User login
Pre-Operative Beta Blockers May Benefit Some Cardiac Patients
Clinical question: In patients with ischemic heart disease (IHD) undergoing non-cardiac surgery, do pre-operative beta blockers reduce post-operative major cardiovascular events (MACE) or mortality at 30 days?
Background: Pre-operative beta blocker use has become more restricted, as evidence about which patients derive benefit has become clearer. Opinions and practice vary regarding whether all patients with IHD, or only certain populations within this group, benefit from pre-operative beta blockers.
Study design: Retrospective, national registry-based cohort study.
Setting: Denmark, 2004-2009.
Synopsis: No benefit was found for the overall cohort of 28,263 patients. Patients with IHD and heart failure (n=7990) had lower risk of MACE (HR=0.75, 95% CI, 0.70-0.87) and mortality (HR=0.80, 95% CI, 0.70-0.92). Patients with IHD and myocardial infarction within two years (n=1664) had lower risk of MACE (HR=0.54, 95% CI, 0.37-0.78) but not mortality. Beta blocker dose and compliance were unknown. Whether patients had symptoms or inducible ischemia was not clear. This study supports the concept that higher-risk patients benefit more from pre-operative beta blockers, but it is not high-grade evidence.
Bottom line: Not all patients with IHD benefit from pre-operative beta blockers; those with concomitant heart failure or recent MI have a lower risk of MACE and/or mortality at 30 days with beta blockers.
Citation: Andersson C, Merie C, Jorgensen M, et al. Association of ß-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing non-cardiac surgery: A Danish nationwide cohort study JAMA Intern Med. 2014;174(3):336-344.
Clinical question: In patients with ischemic heart disease (IHD) undergoing non-cardiac surgery, do pre-operative beta blockers reduce post-operative major cardiovascular events (MACE) or mortality at 30 days?
Background: Pre-operative beta blocker use has become more restricted, as evidence about which patients derive benefit has become clearer. Opinions and practice vary regarding whether all patients with IHD, or only certain populations within this group, benefit from pre-operative beta blockers.
Study design: Retrospective, national registry-based cohort study.
Setting: Denmark, 2004-2009.
Synopsis: No benefit was found for the overall cohort of 28,263 patients. Patients with IHD and heart failure (n=7990) had lower risk of MACE (HR=0.75, 95% CI, 0.70-0.87) and mortality (HR=0.80, 95% CI, 0.70-0.92). Patients with IHD and myocardial infarction within two years (n=1664) had lower risk of MACE (HR=0.54, 95% CI, 0.37-0.78) but not mortality. Beta blocker dose and compliance were unknown. Whether patients had symptoms or inducible ischemia was not clear. This study supports the concept that higher-risk patients benefit more from pre-operative beta blockers, but it is not high-grade evidence.
Bottom line: Not all patients with IHD benefit from pre-operative beta blockers; those with concomitant heart failure or recent MI have a lower risk of MACE and/or mortality at 30 days with beta blockers.
Citation: Andersson C, Merie C, Jorgensen M, et al. Association of ß-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing non-cardiac surgery: A Danish nationwide cohort study JAMA Intern Med. 2014;174(3):336-344.
Clinical question: In patients with ischemic heart disease (IHD) undergoing non-cardiac surgery, do pre-operative beta blockers reduce post-operative major cardiovascular events (MACE) or mortality at 30 days?
Background: Pre-operative beta blocker use has become more restricted, as evidence about which patients derive benefit has become clearer. Opinions and practice vary regarding whether all patients with IHD, or only certain populations within this group, benefit from pre-operative beta blockers.
Study design: Retrospective, national registry-based cohort study.
Setting: Denmark, 2004-2009.
Synopsis: No benefit was found for the overall cohort of 28,263 patients. Patients with IHD and heart failure (n=7990) had lower risk of MACE (HR=0.75, 95% CI, 0.70-0.87) and mortality (HR=0.80, 95% CI, 0.70-0.92). Patients with IHD and myocardial infarction within two years (n=1664) had lower risk of MACE (HR=0.54, 95% CI, 0.37-0.78) but not mortality. Beta blocker dose and compliance were unknown. Whether patients had symptoms or inducible ischemia was not clear. This study supports the concept that higher-risk patients benefit more from pre-operative beta blockers, but it is not high-grade evidence.
Bottom line: Not all patients with IHD benefit from pre-operative beta blockers; those with concomitant heart failure or recent MI have a lower risk of MACE and/or mortality at 30 days with beta blockers.
Citation: Andersson C, Merie C, Jorgensen M, et al. Association of ß-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing non-cardiac surgery: A Danish nationwide cohort study JAMA Intern Med. 2014;174(3):336-344.
Hospitalist Reviews on Pre-Operative Beta Blockers, Therapeutic Hypothermia after Cardiac Arrest, Colloids vs. Crystalloids for Hypovolemic Shock
In This Edition
Literature At A Glance
A guide to this month’s studies
- Facecards improve familiarity with physician names, not satisfaction
- Pre-operative beta-blockers may benefit some cardiac patients
- Benefit of therapeutic hypothermia after cardiac arrest unclear
- Patients prefer inpatient boarding to ED boarding
- Triple rule outs for chest pain
- Colloids vs. crystalloids for critically ill patients presenting with hypovolemic shock
- Interdisciplinary intervention improves medication compliance, not blood pressure or LDL-C levels
- Edoxaban is noninferior to warfarin in Afib patients
- Beta blockers lower mortality after acute MI in COPD patients
- Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction
Facecards Improve Familiarity with Physician Names but Not Satisfaction
Clinical question: Do facecards improve patients’ familiarity with physicians and increase satisfaction, trust, and agreement with physicians?
Background: Facecards can improve patients’ knowledge of names and roles of physicians, but their impact on other outcomes is unclear. This pilot trial was designed to assess facecards’ impact on patient satisfaction, trust, or agreement with physicians.
Study design: Cluster, randomized controlled trial (RCT).
Setting: A large teaching hospital in the United States.
Synopsis: Patients (n=138) were randomized to receive either facecards with the name and picture of their hospitalists, as well as a brief description of the hospitalist’s role (n=66), or to receive traditional communication (n=72). There were no significant differences in patient age, sex, or race.
Patients who received a facecard were more likely to correctly identify their hospital physician (89.1% vs. 51.1%; P< 0.01) and were more likely to correctly identify the role of their hospital physician than those in the control group (67.4% vs. 16.3%; P<0.01).
Patients who received a facecard rated satisfaction, trust, and agreement slightly higher compared with those who had not received a card, but the results were not statistically significant (P values 0.27, 0.32, 0.37, respectively.) The authors note that larger studies may be needed to see a difference in these areas.
Bottom line: Facecards improve patients’ knowledge of the names and roles of hospital physicians but have no clear impact on satisfaction with, trust of, or agreement with physicians.
Citation: Simons Y, Caprio T, Furiasse N, Kriss, M, Williams MV, O’Leary KJ. The impact of facecards on patients’ knowledge, satisfaction, trust, and agreement with hospitalist physicians: a pilot study. J Hosp Med. 2014;9(3):137-141.
Pre-Operative Beta Blockers May Benefit Some Cardiac Patients
Clinical question: In patients with ischemic heart disease (IHD) undergoing non-cardiac surgery, do pre-operative beta blockers reduce post-operative major cardiovascular events (MACE) or mortality at 30 days?
Background: Peri-operative beta blocker use has become more restricted, as evidence about which patients derive benefit has become clearer. Opinions and practice vary regarding whether all patients with IHD, or only certain populations within this group, benefit from peri-operative beta blockers.
Study design: Retrospective, national registry-based cohort study.
Setting: Denmark, 2004-2009.
Synopsis: No benefit was found for the overall cohort of 28,263 patients. Patients with IHD and heart failure (n=7990) had lower risk of MACE (HR=0.75, 95% CI, 0.70-0.87) and mortality (HR=0.80, 95% CI, 0.70-0.92). Patients with IHD and myocardial infarction within two years (n=1664) had lower risk of MACE (HR=0.54, 95% CI, 0.37-0.78) but not mortality.
Beta blocker dose and compliance were unknown. Whether patients had symptoms or inducible ischemia was not clear.
This study supports the concept that higher-risk patients benefit more from peri-operative beta blockers, but it is not high-grade evidence.
Bottom line: Not all patients with IHD benefit from pre-operative beta blockers; those with concomitant heart failure or recent MI have a lower risk of MACE and/or mortality at 30 days with beta blockers.
Citation: Andersson C, Merie C, Jorgensen M, et al. Association of ß-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing non-cardiac surgery: a Danish nationwide cohort study. JAMA Intern Med. 2014;174(3):336-344.
Benefit of Therapeutic Hypothermia after Cardiac Arrest Unclear
Clinical question: Does targeted hypothermia (33°C) after cardiac arrest confer benefits compared with targeted temperature management at 36°C?
Background: Therapeutic hypothermia is a current recommendation in resuscitation guidelines after cardiac arrest. Fever develops in many patients after arrest, and it is unclear if the treatment benefit is due to hypothermia or due to the prevention of fever.
Study design: RCT.
Setting: ICUs in Europe and Australia.
Synopsis: The study authors randomized 950 patients who experienced out-of-hospital cardiac arrest to targeted temperature management at either 36°C or 33°C. The goal of this trial was to prevent fever in both groups during the first 36 hours after cardiac arrest. No statistically significant difference in outcomes between these two approaches was found. In the 33°C group, 54% died or had poor neurologic function, compared with 52% in the 36°C group (risk ratio 1.02; 95% CI 0.88 to 1.16; P=0.78).
Given the wide confidence interval, a trial with either more participants or more events might be able to determine whether a true difference in these management approaches exists.
Bottom line: Therapeutic hypothermia at 33°C after out-of-hospital cardiac arrest did not confer a benefit compared with targeted temperature management at 36°C.
Citation: Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197-2206.
Patients Prefer Inpatient Boarding to Emergency Department Boarding
Clinical question: Do patients who experience overcrowding and long waits in the emergency department (ED) prefer boarding within ED hallways or within inpatient medical unit hallways?
Background: Boarding of admitted patients in EDs can be problematic, especially with regard to patient safety and patient satisfaction. Patient satisfaction data comparing boarding in the ED versus boarding in an inpatient unit hallway is limited.
Study design: Post-discharge, structured, telephone satisfaction survey.
Setting: Suburban, university-based teaching hospital.
Synopsis: A group of patients who experienced hallway boarding in the ED and then hallway boarding on the inpatient medical unit were identified. They were contacted by phone and asked to take a survey on their experience; 105 of 110 patients identified agreed. Patients were asked to rate their location preference with regard to various aspects of care. A five-point Likert scale consisting of the following answers was used: ED hallway much better, ED hallway better, no preference, inpatient hallway better, and inpatient hallway much better.
The inpatient hallway was the overall preferred location in 85% of respondents. Respondents preferred inpatient boarding with regard to multiple other parameters: rest, 85%; safety, 83%; confidentiality, 82%; treatment, 78%; comfort, 79%; quiet, 84%; staff availability, 84%; and privacy, 84%. For no item was there a preference for boarding in the ED.
Patient demographics in this hospital may differ from other settings and should be considered when applying the results. With Hospital Consumer Assessment of Healthcare Providers and Systems scores and ED throughput being publicly reported, further studies in this area would be valuable.
Bottom line: In a post-discharge telephone survey, patients preferred boarding in inpatient unit hallways rather than boarding in the ED.
Citation: Viccellio P, Zito JA, Sayage V, et al. Patients overwhelmingly prefer inpatient boarding to emergency department boarding. J Emerg Med. 2013;45(6):942-946.
“Triple Rule Outs” for Chest Pain: A Tool to Evaluate the Coronaries but Not Pulmonary Embolism or Aortic Dissection
Clinical question: How does “triple rule out” (TRO) computed tomographic (CT) angiography compare to other imaging modalities in evaluating coronary and other life-threatening etiologies of chest pain, such as pulmonary embolism (PE) and aortic dissection?
Background: TRO CT angiography is a noninvasive technology that evaluates the coronary arteries, thoracic aorta, and pulmonary vasculature simultaneously. Comparison with other tests in the diagnosis of common clinical conditions is useful information for clinical practice.
Study design: Systematic review and meta-analysis.
Setting: Systematic review of 11 studies (one randomized, 10 observational).
Synopsis: Using an enrolled population of 3,539 patients, TRO CT was compared to other imaging modalities on the basis of image quality, diagnostic accuracy, radiation, and contrast volume. When TRO CT was compared to dedicated CT scans, no significant imaging difference was discovered. TRO CT detected CAD with a sensitivity of 94.3% (95% CI, 89.1% to 97.5%, I2=58.2%) and specificity of 97.4% (95% CI, 96.1% to 98.5%, I2=91.2%).
An insufficient number of patients with PE or aortic dissection were studied to generate diagnostic accuracy for these conditions. TRO CT involved greater radiation exposure and contrast exposure than non-TRO CT.
This study reports high accuracy of TRO CT in the diagnosis of coronary artery disease. Due to the low prevalence of patients with PE or aortic dissection (<1%), the data cannot be extrapolated to these conditions.
Bottom line: Although TRO CT is highly accurate for detecting coronary artery disease, there is insufficient data to recommend its use for the diagnosis of PE or aortic dissection.
Citation: Ayaram D, Bellolio MF, Murad MH, et al. Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med. 2013;20(9):861-871.
Colloids vs. Crystalloids for Critically Ill Patients Presenting with Hypovolemic Shock
Clinical question: In critically ill patients admitted to the ICU with hypovolemic shock, does the use of colloid for fluid resuscitation, compared with crystalloid, improve mortality?
Background: The current Surviving Sepsis Campaign guidelines recommend crystalloids as the preferred fluid for resuscitation of patients with hypovolemic shock; however, evidence supporting the choice of intravenous colloid vs. crystalloid solutions for management of hypovolemic shock is weak.
Study design: RCT.
Setting: International, multi-center study.
Synopsis: Researchers randomized 2,857 adult patients who were admitted to an ICU and required fluid resuscitation for acute hypovolemia to receive either crystalloids or colloids.
At 28 days, there were 359 deaths (25.4%) in the colloids group vs. 390 deaths (27.0%) in the crystalloids group (P=0.26). At 90 days, there were 434 deaths (30.7%) in the colloids group vs. 493 deaths (34.2%) in the crystalloids group (P=0.03).
Renal replacement therapy was used in 11.0% of the colloids group vs. 12.5% of the crystalloids group (P=0.19). There were more days alive without mechanical ventilation in the colloids group vs. the crystalloids group at seven days (P=0.01) and at 28 days (P=0.01), and there were more days alive without vasopressor therapy in the colloids group vs. the crystalloids group at seven days (P=0.04) and at 28 days (P=0.03).
Major limitations of the study included the use of open-labeled fluids during allocation, so the initial investigators were not blinded to the type of fluid. Moreover, the study compared two therapeutic strategies (colloid vs. crystalloids) rather than two types of molecules.
Bottom line: In ICU patients with hypovolemia requiring resuscitation, the use of colloids vs. crystalloids did not result in a significant difference in 28-day mortality; however, 90-day mortality was lower among patients receiving colloids.
Citation: Annane D, Siami S, Jaber S, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality of critically ill patients presenting with hypovolemic shock: the CRISTAL randomization trial. JAMA. 2013;310(17):1809-1817.
Interdisciplinary Intervention Improves Medication Compliance, Not Blood Pressure or LDL-C Levels
Clinical question: Can intervention by pharmacists and physicians improve compliance to cardio-protective medications?
Background: Adherence to cardio-protective medications in the year after hospitalization for acute coronary syndrome is poor.
Study design: RCT.
Setting: Four Department of Veterans Affairs medical centers.
Synopsis: The intervention consisted of pharmacist-led medication reconciliation, patient education, pharmacist and PCP +/- cardiologist collaboration, and voice messaging. The outcome measured was the proportion of patients adherent to medication regimens based on a mean proportion of days covered (PDC) >0.80 in the year after discharge, using pharmacy refill data for clopidogrel, beta blockers, statins, and ACEI/ARBs.
Two hundred forty-one patients (95.3%) completed the study. In the intervention group, 89.3% of patients were adherent vs. 73.9% in the usual care group (P=0.003). Mean PDC was higher in the intervention group (0.94 vs. 0.87; P<0.001). A greater proportion of intervention patients were adherent to clopidogrel (86.8% vs. 70.7%; P=0.03), statins (93.2% vs. 71.3%; P<0.001), and ACEI/ARBs (93.1% vs. 81.7%; P=0.03), but not beta blockers (88.1% vs. 84.8%; P=0.59). There were no statistically significant differences in the proportion of patients who achieved blood pressure and LDL-C level goals.
Bottom line: An interdisciplinary, multi-faceted intervention increased medication compliance in the year after discharge for ACS but did not improve blood pressure or LDL-C levels.
Citation: Ho PM, Lambert-Kerzner A, Carey EP, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge. JAMA Intern Med. 2014;174(2):186-193.
Edoxaban Is Noninferior to Warfarin in Patients with Atrial Fibrillation
Clinical question: What is the long-term efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation (Afib)?
Background: Edoxaban is an oral factor Xa inhibitor approved for use in Japan for the prevention of venous thromboembolism after orthopedic surgery. No specific antidote for edoxaban exists, but hemostatic agents can reverse its anticoagulation effect.
Study design: RCT.
Setting: More than 1,300 centers in 46 countries.
Synopsis: Researchers randomized 21,105 patients in a 1:1:1 ratio to receive warfarin (goal INR of 2-3), low-dose edoxaban, or high-dose edoxoban. All patients received two sets of drugs, either active warfarin with placebo edoxaban or active edoxaban (high- or low-dose) and placebo warfarin (with sham INRs drawn), and were followed for a median of 2.8 years.
The annualized rate of stroke or systemic embolic event was 1.5% in the warfarin group, compared with 1.18% in the high-dose edoxaban group (hazard ratio 0.79; P<0.001) and 1.61% in the low-dose edoxaban group (hazard ratio 1.07; P=0.005). Annualized rate of major bleeding was 3.43% with warfarin, 2.75% with high-dose edoxoban (hazard ratio 0.80; P<0.001), and 1.61% with low-dose edoxaban (hazard ratio 0.47; P<0.001).
Both edoxaban regimens were noninferior to warfarin for the prevention of stroke or systemic emboli. The rates of cardiovascular events, bleeding, or death from any cause was lower with both doses of edoxaban as compared with warfarin.
Bottom line: Once-daily edoxaban is noninferior to warfarin for the prevention of stroke or systemic emboli and is associated with lower rates of bleeding and death.
Citation: Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. New Engl J Med. 2013;369(22):2093-2104.
Beta Blockers Lower Mortality after Acute Myocardial Infarction in COPD Patients
Clinical question: Does the use and timing of beta blockers in COPD patients experiencing a first myocardial infarction (MI) affect survival after the event?
Background: Beta blockers are effective in reducing mortality and reinfarction after an MI; however, concerns regarding the side effects of beta blockers, such as bronchospasm, continue to limit their use in patients with COPD.
Study design: Population-based cohort study.
Setting: The Myocardial Ischemia National Audit Project, linked to the General Practice Research Database, in the United Kingdom.
Synopsis: Researchers identified 1,063 patients over the age of 18 with COPD admitted to the hospital with a first acute MI. Use of beta blockers during hospitalization was associated with increased overall and one-year survival. Initiation of beta blockers during an MI had a mortality-adjusted hazard ratio of 0.50 (95% CI 0.36 to 0.69; P<0.001; median follow-up time=2.9 years).
Patients already on beta blockers prior to the MI had overall survival-adjusted hazard ratio of 0.59 (95% CI 0.44 to 0.79; P<0.001). Both scenarios showed survival benefits compared to COPD patients who were not prescribed beta blockers. Patients given beta blockers with COPD either during the MI hospitalization or before the event were younger and had fewer comorbidities. This may have accounted for some of the survival bias.
Bottom line: The use of beta blockers in patients with COPD started prior to, or at the time of, hospital admission for a first MI is associated with improved survival.
Citation: Quint JK, Herret E, Bhaskaran K, et al. Effect of ß blockers on mortality after myocardial infarction in adults with COPD: population-based cohort study of UK electronic healthcare records. BMJ. 2013;347:f6650.
Neither Low-Dose Dopamine nor Low-Dose Nesiritide Improves Renal Dysfunction in Acute Heart Failure Patients
Clinical question: Does low-dose dopamine or low-dose nesiritide added to diuretic therapy enhance pulmonary volume reduction and preserve renal function in patients with acute heart failure and renal dysfunction, compared to placebo?
Background: Small studies have suggested that low-dose dopamine or low-dose nesiritide may be beneficial in enhancing decongestion and improving renal dysfunction; however, there is ambiguity in overall benefit. Some observational studies suggest that dopamine and nesiritide are associated with higher length of stay, higher costs, and greater mortality.
Study Design: RCT.
Setting: Twenty-six hospital sites in the U.S. and Canada.
Synopsis: Three hundred sixty patients with acute heart failure and renal dysfunction were randomized to receive either nesiritide or dopamine within 24 hours of admission. Within each of these arms, patients were then randomized, in a double-blinded 2:1 fashion, into active treatment versus placebo groups. Treatment groups were compared to the pooled placebo groups.
Two main endpoints were urine output and change in serum cystatin C, from enrollment to 72 hours. Compared with placebo, low-dose dopamine had no significant effect on urine output or serum cystatin C level. Similarly, low-dose nesiritide had no significant effect on 72-hour urine output or serum cystatin C level.
Other studies have shown these drugs to be potentially harmful. Hospitalists should use caution and carefully interpret the relevant evidence when considering their use.
Bottom line: Neither low-dose nesiritide nor low-dose dopamine improved urine output or serum cystatin C levels at 72 hours in patients with acute heart failure and renal dysfunction.
Citation: Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA. 2013;310(23):2533-2543.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Facecards improve familiarity with physician names, not satisfaction
- Pre-operative beta-blockers may benefit some cardiac patients
- Benefit of therapeutic hypothermia after cardiac arrest unclear
- Patients prefer inpatient boarding to ED boarding
- Triple rule outs for chest pain
- Colloids vs. crystalloids for critically ill patients presenting with hypovolemic shock
- Interdisciplinary intervention improves medication compliance, not blood pressure or LDL-C levels
- Edoxaban is noninferior to warfarin in Afib patients
- Beta blockers lower mortality after acute MI in COPD patients
- Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction
Facecards Improve Familiarity with Physician Names but Not Satisfaction
Clinical question: Do facecards improve patients’ familiarity with physicians and increase satisfaction, trust, and agreement with physicians?
Background: Facecards can improve patients’ knowledge of names and roles of physicians, but their impact on other outcomes is unclear. This pilot trial was designed to assess facecards’ impact on patient satisfaction, trust, or agreement with physicians.
Study design: Cluster, randomized controlled trial (RCT).
Setting: A large teaching hospital in the United States.
Synopsis: Patients (n=138) were randomized to receive either facecards with the name and picture of their hospitalists, as well as a brief description of the hospitalist’s role (n=66), or to receive traditional communication (n=72). There were no significant differences in patient age, sex, or race.
Patients who received a facecard were more likely to correctly identify their hospital physician (89.1% vs. 51.1%; P< 0.01) and were more likely to correctly identify the role of their hospital physician than those in the control group (67.4% vs. 16.3%; P<0.01).
Patients who received a facecard rated satisfaction, trust, and agreement slightly higher compared with those who had not received a card, but the results were not statistically significant (P values 0.27, 0.32, 0.37, respectively.) The authors note that larger studies may be needed to see a difference in these areas.
Bottom line: Facecards improve patients’ knowledge of the names and roles of hospital physicians but have no clear impact on satisfaction with, trust of, or agreement with physicians.
Citation: Simons Y, Caprio T, Furiasse N, Kriss, M, Williams MV, O’Leary KJ. The impact of facecards on patients’ knowledge, satisfaction, trust, and agreement with hospitalist physicians: a pilot study. J Hosp Med. 2014;9(3):137-141.
Pre-Operative Beta Blockers May Benefit Some Cardiac Patients
Clinical question: In patients with ischemic heart disease (IHD) undergoing non-cardiac surgery, do pre-operative beta blockers reduce post-operative major cardiovascular events (MACE) or mortality at 30 days?
Background: Peri-operative beta blocker use has become more restricted, as evidence about which patients derive benefit has become clearer. Opinions and practice vary regarding whether all patients with IHD, or only certain populations within this group, benefit from peri-operative beta blockers.
Study design: Retrospective, national registry-based cohort study.
Setting: Denmark, 2004-2009.
Synopsis: No benefit was found for the overall cohort of 28,263 patients. Patients with IHD and heart failure (n=7990) had lower risk of MACE (HR=0.75, 95% CI, 0.70-0.87) and mortality (HR=0.80, 95% CI, 0.70-0.92). Patients with IHD and myocardial infarction within two years (n=1664) had lower risk of MACE (HR=0.54, 95% CI, 0.37-0.78) but not mortality.
Beta blocker dose and compliance were unknown. Whether patients had symptoms or inducible ischemia was not clear.
This study supports the concept that higher-risk patients benefit more from peri-operative beta blockers, but it is not high-grade evidence.
Bottom line: Not all patients with IHD benefit from pre-operative beta blockers; those with concomitant heart failure or recent MI have a lower risk of MACE and/or mortality at 30 days with beta blockers.
Citation: Andersson C, Merie C, Jorgensen M, et al. Association of ß-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing non-cardiac surgery: a Danish nationwide cohort study. JAMA Intern Med. 2014;174(3):336-344.
Benefit of Therapeutic Hypothermia after Cardiac Arrest Unclear
Clinical question: Does targeted hypothermia (33°C) after cardiac arrest confer benefits compared with targeted temperature management at 36°C?
Background: Therapeutic hypothermia is a current recommendation in resuscitation guidelines after cardiac arrest. Fever develops in many patients after arrest, and it is unclear if the treatment benefit is due to hypothermia or due to the prevention of fever.
Study design: RCT.
Setting: ICUs in Europe and Australia.
Synopsis: The study authors randomized 950 patients who experienced out-of-hospital cardiac arrest to targeted temperature management at either 36°C or 33°C. The goal of this trial was to prevent fever in both groups during the first 36 hours after cardiac arrest. No statistically significant difference in outcomes between these two approaches was found. In the 33°C group, 54% died or had poor neurologic function, compared with 52% in the 36°C group (risk ratio 1.02; 95% CI 0.88 to 1.16; P=0.78).
Given the wide confidence interval, a trial with either more participants or more events might be able to determine whether a true difference in these management approaches exists.
Bottom line: Therapeutic hypothermia at 33°C after out-of-hospital cardiac arrest did not confer a benefit compared with targeted temperature management at 36°C.
Citation: Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197-2206.
Patients Prefer Inpatient Boarding to Emergency Department Boarding
Clinical question: Do patients who experience overcrowding and long waits in the emergency department (ED) prefer boarding within ED hallways or within inpatient medical unit hallways?
Background: Boarding of admitted patients in EDs can be problematic, especially with regard to patient safety and patient satisfaction. Patient satisfaction data comparing boarding in the ED versus boarding in an inpatient unit hallway is limited.
Study design: Post-discharge, structured, telephone satisfaction survey.
Setting: Suburban, university-based teaching hospital.
Synopsis: A group of patients who experienced hallway boarding in the ED and then hallway boarding on the inpatient medical unit were identified. They were contacted by phone and asked to take a survey on their experience; 105 of 110 patients identified agreed. Patients were asked to rate their location preference with regard to various aspects of care. A five-point Likert scale consisting of the following answers was used: ED hallway much better, ED hallway better, no preference, inpatient hallway better, and inpatient hallway much better.
The inpatient hallway was the overall preferred location in 85% of respondents. Respondents preferred inpatient boarding with regard to multiple other parameters: rest, 85%; safety, 83%; confidentiality, 82%; treatment, 78%; comfort, 79%; quiet, 84%; staff availability, 84%; and privacy, 84%. For no item was there a preference for boarding in the ED.
Patient demographics in this hospital may differ from other settings and should be considered when applying the results. With Hospital Consumer Assessment of Healthcare Providers and Systems scores and ED throughput being publicly reported, further studies in this area would be valuable.
Bottom line: In a post-discharge telephone survey, patients preferred boarding in inpatient unit hallways rather than boarding in the ED.
Citation: Viccellio P, Zito JA, Sayage V, et al. Patients overwhelmingly prefer inpatient boarding to emergency department boarding. J Emerg Med. 2013;45(6):942-946.
“Triple Rule Outs” for Chest Pain: A Tool to Evaluate the Coronaries but Not Pulmonary Embolism or Aortic Dissection
Clinical question: How does “triple rule out” (TRO) computed tomographic (CT) angiography compare to other imaging modalities in evaluating coronary and other life-threatening etiologies of chest pain, such as pulmonary embolism (PE) and aortic dissection?
Background: TRO CT angiography is a noninvasive technology that evaluates the coronary arteries, thoracic aorta, and pulmonary vasculature simultaneously. Comparison with other tests in the diagnosis of common clinical conditions is useful information for clinical practice.
Study design: Systematic review and meta-analysis.
Setting: Systematic review of 11 studies (one randomized, 10 observational).
Synopsis: Using an enrolled population of 3,539 patients, TRO CT was compared to other imaging modalities on the basis of image quality, diagnostic accuracy, radiation, and contrast volume. When TRO CT was compared to dedicated CT scans, no significant imaging difference was discovered. TRO CT detected CAD with a sensitivity of 94.3% (95% CI, 89.1% to 97.5%, I2=58.2%) and specificity of 97.4% (95% CI, 96.1% to 98.5%, I2=91.2%).
An insufficient number of patients with PE or aortic dissection were studied to generate diagnostic accuracy for these conditions. TRO CT involved greater radiation exposure and contrast exposure than non-TRO CT.
This study reports high accuracy of TRO CT in the diagnosis of coronary artery disease. Due to the low prevalence of patients with PE or aortic dissection (<1%), the data cannot be extrapolated to these conditions.
Bottom line: Although TRO CT is highly accurate for detecting coronary artery disease, there is insufficient data to recommend its use for the diagnosis of PE or aortic dissection.
Citation: Ayaram D, Bellolio MF, Murad MH, et al. Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med. 2013;20(9):861-871.
Colloids vs. Crystalloids for Critically Ill Patients Presenting with Hypovolemic Shock
Clinical question: In critically ill patients admitted to the ICU with hypovolemic shock, does the use of colloid for fluid resuscitation, compared with crystalloid, improve mortality?
Background: The current Surviving Sepsis Campaign guidelines recommend crystalloids as the preferred fluid for resuscitation of patients with hypovolemic shock; however, evidence supporting the choice of intravenous colloid vs. crystalloid solutions for management of hypovolemic shock is weak.
Study design: RCT.
Setting: International, multi-center study.
Synopsis: Researchers randomized 2,857 adult patients who were admitted to an ICU and required fluid resuscitation for acute hypovolemia to receive either crystalloids or colloids.
At 28 days, there were 359 deaths (25.4%) in the colloids group vs. 390 deaths (27.0%) in the crystalloids group (P=0.26). At 90 days, there were 434 deaths (30.7%) in the colloids group vs. 493 deaths (34.2%) in the crystalloids group (P=0.03).
Renal replacement therapy was used in 11.0% of the colloids group vs. 12.5% of the crystalloids group (P=0.19). There were more days alive without mechanical ventilation in the colloids group vs. the crystalloids group at seven days (P=0.01) and at 28 days (P=0.01), and there were more days alive without vasopressor therapy in the colloids group vs. the crystalloids group at seven days (P=0.04) and at 28 days (P=0.03).
Major limitations of the study included the use of open-labeled fluids during allocation, so the initial investigators were not blinded to the type of fluid. Moreover, the study compared two therapeutic strategies (colloid vs. crystalloids) rather than two types of molecules.
Bottom line: In ICU patients with hypovolemia requiring resuscitation, the use of colloids vs. crystalloids did not result in a significant difference in 28-day mortality; however, 90-day mortality was lower among patients receiving colloids.
Citation: Annane D, Siami S, Jaber S, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality of critically ill patients presenting with hypovolemic shock: the CRISTAL randomization trial. JAMA. 2013;310(17):1809-1817.
Interdisciplinary Intervention Improves Medication Compliance, Not Blood Pressure or LDL-C Levels
Clinical question: Can intervention by pharmacists and physicians improve compliance to cardio-protective medications?
Background: Adherence to cardio-protective medications in the year after hospitalization for acute coronary syndrome is poor.
Study design: RCT.
Setting: Four Department of Veterans Affairs medical centers.
Synopsis: The intervention consisted of pharmacist-led medication reconciliation, patient education, pharmacist and PCP +/- cardiologist collaboration, and voice messaging. The outcome measured was the proportion of patients adherent to medication regimens based on a mean proportion of days covered (PDC) >0.80 in the year after discharge, using pharmacy refill data for clopidogrel, beta blockers, statins, and ACEI/ARBs.
Two hundred forty-one patients (95.3%) completed the study. In the intervention group, 89.3% of patients were adherent vs. 73.9% in the usual care group (P=0.003). Mean PDC was higher in the intervention group (0.94 vs. 0.87; P<0.001). A greater proportion of intervention patients were adherent to clopidogrel (86.8% vs. 70.7%; P=0.03), statins (93.2% vs. 71.3%; P<0.001), and ACEI/ARBs (93.1% vs. 81.7%; P=0.03), but not beta blockers (88.1% vs. 84.8%; P=0.59). There were no statistically significant differences in the proportion of patients who achieved blood pressure and LDL-C level goals.
Bottom line: An interdisciplinary, multi-faceted intervention increased medication compliance in the year after discharge for ACS but did not improve blood pressure or LDL-C levels.
Citation: Ho PM, Lambert-Kerzner A, Carey EP, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge. JAMA Intern Med. 2014;174(2):186-193.
Edoxaban Is Noninferior to Warfarin in Patients with Atrial Fibrillation
Clinical question: What is the long-term efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation (Afib)?
Background: Edoxaban is an oral factor Xa inhibitor approved for use in Japan for the prevention of venous thromboembolism after orthopedic surgery. No specific antidote for edoxaban exists, but hemostatic agents can reverse its anticoagulation effect.
Study design: RCT.
Setting: More than 1,300 centers in 46 countries.
Synopsis: Researchers randomized 21,105 patients in a 1:1:1 ratio to receive warfarin (goal INR of 2-3), low-dose edoxaban, or high-dose edoxoban. All patients received two sets of drugs, either active warfarin with placebo edoxaban or active edoxaban (high- or low-dose) and placebo warfarin (with sham INRs drawn), and were followed for a median of 2.8 years.
The annualized rate of stroke or systemic embolic event was 1.5% in the warfarin group, compared with 1.18% in the high-dose edoxaban group (hazard ratio 0.79; P<0.001) and 1.61% in the low-dose edoxaban group (hazard ratio 1.07; P=0.005). Annualized rate of major bleeding was 3.43% with warfarin, 2.75% with high-dose edoxoban (hazard ratio 0.80; P<0.001), and 1.61% with low-dose edoxaban (hazard ratio 0.47; P<0.001).
Both edoxaban regimens were noninferior to warfarin for the prevention of stroke or systemic emboli. The rates of cardiovascular events, bleeding, or death from any cause was lower with both doses of edoxaban as compared with warfarin.
Bottom line: Once-daily edoxaban is noninferior to warfarin for the prevention of stroke or systemic emboli and is associated with lower rates of bleeding and death.
Citation: Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. New Engl J Med. 2013;369(22):2093-2104.
Beta Blockers Lower Mortality after Acute Myocardial Infarction in COPD Patients
Clinical question: Does the use and timing of beta blockers in COPD patients experiencing a first myocardial infarction (MI) affect survival after the event?
Background: Beta blockers are effective in reducing mortality and reinfarction after an MI; however, concerns regarding the side effects of beta blockers, such as bronchospasm, continue to limit their use in patients with COPD.
Study design: Population-based cohort study.
Setting: The Myocardial Ischemia National Audit Project, linked to the General Practice Research Database, in the United Kingdom.
Synopsis: Researchers identified 1,063 patients over the age of 18 with COPD admitted to the hospital with a first acute MI. Use of beta blockers during hospitalization was associated with increased overall and one-year survival. Initiation of beta blockers during an MI had a mortality-adjusted hazard ratio of 0.50 (95% CI 0.36 to 0.69; P<0.001; median follow-up time=2.9 years).
Patients already on beta blockers prior to the MI had overall survival-adjusted hazard ratio of 0.59 (95% CI 0.44 to 0.79; P<0.001). Both scenarios showed survival benefits compared to COPD patients who were not prescribed beta blockers. Patients given beta blockers with COPD either during the MI hospitalization or before the event were younger and had fewer comorbidities. This may have accounted for some of the survival bias.
Bottom line: The use of beta blockers in patients with COPD started prior to, or at the time of, hospital admission for a first MI is associated with improved survival.
Citation: Quint JK, Herret E, Bhaskaran K, et al. Effect of ß blockers on mortality after myocardial infarction in adults with COPD: population-based cohort study of UK electronic healthcare records. BMJ. 2013;347:f6650.
Neither Low-Dose Dopamine nor Low-Dose Nesiritide Improves Renal Dysfunction in Acute Heart Failure Patients
Clinical question: Does low-dose dopamine or low-dose nesiritide added to diuretic therapy enhance pulmonary volume reduction and preserve renal function in patients with acute heart failure and renal dysfunction, compared to placebo?
Background: Small studies have suggested that low-dose dopamine or low-dose nesiritide may be beneficial in enhancing decongestion and improving renal dysfunction; however, there is ambiguity in overall benefit. Some observational studies suggest that dopamine and nesiritide are associated with higher length of stay, higher costs, and greater mortality.
Study Design: RCT.
Setting: Twenty-six hospital sites in the U.S. and Canada.
Synopsis: Three hundred sixty patients with acute heart failure and renal dysfunction were randomized to receive either nesiritide or dopamine within 24 hours of admission. Within each of these arms, patients were then randomized, in a double-blinded 2:1 fashion, into active treatment versus placebo groups. Treatment groups were compared to the pooled placebo groups.
Two main endpoints were urine output and change in serum cystatin C, from enrollment to 72 hours. Compared with placebo, low-dose dopamine had no significant effect on urine output or serum cystatin C level. Similarly, low-dose nesiritide had no significant effect on 72-hour urine output or serum cystatin C level.
Other studies have shown these drugs to be potentially harmful. Hospitalists should use caution and carefully interpret the relevant evidence when considering their use.
Bottom line: Neither low-dose nesiritide nor low-dose dopamine improved urine output or serum cystatin C levels at 72 hours in patients with acute heart failure and renal dysfunction.
Citation: Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA. 2013;310(23):2533-2543.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Facecards improve familiarity with physician names, not satisfaction
- Pre-operative beta-blockers may benefit some cardiac patients
- Benefit of therapeutic hypothermia after cardiac arrest unclear
- Patients prefer inpatient boarding to ED boarding
- Triple rule outs for chest pain
- Colloids vs. crystalloids for critically ill patients presenting with hypovolemic shock
- Interdisciplinary intervention improves medication compliance, not blood pressure or LDL-C levels
- Edoxaban is noninferior to warfarin in Afib patients
- Beta blockers lower mortality after acute MI in COPD patients
- Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction
Facecards Improve Familiarity with Physician Names but Not Satisfaction
Clinical question: Do facecards improve patients’ familiarity with physicians and increase satisfaction, trust, and agreement with physicians?
Background: Facecards can improve patients’ knowledge of names and roles of physicians, but their impact on other outcomes is unclear. This pilot trial was designed to assess facecards’ impact on patient satisfaction, trust, or agreement with physicians.
Study design: Cluster, randomized controlled trial (RCT).
Setting: A large teaching hospital in the United States.
Synopsis: Patients (n=138) were randomized to receive either facecards with the name and picture of their hospitalists, as well as a brief description of the hospitalist’s role (n=66), or to receive traditional communication (n=72). There were no significant differences in patient age, sex, or race.
Patients who received a facecard were more likely to correctly identify their hospital physician (89.1% vs. 51.1%; P< 0.01) and were more likely to correctly identify the role of their hospital physician than those in the control group (67.4% vs. 16.3%; P<0.01).
Patients who received a facecard rated satisfaction, trust, and agreement slightly higher compared with those who had not received a card, but the results were not statistically significant (P values 0.27, 0.32, 0.37, respectively.) The authors note that larger studies may be needed to see a difference in these areas.
Bottom line: Facecards improve patients’ knowledge of the names and roles of hospital physicians but have no clear impact on satisfaction with, trust of, or agreement with physicians.
Citation: Simons Y, Caprio T, Furiasse N, Kriss, M, Williams MV, O’Leary KJ. The impact of facecards on patients’ knowledge, satisfaction, trust, and agreement with hospitalist physicians: a pilot study. J Hosp Med. 2014;9(3):137-141.
Pre-Operative Beta Blockers May Benefit Some Cardiac Patients
Clinical question: In patients with ischemic heart disease (IHD) undergoing non-cardiac surgery, do pre-operative beta blockers reduce post-operative major cardiovascular events (MACE) or mortality at 30 days?
Background: Peri-operative beta blocker use has become more restricted, as evidence about which patients derive benefit has become clearer. Opinions and practice vary regarding whether all patients with IHD, or only certain populations within this group, benefit from peri-operative beta blockers.
Study design: Retrospective, national registry-based cohort study.
Setting: Denmark, 2004-2009.
Synopsis: No benefit was found for the overall cohort of 28,263 patients. Patients with IHD and heart failure (n=7990) had lower risk of MACE (HR=0.75, 95% CI, 0.70-0.87) and mortality (HR=0.80, 95% CI, 0.70-0.92). Patients with IHD and myocardial infarction within two years (n=1664) had lower risk of MACE (HR=0.54, 95% CI, 0.37-0.78) but not mortality.
Beta blocker dose and compliance were unknown. Whether patients had symptoms or inducible ischemia was not clear.
This study supports the concept that higher-risk patients benefit more from peri-operative beta blockers, but it is not high-grade evidence.
Bottom line: Not all patients with IHD benefit from pre-operative beta blockers; those with concomitant heart failure or recent MI have a lower risk of MACE and/or mortality at 30 days with beta blockers.
Citation: Andersson C, Merie C, Jorgensen M, et al. Association of ß-blocker therapy with risks of adverse cardiovascular events and deaths in patients with ischemic heart disease undergoing non-cardiac surgery: a Danish nationwide cohort study. JAMA Intern Med. 2014;174(3):336-344.
Benefit of Therapeutic Hypothermia after Cardiac Arrest Unclear
Clinical question: Does targeted hypothermia (33°C) after cardiac arrest confer benefits compared with targeted temperature management at 36°C?
Background: Therapeutic hypothermia is a current recommendation in resuscitation guidelines after cardiac arrest. Fever develops in many patients after arrest, and it is unclear if the treatment benefit is due to hypothermia or due to the prevention of fever.
Study design: RCT.
Setting: ICUs in Europe and Australia.
Synopsis: The study authors randomized 950 patients who experienced out-of-hospital cardiac arrest to targeted temperature management at either 36°C or 33°C. The goal of this trial was to prevent fever in both groups during the first 36 hours after cardiac arrest. No statistically significant difference in outcomes between these two approaches was found. In the 33°C group, 54% died or had poor neurologic function, compared with 52% in the 36°C group (risk ratio 1.02; 95% CI 0.88 to 1.16; P=0.78).
Given the wide confidence interval, a trial with either more participants or more events might be able to determine whether a true difference in these management approaches exists.
Bottom line: Therapeutic hypothermia at 33°C after out-of-hospital cardiac arrest did not confer a benefit compared with targeted temperature management at 36°C.
Citation: Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369(23):2197-2206.
Patients Prefer Inpatient Boarding to Emergency Department Boarding
Clinical question: Do patients who experience overcrowding and long waits in the emergency department (ED) prefer boarding within ED hallways or within inpatient medical unit hallways?
Background: Boarding of admitted patients in EDs can be problematic, especially with regard to patient safety and patient satisfaction. Patient satisfaction data comparing boarding in the ED versus boarding in an inpatient unit hallway is limited.
Study design: Post-discharge, structured, telephone satisfaction survey.
Setting: Suburban, university-based teaching hospital.
Synopsis: A group of patients who experienced hallway boarding in the ED and then hallway boarding on the inpatient medical unit were identified. They were contacted by phone and asked to take a survey on their experience; 105 of 110 patients identified agreed. Patients were asked to rate their location preference with regard to various aspects of care. A five-point Likert scale consisting of the following answers was used: ED hallway much better, ED hallway better, no preference, inpatient hallway better, and inpatient hallway much better.
The inpatient hallway was the overall preferred location in 85% of respondents. Respondents preferred inpatient boarding with regard to multiple other parameters: rest, 85%; safety, 83%; confidentiality, 82%; treatment, 78%; comfort, 79%; quiet, 84%; staff availability, 84%; and privacy, 84%. For no item was there a preference for boarding in the ED.
Patient demographics in this hospital may differ from other settings and should be considered when applying the results. With Hospital Consumer Assessment of Healthcare Providers and Systems scores and ED throughput being publicly reported, further studies in this area would be valuable.
Bottom line: In a post-discharge telephone survey, patients preferred boarding in inpatient unit hallways rather than boarding in the ED.
Citation: Viccellio P, Zito JA, Sayage V, et al. Patients overwhelmingly prefer inpatient boarding to emergency department boarding. J Emerg Med. 2013;45(6):942-946.
“Triple Rule Outs” for Chest Pain: A Tool to Evaluate the Coronaries but Not Pulmonary Embolism or Aortic Dissection
Clinical question: How does “triple rule out” (TRO) computed tomographic (CT) angiography compare to other imaging modalities in evaluating coronary and other life-threatening etiologies of chest pain, such as pulmonary embolism (PE) and aortic dissection?
Background: TRO CT angiography is a noninvasive technology that evaluates the coronary arteries, thoracic aorta, and pulmonary vasculature simultaneously. Comparison with other tests in the diagnosis of common clinical conditions is useful information for clinical practice.
Study design: Systematic review and meta-analysis.
Setting: Systematic review of 11 studies (one randomized, 10 observational).
Synopsis: Using an enrolled population of 3,539 patients, TRO CT was compared to other imaging modalities on the basis of image quality, diagnostic accuracy, radiation, and contrast volume. When TRO CT was compared to dedicated CT scans, no significant imaging difference was discovered. TRO CT detected CAD with a sensitivity of 94.3% (95% CI, 89.1% to 97.5%, I2=58.2%) and specificity of 97.4% (95% CI, 96.1% to 98.5%, I2=91.2%).
An insufficient number of patients with PE or aortic dissection were studied to generate diagnostic accuracy for these conditions. TRO CT involved greater radiation exposure and contrast exposure than non-TRO CT.
This study reports high accuracy of TRO CT in the diagnosis of coronary artery disease. Due to the low prevalence of patients with PE or aortic dissection (<1%), the data cannot be extrapolated to these conditions.
Bottom line: Although TRO CT is highly accurate for detecting coronary artery disease, there is insufficient data to recommend its use for the diagnosis of PE or aortic dissection.
Citation: Ayaram D, Bellolio MF, Murad MH, et al. Triple rule-out computed tomographic angiography for chest pain: a diagnostic systematic review and meta-analysis. Acad Emerg Med. 2013;20(9):861-871.
Colloids vs. Crystalloids for Critically Ill Patients Presenting with Hypovolemic Shock
Clinical question: In critically ill patients admitted to the ICU with hypovolemic shock, does the use of colloid for fluid resuscitation, compared with crystalloid, improve mortality?
Background: The current Surviving Sepsis Campaign guidelines recommend crystalloids as the preferred fluid for resuscitation of patients with hypovolemic shock; however, evidence supporting the choice of intravenous colloid vs. crystalloid solutions for management of hypovolemic shock is weak.
Study design: RCT.
Setting: International, multi-center study.
Synopsis: Researchers randomized 2,857 adult patients who were admitted to an ICU and required fluid resuscitation for acute hypovolemia to receive either crystalloids or colloids.
At 28 days, there were 359 deaths (25.4%) in the colloids group vs. 390 deaths (27.0%) in the crystalloids group (P=0.26). At 90 days, there were 434 deaths (30.7%) in the colloids group vs. 493 deaths (34.2%) in the crystalloids group (P=0.03).
Renal replacement therapy was used in 11.0% of the colloids group vs. 12.5% of the crystalloids group (P=0.19). There were more days alive without mechanical ventilation in the colloids group vs. the crystalloids group at seven days (P=0.01) and at 28 days (P=0.01), and there were more days alive without vasopressor therapy in the colloids group vs. the crystalloids group at seven days (P=0.04) and at 28 days (P=0.03).
Major limitations of the study included the use of open-labeled fluids during allocation, so the initial investigators were not blinded to the type of fluid. Moreover, the study compared two therapeutic strategies (colloid vs. crystalloids) rather than two types of molecules.
Bottom line: In ICU patients with hypovolemia requiring resuscitation, the use of colloids vs. crystalloids did not result in a significant difference in 28-day mortality; however, 90-day mortality was lower among patients receiving colloids.
Citation: Annane D, Siami S, Jaber S, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality of critically ill patients presenting with hypovolemic shock: the CRISTAL randomization trial. JAMA. 2013;310(17):1809-1817.
Interdisciplinary Intervention Improves Medication Compliance, Not Blood Pressure or LDL-C Levels
Clinical question: Can intervention by pharmacists and physicians improve compliance to cardio-protective medications?
Background: Adherence to cardio-protective medications in the year after hospitalization for acute coronary syndrome is poor.
Study design: RCT.
Setting: Four Department of Veterans Affairs medical centers.
Synopsis: The intervention consisted of pharmacist-led medication reconciliation, patient education, pharmacist and PCP +/- cardiologist collaboration, and voice messaging. The outcome measured was the proportion of patients adherent to medication regimens based on a mean proportion of days covered (PDC) >0.80 in the year after discharge, using pharmacy refill data for clopidogrel, beta blockers, statins, and ACEI/ARBs.
Two hundred forty-one patients (95.3%) completed the study. In the intervention group, 89.3% of patients were adherent vs. 73.9% in the usual care group (P=0.003). Mean PDC was higher in the intervention group (0.94 vs. 0.87; P<0.001). A greater proportion of intervention patients were adherent to clopidogrel (86.8% vs. 70.7%; P=0.03), statins (93.2% vs. 71.3%; P<0.001), and ACEI/ARBs (93.1% vs. 81.7%; P=0.03), but not beta blockers (88.1% vs. 84.8%; P=0.59). There were no statistically significant differences in the proportion of patients who achieved blood pressure and LDL-C level goals.
Bottom line: An interdisciplinary, multi-faceted intervention increased medication compliance in the year after discharge for ACS but did not improve blood pressure or LDL-C levels.
Citation: Ho PM, Lambert-Kerzner A, Carey EP, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge. JAMA Intern Med. 2014;174(2):186-193.
Edoxaban Is Noninferior to Warfarin in Patients with Atrial Fibrillation
Clinical question: What is the long-term efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation (Afib)?
Background: Edoxaban is an oral factor Xa inhibitor approved for use in Japan for the prevention of venous thromboembolism after orthopedic surgery. No specific antidote for edoxaban exists, but hemostatic agents can reverse its anticoagulation effect.
Study design: RCT.
Setting: More than 1,300 centers in 46 countries.
Synopsis: Researchers randomized 21,105 patients in a 1:1:1 ratio to receive warfarin (goal INR of 2-3), low-dose edoxaban, or high-dose edoxoban. All patients received two sets of drugs, either active warfarin with placebo edoxaban or active edoxaban (high- or low-dose) and placebo warfarin (with sham INRs drawn), and were followed for a median of 2.8 years.
The annualized rate of stroke or systemic embolic event was 1.5% in the warfarin group, compared with 1.18% in the high-dose edoxaban group (hazard ratio 0.79; P<0.001) and 1.61% in the low-dose edoxaban group (hazard ratio 1.07; P=0.005). Annualized rate of major bleeding was 3.43% with warfarin, 2.75% with high-dose edoxoban (hazard ratio 0.80; P<0.001), and 1.61% with low-dose edoxaban (hazard ratio 0.47; P<0.001).
Both edoxaban regimens were noninferior to warfarin for the prevention of stroke or systemic emboli. The rates of cardiovascular events, bleeding, or death from any cause was lower with both doses of edoxaban as compared with warfarin.
Bottom line: Once-daily edoxaban is noninferior to warfarin for the prevention of stroke or systemic emboli and is associated with lower rates of bleeding and death.
Citation: Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. New Engl J Med. 2013;369(22):2093-2104.
Beta Blockers Lower Mortality after Acute Myocardial Infarction in COPD Patients
Clinical question: Does the use and timing of beta blockers in COPD patients experiencing a first myocardial infarction (MI) affect survival after the event?
Background: Beta blockers are effective in reducing mortality and reinfarction after an MI; however, concerns regarding the side effects of beta blockers, such as bronchospasm, continue to limit their use in patients with COPD.
Study design: Population-based cohort study.
Setting: The Myocardial Ischemia National Audit Project, linked to the General Practice Research Database, in the United Kingdom.
Synopsis: Researchers identified 1,063 patients over the age of 18 with COPD admitted to the hospital with a first acute MI. Use of beta blockers during hospitalization was associated with increased overall and one-year survival. Initiation of beta blockers during an MI had a mortality-adjusted hazard ratio of 0.50 (95% CI 0.36 to 0.69; P<0.001; median follow-up time=2.9 years).
Patients already on beta blockers prior to the MI had overall survival-adjusted hazard ratio of 0.59 (95% CI 0.44 to 0.79; P<0.001). Both scenarios showed survival benefits compared to COPD patients who were not prescribed beta blockers. Patients given beta blockers with COPD either during the MI hospitalization or before the event were younger and had fewer comorbidities. This may have accounted for some of the survival bias.
Bottom line: The use of beta blockers in patients with COPD started prior to, or at the time of, hospital admission for a first MI is associated with improved survival.
Citation: Quint JK, Herret E, Bhaskaran K, et al. Effect of ß blockers on mortality after myocardial infarction in adults with COPD: population-based cohort study of UK electronic healthcare records. BMJ. 2013;347:f6650.
Neither Low-Dose Dopamine nor Low-Dose Nesiritide Improves Renal Dysfunction in Acute Heart Failure Patients
Clinical question: Does low-dose dopamine or low-dose nesiritide added to diuretic therapy enhance pulmonary volume reduction and preserve renal function in patients with acute heart failure and renal dysfunction, compared to placebo?
Background: Small studies have suggested that low-dose dopamine or low-dose nesiritide may be beneficial in enhancing decongestion and improving renal dysfunction; however, there is ambiguity in overall benefit. Some observational studies suggest that dopamine and nesiritide are associated with higher length of stay, higher costs, and greater mortality.
Study Design: RCT.
Setting: Twenty-six hospital sites in the U.S. and Canada.
Synopsis: Three hundred sixty patients with acute heart failure and renal dysfunction were randomized to receive either nesiritide or dopamine within 24 hours of admission. Within each of these arms, patients were then randomized, in a double-blinded 2:1 fashion, into active treatment versus placebo groups. Treatment groups were compared to the pooled placebo groups.
Two main endpoints were urine output and change in serum cystatin C, from enrollment to 72 hours. Compared with placebo, low-dose dopamine had no significant effect on urine output or serum cystatin C level. Similarly, low-dose nesiritide had no significant effect on 72-hour urine output or serum cystatin C level.
Other studies have shown these drugs to be potentially harmful. Hospitalists should use caution and carefully interpret the relevant evidence when considering their use.
Bottom line: Neither low-dose nesiritide nor low-dose dopamine improved urine output or serum cystatin C levels at 72 hours in patients with acute heart failure and renal dysfunction.
Citation: Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: The ROSE acute heart failure randomized trial. JAMA. 2013;310(23):2533-2543.
How to Manage Pain in Patients with Renal Insufficiency or End-Stage Renal Disease on Dialysis?
Case
A 70-year-old male with ESRD on hemodialysis presents with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and ankle pain after a fall. An MRI of his ankle is negative, and he is started on acetaminophen and lidocaine patches, which result in adequate pain relief of the ankle. He later develops significant neuropathic pain in both arms, and a CT scan of the cervical spine reveals a cervical abscess and osteomyelitis. The patient desires pain relief but adamantly refuses narcotics, stating: “I don’t want to get addicted.” How can his pain be managed?
Overview
Pain is a common problem in patients with renal insufficiency and end-stage renal disease (ESRD) and can have a significant effect on the patient’s quality of life.1 When assessing a patient’s pain, assess both the severity of the pain (such as on an analogue scale, 0-10) and the characteristics of the pain. Pain is most commonly characterized as nociceptive, neuropathic, or both. Nociceptive pain can be further classified as arising from either somatic or visceral sources, and is often described as dull, throbbing, cramping, and/or pressurelike.1 Neuropathic pain is often described as tingling, numbing, burning, and/or stabbing.
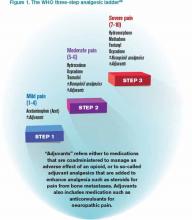
It is a challenge to manage pain in patients with renal insufficiency and dialysis. Renal insufficiency affects the pharmacokinetic properties of most pain medications, including their distribution, clearance, and excretion. The magnitude of the effect of renal insufficiency on drug metabolism varies depending on the agent itself, its metabolite, and the extent of renal failure.3 Multiple factors should be considered when prescribing pain medications for patients on dialysis, including the properties of the parent drug and its metabolites; the physical properties of the dialysis equipment, such as the filter pore size, the flow rate, and the efficiency of the technique used; and the dialysis method (intermittent versus continuous).3 Table 1 provides the recommended dosing of the most commonly prescribed agents, based on the degree of renal impairment. A modified World Health Organization (WHO) ladder has been suggested to treat pain in patients with ESRD, which can lead to effective pain relief in as many as 96% of patients (see Figure 1).2
*Beginning dose: If switching from IR to ER, calculate 24-hour total dose.
**For patients with creatinine clearances (CrCl) of 15 mL/min or less, the daily dosage should be adjusted proportionally (e.g. patients with a CrCl of 7.5 mL/min should receive one-half the dose of a patient with a CrCl of 15 mL/min).
Review of Data
Nonopioid options. Nonopioids, such as acetaminophen and NSAIDs, have no associated tolerance but have a ceiling effect for analgesia, and NSAIDs are associated with dose-dependent acute renal failure, gastrointestinal ulceration and bleeding, and cardiac events. The nonopioids that are considered safe options in patients with renal insufficiency include acetaminophen, ibuprofen, and fenoprofen (Nalfon). However, in the elderly, American Geriatric Society (AGS) guidelines currently recommend avoiding all NSAIDs due to their safety profile in the geriatric population.4 Although all NSAIDs can potentially be used for pain, selected NSAIDs with an FDA indication for acute or chronic pain were included for this review.
Acetaminophen (APAP) is a dialyzable compound that is metabolized in the liver to five inactive metabolites. The terminal elimination half-life of its sulfate and glucuronide metabolites are prolonged in patients with renal failure; therefore, the dosing interval of APAP should be increased to six to eight hours in renally impaired patients.5,6,7 Overall, acetaminophen is considered one of the safest agents to use for the treatment of pain, in renal patients and otherwise, as long as dosing is below the minimal daily dose (see Table 1).
Ibuprofen is metabolized in the liver to inactive compounds. It does not accumulate in renal insufficiency, and two of the inactive compounds are dialyzable.8 It is considered a safe option for the treatment of pain in patients with renal insufficiency or dialysis.9
Fenoprofen is metabolized in the liver to inactive compounds. Renal impairment is likely to cause the accumulation of the inactive metabolites but not the parent compound, so dose reduction is not necessary with the use of this agent in renal insufficiency or dialysis.6
Mefenamic acid (Ponstel) is metabolized in the liver. Mefenamic acid can further deteriorate renal function in patients with underlying renal disease.12 However, the nephrotoxic potential of this agent is of little consideration in ESRD patients on dialysis, and therefore no dosage adjustments are necessary in these patients.6
Ketoprofen is metabolized in the liver, where approximately 80% of the dose is excreted in the urine as a glucuronide metabolite. Dose reduction is recommended in renal insufficiency and dialysis, as it not dialyzable.8
Ketorolac accumulates in renal insufficiency; therefore, it is contraindicated in these patients and in patients at risk for renal failure, including those with volume depletion.10 Ketorolac is unlikely to be removed by dialysis and so should be avoided.10,11
Naproxen is metabolized in the liver to inactive compounds. Use of naproxen is not recommended in patients with moderate to severe renal impairment. If therapy must be initiated, close monitoring of the patient’s renal function is recommended.13
Celecoxib is the only cyclooxygenase-2 (COX-2) inhibitor available in the U.S. It is metabolized extensively by the liver and is unlikely to be removed by dialysis. Therefore, use of COX-2 inhibitors should be avoided in severe renal impairment and in those on dialysis.14,15
Opioid options. The use of opioids in the renally impaired population is challenging, as one must balance opioid-related adverse events with adequate pain control. As such, it is recommended to start with lower-than-recommended doses and slowly titrate up the dose while extending the dosing interval. This will help limit adverse effects, such as respiratory depression and hypotension.3
Hydrocodone is metabolized to hydromorphone (Dilaudid), which is then metabolized to its major metabolite hydromorphine-3-glucuronide (H3G) and minor metabolite hydromorphine-6-hydroxy, all of which are excreted renally along with the parent compound. H3G has no analgesic properties, but it can potentially cause neuroexcitation, agitation, confusion, and hallucination. Hydromorphone has been used safely in patients with renal insufficiency and dialysis, as it is expected to be dialyzable. 16,17
Tramadol is metabolized in the liver, producing one active compound. Approximately 30% of the tramadol dose is excreted unchanged in the urine, whereas 60% of the dose is excreted as metabolites. It is recommended to reduce the dose and increase the dosing interval in patients with renal insufficiency, but tramadol is generally well-tolerated in patients with renal insufficiency and dialysis. It is significantly removed by hemodialysis; therefore, redosing after a session may be necessary.18,19
Oxycodone can be used in patients with mild to moderate renal insufficiency but should be used at reduced dosing; it has been associated with significant sedation with usual doses in renal failure patients.16 Its use is generally not recommended in dialysis patients due to lack of data.3
Methadone and its metabolites are excreted in the urine and feces. Methadone has been used safely in patients with renal insufficiency, but it is poorly removed by dialysis and no specific recommendations are available regarding its dosing in dialysis.3,16
Fentanyl is primarily metabolized in the liver to inactive metabolites. Fentanyl clearance is reduced in patients with moderate to severe uremia (BUN >60 mg/dL). It is not expected that fentanyl be dialyzable because of its pharmacokinetic properties (high protein-binding, low water solubility, high molecular weight, and high volume of distribution). Data suggests that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in dialysis patients, although reduced doses may be prudent. Such patients should be monitored for signs of gradual accumulation of the parent drug.3,16
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), all of which are excreted renally, along with the parent compound. Only M6G has analgesic properties, and when it accumulates, it can lead to CNS depression. M3G is associated with behavioral excitation, a side effect that is further magnified in patients with renal insufficiency. Although morphine is dialyzable, it should generally be avoided in patients with any level of renal insufficiency.16,17,20,21
Codeine is metabolized to several active metabolites, all of which are renally excreted. Lower-than-usual doses are recommended in patients with renal insufficiency, and it should be avoided altogether in dialysis patients.3,16
Meperidine is metabolized in the liver to various metabolites, primarily normeperidine, which is toxic and has a long half-life, five to 10 times longer then meperidine. Meperidine should not be used in patients with renal insufficiency or dialysis.3
Adjunctive therapeutic options. Lidocaine patches currently are only FDA-indicated for postherpetic neuralgia but are used for a wide variety of local pain syndromes. Absorption of lidocaine is determined by the duration of application and the surface area over which it is applied. There is no appreciable accumulation of lidocaine or its metabolites in renal insufficiency; therefore, dose adjustments are not required.22,23
Gabapentin is FDA-indicated for partial seizures and postherpetic neuralgia but is also used for a wide variety of neuropathic pain syndromes, including postoperative pain.24 Gabapentin is not metabolized and is excreted in the urine unchanged. Renal clearance of gabapentin is reduced by 40% and the elimination half-life is increased up to 52 hours in renal insufficiency, but it is dialyzable. Therefore, dose adjustments are required with gabapentin in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.25-27
Pregabalin is structurally related to gabapentin and is indicated for a variety of neuropathic pain conditions. Pregabalin is 90% excreted unchanged in the urine, and approximately 50% of drug is removed after four hours of hemodialysis. Dose adjustments are required in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.28
Antidepressant options. Amitriptyline, nortryptiline, and desipramine are the tricyclic antidepressants (TCAs) commonly used for neuropathic pain. TCAs are metabolized in the liver to inactive metabolites, with the exception of amitriptyline, which is metabolized to nortryptiline. Common side effects reported with TCAs include postural hypotension and anticholinergic side effects, such as constipation, urinary retention, blurred vision, dry mouth, delirium, and sedation. It is unlikely that the TCAs can be removed by dialysis. It is suggested that the dosage be reduced in renal insufficiency and that anticholinergic side effects be monitored.29
Back to the Case
The patient’s ankle pain was controlled with acetaminophen and lidocaine patches. For the neuropathic pain in his upper extremities, tramadol was started at 25 mg oral every 12 hours and increased to 50 mg oral every eight hours (below the maximum of 200 mg a day). The tramadol did not result in adequate pain relief, so gabapentin 100 mg at bedtime was initiated, then increased to twice daily over three days with some relief.
A geriatric consult was obtained to help educate him regarding addiction to opioids, as well as to explore goals of care, but he continued to insist on the use of a non-narcotic regimen for his pain.
Bottom Line
Pain management in patients with renal insufficiency and dialysis can be challenging, but there are a number of safe non-narcotic and narcotic pain regimens that can be safely used in this patient population.
Dr. Harisingani is a board-certified hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y., and Drs. Saad and Cassagnol are assistant clinical professors at St. Johns University College of Pharmacy and Health Sciences in Jamaica, N.Y., and clinical pharmacy coordinators at Long Island Jewish Medical Center.
References
- Mid-Atlantic Renal Coalition and the Kidney End-of-Life Coalition. Clinical algorithm & preferred medications to treat pain in dialysis patients. Coalition for Supportive Care of Kidney Patients website. Available at: http://www.kidneysupportivecare.org/Physicians-Clinicians/Pain—Symptom-Management.aspx. Accessed Nov. 18, 2012.
- Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198-3203.
- Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. Pain Treatment Topics website. Available at: http://pain-topics.org/pdf/Opioids-Renal-Hepatic-Dysfunction.pdf. Accessed Nov. 28, 2012.
- Ferrell B, Argoff CE, Epplin J, et al. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291-297.
- Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain. 2006;6(3):137-148.
- Berg KJ, Djøseland O, Gjellan A, et al. Acute effects of paracetamol on prostaglandin synthesis and renal function in normal man and in patients with renal failure. Clin Nephrol. 1990;34:255-262.
- Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 R.P.) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976;1976(0):45-52.
- Shen CH, Hung CJ, Wu CC, Huang HW, Ho WM. Rhabdomyolysis-induced acute renal failure after morphine overdose—a case report. Acta Anaesthesiol Sin. 1999;37(3):159-162.
- Ketorolac tromethamine oral tablets [package insert]. St. Louis: Ethex Corp.: 2008.
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23:415-427. Erratum in: Clin Pharmacokinet. 1999;24(3):270.
- Ponstel [package insert]. Alpharetta, GA: First Horizon Pharmaceutical Corp.; 2006.
- Naprosyn [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Celebrex [package insert]. New York: G.D. Searle LLC; 2011.
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735-741.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497-504.
- Lee MA, Leng ME, Tiernan EJ. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001;15(1):26-34.
- Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCI. Am J. Med. 1996;101(1A):47S-53S.
- Izzedine H, Launay-Vacher V, Abbara C, Aymard G, Bassilios N, Deray G. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron. 2002;92(3):755-756.
- Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24(4):344-354.
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25(1):74-91.
- Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010.
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447-459.
- Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;15:126(1-3):91-101.
- Neurontin [package insert]. New York: Parke-Davis; 2010.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
- Srivastava U, Kumar A, Saxena S, et al: Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27(N4):331-335.
- Lyrica [package insert]. New York: Pfizer Inc.; 2012.
- Broadbent A, Khor K, Heaney A. Palliation and chronic renal failure: opioid and other palliative medications—dosage guidelines. Progress in Palliative Care. 2003;11(4):183-190(8).
- Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol. 2011;24(1):35-40.
- Wolters Kluwer Health. Facts & comparisons. Wolters Kluwer Health website. Available at: http://www.factsandcomparisons.com. Accessed Jan. 14, 2013.
- Lexicomp. Lexicomp Online. Lexicomp website. Available at: http://www.lexi.com/institutions/products/online/.
Case
A 70-year-old male with ESRD on hemodialysis presents with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and ankle pain after a fall. An MRI of his ankle is negative, and he is started on acetaminophen and lidocaine patches, which result in adequate pain relief of the ankle. He later develops significant neuropathic pain in both arms, and a CT scan of the cervical spine reveals a cervical abscess and osteomyelitis. The patient desires pain relief but adamantly refuses narcotics, stating: “I don’t want to get addicted.” How can his pain be managed?
Overview
Pain is a common problem in patients with renal insufficiency and end-stage renal disease (ESRD) and can have a significant effect on the patient’s quality of life.1 When assessing a patient’s pain, assess both the severity of the pain (such as on an analogue scale, 0-10) and the characteristics of the pain. Pain is most commonly characterized as nociceptive, neuropathic, or both. Nociceptive pain can be further classified as arising from either somatic or visceral sources, and is often described as dull, throbbing, cramping, and/or pressurelike.1 Neuropathic pain is often described as tingling, numbing, burning, and/or stabbing.
It is a challenge to manage pain in patients with renal insufficiency and dialysis. Renal insufficiency affects the pharmacokinetic properties of most pain medications, including their distribution, clearance, and excretion. The magnitude of the effect of renal insufficiency on drug metabolism varies depending on the agent itself, its metabolite, and the extent of renal failure.3 Multiple factors should be considered when prescribing pain medications for patients on dialysis, including the properties of the parent drug and its metabolites; the physical properties of the dialysis equipment, such as the filter pore size, the flow rate, and the efficiency of the technique used; and the dialysis method (intermittent versus continuous).3 Table 1 provides the recommended dosing of the most commonly prescribed agents, based on the degree of renal impairment. A modified World Health Organization (WHO) ladder has been suggested to treat pain in patients with ESRD, which can lead to effective pain relief in as many as 96% of patients (see Figure 1).2
*Beginning dose: If switching from IR to ER, calculate 24-hour total dose.
**For patients with creatinine clearances (CrCl) of 15 mL/min or less, the daily dosage should be adjusted proportionally (e.g. patients with a CrCl of 7.5 mL/min should receive one-half the dose of a patient with a CrCl of 15 mL/min).
Review of Data
Nonopioid options. Nonopioids, such as acetaminophen and NSAIDs, have no associated tolerance but have a ceiling effect for analgesia, and NSAIDs are associated with dose-dependent acute renal failure, gastrointestinal ulceration and bleeding, and cardiac events. The nonopioids that are considered safe options in patients with renal insufficiency include acetaminophen, ibuprofen, and fenoprofen (Nalfon). However, in the elderly, American Geriatric Society (AGS) guidelines currently recommend avoiding all NSAIDs due to their safety profile in the geriatric population.4 Although all NSAIDs can potentially be used for pain, selected NSAIDs with an FDA indication for acute or chronic pain were included for this review.
Acetaminophen (APAP) is a dialyzable compound that is metabolized in the liver to five inactive metabolites. The terminal elimination half-life of its sulfate and glucuronide metabolites are prolonged in patients with renal failure; therefore, the dosing interval of APAP should be increased to six to eight hours in renally impaired patients.5,6,7 Overall, acetaminophen is considered one of the safest agents to use for the treatment of pain, in renal patients and otherwise, as long as dosing is below the minimal daily dose (see Table 1).
Ibuprofen is metabolized in the liver to inactive compounds. It does not accumulate in renal insufficiency, and two of the inactive compounds are dialyzable.8 It is considered a safe option for the treatment of pain in patients with renal insufficiency or dialysis.9
Fenoprofen is metabolized in the liver to inactive compounds. Renal impairment is likely to cause the accumulation of the inactive metabolites but not the parent compound, so dose reduction is not necessary with the use of this agent in renal insufficiency or dialysis.6
Mefenamic acid (Ponstel) is metabolized in the liver. Mefenamic acid can further deteriorate renal function in patients with underlying renal disease.12 However, the nephrotoxic potential of this agent is of little consideration in ESRD patients on dialysis, and therefore no dosage adjustments are necessary in these patients.6
Ketoprofen is metabolized in the liver, where approximately 80% of the dose is excreted in the urine as a glucuronide metabolite. Dose reduction is recommended in renal insufficiency and dialysis, as it not dialyzable.8
Ketorolac accumulates in renal insufficiency; therefore, it is contraindicated in these patients and in patients at risk for renal failure, including those with volume depletion.10 Ketorolac is unlikely to be removed by dialysis and so should be avoided.10,11
Naproxen is metabolized in the liver to inactive compounds. Use of naproxen is not recommended in patients with moderate to severe renal impairment. If therapy must be initiated, close monitoring of the patient’s renal function is recommended.13
Celecoxib is the only cyclooxygenase-2 (COX-2) inhibitor available in the U.S. It is metabolized extensively by the liver and is unlikely to be removed by dialysis. Therefore, use of COX-2 inhibitors should be avoided in severe renal impairment and in those on dialysis.14,15
Opioid options. The use of opioids in the renally impaired population is challenging, as one must balance opioid-related adverse events with adequate pain control. As such, it is recommended to start with lower-than-recommended doses and slowly titrate up the dose while extending the dosing interval. This will help limit adverse effects, such as respiratory depression and hypotension.3
Hydrocodone is metabolized to hydromorphone (Dilaudid), which is then metabolized to its major metabolite hydromorphine-3-glucuronide (H3G) and minor metabolite hydromorphine-6-hydroxy, all of which are excreted renally along with the parent compound. H3G has no analgesic properties, but it can potentially cause neuroexcitation, agitation, confusion, and hallucination. Hydromorphone has been used safely in patients with renal insufficiency and dialysis, as it is expected to be dialyzable. 16,17
Tramadol is metabolized in the liver, producing one active compound. Approximately 30% of the tramadol dose is excreted unchanged in the urine, whereas 60% of the dose is excreted as metabolites. It is recommended to reduce the dose and increase the dosing interval in patients with renal insufficiency, but tramadol is generally well-tolerated in patients with renal insufficiency and dialysis. It is significantly removed by hemodialysis; therefore, redosing after a session may be necessary.18,19
Oxycodone can be used in patients with mild to moderate renal insufficiency but should be used at reduced dosing; it has been associated with significant sedation with usual doses in renal failure patients.16 Its use is generally not recommended in dialysis patients due to lack of data.3
Methadone and its metabolites are excreted in the urine and feces. Methadone has been used safely in patients with renal insufficiency, but it is poorly removed by dialysis and no specific recommendations are available regarding its dosing in dialysis.3,16
Fentanyl is primarily metabolized in the liver to inactive metabolites. Fentanyl clearance is reduced in patients with moderate to severe uremia (BUN >60 mg/dL). It is not expected that fentanyl be dialyzable because of its pharmacokinetic properties (high protein-binding, low water solubility, high molecular weight, and high volume of distribution). Data suggests that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in dialysis patients, although reduced doses may be prudent. Such patients should be monitored for signs of gradual accumulation of the parent drug.3,16
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), all of which are excreted renally, along with the parent compound. Only M6G has analgesic properties, and when it accumulates, it can lead to CNS depression. M3G is associated with behavioral excitation, a side effect that is further magnified in patients with renal insufficiency. Although morphine is dialyzable, it should generally be avoided in patients with any level of renal insufficiency.16,17,20,21
Codeine is metabolized to several active metabolites, all of which are renally excreted. Lower-than-usual doses are recommended in patients with renal insufficiency, and it should be avoided altogether in dialysis patients.3,16
Meperidine is metabolized in the liver to various metabolites, primarily normeperidine, which is toxic and has a long half-life, five to 10 times longer then meperidine. Meperidine should not be used in patients with renal insufficiency or dialysis.3
Adjunctive therapeutic options. Lidocaine patches currently are only FDA-indicated for postherpetic neuralgia but are used for a wide variety of local pain syndromes. Absorption of lidocaine is determined by the duration of application and the surface area over which it is applied. There is no appreciable accumulation of lidocaine or its metabolites in renal insufficiency; therefore, dose adjustments are not required.22,23
Gabapentin is FDA-indicated for partial seizures and postherpetic neuralgia but is also used for a wide variety of neuropathic pain syndromes, including postoperative pain.24 Gabapentin is not metabolized and is excreted in the urine unchanged. Renal clearance of gabapentin is reduced by 40% and the elimination half-life is increased up to 52 hours in renal insufficiency, but it is dialyzable. Therefore, dose adjustments are required with gabapentin in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.25-27
Pregabalin is structurally related to gabapentin and is indicated for a variety of neuropathic pain conditions. Pregabalin is 90% excreted unchanged in the urine, and approximately 50% of drug is removed after four hours of hemodialysis. Dose adjustments are required in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.28
Antidepressant options. Amitriptyline, nortryptiline, and desipramine are the tricyclic antidepressants (TCAs) commonly used for neuropathic pain. TCAs are metabolized in the liver to inactive metabolites, with the exception of amitriptyline, which is metabolized to nortryptiline. Common side effects reported with TCAs include postural hypotension and anticholinergic side effects, such as constipation, urinary retention, blurred vision, dry mouth, delirium, and sedation. It is unlikely that the TCAs can be removed by dialysis. It is suggested that the dosage be reduced in renal insufficiency and that anticholinergic side effects be monitored.29
Back to the Case
The patient’s ankle pain was controlled with acetaminophen and lidocaine patches. For the neuropathic pain in his upper extremities, tramadol was started at 25 mg oral every 12 hours and increased to 50 mg oral every eight hours (below the maximum of 200 mg a day). The tramadol did not result in adequate pain relief, so gabapentin 100 mg at bedtime was initiated, then increased to twice daily over three days with some relief.
A geriatric consult was obtained to help educate him regarding addiction to opioids, as well as to explore goals of care, but he continued to insist on the use of a non-narcotic regimen for his pain.
Bottom Line
Pain management in patients with renal insufficiency and dialysis can be challenging, but there are a number of safe non-narcotic and narcotic pain regimens that can be safely used in this patient population.
Dr. Harisingani is a board-certified hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y., and Drs. Saad and Cassagnol are assistant clinical professors at St. Johns University College of Pharmacy and Health Sciences in Jamaica, N.Y., and clinical pharmacy coordinators at Long Island Jewish Medical Center.
References
- Mid-Atlantic Renal Coalition and the Kidney End-of-Life Coalition. Clinical algorithm & preferred medications to treat pain in dialysis patients. Coalition for Supportive Care of Kidney Patients website. Available at: http://www.kidneysupportivecare.org/Physicians-Clinicians/Pain—Symptom-Management.aspx. Accessed Nov. 18, 2012.
- Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198-3203.
- Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. Pain Treatment Topics website. Available at: http://pain-topics.org/pdf/Opioids-Renal-Hepatic-Dysfunction.pdf. Accessed Nov. 28, 2012.
- Ferrell B, Argoff CE, Epplin J, et al. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291-297.
- Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain. 2006;6(3):137-148.
- Berg KJ, Djøseland O, Gjellan A, et al. Acute effects of paracetamol on prostaglandin synthesis and renal function in normal man and in patients with renal failure. Clin Nephrol. 1990;34:255-262.
- Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 R.P.) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976;1976(0):45-52.
- Shen CH, Hung CJ, Wu CC, Huang HW, Ho WM. Rhabdomyolysis-induced acute renal failure after morphine overdose—a case report. Acta Anaesthesiol Sin. 1999;37(3):159-162.
- Ketorolac tromethamine oral tablets [package insert]. St. Louis: Ethex Corp.: 2008.
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23:415-427. Erratum in: Clin Pharmacokinet. 1999;24(3):270.
- Ponstel [package insert]. Alpharetta, GA: First Horizon Pharmaceutical Corp.; 2006.
- Naprosyn [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Celebrex [package insert]. New York: G.D. Searle LLC; 2011.
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735-741.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497-504.
- Lee MA, Leng ME, Tiernan EJ. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001;15(1):26-34.
- Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCI. Am J. Med. 1996;101(1A):47S-53S.
- Izzedine H, Launay-Vacher V, Abbara C, Aymard G, Bassilios N, Deray G. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron. 2002;92(3):755-756.
- Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24(4):344-354.
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25(1):74-91.
- Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010.
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447-459.
- Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;15:126(1-3):91-101.
- Neurontin [package insert]. New York: Parke-Davis; 2010.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
- Srivastava U, Kumar A, Saxena S, et al: Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27(N4):331-335.
- Lyrica [package insert]. New York: Pfizer Inc.; 2012.
- Broadbent A, Khor K, Heaney A. Palliation and chronic renal failure: opioid and other palliative medications—dosage guidelines. Progress in Palliative Care. 2003;11(4):183-190(8).
- Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol. 2011;24(1):35-40.
- Wolters Kluwer Health. Facts & comparisons. Wolters Kluwer Health website. Available at: http://www.factsandcomparisons.com. Accessed Jan. 14, 2013.
- Lexicomp. Lexicomp Online. Lexicomp website. Available at: http://www.lexi.com/institutions/products/online/.
Case
A 70-year-old male with ESRD on hemodialysis presents with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and ankle pain after a fall. An MRI of his ankle is negative, and he is started on acetaminophen and lidocaine patches, which result in adequate pain relief of the ankle. He later develops significant neuropathic pain in both arms, and a CT scan of the cervical spine reveals a cervical abscess and osteomyelitis. The patient desires pain relief but adamantly refuses narcotics, stating: “I don’t want to get addicted.” How can his pain be managed?
Overview
Pain is a common problem in patients with renal insufficiency and end-stage renal disease (ESRD) and can have a significant effect on the patient’s quality of life.1 When assessing a patient’s pain, assess both the severity of the pain (such as on an analogue scale, 0-10) and the characteristics of the pain. Pain is most commonly characterized as nociceptive, neuropathic, or both. Nociceptive pain can be further classified as arising from either somatic or visceral sources, and is often described as dull, throbbing, cramping, and/or pressurelike.1 Neuropathic pain is often described as tingling, numbing, burning, and/or stabbing.
It is a challenge to manage pain in patients with renal insufficiency and dialysis. Renal insufficiency affects the pharmacokinetic properties of most pain medications, including their distribution, clearance, and excretion. The magnitude of the effect of renal insufficiency on drug metabolism varies depending on the agent itself, its metabolite, and the extent of renal failure.3 Multiple factors should be considered when prescribing pain medications for patients on dialysis, including the properties of the parent drug and its metabolites; the physical properties of the dialysis equipment, such as the filter pore size, the flow rate, and the efficiency of the technique used; and the dialysis method (intermittent versus continuous).3 Table 1 provides the recommended dosing of the most commonly prescribed agents, based on the degree of renal impairment. A modified World Health Organization (WHO) ladder has been suggested to treat pain in patients with ESRD, which can lead to effective pain relief in as many as 96% of patients (see Figure 1).2
*Beginning dose: If switching from IR to ER, calculate 24-hour total dose.
**For patients with creatinine clearances (CrCl) of 15 mL/min or less, the daily dosage should be adjusted proportionally (e.g. patients with a CrCl of 7.5 mL/min should receive one-half the dose of a patient with a CrCl of 15 mL/min).
Review of Data
Nonopioid options. Nonopioids, such as acetaminophen and NSAIDs, have no associated tolerance but have a ceiling effect for analgesia, and NSAIDs are associated with dose-dependent acute renal failure, gastrointestinal ulceration and bleeding, and cardiac events. The nonopioids that are considered safe options in patients with renal insufficiency include acetaminophen, ibuprofen, and fenoprofen (Nalfon). However, in the elderly, American Geriatric Society (AGS) guidelines currently recommend avoiding all NSAIDs due to their safety profile in the geriatric population.4 Although all NSAIDs can potentially be used for pain, selected NSAIDs with an FDA indication for acute or chronic pain were included for this review.
Acetaminophen (APAP) is a dialyzable compound that is metabolized in the liver to five inactive metabolites. The terminal elimination half-life of its sulfate and glucuronide metabolites are prolonged in patients with renal failure; therefore, the dosing interval of APAP should be increased to six to eight hours in renally impaired patients.5,6,7 Overall, acetaminophen is considered one of the safest agents to use for the treatment of pain, in renal patients and otherwise, as long as dosing is below the minimal daily dose (see Table 1).
Ibuprofen is metabolized in the liver to inactive compounds. It does not accumulate in renal insufficiency, and two of the inactive compounds are dialyzable.8 It is considered a safe option for the treatment of pain in patients with renal insufficiency or dialysis.9
Fenoprofen is metabolized in the liver to inactive compounds. Renal impairment is likely to cause the accumulation of the inactive metabolites but not the parent compound, so dose reduction is not necessary with the use of this agent in renal insufficiency or dialysis.6
Mefenamic acid (Ponstel) is metabolized in the liver. Mefenamic acid can further deteriorate renal function in patients with underlying renal disease.12 However, the nephrotoxic potential of this agent is of little consideration in ESRD patients on dialysis, and therefore no dosage adjustments are necessary in these patients.6
Ketoprofen is metabolized in the liver, where approximately 80% of the dose is excreted in the urine as a glucuronide metabolite. Dose reduction is recommended in renal insufficiency and dialysis, as it not dialyzable.8
Ketorolac accumulates in renal insufficiency; therefore, it is contraindicated in these patients and in patients at risk for renal failure, including those with volume depletion.10 Ketorolac is unlikely to be removed by dialysis and so should be avoided.10,11
Naproxen is metabolized in the liver to inactive compounds. Use of naproxen is not recommended in patients with moderate to severe renal impairment. If therapy must be initiated, close monitoring of the patient’s renal function is recommended.13
Celecoxib is the only cyclooxygenase-2 (COX-2) inhibitor available in the U.S. It is metabolized extensively by the liver and is unlikely to be removed by dialysis. Therefore, use of COX-2 inhibitors should be avoided in severe renal impairment and in those on dialysis.14,15
Opioid options. The use of opioids in the renally impaired population is challenging, as one must balance opioid-related adverse events with adequate pain control. As such, it is recommended to start with lower-than-recommended doses and slowly titrate up the dose while extending the dosing interval. This will help limit adverse effects, such as respiratory depression and hypotension.3
Hydrocodone is metabolized to hydromorphone (Dilaudid), which is then metabolized to its major metabolite hydromorphine-3-glucuronide (H3G) and minor metabolite hydromorphine-6-hydroxy, all of which are excreted renally along with the parent compound. H3G has no analgesic properties, but it can potentially cause neuroexcitation, agitation, confusion, and hallucination. Hydromorphone has been used safely in patients with renal insufficiency and dialysis, as it is expected to be dialyzable. 16,17
Tramadol is metabolized in the liver, producing one active compound. Approximately 30% of the tramadol dose is excreted unchanged in the urine, whereas 60% of the dose is excreted as metabolites. It is recommended to reduce the dose and increase the dosing interval in patients with renal insufficiency, but tramadol is generally well-tolerated in patients with renal insufficiency and dialysis. It is significantly removed by hemodialysis; therefore, redosing after a session may be necessary.18,19
Oxycodone can be used in patients with mild to moderate renal insufficiency but should be used at reduced dosing; it has been associated with significant sedation with usual doses in renal failure patients.16 Its use is generally not recommended in dialysis patients due to lack of data.3
Methadone and its metabolites are excreted in the urine and feces. Methadone has been used safely in patients with renal insufficiency, but it is poorly removed by dialysis and no specific recommendations are available regarding its dosing in dialysis.3,16
Fentanyl is primarily metabolized in the liver to inactive metabolites. Fentanyl clearance is reduced in patients with moderate to severe uremia (BUN >60 mg/dL). It is not expected that fentanyl be dialyzable because of its pharmacokinetic properties (high protein-binding, low water solubility, high molecular weight, and high volume of distribution). Data suggests that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in dialysis patients, although reduced doses may be prudent. Such patients should be monitored for signs of gradual accumulation of the parent drug.3,16
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), all of which are excreted renally, along with the parent compound. Only M6G has analgesic properties, and when it accumulates, it can lead to CNS depression. M3G is associated with behavioral excitation, a side effect that is further magnified in patients with renal insufficiency. Although morphine is dialyzable, it should generally be avoided in patients with any level of renal insufficiency.16,17,20,21
Codeine is metabolized to several active metabolites, all of which are renally excreted. Lower-than-usual doses are recommended in patients with renal insufficiency, and it should be avoided altogether in dialysis patients.3,16
Meperidine is metabolized in the liver to various metabolites, primarily normeperidine, which is toxic and has a long half-life, five to 10 times longer then meperidine. Meperidine should not be used in patients with renal insufficiency or dialysis.3
Adjunctive therapeutic options. Lidocaine patches currently are only FDA-indicated for postherpetic neuralgia but are used for a wide variety of local pain syndromes. Absorption of lidocaine is determined by the duration of application and the surface area over which it is applied. There is no appreciable accumulation of lidocaine or its metabolites in renal insufficiency; therefore, dose adjustments are not required.22,23
Gabapentin is FDA-indicated for partial seizures and postherpetic neuralgia but is also used for a wide variety of neuropathic pain syndromes, including postoperative pain.24 Gabapentin is not metabolized and is excreted in the urine unchanged. Renal clearance of gabapentin is reduced by 40% and the elimination half-life is increased up to 52 hours in renal insufficiency, but it is dialyzable. Therefore, dose adjustments are required with gabapentin in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.25-27
Pregabalin is structurally related to gabapentin and is indicated for a variety of neuropathic pain conditions. Pregabalin is 90% excreted unchanged in the urine, and approximately 50% of drug is removed after four hours of hemodialysis. Dose adjustments are required in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.28
Antidepressant options. Amitriptyline, nortryptiline, and desipramine are the tricyclic antidepressants (TCAs) commonly used for neuropathic pain. TCAs are metabolized in the liver to inactive metabolites, with the exception of amitriptyline, which is metabolized to nortryptiline. Common side effects reported with TCAs include postural hypotension and anticholinergic side effects, such as constipation, urinary retention, blurred vision, dry mouth, delirium, and sedation. It is unlikely that the TCAs can be removed by dialysis. It is suggested that the dosage be reduced in renal insufficiency and that anticholinergic side effects be monitored.29
Back to the Case
The patient’s ankle pain was controlled with acetaminophen and lidocaine patches. For the neuropathic pain in his upper extremities, tramadol was started at 25 mg oral every 12 hours and increased to 50 mg oral every eight hours (below the maximum of 200 mg a day). The tramadol did not result in adequate pain relief, so gabapentin 100 mg at bedtime was initiated, then increased to twice daily over three days with some relief.
A geriatric consult was obtained to help educate him regarding addiction to opioids, as well as to explore goals of care, but he continued to insist on the use of a non-narcotic regimen for his pain.
Bottom Line
Pain management in patients with renal insufficiency and dialysis can be challenging, but there are a number of safe non-narcotic and narcotic pain regimens that can be safely used in this patient population.
Dr. Harisingani is a board-certified hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y., and Drs. Saad and Cassagnol are assistant clinical professors at St. Johns University College of Pharmacy and Health Sciences in Jamaica, N.Y., and clinical pharmacy coordinators at Long Island Jewish Medical Center.
References
- Mid-Atlantic Renal Coalition and the Kidney End-of-Life Coalition. Clinical algorithm & preferred medications to treat pain in dialysis patients. Coalition for Supportive Care of Kidney Patients website. Available at: http://www.kidneysupportivecare.org/Physicians-Clinicians/Pain—Symptom-Management.aspx. Accessed Nov. 18, 2012.
- Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198-3203.
- Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. Pain Treatment Topics website. Available at: http://pain-topics.org/pdf/Opioids-Renal-Hepatic-Dysfunction.pdf. Accessed Nov. 28, 2012.
- Ferrell B, Argoff CE, Epplin J, et al. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291-297.
- Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain. 2006;6(3):137-148.
- Berg KJ, Djøseland O, Gjellan A, et al. Acute effects of paracetamol on prostaglandin synthesis and renal function in normal man and in patients with renal failure. Clin Nephrol. 1990;34:255-262.
- Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 R.P.) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976;1976(0):45-52.
- Shen CH, Hung CJ, Wu CC, Huang HW, Ho WM. Rhabdomyolysis-induced acute renal failure after morphine overdose—a case report. Acta Anaesthesiol Sin. 1999;37(3):159-162.
- Ketorolac tromethamine oral tablets [package insert]. St. Louis: Ethex Corp.: 2008.
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23:415-427. Erratum in: Clin Pharmacokinet. 1999;24(3):270.
- Ponstel [package insert]. Alpharetta, GA: First Horizon Pharmaceutical Corp.; 2006.
- Naprosyn [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Celebrex [package insert]. New York: G.D. Searle LLC; 2011.
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735-741.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497-504.
- Lee MA, Leng ME, Tiernan EJ. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001;15(1):26-34.
- Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCI. Am J. Med. 1996;101(1A):47S-53S.
- Izzedine H, Launay-Vacher V, Abbara C, Aymard G, Bassilios N, Deray G. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron. 2002;92(3):755-756.
- Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24(4):344-354.
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25(1):74-91.
- Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010.
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447-459.
- Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;15:126(1-3):91-101.
- Neurontin [package insert]. New York: Parke-Davis; 2010.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
- Srivastava U, Kumar A, Saxena S, et al: Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27(N4):331-335.
- Lyrica [package insert]. New York: Pfizer Inc.; 2012.
- Broadbent A, Khor K, Heaney A. Palliation and chronic renal failure: opioid and other palliative medications—dosage guidelines. Progress in Palliative Care. 2003;11(4):183-190(8).
- Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol. 2011;24(1):35-40.
- Wolters Kluwer Health. Facts & comparisons. Wolters Kluwer Health website. Available at: http://www.factsandcomparisons.com. Accessed Jan. 14, 2013.
- Lexicomp. Lexicomp Online. Lexicomp website. Available at: http://www.lexi.com/institutions/products/online/.
Prices for Common Procedures Not Readily Available
Clinical question: Are patients able to select health-care providers based on price of service?
Background: With health-care costs rising, patients are encouraged to take a more active role in cost containment. Many initiatives call for greater pricing transparency in the health-care system. This study evaluated price availability for a common surgical procedure.
Study design: Telephone inquiries with standardized interview script.
Setting: Twenty top-ranked orthopedic hospitals and 102 non-top-ranked U.S. hospitals.
Synopsis: Hospitals were contacted by phone with a standardized, scripted request for the price of an elective total hip arthroplasty. The script described the patient as a 62-year-old grandmother without insurance who is able to pay out of pocket and wishes to compare hospital prices. On the first or second attempt, 40% of top-ranked and 32% of non-top-ranked hospitals were able to provide their price; after five attempts, authors were unable to obtain full pricing information (both hospital and physician fee) from 40% of top-ranked and 37% of non-top-ranked hospitals. Neither fee was made available by 15% of top-ranked and 16% of non-top-ranked hospitals. Wide variation in pricing was found across hospitals. The authors commented on the difficulties they encountered, such as the transfer of calls between departments and the uncertainty of representatives on how to assist.
Bottom line: For individual patients, applying basic economic principles as a consumer might be tiresome and often impossible, with no major differences between top-ranked and non-top-ranked hospitals.
Citation: Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
Visit our website for more physician reviews of recent HM-relevant literature.
Clinical question: Are patients able to select health-care providers based on price of service?
Background: With health-care costs rising, patients are encouraged to take a more active role in cost containment. Many initiatives call for greater pricing transparency in the health-care system. This study evaluated price availability for a common surgical procedure.
Study design: Telephone inquiries with standardized interview script.
Setting: Twenty top-ranked orthopedic hospitals and 102 non-top-ranked U.S. hospitals.
Synopsis: Hospitals were contacted by phone with a standardized, scripted request for the price of an elective total hip arthroplasty. The script described the patient as a 62-year-old grandmother without insurance who is able to pay out of pocket and wishes to compare hospital prices. On the first or second attempt, 40% of top-ranked and 32% of non-top-ranked hospitals were able to provide their price; after five attempts, authors were unable to obtain full pricing information (both hospital and physician fee) from 40% of top-ranked and 37% of non-top-ranked hospitals. Neither fee was made available by 15% of top-ranked and 16% of non-top-ranked hospitals. Wide variation in pricing was found across hospitals. The authors commented on the difficulties they encountered, such as the transfer of calls between departments and the uncertainty of representatives on how to assist.
Bottom line: For individual patients, applying basic economic principles as a consumer might be tiresome and often impossible, with no major differences between top-ranked and non-top-ranked hospitals.
Citation: Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
Visit our website for more physician reviews of recent HM-relevant literature.
Clinical question: Are patients able to select health-care providers based on price of service?
Background: With health-care costs rising, patients are encouraged to take a more active role in cost containment. Many initiatives call for greater pricing transparency in the health-care system. This study evaluated price availability for a common surgical procedure.
Study design: Telephone inquiries with standardized interview script.
Setting: Twenty top-ranked orthopedic hospitals and 102 non-top-ranked U.S. hospitals.
Synopsis: Hospitals were contacted by phone with a standardized, scripted request for the price of an elective total hip arthroplasty. The script described the patient as a 62-year-old grandmother without insurance who is able to pay out of pocket and wishes to compare hospital prices. On the first or second attempt, 40% of top-ranked and 32% of non-top-ranked hospitals were able to provide their price; after five attempts, authors were unable to obtain full pricing information (both hospital and physician fee) from 40% of top-ranked and 37% of non-top-ranked hospitals. Neither fee was made available by 15% of top-ranked and 16% of non-top-ranked hospitals. Wide variation in pricing was found across hospitals. The authors commented on the difficulties they encountered, such as the transfer of calls between departments and the uncertainty of representatives on how to assist.
Bottom line: For individual patients, applying basic economic principles as a consumer might be tiresome and often impossible, with no major differences between top-ranked and non-top-ranked hospitals.
Citation: Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
Visit our website for more physician reviews of recent HM-relevant literature.
Physician Reviews of Hospital Medicine-Related Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prices for common hospital procedures not readily available
- Antibiotics associated with decreased mortality in acute COPD exacerbation
- Endovascular therapy has no benefit to systemic t-PA in acute stroke
- Net harm observed with rivaroxaban for thromboprophylaxis
- Noninvasive ventilation more effective, safer for AECOPD patients
- Synthetic cannabinoid use and acute kidney injury
- Dabigatran vs. warfarin in the extended treatment of VTE
- Spironolactone improves diastolic function
- Real-time EMR-based prediction tool for clinical deterioration
- Hypothermia protocol and cardiac arrest
Prices for Common Hospital Procedures Not Readily Available
Clinical question: Are patients able to select health-care providers based on price of service?
Background: With health-care costs rising, patients are encouraged to take a more active role in cost containment. Many initiatives call for greater pricing transparency in the health-care system. This study evaluated price availability for a common surgical procedure.
Study design: Telephone inquiries with standardized interview script.
Setting: Twenty top-ranked orthopedic hospitals and 102 non-top-ranked U.S. hospitals.
Synopsis: Hospitals were contacted by phone with a standardized, scripted request for their price of an elective total hip arthroplasty. The script described the patient as a 62-year-old grandmother without insurance who is able to pay out of pocket and wishes to compare hospital prices. On the first or second attempt, 40% of top-ranked and 32% of non-top-ranked hospitals were able to provide their price; after five attempts, authors were unable to obtain full pricing information (both hospital and physician fee) from 40% of top-ranked and 37% of non-top-ranked hospitals. Neither fee was made available by 15% of top-ranked and 16% of non-top-ranked hospitals. Wide variation of pricing was found across hospitals. The authors commented on the difficulties they encountered, such as the transfer of calls between departments and the uncertainty of representatives on how to assist.
Bottom line: For individual patients, applying basic economic principles as a consumer might be tiresome and often impossible, with no major differences between top-ranked and non-top-ranked hospitals.
Citation: Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
Antibiotics Associated with Decreased Mortality in Acute COPD Exacerbation
Clinical question: Do antibiotics when added to systemic steroids provide clinical benefit for patients with acute COPD exacerbation?
Background: Despite widespread use of antibiotics in the treatment of acute COPD, their benefit is not clear.
Study design: Retrospective cohort study.Setting: Four hundred ten U.S. hospitals participating in Perspective, an inpatient administrative database.
Synopsis: More than 50,000 patients treated with systemic steroids for acute COPD exacerbation were included in this study; 85% of them received empiric antibiotics within the first two hospital days. They were compared with those treated with systemic steroids alone. In-hospital mortality was 1.02% for patients on steroids plus antibiotics versus 1.78% on steroids alone. Use of antibiotics was associated with a 40% reduction in the odds of in-hospital mortality (OR, 0.60; 95% CI, 0.50-0.74) and reduced readmissions. In an analysis of matched cohorts, hospital mortality was 1% for patients on antibiotics and 1.8% for patients without antibiotics (OR, 0.53; 95% CI, 0.40-0.71). The risk for readmission for Clostridium difficile colitis was not different between the groups, but other potential adverse effects of antibiotic use, such as development of resistant micro-organisms, were not studied. In a subset analysis, three groups of antibiotics were compared: macrolides, quinolones, and cephalosporins. None was better than another, but those treated with macrolides had a lower readmission rate for C. diff.
Bottom line: Treatment with antibiotics when added to systemic steroids is associated with improved outcomes in acute COPD exacerbations, but there is no clear advantage of one antibiotic class over another.
Citation: Stefan MS, Rothberg MB, Shieh M, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013;143(1):82-90.
Endovascular Therapy Added to Systemic t-PA Has No Benefit in Acute Stroke
Clinical question: Does adding endovascular therapy to intravenous tissue plasminogen activator (t-PA) reduce disability in acute stroke?
Background: Early systemic t-PA is the only proven reperfusion therapy in acute stroke, but it is unknown if adding localized endovascular therapy is beneficial.
Study design: Randomized, open-label, blinded-outcome trial.
Setting: Fifty-eight centers in North America, Europe, and Australia.
Synopsis: Patients aged 18 to 82 with acute ischemic stroke were eligible if they received t-PA within three hours of enrollment and had moderate to severe neurologic deficit (National Institutes of Health Stroke Scale [NIHSS] >10, or NIHSS 8 or 9 with CT angiographic evidence of specific major artery occlusion). All patients received standard-dose t-PA; those randomized to endovascular treatment underwent angiography, and, if indicated, underwent one of the endovascular treatments chosen by the neurointerventionalist. The primary outcome measure was a modified Rankin score of 2 or lower (indicating functional independence) at 90 days (assessed by a neurologist).
After enrollment of 656 patients, the trial was terminated early for futility. There was no significant difference in the primary outcome, with 40.8% of patients in the endovascular intervention group and 38.7% in the t-PA-only group having a modified Rankin score of 2 or lower. There was also no difference in mortality or other secondary outcomes, even when the analysis was limited to patients presenting with more severe neurologic deficits.
Bottom line: The addition of endovascular therapy to systemic t-PA in acute ischemic stroke does not improve functional outcomes or mortality.
Citation: Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903.
Rivaroxaban Compared with Enoxaparin for Thromboprophylaxis
Clinical question: Is extended-duration rivaroxaban more effective than standard-duration enoxaparin in preventing deep venous thrombosis in acutely ill medical patients?
Background: Trials have shown benefits of thromboprophylaxis in acutely ill medical patients at increased risk of venous thrombosis, but the optimal duration and type of anticoagulation is unknown.
Study design: Prospective, randomized, double-blinded, active-comparator controlled trial.
Setting: Five hundred fifty-two centers in 52 countries.
Synopsis: The trial enrolled 8,428 patients hospitalized with an acute medical condition and reduced mobility. Patients were randomized to receive either enoxaparin for 10 (+/-4) days or rivaroxaban for 35 (+/-4) days. The co-primary composite outcomes were the incidence of asymptomatic proximal deep venous thrombosis, symptomatic deep venous thrombosis, pulmonary embolism, or death related to venous thromboembolism over 10 days and over 35 days.
Both groups had a 2.7% incidence of the primary endpoint over 10 days; over 35 days, the extended-duration rivaroxaban group had a reduced incidence of the primary endpoint of 4.4% compared with 5.7% for enoxaparin. However, there was an increase of clinically relevant bleeding in the rivaroxaban group, occurring in 2.8% and 4.1% of patients over 10 and 35 days, respectively, compared with 1.2% and 1.7% for enoxaparin.
Overall, there was net harm with rivaroxaban over both time periods: 6.6% and 9.4% of patients in the rivaroxaban group had a negative outcome at 10 and 35 days, compared with 4.4% and 7.8% with enoxaparin.
Bottom line: There was net harm with extended-duration rivaroxaban versus standard-duration enoxaparin for thromboprophylaxis in hospitalized medical patients.
Citation: Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-523.
Noninvasive Ventilation More Effective, Safer for Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD)
Clinical question: What are the patterns in use of noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV) in patients with AECOPD, and which method produces better outcomes?
Background: Multiple randomized controlled trials and meta-analyses have suggested a mortality benefit with NIV compared to standard medical care in AECOPD. However, little evidence exists on head-to-head comparisons of NIV and IMV.
Study design: Retrospective cohort study.
Setting: Non-federal, short-term, general, and other specialty hospitals nationwide.
Synopsis: A sample of 67,651 ED visits for AECOPD with acute respiratory failure was analyzed from the National Emergency Department Sample (NEDS) database between 2006 and 2008. The study found that NIV use increased to 16% in 2008 from 14% in 2006. Use varied widely between hospitals and was more utilized in higher-case-volume, nonmetropolitan, and Northeastern hospitals. Compared with IMV, NIV was associated with 46% lower inpatient mortality (risk ratio 0.54, 95% confidence interval [CI] 0.50-0.59), shortened hospital length of stay (-3.2 days, 95% CI -3.4 to -2.9), lower hospital charges (-$35,012, 95% CI -$36,848 to -$33,176), and lower risk of iatrogenic pneumothorax (0.05% vs. 0.5%, P<0.001). Causality could not be established given the observational study design.
Bottom line: NIV is associated with better outcomes than IMV in the management of AECOPD, and might be underutilized.
Citation: Tsai CL, Lee WY, Delclos GL, Hanania NA, Camargo CA Jr. Comparative effectiveness of noninvasive ventilation vs. invasive mechanical ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. J Hosp Med. 2013;8(4):165-172.
Synthetic Cannabinoid Use May Cause Acute Kidney Injury
Clinical question: Are synthetic cannabinoids associated with acute kidney injury (AKI)?
Background: Synthetic cannabinoids are designer drugs of abuse with a growing popularity in the U.S.
Study design: Retrospective case series.
Setting: Hospitals in Wyoming, Oklahoma, Rhode Island, New York, Kansas, and Oregon.
Synopsis: The Centers for Disease Control and Prevention (CDC) issued an alert when 16 cases of unexplained AKI after exposure to synthetic cannabinoids were reported between March and December 2012. Synthetic cannabinoid use is associated with neurologic, sympathomimetic, and cardiovascular toxicity; however, this is the first case series reporting renal toxicity. The 16 patients included 15 males and one female, aged 15-33 years, with no pre-existing renal disease or nephrotoxic medication consumption. All used synthetic cannabinoids within hours to days before developing nausea, vomiting, abdominal, and flank and/or back pain.
Creatinine peaked one to six days after symptom onset. Five patients required hemodialysis, and all 16 recovered. There was no finding specific for all cases on ultrasound and/or biopsy. Toxicologic analysis of specimens was possible in seven cases and revealed a previously unreported fluorinated synthetic cannabinoid compound XLR-11 in all clinical specimens of patients who used the drug within two days of being tested.
Overall, the analysis did not reveal any single compound or brand that could explain all cases.
Bottom line: Clinicians should be aware of the potential for renal or other toxicities in users of synthetic cannabinoid products and should ask about their use in cases of unexplained AKI.
Citation: Murphy TD, Weidenbach KN, Van Houten C, et al. Centers for Disease Control and Prevention. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(6):93-98.
Dabigatran versus Warfarin in Extended VTE Treatment
Clinical question: Is dabigatran suitable for extended treatment VTE?
Background: In contrast to warfarin (Coumadin), dabigatran (Pradaxa) is given in a fixed dose and does not require frequent laboratory monitoring. Dabigatran has been shown to be noninferior to warfarin in the initial six-month treatment of VTE.
Study design: Two double-blinded, randomized trials: an active-control study and a placebo-control study.
Setting: Two hundred sixty-five sites in 33 countries for the active-control study, and 147 sites in 21 countries for the placebo-control study.
Synopsis: This study enrolled 4,299 adult patients with objectively confirmed, symptomatic, proximal deep vein thrombosis or pulmonary embolism. In the active-control study comparing warfarin and dabigatran, recurrent objectively confirmed symptomatic or fatal VTE was observed in 1.8% of patients in the dabigatran group compared with 1.3% of patients in the warfarin group (P=0.01 for noninferiority). While major or clinically relevant bleeding was less frequent with dabigatran compared to warfarin (hazard ratio, 0.54), more acute coronary syndromes were observed with dabigatran (0.9% vs. 0.2%, P=0.02). In the placebo-control study, symptomatic or fatal VTE was observed in 0.4% of patients in the dabigatran group compared with 5.6% in the placebo group. Clinically relevant bleeding was more common with dabigatran (5.3% vs. 1.8%; hazard ratio 2.92).
Bottom line: Treatment with dabigatran met the pre-specified noninferiority margin in this study. However, it is worth noting that patients with VTE given extended treatment with dabigatran had significantly higher rates of recurrent symptomatic or fatal VTE than patients treated with warfarin.
Citation: Schulman S, Kearson C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709-718.
Spironolactone Improves Diastolic Function
Clinical question: What is the efficacy of aldosterone receptor blockers on diastolic function and exercise capacity?
Background: Mineralocorticoid receptor activation by aldosterone contributes to the pathophysiology of heart failure (HF) in patients with and without reduced ejection fraction (EF). Aldosterone receptor blockers (spironolactone) reduce overall and cardiovascular mortality in HF patients with reduced EF; however, its effect on HF patients with preserved EF is unknown.
Study design: Prospective, randomized, double-blinded, placebo-controlled trial.
Setting: Ten ambulatory sites in Germany and Austria.
Synopsis: This study enrolled 422 men and women, aged 50 or older, with New York Heart Association (NYHA) Class II or III HF and preserved EF, and randomized them to receive spironolactone 25 mg daily or placebo for one year.
The endpoints included echocardiographic measures of diastolic function and remodeling; N-terminal pro b-type natriuretic peptide (NT pro-BNP) levels; exercise capacity; quality of life; and HF symptoms.
In the spironolactone group, there was significant improvement in echocardiographic measures of diastolic function and remodeling as well as NT pro-BNP levels. However, there was no difference in exercise capacity, quality of life, or HF symptoms when compared to placebo.
The spironolactone group had significantly lower blood pressure than the control group, which may account for some of the remodeling effects. The study may have been underpowered, and the study population might not have had severe enough disease to detect a difference in clinical measures. It remains unknown if structural changes on echocardiography will translate into clinical benefits.
Bottom line: Compared to placebo, spironolactone did improve diastolic function by echo but did not improve exercise capacity.
Citation: Edelmann F, Wachter R, Schmidt A, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
Real-Time, EMR-Based Prediction Tool Accurately Predicts ICU Transfer Risks
Clinical question: Can clinical deterioration be accurately predicted using real-time data from an electronic medical record (EMR), and can it lead to better outcomes using a floor-based intervention?
Background: Research has shown that EMR-based prediction tools can help identify real-time clinical deterioration in ward patients, but an intervention based on these computer-based alerts has not been shown to be effective.
Study design: Randomized controlled crossover study.
Setting: Eight adult medicine wards in an academic medical center in the Midwest.
Synopsis: There were 20,031 patients assigned to intervention versus control based on their floor location. Computerized alerts were generated using a prediction algorithm. For patients admitted to intervention wards, the alerts were sent to the charge nurse via pager. Patients meeting the alert threshold based on the computerized prediction tool had five times higher risk of ICU transfer, and nine times higher risk of death than patients not meeting the alert threshold. Intervention of charge nurse notification via pager did not result in any significant change in length of stay (LOS), ICU transfer, or mortality. Charge nurses in the intervention group were supposed to alert a physician after receiving the computer alert, but the authors did not measure how often this occurred.
Bottom line: A real-time EMR-based prediction tool accurately predicts higher risk of ICU transfer and death, as well as LOS, but a floor-based intervention to alert the charge nurse in real time did not lead to better outcomes.
Citation: Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medicine wards. J Hosp Med. 2013 Feb 25. doi: 10.1002/jhm.2009 [Epub ahead of print].
Hypothermia Protocol and Cardiac Arrest
Clinical question: Is mild therapeutic hypothermia (MTH) following cardiac arrest beneficial and safe?
Background: Those with sudden cardiac arrest often have poor neurologic outcome. There are some studies that show benefit with hypothermia, but information on safety is limited.
Study design: Meta-analysis.
Setting: Europe, the United Kingdom, the U.S., Canada, Japan, and South Korea.
Synopsis: This study pooled data from 63 studies that looked at MTH protocols in the setting of comatose patients as a result of cardiac arrest. The end points included mortality and any complication potentially attributed to the MTH.
When restricting the analysis to include only randomized controlled trials, MTH was associated with decreased risk of in-hospital mortality (RR=0.75, 95% CI: 0.62-0.92), 30-day mortality (RR=0.61, 95% CI 0.45-0.81), and six-month mortality (RR=0.73, 95% CI 0.61-0.88). MTH was associated with increased risk of arrhythmia (RR=1.25, 95% CI: 1.00-1.55) and hypokalemia (RR=2.35, 95% CI: 1.35-4.11); all other complications were similar between groups.
There were inconsistent results regarding benefit in pediatric patients, as well as comatose patients with non-ventricular fibrillation (non-v-fib) arrest (i.e. asystole or pulseless electrical activity).
Bottom line: Mild therapeutic hypothermia can improve survival of comatose patients after v-fib cardiac arrest and is generally safe.
Citation: Xiao G, Guo Q, Xie X, et al. Safety profile and outcome of mild therapeutic hypothermia in patients following cardiac arrest: systematic review and meta-analysis. Emerg Med J. 2013;30:90-100.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prices for common hospital procedures not readily available
- Antibiotics associated with decreased mortality in acute COPD exacerbation
- Endovascular therapy has no benefit to systemic t-PA in acute stroke
- Net harm observed with rivaroxaban for thromboprophylaxis
- Noninvasive ventilation more effective, safer for AECOPD patients
- Synthetic cannabinoid use and acute kidney injury
- Dabigatran vs. warfarin in the extended treatment of VTE
- Spironolactone improves diastolic function
- Real-time EMR-based prediction tool for clinical deterioration
- Hypothermia protocol and cardiac arrest
Prices for Common Hospital Procedures Not Readily Available
Clinical question: Are patients able to select health-care providers based on price of service?
Background: With health-care costs rising, patients are encouraged to take a more active role in cost containment. Many initiatives call for greater pricing transparency in the health-care system. This study evaluated price availability for a common surgical procedure.
Study design: Telephone inquiries with standardized interview script.
Setting: Twenty top-ranked orthopedic hospitals and 102 non-top-ranked U.S. hospitals.
Synopsis: Hospitals were contacted by phone with a standardized, scripted request for their price of an elective total hip arthroplasty. The script described the patient as a 62-year-old grandmother without insurance who is able to pay out of pocket and wishes to compare hospital prices. On the first or second attempt, 40% of top-ranked and 32% of non-top-ranked hospitals were able to provide their price; after five attempts, authors were unable to obtain full pricing information (both hospital and physician fee) from 40% of top-ranked and 37% of non-top-ranked hospitals. Neither fee was made available by 15% of top-ranked and 16% of non-top-ranked hospitals. Wide variation of pricing was found across hospitals. The authors commented on the difficulties they encountered, such as the transfer of calls between departments and the uncertainty of representatives on how to assist.
Bottom line: For individual patients, applying basic economic principles as a consumer might be tiresome and often impossible, with no major differences between top-ranked and non-top-ranked hospitals.
Citation: Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
Antibiotics Associated with Decreased Mortality in Acute COPD Exacerbation
Clinical question: Do antibiotics when added to systemic steroids provide clinical benefit for patients with acute COPD exacerbation?
Background: Despite widespread use of antibiotics in the treatment of acute COPD, their benefit is not clear.
Study design: Retrospective cohort study.Setting: Four hundred ten U.S. hospitals participating in Perspective, an inpatient administrative database.
Synopsis: More than 50,000 patients treated with systemic steroids for acute COPD exacerbation were included in this study; 85% of them received empiric antibiotics within the first two hospital days. They were compared with those treated with systemic steroids alone. In-hospital mortality was 1.02% for patients on steroids plus antibiotics versus 1.78% on steroids alone. Use of antibiotics was associated with a 40% reduction in the odds of in-hospital mortality (OR, 0.60; 95% CI, 0.50-0.74) and reduced readmissions. In an analysis of matched cohorts, hospital mortality was 1% for patients on antibiotics and 1.8% for patients without antibiotics (OR, 0.53; 95% CI, 0.40-0.71). The risk for readmission for Clostridium difficile colitis was not different between the groups, but other potential adverse effects of antibiotic use, such as development of resistant micro-organisms, were not studied. In a subset analysis, three groups of antibiotics were compared: macrolides, quinolones, and cephalosporins. None was better than another, but those treated with macrolides had a lower readmission rate for C. diff.
Bottom line: Treatment with antibiotics when added to systemic steroids is associated with improved outcomes in acute COPD exacerbations, but there is no clear advantage of one antibiotic class over another.
Citation: Stefan MS, Rothberg MB, Shieh M, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013;143(1):82-90.
Endovascular Therapy Added to Systemic t-PA Has No Benefit in Acute Stroke
Clinical question: Does adding endovascular therapy to intravenous tissue plasminogen activator (t-PA) reduce disability in acute stroke?
Background: Early systemic t-PA is the only proven reperfusion therapy in acute stroke, but it is unknown if adding localized endovascular therapy is beneficial.
Study design: Randomized, open-label, blinded-outcome trial.
Setting: Fifty-eight centers in North America, Europe, and Australia.
Synopsis: Patients aged 18 to 82 with acute ischemic stroke were eligible if they received t-PA within three hours of enrollment and had moderate to severe neurologic deficit (National Institutes of Health Stroke Scale [NIHSS] >10, or NIHSS 8 or 9 with CT angiographic evidence of specific major artery occlusion). All patients received standard-dose t-PA; those randomized to endovascular treatment underwent angiography, and, if indicated, underwent one of the endovascular treatments chosen by the neurointerventionalist. The primary outcome measure was a modified Rankin score of 2 or lower (indicating functional independence) at 90 days (assessed by a neurologist).
After enrollment of 656 patients, the trial was terminated early for futility. There was no significant difference in the primary outcome, with 40.8% of patients in the endovascular intervention group and 38.7% in the t-PA-only group having a modified Rankin score of 2 or lower. There was also no difference in mortality or other secondary outcomes, even when the analysis was limited to patients presenting with more severe neurologic deficits.
Bottom line: The addition of endovascular therapy to systemic t-PA in acute ischemic stroke does not improve functional outcomes or mortality.
Citation: Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903.
Rivaroxaban Compared with Enoxaparin for Thromboprophylaxis
Clinical question: Is extended-duration rivaroxaban more effective than standard-duration enoxaparin in preventing deep venous thrombosis in acutely ill medical patients?
Background: Trials have shown benefits of thromboprophylaxis in acutely ill medical patients at increased risk of venous thrombosis, but the optimal duration and type of anticoagulation is unknown.
Study design: Prospective, randomized, double-blinded, active-comparator controlled trial.
Setting: Five hundred fifty-two centers in 52 countries.
Synopsis: The trial enrolled 8,428 patients hospitalized with an acute medical condition and reduced mobility. Patients were randomized to receive either enoxaparin for 10 (+/-4) days or rivaroxaban for 35 (+/-4) days. The co-primary composite outcomes were the incidence of asymptomatic proximal deep venous thrombosis, symptomatic deep venous thrombosis, pulmonary embolism, or death related to venous thromboembolism over 10 days and over 35 days.
Both groups had a 2.7% incidence of the primary endpoint over 10 days; over 35 days, the extended-duration rivaroxaban group had a reduced incidence of the primary endpoint of 4.4% compared with 5.7% for enoxaparin. However, there was an increase of clinically relevant bleeding in the rivaroxaban group, occurring in 2.8% and 4.1% of patients over 10 and 35 days, respectively, compared with 1.2% and 1.7% for enoxaparin.
Overall, there was net harm with rivaroxaban over both time periods: 6.6% and 9.4% of patients in the rivaroxaban group had a negative outcome at 10 and 35 days, compared with 4.4% and 7.8% with enoxaparin.
Bottom line: There was net harm with extended-duration rivaroxaban versus standard-duration enoxaparin for thromboprophylaxis in hospitalized medical patients.
Citation: Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-523.
Noninvasive Ventilation More Effective, Safer for Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD)
Clinical question: What are the patterns in use of noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV) in patients with AECOPD, and which method produces better outcomes?
Background: Multiple randomized controlled trials and meta-analyses have suggested a mortality benefit with NIV compared to standard medical care in AECOPD. However, little evidence exists on head-to-head comparisons of NIV and IMV.
Study design: Retrospective cohort study.
Setting: Non-federal, short-term, general, and other specialty hospitals nationwide.
Synopsis: A sample of 67,651 ED visits for AECOPD with acute respiratory failure was analyzed from the National Emergency Department Sample (NEDS) database between 2006 and 2008. The study found that NIV use increased to 16% in 2008 from 14% in 2006. Use varied widely between hospitals and was more utilized in higher-case-volume, nonmetropolitan, and Northeastern hospitals. Compared with IMV, NIV was associated with 46% lower inpatient mortality (risk ratio 0.54, 95% confidence interval [CI] 0.50-0.59), shortened hospital length of stay (-3.2 days, 95% CI -3.4 to -2.9), lower hospital charges (-$35,012, 95% CI -$36,848 to -$33,176), and lower risk of iatrogenic pneumothorax (0.05% vs. 0.5%, P<0.001). Causality could not be established given the observational study design.
Bottom line: NIV is associated with better outcomes than IMV in the management of AECOPD, and might be underutilized.
Citation: Tsai CL, Lee WY, Delclos GL, Hanania NA, Camargo CA Jr. Comparative effectiveness of noninvasive ventilation vs. invasive mechanical ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. J Hosp Med. 2013;8(4):165-172.
Synthetic Cannabinoid Use May Cause Acute Kidney Injury
Clinical question: Are synthetic cannabinoids associated with acute kidney injury (AKI)?
Background: Synthetic cannabinoids are designer drugs of abuse with a growing popularity in the U.S.
Study design: Retrospective case series.
Setting: Hospitals in Wyoming, Oklahoma, Rhode Island, New York, Kansas, and Oregon.
Synopsis: The Centers for Disease Control and Prevention (CDC) issued an alert when 16 cases of unexplained AKI after exposure to synthetic cannabinoids were reported between March and December 2012. Synthetic cannabinoid use is associated with neurologic, sympathomimetic, and cardiovascular toxicity; however, this is the first case series reporting renal toxicity. The 16 patients included 15 males and one female, aged 15-33 years, with no pre-existing renal disease or nephrotoxic medication consumption. All used synthetic cannabinoids within hours to days before developing nausea, vomiting, abdominal, and flank and/or back pain.
Creatinine peaked one to six days after symptom onset. Five patients required hemodialysis, and all 16 recovered. There was no finding specific for all cases on ultrasound and/or biopsy. Toxicologic analysis of specimens was possible in seven cases and revealed a previously unreported fluorinated synthetic cannabinoid compound XLR-11 in all clinical specimens of patients who used the drug within two days of being tested.
Overall, the analysis did not reveal any single compound or brand that could explain all cases.
Bottom line: Clinicians should be aware of the potential for renal or other toxicities in users of synthetic cannabinoid products and should ask about their use in cases of unexplained AKI.
Citation: Murphy TD, Weidenbach KN, Van Houten C, et al. Centers for Disease Control and Prevention. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(6):93-98.
Dabigatran versus Warfarin in Extended VTE Treatment
Clinical question: Is dabigatran suitable for extended treatment VTE?
Background: In contrast to warfarin (Coumadin), dabigatran (Pradaxa) is given in a fixed dose and does not require frequent laboratory monitoring. Dabigatran has been shown to be noninferior to warfarin in the initial six-month treatment of VTE.
Study design: Two double-blinded, randomized trials: an active-control study and a placebo-control study.
Setting: Two hundred sixty-five sites in 33 countries for the active-control study, and 147 sites in 21 countries for the placebo-control study.
Synopsis: This study enrolled 4,299 adult patients with objectively confirmed, symptomatic, proximal deep vein thrombosis or pulmonary embolism. In the active-control study comparing warfarin and dabigatran, recurrent objectively confirmed symptomatic or fatal VTE was observed in 1.8% of patients in the dabigatran group compared with 1.3% of patients in the warfarin group (P=0.01 for noninferiority). While major or clinically relevant bleeding was less frequent with dabigatran compared to warfarin (hazard ratio, 0.54), more acute coronary syndromes were observed with dabigatran (0.9% vs. 0.2%, P=0.02). In the placebo-control study, symptomatic or fatal VTE was observed in 0.4% of patients in the dabigatran group compared with 5.6% in the placebo group. Clinically relevant bleeding was more common with dabigatran (5.3% vs. 1.8%; hazard ratio 2.92).
Bottom line: Treatment with dabigatran met the pre-specified noninferiority margin in this study. However, it is worth noting that patients with VTE given extended treatment with dabigatran had significantly higher rates of recurrent symptomatic or fatal VTE than patients treated with warfarin.
Citation: Schulman S, Kearson C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709-718.
Spironolactone Improves Diastolic Function
Clinical question: What is the efficacy of aldosterone receptor blockers on diastolic function and exercise capacity?
Background: Mineralocorticoid receptor activation by aldosterone contributes to the pathophysiology of heart failure (HF) in patients with and without reduced ejection fraction (EF). Aldosterone receptor blockers (spironolactone) reduce overall and cardiovascular mortality in HF patients with reduced EF; however, its effect on HF patients with preserved EF is unknown.
Study design: Prospective, randomized, double-blinded, placebo-controlled trial.
Setting: Ten ambulatory sites in Germany and Austria.
Synopsis: This study enrolled 422 men and women, aged 50 or older, with New York Heart Association (NYHA) Class II or III HF and preserved EF, and randomized them to receive spironolactone 25 mg daily or placebo for one year.
The endpoints included echocardiographic measures of diastolic function and remodeling; N-terminal pro b-type natriuretic peptide (NT pro-BNP) levels; exercise capacity; quality of life; and HF symptoms.
In the spironolactone group, there was significant improvement in echocardiographic measures of diastolic function and remodeling as well as NT pro-BNP levels. However, there was no difference in exercise capacity, quality of life, or HF symptoms when compared to placebo.
The spironolactone group had significantly lower blood pressure than the control group, which may account for some of the remodeling effects. The study may have been underpowered, and the study population might not have had severe enough disease to detect a difference in clinical measures. It remains unknown if structural changes on echocardiography will translate into clinical benefits.
Bottom line: Compared to placebo, spironolactone did improve diastolic function by echo but did not improve exercise capacity.
Citation: Edelmann F, Wachter R, Schmidt A, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
Real-Time, EMR-Based Prediction Tool Accurately Predicts ICU Transfer Risks
Clinical question: Can clinical deterioration be accurately predicted using real-time data from an electronic medical record (EMR), and can it lead to better outcomes using a floor-based intervention?
Background: Research has shown that EMR-based prediction tools can help identify real-time clinical deterioration in ward patients, but an intervention based on these computer-based alerts has not been shown to be effective.
Study design: Randomized controlled crossover study.
Setting: Eight adult medicine wards in an academic medical center in the Midwest.
Synopsis: There were 20,031 patients assigned to intervention versus control based on their floor location. Computerized alerts were generated using a prediction algorithm. For patients admitted to intervention wards, the alerts were sent to the charge nurse via pager. Patients meeting the alert threshold based on the computerized prediction tool had five times higher risk of ICU transfer, and nine times higher risk of death than patients not meeting the alert threshold. Intervention of charge nurse notification via pager did not result in any significant change in length of stay (LOS), ICU transfer, or mortality. Charge nurses in the intervention group were supposed to alert a physician after receiving the computer alert, but the authors did not measure how often this occurred.
Bottom line: A real-time EMR-based prediction tool accurately predicts higher risk of ICU transfer and death, as well as LOS, but a floor-based intervention to alert the charge nurse in real time did not lead to better outcomes.
Citation: Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medicine wards. J Hosp Med. 2013 Feb 25. doi: 10.1002/jhm.2009 [Epub ahead of print].
Hypothermia Protocol and Cardiac Arrest
Clinical question: Is mild therapeutic hypothermia (MTH) following cardiac arrest beneficial and safe?
Background: Those with sudden cardiac arrest often have poor neurologic outcome. There are some studies that show benefit with hypothermia, but information on safety is limited.
Study design: Meta-analysis.
Setting: Europe, the United Kingdom, the U.S., Canada, Japan, and South Korea.
Synopsis: This study pooled data from 63 studies that looked at MTH protocols in the setting of comatose patients as a result of cardiac arrest. The end points included mortality and any complication potentially attributed to the MTH.
When restricting the analysis to include only randomized controlled trials, MTH was associated with decreased risk of in-hospital mortality (RR=0.75, 95% CI: 0.62-0.92), 30-day mortality (RR=0.61, 95% CI 0.45-0.81), and six-month mortality (RR=0.73, 95% CI 0.61-0.88). MTH was associated with increased risk of arrhythmia (RR=1.25, 95% CI: 1.00-1.55) and hypokalemia (RR=2.35, 95% CI: 1.35-4.11); all other complications were similar between groups.
There were inconsistent results regarding benefit in pediatric patients, as well as comatose patients with non-ventricular fibrillation (non-v-fib) arrest (i.e. asystole or pulseless electrical activity).
Bottom line: Mild therapeutic hypothermia can improve survival of comatose patients after v-fib cardiac arrest and is generally safe.
Citation: Xiao G, Guo Q, Xie X, et al. Safety profile and outcome of mild therapeutic hypothermia in patients following cardiac arrest: systematic review and meta-analysis. Emerg Med J. 2013;30:90-100.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Prices for common hospital procedures not readily available
- Antibiotics associated with decreased mortality in acute COPD exacerbation
- Endovascular therapy has no benefit to systemic t-PA in acute stroke
- Net harm observed with rivaroxaban for thromboprophylaxis
- Noninvasive ventilation more effective, safer for AECOPD patients
- Synthetic cannabinoid use and acute kidney injury
- Dabigatran vs. warfarin in the extended treatment of VTE
- Spironolactone improves diastolic function
- Real-time EMR-based prediction tool for clinical deterioration
- Hypothermia protocol and cardiac arrest
Prices for Common Hospital Procedures Not Readily Available
Clinical question: Are patients able to select health-care providers based on price of service?
Background: With health-care costs rising, patients are encouraged to take a more active role in cost containment. Many initiatives call for greater pricing transparency in the health-care system. This study evaluated price availability for a common surgical procedure.
Study design: Telephone inquiries with standardized interview script.
Setting: Twenty top-ranked orthopedic hospitals and 102 non-top-ranked U.S. hospitals.
Synopsis: Hospitals were contacted by phone with a standardized, scripted request for their price of an elective total hip arthroplasty. The script described the patient as a 62-year-old grandmother without insurance who is able to pay out of pocket and wishes to compare hospital prices. On the first or second attempt, 40% of top-ranked and 32% of non-top-ranked hospitals were able to provide their price; after five attempts, authors were unable to obtain full pricing information (both hospital and physician fee) from 40% of top-ranked and 37% of non-top-ranked hospitals. Neither fee was made available by 15% of top-ranked and 16% of non-top-ranked hospitals. Wide variation of pricing was found across hospitals. The authors commented on the difficulties they encountered, such as the transfer of calls between departments and the uncertainty of representatives on how to assist.
Bottom line: For individual patients, applying basic economic principles as a consumer might be tiresome and often impossible, with no major differences between top-ranked and non-top-ranked hospitals.
Citation: Rosenthal JA, Lu X, Cram P. Availability of consumer prices from US hospitals for a common surgical procedure. JAMA Intern Med. 2013;173(6):427-432.
Antibiotics Associated with Decreased Mortality in Acute COPD Exacerbation
Clinical question: Do antibiotics when added to systemic steroids provide clinical benefit for patients with acute COPD exacerbation?
Background: Despite widespread use of antibiotics in the treatment of acute COPD, their benefit is not clear.
Study design: Retrospective cohort study.Setting: Four hundred ten U.S. hospitals participating in Perspective, an inpatient administrative database.
Synopsis: More than 50,000 patients treated with systemic steroids for acute COPD exacerbation were included in this study; 85% of them received empiric antibiotics within the first two hospital days. They were compared with those treated with systemic steroids alone. In-hospital mortality was 1.02% for patients on steroids plus antibiotics versus 1.78% on steroids alone. Use of antibiotics was associated with a 40% reduction in the odds of in-hospital mortality (OR, 0.60; 95% CI, 0.50-0.74) and reduced readmissions. In an analysis of matched cohorts, hospital mortality was 1% for patients on antibiotics and 1.8% for patients without antibiotics (OR, 0.53; 95% CI, 0.40-0.71). The risk for readmission for Clostridium difficile colitis was not different between the groups, but other potential adverse effects of antibiotic use, such as development of resistant micro-organisms, were not studied. In a subset analysis, three groups of antibiotics were compared: macrolides, quinolones, and cephalosporins. None was better than another, but those treated with macrolides had a lower readmission rate for C. diff.
Bottom line: Treatment with antibiotics when added to systemic steroids is associated with improved outcomes in acute COPD exacerbations, but there is no clear advantage of one antibiotic class over another.
Citation: Stefan MS, Rothberg MB, Shieh M, Pekow PS, Lindenauer PK. Association between antibiotic treatment and outcomes in patients hospitalized with acute exacerbation of COPD treated with systemic steroids. Chest. 2013;143(1):82-90.
Endovascular Therapy Added to Systemic t-PA Has No Benefit in Acute Stroke
Clinical question: Does adding endovascular therapy to intravenous tissue plasminogen activator (t-PA) reduce disability in acute stroke?
Background: Early systemic t-PA is the only proven reperfusion therapy in acute stroke, but it is unknown if adding localized endovascular therapy is beneficial.
Study design: Randomized, open-label, blinded-outcome trial.
Setting: Fifty-eight centers in North America, Europe, and Australia.
Synopsis: Patients aged 18 to 82 with acute ischemic stroke were eligible if they received t-PA within three hours of enrollment and had moderate to severe neurologic deficit (National Institutes of Health Stroke Scale [NIHSS] >10, or NIHSS 8 or 9 with CT angiographic evidence of specific major artery occlusion). All patients received standard-dose t-PA; those randomized to endovascular treatment underwent angiography, and, if indicated, underwent one of the endovascular treatments chosen by the neurointerventionalist. The primary outcome measure was a modified Rankin score of 2 or lower (indicating functional independence) at 90 days (assessed by a neurologist).
After enrollment of 656 patients, the trial was terminated early for futility. There was no significant difference in the primary outcome, with 40.8% of patients in the endovascular intervention group and 38.7% in the t-PA-only group having a modified Rankin score of 2 or lower. There was also no difference in mortality or other secondary outcomes, even when the analysis was limited to patients presenting with more severe neurologic deficits.
Bottom line: The addition of endovascular therapy to systemic t-PA in acute ischemic stroke does not improve functional outcomes or mortality.
Citation: Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893-903.
Rivaroxaban Compared with Enoxaparin for Thromboprophylaxis
Clinical question: Is extended-duration rivaroxaban more effective than standard-duration enoxaparin in preventing deep venous thrombosis in acutely ill medical patients?
Background: Trials have shown benefits of thromboprophylaxis in acutely ill medical patients at increased risk of venous thrombosis, but the optimal duration and type of anticoagulation is unknown.
Study design: Prospective, randomized, double-blinded, active-comparator controlled trial.
Setting: Five hundred fifty-two centers in 52 countries.
Synopsis: The trial enrolled 8,428 patients hospitalized with an acute medical condition and reduced mobility. Patients were randomized to receive either enoxaparin for 10 (+/-4) days or rivaroxaban for 35 (+/-4) days. The co-primary composite outcomes were the incidence of asymptomatic proximal deep venous thrombosis, symptomatic deep venous thrombosis, pulmonary embolism, or death related to venous thromboembolism over 10 days and over 35 days.
Both groups had a 2.7% incidence of the primary endpoint over 10 days; over 35 days, the extended-duration rivaroxaban group had a reduced incidence of the primary endpoint of 4.4% compared with 5.7% for enoxaparin. However, there was an increase of clinically relevant bleeding in the rivaroxaban group, occurring in 2.8% and 4.1% of patients over 10 and 35 days, respectively, compared with 1.2% and 1.7% for enoxaparin.
Overall, there was net harm with rivaroxaban over both time periods: 6.6% and 9.4% of patients in the rivaroxaban group had a negative outcome at 10 and 35 days, compared with 4.4% and 7.8% with enoxaparin.
Bottom line: There was net harm with extended-duration rivaroxaban versus standard-duration enoxaparin for thromboprophylaxis in hospitalized medical patients.
Citation: Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513-523.
Noninvasive Ventilation More Effective, Safer for Acute Exacerbation of Chronic Obstructive Pulmonary Disease (AECOPD)
Clinical question: What are the patterns in use of noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV) in patients with AECOPD, and which method produces better outcomes?
Background: Multiple randomized controlled trials and meta-analyses have suggested a mortality benefit with NIV compared to standard medical care in AECOPD. However, little evidence exists on head-to-head comparisons of NIV and IMV.
Study design: Retrospective cohort study.
Setting: Non-federal, short-term, general, and other specialty hospitals nationwide.
Synopsis: A sample of 67,651 ED visits for AECOPD with acute respiratory failure was analyzed from the National Emergency Department Sample (NEDS) database between 2006 and 2008. The study found that NIV use increased to 16% in 2008 from 14% in 2006. Use varied widely between hospitals and was more utilized in higher-case-volume, nonmetropolitan, and Northeastern hospitals. Compared with IMV, NIV was associated with 46% lower inpatient mortality (risk ratio 0.54, 95% confidence interval [CI] 0.50-0.59), shortened hospital length of stay (-3.2 days, 95% CI -3.4 to -2.9), lower hospital charges (-$35,012, 95% CI -$36,848 to -$33,176), and lower risk of iatrogenic pneumothorax (0.05% vs. 0.5%, P<0.001). Causality could not be established given the observational study design.
Bottom line: NIV is associated with better outcomes than IMV in the management of AECOPD, and might be underutilized.
Citation: Tsai CL, Lee WY, Delclos GL, Hanania NA, Camargo CA Jr. Comparative effectiveness of noninvasive ventilation vs. invasive mechanical ventilation in chronic obstructive pulmonary disease patients with acute respiratory failure. J Hosp Med. 2013;8(4):165-172.
Synthetic Cannabinoid Use May Cause Acute Kidney Injury
Clinical question: Are synthetic cannabinoids associated with acute kidney injury (AKI)?
Background: Synthetic cannabinoids are designer drugs of abuse with a growing popularity in the U.S.
Study design: Retrospective case series.
Setting: Hospitals in Wyoming, Oklahoma, Rhode Island, New York, Kansas, and Oregon.
Synopsis: The Centers for Disease Control and Prevention (CDC) issued an alert when 16 cases of unexplained AKI after exposure to synthetic cannabinoids were reported between March and December 2012. Synthetic cannabinoid use is associated with neurologic, sympathomimetic, and cardiovascular toxicity; however, this is the first case series reporting renal toxicity. The 16 patients included 15 males and one female, aged 15-33 years, with no pre-existing renal disease or nephrotoxic medication consumption. All used synthetic cannabinoids within hours to days before developing nausea, vomiting, abdominal, and flank and/or back pain.
Creatinine peaked one to six days after symptom onset. Five patients required hemodialysis, and all 16 recovered. There was no finding specific for all cases on ultrasound and/or biopsy. Toxicologic analysis of specimens was possible in seven cases and revealed a previously unreported fluorinated synthetic cannabinoid compound XLR-11 in all clinical specimens of patients who used the drug within two days of being tested.
Overall, the analysis did not reveal any single compound or brand that could explain all cases.
Bottom line: Clinicians should be aware of the potential for renal or other toxicities in users of synthetic cannabinoid products and should ask about their use in cases of unexplained AKI.
Citation: Murphy TD, Weidenbach KN, Van Houten C, et al. Centers for Disease Control and Prevention. Acute kidney injury associated with synthetic cannabinoid use—multiple states, 2012. MMWR Morb Mortal Wkly Rep. 2013;62(6):93-98.
Dabigatran versus Warfarin in Extended VTE Treatment
Clinical question: Is dabigatran suitable for extended treatment VTE?
Background: In contrast to warfarin (Coumadin), dabigatran (Pradaxa) is given in a fixed dose and does not require frequent laboratory monitoring. Dabigatran has been shown to be noninferior to warfarin in the initial six-month treatment of VTE.
Study design: Two double-blinded, randomized trials: an active-control study and a placebo-control study.
Setting: Two hundred sixty-five sites in 33 countries for the active-control study, and 147 sites in 21 countries for the placebo-control study.
Synopsis: This study enrolled 4,299 adult patients with objectively confirmed, symptomatic, proximal deep vein thrombosis or pulmonary embolism. In the active-control study comparing warfarin and dabigatran, recurrent objectively confirmed symptomatic or fatal VTE was observed in 1.8% of patients in the dabigatran group compared with 1.3% of patients in the warfarin group (P=0.01 for noninferiority). While major or clinically relevant bleeding was less frequent with dabigatran compared to warfarin (hazard ratio, 0.54), more acute coronary syndromes were observed with dabigatran (0.9% vs. 0.2%, P=0.02). In the placebo-control study, symptomatic or fatal VTE was observed in 0.4% of patients in the dabigatran group compared with 5.6% in the placebo group. Clinically relevant bleeding was more common with dabigatran (5.3% vs. 1.8%; hazard ratio 2.92).
Bottom line: Treatment with dabigatran met the pre-specified noninferiority margin in this study. However, it is worth noting that patients with VTE given extended treatment with dabigatran had significantly higher rates of recurrent symptomatic or fatal VTE than patients treated with warfarin.
Citation: Schulman S, Kearson C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709-718.
Spironolactone Improves Diastolic Function
Clinical question: What is the efficacy of aldosterone receptor blockers on diastolic function and exercise capacity?
Background: Mineralocorticoid receptor activation by aldosterone contributes to the pathophysiology of heart failure (HF) in patients with and without reduced ejection fraction (EF). Aldosterone receptor blockers (spironolactone) reduce overall and cardiovascular mortality in HF patients with reduced EF; however, its effect on HF patients with preserved EF is unknown.
Study design: Prospective, randomized, double-blinded, placebo-controlled trial.
Setting: Ten ambulatory sites in Germany and Austria.
Synopsis: This study enrolled 422 men and women, aged 50 or older, with New York Heart Association (NYHA) Class II or III HF and preserved EF, and randomized them to receive spironolactone 25 mg daily or placebo for one year.
The endpoints included echocardiographic measures of diastolic function and remodeling; N-terminal pro b-type natriuretic peptide (NT pro-BNP) levels; exercise capacity; quality of life; and HF symptoms.
In the spironolactone group, there was significant improvement in echocardiographic measures of diastolic function and remodeling as well as NT pro-BNP levels. However, there was no difference in exercise capacity, quality of life, or HF symptoms when compared to placebo.
The spironolactone group had significantly lower blood pressure than the control group, which may account for some of the remodeling effects. The study may have been underpowered, and the study population might not have had severe enough disease to detect a difference in clinical measures. It remains unknown if structural changes on echocardiography will translate into clinical benefits.
Bottom line: Compared to placebo, spironolactone did improve diastolic function by echo but did not improve exercise capacity.
Citation: Edelmann F, Wachter R, Schmidt A, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781-791.
Real-Time, EMR-Based Prediction Tool Accurately Predicts ICU Transfer Risks
Clinical question: Can clinical deterioration be accurately predicted using real-time data from an electronic medical record (EMR), and can it lead to better outcomes using a floor-based intervention?
Background: Research has shown that EMR-based prediction tools can help identify real-time clinical deterioration in ward patients, but an intervention based on these computer-based alerts has not been shown to be effective.
Study design: Randomized controlled crossover study.
Setting: Eight adult medicine wards in an academic medical center in the Midwest.
Synopsis: There were 20,031 patients assigned to intervention versus control based on their floor location. Computerized alerts were generated using a prediction algorithm. For patients admitted to intervention wards, the alerts were sent to the charge nurse via pager. Patients meeting the alert threshold based on the computerized prediction tool had five times higher risk of ICU transfer, and nine times higher risk of death than patients not meeting the alert threshold. Intervention of charge nurse notification via pager did not result in any significant change in length of stay (LOS), ICU transfer, or mortality. Charge nurses in the intervention group were supposed to alert a physician after receiving the computer alert, but the authors did not measure how often this occurred.
Bottom line: A real-time EMR-based prediction tool accurately predicts higher risk of ICU transfer and death, as well as LOS, but a floor-based intervention to alert the charge nurse in real time did not lead to better outcomes.
Citation: Bailey TC, Chen Y, Mao Y, et al. A trial of a real-time alert for clinical deterioration in patients hospitalized on general medicine wards. J Hosp Med. 2013 Feb 25. doi: 10.1002/jhm.2009 [Epub ahead of print].
Hypothermia Protocol and Cardiac Arrest
Clinical question: Is mild therapeutic hypothermia (MTH) following cardiac arrest beneficial and safe?
Background: Those with sudden cardiac arrest often have poor neurologic outcome. There are some studies that show benefit with hypothermia, but information on safety is limited.
Study design: Meta-analysis.
Setting: Europe, the United Kingdom, the U.S., Canada, Japan, and South Korea.
Synopsis: This study pooled data from 63 studies that looked at MTH protocols in the setting of comatose patients as a result of cardiac arrest. The end points included mortality and any complication potentially attributed to the MTH.
When restricting the analysis to include only randomized controlled trials, MTH was associated with decreased risk of in-hospital mortality (RR=0.75, 95% CI: 0.62-0.92), 30-day mortality (RR=0.61, 95% CI 0.45-0.81), and six-month mortality (RR=0.73, 95% CI 0.61-0.88). MTH was associated with increased risk of arrhythmia (RR=1.25, 95% CI: 1.00-1.55) and hypokalemia (RR=2.35, 95% CI: 1.35-4.11); all other complications were similar between groups.
There were inconsistent results regarding benefit in pediatric patients, as well as comatose patients with non-ventricular fibrillation (non-v-fib) arrest (i.e. asystole or pulseless electrical activity).
Bottom line: Mild therapeutic hypothermia can improve survival of comatose patients after v-fib cardiac arrest and is generally safe.
Citation: Xiao G, Guo Q, Xie X, et al. Safety profile and outcome of mild therapeutic hypothermia in patients following cardiac arrest: systematic review and meta-analysis. Emerg Med J. 2013;30:90-100.