User login
The Authors Reply: “Cost and Utility of Thrombophilia Testing”
We thank Dr. Berse and colleagues for their correspondence about our paper.1,2 We are pleased they agreed with our conclusion: Thrombophilia testing has limited clinical utility in most inpatient settings.
Berse and colleagues critiqued details of our methodology in calculating payer cost, including how we estimated the number of Medicare claims for thrombophilia testing. We estimated that there were at least 280,000 Medicare claims in 2014 using CodeMap® (Wheaton Partners, LLC, Schaumburg, IL), a dataset of utilization data from the Physician Supplier Procedure Summary Master File from all Medicare Part B carriers.3 This estimate was similar to that reported in a previous publication.4
Thus, regardless of the precise estimates, even a conservative estimate of 33 to 80 million dollars of unnecessary spending is far too much. Rather, it is a perfect example of “Things We Do for No Reason.”
Disclosure
Nothing to report.
1. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. DOI:10.1136/bmjopen-2014-006578. PubMed
2. Berse B, Lynch JA, Bowen S, Grosse SD. In Reference to: “Cost and Utility of Thrombophilia Testing.” J Hosp Med. 2017;12(9):783.
3. CodeMap® https://www.codemap.com/. Accessed March 2, 2017.
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-7. DOI:10.1309/KV06-32LJ-8EDM-EWQT. PubMed
We thank Dr. Berse and colleagues for their correspondence about our paper.1,2 We are pleased they agreed with our conclusion: Thrombophilia testing has limited clinical utility in most inpatient settings.
Berse and colleagues critiqued details of our methodology in calculating payer cost, including how we estimated the number of Medicare claims for thrombophilia testing. We estimated that there were at least 280,000 Medicare claims in 2014 using CodeMap® (Wheaton Partners, LLC, Schaumburg, IL), a dataset of utilization data from the Physician Supplier Procedure Summary Master File from all Medicare Part B carriers.3 This estimate was similar to that reported in a previous publication.4
Thus, regardless of the precise estimates, even a conservative estimate of 33 to 80 million dollars of unnecessary spending is far too much. Rather, it is a perfect example of “Things We Do for No Reason.”
Disclosure
Nothing to report.
We thank Dr. Berse and colleagues for their correspondence about our paper.1,2 We are pleased they agreed with our conclusion: Thrombophilia testing has limited clinical utility in most inpatient settings.
Berse and colleagues critiqued details of our methodology in calculating payer cost, including how we estimated the number of Medicare claims for thrombophilia testing. We estimated that there were at least 280,000 Medicare claims in 2014 using CodeMap® (Wheaton Partners, LLC, Schaumburg, IL), a dataset of utilization data from the Physician Supplier Procedure Summary Master File from all Medicare Part B carriers.3 This estimate was similar to that reported in a previous publication.4
Thus, regardless of the precise estimates, even a conservative estimate of 33 to 80 million dollars of unnecessary spending is far too much. Rather, it is a perfect example of “Things We Do for No Reason.”
Disclosure
Nothing to report.
1. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. DOI:10.1136/bmjopen-2014-006578. PubMed
2. Berse B, Lynch JA, Bowen S, Grosse SD. In Reference to: “Cost and Utility of Thrombophilia Testing.” J Hosp Med. 2017;12(9):783.
3. CodeMap® https://www.codemap.com/. Accessed March 2, 2017.
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-7. DOI:10.1309/KV06-32LJ-8EDM-EWQT. PubMed
1. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. DOI:10.1136/bmjopen-2014-006578. PubMed
2. Berse B, Lynch JA, Bowen S, Grosse SD. In Reference to: “Cost and Utility of Thrombophilia Testing.” J Hosp Med. 2017;12(9):783.
3. CodeMap® https://www.codemap.com/. Accessed March 2, 2017.
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-7. DOI:10.1309/KV06-32LJ-8EDM-EWQT. PubMed
© 2017 Society of Hospital Medicine
Inherited Thrombophilia Testing
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of venous thromboembolism (VTE). This disorder is prevalent in approximately 7% of the population and includes mutations such as factor V Leiden, prothrombin 20210, protein C deficiency, protein S deficiency, antithrombin deficiency, and methylene tetrahydrofolate reductase. The relative risk of VTE is 3‐ to 20‐fold greater in patients with inherited thrombophilia compared with the general population. Is testing for inherited thrombophilia recommended? The available evidence suggests that testing for inherited thrombophilia is not recommended in most clinical settings. In patients without a personal history of VTE, thrombophilia results do not change management, as there is no evidence to support thromboprophylaxis in this setting. In patients with a personal history of provoked or unprovoked VTE, inpatient testing is not indicated, as results do not influence management, testing is not cost‐effective, and a positive test result may lead to unnecessary patient anxiety or may result in unnecessary involvement of consultants. Testing in hospitalized patients has even more limitations because many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation.
CASE PRESENTATION
A 23‐year‐old man presents to the emergency room with pleuritic chest pain and new oxygen requirement of 2 L nasal cannula. He has a history of unprovoked lower extremity deep venous thrombosis (DVT) diagnosed at age 20 and completed 3 months of systemic anticoagulation without complications. He reports no family history of clotting disorders or venous thromboembolism (VTE) and no reversible risk factors for VTE such as prolonged immobility, recent surgery, or high‐risk medications. A computed tomogram pulmonary embolism protocol shows multiple right lower lobe, segmental pulmonary emboli. Anticoagulation is initiated, and the patient is admitted to the hospital. Will inpatient inherited thrombophilia testing impact management for this case?
WHY MAY INHERITED THROMBOPHILIA TESTING PROVE HELPFUL?
The annual incidence rate of a first VTE event is estimated as 117 per 100,000 individuals per year.[1] The most common presentations are symptomatic DVT of the leg (annual incidence approximately 48 per 100,000 people), or a pulmonary embolism (annual incidence approximately 69 per 100,000 people).[1] Pulmonary embolism results in death in up to 30% of untreated patients and 2.5% of patients who receive systemic anticoagulation.[2] Principal in the pathogenesis of VTE are factors described by Virchow's triad: venous stasis, endothelial injury, and systemic hypercoagulability. By identifying a mutation in 1 or more of the factors in the clotting pathway, an evaluation for inherited thrombophilia theoretically may unearth factors that drive systemic hypercoagulability and inform decision making so as to prevent future events.
Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of VTE.[3] Approximately 7% of the general population has inherited thrombophilia, which includes factor V Leiden (FVL) mutation, prothrombin 20210 mutation (PT20210), protein C deficiency, protein S deficiency, antithrombin III (ATIII) deficiency, and methylene tetrahydrofolate reductase mutation (MTHFR).[4] Of note, the definition does not include acquired etiologies, such as antiphospholipid antibody syndrome. Depending on the underlying condition and expression of the genetic abnormality, the relative risk of VTE in patients with inherited thrombophilia is 3‐ to 20‐fold greater than that of the general population.[5] Therefore, it is logical to consider that testing for inherited thrombophilia might be clinically useful. However, the evidence for doing so is very limited.
DOES INHERITED THROMBOPHILIA TESTING CHANGE MANAGEMENT?
An inherited thrombophilia evaluation is unlikely to affect management in most clinical settings. There is no current evidence to support primary prophylaxis[6] nor is there evidence that management of patients with recurrent VTE should be altered in the setting of inherited thrombophilia.
To date, no prospective trials have evaluated the efficacy of anticoagulant use for primary prevention of VTE in patients with inherited thrombophilia.[6] Given the limited evidence for thromboprophylaxis and risks of anticoagulation, primary prevention for patients with inherited thrombophilia that remain asymptomatic is not recommended by the current American College of Chest Physicians guidelines.[7, 8]
Similarly, in patients with a first VTE or recurrent VTE, diagnosis of inherited thrombophilia is often not associated with recurrent events, which suggests that other nongenetic factors may be just as important, if not more important, in determining the risk of recurrence.[9] Although no randomized controlled or controlled clinical trials have evaluated the effects of testing for inherited thrombophilia on recurrent VTE,[10, 11] several prospective studies have assessed risk factors for recurrence. Data from these studies suggest that recurrence rates after unprovoked VTE are only weakly correlated with inherited thrombophilia status.[12, 13] Rather, it is postulated that patients with recurrent VTE may exhibit a prothrombotic tendency regardless of underlying genetic predisposition. In this case, decisions regarding anticoagulation do not vary by thrombophilia status. Instead, thrombophilia testing may divert attention away from the management of more prevalent, potentially modifiable risk factors such as immobility, oral contraceptive use, or malignancy, all of which are associated with recurrent VTE.[14] These provoking factors are the most important determinants of the chance of VTE recurrence as well as the most significant factors to take into account when deciding duration of anticoagulation.
Christiansen et al. performed a prospective study evaluating the association between recurrent VTE and thrombophilia status. After following 474 patients with confirmed first episode VTE for a mean of 7.3 years, no statistically significant risk of VTE was found for patients with FVL (hazard ratio [HR]: 1.2, 95% confidence interval [CI]: 0.7‐1.9), PT20210 (HR: 0.7, 95% CI: 0.3‐2.0), or an anticoagulant (protein C, protein S or ATIII) deficiency (HR: 1.8, 95% CI: 0.9‐3.7).[15] Although unexplained VTE was statistically associated with VTE recurrence, heritable thrombophilia status was not.
In a systematic review and meta‐analysis investigating the association of FVL and PT20210 with recurrent VTE, Ho and colleagues found a statistically significant risk of recurrent VTE in patients with inherited thrombophilia due to FVL (odds ratio [OR]: 1.41, 95% CI: 1.14‐1.75) and PT20210 (OR: 1.72, 95% CI: 1.27‐2.31), and reported that at most, only up to 1 in 6 recurrent VTEs may be attributable to these mutations.[16] Based on this relatively modest effect, the authors question the utility of testing for inherited thrombophilia, as thrombophilia status is unlikely to warrant a change in type or duration of treatment.
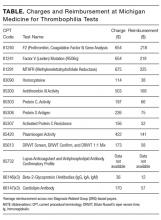
Regardless of whether an underlying inherited thrombophilia is identified, patients with history of recurrent VTE are often candidates for long‐term anticoagulation. Testing for inherited thrombophilia in patients with prior VTE events will therefore not influence decisions regarding clinical management. Additionally, such testing may be confounded by ongoing disease or treatment (Table 1). For example, protein C, protein S antigen, and ATIII levels are low in the setting of acute VTE.[17, 18] Likewise, protein C and S (vitamin Kdependent proteins) will be low in the setting of anticoagulation with warfarin.[19] Moreover, ATIII activity and antigen levels are low in the setting of heparin use.[20] Lack of provider awareness regarding these interactions may have important negative consequences, including a spurious diagnosis of thrombophilia,[21, 22] unnecessary hematology consultation, and psychological distress to patients in the form of ongoing unwarranted testing or apprehension regarding recurrence.[23]
| Acute VTE | Anticoagulation With Warfarin | Anticoagulation With NOACs | Anticoagulation With Heparin/LMWH | |
|---|---|---|---|---|
| ||||
| FVL/PT20210/MTHFR gene mutations | No Impact | No Impact | No Impact | No Impact |
| Protein C* | Decreased | Decreased | No impact | No impact |
| Protein S* | Decreased | Decreased | No impact | No impact |
| ATIII activity | Decreased | Slight increase | Slight increase | Decreased |
| ATIII antigen | Decreased | Slight increase | Slight increase | Decreased |
Additionally, this expensive evaluation has estimated direct costs of $1100 to $2400 per thrombophilia panel based on estimation of charges billed by a large commercial laboratory.[24, 25] In 2014, over 280,000 claims were submitted under Medicare Part B across all care settings for a thrombophilia analysis including FVL, PT20210, and MTHFR gene mutations,[24] which would equate to between $300 million to $672 million.[26] Unfortunately, there have been no large‐scale trials to assess cost‐effectiveness. However, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group stated that cost‐effectiveness modeling studies in this area require updating with current VTE risk estimates but are suggestive that routine FVL/PT20210 testing is not cost‐effective.[27]
ARE THERE CIRCUMSTANCES IN WHICH INPATIENT INHERITED THROMBOPHILIA TESTING PROVES BENEFICIAL?
The evidence for when to test for inherited thrombophilia is very limited and is often based on individualized risk. The current EGAPP guidelines acknowledge this limitation, specifically noting that there is a paucity of data evaluating management or prophylaxis of patients with homozygous or compound heterozygous FVL or P20210 mutation, and a lack of data surrounding whether or not knowledge of thrombophilia mutation should affect anticoagulation treatment.[27] This is why an individualized approach is deemed necessary. For example, the decision to prescribe hormone replacement therapy in women with a family history of inherited thrombophilia may be better informed by testing prior to treatment. Similarly, pregnant women with a family history or personal history of VTE may also benefit from inherited thrombophilia testing, as this may influence antepartum or postpartum management.[28, 29] The National Institute for Health and Clinical Excellence (NICE) guidelines recommend consideration of testing for hereditary thrombophilia in patients with unprovoked VTE and a first‐degree relative with VTE, if stopping anticoagulation treatment is planned; however, these recommendations are based solely on Guideline Development Group's experience and opinion.[30] Regardless, testing for inherited thrombophilia has significant potential consequences. Patients at risk should meet with an outpatient hematologist and/or a genetic counselor, if available, to determine the risks and benefits of testing.
WHAT DO GUIDELINES SAY ABOUT INHERITED THROMBOPHILIA TESTING?
The most recent NICE guidelines recommend against offering inherited thrombophilia testing to patients presenting with a provoked VTE in any clinical setting.[30] In patients diagnosed with unprovoked VTE, testing should not be considered unless a first degree relative with a history of VTE exists.[30] The NICE guidelines also recommend against routinely offering thrombophilia testing to asymptomatic first‐degree relatives of patients with a history of VTE or known inherited thrombophilia. This recommendation is reflected in the American Society of Hematology's Choosing Wisely recommendations since 2013.[31] Further, The American College of Medical Genetics and Genomics' Choosing Wisely recommendations from 2015 state that MTHFR mutations should never be included in any thrombophilia workup, as recent meta‐analyses have disproven an association between the presence of these variants and venous thromboembolism.[32]
The EGAPP Working Group recommends against routine testing for FVL or PT20210 in patients who present with an idiopathic VTE, as longer‐term anticoagulation offers similar benefits to patients with or without these mutations.[27] EGAPP also recommends against testing asymptomatic adult family members of patients with VTE and/or an FVL or PT20210 mutation for the purpose of considering primary prophylactic anticoagulation. In these circumstances, it is felt that the potential risks of thrombophilia testing outweigh any potential benefits.
HOW SHOULD HOSPITALISTS APPROACH TESTING OF INHERITED THROMBOPHILIA?
The providers in our case presentation are challenged with determining whether inpatient thrombophilia evaluation will add value to the evaluation of patients with unprovoked VTE. The available evidence suggests that clinicians should avoid ordering thrombophilia testing for hospitalized patients with unprovoked VTE because (1) many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation, (2) results of testing often do not influence management, (3) testing is not cost‐effective, (4) a positive test result may lead to unnecessary patient anxiety, and (5) testing may result in inappropriately prolonged anticoagulation courses or unnecessary involvement of inpatient consultants. For these reasons, the patient in our case presentation should not be tested for inherited thrombophilia. In patients with personal or family histories of recurrent thromboembolism, modifiable clinical risk factors should be addressed, as these are more likely to influence treatment decisions compared to genetic testing. Finally, patients may be referred to an outpatient hematologist or geneticist for individualized discussions of risks and benefits of testing for inherited thrombophilia.
CONCLUSION
Inpatient evaluation for inherited thrombophilia for VTE is not clinically useful, cost‐effective, or reliable in the setting of VTE. The result of such testing does not affect management of acute primary or recurrent VTE. Testing should only be considered using an individualized approach in the outpatient setting with appropriate genetic counseling.
Disclosure: Christopher M. Petrilli, MD, and Lauren Heidemann, MD, contributed equally to this work. The authors report no conflicts of interest.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing TWDFNR@hospitalmedicine.org.
- , , , , , . Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158(6):585–593.
- , , , et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240–1245.
- , . Hereditary thrombophilia. Thromb J. 2006;4:15.
- , , , . Deep‐vein thrombosis. Lancet. 1999;353(9151):479–485.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S–e736S.
- , . Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. 2013;110(4):697–705.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–2485.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2009;(1):CD007069.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:CD007069.
- , , , . Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362(9383):523–526.
- , , , et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112(12):4432–4436.
- , . Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699–704.
- , , , , . Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352–2361.
- , , , . Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166(7):729–736.
- , , , . Relationship between protein C antigen and anticoagulant activity during oral anticoagulation and in selected disease states. J Clin Invest. 1986;77(2):416–425.
- , . Inherited antithrombin deficiency: a review. Haemophilia. 2008;14(6):1229–1239.
- , , , . Decline of proteins C and S and factors II, VII, IX and X during the initiation of warfarin therapy. Thromb Res. 1987;45(6):783–790.
- . Thrombophilia: common questions on laboratory assessment and management. Hematology Am Soc Hematol Educ Program. 2007:127–135.
- , , . Activated protein C resistance testing for factor V Leiden. Am J Hematol. 2014;89(12):1147–1150.
- , . Quantitation of human protein S in the plasma of normal and warfarin‐treated individuals by radioimmunoassay. Thromb Res. 1984;36(6):527–535.
- , , , . Social aspects of genetic testing for factor V Leiden mutation in healthy individuals and their importance for daily practice. Thromb Res. 2004;113(1):7–12.
- , . Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013–1020.
- , , . An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120–127.
- CodeMap. Available at: https://www.codemap.com. Accessed January 18, 2016.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67–76.
- , , , et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343(20):1439–1444.
- , , , et al. Frequency of pregnancy‐related venous thromboembolism in anticoagulant factor‐deficient women: implications for prophylaxis. Ann Intern Med. 1996;125(12):955–960.
- , , , ; Guideline Development Group. Management of venous thromboembolic diseases and the role of thrombophilia testing: summary of NICE guidance. BMJ. 2012;344:e3979.
- American Society of Hematology. Ten things physicians and patients should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐society‐of‐hematology. Published December 4, 2013. Accessed January 18, 2016.
- American College of Medical Genetics and Genomics. Five Things patients and providers should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐college‐of‐medical‐genetics‐and‐genomics. Published July 10, 2015. Accessed March 13, 2016.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of venous thromboembolism (VTE). This disorder is prevalent in approximately 7% of the population and includes mutations such as factor V Leiden, prothrombin 20210, protein C deficiency, protein S deficiency, antithrombin deficiency, and methylene tetrahydrofolate reductase. The relative risk of VTE is 3‐ to 20‐fold greater in patients with inherited thrombophilia compared with the general population. Is testing for inherited thrombophilia recommended? The available evidence suggests that testing for inherited thrombophilia is not recommended in most clinical settings. In patients without a personal history of VTE, thrombophilia results do not change management, as there is no evidence to support thromboprophylaxis in this setting. In patients with a personal history of provoked or unprovoked VTE, inpatient testing is not indicated, as results do not influence management, testing is not cost‐effective, and a positive test result may lead to unnecessary patient anxiety or may result in unnecessary involvement of consultants. Testing in hospitalized patients has even more limitations because many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation.
CASE PRESENTATION
A 23‐year‐old man presents to the emergency room with pleuritic chest pain and new oxygen requirement of 2 L nasal cannula. He has a history of unprovoked lower extremity deep venous thrombosis (DVT) diagnosed at age 20 and completed 3 months of systemic anticoagulation without complications. He reports no family history of clotting disorders or venous thromboembolism (VTE) and no reversible risk factors for VTE such as prolonged immobility, recent surgery, or high‐risk medications. A computed tomogram pulmonary embolism protocol shows multiple right lower lobe, segmental pulmonary emboli. Anticoagulation is initiated, and the patient is admitted to the hospital. Will inpatient inherited thrombophilia testing impact management for this case?
WHY MAY INHERITED THROMBOPHILIA TESTING PROVE HELPFUL?
The annual incidence rate of a first VTE event is estimated as 117 per 100,000 individuals per year.[1] The most common presentations are symptomatic DVT of the leg (annual incidence approximately 48 per 100,000 people), or a pulmonary embolism (annual incidence approximately 69 per 100,000 people).[1] Pulmonary embolism results in death in up to 30% of untreated patients and 2.5% of patients who receive systemic anticoagulation.[2] Principal in the pathogenesis of VTE are factors described by Virchow's triad: venous stasis, endothelial injury, and systemic hypercoagulability. By identifying a mutation in 1 or more of the factors in the clotting pathway, an evaluation for inherited thrombophilia theoretically may unearth factors that drive systemic hypercoagulability and inform decision making so as to prevent future events.
Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of VTE.[3] Approximately 7% of the general population has inherited thrombophilia, which includes factor V Leiden (FVL) mutation, prothrombin 20210 mutation (PT20210), protein C deficiency, protein S deficiency, antithrombin III (ATIII) deficiency, and methylene tetrahydrofolate reductase mutation (MTHFR).[4] Of note, the definition does not include acquired etiologies, such as antiphospholipid antibody syndrome. Depending on the underlying condition and expression of the genetic abnormality, the relative risk of VTE in patients with inherited thrombophilia is 3‐ to 20‐fold greater than that of the general population.[5] Therefore, it is logical to consider that testing for inherited thrombophilia might be clinically useful. However, the evidence for doing so is very limited.
DOES INHERITED THROMBOPHILIA TESTING CHANGE MANAGEMENT?
An inherited thrombophilia evaluation is unlikely to affect management in most clinical settings. There is no current evidence to support primary prophylaxis[6] nor is there evidence that management of patients with recurrent VTE should be altered in the setting of inherited thrombophilia.
To date, no prospective trials have evaluated the efficacy of anticoagulant use for primary prevention of VTE in patients with inherited thrombophilia.[6] Given the limited evidence for thromboprophylaxis and risks of anticoagulation, primary prevention for patients with inherited thrombophilia that remain asymptomatic is not recommended by the current American College of Chest Physicians guidelines.[7, 8]
Similarly, in patients with a first VTE or recurrent VTE, diagnosis of inherited thrombophilia is often not associated with recurrent events, which suggests that other nongenetic factors may be just as important, if not more important, in determining the risk of recurrence.[9] Although no randomized controlled or controlled clinical trials have evaluated the effects of testing for inherited thrombophilia on recurrent VTE,[10, 11] several prospective studies have assessed risk factors for recurrence. Data from these studies suggest that recurrence rates after unprovoked VTE are only weakly correlated with inherited thrombophilia status.[12, 13] Rather, it is postulated that patients with recurrent VTE may exhibit a prothrombotic tendency regardless of underlying genetic predisposition. In this case, decisions regarding anticoagulation do not vary by thrombophilia status. Instead, thrombophilia testing may divert attention away from the management of more prevalent, potentially modifiable risk factors such as immobility, oral contraceptive use, or malignancy, all of which are associated with recurrent VTE.[14] These provoking factors are the most important determinants of the chance of VTE recurrence as well as the most significant factors to take into account when deciding duration of anticoagulation.
Christiansen et al. performed a prospective study evaluating the association between recurrent VTE and thrombophilia status. After following 474 patients with confirmed first episode VTE for a mean of 7.3 years, no statistically significant risk of VTE was found for patients with FVL (hazard ratio [HR]: 1.2, 95% confidence interval [CI]: 0.7‐1.9), PT20210 (HR: 0.7, 95% CI: 0.3‐2.0), or an anticoagulant (protein C, protein S or ATIII) deficiency (HR: 1.8, 95% CI: 0.9‐3.7).[15] Although unexplained VTE was statistically associated with VTE recurrence, heritable thrombophilia status was not.
In a systematic review and meta‐analysis investigating the association of FVL and PT20210 with recurrent VTE, Ho and colleagues found a statistically significant risk of recurrent VTE in patients with inherited thrombophilia due to FVL (odds ratio [OR]: 1.41, 95% CI: 1.14‐1.75) and PT20210 (OR: 1.72, 95% CI: 1.27‐2.31), and reported that at most, only up to 1 in 6 recurrent VTEs may be attributable to these mutations.[16] Based on this relatively modest effect, the authors question the utility of testing for inherited thrombophilia, as thrombophilia status is unlikely to warrant a change in type or duration of treatment.
Regardless of whether an underlying inherited thrombophilia is identified, patients with history of recurrent VTE are often candidates for long‐term anticoagulation. Testing for inherited thrombophilia in patients with prior VTE events will therefore not influence decisions regarding clinical management. Additionally, such testing may be confounded by ongoing disease or treatment (Table 1). For example, protein C, protein S antigen, and ATIII levels are low in the setting of acute VTE.[17, 18] Likewise, protein C and S (vitamin Kdependent proteins) will be low in the setting of anticoagulation with warfarin.[19] Moreover, ATIII activity and antigen levels are low in the setting of heparin use.[20] Lack of provider awareness regarding these interactions may have important negative consequences, including a spurious diagnosis of thrombophilia,[21, 22] unnecessary hematology consultation, and psychological distress to patients in the form of ongoing unwarranted testing or apprehension regarding recurrence.[23]
| Acute VTE | Anticoagulation With Warfarin | Anticoagulation With NOACs | Anticoagulation With Heparin/LMWH | |
|---|---|---|---|---|
| ||||
| FVL/PT20210/MTHFR gene mutations | No Impact | No Impact | No Impact | No Impact |
| Protein C* | Decreased | Decreased | No impact | No impact |
| Protein S* | Decreased | Decreased | No impact | No impact |
| ATIII activity | Decreased | Slight increase | Slight increase | Decreased |
| ATIII antigen | Decreased | Slight increase | Slight increase | Decreased |
Additionally, this expensive evaluation has estimated direct costs of $1100 to $2400 per thrombophilia panel based on estimation of charges billed by a large commercial laboratory.[24, 25] In 2014, over 280,000 claims were submitted under Medicare Part B across all care settings for a thrombophilia analysis including FVL, PT20210, and MTHFR gene mutations,[24] which would equate to between $300 million to $672 million.[26] Unfortunately, there have been no large‐scale trials to assess cost‐effectiveness. However, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group stated that cost‐effectiveness modeling studies in this area require updating with current VTE risk estimates but are suggestive that routine FVL/PT20210 testing is not cost‐effective.[27]
ARE THERE CIRCUMSTANCES IN WHICH INPATIENT INHERITED THROMBOPHILIA TESTING PROVES BENEFICIAL?
The evidence for when to test for inherited thrombophilia is very limited and is often based on individualized risk. The current EGAPP guidelines acknowledge this limitation, specifically noting that there is a paucity of data evaluating management or prophylaxis of patients with homozygous or compound heterozygous FVL or P20210 mutation, and a lack of data surrounding whether or not knowledge of thrombophilia mutation should affect anticoagulation treatment.[27] This is why an individualized approach is deemed necessary. For example, the decision to prescribe hormone replacement therapy in women with a family history of inherited thrombophilia may be better informed by testing prior to treatment. Similarly, pregnant women with a family history or personal history of VTE may also benefit from inherited thrombophilia testing, as this may influence antepartum or postpartum management.[28, 29] The National Institute for Health and Clinical Excellence (NICE) guidelines recommend consideration of testing for hereditary thrombophilia in patients with unprovoked VTE and a first‐degree relative with VTE, if stopping anticoagulation treatment is planned; however, these recommendations are based solely on Guideline Development Group's experience and opinion.[30] Regardless, testing for inherited thrombophilia has significant potential consequences. Patients at risk should meet with an outpatient hematologist and/or a genetic counselor, if available, to determine the risks and benefits of testing.
WHAT DO GUIDELINES SAY ABOUT INHERITED THROMBOPHILIA TESTING?
The most recent NICE guidelines recommend against offering inherited thrombophilia testing to patients presenting with a provoked VTE in any clinical setting.[30] In patients diagnosed with unprovoked VTE, testing should not be considered unless a first degree relative with a history of VTE exists.[30] The NICE guidelines also recommend against routinely offering thrombophilia testing to asymptomatic first‐degree relatives of patients with a history of VTE or known inherited thrombophilia. This recommendation is reflected in the American Society of Hematology's Choosing Wisely recommendations since 2013.[31] Further, The American College of Medical Genetics and Genomics' Choosing Wisely recommendations from 2015 state that MTHFR mutations should never be included in any thrombophilia workup, as recent meta‐analyses have disproven an association between the presence of these variants and venous thromboembolism.[32]
The EGAPP Working Group recommends against routine testing for FVL or PT20210 in patients who present with an idiopathic VTE, as longer‐term anticoagulation offers similar benefits to patients with or without these mutations.[27] EGAPP also recommends against testing asymptomatic adult family members of patients with VTE and/or an FVL or PT20210 mutation for the purpose of considering primary prophylactic anticoagulation. In these circumstances, it is felt that the potential risks of thrombophilia testing outweigh any potential benefits.
HOW SHOULD HOSPITALISTS APPROACH TESTING OF INHERITED THROMBOPHILIA?
The providers in our case presentation are challenged with determining whether inpatient thrombophilia evaluation will add value to the evaluation of patients with unprovoked VTE. The available evidence suggests that clinicians should avoid ordering thrombophilia testing for hospitalized patients with unprovoked VTE because (1) many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation, (2) results of testing often do not influence management, (3) testing is not cost‐effective, (4) a positive test result may lead to unnecessary patient anxiety, and (5) testing may result in inappropriately prolonged anticoagulation courses or unnecessary involvement of inpatient consultants. For these reasons, the patient in our case presentation should not be tested for inherited thrombophilia. In patients with personal or family histories of recurrent thromboembolism, modifiable clinical risk factors should be addressed, as these are more likely to influence treatment decisions compared to genetic testing. Finally, patients may be referred to an outpatient hematologist or geneticist for individualized discussions of risks and benefits of testing for inherited thrombophilia.
CONCLUSION
Inpatient evaluation for inherited thrombophilia for VTE is not clinically useful, cost‐effective, or reliable in the setting of VTE. The result of such testing does not affect management of acute primary or recurrent VTE. Testing should only be considered using an individualized approach in the outpatient setting with appropriate genetic counseling.
Disclosure: Christopher M. Petrilli, MD, and Lauren Heidemann, MD, contributed equally to this work. The authors report no conflicts of interest.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing TWDFNR@hospitalmedicine.org.
The Things We Do for No Reason (TWDFNR) series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent black and white conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of venous thromboembolism (VTE). This disorder is prevalent in approximately 7% of the population and includes mutations such as factor V Leiden, prothrombin 20210, protein C deficiency, protein S deficiency, antithrombin deficiency, and methylene tetrahydrofolate reductase. The relative risk of VTE is 3‐ to 20‐fold greater in patients with inherited thrombophilia compared with the general population. Is testing for inherited thrombophilia recommended? The available evidence suggests that testing for inherited thrombophilia is not recommended in most clinical settings. In patients without a personal history of VTE, thrombophilia results do not change management, as there is no evidence to support thromboprophylaxis in this setting. In patients with a personal history of provoked or unprovoked VTE, inpatient testing is not indicated, as results do not influence management, testing is not cost‐effective, and a positive test result may lead to unnecessary patient anxiety or may result in unnecessary involvement of consultants. Testing in hospitalized patients has even more limitations because many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation.
CASE PRESENTATION
A 23‐year‐old man presents to the emergency room with pleuritic chest pain and new oxygen requirement of 2 L nasal cannula. He has a history of unprovoked lower extremity deep venous thrombosis (DVT) diagnosed at age 20 and completed 3 months of systemic anticoagulation without complications. He reports no family history of clotting disorders or venous thromboembolism (VTE) and no reversible risk factors for VTE such as prolonged immobility, recent surgery, or high‐risk medications. A computed tomogram pulmonary embolism protocol shows multiple right lower lobe, segmental pulmonary emboli. Anticoagulation is initiated, and the patient is admitted to the hospital. Will inpatient inherited thrombophilia testing impact management for this case?
WHY MAY INHERITED THROMBOPHILIA TESTING PROVE HELPFUL?
The annual incidence rate of a first VTE event is estimated as 117 per 100,000 individuals per year.[1] The most common presentations are symptomatic DVT of the leg (annual incidence approximately 48 per 100,000 people), or a pulmonary embolism (annual incidence approximately 69 per 100,000 people).[1] Pulmonary embolism results in death in up to 30% of untreated patients and 2.5% of patients who receive systemic anticoagulation.[2] Principal in the pathogenesis of VTE are factors described by Virchow's triad: venous stasis, endothelial injury, and systemic hypercoagulability. By identifying a mutation in 1 or more of the factors in the clotting pathway, an evaluation for inherited thrombophilia theoretically may unearth factors that drive systemic hypercoagulability and inform decision making so as to prevent future events.
Inherited thrombophilia refers to a genetic condition that predisposes to an increased risk of VTE.[3] Approximately 7% of the general population has inherited thrombophilia, which includes factor V Leiden (FVL) mutation, prothrombin 20210 mutation (PT20210), protein C deficiency, protein S deficiency, antithrombin III (ATIII) deficiency, and methylene tetrahydrofolate reductase mutation (MTHFR).[4] Of note, the definition does not include acquired etiologies, such as antiphospholipid antibody syndrome. Depending on the underlying condition and expression of the genetic abnormality, the relative risk of VTE in patients with inherited thrombophilia is 3‐ to 20‐fold greater than that of the general population.[5] Therefore, it is logical to consider that testing for inherited thrombophilia might be clinically useful. However, the evidence for doing so is very limited.
DOES INHERITED THROMBOPHILIA TESTING CHANGE MANAGEMENT?
An inherited thrombophilia evaluation is unlikely to affect management in most clinical settings. There is no current evidence to support primary prophylaxis[6] nor is there evidence that management of patients with recurrent VTE should be altered in the setting of inherited thrombophilia.
To date, no prospective trials have evaluated the efficacy of anticoagulant use for primary prevention of VTE in patients with inherited thrombophilia.[6] Given the limited evidence for thromboprophylaxis and risks of anticoagulation, primary prevention for patients with inherited thrombophilia that remain asymptomatic is not recommended by the current American College of Chest Physicians guidelines.[7, 8]
Similarly, in patients with a first VTE or recurrent VTE, diagnosis of inherited thrombophilia is often not associated with recurrent events, which suggests that other nongenetic factors may be just as important, if not more important, in determining the risk of recurrence.[9] Although no randomized controlled or controlled clinical trials have evaluated the effects of testing for inherited thrombophilia on recurrent VTE,[10, 11] several prospective studies have assessed risk factors for recurrence. Data from these studies suggest that recurrence rates after unprovoked VTE are only weakly correlated with inherited thrombophilia status.[12, 13] Rather, it is postulated that patients with recurrent VTE may exhibit a prothrombotic tendency regardless of underlying genetic predisposition. In this case, decisions regarding anticoagulation do not vary by thrombophilia status. Instead, thrombophilia testing may divert attention away from the management of more prevalent, potentially modifiable risk factors such as immobility, oral contraceptive use, or malignancy, all of which are associated with recurrent VTE.[14] These provoking factors are the most important determinants of the chance of VTE recurrence as well as the most significant factors to take into account when deciding duration of anticoagulation.
Christiansen et al. performed a prospective study evaluating the association between recurrent VTE and thrombophilia status. After following 474 patients with confirmed first episode VTE for a mean of 7.3 years, no statistically significant risk of VTE was found for patients with FVL (hazard ratio [HR]: 1.2, 95% confidence interval [CI]: 0.7‐1.9), PT20210 (HR: 0.7, 95% CI: 0.3‐2.0), or an anticoagulant (protein C, protein S or ATIII) deficiency (HR: 1.8, 95% CI: 0.9‐3.7).[15] Although unexplained VTE was statistically associated with VTE recurrence, heritable thrombophilia status was not.
In a systematic review and meta‐analysis investigating the association of FVL and PT20210 with recurrent VTE, Ho and colleagues found a statistically significant risk of recurrent VTE in patients with inherited thrombophilia due to FVL (odds ratio [OR]: 1.41, 95% CI: 1.14‐1.75) and PT20210 (OR: 1.72, 95% CI: 1.27‐2.31), and reported that at most, only up to 1 in 6 recurrent VTEs may be attributable to these mutations.[16] Based on this relatively modest effect, the authors question the utility of testing for inherited thrombophilia, as thrombophilia status is unlikely to warrant a change in type or duration of treatment.
Regardless of whether an underlying inherited thrombophilia is identified, patients with history of recurrent VTE are often candidates for long‐term anticoagulation. Testing for inherited thrombophilia in patients with prior VTE events will therefore not influence decisions regarding clinical management. Additionally, such testing may be confounded by ongoing disease or treatment (Table 1). For example, protein C, protein S antigen, and ATIII levels are low in the setting of acute VTE.[17, 18] Likewise, protein C and S (vitamin Kdependent proteins) will be low in the setting of anticoagulation with warfarin.[19] Moreover, ATIII activity and antigen levels are low in the setting of heparin use.[20] Lack of provider awareness regarding these interactions may have important negative consequences, including a spurious diagnosis of thrombophilia,[21, 22] unnecessary hematology consultation, and psychological distress to patients in the form of ongoing unwarranted testing or apprehension regarding recurrence.[23]
| Acute VTE | Anticoagulation With Warfarin | Anticoagulation With NOACs | Anticoagulation With Heparin/LMWH | |
|---|---|---|---|---|
| ||||
| FVL/PT20210/MTHFR gene mutations | No Impact | No Impact | No Impact | No Impact |
| Protein C* | Decreased | Decreased | No impact | No impact |
| Protein S* | Decreased | Decreased | No impact | No impact |
| ATIII activity | Decreased | Slight increase | Slight increase | Decreased |
| ATIII antigen | Decreased | Slight increase | Slight increase | Decreased |
Additionally, this expensive evaluation has estimated direct costs of $1100 to $2400 per thrombophilia panel based on estimation of charges billed by a large commercial laboratory.[24, 25] In 2014, over 280,000 claims were submitted under Medicare Part B across all care settings for a thrombophilia analysis including FVL, PT20210, and MTHFR gene mutations,[24] which would equate to between $300 million to $672 million.[26] Unfortunately, there have been no large‐scale trials to assess cost‐effectiveness. However, the Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group stated that cost‐effectiveness modeling studies in this area require updating with current VTE risk estimates but are suggestive that routine FVL/PT20210 testing is not cost‐effective.[27]
ARE THERE CIRCUMSTANCES IN WHICH INPATIENT INHERITED THROMBOPHILIA TESTING PROVES BENEFICIAL?
The evidence for when to test for inherited thrombophilia is very limited and is often based on individualized risk. The current EGAPP guidelines acknowledge this limitation, specifically noting that there is a paucity of data evaluating management or prophylaxis of patients with homozygous or compound heterozygous FVL or P20210 mutation, and a lack of data surrounding whether or not knowledge of thrombophilia mutation should affect anticoagulation treatment.[27] This is why an individualized approach is deemed necessary. For example, the decision to prescribe hormone replacement therapy in women with a family history of inherited thrombophilia may be better informed by testing prior to treatment. Similarly, pregnant women with a family history or personal history of VTE may also benefit from inherited thrombophilia testing, as this may influence antepartum or postpartum management.[28, 29] The National Institute for Health and Clinical Excellence (NICE) guidelines recommend consideration of testing for hereditary thrombophilia in patients with unprovoked VTE and a first‐degree relative with VTE, if stopping anticoagulation treatment is planned; however, these recommendations are based solely on Guideline Development Group's experience and opinion.[30] Regardless, testing for inherited thrombophilia has significant potential consequences. Patients at risk should meet with an outpatient hematologist and/or a genetic counselor, if available, to determine the risks and benefits of testing.
WHAT DO GUIDELINES SAY ABOUT INHERITED THROMBOPHILIA TESTING?
The most recent NICE guidelines recommend against offering inherited thrombophilia testing to patients presenting with a provoked VTE in any clinical setting.[30] In patients diagnosed with unprovoked VTE, testing should not be considered unless a first degree relative with a history of VTE exists.[30] The NICE guidelines also recommend against routinely offering thrombophilia testing to asymptomatic first‐degree relatives of patients with a history of VTE or known inherited thrombophilia. This recommendation is reflected in the American Society of Hematology's Choosing Wisely recommendations since 2013.[31] Further, The American College of Medical Genetics and Genomics' Choosing Wisely recommendations from 2015 state that MTHFR mutations should never be included in any thrombophilia workup, as recent meta‐analyses have disproven an association between the presence of these variants and venous thromboembolism.[32]
The EGAPP Working Group recommends against routine testing for FVL or PT20210 in patients who present with an idiopathic VTE, as longer‐term anticoagulation offers similar benefits to patients with or without these mutations.[27] EGAPP also recommends against testing asymptomatic adult family members of patients with VTE and/or an FVL or PT20210 mutation for the purpose of considering primary prophylactic anticoagulation. In these circumstances, it is felt that the potential risks of thrombophilia testing outweigh any potential benefits.
HOW SHOULD HOSPITALISTS APPROACH TESTING OF INHERITED THROMBOPHILIA?
The providers in our case presentation are challenged with determining whether inpatient thrombophilia evaluation will add value to the evaluation of patients with unprovoked VTE. The available evidence suggests that clinicians should avoid ordering thrombophilia testing for hospitalized patients with unprovoked VTE because (1) many thrombophilia tests are inaccurate in the setting of acute VTE and/or anticoagulation, (2) results of testing often do not influence management, (3) testing is not cost‐effective, (4) a positive test result may lead to unnecessary patient anxiety, and (5) testing may result in inappropriately prolonged anticoagulation courses or unnecessary involvement of inpatient consultants. For these reasons, the patient in our case presentation should not be tested for inherited thrombophilia. In patients with personal or family histories of recurrent thromboembolism, modifiable clinical risk factors should be addressed, as these are more likely to influence treatment decisions compared to genetic testing. Finally, patients may be referred to an outpatient hematologist or geneticist for individualized discussions of risks and benefits of testing for inherited thrombophilia.
CONCLUSION
Inpatient evaluation for inherited thrombophilia for VTE is not clinically useful, cost‐effective, or reliable in the setting of VTE. The result of such testing does not affect management of acute primary or recurrent VTE. Testing should only be considered using an individualized approach in the outpatient setting with appropriate genetic counseling.
Disclosure: Christopher M. Petrilli, MD, and Lauren Heidemann, MD, contributed equally to this work. The authors report no conflicts of interest.
Do you think this is a low‐value practice? Is this truly a Thing We Do for No Reason? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other Things We Do for No Reason topics by emailing TWDFNR@hospitalmedicine.org.
- , , , , , . Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158(6):585–593.
- , , , et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240–1245.
- , . Hereditary thrombophilia. Thromb J. 2006;4:15.
- , , , . Deep‐vein thrombosis. Lancet. 1999;353(9151):479–485.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S–e736S.
- , . Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. 2013;110(4):697–705.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–2485.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2009;(1):CD007069.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:CD007069.
- , , , . Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362(9383):523–526.
- , , , et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112(12):4432–4436.
- , . Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699–704.
- , , , , . Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352–2361.
- , , , . Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166(7):729–736.
- , , , . Relationship between protein C antigen and anticoagulant activity during oral anticoagulation and in selected disease states. J Clin Invest. 1986;77(2):416–425.
- , . Inherited antithrombin deficiency: a review. Haemophilia. 2008;14(6):1229–1239.
- , , , . Decline of proteins C and S and factors II, VII, IX and X during the initiation of warfarin therapy. Thromb Res. 1987;45(6):783–790.
- . Thrombophilia: common questions on laboratory assessment and management. Hematology Am Soc Hematol Educ Program. 2007:127–135.
- , , . Activated protein C resistance testing for factor V Leiden. Am J Hematol. 2014;89(12):1147–1150.
- , . Quantitation of human protein S in the plasma of normal and warfarin‐treated individuals by radioimmunoassay. Thromb Res. 1984;36(6):527–535.
- , , , . Social aspects of genetic testing for factor V Leiden mutation in healthy individuals and their importance for daily practice. Thromb Res. 2004;113(1):7–12.
- , . Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013–1020.
- , , . An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120–127.
- CodeMap. Available at: https://www.codemap.com. Accessed January 18, 2016.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67–76.
- , , , et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343(20):1439–1444.
- , , , et al. Frequency of pregnancy‐related venous thromboembolism in anticoagulant factor‐deficient women: implications for prophylaxis. Ann Intern Med. 1996;125(12):955–960.
- , , , ; Guideline Development Group. Management of venous thromboembolic diseases and the role of thrombophilia testing: summary of NICE guidance. BMJ. 2012;344:e3979.
- American Society of Hematology. Ten things physicians and patients should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐society‐of‐hematology. Published December 4, 2013. Accessed January 18, 2016.
- American College of Medical Genetics and Genomics. Five Things patients and providers should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐college‐of‐medical‐genetics‐and‐genomics. Published July 10, 2015. Accessed March 13, 2016.
- , , , , , . Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158(6):585–593.
- , , , et al. The clinical course of pulmonary embolism. N Engl J Med. 1992;326(19):1240–1245.
- , . Hereditary thrombophilia. Thromb J. 2006;4:15.
- , , , . Deep‐vein thrombosis. Lancet. 1999;353(9151):479–485.
- , , , et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8(11):2450–2457.
- , , , et al. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e691S–e736S.
- , . Testing for inherited thrombophilia and consequences for antithrombotic prophylaxis in patients with venous thromboembolism and their relatives. A review of the Guidelines from Scientific Societies and Working Groups. Thromb Haemost. 2013;110(4):697–705.
- , , , et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e195S–e226S.
- , , , et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301(23):2472–2485.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2009;(1):CD007069.
- , , , . Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev. 2012;12:CD007069.
- , , , . Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362(9383):523–526.
- , , , et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112(12):4432–4436.
- , . Testing for thrombophilia: an evidence‐based approach. Postgrad Med J. 2006;82(973):699–704.
- , , , , . Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352–2361.
- , , , . Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166(7):729–736.
- , , , . Relationship between protein C antigen and anticoagulant activity during oral anticoagulation and in selected disease states. J Clin Invest. 1986;77(2):416–425.
- , . Inherited antithrombin deficiency: a review. Haemophilia. 2008;14(6):1229–1239.
- , , , . Decline of proteins C and S and factors II, VII, IX and X during the initiation of warfarin therapy. Thromb Res. 1987;45(6):783–790.
- . Thrombophilia: common questions on laboratory assessment and management. Hematology Am Soc Hematol Educ Program. 2007:127–135.
- , , . Activated protein C resistance testing for factor V Leiden. Am J Hematol. 2014;89(12):1147–1150.
- , . Quantitation of human protein S in the plasma of normal and warfarin‐treated individuals by radioimmunoassay. Thromb Res. 1984;36(6):527–535.
- , , , . Social aspects of genetic testing for factor V Leiden mutation in healthy individuals and their importance for daily practice. Thromb Res. 2004;113(1):7–12.
- , . Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013–1020.
- , , . An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120–127.
- CodeMap. Available at: https://www.codemap.com. Accessed January 18, 2016.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: routine testing for Factor V Leiden (R506Q) and prothrombin (20210G>A) mutations in adults with a history of idiopathic venous thromboembolism and their adult family members. Genet Med. 2011;13(1):67–76.
- , , , et al. Safety of withholding heparin in pregnant women with a history of venous thromboembolism. Recurrence of Clot in This Pregnancy Study Group. N Engl J Med. 2000;343(20):1439–1444.
- , , , et al. Frequency of pregnancy‐related venous thromboembolism in anticoagulant factor‐deficient women: implications for prophylaxis. Ann Intern Med. 1996;125(12):955–960.
- , , , ; Guideline Development Group. Management of venous thromboembolic diseases and the role of thrombophilia testing: summary of NICE guidance. BMJ. 2012;344:e3979.
- American Society of Hematology. Ten things physicians and patients should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐society‐of‐hematology. Published December 4, 2013. Accessed January 18, 2016.
- American College of Medical Genetics and Genomics. Five Things patients and providers should question. Choosing Wisely website. Available at: http://www.choosingwisely.org/societies/american‐college‐of‐medical‐genetics‐and‐genomics. Published July 10, 2015. Accessed March 13, 2016.
© 2016 Society of Hospital Medicine
A3 to Improve STAT
STAT is an abbreviation of the Latin word statim, meaning immediately,[1] and has been a part of healthcare's lexicon for almost as long as there have been hospitals. STAT conveys a sense of urgency, compelling those who hear STAT to act quickly. Unfortunately, given the lack of a consistent understanding of STAT, the term in reality often has an alternate use: to hurry up or to complete sooner than routine, and is sometimes used to circumvent a system that is perceived to be too slow to accomplish a routine task in a timely manner.
As part of a larger systems redesign effort to improve patient safety and quality of care, an institutional review board (IRB)‐approved qualitative study was conducted on 2 medical‐surgical units in a US Department of Veterans Affairs (VA) hospital to explore communication patterns between physicians and nurses.[2] The study revealed wide variation in understanding between physicians and nurses on the ordering and administration of STAT medication. Physicians were unaware that when they placed a STAT order into the computerized patient record system (CPRS), nurses were not automatically alerted about the order. At this facility, nurses did not carry pagers. Although each unit had a supply of wireless telephones, they were often unreliable and therefore not used consistently. Nurses were required by policy to check the CPRS for new orders every 2 hours. This was an inefficient and possibly dangerous process,[3] because if a nurse was not expecting a STAT order, 2 hours could elapse before she or he saw the order in the CPRS and began to look for the medication. A follow‐up survey completed by physicians, nurses, pharmacists, and pharmacy technicians demonstrated stark differences on the definition of STAT and overlap with similar terms such as NOW and ASAP. Interviews with ordering providers indicated that 36% of the time a STAT was ordered it was not clinically urgent, but instead ordered STAT to speed up the process.
The STAT medication process was clearly in need of improvement, but previous quality improvement projects in our organization had varying degrees of success. For example, we used Lean methodology in an attempt to improve our discharge process. We conducted a modified rapid process discharge improvement workshop[4] structured in phases over 4 weeks. During the workshops, a strong emphasis remained on the solutions to the problem, and we were unable to help the team move from a mindset of fix it to create it. This limited the buy‐in of team members, the creativity of their ideas for improvement, and ultimately the momentum to improve the process.
In this article we describe our adaptation of A3 Thinking,[5, 6] a structure for guiding quality improvement based in Lean methodology, to improve the STAT medication process. We chose A3 Thinking for several reasons. A3 Thinking focuses on process improvement and thus aligned well with our interest in improving the STAT medication process. A3 Thinking also reveals otherwise hidden nonvalue‐added activities that should be eliminated.[7] Finally A3 Thinking reinforces a deeper understanding of the way the work is currently being done, providing critical information needed before making a change. This provides a tremendous opportunity to look at work differently and see opportunities for improvement.[8] Given these strengths as well as the lack of congruence between what the STAT process should consist of and how the STAT process was actually being used in our organization, A3 Thinking offered the best fit between an improvement process and the problem to be solved.
METHODS
A search of healthcare literature yielded very few studies on the STAT process.[9, 10] Only 1 intervention to improve the process was found, and this focused on a specific procedure.[10] An informal survey of local VA and non‐VA hospitals regarding their experiences with the STAT medication process revealed insufficient information to aid our efforts. We next searched the business and manufacturing literature and found examples of how the Lean methodology was successfully applied to other problems in healthcare, including improving pediatric surgery workflow and decreasing ventilator‐associated pneumonia.[11, 12]
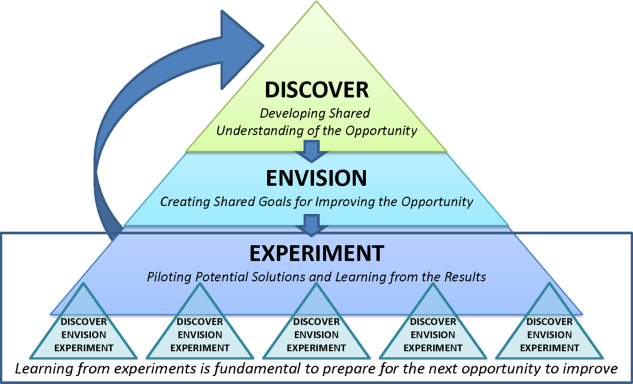
Therefore, the STAT project was structured to adapt a problem‐solving process commonly used in Lean organizationsA3 Thinkingwhich challenges team members to work through a discovery phase to develop a shared understanding of the process, an envisioning phase to conceptualize an ideal process experience, and finally an experimentation phase to identify and trial possible solutions through prioritization, iterative testing, structured reflection, and adjustment on resulting changes. Our application of the term experimentation in this context is distinct from that of controlled experimentation in clinical research; the term is intended to convey iterative learning as changes are tested, evaluated, and modified during this quality improvement project. Figure 1 displays a conceptual model of our adaptation of A3 Thinking. As this was a quality‐improvement project, it was exempt from IRB review.
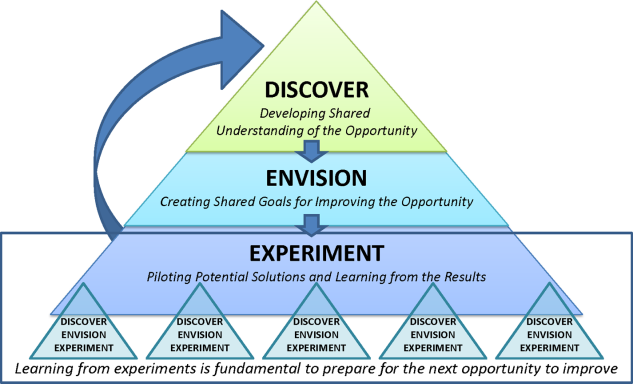
DISCOVERY
To begin the discovery phase, a workgroup consisting of representatives of all groups that had a role in the STAT process (ie, physician, pharmacist, nurse, pharmacy technician, clerk) gathered to identify the opportunity we are looking to address and learn from each other's individual experiences with the STAT medication process. The group was facilitated by an industrial engineer familiar with the A3 Thinking process. The team completed a mapping exercise to lay out, step‐by‐step, the current STAT medication process. This activity allowed the team to build shared empathy with others' experiences and to appreciate the challenges experienced by others through their individual responsibilities in the process. The current process was found to consist of 4 overarching components: a provider entered the STAT order into the CPRS; the order was verified by a pharmacist; a pharmacy technician delivered the medication to the unit (or a nurse retrieved the medication from the Omnicell (Omnicell Inc., Mountain View, CA), a proprietary automated medication dispensing system); and finally the nurse administered the medication to a patient.
A large, color‐coded flow map of the STAT medication process was constructed over several meetings to capture all perspectives and allow team members to gather feedback from their peers. To further our understanding of the current process, the team participated in a modified Go to the Gemba (ie, go to where the work is done)[13] on a real‐time STAT order. Once all workgroup members were satisfied that the flow map represented the current state of the STAT medication process, we came to a consensus on the goals needed to meet our main objective.
We agreed that our main objective was that STAT medication orders should be recognized, verified, and administered to patients in a timely and appropriate manner to ensure quality care. We identified 3 goals to meet this objective: (1) STAT should be consistently defined and understood by everyone; (2) an easy, intuitive STAT process should be available for all stakeholders; and (3) the STAT process should be transparent and ideally visual so that everyone involved can understand at which point in the process a specific STAT order is currently situated. We also identified additional information we would need to reach the goals.
Shortly after the process‐mapping sessions, 2 workgroup members conducted real‐time STAT order time studies to track medications from order to administration. Three time periods in the STAT process were identified for observation and measurement: the time from physician order entry in the CPRS to the time a pharmacist verified the medication, the time from verification to when the medication arrived on the nursing unit, and the time from arrival on the nursing unit to when that medication was administered. Using a data‐collection template, each time period was recorded, and 28 time studies were collected over 1 month. To monitor the progress of our initiatives, the time study was repeated 3 months into the project.
ENVISIONING
Following the discovery phase, the team was better equipped to identify the specific changes needed to achieve an improved process. The envisioning phase allowed the team freedom to imagine an ideal process barring any preconceived notion of constraints within the current process.
In 2 meetings we brainstormed as many improvement ideas as possible. To prioritize and focus our ideas, we developed a matrix (see Supporting Information, Appendix A, in the online version of this article), placing our ideas in 1 of 4 quadrants based on the anticipated effort to implement the change (x‐axis) and impact of making the change (y‐axis). The matrix helped us see that some ideas would be relatively simple to implement (eg, color‐coded bags for STAT medication delivery), whereas others would require more sophisticated efforts and involvement of other people (eg, monthly education sessions to resident physicians).
EXPERIMENTING
Experiments were conducted to meet each of the 3 goals identified above. The team used the outcomes of the prioritization exercise to identify initial experiments to test. To build momentum by showing progress and improvement with a few quick wins, the team began with low‐effort/high‐impact opportunities. Each experiment followed a standard Plan‐Do‐Study‐Act (PDSA) cycle to encourage reflection, learning, adaptation, and adjustment as a result of the experiential learning process.[5]
Goal 1: STAT Should Be Consistently Defined and Understood by Everyone
To address the first goal, a subgroup collected policies and procedures related to the STAT medication administration process. The policy defined a STAT medication as a medication that has the potential to significantly and negatively impact a patient's clinical condition if not given within 30 minutes. The group found that the policy requiring a 30‐minute time to administration was clinically appropriate, reinforcing our goals to create a practice congruent with the policy.
A subgroup led by the pharmacy department collected data related to STAT medications on the 3 medical‐surgical units. Within 1 month, 550 STAT medications were ordered, consisting of medications ranging from furosemide to nicotine lozenges, the latter being a medication clearly outside of the policy definition of STAT. The workgroup reviewed the information and realized education would be required to align practice with policy. According to our matrix, education was a high‐impact/high‐effort activity, so efforts were focused on the high‐impact/low‐effort activities initially. We addressed educational opportunities in later PDSA cycles.
Goal 2: An Easy, Intuitive STAT Process for All Stakeholders
The CPRS contains prefabricated templates that conform to regulatory requirements and ensure completeness. However, the CPRS does not intuitively enable ordering providers to choose the time for the first dose of a new routine medication. This often creates a situation where a provider orders the medication STAT, so that the medication can be given earlier than the CPRS would otherwise allow. Although there is a check box, Give additional dose now, it was not being used because it was visually obscure in the interface. The CPRS restricted our ability to change the template for ordering medications to include a specific time for first‐dose administration before defaulting to the routine order; thus, complementary countermeasures were trialed first. These are outlined in Table 1.
| Countermeasure | Intended Outcome |
|---|---|
| Remove duplicate dosing frequencies from medication order template | Reduce list of dosing frequencies to sort through to find desired selection |
| Develop 1‐page job aid for ordering providers to utilize | Assist in the correct methods of ordering STAT, NOW, and routine medications |
| Added STAT ONCE as a dosing frequency selection | Clarify the medication, if ordered STAT, will only be a 1‐time administration to avoid the recurrence of a STAT order should the orders be transferred to a new unit with the patient |
| Modify existing policies to add STAT ONCE option | Ensure documentation is congruent with new expectations |
| Educate interns and residents with the job aid and a hands‐on how to ordering exercise | Inform ordering physicians on the available references for ordering and educate according to desired practice |
| Provide interns and residents with a visual job aid at their workstation and a hands‐on how to ordering exercise | In addition to providing information and educating according to desired practice, provide a just‐in‐time reference resource |
Goal 3: The STAT Process Should Be Transparent and Ideally Visual
During the time studies, the time period from when the medication arrived on the unit to the time it was administered to the patient averaged 34 minutes. Of 28 STAT orders followed through the entire process, 5 pharmacy technicians (26%) were not informed of 19 STAT medication orders requiring delivery, and 12 nurses (63%) were not notified of the delivery of those 19 medications. The remaining 9 STAT medications were stocked in the Omnicell. Informal interviews with nurses and pharmacy technicians, as well as input from the nurses and pharmacy technicians in our workgroup, revealed several explanations for these findings.
First, the delivering technicians could not always find the patient's nurse, and because the delivery procedure was not standardized, there was no consistency between technicians in where medications were delivered. Second, each unit had a different medication inventory stored in the Omnicell, and the inventory was frequently changed (eg, due to unit‐specific needs, backorders), which made it difficult for nurses to keep track of what was available in Omnicell at any given time. Finally, the STAT medication was not consistently labeled with a visual STAT notation, so even if a nurse saw that new medications had been delivered, he or she would not be able to easily identify which was STAT. The team made several low‐tech process changes to improve the visibility of a STAT medication and ensure reliable communication upon delivery. A subgroup of pharmacists, technicians, and nurses developed and implemented the countermeasures described in Table 2.
| Countermeasure | Intended Outcome |
|---|---|
| Designate delivery preferences with the patient's nurse as the first preference and a set location in the med room as the only alternative preference | Attempt to deliver medications directly to the patient's nurse as frequently as possible to eliminate any unnecessary delays and avoid miscommunication |
| Identify a location in each unit's med room to place a red bin to deliver the STAT medications that are unable to be delivered to the patient's nurse directly | Provide 1 alternate location to retrieve STAT medications if the technician is unable to locate the patient's nurse to deliver the medication directly |
| Utilize a plastic bag with a red STAT indication for transportation of STAT medications to the units | Provide a visual to assist in pharmacy technicians prioritizing their deliveries to the inpatient units |
| Utilize red STAT magnets on the patient's door frame to signal nurses a medication had been delivered to the med room | Provide a visual to assist in timely recognition of a STAT medication delivery given the technician was unable to find the nurse to hand it off directly |
RESULTS
At the start of our project, the average time from STAT order to medication administration was 1 hour and 7 minutes (range, 6 minutes 2 hours and 22 minutes). As a result of the 2 sets of countermeasures outlined in Tables 1 and 2, the average total time from STAT order entry to administration decreased by 21% to an average of 53 minutes. The total time from medication delivery to administration decreased by 26% from 34 minutes to 25 minutes postimplementation. On average, 391 STAT medications were ordered per month during the project period, which represents a decrease of 9.5% from the 432 orders per month for the same time period the previous year. After implementing the countermeasures in Table 2, we followed another 26 STAT medications through the process to evaluate our efforts. Of 15 STAT medications requiring delivery, only 1 nurse (7%) was not notified of the delivery of a STAT medication, and 1 pharmacy technician (7%) was not informed the medication was STAT. The 151% increase in notification of nurses to delivery of a STAT medication suggests that use of the STAT bags, STAT magnets on patient doors, and whenever possible direct delivery of STAT medications to the nurse has improved communication between the technicians and nurses. Similarly, the 27% increase in technician awareness of a STAT designation suggests STAT is being better communicated to them. The improvement in awareness and notification of a STAT medication is summarized in Figure 2.
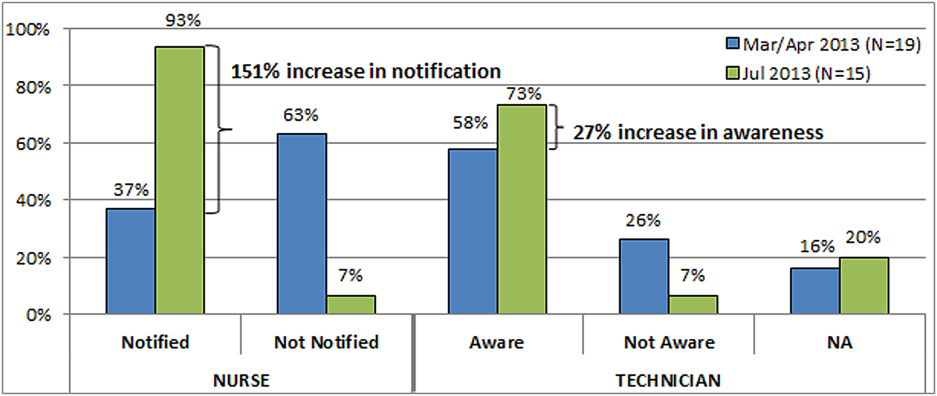
Due to time and financial constraints, the following limitations may have affected our findings. First, resident physicians were not directly represented in our discussions. Attending medicine hospitalists provided the physician perspective, which provides a biased view given their intimate knowledge of the CPRS and additional years of experience. Similarly, nurse perspectives were limited to staff and clinical nurse leaders. Last, our low‐cost approach was mandated by limited resources; a more resource‐rich environment may have devised alternative approaches.
CONCLUSIONS
Adapting A3 Thinking for process improvement was a low‐cost/low‐tech option for a VA facility. Having buy‐in from all levels was crucial to the success of the project. The size and diversity of the group was also very important, as different opinions and aspects of the process were represented. Cross‐discipline relationships and respect were formed, which will be valuable for collaboration in future projects. Although we focused on the STAT medication process, other quality‐improvement projects could also benefit from A3 Thinking. Moreover, there were enough people to serve as ambassadors, taking the project back to their work areas to share with their peers, gather consensus, and elicit additional feedback. The collaboration led to comprehensive understanding of the process, the nature of the problems within the process, and the complexity of solving the problem. For example, although the number of STAT orders did not decrease dramatically, we have learned from these experiments that we may need to change how we approach structuring additional experiments. Future work will focus on increasing communication between physicians and nurses when placing STAT medication orders, enhancing resident education to ensure appropriate use of the STAT designation, and continuing our efforts to improve the delivery process of STAT medications.
Other quality‐improvement methodologies we could have used include: total quality management (TQM), continuous quality improvement (CQI), business process redesign, Lean, Six Sigma, and others.[14] Differences between these can be broadly classified as putting an emphasis on people (eg, inclusion of front line staff in CQI or leadership in TQM) or on process (eg, understanding process function to reduce waste in Lean or statistical process control in Six Sigma).[14] Using A3 Thinking methodology was more useful than these others for the STAT medication process for some very important reasons. The A3 process not only led to a better understanding of the meaning of STAT across disciplines, increasing the intuitive nature, transparency and visual aspects of the whole process, but also promoted a collaborative, multidisciplinary, integrative culture, in which other hospital‐wide problems may be addressed in the future.
Acknowledgements
This work could not have been done without the contribution of all members of the STAT Improvement Workgroup, including Charles Alday; Allison Brenner, PharmD; Paula Carroll; Garry Davis; Michele Delaney, RN, MSN, CWCN; Mary East, MD; Stacy Frick, MSN, RN, CNL; Corry Gessner, CPhT; Kenya Harbin, MSN, RN, CNL; Crystal Heath, MS, RN‐BC; Tom Kerr, MPH; Diane Klemer, RPh; Diane Kohmescher, PharmD, BCPS; Sara Oberdick; Antanita Pickett; Ana Preda, CPhT; Joseph Pugh, RPh, MS; Gloria Salazar, CPhT; Samar Sheth, MD; Andrea Starnes, RN; Christine Wagner, PharmD; Leo Wallace; Roderick Williams; and Marilyn Woodruff.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability Grant and the Veterans in Partnership (VISN11) Healthcare Network. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the US Department of Veterans Affairs. The authors have no other disclosures or conflicts to report.
- The American Heritage Medical Dictionary of the English Language website. 2011. Available at: http://ahdictionary.com/word/search.html?q=STAT. Accessed December 22, 2013.
- , , , , , . The use of multiple qualitative methods to characterize communication events between physicians and nurses [published online ahead of print January 31, 2014]. Health Commun. doi: 10.1080/10410236.2013.835894.
- , , . Fifteen best practice recommendations for bar‐code medication administration in the Veterans Health Administration. Jt Comm J Qual Saf. 2004;30(7):355–365.
- , , , , . Going lean in health care. Cambridge, MA: Institute for Healthcare Improvement; 2005. Available at: http://www.ihi.org. Accessed March 19, 2014.
- , . Understanding A3 Thinking: A Critical Component of Toyota's PDCA Management System. New York, NY: Productivity Press, Taylor 2008.
- . Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor and Lead. Cambridge, MA: Lean Enterprise Institute; 2008.
- , , . Basics of quality improvement in health care. Mayo Clin Proc. 2007;82(6):735–739.
- , . A3 problem solving: unique features of the A3 problem solving method. Available at: http://leanhealthcarewest.com/Page/A3‐Problem‐Solving. Accessed March 27, 2014.
- , , . Evaluation of stat orders in a teaching hospital: a chart review. Clin Drug Investig. 2011;31(4):231–235.
- . Using STAT properly. Radiol Manage. 2006;28(1):26–30; quiz 31–33.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , , . Lean health care: what can hospitals learn from a world‐class automaker? J Hosp Med. 2006;1(3):191–199.
- . Gemba Kaizen: A Commonsense Approach to a Continuous Improvement Strategy. 2nd ed. New York, NY: McGraw‐Hill; 2012.
- . Pseudoinnovation: the development and spread of healthcare quality improvement methodologies. Int J Qual Health Care. 2009;21(3):153–159.
STAT is an abbreviation of the Latin word statim, meaning immediately,[1] and has been a part of healthcare's lexicon for almost as long as there have been hospitals. STAT conveys a sense of urgency, compelling those who hear STAT to act quickly. Unfortunately, given the lack of a consistent understanding of STAT, the term in reality often has an alternate use: to hurry up or to complete sooner than routine, and is sometimes used to circumvent a system that is perceived to be too slow to accomplish a routine task in a timely manner.
As part of a larger systems redesign effort to improve patient safety and quality of care, an institutional review board (IRB)‐approved qualitative study was conducted on 2 medical‐surgical units in a US Department of Veterans Affairs (VA) hospital to explore communication patterns between physicians and nurses.[2] The study revealed wide variation in understanding between physicians and nurses on the ordering and administration of STAT medication. Physicians were unaware that when they placed a STAT order into the computerized patient record system (CPRS), nurses were not automatically alerted about the order. At this facility, nurses did not carry pagers. Although each unit had a supply of wireless telephones, they were often unreliable and therefore not used consistently. Nurses were required by policy to check the CPRS for new orders every 2 hours. This was an inefficient and possibly dangerous process,[3] because if a nurse was not expecting a STAT order, 2 hours could elapse before she or he saw the order in the CPRS and began to look for the medication. A follow‐up survey completed by physicians, nurses, pharmacists, and pharmacy technicians demonstrated stark differences on the definition of STAT and overlap with similar terms such as NOW and ASAP. Interviews with ordering providers indicated that 36% of the time a STAT was ordered it was not clinically urgent, but instead ordered STAT to speed up the process.
The STAT medication process was clearly in need of improvement, but previous quality improvement projects in our organization had varying degrees of success. For example, we used Lean methodology in an attempt to improve our discharge process. We conducted a modified rapid process discharge improvement workshop[4] structured in phases over 4 weeks. During the workshops, a strong emphasis remained on the solutions to the problem, and we were unable to help the team move from a mindset of fix it to create it. This limited the buy‐in of team members, the creativity of their ideas for improvement, and ultimately the momentum to improve the process.
In this article we describe our adaptation of A3 Thinking,[5, 6] a structure for guiding quality improvement based in Lean methodology, to improve the STAT medication process. We chose A3 Thinking for several reasons. A3 Thinking focuses on process improvement and thus aligned well with our interest in improving the STAT medication process. A3 Thinking also reveals otherwise hidden nonvalue‐added activities that should be eliminated.[7] Finally A3 Thinking reinforces a deeper understanding of the way the work is currently being done, providing critical information needed before making a change. This provides a tremendous opportunity to look at work differently and see opportunities for improvement.[8] Given these strengths as well as the lack of congruence between what the STAT process should consist of and how the STAT process was actually being used in our organization, A3 Thinking offered the best fit between an improvement process and the problem to be solved.
METHODS
A search of healthcare literature yielded very few studies on the STAT process.[9, 10] Only 1 intervention to improve the process was found, and this focused on a specific procedure.[10] An informal survey of local VA and non‐VA hospitals regarding their experiences with the STAT medication process revealed insufficient information to aid our efforts. We next searched the business and manufacturing literature and found examples of how the Lean methodology was successfully applied to other problems in healthcare, including improving pediatric surgery workflow and decreasing ventilator‐associated pneumonia.[11, 12]
Therefore, the STAT project was structured to adapt a problem‐solving process commonly used in Lean organizationsA3 Thinkingwhich challenges team members to work through a discovery phase to develop a shared understanding of the process, an envisioning phase to conceptualize an ideal process experience, and finally an experimentation phase to identify and trial possible solutions through prioritization, iterative testing, structured reflection, and adjustment on resulting changes. Our application of the term experimentation in this context is distinct from that of controlled experimentation in clinical research; the term is intended to convey iterative learning as changes are tested, evaluated, and modified during this quality improvement project. Figure 1 displays a conceptual model of our adaptation of A3 Thinking. As this was a quality‐improvement project, it was exempt from IRB review.

DISCOVERY
To begin the discovery phase, a workgroup consisting of representatives of all groups that had a role in the STAT process (ie, physician, pharmacist, nurse, pharmacy technician, clerk) gathered to identify the opportunity we are looking to address and learn from each other's individual experiences with the STAT medication process. The group was facilitated by an industrial engineer familiar with the A3 Thinking process. The team completed a mapping exercise to lay out, step‐by‐step, the current STAT medication process. This activity allowed the team to build shared empathy with others' experiences and to appreciate the challenges experienced by others through their individual responsibilities in the process. The current process was found to consist of 4 overarching components: a provider entered the STAT order into the CPRS; the order was verified by a pharmacist; a pharmacy technician delivered the medication to the unit (or a nurse retrieved the medication from the Omnicell (Omnicell Inc., Mountain View, CA), a proprietary automated medication dispensing system); and finally the nurse administered the medication to a patient.
A large, color‐coded flow map of the STAT medication process was constructed over several meetings to capture all perspectives and allow team members to gather feedback from their peers. To further our understanding of the current process, the team participated in a modified Go to the Gemba (ie, go to where the work is done)[13] on a real‐time STAT order. Once all workgroup members were satisfied that the flow map represented the current state of the STAT medication process, we came to a consensus on the goals needed to meet our main objective.
We agreed that our main objective was that STAT medication orders should be recognized, verified, and administered to patients in a timely and appropriate manner to ensure quality care. We identified 3 goals to meet this objective: (1) STAT should be consistently defined and understood by everyone; (2) an easy, intuitive STAT process should be available for all stakeholders; and (3) the STAT process should be transparent and ideally visual so that everyone involved can understand at which point in the process a specific STAT order is currently situated. We also identified additional information we would need to reach the goals.
Shortly after the process‐mapping sessions, 2 workgroup members conducted real‐time STAT order time studies to track medications from order to administration. Three time periods in the STAT process were identified for observation and measurement: the time from physician order entry in the CPRS to the time a pharmacist verified the medication, the time from verification to when the medication arrived on the nursing unit, and the time from arrival on the nursing unit to when that medication was administered. Using a data‐collection template, each time period was recorded, and 28 time studies were collected over 1 month. To monitor the progress of our initiatives, the time study was repeated 3 months into the project.
ENVISIONING
Following the discovery phase, the team was better equipped to identify the specific changes needed to achieve an improved process. The envisioning phase allowed the team freedom to imagine an ideal process barring any preconceived notion of constraints within the current process.
In 2 meetings we brainstormed as many improvement ideas as possible. To prioritize and focus our ideas, we developed a matrix (see Supporting Information, Appendix A, in the online version of this article), placing our ideas in 1 of 4 quadrants based on the anticipated effort to implement the change (x‐axis) and impact of making the change (y‐axis). The matrix helped us see that some ideas would be relatively simple to implement (eg, color‐coded bags for STAT medication delivery), whereas others would require more sophisticated efforts and involvement of other people (eg, monthly education sessions to resident physicians).
EXPERIMENTING
Experiments were conducted to meet each of the 3 goals identified above. The team used the outcomes of the prioritization exercise to identify initial experiments to test. To build momentum by showing progress and improvement with a few quick wins, the team began with low‐effort/high‐impact opportunities. Each experiment followed a standard Plan‐Do‐Study‐Act (PDSA) cycle to encourage reflection, learning, adaptation, and adjustment as a result of the experiential learning process.[5]
Goal 1: STAT Should Be Consistently Defined and Understood by Everyone
To address the first goal, a subgroup collected policies and procedures related to the STAT medication administration process. The policy defined a STAT medication as a medication that has the potential to significantly and negatively impact a patient's clinical condition if not given within 30 minutes. The group found that the policy requiring a 30‐minute time to administration was clinically appropriate, reinforcing our goals to create a practice congruent with the policy.
A subgroup led by the pharmacy department collected data related to STAT medications on the 3 medical‐surgical units. Within 1 month, 550 STAT medications were ordered, consisting of medications ranging from furosemide to nicotine lozenges, the latter being a medication clearly outside of the policy definition of STAT. The workgroup reviewed the information and realized education would be required to align practice with policy. According to our matrix, education was a high‐impact/high‐effort activity, so efforts were focused on the high‐impact/low‐effort activities initially. We addressed educational opportunities in later PDSA cycles.
Goal 2: An Easy, Intuitive STAT Process for All Stakeholders
The CPRS contains prefabricated templates that conform to regulatory requirements and ensure completeness. However, the CPRS does not intuitively enable ordering providers to choose the time for the first dose of a new routine medication. This often creates a situation where a provider orders the medication STAT, so that the medication can be given earlier than the CPRS would otherwise allow. Although there is a check box, Give additional dose now, it was not being used because it was visually obscure in the interface. The CPRS restricted our ability to change the template for ordering medications to include a specific time for first‐dose administration before defaulting to the routine order; thus, complementary countermeasures were trialed first. These are outlined in Table 1.
| Countermeasure | Intended Outcome |
|---|---|
| Remove duplicate dosing frequencies from medication order template | Reduce list of dosing frequencies to sort through to find desired selection |
| Develop 1‐page job aid for ordering providers to utilize | Assist in the correct methods of ordering STAT, NOW, and routine medications |
| Added STAT ONCE as a dosing frequency selection | Clarify the medication, if ordered STAT, will only be a 1‐time administration to avoid the recurrence of a STAT order should the orders be transferred to a new unit with the patient |
| Modify existing policies to add STAT ONCE option | Ensure documentation is congruent with new expectations |
| Educate interns and residents with the job aid and a hands‐on how to ordering exercise | Inform ordering physicians on the available references for ordering and educate according to desired practice |
| Provide interns and residents with a visual job aid at their workstation and a hands‐on how to ordering exercise | In addition to providing information and educating according to desired practice, provide a just‐in‐time reference resource |
Goal 3: The STAT Process Should Be Transparent and Ideally Visual
During the time studies, the time period from when the medication arrived on the unit to the time it was administered to the patient averaged 34 minutes. Of 28 STAT orders followed through the entire process, 5 pharmacy technicians (26%) were not informed of 19 STAT medication orders requiring delivery, and 12 nurses (63%) were not notified of the delivery of those 19 medications. The remaining 9 STAT medications were stocked in the Omnicell. Informal interviews with nurses and pharmacy technicians, as well as input from the nurses and pharmacy technicians in our workgroup, revealed several explanations for these findings.
First, the delivering technicians could not always find the patient's nurse, and because the delivery procedure was not standardized, there was no consistency between technicians in where medications were delivered. Second, each unit had a different medication inventory stored in the Omnicell, and the inventory was frequently changed (eg, due to unit‐specific needs, backorders), which made it difficult for nurses to keep track of what was available in Omnicell at any given time. Finally, the STAT medication was not consistently labeled with a visual STAT notation, so even if a nurse saw that new medications had been delivered, he or she would not be able to easily identify which was STAT. The team made several low‐tech process changes to improve the visibility of a STAT medication and ensure reliable communication upon delivery. A subgroup of pharmacists, technicians, and nurses developed and implemented the countermeasures described in Table 2.
| Countermeasure | Intended Outcome |
|---|---|
| Designate delivery preferences with the patient's nurse as the first preference and a set location in the med room as the only alternative preference | Attempt to deliver medications directly to the patient's nurse as frequently as possible to eliminate any unnecessary delays and avoid miscommunication |
| Identify a location in each unit's med room to place a red bin to deliver the STAT medications that are unable to be delivered to the patient's nurse directly | Provide 1 alternate location to retrieve STAT medications if the technician is unable to locate the patient's nurse to deliver the medication directly |
| Utilize a plastic bag with a red STAT indication for transportation of STAT medications to the units | Provide a visual to assist in pharmacy technicians prioritizing their deliveries to the inpatient units |
| Utilize red STAT magnets on the patient's door frame to signal nurses a medication had been delivered to the med room | Provide a visual to assist in timely recognition of a STAT medication delivery given the technician was unable to find the nurse to hand it off directly |
RESULTS
At the start of our project, the average time from STAT order to medication administration was 1 hour and 7 minutes (range, 6 minutes 2 hours and 22 minutes). As a result of the 2 sets of countermeasures outlined in Tables 1 and 2, the average total time from STAT order entry to administration decreased by 21% to an average of 53 minutes. The total time from medication delivery to administration decreased by 26% from 34 minutes to 25 minutes postimplementation. On average, 391 STAT medications were ordered per month during the project period, which represents a decrease of 9.5% from the 432 orders per month for the same time period the previous year. After implementing the countermeasures in Table 2, we followed another 26 STAT medications through the process to evaluate our efforts. Of 15 STAT medications requiring delivery, only 1 nurse (7%) was not notified of the delivery of a STAT medication, and 1 pharmacy technician (7%) was not informed the medication was STAT. The 151% increase in notification of nurses to delivery of a STAT medication suggests that use of the STAT bags, STAT magnets on patient doors, and whenever possible direct delivery of STAT medications to the nurse has improved communication between the technicians and nurses. Similarly, the 27% increase in technician awareness of a STAT designation suggests STAT is being better communicated to them. The improvement in awareness and notification of a STAT medication is summarized in Figure 2.

Due to time and financial constraints, the following limitations may have affected our findings. First, resident physicians were not directly represented in our discussions. Attending medicine hospitalists provided the physician perspective, which provides a biased view given their intimate knowledge of the CPRS and additional years of experience. Similarly, nurse perspectives were limited to staff and clinical nurse leaders. Last, our low‐cost approach was mandated by limited resources; a more resource‐rich environment may have devised alternative approaches.
CONCLUSIONS
Adapting A3 Thinking for process improvement was a low‐cost/low‐tech option for a VA facility. Having buy‐in from all levels was crucial to the success of the project. The size and diversity of the group was also very important, as different opinions and aspects of the process were represented. Cross‐discipline relationships and respect were formed, which will be valuable for collaboration in future projects. Although we focused on the STAT medication process, other quality‐improvement projects could also benefit from A3 Thinking. Moreover, there were enough people to serve as ambassadors, taking the project back to their work areas to share with their peers, gather consensus, and elicit additional feedback. The collaboration led to comprehensive understanding of the process, the nature of the problems within the process, and the complexity of solving the problem. For example, although the number of STAT orders did not decrease dramatically, we have learned from these experiments that we may need to change how we approach structuring additional experiments. Future work will focus on increasing communication between physicians and nurses when placing STAT medication orders, enhancing resident education to ensure appropriate use of the STAT designation, and continuing our efforts to improve the delivery process of STAT medications.
Other quality‐improvement methodologies we could have used include: total quality management (TQM), continuous quality improvement (CQI), business process redesign, Lean, Six Sigma, and others.[14] Differences between these can be broadly classified as putting an emphasis on people (eg, inclusion of front line staff in CQI or leadership in TQM) or on process (eg, understanding process function to reduce waste in Lean or statistical process control in Six Sigma).[14] Using A3 Thinking methodology was more useful than these others for the STAT medication process for some very important reasons. The A3 process not only led to a better understanding of the meaning of STAT across disciplines, increasing the intuitive nature, transparency and visual aspects of the whole process, but also promoted a collaborative, multidisciplinary, integrative culture, in which other hospital‐wide problems may be addressed in the future.
Acknowledgements
This work could not have been done without the contribution of all members of the STAT Improvement Workgroup, including Charles Alday; Allison Brenner, PharmD; Paula Carroll; Garry Davis; Michele Delaney, RN, MSN, CWCN; Mary East, MD; Stacy Frick, MSN, RN, CNL; Corry Gessner, CPhT; Kenya Harbin, MSN, RN, CNL; Crystal Heath, MS, RN‐BC; Tom Kerr, MPH; Diane Klemer, RPh; Diane Kohmescher, PharmD, BCPS; Sara Oberdick; Antanita Pickett; Ana Preda, CPhT; Joseph Pugh, RPh, MS; Gloria Salazar, CPhT; Samar Sheth, MD; Andrea Starnes, RN; Christine Wagner, PharmD; Leo Wallace; Roderick Williams; and Marilyn Woodruff.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability Grant and the Veterans in Partnership (VISN11) Healthcare Network. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the US Department of Veterans Affairs. The authors have no other disclosures or conflicts to report.
STAT is an abbreviation of the Latin word statim, meaning immediately,[1] and has been a part of healthcare's lexicon for almost as long as there have been hospitals. STAT conveys a sense of urgency, compelling those who hear STAT to act quickly. Unfortunately, given the lack of a consistent understanding of STAT, the term in reality often has an alternate use: to hurry up or to complete sooner than routine, and is sometimes used to circumvent a system that is perceived to be too slow to accomplish a routine task in a timely manner.
As part of a larger systems redesign effort to improve patient safety and quality of care, an institutional review board (IRB)‐approved qualitative study was conducted on 2 medical‐surgical units in a US Department of Veterans Affairs (VA) hospital to explore communication patterns between physicians and nurses.[2] The study revealed wide variation in understanding between physicians and nurses on the ordering and administration of STAT medication. Physicians were unaware that when they placed a STAT order into the computerized patient record system (CPRS), nurses were not automatically alerted about the order. At this facility, nurses did not carry pagers. Although each unit had a supply of wireless telephones, they were often unreliable and therefore not used consistently. Nurses were required by policy to check the CPRS for new orders every 2 hours. This was an inefficient and possibly dangerous process,[3] because if a nurse was not expecting a STAT order, 2 hours could elapse before she or he saw the order in the CPRS and began to look for the medication. A follow‐up survey completed by physicians, nurses, pharmacists, and pharmacy technicians demonstrated stark differences on the definition of STAT and overlap with similar terms such as NOW and ASAP. Interviews with ordering providers indicated that 36% of the time a STAT was ordered it was not clinically urgent, but instead ordered STAT to speed up the process.
The STAT medication process was clearly in need of improvement, but previous quality improvement projects in our organization had varying degrees of success. For example, we used Lean methodology in an attempt to improve our discharge process. We conducted a modified rapid process discharge improvement workshop[4] structured in phases over 4 weeks. During the workshops, a strong emphasis remained on the solutions to the problem, and we were unable to help the team move from a mindset of fix it to create it. This limited the buy‐in of team members, the creativity of their ideas for improvement, and ultimately the momentum to improve the process.
In this article we describe our adaptation of A3 Thinking,[5, 6] a structure for guiding quality improvement based in Lean methodology, to improve the STAT medication process. We chose A3 Thinking for several reasons. A3 Thinking focuses on process improvement and thus aligned well with our interest in improving the STAT medication process. A3 Thinking also reveals otherwise hidden nonvalue‐added activities that should be eliminated.[7] Finally A3 Thinking reinforces a deeper understanding of the way the work is currently being done, providing critical information needed before making a change. This provides a tremendous opportunity to look at work differently and see opportunities for improvement.[8] Given these strengths as well as the lack of congruence between what the STAT process should consist of and how the STAT process was actually being used in our organization, A3 Thinking offered the best fit between an improvement process and the problem to be solved.
METHODS
A search of healthcare literature yielded very few studies on the STAT process.[9, 10] Only 1 intervention to improve the process was found, and this focused on a specific procedure.[10] An informal survey of local VA and non‐VA hospitals regarding their experiences with the STAT medication process revealed insufficient information to aid our efforts. We next searched the business and manufacturing literature and found examples of how the Lean methodology was successfully applied to other problems in healthcare, including improving pediatric surgery workflow and decreasing ventilator‐associated pneumonia.[11, 12]
Therefore, the STAT project was structured to adapt a problem‐solving process commonly used in Lean organizationsA3 Thinkingwhich challenges team members to work through a discovery phase to develop a shared understanding of the process, an envisioning phase to conceptualize an ideal process experience, and finally an experimentation phase to identify and trial possible solutions through prioritization, iterative testing, structured reflection, and adjustment on resulting changes. Our application of the term experimentation in this context is distinct from that of controlled experimentation in clinical research; the term is intended to convey iterative learning as changes are tested, evaluated, and modified during this quality improvement project. Figure 1 displays a conceptual model of our adaptation of A3 Thinking. As this was a quality‐improvement project, it was exempt from IRB review.

DISCOVERY
To begin the discovery phase, a workgroup consisting of representatives of all groups that had a role in the STAT process (ie, physician, pharmacist, nurse, pharmacy technician, clerk) gathered to identify the opportunity we are looking to address and learn from each other's individual experiences with the STAT medication process. The group was facilitated by an industrial engineer familiar with the A3 Thinking process. The team completed a mapping exercise to lay out, step‐by‐step, the current STAT medication process. This activity allowed the team to build shared empathy with others' experiences and to appreciate the challenges experienced by others through their individual responsibilities in the process. The current process was found to consist of 4 overarching components: a provider entered the STAT order into the CPRS; the order was verified by a pharmacist; a pharmacy technician delivered the medication to the unit (or a nurse retrieved the medication from the Omnicell (Omnicell Inc., Mountain View, CA), a proprietary automated medication dispensing system); and finally the nurse administered the medication to a patient.
A large, color‐coded flow map of the STAT medication process was constructed over several meetings to capture all perspectives and allow team members to gather feedback from their peers. To further our understanding of the current process, the team participated in a modified Go to the Gemba (ie, go to where the work is done)[13] on a real‐time STAT order. Once all workgroup members were satisfied that the flow map represented the current state of the STAT medication process, we came to a consensus on the goals needed to meet our main objective.
We agreed that our main objective was that STAT medication orders should be recognized, verified, and administered to patients in a timely and appropriate manner to ensure quality care. We identified 3 goals to meet this objective: (1) STAT should be consistently defined and understood by everyone; (2) an easy, intuitive STAT process should be available for all stakeholders; and (3) the STAT process should be transparent and ideally visual so that everyone involved can understand at which point in the process a specific STAT order is currently situated. We also identified additional information we would need to reach the goals.
Shortly after the process‐mapping sessions, 2 workgroup members conducted real‐time STAT order time studies to track medications from order to administration. Three time periods in the STAT process were identified for observation and measurement: the time from physician order entry in the CPRS to the time a pharmacist verified the medication, the time from verification to when the medication arrived on the nursing unit, and the time from arrival on the nursing unit to when that medication was administered. Using a data‐collection template, each time period was recorded, and 28 time studies were collected over 1 month. To monitor the progress of our initiatives, the time study was repeated 3 months into the project.
ENVISIONING
Following the discovery phase, the team was better equipped to identify the specific changes needed to achieve an improved process. The envisioning phase allowed the team freedom to imagine an ideal process barring any preconceived notion of constraints within the current process.
In 2 meetings we brainstormed as many improvement ideas as possible. To prioritize and focus our ideas, we developed a matrix (see Supporting Information, Appendix A, in the online version of this article), placing our ideas in 1 of 4 quadrants based on the anticipated effort to implement the change (x‐axis) and impact of making the change (y‐axis). The matrix helped us see that some ideas would be relatively simple to implement (eg, color‐coded bags for STAT medication delivery), whereas others would require more sophisticated efforts and involvement of other people (eg, monthly education sessions to resident physicians).
EXPERIMENTING
Experiments were conducted to meet each of the 3 goals identified above. The team used the outcomes of the prioritization exercise to identify initial experiments to test. To build momentum by showing progress and improvement with a few quick wins, the team began with low‐effort/high‐impact opportunities. Each experiment followed a standard Plan‐Do‐Study‐Act (PDSA) cycle to encourage reflection, learning, adaptation, and adjustment as a result of the experiential learning process.[5]
Goal 1: STAT Should Be Consistently Defined and Understood by Everyone
To address the first goal, a subgroup collected policies and procedures related to the STAT medication administration process. The policy defined a STAT medication as a medication that has the potential to significantly and negatively impact a patient's clinical condition if not given within 30 minutes. The group found that the policy requiring a 30‐minute time to administration was clinically appropriate, reinforcing our goals to create a practice congruent with the policy.
A subgroup led by the pharmacy department collected data related to STAT medications on the 3 medical‐surgical units. Within 1 month, 550 STAT medications were ordered, consisting of medications ranging from furosemide to nicotine lozenges, the latter being a medication clearly outside of the policy definition of STAT. The workgroup reviewed the information and realized education would be required to align practice with policy. According to our matrix, education was a high‐impact/high‐effort activity, so efforts were focused on the high‐impact/low‐effort activities initially. We addressed educational opportunities in later PDSA cycles.
Goal 2: An Easy, Intuitive STAT Process for All Stakeholders
The CPRS contains prefabricated templates that conform to regulatory requirements and ensure completeness. However, the CPRS does not intuitively enable ordering providers to choose the time for the first dose of a new routine medication. This often creates a situation where a provider orders the medication STAT, so that the medication can be given earlier than the CPRS would otherwise allow. Although there is a check box, Give additional dose now, it was not being used because it was visually obscure in the interface. The CPRS restricted our ability to change the template for ordering medications to include a specific time for first‐dose administration before defaulting to the routine order; thus, complementary countermeasures were trialed first. These are outlined in Table 1.
| Countermeasure | Intended Outcome |
|---|---|
| Remove duplicate dosing frequencies from medication order template | Reduce list of dosing frequencies to sort through to find desired selection |
| Develop 1‐page job aid for ordering providers to utilize | Assist in the correct methods of ordering STAT, NOW, and routine medications |
| Added STAT ONCE as a dosing frequency selection | Clarify the medication, if ordered STAT, will only be a 1‐time administration to avoid the recurrence of a STAT order should the orders be transferred to a new unit with the patient |
| Modify existing policies to add STAT ONCE option | Ensure documentation is congruent with new expectations |
| Educate interns and residents with the job aid and a hands‐on how to ordering exercise | Inform ordering physicians on the available references for ordering and educate according to desired practice |
| Provide interns and residents with a visual job aid at their workstation and a hands‐on how to ordering exercise | In addition to providing information and educating according to desired practice, provide a just‐in‐time reference resource |
Goal 3: The STAT Process Should Be Transparent and Ideally Visual
During the time studies, the time period from when the medication arrived on the unit to the time it was administered to the patient averaged 34 minutes. Of 28 STAT orders followed through the entire process, 5 pharmacy technicians (26%) were not informed of 19 STAT medication orders requiring delivery, and 12 nurses (63%) were not notified of the delivery of those 19 medications. The remaining 9 STAT medications were stocked in the Omnicell. Informal interviews with nurses and pharmacy technicians, as well as input from the nurses and pharmacy technicians in our workgroup, revealed several explanations for these findings.
First, the delivering technicians could not always find the patient's nurse, and because the delivery procedure was not standardized, there was no consistency between technicians in where medications were delivered. Second, each unit had a different medication inventory stored in the Omnicell, and the inventory was frequently changed (eg, due to unit‐specific needs, backorders), which made it difficult for nurses to keep track of what was available in Omnicell at any given time. Finally, the STAT medication was not consistently labeled with a visual STAT notation, so even if a nurse saw that new medications had been delivered, he or she would not be able to easily identify which was STAT. The team made several low‐tech process changes to improve the visibility of a STAT medication and ensure reliable communication upon delivery. A subgroup of pharmacists, technicians, and nurses developed and implemented the countermeasures described in Table 2.
| Countermeasure | Intended Outcome |
|---|---|
| Designate delivery preferences with the patient's nurse as the first preference and a set location in the med room as the only alternative preference | Attempt to deliver medications directly to the patient's nurse as frequently as possible to eliminate any unnecessary delays and avoid miscommunication |
| Identify a location in each unit's med room to place a red bin to deliver the STAT medications that are unable to be delivered to the patient's nurse directly | Provide 1 alternate location to retrieve STAT medications if the technician is unable to locate the patient's nurse to deliver the medication directly |
| Utilize a plastic bag with a red STAT indication for transportation of STAT medications to the units | Provide a visual to assist in pharmacy technicians prioritizing their deliveries to the inpatient units |
| Utilize red STAT magnets on the patient's door frame to signal nurses a medication had been delivered to the med room | Provide a visual to assist in timely recognition of a STAT medication delivery given the technician was unable to find the nurse to hand it off directly |
RESULTS
At the start of our project, the average time from STAT order to medication administration was 1 hour and 7 minutes (range, 6 minutes 2 hours and 22 minutes). As a result of the 2 sets of countermeasures outlined in Tables 1 and 2, the average total time from STAT order entry to administration decreased by 21% to an average of 53 minutes. The total time from medication delivery to administration decreased by 26% from 34 minutes to 25 minutes postimplementation. On average, 391 STAT medications were ordered per month during the project period, which represents a decrease of 9.5% from the 432 orders per month for the same time period the previous year. After implementing the countermeasures in Table 2, we followed another 26 STAT medications through the process to evaluate our efforts. Of 15 STAT medications requiring delivery, only 1 nurse (7%) was not notified of the delivery of a STAT medication, and 1 pharmacy technician (7%) was not informed the medication was STAT. The 151% increase in notification of nurses to delivery of a STAT medication suggests that use of the STAT bags, STAT magnets on patient doors, and whenever possible direct delivery of STAT medications to the nurse has improved communication between the technicians and nurses. Similarly, the 27% increase in technician awareness of a STAT designation suggests STAT is being better communicated to them. The improvement in awareness and notification of a STAT medication is summarized in Figure 2.

Due to time and financial constraints, the following limitations may have affected our findings. First, resident physicians were not directly represented in our discussions. Attending medicine hospitalists provided the physician perspective, which provides a biased view given their intimate knowledge of the CPRS and additional years of experience. Similarly, nurse perspectives were limited to staff and clinical nurse leaders. Last, our low‐cost approach was mandated by limited resources; a more resource‐rich environment may have devised alternative approaches.
CONCLUSIONS
Adapting A3 Thinking for process improvement was a low‐cost/low‐tech option for a VA facility. Having buy‐in from all levels was crucial to the success of the project. The size and diversity of the group was also very important, as different opinions and aspects of the process were represented. Cross‐discipline relationships and respect were formed, which will be valuable for collaboration in future projects. Although we focused on the STAT medication process, other quality‐improvement projects could also benefit from A3 Thinking. Moreover, there were enough people to serve as ambassadors, taking the project back to their work areas to share with their peers, gather consensus, and elicit additional feedback. The collaboration led to comprehensive understanding of the process, the nature of the problems within the process, and the complexity of solving the problem. For example, although the number of STAT orders did not decrease dramatically, we have learned from these experiments that we may need to change how we approach structuring additional experiments. Future work will focus on increasing communication between physicians and nurses when placing STAT medication orders, enhancing resident education to ensure appropriate use of the STAT designation, and continuing our efforts to improve the delivery process of STAT medications.
Other quality‐improvement methodologies we could have used include: total quality management (TQM), continuous quality improvement (CQI), business process redesign, Lean, Six Sigma, and others.[14] Differences between these can be broadly classified as putting an emphasis on people (eg, inclusion of front line staff in CQI or leadership in TQM) or on process (eg, understanding process function to reduce waste in Lean or statistical process control in Six Sigma).[14] Using A3 Thinking methodology was more useful than these others for the STAT medication process for some very important reasons. The A3 process not only led to a better understanding of the meaning of STAT across disciplines, increasing the intuitive nature, transparency and visual aspects of the whole process, but also promoted a collaborative, multidisciplinary, integrative culture, in which other hospital‐wide problems may be addressed in the future.
Acknowledgements
This work could not have been done without the contribution of all members of the STAT Improvement Workgroup, including Charles Alday; Allison Brenner, PharmD; Paula Carroll; Garry Davis; Michele Delaney, RN, MSN, CWCN; Mary East, MD; Stacy Frick, MSN, RN, CNL; Corry Gessner, CPhT; Kenya Harbin, MSN, RN, CNL; Crystal Heath, MS, RN‐BC; Tom Kerr, MPH; Diane Klemer, RPh; Diane Kohmescher, PharmD, BCPS; Sara Oberdick; Antanita Pickett; Ana Preda, CPhT; Joseph Pugh, RPh, MS; Gloria Salazar, CPhT; Samar Sheth, MD; Andrea Starnes, RN; Christine Wagner, PharmD; Leo Wallace; Roderick Williams; and Marilyn Woodruff.
Disclosures: This work was funded by a US Department of Veterans Affairs, Office of Systems Redesign Improvement Capability Grant and the Veterans in Partnership (VISN11) Healthcare Network. The findings and conclusions in this report are those of the authors and do not necessarily represent the position or policy of the US Department of Veterans Affairs. The authors have no other disclosures or conflicts to report.
- The American Heritage Medical Dictionary of the English Language website. 2011. Available at: http://ahdictionary.com/word/search.html?q=STAT. Accessed December 22, 2013.
- , , , , , . The use of multiple qualitative methods to characterize communication events between physicians and nurses [published online ahead of print January 31, 2014]. Health Commun. doi: 10.1080/10410236.2013.835894.
- , , . Fifteen best practice recommendations for bar‐code medication administration in the Veterans Health Administration. Jt Comm J Qual Saf. 2004;30(7):355–365.
- , , , , . Going lean in health care. Cambridge, MA: Institute for Healthcare Improvement; 2005. Available at: http://www.ihi.org. Accessed March 19, 2014.
- , . Understanding A3 Thinking: A Critical Component of Toyota's PDCA Management System. New York, NY: Productivity Press, Taylor 2008.
- . Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor and Lead. Cambridge, MA: Lean Enterprise Institute; 2008.
- , , . Basics of quality improvement in health care. Mayo Clin Proc. 2007;82(6):735–739.
- , . A3 problem solving: unique features of the A3 problem solving method. Available at: http://leanhealthcarewest.com/Page/A3‐Problem‐Solving. Accessed March 27, 2014.
- , , . Evaluation of stat orders in a teaching hospital: a chart review. Clin Drug Investig. 2011;31(4):231–235.
- . Using STAT properly. Radiol Manage. 2006;28(1):26–30; quiz 31–33.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , , . Lean health care: what can hospitals learn from a world‐class automaker? J Hosp Med. 2006;1(3):191–199.
- . Gemba Kaizen: A Commonsense Approach to a Continuous Improvement Strategy. 2nd ed. New York, NY: McGraw‐Hill; 2012.
- . Pseudoinnovation: the development and spread of healthcare quality improvement methodologies. Int J Qual Health Care. 2009;21(3):153–159.
- The American Heritage Medical Dictionary of the English Language website. 2011. Available at: http://ahdictionary.com/word/search.html?q=STAT. Accessed December 22, 2013.
- , , , , , . The use of multiple qualitative methods to characterize communication events between physicians and nurses [published online ahead of print January 31, 2014]. Health Commun. doi: 10.1080/10410236.2013.835894.
- , , . Fifteen best practice recommendations for bar‐code medication administration in the Veterans Health Administration. Jt Comm J Qual Saf. 2004;30(7):355–365.
- , , , , . Going lean in health care. Cambridge, MA: Institute for Healthcare Improvement; 2005. Available at: http://www.ihi.org. Accessed March 19, 2014.
- , . Understanding A3 Thinking: A Critical Component of Toyota's PDCA Management System. New York, NY: Productivity Press, Taylor 2008.
- . Managing to Learn: Using the A3 Management Process to Solve Problems, Gain Agreement, Mentor and Lead. Cambridge, MA: Lean Enterprise Institute; 2008.
- , , . Basics of quality improvement in health care. Mayo Clin Proc. 2007;82(6):735–739.
- , . A3 problem solving: unique features of the A3 problem solving method. Available at: http://leanhealthcarewest.com/Page/A3‐Problem‐Solving. Accessed March 27, 2014.
- , , . Evaluation of stat orders in a teaching hospital: a chart review. Clin Drug Investig. 2011;31(4):231–235.
- . Using STAT properly. Radiol Manage. 2006;28(1):26–30; quiz 31–33.
- , . The promise of Lean in health care. Mayo Clin Proc. 2013;88(1):74–82.
- , , , . Lean health care: what can hospitals learn from a world‐class automaker? J Hosp Med. 2006;1(3):191–199.
- . Gemba Kaizen: A Commonsense Approach to a Continuous Improvement Strategy. 2nd ed. New York, NY: McGraw‐Hill; 2012.
- . Pseudoinnovation: the development and spread of healthcare quality improvement methodologies. Int J Qual Health Care. 2009;21(3):153–159.
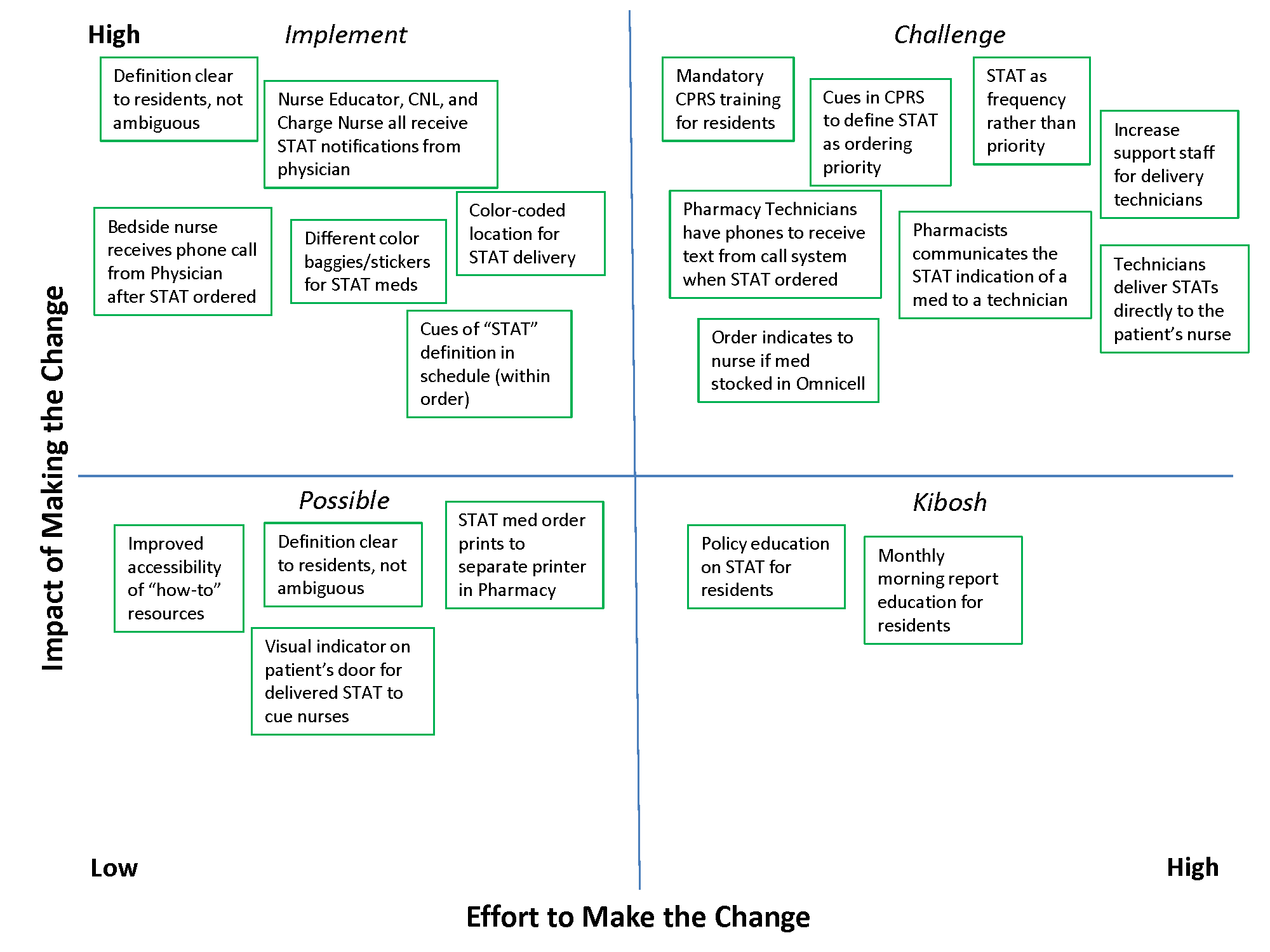
How Should a Patient with Cocaine-Associated Chest Pain be Treated?
Case
A 38-year-old man with a history of tobacco use presents to the emergency department complaining of constant substernal chest pain for three hours. His temperature is 37.7°C, his heart rate is 110 beats per minute, and his blood pressure is 155/95 mmHg. He appears anxious and diaphoretic but examination is otherwise unremarkable. He admits to cocaine use one hour before the onset of symptoms. What are the appropriate treatments for his condition?
Overview
Cocaine is the second-most-commonly used illicit drug in the U.S. and represents 31% of all ED visits related to substance abuse.1,2 According to recent survey results, 2.1 million people report recent cocaine use, and 1.6 million engage in cocaine abuse or dependence.2 Acute cardiopulmonary complaints are common in individuals who present to the ED after cocaine use, with chest pain being the most frequently reported symptom in 40%.3
Numerous etiologies for cocaine-associated chest pain (CACP) have been discovered, including musculoskeletal pain, pulmonary hypertension, cardiomyopathy, arrhythmias, and endocarditis.4 Only 0.5% of patients with aortic dissection over a four-year period had a recent history of cocaine use, making cocaine a rare cause of a rare condition.5 Cardiac chest pain remains the most frequent underlying etiology, resulting in the most common complication of myocardial infarction (MI) in up to 6% of patients.6,7
The ways in which cocaine use can cause myocardial ischemia and MI are multifactorial. A vigorous central sympathomimetic effect, coronary artery vasoconstriction, stimulation of platelets, and enhanced atherosclerosis all lead to a myocardial oxygen supply-demand imbalance.8 Other key interactions in the cardiovascular system are displayed in Figure 1. Understanding the role of these mechanisms in CACP is crucial to patient care.
Clinician goals in the management of CACP are to rapidly and accurately exclude life-threatening etiologies; assess the need for urgent acute coronary syndrome (ACS) evaluation; risk-stratify patients and ensure appropriate disposition; normalize the toxic effects of cocaine; treat resultant organ damage; and prevent long-term complications. An algorithm detailing this approach is provided in Figure 2.
Review of the Data
Diagnostic evaluation. Given potential differences in treatment regimens, it is imperative to differentiate patients who present with CACP from those whose chest pain is not associated with cocaine either by direct questioning or by screening of urine for cocaine metabolites. Once the presence of cocaine has been confirmed, guideline-based evaluation for potential ACS with serial electrocardiograms (ECG), cardiac biomarkers, and close monitoring of cardiac rhythms and hemodynamics is largely similar to standard management of all patients presenting with chest pain, with a few caveats.
Interpretation of the ECG can be challenging in the setting of cocaine. Studies have shown “abnormal” ECGs in 56% to 84% of patients, with many representing early repolarization or left ventricular hypertrophy.9,10 Likewise, patients with MI are as likely to present with normal or nonspecific ECG findings as with ischemic findings.7,11 ECG interpretation to diagnose ischemia or infarction in patients with CACP yields a sensitivity of 36% and specificity of 90%.7
Creatine kinase (CK), CK-MB fraction, and myoglobin have low specificity for the diagnosis of ischemia, as cocaine can induce skeletal muscle injury and rhabdomyolysis.9,12 Cardiac troponins demonstrate a superior specificity compared to CK and CK-MB and are thus the preferred cardiac biomarkers in diagnosing cocaine-associated MI.12
Initial management and disposition. Patients at high risk for cardiovascular events are generally admitted to a monitored bed.13 Immediate reperfusion therapy with primary percutaneous coronary intervention is recommended in patients with ST-elevation MI (STEMI). Treatment with thrombolytic agents is associated with an increased risk of intracerebral hemorrhage and lacks documented efficacy in patients with CACP. Thrombolysis should therefore only be utilized if the diagnosis of STEMI is unequivocal and an experienced cardiac catheterization laboratory is unavailable.14,15
Patients with unstable angina (UA) or non-ST-elevation MI (NSTEMI) are at higher risk for further cardiac events in a similar manner to those with ACS unrelated to cocaine. These cases might benefit from early cardiac catheterization and revascularization.16 Because of the increased risk of stent thrombosis in cocaine-users, thought to be due to recidivism, a detailed risk-benefit analysis should be undertaken prior to the implantation of cardiac stents.
Other diagnostic tests, such as stress testing and myocardial imaging, have not shown significant accuracy in diagnosing MI in this setting; moreover, these patients are at low overall risk for cardiac events and mortality. Consequently, an extensive diagnostic evaluation might not be cost-effective.7,10,13,17 Patients who have CACP without MI have a very low frequency of delayed complications.3,17 As such, cost-effective evaluation strategies, such as nine- or 12-hour observation periods in a chest pain unit, are appropriate for many of these low- to moderate-risk patients.13 For all CACP patients, the most critical post-discharge interventions are cardiac risk modification and cocaine cessation.13
Normalizing the toxic effects of cocaine with medications.
Aspirin: While no specific study has been performed in patients with CACP and aspirin, CACP guidelines, based on data supporting ACS guidelines for all patients, recommend administration of full-dose aspirin given its associated reduction in morbidity and mortality.18,19 Furthermore, given the platelet-stimulating effects of cocaine, using aspirin in this setting seems very reasonable.
Benzodiazepines: CACP guidelines support the use of benzodiazepines early in management to indirectly combat the agitation, hypertension, and tachycardia resulting from the stimulatory effects of cocaine.18,20 These recommendations are based on several animal and human studies that demonstrate significant reduction in heart rate and systemic arterial pressure with the use of these agents.21,22
Nitroglycerin: Cardiac catheterization studies have shown reversal of vasoconstriction with administration of nitroglycerin. One study demonstrated a benefit of the drug in 49% of participants.23 Additional investigation into the benefit of benzodiazepine and nitroglycerin combination therapy revealed mixed results. In one study, lorazepam plus nitroglycerin was found to be more efficacious than nitroglycerin alone.24 In another, however, use of diazepam in combination with nitroglycerin did not show benefit when evaluating pain relief, cardiac dynamics, and left ventricular function.25
Phentolamine: Phentolamine administration has been studied much less in the literature. This nonselective alpha-adrenergic antagonist exerts a dose-dependent reversal of cocaine’s vasoconstrictive properties in monkeys and humans.26,27 International guidelines for Emergency Cardiovascular Care recommend its use in treatment of cocaine-associated ACS;27 however, the AHA recommends it less strongly.18
Calcium channel blockers: Calcium channel blockers (CCBs) have not shown promise as first-line agents. While catheterization studies demonstrate the vasodilatory properties of verapamil, larger studies looking at all-cause mortality conclude that CCBs might worsen mortality rates,28 and animal studies indicate an increased risk of seizures.29 At this time, CCBs are recommended only if cardiac symptoms continue after both benzodiazepines and nitroglycerin are administered.18
The beta-blocker controversy: The use of beta-blockers in patients with CACP remains controversial given the theoretical risk of unopposed alpha-adrenergic activation. Coronary vasospasm, decreased myocardial oxygen delivery, and increased systemic vascular resistance can result from their use.30
Propranolol, a nonselective beta-blocker, was shown in catheterization studies to potentiate the coronary vasoconstriction of cocaine.31 Labetalol, a combined alpha/beta-blocker, reduced mean arterial pressure after cocaine administration during cardiac catheterization but did not reverse coronary vasoconstriction.32 This was attributed to the predominating beta greater than alpha blockade at doses administered. The selective beta-1 antagonists esmolol and metoprolol have shown no benefit in CACP.33 Carvedilol, a combined alpha/beta-blocker with both peripheral and central nervous system activity, has potential to attenuate both physiologic and behavioral response to cocaine, but it has not been well studied in this patient subset.34
The 2005 ACC/AHA STEMI guidelines recommended against beta-blockers in the setting of STEMI precipitated by cocaine use due to the potential of exacerbating coronary vasoconstriction.35 The 2007 ACC/AHA UA/NSTEMI guidelines stated that the use of a combined alpha/beta-blocker in patients with cocaine-induced ACS may be reasonable for patients with hypertension or tachycardia if pre-treated with a vasodilator.19 The 2008 ACC/AHA guidelines on the management of cocaine-related chest pain and MI recommended against the use of beta-blockers in the acute setting given the low incidence of cocaine-related MI and death.18
In a more recent study, Dattilo et al showed that beta-blockers administered to patients admitted with positive urine toxicology for cocaine significantly reduced MI and in-hospital mortality. Reduction of MI was of borderline significance in those admitted with a chief complaint of chest pain.36 Limitations of this study include unknown time of cocaine ingestion, lack of follow-up on discharge mortality, and a small sample size of 348 patients lacking statistical power.
Another retrospective cohort study examined patients admitted with chest pain and urine toxicology positive for cocaine and found that beta-blocker administration during hospitalization was not associated with increased incident mortality. Further, after a mean follow-up of 2.5 years, there was a statistically significant decrease in cardiovascular death.37 Drawbacks of this study included an older patient population, greater proportion of coronary artery disease, and higher follow-up of cardiovascular mortality rates than in previous studies, suggesting this subset might have received greater benefit from beta-blockers as a result of these characteristics.
The 2008 ACC/AHA guidelines instruct individualized consideration of the risk/benefit ratio for beta-blocker use in patients with CACP given the high rate of recidivism in cocaine abusers. The strongest indication is given to those with documented MI, left ventricular systolic dysfunction, or ventricular arrhythmias.18
It is important to note that these recommendations are based on cardiac catheterization laboratory studies, case reports, retrospective analyses, and animal experiments. No prospective controlled trials evaluating the role of beta-blockers in CACP and MI exist, and no trials regarding therapies to improve outcomes of patients sustaining a cocaine-associated MI have been reported.18
Back to the Case
This patient was experiencing cocaine-associated chest pain, which was confirmed with positive urine toxicology. Initial diagnostic workup with basic laboratory studies and cardiac biomarkers showed mild elevation in CK with troponin levels within normal limits. His ECG showed changes consistent with left ventricular hypertrophy. Chest radiograph was unremarkable.
He received aspirin, benzodiazepines, and nitroglycerin with normalization of vital signs, as well as subjective improvement in chest pain and anxiety. He was deemed to be at low risk for potential cardiac complications; thus, further cardiac testing was not pursued. Rather, he was admitted to an overnight observation unit with telemetry monitoring, where his chest pain did not recur.
He was seen in consultation with social work staff who arranged for drug abuse counseling after discharge. Given the uncertainty of relapse to cocaine use, as well as lack of known cardiac risk factors, he was not discharged on any new medications.
Bottom Line
The treatment of CACP includes normalizing the toxic systemic effects of the drug and minimizing the direct ischemic damage to the myocardium. Management varies slightly from traditional chest pain algorithms and includes benzodiazepines as well as antiplatelet agents and vasodilators to achieve this goal. Initial therapy with beta-blockers remains undefined and is largely discouraged in the acute setting. The role of beta-blockade upon discharge, however, can be beneficial in specific populations, especially those found to have underlying coronary disease.
Dr. Houchens and Dr. Czarnik are clinical instructors and Dr. Mack is a clinical lecturer at the University of Michigan Health System in Ann Arbor.
References
- Hughes A, Sathe N, Spagnola K. State Estimates of Substance Use from the 2005-2006 National Surveys on Drug Use and Health. DHHS Publication No. SMA 08-4311, NSDUH Series H-33. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2008.
- Volkow ND. Cocaine: Abuse and Addiction. National Institute on Drug Abuse. Washington, DC: U.S. Department of Health and Human Services; 2009.
- Brody SL, Slovis CM, Wrenn KD. Cocaine-related medical problems: consecutive series of 233 patients. Am J Med. 1990;88:325-331.
- Levis JT, Garmel GM. Cocaine-associated chest pain. Emerg Med Clin North Am. 2005;23:1083-1103.
- Eagle KA, Isselbacher EM, DeSanctis RW. Cocaine-related aortic dissection in perspective. Circulation. 2002;105:1529-1530.
- Feldman JA, Fish SS, Beshansky JR, Griffith JL, Woolard RH, Selker HP. Acute cardiac ischemia in patients with cocaine-associated complaints: results of a multicenter trial. Ann Emerg Med. 2000;36:469-476.
- Hollander JE, Hoffman RS, Gennis P, et al. Prospective multicenter evaluation of cocaine associated chest pain. Cocaine Associated Chest Pain (COCHPA) Study Group. Acad Emerg Med. 1994;1:330-339.
- Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122:2558-2569.
- Gitter MJ, Goldsmith SR, Dunbar DN, et al. Cocaine and chest pain: clinical features and outcomes of patients hospitalized to rule out myocardial infarction. Ann Intern Med. 1991;115:277-282.
- Amin M, Gabelman G, Karpel J, et al. Acute myocardial infarction and chest pain syndromes after cocaine use. Am J Cardiol. 1990;66:1434-1437.
- Tokarski GF, Paganussi P, Urbanski R, et al. An evaluation of cocaine-induced chest pain. Ann Emerg Med. 1990;19:1088-1092.
- Hollander JE, Levitt MA, Young GP, Briglia E, Wetli CV, Gawad Y. Effect of recent cocaine use on the specificity of cardiac markers for diagnosis of acute myocardial infarction. Am Heart J. 1998;135(2 Pt 1):245-252.
- Weber JE, Shofer FS, Larkin GL, Kalaria AS, Hollander JE. Validation of a brief observation period for patients with cocaine-associated chest pain. N Engl J Med. 2003;348:510-517.
- Hahn IH, Hoffman RS. Diagnosis and treatment of acute myocardial infarction: cocaine use and acute myocardial infarction. Emerg Med Clin North Am. 2001;19(2):1-18.
- Hoffman RS, Hollander JE. Evaluation of patients with chest pain after cocaine use. Crit Care Clin. 1997;13:809-828. Cannon CP, Weintraub WS, Demopoulos LA, et al.
- Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879-1887.
- Hollander JE, Hoffman RS. Cocaine-induced myocardial infarction: an analysis and review of the literature. J Emerg Med. 1992;10:169-177.
- McCord J, Jneid H, Hollander JE, et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117:1897-1907.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:E1-E157.
- Hollander JE. Management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
- Brubacher JR, Hoffman RS. Cocaine toxicity. Top Emerg Med. 1997;19(4):1-16.
- Catavas JD, Waters IW. Acute cocaine intoxication in the conscious dog: studies on the mechanism of lethality. J Pharmacol Exp Ther. 1981;217:350-356.
- Hollander JE, Hoffman RS, Gennis P, et al. Nitroglycerin in the treatment of cocaine associated chest pain—clinical safety and efficacy. J Toxicol Clin Toxicol. 1994;32(3): 243-256.
- Honderick T, Williams D, Seaberg D, Wears R. A prospective, randomized, controlled trial of benzodiazepines and nitroglycerin or nitroglycerin alone in the treatment of cocaine-associated acute coronary syndromes. Am J Emerg Med. 2003;21(1):39-42.
- Baumann BM, Perrone J, Hornig SE, Shofer FS, Hollander JE. Randomized, double-blind, placebo-controlled trial of diazepam, nitroglycerin, or both for treatment of patients with potential cocaine-associated acute coronary syndromes. Acad Emerg Med. 2000;7:878-885.
- Schindler CW, Tella SR, Goldberg SR. Adrenoceptor mechanisms in the cardiovascular effects of cocaine in conscious squirrel monkeys. Life Sci. 1992;51(9):653-660.
- Lange RA, Cigarroa RG, Yancy CW Jr., et al. Cocaine-induced coronary-artery vasoconstriction. N Engl J Med. 1989;321(23):1557-1562.
- Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326-1331.
- Derlet RW, Albertson TE. Potentiation of cocaine toxicity with calcium channel blockers. Am J Emerg Med. 1989;7:464-468.
- Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351-358.
- Lange RA, Cigarroa RG, Flores ED, et al. Potentiation of cocaine-induced coronary vasoconstriction by beta-adrenergic blockade. Ann Intern Med. 1990;112:897-903.
- Boehrer JD, Moliterno DJ, Willard JE, Hillis LD, Lange RA. Influence of labetalol on cocaine-induced coronary vasoconstriction in humans. Am J Med. 1993;94:608-610.
- Sand IC, Brody SL, Wrenn KD, Slovis CM. Experience with esmolol for the treatment of cocaine-associated cardiovascular complications. Am J Emerg Med. 1991;9:161-163.
- Sofuoglo M, Brown S, Babb DA, Pentel PR, Hatsukami DK. Carvedilol affects the physiological and behavioral response to smoked cocaine in humans. Drug Alcohol Depend. 2000;60:69-76.
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force of Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:E1-E211.
- Dattilo PB, Hailpern SM, Fearon K, Sohal D, Nordin C. Beta-blockers are associated with reduced risk of myocardial infarction after cocaine use. Ann Emerg Med. 2008;51:117-125.
- Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med. 2010;170:874-879.
Case
A 38-year-old man with a history of tobacco use presents to the emergency department complaining of constant substernal chest pain for three hours. His temperature is 37.7°C, his heart rate is 110 beats per minute, and his blood pressure is 155/95 mmHg. He appears anxious and diaphoretic but examination is otherwise unremarkable. He admits to cocaine use one hour before the onset of symptoms. What are the appropriate treatments for his condition?
Overview
Cocaine is the second-most-commonly used illicit drug in the U.S. and represents 31% of all ED visits related to substance abuse.1,2 According to recent survey results, 2.1 million people report recent cocaine use, and 1.6 million engage in cocaine abuse or dependence.2 Acute cardiopulmonary complaints are common in individuals who present to the ED after cocaine use, with chest pain being the most frequently reported symptom in 40%.3
Numerous etiologies for cocaine-associated chest pain (CACP) have been discovered, including musculoskeletal pain, pulmonary hypertension, cardiomyopathy, arrhythmias, and endocarditis.4 Only 0.5% of patients with aortic dissection over a four-year period had a recent history of cocaine use, making cocaine a rare cause of a rare condition.5 Cardiac chest pain remains the most frequent underlying etiology, resulting in the most common complication of myocardial infarction (MI) in up to 6% of patients.6,7
The ways in which cocaine use can cause myocardial ischemia and MI are multifactorial. A vigorous central sympathomimetic effect, coronary artery vasoconstriction, stimulation of platelets, and enhanced atherosclerosis all lead to a myocardial oxygen supply-demand imbalance.8 Other key interactions in the cardiovascular system are displayed in Figure 1. Understanding the role of these mechanisms in CACP is crucial to patient care.
Clinician goals in the management of CACP are to rapidly and accurately exclude life-threatening etiologies; assess the need for urgent acute coronary syndrome (ACS) evaluation; risk-stratify patients and ensure appropriate disposition; normalize the toxic effects of cocaine; treat resultant organ damage; and prevent long-term complications. An algorithm detailing this approach is provided in Figure 2.
Review of the Data
Diagnostic evaluation. Given potential differences in treatment regimens, it is imperative to differentiate patients who present with CACP from those whose chest pain is not associated with cocaine either by direct questioning or by screening of urine for cocaine metabolites. Once the presence of cocaine has been confirmed, guideline-based evaluation for potential ACS with serial electrocardiograms (ECG), cardiac biomarkers, and close monitoring of cardiac rhythms and hemodynamics is largely similar to standard management of all patients presenting with chest pain, with a few caveats.
Interpretation of the ECG can be challenging in the setting of cocaine. Studies have shown “abnormal” ECGs in 56% to 84% of patients, with many representing early repolarization or left ventricular hypertrophy.9,10 Likewise, patients with MI are as likely to present with normal or nonspecific ECG findings as with ischemic findings.7,11 ECG interpretation to diagnose ischemia or infarction in patients with CACP yields a sensitivity of 36% and specificity of 90%.7
Creatine kinase (CK), CK-MB fraction, and myoglobin have low specificity for the diagnosis of ischemia, as cocaine can induce skeletal muscle injury and rhabdomyolysis.9,12 Cardiac troponins demonstrate a superior specificity compared to CK and CK-MB and are thus the preferred cardiac biomarkers in diagnosing cocaine-associated MI.12
Initial management and disposition. Patients at high risk for cardiovascular events are generally admitted to a monitored bed.13 Immediate reperfusion therapy with primary percutaneous coronary intervention is recommended in patients with ST-elevation MI (STEMI). Treatment with thrombolytic agents is associated with an increased risk of intracerebral hemorrhage and lacks documented efficacy in patients with CACP. Thrombolysis should therefore only be utilized if the diagnosis of STEMI is unequivocal and an experienced cardiac catheterization laboratory is unavailable.14,15
Patients with unstable angina (UA) or non-ST-elevation MI (NSTEMI) are at higher risk for further cardiac events in a similar manner to those with ACS unrelated to cocaine. These cases might benefit from early cardiac catheterization and revascularization.16 Because of the increased risk of stent thrombosis in cocaine-users, thought to be due to recidivism, a detailed risk-benefit analysis should be undertaken prior to the implantation of cardiac stents.
Other diagnostic tests, such as stress testing and myocardial imaging, have not shown significant accuracy in diagnosing MI in this setting; moreover, these patients are at low overall risk for cardiac events and mortality. Consequently, an extensive diagnostic evaluation might not be cost-effective.7,10,13,17 Patients who have CACP without MI have a very low frequency of delayed complications.3,17 As such, cost-effective evaluation strategies, such as nine- or 12-hour observation periods in a chest pain unit, are appropriate for many of these low- to moderate-risk patients.13 For all CACP patients, the most critical post-discharge interventions are cardiac risk modification and cocaine cessation.13
Normalizing the toxic effects of cocaine with medications.
Aspirin: While no specific study has been performed in patients with CACP and aspirin, CACP guidelines, based on data supporting ACS guidelines for all patients, recommend administration of full-dose aspirin given its associated reduction in morbidity and mortality.18,19 Furthermore, given the platelet-stimulating effects of cocaine, using aspirin in this setting seems very reasonable.
Benzodiazepines: CACP guidelines support the use of benzodiazepines early in management to indirectly combat the agitation, hypertension, and tachycardia resulting from the stimulatory effects of cocaine.18,20 These recommendations are based on several animal and human studies that demonstrate significant reduction in heart rate and systemic arterial pressure with the use of these agents.21,22
Nitroglycerin: Cardiac catheterization studies have shown reversal of vasoconstriction with administration of nitroglycerin. One study demonstrated a benefit of the drug in 49% of participants.23 Additional investigation into the benefit of benzodiazepine and nitroglycerin combination therapy revealed mixed results. In one study, lorazepam plus nitroglycerin was found to be more efficacious than nitroglycerin alone.24 In another, however, use of diazepam in combination with nitroglycerin did not show benefit when evaluating pain relief, cardiac dynamics, and left ventricular function.25
Phentolamine: Phentolamine administration has been studied much less in the literature. This nonselective alpha-adrenergic antagonist exerts a dose-dependent reversal of cocaine’s vasoconstrictive properties in monkeys and humans.26,27 International guidelines for Emergency Cardiovascular Care recommend its use in treatment of cocaine-associated ACS;27 however, the AHA recommends it less strongly.18
Calcium channel blockers: Calcium channel blockers (CCBs) have not shown promise as first-line agents. While catheterization studies demonstrate the vasodilatory properties of verapamil, larger studies looking at all-cause mortality conclude that CCBs might worsen mortality rates,28 and animal studies indicate an increased risk of seizures.29 At this time, CCBs are recommended only if cardiac symptoms continue after both benzodiazepines and nitroglycerin are administered.18
The beta-blocker controversy: The use of beta-blockers in patients with CACP remains controversial given the theoretical risk of unopposed alpha-adrenergic activation. Coronary vasospasm, decreased myocardial oxygen delivery, and increased systemic vascular resistance can result from their use.30
Propranolol, a nonselective beta-blocker, was shown in catheterization studies to potentiate the coronary vasoconstriction of cocaine.31 Labetalol, a combined alpha/beta-blocker, reduced mean arterial pressure after cocaine administration during cardiac catheterization but did not reverse coronary vasoconstriction.32 This was attributed to the predominating beta greater than alpha blockade at doses administered. The selective beta-1 antagonists esmolol and metoprolol have shown no benefit in CACP.33 Carvedilol, a combined alpha/beta-blocker with both peripheral and central nervous system activity, has potential to attenuate both physiologic and behavioral response to cocaine, but it has not been well studied in this patient subset.34
The 2005 ACC/AHA STEMI guidelines recommended against beta-blockers in the setting of STEMI precipitated by cocaine use due to the potential of exacerbating coronary vasoconstriction.35 The 2007 ACC/AHA UA/NSTEMI guidelines stated that the use of a combined alpha/beta-blocker in patients with cocaine-induced ACS may be reasonable for patients with hypertension or tachycardia if pre-treated with a vasodilator.19 The 2008 ACC/AHA guidelines on the management of cocaine-related chest pain and MI recommended against the use of beta-blockers in the acute setting given the low incidence of cocaine-related MI and death.18
In a more recent study, Dattilo et al showed that beta-blockers administered to patients admitted with positive urine toxicology for cocaine significantly reduced MI and in-hospital mortality. Reduction of MI was of borderline significance in those admitted with a chief complaint of chest pain.36 Limitations of this study include unknown time of cocaine ingestion, lack of follow-up on discharge mortality, and a small sample size of 348 patients lacking statistical power.
Another retrospective cohort study examined patients admitted with chest pain and urine toxicology positive for cocaine and found that beta-blocker administration during hospitalization was not associated with increased incident mortality. Further, after a mean follow-up of 2.5 years, there was a statistically significant decrease in cardiovascular death.37 Drawbacks of this study included an older patient population, greater proportion of coronary artery disease, and higher follow-up of cardiovascular mortality rates than in previous studies, suggesting this subset might have received greater benefit from beta-blockers as a result of these characteristics.
The 2008 ACC/AHA guidelines instruct individualized consideration of the risk/benefit ratio for beta-blocker use in patients with CACP given the high rate of recidivism in cocaine abusers. The strongest indication is given to those with documented MI, left ventricular systolic dysfunction, or ventricular arrhythmias.18
It is important to note that these recommendations are based on cardiac catheterization laboratory studies, case reports, retrospective analyses, and animal experiments. No prospective controlled trials evaluating the role of beta-blockers in CACP and MI exist, and no trials regarding therapies to improve outcomes of patients sustaining a cocaine-associated MI have been reported.18
Back to the Case
This patient was experiencing cocaine-associated chest pain, which was confirmed with positive urine toxicology. Initial diagnostic workup with basic laboratory studies and cardiac biomarkers showed mild elevation in CK with troponin levels within normal limits. His ECG showed changes consistent with left ventricular hypertrophy. Chest radiograph was unremarkable.
He received aspirin, benzodiazepines, and nitroglycerin with normalization of vital signs, as well as subjective improvement in chest pain and anxiety. He was deemed to be at low risk for potential cardiac complications; thus, further cardiac testing was not pursued. Rather, he was admitted to an overnight observation unit with telemetry monitoring, where his chest pain did not recur.
He was seen in consultation with social work staff who arranged for drug abuse counseling after discharge. Given the uncertainty of relapse to cocaine use, as well as lack of known cardiac risk factors, he was not discharged on any new medications.
Bottom Line
The treatment of CACP includes normalizing the toxic systemic effects of the drug and minimizing the direct ischemic damage to the myocardium. Management varies slightly from traditional chest pain algorithms and includes benzodiazepines as well as antiplatelet agents and vasodilators to achieve this goal. Initial therapy with beta-blockers remains undefined and is largely discouraged in the acute setting. The role of beta-blockade upon discharge, however, can be beneficial in specific populations, especially those found to have underlying coronary disease.
Dr. Houchens and Dr. Czarnik are clinical instructors and Dr. Mack is a clinical lecturer at the University of Michigan Health System in Ann Arbor.
References
- Hughes A, Sathe N, Spagnola K. State Estimates of Substance Use from the 2005-2006 National Surveys on Drug Use and Health. DHHS Publication No. SMA 08-4311, NSDUH Series H-33. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2008.
- Volkow ND. Cocaine: Abuse and Addiction. National Institute on Drug Abuse. Washington, DC: U.S. Department of Health and Human Services; 2009.
- Brody SL, Slovis CM, Wrenn KD. Cocaine-related medical problems: consecutive series of 233 patients. Am J Med. 1990;88:325-331.
- Levis JT, Garmel GM. Cocaine-associated chest pain. Emerg Med Clin North Am. 2005;23:1083-1103.
- Eagle KA, Isselbacher EM, DeSanctis RW. Cocaine-related aortic dissection in perspective. Circulation. 2002;105:1529-1530.
- Feldman JA, Fish SS, Beshansky JR, Griffith JL, Woolard RH, Selker HP. Acute cardiac ischemia in patients with cocaine-associated complaints: results of a multicenter trial. Ann Emerg Med. 2000;36:469-476.
- Hollander JE, Hoffman RS, Gennis P, et al. Prospective multicenter evaluation of cocaine associated chest pain. Cocaine Associated Chest Pain (COCHPA) Study Group. Acad Emerg Med. 1994;1:330-339.
- Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122:2558-2569.
- Gitter MJ, Goldsmith SR, Dunbar DN, et al. Cocaine and chest pain: clinical features and outcomes of patients hospitalized to rule out myocardial infarction. Ann Intern Med. 1991;115:277-282.
- Amin M, Gabelman G, Karpel J, et al. Acute myocardial infarction and chest pain syndromes after cocaine use. Am J Cardiol. 1990;66:1434-1437.
- Tokarski GF, Paganussi P, Urbanski R, et al. An evaluation of cocaine-induced chest pain. Ann Emerg Med. 1990;19:1088-1092.
- Hollander JE, Levitt MA, Young GP, Briglia E, Wetli CV, Gawad Y. Effect of recent cocaine use on the specificity of cardiac markers for diagnosis of acute myocardial infarction. Am Heart J. 1998;135(2 Pt 1):245-252.
- Weber JE, Shofer FS, Larkin GL, Kalaria AS, Hollander JE. Validation of a brief observation period for patients with cocaine-associated chest pain. N Engl J Med. 2003;348:510-517.
- Hahn IH, Hoffman RS. Diagnosis and treatment of acute myocardial infarction: cocaine use and acute myocardial infarction. Emerg Med Clin North Am. 2001;19(2):1-18.
- Hoffman RS, Hollander JE. Evaluation of patients with chest pain after cocaine use. Crit Care Clin. 1997;13:809-828. Cannon CP, Weintraub WS, Demopoulos LA, et al.
- Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879-1887.
- Hollander JE, Hoffman RS. Cocaine-induced myocardial infarction: an analysis and review of the literature. J Emerg Med. 1992;10:169-177.
- McCord J, Jneid H, Hollander JE, et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117:1897-1907.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:E1-E157.
- Hollander JE. Management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
- Brubacher JR, Hoffman RS. Cocaine toxicity. Top Emerg Med. 1997;19(4):1-16.
- Catavas JD, Waters IW. Acute cocaine intoxication in the conscious dog: studies on the mechanism of lethality. J Pharmacol Exp Ther. 1981;217:350-356.
- Hollander JE, Hoffman RS, Gennis P, et al. Nitroglycerin in the treatment of cocaine associated chest pain—clinical safety and efficacy. J Toxicol Clin Toxicol. 1994;32(3): 243-256.
- Honderick T, Williams D, Seaberg D, Wears R. A prospective, randomized, controlled trial of benzodiazepines and nitroglycerin or nitroglycerin alone in the treatment of cocaine-associated acute coronary syndromes. Am J Emerg Med. 2003;21(1):39-42.
- Baumann BM, Perrone J, Hornig SE, Shofer FS, Hollander JE. Randomized, double-blind, placebo-controlled trial of diazepam, nitroglycerin, or both for treatment of patients with potential cocaine-associated acute coronary syndromes. Acad Emerg Med. 2000;7:878-885.
- Schindler CW, Tella SR, Goldberg SR. Adrenoceptor mechanisms in the cardiovascular effects of cocaine in conscious squirrel monkeys. Life Sci. 1992;51(9):653-660.
- Lange RA, Cigarroa RG, Yancy CW Jr., et al. Cocaine-induced coronary-artery vasoconstriction. N Engl J Med. 1989;321(23):1557-1562.
- Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326-1331.
- Derlet RW, Albertson TE. Potentiation of cocaine toxicity with calcium channel blockers. Am J Emerg Med. 1989;7:464-468.
- Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351-358.
- Lange RA, Cigarroa RG, Flores ED, et al. Potentiation of cocaine-induced coronary vasoconstriction by beta-adrenergic blockade. Ann Intern Med. 1990;112:897-903.
- Boehrer JD, Moliterno DJ, Willard JE, Hillis LD, Lange RA. Influence of labetalol on cocaine-induced coronary vasoconstriction in humans. Am J Med. 1993;94:608-610.
- Sand IC, Brody SL, Wrenn KD, Slovis CM. Experience with esmolol for the treatment of cocaine-associated cardiovascular complications. Am J Emerg Med. 1991;9:161-163.
- Sofuoglo M, Brown S, Babb DA, Pentel PR, Hatsukami DK. Carvedilol affects the physiological and behavioral response to smoked cocaine in humans. Drug Alcohol Depend. 2000;60:69-76.
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force of Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:E1-E211.
- Dattilo PB, Hailpern SM, Fearon K, Sohal D, Nordin C. Beta-blockers are associated with reduced risk of myocardial infarction after cocaine use. Ann Emerg Med. 2008;51:117-125.
- Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med. 2010;170:874-879.
Case
A 38-year-old man with a history of tobacco use presents to the emergency department complaining of constant substernal chest pain for three hours. His temperature is 37.7°C, his heart rate is 110 beats per minute, and his blood pressure is 155/95 mmHg. He appears anxious and diaphoretic but examination is otherwise unremarkable. He admits to cocaine use one hour before the onset of symptoms. What are the appropriate treatments for his condition?
Overview
Cocaine is the second-most-commonly used illicit drug in the U.S. and represents 31% of all ED visits related to substance abuse.1,2 According to recent survey results, 2.1 million people report recent cocaine use, and 1.6 million engage in cocaine abuse or dependence.2 Acute cardiopulmonary complaints are common in individuals who present to the ED after cocaine use, with chest pain being the most frequently reported symptom in 40%.3
Numerous etiologies for cocaine-associated chest pain (CACP) have been discovered, including musculoskeletal pain, pulmonary hypertension, cardiomyopathy, arrhythmias, and endocarditis.4 Only 0.5% of patients with aortic dissection over a four-year period had a recent history of cocaine use, making cocaine a rare cause of a rare condition.5 Cardiac chest pain remains the most frequent underlying etiology, resulting in the most common complication of myocardial infarction (MI) in up to 6% of patients.6,7
The ways in which cocaine use can cause myocardial ischemia and MI are multifactorial. A vigorous central sympathomimetic effect, coronary artery vasoconstriction, stimulation of platelets, and enhanced atherosclerosis all lead to a myocardial oxygen supply-demand imbalance.8 Other key interactions in the cardiovascular system are displayed in Figure 1. Understanding the role of these mechanisms in CACP is crucial to patient care.
Clinician goals in the management of CACP are to rapidly and accurately exclude life-threatening etiologies; assess the need for urgent acute coronary syndrome (ACS) evaluation; risk-stratify patients and ensure appropriate disposition; normalize the toxic effects of cocaine; treat resultant organ damage; and prevent long-term complications. An algorithm detailing this approach is provided in Figure 2.
Review of the Data
Diagnostic evaluation. Given potential differences in treatment regimens, it is imperative to differentiate patients who present with CACP from those whose chest pain is not associated with cocaine either by direct questioning or by screening of urine for cocaine metabolites. Once the presence of cocaine has been confirmed, guideline-based evaluation for potential ACS with serial electrocardiograms (ECG), cardiac biomarkers, and close monitoring of cardiac rhythms and hemodynamics is largely similar to standard management of all patients presenting with chest pain, with a few caveats.
Interpretation of the ECG can be challenging in the setting of cocaine. Studies have shown “abnormal” ECGs in 56% to 84% of patients, with many representing early repolarization or left ventricular hypertrophy.9,10 Likewise, patients with MI are as likely to present with normal or nonspecific ECG findings as with ischemic findings.7,11 ECG interpretation to diagnose ischemia or infarction in patients with CACP yields a sensitivity of 36% and specificity of 90%.7
Creatine kinase (CK), CK-MB fraction, and myoglobin have low specificity for the diagnosis of ischemia, as cocaine can induce skeletal muscle injury and rhabdomyolysis.9,12 Cardiac troponins demonstrate a superior specificity compared to CK and CK-MB and are thus the preferred cardiac biomarkers in diagnosing cocaine-associated MI.12
Initial management and disposition. Patients at high risk for cardiovascular events are generally admitted to a monitored bed.13 Immediate reperfusion therapy with primary percutaneous coronary intervention is recommended in patients with ST-elevation MI (STEMI). Treatment with thrombolytic agents is associated with an increased risk of intracerebral hemorrhage and lacks documented efficacy in patients with CACP. Thrombolysis should therefore only be utilized if the diagnosis of STEMI is unequivocal and an experienced cardiac catheterization laboratory is unavailable.14,15
Patients with unstable angina (UA) or non-ST-elevation MI (NSTEMI) are at higher risk for further cardiac events in a similar manner to those with ACS unrelated to cocaine. These cases might benefit from early cardiac catheterization and revascularization.16 Because of the increased risk of stent thrombosis in cocaine-users, thought to be due to recidivism, a detailed risk-benefit analysis should be undertaken prior to the implantation of cardiac stents.
Other diagnostic tests, such as stress testing and myocardial imaging, have not shown significant accuracy in diagnosing MI in this setting; moreover, these patients are at low overall risk for cardiac events and mortality. Consequently, an extensive diagnostic evaluation might not be cost-effective.7,10,13,17 Patients who have CACP without MI have a very low frequency of delayed complications.3,17 As such, cost-effective evaluation strategies, such as nine- or 12-hour observation periods in a chest pain unit, are appropriate for many of these low- to moderate-risk patients.13 For all CACP patients, the most critical post-discharge interventions are cardiac risk modification and cocaine cessation.13
Normalizing the toxic effects of cocaine with medications.
Aspirin: While no specific study has been performed in patients with CACP and aspirin, CACP guidelines, based on data supporting ACS guidelines for all patients, recommend administration of full-dose aspirin given its associated reduction in morbidity and mortality.18,19 Furthermore, given the platelet-stimulating effects of cocaine, using aspirin in this setting seems very reasonable.
Benzodiazepines: CACP guidelines support the use of benzodiazepines early in management to indirectly combat the agitation, hypertension, and tachycardia resulting from the stimulatory effects of cocaine.18,20 These recommendations are based on several animal and human studies that demonstrate significant reduction in heart rate and systemic arterial pressure with the use of these agents.21,22
Nitroglycerin: Cardiac catheterization studies have shown reversal of vasoconstriction with administration of nitroglycerin. One study demonstrated a benefit of the drug in 49% of participants.23 Additional investigation into the benefit of benzodiazepine and nitroglycerin combination therapy revealed mixed results. In one study, lorazepam plus nitroglycerin was found to be more efficacious than nitroglycerin alone.24 In another, however, use of diazepam in combination with nitroglycerin did not show benefit when evaluating pain relief, cardiac dynamics, and left ventricular function.25
Phentolamine: Phentolamine administration has been studied much less in the literature. This nonselective alpha-adrenergic antagonist exerts a dose-dependent reversal of cocaine’s vasoconstrictive properties in monkeys and humans.26,27 International guidelines for Emergency Cardiovascular Care recommend its use in treatment of cocaine-associated ACS;27 however, the AHA recommends it less strongly.18
Calcium channel blockers: Calcium channel blockers (CCBs) have not shown promise as first-line agents. While catheterization studies demonstrate the vasodilatory properties of verapamil, larger studies looking at all-cause mortality conclude that CCBs might worsen mortality rates,28 and animal studies indicate an increased risk of seizures.29 At this time, CCBs are recommended only if cardiac symptoms continue after both benzodiazepines and nitroglycerin are administered.18
The beta-blocker controversy: The use of beta-blockers in patients with CACP remains controversial given the theoretical risk of unopposed alpha-adrenergic activation. Coronary vasospasm, decreased myocardial oxygen delivery, and increased systemic vascular resistance can result from their use.30
Propranolol, a nonselective beta-blocker, was shown in catheterization studies to potentiate the coronary vasoconstriction of cocaine.31 Labetalol, a combined alpha/beta-blocker, reduced mean arterial pressure after cocaine administration during cardiac catheterization but did not reverse coronary vasoconstriction.32 This was attributed to the predominating beta greater than alpha blockade at doses administered. The selective beta-1 antagonists esmolol and metoprolol have shown no benefit in CACP.33 Carvedilol, a combined alpha/beta-blocker with both peripheral and central nervous system activity, has potential to attenuate both physiologic and behavioral response to cocaine, but it has not been well studied in this patient subset.34
The 2005 ACC/AHA STEMI guidelines recommended against beta-blockers in the setting of STEMI precipitated by cocaine use due to the potential of exacerbating coronary vasoconstriction.35 The 2007 ACC/AHA UA/NSTEMI guidelines stated that the use of a combined alpha/beta-blocker in patients with cocaine-induced ACS may be reasonable for patients with hypertension or tachycardia if pre-treated with a vasodilator.19 The 2008 ACC/AHA guidelines on the management of cocaine-related chest pain and MI recommended against the use of beta-blockers in the acute setting given the low incidence of cocaine-related MI and death.18
In a more recent study, Dattilo et al showed that beta-blockers administered to patients admitted with positive urine toxicology for cocaine significantly reduced MI and in-hospital mortality. Reduction of MI was of borderline significance in those admitted with a chief complaint of chest pain.36 Limitations of this study include unknown time of cocaine ingestion, lack of follow-up on discharge mortality, and a small sample size of 348 patients lacking statistical power.
Another retrospective cohort study examined patients admitted with chest pain and urine toxicology positive for cocaine and found that beta-blocker administration during hospitalization was not associated with increased incident mortality. Further, after a mean follow-up of 2.5 years, there was a statistically significant decrease in cardiovascular death.37 Drawbacks of this study included an older patient population, greater proportion of coronary artery disease, and higher follow-up of cardiovascular mortality rates than in previous studies, suggesting this subset might have received greater benefit from beta-blockers as a result of these characteristics.
The 2008 ACC/AHA guidelines instruct individualized consideration of the risk/benefit ratio for beta-blocker use in patients with CACP given the high rate of recidivism in cocaine abusers. The strongest indication is given to those with documented MI, left ventricular systolic dysfunction, or ventricular arrhythmias.18
It is important to note that these recommendations are based on cardiac catheterization laboratory studies, case reports, retrospective analyses, and animal experiments. No prospective controlled trials evaluating the role of beta-blockers in CACP and MI exist, and no trials regarding therapies to improve outcomes of patients sustaining a cocaine-associated MI have been reported.18
Back to the Case
This patient was experiencing cocaine-associated chest pain, which was confirmed with positive urine toxicology. Initial diagnostic workup with basic laboratory studies and cardiac biomarkers showed mild elevation in CK with troponin levels within normal limits. His ECG showed changes consistent with left ventricular hypertrophy. Chest radiograph was unremarkable.
He received aspirin, benzodiazepines, and nitroglycerin with normalization of vital signs, as well as subjective improvement in chest pain and anxiety. He was deemed to be at low risk for potential cardiac complications; thus, further cardiac testing was not pursued. Rather, he was admitted to an overnight observation unit with telemetry monitoring, where his chest pain did not recur.
He was seen in consultation with social work staff who arranged for drug abuse counseling after discharge. Given the uncertainty of relapse to cocaine use, as well as lack of known cardiac risk factors, he was not discharged on any new medications.
Bottom Line
The treatment of CACP includes normalizing the toxic systemic effects of the drug and minimizing the direct ischemic damage to the myocardium. Management varies slightly from traditional chest pain algorithms and includes benzodiazepines as well as antiplatelet agents and vasodilators to achieve this goal. Initial therapy with beta-blockers remains undefined and is largely discouraged in the acute setting. The role of beta-blockade upon discharge, however, can be beneficial in specific populations, especially those found to have underlying coronary disease.
Dr. Houchens and Dr. Czarnik are clinical instructors and Dr. Mack is a clinical lecturer at the University of Michigan Health System in Ann Arbor.
References
- Hughes A, Sathe N, Spagnola K. State Estimates of Substance Use from the 2005-2006 National Surveys on Drug Use and Health. DHHS Publication No. SMA 08-4311, NSDUH Series H-33. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2008.
- Volkow ND. Cocaine: Abuse and Addiction. National Institute on Drug Abuse. Washington, DC: U.S. Department of Health and Human Services; 2009.
- Brody SL, Slovis CM, Wrenn KD. Cocaine-related medical problems: consecutive series of 233 patients. Am J Med. 1990;88:325-331.
- Levis JT, Garmel GM. Cocaine-associated chest pain. Emerg Med Clin North Am. 2005;23:1083-1103.
- Eagle KA, Isselbacher EM, DeSanctis RW. Cocaine-related aortic dissection in perspective. Circulation. 2002;105:1529-1530.
- Feldman JA, Fish SS, Beshansky JR, Griffith JL, Woolard RH, Selker HP. Acute cardiac ischemia in patients with cocaine-associated complaints: results of a multicenter trial. Ann Emerg Med. 2000;36:469-476.
- Hollander JE, Hoffman RS, Gennis P, et al. Prospective multicenter evaluation of cocaine associated chest pain. Cocaine Associated Chest Pain (COCHPA) Study Group. Acad Emerg Med. 1994;1:330-339.
- Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122:2558-2569.
- Gitter MJ, Goldsmith SR, Dunbar DN, et al. Cocaine and chest pain: clinical features and outcomes of patients hospitalized to rule out myocardial infarction. Ann Intern Med. 1991;115:277-282.
- Amin M, Gabelman G, Karpel J, et al. Acute myocardial infarction and chest pain syndromes after cocaine use. Am J Cardiol. 1990;66:1434-1437.
- Tokarski GF, Paganussi P, Urbanski R, et al. An evaluation of cocaine-induced chest pain. Ann Emerg Med. 1990;19:1088-1092.
- Hollander JE, Levitt MA, Young GP, Briglia E, Wetli CV, Gawad Y. Effect of recent cocaine use on the specificity of cardiac markers for diagnosis of acute myocardial infarction. Am Heart J. 1998;135(2 Pt 1):245-252.
- Weber JE, Shofer FS, Larkin GL, Kalaria AS, Hollander JE. Validation of a brief observation period for patients with cocaine-associated chest pain. N Engl J Med. 2003;348:510-517.
- Hahn IH, Hoffman RS. Diagnosis and treatment of acute myocardial infarction: cocaine use and acute myocardial infarction. Emerg Med Clin North Am. 2001;19(2):1-18.
- Hoffman RS, Hollander JE. Evaluation of patients with chest pain after cocaine use. Crit Care Clin. 1997;13:809-828. Cannon CP, Weintraub WS, Demopoulos LA, et al.
- Comparison of early invasive and conservative strategies in patients with unstable coronary syndromes treated with the glycoprotein IIb/IIIa inhibitor tirofiban. N Engl J Med. 2001;344:1879-1887.
- Hollander JE, Hoffman RS. Cocaine-induced myocardial infarction: an analysis and review of the literature. J Emerg Med. 1992;10:169-177.
- McCord J, Jneid H, Hollander JE, et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117:1897-1907.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:E1-E157.
- Hollander JE. Management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333:1267-1272.
- Brubacher JR, Hoffman RS. Cocaine toxicity. Top Emerg Med. 1997;19(4):1-16.
- Catavas JD, Waters IW. Acute cocaine intoxication in the conscious dog: studies on the mechanism of lethality. J Pharmacol Exp Ther. 1981;217:350-356.
- Hollander JE, Hoffman RS, Gennis P, et al. Nitroglycerin in the treatment of cocaine associated chest pain—clinical safety and efficacy. J Toxicol Clin Toxicol. 1994;32(3): 243-256.
- Honderick T, Williams D, Seaberg D, Wears R. A prospective, randomized, controlled trial of benzodiazepines and nitroglycerin or nitroglycerin alone in the treatment of cocaine-associated acute coronary syndromes. Am J Emerg Med. 2003;21(1):39-42.
- Baumann BM, Perrone J, Hornig SE, Shofer FS, Hollander JE. Randomized, double-blind, placebo-controlled trial of diazepam, nitroglycerin, or both for treatment of patients with potential cocaine-associated acute coronary syndromes. Acad Emerg Med. 2000;7:878-885.
- Schindler CW, Tella SR, Goldberg SR. Adrenoceptor mechanisms in the cardiovascular effects of cocaine in conscious squirrel monkeys. Life Sci. 1992;51(9):653-660.
- Lange RA, Cigarroa RG, Yancy CW Jr., et al. Cocaine-induced coronary-artery vasoconstriction. N Engl J Med. 1989;321(23):1557-1562.
- Furberg CD, Psaty BM, Meyer JV. Nifedipine. Dose-related increase in mortality in patients with coronary heart disease. Circulation. 1995;92:1326-1331.
- Derlet RW, Albertson TE. Potentiation of cocaine toxicity with calcium channel blockers. Am J Emerg Med. 1989;7:464-468.
- Lange RA, Hillis LD. Cardiovascular complications of cocaine use. N Engl J Med. 2001;345:351-358.
- Lange RA, Cigarroa RG, Flores ED, et al. Potentiation of cocaine-induced coronary vasoconstriction by beta-adrenergic blockade. Ann Intern Med. 1990;112:897-903.
- Boehrer JD, Moliterno DJ, Willard JE, Hillis LD, Lange RA. Influence of labetalol on cocaine-induced coronary vasoconstriction in humans. Am J Med. 1993;94:608-610.
- Sand IC, Brody SL, Wrenn KD, Slovis CM. Experience with esmolol for the treatment of cocaine-associated cardiovascular complications. Am J Emerg Med. 1991;9:161-163.
- Sofuoglo M, Brown S, Babb DA, Pentel PR, Hatsukami DK. Carvedilol affects the physiological and behavioral response to smoked cocaine in humans. Drug Alcohol Depend. 2000;60:69-76.
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force of Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol. 2004;44:E1-E211.
- Dattilo PB, Hailpern SM, Fearon K, Sohal D, Nordin C. Beta-blockers are associated with reduced risk of myocardial infarction after cocaine use. Ann Emerg Med. 2008;51:117-125.
- Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med. 2010;170:874-879.