User login
How to perform a vulvar biopsy
Many benign, premalignant, and malignant lesions can occur on the vulva. These can be challenging to differentiate by examination alone. A vulvar biopsy often is needed to appropriately diagnose—and ultimately treat—these various conditions.
In this article, we review vulvar biopsy procedures, describe how to prepare tissue specimens for the pathologist, and provide some brief case examples in which biopsy established the diagnosis.
Ask questions first
Prior to examining a patient with a vulvar lesion, obtain a detailed history. Asking specific questions may aid in making the correct diagnosis, such as:
- How long has the lesion been present? Has it changed? What color is it?
- Was any trigger, or trauma, associated with onset of the lesion?
- Does the lesion itch, burn, or cause pain? Is there any associated bleeding or discharge?
- Are other lesions present in the vagina, anus, or mouth, or are other skin lesions present?
- Are any systemic symptoms present, such as fever, lymphadenopathy, weight loss, or joint pain?
- What is the patient’s previous treatment history, including over-the-counter medications and prescribed medications?
- Has there been any incontinence of urine or stool? Does the patient use a pad?
- Is the patient scratching? Is there any nighttime scratching? It also can be useful to ask her partner, if she has one, about nighttime scratching.
- Is there a family history of vulvar conditions?
- Has there been any change in her use of products like soap, lotions, cleansing wipes, sprays, lubricants, or laundry detergent?
- Has the patient had any new partners or significant travel history?
Preprocedure counseling points
Prior to proceeding with a vulvar biopsy, review with the patient the risks, benefits, and alternatives and obtain patient consent for the procedure. Vulvar biopsy risks include pain, bleeding, infection, injury to surrounding tissue, and the need for further surgery. Make patients aware that some biopsies are nondiagnostic. We recommend that clinicians perform a time-out verification to ensure that the patient’s identity and planned procedure are correct.
Assess the biopsy site
A wide variety of lesions may require a biopsy for diagnosis. While it can be challenging to know where to biopsy, taking the time to determine the proper biopsy site may enhance pathology results.
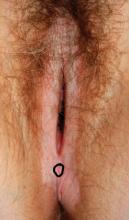
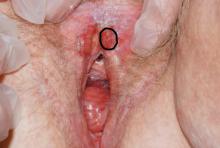
When considering colored lesions, depth is the important factor, and a punch biopsy often is sufficient. A tumor should be biopsied in the thickest area. Lesions that are concerning for malignancy may require multiple biopsies. An erosion or ulcer is best biopsied on the edge, including a small amount of surrounding tissue. For most patients, biopsy of normal-appearing tissue is of low diagnostic yield. Lastly, we try to avoid biopsies directly on the midline to facilitate better healing.1
A photograph of the vulva prior to biopsy may be helpful for the pathologist to see the tissue. Some electronic medical records have the capability to include photographs. Due to the sensitive nature of these photographs, we prefer that a separate written patient consent be obtained prior to taking photographs. We find also that photos are a useful reference for progression of disease at follow-up in a shared care team.
Continue to: Anesthesia procedure and instrument kit...
Anesthesia procedure and instrument kit
Some patients may benefit from the application of topical lidocaine 4% cream (L.M.X.4) prior to the injection of a local anesthetic for tissue biopsy. Ideally, topical lidocaine should be placed on the vulva and covered with a dressing such as Tegaderm or cellophane up to 30 minutes before the anticipated biopsy procedure. The anesthetic effect generally lasts for about 60 minutes. Many patients report stinging for several seconds upon application. Due to clinic time restrictions, we tend to reserve this method for a limited subset of patients. If planning a return visit for a biopsy, the patient can place the topical anesthetic herself.
For the anesthetic injection, we recommend lidocaine 1% or 2% with epinephrine in all areas of the vulva except for the glans clitoris. For a punch biopsy, we draw up 1 to 3 mL in a 3-mL syringe and inject with a 21- to 30-gauge needle, using a lower gauge for thicker tissue. We have not found buffering the anesthetic with sodium bicarbonate to be of particular use. For the glans clitoris, lidocaine without epinephrine should be utilized.
Equipment. Depending on your office setting, having a premade instrument kit may be preferred to peel-pack equipment. We prefer a premade tray that contains sterile gauze, a hemostat, iris scissors, a needle driver, a scalpel handle, and Adson forceps (FIGURE 1).

Types of biopsy procedures
Punch biopsy. We recommend a 4-mm Keyes biopsy punch. As mentioned, we use a biopsy kit to facilitate the procedure. After the tissue is properly anesthetized and prepped, we test the area via gentle touch to the skin with the hemostat or Adson forceps. To perform the punch biopsy, gentle, consistent pressure in a clockwise-counterclockwise fashion yields the best results. The goal is to obtain a 5-mm depth for hair-bearing skin and a 3-mm depth for all other tissue.2 The tissue should then be excised at the base with scissors, taking care not to crush the specimen with forceps.
Punch biopsy permits sampling of the epidermis, dermis, and subcutaneous tissue. Hemostasis is maintained with either silver nitrate, Monsel’s solution (ferric sulfate), or a dissolvable suture such as 4-0 Monocryl (poliglecaprone 25) or Vicryl Rapide (polyglactin 910).
Stitch biopsy. We find the stitch biopsy to be very useful given the architecture of the vulva. A modification of the shave biopsy, the stitch biopsy is depicted in FIGURE 2. A 3-0 or 4-0 dissolvable suture is placed through the intended area of biopsy. Iris scissors are used to undermine the tissue while the suture is held on tension. The goal is to remove the suture with the specimen. Separate sutures are used for hemostasis. The stitch does not cause the crushing artifacts on prepared specimens. Depending on the proceduralist’s comfort, a relatively large sample can be obtained in this fashion. If the suture held on tension is inadvertently cut, a second pass can be made with suture; alternatively, care can be used to remove remaining tissue with forceps and scissors, again avoiding crush injury to the tissue.

Excisional biopsy. Often, a larger area or margins are desired. We find that with adequate preparation, patients tolerate excisions in the office quite well. The planned area for excision can be marked with ink to ensure margins. Adequate anesthesia is instilled. A No. 15 blade scalpel is often the best size used to excise vulvar tissue in an elliptical fashion. Depending on depth of incision, the tissue may need to be approximated in layers for cosmesis and healing.
When planning an excisional biopsy, place a stitch on the excised tissue to mark orientation or pin out the entire specimen to a foam board to help your pathologist interpret tissue orientation.
The box "Vulvar biopsy established the diagnosis" at the end of this discussion provides 6 case examples of vulvar lesions and the respective diagnoses confirmed by biopsy.
Continue to: Preparing tissue for the pathologist...
Preparing tissue for the pathologist
Here are 5 tips for preparing the biopsied specimen for pathology:
- Include a question for the pathologist, such as “rule out lichen sclerosus or lichen simplex chronicus.” The majority of specimens should be sent in formalin. At times, frozen sections are done in the operating room.
- Double-check that the proper paperwork is included with every specimen and be very specific regarding the exact location of the lesion on the vulva. Include photographs whenever possible.
- Request that a dermatopathologist or a gynecologic pathologist with a special interest in vulvar dermatology, when feasible, review the tissue.
- Check your laboratory’s protocol for sending biopsies from areas around ulcerated tissue. Often, special medium is required for immunohistochemistry stains.
- Call your pathologist with questions about results; he or she often is happy to clarify, and together you may be able to arrive at a diagnosis to better serve your patient.3
Complications and how to avoid them
Bleeding. Any procedure has bleeding risks. To avoid bleeding, review the patient’s medication list and medical history prior to biopsy, as certain medications, such as blood thinners, increase risk for bleeding. Counseling a patient on applying direct pressure to the biopsy site for 2 minutes is generally sufficient for any bleeding that may occur once she is discharged from the clinic.
Infection. With aseptic technique, infection of a biopsy site is rare. We use nonsterile gloves for biopsy procedures. This does not increase the risk of infection.4 If a patient has iodine allergy, dilute chlorhexidine is a reasonable alternative for skin cleansing. Instruct the patient to keep the site clean and dry; if the biopsy proximity is close to the urethra or anus, use of a peri-bottle may be preferred after toileting. Instruct patients not to pull sutures. While instructions are specific for each patient, we generally advise that patients wait 4 to 7 days before resuming use of topical medications.
Scarring or tattooing. Avoid using dyed suture on skin surfaces and counsel the patient that silver nitrate can permanently stain tissue. Usually, small biopsies heal well but a small scar is possible.
Key points to keep in mind
- Counsel patients on biopsy risks, benefits, and alternatives. Counsel regarding possible inconclusive results.
- Take time in choosing the biopsy site and consider multiple biopsies.
- Have all anticipated equipment available; consider using premade biopsy kits.
- Consider performing a stitch biopsy to avoid crush injury.
- Take photographs of the area to be biopsied and communicate with your pathologist to facilitate diagnosis.
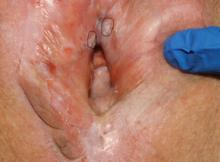
Case 1
Biopsies were obtained of the areas highlighted in the photo. Pathology shows dVIN.
Image courtesy of Hope Haefner, MD.
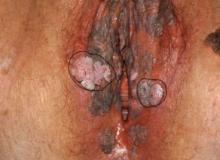
Case 2
The examination is consistent with condylomata acuminata and biopsy is recommended with a 4-mm punch. Biopsy results are consistent with condylomata acuminata.
Image courtesy of Hope Haefner, MD.
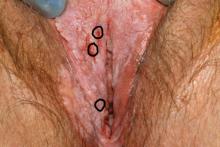
Case 3
The final pathology shows high-grade squamous intraepithelial lesions (HSIL) of the vulva.
Image courtesy of Hope Haefner, MD.
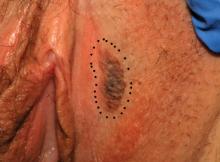
Case 4
This presentation is an excellent opportunity for an excisional biopsy of the vulva. A marking pen is used to draw margins. A No. 15 blade is used to outline and then undermine the lesion, removing it in its entirety.
Final pathology shows a compound nevus of the vulva.
Image courtesy of Hope Haefner, MD.
Case 5
A 4-mm punch biopsy result is consistent with a diagnosis of lichen sclerosus.
Image courtesy of Hope Haefner, MD.
Case 6
A 4-mm punch biopsy result reveals that the pathology is significant for squamous cell carcinoma.
Image courtesy of Hope Haefner, MD.
- Edwards L, Lynch PJ. Genital Dermatology Atlas and Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2018.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 93: Diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111:1243-1253.
- Heller DS. Areas of confusion in pathologist-clinician communication as it relates to understanding the vulvar pathology report. J Low Genit Tract Dis. 2017;21:327-328.
- Rietz A, Barzin A, Jones K, et al. Sterile or non-sterile gloves for minor skin excisions? J Fam Pract. 2015;64:723-727.
Many benign, premalignant, and malignant lesions can occur on the vulva. These can be challenging to differentiate by examination alone. A vulvar biopsy often is needed to appropriately diagnose—and ultimately treat—these various conditions.
In this article, we review vulvar biopsy procedures, describe how to prepare tissue specimens for the pathologist, and provide some brief case examples in which biopsy established the diagnosis.
Ask questions first
Prior to examining a patient with a vulvar lesion, obtain a detailed history. Asking specific questions may aid in making the correct diagnosis, such as:
- How long has the lesion been present? Has it changed? What color is it?
- Was any trigger, or trauma, associated with onset of the lesion?
- Does the lesion itch, burn, or cause pain? Is there any associated bleeding or discharge?
- Are other lesions present in the vagina, anus, or mouth, or are other skin lesions present?
- Are any systemic symptoms present, such as fever, lymphadenopathy, weight loss, or joint pain?
- What is the patient’s previous treatment history, including over-the-counter medications and prescribed medications?
- Has there been any incontinence of urine or stool? Does the patient use a pad?
- Is the patient scratching? Is there any nighttime scratching? It also can be useful to ask her partner, if she has one, about nighttime scratching.
- Is there a family history of vulvar conditions?
- Has there been any change in her use of products like soap, lotions, cleansing wipes, sprays, lubricants, or laundry detergent?
- Has the patient had any new partners or significant travel history?
Preprocedure counseling points
Prior to proceeding with a vulvar biopsy, review with the patient the risks, benefits, and alternatives and obtain patient consent for the procedure. Vulvar biopsy risks include pain, bleeding, infection, injury to surrounding tissue, and the need for further surgery. Make patients aware that some biopsies are nondiagnostic. We recommend that clinicians perform a time-out verification to ensure that the patient’s identity and planned procedure are correct.
Assess the biopsy site
A wide variety of lesions may require a biopsy for diagnosis. While it can be challenging to know where to biopsy, taking the time to determine the proper biopsy site may enhance pathology results.
When considering colored lesions, depth is the important factor, and a punch biopsy often is sufficient. A tumor should be biopsied in the thickest area. Lesions that are concerning for malignancy may require multiple biopsies. An erosion or ulcer is best biopsied on the edge, including a small amount of surrounding tissue. For most patients, biopsy of normal-appearing tissue is of low diagnostic yield. Lastly, we try to avoid biopsies directly on the midline to facilitate better healing.1
A photograph of the vulva prior to biopsy may be helpful for the pathologist to see the tissue. Some electronic medical records have the capability to include photographs. Due to the sensitive nature of these photographs, we prefer that a separate written patient consent be obtained prior to taking photographs. We find also that photos are a useful reference for progression of disease at follow-up in a shared care team.
Continue to: Anesthesia procedure and instrument kit...
Anesthesia procedure and instrument kit
Some patients may benefit from the application of topical lidocaine 4% cream (L.M.X.4) prior to the injection of a local anesthetic for tissue biopsy. Ideally, topical lidocaine should be placed on the vulva and covered with a dressing such as Tegaderm or cellophane up to 30 minutes before the anticipated biopsy procedure. The anesthetic effect generally lasts for about 60 minutes. Many patients report stinging for several seconds upon application. Due to clinic time restrictions, we tend to reserve this method for a limited subset of patients. If planning a return visit for a biopsy, the patient can place the topical anesthetic herself.
For the anesthetic injection, we recommend lidocaine 1% or 2% with epinephrine in all areas of the vulva except for the glans clitoris. For a punch biopsy, we draw up 1 to 3 mL in a 3-mL syringe and inject with a 21- to 30-gauge needle, using a lower gauge for thicker tissue. We have not found buffering the anesthetic with sodium bicarbonate to be of particular use. For the glans clitoris, lidocaine without epinephrine should be utilized.
Equipment. Depending on your office setting, having a premade instrument kit may be preferred to peel-pack equipment. We prefer a premade tray that contains sterile gauze, a hemostat, iris scissors, a needle driver, a scalpel handle, and Adson forceps (FIGURE 1).

Types of biopsy procedures
Punch biopsy. We recommend a 4-mm Keyes biopsy punch. As mentioned, we use a biopsy kit to facilitate the procedure. After the tissue is properly anesthetized and prepped, we test the area via gentle touch to the skin with the hemostat or Adson forceps. To perform the punch biopsy, gentle, consistent pressure in a clockwise-counterclockwise fashion yields the best results. The goal is to obtain a 5-mm depth for hair-bearing skin and a 3-mm depth for all other tissue.2 The tissue should then be excised at the base with scissors, taking care not to crush the specimen with forceps.
Punch biopsy permits sampling of the epidermis, dermis, and subcutaneous tissue. Hemostasis is maintained with either silver nitrate, Monsel’s solution (ferric sulfate), or a dissolvable suture such as 4-0 Monocryl (poliglecaprone 25) or Vicryl Rapide (polyglactin 910).
Stitch biopsy. We find the stitch biopsy to be very useful given the architecture of the vulva. A modification of the shave biopsy, the stitch biopsy is depicted in FIGURE 2. A 3-0 or 4-0 dissolvable suture is placed through the intended area of biopsy. Iris scissors are used to undermine the tissue while the suture is held on tension. The goal is to remove the suture with the specimen. Separate sutures are used for hemostasis. The stitch does not cause the crushing artifacts on prepared specimens. Depending on the proceduralist’s comfort, a relatively large sample can be obtained in this fashion. If the suture held on tension is inadvertently cut, a second pass can be made with suture; alternatively, care can be used to remove remaining tissue with forceps and scissors, again avoiding crush injury to the tissue.

Excisional biopsy. Often, a larger area or margins are desired. We find that with adequate preparation, patients tolerate excisions in the office quite well. The planned area for excision can be marked with ink to ensure margins. Adequate anesthesia is instilled. A No. 15 blade scalpel is often the best size used to excise vulvar tissue in an elliptical fashion. Depending on depth of incision, the tissue may need to be approximated in layers for cosmesis and healing.
When planning an excisional biopsy, place a stitch on the excised tissue to mark orientation or pin out the entire specimen to a foam board to help your pathologist interpret tissue orientation.
The box "Vulvar biopsy established the diagnosis" at the end of this discussion provides 6 case examples of vulvar lesions and the respective diagnoses confirmed by biopsy.
Continue to: Preparing tissue for the pathologist...
Preparing tissue for the pathologist
Here are 5 tips for preparing the biopsied specimen for pathology:
- Include a question for the pathologist, such as “rule out lichen sclerosus or lichen simplex chronicus.” The majority of specimens should be sent in formalin. At times, frozen sections are done in the operating room.
- Double-check that the proper paperwork is included with every specimen and be very specific regarding the exact location of the lesion on the vulva. Include photographs whenever possible.
- Request that a dermatopathologist or a gynecologic pathologist with a special interest in vulvar dermatology, when feasible, review the tissue.
- Check your laboratory’s protocol for sending biopsies from areas around ulcerated tissue. Often, special medium is required for immunohistochemistry stains.
- Call your pathologist with questions about results; he or she often is happy to clarify, and together you may be able to arrive at a diagnosis to better serve your patient.3
Complications and how to avoid them
Bleeding. Any procedure has bleeding risks. To avoid bleeding, review the patient’s medication list and medical history prior to biopsy, as certain medications, such as blood thinners, increase risk for bleeding. Counseling a patient on applying direct pressure to the biopsy site for 2 minutes is generally sufficient for any bleeding that may occur once she is discharged from the clinic.
Infection. With aseptic technique, infection of a biopsy site is rare. We use nonsterile gloves for biopsy procedures. This does not increase the risk of infection.4 If a patient has iodine allergy, dilute chlorhexidine is a reasonable alternative for skin cleansing. Instruct the patient to keep the site clean and dry; if the biopsy proximity is close to the urethra or anus, use of a peri-bottle may be preferred after toileting. Instruct patients not to pull sutures. While instructions are specific for each patient, we generally advise that patients wait 4 to 7 days before resuming use of topical medications.
Scarring or tattooing. Avoid using dyed suture on skin surfaces and counsel the patient that silver nitrate can permanently stain tissue. Usually, small biopsies heal well but a small scar is possible.
Key points to keep in mind
- Counsel patients on biopsy risks, benefits, and alternatives. Counsel regarding possible inconclusive results.
- Take time in choosing the biopsy site and consider multiple biopsies.
- Have all anticipated equipment available; consider using premade biopsy kits.
- Consider performing a stitch biopsy to avoid crush injury.
- Take photographs of the area to be biopsied and communicate with your pathologist to facilitate diagnosis.
Case 1
Biopsies were obtained of the areas highlighted in the photo. Pathology shows dVIN.
Image courtesy of Hope Haefner, MD.
Case 2
The examination is consistent with condylomata acuminata and biopsy is recommended with a 4-mm punch. Biopsy results are consistent with condylomata acuminata.
Image courtesy of Hope Haefner, MD.
Case 3
The final pathology shows high-grade squamous intraepithelial lesions (HSIL) of the vulva.
Image courtesy of Hope Haefner, MD.
Case 4
This presentation is an excellent opportunity for an excisional biopsy of the vulva. A marking pen is used to draw margins. A No. 15 blade is used to outline and then undermine the lesion, removing it in its entirety.
Final pathology shows a compound nevus of the vulva.
Image courtesy of Hope Haefner, MD.
Case 5
A 4-mm punch biopsy result is consistent with a diagnosis of lichen sclerosus.
Image courtesy of Hope Haefner, MD.
Case 6
A 4-mm punch biopsy result reveals that the pathology is significant for squamous cell carcinoma.
Image courtesy of Hope Haefner, MD.
Many benign, premalignant, and malignant lesions can occur on the vulva. These can be challenging to differentiate by examination alone. A vulvar biopsy often is needed to appropriately diagnose—and ultimately treat—these various conditions.
In this article, we review vulvar biopsy procedures, describe how to prepare tissue specimens for the pathologist, and provide some brief case examples in which biopsy established the diagnosis.
Ask questions first
Prior to examining a patient with a vulvar lesion, obtain a detailed history. Asking specific questions may aid in making the correct diagnosis, such as:
- How long has the lesion been present? Has it changed? What color is it?
- Was any trigger, or trauma, associated with onset of the lesion?
- Does the lesion itch, burn, or cause pain? Is there any associated bleeding or discharge?
- Are other lesions present in the vagina, anus, or mouth, or are other skin lesions present?
- Are any systemic symptoms present, such as fever, lymphadenopathy, weight loss, or joint pain?
- What is the patient’s previous treatment history, including over-the-counter medications and prescribed medications?
- Has there been any incontinence of urine or stool? Does the patient use a pad?
- Is the patient scratching? Is there any nighttime scratching? It also can be useful to ask her partner, if she has one, about nighttime scratching.
- Is there a family history of vulvar conditions?
- Has there been any change in her use of products like soap, lotions, cleansing wipes, sprays, lubricants, or laundry detergent?
- Has the patient had any new partners or significant travel history?
Preprocedure counseling points
Prior to proceeding with a vulvar biopsy, review with the patient the risks, benefits, and alternatives and obtain patient consent for the procedure. Vulvar biopsy risks include pain, bleeding, infection, injury to surrounding tissue, and the need for further surgery. Make patients aware that some biopsies are nondiagnostic. We recommend that clinicians perform a time-out verification to ensure that the patient’s identity and planned procedure are correct.
Assess the biopsy site
A wide variety of lesions may require a biopsy for diagnosis. While it can be challenging to know where to biopsy, taking the time to determine the proper biopsy site may enhance pathology results.
When considering colored lesions, depth is the important factor, and a punch biopsy often is sufficient. A tumor should be biopsied in the thickest area. Lesions that are concerning for malignancy may require multiple biopsies. An erosion or ulcer is best biopsied on the edge, including a small amount of surrounding tissue. For most patients, biopsy of normal-appearing tissue is of low diagnostic yield. Lastly, we try to avoid biopsies directly on the midline to facilitate better healing.1
A photograph of the vulva prior to biopsy may be helpful for the pathologist to see the tissue. Some electronic medical records have the capability to include photographs. Due to the sensitive nature of these photographs, we prefer that a separate written patient consent be obtained prior to taking photographs. We find also that photos are a useful reference for progression of disease at follow-up in a shared care team.
Continue to: Anesthesia procedure and instrument kit...
Anesthesia procedure and instrument kit
Some patients may benefit from the application of topical lidocaine 4% cream (L.M.X.4) prior to the injection of a local anesthetic for tissue biopsy. Ideally, topical lidocaine should be placed on the vulva and covered with a dressing such as Tegaderm or cellophane up to 30 minutes before the anticipated biopsy procedure. The anesthetic effect generally lasts for about 60 minutes. Many patients report stinging for several seconds upon application. Due to clinic time restrictions, we tend to reserve this method for a limited subset of patients. If planning a return visit for a biopsy, the patient can place the topical anesthetic herself.
For the anesthetic injection, we recommend lidocaine 1% or 2% with epinephrine in all areas of the vulva except for the glans clitoris. For a punch biopsy, we draw up 1 to 3 mL in a 3-mL syringe and inject with a 21- to 30-gauge needle, using a lower gauge for thicker tissue. We have not found buffering the anesthetic with sodium bicarbonate to be of particular use. For the glans clitoris, lidocaine without epinephrine should be utilized.
Equipment. Depending on your office setting, having a premade instrument kit may be preferred to peel-pack equipment. We prefer a premade tray that contains sterile gauze, a hemostat, iris scissors, a needle driver, a scalpel handle, and Adson forceps (FIGURE 1).

Types of biopsy procedures
Punch biopsy. We recommend a 4-mm Keyes biopsy punch. As mentioned, we use a biopsy kit to facilitate the procedure. After the tissue is properly anesthetized and prepped, we test the area via gentle touch to the skin with the hemostat or Adson forceps. To perform the punch biopsy, gentle, consistent pressure in a clockwise-counterclockwise fashion yields the best results. The goal is to obtain a 5-mm depth for hair-bearing skin and a 3-mm depth for all other tissue.2 The tissue should then be excised at the base with scissors, taking care not to crush the specimen with forceps.
Punch biopsy permits sampling of the epidermis, dermis, and subcutaneous tissue. Hemostasis is maintained with either silver nitrate, Monsel’s solution (ferric sulfate), or a dissolvable suture such as 4-0 Monocryl (poliglecaprone 25) or Vicryl Rapide (polyglactin 910).
Stitch biopsy. We find the stitch biopsy to be very useful given the architecture of the vulva. A modification of the shave biopsy, the stitch biopsy is depicted in FIGURE 2. A 3-0 or 4-0 dissolvable suture is placed through the intended area of biopsy. Iris scissors are used to undermine the tissue while the suture is held on tension. The goal is to remove the suture with the specimen. Separate sutures are used for hemostasis. The stitch does not cause the crushing artifacts on prepared specimens. Depending on the proceduralist’s comfort, a relatively large sample can be obtained in this fashion. If the suture held on tension is inadvertently cut, a second pass can be made with suture; alternatively, care can be used to remove remaining tissue with forceps and scissors, again avoiding crush injury to the tissue.

Excisional biopsy. Often, a larger area or margins are desired. We find that with adequate preparation, patients tolerate excisions in the office quite well. The planned area for excision can be marked with ink to ensure margins. Adequate anesthesia is instilled. A No. 15 blade scalpel is often the best size used to excise vulvar tissue in an elliptical fashion. Depending on depth of incision, the tissue may need to be approximated in layers for cosmesis and healing.
When planning an excisional biopsy, place a stitch on the excised tissue to mark orientation or pin out the entire specimen to a foam board to help your pathologist interpret tissue orientation.
The box "Vulvar biopsy established the diagnosis" at the end of this discussion provides 6 case examples of vulvar lesions and the respective diagnoses confirmed by biopsy.
Continue to: Preparing tissue for the pathologist...
Preparing tissue for the pathologist
Here are 5 tips for preparing the biopsied specimen for pathology:
- Include a question for the pathologist, such as “rule out lichen sclerosus or lichen simplex chronicus.” The majority of specimens should be sent in formalin. At times, frozen sections are done in the operating room.
- Double-check that the proper paperwork is included with every specimen and be very specific regarding the exact location of the lesion on the vulva. Include photographs whenever possible.
- Request that a dermatopathologist or a gynecologic pathologist with a special interest in vulvar dermatology, when feasible, review the tissue.
- Check your laboratory’s protocol for sending biopsies from areas around ulcerated tissue. Often, special medium is required for immunohistochemistry stains.
- Call your pathologist with questions about results; he or she often is happy to clarify, and together you may be able to arrive at a diagnosis to better serve your patient.3
Complications and how to avoid them
Bleeding. Any procedure has bleeding risks. To avoid bleeding, review the patient’s medication list and medical history prior to biopsy, as certain medications, such as blood thinners, increase risk for bleeding. Counseling a patient on applying direct pressure to the biopsy site for 2 minutes is generally sufficient for any bleeding that may occur once she is discharged from the clinic.
Infection. With aseptic technique, infection of a biopsy site is rare. We use nonsterile gloves for biopsy procedures. This does not increase the risk of infection.4 If a patient has iodine allergy, dilute chlorhexidine is a reasonable alternative for skin cleansing. Instruct the patient to keep the site clean and dry; if the biopsy proximity is close to the urethra or anus, use of a peri-bottle may be preferred after toileting. Instruct patients not to pull sutures. While instructions are specific for each patient, we generally advise that patients wait 4 to 7 days before resuming use of topical medications.
Scarring or tattooing. Avoid using dyed suture on skin surfaces and counsel the patient that silver nitrate can permanently stain tissue. Usually, small biopsies heal well but a small scar is possible.
Key points to keep in mind
- Counsel patients on biopsy risks, benefits, and alternatives. Counsel regarding possible inconclusive results.
- Take time in choosing the biopsy site and consider multiple biopsies.
- Have all anticipated equipment available; consider using premade biopsy kits.
- Consider performing a stitch biopsy to avoid crush injury.
- Take photographs of the area to be biopsied and communicate with your pathologist to facilitate diagnosis.
Case 1
Biopsies were obtained of the areas highlighted in the photo. Pathology shows dVIN.
Image courtesy of Hope Haefner, MD.
Case 2
The examination is consistent with condylomata acuminata and biopsy is recommended with a 4-mm punch. Biopsy results are consistent with condylomata acuminata.
Image courtesy of Hope Haefner, MD.
Case 3
The final pathology shows high-grade squamous intraepithelial lesions (HSIL) of the vulva.
Image courtesy of Hope Haefner, MD.
Case 4
This presentation is an excellent opportunity for an excisional biopsy of the vulva. A marking pen is used to draw margins. A No. 15 blade is used to outline and then undermine the lesion, removing it in its entirety.
Final pathology shows a compound nevus of the vulva.
Image courtesy of Hope Haefner, MD.
Case 5
A 4-mm punch biopsy result is consistent with a diagnosis of lichen sclerosus.
Image courtesy of Hope Haefner, MD.
Case 6
A 4-mm punch biopsy result reveals that the pathology is significant for squamous cell carcinoma.
Image courtesy of Hope Haefner, MD.
- Edwards L, Lynch PJ. Genital Dermatology Atlas and Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2018.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 93: Diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111:1243-1253.
- Heller DS. Areas of confusion in pathologist-clinician communication as it relates to understanding the vulvar pathology report. J Low Genit Tract Dis. 2017;21:327-328.
- Rietz A, Barzin A, Jones K, et al. Sterile or non-sterile gloves for minor skin excisions? J Fam Pract. 2015;64:723-727.
- Edwards L, Lynch PJ. Genital Dermatology Atlas and Manual. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2018.
- American College of Obstetricians and Gynecologists. Practice bulletin no. 93: Diagnosis and management of vulvar skin disorders. Obstet Gynecol. 2008;111:1243-1253.
- Heller DS. Areas of confusion in pathologist-clinician communication as it relates to understanding the vulvar pathology report. J Low Genit Tract Dis. 2017;21:327-328.
- Rietz A, Barzin A, Jones K, et al. Sterile or non-sterile gloves for minor skin excisions? J Fam Pract. 2015;64:723-727.
Vulvar pain syndromes: Making the correct diagnosis
Although the incidence of vulvar pain has increased over the past decade—thanks to both greater awareness and increasing numbers of affected women—the phenomenon is not a recent development. As early as 1874, T. Galliard Thomas wrote, “[T]his disorder, although fortunately not very frequent, is by no means very rare.”1 He went on to express “surprise” that it had not been “more generally and fully described.”
Despite the focus Thomas directed to the issue, vulvar pain did not get much attention until the 21st century, when a number of studies began to gauge its prevalence. For example, in a study in Boston of about 5,000 women, the lifetime prevalence of chronic vulvar pain was 16%.2 And in a study in Texas, the prevalence of vulvar pain in an urban, largely minority population was estimated to be 11%.3 The Boston study also reported that “nearly 40% of women chose not to seek treatment, and, of those who did, 60% saw three or more doctors, many of whom could not provide a diagnosis.”2
Clearly, there is a need for comprehensive information on vulvar pain and its causes, symptoms, diagnosis, and treatment. To address the lack of guidance, OBG Management Contributing Editor Neal M. Lonky, MD, assembled a panel of experts on vulvar pain syndromes and invited them to share their considerable knowledge. The ensuing discussion, presented in three parts, offers a gold mine of information. In this opening article, the panel focuses on causes, symptomatology, and diagnosis of this common complaint.
|
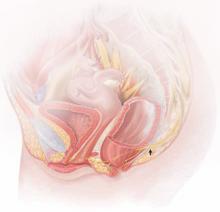
The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain. |
![]()
Dr. Lonky: What are the most common diagnoses when vulvar pain is the complaint?
Dr. Gunter: The most common cause of chronic vulvar pain is vulvodynia, although lichen simplex chronicus, chronic yeast infections, and non-neoplastic epithelial disorders, such as lichen sclerosus and lichen planus, can also produce irritation and pain. In postmenopausal women, atrophic vaginitis can also cause a burning pain, although symptoms are typically more vaginal than vulvar. Yeast and lichen simplex chronicus typically produce itching, although sometimes they can present with irritation and pain, so they must be considered in the differential diagnosis. It is important to remember that many women with vulvodynia have used multiple topical agents and may have developed complex hygiene rituals in an attempt to treat their symptoms, which can result in a secondary lichen simplex chronicus.
That said, there is a high frequency of misdiagnosis with yeast. For example, in a study by Nyirjesy and colleagues, two thirds of women who were referred to a tertiary clinic for chronic vulvovaginal candidiasis were found to have a noninfectious entity instead—most commonly lichen simplex chronicus and vulvodynia.4
Dr. Edwards: The most common “diagnosis” for vulvar pain is vulvodynia. However, the definition of vulvodynia is pain—i.e., burning, rawness, irritation, soreness, aching, or stabbing or stinging sensations—in the absence of skin disease, infection, or specific neurologic disease. Therefore, even though the usual cause of vulvar pain is vulvodynia, it is a diagnosis of exclusion, and skin disease, infection, and neurologic disease must be ruled out.
In regard to infection, Candida albicans and bacterial vaginosis (BV) are usually the first conditions that are considered when a patient complains of vulvar pain, but they are not common causes of vulvar pain and are never causes of chronic vulvar pain. Very rarely they may cause recurrent pain that clears, at least briefly, with treatment.
Candida albicans is usually primarily pruritic, and BV produces discharge and odor, sometimes with minor symptoms. Non-albicans Candida (e.g., Candida glabrata) is nearly always asymptomatic, but it occasionally causes irritation and burning.
Group B streptococcus is another infectious entity that very, very occasionally causes irritation and dyspareunia but is usually only a colonizer.
Herpes simplex virus is a cause of recurrent but not chronic pain.
Chronic pain is more likely to be caused by skin disease than by infection. Lichen simplex chronicus causes itching; any pain is due to erosions from scratching.
Dr. Haefner: Several other infectious conditions or their treatments can cause vulvar pain. For example, herpes (particularly primary herpes infection) is classically associated with vulvar pain. The pain is so great that, at times, the patient requires admission for pain control. Surprisingly, despite the known pain of herpes, approximately 80% of patients who have it are unaware of their diagnosis.
Although condyloma is generally a painless condition, many patients complain of pain following treatment for it, whether treatment involves topical medications or laser surgery.
Chancroid is a painful vulvar ulcer. Trichomonas can sometimes be associated with vulvar pain.
Dr. Lonky: What terminology do we use when we discuss vulvar pain?
Dr. Haefner: The current terminology used to describe vulvar pain was published in 2004, after years of debate over nomenclature within the International Society for the Study of Vulvovaginal Disease.5 The terminology lists two major categories of vulvar pain:
-
pain related to a specific disorder. This category encompasses numerous conditions that feature an abnormal appearance of the vulva (Table 1).
TABLE 1
Terminology and classification of vulvar pain from the International Society for the Study of Vulvovaginal Disease
| SOURCE: Moyal-Barracco and Lynch.5 Reproduced with permission from the Journal of Reproductive Medicine. |
|
-
vulvodynia, in which the vulva appears normal, other than occasional erythema, which is most prominent at the duct openings (vestibular ducts—Bartholin’s and Skene’s).
As for vulvar pain, there are two major forms:
-
hyperalgesia (a low threshold for pain)
-
allodynia (pain in response to light touch).
Some diseases that are associated with vulvar pain do not qualify for the diagnosis of vulvodynia (Table 2) because they are associated with an abnormal appearance of the vulva.
TABLE 2
Conditions other than vulvodynia that are associated with vulvar pain
| Acute irritant contact dermatitis (e.g., erosion due to podofilox, imiquimod, cantharidin, fluorouracil, or podophyllin toxin) |
| Aphthous ulcer |
| Atrophy |
| Bartholin’s abscess |
| Candidiasis |
| Carcinoma |
| Chronic irritant contact dermatitis |
| Endometriosis |
| Herpes (simplex and zoster) |
| Immunobullous diseases (including cicatricial pemphigoid, pemphigus vulgaris, linear immunoglobulin A disease, etc.) |
| Lichen planus |
| Lichen sclerosus |
| Podophyllin overdose (see above) |
| Prolapsed urethra |
| Sjögren’s syndrome |
| Trauma |
| Trichomoniasis |
| Vulvar intraepithelial neoplasia |
![]()
Dr. Lonky: What skin diseases need to be ruled out before vulvodynia can be diagnosed?
Dr. Edwards: Skin diseases that affect the vulva are usually pruritic—pain is a later sign. Lichen simplex chronicus (also known as eczema) is pruritus caused by any irritant; any pain that arises is produced by visible excoriations from scratching.
Lichen sclerosus manifests as white epithelium that has a crinkling, shiny, or waxy texture. It can produce pain, especially dyspareunia. The pain is caused by erosions that arise from fragility and introital narrowing and inelasticity.
Vulvovaginal lichen planus is usually erosive and preferentially affects mucous membranes, especially the vestibule; it sometimes affects the vagina and mouth, as well.
Desquamative inflammatory vaginitis is most likely a skin disease that affects only the vagina. It involves introital redness and a clinically and microscopically purulent vaginal discharge that also reveals parabasal cells and absent lactobacilli.
Dr. Lonky: You mentioned that neurologic diseases can sometimes cause vulvar pain. Which ones?
Dr. Edwards: Pudendal neuralgia, diabetic neuropathy, and post-herpetic neuralgia are the most common specific neurologic causes of vulvar pain. Multiple sclerosis can also produce pain syndromes. Post-herpetic neuralgia follows herpes zoster—not herpes simplex—virus infection.
Dr. Lonky: Any other conditions that can cause vulvar pain?
Dr. Haefner: Aphthous ulcers are common and are often flared by stress.
Non-neoplastic epithelial disorders are also seen frequently in health-care providers’ offices; many patients who experience them report pain on the vulva.
It is always important to consider cancer when a patient has an abnormal vulvar appearance and pain that has persisted despite treatment.
![]()
Dr. Lonky: If you were to rank vulvar pain syndromes according to their prevalence, what would the most common syndromes be?
Dr. Gunter: Given the misdiagnosis of many women, who are told they have chronic yeast infection, as I mentioned, it’s hard to know which vulvar pain syndromes are most prevalent. I suspect that lichen simplex chronicus is most common, followed by vulvodynia, with chronic yeast infection a distant third.
My experience reflects what Nyirjesy and colleagues4 found: 65% to 75% of women referred to my clinic with chronic yeast actually have lichen simplex chronicus or vulvodynia. In postmenopausal women, atrophic vaginitis is also a consideration; it’s becoming more common now that the use of systemic hormone replacement therapy is decreasing.
Dr. Lonky: What about subsets of vulvodynia? Which ones are most common?
Dr. Edwards: There is good evidence of marked overlap among subsets of vulvodynia. The vast majority of women who have vulvodynia experience primarily provoked vestibular pain, regardless of age. However, I find that almost all patients also report pain that extends beyond the vestibule at times, as well as occasional unprovoked pain.
The diagnosis requires the exclusion of other causes of vulvar pain, and the subset is identified by the location of pain (that is, is it strictly localized or generalized or even migratory?) and its provoked or unprovoked nature.
Localized clitoral pain and vulvar pain localized to one side of the vulva are extremely uncommon, but they do occur. And although I rarely encounter teenagers and prepubertal children who have vulvodynia, I do have patients in both age groups who have vulvodynia.
Dr. Lonky: Are there racial differences in the prevalence of vulvodynia?
Dr. Edwards: Although several good studies show that women of African descent and white patients are equally likely to experience vulvodynia, the vast majority (99%) of my patients who have vulvodynia are white. My patients of African descent consult me primarily for itching or discharge.
My local demographics prevent me from judging the likelihood of Asians having vulvodynia, and our Hispanic population has limited access to health care.
In general, I don’t think that demographics are useful in making the diagnosis of vulvodynia.
![]()
Dr. Lonky: Do your patients who have vulvodynia or another vulvar pain syndrome tend to have comorbidities? If so, is this information helpful in establishing the diagnosis and planning therapy?
Dr. Haefner: Women who have vulvodynia often have other medical problems as well. In my practice, when new patients who have vulvodynia complete their intake survey, they often report a history of headache, irritable bowel syndrome, interstitial cystitis, fibromyalgia,6 chronic fatigue syndrome, back pain, and temporomandibular joint (TMJ) disorder. These comorbidities are not particularly helpful in establishing the diagnosis of vulvodynia, but they are an important consideration when choosing therapy for the patient. Often, the medications chosen to treat one condition will also benefit another condition. However, it’s important to check for potential interactions between drugs before prescribing a new treatment.
Dr. Gunter: A significant number of women who have vulvodynia also have other chronic pain syndromes. For example, the incidence of bladder pain syndrome–interstitial cystitis is 68% to 82% among women who have vulvodynia, compared with a baseline rate among all women of 6% to 11%.7-10 The rate of irritable bowel syndrome is more than doubled among women who have vulvodynia, compared with the general population (27% versus 12%).8 Another common comorbidity, hypertonic somatic dysfunction of the pelvic floor, is identified in 10% to 90% of women who have chronic vulvar pain.8,11,12 These women also have a higher incidence of nongenital pain syndromes, such as fibromyalgia, migraine, and TMJ dysfunction, than the general population, as Dr. Haefner noted.8,12,13
Many studies have evaluated psychological and emotional contributions to chronic vulvar pain. Pain and depression are intimately related—the incidence of depression among all people who experience chronic pain ranges from 27% to 54%, compared with 5% to 17% among the general population.14-16 The relationship is complex because chronic illness in general is associated with depression. Nevertheless, several studies have noted an increase in anxiety, stress, and depression among women who have vulvodynia.17-19
I screen every patient for depression using a Patient Health Questionnaire (PHQ-9); I also screen for anxiety. I find that a significant percentage of patients in my clinic are depressed or have an anxiety disorder. Failure to address these comorbidities makes treatment very difficult. I typically prescribe citalopram (Celexa), although there is some question whether it can safely be combined with a tricyclic antidepressant. We also offer stress-reduction classes, teach every patient the value of diaphragmatic breathing, offer mind-body classes for anxiety and stress, and provide intensive programs where the patient can learn important self-care skills, such as pacing (spacing activities throughout the day in a manner that avoids aggravating the pain), and address her anxiety and stress in a more guided manner. We also have a psychologist who specializes in pain for any patient who may need one-on-one counseling.
Dr. Edwards: The presence of comorbidities is somewhat useful in making the diagnosis of vulvodynia. I question my diagnosis, in fact, when a patient who has vulvodynia does not have headaches, low energy, depression, anxiety, irritable bowel syndrome, constipation, fibromyalgia, chronic fatigue, sensitivity to medications, TMJ dysfunction, or urinary symptoms.
![]()
Dr. Lonky: How prevalent is a finding of pudendal neuralgia?
Dr. Edwards: The prevalence and incidence of pudendal neuralgia are not known. Those who specialize in this condition think it is relatively common. I do not identify or suspect it very often. Its definitive diagnosis and management are outside the purview of the general gynecologist, but the general gynecologist should recognize the symptoms of pudendal neuralgia and refer the patient for evaluation and therapy.
Dr. Lonky: What are those symptoms?
Dr. Haefner: Pudendal neuralgia often occurs following trauma to the pudendal nerve. The pudendal nerve arises from sacral nerves, generally sacral nerves 2 to 4. Several tests can be utilized to diagnose this condition, including quantitative sensor tests, pudendal nerve motor latency tests, electromyography (EMG), and pudendal nerve blocks.20
Nantes Criteria allow for making a diagnosis of pudendal neuralgia (Table 3).21
TABLE 3
Nantes Criteria for pudendal neuralgia by pudendal nerve entrapment
| SOURCE: Labat et al.21 Reproduced with permission from Neurology and Urodynamics. |
Essential criteria
|
Complementary diagnostic criteria
|
Exclusion criteria
|
Associated signs not excluding the diagnosis
|
Initial treatments for pudendal neuralgia should be conservative. Treatments consist of lifestyle changes to prevent flare of disease. Physical therapy, medical management, nerve blocks, and alternative treatments may be beneficial.
Pudendal nerve entrapment is often exacerbated by sitting (not on a toilet seat, however) and is reduced in a standing position. It tends to increase in intensity throughout the day.22 The final treatment for pudendal nerve entrapment is surgery if the nerve is compressed. By this time, the generalist is not generally the provider who performs the surgery.
Dr. Gunter: I believe pudendal neuralgia is sometimes overdiagnosed. EMG studies of the pudendal nerve, often touted as a diagnostic tool, are unreliable (they can be abnormal after vaginal delivery or vaginal hysterectomy, for example). In my experience, bilateral pain is less likely to be pudendal neuralgia; spontaneous bilateral compression neuropathy at exactly the same level is not a common phenomenon in chronic pain.
I reserve the diagnosis of pudendal neuralgia for women who have allodynia in the distribution of the pudendal nerve with severe pain on sitting, and who have exquisite tenderness when pressure is applied over the pudendal nerve (at the level of the ischial spine on vaginal examination). Typically, the vaginal sidewall on the affected side is very sensitive to light touch. I do see pudendal nerve pain after vaginal surgery when there has been some compromise of the pudendal nerve or the sacral plexus. This is typically unilateral pain.
Dr. Lonky: Thank you all. We’ll continue our discussion, with a focus on treatment.
|
MORE TO COME
|
References
1. Thomas TG. Practical Treatise on the Diseases of Women. Philadelphia Pa: Henry C. Lea; 1874.
2. Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88.
3. Lavy RJ, Hynan LS, Haley RW. Prevalence of vulvar pain in an urban minority population. J Reprod Med. 2007;52(1):59–62.
4. Nyirjesy P, Peyton C, Weitz MV, Mathew L, Culhane JF. Causes of chronic vaginitis: analysis of a prospective database of affected women. Obstet Gynecol. 2006;108(5):1185–1191.
5. Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. J Reprod Med. 2004;49(10):772–777.
6. Yunas MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36(6):339–356.
7. Kahn BS, Tatro C, Parsons CL, Willems JJ. Prevalence of interstitial cystitis in vulvodynia patients detected by bladder potassium sensitivity. J Sex Med. 2010;7(2 Pt 2):996–1002.
8. Arnold JD, Bachman GS, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107(3):617–624.
9. Parsons CL, Dell J, Stanford EJ, et al. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187(5):1395–1400.
10. Clemens JQ, Meenan RT, O’Keefe Rosetti MC, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174(2):576–580.
11. Engman M, Lindehammar H, Wijma B. Surface electromyography diagnostics in women with partial vaginismus with or without vulvar vestibulitis and in asymptomatic women. J Psychosom Obstet Gynecol. 2004;25(3-4):281–294.
12. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812–819.
13. Gordon AS, Panahlan-Jand M, McComb F, Melegari C, Sharp S. Characteristics of women with vulvar pain disorders: a Web-based survey. J Sex Marital Ther. 2003;29(suppl 1):45.
14. Whitten CE, Cristobal K. Chronic pain is a chronic condition not just a symptom. Permanente J. 2005;9(3):43.
15. Manchikanti L, Fellows B, Pampati V, et al. Comparison of psychological status of chronic pain patients and the general population. Pain Physician. 2002;5(1):40–48.
16. Banks SM, Kerns RD. Explaining the high rates of depression in chronic pain: a diathesis-stress framework. Psychological Bulletin. 1996;119(1):95–110.
17. Sadownik LA. Clinical correlates of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;5:40–48. Editor found in PubMed: Sadownik LA. Clinical profile of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;45(8):679–684. Could not find the citation listed. Please confirm.
18. Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624–632.
19. Landry T, Bergeron S. Biopsychosocial factors associated with dyspareunia in a community sample of adolescent girls. Arch Sex Behav. 2011; June 22.
20. Goldstein A, Pukall C, Goldstein I. When Sex Hurts: A Woman’s Guide to Banishing Sexual Pain. Cambridge Mass: Da Capo Lifelong Books; 2011;117–126.
21. Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurol Urodyn. 2008;27(4):306–310.
22. Popeney C, Answell V, Renney K. Pudendal entrapment as an etiology of chronic perineal pain: Diagnosis and treatment. Neurol Urodyn. 2007;26(6):820–827.
Although the incidence of vulvar pain has increased over the past decade—thanks to both greater awareness and increasing numbers of affected women—the phenomenon is not a recent development. As early as 1874, T. Galliard Thomas wrote, “[T]his disorder, although fortunately not very frequent, is by no means very rare.”1 He went on to express “surprise” that it had not been “more generally and fully described.”
Despite the focus Thomas directed to the issue, vulvar pain did not get much attention until the 21st century, when a number of studies began to gauge its prevalence. For example, in a study in Boston of about 5,000 women, the lifetime prevalence of chronic vulvar pain was 16%.2 And in a study in Texas, the prevalence of vulvar pain in an urban, largely minority population was estimated to be 11%.3 The Boston study also reported that “nearly 40% of women chose not to seek treatment, and, of those who did, 60% saw three or more doctors, many of whom could not provide a diagnosis.”2
Clearly, there is a need for comprehensive information on vulvar pain and its causes, symptoms, diagnosis, and treatment. To address the lack of guidance, OBG Management Contributing Editor Neal M. Lonky, MD, assembled a panel of experts on vulvar pain syndromes and invited them to share their considerable knowledge. The ensuing discussion, presented in three parts, offers a gold mine of information. In this opening article, the panel focuses on causes, symptomatology, and diagnosis of this common complaint.
|
The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain. |
![]()
Dr. Lonky: What are the most common diagnoses when vulvar pain is the complaint?
Dr. Gunter: The most common cause of chronic vulvar pain is vulvodynia, although lichen simplex chronicus, chronic yeast infections, and non-neoplastic epithelial disorders, such as lichen sclerosus and lichen planus, can also produce irritation and pain. In postmenopausal women, atrophic vaginitis can also cause a burning pain, although symptoms are typically more vaginal than vulvar. Yeast and lichen simplex chronicus typically produce itching, although sometimes they can present with irritation and pain, so they must be considered in the differential diagnosis. It is important to remember that many women with vulvodynia have used multiple topical agents and may have developed complex hygiene rituals in an attempt to treat their symptoms, which can result in a secondary lichen simplex chronicus.
That said, there is a high frequency of misdiagnosis with yeast. For example, in a study by Nyirjesy and colleagues, two thirds of women who were referred to a tertiary clinic for chronic vulvovaginal candidiasis were found to have a noninfectious entity instead—most commonly lichen simplex chronicus and vulvodynia.4
Dr. Edwards: The most common “diagnosis” for vulvar pain is vulvodynia. However, the definition of vulvodynia is pain—i.e., burning, rawness, irritation, soreness, aching, or stabbing or stinging sensations—in the absence of skin disease, infection, or specific neurologic disease. Therefore, even though the usual cause of vulvar pain is vulvodynia, it is a diagnosis of exclusion, and skin disease, infection, and neurologic disease must be ruled out.
In regard to infection, Candida albicans and bacterial vaginosis (BV) are usually the first conditions that are considered when a patient complains of vulvar pain, but they are not common causes of vulvar pain and are never causes of chronic vulvar pain. Very rarely they may cause recurrent pain that clears, at least briefly, with treatment.
Candida albicans is usually primarily pruritic, and BV produces discharge and odor, sometimes with minor symptoms. Non-albicans Candida (e.g., Candida glabrata) is nearly always asymptomatic, but it occasionally causes irritation and burning.
Group B streptococcus is another infectious entity that very, very occasionally causes irritation and dyspareunia but is usually only a colonizer.
Herpes simplex virus is a cause of recurrent but not chronic pain.
Chronic pain is more likely to be caused by skin disease than by infection. Lichen simplex chronicus causes itching; any pain is due to erosions from scratching.
Dr. Haefner: Several other infectious conditions or their treatments can cause vulvar pain. For example, herpes (particularly primary herpes infection) is classically associated with vulvar pain. The pain is so great that, at times, the patient requires admission for pain control. Surprisingly, despite the known pain of herpes, approximately 80% of patients who have it are unaware of their diagnosis.
Although condyloma is generally a painless condition, many patients complain of pain following treatment for it, whether treatment involves topical medications or laser surgery.
Chancroid is a painful vulvar ulcer. Trichomonas can sometimes be associated with vulvar pain.
Dr. Lonky: What terminology do we use when we discuss vulvar pain?
Dr. Haefner: The current terminology used to describe vulvar pain was published in 2004, after years of debate over nomenclature within the International Society for the Study of Vulvovaginal Disease.5 The terminology lists two major categories of vulvar pain:
-
pain related to a specific disorder. This category encompasses numerous conditions that feature an abnormal appearance of the vulva (Table 1).
TABLE 1
Terminology and classification of vulvar pain from the International Society for the Study of Vulvovaginal Disease
| SOURCE: Moyal-Barracco and Lynch.5 Reproduced with permission from the Journal of Reproductive Medicine. |
|
-
vulvodynia, in which the vulva appears normal, other than occasional erythema, which is most prominent at the duct openings (vestibular ducts—Bartholin’s and Skene’s).
As for vulvar pain, there are two major forms:
-
hyperalgesia (a low threshold for pain)
-
allodynia (pain in response to light touch).
Some diseases that are associated with vulvar pain do not qualify for the diagnosis of vulvodynia (Table 2) because they are associated with an abnormal appearance of the vulva.
TABLE 2
Conditions other than vulvodynia that are associated with vulvar pain
| Acute irritant contact dermatitis (e.g., erosion due to podofilox, imiquimod, cantharidin, fluorouracil, or podophyllin toxin) |
| Aphthous ulcer |
| Atrophy |
| Bartholin’s abscess |
| Candidiasis |
| Carcinoma |
| Chronic irritant contact dermatitis |
| Endometriosis |
| Herpes (simplex and zoster) |
| Immunobullous diseases (including cicatricial pemphigoid, pemphigus vulgaris, linear immunoglobulin A disease, etc.) |
| Lichen planus |
| Lichen sclerosus |
| Podophyllin overdose (see above) |
| Prolapsed urethra |
| Sjögren’s syndrome |
| Trauma |
| Trichomoniasis |
| Vulvar intraepithelial neoplasia |
![]()
Dr. Lonky: What skin diseases need to be ruled out before vulvodynia can be diagnosed?
Dr. Edwards: Skin diseases that affect the vulva are usually pruritic—pain is a later sign. Lichen simplex chronicus (also known as eczema) is pruritus caused by any irritant; any pain that arises is produced by visible excoriations from scratching.
Lichen sclerosus manifests as white epithelium that has a crinkling, shiny, or waxy texture. It can produce pain, especially dyspareunia. The pain is caused by erosions that arise from fragility and introital narrowing and inelasticity.
Vulvovaginal lichen planus is usually erosive and preferentially affects mucous membranes, especially the vestibule; it sometimes affects the vagina and mouth, as well.
Desquamative inflammatory vaginitis is most likely a skin disease that affects only the vagina. It involves introital redness and a clinically and microscopically purulent vaginal discharge that also reveals parabasal cells and absent lactobacilli.
Dr. Lonky: You mentioned that neurologic diseases can sometimes cause vulvar pain. Which ones?
Dr. Edwards: Pudendal neuralgia, diabetic neuropathy, and post-herpetic neuralgia are the most common specific neurologic causes of vulvar pain. Multiple sclerosis can also produce pain syndromes. Post-herpetic neuralgia follows herpes zoster—not herpes simplex—virus infection.
Dr. Lonky: Any other conditions that can cause vulvar pain?
Dr. Haefner: Aphthous ulcers are common and are often flared by stress.
Non-neoplastic epithelial disorders are also seen frequently in health-care providers’ offices; many patients who experience them report pain on the vulva.
It is always important to consider cancer when a patient has an abnormal vulvar appearance and pain that has persisted despite treatment.
![]()
Dr. Lonky: If you were to rank vulvar pain syndromes according to their prevalence, what would the most common syndromes be?
Dr. Gunter: Given the misdiagnosis of many women, who are told they have chronic yeast infection, as I mentioned, it’s hard to know which vulvar pain syndromes are most prevalent. I suspect that lichen simplex chronicus is most common, followed by vulvodynia, with chronic yeast infection a distant third.
My experience reflects what Nyirjesy and colleagues4 found: 65% to 75% of women referred to my clinic with chronic yeast actually have lichen simplex chronicus or vulvodynia. In postmenopausal women, atrophic vaginitis is also a consideration; it’s becoming more common now that the use of systemic hormone replacement therapy is decreasing.
Dr. Lonky: What about subsets of vulvodynia? Which ones are most common?
Dr. Edwards: There is good evidence of marked overlap among subsets of vulvodynia. The vast majority of women who have vulvodynia experience primarily provoked vestibular pain, regardless of age. However, I find that almost all patients also report pain that extends beyond the vestibule at times, as well as occasional unprovoked pain.
The diagnosis requires the exclusion of other causes of vulvar pain, and the subset is identified by the location of pain (that is, is it strictly localized or generalized or even migratory?) and its provoked or unprovoked nature.
Localized clitoral pain and vulvar pain localized to one side of the vulva are extremely uncommon, but they do occur. And although I rarely encounter teenagers and prepubertal children who have vulvodynia, I do have patients in both age groups who have vulvodynia.
Dr. Lonky: Are there racial differences in the prevalence of vulvodynia?
Dr. Edwards: Although several good studies show that women of African descent and white patients are equally likely to experience vulvodynia, the vast majority (99%) of my patients who have vulvodynia are white. My patients of African descent consult me primarily for itching or discharge.
My local demographics prevent me from judging the likelihood of Asians having vulvodynia, and our Hispanic population has limited access to health care.
In general, I don’t think that demographics are useful in making the diagnosis of vulvodynia.
![]()
Dr. Lonky: Do your patients who have vulvodynia or another vulvar pain syndrome tend to have comorbidities? If so, is this information helpful in establishing the diagnosis and planning therapy?
Dr. Haefner: Women who have vulvodynia often have other medical problems as well. In my practice, when new patients who have vulvodynia complete their intake survey, they often report a history of headache, irritable bowel syndrome, interstitial cystitis, fibromyalgia,6 chronic fatigue syndrome, back pain, and temporomandibular joint (TMJ) disorder. These comorbidities are not particularly helpful in establishing the diagnosis of vulvodynia, but they are an important consideration when choosing therapy for the patient. Often, the medications chosen to treat one condition will also benefit another condition. However, it’s important to check for potential interactions between drugs before prescribing a new treatment.
Dr. Gunter: A significant number of women who have vulvodynia also have other chronic pain syndromes. For example, the incidence of bladder pain syndrome–interstitial cystitis is 68% to 82% among women who have vulvodynia, compared with a baseline rate among all women of 6% to 11%.7-10 The rate of irritable bowel syndrome is more than doubled among women who have vulvodynia, compared with the general population (27% versus 12%).8 Another common comorbidity, hypertonic somatic dysfunction of the pelvic floor, is identified in 10% to 90% of women who have chronic vulvar pain.8,11,12 These women also have a higher incidence of nongenital pain syndromes, such as fibromyalgia, migraine, and TMJ dysfunction, than the general population, as Dr. Haefner noted.8,12,13
Many studies have evaluated psychological and emotional contributions to chronic vulvar pain. Pain and depression are intimately related—the incidence of depression among all people who experience chronic pain ranges from 27% to 54%, compared with 5% to 17% among the general population.14-16 The relationship is complex because chronic illness in general is associated with depression. Nevertheless, several studies have noted an increase in anxiety, stress, and depression among women who have vulvodynia.17-19
I screen every patient for depression using a Patient Health Questionnaire (PHQ-9); I also screen for anxiety. I find that a significant percentage of patients in my clinic are depressed or have an anxiety disorder. Failure to address these comorbidities makes treatment very difficult. I typically prescribe citalopram (Celexa), although there is some question whether it can safely be combined with a tricyclic antidepressant. We also offer stress-reduction classes, teach every patient the value of diaphragmatic breathing, offer mind-body classes for anxiety and stress, and provide intensive programs where the patient can learn important self-care skills, such as pacing (spacing activities throughout the day in a manner that avoids aggravating the pain), and address her anxiety and stress in a more guided manner. We also have a psychologist who specializes in pain for any patient who may need one-on-one counseling.
Dr. Edwards: The presence of comorbidities is somewhat useful in making the diagnosis of vulvodynia. I question my diagnosis, in fact, when a patient who has vulvodynia does not have headaches, low energy, depression, anxiety, irritable bowel syndrome, constipation, fibromyalgia, chronic fatigue, sensitivity to medications, TMJ dysfunction, or urinary symptoms.
![]()
Dr. Lonky: How prevalent is a finding of pudendal neuralgia?
Dr. Edwards: The prevalence and incidence of pudendal neuralgia are not known. Those who specialize in this condition think it is relatively common. I do not identify or suspect it very often. Its definitive diagnosis and management are outside the purview of the general gynecologist, but the general gynecologist should recognize the symptoms of pudendal neuralgia and refer the patient for evaluation and therapy.
Dr. Lonky: What are those symptoms?
Dr. Haefner: Pudendal neuralgia often occurs following trauma to the pudendal nerve. The pudendal nerve arises from sacral nerves, generally sacral nerves 2 to 4. Several tests can be utilized to diagnose this condition, including quantitative sensor tests, pudendal nerve motor latency tests, electromyography (EMG), and pudendal nerve blocks.20
Nantes Criteria allow for making a diagnosis of pudendal neuralgia (Table 3).21
TABLE 3
Nantes Criteria for pudendal neuralgia by pudendal nerve entrapment
| SOURCE: Labat et al.21 Reproduced with permission from Neurology and Urodynamics. |
Essential criteria
|
Complementary diagnostic criteria
|
Exclusion criteria
|
Associated signs not excluding the diagnosis
|
Initial treatments for pudendal neuralgia should be conservative. Treatments consist of lifestyle changes to prevent flare of disease. Physical therapy, medical management, nerve blocks, and alternative treatments may be beneficial.
Pudendal nerve entrapment is often exacerbated by sitting (not on a toilet seat, however) and is reduced in a standing position. It tends to increase in intensity throughout the day.22 The final treatment for pudendal nerve entrapment is surgery if the nerve is compressed. By this time, the generalist is not generally the provider who performs the surgery.
Dr. Gunter: I believe pudendal neuralgia is sometimes overdiagnosed. EMG studies of the pudendal nerve, often touted as a diagnostic tool, are unreliable (they can be abnormal after vaginal delivery or vaginal hysterectomy, for example). In my experience, bilateral pain is less likely to be pudendal neuralgia; spontaneous bilateral compression neuropathy at exactly the same level is not a common phenomenon in chronic pain.
I reserve the diagnosis of pudendal neuralgia for women who have allodynia in the distribution of the pudendal nerve with severe pain on sitting, and who have exquisite tenderness when pressure is applied over the pudendal nerve (at the level of the ischial spine on vaginal examination). Typically, the vaginal sidewall on the affected side is very sensitive to light touch. I do see pudendal nerve pain after vaginal surgery when there has been some compromise of the pudendal nerve or the sacral plexus. This is typically unilateral pain.
Dr. Lonky: Thank you all. We’ll continue our discussion, with a focus on treatment.
|
MORE TO COME
|
References
1. Thomas TG. Practical Treatise on the Diseases of Women. Philadelphia Pa: Henry C. Lea; 1874.
2. Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88.
3. Lavy RJ, Hynan LS, Haley RW. Prevalence of vulvar pain in an urban minority population. J Reprod Med. 2007;52(1):59–62.
4. Nyirjesy P, Peyton C, Weitz MV, Mathew L, Culhane JF. Causes of chronic vaginitis: analysis of a prospective database of affected women. Obstet Gynecol. 2006;108(5):1185–1191.
5. Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. J Reprod Med. 2004;49(10):772–777.
6. Yunas MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36(6):339–356.
7. Kahn BS, Tatro C, Parsons CL, Willems JJ. Prevalence of interstitial cystitis in vulvodynia patients detected by bladder potassium sensitivity. J Sex Med. 2010;7(2 Pt 2):996–1002.
8. Arnold JD, Bachman GS, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107(3):617–624.
9. Parsons CL, Dell J, Stanford EJ, et al. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187(5):1395–1400.
10. Clemens JQ, Meenan RT, O’Keefe Rosetti MC, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174(2):576–580.
11. Engman M, Lindehammar H, Wijma B. Surface electromyography diagnostics in women with partial vaginismus with or without vulvar vestibulitis and in asymptomatic women. J Psychosom Obstet Gynecol. 2004;25(3-4):281–294.
12. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812–819.
13. Gordon AS, Panahlan-Jand M, McComb F, Melegari C, Sharp S. Characteristics of women with vulvar pain disorders: a Web-based survey. J Sex Marital Ther. 2003;29(suppl 1):45.
14. Whitten CE, Cristobal K. Chronic pain is a chronic condition not just a symptom. Permanente J. 2005;9(3):43.
15. Manchikanti L, Fellows B, Pampati V, et al. Comparison of psychological status of chronic pain patients and the general population. Pain Physician. 2002;5(1):40–48.
16. Banks SM, Kerns RD. Explaining the high rates of depression in chronic pain: a diathesis-stress framework. Psychological Bulletin. 1996;119(1):95–110.
17. Sadownik LA. Clinical correlates of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;5:40–48. Editor found in PubMed: Sadownik LA. Clinical profile of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;45(8):679–684. Could not find the citation listed. Please confirm.
18. Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624–632.
19. Landry T, Bergeron S. Biopsychosocial factors associated with dyspareunia in a community sample of adolescent girls. Arch Sex Behav. 2011; June 22.
20. Goldstein A, Pukall C, Goldstein I. When Sex Hurts: A Woman’s Guide to Banishing Sexual Pain. Cambridge Mass: Da Capo Lifelong Books; 2011;117–126.
21. Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurol Urodyn. 2008;27(4):306–310.
22. Popeney C, Answell V, Renney K. Pudendal entrapment as an etiology of chronic perineal pain: Diagnosis and treatment. Neurol Urodyn. 2007;26(6):820–827.
Although the incidence of vulvar pain has increased over the past decade—thanks to both greater awareness and increasing numbers of affected women—the phenomenon is not a recent development. As early as 1874, T. Galliard Thomas wrote, “[T]his disorder, although fortunately not very frequent, is by no means very rare.”1 He went on to express “surprise” that it had not been “more generally and fully described.”
Despite the focus Thomas directed to the issue, vulvar pain did not get much attention until the 21st century, when a number of studies began to gauge its prevalence. For example, in a study in Boston of about 5,000 women, the lifetime prevalence of chronic vulvar pain was 16%.2 And in a study in Texas, the prevalence of vulvar pain in an urban, largely minority population was estimated to be 11%.3 The Boston study also reported that “nearly 40% of women chose not to seek treatment, and, of those who did, 60% saw three or more doctors, many of whom could not provide a diagnosis.”2
Clearly, there is a need for comprehensive information on vulvar pain and its causes, symptoms, diagnosis, and treatment. To address the lack of guidance, OBG Management Contributing Editor Neal M. Lonky, MD, assembled a panel of experts on vulvar pain syndromes and invited them to share their considerable knowledge. The ensuing discussion, presented in three parts, offers a gold mine of information. In this opening article, the panel focuses on causes, symptomatology, and diagnosis of this common complaint.
|
The lower vagina and vulva are richly supplied with peripheral nerves and are, therefore, sensitive to pain, particularly the region of the hymeneal ring. Although the pudendal nerve (arrow) courses through the area, it is an uncommon source of vulvar pain. |
![]()
Dr. Lonky: What are the most common diagnoses when vulvar pain is the complaint?
Dr. Gunter: The most common cause of chronic vulvar pain is vulvodynia, although lichen simplex chronicus, chronic yeast infections, and non-neoplastic epithelial disorders, such as lichen sclerosus and lichen planus, can also produce irritation and pain. In postmenopausal women, atrophic vaginitis can also cause a burning pain, although symptoms are typically more vaginal than vulvar. Yeast and lichen simplex chronicus typically produce itching, although sometimes they can present with irritation and pain, so they must be considered in the differential diagnosis. It is important to remember that many women with vulvodynia have used multiple topical agents and may have developed complex hygiene rituals in an attempt to treat their symptoms, which can result in a secondary lichen simplex chronicus.
That said, there is a high frequency of misdiagnosis with yeast. For example, in a study by Nyirjesy and colleagues, two thirds of women who were referred to a tertiary clinic for chronic vulvovaginal candidiasis were found to have a noninfectious entity instead—most commonly lichen simplex chronicus and vulvodynia.4
Dr. Edwards: The most common “diagnosis” for vulvar pain is vulvodynia. However, the definition of vulvodynia is pain—i.e., burning, rawness, irritation, soreness, aching, or stabbing or stinging sensations—in the absence of skin disease, infection, or specific neurologic disease. Therefore, even though the usual cause of vulvar pain is vulvodynia, it is a diagnosis of exclusion, and skin disease, infection, and neurologic disease must be ruled out.
In regard to infection, Candida albicans and bacterial vaginosis (BV) are usually the first conditions that are considered when a patient complains of vulvar pain, but they are not common causes of vulvar pain and are never causes of chronic vulvar pain. Very rarely they may cause recurrent pain that clears, at least briefly, with treatment.
Candida albicans is usually primarily pruritic, and BV produces discharge and odor, sometimes with minor symptoms. Non-albicans Candida (e.g., Candida glabrata) is nearly always asymptomatic, but it occasionally causes irritation and burning.
Group B streptococcus is another infectious entity that very, very occasionally causes irritation and dyspareunia but is usually only a colonizer.
Herpes simplex virus is a cause of recurrent but not chronic pain.
Chronic pain is more likely to be caused by skin disease than by infection. Lichen simplex chronicus causes itching; any pain is due to erosions from scratching.
Dr. Haefner: Several other infectious conditions or their treatments can cause vulvar pain. For example, herpes (particularly primary herpes infection) is classically associated with vulvar pain. The pain is so great that, at times, the patient requires admission for pain control. Surprisingly, despite the known pain of herpes, approximately 80% of patients who have it are unaware of their diagnosis.
Although condyloma is generally a painless condition, many patients complain of pain following treatment for it, whether treatment involves topical medications or laser surgery.
Chancroid is a painful vulvar ulcer. Trichomonas can sometimes be associated with vulvar pain.
Dr. Lonky: What terminology do we use when we discuss vulvar pain?
Dr. Haefner: The current terminology used to describe vulvar pain was published in 2004, after years of debate over nomenclature within the International Society for the Study of Vulvovaginal Disease.5 The terminology lists two major categories of vulvar pain:
-
pain related to a specific disorder. This category encompasses numerous conditions that feature an abnormal appearance of the vulva (Table 1).
TABLE 1
Terminology and classification of vulvar pain from the International Society for the Study of Vulvovaginal Disease
| SOURCE: Moyal-Barracco and Lynch.5 Reproduced with permission from the Journal of Reproductive Medicine. |
|
-
vulvodynia, in which the vulva appears normal, other than occasional erythema, which is most prominent at the duct openings (vestibular ducts—Bartholin’s and Skene’s).
As for vulvar pain, there are two major forms:
-
hyperalgesia (a low threshold for pain)
-
allodynia (pain in response to light touch).
Some diseases that are associated with vulvar pain do not qualify for the diagnosis of vulvodynia (Table 2) because they are associated with an abnormal appearance of the vulva.
TABLE 2
Conditions other than vulvodynia that are associated with vulvar pain
| Acute irritant contact dermatitis (e.g., erosion due to podofilox, imiquimod, cantharidin, fluorouracil, or podophyllin toxin) |
| Aphthous ulcer |
| Atrophy |
| Bartholin’s abscess |
| Candidiasis |
| Carcinoma |
| Chronic irritant contact dermatitis |
| Endometriosis |
| Herpes (simplex and zoster) |
| Immunobullous diseases (including cicatricial pemphigoid, pemphigus vulgaris, linear immunoglobulin A disease, etc.) |
| Lichen planus |
| Lichen sclerosus |
| Podophyllin overdose (see above) |
| Prolapsed urethra |
| Sjögren’s syndrome |
| Trauma |
| Trichomoniasis |
| Vulvar intraepithelial neoplasia |
![]()
Dr. Lonky: What skin diseases need to be ruled out before vulvodynia can be diagnosed?
Dr. Edwards: Skin diseases that affect the vulva are usually pruritic—pain is a later sign. Lichen simplex chronicus (also known as eczema) is pruritus caused by any irritant; any pain that arises is produced by visible excoriations from scratching.
Lichen sclerosus manifests as white epithelium that has a crinkling, shiny, or waxy texture. It can produce pain, especially dyspareunia. The pain is caused by erosions that arise from fragility and introital narrowing and inelasticity.
Vulvovaginal lichen planus is usually erosive and preferentially affects mucous membranes, especially the vestibule; it sometimes affects the vagina and mouth, as well.
Desquamative inflammatory vaginitis is most likely a skin disease that affects only the vagina. It involves introital redness and a clinically and microscopically purulent vaginal discharge that also reveals parabasal cells and absent lactobacilli.
Dr. Lonky: You mentioned that neurologic diseases can sometimes cause vulvar pain. Which ones?
Dr. Edwards: Pudendal neuralgia, diabetic neuropathy, and post-herpetic neuralgia are the most common specific neurologic causes of vulvar pain. Multiple sclerosis can also produce pain syndromes. Post-herpetic neuralgia follows herpes zoster—not herpes simplex—virus infection.
Dr. Lonky: Any other conditions that can cause vulvar pain?
Dr. Haefner: Aphthous ulcers are common and are often flared by stress.
Non-neoplastic epithelial disorders are also seen frequently in health-care providers’ offices; many patients who experience them report pain on the vulva.
It is always important to consider cancer when a patient has an abnormal vulvar appearance and pain that has persisted despite treatment.
![]()
Dr. Lonky: If you were to rank vulvar pain syndromes according to their prevalence, what would the most common syndromes be?
Dr. Gunter: Given the misdiagnosis of many women, who are told they have chronic yeast infection, as I mentioned, it’s hard to know which vulvar pain syndromes are most prevalent. I suspect that lichen simplex chronicus is most common, followed by vulvodynia, with chronic yeast infection a distant third.
My experience reflects what Nyirjesy and colleagues4 found: 65% to 75% of women referred to my clinic with chronic yeast actually have lichen simplex chronicus or vulvodynia. In postmenopausal women, atrophic vaginitis is also a consideration; it’s becoming more common now that the use of systemic hormone replacement therapy is decreasing.
Dr. Lonky: What about subsets of vulvodynia? Which ones are most common?
Dr. Edwards: There is good evidence of marked overlap among subsets of vulvodynia. The vast majority of women who have vulvodynia experience primarily provoked vestibular pain, regardless of age. However, I find that almost all patients also report pain that extends beyond the vestibule at times, as well as occasional unprovoked pain.
The diagnosis requires the exclusion of other causes of vulvar pain, and the subset is identified by the location of pain (that is, is it strictly localized or generalized or even migratory?) and its provoked or unprovoked nature.
Localized clitoral pain and vulvar pain localized to one side of the vulva are extremely uncommon, but they do occur. And although I rarely encounter teenagers and prepubertal children who have vulvodynia, I do have patients in both age groups who have vulvodynia.
Dr. Lonky: Are there racial differences in the prevalence of vulvodynia?
Dr. Edwards: Although several good studies show that women of African descent and white patients are equally likely to experience vulvodynia, the vast majority (99%) of my patients who have vulvodynia are white. My patients of African descent consult me primarily for itching or discharge.
My local demographics prevent me from judging the likelihood of Asians having vulvodynia, and our Hispanic population has limited access to health care.
In general, I don’t think that demographics are useful in making the diagnosis of vulvodynia.
![]()
Dr. Lonky: Do your patients who have vulvodynia or another vulvar pain syndrome tend to have comorbidities? If so, is this information helpful in establishing the diagnosis and planning therapy?
Dr. Haefner: Women who have vulvodynia often have other medical problems as well. In my practice, when new patients who have vulvodynia complete their intake survey, they often report a history of headache, irritable bowel syndrome, interstitial cystitis, fibromyalgia,6 chronic fatigue syndrome, back pain, and temporomandibular joint (TMJ) disorder. These comorbidities are not particularly helpful in establishing the diagnosis of vulvodynia, but they are an important consideration when choosing therapy for the patient. Often, the medications chosen to treat one condition will also benefit another condition. However, it’s important to check for potential interactions between drugs before prescribing a new treatment.
Dr. Gunter: A significant number of women who have vulvodynia also have other chronic pain syndromes. For example, the incidence of bladder pain syndrome–interstitial cystitis is 68% to 82% among women who have vulvodynia, compared with a baseline rate among all women of 6% to 11%.7-10 The rate of irritable bowel syndrome is more than doubled among women who have vulvodynia, compared with the general population (27% versus 12%).8 Another common comorbidity, hypertonic somatic dysfunction of the pelvic floor, is identified in 10% to 90% of women who have chronic vulvar pain.8,11,12 These women also have a higher incidence of nongenital pain syndromes, such as fibromyalgia, migraine, and TMJ dysfunction, than the general population, as Dr. Haefner noted.8,12,13
Many studies have evaluated psychological and emotional contributions to chronic vulvar pain. Pain and depression are intimately related—the incidence of depression among all people who experience chronic pain ranges from 27% to 54%, compared with 5% to 17% among the general population.14-16 The relationship is complex because chronic illness in general is associated with depression. Nevertheless, several studies have noted an increase in anxiety, stress, and depression among women who have vulvodynia.17-19
I screen every patient for depression using a Patient Health Questionnaire (PHQ-9); I also screen for anxiety. I find that a significant percentage of patients in my clinic are depressed or have an anxiety disorder. Failure to address these comorbidities makes treatment very difficult. I typically prescribe citalopram (Celexa), although there is some question whether it can safely be combined with a tricyclic antidepressant. We also offer stress-reduction classes, teach every patient the value of diaphragmatic breathing, offer mind-body classes for anxiety and stress, and provide intensive programs where the patient can learn important self-care skills, such as pacing (spacing activities throughout the day in a manner that avoids aggravating the pain), and address her anxiety and stress in a more guided manner. We also have a psychologist who specializes in pain for any patient who may need one-on-one counseling.
Dr. Edwards: The presence of comorbidities is somewhat useful in making the diagnosis of vulvodynia. I question my diagnosis, in fact, when a patient who has vulvodynia does not have headaches, low energy, depression, anxiety, irritable bowel syndrome, constipation, fibromyalgia, chronic fatigue, sensitivity to medications, TMJ dysfunction, or urinary symptoms.
![]()
Dr. Lonky: How prevalent is a finding of pudendal neuralgia?
Dr. Edwards: The prevalence and incidence of pudendal neuralgia are not known. Those who specialize in this condition think it is relatively common. I do not identify or suspect it very often. Its definitive diagnosis and management are outside the purview of the general gynecologist, but the general gynecologist should recognize the symptoms of pudendal neuralgia and refer the patient for evaluation and therapy.
Dr. Lonky: What are those symptoms?
Dr. Haefner: Pudendal neuralgia often occurs following trauma to the pudendal nerve. The pudendal nerve arises from sacral nerves, generally sacral nerves 2 to 4. Several tests can be utilized to diagnose this condition, including quantitative sensor tests, pudendal nerve motor latency tests, electromyography (EMG), and pudendal nerve blocks.20
Nantes Criteria allow for making a diagnosis of pudendal neuralgia (Table 3).21
TABLE 3
Nantes Criteria for pudendal neuralgia by pudendal nerve entrapment
| SOURCE: Labat et al.21 Reproduced with permission from Neurology and Urodynamics. |
Essential criteria
|
Complementary diagnostic criteria
|
Exclusion criteria
|
Associated signs not excluding the diagnosis
|
Initial treatments for pudendal neuralgia should be conservative. Treatments consist of lifestyle changes to prevent flare of disease. Physical therapy, medical management, nerve blocks, and alternative treatments may be beneficial.
Pudendal nerve entrapment is often exacerbated by sitting (not on a toilet seat, however) and is reduced in a standing position. It tends to increase in intensity throughout the day.22 The final treatment for pudendal nerve entrapment is surgery if the nerve is compressed. By this time, the generalist is not generally the provider who performs the surgery.
Dr. Gunter: I believe pudendal neuralgia is sometimes overdiagnosed. EMG studies of the pudendal nerve, often touted as a diagnostic tool, are unreliable (they can be abnormal after vaginal delivery or vaginal hysterectomy, for example). In my experience, bilateral pain is less likely to be pudendal neuralgia; spontaneous bilateral compression neuropathy at exactly the same level is not a common phenomenon in chronic pain.
I reserve the diagnosis of pudendal neuralgia for women who have allodynia in the distribution of the pudendal nerve with severe pain on sitting, and who have exquisite tenderness when pressure is applied over the pudendal nerve (at the level of the ischial spine on vaginal examination). Typically, the vaginal sidewall on the affected side is very sensitive to light touch. I do see pudendal nerve pain after vaginal surgery when there has been some compromise of the pudendal nerve or the sacral plexus. This is typically unilateral pain.
Dr. Lonky: Thank you all. We’ll continue our discussion, with a focus on treatment.
|
MORE TO COME
|
References
1. Thomas TG. Practical Treatise on the Diseases of Women. Philadelphia Pa: Henry C. Lea; 1874.
2. Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc. 2003;58(2):82–88.
3. Lavy RJ, Hynan LS, Haley RW. Prevalence of vulvar pain in an urban minority population. J Reprod Med. 2007;52(1):59–62.
4. Nyirjesy P, Peyton C, Weitz MV, Mathew L, Culhane JF. Causes of chronic vaginitis: analysis of a prospective database of affected women. Obstet Gynecol. 2006;108(5):1185–1191.
5. Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. J Reprod Med. 2004;49(10):772–777.
6. Yunas MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36(6):339–356.
7. Kahn BS, Tatro C, Parsons CL, Willems JJ. Prevalence of interstitial cystitis in vulvodynia patients detected by bladder potassium sensitivity. J Sex Med. 2010;7(2 Pt 2):996–1002.
8. Arnold JD, Bachman GS, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstet Gynecol. 2006;107(3):617–624.
9. Parsons CL, Dell J, Stanford EJ, et al. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187(5):1395–1400.
10. Clemens JQ, Meenan RT, O’Keefe Rosetti MC, et al. Prevalence of interstitial cystitis symptoms in a managed care population. J Urol. 2005;174(2):576–580.
11. Engman M, Lindehammar H, Wijma B. Surface electromyography diagnostics in women with partial vaginismus with or without vulvar vestibulitis and in asymptomatic women. J Psychosom Obstet Gynecol. 2004;25(3-4):281–294.
12. Gunter J. Vulvodynia: new thoughts on a devastating condition. Obstet Gynecol Surv. 2007;62(12):812–819.
13. Gordon AS, Panahlan-Jand M, McComb F, Melegari C, Sharp S. Characteristics of women with vulvar pain disorders: a Web-based survey. J Sex Marital Ther. 2003;29(suppl 1):45.
14. Whitten CE, Cristobal K. Chronic pain is a chronic condition not just a symptom. Permanente J. 2005;9(3):43.
15. Manchikanti L, Fellows B, Pampati V, et al. Comparison of psychological status of chronic pain patients and the general population. Pain Physician. 2002;5(1):40–48.
16. Banks SM, Kerns RD. Explaining the high rates of depression in chronic pain: a diathesis-stress framework. Psychological Bulletin. 1996;119(1):95–110.
17. Sadownik LA. Clinical correlates of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;5:40–48. Editor found in PubMed: Sadownik LA. Clinical profile of vulvodynia patients. A prospective study of 300 patients. J Reprod Med. 2000;45(8):679–684. Could not find the citation listed. Please confirm.
18. Reed BD, Haefner HK, Punch MR, Roth RS, Gorenflo DW, Gillespie BW. Psychosocial and sexual functioning in women with vulvodynia and chronic pelvic pain. A comparative evaluation. J Reprod Med. 2000;45(8):624–632.
19. Landry T, Bergeron S. Biopsychosocial factors associated with dyspareunia in a community sample of adolescent girls. Arch Sex Behav. 2011; June 22.
20. Goldstein A, Pukall C, Goldstein I. When Sex Hurts: A Woman’s Guide to Banishing Sexual Pain. Cambridge Mass: Da Capo Lifelong Books; 2011;117–126.
21. Labat JJ, Riant T, Robert R, Amarenco G, Lefaucheur JP, Rigaud J. Diagnostic criteria for pudendal neuralgia by pudendal nerve entrapment (Nantes criteria). Neurol Urodyn. 2008;27(4):306–310.
22. Popeney C, Answell V, Renney K. Pudendal entrapment as an etiology of chronic perineal pain: Diagnosis and treatment. Neurol Urodyn. 2007;26(6):820–827.