User login
Arthroscopic Management of Full-Thickness Rotator Cuff Tears in Major League Baseball Pitchers: The Lateralized Footprint Repair Technique
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
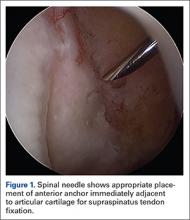
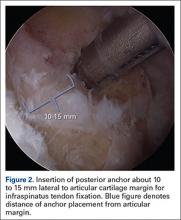
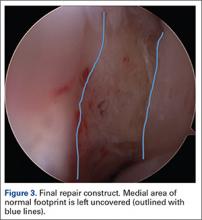
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Does a Prior Hip Arthroscopy Affect Clinical Outcomes in Metal-on-Metal Hip Resurfacing Arthroplasty?
Metal-on-metal hip resurfacing arthroplasty (HRA) remains an alternative to total hip arthroplasty (THA) in appropriately selected, younger, active adults with degenerative hip disease.1-4 While concerns remain regarding the potential for adverse local tissue reactions from wear of the metal-on-metal bearing surface,5-8 10-year data from the Australian Orthopaedic Association National Joint Replacement Registry Annual Report9 showed a revision rate of only 6.3% when the Birmingham Hip Resurfacing (BHR) System was used (Smith & Nephew Inc, Memphis, Tennessee).In addition, in an independent review of 230 consecutive BHRs at a mean follow-up of 10.4 years, Coulter and colleagues10 showed encouraging clinical results, with a mean Oxford Hip Score of 45.0 and a mean University of California at Los Angeles (UCLA) activity score of 7.4.
Similar to the prior increase in popularity of HRA, hip arthroscopy has also become much more commonplace, and its indications continue to evolve.11 Hip arthroscopy has been used in the native hip joint to manage femoroacetabular impingement, labral tears, and iliopsoas tendinopathy, among other conditions.12 In addition, the use of hip arthroscopy has not been limited to the native hip but also has increased as a diagnostic and therapeutic procedure after hip arthroplasties. Bajwa and Villar12 found hip arthroscopy to be diagnostic in 23 of 24 patients who underwent the procedure after a hip arthroplasty, concluding that arthroscopy is a useful adjunct in the diagnosis of symptomatic arthroplasties.
Therefore, hip arthroscopy has been shown to be an effective modality to treat pathology in both the native hip and after hip arthroplasties. However, the effect of a prior hip arthroscopy on the outcome of a subsequent metal-on-metal HRA has not been determined. Piedade and colleagues13 showed a prior knee arthroscopy to increase the risk of postoperative complications and subsequent revision after total knee arthroplasty. Complications included reflex sympathetic dystrophy, undiagnosed pain, infection, stiffness, and component loosening. A prior osteochondroplasty at the femoral head-neck junction could increase the risk of femoral neck fracture after a subsequent HRA. Thus, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy and to compare these results with a cohort of patients who received an HRA with no prior hip surgeries. Our hypothesis is that a prior hip arthroscopy will lead to inferior outcomes in patients undergoing HRA.
Materials and Methods
This study is a retrospective, case-control study using a 1:2 matching analysis. Dr. Su performed all HRAs, which were enrolled in an institutional review board–approved arthroplasty registry. All HRAs were performed using the BHR System.
The surgical technique for hip resurfacing arthroplasty has been described.1 All procedures were performed via a posterior approach with the patient in the lateral decubitus position. All patients received a hybrid metal-on-metal hip resurfacing, with an uncemented acetabular component and cemented femoral component. Intraoperative anesthesia for all patients was performed via a combined spinal-epidural anesthetic, and an epidural patient-controlled analgesic was used for the first day postoperatively, followed by a transition to oral analgesics. The sizes of the acetabular and femoral components were recorded for each hip resurfacing. Postoperatively, intermittent pneumatic compression devices were placed upon arrival in the recovery room, and active ankle flexion and extension exercises were initiated immediately after the patient’s neurologic function returned.14 Aspirin was used for chemical deep venous thrombosis prophylaxis in all patients postoperatively for a period of 6 weeks. Full weight-bearing, with the use of crutches for assistance with balance, was permitted immediately. Crutches were used for a period of 3 weeks prior to being discontinued.
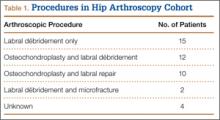
From a database of 1357 HRAs (all BHR implants) performed between June 2006 and June 2012, 51 patients were identified who received an HRA after a prior hip arthroscopy. Eight patients were excluded because they did not possess adequate clinical documentation or were lost to follow-up. In the remaining 43 patients, there were 32 men and 11 women (21 right hips, 22 left hips), which formed the arthroscopy cohort. Two patients had a history of multiple hip arthroscopies (1 patient with 2 prior procedures, 1 patient with 3 prior procedures). The mean (SD) time from the most recent hip arthroscopy to the HRA was 2.5 (2.5) years. Table 1 presents a summary of the hip arthroscopy procedures (including only the most recent hip arthroscopy procedure in those with multiple arthroscopies).
Patient demographic variables (age, body mass index [BMI]) were recorded preoperatively, along with the Harris Hip Score (HHS),15 UCLA activity score,16 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score,17 and preoperative hip range of motion (flexion, extension, abduction, adduction, internal rotation, and external rotation). The same clinical indices were assessed postoperatively along with the Short Form-12 (SF-12) Health Survey Score,18 at the 6-week, 3-month, 6-month, 1-year, and most recent follow-up visits.
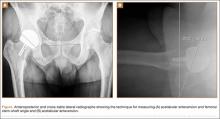
Radiographic assessment consisted of a low anteroposterior (AP) pelvic radiograph (with the radiographic beam centered on the pubic symphysis) and a cross-table lateral radiograph obtained at the most recent follow-up visit. Both the acetabular component abduction relative to the inter-teardrop line, and the angle between the femoral stem and the anatomic axis of the femoral shaft (stem-shaft angle) were measured on AP radiographs.19,20 Acetabular component anteversion was measured on the cross-table lateral radiographs as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the long axis plane of the body (Figures A, B).21
The same registry database was used to identify patients who received an HRA without a prior history of arthroscopy or hip surgery. A 1:2 matching analysis for those patients with a prior hip arthroscopy to those without a prior hip arthroscopy was performed to formulate a control group (control cohort) of 86 patients. Each patient in the arthroscopy cohort was matched with 2 patients in the control cohort based on the following parameters: age (± 6 years), sex (same), BMI (± 4 kg/m2), femoral head size (± 4 mm), and preoperative HHS and WOMAC scores (± 7 points). In the event an arthroscopy patient matched to 2 or more control patients, the patients who minimized the least squared error among the matching variables were selected.
Statistical Analysis
All data were collected and analyzed using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Statistical comparisons between the 2 cohorts regarding demographic variables, clinical outcomes, and radiographic alignment were performed using an unpaired, Student 2-tailed t test, with statistical significance set at P ≤ .05.
Results
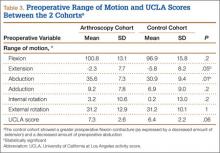
A comparison of the results of the 1:2 matching analysis between the arthroscopy and control cohorts is presented in Table 2. There was no significant difference in the preoperative age, BMI, femoral head size, HHS, or WOMAC score between the 2 cohorts. However, the control cohort did show a more severe, preoperative flexion contracture (as expressed by a decreased amount of extension) and a decreased amount of preoperative abduction (Table 3). The preoperative UCLA activity score was also decreased in the control cohort, but this was not statistically significant.
The mean (SD) follow-up was 2.0 (1.0) years in the arthroscopy cohort and 2.1 (1.1) years in the control cohort. There was no significant difference in radiographic alignment between the 2 cohorts. The stem-shaft angle was 139.3° (SD, 5.4°) in the arthroscopy cohort (vs 138.3° [SD, 5.5°] in the control cohort; P = .3), the acetabular abduction was 43.9° (SD, 5.8°) in the arthroscopy cohort (vs 42.9° [SD, 6.1°] in the control cohort; P = .4), and the acetabular anteversion was 21.1° (SD, 7.5°) in the arthroscopy cohort (vs 20.8° [SD, 7.1°] in the control cohort; P = .8).
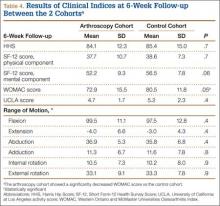
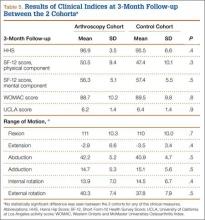
At 6-week follow-up, the arthroscopy cohort showed a significantly decreased WOMAC score compared with the control cohort (72.9 [SD, 15.5] vs 80.5 [SD, 11.8], respectively; P = .05). In addition, there was a trend towards a decreased SF-12 mental component score in the arthroscopy cohort (52.2 [SD, 9.3] vs 56.5 [SD, 7.8] in the control cohort; P = .06). However, none of the remaining clinical indices showed a significant difference between the 2 cohorts, and there was no difference in range of motion between the 2 cohorts at the 6-week follow-up visit (Table 4).
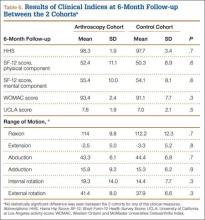
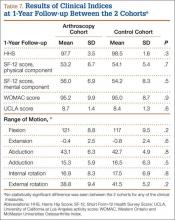
In addition, at 3-month follow-up, no statistically significant differences were seen between the 2 cohorts for any of the clinical indices or range of motion values. Both groups continued to improve rapidly, with HHS of 96.9 (SD, 3.5) in the arthroscopy cohort and 95.5 (SD, 6.6) in the control cohort, and WOMAC scores of 88.7 (SD, 10.2) and 89.5 (SD, 9.8), respectively (Table 5). Similarly, at the 6-month and 1-year follow-up intervals, the 2 cohorts showed continued improvement in their clinical measures, with no statistically significant differences between the 2 cohorts (Tables 6, 7).
At the most recent follow-up visit, more than 1 year after surgery, the HHS was 99.5 (SD, 1.3) in the arthroscopy cohort and 99.2 (SD, 9.7) in the control cohort (P = .9), and the WOMAC score was 93.5 (SD, 11.3) and 92.4 (SD, 12.2), respectively (P = .8). No significant perioperative complications were seen in the arthroscopy cohort. In the arthroscopy cohort, 1 patient was diagnosed with a deep venous thrombosis 2 weeks after the procedure and was placed on low-molecular-weight heparin and coumadin for treatment. A second patient in the arthroscopy cohort had continued serosanguinous drainage for 4 days postoperatively, which resolved with continued compressive dressings. To date, no patients in the arthroscopy or control cohorts have required a second operation or revision of their components.
Discussion
Given the increasing prevalence of hip arthroscopies to treat multiple disorders of the native joint, it is important to assess the potential consequences of these procedures on future arthroplasties. Piedade and colleagues,13 in a retrospective review of 1474 primary total knee arthroplasties, showed a prior bony procedure (high tibial osteotomy, tibial plateau fracture, patellar realignment) to be predictive of decreased range of motion postoperatively. In addition, a prior knee arthroscopy was associated with a higher rate of postoperative complications, with 30% of the complications requiring a reoperation, and 8.3% of the complications requiring a revision total knee arthroplasty. Kaplan-Meier survival curves showed a survival rate of only 86.8% in those patients with a prior knee arthroscopy (vs 98.1% in those without a prior knee surgery).22 Therefore, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy. After the initial 6-week follow-up visit, no significant difference was seen in the functional outcomes between those patients with or without a history of prior hip arthroscopy who received an HRA.
After analysis of patient outcomes using multiple clinical measurement tools, at 6-week, 3-month, 6-month, 1-year, and most recent follow-up intervals, the only significant difference between the 2 cohorts was the WOMAC score at 6-week follow-up. Interestingly, there was no significant difference seen in the other clinical assessments, including the SF-12 score, HHS, range of motion, or UCLA activity score (although this did trend towards significance). This can be explained by the difference in both the mode of administration and various metrics assessed by these instruments. In comparison to the HHS evaluation, the patient completes the WOMAC (rather than the clinician) and also provides a more detailed assessment of symptoms, pain, stiffness, and activities of daily living.17 Therefore, this study suggests that patients with a prior hip arthroscopy may require more time to return to their activities of daily living after an HRA. However, whether the statistically significant difference between the 2 scores translates into a clinically significant difference can be questioned.
The clinical outcomes of this series of patients were excellent at the short-term follow-up, and both groups achieved clinical results comparable to prior reported results of HRA.1,10,23,24 However, despite these results, there are several limitations to this study. First, longer-term follow-up is required to determine if any significant differences (such as aseptic loosening, infection, and prosthesis survival) are associated with a prior hip arthroscopy. In addition, this study included a relatively small cohort of patients who had a prior hip arthroscopy. However, a relatively large, single-surgeon database of 1357 HRAs was reviewed, with only 51 cases being reported (3.7%). With the increasing popularity of hip arthroscopy, the number of patients presenting for HRA will likely continue to increase. However, despite these limitations, this study shows that a prior hip arthroscopy does not appear to affect the short-term, clinical outcomes of a metal-on-metal HRA.
1. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty. Surgical Technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):234-249.
2. Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86(2):177-184.
3. Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88(5):592-600.
4. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
5. Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92(16):2663-2671.
6. Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study.
J Bone Joint Surg Br. 2007;89(2):169-173.
7. deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92(12):1642-1647.
8. Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-450.
9. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. Adelaide: Australian Orthopaedic Association; 2011. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2011. Accessed September 16, 2014.
10. Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg Br. 2012;94(3):315-321.
11. McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop. 2011;469(2):362-371.
12. Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93(7):890-896.
13. Piedade SR, Pinaroli A, Servien E, Neyret P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2009;17(4):328-333.
14. Gonzalez Della Valle A, Serota A, Go G, et al. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop. 2006;(444):146-153.
15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
16. Kershaw CJ, Atkins RM, Dodd CA, Bulstrode CJ. Revision total hip arthroplasty for aseptic failure. A review of 276 cases. J Bone Joint Surg Br. 1991;73(4):564-568.
17. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473-2476.
18. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
19. Clark JM, Freeman MA, Witham D. The relationship of neck orientation to the shape of the proximal femur. J Arthroplasty. 1987;2(2):99-109.
20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
21. Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop. 1995;(316):106-111.
22. Piedade SR, Pinaroli A, Servien E, Neyret P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2737-2743.
23. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86(1):28-39.
24. Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br. 2008;90(4):436-441.
Metal-on-metal hip resurfacing arthroplasty (HRA) remains an alternative to total hip arthroplasty (THA) in appropriately selected, younger, active adults with degenerative hip disease.1-4 While concerns remain regarding the potential for adverse local tissue reactions from wear of the metal-on-metal bearing surface,5-8 10-year data from the Australian Orthopaedic Association National Joint Replacement Registry Annual Report9 showed a revision rate of only 6.3% when the Birmingham Hip Resurfacing (BHR) System was used (Smith & Nephew Inc, Memphis, Tennessee).In addition, in an independent review of 230 consecutive BHRs at a mean follow-up of 10.4 years, Coulter and colleagues10 showed encouraging clinical results, with a mean Oxford Hip Score of 45.0 and a mean University of California at Los Angeles (UCLA) activity score of 7.4.
Similar to the prior increase in popularity of HRA, hip arthroscopy has also become much more commonplace, and its indications continue to evolve.11 Hip arthroscopy has been used in the native hip joint to manage femoroacetabular impingement, labral tears, and iliopsoas tendinopathy, among other conditions.12 In addition, the use of hip arthroscopy has not been limited to the native hip but also has increased as a diagnostic and therapeutic procedure after hip arthroplasties. Bajwa and Villar12 found hip arthroscopy to be diagnostic in 23 of 24 patients who underwent the procedure after a hip arthroplasty, concluding that arthroscopy is a useful adjunct in the diagnosis of symptomatic arthroplasties.
Therefore, hip arthroscopy has been shown to be an effective modality to treat pathology in both the native hip and after hip arthroplasties. However, the effect of a prior hip arthroscopy on the outcome of a subsequent metal-on-metal HRA has not been determined. Piedade and colleagues13 showed a prior knee arthroscopy to increase the risk of postoperative complications and subsequent revision after total knee arthroplasty. Complications included reflex sympathetic dystrophy, undiagnosed pain, infection, stiffness, and component loosening. A prior osteochondroplasty at the femoral head-neck junction could increase the risk of femoral neck fracture after a subsequent HRA. Thus, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy and to compare these results with a cohort of patients who received an HRA with no prior hip surgeries. Our hypothesis is that a prior hip arthroscopy will lead to inferior outcomes in patients undergoing HRA.
Materials and Methods
This study is a retrospective, case-control study using a 1:2 matching analysis. Dr. Su performed all HRAs, which were enrolled in an institutional review board–approved arthroplasty registry. All HRAs were performed using the BHR System.
The surgical technique for hip resurfacing arthroplasty has been described.1 All procedures were performed via a posterior approach with the patient in the lateral decubitus position. All patients received a hybrid metal-on-metal hip resurfacing, with an uncemented acetabular component and cemented femoral component. Intraoperative anesthesia for all patients was performed via a combined spinal-epidural anesthetic, and an epidural patient-controlled analgesic was used for the first day postoperatively, followed by a transition to oral analgesics. The sizes of the acetabular and femoral components were recorded for each hip resurfacing. Postoperatively, intermittent pneumatic compression devices were placed upon arrival in the recovery room, and active ankle flexion and extension exercises were initiated immediately after the patient’s neurologic function returned.14 Aspirin was used for chemical deep venous thrombosis prophylaxis in all patients postoperatively for a period of 6 weeks. Full weight-bearing, with the use of crutches for assistance with balance, was permitted immediately. Crutches were used for a period of 3 weeks prior to being discontinued.
From a database of 1357 HRAs (all BHR implants) performed between June 2006 and June 2012, 51 patients were identified who received an HRA after a prior hip arthroscopy. Eight patients were excluded because they did not possess adequate clinical documentation or were lost to follow-up. In the remaining 43 patients, there were 32 men and 11 women (21 right hips, 22 left hips), which formed the arthroscopy cohort. Two patients had a history of multiple hip arthroscopies (1 patient with 2 prior procedures, 1 patient with 3 prior procedures). The mean (SD) time from the most recent hip arthroscopy to the HRA was 2.5 (2.5) years. Table 1 presents a summary of the hip arthroscopy procedures (including only the most recent hip arthroscopy procedure in those with multiple arthroscopies).
Patient demographic variables (age, body mass index [BMI]) were recorded preoperatively, along with the Harris Hip Score (HHS),15 UCLA activity score,16 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score,17 and preoperative hip range of motion (flexion, extension, abduction, adduction, internal rotation, and external rotation). The same clinical indices were assessed postoperatively along with the Short Form-12 (SF-12) Health Survey Score,18 at the 6-week, 3-month, 6-month, 1-year, and most recent follow-up visits.
Radiographic assessment consisted of a low anteroposterior (AP) pelvic radiograph (with the radiographic beam centered on the pubic symphysis) and a cross-table lateral radiograph obtained at the most recent follow-up visit. Both the acetabular component abduction relative to the inter-teardrop line, and the angle between the femoral stem and the anatomic axis of the femoral shaft (stem-shaft angle) were measured on AP radiographs.19,20 Acetabular component anteversion was measured on the cross-table lateral radiographs as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the long axis plane of the body (Figures A, B).21
The same registry database was used to identify patients who received an HRA without a prior history of arthroscopy or hip surgery. A 1:2 matching analysis for those patients with a prior hip arthroscopy to those without a prior hip arthroscopy was performed to formulate a control group (control cohort) of 86 patients. Each patient in the arthroscopy cohort was matched with 2 patients in the control cohort based on the following parameters: age (± 6 years), sex (same), BMI (± 4 kg/m2), femoral head size (± 4 mm), and preoperative HHS and WOMAC scores (± 7 points). In the event an arthroscopy patient matched to 2 or more control patients, the patients who minimized the least squared error among the matching variables were selected.
Statistical Analysis
All data were collected and analyzed using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Statistical comparisons between the 2 cohorts regarding demographic variables, clinical outcomes, and radiographic alignment were performed using an unpaired, Student 2-tailed t test, with statistical significance set at P ≤ .05.
Results
A comparison of the results of the 1:2 matching analysis between the arthroscopy and control cohorts is presented in Table 2. There was no significant difference in the preoperative age, BMI, femoral head size, HHS, or WOMAC score between the 2 cohorts. However, the control cohort did show a more severe, preoperative flexion contracture (as expressed by a decreased amount of extension) and a decreased amount of preoperative abduction (Table 3). The preoperative UCLA activity score was also decreased in the control cohort, but this was not statistically significant.
The mean (SD) follow-up was 2.0 (1.0) years in the arthroscopy cohort and 2.1 (1.1) years in the control cohort. There was no significant difference in radiographic alignment between the 2 cohorts. The stem-shaft angle was 139.3° (SD, 5.4°) in the arthroscopy cohort (vs 138.3° [SD, 5.5°] in the control cohort; P = .3), the acetabular abduction was 43.9° (SD, 5.8°) in the arthroscopy cohort (vs 42.9° [SD, 6.1°] in the control cohort; P = .4), and the acetabular anteversion was 21.1° (SD, 7.5°) in the arthroscopy cohort (vs 20.8° [SD, 7.1°] in the control cohort; P = .8).
At 6-week follow-up, the arthroscopy cohort showed a significantly decreased WOMAC score compared with the control cohort (72.9 [SD, 15.5] vs 80.5 [SD, 11.8], respectively; P = .05). In addition, there was a trend towards a decreased SF-12 mental component score in the arthroscopy cohort (52.2 [SD, 9.3] vs 56.5 [SD, 7.8] in the control cohort; P = .06). However, none of the remaining clinical indices showed a significant difference between the 2 cohorts, and there was no difference in range of motion between the 2 cohorts at the 6-week follow-up visit (Table 4).
In addition, at 3-month follow-up, no statistically significant differences were seen between the 2 cohorts for any of the clinical indices or range of motion values. Both groups continued to improve rapidly, with HHS of 96.9 (SD, 3.5) in the arthroscopy cohort and 95.5 (SD, 6.6) in the control cohort, and WOMAC scores of 88.7 (SD, 10.2) and 89.5 (SD, 9.8), respectively (Table 5). Similarly, at the 6-month and 1-year follow-up intervals, the 2 cohorts showed continued improvement in their clinical measures, with no statistically significant differences between the 2 cohorts (Tables 6, 7).
At the most recent follow-up visit, more than 1 year after surgery, the HHS was 99.5 (SD, 1.3) in the arthroscopy cohort and 99.2 (SD, 9.7) in the control cohort (P = .9), and the WOMAC score was 93.5 (SD, 11.3) and 92.4 (SD, 12.2), respectively (P = .8). No significant perioperative complications were seen in the arthroscopy cohort. In the arthroscopy cohort, 1 patient was diagnosed with a deep venous thrombosis 2 weeks after the procedure and was placed on low-molecular-weight heparin and coumadin for treatment. A second patient in the arthroscopy cohort had continued serosanguinous drainage for 4 days postoperatively, which resolved with continued compressive dressings. To date, no patients in the arthroscopy or control cohorts have required a second operation or revision of their components.
Discussion
Given the increasing prevalence of hip arthroscopies to treat multiple disorders of the native joint, it is important to assess the potential consequences of these procedures on future arthroplasties. Piedade and colleagues,13 in a retrospective review of 1474 primary total knee arthroplasties, showed a prior bony procedure (high tibial osteotomy, tibial plateau fracture, patellar realignment) to be predictive of decreased range of motion postoperatively. In addition, a prior knee arthroscopy was associated with a higher rate of postoperative complications, with 30% of the complications requiring a reoperation, and 8.3% of the complications requiring a revision total knee arthroplasty. Kaplan-Meier survival curves showed a survival rate of only 86.8% in those patients with a prior knee arthroscopy (vs 98.1% in those without a prior knee surgery).22 Therefore, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy. After the initial 6-week follow-up visit, no significant difference was seen in the functional outcomes between those patients with or without a history of prior hip arthroscopy who received an HRA.
After analysis of patient outcomes using multiple clinical measurement tools, at 6-week, 3-month, 6-month, 1-year, and most recent follow-up intervals, the only significant difference between the 2 cohorts was the WOMAC score at 6-week follow-up. Interestingly, there was no significant difference seen in the other clinical assessments, including the SF-12 score, HHS, range of motion, or UCLA activity score (although this did trend towards significance). This can be explained by the difference in both the mode of administration and various metrics assessed by these instruments. In comparison to the HHS evaluation, the patient completes the WOMAC (rather than the clinician) and also provides a more detailed assessment of symptoms, pain, stiffness, and activities of daily living.17 Therefore, this study suggests that patients with a prior hip arthroscopy may require more time to return to their activities of daily living after an HRA. However, whether the statistically significant difference between the 2 scores translates into a clinically significant difference can be questioned.
The clinical outcomes of this series of patients were excellent at the short-term follow-up, and both groups achieved clinical results comparable to prior reported results of HRA.1,10,23,24 However, despite these results, there are several limitations to this study. First, longer-term follow-up is required to determine if any significant differences (such as aseptic loosening, infection, and prosthesis survival) are associated with a prior hip arthroscopy. In addition, this study included a relatively small cohort of patients who had a prior hip arthroscopy. However, a relatively large, single-surgeon database of 1357 HRAs was reviewed, with only 51 cases being reported (3.7%). With the increasing popularity of hip arthroscopy, the number of patients presenting for HRA will likely continue to increase. However, despite these limitations, this study shows that a prior hip arthroscopy does not appear to affect the short-term, clinical outcomes of a metal-on-metal HRA.
Metal-on-metal hip resurfacing arthroplasty (HRA) remains an alternative to total hip arthroplasty (THA) in appropriately selected, younger, active adults with degenerative hip disease.1-4 While concerns remain regarding the potential for adverse local tissue reactions from wear of the metal-on-metal bearing surface,5-8 10-year data from the Australian Orthopaedic Association National Joint Replacement Registry Annual Report9 showed a revision rate of only 6.3% when the Birmingham Hip Resurfacing (BHR) System was used (Smith & Nephew Inc, Memphis, Tennessee).In addition, in an independent review of 230 consecutive BHRs at a mean follow-up of 10.4 years, Coulter and colleagues10 showed encouraging clinical results, with a mean Oxford Hip Score of 45.0 and a mean University of California at Los Angeles (UCLA) activity score of 7.4.
Similar to the prior increase in popularity of HRA, hip arthroscopy has also become much more commonplace, and its indications continue to evolve.11 Hip arthroscopy has been used in the native hip joint to manage femoroacetabular impingement, labral tears, and iliopsoas tendinopathy, among other conditions.12 In addition, the use of hip arthroscopy has not been limited to the native hip but also has increased as a diagnostic and therapeutic procedure after hip arthroplasties. Bajwa and Villar12 found hip arthroscopy to be diagnostic in 23 of 24 patients who underwent the procedure after a hip arthroplasty, concluding that arthroscopy is a useful adjunct in the diagnosis of symptomatic arthroplasties.
Therefore, hip arthroscopy has been shown to be an effective modality to treat pathology in both the native hip and after hip arthroplasties. However, the effect of a prior hip arthroscopy on the outcome of a subsequent metal-on-metal HRA has not been determined. Piedade and colleagues13 showed a prior knee arthroscopy to increase the risk of postoperative complications and subsequent revision after total knee arthroplasty. Complications included reflex sympathetic dystrophy, undiagnosed pain, infection, stiffness, and component loosening. A prior osteochondroplasty at the femoral head-neck junction could increase the risk of femoral neck fracture after a subsequent HRA. Thus, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy and to compare these results with a cohort of patients who received an HRA with no prior hip surgeries. Our hypothesis is that a prior hip arthroscopy will lead to inferior outcomes in patients undergoing HRA.
Materials and Methods
This study is a retrospective, case-control study using a 1:2 matching analysis. Dr. Su performed all HRAs, which were enrolled in an institutional review board–approved arthroplasty registry. All HRAs were performed using the BHR System.
The surgical technique for hip resurfacing arthroplasty has been described.1 All procedures were performed via a posterior approach with the patient in the lateral decubitus position. All patients received a hybrid metal-on-metal hip resurfacing, with an uncemented acetabular component and cemented femoral component. Intraoperative anesthesia for all patients was performed via a combined spinal-epidural anesthetic, and an epidural patient-controlled analgesic was used for the first day postoperatively, followed by a transition to oral analgesics. The sizes of the acetabular and femoral components were recorded for each hip resurfacing. Postoperatively, intermittent pneumatic compression devices were placed upon arrival in the recovery room, and active ankle flexion and extension exercises were initiated immediately after the patient’s neurologic function returned.14 Aspirin was used for chemical deep venous thrombosis prophylaxis in all patients postoperatively for a period of 6 weeks. Full weight-bearing, with the use of crutches for assistance with balance, was permitted immediately. Crutches were used for a period of 3 weeks prior to being discontinued.
From a database of 1357 HRAs (all BHR implants) performed between June 2006 and June 2012, 51 patients were identified who received an HRA after a prior hip arthroscopy. Eight patients were excluded because they did not possess adequate clinical documentation or were lost to follow-up. In the remaining 43 patients, there were 32 men and 11 women (21 right hips, 22 left hips), which formed the arthroscopy cohort. Two patients had a history of multiple hip arthroscopies (1 patient with 2 prior procedures, 1 patient with 3 prior procedures). The mean (SD) time from the most recent hip arthroscopy to the HRA was 2.5 (2.5) years. Table 1 presents a summary of the hip arthroscopy procedures (including only the most recent hip arthroscopy procedure in those with multiple arthroscopies).
Patient demographic variables (age, body mass index [BMI]) were recorded preoperatively, along with the Harris Hip Score (HHS),15 UCLA activity score,16 Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score,17 and preoperative hip range of motion (flexion, extension, abduction, adduction, internal rotation, and external rotation). The same clinical indices were assessed postoperatively along with the Short Form-12 (SF-12) Health Survey Score,18 at the 6-week, 3-month, 6-month, 1-year, and most recent follow-up visits.
Radiographic assessment consisted of a low anteroposterior (AP) pelvic radiograph (with the radiographic beam centered on the pubic symphysis) and a cross-table lateral radiograph obtained at the most recent follow-up visit. Both the acetabular component abduction relative to the inter-teardrop line, and the angle between the femoral stem and the anatomic axis of the femoral shaft (stem-shaft angle) were measured on AP radiographs.19,20 Acetabular component anteversion was measured on the cross-table lateral radiographs as the angle between the projected long axis of the acetabular opening and a line drawn perpendicular to the long axis plane of the body (Figures A, B).21
The same registry database was used to identify patients who received an HRA without a prior history of arthroscopy or hip surgery. A 1:2 matching analysis for those patients with a prior hip arthroscopy to those without a prior hip arthroscopy was performed to formulate a control group (control cohort) of 86 patients. Each patient in the arthroscopy cohort was matched with 2 patients in the control cohort based on the following parameters: age (± 6 years), sex (same), BMI (± 4 kg/m2), femoral head size (± 4 mm), and preoperative HHS and WOMAC scores (± 7 points). In the event an arthroscopy patient matched to 2 or more control patients, the patients who minimized the least squared error among the matching variables were selected.
Statistical Analysis
All data were collected and analyzed using Microsoft Excel software (Microsoft Corporation, Redmond, Washington). Statistical comparisons between the 2 cohorts regarding demographic variables, clinical outcomes, and radiographic alignment were performed using an unpaired, Student 2-tailed t test, with statistical significance set at P ≤ .05.
Results
A comparison of the results of the 1:2 matching analysis between the arthroscopy and control cohorts is presented in Table 2. There was no significant difference in the preoperative age, BMI, femoral head size, HHS, or WOMAC score between the 2 cohorts. However, the control cohort did show a more severe, preoperative flexion contracture (as expressed by a decreased amount of extension) and a decreased amount of preoperative abduction (Table 3). The preoperative UCLA activity score was also decreased in the control cohort, but this was not statistically significant.
The mean (SD) follow-up was 2.0 (1.0) years in the arthroscopy cohort and 2.1 (1.1) years in the control cohort. There was no significant difference in radiographic alignment between the 2 cohorts. The stem-shaft angle was 139.3° (SD, 5.4°) in the arthroscopy cohort (vs 138.3° [SD, 5.5°] in the control cohort; P = .3), the acetabular abduction was 43.9° (SD, 5.8°) in the arthroscopy cohort (vs 42.9° [SD, 6.1°] in the control cohort; P = .4), and the acetabular anteversion was 21.1° (SD, 7.5°) in the arthroscopy cohort (vs 20.8° [SD, 7.1°] in the control cohort; P = .8).
At 6-week follow-up, the arthroscopy cohort showed a significantly decreased WOMAC score compared with the control cohort (72.9 [SD, 15.5] vs 80.5 [SD, 11.8], respectively; P = .05). In addition, there was a trend towards a decreased SF-12 mental component score in the arthroscopy cohort (52.2 [SD, 9.3] vs 56.5 [SD, 7.8] in the control cohort; P = .06). However, none of the remaining clinical indices showed a significant difference between the 2 cohorts, and there was no difference in range of motion between the 2 cohorts at the 6-week follow-up visit (Table 4).
In addition, at 3-month follow-up, no statistically significant differences were seen between the 2 cohorts for any of the clinical indices or range of motion values. Both groups continued to improve rapidly, with HHS of 96.9 (SD, 3.5) in the arthroscopy cohort and 95.5 (SD, 6.6) in the control cohort, and WOMAC scores of 88.7 (SD, 10.2) and 89.5 (SD, 9.8), respectively (Table 5). Similarly, at the 6-month and 1-year follow-up intervals, the 2 cohorts showed continued improvement in their clinical measures, with no statistically significant differences between the 2 cohorts (Tables 6, 7).
At the most recent follow-up visit, more than 1 year after surgery, the HHS was 99.5 (SD, 1.3) in the arthroscopy cohort and 99.2 (SD, 9.7) in the control cohort (P = .9), and the WOMAC score was 93.5 (SD, 11.3) and 92.4 (SD, 12.2), respectively (P = .8). No significant perioperative complications were seen in the arthroscopy cohort. In the arthroscopy cohort, 1 patient was diagnosed with a deep venous thrombosis 2 weeks after the procedure and was placed on low-molecular-weight heparin and coumadin for treatment. A second patient in the arthroscopy cohort had continued serosanguinous drainage for 4 days postoperatively, which resolved with continued compressive dressings. To date, no patients in the arthroscopy or control cohorts have required a second operation or revision of their components.
Discussion
Given the increasing prevalence of hip arthroscopies to treat multiple disorders of the native joint, it is important to assess the potential consequences of these procedures on future arthroplasties. Piedade and colleagues,13 in a retrospective review of 1474 primary total knee arthroplasties, showed a prior bony procedure (high tibial osteotomy, tibial plateau fracture, patellar realignment) to be predictive of decreased range of motion postoperatively. In addition, a prior knee arthroscopy was associated with a higher rate of postoperative complications, with 30% of the complications requiring a reoperation, and 8.3% of the complications requiring a revision total knee arthroplasty. Kaplan-Meier survival curves showed a survival rate of only 86.8% in those patients with a prior knee arthroscopy (vs 98.1% in those without a prior knee surgery).22 Therefore, the purpose of this study was to evaluate the clinical outcomes of a series of patients who received an HRA after a prior hip arthroscopy. After the initial 6-week follow-up visit, no significant difference was seen in the functional outcomes between those patients with or without a history of prior hip arthroscopy who received an HRA.
After analysis of patient outcomes using multiple clinical measurement tools, at 6-week, 3-month, 6-month, 1-year, and most recent follow-up intervals, the only significant difference between the 2 cohorts was the WOMAC score at 6-week follow-up. Interestingly, there was no significant difference seen in the other clinical assessments, including the SF-12 score, HHS, range of motion, or UCLA activity score (although this did trend towards significance). This can be explained by the difference in both the mode of administration and various metrics assessed by these instruments. In comparison to the HHS evaluation, the patient completes the WOMAC (rather than the clinician) and also provides a more detailed assessment of symptoms, pain, stiffness, and activities of daily living.17 Therefore, this study suggests that patients with a prior hip arthroscopy may require more time to return to their activities of daily living after an HRA. However, whether the statistically significant difference between the 2 scores translates into a clinically significant difference can be questioned.
The clinical outcomes of this series of patients were excellent at the short-term follow-up, and both groups achieved clinical results comparable to prior reported results of HRA.1,10,23,24 However, despite these results, there are several limitations to this study. First, longer-term follow-up is required to determine if any significant differences (such as aseptic loosening, infection, and prosthesis survival) are associated with a prior hip arthroscopy. In addition, this study included a relatively small cohort of patients who had a prior hip arthroscopy. However, a relatively large, single-surgeon database of 1357 HRAs was reviewed, with only 51 cases being reported (3.7%). With the increasing popularity of hip arthroscopy, the number of patients presenting for HRA will likely continue to increase. However, despite these limitations, this study shows that a prior hip arthroscopy does not appear to affect the short-term, clinical outcomes of a metal-on-metal HRA.
1. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty. Surgical Technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):234-249.
2. Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86(2):177-184.
3. Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88(5):592-600.
4. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
5. Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92(16):2663-2671.
6. Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study.
J Bone Joint Surg Br. 2007;89(2):169-173.
7. deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92(12):1642-1647.
8. Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-450.
9. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. Adelaide: Australian Orthopaedic Association; 2011. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2011. Accessed September 16, 2014.
10. Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg Br. 2012;94(3):315-321.
11. McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop. 2011;469(2):362-371.
12. Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93(7):890-896.
13. Piedade SR, Pinaroli A, Servien E, Neyret P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2009;17(4):328-333.
14. Gonzalez Della Valle A, Serota A, Go G, et al. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop. 2006;(444):146-153.
15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
16. Kershaw CJ, Atkins RM, Dodd CA, Bulstrode CJ. Revision total hip arthroplasty for aseptic failure. A review of 276 cases. J Bone Joint Surg Br. 1991;73(4):564-568.
17. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473-2476.
18. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
19. Clark JM, Freeman MA, Witham D. The relationship of neck orientation to the shape of the proximal femur. J Arthroplasty. 1987;2(2):99-109.
20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
21. Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop. 1995;(316):106-111.
22. Piedade SR, Pinaroli A, Servien E, Neyret P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2737-2743.
23. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86(1):28-39.
24. Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br. 2008;90(4):436-441.
1. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty. Surgical Technique. J Bone Joint Surg Am. 2006;88(suppl 1 Pt 2):234-249.
2. Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86(2):177-184.
3. Pollard TC, Baker RP, Eastaugh-Waring SJ, Bannister GC. Treatment of the young active patient with osteoarthritis of the hip. A five- to seven-year comparison of hybrid total hip arthroplasty and metal-on-metal resurfacing. J Bone Joint Surg Br. 2006;88(5):592-600.
4. Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty. A minimum follow-up of five years. J Bone Joint Surg Br. 2005;87(2):167-170.
5. Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;92(16):2663-2671.
6. Daniel J, Ziaee H, Pradhan C, Pynsent PB, McMinn DJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study.
J Bone Joint Surg Br. 2007;89(2):169-173.
7. deSouza RM, Parsons NR, Oni T, Dalton P, Costa M, Krikler S. Metal ion levels following resurfacing arthroplasty of the hip: serial results over a ten-year period. J Bone Joint Surg Br. 2010;92(12):1642-1647.
8. Kwon YM, Thomas P, Summer B, et al. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;28(4):444-450.
9. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2011. Adelaide: Australian Orthopaedic Association; 2011. https://aoanjrr.dmac.adelaide.edu.au/annual-reports-2011. Accessed September 16, 2014.
10. Coulter G, Young DA, Dalziel RE, Shimmin AJ. Birmingham hip resurfacing at a mean of ten years: results from an independent centre. J Bone Joint Surg Br. 2012;94(3):315-321.
11. McCarthy JC, Jarrett BT, Ojeifo O, Lee JA, Bragdon CR. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop. 2011;469(2):362-371.
12. Bajwa AS, Villar RN. Arthroscopy of the hip in patients following joint replacement. J Bone Joint Surg Br. 2011;93(7):890-896.
13. Piedade SR, Pinaroli A, Servien E, Neyret P. Is previous knee arthroscopy related to worse results in primary total knee arthroplasty? Knee Surg Sports Traumatol Arthrosc. 2009;17(4):328-333.
14. Gonzalez Della Valle A, Serota A, Go G, et al. Venous thromboembolism is rare with a multimodal prophylaxis protocol after total hip arthroplasty. Clin Orthop. 2006;(444):146-153.
15. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
16. Kershaw CJ, Atkins RM, Dodd CA, Bulstrode CJ. Revision total hip arthroplasty for aseptic failure. A review of 276 cases. J Bone Joint Surg Br. 1991;73(4):564-568.
17. Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29(12):2473-2476.
18. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
19. Clark JM, Freeman MA, Witham D. The relationship of neck orientation to the shape of the proximal femur. J Arthroplasty. 1987;2(2):99-109.
20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217-220.
21. Yao L, Yao J, Gold RH. Measurement of acetabular version on the axiolateral radiograph. Clin Orthop. 1995;(316):106-111.
22. Piedade SR, Pinaroli A, Servien E, Neyret P. TKA outcomes after prior bone and soft tissue knee surgery. Knee Surg Sports Traumatol Arthrosc. 2013;21(12):2737-2743.
23. Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86(1):28-39.
24. Steffen RT, Pandit HP, Palan J, et al. The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg Br. 2008;90(4):436-441.