristomal pyoderma gangrenosum (PPG) is a rare entity first described in 1984.1 Lesions usually begin as pustules that coalesce into an erythematous skin ulceration that contains purulent material. The lesion appears on the skin that surrounds an abdominal stoma. Peristomal pyoderma gangrenosum typically is associated with Crohn disease and ulcerative colitis, cancer, blood dyscrasia, diabetes mellitus, and hepatitis.2 We describe a case of PPG following an ileostomy in a patient with colon cancer and a related history of Crohn disease.
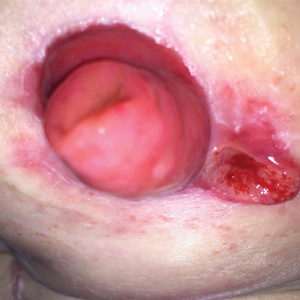
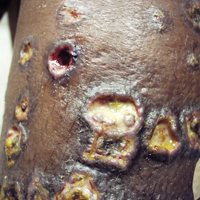
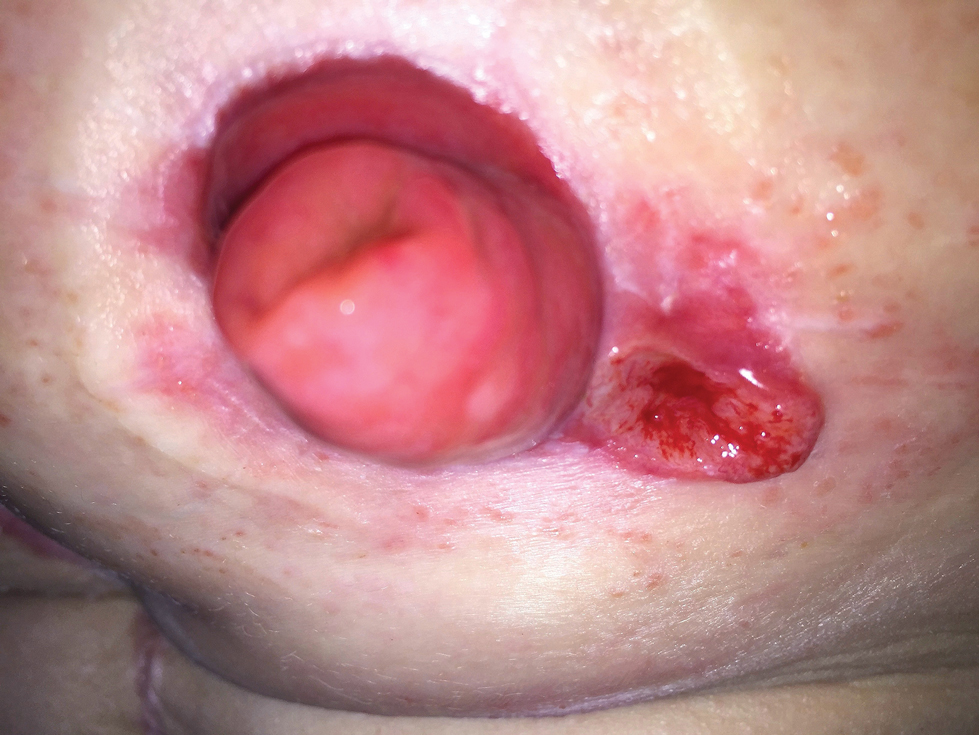
A 32-year-old woman presented to a dermatology office with a spontaneously painful, 3.2-cm ulceration that was extremely tender to palpation, located immediately adjacent to the site of an ileostomy (Figure). The patient had a history of refractory constipation that failed to respond to standard conservative measures 4 years prior. She underwent a colonoscopy, which revealed a 6.5-cm, irregularly shaped, exophytic mass in the rectosigmoid portion of the colon. Histopathologic examination of several biopsies confirmed the diagnosis of moderately well-differentiated adenocarcinoma, and additional evaluation determined the cancer to be stage IIB. She had a medical history of pancolonic Crohn disease since high school that was treated with periodic infusions of infliximab at the standard dose of 5 mg/kg. Colon cancer treatment consisted of preoperative radiotherapy, complete colectomy with ileoanal anastomosis, and creation of a J-pouch and formation of a temporary ileostomy, along with postoperative capecitabine chemotherapy.
The ileostomy eventually was reversed, and the patient did well for 3 years. When the patient developed severe abdominal pain, the J-pouch was examined and found to be remarkably involved with Crohn disease. However, during the colonoscopy, the J-pouch was inadvertently punctured, leading to the formation of a large pelvic abscess. The latter necessitated diversion of stool, and the patient had the original ileostomy recreated.
Prior to presentation to dermatology, various consultants suspected the ulceration was possibly a deep fungal infection, cutaneous Crohn disease, a factitious ulceration, or acute allergic contact dermatitis related to some element of ostomy care. However, dermatologic consultation suggested that the troublesome lesion was classic PPG and recommended administration of a tumor necrosis factor (TNF) α–blocking agent and concomitant intralesional injections of dilute triamcinolone acetonide.
The patient was treated with subcutaneous adalimumab 40 mg once weekly, and received near weekly subcutaneous injections of triamcinolone acetonide 10 mg/mL. After 2 months, the discomfort subsided, and the ulceration gradually resolved into a depressed scar. Eighteen months later, the scar was barely perceptible as a minimally erythematous depression. Adalimumab ultimately was discontinued, as the residual J-pouch was removed, and the biologic drug was associated with extensive alopecia areata–like hair loss. There has been no recurrence of PPG in the 40 months since clinical resolution.
Peristomal pyoderma gangrenosum is an uncommon subtype of pyoderma gangrenosum, which is characterized by chronic, persistent, or recurrent painful ulceration(s) close to an abdominal stoma. In total, fewer than 100 cases of PPG have been reported thus far in the readily available medical literature.3 Inflammatory bowel disease (IBD) is the most frequently diagnosed systemic condition associated with PPG, though other associated conditions include diverticular disease, abdominal malignancy, and neurologic dysfunction. Approximately 2% to 4.3% of all patients who have stoma creation surgery related to underlying IBD develop PPG. It is estimated that the yearly incidence rate of PPG in all abdominal stomas is quite low (approximately 0.6%).4
Peristomal pyoderma gangrenosum can occur at any age, but it tends to predominate in young to middle-aged adults, with a slight female predilection. The etiology and pathogenesis of PPG are largely unknown, though studies have shown that an abnormal immune response may be critical to its development. Risk factors for PPG are not well defined but potentially include autoimmune disorders, a high body mass index, and females or African Americans with IBD.4 Because PPG does not have characteristic histopathologic features, it is a diagnosis of exclusion that is based on the clinical examination and histologic findings that rule out other potential disorders.
There are 4 types of PPG based on the clinical and histopathologic characteristics: ulcerative, pustular, bullous, and vegetative. Peristomal pyoderma gangrenosum tends to be either ulcerative or vegetative, with ulcerative being by far the predominant type. The onset of PPG is quite variable, occurring a few weeks to several years after stoma formation.5 Ulcer size can range from less than 3 cm to 30 cm.4 Lesions begin as deep painful nodules or as superficial hemorrhagic pustules, either idiopathic or following ostensibly minimal trauma. Subsequently, they become necrotic and form an ulceration. The ulcers can be single or multiple lesions, typically with erythematous raised borders and purulent discharge. The ulcers are extremely painful and rapidly progressive. After the ulcers heal, they often leave a characteristic weblike atrophic scar that can break down further following any form of irritation or trauma.5
A prompt diagnosis of PPG is important. A diagnosis of PPG should be considered when dealing with a noninfectious ulcer surrounding a stoma in patients with IBD or other autoimmune conditions.6 Because PPG is a rare skin disorder, it is likely to be missed and lead to unnecessary diagnostic workup and a delay in proper therapy. In our patient, a diagnosis of PPG was overlooked for other infectious and autoimmune causes. The diagnostic evaluation of a patient with PPG is based on 3 principles: (1) ruling out other causes of a peristomal ulcer, such as an abscess, contact dermatitis, or wound infection; (2) determining whether there is an underlying intestinal bowel disease in the stoma; and (3) identifying associated systemic disorders such as vasculitis, erythema nodosum, or similar processes.4 The differential diagnosis depends on the type and stage of PPG and can include malignancy, vasculitis, extraintestinal IBD, infectious disease, and insect bites. A review of the history of the ulcer is helpful in ruling out other diseases, and a colonoscopy or ileoscopy can identify if patients have an underlying active IBD. Swabs for smear and both bacterial and fungal cultures should be taken from the exudate and directly from the ulcer base. Biopsy of the ulcer also helps to exclude alternative diagnoses.6
The primary goals of treating PPG include to reduce pain and the risk for secondary infection, increase pouch adherence, and decrease purulent exudate.7 Although there is not one well-defined optimal therapeutic intervention, there are a variety of effective approaches that may be considered and used. In mild cases, management methods such as dressings, topical agents, or intralesional steroids may be capable of controlling the disease. Daily wound care is important. Moisture-retentive dressings can control pain, induce collagen formation, promote angiogenesis, and prevent contamination. Cleaning the wound with sterile saline and applying an anti-infective agent also may be effective. Application of ultrapotent topical steroids and tacrolimus ointment 0.3% can be used in patients without concomitant secondary infection. In patients who are in remission, human platelet-derived growth factor may be used. Intralesional injections of dilute triamcinolone acetonide or cyclosporine solution also can be helpful. Cyclosporin A was used as a systemic monotherapy to treat a 48-year-old man and 50-year-old woman with the idiopathic form of PPG. After 3 months of treatment, PPG had completely resolved and there were no major side effects.8 Other potential topical therapies that control inflammation and promote wound healing include benzoyl peroxide, chlormethine (topical alkylating agent and nitrogen mustard that has anti-inflammatory properties), nicotine, and 5-aminosalicylic acid. If an ulcer becomes infected, empiric antibiotic therapy should be given immediately and adjusted based on culture and sensitivity results.4
Systemic therapy should be considered in patients who do not respond to topical or local interventions, have a rapid and severe course, or have an active underlying bowel disease. Oral prednisone (1 mg/kg/d) has proved to be one of the most successful drugs used to treat PPG. Treatment should be continued until complete lesion healing, and low-dose maintenance therapy should be administered in recurrent cases. Intravenous corticosteroid therapy—hydrocortisone 100 mg 4 times daily or pulse therapy with intravenous methylprednisolone 1 g/d)—can be used for up to 5 days and may be effective. Oral minocycline 100 mg twice daily may be helpful as an adjunctive therapy to corticosteroids. When corticosteroids fail, oral cyclosporine 3 to 5 mg/kg/d often is prescribed. Studies have shown that patients demonstrate clinical improvement within 3 weeks of cyclosporine initiation, and it has been shown further to be more effective than either azathioprine or methotrexate.4,8
Infliximab, a chimeric antibody that binds both circulating and tissue-bound TNF-α, has been shown to effectively treat PPG. A clinical trial conducted by Brooklyn et al9 found that 46% of patients (6/13) treated with infliximab responded compared with only 6% in a placebo control group (1/17). Although infliximab may result in sepsis, the benefits far outweigh the risks, especially for patients with steroid-refractory PPG.4 Adalimumab is a human monoclonal IgG1 antibody to TNF-α that neutralizes its function by blocking the interaction between the molecule and its receptor. Many clinical studies have shown that adalimumab induces and maintains a clinical response in patients with active Crohn disease. The biologic proved to be effective in our patient, but it is associated with potential side effects that should be monitored including injection-site reactions, pruritus, leukopenia, urticaria, and rare instances of alopecia.10 Etanercept is another potentially effective biologic agent.7 Plasma exchange, immunoglobulin infusion, and interferon-alfa therapy also can be used in refractory PPG cases, though data on these treatments are very limited.4
Unlike routine pyoderma gangrenosum—for which surgical intervention is contraindicated—surgical intervention may be appropriate for the peristomal variant. Surgical treatment options include stoma revision and/or relocation; however, both of these procedures are accompanied by failure rates ranging from 40% to 100%.5 Removal of a diseased intestinal segment, especially one with active IBD, may result in healing of the skin lesion. In our patient, removal of the residual and diseased J-pouch was part of the management plan. However,it generally is recommended that any surgical intervention be accompanied by medical therapy including oral metronidazole 500 mg/d and concomitant administration of an immunosuppressant.1,3
Because PPG tends to recur, long-term maintenance therapy should always be considered. Pain reduction, anemia correction, proper nutrition, and management of associated and underlying diseases should be performed. Meticulous care of the stoma and prevention of leaks also should be emphasized. Overall, if PPG is detected and diagnosed early as well as treated appropriately and aggressively, the patient likely will have a good prognosis.4
- Sheldon DG, Sawchuk LL, Kozarek RA, et al. Twenty cases of peristomal pyoderma gangrenosum: diagnostic implications and management. Arch Surg. 2000;135:564-569.
- Hughes AP, Jackson JM, Callen JP. Clinical features and treatment of peristomal pyoderma gangrenosum. JAMA. 2000;284:1546-1548.
- Afifi L, Sanchez IM, Wallace MM, et al. Diagnosis and management of peristomal pyoderma gangrenosum: a systematic review. J Am Acad Dermatol. 2018;78:1195-1204.
- Wu XR, Shen B. Diagnosis and management of parastomal pyoderma gangrenosum. Gastroenterol Rep (Oxf). 2013;1:1-8.
- Javed A, Pal S, Ahuja V, et al. Management of peristomal pyoderma gangrenosum: two different approaches for the same clinical problem. Trop Gastroenterol. 2011;32:153-156.
- Toh JW, Whiteley I. Devastating peristomal pyoderma gangrenosum: challenges in diagnosis and management. Clin Gastroenterol Hepatol. 2017;15:A19-A20.
- DeMartyn LE, Faller NA, Miller L. Treating peristomal pyoderma gangrenosum with topical crushed prednisone: a report of three cases. Ostomy Wound Manage. 2014;60:50-54.
- V’lckova-Laskoska MT, Laskoski DS, Caca-Biljanovska NG, et al. Pyoderma gangrenosum successfully treated with cyclosporin A.Adv Exp Med Biol. 1999;455:541-555.
- Brooklyn TN, Dunnill MGS, Shetty A, at al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Alkhouri N, Hupertz V, Mahajan L. Adalimumab treatment for peristomal pyoderma gangrenosum associated with Crohn’s disease. Inflamm Bowel Dis. 2009;15:803-806.
To the Editor:
Peristomal pyoderma gangrenosum (PPG) is a rare entity first described in 1984.1 Lesions usually begin as pustules that coalesce into an erythematous skin ulceration that contains purulent material. The lesion appears on the skin that surrounds an abdominal stoma. Peristomal pyoderma gangrenosum typically is associated with Crohn disease and ulcerative colitis, cancer, blood dyscrasia, diabetes mellitus, and hepatitis.2 We describe a case of PPG following an ileostomy in a patient with colon cancer and a related history of Crohn disease.
A 32-year-old woman presented to a dermatology office with a spontaneously painful, 3.2-cm ulceration that was extremely tender to palpation, located immediately adjacent to the site of an ileostomy (Figure). The patient had a history of refractory constipation that failed to respond to standard conservative measures 4 years prior. She underwent a colonoscopy, which revealed a 6.5-cm, irregularly shaped, exophytic mass in the rectosigmoid portion of the colon. Histopathologic examination of several biopsies confirmed the diagnosis of moderately well-differentiated adenocarcinoma, and additional evaluation determined the cancer to be stage IIB. She had a medical history of pancolonic Crohn disease since high school that was treated with periodic infusions of infliximab at the standard dose of 5 mg/kg. Colon cancer treatment consisted of preoperative radiotherapy, complete colectomy with ileoanal anastomosis, and creation of a J-pouch and formation of a temporary ileostomy, along with postoperative capecitabine chemotherapy.

The ileostomy eventually was reversed, and the patient did well for 3 years. When the patient developed severe abdominal pain, the J-pouch was examined and found to be remarkably involved with Crohn disease. However, during the colonoscopy, the J-pouch was inadvertently punctured, leading to the formation of a large pelvic abscess. The latter necessitated diversion of stool, and the patient had the original ileostomy recreated.
Prior to presentation to dermatology, various consultants suspected the ulceration was possibly a deep fungal infection, cutaneous Crohn disease, a factitious ulceration, or acute allergic contact dermatitis related to some element of ostomy care. However, dermatologic consultation suggested that the troublesome lesion was classic PPG and recommended administration of a tumor necrosis factor (TNF) α–blocking agent and concomitant intralesional injections of dilute triamcinolone acetonide.
The patient was treated with subcutaneous adalimumab 40 mg once weekly, and received near weekly subcutaneous injections of triamcinolone acetonide 10 mg/mL. After 2 months, the discomfort subsided, and the ulceration gradually resolved into a depressed scar. Eighteen months later, the scar was barely perceptible as a minimally erythematous depression. Adalimumab ultimately was discontinued, as the residual J-pouch was removed, and the biologic drug was associated with extensive alopecia areata–like hair loss. There has been no recurrence of PPG in the 40 months since clinical resolution.
Peristomal pyoderma gangrenosum is an uncommon subtype of pyoderma gangrenosum, which is characterized by chronic, persistent, or recurrent painful ulceration(s) close to an abdominal stoma. In total, fewer than 100 cases of PPG have been reported thus far in the readily available medical literature.3 Inflammatory bowel disease (IBD) is the most frequently diagnosed systemic condition associated with PPG, though other associated conditions include diverticular disease, abdominal malignancy, and neurologic dysfunction. Approximately 2% to 4.3% of all patients who have stoma creation surgery related to underlying IBD develop PPG. It is estimated that the yearly incidence rate of PPG in all abdominal stomas is quite low (approximately 0.6%).4
Peristomal pyoderma gangrenosum can occur at any age, but it tends to predominate in young to middle-aged adults, with a slight female predilection. The etiology and pathogenesis of PPG are largely unknown, though studies have shown that an abnormal immune response may be critical to its development. Risk factors for PPG are not well defined but potentially include autoimmune disorders, a high body mass index, and females or African Americans with IBD.4 Because PPG does not have characteristic histopathologic features, it is a diagnosis of exclusion that is based on the clinical examination and histologic findings that rule out other potential disorders.
There are 4 types of PPG based on the clinical and histopathologic characteristics: ulcerative, pustular, bullous, and vegetative. Peristomal pyoderma gangrenosum tends to be either ulcerative or vegetative, with ulcerative being by far the predominant type. The onset of PPG is quite variable, occurring a few weeks to several years after stoma formation.5 Ulcer size can range from less than 3 cm to 30 cm.4 Lesions begin as deep painful nodules or as superficial hemorrhagic pustules, either idiopathic or following ostensibly minimal trauma. Subsequently, they become necrotic and form an ulceration. The ulcers can be single or multiple lesions, typically with erythematous raised borders and purulent discharge. The ulcers are extremely painful and rapidly progressive. After the ulcers heal, they often leave a characteristic weblike atrophic scar that can break down further following any form of irritation or trauma.5
A prompt diagnosis of PPG is important. A diagnosis of PPG should be considered when dealing with a noninfectious ulcer surrounding a stoma in patients with IBD or other autoimmune conditions.6 Because PPG is a rare skin disorder, it is likely to be missed and lead to unnecessary diagnostic workup and a delay in proper therapy. In our patient, a diagnosis of PPG was overlooked for other infectious and autoimmune causes. The diagnostic evaluation of a patient with PPG is based on 3 principles: (1) ruling out other causes of a peristomal ulcer, such as an abscess, contact dermatitis, or wound infection; (2) determining whether there is an underlying intestinal bowel disease in the stoma; and (3) identifying associated systemic disorders such as vasculitis, erythema nodosum, or similar processes.4 The differential diagnosis depends on the type and stage of PPG and can include malignancy, vasculitis, extraintestinal IBD, infectious disease, and insect bites. A review of the history of the ulcer is helpful in ruling out other diseases, and a colonoscopy or ileoscopy can identify if patients have an underlying active IBD. Swabs for smear and both bacterial and fungal cultures should be taken from the exudate and directly from the ulcer base. Biopsy of the ulcer also helps to exclude alternative diagnoses.6
The primary goals of treating PPG include to reduce pain and the risk for secondary infection, increase pouch adherence, and decrease purulent exudate.7 Although there is not one well-defined optimal therapeutic intervention, there are a variety of effective approaches that may be considered and used. In mild cases, management methods such as dressings, topical agents, or intralesional steroids may be capable of controlling the disease. Daily wound care is important. Moisture-retentive dressings can control pain, induce collagen formation, promote angiogenesis, and prevent contamination. Cleaning the wound with sterile saline and applying an anti-infective agent also may be effective. Application of ultrapotent topical steroids and tacrolimus ointment 0.3% can be used in patients without concomitant secondary infection. In patients who are in remission, human platelet-derived growth factor may be used. Intralesional injections of dilute triamcinolone acetonide or cyclosporine solution also can be helpful. Cyclosporin A was used as a systemic monotherapy to treat a 48-year-old man and 50-year-old woman with the idiopathic form of PPG. After 3 months of treatment, PPG had completely resolved and there were no major side effects.8 Other potential topical therapies that control inflammation and promote wound healing include benzoyl peroxide, chlormethine (topical alkylating agent and nitrogen mustard that has anti-inflammatory properties), nicotine, and 5-aminosalicylic acid. If an ulcer becomes infected, empiric antibiotic therapy should be given immediately and adjusted based on culture and sensitivity results.4
Systemic therapy should be considered in patients who do not respond to topical or local interventions, have a rapid and severe course, or have an active underlying bowel disease. Oral prednisone (1 mg/kg/d) has proved to be one of the most successful drugs used to treat PPG. Treatment should be continued until complete lesion healing, and low-dose maintenance therapy should be administered in recurrent cases. Intravenous corticosteroid therapy—hydrocortisone 100 mg 4 times daily or pulse therapy with intravenous methylprednisolone 1 g/d)—can be used for up to 5 days and may be effective. Oral minocycline 100 mg twice daily may be helpful as an adjunctive therapy to corticosteroids. When corticosteroids fail, oral cyclosporine 3 to 5 mg/kg/d often is prescribed. Studies have shown that patients demonstrate clinical improvement within 3 weeks of cyclosporine initiation, and it has been shown further to be more effective than either azathioprine or methotrexate.4,8
Infliximab, a chimeric antibody that binds both circulating and tissue-bound TNF-α, has been shown to effectively treat PPG. A clinical trial conducted by Brooklyn et al9 found that 46% of patients (6/13) treated with infliximab responded compared with only 6% in a placebo control group (1/17). Although infliximab may result in sepsis, the benefits far outweigh the risks, especially for patients with steroid-refractory PPG.4 Adalimumab is a human monoclonal IgG1 antibody to TNF-α that neutralizes its function by blocking the interaction between the molecule and its receptor. Many clinical studies have shown that adalimumab induces and maintains a clinical response in patients with active Crohn disease. The biologic proved to be effective in our patient, but it is associated with potential side effects that should be monitored including injection-site reactions, pruritus, leukopenia, urticaria, and rare instances of alopecia.10 Etanercept is another potentially effective biologic agent.7 Plasma exchange, immunoglobulin infusion, and interferon-alfa therapy also can be used in refractory PPG cases, though data on these treatments are very limited.4
Unlike routine pyoderma gangrenosum—for which surgical intervention is contraindicated—surgical intervention may be appropriate for the peristomal variant. Surgical treatment options include stoma revision and/or relocation; however, both of these procedures are accompanied by failure rates ranging from 40% to 100%.5 Removal of a diseased intestinal segment, especially one with active IBD, may result in healing of the skin lesion. In our patient, removal of the residual and diseased J-pouch was part of the management plan. However,it generally is recommended that any surgical intervention be accompanied by medical therapy including oral metronidazole 500 mg/d and concomitant administration of an immunosuppressant.1,3
Because PPG tends to recur, long-term maintenance therapy should always be considered. Pain reduction, anemia correction, proper nutrition, and management of associated and underlying diseases should be performed. Meticulous care of the stoma and prevention of leaks also should be emphasized. Overall, if PPG is detected and diagnosed early as well as treated appropriately and aggressively, the patient likely will have a good prognosis.4
To the Editor:
Peristomal pyoderma gangrenosum (PPG) is a rare entity first described in 1984.1 Lesions usually begin as pustules that coalesce into an erythematous skin ulceration that contains purulent material. The lesion appears on the skin that surrounds an abdominal stoma. Peristomal pyoderma gangrenosum typically is associated with Crohn disease and ulcerative colitis, cancer, blood dyscrasia, diabetes mellitus, and hepatitis.2 We describe a case of PPG following an ileostomy in a patient with colon cancer and a related history of Crohn disease.
A 32-year-old woman presented to a dermatology office with a spontaneously painful, 3.2-cm ulceration that was extremely tender to palpation, located immediately adjacent to the site of an ileostomy (Figure). The patient had a history of refractory constipation that failed to respond to standard conservative measures 4 years prior. She underwent a colonoscopy, which revealed a 6.5-cm, irregularly shaped, exophytic mass in the rectosigmoid portion of the colon. Histopathologic examination of several biopsies confirmed the diagnosis of moderately well-differentiated adenocarcinoma, and additional evaluation determined the cancer to be stage IIB. She had a medical history of pancolonic Crohn disease since high school that was treated with periodic infusions of infliximab at the standard dose of 5 mg/kg. Colon cancer treatment consisted of preoperative radiotherapy, complete colectomy with ileoanal anastomosis, and creation of a J-pouch and formation of a temporary ileostomy, along with postoperative capecitabine chemotherapy.

The ileostomy eventually was reversed, and the patient did well for 3 years. When the patient developed severe abdominal pain, the J-pouch was examined and found to be remarkably involved with Crohn disease. However, during the colonoscopy, the J-pouch was inadvertently punctured, leading to the formation of a large pelvic abscess. The latter necessitated diversion of stool, and the patient had the original ileostomy recreated.
Prior to presentation to dermatology, various consultants suspected the ulceration was possibly a deep fungal infection, cutaneous Crohn disease, a factitious ulceration, or acute allergic contact dermatitis related to some element of ostomy care. However, dermatologic consultation suggested that the troublesome lesion was classic PPG and recommended administration of a tumor necrosis factor (TNF) α–blocking agent and concomitant intralesional injections of dilute triamcinolone acetonide.
The patient was treated with subcutaneous adalimumab 40 mg once weekly, and received near weekly subcutaneous injections of triamcinolone acetonide 10 mg/mL. After 2 months, the discomfort subsided, and the ulceration gradually resolved into a depressed scar. Eighteen months later, the scar was barely perceptible as a minimally erythematous depression. Adalimumab ultimately was discontinued, as the residual J-pouch was removed, and the biologic drug was associated with extensive alopecia areata–like hair loss. There has been no recurrence of PPG in the 40 months since clinical resolution.
Peristomal pyoderma gangrenosum is an uncommon subtype of pyoderma gangrenosum, which is characterized by chronic, persistent, or recurrent painful ulceration(s) close to an abdominal stoma. In total, fewer than 100 cases of PPG have been reported thus far in the readily available medical literature.3 Inflammatory bowel disease (IBD) is the most frequently diagnosed systemic condition associated with PPG, though other associated conditions include diverticular disease, abdominal malignancy, and neurologic dysfunction. Approximately 2% to 4.3% of all patients who have stoma creation surgery related to underlying IBD develop PPG. It is estimated that the yearly incidence rate of PPG in all abdominal stomas is quite low (approximately 0.6%).4
Peristomal pyoderma gangrenosum can occur at any age, but it tends to predominate in young to middle-aged adults, with a slight female predilection. The etiology and pathogenesis of PPG are largely unknown, though studies have shown that an abnormal immune response may be critical to its development. Risk factors for PPG are not well defined but potentially include autoimmune disorders, a high body mass index, and females or African Americans with IBD.4 Because PPG does not have characteristic histopathologic features, it is a diagnosis of exclusion that is based on the clinical examination and histologic findings that rule out other potential disorders.
There are 4 types of PPG based on the clinical and histopathologic characteristics: ulcerative, pustular, bullous, and vegetative. Peristomal pyoderma gangrenosum tends to be either ulcerative or vegetative, with ulcerative being by far the predominant type. The onset of PPG is quite variable, occurring a few weeks to several years after stoma formation.5 Ulcer size can range from less than 3 cm to 30 cm.4 Lesions begin as deep painful nodules or as superficial hemorrhagic pustules, either idiopathic or following ostensibly minimal trauma. Subsequently, they become necrotic and form an ulceration. The ulcers can be single or multiple lesions, typically with erythematous raised borders and purulent discharge. The ulcers are extremely painful and rapidly progressive. After the ulcers heal, they often leave a characteristic weblike atrophic scar that can break down further following any form of irritation or trauma.5
A prompt diagnosis of PPG is important. A diagnosis of PPG should be considered when dealing with a noninfectious ulcer surrounding a stoma in patients with IBD or other autoimmune conditions.6 Because PPG is a rare skin disorder, it is likely to be missed and lead to unnecessary diagnostic workup and a delay in proper therapy. In our patient, a diagnosis of PPG was overlooked for other infectious and autoimmune causes. The diagnostic evaluation of a patient with PPG is based on 3 principles: (1) ruling out other causes of a peristomal ulcer, such as an abscess, contact dermatitis, or wound infection; (2) determining whether there is an underlying intestinal bowel disease in the stoma; and (3) identifying associated systemic disorders such as vasculitis, erythema nodosum, or similar processes.4 The differential diagnosis depends on the type and stage of PPG and can include malignancy, vasculitis, extraintestinal IBD, infectious disease, and insect bites. A review of the history of the ulcer is helpful in ruling out other diseases, and a colonoscopy or ileoscopy can identify if patients have an underlying active IBD. Swabs for smear and both bacterial and fungal cultures should be taken from the exudate and directly from the ulcer base. Biopsy of the ulcer also helps to exclude alternative diagnoses.6
The primary goals of treating PPG include to reduce pain and the risk for secondary infection, increase pouch adherence, and decrease purulent exudate.7 Although there is not one well-defined optimal therapeutic intervention, there are a variety of effective approaches that may be considered and used. In mild cases, management methods such as dressings, topical agents, or intralesional steroids may be capable of controlling the disease. Daily wound care is important. Moisture-retentive dressings can control pain, induce collagen formation, promote angiogenesis, and prevent contamination. Cleaning the wound with sterile saline and applying an anti-infective agent also may be effective. Application of ultrapotent topical steroids and tacrolimus ointment 0.3% can be used in patients without concomitant secondary infection. In patients who are in remission, human platelet-derived growth factor may be used. Intralesional injections of dilute triamcinolone acetonide or cyclosporine solution also can be helpful. Cyclosporin A was used as a systemic monotherapy to treat a 48-year-old man and 50-year-old woman with the idiopathic form of PPG. After 3 months of treatment, PPG had completely resolved and there were no major side effects.8 Other potential topical therapies that control inflammation and promote wound healing include benzoyl peroxide, chlormethine (topical alkylating agent and nitrogen mustard that has anti-inflammatory properties), nicotine, and 5-aminosalicylic acid. If an ulcer becomes infected, empiric antibiotic therapy should be given immediately and adjusted based on culture and sensitivity results.4
Systemic therapy should be considered in patients who do not respond to topical or local interventions, have a rapid and severe course, or have an active underlying bowel disease. Oral prednisone (1 mg/kg/d) has proved to be one of the most successful drugs used to treat PPG. Treatment should be continued until complete lesion healing, and low-dose maintenance therapy should be administered in recurrent cases. Intravenous corticosteroid therapy—hydrocortisone 100 mg 4 times daily or pulse therapy with intravenous methylprednisolone 1 g/d)—can be used for up to 5 days and may be effective. Oral minocycline 100 mg twice daily may be helpful as an adjunctive therapy to corticosteroids. When corticosteroids fail, oral cyclosporine 3 to 5 mg/kg/d often is prescribed. Studies have shown that patients demonstrate clinical improvement within 3 weeks of cyclosporine initiation, and it has been shown further to be more effective than either azathioprine or methotrexate.4,8
Infliximab, a chimeric antibody that binds both circulating and tissue-bound TNF-α, has been shown to effectively treat PPG. A clinical trial conducted by Brooklyn et al9 found that 46% of patients (6/13) treated with infliximab responded compared with only 6% in a placebo control group (1/17). Although infliximab may result in sepsis, the benefits far outweigh the risks, especially for patients with steroid-refractory PPG.4 Adalimumab is a human monoclonal IgG1 antibody to TNF-α that neutralizes its function by blocking the interaction between the molecule and its receptor. Many clinical studies have shown that adalimumab induces and maintains a clinical response in patients with active Crohn disease. The biologic proved to be effective in our patient, but it is associated with potential side effects that should be monitored including injection-site reactions, pruritus, leukopenia, urticaria, and rare instances of alopecia.10 Etanercept is another potentially effective biologic agent.7 Plasma exchange, immunoglobulin infusion, and interferon-alfa therapy also can be used in refractory PPG cases, though data on these treatments are very limited.4
Unlike routine pyoderma gangrenosum—for which surgical intervention is contraindicated—surgical intervention may be appropriate for the peristomal variant. Surgical treatment options include stoma revision and/or relocation; however, both of these procedures are accompanied by failure rates ranging from 40% to 100%.5 Removal of a diseased intestinal segment, especially one with active IBD, may result in healing of the skin lesion. In our patient, removal of the residual and diseased J-pouch was part of the management plan. However,it generally is recommended that any surgical intervention be accompanied by medical therapy including oral metronidazole 500 mg/d and concomitant administration of an immunosuppressant.1,3
Because PPG tends to recur, long-term maintenance therapy should always be considered. Pain reduction, anemia correction, proper nutrition, and management of associated and underlying diseases should be performed. Meticulous care of the stoma and prevention of leaks also should be emphasized. Overall, if PPG is detected and diagnosed early as well as treated appropriately and aggressively, the patient likely will have a good prognosis.4
- Sheldon DG, Sawchuk LL, Kozarek RA, et al. Twenty cases of peristomal pyoderma gangrenosum: diagnostic implications and management. Arch Surg. 2000;135:564-569.
- Hughes AP, Jackson JM, Callen JP. Clinical features and treatment of peristomal pyoderma gangrenosum. JAMA. 2000;284:1546-1548.
- Afifi L, Sanchez IM, Wallace MM, et al. Diagnosis and management of peristomal pyoderma gangrenosum: a systematic review. J Am Acad Dermatol. 2018;78:1195-1204.
- Wu XR, Shen B. Diagnosis and management of parastomal pyoderma gangrenosum. Gastroenterol Rep (Oxf). 2013;1:1-8.
- Javed A, Pal S, Ahuja V, et al. Management of peristomal pyoderma gangrenosum: two different approaches for the same clinical problem. Trop Gastroenterol. 2011;32:153-156.
- Toh JW, Whiteley I. Devastating peristomal pyoderma gangrenosum: challenges in diagnosis and management. Clin Gastroenterol Hepatol. 2017;15:A19-A20.
- DeMartyn LE, Faller NA, Miller L. Treating peristomal pyoderma gangrenosum with topical crushed prednisone: a report of three cases. Ostomy Wound Manage. 2014;60:50-54.
- V’lckova-Laskoska MT, Laskoski DS, Caca-Biljanovska NG, et al. Pyoderma gangrenosum successfully treated with cyclosporin A.Adv Exp Med Biol. 1999;455:541-555.
- Brooklyn TN, Dunnill MGS, Shetty A, at al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Alkhouri N, Hupertz V, Mahajan L. Adalimumab treatment for peristomal pyoderma gangrenosum associated with Crohn’s disease. Inflamm Bowel Dis. 2009;15:803-806.
- Sheldon DG, Sawchuk LL, Kozarek RA, et al. Twenty cases of peristomal pyoderma gangrenosum: diagnostic implications and management. Arch Surg. 2000;135:564-569.
- Hughes AP, Jackson JM, Callen JP. Clinical features and treatment of peristomal pyoderma gangrenosum. JAMA. 2000;284:1546-1548.
- Afifi L, Sanchez IM, Wallace MM, et al. Diagnosis and management of peristomal pyoderma gangrenosum: a systematic review. J Am Acad Dermatol. 2018;78:1195-1204.
- Wu XR, Shen B. Diagnosis and management of parastomal pyoderma gangrenosum. Gastroenterol Rep (Oxf). 2013;1:1-8.
- Javed A, Pal S, Ahuja V, et al. Management of peristomal pyoderma gangrenosum: two different approaches for the same clinical problem. Trop Gastroenterol. 2011;32:153-156.
- Toh JW, Whiteley I. Devastating peristomal pyoderma gangrenosum: challenges in diagnosis and management. Clin Gastroenterol Hepatol. 2017;15:A19-A20.
- DeMartyn LE, Faller NA, Miller L. Treating peristomal pyoderma gangrenosum with topical crushed prednisone: a report of three cases. Ostomy Wound Manage. 2014;60:50-54.
- V’lckova-Laskoska MT, Laskoski DS, Caca-Biljanovska NG, et al. Pyoderma gangrenosum successfully treated with cyclosporin A.Adv Exp Med Biol. 1999;455:541-555.
- Brooklyn TN, Dunnill MGS, Shetty A, at al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Alkhouri N, Hupertz V, Mahajan L. Adalimumab treatment for peristomal pyoderma gangrenosum associated with Crohn’s disease. Inflamm Bowel Dis. 2009;15:803-806.
Practice Points
- A pyoderma gangrenosum subtype occurs in close proximity to an abdominal stoma.
- Peristomal pyoderma gangrenosum is a diagnosis of exclusion.
- Peristomal pyoderma gangrenosum typically responds best to tumor necrosis factor α blockers and corticosteroid therapy (intralesional and systemic).
Dog Walking Can Be Hazardous to Cutaneous Health
Studies have recommended dog walking as an activity designed to improve the overall health of older adults.1,2 Benefits purportedly associated with dog walking include lower body mass index, fewer chronic diseases, reduction in the number of physician visits, and decreased limitations of activities of daily living.2 The Arthritis Foundation even recommends dog walking to relieve arthritis symptoms.3 Of course, dogs also provide comfort in companionship, and dog walking can be an enjoyable way for a pet and owner to spend time together.
However, this seemingly benign activity poses a notable and perhaps grossly underrecognized risk for injury in older adults. The annual number of patients 65 years and older who presented to US emergency departments (EDs) for fractures directly associated with walking leashed dogs more than doubled from 2004 to 2017.4 Interestingly, this dramatic increase parallels a nationwide trend in dog ownership demographics. Between 2006 and 2016, the median age of dog owners in the United States rose from 46 to 49 years.5
These trends raise concern for more than just the health of older Americans’ bones. Intuitively, a dog- walking accident that results in a bone fracture will likely also lead to some degree of skin trauma. Older adults have thin fragile skin due to flattening of the dermoepidermal junction and disintegration or degeneration of dermal collagen and elastin.6 This loss of connective tissue as well as subcutaneous tissue in some body areas facilitates shearing injury; concurrently, weakened perivascular support increases the risk for vascular injury and bruising.7 Therefore, when an older person falls while walking a dog, trauma can easily damage delicate aged skin.
Older adults are particularly susceptible to falls, the leading cause of fatal and nonfatal injuries in this age group.8 There are multiple risk factors for falls, including polypharmacy, impaired balance and gait, visual impairments, and cognitive decline, among others.9
Also, many older adults with atrial fibrillation or venous thromboembolism take an anticoagulant drug to prevent stroke. The use of anticoagulants is associated with an increased risk for bleeding, ranging from minor cutaneous bleeding to fatal intracranial hemorrhage.10
A predisposition to falling and bleeding can be hazardous for a dog owner whose excited pet suddenly jumps, runs, or scratches. The use of a leash, mandatory in many urban jurisdictions, tethers the human to the dog, which expedites a fall associated with any sudden, forceful forward or lateral movement by the dog. The following case reports describe a variety of cutaneous injuries experienced by older adults while dog walking.
Case Reports
Patient 1
A 79-year-old woman was quietly walking her dog when the dog spotted a squirrel climbing a tree. The dog became excited, turned to the owner, and jumped on her, which caused the dog’s claws to dig into the owner’s fragile forearm skin, creating several superficial but painful abrasions and lacerations (Figure 1). These injuries healed well with conservative therapy including application of an occlusive ointment.
Patient 2
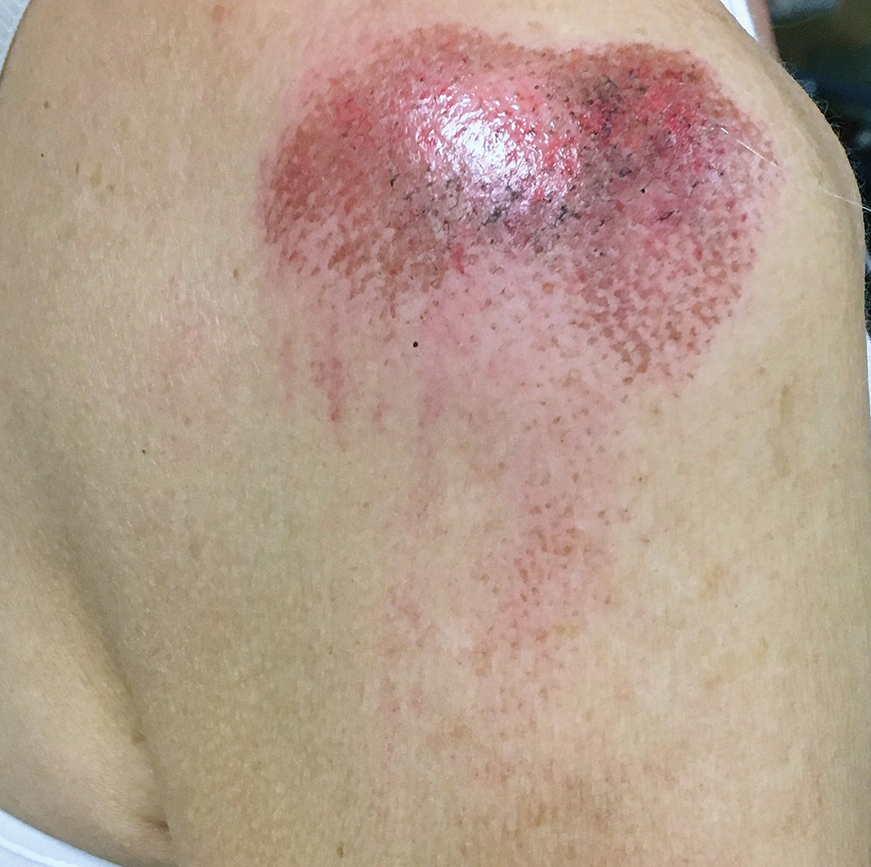
A 68-year-old woman was walking her dog when the dog saw a cat running across the street. The dog suddenly leaped toward the cat, causing the owner to fall forward as the animal’s momentum was transferred through the leash. The owner fell awkwardly on her side, leading to an extensive abrasion and contusion of the shoulder (Figure 2). The lesion healed well with conservative management, albeit with moderate postinflammatory hypochromia.
Patient 3
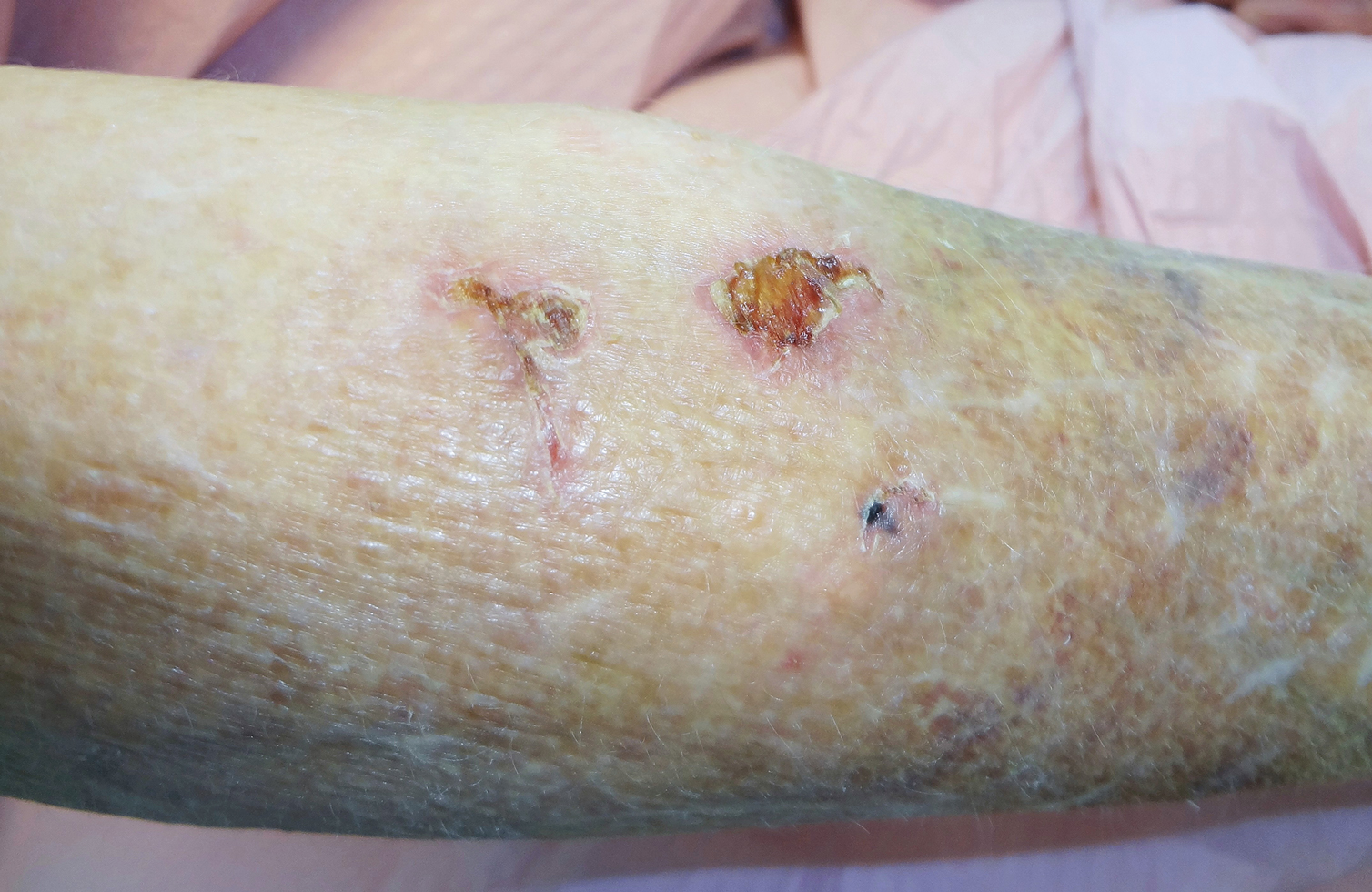
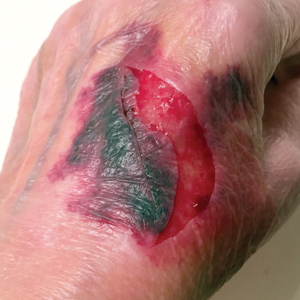
A 65-year-old woman was walking her dog and they heard a loud noise. The dog started to run forward—likely, startled. The owner did not fall, but the leash, which was wrapped around her hand, exerted enough force to avulse a 5×3-cm piece of skin from the dorsum of the hand (Figure 3). The painful abrasion and concomitant bruise eventually healed with conservative management but left a noticeable hemosiderin stain.
Patient 4
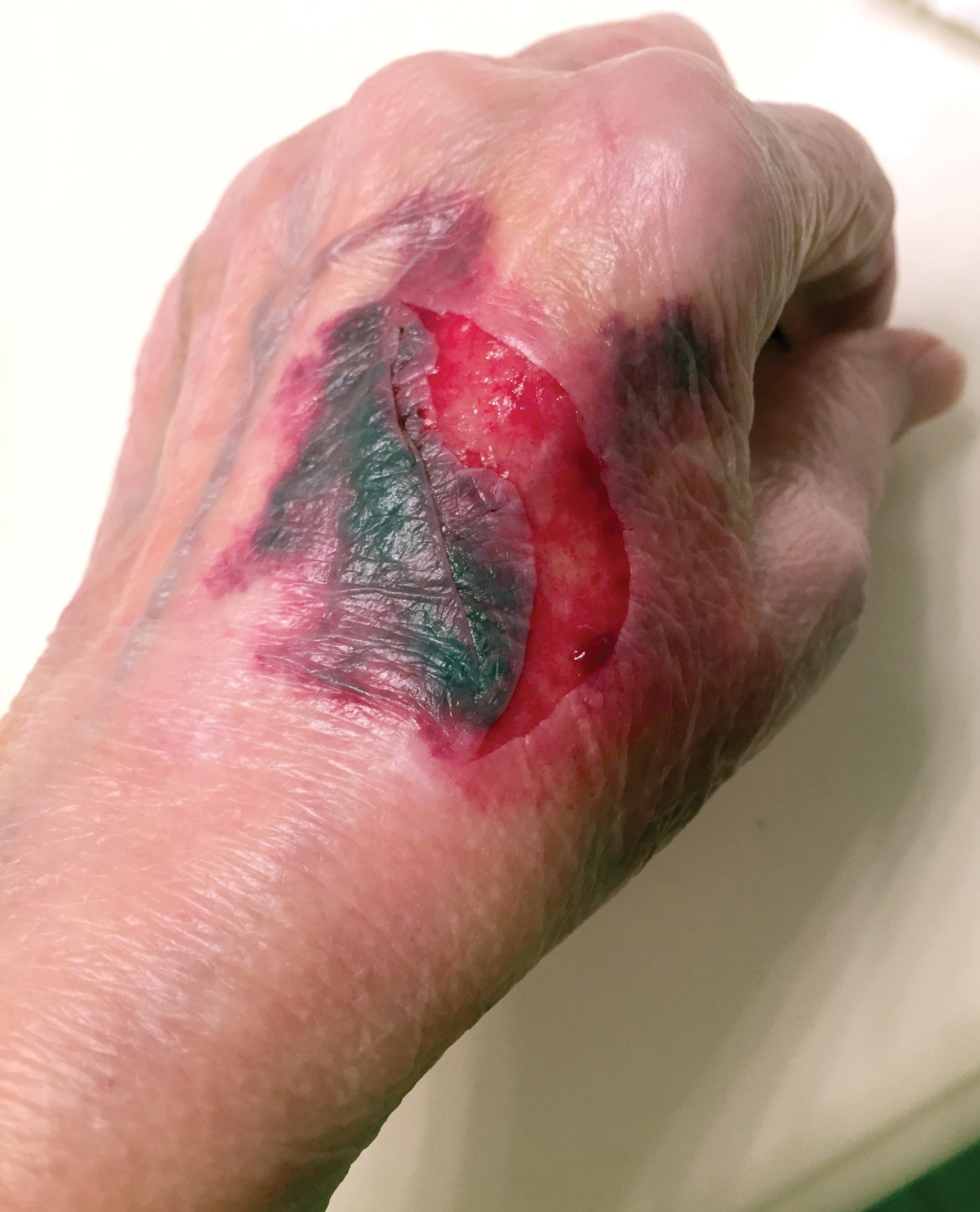
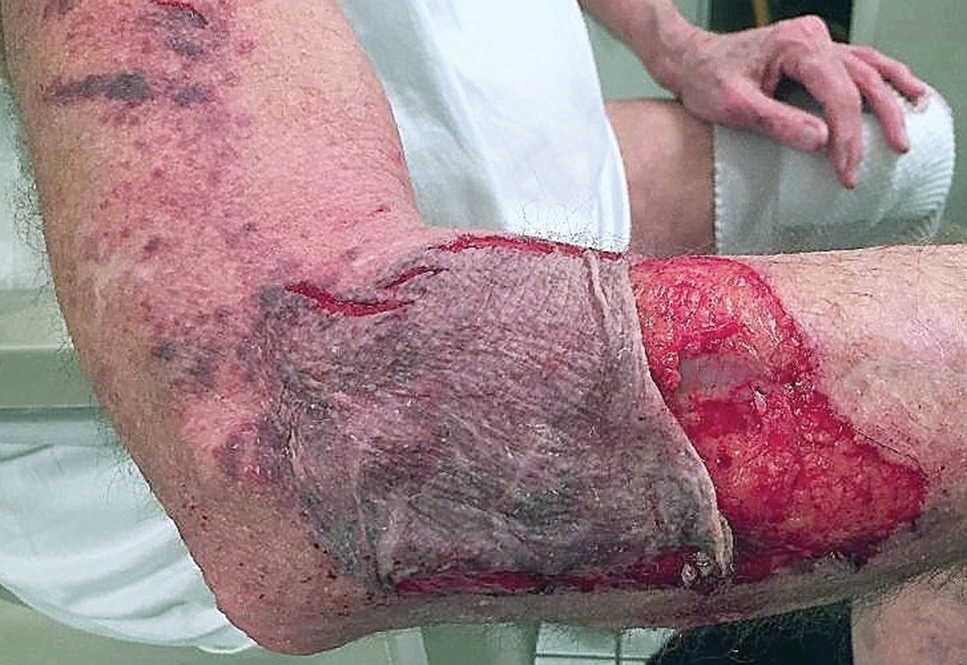
A 66-year-old man was walking a large Rottweiler when the dog lurched toward another dog that was being walked across the street. The owner, taken by surprise by this sudden motion, fell on the concrete sidewalk and was dragged several feet by the dog. This unexpected and off-balance fall caused multiple injuries, including bruises on the upper arm, a large avulsion of epidermal forearm skin (Figure 4), a gouge in the dermis down to fat, and a large abrasion of the contralateral knee. The patient received a tetanus booster and conservative therapy. The affected area healed with an atrophic hypopigmented scar.
Patient 5
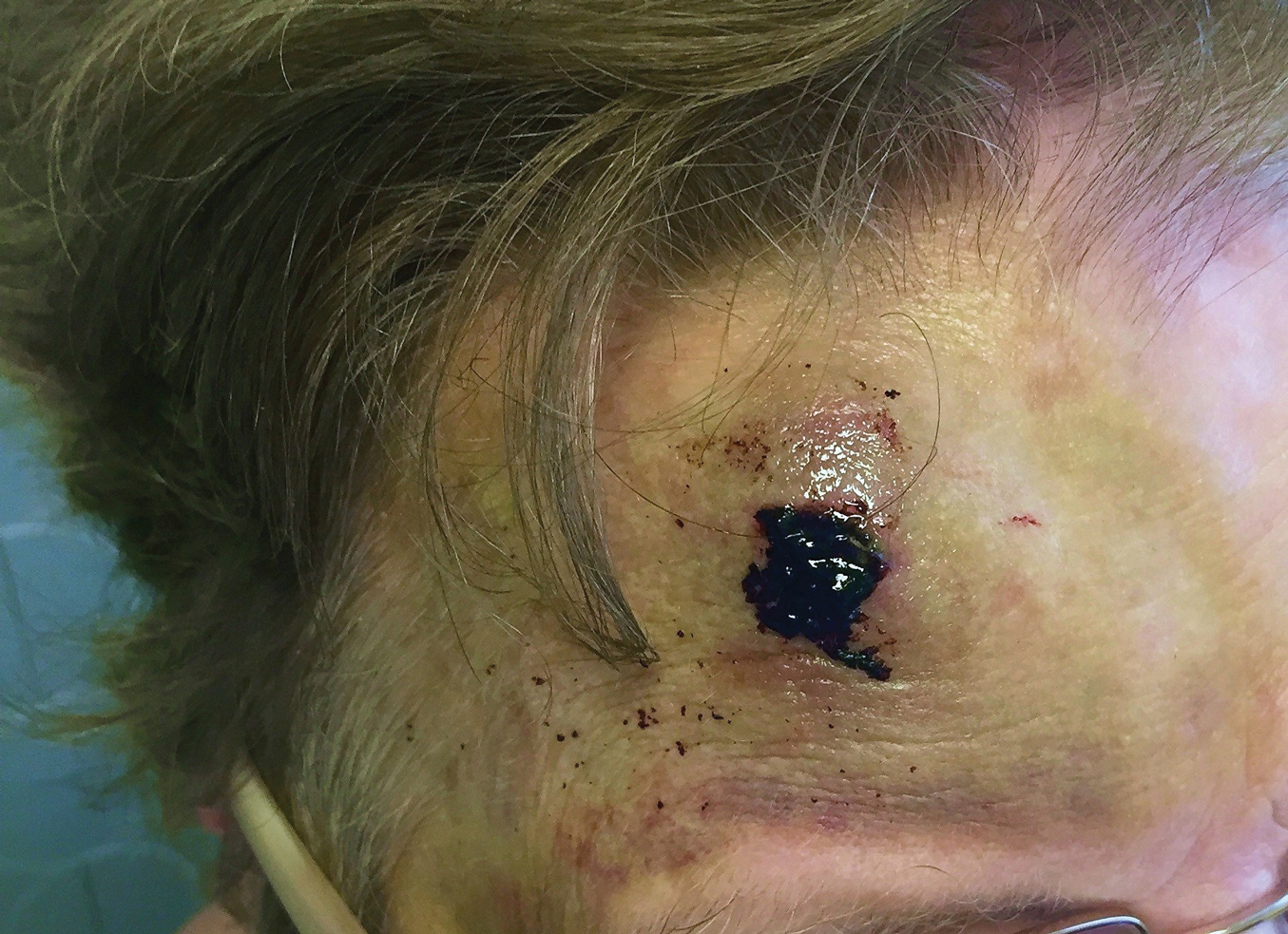
An 82-year-old woman with known atrial fibrillation who was taking chronic anticoagulation medication was walking her dog. For no apparent reason, the dog sped up the pace. The woman lost her balance and fell face first onto the sidewalk. She did not lose consciousness but did develop a large bruise on the forehead with a tender fluctuant nodule in the center (Figure 5).
The patient presented the next day, requesting drainage of the forehead hematoma. However, a brief review of systems revealed a persistent severe headache and nausea with vomiting since the prior day. She was immediately transported to the nearest ED where complete neurologic workup revealed a moderate-sized subdural hematoma that was treated by trephination. Recovery was uneventful.
Comment
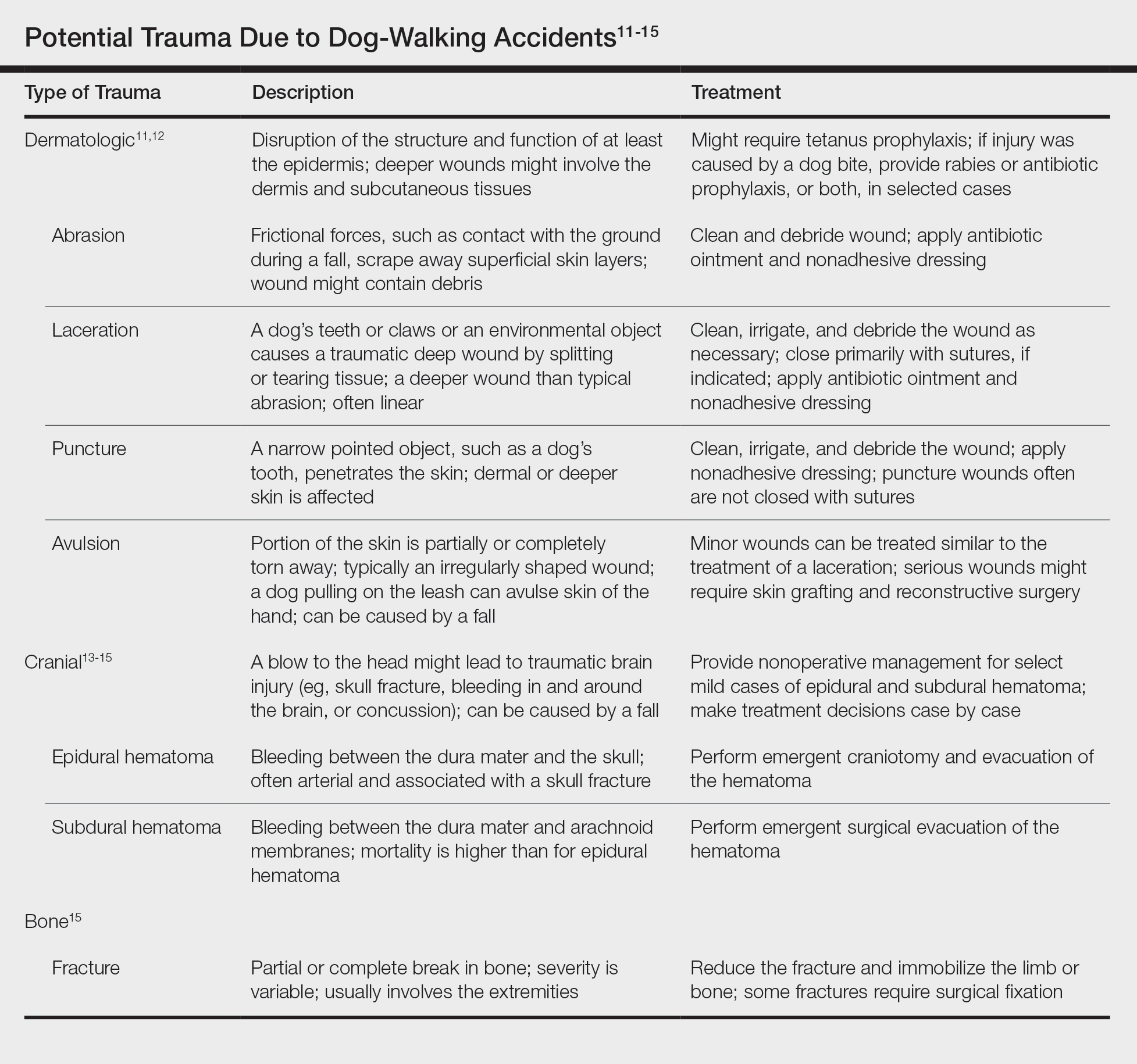
These 5 cases illustrate the notable skin (and neurologic) trauma that can occur due to a dog-walking accident (Table).11-15
Regrettably, obtaining an accurate national estimate of the annual incidence of cutaneous dog-walking injuries is difficult. Researchers who have described the rise in dog walking–associated bone fractures queried the US Consumer Product Safety Commission’s National Electronic Injury Surveillance System database for its numbers.4 This public database generates incidence estimates of activity- or product-related injuries based on data from a nationally representative sample of approximately 100 hospital EDs.16
We queried the same database for the diagnoses avulsion, abrasion or contusion, and laceration.17 These terms were searched in association with pet supplies, including leashes, and patients 65 years and older. This search yielded fewer than 800 total cases from 2008 to 2017, resulting in unreliable estimates for each year.
The National Electronic Injury Surveillance System database no doubt underestimates the true incidence of dog walking–related skin trauma; the great majority of patients with cutaneous injury, as illustrated here, likely never present to the ED, unlike patients with bone fracture. Moreover, data do not capture cases handled by providers outside the ED and self-treated injuries.
In the absence of accurate estimates of cutaneous morbidity related to dog-walking injury, the case reports here are clearly a cautionary tale. Physicians and older adults need to be cognizant of the hazards of this activity. Providers should discuss with older patients the potential risks of dog walking before recommending or condoning this exercise.
The presence of other comorbidities that could hamper a person’s ability to control a leashed dog warrants special consideration. Older prospective dog owners might consider adopting a small, easily manageable breed. These measures can help protect older adults’ fragile skin (and bones) from avoidable minor to potentially life-threatening trauma.
- Christian H, Bauman A, Epping JN, et al. Encouraging dog walking for health promotion and disease prevention. Am J Lifestyle Med. 2016;12:233-243.
- Curl AL, Bibbo J, Johnson RA. Dog walking, the human–animal bond and older adults’ physical health. Gerontologist. 2017;57:930-939.
- Dunkin MA. Walking strategies. Arthritis Foundation website. https://arthritis.org/health-wellness/healthy-living/physical-activity/walking/5-walking-strategies. Accessed March 16, 2020.
- Pirruccio K, Yoon YM, Ahn J. Fractures in elderly Americans associated with walking leashed dogs. JAMA Surg. 2019;154:458-459.
- Sprinkle D. Pet owner demographics get grayer, more golden. Petfood Industry website. https://www.petfoodindustry.com/articles/6315-pet-owner-demographics-get-grayer-more-golden?v=preview. Published March 10, 2017. Accessed March 16, 2020.
- Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61:427-434.
- Aging & painful skin. Cleveland Clinic website. https://my.clevelandclinic.org/health/diseases/16725-aging--painful-skin. Accessed March 16, 2020.
- Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
- Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61.
- January CT, Wann LS, Alpert JS, et al; . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:E1-E76.
- Armstrong DG, Meyr AJ. Basic principles of wound management. UpToDate. https://www.uptodate.com/contents/basic-principles-of-wound-management. Accessed March 18, 2020.
- Trott AT. Wounds and Lacerations: Emergency Care and Closure. 4th ed. Philadelphia, PA: Saunders; 2012.
- Head injuries in adults: what is it? Harvard Health Publishing website. www.health.harvard.edu/a_to_z/head-injury-in-adults-a-to-z. Published October 2018. Accessed January 30, 2020.
- McBride W. Intracranial epidural hematoma in adults. UpToDate. https://www.uptodate.com/contents/intracranial-epidural-hematoma-in-adults. Updated July 23, 2018. Accessed March 18, 2020.
- McBride W. Subdural hematoma in adults: prognosis and management. UpToDate. https://www.uptodate.com/contents/subdural-hematoma-in-adults-prognosis-and-management. Updated July 11, 2019. Accessed March 18, 2020.
- Schroeder T, Ault K. The NEISS sample: design and implementation. Washington, DC: US Consumer Product Safety Commission, Division of Hazard and Injury Data Systems; June 2001. https://cpsc.gov/s3fs-public/pdfs/blk_media_2001d011-6b6.pdf. Accessed January 30, 2020.
- National Electronic Injury Surveillance System (NEISS). Bethesda, MD: US Consumer Product Safety Commission; 2018. https://www.cpsc.gov/Research--Statistics/NEISS-Injury-Data. Accessed March 16, 2020.
Studies have recommended dog walking as an activity designed to improve the overall health of older adults.1,2 Benefits purportedly associated with dog walking include lower body mass index, fewer chronic diseases, reduction in the number of physician visits, and decreased limitations of activities of daily living.2 The Arthritis Foundation even recommends dog walking to relieve arthritis symptoms.3 Of course, dogs also provide comfort in companionship, and dog walking can be an enjoyable way for a pet and owner to spend time together.
However, this seemingly benign activity poses a notable and perhaps grossly underrecognized risk for injury in older adults. The annual number of patients 65 years and older who presented to US emergency departments (EDs) for fractures directly associated with walking leashed dogs more than doubled from 2004 to 2017.4 Interestingly, this dramatic increase parallels a nationwide trend in dog ownership demographics. Between 2006 and 2016, the median age of dog owners in the United States rose from 46 to 49 years.5
These trends raise concern for more than just the health of older Americans’ bones. Intuitively, a dog- walking accident that results in a bone fracture will likely also lead to some degree of skin trauma. Older adults have thin fragile skin due to flattening of the dermoepidermal junction and disintegration or degeneration of dermal collagen and elastin.6 This loss of connective tissue as well as subcutaneous tissue in some body areas facilitates shearing injury; concurrently, weakened perivascular support increases the risk for vascular injury and bruising.7 Therefore, when an older person falls while walking a dog, trauma can easily damage delicate aged skin.
Older adults are particularly susceptible to falls, the leading cause of fatal and nonfatal injuries in this age group.8 There are multiple risk factors for falls, including polypharmacy, impaired balance and gait, visual impairments, and cognitive decline, among others.9
Also, many older adults with atrial fibrillation or venous thromboembolism take an anticoagulant drug to prevent stroke. The use of anticoagulants is associated with an increased risk for bleeding, ranging from minor cutaneous bleeding to fatal intracranial hemorrhage.10
A predisposition to falling and bleeding can be hazardous for a dog owner whose excited pet suddenly jumps, runs, or scratches. The use of a leash, mandatory in many urban jurisdictions, tethers the human to the dog, which expedites a fall associated with any sudden, forceful forward or lateral movement by the dog. The following case reports describe a variety of cutaneous injuries experienced by older adults while dog walking.
Case Reports
Patient 1
A 79-year-old woman was quietly walking her dog when the dog spotted a squirrel climbing a tree. The dog became excited, turned to the owner, and jumped on her, which caused the dog’s claws to dig into the owner’s fragile forearm skin, creating several superficial but painful abrasions and lacerations (Figure 1). These injuries healed well with conservative therapy including application of an occlusive ointment.
Patient 2
A 68-year-old woman was walking her dog when the dog saw a cat running across the street. The dog suddenly leaped toward the cat, causing the owner to fall forward as the animal’s momentum was transferred through the leash. The owner fell awkwardly on her side, leading to an extensive abrasion and contusion of the shoulder (Figure 2). The lesion healed well with conservative management, albeit with moderate postinflammatory hypochromia.
Patient 3
A 65-year-old woman was walking her dog and they heard a loud noise. The dog started to run forward—likely, startled. The owner did not fall, but the leash, which was wrapped around her hand, exerted enough force to avulse a 5×3-cm piece of skin from the dorsum of the hand (Figure 3). The painful abrasion and concomitant bruise eventually healed with conservative management but left a noticeable hemosiderin stain.
Patient 4
A 66-year-old man was walking a large Rottweiler when the dog lurched toward another dog that was being walked across the street. The owner, taken by surprise by this sudden motion, fell on the concrete sidewalk and was dragged several feet by the dog. This unexpected and off-balance fall caused multiple injuries, including bruises on the upper arm, a large avulsion of epidermal forearm skin (Figure 4), a gouge in the dermis down to fat, and a large abrasion of the contralateral knee. The patient received a tetanus booster and conservative therapy. The affected area healed with an atrophic hypopigmented scar.
Patient 5
An 82-year-old woman with known atrial fibrillation who was taking chronic anticoagulation medication was walking her dog. For no apparent reason, the dog sped up the pace. The woman lost her balance and fell face first onto the sidewalk. She did not lose consciousness but did develop a large bruise on the forehead with a tender fluctuant nodule in the center (Figure 5).
The patient presented the next day, requesting drainage of the forehead hematoma. However, a brief review of systems revealed a persistent severe headache and nausea with vomiting since the prior day. She was immediately transported to the nearest ED where complete neurologic workup revealed a moderate-sized subdural hematoma that was treated by trephination. Recovery was uneventful.
Comment
These 5 cases illustrate the notable skin (and neurologic) trauma that can occur due to a dog-walking accident (Table).11-15
Regrettably, obtaining an accurate national estimate of the annual incidence of cutaneous dog-walking injuries is difficult. Researchers who have described the rise in dog walking–associated bone fractures queried the US Consumer Product Safety Commission’s National Electronic Injury Surveillance System database for its numbers.4 This public database generates incidence estimates of activity- or product-related injuries based on data from a nationally representative sample of approximately 100 hospital EDs.16
We queried the same database for the diagnoses avulsion, abrasion or contusion, and laceration.17 These terms were searched in association with pet supplies, including leashes, and patients 65 years and older. This search yielded fewer than 800 total cases from 2008 to 2017, resulting in unreliable estimates for each year.
The National Electronic Injury Surveillance System database no doubt underestimates the true incidence of dog walking–related skin trauma; the great majority of patients with cutaneous injury, as illustrated here, likely never present to the ED, unlike patients with bone fracture. Moreover, data do not capture cases handled by providers outside the ED and self-treated injuries.
In the absence of accurate estimates of cutaneous morbidity related to dog-walking injury, the case reports here are clearly a cautionary tale. Physicians and older adults need to be cognizant of the hazards of this activity. Providers should discuss with older patients the potential risks of dog walking before recommending or condoning this exercise.
The presence of other comorbidities that could hamper a person’s ability to control a leashed dog warrants special consideration. Older prospective dog owners might consider adopting a small, easily manageable breed. These measures can help protect older adults’ fragile skin (and bones) from avoidable minor to potentially life-threatening trauma.
Studies have recommended dog walking as an activity designed to improve the overall health of older adults.1,2 Benefits purportedly associated with dog walking include lower body mass index, fewer chronic diseases, reduction in the number of physician visits, and decreased limitations of activities of daily living.2 The Arthritis Foundation even recommends dog walking to relieve arthritis symptoms.3 Of course, dogs also provide comfort in companionship, and dog walking can be an enjoyable way for a pet and owner to spend time together.
However, this seemingly benign activity poses a notable and perhaps grossly underrecognized risk for injury in older adults. The annual number of patients 65 years and older who presented to US emergency departments (EDs) for fractures directly associated with walking leashed dogs more than doubled from 2004 to 2017.4 Interestingly, this dramatic increase parallels a nationwide trend in dog ownership demographics. Between 2006 and 2016, the median age of dog owners in the United States rose from 46 to 49 years.5
These trends raise concern for more than just the health of older Americans’ bones. Intuitively, a dog- walking accident that results in a bone fracture will likely also lead to some degree of skin trauma. Older adults have thin fragile skin due to flattening of the dermoepidermal junction and disintegration or degeneration of dermal collagen and elastin.6 This loss of connective tissue as well as subcutaneous tissue in some body areas facilitates shearing injury; concurrently, weakened perivascular support increases the risk for vascular injury and bruising.7 Therefore, when an older person falls while walking a dog, trauma can easily damage delicate aged skin.
Older adults are particularly susceptible to falls, the leading cause of fatal and nonfatal injuries in this age group.8 There are multiple risk factors for falls, including polypharmacy, impaired balance and gait, visual impairments, and cognitive decline, among others.9
Also, many older adults with atrial fibrillation or venous thromboembolism take an anticoagulant drug to prevent stroke. The use of anticoagulants is associated with an increased risk for bleeding, ranging from minor cutaneous bleeding to fatal intracranial hemorrhage.10
A predisposition to falling and bleeding can be hazardous for a dog owner whose excited pet suddenly jumps, runs, or scratches. The use of a leash, mandatory in many urban jurisdictions, tethers the human to the dog, which expedites a fall associated with any sudden, forceful forward or lateral movement by the dog. The following case reports describe a variety of cutaneous injuries experienced by older adults while dog walking.
Case Reports
Patient 1
A 79-year-old woman was quietly walking her dog when the dog spotted a squirrel climbing a tree. The dog became excited, turned to the owner, and jumped on her, which caused the dog’s claws to dig into the owner’s fragile forearm skin, creating several superficial but painful abrasions and lacerations (Figure 1). These injuries healed well with conservative therapy including application of an occlusive ointment.
Patient 2
A 68-year-old woman was walking her dog when the dog saw a cat running across the street. The dog suddenly leaped toward the cat, causing the owner to fall forward as the animal’s momentum was transferred through the leash. The owner fell awkwardly on her side, leading to an extensive abrasion and contusion of the shoulder (Figure 2). The lesion healed well with conservative management, albeit with moderate postinflammatory hypochromia.
Patient 3
A 65-year-old woman was walking her dog and they heard a loud noise. The dog started to run forward—likely, startled. The owner did not fall, but the leash, which was wrapped around her hand, exerted enough force to avulse a 5×3-cm piece of skin from the dorsum of the hand (Figure 3). The painful abrasion and concomitant bruise eventually healed with conservative management but left a noticeable hemosiderin stain.
Patient 4
A 66-year-old man was walking a large Rottweiler when the dog lurched toward another dog that was being walked across the street. The owner, taken by surprise by this sudden motion, fell on the concrete sidewalk and was dragged several feet by the dog. This unexpected and off-balance fall caused multiple injuries, including bruises on the upper arm, a large avulsion of epidermal forearm skin (Figure 4), a gouge in the dermis down to fat, and a large abrasion of the contralateral knee. The patient received a tetanus booster and conservative therapy. The affected area healed with an atrophic hypopigmented scar.
Patient 5
An 82-year-old woman with known atrial fibrillation who was taking chronic anticoagulation medication was walking her dog. For no apparent reason, the dog sped up the pace. The woman lost her balance and fell face first onto the sidewalk. She did not lose consciousness but did develop a large bruise on the forehead with a tender fluctuant nodule in the center (Figure 5).
The patient presented the next day, requesting drainage of the forehead hematoma. However, a brief review of systems revealed a persistent severe headache and nausea with vomiting since the prior day. She was immediately transported to the nearest ED where complete neurologic workup revealed a moderate-sized subdural hematoma that was treated by trephination. Recovery was uneventful.
Comment
These 5 cases illustrate the notable skin (and neurologic) trauma that can occur due to a dog-walking accident (Table).11-15
Regrettably, obtaining an accurate national estimate of the annual incidence of cutaneous dog-walking injuries is difficult. Researchers who have described the rise in dog walking–associated bone fractures queried the US Consumer Product Safety Commission’s National Electronic Injury Surveillance System database for its numbers.4 This public database generates incidence estimates of activity- or product-related injuries based on data from a nationally representative sample of approximately 100 hospital EDs.16
We queried the same database for the diagnoses avulsion, abrasion or contusion, and laceration.17 These terms were searched in association with pet supplies, including leashes, and patients 65 years and older. This search yielded fewer than 800 total cases from 2008 to 2017, resulting in unreliable estimates for each year.
The National Electronic Injury Surveillance System database no doubt underestimates the true incidence of dog walking–related skin trauma; the great majority of patients with cutaneous injury, as illustrated here, likely never present to the ED, unlike patients with bone fracture. Moreover, data do not capture cases handled by providers outside the ED and self-treated injuries.
In the absence of accurate estimates of cutaneous morbidity related to dog-walking injury, the case reports here are clearly a cautionary tale. Physicians and older adults need to be cognizant of the hazards of this activity. Providers should discuss with older patients the potential risks of dog walking before recommending or condoning this exercise.
The presence of other comorbidities that could hamper a person’s ability to control a leashed dog warrants special consideration. Older prospective dog owners might consider adopting a small, easily manageable breed. These measures can help protect older adults’ fragile skin (and bones) from avoidable minor to potentially life-threatening trauma.
- Christian H, Bauman A, Epping JN, et al. Encouraging dog walking for health promotion and disease prevention. Am J Lifestyle Med. 2016;12:233-243.
- Curl AL, Bibbo J, Johnson RA. Dog walking, the human–animal bond and older adults’ physical health. Gerontologist. 2017;57:930-939.
- Dunkin MA. Walking strategies. Arthritis Foundation website. https://arthritis.org/health-wellness/healthy-living/physical-activity/walking/5-walking-strategies. Accessed March 16, 2020.
- Pirruccio K, Yoon YM, Ahn J. Fractures in elderly Americans associated with walking leashed dogs. JAMA Surg. 2019;154:458-459.
- Sprinkle D. Pet owner demographics get grayer, more golden. Petfood Industry website. https://www.petfoodindustry.com/articles/6315-pet-owner-demographics-get-grayer-more-golden?v=preview. Published March 10, 2017. Accessed March 16, 2020.
- Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61:427-434.
- Aging & painful skin. Cleveland Clinic website. https://my.clevelandclinic.org/health/diseases/16725-aging--painful-skin. Accessed March 16, 2020.
- Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
- Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61.
- January CT, Wann LS, Alpert JS, et al; . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:E1-E76.
- Armstrong DG, Meyr AJ. Basic principles of wound management. UpToDate. https://www.uptodate.com/contents/basic-principles-of-wound-management. Accessed March 18, 2020.
- Trott AT. Wounds and Lacerations: Emergency Care and Closure. 4th ed. Philadelphia, PA: Saunders; 2012.
- Head injuries in adults: what is it? Harvard Health Publishing website. www.health.harvard.edu/a_to_z/head-injury-in-adults-a-to-z. Published October 2018. Accessed January 30, 2020.
- McBride W. Intracranial epidural hematoma in adults. UpToDate. https://www.uptodate.com/contents/intracranial-epidural-hematoma-in-adults. Updated July 23, 2018. Accessed March 18, 2020.
- McBride W. Subdural hematoma in adults: prognosis and management. UpToDate. https://www.uptodate.com/contents/subdural-hematoma-in-adults-prognosis-and-management. Updated July 11, 2019. Accessed March 18, 2020.
- Schroeder T, Ault K. The NEISS sample: design and implementation. Washington, DC: US Consumer Product Safety Commission, Division of Hazard and Injury Data Systems; June 2001. https://cpsc.gov/s3fs-public/pdfs/blk_media_2001d011-6b6.pdf. Accessed January 30, 2020.
- National Electronic Injury Surveillance System (NEISS). Bethesda, MD: US Consumer Product Safety Commission; 2018. https://www.cpsc.gov/Research--Statistics/NEISS-Injury-Data. Accessed March 16, 2020.
- Christian H, Bauman A, Epping JN, et al. Encouraging dog walking for health promotion and disease prevention. Am J Lifestyle Med. 2016;12:233-243.
- Curl AL, Bibbo J, Johnson RA. Dog walking, the human–animal bond and older adults’ physical health. Gerontologist. 2017;57:930-939.
- Dunkin MA. Walking strategies. Arthritis Foundation website. https://arthritis.org/health-wellness/healthy-living/physical-activity/walking/5-walking-strategies. Accessed March 16, 2020.
- Pirruccio K, Yoon YM, Ahn J. Fractures in elderly Americans associated with walking leashed dogs. JAMA Surg. 2019;154:458-459.
- Sprinkle D. Pet owner demographics get grayer, more golden. Petfood Industry website. https://www.petfoodindustry.com/articles/6315-pet-owner-demographics-get-grayer-more-golden?v=preview. Published March 10, 2017. Accessed March 16, 2020.
- Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61:427-434.
- Aging & painful skin. Cleveland Clinic website. https://my.clevelandclinic.org/health/diseases/16725-aging--painful-skin. Accessed March 16, 2020.
- Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:993-998.
- Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75:51-61.
- January CT, Wann LS, Alpert JS, et al; . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:E1-E76.
- Armstrong DG, Meyr AJ. Basic principles of wound management. UpToDate. https://www.uptodate.com/contents/basic-principles-of-wound-management. Accessed March 18, 2020.
- Trott AT. Wounds and Lacerations: Emergency Care and Closure. 4th ed. Philadelphia, PA: Saunders; 2012.
- Head injuries in adults: what is it? Harvard Health Publishing website. www.health.harvard.edu/a_to_z/head-injury-in-adults-a-to-z. Published October 2018. Accessed January 30, 2020.
- McBride W. Intracranial epidural hematoma in adults. UpToDate. https://www.uptodate.com/contents/intracranial-epidural-hematoma-in-adults. Updated July 23, 2018. Accessed March 18, 2020.
- McBride W. Subdural hematoma in adults: prognosis and management. UpToDate. https://www.uptodate.com/contents/subdural-hematoma-in-adults-prognosis-and-management. Updated July 11, 2019. Accessed March 18, 2020.
- Schroeder T, Ault K. The NEISS sample: design and implementation. Washington, DC: US Consumer Product Safety Commission, Division of Hazard and Injury Data Systems; June 2001. https://cpsc.gov/s3fs-public/pdfs/blk_media_2001d011-6b6.pdf. Accessed January 30, 2020.
- National Electronic Injury Surveillance System (NEISS). Bethesda, MD: US Consumer Product Safety Commission; 2018. https://www.cpsc.gov/Research--Statistics/NEISS-Injury-Data. Accessed March 16, 2020.
Practice Points
- Dog walking is a good source of exercise but can lead to serious skin/soft tissue injury.
- When evaluating cutaneous trauma related to dog walking, remember to consider the possibility of an underlying bone fracture.
- Cutaneous trauma may overlay serious internal injury, such as epidural or subdural hematoma.
The Syphilis Epidemic: Dermatologists on the Frontline of Treatment and Diagnosis
Ulcerative Sarcoidosis: A Prototypical Presentation and Review
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
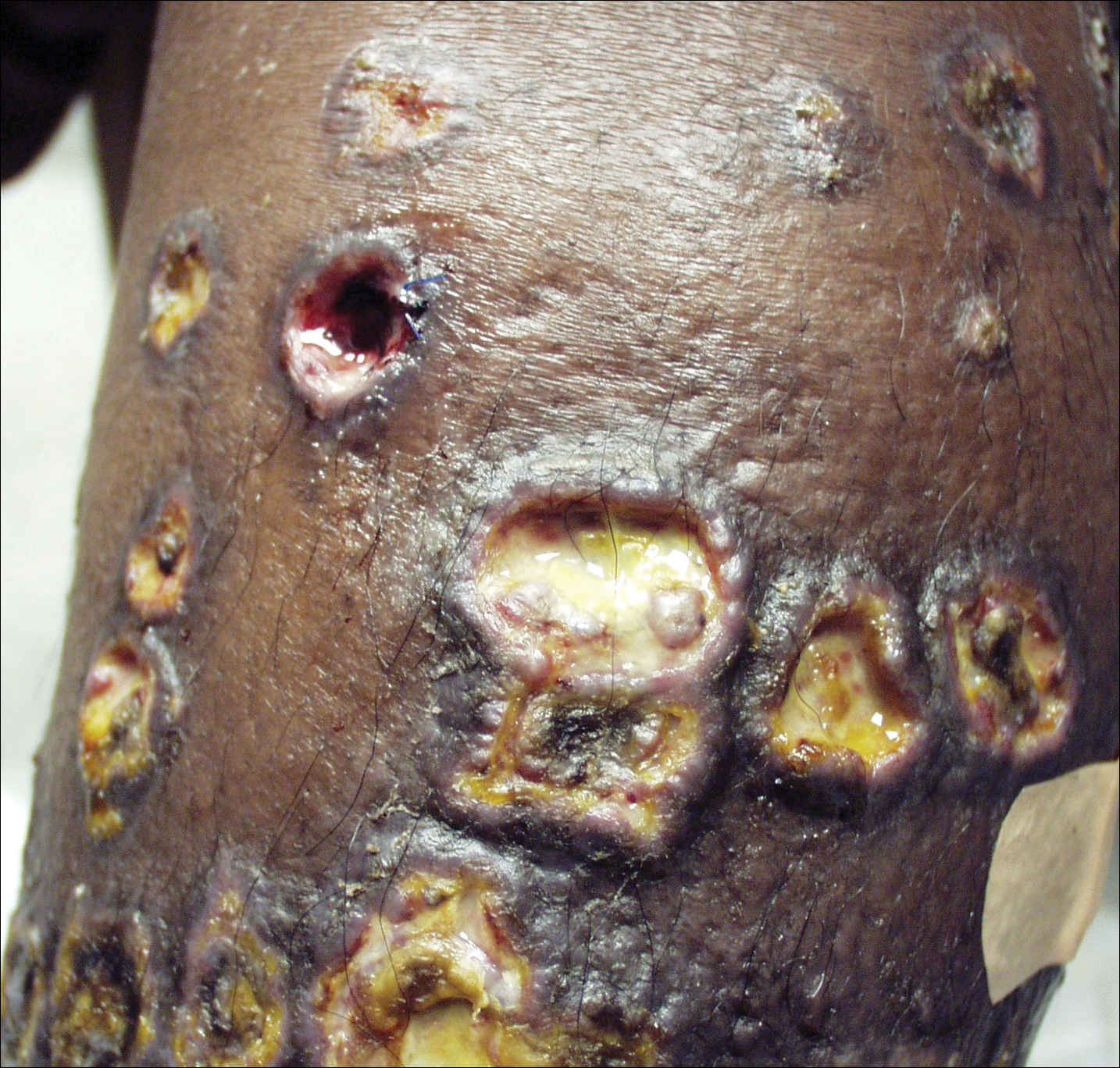
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).
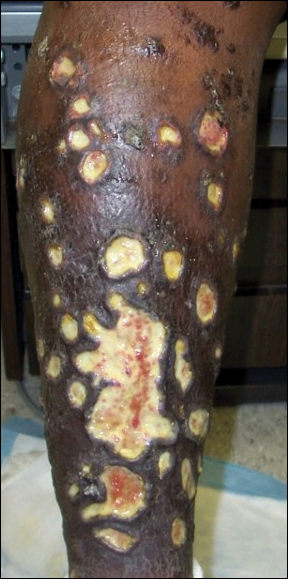
Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.
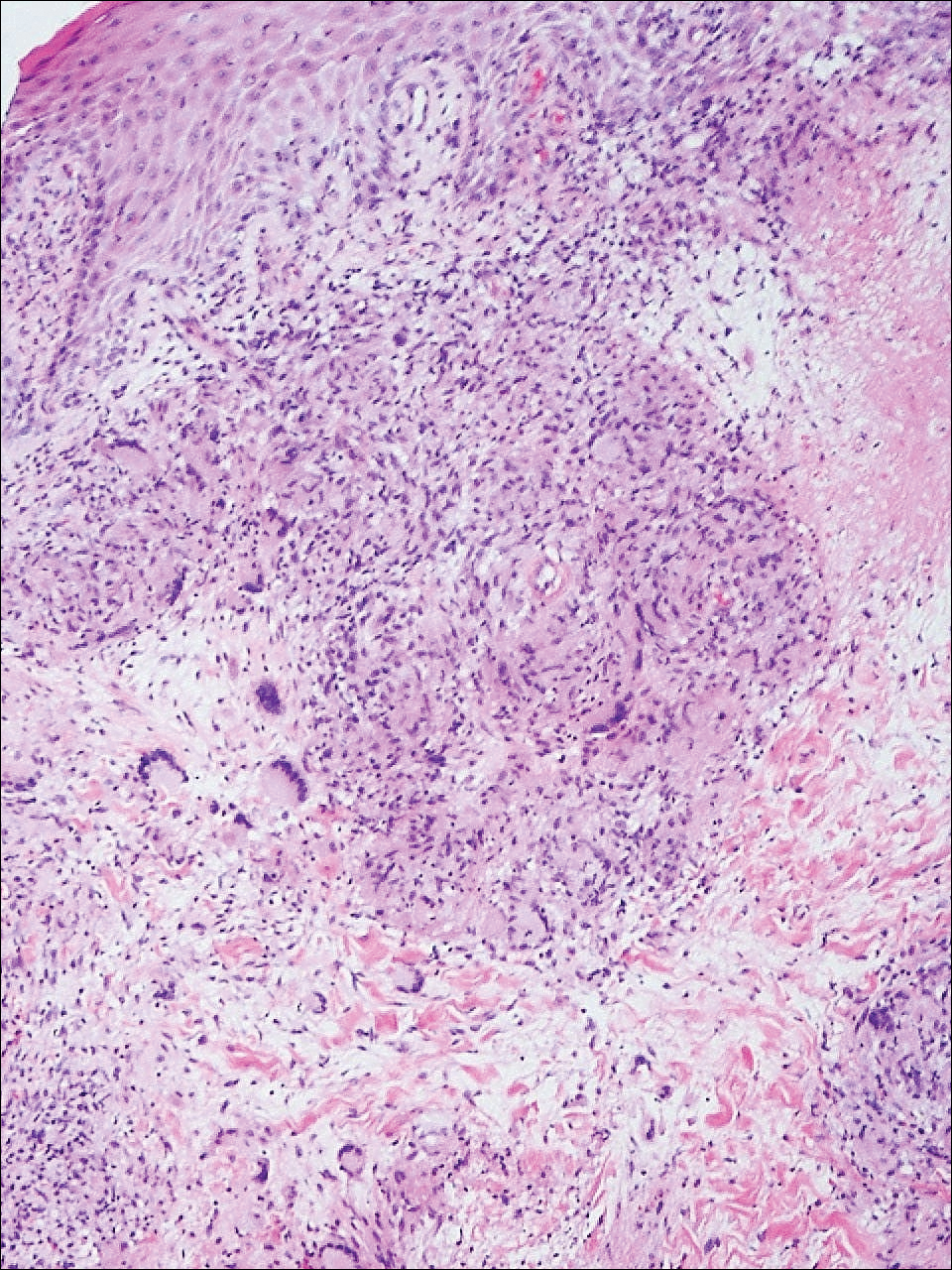
Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.
Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.
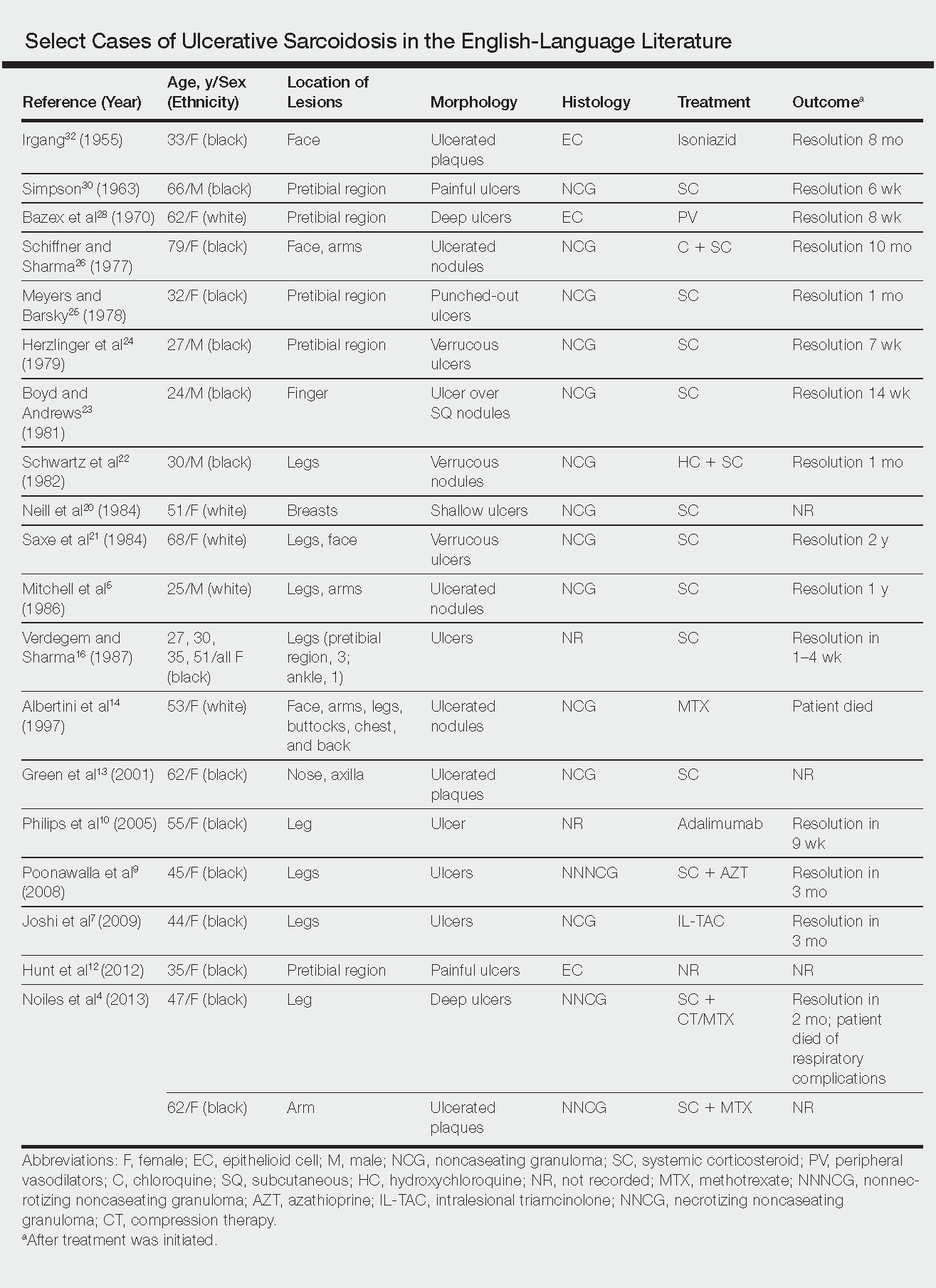
The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).

Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.

Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.

Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.

The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
Sarcoidosis is a multisystem granulomatous disorder of unknown etiology that primarily affects the lungs and lymphatic system but also may involve the skin, eyes, liver, spleen, muscles, bones, and nervous system.1 Cutaneous symptoms of sarcoidosis occur in approximately 25% of patients and are classified as specific and nonspecific, with specific lesions demonstrating noncaseating granuloma formation, which is typical of sarcoidosis.2 Nonspecific lesions primarily include erythema nodosum and calcinosis cutis. Specific lesions commonly present as reddish brown infiltrated plaques that may be annular, polycyclic, or serpiginous.1,3 They also may appear as yellowish brown or violaceous maculopapular lesions. However, specific lesions may present in a wide variety of morphologies, most often papules, nodules, subcutaneous infiltrates, and lupus pernio.4 Additionally, atypical cutaneous manifestations of sarcoidosis include erythroderma; scarring alopecia; nail dystrophy; and verrucous, ichthyosiform, psoriasiform, hypopigmented, or ulcerative skin lesions.3-5 Among these many potential clinical presentations, ulcerative sarcoidosis is quite uncommon.
We report a case of a patient who presented with classic clinical and histopathological findings of ulcerative sarcoidosis to highlight the prototypical presentation of a rare condition. We also review 34 additional cases of ulcerative sarcoidosis published in the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid.4-32 Analyzing this historical information, the scope of this unusual form of cutaneous sarcoidosis can be better understood, recognized, and treated. Although current standard-of-care treatments are most often successful, there is a paucity of definitive clinical trials to justify and verify comparative therapeutic efficacy.
Case Report
A 49-year-old black man with known pulmonary sarcoidosis, idiopathic (human immunodeficiency virus–negative) CD4 depletion syndrome, and chronic kidney disease presented with persistent bilateral ulcers of the legs of 1 month’s duration. The lesions first appeared as multiple “dark spots” on the legs. After the patient applied homemade aloe vera extract under occlusion for 1 to 2 days, the lesions became painful and began to ulcerate approximately 3 months prior to presentation. The patient applied a combination of a topical first aid antibiotic ointment, Epsom salts, and hydrogen peroxide without any improvement. A current review of systems was negative.
The patient’s medical history was notable for sarcoidosis diagnosed more than 10 years prior. During this time, he had intermittently been treated elsewhere with low-dose oral prednisone (5 mg once daily), hydroxychloroquine (200 mg twice daily), and an inhaled steroid as needed. He had a history of human immunodeficiency virus–negative, idiopathic CD4 depletion syndrome, which had been complicated by cryptococcal meningitis 7 years prior to presentation. He also had renal insufficiency, with baseline creatinine levels ranging from 1.4 to 1.7 mg/dL (reference range, 0.6–1.2 mg/dL). There was no personal or family history of known or suspected inflammatory bowel disease.
On physical examination, numerous discrete, coalescing, punched out–appearing ulcerations with foul-smelling, greenish yellow, purulent drainage were present bilaterally on the legs (Figure 1). The ulcers had a rolled border with a moderate amount of seemingly nonviable necrotic tissue. A number of hyperpigmented round papules, patches, and plaques also were present on the proximal legs. Laboratory evaluation revealed a CD4 count of 151 cc/mm3 (reference range, 500–1600 cc/mm3) and mildly elevated calcium of 10.7 mg/dL (reference range, 8.2–10.2 mg/dL).

Aerobic, anaerobic, mycobacterial, and fungal cultures of the purulent exudate were obtained. Given a high suspicion for secondary infection of the exogenous wound sites, doxycycline (100 mg twice daily) and topical mupir-ocin were initiated. Gram stain revealed few to moderate polymorphonuclear cells and many gram-positive cocci in pairs, chains, and clusters, along with many gram-negative rods. Bacterial culture grew Pseudomonas aeruginosa, Enterococcus species group G streptococci, and methicillin-resistant Staphylococcus aureus–positive staphylococci. Ciprofloxacin (500 mg twice daily) was then initiated, but the ulcers showed absolutely no clinical improvement and in fact worsened both in number and depth (Figure 2) over subsequent clinic visits during the next 3 months, even after amoxicillin (500 mg 3 times daily) was added. The patient was admitted for treatment with intravenous antibiotics after additional wound cultures revealed fluoroquinolone-resistant Pseudomonas.

Punch biopsies of the ulcers showed nonspecific acute inflammation and tissue necrosis in the active ulcers with nonnecrotizing granulomatous inflammation extending into the deep dermis, with many Langerhans-type giant cells present in the palpable ulcer borders (Figure 3). Neither birefringent particles nor asteroid bodies were observed. Tissue Gram stains did not reveal evidence of bacterial infection. Special stains for acid-fast and fungal organisms (ie, periodic acid–Schiff, Gomori methenamine-silver, Fite, acid-fast bacilli) were similarly negative. Tissue cultures obtained on deep biopsy revealed only rare colonies of P aeruginosa and no isolates on anaerobic, mycobacterial, or fungal cultures. Polymerase chain reaction for mycobacteria and common endemic fungi also was negative. In the absence of infection and considering his history of known sarcoidosis, these histologic features were consistent with ulcerative sarcoidosis. The patient was started on prednisone (60 mg once daily) and hydroxychloroquine (200 mg twice daily). The prednisone was tapered to 20 mg once daily over a 2-year period, at which point 90% of the ulcers had healed. He was continued on hydroxychloroquine at the initial dose, and at a 3-year follow-up his ulcers had healed completely without relapse.

Comment
Ulcerative sarcoidosis is rare, seen worldwide in only 5% of patients with cutaneous sarcoidosis.33 However, cases have been encountered worldwide, with reports emanating from Japan, China, Germany, France, and Russia, among others.6,34-55 We reviewed 34 cases from the English-language literature based on a PubMed search of articles indexed for MEDLINE using the term ulcerative sarcoid and examined patient demographics, clinical presentation, histological findings, treatment type, and outcome. Key references are presented in the Table. Disease prevalence previously has been estimated as being 3-times more common in women than men1; in our literature review, we found a female to male ratio of 3.25 to 1. Additionally, ulcerative sarcoidosis is reported to be twice as common in black versus white individuals.33 In our literature review, when race was reported, 66% (21/32) of patients were black. Disease prevalence has been reported to peak at 20 to 40 years of age.3 In this review, the average age of presentation was 45 years (age range, 24–79 years).
Ulceration may arise de novo but more commonly arises in preexisting scars or cutaneous lesions. There are 2 distinct patterns seen in ulcerative sarcoidosis.4 The first is characterized by ulceration within necrotic yellow plaques.2 The second pattern is characterized by violaceous nodules arising in an annular confluent pattern that eventually ulcerate.4 This presentation commonly mimics or may be mimicked by multiple disease states, including sporotrichosis, tuberculosis, stasis dermatitis with venous ulceration, and even metastatic breast cancer.7,46,55,56 Regardless of presentation, the legs are the most common location of ulcer formation.1,33 In our review, 85% (29/34) of cases presented with involvement of the legs, including our own case. Other locations of ulcer formation have included the face, arms, trunk, and genital area.
On histologic examination of ulcerative sarcoidosis, epithelioid granulomas composed of multinucleated giant cells, histiocytes, and scant numbers of lymphocytes are present.1,3 These formations are the noncaseating granulomas typical of sarcoidosis (Table). All of the cases in our review of the literature were described as either a collection of epithelioid granulomas with giant cell formation or noncaseating granulomas. There also have been reports of atypical features including necrotizing granulomas and granulomatous vasculitis.4,8,9,50 The histologic differential diagnosis in this case also would primarily include an infectious granulomatous process and less so an id reaction, rosacea, a paraneoplastic phenomenon, foreign body granulomas, and metastatic Crohn disease. The presence of ulceration, the large number of lesions, and the anatomic distribution help rule out most of these alternate diagnostic considerations. Diligent extensive workup was done in our patient to insure it was not an infection.

The goals of treatment include symptomatic relief, improvement in objective parameters of disease activity, and prevention of disease progression and subsequent disability.33,57 Fortunately, the majority of sarcoidosis patients with cutaneous symptoms achieve full recovery within months to years.33 Our literature review indicated that 81% (22/27) of patients with ulcerative lesions experienced full resolution within 1 year of treatment. Of those that did not (19% [5/27]), the patients were either lost to follow-up or died from other complications of sarcoidosis.
The widely accepted standard therapy for cutaneous sarcoidosis includes topical, intralesional, and systemic corticosteroids; antimalarials; and methotrexate.33,57 Steroids and methotrexate act by suppressing granuloma formation, while antimalarials prevent antigen presentation (presumably part of the pathogenesis).33 For mild to moderate disease, topical and intralesional steroids may be all that is necessary.33,57 Systemic steroids are used for disfiguring, destructive, and widespread lesions that have been refractory to local and other systemic therapies.33,57 Steroids are tapered gradually depending on the patient’s response, as it is common for patients to relapse below a certain dose.33,57 Antimalarials (chloroquine or hydroxychloroquine) and methotrexate are considered adjunct treatments for patients who are either steroid unresponsive or who are unable to tolerate corticosteroid treatment due to adverse events.33,57
Standard therapy is complicated by the side effects of treatment. Use of corticosteroids may lead to gastrointestinal tract upset, increased appetite, mood disturbances, impaired wound healing, hyperglycemia, hypertension, cushingoid features, and acne.57 Antimalarials can cause nausea, anorexia, and agranulocytosis, and chloroquine therapy in particular can lead to blurred vision, corneal deposits, and central retinopathy.33,57 Methotrexate is associated with hematologic, gastrointestinal tract, pulmonary, and hepatic toxicities well known to most practitioners.
Because of the variable clinical response of patients to standard therapy and their associated toxicities, other treatment options have been used including pentoxifylline, tetracyclines, isotretinoin, leflunomide, thalidomide, infliximab, adalimumab, allopurinol, and the pulsed dye or CO2 laser.10,33,57 In nonhealing ulcers, split-thickness grafting and a bilayered bioengineered skin substitute have been used with good results in conjunction with ongoing systemic therapy.11,47 Additionally, nanoparticle silver burn paste has been used successfully, with resolution of ulcers within 2 weeks in the Chinese literature.53
All of these treatment recommendations are based on historically accepted modalities. Controlled trials with longitudinal follow-up are needed to provide justification for the current standard of care.34
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
- Howard A, White CR Jr. Non-infectious granulomas. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Spain: Elsevier; 2008:1421-1435.
- Doherty CB, Rosen T. Evidence-based therapy for cutaneous sarcoidosis. Drugs. 2008;68:1361-1383.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Noiles K, Beleznay K, Crawford RI, et al. Sarcoidosis can present with necrotizing granulomas histologically: two cases of ulcerated sarcoidosis and review of the literature. J Cutan Med Surg. 2013;17:377-378.
- Mitchell IC, Sweatman MC, Rustin MH, et al. Ulcerative and hypopigmented sarcoidosis. J Am Acad Dermatol. 1986;15:1062-1065.
- Yoo SS, Mimouni D, Nikolskaia OV, et al. Clinicopathologic features of ulcerative-atrophic sarcoidosis. Int J Dermatol. 2004;43:108-112.
- Joshi SS, Romanelli R, Kirsner RS. Sarcoidosis mimicking a venous ulcer: a case report. Ostomy Wound Manage. 2009;55:46-48.
- Petri M, Barr E, Cho K, et al. Overlap of granulomatous vasculitis and sarcoidosis: presentation with uveitis, eosinophilia, leg ulcers, sinusitis and past foot drop. J Rheumatol. 1988;15:1171-1173.
- Poonawalla T, Colome-Grimmer MI, Kelly B. Ulcerative sarcoidosis in the legs with granulomatous vasculitis. Clin Exp Dermatol. 2008;33:282-286.
- Philips MA, Lynch J, Azmi FH. Ulcerative sarcoidosis responding to adalimumab. J Am Acad Dermatol. 2005;53:917.
- Collison DW, Novice F, Banse L, et al. Split-thickness skin grafting in extensive ulcerative sarcoidosis. J Dermatol Surg Oncol. 1989;15:679-683.
- Hunt RD, Gonzalez ME, Robinson M, et al. Ulcerative sarcoidosis. Dermatol Online J. 2012;18:29.
- Green JJ, Lawrence N, Heymann WR. Generalized ulcerative sarcoidosis induced by therapy with the flashlamp-pumped pulsed dye. Arch Dermatol. 2001;137:507-508.
- Albertini JG, Tyler W, Miller OF. Ulcerative sarcoidosis. case report and review of the literature. Arch Dermatol. 1997;133:215-219.
- Thomas J, Williams DW. Peritoneal involvement and ulcerative skin plaques in sarcoidosis: a case report. Sarcoidosis. 1989;6:161-162.
- Verdegem TD, Sharma OP. Cutaneous ulcers in sarcoidosis. Arch Dermatol. 1987;123:1531-1534.
- Gupta AK, Haberman HF, From GL, et al. Sarcoidosis with extensive cutaneous ulceration. unusual clinical presentation. Dermatologica. 1987;174:135-139.
- Hruza GJ, Kerdel FA. Generalized atrophic sarcoidosis with ulcerations. Arch Dermatol. 1986;122:320-322.
- Muhlemann MF, Walker NP, Tan LB, et al. Elephantine sarcoidosis presenting as ulcerating lymphoedema. J R Soc Med. 1985;78:260-261.
- Neill SM, Smith NP, Eady RA. Ulcerative sarcoidosis: a rare manifestation of a common disease. Clin Exp Dermatol. 1984;9:277-279.
- Saxe N, Benatar SR, Bok L, et al. Sarcoidosis with leg ulcers and annular facial lesions. Arch Dermatol. 1984;120:93-96.
- Schwartz RA, Robertson DB, Tierney LM, et al. Generalized ulcerative sarcoidosis. Arch Dermatol. 1982;118:931-933.
- Boyd RE, Andrews BS. Sarcoidosis presenting as cutaneous ulceration, subcutaneous nodules and chronic arthritis. J Rheumatol. 1981;8:311-316.
- Herzlinger DC, Marland AM, Barr RJ. Verrucous ulcerative skin lesions in sarcoidosis. an unusual clinical presentation. Cutis. 1979;23:569-572.
- Meyers M, Barsky S. Ulcerative sarcoidosis. Arch Dermatol. 1978;114:447.
- Schiffner J, Sharma OP. Ulcerative sarcoidosis. report of an unusual case. Arch Dermatol. 1977;113:676-677.
- Williamson DM. Sarcoidosis with atrophic lesions and ulcers of the legs. Br J Dermatol. 1971;84:92-93.
- Bazex A, Dupre A, Christol B, et al. Sarcoidosis with atrophic lesions and ulcers and the presence in some sarcoid granulomata of orceinophil fibres. Br J Dermatol. 1970;83:255-262.
- Brodkin RH. Leg ulcers. a report of two cases caused by sarcoidosis. Acta Derm Venereol. 1969;49:584-587.
- Simpson JR. Sarcoidosis with erythrodermia and ulceration. Br J Dermatol. 1963;75:193-198.
- Irgang S. Ulcerative cutaneous lesion in sarcoidosis; report of a case with clinical resemblance to lupus vulgaris. Harlem Hosp Bull. 1956;8:134-139.
- Irgang S. Ulcerative cutaneous lesions in sarcoidosis; report of a case with clinical resemblance to papulonecrotic tuberculide. Br J Dermatol. 1955;67:255-260.
- Hoffman MD. Atypical ulcers. Dermatol Ther. 2013;26:222-235.
- Hopf B, Krebs A. Ulcera cruris as a rare manifestation of sarcoidosis. Dermatologica. 1974;113:55-62.
- Metz J, Hartmann A, Hautkr Z. Ulcerative form of skin sarcoidosis. Z Hautkr. 1977;52:890-896.
- Berenbeĭn BA, Malygina LA, Tiutiunnikova IA. Ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1984;4:50-53.
- Takahashi N, Hoshino M, Takase T, et al. A case of ulcerative sarcoidosis [in Japanese]. Nihon Hifuka Gakkai Zasshi. 1985;95:1049-1054.
- Schamroth JM. Sarcoidosis with severe extensive skin ulceration. Int J Dermatol. 1985;24:451-452.
- Porteau L, Dromer C, Le Guennec P, et al. Ulcer lesions in sarcoidosis: apropos of a case [in French]. Ann Med Interne (Paris). 1997;148:105-106.
- de La Blanchardière A, Bachmeyer C, Toutous L, et al. Cutaneous ulcerations in sarcoidosis [in French]. Rev Med Interne. 1995;16:927-929.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol. 1992;31:339-340.
- Rodionov AN, Samtsov AV. The ulcerative form of skin sarcoidosis [in Russian]. Vestn Dermatol Venerol. 1990;7:68-71.
- Jacyk WK. Cutaneous sarcoidosis in black South Africans. Int J Dermatol. 1999;38:841-845.
- Gungor E, Artuz F, Alli N, et al. Ulcerative sarcoidosis. J Eur Acad Dermatol Venereol. 1999;12:78-79.
- Schleinitz N, Luc M, Genot S, et al. Ulcerative cutaneous lesions: a rare manifestation of sarcoidosis [in French]. Rev Med Interne. 2005;26:758-759.
- Klocker J, Duckers J, Morse R, et al. Ulcerative cutaneous sarcoidosis masquerading as metastatic carcinoma of the breast. Age Ageing. 2002;31:77-79.
- Streit M, Bohlen LM, Braathen LR. Ulcerative sarcoidosis successfully treated with apligraf. Dermatology. 2001;202:367-370.
- Ichiki Y, Kitajima Y. Ulcerative sarcoidosis: case report and review of the Japanese literature. Acta Derm Venereol. 2008;88:526-528
- Meyersburg D, Schön MP, Bertsch HP, et al. Uncommon cutaneous ulcerative and systemic sarcoidosis. successful treatment with hydroxychloroquine and compression therapy [in German]. Hautarzt. 2011;62:691-695.
- Wei CH, Huang YH, Shih YC, et al. Sarcoidosis with cutaneous granulomatous vasculitis. Australas J Dermatol. 2010;51:198-201.
- Kluger N, Girard C, Durand L, et al. Leg ulcers revealing systemic sarcoidosis with splenomegaly and thrombocytopenia. Int J Dermatol. 2013;52:1425-1427.
- Jun L, Jia-Wei L, Hong-Zhong J. Ulcerative sarcoidosis. Int J Dermatol. 2014;53:E315-E316.
- Chen JH, Wang TT, Lin ZQ. Successful application of a novel dressing for the treatment of ulcerative cutaneous sarcoidosis. Chin Med J. 2013;126:3400.
- Ri G, Yoshikawa E, Shigekiyo T, et al. Takayasu artertitis and ulcerative sarcoidosis. Intern Med. 2015;54:1075-1080.
- Spiliopoulou I, Foka A, Bounas A, et al. Mycobacterium kansasii cutaneous infection in a patient with sarcoidosis treated with anti-TNF agents. Acta Clin Belg. 2014;69:229-231.
- Yang DJ, Krishnan RS, Guillen DR, et al. Disseminated sporotrichosis mimicking sarcoidosis. Int J Dermatol. 2006;45:450-453.
- Badgwell C, Rosen T. Cutaneous sarcoidosis therapy updated. J Am Acad Dermatol. 2007;56:69-83.
Practice Points
- Sarcoidosis can present as a primary ulcerative disease.
- Suspect ulcerative sarcoidosis when ulcerations are seen on the leg.
- Systemic corticosteroids may be the most effective treatment of ulcerative sarcoidosis.
Syphilis and the Dermatologist
Once upon a time, and long ago, dermatology journals included “syphilology” in their names. The first dermatologic journal published in the United States was the American Journal of Syphilology and Dermatology.1 In October 1882 the Journal of Cutaneous and Venereal Diseases appeared and subsequently renamed several times from 1882 to 1919: Journal of Cutaneous Diseases and Genitourinary Diseases and the Journal of Cutaneous Diseases, Including Syphilis. When the American Medical Association (AMA) assumed control, this publication obtained a new name: Archives of Dermatology and Syphilology; in January 1955 syphilology was deleted from the title. According to an editorial in that issue, the rationale for dropping the word syphilology was as follows: “The diagnosis and treatment of patients with syphilis is no longer an important part of dermatologic practice. . . . Few dermatologists now have patients with syphilis; in fact, there are decidedly fewer patients with syphilis, and so continuance of the old label, ‘Syphilology,’ on this publication seems no longer warranted.”1 Needless to say, this decision ignored the obvious fact that the majority of dermatologists traditionally were well trained in and clinically practiced venereology, particularly the management of syphilis,2,3 which makes sense, considering that many of the clinical manifestations of syphilis involve the skin, hair, and oral mucosa. My own mentor and former Baylor College of Medicine dermatology department chair, Dr. John Knox, authored 3 dozen major publications regarding the diagnosis, treatment, and immunology of syphilis. During his chairmanship, all residents were required to rotate in the Harris County sexually transmitted disease (STD) clinic on a weekly basis.
I am confident that the decision to drop “syphilology” from the journal title also was based on the unduly optimistic assumption that syphilis would soon become a rare disease due to the availability of penicillin. Indeed, the Centers for Disease Control and Prevention in the United States has periodically announced strategic programs designed to eradicate syphilis!4 This rosy outlook reached a fever pitch in 2000 when the number of cases (5979) and the incidence (2.1 cases per 100,000 population) of primary and secondary syphilis reached an all-time low in the United States.5
Unfortunately, no one could accurately predict the future. Although the number of cases and incidence of early infectious syphilis have fluctuated widely since the 1940s, we currently are in a dire period of syphilis resurgence; the largest number of cases (27,814) and the highest incidence rate of primary and secondary syphilis (8.7 cases per 100,000 population) since 1994 were reported in 2016,6 which illustrates the inability of public health initiatives to eliminate syphilis, largely due to the inability of health authorities, health care providers, teachers, parents, clergy, and peer groups to alter sexual behaviors or modify other socioeconomic factors.7 Thus, syphilis lives on! Nobody could have predicted the easy availability of oral contraceptives and the ensuing sexual revolution of the 1960s or the advent of erectile dysfunction drugs decades later that led to increasing STDs among older patients.8 Nobody could have predicted the wholesale acceptance of casual sexual intercourse as popularized on television and in the movies or the pervasive use of sexual images in advertising. Nobody could have predicted the modern phenomena of “booty-call relationships,” “friends with benefits,” and “sexting,” or the nearly ubiquitous and increasingly legal use of noninjectable mind-altering drugs, all of which facilitate the perpetuation of STDs.9-11 Finally, those who removed “syphilology” from that journal title certainly did not foresee the worldwide epidemic now known as human immunodeficiency virus/AIDS, which has most assuredly helped keep syphilis a modern day menace.12-14
How have dermatologists been impacted? Our journals and our teachers have deemphasized STDs, including syphilis, in modern times, yet we are faced with a disease carrying serious, if not often fatal, consequences that is simply refusing to disappear (contrary to wishful thinking). Dermatologists are, however, in a perfect epidemiological position to help in the war against Treponema pallidum, the bacterium that causes syphilis. We frequently see adolescent patients for warts and acne, and we often diagnose and help care for patients with human immunodeficiency virus. We obliterate actinic keratoses and perform cosmetic procedures on those who rely on erectile dysfunction drugs (or their partners do). Who better than a dermatologist to recognize in these high-risk constituencies, and others, that patchy hair loss may represent syphilitic alopecia and that extragenital chancres can mimic nonmelanoma skin cancer? Who better than the dermatologist to distinguish between oral mucous patches and orolabial herpes? Who better than the dermatologist to diagnose the annular syphilid of the face, or ostraceous, florid nodular, or ulceronecrotic lesions of lues maligna? Who better than the dermatologist to differentiate condylomata lata from external genital warts?
I would suggest that the responsible dermatologist become reacquainted with syphilis, in all its various manifestations. I would further suggest that our dermatology training centers spend more time diligently teaching residents about syphilis and other STDs. In conclusion, I fervently hope that organized dermatology will once again dutifully consider venereal disease to be a critical part of our specialty’s skill set.
- Editorial. AMA Arch Dermatol. 1955;71:1.
- Shelley WB. Major contributors to American dermatology—1876 to 1926. Arch Dermatol. 1976;112:1642-1646.
- Lobitz WC Jr. Major contributions of American dermatologists—1926 to 1976. Arch Dermatol. 1976;112:1646-1650.
- Hook EW 3rd. Elimination of syphilis transmission in the United States: historic perspectives and practical considerations. Trans Am ClinClimatol Assoc. 1999;110:195-203.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2000. Atlanta, GA: Department of Health and Human Services; 2001.
- 2016 Sexually transmitted diseases surveillance: syphilis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/stats16/syphilis.htm. Updated September 26, 2017. Accessed October 20, 2017.
- Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clin Dermatol. 2014;32:213-218.
- Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: sexual but more emotional than one-night stands. J Sex Res. 2011;48:486-495.
- Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134:E1287-E1292.
- Regan R, Dyer TP, Gooding T, et al. Associations between drug use and sexual risks among heterosexual men in the Philippines [published online July 22, 2013]. Int J STD AIDS. 2013;24:969-976.
- Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42:171-179.
- Shilaih M, Marzel A, Braun DL, et al; Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96:E5849.
- Salado-Rasmussen K. Syphilis and HIV co-infection. epidemiology, treatment and molecular typing of Treponema pallidum. Dan Med J. 2015;62:B5176.
Once upon a time, and long ago, dermatology journals included “syphilology” in their names. The first dermatologic journal published in the United States was the American Journal of Syphilology and Dermatology.1 In October 1882 the Journal of Cutaneous and Venereal Diseases appeared and subsequently renamed several times from 1882 to 1919: Journal of Cutaneous Diseases and Genitourinary Diseases and the Journal of Cutaneous Diseases, Including Syphilis. When the American Medical Association (AMA) assumed control, this publication obtained a new name: Archives of Dermatology and Syphilology; in January 1955 syphilology was deleted from the title. According to an editorial in that issue, the rationale for dropping the word syphilology was as follows: “The diagnosis and treatment of patients with syphilis is no longer an important part of dermatologic practice. . . . Few dermatologists now have patients with syphilis; in fact, there are decidedly fewer patients with syphilis, and so continuance of the old label, ‘Syphilology,’ on this publication seems no longer warranted.”1 Needless to say, this decision ignored the obvious fact that the majority of dermatologists traditionally were well trained in and clinically practiced venereology, particularly the management of syphilis,2,3 which makes sense, considering that many of the clinical manifestations of syphilis involve the skin, hair, and oral mucosa. My own mentor and former Baylor College of Medicine dermatology department chair, Dr. John Knox, authored 3 dozen major publications regarding the diagnosis, treatment, and immunology of syphilis. During his chairmanship, all residents were required to rotate in the Harris County sexually transmitted disease (STD) clinic on a weekly basis.
I am confident that the decision to drop “syphilology” from the journal title also was based on the unduly optimistic assumption that syphilis would soon become a rare disease due to the availability of penicillin. Indeed, the Centers for Disease Control and Prevention in the United States has periodically announced strategic programs designed to eradicate syphilis!4 This rosy outlook reached a fever pitch in 2000 when the number of cases (5979) and the incidence (2.1 cases per 100,000 population) of primary and secondary syphilis reached an all-time low in the United States.5
Unfortunately, no one could accurately predict the future. Although the number of cases and incidence of early infectious syphilis have fluctuated widely since the 1940s, we currently are in a dire period of syphilis resurgence; the largest number of cases (27,814) and the highest incidence rate of primary and secondary syphilis (8.7 cases per 100,000 population) since 1994 were reported in 2016,6 which illustrates the inability of public health initiatives to eliminate syphilis, largely due to the inability of health authorities, health care providers, teachers, parents, clergy, and peer groups to alter sexual behaviors or modify other socioeconomic factors.7 Thus, syphilis lives on! Nobody could have predicted the easy availability of oral contraceptives and the ensuing sexual revolution of the 1960s or the advent of erectile dysfunction drugs decades later that led to increasing STDs among older patients.8 Nobody could have predicted the wholesale acceptance of casual sexual intercourse as popularized on television and in the movies or the pervasive use of sexual images in advertising. Nobody could have predicted the modern phenomena of “booty-call relationships,” “friends with benefits,” and “sexting,” or the nearly ubiquitous and increasingly legal use of noninjectable mind-altering drugs, all of which facilitate the perpetuation of STDs.9-11 Finally, those who removed “syphilology” from that journal title certainly did not foresee the worldwide epidemic now known as human immunodeficiency virus/AIDS, which has most assuredly helped keep syphilis a modern day menace.12-14
How have dermatologists been impacted? Our journals and our teachers have deemphasized STDs, including syphilis, in modern times, yet we are faced with a disease carrying serious, if not often fatal, consequences that is simply refusing to disappear (contrary to wishful thinking). Dermatologists are, however, in a perfect epidemiological position to help in the war against Treponema pallidum, the bacterium that causes syphilis. We frequently see adolescent patients for warts and acne, and we often diagnose and help care for patients with human immunodeficiency virus. We obliterate actinic keratoses and perform cosmetic procedures on those who rely on erectile dysfunction drugs (or their partners do). Who better than a dermatologist to recognize in these high-risk constituencies, and others, that patchy hair loss may represent syphilitic alopecia and that extragenital chancres can mimic nonmelanoma skin cancer? Who better than the dermatologist to distinguish between oral mucous patches and orolabial herpes? Who better than the dermatologist to diagnose the annular syphilid of the face, or ostraceous, florid nodular, or ulceronecrotic lesions of lues maligna? Who better than the dermatologist to differentiate condylomata lata from external genital warts?
I would suggest that the responsible dermatologist become reacquainted with syphilis, in all its various manifestations. I would further suggest that our dermatology training centers spend more time diligently teaching residents about syphilis and other STDs. In conclusion, I fervently hope that organized dermatology will once again dutifully consider venereal disease to be a critical part of our specialty’s skill set.
Once upon a time, and long ago, dermatology journals included “syphilology” in their names. The first dermatologic journal published in the United States was the American Journal of Syphilology and Dermatology.1 In October 1882 the Journal of Cutaneous and Venereal Diseases appeared and subsequently renamed several times from 1882 to 1919: Journal of Cutaneous Diseases and Genitourinary Diseases and the Journal of Cutaneous Diseases, Including Syphilis. When the American Medical Association (AMA) assumed control, this publication obtained a new name: Archives of Dermatology and Syphilology; in January 1955 syphilology was deleted from the title. According to an editorial in that issue, the rationale for dropping the word syphilology was as follows: “The diagnosis and treatment of patients with syphilis is no longer an important part of dermatologic practice. . . . Few dermatologists now have patients with syphilis; in fact, there are decidedly fewer patients with syphilis, and so continuance of the old label, ‘Syphilology,’ on this publication seems no longer warranted.”1 Needless to say, this decision ignored the obvious fact that the majority of dermatologists traditionally were well trained in and clinically practiced venereology, particularly the management of syphilis,2,3 which makes sense, considering that many of the clinical manifestations of syphilis involve the skin, hair, and oral mucosa. My own mentor and former Baylor College of Medicine dermatology department chair, Dr. John Knox, authored 3 dozen major publications regarding the diagnosis, treatment, and immunology of syphilis. During his chairmanship, all residents were required to rotate in the Harris County sexually transmitted disease (STD) clinic on a weekly basis.
I am confident that the decision to drop “syphilology” from the journal title also was based on the unduly optimistic assumption that syphilis would soon become a rare disease due to the availability of penicillin. Indeed, the Centers for Disease Control and Prevention in the United States has periodically announced strategic programs designed to eradicate syphilis!4 This rosy outlook reached a fever pitch in 2000 when the number of cases (5979) and the incidence (2.1 cases per 100,000 population) of primary and secondary syphilis reached an all-time low in the United States.5
Unfortunately, no one could accurately predict the future. Although the number of cases and incidence of early infectious syphilis have fluctuated widely since the 1940s, we currently are in a dire period of syphilis resurgence; the largest number of cases (27,814) and the highest incidence rate of primary and secondary syphilis (8.7 cases per 100,000 population) since 1994 were reported in 2016,6 which illustrates the inability of public health initiatives to eliminate syphilis, largely due to the inability of health authorities, health care providers, teachers, parents, clergy, and peer groups to alter sexual behaviors or modify other socioeconomic factors.7 Thus, syphilis lives on! Nobody could have predicted the easy availability of oral contraceptives and the ensuing sexual revolution of the 1960s or the advent of erectile dysfunction drugs decades later that led to increasing STDs among older patients.8 Nobody could have predicted the wholesale acceptance of casual sexual intercourse as popularized on television and in the movies or the pervasive use of sexual images in advertising. Nobody could have predicted the modern phenomena of “booty-call relationships,” “friends with benefits,” and “sexting,” or the nearly ubiquitous and increasingly legal use of noninjectable mind-altering drugs, all of which facilitate the perpetuation of STDs.9-11 Finally, those who removed “syphilology” from that journal title certainly did not foresee the worldwide epidemic now known as human immunodeficiency virus/AIDS, which has most assuredly helped keep syphilis a modern day menace.12-14
How have dermatologists been impacted? Our journals and our teachers have deemphasized STDs, including syphilis, in modern times, yet we are faced with a disease carrying serious, if not often fatal, consequences that is simply refusing to disappear (contrary to wishful thinking). Dermatologists are, however, in a perfect epidemiological position to help in the war against Treponema pallidum, the bacterium that causes syphilis. We frequently see adolescent patients for warts and acne, and we often diagnose and help care for patients with human immunodeficiency virus. We obliterate actinic keratoses and perform cosmetic procedures on those who rely on erectile dysfunction drugs (or their partners do). Who better than a dermatologist to recognize in these high-risk constituencies, and others, that patchy hair loss may represent syphilitic alopecia and that extragenital chancres can mimic nonmelanoma skin cancer? Who better than the dermatologist to distinguish between oral mucous patches and orolabial herpes? Who better than the dermatologist to diagnose the annular syphilid of the face, or ostraceous, florid nodular, or ulceronecrotic lesions of lues maligna? Who better than the dermatologist to differentiate condylomata lata from external genital warts?
I would suggest that the responsible dermatologist become reacquainted with syphilis, in all its various manifestations. I would further suggest that our dermatology training centers spend more time diligently teaching residents about syphilis and other STDs. In conclusion, I fervently hope that organized dermatology will once again dutifully consider venereal disease to be a critical part of our specialty’s skill set.
- Editorial. AMA Arch Dermatol. 1955;71:1.
- Shelley WB. Major contributors to American dermatology—1876 to 1926. Arch Dermatol. 1976;112:1642-1646.
- Lobitz WC Jr. Major contributions of American dermatologists—1926 to 1976. Arch Dermatol. 1976;112:1646-1650.
- Hook EW 3rd. Elimination of syphilis transmission in the United States: historic perspectives and practical considerations. Trans Am ClinClimatol Assoc. 1999;110:195-203.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2000. Atlanta, GA: Department of Health and Human Services; 2001.
- 2016 Sexually transmitted diseases surveillance: syphilis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/stats16/syphilis.htm. Updated September 26, 2017. Accessed October 20, 2017.
- Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clin Dermatol. 2014;32:213-218.
- Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: sexual but more emotional than one-night stands. J Sex Res. 2011;48:486-495.
- Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134:E1287-E1292.
- Regan R, Dyer TP, Gooding T, et al. Associations between drug use and sexual risks among heterosexual men in the Philippines [published online July 22, 2013]. Int J STD AIDS. 2013;24:969-976.
- Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42:171-179.
- Shilaih M, Marzel A, Braun DL, et al; Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96:E5849.
- Salado-Rasmussen K. Syphilis and HIV co-infection. epidemiology, treatment and molecular typing of Treponema pallidum. Dan Med J. 2015;62:B5176.
- Editorial. AMA Arch Dermatol. 1955;71:1.
- Shelley WB. Major contributors to American dermatology—1876 to 1926. Arch Dermatol. 1976;112:1642-1646.
- Lobitz WC Jr. Major contributions of American dermatologists—1926 to 1976. Arch Dermatol. 1976;112:1646-1650.
- Hook EW 3rd. Elimination of syphilis transmission in the United States: historic perspectives and practical considerations. Trans Am ClinClimatol Assoc. 1999;110:195-203.
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2000. Atlanta, GA: Department of Health and Human Services; 2001.
- 2016 Sexually transmitted diseases surveillance: syphilis. Centers for Disease Control and Prevention website. https://www.cdc.gov/std/stats16/syphilis.htm. Updated September 26, 2017. Accessed October 20, 2017.
- Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clin Dermatol. 2014;32:213-218.
- Jena AB, Goldman DP, Kamdar A, et al. Sexually transmitted diseases among users of erectile dysfunction drugs: analysis of claims data. Ann Intern Med. 2010;153:1-7.
- Jonason PK, Li NP, Richardson J. Positioning the booty-call relationship on the spectrum of relationships: sexual but more emotional than one-night stands. J Sex Res. 2011;48:486-495.
- Temple JR, Choi H. Longitudinal association between teen sexting and sexual behavior. Pediatrics. 2014;134:E1287-E1292.
- Regan R, Dyer TP, Gooding T, et al. Associations between drug use and sexual risks among heterosexual men in the Philippines [published online July 22, 2013]. Int J STD AIDS. 2013;24:969-976.
- Flagg EW, Weinstock HS, Frazier EL, et al. Bacterial sexually transmitted infections among HIV-infected patients in the United States: estimates from the Medical Monitoring Project. Sex Transm Dis. 2015;42:171-179.
- Shilaih M, Marzel A, Braun DL, et al; Swiss HIV Cohort Study. Factors associated with syphilis incidence in the HIV-infected in the era of highly active antiretrovirals. Medicine (Baltimore). 2017;96:E5849.
- Salado-Rasmussen K. Syphilis and HIV co-infection. epidemiology, treatment and molecular typing of Treponema pallidum. Dan Med J. 2015;62:B5176.
Innovative Pearls for Therapeutic Success: Report From the AAD Meeting
At the Summer Meeting of the American Academy of Dermatology, Dr. Ted Rosen provides therapeutic pearls on vitamin D for chronic idiopathic urticaria and the quadrivalent human papillomavirus vaccine as a treatment of chronic refractory common warts. Here he reviews anecdotes about successes with both and recommended amounts of vitamin D.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the Summer Meeting of the American Academy of Dermatology, Dr. Ted Rosen provides therapeutic pearls on vitamin D for chronic idiopathic urticaria and the quadrivalent human papillomavirus vaccine as a treatment of chronic refractory common warts. Here he reviews anecdotes about successes with both and recommended amounts of vitamin D.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
At the Summer Meeting of the American Academy of Dermatology, Dr. Ted Rosen provides therapeutic pearls on vitamin D for chronic idiopathic urticaria and the quadrivalent human papillomavirus vaccine as a treatment of chronic refractory common warts. Here he reviews anecdotes about successes with both and recommended amounts of vitamin D.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Judicious Use of Antibiotics in Dermatology
What does your patient need to know at the first visit? Does it apply to patients of all genders, ages, and races?
There are 3 scenarios in which antibiotics are used in dermatology. First, there is the treatment of a bona fide, verified skin infection, which may range from the relatively simple (impetigo) to the complex (botryomycosis) to the exotic (fish tank granuloma). The second scenario is antibiotic administration, often due to ancillary properties such as anti-inflammatory effects, in the management of noninfectious disorders, such as familial benign pemphigus or pityriasis lichenoides et varioliformis acuta. I try hard to avoid antibiotic use in these situations unless all else fails. The third scenario involves use of antibiotics at the patient’s request, usually associated with the phrase “just in case it’s infected.” In my opinion, this practice is completely ill advised.
Male and female patients of all ages, ethnic origins, and socioeconomic backgrounds are woefully uninformed regarding the promise and peril of antibiotics. I want patients to buy into the concept of good antibiotic stewardship. Thus, patients should understand that there must be a specific and justifiable reason for antibiotic use and that the recommended dose and duration of treatment should not be altered. In some situations, antibiotic therapy is intended to be of short duration, while in other situations, such therapy may be quite protracted. Patients also need to know at the outset of treatment when we plan to transition from a short-term, antibiotic-based modality to a long-term nonantibiotic maintenance regimen, which is especially true for acne and rosacea. I try to limit antibiotic use in these disorders to 3 months. Furthermore, patients should always be educated about the potential side effects associated with the particular antibiotic being prescribed. Hoarding and sharing leftover antibiotics should be strongly and explicitly discouraged.
Finally, patients must be educated that taking shortcuts when prescribing antibiotics may lead to therapeutic failure, worsening disease, or serious long-term adverse consequences. For example, rational antibiotic use may require the added expense of an initial and/or subsequent test-of-cure culture and sensitivity. Is that swollen and tender hand following a cat bite due to Pasteurella multocida or methicillin-resistant Staphylococcus aureus? Is that new eruption in an atopic patient due to secondary impetigo or eczema herpeticum? Other laboratory testing also may be required, such as a follow-up serology after treating syphilis. Patients need to know why laboratory tests are being ordered and how the tests complement direct antibiotic intervention.
What are your go-to treatments? What are the side effects?
I am a fan of subantimicrobial-dose doxycycline for both rosacea (on label) and acne (off label). Studies have shown that neither quantitative nor qualitative changes occur in the cutaneous, oral, or gastrointestinal flora. Thus, I avoid contributing to the emerging global crisis of antimicrobial resistance. I am also a proponent of topical antibiotics whenever appropriate and reasonable. Mupirocin and retapamulin, for example, are quite effective for routine cases of impetigo. When incision and drainage alone are insufficient to resolve methicillin-resistant S aureus furunculosis, I prefer either trimethoprim-sulfamethoxazole or doxycycline. Of course, other specific oral and even parenteral antibiotics are appropriate for select disease states.
Although antibiotics generally are well tolerated, there are many possible side effects. Hypersensitivity reactions, ranging from self-limited fixed drug and pruritic maculopapular eruptions through acute urticaria to anaphylaxis, may occur with any antibiotic. Clostridium difficile–associated diarrhea also may occur in conjunction with the use of any antibacterial drug, especially those with a broad spectrum of activity. Nausea and headache are mild but common side effects of these agents. All tetracycline derivatives may be photosensitizers and may provoke intracranial hypertension. Minocycline may lead to hyperpigmentation of skin and teeth, vestibular disturbances (ie, dizziness, ataxia, vertigo, tinnitus) and rarely autoimmune hepatitis. Macrolide antibiotics have been linked to serious cardiotoxicity, and quinolone antibiotics have been linked to tendonitis/tendon rupture, cardiotoxicity, and insomnia. Many antibiotics can result in vaginal yeast infections. There is some evidence that prolonged antibiotic use may precipitate inflammatory bowel disease, especially in those who are genetically predisposed.
Finally, keep in mind that antibiotic administration changes the normal cutaneous flora, which may interfere with the normal antimicrobial and anti-inflammatory homeostatic roles played by resident skin microflora. Antibiotic administration also changes the gut flora and, in this manner, may help promote the development of resistant microbes.
How do you keep patients compliant with treatment?
The most important step to assure adherence is adequate pretreatment education. Whether short-term or long-term antibiotic treatment is anticipated, I always schedule a follow-up office visit in approximately 2 weeks to check on clinical progress and reinforce good habits. Younger patients benefit from periodic reminders using emails, text messages, and tweets.
What do you do if they refuse treatment?
In some instances, antibiotic phobia in patients can be totally accepted and alternative treatments explored. As an example, laser and light therapy, hormonal manipulation, zinc-based nutritional supplements, and intensive nonantibiotic topical combination drugs can supplant antibiotics for the management of acne.
What resources do you recommend to patients for more information?
There are some excellent resources online for patients such as “Using Antibiotics Wisely” and “Get Smart: Know When Antibiotics Work.”
Suggested Readings
Chon SY, Doan HQ, Mays RM. Antibiotic overuse and resistance in dermatology. Dermatol Ther. 2012;25:55-69.
Eichenfield LF, Del Rosso JQ, Mancini AJ, et al. Evolving perspectives on the etiology and pathogenesis of acne vulgaris. J Drugs Dermatol. 2015;14:263-272.
Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974-1980.
Gelband H, Miller-Petrie M, Pant S, et al. The State of the World’s Antibiotics, 2015. Washington, DC: Center for Disease Dynamics, Economics & Policy; 2015. http://cddep.org/publications/state_worlds_antibiotics_2015. Accessed February 11, 2016.
Get smart: know when antibiotics work. Centers for Disease Control and Prevention website. http://www.cdc.gov/getsmart/community/about/index.html. Updated April 17, 2015. Accessed February 11, 2016.
Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention [published online January 19, 2016]. Ann Intern Med. doi:10.7326/M15-1840.
Kirchner M, Mafura M, Hunt T, et al. Antimicrobial resistance characteristics and fitness of Gram-negative fecal bacteria from volunteers treated with minocycline or amoxicillin. Front Microbiol. 2014;5:722. doi:10.3389/fmicb.2014.00722.
Muhammad M, Rosen T. A controversial proposal: no more antibiotics for acne! Skin Therapy Lett. 2013;18:1-4.
Using antibiotics wisely. WedMD Medical Reference. http://www.webmd.com/a-to-z-guides/using-antibiotics-wisely-topic-overview. Updated November 14, 2014. Accessed February 11, 2016.
What does your patient need to know at the first visit? Does it apply to patients of all genders, ages, and races?
There are 3 scenarios in which antibiotics are used in dermatology. First, there is the treatment of a bona fide, verified skin infection, which may range from the relatively simple (impetigo) to the complex (botryomycosis) to the exotic (fish tank granuloma). The second scenario is antibiotic administration, often due to ancillary properties such as anti-inflammatory effects, in the management of noninfectious disorders, such as familial benign pemphigus or pityriasis lichenoides et varioliformis acuta. I try hard to avoid antibiotic use in these situations unless all else fails. The third scenario involves use of antibiotics at the patient’s request, usually associated with the phrase “just in case it’s infected.” In my opinion, this practice is completely ill advised.
Male and female patients of all ages, ethnic origins, and socioeconomic backgrounds are woefully uninformed regarding the promise and peril of antibiotics. I want patients to buy into the concept of good antibiotic stewardship. Thus, patients should understand that there must be a specific and justifiable reason for antibiotic use and that the recommended dose and duration of treatment should not be altered. In some situations, antibiotic therapy is intended to be of short duration, while in other situations, such therapy may be quite protracted. Patients also need to know at the outset of treatment when we plan to transition from a short-term, antibiotic-based modality to a long-term nonantibiotic maintenance regimen, which is especially true for acne and rosacea. I try to limit antibiotic use in these disorders to 3 months. Furthermore, patients should always be educated about the potential side effects associated with the particular antibiotic being prescribed. Hoarding and sharing leftover antibiotics should be strongly and explicitly discouraged.
Finally, patients must be educated that taking shortcuts when prescribing antibiotics may lead to therapeutic failure, worsening disease, or serious long-term adverse consequences. For example, rational antibiotic use may require the added expense of an initial and/or subsequent test-of-cure culture and sensitivity. Is that swollen and tender hand following a cat bite due to Pasteurella multocida or methicillin-resistant Staphylococcus aureus? Is that new eruption in an atopic patient due to secondary impetigo or eczema herpeticum? Other laboratory testing also may be required, such as a follow-up serology after treating syphilis. Patients need to know why laboratory tests are being ordered and how the tests complement direct antibiotic intervention.
What are your go-to treatments? What are the side effects?
I am a fan of subantimicrobial-dose doxycycline for both rosacea (on label) and acne (off label). Studies have shown that neither quantitative nor qualitative changes occur in the cutaneous, oral, or gastrointestinal flora. Thus, I avoid contributing to the emerging global crisis of antimicrobial resistance. I am also a proponent of topical antibiotics whenever appropriate and reasonable. Mupirocin and retapamulin, for example, are quite effective for routine cases of impetigo. When incision and drainage alone are insufficient to resolve methicillin-resistant S aureus furunculosis, I prefer either trimethoprim-sulfamethoxazole or doxycycline. Of course, other specific oral and even parenteral antibiotics are appropriate for select disease states.
Although antibiotics generally are well tolerated, there are many possible side effects. Hypersensitivity reactions, ranging from self-limited fixed drug and pruritic maculopapular eruptions through acute urticaria to anaphylaxis, may occur with any antibiotic. Clostridium difficile–associated diarrhea also may occur in conjunction with the use of any antibacterial drug, especially those with a broad spectrum of activity. Nausea and headache are mild but common side effects of these agents. All tetracycline derivatives may be photosensitizers and may provoke intracranial hypertension. Minocycline may lead to hyperpigmentation of skin and teeth, vestibular disturbances (ie, dizziness, ataxia, vertigo, tinnitus) and rarely autoimmune hepatitis. Macrolide antibiotics have been linked to serious cardiotoxicity, and quinolone antibiotics have been linked to tendonitis/tendon rupture, cardiotoxicity, and insomnia. Many antibiotics can result in vaginal yeast infections. There is some evidence that prolonged antibiotic use may precipitate inflammatory bowel disease, especially in those who are genetically predisposed.
Finally, keep in mind that antibiotic administration changes the normal cutaneous flora, which may interfere with the normal antimicrobial and anti-inflammatory homeostatic roles played by resident skin microflora. Antibiotic administration also changes the gut flora and, in this manner, may help promote the development of resistant microbes.
How do you keep patients compliant with treatment?
The most important step to assure adherence is adequate pretreatment education. Whether short-term or long-term antibiotic treatment is anticipated, I always schedule a follow-up office visit in approximately 2 weeks to check on clinical progress and reinforce good habits. Younger patients benefit from periodic reminders using emails, text messages, and tweets.
What do you do if they refuse treatment?
In some instances, antibiotic phobia in patients can be totally accepted and alternative treatments explored. As an example, laser and light therapy, hormonal manipulation, zinc-based nutritional supplements, and intensive nonantibiotic topical combination drugs can supplant antibiotics for the management of acne.
What resources do you recommend to patients for more information?
There are some excellent resources online for patients such as “Using Antibiotics Wisely” and “Get Smart: Know When Antibiotics Work.”
What does your patient need to know at the first visit? Does it apply to patients of all genders, ages, and races?
There are 3 scenarios in which antibiotics are used in dermatology. First, there is the treatment of a bona fide, verified skin infection, which may range from the relatively simple (impetigo) to the complex (botryomycosis) to the exotic (fish tank granuloma). The second scenario is antibiotic administration, often due to ancillary properties such as anti-inflammatory effects, in the management of noninfectious disorders, such as familial benign pemphigus or pityriasis lichenoides et varioliformis acuta. I try hard to avoid antibiotic use in these situations unless all else fails. The third scenario involves use of antibiotics at the patient’s request, usually associated with the phrase “just in case it’s infected.” In my opinion, this practice is completely ill advised.
Male and female patients of all ages, ethnic origins, and socioeconomic backgrounds are woefully uninformed regarding the promise and peril of antibiotics. I want patients to buy into the concept of good antibiotic stewardship. Thus, patients should understand that there must be a specific and justifiable reason for antibiotic use and that the recommended dose and duration of treatment should not be altered. In some situations, antibiotic therapy is intended to be of short duration, while in other situations, such therapy may be quite protracted. Patients also need to know at the outset of treatment when we plan to transition from a short-term, antibiotic-based modality to a long-term nonantibiotic maintenance regimen, which is especially true for acne and rosacea. I try to limit antibiotic use in these disorders to 3 months. Furthermore, patients should always be educated about the potential side effects associated with the particular antibiotic being prescribed. Hoarding and sharing leftover antibiotics should be strongly and explicitly discouraged.
Finally, patients must be educated that taking shortcuts when prescribing antibiotics may lead to therapeutic failure, worsening disease, or serious long-term adverse consequences. For example, rational antibiotic use may require the added expense of an initial and/or subsequent test-of-cure culture and sensitivity. Is that swollen and tender hand following a cat bite due to Pasteurella multocida or methicillin-resistant Staphylococcus aureus? Is that new eruption in an atopic patient due to secondary impetigo or eczema herpeticum? Other laboratory testing also may be required, such as a follow-up serology after treating syphilis. Patients need to know why laboratory tests are being ordered and how the tests complement direct antibiotic intervention.
What are your go-to treatments? What are the side effects?
I am a fan of subantimicrobial-dose doxycycline for both rosacea (on label) and acne (off label). Studies have shown that neither quantitative nor qualitative changes occur in the cutaneous, oral, or gastrointestinal flora. Thus, I avoid contributing to the emerging global crisis of antimicrobial resistance. I am also a proponent of topical antibiotics whenever appropriate and reasonable. Mupirocin and retapamulin, for example, are quite effective for routine cases of impetigo. When incision and drainage alone are insufficient to resolve methicillin-resistant S aureus furunculosis, I prefer either trimethoprim-sulfamethoxazole or doxycycline. Of course, other specific oral and even parenteral antibiotics are appropriate for select disease states.
Although antibiotics generally are well tolerated, there are many possible side effects. Hypersensitivity reactions, ranging from self-limited fixed drug and pruritic maculopapular eruptions through acute urticaria to anaphylaxis, may occur with any antibiotic. Clostridium difficile–associated diarrhea also may occur in conjunction with the use of any antibacterial drug, especially those with a broad spectrum of activity. Nausea and headache are mild but common side effects of these agents. All tetracycline derivatives may be photosensitizers and may provoke intracranial hypertension. Minocycline may lead to hyperpigmentation of skin and teeth, vestibular disturbances (ie, dizziness, ataxia, vertigo, tinnitus) and rarely autoimmune hepatitis. Macrolide antibiotics have been linked to serious cardiotoxicity, and quinolone antibiotics have been linked to tendonitis/tendon rupture, cardiotoxicity, and insomnia. Many antibiotics can result in vaginal yeast infections. There is some evidence that prolonged antibiotic use may precipitate inflammatory bowel disease, especially in those who are genetically predisposed.
Finally, keep in mind that antibiotic administration changes the normal cutaneous flora, which may interfere with the normal antimicrobial and anti-inflammatory homeostatic roles played by resident skin microflora. Antibiotic administration also changes the gut flora and, in this manner, may help promote the development of resistant microbes.
How do you keep patients compliant with treatment?
The most important step to assure adherence is adequate pretreatment education. Whether short-term or long-term antibiotic treatment is anticipated, I always schedule a follow-up office visit in approximately 2 weeks to check on clinical progress and reinforce good habits. Younger patients benefit from periodic reminders using emails, text messages, and tweets.
What do you do if they refuse treatment?
In some instances, antibiotic phobia in patients can be totally accepted and alternative treatments explored. As an example, laser and light therapy, hormonal manipulation, zinc-based nutritional supplements, and intensive nonantibiotic topical combination drugs can supplant antibiotics for the management of acne.
What resources do you recommend to patients for more information?
There are some excellent resources online for patients such as “Using Antibiotics Wisely” and “Get Smart: Know When Antibiotics Work.”
Suggested Readings
Chon SY, Doan HQ, Mays RM. Antibiotic overuse and resistance in dermatology. Dermatol Ther. 2012;25:55-69.
Eichenfield LF, Del Rosso JQ, Mancini AJ, et al. Evolving perspectives on the etiology and pathogenesis of acne vulgaris. J Drugs Dermatol. 2015;14:263-272.
Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974-1980.
Gelband H, Miller-Petrie M, Pant S, et al. The State of the World’s Antibiotics, 2015. Washington, DC: Center for Disease Dynamics, Economics & Policy; 2015. http://cddep.org/publications/state_worlds_antibiotics_2015. Accessed February 11, 2016.
Get smart: know when antibiotics work. Centers for Disease Control and Prevention website. http://www.cdc.gov/getsmart/community/about/index.html. Updated April 17, 2015. Accessed February 11, 2016.
Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention [published online January 19, 2016]. Ann Intern Med. doi:10.7326/M15-1840.
Kirchner M, Mafura M, Hunt T, et al. Antimicrobial resistance characteristics and fitness of Gram-negative fecal bacteria from volunteers treated with minocycline or amoxicillin. Front Microbiol. 2014;5:722. doi:10.3389/fmicb.2014.00722.
Muhammad M, Rosen T. A controversial proposal: no more antibiotics for acne! Skin Therapy Lett. 2013;18:1-4.
Using antibiotics wisely. WedMD Medical Reference. http://www.webmd.com/a-to-z-guides/using-antibiotics-wisely-topic-overview. Updated November 14, 2014. Accessed February 11, 2016.
Suggested Readings
Chon SY, Doan HQ, Mays RM. Antibiotic overuse and resistance in dermatology. Dermatol Ther. 2012;25:55-69.
Eichenfield LF, Del Rosso JQ, Mancini AJ, et al. Evolving perspectives on the etiology and pathogenesis of acne vulgaris. J Drugs Dermatol. 2015;14:263-272.
Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974-1980.
Gelband H, Miller-Petrie M, Pant S, et al. The State of the World’s Antibiotics, 2015. Washington, DC: Center for Disease Dynamics, Economics & Policy; 2015. http://cddep.org/publications/state_worlds_antibiotics_2015. Accessed February 11, 2016.
Get smart: know when antibiotics work. Centers for Disease Control and Prevention website. http://www.cdc.gov/getsmart/community/about/index.html. Updated April 17, 2015. Accessed February 11, 2016.
Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: Advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention [published online January 19, 2016]. Ann Intern Med. doi:10.7326/M15-1840.
Kirchner M, Mafura M, Hunt T, et al. Antimicrobial resistance characteristics and fitness of Gram-negative fecal bacteria from volunteers treated with minocycline or amoxicillin. Front Microbiol. 2014;5:722. doi:10.3389/fmicb.2014.00722.
Muhammad M, Rosen T. A controversial proposal: no more antibiotics for acne! Skin Therapy Lett. 2013;18:1-4.
Using antibiotics wisely. WedMD Medical Reference. http://www.webmd.com/a-to-z-guides/using-antibiotics-wisely-topic-overview. Updated November 14, 2014. Accessed February 11, 2016.
Imiquimod Cream 2.5% and 3.75% Applied Once Daily to Treat External Genital Warts in Men
External genital warts (EGWs), which are caused by infection with select types of human papillomavirus (HPV), are one of the most prevalent and fastest growing sexually transmitted infections.1 External genital warts affect approximately 1% of sexually active adults in the United States and Europe, with another 15% having subclinical infections; more than 1 million new cases of EGWs are diagnosed annually.2-4 Although the condition is not life threatening, lesions can cause symptoms, such as burning, itching, bleeding, pain and dyspareunia, and potential urethral or rectal obstruction. External genital warts also have been associated with adverse psychological effects.5-8
The time between exposure to HPV and development of EGWs can vary from a few weeks to several months or years (median, 2.9 months).9 Many HPV infections are mild and transient, resolving spontaneously.10 As many as 30% of EGWs will regress over 4 months and approximately 90% clear within 2 years.11,12 However, even with treatment, the median time to resolution is 5.9 months.9
Imiquimod cream 5%, which has been successfully used to treat EGWs since it was approved by the US Food and Drug Administration in 1997, is applied to lesions 3 times weekly at bedtime until clearance is achieved or for a maximum of 16 weeks.13 In clinical studies, complete clearance has been reported in 35% to 75% of participants.14-21 However, it is important to note that not all anogenital regions with warts were required to be treated in these studies,14-21 and newly arising warts were not included in the analysis.17 Reported clearance rates were higher and median clearance time was shorter in women.17 Relatively low recurrence rates (6%–26%) have been reported after successful clearance of EGWs.16,17,20,21
Long treatment durations are always a concern for patient adherence. Although increasing the dosing frequency with imiquimod cream 5% might be considered an attractive option to reduce the length of the treatment course, it has resulted in greater incidence and severity of local adverse events (AEs) in some studies without improved efficacy.18,22,23 Thus lower concentrations of imiquimod (ie, 2.5% and 3.75% formulations) were developed to potentially decrease treatment duration and provide a daily dosing regimen.
We report the results of 2 identical, placebo-controlled, phase 3 studies evaluating the safety and efficacy of imiquimod cream 2.5% and 3.75% in treating EGWs in men. Pooled results from a female subgroup previously have been reported.24 Although the percentage of women who reported ever being diagnosed with EGWs was higher than in men (7.2% vs 4%) in one survey,25 other assessments have found a similar prevalence of EGWs among both genders.26-28 We provide important insights herein by reporting efficacy and tolerability data for imiquimod cream 2.5% and 3.75% in the treatment of EGWs in males.
Methods
Study Design
Male patients aged 12 years and older with 2 to 30 EGWs in the inguinal, perineal, and/or perianal areas as well as on the glans penis, penile shaft, scrotum, and/or foreskin were enrolled in 2 identical, multicenter, randomized, parallel-group, double-blind, placebo-controlled studies. Participants were randomized (2:2:1) to self-treatment with imiquimod cream 3.75% or 2.5% or placebo once daily until complete clearance was achieved or for a maximum of 8 weeks (end of treatment [EOT]). There was a follow-up period of up to 8 weeks (end of study [EOS]) in participants who did not achieve complete clearance by EOT. All participants who achieved complete clearance by EOS entered a 12-week observational follow-up period to assess recurrence.
Primary and Secondary Efficacy Criteria
The primary efficacy end point was complete clearance rate, which was defined as the proportion of participants by the EOS visit with zero EGWs (that either existed at baseline and any warts developing during the study) in all anogenital anatomic areas. It is important to note that this primary efficacy end point was very conservative in that it included any new warts occurring during the study that may not have received a full treatment course. Lesions were counted in all assessed anatomic areas without distinction between those that were identified at baseline or those that were newly identified during the study period. If new EGWs appeared during the study in new anatomic areas, such lesions were treated with the study medication as they appeared. Therefore, any newly arising EGWs received less than the full course of treatment, as therapy was not extended beyond the 8-week study period. Participants were evaluated for the presence of any EGWs in all anatomic areas without distinction between lesions that were present at baseline and newly arising EGWs. Therefore, development of new EGWs during the study period could potentially lower clearance rates.
Secondary end points were 75% or more and 50% or more reduction in EGW count, change in EGW count from baseline, and 12-week sustained clearance rate.
Safety
Safety assessments of AEs, both volunteered and elicited, were made throughout the study.
Study Oversight
The study was conducted in accordance with the ethical principles specified in the Declaration of Helsinki and in compliance with the requirements of local regulatory committees. All participants provided written informed consent.
Statistical Analysis
Statistical analysis for intention-to-treat (ITT) imputations was made for missing data points using last observation carried forward (LOCF). Complete clearance rates and partial clearance rates were analyzed using Cochran-Mantel-Haenszel statistics stratified by center and by gender for the overall population analyses. The percentage change in EGW count was analyzed using analysis of covariance. All statistical analyses were performed using SAS software (version 9.1.3).
Results
Study Population
Study characteristics by treatment group are summarized in the Table. Overall, 447 male participants (225 from study 1 and 222 from study 2) were included in the study. The majority of participants (84.1%) had EGWs on the penile shaft with only 1 affected region in just over half of participants (51.9%). Most participants (70.2%) were 35 years or younger, and approximately half had a baseline EGW count of 7 or less (50.6%). More than 20% of participants had an affected wart surface area greater than 150 mm2 at baseline, and in more than 60% of participants, the duration from first diagnosis of EGWs to enrollment in the study was more than 1 year.
Primary Efficacy End Point
Imiquimod cream 3.75% was statistically superior to placebo in study 1 and study 2 (P=.015 and P=.019, respectively)(Figure) in providing complete clearance of all EGWs (baseline or new) at EOS. Imiquimod cream 2.5% was only statistically superior to placebo in study 2 (P=.034). Importantly, there were a number of participants who did not achieve complete clearance at EOT who continued to improve posttreatment. The percentage of participants treated with imiquimod cream 3.75% and 2.5% who were completely cleared at EOT was 12.0% (14.7% study 1 and 9.1% study 2) and 7.1% (7.2% study 1 and 7.1% study 2), respectively, compared to complete clearance rates of 18.6% (20.0% study 1 and 17.0% study 2) and 14.3% (13.3% study 1 and 15.3% study 2), respectively, at EOS (ITT population).
 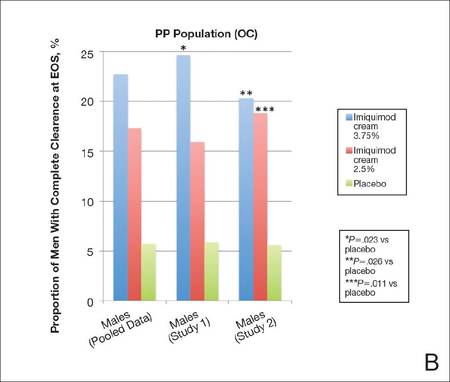 |
Complete clearance rates (defined as the proportion of participants by the end-of-study [EOS] visit with zero external genital warts in all anogenital anatomic areas) in the intention-to-treat (ITT) population (last observation carried forward [LOCF])(A) and per-protocol (PP) population (OC [observed case])(B), including both individual and pooled study data. |
In both studies complete clearance rates were significantly higher (P<.019 both studies) with imiquimod cream 3.75% compared with placebo at weeks 10 through 16 (EOS). In study 2, complete clearance rates were significantly higher (P<.049) with imiquimod cream 2.5% compared to placebo from week 14 to week 16 (EOS). Complete clearance rates were highest in participants treated with imiquimod cream 3.75% who had EGWs in the perianal region or on the glans penis (28.6% and 33.3%, respectively); however, the number of participants in both groups was relatively small.
Overall, 18.8% of participants took rest periods. Complete clearance rates were higher in men who took a rest period (26.5% and 27.3% for imiquimod cream 3.75% and 2.5%, respectively), perhaps reflecting a more brisk immunological response. The frequency, duration, and number of dosing days prior to the rest period were similar in the active treatment groups and lower in the placebo group.
There was a tendency for older participants (ie, >35 years) and those with lower baseline EGW counts (ie, ≤7) to respond better. Participants treated with imiquimod cream 3.75% also tended to respond best to treatment when only 1 anatomic area was affected.
Secondary Efficacy End Points
The proportion of male participants with at least a 75% reduction in EGW count from baseline at EOS was statistically superior with imiquimod 3.75% compared to placebo in study 1 and study 2 (P=.001 and P=.008, respectively). Statistical superiority also was apparent with imiquimod cream 2.5% versus placebo in study 2 (P=.013). Overall, 20.2% (18.1% study 1 and 22.4% study 2) and 27.3% (30.5% study 1 and 23.9% study 2) of participants achieved at least a 75% reduction in wart count at EOS with imiquimod cream 2.5% and 3.75%, respectively (pooled data).
Percentage change in EGW count from baseline at EOS was 35.8% and 24.1% with imiquimod cream 3.75% in study 1 and study 2, respectively, both significantly better than placebo (P=.002 and P=.011, respectively). Change in EGW count following treatment with imiquimod cream 2.5% was only significant in study 2 (P=.001).
The median time to complete clearance was shorter in the 2 active treatment groups compared with placebo. For those participants who attained complete clearance, the median time to complete clearance ranged from 57 to 59 days in the imiquimod cream 3.75% groups (studies 1 and 2, respectively), 60 to 74 days in the imiquimod 2.5% cream groups (studies 1 and 2, respectively), and 76 to 81 days with placebo (studies 2 and 1, respectively).
Safety
Less than one-third of male participants in each treatment group experienced AEs during the studies. The incidence of serious adverse events (SAEs) and AEs leading to study discontinuation was low. In total, 4 participants (0.9% [3 in the imiquimod cream 2.5% group and 1 in the imiquimod cream 3.75% group]) had AEs that led to study discontinuation. Application-site reactions were reported in a total of 46 participants (10.3%). The incidence and severity of local skin reactions was mostly mild or moderate, similar in both active treatment groups, and higher than in the placebo group. Local skin reactions were coincident with the treatment period and rapidly decreased when treatment was concluded. There were no clinically meaningful trends in vital sign measurements or laboratory measurements.
Comment
Imiquimod cream 5% has been shown to be a safe and effective treatment of EGWs. Our study was designed to evaluate lower concentrations of imiquimod cream (2.5% and 3.75%), which may permit daily dosing and a shortened treatment course in men with EGWs.
Efficacy of imiquimod cream 2.5% and 3.75% was established through both primary and secondary end points, though only the higher concentration was significantly more effective than placebo in both studies. In addition, a number of participants who were not completely cleared following 8 weeks of treatment went on to be completely cleared at EOS, demonstrating continued activity of imiquimod despite cessation of active treatment.
Imiquimod cream 3.75% was particularly effective when compared to placebo, with 18.6% of participants completely cleared at EOS, though the PP (observed case) results (22.7%) may be more encouraging and can be used to motivate patients.
Although there are limitations in making direct comparisons between studies, complete clearance rates in our studies were lower than those reported previously with imiquimod cream 5%.17 Lower efficacy rates might be expected given the differences in methodology. In the 2 studies reported here, participants had to have no EGWs (baseline or new, treated or untreated) in any of the anogenital areas specified to be reported as having achieved complete clearance. In earlier studies with imiquimod cream 5%, not all anogenital regions were required to be treated, and any new EGWs arising during treatment were not included in the analysis.17 Also, our analysis focused purely on a male patient population in which efficacy results tend to be lower regardless of treatment modality employed.
Recurrence is another important issue in the treatment of EGWs. Although not studied specifically in a male population, recurrence rates of 16.7% to 17.7% were seen in the 3 months following successful treatment with imiquimod cream 2.5% and 3.75% in the 2 pivotal studies. These results were consistent with the recurrence rates reported following successful treatment with imiquimod cream 5%.17
In general, complete clearance rates increased in a dose-dependent manner. Complete clearance rates were lower in the male subpopulation across all treatment groups compared to those previously reported in females,24 which was consistent with prior results reported for imiquimod cream 5% as well as other topical treatments.17 It has been suggested that this difference may be due in part to the distribution of female EGWs in areas of less keratinization. Complete clearance rates in the current analysis tended to be higher in male participants with baseline EGWs in anatomic sites with less keratinized skin such as the perianal, perineal, or glans penis areas.
Daily application of imiquimod cream 2.5% and 3.75% generally was well tolerated. Most reported AEs were mild or moderate, and few participants discontinued because of AEs. Few SAEs were reported and none were considered to be treatment related. There was no difference in the incidence rates of AEs between the 2 active treatments. The incidence of SAEs and study discontinuations was much lower than previously reported in the female cohort of these 2 studies.24
Conclusion
In conclusion, 2 well-controlled studies of males with EGWs who were treated for up to 8 weeks with imiquimod cream 2.5% and 3.75% applied daily demonstrated good tolerability and superior efficacy to placebo in complete clearance of all baseline and newly arising warts in addition to reducing EGW counts.
Acknowledgments—The authors thank Christina Cognata Smith, PharmD, and Mandeep Kaur, MD (both previously of Valeant Pharmaceuticals North America, LLC, Bridgewater, New Jersey), as well as Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for assistance with the preparation of the manuscript. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis.
1. Weinstock H, Berman S, Cates W. Sexually transmitted infections in American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6-10.
2. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813-819.
3. Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3-8.
4. Kjaer SK, Tran TN, Sparen P, et al. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196:1447-1454.
5. Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect. 2008;84:161-166.
6. Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health. 2010;10:113.
7. Wang KL, Jeng CJ, Yang YC, et al. The psychological impact of illness among women experiencing human papillomavirus-related illness or screening interventions. J Psychsom Obstet Gynaecol. 2010;31:16-23.
8. Lawrence S, Walzman M, Sheppard S, et al. The psychological impact caused by genital warts: has the Department of Health’s choice of vaccination missed the opportunity to prevent such morbidity? Int J STD AIDS. 2009;20:696-700.
9. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
10. Centers for Disease Control and Prevention. Human papillomavirus: HPV information for clinicians. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; April 2007.
11. Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: disease, diagnosis, management, and prevention. Dermatol Ther. 2010;23:458-476.
12. Scheinfeld N, Lehman DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12:5.
13. Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2010.
14. Komericki P, Akkilic-Materna M, Strimitzer T, et al. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of genital warts. Sex Transm Dis. 2011;38:216-218.
15. Beutner KR, Tyring SK, Trofatter KF Jr, et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother. 1998;42:789-794.
16. Beutner KR, Spruance SL, Hougham AJ, et al. Treatment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol. 1998;38:230-239.
17. Edwards L, Ferenczy A, Eron L, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. Arch Dermatol. 1998;134:25-30.
18. Fife KH, Ferenczy A, Douglas JM, et al. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily, or three times a day. Sex Transm Dis. 2001;28:226-231.
19. Garland SM, Waddell R, Mindel A, et al. An open-label phase II pilot study investigating the optimal duration of imiquimod 5% cream for the treatment of external genital warts in women. Int J STD AIDS. 2006;17:448-452.
20. Schofer H, Van Ophoven A, Henke U, et al. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol. 2006;16:642-648.
21. Arican O, Guneri F, Bilgic K, et al. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31:627-631.
22. Gollnick H, Barasso R, Jappe U, et al. Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or once per day. Int J STD AIDS. 2001;12:22-28.
23. Trofatter KF Jr, Ferenczy A, Fife KH. Increased frequency of dosing of imiquimod 5% cream in the treatment of external genital warts in women. Int J Gynecol Obstet. 2002;76:191-193.
24. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies [published online ahead of print August 24, 2011]. Infect Dis Obstet Gynecol. 2011;2011:806105.
25. Dinh TH, Sternberg M, Dunne EF, et al. Genital warts among 18- to 59-year-olds in the US, National Health and Nutrition Examination Survey, 1999-2004. Sex Transm Dis. 2008;35:357-360.
26. Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107-1122.
27. Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis. 2004;31:748-752.
28. Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191:114-120.
External genital warts (EGWs), which are caused by infection with select types of human papillomavirus (HPV), are one of the most prevalent and fastest growing sexually transmitted infections.1 External genital warts affect approximately 1% of sexually active adults in the United States and Europe, with another 15% having subclinical infections; more than 1 million new cases of EGWs are diagnosed annually.2-4 Although the condition is not life threatening, lesions can cause symptoms, such as burning, itching, bleeding, pain and dyspareunia, and potential urethral or rectal obstruction. External genital warts also have been associated with adverse psychological effects.5-8
The time between exposure to HPV and development of EGWs can vary from a few weeks to several months or years (median, 2.9 months).9 Many HPV infections are mild and transient, resolving spontaneously.10 As many as 30% of EGWs will regress over 4 months and approximately 90% clear within 2 years.11,12 However, even with treatment, the median time to resolution is 5.9 months.9
Imiquimod cream 5%, which has been successfully used to treat EGWs since it was approved by the US Food and Drug Administration in 1997, is applied to lesions 3 times weekly at bedtime until clearance is achieved or for a maximum of 16 weeks.13 In clinical studies, complete clearance has been reported in 35% to 75% of participants.14-21 However, it is important to note that not all anogenital regions with warts were required to be treated in these studies,14-21 and newly arising warts were not included in the analysis.17 Reported clearance rates were higher and median clearance time was shorter in women.17 Relatively low recurrence rates (6%–26%) have been reported after successful clearance of EGWs.16,17,20,21
Long treatment durations are always a concern for patient adherence. Although increasing the dosing frequency with imiquimod cream 5% might be considered an attractive option to reduce the length of the treatment course, it has resulted in greater incidence and severity of local adverse events (AEs) in some studies without improved efficacy.18,22,23 Thus lower concentrations of imiquimod (ie, 2.5% and 3.75% formulations) were developed to potentially decrease treatment duration and provide a daily dosing regimen.
We report the results of 2 identical, placebo-controlled, phase 3 studies evaluating the safety and efficacy of imiquimod cream 2.5% and 3.75% in treating EGWs in men. Pooled results from a female subgroup previously have been reported.24 Although the percentage of women who reported ever being diagnosed with EGWs was higher than in men (7.2% vs 4%) in one survey,25 other assessments have found a similar prevalence of EGWs among both genders.26-28 We provide important insights herein by reporting efficacy and tolerability data for imiquimod cream 2.5% and 3.75% in the treatment of EGWs in males.
Methods
Study Design
Male patients aged 12 years and older with 2 to 30 EGWs in the inguinal, perineal, and/or perianal areas as well as on the glans penis, penile shaft, scrotum, and/or foreskin were enrolled in 2 identical, multicenter, randomized, parallel-group, double-blind, placebo-controlled studies. Participants were randomized (2:2:1) to self-treatment with imiquimod cream 3.75% or 2.5% or placebo once daily until complete clearance was achieved or for a maximum of 8 weeks (end of treatment [EOT]). There was a follow-up period of up to 8 weeks (end of study [EOS]) in participants who did not achieve complete clearance by EOT. All participants who achieved complete clearance by EOS entered a 12-week observational follow-up period to assess recurrence.
Primary and Secondary Efficacy Criteria
The primary efficacy end point was complete clearance rate, which was defined as the proportion of participants by the EOS visit with zero EGWs (that either existed at baseline and any warts developing during the study) in all anogenital anatomic areas. It is important to note that this primary efficacy end point was very conservative in that it included any new warts occurring during the study that may not have received a full treatment course. Lesions were counted in all assessed anatomic areas without distinction between those that were identified at baseline or those that were newly identified during the study period. If new EGWs appeared during the study in new anatomic areas, such lesions were treated with the study medication as they appeared. Therefore, any newly arising EGWs received less than the full course of treatment, as therapy was not extended beyond the 8-week study period. Participants were evaluated for the presence of any EGWs in all anatomic areas without distinction between lesions that were present at baseline and newly arising EGWs. Therefore, development of new EGWs during the study period could potentially lower clearance rates.
Secondary end points were 75% or more and 50% or more reduction in EGW count, change in EGW count from baseline, and 12-week sustained clearance rate.
Safety
Safety assessments of AEs, both volunteered and elicited, were made throughout the study.
Study Oversight
The study was conducted in accordance with the ethical principles specified in the Declaration of Helsinki and in compliance with the requirements of local regulatory committees. All participants provided written informed consent.
Statistical Analysis
Statistical analysis for intention-to-treat (ITT) imputations was made for missing data points using last observation carried forward (LOCF). Complete clearance rates and partial clearance rates were analyzed using Cochran-Mantel-Haenszel statistics stratified by center and by gender for the overall population analyses. The percentage change in EGW count was analyzed using analysis of covariance. All statistical analyses were performed using SAS software (version 9.1.3).
Results
Study Population
Study characteristics by treatment group are summarized in the Table. Overall, 447 male participants (225 from study 1 and 222 from study 2) were included in the study. The majority of participants (84.1%) had EGWs on the penile shaft with only 1 affected region in just over half of participants (51.9%). Most participants (70.2%) were 35 years or younger, and approximately half had a baseline EGW count of 7 or less (50.6%). More than 20% of participants had an affected wart surface area greater than 150 mm2 at baseline, and in more than 60% of participants, the duration from first diagnosis of EGWs to enrollment in the study was more than 1 year.
Primary Efficacy End Point
Imiquimod cream 3.75% was statistically superior to placebo in study 1 and study 2 (P=.015 and P=.019, respectively)(Figure) in providing complete clearance of all EGWs (baseline or new) at EOS. Imiquimod cream 2.5% was only statistically superior to placebo in study 2 (P=.034). Importantly, there were a number of participants who did not achieve complete clearance at EOT who continued to improve posttreatment. The percentage of participants treated with imiquimod cream 3.75% and 2.5% who were completely cleared at EOT was 12.0% (14.7% study 1 and 9.1% study 2) and 7.1% (7.2% study 1 and 7.1% study 2), respectively, compared to complete clearance rates of 18.6% (20.0% study 1 and 17.0% study 2) and 14.3% (13.3% study 1 and 15.3% study 2), respectively, at EOS (ITT population).
  |
Complete clearance rates (defined as the proportion of participants by the end-of-study [EOS] visit with zero external genital warts in all anogenital anatomic areas) in the intention-to-treat (ITT) population (last observation carried forward [LOCF])(A) and per-protocol (PP) population (OC [observed case])(B), including both individual and pooled study data. |
In both studies complete clearance rates were significantly higher (P<.019 both studies) with imiquimod cream 3.75% compared with placebo at weeks 10 through 16 (EOS). In study 2, complete clearance rates were significantly higher (P<.049) with imiquimod cream 2.5% compared to placebo from week 14 to week 16 (EOS). Complete clearance rates were highest in participants treated with imiquimod cream 3.75% who had EGWs in the perianal region or on the glans penis (28.6% and 33.3%, respectively); however, the number of participants in both groups was relatively small.
Overall, 18.8% of participants took rest periods. Complete clearance rates were higher in men who took a rest period (26.5% and 27.3% for imiquimod cream 3.75% and 2.5%, respectively), perhaps reflecting a more brisk immunological response. The frequency, duration, and number of dosing days prior to the rest period were similar in the active treatment groups and lower in the placebo group.
There was a tendency for older participants (ie, >35 years) and those with lower baseline EGW counts (ie, ≤7) to respond better. Participants treated with imiquimod cream 3.75% also tended to respond best to treatment when only 1 anatomic area was affected.
Secondary Efficacy End Points
The proportion of male participants with at least a 75% reduction in EGW count from baseline at EOS was statistically superior with imiquimod 3.75% compared to placebo in study 1 and study 2 (P=.001 and P=.008, respectively). Statistical superiority also was apparent with imiquimod cream 2.5% versus placebo in study 2 (P=.013). Overall, 20.2% (18.1% study 1 and 22.4% study 2) and 27.3% (30.5% study 1 and 23.9% study 2) of participants achieved at least a 75% reduction in wart count at EOS with imiquimod cream 2.5% and 3.75%, respectively (pooled data).
Percentage change in EGW count from baseline at EOS was 35.8% and 24.1% with imiquimod cream 3.75% in study 1 and study 2, respectively, both significantly better than placebo (P=.002 and P=.011, respectively). Change in EGW count following treatment with imiquimod cream 2.5% was only significant in study 2 (P=.001).
The median time to complete clearance was shorter in the 2 active treatment groups compared with placebo. For those participants who attained complete clearance, the median time to complete clearance ranged from 57 to 59 days in the imiquimod cream 3.75% groups (studies 1 and 2, respectively), 60 to 74 days in the imiquimod 2.5% cream groups (studies 1 and 2, respectively), and 76 to 81 days with placebo (studies 2 and 1, respectively).
Safety
Less than one-third of male participants in each treatment group experienced AEs during the studies. The incidence of serious adverse events (SAEs) and AEs leading to study discontinuation was low. In total, 4 participants (0.9% [3 in the imiquimod cream 2.5% group and 1 in the imiquimod cream 3.75% group]) had AEs that led to study discontinuation. Application-site reactions were reported in a total of 46 participants (10.3%). The incidence and severity of local skin reactions was mostly mild or moderate, similar in both active treatment groups, and higher than in the placebo group. Local skin reactions were coincident with the treatment period and rapidly decreased when treatment was concluded. There were no clinically meaningful trends in vital sign measurements or laboratory measurements.
Comment
Imiquimod cream 5% has been shown to be a safe and effective treatment of EGWs. Our study was designed to evaluate lower concentrations of imiquimod cream (2.5% and 3.75%), which may permit daily dosing and a shortened treatment course in men with EGWs.
Efficacy of imiquimod cream 2.5% and 3.75% was established through both primary and secondary end points, though only the higher concentration was significantly more effective than placebo in both studies. In addition, a number of participants who were not completely cleared following 8 weeks of treatment went on to be completely cleared at EOS, demonstrating continued activity of imiquimod despite cessation of active treatment.
Imiquimod cream 3.75% was particularly effective when compared to placebo, with 18.6% of participants completely cleared at EOS, though the PP (observed case) results (22.7%) may be more encouraging and can be used to motivate patients.
Although there are limitations in making direct comparisons between studies, complete clearance rates in our studies were lower than those reported previously with imiquimod cream 5%.17 Lower efficacy rates might be expected given the differences in methodology. In the 2 studies reported here, participants had to have no EGWs (baseline or new, treated or untreated) in any of the anogenital areas specified to be reported as having achieved complete clearance. In earlier studies with imiquimod cream 5%, not all anogenital regions were required to be treated, and any new EGWs arising during treatment were not included in the analysis.17 Also, our analysis focused purely on a male patient population in which efficacy results tend to be lower regardless of treatment modality employed.
Recurrence is another important issue in the treatment of EGWs. Although not studied specifically in a male population, recurrence rates of 16.7% to 17.7% were seen in the 3 months following successful treatment with imiquimod cream 2.5% and 3.75% in the 2 pivotal studies. These results were consistent with the recurrence rates reported following successful treatment with imiquimod cream 5%.17
In general, complete clearance rates increased in a dose-dependent manner. Complete clearance rates were lower in the male subpopulation across all treatment groups compared to those previously reported in females,24 which was consistent with prior results reported for imiquimod cream 5% as well as other topical treatments.17 It has been suggested that this difference may be due in part to the distribution of female EGWs in areas of less keratinization. Complete clearance rates in the current analysis tended to be higher in male participants with baseline EGWs in anatomic sites with less keratinized skin such as the perianal, perineal, or glans penis areas.
Daily application of imiquimod cream 2.5% and 3.75% generally was well tolerated. Most reported AEs were mild or moderate, and few participants discontinued because of AEs. Few SAEs were reported and none were considered to be treatment related. There was no difference in the incidence rates of AEs between the 2 active treatments. The incidence of SAEs and study discontinuations was much lower than previously reported in the female cohort of these 2 studies.24
Conclusion
In conclusion, 2 well-controlled studies of males with EGWs who were treated for up to 8 weeks with imiquimod cream 2.5% and 3.75% applied daily demonstrated good tolerability and superior efficacy to placebo in complete clearance of all baseline and newly arising warts in addition to reducing EGW counts.
Acknowledgments—The authors thank Christina Cognata Smith, PharmD, and Mandeep Kaur, MD (both previously of Valeant Pharmaceuticals North America, LLC, Bridgewater, New Jersey), as well as Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for assistance with the preparation of the manuscript. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis.
External genital warts (EGWs), which are caused by infection with select types of human papillomavirus (HPV), are one of the most prevalent and fastest growing sexually transmitted infections.1 External genital warts affect approximately 1% of sexually active adults in the United States and Europe, with another 15% having subclinical infections; more than 1 million new cases of EGWs are diagnosed annually.2-4 Although the condition is not life threatening, lesions can cause symptoms, such as burning, itching, bleeding, pain and dyspareunia, and potential urethral or rectal obstruction. External genital warts also have been associated with adverse psychological effects.5-8
The time between exposure to HPV and development of EGWs can vary from a few weeks to several months or years (median, 2.9 months).9 Many HPV infections are mild and transient, resolving spontaneously.10 As many as 30% of EGWs will regress over 4 months and approximately 90% clear within 2 years.11,12 However, even with treatment, the median time to resolution is 5.9 months.9
Imiquimod cream 5%, which has been successfully used to treat EGWs since it was approved by the US Food and Drug Administration in 1997, is applied to lesions 3 times weekly at bedtime until clearance is achieved or for a maximum of 16 weeks.13 In clinical studies, complete clearance has been reported in 35% to 75% of participants.14-21 However, it is important to note that not all anogenital regions with warts were required to be treated in these studies,14-21 and newly arising warts were not included in the analysis.17 Reported clearance rates were higher and median clearance time was shorter in women.17 Relatively low recurrence rates (6%–26%) have been reported after successful clearance of EGWs.16,17,20,21
Long treatment durations are always a concern for patient adherence. Although increasing the dosing frequency with imiquimod cream 5% might be considered an attractive option to reduce the length of the treatment course, it has resulted in greater incidence and severity of local adverse events (AEs) in some studies without improved efficacy.18,22,23 Thus lower concentrations of imiquimod (ie, 2.5% and 3.75% formulations) were developed to potentially decrease treatment duration and provide a daily dosing regimen.
We report the results of 2 identical, placebo-controlled, phase 3 studies evaluating the safety and efficacy of imiquimod cream 2.5% and 3.75% in treating EGWs in men. Pooled results from a female subgroup previously have been reported.24 Although the percentage of women who reported ever being diagnosed with EGWs was higher than in men (7.2% vs 4%) in one survey,25 other assessments have found a similar prevalence of EGWs among both genders.26-28 We provide important insights herein by reporting efficacy and tolerability data for imiquimod cream 2.5% and 3.75% in the treatment of EGWs in males.
Methods
Study Design
Male patients aged 12 years and older with 2 to 30 EGWs in the inguinal, perineal, and/or perianal areas as well as on the glans penis, penile shaft, scrotum, and/or foreskin were enrolled in 2 identical, multicenter, randomized, parallel-group, double-blind, placebo-controlled studies. Participants were randomized (2:2:1) to self-treatment with imiquimod cream 3.75% or 2.5% or placebo once daily until complete clearance was achieved or for a maximum of 8 weeks (end of treatment [EOT]). There was a follow-up period of up to 8 weeks (end of study [EOS]) in participants who did not achieve complete clearance by EOT. All participants who achieved complete clearance by EOS entered a 12-week observational follow-up period to assess recurrence.
Primary and Secondary Efficacy Criteria
The primary efficacy end point was complete clearance rate, which was defined as the proportion of participants by the EOS visit with zero EGWs (that either existed at baseline and any warts developing during the study) in all anogenital anatomic areas. It is important to note that this primary efficacy end point was very conservative in that it included any new warts occurring during the study that may not have received a full treatment course. Lesions were counted in all assessed anatomic areas without distinction between those that were identified at baseline or those that were newly identified during the study period. If new EGWs appeared during the study in new anatomic areas, such lesions were treated with the study medication as they appeared. Therefore, any newly arising EGWs received less than the full course of treatment, as therapy was not extended beyond the 8-week study period. Participants were evaluated for the presence of any EGWs in all anatomic areas without distinction between lesions that were present at baseline and newly arising EGWs. Therefore, development of new EGWs during the study period could potentially lower clearance rates.
Secondary end points were 75% or more and 50% or more reduction in EGW count, change in EGW count from baseline, and 12-week sustained clearance rate.
Safety
Safety assessments of AEs, both volunteered and elicited, were made throughout the study.
Study Oversight
The study was conducted in accordance with the ethical principles specified in the Declaration of Helsinki and in compliance with the requirements of local regulatory committees. All participants provided written informed consent.
Statistical Analysis
Statistical analysis for intention-to-treat (ITT) imputations was made for missing data points using last observation carried forward (LOCF). Complete clearance rates and partial clearance rates were analyzed using Cochran-Mantel-Haenszel statistics stratified by center and by gender for the overall population analyses. The percentage change in EGW count was analyzed using analysis of covariance. All statistical analyses were performed using SAS software (version 9.1.3).
Results
Study Population
Study characteristics by treatment group are summarized in the Table. Overall, 447 male participants (225 from study 1 and 222 from study 2) were included in the study. The majority of participants (84.1%) had EGWs on the penile shaft with only 1 affected region in just over half of participants (51.9%). Most participants (70.2%) were 35 years or younger, and approximately half had a baseline EGW count of 7 or less (50.6%). More than 20% of participants had an affected wart surface area greater than 150 mm2 at baseline, and in more than 60% of participants, the duration from first diagnosis of EGWs to enrollment in the study was more than 1 year.
Primary Efficacy End Point
Imiquimod cream 3.75% was statistically superior to placebo in study 1 and study 2 (P=.015 and P=.019, respectively)(Figure) in providing complete clearance of all EGWs (baseline or new) at EOS. Imiquimod cream 2.5% was only statistically superior to placebo in study 2 (P=.034). Importantly, there were a number of participants who did not achieve complete clearance at EOT who continued to improve posttreatment. The percentage of participants treated with imiquimod cream 3.75% and 2.5% who were completely cleared at EOT was 12.0% (14.7% study 1 and 9.1% study 2) and 7.1% (7.2% study 1 and 7.1% study 2), respectively, compared to complete clearance rates of 18.6% (20.0% study 1 and 17.0% study 2) and 14.3% (13.3% study 1 and 15.3% study 2), respectively, at EOS (ITT population).
  |
Complete clearance rates (defined as the proportion of participants by the end-of-study [EOS] visit with zero external genital warts in all anogenital anatomic areas) in the intention-to-treat (ITT) population (last observation carried forward [LOCF])(A) and per-protocol (PP) population (OC [observed case])(B), including both individual and pooled study data. |
In both studies complete clearance rates were significantly higher (P<.019 both studies) with imiquimod cream 3.75% compared with placebo at weeks 10 through 16 (EOS). In study 2, complete clearance rates were significantly higher (P<.049) with imiquimod cream 2.5% compared to placebo from week 14 to week 16 (EOS). Complete clearance rates were highest in participants treated with imiquimod cream 3.75% who had EGWs in the perianal region or on the glans penis (28.6% and 33.3%, respectively); however, the number of participants in both groups was relatively small.
Overall, 18.8% of participants took rest periods. Complete clearance rates were higher in men who took a rest period (26.5% and 27.3% for imiquimod cream 3.75% and 2.5%, respectively), perhaps reflecting a more brisk immunological response. The frequency, duration, and number of dosing days prior to the rest period were similar in the active treatment groups and lower in the placebo group.
There was a tendency for older participants (ie, >35 years) and those with lower baseline EGW counts (ie, ≤7) to respond better. Participants treated with imiquimod cream 3.75% also tended to respond best to treatment when only 1 anatomic area was affected.
Secondary Efficacy End Points
The proportion of male participants with at least a 75% reduction in EGW count from baseline at EOS was statistically superior with imiquimod 3.75% compared to placebo in study 1 and study 2 (P=.001 and P=.008, respectively). Statistical superiority also was apparent with imiquimod cream 2.5% versus placebo in study 2 (P=.013). Overall, 20.2% (18.1% study 1 and 22.4% study 2) and 27.3% (30.5% study 1 and 23.9% study 2) of participants achieved at least a 75% reduction in wart count at EOS with imiquimod cream 2.5% and 3.75%, respectively (pooled data).
Percentage change in EGW count from baseline at EOS was 35.8% and 24.1% with imiquimod cream 3.75% in study 1 and study 2, respectively, both significantly better than placebo (P=.002 and P=.011, respectively). Change in EGW count following treatment with imiquimod cream 2.5% was only significant in study 2 (P=.001).
The median time to complete clearance was shorter in the 2 active treatment groups compared with placebo. For those participants who attained complete clearance, the median time to complete clearance ranged from 57 to 59 days in the imiquimod cream 3.75% groups (studies 1 and 2, respectively), 60 to 74 days in the imiquimod 2.5% cream groups (studies 1 and 2, respectively), and 76 to 81 days with placebo (studies 2 and 1, respectively).
Safety
Less than one-third of male participants in each treatment group experienced AEs during the studies. The incidence of serious adverse events (SAEs) and AEs leading to study discontinuation was low. In total, 4 participants (0.9% [3 in the imiquimod cream 2.5% group and 1 in the imiquimod cream 3.75% group]) had AEs that led to study discontinuation. Application-site reactions were reported in a total of 46 participants (10.3%). The incidence and severity of local skin reactions was mostly mild or moderate, similar in both active treatment groups, and higher than in the placebo group. Local skin reactions were coincident with the treatment period and rapidly decreased when treatment was concluded. There were no clinically meaningful trends in vital sign measurements or laboratory measurements.
Comment
Imiquimod cream 5% has been shown to be a safe and effective treatment of EGWs. Our study was designed to evaluate lower concentrations of imiquimod cream (2.5% and 3.75%), which may permit daily dosing and a shortened treatment course in men with EGWs.
Efficacy of imiquimod cream 2.5% and 3.75% was established through both primary and secondary end points, though only the higher concentration was significantly more effective than placebo in both studies. In addition, a number of participants who were not completely cleared following 8 weeks of treatment went on to be completely cleared at EOS, demonstrating continued activity of imiquimod despite cessation of active treatment.
Imiquimod cream 3.75% was particularly effective when compared to placebo, with 18.6% of participants completely cleared at EOS, though the PP (observed case) results (22.7%) may be more encouraging and can be used to motivate patients.
Although there are limitations in making direct comparisons between studies, complete clearance rates in our studies were lower than those reported previously with imiquimod cream 5%.17 Lower efficacy rates might be expected given the differences in methodology. In the 2 studies reported here, participants had to have no EGWs (baseline or new, treated or untreated) in any of the anogenital areas specified to be reported as having achieved complete clearance. In earlier studies with imiquimod cream 5%, not all anogenital regions were required to be treated, and any new EGWs arising during treatment were not included in the analysis.17 Also, our analysis focused purely on a male patient population in which efficacy results tend to be lower regardless of treatment modality employed.
Recurrence is another important issue in the treatment of EGWs. Although not studied specifically in a male population, recurrence rates of 16.7% to 17.7% were seen in the 3 months following successful treatment with imiquimod cream 2.5% and 3.75% in the 2 pivotal studies. These results were consistent with the recurrence rates reported following successful treatment with imiquimod cream 5%.17
In general, complete clearance rates increased in a dose-dependent manner. Complete clearance rates were lower in the male subpopulation across all treatment groups compared to those previously reported in females,24 which was consistent with prior results reported for imiquimod cream 5% as well as other topical treatments.17 It has been suggested that this difference may be due in part to the distribution of female EGWs in areas of less keratinization. Complete clearance rates in the current analysis tended to be higher in male participants with baseline EGWs in anatomic sites with less keratinized skin such as the perianal, perineal, or glans penis areas.
Daily application of imiquimod cream 2.5% and 3.75% generally was well tolerated. Most reported AEs were mild or moderate, and few participants discontinued because of AEs. Few SAEs were reported and none were considered to be treatment related. There was no difference in the incidence rates of AEs between the 2 active treatments. The incidence of SAEs and study discontinuations was much lower than previously reported in the female cohort of these 2 studies.24
Conclusion
In conclusion, 2 well-controlled studies of males with EGWs who were treated for up to 8 weeks with imiquimod cream 2.5% and 3.75% applied daily demonstrated good tolerability and superior efficacy to placebo in complete clearance of all baseline and newly arising warts in addition to reducing EGW counts.
Acknowledgments—The authors thank Christina Cognata Smith, PharmD, and Mandeep Kaur, MD (both previously of Valeant Pharmaceuticals North America, LLC, Bridgewater, New Jersey), as well as Brian Bulley, MSc (Inergy Limited, Lindfield, West Sussex, United Kingdom), for assistance with the preparation of the manuscript. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to this analysis.
1. Weinstock H, Berman S, Cates W. Sexually transmitted infections in American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6-10.
2. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813-819.
3. Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3-8.
4. Kjaer SK, Tran TN, Sparen P, et al. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196:1447-1454.
5. Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect. 2008;84:161-166.
6. Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health. 2010;10:113.
7. Wang KL, Jeng CJ, Yang YC, et al. The psychological impact of illness among women experiencing human papillomavirus-related illness or screening interventions. J Psychsom Obstet Gynaecol. 2010;31:16-23.
8. Lawrence S, Walzman M, Sheppard S, et al. The psychological impact caused by genital warts: has the Department of Health’s choice of vaccination missed the opportunity to prevent such morbidity? Int J STD AIDS. 2009;20:696-700.
9. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
10. Centers for Disease Control and Prevention. Human papillomavirus: HPV information for clinicians. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; April 2007.
11. Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: disease, diagnosis, management, and prevention. Dermatol Ther. 2010;23:458-476.
12. Scheinfeld N, Lehman DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12:5.
13. Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2010.
14. Komericki P, Akkilic-Materna M, Strimitzer T, et al. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of genital warts. Sex Transm Dis. 2011;38:216-218.
15. Beutner KR, Tyring SK, Trofatter KF Jr, et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother. 1998;42:789-794.
16. Beutner KR, Spruance SL, Hougham AJ, et al. Treatment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol. 1998;38:230-239.
17. Edwards L, Ferenczy A, Eron L, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. Arch Dermatol. 1998;134:25-30.
18. Fife KH, Ferenczy A, Douglas JM, et al. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily, or three times a day. Sex Transm Dis. 2001;28:226-231.
19. Garland SM, Waddell R, Mindel A, et al. An open-label phase II pilot study investigating the optimal duration of imiquimod 5% cream for the treatment of external genital warts in women. Int J STD AIDS. 2006;17:448-452.
20. Schofer H, Van Ophoven A, Henke U, et al. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol. 2006;16:642-648.
21. Arican O, Guneri F, Bilgic K, et al. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31:627-631.
22. Gollnick H, Barasso R, Jappe U, et al. Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or once per day. Int J STD AIDS. 2001;12:22-28.
23. Trofatter KF Jr, Ferenczy A, Fife KH. Increased frequency of dosing of imiquimod 5% cream in the treatment of external genital warts in women. Int J Gynecol Obstet. 2002;76:191-193.
24. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies [published online ahead of print August 24, 2011]. Infect Dis Obstet Gynecol. 2011;2011:806105.
25. Dinh TH, Sternberg M, Dunne EF, et al. Genital warts among 18- to 59-year-olds in the US, National Health and Nutrition Examination Survey, 1999-2004. Sex Transm Dis. 2008;35:357-360.
26. Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107-1122.
27. Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis. 2004;31:748-752.
28. Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191:114-120.
1. Weinstock H, Berman S, Cates W. Sexually transmitted infections in American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36:6-10.
2. Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297:813-819.
3. Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3-8.
4. Kjaer SK, Tran TN, Sparen P, et al. The burden of genital warts: a study of nearly 70,000 women from the general female population in the 4 Nordic countries. J Infect Dis. 2007;196:1447-1454.
5. Woodhall S, Ramsey T, Cai C, et al. Estimation of the impact of genital warts on health-related quality of life. Sex Transm Infect. 2008;84:161-166.
6. Mortensen GL, Larsen HK. The quality of life of patients with genital warts: a qualitative study. BMC Public Health. 2010;10:113.
7. Wang KL, Jeng CJ, Yang YC, et al. The psychological impact of illness among women experiencing human papillomavirus-related illness or screening interventions. J Psychsom Obstet Gynaecol. 2010;31:16-23.
8. Lawrence S, Walzman M, Sheppard S, et al. The psychological impact caused by genital warts: has the Department of Health’s choice of vaccination missed the opportunity to prevent such morbidity? Int J STD AIDS. 2009;20:696-700.
9. Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191:731-738.
10. Centers for Disease Control and Prevention. Human papillomavirus: HPV information for clinicians. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; April 2007.
11. Forcier M, Musacchio N. An overview of human papillomavirus infection for the dermatologist: disease, diagnosis, management, and prevention. Dermatol Ther. 2010;23:458-476.
12. Scheinfeld N, Lehman DS. An evidence-based review of medical and surgical treatments of genital warts. Dermatol Online J. 2006;12:5.
13. Aldara [package insert]. Bristol, TN: Graceway Pharmaceuticals, LLC; 2010.
14. Komericki P, Akkilic-Materna M, Strimitzer T, et al. Efficacy and safety of imiquimod versus podophyllotoxin in the treatment of genital warts. Sex Transm Dis. 2011;38:216-218.
15. Beutner KR, Tyring SK, Trofatter KF Jr, et al. Imiquimod, a patient-applied immune-response modifier for treatment of external genital warts. Antimicrob Agents Chemother. 1998;42:789-794.
16. Beutner KR, Spruance SL, Hougham AJ, et al. Treatment of genital warts with an immune-response modifier (imiquimod). J Am Acad Dermatol. 1998;38:230-239.
17. Edwards L, Ferenczy A, Eron L, et al. Self-administered topical 5% imiquimod cream for external anogenital warts. Arch Dermatol. 1998;134:25-30.
18. Fife KH, Ferenczy A, Douglas JM, et al. Treatment of external genital warts in men using 5% imiquimod cream applied three times a week, once daily, twice daily, or three times a day. Sex Transm Dis. 2001;28:226-231.
19. Garland SM, Waddell R, Mindel A, et al. An open-label phase II pilot study investigating the optimal duration of imiquimod 5% cream for the treatment of external genital warts in women. Int J STD AIDS. 2006;17:448-452.
20. Schofer H, Van Ophoven A, Henke U, et al. Randomized, comparative trial on the sustained efficacy of topical imiquimod 5% cream versus conventional ablative methods in external anogenital warts. Eur J Dermatol. 2006;16:642-648.
21. Arican O, Guneri F, Bilgic K, et al. Topical imiquimod 5% cream in external anogenital warts: a randomized, double-blind, placebo-controlled study. J Dermatol. 2004;31:627-631.
22. Gollnick H, Barasso R, Jappe U, et al. Safety and efficacy of imiquimod 5% cream in the treatment of penile genital warts in uncircumcised men when applied three times weekly or once per day. Int J STD AIDS. 2001;12:22-28.
23. Trofatter KF Jr, Ferenczy A, Fife KH. Increased frequency of dosing of imiquimod 5% cream in the treatment of external genital warts in women. Int J Gynecol Obstet. 2002;76:191-193.
24. Baker DA, Ferris DG, Martens MG, et al. Imiquimod 3.75% cream applied daily to treat anogenital warts: combined results from women in two randomized, placebo-controlled studies [published online ahead of print August 24, 2011]. Infect Dis Obstet Gynecol. 2011;2011:806105.
25. Dinh TH, Sternberg M, Dunne EF, et al. Genital warts among 18- to 59-year-olds in the US, National Health and Nutrition Examination Survey, 1999-2004. Sex Transm Dis. 2008;35:357-360.
26. Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23:1107-1122.
27. Koshiol JE, Laurent SA, Pimenta JM. Rate and predictors of new genital warts claims and genital warts-related healthcare utilization among privately insured patients in the United States. Sex Transm Dis. 2004;31:748-752.
28. Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus-related disease. Am J Obstet Gynecol. 2004;191:114-120.
Practice Points
- Imiquimod cream, both 2.5% and 3.75% concentrations, is more effective than placebo in treating external genital warts (EGWs) in men.
- Imiquimod cream, in both concentrations tested, is somewhat less effective in men than in women in the same protocol.
- Imiquimod cream treatment of EGWs is better tolerated in men than in women in the same protocol.
Evaluation of Gender as a Clinically Relevant Outcome Variable in the Treatment of Onychomycosis With Efinaconazole Topical Solution 10%
Onychomycosis is the most common nail disease in adults, representing up to 50% of all nail disorders, and is nearly always associated with tinea pedis.1,2 Moreover, toenail onychomycosis frequently involves several nails3 and can be more challenging to treat because of the slow growth rate of nails and the difficult delivery of antifungal agents to the nail bed.3,4
The most prevalent predisposing risk factor for developing onychomycosis is advanced age, with a reported prevalence of 18.2% in patients aged 60 to 79 years compared to 0.7% in patients younger than 19 years.2 Men are up to 3 times more likely to develop onychomycosis than women, though the reasons for this gender difference are less clear.2,5 It has been hypothesized that occupational factors may play a role,2 with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.6
Differences in hormone levels associated with gender also may result in different capacities to inhibit the growth of dermatophytes.2 The risk for developing onychomycosis increases with age at a similar rate in both genders.7
Although onychomycosis is more common in men, the disease has been shown to have a greater impact on quality of life (QOL) in women. Studies have shown that onychomycosis was more likely to cause embarrassment in women than in men (83% vs 71%; N=258), and women with onychomycosis felt severely embarrassed more often than men (44% vs 26%; N=258).8,9 Additionally, one study (N=43,593) showed statistically significant differences associated with gender among onychomycosis patients who reported experiencing pain (33.7% of women vs 26.7% of men; P<.001), discomfort in walking (43.1% vs 36.4%; P<.001), and embarrassment (28.8% vs 25.1%; P<.001).10 Severe cases of onychomycosis even appear to have a negative impact on patients’ intimate relationships, and lower self-esteem has been reported in female patients due to unsightly and contagious-looking nail plates.11,12 Socks and stockings frequently may be damaged due to the constant friction from diseased nails that are sharp and dystrophic.13,14 In one study, treatment satisfaction was related to improvement in nail condition; however, males tended to be more satisfied with the improvement than females. Females were significantly less satisfied than males based on QOL scores for discomfort in wearing shoes (61.5 vs 86.3; P=.001), restrictions in shoe options (59.0 vs 82.8; P=.001), and the need to conceal toenails (73.3 vs 89.3; P<.01).15
Numerous studies have assessed the effectiveness of antifungal drugs in treating onychomycosis; however, there are limited data available on the impact of gender on outcome variables. Results from 2 identical 52-week, prospective, multicenter, randomized, double-blind studies of a total of 1655 participants (age range, 18–70 years) assessing the safety and efficacy of efinaconazole topical solution 10% in the treatment of onychomycosis were reported in 2013.16 Here, a gender subgroup analysis for male and female participants with mild to moderate onychomycosis is presented.
Methods
Two 52-week, prospective, multicenter, randomized, double-blind, vehicle-controlled studies were designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution 10% versus vehicle in 1655 participants aged 18 to 70 years with mild to moderate toenail onychomycosis. Participants who presented with 20% to 50% clinical involvement of the target toenail were randomized (3:1 ratio) to once-daily application of a blinded study drug on the toenails for 48 weeks, followed by a 4-week follow-up period.16
Efficacy Evaluation
The primary efficacy end point was complete cure, defined as 0% clinical involvement of target toenail and mycologic cure based on negative potassium hydroxide examination and negative fungal culture at week 52.16 Secondary and supportive efficacy end points included mycologic cure, treatment success (<10% clinical involvement of the target toenail), complete or almost complete cure (≤5% clinical involvement and mycologic cure), and change in QOL based on a self-administered QOL questionnaire. All secondary end points were assessed at week 52.16 All items in the QOL questionnaire were transferred to a 0 to 100 scale, with higher scores indicating better functioning.17
In both studies, treatment compliance was assessed through participant diaries that detailed all drug applications as well as the weight of returned product bottles. Participants were considered noncompliant if they missed more than 14 cumulative applications of the study drug in the 28 days leading up to the visit at week 48, if they missed more than 20% of the total number of expected study drug applications during the treatment period, and/or if they missed 28 or more consecutive applications of the study drug during the total treatment period.
Safety Evaluation
Safety assessments included monitoring and recording adverse events (AEs) until week 52.16
Results
The 2 studies included a total of 1275 (77.2%) male and 376 (22.8%) female participants with mild to moderate onychomycosis (intention-to-treat population). Pooled results are provided in this analysis.
At baseline, the mean area of target toenail involvement among male and female participants in the efinaconazole treatment group was 36.7% and 35.6%, respectively, compared to 36.4% and 37.9%, respectively, in the vehicle group. The mean number of affected nontarget toenails was 2.8 and 2.7 among male and female participants, respectively, in the efinaconazole group compared to 2.9 and 2.4, respectively, in the vehicle group (Table 1).
Female participants tended to be somewhat more compliant with treatment than male participants at study end. At week 52, 93.0% and 93.4% of female participants in the efinaconazole and vehicle groups, respectively, were considered compliant with treatment compared to 91.1% and 88.6% of male participants, respectively (Table 1).
Primary Efficacy End Point (Observed Case)
At week 52, 15.8% of male and 27.1% of female participants in the efinaconazole treatment group had a complete cure compared to 4.2% and 6.3%, respectively, of those in the vehicle group (both P<.001). Efinaconazole topical solution 10% was significantly more effective than vehicle from week 48 (P<.001 male and P=.004 female).
The differences in complete cure rates reported for male (15.8%) and female (27.1%) participants treated with efinaconazole topical solution 10% were significant at week 52 (P=.001)(Figure 1).
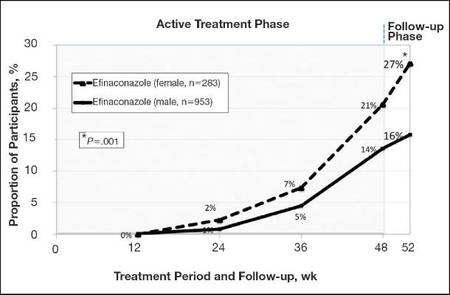 |
| Figure 1. Proportion of male and female participants treated with once-daily application of efinaconazole topical solution 10% who achieved complete cure from weeks 12 to 52 (observed case; intention-to-treat population; pooled data). |
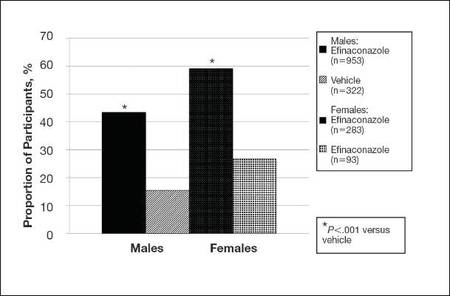 |
| Figure 2. Treatment success (defined as ≤10% clinical involvement of the target toenail) at week 52. Comparison of results with efinaconazole topical solution 10% and vehicle (observed case; intention-to-treat population; pooled data). |
Secondary and Supportive Efficacy End Points (Observed Case)
At week 52, 53.7% of male participants and 64.8% of female participants in the efinaconazole group achieved mycologic cure compared to 14.8% and 22.5%, respectively, of those in the vehicle group (both P<.001). Mycologic cure in the efinaconazole group versus the vehicle group became statistically significant at week 12 in male participants (P=.002) and at week 24 in female participants (P<.001).
At week 52, more male and female participants in the efinaconazole group (24.9% and 36.8%, respectively) achieved complete or almost complete cure compared to those in the vehicle group (6.8% and 11.3%, respectively), and 43.5% and 59.1% of male and female participants, respectively, were considered treatment successes (≤10% clinical involvement of the target toenail) compared to 15.5% and 26.8%, respectively, in the vehicle group (all P<.001)(Figure 2).
Treatment satisfaction scores were higher among female participants. At week 52, the mean QOL assessment score among female participants in the efinaconazole group was 77.2 compared to 70.3 among male participants in the same group (43.0 and 41.2, respectively, in the vehicle group). All QOL assessment scores were lower (ie, worse) in female onychomycosis participants at baseline. Improvements in all QOL scores were much greater in female participants at week 52 (Table 2).
The total number of efinaconazole applications was similar among male and female participants (315.1 vs 316.7). The mean amount of efina- conazole applied was greater in male participants (50.4 g vs 45.6 g), and overall compliance rates, though similar, were slightly higher in females compared to males (efinaconazole only)(93.0% vs 91.1%).
Safety
Overall, AE rates for efinaconazole were similar to those reported for vehicle (65.3% vs 59.8%).16 Slightly more female participants reported 1 or more AE than males (71.3% vs 63.5%). Adverse events were generally mild (50.0% in females; 53.7% in males) or moderate (46.7% in females; 41.8% in males) in severity, were not related to the study drug (89.9% in females; 93.1% in males), and resolved without sequelae. The rate of discontinuation from AEs was low (2.8% in females; 2.5% in males).
Comment
Efinaconazole topical solution 10% was significantly more effective than vehicle in both male and female participants with mild to moderate onychomycosis. It appears to be especially effective in female participants, with more than 27% of female participants achieving complete cure at week 52, and nearly 37% of female participants achieving complete or almost complete cure at week 52.
Mycologic cure is the only consistently defined efficacy parameter reported in toenail onychomycosis studies.18 It often is considered the main treatment goal, with complete cure occurring somewhat later as the nails grow out.19 Indeed, in this subgroup analysis the differences seen between the active and vehicle groups correlated well with the cure rates seen at week 52. Interestingly, significantly better mycologic cure rates (P=.002, active vs vehicle) were seen as early as week 12 in the male subgroup.
The current analysis suggests that male onychomycosis patients may be more difficult to treat, a finding noted by other investigators, though the reason is not clear.20 It is known that the prevalence of onychomycosis is higher in males,2,5 but data comparing cure rates by gender is lacking. It has been suggested that men more frequently undergo nail trauma and tend to seek help for more advanced disease.20 Treatment compliance also may be an issue. In our study, mean nail involvement was similar among male and female participants treated with efinaconazole (36.7% and 35.6%, respectively). Treatment compliance was higher among females compared to males (93.0% vs 91.1%), with the lowest compliance rates seen in males in the vehicle group (where complete cure rates also were the lowest). The amount of study drug used was greater in males, possibly due to larger toenails, though toenail surface area was not measured. Although there is no evidence to suggest that male toenails grow quicker, as many factors can impact nail growth, they tend to be thicker. Patients with thick toenails may be less likely to achieve complete cure.20 It also is possible that male toenails take longer to grow out fully, and they may require a longer treatment course. The 52-week duration of these studies may not have allowed for full regrowth of the nails, despite mycologic cure. Indeed, continued improvement in cure rates in onychomycosis patients with longer treatment courses have been noted by other investigators.21
The current analysis revealed much lower baseline QOL scores in female onychomycosis patients compared to male patients. Given that target nail involvement at baseline was similar across both groups, this finding may be indicative of greater concern about their condition among females, supporting other views that onychomycosis has a greater impact on QOL in female patients. Similar scores reported across genders at week 52 likely reflects the greater efficacy seen in females.
Conclusion
Based on this subgroup analysis, once-daily application of efinaconazole topical solution 10% may provide a useful option in the treatment of mild to moderate onychomycosis, particularly in female patients. The greater improvement in nail condition concomitantly among females translates to higher overall treatment satisfaction.
Acknowledgment—The author thanks Brian Bulley, MSc, of Inergy Limited, Lindfield, West Sussex, United Kingdom, for medical writing support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to the manuscript.
1. Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
2. Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
3. Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31-46.
4. Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
5. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
6. Araujo AJG, Bastos OMP, Souza MAJ, et al. Occurrence of onychomycosis among patients attended in dermatology offices in the city of Rio de Janeiro, Brazil. An Bras Dermatol. 2003;78:299-308.
7. Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a Pan-European Survey. Dermatology. 2001;202:220-224.
8. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5, pt 1):702-704.
9. Kowalczuk-Zieleniec E, Nowicki E, Majkowicz M. Onychomycosis changes quality of life. J Eur Acad Dermatol Venereol. 2002;16(suppl 1):248.
10. Katsambas A, Abeck D, Haneke E, et al. The effects of foot disease on quality of life: results of the Achilles Project. J Eur Acad Dermatol Venereol. 2005;19:191-195.
11. Salgo PL, Daniel CR, Gupta AK, et al. Onychomycosis disease management. Medical Crossfire: Debates, Peer Exchange and Insights in Medicine. 2003;4:1-17.
12. Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754-756.
13. Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br J Dermatol. 2001;145:3-8.
14. Whittam LR, Hay RJ. The impact of onychomycosis on quality of life. Clin Exp Dermatol. 1997;22:87-89.
15. Stier DM, Gause D, Joseph WS, et al. Patient satisfaction with oral versus nonoral therapeutic approaches in onychomycosis. J Am Podiatr Med Assoc. 2001;91:521-527.
16. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
17. Tosti A, Elewski BE. Treatment of onychomycosis with efinaconazole 10% topical solution and quality of life. J Clin Aesthet Dermatol. 2014;7:25-30.
18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol. 2004;5:145-152.
19. Gupta AK. Treatment of dermatophyte toenail onychomycosis in the United States: a pharmacoeconomic analysis. J Am Podiatr Med Assoc. 2002;92:272-286.
20. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010;24:679-684.
21. Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? an analysis of published data. Arch Dermatol. 1998;134:1551-1554.
Onychomycosis is the most common nail disease in adults, representing up to 50% of all nail disorders, and is nearly always associated with tinea pedis.1,2 Moreover, toenail onychomycosis frequently involves several nails3 and can be more challenging to treat because of the slow growth rate of nails and the difficult delivery of antifungal agents to the nail bed.3,4
The most prevalent predisposing risk factor for developing onychomycosis is advanced age, with a reported prevalence of 18.2% in patients aged 60 to 79 years compared to 0.7% in patients younger than 19 years.2 Men are up to 3 times more likely to develop onychomycosis than women, though the reasons for this gender difference are less clear.2,5 It has been hypothesized that occupational factors may play a role,2 with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.6
Differences in hormone levels associated with gender also may result in different capacities to inhibit the growth of dermatophytes.2 The risk for developing onychomycosis increases with age at a similar rate in both genders.7
Although onychomycosis is more common in men, the disease has been shown to have a greater impact on quality of life (QOL) in women. Studies have shown that onychomycosis was more likely to cause embarrassment in women than in men (83% vs 71%; N=258), and women with onychomycosis felt severely embarrassed more often than men (44% vs 26%; N=258).8,9 Additionally, one study (N=43,593) showed statistically significant differences associated with gender among onychomycosis patients who reported experiencing pain (33.7% of women vs 26.7% of men; P<.001), discomfort in walking (43.1% vs 36.4%; P<.001), and embarrassment (28.8% vs 25.1%; P<.001).10 Severe cases of onychomycosis even appear to have a negative impact on patients’ intimate relationships, and lower self-esteem has been reported in female patients due to unsightly and contagious-looking nail plates.11,12 Socks and stockings frequently may be damaged due to the constant friction from diseased nails that are sharp and dystrophic.13,14 In one study, treatment satisfaction was related to improvement in nail condition; however, males tended to be more satisfied with the improvement than females. Females were significantly less satisfied than males based on QOL scores for discomfort in wearing shoes (61.5 vs 86.3; P=.001), restrictions in shoe options (59.0 vs 82.8; P=.001), and the need to conceal toenails (73.3 vs 89.3; P<.01).15
Numerous studies have assessed the effectiveness of antifungal drugs in treating onychomycosis; however, there are limited data available on the impact of gender on outcome variables. Results from 2 identical 52-week, prospective, multicenter, randomized, double-blind studies of a total of 1655 participants (age range, 18–70 years) assessing the safety and efficacy of efinaconazole topical solution 10% in the treatment of onychomycosis were reported in 2013.16 Here, a gender subgroup analysis for male and female participants with mild to moderate onychomycosis is presented.
Methods
Two 52-week, prospective, multicenter, randomized, double-blind, vehicle-controlled studies were designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution 10% versus vehicle in 1655 participants aged 18 to 70 years with mild to moderate toenail onychomycosis. Participants who presented with 20% to 50% clinical involvement of the target toenail were randomized (3:1 ratio) to once-daily application of a blinded study drug on the toenails for 48 weeks, followed by a 4-week follow-up period.16
Efficacy Evaluation
The primary efficacy end point was complete cure, defined as 0% clinical involvement of target toenail and mycologic cure based on negative potassium hydroxide examination and negative fungal culture at week 52.16 Secondary and supportive efficacy end points included mycologic cure, treatment success (<10% clinical involvement of the target toenail), complete or almost complete cure (≤5% clinical involvement and mycologic cure), and change in QOL based on a self-administered QOL questionnaire. All secondary end points were assessed at week 52.16 All items in the QOL questionnaire were transferred to a 0 to 100 scale, with higher scores indicating better functioning.17
In both studies, treatment compliance was assessed through participant diaries that detailed all drug applications as well as the weight of returned product bottles. Participants were considered noncompliant if they missed more than 14 cumulative applications of the study drug in the 28 days leading up to the visit at week 48, if they missed more than 20% of the total number of expected study drug applications during the treatment period, and/or if they missed 28 or more consecutive applications of the study drug during the total treatment period.
Safety Evaluation
Safety assessments included monitoring and recording adverse events (AEs) until week 52.16
Results
The 2 studies included a total of 1275 (77.2%) male and 376 (22.8%) female participants with mild to moderate onychomycosis (intention-to-treat population). Pooled results are provided in this analysis.
At baseline, the mean area of target toenail involvement among male and female participants in the efinaconazole treatment group was 36.7% and 35.6%, respectively, compared to 36.4% and 37.9%, respectively, in the vehicle group. The mean number of affected nontarget toenails was 2.8 and 2.7 among male and female participants, respectively, in the efinaconazole group compared to 2.9 and 2.4, respectively, in the vehicle group (Table 1).
Female participants tended to be somewhat more compliant with treatment than male participants at study end. At week 52, 93.0% and 93.4% of female participants in the efinaconazole and vehicle groups, respectively, were considered compliant with treatment compared to 91.1% and 88.6% of male participants, respectively (Table 1).
Primary Efficacy End Point (Observed Case)
At week 52, 15.8% of male and 27.1% of female participants in the efinaconazole treatment group had a complete cure compared to 4.2% and 6.3%, respectively, of those in the vehicle group (both P<.001). Efinaconazole topical solution 10% was significantly more effective than vehicle from week 48 (P<.001 male and P=.004 female).
The differences in complete cure rates reported for male (15.8%) and female (27.1%) participants treated with efinaconazole topical solution 10% were significant at week 52 (P=.001)(Figure 1).
 |
| Figure 1. Proportion of male and female participants treated with once-daily application of efinaconazole topical solution 10% who achieved complete cure from weeks 12 to 52 (observed case; intention-to-treat population; pooled data). |
 |
| Figure 2. Treatment success (defined as ≤10% clinical involvement of the target toenail) at week 52. Comparison of results with efinaconazole topical solution 10% and vehicle (observed case; intention-to-treat population; pooled data). |
Secondary and Supportive Efficacy End Points (Observed Case)
At week 52, 53.7% of male participants and 64.8% of female participants in the efinaconazole group achieved mycologic cure compared to 14.8% and 22.5%, respectively, of those in the vehicle group (both P<.001). Mycologic cure in the efinaconazole group versus the vehicle group became statistically significant at week 12 in male participants (P=.002) and at week 24 in female participants (P<.001).
At week 52, more male and female participants in the efinaconazole group (24.9% and 36.8%, respectively) achieved complete or almost complete cure compared to those in the vehicle group (6.8% and 11.3%, respectively), and 43.5% and 59.1% of male and female participants, respectively, were considered treatment successes (≤10% clinical involvement of the target toenail) compared to 15.5% and 26.8%, respectively, in the vehicle group (all P<.001)(Figure 2).
Treatment satisfaction scores were higher among female participants. At week 52, the mean QOL assessment score among female participants in the efinaconazole group was 77.2 compared to 70.3 among male participants in the same group (43.0 and 41.2, respectively, in the vehicle group). All QOL assessment scores were lower (ie, worse) in female onychomycosis participants at baseline. Improvements in all QOL scores were much greater in female participants at week 52 (Table 2).
The total number of efinaconazole applications was similar among male and female participants (315.1 vs 316.7). The mean amount of efina- conazole applied was greater in male participants (50.4 g vs 45.6 g), and overall compliance rates, though similar, were slightly higher in females compared to males (efinaconazole only)(93.0% vs 91.1%).
Safety
Overall, AE rates for efinaconazole were similar to those reported for vehicle (65.3% vs 59.8%).16 Slightly more female participants reported 1 or more AE than males (71.3% vs 63.5%). Adverse events were generally mild (50.0% in females; 53.7% in males) or moderate (46.7% in females; 41.8% in males) in severity, were not related to the study drug (89.9% in females; 93.1% in males), and resolved without sequelae. The rate of discontinuation from AEs was low (2.8% in females; 2.5% in males).
Comment
Efinaconazole topical solution 10% was significantly more effective than vehicle in both male and female participants with mild to moderate onychomycosis. It appears to be especially effective in female participants, with more than 27% of female participants achieving complete cure at week 52, and nearly 37% of female participants achieving complete or almost complete cure at week 52.
Mycologic cure is the only consistently defined efficacy parameter reported in toenail onychomycosis studies.18 It often is considered the main treatment goal, with complete cure occurring somewhat later as the nails grow out.19 Indeed, in this subgroup analysis the differences seen between the active and vehicle groups correlated well with the cure rates seen at week 52. Interestingly, significantly better mycologic cure rates (P=.002, active vs vehicle) were seen as early as week 12 in the male subgroup.
The current analysis suggests that male onychomycosis patients may be more difficult to treat, a finding noted by other investigators, though the reason is not clear.20 It is known that the prevalence of onychomycosis is higher in males,2,5 but data comparing cure rates by gender is lacking. It has been suggested that men more frequently undergo nail trauma and tend to seek help for more advanced disease.20 Treatment compliance also may be an issue. In our study, mean nail involvement was similar among male and female participants treated with efinaconazole (36.7% and 35.6%, respectively). Treatment compliance was higher among females compared to males (93.0% vs 91.1%), with the lowest compliance rates seen in males in the vehicle group (where complete cure rates also were the lowest). The amount of study drug used was greater in males, possibly due to larger toenails, though toenail surface area was not measured. Although there is no evidence to suggest that male toenails grow quicker, as many factors can impact nail growth, they tend to be thicker. Patients with thick toenails may be less likely to achieve complete cure.20 It also is possible that male toenails take longer to grow out fully, and they may require a longer treatment course. The 52-week duration of these studies may not have allowed for full regrowth of the nails, despite mycologic cure. Indeed, continued improvement in cure rates in onychomycosis patients with longer treatment courses have been noted by other investigators.21
The current analysis revealed much lower baseline QOL scores in female onychomycosis patients compared to male patients. Given that target nail involvement at baseline was similar across both groups, this finding may be indicative of greater concern about their condition among females, supporting other views that onychomycosis has a greater impact on QOL in female patients. Similar scores reported across genders at week 52 likely reflects the greater efficacy seen in females.
Conclusion
Based on this subgroup analysis, once-daily application of efinaconazole topical solution 10% may provide a useful option in the treatment of mild to moderate onychomycosis, particularly in female patients. The greater improvement in nail condition concomitantly among females translates to higher overall treatment satisfaction.
Acknowledgment—The author thanks Brian Bulley, MSc, of Inergy Limited, Lindfield, West Sussex, United Kingdom, for medical writing support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to the manuscript.
Onychomycosis is the most common nail disease in adults, representing up to 50% of all nail disorders, and is nearly always associated with tinea pedis.1,2 Moreover, toenail onychomycosis frequently involves several nails3 and can be more challenging to treat because of the slow growth rate of nails and the difficult delivery of antifungal agents to the nail bed.3,4
The most prevalent predisposing risk factor for developing onychomycosis is advanced age, with a reported prevalence of 18.2% in patients aged 60 to 79 years compared to 0.7% in patients younger than 19 years.2 Men are up to 3 times more likely to develop onychomycosis than women, though the reasons for this gender difference are less clear.2,5 It has been hypothesized that occupational factors may play a role,2 with increased use of occlusive footwear and more frequent nail injuries contributing to a higher incidence of onychomycosis in males.6
Differences in hormone levels associated with gender also may result in different capacities to inhibit the growth of dermatophytes.2 The risk for developing onychomycosis increases with age at a similar rate in both genders.7
Although onychomycosis is more common in men, the disease has been shown to have a greater impact on quality of life (QOL) in women. Studies have shown that onychomycosis was more likely to cause embarrassment in women than in men (83% vs 71%; N=258), and women with onychomycosis felt severely embarrassed more often than men (44% vs 26%; N=258).8,9 Additionally, one study (N=43,593) showed statistically significant differences associated with gender among onychomycosis patients who reported experiencing pain (33.7% of women vs 26.7% of men; P<.001), discomfort in walking (43.1% vs 36.4%; P<.001), and embarrassment (28.8% vs 25.1%; P<.001).10 Severe cases of onychomycosis even appear to have a negative impact on patients’ intimate relationships, and lower self-esteem has been reported in female patients due to unsightly and contagious-looking nail plates.11,12 Socks and stockings frequently may be damaged due to the constant friction from diseased nails that are sharp and dystrophic.13,14 In one study, treatment satisfaction was related to improvement in nail condition; however, males tended to be more satisfied with the improvement than females. Females were significantly less satisfied than males based on QOL scores for discomfort in wearing shoes (61.5 vs 86.3; P=.001), restrictions in shoe options (59.0 vs 82.8; P=.001), and the need to conceal toenails (73.3 vs 89.3; P<.01).15
Numerous studies have assessed the effectiveness of antifungal drugs in treating onychomycosis; however, there are limited data available on the impact of gender on outcome variables. Results from 2 identical 52-week, prospective, multicenter, randomized, double-blind studies of a total of 1655 participants (age range, 18–70 years) assessing the safety and efficacy of efinaconazole topical solution 10% in the treatment of onychomycosis were reported in 2013.16 Here, a gender subgroup analysis for male and female participants with mild to moderate onychomycosis is presented.
Methods
Two 52-week, prospective, multicenter, randomized, double-blind, vehicle-controlled studies were designed to evaluate the efficacy, safety, and tolerability of efinaconazole topical solution 10% versus vehicle in 1655 participants aged 18 to 70 years with mild to moderate toenail onychomycosis. Participants who presented with 20% to 50% clinical involvement of the target toenail were randomized (3:1 ratio) to once-daily application of a blinded study drug on the toenails for 48 weeks, followed by a 4-week follow-up period.16
Efficacy Evaluation
The primary efficacy end point was complete cure, defined as 0% clinical involvement of target toenail and mycologic cure based on negative potassium hydroxide examination and negative fungal culture at week 52.16 Secondary and supportive efficacy end points included mycologic cure, treatment success (<10% clinical involvement of the target toenail), complete or almost complete cure (≤5% clinical involvement and mycologic cure), and change in QOL based on a self-administered QOL questionnaire. All secondary end points were assessed at week 52.16 All items in the QOL questionnaire were transferred to a 0 to 100 scale, with higher scores indicating better functioning.17
In both studies, treatment compliance was assessed through participant diaries that detailed all drug applications as well as the weight of returned product bottles. Participants were considered noncompliant if they missed more than 14 cumulative applications of the study drug in the 28 days leading up to the visit at week 48, if they missed more than 20% of the total number of expected study drug applications during the treatment period, and/or if they missed 28 or more consecutive applications of the study drug during the total treatment period.
Safety Evaluation
Safety assessments included monitoring and recording adverse events (AEs) until week 52.16
Results
The 2 studies included a total of 1275 (77.2%) male and 376 (22.8%) female participants with mild to moderate onychomycosis (intention-to-treat population). Pooled results are provided in this analysis.
At baseline, the mean area of target toenail involvement among male and female participants in the efinaconazole treatment group was 36.7% and 35.6%, respectively, compared to 36.4% and 37.9%, respectively, in the vehicle group. The mean number of affected nontarget toenails was 2.8 and 2.7 among male and female participants, respectively, in the efinaconazole group compared to 2.9 and 2.4, respectively, in the vehicle group (Table 1).
Female participants tended to be somewhat more compliant with treatment than male participants at study end. At week 52, 93.0% and 93.4% of female participants in the efinaconazole and vehicle groups, respectively, were considered compliant with treatment compared to 91.1% and 88.6% of male participants, respectively (Table 1).
Primary Efficacy End Point (Observed Case)
At week 52, 15.8% of male and 27.1% of female participants in the efinaconazole treatment group had a complete cure compared to 4.2% and 6.3%, respectively, of those in the vehicle group (both P<.001). Efinaconazole topical solution 10% was significantly more effective than vehicle from week 48 (P<.001 male and P=.004 female).
The differences in complete cure rates reported for male (15.8%) and female (27.1%) participants treated with efinaconazole topical solution 10% were significant at week 52 (P=.001)(Figure 1).
 |
| Figure 1. Proportion of male and female participants treated with once-daily application of efinaconazole topical solution 10% who achieved complete cure from weeks 12 to 52 (observed case; intention-to-treat population; pooled data). |
 |
| Figure 2. Treatment success (defined as ≤10% clinical involvement of the target toenail) at week 52. Comparison of results with efinaconazole topical solution 10% and vehicle (observed case; intention-to-treat population; pooled data). |
Secondary and Supportive Efficacy End Points (Observed Case)
At week 52, 53.7% of male participants and 64.8% of female participants in the efinaconazole group achieved mycologic cure compared to 14.8% and 22.5%, respectively, of those in the vehicle group (both P<.001). Mycologic cure in the efinaconazole group versus the vehicle group became statistically significant at week 12 in male participants (P=.002) and at week 24 in female participants (P<.001).
At week 52, more male and female participants in the efinaconazole group (24.9% and 36.8%, respectively) achieved complete or almost complete cure compared to those in the vehicle group (6.8% and 11.3%, respectively), and 43.5% and 59.1% of male and female participants, respectively, were considered treatment successes (≤10% clinical involvement of the target toenail) compared to 15.5% and 26.8%, respectively, in the vehicle group (all P<.001)(Figure 2).
Treatment satisfaction scores were higher among female participants. At week 52, the mean QOL assessment score among female participants in the efinaconazole group was 77.2 compared to 70.3 among male participants in the same group (43.0 and 41.2, respectively, in the vehicle group). All QOL assessment scores were lower (ie, worse) in female onychomycosis participants at baseline. Improvements in all QOL scores were much greater in female participants at week 52 (Table 2).
The total number of efinaconazole applications was similar among male and female participants (315.1 vs 316.7). The mean amount of efina- conazole applied was greater in male participants (50.4 g vs 45.6 g), and overall compliance rates, though similar, were slightly higher in females compared to males (efinaconazole only)(93.0% vs 91.1%).
Safety
Overall, AE rates for efinaconazole were similar to those reported for vehicle (65.3% vs 59.8%).16 Slightly more female participants reported 1 or more AE than males (71.3% vs 63.5%). Adverse events were generally mild (50.0% in females; 53.7% in males) or moderate (46.7% in females; 41.8% in males) in severity, were not related to the study drug (89.9% in females; 93.1% in males), and resolved without sequelae. The rate of discontinuation from AEs was low (2.8% in females; 2.5% in males).
Comment
Efinaconazole topical solution 10% was significantly more effective than vehicle in both male and female participants with mild to moderate onychomycosis. It appears to be especially effective in female participants, with more than 27% of female participants achieving complete cure at week 52, and nearly 37% of female participants achieving complete or almost complete cure at week 52.
Mycologic cure is the only consistently defined efficacy parameter reported in toenail onychomycosis studies.18 It often is considered the main treatment goal, with complete cure occurring somewhat later as the nails grow out.19 Indeed, in this subgroup analysis the differences seen between the active and vehicle groups correlated well with the cure rates seen at week 52. Interestingly, significantly better mycologic cure rates (P=.002, active vs vehicle) were seen as early as week 12 in the male subgroup.
The current analysis suggests that male onychomycosis patients may be more difficult to treat, a finding noted by other investigators, though the reason is not clear.20 It is known that the prevalence of onychomycosis is higher in males,2,5 but data comparing cure rates by gender is lacking. It has been suggested that men more frequently undergo nail trauma and tend to seek help for more advanced disease.20 Treatment compliance also may be an issue. In our study, mean nail involvement was similar among male and female participants treated with efinaconazole (36.7% and 35.6%, respectively). Treatment compliance was higher among females compared to males (93.0% vs 91.1%), with the lowest compliance rates seen in males in the vehicle group (where complete cure rates also were the lowest). The amount of study drug used was greater in males, possibly due to larger toenails, though toenail surface area was not measured. Although there is no evidence to suggest that male toenails grow quicker, as many factors can impact nail growth, they tend to be thicker. Patients with thick toenails may be less likely to achieve complete cure.20 It also is possible that male toenails take longer to grow out fully, and they may require a longer treatment course. The 52-week duration of these studies may not have allowed for full regrowth of the nails, despite mycologic cure. Indeed, continued improvement in cure rates in onychomycosis patients with longer treatment courses have been noted by other investigators.21
The current analysis revealed much lower baseline QOL scores in female onychomycosis patients compared to male patients. Given that target nail involvement at baseline was similar across both groups, this finding may be indicative of greater concern about their condition among females, supporting other views that onychomycosis has a greater impact on QOL in female patients. Similar scores reported across genders at week 52 likely reflects the greater efficacy seen in females.
Conclusion
Based on this subgroup analysis, once-daily application of efinaconazole topical solution 10% may provide a useful option in the treatment of mild to moderate onychomycosis, particularly in female patients. The greater improvement in nail condition concomitantly among females translates to higher overall treatment satisfaction.
Acknowledgment—The author thanks Brian Bulley, MSc, of Inergy Limited, Lindfield, West Sussex, United Kingdom, for medical writing support. Valeant Pharmaceuticals North America, LLC, funded Inergy’s activities pertaining to the manuscript.
1. Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
2. Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
3. Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31-46.
4. Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
5. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
6. Araujo AJG, Bastos OMP, Souza MAJ, et al. Occurrence of onychomycosis among patients attended in dermatology offices in the city of Rio de Janeiro, Brazil. An Bras Dermatol. 2003;78:299-308.
7. Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a Pan-European Survey. Dermatology. 2001;202:220-224.
8. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5, pt 1):702-704.
9. Kowalczuk-Zieleniec E, Nowicki E, Majkowicz M. Onychomycosis changes quality of life. J Eur Acad Dermatol Venereol. 2002;16(suppl 1):248.
10. Katsambas A, Abeck D, Haneke E, et al. The effects of foot disease on quality of life: results of the Achilles Project. J Eur Acad Dermatol Venereol. 2005;19:191-195.
11. Salgo PL, Daniel CR, Gupta AK, et al. Onychomycosis disease management. Medical Crossfire: Debates, Peer Exchange and Insights in Medicine. 2003;4:1-17.
12. Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754-756.
13. Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br J Dermatol. 2001;145:3-8.
14. Whittam LR, Hay RJ. The impact of onychomycosis on quality of life. Clin Exp Dermatol. 1997;22:87-89.
15. Stier DM, Gause D, Joseph WS, et al. Patient satisfaction with oral versus nonoral therapeutic approaches in onychomycosis. J Am Podiatr Med Assoc. 2001;91:521-527.
16. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
17. Tosti A, Elewski BE. Treatment of onychomycosis with efinaconazole 10% topical solution and quality of life. J Clin Aesthet Dermatol. 2014;7:25-30.
18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol. 2004;5:145-152.
19. Gupta AK. Treatment of dermatophyte toenail onychomycosis in the United States: a pharmacoeconomic analysis. J Am Podiatr Med Assoc. 2002;92:272-286.
20. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010;24:679-684.
21. Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? an analysis of published data. Arch Dermatol. 1998;134:1551-1554.
1. Scher RK, Coppa LM. Advances in the diagnosis and treatment of onychomycosis. Hosp Med. 1998;34:11-20.
2. Gupta AK, Jain HC, Lynde CW, et al. Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. J Am Acad Dermatol. 2000;43:244-248.
3. Finch JJ, Warshaw EM. Toenail onychomycosis: current and future treatment options. Dermatol Ther. 2007;20:31-46.
4. Kumar S, Kimball AB. New antifungal therapies for the treatment of onychomycosis. Expert Opin Investig Drugs. 2009;18:727-734.
5. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
6. Araujo AJG, Bastos OMP, Souza MAJ, et al. Occurrence of onychomycosis among patients attended in dermatology offices in the city of Rio de Janeiro, Brazil. An Bras Dermatol. 2003;78:299-308.
7. Pierard G. Onychomycosis and other superficial fungal infections of the foot in the elderly: a Pan-European Survey. Dermatology. 2001;202:220-224.
8. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5, pt 1):702-704.
9. Kowalczuk-Zieleniec E, Nowicki E, Majkowicz M. Onychomycosis changes quality of life. J Eur Acad Dermatol Venereol. 2002;16(suppl 1):248.
10. Katsambas A, Abeck D, Haneke E, et al. The effects of foot disease on quality of life: results of the Achilles Project. J Eur Acad Dermatol Venereol. 2005;19:191-195.
11. Salgo PL, Daniel CR, Gupta AK, et al. Onychomycosis disease management. Medical Crossfire: Debates, Peer Exchange and Insights in Medicine. 2003;4:1-17.
12. Elewski BE. The effect of toenail onychomycosis on patient quality of life. Int J Dermatol. 1997;36:754-756.
13. Hay RJ. The future of onychomycosis therapy may involve a combination of approaches. Br J Dermatol. 2001;145:3-8.
14. Whittam LR, Hay RJ. The impact of onychomycosis on quality of life. Clin Exp Dermatol. 1997;22:87-89.
15. Stier DM, Gause D, Joseph WS, et al. Patient satisfaction with oral versus nonoral therapeutic approaches in onychomycosis. J Am Podiatr Med Assoc. 2001;91:521-527.
16. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase 3 multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
17. Tosti A, Elewski BE. Treatment of onychomycosis with efinaconazole 10% topical solution and quality of life. J Clin Aesthet Dermatol. 2014;7:25-30.
18. Werschler WP, Bondar G, Armstrong D. Assessing treatment outcomes in toenail onychomycosis clinical trials. Am J Clin Dermatol. 2004;5:145-152.
19. Gupta AK. Treatment of dermatophyte toenail onychomycosis in the United States: a pharmacoeconomic analysis. J Am Podiatr Med Assoc. 2002;92:272-286.
20. Sigurgeirsson B. Prognostic factors for cure following treatment of onychomycosis. J Eur Acad Dermatol Venereol. 2010;24:679-684.
21. Epstein E. How often does oral treatment of toenail onychomycosis produce a disease-free nail? an analysis of published data. Arch Dermatol. 1998;134:1551-1554.
Practice Points
- Men, particularly as they age, are more likely to develop onychomycosis.
- Treatment adherence may be a bigger issue among male patients.
- Onychomycosis in males may be more difficult to treat for a variety of reasons.
Dermatologic Complications From Sojourns Abroad

Global travel has become ubiquitous for recreational, occupational, educational, humanitarian, and other purposes. For this reason, those who travel may encounter and acquire diseases from countries outside their normal habitat. A recent fascinating session from the Summer American Academy of Dermatology Meeting, “Infectious Disease and Infestations in Returned Travelers: The Americas,” highlighted current trends relating to this phenomenon.
Dr. Natasha Mesinkovska noted that Americans are most likely to travel within the Americas, particularly to Mexico, Canada, and the Dominican Republic, but also to England and France. Conversely, approximately 66 million visitors from other regions visit the United States annually. GeoSentinel surveillance clinics suggest that diarrhea and febrile illness, such as malaria, are the most common concerns reported among returning travelers who are ill. Dermatologic concerns account for approximately 18% of all problems (Int J Infect Dis. 2008;12:593-602), and many of these are either cosmopolitan infections (eg, scabies, herpes, staphylococcal) or specific tropical infections (eg, cutaneous larva migrans, tungiasis, leishmaniasis, myiasis). Because many tropical infections are associated with insect vectors, particularly mosquitoes, the prophylactic use of repellant products such as 30% to 35% DEET is advisable. Also, travelers should avoid contact with sand, soil, stagnant water, and farm animals; drink only pasteurized liquids; close hotel windows; and sleep under mosquito netting, where appropriate.
Dr. Ron (Ronald) Rapini reviewed the various diagnostic procedures that may be used for unusual infections associated with the Americas. Diagnostic techniques include potassium hydroxide preparation, routine biopsy, biopsy with special stains, culture, and serologic testing. For example, South American blastomycosis is associated with 90% sensitive and 80% specific serologic test, and Gomori methenamine-silver or Periodic acid–Schiff stains may reveal the characteristic mariner’s wheel organisms in tissue.
Dr. Dirk Elston reviewed the nuances of dealing with worms and insects. Ivermectin may be given to kill the maggots that cause myiasis; the dead maggots can then be easily removed about a week later, as spines that lodge them into the tissue have retracted. A snake venom extractor may be utilized to suck these maggots out of infested tissue. Recurring scabies may lead to chronic impetigo and thence to glomerulonephritis and renal failure. Use of the dermatoscope may aid in the diagnosis of scabies. Many cases of so-called prurigo nodularis may actually be due to repeated bed bug bites. Both reduviid bugs and wild and domestic mammals have been found to harbor the etiologic trypanosomes of Chagas disease within the continental United States (Clin Microbiol Rev. 2011;24:655-681). Cyclic fevers should at least suggest the diagnosis of the tick-borne illness babesiosis.
Dr. Jose Dario Martinez reviewed the problem of “rash and fever.” Among the common culprits associated with such a presentation are dengue and chikungunya. Both are mosquito borne and both have now been seen in the United States, most commonly in returning travelers but also rarely as autochthonous infection. “Islands of normal in a sea of red”–appearing skin associated with fever, headache, retro-orbital pain, any sign of bleeding diathesis, and thrombocytopenia suggests dengue hemorrhagic fever. IgM serologic tests may be positive approximately 5 days into the infection, but polymerase chain reaction tests may be even more rapid. Promising work on a 3-dose quadrivalent dengue vaccine recently has been published (Lancet. July 10, 2014. doi:10.1016/S0140-6736(14)61142-9). Chikungunya, once previously limited to parts of Asia and Africa, is now present in the Americas. As of August 2014, local transmission had been identified in 31 countries or territories in the Caribbean, Central America, South America, or North America including the United States. Clinically, this disorder resembles dengue with less bleeding diathesis and more severe polyarthralgia. Reverse transcription–polymerase chain reaction testing can confirm the diagnosis in less than a week after infection. Treatment is supportive only, and a vaccine is under testing.
I discussed the ever-expanding worldwide epidemic of bed bug infestation, as verified by reports on a global bed bug registry Web site. Although bed bugs have not yet been reported to transmit disease to humans, the bite is pruritic and may be associated with severe emotional ramifications (Psychosomatics. 2012;53:85-91). Bed bugs can be detected by visual inspection of typical hiding places within 3 feet of the bed; expensive but very sensitive lures and bed bug–detecting canines also can be used to verify an infestation. Clearing bed bug infestations may require a complex multipronged approach, including thorough vacuuming and steaming, placing traps and desiccants, spreading insecticides, and thermal remediation. To avoid bringing bed bugs into the home following a trip, travelers are advised to check for bed bug infestation of hotel rooms, keep luggage off the floor and hang clothing up high, and launder clothes immediately upon returning home.
What’s the issue?
Travelers are potentially subject to many complications relating to their sojourn, particularly those of an infectious nature. Bugs and worms and unicellular parasites account for many of these complications. This situation is not static but remains perpetually evolving, as evidenced by the recent and rapid appearance of chikungunya, a virus in North America. To whom would you turn for advice or consultation if your patient returned from an exotic destination with a fever and skin abnormalities with which you have no familiarity?

Global travel has become ubiquitous for recreational, occupational, educational, humanitarian, and other purposes. For this reason, those who travel may encounter and acquire diseases from countries outside their normal habitat. A recent fascinating session from the Summer American Academy of Dermatology Meeting, “Infectious Disease and Infestations in Returned Travelers: The Americas,” highlighted current trends relating to this phenomenon.
Dr. Natasha Mesinkovska noted that Americans are most likely to travel within the Americas, particularly to Mexico, Canada, and the Dominican Republic, but also to England and France. Conversely, approximately 66 million visitors from other regions visit the United States annually. GeoSentinel surveillance clinics suggest that diarrhea and febrile illness, such as malaria, are the most common concerns reported among returning travelers who are ill. Dermatologic concerns account for approximately 18% of all problems (Int J Infect Dis. 2008;12:593-602), and many of these are either cosmopolitan infections (eg, scabies, herpes, staphylococcal) or specific tropical infections (eg, cutaneous larva migrans, tungiasis, leishmaniasis, myiasis). Because many tropical infections are associated with insect vectors, particularly mosquitoes, the prophylactic use of repellant products such as 30% to 35% DEET is advisable. Also, travelers should avoid contact with sand, soil, stagnant water, and farm animals; drink only pasteurized liquids; close hotel windows; and sleep under mosquito netting, where appropriate.
Dr. Ron (Ronald) Rapini reviewed the various diagnostic procedures that may be used for unusual infections associated with the Americas. Diagnostic techniques include potassium hydroxide preparation, routine biopsy, biopsy with special stains, culture, and serologic testing. For example, South American blastomycosis is associated with 90% sensitive and 80% specific serologic test, and Gomori methenamine-silver or Periodic acid–Schiff stains may reveal the characteristic mariner’s wheel organisms in tissue.
Dr. Dirk Elston reviewed the nuances of dealing with worms and insects. Ivermectin may be given to kill the maggots that cause myiasis; the dead maggots can then be easily removed about a week later, as spines that lodge them into the tissue have retracted. A snake venom extractor may be utilized to suck these maggots out of infested tissue. Recurring scabies may lead to chronic impetigo and thence to glomerulonephritis and renal failure. Use of the dermatoscope may aid in the diagnosis of scabies. Many cases of so-called prurigo nodularis may actually be due to repeated bed bug bites. Both reduviid bugs and wild and domestic mammals have been found to harbor the etiologic trypanosomes of Chagas disease within the continental United States (Clin Microbiol Rev. 2011;24:655-681). Cyclic fevers should at least suggest the diagnosis of the tick-borne illness babesiosis.
Dr. Jose Dario Martinez reviewed the problem of “rash and fever.” Among the common culprits associated with such a presentation are dengue and chikungunya. Both are mosquito borne and both have now been seen in the United States, most commonly in returning travelers but also rarely as autochthonous infection. “Islands of normal in a sea of red”–appearing skin associated with fever, headache, retro-orbital pain, any sign of bleeding diathesis, and thrombocytopenia suggests dengue hemorrhagic fever. IgM serologic tests may be positive approximately 5 days into the infection, but polymerase chain reaction tests may be even more rapid. Promising work on a 3-dose quadrivalent dengue vaccine recently has been published (Lancet. July 10, 2014. doi:10.1016/S0140-6736(14)61142-9). Chikungunya, once previously limited to parts of Asia and Africa, is now present in the Americas. As of August 2014, local transmission had been identified in 31 countries or territories in the Caribbean, Central America, South America, or North America including the United States. Clinically, this disorder resembles dengue with less bleeding diathesis and more severe polyarthralgia. Reverse transcription–polymerase chain reaction testing can confirm the diagnosis in less than a week after infection. Treatment is supportive only, and a vaccine is under testing.
I discussed the ever-expanding worldwide epidemic of bed bug infestation, as verified by reports on a global bed bug registry Web site. Although bed bugs have not yet been reported to transmit disease to humans, the bite is pruritic and may be associated with severe emotional ramifications (Psychosomatics. 2012;53:85-91). Bed bugs can be detected by visual inspection of typical hiding places within 3 feet of the bed; expensive but very sensitive lures and bed bug–detecting canines also can be used to verify an infestation. Clearing bed bug infestations may require a complex multipronged approach, including thorough vacuuming and steaming, placing traps and desiccants, spreading insecticides, and thermal remediation. To avoid bringing bed bugs into the home following a trip, travelers are advised to check for bed bug infestation of hotel rooms, keep luggage off the floor and hang clothing up high, and launder clothes immediately upon returning home.
What’s the issue?
Travelers are potentially subject to many complications relating to their sojourn, particularly those of an infectious nature. Bugs and worms and unicellular parasites account for many of these complications. This situation is not static but remains perpetually evolving, as evidenced by the recent and rapid appearance of chikungunya, a virus in North America. To whom would you turn for advice or consultation if your patient returned from an exotic destination with a fever and skin abnormalities with which you have no familiarity?

Global travel has become ubiquitous for recreational, occupational, educational, humanitarian, and other purposes. For this reason, those who travel may encounter and acquire diseases from countries outside their normal habitat. A recent fascinating session from the Summer American Academy of Dermatology Meeting, “Infectious Disease and Infestations in Returned Travelers: The Americas,” highlighted current trends relating to this phenomenon.
Dr. Natasha Mesinkovska noted that Americans are most likely to travel within the Americas, particularly to Mexico, Canada, and the Dominican Republic, but also to England and France. Conversely, approximately 66 million visitors from other regions visit the United States annually. GeoSentinel surveillance clinics suggest that diarrhea and febrile illness, such as malaria, are the most common concerns reported among returning travelers who are ill. Dermatologic concerns account for approximately 18% of all problems (Int J Infect Dis. 2008;12:593-602), and many of these are either cosmopolitan infections (eg, scabies, herpes, staphylococcal) or specific tropical infections (eg, cutaneous larva migrans, tungiasis, leishmaniasis, myiasis). Because many tropical infections are associated with insect vectors, particularly mosquitoes, the prophylactic use of repellant products such as 30% to 35% DEET is advisable. Also, travelers should avoid contact with sand, soil, stagnant water, and farm animals; drink only pasteurized liquids; close hotel windows; and sleep under mosquito netting, where appropriate.
Dr. Ron (Ronald) Rapini reviewed the various diagnostic procedures that may be used for unusual infections associated with the Americas. Diagnostic techniques include potassium hydroxide preparation, routine biopsy, biopsy with special stains, culture, and serologic testing. For example, South American blastomycosis is associated with 90% sensitive and 80% specific serologic test, and Gomori methenamine-silver or Periodic acid–Schiff stains may reveal the characteristic mariner’s wheel organisms in tissue.
Dr. Dirk Elston reviewed the nuances of dealing with worms and insects. Ivermectin may be given to kill the maggots that cause myiasis; the dead maggots can then be easily removed about a week later, as spines that lodge them into the tissue have retracted. A snake venom extractor may be utilized to suck these maggots out of infested tissue. Recurring scabies may lead to chronic impetigo and thence to glomerulonephritis and renal failure. Use of the dermatoscope may aid in the diagnosis of scabies. Many cases of so-called prurigo nodularis may actually be due to repeated bed bug bites. Both reduviid bugs and wild and domestic mammals have been found to harbor the etiologic trypanosomes of Chagas disease within the continental United States (Clin Microbiol Rev. 2011;24:655-681). Cyclic fevers should at least suggest the diagnosis of the tick-borne illness babesiosis.
Dr. Jose Dario Martinez reviewed the problem of “rash and fever.” Among the common culprits associated with such a presentation are dengue and chikungunya. Both are mosquito borne and both have now been seen in the United States, most commonly in returning travelers but also rarely as autochthonous infection. “Islands of normal in a sea of red”–appearing skin associated with fever, headache, retro-orbital pain, any sign of bleeding diathesis, and thrombocytopenia suggests dengue hemorrhagic fever. IgM serologic tests may be positive approximately 5 days into the infection, but polymerase chain reaction tests may be even more rapid. Promising work on a 3-dose quadrivalent dengue vaccine recently has been published (Lancet. July 10, 2014. doi:10.1016/S0140-6736(14)61142-9). Chikungunya, once previously limited to parts of Asia and Africa, is now present in the Americas. As of August 2014, local transmission had been identified in 31 countries or territories in the Caribbean, Central America, South America, or North America including the United States. Clinically, this disorder resembles dengue with less bleeding diathesis and more severe polyarthralgia. Reverse transcription–polymerase chain reaction testing can confirm the diagnosis in less than a week after infection. Treatment is supportive only, and a vaccine is under testing.
I discussed the ever-expanding worldwide epidemic of bed bug infestation, as verified by reports on a global bed bug registry Web site. Although bed bugs have not yet been reported to transmit disease to humans, the bite is pruritic and may be associated with severe emotional ramifications (Psychosomatics. 2012;53:85-91). Bed bugs can be detected by visual inspection of typical hiding places within 3 feet of the bed; expensive but very sensitive lures and bed bug–detecting canines also can be used to verify an infestation. Clearing bed bug infestations may require a complex multipronged approach, including thorough vacuuming and steaming, placing traps and desiccants, spreading insecticides, and thermal remediation. To avoid bringing bed bugs into the home following a trip, travelers are advised to check for bed bug infestation of hotel rooms, keep luggage off the floor and hang clothing up high, and launder clothes immediately upon returning home.
What’s the issue?
Travelers are potentially subject to many complications relating to their sojourn, particularly those of an infectious nature. Bugs and worms and unicellular parasites account for many of these complications. This situation is not static but remains perpetually evolving, as evidenced by the recent and rapid appearance of chikungunya, a virus in North America. To whom would you turn for advice or consultation if your patient returned from an exotic destination with a fever and skin abnormalities with which you have no familiarity?