User login
Disease Education
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Updates on Kidney Donation
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Kidney Donation & HIV
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Anemia, A1C, and Rhabdomyolysis
Q) Does anemia in CKD patients affect their A1C? Is A1C accurate in CKD patients?
Tight glycemic control is imperative for patients with chronic kidney disease (CKD), but the management of diabetes in CKD can be complex due to factors including anemia and changes in glucose and insulin homeostasis.
A1C is directly proportionate to the ambient blood glucose concentration and in the general diabetic population has proven to be a reliable marker.1 However, it may not be valid in patients with diabetes and CKD. Reduced red blood cell (RBC) lifespan, rapid hemolysis, and iron deficiency may lead to falsely decreased results.2 Decreased RBC survival results from an increase in hemoglobin turnover, which decreases glycemic exposure time.1 This process then lowers the amount of nonenzymatic glucose binding to hemoglobin.1 Folate deficiency caused by impaired intestinal absorption in CKD also affects RBC survival.3 Falsely increased results may be related to carbamylation of the hemoglobin and acidosis, both of which are influenced by uremia.2
Special considerations should be made for dialysis patients with diabetes. In hemodialysis patients, A1C may be falsely decreased due to blood loss, RBC transfusion, and erythropoietin therapy.3 Observational studies have shown that erythropoietin therapy is associated with lower A1C due to the increased number of immature RBCs that have a decreased glycemic exposure time.1 In peritoneal dialysis patients, A1C may increase after the start of therapy as a result of dialysate absorption.3
Research suggests that glycated albumin (GA) provides a short-term index of glycemic control (typically two to three weeks) and is not influenced by albumin concentration, RBC lifespan, or erythropoietin administration.1 A clear consensus on optimal levels of GA has not been established, but GA may be a more reliable marker of glycemic control in patients with diabetes and CKD. Further research is needed to establish a target GA level that predicts the best prognosis for patients with both conditions.1
A1C is the most reliable marker at this time, but special considerations should be made for the patient with CKD. Rather than focus on a single measurement, clinicians need to consider the patient’s symptoms and results from all labwork, along with A1C, to best evaluate glycemic control.4 Further research is needed in patients with diabetes and CKD to explore other reliable markers to help maintain tight glycemic control.
Continued on next page >>
Q) One of my patients developed severe leg cramps while taking statins. I felt it was questionable rhabdomyolysis and stopped the medication; the leg pain went away. Is there a way to know if the rhabdomyolysis is progressive?
Rhabdomyolysis is a serious condition caused by the breakdown of muscle tissue that leads to the release of myoglobin into the bloodstream. This condition can lead to severe kidney failure and death.
Previously, there has been no easy method to predict progressive rhabdomyolysis. But researchers from Brigham and Women’s Hospital recently developed the Rhabdomyolysis Risk Calculator, a prediction score that can help determine whether a patient with rhabdomyolysis is at risk for severe kidney failure or death.
The researchers conducted a retrospective cohort study of 2,371 patients admitted between 2000 and 2011 and examined variables that may be associated with kidney failure and death.5 They identified independent predictors for these outcomes, including age; gender; initial levels of phosphate, calcium, creatinine, carbon dioxide, and creatine kinase; and etiology of rhabdomyolysis (myositis, exercise, statin use, or seizure).5
This tool can assist providers in developing a patient-specific treatment plan. However, further research is needed to validate the current variables, verify the risk prediction score in other populations, and examine its ability to guide individualized treatment plans.
The Rhabdomyolysis Risk Calculator is available at www.brighamandwomens.org/research/rhabdo/default.aspx
Kristy Washinger, MSN, CRNP
Nephrology Associates of Central PA
Camp Hill, PA
REFERENCES
1. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. Nephrol Dial Transplant Plus. 2011; 4(6):368-375.
2. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Guideline 2: management of hyperglycemia and general diabetes care in chronic kidney disease. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed April 15, 2014.
3. Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21(3):383-385.
4. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
5. McMahon GM, Zeng X, Walker SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
Q) Does anemia in CKD patients affect their A1C? Is A1C accurate in CKD patients?
Tight glycemic control is imperative for patients with chronic kidney disease (CKD), but the management of diabetes in CKD can be complex due to factors including anemia and changes in glucose and insulin homeostasis.
A1C is directly proportionate to the ambient blood glucose concentration and in the general diabetic population has proven to be a reliable marker.1 However, it may not be valid in patients with diabetes and CKD. Reduced red blood cell (RBC) lifespan, rapid hemolysis, and iron deficiency may lead to falsely decreased results.2 Decreased RBC survival results from an increase in hemoglobin turnover, which decreases glycemic exposure time.1 This process then lowers the amount of nonenzymatic glucose binding to hemoglobin.1 Folate deficiency caused by impaired intestinal absorption in CKD also affects RBC survival.3 Falsely increased results may be related to carbamylation of the hemoglobin and acidosis, both of which are influenced by uremia.2
Special considerations should be made for dialysis patients with diabetes. In hemodialysis patients, A1C may be falsely decreased due to blood loss, RBC transfusion, and erythropoietin therapy.3 Observational studies have shown that erythropoietin therapy is associated with lower A1C due to the increased number of immature RBCs that have a decreased glycemic exposure time.1 In peritoneal dialysis patients, A1C may increase after the start of therapy as a result of dialysate absorption.3
Research suggests that glycated albumin (GA) provides a short-term index of glycemic control (typically two to three weeks) and is not influenced by albumin concentration, RBC lifespan, or erythropoietin administration.1 A clear consensus on optimal levels of GA has not been established, but GA may be a more reliable marker of glycemic control in patients with diabetes and CKD. Further research is needed to establish a target GA level that predicts the best prognosis for patients with both conditions.1
A1C is the most reliable marker at this time, but special considerations should be made for the patient with CKD. Rather than focus on a single measurement, clinicians need to consider the patient’s symptoms and results from all labwork, along with A1C, to best evaluate glycemic control.4 Further research is needed in patients with diabetes and CKD to explore other reliable markers to help maintain tight glycemic control.
Continued on next page >>
Q) One of my patients developed severe leg cramps while taking statins. I felt it was questionable rhabdomyolysis and stopped the medication; the leg pain went away. Is there a way to know if the rhabdomyolysis is progressive?
Rhabdomyolysis is a serious condition caused by the breakdown of muscle tissue that leads to the release of myoglobin into the bloodstream. This condition can lead to severe kidney failure and death.
Previously, there has been no easy method to predict progressive rhabdomyolysis. But researchers from Brigham and Women’s Hospital recently developed the Rhabdomyolysis Risk Calculator, a prediction score that can help determine whether a patient with rhabdomyolysis is at risk for severe kidney failure or death.
The researchers conducted a retrospective cohort study of 2,371 patients admitted between 2000 and 2011 and examined variables that may be associated with kidney failure and death.5 They identified independent predictors for these outcomes, including age; gender; initial levels of phosphate, calcium, creatinine, carbon dioxide, and creatine kinase; and etiology of rhabdomyolysis (myositis, exercise, statin use, or seizure).5
This tool can assist providers in developing a patient-specific treatment plan. However, further research is needed to validate the current variables, verify the risk prediction score in other populations, and examine its ability to guide individualized treatment plans.
The Rhabdomyolysis Risk Calculator is available at www.brighamandwomens.org/research/rhabdo/default.aspx
Kristy Washinger, MSN, CRNP
Nephrology Associates of Central PA
Camp Hill, PA
REFERENCES
1. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. Nephrol Dial Transplant Plus. 2011; 4(6):368-375.
2. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Guideline 2: management of hyperglycemia and general diabetes care in chronic kidney disease. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed April 15, 2014.
3. Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21(3):383-385.
4. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
5. McMahon GM, Zeng X, Walker SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
Q) Does anemia in CKD patients affect their A1C? Is A1C accurate in CKD patients?
Tight glycemic control is imperative for patients with chronic kidney disease (CKD), but the management of diabetes in CKD can be complex due to factors including anemia and changes in glucose and insulin homeostasis.
A1C is directly proportionate to the ambient blood glucose concentration and in the general diabetic population has proven to be a reliable marker.1 However, it may not be valid in patients with diabetes and CKD. Reduced red blood cell (RBC) lifespan, rapid hemolysis, and iron deficiency may lead to falsely decreased results.2 Decreased RBC survival results from an increase in hemoglobin turnover, which decreases glycemic exposure time.1 This process then lowers the amount of nonenzymatic glucose binding to hemoglobin.1 Folate deficiency caused by impaired intestinal absorption in CKD also affects RBC survival.3 Falsely increased results may be related to carbamylation of the hemoglobin and acidosis, both of which are influenced by uremia.2
Special considerations should be made for dialysis patients with diabetes. In hemodialysis patients, A1C may be falsely decreased due to blood loss, RBC transfusion, and erythropoietin therapy.3 Observational studies have shown that erythropoietin therapy is associated with lower A1C due to the increased number of immature RBCs that have a decreased glycemic exposure time.1 In peritoneal dialysis patients, A1C may increase after the start of therapy as a result of dialysate absorption.3
Research suggests that glycated albumin (GA) provides a short-term index of glycemic control (typically two to three weeks) and is not influenced by albumin concentration, RBC lifespan, or erythropoietin administration.1 A clear consensus on optimal levels of GA has not been established, but GA may be a more reliable marker of glycemic control in patients with diabetes and CKD. Further research is needed to establish a target GA level that predicts the best prognosis for patients with both conditions.1
A1C is the most reliable marker at this time, but special considerations should be made for the patient with CKD. Rather than focus on a single measurement, clinicians need to consider the patient’s symptoms and results from all labwork, along with A1C, to best evaluate glycemic control.4 Further research is needed in patients with diabetes and CKD to explore other reliable markers to help maintain tight glycemic control.
Continued on next page >>
Q) One of my patients developed severe leg cramps while taking statins. I felt it was questionable rhabdomyolysis and stopped the medication; the leg pain went away. Is there a way to know if the rhabdomyolysis is progressive?
Rhabdomyolysis is a serious condition caused by the breakdown of muscle tissue that leads to the release of myoglobin into the bloodstream. This condition can lead to severe kidney failure and death.
Previously, there has been no easy method to predict progressive rhabdomyolysis. But researchers from Brigham and Women’s Hospital recently developed the Rhabdomyolysis Risk Calculator, a prediction score that can help determine whether a patient with rhabdomyolysis is at risk for severe kidney failure or death.
The researchers conducted a retrospective cohort study of 2,371 patients admitted between 2000 and 2011 and examined variables that may be associated with kidney failure and death.5 They identified independent predictors for these outcomes, including age; gender; initial levels of phosphate, calcium, creatinine, carbon dioxide, and creatine kinase; and etiology of rhabdomyolysis (myositis, exercise, statin use, or seizure).5
This tool can assist providers in developing a patient-specific treatment plan. However, further research is needed to validate the current variables, verify the risk prediction score in other populations, and examine its ability to guide individualized treatment plans.
The Rhabdomyolysis Risk Calculator is available at www.brighamandwomens.org/research/rhabdo/default.aspx
Kristy Washinger, MSN, CRNP
Nephrology Associates of Central PA
Camp Hill, PA
REFERENCES
1. Vos FE, Schollum JB, Walker RJ. Glycated albumin is the preferred marker for assessing glycaemic control in advanced chronic kidney disease. Nephrol Dial Transplant Plus. 2011; 4(6):368-375.
2. National Kidney Foundation Kidney Disease Outcomes Quality Initiative. Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Guideline 2: management of hyperglycemia and general diabetes care in chronic kidney disease. www.kidney.org/professionals/kdoqi/guideline_diabetes/guide2.htm. Accessed April 15, 2014.
3. Sharif A, Baboolal K. Diagnostic application of the A1c assay in renal disease. J Am Soc Nephrol. 2010;21(3):383-385.
4. American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
5. McMahon GM, Zeng X, Walker SS. A risk prediction score for kidney failure or mortality in rhabdomyolysis. JAMA Intern Med. 2013;173(19):1821-1828.
Determining Renal Function: What Those Test Results Mean
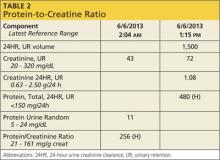
Q: Even though you suggested a random urine ACR (albumin-to-creatinine ratio), the internal medicine group ordered a 24-hour urine test for protein. As you can see from the results (see Table 2), the PCR (protein-to-creatinine ratio) is high. What does this mean? Does my patient have more severe kidney disease than I thought for her age?
Advanced age is a risk factor for CKD, and the patient has also had weight loss that can affect her serum creatinine. Because of her femur fracture, she has likely been in pain and probably has been taking nephrotoxic analgesics, such as NSAIDs or a ketorolac injection, commonly given postoperatively.
The patient’s weight does not appear to be stable, and she may have a degree of malnutrition. Both malnutrition and reduced muscle mass are known to decrease serum creatinine, which can mask worsening kidney disease. Thus she may have a lower true GFR than predicted by CG, which tends to overestimate renal function in the case of lower levels of creatinine production.6
Looking at all of these factors, it is likely that she has some degree of renal disease; however, it is important to determine if this is an acute change or a chronic issue. Looking closely at the higher-than-normal urinary protein result requires some out-of-the-box thinking.
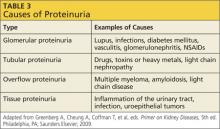
Proteinuria has four types; each indicates a particular disorder.5 Table 3 provides examples of causative factors for each type.
Based on the data provided (Table 2), you have a high urinary protein result and are unsure if it is albumin. It is important to determine if this is albumin—and therefore pathognomonic for progressive kidney disease—or if the protein is of a nonalbumin type that will require further evaluation. What started as just an elderly female with a femur fracture and decreased GFR can turn into a diagnosis of multiple myeloma (which is more common in this age-group), kidney damage from postoperative medications, or another form of kidney disease. Only by looking at urinary protein type can one “tease out” what this might be.
In conclusion, there are many different ways to determine renal function, either by creatinine clearance or by using an estimation formula. Each one, used correctly, can offer advantages in certain populations. It is extremely important to determine whether an individual has diminished kidney function in order to be able to delay the progression of CKD.
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: Even though you suggested a random urine ACR (albumin-to-creatinine ratio), the internal medicine group ordered a 24-hour urine test for protein. As you can see from the results (see Table 2), the PCR (protein-to-creatinine ratio) is high. What does this mean? Does my patient have more severe kidney disease than I thought for her age?
Advanced age is a risk factor for CKD, and the patient has also had weight loss that can affect her serum creatinine. Because of her femur fracture, she has likely been in pain and probably has been taking nephrotoxic analgesics, such as NSAIDs or a ketorolac injection, commonly given postoperatively.
The patient’s weight does not appear to be stable, and she may have a degree of malnutrition. Both malnutrition and reduced muscle mass are known to decrease serum creatinine, which can mask worsening kidney disease. Thus she may have a lower true GFR than predicted by CG, which tends to overestimate renal function in the case of lower levels of creatinine production.6
Looking at all of these factors, it is likely that she has some degree of renal disease; however, it is important to determine if this is an acute change or a chronic issue. Looking closely at the higher-than-normal urinary protein result requires some out-of-the-box thinking.
Proteinuria has four types; each indicates a particular disorder.5 Table 3 provides examples of causative factors for each type.
Based on the data provided (Table 2), you have a high urinary protein result and are unsure if it is albumin. It is important to determine if this is albumin—and therefore pathognomonic for progressive kidney disease—or if the protein is of a nonalbumin type that will require further evaluation. What started as just an elderly female with a femur fracture and decreased GFR can turn into a diagnosis of multiple myeloma (which is more common in this age-group), kidney damage from postoperative medications, or another form of kidney disease. Only by looking at urinary protein type can one “tease out” what this might be.
In conclusion, there are many different ways to determine renal function, either by creatinine clearance or by using an estimation formula. Each one, used correctly, can offer advantages in certain populations. It is extremely important to determine whether an individual has diminished kidney function in order to be able to delay the progression of CKD.
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: Even though you suggested a random urine ACR (albumin-to-creatinine ratio), the internal medicine group ordered a 24-hour urine test for protein. As you can see from the results (see Table 2), the PCR (protein-to-creatinine ratio) is high. What does this mean? Does my patient have more severe kidney disease than I thought for her age?
Advanced age is a risk factor for CKD, and the patient has also had weight loss that can affect her serum creatinine. Because of her femur fracture, she has likely been in pain and probably has been taking nephrotoxic analgesics, such as NSAIDs or a ketorolac injection, commonly given postoperatively.
The patient’s weight does not appear to be stable, and she may have a degree of malnutrition. Both malnutrition and reduced muscle mass are known to decrease serum creatinine, which can mask worsening kidney disease. Thus she may have a lower true GFR than predicted by CG, which tends to overestimate renal function in the case of lower levels of creatinine production.6
Looking at all of these factors, it is likely that she has some degree of renal disease; however, it is important to determine if this is an acute change or a chronic issue. Looking closely at the higher-than-normal urinary protein result requires some out-of-the-box thinking.
Proteinuria has four types; each indicates a particular disorder.5 Table 3 provides examples of causative factors for each type.
Based on the data provided (Table 2), you have a high urinary protein result and are unsure if it is albumin. It is important to determine if this is albumin—and therefore pathognomonic for progressive kidney disease—or if the protein is of a nonalbumin type that will require further evaluation. What started as just an elderly female with a femur fracture and decreased GFR can turn into a diagnosis of multiple myeloma (which is more common in this age-group), kidney damage from postoperative medications, or another form of kidney disease. Only by looking at urinary protein type can one “tease out” what this might be.
In conclusion, there are many different ways to determine renal function, either by creatinine clearance or by using an estimation formula. Each one, used correctly, can offer advantages in certain populations. It is extremely important to determine whether an individual has diminished kidney function in order to be able to delay the progression of CKD.
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Determining Renal Function: What’s the Best Way to Evaluate?
Q: One of my patients is a 72-year-old woman who weighs 59 kg. Her creatinine clearance by Cockcroft-Gault (CG) came back low (49 mL/min). Is this due to her age, gender, and weight loss during the past five months (subsequent to a femur fracture), or does she have underlying kidney disease? Would a 24-hour urine creatinine test be the best way to determine her level of kidney function—and would it be appropriate for someone her age? Is there a better way to evaluate her kidney function?
Accurate measurement of renal function is vital for any patient suspected of having chronic kidney disease (CKD). More than 20 million adults in the United States, or more than 10% of the adult population, have CKD.1 The 2012 US Renal Data System (USRDS) Annual Data Report states that the prevalence of chronic kidney disease in the Medicare population alone rose more than three-fold between 2000 and 2010, from 2.7% to 9.2%.2
CKD consumes a large proportion of Medicare dollars: more than $23,000 per person per year (PPPY) annually. For end-stage renal disease (ESRD) patients on hemodialysis, the cost is an astounding $88,000 PPPY.2 The cost of treating 871,000 ESRD patients was more than $40 billion in both public and private funds in 2009.3
Risk factors for CKD include but are not limited to: advancing age, male sex, race, hypertension, diabetes mellitus, smoking, family history of kidney disease, proteinuria, exposure to nephrotoxins, and atherosclerosis.4
In the US, the most common methods used to estimate renal function are the CG (Cockcroft-Gault) equation, Modification of Diet in Renal Disease (MDRD) study equations, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It is often difficult to determine which test is best suited for a patient, because there are pros and cons to each formula and no one test is perfectly suited for every clinical application.4
Since we know this patient’s renal function is low via CG (49 mL/min), the next important question to ask is, “Is it progressive?” I would recommend obtaining a urinalysis to look for hematuria and albuminuria. Proteinuria is an all-encompassing term. Albumin is only one type of protein and is the single most predictive risk factor for kidney disease progression. Persistent albuminuria alone is diagnostic of renal disease.5 The recommended test is a random urine albumin-to-creatinine ratio (ACR; see Table 1).6
You asked if a 24-hour urine creatinine clearance might evaluate her renal function better. Creatinine clearance can be determined by a 24-hour urine test and a serum blood sample in a steady state. However, this test should be interpreted with caution due to both collection errors and the fact that creatinine clearance overestimates true glomerular filtration rate (GFR) due to tubular secretion of creatinine.7,8 Thus, this test is no longer routinely recommended to determine kidney function.8
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: One of my patients is a 72-year-old woman who weighs 59 kg. Her creatinine clearance by Cockcroft-Gault (CG) came back low (49 mL/min). Is this due to her age, gender, and weight loss during the past five months (subsequent to a femur fracture), or does she have underlying kidney disease? Would a 24-hour urine creatinine test be the best way to determine her level of kidney function—and would it be appropriate for someone her age? Is there a better way to evaluate her kidney function?
Accurate measurement of renal function is vital for any patient suspected of having chronic kidney disease (CKD). More than 20 million adults in the United States, or more than 10% of the adult population, have CKD.1 The 2012 US Renal Data System (USRDS) Annual Data Report states that the prevalence of chronic kidney disease in the Medicare population alone rose more than three-fold between 2000 and 2010, from 2.7% to 9.2%.2
CKD consumes a large proportion of Medicare dollars: more than $23,000 per person per year (PPPY) annually. For end-stage renal disease (ESRD) patients on hemodialysis, the cost is an astounding $88,000 PPPY.2 The cost of treating 871,000 ESRD patients was more than $40 billion in both public and private funds in 2009.3
Risk factors for CKD include but are not limited to: advancing age, male sex, race, hypertension, diabetes mellitus, smoking, family history of kidney disease, proteinuria, exposure to nephrotoxins, and atherosclerosis.4
In the US, the most common methods used to estimate renal function are the CG (Cockcroft-Gault) equation, Modification of Diet in Renal Disease (MDRD) study equations, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It is often difficult to determine which test is best suited for a patient, because there are pros and cons to each formula and no one test is perfectly suited for every clinical application.4
Since we know this patient’s renal function is low via CG (49 mL/min), the next important question to ask is, “Is it progressive?” I would recommend obtaining a urinalysis to look for hematuria and albuminuria. Proteinuria is an all-encompassing term. Albumin is only one type of protein and is the single most predictive risk factor for kidney disease progression. Persistent albuminuria alone is diagnostic of renal disease.5 The recommended test is a random urine albumin-to-creatinine ratio (ACR; see Table 1).6
You asked if a 24-hour urine creatinine clearance might evaluate her renal function better. Creatinine clearance can be determined by a 24-hour urine test and a serum blood sample in a steady state. However, this test should be interpreted with caution due to both collection errors and the fact that creatinine clearance overestimates true glomerular filtration rate (GFR) due to tubular secretion of creatinine.7,8 Thus, this test is no longer routinely recommended to determine kidney function.8
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Q: One of my patients is a 72-year-old woman who weighs 59 kg. Her creatinine clearance by Cockcroft-Gault (CG) came back low (49 mL/min). Is this due to her age, gender, and weight loss during the past five months (subsequent to a femur fracture), or does she have underlying kidney disease? Would a 24-hour urine creatinine test be the best way to determine her level of kidney function—and would it be appropriate for someone her age? Is there a better way to evaluate her kidney function?
Accurate measurement of renal function is vital for any patient suspected of having chronic kidney disease (CKD). More than 20 million adults in the United States, or more than 10% of the adult population, have CKD.1 The 2012 US Renal Data System (USRDS) Annual Data Report states that the prevalence of chronic kidney disease in the Medicare population alone rose more than three-fold between 2000 and 2010, from 2.7% to 9.2%.2
CKD consumes a large proportion of Medicare dollars: more than $23,000 per person per year (PPPY) annually. For end-stage renal disease (ESRD) patients on hemodialysis, the cost is an astounding $88,000 PPPY.2 The cost of treating 871,000 ESRD patients was more than $40 billion in both public and private funds in 2009.3
Risk factors for CKD include but are not limited to: advancing age, male sex, race, hypertension, diabetes mellitus, smoking, family history of kidney disease, proteinuria, exposure to nephrotoxins, and atherosclerosis.4
In the US, the most common methods used to estimate renal function are the CG (Cockcroft-Gault) equation, Modification of Diet in Renal Disease (MDRD) study equations, and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. It is often difficult to determine which test is best suited for a patient, because there are pros and cons to each formula and no one test is perfectly suited for every clinical application.4
Since we know this patient’s renal function is low via CG (49 mL/min), the next important question to ask is, “Is it progressive?” I would recommend obtaining a urinalysis to look for hematuria and albuminuria. Proteinuria is an all-encompassing term. Albumin is only one type of protein and is the single most predictive risk factor for kidney disease progression. Persistent albuminuria alone is diagnostic of renal disease.5 The recommended test is a random urine albumin-to-creatinine ratio (ACR; see Table 1).6
You asked if a 24-hour urine creatinine clearance might evaluate her renal function better. Creatinine clearance can be determined by a 24-hour urine test and a serum blood sample in a steady state. However, this test should be interpreted with caution due to both collection errors and the fact that creatinine clearance overestimates true glomerular filtration rate (GFR) due to tubular secretion of creatinine.7,8 Thus, this test is no longer routinely recommended to determine kidney function.8
Catherine B. York, MSN, APRN-BC
Springfield Nephrology
Associates, Springfield, MO
References
1. CDC. National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, 2010. Atlanta, GA: US Department of Health and Human Services, CDC; 2010.
2. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
3. US Renal Data System. USRDS 2012 annual data report: atlas of end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011.
4. Clarkson MR, Brenner BM. Clinical assessment of the patient with kidney disease. In: Clarkson MR, Brenner BM. Pocket Companion to Brenner & Rector’s The Kidney. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005: 3-19.5.
5. Hsu C. Clinical evaluation of kidney function. In: Greenberg A, Cheung A, Coffman T, et al, eds. Primer on Kidney Diseases, 5th ed. Philadelphia, PA; Saunders Elsevier; 2009:19-237.
6. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1-150.
7. National Kidney Foundation. Guideline 5: assessment of proteinuria. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification; 2000.
8. Stevens LA, Coresh J, Greene T, et al. Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483.
Why Take This Patient Off Her ACEI?
Q: I sent a 68-year-old, hypertensive, diabetic woman with stage 4 kidney disease (estimated glomerular filtration rate [eGFR], 25 mL/min/1.73 m2; serum creatinine [SCr], 2 mg/mL) to a local nephrology group. Since she was diabetic, we had had her taking an angiotensin-converting enzyme inhibitor (ACEI) for years. The first thing the nephrology group did was take her off the ACEI. Why would they do that? I thought the hypertensive drug of choice for all diabetic patients is an ACEI or an angiotensin receptor blocker (ARB). Am I wrong?
A: The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of blood pressure and intravascular volume through its effects on renin, angiotensin, and aldosterone production. Activation of RAAS causes an increase in blood pressure through vasoconstriction (angiotensin II effects) and the fluid retention associated with reabsorption of sodium and water (aldosterone effects). As such, the physiologic effects of RAAS have been implicated in the pathophysiology of cardiovascular diseases, such as heart failure, kidney disease, and hypertension.1
The JNC 7 guidelines2 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) recommend the use of ACEIs or ARBs for the treatment of hypertension in patients with chronic kidney disease (CKD) or diabetes to slow progression of kidney disease. Given the benefits of ACEI or ARB therapy in reducing mortality in cardiovascular disease (for which patients with CKD are at increased risk3), these medications are indicated in patients with CKD.
Angiotensin II causes systemic vasoconstriction and also acts on the efferent arterioles of the glomerulus. ACEI/ARB therapy causes vasodilation of the efferent arterioles, thus lowering the intraglomerular capillary pressure.1 This mechanism accounts for the antiproteinuric effect and the subsequent decrease in GFR when these agents are initiated.
Proteinuria is a marker that may indicate nephropathy and may lead to further kidney damage.1 Slowing renal disease progression depends on controlling blood pressure and proteinuria.3
To date, a number of trials have been conducted to explore the benefit of the antiproteinuric effects of ACEI/ARB therapy. The antiproteinuric effect is more pronounced in patients with more severe proteinuria. Studies of these agents in patients with diabetic and nondiabetic nephropathy have shown that besides reducing proteinuria, they slow progression to end-stage renal disease (ESRD), thus delaying the need for renal replacement therapy.4 As such, ACEI/ARB therapy is widely used to reduce proteinuria, independent of the blood pressure–lowering effects.
Initiation of ACEI/ARB therapy is associated with an increase in SCr due to the drugs’ effects on the efferent arterioles, resulting in a decrease in intraglomerular pressure.3 However, discontinuing therapy is not warranted unless the SCr rises to more than 30% above baseline. According to findings from one meta-analysis, the degree of loss in renal function when ACEI therapy was initiated was inversely related to the rate of annual decline in renal function.5 Thus, patients with higher SCr levels at the start of therapy had poorer renal function initially, but they received the greatest benefit in long-term renal preservation.
The rise in SCr typically occurs within a few days of therapy initiation; thus, SCr should be measured within the first seven days of therapy.3 The SCr level is expected to stabilize within six to eight weeks of therapy. Patients whose SCr level continues to rise more than 30% to 35% above baseline may need to discontinue ACEI/ARB therapy; this rise may be attributed to kidney hypoperfusion. Kidney hypoperfusion can also occur when diuretics are initiated or their dosage increased, when NSAIDs are used, or in patients with bilateral renal artery stenosis or volume depletion resulting from gastroenteritis.3
Hyperkalemia due to decreased urinary excretion of potassium (K+) may present yet another reason to discontinue ACEI/ARB therapy in patients with CKD. Incidence of hyperkalemia in those with CKD stages 3 through 5 who receive either an ACEI or an ARB ranges from 5% to 50%.3 Hyperkalemia can occur when a long-acting ACEI is prescribed or when ACEI/ARB therapy is used concurrently with NSAIDs or potassium-sparing diuretics; thiazides or loop diuretics, by contrast, can reduce the risk for hyperkalemia.
Risk factors for moderate hyperkalemia (serum K+ ≥ 5.6 mmol/L) include age older than 65, congestive heart failure, SCr level greater than 1.6 mg/dL, and a blood urea nitrogen level exceeding 18 mg/dL.5
A dosing reduction or discontinuation of ACEI/ARB therapy should be considered when serum K+ levels are 5.6 mmol/L or greater.3 Patients who are older than 70 or whose serum urea nitrogen level exceeds 25 mg/dL are at increased risk for severe hyperkalemia (K+ > 6.0 mmol/L). Termination of an ACEI or an ARB may be warranted in patients with a serum K+ level exceeding 6.0 mmol/L or in those considered at increased risk for severe hyperkalemia.3
Other patients may also benefit from discontinuing ACEI/ARB therapy. Few data exist to support their use in patients with kidney function at 25% or less, or those with a GFR below 30 mL/min/1.73 m2.3 Onuigbo6 suggests that ESRD in patients with CKD can either progress gradually and steadily or rapidly as a result of acute kidney injury (AKI). Patients with CKD who are at risk for ESRD because of AKI include those currently taking an ACEI or an ARB, older patients, and those who experience unexplained decreases in GFR.
To avoid any worsening of renal function, Onuigbo6 suggests temporarily discontinuing ACEI/ARB therapy in the following patients:
• Those older than 65 who are scheduled for colonoscopy, administration of IV radiocontrast, or surgery (especially cardiovascular surgery); or
• Patients hospitalized for an acute ailment.
Discontinuing these agents may prevent progression to ESRD in such patients.
In summary, use of ACEIs or ARBs to delay progression of renal disease may be continued if a clear therapeutic benefit exists. However, discontinuation should be considered if a patient with CKD is at risk for hyperkalemia or if the K+ level remains higher than 5.6 mmol/L, if SCr levels increase more than 30% above baseline, or if patients (especially those older than 65) are at risk for AKI.
References
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-S65.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168-175.
4. St Peter WL, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013 Apr 9.
5. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
6. Onuigbo MA. Is renoprotection with RAAS blockade a failed paradigm? Have we learnt any lessons so far? Int J Clin Pract. 2010;64:1341-1346.
Q: I sent a 68-year-old, hypertensive, diabetic woman with stage 4 kidney disease (estimated glomerular filtration rate [eGFR], 25 mL/min/1.73 m2; serum creatinine [SCr], 2 mg/mL) to a local nephrology group. Since she was diabetic, we had had her taking an angiotensin-converting enzyme inhibitor (ACEI) for years. The first thing the nephrology group did was take her off the ACEI. Why would they do that? I thought the hypertensive drug of choice for all diabetic patients is an ACEI or an angiotensin receptor blocker (ARB). Am I wrong?
A: The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of blood pressure and intravascular volume through its effects on renin, angiotensin, and aldosterone production. Activation of RAAS causes an increase in blood pressure through vasoconstriction (angiotensin II effects) and the fluid retention associated with reabsorption of sodium and water (aldosterone effects). As such, the physiologic effects of RAAS have been implicated in the pathophysiology of cardiovascular diseases, such as heart failure, kidney disease, and hypertension.1
The JNC 7 guidelines2 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) recommend the use of ACEIs or ARBs for the treatment of hypertension in patients with chronic kidney disease (CKD) or diabetes to slow progression of kidney disease. Given the benefits of ACEI or ARB therapy in reducing mortality in cardiovascular disease (for which patients with CKD are at increased risk3), these medications are indicated in patients with CKD.
Angiotensin II causes systemic vasoconstriction and also acts on the efferent arterioles of the glomerulus. ACEI/ARB therapy causes vasodilation of the efferent arterioles, thus lowering the intraglomerular capillary pressure.1 This mechanism accounts for the antiproteinuric effect and the subsequent decrease in GFR when these agents are initiated.
Proteinuria is a marker that may indicate nephropathy and may lead to further kidney damage.1 Slowing renal disease progression depends on controlling blood pressure and proteinuria.3
To date, a number of trials have been conducted to explore the benefit of the antiproteinuric effects of ACEI/ARB therapy. The antiproteinuric effect is more pronounced in patients with more severe proteinuria. Studies of these agents in patients with diabetic and nondiabetic nephropathy have shown that besides reducing proteinuria, they slow progression to end-stage renal disease (ESRD), thus delaying the need for renal replacement therapy.4 As such, ACEI/ARB therapy is widely used to reduce proteinuria, independent of the blood pressure–lowering effects.
Initiation of ACEI/ARB therapy is associated with an increase in SCr due to the drugs’ effects on the efferent arterioles, resulting in a decrease in intraglomerular pressure.3 However, discontinuing therapy is not warranted unless the SCr rises to more than 30% above baseline. According to findings from one meta-analysis, the degree of loss in renal function when ACEI therapy was initiated was inversely related to the rate of annual decline in renal function.5 Thus, patients with higher SCr levels at the start of therapy had poorer renal function initially, but they received the greatest benefit in long-term renal preservation.
The rise in SCr typically occurs within a few days of therapy initiation; thus, SCr should be measured within the first seven days of therapy.3 The SCr level is expected to stabilize within six to eight weeks of therapy. Patients whose SCr level continues to rise more than 30% to 35% above baseline may need to discontinue ACEI/ARB therapy; this rise may be attributed to kidney hypoperfusion. Kidney hypoperfusion can also occur when diuretics are initiated or their dosage increased, when NSAIDs are used, or in patients with bilateral renal artery stenosis or volume depletion resulting from gastroenteritis.3
Hyperkalemia due to decreased urinary excretion of potassium (K+) may present yet another reason to discontinue ACEI/ARB therapy in patients with CKD. Incidence of hyperkalemia in those with CKD stages 3 through 5 who receive either an ACEI or an ARB ranges from 5% to 50%.3 Hyperkalemia can occur when a long-acting ACEI is prescribed or when ACEI/ARB therapy is used concurrently with NSAIDs or potassium-sparing diuretics; thiazides or loop diuretics, by contrast, can reduce the risk for hyperkalemia.
Risk factors for moderate hyperkalemia (serum K+ ≥ 5.6 mmol/L) include age older than 65, congestive heart failure, SCr level greater than 1.6 mg/dL, and a blood urea nitrogen level exceeding 18 mg/dL.5
A dosing reduction or discontinuation of ACEI/ARB therapy should be considered when serum K+ levels are 5.6 mmol/L or greater.3 Patients who are older than 70 or whose serum urea nitrogen level exceeds 25 mg/dL are at increased risk for severe hyperkalemia (K+ > 6.0 mmol/L). Termination of an ACEI or an ARB may be warranted in patients with a serum K+ level exceeding 6.0 mmol/L or in those considered at increased risk for severe hyperkalemia.3
Other patients may also benefit from discontinuing ACEI/ARB therapy. Few data exist to support their use in patients with kidney function at 25% or less, or those with a GFR below 30 mL/min/1.73 m2.3 Onuigbo6 suggests that ESRD in patients with CKD can either progress gradually and steadily or rapidly as a result of acute kidney injury (AKI). Patients with CKD who are at risk for ESRD because of AKI include those currently taking an ACEI or an ARB, older patients, and those who experience unexplained decreases in GFR.
To avoid any worsening of renal function, Onuigbo6 suggests temporarily discontinuing ACEI/ARB therapy in the following patients:
• Those older than 65 who are scheduled for colonoscopy, administration of IV radiocontrast, or surgery (especially cardiovascular surgery); or
• Patients hospitalized for an acute ailment.
Discontinuing these agents may prevent progression to ESRD in such patients.
In summary, use of ACEIs or ARBs to delay progression of renal disease may be continued if a clear therapeutic benefit exists. However, discontinuation should be considered if a patient with CKD is at risk for hyperkalemia or if the K+ level remains higher than 5.6 mmol/L, if SCr levels increase more than 30% above baseline, or if patients (especially those older than 65) are at risk for AKI.
References
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-S65.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168-175.
4. St Peter WL, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013 Apr 9.
5. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
6. Onuigbo MA. Is renoprotection with RAAS blockade a failed paradigm? Have we learnt any lessons so far? Int J Clin Pract. 2010;64:1341-1346.
Q: I sent a 68-year-old, hypertensive, diabetic woman with stage 4 kidney disease (estimated glomerular filtration rate [eGFR], 25 mL/min/1.73 m2; serum creatinine [SCr], 2 mg/mL) to a local nephrology group. Since she was diabetic, we had had her taking an angiotensin-converting enzyme inhibitor (ACEI) for years. The first thing the nephrology group did was take her off the ACEI. Why would they do that? I thought the hypertensive drug of choice for all diabetic patients is an ACEI or an angiotensin receptor blocker (ARB). Am I wrong?
A: The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation of blood pressure and intravascular volume through its effects on renin, angiotensin, and aldosterone production. Activation of RAAS causes an increase in blood pressure through vasoconstriction (angiotensin II effects) and the fluid retention associated with reabsorption of sodium and water (aldosterone effects). As such, the physiologic effects of RAAS have been implicated in the pathophysiology of cardiovascular diseases, such as heart failure, kidney disease, and hypertension.1
The JNC 7 guidelines2 (Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure) recommend the use of ACEIs or ARBs for the treatment of hypertension in patients with chronic kidney disease (CKD) or diabetes to slow progression of kidney disease. Given the benefits of ACEI or ARB therapy in reducing mortality in cardiovascular disease (for which patients with CKD are at increased risk3), these medications are indicated in patients with CKD.
Angiotensin II causes systemic vasoconstriction and also acts on the efferent arterioles of the glomerulus. ACEI/ARB therapy causes vasodilation of the efferent arterioles, thus lowering the intraglomerular capillary pressure.1 This mechanism accounts for the antiproteinuric effect and the subsequent decrease in GFR when these agents are initiated.
Proteinuria is a marker that may indicate nephropathy and may lead to further kidney damage.1 Slowing renal disease progression depends on controlling blood pressure and proteinuria.3
To date, a number of trials have been conducted to explore the benefit of the antiproteinuric effects of ACEI/ARB therapy. The antiproteinuric effect is more pronounced in patients with more severe proteinuria. Studies of these agents in patients with diabetic and nondiabetic nephropathy have shown that besides reducing proteinuria, they slow progression to end-stage renal disease (ESRD), thus delaying the need for renal replacement therapy.4 As such, ACEI/ARB therapy is widely used to reduce proteinuria, independent of the blood pressure–lowering effects.
Initiation of ACEI/ARB therapy is associated with an increase in SCr due to the drugs’ effects on the efferent arterioles, resulting in a decrease in intraglomerular pressure.3 However, discontinuing therapy is not warranted unless the SCr rises to more than 30% above baseline. According to findings from one meta-analysis, the degree of loss in renal function when ACEI therapy was initiated was inversely related to the rate of annual decline in renal function.5 Thus, patients with higher SCr levels at the start of therapy had poorer renal function initially, but they received the greatest benefit in long-term renal preservation.
The rise in SCr typically occurs within a few days of therapy initiation; thus, SCr should be measured within the first seven days of therapy.3 The SCr level is expected to stabilize within six to eight weeks of therapy. Patients whose SCr level continues to rise more than 30% to 35% above baseline may need to discontinue ACEI/ARB therapy; this rise may be attributed to kidney hypoperfusion. Kidney hypoperfusion can also occur when diuretics are initiated or their dosage increased, when NSAIDs are used, or in patients with bilateral renal artery stenosis or volume depletion resulting from gastroenteritis.3
Hyperkalemia due to decreased urinary excretion of potassium (K+) may present yet another reason to discontinue ACEI/ARB therapy in patients with CKD. Incidence of hyperkalemia in those with CKD stages 3 through 5 who receive either an ACEI or an ARB ranges from 5% to 50%.3 Hyperkalemia can occur when a long-acting ACEI is prescribed or when ACEI/ARB therapy is used concurrently with NSAIDs or potassium-sparing diuretics; thiazides or loop diuretics, by contrast, can reduce the risk for hyperkalemia.
Risk factors for moderate hyperkalemia (serum K+ ≥ 5.6 mmol/L) include age older than 65, congestive heart failure, SCr level greater than 1.6 mg/dL, and a blood urea nitrogen level exceeding 18 mg/dL.5
A dosing reduction or discontinuation of ACEI/ARB therapy should be considered when serum K+ levels are 5.6 mmol/L or greater.3 Patients who are older than 70 or whose serum urea nitrogen level exceeds 25 mg/dL are at increased risk for severe hyperkalemia (K+ > 6.0 mmol/L). Termination of an ACEI or an ARB may be warranted in patients with a serum K+ level exceeding 6.0 mmol/L or in those considered at increased risk for severe hyperkalemia.3
Other patients may also benefit from discontinuing ACEI/ARB therapy. Few data exist to support their use in patients with kidney function at 25% or less, or those with a GFR below 30 mL/min/1.73 m2.3 Onuigbo6 suggests that ESRD in patients with CKD can either progress gradually and steadily or rapidly as a result of acute kidney injury (AKI). Patients with CKD who are at risk for ESRD because of AKI include those currently taking an ACEI or an ARB, older patients, and those who experience unexplained decreases in GFR.
To avoid any worsening of renal function, Onuigbo6 suggests temporarily discontinuing ACEI/ARB therapy in the following patients:
• Those older than 65 who are scheduled for colonoscopy, administration of IV radiocontrast, or surgery (especially cardiovascular surgery); or
• Patients hospitalized for an acute ailment.
Discontinuing these agents may prevent progression to ESRD in such patients.
In summary, use of ACEIs or ARBs to delay progression of renal disease may be continued if a clear therapeutic benefit exists. However, discontinuation should be considered if a patient with CKD is at risk for hyperkalemia or if the K+ level remains higher than 5.6 mmol/L, if SCr levels increase more than 30% above baseline, or if patients (especially those older than 65) are at risk for AKI.
References
1. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005;99:S57-S65.
2. Chobanian AV, Bakris GL, Black HR, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
3. Mangrum AJ, Bakris GL. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in chronic renal disease: safety issues. Semin Nephrol. 2004;24:168-175.
4. St Peter WL, Odum LE, Whaley-Connell AT. To RAS or not to RAS? The evidence for and cautions with renin-angiotensin system inhibition in patients with diabetic kidney disease. Pharmacotherapy. 2013 Apr 9.
5. Bakris GL, Weir MR. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685-693.
6. Onuigbo MA. Is renoprotection with RAAS blockade a failed paradigm? Have we learnt any lessons so far? Int J Clin Pract. 2010;64:1341-1346.
Does Combination ACEi/ARB Therapy Benefit Patients With Proteinuria?
Q: I have a diabetic patient with microscopic proteinuria. I put her on an ACE inhibitor, but she still has the same albumin–creatinine ratio. My supervising physician suggested I add an ARB to her regimen, but I seem to remember reading that this does not work. Is that true? Or should I prescribe an ACE inhibitor/ARB combination?
This is a common question, but there is no consensus regarding the correct answer. The question should be: Are two drugs better than one when it comes to reducing proteinuria and progression to end-stage renal disease? Researchers have demonstrated a decrease in proteinuria in patients given combination ACE inhibitor/angiotension receptor blocker (ARB) therapy; however, the studies involved were found to have flaws, including small sample sizes and relatively short follow-up, once treatment was initiated.1,2
A larger study, known as Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET),3,4 is a multiyear study with more than 25,000 patients enrolled. ONTARGET (which excludes patients with heart failure) addresses the question of whether an ACE inhibitor in combination with an ARB, or either agent alone, is more effective in the reduction of proteinuria. In ONTARGET, combination therapy was associated with a decrease in proteinuria; however, the incidence of renal impairment was much higher.3
ONTARGET was the first trial to cast doubt on the belief that proteinuria is an accurate marker for progressive renal dysfunction. Combination treatment led to an advanced risk for increased serum creatinine and need for dialysis, despite the reduction in proteinuria. Further, combination therapy was more likely than either agent alone to cause adverse effects, including hypotension and hyperkalemia.3,4
Finally, the study also demonstrated that ACE inhibitors are not superior to ARBs. Both drugs reduce proteinuria, and each one taken alone decreases progression to end-stage renal disease. Therefore, the conclusion is that either an ACE inhibitor or an ARB alone is more efficacious than the two drugs combined.
Tricia A. Howard, MHS, PA-C, South University PA Program, Tampa, FL
References
1. Misra S, Stevermer JJ. ACE inhibitors and ARBs: one or the other—not both—for high-risk patients. J Fam Pract. 2009;58:24-26.
2. Jennings DL, Kalus JS, Coleman CI, et al. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet Med. 2007;24:486-493.
3. Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547-553.
4. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
Q: I have a diabetic patient with microscopic proteinuria. I put her on an ACE inhibitor, but she still has the same albumin–creatinine ratio. My supervising physician suggested I add an ARB to her regimen, but I seem to remember reading that this does not work. Is that true? Or should I prescribe an ACE inhibitor/ARB combination?
This is a common question, but there is no consensus regarding the correct answer. The question should be: Are two drugs better than one when it comes to reducing proteinuria and progression to end-stage renal disease? Researchers have demonstrated a decrease in proteinuria in patients given combination ACE inhibitor/angiotension receptor blocker (ARB) therapy; however, the studies involved were found to have flaws, including small sample sizes and relatively short follow-up, once treatment was initiated.1,2
A larger study, known as Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET),3,4 is a multiyear study with more than 25,000 patients enrolled. ONTARGET (which excludes patients with heart failure) addresses the question of whether an ACE inhibitor in combination with an ARB, or either agent alone, is more effective in the reduction of proteinuria. In ONTARGET, combination therapy was associated with a decrease in proteinuria; however, the incidence of renal impairment was much higher.3
ONTARGET was the first trial to cast doubt on the belief that proteinuria is an accurate marker for progressive renal dysfunction. Combination treatment led to an advanced risk for increased serum creatinine and need for dialysis, despite the reduction in proteinuria. Further, combination therapy was more likely than either agent alone to cause adverse effects, including hypotension and hyperkalemia.3,4
Finally, the study also demonstrated that ACE inhibitors are not superior to ARBs. Both drugs reduce proteinuria, and each one taken alone decreases progression to end-stage renal disease. Therefore, the conclusion is that either an ACE inhibitor or an ARB alone is more efficacious than the two drugs combined.
Tricia A. Howard, MHS, PA-C, South University PA Program, Tampa, FL
References
1. Misra S, Stevermer JJ. ACE inhibitors and ARBs: one or the other—not both—for high-risk patients. J Fam Pract. 2009;58:24-26.
2. Jennings DL, Kalus JS, Coleman CI, et al. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet Med. 2007;24:486-493.
3. Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547-553.
4. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
Q: I have a diabetic patient with microscopic proteinuria. I put her on an ACE inhibitor, but she still has the same albumin–creatinine ratio. My supervising physician suggested I add an ARB to her regimen, but I seem to remember reading that this does not work. Is that true? Or should I prescribe an ACE inhibitor/ARB combination?
This is a common question, but there is no consensus regarding the correct answer. The question should be: Are two drugs better than one when it comes to reducing proteinuria and progression to end-stage renal disease? Researchers have demonstrated a decrease in proteinuria in patients given combination ACE inhibitor/angiotension receptor blocker (ARB) therapy; however, the studies involved were found to have flaws, including small sample sizes and relatively short follow-up, once treatment was initiated.1,2
A larger study, known as Ongoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial (ONTARGET),3,4 is a multiyear study with more than 25,000 patients enrolled. ONTARGET (which excludes patients with heart failure) addresses the question of whether an ACE inhibitor in combination with an ARB, or either agent alone, is more effective in the reduction of proteinuria. In ONTARGET, combination therapy was associated with a decrease in proteinuria; however, the incidence of renal impairment was much higher.3
ONTARGET was the first trial to cast doubt on the belief that proteinuria is an accurate marker for progressive renal dysfunction. Combination treatment led to an advanced risk for increased serum creatinine and need for dialysis, despite the reduction in proteinuria. Further, combination therapy was more likely than either agent alone to cause adverse effects, including hypotension and hyperkalemia.3,4
Finally, the study also demonstrated that ACE inhibitors are not superior to ARBs. Both drugs reduce proteinuria, and each one taken alone decreases progression to end-stage renal disease. Therefore, the conclusion is that either an ACE inhibitor or an ARB alone is more efficacious than the two drugs combined.
Tricia A. Howard, MHS, PA-C, South University PA Program, Tampa, FL
References
1. Misra S, Stevermer JJ. ACE inhibitors and ARBs: one or the other—not both—for high-risk patients. J Fam Pract. 2009;58:24-26.
2. Jennings DL, Kalus JS, Coleman CI, et al. Combination therapy with an ACE inhibitor and an angiotensin receptor blocker for diabetic nephropathy: a meta-analysis. Diabet Med. 2007;24:486-493.
3. Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547-553.
4. Yusuf S, Teo KK, Pogue J, et al; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547-1559.
Acute Kidney Injury in an Unexpected Patient Population
Q: When I was doing sports physicals at the high school this week, several students asked if it was true that marijuana causes kidney failure. I had not heard this. Is it true? What should I tell teens who ask about this?
Synthetic marijuana, which goes by the street names of Spice, K2, Black Mamba, Fake Weed, Genie, and Zohai, is a mixture of herbs and spices that is sprayed with a synthetic THC-type compound.1 These can be sold over the Internet as "incense" or "bath salts." However, as is often the case with drugs purchased online or from a neighborhood dealer, other compounds toxic to humans can be cut and mixed in with these substances. While hypertension, nausea, cognitive dysfunction, and dizziness have all been associated with Spice, there has been a recent flurry of reports of severe and lasting cardiac and renal damage following use of these drugs.
In 2011, three cases of Spice-associated acute coronary syndrome were reported in the pediatric literature.2 In late 2012, four residents of the same Alabama community developed AKI after using Spice. While all four eventually recovered kidney function, they now have some permanent chronic kidney damage, and all four patients required kidney biopsies.3 Similarly, the CDC recently reported 14 cases of AKI in Wyoming that developed in patients who had smoked Spice.4 Six cases were reported from Oregon, two each from New York and Oklahoma, and one each from Rhode Island and Kansas. Half of the case patients required hemodialysis and kidney biopsy. All had residual chronic kidney disease after recovery.4
The patients' presentations were similar: they were all young and healthy with no history of kidney problems—then, wham! After they had smoked Spice, severe nausea and vomiting with flank pain took them to the ER. On admission, serum creatinine (SCr) was mildly abnormal, but it rose to an average of 8 mg/dL, with one patient's SCr peaking at 21 mg/dL.4
While there have been no Spice-associated deaths reported, the critical care needed for these young people included hemodialysis. Perhaps a graphic description of the standard 15-gauge needles we use for dialysis would be helpful during a discussion of drug use with teens.
Kim Zuber, PA-C, MSPS, DFAAPA, Metropolitan Nephrology, Alexandria, VA, and Clinton, MD
REFERENCES
1. US Drug Enforcement Administration. Drug Fact Sheet: K2 or Spice. www.justice.gov/dea/druginfo/drug_data_sheets/K2_Spice.pdf. Accessed March 15, 2013.
2. Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128:e1622-e1627.
3. Bhanushali GK, Jain G, Fatima H, et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2012 Dec 14. [Epub ahead of print]
4. CDC. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93-98.
Q: When I was doing sports physicals at the high school this week, several students asked if it was true that marijuana causes kidney failure. I had not heard this. Is it true? What should I tell teens who ask about this?
Synthetic marijuana, which goes by the street names of Spice, K2, Black Mamba, Fake Weed, Genie, and Zohai, is a mixture of herbs and spices that is sprayed with a synthetic THC-type compound.1 These can be sold over the Internet as "incense" or "bath salts." However, as is often the case with drugs purchased online or from a neighborhood dealer, other compounds toxic to humans can be cut and mixed in with these substances. While hypertension, nausea, cognitive dysfunction, and dizziness have all been associated with Spice, there has been a recent flurry of reports of severe and lasting cardiac and renal damage following use of these drugs.
In 2011, three cases of Spice-associated acute coronary syndrome were reported in the pediatric literature.2 In late 2012, four residents of the same Alabama community developed AKI after using Spice. While all four eventually recovered kidney function, they now have some permanent chronic kidney damage, and all four patients required kidney biopsies.3 Similarly, the CDC recently reported 14 cases of AKI in Wyoming that developed in patients who had smoked Spice.4 Six cases were reported from Oregon, two each from New York and Oklahoma, and one each from Rhode Island and Kansas. Half of the case patients required hemodialysis and kidney biopsy. All had residual chronic kidney disease after recovery.4
The patients' presentations were similar: they were all young and healthy with no history of kidney problems—then, wham! After they had smoked Spice, severe nausea and vomiting with flank pain took them to the ER. On admission, serum creatinine (SCr) was mildly abnormal, but it rose to an average of 8 mg/dL, with one patient's SCr peaking at 21 mg/dL.4
While there have been no Spice-associated deaths reported, the critical care needed for these young people included hemodialysis. Perhaps a graphic description of the standard 15-gauge needles we use for dialysis would be helpful during a discussion of drug use with teens.
Kim Zuber, PA-C, MSPS, DFAAPA, Metropolitan Nephrology, Alexandria, VA, and Clinton, MD
REFERENCES
1. US Drug Enforcement Administration. Drug Fact Sheet: K2 or Spice. www.justice.gov/dea/druginfo/drug_data_sheets/K2_Spice.pdf. Accessed March 15, 2013.
2. Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128:e1622-e1627.
3. Bhanushali GK, Jain G, Fatima H, et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2012 Dec 14. [Epub ahead of print]
4. CDC. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93-98.
Q: When I was doing sports physicals at the high school this week, several students asked if it was true that marijuana causes kidney failure. I had not heard this. Is it true? What should I tell teens who ask about this?
Synthetic marijuana, which goes by the street names of Spice, K2, Black Mamba, Fake Weed, Genie, and Zohai, is a mixture of herbs and spices that is sprayed with a synthetic THC-type compound.1 These can be sold over the Internet as "incense" or "bath salts." However, as is often the case with drugs purchased online or from a neighborhood dealer, other compounds toxic to humans can be cut and mixed in with these substances. While hypertension, nausea, cognitive dysfunction, and dizziness have all been associated with Spice, there has been a recent flurry of reports of severe and lasting cardiac and renal damage following use of these drugs.
In 2011, three cases of Spice-associated acute coronary syndrome were reported in the pediatric literature.2 In late 2012, four residents of the same Alabama community developed AKI after using Spice. While all four eventually recovered kidney function, they now have some permanent chronic kidney damage, and all four patients required kidney biopsies.3 Similarly, the CDC recently reported 14 cases of AKI in Wyoming that developed in patients who had smoked Spice.4 Six cases were reported from Oregon, two each from New York and Oklahoma, and one each from Rhode Island and Kansas. Half of the case patients required hemodialysis and kidney biopsy. All had residual chronic kidney disease after recovery.4
The patients' presentations were similar: they were all young and healthy with no history of kidney problems—then, wham! After they had smoked Spice, severe nausea and vomiting with flank pain took them to the ER. On admission, serum creatinine (SCr) was mildly abnormal, but it rose to an average of 8 mg/dL, with one patient's SCr peaking at 21 mg/dL.4
While there have been no Spice-associated deaths reported, the critical care needed for these young people included hemodialysis. Perhaps a graphic description of the standard 15-gauge needles we use for dialysis would be helpful during a discussion of drug use with teens.
Kim Zuber, PA-C, MSPS, DFAAPA, Metropolitan Nephrology, Alexandria, VA, and Clinton, MD
REFERENCES
1. US Drug Enforcement Administration. Drug Fact Sheet: K2 or Spice. www.justice.gov/dea/druginfo/drug_data_sheets/K2_Spice.pdf. Accessed March 15, 2013.
2. Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128:e1622-e1627.
3. Bhanushali GK, Jain G, Fatima H, et al. AKI associated with synthetic cannabinoids: a case series. Clin J Am Soc Nephrol. 2012 Dec 14. [Epub ahead of print]
4. CDC. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:93-98.
Guidelines on Hematuria: Determing the Cause
The American Urological Association (AUA) published guidelines for asymptomatic microhematuria. The document includes 19 guidelines with recommendation levels ranging from A to C (high to low) and some expert opinion recommendations included. The full guidelines can be accessed at http://www.auanet.org/common/pdf/education/clinical-guidance/Asymptomatic-Microhematuria.pdf.
Q: A 45-year-old man came into my office complaining that he had seen “pink” in his urine. I dipped the urine in the office, and it was positive for blood. What should I do now? Should I send him directly to nephrology or urology? Or should I do a work-up myself? And if I do the work-up, what tests should I order?
When treating the patient with hematuria, it is important to keep in mind both the most common benign causes and the more serious causes of hematuria. The most common benign causes, according to the AUA guideline 6,1 include infection, menstruation, vigorous exercise, trauma, anticoagulant use, and a recent urologic procedure. The potentially serious causes include glomerulonephritis (which can be rapidly progressive) and malignancy.
The AUA (guidelines 1 to 4, based on expert opinion)1 recommends confirming hematuria with a microscopic exam rather than relying on a urine dipstick.
The common benign causes of hematuria can usually be identified in the course of a thorough history and physical. Because hematuria can be a harbinger of renal disease, however, serum creatinine and blood urea nitrogen (BUN) should be ordered at the initial evaluation in the primary care setting.
If a benign cause of hematuria is identified and renal function is normal, the patient should be treated by the primary care provider and re-evaluated as indicated, based on the underlying diagnosis. If there is a rise in serum creatinine or a reduction in estimated glomerular filtration rate (eGFR) in conjunction with the hematuria, the patient should be referred to nephrology for further evaluation.
If no benign cause of hematuria is identified and renal function is unaffected, the patient should be referred to urology for urologic evaluation.1
Alexis Chettiar, ACNP, East Bay Nephrology Medical Group, Oakland, CA
References
1. Davis R, Jones JS, Barocas DA, et al; American Urological Association. Diagnosis, Evaluation, and Follow-up of Asymptomatic Microhematuria (AMH) in Adults: AUA Guideline. Linthicum, MD: American Urological Association Education and Research, Inc; 2012. http://www.auanet.org/common/pdf/education/clinical-guidance/Asymptomatic-Microhematuria.pdf. Accessed January 24, 2013.
2. National Kidney and Urologic Diseases Information Clearinghouse. Hematuria: blood in the urine (2012). http://kidney.niddk.nih.gov/kudiseases/pubs/hematuria. Accessed January 17, 2013.
3. Geavlete B, Jecu M, Multescu R, et al. HAL blue-light cystoscopy in high-risk nonmuscle-invasive bladder cancer: re-TURBT recurrence rates in a prospective, randomized study. Urology. 2010;76(3):664-669.
Suggested Reading
Feldman AS, Hsu C-Y, Kurtz M, Cho KC. Etiology and evaluation of hematuria in adults (2012). www.uptodate.com/contents/etiology-and-evaluation-of-hematuria-in-adults. Accessed January 17, 2013.
Jayne D. Hematuria and proteinuria. In: Greenberg A, ed; National Kidney Foundation. Primer on Kidney Diseases. 5th ed. Saunders; 2009:33-42.
The American Urological Association (AUA) published guidelines for asymptomatic microhematuria. The document includes 19 guidelines with recommendation levels ranging from A to C (high to low) and some expert opinion recommendations included. The full guidelines can be accessed at http://www.auanet.org/common/pdf/education/clinical-guidance/Asymptomatic-Microhematuria.pdf.
Q: A 45-year-old man came into my office complaining that he had seen “pink” in his urine. I dipped the urine in the office, and it was positive for blood. What should I do now? Should I send him directly to nephrology or urology? Or should I do a work-up myself? And if I do the work-up, what tests should I order?
When treating the patient with hematuria, it is important to keep in mind both the most common benign causes and the more serious causes of hematuria. The most common benign causes, according to the AUA guideline 6,1 include infection, menstruation, vigorous exercise, trauma, anticoagulant use, and a recent urologic procedure. The potentially serious causes include glomerulonephritis (which can be rapidly progressive) and malignancy.
The AUA (guidelines 1 to 4, based on expert opinion)1 recommends confirming hematuria with a microscopic exam rather than relying on a urine dipstick.
The common benign causes of hematuria can usually be identified in the course of a thorough history and physical. Because hematuria can be a harbinger of renal disease, however, serum creatinine and blood urea nitrogen (BUN) should be ordered at the initial evaluation in the primary care setting.
If a benign cause of hematuria is identified and renal function is normal, the patient should be treated by the primary care provider and re-evaluated as indicated, based on the underlying diagnosis. If there is a rise in serum creatinine or a reduction in estimated glomerular filtration rate (eGFR) in conjunction with the hematuria, the patient should be referred to nephrology for further evaluation.
If no benign cause of hematuria is identified and renal function is unaffected, the patient should be referred to urology for urologic evaluation.1
Alexis Chettiar, ACNP, East Bay Nephrology Medical Group, Oakland, CA
References
1. Davis R, Jones JS, Barocas DA, et al; American Urological Association. Diagnosis, Evaluation, and Follow-up of Asymptomatic Microhematuria (AMH) in Adults: AUA Guideline. Linthicum, MD: American Urological Association Education and Research, Inc; 2012. http://www.auanet.org/common/pdf/education/clinical-guidance/Asymptomatic-Microhematuria.pdf. Accessed January 24, 2013.
2. National Kidney and Urologic Diseases Information Clearinghouse. Hematuria: blood in the urine (2012). http://kidney.niddk.nih.gov/kudiseases/pubs/hematuria. Accessed January 17, 2013.
3. Geavlete B, Jecu M, Multescu R, et al. HAL blue-light cystoscopy in high-risk nonmuscle-invasive bladder cancer: re-TURBT recurrence rates in a prospective, randomized study. Urology. 2010;76(3):664-669.
Suggested Reading
Feldman AS, Hsu C-Y, Kurtz M, Cho KC. Etiology and evaluation of hematuria in adults (2012). www.uptodate.com/contents/etiology-and-evaluation-of-hematuria-in-adults. Accessed January 17, 2013.
Jayne D. Hematuria and proteinuria. In: Greenberg A, ed; National Kidney Foundation. Primer on Kidney Diseases. 5th ed. Saunders; 2009:33-42.
The American Urological Association (AUA) published guidelines for asymptomatic microhematuria. The document includes 19 guidelines with recommendation levels ranging from A to C (high to low) and some expert opinion recommendations included. The full guidelines can be accessed at http://www.auanet.org/common/pdf/education/clinical-guidance/Asymptomatic-Microhematuria.pdf.
Q: A 45-year-old man came into my office complaining that he had seen “pink” in his urine. I dipped the urine in the office, and it was positive for blood. What should I do now? Should I send him directly to nephrology or urology? Or should I do a work-up myself? And if I do the work-up, what tests should I order?
When treating the patient with hematuria, it is important to keep in mind both the most common benign causes and the more serious causes of hematuria. The most common benign causes, according to the AUA guideline 6,1 include infection, menstruation, vigorous exercise, trauma, anticoagulant use, and a recent urologic procedure. The potentially serious causes include glomerulonephritis (which can be rapidly progressive) and malignancy.
The AUA (guidelines 1 to 4, based on expert opinion)1 recommends confirming hematuria with a microscopic exam rather than relying on a urine dipstick.
The common benign causes of hematuria can usually be identified in the course of a thorough history and physical. Because hematuria can be a harbinger of renal disease, however, serum creatinine and blood urea nitrogen (BUN) should be ordered at the initial evaluation in the primary care setting.
If a benign cause of hematuria is identified and renal function is normal, the patient should be treated by the primary care provider and re-evaluated as indicated, based on the underlying diagnosis. If there is a rise in serum creatinine or a reduction in estimated glomerular filtration rate (eGFR) in conjunction with the hematuria, the patient should be referred to nephrology for further evaluation.
If no benign cause of hematuria is identified and renal function is unaffected, the patient should be referred to urology for urologic evaluation.1
Alexis Chettiar, ACNP, East Bay Nephrology Medical Group, Oakland, CA
References
1. Davis R, Jones JS, Barocas DA, et al; American Urological Association. Diagnosis, Evaluation, and Follow-up of Asymptomatic Microhematuria (AMH) in Adults: AUA Guideline. Linthicum, MD: American Urological Association Education and Research, Inc; 2012. http://www.auanet.org/common/pdf/education/clinical-guidance/Asymptomatic-Microhematuria.pdf. Accessed January 24, 2013.
2. National Kidney and Urologic Diseases Information Clearinghouse. Hematuria: blood in the urine (2012). http://kidney.niddk.nih.gov/kudiseases/pubs/hematuria. Accessed January 17, 2013.
3. Geavlete B, Jecu M, Multescu R, et al. HAL blue-light cystoscopy in high-risk nonmuscle-invasive bladder cancer: re-TURBT recurrence rates in a prospective, randomized study. Urology. 2010;76(3):664-669.
Suggested Reading
Feldman AS, Hsu C-Y, Kurtz M, Cho KC. Etiology and evaluation of hematuria in adults (2012). www.uptodate.com/contents/etiology-and-evaluation-of-hematuria-in-adults. Accessed January 17, 2013.
Jayne D. Hematuria and proteinuria. In: Greenberg A, ed; National Kidney Foundation. Primer on Kidney Diseases. 5th ed. Saunders; 2009:33-42.