User login
Mark Lesney is the editor of MDedge.com/IDPractioner and of Hematology News. He has been at Frontline Medical Communications since 2005, before which he worked as an editor/writer for the American Chemical Society. He has a PhD in plant virology and a PhD in the history of science. He has served as an adjunct assistant professor in the department of biochemistry and molecular & celluar biology at Georgetown University, Washington, and an assistant professor in the department of forestry at the University of Florida, Gainesville.
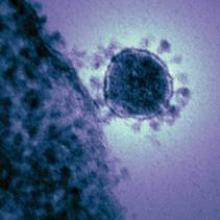
Pathways to new therapeutic agents for human coronaviruses
No specific treatment is currently available for human coronaviruses to date, but numerous antiviral agents are being identified through a variety of approaches, according to Thanigaimalai Pillaiyar, PhD, and colleagues in a review published in Drug Discovery Today.
Using the six previously discovered human coronaviruses – human CoV 229E (HCoV-229E), OC43 (HCoV-OC43), NL63 (HCoV-NL63), HKU1 (HCoV-HKU1); severe acute respiratory syndrome (SARS) CoV; and Middle East respiratory syndrome (MERS) CoV – the investigators examined progress in the use and development of therapeutic drugs, focusing on the potential roles of virus inhibitors.
“Research has mainly been focused on SARS- and MERS-CoV infections, because they were responsible for severe illness when compared with other CoVs,” Dr. Pillaiyar, of the department of pharmaceutical and medicinal chemistry at the University of Bonn (Germany), and colleagues wrote.
2019-nCov has been linked genomically as most closely related to SARS, and the Coronavirus Study Group of the International Committee on Virus Taxonomy, which has the responsibility for naming viruses, has designated the new virus SARS-CoV-2.
Examining extant drugs
The first approach to identifying possible antiviral agents reevaluates known, broadly acting antiviral drugs that have been used for other viral infections or other indications. The initial research into coronavirus therapeutics, in particular, has examined current antiviral therapeutics for their effectiveness against both SARS-CoV and MERS-CoV, but with mixed results.
For example, in a search of potential antiviral agents against CoVs, researchers identified four drugs – chloroquine, chlorpromazine, loperamide, and lopinavir – by screening drug libraries approved by the Food and Drug Administration. They were all able to inhibit the replication of MERS-CoV, SARS-CoV, and HCoV-229E in the low-micromolar range, which suggested that they could be used for broad-spectrum antiviral activity, according to Dr. Pillaiyar and colleagues.
Other research groups have also reported the discovery of antiviral drugs using this drug-repurposing approach, which included a number of broad-spectrum inhibitors of HCoVs (lycorine, emetine, monensin sodium, mycophenolate mofetil, mycophenolic acid, phenazopyridine, and pyrvinium pamoate) that showed strong inhibition of replication by four CoVs in vitro at low-micromolar concentrations and suppressed the replication of all CoVs in a dose-dependent manner. Findings from in vivo studies showed lycorine protected mice against lethal HCoV-OC43 infection.
Along with the aforementioned drugs, a number of others have also shown potential usefulness, but, as yet, none has been validated for use in humans.
Developing new antivirals
The second approach for anti-CoV drug discovery involves the development of new therapeutics based on the genomic and biophysical understanding of the individual CoV in order to interfere with the virus itself or to disrupt its direct metabolic requirements. This can take several approaches.
MERS-CoV and SARS-CoV PL protease inhibitors
Of particular interest are antiviral therapies that attack papain-like protease, which is an important target because it is a multifunctional protein involved in proteolytic deubiquitination and viral evasion of the innate immune response. One such potential therapeutic that takes advantage of this target is disulfiram, an FDA-approved drug for use in alcohol-aversion therapy. Disulfiram has been reported as an allosteric inhibitor of MERS-CoV papain-like protease. Numerous other drug categories are being examined, with promising results in targeting the papain-like protease enzymes of both SARS and MERS.
Replicase inhibitors
Helicase (nsP13) protein is a crucial component required for virus replication in host cells and could serve as a feasible target for anti-MERS and anti-SARS chemical therapies, the review authors wrote, citing as an example, the recent development of a small 1,2,4-triazole derivative that inhibited the viral NTPase/helicase of SARS- and MERS-CoVs and demonstrated high antiviral activity and low cytotoxicity.
Membrane-bound viral RNA synthesis inhibitors
Antiviral agents that target membrane-bound coronaviral RNA synthesis represent a novel and attractive approach, according to Dr. Pillaiyar and colleagues. And recently, an inhibitor was developed that targets membrane-bound coronaviral RNA synthesis and “showed potent antiviral activity of MERS-CoV infection with remarkable efficacy.”
Host-based, anti-CoV treatment options
An alternate therapeutic tactic is to bolster host defenses or to modify host susceptibilities to prevent virus infection or replication. The innate interferon response of the host is crucial for the control of viral replication after infection, and the addition of exogenous recombinant interferon or use of drugs to stimulate the normal host interferon response are both potential therapeutic avenues. For example, nitazoxanide is a potent type I interferon inducer that has been used in humans for parasitic infections, and a synthetic nitrothiazolyl-salicylamide derivative was found to exhibit broad-spectrum antiviral activities against RNA and DNA viruses, including some coronaviruses.
Numerous other host pathways are being investigated as potential areas to enhance defense against infection and replication, for example, using inhibitors to block nucleic acid synthesis has been shown to provide broad-spectrum activity against SARS-CoV and MERS-CoV.
One particular example is remdesivir, a novel nucleotide analog antiviral drug, that was developed as a therapy for Ebola virus disease and Marburg virus infections. It was later shown to provide “reasonable antiviral activity against more distantly related viruses, such as respiratory syncytial virus, Junin virus, Lassa fever virus, and MERS-CoV,” the authors wrote.
Also of interest regarding remdesivir’s potential broad-spectrum use is that it has shown potent in vitro “antiviral activity against Malaysian and Bangladesh genotypes of Nipah virus (an RNA virus, although not a coronavirus, that infects both humans and animals) and reduced replication of Malaysian Nipah virus in primary human lung microvascular endothelial cells by more than four orders of magnitude,” Dr. Pillaiyar and colleagues added. Of particular note, all remdesivir-treated, Nipah virus–infected animals “survived the lethal challenge, indicating that remdesivir represents a promising antiviral treatment.”
In a press briefing earlier this month, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, reported that a randomized, controlled, phase 3 trial of the antiviral drug remdesivir is currently underway in China to establish whether the drug would be an effective and safe treatment for adults patients with mild or moderate 2019 Novel Coronavirus (2019-nCoV) disease.
“Our increasing understanding of novel emerging coronaviruses will be accompanied by increasing opportunities for the reasonable design of therapeutics. Importantly, understanding this basic information about CoV protease targets will not only aid the public health against SARS-CoV and MERS-CoV but also help in advance to target new coronaviruses that might emerge in the future,” the authors concluded.
Dr. Pillaiyar and colleagues reported that they had no financial conflicts of interest.
SOURCE: Pillaiyar T et al. Drug Discov Today. 2020 Jan 30. doi: 10.1016/j.drudis.2020.01.015.
No specific treatment is currently available for human coronaviruses to date, but numerous antiviral agents are being identified through a variety of approaches, according to Thanigaimalai Pillaiyar, PhD, and colleagues in a review published in Drug Discovery Today.
Using the six previously discovered human coronaviruses – human CoV 229E (HCoV-229E), OC43 (HCoV-OC43), NL63 (HCoV-NL63), HKU1 (HCoV-HKU1); severe acute respiratory syndrome (SARS) CoV; and Middle East respiratory syndrome (MERS) CoV – the investigators examined progress in the use and development of therapeutic drugs, focusing on the potential roles of virus inhibitors.
“Research has mainly been focused on SARS- and MERS-CoV infections, because they were responsible for severe illness when compared with other CoVs,” Dr. Pillaiyar, of the department of pharmaceutical and medicinal chemistry at the University of Bonn (Germany), and colleagues wrote.
2019-nCov has been linked genomically as most closely related to SARS, and the Coronavirus Study Group of the International Committee on Virus Taxonomy, which has the responsibility for naming viruses, has designated the new virus SARS-CoV-2.
Examining extant drugs
The first approach to identifying possible antiviral agents reevaluates known, broadly acting antiviral drugs that have been used for other viral infections or other indications. The initial research into coronavirus therapeutics, in particular, has examined current antiviral therapeutics for their effectiveness against both SARS-CoV and MERS-CoV, but with mixed results.
For example, in a search of potential antiviral agents against CoVs, researchers identified four drugs – chloroquine, chlorpromazine, loperamide, and lopinavir – by screening drug libraries approved by the Food and Drug Administration. They were all able to inhibit the replication of MERS-CoV, SARS-CoV, and HCoV-229E in the low-micromolar range, which suggested that they could be used for broad-spectrum antiviral activity, according to Dr. Pillaiyar and colleagues.
Other research groups have also reported the discovery of antiviral drugs using this drug-repurposing approach, which included a number of broad-spectrum inhibitors of HCoVs (lycorine, emetine, monensin sodium, mycophenolate mofetil, mycophenolic acid, phenazopyridine, and pyrvinium pamoate) that showed strong inhibition of replication by four CoVs in vitro at low-micromolar concentrations and suppressed the replication of all CoVs in a dose-dependent manner. Findings from in vivo studies showed lycorine protected mice against lethal HCoV-OC43 infection.
Along with the aforementioned drugs, a number of others have also shown potential usefulness, but, as yet, none has been validated for use in humans.
Developing new antivirals
The second approach for anti-CoV drug discovery involves the development of new therapeutics based on the genomic and biophysical understanding of the individual CoV in order to interfere with the virus itself or to disrupt its direct metabolic requirements. This can take several approaches.
MERS-CoV and SARS-CoV PL protease inhibitors
Of particular interest are antiviral therapies that attack papain-like protease, which is an important target because it is a multifunctional protein involved in proteolytic deubiquitination and viral evasion of the innate immune response. One such potential therapeutic that takes advantage of this target is disulfiram, an FDA-approved drug for use in alcohol-aversion therapy. Disulfiram has been reported as an allosteric inhibitor of MERS-CoV papain-like protease. Numerous other drug categories are being examined, with promising results in targeting the papain-like protease enzymes of both SARS and MERS.
Replicase inhibitors
Helicase (nsP13) protein is a crucial component required for virus replication in host cells and could serve as a feasible target for anti-MERS and anti-SARS chemical therapies, the review authors wrote, citing as an example, the recent development of a small 1,2,4-triazole derivative that inhibited the viral NTPase/helicase of SARS- and MERS-CoVs and demonstrated high antiviral activity and low cytotoxicity.
Membrane-bound viral RNA synthesis inhibitors
Antiviral agents that target membrane-bound coronaviral RNA synthesis represent a novel and attractive approach, according to Dr. Pillaiyar and colleagues. And recently, an inhibitor was developed that targets membrane-bound coronaviral RNA synthesis and “showed potent antiviral activity of MERS-CoV infection with remarkable efficacy.”
Host-based, anti-CoV treatment options
An alternate therapeutic tactic is to bolster host defenses or to modify host susceptibilities to prevent virus infection or replication. The innate interferon response of the host is crucial for the control of viral replication after infection, and the addition of exogenous recombinant interferon or use of drugs to stimulate the normal host interferon response are both potential therapeutic avenues. For example, nitazoxanide is a potent type I interferon inducer that has been used in humans for parasitic infections, and a synthetic nitrothiazolyl-salicylamide derivative was found to exhibit broad-spectrum antiviral activities against RNA and DNA viruses, including some coronaviruses.
Numerous other host pathways are being investigated as potential areas to enhance defense against infection and replication, for example, using inhibitors to block nucleic acid synthesis has been shown to provide broad-spectrum activity against SARS-CoV and MERS-CoV.
One particular example is remdesivir, a novel nucleotide analog antiviral drug, that was developed as a therapy for Ebola virus disease and Marburg virus infections. It was later shown to provide “reasonable antiviral activity against more distantly related viruses, such as respiratory syncytial virus, Junin virus, Lassa fever virus, and MERS-CoV,” the authors wrote.
Also of interest regarding remdesivir’s potential broad-spectrum use is that it has shown potent in vitro “antiviral activity against Malaysian and Bangladesh genotypes of Nipah virus (an RNA virus, although not a coronavirus, that infects both humans and animals) and reduced replication of Malaysian Nipah virus in primary human lung microvascular endothelial cells by more than four orders of magnitude,” Dr. Pillaiyar and colleagues added. Of particular note, all remdesivir-treated, Nipah virus–infected animals “survived the lethal challenge, indicating that remdesivir represents a promising antiviral treatment.”
In a press briefing earlier this month, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, reported that a randomized, controlled, phase 3 trial of the antiviral drug remdesivir is currently underway in China to establish whether the drug would be an effective and safe treatment for adults patients with mild or moderate 2019 Novel Coronavirus (2019-nCoV) disease.
“Our increasing understanding of novel emerging coronaviruses will be accompanied by increasing opportunities for the reasonable design of therapeutics. Importantly, understanding this basic information about CoV protease targets will not only aid the public health against SARS-CoV and MERS-CoV but also help in advance to target new coronaviruses that might emerge in the future,” the authors concluded.
Dr. Pillaiyar and colleagues reported that they had no financial conflicts of interest.
SOURCE: Pillaiyar T et al. Drug Discov Today. 2020 Jan 30. doi: 10.1016/j.drudis.2020.01.015.
No specific treatment is currently available for human coronaviruses to date, but numerous antiviral agents are being identified through a variety of approaches, according to Thanigaimalai Pillaiyar, PhD, and colleagues in a review published in Drug Discovery Today.
Using the six previously discovered human coronaviruses – human CoV 229E (HCoV-229E), OC43 (HCoV-OC43), NL63 (HCoV-NL63), HKU1 (HCoV-HKU1); severe acute respiratory syndrome (SARS) CoV; and Middle East respiratory syndrome (MERS) CoV – the investigators examined progress in the use and development of therapeutic drugs, focusing on the potential roles of virus inhibitors.
“Research has mainly been focused on SARS- and MERS-CoV infections, because they were responsible for severe illness when compared with other CoVs,” Dr. Pillaiyar, of the department of pharmaceutical and medicinal chemistry at the University of Bonn (Germany), and colleagues wrote.
2019-nCov has been linked genomically as most closely related to SARS, and the Coronavirus Study Group of the International Committee on Virus Taxonomy, which has the responsibility for naming viruses, has designated the new virus SARS-CoV-2.
Examining extant drugs
The first approach to identifying possible antiviral agents reevaluates known, broadly acting antiviral drugs that have been used for other viral infections or other indications. The initial research into coronavirus therapeutics, in particular, has examined current antiviral therapeutics for their effectiveness against both SARS-CoV and MERS-CoV, but with mixed results.
For example, in a search of potential antiviral agents against CoVs, researchers identified four drugs – chloroquine, chlorpromazine, loperamide, and lopinavir – by screening drug libraries approved by the Food and Drug Administration. They were all able to inhibit the replication of MERS-CoV, SARS-CoV, and HCoV-229E in the low-micromolar range, which suggested that they could be used for broad-spectrum antiviral activity, according to Dr. Pillaiyar and colleagues.
Other research groups have also reported the discovery of antiviral drugs using this drug-repurposing approach, which included a number of broad-spectrum inhibitors of HCoVs (lycorine, emetine, monensin sodium, mycophenolate mofetil, mycophenolic acid, phenazopyridine, and pyrvinium pamoate) that showed strong inhibition of replication by four CoVs in vitro at low-micromolar concentrations and suppressed the replication of all CoVs in a dose-dependent manner. Findings from in vivo studies showed lycorine protected mice against lethal HCoV-OC43 infection.
Along with the aforementioned drugs, a number of others have also shown potential usefulness, but, as yet, none has been validated for use in humans.
Developing new antivirals
The second approach for anti-CoV drug discovery involves the development of new therapeutics based on the genomic and biophysical understanding of the individual CoV in order to interfere with the virus itself or to disrupt its direct metabolic requirements. This can take several approaches.
MERS-CoV and SARS-CoV PL protease inhibitors
Of particular interest are antiviral therapies that attack papain-like protease, which is an important target because it is a multifunctional protein involved in proteolytic deubiquitination and viral evasion of the innate immune response. One such potential therapeutic that takes advantage of this target is disulfiram, an FDA-approved drug for use in alcohol-aversion therapy. Disulfiram has been reported as an allosteric inhibitor of MERS-CoV papain-like protease. Numerous other drug categories are being examined, with promising results in targeting the papain-like protease enzymes of both SARS and MERS.
Replicase inhibitors
Helicase (nsP13) protein is a crucial component required for virus replication in host cells and could serve as a feasible target for anti-MERS and anti-SARS chemical therapies, the review authors wrote, citing as an example, the recent development of a small 1,2,4-triazole derivative that inhibited the viral NTPase/helicase of SARS- and MERS-CoVs and demonstrated high antiviral activity and low cytotoxicity.
Membrane-bound viral RNA synthesis inhibitors
Antiviral agents that target membrane-bound coronaviral RNA synthesis represent a novel and attractive approach, according to Dr. Pillaiyar and colleagues. And recently, an inhibitor was developed that targets membrane-bound coronaviral RNA synthesis and “showed potent antiviral activity of MERS-CoV infection with remarkable efficacy.”
Host-based, anti-CoV treatment options
An alternate therapeutic tactic is to bolster host defenses or to modify host susceptibilities to prevent virus infection or replication. The innate interferon response of the host is crucial for the control of viral replication after infection, and the addition of exogenous recombinant interferon or use of drugs to stimulate the normal host interferon response are both potential therapeutic avenues. For example, nitazoxanide is a potent type I interferon inducer that has been used in humans for parasitic infections, and a synthetic nitrothiazolyl-salicylamide derivative was found to exhibit broad-spectrum antiviral activities against RNA and DNA viruses, including some coronaviruses.
Numerous other host pathways are being investigated as potential areas to enhance defense against infection and replication, for example, using inhibitors to block nucleic acid synthesis has been shown to provide broad-spectrum activity against SARS-CoV and MERS-CoV.
One particular example is remdesivir, a novel nucleotide analog antiviral drug, that was developed as a therapy for Ebola virus disease and Marburg virus infections. It was later shown to provide “reasonable antiviral activity against more distantly related viruses, such as respiratory syncytial virus, Junin virus, Lassa fever virus, and MERS-CoV,” the authors wrote.
Also of interest regarding remdesivir’s potential broad-spectrum use is that it has shown potent in vitro “antiviral activity against Malaysian and Bangladesh genotypes of Nipah virus (an RNA virus, although not a coronavirus, that infects both humans and animals) and reduced replication of Malaysian Nipah virus in primary human lung microvascular endothelial cells by more than four orders of magnitude,” Dr. Pillaiyar and colleagues added. Of particular note, all remdesivir-treated, Nipah virus–infected animals “survived the lethal challenge, indicating that remdesivir represents a promising antiviral treatment.”
In a press briefing earlier this month, Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, reported that a randomized, controlled, phase 3 trial of the antiviral drug remdesivir is currently underway in China to establish whether the drug would be an effective and safe treatment for adults patients with mild or moderate 2019 Novel Coronavirus (2019-nCoV) disease.
“Our increasing understanding of novel emerging coronaviruses will be accompanied by increasing opportunities for the reasonable design of therapeutics. Importantly, understanding this basic information about CoV protease targets will not only aid the public health against SARS-CoV and MERS-CoV but also help in advance to target new coronaviruses that might emerge in the future,” the authors concluded.
Dr. Pillaiyar and colleagues reported that they had no financial conflicts of interest.
SOURCE: Pillaiyar T et al. Drug Discov Today. 2020 Jan 30. doi: 10.1016/j.drudis.2020.01.015.
FROM DRUG DISCOVERY TODAY
FDA approves rituximab to treat children with rare vasculitis
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
The Food and Drug Administration approved rituximab (Rituxan) by injection to treat granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) in children 2 years of age and older in combination with glucocorticoid treatment, according to an FDA news release.
These rare forms of vasculitis damage small blood vessels through inflammation and can lead to serious organ failure, including lungs and kidneys.
The Genentech drug received priority review and an orphan drug designation based on the results of a pediatric clinical trial of 25 patients aged 6-17 years with active GPA or MPA who were treated with rituximab in an international multicenter, open-label, uncontrolled study. Patients in the trial were also given methylprednisolone prior to starting treatment.
The trial consisted of a 6-month remission induction phase where patients were treated only with rituximab and glucocorticoids. In addition, patients who had not achieved remission could receive additional treatment, including other therapies, at the discretion of the investigator, according to the FDA. By 6 months, 14 of the patients were in remission, and after 18 months, all 25 patients were in remission.
Rituximab contains a boxed warning regarding increased risks of fatal infusion reactions, potentially fatal severe skin and mouth reactions, hepatitis B virus reactivation that may cause serious or lethal liver problems, and progressive multifocal leukoencephalopathy, a rare, potentially lethal brain infection.
The trial was conducted and sponsored by F. Hoffmann-La Roche, which owns Genentech.
VIDEO: Preventing healthcare acquired infections after CT surgery
PHOENIX – More and more attention is being paid to preventing healthcare acquired infections (HAIs) in the hospital setting, and the role of HAIs in cardiothoracic surgery is a particlularly important area of focus.
“The good news is that cardiothoracic surgeons are really good at preventing infections. There’s been a lot of pressure over the past many years to report infections after cardiothoracic surgery, and so they’ve gotten a lot of things right,” Dr. Emily Landon said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
“However, patients that undergo cardiothoracic surgery are still at risk of the infections that plague everyone in hospitals ... all of these are a problem based on whatever the hospital’s current situation is.”
Dr. Landon, who is the medical director of antimicrobial stewardship and infection control at University of Chicago Medicine, Chicago, discussed how cardiothroacic surgeons can maintain their own good outcomes and how they can have a postive impact outside the OR on protecting their patients after surgery.
Dr. Landon reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – More and more attention is being paid to preventing healthcare acquired infections (HAIs) in the hospital setting, and the role of HAIs in cardiothoracic surgery is a particlularly important area of focus.
“The good news is that cardiothoracic surgeons are really good at preventing infections. There’s been a lot of pressure over the past many years to report infections after cardiothoracic surgery, and so they’ve gotten a lot of things right,” Dr. Emily Landon said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
“However, patients that undergo cardiothoracic surgery are still at risk of the infections that plague everyone in hospitals ... all of these are a problem based on whatever the hospital’s current situation is.”
Dr. Landon, who is the medical director of antimicrobial stewardship and infection control at University of Chicago Medicine, Chicago, discussed how cardiothroacic surgeons can maintain their own good outcomes and how they can have a postive impact outside the OR on protecting their patients after surgery.
Dr. Landon reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – More and more attention is being paid to preventing healthcare acquired infections (HAIs) in the hospital setting, and the role of HAIs in cardiothoracic surgery is a particlularly important area of focus.
“The good news is that cardiothoracic surgeons are really good at preventing infections. There’s been a lot of pressure over the past many years to report infections after cardiothoracic surgery, and so they’ve gotten a lot of things right,” Dr. Emily Landon said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
“However, patients that undergo cardiothoracic surgery are still at risk of the infections that plague everyone in hospitals ... all of these are a problem based on whatever the hospital’s current situation is.”
Dr. Landon, who is the medical director of antimicrobial stewardship and infection control at University of Chicago Medicine, Chicago, discussed how cardiothroacic surgeons can maintain their own good outcomes and how they can have a postive impact outside the OR on protecting their patients after surgery.
Dr. Landon reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING
Confronting aspirin unresponsiveness in congenital heart surgery
Thrombosis occurs in up to 15% of pediatric patients following cardiac surgery, and is associated with increased mortality. Although aspirin is commonly administered to pediatric patients after high-risk congenital cardiac surgery to reduce thrombosis risk, aspirin responsiveness is rarely assessed, according to Dr. Sirisha Emani and colleagues .
“In our observational study, aspirin unresponsiveness occurred in approximately 11% of patients undergoing specific high-risk cardiac procedures, and postoperative thrombosis was associated with aspirin unresponsiveness in this patient population,” said Dr. Emani.
In order to determine whether inadequate response to aspirin was associated with increased risk of thrombosis following high-risk procedures, the researchers performed a prospective analysis of 62 patients undergoing congenital cardiac surgical procedures involving placement of prosthetic material into the circulation or coronary artery manipulation who received aspirin.
Response to aspirin was determined using the Verify Now system at least 48 hours following administration. Patients were prospectively monitored for development of thrombosis events by imaging (echocardiogram, cardiac catheterization, MRI) and review of clinical events (shunt thrombosis, stroke, or limb ischemia) until the time of hospital discharge.
Aspirin responsiveness was tested a median of 2 days after initiation of therapy. The rate of aspirin unresponsiveness (Aspirin Responsive Unit, ARU greater than 550) was 7/62 (11.3%) in all patients and was highest in patients less than 5 kg who received 20.25 mg aspirin. Thrombosis events were demonstrated in 7 patients (11.3%). Thrombosis was observed in 6 (86%) of 7 patients who were unresponsive to aspirin as opposed to 1 (2%) of 54 patients who were responsive to aspirin, a significant difference. In two neonates who were unresponsive at 20.25 and 40.5 mg of aspirin, increase in dosage to 40.5 and 81 mg, respectively, resulted in an aspirin response, suggesting insufficiency rather than true unresponsiveness.
“Monitoring of aspirin therapy and consideration of dose adjustment or alternative agents for unresponsive patients may be justified and warrants further investigation in a prospective trial,” concluded Dr. Emani.
 |
Dr. Robert Jaquiss |
Aspirin is sometimes used in pediatric cardiac surgical patients with either therapeutic or prophylactic intent, and the anticipated anti-platelet activity is simply assumed to follow. This study demonstrates that in children, this assumption may be flawed in as many as 11% of patients. Furthermore, in those patients in whom the assumption of efficacy was wrong, thrombosis was alarmingly common. This information should be of concern to physicians and surgeons who prescribe aspirin for children with cardiovascular abnormalities, and certainly merits further study.
Dr. Robert Jaquiss is chief of pediatric heart surgery, Duke University Medical Center, Durham, N.C.
 |
Dr. Robert Jaquiss |
Aspirin is sometimes used in pediatric cardiac surgical patients with either therapeutic or prophylactic intent, and the anticipated anti-platelet activity is simply assumed to follow. This study demonstrates that in children, this assumption may be flawed in as many as 11% of patients. Furthermore, in those patients in whom the assumption of efficacy was wrong, thrombosis was alarmingly common. This information should be of concern to physicians and surgeons who prescribe aspirin for children with cardiovascular abnormalities, and certainly merits further study.
Dr. Robert Jaquiss is chief of pediatric heart surgery, Duke University Medical Center, Durham, N.C.
 |
Dr. Robert Jaquiss |
Aspirin is sometimes used in pediatric cardiac surgical patients with either therapeutic or prophylactic intent, and the anticipated anti-platelet activity is simply assumed to follow. This study demonstrates that in children, this assumption may be flawed in as many as 11% of patients. Furthermore, in those patients in whom the assumption of efficacy was wrong, thrombosis was alarmingly common. This information should be of concern to physicians and surgeons who prescribe aspirin for children with cardiovascular abnormalities, and certainly merits further study.
Dr. Robert Jaquiss is chief of pediatric heart surgery, Duke University Medical Center, Durham, N.C.
Thrombosis occurs in up to 15% of pediatric patients following cardiac surgery, and is associated with increased mortality. Although aspirin is commonly administered to pediatric patients after high-risk congenital cardiac surgery to reduce thrombosis risk, aspirin responsiveness is rarely assessed, according to Dr. Sirisha Emani and colleagues .
“In our observational study, aspirin unresponsiveness occurred in approximately 11% of patients undergoing specific high-risk cardiac procedures, and postoperative thrombosis was associated with aspirin unresponsiveness in this patient population,” said Dr. Emani.
In order to determine whether inadequate response to aspirin was associated with increased risk of thrombosis following high-risk procedures, the researchers performed a prospective analysis of 62 patients undergoing congenital cardiac surgical procedures involving placement of prosthetic material into the circulation or coronary artery manipulation who received aspirin.
Response to aspirin was determined using the Verify Now system at least 48 hours following administration. Patients were prospectively monitored for development of thrombosis events by imaging (echocardiogram, cardiac catheterization, MRI) and review of clinical events (shunt thrombosis, stroke, or limb ischemia) until the time of hospital discharge.
Aspirin responsiveness was tested a median of 2 days after initiation of therapy. The rate of aspirin unresponsiveness (Aspirin Responsive Unit, ARU greater than 550) was 7/62 (11.3%) in all patients and was highest in patients less than 5 kg who received 20.25 mg aspirin. Thrombosis events were demonstrated in 7 patients (11.3%). Thrombosis was observed in 6 (86%) of 7 patients who were unresponsive to aspirin as opposed to 1 (2%) of 54 patients who were responsive to aspirin, a significant difference. In two neonates who were unresponsive at 20.25 and 40.5 mg of aspirin, increase in dosage to 40.5 and 81 mg, respectively, resulted in an aspirin response, suggesting insufficiency rather than true unresponsiveness.
“Monitoring of aspirin therapy and consideration of dose adjustment or alternative agents for unresponsive patients may be justified and warrants further investigation in a prospective trial,” concluded Dr. Emani.
Thrombosis occurs in up to 15% of pediatric patients following cardiac surgery, and is associated with increased mortality. Although aspirin is commonly administered to pediatric patients after high-risk congenital cardiac surgery to reduce thrombosis risk, aspirin responsiveness is rarely assessed, according to Dr. Sirisha Emani and colleagues .
“In our observational study, aspirin unresponsiveness occurred in approximately 11% of patients undergoing specific high-risk cardiac procedures, and postoperative thrombosis was associated with aspirin unresponsiveness in this patient population,” said Dr. Emani.
In order to determine whether inadequate response to aspirin was associated with increased risk of thrombosis following high-risk procedures, the researchers performed a prospective analysis of 62 patients undergoing congenital cardiac surgical procedures involving placement of prosthetic material into the circulation or coronary artery manipulation who received aspirin.
Response to aspirin was determined using the Verify Now system at least 48 hours following administration. Patients were prospectively monitored for development of thrombosis events by imaging (echocardiogram, cardiac catheterization, MRI) and review of clinical events (shunt thrombosis, stroke, or limb ischemia) until the time of hospital discharge.
Aspirin responsiveness was tested a median of 2 days after initiation of therapy. The rate of aspirin unresponsiveness (Aspirin Responsive Unit, ARU greater than 550) was 7/62 (11.3%) in all patients and was highest in patients less than 5 kg who received 20.25 mg aspirin. Thrombosis events were demonstrated in 7 patients (11.3%). Thrombosis was observed in 6 (86%) of 7 patients who were unresponsive to aspirin as opposed to 1 (2%) of 54 patients who were responsive to aspirin, a significant difference. In two neonates who were unresponsive at 20.25 and 40.5 mg of aspirin, increase in dosage to 40.5 and 81 mg, respectively, resulted in an aspirin response, suggesting insufficiency rather than true unresponsiveness.
“Monitoring of aspirin therapy and consideration of dose adjustment or alternative agents for unresponsive patients may be justified and warrants further investigation in a prospective trial,” concluded Dr. Emani.
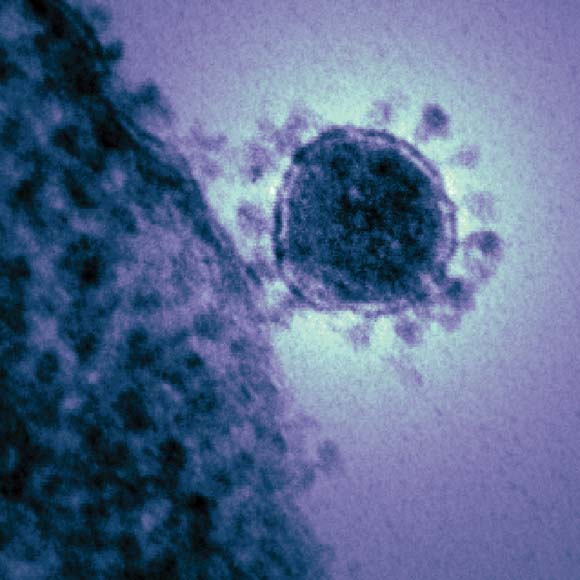
WHO, CDC continue monitoring deadly new coronavirus
The World Health Organization and the Centers for Disease Control and Prevention are closely following the spread of a new coronavirus that emerged in the Middle East last fall and currently demonstrates a mortality rate of more than 50%.
Widely referred to as the "novel coronavirus" (NCoV), the new agent is very similar to the severe acute respiratory syndrome (SARS)-associated coronavirus. By mid May 2013, the new virus had been identified in 38 patients worldwide – in Saudi Arabia, Jordan, France, the United Kingdom, the United Arab Emirates, and Qatar – causing 20 deaths.
"Health care providers are advised to be vigilant among recent travelers returning from areas affected by the virus who develop severe SARI [severe acute respiratory infections]. Specimens from patients’ lower respiratory tracts should be obtained for diagnosis where possible. Clinicians are reminded that NCoV infection should be considered even with atypical signs and symptoms in patients who are significantly immune compromised," WHO said in a written statement May 14.
In a May 13 statement the CDC noted: "So far, there are no reports of anyone in the U.S. getting infected and sick with the novel coronavirus." The CDC added that investigations are underway to determine the source of the novel coronavirus and how it spreads.
The World Health Organization and the Centers for Disease Control and Prevention are closely following the spread of a new coronavirus that emerged in the Middle East last fall and currently demonstrates a mortality rate of more than 50%.
Widely referred to as the "novel coronavirus" (NCoV), the new agent is very similar to the severe acute respiratory syndrome (SARS)-associated coronavirus. By mid May 2013, the new virus had been identified in 38 patients worldwide – in Saudi Arabia, Jordan, France, the United Kingdom, the United Arab Emirates, and Qatar – causing 20 deaths.
"Health care providers are advised to be vigilant among recent travelers returning from areas affected by the virus who develop severe SARI [severe acute respiratory infections]. Specimens from patients’ lower respiratory tracts should be obtained for diagnosis where possible. Clinicians are reminded that NCoV infection should be considered even with atypical signs and symptoms in patients who are significantly immune compromised," WHO said in a written statement May 14.
In a May 13 statement the CDC noted: "So far, there are no reports of anyone in the U.S. getting infected and sick with the novel coronavirus." The CDC added that investigations are underway to determine the source of the novel coronavirus and how it spreads.
The World Health Organization and the Centers for Disease Control and Prevention are closely following the spread of a new coronavirus that emerged in the Middle East last fall and currently demonstrates a mortality rate of more than 50%.
Widely referred to as the "novel coronavirus" (NCoV), the new agent is very similar to the severe acute respiratory syndrome (SARS)-associated coronavirus. By mid May 2013, the new virus had been identified in 38 patients worldwide – in Saudi Arabia, Jordan, France, the United Kingdom, the United Arab Emirates, and Qatar – causing 20 deaths.
"Health care providers are advised to be vigilant among recent travelers returning from areas affected by the virus who develop severe SARI [severe acute respiratory infections]. Specimens from patients’ lower respiratory tracts should be obtained for diagnosis where possible. Clinicians are reminded that NCoV infection should be considered even with atypical signs and symptoms in patients who are significantly immune compromised," WHO said in a written statement May 14.
In a May 13 statement the CDC noted: "So far, there are no reports of anyone in the U.S. getting infected and sick with the novel coronavirus." The CDC added that investigations are underway to determine the source of the novel coronavirus and how it spreads.
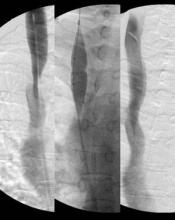
Translumbar Catheters Deemed Safe for Some Patients
About 23%-41% of the dialysis population uses central venous catheters for access. For some catheter-dependent patients, percutaneous translumbar access has been considered a good alternative when traditional access sites have been exhausted.
To address safety concerns about translumbar catheters, Dr. Gregory J. Nadolski and his colleagues retrospectively studied all translumbar tunneled hemodialysis catheters (TDCs) placed between January 2002 and July 2011 at the Hospital of the University of Pennsylvania in Philadelphia. A total of 33 patients were included – 18 with a normal body mass index and 15 with a BMI greater than 25 kg/m2. All patients had central venous occlusion. The study included 92 catheters (33 initial placements) with a total of 7,825 catheter-days and a mean number of 2.8 exchanges.
All catheters were placed successfully, and over-the-wire exchange was never precluded by the presence of retroperitoneal fibrosis around the catheters. The major indications for exchanges or removal were catheter-related infection (n = 39), followed by malposition (n = 14), catheter malfunction secondary to occlusion (n = 11), and mature permanent vascular access (n = 7). Conversion to peritoneal dialysis (n = 3), functioning transplant (n = 2), malfunction and infection (n = 1), and unknown (n = 1) accounted for the rest of the patients, according to Dr. Nadolski, who presented the results at the Veith symposium on vascular medicine sponsored by the Cleveland Clinic.
At the time of the review, three translumbar dialysis catheters remained in use. Nine patients had died of non–catheter-related causes, and two were lost to follow-up. The catheter-associated infection rate was 0.51 per 100 catheter-days (n = 40, 19 normal BMI, 19 abnormal BMI, 2 LTFU [long-term follow-up]), Dr. Nadolski said.
The frequency of catheter infection was not significantly different between patients with normal and abnormal BMIs. The frequency of catheter exchange for malposition also was not significantly different. The median time catheter in situ (quartile range) between all patients – those with a normal BMI and those with an abnormal BMI – was not significantly different. The same held true for the median total access site interval (quartile range). There was also no significant difference between the three groups in the primary or secondary device service interval.
"The median time catheter in situ, median total access site interval, median primary device service interval, and median secondary device service interval were not statistically different between normal BMI and overweight patients," Dr. Nadolski noted. "In all, five complications occurred during 92 procedures, for a total complication rate of 5.5%. Two complications occurred in patients with normal BMIs and three in patients with abnormal BMIs, supporting the safety of translumbar TDCs as a whole as well as in overweight patients. Overall, we found the frequency of catheter exchange for infection and malposition to be similar for both normal and abnormal BMI patients. These findings support our hypothesis that translumbar access is no less effective in overweight and obese patients," he said.
"Obtaining durable functioning access for patients with limited central venous access is difficult. We prefer translumbar access over transhepatic access because of their propensity for malposition and migration related to respiratory motion. We found translumbar TDCs to be complicated by catheter migration/malposition in 19% of cases (n = 15). Compared to transfemoral catheters, we prefer to use translumbar access to leave the femoral and iliac veins available for surgical access creation or renal transplant, respectively," he added.
About 23%-41% of the dialysis population uses central venous catheters for access. For some catheter-dependent patients, percutaneous translumbar access has been considered a good alternative when traditional access sites have been exhausted.
To address safety concerns about translumbar catheters, Dr. Gregory J. Nadolski and his colleagues retrospectively studied all translumbar tunneled hemodialysis catheters (TDCs) placed between January 2002 and July 2011 at the Hospital of the University of Pennsylvania in Philadelphia. A total of 33 patients were included – 18 with a normal body mass index and 15 with a BMI greater than 25 kg/m2. All patients had central venous occlusion. The study included 92 catheters (33 initial placements) with a total of 7,825 catheter-days and a mean number of 2.8 exchanges.
All catheters were placed successfully, and over-the-wire exchange was never precluded by the presence of retroperitoneal fibrosis around the catheters. The major indications for exchanges or removal were catheter-related infection (n = 39), followed by malposition (n = 14), catheter malfunction secondary to occlusion (n = 11), and mature permanent vascular access (n = 7). Conversion to peritoneal dialysis (n = 3), functioning transplant (n = 2), malfunction and infection (n = 1), and unknown (n = 1) accounted for the rest of the patients, according to Dr. Nadolski, who presented the results at the Veith symposium on vascular medicine sponsored by the Cleveland Clinic.
At the time of the review, three translumbar dialysis catheters remained in use. Nine patients had died of non–catheter-related causes, and two were lost to follow-up. The catheter-associated infection rate was 0.51 per 100 catheter-days (n = 40, 19 normal BMI, 19 abnormal BMI, 2 LTFU [long-term follow-up]), Dr. Nadolski said.
The frequency of catheter infection was not significantly different between patients with normal and abnormal BMIs. The frequency of catheter exchange for malposition also was not significantly different. The median time catheter in situ (quartile range) between all patients – those with a normal BMI and those with an abnormal BMI – was not significantly different. The same held true for the median total access site interval (quartile range). There was also no significant difference between the three groups in the primary or secondary device service interval.
"The median time catheter in situ, median total access site interval, median primary device service interval, and median secondary device service interval were not statistically different between normal BMI and overweight patients," Dr. Nadolski noted. "In all, five complications occurred during 92 procedures, for a total complication rate of 5.5%. Two complications occurred in patients with normal BMIs and three in patients with abnormal BMIs, supporting the safety of translumbar TDCs as a whole as well as in overweight patients. Overall, we found the frequency of catheter exchange for infection and malposition to be similar for both normal and abnormal BMI patients. These findings support our hypothesis that translumbar access is no less effective in overweight and obese patients," he said.
"Obtaining durable functioning access for patients with limited central venous access is difficult. We prefer translumbar access over transhepatic access because of their propensity for malposition and migration related to respiratory motion. We found translumbar TDCs to be complicated by catheter migration/malposition in 19% of cases (n = 15). Compared to transfemoral catheters, we prefer to use translumbar access to leave the femoral and iliac veins available for surgical access creation or renal transplant, respectively," he added.
About 23%-41% of the dialysis population uses central venous catheters for access. For some catheter-dependent patients, percutaneous translumbar access has been considered a good alternative when traditional access sites have been exhausted.
To address safety concerns about translumbar catheters, Dr. Gregory J. Nadolski and his colleagues retrospectively studied all translumbar tunneled hemodialysis catheters (TDCs) placed between January 2002 and July 2011 at the Hospital of the University of Pennsylvania in Philadelphia. A total of 33 patients were included – 18 with a normal body mass index and 15 with a BMI greater than 25 kg/m2. All patients had central venous occlusion. The study included 92 catheters (33 initial placements) with a total of 7,825 catheter-days and a mean number of 2.8 exchanges.
All catheters were placed successfully, and over-the-wire exchange was never precluded by the presence of retroperitoneal fibrosis around the catheters. The major indications for exchanges or removal were catheter-related infection (n = 39), followed by malposition (n = 14), catheter malfunction secondary to occlusion (n = 11), and mature permanent vascular access (n = 7). Conversion to peritoneal dialysis (n = 3), functioning transplant (n = 2), malfunction and infection (n = 1), and unknown (n = 1) accounted for the rest of the patients, according to Dr. Nadolski, who presented the results at the Veith symposium on vascular medicine sponsored by the Cleveland Clinic.
At the time of the review, three translumbar dialysis catheters remained in use. Nine patients had died of non–catheter-related causes, and two were lost to follow-up. The catheter-associated infection rate was 0.51 per 100 catheter-days (n = 40, 19 normal BMI, 19 abnormal BMI, 2 LTFU [long-term follow-up]), Dr. Nadolski said.
The frequency of catheter infection was not significantly different between patients with normal and abnormal BMIs. The frequency of catheter exchange for malposition also was not significantly different. The median time catheter in situ (quartile range) between all patients – those with a normal BMI and those with an abnormal BMI – was not significantly different. The same held true for the median total access site interval (quartile range). There was also no significant difference between the three groups in the primary or secondary device service interval.
"The median time catheter in situ, median total access site interval, median primary device service interval, and median secondary device service interval were not statistically different between normal BMI and overweight patients," Dr. Nadolski noted. "In all, five complications occurred during 92 procedures, for a total complication rate of 5.5%. Two complications occurred in patients with normal BMIs and three in patients with abnormal BMIs, supporting the safety of translumbar TDCs as a whole as well as in overweight patients. Overall, we found the frequency of catheter exchange for infection and malposition to be similar for both normal and abnormal BMI patients. These findings support our hypothesis that translumbar access is no less effective in overweight and obese patients," he said.
"Obtaining durable functioning access for patients with limited central venous access is difficult. We prefer translumbar access over transhepatic access because of their propensity for malposition and migration related to respiratory motion. We found translumbar TDCs to be complicated by catheter migration/malposition in 19% of cases (n = 15). Compared to transfemoral catheters, we prefer to use translumbar access to leave the femoral and iliac veins available for surgical access creation or renal transplant, respectively," he added.
FROM THE VEITH SYMPOSIUM
Angioplasty Improved Venous Flow in MS
NATIONAL HARBOR, MD. -- Percutaneous balloon angioplasty improved flow dynamics in multiple sclerosis patients with chronic cerebrospinal venous insufficiency in a pilot study.
An association has been made recently between multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) that is characterized by stenosis and reflux of the principal extracranial venous drainage, including the internal jugular veins and the azygous veins. But there has been considerable debate about the validity of percutaneous balloon angioplasty in the treatment of this stenosis.
Dr. Manish Mehta of Albany (N.Y.) Medical College and his colleagues conducted the first angiographic study to quantitatively analyze the impact of percutaneous balloon angioplasty on flow dynamics across these lesions. Dr. Mehta shared their results at the Vascular Annual Meeting.
The researchers assessed 50 internal jugular veins (IJVs) from MS patients with CCSVI, as well as 12 IJVs from healthy volunteers, all of whom underwent detailed angiographic evaluation. The technical components of all venograms were standardized.
Quantitative analysis included the contrast time of flight from the mid-IJV to the superior vena cava, and the primary venous emptying time (PVET), quantified as greater than 50% of venous emptying from the IJV. The time of flight and PVET were recorded in patients with CCSVI prior to and subsequent to balloon angioplasty. The same data were recorded in the healthy controls. All data were collected prospectively, and statistical analysis was performed using two-tailed Student's test.
Of the 50 CCSVI-MS patients who had IJV stenosis greater than 70% and reflux and who underwent balloon angioplasty, technical success (defined as less than 20% residual IJV stenosis) was achieved in 44 (78%). CCSVI patients were observed to have a significant improvement in both the time of flight and PVET following balloon angioplasty that paralleled those of healthy subjects without MS.
"The results of this prospective pilot study suggest an association between MS and CCSVI, which results in abnormally elevated time of flight and PVET through the IJV," Dr. Mehta said. "Furthermore, balloon angioplasty of these lesions improves the hemodynamic parameters so that they are comparable to those of healthy non-MS patients."
Dr. Mehta stressed the need for randomized studies to further investigate this issue and said that this patient population is at high risk for a placebo effect with regard to their reported symptoms. He also stated that this treatment is being provided to many patients around the world and that the U.S. Food and Drug Administration has cautioned against its use outside of well-regulated trials due to lack of safety and efficacy data.
Dr. Mehta reported that he had nothing to disclose.
Manish Mehta
NATIONAL HARBOR, MD. -- Percutaneous balloon angioplasty improved flow dynamics in multiple sclerosis patients with chronic cerebrospinal venous insufficiency in a pilot study.
An association has been made recently between multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) that is characterized by stenosis and reflux of the principal extracranial venous drainage, including the internal jugular veins and the azygous veins. But there has been considerable debate about the validity of percutaneous balloon angioplasty in the treatment of this stenosis.
Dr. Manish Mehta of Albany (N.Y.) Medical College and his colleagues conducted the first angiographic study to quantitatively analyze the impact of percutaneous balloon angioplasty on flow dynamics across these lesions. Dr. Mehta shared their results at the Vascular Annual Meeting.
The researchers assessed 50 internal jugular veins (IJVs) from MS patients with CCSVI, as well as 12 IJVs from healthy volunteers, all of whom underwent detailed angiographic evaluation. The technical components of all venograms were standardized.
Quantitative analysis included the contrast time of flight from the mid-IJV to the superior vena cava, and the primary venous emptying time (PVET), quantified as greater than 50% of venous emptying from the IJV. The time of flight and PVET were recorded in patients with CCSVI prior to and subsequent to balloon angioplasty. The same data were recorded in the healthy controls. All data were collected prospectively, and statistical analysis was performed using two-tailed Student's test.
Of the 50 CCSVI-MS patients who had IJV stenosis greater than 70% and reflux and who underwent balloon angioplasty, technical success (defined as less than 20% residual IJV stenosis) was achieved in 44 (78%). CCSVI patients were observed to have a significant improvement in both the time of flight and PVET following balloon angioplasty that paralleled those of healthy subjects without MS.
"The results of this prospective pilot study suggest an association between MS and CCSVI, which results in abnormally elevated time of flight and PVET through the IJV," Dr. Mehta said. "Furthermore, balloon angioplasty of these lesions improves the hemodynamic parameters so that they are comparable to those of healthy non-MS patients."
Dr. Mehta stressed the need for randomized studies to further investigate this issue and said that this patient population is at high risk for a placebo effect with regard to their reported symptoms. He also stated that this treatment is being provided to many patients around the world and that the U.S. Food and Drug Administration has cautioned against its use outside of well-regulated trials due to lack of safety and efficacy data.
Dr. Mehta reported that he had nothing to disclose.
NATIONAL HARBOR, MD. -- Percutaneous balloon angioplasty improved flow dynamics in multiple sclerosis patients with chronic cerebrospinal venous insufficiency in a pilot study.
An association has been made recently between multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) that is characterized by stenosis and reflux of the principal extracranial venous drainage, including the internal jugular veins and the azygous veins. But there has been considerable debate about the validity of percutaneous balloon angioplasty in the treatment of this stenosis.
Dr. Manish Mehta of Albany (N.Y.) Medical College and his colleagues conducted the first angiographic study to quantitatively analyze the impact of percutaneous balloon angioplasty on flow dynamics across these lesions. Dr. Mehta shared their results at the Vascular Annual Meeting.
The researchers assessed 50 internal jugular veins (IJVs) from MS patients with CCSVI, as well as 12 IJVs from healthy volunteers, all of whom underwent detailed angiographic evaluation. The technical components of all venograms were standardized.
Quantitative analysis included the contrast time of flight from the mid-IJV to the superior vena cava, and the primary venous emptying time (PVET), quantified as greater than 50% of venous emptying from the IJV. The time of flight and PVET were recorded in patients with CCSVI prior to and subsequent to balloon angioplasty. The same data were recorded in the healthy controls. All data were collected prospectively, and statistical analysis was performed using two-tailed Student's test.
Of the 50 CCSVI-MS patients who had IJV stenosis greater than 70% and reflux and who underwent balloon angioplasty, technical success (defined as less than 20% residual IJV stenosis) was achieved in 44 (78%). CCSVI patients were observed to have a significant improvement in both the time of flight and PVET following balloon angioplasty that paralleled those of healthy subjects without MS.
"The results of this prospective pilot study suggest an association between MS and CCSVI, which results in abnormally elevated time of flight and PVET through the IJV," Dr. Mehta said. "Furthermore, balloon angioplasty of these lesions improves the hemodynamic parameters so that they are comparable to those of healthy non-MS patients."
Dr. Mehta stressed the need for randomized studies to further investigate this issue and said that this patient population is at high risk for a placebo effect with regard to their reported symptoms. He also stated that this treatment is being provided to many patients around the world and that the U.S. Food and Drug Administration has cautioned against its use outside of well-regulated trials due to lack of safety and efficacy data.
Dr. Mehta reported that he had nothing to disclose.
Manish Mehta
Manish Mehta
AT THE VASCULAR ANNUAL MEETING
Major Finding: Of the 50 CCSVI-MS patients with IJV stenosis greater than 70% and reflux who underwent balloon angioplasty, technical success was achieved in 78%. CCSVI patients were observed to have a significant improvement in venous flow characteristics following balloon angioplasty that paralleled those of healthy subjects.
Data Source: The researchers performed a comparative pilot study of 50 internal jugular veins (IJVs) from MS patients with chronic cerebrospinal venous insufficiency who underwent balloon angioplasty with 12 IJVs from healthy volunteers who underwent detailed angiographic analysis.
Disclosures: Dr. Mehta reported that he had nothing to disclose.
Predicted Survival Improves Patient Selection for CEA
NATIONAL HARBOR, MD. – The 5-year survival rates for patients undergoing carotid endarterectomy for asymptomatic stenosis differ significantly depending on whether they are classified as low, medium, or high risk, according to an analysis of prospectively collected data on more than 4,000 patients.
"Our study is the first of its kind to describe independent predictors of 5-year mortality in this population of patients," Dr. Jessica B. Wallaert said at the Vascular Annual Meeting.
Dr. Wallaert of Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and her colleagues studied factors associated with 5-year survival after carotid endarterectomy (CEA) in patients with asymptomatic internal carotid artery (ICA) stenosis. Whether such patients will benefit from the procedure – especially when they are asymptomatic – depends on their ability to achieve long-term survival. The researchers’ goal was to improve patient selection and create user-friendly tools for surgeons and patients alike to predict the probability of benefiting from this prophylactic surgery.
They analyzed prospectively collected data from 4,294 isolated CEAs that were performed for asymptomatic stenosis across 24 centers in the Vascular Study Group of New England (VSGNE) in 2003-2011. Mortality was determined from the Social Security Death Index. Cox proportional hazard models were used to identify risk factors for mortality within the 5 years after CEA.
Overall 5-year survival was 82%. In multivariate analysis, increasing strata of age, diabetes, smoking history, heart failure, chronic obstructive pulmonary disease, poor renal function (defined as an estimated glomerular filtration rate less than 60 mL/min, or dialysis dependence), and degree of contralateral ICA stenosis were all associated with poorer survival. Statin use, however, predicted improved survival.
Patients who were classified as low (27%), medium (68%), and high (5%) risk on the basis of their number of risk factors had significantly different 5-year survival rates: 94%, 80%, and 51%, respectively.
"More than four out of five asymptomatic patients selected for CEA in the Vascular Study Group of New England achieved 5-year survival, demonstrating appropriate patient selection in our region," she said. However, there are patients with high-risk profiles who are unlikely to survive long enough to realize a benefit of CEA for asymptomatic stenosis.
Dr. Wallaert reported that she had nothing to disclose.
NATIONAL HARBOR, MD. – The 5-year survival rates for patients undergoing carotid endarterectomy for asymptomatic stenosis differ significantly depending on whether they are classified as low, medium, or high risk, according to an analysis of prospectively collected data on more than 4,000 patients.
"Our study is the first of its kind to describe independent predictors of 5-year mortality in this population of patients," Dr. Jessica B. Wallaert said at the Vascular Annual Meeting.
Dr. Wallaert of Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and her colleagues studied factors associated with 5-year survival after carotid endarterectomy (CEA) in patients with asymptomatic internal carotid artery (ICA) stenosis. Whether such patients will benefit from the procedure – especially when they are asymptomatic – depends on their ability to achieve long-term survival. The researchers’ goal was to improve patient selection and create user-friendly tools for surgeons and patients alike to predict the probability of benefiting from this prophylactic surgery.
They analyzed prospectively collected data from 4,294 isolated CEAs that were performed for asymptomatic stenosis across 24 centers in the Vascular Study Group of New England (VSGNE) in 2003-2011. Mortality was determined from the Social Security Death Index. Cox proportional hazard models were used to identify risk factors for mortality within the 5 years after CEA.
Overall 5-year survival was 82%. In multivariate analysis, increasing strata of age, diabetes, smoking history, heart failure, chronic obstructive pulmonary disease, poor renal function (defined as an estimated glomerular filtration rate less than 60 mL/min, or dialysis dependence), and degree of contralateral ICA stenosis were all associated with poorer survival. Statin use, however, predicted improved survival.
Patients who were classified as low (27%), medium (68%), and high (5%) risk on the basis of their number of risk factors had significantly different 5-year survival rates: 94%, 80%, and 51%, respectively.
"More than four out of five asymptomatic patients selected for CEA in the Vascular Study Group of New England achieved 5-year survival, demonstrating appropriate patient selection in our region," she said. However, there are patients with high-risk profiles who are unlikely to survive long enough to realize a benefit of CEA for asymptomatic stenosis.
Dr. Wallaert reported that she had nothing to disclose.
NATIONAL HARBOR, MD. – The 5-year survival rates for patients undergoing carotid endarterectomy for asymptomatic stenosis differ significantly depending on whether they are classified as low, medium, or high risk, according to an analysis of prospectively collected data on more than 4,000 patients.
"Our study is the first of its kind to describe independent predictors of 5-year mortality in this population of patients," Dr. Jessica B. Wallaert said at the Vascular Annual Meeting.
Dr. Wallaert of Dartmouth-Hitchcock Medical Center, Lebanon, N.H., and her colleagues studied factors associated with 5-year survival after carotid endarterectomy (CEA) in patients with asymptomatic internal carotid artery (ICA) stenosis. Whether such patients will benefit from the procedure – especially when they are asymptomatic – depends on their ability to achieve long-term survival. The researchers’ goal was to improve patient selection and create user-friendly tools for surgeons and patients alike to predict the probability of benefiting from this prophylactic surgery.
They analyzed prospectively collected data from 4,294 isolated CEAs that were performed for asymptomatic stenosis across 24 centers in the Vascular Study Group of New England (VSGNE) in 2003-2011. Mortality was determined from the Social Security Death Index. Cox proportional hazard models were used to identify risk factors for mortality within the 5 years after CEA.
Overall 5-year survival was 82%. In multivariate analysis, increasing strata of age, diabetes, smoking history, heart failure, chronic obstructive pulmonary disease, poor renal function (defined as an estimated glomerular filtration rate less than 60 mL/min, or dialysis dependence), and degree of contralateral ICA stenosis were all associated with poorer survival. Statin use, however, predicted improved survival.
Patients who were classified as low (27%), medium (68%), and high (5%) risk on the basis of their number of risk factors had significantly different 5-year survival rates: 94%, 80%, and 51%, respectively.
"More than four out of five asymptomatic patients selected for CEA in the Vascular Study Group of New England achieved 5-year survival, demonstrating appropriate patient selection in our region," she said. However, there are patients with high-risk profiles who are unlikely to survive long enough to realize a benefit of CEA for asymptomatic stenosis.
Dr. Wallaert reported that she had nothing to disclose.
FROM THE VASCULAR ANNUAL MEETING
Major Finding: Patients classified as low (27%), medium (68%), and high risk (5%) on the basis of their number of risk factors had significantly different 5-year survival rates (94%, 80%, and 51%, respectively) after undergoing carotid endarterectomy.
Data Source: Researchers prospectively collected data from 4,294 isolated CEAs performed for asymptomatic stenosis across 24 centers in the VSGNE in 2003-2011.
Disclosures: Dr. Wallaert reported that she had nothing to disclose.
Balloon Angioplasty Improves Venous Flow in MS Patients
NATIONAL HARBOR, MD. – Percutaneous balloon angioplasty improved flow dynamics in multiple sclerosis patients with chronic cerebrospinal venous insufficiency in a pilot study.
An association has been made recently between multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) that is characterized by stenosis and reflux of the principal extracranial venous drainage, including the internal jugular veins and the azygous veins. But there has been considerable debate about the validity of percutaneous balloon angioplasty in the treatment of this stenosis.
Dr. Manish Mehta of Albany (N.Y.) Medical College and his colleagues conducted the first angiographic study to quantitatively analyze the impact of percutaneous balloon angioplasty on flow dynamics across these lesions. Dr. Mehta shared their results at the Vascular Annual Meeting.
The researchers assessed 50 internal jugular veins (IJVs) from MS patients with CCSVI, as well as 12 IJVs from healthy volunteers, all of whom underwent detailed angiographic evaluation. The technical components of all venograms were standardized.
Quantitative analysis included the contrast time of flight from the mid-IJV to the superior vena cava, and the primary venous emptying time (PVET), quantified as greater than 50% of venous emptying from the IJV. The time of flight and PVET were recorded in patients with CCSVI prior to and subsequent to balloon angioplasty. The same data were recorded in the healthy controls. All data were collected prospectively, and statistical analysis was performed using two-tailed Student’s test.
Of the 50 CCSVI-MS patients who had IJV stenosis greater than 70% and reflux and who underwent balloon angioplasty, technical success (defined as less than 20% residual IJV stenosis) was achieved in 44 (78%). CCSVI patients were observed to have a significant improvement in both the time of flight and PVET following balloon angioplasty that paralleled those of healthy subjects without MS.
"The results of this prospective pilot study suggest an association between MS and CCSVI, which results in abnormally elevated time of flight and PVET through the IJV," Dr. Mehta said. "Furthermore, balloon angioplasty of these lesions improves the hemodynamic parameters so that they are comparable to" those of healthy non-MS patients."
Dr. Mehta stressed the need for randomized studies to further investigate this issue and said that this patient population is at high risk for a placebo effect with regard to their reported symptoms. He also stated that this treatment is being provided to many patients around the world and that the U.S. Food and Drug Administration has cautioned against its use outside of well-regulated trials due to lack of safety and efficacy data.
Dr. Mehta reported that he had nothing to disclose.
NATIONAL HARBOR, MD. – Percutaneous balloon angioplasty improved flow dynamics in multiple sclerosis patients with chronic cerebrospinal venous insufficiency in a pilot study.
An association has been made recently between multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) that is characterized by stenosis and reflux of the principal extracranial venous drainage, including the internal jugular veins and the azygous veins. But there has been considerable debate about the validity of percutaneous balloon angioplasty in the treatment of this stenosis.
Dr. Manish Mehta of Albany (N.Y.) Medical College and his colleagues conducted the first angiographic study to quantitatively analyze the impact of percutaneous balloon angioplasty on flow dynamics across these lesions. Dr. Mehta shared their results at the Vascular Annual Meeting.
The researchers assessed 50 internal jugular veins (IJVs) from MS patients with CCSVI, as well as 12 IJVs from healthy volunteers, all of whom underwent detailed angiographic evaluation. The technical components of all venograms were standardized.
Quantitative analysis included the contrast time of flight from the mid-IJV to the superior vena cava, and the primary venous emptying time (PVET), quantified as greater than 50% of venous emptying from the IJV. The time of flight and PVET were recorded in patients with CCSVI prior to and subsequent to balloon angioplasty. The same data were recorded in the healthy controls. All data were collected prospectively, and statistical analysis was performed using two-tailed Student’s test.
Of the 50 CCSVI-MS patients who had IJV stenosis greater than 70% and reflux and who underwent balloon angioplasty, technical success (defined as less than 20% residual IJV stenosis) was achieved in 44 (78%). CCSVI patients were observed to have a significant improvement in both the time of flight and PVET following balloon angioplasty that paralleled those of healthy subjects without MS.
"The results of this prospective pilot study suggest an association between MS and CCSVI, which results in abnormally elevated time of flight and PVET through the IJV," Dr. Mehta said. "Furthermore, balloon angioplasty of these lesions improves the hemodynamic parameters so that they are comparable to" those of healthy non-MS patients."
Dr. Mehta stressed the need for randomized studies to further investigate this issue and said that this patient population is at high risk for a placebo effect with regard to their reported symptoms. He also stated that this treatment is being provided to many patients around the world and that the U.S. Food and Drug Administration has cautioned against its use outside of well-regulated trials due to lack of safety and efficacy data.
Dr. Mehta reported that he had nothing to disclose.
NATIONAL HARBOR, MD. – Percutaneous balloon angioplasty improved flow dynamics in multiple sclerosis patients with chronic cerebrospinal venous insufficiency in a pilot study.
An association has been made recently between multiple sclerosis and chronic cerebrospinal venous insufficiency (CCSVI) that is characterized by stenosis and reflux of the principal extracranial venous drainage, including the internal jugular veins and the azygous veins. But there has been considerable debate about the validity of percutaneous balloon angioplasty in the treatment of this stenosis.
Dr. Manish Mehta of Albany (N.Y.) Medical College and his colleagues conducted the first angiographic study to quantitatively analyze the impact of percutaneous balloon angioplasty on flow dynamics across these lesions. Dr. Mehta shared their results at the Vascular Annual Meeting.
The researchers assessed 50 internal jugular veins (IJVs) from MS patients with CCSVI, as well as 12 IJVs from healthy volunteers, all of whom underwent detailed angiographic evaluation. The technical components of all venograms were standardized.
Quantitative analysis included the contrast time of flight from the mid-IJV to the superior vena cava, and the primary venous emptying time (PVET), quantified as greater than 50% of venous emptying from the IJV. The time of flight and PVET were recorded in patients with CCSVI prior to and subsequent to balloon angioplasty. The same data were recorded in the healthy controls. All data were collected prospectively, and statistical analysis was performed using two-tailed Student’s test.
Of the 50 CCSVI-MS patients who had IJV stenosis greater than 70% and reflux and who underwent balloon angioplasty, technical success (defined as less than 20% residual IJV stenosis) was achieved in 44 (78%). CCSVI patients were observed to have a significant improvement in both the time of flight and PVET following balloon angioplasty that paralleled those of healthy subjects without MS.
"The results of this prospective pilot study suggest an association between MS and CCSVI, which results in abnormally elevated time of flight and PVET through the IJV," Dr. Mehta said. "Furthermore, balloon angioplasty of these lesions improves the hemodynamic parameters so that they are comparable to" those of healthy non-MS patients."
Dr. Mehta stressed the need for randomized studies to further investigate this issue and said that this patient population is at high risk for a placebo effect with regard to their reported symptoms. He also stated that this treatment is being provided to many patients around the world and that the U.S. Food and Drug Administration has cautioned against its use outside of well-regulated trials due to lack of safety and efficacy data.
Dr. Mehta reported that he had nothing to disclose.
FROM THE VASCULAR ANNUAL MEETING
Major Finding: Of the 50 CCSVI-MS patients with IJV stenosis greater than 70% and reflux who underwent balloon angioplasty, technical success was achieved in 78%. CCSVI patients were observed to have a significant improvement in venous flow characteristics following balloon angioplasty that paralleled those of healthy subjects.
Data Source: The researchers performed a comparative pilot study of 50 internal jugular veins (IJVs) from MS patients with chronic cerebrospinal venous insufficiency who underwent balloon angioplasty with 12 IJVs from healthy volunteers who underwent detailed angiographic analysis.
Disclosures: Dr. Mehta reported that he had nothing to disclose.
Hemodialysis Access and Age-Related Outcomes
NATIONAL HARBOR, MD. – The National Kidney Foundation recommends the preferential creation of radiocephalic fistulas over that of brachiocephalic fistulas for hemodialysis access, but in patients older than 68 years, radiocephalic fistulas are associated with more postoperative complications, according to a retrospective analysis of 287 patients.
Dr. Steven Abramowitz and his colleagues at the Mount Sinai Medical Center, New York, studied patients older than 68 years of age who had preoperative vein mapping and regular follow-up after creation of arteriovenous fistulas. Within this group of 287 patients, 164 underwent radiocephalic fistula (RCF) creation and 123 underwent brachiocephalic fistula (BCF) creation. Dr. Abramowitz presented their findings at the Vascular Annual Meeting.
The researchers analyzed medical records to determine the number of central venous catheter days, the number of fistula-related procedures recorded, and the number of access-related hospitalizations for each patient. Bivariate analysis using linear modeling and one-way analysis of variance was used to assess cohort differences.
Among those patients who had a BCF, the average number of central venous catheter days was 53.3 days per patient, the average number of fistula-related procedures recorded was 0.6 per patient, and the number of hemodialysis-related hospitalizations was 0.3 per patient.
In comparison, among those patients who had an RCF, the average number of central venous catheter days was 83.4 days per patient, the average number of fistula-related procedures recorded was 1.8 per patient, and the average number of hemodialysis-related hospitalizations was 0.8 per patient.
These results indicated a significant difference in postoperative course between those patients who underwent BCF vs. RCF creation.
Patients older than 68 years of age who undergo RCF creation "may have a greater likelihood of increased central venous catheter days, a greater number of hospitalizations related to hemodialysis access, and a greater number of postoperative procedures than those who undergo BCF creation," said Dr. Abramowitz.
He reported that he had no disclosures.
NATIONAL HARBOR, MD. – The National Kidney Foundation recommends the preferential creation of radiocephalic fistulas over that of brachiocephalic fistulas for hemodialysis access, but in patients older than 68 years, radiocephalic fistulas are associated with more postoperative complications, according to a retrospective analysis of 287 patients.
Dr. Steven Abramowitz and his colleagues at the Mount Sinai Medical Center, New York, studied patients older than 68 years of age who had preoperative vein mapping and regular follow-up after creation of arteriovenous fistulas. Within this group of 287 patients, 164 underwent radiocephalic fistula (RCF) creation and 123 underwent brachiocephalic fistula (BCF) creation. Dr. Abramowitz presented their findings at the Vascular Annual Meeting.
The researchers analyzed medical records to determine the number of central venous catheter days, the number of fistula-related procedures recorded, and the number of access-related hospitalizations for each patient. Bivariate analysis using linear modeling and one-way analysis of variance was used to assess cohort differences.
Among those patients who had a BCF, the average number of central venous catheter days was 53.3 days per patient, the average number of fistula-related procedures recorded was 0.6 per patient, and the number of hemodialysis-related hospitalizations was 0.3 per patient.
In comparison, among those patients who had an RCF, the average number of central venous catheter days was 83.4 days per patient, the average number of fistula-related procedures recorded was 1.8 per patient, and the average number of hemodialysis-related hospitalizations was 0.8 per patient.
These results indicated a significant difference in postoperative course between those patients who underwent BCF vs. RCF creation.
Patients older than 68 years of age who undergo RCF creation "may have a greater likelihood of increased central venous catheter days, a greater number of hospitalizations related to hemodialysis access, and a greater number of postoperative procedures than those who undergo BCF creation," said Dr. Abramowitz.
He reported that he had no disclosures.
NATIONAL HARBOR, MD. – The National Kidney Foundation recommends the preferential creation of radiocephalic fistulas over that of brachiocephalic fistulas for hemodialysis access, but in patients older than 68 years, radiocephalic fistulas are associated with more postoperative complications, according to a retrospective analysis of 287 patients.
Dr. Steven Abramowitz and his colleagues at the Mount Sinai Medical Center, New York, studied patients older than 68 years of age who had preoperative vein mapping and regular follow-up after creation of arteriovenous fistulas. Within this group of 287 patients, 164 underwent radiocephalic fistula (RCF) creation and 123 underwent brachiocephalic fistula (BCF) creation. Dr. Abramowitz presented their findings at the Vascular Annual Meeting.
The researchers analyzed medical records to determine the number of central venous catheter days, the number of fistula-related procedures recorded, and the number of access-related hospitalizations for each patient. Bivariate analysis using linear modeling and one-way analysis of variance was used to assess cohort differences.
Among those patients who had a BCF, the average number of central venous catheter days was 53.3 days per patient, the average number of fistula-related procedures recorded was 0.6 per patient, and the number of hemodialysis-related hospitalizations was 0.3 per patient.
In comparison, among those patients who had an RCF, the average number of central venous catheter days was 83.4 days per patient, the average number of fistula-related procedures recorded was 1.8 per patient, and the average number of hemodialysis-related hospitalizations was 0.8 per patient.
These results indicated a significant difference in postoperative course between those patients who underwent BCF vs. RCF creation.
Patients older than 68 years of age who undergo RCF creation "may have a greater likelihood of increased central venous catheter days, a greater number of hospitalizations related to hemodialysis access, and a greater number of postoperative procedures than those who undergo BCF creation," said Dr. Abramowitz.
He reported that he had no disclosures.
FROM THE VASCULAR ANNUAL MEETING
Major Finding: Patients older than 68 years of age with radiocephalic fistulas had significantly increased central venous catheter days, more hospitalizations related to hemodialysis access, and more postoperative procedures than did those who underwent brachiocephalic fistula creation.
Data Source: Researchers retrospectively analyzed 287 patients older than 68 years of age, 164 of whom underwent radiocephalic fistula creation and 123 of whom underwent brachiocephalic fistula creation.
Disclosures: Dr. Abramowitz reported that he had no disclosures.