User login
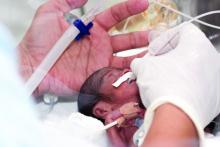
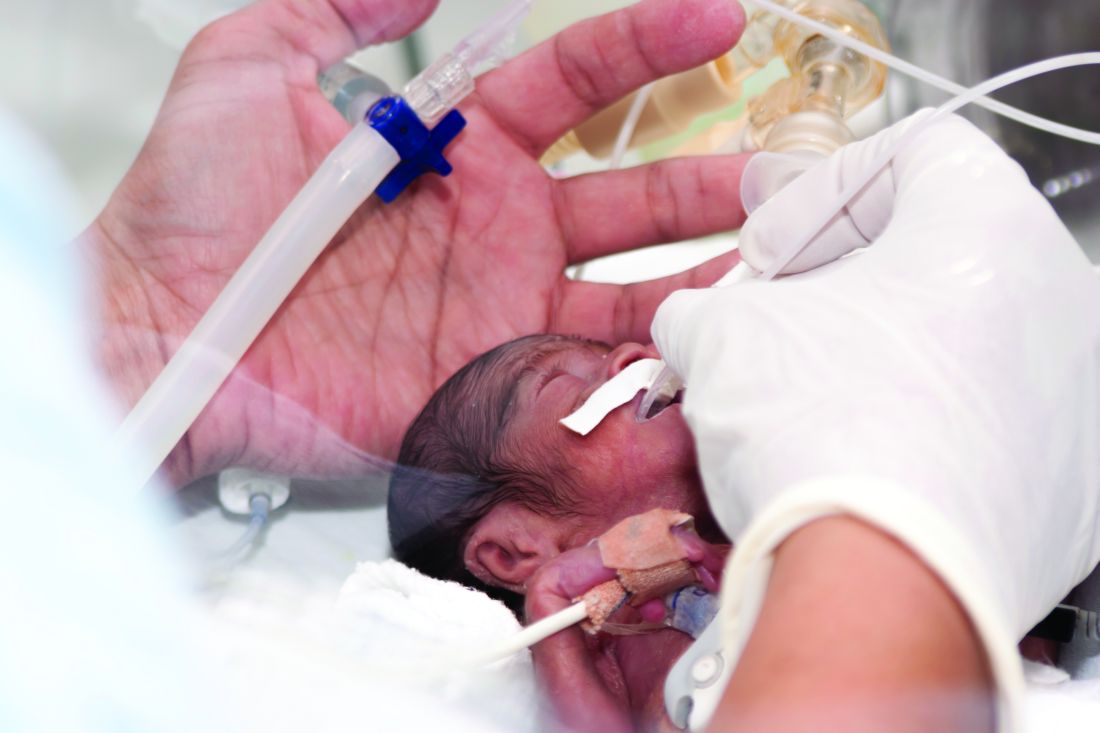
ASDS issues first filler safety recommendations
The .
The recommendations, published in the February issue of Dermatologic Surgery, are from a multidisciplinary task force convened by the ASDS, comprising 11 physicians – 8 board-certified in dermatology, 2 board-certified in plastic and reconstructive surgery, and 1 board-certified in oculoplastic surgery (all ASDS members) – and 2 patient representatives.
While redness, swelling, and other injection site reactions associated with injectable fillers are common, and usually resolve within 1-2 weeks, “rare but more serious adverse events from injectable fillers include vascular occlusion leading to skin necrosis or blindness, inflammatory events, and nodule formation, among others,” the authors wrote. They are “likely underreported” and cases are increasing as injectable fillers become more popular.
“Truthfully, a lot of people don’t know what they’re doing,” lead author Derek Jones, MD, of Skin Care and Laser Physicians of Beverly Hills, Los Angeles, said in an interview. Worldwide, he said that the absolute number of injectable filler treatments “has exploded,” particularly in the United States – and over the last decade. Moreover, “a lot of these treatments are being relegated to the nurse practitioner or the registered nurse who may take a weekend course,” he added. “There is this lack of knowledge and lack of recognition of some of these basic principles that we are publishing.”
About half of the document focuses on the potentially devastating complications of filler embolization and vascular occlusion of facial arteries, which include tissue ischemia, necrosis, visual abnormalities, blindness, and stroke. While these complications are considered rare, there is actually little information on their prevalence, Dr. Jones pointed out. “We think there is massive underreporting … so we view it as just the tip of the iceberg.”
Almost 200 unique cases of injection-related visual compromise (IRVC) have been reported in the literature, but not enough to provide strong evidence-based treatment protocols, he said.
“There are really no randomized clinical trials on how to treat them – they’re just not common enough – but there’s still some very good evidence that points the way. Most of what we’re relying on is in that low- to moderate-certainty range, but nevertheless, it points in a direction most of us can hang our hat on.”
He described the two most important cornerstones for preventing vascular occlusion. The first, he said, is “having impeccable knowledge and understanding of vascular anatomy and the cutaneous landmarks for blood vessels that are at risk.” The second pertains to injection techniques, he said, noting that “it’s becoming clearer – although it’s somewhat controversial – that cannulas are safer than needles.”
While anatomical knowledge might seem like the most basic requirement for practitioners who inject fillers, Dr. Jones said it is not. “It is true that we study anatomy in great detail in medical school, but injection anatomy is a completely different bird. Of course someone can study the facial arteries and have a basic understanding, but understanding it in a way that it relates to safe filler injections is a completely different thing. Our understanding of this has evolved over the last couple of decades ... and there are new papers that come out all the time that refine our knowledge.”
In terms of treating an IRVC resulting from hyaluronic acid (HA) filler, “the take-home point is the hyaluronidase is the mainstay of treatment, and not a little bit of hyaluronidase, but a lot of hyaluronidase – hundreds of units injected into the area of ischemia,” he said. However, while the new guidelines emphasize that time is of the essence – “the most cited window of time for reperfusion is 90 minutes” – the authors also strongly advise practitioners to evaluate immediate post-event visual status first, before attempting any intervention.
Dr. Jones said the goal is to untangle some confusion about whether it is the filler or the rescue that does more damage. “There is a lot of controversy with ophthalmology, ophthalmologic surgeons, plastic surgeons, and dermatologists,” he said, and “there has been finger-pointing between specialists when people make an intervention … that the actual rescue procedure created the problem,” which is why “it is imperative to document the visual status prior to doing anything.”
Beyond IRVC, and nonvisual skin ischemia due to vascular occlusion, the document addresses prevention and treatment of nodules, both inflammatory and noninflammatory, that occur either early or more than a month after treatment with HA fillers, as well with semi-permanent and permanent fillers.
For HA-related nodules, “the mainstay of treatment is steroids – either oral or intralesional – antibiotics, and hyaluronidase, which erases the substance,” said Dr. Jones. As for nodules related to permanent fillers, he said that they are difficult to treat, and “tend to respond best to repetitive monthly injections of 5-fluououracil combined with small amounts of triamcinolone.”
Dr. Jones is an investigator or consultant for Allergan, Galderma, Merz, and Revance; other authors had disclosures that included serving as a consultant, investigator, and/or trainer for these and/or other companies; one author received partial funding from ASDS to do this work; and one author had no disclosures.
The .
The recommendations, published in the February issue of Dermatologic Surgery, are from a multidisciplinary task force convened by the ASDS, comprising 11 physicians – 8 board-certified in dermatology, 2 board-certified in plastic and reconstructive surgery, and 1 board-certified in oculoplastic surgery (all ASDS members) – and 2 patient representatives.
While redness, swelling, and other injection site reactions associated with injectable fillers are common, and usually resolve within 1-2 weeks, “rare but more serious adverse events from injectable fillers include vascular occlusion leading to skin necrosis or blindness, inflammatory events, and nodule formation, among others,” the authors wrote. They are “likely underreported” and cases are increasing as injectable fillers become more popular.
“Truthfully, a lot of people don’t know what they’re doing,” lead author Derek Jones, MD, of Skin Care and Laser Physicians of Beverly Hills, Los Angeles, said in an interview. Worldwide, he said that the absolute number of injectable filler treatments “has exploded,” particularly in the United States – and over the last decade. Moreover, “a lot of these treatments are being relegated to the nurse practitioner or the registered nurse who may take a weekend course,” he added. “There is this lack of knowledge and lack of recognition of some of these basic principles that we are publishing.”
About half of the document focuses on the potentially devastating complications of filler embolization and vascular occlusion of facial arteries, which include tissue ischemia, necrosis, visual abnormalities, blindness, and stroke. While these complications are considered rare, there is actually little information on their prevalence, Dr. Jones pointed out. “We think there is massive underreporting … so we view it as just the tip of the iceberg.”
Almost 200 unique cases of injection-related visual compromise (IRVC) have been reported in the literature, but not enough to provide strong evidence-based treatment protocols, he said.
“There are really no randomized clinical trials on how to treat them – they’re just not common enough – but there’s still some very good evidence that points the way. Most of what we’re relying on is in that low- to moderate-certainty range, but nevertheless, it points in a direction most of us can hang our hat on.”
He described the two most important cornerstones for preventing vascular occlusion. The first, he said, is “having impeccable knowledge and understanding of vascular anatomy and the cutaneous landmarks for blood vessels that are at risk.” The second pertains to injection techniques, he said, noting that “it’s becoming clearer – although it’s somewhat controversial – that cannulas are safer than needles.”
While anatomical knowledge might seem like the most basic requirement for practitioners who inject fillers, Dr. Jones said it is not. “It is true that we study anatomy in great detail in medical school, but injection anatomy is a completely different bird. Of course someone can study the facial arteries and have a basic understanding, but understanding it in a way that it relates to safe filler injections is a completely different thing. Our understanding of this has evolved over the last couple of decades ... and there are new papers that come out all the time that refine our knowledge.”
In terms of treating an IRVC resulting from hyaluronic acid (HA) filler, “the take-home point is the hyaluronidase is the mainstay of treatment, and not a little bit of hyaluronidase, but a lot of hyaluronidase – hundreds of units injected into the area of ischemia,” he said. However, while the new guidelines emphasize that time is of the essence – “the most cited window of time for reperfusion is 90 minutes” – the authors also strongly advise practitioners to evaluate immediate post-event visual status first, before attempting any intervention.
Dr. Jones said the goal is to untangle some confusion about whether it is the filler or the rescue that does more damage. “There is a lot of controversy with ophthalmology, ophthalmologic surgeons, plastic surgeons, and dermatologists,” he said, and “there has been finger-pointing between specialists when people make an intervention … that the actual rescue procedure created the problem,” which is why “it is imperative to document the visual status prior to doing anything.”
Beyond IRVC, and nonvisual skin ischemia due to vascular occlusion, the document addresses prevention and treatment of nodules, both inflammatory and noninflammatory, that occur either early or more than a month after treatment with HA fillers, as well with semi-permanent and permanent fillers.
For HA-related nodules, “the mainstay of treatment is steroids – either oral or intralesional – antibiotics, and hyaluronidase, which erases the substance,” said Dr. Jones. As for nodules related to permanent fillers, he said that they are difficult to treat, and “tend to respond best to repetitive monthly injections of 5-fluououracil combined with small amounts of triamcinolone.”
Dr. Jones is an investigator or consultant for Allergan, Galderma, Merz, and Revance; other authors had disclosures that included serving as a consultant, investigator, and/or trainer for these and/or other companies; one author received partial funding from ASDS to do this work; and one author had no disclosures.
The .
The recommendations, published in the February issue of Dermatologic Surgery, are from a multidisciplinary task force convened by the ASDS, comprising 11 physicians – 8 board-certified in dermatology, 2 board-certified in plastic and reconstructive surgery, and 1 board-certified in oculoplastic surgery (all ASDS members) – and 2 patient representatives.
While redness, swelling, and other injection site reactions associated with injectable fillers are common, and usually resolve within 1-2 weeks, “rare but more serious adverse events from injectable fillers include vascular occlusion leading to skin necrosis or blindness, inflammatory events, and nodule formation, among others,” the authors wrote. They are “likely underreported” and cases are increasing as injectable fillers become more popular.
“Truthfully, a lot of people don’t know what they’re doing,” lead author Derek Jones, MD, of Skin Care and Laser Physicians of Beverly Hills, Los Angeles, said in an interview. Worldwide, he said that the absolute number of injectable filler treatments “has exploded,” particularly in the United States – and over the last decade. Moreover, “a lot of these treatments are being relegated to the nurse practitioner or the registered nurse who may take a weekend course,” he added. “There is this lack of knowledge and lack of recognition of some of these basic principles that we are publishing.”
About half of the document focuses on the potentially devastating complications of filler embolization and vascular occlusion of facial arteries, which include tissue ischemia, necrosis, visual abnormalities, blindness, and stroke. While these complications are considered rare, there is actually little information on their prevalence, Dr. Jones pointed out. “We think there is massive underreporting … so we view it as just the tip of the iceberg.”
Almost 200 unique cases of injection-related visual compromise (IRVC) have been reported in the literature, but not enough to provide strong evidence-based treatment protocols, he said.
“There are really no randomized clinical trials on how to treat them – they’re just not common enough – but there’s still some very good evidence that points the way. Most of what we’re relying on is in that low- to moderate-certainty range, but nevertheless, it points in a direction most of us can hang our hat on.”
He described the two most important cornerstones for preventing vascular occlusion. The first, he said, is “having impeccable knowledge and understanding of vascular anatomy and the cutaneous landmarks for blood vessels that are at risk.” The second pertains to injection techniques, he said, noting that “it’s becoming clearer – although it’s somewhat controversial – that cannulas are safer than needles.”
While anatomical knowledge might seem like the most basic requirement for practitioners who inject fillers, Dr. Jones said it is not. “It is true that we study anatomy in great detail in medical school, but injection anatomy is a completely different bird. Of course someone can study the facial arteries and have a basic understanding, but understanding it in a way that it relates to safe filler injections is a completely different thing. Our understanding of this has evolved over the last couple of decades ... and there are new papers that come out all the time that refine our knowledge.”
In terms of treating an IRVC resulting from hyaluronic acid (HA) filler, “the take-home point is the hyaluronidase is the mainstay of treatment, and not a little bit of hyaluronidase, but a lot of hyaluronidase – hundreds of units injected into the area of ischemia,” he said. However, while the new guidelines emphasize that time is of the essence – “the most cited window of time for reperfusion is 90 minutes” – the authors also strongly advise practitioners to evaluate immediate post-event visual status first, before attempting any intervention.
Dr. Jones said the goal is to untangle some confusion about whether it is the filler or the rescue that does more damage. “There is a lot of controversy with ophthalmology, ophthalmologic surgeons, plastic surgeons, and dermatologists,” he said, and “there has been finger-pointing between specialists when people make an intervention … that the actual rescue procedure created the problem,” which is why “it is imperative to document the visual status prior to doing anything.”
Beyond IRVC, and nonvisual skin ischemia due to vascular occlusion, the document addresses prevention and treatment of nodules, both inflammatory and noninflammatory, that occur either early or more than a month after treatment with HA fillers, as well with semi-permanent and permanent fillers.
For HA-related nodules, “the mainstay of treatment is steroids – either oral or intralesional – antibiotics, and hyaluronidase, which erases the substance,” said Dr. Jones. As for nodules related to permanent fillers, he said that they are difficult to treat, and “tend to respond best to repetitive monthly injections of 5-fluououracil combined with small amounts of triamcinolone.”
Dr. Jones is an investigator or consultant for Allergan, Galderma, Merz, and Revance; other authors had disclosures that included serving as a consultant, investigator, and/or trainer for these and/or other companies; one author received partial funding from ASDS to do this work; and one author had no disclosures.
Less pain, same gain with tirbanibulin for actinic keratosis
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
“with transient local reactions,” according to the results of two identically designed trials.
However, the results, assessed at day 57 and out to 1 year of follow-up, were associated with recurrence of lesions at 1 year, noted lead author Andrew Blauvelt, MD, president of the Oregon Medical Research Center, Portland, and colleagues.
“The incidence of recurrence with conventional treatment has ranged from 20% to 96%,” they noted. “Among patients who had complete clearance at day 57 in the current trials, the estimated incidence of recurrence of previously cleared lesions was 47% at 1 year.” At 1 year, they added, “the estimated incidence of any lesions (new or recurrent) within the application area was 73%” and the estimate of sustained complete clearance was 27%.
A total of 700 adults completed the two multicenter, double-blind, parallel-group, vehicle-controlled trials, conducted concurrently between September 2017 and April 2019 at 62 U.S. sites. The results were published in the New England Journal of Medicine.
To be eligible, patients, mostly White men, had to have four to eight clinically typical, visible, and discrete AK lesions on the face or scalp within a contiguous area measuring 25 cm2. They were randomly assigned to treatment with either tirbanibulin 1% ointment or vehicle ointment (containing monoglycerides, diglycerides, and propylene glycol), which they applied once daily to the entire contiguous area for 5 days.
Pooled data across the two trials showed that the primary outcome, complete clearance of all lesions at day 57, occurred in 49% of the tirbanibulin groups versus 9% of the vehicle groups, and partial clearance (the secondary outcome) occurred in 72% versus 18% respectively. For both outcomes, and in both trials, all results were statistically significant.
Of the 174 patients who received tirbanibulin and had complete clearance, 124 had one or more lesions develop within the application area during follow-up, the authors reported. Of these, 58% had recurrences, while 42% had new lesions.
While individual AK lesions are typically treated with cryosurgery, the study authors noted that treatment of multiple lesions involves topical agents, such as fluorouracil, diclofenac, imiquimod, or ingenol mebutate, and photodynamic therapy, some of which have to be administered over periods of weeks or months and “may be associated with local reactions of pain, irritation, erosions, ulcerations, and irreversible skin changes of pigmentation and scarring,” which may reduce adherence.
In contrast, the current studies showed the most common local reactions to tirbanibulin were erythema in 91% of patients and flaking or scaling in 82%, with transient adverse events including application-site pain in 10% and pruritus in 9%.
“Unlike with most topical treatments for actinic keratosis ... severe local reactions, including vesiculation or pustulation and erosion or ulceration, were infrequent with tirbanibulin ointment,” the authors noted. “This could be due to the relatively short, 5-day course of once-daily treatment.”
They concluded that “larger and longer trials are necessary to determine the effects and risks” of treatment with tirbanibulin for treating AK.
Tirbanibulin, a synthetic inhibitor of tubulin polymerization and Src kinase signaling, was approved by the Food and Drug Administration in December 2020, for the topical treatment of AK of the face or scalp.
Asked to comment on the findings, Neal Bhatia, MD, a dermatologist and researcher at Therapeutics Dermatology, San Diego, who was not involved with the study, said that “a treatment with a 5-day course and excellent tolerability will make dermatologists rethink the old practice of ‘freeze and go.’ ”
In an interview, he added, “tirbanibulin comes to the U.S. market for treating AKs at a great time, as ingenol mebutate has been withdrawn and the others are not widely supported. The mechanism of promoting apoptosis and inducing cell cycle arrest directly correlates to the local skin reaction profile of less crusting, vesiculation, and overall signs of skin necrosis as compared to [5-fluorouracil] and ingenol mebutate, which work via that pathway. As a result, there is a direct impact on the hyperproliferation of atypical keratinocytes that will treat visible and subclinical disease.”
“The ointment vehicle is also novel as previous therapies have been in either creams or gels,” he said.
The two trials were funded by tirbanibulin manufacturer Athenex. Dr. Blauvelt reported receiving consulting fees from Athenex and other pharmaceutical companies, including Almirall, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Dermavant Sciences. Other author disclosures included serving as a consultant to Athenex and other companies. Several authors are Athenex employees. Dr. Bhatia disclosed that he is an adviser and consultant for Almirall and has been an investigator for multiple other AK treatments.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Psoriasis registry study finds normal pregnancy outcomes
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
according to one of the largest studies to examine the issue to date.
However, “pregnancy-specific registries that include a larger number of pregnant women with psoriasis ... are needed to more fully characterize the association between psoriasis and treatment and birth outcomes,” acknowledged first author Alexa B. Kimball, MD, MPH, professor of dermatology, Harvard Medical School, Boston, and colleagues.
The cohort study, published in JAMA Dermatology, used data from the Psoriasis Longitudinal Assessment and Registry (PSOLAR), which “is not a pregnancy specific registry, and medical history is captured only at baseline,” they noted.
Their findings showed pregnancy outcomes such as spontaneous abortion, neonatal problems, and congenital anomalies among women with moderate to severe psoriasis were similar to rates in the general U.S. population, and are “consistent with previously reported data,” they reported. “And pregnancy outcomes for women exposed to biologics were similar to those for women with exposure to nonbiologics.”
The study “provides further reassurance that the biologics appear safe at least related to pregnancy outcomes,” commented Jenny Murase, MD, associate professor of dermatology at the University of California, San Francisco, who was not involved in the study. In an interview, she noted that the study “did not examine any potential immunosuppression of the fetus in the first 6 months of life,” which she described as “the heart of the concern, more than whether or not the psoriasis or the biologic affects the pregnancy itself.”
The study used data from the PSOLAR registry collected from June 20, 2007, to Aug.23, 2019, which included 2,224 women of childbearing age (18-45 years) who were collectively followed up for 12,929 patient-years. Among these women, 220 had 298 pregnancies, with 244 live births (81.9%).
“Birth outcomes among all 244 births included 231 healthy newborns (94.7%), 10 infants with a neonatal problem (4.1%), 1 stillbirth (0.4%), and 2 congenital anomalies (0.8%),” the authors reported.
There were also 41 spontaneous abortions (13.8%), and 13 elective terminations (4.4%). “No elective terminations were known to derive from a congenital anomaly or other medical issue,” they added.
Among the documented pregnancies, 252 occurred in women with exposure to biologic therapy either before or during pregnancy, including 168 (56.4%) during the prenatal period, while 46 pregnancies occurred in women with no exposure to biologic therapy.
Dr. Murase, director of medical consultative dermatology for the Palo Alto Foundation Medical Group in Mountain View, Calif., said that a more detailed comparison of the different psoriasis treatments, as well as the offspring outcomes during the first 6 months of life, might offer some further important insight,.
Infants born after exposure to infliximab “and potentially other anti–tumor necrosis factor–alpha agents during the third trimester may be unable to develop an appropriate immune response to live vaccines,” she and her coauthors cautioned in a letter published in 2011, which referred to a case of an infant with disseminated bacillus Calmette-Guérin infection, whose mother had received infliximab for Crohn’s disease throughout pregnancy.
Dr. Murase pointed out that, in the registry study, exposures to certolizumab, which is pegylated and does not cross the placental barrier, were not separated from other cases. It is important to consider “the cross over late in the second trimester and especially third trimester as the infant is getting the ‘antibody boost’ from the mother as it gets ready to set foot in this world and needs the maternal antibodies to prepare its immune system. If the IgG biologics cross third trimester and immunosuppress the infant ... then I think a medication that does not cross the placental barrier is important to consider.”
The study was sponsored by Janssen Scientific Affairs. Dr. Kimball’s disclosures included serving as a consultant and investigator for companies that included AbbVie, Bristol-Myers Squibb, and Janssen; several other authors also had disclosures related to multiple pharmaceutical companies. Dr. Murase’s disclosures included serving as a consultant for Dermira, UCB Pharma, Sanofi, Ferndale, and Regeneron.
FROM JAMA DERMATOLOGY
Are more female physicians leaving medicine as pandemic surges?
For mid-career oncologist Tanya Wildes, MD, the pandemic was the last straw. In late September, she tweeted: “I have done the academically unfathomable: I am resigning my faculty position without another job lined up.”
She wasn’t burned out, she insisted. She loved her patients and her research. But she was also “100% confident” in her decision and “also 100% sad. This did not have to happen,” she lamented, asking not to disclose her workplace for fear of retribution.
Being a woman in medicine “is a hard life to start with,” Dr. Wildes said in an interview. “We all have that tenuous balance going on and the pandemic made everything just a little bit harder.”
She describes her prepandemic work-life balance as a “Jenga tower, with everything only just in place.” But she realized that the balance had tipped, when after a difficult clinic she felt emotionally wrung out. Her 11-year-old son had asked her to help him fly his model airplane. “I told him, ‘Honey, I can’t do it because if it crashes or gets stuck in a tree ... you’re going to be devastated and I have nothing left for you.’ “
This was a eureka moment, as “I realized, this is not who I want to be,” she said, holding back tears. “Seventy years from now my son is going to tell his grandchildren about the pandemic and I don’t want his memory of his mom to be that she couldn’t be there for him because she was too spent.”
When Dr. Wildes shared her story on Twitter, other female oncologists and physicians responded that they too have felt they’re under increased pressure this year, with the extra stress of the pandemic leading others to quit as well.
The trend of doctors leaving medicine has been noticeable. A July survey from the Physicians Foundation found that roughly 16,000 medical practices had already closed during the pandemic, with another 8,000 predicted to close within the next year.
“Similar patterns” were evident in another analysis by the Larry A. Green Center and the Primary Care Collaborative, as reported in The New York Times. In that survey, nearly one-fifth of primary care clinicians said “someone in their practice plans to retire early or has already retired because of COVID-19,” and 15% say “someone has left or plans to leave the practice.” About half said their mental exhaustion was at an all-time high, the survey found.
“COVID-19 is a burden, and that added burden has tipped people over the edge of many things,” said Monica Bertagnolli, MD, chief of the division of surgical oncology at Brigham and Women’s Hospital, Boston, and former president of the American Society of Clinical Oncology.
“It has illustrated that we do have a lot of people who are working kind of on the edge of not being able to handle everything,” she said.
While many in medicine are struggling, the pandemic seems to be pushing more women to leave, highlighting longtime gender disparities and increased caregiving burdens. And their absence may be felt for years to come.
Firm numbers are hard to come by, said Julie Silver, MD, associate professor, associate chair, and director of cancer rehabilitation in the department of physical medicine and rehabilitation at Harvard Medical School, Boston, and an expert in gender equity in medicine. But she sees some troubling trends.
“There are many indications that women are leaving medicine in disproportionately high numbers,” Dr. Silver said in an interview. “A lack of fair pay and promotion opportunities that were present before COVID-19 are now combined with a host of pandemic-related challenges.”
A survey of 1,809 women conducted in mid-April with the Physician Moms Facebook Group and accepted for online publication by the American Journal of Psychiatry found that 41% scored over the cutoff points for moderate or severe anxiety, with 46% meeting these criteria among front-line workers.
“It’s really important for society to recognize the extraordinary impact this pandemic is having on physician mothers, as there will be profound ripple effects on the ability of this key segment of the health care workforce to serve others if we do not address this problem urgently,” co-senior author Reshma Jagsi, MD, DPhil, a radiation oncologist at the University of Michigan, Ann Arbor, said in an interview.
Women weighed in on Twitter, in response to Dr. Silver’s tweet to #WomenInMedicine: “If you are thinking of leaving #medicine & need a reason to stay: we value you & need you.”
In reply, Emmy Betz, MD, MPH, associate professor of emergency medicine at the University of Colorado at Denver, Aurora, said via Twitter, “I’ve had lots of conversations with women considering leaving medicine.”
“I have thought about leaving many times. I love what I do, but medicine can be an unkind world at times,” responded Valerie Fitzhugh MD, associate professor and pathologist at New Jersey Medical School, Newark.
“Too late. Left at the end of July and it was the best decision ever,” wrote Michelle Gordon, DO, who was previously a board-certified general surgeon at Northern Westchester Surgical Associates in Putnam Valley, N.Y.
Prepandemic disparities accentuated
The pandemic “has merely accentuated – or made more apparent – some of the longstanding issues and struggles of women in oncology, women in medicine, women in academia,” said Sarah Holstein, MD, PhD, another mid-career oncologist and associate professor at the University of Nebraska Medical Center, Omaha.
“There are disparities in first-author/last-author publications, disparities in being asked to give speaking engagements, disparities in leadership,” Dr. Holstein said in an interview. “And then ... put on top of that the various surges with the pandemic where you are being asked to do clinical responsibilities you don’t normally do, perhaps some things you haven’t done since your training 10 or 20 years ago.”
This is backed up with data: There is already a “robust” body of prepandemic literature demonstrating pay gaps for female physicians and scientists, noted Dr. Silver, who founded the Her Time Is Now campaign for gender equity in medicine and runs a women’s leadership course at Harvard.
In addition, female physicians are more likely to be involved in “nonpromotable” work, group projects and educator roles that are often underappreciated and undercompensated, she said.
Writing recently in a blog post for the BMJ, Dr. Silver and colleagues predict that as a result of the pandemic, female physicians will “face disadvantages from unconscious bias in decisions about whose pay should be cut, whose operating schedules should take priority when resources are limited, and whose contributions merit retention ... The ground that women lose now will likely have a profound effect for many years to come, perhaps putting them at a disadvantage for the rest of their careers.”
There is already evidence of reduced publishing by female scientists during the pandemic, something that “could undermine the careers of an entire generation of women scholars,” noted Caitlyn Collins, PhD, assistant professor of sociology at Washington University in St. Louis.
“Science needs to address the culture of overwork,” Dr. Collins said in an interview. “Parents and other caregivers deserve support. The stress and ‘overwhelm’ they feel is not inevitable. A more fair, just, and humane approach to combining work and family is possible – what we need is the political will to pass better policies and a massive shift in our cultural understandings about how work should fit into family life, not the other way around.”
Lack of support for “vulnerable scientists,” particularly “junior scientists who are parents, women, or minorities” could lead to “severe attrition in cancer research in the coming years,” Cullen Taniguchi, MD, PhD, a radiation oncologist and associate professor at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, warned in a recent letter to the journal Cancer Cell.
“The biggest worries of attrition will come from young faculty who started just before or after the pandemic,” Dr. Taniguchi said in an interview. “The first year in an academic setting is incredibly challenging but also important for establishing research efforts and building networks of colleagues to collaborate with. While completely necessary, the restrictions put in place during the pandemic made doing these things even more difficult.”
Another stressor: Caregiving at home
Another reason female physicians may be marginalized during the pandemic is that they are more often the primary caregivers at home.
“Anyone who is a caregiver, be it to kids, parents, or spouses, can relate to the challenges brought [on] by the pandemic,” said Ishwaria Subbiah, MD, a palliative care physician and medical oncologist at MD Anderson.
“Most of us work toward meeting our responsibilities by engaging a network of support, whether it’s home care workers, center-based or at-home child care, schools, or activities outside of school. The pandemic led to a level of disruption that brought most (if not all) of those responsibilities onto the caregivers themselves,” she said in an interview.
As the mother of an adult son with severe epilepsy, Dr. Bertagnolli has certainly experienced the challenges of parenting during the pandemic. “Our son is now 24 but he is handicapped, and lives with us. The care issues we have to deal with as professionals have been enormously magnified by COVID,” she said.
But she cautions against making gender distinctions when it comes to caregiving. “Has it fallen on the women? Well, this kind of stuff generally falls on the women, but I am certain it has fallen on an awful lot of men as well, because I think the world is changing that way, so it’s fallen on all of us.”
There is no question that female oncologists are bearing the brunt, both at work and at home, contended Dr. Taniguchi. “Absolutely. I have seen this first-hand,” he said.
“If it was difficult for women, underrepresented minorities, and junior faculty to find a voice in the room prepandemic, I think it can be harder in the times of virtual meetings when it is difficult to engage audiences,” he said.
Dr. Holstein said she is lucky to be well-supported at her institution, with both a female chief of hematology/oncology and a female chair of internal medicine, but still, she worries about the long-term consequences of the pandemic on the gender landscape of medicine.
“If you’re having to put aside research projects because you have extra responsibilities – again because women just tend to have a lot of other things going on – that might not be a big deal for 3 months, 6 months, but this is going to be a year or 2 years before ‘normal’ comes back,” she says. “One to two years of underpublishing or not getting the grants could be career killers for women in academic oncology.”
Cancer COVID-19 combo
As Dr. Wildes completed her final weeks of seeing cancer patients, she received an outpouring of support, which she says convinced her of the shared experience of all doctors, and especially female doctors, during the pandemic. But even more specifically, she feels that she has tapped into the unique burden shouldered by oncologists during this time.
“It’s intimidating being an oncologist; we are literally giving people poison for a living. Then throw into it a pandemic where early in March we had so little data. I was helping my patients make decisions about their cancer care based on a case series of four patients in China. The burden of those conversations is something I never want to have to live through again,” she said.
“Oncology is a particularly intense subspecialty within medicine,” agreed Dr. Subbiah. “The people we care for have received a life-altering and potentially life-limiting diagnosis. Coupled with that, the COVID-19 pandemic has brought an unprecedented cloud of uncertainty ... Whether the patients can see it overtly or not, oncologists carry the weight of this worry with them for not just one but all of their patients.”
Dr. Wildes said she plans to return to academic medicine and clinical care “in time,” but for now, the gap that she and others like her leave is troubling to those who have stayed on.
“We need these women in medicine,” said Dr. Holstein. “We have data suggesting that women take more time with their patients than men, that patient outcomes are better if they have a female physician. But also for the generations coming up, we need the mid-career and senior women to be in place to mentor and guide and make sure we continue to increase women in leadership.”
A version of this article originally appeared on Medscape.com.
For mid-career oncologist Tanya Wildes, MD, the pandemic was the last straw. In late September, she tweeted: “I have done the academically unfathomable: I am resigning my faculty position without another job lined up.”
She wasn’t burned out, she insisted. She loved her patients and her research. But she was also “100% confident” in her decision and “also 100% sad. This did not have to happen,” she lamented, asking not to disclose her workplace for fear of retribution.
Being a woman in medicine “is a hard life to start with,” Dr. Wildes said in an interview. “We all have that tenuous balance going on and the pandemic made everything just a little bit harder.”
She describes her prepandemic work-life balance as a “Jenga tower, with everything only just in place.” But she realized that the balance had tipped, when after a difficult clinic she felt emotionally wrung out. Her 11-year-old son had asked her to help him fly his model airplane. “I told him, ‘Honey, I can’t do it because if it crashes or gets stuck in a tree ... you’re going to be devastated and I have nothing left for you.’ “
This was a eureka moment, as “I realized, this is not who I want to be,” she said, holding back tears. “Seventy years from now my son is going to tell his grandchildren about the pandemic and I don’t want his memory of his mom to be that she couldn’t be there for him because she was too spent.”
When Dr. Wildes shared her story on Twitter, other female oncologists and physicians responded that they too have felt they’re under increased pressure this year, with the extra stress of the pandemic leading others to quit as well.
The trend of doctors leaving medicine has been noticeable. A July survey from the Physicians Foundation found that roughly 16,000 medical practices had already closed during the pandemic, with another 8,000 predicted to close within the next year.
“Similar patterns” were evident in another analysis by the Larry A. Green Center and the Primary Care Collaborative, as reported in The New York Times. In that survey, nearly one-fifth of primary care clinicians said “someone in their practice plans to retire early or has already retired because of COVID-19,” and 15% say “someone has left or plans to leave the practice.” About half said their mental exhaustion was at an all-time high, the survey found.
“COVID-19 is a burden, and that added burden has tipped people over the edge of many things,” said Monica Bertagnolli, MD, chief of the division of surgical oncology at Brigham and Women’s Hospital, Boston, and former president of the American Society of Clinical Oncology.
“It has illustrated that we do have a lot of people who are working kind of on the edge of not being able to handle everything,” she said.
While many in medicine are struggling, the pandemic seems to be pushing more women to leave, highlighting longtime gender disparities and increased caregiving burdens. And their absence may be felt for years to come.
Firm numbers are hard to come by, said Julie Silver, MD, associate professor, associate chair, and director of cancer rehabilitation in the department of physical medicine and rehabilitation at Harvard Medical School, Boston, and an expert in gender equity in medicine. But she sees some troubling trends.
“There are many indications that women are leaving medicine in disproportionately high numbers,” Dr. Silver said in an interview. “A lack of fair pay and promotion opportunities that were present before COVID-19 are now combined with a host of pandemic-related challenges.”
A survey of 1,809 women conducted in mid-April with the Physician Moms Facebook Group and accepted for online publication by the American Journal of Psychiatry found that 41% scored over the cutoff points for moderate or severe anxiety, with 46% meeting these criteria among front-line workers.
“It’s really important for society to recognize the extraordinary impact this pandemic is having on physician mothers, as there will be profound ripple effects on the ability of this key segment of the health care workforce to serve others if we do not address this problem urgently,” co-senior author Reshma Jagsi, MD, DPhil, a radiation oncologist at the University of Michigan, Ann Arbor, said in an interview.
Women weighed in on Twitter, in response to Dr. Silver’s tweet to #WomenInMedicine: “If you are thinking of leaving #medicine & need a reason to stay: we value you & need you.”
In reply, Emmy Betz, MD, MPH, associate professor of emergency medicine at the University of Colorado at Denver, Aurora, said via Twitter, “I’ve had lots of conversations with women considering leaving medicine.”
“I have thought about leaving many times. I love what I do, but medicine can be an unkind world at times,” responded Valerie Fitzhugh MD, associate professor and pathologist at New Jersey Medical School, Newark.
“Too late. Left at the end of July and it was the best decision ever,” wrote Michelle Gordon, DO, who was previously a board-certified general surgeon at Northern Westchester Surgical Associates in Putnam Valley, N.Y.
Prepandemic disparities accentuated
The pandemic “has merely accentuated – or made more apparent – some of the longstanding issues and struggles of women in oncology, women in medicine, women in academia,” said Sarah Holstein, MD, PhD, another mid-career oncologist and associate professor at the University of Nebraska Medical Center, Omaha.
“There are disparities in first-author/last-author publications, disparities in being asked to give speaking engagements, disparities in leadership,” Dr. Holstein said in an interview. “And then ... put on top of that the various surges with the pandemic where you are being asked to do clinical responsibilities you don’t normally do, perhaps some things you haven’t done since your training 10 or 20 years ago.”
This is backed up with data: There is already a “robust” body of prepandemic literature demonstrating pay gaps for female physicians and scientists, noted Dr. Silver, who founded the Her Time Is Now campaign for gender equity in medicine and runs a women’s leadership course at Harvard.
In addition, female physicians are more likely to be involved in “nonpromotable” work, group projects and educator roles that are often underappreciated and undercompensated, she said.
Writing recently in a blog post for the BMJ, Dr. Silver and colleagues predict that as a result of the pandemic, female physicians will “face disadvantages from unconscious bias in decisions about whose pay should be cut, whose operating schedules should take priority when resources are limited, and whose contributions merit retention ... The ground that women lose now will likely have a profound effect for many years to come, perhaps putting them at a disadvantage for the rest of their careers.”
There is already evidence of reduced publishing by female scientists during the pandemic, something that “could undermine the careers of an entire generation of women scholars,” noted Caitlyn Collins, PhD, assistant professor of sociology at Washington University in St. Louis.
“Science needs to address the culture of overwork,” Dr. Collins said in an interview. “Parents and other caregivers deserve support. The stress and ‘overwhelm’ they feel is not inevitable. A more fair, just, and humane approach to combining work and family is possible – what we need is the political will to pass better policies and a massive shift in our cultural understandings about how work should fit into family life, not the other way around.”
Lack of support for “vulnerable scientists,” particularly “junior scientists who are parents, women, or minorities” could lead to “severe attrition in cancer research in the coming years,” Cullen Taniguchi, MD, PhD, a radiation oncologist and associate professor at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, warned in a recent letter to the journal Cancer Cell.
“The biggest worries of attrition will come from young faculty who started just before or after the pandemic,” Dr. Taniguchi said in an interview. “The first year in an academic setting is incredibly challenging but also important for establishing research efforts and building networks of colleagues to collaborate with. While completely necessary, the restrictions put in place during the pandemic made doing these things even more difficult.”
Another stressor: Caregiving at home
Another reason female physicians may be marginalized during the pandemic is that they are more often the primary caregivers at home.
“Anyone who is a caregiver, be it to kids, parents, or spouses, can relate to the challenges brought [on] by the pandemic,” said Ishwaria Subbiah, MD, a palliative care physician and medical oncologist at MD Anderson.
“Most of us work toward meeting our responsibilities by engaging a network of support, whether it’s home care workers, center-based or at-home child care, schools, or activities outside of school. The pandemic led to a level of disruption that brought most (if not all) of those responsibilities onto the caregivers themselves,” she said in an interview.
As the mother of an adult son with severe epilepsy, Dr. Bertagnolli has certainly experienced the challenges of parenting during the pandemic. “Our son is now 24 but he is handicapped, and lives with us. The care issues we have to deal with as professionals have been enormously magnified by COVID,” she said.
But she cautions against making gender distinctions when it comes to caregiving. “Has it fallen on the women? Well, this kind of stuff generally falls on the women, but I am certain it has fallen on an awful lot of men as well, because I think the world is changing that way, so it’s fallen on all of us.”
There is no question that female oncologists are bearing the brunt, both at work and at home, contended Dr. Taniguchi. “Absolutely. I have seen this first-hand,” he said.
“If it was difficult for women, underrepresented minorities, and junior faculty to find a voice in the room prepandemic, I think it can be harder in the times of virtual meetings when it is difficult to engage audiences,” he said.
Dr. Holstein said she is lucky to be well-supported at her institution, with both a female chief of hematology/oncology and a female chair of internal medicine, but still, she worries about the long-term consequences of the pandemic on the gender landscape of medicine.
“If you’re having to put aside research projects because you have extra responsibilities – again because women just tend to have a lot of other things going on – that might not be a big deal for 3 months, 6 months, but this is going to be a year or 2 years before ‘normal’ comes back,” she says. “One to two years of underpublishing or not getting the grants could be career killers for women in academic oncology.”
Cancer COVID-19 combo
As Dr. Wildes completed her final weeks of seeing cancer patients, she received an outpouring of support, which she says convinced her of the shared experience of all doctors, and especially female doctors, during the pandemic. But even more specifically, she feels that she has tapped into the unique burden shouldered by oncologists during this time.
“It’s intimidating being an oncologist; we are literally giving people poison for a living. Then throw into it a pandemic where early in March we had so little data. I was helping my patients make decisions about their cancer care based on a case series of four patients in China. The burden of those conversations is something I never want to have to live through again,” she said.
“Oncology is a particularly intense subspecialty within medicine,” agreed Dr. Subbiah. “The people we care for have received a life-altering and potentially life-limiting diagnosis. Coupled with that, the COVID-19 pandemic has brought an unprecedented cloud of uncertainty ... Whether the patients can see it overtly or not, oncologists carry the weight of this worry with them for not just one but all of their patients.”
Dr. Wildes said she plans to return to academic medicine and clinical care “in time,” but for now, the gap that she and others like her leave is troubling to those who have stayed on.
“We need these women in medicine,” said Dr. Holstein. “We have data suggesting that women take more time with their patients than men, that patient outcomes are better if they have a female physician. But also for the generations coming up, we need the mid-career and senior women to be in place to mentor and guide and make sure we continue to increase women in leadership.”
A version of this article originally appeared on Medscape.com.
For mid-career oncologist Tanya Wildes, MD, the pandemic was the last straw. In late September, she tweeted: “I have done the academically unfathomable: I am resigning my faculty position without another job lined up.”
She wasn’t burned out, she insisted. She loved her patients and her research. But she was also “100% confident” in her decision and “also 100% sad. This did not have to happen,” she lamented, asking not to disclose her workplace for fear of retribution.
Being a woman in medicine “is a hard life to start with,” Dr. Wildes said in an interview. “We all have that tenuous balance going on and the pandemic made everything just a little bit harder.”
She describes her prepandemic work-life balance as a “Jenga tower, with everything only just in place.” But she realized that the balance had tipped, when after a difficult clinic she felt emotionally wrung out. Her 11-year-old son had asked her to help him fly his model airplane. “I told him, ‘Honey, I can’t do it because if it crashes or gets stuck in a tree ... you’re going to be devastated and I have nothing left for you.’ “
This was a eureka moment, as “I realized, this is not who I want to be,” she said, holding back tears. “Seventy years from now my son is going to tell his grandchildren about the pandemic and I don’t want his memory of his mom to be that she couldn’t be there for him because she was too spent.”
When Dr. Wildes shared her story on Twitter, other female oncologists and physicians responded that they too have felt they’re under increased pressure this year, with the extra stress of the pandemic leading others to quit as well.
The trend of doctors leaving medicine has been noticeable. A July survey from the Physicians Foundation found that roughly 16,000 medical practices had already closed during the pandemic, with another 8,000 predicted to close within the next year.
“Similar patterns” were evident in another analysis by the Larry A. Green Center and the Primary Care Collaborative, as reported in The New York Times. In that survey, nearly one-fifth of primary care clinicians said “someone in their practice plans to retire early or has already retired because of COVID-19,” and 15% say “someone has left or plans to leave the practice.” About half said their mental exhaustion was at an all-time high, the survey found.
“COVID-19 is a burden, and that added burden has tipped people over the edge of many things,” said Monica Bertagnolli, MD, chief of the division of surgical oncology at Brigham and Women’s Hospital, Boston, and former president of the American Society of Clinical Oncology.
“It has illustrated that we do have a lot of people who are working kind of on the edge of not being able to handle everything,” she said.
While many in medicine are struggling, the pandemic seems to be pushing more women to leave, highlighting longtime gender disparities and increased caregiving burdens. And their absence may be felt for years to come.
Firm numbers are hard to come by, said Julie Silver, MD, associate professor, associate chair, and director of cancer rehabilitation in the department of physical medicine and rehabilitation at Harvard Medical School, Boston, and an expert in gender equity in medicine. But she sees some troubling trends.
“There are many indications that women are leaving medicine in disproportionately high numbers,” Dr. Silver said in an interview. “A lack of fair pay and promotion opportunities that were present before COVID-19 are now combined with a host of pandemic-related challenges.”
A survey of 1,809 women conducted in mid-April with the Physician Moms Facebook Group and accepted for online publication by the American Journal of Psychiatry found that 41% scored over the cutoff points for moderate or severe anxiety, with 46% meeting these criteria among front-line workers.
“It’s really important for society to recognize the extraordinary impact this pandemic is having on physician mothers, as there will be profound ripple effects on the ability of this key segment of the health care workforce to serve others if we do not address this problem urgently,” co-senior author Reshma Jagsi, MD, DPhil, a radiation oncologist at the University of Michigan, Ann Arbor, said in an interview.
Women weighed in on Twitter, in response to Dr. Silver’s tweet to #WomenInMedicine: “If you are thinking of leaving #medicine & need a reason to stay: we value you & need you.”
In reply, Emmy Betz, MD, MPH, associate professor of emergency medicine at the University of Colorado at Denver, Aurora, said via Twitter, “I’ve had lots of conversations with women considering leaving medicine.”
“I have thought about leaving many times. I love what I do, but medicine can be an unkind world at times,” responded Valerie Fitzhugh MD, associate professor and pathologist at New Jersey Medical School, Newark.
“Too late. Left at the end of July and it was the best decision ever,” wrote Michelle Gordon, DO, who was previously a board-certified general surgeon at Northern Westchester Surgical Associates in Putnam Valley, N.Y.
Prepandemic disparities accentuated
The pandemic “has merely accentuated – or made more apparent – some of the longstanding issues and struggles of women in oncology, women in medicine, women in academia,” said Sarah Holstein, MD, PhD, another mid-career oncologist and associate professor at the University of Nebraska Medical Center, Omaha.
“There are disparities in first-author/last-author publications, disparities in being asked to give speaking engagements, disparities in leadership,” Dr. Holstein said in an interview. “And then ... put on top of that the various surges with the pandemic where you are being asked to do clinical responsibilities you don’t normally do, perhaps some things you haven’t done since your training 10 or 20 years ago.”
This is backed up with data: There is already a “robust” body of prepandemic literature demonstrating pay gaps for female physicians and scientists, noted Dr. Silver, who founded the Her Time Is Now campaign for gender equity in medicine and runs a women’s leadership course at Harvard.
In addition, female physicians are more likely to be involved in “nonpromotable” work, group projects and educator roles that are often underappreciated and undercompensated, she said.
Writing recently in a blog post for the BMJ, Dr. Silver and colleagues predict that as a result of the pandemic, female physicians will “face disadvantages from unconscious bias in decisions about whose pay should be cut, whose operating schedules should take priority when resources are limited, and whose contributions merit retention ... The ground that women lose now will likely have a profound effect for many years to come, perhaps putting them at a disadvantage for the rest of their careers.”
There is already evidence of reduced publishing by female scientists during the pandemic, something that “could undermine the careers of an entire generation of women scholars,” noted Caitlyn Collins, PhD, assistant professor of sociology at Washington University in St. Louis.
“Science needs to address the culture of overwork,” Dr. Collins said in an interview. “Parents and other caregivers deserve support. The stress and ‘overwhelm’ they feel is not inevitable. A more fair, just, and humane approach to combining work and family is possible – what we need is the political will to pass better policies and a massive shift in our cultural understandings about how work should fit into family life, not the other way around.”
Lack of support for “vulnerable scientists,” particularly “junior scientists who are parents, women, or minorities” could lead to “severe attrition in cancer research in the coming years,” Cullen Taniguchi, MD, PhD, a radiation oncologist and associate professor at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, warned in a recent letter to the journal Cancer Cell.
“The biggest worries of attrition will come from young faculty who started just before or after the pandemic,” Dr. Taniguchi said in an interview. “The first year in an academic setting is incredibly challenging but also important for establishing research efforts and building networks of colleagues to collaborate with. While completely necessary, the restrictions put in place during the pandemic made doing these things even more difficult.”
Another stressor: Caregiving at home
Another reason female physicians may be marginalized during the pandemic is that they are more often the primary caregivers at home.
“Anyone who is a caregiver, be it to kids, parents, or spouses, can relate to the challenges brought [on] by the pandemic,” said Ishwaria Subbiah, MD, a palliative care physician and medical oncologist at MD Anderson.
“Most of us work toward meeting our responsibilities by engaging a network of support, whether it’s home care workers, center-based or at-home child care, schools, or activities outside of school. The pandemic led to a level of disruption that brought most (if not all) of those responsibilities onto the caregivers themselves,” she said in an interview.
As the mother of an adult son with severe epilepsy, Dr. Bertagnolli has certainly experienced the challenges of parenting during the pandemic. “Our son is now 24 but he is handicapped, and lives with us. The care issues we have to deal with as professionals have been enormously magnified by COVID,” she said.
But she cautions against making gender distinctions when it comes to caregiving. “Has it fallen on the women? Well, this kind of stuff generally falls on the women, but I am certain it has fallen on an awful lot of men as well, because I think the world is changing that way, so it’s fallen on all of us.”
There is no question that female oncologists are bearing the brunt, both at work and at home, contended Dr. Taniguchi. “Absolutely. I have seen this first-hand,” he said.
“If it was difficult for women, underrepresented minorities, and junior faculty to find a voice in the room prepandemic, I think it can be harder in the times of virtual meetings when it is difficult to engage audiences,” he said.
Dr. Holstein said she is lucky to be well-supported at her institution, with both a female chief of hematology/oncology and a female chair of internal medicine, but still, she worries about the long-term consequences of the pandemic on the gender landscape of medicine.
“If you’re having to put aside research projects because you have extra responsibilities – again because women just tend to have a lot of other things going on – that might not be a big deal for 3 months, 6 months, but this is going to be a year or 2 years before ‘normal’ comes back,” she says. “One to two years of underpublishing or not getting the grants could be career killers for women in academic oncology.”
Cancer COVID-19 combo
As Dr. Wildes completed her final weeks of seeing cancer patients, she received an outpouring of support, which she says convinced her of the shared experience of all doctors, and especially female doctors, during the pandemic. But even more specifically, she feels that she has tapped into the unique burden shouldered by oncologists during this time.
“It’s intimidating being an oncologist; we are literally giving people poison for a living. Then throw into it a pandemic where early in March we had so little data. I was helping my patients make decisions about their cancer care based on a case series of four patients in China. The burden of those conversations is something I never want to have to live through again,” she said.
“Oncology is a particularly intense subspecialty within medicine,” agreed Dr. Subbiah. “The people we care for have received a life-altering and potentially life-limiting diagnosis. Coupled with that, the COVID-19 pandemic has brought an unprecedented cloud of uncertainty ... Whether the patients can see it overtly or not, oncologists carry the weight of this worry with them for not just one but all of their patients.”
Dr. Wildes said she plans to return to academic medicine and clinical care “in time,” but for now, the gap that she and others like her leave is troubling to those who have stayed on.
“We need these women in medicine,” said Dr. Holstein. “We have data suggesting that women take more time with their patients than men, that patient outcomes are better if they have a female physician. But also for the generations coming up, we need the mid-career and senior women to be in place to mentor and guide and make sure we continue to increase women in leadership.”
A version of this article originally appeared on Medscape.com.
Semaglutide shows promise in NASH phase 2 study
according to a phase 2, double-blind, randomized, placebo-controlled trial published in the New England Journal of Medicine and presented at the 2020 American Association for the Study of Liver Diseases (AASLD) meeting.
“This bodes well for further study of semaglutide and is supported further by marked improvements in weight, glycemic control and lipid profile,” commented the study’s senior author Philip N. Newsome, PhD, FRCPE, of the University of Birmingham (England), in an interview.
The highest daily dose (0.4 mg) of the glucagonlike peptide-1 (GLP-1) receptor agonist, semaglutide, which is approved for the treatment of type 2 diabetes, led to levels of NASH resolution “which are higher than any previously demonstrated,” noted Dr. Newsome. “This was also accompanied by improvement in noninvasive markers of liver fibrosis and also less fibrosis progression, compared to placebo.”
“I think this represents an exciting advance and will, if confirmed in further studies, mark a step-change in our management of patients with NASH,” he added.
The multicenter study, conducted at 143 sites in 16 countries, included 320 patients, aged 18-75 years, with or without type 2 diabetes, who had histologic evidence of NASH and stage 1-3 liver fibrosis.
They were randomized in a 3:3:3:1:1:1 ratio to receive once-daily subcutaneous semaglutide at a dose of 0.1, 0.2, or 0.4 mg, or placebo for 72 weeks.
The primary endpoint was resolution of NASH and no worsening of fibrosis, with a secondary endpoint being improvement of fibrosis by at least one stage without worsening of NASH.
The study found 40% of patients in the 0.1-mg semaglutide group, 36% in the 0.2-mg group, and 59% in the 0.4-mg group achieved NASH resolution with no worsening of fibrosis, compared with 17% of the placebo group (odds ratio, 6.87; P < .001 for the highest semaglutide dose). However, the treatment did not lead to significant between-group differences in the secondary endpoint, which occurred in 43% of patients on the highest semaglutide dose compared to 33% in the placebo group (OR, 1.42; P = .48).
Treatment with semaglutide also resulted in dose-dependent reductions in body weight, as well as in glycated hemoglobin levels. Bodyweight was reduced by a mean of 5% in the 0.1-mg semaglutide group, followed by mean reductions of 9% and 13% in the 0.2-mg and 0.4-mg groups respectively. This compared to a mean reduction of 1% in the placebo group.
Similarly, glycated hemoglobin levels among patients with type 2 diabetes dropped by 0.63, 1.07, and 1.15 percentage points in the 0.1-mg, 0.2-mg, and 0.4-mg semaglutide groups respectively, compared with a drop of 0.01 percentage point in the placebo group.
“The fact that the percentage of patients who had an improvement in fibrosis stage was not significantly higher with semaglutide than with placebo – despite a greater benefit with respect to NASH resolution and dose-dependent weight loss – was unexpected, given that previous studies have suggested that resolution of NASH and improvements in activity scores for the components of nonalcoholic fatty liver disease are associated with regression of fibrosis,” wrote the authors. “However, the temporal association among NASH resolution, weight loss, and improvement in fibrosis stage is not fully understood. It is possible that the current trial was not of sufficient duration for improvements in fibrosis stage to become apparent.”
The authors also noted that the safety profile of semaglutide was “consistent with that observed in patients with type 2 diabetes in other trials and with the known effects of GLP-1 receptor agonists,” with gastrointestinal disorders being the most commonly reported.
Nausea, constipation, and vomiting were reported more often in the 0.4-mg semaglutide group than in the placebo group (nausea, 42% vs. 11%; constipation, 22% vs. 12%; and vomiting, 15% vs. 2%).
The overall incidence of benign, malignant, or unspecified neoplasms was 15% in the treatment groups versus 8% in the placebo group.
Rowen K. Zetterman, MD, who was not involved with the study, noted that “treatment of NASH is currently limited, and no therapies have yet been approved by the Food and Drug Administration.”
The findings are “important but not yet exciting,” added Dr. Zetterman, who is professor emeritus of internal medicine and associate vice chancellor for strategic planning for the University of Nebraska Medical Center, Omaha.
“Though reversal of liver fibrosis was not noted, the resolution of hepatic inflammation and liver cell injury by semaglutide suggests it may be slowing disease progression,” said Dr. Zetterman, who also serves on the editorial advisory board of Internal Medicine News. This “warrants additional studies where longer treatment with semaglutide may prove reversal of fibrosis and/or prevention of progression to cirrhosis.”
The study was sponsored by Novo Nordisk. Dr. Newsome reported disclosures related to Novo Nordisk during the conduct of the study, and to Boehringer Ingelheim, Bristol-Myers Squibb, Echosens, Gilead, Pfizer, Pharmaxis, and Poxel. Several of the other study authors reported receiving fees and grants from various pharmaceutical companies, including Novo Nordisk One author reported pending patents for the use of semaglutide. Dr. Zetterman had no relevant disclosures.
SOURCE: Newsome PN et al. N Engl J Med. 2020 Nov 13. doi: 10.1056/NEJMoa2028395.
according to a phase 2, double-blind, randomized, placebo-controlled trial published in the New England Journal of Medicine and presented at the 2020 American Association for the Study of Liver Diseases (AASLD) meeting.
“This bodes well for further study of semaglutide and is supported further by marked improvements in weight, glycemic control and lipid profile,” commented the study’s senior author Philip N. Newsome, PhD, FRCPE, of the University of Birmingham (England), in an interview.
The highest daily dose (0.4 mg) of the glucagonlike peptide-1 (GLP-1) receptor agonist, semaglutide, which is approved for the treatment of type 2 diabetes, led to levels of NASH resolution “which are higher than any previously demonstrated,” noted Dr. Newsome. “This was also accompanied by improvement in noninvasive markers of liver fibrosis and also less fibrosis progression, compared to placebo.”
“I think this represents an exciting advance and will, if confirmed in further studies, mark a step-change in our management of patients with NASH,” he added.
The multicenter study, conducted at 143 sites in 16 countries, included 320 patients, aged 18-75 years, with or without type 2 diabetes, who had histologic evidence of NASH and stage 1-3 liver fibrosis.
They were randomized in a 3:3:3:1:1:1 ratio to receive once-daily subcutaneous semaglutide at a dose of 0.1, 0.2, or 0.4 mg, or placebo for 72 weeks.
The primary endpoint was resolution of NASH and no worsening of fibrosis, with a secondary endpoint being improvement of fibrosis by at least one stage without worsening of NASH.
The study found 40% of patients in the 0.1-mg semaglutide group, 36% in the 0.2-mg group, and 59% in the 0.4-mg group achieved NASH resolution with no worsening of fibrosis, compared with 17% of the placebo group (odds ratio, 6.87; P < .001 for the highest semaglutide dose). However, the treatment did not lead to significant between-group differences in the secondary endpoint, which occurred in 43% of patients on the highest semaglutide dose compared to 33% in the placebo group (OR, 1.42; P = .48).
Treatment with semaglutide also resulted in dose-dependent reductions in body weight, as well as in glycated hemoglobin levels. Bodyweight was reduced by a mean of 5% in the 0.1-mg semaglutide group, followed by mean reductions of 9% and 13% in the 0.2-mg and 0.4-mg groups respectively. This compared to a mean reduction of 1% in the placebo group.
Similarly, glycated hemoglobin levels among patients with type 2 diabetes dropped by 0.63, 1.07, and 1.15 percentage points in the 0.1-mg, 0.2-mg, and 0.4-mg semaglutide groups respectively, compared with a drop of 0.01 percentage point in the placebo group.
“The fact that the percentage of patients who had an improvement in fibrosis stage was not significantly higher with semaglutide than with placebo – despite a greater benefit with respect to NASH resolution and dose-dependent weight loss – was unexpected, given that previous studies have suggested that resolution of NASH and improvements in activity scores for the components of nonalcoholic fatty liver disease are associated with regression of fibrosis,” wrote the authors. “However, the temporal association among NASH resolution, weight loss, and improvement in fibrosis stage is not fully understood. It is possible that the current trial was not of sufficient duration for improvements in fibrosis stage to become apparent.”
The authors also noted that the safety profile of semaglutide was “consistent with that observed in patients with type 2 diabetes in other trials and with the known effects of GLP-1 receptor agonists,” with gastrointestinal disorders being the most commonly reported.
Nausea, constipation, and vomiting were reported more often in the 0.4-mg semaglutide group than in the placebo group (nausea, 42% vs. 11%; constipation, 22% vs. 12%; and vomiting, 15% vs. 2%).
The overall incidence of benign, malignant, or unspecified neoplasms was 15% in the treatment groups versus 8% in the placebo group.
Rowen K. Zetterman, MD, who was not involved with the study, noted that “treatment of NASH is currently limited, and no therapies have yet been approved by the Food and Drug Administration.”
The findings are “important but not yet exciting,” added Dr. Zetterman, who is professor emeritus of internal medicine and associate vice chancellor for strategic planning for the University of Nebraska Medical Center, Omaha.
“Though reversal of liver fibrosis was not noted, the resolution of hepatic inflammation and liver cell injury by semaglutide suggests it may be slowing disease progression,” said Dr. Zetterman, who also serves on the editorial advisory board of Internal Medicine News. This “warrants additional studies where longer treatment with semaglutide may prove reversal of fibrosis and/or prevention of progression to cirrhosis.”
The study was sponsored by Novo Nordisk. Dr. Newsome reported disclosures related to Novo Nordisk during the conduct of the study, and to Boehringer Ingelheim, Bristol-Myers Squibb, Echosens, Gilead, Pfizer, Pharmaxis, and Poxel. Several of the other study authors reported receiving fees and grants from various pharmaceutical companies, including Novo Nordisk One author reported pending patents for the use of semaglutide. Dr. Zetterman had no relevant disclosures.
SOURCE: Newsome PN et al. N Engl J Med. 2020 Nov 13. doi: 10.1056/NEJMoa2028395.
according to a phase 2, double-blind, randomized, placebo-controlled trial published in the New England Journal of Medicine and presented at the 2020 American Association for the Study of Liver Diseases (AASLD) meeting.
“This bodes well for further study of semaglutide and is supported further by marked improvements in weight, glycemic control and lipid profile,” commented the study’s senior author Philip N. Newsome, PhD, FRCPE, of the University of Birmingham (England), in an interview.
The highest daily dose (0.4 mg) of the glucagonlike peptide-1 (GLP-1) receptor agonist, semaglutide, which is approved for the treatment of type 2 diabetes, led to levels of NASH resolution “which are higher than any previously demonstrated,” noted Dr. Newsome. “This was also accompanied by improvement in noninvasive markers of liver fibrosis and also less fibrosis progression, compared to placebo.”
“I think this represents an exciting advance and will, if confirmed in further studies, mark a step-change in our management of patients with NASH,” he added.
The multicenter study, conducted at 143 sites in 16 countries, included 320 patients, aged 18-75 years, with or without type 2 diabetes, who had histologic evidence of NASH and stage 1-3 liver fibrosis.
They were randomized in a 3:3:3:1:1:1 ratio to receive once-daily subcutaneous semaglutide at a dose of 0.1, 0.2, or 0.4 mg, or placebo for 72 weeks.
The primary endpoint was resolution of NASH and no worsening of fibrosis, with a secondary endpoint being improvement of fibrosis by at least one stage without worsening of NASH.
The study found 40% of patients in the 0.1-mg semaglutide group, 36% in the 0.2-mg group, and 59% in the 0.4-mg group achieved NASH resolution with no worsening of fibrosis, compared with 17% of the placebo group (odds ratio, 6.87; P < .001 for the highest semaglutide dose). However, the treatment did not lead to significant between-group differences in the secondary endpoint, which occurred in 43% of patients on the highest semaglutide dose compared to 33% in the placebo group (OR, 1.42; P = .48).
Treatment with semaglutide also resulted in dose-dependent reductions in body weight, as well as in glycated hemoglobin levels. Bodyweight was reduced by a mean of 5% in the 0.1-mg semaglutide group, followed by mean reductions of 9% and 13% in the 0.2-mg and 0.4-mg groups respectively. This compared to a mean reduction of 1% in the placebo group.
Similarly, glycated hemoglobin levels among patients with type 2 diabetes dropped by 0.63, 1.07, and 1.15 percentage points in the 0.1-mg, 0.2-mg, and 0.4-mg semaglutide groups respectively, compared with a drop of 0.01 percentage point in the placebo group.
“The fact that the percentage of patients who had an improvement in fibrosis stage was not significantly higher with semaglutide than with placebo – despite a greater benefit with respect to NASH resolution and dose-dependent weight loss – was unexpected, given that previous studies have suggested that resolution of NASH and improvements in activity scores for the components of nonalcoholic fatty liver disease are associated with regression of fibrosis,” wrote the authors. “However, the temporal association among NASH resolution, weight loss, and improvement in fibrosis stage is not fully understood. It is possible that the current trial was not of sufficient duration for improvements in fibrosis stage to become apparent.”
The authors also noted that the safety profile of semaglutide was “consistent with that observed in patients with type 2 diabetes in other trials and with the known effects of GLP-1 receptor agonists,” with gastrointestinal disorders being the most commonly reported.
Nausea, constipation, and vomiting were reported more often in the 0.4-mg semaglutide group than in the placebo group (nausea, 42% vs. 11%; constipation, 22% vs. 12%; and vomiting, 15% vs. 2%).
The overall incidence of benign, malignant, or unspecified neoplasms was 15% in the treatment groups versus 8% in the placebo group.
Rowen K. Zetterman, MD, who was not involved with the study, noted that “treatment of NASH is currently limited, and no therapies have yet been approved by the Food and Drug Administration.”
The findings are “important but not yet exciting,” added Dr. Zetterman, who is professor emeritus of internal medicine and associate vice chancellor for strategic planning for the University of Nebraska Medical Center, Omaha.
“Though reversal of liver fibrosis was not noted, the resolution of hepatic inflammation and liver cell injury by semaglutide suggests it may be slowing disease progression,” said Dr. Zetterman, who also serves on the editorial advisory board of Internal Medicine News. This “warrants additional studies where longer treatment with semaglutide may prove reversal of fibrosis and/or prevention of progression to cirrhosis.”
The study was sponsored by Novo Nordisk. Dr. Newsome reported disclosures related to Novo Nordisk during the conduct of the study, and to Boehringer Ingelheim, Bristol-Myers Squibb, Echosens, Gilead, Pfizer, Pharmaxis, and Poxel. Several of the other study authors reported receiving fees and grants from various pharmaceutical companies, including Novo Nordisk One author reported pending patents for the use of semaglutide. Dr. Zetterman had no relevant disclosures.
SOURCE: Newsome PN et al. N Engl J Med. 2020 Nov 13. doi: 10.1056/NEJMoa2028395.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Proposed withdrawal of approval of preterm drug: Two opposing views
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
The Oct. 5, 2020 move by the Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) suggesting the withdrawal of the approval of Makena incited some opposition.
Amag Pharmaceuticals’ 17 alpha-hydroxyprogesterone caproate (17OHP) injection received accelerated approval in 2011 to reduce the risk of recurrent preterm birth in women with previous unexplained preterm birth. Makena is the only drug approved for preventing recurrent preterm birth.
The back story
The approval was based on findings from a randomized, placebo-controlled trial that demonstrated a 34% relative risk reduction in births before 37 weeks – from 55% in the placebo arm to 36% in the 17OHP-treated arm.
The trial was not designed to measure neonatal outcomes, with the surrogate outcome of recurrent preterm birth being determined as “reasonably likely” to predict benefit to the neonate.
Subsequently, results of the required postapproval confirmatory PROLONG trialproduced conflicting results, failing to show a benefit of 17OHP on either preterm birth or neonatal outcome, which prompted the proposed withdrawal of the drug’s approval.
The CDER advisory committee agreed unanimously that the PROLONG trial did not support the clinical benefit of 17OHP, but the committee was not unanimous in deciding what to do. Of the 16 members, 9 voted to withdraw the drug’s approval, while seven voted to retain it and require another confirmatory trial.
When CDER recommends withdrawal, the company can request a public hearing, which it has done. The FDA commissioner will recommend whether to grant this request.
In the meantime, one from a group of three doctors who are against it and the other from the CDER.
Arguments from the opposing views
“We sympathize with women who are at risk for recurrent preterm birth that could result in death or significant lifelong health effects in neonates, but retaining on the market a drug not shown to be effective for this use does not protect or promote their health,” wrote Christina Chang, MD, MPH and associates from CDER.
On the other hand, “the widespread use of 17OHP after accelerated approval has not uncovered important safety signals,” countered Michael F. Greene, MD, from Massachusetts General Hospital, Boston; David Harrington, PhD, from the Harvard T. Chan School of Public Health, Boston; and Mark A. Klebanoff, MD, MPH, who was coauthor on the original preapproval study and is with Nationwide Children’s Hospital, the Ohio State University College of Medicine, and Ohio State University College of Public Health, all in Columbus. “Withdrawal of the approval for 17OHP, as imperfect as it may be, will leave a very vulnerable demographic group of U.S. women at high risk for this complication of pregnancy with absolutely no available therapeutic option.”
While both the preapproval study and postapproval PROLONG trial had the same enrollment criteria – namely women with a singleton pregnancy and previous singleton spontaneous preterm birth – all parties acknowledged that the studies ended up with very different cohorts. Approval of the drug in the United States made it difficult to recruit U.S. participants for the second trial “because of a lack of equipoise perceived by health care providers and patients,” noted Dr. Greene and associates, resulting in 75% of the PROLONG study’s cohort coming from Europe. This meant that 59% of those in the first study were non-Hispanic black compared with just 6.6% in the PROLONG study, a difference that is important because of the increased risk of preterm birth in Black women.
“Black women are generally underrepresented in U.S. clinical trials, and they are clearly underrepresented in the PROLONG study,” noted Dr. Greene and colleagues, adding that “the total number of qualifying composite neonatal outcome events among Blacks or African Americans in the entire PROLONG study population of 1,700 participants was 9 (6 of 69 in the 17OHP group and 3 of 40 in the placebo group). This is not a robust database from which to conclude that there is no effect in Black women.”
But, Dr. Chang and the CDER group argued, while the first study showed 17OHP “reduced the risk of recurrent preterm birth in both Black and non-Black participants, the lack of even a trend toward efficacy among either Black or non-Black women in [the PROLONG study] argues that the smaller proportion of Black women [in the PROLONG study] does not explain the lack of efficacy.”
In addition to race, there were other risk factors for preterm birth, such as tobacco, alcohol, and street drug use; marital status; and age that differed between the two study cohorts. Even after subcategorizing PROLONG trial participants into higher or lower risk for preterm birth based on these risk factors, Dr. Chang and associates still found no evidence of benefit to 17OHP treatment in any risk group.
Withdrawing approval of 17OHP for a recurrent preterm indication would still allow off-label prescribing, but would most likely end insurance coverage and eventually manufacturing of the drug, noted Dr. Greene and associates.
“When the majority of a population achieves little benefit from a drug, but a minority demographic group at greatest risk for a serious medical problem appears to obtain significant benefit, any decision that will ultimately make it impossible to obtain the drug should be undertaken cautiously,” they warned. “This issue is particularly pressing when that minority group may be the least able to find and financially afford work-arounds to obtain the needed medication in our complex medical system that has a history of failing to serve them well.”
Dr. Chang and associates reported they had no relevant financial disclosures. Dr. Greene and associates reported that they had no relevant conflicts of interest or financial disclosures. Dr. Greene reported he is employed by the New England Journal of Medicine as associate editor. Dr. Harrington reported being employed by the journal as statistical consultant. Dr. Klebanoff reported he was an author of the original article about 17OHP published in the journal and referenced in this article.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Study: 10% of pregnant women test positive for COVID-19, with most asymptomatic
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
according to a living systematic review from the PregCOV-19 Living Systematic Review Consortium.
The study, published in BMJ, shows an increased risk of preterm delivery, as well as the need for invasive ventilation in these women, wrote John Allotey, PhD, of the University of Birmingham (England) and colleagues. The findings “will produce a strong evidence base for living guidelines on COVID-19 and pregnancy,” they noted.
The systematic review included 77 studies, one-third each from the United States and China, with the remaining studies from Belgium, Brazil, Denmark, France, Israel, Italy, Japan, Mexico, the Netherlands Portugal, Spain, and the United Kingdom.
The studies included women with COVID-19, of whom 13,118 were either pregnant or in the postpartum or postabortion period and 83,486 were of reproductive age but not pregnant. Some studies also included healthy pregnant women for comparison.
In the pregnant and recently pregnant women, the most common COVID-19 symptoms were fever (40%) and cough (39%), with lymphopenia (35%) and raised C reactive protein levels (49%) being the most common laboratory findings. Pregnant and recently pregnant women with COVID-19 were less likely to have fever (odds ratio, 0.43) and myalgia (OR, 0.48), compared with nonpregnant women of reproductive age with COVID-19, reported the authors.
The overall preterm and spontaneous preterm birth rates in the COVID-19–positive women were 17% and 6% respectively. Dr. Allotey and authors noted that “these preterm births could be medically indicated, as the overall rates of spontaneous preterm births in pregnant women with COVID-19 was broadly similar to those observed in the pre-pandemic period.” There were 18 stillbirths and 6 neonatal deaths in the COVID-19 cohort.
Overall, 73 (0.1%) of pregnant women with confirmed COVID-19 died from any cause, and severe COVID-19 infection was diagnosed in 13%. Maternal risk factors associated with severe infection included older age (OR, 1.78), high body mass index (OR, 2.3), chronic hypertension (OR, 2.0), and preexisting diabetes (OR, 2.51). Compared with nonpregnant women with COVID-19, pregnant or recently pregnant women with the infection were at increased risk of admission to intensive care (OR, 1.62) and needing invasive ventilation (OR, 1.88).
The report included studies published between December 1, 2019, and June 26, 2020, but the living systematic review will involve weekly search updates, with analysis performed every 2-4 weeks and reported through a dedicated website.
The value of a living meta-analysis
Asked to comment on the findings, Torri Metz, MD, a maternal-fetal medicine subspecialist at the University of Utah, Salt Lake City, expressed surprise at the 10% rate of infection in the pregnant or recently pregnant population. “This is higher than currently observed at many hospitals in the United States,” she said in an interview. “This may overestimate the actual risk as many of these studies were published early in the pandemic and did not universally sample women who were pregnant for SARS-CoV-2.”
She noted the value of a living meta-analysis in that it will be updated on a regular basis as new evidence emerges. “During this time of rapidly accumulating publications about COVID-19 infection, clinicians will find it useful to have a resource in which the available data can be combined in one source.”
And there are still some outstanding questions that new studies hopefully will shed light on, she added. “The authors found that many of the risk factors for severe disease, like diabetes, obesity and high blood pressure, in nonpregnant adults are the same in the pregnant population. What remains unknown is if pregnant patients with COVID-19 infection are at higher risk than those who are not pregnant. The authors note that this information is still limited and largely influenced in this published analysis by a CDC [Centers for Disease Control and Prevention] study in which the majority of patients had unknown pregnancy status. We also do not know if COVID-19 infection is associated with any birth defects since the majority of women with COVID-19 infection in the first trimester have not yet delivered.”
Malavika Prabhu, MD, an obstetetrician/gyneologist at Weill Cornell Medicine in New York City added that “this systematic review and meta analysis, which is a compilation of other studies done around the globe, confirms that pregnant women with preexisting medical conditions such as diabetes, hypertension, and obesity, are at increased risk of severe COVID-19 and that pregnant women with COVID-19 are at increased risk of invasive ventilation, compared to nonpregnant women with COVID-19, particularly if they have a preexisting medical condition.”
She said the preterm delivery rate of COVID-positive women is “challenging to interpret given that the total preterm birth rate potentially included many medically indicated preterm deliveries – which is to be expected – and there is no comparison group for spontaneous preterm birth presented”.
Other outstanding questions about COVID-19 pregnancies include whether they are associated with preeclampsia or smaller/growth restricted infants and why the cesarean delivery rate is high, she said. “But some of these questions are tough to answer with this data because it primarily reflects a COVID infection close to the delivery, not one that occurred several months prior to a delivery.”
Deborah Money, MD, professor of obstetrics and gynecology, medicine, and the school of population and public health, University of British Columbia, Vancouver, commented that “this is a group that have been doing ongoing living systematic reviews of the literature scanning for pregnancy outcomes. They post their information in real time on their website, so many of us in this area follow these postings as their methodology is robust and they work hard to only include high-quality literature and avoid duplication of cases in multiple papers. There has been a problem of re-reporting the same severe cases of COVID-19 in the literature.”
This “amplifies the importance of collecting Canadian-specific data to ensure that we understand if these kind of outcomes will also be found in Canada. The data presented in this paper represent outcomes from a broad range of countries with different methods of collecting information on pregnancy and highly variable prenatal care systems. This makes our pan-Canadian study of outcomes of COVID-19 for pregnant women and their infants, CANCOVID-Preg, even more important,” she said.
“Globally, we all must continue to monitor outcomes of COVID-19 in pregnancy to minimize adverse impact on women and their infants,” said Dr. Money, who was not involved in the study.
The study was partially funded by the World Health Organization and supported by Katie’s Team, a dedicated patient and public involvement group in Women’s Health. Dr. Metz is principal investigator for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network COVID-19 study; the study is funded by NICHD and enrollment is ongoing. Dr. Prabhu had no relevant financial disclosures. Dr. Money received funding from the Canadian Institutes for Health Research and the Public Health Agency of Canada and received a small grant from theBC Women’s Foundation for COVID-19 in pregnancy research.
SOURCE: Allotey J et al. BMJ. 2020;370:m3320.
FROM BMJ
Tailored messaging needed to get cancer screening back on track
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
In late June, Lisa Richardson, MD, emerged from Atlanta, Georgia’s initial COVID-19 lockdown, and “got back out there” for some overdue doctor’s appointments, including a mammogram.
The mammogram was a particular priority for her, since she is director of the CDC’s Division of Cancer Prevention and Control. But she knows that cancer screening is going to be a much tougher sell for the average person going forward in the pandemic era.
“It really is a challenge trying to get people to feel comfortable coming back in to be screened,” she said. Richardson was speaking recently at the AACR virtual meeting: COVID-19 and Cancer, a virtual symposium on cancer prevention and early detection in the COVID-19 pandemic organized by the American Association for Cancer Research.
While health service shutdowns and stay-at-home orders forced the country’s initial precipitous decline in cancer screening, fear of contracting COVID-19 is a big part of what is preventing patients from returning.
“We’ve known even pre-pandemic that people were hesitant to do cancer screening and in some ways this has really given them an out to say, ‘Well, I’m going to hold off on that colonoscopy,’ ” Amy Leader, MD, from Thomas Jefferson University’s Kimmel Cancer Center in Philadelphia, Pennsylvania, said during the symposium.
Estimating the pandemic’s impact on cancer care
While the impact of the pandemic on cancer can only be estimated at the moment, the prospects are already daunting, said Richardson, speculating that the hard-won 26% drop in cancer mortality over the past two decades “may be put on hold or reversed” by COVID-19.
There could be as many as 10,000 excess deaths in the US from colorectal and breast cancer alone because of COVID-19 delays, predicted Norman E. Sharpless, director of the US National Cancer Institute in Bethesda, Maryland.
But even Sharpless acknowledges that his modeling gives a conservative estimate, “as it does not consider other cancer types, it does not account for the additional nonlethal morbidity from upstaging, and it assumes a moderate disruption in care that completely resolves after 6 months.”
With still no end to the pandemic in sight, the true scope of cancer screening and treatment disruptions will take a long time to assess, but several studies presented during the symposium revealed some early indications.
A national survey launched in mid-May, which involved 534 women either diagnosed with breast cancer or undergoing screening or diagnostic evaluation for it, found that delays in screening were reported by 31.7% of those with breast cancer, and 26.7% of those without. Additionally, 21% of those on active treatment for breast cancer reported treatment delays.
“It’s going to be really important to implement strategies to help patients return to care ... creating a culture and a feeling of safety among patients and communicating through the uncertainty that exists in the pandemic,” said study investigator Erica T. Warner, ScD MPH, from Massachusetts General Hospital, Boston.
Screening for prostate cancer (via prostate-specific antigen testing) also declined, though not as dramatically as that for breast cancer, noted Mara Epstein, ScD, from The Meyers Primary Care Institute, University of Massachusetts Medical School, Worcester. Her study at a large healthcare provider group compared rates of both screening and diagnostic mammographies, and also PSA testing, as well as breast and prostate biopsies in the first five months of 2020 vs the same months in 2019.
While a decrease from 2019 to 2020 was seen in all procedures over the entire study period, the greatest decline was seen in April for screening mammography (down 98%), and tomosynthesis (down 96%), as well as PSA testing (down 83%), she said.
More recent figures are hard to come by, but a recent weekly survey from the Primary Care Collaborative shows 46% of practices are offering preventive and chronic care management visits, but patients are not scheduling them, and 44% report that in-person visit volume is between 30%-50% below normal over the last 4 weeks.
Will COVID-19 exacerbate racial disparities in cancer?
Neither of the studies presented at the symposium analyzed cancer care disruptions by race, but there was concern among some panelists that cancer care disparities that existed before the pandemic will be magnified further.
“Over the next several months and into the next year there’s going to be some catch-up in screening and treatment, and one of my concerns is minority and underserved populations will not partake in that catch-up the way many middle-class Americans will,” said Otis Brawley, MD, from Johns Hopkins University, Baltimore, Maryland.
There is ample evidence that minority populations have been disproportionately hit by COVID-19, job losses, and lost health insurance, said the CDC’s Richardson, and all these factors could widen the cancer gap.
“It’s not a race thing, it’s a ‘what do you do thing,’ and an access to care thing, and what your socioeconomic status is,” Richardson said in an interview. “People who didn’t have sick leave before the pandemic still don’t have sick leave; if they didn’t have time to get their mammogram they still don’t have time.”
But she acknowledges that evidence is still lacking. Could some minority populations actually be less fearful of medical encounters because their work has already prevented them from sheltering in place? “It could go either way,” she said. “They might be less wary of venturing out into the clinic, but they also might reason that they’ve exposed themselves enough already at work and don’t want any additional exposure.”
In that regard, Richardson suggests population-specific messaging will be an important way of communicating with under-served populations to restart screening.
“We’re struggling at CDC with how to develop messages that resonate within different communities, because we’re missing the point of actually speaking to people within their culture and within the places that they live,” she said. “Just saying the same thing and putting a black face on it is not going to make a difference; you actually have to speak the language of the people you’re trying to reach — the same message in different packages.”
To that end, even before the pandemic, the CDC supported the development of Make It Your Own, a website that uses “evidence-based strategies” to assist healthcare organizations in customizing health information “by race, ethnicity, age, gender and location”, and target messages to “specific populations, cultural groups and languages”.
But Mass General’s Warner says she’s not sure she would argue for messages to be tailored by race, “at least not without evidence that values and priorities regarding returning to care differ between racial/ethnic groups.”
“Tailoring in the absence of data requires assumptions that may or may not be correct and ignores within-group heterogeneity,” Warner told Medscape Medical News. “However, I do believe that messaging about return to cancer screening and care should be multifaceted and use diverse imagery. This recognizes that some messages will resonate more or less with individuals based on their own characteristics, of which race may be one.”
Warner does believe in the power of tailored messaging though. “Part of the onus for healthcare institutions and providers is to make some decisions about who it is really important to bring back in soonest,” she said.
“Those are the ones we want to prioritize, as opposed to those who we want to get back into care but we don’t need to get them in right now,” Warner emphasized. “As they are balancing all the needs of their family and their community and their other needs, messaging that adds additional stress, worry, anxiety and shame is not what we want to do. So really we need to distinguish between these populations, identify the priorities, hit the hard message to people who really need it now, and encourage others to come back in as they can.”
Building trust
All the panelists agreed that building trust with the public will be key to getting cancer care back on track.
“I don’t think anyone trusts the healthcare community right now, but we already had this baseline distrust of healthcare among many minority communities, and now with COVID-19, the African American community in particular is seeing people go into the hospital and never come back,” said Richardson.
For Warner, the onus really falls on healthcare institutions. “We have to be proactive and not leave the burden of deciding when and how to return to care up to patients,” she said.
“What we need to focus on as much as possible is to get people to realize it is safe to come see the doctor,” said Johns Hopkins oncologist Brawley. “We have to make it safe for them to come see us, and then we have to convince them it is safe to come see us.”
Venturing out to her mammography appointment in early June, Richardson said she felt safe. “Everything was just the way it was supposed to be, everyone was masked, everyone was washing their hands,” she said.
Yet, by mid-June she had contracted COVID-19. “I don’t know where I got it,” she said. “No matter how careful you are, understand that if you’re in a total red spot, as I am, you can just get it.”
This article first appeared on Medscape.com.
Does stirrup choice influence vaginal surgery outcome?
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
according to the first randomized controlled trial comparing both types of lithotomy stirrups.
“Participants positioned in candy cane stirrups had greater hip abduction than those positioned in boot stirrups, which could provide a rationale for our findings,” suggested Ankita Gupta, MD, MPH, of the University of Louisville (Ky.), and colleagues. Their report is in Obstetrics & Gynecology.
But one expert questions this interpretation, calling it a major limitation of the study.
“The only difference between the two arms of the study is associated with the angles between the femurs,” said Rosanne M. Kho, MD, a gynecologic surgeon at Cleveland Clinic, who was not involved in the study. “The difference of the angles at the femur is not inherent to the type of stirrup but in the method in which the patients were positioned using the two different types of stirrups,” she said. “The same wide angle between the femurs can be attained with the boot stirrups if the patient is not positioned properly. To determine if the same benefit in physical function is achieved with a lesser angle between the femur, the investigators should use only one type of stirrup (whether the candy cane or the boot stirrups) and change only the angles of the femur.”
The study was a single-masked, randomized controlled trial of women undergoing vaginal surgery at the University of Louisville’s division of urogynecology between March 2018 and Oct. 2019. Surgeries included any combination of vaginal hysterectomy, vaginal vault suspension (uterosacral or sacrospinous ligament fixation), vaginectomy (partial or total), mid-urethral slings, or other surgeries such as urethral diverticulectomy, fistula repair, or mesh excision.
Among the 138 women included in the intention-to-treat analysis, 72 were randomized to candy cane, and 66 to boot (Yellofin) stirrups. They were positioned in the assigned stirrup by the attending surgeon, with assistance from the surgical team, after administration of anesthesia and were not informed of their allocation until the end of the study at 6 weeks post surgery.
On day 1 post surgery, a 100-point visual analog scale (VAS) questionnaire was administered for pain in the lower back, hips, buttocks, thighs, knees, calves, and feet, followed by a series of questionnaires at 6 weeks post surgery, including the PROMIS (Patient-Reported Outcomes Measurement Information System) forms on physical function, pain intensity, and pain interference, as well as the Pelvic Floor Disability Index (PFDI-20) and the Patient Global Impression of Improvement forms.
While the authors acknowledged that neurologic injuries following vaginal surgery are rare, and therefore difficult to measure, physical function is a “prudent” alternative measurement.
Although the study was designed to compare lithotomy stirrups, patient positioning also was measured. Once the patient was anesthetized, the surgeon used a goniometer to measure flexion at the hip and knee joints, the angle of abduction and external rotation at the hip. The “angle between the femurs” was measured by placing the fulcrum of the goniometer at the anal opening.
While the angles of flexion at the hips and knees were similar between groups, the study found a significant difference between groups in the angle between the femurs (mean ± standard deviation, 88.7 ± 13.4 candy cane vs. 77.2 ± 13.3 boot, P < .01).
In addition, the primary outcome, change in physical function based on the PROMIS physical function shortform-20a, was significantly different between the two groups: While subjects in the candy cane group demonstrated a decline of 1.9 in mean physical function score at 6 weeks compared to baseline, those in the boot stirrup group showed an increase of 1.9 from baseline. The mean 6-week postoperative scores were 45.8 versus 49.8 for the candy cane and boot stirrup groups respectively (P < .01).
Although it was “well executed by a well-respected group of vaginal surgeons at a major academic institution,” the study has other limitations, noted Dr. Kho.
“Though the measurements were obtained with the goniometer at the beginning of the surgery, it does not appear that a repeat measurement was performed at the end of the case. Is it possible that positioning could have shifted and resulted in further change in the angle of the femur/hip/knees compared to the beginning of the surgery?” she asked.
In addition, “compared to the candy canes, the boot stirrup has bulky boots that could limit opportunities for bedside assistants who were standing next to the primary surgeon to lean against the patient’s thighs during the surgery. Were there measures done to ensure that assistants were not leaning against the [candy cane] patients?”
In terms of the 6-week outcome measure, Dr. Kho suggested PROMIS outcomes measured at 2 weeks and at 4 or 6 weeks “would have provided greater insight to the study question.
“The authors acknowledge that neuropathies due to patient positioning manifest soon after surgery and tend to be transient. Incidence of neuropathy is extremely low in both groups and is equivalent. Factors that could impair quick return to normal activity as a result of the neuromuscular effects due to patient positioning should have been measured earlier,” she suggested.
Finally, Dr. Kho noted that the authors “fail to provide any likely rationale for the impaired physical function measured at 6 weeks that can be attributed to the difference in the angles at the femur. The findings of decreased physical function at 6 weeks in the candy cane group may be incidental, and may be different if measured at an earlier time (which would be more pertinent for this study) or at a later time such as 3 months.”
Individual authors acknowledged personal funds from Society of Gynecologic Surgeons, Elsevier publishing, RBI Medical, and AMAG Pharmaceuticals. Dr. Kho had no relevant financial disclosures.
SOURCE: Gupta A et al. Obstet Gynecol. 2020 July 8. doi: 10.1097/AOG.0000000000003954.
FROM OBSTETRICS & GYNECOLOGY
Postpartum tubal ligation safe in obese women
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
Women with a high body mass index who request tubal ligation immediately post partum face no increased risk of complications, compared with normal-weight woman, according to a large, single-institution, retrospective study.
“Our study underscores the overall safety of postpartum tubal ligation among overweight and obese women,” John J. Byrne, MD, MPH, and colleagues at the University of Texas, Dallas, reported in Obstetrics & Gynecology.
“Even among women in the highest BMI category, this procedure is safe and effective,” they noted, despite previous studies identifying body mass index (BMI) higher than 40 kg/m2 “as a significant barrier to this procedure.”
“For the woman who is appropriately counseled and desires permanent contraception, BMI should not impede her access to the procedure,” Dr. Byrne and associates said.
The study included 3,670 women undergoing postpartum tubal ligation after a vaginal delivery between August 2015 and March 2019 at Parkland Hospital, which is operated by the Dallas County Hospital District.
The method used was the Parkland-type tubal ligation – a bilateral midsegment partial salpingectomy performed through a 2-3 cm infraumbilical incision. Women were excluded if they were planning additional surgery, such as ovarian cyst removal or hernia repair at the same time.
Comparing a composite outcome of surgical complications and subsequent pregnancies over a 5-year follow-up, the study found no differences across all maternal BMI categories, which were stratified as: underweight or normal weight (BMI, 24.9 or lower), overweight (25-29.9), class I obesity (30-34.9), class II obesity (35-39.9), and class III obesity (40 or higher).
A full breakdown of the composite morbidity included “blood transfusion, aborted procedure, intraoperative complications (bleeding requiring additional surgery, extension of incision), anesthetic complication (high spinal, bronchospasm, postdural puncture headaches requiring blood patch, and allergic reaction to anesthetic), postoperative complication (deep wound infection, venous thromboembolism, ileus, small bowel obstruction, acute intestinal herniation, peritonitis), return to operating room, incomplete transection of fallopian tube, and subsequent pregnancy,” they reported.
Among the study subjects, the mean BMI was 32.2, with 263 being underweight or normal weight at the time of admission, 1,044 being overweight, 1,371 having class I obesity, 689 having class II obesity, 303 having class III obesity, and 11 patients classified as supermorbidly obese (a BMI of 50 or higher).
Overall, “composite morbidity occurred in 49 (1.3%) women and was not significantly different across BMI categories (P = .07),” noted the authors.
More specifically, there were 19 (1.5%) composite morbidity events in the nonobese cohort and 30 (1.3%) in the obese cohort. “Even among women who had undergone prior abdominal surgery, there was no association of BMI with the rate of procedural complication,” Dr. Byrne and associates added.
The subsequent pregnancy rate was 1.63 per 1,000 procedures performed, which is “significantly lower than previously reported estimates,” they noted. In total, there were six subsequent pregnancies in the cohort: three full term, two ectopic, and one of unknown location.
“Although there was variability in operative time in all BMI categories, this is likely not clinically relevant as the range in operative time overlapped across groups,” reported the authors. “Other surgical metrics, such as estimated blood loss and length of hospitalization after tubal ligation, were found to be no different between BMI categories.”
Their findings “can be generalized to other tubal ligation forms, such as modified Pomeroy and even possibly salpingectomy, if the minilaparotomy incision is the same,” Dr. Byrne and colleagues suggested.
“This innovative study adds an important practical perspective to the literature on postpartum permanent contraception – a finding that should be reassuring for obstetrician/gynecologists,” commented Eve Espey, MD MPH, who was not involved in the research.
“Women with high BMI are significantly less likely to receive desired postvaginal delivery tubal ligation, compared to lower-BMI women, as documented in several prior studies,” said Dr. Espey, who is professor and chair of the department of obstetrics and gynecology at the University of New Mexico, Albuquerque.
“Although those studies did not explore the reasons for nonfulfillment, intuitively concerns about complications or inability to complete the procedure are the most likely explanations,” she added.
“Although this study is limited by its retrospective nature, the smaller number of women in the highest BMI category, and lack of information on patients with unfulfilled requests for tubal ligation, it is overall well designed and should serve to encourage physicians to proceed with postvaginal delivery tubal ligation in patients across all BMI categories,” Dr. Espey concluded.
The study received no external funding; Dr. Byrne and associates reported no relevant financial disclosures. Dr. Espey is a member of the Ob.Gyn. News editorial advisory board, and said she has no relevant financial disclosures.
SOURCE: Byrne JJ et al. Obstet Gynecol. 2020;136:342-8.
FROM OBSTETRICS & GYNECOLOGY