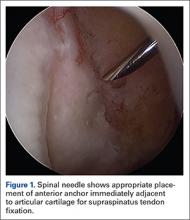
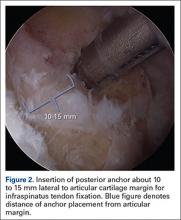
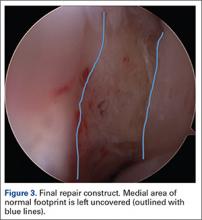
User login
Platelet-Rich Plasma Can Be Used to Successfully Treat Elbow Ulnar Collateral Ligament Insufficiency in High-Level Throwers
For overhead athletes, elbow ulnar collateral ligament (UCL) insufficiency is a potential career-ending injury. Baseball players with UCL insufficiency typically complain of medial-sided elbow pain that affects their ability to throw. Loss of velocity, loss of control, difficulty warming up, and pain while throwing are all symptoms of UCL injury.
Classically, nonoperative treatment of UCL injuries involves activity modification, use of anti-inflammatory medication, and a structured physical therapy program. Asymptomatic players can return to throwing after a structured interval throwing program. Rettig and colleagues1 found a 42% rate of success in conservatively treating UCL injuries in throwing athletes. UCL reconstruction is reserved for players with complete tears of the UCL or with partial tears after failed conservative treatment. Several techniques have been used to reconstruct the ligament, but successful outcomes depend on a long rehabilitation process. According to most published series, 85% to 90% of athletes who had UCL reconstruction returned to their previous level of play, but it took, on average, 9 to 12 months.2,3 This prolonged recovery period is one reason that some older professional baseball players, as well as casual high school and college players, elect to forgo surgery.
Over the past few years, platelet-rich plasma (PRP) has garnered attention as a bridge between conservative treatment and surgery. PRP refers to a sample of autologous blood that contains a platelet concentration higher than baseline levels. This sample often has a 3 to 5 times increase in growth factor concentration.4-6 Initial studies focused on its ability to successfully treat lateral epicondylitis.7-9 More recent clinical work has shown that PRP can potentially enhance healing after anterior cruciate ligament reconstruction,10-14 rotator cuff repair,15-17 and subacromial decompression.11,18-23 If PRP could be used to successfully treat UCL insufficiency that is refractory to conservative treatment, then year-long recovery periods could be avoided. This could potentially prolong certain athletes’ careers or, at the very least, allow them to return to play much sooner. In the present case series, we hypothesized that PRP injections could be used to successfully treat partial UCL tears in high-level throwing athletes, obviating the need for surgery and its associated prolonged recovery period.
Materials and Methods
Institutional Review Board approval was obtained for this retrospective study of 44 baseball players treated with PRP injections for partial-thickness UCL tears.
Patients provided written informed consent. They were diagnosed with UCL insufficiency by physical examination, and findings were confirmed by magnetic resonance imaging (MRI). After diagnosis, all throwers underwent a trial of conservative treatment that included rest, activity modification, use of anti-inflammatory medication, and physical therapy followed by an attempt to return to throwing using an interval throwing program.
Study inclusion criteria were physical examinations and MRI results consistent with UCL insufficiency, and failure of the conservative treatment plan described.
Patients were injected using the Autologous Conditioned Plasma system (Arthrex). PRP solutions were prepared according to manufacturer guidelines. After the elbow was prepared sterilely, the UCL was injected at the location of the tear. Typically, 3 mL of PRP was injected into the elbow. Sixteen patients had 1 injection, 6 had 2, and 22 had 3. Repeat injections were considered for recalcitrant pain after 3 weeks.
After injection, patients used acetaminophen and ice for pain control. Anti-inflammatory medications were avoided for a minimum of 2 weeks after injection. Typical postinjection therapy protocol consisted of rest followed by progressive stretching and strengthening for about 4 to 6 weeks before the start of an interval throwing program. Although there is no well-defined postinjection recovery protocol, as a general rule rest was prescribed for the first 2 weeks, followed by a progressive stretching and strengthening program for the next month. Patients who were asymptomatic subjectively and clinically—negative moving valgus stress test, negative milking maneuver, no pain with valgus stress—were started on an interval throwing program.
Final follow-up involved a physical examination. Results were classified according to a modified version of the Conway Scale12,24-26: excellent (return to preinjury level of competition or performance), good (return to play at a lower level of competition or performance or, specifically for baseball players, ability to throw in daily batting practice), fair (able to play recreationally), and poor (unable to return to previous sport at any level).
By final follow-up, all patients had completed their postoperative rehabilitation protocol, and all had at least tried to return to their previous activities. No patients were lost to follow-up.
Results
Of the 44 baseball players, 6 were professional, 14 were in college, and 24 were in high school. There were 36 pitchers and 8 position players. Mean age was 17.3 years (range, 16-28 years). All patients were available for follow-up after injection (mean, 11 months). Fifteen of the 44 players had an excellent outcome (34%), 17 had a good outcome, 2 had a fair outcome, and 10 had a poor outcome. After injection, 4 (67%) of the 6 professional baseball players returned to professional play. Five (36%) of the 14 college players had an excellent outcome, and 4 (17%) of the 24 high school players had an excellent outcome. Of the 8 position players, 4 had an excellent outcome, 3 had a good outcome, and 1 had a poor outcome.
Before treatment, all patients had medial-sided elbow pain over the UCL inhibiting their ability to throw. Mean duration of symptoms before injection was 8.8 months (range, 1-36 months). There was no correlation between symptom duration and any outcome measure. On MRI, 29 patients showed partial tears: 22 proximally based and 7 distally based. The other 15 patients had diffuse signal without partial tear. All 7 patients with distally based partial tears and 3 of the patients with proximally based partial tears had a poor outcome. Overall, there were 6 excellent, 7 good, and 2 fair outcomes in the partial-tear group. In the patients with diffuse signal without partial tear, there were 9 excellent and 10 good outcomes.
Mean time from injection to return to throwing was 5 weeks, and mean time to return to competition was 12 weeks (range, 5-24 weeks). The 1 player who returned at 5 weeks was a professional relief pitcher whose team was in the playoffs. He has now pitched for an additional 2 baseball seasons without elbow difficulty.
There were no injection-related complications.
Discussion
To our knowledge, this is the first report documenting successful PRP treatment of UCL insufficiency. In this study, 73% of players who had failed a course of conservative treatment had good to excellent outcomes with PRP injection.
Data on successful nonoperative treatment of UCL injuries are limited. Rettig and colleagues1 treated 31 throwing athletes’ UCL injuries with a supervised rehabilitation program. Treatment included rest, use of anti-inflammatory medication, progressive strengthening, and an interval throwing program. Only 41% of the athletes returned to their previous level of play, and it took, on average, 24.5 weeks. There was no significant difference in age or in duration or acuity of symptoms between those who returned to play and those whose conservative treatment failed.
Surgical reconstruction of UCL injuries has been very successful, with upward of 90% of athletes returning to previous level of play.3,27The procedure, however, is not without associated complications, including retear of the ligament, stiffness, ulnar nerve injury, and fracture.27-29 In addition, even when successful, the procedure requires that athletes take 9 to 12 months to recover before returning to competition at their previous level.
Savoie and colleagues,30 in their recent study on UCL repairs, highlighted an important fact that is often overlooked when reviewing the literature on UCL tears. Most of the literature on these injuries focuses on college and professional baseball players in whom ligament damage is often extensive, precluding repair. In contrast to prior reports, Savoie and colleagues30 found excellent results in 93% of their young athletes who underwent UCL repair. It is possible that their results can be attributed to the fact that many of their athletes had tears isolated to one area of the ligament, as opposed to generalized ligament incompetence. Our improved results vis-à-vis other reports on conservative management may be attributable to the same phenomenon.
PRP has garnered much attention in the literature and media because of its potential to enhance healing of tendons and ligaments; in some cases, it can obviate the need for surgery. After failure of other nonoperative measures in 15 patients with elbow epicondylitis, Mishra and Pavelko8 treated each patient with a single PRP injection. They prepared the PRP using the GPS III system (Biomet). At final follow-up, 93% improvement was seen. Clearly, their experiment had design flaws: It was nonblinded, and 3 of the 5 patients in the control group treated with bupivacaine injection withdrew from the experiment. Despite its shortcomings, their study became the impetus for several other studies.
A larger, double-blinded, randomized controlled trial comparing PRP and cortisone injections for lateral epicondylitis in 100 patients is under way, and preliminary results have been published.9 A minimum of 6 months after injection, patients who received PRP showed more improvement in visual analog scale (VAS) pain scores and Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire scores. In another large, double-blinded, randomized controlled trial, patients with chronic lateral epicondylitis had significant improvements in VAS pain scores and DASH scores relative to patients injected with corticosteroids with a 2-year follow-up.31 Similarly, Thanasas and colleagues32 found significantly reduced VAS pain scores in patients injected with PRP versus autologous whole blood. Another study demonstrated improved tendon morphology using ultrasound imaging 6 months after PRP injection.33
Contrary to these positive results, Krogh and colleagues34 found that a single injection of PRP or glucocorticoid was not significantly superior to a saline injection for reducing pain and disability over a 3-month period in patients with lateral epicondylitis. Their study, however, had major flaws. Its original design called for a 12-month follow-up, but there was massive dropout in all 3 treatment arms, necessitating reporting of only 3-month data. In addition, 60% of the patients in the glucocorticoid group were not naïve to this treatment, so definitive conclusions about the efficacy of glucocorticoids could not be made.
In the present study, we successfully treated partial ligament tears with PRP injections. Sixty-seven percent of our baseball players returned to play at a mean of 4 months, much earlier than the 9 to 12 months typically required after ligament reconstruction. Many athletes, such as high school baseball players or aging veteran professional baseball players, do not have the luxury of 12 months for recovery. Therefore, this select group of patients clearly has a limited window of opportunity to return to play. In fact, these patients might be ideal candidates for PRP injections for UCL injuries. Return-to-play rates, however, differed significantly among professional players and nonprofessional players. The difference may be attributable to professional players’ conditioning, quality of physical therapy, extrinsic motivation, and other intangible factors. Four (67%) of our 6 professional baseball players returned to professional play after injection, whereas only 36% of college players and 17% of high school players had excellent outcomes.
Limitations
The present study had several weaknesses, several of which are inherent to PRP studies conducted so far. It was not a prospective, randomized controlled trial. It is important to note that PRP treatment in diseased tissue may have some drawbacks, as its success depends on the ability of healing tissue to use concentrated growth factors and cytokines to proliferate.35 Thus, a chronically injured ligament with depleted active cells may have a diminished response to PRP. Another limitation of this study is that we evaluated outcomes based on return to play using the Conway Scale, which is well reported but not validated. Despite the potential weaknesses of this outcome scale, it has become the benchmark for measuring the success of outcomes of UCL reconstruction. Furthermore, we did not measure patients’ satisfaction with the treatment. Players who could not return to their preinjury level of play may have considered the treatment a failure regardless of their ability to continue throwing. Last, MRI was not repeated to document ligament healing. We did not routinely perform a second MRI because we thought it would not affect treatment. Several series have found a high incidence of abnormal signal in baseball players’ UCLs. In this group of patients, the most important outcome is return to previous level of competition.
This study raised several questions. Is one PRP brand better than another? Should more than 1 injection be given? What is the ideal postinjection protocol? Clearly, larger, prospective, randomized controlled studies are needed to truly elucidate the potential role of PRP in the treatment algorithm for UCL injury. Nevertheless, in certain cases in which traditional conservative measures have failed and patients do not have the luxury of rehabilitating for 9 to 12 months after surgery, PRP may be a viable treatment option.
Conclusion
In this study, use of PRP in the treatment of UCL insufficiency produced outcomes much better than earlier reported outcomes of conservative treatment of these injuries. PRP injections may be particularly beneficial in young athletes who have sustained acute damage to an isolated part of the ligament and in athletes unwilling or unable to undergo the extended rehabilitation required after surgical reconstruction of the ligament.
1. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
2. Eygendaal D, Rahussen FT, Diercks RL. Biomechanics of the elbow joint in tennis players and relation to pathology. Br J Sports Med. 2007;41(11):820-823.
3. Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19(2):110-117.
5. Kibler WB. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
7. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
8. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
9. Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113-125.
10. Kovacs MS. Applied physiology of tennis performance. Br J Sports Med. 2006;40(5):381-386.
11. Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180(1):80-88.
12. Pluim BM, Staal JB, Windler GE, Jayanthi N. Tennis injuries: occurrence, aetiology, and prevention. Br J Sports Med. 2006;40(5):415-423.
13. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214-222.
14. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269-278.
15. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102-2110.
16. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blinded, parallel-group trial. Am J Sports Med. 2013;41(10):2240-2248.
17. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet-rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
18. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
19. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
20. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
21. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035-1045.
22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718-1727.
23. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244-250.
24. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
25. Hutchinson MR, Laprade RF, Burnett QM 2nd, Moss R, Terpstra J. Injury surveillance at the USTA boys’ tennis championships: a 6-yr study. Med Sci Sports Exerc. 1995;27(6):826-830.
26. Winge S, Jørgensen U, Nielsen A. Epidemiology of injuries in Danish championship tennis. Int J Sports Med. 1989;10(5):368-371.
27. Safran MR, Hutchinson MR, Moss R, Albrandt J. A comparison of injuries in elite boys and girls tennis players. Paper presented at: 9th Annual Meeting of the Society of Tennis Medicine and Science; March 1999; Indian Wells, CA.
28. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
29. Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36(6):1061-1065.
30. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
31. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
32. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130-2134.
33. Chaudhury S, La Lama de M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
34. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
35. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
For overhead athletes, elbow ulnar collateral ligament (UCL) insufficiency is a potential career-ending injury. Baseball players with UCL insufficiency typically complain of medial-sided elbow pain that affects their ability to throw. Loss of velocity, loss of control, difficulty warming up, and pain while throwing are all symptoms of UCL injury.
Classically, nonoperative treatment of UCL injuries involves activity modification, use of anti-inflammatory medication, and a structured physical therapy program. Asymptomatic players can return to throwing after a structured interval throwing program. Rettig and colleagues1 found a 42% rate of success in conservatively treating UCL injuries in throwing athletes. UCL reconstruction is reserved for players with complete tears of the UCL or with partial tears after failed conservative treatment. Several techniques have been used to reconstruct the ligament, but successful outcomes depend on a long rehabilitation process. According to most published series, 85% to 90% of athletes who had UCL reconstruction returned to their previous level of play, but it took, on average, 9 to 12 months.2,3 This prolonged recovery period is one reason that some older professional baseball players, as well as casual high school and college players, elect to forgo surgery.
Over the past few years, platelet-rich plasma (PRP) has garnered attention as a bridge between conservative treatment and surgery. PRP refers to a sample of autologous blood that contains a platelet concentration higher than baseline levels. This sample often has a 3 to 5 times increase in growth factor concentration.4-6 Initial studies focused on its ability to successfully treat lateral epicondylitis.7-9 More recent clinical work has shown that PRP can potentially enhance healing after anterior cruciate ligament reconstruction,10-14 rotator cuff repair,15-17 and subacromial decompression.11,18-23 If PRP could be used to successfully treat UCL insufficiency that is refractory to conservative treatment, then year-long recovery periods could be avoided. This could potentially prolong certain athletes’ careers or, at the very least, allow them to return to play much sooner. In the present case series, we hypothesized that PRP injections could be used to successfully treat partial UCL tears in high-level throwing athletes, obviating the need for surgery and its associated prolonged recovery period.
Materials and Methods
Institutional Review Board approval was obtained for this retrospective study of 44 baseball players treated with PRP injections for partial-thickness UCL tears.
Patients provided written informed consent. They were diagnosed with UCL insufficiency by physical examination, and findings were confirmed by magnetic resonance imaging (MRI). After diagnosis, all throwers underwent a trial of conservative treatment that included rest, activity modification, use of anti-inflammatory medication, and physical therapy followed by an attempt to return to throwing using an interval throwing program.
Study inclusion criteria were physical examinations and MRI results consistent with UCL insufficiency, and failure of the conservative treatment plan described.
Patients were injected using the Autologous Conditioned Plasma system (Arthrex). PRP solutions were prepared according to manufacturer guidelines. After the elbow was prepared sterilely, the UCL was injected at the location of the tear. Typically, 3 mL of PRP was injected into the elbow. Sixteen patients had 1 injection, 6 had 2, and 22 had 3. Repeat injections were considered for recalcitrant pain after 3 weeks.
After injection, patients used acetaminophen and ice for pain control. Anti-inflammatory medications were avoided for a minimum of 2 weeks after injection. Typical postinjection therapy protocol consisted of rest followed by progressive stretching and strengthening for about 4 to 6 weeks before the start of an interval throwing program. Although there is no well-defined postinjection recovery protocol, as a general rule rest was prescribed for the first 2 weeks, followed by a progressive stretching and strengthening program for the next month. Patients who were asymptomatic subjectively and clinically—negative moving valgus stress test, negative milking maneuver, no pain with valgus stress—were started on an interval throwing program.
Final follow-up involved a physical examination. Results were classified according to a modified version of the Conway Scale12,24-26: excellent (return to preinjury level of competition or performance), good (return to play at a lower level of competition or performance or, specifically for baseball players, ability to throw in daily batting practice), fair (able to play recreationally), and poor (unable to return to previous sport at any level).
By final follow-up, all patients had completed their postoperative rehabilitation protocol, and all had at least tried to return to their previous activities. No patients were lost to follow-up.
Results
Of the 44 baseball players, 6 were professional, 14 were in college, and 24 were in high school. There were 36 pitchers and 8 position players. Mean age was 17.3 years (range, 16-28 years). All patients were available for follow-up after injection (mean, 11 months). Fifteen of the 44 players had an excellent outcome (34%), 17 had a good outcome, 2 had a fair outcome, and 10 had a poor outcome. After injection, 4 (67%) of the 6 professional baseball players returned to professional play. Five (36%) of the 14 college players had an excellent outcome, and 4 (17%) of the 24 high school players had an excellent outcome. Of the 8 position players, 4 had an excellent outcome, 3 had a good outcome, and 1 had a poor outcome.
Before treatment, all patients had medial-sided elbow pain over the UCL inhibiting their ability to throw. Mean duration of symptoms before injection was 8.8 months (range, 1-36 months). There was no correlation between symptom duration and any outcome measure. On MRI, 29 patients showed partial tears: 22 proximally based and 7 distally based. The other 15 patients had diffuse signal without partial tear. All 7 patients with distally based partial tears and 3 of the patients with proximally based partial tears had a poor outcome. Overall, there were 6 excellent, 7 good, and 2 fair outcomes in the partial-tear group. In the patients with diffuse signal without partial tear, there were 9 excellent and 10 good outcomes.
Mean time from injection to return to throwing was 5 weeks, and mean time to return to competition was 12 weeks (range, 5-24 weeks). The 1 player who returned at 5 weeks was a professional relief pitcher whose team was in the playoffs. He has now pitched for an additional 2 baseball seasons without elbow difficulty.
There were no injection-related complications.
Discussion
To our knowledge, this is the first report documenting successful PRP treatment of UCL insufficiency. In this study, 73% of players who had failed a course of conservative treatment had good to excellent outcomes with PRP injection.
Data on successful nonoperative treatment of UCL injuries are limited. Rettig and colleagues1 treated 31 throwing athletes’ UCL injuries with a supervised rehabilitation program. Treatment included rest, use of anti-inflammatory medication, progressive strengthening, and an interval throwing program. Only 41% of the athletes returned to their previous level of play, and it took, on average, 24.5 weeks. There was no significant difference in age or in duration or acuity of symptoms between those who returned to play and those whose conservative treatment failed.
Surgical reconstruction of UCL injuries has been very successful, with upward of 90% of athletes returning to previous level of play.3,27The procedure, however, is not without associated complications, including retear of the ligament, stiffness, ulnar nerve injury, and fracture.27-29 In addition, even when successful, the procedure requires that athletes take 9 to 12 months to recover before returning to competition at their previous level.
Savoie and colleagues,30 in their recent study on UCL repairs, highlighted an important fact that is often overlooked when reviewing the literature on UCL tears. Most of the literature on these injuries focuses on college and professional baseball players in whom ligament damage is often extensive, precluding repair. In contrast to prior reports, Savoie and colleagues30 found excellent results in 93% of their young athletes who underwent UCL repair. It is possible that their results can be attributed to the fact that many of their athletes had tears isolated to one area of the ligament, as opposed to generalized ligament incompetence. Our improved results vis-à-vis other reports on conservative management may be attributable to the same phenomenon.
PRP has garnered much attention in the literature and media because of its potential to enhance healing of tendons and ligaments; in some cases, it can obviate the need for surgery. After failure of other nonoperative measures in 15 patients with elbow epicondylitis, Mishra and Pavelko8 treated each patient with a single PRP injection. They prepared the PRP using the GPS III system (Biomet). At final follow-up, 93% improvement was seen. Clearly, their experiment had design flaws: It was nonblinded, and 3 of the 5 patients in the control group treated with bupivacaine injection withdrew from the experiment. Despite its shortcomings, their study became the impetus for several other studies.
A larger, double-blinded, randomized controlled trial comparing PRP and cortisone injections for lateral epicondylitis in 100 patients is under way, and preliminary results have been published.9 A minimum of 6 months after injection, patients who received PRP showed more improvement in visual analog scale (VAS) pain scores and Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire scores. In another large, double-blinded, randomized controlled trial, patients with chronic lateral epicondylitis had significant improvements in VAS pain scores and DASH scores relative to patients injected with corticosteroids with a 2-year follow-up.31 Similarly, Thanasas and colleagues32 found significantly reduced VAS pain scores in patients injected with PRP versus autologous whole blood. Another study demonstrated improved tendon morphology using ultrasound imaging 6 months after PRP injection.33
Contrary to these positive results, Krogh and colleagues34 found that a single injection of PRP or glucocorticoid was not significantly superior to a saline injection for reducing pain and disability over a 3-month period in patients with lateral epicondylitis. Their study, however, had major flaws. Its original design called for a 12-month follow-up, but there was massive dropout in all 3 treatment arms, necessitating reporting of only 3-month data. In addition, 60% of the patients in the glucocorticoid group were not naïve to this treatment, so definitive conclusions about the efficacy of glucocorticoids could not be made.
In the present study, we successfully treated partial ligament tears with PRP injections. Sixty-seven percent of our baseball players returned to play at a mean of 4 months, much earlier than the 9 to 12 months typically required after ligament reconstruction. Many athletes, such as high school baseball players or aging veteran professional baseball players, do not have the luxury of 12 months for recovery. Therefore, this select group of patients clearly has a limited window of opportunity to return to play. In fact, these patients might be ideal candidates for PRP injections for UCL injuries. Return-to-play rates, however, differed significantly among professional players and nonprofessional players. The difference may be attributable to professional players’ conditioning, quality of physical therapy, extrinsic motivation, and other intangible factors. Four (67%) of our 6 professional baseball players returned to professional play after injection, whereas only 36% of college players and 17% of high school players had excellent outcomes.
Limitations
The present study had several weaknesses, several of which are inherent to PRP studies conducted so far. It was not a prospective, randomized controlled trial. It is important to note that PRP treatment in diseased tissue may have some drawbacks, as its success depends on the ability of healing tissue to use concentrated growth factors and cytokines to proliferate.35 Thus, a chronically injured ligament with depleted active cells may have a diminished response to PRP. Another limitation of this study is that we evaluated outcomes based on return to play using the Conway Scale, which is well reported but not validated. Despite the potential weaknesses of this outcome scale, it has become the benchmark for measuring the success of outcomes of UCL reconstruction. Furthermore, we did not measure patients’ satisfaction with the treatment. Players who could not return to their preinjury level of play may have considered the treatment a failure regardless of their ability to continue throwing. Last, MRI was not repeated to document ligament healing. We did not routinely perform a second MRI because we thought it would not affect treatment. Several series have found a high incidence of abnormal signal in baseball players’ UCLs. In this group of patients, the most important outcome is return to previous level of competition.
This study raised several questions. Is one PRP brand better than another? Should more than 1 injection be given? What is the ideal postinjection protocol? Clearly, larger, prospective, randomized controlled studies are needed to truly elucidate the potential role of PRP in the treatment algorithm for UCL injury. Nevertheless, in certain cases in which traditional conservative measures have failed and patients do not have the luxury of rehabilitating for 9 to 12 months after surgery, PRP may be a viable treatment option.
Conclusion
In this study, use of PRP in the treatment of UCL insufficiency produced outcomes much better than earlier reported outcomes of conservative treatment of these injuries. PRP injections may be particularly beneficial in young athletes who have sustained acute damage to an isolated part of the ligament and in athletes unwilling or unable to undergo the extended rehabilitation required after surgical reconstruction of the ligament.
For overhead athletes, elbow ulnar collateral ligament (UCL) insufficiency is a potential career-ending injury. Baseball players with UCL insufficiency typically complain of medial-sided elbow pain that affects their ability to throw. Loss of velocity, loss of control, difficulty warming up, and pain while throwing are all symptoms of UCL injury.
Classically, nonoperative treatment of UCL injuries involves activity modification, use of anti-inflammatory medication, and a structured physical therapy program. Asymptomatic players can return to throwing after a structured interval throwing program. Rettig and colleagues1 found a 42% rate of success in conservatively treating UCL injuries in throwing athletes. UCL reconstruction is reserved for players with complete tears of the UCL or with partial tears after failed conservative treatment. Several techniques have been used to reconstruct the ligament, but successful outcomes depend on a long rehabilitation process. According to most published series, 85% to 90% of athletes who had UCL reconstruction returned to their previous level of play, but it took, on average, 9 to 12 months.2,3 This prolonged recovery period is one reason that some older professional baseball players, as well as casual high school and college players, elect to forgo surgery.
Over the past few years, platelet-rich plasma (PRP) has garnered attention as a bridge between conservative treatment and surgery. PRP refers to a sample of autologous blood that contains a platelet concentration higher than baseline levels. This sample often has a 3 to 5 times increase in growth factor concentration.4-6 Initial studies focused on its ability to successfully treat lateral epicondylitis.7-9 More recent clinical work has shown that PRP can potentially enhance healing after anterior cruciate ligament reconstruction,10-14 rotator cuff repair,15-17 and subacromial decompression.11,18-23 If PRP could be used to successfully treat UCL insufficiency that is refractory to conservative treatment, then year-long recovery periods could be avoided. This could potentially prolong certain athletes’ careers or, at the very least, allow them to return to play much sooner. In the present case series, we hypothesized that PRP injections could be used to successfully treat partial UCL tears in high-level throwing athletes, obviating the need for surgery and its associated prolonged recovery period.
Materials and Methods
Institutional Review Board approval was obtained for this retrospective study of 44 baseball players treated with PRP injections for partial-thickness UCL tears.
Patients provided written informed consent. They were diagnosed with UCL insufficiency by physical examination, and findings were confirmed by magnetic resonance imaging (MRI). After diagnosis, all throwers underwent a trial of conservative treatment that included rest, activity modification, use of anti-inflammatory medication, and physical therapy followed by an attempt to return to throwing using an interval throwing program.
Study inclusion criteria were physical examinations and MRI results consistent with UCL insufficiency, and failure of the conservative treatment plan described.
Patients were injected using the Autologous Conditioned Plasma system (Arthrex). PRP solutions were prepared according to manufacturer guidelines. After the elbow was prepared sterilely, the UCL was injected at the location of the tear. Typically, 3 mL of PRP was injected into the elbow. Sixteen patients had 1 injection, 6 had 2, and 22 had 3. Repeat injections were considered for recalcitrant pain after 3 weeks.
After injection, patients used acetaminophen and ice for pain control. Anti-inflammatory medications were avoided for a minimum of 2 weeks after injection. Typical postinjection therapy protocol consisted of rest followed by progressive stretching and strengthening for about 4 to 6 weeks before the start of an interval throwing program. Although there is no well-defined postinjection recovery protocol, as a general rule rest was prescribed for the first 2 weeks, followed by a progressive stretching and strengthening program for the next month. Patients who were asymptomatic subjectively and clinically—negative moving valgus stress test, negative milking maneuver, no pain with valgus stress—were started on an interval throwing program.
Final follow-up involved a physical examination. Results were classified according to a modified version of the Conway Scale12,24-26: excellent (return to preinjury level of competition or performance), good (return to play at a lower level of competition or performance or, specifically for baseball players, ability to throw in daily batting practice), fair (able to play recreationally), and poor (unable to return to previous sport at any level).
By final follow-up, all patients had completed their postoperative rehabilitation protocol, and all had at least tried to return to their previous activities. No patients were lost to follow-up.
Results
Of the 44 baseball players, 6 were professional, 14 were in college, and 24 were in high school. There were 36 pitchers and 8 position players. Mean age was 17.3 years (range, 16-28 years). All patients were available for follow-up after injection (mean, 11 months). Fifteen of the 44 players had an excellent outcome (34%), 17 had a good outcome, 2 had a fair outcome, and 10 had a poor outcome. After injection, 4 (67%) of the 6 professional baseball players returned to professional play. Five (36%) of the 14 college players had an excellent outcome, and 4 (17%) of the 24 high school players had an excellent outcome. Of the 8 position players, 4 had an excellent outcome, 3 had a good outcome, and 1 had a poor outcome.
Before treatment, all patients had medial-sided elbow pain over the UCL inhibiting their ability to throw. Mean duration of symptoms before injection was 8.8 months (range, 1-36 months). There was no correlation between symptom duration and any outcome measure. On MRI, 29 patients showed partial tears: 22 proximally based and 7 distally based. The other 15 patients had diffuse signal without partial tear. All 7 patients with distally based partial tears and 3 of the patients with proximally based partial tears had a poor outcome. Overall, there were 6 excellent, 7 good, and 2 fair outcomes in the partial-tear group. In the patients with diffuse signal without partial tear, there were 9 excellent and 10 good outcomes.
Mean time from injection to return to throwing was 5 weeks, and mean time to return to competition was 12 weeks (range, 5-24 weeks). The 1 player who returned at 5 weeks was a professional relief pitcher whose team was in the playoffs. He has now pitched for an additional 2 baseball seasons without elbow difficulty.
There were no injection-related complications.
Discussion
To our knowledge, this is the first report documenting successful PRP treatment of UCL insufficiency. In this study, 73% of players who had failed a course of conservative treatment had good to excellent outcomes with PRP injection.
Data on successful nonoperative treatment of UCL injuries are limited. Rettig and colleagues1 treated 31 throwing athletes’ UCL injuries with a supervised rehabilitation program. Treatment included rest, use of anti-inflammatory medication, progressive strengthening, and an interval throwing program. Only 41% of the athletes returned to their previous level of play, and it took, on average, 24.5 weeks. There was no significant difference in age or in duration or acuity of symptoms between those who returned to play and those whose conservative treatment failed.
Surgical reconstruction of UCL injuries has been very successful, with upward of 90% of athletes returning to previous level of play.3,27The procedure, however, is not without associated complications, including retear of the ligament, stiffness, ulnar nerve injury, and fracture.27-29 In addition, even when successful, the procedure requires that athletes take 9 to 12 months to recover before returning to competition at their previous level.
Savoie and colleagues,30 in their recent study on UCL repairs, highlighted an important fact that is often overlooked when reviewing the literature on UCL tears. Most of the literature on these injuries focuses on college and professional baseball players in whom ligament damage is often extensive, precluding repair. In contrast to prior reports, Savoie and colleagues30 found excellent results in 93% of their young athletes who underwent UCL repair. It is possible that their results can be attributed to the fact that many of their athletes had tears isolated to one area of the ligament, as opposed to generalized ligament incompetence. Our improved results vis-à-vis other reports on conservative management may be attributable to the same phenomenon.
PRP has garnered much attention in the literature and media because of its potential to enhance healing of tendons and ligaments; in some cases, it can obviate the need for surgery. After failure of other nonoperative measures in 15 patients with elbow epicondylitis, Mishra and Pavelko8 treated each patient with a single PRP injection. They prepared the PRP using the GPS III system (Biomet). At final follow-up, 93% improvement was seen. Clearly, their experiment had design flaws: It was nonblinded, and 3 of the 5 patients in the control group treated with bupivacaine injection withdrew from the experiment. Despite its shortcomings, their study became the impetus for several other studies.
A larger, double-blinded, randomized controlled trial comparing PRP and cortisone injections for lateral epicondylitis in 100 patients is under way, and preliminary results have been published.9 A minimum of 6 months after injection, patients who received PRP showed more improvement in visual analog scale (VAS) pain scores and Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire scores. In another large, double-blinded, randomized controlled trial, patients with chronic lateral epicondylitis had significant improvements in VAS pain scores and DASH scores relative to patients injected with corticosteroids with a 2-year follow-up.31 Similarly, Thanasas and colleagues32 found significantly reduced VAS pain scores in patients injected with PRP versus autologous whole blood. Another study demonstrated improved tendon morphology using ultrasound imaging 6 months after PRP injection.33
Contrary to these positive results, Krogh and colleagues34 found that a single injection of PRP or glucocorticoid was not significantly superior to a saline injection for reducing pain and disability over a 3-month period in patients with lateral epicondylitis. Their study, however, had major flaws. Its original design called for a 12-month follow-up, but there was massive dropout in all 3 treatment arms, necessitating reporting of only 3-month data. In addition, 60% of the patients in the glucocorticoid group were not naïve to this treatment, so definitive conclusions about the efficacy of glucocorticoids could not be made.
In the present study, we successfully treated partial ligament tears with PRP injections. Sixty-seven percent of our baseball players returned to play at a mean of 4 months, much earlier than the 9 to 12 months typically required after ligament reconstruction. Many athletes, such as high school baseball players or aging veteran professional baseball players, do not have the luxury of 12 months for recovery. Therefore, this select group of patients clearly has a limited window of opportunity to return to play. In fact, these patients might be ideal candidates for PRP injections for UCL injuries. Return-to-play rates, however, differed significantly among professional players and nonprofessional players. The difference may be attributable to professional players’ conditioning, quality of physical therapy, extrinsic motivation, and other intangible factors. Four (67%) of our 6 professional baseball players returned to professional play after injection, whereas only 36% of college players and 17% of high school players had excellent outcomes.
Limitations
The present study had several weaknesses, several of which are inherent to PRP studies conducted so far. It was not a prospective, randomized controlled trial. It is important to note that PRP treatment in diseased tissue may have some drawbacks, as its success depends on the ability of healing tissue to use concentrated growth factors and cytokines to proliferate.35 Thus, a chronically injured ligament with depleted active cells may have a diminished response to PRP. Another limitation of this study is that we evaluated outcomes based on return to play using the Conway Scale, which is well reported but not validated. Despite the potential weaknesses of this outcome scale, it has become the benchmark for measuring the success of outcomes of UCL reconstruction. Furthermore, we did not measure patients’ satisfaction with the treatment. Players who could not return to their preinjury level of play may have considered the treatment a failure regardless of their ability to continue throwing. Last, MRI was not repeated to document ligament healing. We did not routinely perform a second MRI because we thought it would not affect treatment. Several series have found a high incidence of abnormal signal in baseball players’ UCLs. In this group of patients, the most important outcome is return to previous level of competition.
This study raised several questions. Is one PRP brand better than another? Should more than 1 injection be given? What is the ideal postinjection protocol? Clearly, larger, prospective, randomized controlled studies are needed to truly elucidate the potential role of PRP in the treatment algorithm for UCL injury. Nevertheless, in certain cases in which traditional conservative measures have failed and patients do not have the luxury of rehabilitating for 9 to 12 months after surgery, PRP may be a viable treatment option.
Conclusion
In this study, use of PRP in the treatment of UCL insufficiency produced outcomes much better than earlier reported outcomes of conservative treatment of these injuries. PRP injections may be particularly beneficial in young athletes who have sustained acute damage to an isolated part of the ligament and in athletes unwilling or unable to undergo the extended rehabilitation required after surgical reconstruction of the ligament.
1. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
2. Eygendaal D, Rahussen FT, Diercks RL. Biomechanics of the elbow joint in tennis players and relation to pathology. Br J Sports Med. 2007;41(11):820-823.
3. Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19(2):110-117.
5. Kibler WB. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
7. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
8. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
9. Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113-125.
10. Kovacs MS. Applied physiology of tennis performance. Br J Sports Med. 2006;40(5):381-386.
11. Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180(1):80-88.
12. Pluim BM, Staal JB, Windler GE, Jayanthi N. Tennis injuries: occurrence, aetiology, and prevention. Br J Sports Med. 2006;40(5):415-423.
13. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214-222.
14. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269-278.
15. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102-2110.
16. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blinded, parallel-group trial. Am J Sports Med. 2013;41(10):2240-2248.
17. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet-rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
18. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
19. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
20. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
21. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035-1045.
22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718-1727.
23. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244-250.
24. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
25. Hutchinson MR, Laprade RF, Burnett QM 2nd, Moss R, Terpstra J. Injury surveillance at the USTA boys’ tennis championships: a 6-yr study. Med Sci Sports Exerc. 1995;27(6):826-830.
26. Winge S, Jørgensen U, Nielsen A. Epidemiology of injuries in Danish championship tennis. Int J Sports Med. 1989;10(5):368-371.
27. Safran MR, Hutchinson MR, Moss R, Albrandt J. A comparison of injuries in elite boys and girls tennis players. Paper presented at: 9th Annual Meeting of the Society of Tennis Medicine and Science; March 1999; Indian Wells, CA.
28. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
29. Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36(6):1061-1065.
30. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
31. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
32. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130-2134.
33. Chaudhury S, La Lama de M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
34. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
35. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
1. Rettig AC, Sherrill C, Snead DS, Mendler JC, Mieling P. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29(1):15-17.
2. Eygendaal D, Rahussen FT, Diercks RL. Biomechanics of the elbow joint in tennis players and relation to pathology. Br J Sports Med. 2007;41(11):820-823.
3. Bowers AL, Dines JS, Dines DM, Altchek DW. Elbow medial ulnar collateral ligament reconstruction: clinical relevance and the docking technique. J Shoulder Elbow Surg. 2010;19(2):110-117.
5. Kibler WB. Biomechanical analysis of the shoulder during tennis activities. Clin Sports Med. 1995;14(1):79-85.
5. Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489-496.
6. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
7. Elliott B, Fleisig G, Nicholls R, Escamilia R. Technique effects on upper limb loading in the tennis serve. J Sci Med Sport. 2003;6(1):76-87.
8. Mishra A, Pavelko T. Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med. 2006;34(11):1774-1778.
9. Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med. 2009;28(1):113-125.
10. Kovacs MS. Applied physiology of tennis performance. Br J Sports Med. 2006;40(5):381-386.
11. Xie X, Wu H, Zhao S, Xie G, Huangfu X, Zhao J. The effect of platelet-rich plasma on patterns of gene expression in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;180(1):80-88.
12. Pluim BM, Staal JB, Windler GE, Jayanthi N. Tennis injuries: occurrence, aetiology, and prevention. Br J Sports Med. 2006;40(5):415-423.
13. Xie X, Zhao S, Wu H, et al. Platelet-rich plasma enhances autograft revascularization and reinnervation in a dog model of anterior cruciate ligament reconstruction. J Surg Res. 2013;183(1):214-222.
14. Lopez-Vidriero E, Goulding KA, Simon DA, Sanchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26(2):269-278.
15. Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. Am J Sports Med. 2015;43(9):2102-2110.
16. Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blinded, parallel-group trial. Am J Sports Med. 2013;41(10):2240-2248.
17. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet-rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
18. Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518-528.
19. Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy. 2011;27(8):1029-1035.
20. Jo CH, Kim JE, Yoon KS, et al. Does platelet-rich plasma accelerate recovery after rotator cuff repair? A prospective cohort study. Am J Sports Med. 2011;39(10):2082-2090.
21. Jo CH, Kim JE, Yoon KS, Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am J Sports Med. 2012;40(5):1035-1045.
22. Chahal J, Van Thiel GS, Mall N, et al. The role of platelet-rich plasma in arthroscopic rotator cuff repair: a systematic review with quantitative synthesis. Arthroscopy. 2012;28(11):1718-1727.
23. Mei-Dan O, Carmont MR. The role of platelet-rich plasma in rotator cuff repair. Sports Med Arthrosc Rev. 2011;19(3):244-250.
24. Dines JS, ElAttrache NS, Conway JE, Smith W, Ahmad CS. Clinical outcomes of the DANE TJ technique to treat ulnar collateral ligament insufficiency of the elbow. Am J Sports Med. 2007;35(12):2039-2044.
25. Hutchinson MR, Laprade RF, Burnett QM 2nd, Moss R, Terpstra J. Injury surveillance at the USTA boys’ tennis championships: a 6-yr study. Med Sci Sports Exerc. 1995;27(6):826-830.
26. Winge S, Jørgensen U, Nielsen A. Epidemiology of injuries in Danish championship tennis. Int J Sports Med. 1989;10(5):368-371.
27. Safran MR, Hutchinson MR, Moss R, Albrandt J. A comparison of injuries in elite boys and girls tennis players. Paper presented at: 9th Annual Meeting of the Society of Tennis Medicine and Science; March 1999; Indian Wells, CA.
28. Cain EL, Andrews JR, Dugas JR, et al. Outcome of ulnar collateral ligament reconstruction of the elbow in 1281 athletes: results in 743 athletes with minimum 2-year follow-up. Am J Sports Med. 2010;38(12):2426-2434.
29. Dines JS, Yocum LA, Frank JB, ElAttrache NS, Gambardella RA, Jobe FW. Revision surgery for failed elbow medial collateral ligament reconstruction. Am J Sports Med. 2008;36(6):1061-1065.
30. Savoie FH, Trenhaile SW, Roberts J, Field LD, Ramsey JR. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: a case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36(6):1066-1072.
31. Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
32. Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39(10):2130-2134.
33. Chaudhury S, La Lama de M, Adler RS, et al. Platelet-rich plasma for the treatment of lateral epicondylitis: sonographic assessment of tendon morphology and vascularity (pilot study). Skeletal Radiol. 2013;42(1):91-97.
34. Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41(3):625-635.
35. Anz AW, Hackel JG, Nilssen EC, Andrews JR. Application of biologics in the treatment of the rotator cuff, meniscus, cartilage, and osteoarthritis. J Am Acad Orthop Surg. 2014;22(2):68-79.
Shoulder Instability Management: A Survey of the American Shoulder and Elbow Surgeons
Despite an abundance of peer-reviewed resources, there is wide variation in the surgical management of shoulder instability.1,2 Current American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines regarding the shoulder address only generalized shoulder pain, glenohumeral osteoarthritis, and rotator cuff injuries,3,4 and treatment algorithms focus on conservative treatment, rather than surgical recommendations.4-7
Shoulder instability most commonly results from 1 or more of 4 common lesions (capsular laxity, glenoid bone loss, humeral bone loss, and capsulolabral insufficiency).8 While it is a relatively common condition that represents 1% to 2% of all athletic injuries,9,10 little consensus exists about surgical indications, ideal treatment algorithms, or optimal operative technique. This is a critical issue because more than 50% of patients with glenohumeral instability will undergo surgical intervention.11 Chahal and associates6 surveyed 44 shoulder experts and reported strong consensus about diagnosis, but little agreement regarding surgical management. Owens and colleagues1 have also evaluated current trends for surgical treatment of this pathology. Randelli and associates5 attempted to categorize operative management based upon case-specific shoulder scenarios through online surveys. Their survey, however, covered a broad range of shoulder injuries rather than instability in particular. In this study, we assess trends for surgical management of glenohumeral instability in a case-based survey of shoulder experts.
Materials and Methods
Survey Information
An online survey (Survey Monkey) of 417 active members of the American Shoulder and Elbow Surgeons (ASES) was administered on May 1, 2014. Respondents were blinded to the institution and co-investigators conducting the survey. The survey link was distributed via email because it has been shown to be a more efficacious conduit than standard postal mail.12 The case-based, 25-question survey (Appendix) was designed to assess respondents’ selection of surgical intervention. Section 1 determined member demographics, including fellowship training, arthroscopy experience, and years of practice. Section 2 involved the presentation of 5 case scenarios. For each case, respondents were asked to identify the optimal surgical procedure in both primary and revision settings. Section 3 posed several general questions regarding shoulder-instability management.
Statistical Analysis
Data were stored using Microsoft Excel (Microsoft) and analyzed using SAS Software version 9.3 (SAS Institute, Inc.). Demographic survey responses were reported using descriptive statistics. Responses to clinical survey questions were reported using frequencies and percentages. To identify when a majority consensus was achieved for a given question, responses were flagged as reaching consensus when more than 50% of participants gave the same response.13In the event that only 2 response options were available, reaching consensus required 67% of respondents to choose a single answer (since, by default, a consensus would be reached with only 2 response options). Because this was an analysis of all respondents, an a priori power calculation was not performed. Associations between training and practice demographics and responses to clinical questions were investigated using chi-square analyses. All comparative analyses were two-tailed and used P = .05 as the threshold for statistical significance.
Results
Demographics
One hundred and twenty-five (29.9%) ASES members responded to the survey. Of the respondents, 71.2% reported at least 15 years of experience, and 71% performed more than 150 shoulder cases annually. Surgeons came from academic institutions (41.6%), private practice (24.8%), or mixed (33.6%). The majority of respondents were fellowship-trained in shoulder/elbow surgery (52.8%), while fewer had completed a sports-medicine fellowship (24.0%). For arthroscopic procedures, responses were nearly divided between those who preferred beach-chair positioning (47.2%) and those who preferred the lateral decubitus position (46.4%). The majority (70.4%) of respondents practiced in the United States and with a relatively even distribution among states and region. The remaining 29.6% of those surveyed practiced abroad.
Degree of Consensus Responses and Cases
Of the 25 survey questions, 6 questions were omitted from consensus calculations because these were designed for demographic categorization rather than professional opinion (questions 1-5, 8). Thirteen of the remaining 19 questions (68%) reached consensus response. All clinical case scenarios (5 of 5) reached consensus for selection of technique for the primary procedure; however, only 40% (2 of 5) of cases had a consensus in the revision setting.
In case 1, a young soccer player (noncontact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 81.6% of respondents. In the event of revision surgery, only 22.4% recommended arthroscopic Bankart repair, and the remainder split between open Bankart repair with possible capsular shift (36%) or Latarjet procedure (32.8%).
In case 2, a college American football player (contact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 56.8%. In the event of revision surgery, a majority of members (51.2%) suggested a Latarjet procedure.
In case 3, the weekend warrior with significant bone loss, most respondents recommended a Latarjet procedure for both primary (72.8%) and revision surgery (79.0%).
In case 4, a weekend warrior with multidirectional instability, 60% of respondents suggested arthroscopic Bankart repair, 21.6% recommended rotator interval closure, and 10.4% chose a capsular shift. As a revision procedure, there was less agreement, with a split between open Bankart repair (39.2%) and capsular shift only (39.2%).
In case 5, the weekend warrior with large engaging Hill-Sachs lesions, 60% of respondent selected a remplissage procedure. If revision was required, a Latarjet procedure was the choice of 48.8% of respondents (Table).
General Questions
For contact athletes, most respondents (87.2%) would allow return to play in the same season and recommended surgery after the end of the season. After surgical intervention, 56.8% prescribed 4 weeks of immobilization. When counseling a return to contact sports, 51.2% recommended waiting for 4 to 6 months.
The ASES members were divided on conservative management of instability injuries. Responses included immobilization in internal rotation (39.2%), no immobilization (39.2%), and external-rotation bracing (21.6%).
Finally, members thought the most important factor in choosing surgical technique was the patient’s pathology, then age; the least influential criteria was the patient’s sports participation.
Analysis of Training Demographics and Surgical Technique Preferences
Chi-square analyses demonstrated that respondents who completed a sports fellowship were more likely to do at least 50% of cases arthroscopically (odds ratio [OR], 15.3; P < .001) and were more likely to use the lateral decubitus position (OR, 2.8; P < .021). Furthermore, American respondents had a higher likelihood of having completed either a sports fellowship (OR, 12.8; P < .001) or a shoulder/elbow fellowship (OR, 4.6; P = .002) when compared with foreign respondents.
Discussion
In the absence of formal clinical practice guidelines, most surgeons formulate treatment strategy based upon a combination of experience and peer-reviewed evidence. The cohort analyzed in the current study was highly experienced, with more than 70% performing 150 shoulder cases annually and having more than 15 years of experience. We found a consensus response in 68% of questions and all primary surgical techniques for our shoulder instability scenarios. While expert consensus reported here is not equivalent to evidence-based clinical practice guidelines, it does provide important information to consider when treating anterior shoulder instability.
Specific responses to our case scenarios invite further reflection. Considering young (both noncontact and contact) athletes without bony pathology (cases 1 and 2, respectively), the ASES surgeons recommended arthroscopic Bankart repair for both. Randelli and associates5 found 71% of survey respondents recommended arthroscopic Bankart repair in a similar setting. It is interesting to note that consensus persisted regardless of the sport in which they engaged. Contact athletes have the highest rates of dislocation (up to 7 times higher incidence) compared with the general population.14 In addition, they have a higher recurrence rate after surgery.15 It should be noted, however, that although both cases reached consensus, the percentage of experts who recommended an arthroscopic procedure fell from 82% in the noncontact athlete to 57% in the contact athlete. This concurs with a recent review by Harris and Romeo,16 who recommended similar treatments for athletes without bony defects. In an older patient population with recurrent instability (case 3), responses varied more widely but still reached a consensus on primary surgical techniques. Respondents agreed that, even for patients with multidirectional instability, initial management should consist of arthroscopic capsulolabral repair. Overall, the agreement for arthroscopy for cases 1 through 3 mimics recent US practice patterns, showing 90% of stabilizations are being performed arthroscopically.17 Additionally, a recent meta-analysis by Harris and associates18 favored arthroscopic Bankart repair, showing no significant difference vs open stabilization even on long-term follow-up.
Glenoid bone loss is a difficult clinical scenario and that is reflected in this study’s findings. The literature suggests that arthroscopic Bankart repair, in this setting, is usually not sufficient and may result in a recurrence rate up to 75%, if bone loss greater than 20% is unaddressed.19 Our study supports this trend because ASES members recommended a Latarjet procedure when there is substantial bone loss.
While open Latarjet procedure was the consensus for dealing with glenoid bone loss, arthroscopic techniques were strongly favored for humeral head defects. This change in practice patterns results from the introduction of the arthroscopic remplissage technique.20 Two recent systemic reviews have supported this technique, reporting good functional outcomes for engaging Hill-Sachs lesions.21,22 Our study had similar agreement, with most respondents recommending remplissage for these patients.
This study found the lowest rates of expert consensus in the setting of revision surgery, likely caused, in part, by the paucity of available large cohort studies. This is a major void in the literature, and more studies are needed to help guide surgeons on the best techniques to deal with this difficult patient population.
Conservative bracing technique was 1 of the survey questions lacking a consensus response. Interestingly, 39% of members recommended no immobilization after an instability event. This contrasts with recent literature concerning the best position for bracing. We also found twice as many surgeons recommended internal rotation immobilization over external rotation. This is a subject of debate, with some studies stating improvement with external rotation immobilization,23 while other studies reported no difference.24 Overall, recommendations regarding type of immobilization are unclear, which will likely continue until larger studies can be performed.
The literature describing surgical trends in the treatment of shoulder instability is sparse and variable. With regard to other shoulder etiologies, only rotator cuff pathology has used expert consensus. Acevedo and colleagues13 reported agreement of ASES members surveyed regarding rotator cuff management. There was no consensus among surgeons in more than 50% of questions, despite AAOS published guidelines for rotator cuff treatment.25 Despite the lack of guidelines for our topic, we found a consensus among respondents with 68% of survey questions.
To date, only 2 studies of shoulder instability management have elicited the opinion of experts in shoulder surgery. Chahal and associates6 surveyed 42 members of ASES and JOINTS (Joined Orthopaedic Initiatives for National Trials of the Shoulder) Canada on shoulder instability cases and found substantial agreement on diagnosis but little consensus regarding surgical technique. This lack of agreement on procedures differs from our findings and may be related to their complicated case scenarios that generated a wide array of treatment recommendations. Randelli and colleagues5 surveyed more than 1000 European Society of Sports Traumatology, Knee Surgery, and Arthroscopy members and reported similar agreement on arthroscopic Bankart repair in young male shoulder-dislocation patients, although no other instability scenarios were investigated. Our study is the first to report responses from expert shoulder surgeons on surgical-treatment strategies for an array of common shoulder instability pathologies.
This study had several limitations. First, while our study suffered from a low response rate (29.9%), it was similar to other published studies.5,13 Second, because the case series included in the survey attempted to capture the most common instability scenarios, they were limited in their scope and failed to address additional etiologies or pathologic permutations. We believe, however, that a more comprehensive survey would have resulted in respondent fatigue and lowered the response rate. It is unlikely that any survey could capture all variables that come into play during clinical decision-making, and we sought to evaluate the most common shoulder instability scenarios. Third, 30% of respondents were from outside the United States, where the Latarjet procedure is much more popular. While this was not a majority, Latarjet’s regional preference may have decreased the consensus response in some scenarios if only the United States was included. Finally, there is inherent bias in a respondent pool that is heavily weighted to shoulder-surgery experts (ASES members) and does not consider the responses of the general orthopedic surgery community as have other studies.7
Conclusion
This study demonstrates that expert shoulder surgeons often agreed on shoulder-treatment principles for anterior shoulder instability. In the setting of primary repair, arthroscopic Bankart repair was favored in the absence of bony pathology, regardless of age (20 to 35 years) or nature of sport (contact versus noncontact). Latarjet procedures were favored in the setting of glenoid bone loss, and remplissage for an engaging Hill-Sachs lesion. Less agreement was observed for revision stabilization. It should be noted that, while consensus was often reached for our cases, there was a wide distribution of technical considerations and surgical preferences even among those who are fellowship-trained and high-volume surgeons, and who can be considered experts in the field of shoulder surgery.
1. Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-1869.
2. Loebenberg MI, Rosen JE, Ishak C, Jazrawi LM, Zuckerman JD. A survey of decision-making processes in the treatment of common shoulder ailments among primary care physicians. Bull Hosp Jt Dis. 2006;63(3-4):137-144.
3. American Academy of Orthopaedic Surgeons. AAOS clinical practice guidelines (CPG). www.aaos.org/research/guidelines/guide.asp. Updated December 30, 2013. Accessed May 1, 2015.
4. Sanders JO, Bozic KJ, Glassman SD, Jevsevar DS, Weber KL. Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg. 2014;22(3):135-144.
5. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2011;20(5):803-815.
6. Chahal J, Kassiri K, Dion A, MacDonald P, Leiter J. Diagnostic and treatment differences among experienced shoulder surgeons for instability conditions of the shoulder. Clin J Sport Med. 2007;17(1):5-9.
7. Redfern J, Burks R. 2009 survey results: surgeon practice patterns regarding arthroscopic surgery. Arthroscopy. 2009;25(12):1447-1452.
8. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic bankart repairs: significance of the inverted-pear glenoid and the humeral engaging hill-sachs lesion. Arthroscopy. 2000;16(7):677-694.
9. Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750-1754.
10. Owens MBD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
11. Hovelius L, Olofsson A, Sandström B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-952.
12. Raziano DB, Jayadevappa R, Valenzula D, Weiner M, Lavizzo-Mourey R. E-mail versus conventional postal mail survey of geriatric chiefs. Gerontologist. 2001;41(6):799-804.
13. Acevedo DC, Paxton ES, Williams GR, Abboud JA. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96(14):e123.
14. Kaplan LD, Flanigan DC, Norwig J, Jost P, Bradley J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005;33(8):1142-1146.
15. Petrera M, Dwyer T, Tsuji MR, Theodoropoulos JS. Outcomes of arthroscopic Bankart repair in collision versus noncollision athletes. Orthopedics. 2013;36(5):e621-e626.
16. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
17. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the united states. Arthroscopy. 2014;30(4):436-443.
18. Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-933.
19. Boileau P, Villalba M, Héry J, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755-1763.
20. Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs ”remplissage”: an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-726.
21. Buza JA 3rd, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549-555.
22. Rashid MS, Crichton J, Butt U, Akimau PI, Charalambous CP. Arthroscopic “Remplissage” for shoulder instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014:1-7.
23. Itoi E, Hatakeyama Y, Kido T, et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12(5):413-415.
24. Whelan DB, Litchfield R, Wambolt E, Dainty KN; Joint Orthopaedic Initiative for National Trials of the Shoulder (JOINTS). External rotation immobilization for primary shoulder dislocation: A randomized controlled trial. Clin Orthop Relat Res. 2014;472(8):2380-2386.
25. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
Despite an abundance of peer-reviewed resources, there is wide variation in the surgical management of shoulder instability.1,2 Current American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines regarding the shoulder address only generalized shoulder pain, glenohumeral osteoarthritis, and rotator cuff injuries,3,4 and treatment algorithms focus on conservative treatment, rather than surgical recommendations.4-7
Shoulder instability most commonly results from 1 or more of 4 common lesions (capsular laxity, glenoid bone loss, humeral bone loss, and capsulolabral insufficiency).8 While it is a relatively common condition that represents 1% to 2% of all athletic injuries,9,10 little consensus exists about surgical indications, ideal treatment algorithms, or optimal operative technique. This is a critical issue because more than 50% of patients with glenohumeral instability will undergo surgical intervention.11 Chahal and associates6 surveyed 44 shoulder experts and reported strong consensus about diagnosis, but little agreement regarding surgical management. Owens and colleagues1 have also evaluated current trends for surgical treatment of this pathology. Randelli and associates5 attempted to categorize operative management based upon case-specific shoulder scenarios through online surveys. Their survey, however, covered a broad range of shoulder injuries rather than instability in particular. In this study, we assess trends for surgical management of glenohumeral instability in a case-based survey of shoulder experts.
Materials and Methods
Survey Information
An online survey (Survey Monkey) of 417 active members of the American Shoulder and Elbow Surgeons (ASES) was administered on May 1, 2014. Respondents were blinded to the institution and co-investigators conducting the survey. The survey link was distributed via email because it has been shown to be a more efficacious conduit than standard postal mail.12 The case-based, 25-question survey (Appendix) was designed to assess respondents’ selection of surgical intervention. Section 1 determined member demographics, including fellowship training, arthroscopy experience, and years of practice. Section 2 involved the presentation of 5 case scenarios. For each case, respondents were asked to identify the optimal surgical procedure in both primary and revision settings. Section 3 posed several general questions regarding shoulder-instability management.
Statistical Analysis
Data were stored using Microsoft Excel (Microsoft) and analyzed using SAS Software version 9.3 (SAS Institute, Inc.). Demographic survey responses were reported using descriptive statistics. Responses to clinical survey questions were reported using frequencies and percentages. To identify when a majority consensus was achieved for a given question, responses were flagged as reaching consensus when more than 50% of participants gave the same response.13In the event that only 2 response options were available, reaching consensus required 67% of respondents to choose a single answer (since, by default, a consensus would be reached with only 2 response options). Because this was an analysis of all respondents, an a priori power calculation was not performed. Associations between training and practice demographics and responses to clinical questions were investigated using chi-square analyses. All comparative analyses were two-tailed and used P = .05 as the threshold for statistical significance.
Results
Demographics
One hundred and twenty-five (29.9%) ASES members responded to the survey. Of the respondents, 71.2% reported at least 15 years of experience, and 71% performed more than 150 shoulder cases annually. Surgeons came from academic institutions (41.6%), private practice (24.8%), or mixed (33.6%). The majority of respondents were fellowship-trained in shoulder/elbow surgery (52.8%), while fewer had completed a sports-medicine fellowship (24.0%). For arthroscopic procedures, responses were nearly divided between those who preferred beach-chair positioning (47.2%) and those who preferred the lateral decubitus position (46.4%). The majority (70.4%) of respondents practiced in the United States and with a relatively even distribution among states and region. The remaining 29.6% of those surveyed practiced abroad.
Degree of Consensus Responses and Cases
Of the 25 survey questions, 6 questions were omitted from consensus calculations because these were designed for demographic categorization rather than professional opinion (questions 1-5, 8). Thirteen of the remaining 19 questions (68%) reached consensus response. All clinical case scenarios (5 of 5) reached consensus for selection of technique for the primary procedure; however, only 40% (2 of 5) of cases had a consensus in the revision setting.
In case 1, a young soccer player (noncontact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 81.6% of respondents. In the event of revision surgery, only 22.4% recommended arthroscopic Bankart repair, and the remainder split between open Bankart repair with possible capsular shift (36%) or Latarjet procedure (32.8%).
In case 2, a college American football player (contact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 56.8%. In the event of revision surgery, a majority of members (51.2%) suggested a Latarjet procedure.
In case 3, the weekend warrior with significant bone loss, most respondents recommended a Latarjet procedure for both primary (72.8%) and revision surgery (79.0%).
In case 4, a weekend warrior with multidirectional instability, 60% of respondents suggested arthroscopic Bankart repair, 21.6% recommended rotator interval closure, and 10.4% chose a capsular shift. As a revision procedure, there was less agreement, with a split between open Bankart repair (39.2%) and capsular shift only (39.2%).
In case 5, the weekend warrior with large engaging Hill-Sachs lesions, 60% of respondent selected a remplissage procedure. If revision was required, a Latarjet procedure was the choice of 48.8% of respondents (Table).
General Questions
For contact athletes, most respondents (87.2%) would allow return to play in the same season and recommended surgery after the end of the season. After surgical intervention, 56.8% prescribed 4 weeks of immobilization. When counseling a return to contact sports, 51.2% recommended waiting for 4 to 6 months.
The ASES members were divided on conservative management of instability injuries. Responses included immobilization in internal rotation (39.2%), no immobilization (39.2%), and external-rotation bracing (21.6%).
Finally, members thought the most important factor in choosing surgical technique was the patient’s pathology, then age; the least influential criteria was the patient’s sports participation.
Analysis of Training Demographics and Surgical Technique Preferences
Chi-square analyses demonstrated that respondents who completed a sports fellowship were more likely to do at least 50% of cases arthroscopically (odds ratio [OR], 15.3; P < .001) and were more likely to use the lateral decubitus position (OR, 2.8; P < .021). Furthermore, American respondents had a higher likelihood of having completed either a sports fellowship (OR, 12.8; P < .001) or a shoulder/elbow fellowship (OR, 4.6; P = .002) when compared with foreign respondents.
Discussion
In the absence of formal clinical practice guidelines, most surgeons formulate treatment strategy based upon a combination of experience and peer-reviewed evidence. The cohort analyzed in the current study was highly experienced, with more than 70% performing 150 shoulder cases annually and having more than 15 years of experience. We found a consensus response in 68% of questions and all primary surgical techniques for our shoulder instability scenarios. While expert consensus reported here is not equivalent to evidence-based clinical practice guidelines, it does provide important information to consider when treating anterior shoulder instability.
Specific responses to our case scenarios invite further reflection. Considering young (both noncontact and contact) athletes without bony pathology (cases 1 and 2, respectively), the ASES surgeons recommended arthroscopic Bankart repair for both. Randelli and associates5 found 71% of survey respondents recommended arthroscopic Bankart repair in a similar setting. It is interesting to note that consensus persisted regardless of the sport in which they engaged. Contact athletes have the highest rates of dislocation (up to 7 times higher incidence) compared with the general population.14 In addition, they have a higher recurrence rate after surgery.15 It should be noted, however, that although both cases reached consensus, the percentage of experts who recommended an arthroscopic procedure fell from 82% in the noncontact athlete to 57% in the contact athlete. This concurs with a recent review by Harris and Romeo,16 who recommended similar treatments for athletes without bony defects. In an older patient population with recurrent instability (case 3), responses varied more widely but still reached a consensus on primary surgical techniques. Respondents agreed that, even for patients with multidirectional instability, initial management should consist of arthroscopic capsulolabral repair. Overall, the agreement for arthroscopy for cases 1 through 3 mimics recent US practice patterns, showing 90% of stabilizations are being performed arthroscopically.17 Additionally, a recent meta-analysis by Harris and associates18 favored arthroscopic Bankart repair, showing no significant difference vs open stabilization even on long-term follow-up.
Glenoid bone loss is a difficult clinical scenario and that is reflected in this study’s findings. The literature suggests that arthroscopic Bankart repair, in this setting, is usually not sufficient and may result in a recurrence rate up to 75%, if bone loss greater than 20% is unaddressed.19 Our study supports this trend because ASES members recommended a Latarjet procedure when there is substantial bone loss.
While open Latarjet procedure was the consensus for dealing with glenoid bone loss, arthroscopic techniques were strongly favored for humeral head defects. This change in practice patterns results from the introduction of the arthroscopic remplissage technique.20 Two recent systemic reviews have supported this technique, reporting good functional outcomes for engaging Hill-Sachs lesions.21,22 Our study had similar agreement, with most respondents recommending remplissage for these patients.
This study found the lowest rates of expert consensus in the setting of revision surgery, likely caused, in part, by the paucity of available large cohort studies. This is a major void in the literature, and more studies are needed to help guide surgeons on the best techniques to deal with this difficult patient population.
Conservative bracing technique was 1 of the survey questions lacking a consensus response. Interestingly, 39% of members recommended no immobilization after an instability event. This contrasts with recent literature concerning the best position for bracing. We also found twice as many surgeons recommended internal rotation immobilization over external rotation. This is a subject of debate, with some studies stating improvement with external rotation immobilization,23 while other studies reported no difference.24 Overall, recommendations regarding type of immobilization are unclear, which will likely continue until larger studies can be performed.
The literature describing surgical trends in the treatment of shoulder instability is sparse and variable. With regard to other shoulder etiologies, only rotator cuff pathology has used expert consensus. Acevedo and colleagues13 reported agreement of ASES members surveyed regarding rotator cuff management. There was no consensus among surgeons in more than 50% of questions, despite AAOS published guidelines for rotator cuff treatment.25 Despite the lack of guidelines for our topic, we found a consensus among respondents with 68% of survey questions.
To date, only 2 studies of shoulder instability management have elicited the opinion of experts in shoulder surgery. Chahal and associates6 surveyed 42 members of ASES and JOINTS (Joined Orthopaedic Initiatives for National Trials of the Shoulder) Canada on shoulder instability cases and found substantial agreement on diagnosis but little consensus regarding surgical technique. This lack of agreement on procedures differs from our findings and may be related to their complicated case scenarios that generated a wide array of treatment recommendations. Randelli and colleagues5 surveyed more than 1000 European Society of Sports Traumatology, Knee Surgery, and Arthroscopy members and reported similar agreement on arthroscopic Bankart repair in young male shoulder-dislocation patients, although no other instability scenarios were investigated. Our study is the first to report responses from expert shoulder surgeons on surgical-treatment strategies for an array of common shoulder instability pathologies.
This study had several limitations. First, while our study suffered from a low response rate (29.9%), it was similar to other published studies.5,13 Second, because the case series included in the survey attempted to capture the most common instability scenarios, they were limited in their scope and failed to address additional etiologies or pathologic permutations. We believe, however, that a more comprehensive survey would have resulted in respondent fatigue and lowered the response rate. It is unlikely that any survey could capture all variables that come into play during clinical decision-making, and we sought to evaluate the most common shoulder instability scenarios. Third, 30% of respondents were from outside the United States, where the Latarjet procedure is much more popular. While this was not a majority, Latarjet’s regional preference may have decreased the consensus response in some scenarios if only the United States was included. Finally, there is inherent bias in a respondent pool that is heavily weighted to shoulder-surgery experts (ASES members) and does not consider the responses of the general orthopedic surgery community as have other studies.7
Conclusion
This study demonstrates that expert shoulder surgeons often agreed on shoulder-treatment principles for anterior shoulder instability. In the setting of primary repair, arthroscopic Bankart repair was favored in the absence of bony pathology, regardless of age (20 to 35 years) or nature of sport (contact versus noncontact). Latarjet procedures were favored in the setting of glenoid bone loss, and remplissage for an engaging Hill-Sachs lesion. Less agreement was observed for revision stabilization. It should be noted that, while consensus was often reached for our cases, there was a wide distribution of technical considerations and surgical preferences even among those who are fellowship-trained and high-volume surgeons, and who can be considered experts in the field of shoulder surgery.
Despite an abundance of peer-reviewed resources, there is wide variation in the surgical management of shoulder instability.1,2 Current American Academy of Orthopaedic Surgeons (AAOS) clinical practice guidelines regarding the shoulder address only generalized shoulder pain, glenohumeral osteoarthritis, and rotator cuff injuries,3,4 and treatment algorithms focus on conservative treatment, rather than surgical recommendations.4-7
Shoulder instability most commonly results from 1 or more of 4 common lesions (capsular laxity, glenoid bone loss, humeral bone loss, and capsulolabral insufficiency).8 While it is a relatively common condition that represents 1% to 2% of all athletic injuries,9,10 little consensus exists about surgical indications, ideal treatment algorithms, or optimal operative technique. This is a critical issue because more than 50% of patients with glenohumeral instability will undergo surgical intervention.11 Chahal and associates6 surveyed 44 shoulder experts and reported strong consensus about diagnosis, but little agreement regarding surgical management. Owens and colleagues1 have also evaluated current trends for surgical treatment of this pathology. Randelli and associates5 attempted to categorize operative management based upon case-specific shoulder scenarios through online surveys. Their survey, however, covered a broad range of shoulder injuries rather than instability in particular. In this study, we assess trends for surgical management of glenohumeral instability in a case-based survey of shoulder experts.
Materials and Methods
Survey Information
An online survey (Survey Monkey) of 417 active members of the American Shoulder and Elbow Surgeons (ASES) was administered on May 1, 2014. Respondents were blinded to the institution and co-investigators conducting the survey. The survey link was distributed via email because it has been shown to be a more efficacious conduit than standard postal mail.12 The case-based, 25-question survey (Appendix) was designed to assess respondents’ selection of surgical intervention. Section 1 determined member demographics, including fellowship training, arthroscopy experience, and years of practice. Section 2 involved the presentation of 5 case scenarios. For each case, respondents were asked to identify the optimal surgical procedure in both primary and revision settings. Section 3 posed several general questions regarding shoulder-instability management.
Statistical Analysis
Data were stored using Microsoft Excel (Microsoft) and analyzed using SAS Software version 9.3 (SAS Institute, Inc.). Demographic survey responses were reported using descriptive statistics. Responses to clinical survey questions were reported using frequencies and percentages. To identify when a majority consensus was achieved for a given question, responses were flagged as reaching consensus when more than 50% of participants gave the same response.13In the event that only 2 response options were available, reaching consensus required 67% of respondents to choose a single answer (since, by default, a consensus would be reached with only 2 response options). Because this was an analysis of all respondents, an a priori power calculation was not performed. Associations between training and practice demographics and responses to clinical questions were investigated using chi-square analyses. All comparative analyses were two-tailed and used P = .05 as the threshold for statistical significance.
Results
Demographics
One hundred and twenty-five (29.9%) ASES members responded to the survey. Of the respondents, 71.2% reported at least 15 years of experience, and 71% performed more than 150 shoulder cases annually. Surgeons came from academic institutions (41.6%), private practice (24.8%), or mixed (33.6%). The majority of respondents were fellowship-trained in shoulder/elbow surgery (52.8%), while fewer had completed a sports-medicine fellowship (24.0%). For arthroscopic procedures, responses were nearly divided between those who preferred beach-chair positioning (47.2%) and those who preferred the lateral decubitus position (46.4%). The majority (70.4%) of respondents practiced in the United States and with a relatively even distribution among states and region. The remaining 29.6% of those surveyed practiced abroad.
Degree of Consensus Responses and Cases
Of the 25 survey questions, 6 questions were omitted from consensus calculations because these were designed for demographic categorization rather than professional opinion (questions 1-5, 8). Thirteen of the remaining 19 questions (68%) reached consensus response. All clinical case scenarios (5 of 5) reached consensus for selection of technique for the primary procedure; however, only 40% (2 of 5) of cases had a consensus in the revision setting.
In case 1, a young soccer player (noncontact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 81.6% of respondents. In the event of revision surgery, only 22.4% recommended arthroscopic Bankart repair, and the remainder split between open Bankart repair with possible capsular shift (36%) or Latarjet procedure (32.8%).
In case 2, a college American football player (contact athlete) with negligible bone loss, arthroscopic Bankart repair was recommended by 56.8%. In the event of revision surgery, a majority of members (51.2%) suggested a Latarjet procedure.
In case 3, the weekend warrior with significant bone loss, most respondents recommended a Latarjet procedure for both primary (72.8%) and revision surgery (79.0%).
In case 4, a weekend warrior with multidirectional instability, 60% of respondents suggested arthroscopic Bankart repair, 21.6% recommended rotator interval closure, and 10.4% chose a capsular shift. As a revision procedure, there was less agreement, with a split between open Bankart repair (39.2%) and capsular shift only (39.2%).
In case 5, the weekend warrior with large engaging Hill-Sachs lesions, 60% of respondent selected a remplissage procedure. If revision was required, a Latarjet procedure was the choice of 48.8% of respondents (Table).
General Questions
For contact athletes, most respondents (87.2%) would allow return to play in the same season and recommended surgery after the end of the season. After surgical intervention, 56.8% prescribed 4 weeks of immobilization. When counseling a return to contact sports, 51.2% recommended waiting for 4 to 6 months.
The ASES members were divided on conservative management of instability injuries. Responses included immobilization in internal rotation (39.2%), no immobilization (39.2%), and external-rotation bracing (21.6%).
Finally, members thought the most important factor in choosing surgical technique was the patient’s pathology, then age; the least influential criteria was the patient’s sports participation.
Analysis of Training Demographics and Surgical Technique Preferences
Chi-square analyses demonstrated that respondents who completed a sports fellowship were more likely to do at least 50% of cases arthroscopically (odds ratio [OR], 15.3; P < .001) and were more likely to use the lateral decubitus position (OR, 2.8; P < .021). Furthermore, American respondents had a higher likelihood of having completed either a sports fellowship (OR, 12.8; P < .001) or a shoulder/elbow fellowship (OR, 4.6; P = .002) when compared with foreign respondents.
Discussion
In the absence of formal clinical practice guidelines, most surgeons formulate treatment strategy based upon a combination of experience and peer-reviewed evidence. The cohort analyzed in the current study was highly experienced, with more than 70% performing 150 shoulder cases annually and having more than 15 years of experience. We found a consensus response in 68% of questions and all primary surgical techniques for our shoulder instability scenarios. While expert consensus reported here is not equivalent to evidence-based clinical practice guidelines, it does provide important information to consider when treating anterior shoulder instability.
Specific responses to our case scenarios invite further reflection. Considering young (both noncontact and contact) athletes without bony pathology (cases 1 and 2, respectively), the ASES surgeons recommended arthroscopic Bankart repair for both. Randelli and associates5 found 71% of survey respondents recommended arthroscopic Bankart repair in a similar setting. It is interesting to note that consensus persisted regardless of the sport in which they engaged. Contact athletes have the highest rates of dislocation (up to 7 times higher incidence) compared with the general population.14 In addition, they have a higher recurrence rate after surgery.15 It should be noted, however, that although both cases reached consensus, the percentage of experts who recommended an arthroscopic procedure fell from 82% in the noncontact athlete to 57% in the contact athlete. This concurs with a recent review by Harris and Romeo,16 who recommended similar treatments for athletes without bony defects. In an older patient population with recurrent instability (case 3), responses varied more widely but still reached a consensus on primary surgical techniques. Respondents agreed that, even for patients with multidirectional instability, initial management should consist of arthroscopic capsulolabral repair. Overall, the agreement for arthroscopy for cases 1 through 3 mimics recent US practice patterns, showing 90% of stabilizations are being performed arthroscopically.17 Additionally, a recent meta-analysis by Harris and associates18 favored arthroscopic Bankart repair, showing no significant difference vs open stabilization even on long-term follow-up.
Glenoid bone loss is a difficult clinical scenario and that is reflected in this study’s findings. The literature suggests that arthroscopic Bankart repair, in this setting, is usually not sufficient and may result in a recurrence rate up to 75%, if bone loss greater than 20% is unaddressed.19 Our study supports this trend because ASES members recommended a Latarjet procedure when there is substantial bone loss.
While open Latarjet procedure was the consensus for dealing with glenoid bone loss, arthroscopic techniques were strongly favored for humeral head defects. This change in practice patterns results from the introduction of the arthroscopic remplissage technique.20 Two recent systemic reviews have supported this technique, reporting good functional outcomes for engaging Hill-Sachs lesions.21,22 Our study had similar agreement, with most respondents recommending remplissage for these patients.
This study found the lowest rates of expert consensus in the setting of revision surgery, likely caused, in part, by the paucity of available large cohort studies. This is a major void in the literature, and more studies are needed to help guide surgeons on the best techniques to deal with this difficult patient population.
Conservative bracing technique was 1 of the survey questions lacking a consensus response. Interestingly, 39% of members recommended no immobilization after an instability event. This contrasts with recent literature concerning the best position for bracing. We also found twice as many surgeons recommended internal rotation immobilization over external rotation. This is a subject of debate, with some studies stating improvement with external rotation immobilization,23 while other studies reported no difference.24 Overall, recommendations regarding type of immobilization are unclear, which will likely continue until larger studies can be performed.
The literature describing surgical trends in the treatment of shoulder instability is sparse and variable. With regard to other shoulder etiologies, only rotator cuff pathology has used expert consensus. Acevedo and colleagues13 reported agreement of ASES members surveyed regarding rotator cuff management. There was no consensus among surgeons in more than 50% of questions, despite AAOS published guidelines for rotator cuff treatment.25 Despite the lack of guidelines for our topic, we found a consensus among respondents with 68% of survey questions.
To date, only 2 studies of shoulder instability management have elicited the opinion of experts in shoulder surgery. Chahal and associates6 surveyed 42 members of ASES and JOINTS (Joined Orthopaedic Initiatives for National Trials of the Shoulder) Canada on shoulder instability cases and found substantial agreement on diagnosis but little consensus regarding surgical technique. This lack of agreement on procedures differs from our findings and may be related to their complicated case scenarios that generated a wide array of treatment recommendations. Randelli and colleagues5 surveyed more than 1000 European Society of Sports Traumatology, Knee Surgery, and Arthroscopy members and reported similar agreement on arthroscopic Bankart repair in young male shoulder-dislocation patients, although no other instability scenarios were investigated. Our study is the first to report responses from expert shoulder surgeons on surgical-treatment strategies for an array of common shoulder instability pathologies.
This study had several limitations. First, while our study suffered from a low response rate (29.9%), it was similar to other published studies.5,13 Second, because the case series included in the survey attempted to capture the most common instability scenarios, they were limited in their scope and failed to address additional etiologies or pathologic permutations. We believe, however, that a more comprehensive survey would have resulted in respondent fatigue and lowered the response rate. It is unlikely that any survey could capture all variables that come into play during clinical decision-making, and we sought to evaluate the most common shoulder instability scenarios. Third, 30% of respondents were from outside the United States, where the Latarjet procedure is much more popular. While this was not a majority, Latarjet’s regional preference may have decreased the consensus response in some scenarios if only the United States was included. Finally, there is inherent bias in a respondent pool that is heavily weighted to shoulder-surgery experts (ASES members) and does not consider the responses of the general orthopedic surgery community as have other studies.7
Conclusion
This study demonstrates that expert shoulder surgeons often agreed on shoulder-treatment principles for anterior shoulder instability. In the setting of primary repair, arthroscopic Bankart repair was favored in the absence of bony pathology, regardless of age (20 to 35 years) or nature of sport (contact versus noncontact). Latarjet procedures were favored in the setting of glenoid bone loss, and remplissage for an engaging Hill-Sachs lesion. Less agreement was observed for revision stabilization. It should be noted that, while consensus was often reached for our cases, there was a wide distribution of technical considerations and surgical preferences even among those who are fellowship-trained and high-volume surgeons, and who can be considered experts in the field of shoulder surgery.
1. Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-1869.
2. Loebenberg MI, Rosen JE, Ishak C, Jazrawi LM, Zuckerman JD. A survey of decision-making processes in the treatment of common shoulder ailments among primary care physicians. Bull Hosp Jt Dis. 2006;63(3-4):137-144.
3. American Academy of Orthopaedic Surgeons. AAOS clinical practice guidelines (CPG). www.aaos.org/research/guidelines/guide.asp. Updated December 30, 2013. Accessed May 1, 2015.
4. Sanders JO, Bozic KJ, Glassman SD, Jevsevar DS, Weber KL. Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg. 2014;22(3):135-144.
5. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2011;20(5):803-815.
6. Chahal J, Kassiri K, Dion A, MacDonald P, Leiter J. Diagnostic and treatment differences among experienced shoulder surgeons for instability conditions of the shoulder. Clin J Sport Med. 2007;17(1):5-9.
7. Redfern J, Burks R. 2009 survey results: surgeon practice patterns regarding arthroscopic surgery. Arthroscopy. 2009;25(12):1447-1452.
8. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic bankart repairs: significance of the inverted-pear glenoid and the humeral engaging hill-sachs lesion. Arthroscopy. 2000;16(7):677-694.
9. Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750-1754.
10. Owens MBD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
11. Hovelius L, Olofsson A, Sandström B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-952.
12. Raziano DB, Jayadevappa R, Valenzula D, Weiner M, Lavizzo-Mourey R. E-mail versus conventional postal mail survey of geriatric chiefs. Gerontologist. 2001;41(6):799-804.
13. Acevedo DC, Paxton ES, Williams GR, Abboud JA. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96(14):e123.
14. Kaplan LD, Flanigan DC, Norwig J, Jost P, Bradley J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005;33(8):1142-1146.
15. Petrera M, Dwyer T, Tsuji MR, Theodoropoulos JS. Outcomes of arthroscopic Bankart repair in collision versus noncollision athletes. Orthopedics. 2013;36(5):e621-e626.
16. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
17. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the united states. Arthroscopy. 2014;30(4):436-443.
18. Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-933.
19. Boileau P, Villalba M, Héry J, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755-1763.
20. Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs ”remplissage”: an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-726.
21. Buza JA 3rd, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549-555.
22. Rashid MS, Crichton J, Butt U, Akimau PI, Charalambous CP. Arthroscopic “Remplissage” for shoulder instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014:1-7.
23. Itoi E, Hatakeyama Y, Kido T, et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12(5):413-415.
24. Whelan DB, Litchfield R, Wambolt E, Dainty KN; Joint Orthopaedic Initiative for National Trials of the Shoulder (JOINTS). External rotation immobilization for primary shoulder dislocation: A randomized controlled trial. Clin Orthop Relat Res. 2014;472(8):2380-2386.
25. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
1. Owens BD, Harrast JJ, Hurwitz SR, Thompson TL, Wolf JM. Surgical trends in bankart repair: an analysis of data from the American Board of Orthopaedic Surgery certification examination. Am J Sports Med. 2011;39(9):1865-1869.
2. Loebenberg MI, Rosen JE, Ishak C, Jazrawi LM, Zuckerman JD. A survey of decision-making processes in the treatment of common shoulder ailments among primary care physicians. Bull Hosp Jt Dis. 2006;63(3-4):137-144.
3. American Academy of Orthopaedic Surgeons. AAOS clinical practice guidelines (CPG). www.aaos.org/research/guidelines/guide.asp. Updated December 30, 2013. Accessed May 1, 2015.
4. Sanders JO, Bozic KJ, Glassman SD, Jevsevar DS, Weber KL. Clinical practice guidelines: their use, misuse, and future directions. J Am Acad Orthop Surg. 2014;22(3):135-144.
5. Randelli P, Arrigoni P, Cabitza F, Ragone V, Cabitza P. Current practice in shoulder pathology: results of a web-based survey among a community of 1,084 orthopedic surgeons. Knee Surg Sports Traumatol Arthrosc. 2011;20(5):803-815.
6. Chahal J, Kassiri K, Dion A, MacDonald P, Leiter J. Diagnostic and treatment differences among experienced shoulder surgeons for instability conditions of the shoulder. Clin J Sport Med. 2007;17(1):5-9.
7. Redfern J, Burks R. 2009 survey results: surgeon practice patterns regarding arthroscopic surgery. Arthroscopy. 2009;25(12):1447-1452.
8. Burkhart SS, De Beer JF. Traumatic glenohumeral bone defects and their relationship to failure of arthroscopic bankart repairs: significance of the inverted-pear glenoid and the humeral engaging hill-sachs lesion. Arthroscopy. 2000;16(7):677-694.
9. Owens BD, Agel J, Mountcastle SB, Cameron KL, Nelson BJ. Incidence of glenohumeral instability in collegiate athletics. Am J Sports Med. 2009;37(9):1750-1754.
10. Owens MBD, Duffey ML, Nelson BJ, et al. The incidence and characteristics of shoulder instability at the United States Military Academy. Am J Sports Med. 2007;35(7):1168-1173.
11. Hovelius L, Olofsson A, Sandström B, et al. Nonoperative treatment of primary anterior shoulder dislocation in patients forty years of age and younger. A prospective twenty-five-year follow-up. J Bone Joint Surg Am. 2008;90(5):945-952.
12. Raziano DB, Jayadevappa R, Valenzula D, Weiner M, Lavizzo-Mourey R. E-mail versus conventional postal mail survey of geriatric chiefs. Gerontologist. 2001;41(6):799-804.
13. Acevedo DC, Paxton ES, Williams GR, Abboud JA. A survey of expert opinion regarding rotator cuff repair. J Bone Joint Surg Am. 2014;96(14):e123.
14. Kaplan LD, Flanigan DC, Norwig J, Jost P, Bradley J. Prevalence and variance of shoulder injuries in elite collegiate football players. Am J Sports Med. 2005;33(8):1142-1146.
15. Petrera M, Dwyer T, Tsuji MR, Theodoropoulos JS. Outcomes of arthroscopic Bankart repair in collision versus noncollision athletes. Orthopedics. 2013;36(5):e621-e626.
16. Harris JD, Romeo AA. Arthroscopic management of the contact athlete with instability. Clin Sports Med. 2013;32(4):709-730.
17. Zhang AL, Montgomery SR, Ngo SS, Hame SL, Wang JC, Gamradt SC. Arthroscopic versus open shoulder stabilization: current practice patterns in the united states. Arthroscopy. 2014;30(4):436-443.
18. Harris JD, Gupta AK, Mall NA, et al. Long-term outcomes after Bankart shoulder stabilization. Arthroscopy. 2013;29(5):920-933.
19. Boileau P, Villalba M, Héry J, Balg F, Ahrens P, Neyton L. Risk factors for recurrence of shoulder instability after arthroscopic Bankart repair. J Bone Joint Surg Am. 2006;88(8):1755-1763.
20. Purchase RJ, Wolf EM, Hobgood ER, Pollock ME, Smalley CC. Hill-sachs ”remplissage”: an arthroscopic solution for the engaging hill-sachs lesion. Arthroscopy. 2008;24(6):723-726.
21. Buza JA 3rd, Iyengar JJ, Anakwenze OA, Ahmad CS, Levine WN. Arthroscopic Hill-Sachs remplissage: a systematic review. J Bone Joint Surg Am. 2014;96(7):549-555.
22. Rashid MS, Crichton J, Butt U, Akimau PI, Charalambous CP. Arthroscopic “Remplissage” for shoulder instability: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2014:1-7.
23. Itoi E, Hatakeyama Y, Kido T, et al. A new method of immobilization after traumatic anterior dislocation of the shoulder: a preliminary study. J Shoulder Elbow Surg. 2003;12(5):413-415.
24. Whelan DB, Litchfield R, Wambolt E, Dainty KN; Joint Orthopaedic Initiative for National Trials of the Shoulder (JOINTS). External rotation immobilization for primary shoulder dislocation: A randomized controlled trial. Clin Orthop Relat Res. 2014;472(8):2380-2386.
25. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons Clinical Practice Guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
Arthroscopic Management of Full-Thickness Rotator Cuff Tears in Major League Baseball Pitchers: The Lateralized Footprint Repair Technique
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
Rotator cuff injuries can be a source of debilitating pain and dysfunction in athletes at all levels, occasionally precluding return to competitive sport. Overhead athletes place extraordinary physiologic demands on the shoulder, as humeral angular velocities of 7000° to 8000° per second and rotational torques higher than 70 Nm have been measured during the baseball pitch.1 Repetitive supraphysiologic loading of the rotator cuff throughout the coordinated phases of throwing can result in a characteristic spectrum of shoulder pathology in overhead throwers. Several studies have demonstrated partial-thickness articular-sided rotator cuff tears (RCTs) in the area of the posterior supraspinatus and anterior infraspinatus tendons.2-4 Although the precise mechanism remains unclear, plausible explanations for the pathogenesis of these injuries include eccentric tensile and shear forces that lead to tendon failure with repetitive throwing, as well as internal impingement (mechanical impingement of the aforementioned tendons against the posterosuperior glenoid at 90° of shoulder abduction and maximum external rotation).5,6
Whereas partial-thickness articular-sided RCTs have been described in overhead athletes with rotator cuff pathology, full-thickness tears are encountered less often.7,8 Accordingly, there is a paucity of literature on clinical outcomes in professional baseball players with these injuries. To our knowledge, only 2 studies have investigated functional outcomes of open surgical repair of full-thickness tears in this population, and the outcomes have been uniformly poor.8,9
An anatomical description of rotator cuff anatomy has demonstrated a consistent pattern of supraspinatus and infraspinatus tendon insertion relative to the articular surface, biceps groove, and the bare area of the humerus.10 Using gross and microscopic analyses, the authors noted that the supraspinatus tendon inserted immediately adjacent to the articular margin, and the infraspinatus and teres minor tapered laterally away from the margin to form the bare area. Detailed knowledge of the insertional anatomy of the rotator cuff is important, as surgical repair should recreate the broad footprint to restore normal biomechanics and increase the surface area available for healing.11,12 Medial advancement of the rotator cuff insertion during surgical repair can have deleterious biomechanical effects on glenohumeral motion.11
Given the unfavorable results found after routine open repair of full-thickness tears, we altered our approach to these injuries and adopted an arthroscopic technique in which the tendon is repaired immediately lateral to the anatomical footprint. Research studies have demonstrated that chronic stress from repetitive throwing can lead to attenuation of soft-tissue restraints, and we think preservation of these adaptive changes after surgical repair may be important for these athletes to maintain extraordinary glenohumeral rotation and achieve high throwing velocities.13 We conducted a study to describe the lateralized repair technique for full-thickness RCTs and to report functional outcomes in Major League Baseball (MLB) pitchers treated with this procedure at minimum 2-year follow-up. We hypothesized that use of this novel technique would result in a higher rate of return to preinjury level of play in comparison with open rotator cuff repair in comparable cohorts, as reported in other studies.8,9
Materials and Methods
After obtaining Institutional Review Board approval for this study, we performed a retrospective chart review of MLB players treated by Dr. Altchek. We identified all professional baseball players who received a diagnosis of full-thickness RCT after preoperative magnetic resonance imaging with subsequent confirmation during surgery. Any patient who underwent arthroscopic repair using the lateralized footprint technique was included in the study. Demographic and preoperative injury information was collected from the chart, and final follow-up data were collected at the last available clinic visit. From available team records, we also obtained return-to-play data and objective pitching statistics: seasons played, games played, innings pitched, strikeouts per 9 innings, walks per 9 innings, and earned run average.
Surgical Technique
We routinely perform arthroscopic rotator cuff repairs with the patient under regional anesthesia in the beach-chair position. The operative extremity is placed in a Spider Limb Positioner (Smith & Nephew) to facilitate easy manipulation of the arm throughout the procedure. A standard posterior portal is established, and then an anterior portal is placed in the superolateral aspect of the rotator interval directly anterior to the leading edge of the supraspinatus tendon. A lateral portal created 2 to 3 cm distal to the anterolateral margin of the acromion may be used as an additional working portal. A thorough diagnostic arthroscopy is performed to evaluate the glenohumeral joint for any concomitant intra-articular pathology. Particular attention is directed to inspection of the superior labrum, biceps tendon, and capsuloligamentous structures, as injuries to these structures are often associated with rotator cuff pathology in overhead athletes.
Once presence of an RCT is confirmed, a thorough subacromial bursectomy is performed to help with visualization and inspection of the injury. The tissue is provisionally grasped and mobilized to measure the amount of available tendon excursion. In this unique population, the vast majority of injuries are diagnosed in an expeditious manner, thereby precluding the presence of significant retraction, poor tissue quality, and inadequate mobilization of the tendons. The greater tuberosity is identified, and the area immediately adjacent to the articular margin is abraded with a mechanical shaver to enhance healing potential. For supraspinatus tears, an anchor is placed immediately lateral to the articular margin in the region of the anterior attachment of the rotator cable (Figure 1). The posterior anchor is placed about 10 to 15 mm lateral to the articular margin to reattach the infraspinatus tendon (Figure 2). When the medial row sutures are tied down, anatomical placement of these anchors effectively re-creates the bare area described by Curtis and colleagues10 (Figure 3). In most cases, the medial row sutures are left intact and fixed laterally with a knotless anchor to provide a transosseous equivalent (double-row) repair.
Results
We identified 6 MLB pitchers who underwent arthroscopic rotator cuff repair using the aforementioned technique over an 8-year period. Each patient presented with complaints of debilitating shoulder pain and decreased pitching performance, including loss of throwing accuracy and velocity. There were 4 right-hand–dominant pitchers and 2 left-hand–dominant pitchers; rotator cuff pathology was observed in the dominant pitching arm in each case. Three players were classified as starting pitchers; the other 3 pitched in a relief role. Mean age of all pitchers at time of surgery was 29.8 years (range, 25-37 years). According to records, 2 patients (33%) underwent previous rotator cuff débridement for partial-thickness RCTs before surgical intervention at our institution. Operative information on the depth of the partial-thickness tears observed during the previous procedures was not available for review. At time of rotator cuff repair, 3 patients (50%) underwent concomitant procedures, including superior labrum anterior-posterior (SLAP) lesion repair (1 patient) and posterior labrum débridement (2 patients). A double-row fixation construct was achieved in each case. Review of operative records revealed a mean tear size of 2.1 cm (range, 1.5-3.0 cm) measured anterior to posterior, and all tears involved the supraspinatus and/or infraspinatus tendons. Postoperative rehabilitation included immobilization in a sling for 4 weeks. Hand, wrist, and elbow range-of-motion (ROM) exercises were started immediately to help reduce inflammation. Passive ROM exercises in the plane of the scapula were begun 4 weeks after surgery. Isometric scapular stabilization exercises were also incorporated at that time. Active-assisted ROM exercises were started at about 6 weeks, and isometric strengthening exercises were started at week 8 with progression to eccentric strengthening and weight training at about 3 months. Most pitchers were allowed to begin an interval throwing program at 24 weeks. There were no significant differences in the therapy programs for pitchers who underwent concomitant labral procedures, but the patient who underwent SLAP repair was limited to 30° of external rotation and 90° of forward flexion, with avoidance of active biceps contractions, for the first 6 weeks of rehabilitation.
By mean follow-up of 66.7 months (range, 23.2-94.6 months), 5 pitchers (83%) returned to their preinjury level of competition for at least 1 full season. One player pitched at Minor League Class AA level for about 1 season but was forced to retire because of persistent symptoms related to the shoulder. This pitcher underwent simultaneous rotator cuff and SLAP lesion repair. Of the 5 pitchers who resumed MLB play, none returned to their preoperative pitching productivity; mean number of innings pitched decreased from 1806.5 to 183.7. Three (60%) of these 5 pitchers experienced a slight reduction in performance as measured by earned run average. Interestingly, both players over age 30 years at time of surgery, versus 3 of the 4 pitchers under age 30 years, returned to their preoperative level of competition for at least 1 season. The Table summarizes MLB player data and objective pitching statistics. There were no perioperative complications related to this arthroscopic technique, and there were no glenohumeral ROM deficits at final follow-up.
Discussion
Although the incidence of full-thickness RCTs in professional baseball players is presumably low, available studies suggest that it is a debilitating injury with a poor prognosis for return to high-level athletics. Mazoué and Andrews9 reviewed the outcomes of 16 professional baseball players (12 pitchers, 4 position players) who underwent mini-open repair of full-thickness RCTs that involved more than 90% of the rotator cuff. Fifteen patients underwent mini-open rotator cuff repair using suture anchors in the anatomical footprint along with bone tunnels established near the lateral margin of the greater tuberosity to create a 2-level anatomical repair. One patient was treated with a mini-open repair using suture anchors in the greater tuberosity with a side-side repair of a longitudinal split within the rotator cuff. In the evaluation of outcomes by player position, only 1 pitcher (8%) returned to a competitive level of pitching at a mean follow-up of 67 months. On review of 2 position players with a full-thickness RCT in the dominant shoulder, only 1 (50%) returned to Major League play at a mean follow-up of 62.5 months. The remaining 2 position players underwent surgical repair of the nondominant shoulder, and, not surprisingly, both returned to their previous level of athletic activity without any difficulty. These results should be examined carefully, as the associated pathology in this high-demand cohort should not be discounted. Eleven (almost 92%) of the 12 pitchers had undergone at least 1 previous procedure on the shoulder. Furthermore, at time of full-thickness rotator cuff repair, 9 (75%) of the 12 pitchers were treated for concomitant intra-articular pathology, including SLAP tears, capsular attenuation, and/or labral fraying. In our study, 50% of pitchers underwent an associated labral procedure. Although labral débridement did not have a significant effect on return to play, the 1 pitcher who underwent SLAP repair was not able to return to preinjury level of play.
Tibone and colleagues8 reviewed postoperative outcomes in 45 athletes with rotator cuff pathology. Within their series, 5 professional baseball pitchers with full-thickness tears were treated with open subacromial decompression and rotator cuff repair. Two baseball pitchers with RCTs larger than 2 cm underwent open transosseous footprint repair in which the cuff was reinserted using bone tunnels created within the greater tuberosity. At long-term follow-up, only 2 (40%) of the 5 pitchers returned to competitive pitching. Interestingly, both pitchers who underwent transosseous footprint fixation were unable to return to professional baseball.
Overhead athletes require a delicate balance of shoulder mobility and stability to meet the high functional demands of their sports. Significant debate continues as to whether innate alterations in glenohumeral mobility preselect individuals for overhead sports, or if these changes are acquired through adaptations in supporting soft-tissue and osseous structures. Sethi and colleagues14 used an instrumented manual laxity examination to compare anterior-posterior laxity in asymptomatic professional and Division I college baseball players. The authors noted asymmetric anterior-posterior translation (>3 mm) between the throwing shoulder and the nondominant shoulder in 12 (60%) of 20 professional pitchers and 10 (59%) of 17 college pitchers. Although the authors did not correlate translational differences with corresponding shoulder pathology, the observed asymmetry supported the idea that these athletes may experience adaptive glenohumeral changes with repetitive throwing. The association between adaptive changes and shoulder biomechanics has been studied. Burkhart and Lo15 used a cadaveric model to describe the cam effect of the proximal humerus and the biomechanical consequences of a relative reduction in this effect after pathologic changes within the glenohumeral joint (constriction of posteroinferior capsule). They noted that a posterosuperior shift in the glenohumeral contact point in the throwing position can result in anterior capsular redundancy that may contribute to microinstability of the shoulder. This relative laxity increases external rotation, resulting in increased torsional and shear forces at the rotator cuff insertion.16 Ultimately, these abnormal forces may predispose overhead athletes to rotator cuff injury.
Given the available literature, it is clear that full-thickness RCTs are potentially career-ending injuries for professional baseball players. The question arises as to why the results are so poor. Ultimately, the high incidence of concomitant intra-articular pathology associated with full-thickness RCTs underscores the severity of soft-tissue damage sustained with repetitive overhead throwing. Mazoué and Andrews9 proposed the presence of associated labral and capsular pathology as a potential explanation for poor outcomes of surgical repair. Given the myriad of additional pathology observed in each patient, it is difficult to ascertain the precise impact of these injuries on postoperative outcome. However, early diagnosis and aggressive surgical intervention are clearly necessary to prevent accumulative injury. Regarding surgical intervention, both Tibone and colleagues8 and Mazoué and Andrews9 reported use of an open surgical repair technique in which the tendon was repaired to the anatomical footprint. Certainly, the benefits of an all-arthroscopic technique include optimal visualization of the RCT, less perioperative morbidity, and minimal soft-tissue injury. With our arthroscopic technique, the rotator cuff was fixed immediately lateral to the anatomical footprint, thereby leaving the medial aspect of the footprint uncovered. Functionally, the goal of this procedure is to restore the integrity of the rotator cuff without compromising glenohumeral mobility acquired through soft-tissue adaptation. Investigation of the insertional anatomy of the rotator cuff has demonstrated that the supraspinatus tendon inserts about 0.9 mm from the edge of the articular surface, and the infraspinatus insertional footprint tapers away from the articular surface to form the bare area as it extends inferiorly on the greater tuberosity.10 We think preexisting adaptations in glenohumeral anatomy are important for peak performance in this unique population, and even small alterations in the repair location can have deleterious effects on throwing mechanics. Lateralized repair of the cuff precludes potential medialization of the cuff insertion and may facilitate preservation of soft-tissue adaptations that these athletes rely on to achieve extraordinary glenohumeral motion.
Interestingly, with this technique we noted a higher rate of return to MLB play in pitchers over age 30 years. Although several individual factors (eg, player talent level, work ethics, compliance with rehabilitation) may play a role in this finding, it is possible that older, more mature patients may be more willing to assume diminished roles to continue to play. Jones and colleagues17 recently reported similar findings in older MLB pitchers after revision ulnar collateral ligament reconstruction.
This study had several limitations. First, the patient cohort was small (a result of the nature and relatively infrequent incidence of the clinical problem). Second, clinical information was collected retrospectively, which limited our ability to determine precise differences between preoperative and postoperative glenohumeral ROM with this technique. Third, the cohort included patients who demonstrated additional intra-articular (labral) pathology. Although associated pathology is common in this high-demand athletic population, it is clear that advanced pathology (eg, SLAP tears) may affect clinical outcomes, as in our study. Despite these limitations, our study is the largest review of professional baseball players treated for full-thickness rotator cuff injuries with an arthroscopic technique. Overall, the results of this study are promising and call for further clinical and biomechanical evaluation.
Conclusion
Surgical management of rotator cuff injuries in professional baseball players remains an extremely difficult problem. Current studies of full-thickness RCTs highlight these athletes’ poor functional outcomes. These unfavorable results prompted us to alter our surgical technique. Initial outcomes have been encouraging, and extended follow-up in this cohort of patients will provide a more definitive assessment of the success of this technique.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
1. Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18(2):402-408.
2. Andrews JR, Broussard TS, Carson WG. Arthroscopy of the shoulder in the management of partial tears of the rotator cuff: a preliminary report. Arthroscopy. 1985;1(2):117-122.
3. Paley KJ, Jobe FW, Pink MM, Kvitne RS, ElAttrache NS. Arthroscopic findings in the overhead throwing athlete: evidence for posterior internal impingement of the rotator cuff. Arthroscopy. 2000;16(1):35-40.
4. Nakagawa S, Yoneda M, Hayashida K, Wakitani S, Okamura K. Greater tuberosity notch: an important indicator of articular-side partial rotator cuff tears in the shoulders of throwing athletes. Am J Sports Med. 2001;29(6):762-770.
5. Walch G, Boileau P, Noel E, Donell ST. Impingement of the deep surface of the supraspinatus tendon on the posterosuperior glenoid rim: an arthroscopic study. J Shoulder Elbow Surg. 1992;1(5):238-245.
6. Halbrecht JL, Tirman P, Atkin D. Internal impingement of the shoulder: comparison of findings between the throwing and nonthrowing shoulders of college baseball players. Arthroscopy. 1999;15(3):253-258.
7. Reynolds SB, Dugas JR, Cain EL, McMichael CS, Andrews JR. Debridement of small partial-thickness rotator cuff tears in elite overhead throwers. Clin Orthop Relat Res. 2008;466(3):614-621.
8. Tibone JE, Elrod B, Jobe FW, et al. Surgical treatment of tears of the rotator cuff in athletes. J Bone Joint Surg Am. 1986;68(6):887-891.
9. Mazoué C, Andrews JR. Repair of full-thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34():182-189.
10. Curtis AS, Burbank KM, Tierney JJ, Scheller AD, Curran AR. The insertional footprint of the rotator cuff: an anatomic study. Arthroscopy. 2006;22(6):603-609.
11. Liu J, Hughes RE, O’Driscoll SW, An K. Biomechanical effect of medial advancement of the supraspinatus tendon. J Bone Joint Surg Am. 1998;80(6):853-859.
12. Lo IK, Burkhart SS. Double row arthroscopic rotator cuff repair: re-establishing the footprint of the rotator cuff. Arthroscopy. 2003;19(9):1035-1042.
13. Borsa PA, Laudner KG, Sauers EL. Mobility and stability adaptations in the shoulder of the overhead athlete: a theoretical and evidence-based perspective. Sports Med. 2008;38(1):17-36.
14. Sethi PM, Tibone JE, Lee TQ. Quantitative assessment of glenohumeral translation in baseball players: a comparison of pitchers versus nonpitching athletes. Am J Sports Med. 2004;32(7):1711-1715.
15. Burkhart SS, Lo IK. The cam effect of the proximal humerus: its role in the production of relative capsular redundancy of the shoulder. Arthroscopy. 2007;23(3):241-246.
16. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19(4):404-420.
17. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elbow Surg. 2013;22(5):642-646.
Injury Trends in Major League Baseball Over 18 Seasons: 1998-2015
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
Epidemiology, Treatment, and Prevention of Lumbar Spine Injuries in Major League Baseball Players
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.