User login
FDA OKs first fecal transplant therapy for recurrent C. difficile
Rebyota (fecal microbiota, live-jslm), from Ferring Pharmaceuticals, is intended for use after an individual has completed antibiotic treatment for recurrent CDI. It is not indicated for the first occurrence of CDI.
“Recurrent CDI impacts an individual’s quality of life and can also potentially be life-threatening,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing approval.
As the first FDA-approved fecal microbiota product, this approval “represents an important milestone, as it provides an additional approved option to prevent recurrent CDI,” Dr. Marks added.
A panel of FDA advisors recommended approval of Rebyota in September.
The application for Rebyota received priority review and had orphan drug and breakthrough therapy designation.
A vicious cycle
Treatment options for recurrent CDI are limited. It’s been estimated that up to one-third of CDI cases recur, and people who suffer a recurrent bout of CDI are at a significantly higher risk for further infections.
Following the first recurrence, up to two-thirds of patients may experience a subsequent recurrence. Antibiotics used to treat CDI may contribute to a cycle of recurrence by altering the gut flora. The administration of fecal microbiota helps restore the gut flora to prevent further episodes of CDI.
Rebyota is a microbiota-based live biotherapeutic prepared from human stool collected from prescreened, qualified donors. It comes prepackaged in a single dose that is administered rectally.
The safety and efficacy of Rebyota were assessed in five clinical trials with more than 1,000 participants, the company notes in a press release.
In one trial, following a standard course of antibiotics, a one-time treatment with Rebyota was successful for three-quarters of participants at 8 weeks.
The treatment also prevented additional bouts; 84% of these initial responders remaining free of CDI at 6 months.
Two-thirds of participants reported treatment-emergent adverse events. Most events were mild to moderate in severity. Diarrhea and abdominal pain were the most common.
The data, from the ongoing PUNCH CD3-OLS study, were presented in October at the annual meeting of the American College of Gastroenterology and were published simultaneously in the journal Drugs.
“This is a positive adjunct to our current therapies for C. difficile in terms of trying to knock it out once a standard course of antibiotics has been administered,” Lisa Malter, MD, a gastroenterologist and professor of medicine at New York University Langone Health, said in an interview.
Dr. Malter acknowledged that because it’s delivered rectally, there could be “some hesitation” on the patient’s part to undergo the therapy.
However, C. difficile can be “excruciating” for patients, and they “may be more than willing to take [this agent] because it gets them feeling better,” Dr. Malter said.
Full prescribing information for Rebyota is available online.
Dr. Malter reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rebyota (fecal microbiota, live-jslm), from Ferring Pharmaceuticals, is intended for use after an individual has completed antibiotic treatment for recurrent CDI. It is not indicated for the first occurrence of CDI.
“Recurrent CDI impacts an individual’s quality of life and can also potentially be life-threatening,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing approval.
As the first FDA-approved fecal microbiota product, this approval “represents an important milestone, as it provides an additional approved option to prevent recurrent CDI,” Dr. Marks added.
A panel of FDA advisors recommended approval of Rebyota in September.
The application for Rebyota received priority review and had orphan drug and breakthrough therapy designation.
A vicious cycle
Treatment options for recurrent CDI are limited. It’s been estimated that up to one-third of CDI cases recur, and people who suffer a recurrent bout of CDI are at a significantly higher risk for further infections.
Following the first recurrence, up to two-thirds of patients may experience a subsequent recurrence. Antibiotics used to treat CDI may contribute to a cycle of recurrence by altering the gut flora. The administration of fecal microbiota helps restore the gut flora to prevent further episodes of CDI.
Rebyota is a microbiota-based live biotherapeutic prepared from human stool collected from prescreened, qualified donors. It comes prepackaged in a single dose that is administered rectally.
The safety and efficacy of Rebyota were assessed in five clinical trials with more than 1,000 participants, the company notes in a press release.
In one trial, following a standard course of antibiotics, a one-time treatment with Rebyota was successful for three-quarters of participants at 8 weeks.
The treatment also prevented additional bouts; 84% of these initial responders remaining free of CDI at 6 months.
Two-thirds of participants reported treatment-emergent adverse events. Most events were mild to moderate in severity. Diarrhea and abdominal pain were the most common.
The data, from the ongoing PUNCH CD3-OLS study, were presented in October at the annual meeting of the American College of Gastroenterology and were published simultaneously in the journal Drugs.
“This is a positive adjunct to our current therapies for C. difficile in terms of trying to knock it out once a standard course of antibiotics has been administered,” Lisa Malter, MD, a gastroenterologist and professor of medicine at New York University Langone Health, said in an interview.
Dr. Malter acknowledged that because it’s delivered rectally, there could be “some hesitation” on the patient’s part to undergo the therapy.
However, C. difficile can be “excruciating” for patients, and they “may be more than willing to take [this agent] because it gets them feeling better,” Dr. Malter said.
Full prescribing information for Rebyota is available online.
Dr. Malter reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rebyota (fecal microbiota, live-jslm), from Ferring Pharmaceuticals, is intended for use after an individual has completed antibiotic treatment for recurrent CDI. It is not indicated for the first occurrence of CDI.
“Recurrent CDI impacts an individual’s quality of life and can also potentially be life-threatening,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing approval.
As the first FDA-approved fecal microbiota product, this approval “represents an important milestone, as it provides an additional approved option to prevent recurrent CDI,” Dr. Marks added.
A panel of FDA advisors recommended approval of Rebyota in September.
The application for Rebyota received priority review and had orphan drug and breakthrough therapy designation.
A vicious cycle
Treatment options for recurrent CDI are limited. It’s been estimated that up to one-third of CDI cases recur, and people who suffer a recurrent bout of CDI are at a significantly higher risk for further infections.
Following the first recurrence, up to two-thirds of patients may experience a subsequent recurrence. Antibiotics used to treat CDI may contribute to a cycle of recurrence by altering the gut flora. The administration of fecal microbiota helps restore the gut flora to prevent further episodes of CDI.
Rebyota is a microbiota-based live biotherapeutic prepared from human stool collected from prescreened, qualified donors. It comes prepackaged in a single dose that is administered rectally.
The safety and efficacy of Rebyota were assessed in five clinical trials with more than 1,000 participants, the company notes in a press release.
In one trial, following a standard course of antibiotics, a one-time treatment with Rebyota was successful for three-quarters of participants at 8 weeks.
The treatment also prevented additional bouts; 84% of these initial responders remaining free of CDI at 6 months.
Two-thirds of participants reported treatment-emergent adverse events. Most events were mild to moderate in severity. Diarrhea and abdominal pain were the most common.
The data, from the ongoing PUNCH CD3-OLS study, were presented in October at the annual meeting of the American College of Gastroenterology and were published simultaneously in the journal Drugs.
“This is a positive adjunct to our current therapies for C. difficile in terms of trying to knock it out once a standard course of antibiotics has been administered,” Lisa Malter, MD, a gastroenterologist and professor of medicine at New York University Langone Health, said in an interview.
Dr. Malter acknowledged that because it’s delivered rectally, there could be “some hesitation” on the patient’s part to undergo the therapy.
However, C. difficile can be “excruciating” for patients, and they “may be more than willing to take [this agent] because it gets them feeling better,” Dr. Malter said.
Full prescribing information for Rebyota is available online.
Dr. Malter reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of health care use after bariatric surgery in teens
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.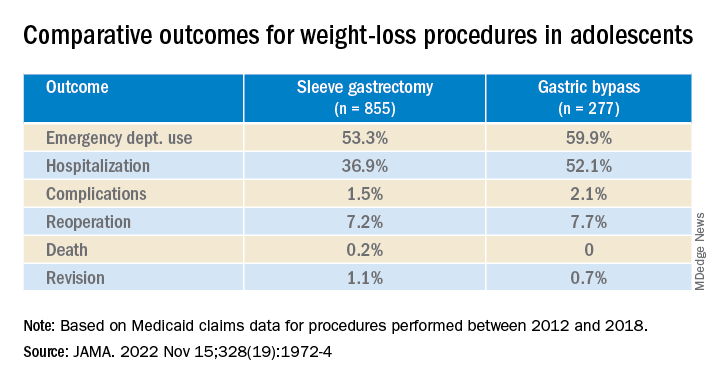
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers found significantly lower rates of both emergency department (ED) use and hospitalization 5 years after sleeve gastrectomy compared with gastric bypass, and similarly low rates of adverse events.
The study, by researchers with the department of surgery and Center for Health Outcomes and Policy, University of Michigan, Ann Arbor, was published in JAMA.
Studies have shown that sleeve gastrectomy and gastric bypass both lead to significant weight loss and are associated with low complication rates among adolescents with severe obesity.
Until now, however, comparative outcomes for these two weight-loss procedures have not been described for adolescents insured by Medicaid, the largest insurer of adolescents in the United States.
Using Medicaid claims data, Ryan Howard, MD, and colleagues identified 855 adolescents who underwent sleeve gastrectomy and 277 who underwent Roux-en-Y gastric bypass between 2012 and 2018.
Adolescents in both groups were about 18 years old on average at the time of surgery, and about three-quarters were female.
Sleeve gastrectomy became more common over the study period. The annual percentage of sleeve gastrectomy relative to gastric bypass increased from 48.8% in 2012 to 82.6% in 2018.
There was no significant difference in rates of complications (P = .31) or reoperation (P = .78), defined as abdominal operation potentially related to the index procedure, including biliary procedures and abdominal wall, internal, and paraesophageal hernia repair.
Researchers also found no difference between sleeve gastrectomy and gastric bypass in rates of death (P = .42) or revision (P = .63), which included any operation that directly modified the index procedure.
The results “may help inform the treatment of severe obesity in adolescents insured by Medicaid, although future studies should also evaluate long-term weight loss and comorbidity resolution in this population,” Dr. Howard and colleagues write.
They caution that their analysis is subject to selection bias because patient characteristics may influence the choice of procedure, although appropriate statistical adjustment was used.
Other limitations include the small sample size, which increases the possibility of type II error; the relatively short follow-up period; and the inability to directly attribute outcomes to the index procedure.
Funding for the study was provided by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Cardiovascular societies less apt to recognize women, minorities
Major cardiovascular societies are more apt to give out awards to men and White individuals than to women and minorities, according to a look at 2 decades’ worth of data.
“Women received significantly fewer awards than men in all societies, countries, and award categories,” author Martha Gulati, MD, director of preventive cardiology at Smidt Heart Institute at Cedars-Sinai, Los Angeles, said in a news release. “This bias may be responsible for preventing underrepresented groups from ascending the academic ladder and receiving senior awards like lifetime achievement awards.”
The study was published online in the Journal of the American College of Cardiology.
A slow climb
The findings are based on a review of honors given from 2000 to 2021 by the ACC, the American Heart Association, the American Society of Echocardiography, the Society for Cardiovascular Angiography and Interventions, the Heart Rhythm Society, the European Society of Cardiology, and the Canadian Cardiovascular Society.
Among the 173 unique awards, 94 were given by the AHA, 27 by the HRS, 17 by the ACC, 16 by the CCS, 8 by the ASE, 7 by the ESC, and 4 by the SCAI. There were 3,044 recipients of these awards, including 2,830 unique awardees.
The vast majority of the awardees were White (75.2%), with Asian, Hispanic/Latino, and Black awardees representing just 18.9%, 4.5%, and 1.4% of the total awardees, respectively.
In a gender analysis, the researchers looked at 169 awards after excluding female-specific awards. These 169 awards were distributed to 2,995 recipients. More than three-quarters of these awardees (76.2%) were men, with women making up less than one-quarter (23.8%).
Encouragingly, there was an increasing trend in recognition of women over time, with 7.7% of female awardees in 2000 and climbing to 31.2% in 2021 (average annual percentage change, 6.6%; P < .05).
The distribution of awards also became more racially/ethnically diverse over time; in 2000, 92.3% of awardees were White versus 62.8% in 2021 (AAPC, –1.4%; P < .001).
There was also a significant increase in Asian (AAPC, 5.7%; P < .001), Hispanic/Latino (AAPC, 4.8%; P = .040), and Black (AAPC, 7.8%; P < .05) honorees.
Core influencers
By award type, women received fewer leadership awards than men, “which can be attributed to fewer leadership opportunities for women and a lack of acknowledgment of leadership responsibilities fulfilled by women,” the researchers said.
Award recipients with a PhD degree were nearly gender balanced (48.2% women), whereas men formed an overwhelming majority of awardees with an MD (84.7%).
Awards with male eponyms had fewer women recipients than did noneponymous awards (20.9% vs. 23.2%; P < .01).
“Male-eponymous awards can deter women applicants and give a subtle hint to selection committees to favor men as winners, creating an implicit bias,” the researchers said.
“Given the increased emphasis on redesigning cardiovascular health care delivery by incorporating the tenets of diversity, equity, and inclusion (DEI), cardiovascular societies have a significant role as core influencers,” Dr. Gulati and colleagues wrote.
They said that equitable award distribution can be a “key strategy to celebrate women and diverse members of the cardiovascular workforce and promulgate DEI.”
“Recognition of their contributions is pivotal to enhancing their self-perception. In addition to boosting confidence, receiving an award can also catalyze their career trajectory,” the authors added.
The study had no specific funding. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Major cardiovascular societies are more apt to give out awards to men and White individuals than to women and minorities, according to a look at 2 decades’ worth of data.
“Women received significantly fewer awards than men in all societies, countries, and award categories,” author Martha Gulati, MD, director of preventive cardiology at Smidt Heart Institute at Cedars-Sinai, Los Angeles, said in a news release. “This bias may be responsible for preventing underrepresented groups from ascending the academic ladder and receiving senior awards like lifetime achievement awards.”
The study was published online in the Journal of the American College of Cardiology.
A slow climb
The findings are based on a review of honors given from 2000 to 2021 by the ACC, the American Heart Association, the American Society of Echocardiography, the Society for Cardiovascular Angiography and Interventions, the Heart Rhythm Society, the European Society of Cardiology, and the Canadian Cardiovascular Society.
Among the 173 unique awards, 94 were given by the AHA, 27 by the HRS, 17 by the ACC, 16 by the CCS, 8 by the ASE, 7 by the ESC, and 4 by the SCAI. There were 3,044 recipients of these awards, including 2,830 unique awardees.
The vast majority of the awardees were White (75.2%), with Asian, Hispanic/Latino, and Black awardees representing just 18.9%, 4.5%, and 1.4% of the total awardees, respectively.
In a gender analysis, the researchers looked at 169 awards after excluding female-specific awards. These 169 awards were distributed to 2,995 recipients. More than three-quarters of these awardees (76.2%) were men, with women making up less than one-quarter (23.8%).
Encouragingly, there was an increasing trend in recognition of women over time, with 7.7% of female awardees in 2000 and climbing to 31.2% in 2021 (average annual percentage change, 6.6%; P < .05).
The distribution of awards also became more racially/ethnically diverse over time; in 2000, 92.3% of awardees were White versus 62.8% in 2021 (AAPC, –1.4%; P < .001).
There was also a significant increase in Asian (AAPC, 5.7%; P < .001), Hispanic/Latino (AAPC, 4.8%; P = .040), and Black (AAPC, 7.8%; P < .05) honorees.
Core influencers
By award type, women received fewer leadership awards than men, “which can be attributed to fewer leadership opportunities for women and a lack of acknowledgment of leadership responsibilities fulfilled by women,” the researchers said.
Award recipients with a PhD degree were nearly gender balanced (48.2% women), whereas men formed an overwhelming majority of awardees with an MD (84.7%).
Awards with male eponyms had fewer women recipients than did noneponymous awards (20.9% vs. 23.2%; P < .01).
“Male-eponymous awards can deter women applicants and give a subtle hint to selection committees to favor men as winners, creating an implicit bias,” the researchers said.
“Given the increased emphasis on redesigning cardiovascular health care delivery by incorporating the tenets of diversity, equity, and inclusion (DEI), cardiovascular societies have a significant role as core influencers,” Dr. Gulati and colleagues wrote.
They said that equitable award distribution can be a “key strategy to celebrate women and diverse members of the cardiovascular workforce and promulgate DEI.”
“Recognition of their contributions is pivotal to enhancing their self-perception. In addition to boosting confidence, receiving an award can also catalyze their career trajectory,” the authors added.
The study had no specific funding. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Major cardiovascular societies are more apt to give out awards to men and White individuals than to women and minorities, according to a look at 2 decades’ worth of data.
“Women received significantly fewer awards than men in all societies, countries, and award categories,” author Martha Gulati, MD, director of preventive cardiology at Smidt Heart Institute at Cedars-Sinai, Los Angeles, said in a news release. “This bias may be responsible for preventing underrepresented groups from ascending the academic ladder and receiving senior awards like lifetime achievement awards.”
The study was published online in the Journal of the American College of Cardiology.
A slow climb
The findings are based on a review of honors given from 2000 to 2021 by the ACC, the American Heart Association, the American Society of Echocardiography, the Society for Cardiovascular Angiography and Interventions, the Heart Rhythm Society, the European Society of Cardiology, and the Canadian Cardiovascular Society.
Among the 173 unique awards, 94 were given by the AHA, 27 by the HRS, 17 by the ACC, 16 by the CCS, 8 by the ASE, 7 by the ESC, and 4 by the SCAI. There were 3,044 recipients of these awards, including 2,830 unique awardees.
The vast majority of the awardees were White (75.2%), with Asian, Hispanic/Latino, and Black awardees representing just 18.9%, 4.5%, and 1.4% of the total awardees, respectively.
In a gender analysis, the researchers looked at 169 awards after excluding female-specific awards. These 169 awards were distributed to 2,995 recipients. More than three-quarters of these awardees (76.2%) were men, with women making up less than one-quarter (23.8%).
Encouragingly, there was an increasing trend in recognition of women over time, with 7.7% of female awardees in 2000 and climbing to 31.2% in 2021 (average annual percentage change, 6.6%; P < .05).
The distribution of awards also became more racially/ethnically diverse over time; in 2000, 92.3% of awardees were White versus 62.8% in 2021 (AAPC, –1.4%; P < .001).
There was also a significant increase in Asian (AAPC, 5.7%; P < .001), Hispanic/Latino (AAPC, 4.8%; P = .040), and Black (AAPC, 7.8%; P < .05) honorees.
Core influencers
By award type, women received fewer leadership awards than men, “which can be attributed to fewer leadership opportunities for women and a lack of acknowledgment of leadership responsibilities fulfilled by women,” the researchers said.
Award recipients with a PhD degree were nearly gender balanced (48.2% women), whereas men formed an overwhelming majority of awardees with an MD (84.7%).
Awards with male eponyms had fewer women recipients than did noneponymous awards (20.9% vs. 23.2%; P < .01).
“Male-eponymous awards can deter women applicants and give a subtle hint to selection committees to favor men as winners, creating an implicit bias,” the researchers said.
“Given the increased emphasis on redesigning cardiovascular health care delivery by incorporating the tenets of diversity, equity, and inclusion (DEI), cardiovascular societies have a significant role as core influencers,” Dr. Gulati and colleagues wrote.
They said that equitable award distribution can be a “key strategy to celebrate women and diverse members of the cardiovascular workforce and promulgate DEI.”
“Recognition of their contributions is pivotal to enhancing their self-perception. In addition to boosting confidence, receiving an award can also catalyze their career trajectory,” the authors added.
The study had no specific funding. The authors have declared no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Bloating common but often ignored: Survey
, some of whom said they weren’t comfortable discussing it with their doctor, according to a large national survey.
The findings suggest doctors should “proactively” ask about bloating, especially in adults at increased risk, including women and those with irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD), the researchers say.
“Bloating is common because it usually has multifactorial causes and can also be a secondary symptom to another gastrointestinal (GI) symptom or condition. Its mechanisms are complex and individualized, making it difficult for providers to identify and treat each patient,” Janice E. Oh, MD, department of medicine, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“Thus, many adults may be persistently suffering without proper diagnosis or management,” Dr. Oh added.
Results of the survey are published online in Clinical Gastroenterology and Hepatology.
Common problem, incompletely understood
To get a better handle on the nationwide prevalence and health-related impact of bloating in the United States, Dr. Oh and her colleagues conducted an online survey of a nationally representative group of 88,795 adults aged 18 years or older.
Altogether, 12,324 (14%) respondents reported bloating in the past week.
The likelihood of bloating was significantly higher in women (odds ratio, 2.56) and in those with certain comorbid conditions, especially IBS, chronic constipation, and ulcerative colitis, the authors write.
The odds of bloating were also higher in adults with other concomitant GI symptoms, especially abdominal pain and excess gas.
Factors associated with more severe bloating included the presence of IBS, IBD, celiac disease, bowel incontinence, abdominal pain, constipation (functional and opioid-induced), and excess gas.
Bloating severity increased with age up to 59 years and then decreased in people aged 60 years or older.
Suffering in silence?
Notably, more than half (59%) of people who reported recent bloating never sought care for the problem. About one-third of them reported that bloating resolved on its own, and 30% said the symptoms were not bothersome.
About 1 in 5 adults who did not seek care said that they were managing symptoms on their own with over-the-counter medications or lifestyle modifications. And 9% of those who did not seek care said that they were uncomfortable discussing the problem with their doctor.
“The hesitancy in seeking health care or discussing bloating in patients may be attributed to lack of routine screening for bloating, lack of focus on bloating complaints by providers, or patients’ dissatisfaction with management of bloating symptoms,” the researchers say.
Adults most apt to seek care for bloating were those older than 29 years; non-Hispanic Black persons; those with comorbid conditions, such as celiac disease, IBD, and IBS; and those with more severe bloating symptoms.
A limitation is that individuals with GI symptoms or conditions may be more likely to participate in a GI-focused survey, leading to a possible overestimation of the prevalence of bloating.
Also, the survey was conducted during the COVID-19 pandemic, which has the potential to overestimate the prevalence or severity of bloating because COVID-19 is known to affect the GI system.
Despite these limitations, the researchers encourage health care professionals to routinely ask their patients about bloating as a first step in appropriate management.
“Bloating can be associated with nutrition/diet, the gut microbiome, anatomical issues, or underlying conditions that range from neurologic to gynecologic disorders. And, the majority of the time, it is usually more than one distinct issue that is attributing to the bloating,” Dr. Oh said.
“Understanding the patterns of bloating occurrence, psychosocial factors, past medical history, and nutrition can help providers determine the causes. We hope to identify a more standardized method to identify causes of bloating,” Dr. Oh added.
Support for the survey was provided by Ironwood Pharmaceuticals in the form of an institutional research grant to Cedars-Sinai. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, some of whom said they weren’t comfortable discussing it with their doctor, according to a large national survey.
The findings suggest doctors should “proactively” ask about bloating, especially in adults at increased risk, including women and those with irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD), the researchers say.
“Bloating is common because it usually has multifactorial causes and can also be a secondary symptom to another gastrointestinal (GI) symptom or condition. Its mechanisms are complex and individualized, making it difficult for providers to identify and treat each patient,” Janice E. Oh, MD, department of medicine, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“Thus, many adults may be persistently suffering without proper diagnosis or management,” Dr. Oh added.
Results of the survey are published online in Clinical Gastroenterology and Hepatology.
Common problem, incompletely understood
To get a better handle on the nationwide prevalence and health-related impact of bloating in the United States, Dr. Oh and her colleagues conducted an online survey of a nationally representative group of 88,795 adults aged 18 years or older.
Altogether, 12,324 (14%) respondents reported bloating in the past week.
The likelihood of bloating was significantly higher in women (odds ratio, 2.56) and in those with certain comorbid conditions, especially IBS, chronic constipation, and ulcerative colitis, the authors write.
The odds of bloating were also higher in adults with other concomitant GI symptoms, especially abdominal pain and excess gas.
Factors associated with more severe bloating included the presence of IBS, IBD, celiac disease, bowel incontinence, abdominal pain, constipation (functional and opioid-induced), and excess gas.
Bloating severity increased with age up to 59 years and then decreased in people aged 60 years or older.
Suffering in silence?
Notably, more than half (59%) of people who reported recent bloating never sought care for the problem. About one-third of them reported that bloating resolved on its own, and 30% said the symptoms were not bothersome.
About 1 in 5 adults who did not seek care said that they were managing symptoms on their own with over-the-counter medications or lifestyle modifications. And 9% of those who did not seek care said that they were uncomfortable discussing the problem with their doctor.
“The hesitancy in seeking health care or discussing bloating in patients may be attributed to lack of routine screening for bloating, lack of focus on bloating complaints by providers, or patients’ dissatisfaction with management of bloating symptoms,” the researchers say.
Adults most apt to seek care for bloating were those older than 29 years; non-Hispanic Black persons; those with comorbid conditions, such as celiac disease, IBD, and IBS; and those with more severe bloating symptoms.
A limitation is that individuals with GI symptoms or conditions may be more likely to participate in a GI-focused survey, leading to a possible overestimation of the prevalence of bloating.
Also, the survey was conducted during the COVID-19 pandemic, which has the potential to overestimate the prevalence or severity of bloating because COVID-19 is known to affect the GI system.
Despite these limitations, the researchers encourage health care professionals to routinely ask their patients about bloating as a first step in appropriate management.
“Bloating can be associated with nutrition/diet, the gut microbiome, anatomical issues, or underlying conditions that range from neurologic to gynecologic disorders. And, the majority of the time, it is usually more than one distinct issue that is attributing to the bloating,” Dr. Oh said.
“Understanding the patterns of bloating occurrence, psychosocial factors, past medical history, and nutrition can help providers determine the causes. We hope to identify a more standardized method to identify causes of bloating,” Dr. Oh added.
Support for the survey was provided by Ironwood Pharmaceuticals in the form of an institutional research grant to Cedars-Sinai. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
, some of whom said they weren’t comfortable discussing it with their doctor, according to a large national survey.
The findings suggest doctors should “proactively” ask about bloating, especially in adults at increased risk, including women and those with irritable bowel syndrome (IBS) or inflammatory bowel disease (IBD), the researchers say.
“Bloating is common because it usually has multifactorial causes and can also be a secondary symptom to another gastrointestinal (GI) symptom or condition. Its mechanisms are complex and individualized, making it difficult for providers to identify and treat each patient,” Janice E. Oh, MD, department of medicine, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“Thus, many adults may be persistently suffering without proper diagnosis or management,” Dr. Oh added.
Results of the survey are published online in Clinical Gastroenterology and Hepatology.
Common problem, incompletely understood
To get a better handle on the nationwide prevalence and health-related impact of bloating in the United States, Dr. Oh and her colleagues conducted an online survey of a nationally representative group of 88,795 adults aged 18 years or older.
Altogether, 12,324 (14%) respondents reported bloating in the past week.
The likelihood of bloating was significantly higher in women (odds ratio, 2.56) and in those with certain comorbid conditions, especially IBS, chronic constipation, and ulcerative colitis, the authors write.
The odds of bloating were also higher in adults with other concomitant GI symptoms, especially abdominal pain and excess gas.
Factors associated with more severe bloating included the presence of IBS, IBD, celiac disease, bowel incontinence, abdominal pain, constipation (functional and opioid-induced), and excess gas.
Bloating severity increased with age up to 59 years and then decreased in people aged 60 years or older.
Suffering in silence?
Notably, more than half (59%) of people who reported recent bloating never sought care for the problem. About one-third of them reported that bloating resolved on its own, and 30% said the symptoms were not bothersome.
About 1 in 5 adults who did not seek care said that they were managing symptoms on their own with over-the-counter medications or lifestyle modifications. And 9% of those who did not seek care said that they were uncomfortable discussing the problem with their doctor.
“The hesitancy in seeking health care or discussing bloating in patients may be attributed to lack of routine screening for bloating, lack of focus on bloating complaints by providers, or patients’ dissatisfaction with management of bloating symptoms,” the researchers say.
Adults most apt to seek care for bloating were those older than 29 years; non-Hispanic Black persons; those with comorbid conditions, such as celiac disease, IBD, and IBS; and those with more severe bloating symptoms.
A limitation is that individuals with GI symptoms or conditions may be more likely to participate in a GI-focused survey, leading to a possible overestimation of the prevalence of bloating.
Also, the survey was conducted during the COVID-19 pandemic, which has the potential to overestimate the prevalence or severity of bloating because COVID-19 is known to affect the GI system.
Despite these limitations, the researchers encourage health care professionals to routinely ask their patients about bloating as a first step in appropriate management.
“Bloating can be associated with nutrition/diet, the gut microbiome, anatomical issues, or underlying conditions that range from neurologic to gynecologic disorders. And, the majority of the time, it is usually more than one distinct issue that is attributing to the bloating,” Dr. Oh said.
“Understanding the patterns of bloating occurrence, psychosocial factors, past medical history, and nutrition can help providers determine the causes. We hope to identify a more standardized method to identify causes of bloating,” Dr. Oh added.
Support for the survey was provided by Ironwood Pharmaceuticals in the form of an institutional research grant to Cedars-Sinai. The authors have no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Fentanyl vaccine a potential ‘game changer’ for opioid crisis
Texas-based researchers have developed a vaccine that blocks the euphoric effects of fentanyl, a potent synthetic opioid that is increasingly involved in opioid overdose deaths in the United States.
In studies in male and female mice, the vaccine generated significant and long-lasting levels of anti-fentanyl antibodies that were highly effective at reducing the antinociceptive, behavioral, and physiological effects of the drug.
“Thus, the individual will not feel the euphoric effects and can ‘get back on the wagon’ to sobriety,” lead investigator Colin Haile, MD, PhD, with University of Houston and founding member of the UH Drug Discovery Institute, said in a news release. The study was published online in the journal Pharmaceutics.
“The anti-fentanyl antibodies were specific to fentanyl and a fentanyl derivative and did not cross-react with other opioids, such as morphine. That means a vaccinated person would still be able to be treated for pain relief with other opioids,” said Dr. Haile.
The vaccine did not cause any adverse effects in the immunized mice. The research team plans to start manufacturing clinical-grade vaccine in the coming months with clinical trials in humans planned soon.
If proven safe and effective in clinical testing, the vaccine could have major implications for the nation’s opioid epidemic by becoming a relapse prevention agent for people trying to quit using opioids, the researchers note.
The United States in 2021 recorded more than 107,000 drug overdose deaths – a record high, according to federal health officials – and fentanyl was involved in most of these deaths.
Senior author Therese Kosten, PhD, director of the UH Developmental, Cognitive & Behavioral Neuroscience program, calls the new fentanyl vaccine a potential “game changer.”
“Fentanyl use and overdose is a particular treatment challenge that is not adequately addressed with current medications because of its pharmacodynamics, and managing acute overdose with the short-acting naloxone [Narcan] is not appropriately effective as multiple doses of naloxone are often needed to reverse fentanyl’s fatal effects,” said Dr. Kosten.
Funding for the study was provided by the Department of Defense through the Alcohol and Substance Abuse Disorders Program managed by RTI International’s Pharmacotherapies for Alcohol and Substance Use Disorders Alliance, which has funded Dr. Haile’s lab for several years to develop the anti-fentanyl vaccine. The authors have no relevant conflicts of interest. A provisional patent has been submitted by the University of Houston on behalf of four of the investigators containing technology related to the fentanyl vaccine.
A version of this article first appeared on Medscape.com.
Texas-based researchers have developed a vaccine that blocks the euphoric effects of fentanyl, a potent synthetic opioid that is increasingly involved in opioid overdose deaths in the United States.
In studies in male and female mice, the vaccine generated significant and long-lasting levels of anti-fentanyl antibodies that were highly effective at reducing the antinociceptive, behavioral, and physiological effects of the drug.
“Thus, the individual will not feel the euphoric effects and can ‘get back on the wagon’ to sobriety,” lead investigator Colin Haile, MD, PhD, with University of Houston and founding member of the UH Drug Discovery Institute, said in a news release. The study was published online in the journal Pharmaceutics.
“The anti-fentanyl antibodies were specific to fentanyl and a fentanyl derivative and did not cross-react with other opioids, such as morphine. That means a vaccinated person would still be able to be treated for pain relief with other opioids,” said Dr. Haile.
The vaccine did not cause any adverse effects in the immunized mice. The research team plans to start manufacturing clinical-grade vaccine in the coming months with clinical trials in humans planned soon.
If proven safe and effective in clinical testing, the vaccine could have major implications for the nation’s opioid epidemic by becoming a relapse prevention agent for people trying to quit using opioids, the researchers note.
The United States in 2021 recorded more than 107,000 drug overdose deaths – a record high, according to federal health officials – and fentanyl was involved in most of these deaths.
Senior author Therese Kosten, PhD, director of the UH Developmental, Cognitive & Behavioral Neuroscience program, calls the new fentanyl vaccine a potential “game changer.”
“Fentanyl use and overdose is a particular treatment challenge that is not adequately addressed with current medications because of its pharmacodynamics, and managing acute overdose with the short-acting naloxone [Narcan] is not appropriately effective as multiple doses of naloxone are often needed to reverse fentanyl’s fatal effects,” said Dr. Kosten.
Funding for the study was provided by the Department of Defense through the Alcohol and Substance Abuse Disorders Program managed by RTI International’s Pharmacotherapies for Alcohol and Substance Use Disorders Alliance, which has funded Dr. Haile’s lab for several years to develop the anti-fentanyl vaccine. The authors have no relevant conflicts of interest. A provisional patent has been submitted by the University of Houston on behalf of four of the investigators containing technology related to the fentanyl vaccine.
A version of this article first appeared on Medscape.com.
Texas-based researchers have developed a vaccine that blocks the euphoric effects of fentanyl, a potent synthetic opioid that is increasingly involved in opioid overdose deaths in the United States.
In studies in male and female mice, the vaccine generated significant and long-lasting levels of anti-fentanyl antibodies that were highly effective at reducing the antinociceptive, behavioral, and physiological effects of the drug.
“Thus, the individual will not feel the euphoric effects and can ‘get back on the wagon’ to sobriety,” lead investigator Colin Haile, MD, PhD, with University of Houston and founding member of the UH Drug Discovery Institute, said in a news release. The study was published online in the journal Pharmaceutics.
“The anti-fentanyl antibodies were specific to fentanyl and a fentanyl derivative and did not cross-react with other opioids, such as morphine. That means a vaccinated person would still be able to be treated for pain relief with other opioids,” said Dr. Haile.
The vaccine did not cause any adverse effects in the immunized mice. The research team plans to start manufacturing clinical-grade vaccine in the coming months with clinical trials in humans planned soon.
If proven safe and effective in clinical testing, the vaccine could have major implications for the nation’s opioid epidemic by becoming a relapse prevention agent for people trying to quit using opioids, the researchers note.
The United States in 2021 recorded more than 107,000 drug overdose deaths – a record high, according to federal health officials – and fentanyl was involved in most of these deaths.
Senior author Therese Kosten, PhD, director of the UH Developmental, Cognitive & Behavioral Neuroscience program, calls the new fentanyl vaccine a potential “game changer.”
“Fentanyl use and overdose is a particular treatment challenge that is not adequately addressed with current medications because of its pharmacodynamics, and managing acute overdose with the short-acting naloxone [Narcan] is not appropriately effective as multiple doses of naloxone are often needed to reverse fentanyl’s fatal effects,” said Dr. Kosten.
Funding for the study was provided by the Department of Defense through the Alcohol and Substance Abuse Disorders Program managed by RTI International’s Pharmacotherapies for Alcohol and Substance Use Disorders Alliance, which has funded Dr. Haile’s lab for several years to develop the anti-fentanyl vaccine. The authors have no relevant conflicts of interest. A provisional patent has been submitted by the University of Houston on behalf of four of the investigators containing technology related to the fentanyl vaccine.
A version of this article first appeared on Medscape.com.
FROM PHARMACEUTICS
Major life stressors ‘strongly predictive’ of long COVID symptoms
new research suggests.
Major life stressors in the year after hospital discharge for COVID-19 are “strongly predictive of a lot of the important outcomes that people may face after COVID,” lead investigator Jennifer A. Frontera, MD, a professor in the department of neurology at New York University Langone Health, said in an interview.
These outcomes include depression, brain fog, fatigue, trouble sleeping, and other long COVID symptoms.
The findings were published online in the Journal of the Neurological Sciences.
Major stressful events common
Dr. Frontera and the NYU Neurology COVID-19 study team evaluated 451 adults who survived a COVID hospital stay. Of these, 383 completed a 6-month follow-up, 242 completed a 12-month follow-up, and 174 completed follow-up at both time points.
Within 1 year of discharge, 77 (17%) patients died and 51% suffered a major stressful life event.
In multivariable analyses, major life stressors – including financial insecurity, food insecurity, death of a close contact, and new disability – were strong independent predictors of disability, trouble with activities of daily living, depression, fatigue, sleep problems, and prolonged post-acute COVID symptoms. The adjusted odds ratios for these outcomes ranged from 2.5 to 20.8.
The research also confirmed the contribution of traditional risk factors for long COVID symptoms, as shown in past studies. These include older age, poor pre-COVID functional status, and more severe initial COVID-19 infection.
Long-term sequelae of COVID are increasingly recognized as major public health issues.
It has been estimated that roughly 16 million U.S. adults aged 18-65 years ave long COVID, with the often debilitating symptoms keeping up to 4 million out of work.
Holistic approach
Dr. Frontera said it’s important to realize that “sleep, fatigue, anxiety, depression, even cognition are so interwoven with each other that anything that impacts any one of them could have repercussions on the other.”
She added that it “certainly makes sense that there is an interplay or even a bidirectional relationship between the stressors that people face and how well they can recover after COVID.”
Therapies that lessen the trauma of the most stress-inducing life events need to be a central part of treatment for long COVID, with more research needed to validate the best approaches, Dr. Frontera said.
She also noted that social services or case management resources may be able to help address at least some of the stressors that individuals are under – and it is important to refer them to these resources. Referral to mental health services is also important.
“I think it’s really important to take a holistic approach and try to deal with whatever the problem may be,” said Dr. Frontera.
“I’m a neurologist, but as part of my evaluation, I really need to address if there are life stressors or mental health issues that may be impacting this person’s function,” she added.
The study had no commercial funding. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Major life stressors in the year after hospital discharge for COVID-19 are “strongly predictive of a lot of the important outcomes that people may face after COVID,” lead investigator Jennifer A. Frontera, MD, a professor in the department of neurology at New York University Langone Health, said in an interview.
These outcomes include depression, brain fog, fatigue, trouble sleeping, and other long COVID symptoms.
The findings were published online in the Journal of the Neurological Sciences.
Major stressful events common
Dr. Frontera and the NYU Neurology COVID-19 study team evaluated 451 adults who survived a COVID hospital stay. Of these, 383 completed a 6-month follow-up, 242 completed a 12-month follow-up, and 174 completed follow-up at both time points.
Within 1 year of discharge, 77 (17%) patients died and 51% suffered a major stressful life event.
In multivariable analyses, major life stressors – including financial insecurity, food insecurity, death of a close contact, and new disability – were strong independent predictors of disability, trouble with activities of daily living, depression, fatigue, sleep problems, and prolonged post-acute COVID symptoms. The adjusted odds ratios for these outcomes ranged from 2.5 to 20.8.
The research also confirmed the contribution of traditional risk factors for long COVID symptoms, as shown in past studies. These include older age, poor pre-COVID functional status, and more severe initial COVID-19 infection.
Long-term sequelae of COVID are increasingly recognized as major public health issues.
It has been estimated that roughly 16 million U.S. adults aged 18-65 years ave long COVID, with the often debilitating symptoms keeping up to 4 million out of work.
Holistic approach
Dr. Frontera said it’s important to realize that “sleep, fatigue, anxiety, depression, even cognition are so interwoven with each other that anything that impacts any one of them could have repercussions on the other.”
She added that it “certainly makes sense that there is an interplay or even a bidirectional relationship between the stressors that people face and how well they can recover after COVID.”
Therapies that lessen the trauma of the most stress-inducing life events need to be a central part of treatment for long COVID, with more research needed to validate the best approaches, Dr. Frontera said.
She also noted that social services or case management resources may be able to help address at least some of the stressors that individuals are under – and it is important to refer them to these resources. Referral to mental health services is also important.
“I think it’s really important to take a holistic approach and try to deal with whatever the problem may be,” said Dr. Frontera.
“I’m a neurologist, but as part of my evaluation, I really need to address if there are life stressors or mental health issues that may be impacting this person’s function,” she added.
The study had no commercial funding. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Major life stressors in the year after hospital discharge for COVID-19 are “strongly predictive of a lot of the important outcomes that people may face after COVID,” lead investigator Jennifer A. Frontera, MD, a professor in the department of neurology at New York University Langone Health, said in an interview.
These outcomes include depression, brain fog, fatigue, trouble sleeping, and other long COVID symptoms.
The findings were published online in the Journal of the Neurological Sciences.
Major stressful events common
Dr. Frontera and the NYU Neurology COVID-19 study team evaluated 451 adults who survived a COVID hospital stay. Of these, 383 completed a 6-month follow-up, 242 completed a 12-month follow-up, and 174 completed follow-up at both time points.
Within 1 year of discharge, 77 (17%) patients died and 51% suffered a major stressful life event.
In multivariable analyses, major life stressors – including financial insecurity, food insecurity, death of a close contact, and new disability – were strong independent predictors of disability, trouble with activities of daily living, depression, fatigue, sleep problems, and prolonged post-acute COVID symptoms. The adjusted odds ratios for these outcomes ranged from 2.5 to 20.8.
The research also confirmed the contribution of traditional risk factors for long COVID symptoms, as shown in past studies. These include older age, poor pre-COVID functional status, and more severe initial COVID-19 infection.
Long-term sequelae of COVID are increasingly recognized as major public health issues.
It has been estimated that roughly 16 million U.S. adults aged 18-65 years ave long COVID, with the often debilitating symptoms keeping up to 4 million out of work.
Holistic approach
Dr. Frontera said it’s important to realize that “sleep, fatigue, anxiety, depression, even cognition are so interwoven with each other that anything that impacts any one of them could have repercussions on the other.”
She added that it “certainly makes sense that there is an interplay or even a bidirectional relationship between the stressors that people face and how well they can recover after COVID.”
Therapies that lessen the trauma of the most stress-inducing life events need to be a central part of treatment for long COVID, with more research needed to validate the best approaches, Dr. Frontera said.
She also noted that social services or case management resources may be able to help address at least some of the stressors that individuals are under – and it is important to refer them to these resources. Referral to mental health services is also important.
“I think it’s really important to take a holistic approach and try to deal with whatever the problem may be,” said Dr. Frontera.
“I’m a neurologist, but as part of my evaluation, I really need to address if there are life stressors or mental health issues that may be impacting this person’s function,” she added.
The study had no commercial funding. The investigators reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE NEUROLOGICAL SCIENCES
Bepirovirsen: Is a ‘functional cure’ for HBV on the horizon?
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment with bepirovirsen led to sustained clearance of hepatitis B surface antigen (HBsAg) and hepatitis B virus (HBV) DNA for 24 weeks after the end of treatment for adults with chronic HBV in the phase 2b B-Clear study.
The study results were presented at the annual meeting of the American Association for the Study of Liver Diseases and were simultaneously published in The New England Journal of Medicine.
Currently, nucleoside/nucleotide analogue (NA) therapy is the recommended first-line therapy for patients with chronic HBV because it can inhibit viral replication.
However, fewer than 5% of patients have HBsAg loss after 12 months of NA therapy, which underscores the need for therapies that can achieve a “functional” cure, largely defined as sustained, undetectable levels of HBV DNA and HBsAg in the blood, with or without generation of protective antibodies against HBsAg, the researchers noted.
Bepirovirsen is a potential first-in-class antisense oligonucleotide that targets all HBV messenger RNA and acts to decrease levels of viral proteins.
The phase 2b B-Clear study enrolled 457 patients with chronic HBV; 227 were receiving NA therapy, and 230 were not.
Participants were randomly assigned to receive weekly subcutaneous injections of bepirovirsen 300 mg for 24 weeks; bepirovirsen 300 mg for 12 weeks, then 150 mg for 12 weeks; bepirovirsen 300 mg for 12 weeks, then placebo for 12 weeks; or placebo for 12 weeks, then bepirovirsen 300 mg for 12 weeks (groups 1, 2, 3, and 4, respectively).
The composite primary outcome was HBsAg level below the limit of detection and HBV DNA level below the limit of quantification maintained for 24 weeks after the end of bepirovirsen treatment, without newly initiated antiviral medication.
Bepirovirsen 300 mg weekly for 24 weeks (group 1) led to HBsAg and HBV DNA loss in 9% of patients receiving NA therapy and 10% of patients not receiving NA treatment, which was sustained for 24 weeks after the last dose.
For groups 2, 3, and 4, HBsAg and HBV DNA loss occurred in 9%, 3%, and 0%, respectively, of patients receiving NA therapy and 6%, 1%, and 0%, respectively, of patients not receiving NA treatment.
Patients with low baseline HBsAg levels (< 1,000 IU/mL) responded best to treatment with bepirovirsen. Among patients who received bepirovirsen 300 mg weekly for 24 weeks, the primary outcome was achieved by 16% of patients taking NA therapy and by 25% of patients not taking NA therapy.
Although a “relatively low percentage” of patients overall achieved the primary outcome, the study “indicates the possibility of enhanced efficacy with the selection of patients according to baseline characteristics (low HBsAg level at baseline), with combination therapies, or both,” the researchers wrote.
Adverse events with bepirovirsen included injection-site reactions, pyrexia, fatigue, and increased alanine aminotransferase (ALT) levels. Increases in ALT levels, which were more common in those not receiving NA therapy than in those receiving NA therapy (41% vs. 17%), led to two serious adverse events.
On the basis of phase 2b data, GlaxoSmithKline (GSK) plans to advance bepirovirsen into phase 3 development, according to a news release.
Further pursuit of bepirovirsen therapy is “certainly warranted, with the use of a dose of 300 mg per week for at least 24 weeks; indeed, the duration of therapy might be dictated best by HBsAg levels at baseline,” Jay H. Hoofnagle, MD, director of the liver disease research branch at the National Institute of Diabetes and Digestive and Kidney Diseases, wrote in an editorial in the New England Journal of Medicine.
Several critical questions remain, including whether HBsAg negativity will persist beyond 24 weeks, wrote Dr. Hoofnagle, who was not involved in the study.
It’s a question GSK is addressing in the B-Sure trial, which will follow participants for an additional 33 months, the study noted.
Other questions include when NA therapy can be safely stopped, what other factors predict response, and whether RNA therapy–induced loss of HBsAg materially improves long-term outcomes, Dr. Hoofnagle wrote.
“Bepirovirsen is just one RNA-based HBV therapy now being pursued. Several other antisense RNAs as well as the more malleable small interfering RNA molecules (‘-sirans’) are currently in early-phase clinical trials. A new era in the control of hepatitis B may be at hand with these most modern of therapies for this most ancient disease,” Dr. Hoofnagle noted.
The B-Clear study was supported by GSK. Several authors have disclosed relationships with the company. A complete list of author disclosures is available with the original article. Dr. Hoofnagle has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LIVER MEETING
Vonoprazan promising for erosive esophagitis
, according to results of the phase 3 PHALCON-EE trial.
Vonoprazan achieved higher rates of healing and maintenance of healing than lansoprazole, with the benefit seen primarily in patients with more severe esophagitis.
The differences in healing rates were evident after 2 weeks of therapy and were maintained throughout the 24-week study, report Loren Laine, MD, Yale University, New Haven, Conn., and colleagues.
The study was published online in Gastroenterology.
More potent acid suppression
Gastroesophageal reflux disease is one of the most common disorders of the gastrointestinal tract, and erosive esophagitis is its most common complication.
Although standard PPI therapy is effective for healing erosive esophagitis, some patients do not achieve success with this conventional treatment.
Studies suggest that lack of healing of erosive esophagitis with 8 weeks of PPI therapy can be expected in roughly 5%-20% of patients, with rates up to 30% reported in patients with more severe esophagitis.
The PCAB vonoprazan provides more potent inhibition of gastric acid than PPIs and is seen as a potential alternative. However, data on its efficacy for erosive esophagitis are limited, the authors note.
The PHALCON-EE trial enrolled 1,024 adults from the United States and Europe with erosive esophagitis without Helicobacter pylori infection or Barrett esophagus.
Participants were randomized to receive once-daily vonoprazan 20 mg or lansoprazole 30 mg for up to 8 weeks in the healing phase.
The 878 patients with healing were then rerandomized to receive once-daily vonoprazan 10 mg, vonoprazan 20 mg, or lansoprazole 15 mg for 24 weeks in the maintenance phase.
For healing by week 8, vonoprazan was noninferior to lansoprazole in the primary analysis and superior to lansoprazole in a predefined exploratory analysis (92.9% vs. 84.6%; P < .0001).
Secondary analyses showed that vonoprazan was noninferior to lansoprazole in mean 24-hour heartburn-free days and superior in healing at week 2 for grade C/D esophagitis (70.2% vs. 52.6%; P = .0008).
For maintenance of healing at week 24, vonoprazan was noninferior to lansoprazole in the primary analysis and superior on secondary analysis of healing (80.7% for vonoprazan 20 mg and 79.2% for vonoprazan 10 mg vs. 72.0% for lansoprazole; P < .0001 for both comparisons).
The most common adverse event reported in the healing phase was diarrhea and in the maintenance phase was COVID-19. Two deaths occurred, both from COVID-19, during the maintenance phase in the vonoprazan 20-mg group.
As expected, serum gastrin increased to a greater extent with vonoprazan than lansoprazole, with levels > 500 pg/mL in 16% of those taking 20 mg at the end of maintenance therapy, the authors report. After stopping vonoprazan, gastrin levels dropped by roughly 60%-65% within 4 weeks.
Promising new option
“PCABs are a promising new option,” Avin Aggarwal, MD, who was not involved in the study, told this news organization.
They have a “more potent acid inhibitory effect” and have shown “superior healing of erosive esophagitis,” said Dr. Aggarwal, a gastroenterologist and medical director of Banner Health’s South Campus endoscopy services and clinical assistant professor at the University of Arizona in Tucson.
The results of the PHALCON-EE trial “validate noninferiority of PCABs compared to standard PPI therapy in the Western population after being proven in multiple Asian studies,” he said.
Dr. Aggarwal noted that PCABs work the same way as PPIs, by blocking the proton pumps, but “the longer half-life of PCABs and action on both active and inactive proton channels result in greater acid inhibition.”
Long-term effects of PCAB therapy from stronger acid inhibition and resulting hypergastrinemia still remain to be determined, he said.
Earlier this year, the U.S. Food and Drug Administration accepted Phathom Pharmaceuticals’ new drug application for vonoprazan for the treatment of erosive esophagitis.
Last May, the FDA approved two vonoprazan-based therapies for the treatment of H. pylori infection.
The study was funded by Phathom Pharmaceuticals. Dr. Laine and several coauthors have disclosed financial relationships with the company. Dr. Aggarwal reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results of the phase 3 PHALCON-EE trial.
Vonoprazan achieved higher rates of healing and maintenance of healing than lansoprazole, with the benefit seen primarily in patients with more severe esophagitis.
The differences in healing rates were evident after 2 weeks of therapy and were maintained throughout the 24-week study, report Loren Laine, MD, Yale University, New Haven, Conn., and colleagues.
The study was published online in Gastroenterology.
More potent acid suppression
Gastroesophageal reflux disease is one of the most common disorders of the gastrointestinal tract, and erosive esophagitis is its most common complication.
Although standard PPI therapy is effective for healing erosive esophagitis, some patients do not achieve success with this conventional treatment.
Studies suggest that lack of healing of erosive esophagitis with 8 weeks of PPI therapy can be expected in roughly 5%-20% of patients, with rates up to 30% reported in patients with more severe esophagitis.
The PCAB vonoprazan provides more potent inhibition of gastric acid than PPIs and is seen as a potential alternative. However, data on its efficacy for erosive esophagitis are limited, the authors note.
The PHALCON-EE trial enrolled 1,024 adults from the United States and Europe with erosive esophagitis without Helicobacter pylori infection or Barrett esophagus.
Participants were randomized to receive once-daily vonoprazan 20 mg or lansoprazole 30 mg for up to 8 weeks in the healing phase.
The 878 patients with healing were then rerandomized to receive once-daily vonoprazan 10 mg, vonoprazan 20 mg, or lansoprazole 15 mg for 24 weeks in the maintenance phase.
For healing by week 8, vonoprazan was noninferior to lansoprazole in the primary analysis and superior to lansoprazole in a predefined exploratory analysis (92.9% vs. 84.6%; P < .0001).
Secondary analyses showed that vonoprazan was noninferior to lansoprazole in mean 24-hour heartburn-free days and superior in healing at week 2 for grade C/D esophagitis (70.2% vs. 52.6%; P = .0008).
For maintenance of healing at week 24, vonoprazan was noninferior to lansoprazole in the primary analysis and superior on secondary analysis of healing (80.7% for vonoprazan 20 mg and 79.2% for vonoprazan 10 mg vs. 72.0% for lansoprazole; P < .0001 for both comparisons).
The most common adverse event reported in the healing phase was diarrhea and in the maintenance phase was COVID-19. Two deaths occurred, both from COVID-19, during the maintenance phase in the vonoprazan 20-mg group.
As expected, serum gastrin increased to a greater extent with vonoprazan than lansoprazole, with levels > 500 pg/mL in 16% of those taking 20 mg at the end of maintenance therapy, the authors report. After stopping vonoprazan, gastrin levels dropped by roughly 60%-65% within 4 weeks.
Promising new option
“PCABs are a promising new option,” Avin Aggarwal, MD, who was not involved in the study, told this news organization.
They have a “more potent acid inhibitory effect” and have shown “superior healing of erosive esophagitis,” said Dr. Aggarwal, a gastroenterologist and medical director of Banner Health’s South Campus endoscopy services and clinical assistant professor at the University of Arizona in Tucson.
The results of the PHALCON-EE trial “validate noninferiority of PCABs compared to standard PPI therapy in the Western population after being proven in multiple Asian studies,” he said.
Dr. Aggarwal noted that PCABs work the same way as PPIs, by blocking the proton pumps, but “the longer half-life of PCABs and action on both active and inactive proton channels result in greater acid inhibition.”
Long-term effects of PCAB therapy from stronger acid inhibition and resulting hypergastrinemia still remain to be determined, he said.
Earlier this year, the U.S. Food and Drug Administration accepted Phathom Pharmaceuticals’ new drug application for vonoprazan for the treatment of erosive esophagitis.
Last May, the FDA approved two vonoprazan-based therapies for the treatment of H. pylori infection.
The study was funded by Phathom Pharmaceuticals. Dr. Laine and several coauthors have disclosed financial relationships with the company. Dr. Aggarwal reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, according to results of the phase 3 PHALCON-EE trial.
Vonoprazan achieved higher rates of healing and maintenance of healing than lansoprazole, with the benefit seen primarily in patients with more severe esophagitis.
The differences in healing rates were evident after 2 weeks of therapy and were maintained throughout the 24-week study, report Loren Laine, MD, Yale University, New Haven, Conn., and colleagues.
The study was published online in Gastroenterology.
More potent acid suppression
Gastroesophageal reflux disease is one of the most common disorders of the gastrointestinal tract, and erosive esophagitis is its most common complication.
Although standard PPI therapy is effective for healing erosive esophagitis, some patients do not achieve success with this conventional treatment.
Studies suggest that lack of healing of erosive esophagitis with 8 weeks of PPI therapy can be expected in roughly 5%-20% of patients, with rates up to 30% reported in patients with more severe esophagitis.
The PCAB vonoprazan provides more potent inhibition of gastric acid than PPIs and is seen as a potential alternative. However, data on its efficacy for erosive esophagitis are limited, the authors note.
The PHALCON-EE trial enrolled 1,024 adults from the United States and Europe with erosive esophagitis without Helicobacter pylori infection or Barrett esophagus.
Participants were randomized to receive once-daily vonoprazan 20 mg or lansoprazole 30 mg for up to 8 weeks in the healing phase.
The 878 patients with healing were then rerandomized to receive once-daily vonoprazan 10 mg, vonoprazan 20 mg, or lansoprazole 15 mg for 24 weeks in the maintenance phase.
For healing by week 8, vonoprazan was noninferior to lansoprazole in the primary analysis and superior to lansoprazole in a predefined exploratory analysis (92.9% vs. 84.6%; P < .0001).
Secondary analyses showed that vonoprazan was noninferior to lansoprazole in mean 24-hour heartburn-free days and superior in healing at week 2 for grade C/D esophagitis (70.2% vs. 52.6%; P = .0008).
For maintenance of healing at week 24, vonoprazan was noninferior to lansoprazole in the primary analysis and superior on secondary analysis of healing (80.7% for vonoprazan 20 mg and 79.2% for vonoprazan 10 mg vs. 72.0% for lansoprazole; P < .0001 for both comparisons).
The most common adverse event reported in the healing phase was diarrhea and in the maintenance phase was COVID-19. Two deaths occurred, both from COVID-19, during the maintenance phase in the vonoprazan 20-mg group.
As expected, serum gastrin increased to a greater extent with vonoprazan than lansoprazole, with levels > 500 pg/mL in 16% of those taking 20 mg at the end of maintenance therapy, the authors report. After stopping vonoprazan, gastrin levels dropped by roughly 60%-65% within 4 weeks.
Promising new option
“PCABs are a promising new option,” Avin Aggarwal, MD, who was not involved in the study, told this news organization.
They have a “more potent acid inhibitory effect” and have shown “superior healing of erosive esophagitis,” said Dr. Aggarwal, a gastroenterologist and medical director of Banner Health’s South Campus endoscopy services and clinical assistant professor at the University of Arizona in Tucson.
The results of the PHALCON-EE trial “validate noninferiority of PCABs compared to standard PPI therapy in the Western population after being proven in multiple Asian studies,” he said.
Dr. Aggarwal noted that PCABs work the same way as PPIs, by blocking the proton pumps, but “the longer half-life of PCABs and action on both active and inactive proton channels result in greater acid inhibition.”
Long-term effects of PCAB therapy from stronger acid inhibition and resulting hypergastrinemia still remain to be determined, he said.
Earlier this year, the U.S. Food and Drug Administration accepted Phathom Pharmaceuticals’ new drug application for vonoprazan for the treatment of erosive esophagitis.
Last May, the FDA approved two vonoprazan-based therapies for the treatment of H. pylori infection.
The study was funded by Phathom Pharmaceuticals. Dr. Laine and several coauthors have disclosed financial relationships with the company. Dr. Aggarwal reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM GASTROENTEROLOGY
Electrolyte disturbances a harbinger of eating disorders?
Electrolyte abnormalities may serve as a precursor to a future eating disorder diagnosis, a finding that may help pinpoint candidates for screening.
Researchers found that adolescents and adults with electrolyte abnormalities on routine outpatient lab work were twice as likely as those without these disturbances to be subsequently diagnosed with an eating disorder.
“These electrolyte abnormalities were in fact seen well ahead (> 1 year on average) of the time when patients were diagnosed with eating disorders,” study investigator Gregory Hundemer, MD, department of nephrology, University of Ottawa, told this news organization.
“Incidentally discovered outpatient electrolyte abnormalities may help to identify individuals who may benefit from more targeted screening into an underlying eating disorder. This, in turn, may allow for earlier diagnosis and therapeutic intervention,” Dr. Hundemer said.
The study was published online in JAMA Network Open.
Tailored screening?
Electrolyte abnormalities are often found when an individual is diagnosed with an eating disorder, but it’s largely unknown whether electrolyte abnormalities prior to the acute presentation of an eating disorder are associated with the future diagnosis of an eating disorder.
To investigate, the researchers used administrative health data to match 6,970 individuals (mean age, 28 years; 13% male) with an eating disorder diagnosis to 27,878 controls without an eating disorder diagnosis.
They found that individuals with an eating disorder were more likely to have a preceding electrolyte abnormality, compared with peers without an eating disorder (18.4% vs. 7.5%).
An outpatient electrolyte abnormality present 3 years to 30 days prior to diagnosis was associated with about a twofold higher odds for subsequent eating disorder diagnosis (adjusted odds ratio, 2.12; 95% confidence interval, 1.86-2.41).
The median time from the earliest electrolyte abnormality to eating disorder diagnosis was 386 days (range, 157-716 days).
Hypokalemia was the most common electrolyte abnormality (present in 12% of cases vs. 5% of controls), while hyponatremia, hypernatremia, hypophosphatemia, and metabolic alkalosis were the most specific for a subsequent eating disorder diagnosis.
Severe hypokalemia (serum potassium levels of 3.0 mmol/L or lower) and severe hyponatremia (serum sodium, 128 mmol/L or lower) were associated with over sevenfold and fivefold higher odds for the diagnosis of an eating disorder, respectively.
The U.S. Preventive Services Task Force issued its first-ever statement on screening for eating disorders earlier this year.
The task force concluded that there is insufficient evidence to weigh the balance of benefits and harms of screening for eating disorders in adolescents and adults with no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder.
Dr. Hundemer and colleagues believe an incidental electrolyte abnormality may identify candidates at high risk for an underlying eating disorder who many benefit from screening.
Several screening tools of varying complexity have been developed that are validated and accurate in identifying individuals with a potential eating disorder.
They include the SCOFF questionnaire, the Eating Disorder Screen for Primary Care, the Eating Attitudes Test, and the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire.
Underdiagnosed, undertreated
Offering perspective on the findings, Kamryn T. Eddy, PhD, codirector, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, said the notion “that a physical sign may help to promote eating disorder assessment is important particularly given that early detection can improve outcomes.”
“But this finding appears in the current context of eating disorders going largely underdetected, underdiagnosed, and undertreated across medical and psychiatric settings,” said Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston.
“Indeed, eating disorders are prevalent and cut across age, sex, gender, weight, race, ethnicity, and socioeconomic strata, and still, many providers do not routinely assess for eating disorders,” Dr. Eddy said.
“I might suggest that perhaps in addition to letting electrolyte abnormalities be a cue to screen for eating disorders, an even more powerful shift toward routine screening and assessment of eating disorders by medical providers be made,” Dr. Eddy said in an interview.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Dr. Hundemer and Dr. Eddy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Electrolyte abnormalities may serve as a precursor to a future eating disorder diagnosis, a finding that may help pinpoint candidates for screening.
Researchers found that adolescents and adults with electrolyte abnormalities on routine outpatient lab work were twice as likely as those without these disturbances to be subsequently diagnosed with an eating disorder.
“These electrolyte abnormalities were in fact seen well ahead (> 1 year on average) of the time when patients were diagnosed with eating disorders,” study investigator Gregory Hundemer, MD, department of nephrology, University of Ottawa, told this news organization.
“Incidentally discovered outpatient electrolyte abnormalities may help to identify individuals who may benefit from more targeted screening into an underlying eating disorder. This, in turn, may allow for earlier diagnosis and therapeutic intervention,” Dr. Hundemer said.
The study was published online in JAMA Network Open.
Tailored screening?
Electrolyte abnormalities are often found when an individual is diagnosed with an eating disorder, but it’s largely unknown whether electrolyte abnormalities prior to the acute presentation of an eating disorder are associated with the future diagnosis of an eating disorder.
To investigate, the researchers used administrative health data to match 6,970 individuals (mean age, 28 years; 13% male) with an eating disorder diagnosis to 27,878 controls without an eating disorder diagnosis.
They found that individuals with an eating disorder were more likely to have a preceding electrolyte abnormality, compared with peers without an eating disorder (18.4% vs. 7.5%).
An outpatient electrolyte abnormality present 3 years to 30 days prior to diagnosis was associated with about a twofold higher odds for subsequent eating disorder diagnosis (adjusted odds ratio, 2.12; 95% confidence interval, 1.86-2.41).
The median time from the earliest electrolyte abnormality to eating disorder diagnosis was 386 days (range, 157-716 days).
Hypokalemia was the most common electrolyte abnormality (present in 12% of cases vs. 5% of controls), while hyponatremia, hypernatremia, hypophosphatemia, and metabolic alkalosis were the most specific for a subsequent eating disorder diagnosis.
Severe hypokalemia (serum potassium levels of 3.0 mmol/L or lower) and severe hyponatremia (serum sodium, 128 mmol/L or lower) were associated with over sevenfold and fivefold higher odds for the diagnosis of an eating disorder, respectively.
The U.S. Preventive Services Task Force issued its first-ever statement on screening for eating disorders earlier this year.
The task force concluded that there is insufficient evidence to weigh the balance of benefits and harms of screening for eating disorders in adolescents and adults with no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder.
Dr. Hundemer and colleagues believe an incidental electrolyte abnormality may identify candidates at high risk for an underlying eating disorder who many benefit from screening.
Several screening tools of varying complexity have been developed that are validated and accurate in identifying individuals with a potential eating disorder.
They include the SCOFF questionnaire, the Eating Disorder Screen for Primary Care, the Eating Attitudes Test, and the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire.
Underdiagnosed, undertreated
Offering perspective on the findings, Kamryn T. Eddy, PhD, codirector, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, said the notion “that a physical sign may help to promote eating disorder assessment is important particularly given that early detection can improve outcomes.”
“But this finding appears in the current context of eating disorders going largely underdetected, underdiagnosed, and undertreated across medical and psychiatric settings,” said Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston.
“Indeed, eating disorders are prevalent and cut across age, sex, gender, weight, race, ethnicity, and socioeconomic strata, and still, many providers do not routinely assess for eating disorders,” Dr. Eddy said.
“I might suggest that perhaps in addition to letting electrolyte abnormalities be a cue to screen for eating disorders, an even more powerful shift toward routine screening and assessment of eating disorders by medical providers be made,” Dr. Eddy said in an interview.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Dr. Hundemer and Dr. Eddy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Electrolyte abnormalities may serve as a precursor to a future eating disorder diagnosis, a finding that may help pinpoint candidates for screening.
Researchers found that adolescents and adults with electrolyte abnormalities on routine outpatient lab work were twice as likely as those without these disturbances to be subsequently diagnosed with an eating disorder.
“These electrolyte abnormalities were in fact seen well ahead (> 1 year on average) of the time when patients were diagnosed with eating disorders,” study investigator Gregory Hundemer, MD, department of nephrology, University of Ottawa, told this news organization.
“Incidentally discovered outpatient electrolyte abnormalities may help to identify individuals who may benefit from more targeted screening into an underlying eating disorder. This, in turn, may allow for earlier diagnosis and therapeutic intervention,” Dr. Hundemer said.
The study was published online in JAMA Network Open.
Tailored screening?
Electrolyte abnormalities are often found when an individual is diagnosed with an eating disorder, but it’s largely unknown whether electrolyte abnormalities prior to the acute presentation of an eating disorder are associated with the future diagnosis of an eating disorder.
To investigate, the researchers used administrative health data to match 6,970 individuals (mean age, 28 years; 13% male) with an eating disorder diagnosis to 27,878 controls without an eating disorder diagnosis.
They found that individuals with an eating disorder were more likely to have a preceding electrolyte abnormality, compared with peers without an eating disorder (18.4% vs. 7.5%).
An outpatient electrolyte abnormality present 3 years to 30 days prior to diagnosis was associated with about a twofold higher odds for subsequent eating disorder diagnosis (adjusted odds ratio, 2.12; 95% confidence interval, 1.86-2.41).
The median time from the earliest electrolyte abnormality to eating disorder diagnosis was 386 days (range, 157-716 days).
Hypokalemia was the most common electrolyte abnormality (present in 12% of cases vs. 5% of controls), while hyponatremia, hypernatremia, hypophosphatemia, and metabolic alkalosis were the most specific for a subsequent eating disorder diagnosis.
Severe hypokalemia (serum potassium levels of 3.0 mmol/L or lower) and severe hyponatremia (serum sodium, 128 mmol/L or lower) were associated with over sevenfold and fivefold higher odds for the diagnosis of an eating disorder, respectively.
The U.S. Preventive Services Task Force issued its first-ever statement on screening for eating disorders earlier this year.
The task force concluded that there is insufficient evidence to weigh the balance of benefits and harms of screening for eating disorders in adolescents and adults with no signs or symptoms of an eating disorder or concerns about their eating and who have not previously been diagnosed with an eating disorder.
Dr. Hundemer and colleagues believe an incidental electrolyte abnormality may identify candidates at high risk for an underlying eating disorder who many benefit from screening.
Several screening tools of varying complexity have been developed that are validated and accurate in identifying individuals with a potential eating disorder.
They include the SCOFF questionnaire, the Eating Disorder Screen for Primary Care, the Eating Attitudes Test, and the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire.
Underdiagnosed, undertreated
Offering perspective on the findings, Kamryn T. Eddy, PhD, codirector, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, Boston, said the notion “that a physical sign may help to promote eating disorder assessment is important particularly given that early detection can improve outcomes.”
“But this finding appears in the current context of eating disorders going largely underdetected, underdiagnosed, and undertreated across medical and psychiatric settings,” said Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston.
“Indeed, eating disorders are prevalent and cut across age, sex, gender, weight, race, ethnicity, and socioeconomic strata, and still, many providers do not routinely assess for eating disorders,” Dr. Eddy said.
“I might suggest that perhaps in addition to letting electrolyte abnormalities be a cue to screen for eating disorders, an even more powerful shift toward routine screening and assessment of eating disorders by medical providers be made,” Dr. Eddy said in an interview.
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Dr. Hundemer and Dr. Eddy have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Hiccups in patients with cancer often overlooked, undertreated
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
But even if recognized, hiccups may not be treated effectively, according to a national survey of cancer care clinicians.
When poorly controlled, persistent hiccups can affect a patient’s quality of life, with 40% of survey respondents considering chronic hiccups “much more” or “somewhat more” severe than nausea and vomiting.
Overall, the findings indicate that patients with cancer who develop persistent hiccups are “truly suffering,” the authors wrote.
The survey results were published online recently in the American Journal of Hospice and Palliative Medicine.
Hiccups may simply be a nuisance for most, but these spasms can become problematic for patients with cancer, leading to sleep deprivation, fatigue, aspiration pneumonia, compromised food intake, weight loss, pain, and even death.
Hiccups can develop when the nerve that controls the diaphragm becomes irritated, which can be triggered by certain chemotherapy drugs.
Yet few studies have focused on hiccups in patients with cancer and none, until now, has sought the perspectives of cancer care clinicians.
Aminah Jatoi, MD, medical oncologist with the Mayo Clinic in Rochester, Minn., and two Mayo colleagues developed a survey, alongside MeterHealth, which this news organization distributed to clinicians with an interest in cancer care.
The survey gauged clinicians’ awareness or lack of awareness about clinically significant hiccups as well as treatments for hiccups and whether they consider hiccups an unmet palliative need.
A total of 684 clinicians completed two eligibility screening questions, which required them to have cared for more than 10 patients with cancer in the past 6 months with clinically significant hiccups (defined as hiccups that lasted more than 48 hours or occurred from cancer or cancer care).
Among 113 eligible health care professionals, 90 completed the survey: 42 physicians, 29 nurses, 15 nurse practitioners, and 4 physician assistants.
The survey revealed three key issues.
The first is that hiccups appear to be an underrecognized issue.
Among health care professionals who answered the eligibility screening questions, fewer than 20% reported caring for more than 10 patients with cancer in the past 6 months who had persistent hiccups. Most of these clinicians reported caring for more than 1,000 patients per year.
Given that 15%-40% of patients with cancer report hiccups, this finding suggests that hiccups are not widely recognized by health care professionals.
Second: The survey data showed that hiccups often increase patients’ anxiety, fatigue, and sleep problems and can decrease productivity at work or school.
In fact, when comparing hiccups to nausea and vomiting – sometimes described as one of the most severe side effects of cancer care – 40% of respondents rated hiccups as “much more” or “somewhat more” severe than nausea and vomiting for their patients and 38% rated the severity of the two issues as “about the same.”
Finally, even when hiccups are recognized and treated, about 20% of respondents said that current therapies are not very effective, and more treatment options are needed.
Among the survey respondents, the most frequently prescribed medications for chronic hiccups were the antipsychotic chlorpromazine, the muscle relaxant baclofen (Lioresal), the antiemetic metoclopramide (Metozolv ODT, Reglan), and the anticonvulsants gabapentin (Neurontin) and carbamazepine (Tegretol).
Survey respondents who provided comments about current treatments for hiccups highlighted a range of challenges. One respondent said, “When current therapies do not work, it can be very demoralizing to our patients.” Another said, “I feel like it is a gamble whether treatment for hiccups will work or not.”
Still another felt that while current treatments work “quite well to halt hiccups,” they come with side effects which can be “quite severe.”
These results “clearly point to the unmet needs of hiccups in patients with cancer and should prompt more research aimed at generating more palliative options,” the authors said.
This research had no commercial funding. MeterHealth reviewed the manuscript and provided input on the accuracy of methods and results. Dr. Jatoi reports serving on an advisory board for MeterHealth (honoraria to institution).
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF HOSPICE AND PALLIATIVE MEDICINE

