User login
Painful, Nonhealing, Violaceus Plaque on the Right Breast
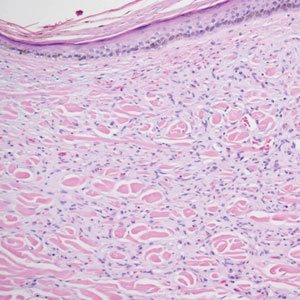
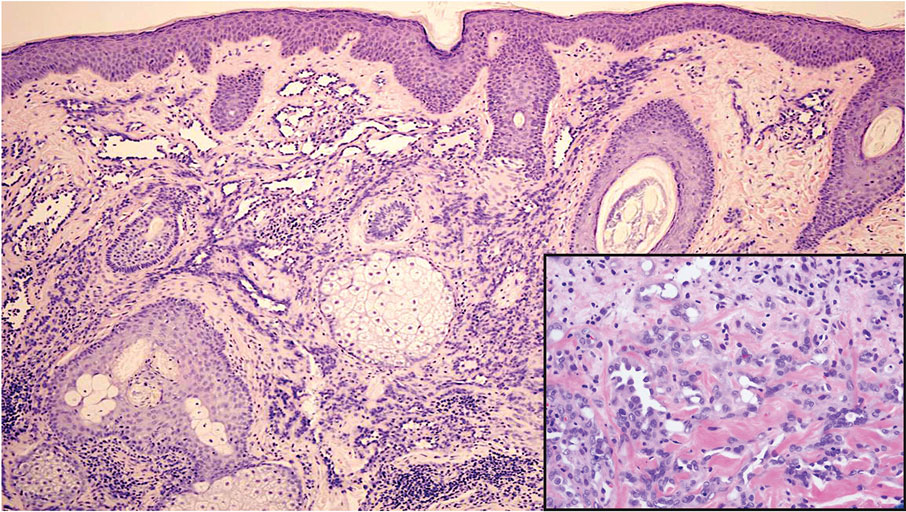
The Diagnosis: Diffuse Dermal Angiomatosis
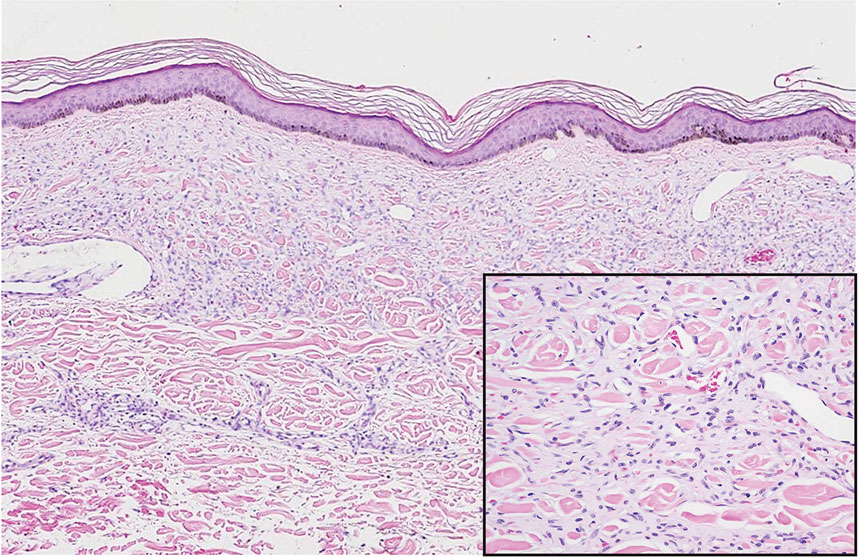
Diffuse dermal angiomatosis (DDA) is an acquired reactive vascular proliferation in the spectrum of cutaneous reactive angioendotheliomatoses. Clinically, DDA presents as violaceous reticulated plaques, often with secondary ulceration and sometimes necrosis.1-3 Diffuse dermal angiomatosis more commonly presents in patients with a history of severe peripheral vascular disease, coagulopathies, or infection, and it frequently arises on the extremities. Diffuse dermal angiomatosis also has been shown to develop on the breasts, particularly in patients with pendulous breast tissue. Vascular proliferation in DDA is hypothesized to be from ischemia and hypoxia, leading to angiogenesis.1-3 Diffuse dermal angiomatosis is characterized histologically by the presence of a diffuse proliferation of spindled endothelial cells distributed between the collagen bundles throughout the dermis (quiz image and Figure 1). Spindle-shaped endothelial cells exhibit a vacuolated cytoplasm. On immunohistochemistry, these dermal spindle cells classically stain positive for CD31, CD34, and erythroblast transformation specific–related gene (Erg) and stain negative for both human herpesvirus 8 (HHV-8) and factor XIIIa.
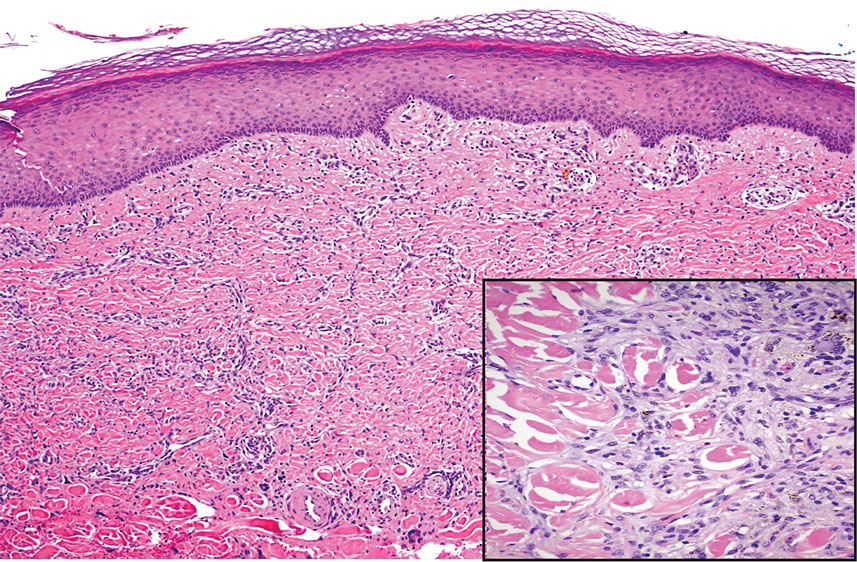
Cutaneous fibrous histiocytoma, more commonly referred to as dermatofibroma, is a common benign lesion that presents clinically as a solitary firm nodule most commonly on the extremities in areas of repetitive trauma or pressure. It classically exhibits dimpling of the overlaying skin with lateral pressure on the lesion, known as the dimple sign.4 Histologically, dermatofibromas share similar features to DDA and demonstrate the presence of bland-appearing spindle cells within the dermis between the collagen bundles, resulting in collagen trapping. However, a distinguishing histologic feature of a dermatofibroma in comparison to DDA is the presence of epidermal hyperplasia overlying the dermatofibroma, leading to tabled rete ridges (Figure 2). Spindle cells in dermatofibromas are fibroblasts and have a distinct immunophenotype that includes factor XIIIa positivity and negative staining for CD31, CD34, and Erg.4,5
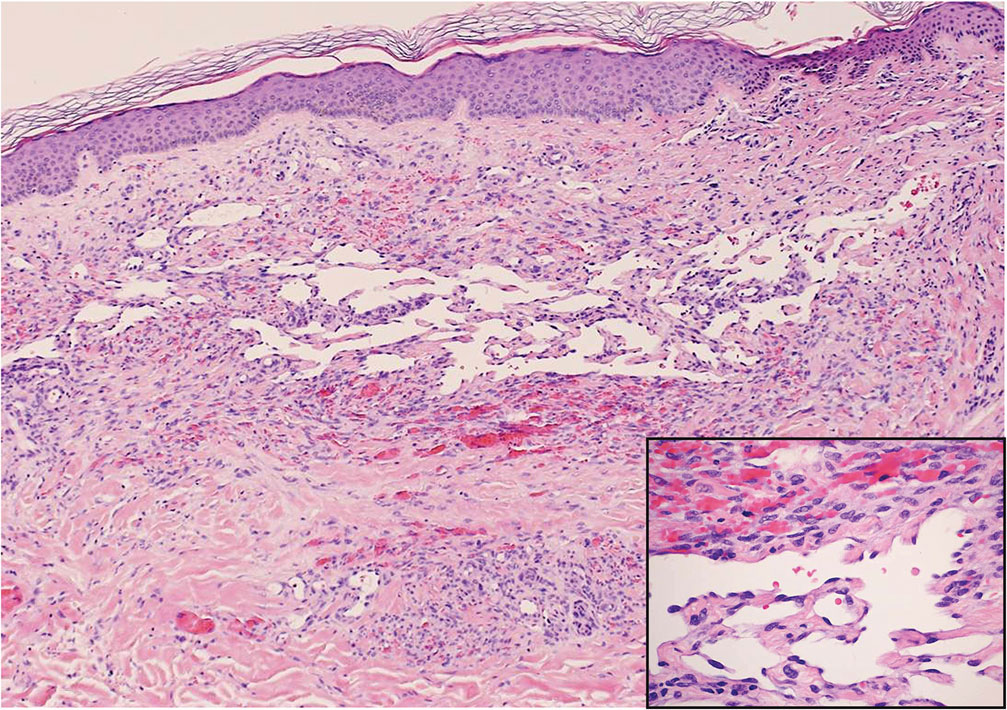
Dermatofibrosarcoma protuberans (DFSP) is a rare malignant soft-tissue sarcoma that clinically presents as a firm, flesh-colored, dermal plaque on the trunk, proximal extremities, head, or neck.5 Histologically, DFSP can be distinguished from DDA by the high density of spindle cells that are arranged in a storiform pattern, extending and infiltrating the underlying subcutaneous fat in a honeycomblike pattern (Figure 3). Spindle cells in DFSP typically show expression of CD34 but are negative for CD31, Erg, and factor XIIIa.5

Kaposi sarcoma (KS) is an endothelial cell–driven angioproliferative neoplasm that is associated with HHV-8 infection.6 The clinical presentation of KS can range from isolated pink or purple papules and patches to more extensive ulcerated plaques or nodules. Histopathology exhibits proliferation of monomorphic spindled endothelial cells within the dermis staining positive for HHV-8, Erg, CD31, and CD34, in conjunction with extravasated erythrocytes arranged within slitlike vascular spaces (Figure 4). Additionally, KS classically exhibits aberrant endothelial cell proliferation and vessel formation around preexisting vessels, which is referred to as the promontory sign (Figure 4).
Angiosarcoma is a rare and highly aggressive vascular tumor arising from endothelial cells lining the blood vessels and lymphatics.7,8 Clinically, angiosarcoma presents as ulcerated violaceous nodules or plaques on the head, neck, or trunk. Histologic evaluation of angiosarcoma reveals a complex and poorly demarcated vascular network dissecting between collagen bundles in the dermis (Figure 5). Multilayering of endothelial cells, papillary projections extending into the vessel lumina, and mitoses frequently are seen. On immunohistochemistry, endothelial cells demonstrate prominent cellular atypia and stain positive with CD31, CD34, and Erg.
- Touloei K, Tongdee E, Smirnov B, et al. Diffuse dermal angiomatosis. Cutis. 2019;103:181-184.
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Baylor Univ Med Cent Proc. 2020;33:273-275.
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505-517.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
The Diagnosis: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is an acquired reactive vascular proliferation in the spectrum of cutaneous reactive angioendotheliomatoses. Clinically, DDA presents as violaceous reticulated plaques, often with secondary ulceration and sometimes necrosis.1-3 Diffuse dermal angiomatosis more commonly presents in patients with a history of severe peripheral vascular disease, coagulopathies, or infection, and it frequently arises on the extremities. Diffuse dermal angiomatosis also has been shown to develop on the breasts, particularly in patients with pendulous breast tissue. Vascular proliferation in DDA is hypothesized to be from ischemia and hypoxia, leading to angiogenesis.1-3 Diffuse dermal angiomatosis is characterized histologically by the presence of a diffuse proliferation of spindled endothelial cells distributed between the collagen bundles throughout the dermis (quiz image and Figure 1). Spindle-shaped endothelial cells exhibit a vacuolated cytoplasm. On immunohistochemistry, these dermal spindle cells classically stain positive for CD31, CD34, and erythroblast transformation specific–related gene (Erg) and stain negative for both human herpesvirus 8 (HHV-8) and factor XIIIa.

Cutaneous fibrous histiocytoma, more commonly referred to as dermatofibroma, is a common benign lesion that presents clinically as a solitary firm nodule most commonly on the extremities in areas of repetitive trauma or pressure. It classically exhibits dimpling of the overlaying skin with lateral pressure on the lesion, known as the dimple sign.4 Histologically, dermatofibromas share similar features to DDA and demonstrate the presence of bland-appearing spindle cells within the dermis between the collagen bundles, resulting in collagen trapping. However, a distinguishing histologic feature of a dermatofibroma in comparison to DDA is the presence of epidermal hyperplasia overlying the dermatofibroma, leading to tabled rete ridges (Figure 2). Spindle cells in dermatofibromas are fibroblasts and have a distinct immunophenotype that includes factor XIIIa positivity and negative staining for CD31, CD34, and Erg.4,5

Dermatofibrosarcoma protuberans (DFSP) is a rare malignant soft-tissue sarcoma that clinically presents as a firm, flesh-colored, dermal plaque on the trunk, proximal extremities, head, or neck.5 Histologically, DFSP can be distinguished from DDA by the high density of spindle cells that are arranged in a storiform pattern, extending and infiltrating the underlying subcutaneous fat in a honeycomblike pattern (Figure 3). Spindle cells in DFSP typically show expression of CD34 but are negative for CD31, Erg, and factor XIIIa.5

Kaposi sarcoma (KS) is an endothelial cell–driven angioproliferative neoplasm that is associated with HHV-8 infection.6 The clinical presentation of KS can range from isolated pink or purple papules and patches to more extensive ulcerated plaques or nodules. Histopathology exhibits proliferation of monomorphic spindled endothelial cells within the dermis staining positive for HHV-8, Erg, CD31, and CD34, in conjunction with extravasated erythrocytes arranged within slitlike vascular spaces (Figure 4). Additionally, KS classically exhibits aberrant endothelial cell proliferation and vessel formation around preexisting vessels, which is referred to as the promontory sign (Figure 4).

Angiosarcoma is a rare and highly aggressive vascular tumor arising from endothelial cells lining the blood vessels and lymphatics.7,8 Clinically, angiosarcoma presents as ulcerated violaceous nodules or plaques on the head, neck, or trunk. Histologic evaluation of angiosarcoma reveals a complex and poorly demarcated vascular network dissecting between collagen bundles in the dermis (Figure 5). Multilayering of endothelial cells, papillary projections extending into the vessel lumina, and mitoses frequently are seen. On immunohistochemistry, endothelial cells demonstrate prominent cellular atypia and stain positive with CD31, CD34, and Erg.

The Diagnosis: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is an acquired reactive vascular proliferation in the spectrum of cutaneous reactive angioendotheliomatoses. Clinically, DDA presents as violaceous reticulated plaques, often with secondary ulceration and sometimes necrosis.1-3 Diffuse dermal angiomatosis more commonly presents in patients with a history of severe peripheral vascular disease, coagulopathies, or infection, and it frequently arises on the extremities. Diffuse dermal angiomatosis also has been shown to develop on the breasts, particularly in patients with pendulous breast tissue. Vascular proliferation in DDA is hypothesized to be from ischemia and hypoxia, leading to angiogenesis.1-3 Diffuse dermal angiomatosis is characterized histologically by the presence of a diffuse proliferation of spindled endothelial cells distributed between the collagen bundles throughout the dermis (quiz image and Figure 1). Spindle-shaped endothelial cells exhibit a vacuolated cytoplasm. On immunohistochemistry, these dermal spindle cells classically stain positive for CD31, CD34, and erythroblast transformation specific–related gene (Erg) and stain negative for both human herpesvirus 8 (HHV-8) and factor XIIIa.

Cutaneous fibrous histiocytoma, more commonly referred to as dermatofibroma, is a common benign lesion that presents clinically as a solitary firm nodule most commonly on the extremities in areas of repetitive trauma or pressure. It classically exhibits dimpling of the overlaying skin with lateral pressure on the lesion, known as the dimple sign.4 Histologically, dermatofibromas share similar features to DDA and demonstrate the presence of bland-appearing spindle cells within the dermis between the collagen bundles, resulting in collagen trapping. However, a distinguishing histologic feature of a dermatofibroma in comparison to DDA is the presence of epidermal hyperplasia overlying the dermatofibroma, leading to tabled rete ridges (Figure 2). Spindle cells in dermatofibromas are fibroblasts and have a distinct immunophenotype that includes factor XIIIa positivity and negative staining for CD31, CD34, and Erg.4,5

Dermatofibrosarcoma protuberans (DFSP) is a rare malignant soft-tissue sarcoma that clinically presents as a firm, flesh-colored, dermal plaque on the trunk, proximal extremities, head, or neck.5 Histologically, DFSP can be distinguished from DDA by the high density of spindle cells that are arranged in a storiform pattern, extending and infiltrating the underlying subcutaneous fat in a honeycomblike pattern (Figure 3). Spindle cells in DFSP typically show expression of CD34 but are negative for CD31, Erg, and factor XIIIa.5

Kaposi sarcoma (KS) is an endothelial cell–driven angioproliferative neoplasm that is associated with HHV-8 infection.6 The clinical presentation of KS can range from isolated pink or purple papules and patches to more extensive ulcerated plaques or nodules. Histopathology exhibits proliferation of monomorphic spindled endothelial cells within the dermis staining positive for HHV-8, Erg, CD31, and CD34, in conjunction with extravasated erythrocytes arranged within slitlike vascular spaces (Figure 4). Additionally, KS classically exhibits aberrant endothelial cell proliferation and vessel formation around preexisting vessels, which is referred to as the promontory sign (Figure 4).

Angiosarcoma is a rare and highly aggressive vascular tumor arising from endothelial cells lining the blood vessels and lymphatics.7,8 Clinically, angiosarcoma presents as ulcerated violaceous nodules or plaques on the head, neck, or trunk. Histologic evaluation of angiosarcoma reveals a complex and poorly demarcated vascular network dissecting between collagen bundles in the dermis (Figure 5). Multilayering of endothelial cells, papillary projections extending into the vessel lumina, and mitoses frequently are seen. On immunohistochemistry, endothelial cells demonstrate prominent cellular atypia and stain positive with CD31, CD34, and Erg.

- Touloei K, Tongdee E, Smirnov B, et al. Diffuse dermal angiomatosis. Cutis. 2019;103:181-184.
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Baylor Univ Med Cent Proc. 2020;33:273-275.
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505-517.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
- Touloei K, Tongdee E, Smirnov B, et al. Diffuse dermal angiomatosis. Cutis. 2019;103:181-184.
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Baylor Univ Med Cent Proc. 2020;33:273-275.
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505-517.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
A 42-year-old woman with a medical history of hypertension and smoking tobacco (5 pack years) presented with a painful, nonhealing, violaceous, reticulated plaque with ulceration on the right breast of 3 months’ duration. Histopathology revealed diffuse, interstitial, bland-appearing spindle cells throughout the papillary and reticular dermis that were distributed between the collagen bundles. Dermal interstitial spindle cells were positive for CD31, CD34, and erythroblast transformation specific–related gene immunostains. Factor XIIIa and human herpesvirus 8 immunostaining was negative.
Firm Abdominal Papule
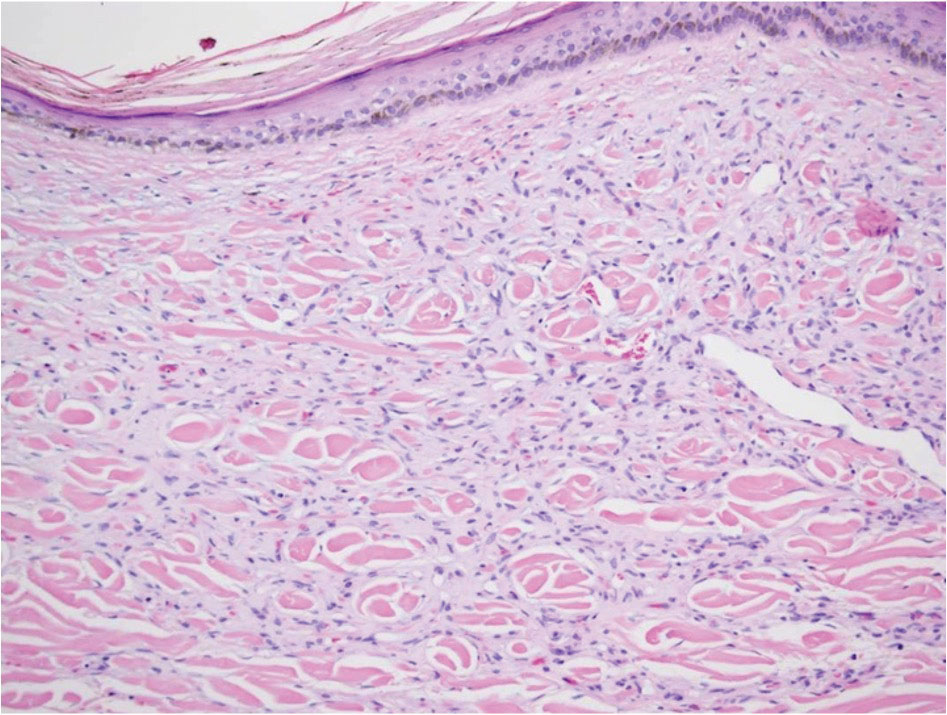
The Diagnosis: Cutaneous Metastatic Gastric Carcinoma
Cutaneous metastasis of primary gastric carcinoma is a rare occurrence, with the more common metastatic sites being the lymph nodes, liver, and peritoneal cavity. The incidence of visceral neoplasm metastasis to the skin ranges from 0.7% to 9% and is less than 1% for upper digestive tract carcinomas.1 Cutaneous metastases make up 2% of all tumors of the skin and commonly are located near the site of the primary tumor.2 The most common cutaneous metastasis sites for gastric carcinoma include the neck, chest, and head.3 One of the more typical sites of cutaneous metastasis from gastric cancer is the umbilicus (ie, Sister Mary Joseph nodule). Cutaneous metastases from gastric carcinoma commonly present as asymptomatic hyperpigmented nodules.1,3
In our patient, histopathologic sections showed diffuse infiltration of the dermis by atypical polygonal/round cells arranged in cords and small aggregates. Some of the neoplastic cells had signet ring morphology (Figure). Tumor cells demonstrated positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen; they were negative for S-100, MART-1 (melanoma-associated antigen recognized by T cells 1), leukocyte common antigen, gross cystic disease fluid protein 15, estrogen and progesterone receptor, and HER2/neu (human epidermal growth factor receptor 2).
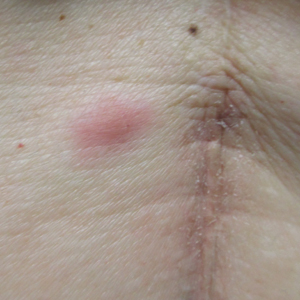
Our patient's presentation was rare in that she developed an asymptomatic erythematous papule on the skin of the abdomen. However, her history of stage IIIB gastric adenocarcinoma in conjunction with the clinical picture and microscopic findings were most consistent with metastatic carcinoma of gastrointestinal origin. The histologic hallmarks of cutaneous metastatic gastric carcinoma include aggregates of neoplastic cells arranged in cords, sometimes forming glands, embedded in a fibrous stroma. Tumor cells may demonstrate signet ring morphology. These unique histologic findings, as well as positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen, rule out other potential diagnoses for an asymptomatic solitary papule.
Dermatofibrosarcoma protuberans presents as an asymptomatic, slow-growing, indurated papule or plaque that develops into a red or brownish nodule. Histologically, dermatofibrosarcoma protuberans is characterized by spindled cells, few mitotic figures, infiltration of the subcutaneous tissue in a honeycomblike pattern, and obliteration of the adnexal structures.4
Cutaneous B-cell lymphoma (CBCL) can present as single or multiple red papules or nodules located on the trunk, face, or extremities. Histologically, CBCL would show a nodular or diffuse infiltrate throughout the dermis, frequently with accentuation in the deep reticular dermis, sparing of the epidermis, and the presence of a grenz zone. The infiltrate in CBCL consists of CD20+, CD19+, and CD79a+ B cells. Identification of a monoclonal B-cell population either by immunohistochemistry or polymerase chain reaction would further support a diagnosis of CBCL.4 These specific histologic findings and the immunohistochemical staining pattern helped rule out CBCL as the diagnosis in our patient.
Amelanotic melanomas present as flesh-colored to light pink papules, making them especially challenging to diagnose clinically. Asymmetrical, poorly circumscribed nests of atypical melanocytes as well as single melanocytes within the epidermis and dermis are seen histologically; mitotic figures are common. Immunohistochemical staining for melanoma includes S-100, human melanoma black 45, MART-1/Melan-A, tyrosinase, and microphthalmia-associated transcription factor 1.4
Neurothekeomas can present as asymptomatic, solitary, flesh-colored papules located on the head, neck, and upper trunk. Histologically, neurothekeomas have a distinct appearance consisting of a well-defined mass composed of variable-sized lobules of spindled and epithelioid cells dispersed in a myxoid stroma within the reticular dermis.4 These specific histologic findings helped rule out neurothekeoma in our patient.
Following the diagnosis of cutaneous metastatic gastric carcinoma in our patient, positron emission tomography and computed tomography of the chest, abdomen, and pelvis were unremarkable for distant disease. Subsequently, the patient underwent surgical excision of the papule with clear margins, followed by a short course of radiation therapy. She currently is under close monitoring but remains in remission with no new cutaneous manifestations of the gastric carcinoma.
- Erdemir A, Atilganoglu U, Onsun N, et al. Cutaneous metastases from gastric adenocarcinoma. Indian J Dermatol. 2011;56:236-237.
- Junqueira AL, Corbett AM, Oliveira Filho Jd, et al. Cutaneous metastasis from gastrointestinal adenocarcinoma of unknown primary origin. An Bras Dermatol. 2015;90:564-566.
- Cesaretti M, Malerba M, Basso V, et al. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
The Diagnosis: Cutaneous Metastatic Gastric Carcinoma
Cutaneous metastasis of primary gastric carcinoma is a rare occurrence, with the more common metastatic sites being the lymph nodes, liver, and peritoneal cavity. The incidence of visceral neoplasm metastasis to the skin ranges from 0.7% to 9% and is less than 1% for upper digestive tract carcinomas.1 Cutaneous metastases make up 2% of all tumors of the skin and commonly are located near the site of the primary tumor.2 The most common cutaneous metastasis sites for gastric carcinoma include the neck, chest, and head.3 One of the more typical sites of cutaneous metastasis from gastric cancer is the umbilicus (ie, Sister Mary Joseph nodule). Cutaneous metastases from gastric carcinoma commonly present as asymptomatic hyperpigmented nodules.1,3
In our patient, histopathologic sections showed diffuse infiltration of the dermis by atypical polygonal/round cells arranged in cords and small aggregates. Some of the neoplastic cells had signet ring morphology (Figure). Tumor cells demonstrated positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen; they were negative for S-100, MART-1 (melanoma-associated antigen recognized by T cells 1), leukocyte common antigen, gross cystic disease fluid protein 15, estrogen and progesterone receptor, and HER2/neu (human epidermal growth factor receptor 2).
Our patient's presentation was rare in that she developed an asymptomatic erythematous papule on the skin of the abdomen. However, her history of stage IIIB gastric adenocarcinoma in conjunction with the clinical picture and microscopic findings were most consistent with metastatic carcinoma of gastrointestinal origin. The histologic hallmarks of cutaneous metastatic gastric carcinoma include aggregates of neoplastic cells arranged in cords, sometimes forming glands, embedded in a fibrous stroma. Tumor cells may demonstrate signet ring morphology. These unique histologic findings, as well as positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen, rule out other potential diagnoses for an asymptomatic solitary papule.
Dermatofibrosarcoma protuberans presents as an asymptomatic, slow-growing, indurated papule or plaque that develops into a red or brownish nodule. Histologically, dermatofibrosarcoma protuberans is characterized by spindled cells, few mitotic figures, infiltration of the subcutaneous tissue in a honeycomblike pattern, and obliteration of the adnexal structures.4
Cutaneous B-cell lymphoma (CBCL) can present as single or multiple red papules or nodules located on the trunk, face, or extremities. Histologically, CBCL would show a nodular or diffuse infiltrate throughout the dermis, frequently with accentuation in the deep reticular dermis, sparing of the epidermis, and the presence of a grenz zone. The infiltrate in CBCL consists of CD20+, CD19+, and CD79a+ B cells. Identification of a monoclonal B-cell population either by immunohistochemistry or polymerase chain reaction would further support a diagnosis of CBCL.4 These specific histologic findings and the immunohistochemical staining pattern helped rule out CBCL as the diagnosis in our patient.
Amelanotic melanomas present as flesh-colored to light pink papules, making them especially challenging to diagnose clinically. Asymmetrical, poorly circumscribed nests of atypical melanocytes as well as single melanocytes within the epidermis and dermis are seen histologically; mitotic figures are common. Immunohistochemical staining for melanoma includes S-100, human melanoma black 45, MART-1/Melan-A, tyrosinase, and microphthalmia-associated transcription factor 1.4
Neurothekeomas can present as asymptomatic, solitary, flesh-colored papules located on the head, neck, and upper trunk. Histologically, neurothekeomas have a distinct appearance consisting of a well-defined mass composed of variable-sized lobules of spindled and epithelioid cells dispersed in a myxoid stroma within the reticular dermis.4 These specific histologic findings helped rule out neurothekeoma in our patient.
Following the diagnosis of cutaneous metastatic gastric carcinoma in our patient, positron emission tomography and computed tomography of the chest, abdomen, and pelvis were unremarkable for distant disease. Subsequently, the patient underwent surgical excision of the papule with clear margins, followed by a short course of radiation therapy. She currently is under close monitoring but remains in remission with no new cutaneous manifestations of the gastric carcinoma.
The Diagnosis: Cutaneous Metastatic Gastric Carcinoma
Cutaneous metastasis of primary gastric carcinoma is a rare occurrence, with the more common metastatic sites being the lymph nodes, liver, and peritoneal cavity. The incidence of visceral neoplasm metastasis to the skin ranges from 0.7% to 9% and is less than 1% for upper digestive tract carcinomas.1 Cutaneous metastases make up 2% of all tumors of the skin and commonly are located near the site of the primary tumor.2 The most common cutaneous metastasis sites for gastric carcinoma include the neck, chest, and head.3 One of the more typical sites of cutaneous metastasis from gastric cancer is the umbilicus (ie, Sister Mary Joseph nodule). Cutaneous metastases from gastric carcinoma commonly present as asymptomatic hyperpigmented nodules.1,3
In our patient, histopathologic sections showed diffuse infiltration of the dermis by atypical polygonal/round cells arranged in cords and small aggregates. Some of the neoplastic cells had signet ring morphology (Figure). Tumor cells demonstrated positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen; they were negative for S-100, MART-1 (melanoma-associated antigen recognized by T cells 1), leukocyte common antigen, gross cystic disease fluid protein 15, estrogen and progesterone receptor, and HER2/neu (human epidermal growth factor receptor 2).
Our patient's presentation was rare in that she developed an asymptomatic erythematous papule on the skin of the abdomen. However, her history of stage IIIB gastric adenocarcinoma in conjunction with the clinical picture and microscopic findings were most consistent with metastatic carcinoma of gastrointestinal origin. The histologic hallmarks of cutaneous metastatic gastric carcinoma include aggregates of neoplastic cells arranged in cords, sometimes forming glands, embedded in a fibrous stroma. Tumor cells may demonstrate signet ring morphology. These unique histologic findings, as well as positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen, rule out other potential diagnoses for an asymptomatic solitary papule.
Dermatofibrosarcoma protuberans presents as an asymptomatic, slow-growing, indurated papule or plaque that develops into a red or brownish nodule. Histologically, dermatofibrosarcoma protuberans is characterized by spindled cells, few mitotic figures, infiltration of the subcutaneous tissue in a honeycomblike pattern, and obliteration of the adnexal structures.4
Cutaneous B-cell lymphoma (CBCL) can present as single or multiple red papules or nodules located on the trunk, face, or extremities. Histologically, CBCL would show a nodular or diffuse infiltrate throughout the dermis, frequently with accentuation in the deep reticular dermis, sparing of the epidermis, and the presence of a grenz zone. The infiltrate in CBCL consists of CD20+, CD19+, and CD79a+ B cells. Identification of a monoclonal B-cell population either by immunohistochemistry or polymerase chain reaction would further support a diagnosis of CBCL.4 These specific histologic findings and the immunohistochemical staining pattern helped rule out CBCL as the diagnosis in our patient.
Amelanotic melanomas present as flesh-colored to light pink papules, making them especially challenging to diagnose clinically. Asymmetrical, poorly circumscribed nests of atypical melanocytes as well as single melanocytes within the epidermis and dermis are seen histologically; mitotic figures are common. Immunohistochemical staining for melanoma includes S-100, human melanoma black 45, MART-1/Melan-A, tyrosinase, and microphthalmia-associated transcription factor 1.4
Neurothekeomas can present as asymptomatic, solitary, flesh-colored papules located on the head, neck, and upper trunk. Histologically, neurothekeomas have a distinct appearance consisting of a well-defined mass composed of variable-sized lobules of spindled and epithelioid cells dispersed in a myxoid stroma within the reticular dermis.4 These specific histologic findings helped rule out neurothekeoma in our patient.
Following the diagnosis of cutaneous metastatic gastric carcinoma in our patient, positron emission tomography and computed tomography of the chest, abdomen, and pelvis were unremarkable for distant disease. Subsequently, the patient underwent surgical excision of the papule with clear margins, followed by a short course of radiation therapy. She currently is under close monitoring but remains in remission with no new cutaneous manifestations of the gastric carcinoma.
- Erdemir A, Atilganoglu U, Onsun N, et al. Cutaneous metastases from gastric adenocarcinoma. Indian J Dermatol. 2011;56:236-237.
- Junqueira AL, Corbett AM, Oliveira Filho Jd, et al. Cutaneous metastasis from gastrointestinal adenocarcinoma of unknown primary origin. An Bras Dermatol. 2015;90:564-566.
- Cesaretti M, Malerba M, Basso V, et al. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
- Erdemir A, Atilganoglu U, Onsun N, et al. Cutaneous metastases from gastric adenocarcinoma. Indian J Dermatol. 2011;56:236-237.
- Junqueira AL, Corbett AM, Oliveira Filho Jd, et al. Cutaneous metastasis from gastrointestinal adenocarcinoma of unknown primary origin. An Bras Dermatol. 2015;90:564-566.
- Cesaretti M, Malerba M, Basso V, et al. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
A 53-year-old woman with a history of melanoma on the right thigh, stage II Hodgkin lymphoma, and stage IIIB gastric adenocarcinoma treated with a distal gastrectomy presented with an asymptomatic but persistent skin lesion on the abdomen of 2 months' duration. The lesion arose spontaneously 6 months prior and had increased in size during that time. Physical examination revealed a 6-mm, solitary, firm, erythematous papule on the skin of the right upper quadrant of the abdomen. The patient was otherwise healthy, and a review of systems did not reveal any abnormalities. A punch biopsy was submitted for histopathologic review.
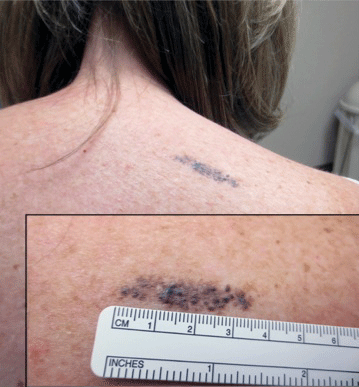
Linear Bluish Black Papules on the Shoulder
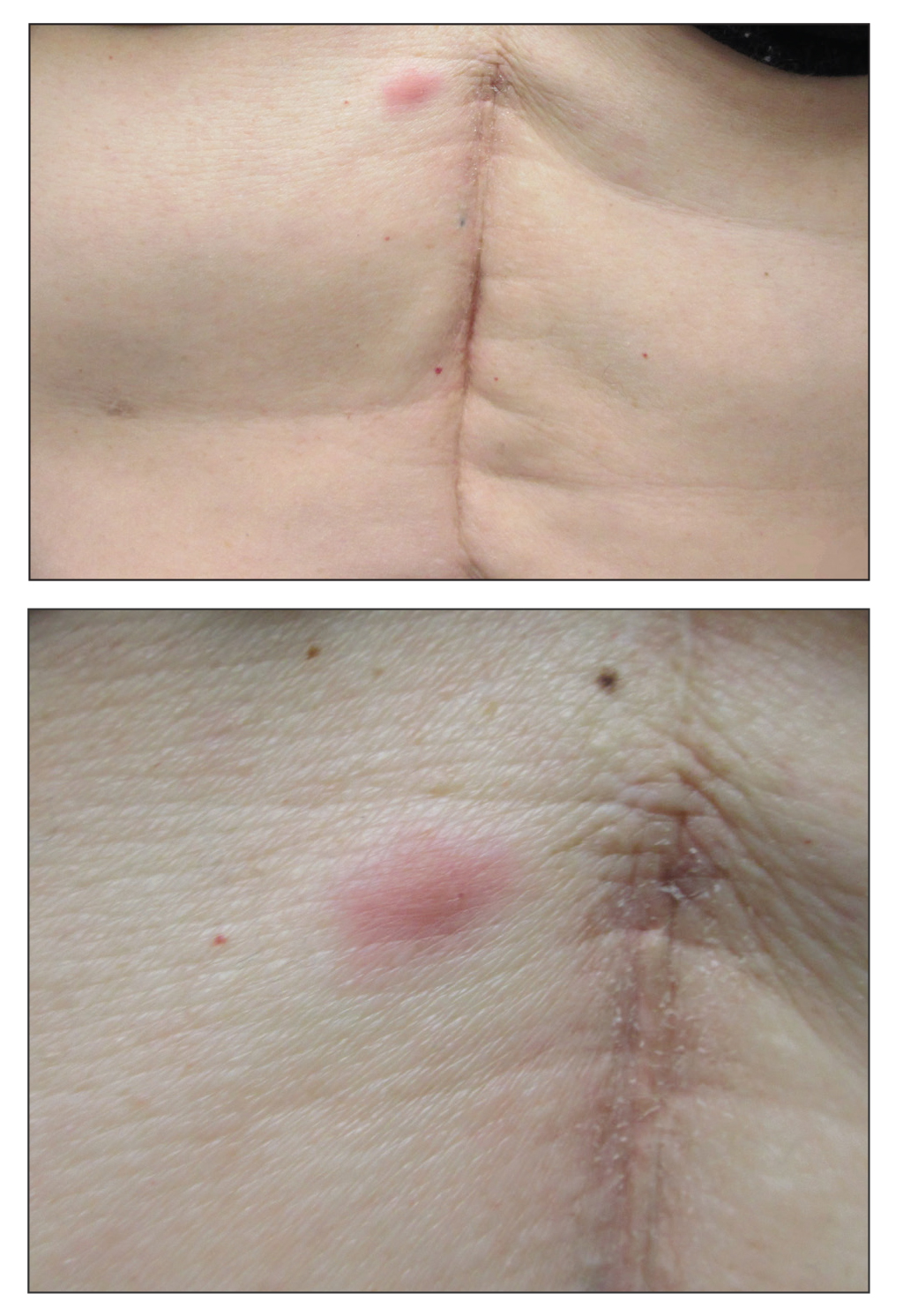
The Diagnosis: Agminated Blue Nevus
Agminated blue nevus is a rare melanocytic nevus that characteristically presents as a group of multiple small, bluish papules occurring in a well-circumscribed area.1 It also has been referred to as a plaque-type nevus2,3 and an eruptive blue nevus.4 Originally described by Upshaw et al2 in 1947, agminated blue nevi may be congenital or arise in early childhood and almost always occur on the trunk. The skin between the papules often is unaffected or sometimes may show bluish or brown pigmentation.4 Agminated blue nevi usually are smaller than 10 cm in diameter; however, rare cases have measured up to 24 cm.1,4-6 The incidence of agminated blue nevi is 2 times higher in males than in females.1
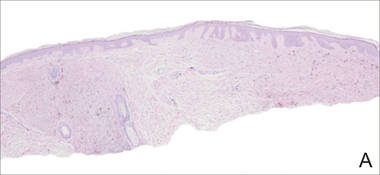 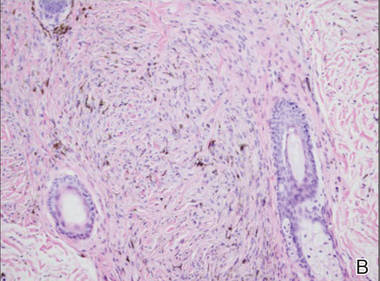 |
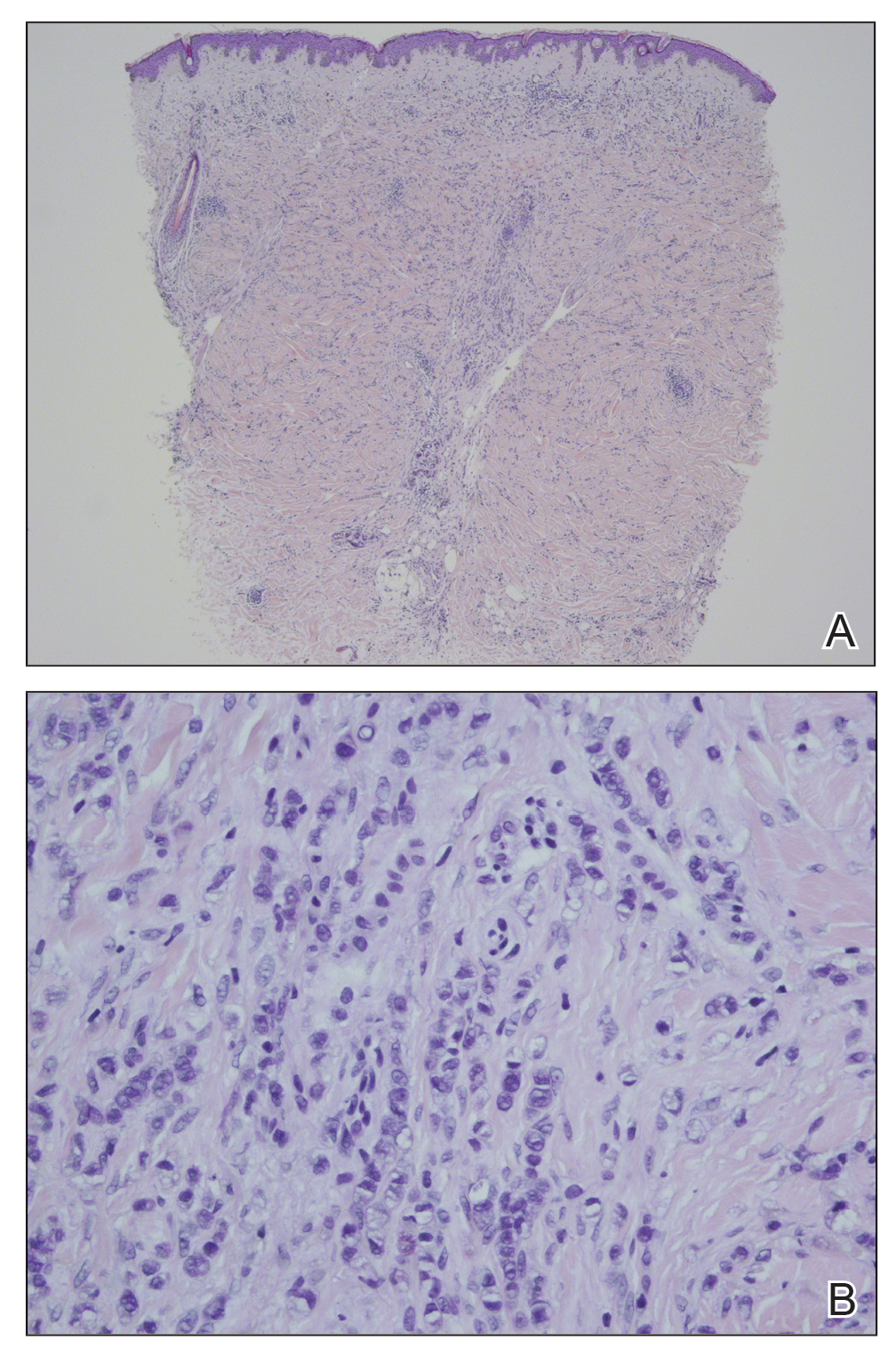
A biopsy of the lesion revealed pigmented dendritic melanocytes admixed with melanophages, forming fascicles and bundles (A)(H&E, original magnification ×10). In some areas the dermis was uninvolved. Higher magnification showed dendritic and epithelioid melanocytes extending down along the adnexal structures (B)(H&E, original magnification ×40). |
Histopathologically, agminated blue nevi typically demonstrate the features of common and/or cellular blue nevi. Cytologic atypia and mitoses are rare.1 The degree of cellularity and pigmentation of the lesions is variable, and the presence of subcutaneous cellular nodules also has been described.5
In our patient, histologic evaluation revealed foci of diffuse dermal spindle cell proliferation composed of heavily pigmented dendritic melanocytes admixed with melanophages in a fibrotic stroma (Figure, A). The dermis was uninvolved in some areas and the melanocytes were epithelioid and formed fascicles and bundles that extended down adnexal structures in other areas (Figure, B). Junctional involvement of melanocytes, cellular atypia, and mitoses were not identified. Our case demonstrated a combination of histologic findings of a cellular blue nevus as well as features reminiscent of a deep penetrating nevus. The differential diagnosis of agminated blue nevus includes agminated Spitz nevus arising in a speckled lentiginous nevus,7 dermal melanocytosis, melanoma, and pilar neurocristic hamartoma. Pilar neurocristic hamartomas may resemble plaque-type blue nevi; however, the former show a predilection for the scalp, histologically demonstrate features that overlap with blue nevi and congenital nevi, and are associated with neural structures that show Schwannian differentiation.8 Agminated blue nevi usually are characterized by a benign clinical course, but few cases describing malignant changes with development of malignant melanoma have been reported.9,10 Therefore, recognition of the clinical and histopathologic spectrum of agminated blue nevus is critical in order to avoid diagnostic pitfalls and confusion with melanoma.
- Vélez A, del-Río E, Martín-de-Hijas C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186:144-148.
- Upshaw BY, Ghormley RK, Montgomery H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery. 1947;22:761-765.
- Pittman JL, Fisher BK. Plaque-type blue nevus. Arch Dermatol. 1976;112:1127-1128.
- Hendricks WM. Eruptive blue nevi. J Am Acad Dermatol.1981;4:50-53.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Shenfield HT, Maize JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6:725-728.
- Misago N, Narisawa Y, Kohda H. A combination of speckled lentiginous nevus with patch-type blue nevus. J Dermatol. 1993;20:643-647.
- Bevona C, Tannous Z, Tsao H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49:924-929.
- Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258-1263.
- Zattra E, Salmaso R, Montesco MC, et al. Large plaque type blue nevus with subcutaneous cellular nodules. Eur J Dermatol. 2009;19:287-288.
The Diagnosis: Agminated Blue Nevus
Agminated blue nevus is a rare melanocytic nevus that characteristically presents as a group of multiple small, bluish papules occurring in a well-circumscribed area.1 It also has been referred to as a plaque-type nevus2,3 and an eruptive blue nevus.4 Originally described by Upshaw et al2 in 1947, agminated blue nevi may be congenital or arise in early childhood and almost always occur on the trunk. The skin between the papules often is unaffected or sometimes may show bluish or brown pigmentation.4 Agminated blue nevi usually are smaller than 10 cm in diameter; however, rare cases have measured up to 24 cm.1,4-6 The incidence of agminated blue nevi is 2 times higher in males than in females.1
  |
A biopsy of the lesion revealed pigmented dendritic melanocytes admixed with melanophages, forming fascicles and bundles (A)(H&E, original magnification ×10). In some areas the dermis was uninvolved. Higher magnification showed dendritic and epithelioid melanocytes extending down along the adnexal structures (B)(H&E, original magnification ×40). |
Histopathologically, agminated blue nevi typically demonstrate the features of common and/or cellular blue nevi. Cytologic atypia and mitoses are rare.1 The degree of cellularity and pigmentation of the lesions is variable, and the presence of subcutaneous cellular nodules also has been described.5
In our patient, histologic evaluation revealed foci of diffuse dermal spindle cell proliferation composed of heavily pigmented dendritic melanocytes admixed with melanophages in a fibrotic stroma (Figure, A). The dermis was uninvolved in some areas and the melanocytes were epithelioid and formed fascicles and bundles that extended down adnexal structures in other areas (Figure, B). Junctional involvement of melanocytes, cellular atypia, and mitoses were not identified. Our case demonstrated a combination of histologic findings of a cellular blue nevus as well as features reminiscent of a deep penetrating nevus. The differential diagnosis of agminated blue nevus includes agminated Spitz nevus arising in a speckled lentiginous nevus,7 dermal melanocytosis, melanoma, and pilar neurocristic hamartoma. Pilar neurocristic hamartomas may resemble plaque-type blue nevi; however, the former show a predilection for the scalp, histologically demonstrate features that overlap with blue nevi and congenital nevi, and are associated with neural structures that show Schwannian differentiation.8 Agminated blue nevi usually are characterized by a benign clinical course, but few cases describing malignant changes with development of malignant melanoma have been reported.9,10 Therefore, recognition of the clinical and histopathologic spectrum of agminated blue nevus is critical in order to avoid diagnostic pitfalls and confusion with melanoma.
The Diagnosis: Agminated Blue Nevus
Agminated blue nevus is a rare melanocytic nevus that characteristically presents as a group of multiple small, bluish papules occurring in a well-circumscribed area.1 It also has been referred to as a plaque-type nevus2,3 and an eruptive blue nevus.4 Originally described by Upshaw et al2 in 1947, agminated blue nevi may be congenital or arise in early childhood and almost always occur on the trunk. The skin between the papules often is unaffected or sometimes may show bluish or brown pigmentation.4 Agminated blue nevi usually are smaller than 10 cm in diameter; however, rare cases have measured up to 24 cm.1,4-6 The incidence of agminated blue nevi is 2 times higher in males than in females.1
  |
A biopsy of the lesion revealed pigmented dendritic melanocytes admixed with melanophages, forming fascicles and bundles (A)(H&E, original magnification ×10). In some areas the dermis was uninvolved. Higher magnification showed dendritic and epithelioid melanocytes extending down along the adnexal structures (B)(H&E, original magnification ×40). |
Histopathologically, agminated blue nevi typically demonstrate the features of common and/or cellular blue nevi. Cytologic atypia and mitoses are rare.1 The degree of cellularity and pigmentation of the lesions is variable, and the presence of subcutaneous cellular nodules also has been described.5
In our patient, histologic evaluation revealed foci of diffuse dermal spindle cell proliferation composed of heavily pigmented dendritic melanocytes admixed with melanophages in a fibrotic stroma (Figure, A). The dermis was uninvolved in some areas and the melanocytes were epithelioid and formed fascicles and bundles that extended down adnexal structures in other areas (Figure, B). Junctional involvement of melanocytes, cellular atypia, and mitoses were not identified. Our case demonstrated a combination of histologic findings of a cellular blue nevus as well as features reminiscent of a deep penetrating nevus. The differential diagnosis of agminated blue nevus includes agminated Spitz nevus arising in a speckled lentiginous nevus,7 dermal melanocytosis, melanoma, and pilar neurocristic hamartoma. Pilar neurocristic hamartomas may resemble plaque-type blue nevi; however, the former show a predilection for the scalp, histologically demonstrate features that overlap with blue nevi and congenital nevi, and are associated with neural structures that show Schwannian differentiation.8 Agminated blue nevi usually are characterized by a benign clinical course, but few cases describing malignant changes with development of malignant melanoma have been reported.9,10 Therefore, recognition of the clinical and histopathologic spectrum of agminated blue nevus is critical in order to avoid diagnostic pitfalls and confusion with melanoma.
- Vélez A, del-Río E, Martín-de-Hijas C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186:144-148.
- Upshaw BY, Ghormley RK, Montgomery H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery. 1947;22:761-765.
- Pittman JL, Fisher BK. Plaque-type blue nevus. Arch Dermatol. 1976;112:1127-1128.
- Hendricks WM. Eruptive blue nevi. J Am Acad Dermatol.1981;4:50-53.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Shenfield HT, Maize JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6:725-728.
- Misago N, Narisawa Y, Kohda H. A combination of speckled lentiginous nevus with patch-type blue nevus. J Dermatol. 1993;20:643-647.
- Bevona C, Tannous Z, Tsao H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49:924-929.
- Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258-1263.
- Zattra E, Salmaso R, Montesco MC, et al. Large plaque type blue nevus with subcutaneous cellular nodules. Eur J Dermatol. 2009;19:287-288.
- Vélez A, del-Río E, Martín-de-Hijas C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186:144-148.
- Upshaw BY, Ghormley RK, Montgomery H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery. 1947;22:761-765.
- Pittman JL, Fisher BK. Plaque-type blue nevus. Arch Dermatol. 1976;112:1127-1128.
- Hendricks WM. Eruptive blue nevi. J Am Acad Dermatol.1981;4:50-53.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Shenfield HT, Maize JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6:725-728.
- Misago N, Narisawa Y, Kohda H. A combination of speckled lentiginous nevus with patch-type blue nevus. J Dermatol. 1993;20:643-647.
- Bevona C, Tannous Z, Tsao H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49:924-929.
- Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258-1263.
- Zattra E, Salmaso R, Montesco MC, et al. Large plaque type blue nevus with subcutaneous cellular nodules. Eur J Dermatol. 2009;19:287-288.
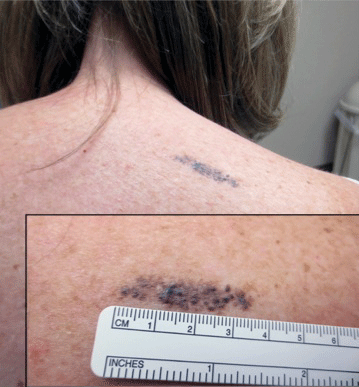
A 57-year-old woman presented with an asymptomatic, unchanging, 3.5×0.7-cm linear plaque on the right shoulder composed of dozens of clustered, bluish black papules that had been present for several decades. The skin between the papules was unaffected. The patient’s medical and family histories were unremarkable. A deep shave biopsy from the center of the plaque was performed.