User login
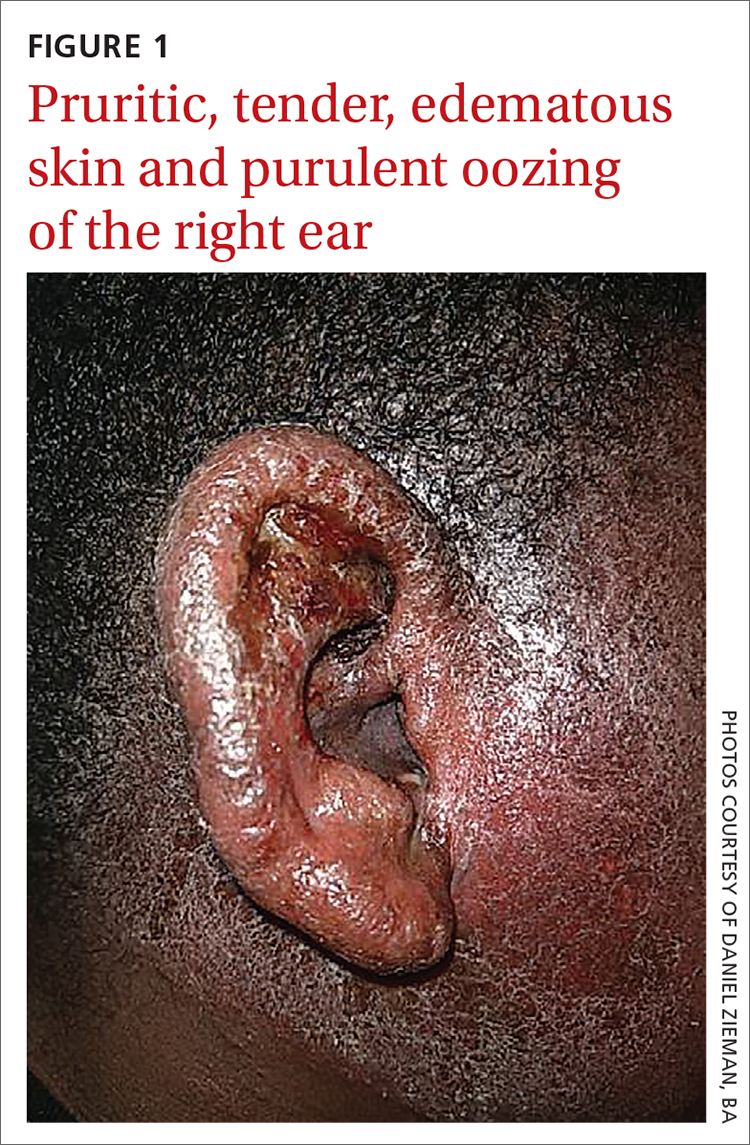
Erythematous ear with drainage
A 6-year-old boy was seen in the hospital in consultation for a 3-week history of suspected cellulitis of the right ear. Drainage from the right ear was refractory to treatment with a 7-day course of cephalexin 15 mL po bid of 250 mg/5 mL solution and clindamycin 24.4 mL po tid of 75 mg/5 mL solution. Treatment was followed by admission to the hospital for treatment with intravenous (IV) cefazolin 1000 mg q6h and IV vancomycin 825 mg q6h for 1 week.
The patient had a significant past medical history for asthma, allergic rhinitis, and severe atopic dermatitis that had been treated with methotrexate 10 mg per week for 6 months beginning when the child was 5 years of age. When the methotrexate proved to be ineffective, the patient was started on Aquaphor and mometasone 0.1% ointment. A 6-month trial of these agents failed as well.
Physical examination revealed that the right ear and skin around it were edematous, erythematous, pruritic, and tender. There was also purulent drainage coming from the ear (FIGURE 1).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infectious eczematoid dermatitis
The patient was referred to a dermatologist after seeing an ear, nose, and throat (ENT) specialist who made the diagnosis of otitis externa when the rash failed to respond to topical and systemic antibiotics. The patient’s tender, pruritic, oozing, edematous eruption was recognized as an infectious eczematoid dermatitis (IED).
Although it is not an uncommon condition, IED may be underrecognized. It accounted for 2.9% of admissions to a dermatology-run inpatient service between 2000 and 2010.1 IED results from cutaneous sensitization to purulent drainage secondary to acute otitis externa or another primary infection.2 In fact, cultures from the purulent drainage in this patient grew methicillin-resistant Staphylococcus aureus. The patient’s right otitis externa drainage may have been associated with the previous history of atopic dermatitis. Atopic dermatitis is associated with an increased risk of skin infections due to decreased inflammatory mediators (defensins).
Cellulitis and herpes zoster oticus are part of the differential
The differential diagnosis in this case includes bacterial cellulitis, acute otitis media, and herpes zoster oticus.
Bacterial cellulitis manifests with erythema, edema, and tenderness with blistering when associated with bullous impetigo rather than pruritus. The clinical appearance of the patient’s diffuse, weeping, edematous external ear, the lack of response to guided antibiotic therapy, and the pruritus experienced by the patient argue against the diagnosis of bacterial cellulitis.
Acute otitis media, like otitis externa, produces ear discharge usually associated with significant pain. Thus, it is important when working through the differential to define the source of the ear discharge. In this case, a consultation with an ENT specialist confirmed that there was an intact tympanic membrane with no middle ear involvement, ruling out the diagnosis of acute otitis media.
Continue to: Herpes zoster oticus
Herpes zoster oticus. The absence of grouped vesicles at any point during the eruption, itching rather than pain, and negative viral culture and polymerase chain reaction studies for herpes simplex and varicella zoster virus excluded the diagnosis of herpes zoster oticus.
Diagnostic criteria were met
This case was compatible with the characterizations of IED as initially described by Engman3 in 1902 and further detailed by Sutton,4 who provided the following criteria for diagnosis:
- an initial eczematous or pustular lesion
- extension peripherally by autoinoculation
- an absence of central clearing
- Staphylococcus on culture of the initial lesion
- a history of infection.
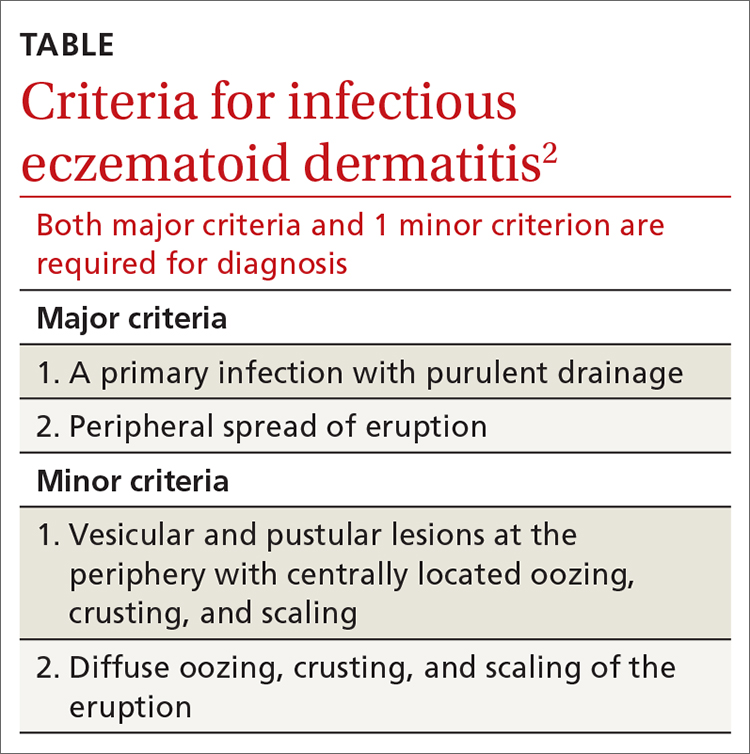
Case reports have added to our understanding of the mechanism of autosensitization of surrounding skin.5 Yamany and Schwartz have proposed the diagnostic criteria summarized in the TABLE.2
Age factors into location. The ears, nose, and face are predominantly involved in cases of IED in the pediatric population, while the lower extremities are predominantly involved in adults.6 Laboratory tests and imaging may aid in excluding other potential diagnoses or complications, but the diagnosis remains clinical and requires the clinician to avoid jumping to the conclusion that every moist, erythematous crusting eruption is purely infectious in nature.
Tx and prevention hinge on a combination of antibiotics, steroids
The management of IED should be aimed at fighting the infection, eliminating the allergic contact dermatitis associated with infectious products, and improving barrier protection. Topical and/or systemic antibiotics guided by culture focus on killing bacteria. The allergic immune response is dampened by systemic steroids. Topical steroids, however, are difficult to utilize on moist, draining skin. In the case of otitis externa, a combination topical antibiotic and steroid otic drop can be utilized. As healing begins, emollients are applied to aid in skin repair.2 Topical antibiotics containing neomycin or polymyxin should be avoided to eliminate the possibility of developing contact sensitivity to these agents.
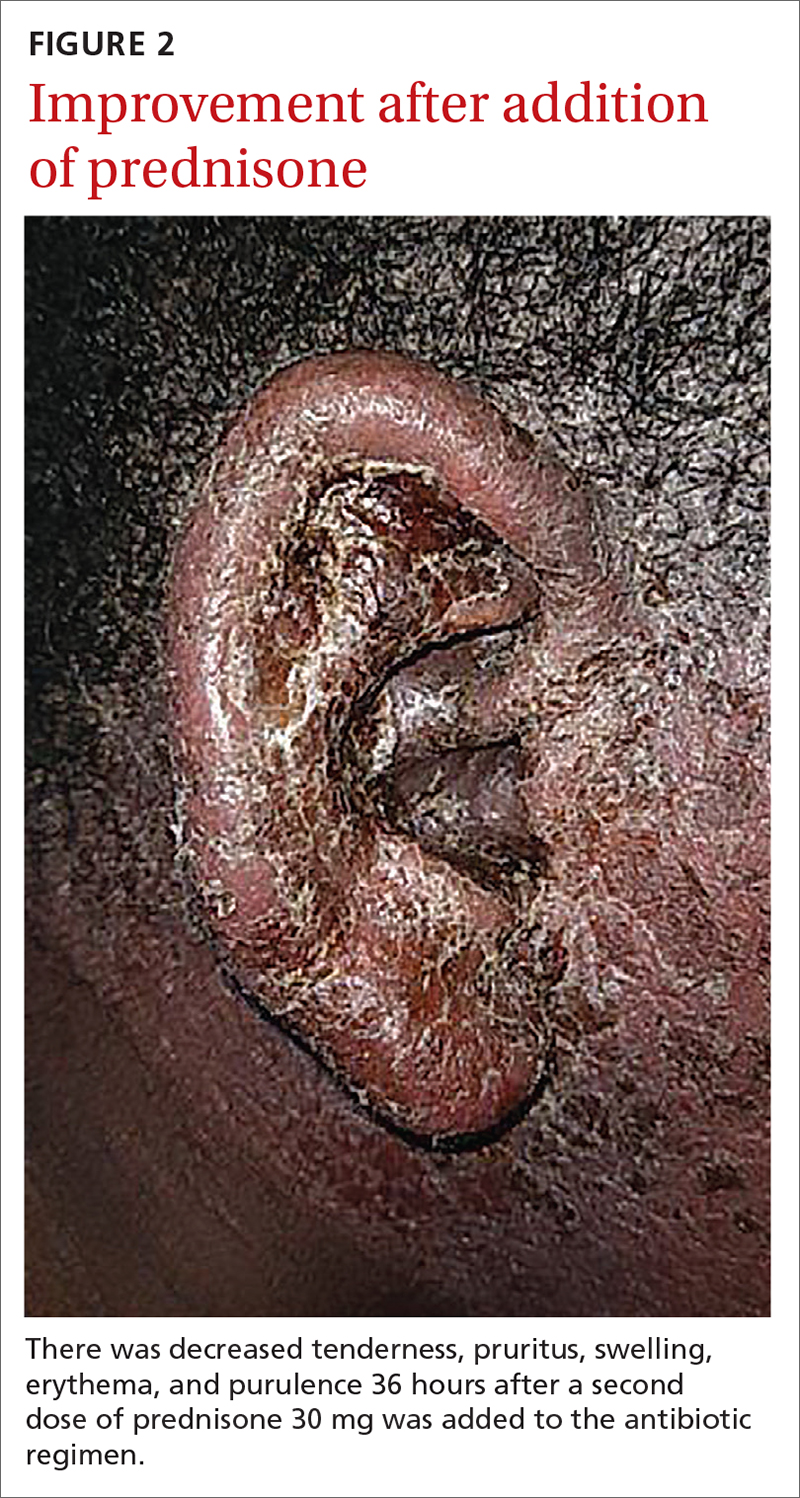
For our patient, inpatient wound cultures demonstrated methicillin-resistant S aureus, and empiric treatment with IV cefepime and vancomycin was transitioned to IV clindamycin based on sensitivities and then transitioned to a 12-day course of oral clindamycin 150 mg bid. In addition, the patient received ciprofloxacin/dexamethasone otic drops 3 times/d to treat his otitis externa. After initiating prednisone 30 mg (1 mg/kg/d) for 10 days to cover the allergic component, the patient showed prompt clinical improvement. Gentle cleansing of the right ear with hypoallergenic soap and water followed by application of petrolatum ointment 4 times/d was used to promote healing and improve barrier function (FIGURE 2). The patient’s mother indicated during a follow-up call that the affected area had dramatically improved.
1. Storan ER, McEvoy MT, Wetter DA, et al. Experience with the dermatology inpatient hospital service for adults: Mayo Clinic, 2000–2010. J Eur Acad Dermatol Venereol. 2013;27:1360-1365. doi: 10.1111/jdv.12010
2. Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208. doi: 10.1111/jdv.12715
. An infectious form of an eczematoid dermatitis. St. Louis Courier of Med. 1902;27:401414.
4. Infectious eczematoid dermatitis. J Am Med Assoc. 1920;75:976-979.
, , . Autosensitization dermatitis: report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77. doi: 10.1001/archderm.1949.01520260072010
, . Autosensitization in infectious eczematoid dermatitis. AMA Arch Derm Syphilol. 1950;62:703-704. doi: 10.1001/archderm.1950.01530180092021
A 6-year-old boy was seen in the hospital in consultation for a 3-week history of suspected cellulitis of the right ear. Drainage from the right ear was refractory to treatment with a 7-day course of cephalexin 15 mL po bid of 250 mg/5 mL solution and clindamycin 24.4 mL po tid of 75 mg/5 mL solution. Treatment was followed by admission to the hospital for treatment with intravenous (IV) cefazolin 1000 mg q6h and IV vancomycin 825 mg q6h for 1 week.
The patient had a significant past medical history for asthma, allergic rhinitis, and severe atopic dermatitis that had been treated with methotrexate 10 mg per week for 6 months beginning when the child was 5 years of age. When the methotrexate proved to be ineffective, the patient was started on Aquaphor and mometasone 0.1% ointment. A 6-month trial of these agents failed as well.
Physical examination revealed that the right ear and skin around it were edematous, erythematous, pruritic, and tender. There was also purulent drainage coming from the ear (FIGURE 1).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infectious eczematoid dermatitis
The patient was referred to a dermatologist after seeing an ear, nose, and throat (ENT) specialist who made the diagnosis of otitis externa when the rash failed to respond to topical and systemic antibiotics. The patient’s tender, pruritic, oozing, edematous eruption was recognized as an infectious eczematoid dermatitis (IED).
Although it is not an uncommon condition, IED may be underrecognized. It accounted for 2.9% of admissions to a dermatology-run inpatient service between 2000 and 2010.1 IED results from cutaneous sensitization to purulent drainage secondary to acute otitis externa or another primary infection.2 In fact, cultures from the purulent drainage in this patient grew methicillin-resistant Staphylococcus aureus. The patient’s right otitis externa drainage may have been associated with the previous history of atopic dermatitis. Atopic dermatitis is associated with an increased risk of skin infections due to decreased inflammatory mediators (defensins).
Cellulitis and herpes zoster oticus are part of the differential
The differential diagnosis in this case includes bacterial cellulitis, acute otitis media, and herpes zoster oticus.
Bacterial cellulitis manifests with erythema, edema, and tenderness with blistering when associated with bullous impetigo rather than pruritus. The clinical appearance of the patient’s diffuse, weeping, edematous external ear, the lack of response to guided antibiotic therapy, and the pruritus experienced by the patient argue against the diagnosis of bacterial cellulitis.
Acute otitis media, like otitis externa, produces ear discharge usually associated with significant pain. Thus, it is important when working through the differential to define the source of the ear discharge. In this case, a consultation with an ENT specialist confirmed that there was an intact tympanic membrane with no middle ear involvement, ruling out the diagnosis of acute otitis media.
Continue to: Herpes zoster oticus
Herpes zoster oticus. The absence of grouped vesicles at any point during the eruption, itching rather than pain, and negative viral culture and polymerase chain reaction studies for herpes simplex and varicella zoster virus excluded the diagnosis of herpes zoster oticus.
Diagnostic criteria were met
This case was compatible with the characterizations of IED as initially described by Engman3 in 1902 and further detailed by Sutton,4 who provided the following criteria for diagnosis:
- an initial eczematous or pustular lesion
- extension peripherally by autoinoculation
- an absence of central clearing
- Staphylococcus on culture of the initial lesion
- a history of infection.
Case reports have added to our understanding of the mechanism of autosensitization of surrounding skin.5 Yamany and Schwartz have proposed the diagnostic criteria summarized in the TABLE.2
Age factors into location. The ears, nose, and face are predominantly involved in cases of IED in the pediatric population, while the lower extremities are predominantly involved in adults.6 Laboratory tests and imaging may aid in excluding other potential diagnoses or complications, but the diagnosis remains clinical and requires the clinician to avoid jumping to the conclusion that every moist, erythematous crusting eruption is purely infectious in nature.
Tx and prevention hinge on a combination of antibiotics, steroids
The management of IED should be aimed at fighting the infection, eliminating the allergic contact dermatitis associated with infectious products, and improving barrier protection. Topical and/or systemic antibiotics guided by culture focus on killing bacteria. The allergic immune response is dampened by systemic steroids. Topical steroids, however, are difficult to utilize on moist, draining skin. In the case of otitis externa, a combination topical antibiotic and steroid otic drop can be utilized. As healing begins, emollients are applied to aid in skin repair.2 Topical antibiotics containing neomycin or polymyxin should be avoided to eliminate the possibility of developing contact sensitivity to these agents.
For our patient, inpatient wound cultures demonstrated methicillin-resistant S aureus, and empiric treatment with IV cefepime and vancomycin was transitioned to IV clindamycin based on sensitivities and then transitioned to a 12-day course of oral clindamycin 150 mg bid. In addition, the patient received ciprofloxacin/dexamethasone otic drops 3 times/d to treat his otitis externa. After initiating prednisone 30 mg (1 mg/kg/d) for 10 days to cover the allergic component, the patient showed prompt clinical improvement. Gentle cleansing of the right ear with hypoallergenic soap and water followed by application of petrolatum ointment 4 times/d was used to promote healing and improve barrier function (FIGURE 2). The patient’s mother indicated during a follow-up call that the affected area had dramatically improved.
A 6-year-old boy was seen in the hospital in consultation for a 3-week history of suspected cellulitis of the right ear. Drainage from the right ear was refractory to treatment with a 7-day course of cephalexin 15 mL po bid of 250 mg/5 mL solution and clindamycin 24.4 mL po tid of 75 mg/5 mL solution. Treatment was followed by admission to the hospital for treatment with intravenous (IV) cefazolin 1000 mg q6h and IV vancomycin 825 mg q6h for 1 week.
The patient had a significant past medical history for asthma, allergic rhinitis, and severe atopic dermatitis that had been treated with methotrexate 10 mg per week for 6 months beginning when the child was 5 years of age. When the methotrexate proved to be ineffective, the patient was started on Aquaphor and mometasone 0.1% ointment. A 6-month trial of these agents failed as well.
Physical examination revealed that the right ear and skin around it were edematous, erythematous, pruritic, and tender. There was also purulent drainage coming from the ear (FIGURE 1).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Infectious eczematoid dermatitis
The patient was referred to a dermatologist after seeing an ear, nose, and throat (ENT) specialist who made the diagnosis of otitis externa when the rash failed to respond to topical and systemic antibiotics. The patient’s tender, pruritic, oozing, edematous eruption was recognized as an infectious eczematoid dermatitis (IED).
Although it is not an uncommon condition, IED may be underrecognized. It accounted for 2.9% of admissions to a dermatology-run inpatient service between 2000 and 2010.1 IED results from cutaneous sensitization to purulent drainage secondary to acute otitis externa or another primary infection.2 In fact, cultures from the purulent drainage in this patient grew methicillin-resistant Staphylococcus aureus. The patient’s right otitis externa drainage may have been associated with the previous history of atopic dermatitis. Atopic dermatitis is associated with an increased risk of skin infections due to decreased inflammatory mediators (defensins).
Cellulitis and herpes zoster oticus are part of the differential
The differential diagnosis in this case includes bacterial cellulitis, acute otitis media, and herpes zoster oticus.
Bacterial cellulitis manifests with erythema, edema, and tenderness with blistering when associated with bullous impetigo rather than pruritus. The clinical appearance of the patient’s diffuse, weeping, edematous external ear, the lack of response to guided antibiotic therapy, and the pruritus experienced by the patient argue against the diagnosis of bacterial cellulitis.
Acute otitis media, like otitis externa, produces ear discharge usually associated with significant pain. Thus, it is important when working through the differential to define the source of the ear discharge. In this case, a consultation with an ENT specialist confirmed that there was an intact tympanic membrane with no middle ear involvement, ruling out the diagnosis of acute otitis media.
Continue to: Herpes zoster oticus
Herpes zoster oticus. The absence of grouped vesicles at any point during the eruption, itching rather than pain, and negative viral culture and polymerase chain reaction studies for herpes simplex and varicella zoster virus excluded the diagnosis of herpes zoster oticus.
Diagnostic criteria were met
This case was compatible with the characterizations of IED as initially described by Engman3 in 1902 and further detailed by Sutton,4 who provided the following criteria for diagnosis:
- an initial eczematous or pustular lesion
- extension peripherally by autoinoculation
- an absence of central clearing
- Staphylococcus on culture of the initial lesion
- a history of infection.
Case reports have added to our understanding of the mechanism of autosensitization of surrounding skin.5 Yamany and Schwartz have proposed the diagnostic criteria summarized in the TABLE.2
Age factors into location. The ears, nose, and face are predominantly involved in cases of IED in the pediatric population, while the lower extremities are predominantly involved in adults.6 Laboratory tests and imaging may aid in excluding other potential diagnoses or complications, but the diagnosis remains clinical and requires the clinician to avoid jumping to the conclusion that every moist, erythematous crusting eruption is purely infectious in nature.
Tx and prevention hinge on a combination of antibiotics, steroids
The management of IED should be aimed at fighting the infection, eliminating the allergic contact dermatitis associated with infectious products, and improving barrier protection. Topical and/or systemic antibiotics guided by culture focus on killing bacteria. The allergic immune response is dampened by systemic steroids. Topical steroids, however, are difficult to utilize on moist, draining skin. In the case of otitis externa, a combination topical antibiotic and steroid otic drop can be utilized. As healing begins, emollients are applied to aid in skin repair.2 Topical antibiotics containing neomycin or polymyxin should be avoided to eliminate the possibility of developing contact sensitivity to these agents.
For our patient, inpatient wound cultures demonstrated methicillin-resistant S aureus, and empiric treatment with IV cefepime and vancomycin was transitioned to IV clindamycin based on sensitivities and then transitioned to a 12-day course of oral clindamycin 150 mg bid. In addition, the patient received ciprofloxacin/dexamethasone otic drops 3 times/d to treat his otitis externa. After initiating prednisone 30 mg (1 mg/kg/d) for 10 days to cover the allergic component, the patient showed prompt clinical improvement. Gentle cleansing of the right ear with hypoallergenic soap and water followed by application of petrolatum ointment 4 times/d was used to promote healing and improve barrier function (FIGURE 2). The patient’s mother indicated during a follow-up call that the affected area had dramatically improved.
1. Storan ER, McEvoy MT, Wetter DA, et al. Experience with the dermatology inpatient hospital service for adults: Mayo Clinic, 2000–2010. J Eur Acad Dermatol Venereol. 2013;27:1360-1365. doi: 10.1111/jdv.12010
2. Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208. doi: 10.1111/jdv.12715
. An infectious form of an eczematoid dermatitis. St. Louis Courier of Med. 1902;27:401414.
4. Infectious eczematoid dermatitis. J Am Med Assoc. 1920;75:976-979.
, , . Autosensitization dermatitis: report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77. doi: 10.1001/archderm.1949.01520260072010
, . Autosensitization in infectious eczematoid dermatitis. AMA Arch Derm Syphilol. 1950;62:703-704. doi: 10.1001/archderm.1950.01530180092021
1. Storan ER, McEvoy MT, Wetter DA, et al. Experience with the dermatology inpatient hospital service for adults: Mayo Clinic, 2000–2010. J Eur Acad Dermatol Venereol. 2013;27:1360-1365. doi: 10.1111/jdv.12010
2. Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208. doi: 10.1111/jdv.12715
. An infectious form of an eczematoid dermatitis. St. Louis Courier of Med. 1902;27:401414.
4. Infectious eczematoid dermatitis. J Am Med Assoc. 1920;75:976-979.
, , . Autosensitization dermatitis: report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77. doi: 10.1001/archderm.1949.01520260072010
, . Autosensitization in infectious eczematoid dermatitis. AMA Arch Derm Syphilol. 1950;62:703-704. doi: 10.1001/archderm.1950.01530180092021
Diffuse skin rash, altered mental status
A 74-year-old Caucasian man presented to the hospital with intractable back and chest pain, a diffuse skin rash, and altered mental status. He said that 2 days ago, he’d gone to a different local hospital for treatment of back pain and a headache that had begun 3 days earlier. He was treated with intravenous hydromorphone and sent home with a prescription for meperidine. He said that several hours after being treated with the hydromorphone, the rash developed on his head and then spread to his trunk and upper extremities.
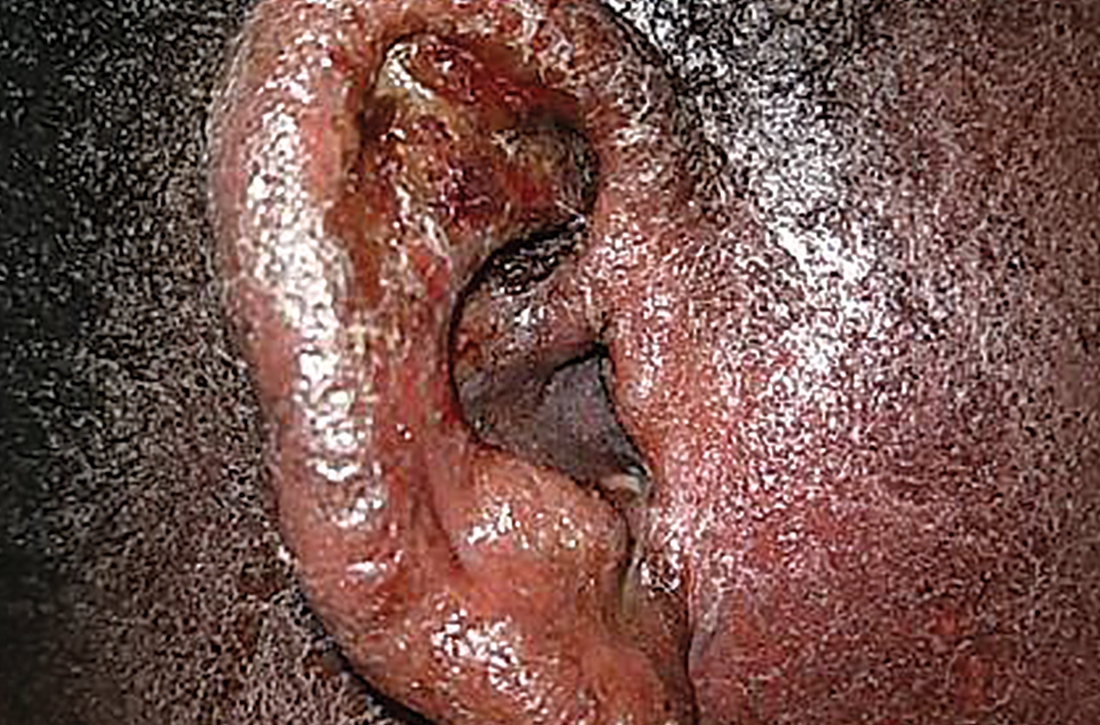
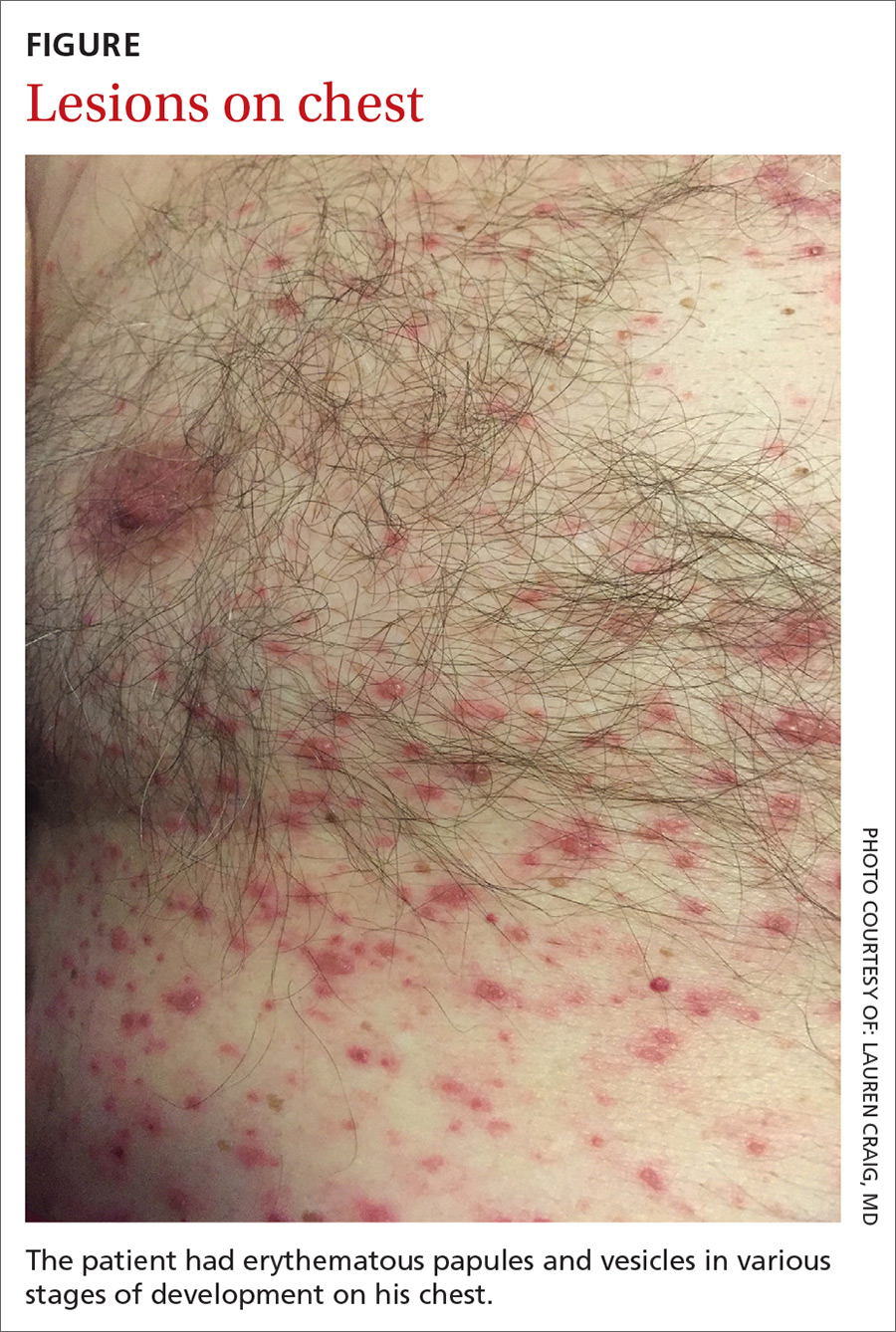
On physical examination, the patient was afebrile. He had numerous erythematous papules and vesicles in various stages of development on his scalp, face, neck, chest (FIGURE), abdomen, back, upper extremities, and groin. The lesions continued to spread and eventually involved his posterior oropharynx. The patient also developed conjunctivitis.
Laboratory findings included a white blood cell count of 4000/mcL (normal: 4500-11,000/mcL) with 65.9% segmented neutrophils (normal: 40%-60%), and 16.7% lymphocytes (normal: 20%-40%). Lab tests also revealed an aspartate aminotransferase level of 263 U/L (normal: 10-40 U/L), alanine aminotransferase of 236 U/L (normal: 7-56 U/L), and lactate dehydrogenase of 628 U/L (normal: 140-280 U/L).
The patient’s medical history was significant for hypertension, osteoarthritis, and IgG-kappa multiple myeloma, which had been treated with multiple chemotherapy regimens that included lenalidomide. Five years earlier, he’d undergone an autologous bone marrow transplant (BMT). At the time of presentation, the patient was being treated with daratumumab; he received his most recent treatment approximately one month earlier. Other medications included amlodipine, esomeprazole, and escitalopram.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated varicella-zoster virus infection
Because of the patient’s immunocompromised state, his presentation with altered mental status and diffuse rash was concerning. On hospital Day 2, a sample was taken from one of his skin lesions. Polymerase chain reaction (PCR) detected varicella-zoster virus (VZV), and we diagnosed disseminated VZV infection. On hospital Day 3, we performed a lumbar puncture because of worsening confusion and discovered that the cerebrospinal fluid was also positive for VZV.
Disseminated VZV is the most common cause of late infection in patients who have received an allogenic BMT; it is usually due to reactivation of the virus.1 In one study of 1186 patients who underwent BMT, 52% developed VZV infection within 5 years.2 Disseminated VZV may also involve visceral organs, causing pneumonitis, pancreatitis, hepatitis, or encephalitis. Mortality rates for disseminated VZV are as high as 50%.3 Because of this, physicians should be vigilant when patients who have received a BMT present with a rash and signs of systemic involvement.
Two reliable tests. Even when lesions are classic for VZV, the diagnosis must be confirmed by laboratory testing. Real-time PCR assay is a rapid and highly sensitive test for diagnosing VZV.4 Another rapid test that can be used to confirm the clinical diagnosis of VZV is a direct fluorescent antibody assay, which is becoming more widely available.
In contrast, the sensitivity of viral culture for VZV has been reported to be as low as 20%.5 Viral culture also takes much longer and has a significantly lower yield compared with newer methods.6 A biopsy of skin lesions will reveal multinucleated giant cells, but cannot differentiate between herpes simplex virus (HSV) and VZV.7
These lesions can be mimicked
When a rash develops following the use of intravenous hydromorphone, as occurred with our patient, a drug reaction must be ruled out. A drug reaction can cause almost any skin manifestation and may present as vesicles, a macular rash, a papular rash, or diffuse erythema. In this case, drug rash was ruled out by the positive VZV PCR.
Viral exanthems can also present in a variety of ways. They may cause a macular, papular, or vesicular rash.
Prompt management is crucial
Prompt treatment of VZV with acyclovir improves outcomes, but death may still occur, even with early diagnosis.3 Immunocompromised patients with VZV should be closely monitored for secondary infections, which may rapidly progress and become fatal.8 The Centers for Disease Control and Prevention recommends both airborne and contact precautions for patients with disseminated VZV until all lesions are dry and crusted.9
While the live zoster vaccine is approved for prevention of shingles in patients <60 years of age, it is contraindicated in patients with a history of primary or acquired immunodeficiency states including leukemia, lymphoma, or other malignant neoplasms affecting bone marrow.
Our patient. On admission, he was treated with intravenous (IV) acyclovir 10 mg/kg TID; IV vancomycin 15 mg/kg every 12 hours; and IV ceftriaxone 2 g/d. Slowly, his mental status returned to baseline, and his rash and conjunctivitis resolved. We discharged him on hospital Day 12. He was transitioned to oral valacyclovir 1000 mg TID. Including both inpatient and outpatient treatment, the patient received 3 weeks (total) of acyclovir/valacyclovir therapy.
CORRESPONDENCE
Caitlyn T. Reed, MD, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; caitreed11@gmail.com.
1. Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172-1181.
2. Han CS, Miller W, Haake R, et al. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277-283.
3. David DS, Tegtmeier BR, O’Donnell MR, at el. Visceral varicella-zoster after bone marrow transplantation: report of a case series and review of the literature. Am J Gastroenterol. 1998;93:810-813.
4. Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
5. Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347;340-346.
7. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd edition. China: Elsevier Limited; 2012:1321-1343.
8. Woznowski M, Quack I, Bölke E, et al. Fulminant staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15:410-414.
9. Centers for Disease Control and Prevention. Preventing varicella in healthcare settings. Available at: http://www.cdc.gov/chickenpox/hcp/healthcare-setting.html. Accessed October 6,2017.
A 74-year-old Caucasian man presented to the hospital with intractable back and chest pain, a diffuse skin rash, and altered mental status. He said that 2 days ago, he’d gone to a different local hospital for treatment of back pain and a headache that had begun 3 days earlier. He was treated with intravenous hydromorphone and sent home with a prescription for meperidine. He said that several hours after being treated with the hydromorphone, the rash developed on his head and then spread to his trunk and upper extremities.
On physical examination, the patient was afebrile. He had numerous erythematous papules and vesicles in various stages of development on his scalp, face, neck, chest (FIGURE), abdomen, back, upper extremities, and groin. The lesions continued to spread and eventually involved his posterior oropharynx. The patient also developed conjunctivitis.
Laboratory findings included a white blood cell count of 4000/mcL (normal: 4500-11,000/mcL) with 65.9% segmented neutrophils (normal: 40%-60%), and 16.7% lymphocytes (normal: 20%-40%). Lab tests also revealed an aspartate aminotransferase level of 263 U/L (normal: 10-40 U/L), alanine aminotransferase of 236 U/L (normal: 7-56 U/L), and lactate dehydrogenase of 628 U/L (normal: 140-280 U/L).
The patient’s medical history was significant for hypertension, osteoarthritis, and IgG-kappa multiple myeloma, which had been treated with multiple chemotherapy regimens that included lenalidomide. Five years earlier, he’d undergone an autologous bone marrow transplant (BMT). At the time of presentation, the patient was being treated with daratumumab; he received his most recent treatment approximately one month earlier. Other medications included amlodipine, esomeprazole, and escitalopram.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated varicella-zoster virus infection
Because of the patient’s immunocompromised state, his presentation with altered mental status and diffuse rash was concerning. On hospital Day 2, a sample was taken from one of his skin lesions. Polymerase chain reaction (PCR) detected varicella-zoster virus (VZV), and we diagnosed disseminated VZV infection. On hospital Day 3, we performed a lumbar puncture because of worsening confusion and discovered that the cerebrospinal fluid was also positive for VZV.
Disseminated VZV is the most common cause of late infection in patients who have received an allogenic BMT; it is usually due to reactivation of the virus.1 In one study of 1186 patients who underwent BMT, 52% developed VZV infection within 5 years.2 Disseminated VZV may also involve visceral organs, causing pneumonitis, pancreatitis, hepatitis, or encephalitis. Mortality rates for disseminated VZV are as high as 50%.3 Because of this, physicians should be vigilant when patients who have received a BMT present with a rash and signs of systemic involvement.
Two reliable tests. Even when lesions are classic for VZV, the diagnosis must be confirmed by laboratory testing. Real-time PCR assay is a rapid and highly sensitive test for diagnosing VZV.4 Another rapid test that can be used to confirm the clinical diagnosis of VZV is a direct fluorescent antibody assay, which is becoming more widely available.
In contrast, the sensitivity of viral culture for VZV has been reported to be as low as 20%.5 Viral culture also takes much longer and has a significantly lower yield compared with newer methods.6 A biopsy of skin lesions will reveal multinucleated giant cells, but cannot differentiate between herpes simplex virus (HSV) and VZV.7
These lesions can be mimicked
When a rash develops following the use of intravenous hydromorphone, as occurred with our patient, a drug reaction must be ruled out. A drug reaction can cause almost any skin manifestation and may present as vesicles, a macular rash, a papular rash, or diffuse erythema. In this case, drug rash was ruled out by the positive VZV PCR.
Viral exanthems can also present in a variety of ways. They may cause a macular, papular, or vesicular rash.
Prompt management is crucial
Prompt treatment of VZV with acyclovir improves outcomes, but death may still occur, even with early diagnosis.3 Immunocompromised patients with VZV should be closely monitored for secondary infections, which may rapidly progress and become fatal.8 The Centers for Disease Control and Prevention recommends both airborne and contact precautions for patients with disseminated VZV until all lesions are dry and crusted.9
While the live zoster vaccine is approved for prevention of shingles in patients <60 years of age, it is contraindicated in patients with a history of primary or acquired immunodeficiency states including leukemia, lymphoma, or other malignant neoplasms affecting bone marrow.
Our patient. On admission, he was treated with intravenous (IV) acyclovir 10 mg/kg TID; IV vancomycin 15 mg/kg every 12 hours; and IV ceftriaxone 2 g/d. Slowly, his mental status returned to baseline, and his rash and conjunctivitis resolved. We discharged him on hospital Day 12. He was transitioned to oral valacyclovir 1000 mg TID. Including both inpatient and outpatient treatment, the patient received 3 weeks (total) of acyclovir/valacyclovir therapy.
CORRESPONDENCE
Caitlyn T. Reed, MD, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; caitreed11@gmail.com.
A 74-year-old Caucasian man presented to the hospital with intractable back and chest pain, a diffuse skin rash, and altered mental status. He said that 2 days ago, he’d gone to a different local hospital for treatment of back pain and a headache that had begun 3 days earlier. He was treated with intravenous hydromorphone and sent home with a prescription for meperidine. He said that several hours after being treated with the hydromorphone, the rash developed on his head and then spread to his trunk and upper extremities.
On physical examination, the patient was afebrile. He had numerous erythematous papules and vesicles in various stages of development on his scalp, face, neck, chest (FIGURE), abdomen, back, upper extremities, and groin. The lesions continued to spread and eventually involved his posterior oropharynx. The patient also developed conjunctivitis.
Laboratory findings included a white blood cell count of 4000/mcL (normal: 4500-11,000/mcL) with 65.9% segmented neutrophils (normal: 40%-60%), and 16.7% lymphocytes (normal: 20%-40%). Lab tests also revealed an aspartate aminotransferase level of 263 U/L (normal: 10-40 U/L), alanine aminotransferase of 236 U/L (normal: 7-56 U/L), and lactate dehydrogenase of 628 U/L (normal: 140-280 U/L).
The patient’s medical history was significant for hypertension, osteoarthritis, and IgG-kappa multiple myeloma, which had been treated with multiple chemotherapy regimens that included lenalidomide. Five years earlier, he’d undergone an autologous bone marrow transplant (BMT). At the time of presentation, the patient was being treated with daratumumab; he received his most recent treatment approximately one month earlier. Other medications included amlodipine, esomeprazole, and escitalopram.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Disseminated varicella-zoster virus infection
Because of the patient’s immunocompromised state, his presentation with altered mental status and diffuse rash was concerning. On hospital Day 2, a sample was taken from one of his skin lesions. Polymerase chain reaction (PCR) detected varicella-zoster virus (VZV), and we diagnosed disseminated VZV infection. On hospital Day 3, we performed a lumbar puncture because of worsening confusion and discovered that the cerebrospinal fluid was also positive for VZV.
Disseminated VZV is the most common cause of late infection in patients who have received an allogenic BMT; it is usually due to reactivation of the virus.1 In one study of 1186 patients who underwent BMT, 52% developed VZV infection within 5 years.2 Disseminated VZV may also involve visceral organs, causing pneumonitis, pancreatitis, hepatitis, or encephalitis. Mortality rates for disseminated VZV are as high as 50%.3 Because of this, physicians should be vigilant when patients who have received a BMT present with a rash and signs of systemic involvement.
Two reliable tests. Even when lesions are classic for VZV, the diagnosis must be confirmed by laboratory testing. Real-time PCR assay is a rapid and highly sensitive test for diagnosing VZV.4 Another rapid test that can be used to confirm the clinical diagnosis of VZV is a direct fluorescent antibody assay, which is becoming more widely available.
In contrast, the sensitivity of viral culture for VZV has been reported to be as low as 20%.5 Viral culture also takes much longer and has a significantly lower yield compared with newer methods.6 A biopsy of skin lesions will reveal multinucleated giant cells, but cannot differentiate between herpes simplex virus (HSV) and VZV.7
These lesions can be mimicked
When a rash develops following the use of intravenous hydromorphone, as occurred with our patient, a drug reaction must be ruled out. A drug reaction can cause almost any skin manifestation and may present as vesicles, a macular rash, a papular rash, or diffuse erythema. In this case, drug rash was ruled out by the positive VZV PCR.
Viral exanthems can also present in a variety of ways. They may cause a macular, papular, or vesicular rash.
Prompt management is crucial
Prompt treatment of VZV with acyclovir improves outcomes, but death may still occur, even with early diagnosis.3 Immunocompromised patients with VZV should be closely monitored for secondary infections, which may rapidly progress and become fatal.8 The Centers for Disease Control and Prevention recommends both airborne and contact precautions for patients with disseminated VZV until all lesions are dry and crusted.9
While the live zoster vaccine is approved for prevention of shingles in patients <60 years of age, it is contraindicated in patients with a history of primary or acquired immunodeficiency states including leukemia, lymphoma, or other malignant neoplasms affecting bone marrow.
Our patient. On admission, he was treated with intravenous (IV) acyclovir 10 mg/kg TID; IV vancomycin 15 mg/kg every 12 hours; and IV ceftriaxone 2 g/d. Slowly, his mental status returned to baseline, and his rash and conjunctivitis resolved. We discharged him on hospital Day 12. He was transitioned to oral valacyclovir 1000 mg TID. Including both inpatient and outpatient treatment, the patient received 3 weeks (total) of acyclovir/valacyclovir therapy.
CORRESPONDENCE
Caitlyn T. Reed, MD, School of Medicine, University of Mississippi Medical Center, 2500 North State Street, Jackson, MS 39216; caitreed11@gmail.com.
1. Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172-1181.
2. Han CS, Miller W, Haake R, et al. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277-283.
3. David DS, Tegtmeier BR, O’Donnell MR, at el. Visceral varicella-zoster after bone marrow transplantation: report of a case series and review of the literature. Am J Gastroenterol. 1998;93:810-813.
4. Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
5. Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347;340-346.
7. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd edition. China: Elsevier Limited; 2012:1321-1343.
8. Woznowski M, Quack I, Bölke E, et al. Fulminant staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15:410-414.
9. Centers for Disease Control and Prevention. Preventing varicella in healthcare settings. Available at: http://www.cdc.gov/chickenpox/hcp/healthcare-setting.html. Accessed October 6,2017.
1. Locksley RM, Flournoy N, Sullivan KM, et al. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985;152:1172-1181.
2. Han CS, Miller W, Haake R, et al. Varicella zoster infection after bone marrow transplantation: incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277-283.
3. David DS, Tegtmeier BR, O’Donnell MR, at el. Visceral varicella-zoster after bone marrow transplantation: report of a case series and review of the literature. Am J Gastroenterol. 1998;93:810-813.
4. Harbecke R, Oxman MN, Arnold BA, et al. A real-time PCR assay to identify and discriminate among wild-type and vaccine strains of varicella-zoster virus and herpes simplex virus in clinical specimens, and comparison with the clinical diagnoses. J Med Virol. 2009;81:1310-1322.
5. Sauerbrei A, Eichhorn U, Schacke M, et al. Laboratory diagnosis of herpes zoster. J Clin Virol. 1999;14:31-36.
6. Gnann JW Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347;340-346.
7. Mendoza N, Madkan V, Sra K, et al. Human herpesviruses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd edition. China: Elsevier Limited; 2012:1321-1343.
8. Woznowski M, Quack I, Bölke E, et al. Fulminant staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 2010;15:410-414.
9. Centers for Disease Control and Prevention. Preventing varicella in healthcare settings. Available at: http://www.cdc.gov/chickenpox/hcp/healthcare-setting.html. Accessed October 6,2017.
The Clinical Learning Environment Review as a Model for Impactful Self-directed Quality Control Initiatives in Clinical Practice
As part of its Next Accreditation System, the Accreditation Council for Graduate Medical Education (ACGME) has introduced the Clinical Learning Environment Review (CLER) program, designed to assess the learning environment of institutions that have ACGME residency and fellowship programs.1 The CLER program emphasizes the responsibility of these hospitals, multispecialty groups, and other organizations to focus on quality and safety in the health care environment of resident learning and patient care. The expectation is that emphasis on quality of care in a residency training program will influence these physicians’ approach to quality of care after graduation.2,3 The Department of Dermatology at the University of Mississippi Medical Center (UMMC)(Jackson, Mississippi) saw CLER as an opportunity to demonstrate leadership in the patient safety movement.
CLER Program at UMMC
As a model CLER program at our institution, our project at the outset concentrated resident efforts on the focus areas specified by the ACGME (Table 1). We also were aware that our ACGME committee would need to answer questions during CLER site visits (Table 2). Because the data generated would not be used for accreditation decisions, there was no concern that exposing errors would jeopardize our postgraduate training certification.
The first 15 minutes of monthly faculty meetings were devoted to the presentation of a resident project, called a QA/QI (quality assurance/quality improvement) moment, that addressed ACGME focus areas 1, 2, 3, or 6 (Table 1). (Transitions in care [focus area 4] and work hours and fatigue [focus area 5] generally are less important issues in a predominantly outpatient specialty such as dermatology.) The residents were encouraged to identify areas where patient harm could occur due to poorly designed systems and to report situations in which patients actually were harmed.
Each project had to be approved by the department chairperson based on the following 4 requirements: First, the initiative must have the potential to notably impact patient safety and reduce harm. Second, residents with faculty support had to design methods to assess the identified problem. Third, participants had to design (to the best of their abilities) cost-effective and achievable interventions in a manner that would not produce unintended consequences. Fourth, residents were asked to devise a system to close the loop, ensuring that the effort put into the process was not wasted.
Findings From the CLER Program
The CLER program generates data on program and institutional attributes that have a salutatory effect on quality and safety, specifically involving 6 focus areas highlighted in Table 1. Putting residents at the center of efforts to improve the quality of care in our department proved critical to improving patient safety.
Involving residents in a series of QA/QI initiatives was logical because they rotate with faculty members. They also are in a position to view inconsistencies and to work to establish consistent patterns of patient care. In addition, our busy faculty members are charged with a variety of other clinical, educational, and administrative duties complicated by requirements in the design of a new residency training program. Faculty and residents working together were able to find problem areas in our department and devise solutions to improve those problems.
The CLER program involved a series of steps. Residents were charged with identifying errors (QA) and then devising a system to prevent similar errors from being repeated (QI)(Table 3). Efforts focused on preventing needless harm in our department. Initiatives developed by residents, who are closest to patients, have advantages over safety programs developed by the hospital’s administration. Residents became passionate about error prevention when they determined that their efforts could make a difference to patients.
Forward Thinking for Dermatology Practices
Perhaps there are lessons here that could apply to safety promotion in the practicing dermatologist’s office. The American Board of Dermatology, within the framework established by the American Board of Medical Specialties, requires physicians seeking recertification to participate in preapproved practice assessment QI exercises twice every 10 years.17 Six programs sponsored by the American Academy of Dermatology have now been approved in the areas of melanoma, biopsy follow-up measure, psoriasis, chronic urticaria, venous insufficiency, and laser- and light-based therapy for rejuvenation.18 An additional program has been approved for dermatopathologists through the American Society of Dermatopathology.19 None of these programs match the topics chosen by our residents in consultation with faculty to meet safety gaps identified in clinics at UMMC. Perhaps the next generation of performance improvement continuing medical education programs could include a pilot program for part 4 of Maintenance of Certification credit that is nonpunitive, patient focused, and allows dermatologists to design specific error-prevention solutions tailored to their individual practice in the same way residency programs are taking up this task.
- Nasca TJ, Philibert I, Brigham T, et al. The Next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Philibert I, Gonzalez del Rey JA, Lannon C, et al. Quality improvement skills for pediatric residents: from lecture to implementation and sustainability. Acad Pediatr. 2014;14:40-46.
- Vidyarthi AR, Green AL, Rosenbluth G, et al. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89:460-468.
- Brodell RT, Elewski B. Antifungal drug interactions. avoidance requires more than memorization. Postgrad Med. 2000;107:41-43.
- Kerr IG, Jolivet J, Collin JM, et al. Test dose for predicting high-dose methotrexate infusions. Clin Pharmacol Ther. 1983;33:44-51.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Saporito FC, Menter MA. Methotrexate and psoriasis in the era of new biologic agents. J Am Acad Dermatol. 2004;50:301-309.
- Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138-147.
- Hunter KM. Implementation of an electronic medication administration record and bedside verification system. Online J Nurs Inform (OJNI). 2011;15:672.
- Nanji KC, Slight SP, Seger DL, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21:487-491.
- Schedlbauer A, Prasad V, Mulvaney C, et al. What evidence supports the use of computerized alerts and prompts to improve clinicians’ prescribing behavior? J Am Med Inform Assoc. 2009;16:531-538.
- Lee EK, Mejia AF, Senior T, et al. Improving patient safety through medical alert management: an automated decision tool to reduce alert fatigue. AMIA Annu Symp Proc. 2010;2010:417-421.
- Brenner AB. Physician and nurse relationships, a key to patient safety. J Ky Med Assoc. 2007;105:165-169.
- Rush JL, Flowers RH, Casamiquela KM, et al. Research letter: the knock: an adjunct to education opening the door to improved outpatient hand hygiene. J Am Acad Dermatol. In press.
- Lee SL. The extended surgical time-out: does it improve quality and prevent wrong-site surgery? Perm J. 2010;14:19-23.
- Altpeter T, Luckhardt K, Lewis JN, et al. Expanded surgical time out: a key to real-time data collection and quality improvement. J Am Coll Surg. 2007;204:527-532.
- MOC requirements. American Board of Dermatology Web site. https://www.abderm.org/diplomates/fulfilling-moc-requirements/moc-requirements.aspx#PI. Accessed January 18, 2016.
- How AAD develops measures. American Academy of Dermatology Web site. https://www.aad.org/practice-tools/quality-care/quality-measures. Accessed January 20, 2016.
- Quality assurance programs. The American Society of Dermatopathology Web site. http://www.asdp.org/education/quality-assurance-programs. Accessed January 20, 2016.
As part of its Next Accreditation System, the Accreditation Council for Graduate Medical Education (ACGME) has introduced the Clinical Learning Environment Review (CLER) program, designed to assess the learning environment of institutions that have ACGME residency and fellowship programs.1 The CLER program emphasizes the responsibility of these hospitals, multispecialty groups, and other organizations to focus on quality and safety in the health care environment of resident learning and patient care. The expectation is that emphasis on quality of care in a residency training program will influence these physicians’ approach to quality of care after graduation.2,3 The Department of Dermatology at the University of Mississippi Medical Center (UMMC)(Jackson, Mississippi) saw CLER as an opportunity to demonstrate leadership in the patient safety movement.
CLER Program at UMMC
As a model CLER program at our institution, our project at the outset concentrated resident efforts on the focus areas specified by the ACGME (Table 1). We also were aware that our ACGME committee would need to answer questions during CLER site visits (Table 2). Because the data generated would not be used for accreditation decisions, there was no concern that exposing errors would jeopardize our postgraduate training certification.
The first 15 minutes of monthly faculty meetings were devoted to the presentation of a resident project, called a QA/QI (quality assurance/quality improvement) moment, that addressed ACGME focus areas 1, 2, 3, or 6 (Table 1). (Transitions in care [focus area 4] and work hours and fatigue [focus area 5] generally are less important issues in a predominantly outpatient specialty such as dermatology.) The residents were encouraged to identify areas where patient harm could occur due to poorly designed systems and to report situations in which patients actually were harmed.
Each project had to be approved by the department chairperson based on the following 4 requirements: First, the initiative must have the potential to notably impact patient safety and reduce harm. Second, residents with faculty support had to design methods to assess the identified problem. Third, participants had to design (to the best of their abilities) cost-effective and achievable interventions in a manner that would not produce unintended consequences. Fourth, residents were asked to devise a system to close the loop, ensuring that the effort put into the process was not wasted.
Findings From the CLER Program
The CLER program generates data on program and institutional attributes that have a salutatory effect on quality and safety, specifically involving 6 focus areas highlighted in Table 1. Putting residents at the center of efforts to improve the quality of care in our department proved critical to improving patient safety.
Involving residents in a series of QA/QI initiatives was logical because they rotate with faculty members. They also are in a position to view inconsistencies and to work to establish consistent patterns of patient care. In addition, our busy faculty members are charged with a variety of other clinical, educational, and administrative duties complicated by requirements in the design of a new residency training program. Faculty and residents working together were able to find problem areas in our department and devise solutions to improve those problems.
The CLER program involved a series of steps. Residents were charged with identifying errors (QA) and then devising a system to prevent similar errors from being repeated (QI)(Table 3). Efforts focused on preventing needless harm in our department. Initiatives developed by residents, who are closest to patients, have advantages over safety programs developed by the hospital’s administration. Residents became passionate about error prevention when they determined that their efforts could make a difference to patients.
Forward Thinking for Dermatology Practices
Perhaps there are lessons here that could apply to safety promotion in the practicing dermatologist’s office. The American Board of Dermatology, within the framework established by the American Board of Medical Specialties, requires physicians seeking recertification to participate in preapproved practice assessment QI exercises twice every 10 years.17 Six programs sponsored by the American Academy of Dermatology have now been approved in the areas of melanoma, biopsy follow-up measure, psoriasis, chronic urticaria, venous insufficiency, and laser- and light-based therapy for rejuvenation.18 An additional program has been approved for dermatopathologists through the American Society of Dermatopathology.19 None of these programs match the topics chosen by our residents in consultation with faculty to meet safety gaps identified in clinics at UMMC. Perhaps the next generation of performance improvement continuing medical education programs could include a pilot program for part 4 of Maintenance of Certification credit that is nonpunitive, patient focused, and allows dermatologists to design specific error-prevention solutions tailored to their individual practice in the same way residency programs are taking up this task.
As part of its Next Accreditation System, the Accreditation Council for Graduate Medical Education (ACGME) has introduced the Clinical Learning Environment Review (CLER) program, designed to assess the learning environment of institutions that have ACGME residency and fellowship programs.1 The CLER program emphasizes the responsibility of these hospitals, multispecialty groups, and other organizations to focus on quality and safety in the health care environment of resident learning and patient care. The expectation is that emphasis on quality of care in a residency training program will influence these physicians’ approach to quality of care after graduation.2,3 The Department of Dermatology at the University of Mississippi Medical Center (UMMC)(Jackson, Mississippi) saw CLER as an opportunity to demonstrate leadership in the patient safety movement.
CLER Program at UMMC
As a model CLER program at our institution, our project at the outset concentrated resident efforts on the focus areas specified by the ACGME (Table 1). We also were aware that our ACGME committee would need to answer questions during CLER site visits (Table 2). Because the data generated would not be used for accreditation decisions, there was no concern that exposing errors would jeopardize our postgraduate training certification.
The first 15 minutes of monthly faculty meetings were devoted to the presentation of a resident project, called a QA/QI (quality assurance/quality improvement) moment, that addressed ACGME focus areas 1, 2, 3, or 6 (Table 1). (Transitions in care [focus area 4] and work hours and fatigue [focus area 5] generally are less important issues in a predominantly outpatient specialty such as dermatology.) The residents were encouraged to identify areas where patient harm could occur due to poorly designed systems and to report situations in which patients actually were harmed.
Each project had to be approved by the department chairperson based on the following 4 requirements: First, the initiative must have the potential to notably impact patient safety and reduce harm. Second, residents with faculty support had to design methods to assess the identified problem. Third, participants had to design (to the best of their abilities) cost-effective and achievable interventions in a manner that would not produce unintended consequences. Fourth, residents were asked to devise a system to close the loop, ensuring that the effort put into the process was not wasted.
Findings From the CLER Program
The CLER program generates data on program and institutional attributes that have a salutatory effect on quality and safety, specifically involving 6 focus areas highlighted in Table 1. Putting residents at the center of efforts to improve the quality of care in our department proved critical to improving patient safety.
Involving residents in a series of QA/QI initiatives was logical because they rotate with faculty members. They also are in a position to view inconsistencies and to work to establish consistent patterns of patient care. In addition, our busy faculty members are charged with a variety of other clinical, educational, and administrative duties complicated by requirements in the design of a new residency training program. Faculty and residents working together were able to find problem areas in our department and devise solutions to improve those problems.
The CLER program involved a series of steps. Residents were charged with identifying errors (QA) and then devising a system to prevent similar errors from being repeated (QI)(Table 3). Efforts focused on preventing needless harm in our department. Initiatives developed by residents, who are closest to patients, have advantages over safety programs developed by the hospital’s administration. Residents became passionate about error prevention when they determined that their efforts could make a difference to patients.
Forward Thinking for Dermatology Practices
Perhaps there are lessons here that could apply to safety promotion in the practicing dermatologist’s office. The American Board of Dermatology, within the framework established by the American Board of Medical Specialties, requires physicians seeking recertification to participate in preapproved practice assessment QI exercises twice every 10 years.17 Six programs sponsored by the American Academy of Dermatology have now been approved in the areas of melanoma, biopsy follow-up measure, psoriasis, chronic urticaria, venous insufficiency, and laser- and light-based therapy for rejuvenation.18 An additional program has been approved for dermatopathologists through the American Society of Dermatopathology.19 None of these programs match the topics chosen by our residents in consultation with faculty to meet safety gaps identified in clinics at UMMC. Perhaps the next generation of performance improvement continuing medical education programs could include a pilot program for part 4 of Maintenance of Certification credit that is nonpunitive, patient focused, and allows dermatologists to design specific error-prevention solutions tailored to their individual practice in the same way residency programs are taking up this task.
- Nasca TJ, Philibert I, Brigham T, et al. The Next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Philibert I, Gonzalez del Rey JA, Lannon C, et al. Quality improvement skills for pediatric residents: from lecture to implementation and sustainability. Acad Pediatr. 2014;14:40-46.
- Vidyarthi AR, Green AL, Rosenbluth G, et al. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89:460-468.
- Brodell RT, Elewski B. Antifungal drug interactions. avoidance requires more than memorization. Postgrad Med. 2000;107:41-43.
- Kerr IG, Jolivet J, Collin JM, et al. Test dose for predicting high-dose methotrexate infusions. Clin Pharmacol Ther. 1983;33:44-51.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Saporito FC, Menter MA. Methotrexate and psoriasis in the era of new biologic agents. J Am Acad Dermatol. 2004;50:301-309.
- Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138-147.
- Hunter KM. Implementation of an electronic medication administration record and bedside verification system. Online J Nurs Inform (OJNI). 2011;15:672.
- Nanji KC, Slight SP, Seger DL, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21:487-491.
- Schedlbauer A, Prasad V, Mulvaney C, et al. What evidence supports the use of computerized alerts and prompts to improve clinicians’ prescribing behavior? J Am Med Inform Assoc. 2009;16:531-538.
- Lee EK, Mejia AF, Senior T, et al. Improving patient safety through medical alert management: an automated decision tool to reduce alert fatigue. AMIA Annu Symp Proc. 2010;2010:417-421.
- Brenner AB. Physician and nurse relationships, a key to patient safety. J Ky Med Assoc. 2007;105:165-169.
- Rush JL, Flowers RH, Casamiquela KM, et al. Research letter: the knock: an adjunct to education opening the door to improved outpatient hand hygiene. J Am Acad Dermatol. In press.
- Lee SL. The extended surgical time-out: does it improve quality and prevent wrong-site surgery? Perm J. 2010;14:19-23.
- Altpeter T, Luckhardt K, Lewis JN, et al. Expanded surgical time out: a key to real-time data collection and quality improvement. J Am Coll Surg. 2007;204:527-532.
- MOC requirements. American Board of Dermatology Web site. https://www.abderm.org/diplomates/fulfilling-moc-requirements/moc-requirements.aspx#PI. Accessed January 18, 2016.
- How AAD develops measures. American Academy of Dermatology Web site. https://www.aad.org/practice-tools/quality-care/quality-measures. Accessed January 20, 2016.
- Quality assurance programs. The American Society of Dermatopathology Web site. http://www.asdp.org/education/quality-assurance-programs. Accessed January 20, 2016.
- Nasca TJ, Philibert I, Brigham T, et al. The Next GME accreditation system—rationale and benefits. N Engl J Med. 2012;366:1051-1056.
- Philibert I, Gonzalez del Rey JA, Lannon C, et al. Quality improvement skills for pediatric residents: from lecture to implementation and sustainability. Acad Pediatr. 2014;14:40-46.
- Vidyarthi AR, Green AL, Rosenbluth G, et al. Engaging residents and fellows to improve institution-wide quality: the first six years of a novel financial incentive program. Acad Med. 2014;89:460-468.
- Brodell RT, Elewski B. Antifungal drug interactions. avoidance requires more than memorization. Postgrad Med. 2000;107:41-43.
- Kerr IG, Jolivet J, Collin JM, et al. Test dose for predicting high-dose methotrexate infusions. Clin Pharmacol Ther. 1983;33:44-51.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Saporito FC, Menter MA. Methotrexate and psoriasis in the era of new biologic agents. J Am Acad Dermatol. 2004;50:301-309.
- Van Der Sijs H, Aarts J, Vulto A, et al. Overriding of drug safety alerts in computerized physician order entry. J Am Med Inform Assoc. 2006;13:138-147.
- Hunter KM. Implementation of an electronic medication administration record and bedside verification system. Online J Nurs Inform (OJNI). 2011;15:672.
- Nanji KC, Slight SP, Seger DL, et al. Overrides of medication-related clinical decision support alerts in outpatients. J Am Med Inform Assoc. 2014;21:487-491.
- Schedlbauer A, Prasad V, Mulvaney C, et al. What evidence supports the use of computerized alerts and prompts to improve clinicians’ prescribing behavior? J Am Med Inform Assoc. 2009;16:531-538.
- Lee EK, Mejia AF, Senior T, et al. Improving patient safety through medical alert management: an automated decision tool to reduce alert fatigue. AMIA Annu Symp Proc. 2010;2010:417-421.
- Brenner AB. Physician and nurse relationships, a key to patient safety. J Ky Med Assoc. 2007;105:165-169.
- Rush JL, Flowers RH, Casamiquela KM, et al. Research letter: the knock: an adjunct to education opening the door to improved outpatient hand hygiene. J Am Acad Dermatol. In press.
- Lee SL. The extended surgical time-out: does it improve quality and prevent wrong-site surgery? Perm J. 2010;14:19-23.
- Altpeter T, Luckhardt K, Lewis JN, et al. Expanded surgical time out: a key to real-time data collection and quality improvement. J Am Coll Surg. 2007;204:527-532.
- MOC requirements. American Board of Dermatology Web site. https://www.abderm.org/diplomates/fulfilling-moc-requirements/moc-requirements.aspx#PI. Accessed January 18, 2016.
- How AAD develops measures. American Academy of Dermatology Web site. https://www.aad.org/practice-tools/quality-care/quality-measures. Accessed January 20, 2016.
- Quality assurance programs. The American Society of Dermatopathology Web site. http://www.asdp.org/education/quality-assurance-programs. Accessed January 20, 2016.
Practice Points
- The Clinical Learning Environment Review mobilizes residency and fellowship training programs in the movement to improve the quality of patient care.
- Quality assessment/quality improvement (QA/QI) projects enhance communication between residents and faculty and promote systems that improve patient safety.
- Emphasis on resident-initiated QA/QI impacts quality of care in clinical practice long after graduation.