User login
Management of the Biconcave (B2) Glenoid in Shoulder Arthroplasty: Technical Considerations
Total shoulder arthroplasty (TSA) has demonstrated excellent long-term clinical outcomes for the treatment of advanced glenohumeral osteoarthritis (OA).1-5 Glenohumeral OA is characterized by a broad spectrum of glenoid pathology. Both the morphology of the glenoid and humeral head subluxation are important preoperative factors to evaluate, as these have been shown to adversely impact shoulder arthroplasty outcomes.6,7
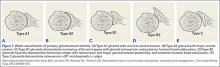
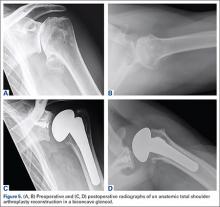
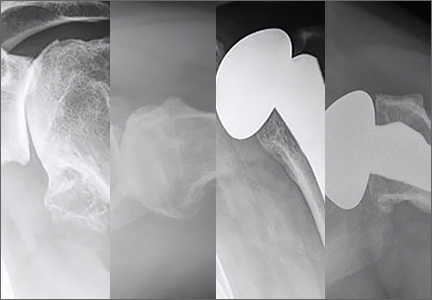
Walch and colleagues8 have previously classified glenoid morphology in cases of advanced glenohumeral arthritis based on the preoperative computed tomography (CT) scans of individuals undergoing shoulder arthroplasty (Figures 1A-1E). The biconcave (B2) glenoid is characterized by asymmetric posterior bone loss and a posterior translated humeral head that is seated in a biconcave glenoid. The degree and extent of bone loss in the B2 glenoid can be highly variable, ranging from the classic interpretation, in which 50% of the native glenoid fossa is preserved, to the more extreme case with little remaining native anterior glenoid. Scalise and colleagues9 have reported that determining the premorbid native glenoid version with a 3-dimensional (3D) glenoid vault model can aid in differentiating a pathologic B2 glenoid from a nonpathologic type C glenoid.
The B2 glenoid in particular has been associated with poor shoulder arthroplasty outcomes and component survivorship.6,10-12 There are many factors that are thought to contribute to this problem, such as glenoid component malposition, or undercorrection of the pathologic retroversion.6,13,14 Walch and colleagues10 reported that if the neoglenoid retroversion was greater than 27°, there was a 44% incidence of loosening and/or instability and 60% of the dislocations were observed when the humeral head subluxation was greater than 80%. Cases with severe posterior glenoid bone deficiency present a unique challenge to the surgeon, and the ability to accurately and securely place an implant in the correct anatomic position can be compromised. Standard TSA has proven excellent outcomes in the setting of typical glenohumeral OA, but in the B2 glenoid with significant posterior bone erosion, additional attention must be given to ensure adequate correction of the bony deformity, soft tissue balancing, and implant stability.
Several strategies that have been proposed to address extreme bone loss in the B2 glenoid will be discussed in this review. These include hemiarthroplasty, TSA with asymmetric reaming of the high side, TSA with bone grafting of the posterior glenoid bone loss, TSA with an augmented glenoid component, and reverse shoulder arthroplasty (RSA). Importantly, while these techniques have been proposed for managing the B2 glenoid, currently there is no gold standard consensus for the treatment of this condition. The purpose of this review is to highlight important characteristics of the B2 glenoid morphology on clinical outcomes and discuss the current surgical management options for this condition.
Preoperative Planning
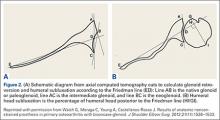
Being able to accurately determine the amount of retroversion is critical for preoperative planning. Friedman and colleagues15 initially described a method to measure glenoid retroversion; however, this is less accurate in B2 glenoids (Figures 2A, 2B). More recently, Rouleau and colleagues16 have validated and published methods to measure glenoid retroversion and subluxation in the B2 glenoid using 3 reference lines: the paleoglenoid (native glenoid surface), intermediate glenoid (line from anterior and posterior edge), and neoglenoid (eroded posterior surface) (Figure 2).
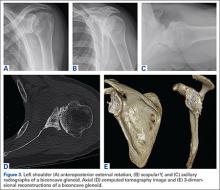
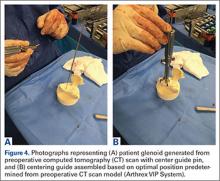
Preoperative evaluation starts with plain radiographs; however, additional imaging is needed, as the axillary view has shown to overestimate retroversion in 86% of patients (Figures 3A-3E).17 For a detailed evaluation of the glenoid retroversion and bone deficiency, CT scans with 3D reconstructions are useful.18,19 The surgical plan should be guided by the location and extent of glenoid bone loss. One tool that has been developed to help in predicting premorbid glenoid version, inclination, and position of the joint line is the 3D virtual glenoid vault model.9,20,21 This helps determine accurate premorbid glenoid anatomy and has been shown to assist in the selection of the optimal implant in an attempt to restore native glenoid anatomy, and avoid peg perforation.21 Patient-specific instrumentation (PSI) for shoulder arthroplasty is being used more frequently and has shown promise for more accurate glenoid component placement, particularly in the complex glenoid with severe bone deficiency. PSI involves creating a custom-fitted guide that is referenced to surface anatomy derived from the preoperative CT scan, which can then direct the surgeon toward optimal implant position with regard to glenoid component location, version and inclination (Figures 4A, 4B). Early reports show that PSI has resulted in a significant reduction in the frequency of malpositioned glenoid implants, with the greatest benefit observed in patients with retroversion in excess of 16°.22
Surgical Management
Hemiarthroplasty
Shoulder hemiarthroplasty has been traditionally described as an option for younger, more active patients in whom longevity of the glenoid component is a concern, or in patients with inadequate glenoid bone stock to tolerate a glenoid component. While there are no reports of hemiarthroplasty specifically for patients with B2 glenoids, one study has examined the effect of glenoid morphology on the outcomes of hemiarthroplasty for shoulder osteoarthritis. Levine and colleagues7 reported inferior clinical outcomes after shoulder hemiarthroplasty in patients with eccentric posterior glenoid wear. Several authors have advocated a “ream-and-run” technique to create a concentric glenoid and re-center the humeral head while still maintaining the native glenoid.23,24 However, in a recent series of 162 ream-and-run procedures, Gilmer and colleagues25 reported that only 23% of patients with B2 glenoid geometry achieved a minimal clinically important change in patient-reported outcome scores and 14% required revision. Furthermore, Lynch and colleagues26 found that progressive medial erosion and recurrent posterior glenoid erosion occur in a significant percentage of patients at early follow-up. Given these recent findings, the use of hemiarthroplasty alone or a ream-and-run procedure for patients with B2 glenoid morphology should be approached with caution.
Total Shoulder Arthroplasty
As with any TSA, the primary goals in treating patients with B2 glenoid defects are to provide the patient with a pain-free, stable, and functional shoulder (Figures 5A-5D). There are, however, a few challenges that are unique to TSA in the setting of B2 glenoid defects. Because the humeral head is often subluxated posteriorly into the defect, the anterior capsule and rotator cuff can tighten while the posterior aspect of the joint becomes lax. These soft tissues must be balanced during TSA in order to stabilize the shoulder and restore the appropriate length-tension relationship of the rotator cuff. The other primary concern is restoration of appropriate glenoid version and lateralization. To accomplish this, the most common techniques utilized are asymmetric reaming, bone graft augmentation, and glenoid component augmentation.27,28
Asymmetric Reaming. One of the more readily utilized techniques for addressing the B2 glenoid during TSA is eccentric or asymmetric reaming. During this process, the anterior glenoid is preferentially reamed while little to no bone is removed posteriorly. This technique is generally felt to be sufficient to treat posterior defects up to 5 mm to 8 mm or retroversion up to 15°.28 These upper limits have been confirmed in a number of cadaveric and simulated models.29-31
The success of this technique hinges on excellent glenoid exposure. With appropriate retractors in place, the anterior capsulolabral complex, including the biceps insertion, is resected to improve visualization. The inferior capsule must be resected carefully to ensure exposure and better motion postoperatively. On the other hand, it is imperative to protect the posterior capsulolabral attachments because of the increased risk of posterior instability in patients with B2 glenoids.
Detailed imaging such as CT scans with 3D reconstructions have improved our understanding of the degree of the deformities in all directions, which can better guide the reaming. PSI and planning software developed to improve the surgeon’s ability to place the glenoid component centrally in the best possible position after version correction can be even more helpful. We find that using a burr to provisionally lower the high side (anterior) provides a more en face view, which subsequently makes the eccentric reaming easier. As a guide, we will not ream more than 1 cm of anterior bone or attempt to correct more than ~20° of retroversion. The goal should be to create a glenoid surface that is more neutral and congruent to the posterior surface of the glenoid component while not overmedializing the component.
Although eccentric reaming may be one of the more straightforward methods for addressing posterior glenoid erosion, it is not without a number of potential downsides. When attempting to correct defects >10 mm or retroversion beyond 15°, excessive medialization of the implant can occur. Although increasing the thickness of the glenoid component can compensate for small amounts of medialization, excessive medialization can lead to a number of issues.27,28,32 As reaming progresses medially, the risk of keel penetration increases as the glenoid vault narrows.30,32 Further medialization decreases posterior cortical support for the implant, which increases the risk of component loosening and subsidence.33-35 The more medial the implant is placed, the smaller the surface of available bone for implant fixation. This often requires utilization of a smaller sized glenoid component that may result in component mismatch with the humeral implant. Finally, excessive medialization has the potential to under tension the rotator cuff, leading to decreased shoulder stability, strength, and function.
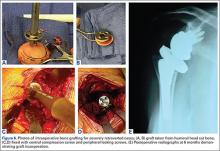
Bone Graft Augmentation. When posterior erosion becomes too excessive to address with eccentric reaming alone, defect augmentation is another option to consider (Figures 6A-6E). While technically more demanding, bone graft also provides the advantage of better re-creating the natural joint line and center of rotation of the glenohumeral joint.
For most defects, the resected humeral head provides the ideal source of graft. After initial reaming of the anterior glenoid, the defect must be sized and measured. We then recommend using a guided, cannulated system to place a central pin, lying perpendicular to the glenoid axis in neutral position. The anterior glenoid is then reamed enough to create a flat surface on which to attach the bone graft. The posterior surface is then gently burred to create a bleeding surface to enhance graft incorporation. The graft is then contoured to the defect and placed flush with the anterior glenoid. Cannulated screws are placed over guidewires to fix the graft. Using an arthroscopic cannula inserted posteriorly allows for easier placement of the guidewires and easier implantation of the screws. Although a reamer or burr can be used to contour the graft once it is fixed in place, this should be minimized to prevent loss of fixation. When the graft is fixed, we then cement the glenoid component into place.
Although good clinical results have been obtained with this technique, there is concern of incomplete graft healing and component loosening in the long term. Even in clinically asymptomatic and well functioning patients, some degree of radiographic lucency may be present in over 50% of cases.31,36,37 Glenoid Component Augmentation. To address the issues related to lucency and nonunion of bone graft augmentation, several augmented glenoid components have been developed. Augmented glenoid components have the benefit of filling posterior defects and stabilizing the shoulder without requiring excessive medialization (as often occurs with eccentric reaming) or union of a bone-to-bone interface (as is required in bone graft augmentation).38 Although many of the metal back designs experienced undesirably high failure rates and have since been recalled,39 more modern all-polyethylene components hold promise. The 2 most commonly utilized designs are the posterior step augment (DePuy) and the posterior wedge (Exactech). Although biomechanical analyses of both designs have demonstrated increased stability during loading in cadaveric and simulation models, the step augment (DePuy) has demonstrated increased stability and resistance to loosening.40,41 Although midterm results are not yet available for this newest generation of augmented components, short-term results with 2 to 3 years of follow-up have demonstrated excellent clinical outcomes.28
Reverse Total Shoulder Arthroplasty
While most commonly indicated for patients with rotator cuff tear arthropathy, RSA has recently been advocated for older patients with osteoarthritis and B2 glenoids in the setting of an intact rotator cuff. The semi-constrained design of the RSA is a potential solution to the static posterior humeral head subluxation seen in patients with B2 glenoid geometry (Figure 6E).
Technically, RSA is often an easier solution than a TSA with bone grafting because there is usually enough glenoid bone stock for fixation. That said, we always get a CT scan with 3D reconstructions to better appreciate the anatomy. Note that in B2 glenoids, the bone loss is typically posterior and inferior. RSA in the setting of a B2 glenoid is one of the ideal indications to use PSI to ensure ideal placement of the central pin, which is the key to glenoid baseplate positioning. Even when using a RSA, eccentric reaming and/or bone grafting allow for more ideal component placement. Using the same eccentric reaming techniques described above, one should try to ream to place the baseplate at 10° of retroversion. In cases where retroversion cannot be corrected to 10°, graft can be taken from the humeral head, iliac crest, or allograft. A benefit to using bone graft with RSA as opposed to TSA is that the graft can be fashioned to the baseplate, impacted/compressed into the B2 glenoid, and then secured with a central compression screw and peripheral locking screws.
Mizuno and colleagues41 reported a retrospective series of 27 RSAs performed for primary glenohumeral osteoarthritis and biconcave glenoid. At a mean follow-up of nearly 5 years, the authors noted significant improvement in Constant scores and shoulder motion with minimal complications. There was no recurrence of posterior instability observed by the time of final follow-up.41
RSA is a promising treatment for primary glenohumeral arthritis with posterior glenoid bone loss and static posterior subluxation in elderly or less active patients, but the longevity of these implants has yet to be established for younger, more active patients and requires further study.
Conclusion
Reconstruction of the B2 glenoid presents a challenging clinical problem that has been associated with poor clinical outcomes and implant survivorship. The high failure rate from glenoid component loosening and subsequent premature implant failure can be substantially decreased with accurate glenoid component positioning and appropriate correction of the pathologic glenoid retroversion. Careful preoperative planning is essential for accurate preparation and execution of the optimal surgical plan. There are many surgical strategies to address the B2 glenoid, but no consensus on the optimal method exists, as the technique should be uniquely customized to the individual’s pathology and surgeon preference (Table). Cases with mild deformity may be corrected with eccentric reaming and TSA, while the more severe deformities may require posterior glenoid bone grafting and/or augmented implants to restore native version. Finally, the RSA is a reliable option to restore stability and address bone deficiency for the severe B2 glenoid in an older, lower demand patient.
1. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
2. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
3. Matsen FA 3rd. Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am. 1996;78(2):260-264.
4. Fenlin JM Jr, Frieman BG. Indications, technique, and results of total shoulder arthroplasty in osteoarthritis. Orthop Clin North Am. 1998;29(3):423-434.
5. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: Analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517.
6. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
7. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
8. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
9. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
10. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-1533.
11. Kany J, Katz D. How to deal with glenoid type B2 or C? How to prevent mistakes in implantation of glenoid component? Eur J Orthop Surg Traumatol. 2013;23(4):379-385.
12. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82.
15. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
16. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230-1237.
17. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
18. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
19. Bokor DJ, O’Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg. 1999;8(6):595-598.
20. Ganapathi A, McCarron JA, Chen X, Iannotti JP. Predicting normal glenoid version from the pathologic scapula: A comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg. 2011;20(2):234-244.
21. Ricchetti ET, Hendel MD, Collins DN, Iannotti JP. Is premorbid glenoid anatomy altered in patients with glenohumeral osteoarthritis? Clin Orthop Relat Res. 2013;471(9):2932-2939.
22. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94(23):2167-2175.
23. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
24. Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA 3rd. Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20(4):609-615.
25. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: An analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
26. Lynch JR, Franta AK, Montgomery WH Jr, Lenters TR, Mounce D, Matsen FA 3rd. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292.
27. Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016;9(1):30-39.
28. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-848.
29. Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics. 2009;32(1):21.
30. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
31. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
32. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833.
33. Walch G, Young AA, Boileau P, Loew M, Gazielly D, Mole D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: Results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(2):145-150.
34. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: Multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
35. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072.
36. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973.
37. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
38. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157.
39. Iannotti JP, Lappin KE, Klotz CL, Reber EW, Swope SW. Liftoff resistance of augmented glenoid components during cyclic fatigue loading in the posterior-superior direction. J Shoulder Elbow Surg. 2013;22(11):1530-1536.
40. Knowles NK, Ferreira LM, Athwal GS. Augmented glenoid component designs for type B2 erosions: A computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg. 2015;24(8):1218-1226.
41. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
Total shoulder arthroplasty (TSA) has demonstrated excellent long-term clinical outcomes for the treatment of advanced glenohumeral osteoarthritis (OA).1-5 Glenohumeral OA is characterized by a broad spectrum of glenoid pathology. Both the morphology of the glenoid and humeral head subluxation are important preoperative factors to evaluate, as these have been shown to adversely impact shoulder arthroplasty outcomes.6,7
Walch and colleagues8 have previously classified glenoid morphology in cases of advanced glenohumeral arthritis based on the preoperative computed tomography (CT) scans of individuals undergoing shoulder arthroplasty (Figures 1A-1E). The biconcave (B2) glenoid is characterized by asymmetric posterior bone loss and a posterior translated humeral head that is seated in a biconcave glenoid. The degree and extent of bone loss in the B2 glenoid can be highly variable, ranging from the classic interpretation, in which 50% of the native glenoid fossa is preserved, to the more extreme case with little remaining native anterior glenoid. Scalise and colleagues9 have reported that determining the premorbid native glenoid version with a 3-dimensional (3D) glenoid vault model can aid in differentiating a pathologic B2 glenoid from a nonpathologic type C glenoid.
The B2 glenoid in particular has been associated with poor shoulder arthroplasty outcomes and component survivorship.6,10-12 There are many factors that are thought to contribute to this problem, such as glenoid component malposition, or undercorrection of the pathologic retroversion.6,13,14 Walch and colleagues10 reported that if the neoglenoid retroversion was greater than 27°, there was a 44% incidence of loosening and/or instability and 60% of the dislocations were observed when the humeral head subluxation was greater than 80%. Cases with severe posterior glenoid bone deficiency present a unique challenge to the surgeon, and the ability to accurately and securely place an implant in the correct anatomic position can be compromised. Standard TSA has proven excellent outcomes in the setting of typical glenohumeral OA, but in the B2 glenoid with significant posterior bone erosion, additional attention must be given to ensure adequate correction of the bony deformity, soft tissue balancing, and implant stability.
Several strategies that have been proposed to address extreme bone loss in the B2 glenoid will be discussed in this review. These include hemiarthroplasty, TSA with asymmetric reaming of the high side, TSA with bone grafting of the posterior glenoid bone loss, TSA with an augmented glenoid component, and reverse shoulder arthroplasty (RSA). Importantly, while these techniques have been proposed for managing the B2 glenoid, currently there is no gold standard consensus for the treatment of this condition. The purpose of this review is to highlight important characteristics of the B2 glenoid morphology on clinical outcomes and discuss the current surgical management options for this condition.
Preoperative Planning
Being able to accurately determine the amount of retroversion is critical for preoperative planning. Friedman and colleagues15 initially described a method to measure glenoid retroversion; however, this is less accurate in B2 glenoids (Figures 2A, 2B). More recently, Rouleau and colleagues16 have validated and published methods to measure glenoid retroversion and subluxation in the B2 glenoid using 3 reference lines: the paleoglenoid (native glenoid surface), intermediate glenoid (line from anterior and posterior edge), and neoglenoid (eroded posterior surface) (Figure 2).
Preoperative evaluation starts with plain radiographs; however, additional imaging is needed, as the axillary view has shown to overestimate retroversion in 86% of patients (Figures 3A-3E).17 For a detailed evaluation of the glenoid retroversion and bone deficiency, CT scans with 3D reconstructions are useful.18,19 The surgical plan should be guided by the location and extent of glenoid bone loss. One tool that has been developed to help in predicting premorbid glenoid version, inclination, and position of the joint line is the 3D virtual glenoid vault model.9,20,21 This helps determine accurate premorbid glenoid anatomy and has been shown to assist in the selection of the optimal implant in an attempt to restore native glenoid anatomy, and avoid peg perforation.21 Patient-specific instrumentation (PSI) for shoulder arthroplasty is being used more frequently and has shown promise for more accurate glenoid component placement, particularly in the complex glenoid with severe bone deficiency. PSI involves creating a custom-fitted guide that is referenced to surface anatomy derived from the preoperative CT scan, which can then direct the surgeon toward optimal implant position with regard to glenoid component location, version and inclination (Figures 4A, 4B). Early reports show that PSI has resulted in a significant reduction in the frequency of malpositioned glenoid implants, with the greatest benefit observed in patients with retroversion in excess of 16°.22
Surgical Management
Hemiarthroplasty
Shoulder hemiarthroplasty has been traditionally described as an option for younger, more active patients in whom longevity of the glenoid component is a concern, or in patients with inadequate glenoid bone stock to tolerate a glenoid component. While there are no reports of hemiarthroplasty specifically for patients with B2 glenoids, one study has examined the effect of glenoid morphology on the outcomes of hemiarthroplasty for shoulder osteoarthritis. Levine and colleagues7 reported inferior clinical outcomes after shoulder hemiarthroplasty in patients with eccentric posterior glenoid wear. Several authors have advocated a “ream-and-run” technique to create a concentric glenoid and re-center the humeral head while still maintaining the native glenoid.23,24 However, in a recent series of 162 ream-and-run procedures, Gilmer and colleagues25 reported that only 23% of patients with B2 glenoid geometry achieved a minimal clinically important change in patient-reported outcome scores and 14% required revision. Furthermore, Lynch and colleagues26 found that progressive medial erosion and recurrent posterior glenoid erosion occur in a significant percentage of patients at early follow-up. Given these recent findings, the use of hemiarthroplasty alone or a ream-and-run procedure for patients with B2 glenoid morphology should be approached with caution.
Total Shoulder Arthroplasty
As with any TSA, the primary goals in treating patients with B2 glenoid defects are to provide the patient with a pain-free, stable, and functional shoulder (Figures 5A-5D). There are, however, a few challenges that are unique to TSA in the setting of B2 glenoid defects. Because the humeral head is often subluxated posteriorly into the defect, the anterior capsule and rotator cuff can tighten while the posterior aspect of the joint becomes lax. These soft tissues must be balanced during TSA in order to stabilize the shoulder and restore the appropriate length-tension relationship of the rotator cuff. The other primary concern is restoration of appropriate glenoid version and lateralization. To accomplish this, the most common techniques utilized are asymmetric reaming, bone graft augmentation, and glenoid component augmentation.27,28
Asymmetric Reaming. One of the more readily utilized techniques for addressing the B2 glenoid during TSA is eccentric or asymmetric reaming. During this process, the anterior glenoid is preferentially reamed while little to no bone is removed posteriorly. This technique is generally felt to be sufficient to treat posterior defects up to 5 mm to 8 mm or retroversion up to 15°.28 These upper limits have been confirmed in a number of cadaveric and simulated models.29-31
The success of this technique hinges on excellent glenoid exposure. With appropriate retractors in place, the anterior capsulolabral complex, including the biceps insertion, is resected to improve visualization. The inferior capsule must be resected carefully to ensure exposure and better motion postoperatively. On the other hand, it is imperative to protect the posterior capsulolabral attachments because of the increased risk of posterior instability in patients with B2 glenoids.
Detailed imaging such as CT scans with 3D reconstructions have improved our understanding of the degree of the deformities in all directions, which can better guide the reaming. PSI and planning software developed to improve the surgeon’s ability to place the glenoid component centrally in the best possible position after version correction can be even more helpful. We find that using a burr to provisionally lower the high side (anterior) provides a more en face view, which subsequently makes the eccentric reaming easier. As a guide, we will not ream more than 1 cm of anterior bone or attempt to correct more than ~20° of retroversion. The goal should be to create a glenoid surface that is more neutral and congruent to the posterior surface of the glenoid component while not overmedializing the component.
Although eccentric reaming may be one of the more straightforward methods for addressing posterior glenoid erosion, it is not without a number of potential downsides. When attempting to correct defects >10 mm or retroversion beyond 15°, excessive medialization of the implant can occur. Although increasing the thickness of the glenoid component can compensate for small amounts of medialization, excessive medialization can lead to a number of issues.27,28,32 As reaming progresses medially, the risk of keel penetration increases as the glenoid vault narrows.30,32 Further medialization decreases posterior cortical support for the implant, which increases the risk of component loosening and subsidence.33-35 The more medial the implant is placed, the smaller the surface of available bone for implant fixation. This often requires utilization of a smaller sized glenoid component that may result in component mismatch with the humeral implant. Finally, excessive medialization has the potential to under tension the rotator cuff, leading to decreased shoulder stability, strength, and function.
Bone Graft Augmentation. When posterior erosion becomes too excessive to address with eccentric reaming alone, defect augmentation is another option to consider (Figures 6A-6E). While technically more demanding, bone graft also provides the advantage of better re-creating the natural joint line and center of rotation of the glenohumeral joint.
For most defects, the resected humeral head provides the ideal source of graft. After initial reaming of the anterior glenoid, the defect must be sized and measured. We then recommend using a guided, cannulated system to place a central pin, lying perpendicular to the glenoid axis in neutral position. The anterior glenoid is then reamed enough to create a flat surface on which to attach the bone graft. The posterior surface is then gently burred to create a bleeding surface to enhance graft incorporation. The graft is then contoured to the defect and placed flush with the anterior glenoid. Cannulated screws are placed over guidewires to fix the graft. Using an arthroscopic cannula inserted posteriorly allows for easier placement of the guidewires and easier implantation of the screws. Although a reamer or burr can be used to contour the graft once it is fixed in place, this should be minimized to prevent loss of fixation. When the graft is fixed, we then cement the glenoid component into place.
Although good clinical results have been obtained with this technique, there is concern of incomplete graft healing and component loosening in the long term. Even in clinically asymptomatic and well functioning patients, some degree of radiographic lucency may be present in over 50% of cases.31,36,37 Glenoid Component Augmentation. To address the issues related to lucency and nonunion of bone graft augmentation, several augmented glenoid components have been developed. Augmented glenoid components have the benefit of filling posterior defects and stabilizing the shoulder without requiring excessive medialization (as often occurs with eccentric reaming) or union of a bone-to-bone interface (as is required in bone graft augmentation).38 Although many of the metal back designs experienced undesirably high failure rates and have since been recalled,39 more modern all-polyethylene components hold promise. The 2 most commonly utilized designs are the posterior step augment (DePuy) and the posterior wedge (Exactech). Although biomechanical analyses of both designs have demonstrated increased stability during loading in cadaveric and simulation models, the step augment (DePuy) has demonstrated increased stability and resistance to loosening.40,41 Although midterm results are not yet available for this newest generation of augmented components, short-term results with 2 to 3 years of follow-up have demonstrated excellent clinical outcomes.28
Reverse Total Shoulder Arthroplasty
While most commonly indicated for patients with rotator cuff tear arthropathy, RSA has recently been advocated for older patients with osteoarthritis and B2 glenoids in the setting of an intact rotator cuff. The semi-constrained design of the RSA is a potential solution to the static posterior humeral head subluxation seen in patients with B2 glenoid geometry (Figure 6E).
Technically, RSA is often an easier solution than a TSA with bone grafting because there is usually enough glenoid bone stock for fixation. That said, we always get a CT scan with 3D reconstructions to better appreciate the anatomy. Note that in B2 glenoids, the bone loss is typically posterior and inferior. RSA in the setting of a B2 glenoid is one of the ideal indications to use PSI to ensure ideal placement of the central pin, which is the key to glenoid baseplate positioning. Even when using a RSA, eccentric reaming and/or bone grafting allow for more ideal component placement. Using the same eccentric reaming techniques described above, one should try to ream to place the baseplate at 10° of retroversion. In cases where retroversion cannot be corrected to 10°, graft can be taken from the humeral head, iliac crest, or allograft. A benefit to using bone graft with RSA as opposed to TSA is that the graft can be fashioned to the baseplate, impacted/compressed into the B2 glenoid, and then secured with a central compression screw and peripheral locking screws.
Mizuno and colleagues41 reported a retrospective series of 27 RSAs performed for primary glenohumeral osteoarthritis and biconcave glenoid. At a mean follow-up of nearly 5 years, the authors noted significant improvement in Constant scores and shoulder motion with minimal complications. There was no recurrence of posterior instability observed by the time of final follow-up.41
RSA is a promising treatment for primary glenohumeral arthritis with posterior glenoid bone loss and static posterior subluxation in elderly or less active patients, but the longevity of these implants has yet to be established for younger, more active patients and requires further study.
Conclusion
Reconstruction of the B2 glenoid presents a challenging clinical problem that has been associated with poor clinical outcomes and implant survivorship. The high failure rate from glenoid component loosening and subsequent premature implant failure can be substantially decreased with accurate glenoid component positioning and appropriate correction of the pathologic glenoid retroversion. Careful preoperative planning is essential for accurate preparation and execution of the optimal surgical plan. There are many surgical strategies to address the B2 glenoid, but no consensus on the optimal method exists, as the technique should be uniquely customized to the individual’s pathology and surgeon preference (Table). Cases with mild deformity may be corrected with eccentric reaming and TSA, while the more severe deformities may require posterior glenoid bone grafting and/or augmented implants to restore native version. Finally, the RSA is a reliable option to restore stability and address bone deficiency for the severe B2 glenoid in an older, lower demand patient.
Total shoulder arthroplasty (TSA) has demonstrated excellent long-term clinical outcomes for the treatment of advanced glenohumeral osteoarthritis (OA).1-5 Glenohumeral OA is characterized by a broad spectrum of glenoid pathology. Both the morphology of the glenoid and humeral head subluxation are important preoperative factors to evaluate, as these have been shown to adversely impact shoulder arthroplasty outcomes.6,7
Walch and colleagues8 have previously classified glenoid morphology in cases of advanced glenohumeral arthritis based on the preoperative computed tomography (CT) scans of individuals undergoing shoulder arthroplasty (Figures 1A-1E). The biconcave (B2) glenoid is characterized by asymmetric posterior bone loss and a posterior translated humeral head that is seated in a biconcave glenoid. The degree and extent of bone loss in the B2 glenoid can be highly variable, ranging from the classic interpretation, in which 50% of the native glenoid fossa is preserved, to the more extreme case with little remaining native anterior glenoid. Scalise and colleagues9 have reported that determining the premorbid native glenoid version with a 3-dimensional (3D) glenoid vault model can aid in differentiating a pathologic B2 glenoid from a nonpathologic type C glenoid.
The B2 glenoid in particular has been associated with poor shoulder arthroplasty outcomes and component survivorship.6,10-12 There are many factors that are thought to contribute to this problem, such as glenoid component malposition, or undercorrection of the pathologic retroversion.6,13,14 Walch and colleagues10 reported that if the neoglenoid retroversion was greater than 27°, there was a 44% incidence of loosening and/or instability and 60% of the dislocations were observed when the humeral head subluxation was greater than 80%. Cases with severe posterior glenoid bone deficiency present a unique challenge to the surgeon, and the ability to accurately and securely place an implant in the correct anatomic position can be compromised. Standard TSA has proven excellent outcomes in the setting of typical glenohumeral OA, but in the B2 glenoid with significant posterior bone erosion, additional attention must be given to ensure adequate correction of the bony deformity, soft tissue balancing, and implant stability.
Several strategies that have been proposed to address extreme bone loss in the B2 glenoid will be discussed in this review. These include hemiarthroplasty, TSA with asymmetric reaming of the high side, TSA with bone grafting of the posterior glenoid bone loss, TSA with an augmented glenoid component, and reverse shoulder arthroplasty (RSA). Importantly, while these techniques have been proposed for managing the B2 glenoid, currently there is no gold standard consensus for the treatment of this condition. The purpose of this review is to highlight important characteristics of the B2 glenoid morphology on clinical outcomes and discuss the current surgical management options for this condition.
Preoperative Planning
Being able to accurately determine the amount of retroversion is critical for preoperative planning. Friedman and colleagues15 initially described a method to measure glenoid retroversion; however, this is less accurate in B2 glenoids (Figures 2A, 2B). More recently, Rouleau and colleagues16 have validated and published methods to measure glenoid retroversion and subluxation in the B2 glenoid using 3 reference lines: the paleoglenoid (native glenoid surface), intermediate glenoid (line from anterior and posterior edge), and neoglenoid (eroded posterior surface) (Figure 2).
Preoperative evaluation starts with plain radiographs; however, additional imaging is needed, as the axillary view has shown to overestimate retroversion in 86% of patients (Figures 3A-3E).17 For a detailed evaluation of the glenoid retroversion and bone deficiency, CT scans with 3D reconstructions are useful.18,19 The surgical plan should be guided by the location and extent of glenoid bone loss. One tool that has been developed to help in predicting premorbid glenoid version, inclination, and position of the joint line is the 3D virtual glenoid vault model.9,20,21 This helps determine accurate premorbid glenoid anatomy and has been shown to assist in the selection of the optimal implant in an attempt to restore native glenoid anatomy, and avoid peg perforation.21 Patient-specific instrumentation (PSI) for shoulder arthroplasty is being used more frequently and has shown promise for more accurate glenoid component placement, particularly in the complex glenoid with severe bone deficiency. PSI involves creating a custom-fitted guide that is referenced to surface anatomy derived from the preoperative CT scan, which can then direct the surgeon toward optimal implant position with regard to glenoid component location, version and inclination (Figures 4A, 4B). Early reports show that PSI has resulted in a significant reduction in the frequency of malpositioned glenoid implants, with the greatest benefit observed in patients with retroversion in excess of 16°.22
Surgical Management
Hemiarthroplasty
Shoulder hemiarthroplasty has been traditionally described as an option for younger, more active patients in whom longevity of the glenoid component is a concern, or in patients with inadequate glenoid bone stock to tolerate a glenoid component. While there are no reports of hemiarthroplasty specifically for patients with B2 glenoids, one study has examined the effect of glenoid morphology on the outcomes of hemiarthroplasty for shoulder osteoarthritis. Levine and colleagues7 reported inferior clinical outcomes after shoulder hemiarthroplasty in patients with eccentric posterior glenoid wear. Several authors have advocated a “ream-and-run” technique to create a concentric glenoid and re-center the humeral head while still maintaining the native glenoid.23,24 However, in a recent series of 162 ream-and-run procedures, Gilmer and colleagues25 reported that only 23% of patients with B2 glenoid geometry achieved a minimal clinically important change in patient-reported outcome scores and 14% required revision. Furthermore, Lynch and colleagues26 found that progressive medial erosion and recurrent posterior glenoid erosion occur in a significant percentage of patients at early follow-up. Given these recent findings, the use of hemiarthroplasty alone or a ream-and-run procedure for patients with B2 glenoid morphology should be approached with caution.
Total Shoulder Arthroplasty
As with any TSA, the primary goals in treating patients with B2 glenoid defects are to provide the patient with a pain-free, stable, and functional shoulder (Figures 5A-5D). There are, however, a few challenges that are unique to TSA in the setting of B2 glenoid defects. Because the humeral head is often subluxated posteriorly into the defect, the anterior capsule and rotator cuff can tighten while the posterior aspect of the joint becomes lax. These soft tissues must be balanced during TSA in order to stabilize the shoulder and restore the appropriate length-tension relationship of the rotator cuff. The other primary concern is restoration of appropriate glenoid version and lateralization. To accomplish this, the most common techniques utilized are asymmetric reaming, bone graft augmentation, and glenoid component augmentation.27,28
Asymmetric Reaming. One of the more readily utilized techniques for addressing the B2 glenoid during TSA is eccentric or asymmetric reaming. During this process, the anterior glenoid is preferentially reamed while little to no bone is removed posteriorly. This technique is generally felt to be sufficient to treat posterior defects up to 5 mm to 8 mm or retroversion up to 15°.28 These upper limits have been confirmed in a number of cadaveric and simulated models.29-31
The success of this technique hinges on excellent glenoid exposure. With appropriate retractors in place, the anterior capsulolabral complex, including the biceps insertion, is resected to improve visualization. The inferior capsule must be resected carefully to ensure exposure and better motion postoperatively. On the other hand, it is imperative to protect the posterior capsulolabral attachments because of the increased risk of posterior instability in patients with B2 glenoids.
Detailed imaging such as CT scans with 3D reconstructions have improved our understanding of the degree of the deformities in all directions, which can better guide the reaming. PSI and planning software developed to improve the surgeon’s ability to place the glenoid component centrally in the best possible position after version correction can be even more helpful. We find that using a burr to provisionally lower the high side (anterior) provides a more en face view, which subsequently makes the eccentric reaming easier. As a guide, we will not ream more than 1 cm of anterior bone or attempt to correct more than ~20° of retroversion. The goal should be to create a glenoid surface that is more neutral and congruent to the posterior surface of the glenoid component while not overmedializing the component.
Although eccentric reaming may be one of the more straightforward methods for addressing posterior glenoid erosion, it is not without a number of potential downsides. When attempting to correct defects >10 mm or retroversion beyond 15°, excessive medialization of the implant can occur. Although increasing the thickness of the glenoid component can compensate for small amounts of medialization, excessive medialization can lead to a number of issues.27,28,32 As reaming progresses medially, the risk of keel penetration increases as the glenoid vault narrows.30,32 Further medialization decreases posterior cortical support for the implant, which increases the risk of component loosening and subsidence.33-35 The more medial the implant is placed, the smaller the surface of available bone for implant fixation. This often requires utilization of a smaller sized glenoid component that may result in component mismatch with the humeral implant. Finally, excessive medialization has the potential to under tension the rotator cuff, leading to decreased shoulder stability, strength, and function.
Bone Graft Augmentation. When posterior erosion becomes too excessive to address with eccentric reaming alone, defect augmentation is another option to consider (Figures 6A-6E). While technically more demanding, bone graft also provides the advantage of better re-creating the natural joint line and center of rotation of the glenohumeral joint.
For most defects, the resected humeral head provides the ideal source of graft. After initial reaming of the anterior glenoid, the defect must be sized and measured. We then recommend using a guided, cannulated system to place a central pin, lying perpendicular to the glenoid axis in neutral position. The anterior glenoid is then reamed enough to create a flat surface on which to attach the bone graft. The posterior surface is then gently burred to create a bleeding surface to enhance graft incorporation. The graft is then contoured to the defect and placed flush with the anterior glenoid. Cannulated screws are placed over guidewires to fix the graft. Using an arthroscopic cannula inserted posteriorly allows for easier placement of the guidewires and easier implantation of the screws. Although a reamer or burr can be used to contour the graft once it is fixed in place, this should be minimized to prevent loss of fixation. When the graft is fixed, we then cement the glenoid component into place.
Although good clinical results have been obtained with this technique, there is concern of incomplete graft healing and component loosening in the long term. Even in clinically asymptomatic and well functioning patients, some degree of radiographic lucency may be present in over 50% of cases.31,36,37 Glenoid Component Augmentation. To address the issues related to lucency and nonunion of bone graft augmentation, several augmented glenoid components have been developed. Augmented glenoid components have the benefit of filling posterior defects and stabilizing the shoulder without requiring excessive medialization (as often occurs with eccentric reaming) or union of a bone-to-bone interface (as is required in bone graft augmentation).38 Although many of the metal back designs experienced undesirably high failure rates and have since been recalled,39 more modern all-polyethylene components hold promise. The 2 most commonly utilized designs are the posterior step augment (DePuy) and the posterior wedge (Exactech). Although biomechanical analyses of both designs have demonstrated increased stability during loading in cadaveric and simulation models, the step augment (DePuy) has demonstrated increased stability and resistance to loosening.40,41 Although midterm results are not yet available for this newest generation of augmented components, short-term results with 2 to 3 years of follow-up have demonstrated excellent clinical outcomes.28
Reverse Total Shoulder Arthroplasty
While most commonly indicated for patients with rotator cuff tear arthropathy, RSA has recently been advocated for older patients with osteoarthritis and B2 glenoids in the setting of an intact rotator cuff. The semi-constrained design of the RSA is a potential solution to the static posterior humeral head subluxation seen in patients with B2 glenoid geometry (Figure 6E).
Technically, RSA is often an easier solution than a TSA with bone grafting because there is usually enough glenoid bone stock for fixation. That said, we always get a CT scan with 3D reconstructions to better appreciate the anatomy. Note that in B2 glenoids, the bone loss is typically posterior and inferior. RSA in the setting of a B2 glenoid is one of the ideal indications to use PSI to ensure ideal placement of the central pin, which is the key to glenoid baseplate positioning. Even when using a RSA, eccentric reaming and/or bone grafting allow for more ideal component placement. Using the same eccentric reaming techniques described above, one should try to ream to place the baseplate at 10° of retroversion. In cases where retroversion cannot be corrected to 10°, graft can be taken from the humeral head, iliac crest, or allograft. A benefit to using bone graft with RSA as opposed to TSA is that the graft can be fashioned to the baseplate, impacted/compressed into the B2 glenoid, and then secured with a central compression screw and peripheral locking screws.
Mizuno and colleagues41 reported a retrospective series of 27 RSAs performed for primary glenohumeral osteoarthritis and biconcave glenoid. At a mean follow-up of nearly 5 years, the authors noted significant improvement in Constant scores and shoulder motion with minimal complications. There was no recurrence of posterior instability observed by the time of final follow-up.41
RSA is a promising treatment for primary glenohumeral arthritis with posterior glenoid bone loss and static posterior subluxation in elderly or less active patients, but the longevity of these implants has yet to be established for younger, more active patients and requires further study.
Conclusion
Reconstruction of the B2 glenoid presents a challenging clinical problem that has been associated with poor clinical outcomes and implant survivorship. The high failure rate from glenoid component loosening and subsequent premature implant failure can be substantially decreased with accurate glenoid component positioning and appropriate correction of the pathologic glenoid retroversion. Careful preoperative planning is essential for accurate preparation and execution of the optimal surgical plan. There are many surgical strategies to address the B2 glenoid, but no consensus on the optimal method exists, as the technique should be uniquely customized to the individual’s pathology and surgeon preference (Table). Cases with mild deformity may be corrected with eccentric reaming and TSA, while the more severe deformities may require posterior glenoid bone grafting and/or augmented implants to restore native version. Finally, the RSA is a reliable option to restore stability and address bone deficiency for the severe B2 glenoid in an older, lower demand patient.
1. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
2. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
3. Matsen FA 3rd. Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am. 1996;78(2):260-264.
4. Fenlin JM Jr, Frieman BG. Indications, technique, and results of total shoulder arthroplasty in osteoarthritis. Orthop Clin North Am. 1998;29(3):423-434.
5. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: Analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517.
6. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
7. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
8. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
9. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
10. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-1533.
11. Kany J, Katz D. How to deal with glenoid type B2 or C? How to prevent mistakes in implantation of glenoid component? Eur J Orthop Surg Traumatol. 2013;23(4):379-385.
12. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82.
15. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
16. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230-1237.
17. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
18. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
19. Bokor DJ, O’Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg. 1999;8(6):595-598.
20. Ganapathi A, McCarron JA, Chen X, Iannotti JP. Predicting normal glenoid version from the pathologic scapula: A comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg. 2011;20(2):234-244.
21. Ricchetti ET, Hendel MD, Collins DN, Iannotti JP. Is premorbid glenoid anatomy altered in patients with glenohumeral osteoarthritis? Clin Orthop Relat Res. 2013;471(9):2932-2939.
22. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94(23):2167-2175.
23. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
24. Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA 3rd. Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20(4):609-615.
25. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: An analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
26. Lynch JR, Franta AK, Montgomery WH Jr, Lenters TR, Mounce D, Matsen FA 3rd. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292.
27. Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016;9(1):30-39.
28. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-848.
29. Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics. 2009;32(1):21.
30. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
31. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
32. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833.
33. Walch G, Young AA, Boileau P, Loew M, Gazielly D, Mole D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: Results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(2):145-150.
34. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: Multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
35. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072.
36. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973.
37. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
38. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157.
39. Iannotti JP, Lappin KE, Klotz CL, Reber EW, Swope SW. Liftoff resistance of augmented glenoid components during cyclic fatigue loading in the posterior-superior direction. J Shoulder Elbow Surg. 2013;22(11):1530-1536.
40. Knowles NK, Ferreira LM, Athwal GS. Augmented glenoid component designs for type B2 erosions: A computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg. 2015;24(8):1218-1226.
41. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
1. Barrett WP, Franklin JL, Jackins SE, Wyss CR, Matsen FA 3rd. Total shoulder arthroplasty. J Bone Joint Surg Am. 1987;69(6):865-872.
2. Bryant D, Litchfield R, Sandow M, Gartsman GM, Guyatt G, Kirkley A. A comparison of pain, strength, range of motion, and functional outcomes after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. A systematic review and meta-analysis. J Bone Joint Surg Am. 2005;87(9):1947-1956.
3. Matsen FA 3rd. Early effectiveness of shoulder arthroplasty for patients who have primary glenohumeral degenerative joint disease. J Bone Joint Surg Am. 1996;78(2):260-264.
4. Fenlin JM Jr, Frieman BG. Indications, technique, and results of total shoulder arthroplasty in osteoarthritis. Orthop Clin North Am. 1998;29(3):423-434.
5. Singh JA, Sperling JW, Cofield RH. Revision surgery following total shoulder arthroplasty: Analysis of 2588 shoulders over three decades (1976 to 2008). J Bone Joint Surg Br. 2011;93(11):1513-1517.
6. Iannotti JP, Norris TR. Influence of preoperative factors on outcome of shoulder arthroplasty for glenohumeral osteoarthritis. J Bone Joint Surg Am. 2003;85-A(2):251-258.
7. Levine WN, Djurasovic M, Glasson JM, Pollock RG, Flatow EL, Bigliani LU. Hemiarthroplasty for glenohumeral osteoarthritis: Results correlated to degree of glenoid wear. J Shoulder Elbow Surg. 1997;6(5):449-454.
8. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756-760.
9. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491.
10. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg. 2012;21(11):1526-1533.
11. Kany J, Katz D. How to deal with glenoid type B2 or C? How to prevent mistakes in implantation of glenoid component? Eur J Orthop Surg Traumatol. 2013;23(4):379-385.
12. Denard PJ, Walch G. Current concepts in the surgical management of primary glenohumeral arthritis with a biconcave glenoid. J Shoulder Elbow Surg. 2013;22(11):1589-1598.
13. Iannotti JP, Greeson C, Downing D, Sabesan V, Bryan JA. Effect of glenoid deformity on glenoid component placement in primary shoulder arthroplasty. J Shoulder Elbow Surg. 2012;21(1):48-55.
14. Ho JC, Sabesan VJ, Iannotti JP. Glenoid component retroversion is associated with osteolysis. J Bone Joint Surg Am. 2013;95(12):e82.
15. Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032-1037.
16. Rouleau DM, Kidder JF, Pons-Villanueva J, Dynamidis S, Defranco M, Walch G. Glenoid version: How to measure it? Validity of different methods in two-dimensional computed tomography scans. J Shoulder Elbow Surg. 2010;19(8):1230-1237.
17. Nyffeler RW, Jost B, Pfirrmann CW, Gerber C. Measurement of glenoid version: Conventional radiographs versus computed tomography scans. J Shoulder Elbow Surg. 2003;12(5):493-496.
18. Budge MD, Lewis GS, Schaefer E, Coquia S, Flemming DJ, Armstrong AD. Comparison of standard two-dimensional and three-dimensional corrected glenoid version measurements. J Shoulder Elbow Surg. 2011;20(4):577-583.
19. Bokor DJ, O’Sullivan MD, Hazan GJ. Variability of measurement of glenoid version on computed tomography scan. J Shoulder Elbow Surg. 1999;8(6):595-598.
20. Ganapathi A, McCarron JA, Chen X, Iannotti JP. Predicting normal glenoid version from the pathologic scapula: A comparison of 4 methods in 2- and 3-dimensional models. J Shoulder Elbow Surg. 2011;20(2):234-244.
21. Ricchetti ET, Hendel MD, Collins DN, Iannotti JP. Is premorbid glenoid anatomy altered in patients with glenohumeral osteoarthritis? Clin Orthop Relat Res. 2013;471(9):2932-2939.
22. Hendel MD, Bryan JA, Barsoum WK, et al. Comparison of patient-specific instruments with standard surgical instruments in determining glenoid component position: A randomized prospective clinical trial. J Bone Joint Surg Am. 2012;94(23):2167-2175.
23. Matsen FA 3rd, Warme WJ, Jackins SE. Can the ream and run procedure improve glenohumeral relationships and function for shoulders with the arthritic triad? Clin Orthop Relat Res. 2015;473(6):2088-2096.
24. Saltzman MD, Chamberlain AM, Mercer DM, Warme WJ, Bertelsen AL, Matsen FA 3rd. Shoulder hemiarthroplasty with concentric glenoid reaming in patients 55 years old or less. J Shoulder Elbow Surg. 2011;20(4):609-615.
25. Gilmer BB, Comstock BA, Jette JL, Warme WJ, Jackins SE, Matsen FA. The prognosis for improvement in comfort and function after the ream-and-run arthroplasty for glenohumeral arthritis: An analysis of 176 consecutive cases. J Bone Joint Surg Am. 2012;94(14):e102.
26. Lynch JR, Franta AK, Montgomery WH Jr, Lenters TR, Mounce D, Matsen FA 3rd. Self-assessed outcome at two to four years after shoulder hemiarthroplasty with concentric glenoid reaming. J Bone Joint Surg Am. 2007;89(6):1284-1292.
27. Donohue KW, Ricchetti ET, Iannotti JP. Surgical management of the biconcave (B2) glenoid. Curr Rev Musculoskelet Med. 2016;9(1):30-39.
28. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: What are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg. 2007;16(6):843-848.
29. Gillespie R, Lyons R, Lazarus M. Eccentric reaming in total shoulder arthroplasty: A cadaveric study. Orthopedics. 2009;32(1):21.
30. Neer CS 2nd, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg Am. 1988;70(8):1154-1162.
31. Nowak DD, Bahu MJ, Gardner TR, et al. Simulation of surgical glenoid resurfacing using three-dimensional computed tomography of the arthritic glenohumeral joint: The amount of glenoid retroversion that can be corrected. J Shoulder Elbow Surg. 2009;18(5):680-688.
32. Strauss EJ, Roche C, Flurin PH, Wright T, Zuckerman JD. The glenoid in shoulder arthroplasty. J Shoulder Elbow Surg. 2009;18(5):819-833.
33. Walch G, Young AA, Boileau P, Loew M, Gazielly D, Mole D. Patterns of loosening of polyethylene keeled glenoid components after shoulder arthroplasty for primary osteoarthritis: Results of a multicenter study with more than five years of follow-up. J Bone Joint Surg Am. 2012;94(2):145-150.
34. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: Multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
35. Klika BJ, Wooten CW, Sperling JW, et al. Structural bone grafting for glenoid deficiency in primary total shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):1066-1072.
36. Sabesan V, Callanan M, Sharma V, Iannotti JP. Correction of acquired glenoid bone loss in osteoarthritis with a standard versus an augmented glenoid component. J Shoulder Elbow Surg. 2014;23(7):964-973.
37. Steinmann SP, Cofield RH. Bone grafting for glenoid deficiency in total shoulder replacement. J Shoulder Elbow Surg. 2000;9(5):361-367.
38. Cil A, Sperling JW, Cofield RH. Nonstandard glenoid components for bone deficiencies in shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(7):e149-e157.
39. Iannotti JP, Lappin KE, Klotz CL, Reber EW, Swope SW. Liftoff resistance of augmented glenoid components during cyclic fatigue loading in the posterior-superior direction. J Shoulder Elbow Surg. 2013;22(11):1530-1536.
40. Knowles NK, Ferreira LM, Athwal GS. Augmented glenoid component designs for type B2 erosions: A computational comparison by volume of bone removal and quality of remaining bone. J Shoulder Elbow Surg. 2015;24(8):1218-1226.
41. Mizuno N, Denard PJ, Raiss P, Walch G. Reverse total shoulder arthroplasty for primary glenohumeral osteoarthritis in patients with a biconcave glenoid. J Bone Joint Surg Am. 2013;95(14):1297-1304.
Epidemiology and Impact of Knee Injuries in Major and Minor League Baseball Players
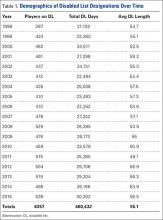
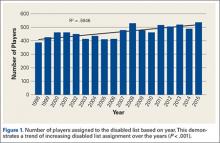
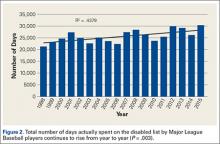
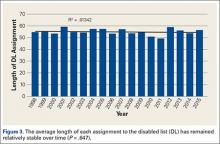
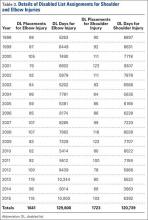
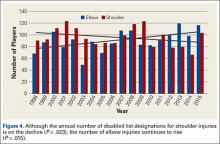
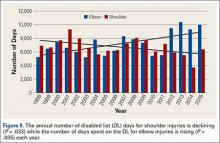
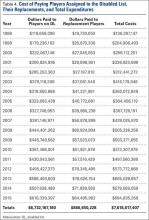
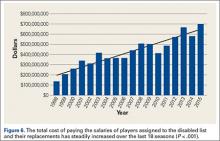
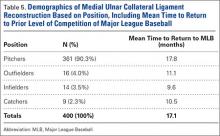
Injuries among professional baseball players have been on the rise for several years.1,2 From 1989 to 1999, the number of disabled list (DL) reports increased 38% (266 to 367 annual reports),1 and a similar increase in injury rates was noted from the 2002 to the 2008 seasons (37%).2 These injuries have important implications for future injury risk and time away from play. Identifying these injuries and determining correlates and risk factors is important for targeted prevention efforts.
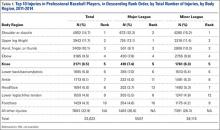
Several studies have explored the prevalence of upper extremity injuries in professional and collegiate baseball players;2-4 however, detailed epidemiology of knee injuries in Major League Baseball (MLB) and Minor League Baseball (MiLB) players is lacking. Much more is known about the prevalence, treatment, and outcomes of knee injuries in other professional sporting organizations, such as the National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL).4-12 A recent meta-analysis exploring injuries in professional athletes found that studies on lower extremity injuries comprised approximately 12% of the literature reporting injuries in MLB players.4 In other professional leagues, publications on lower extremity injuries comprise approximately 56% of the sports medicine literature in the NFL, 54% in the NBA, and 62% in the NHL.4 Since few studies have investigated lower extremity injuries among professional baseball players, there is an opportunity for additional research to guide evidence-based prevention strategies.
A better understanding of the nature of these injuries is one of the first steps towards developing targeted injury prevention programs and treatment algorithms. The study of injury epidemiology among professional baseball players has been aided by the creation of an injury tracking system initiated by the MLB, its minor league affiliates, and the Major League Baseball Players Association.5,13,14 This surveillance system allows for the tracking of medical histories and injuries to players as they move across major and minor league organizations. Similar systems have been utilized in the National Collegiate Athletic Association and other professional sports organizations.3,15-17 A unique advantage of the MLB surveillance system is the required participation of all major and minor league teams, which allows for investigation of the entire population of players rather than simply a sample of players from select teams. This system has propelled an effort to identify injury patterns as a means of developing appropriate targets for potential preventative measures.5
The purpose of this descriptive epidemiologic study is to better understand the distribution and characteristics of knee injuries in these elite athletes by reporting on all knee injuries occurring over a span of 4 seasons (2011-2014). Additionally, this study seeks to characterize the impact of these injuries by analyzing the time required for return to play and the treatments rendered (surgical and nonsurgical).
Materials and Methods
After approval from the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, detailed data regarding knee injuries in both MLB and MiLB baseball players were extracted from the de-identified MLB Health and Injury Tracking System (HITS). The HITS database is a centralized database that contains data on injuries from an electronic medical record (EMR). All players provided consent to have their data included in this EMR. HITS system captures injuries reported by the athletic trainers for all professional baseball players from 30 MLB clubs and their 230 minor league affiliates. Additional details on this population of professional baseball players have been published elsewhere.5 Only injuries that result in time out of play (≥1 day missed) are included in the database, and they are logged with basic information such as region of the body, diagnosis, date, player position, activity leading to injury, and general treatment. Any injury that affects participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training) is captured in HITS.
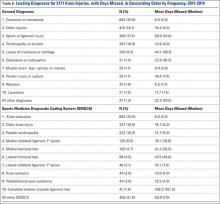
All baseball-related knee injuries occurring during the 2011-2014 seasons that resulted in time out of sport were included in the study. These injuries were identified based on the Sports Medicine Diagnostic Coding System (SMDCS) to capture injuries by diagnostic groups.18 Knee injuries were included if they occurred during spring training, regular season, or postseason play. Offseason injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of days missed because many of these players may not have been cleared to play until the beginning of the following season. To determine the proportion of knee injuries during the study period, all injuries were included for comparative purposes (subdivided based on 30 anatomic regions or types).
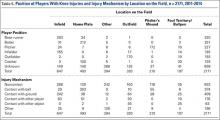
For each knee injury, a number of variables were analyzed, including diagnosis, level of play (MLB vs. MiLB), age, player position at the time of injury (pitcher, catcher, infield, outfield, base runner, or batter), field location where the injury occurred (home plate, pitcher’s mound, infield, outfield, foul territory or bullpen, or other), mechanism of injury, days missed, and treatment rendered (conservative vs surgical). The classification used to describe the mechanism of injury consisted of contact with ball, contact with ground, contact with another player, contact with another object, or noncontact.
Statistical Analysis Epidemiologic data are presented with descriptive statistics such as mean, median, frequency, and percentage where appropriate. When comparing player age, days missed, and surgical vs nonsurgical treatment between MLB and MiLB players, t-tests and tests for difference in proportions were applied as appropriate. Statistical significance was established for P values < .05.
The distribution of days missed for the variables considered was often skewed to the right (ie, days missed mostly concentrated on the low to moderate number of days, with fewer values in the much higher days missed range), even after excluding the season-ending injuries; hence the mean (or average) days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when knee injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those knee injuries that occurred during the regular season. It should be noted that the number of regular season knee injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training knee injuries.
RESULTS
Overall Summary
Of the 30 general body regions/systems included in the HITS database, injuries to the knee were the fifth most common reason for days missed in all of professional baseball from 2011-2014 (Table 1). Injuries to the knee represented 6.5% of the nearly 34,000 injuries sustained during the study period. Knee injuries were the fifth most common reason for time out of play for players in both the MiLB and MLB.
A total of 2171 isolated knee injuries resulted in time out of sport for professional baseball players (Table 2). Of these, 410 (19%) occurred in MLB players and 1761 (81%) occurred in MiLB players. MLB players were older than MiLB players at the time of injury (29.5 vs 22.8 years, respectively). Overall mean number of days missed was 16.2 days per knee injury, with MLB players missing an approximately 7 days more per injury than MiLB athletes (21.8 vs. 14.9 days respectively; P = .001).Over the course of the 4 seasons, a total of 30,449 days were missed due to knee injuries in professional baseball, giving an average rate of 7612 days lost per season. Surgery was performed for 263 (12.1%) of the 2171 knee injuries, with a greater proportion of MLB players requiring surgery than MiLB players (17.3% vs 10.9%) (P < .001). With respect to number of days missed per injury, 26% of knee injuries in the minor leagues resulted in greater than 30 days missed, while this number rose to 32% for knee injuries in MLB players (Table 3).
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE, respectively, over the course of the 4 seasons (2011-2014). The overall knee injury rate across both the MiLB and MLB was 1.2 per 1000 AE, based on the subset of 308 and 1473 regular season knee injuries in MiLB and MLB, respectively. The rate of knee injury was similar and not significantly different between the MiLB and MLB (1.2 per 1000 AE in the MiLB and 1.1 per 1000 AE in the MLB).
Characteristics of Injuries
When considering the position of the player during injury, defensive players were most frequently injured (n = 742, 56.5%), with pitchers (n = 227, 17.3%), infielders (n =193, 14.7%), outfielders (n = 193, 14.7%), and catchers (n = 129, 9.8%) sustaining injuries in decreasing frequency. Injuries while on offense (n = 571, 43.5%) were most frequent in base runners (n = 320, 24.4%) followed by batters (n = 251, 19.1%) (Table 4). Injuries while on defense occurring in infielders and catchers resulted in the longest period of time away from play (average of 22.4 and 20.8 days missed, respectively), while those occurring in batters resulted in the least average days missed (8.9 days).
The most common field location for knee injuries to occur was the infield, which was responsible for n = 647 (29.8%) of the total knee injuries (Table 4). This was followed by home plate (n = 493, 22.7%), other locations outside those specified (n = 394, 18.1%), outfield (n = 320, 14.7%), pitcher’s mound (n = 210, 9.7%), and foul territory or the bullpen (n = 107, 4.9%). Of the knee injuries with a specified location, those occurring in foul territory or the bullpen resulted in the highest mean days missed (18.4), while those occurring at home plate resulted in the least mean days missed (13.4 days).
When analyzed by mechanism of injury, noncontact injuries (n = 953, 43.9%) were more common than being hit with the ball (n = 374, 17.2%), striking the ground (n = 409, 18.8%), other mechanisms not listed (n = 196, 9%), contact with another player (n = 176, 8.1%), or contact with other objects (n = 63, 2.9%) (Table 4). Noncontact injuries and player to player collisions resulted in the greatest number of missed days (21.6 and 17.1 days, respectively) while being struck by the ball resulted in the least mean days missed (5.1).
Of the n = 493 knee injuries occurring at home plate, n = 212 (43%) occurred to the batter, n = 100 (20%) to the catcher, n = 34 (6.9%) to base runners, and n = 7 (1.4%) to pitchers (Table 5). The majority of knee injuries in the infield occurred to base runners (n = 283, 43.7%). Player-to-player collisions at home plate were responsible for 51 (2.3%) knee injuries, while 163 (24%) were noncontact injuries and 376 (56%) were the result of a player being hit by the ball (Table 5).
Injury Diagnosis
By diagnosis, the most common knee injuries observed were contusions or hematomas (n = 662, 30.5%), other injuries (n = 415, 19.1%), sprains or ligament injuries (n = 380, 17.5%), tendinopathies or bursitis (n = 367, 16.9%), and meniscal or cartilage injury (n = 200, 9.2%) (Table 6). Injuries resulting in the greatest mean number of days missed included meniscal or cartilage injuries (44 days), sprains or ligament injuries (30 days), or dislocations (22 days).
Based on specific SMDCS descriptors, the most frequent knee injuries reported were contusion (n = 662, 30.5%), patella tendinopathy (n = 222, 10.2%), and meniscal tears (n = 200, 9.2%) (Table 6). Complete anterior cruciate ligament tears, although infrequent, were responsible for the greatest mean days missed (156.2 days). This was followed by lateral meniscus tears (47.5 days) and medial meniscus tears (41.2 days). Knee contusions, although very common, resulted in the least number of days missed (6.0 days).
Discussion
Although much is known about knee injuries in other professional athletic leagues, little is known about knee injuries in professional baseball players.2-4 The majority of epidemiologic studies regarding baseball players at any level emphasizes the study of shoulder and elbow injuries.3,4,19 Since the implementation of the electronic medical record and the HITS database in professional baseball, there has been increased effort to document injuries that have received less attention in the existing literature. Understanding the epidemiology of these injuries is important for the development of targeted prevention efforts.
Prior studies of injuries in professional baseball relied on data captured by the publicly available DL. Posner and colleagues2 provide one of the most comprehensive reports on MLB injuries in a report utilizing DL assignment data over a period of 7 seasons.They demonstrated that knee injuries were responsible for 7.7% (12.5% for fielders and 3.7% for pitchers) of assignments to the DL. The current study utilized a comprehensive surveillance and builds on this existing knowledge. The present study found similar trends to Posner and colleagues2 in that knee injuries were responsible for 6.5% of injuries in professional baseball players that resulted in missed games. From the 2002 season to the 2008 season, knee injuries were the fifth most common reason MLB players were placed on the DL,2 and the current study indicates that they remain the fifth most common reason for missed time from play based on the HITS data. Since the prevalence of these injuries have remained constant since the 2002 season, efforts to better understand these injuries are warranted in order to identify strategies to prevent them. These analyses have generated important data towards achieving this understanding.
As with most injuries in professional sports, goals for treatment are aimed at maximizing patient function and performance while minimizing time out of play. For the 2011-2014 professional baseball seasons, a total of 2171 players sustained knee injuries and missed an average of 16.2 days per injury. Knee injuries were responsible for a total of 7612 days of missed work for MLB and MiLB players per season (30,449 days over the 4-season study period). This is equivalent to a total of 20.9 years of players’ time lost in professional baseball per season over the last 4 years. The implications of this amount of time away from sport are significant, and further study should be targeted at prevention of these injuries and optimizing return to play times.
When attempting to reduce the burden of knee injuries in professional baseball, it may prove beneficial to first understand how the injuries occur, where on the field, and who is at greatest risk. From 2011 to 2014, nearly 44% of knee injuries occurred by noncontact mechanisms. Among all locations on the field where knee injuries occurred, those occurring in the infield were responsible for the greatest mean days missed. The players who seem to be at greatest risk for knee injuries appear to be base runners. These data suggest the need for prevention efforts targeting base runners and infield players, as well as players in MiLB, where the largest number of injuries occurred.
Recently, playing rules implemented by MLB after consultation with players have focused on reducing the number of player-to-player collisions at home plate in an attempt to decrease the injury burden to catchers and base runners.20 This present analysis suggests that this rule change may also reduce the occurrence of knee injuries, as player collisions at home plate were responsible for a total of 51 knee injuries during the study period. The impact of this rule change on injury rates should also be explored. Interestingly, of the 51 knees injuries occurring due to contact at home plate, 23 occurred in 2011, and only 2 occurred in 2014—the first year of the new rule. Additional areas that resulted in high numbers of knee injuries were player-to-player contact in the infield and player contact with the ground in the infield.
Attempting to reduce injury burden and time out of play related to knee injuries in professional baseball players will likely prove to be a difficult task. In order to generate meaningful improvement, a comprehensive approach that involves players, management, trainers, therapists, and physicians will likely be required. As the first report of the epidemiology of knee injuries in professional baseball players, this study is one important step in that process. The strengths of this study are its comprehensive nature that analyzes injuries from an entire population of players on more than 200 teams over a 3-year period. Also, this research is strengthened by its focus on one particular region of the body that has received limited attention in the empirical literature, but represents a significant source of lost time during the baseball season.
There are some limitations to this study. As with any injury surveillance system, there is the possibility that not all cases were captured. Additionally, since the surveillance system is based on data from multiple teams, data entry discrepancy is possible; however, the presence of dropdown boxes and systematic definitions for injuries reduces this risk. Finally, this study did not investigate the various treatments for knee injuries beyond whether or not the injury required surgery. Since this was the first comprehensive exploration of knee injuries in professional baseball, future studies are needed to explore additional facets including outcomes related to treatment, return to play, and performance.
Conclusion
Knee injuries represent 6.5% of all injuries in professional baseball, occurring at a rate of 1.3 per 1000 AE. The burden of these injuries is significant for professional baseball players. This study fills a critical gap in sports injury research by contributing to the knowledge about the effect of knee injuries in professional baseball. It also provides an important foundation for future epidemiologic inquiry to identify modifiable risk factors and interventions that may reduce the impact of these injuries in athletes.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Dick R, Sauers EL, Agel J, et al. Descriptive epidemiology of collegiate men’s baseball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athletic Training. 2007;42(2):183-193.
4. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: A comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
5. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
6. Aune KT, Andrews JR, Dugas JR, Cain EL Jr. Return to play after partial lateral meniscectomy in National Football League Athletes. Am J Sports Med. 2014;42(8):1865-1872.
7. Brophy RH, Gill CS, Lyman S, Barnes RP, Rodeo SA, Warren RF. Effect of anterior cruciate ligament reconstruction and meniscectomy on length of career in National Football League athletes: a case control study. Am J Sports Med. 2009;37(11):2102-2107.
8. Brophy RH, Rodeo SA, Barnes RP, Powell JW, Warren RF. Knee articular cartilage injuries in the National Football League: epidemiology and treatment approach by team physicians. J Knee Surg. 2009;22(4):331-338.
9. Cerynik DL, Lewullis GE, Joves BC, Palmer MP, Tom JA. Outcomes of microfracture in professional basketball players. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1135-1139.
10. Hershman EB, Anderson R, Bergfeld JA, et al; National Football League Injury and Safety Panel. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
11. Namdari S, Baldwin K, Anakwenze O, Park MJ, Huffman GR, Sennett BJ. Results and performance after microfracture in National Basketball Association athletes. Am J Sports Med. 2009;37(5):943-948.
12. Yeh PC, Starkey C, Lombardo S, Vitti G, Kharrazi FD. Epidemiology of isolated meniscal injury and its effect on performance in athletes from the National Basketball Association. Am J Sports Med. 2012;40(3):589-594.
13. Pollack KM, D’Angelo J, Green G, et al. Developing and implementing major league baseball’s health and injury tracking system. Am J Epidem. (accepted), 2016.
14. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
15. Dick R, Agel J, Marshall SW. National Collegiate Athletic Association Injury Surveillance System commentaries: introduction and methods. J Athletic Training. 2007;42(2):173-182.
16. Pellman EJ, Viano DC, Casson IR, Arfken C, Feuer H. Concussion in professional football players returning to the same game—part 7. Neurosurg. 2005;56(1):79-90.
17. Stevens ST, Lassonde M, De Beaumont L, Keenan JP. The effect of visors on head and facial injury in national hockey league players. J Sci Med Sport. 2006;9(3):238-242.
18. Meeuwisse WH, Wiley JP. The sport medicine diagnostic coding system. Clin J Sport Med. 2007;17(3):205-207.
19. Mcfarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
20. Hagen P. New rule on home-plate collisions put into effect. Major League Baseball website. http://m.mlb.com/news/article/68267610/mlb-institutes-new-rule-on-home-plate-collisions. Accessed December 5, 2014.
Injuries among professional baseball players have been on the rise for several years.1,2 From 1989 to 1999, the number of disabled list (DL) reports increased 38% (266 to 367 annual reports),1 and a similar increase in injury rates was noted from the 2002 to the 2008 seasons (37%).2 These injuries have important implications for future injury risk and time away from play. Identifying these injuries and determining correlates and risk factors is important for targeted prevention efforts.
Several studies have explored the prevalence of upper extremity injuries in professional and collegiate baseball players;2-4 however, detailed epidemiology of knee injuries in Major League Baseball (MLB) and Minor League Baseball (MiLB) players is lacking. Much more is known about the prevalence, treatment, and outcomes of knee injuries in other professional sporting organizations, such as the National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL).4-12 A recent meta-analysis exploring injuries in professional athletes found that studies on lower extremity injuries comprised approximately 12% of the literature reporting injuries in MLB players.4 In other professional leagues, publications on lower extremity injuries comprise approximately 56% of the sports medicine literature in the NFL, 54% in the NBA, and 62% in the NHL.4 Since few studies have investigated lower extremity injuries among professional baseball players, there is an opportunity for additional research to guide evidence-based prevention strategies.
A better understanding of the nature of these injuries is one of the first steps towards developing targeted injury prevention programs and treatment algorithms. The study of injury epidemiology among professional baseball players has been aided by the creation of an injury tracking system initiated by the MLB, its minor league affiliates, and the Major League Baseball Players Association.5,13,14 This surveillance system allows for the tracking of medical histories and injuries to players as they move across major and minor league organizations. Similar systems have been utilized in the National Collegiate Athletic Association and other professional sports organizations.3,15-17 A unique advantage of the MLB surveillance system is the required participation of all major and minor league teams, which allows for investigation of the entire population of players rather than simply a sample of players from select teams. This system has propelled an effort to identify injury patterns as a means of developing appropriate targets for potential preventative measures.5
The purpose of this descriptive epidemiologic study is to better understand the distribution and characteristics of knee injuries in these elite athletes by reporting on all knee injuries occurring over a span of 4 seasons (2011-2014). Additionally, this study seeks to characterize the impact of these injuries by analyzing the time required for return to play and the treatments rendered (surgical and nonsurgical).
Materials and Methods
After approval from the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, detailed data regarding knee injuries in both MLB and MiLB baseball players were extracted from the de-identified MLB Health and Injury Tracking System (HITS). The HITS database is a centralized database that contains data on injuries from an electronic medical record (EMR). All players provided consent to have their data included in this EMR. HITS system captures injuries reported by the athletic trainers for all professional baseball players from 30 MLB clubs and their 230 minor league affiliates. Additional details on this population of professional baseball players have been published elsewhere.5 Only injuries that result in time out of play (≥1 day missed) are included in the database, and they are logged with basic information such as region of the body, diagnosis, date, player position, activity leading to injury, and general treatment. Any injury that affects participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training) is captured in HITS.
All baseball-related knee injuries occurring during the 2011-2014 seasons that resulted in time out of sport were included in the study. These injuries were identified based on the Sports Medicine Diagnostic Coding System (SMDCS) to capture injuries by diagnostic groups.18 Knee injuries were included if they occurred during spring training, regular season, or postseason play. Offseason injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of days missed because many of these players may not have been cleared to play until the beginning of the following season. To determine the proportion of knee injuries during the study period, all injuries were included for comparative purposes (subdivided based on 30 anatomic regions or types).
For each knee injury, a number of variables were analyzed, including diagnosis, level of play (MLB vs. MiLB), age, player position at the time of injury (pitcher, catcher, infield, outfield, base runner, or batter), field location where the injury occurred (home plate, pitcher’s mound, infield, outfield, foul territory or bullpen, or other), mechanism of injury, days missed, and treatment rendered (conservative vs surgical). The classification used to describe the mechanism of injury consisted of contact with ball, contact with ground, contact with another player, contact with another object, or noncontact.
Statistical Analysis Epidemiologic data are presented with descriptive statistics such as mean, median, frequency, and percentage where appropriate. When comparing player age, days missed, and surgical vs nonsurgical treatment between MLB and MiLB players, t-tests and tests for difference in proportions were applied as appropriate. Statistical significance was established for P values < .05.
The distribution of days missed for the variables considered was often skewed to the right (ie, days missed mostly concentrated on the low to moderate number of days, with fewer values in the much higher days missed range), even after excluding the season-ending injuries; hence the mean (or average) days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when knee injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those knee injuries that occurred during the regular season. It should be noted that the number of regular season knee injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training knee injuries.
RESULTS
Overall Summary
Of the 30 general body regions/systems included in the HITS database, injuries to the knee were the fifth most common reason for days missed in all of professional baseball from 2011-2014 (Table 1). Injuries to the knee represented 6.5% of the nearly 34,000 injuries sustained during the study period. Knee injuries were the fifth most common reason for time out of play for players in both the MiLB and MLB.
A total of 2171 isolated knee injuries resulted in time out of sport for professional baseball players (Table 2). Of these, 410 (19%) occurred in MLB players and 1761 (81%) occurred in MiLB players. MLB players were older than MiLB players at the time of injury (29.5 vs 22.8 years, respectively). Overall mean number of days missed was 16.2 days per knee injury, with MLB players missing an approximately 7 days more per injury than MiLB athletes (21.8 vs. 14.9 days respectively; P = .001).Over the course of the 4 seasons, a total of 30,449 days were missed due to knee injuries in professional baseball, giving an average rate of 7612 days lost per season. Surgery was performed for 263 (12.1%) of the 2171 knee injuries, with a greater proportion of MLB players requiring surgery than MiLB players (17.3% vs 10.9%) (P < .001). With respect to number of days missed per injury, 26% of knee injuries in the minor leagues resulted in greater than 30 days missed, while this number rose to 32% for knee injuries in MLB players (Table 3).
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE, respectively, over the course of the 4 seasons (2011-2014). The overall knee injury rate across both the MiLB and MLB was 1.2 per 1000 AE, based on the subset of 308 and 1473 regular season knee injuries in MiLB and MLB, respectively. The rate of knee injury was similar and not significantly different between the MiLB and MLB (1.2 per 1000 AE in the MiLB and 1.1 per 1000 AE in the MLB).
Characteristics of Injuries
When considering the position of the player during injury, defensive players were most frequently injured (n = 742, 56.5%), with pitchers (n = 227, 17.3%), infielders (n =193, 14.7%), outfielders (n = 193, 14.7%), and catchers (n = 129, 9.8%) sustaining injuries in decreasing frequency. Injuries while on offense (n = 571, 43.5%) were most frequent in base runners (n = 320, 24.4%) followed by batters (n = 251, 19.1%) (Table 4). Injuries while on defense occurring in infielders and catchers resulted in the longest period of time away from play (average of 22.4 and 20.8 days missed, respectively), while those occurring in batters resulted in the least average days missed (8.9 days).
The most common field location for knee injuries to occur was the infield, which was responsible for n = 647 (29.8%) of the total knee injuries (Table 4). This was followed by home plate (n = 493, 22.7%), other locations outside those specified (n = 394, 18.1%), outfield (n = 320, 14.7%), pitcher’s mound (n = 210, 9.7%), and foul territory or the bullpen (n = 107, 4.9%). Of the knee injuries with a specified location, those occurring in foul territory or the bullpen resulted in the highest mean days missed (18.4), while those occurring at home plate resulted in the least mean days missed (13.4 days).
When analyzed by mechanism of injury, noncontact injuries (n = 953, 43.9%) were more common than being hit with the ball (n = 374, 17.2%), striking the ground (n = 409, 18.8%), other mechanisms not listed (n = 196, 9%), contact with another player (n = 176, 8.1%), or contact with other objects (n = 63, 2.9%) (Table 4). Noncontact injuries and player to player collisions resulted in the greatest number of missed days (21.6 and 17.1 days, respectively) while being struck by the ball resulted in the least mean days missed (5.1).
Of the n = 493 knee injuries occurring at home plate, n = 212 (43%) occurred to the batter, n = 100 (20%) to the catcher, n = 34 (6.9%) to base runners, and n = 7 (1.4%) to pitchers (Table 5). The majority of knee injuries in the infield occurred to base runners (n = 283, 43.7%). Player-to-player collisions at home plate were responsible for 51 (2.3%) knee injuries, while 163 (24%) were noncontact injuries and 376 (56%) were the result of a player being hit by the ball (Table 5).
Injury Diagnosis
By diagnosis, the most common knee injuries observed were contusions or hematomas (n = 662, 30.5%), other injuries (n = 415, 19.1%), sprains or ligament injuries (n = 380, 17.5%), tendinopathies or bursitis (n = 367, 16.9%), and meniscal or cartilage injury (n = 200, 9.2%) (Table 6). Injuries resulting in the greatest mean number of days missed included meniscal or cartilage injuries (44 days), sprains or ligament injuries (30 days), or dislocations (22 days).
Based on specific SMDCS descriptors, the most frequent knee injuries reported were contusion (n = 662, 30.5%), patella tendinopathy (n = 222, 10.2%), and meniscal tears (n = 200, 9.2%) (Table 6). Complete anterior cruciate ligament tears, although infrequent, were responsible for the greatest mean days missed (156.2 days). This was followed by lateral meniscus tears (47.5 days) and medial meniscus tears (41.2 days). Knee contusions, although very common, resulted in the least number of days missed (6.0 days).
Discussion
Although much is known about knee injuries in other professional athletic leagues, little is known about knee injuries in professional baseball players.2-4 The majority of epidemiologic studies regarding baseball players at any level emphasizes the study of shoulder and elbow injuries.3,4,19 Since the implementation of the electronic medical record and the HITS database in professional baseball, there has been increased effort to document injuries that have received less attention in the existing literature. Understanding the epidemiology of these injuries is important for the development of targeted prevention efforts.
Prior studies of injuries in professional baseball relied on data captured by the publicly available DL. Posner and colleagues2 provide one of the most comprehensive reports on MLB injuries in a report utilizing DL assignment data over a period of 7 seasons.They demonstrated that knee injuries were responsible for 7.7% (12.5% for fielders and 3.7% for pitchers) of assignments to the DL. The current study utilized a comprehensive surveillance and builds on this existing knowledge. The present study found similar trends to Posner and colleagues2 in that knee injuries were responsible for 6.5% of injuries in professional baseball players that resulted in missed games. From the 2002 season to the 2008 season, knee injuries were the fifth most common reason MLB players were placed on the DL,2 and the current study indicates that they remain the fifth most common reason for missed time from play based on the HITS data. Since the prevalence of these injuries have remained constant since the 2002 season, efforts to better understand these injuries are warranted in order to identify strategies to prevent them. These analyses have generated important data towards achieving this understanding.
As with most injuries in professional sports, goals for treatment are aimed at maximizing patient function and performance while minimizing time out of play. For the 2011-2014 professional baseball seasons, a total of 2171 players sustained knee injuries and missed an average of 16.2 days per injury. Knee injuries were responsible for a total of 7612 days of missed work for MLB and MiLB players per season (30,449 days over the 4-season study period). This is equivalent to a total of 20.9 years of players’ time lost in professional baseball per season over the last 4 years. The implications of this amount of time away from sport are significant, and further study should be targeted at prevention of these injuries and optimizing return to play times.
When attempting to reduce the burden of knee injuries in professional baseball, it may prove beneficial to first understand how the injuries occur, where on the field, and who is at greatest risk. From 2011 to 2014, nearly 44% of knee injuries occurred by noncontact mechanisms. Among all locations on the field where knee injuries occurred, those occurring in the infield were responsible for the greatest mean days missed. The players who seem to be at greatest risk for knee injuries appear to be base runners. These data suggest the need for prevention efforts targeting base runners and infield players, as well as players in MiLB, where the largest number of injuries occurred.
Recently, playing rules implemented by MLB after consultation with players have focused on reducing the number of player-to-player collisions at home plate in an attempt to decrease the injury burden to catchers and base runners.20 This present analysis suggests that this rule change may also reduce the occurrence of knee injuries, as player collisions at home plate were responsible for a total of 51 knee injuries during the study period. The impact of this rule change on injury rates should also be explored. Interestingly, of the 51 knees injuries occurring due to contact at home plate, 23 occurred in 2011, and only 2 occurred in 2014—the first year of the new rule. Additional areas that resulted in high numbers of knee injuries were player-to-player contact in the infield and player contact with the ground in the infield.
Attempting to reduce injury burden and time out of play related to knee injuries in professional baseball players will likely prove to be a difficult task. In order to generate meaningful improvement, a comprehensive approach that involves players, management, trainers, therapists, and physicians will likely be required. As the first report of the epidemiology of knee injuries in professional baseball players, this study is one important step in that process. The strengths of this study are its comprehensive nature that analyzes injuries from an entire population of players on more than 200 teams over a 3-year period. Also, this research is strengthened by its focus on one particular region of the body that has received limited attention in the empirical literature, but represents a significant source of lost time during the baseball season.
There are some limitations to this study. As with any injury surveillance system, there is the possibility that not all cases were captured. Additionally, since the surveillance system is based on data from multiple teams, data entry discrepancy is possible; however, the presence of dropdown boxes and systematic definitions for injuries reduces this risk. Finally, this study did not investigate the various treatments for knee injuries beyond whether or not the injury required surgery. Since this was the first comprehensive exploration of knee injuries in professional baseball, future studies are needed to explore additional facets including outcomes related to treatment, return to play, and performance.
Conclusion
Knee injuries represent 6.5% of all injuries in professional baseball, occurring at a rate of 1.3 per 1000 AE. The burden of these injuries is significant for professional baseball players. This study fills a critical gap in sports injury research by contributing to the knowledge about the effect of knee injuries in professional baseball. It also provides an important foundation for future epidemiologic inquiry to identify modifiable risk factors and interventions that may reduce the impact of these injuries in athletes.
Injuries among professional baseball players have been on the rise for several years.1,2 From 1989 to 1999, the number of disabled list (DL) reports increased 38% (266 to 367 annual reports),1 and a similar increase in injury rates was noted from the 2002 to the 2008 seasons (37%).2 These injuries have important implications for future injury risk and time away from play. Identifying these injuries and determining correlates and risk factors is important for targeted prevention efforts.
Several studies have explored the prevalence of upper extremity injuries in professional and collegiate baseball players;2-4 however, detailed epidemiology of knee injuries in Major League Baseball (MLB) and Minor League Baseball (MiLB) players is lacking. Much more is known about the prevalence, treatment, and outcomes of knee injuries in other professional sporting organizations, such as the National Basketball Association (NBA), National Football League (NFL), and National Hockey League (NHL).4-12 A recent meta-analysis exploring injuries in professional athletes found that studies on lower extremity injuries comprised approximately 12% of the literature reporting injuries in MLB players.4 In other professional leagues, publications on lower extremity injuries comprise approximately 56% of the sports medicine literature in the NFL, 54% in the NBA, and 62% in the NHL.4 Since few studies have investigated lower extremity injuries among professional baseball players, there is an opportunity for additional research to guide evidence-based prevention strategies.
A better understanding of the nature of these injuries is one of the first steps towards developing targeted injury prevention programs and treatment algorithms. The study of injury epidemiology among professional baseball players has been aided by the creation of an injury tracking system initiated by the MLB, its minor league affiliates, and the Major League Baseball Players Association.5,13,14 This surveillance system allows for the tracking of medical histories and injuries to players as they move across major and minor league organizations. Similar systems have been utilized in the National Collegiate Athletic Association and other professional sports organizations.3,15-17 A unique advantage of the MLB surveillance system is the required participation of all major and minor league teams, which allows for investigation of the entire population of players rather than simply a sample of players from select teams. This system has propelled an effort to identify injury patterns as a means of developing appropriate targets for potential preventative measures.5
The purpose of this descriptive epidemiologic study is to better understand the distribution and characteristics of knee injuries in these elite athletes by reporting on all knee injuries occurring over a span of 4 seasons (2011-2014). Additionally, this study seeks to characterize the impact of these injuries by analyzing the time required for return to play and the treatments rendered (surgical and nonsurgical).
Materials and Methods
After approval from the Johns Hopkins Bloomberg School of Public Health Institutional Review Board, detailed data regarding knee injuries in both MLB and MiLB baseball players were extracted from the de-identified MLB Health and Injury Tracking System (HITS). The HITS database is a centralized database that contains data on injuries from an electronic medical record (EMR). All players provided consent to have their data included in this EMR. HITS system captures injuries reported by the athletic trainers for all professional baseball players from 30 MLB clubs and their 230 minor league affiliates. Additional details on this population of professional baseball players have been published elsewhere.5 Only injuries that result in time out of play (≥1 day missed) are included in the database, and they are logged with basic information such as region of the body, diagnosis, date, player position, activity leading to injury, and general treatment. Any injury that affects participation in any aspect of baseball-related activity (eg, game, practice, warm-up, conditioning, weight training) is captured in HITS.
All baseball-related knee injuries occurring during the 2011-2014 seasons that resulted in time out of sport were included in the study. These injuries were identified based on the Sports Medicine Diagnostic Coding System (SMDCS) to capture injuries by diagnostic groups.18 Knee injuries were included if they occurred during spring training, regular season, or postseason play. Offseason injuries were not included. Injury events that were classified as “season-ending” were not included in the analysis of days missed because many of these players may not have been cleared to play until the beginning of the following season. To determine the proportion of knee injuries during the study period, all injuries were included for comparative purposes (subdivided based on 30 anatomic regions or types).
For each knee injury, a number of variables were analyzed, including diagnosis, level of play (MLB vs. MiLB), age, player position at the time of injury (pitcher, catcher, infield, outfield, base runner, or batter), field location where the injury occurred (home plate, pitcher’s mound, infield, outfield, foul territory or bullpen, or other), mechanism of injury, days missed, and treatment rendered (conservative vs surgical). The classification used to describe the mechanism of injury consisted of contact with ball, contact with ground, contact with another player, contact with another object, or noncontact.
Statistical Analysis Epidemiologic data are presented with descriptive statistics such as mean, median, frequency, and percentage where appropriate. When comparing player age, days missed, and surgical vs nonsurgical treatment between MLB and MiLB players, t-tests and tests for difference in proportions were applied as appropriate. Statistical significance was established for P values < .05.
The distribution of days missed for the variables considered was often skewed to the right (ie, days missed mostly concentrated on the low to moderate number of days, with fewer values in the much higher days missed range), even after excluding the season-ending injuries; hence the mean (or average) days missed was often larger than the median days missed. Reporting the median would allow for a robust estimate of the expected number of days missed, but would down weight those instances when knee injuries result in much longer missed days, as reflected by the mean. Because of the importance of the days missed measure for professional baseball, both the mean and median are presented.
In order to estimate exposure, the average number of players per team per game was calculated based on analysis of regular season game participation via box scores. This average number over a season, multiplied by the number of team games at each professional level of baseball, was used as an estimate of athlete exposures in order to provide rates comparable to those of other injury surveillance systems. Injury rates were reported as injuries per 1000 athlete-exposures (AE) for those knee injuries that occurred during the regular season. It should be noted that the number of regular season knee injuries and the subsequent AE rates are based on injuries that were deemed work-related during the regular season. This does not necessarily only include injuries occurring during the course of a game, but injuries in game preparation as well. Due to the variations in spring training games and fluctuating rosters, an exposure rate could not be calculated for spring training knee injuries.
RESULTS
Overall Summary
Of the 30 general body regions/systems included in the HITS database, injuries to the knee were the fifth most common reason for days missed in all of professional baseball from 2011-2014 (Table 1). Injuries to the knee represented 6.5% of the nearly 34,000 injuries sustained during the study period. Knee injuries were the fifth most common reason for time out of play for players in both the MiLB and MLB.
A total of 2171 isolated knee injuries resulted in time out of sport for professional baseball players (Table 2). Of these, 410 (19%) occurred in MLB players and 1761 (81%) occurred in MiLB players. MLB players were older than MiLB players at the time of injury (29.5 vs 22.8 years, respectively). Overall mean number of days missed was 16.2 days per knee injury, with MLB players missing an approximately 7 days more per injury than MiLB athletes (21.8 vs. 14.9 days respectively; P = .001).Over the course of the 4 seasons, a total of 30,449 days were missed due to knee injuries in professional baseball, giving an average rate of 7612 days lost per season. Surgery was performed for 263 (12.1%) of the 2171 knee injuries, with a greater proportion of MLB players requiring surgery than MiLB players (17.3% vs 10.9%) (P < .001). With respect to number of days missed per injury, 26% of knee injuries in the minor leagues resulted in greater than 30 days missed, while this number rose to 32% for knee injuries in MLB players (Table 3).
For regular season games, it was estimated that there were 1,197,738 MiLB and 276,608 MLB AE, respectively, over the course of the 4 seasons (2011-2014). The overall knee injury rate across both the MiLB and MLB was 1.2 per 1000 AE, based on the subset of 308 and 1473 regular season knee injuries in MiLB and MLB, respectively. The rate of knee injury was similar and not significantly different between the MiLB and MLB (1.2 per 1000 AE in the MiLB and 1.1 per 1000 AE in the MLB).
Characteristics of Injuries
When considering the position of the player during injury, defensive players were most frequently injured (n = 742, 56.5%), with pitchers (n = 227, 17.3%), infielders (n =193, 14.7%), outfielders (n = 193, 14.7%), and catchers (n = 129, 9.8%) sustaining injuries in decreasing frequency. Injuries while on offense (n = 571, 43.5%) were most frequent in base runners (n = 320, 24.4%) followed by batters (n = 251, 19.1%) (Table 4). Injuries while on defense occurring in infielders and catchers resulted in the longest period of time away from play (average of 22.4 and 20.8 days missed, respectively), while those occurring in batters resulted in the least average days missed (8.9 days).
The most common field location for knee injuries to occur was the infield, which was responsible for n = 647 (29.8%) of the total knee injuries (Table 4). This was followed by home plate (n = 493, 22.7%), other locations outside those specified (n = 394, 18.1%), outfield (n = 320, 14.7%), pitcher’s mound (n = 210, 9.7%), and foul territory or the bullpen (n = 107, 4.9%). Of the knee injuries with a specified location, those occurring in foul territory or the bullpen resulted in the highest mean days missed (18.4), while those occurring at home plate resulted in the least mean days missed (13.4 days).
When analyzed by mechanism of injury, noncontact injuries (n = 953, 43.9%) were more common than being hit with the ball (n = 374, 17.2%), striking the ground (n = 409, 18.8%), other mechanisms not listed (n = 196, 9%), contact with another player (n = 176, 8.1%), or contact with other objects (n = 63, 2.9%) (Table 4). Noncontact injuries and player to player collisions resulted in the greatest number of missed days (21.6 and 17.1 days, respectively) while being struck by the ball resulted in the least mean days missed (5.1).
Of the n = 493 knee injuries occurring at home plate, n = 212 (43%) occurred to the batter, n = 100 (20%) to the catcher, n = 34 (6.9%) to base runners, and n = 7 (1.4%) to pitchers (Table 5). The majority of knee injuries in the infield occurred to base runners (n = 283, 43.7%). Player-to-player collisions at home plate were responsible for 51 (2.3%) knee injuries, while 163 (24%) were noncontact injuries and 376 (56%) were the result of a player being hit by the ball (Table 5).
Injury Diagnosis
By diagnosis, the most common knee injuries observed were contusions or hematomas (n = 662, 30.5%), other injuries (n = 415, 19.1%), sprains or ligament injuries (n = 380, 17.5%), tendinopathies or bursitis (n = 367, 16.9%), and meniscal or cartilage injury (n = 200, 9.2%) (Table 6). Injuries resulting in the greatest mean number of days missed included meniscal or cartilage injuries (44 days), sprains or ligament injuries (30 days), or dislocations (22 days).
Based on specific SMDCS descriptors, the most frequent knee injuries reported were contusion (n = 662, 30.5%), patella tendinopathy (n = 222, 10.2%), and meniscal tears (n = 200, 9.2%) (Table 6). Complete anterior cruciate ligament tears, although infrequent, were responsible for the greatest mean days missed (156.2 days). This was followed by lateral meniscus tears (47.5 days) and medial meniscus tears (41.2 days). Knee contusions, although very common, resulted in the least number of days missed (6.0 days).
Discussion
Although much is known about knee injuries in other professional athletic leagues, little is known about knee injuries in professional baseball players.2-4 The majority of epidemiologic studies regarding baseball players at any level emphasizes the study of shoulder and elbow injuries.3,4,19 Since the implementation of the electronic medical record and the HITS database in professional baseball, there has been increased effort to document injuries that have received less attention in the existing literature. Understanding the epidemiology of these injuries is important for the development of targeted prevention efforts.
Prior studies of injuries in professional baseball relied on data captured by the publicly available DL. Posner and colleagues2 provide one of the most comprehensive reports on MLB injuries in a report utilizing DL assignment data over a period of 7 seasons.They demonstrated that knee injuries were responsible for 7.7% (12.5% for fielders and 3.7% for pitchers) of assignments to the DL. The current study utilized a comprehensive surveillance and builds on this existing knowledge. The present study found similar trends to Posner and colleagues2 in that knee injuries were responsible for 6.5% of injuries in professional baseball players that resulted in missed games. From the 2002 season to the 2008 season, knee injuries were the fifth most common reason MLB players were placed on the DL,2 and the current study indicates that they remain the fifth most common reason for missed time from play based on the HITS data. Since the prevalence of these injuries have remained constant since the 2002 season, efforts to better understand these injuries are warranted in order to identify strategies to prevent them. These analyses have generated important data towards achieving this understanding.
As with most injuries in professional sports, goals for treatment are aimed at maximizing patient function and performance while minimizing time out of play. For the 2011-2014 professional baseball seasons, a total of 2171 players sustained knee injuries and missed an average of 16.2 days per injury. Knee injuries were responsible for a total of 7612 days of missed work for MLB and MiLB players per season (30,449 days over the 4-season study period). This is equivalent to a total of 20.9 years of players’ time lost in professional baseball per season over the last 4 years. The implications of this amount of time away from sport are significant, and further study should be targeted at prevention of these injuries and optimizing return to play times.
When attempting to reduce the burden of knee injuries in professional baseball, it may prove beneficial to first understand how the injuries occur, where on the field, and who is at greatest risk. From 2011 to 2014, nearly 44% of knee injuries occurred by noncontact mechanisms. Among all locations on the field where knee injuries occurred, those occurring in the infield were responsible for the greatest mean days missed. The players who seem to be at greatest risk for knee injuries appear to be base runners. These data suggest the need for prevention efforts targeting base runners and infield players, as well as players in MiLB, where the largest number of injuries occurred.
Recently, playing rules implemented by MLB after consultation with players have focused on reducing the number of player-to-player collisions at home plate in an attempt to decrease the injury burden to catchers and base runners.20 This present analysis suggests that this rule change may also reduce the occurrence of knee injuries, as player collisions at home plate were responsible for a total of 51 knee injuries during the study period. The impact of this rule change on injury rates should also be explored. Interestingly, of the 51 knees injuries occurring due to contact at home plate, 23 occurred in 2011, and only 2 occurred in 2014—the first year of the new rule. Additional areas that resulted in high numbers of knee injuries were player-to-player contact in the infield and player contact with the ground in the infield.
Attempting to reduce injury burden and time out of play related to knee injuries in professional baseball players will likely prove to be a difficult task. In order to generate meaningful improvement, a comprehensive approach that involves players, management, trainers, therapists, and physicians will likely be required. As the first report of the epidemiology of knee injuries in professional baseball players, this study is one important step in that process. The strengths of this study are its comprehensive nature that analyzes injuries from an entire population of players on more than 200 teams over a 3-year period. Also, this research is strengthened by its focus on one particular region of the body that has received limited attention in the empirical literature, but represents a significant source of lost time during the baseball season.
There are some limitations to this study. As with any injury surveillance system, there is the possibility that not all cases were captured. Additionally, since the surveillance system is based on data from multiple teams, data entry discrepancy is possible; however, the presence of dropdown boxes and systematic definitions for injuries reduces this risk. Finally, this study did not investigate the various treatments for knee injuries beyond whether or not the injury required surgery. Since this was the first comprehensive exploration of knee injuries in professional baseball, future studies are needed to explore additional facets including outcomes related to treatment, return to play, and performance.
Conclusion
Knee injuries represent 6.5% of all injuries in professional baseball, occurring at a rate of 1.3 per 1000 AE. The burden of these injuries is significant for professional baseball players. This study fills a critical gap in sports injury research by contributing to the knowledge about the effect of knee injuries in professional baseball. It also provides an important foundation for future epidemiologic inquiry to identify modifiable risk factors and interventions that may reduce the impact of these injuries in athletes.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Dick R, Sauers EL, Agel J, et al. Descriptive epidemiology of collegiate men’s baseball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athletic Training. 2007;42(2):183-193.
4. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: A comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
5. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
6. Aune KT, Andrews JR, Dugas JR, Cain EL Jr. Return to play after partial lateral meniscectomy in National Football League Athletes. Am J Sports Med. 2014;42(8):1865-1872.
7. Brophy RH, Gill CS, Lyman S, Barnes RP, Rodeo SA, Warren RF. Effect of anterior cruciate ligament reconstruction and meniscectomy on length of career in National Football League athletes: a case control study. Am J Sports Med. 2009;37(11):2102-2107.
8. Brophy RH, Rodeo SA, Barnes RP, Powell JW, Warren RF. Knee articular cartilage injuries in the National Football League: epidemiology and treatment approach by team physicians. J Knee Surg. 2009;22(4):331-338.
9. Cerynik DL, Lewullis GE, Joves BC, Palmer MP, Tom JA. Outcomes of microfracture in professional basketball players. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1135-1139.
10. Hershman EB, Anderson R, Bergfeld JA, et al; National Football League Injury and Safety Panel. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
11. Namdari S, Baldwin K, Anakwenze O, Park MJ, Huffman GR, Sennett BJ. Results and performance after microfracture in National Basketball Association athletes. Am J Sports Med. 2009;37(5):943-948.
12. Yeh PC, Starkey C, Lombardo S, Vitti G, Kharrazi FD. Epidemiology of isolated meniscal injury and its effect on performance in athletes from the National Basketball Association. Am J Sports Med. 2012;40(3):589-594.
13. Pollack KM, D’Angelo J, Green G, et al. Developing and implementing major league baseball’s health and injury tracking system. Am J Epidem. (accepted), 2016.
14. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
15. Dick R, Agel J, Marshall SW. National Collegiate Athletic Association Injury Surveillance System commentaries: introduction and methods. J Athletic Training. 2007;42(2):173-182.
16. Pellman EJ, Viano DC, Casson IR, Arfken C, Feuer H. Concussion in professional football players returning to the same game—part 7. Neurosurg. 2005;56(1):79-90.
17. Stevens ST, Lassonde M, De Beaumont L, Keenan JP. The effect of visors on head and facial injury in national hockey league players. J Sci Med Sport. 2006;9(3):238-242.
18. Meeuwisse WH, Wiley JP. The sport medicine diagnostic coding system. Clin J Sport Med. 2007;17(3):205-207.
19. Mcfarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
20. Hagen P. New rule on home-plate collisions put into effect. Major League Baseball website. http://m.mlb.com/news/article/68267610/mlb-institutes-new-rule-on-home-plate-collisions. Accessed December 5, 2014.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ Jr, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Dick R, Sauers EL, Agel J, et al. Descriptive epidemiology of collegiate men’s baseball injuries: National Collegiate Athletic Association Injury Surveillance System, 1988-1989 through 2003-2004. J Athletic Training. 2007;42(2):183-193.
4. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: A comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
5. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
6. Aune KT, Andrews JR, Dugas JR, Cain EL Jr. Return to play after partial lateral meniscectomy in National Football League Athletes. Am J Sports Med. 2014;42(8):1865-1872.
7. Brophy RH, Gill CS, Lyman S, Barnes RP, Rodeo SA, Warren RF. Effect of anterior cruciate ligament reconstruction and meniscectomy on length of career in National Football League athletes: a case control study. Am J Sports Med. 2009;37(11):2102-2107.
8. Brophy RH, Rodeo SA, Barnes RP, Powell JW, Warren RF. Knee articular cartilage injuries in the National Football League: epidemiology and treatment approach by team physicians. J Knee Surg. 2009;22(4):331-338.
9. Cerynik DL, Lewullis GE, Joves BC, Palmer MP, Tom JA. Outcomes of microfracture in professional basketball players. Knee Surg Sports Traumatol Arthrosc. 2009;17(9):1135-1139.
10. Hershman EB, Anderson R, Bergfeld JA, et al; National Football League Injury and Safety Panel. An analysis of specific lower extremity injury rates on grass and FieldTurf playing surfaces in National Football League Games: 2000-2009 seasons. Am J Sports Med. 2012;40(10):2200-2205.
11. Namdari S, Baldwin K, Anakwenze O, Park MJ, Huffman GR, Sennett BJ. Results and performance after microfracture in National Basketball Association athletes. Am J Sports Med. 2009;37(5):943-948.
12. Yeh PC, Starkey C, Lombardo S, Vitti G, Kharrazi FD. Epidemiology of isolated meniscal injury and its effect on performance in athletes from the National Basketball Association. Am J Sports Med. 2012;40(3):589-594.
13. Pollack KM, D’Angelo J, Green G, et al. Developing and implementing major league baseball’s health and injury tracking system. Am J Epidem. (accepted), 2016.
14. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
15. Dick R, Agel J, Marshall SW. National Collegiate Athletic Association Injury Surveillance System commentaries: introduction and methods. J Athletic Training. 2007;42(2):173-182.
16. Pellman EJ, Viano DC, Casson IR, Arfken C, Feuer H. Concussion in professional football players returning to the same game—part 7. Neurosurg. 2005;56(1):79-90.
17. Stevens ST, Lassonde M, De Beaumont L, Keenan JP. The effect of visors on head and facial injury in national hockey league players. J Sci Med Sport. 2006;9(3):238-242.
18. Meeuwisse WH, Wiley JP. The sport medicine diagnostic coding system. Clin J Sport Med. 2007;17(3):205-207.
19. Mcfarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
20. Hagen P. New rule on home-plate collisions put into effect. Major League Baseball website. http://m.mlb.com/news/article/68267610/mlb-institutes-new-rule-on-home-plate-collisions. Accessed December 5, 2014.
Injury Trends in Major League Baseball Over 18 Seasons: 1998-2015
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
While the exact origins of the game of baseball are commonly debated, one thing is certain: statistics have been an integral part of the game since its existence.1-3 This is true at nearly every level of baseball, especially in Major League Baseball (MLB). As our knowledge and technical capabilities advance, new statistical measures of baseball performance are added at a rapid pace.1,3 One example is the Pitch f/x video tracking system (Sportvision, Inc.), which now analyzes over 60 variables on each of the estimated 660,000 pitches thrown in the MLB annually. In addition to measuring performance and production, these advancements are being leveraged to better understand the epidemiology and impact of injuries in MLB players.4,5 As with any sport, performance at the most elite level is highly dependent upon player health and functional capacity. Accordingly, player injuries can have a profound impact not only on individual performance but also on the success of the team as a whole.
The first epidemiologic study of injuries in professional baseball was published by Conte and colleagues4 in 2001. This work utilized publically available disabled list (DL) data to perform a comprehensive review of injury patterns in MLB from 1989 to 1999. They demonstrated that injuries were on the rise and that pitchers were more commonly injured (48.4% of all DL reports) and had greater time out of play compared to players of other positions.4 Shoulder and elbow injuries were responsible for 49.8% of all DL assignments, distantly followed by knee (7.3%), wrist/hand (6.1%), and back (5.0%).4 In a later study, Posner and colleagues5 analyzed DL data spanning the 2002 to 2008 seasons. Similarly, they found that injuries continued to increase, and over half (51.2%) of DL assignments occurred secondary to upper extremity injuries.5 Although the DL is primarily designed as a roster management tool rather than an injury database, it has provided valuable epidemiologic injury information through the years. Out of concern for player health and well-being, MLB and the MLB Players Association (MLBPA) worked together to create and implement an electronic medical record and Health and Injury Tracking System (HITS) for all MLB and Minor League Baseball (MiLB) players. Now active for over 5 seasons, this database has provided valuable, detailed reports regarding specific injuries occurring in professional baseball, such as hamstring strains and concussions.6,7
With shoulder and elbow injuries in pitchers representing the greatest proportion of DL assignments in recent years, a large body of literature on these injuries, particularly medial ulnar collateral ligament (MUCL) injuries, has been published.8-13 Since the initial description of MUCL reconstruction, or “Tommy John surgery,” by Dr. Frank Jobe in 1986, much has been done to improve the technique and rehabilitation to maximize player performance following surgery.10,14-16 Despite this increased attention, large-scale epidemiologic reporting of MUCL injuries in MLB is lacking, but such a report is desirable. The purpose of this work is to: 1) provide a large-scale analysis of injuries occurring in MLB baseball over the course of 18 seasons (1998-2015); 2) highlight the financial implications of these injuries; and 3) detail the evolution of MUCL injuries and reconstructive surgery since it was first performed on a MLB pitcher in 1974. Our study represents the largest longitudinal analysis of MLB injuries since the league expanded to its current level of 30 teams in 1998. It is our hope that this work will serve as a framework for future study of the most common and highest impact injuries occurring in baseball.
Materials And Methods
We performed a retrospective review of the MLB DL from 1998 to 2015. Data analyzed included player demographics such as club, year of placement, age, and position. Injury-specific variables included date of placement on DL, length of time on DL, date of reinstatement, body part injured, diagnosis, and cost of replacement. If a player was put on the DL multiple times during a season, each placement was viewed as a different injury, even if it was to the same body part. If a player was put on the DL for injuries to multiple body parts, the primary injury was analyzed.
Disabled List Data
Although the DL has existed since 1916, this current study covers 18 seasons from 1998 to 2015. The 1998 season was chosen as a starting point because this is the year when MLB expanded to 30 teams. Since then, the number of teams and the active roster limits (25 players) have remained constant, allowing for reliable comparisons across seasons. Initially designed as a roster management tool to allow injured players to temporarily be replaced with healthy players, the DL was not created as an injury database. However, the rules and regulations of the DL have remained fairly constant over the last 18 years, allowing reasonable comparisons of injury data and trends across this timespan. In order for a player to be assigned to the DL, the nature and extent of injury must be certified by a physician. Once designated for the DL, a player cannot return to the major league team for a minimum of 15 days. If the injury is severe, the player can remain on the DL for the remainder of the season or until he is deemed healthy enough to return to play by a physician. One notable exception is the treatment of concussions. Since 2011, a player diagnosed with a concussion may be placed on the DL for a minimum of 7 days rather than 15. The introduction of the HITS database in 2010 should allow for more detailed and reliable study of injuries in baseball moving forward. Although it contains robust data for every injury that has occurred in MLB and MiLB over the last 5 seasons, it does not allow for epidemiologic and longitudinal study of injury patterns and trends in baseball prior to 2010.
Cost of Placing Players on the DL
The dollars lost were calculated by prorating the injured player’s daily salary and multiplying by the number of days missed on the DL. For example, if a player’s annual salary is $1,820,000, his daily salary for the 182 day season is $10,000. If assigned to the DL for 15 days, $150,000 is paid to that player while he is inactive and unable to play. An additional cost is the salary of the replacement player who fills the roster spot. For this work, the replacement player’s prorated, daily salary was assumed to be the league minimum for that specific year. For example, if the league minimum for a given season is $182,000, and the season is 182 days long, a replacement player earns a minimum of $1,000 per day while he is on the 25-man active roster. Thus, the dollars paid to the replacement would be $15,000. In this scenario, that brings the team’s total cost to $165,000 ($150,000 plus $15,000). Because the league minimum salary changes year to year, salaries specific to the year of injury were utilized in this analysis.
MUCL Injury Analysis
In order to better understand the evaluation of MUCL injuries over time, all MLB players undergoing MUCL reconstruction (“Tommy John surgery”) were analyzed separately. Similar to prior studies of UCL injuries, these players were identified using DL data, team websites, and publically available internet databases (primarily www.heatmaps.com).9,12,17-19 Variables studied include the number of procedures, year of surgery, player position, and mean time until return to play at the MLB level. All MLB players undergoing MUCL reconstruction since 1974 (the year the first procedure was performed) were included.
Statistical Methods
Epidemiologic data are reported using descriptive statistics (mean, range, and percentage) where indicated. To determine the significance of trends over time, a best-fit line was generated to illustrate the change over the years. These lines are reported with corresponding R2 values. To assess the trend for significance, the slope was compared to a line with a slope of zero (no change over time) using t tests. For all statistical comparisons, the threshold for alpha was set to P < .05.
Results
Between 1998 and 2015, there were 8357 placements of players on the DL, at an average rate of 464 designations per year (Table 1, Figure 1). This resulted in 460,432 days lost to injury, with a mean of 25,186 days out of play per season (Table 1, Figure 2). The mean length of DL assignment per year was 55.1 days per injury, with a low of 49.1 days in 2011 and a high of 59.2 days in 2001 (Table 1, Figure 3). During the study period, the number of players placed on the DL and the total number of DL days steadily increased (P < .001 and P = .003, respectively), while the average length of DL assignments remained steady (P = .647). When analyzing the data by body region injured, the shoulder (20.6%) and elbow (19.6%) were the 2 leading causes of time out of play (Table 2). This was followed distantly by the chest/back/spine (13.7%), wrist/hand/fingers (10.1%), lower leg/knee (9.8%), and the upper leg/thigh (9.5%). Although the percentage of injuries occurring to the upper extremity remained stable, the rate of shoulder injuries steadily decreased (P = .023) as elbow injuries increased (P = .015) (Table 3, Figure 4). This inverse relationship was also demonstrated for the annual number of DL days for shoulder (P = .033) and elbow (P = 0.005) injuries (Figure 5).
Regarding the financial impact of these injuries, the mean annual cost of replacing players on the DL was $423,267,633.78 (Table 4). This ranged from a low of $136,397,147 in 1998 to a high of $694,835,359 in 2015. There was a steady increase in the cost of replacement during the study period (P < .001) that coincides with the increasing salaries during that time span (Figure 6). In total, $6,732,167,180 was paid to players assigned to the DL and $886,650,228 was spent to fill their positions. This brings the total cost of DL assignments to $7,618,817,407 for the study period.
Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed on MLB players since the procedure was first developed in 1974. The vast majority of these were performed in pitchers (n = 361, 90.3%) followed by outfielders (n = 16, 4.0%), infielders (n = 14, 3.5%) and catchers (n = 9, 2.3%) (Table 5). The mean time to return to competition at the MLB level was 17.8 months for pitchers, 11.1 months for outfielders, 9.6 months for infielders, and 10.5 months for catchers. The overall mean time to return was 17.1 months. The annual number of MUCL reconstructions continues to rise dramatically (P < .001) (Figure 7). During the first 12 years (1974-1985), a total of 8 (2.0%) MUCL reconstructions were performed on MLB players. In subsequent decades, this number increased to 44 (11.0%) from 1986-1995, 123 (30.8%) from 1996-2005, and 225 (56.3%) from 2006-2015. Of all Tommy John surgeries performed over 42 years, nearly one-third (n = 131, 32.75%) were performed in the last 5 years alone (2011-2015).
Discussion
To date, a number of studies have been published on injuries in professional baseball. These can primarily be categorized as either studies with a detailed focus on a single injury type or body region6-13,17,19 or broader reviews that are limited by the relatively short time span covered.4,5 The purpose of this work was to provide a comprehensive review of injury trends in MLB since the league expanded to 30 teams in 1998 while paying special attention to the financial impact of those injuries. Additionally, we sought to provide an up-to-date review of MUCL injuries and surgeries since the procedure was first developed in 1974. Ultimately, this data demonstrates that injuries continue to rise in MLB and this increase is accompanied by increased expense for teams. Thankfully, the rates of DL assignments for shoulder injuries are on the decline; however, this decrease is countered by a reciprocal increase in elbow injuries. Similarly, the rates of MUCL reconstruction have also risen dramatically in recent years.
The fact that injury rates are on the rise is confirmed by other published reports. This trend was demonstrated in prior analyses of DL data from the 1989 to 19984 and 2002 to 2008 seasons.5 These 2 studies represent the only comprehensive reviews of MLB injury trends to date, and each provides valuable information. Both are consistent with the current study findings that pitchers are the most commonly injured players and that shoulder and elbow injuries represent about half of all injuries.4,5 Similar injury rates and characteristics have been reported at the collegiate20 and minor league levels.21 Despite this consistency, this analysis of injuries from 1998 to 2015 is the first to report that DL designations for shoulder injuries are on the decline while designations for elbow injuries continue to rise. Although the exact etiology of this decline in shoulder injuries remains unknown, there are a number of possible explanations. In recent years, increased emphasis has been placed on shoulder rehabilitation, reduction of glenohumeral internal rotation deficits, scapular stabilization, and overall kinetic chain balance and coordination. However, this does not explain why elbow injuries continue to rise annually.
With this increase in injuries, the cost of maintaining an active 25-man roster is also climbing. As expected, this growing expense is primarily due to the increased number of DL days each year as well as the increase in league salaries. Fortunately, this increased financial strain has been met with steadily increased annual revenues in professional baseball. In 2014, the prorated salary cost to players designated to the DL and their replacements was $579,568,059. This figure represents an estimated 6.4% of the $9 billion in total revenue for MLB that same year.22 Although this may represent a small percentage of the whole, it still embodies an exceptionally large financial responsibility. This does not include the medical expenses incurred to treat and rehabilitate the players’ injuries.
Every injury that occurs in MLB players has the potential to adversely affect players, teams, and MLB as a whole. With its increasing prevalence, need for surgical treatment, and prolonged return to play, injuries to the MUCL of the elbow may represent the most costly of all injuries. Although a multitude of reports on MUCL injuries, treatments, techniques, rehabilitation, and outcomes have been reported,8,9,12,14-19,23-25 to our knowledge, a comprehensive and longitudinal incidence study in MLB players has not yet been published. By including every MUCL reconstruction that has been performed on a MLB player, our study demonstrates the dramatic increase in the annual incidence of MUCL surgeries. Studies performed over shorter time intervals corroborate these findings. A recent review of a privately insured patient database revealed an annual increase in MUCL reconstructions of 4.2% in that cohort.26 When looking specifically at the MLB, a recent survey of all 30 clubs found that 25% (96 of 382) of MLB pitchers and 15% (341 of 2324) of minor league pitchers have undergone MUCL reconstruction.8 Because it occurs so frequently and requires a mean of 17 months to return to sport, MUCL injuries represent a very significant cause of time out of play.
While this study represents a unique epidemiologic report on injuries in baseball, it is certainly not without its limitations. As stated previously, it relies on DL data that was initially intended to serve as a roster management tool rather than an injury database. Accordingly, detailed and specific information about every injury is not always available. The limitations of DL data will largely be overcome in future studies thanks to the implementation of the HITS database in 2010. Moving forward, this system will allow for more detailed analysis of injury patterns, characteristics, time out of play, treatments rendered, etc. Its main limitation is that the earliest data dates back to 2010, making it less applicable for longitudinal studies like the present one. Another limitation of this study is the estimations used for the cost of replacing players designated to the DL. For each injury, it was assumed that the replacement player was paid a prorated portion of the league minimum salary while on the major league roster, but in some instances, that may not have been the case. It is possible that some players filling roster spots were already under contract for amounts higher than the league minimum. Since that player would be making that amount regardless of the level of play, the team may not have paid them any additional salary while filling the position of the injured player. The strengths of this study are its comprehensive nature and inclusion of 18 years of data, making it the longest such study of injuries in MLB. It also represents the first report of cost of replacement for players designated to the DL. To our knowledge, this study also represents the first comprehensive report of every MUCL surgery that has been performed on MLB players.
Conclusion
Injury rates continue to rise in MLB, and upper extremity injuries continue to represent approximately half of all injuries resulting in time out of play. Although shoulder injuries have been on the decline in recent years, this decline is offset by a steady increase in elbow injuries. Each year, MLB players are designated to the DL an average of 464 times for a total of 25,579.6 days. This results in a mean annual cost of over $400 million dollars to replace players lost to injury. Looking specifically at MUCL injuries, a total of 400 MUCL reconstructions have been performed in the MLB since 1974, and nearly one-third of these were performed in the last 5 years. Pitchers represent 90.3% of players requiring MUCL surgery, and the average time to return to sport for all players is 17 months. These data may serve as a foundation for identifying appropriate targets for continued study into the etiologies, strategies for prevention, and optimal treatments of injuries commonly affecting professional baseball players.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
1. Lewis M. Moneyball: The Art of Winning an Unfair Game. Vol 1. New York, NY: W. W. Norton & Company; 2004.
2. Block D. Baseball Before We Knew It: A Search for the Roots of the Game. Vol 1. Lincoln, NE: Bison Books; 2006.
3. James B. The New Bill James Historical Baseball Abstract. Vol 2. Detroit, MI: Free Press; 2003.
4. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
5. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
6. Ahmad CS, Dick RW, Snell E, et al. Major and Minor League Baseball hamstring injuries: epidemiologic findings from the Major League Baseball Injury Surveillance System. Am J Sports Med. 2014;42(6):1464-1470.
7. Green GA, Pollack KM, D’Angelo J, et al. Mild traumatic brain injury in major and Minor League Baseball players. Am J Sports Med. 2015;43(5):1118-1126.
8. Conte SA, Fleisig GS, Dines JS, et al. Prevalence of ulnar collateral ligament surgery in professional baseball players. Am J Sports Med. 2015;43(7):1764-1769.
9. Jones KJ, Conte S, Patterson N, ElAttrache NS, Dines JS. Functional outcomes following revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. J Shoulder Elb Surg. 2013;22(5):642-646.
10. Jones KJ, Osbahr DC, Schrumpf MA, Dines JS, Altchek DW. Ulnar collateral ligament reconstruction in throwing athletes: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(8):e49.
11. Dodson CC, Thomas A, Dines JS, Nho SJ, Williams RJ 3rd, Altchek DW. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12. Erickson BJ, Gupta AK, Harris JD, et al. Rate of return to pitching and performance after Tommy John surgery in Major League Baseball pitchers. Am J Sports Med. 2014;42(3):536-543.
13. Makhni EC, Lee RW, Morrow ZS, Gualtieri AP, Gorroochurn P, Ahmad CS. Performance, return to competition, and reinjury after Tommy John surgery in Major League Baseball pitchers: A review of 147 cases. Am J Sports Med. 2014;42(6):
1323-1332.
14. Jobe FW, Stark H, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg Am. 1986;68(8):1158-1163.
15. Rohrbough JT, Altchek DW, Hyman J, Williams RJ 3rd, Botts JD. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30(4):541-548.
16. Andrews JR, Jost PW, Cain EL. The ulnar collateral ligament procedure revisited: the procedure we use. Sports Health. 2012;4(5):438-441.
17. Keller RA, Steffes MJ, Zhuo D, Bey MJ, Moutzouros V. The effects of medial ulnar collateral ligament reconstruction on Major League pitching performance. J Shoulder Elbow Surg. 2014;23(11):1591-1598.
18. Marshall NE, Keller RA, Lynch JR, Bey MJ, Moutzouros V. Pitching performance and longevity after revision ulnar collateral ligament reconstruction in Major League Baseball pitchers. Am J Sports Med. 2015;43(5):1051-1056.
19. Liu JN, Garcia GH, Conte S, ElAttrache N, Altchek DW, Dines JS. Outcomes in revision Tommy John surgery in Major League Baseball pitchers. J Shoulder Elbow Surg. 2016;25(1):90-97.
20. McFarland EG, Wasik M. Epidemiology of collegiate baseball injuries. Clin J Sport Med. 1998;8(1):10-13.
21. Chambless KM, Knudtson J, Eck JC, Covington LA. Rate of injury in minor league baseball by level of play. Am J Orthop. 2000;29(11):869-872.
22. Brown M. Major League Baseball Sees Record $9 Billion In Revenues For 2014. Forbes. http://www.forbes.com/sites/maurybrown/2014/12/10/major-league-baseball-sees-record-9-billion-in-revenues-for-2014/. Published December 10, 2014. Accessed February 3, 2016.
23. Jones KJ, Dines JS, Rebolledo BJ, et al. Operative management of ulnar collateral ligament insufficiency in adolescent athletes. Am J Sports Med. 2014;42(1):117-121.
24. Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes: a systematic review. Am J Sports Med. 2008;36(6):1193-1205.
25. Wilk KE, Meister K, Andrews JR. Current concepts in the rehabilitation of the overhead throwing athlete. Am J Sports Med. 2002;30(1):136-151.
26. Erickson BJ, Nwachukwu BU, Rosas S, et al. Trends in medial ulnar collateral ligament reconstruction in the United States: A retrospective review of a large private-payer database from 2007 to 2011. Am J Sports Med. 2015;43(7):1770-1774.
Epidemiology, Treatment, and Prevention of Lumbar Spine Injuries in Major League Baseball Players
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
For the last 20 years, injuries resulting in time out of play have been on the rise in Major League Baseball (MLB), and those affecting the back are no exception.1,2 In the first comprehensive report on injuries in MLB players, back injuries resulted in a mean of 1016 disabled list days per season from 1995 to 1999.1 Similarly, core and back injuries were responsible for 359 disabled list designations from 2002 to 2008. This represented 11.7% of all injuries resulting in time out of play during that time span.2 During that time, back injury prevalence ranked 6th highest of all possible body regions (out of 17), and both position players and pitchers were similarly affected (7.8% and 7.4% of all injuries, respectively).2 These injuries often result in a significant time out of play and can have a tremendous impact on player health. A healthy, stable, and well-functioning lumbar spine is a prerequisite for nearly all baseball-related activities, including pitching, throwing, batting, and running. Accordingly, even minor lumbar spine injuries may profoundly influence baseball performance. Despite this, less is currently known about the true epidemiology and impact of back injuries in professional baseball compared to other professional sporting organizations.3
The most common causes of low back pain and injury in elite baseball players include muscle strains, stress fractures (spondylolysis), annular tears, disc herniation, stenosis, transverse process fractures, facetogenic pain, and sacroiliac (SI) joint arthropathy.4-8 These injuries present in a variety of ways with varying symptomatology. Accordingly, a thorough understanding and comprehensive approach to the diagnosis and treatment of these injuries is necessary. The purpose of this article is to discuss the current state of lumbar spine injuries in professional baseball players. Specifically, we will discuss the critical role of the spine in baseball activities, common causes of injury, tips for making the diagnosis, treatment options, outcomes, and injury prevention and rehabilitation strategies.
Role of the Spine in Baseball
The spine and core musculature are responsible for positioning the head, shoulders, and upper extremities in space over the hips and lower extremities. Proper maintenance of this relationship is required during all phases of throwing, pitching, running, and hitting. During these activities, the spine may dynamically flex, extend, rotate, and laterally bend as needed to keep the body balanced with the head centered over the trunk.
Pitching and Throwing
Whether pitching from the wind-up or the stretch, the head begins centered over the hips and pelvis. As the pitching motion progresses, the hips undergo rotation, flexion, extension, abduction, and circumduction. While this is occurring, the shoulders and upper truck must bend, rotate, and translate toward home plate with the body. Just prior to front foot contact, trunk rotation averages 55 ± 6° with a maximal mean angular acceleration of 11,600 ± 3100°/s2. 9 In order for the body to remain balanced, controlled, and synchronized throughout this delivery, the lumbar spine and core musculature must work diligently to stabilize the entire kinetic chain. Of all the trunk muscles (paraspinal, rectus abdominis, obliques, and glutei), the lumbar paraspinal muscles often work the hardest during the pitching motion, demonstrating activity increases ranging from 100% to 400%.10 Accordingly, it is not uncommon for pitchers to develop SI joint or lumbar facet joint pain due to this high degree of torsional strain exerted on the low back.4 Poor lumbopelvic control has been shown to be a predictor of subsequent injury, and the degree of lumbopelvic dysfunction is proportional to injury severity in MLB pitchers.5
Hitting
Similar to pitching, hitting involves a complex combination of movements from the upper and lower extremities that must be balanced by the core and spine. Although numerous movements occur simultaneously, rotational motion is primarily responsible for generating power. The trunk rotates an average of 46 ± 9° during the swing and reaches a maximal angular acceleration of 7200 ± 2800°/s2 just after contact.9 During this period of rapid torsion, the spine must rotate in conjunction with the hips and shoulders to create a stable cylinder and axis of rotation. The spine and core are responsible for synchronizing rotation to ensure that hip and shoulder parallelism is maintained from swing initiation to ball contact. If the body does not rotate as a unit, the position of the head is affected and the batter’s ability to see the ball may be compromised. Additionally, if delivery of the shoulders lags too far behind that of the hips, the position of the hands (and bat) in space is adversely affected. The entire kinetic chain must remain balanced, coordinated, precisely timed, and standardized throughout the entire swing from initial trigger to final follow-through. The lumbar spine plays a critical role in each of these steps. If lumbar spine mechanics are not sound, this can have significant adverse effects on batting performance and may predispose hitters to injury.4
Common Etiologies for Spinal Injury
The vast majority of baseball players who experience lumbar pain will have injuries that can be classified as mechanical back pain (ie, spondylolysis, annular tears, facetogenic pain, SI joint arthropathy, or muscle injuries) (Table). Although less likely to occur, nerve entrapment or impingement syndromes (ie, disc herniation, stenosis, and peripheral nerve entrapment) have been observed in professional baseball players. Finally, more concerning pathologies such as infection and tumor are extremely rare, but they must not be overlooked in this high-demand patient population.
Stress Fracture or Spondylolysis
In young athletic patients, up to one-third of those with low back pain may have evidence of a lumbar stress fracture on bone scan.11,12 This is particularly true for athletes who undergo repetitive lumbar extension and rotation, such as linemen, gymnasts, wrestlers, weight lifters, and baseball players.4,13 Although the majority of lumbar stress fractures occur at the pars interarticularis, they can occur in the pedicle or articular process (Figure 1). Most spondylolytic lesions do not progress to spondylolisthesis, especially once patients reach skeletal maturity. Because the fifth lumbar vertebra represents the transition from the lumbar to the sacral spine, most stress fractures occur at L5. These typically present as localized low back pain that worsens with flexion, extension, and rotation.
Muscle Injury
One of the most common causes of low back pain in athletes is muscle strains and spasms. Because the lumbar paraspinal muscles are extremely active during throwing and hitting,10 they are particularly susceptible to injury. This is particularly true in deconditioned athletes or those who report to spring training having not adequately maintained strength and flexibility through the off-season.4,5 These injuries typically present in an acute fashion with an obvious inciting incident. Players may have a history of similar muscle injuries in the past. On examination, they tend to have difficulty maintaining normal posture or ranging the spine through a full arc of motion. Localized, superficial tenderness to palpation in the injured muscle is a key component of the diagnosis.
Annular Tears and Disc Herniation
These injuries typically occur as the result of a combination of compressive and rotary forces on the lumbar spine that overcome the ability of the annulus fibrosus to resist hoop stresses. Patients with annular tears typically present with severe lower back pain that may be accompanied by spasm and pain radiation into the buttock or lower extremities. Pain is usually worsened by valsalva, coughing, sneezing, or bearing down.4 Although annular tears can occur in isolation, they can also lead to herniation of the nucleus pulposus into the spinal canal (Figure 2). Depending on the location and severity of the herniation, nerve entrapment or impingement can occur. This may initially present as pain that radiates into the lower extremities in a dermatomal fashion. As the herniation progresses, decreased sensation and weakness may develop.
Facet Joint Pain
Facetogenic pain can occur as the result of degenerative changes, trauma, or joint inflammation. Facet injury typically occurs during rotation while the back is extended.4 This results in localized pain and tenderness that can be reproduced by loading the facet joint (lumbar extension) during the examination, and patients will often demonstrate discomfort and altered motion when extending the flexed back.
Sacroiliac Joint Pain
Although pain in the region of the SI joint is very common, much of this may actually be referred from more centrally located neuromotion segments.4 SI joint pathology can occur as a result of trauma, degeneration, or inflammatory processes as is seen in ankylosing spondylitis (AS). Patients with AS typically present with a gradual onset of progressive stiffness and pain in the low back and hips that is worse in the morning or following periods of inactivity. It is most common in Caucasian males in their second to fourth decades.14 Although 80% to 95% of patients with AS will test positive for human leukocyte antigen B27 (HLA-B27), it is important to note that the vast majority of people with HLA-B27 do not go on to develop AS.14 Regardless of the cause, SI joint pain can be very debilitating and negatively impact all baseball-related activities.
Stenosis
Lumbar stenosis may develop from arthritic changes, disc protrusion, facet hypertrophy, or ligament ossification. In this young, athletic population, congenital stenosis should also be a consideration. Patients with congenital stenosis at baseline are at increased risk for developing neurologic symptoms from disc protrusion or other acquired spinal pathology. Lumbar stenosis generally manifests as a gradual onset of progressive low back pain with radicular symptoms or neurogenic claudication.4
Making the Diagnosis
History
When identifying the cause of any musculoskeletal complaint, the diagnosis begins with a thorough history. In addition to the standard components of the history, such as timing, severity, relation to activity, exacerbating factors, associated symptoms, and prior treatments, Watkins and colleagues4 have outlined a number of key factors that should be determined when specifically evaluating the athlete with low back pain.These include quantification of the morbidity, identification of contributing psychosocial factors, ruling out of urgent diagnoses (ie, neoplasm, infection, rapidly progressive neurologic deficits, cauda equina, and paralysis), determination of injury type and duration, identification of the clinical syndrome/etiology, pinpointing the location of the pathology (what nerve at what level?), and quantification of back versus leg symptoms. Answers to these questions will set the framework for an appropriately directed physical examination, imaging, and diagnostic tests.
Physical Examination
The physical examination begins by observing the patient or player walk across the playing field, training room, or examination room, paying attention to posture, gait, and overall body movement. Many patients with lumbar injuries will demonstrate adaptive patterns of motion in an attempt to accommodate their pain. This may be seen during baseball-related activities such as throwing, batting, or running. The spine should be visualized and palpated for malalignment while standing erect and during forward bending. If possible, motion should be assessed in rotation, lateral bending, and the flexion and extension planes. Special attention should be paid to any positions or maneuvers that reproduce pain or neurologic symptoms. Areas of tenderness and radiating pain should be fully palpated. A full neurologic examination consisting of manual muscle testing, sensory examination, and reflex evaluation of both the upper and lower extremities should be performed. Numerous special tests and neurologic stretch maneuvers that assess specific lumbar nerve roots have been described.15
Imaging and Diagnostic Tests
Depending on the history and physical examination, imaging of the lumbar spine is not always warranted in the acute setting. This is especially the case if muscle injury, herniation, or annular tears are suspected. In cases of persistent pain, trauma, or suspected neoplasia, imaging is generally warranted. When x-rays are negative and spondylolysis is suspected, bone scan with lumbar single photon emission computed tomography (SPECT) is the most sensitive test.16 SPECT scans are positive in active spondylolysis because the radio-nucleotide is taken up by active, bone-forming osteoblasts. Quiescent stress fractures that are not apparent on SPECT scans are generally chronic and painless.4 If the SPECT scan is positive, the injury can be further characterized by computed tomography (CT) (Figure 1), which can distinguish between spondylolysis, osteoid osteoma, osteoblastoma, acute fracture, or arthritic degeneration. When the SPECT is negative, or if neural impingement is suspected, magnetic resonance imaging (MRI) (Figure 2) is likely the best diagnostic imaging tool. MRI allows identification of bone edema, disc herniation, annular disruption, disc desiccation, stenosis, and nerve entrapment. Finally, when attempting to distinguish between central and peripheral nerve entrapment syndromes, an electromyogram (EMG) or nerve conduction study (NCS) is a reliable way to identify the location of injury.
Treatment and Outcomes
The approach to a patient with low back pain begins with identification of the etiology and discontinuation of the activities that reproduce pain.4 Trunk stabilization exercises and anti-inflammatory medications are the mainstays of treatment regardless of the cause of the lumbar spinal injury in the baseball player.4
Stress Fracture or Spondylolysis
Management of symptomatic spondylolysis or spondylolisthesis in the athlete initially consists of conservative treatment, which achieves good to excellent long-term outcomes and return to play in 70% to 90% of athletes, especially for acute injuries.17-19 After stopping the activity that causes the pain, trunk stabilization exercises should be started as soon as tolerated with the use of non-steroidal anti-inflammatory medications (NSAIDs), oral steroids, and spinal injections to control symptoms and permit initiation of the rehabilitation program.4 Although bracing is a commonly used adjunctive treatment, a recent meta-analysis did not demonstrate any difference in clinical outcomes between patients treated with a brace compared to non-braced controls.20
Surgical indications for the treatment of spondylolysis or spondylolisthesis are limited; however, failure of nonoperative treatment after 6 months is a reasonable time to consider surgery.17 The spondylolytic defect can often be repaired directly using hook screws, translaminar screws, wiring, pedicle screws, or image-guided lag screws across the lesion with grafting.4 Lumbar spinal fusion is less successful in professional athletes due to the high demands placed on adjacent levels as well as the time required for the fusion to heal.4 Bony union can be determined by a CT scan at 6 months postoperatively if the patient has met appropriate return to play criteria.4
Muscle Injury
Management of lumbar sprains and strains typically includes restricting painful postures and a rehabilitation program that focuses on core strengthening within a pain-free arc of motion.21 Because acute injuries typically resolve quickly and spontaneously, a short interval of decreased activity, icing, NSAIDs, and stretching followed by focused strength training is appropriate before return to sports activity.22
Annular Tears and Disc Herniation
Initial management of baseball players with acute lumbar disc herniation and/or annular tears consists of rest for up to 5 days followed by physical therapy and NSAIDs, Medrol Dose pak, or epidural injections.4 Professional baseball players return to play at high rates following a herniated lumbar disc.6 Earhart and colleagues6 found that 97.1% of players returned to play at an average time of 6.6 months from the time of injury. When stratified by position, all pitchers (29 of 29) returned to competitive play after operative or nonoperative management, while 38 of 40 hitters returned.6 The average career length after lumbar disc herniation in the professional baseball player is between 4.1 and 5.3 years or between 256 and 471 games.6,23 Other work has suggested that players undergoing operative treatment for lumbar herniation had shorter career lengths; however, patients in the operative group tended to be older at the time of injury.23
Emphasis should be placed on nonoperative management of baseball players with disc pathology except in cases of cauda equina syndrome.4 Hitters and pitchers who require surgery have demonstrated decreased 1-year and 3-year postoperative statistical performance compared to preinjury levels.6 No significant changes in any performance statistic were seen in baseball following nonoperative management.6 Consequently, indications for surgery in the baseball player with lumbar disc pathology includes cauda equina syndrome, progressive neurologic deficit, sufficient morbidity, failure of conservative care, a lesion that can be corrected safely with surgery, and the ability for the patient to comply with a comprehensive postoperative rehabilitation program.4 Operative treatment typically consists of a lumbar microdiscectomy and/or laminotomy. 4,6
Facet Joint Pain
The mainstay of therapy in patients with facet joint pain consists of analgesia and a trunk stabilization program.24 Lumbar zygapophysial joint injections and radiofrequency denervation can be considered if the patient fails 4 weeks of directed conservative treatment.24,25 Injections may be useful in select patients; however, the literature supporting the use of lumbar facet joint injections or radiofrequency denervation for facetogenic pain is limited.24,25
Sacroiliac Joint Pain
Acute injury of the SI joint can be treated with NSAIDs, icing, and relative rest.26 Mobilization of the SI joint in addition to correcting any asymmetries in muscle length or stiffness should be started and progressed as soon as tolerated within a pain-free range of motion.26 Rehabilitation should correct biomechanical deficits and maladaptation with a special focus on agonist and antagonist muscle groups across the sacrum and ilium.26 Treatment of AS in the athlete should emphasize symptom control, as there is no definite treatment. For patients with AS, other long-term therapeutic options include sulfasalazine, methotrexate, thalidomide, and anti-tumor necrosis factor therapies.14
Stenosis
Lumbar spinal stenosis, whether congenital or acquired, should initially be managed conservatively.27 Although they do not alter the progression of the disease, epidural steroids and local injections may temporarily decrease symptoms in approximately 40% of cases.27 Those who fail conservative therapy after 3 months may be candidates for surgical decompression and/or fusion.27,28 However, surgical treatment for lumbar spinal stenosis in elite baseball players has not been thoroughly studied, so the long-term prognosis is not well documented.27
Rehabilitation and Prevention of Injuries
After an appropriate diagnosis has been made, a structured rehabilitation process should commence. During rehabilitation, it is of primary importance that deep core stabilization is established. As an initial step in this process, athletes are trained to initiate deep core stabilization with breathing techniques in a static, supine position.29 Proper diaphragm activation with co-contractions of the transverse abdominis (TA) and pelvic floor has been shown to increase lumbar spine stability.30 This will allow for an increase in intra-abdominal pressure (IAP) and improved stabilization of the lumbar spine, creating a muscular cylinder between the bottom of the rib cage and top of the pelvis. These activities are initiated in the supine position but are soon advanced as upper and lower extremity movement against resistance is added. It is important to make sure IAP and contraction of the TA is maintained throughout this sequence of progression.
Once deep core stabilization has been established, athletes are progressed to global muscle training and kinetic linking in all 3 planes of movement. This is an important phase, as lumbar stability is a result of coordinated muscle activation involving many muscles.31 This program progresses from supine breathing exercises to a modified side bridge position to enhance core activation along with frontal plane stability. Next, athletes are progressed to a half kneeling position and then on to standing. Rotational activities are introduced starting with isometric holds progressing to chops/lifts and rotational medicine ball toss. During these tasks, focus should be on quality of movement and maintenance of core activation. Endurance of these muscles should be trained during this process. Appropriate pain-free and safe cardiovascular exercise, such as walking, biking, swimming, and jogging, should be performed throughout each stage in the rehabilitation process. Activities should be halted with any increase in pain. At the completion of the rehabilitation process, it is important to observe the athlete while performing sport-specific tasks. Spinal stabilization must be translational and monitored by observing maintenance of the “cylinder” from the training room to sports specific movements.
Since poor lumbar control has been associated with increased amount of time on the disabled list,5 it would be ideal to identify those at risk of injury before problems arise. Conte and colleagues32 have shown that core muscle strains could be a result of muscle imbalance or improper pitching or hitting technique. Other work has demonstrated that pitchers with poor lumbopelvic control did not perform as well as those with superior control.33 By assessing spinal stability and biomechanics at baseline, we may be able to identify those at risk. Pitchers with suboptimal spinal stabilization can present with an unstable balance phase, increased amounts of hyperextension of the lumbar spine from the moment of max cocking through ball release, as well as increased lateral trunk tilt at ball release. Correcting these flaws and increasing deep core stabilization can prevent injuries and improve performance.
Summary
A stable, well-functioning lumbar spine is vital to nearly every baseball-related activity, including pitching, throwing, batting, fielding, and running. The spine serves as a critical link in the kinetic chain between the upper and lower extremities. Due to the high demand on the lumbar spine, injuries to this area represent a significant amount of time out of play in MLB. Initial treatment typically consists of a comprehensive nonoperative rehabilitation process involving analgesics, rest, and therapy focusing on core stabilization. Because poor lumbopelvic control and mechanics have been demonstrated to increase injury risk, preemptive spinal and core stabilization is likely an appropriate step towards injury prevention.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.
1. Conte S, Requa RK, Garrick JG. Disability days in major league baseball. Am J Sports Med. 2001;29(4):431-436.
2. Posner M, Cameron KL, Wolf JM, Belmont PJ, Owens BD. Epidemiology of Major League Baseball injuries. Am J Sports Med. 2011;39(8):1676-1680.
3. Makhni EC, Buza JA, Byram I, Ahmad CS. Sports reporting: a comprehensive review of the medical literature regarding North American professional sports. Phys Sportsmed. 2014;42(2):154-162.
4. Watkins RG III, Watkins RG IV. Chapter 36: Lumbar injuries. In: Sports Medicine of Baseball. Dines JS, Altchek DW, Andrews JR, ElAttrache NS, Wilk KE, Yocum LA, eds. Philadelphia, PA: Lippincott Williams & Wilkins; 2012; 383-398.
5. Chaudhari AMW, McKenzie CS, Pan X, Oñate JA. Lumbopelvic control and days missed because of injury in professional baseball pitchers. Am J Sports Med. 2014;42(11):2734-2740.
6. Earhart JS, Roberts D, Roc G, Gryzlo S, Hsu W. Effects of lumbar disk herniation on the careers of professional baseball players. Orthopedics. 2012;35(1):43-49.
7. Hamid KS, Nwachukwu BU, Hsu E, Edgerton CA, Hobson DR, Lang JE. Orthopedic resident work-shift analysis: Are we making the best use of resident work hours? J Surg Educ. 2014;71(2):205-210.
8. Nair R, Kahlenberg CA, Hsu WK. Outcomes of lumbar discectomy in elite athletes: the need for high-level evidence. Clin Orthop Relat Res. 2015;473(6):1971-1977.
9. Fleisig GS, Hsu WK, Fortenbaugh D, Cordover A, Press JM. Trunk axial rotation in baseball pitching and batting. Sports Biomech. 2013;12(4):324-333.
10. Watkins RG, Dennis S, Dillin WH, et al. Dynamic EMG analysis of torque transfer in professional baseball pitchers. Spine (Phila Pa 1976). 1989;14(4):404-408.
11. Micheli LJ. Back injuries in gymnastics. Clin Sports Med. 1985;4(1):85-93.
12. Papanicolaou N, Wilkinson RH, Emans JB, Treves S, Micheli LJ. Bone scintigraphy and radiography in young athletes with low back pain. AJR Am J Roentgenol. 1985;145(5):1039-1044.
13. Elliott S, Hutson MA, Wastie ML. Bone scintigraphy in the assessment of spondylolysis in patients attending a sports injury clinic. Clin Radiol. 1988;39(3):269-272.
14. Kubiak EN, Moskovich R, Errico TJ, Di Cesare PE. Orthopaedic management of ankylosing spondylitis. J Am Acad Orthop Surg. 2005;13(4):267-278.
15. Miller KJ. Physical assessment of lower extremity radiculopathy and sciatica. J Chiropr Med. 2007;6(2):75-82.
16. Bellah RD, Summerville DA, Treves ST, Micheli LJ. Low-back pain in adolescent athletes: detection of stress injury to the pars interarticularis with SPECT. Radiology. 1991;180(2):509-512.
17. Radcliff KE, Kalantar SB, Reitman CA. Surgical management of spondylolysis and spondylolisthesis in athletes: indications and return to play. Curr Sports Med Rep. 8(1):35-40.
18. Morita T, Ikata T, Katoh S, Miyake R. Lumbar spondylolysis in children and adolescents. J Bone Joint Surg Br. 1995;77(4):620-625.
19. Hu SS, Tribus CB, Diab M, Ghanayem AJ. Spondylolisthesis and spondylolysis. J Bone Joint Surg Am. 2008;90(3):656-671.
20. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. J Pediatr Orthop. 2009;29(2):146-156.
21. Bono CM. Low-back pain in athletes. J Bone Joint Surg Am. 2004;86-A(2):382-396.
22. Dreisinger TE, Nelson B. Management of back pain in athletes. Sports Med. 1996;21(4):313-320.
23. Hsu WK, McCarthy KJ, Savage JW, et al. The Professional Athlete Spine Initiative: outcomes after lumbar disc herniation in 342 elite professional athletes. Spine J. 2011;11(3):180-186.
24. Dreyfuss PH, Dreyer SJ; NASS. Lumbar zygapophysial (facet) joint injections. Spine J. 2003;3(3 Suppl):50S-59S.
25. Slipman CW, Bhat AL, Gilchrist R V, Issac Z, Chou L, Lenrow DA. A critical review of the evidence for the use of zygapophysial injections and radiofrequency denervation in the treatment of low back pain. Spine J. 2003;3(4):310-316.
26. Prather H. Sacroiliac joint pain: practical management. Clin J Sport Med. 2003;13(4):252-255.
27. Graw BP, Wiesel SW. Low back pain in the aging athlete. Sports Med Arthrosc. 2008;16(1):39-46.
28. Melancia JL, Francisco AF, Antunes JL. Spinal stenosis. Handb Clin Neurol. 2014;119:541-549.
29. Frank C, Kobesova A, Kolar P. Dynamic neuromuscular stabilization & sports rehabilitation. Int J Sports Phys Ther. 2013;8(1):62-73.
30. Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J Biomech. 1999;32(1):13-17.
31. McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-359.
32. Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991-2010. Am J Sports Med. 2012;40(3):650-656.
33. Chaudhari AMW, McKenzie CS, Borchers JR, Best TM. Lumbopelvic control and pitching performance of professional baseball pitchers. J Strength Cond Res. 2011;25(8):2127-2132.