User login
PMI After Hip Fracture Surgery
Perioperative myocardial infarction (PMI) often remains unrecognized with higher mortality in the aged.13 Perioperative ischemic symptoms are often masked by analgesia, sedation, and transient and subtle electrocardiographic (ECG) changes. Postoperative troponin measurement is not routinely done for PMI diagnosis. Hip fracture surgery is the most common non‐cardiac surgical procedure in the elderly, with limited data on clinical presentation of PMI.46 Moreover, the elderly are significantly underrepresented in clinical studies.7 We therefore examined the clinical presentation of PMI and its outcomes among elderly patients admitted for hip fracture repair.
METHODS
Study Population
A population‐based, retrospective, case‐control study was conducted of all residents in Olmsted County, Minnesota undergoing surgery for hip fracture repair from January 1, 1988 through December 31, 2002. Primary indication for the surgery was proximal femur (femoral neck or subtrochanteric) fracture. Patients who were <65 years old, had a pathological hip fracture, multiple injuries or fractures, surgery >72 hours after injury (due to higher mortality with delayed surgery),8 nonsurgical management of hip fracture repair, or incomplete data were excluded. All patients provided prior authorization to use their medical records for research, per institutional protocols.9
Criteria for Perioperative Myocardial Infarction and Death
We utilized the universal definition of acute myocardial infarction10 to define PMI within the first 7 days following hip fracture surgery. We included creatine kinase‐MB fraction (CK‐MB) as the biomarker for 1988July 2000, and troponin as the biomarker for August 20002002. Mortality was defined as death from any cause within the first year following hip fracture repair. Deaths were identified through the National Death Index.
Statistical Analysis
For each case of PMI, we identified 2 control patients who were selected at random from the non‐PMI patient population. These controls were matched to cases based on age at the time of surgery (5 years) and gender in 1:2 ratios. Baseline characteristics across PMI and non‐PMI groups were compared using the Kruskal‐Wallis test (for continuous data) and the chi‐square or Fisher's exact tests (for categorical data). Mean values were utilized in place of the missing values for the following variables: preoperative troponin (missing values 88 [17.5%]), CK‐MB (8 [1.6%]), troponin (21 [5.4%]), and postoperative hemoglobin (17 [3.4%]). Univariate predictors of PMI with P 0.2 baseline characteristics were entered into a multivariate, conditional, logistic regression analysis. Rates of outcomes were calculated using the Kaplan‐Meier method, and by a landmark survival curve for those with and without PMI. Cox proportional hazards analysis was utilized for survival analysis at 30 days and 1 year. All statistical tests were 2‐sided, and P values <0.05 were considered significant. All analyses were performed using SAS for UNIX (version 9.1.3; SAS Institute, Inc, Cary, NC).
RESULTS
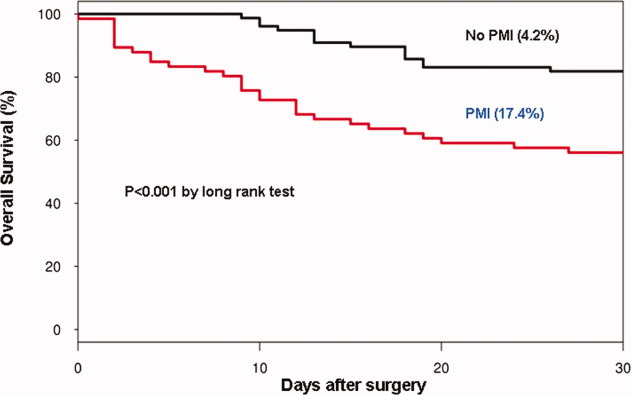
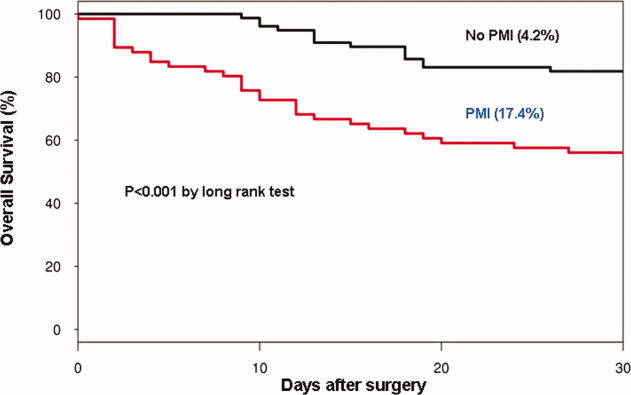
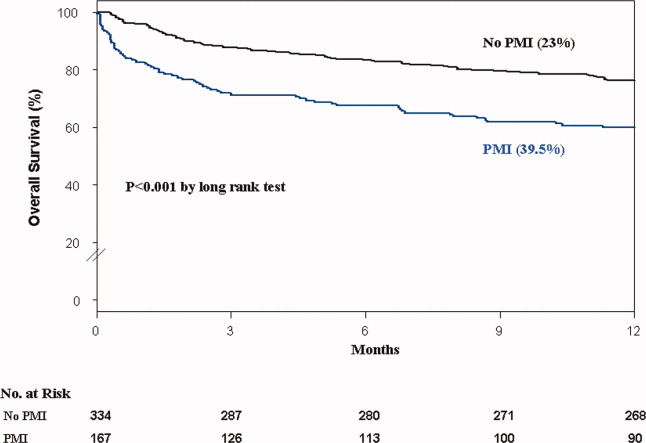
In the cohort of 1212 with hip fracture surgeries, 167 (13.8%) cases of PMI occurred in the first 7 days, of which 153 (92%) occurred within the first 48 hours. A total of 334 controls were matched with 167 cases of PMI. Table 1 summarizes the demographic characteristics of the study participants. Of the patients with PMI, 25.2% experienced symptoms of ischemia; 7% reported chest pain, and 12% reported dyspnea. Only 22.8% of patients with PMI had ECG changes consistent with ischemia. ST elevation MI was present in 7.2% patients. PMI patients had a lower mean hemoglobin compared to the patients without PMI (8.9 mg/dL vs 9.4 mg/dL, P < 0.001). Median length of stay (LOS) in the hospital was higher among patients who experienced PMI (11.6 vs 7.4 days, P < 0.001). Overall in‐hospital mortality was 5.6%. There were 24 deaths (14.4%) in the PMI group compared to 4 (1.2%) in‐hospital deaths in patients without PMI (P < 0.001). A total of 473 (94%) patients survived to discharge. At 30‐day follow‐up, there were 29 (17.4%) deaths in the PMI group and 14 (4.2%) deaths in non‐PMI group. During the follow‐up for 1 year, there were 143 (29%) deaths: PMI 66 (39.5%) and 77 (23%) non‐PMI group (P < 0.01).
| Characteristics, n (%) | Patients With PMI | Patients Without PMI | P Value* |
|---|---|---|---|
| (N = 167) | (N = 334) | ||
| |||
| Age mean SD | 85.3 7.4 | 85.2 7.1 | 0.5 |
| Weight (kg) mean SD | 59.98 16.7 | 59.80 13.9 | 0.5 |
| Women | 127 (76.4) | 254 (76) | 0.5 |
| Any symptom of ischemia, n (%) | |||
| Chest/arm pain | 11 (7) | 4 (1) | 0.002 |
| Dyspnea | 20 (12) | 14 (4) | 0.001 |
| Nausea/vomiting | 8 (5) | 6 (2) | 0.08 |
| Diaphoresis | 1 (1) | 1 (0.3) | 1.0 |
| PND | 3 (2) | 1 (0.3) | 0.3 |
| ECG changes, n (%) | |||
| ST‐segment elevation MI | 12 (7.2) | 0 | 0.01 |
| New ECG changes consistent with ischemia | 38 (22.8) | 1(0.3) | 0.01 |
| Biochemical evidence of ischemia, n (%) | |||
| CK‐MB | 147 (88) | 20 (6) | 0.01 |
| Troponin | 52 (33) | 9 (3) | 0.001 |
| Laboratory markers | |||
| Hemoglobin gm/dL mean (SD) | 8.9 1.0 | 9.4 1.2 | 0.001 |
| Postoperative anemia (<8.0 gm/dL), n (%) | 22 (13.2) | 37 (11.1) | 0.5 |
| Length of stay (days), mean SD | 11.6 7.7 | 7.4 6.4 | 0.001 |
| In‐hospital outcome | <0.001 | ||
| Dead | 24 (14.4) | 4 (1.2) | |
| Alive | 143 (85.6) | 330 (98.8) | |
| 30‐Day outcome | <0.001 | ||
| Dead | 29 (17.4) | 14 (4.2) | |
| Alive | 138 (82.6) | 320 (95.8) | |
| 1‐Year outcome | <0.001 | ||
| Dead | 66 (39.5) | 77 (23) | |
| Alive | 101 (60.4) | 257 (77) | |
Table 2 describes the risk factors associated with PMI in‐hospital, 30‐day, and 1‐year mortality. Risk factors for PMI were coronary artery disease (CAD) (odds ratio [OR], 3.5; confidence interval [CI], 2.25.6), and serum creatinine >2 mg/dL (OR, 2.4; CI, 1.34.4). Risk factors for in‐hospital mortality were age 8589 (OR, 5.3; CI, 1.617.7), age 90 (OR, 8.9; CI, 2.630.8), PMI (OR 15.1; CI, 4.648.8), male gender (OR 5.8; CI, 2.215.2), dyspnea (OR 5.4; CI, 1.816.9), and hemoglobin <8.0 gm/dL (OR, 3.5; CI, 1.29.9). PMI was a strong predictor for 30‐day mortality (hazard ratio [HR], 4.3; CI, 2.18.9). Risk factors for 1‐year mortality were: age 90 (HR, 2.0; CI, 1.43.1), male gender (HR, 2.1; CI, 1.53.0), and PMI (HR, 1.9; CI, 1.42.7). Figures 1 and 2 describe the Kaplan‐Meier survival curves for patients with and without PMI.
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P Value | |
|---|---|---|---|
| |||
| Perioperative myocardial infarction | |||
| Coronary artery disease | 3.0 (2.14.5) | 3.5 (2.25.6) | <0.001 |
| Serum creatinine >2.0 mg/dL | 2.7 (1.64.8) | 2.4 (1.34.4) | 0.003 |
| In‐hospital mortality | |||
| Age 8589 | 1.7 (0.83.7) | 5.3 (1.617.7) | 0.01 |
| Age 90 | 2.2 (1.04.8) | 8.9 (2.630.8) | <0.001 |
| Male gender | 3.0 (1.46.4) | 5.8 (2.215.2) | <0.001 |
| Postoperative anemia (<8.0 gm/dL) | 4.2 (1.710.0) | 3.5 (1.29.9) | 0.02 |
| Perioperative myocardial infarction | 14.0 (5.248.0) | 15.1 (4.649.0) | <0.001 |
| 30‐Day mortality | |||
| Perioperative myocardial infarction | 4.1 (2.27.8) | 4.3 (2.18.9) | <0.001 |
| 1‐Year mortality | |||
| Age 8589 | 1.3 (0.81.9) | 1.6 (1.02.4) | <0.03 |
| Age 90 | 1.9 (1.32.9) | 2.0 (1.43.1) | 0.001 |
| Male gender | 1.9 (1.32.6) | 2.1 (1.53.0) | <0.001 |
| Dementia | 2.5 (1.83.6) | 2.7 (1.93.8) | <0.001 |
| Perioperative myocardial infarction | 2.0 (1.52.8) | 1.9 (1.42.7) | 0.001 |
DISCUSSION
We report the high incidence of PMI (13.8%) in the cohort of 1212 elderly patients (mean age 85 years) undergoing hip fracture surgery. Most PMI events (92%) occurred within the first 48 hours of surgery. Most of the events (75%) were asymptomatic. Elderly patients with PMI had an increased hospital LOS by 4.2 days, with high in‐hospital mortality (13.8%), 30‐day mortality (17.4%), and 1‐year mortality (39.5%).
Most of the PMI patients were identified with cardiac biomarkers on the basis of universal definition of MI within the first 48 hours. Although universal definition of MI does not define PMI as a separate type, PMI shares common pathophysiological pathways of Type 1 MI (primary coronary event) and Type 2 MI (myocardial oxygen supplydemand imbalance). Postoperative tachycardia, hemodynamic instability, anemia, and hypoxemia may initiate pathways causing more Type 2 MI. Our study highlights the continued need for active surveillance of clinical symptoms, postoperative ECG monitoring for STT changes, and utilizing cardiac troponin in older postoperative patients to improve diagnostic accuracy of PMI.
The current study has higher asymptomatic PMI events when compared to a study of Devereaux et al.11 The current study had an older population undergoing urgent hip fracture surgery, with a higher burden of CAD (60%) and renal failure (20%) with serum creatinine >2 gm/dL (see Supporting Information, Appendix 1, in the online version of this article). Older age and a higher burden of these risk factors may explain the higher incidence of PMI in the current study. Perioperative liberal use of analgesics in hip fracture surgery may explain more asymptomatic patients.
In light of the recently published FOCUS12 trial, an important finding from our study is that postoperative anemia among elderly (<8.0 gm/dL) is associated with a 3.5‐fold increased in‐hospital mortality. It is critical to maintain perioperative hemoglobin above 8.0 gm/dL in very elderly patients, due to asymptomatic presentation of PMI.
In the current study, PMI is associated with a 15‐fold increased risk of in‐hospital death and a 4.3‐fold increased risk of 30‐day mortality in the elderly. Advanced age (85 years) is a well known strong predictor of initial hospital admission and death in elderly patients after outpatient surgery.13 Furthermore, the odds for an in‐hospital death increase by 70% for each 10‐year increase in age.14 Therefore, early detection of silent PMI among at‐risk elderly patients by cardiac biomarkers may help in optimization of cardiac pharmacotherapy known to decrease short‐ and long‐term mortality.
There are limitations inherent to the retrospective design and methodology. Data collection was done through the year 2002. CK was used for the period that spans from 1988 to mid‐2000. Troponin was used from 2000 to 2002. Statin use was not analyzed for lack of significant data. Limited use of beta‐blockers (15%) and angiotensin‐converting‐enzyme (ACE) inhibitors (25%) may also contribute to higher events (see Supporting Information, Appendix 1, in the online version of this article).
CONCLUSIONS
Elderly patients have a higher incidence of PMI and mortality after hip fracture surgery than what guidelines indicate. The majority of the elderly patients with PMI did not experience ischemic symptoms and required cardiac biomarkers for diagnosis. The results of our study support the measurement of troponin in postoperative elderly patients for the diagnosis of PMI to implement in‐hospital preventive strategies to reduce PMI‐associated mortality.
Acknowledgements
The authors gratefully acknowledge the assistance of Ms Dawn Bergen in drafting and editing the manuscript.
Disclosures: This research was supported by funding from AHA grant 03‐30103N‐04, Rochester Epidemiology Project (grant RO1‐AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases). The project was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
- , , , et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- , , , et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390.
- , , , et al. Body mass index (BMI) and risk of noncardiac postoperative medical complications in elderly hip fracture patients: a population‐based study. J Hosp Med. 2009;4(8):E1–E9.
- . History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274.
- , , , . Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579.
- , , , et al. Body mass index and risk of adverse cardiac events in elderly patients with hip fracture: a population‐based study. J Am Geriatr Soc. 2009;57(3):419–426.
- , , , et al. Acute coronary care in the elderly, part I. Non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569.
- , . Hip fracture mortality. A prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;280:214–222.
- , , , et al. ACC/AHA/ACP‐ASIM guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 1999;33(7):2092–2190.
- , , ; for the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery. Ann Intern Med. 2011;154(8):523–528.
- , , , et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , . Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg. 2004;139(1):67–72.
- , , , et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353.
Perioperative myocardial infarction (PMI) often remains unrecognized with higher mortality in the aged.13 Perioperative ischemic symptoms are often masked by analgesia, sedation, and transient and subtle electrocardiographic (ECG) changes. Postoperative troponin measurement is not routinely done for PMI diagnosis. Hip fracture surgery is the most common non‐cardiac surgical procedure in the elderly, with limited data on clinical presentation of PMI.46 Moreover, the elderly are significantly underrepresented in clinical studies.7 We therefore examined the clinical presentation of PMI and its outcomes among elderly patients admitted for hip fracture repair.
METHODS
Study Population
A population‐based, retrospective, case‐control study was conducted of all residents in Olmsted County, Minnesota undergoing surgery for hip fracture repair from January 1, 1988 through December 31, 2002. Primary indication for the surgery was proximal femur (femoral neck or subtrochanteric) fracture. Patients who were <65 years old, had a pathological hip fracture, multiple injuries or fractures, surgery >72 hours after injury (due to higher mortality with delayed surgery),8 nonsurgical management of hip fracture repair, or incomplete data were excluded. All patients provided prior authorization to use their medical records for research, per institutional protocols.9
Criteria for Perioperative Myocardial Infarction and Death
We utilized the universal definition of acute myocardial infarction10 to define PMI within the first 7 days following hip fracture surgery. We included creatine kinase‐MB fraction (CK‐MB) as the biomarker for 1988July 2000, and troponin as the biomarker for August 20002002. Mortality was defined as death from any cause within the first year following hip fracture repair. Deaths were identified through the National Death Index.
Statistical Analysis
For each case of PMI, we identified 2 control patients who were selected at random from the non‐PMI patient population. These controls were matched to cases based on age at the time of surgery (5 years) and gender in 1:2 ratios. Baseline characteristics across PMI and non‐PMI groups were compared using the Kruskal‐Wallis test (for continuous data) and the chi‐square or Fisher's exact tests (for categorical data). Mean values were utilized in place of the missing values for the following variables: preoperative troponin (missing values 88 [17.5%]), CK‐MB (8 [1.6%]), troponin (21 [5.4%]), and postoperative hemoglobin (17 [3.4%]). Univariate predictors of PMI with P 0.2 baseline characteristics were entered into a multivariate, conditional, logistic regression analysis. Rates of outcomes were calculated using the Kaplan‐Meier method, and by a landmark survival curve for those with and without PMI. Cox proportional hazards analysis was utilized for survival analysis at 30 days and 1 year. All statistical tests were 2‐sided, and P values <0.05 were considered significant. All analyses were performed using SAS for UNIX (version 9.1.3; SAS Institute, Inc, Cary, NC).
RESULTS
In the cohort of 1212 with hip fracture surgeries, 167 (13.8%) cases of PMI occurred in the first 7 days, of which 153 (92%) occurred within the first 48 hours. A total of 334 controls were matched with 167 cases of PMI. Table 1 summarizes the demographic characteristics of the study participants. Of the patients with PMI, 25.2% experienced symptoms of ischemia; 7% reported chest pain, and 12% reported dyspnea. Only 22.8% of patients with PMI had ECG changes consistent with ischemia. ST elevation MI was present in 7.2% patients. PMI patients had a lower mean hemoglobin compared to the patients without PMI (8.9 mg/dL vs 9.4 mg/dL, P < 0.001). Median length of stay (LOS) in the hospital was higher among patients who experienced PMI (11.6 vs 7.4 days, P < 0.001). Overall in‐hospital mortality was 5.6%. There were 24 deaths (14.4%) in the PMI group compared to 4 (1.2%) in‐hospital deaths in patients without PMI (P < 0.001). A total of 473 (94%) patients survived to discharge. At 30‐day follow‐up, there were 29 (17.4%) deaths in the PMI group and 14 (4.2%) deaths in non‐PMI group. During the follow‐up for 1 year, there were 143 (29%) deaths: PMI 66 (39.5%) and 77 (23%) non‐PMI group (P < 0.01).
| Characteristics, n (%) | Patients With PMI | Patients Without PMI | P Value* |
|---|---|---|---|
| (N = 167) | (N = 334) | ||
| |||
| Age mean SD | 85.3 7.4 | 85.2 7.1 | 0.5 |
| Weight (kg) mean SD | 59.98 16.7 | 59.80 13.9 | 0.5 |
| Women | 127 (76.4) | 254 (76) | 0.5 |
| Any symptom of ischemia, n (%) | |||
| Chest/arm pain | 11 (7) | 4 (1) | 0.002 |
| Dyspnea | 20 (12) | 14 (4) | 0.001 |
| Nausea/vomiting | 8 (5) | 6 (2) | 0.08 |
| Diaphoresis | 1 (1) | 1 (0.3) | 1.0 |
| PND | 3 (2) | 1 (0.3) | 0.3 |
| ECG changes, n (%) | |||
| ST‐segment elevation MI | 12 (7.2) | 0 | 0.01 |
| New ECG changes consistent with ischemia | 38 (22.8) | 1(0.3) | 0.01 |
| Biochemical evidence of ischemia, n (%) | |||
| CK‐MB | 147 (88) | 20 (6) | 0.01 |
| Troponin | 52 (33) | 9 (3) | 0.001 |
| Laboratory markers | |||
| Hemoglobin gm/dL mean (SD) | 8.9 1.0 | 9.4 1.2 | 0.001 |
| Postoperative anemia (<8.0 gm/dL), n (%) | 22 (13.2) | 37 (11.1) | 0.5 |
| Length of stay (days), mean SD | 11.6 7.7 | 7.4 6.4 | 0.001 |
| In‐hospital outcome | <0.001 | ||
| Dead | 24 (14.4) | 4 (1.2) | |
| Alive | 143 (85.6) | 330 (98.8) | |
| 30‐Day outcome | <0.001 | ||
| Dead | 29 (17.4) | 14 (4.2) | |
| Alive | 138 (82.6) | 320 (95.8) | |
| 1‐Year outcome | <0.001 | ||
| Dead | 66 (39.5) | 77 (23) | |
| Alive | 101 (60.4) | 257 (77) | |
Table 2 describes the risk factors associated with PMI in‐hospital, 30‐day, and 1‐year mortality. Risk factors for PMI were coronary artery disease (CAD) (odds ratio [OR], 3.5; confidence interval [CI], 2.25.6), and serum creatinine >2 mg/dL (OR, 2.4; CI, 1.34.4). Risk factors for in‐hospital mortality were age 8589 (OR, 5.3; CI, 1.617.7), age 90 (OR, 8.9; CI, 2.630.8), PMI (OR 15.1; CI, 4.648.8), male gender (OR 5.8; CI, 2.215.2), dyspnea (OR 5.4; CI, 1.816.9), and hemoglobin <8.0 gm/dL (OR, 3.5; CI, 1.29.9). PMI was a strong predictor for 30‐day mortality (hazard ratio [HR], 4.3; CI, 2.18.9). Risk factors for 1‐year mortality were: age 90 (HR, 2.0; CI, 1.43.1), male gender (HR, 2.1; CI, 1.53.0), and PMI (HR, 1.9; CI, 1.42.7). Figures 1 and 2 describe the Kaplan‐Meier survival curves for patients with and without PMI.


| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P Value | |
|---|---|---|---|
| |||
| Perioperative myocardial infarction | |||
| Coronary artery disease | 3.0 (2.14.5) | 3.5 (2.25.6) | <0.001 |
| Serum creatinine >2.0 mg/dL | 2.7 (1.64.8) | 2.4 (1.34.4) | 0.003 |
| In‐hospital mortality | |||
| Age 8589 | 1.7 (0.83.7) | 5.3 (1.617.7) | 0.01 |
| Age 90 | 2.2 (1.04.8) | 8.9 (2.630.8) | <0.001 |
| Male gender | 3.0 (1.46.4) | 5.8 (2.215.2) | <0.001 |
| Postoperative anemia (<8.0 gm/dL) | 4.2 (1.710.0) | 3.5 (1.29.9) | 0.02 |
| Perioperative myocardial infarction | 14.0 (5.248.0) | 15.1 (4.649.0) | <0.001 |
| 30‐Day mortality | |||
| Perioperative myocardial infarction | 4.1 (2.27.8) | 4.3 (2.18.9) | <0.001 |
| 1‐Year mortality | |||
| Age 8589 | 1.3 (0.81.9) | 1.6 (1.02.4) | <0.03 |
| Age 90 | 1.9 (1.32.9) | 2.0 (1.43.1) | 0.001 |
| Male gender | 1.9 (1.32.6) | 2.1 (1.53.0) | <0.001 |
| Dementia | 2.5 (1.83.6) | 2.7 (1.93.8) | <0.001 |
| Perioperative myocardial infarction | 2.0 (1.52.8) | 1.9 (1.42.7) | 0.001 |
DISCUSSION
We report the high incidence of PMI (13.8%) in the cohort of 1212 elderly patients (mean age 85 years) undergoing hip fracture surgery. Most PMI events (92%) occurred within the first 48 hours of surgery. Most of the events (75%) were asymptomatic. Elderly patients with PMI had an increased hospital LOS by 4.2 days, with high in‐hospital mortality (13.8%), 30‐day mortality (17.4%), and 1‐year mortality (39.5%).
Most of the PMI patients were identified with cardiac biomarkers on the basis of universal definition of MI within the first 48 hours. Although universal definition of MI does not define PMI as a separate type, PMI shares common pathophysiological pathways of Type 1 MI (primary coronary event) and Type 2 MI (myocardial oxygen supplydemand imbalance). Postoperative tachycardia, hemodynamic instability, anemia, and hypoxemia may initiate pathways causing more Type 2 MI. Our study highlights the continued need for active surveillance of clinical symptoms, postoperative ECG monitoring for STT changes, and utilizing cardiac troponin in older postoperative patients to improve diagnostic accuracy of PMI.
The current study has higher asymptomatic PMI events when compared to a study of Devereaux et al.11 The current study had an older population undergoing urgent hip fracture surgery, with a higher burden of CAD (60%) and renal failure (20%) with serum creatinine >2 gm/dL (see Supporting Information, Appendix 1, in the online version of this article). Older age and a higher burden of these risk factors may explain the higher incidence of PMI in the current study. Perioperative liberal use of analgesics in hip fracture surgery may explain more asymptomatic patients.
In light of the recently published FOCUS12 trial, an important finding from our study is that postoperative anemia among elderly (<8.0 gm/dL) is associated with a 3.5‐fold increased in‐hospital mortality. It is critical to maintain perioperative hemoglobin above 8.0 gm/dL in very elderly patients, due to asymptomatic presentation of PMI.
In the current study, PMI is associated with a 15‐fold increased risk of in‐hospital death and a 4.3‐fold increased risk of 30‐day mortality in the elderly. Advanced age (85 years) is a well known strong predictor of initial hospital admission and death in elderly patients after outpatient surgery.13 Furthermore, the odds for an in‐hospital death increase by 70% for each 10‐year increase in age.14 Therefore, early detection of silent PMI among at‐risk elderly patients by cardiac biomarkers may help in optimization of cardiac pharmacotherapy known to decrease short‐ and long‐term mortality.
There are limitations inherent to the retrospective design and methodology. Data collection was done through the year 2002. CK was used for the period that spans from 1988 to mid‐2000. Troponin was used from 2000 to 2002. Statin use was not analyzed for lack of significant data. Limited use of beta‐blockers (15%) and angiotensin‐converting‐enzyme (ACE) inhibitors (25%) may also contribute to higher events (see Supporting Information, Appendix 1, in the online version of this article).
CONCLUSIONS
Elderly patients have a higher incidence of PMI and mortality after hip fracture surgery than what guidelines indicate. The majority of the elderly patients with PMI did not experience ischemic symptoms and required cardiac biomarkers for diagnosis. The results of our study support the measurement of troponin in postoperative elderly patients for the diagnosis of PMI to implement in‐hospital preventive strategies to reduce PMI‐associated mortality.
Acknowledgements
The authors gratefully acknowledge the assistance of Ms Dawn Bergen in drafting and editing the manuscript.
Disclosures: This research was supported by funding from AHA grant 03‐30103N‐04, Rochester Epidemiology Project (grant RO1‐AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases). The project was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Perioperative myocardial infarction (PMI) often remains unrecognized with higher mortality in the aged.13 Perioperative ischemic symptoms are often masked by analgesia, sedation, and transient and subtle electrocardiographic (ECG) changes. Postoperative troponin measurement is not routinely done for PMI diagnosis. Hip fracture surgery is the most common non‐cardiac surgical procedure in the elderly, with limited data on clinical presentation of PMI.46 Moreover, the elderly are significantly underrepresented in clinical studies.7 We therefore examined the clinical presentation of PMI and its outcomes among elderly patients admitted for hip fracture repair.
METHODS
Study Population
A population‐based, retrospective, case‐control study was conducted of all residents in Olmsted County, Minnesota undergoing surgery for hip fracture repair from January 1, 1988 through December 31, 2002. Primary indication for the surgery was proximal femur (femoral neck or subtrochanteric) fracture. Patients who were <65 years old, had a pathological hip fracture, multiple injuries or fractures, surgery >72 hours after injury (due to higher mortality with delayed surgery),8 nonsurgical management of hip fracture repair, or incomplete data were excluded. All patients provided prior authorization to use their medical records for research, per institutional protocols.9
Criteria for Perioperative Myocardial Infarction and Death
We utilized the universal definition of acute myocardial infarction10 to define PMI within the first 7 days following hip fracture surgery. We included creatine kinase‐MB fraction (CK‐MB) as the biomarker for 1988July 2000, and troponin as the biomarker for August 20002002. Mortality was defined as death from any cause within the first year following hip fracture repair. Deaths were identified through the National Death Index.
Statistical Analysis
For each case of PMI, we identified 2 control patients who were selected at random from the non‐PMI patient population. These controls were matched to cases based on age at the time of surgery (5 years) and gender in 1:2 ratios. Baseline characteristics across PMI and non‐PMI groups were compared using the Kruskal‐Wallis test (for continuous data) and the chi‐square or Fisher's exact tests (for categorical data). Mean values were utilized in place of the missing values for the following variables: preoperative troponin (missing values 88 [17.5%]), CK‐MB (8 [1.6%]), troponin (21 [5.4%]), and postoperative hemoglobin (17 [3.4%]). Univariate predictors of PMI with P 0.2 baseline characteristics were entered into a multivariate, conditional, logistic regression analysis. Rates of outcomes were calculated using the Kaplan‐Meier method, and by a landmark survival curve for those with and without PMI. Cox proportional hazards analysis was utilized for survival analysis at 30 days and 1 year. All statistical tests were 2‐sided, and P values <0.05 were considered significant. All analyses were performed using SAS for UNIX (version 9.1.3; SAS Institute, Inc, Cary, NC).
RESULTS
In the cohort of 1212 with hip fracture surgeries, 167 (13.8%) cases of PMI occurred in the first 7 days, of which 153 (92%) occurred within the first 48 hours. A total of 334 controls were matched with 167 cases of PMI. Table 1 summarizes the demographic characteristics of the study participants. Of the patients with PMI, 25.2% experienced symptoms of ischemia; 7% reported chest pain, and 12% reported dyspnea. Only 22.8% of patients with PMI had ECG changes consistent with ischemia. ST elevation MI was present in 7.2% patients. PMI patients had a lower mean hemoglobin compared to the patients without PMI (8.9 mg/dL vs 9.4 mg/dL, P < 0.001). Median length of stay (LOS) in the hospital was higher among patients who experienced PMI (11.6 vs 7.4 days, P < 0.001). Overall in‐hospital mortality was 5.6%. There were 24 deaths (14.4%) in the PMI group compared to 4 (1.2%) in‐hospital deaths in patients without PMI (P < 0.001). A total of 473 (94%) patients survived to discharge. At 30‐day follow‐up, there were 29 (17.4%) deaths in the PMI group and 14 (4.2%) deaths in non‐PMI group. During the follow‐up for 1 year, there were 143 (29%) deaths: PMI 66 (39.5%) and 77 (23%) non‐PMI group (P < 0.01).
| Characteristics, n (%) | Patients With PMI | Patients Without PMI | P Value* |
|---|---|---|---|
| (N = 167) | (N = 334) | ||
| |||
| Age mean SD | 85.3 7.4 | 85.2 7.1 | 0.5 |
| Weight (kg) mean SD | 59.98 16.7 | 59.80 13.9 | 0.5 |
| Women | 127 (76.4) | 254 (76) | 0.5 |
| Any symptom of ischemia, n (%) | |||
| Chest/arm pain | 11 (7) | 4 (1) | 0.002 |
| Dyspnea | 20 (12) | 14 (4) | 0.001 |
| Nausea/vomiting | 8 (5) | 6 (2) | 0.08 |
| Diaphoresis | 1 (1) | 1 (0.3) | 1.0 |
| PND | 3 (2) | 1 (0.3) | 0.3 |
| ECG changes, n (%) | |||
| ST‐segment elevation MI | 12 (7.2) | 0 | 0.01 |
| New ECG changes consistent with ischemia | 38 (22.8) | 1(0.3) | 0.01 |
| Biochemical evidence of ischemia, n (%) | |||
| CK‐MB | 147 (88) | 20 (6) | 0.01 |
| Troponin | 52 (33) | 9 (3) | 0.001 |
| Laboratory markers | |||
| Hemoglobin gm/dL mean (SD) | 8.9 1.0 | 9.4 1.2 | 0.001 |
| Postoperative anemia (<8.0 gm/dL), n (%) | 22 (13.2) | 37 (11.1) | 0.5 |
| Length of stay (days), mean SD | 11.6 7.7 | 7.4 6.4 | 0.001 |
| In‐hospital outcome | <0.001 | ||
| Dead | 24 (14.4) | 4 (1.2) | |
| Alive | 143 (85.6) | 330 (98.8) | |
| 30‐Day outcome | <0.001 | ||
| Dead | 29 (17.4) | 14 (4.2) | |
| Alive | 138 (82.6) | 320 (95.8) | |
| 1‐Year outcome | <0.001 | ||
| Dead | 66 (39.5) | 77 (23) | |
| Alive | 101 (60.4) | 257 (77) | |
Table 2 describes the risk factors associated with PMI in‐hospital, 30‐day, and 1‐year mortality. Risk factors for PMI were coronary artery disease (CAD) (odds ratio [OR], 3.5; confidence interval [CI], 2.25.6), and serum creatinine >2 mg/dL (OR, 2.4; CI, 1.34.4). Risk factors for in‐hospital mortality were age 8589 (OR, 5.3; CI, 1.617.7), age 90 (OR, 8.9; CI, 2.630.8), PMI (OR 15.1; CI, 4.648.8), male gender (OR 5.8; CI, 2.215.2), dyspnea (OR 5.4; CI, 1.816.9), and hemoglobin <8.0 gm/dL (OR, 3.5; CI, 1.29.9). PMI was a strong predictor for 30‐day mortality (hazard ratio [HR], 4.3; CI, 2.18.9). Risk factors for 1‐year mortality were: age 90 (HR, 2.0; CI, 1.43.1), male gender (HR, 2.1; CI, 1.53.0), and PMI (HR, 1.9; CI, 1.42.7). Figures 1 and 2 describe the Kaplan‐Meier survival curves for patients with and without PMI.


| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P Value | |
|---|---|---|---|
| |||
| Perioperative myocardial infarction | |||
| Coronary artery disease | 3.0 (2.14.5) | 3.5 (2.25.6) | <0.001 |
| Serum creatinine >2.0 mg/dL | 2.7 (1.64.8) | 2.4 (1.34.4) | 0.003 |
| In‐hospital mortality | |||
| Age 8589 | 1.7 (0.83.7) | 5.3 (1.617.7) | 0.01 |
| Age 90 | 2.2 (1.04.8) | 8.9 (2.630.8) | <0.001 |
| Male gender | 3.0 (1.46.4) | 5.8 (2.215.2) | <0.001 |
| Postoperative anemia (<8.0 gm/dL) | 4.2 (1.710.0) | 3.5 (1.29.9) | 0.02 |
| Perioperative myocardial infarction | 14.0 (5.248.0) | 15.1 (4.649.0) | <0.001 |
| 30‐Day mortality | |||
| Perioperative myocardial infarction | 4.1 (2.27.8) | 4.3 (2.18.9) | <0.001 |
| 1‐Year mortality | |||
| Age 8589 | 1.3 (0.81.9) | 1.6 (1.02.4) | <0.03 |
| Age 90 | 1.9 (1.32.9) | 2.0 (1.43.1) | 0.001 |
| Male gender | 1.9 (1.32.6) | 2.1 (1.53.0) | <0.001 |
| Dementia | 2.5 (1.83.6) | 2.7 (1.93.8) | <0.001 |
| Perioperative myocardial infarction | 2.0 (1.52.8) | 1.9 (1.42.7) | 0.001 |
DISCUSSION
We report the high incidence of PMI (13.8%) in the cohort of 1212 elderly patients (mean age 85 years) undergoing hip fracture surgery. Most PMI events (92%) occurred within the first 48 hours of surgery. Most of the events (75%) were asymptomatic. Elderly patients with PMI had an increased hospital LOS by 4.2 days, with high in‐hospital mortality (13.8%), 30‐day mortality (17.4%), and 1‐year mortality (39.5%).
Most of the PMI patients were identified with cardiac biomarkers on the basis of universal definition of MI within the first 48 hours. Although universal definition of MI does not define PMI as a separate type, PMI shares common pathophysiological pathways of Type 1 MI (primary coronary event) and Type 2 MI (myocardial oxygen supplydemand imbalance). Postoperative tachycardia, hemodynamic instability, anemia, and hypoxemia may initiate pathways causing more Type 2 MI. Our study highlights the continued need for active surveillance of clinical symptoms, postoperative ECG monitoring for STT changes, and utilizing cardiac troponin in older postoperative patients to improve diagnostic accuracy of PMI.
The current study has higher asymptomatic PMI events when compared to a study of Devereaux et al.11 The current study had an older population undergoing urgent hip fracture surgery, with a higher burden of CAD (60%) and renal failure (20%) with serum creatinine >2 gm/dL (see Supporting Information, Appendix 1, in the online version of this article). Older age and a higher burden of these risk factors may explain the higher incidence of PMI in the current study. Perioperative liberal use of analgesics in hip fracture surgery may explain more asymptomatic patients.
In light of the recently published FOCUS12 trial, an important finding from our study is that postoperative anemia among elderly (<8.0 gm/dL) is associated with a 3.5‐fold increased in‐hospital mortality. It is critical to maintain perioperative hemoglobin above 8.0 gm/dL in very elderly patients, due to asymptomatic presentation of PMI.
In the current study, PMI is associated with a 15‐fold increased risk of in‐hospital death and a 4.3‐fold increased risk of 30‐day mortality in the elderly. Advanced age (85 years) is a well known strong predictor of initial hospital admission and death in elderly patients after outpatient surgery.13 Furthermore, the odds for an in‐hospital death increase by 70% for each 10‐year increase in age.14 Therefore, early detection of silent PMI among at‐risk elderly patients by cardiac biomarkers may help in optimization of cardiac pharmacotherapy known to decrease short‐ and long‐term mortality.
There are limitations inherent to the retrospective design and methodology. Data collection was done through the year 2002. CK was used for the period that spans from 1988 to mid‐2000. Troponin was used from 2000 to 2002. Statin use was not analyzed for lack of significant data. Limited use of beta‐blockers (15%) and angiotensin‐converting‐enzyme (ACE) inhibitors (25%) may also contribute to higher events (see Supporting Information, Appendix 1, in the online version of this article).
CONCLUSIONS
Elderly patients have a higher incidence of PMI and mortality after hip fracture surgery than what guidelines indicate. The majority of the elderly patients with PMI did not experience ischemic symptoms and required cardiac biomarkers for diagnosis. The results of our study support the measurement of troponin in postoperative elderly patients for the diagnosis of PMI to implement in‐hospital preventive strategies to reduce PMI‐associated mortality.
Acknowledgements
The authors gratefully acknowledge the assistance of Ms Dawn Bergen in drafting and editing the manuscript.
Disclosures: This research was supported by funding from AHA grant 03‐30103N‐04, Rochester Epidemiology Project (grant RO1‐AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases). The project was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
- , , , et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- , , , et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390.
- , , , et al. Body mass index (BMI) and risk of noncardiac postoperative medical complications in elderly hip fracture patients: a population‐based study. J Hosp Med. 2009;4(8):E1–E9.
- . History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274.
- , , , . Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579.
- , , , et al. Body mass index and risk of adverse cardiac events in elderly patients with hip fracture: a population‐based study. J Am Geriatr Soc. 2009;57(3):419–426.
- , , , et al. Acute coronary care in the elderly, part I. Non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569.
- , . Hip fracture mortality. A prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;280:214–222.
- , , , et al. ACC/AHA/ACP‐ASIM guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 1999;33(7):2092–2190.
- , , ; for the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery. Ann Intern Med. 2011;154(8):523–528.
- , , , et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , . Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg. 2004;139(1):67–72.
- , , , et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353.
- , , , et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- , , , et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390.
- , , , et al. Body mass index (BMI) and risk of noncardiac postoperative medical complications in elderly hip fracture patients: a population‐based study. J Hosp Med. 2009;4(8):E1–E9.
- . History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274.
- , , , . Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579.
- , , , et al. Body mass index and risk of adverse cardiac events in elderly patients with hip fracture: a population‐based study. J Am Geriatr Soc. 2009;57(3):419–426.
- , , , et al. Acute coronary care in the elderly, part I. Non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569.
- , . Hip fracture mortality. A prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;280:214–222.
- , , , et al. ACC/AHA/ACP‐ASIM guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 1999;33(7):2092–2190.
- , , ; for the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery. Ann Intern Med. 2011;154(8):523–528.
- , , , et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , . Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg. 2004;139(1):67–72.
- , , , et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353.
Ischemic Stroke After Hip Operation
In the United States, hip operations (internal fixation of fracture or total hip arthroplasty [THA]) are the most common noncardiac major surgical procedures performed in patients age 65 years and older (45.2 procedures per 100,000 persons per year).1 This number of procedures is projected to increase substantially in the coming decades.
Little is known about the clinical predictors of postoperative stroke in patients undergoing hip surgical procedures. Further, recent results of the Perioperative Ischemic Evaluation (POISE) trial have shown that measures taken to reduce cardiac complications postoperatively may adversely affect the risk of stroke.2 The POISE study showed decreases in myocardial infarction and coronary revascularization but accompanying increases in stroke and death with use of ‐blockers in patients undergoing noncardiac surgery.
Prevention of adverse events is one of the top priorities of the U.S. health care system today.35 Risk stratification and therapeutic optimization of underlying chronic diseases may be important in decreasing perioperative risk and improving postoperative outcomes.
Our objective was to determine the rate of postoperative ischemic stroke in all residents of Olmsted County, MN, who underwent hip operation between 1988 and 2002 and to identify clinical predictors of postoperative stroke.
Subjects and Methods
Olmsted County is one of the few places in the world where comprehensive population‐based studies of disease etiology and outcomes are feasible. This feasibility is due to the Rochester Epidemiology Project, a medical records linkage system that provides access to the records of all medical care in the community.1 All medical diagnoses made for a resident of Olmsted County are entered on a master sheet in the patient's medical record, which is then entered into a central computer index.
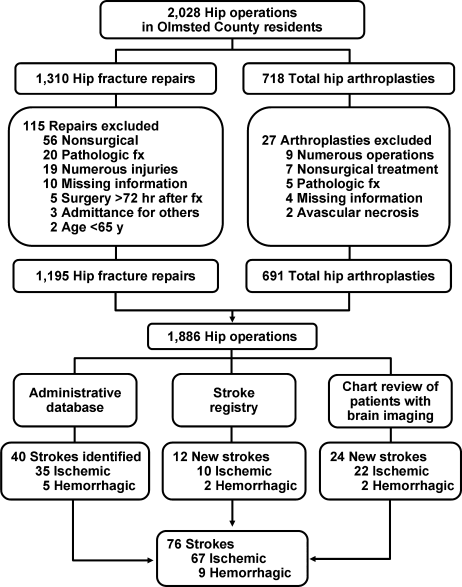
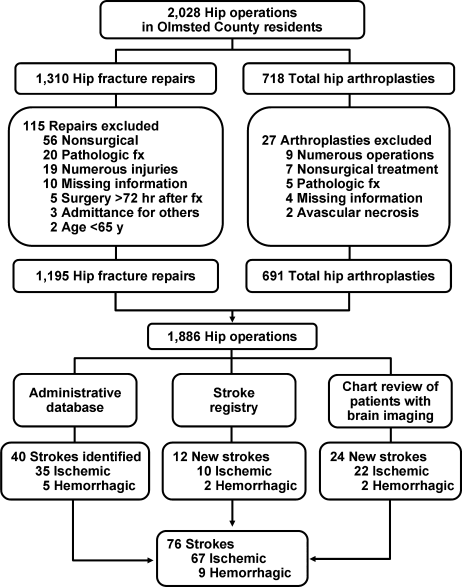
Hip operations were identified using the Surgical Information Recording System data warehouse, where detailed data are stored as International Classification of Diseases, 9th edition (ICD‐9) codes for all surgical procedures performed from January 1, 1988, forward. A total of 2028 THAs and hip fracture repairs (ICD‐9 codes 81.51, 81.52, 81.53, 79.15, and 79.25) performed between 1988 and 2002 in Olmsted County were identified. Of the hip procedures, 142 were excluded (Figure 1). The final analysis cohort contained 1886 hip operations1195 hip fracture repairs and 691 THAs.
The population‐based cohort was assembled and the data were abstracted from complete inpatient and outpatient records from admission for surgical treatment up to 1 year after surgery. Only those patients who had given prior authorization for research were included in the study cohort. The Mayo Clinic Institutional Review Board approved the study.
Case Ascertainment
We used several screening procedures to completely enumerate all postoperative strokes in our study population (Figure 1). The Mayo Clinic administrative database was used to identify all cases with relevant cerebrovascular disease (ICD‐9 codes 430.0‐437.9, 368.12, 781.4, and 784.3) within 1 year after hip operation. The Rochester Stroke Registry identified incident cases of ischemic stroke in Olmsted County from 1988 through 1994. The clinic's administrative database was also used to identify brain imaging studies (brain computed tomography, magnetic resonance imaging, or carotid ultrasonography) between the day of the procedure and 1 year postoperatively. A neurologist reviewed each image and the associated medical record identified during the screening process in detail for the constellation of signs and symptoms consistent with the diagnosis of stroke. Death certificates and autopsy reports were also reviewed to identify persons with the diagnosis of stroke. The outcome (stroke) was masked to the nurse abstractor who reviewed charts for predictors of postoperative stroke (eg, atrial fibrillation, coronary artery disease [CAD], history of stroke, medication use). The exposed or unexposed status of the patients to the predictors of stroke was masked to the physician (A.S.P.) who screened electronic medical records for the outcome measure (stroke).
Cerebral infarction or ischemic stroke was defined as the acute onset of a neurologic deficit that persisted for longer than 24 hours and corresponded to an arterial vascular territory of the cerebral hemispheres, brainstem, or cerebellum, with or without computed tomographic or magnetic resonance imaging documentation. Transient ischemic attack was defined as an episode of focal neurologic symptoms with abrupt onset and rapid resolution, lasting less than 24 hours, and due to altered circulation to a limited region of the brain.
Only patients with ischemic strokes clinically documented by a neurologist were included in the analysis.
Primary Outcomes
Outcomes were the cumulative probability of ischemic stroke and predictors of stroke in the first 12 months after surgical treatment of the hip.
Statistical Analysis
Continuous variables are presented as mean (standard deviation [SD]); categorical variables are presented as number and percentage. Two‐sample t tests or Wilcoxon rank sum tests were used to test for differences between THAs and hip fracture repairs in demographic characteristics, past medical history, and baseline clinical data composed of continuous variables; 2 or Fisher exact tests were used for categorical variables. No patient was lost to follow‐up during the 1 year after the initial surgery. However, the data of patients who died or had a second hip procedure within that period were censored.
The rate of ischemic stroke within 1 year after the incident hip procedure was calculated using the Kaplan‐Meier method. Second hip procedures within that period were counted as additional cases. Rates were calculated for the overall group, as well as for the univariate risk factors of operative procedure type, age, sex, past medical history of stroke, hypertension, atrial fibrillation, CAD, chronic obstructive pulmonary disease (COPD), diabetes mellitus, and chronic renal insufficiency. Use of ‐blockers, hydroxymethylglutaryl‐coenzyme A (HMG‐CoA) reductase inhibitors, or aspirin at hospital admission was also considered. Cox proportional hazards regression models were used to evaluate the risk of ischemic stroke for each of these univariate risk factors. Multivariable Cox proportional hazards models were constructed with adjustments for operative procedure type, age, sex, and comorbid conditions such as atrial fibrillation and hypertension. These covariates were added in a stepwise selection to identify factors significantly associated with the outcome. To account for patients who had a second hip procedure within 1 year of their first operation, we calculated all Cox proportional hazards regression results using the robust sandwich estimate of the covariance matrix. The proportional hazards assumption for all Cox models was evaluated with the methods proposed by Therneau and Grambsch;6 no violations of this assumption were identified. The rate of postoperative stroke after adjusting for the competing risk of death was calculated using the approach of Gooley et al.7 All statistical tests were 2‐sided, and a P value was considered significant if it was less than 0.05. Statistical analyses were performed using statistical software (SAS version 9.1.3; SAS Institute, Inc., Cary, NC).
Results
Among the patients with the 1886 hip procedures, 67 ischemic strokes were identified within 1 year after the index surgical procedure10 (1.4%) among the 691 THAs and 57 (4.8%) among the 1195 hip fracture repairs. Baseline characteristics are summarized in Table 1. Compared with the THA group, patients in the hip fracture repair group were more likely to be older and female. Additionally, such comorbid conditions as a history of stroke, diabetes mellitus, congestive heart failure, atrial fibrillation, or dementia were more prevalent in the hip fracture repair group.
| Characteristics | Surgical Procedure | Total (n = 1,886) | P Value* | |
|---|---|---|---|---|
| THA (n = 691) | Fracture Repair (n = 1,195) | |||
| ||||
| Age, years | 74.9 (6.59) | 84.2 (7.49) | 80.8 (8.46) | <0.001 |
| Sex, male | 258 (37.3) | 234 (19.6) | 492 (26.1) | <0.001 |
| Race, White | 690 (100) | 1,187 (99.3) | 1,877 (99.5) | 0.17 |
| BMI | 27.7 (5.36) | 23.3 (4.93) | 24.9 (5.52) | <0.001 |
| History | ||||
| Hypertension | 424 (61.4) | 695 (58.2) | 1,119 (59.3) | 0.17 |
| Diabetes | 57 (8.2) | 141 (11.8) | 198 (10.5) | 0.02 |
| Stroke | 50 (7.2) | 334 (27.9) | 384 (20.4) | <0.001 |
| CHF | 100 (14.5) | 321 (26.9) | 421 (22.3) | <0.001 |
| Atrial fibrillation | 72 (10.4) | 241 (20.2) | 313 (16.6) | <0.001 |
| Dementia | 16 (2.3) | 407 (34.1) | 423 (22.4) | <0.001 |
| ASA risk classification | <0.001 | |||
| 1 or 2 | 343 (49.6) | 172 (14.4) | 515 (27.3) | |
| 3, 4, or 5 | 348 (50.4) | 1,022 (85.6) | 1,370 (72.7) | |
| Medication on admission | ||||
| Aspirin | 168 (24.3) | 369 (30.9) | 537 (28.5) | 0.002 |
| ‐Blocker | 134 (19.4) | 184 (15.4) | 318 (16.9) | 0.03 |
| Insulin | 12 (1.7) | 48 (4) | 60 (3.2) | 0.007 |
| Length of stay, days | 7.3 (3.9) | 10.0 (7.61) | 9.0 (6.63) | <0.001 |
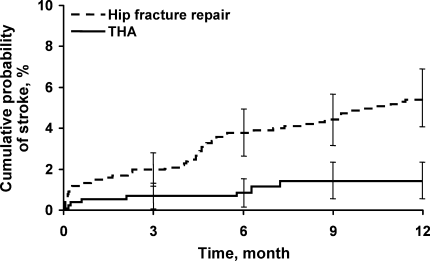
Univariate analyses assessing the rate and risk of postoperative ischemic stroke are shown in Table 2. The rate of stroke was significantly greater among hip fracture repairs than THAs 30 days postoperatively and 1 year postoperatively (1.5% vs. 0.6% and 5.5% vs. 1.5%, respectively; P < 0.001) (Figure 2). In our study we found an annual incidence rate of ischemic stroke of 4093 per 100,000 person‐years (95% confidence interval [CI], 3172‐5198 per 100,000 person‐years). Accounting for death as a competing risk for stroke had little impact on the rate of stroke overall or within the 2 surgical groups (results not shown). Univariate Cox proportional hazards models showed that neither sex nor history of hypertension, diabetes mellitus, COPD, chronic renal insufficiency, or CAD or use of HMG‐CoA reductase inhibitors or ‐blockers were significant predictors of ischemic stroke. However, other clinical risk factors, such as a history of atrial fibrillation (hazard ratio [HR], 2.16; P = 0.005), hip fracture repair vs. THA (HR, 3.80; P < 0.001), increased age (HR, 2.20; P = 0.017), aspirin use (HR, 1.8; P = 0.014), and history of previous stroke (HR, 4.18; P < 0.001), were significantly associated with an increased risk of stroke (Table 2).
| Variable | Number of Patients | Number of Events | Rate (%) | Hazard Ratio | P Value | |
|---|---|---|---|---|---|---|
| 30‐Day (95% CI) | 1‐Year (95% CI) | |||||
| ||||||
| Overall | 1886 | 67 | 1.2 (0.7‐1.7) | 3.9 (3‐4.8) | ||
| Type of operative procedure | ||||||
| THA | 691 | 10 | 0.6 (0.0‐1.1) | 1.5 (0.6‐2.4) | ||
| Hip fracture repair | 1195 | 57 | 1.5 (0.8‐2.2) | 5.5 (4.1‐6.9) | 3.80 (1.94‐7.44) | <0.001 |
| Age at operation, years | ||||||
| <75 | 528 | 11 | 1.0 (0.1‐1.8) | 2.1 (0.9‐3.3) | ||
| 75 | 1358 | 56 | 1.3 (0.7‐1.9) | 4.7 (3.5‐5.8) | 2.20 (1.15‐4.21) | 0.02 |
| Sex | ||||||
| Female | 1394 | 54 | 1.3 (0.7‐1.9) | 4.2 (3.1‐5.3) | ||
| Male | 492 | 13 | 0.8 (0.0‐1.7) | 2.9 (1.3‐4.4) | 0.69 (0.38‐1.27) | 0.24 |
| History of stroke | ||||||
| No | 1502 | 34 | 0.7 (0.3‐1.2) | 2.4 (1.6‐3.3) | ||
| Yes | 384 | 33 | 3.0 (1.2‐4.7) | 9.9 (6.6‐13) | 4.18 (2.59‐6.74) | <0.001 |
| History of hypertension | ||||||
| No | 767 | 23 | 0.8 (0.2‐1.4) | 3.4 (2.0‐4.7) | ||
| Yes | 1119 | 44 | 1.5 (0.7‐2.2) | 4.2 (3.0‐5.5) | 1.29 (0.78‐2.14) | 0.32 |
| History of atrial fibrillation | ||||||
| No | 1573 | 48 | 1.0 (0.5‐1.5) | 3.3 (2.4‐4.2) | ||
| Yes | 313 | 19 | 1.9 (0.4‐3.5) | 7.0 (3.9‐9.9) | 2.16 (1.27‐3.67) | 0.005 |
| History of CAD | ||||||
| No | 1224 | 40 | 1.1 (0.5‐1.6) | 3.5 (2.4‐4.5) | ||
| Yes | 662 | 27 | 1.4 (0.5‐2.3) | 4.7 (2.9‐6.4) | 1.34 (0.82‐2.19) | 0.24 |
| History of COPD | ||||||
| No | 1606 | 62 | 1.4 (0.8‐2.0) | 4.2 (3.1‐5.2) | ||
| Yes | 280 | 5 | 0 (0.0‐0.0) | 2.2 (0.3‐4.1) | 0.49 (0.20‐1.22) | 0.13 |
| History of diabetes mellitus | ||||||
| No | 1688 | 56 | 1.1 (0.6‐1.7) | 3.6 (2.7‐4.5) | ||
| Yes | 198 | 11 | 1.5 (0‐3.3) | 6.3 (2.6‐9.9) | 1.75 (0.92‐3.34) | 0.09 |
| History of renal insufficiency | ||||||
| No | 1718 | 58 | 1.0 (0.5‐1.5) | 3.7 (2.7‐4.6) | ||
| Yes | 168 | 9 | 3.0 (0.4‐5.5) | 5.8 (2‐9.5) | 1.77 (0.88‐3.57) | 0.11 |
| Aspirin use | ||||||
| No | 1349 | 39 | 0.7 (0.2‐1.1) | 3.2 (2.2‐4.2) | ||
| Yes | 537 | 28 | 2.5 (0.1‐3.8) | 5.7 (3.6‐7.7) | 1.86 (1.13‐3.06) | 0.01 |
| ‐Blocker use | ||||||
| No | 1568 | 52 | 1.1 (0.6‐1.6) | 3.6 (2.7‐4.6) | ||
| Yes | 318 | 15 | 1.6 (0.2‐3.0) | 5.1 (2.6‐7.6) | 1.42 (0.81‐2.52) | 0.22 |
| HMG‐CoA reductase inhibitor use | ||||||
| No | 1736 | 63 | 1.2 (0.7‐1.7) | 4.0 (3.0‐4.9) | ||
| Yes (statin/other lipid lowering drugs) | 148 | 4 | 1.4 (0‐3.2) | 2.8 (0.1‐5.4) | 0.70 (0.26‐1.94) | 0.50 |
Because age was associated with the type of surgical procedure (87% of hip fracture repair patients were 75 years or older compared with 45% of THA patients), the effect of hip fracture repair on ischemic stroke was adjusted for age. For similar reasons, sex was also examined as an adjusting factor. Adjustment for age and sex resulted in only a slight attenuation of the HR for hip fracture repair vs. THA, from 3.8 to 3.4. A further analysis also adjusted for history of hypertension and history of atrial fibrillation, both comorbidities commonly associated with ischemic stroke. After adjustment for age, sex, history of hypertension, and history of atrial fibrillation, the risk of ischemic stroke was still significantly greater in the hip fracture repair group than in the THA group (HR, 2.8; 95% CI, 1.4‐5.7; P = 0.005).
To determine the most important predictors of postoperative ischemic stroke, multivariable analysis was conducted with stepwise selection. Potential risk factors included the following: operative procedure type (hip fracture repair vs. THA), age, sex, and history of stroke, hypertension, atrial fibrillation, CAD, COPD, diabetes mellitus, and chronic renal insufficiency, as well as use of ‐blockers, HMG‐CoA reductase inhibitors, and aspirin on hospital admission. Among all these factors, history of stroke (HR, 3.27; P < 0.001) and hip fracture repair vs. THA (HR, 2.74; P = 0.004) were confirmed to be significant predictors of postoperative ischemic stroke; the other factors did not significantly affect the model (Figure 2).
Comment
Our findings contrast those of previous studies that focused on perioperative ischemic stroke rates for specific surgical procedures,2, 8, 9 but do seem concordant with published results for early event rates of cerebrovascular accident or transient ischemic attack (1%) following hip fracture.10 The data from our study suggest that perioperative stroke cumulative probability is relatively high for hip procedures at both 30 days (1.2%) and 1 year (3.9%) after the index surgical procedure compared with general procedures. Subjects with a history of stroke who were undergoing hip operation had a postoperative stroke risk of 3.0% at 30 days and 9.9% at 1 year.
The incidence of stroke was greater in the hip fracture repair group (1.5% at 30 days and 5.5% at 1 year) than in the elective THA group (0.6% at 30 days and 1.5% at 1 year). The increased 1‐year mortality for patients undergoing hip surgery compared with the general population is in part due to cerebrovascular disease,10 and, therefore, the 1‐year stroke incidence is important.
After adjustment for age, sex, and comorbidities (hypertension and atrial fibrillation), the risk of postoperative ischemic stroke was 2.71 times greater in the hip fracture repair group than in the THA group (P = 0.006). These data are important in counseling and caring for patients undergoing different types of hip procedures.
From 1985 through 1989, for the age group (75‐84 years old) that best fits the demographics of our cohort, both men and women had limited variation over time in annual incidence rates of stroke (2149‐1074 strokes per 100,000 population per year) for Olmsted County, MN.11 In our study we found an annual incidence rate of ischemic stroke of 4,093 per 100,000 person‐years (95% CI, 3172‐5198 per 100,000 person‐years). The lower limit of the 95% CI is higher than the rates reported for Olmsted County, suggesting that having hip surgery increases the 1‐year risk of ischemic stroke.
Previous studies have shown that the risk factor most consistently correlated to perioperative ischemic stroke is a history of stroke.9 In our study, history of stroke and type of hip fracture surgery were confirmed to be the strongest predictors of postoperative stroke. History of hypertension, atrial fibrillation, CAD, COPD, diabetes, or chronic renal insufficiency was not correlated to perioperative ischemic stroke.
Nonmodifiable risk factors, such as advanced age, serve as markers of stroke risk and help identify high‐risk populations that may require aggressive intervention. After age adjustment of hip fracture repair, age was no longer significantly associated with postoperative stroke.
Cerebrovascular disease appears to be a marker for CAD, and, therefore, patients with a history of stroke usually have a Revised Cardiac Risk Index that may suggest the use of ‐blockers. According to the recent results of the POISE trial, use of ‐blockers could lead to increased stroke incidence.2 Our results showed no significant correlation between stroke risk and ‐blocker use, but our study period was from 1988 to 2002, when titration of ‐blocker dose to heart rates of 55 to 60 beats per minute was not common practice.
Several studies have confirmed the value of aspirin in decreasing the rate of vascular outcomes after diagnosis of transient ischemic attack or stroke.12 In our study, aspirin use on hospital admission was found in the univariate analysis to be associated with an increased risk of stroke, but this finding was not confirmed after adjustments for age, sex, and comorbid conditions. Aspirin use on admission was not a significant predictor of postoperative stroke, most likely because aspirin use can be considered a marker of increased cardiovascular risk and we adjusted for these comorbid conditions.
The limitations of this study are inherent in its retrospective design. First, we identified all incident cases of stroke after hip operation by reviewing medical records and then abstracting data from those records. We may have missed some mild strokes if they were misclassified as peripheral vestibular neuropathy, migraine, or even seizure. Less likely is that we missed strokes within the first 30 days after the procedure because that is the period in which patients with hip operation are either hospitalized or sent for rehabilitation in skilled nursing facilities. It is known that institutionalization leads to better surveillance and more complete ascertainment of any medical event.
The event rate of postoperative stroke at 30 days after hip operation was low. Therefore, we did not have the statistical power to comment meaningfully on predictors of stroke at 30 days after the hip procedure. Any nonrespondent or volunteer bias was addressed by using data from the Rochester Epidemiology Project, which allowed us to identify all Olmsted County residents who underwent hip operation between 1988 and 2002. The diagnostic suspicion bias was also accounted for in our study design because different physicians provided care and outcome measurement.
Our results apply for the patients who underwent hip operation between 1988 and 2002. The noncardiac surgery guidelines have been revised between 1988 and 2002, and we did not perform a stratified analysis by index year. The next step in our study will be to extend our data collection to 2008 and look at time trends.
Conclusion
In this population‐based historical cohort study, patients undergoing hip operation had a 3.9% cumulative probability of ischemic stroke during the first postoperative year. History of stroke and type of hip procedure (ie, hip fracture repair) were the strongest predictors of this complication. Because history of stroke is such a strong predictor of postoperative stroke, the perioperative management of these patients should probably be tailored, with closely observed blood pressure management and antihypertensive medication adjustment, to avoid compromising cerebral perfusion. Also, to avoid postoperative hypercoagulability that increases the risk of stroke, these patients may need to begin receiving antiplatelets as soon as is surgically acceptable.1315
- .History of the Rochester Epidemiology Project.Mayo Clin Proc.1996;71(3):266–274.
- POISE Study Group;,,,,,, et al.Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial.Lancet.2008;371(9627):1839–1847.
- ,,,,,, et al;American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2006;113(6):e85–e151.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ, eds.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No.43.AHRQ publication no. 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality (AHRQ),U.S. Department of Health and Human Services;2001.668 p.
- ,,.Deaths due to medical errors are exaggerated in Institute of Medicine report.JAMA.2000;284(1):93–95.
- ,.Modeling survival data: extending the Cox model.New York:Springer;2000.
- ,,,.Estimation of failure probabilities in the presence of competing risks: new representations of old estimators.Stat Med.1999;18(6):695–706.
- ,,.Postoperative cerebrovascular accidents in general surgery.Acta Anaesthesiol Scand.1988;32(8):698–701.
- ,,,,,, et al.Perioperative stroke risk in 173 consecutive patients with a past history of stroke.Arch Surg.1990;125(8):986–989.
- ,,,,.Medical complications and outcomes after hip fracture repair.Arch Intern Med.2002;162(18):2053–2057.
- ,,,,.Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989.Stroke.1996;27(3):373–380.
- CAST (Chinese Acute Stroke Trial) Collaborative Group.Randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke.Lancet.1997;349(9066):1641–1649.
- ,,.Coagulation activation and organ dysfunction following cardiac surgery.Chest.2005;128(1):229–236.
- ,,,,,.Intra‐ and postoperative fibrinolysis in patients undergoing cardiopulmonary bypass surgery.Haemostasis.1991;21(1):58–64.
- .Perioperative stroke.N Engl J Med.2007;356(7):706–713.
In the United States, hip operations (internal fixation of fracture or total hip arthroplasty [THA]) are the most common noncardiac major surgical procedures performed in patients age 65 years and older (45.2 procedures per 100,000 persons per year).1 This number of procedures is projected to increase substantially in the coming decades.
Little is known about the clinical predictors of postoperative stroke in patients undergoing hip surgical procedures. Further, recent results of the Perioperative Ischemic Evaluation (POISE) trial have shown that measures taken to reduce cardiac complications postoperatively may adversely affect the risk of stroke.2 The POISE study showed decreases in myocardial infarction and coronary revascularization but accompanying increases in stroke and death with use of ‐blockers in patients undergoing noncardiac surgery.
Prevention of adverse events is one of the top priorities of the U.S. health care system today.35 Risk stratification and therapeutic optimization of underlying chronic diseases may be important in decreasing perioperative risk and improving postoperative outcomes.
Our objective was to determine the rate of postoperative ischemic stroke in all residents of Olmsted County, MN, who underwent hip operation between 1988 and 2002 and to identify clinical predictors of postoperative stroke.
Subjects and Methods
Olmsted County is one of the few places in the world where comprehensive population‐based studies of disease etiology and outcomes are feasible. This feasibility is due to the Rochester Epidemiology Project, a medical records linkage system that provides access to the records of all medical care in the community.1 All medical diagnoses made for a resident of Olmsted County are entered on a master sheet in the patient's medical record, which is then entered into a central computer index.
Hip operations were identified using the Surgical Information Recording System data warehouse, where detailed data are stored as International Classification of Diseases, 9th edition (ICD‐9) codes for all surgical procedures performed from January 1, 1988, forward. A total of 2028 THAs and hip fracture repairs (ICD‐9 codes 81.51, 81.52, 81.53, 79.15, and 79.25) performed between 1988 and 2002 in Olmsted County were identified. Of the hip procedures, 142 were excluded (Figure 1). The final analysis cohort contained 1886 hip operations1195 hip fracture repairs and 691 THAs.

The population‐based cohort was assembled and the data were abstracted from complete inpatient and outpatient records from admission for surgical treatment up to 1 year after surgery. Only those patients who had given prior authorization for research were included in the study cohort. The Mayo Clinic Institutional Review Board approved the study.
Case Ascertainment
We used several screening procedures to completely enumerate all postoperative strokes in our study population (Figure 1). The Mayo Clinic administrative database was used to identify all cases with relevant cerebrovascular disease (ICD‐9 codes 430.0‐437.9, 368.12, 781.4, and 784.3) within 1 year after hip operation. The Rochester Stroke Registry identified incident cases of ischemic stroke in Olmsted County from 1988 through 1994. The clinic's administrative database was also used to identify brain imaging studies (brain computed tomography, magnetic resonance imaging, or carotid ultrasonography) between the day of the procedure and 1 year postoperatively. A neurologist reviewed each image and the associated medical record identified during the screening process in detail for the constellation of signs and symptoms consistent with the diagnosis of stroke. Death certificates and autopsy reports were also reviewed to identify persons with the diagnosis of stroke. The outcome (stroke) was masked to the nurse abstractor who reviewed charts for predictors of postoperative stroke (eg, atrial fibrillation, coronary artery disease [CAD], history of stroke, medication use). The exposed or unexposed status of the patients to the predictors of stroke was masked to the physician (A.S.P.) who screened electronic medical records for the outcome measure (stroke).
Cerebral infarction or ischemic stroke was defined as the acute onset of a neurologic deficit that persisted for longer than 24 hours and corresponded to an arterial vascular territory of the cerebral hemispheres, brainstem, or cerebellum, with or without computed tomographic or magnetic resonance imaging documentation. Transient ischemic attack was defined as an episode of focal neurologic symptoms with abrupt onset and rapid resolution, lasting less than 24 hours, and due to altered circulation to a limited region of the brain.
Only patients with ischemic strokes clinically documented by a neurologist were included in the analysis.
Primary Outcomes
Outcomes were the cumulative probability of ischemic stroke and predictors of stroke in the first 12 months after surgical treatment of the hip.
Statistical Analysis
Continuous variables are presented as mean (standard deviation [SD]); categorical variables are presented as number and percentage. Two‐sample t tests or Wilcoxon rank sum tests were used to test for differences between THAs and hip fracture repairs in demographic characteristics, past medical history, and baseline clinical data composed of continuous variables; 2 or Fisher exact tests were used for categorical variables. No patient was lost to follow‐up during the 1 year after the initial surgery. However, the data of patients who died or had a second hip procedure within that period were censored.
The rate of ischemic stroke within 1 year after the incident hip procedure was calculated using the Kaplan‐Meier method. Second hip procedures within that period were counted as additional cases. Rates were calculated for the overall group, as well as for the univariate risk factors of operative procedure type, age, sex, past medical history of stroke, hypertension, atrial fibrillation, CAD, chronic obstructive pulmonary disease (COPD), diabetes mellitus, and chronic renal insufficiency. Use of ‐blockers, hydroxymethylglutaryl‐coenzyme A (HMG‐CoA) reductase inhibitors, or aspirin at hospital admission was also considered. Cox proportional hazards regression models were used to evaluate the risk of ischemic stroke for each of these univariate risk factors. Multivariable Cox proportional hazards models were constructed with adjustments for operative procedure type, age, sex, and comorbid conditions such as atrial fibrillation and hypertension. These covariates were added in a stepwise selection to identify factors significantly associated with the outcome. To account for patients who had a second hip procedure within 1 year of their first operation, we calculated all Cox proportional hazards regression results using the robust sandwich estimate of the covariance matrix. The proportional hazards assumption for all Cox models was evaluated with the methods proposed by Therneau and Grambsch;6 no violations of this assumption were identified. The rate of postoperative stroke after adjusting for the competing risk of death was calculated using the approach of Gooley et al.7 All statistical tests were 2‐sided, and a P value was considered significant if it was less than 0.05. Statistical analyses were performed using statistical software (SAS version 9.1.3; SAS Institute, Inc., Cary, NC).
Results
Among the patients with the 1886 hip procedures, 67 ischemic strokes were identified within 1 year after the index surgical procedure10 (1.4%) among the 691 THAs and 57 (4.8%) among the 1195 hip fracture repairs. Baseline characteristics are summarized in Table 1. Compared with the THA group, patients in the hip fracture repair group were more likely to be older and female. Additionally, such comorbid conditions as a history of stroke, diabetes mellitus, congestive heart failure, atrial fibrillation, or dementia were more prevalent in the hip fracture repair group.
| Characteristics | Surgical Procedure | Total (n = 1,886) | P Value* | |
|---|---|---|---|---|
| THA (n = 691) | Fracture Repair (n = 1,195) | |||
| ||||
| Age, years | 74.9 (6.59) | 84.2 (7.49) | 80.8 (8.46) | <0.001 |
| Sex, male | 258 (37.3) | 234 (19.6) | 492 (26.1) | <0.001 |
| Race, White | 690 (100) | 1,187 (99.3) | 1,877 (99.5) | 0.17 |
| BMI | 27.7 (5.36) | 23.3 (4.93) | 24.9 (5.52) | <0.001 |
| History | ||||
| Hypertension | 424 (61.4) | 695 (58.2) | 1,119 (59.3) | 0.17 |
| Diabetes | 57 (8.2) | 141 (11.8) | 198 (10.5) | 0.02 |
| Stroke | 50 (7.2) | 334 (27.9) | 384 (20.4) | <0.001 |
| CHF | 100 (14.5) | 321 (26.9) | 421 (22.3) | <0.001 |
| Atrial fibrillation | 72 (10.4) | 241 (20.2) | 313 (16.6) | <0.001 |
| Dementia | 16 (2.3) | 407 (34.1) | 423 (22.4) | <0.001 |
| ASA risk classification | <0.001 | |||
| 1 or 2 | 343 (49.6) | 172 (14.4) | 515 (27.3) | |
| 3, 4, or 5 | 348 (50.4) | 1,022 (85.6) | 1,370 (72.7) | |
| Medication on admission | ||||
| Aspirin | 168 (24.3) | 369 (30.9) | 537 (28.5) | 0.002 |
| ‐Blocker | 134 (19.4) | 184 (15.4) | 318 (16.9) | 0.03 |
| Insulin | 12 (1.7) | 48 (4) | 60 (3.2) | 0.007 |
| Length of stay, days | 7.3 (3.9) | 10.0 (7.61) | 9.0 (6.63) | <0.001 |
Univariate analyses assessing the rate and risk of postoperative ischemic stroke are shown in Table 2. The rate of stroke was significantly greater among hip fracture repairs than THAs 30 days postoperatively and 1 year postoperatively (1.5% vs. 0.6% and 5.5% vs. 1.5%, respectively; P < 0.001) (Figure 2). In our study we found an annual incidence rate of ischemic stroke of 4093 per 100,000 person‐years (95% confidence interval [CI], 3172‐5198 per 100,000 person‐years). Accounting for death as a competing risk for stroke had little impact on the rate of stroke overall or within the 2 surgical groups (results not shown). Univariate Cox proportional hazards models showed that neither sex nor history of hypertension, diabetes mellitus, COPD, chronic renal insufficiency, or CAD or use of HMG‐CoA reductase inhibitors or ‐blockers were significant predictors of ischemic stroke. However, other clinical risk factors, such as a history of atrial fibrillation (hazard ratio [HR], 2.16; P = 0.005), hip fracture repair vs. THA (HR, 3.80; P < 0.001), increased age (HR, 2.20; P = 0.017), aspirin use (HR, 1.8; P = 0.014), and history of previous stroke (HR, 4.18; P < 0.001), were significantly associated with an increased risk of stroke (Table 2).

| Variable | Number of Patients | Number of Events | Rate (%) | Hazard Ratio | P Value | |
|---|---|---|---|---|---|---|
| 30‐Day (95% CI) | 1‐Year (95% CI) | |||||
| ||||||
| Overall | 1886 | 67 | 1.2 (0.7‐1.7) | 3.9 (3‐4.8) | ||
| Type of operative procedure | ||||||
| THA | 691 | 10 | 0.6 (0.0‐1.1) | 1.5 (0.6‐2.4) | ||
| Hip fracture repair | 1195 | 57 | 1.5 (0.8‐2.2) | 5.5 (4.1‐6.9) | 3.80 (1.94‐7.44) | <0.001 |
| Age at operation, years | ||||||
| <75 | 528 | 11 | 1.0 (0.1‐1.8) | 2.1 (0.9‐3.3) | ||
| 75 | 1358 | 56 | 1.3 (0.7‐1.9) | 4.7 (3.5‐5.8) | 2.20 (1.15‐4.21) | 0.02 |
| Sex | ||||||
| Female | 1394 | 54 | 1.3 (0.7‐1.9) | 4.2 (3.1‐5.3) | ||
| Male | 492 | 13 | 0.8 (0.0‐1.7) | 2.9 (1.3‐4.4) | 0.69 (0.38‐1.27) | 0.24 |
| History of stroke | ||||||
| No | 1502 | 34 | 0.7 (0.3‐1.2) | 2.4 (1.6‐3.3) | ||
| Yes | 384 | 33 | 3.0 (1.2‐4.7) | 9.9 (6.6‐13) | 4.18 (2.59‐6.74) | <0.001 |
| History of hypertension | ||||||
| No | 767 | 23 | 0.8 (0.2‐1.4) | 3.4 (2.0‐4.7) | ||
| Yes | 1119 | 44 | 1.5 (0.7‐2.2) | 4.2 (3.0‐5.5) | 1.29 (0.78‐2.14) | 0.32 |
| History of atrial fibrillation | ||||||
| No | 1573 | 48 | 1.0 (0.5‐1.5) | 3.3 (2.4‐4.2) | ||
| Yes | 313 | 19 | 1.9 (0.4‐3.5) | 7.0 (3.9‐9.9) | 2.16 (1.27‐3.67) | 0.005 |
| History of CAD | ||||||
| No | 1224 | 40 | 1.1 (0.5‐1.6) | 3.5 (2.4‐4.5) | ||
| Yes | 662 | 27 | 1.4 (0.5‐2.3) | 4.7 (2.9‐6.4) | 1.34 (0.82‐2.19) | 0.24 |
| History of COPD | ||||||
| No | 1606 | 62 | 1.4 (0.8‐2.0) | 4.2 (3.1‐5.2) | ||
| Yes | 280 | 5 | 0 (0.0‐0.0) | 2.2 (0.3‐4.1) | 0.49 (0.20‐1.22) | 0.13 |
| History of diabetes mellitus | ||||||
| No | 1688 | 56 | 1.1 (0.6‐1.7) | 3.6 (2.7‐4.5) | ||
| Yes | 198 | 11 | 1.5 (0‐3.3) | 6.3 (2.6‐9.9) | 1.75 (0.92‐3.34) | 0.09 |
| History of renal insufficiency | ||||||
| No | 1718 | 58 | 1.0 (0.5‐1.5) | 3.7 (2.7‐4.6) | ||
| Yes | 168 | 9 | 3.0 (0.4‐5.5) | 5.8 (2‐9.5) | 1.77 (0.88‐3.57) | 0.11 |
| Aspirin use | ||||||
| No | 1349 | 39 | 0.7 (0.2‐1.1) | 3.2 (2.2‐4.2) | ||
| Yes | 537 | 28 | 2.5 (0.1‐3.8) | 5.7 (3.6‐7.7) | 1.86 (1.13‐3.06) | 0.01 |
| ‐Blocker use | ||||||
| No | 1568 | 52 | 1.1 (0.6‐1.6) | 3.6 (2.7‐4.6) | ||
| Yes | 318 | 15 | 1.6 (0.2‐3.0) | 5.1 (2.6‐7.6) | 1.42 (0.81‐2.52) | 0.22 |
| HMG‐CoA reductase inhibitor use | ||||||
| No | 1736 | 63 | 1.2 (0.7‐1.7) | 4.0 (3.0‐4.9) | ||
| Yes (statin/other lipid lowering drugs) | 148 | 4 | 1.4 (0‐3.2) | 2.8 (0.1‐5.4) | 0.70 (0.26‐1.94) | 0.50 |
Because age was associated with the type of surgical procedure (87% of hip fracture repair patients were 75 years or older compared with 45% of THA patients), the effect of hip fracture repair on ischemic stroke was adjusted for age. For similar reasons, sex was also examined as an adjusting factor. Adjustment for age and sex resulted in only a slight attenuation of the HR for hip fracture repair vs. THA, from 3.8 to 3.4. A further analysis also adjusted for history of hypertension and history of atrial fibrillation, both comorbidities commonly associated with ischemic stroke. After adjustment for age, sex, history of hypertension, and history of atrial fibrillation, the risk of ischemic stroke was still significantly greater in the hip fracture repair group than in the THA group (HR, 2.8; 95% CI, 1.4‐5.7; P = 0.005).
To determine the most important predictors of postoperative ischemic stroke, multivariable analysis was conducted with stepwise selection. Potential risk factors included the following: operative procedure type (hip fracture repair vs. THA), age, sex, and history of stroke, hypertension, atrial fibrillation, CAD, COPD, diabetes mellitus, and chronic renal insufficiency, as well as use of ‐blockers, HMG‐CoA reductase inhibitors, and aspirin on hospital admission. Among all these factors, history of stroke (HR, 3.27; P < 0.001) and hip fracture repair vs. THA (HR, 2.74; P = 0.004) were confirmed to be significant predictors of postoperative ischemic stroke; the other factors did not significantly affect the model (Figure 2).
Comment
Our findings contrast those of previous studies that focused on perioperative ischemic stroke rates for specific surgical procedures,2, 8, 9 but do seem concordant with published results for early event rates of cerebrovascular accident or transient ischemic attack (1%) following hip fracture.10 The data from our study suggest that perioperative stroke cumulative probability is relatively high for hip procedures at both 30 days (1.2%) and 1 year (3.9%) after the index surgical procedure compared with general procedures. Subjects with a history of stroke who were undergoing hip operation had a postoperative stroke risk of 3.0% at 30 days and 9.9% at 1 year.
The incidence of stroke was greater in the hip fracture repair group (1.5% at 30 days and 5.5% at 1 year) than in the elective THA group (0.6% at 30 days and 1.5% at 1 year). The increased 1‐year mortality for patients undergoing hip surgery compared with the general population is in part due to cerebrovascular disease,10 and, therefore, the 1‐year stroke incidence is important.
After adjustment for age, sex, and comorbidities (hypertension and atrial fibrillation), the risk of postoperative ischemic stroke was 2.71 times greater in the hip fracture repair group than in the THA group (P = 0.006). These data are important in counseling and caring for patients undergoing different types of hip procedures.
From 1985 through 1989, for the age group (75‐84 years old) that best fits the demographics of our cohort, both men and women had limited variation over time in annual incidence rates of stroke (2149‐1074 strokes per 100,000 population per year) for Olmsted County, MN.11 In our study we found an annual incidence rate of ischemic stroke of 4,093 per 100,000 person‐years (95% CI, 3172‐5198 per 100,000 person‐years). The lower limit of the 95% CI is higher than the rates reported for Olmsted County, suggesting that having hip surgery increases the 1‐year risk of ischemic stroke.
Previous studies have shown that the risk factor most consistently correlated to perioperative ischemic stroke is a history of stroke.9 In our study, history of stroke and type of hip fracture surgery were confirmed to be the strongest predictors of postoperative stroke. History of hypertension, atrial fibrillation, CAD, COPD, diabetes, or chronic renal insufficiency was not correlated to perioperative ischemic stroke.
Nonmodifiable risk factors, such as advanced age, serve as markers of stroke risk and help identify high‐risk populations that may require aggressive intervention. After age adjustment of hip fracture repair, age was no longer significantly associated with postoperative stroke.
Cerebrovascular disease appears to be a marker for CAD, and, therefore, patients with a history of stroke usually have a Revised Cardiac Risk Index that may suggest the use of ‐blockers. According to the recent results of the POISE trial, use of ‐blockers could lead to increased stroke incidence.2 Our results showed no significant correlation between stroke risk and ‐blocker use, but our study period was from 1988 to 2002, when titration of ‐blocker dose to heart rates of 55 to 60 beats per minute was not common practice.
Several studies have confirmed the value of aspirin in decreasing the rate of vascular outcomes after diagnosis of transient ischemic attack or stroke.12 In our study, aspirin use on hospital admission was found in the univariate analysis to be associated with an increased risk of stroke, but this finding was not confirmed after adjustments for age, sex, and comorbid conditions. Aspirin use on admission was not a significant predictor of postoperative stroke, most likely because aspirin use can be considered a marker of increased cardiovascular risk and we adjusted for these comorbid conditions.
The limitations of this study are inherent in its retrospective design. First, we identified all incident cases of stroke after hip operation by reviewing medical records and then abstracting data from those records. We may have missed some mild strokes if they were misclassified as peripheral vestibular neuropathy, migraine, or even seizure. Less likely is that we missed strokes within the first 30 days after the procedure because that is the period in which patients with hip operation are either hospitalized or sent for rehabilitation in skilled nursing facilities. It is known that institutionalization leads to better surveillance and more complete ascertainment of any medical event.
The event rate of postoperative stroke at 30 days after hip operation was low. Therefore, we did not have the statistical power to comment meaningfully on predictors of stroke at 30 days after the hip procedure. Any nonrespondent or volunteer bias was addressed by using data from the Rochester Epidemiology Project, which allowed us to identify all Olmsted County residents who underwent hip operation between 1988 and 2002. The diagnostic suspicion bias was also accounted for in our study design because different physicians provided care and outcome measurement.
Our results apply for the patients who underwent hip operation between 1988 and 2002. The noncardiac surgery guidelines have been revised between 1988 and 2002, and we did not perform a stratified analysis by index year. The next step in our study will be to extend our data collection to 2008 and look at time trends.
Conclusion
In this population‐based historical cohort study, patients undergoing hip operation had a 3.9% cumulative probability of ischemic stroke during the first postoperative year. History of stroke and type of hip procedure (ie, hip fracture repair) were the strongest predictors of this complication. Because history of stroke is such a strong predictor of postoperative stroke, the perioperative management of these patients should probably be tailored, with closely observed blood pressure management and antihypertensive medication adjustment, to avoid compromising cerebral perfusion. Also, to avoid postoperative hypercoagulability that increases the risk of stroke, these patients may need to begin receiving antiplatelets as soon as is surgically acceptable.1315
In the United States, hip operations (internal fixation of fracture or total hip arthroplasty [THA]) are the most common noncardiac major surgical procedures performed in patients age 65 years and older (45.2 procedures per 100,000 persons per year).1 This number of procedures is projected to increase substantially in the coming decades.
Little is known about the clinical predictors of postoperative stroke in patients undergoing hip surgical procedures. Further, recent results of the Perioperative Ischemic Evaluation (POISE) trial have shown that measures taken to reduce cardiac complications postoperatively may adversely affect the risk of stroke.2 The POISE study showed decreases in myocardial infarction and coronary revascularization but accompanying increases in stroke and death with use of ‐blockers in patients undergoing noncardiac surgery.
Prevention of adverse events is one of the top priorities of the U.S. health care system today.35 Risk stratification and therapeutic optimization of underlying chronic diseases may be important in decreasing perioperative risk and improving postoperative outcomes.
Our objective was to determine the rate of postoperative ischemic stroke in all residents of Olmsted County, MN, who underwent hip operation between 1988 and 2002 and to identify clinical predictors of postoperative stroke.
Subjects and Methods
Olmsted County is one of the few places in the world where comprehensive population‐based studies of disease etiology and outcomes are feasible. This feasibility is due to the Rochester Epidemiology Project, a medical records linkage system that provides access to the records of all medical care in the community.1 All medical diagnoses made for a resident of Olmsted County are entered on a master sheet in the patient's medical record, which is then entered into a central computer index.
Hip operations were identified using the Surgical Information Recording System data warehouse, where detailed data are stored as International Classification of Diseases, 9th edition (ICD‐9) codes for all surgical procedures performed from January 1, 1988, forward. A total of 2028 THAs and hip fracture repairs (ICD‐9 codes 81.51, 81.52, 81.53, 79.15, and 79.25) performed between 1988 and 2002 in Olmsted County were identified. Of the hip procedures, 142 were excluded (Figure 1). The final analysis cohort contained 1886 hip operations1195 hip fracture repairs and 691 THAs.

The population‐based cohort was assembled and the data were abstracted from complete inpatient and outpatient records from admission for surgical treatment up to 1 year after surgery. Only those patients who had given prior authorization for research were included in the study cohort. The Mayo Clinic Institutional Review Board approved the study.
Case Ascertainment
We used several screening procedures to completely enumerate all postoperative strokes in our study population (Figure 1). The Mayo Clinic administrative database was used to identify all cases with relevant cerebrovascular disease (ICD‐9 codes 430.0‐437.9, 368.12, 781.4, and 784.3) within 1 year after hip operation. The Rochester Stroke Registry identified incident cases of ischemic stroke in Olmsted County from 1988 through 1994. The clinic's administrative database was also used to identify brain imaging studies (brain computed tomography, magnetic resonance imaging, or carotid ultrasonography) between the day of the procedure and 1 year postoperatively. A neurologist reviewed each image and the associated medical record identified during the screening process in detail for the constellation of signs and symptoms consistent with the diagnosis of stroke. Death certificates and autopsy reports were also reviewed to identify persons with the diagnosis of stroke. The outcome (stroke) was masked to the nurse abstractor who reviewed charts for predictors of postoperative stroke (eg, atrial fibrillation, coronary artery disease [CAD], history of stroke, medication use). The exposed or unexposed status of the patients to the predictors of stroke was masked to the physician (A.S.P.) who screened electronic medical records for the outcome measure (stroke).
Cerebral infarction or ischemic stroke was defined as the acute onset of a neurologic deficit that persisted for longer than 24 hours and corresponded to an arterial vascular territory of the cerebral hemispheres, brainstem, or cerebellum, with or without computed tomographic or magnetic resonance imaging documentation. Transient ischemic attack was defined as an episode of focal neurologic symptoms with abrupt onset and rapid resolution, lasting less than 24 hours, and due to altered circulation to a limited region of the brain.
Only patients with ischemic strokes clinically documented by a neurologist were included in the analysis.
Primary Outcomes
Outcomes were the cumulative probability of ischemic stroke and predictors of stroke in the first 12 months after surgical treatment of the hip.
Statistical Analysis
Continuous variables are presented as mean (standard deviation [SD]); categorical variables are presented as number and percentage. Two‐sample t tests or Wilcoxon rank sum tests were used to test for differences between THAs and hip fracture repairs in demographic characteristics, past medical history, and baseline clinical data composed of continuous variables; 2 or Fisher exact tests were used for categorical variables. No patient was lost to follow‐up during the 1 year after the initial surgery. However, the data of patients who died or had a second hip procedure within that period were censored.
The rate of ischemic stroke within 1 year after the incident hip procedure was calculated using the Kaplan‐Meier method. Second hip procedures within that period were counted as additional cases. Rates were calculated for the overall group, as well as for the univariate risk factors of operative procedure type, age, sex, past medical history of stroke, hypertension, atrial fibrillation, CAD, chronic obstructive pulmonary disease (COPD), diabetes mellitus, and chronic renal insufficiency. Use of ‐blockers, hydroxymethylglutaryl‐coenzyme A (HMG‐CoA) reductase inhibitors, or aspirin at hospital admission was also considered. Cox proportional hazards regression models were used to evaluate the risk of ischemic stroke for each of these univariate risk factors. Multivariable Cox proportional hazards models were constructed with adjustments for operative procedure type, age, sex, and comorbid conditions such as atrial fibrillation and hypertension. These covariates were added in a stepwise selection to identify factors significantly associated with the outcome. To account for patients who had a second hip procedure within 1 year of their first operation, we calculated all Cox proportional hazards regression results using the robust sandwich estimate of the covariance matrix. The proportional hazards assumption for all Cox models was evaluated with the methods proposed by Therneau and Grambsch;6 no violations of this assumption were identified. The rate of postoperative stroke after adjusting for the competing risk of death was calculated using the approach of Gooley et al.7 All statistical tests were 2‐sided, and a P value was considered significant if it was less than 0.05. Statistical analyses were performed using statistical software (SAS version 9.1.3; SAS Institute, Inc., Cary, NC).
Results
Among the patients with the 1886 hip procedures, 67 ischemic strokes were identified within 1 year after the index surgical procedure10 (1.4%) among the 691 THAs and 57 (4.8%) among the 1195 hip fracture repairs. Baseline characteristics are summarized in Table 1. Compared with the THA group, patients in the hip fracture repair group were more likely to be older and female. Additionally, such comorbid conditions as a history of stroke, diabetes mellitus, congestive heart failure, atrial fibrillation, or dementia were more prevalent in the hip fracture repair group.
| Characteristics | Surgical Procedure | Total (n = 1,886) | P Value* | |
|---|---|---|---|---|
| THA (n = 691) | Fracture Repair (n = 1,195) | |||
| ||||
| Age, years | 74.9 (6.59) | 84.2 (7.49) | 80.8 (8.46) | <0.001 |
| Sex, male | 258 (37.3) | 234 (19.6) | 492 (26.1) | <0.001 |
| Race, White | 690 (100) | 1,187 (99.3) | 1,877 (99.5) | 0.17 |
| BMI | 27.7 (5.36) | 23.3 (4.93) | 24.9 (5.52) | <0.001 |
| History | ||||
| Hypertension | 424 (61.4) | 695 (58.2) | 1,119 (59.3) | 0.17 |
| Diabetes | 57 (8.2) | 141 (11.8) | 198 (10.5) | 0.02 |
| Stroke | 50 (7.2) | 334 (27.9) | 384 (20.4) | <0.001 |
| CHF | 100 (14.5) | 321 (26.9) | 421 (22.3) | <0.001 |
| Atrial fibrillation | 72 (10.4) | 241 (20.2) | 313 (16.6) | <0.001 |
| Dementia | 16 (2.3) | 407 (34.1) | 423 (22.4) | <0.001 |
| ASA risk classification | <0.001 | |||
| 1 or 2 | 343 (49.6) | 172 (14.4) | 515 (27.3) | |
| 3, 4, or 5 | 348 (50.4) | 1,022 (85.6) | 1,370 (72.7) | |
| Medication on admission | ||||
| Aspirin | 168 (24.3) | 369 (30.9) | 537 (28.5) | 0.002 |
| ‐Blocker | 134 (19.4) | 184 (15.4) | 318 (16.9) | 0.03 |
| Insulin | 12 (1.7) | 48 (4) | 60 (3.2) | 0.007 |
| Length of stay, days | 7.3 (3.9) | 10.0 (7.61) | 9.0 (6.63) | <0.001 |
Univariate analyses assessing the rate and risk of postoperative ischemic stroke are shown in Table 2. The rate of stroke was significantly greater among hip fracture repairs than THAs 30 days postoperatively and 1 year postoperatively (1.5% vs. 0.6% and 5.5% vs. 1.5%, respectively; P < 0.001) (Figure 2). In our study we found an annual incidence rate of ischemic stroke of 4093 per 100,000 person‐years (95% confidence interval [CI], 3172‐5198 per 100,000 person‐years). Accounting for death as a competing risk for stroke had little impact on the rate of stroke overall or within the 2 surgical groups (results not shown). Univariate Cox proportional hazards models showed that neither sex nor history of hypertension, diabetes mellitus, COPD, chronic renal insufficiency, or CAD or use of HMG‐CoA reductase inhibitors or ‐blockers were significant predictors of ischemic stroke. However, other clinical risk factors, such as a history of atrial fibrillation (hazard ratio [HR], 2.16; P = 0.005), hip fracture repair vs. THA (HR, 3.80; P < 0.001), increased age (HR, 2.20; P = 0.017), aspirin use (HR, 1.8; P = 0.014), and history of previous stroke (HR, 4.18; P < 0.001), were significantly associated with an increased risk of stroke (Table 2).

| Variable | Number of Patients | Number of Events | Rate (%) | Hazard Ratio | P Value | |
|---|---|---|---|---|---|---|
| 30‐Day (95% CI) | 1‐Year (95% CI) | |||||
| ||||||
| Overall | 1886 | 67 | 1.2 (0.7‐1.7) | 3.9 (3‐4.8) | ||
| Type of operative procedure | ||||||
| THA | 691 | 10 | 0.6 (0.0‐1.1) | 1.5 (0.6‐2.4) | ||
| Hip fracture repair | 1195 | 57 | 1.5 (0.8‐2.2) | 5.5 (4.1‐6.9) | 3.80 (1.94‐7.44) | <0.001 |
| Age at operation, years | ||||||
| <75 | 528 | 11 | 1.0 (0.1‐1.8) | 2.1 (0.9‐3.3) | ||
| 75 | 1358 | 56 | 1.3 (0.7‐1.9) | 4.7 (3.5‐5.8) | 2.20 (1.15‐4.21) | 0.02 |
| Sex | ||||||
| Female | 1394 | 54 | 1.3 (0.7‐1.9) | 4.2 (3.1‐5.3) | ||
| Male | 492 | 13 | 0.8 (0.0‐1.7) | 2.9 (1.3‐4.4) | 0.69 (0.38‐1.27) | 0.24 |
| History of stroke | ||||||
| No | 1502 | 34 | 0.7 (0.3‐1.2) | 2.4 (1.6‐3.3) | ||
| Yes | 384 | 33 | 3.0 (1.2‐4.7) | 9.9 (6.6‐13) | 4.18 (2.59‐6.74) | <0.001 |
| History of hypertension | ||||||
| No | 767 | 23 | 0.8 (0.2‐1.4) | 3.4 (2.0‐4.7) | ||
| Yes | 1119 | 44 | 1.5 (0.7‐2.2) | 4.2 (3.0‐5.5) | 1.29 (0.78‐2.14) | 0.32 |
| History of atrial fibrillation | ||||||
| No | 1573 | 48 | 1.0 (0.5‐1.5) | 3.3 (2.4‐4.2) | ||
| Yes | 313 | 19 | 1.9 (0.4‐3.5) | 7.0 (3.9‐9.9) | 2.16 (1.27‐3.67) | 0.005 |
| History of CAD | ||||||
| No | 1224 | 40 | 1.1 (0.5‐1.6) | 3.5 (2.4‐4.5) | ||
| Yes | 662 | 27 | 1.4 (0.5‐2.3) | 4.7 (2.9‐6.4) | 1.34 (0.82‐2.19) | 0.24 |
| History of COPD | ||||||
| No | 1606 | 62 | 1.4 (0.8‐2.0) | 4.2 (3.1‐5.2) | ||
| Yes | 280 | 5 | 0 (0.0‐0.0) | 2.2 (0.3‐4.1) | 0.49 (0.20‐1.22) | 0.13 |
| History of diabetes mellitus | ||||||
| No | 1688 | 56 | 1.1 (0.6‐1.7) | 3.6 (2.7‐4.5) | ||
| Yes | 198 | 11 | 1.5 (0‐3.3) | 6.3 (2.6‐9.9) | 1.75 (0.92‐3.34) | 0.09 |
| History of renal insufficiency | ||||||
| No | 1718 | 58 | 1.0 (0.5‐1.5) | 3.7 (2.7‐4.6) | ||
| Yes | 168 | 9 | 3.0 (0.4‐5.5) | 5.8 (2‐9.5) | 1.77 (0.88‐3.57) | 0.11 |
| Aspirin use | ||||||
| No | 1349 | 39 | 0.7 (0.2‐1.1) | 3.2 (2.2‐4.2) | ||
| Yes | 537 | 28 | 2.5 (0.1‐3.8) | 5.7 (3.6‐7.7) | 1.86 (1.13‐3.06) | 0.01 |
| ‐Blocker use | ||||||
| No | 1568 | 52 | 1.1 (0.6‐1.6) | 3.6 (2.7‐4.6) | ||
| Yes | 318 | 15 | 1.6 (0.2‐3.0) | 5.1 (2.6‐7.6) | 1.42 (0.81‐2.52) | 0.22 |
| HMG‐CoA reductase inhibitor use | ||||||
| No | 1736 | 63 | 1.2 (0.7‐1.7) | 4.0 (3.0‐4.9) | ||
| Yes (statin/other lipid lowering drugs) | 148 | 4 | 1.4 (0‐3.2) | 2.8 (0.1‐5.4) | 0.70 (0.26‐1.94) | 0.50 |
Because age was associated with the type of surgical procedure (87% of hip fracture repair patients were 75 years or older compared with 45% of THA patients), the effect of hip fracture repair on ischemic stroke was adjusted for age. For similar reasons, sex was also examined as an adjusting factor. Adjustment for age and sex resulted in only a slight attenuation of the HR for hip fracture repair vs. THA, from 3.8 to 3.4. A further analysis also adjusted for history of hypertension and history of atrial fibrillation, both comorbidities commonly associated with ischemic stroke. After adjustment for age, sex, history of hypertension, and history of atrial fibrillation, the risk of ischemic stroke was still significantly greater in the hip fracture repair group than in the THA group (HR, 2.8; 95% CI, 1.4‐5.7; P = 0.005).
To determine the most important predictors of postoperative ischemic stroke, multivariable analysis was conducted with stepwise selection. Potential risk factors included the following: operative procedure type (hip fracture repair vs. THA), age, sex, and history of stroke, hypertension, atrial fibrillation, CAD, COPD, diabetes mellitus, and chronic renal insufficiency, as well as use of ‐blockers, HMG‐CoA reductase inhibitors, and aspirin on hospital admission. Among all these factors, history of stroke (HR, 3.27; P < 0.001) and hip fracture repair vs. THA (HR, 2.74; P = 0.004) were confirmed to be significant predictors of postoperative ischemic stroke; the other factors did not significantly affect the model (Figure 2).
Comment
Our findings contrast those of previous studies that focused on perioperative ischemic stroke rates for specific surgical procedures,2, 8, 9 but do seem concordant with published results for early event rates of cerebrovascular accident or transient ischemic attack (1%) following hip fracture.10 The data from our study suggest that perioperative stroke cumulative probability is relatively high for hip procedures at both 30 days (1.2%) and 1 year (3.9%) after the index surgical procedure compared with general procedures. Subjects with a history of stroke who were undergoing hip operation had a postoperative stroke risk of 3.0% at 30 days and 9.9% at 1 year.
The incidence of stroke was greater in the hip fracture repair group (1.5% at 30 days and 5.5% at 1 year) than in the elective THA group (0.6% at 30 days and 1.5% at 1 year). The increased 1‐year mortality for patients undergoing hip surgery compared with the general population is in part due to cerebrovascular disease,10 and, therefore, the 1‐year stroke incidence is important.
After adjustment for age, sex, and comorbidities (hypertension and atrial fibrillation), the risk of postoperative ischemic stroke was 2.71 times greater in the hip fracture repair group than in the THA group (P = 0.006). These data are important in counseling and caring for patients undergoing different types of hip procedures.
From 1985 through 1989, for the age group (75‐84 years old) that best fits the demographics of our cohort, both men and women had limited variation over time in annual incidence rates of stroke (2149‐1074 strokes per 100,000 population per year) for Olmsted County, MN.11 In our study we found an annual incidence rate of ischemic stroke of 4,093 per 100,000 person‐years (95% CI, 3172‐5198 per 100,000 person‐years). The lower limit of the 95% CI is higher than the rates reported for Olmsted County, suggesting that having hip surgery increases the 1‐year risk of ischemic stroke.
Previous studies have shown that the risk factor most consistently correlated to perioperative ischemic stroke is a history of stroke.9 In our study, history of stroke and type of hip fracture surgery were confirmed to be the strongest predictors of postoperative stroke. History of hypertension, atrial fibrillation, CAD, COPD, diabetes, or chronic renal insufficiency was not correlated to perioperative ischemic stroke.
Nonmodifiable risk factors, such as advanced age, serve as markers of stroke risk and help identify high‐risk populations that may require aggressive intervention. After age adjustment of hip fracture repair, age was no longer significantly associated with postoperative stroke.
Cerebrovascular disease appears to be a marker for CAD, and, therefore, patients with a history of stroke usually have a Revised Cardiac Risk Index that may suggest the use of ‐blockers. According to the recent results of the POISE trial, use of ‐blockers could lead to increased stroke incidence.2 Our results showed no significant correlation between stroke risk and ‐blocker use, but our study period was from 1988 to 2002, when titration of ‐blocker dose to heart rates of 55 to 60 beats per minute was not common practice.
Several studies have confirmed the value of aspirin in decreasing the rate of vascular outcomes after diagnosis of transient ischemic attack or stroke.12 In our study, aspirin use on hospital admission was found in the univariate analysis to be associated with an increased risk of stroke, but this finding was not confirmed after adjustments for age, sex, and comorbid conditions. Aspirin use on admission was not a significant predictor of postoperative stroke, most likely because aspirin use can be considered a marker of increased cardiovascular risk and we adjusted for these comorbid conditions.
The limitations of this study are inherent in its retrospective design. First, we identified all incident cases of stroke after hip operation by reviewing medical records and then abstracting data from those records. We may have missed some mild strokes if they were misclassified as peripheral vestibular neuropathy, migraine, or even seizure. Less likely is that we missed strokes within the first 30 days after the procedure because that is the period in which patients with hip operation are either hospitalized or sent for rehabilitation in skilled nursing facilities. It is known that institutionalization leads to better surveillance and more complete ascertainment of any medical event.
The event rate of postoperative stroke at 30 days after hip operation was low. Therefore, we did not have the statistical power to comment meaningfully on predictors of stroke at 30 days after the hip procedure. Any nonrespondent or volunteer bias was addressed by using data from the Rochester Epidemiology Project, which allowed us to identify all Olmsted County residents who underwent hip operation between 1988 and 2002. The diagnostic suspicion bias was also accounted for in our study design because different physicians provided care and outcome measurement.
Our results apply for the patients who underwent hip operation between 1988 and 2002. The noncardiac surgery guidelines have been revised between 1988 and 2002, and we did not perform a stratified analysis by index year. The next step in our study will be to extend our data collection to 2008 and look at time trends.
Conclusion
In this population‐based historical cohort study, patients undergoing hip operation had a 3.9% cumulative probability of ischemic stroke during the first postoperative year. History of stroke and type of hip procedure (ie, hip fracture repair) were the strongest predictors of this complication. Because history of stroke is such a strong predictor of postoperative stroke, the perioperative management of these patients should probably be tailored, with closely observed blood pressure management and antihypertensive medication adjustment, to avoid compromising cerebral perfusion. Also, to avoid postoperative hypercoagulability that increases the risk of stroke, these patients may need to begin receiving antiplatelets as soon as is surgically acceptable.1315
- .History of the Rochester Epidemiology Project.Mayo Clin Proc.1996;71(3):266–274.
- POISE Study Group;,,,,,, et al.Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial.Lancet.2008;371(9627):1839–1847.
- ,,,,,, et al;American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2006;113(6):e85–e151.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ, eds.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No.43.AHRQ publication no. 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality (AHRQ),U.S. Department of Health and Human Services;2001.668 p.
- ,,.Deaths due to medical errors are exaggerated in Institute of Medicine report.JAMA.2000;284(1):93–95.
- ,.Modeling survival data: extending the Cox model.New York:Springer;2000.
- ,,,.Estimation of failure probabilities in the presence of competing risks: new representations of old estimators.Stat Med.1999;18(6):695–706.
- ,,.Postoperative cerebrovascular accidents in general surgery.Acta Anaesthesiol Scand.1988;32(8):698–701.
- ,,,,,, et al.Perioperative stroke risk in 173 consecutive patients with a past history of stroke.Arch Surg.1990;125(8):986–989.
- ,,,,.Medical complications and outcomes after hip fracture repair.Arch Intern Med.2002;162(18):2053–2057.
- ,,,,.Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989.Stroke.1996;27(3):373–380.
- CAST (Chinese Acute Stroke Trial) Collaborative Group.Randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke.Lancet.1997;349(9066):1641–1649.
- ,,.Coagulation activation and organ dysfunction following cardiac surgery.Chest.2005;128(1):229–236.
- ,,,,,.Intra‐ and postoperative fibrinolysis in patients undergoing cardiopulmonary bypass surgery.Haemostasis.1991;21(1):58–64.
- .Perioperative stroke.N Engl J Med.2007;356(7):706–713.
- .History of the Rochester Epidemiology Project.Mayo Clin Proc.1996;71(3):266–274.
- POISE Study Group;,,,,,, et al.Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial.Lancet.2008;371(9627):1839–1847.
- ,,,,,, et al;American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2006;113(6):e85–e151.
- Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ, eds.Making health care safer: a critical analysis of patient safety practices. Evidence Report/Technology Assessment No.43.AHRQ publication no. 01‐E058.Rockville, MD:Agency for Healthcare Research and Quality (AHRQ),U.S. Department of Health and Human Services;2001.668 p.
- ,,.Deaths due to medical errors are exaggerated in Institute of Medicine report.JAMA.2000;284(1):93–95.
- ,.Modeling survival data: extending the Cox model.New York:Springer;2000.
- ,,,.Estimation of failure probabilities in the presence of competing risks: new representations of old estimators.Stat Med.1999;18(6):695–706.
- ,,.Postoperative cerebrovascular accidents in general surgery.Acta Anaesthesiol Scand.1988;32(8):698–701.
- ,,,,,, et al.Perioperative stroke risk in 173 consecutive patients with a past history of stroke.Arch Surg.1990;125(8):986–989.
- ,,,,.Medical complications and outcomes after hip fracture repair.Arch Intern Med.2002;162(18):2053–2057.
- ,,,,.Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989.Stroke.1996;27(3):373–380.
- CAST (Chinese Acute Stroke Trial) Collaborative Group.Randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke.Lancet.1997;349(9066):1641–1649.
- ,,.Coagulation activation and organ dysfunction following cardiac surgery.Chest.2005;128(1):229–236.
- ,,,,,.Intra‐ and postoperative fibrinolysis in patients undergoing cardiopulmonary bypass surgery.Haemostasis.1991;21(1):58–64.
- .Perioperative stroke.N Engl J Med.2007;356(7):706–713.
Copyright © 2009 Society of Hospital Medicine
Costs and Arthroplasty
Hospital practices are increasingly responsible for ensuring enhanced patient safety, satisfaction, and cost containment. Recently developed models of care have achieved the necessary efficiency to attain these measures, not only in the use of hospitalists managing general medical1, 2 and postoperative orthopedic patients,3, 4 but also in the use of midlevel providers in busy primary care settings.5 In addition, stroke units6 and geriatric evaluation and management units7, 8 worldwide have demonstrated reduced disability and improved survival and importantly have been proven to provide cost‐effective care. Specialized orthopedic surgery (SOS) units may be a means to reproduce the results observed in these other models.
The economic potential of SOS units will become more significant with changing demographics. The percentage of patients greater than 65 years old will increase, from 12.3% in 2002 to 20% by 2030, with a parallel increase in the prevalence of osteoarthritis (OA).9 The World Health Organization has declared 2000‐2010 the Bone and Joint Decade,10, 11 reflecting that OA affects some 43 million people, with more than 60 million projected to be affected by 2020.12, 13 The National Center for Health Statistics reported that more than 280,000 total knee arthroplasties (TKAs) are performed annually in the United States, which marks an increase in frequency in the last decade that is likely to continue.1419
Approximately 75% of all TKAs are reimbursed under Medicare,17 whereas elective TKA continues to be one of the most common surgeries in the Medicare‐age patient population,20 foreshadowing the prominent cost burden of osteoarthritis in the aging population. The concomitant decreasing reimbursement for arthroplasty in general supports an examination of what constitutes efficient, high‐quality, and cost‐effective care21 for TKA. At our institution, patients undergoing TKA are preferentially triaged to an SOS nursing unit for postoperative care. As hospital bed capacity continues to decline, patients may be triaged to open beds at locations that may not be the optimal choice for nursing care. The primary purpose of this study was to determine the impact of SOS units versus nonorthopedic nursing (NON) units on resource utilization for and outcomes of patients undergoing elective knee arthroplasty. We hypothesized that length of stay would be shorter and cost of inpatient care would be lower for patients cared for on SOS units.
MATERIALS AND METHODS
Study Design and Setting
We conducted a retrospective observational cohort study of all patients undergoing elective primary TKA from January 1, 1996, to December 31, 2004, comparing outcomes of patients assigned to SOS units with those of patients assigned to NON units. Patients were admitted to Rochester Methodist Hospital, Mayo Clinic, a tertiary‐care primary surgical teaching hospital that has 794 beds and more than 15,000 admissions annually. There were 13 faculty orthopedic surgeons performing elective nontraumatic lower‐extremity joint procedures during the study period, each with orthopedic residents rotating as part of the patient care team.
Study Population
All patients at Mayo Clinic who had undergone a joint replacement were followed prospectively, and data were collected using standardized forms and protocols, the methodologies of which have been described previously.22 Follow‐up was greater than 95% complete. Using the joint registry, patients who had undergone a TKA were identified (n = 9798). Postoperative patients initially transferred from the postanesthesia care unit to a general care floor were included. We excluded patients who required urgent, revision, or bilateral arthroplasties; who had been treated at or transferred from another institution; and whose primary surgical indication was trauma or septic arthritis. Subjects admitted to the hospital on the day prior to the procedure and subjects initially transferred directly from the postanesthesia care unit to the intensive care unit (ICU) were excluded, including patients requiring immediate postoperative cardiac monitoring. All primary surgical interventions were performed between Monday and Friday. The study authors identified 5883 eligible patients.
Patient clinical and demographic data including surgical indication; age; sex; height and weight at surgery; and dates of admission, surgery, death, discharge, and last follow‐up were abstracted from the registry. Type of anesthesia (general, regional, combined), American Society of Anesthesiologists (ASA) physical status class, and date and time of ICU admission and discharge were abstracted from individual departmental databases. The Decision Support System (DSS) administrative database (Eclipsys, Boca Raton, FL) was utilized to abstract relevant clinical variables, including major comorbid conditions such as cancer, cerebrovascular disease, chronic pulmonary disease, congestive heart failure, dementia, diabetes, hemiplegia, HIV/AIDS, metastatic solid tumors, myocardial infarction, peripheral vascular disease, renal disease, rheumatologic disease, and ulcers. A composite Charlson comorbidity score was computed as previously described.23, 24 Administrative variables regarding patient encounters including inpatient stay variableslength of stay, costs, patient location, nursing care units, admission times, discharge disposition and datewere also obtained from the DSS database.
Variables and Definitions
Length of stay was defined as the number of days from time of admission for the surgical episode to time of discharge. All costs were based on a provider perspective using standardized 2005 costs based on inflation‐adjusted estimates as previously described.3, 25, 26 We assessed resource utilization among patients who received care on an SOS unit by determining length of stay and total, hospital, and physician costs for the specified surgical episode. We also assessed blood bank, ICU, laboratory, pharmacy, physical therapy, occupational therapy, respiratory therapy, radiology, and room‐and‐board costs. Blood bank costs consisted of the costs of storing, processing, and administering the transfusion. Surgical procedure, anesthesia, and preoperative service costs were excluded from our cost analyses, as our aim was to examine hospital flow and resource utilization from time of transfer from the postanesthesia care unit to hospital discharge in order to specifically examine the impact of an SOS unit. We compared unexpected ICU admissions and stays and the resources utilized of patients in these 2 groups.
State and federal death registries confirmed patient expiration and primary cause of death. In‐hospital mortality was defined as death during the same hospital admission as the indexed surgical episode. Thirty‐day mortality was defined as death occurring within 30 days of the surgical procedure. Readmission at 30 days was defined as any admission to our institutions within a 30‐day period whose purpose was possibly related to the initial surgical episode and not a result of an elective admission. A priori we were aware of the small number of these events in the elective joint population. Therefore, we combined inpatient 30‐day mortality, 30‐day reoperation, and 30‐day readmission rates as a composite endpoint.
Specialized Orthopedic Surgery Units
An SOS unit was defined as a general care nursing unit where patients receive all their postoperative care after elective TKA. Such a unit has a multidisciplinary staff that has orthopedic expertise. The differences between an SOS unit and a NON unit are described in Table 1. Bed availability at the time of discharge from the postanesthesia care unit was the exclusive factor for admission to this unit. Bed availability was dependent on staff availability or whether there was an excess number of operative cases. The number and severity of patient medical comorbidities or complications, the time of discharge from the postanesthesia care unit, and patient room preference had no impact on which unit patients were admitted to. Patients were allocated to the SOS group or the NON unit group according to their physical location the evening of admission. Monitored beds at this facility are solely located in the ICU, and neither SOS nor NON units have this capability. Any patient requiring a monitored bed at any time, regardless of the reason, would be transferred directly to the ICU. Daily rounds were performed on either unit by the primary orthopedic team. The need for either medical or pain service consultation was at the discretion of the primary orthopedic team and not dependent on the patient's physical location.
| Specialized orthopedic surgical unit (SOS) | Nonorthopedic nursing unit (NON) | |
|---|---|---|
| ||
| Type of unit | Orthopedic general care unit. | General surgical care unit. |
| Patient type | Postoperative elective orthopedic only. | Any patientmedical or surgical. |
| Determinants of physical location for orthopedic patient | Primary bed assignment. | Admitted only if SOS units have reached full bed capacity. |
| Orthopedic‐trained nursing staff | Yesrequired to have additional post‐RN* training in orthopedics. These RNs rarely float to nonorthopedic units. | Nomay have additional training or experience in an unrelated medical or surgical discipline. Floating to other units may occur. |
| Orthopedic‐specific physical + occupational therapy | Provided by certified physical therapists trained in lower‐extremity joint procedures. Site‐based therapy available to all patients on SOS units. | Provided by certified physical therapists who do not necessarily have postoperative orthopedic lower‐extremity specialization. Site‐based therapy on NON unit available to all patients. |
| Licensed social workers | Dedicated to postoperative needs of orthopedic patients physically located on SOS units. | Not specifically dedicated to the postoperative elective orthopedic joint patient and not physically located on these units. |
| Interdisciplinary team meetings | Patient care addressed in a interdisciplinary team meeting 3 times weeklyconsists of an RN, physical and occupational therapists, social worker, and physician. | No care team meetings, as patients are off‐service. |
| Physician postoperative order set | Orthopedic‐specific order set that is available hospitalwide. Nursing staff on these units is familiar with these order sets. | Orthopedic‐specific order set available hospitalwide. Nursing staff on these units may not be entirely familiar with these order sets. |
| Rehabilitation protocols | Orthopedic specific. | Not orthopedic specific. |
| Patient‐care instructions | Orthopedic diagnosis‐specific instructions readily available | Orthopedic diagnosis‐specific instructions available but requires staff to obtain information and forms from the SOS inits. |
| Discharge protocol | Specifically targeted to the postarthroplasty patient | Generic hospitalwide protocol. |
| Hospital discharge summary | Yescowritten by primary orthopedic team and primary orthopedic RN. | Yescowritten by primary orthopedic team and nonorthopedic RN. |
| Orthopedic‐specific discharge instructions | Yescowritten by primary orthopedic team and primary orthopedic RN. | No. |
All data were subsequently combined into a single database to facilitate data analysis. We further excluded 44 patients because no cost information was available, 9 patients who had multiple joint replacements performed during the specified surgical hospitalization, 69 patients because they had not authorized their medical records to be used for the purposes of research; 163 patients admitted directly to the ICU, 63 patients admitted the day prior to surgery, and 1 patient whose billing data suggested an outpatient encounter. A final patient cohort of 5534 patients was in the analysis. With the observed sample size and the overall variability, our study had 80% power to detect a difference between the 2 groups as small as 0.22 days in length of stay and $761 in hospital costs. The study was approved by our institutional review board. All study patients had authorized the use of their medical records for the purposes of research. Funding was obtained through an intramurally sponsored Small Grants Program by the Division of General Internal Medicine, which had no impact on the design of the study, reporting, or decision to submit an article on the study for publication.
Statistical Analysis
The statistical analysis compared baseline health and demographic characteristics of the patients cared for on SOS units with those cared for on NON units using chi‐square tests for nominal factors and the 2‐sample Wilcoxon rank sum tests for continuous variables. We used the chi‐square test to test for unadjusted differences in sex, patient residence (local or referred), race, individual Charlson comorbid conditions, anesthesia type, admitting diagnosis, 30‐day readmission rate, and discharge location. The 2‐sample Wilcoxon rank sum test assessed unadjusted differences in length of stay, costs, age, ICU days of stay, number of reoperations, total Charlson score and ASA class. Thirty‐day mortality rates were tested using the Fisher exact test.
Differences between patients in SOS and NON units in length of stay (LOS) and costs were the study's primary outcomes. We adjusted for baseline and surgical covariates using generalized linear regression models for these outcomes. The effect of the nursing unit was based on regression coefficients for age, sex, ASA class, anesthesia type, Charlson comorbidities, and surgical year. Age was analyzed using 5 categories: <55; 55‐64; 70‐74; 54‐69, and >75 years, with 65‐69 years used as the reference group. Each Charlson comorbid condition was treated as an indicator variable. Indicator variables were also assigned to surgical year, with 2004 used as the reference. These variables were subsequently entered into the model to calculate the differences between patients on an SOS unit and those on a NON unit.
Our secondary outcomes included ICU utilization and 30‐day outcomes of mortality, reoperations, and readmissions. We then assessed the effect of treatment on the SOS unit using the entire cohort (n = 5534) for unplanned postoperative ICU stay (yes or no) and on our combined endpoint after adjusting for the variables listed previously, using logistic regression models. A P value < 0.05 was considered statistically significant. All analyses were performed using statistical software (SAS, version 9.1; SAS Institute Inc, Cary, NC).
RESULTS
Baseline patient characteristics are represented in Table 2. Five thousand and eighty‐two patients were admitted to an SOS unit, and 452 patients were admitted to a NON unit. The annual number of patients undergoing TKA increased during our study period, as did the number of patients cared for on NON units. There were no differences between groups in the number of local county patients or in the number of patients primarily referred by other providers for elective arthroplasty. Mean length of stay was 4.9 days in both groups. After adjusting for the specified covariates, including age, sex, year of surgery, Charlson comorbidities, ASA class, and type of anesthesia, LOS was 0.234 days shorter in the SOS group (95% confidence interval [CI]: 0.08, 0.39; P = .002). Overall and hospital costs were significantly lower in the SOS group, as outlined with the other costs in Table 3. Room‐and‐board costs were 5.3% lower for SOS patients than for patients on NON units, representing a per‐patient difference of $244 $87 (95% CI: $72, $415; P = .005).
| Specialized orthopedic surgery unit (n = 5082) | Nonorthopedic nursing unit (n = 452) | P value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| |||||
| Age (years) | |||||
| <55 | 534 | 10.5% | 57 | 12.6% | |
| 55‐64 | 1148 | 22.6% | 101 | 22.4% | |
| 65‐69 | 802 | 15.8% | 66 | 14.6% | |
| 70‐74 | 1106 | 21.8% | 91 | 20.1% | |
| >75 | 1492 | 29.4% | 137 | 30.3% | |
| Mean age ( SD*) | 68.3 10.75 | 67.9 11.5 | .50 | ||
| Sex | .70 | ||||
| Male | 2173 | 42.8% | 189 | 41.8% | |
| Female | 2909 | 57.2% | 263 | 58.2% | |
| Race | .28 | ||||
| White | 4731 | 93.1% | 420 | 92.9% | |
| Other* | 51 | 1.0% | 8 | 1.8% | |
| Unknown | 300 | 5.9% | 24 | 5.3% | |
| Local Olmsted County patients | 772 | 15.2% | 58 | 12.8% | .18 |
| Indication for surgery | .03 | ||||
| Osteoarthritis | 4778 | 94% | 430 | 95.1% | |
| Rheumatologic disease | 184 | 3.6% | 6 | 1.3% | |
| Avascular necrosis | 62 | 1.2% | 5 | 1.1% | |
| Congenital | 6 | 0.1% | 1 | 0.2% | |
| Cancer | 22 | 0.4% | 5 | 1.1% | |
| Other | 30 | 0.6% | 5 | 1.1% | |
| Year of surgery | < .001 | ||||
| 1996 | 497 | 98.8% | 6 | 1.19% | |
| 1997 | 571 | 99.7% | 2 | 0.35% | |
| 1998 | 479 | 98.8% | 6 | 1.24% | |
| 1999 | 487 | 94.8% | 27 | 5.25% | |
| 2000 | 458 | 92.7% | 36 | 7.29% | |
| 2001 | 502 | 86.7% | 77 | 13.3% | |
| 2002 | 593 | 89.2% | 72 | 10.8% | |
| 2003 | 639 | 87.1% | 95 | 12.9% | |
| 2004 | 856 | 86.7% | 131 | 13.3% | |
| Charlson score (mean SD) | 0.256 0.536 | 0.288 0.593 | .23 | ||
| AIDS | 0 | 0% | 1 | 0.22% | 1.00 |
| Cancer | 85 | 1.68% | 7 | 1.55% | .84 |
| Cerebrovascular disease | 32 | 0.63% | 0 | 0% | .09 |
| Chronic pulmonary disease | 28 | 5.63% | 23 | 5.09% | .63 |
| Congestive heart failure | 89 | 1.75% | 22 | 4.87% | < .001 |
| Dementia | 10 | 0.2% | 2 | 0.44% | .28 |
| Diabetes | 603 | 11.9% | 58 | 12.8% | .54 |
| Hemiplegia | 9 | 0.18% | 0 | 0% | .37 |
| Metastatic solid tumor | 11 | 0.22% | 2 | 0.44% | .34 |
| Myocardial infarction | 29 | 0.57% | 4 | 0.88% | .4 |
| Peripheral vascular disease | 67 | 1.32% | 4 | 0.88% | .43 |
| Renal disease | 52 | 1.02% | 5 | 1.11% | .87 |
| Rheumatologic disease | 12 | 0.24% | 2 | 0.44% | .40 |
| Ulcers | 15 | 0.3% | 0 | 0% | .25 |
| ASA class‖ | |||||
| I | 99 | 2.0% | 12 | 2.7% | |
| II | 2891 | 56.9% | 255 | 56.4% | |
| III | 2084 | 41.0% | 183 | 40.5% | |
| IV | 8 | 0.2% | 2 | 0.4% | |
| Average ASA class ( SD) | 2.39 0.53 | 2.39 0.55 | .80 | ||
| Anesthesia type | .02 | ||||
| General | 1644 | 32.4% | 143 | 31.6% | |
| Regional | 2742 | 54% | 226 | 50% | |
| Combined | 696 | 13.7% | 83 | 18.4% | |
| Unadjusted values | Adjusted values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SOS* | SD | NON | SD | P value | Difference | SD | P value | 95% CI | |
| |||||||||
| Total cost | $9989 | $5392 | $10,067 | $5075 | .77 | $600 | $244 | .01 | $122, $1079 |
| Hospital costs | $9789 | $5123 | $ 9805 | $4647 | .23 | $594 | $231 | .01 | $141, $1047 |
| Room & board | $4399 | $1825 | $ 4577 | $1579 | .04 | $244 | $ 87 | .005 | $ 72, $ 415 |
| ICU costs | $ 58 | $1094 | $ 107 | $ 682 | .35 | $ 11 | $ 51 | .82 | $111, $ 88 |
| Pharmacy | $ 851 | $1701 | $ 931 | $1823 | .34 | $ 87 | $ 85 | .30 | $ 79, $253 |
| Laboratory costs | $ 386 | $ 438 | $ 395 | $ 405 | .65 | $ 27 | $ 20 | .18 | $ 12, $ 65 |
| Radiology costs | $ 98 | $ 205 | $ 103 | $ 183 | .61 | $ 1 | $ 10 | .93 | $ 20, $ 19 |
| PT/OT**/RT | $ 739 | $ 505 | $ 682 | $ 394 | .004 | $ 15 | $ 19 | .45 | $ 23, $ 52 |
| Blood bank | $ 159 | $ 306 | $ 178 | $3023 | .22 | $ 6 | $ 15 | .69 | $ 35, $ 23 |
| Physician costs | $ 207 | $ 464 | $ 258 | $ 628 | .09 | $ 20 | $ 22 | .386 | $ 24, $ 63 |
| E&M costs‖ | $ 89 | $ 211 | $ 109 | $ 238 | .09 | $ 4 | $ 9 | .658 | $ 23, $ 14 |
| Physician radiology | $ 63 | $ 158 | $ 38 | $ 192 | .49 | $ 2 | $ 8 | .78 | $ 13, $ 18 |
| Other costs | $ 34 | $ 138 | $ 37 | $ 160 | .61 | $0.64 | $ 6 | .92 | $ 13, $ 12 |
There were 83 patients (1.63%) transferred from SOS units to the ICU, compared with 14 patients (3.1%) transferred from NON units (P = .02), but no differences in the mean number of ICU days or associated costs between groups. A priori, the authors were aware of the small number of postoperative medical events in this population. In examining the combined endpoint of reoperations, readmissions, and mortality, there were no differences observed in our regression analysis between SOS patients and NON unit patients (0.03 events, standard error: 0.1859; odds ratio: 0.976). Table 4 demonstrates a higher percentage of patients discharged with home health on the NON units than on the SOS units (8.41% vs. 4.62%; P < .001).
| Specialized orthopedic surgery unit | Nonorthopedic nursing unit | P value | |||
|---|---|---|---|---|---|
| n* | % | n | % | ||
| |||||
| Home | 3812 | 75% | 328 | 72.6% | .252 |
| Home health | 235 | 4.62% | 38 | 8.41% | < .001 |
| Transferred to skilled nursing facility | 1030 | 20.3% | 86 | 19% | .529 |
DISCUSSION
To the best of our knowledge, this is the first study to examine the impact of specialized orthopedic surgery units on resource utilization in elective knee arthroplasty patients. Our findings demonstrate that patients admitted following elective TKA to SOS units will have a reduced length of stay, lower overall and hospital costs, and fewer unexpected transfers to higher levels of care (ICUs). We believe that these findings are a result in part of the specialized expertise allied health care providers develop by taking care of and focusing on a large volume of patients over time with the same group and type of surgeons. This multidisciplinary setting in which care providers are familiar not only with each other but with this specific population of patients creates the environment necessary for adherence to specialized clinical pathways.27
Patient LOS is an important determinant of resource utilization. In a study by Husted et al., the mean length of stay in Danish hospitals following TKA was 8.6 days in 2003.28 An epidemiological study using the Nationwide Inpatient Sample database of patients in the United States showed that from 1998 to 2000, the mean LOS was 4.3 days.18 In our study, the mean LOS was slightly higher (4.9 days), potentially reflecting referral bias. Achieving additional savings and improved outcomes by further reducing LOS in an environment in which care pathways are already in place is often difficult; hence, alternative approaches and strategies are often necessary.29, 30 Our results suggest that in TKA patients, after adjusting for other factors, there is a decrease in the length of stay of 0.234 days among those cared for on SOS units. However, we cannot state that the existence of the clinical pathway alone is responsible for our data differences because certain components of the care pathway for elective TKA patients are used throughout the hospital regardless of type of postoperative nursing unit. We believe that the interdisciplinary specialty care provided to orthopedic patients on SOS units is a critical component of a successfully implemented care pathway and not just a convenience or practice preference. The same surgeons admitting patients to the same nursing unit, with the same nurses, physical therapists and pharmacists providing care to the same type of patient population over time, leverages the collective experience of all care providers. This integrated, multidisciplinary teamwork may optimize timeliness, achieve incremental cost savings, and improve safety (including a decreased number of unanticipated transfers to an ICU setting).
Clinical pathways are known to reduce overall costs, normally by reducing LOS,29, 3133 and our results suggest approximately an incremental 6% cost reduction with the use of improving patient logistics by using SOS units. An economic evaluation study by Healy et al. suggests that focusing on nursing units may be a means of reducing total costs.29 Our cost savings were slightly lower than the reported savings by other practice assessments; however, we excluded operative and anesthesia costs, both significant contributors to overall and hospital costs. By eliminating these variables, our costs were specifically limited to the postoperative course, which is highly dependent on specialized interdisciplinary care.29
Providing specialized care has a significant impact on society. Although there is a per‐patient savings of only $600 when elective TKA patients are cared for on SOS units, this could be the difference between a positive and negative margin in the setting of fixed reimbursement. With a current average of 90 patients annually triaged postoperatively to NON units, there is a potential loss of $54,000 annually at our institution in just this single patient population with the current mechanisms of perioperative hospital flow. Multiply this potential savings to a national level, and the total is significant. With an aging population, the number of arthroplasties and concomitantly the number of hospitalizations in general are likely to increase, suggesting that changes in hospital flow are required to ensure optimal, cost‐effective care in the best setting available for patients. Such care is often related to surgical volume, and our institution observes such volume. Our results indicate that SOS units are one possible means of achieving this objective of fiscal sustainability, but further studies are needed to determine the indirect and hidden costs of sustaining such units in order to observe the actual cost savings.34 It could be argued that for elective TKA patients to have the most optimal outcomes and most efficient care, the surgical procedure should be performed only if beds are available on the nursing units whose staff has the most specific training.
Thirty‐Day Outcomes
We elected to combine 30‐day mortality, reoperations, and readmissions pertaining to the joint procedure as a composite endpoint and found no differences in outcomes between groups. These results suggest that these longer‐term patient‐specific outcomes are likely not related to the specialty nursing care. We used a 30‐day endpoint assuming that a longer period may have led to the inclusion of deaths that were not directly attributable to the surgical intervention. In addition, a previous study advocated using 30 days as an endpoint for follow‐up, as it adequately accounts for adverse events.35 Our institution is also a referral center; hence, we would likely be unable to capture all events if we were to use the standard 90‐day period used for payment for this procedure, as these data are not canvassed by the joint registry.
Discharge Disposition
NON unit patients tended to have a higher degree of home health arranged at discharge. The NON unit nursing staff cares for other nonorthopedic surgical patients daily and may transfer their patterns of care utilization to the orthopedic patients despite different postoperative needs. In addition, if NON unit nursing staff members care for TKA patients only intermittently, they may not have as clear a working understanding of the particular postoperative requirements of TKA patients and consequently request unnecessary home health services and general community resources. Alternatively, patients cared for on NON units may actually have needed more assistance and more services on discharge. Although purely speculative, patients cared for by dedicated orthopedic surgery staff may develop added confidence from the experience of the allied care staff and feel less of a need for postdismissal services.
Role of Hospitalists in Specialized Care Pathways
Hospitalists are known to improve efficiency without reducing patient satisfaction. Their role has been demonstrated in different patient populations.1, 2, 3638 In a study of hip fracture patients, a hospitalist care model demonstrated a reduction in length of stay and time to surgery, without compromising long‐term outcomes.4, 39 Utilizing a hospitalist/midlevel care provider team approach to reduce LOS in units with a static number of beds can possibly increase bed turnover and prevent triaging of patients onto NON units. This is but one example of how a medical‐surgical partnership can improve outcomes. However, in an era where cost‐effective and regulatory practices require optimal resource allocation, hospitalists are in a key position to foster quality improvement projects, promote patient safety measures, and enhance systems care delivery. Becoming involved in designing specialized clinical units, with an emphasis on a multidisciplinary care approach, and developing their relationships with hospital administrators and nursing staff should be among their priorities. The Society of Hospital Medicine has also been committed to the care of the elderly through its core competencies40 and the orthopedic population that will benefit from such process changes and care pathways. Hospital innovations such as the implementation of SOS‐type units not only for other medical‐surgical partnerships but also for site‐based units caring for geriatric patients can be top priorities for hospitalists.
Strengths and Applicability
Our results are important in that they can likely be applied to both large tertiary‐care centers and smaller community‐based centers that perform specialized orthopedic surgeries. Nurses on specialized orthopedic units are very familiar with this postoperative population and have developed expertise in the care of these patients. These experienced nurses can likely be found on orthopedic units in tertiary‐care centers or surgical units in smaller facilities. Furthermore, our results support the benefits of interdisciplinary advanced teamwork. When an interdisciplinary group of health care providers works together on a daily basis, certain habits and patterns inevitably develop that often are unplanned and may be difficult to measure. This enhanced patient flow may not occur if these patients are cared for by providers unfamiliar with each other's work patterns. The importance of optimized teamwork is not hospital‐size dependent. Only primary elective knee arthroplasties were included to minimize confounding bias by bilateral or revision surgeries or indications such as septic arthritis, which are known to lead to increased length of stay, costs and complications.41
Limitations
Our study has the limitations of its retrospective nonrandomized study design, and only a prospective, randomized investigation could definitively address our aims. By excluding sicker patients, such as those referred with complicated health issues or high‐risk patients who required admission in advance of the proposed surgery for monitoring of perioperative anticoagulation issues, our estimates of possible differences between our comparison groups may have been conservative. We are unaware of how these sicker patients would fare on either nursing unit. Furthermore, what occurs in the hospital setting may not only have an impact on the hospital stay but may also influence long‐term outcomes. This is impossible to assess with analysis of administrative databases.
We relied on the complete and accurate recording of data from various databases, depending on the validity of data entry and collection. With a large cohort of patients, any errors in documentation or abstraction would be expected to be similar in both groups. Furthermore, confounding variables such as patient comorbidities are extracted from administrative data sets whose personnel might not be as familiar with the medical aspects of patient care. We used linear and logistic regression analyses to account for known differences in baseline characteristics despite the sample sizes being proportionally larger in the SOS group. Although we attribute the shortened length of stay in the SOS group to the interdisciplinary team approach, we were unable to determine to what extent this was a result of nursing staff or discharge planning. By using administrative databases, we were unable to abstract the consensus time and date of discharge, when all hospital staff deemed the patient ready for discharge, and hence relied on the actual time of discharge, which can be heavily reliant on availability at skilled nursing facilities. In addition, it was unknown whether patients discharged from SOS units were, by matter of protocol, discharged earlier in the day. Nevertheless, this small difference in length of stay can improve patient flow by opening up postoperative patient beds. Furthermore, such data sets are unable to provide information on patient satisfaction or quality‐of‐life measures, both of which are important determinants in specialized care pathways.42 The patient population served by our institution is generally ethnically homogeneous, thereby limiting potential generalizations to tertiary‐care centers or geographical areas with a population similar to ours. Our study also was not intended as a formal cost‐effectiveness analysis; hence, the impact of possible startup costs to begin a similar nursing unit was not explored. Although differences in practice management can be considered a limitation of not only operative but also perioperative care, we neither expected nor encountered any significant or drastic alterations during the study period, and year of surgery was adjusted for in our analysis. However, prospective randomized controlled studies testing specific clinical pathways and practice‐related innovations are needed to better examine these outcomes.
CONCLUSIONS
In conclusion, postoperative patients after elective knee arthroplasty cared for on specialized orthopedic surgery units have shorter length of stays and cost hospitals less than patients admitted to nonspecialized orthopedic nursing units. In an era in which quality indicators and external reviews are forcing practitioners and health care organizations to become increasingly responsible for their own practices, more research is required to better address specific questions pertaining to different processes of care. Our study is meant to increase the attention paid to patient flow and postoperative logistics in the elective TKA population. SOS units, as a unique model of care, may become an additional step toward ensuring quality care and improved resource utilization.
Acknowledgements
The authors thank Donna K. Lawson, LPN, for her assistance in data collection and management.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,,.The economic benefit for family/general medicine practices employing physician assistants.Am J Manag Care.2002;8:613–620.
- ,,, et al.Economic evaluation of Australian stroke services: a prospective, multicenter study comparing dedicated stroke units with other care modalities.Stroke.2006;37:2790–2795.
- ,,,.Geriatric evaluation and management units in the care of the frail elderly cancer patient.J Gerontol A Biol Sci Med Sci.2005;60:798–803.
- ,,,,,.A randomized, controlled trial of a geriatric assessment unit in a community rehabilitation hospital.N Engl J Med.1990;322:1572–1578.
- ,.The effect of longevity on spending for acute and long‐term care.N Engl J Med.2000;342:1409–1415.
- ,,, et al.The Bone and Joint Decade 2000‐2010.Acta Orthop Scand.1998;69:219–220.
- .The Bone and Joint Decade 2000‐2010— for prevention and treatment of musculoskeletal disease.Osteoarthr Cartil.1998;7:1–4.
- Prevalence of self‐reported arthritis or chronic joint symptoms among adults—United States, 2001.MMWR Morb Mortal Wkly Rep.2002;51:948–950.
- ,,, et al.Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States.Arthritis Rheum.1998;41:778–799.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, 2002. Available at: http://www.ahrq.gov. Accessed January 25,2005.
- ,,,,,.Costs and cost‐effectiveness in hip and knee replacements. A prospective study.Int J Technol Assess Health Care.1997;13:575–588.
- ,,,,.Trends in total knee replacement surgeries and implications for public health, 1990‐2000.Public Health Rep.2005;120:278–282.
- ,,, et al.Demographic variation in the rate of knee replacement: a multi‐year analysis.Health Serv Res.1996;31:125–140.
- ,,,,.Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990‐2000.Arthritis Rheum.2006;55:591–597.
- ,.2001 National Hospital Discharge Survey.Adv Data.2003;332.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004:1–29.
- ,,.Costs of health care administration in the United States and Canada.N Engl J Med2003;349:768–775.
- ,,.Maintaining a hip registry for 25 years. Mayo Clinic experience.Clin Orthop Relat Res.1997:61–68.
- ,,,.Validation of a combined comorbidity index.J Clin Epidemiol.1994;47:1245–1251.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,, et al.A prospective randomized comparison of laparoscopic appendectomy with open appendectomy: Clinical and economic analyses.Surgery.2001;129:390–400.
- ,,, et al.Incremental costs of enrolling cancer patients in clinical trials: a population‐based study.J Natl Cancer Inst.1999;91:847–853.
- ,.Understanding the complexity of redesigning care around the clinical microsystem.Qual Saf Health Care.2006;15(Suppl 1):i10–i16.
- ,,, et al.Length of stay after primary total hip and knee arthroplasty in Denmark, 2001‐2003.Ugeskr Laeger.2006;168:276–279.
- ,,,.Opportunities for control of hospital costs for total joint arthroplasty after initial cost containment.J Arthroplasty.1998;13:504–507.
- ,,,,.Effectiveness of clinical pathways for total knee and total hip arthroplasty: literature review.J Arthroplasty.2003;18:69–74.
- ,,, et al.The effect of a perioperative clinical pathway for knee replacement surgery on hospital costs.Anesth Analg.1998;86:978–984.
- The cost effectiveness of streamlined care pathways and product standardization in total knee arthroplasty.J Arthroplasty.1999;14:182–186.
- ,,,,.Impact of cost reduction programs on short‐term patient outcome and hospital cost of total knee arthroplasty.J Bone Joint Surg Am.2002;84‐A:348–353.
- ,,,.Success of clinical pathways for total joint arthroplasty in a community hospital.Clin Orthop Relat Res.2007;457:133–137.
- ,,,.Optimal timeframe for reporting short‐term complication rates after total knee arthroplasty.J Arthroplasty.2006;21:705–711.
- ,,.Associations with reduced length of stay and costs on an academic hospitalist service.Am J Manag Care.2004;10:561–568.
- ,,.Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs.J Hosp Med.2006;1:29–35.
- .Care of hospitalized older patients: opportunities for hospital‐based physicians.J Hosp Med.2006;1:42–47.
- ,,, et al.Effects of a hospitalist care model on mortality of elderly patients with hip fractures.J Hosp Med.2007;2:219–225.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,,,.Effect of feedback on resource use and morbidity in hip and knee arthroplasty in an integrated group practice setting.Mayo Clin Proc.1996;71:127–133.
- ,,,.Integrated care pathways.BMJ.1998;316:133–137.
Hospital practices are increasingly responsible for ensuring enhanced patient safety, satisfaction, and cost containment. Recently developed models of care have achieved the necessary efficiency to attain these measures, not only in the use of hospitalists managing general medical1, 2 and postoperative orthopedic patients,3, 4 but also in the use of midlevel providers in busy primary care settings.5 In addition, stroke units6 and geriatric evaluation and management units7, 8 worldwide have demonstrated reduced disability and improved survival and importantly have been proven to provide cost‐effective care. Specialized orthopedic surgery (SOS) units may be a means to reproduce the results observed in these other models.
The economic potential of SOS units will become more significant with changing demographics. The percentage of patients greater than 65 years old will increase, from 12.3% in 2002 to 20% by 2030, with a parallel increase in the prevalence of osteoarthritis (OA).9 The World Health Organization has declared 2000‐2010 the Bone and Joint Decade,10, 11 reflecting that OA affects some 43 million people, with more than 60 million projected to be affected by 2020.12, 13 The National Center for Health Statistics reported that more than 280,000 total knee arthroplasties (TKAs) are performed annually in the United States, which marks an increase in frequency in the last decade that is likely to continue.1419
Approximately 75% of all TKAs are reimbursed under Medicare,17 whereas elective TKA continues to be one of the most common surgeries in the Medicare‐age patient population,20 foreshadowing the prominent cost burden of osteoarthritis in the aging population. The concomitant decreasing reimbursement for arthroplasty in general supports an examination of what constitutes efficient, high‐quality, and cost‐effective care21 for TKA. At our institution, patients undergoing TKA are preferentially triaged to an SOS nursing unit for postoperative care. As hospital bed capacity continues to decline, patients may be triaged to open beds at locations that may not be the optimal choice for nursing care. The primary purpose of this study was to determine the impact of SOS units versus nonorthopedic nursing (NON) units on resource utilization for and outcomes of patients undergoing elective knee arthroplasty. We hypothesized that length of stay would be shorter and cost of inpatient care would be lower for patients cared for on SOS units.
MATERIALS AND METHODS
Study Design and Setting
We conducted a retrospective observational cohort study of all patients undergoing elective primary TKA from January 1, 1996, to December 31, 2004, comparing outcomes of patients assigned to SOS units with those of patients assigned to NON units. Patients were admitted to Rochester Methodist Hospital, Mayo Clinic, a tertiary‐care primary surgical teaching hospital that has 794 beds and more than 15,000 admissions annually. There were 13 faculty orthopedic surgeons performing elective nontraumatic lower‐extremity joint procedures during the study period, each with orthopedic residents rotating as part of the patient care team.
Study Population
All patients at Mayo Clinic who had undergone a joint replacement were followed prospectively, and data were collected using standardized forms and protocols, the methodologies of which have been described previously.22 Follow‐up was greater than 95% complete. Using the joint registry, patients who had undergone a TKA were identified (n = 9798). Postoperative patients initially transferred from the postanesthesia care unit to a general care floor were included. We excluded patients who required urgent, revision, or bilateral arthroplasties; who had been treated at or transferred from another institution; and whose primary surgical indication was trauma or septic arthritis. Subjects admitted to the hospital on the day prior to the procedure and subjects initially transferred directly from the postanesthesia care unit to the intensive care unit (ICU) were excluded, including patients requiring immediate postoperative cardiac monitoring. All primary surgical interventions were performed between Monday and Friday. The study authors identified 5883 eligible patients.
Patient clinical and demographic data including surgical indication; age; sex; height and weight at surgery; and dates of admission, surgery, death, discharge, and last follow‐up were abstracted from the registry. Type of anesthesia (general, regional, combined), American Society of Anesthesiologists (ASA) physical status class, and date and time of ICU admission and discharge were abstracted from individual departmental databases. The Decision Support System (DSS) administrative database (Eclipsys, Boca Raton, FL) was utilized to abstract relevant clinical variables, including major comorbid conditions such as cancer, cerebrovascular disease, chronic pulmonary disease, congestive heart failure, dementia, diabetes, hemiplegia, HIV/AIDS, metastatic solid tumors, myocardial infarction, peripheral vascular disease, renal disease, rheumatologic disease, and ulcers. A composite Charlson comorbidity score was computed as previously described.23, 24 Administrative variables regarding patient encounters including inpatient stay variableslength of stay, costs, patient location, nursing care units, admission times, discharge disposition and datewere also obtained from the DSS database.
Variables and Definitions
Length of stay was defined as the number of days from time of admission for the surgical episode to time of discharge. All costs were based on a provider perspective using standardized 2005 costs based on inflation‐adjusted estimates as previously described.3, 25, 26 We assessed resource utilization among patients who received care on an SOS unit by determining length of stay and total, hospital, and physician costs for the specified surgical episode. We also assessed blood bank, ICU, laboratory, pharmacy, physical therapy, occupational therapy, respiratory therapy, radiology, and room‐and‐board costs. Blood bank costs consisted of the costs of storing, processing, and administering the transfusion. Surgical procedure, anesthesia, and preoperative service costs were excluded from our cost analyses, as our aim was to examine hospital flow and resource utilization from time of transfer from the postanesthesia care unit to hospital discharge in order to specifically examine the impact of an SOS unit. We compared unexpected ICU admissions and stays and the resources utilized of patients in these 2 groups.
State and federal death registries confirmed patient expiration and primary cause of death. In‐hospital mortality was defined as death during the same hospital admission as the indexed surgical episode. Thirty‐day mortality was defined as death occurring within 30 days of the surgical procedure. Readmission at 30 days was defined as any admission to our institutions within a 30‐day period whose purpose was possibly related to the initial surgical episode and not a result of an elective admission. A priori we were aware of the small number of these events in the elective joint population. Therefore, we combined inpatient 30‐day mortality, 30‐day reoperation, and 30‐day readmission rates as a composite endpoint.
Specialized Orthopedic Surgery Units
An SOS unit was defined as a general care nursing unit where patients receive all their postoperative care after elective TKA. Such a unit has a multidisciplinary staff that has orthopedic expertise. The differences between an SOS unit and a NON unit are described in Table 1. Bed availability at the time of discharge from the postanesthesia care unit was the exclusive factor for admission to this unit. Bed availability was dependent on staff availability or whether there was an excess number of operative cases. The number and severity of patient medical comorbidities or complications, the time of discharge from the postanesthesia care unit, and patient room preference had no impact on which unit patients were admitted to. Patients were allocated to the SOS group or the NON unit group according to their physical location the evening of admission. Monitored beds at this facility are solely located in the ICU, and neither SOS nor NON units have this capability. Any patient requiring a monitored bed at any time, regardless of the reason, would be transferred directly to the ICU. Daily rounds were performed on either unit by the primary orthopedic team. The need for either medical or pain service consultation was at the discretion of the primary orthopedic team and not dependent on the patient's physical location.
| Specialized orthopedic surgical unit (SOS) | Nonorthopedic nursing unit (NON) | |
|---|---|---|
| ||
| Type of unit | Orthopedic general care unit. | General surgical care unit. |
| Patient type | Postoperative elective orthopedic only. | Any patientmedical or surgical. |
| Determinants of physical location for orthopedic patient | Primary bed assignment. | Admitted only if SOS units have reached full bed capacity. |
| Orthopedic‐trained nursing staff | Yesrequired to have additional post‐RN* training in orthopedics. These RNs rarely float to nonorthopedic units. | Nomay have additional training or experience in an unrelated medical or surgical discipline. Floating to other units may occur. |
| Orthopedic‐specific physical + occupational therapy | Provided by certified physical therapists trained in lower‐extremity joint procedures. Site‐based therapy available to all patients on SOS units. | Provided by certified physical therapists who do not necessarily have postoperative orthopedic lower‐extremity specialization. Site‐based therapy on NON unit available to all patients. |
| Licensed social workers | Dedicated to postoperative needs of orthopedic patients physically located on SOS units. | Not specifically dedicated to the postoperative elective orthopedic joint patient and not physically located on these units. |
| Interdisciplinary team meetings | Patient care addressed in a interdisciplinary team meeting 3 times weeklyconsists of an RN, physical and occupational therapists, social worker, and physician. | No care team meetings, as patients are off‐service. |
| Physician postoperative order set | Orthopedic‐specific order set that is available hospitalwide. Nursing staff on these units is familiar with these order sets. | Orthopedic‐specific order set available hospitalwide. Nursing staff on these units may not be entirely familiar with these order sets. |
| Rehabilitation protocols | Orthopedic specific. | Not orthopedic specific. |
| Patient‐care instructions | Orthopedic diagnosis‐specific instructions readily available | Orthopedic diagnosis‐specific instructions available but requires staff to obtain information and forms from the SOS inits. |
| Discharge protocol | Specifically targeted to the postarthroplasty patient | Generic hospitalwide protocol. |
| Hospital discharge summary | Yescowritten by primary orthopedic team and primary orthopedic RN. | Yescowritten by primary orthopedic team and nonorthopedic RN. |
| Orthopedic‐specific discharge instructions | Yescowritten by primary orthopedic team and primary orthopedic RN. | No. |
All data were subsequently combined into a single database to facilitate data analysis. We further excluded 44 patients because no cost information was available, 9 patients who had multiple joint replacements performed during the specified surgical hospitalization, 69 patients because they had not authorized their medical records to be used for the purposes of research; 163 patients admitted directly to the ICU, 63 patients admitted the day prior to surgery, and 1 patient whose billing data suggested an outpatient encounter. A final patient cohort of 5534 patients was in the analysis. With the observed sample size and the overall variability, our study had 80% power to detect a difference between the 2 groups as small as 0.22 days in length of stay and $761 in hospital costs. The study was approved by our institutional review board. All study patients had authorized the use of their medical records for the purposes of research. Funding was obtained through an intramurally sponsored Small Grants Program by the Division of General Internal Medicine, which had no impact on the design of the study, reporting, or decision to submit an article on the study for publication.
Statistical Analysis
The statistical analysis compared baseline health and demographic characteristics of the patients cared for on SOS units with those cared for on NON units using chi‐square tests for nominal factors and the 2‐sample Wilcoxon rank sum tests for continuous variables. We used the chi‐square test to test for unadjusted differences in sex, patient residence (local or referred), race, individual Charlson comorbid conditions, anesthesia type, admitting diagnosis, 30‐day readmission rate, and discharge location. The 2‐sample Wilcoxon rank sum test assessed unadjusted differences in length of stay, costs, age, ICU days of stay, number of reoperations, total Charlson score and ASA class. Thirty‐day mortality rates were tested using the Fisher exact test.
Differences between patients in SOS and NON units in length of stay (LOS) and costs were the study's primary outcomes. We adjusted for baseline and surgical covariates using generalized linear regression models for these outcomes. The effect of the nursing unit was based on regression coefficients for age, sex, ASA class, anesthesia type, Charlson comorbidities, and surgical year. Age was analyzed using 5 categories: <55; 55‐64; 70‐74; 54‐69, and >75 years, with 65‐69 years used as the reference group. Each Charlson comorbid condition was treated as an indicator variable. Indicator variables were also assigned to surgical year, with 2004 used as the reference. These variables were subsequently entered into the model to calculate the differences between patients on an SOS unit and those on a NON unit.
Our secondary outcomes included ICU utilization and 30‐day outcomes of mortality, reoperations, and readmissions. We then assessed the effect of treatment on the SOS unit using the entire cohort (n = 5534) for unplanned postoperative ICU stay (yes or no) and on our combined endpoint after adjusting for the variables listed previously, using logistic regression models. A P value < 0.05 was considered statistically significant. All analyses were performed using statistical software (SAS, version 9.1; SAS Institute Inc, Cary, NC).
RESULTS
Baseline patient characteristics are represented in Table 2. Five thousand and eighty‐two patients were admitted to an SOS unit, and 452 patients were admitted to a NON unit. The annual number of patients undergoing TKA increased during our study period, as did the number of patients cared for on NON units. There were no differences between groups in the number of local county patients or in the number of patients primarily referred by other providers for elective arthroplasty. Mean length of stay was 4.9 days in both groups. After adjusting for the specified covariates, including age, sex, year of surgery, Charlson comorbidities, ASA class, and type of anesthesia, LOS was 0.234 days shorter in the SOS group (95% confidence interval [CI]: 0.08, 0.39; P = .002). Overall and hospital costs were significantly lower in the SOS group, as outlined with the other costs in Table 3. Room‐and‐board costs were 5.3% lower for SOS patients than for patients on NON units, representing a per‐patient difference of $244 $87 (95% CI: $72, $415; P = .005).
| Specialized orthopedic surgery unit (n = 5082) | Nonorthopedic nursing unit (n = 452) | P value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| |||||
| Age (years) | |||||
| <55 | 534 | 10.5% | 57 | 12.6% | |
| 55‐64 | 1148 | 22.6% | 101 | 22.4% | |
| 65‐69 | 802 | 15.8% | 66 | 14.6% | |
| 70‐74 | 1106 | 21.8% | 91 | 20.1% | |
| >75 | 1492 | 29.4% | 137 | 30.3% | |
| Mean age ( SD*) | 68.3 10.75 | 67.9 11.5 | .50 | ||
| Sex | .70 | ||||
| Male | 2173 | 42.8% | 189 | 41.8% | |
| Female | 2909 | 57.2% | 263 | 58.2% | |
| Race | .28 | ||||
| White | 4731 | 93.1% | 420 | 92.9% | |
| Other* | 51 | 1.0% | 8 | 1.8% | |
| Unknown | 300 | 5.9% | 24 | 5.3% | |
| Local Olmsted County patients | 772 | 15.2% | 58 | 12.8% | .18 |
| Indication for surgery | .03 | ||||
| Osteoarthritis | 4778 | 94% | 430 | 95.1% | |
| Rheumatologic disease | 184 | 3.6% | 6 | 1.3% | |
| Avascular necrosis | 62 | 1.2% | 5 | 1.1% | |
| Congenital | 6 | 0.1% | 1 | 0.2% | |
| Cancer | 22 | 0.4% | 5 | 1.1% | |
| Other | 30 | 0.6% | 5 | 1.1% | |
| Year of surgery | < .001 | ||||
| 1996 | 497 | 98.8% | 6 | 1.19% | |
| 1997 | 571 | 99.7% | 2 | 0.35% | |
| 1998 | 479 | 98.8% | 6 | 1.24% | |
| 1999 | 487 | 94.8% | 27 | 5.25% | |
| 2000 | 458 | 92.7% | 36 | 7.29% | |
| 2001 | 502 | 86.7% | 77 | 13.3% | |
| 2002 | 593 | 89.2% | 72 | 10.8% | |
| 2003 | 639 | 87.1% | 95 | 12.9% | |
| 2004 | 856 | 86.7% | 131 | 13.3% | |
| Charlson score (mean SD) | 0.256 0.536 | 0.288 0.593 | .23 | ||
| AIDS | 0 | 0% | 1 | 0.22% | 1.00 |
| Cancer | 85 | 1.68% | 7 | 1.55% | .84 |
| Cerebrovascular disease | 32 | 0.63% | 0 | 0% | .09 |
| Chronic pulmonary disease | 28 | 5.63% | 23 | 5.09% | .63 |
| Congestive heart failure | 89 | 1.75% | 22 | 4.87% | < .001 |
| Dementia | 10 | 0.2% | 2 | 0.44% | .28 |
| Diabetes | 603 | 11.9% | 58 | 12.8% | .54 |
| Hemiplegia | 9 | 0.18% | 0 | 0% | .37 |
| Metastatic solid tumor | 11 | 0.22% | 2 | 0.44% | .34 |
| Myocardial infarction | 29 | 0.57% | 4 | 0.88% | .4 |
| Peripheral vascular disease | 67 | 1.32% | 4 | 0.88% | .43 |
| Renal disease | 52 | 1.02% | 5 | 1.11% | .87 |
| Rheumatologic disease | 12 | 0.24% | 2 | 0.44% | .40 |
| Ulcers | 15 | 0.3% | 0 | 0% | .25 |
| ASA class‖ | |||||
| I | 99 | 2.0% | 12 | 2.7% | |
| II | 2891 | 56.9% | 255 | 56.4% | |
| III | 2084 | 41.0% | 183 | 40.5% | |
| IV | 8 | 0.2% | 2 | 0.4% | |
| Average ASA class ( SD) | 2.39 0.53 | 2.39 0.55 | .80 | ||
| Anesthesia type | .02 | ||||
| General | 1644 | 32.4% | 143 | 31.6% | |
| Regional | 2742 | 54% | 226 | 50% | |
| Combined | 696 | 13.7% | 83 | 18.4% | |
| Unadjusted values | Adjusted values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SOS* | SD | NON | SD | P value | Difference | SD | P value | 95% CI | |
| |||||||||
| Total cost | $9989 | $5392 | $10,067 | $5075 | .77 | $600 | $244 | .01 | $122, $1079 |
| Hospital costs | $9789 | $5123 | $ 9805 | $4647 | .23 | $594 | $231 | .01 | $141, $1047 |
| Room & board | $4399 | $1825 | $ 4577 | $1579 | .04 | $244 | $ 87 | .005 | $ 72, $ 415 |
| ICU costs | $ 58 | $1094 | $ 107 | $ 682 | .35 | $ 11 | $ 51 | .82 | $111, $ 88 |
| Pharmacy | $ 851 | $1701 | $ 931 | $1823 | .34 | $ 87 | $ 85 | .30 | $ 79, $253 |
| Laboratory costs | $ 386 | $ 438 | $ 395 | $ 405 | .65 | $ 27 | $ 20 | .18 | $ 12, $ 65 |
| Radiology costs | $ 98 | $ 205 | $ 103 | $ 183 | .61 | $ 1 | $ 10 | .93 | $ 20, $ 19 |
| PT/OT**/RT | $ 739 | $ 505 | $ 682 | $ 394 | .004 | $ 15 | $ 19 | .45 | $ 23, $ 52 |
| Blood bank | $ 159 | $ 306 | $ 178 | $3023 | .22 | $ 6 | $ 15 | .69 | $ 35, $ 23 |
| Physician costs | $ 207 | $ 464 | $ 258 | $ 628 | .09 | $ 20 | $ 22 | .386 | $ 24, $ 63 |
| E&M costs‖ | $ 89 | $ 211 | $ 109 | $ 238 | .09 | $ 4 | $ 9 | .658 | $ 23, $ 14 |
| Physician radiology | $ 63 | $ 158 | $ 38 | $ 192 | .49 | $ 2 | $ 8 | .78 | $ 13, $ 18 |
| Other costs | $ 34 | $ 138 | $ 37 | $ 160 | .61 | $0.64 | $ 6 | .92 | $ 13, $ 12 |
There were 83 patients (1.63%) transferred from SOS units to the ICU, compared with 14 patients (3.1%) transferred from NON units (P = .02), but no differences in the mean number of ICU days or associated costs between groups. A priori, the authors were aware of the small number of postoperative medical events in this population. In examining the combined endpoint of reoperations, readmissions, and mortality, there were no differences observed in our regression analysis between SOS patients and NON unit patients (0.03 events, standard error: 0.1859; odds ratio: 0.976). Table 4 demonstrates a higher percentage of patients discharged with home health on the NON units than on the SOS units (8.41% vs. 4.62%; P < .001).
| Specialized orthopedic surgery unit | Nonorthopedic nursing unit | P value | |||
|---|---|---|---|---|---|
| n* | % | n | % | ||
| |||||
| Home | 3812 | 75% | 328 | 72.6% | .252 |
| Home health | 235 | 4.62% | 38 | 8.41% | < .001 |
| Transferred to skilled nursing facility | 1030 | 20.3% | 86 | 19% | .529 |
DISCUSSION
To the best of our knowledge, this is the first study to examine the impact of specialized orthopedic surgery units on resource utilization in elective knee arthroplasty patients. Our findings demonstrate that patients admitted following elective TKA to SOS units will have a reduced length of stay, lower overall and hospital costs, and fewer unexpected transfers to higher levels of care (ICUs). We believe that these findings are a result in part of the specialized expertise allied health care providers develop by taking care of and focusing on a large volume of patients over time with the same group and type of surgeons. This multidisciplinary setting in which care providers are familiar not only with each other but with this specific population of patients creates the environment necessary for adherence to specialized clinical pathways.27
Patient LOS is an important determinant of resource utilization. In a study by Husted et al., the mean length of stay in Danish hospitals following TKA was 8.6 days in 2003.28 An epidemiological study using the Nationwide Inpatient Sample database of patients in the United States showed that from 1998 to 2000, the mean LOS was 4.3 days.18 In our study, the mean LOS was slightly higher (4.9 days), potentially reflecting referral bias. Achieving additional savings and improved outcomes by further reducing LOS in an environment in which care pathways are already in place is often difficult; hence, alternative approaches and strategies are often necessary.29, 30 Our results suggest that in TKA patients, after adjusting for other factors, there is a decrease in the length of stay of 0.234 days among those cared for on SOS units. However, we cannot state that the existence of the clinical pathway alone is responsible for our data differences because certain components of the care pathway for elective TKA patients are used throughout the hospital regardless of type of postoperative nursing unit. We believe that the interdisciplinary specialty care provided to orthopedic patients on SOS units is a critical component of a successfully implemented care pathway and not just a convenience or practice preference. The same surgeons admitting patients to the same nursing unit, with the same nurses, physical therapists and pharmacists providing care to the same type of patient population over time, leverages the collective experience of all care providers. This integrated, multidisciplinary teamwork may optimize timeliness, achieve incremental cost savings, and improve safety (including a decreased number of unanticipated transfers to an ICU setting).
Clinical pathways are known to reduce overall costs, normally by reducing LOS,29, 3133 and our results suggest approximately an incremental 6% cost reduction with the use of improving patient logistics by using SOS units. An economic evaluation study by Healy et al. suggests that focusing on nursing units may be a means of reducing total costs.29 Our cost savings were slightly lower than the reported savings by other practice assessments; however, we excluded operative and anesthesia costs, both significant contributors to overall and hospital costs. By eliminating these variables, our costs were specifically limited to the postoperative course, which is highly dependent on specialized interdisciplinary care.29
Providing specialized care has a significant impact on society. Although there is a per‐patient savings of only $600 when elective TKA patients are cared for on SOS units, this could be the difference between a positive and negative margin in the setting of fixed reimbursement. With a current average of 90 patients annually triaged postoperatively to NON units, there is a potential loss of $54,000 annually at our institution in just this single patient population with the current mechanisms of perioperative hospital flow. Multiply this potential savings to a national level, and the total is significant. With an aging population, the number of arthroplasties and concomitantly the number of hospitalizations in general are likely to increase, suggesting that changes in hospital flow are required to ensure optimal, cost‐effective care in the best setting available for patients. Such care is often related to surgical volume, and our institution observes such volume. Our results indicate that SOS units are one possible means of achieving this objective of fiscal sustainability, but further studies are needed to determine the indirect and hidden costs of sustaining such units in order to observe the actual cost savings.34 It could be argued that for elective TKA patients to have the most optimal outcomes and most efficient care, the surgical procedure should be performed only if beds are available on the nursing units whose staff has the most specific training.
Thirty‐Day Outcomes
We elected to combine 30‐day mortality, reoperations, and readmissions pertaining to the joint procedure as a composite endpoint and found no differences in outcomes between groups. These results suggest that these longer‐term patient‐specific outcomes are likely not related to the specialty nursing care. We used a 30‐day endpoint assuming that a longer period may have led to the inclusion of deaths that were not directly attributable to the surgical intervention. In addition, a previous study advocated using 30 days as an endpoint for follow‐up, as it adequately accounts for adverse events.35 Our institution is also a referral center; hence, we would likely be unable to capture all events if we were to use the standard 90‐day period used for payment for this procedure, as these data are not canvassed by the joint registry.
Discharge Disposition
NON unit patients tended to have a higher degree of home health arranged at discharge. The NON unit nursing staff cares for other nonorthopedic surgical patients daily and may transfer their patterns of care utilization to the orthopedic patients despite different postoperative needs. In addition, if NON unit nursing staff members care for TKA patients only intermittently, they may not have as clear a working understanding of the particular postoperative requirements of TKA patients and consequently request unnecessary home health services and general community resources. Alternatively, patients cared for on NON units may actually have needed more assistance and more services on discharge. Although purely speculative, patients cared for by dedicated orthopedic surgery staff may develop added confidence from the experience of the allied care staff and feel less of a need for postdismissal services.
Role of Hospitalists in Specialized Care Pathways
Hospitalists are known to improve efficiency without reducing patient satisfaction. Their role has been demonstrated in different patient populations.1, 2, 3638 In a study of hip fracture patients, a hospitalist care model demonstrated a reduction in length of stay and time to surgery, without compromising long‐term outcomes.4, 39 Utilizing a hospitalist/midlevel care provider team approach to reduce LOS in units with a static number of beds can possibly increase bed turnover and prevent triaging of patients onto NON units. This is but one example of how a medical‐surgical partnership can improve outcomes. However, in an era where cost‐effective and regulatory practices require optimal resource allocation, hospitalists are in a key position to foster quality improvement projects, promote patient safety measures, and enhance systems care delivery. Becoming involved in designing specialized clinical units, with an emphasis on a multidisciplinary care approach, and developing their relationships with hospital administrators and nursing staff should be among their priorities. The Society of Hospital Medicine has also been committed to the care of the elderly through its core competencies40 and the orthopedic population that will benefit from such process changes and care pathways. Hospital innovations such as the implementation of SOS‐type units not only for other medical‐surgical partnerships but also for site‐based units caring for geriatric patients can be top priorities for hospitalists.
Strengths and Applicability
Our results are important in that they can likely be applied to both large tertiary‐care centers and smaller community‐based centers that perform specialized orthopedic surgeries. Nurses on specialized orthopedic units are very familiar with this postoperative population and have developed expertise in the care of these patients. These experienced nurses can likely be found on orthopedic units in tertiary‐care centers or surgical units in smaller facilities. Furthermore, our results support the benefits of interdisciplinary advanced teamwork. When an interdisciplinary group of health care providers works together on a daily basis, certain habits and patterns inevitably develop that often are unplanned and may be difficult to measure. This enhanced patient flow may not occur if these patients are cared for by providers unfamiliar with each other's work patterns. The importance of optimized teamwork is not hospital‐size dependent. Only primary elective knee arthroplasties were included to minimize confounding bias by bilateral or revision surgeries or indications such as septic arthritis, which are known to lead to increased length of stay, costs and complications.41
Limitations
Our study has the limitations of its retrospective nonrandomized study design, and only a prospective, randomized investigation could definitively address our aims. By excluding sicker patients, such as those referred with complicated health issues or high‐risk patients who required admission in advance of the proposed surgery for monitoring of perioperative anticoagulation issues, our estimates of possible differences between our comparison groups may have been conservative. We are unaware of how these sicker patients would fare on either nursing unit. Furthermore, what occurs in the hospital setting may not only have an impact on the hospital stay but may also influence long‐term outcomes. This is impossible to assess with analysis of administrative databases.
We relied on the complete and accurate recording of data from various databases, depending on the validity of data entry and collection. With a large cohort of patients, any errors in documentation or abstraction would be expected to be similar in both groups. Furthermore, confounding variables such as patient comorbidities are extracted from administrative data sets whose personnel might not be as familiar with the medical aspects of patient care. We used linear and logistic regression analyses to account for known differences in baseline characteristics despite the sample sizes being proportionally larger in the SOS group. Although we attribute the shortened length of stay in the SOS group to the interdisciplinary team approach, we were unable to determine to what extent this was a result of nursing staff or discharge planning. By using administrative databases, we were unable to abstract the consensus time and date of discharge, when all hospital staff deemed the patient ready for discharge, and hence relied on the actual time of discharge, which can be heavily reliant on availability at skilled nursing facilities. In addition, it was unknown whether patients discharged from SOS units were, by matter of protocol, discharged earlier in the day. Nevertheless, this small difference in length of stay can improve patient flow by opening up postoperative patient beds. Furthermore, such data sets are unable to provide information on patient satisfaction or quality‐of‐life measures, both of which are important determinants in specialized care pathways.42 The patient population served by our institution is generally ethnically homogeneous, thereby limiting potential generalizations to tertiary‐care centers or geographical areas with a population similar to ours. Our study also was not intended as a formal cost‐effectiveness analysis; hence, the impact of possible startup costs to begin a similar nursing unit was not explored. Although differences in practice management can be considered a limitation of not only operative but also perioperative care, we neither expected nor encountered any significant or drastic alterations during the study period, and year of surgery was adjusted for in our analysis. However, prospective randomized controlled studies testing specific clinical pathways and practice‐related innovations are needed to better examine these outcomes.
CONCLUSIONS
In conclusion, postoperative patients after elective knee arthroplasty cared for on specialized orthopedic surgery units have shorter length of stays and cost hospitals less than patients admitted to nonspecialized orthopedic nursing units. In an era in which quality indicators and external reviews are forcing practitioners and health care organizations to become increasingly responsible for their own practices, more research is required to better address specific questions pertaining to different processes of care. Our study is meant to increase the attention paid to patient flow and postoperative logistics in the elective TKA population. SOS units, as a unique model of care, may become an additional step toward ensuring quality care and improved resource utilization.
Acknowledgements
The authors thank Donna K. Lawson, LPN, for her assistance in data collection and management.
Hospital practices are increasingly responsible for ensuring enhanced patient safety, satisfaction, and cost containment. Recently developed models of care have achieved the necessary efficiency to attain these measures, not only in the use of hospitalists managing general medical1, 2 and postoperative orthopedic patients,3, 4 but also in the use of midlevel providers in busy primary care settings.5 In addition, stroke units6 and geriatric evaluation and management units7, 8 worldwide have demonstrated reduced disability and improved survival and importantly have been proven to provide cost‐effective care. Specialized orthopedic surgery (SOS) units may be a means to reproduce the results observed in these other models.
The economic potential of SOS units will become more significant with changing demographics. The percentage of patients greater than 65 years old will increase, from 12.3% in 2002 to 20% by 2030, with a parallel increase in the prevalence of osteoarthritis (OA).9 The World Health Organization has declared 2000‐2010 the Bone and Joint Decade,10, 11 reflecting that OA affects some 43 million people, with more than 60 million projected to be affected by 2020.12, 13 The National Center for Health Statistics reported that more than 280,000 total knee arthroplasties (TKAs) are performed annually in the United States, which marks an increase in frequency in the last decade that is likely to continue.1419
Approximately 75% of all TKAs are reimbursed under Medicare,17 whereas elective TKA continues to be one of the most common surgeries in the Medicare‐age patient population,20 foreshadowing the prominent cost burden of osteoarthritis in the aging population. The concomitant decreasing reimbursement for arthroplasty in general supports an examination of what constitutes efficient, high‐quality, and cost‐effective care21 for TKA. At our institution, patients undergoing TKA are preferentially triaged to an SOS nursing unit for postoperative care. As hospital bed capacity continues to decline, patients may be triaged to open beds at locations that may not be the optimal choice for nursing care. The primary purpose of this study was to determine the impact of SOS units versus nonorthopedic nursing (NON) units on resource utilization for and outcomes of patients undergoing elective knee arthroplasty. We hypothesized that length of stay would be shorter and cost of inpatient care would be lower for patients cared for on SOS units.
MATERIALS AND METHODS
Study Design and Setting
We conducted a retrospective observational cohort study of all patients undergoing elective primary TKA from January 1, 1996, to December 31, 2004, comparing outcomes of patients assigned to SOS units with those of patients assigned to NON units. Patients were admitted to Rochester Methodist Hospital, Mayo Clinic, a tertiary‐care primary surgical teaching hospital that has 794 beds and more than 15,000 admissions annually. There were 13 faculty orthopedic surgeons performing elective nontraumatic lower‐extremity joint procedures during the study period, each with orthopedic residents rotating as part of the patient care team.
Study Population
All patients at Mayo Clinic who had undergone a joint replacement were followed prospectively, and data were collected using standardized forms and protocols, the methodologies of which have been described previously.22 Follow‐up was greater than 95% complete. Using the joint registry, patients who had undergone a TKA were identified (n = 9798). Postoperative patients initially transferred from the postanesthesia care unit to a general care floor were included. We excluded patients who required urgent, revision, or bilateral arthroplasties; who had been treated at or transferred from another institution; and whose primary surgical indication was trauma or septic arthritis. Subjects admitted to the hospital on the day prior to the procedure and subjects initially transferred directly from the postanesthesia care unit to the intensive care unit (ICU) were excluded, including patients requiring immediate postoperative cardiac monitoring. All primary surgical interventions were performed between Monday and Friday. The study authors identified 5883 eligible patients.
Patient clinical and demographic data including surgical indication; age; sex; height and weight at surgery; and dates of admission, surgery, death, discharge, and last follow‐up were abstracted from the registry. Type of anesthesia (general, regional, combined), American Society of Anesthesiologists (ASA) physical status class, and date and time of ICU admission and discharge were abstracted from individual departmental databases. The Decision Support System (DSS) administrative database (Eclipsys, Boca Raton, FL) was utilized to abstract relevant clinical variables, including major comorbid conditions such as cancer, cerebrovascular disease, chronic pulmonary disease, congestive heart failure, dementia, diabetes, hemiplegia, HIV/AIDS, metastatic solid tumors, myocardial infarction, peripheral vascular disease, renal disease, rheumatologic disease, and ulcers. A composite Charlson comorbidity score was computed as previously described.23, 24 Administrative variables regarding patient encounters including inpatient stay variableslength of stay, costs, patient location, nursing care units, admission times, discharge disposition and datewere also obtained from the DSS database.
Variables and Definitions
Length of stay was defined as the number of days from time of admission for the surgical episode to time of discharge. All costs were based on a provider perspective using standardized 2005 costs based on inflation‐adjusted estimates as previously described.3, 25, 26 We assessed resource utilization among patients who received care on an SOS unit by determining length of stay and total, hospital, and physician costs for the specified surgical episode. We also assessed blood bank, ICU, laboratory, pharmacy, physical therapy, occupational therapy, respiratory therapy, radiology, and room‐and‐board costs. Blood bank costs consisted of the costs of storing, processing, and administering the transfusion. Surgical procedure, anesthesia, and preoperative service costs were excluded from our cost analyses, as our aim was to examine hospital flow and resource utilization from time of transfer from the postanesthesia care unit to hospital discharge in order to specifically examine the impact of an SOS unit. We compared unexpected ICU admissions and stays and the resources utilized of patients in these 2 groups.
State and federal death registries confirmed patient expiration and primary cause of death. In‐hospital mortality was defined as death during the same hospital admission as the indexed surgical episode. Thirty‐day mortality was defined as death occurring within 30 days of the surgical procedure. Readmission at 30 days was defined as any admission to our institutions within a 30‐day period whose purpose was possibly related to the initial surgical episode and not a result of an elective admission. A priori we were aware of the small number of these events in the elective joint population. Therefore, we combined inpatient 30‐day mortality, 30‐day reoperation, and 30‐day readmission rates as a composite endpoint.
Specialized Orthopedic Surgery Units
An SOS unit was defined as a general care nursing unit where patients receive all their postoperative care after elective TKA. Such a unit has a multidisciplinary staff that has orthopedic expertise. The differences between an SOS unit and a NON unit are described in Table 1. Bed availability at the time of discharge from the postanesthesia care unit was the exclusive factor for admission to this unit. Bed availability was dependent on staff availability or whether there was an excess number of operative cases. The number and severity of patient medical comorbidities or complications, the time of discharge from the postanesthesia care unit, and patient room preference had no impact on which unit patients were admitted to. Patients were allocated to the SOS group or the NON unit group according to their physical location the evening of admission. Monitored beds at this facility are solely located in the ICU, and neither SOS nor NON units have this capability. Any patient requiring a monitored bed at any time, regardless of the reason, would be transferred directly to the ICU. Daily rounds were performed on either unit by the primary orthopedic team. The need for either medical or pain service consultation was at the discretion of the primary orthopedic team and not dependent on the patient's physical location.
| Specialized orthopedic surgical unit (SOS) | Nonorthopedic nursing unit (NON) | |
|---|---|---|
| ||
| Type of unit | Orthopedic general care unit. | General surgical care unit. |
| Patient type | Postoperative elective orthopedic only. | Any patientmedical or surgical. |
| Determinants of physical location for orthopedic patient | Primary bed assignment. | Admitted only if SOS units have reached full bed capacity. |
| Orthopedic‐trained nursing staff | Yesrequired to have additional post‐RN* training in orthopedics. These RNs rarely float to nonorthopedic units. | Nomay have additional training or experience in an unrelated medical or surgical discipline. Floating to other units may occur. |
| Orthopedic‐specific physical + occupational therapy | Provided by certified physical therapists trained in lower‐extremity joint procedures. Site‐based therapy available to all patients on SOS units. | Provided by certified physical therapists who do not necessarily have postoperative orthopedic lower‐extremity specialization. Site‐based therapy on NON unit available to all patients. |
| Licensed social workers | Dedicated to postoperative needs of orthopedic patients physically located on SOS units. | Not specifically dedicated to the postoperative elective orthopedic joint patient and not physically located on these units. |
| Interdisciplinary team meetings | Patient care addressed in a interdisciplinary team meeting 3 times weeklyconsists of an RN, physical and occupational therapists, social worker, and physician. | No care team meetings, as patients are off‐service. |
| Physician postoperative order set | Orthopedic‐specific order set that is available hospitalwide. Nursing staff on these units is familiar with these order sets. | Orthopedic‐specific order set available hospitalwide. Nursing staff on these units may not be entirely familiar with these order sets. |
| Rehabilitation protocols | Orthopedic specific. | Not orthopedic specific. |
| Patient‐care instructions | Orthopedic diagnosis‐specific instructions readily available | Orthopedic diagnosis‐specific instructions available but requires staff to obtain information and forms from the SOS inits. |
| Discharge protocol | Specifically targeted to the postarthroplasty patient | Generic hospitalwide protocol. |
| Hospital discharge summary | Yescowritten by primary orthopedic team and primary orthopedic RN. | Yescowritten by primary orthopedic team and nonorthopedic RN. |
| Orthopedic‐specific discharge instructions | Yescowritten by primary orthopedic team and primary orthopedic RN. | No. |
All data were subsequently combined into a single database to facilitate data analysis. We further excluded 44 patients because no cost information was available, 9 patients who had multiple joint replacements performed during the specified surgical hospitalization, 69 patients because they had not authorized their medical records to be used for the purposes of research; 163 patients admitted directly to the ICU, 63 patients admitted the day prior to surgery, and 1 patient whose billing data suggested an outpatient encounter. A final patient cohort of 5534 patients was in the analysis. With the observed sample size and the overall variability, our study had 80% power to detect a difference between the 2 groups as small as 0.22 days in length of stay and $761 in hospital costs. The study was approved by our institutional review board. All study patients had authorized the use of their medical records for the purposes of research. Funding was obtained through an intramurally sponsored Small Grants Program by the Division of General Internal Medicine, which had no impact on the design of the study, reporting, or decision to submit an article on the study for publication.
Statistical Analysis
The statistical analysis compared baseline health and demographic characteristics of the patients cared for on SOS units with those cared for on NON units using chi‐square tests for nominal factors and the 2‐sample Wilcoxon rank sum tests for continuous variables. We used the chi‐square test to test for unadjusted differences in sex, patient residence (local or referred), race, individual Charlson comorbid conditions, anesthesia type, admitting diagnosis, 30‐day readmission rate, and discharge location. The 2‐sample Wilcoxon rank sum test assessed unadjusted differences in length of stay, costs, age, ICU days of stay, number of reoperations, total Charlson score and ASA class. Thirty‐day mortality rates were tested using the Fisher exact test.
Differences between patients in SOS and NON units in length of stay (LOS) and costs were the study's primary outcomes. We adjusted for baseline and surgical covariates using generalized linear regression models for these outcomes. The effect of the nursing unit was based on regression coefficients for age, sex, ASA class, anesthesia type, Charlson comorbidities, and surgical year. Age was analyzed using 5 categories: <55; 55‐64; 70‐74; 54‐69, and >75 years, with 65‐69 years used as the reference group. Each Charlson comorbid condition was treated as an indicator variable. Indicator variables were also assigned to surgical year, with 2004 used as the reference. These variables were subsequently entered into the model to calculate the differences between patients on an SOS unit and those on a NON unit.
Our secondary outcomes included ICU utilization and 30‐day outcomes of mortality, reoperations, and readmissions. We then assessed the effect of treatment on the SOS unit using the entire cohort (n = 5534) for unplanned postoperative ICU stay (yes or no) and on our combined endpoint after adjusting for the variables listed previously, using logistic regression models. A P value < 0.05 was considered statistically significant. All analyses were performed using statistical software (SAS, version 9.1; SAS Institute Inc, Cary, NC).
RESULTS
Baseline patient characteristics are represented in Table 2. Five thousand and eighty‐two patients were admitted to an SOS unit, and 452 patients were admitted to a NON unit. The annual number of patients undergoing TKA increased during our study period, as did the number of patients cared for on NON units. There were no differences between groups in the number of local county patients or in the number of patients primarily referred by other providers for elective arthroplasty. Mean length of stay was 4.9 days in both groups. After adjusting for the specified covariates, including age, sex, year of surgery, Charlson comorbidities, ASA class, and type of anesthesia, LOS was 0.234 days shorter in the SOS group (95% confidence interval [CI]: 0.08, 0.39; P = .002). Overall and hospital costs were significantly lower in the SOS group, as outlined with the other costs in Table 3. Room‐and‐board costs were 5.3% lower for SOS patients than for patients on NON units, representing a per‐patient difference of $244 $87 (95% CI: $72, $415; P = .005).
| Specialized orthopedic surgery unit (n = 5082) | Nonorthopedic nursing unit (n = 452) | P value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| |||||
| Age (years) | |||||
| <55 | 534 | 10.5% | 57 | 12.6% | |
| 55‐64 | 1148 | 22.6% | 101 | 22.4% | |
| 65‐69 | 802 | 15.8% | 66 | 14.6% | |
| 70‐74 | 1106 | 21.8% | 91 | 20.1% | |
| >75 | 1492 | 29.4% | 137 | 30.3% | |
| Mean age ( SD*) | 68.3 10.75 | 67.9 11.5 | .50 | ||
| Sex | .70 | ||||
| Male | 2173 | 42.8% | 189 | 41.8% | |
| Female | 2909 | 57.2% | 263 | 58.2% | |
| Race | .28 | ||||
| White | 4731 | 93.1% | 420 | 92.9% | |
| Other* | 51 | 1.0% | 8 | 1.8% | |
| Unknown | 300 | 5.9% | 24 | 5.3% | |
| Local Olmsted County patients | 772 | 15.2% | 58 | 12.8% | .18 |
| Indication for surgery | .03 | ||||
| Osteoarthritis | 4778 | 94% | 430 | 95.1% | |
| Rheumatologic disease | 184 | 3.6% | 6 | 1.3% | |
| Avascular necrosis | 62 | 1.2% | 5 | 1.1% | |
| Congenital | 6 | 0.1% | 1 | 0.2% | |
| Cancer | 22 | 0.4% | 5 | 1.1% | |
| Other | 30 | 0.6% | 5 | 1.1% | |
| Year of surgery | < .001 | ||||
| 1996 | 497 | 98.8% | 6 | 1.19% | |
| 1997 | 571 | 99.7% | 2 | 0.35% | |
| 1998 | 479 | 98.8% | 6 | 1.24% | |
| 1999 | 487 | 94.8% | 27 | 5.25% | |
| 2000 | 458 | 92.7% | 36 | 7.29% | |
| 2001 | 502 | 86.7% | 77 | 13.3% | |
| 2002 | 593 | 89.2% | 72 | 10.8% | |
| 2003 | 639 | 87.1% | 95 | 12.9% | |
| 2004 | 856 | 86.7% | 131 | 13.3% | |
| Charlson score (mean SD) | 0.256 0.536 | 0.288 0.593 | .23 | ||
| AIDS | 0 | 0% | 1 | 0.22% | 1.00 |
| Cancer | 85 | 1.68% | 7 | 1.55% | .84 |
| Cerebrovascular disease | 32 | 0.63% | 0 | 0% | .09 |
| Chronic pulmonary disease | 28 | 5.63% | 23 | 5.09% | .63 |
| Congestive heart failure | 89 | 1.75% | 22 | 4.87% | < .001 |
| Dementia | 10 | 0.2% | 2 | 0.44% | .28 |
| Diabetes | 603 | 11.9% | 58 | 12.8% | .54 |
| Hemiplegia | 9 | 0.18% | 0 | 0% | .37 |
| Metastatic solid tumor | 11 | 0.22% | 2 | 0.44% | .34 |
| Myocardial infarction | 29 | 0.57% | 4 | 0.88% | .4 |
| Peripheral vascular disease | 67 | 1.32% | 4 | 0.88% | .43 |
| Renal disease | 52 | 1.02% | 5 | 1.11% | .87 |
| Rheumatologic disease | 12 | 0.24% | 2 | 0.44% | .40 |
| Ulcers | 15 | 0.3% | 0 | 0% | .25 |
| ASA class‖ | |||||
| I | 99 | 2.0% | 12 | 2.7% | |
| II | 2891 | 56.9% | 255 | 56.4% | |
| III | 2084 | 41.0% | 183 | 40.5% | |
| IV | 8 | 0.2% | 2 | 0.4% | |
| Average ASA class ( SD) | 2.39 0.53 | 2.39 0.55 | .80 | ||
| Anesthesia type | .02 | ||||
| General | 1644 | 32.4% | 143 | 31.6% | |
| Regional | 2742 | 54% | 226 | 50% | |
| Combined | 696 | 13.7% | 83 | 18.4% | |
| Unadjusted values | Adjusted values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SOS* | SD | NON | SD | P value | Difference | SD | P value | 95% CI | |
| |||||||||
| Total cost | $9989 | $5392 | $10,067 | $5075 | .77 | $600 | $244 | .01 | $122, $1079 |
| Hospital costs | $9789 | $5123 | $ 9805 | $4647 | .23 | $594 | $231 | .01 | $141, $1047 |
| Room & board | $4399 | $1825 | $ 4577 | $1579 | .04 | $244 | $ 87 | .005 | $ 72, $ 415 |
| ICU costs | $ 58 | $1094 | $ 107 | $ 682 | .35 | $ 11 | $ 51 | .82 | $111, $ 88 |
| Pharmacy | $ 851 | $1701 | $ 931 | $1823 | .34 | $ 87 | $ 85 | .30 | $ 79, $253 |
| Laboratory costs | $ 386 | $ 438 | $ 395 | $ 405 | .65 | $ 27 | $ 20 | .18 | $ 12, $ 65 |
| Radiology costs | $ 98 | $ 205 | $ 103 | $ 183 | .61 | $ 1 | $ 10 | .93 | $ 20, $ 19 |
| PT/OT**/RT | $ 739 | $ 505 | $ 682 | $ 394 | .004 | $ 15 | $ 19 | .45 | $ 23, $ 52 |
| Blood bank | $ 159 | $ 306 | $ 178 | $3023 | .22 | $ 6 | $ 15 | .69 | $ 35, $ 23 |
| Physician costs | $ 207 | $ 464 | $ 258 | $ 628 | .09 | $ 20 | $ 22 | .386 | $ 24, $ 63 |
| E&M costs‖ | $ 89 | $ 211 | $ 109 | $ 238 | .09 | $ 4 | $ 9 | .658 | $ 23, $ 14 |
| Physician radiology | $ 63 | $ 158 | $ 38 | $ 192 | .49 | $ 2 | $ 8 | .78 | $ 13, $ 18 |
| Other costs | $ 34 | $ 138 | $ 37 | $ 160 | .61 | $0.64 | $ 6 | .92 | $ 13, $ 12 |
There were 83 patients (1.63%) transferred from SOS units to the ICU, compared with 14 patients (3.1%) transferred from NON units (P = .02), but no differences in the mean number of ICU days or associated costs between groups. A priori, the authors were aware of the small number of postoperative medical events in this population. In examining the combined endpoint of reoperations, readmissions, and mortality, there were no differences observed in our regression analysis between SOS patients and NON unit patients (0.03 events, standard error: 0.1859; odds ratio: 0.976). Table 4 demonstrates a higher percentage of patients discharged with home health on the NON units than on the SOS units (8.41% vs. 4.62%; P < .001).
| Specialized orthopedic surgery unit | Nonorthopedic nursing unit | P value | |||
|---|---|---|---|---|---|
| n* | % | n | % | ||
| |||||
| Home | 3812 | 75% | 328 | 72.6% | .252 |
| Home health | 235 | 4.62% | 38 | 8.41% | < .001 |
| Transferred to skilled nursing facility | 1030 | 20.3% | 86 | 19% | .529 |
DISCUSSION
To the best of our knowledge, this is the first study to examine the impact of specialized orthopedic surgery units on resource utilization in elective knee arthroplasty patients. Our findings demonstrate that patients admitted following elective TKA to SOS units will have a reduced length of stay, lower overall and hospital costs, and fewer unexpected transfers to higher levels of care (ICUs). We believe that these findings are a result in part of the specialized expertise allied health care providers develop by taking care of and focusing on a large volume of patients over time with the same group and type of surgeons. This multidisciplinary setting in which care providers are familiar not only with each other but with this specific population of patients creates the environment necessary for adherence to specialized clinical pathways.27
Patient LOS is an important determinant of resource utilization. In a study by Husted et al., the mean length of stay in Danish hospitals following TKA was 8.6 days in 2003.28 An epidemiological study using the Nationwide Inpatient Sample database of patients in the United States showed that from 1998 to 2000, the mean LOS was 4.3 days.18 In our study, the mean LOS was slightly higher (4.9 days), potentially reflecting referral bias. Achieving additional savings and improved outcomes by further reducing LOS in an environment in which care pathways are already in place is often difficult; hence, alternative approaches and strategies are often necessary.29, 30 Our results suggest that in TKA patients, after adjusting for other factors, there is a decrease in the length of stay of 0.234 days among those cared for on SOS units. However, we cannot state that the existence of the clinical pathway alone is responsible for our data differences because certain components of the care pathway for elective TKA patients are used throughout the hospital regardless of type of postoperative nursing unit. We believe that the interdisciplinary specialty care provided to orthopedic patients on SOS units is a critical component of a successfully implemented care pathway and not just a convenience or practice preference. The same surgeons admitting patients to the same nursing unit, with the same nurses, physical therapists and pharmacists providing care to the same type of patient population over time, leverages the collective experience of all care providers. This integrated, multidisciplinary teamwork may optimize timeliness, achieve incremental cost savings, and improve safety (including a decreased number of unanticipated transfers to an ICU setting).
Clinical pathways are known to reduce overall costs, normally by reducing LOS,29, 3133 and our results suggest approximately an incremental 6% cost reduction with the use of improving patient logistics by using SOS units. An economic evaluation study by Healy et al. suggests that focusing on nursing units may be a means of reducing total costs.29 Our cost savings were slightly lower than the reported savings by other practice assessments; however, we excluded operative and anesthesia costs, both significant contributors to overall and hospital costs. By eliminating these variables, our costs were specifically limited to the postoperative course, which is highly dependent on specialized interdisciplinary care.29
Providing specialized care has a significant impact on society. Although there is a per‐patient savings of only $600 when elective TKA patients are cared for on SOS units, this could be the difference between a positive and negative margin in the setting of fixed reimbursement. With a current average of 90 patients annually triaged postoperatively to NON units, there is a potential loss of $54,000 annually at our institution in just this single patient population with the current mechanisms of perioperative hospital flow. Multiply this potential savings to a national level, and the total is significant. With an aging population, the number of arthroplasties and concomitantly the number of hospitalizations in general are likely to increase, suggesting that changes in hospital flow are required to ensure optimal, cost‐effective care in the best setting available for patients. Such care is often related to surgical volume, and our institution observes such volume. Our results indicate that SOS units are one possible means of achieving this objective of fiscal sustainability, but further studies are needed to determine the indirect and hidden costs of sustaining such units in order to observe the actual cost savings.34 It could be argued that for elective TKA patients to have the most optimal outcomes and most efficient care, the surgical procedure should be performed only if beds are available on the nursing units whose staff has the most specific training.
Thirty‐Day Outcomes
We elected to combine 30‐day mortality, reoperations, and readmissions pertaining to the joint procedure as a composite endpoint and found no differences in outcomes between groups. These results suggest that these longer‐term patient‐specific outcomes are likely not related to the specialty nursing care. We used a 30‐day endpoint assuming that a longer period may have led to the inclusion of deaths that were not directly attributable to the surgical intervention. In addition, a previous study advocated using 30 days as an endpoint for follow‐up, as it adequately accounts for adverse events.35 Our institution is also a referral center; hence, we would likely be unable to capture all events if we were to use the standard 90‐day period used for payment for this procedure, as these data are not canvassed by the joint registry.
Discharge Disposition
NON unit patients tended to have a higher degree of home health arranged at discharge. The NON unit nursing staff cares for other nonorthopedic surgical patients daily and may transfer their patterns of care utilization to the orthopedic patients despite different postoperative needs. In addition, if NON unit nursing staff members care for TKA patients only intermittently, they may not have as clear a working understanding of the particular postoperative requirements of TKA patients and consequently request unnecessary home health services and general community resources. Alternatively, patients cared for on NON units may actually have needed more assistance and more services on discharge. Although purely speculative, patients cared for by dedicated orthopedic surgery staff may develop added confidence from the experience of the allied care staff and feel less of a need for postdismissal services.
Role of Hospitalists in Specialized Care Pathways
Hospitalists are known to improve efficiency without reducing patient satisfaction. Their role has been demonstrated in different patient populations.1, 2, 3638 In a study of hip fracture patients, a hospitalist care model demonstrated a reduction in length of stay and time to surgery, without compromising long‐term outcomes.4, 39 Utilizing a hospitalist/midlevel care provider team approach to reduce LOS in units with a static number of beds can possibly increase bed turnover and prevent triaging of patients onto NON units. This is but one example of how a medical‐surgical partnership can improve outcomes. However, in an era where cost‐effective and regulatory practices require optimal resource allocation, hospitalists are in a key position to foster quality improvement projects, promote patient safety measures, and enhance systems care delivery. Becoming involved in designing specialized clinical units, with an emphasis on a multidisciplinary care approach, and developing their relationships with hospital administrators and nursing staff should be among their priorities. The Society of Hospital Medicine has also been committed to the care of the elderly through its core competencies40 and the orthopedic population that will benefit from such process changes and care pathways. Hospital innovations such as the implementation of SOS‐type units not only for other medical‐surgical partnerships but also for site‐based units caring for geriatric patients can be top priorities for hospitalists.
Strengths and Applicability
Our results are important in that they can likely be applied to both large tertiary‐care centers and smaller community‐based centers that perform specialized orthopedic surgeries. Nurses on specialized orthopedic units are very familiar with this postoperative population and have developed expertise in the care of these patients. These experienced nurses can likely be found on orthopedic units in tertiary‐care centers or surgical units in smaller facilities. Furthermore, our results support the benefits of interdisciplinary advanced teamwork. When an interdisciplinary group of health care providers works together on a daily basis, certain habits and patterns inevitably develop that often are unplanned and may be difficult to measure. This enhanced patient flow may not occur if these patients are cared for by providers unfamiliar with each other's work patterns. The importance of optimized teamwork is not hospital‐size dependent. Only primary elective knee arthroplasties were included to minimize confounding bias by bilateral or revision surgeries or indications such as septic arthritis, which are known to lead to increased length of stay, costs and complications.41
Limitations
Our study has the limitations of its retrospective nonrandomized study design, and only a prospective, randomized investigation could definitively address our aims. By excluding sicker patients, such as those referred with complicated health issues or high‐risk patients who required admission in advance of the proposed surgery for monitoring of perioperative anticoagulation issues, our estimates of possible differences between our comparison groups may have been conservative. We are unaware of how these sicker patients would fare on either nursing unit. Furthermore, what occurs in the hospital setting may not only have an impact on the hospital stay but may also influence long‐term outcomes. This is impossible to assess with analysis of administrative databases.
We relied on the complete and accurate recording of data from various databases, depending on the validity of data entry and collection. With a large cohort of patients, any errors in documentation or abstraction would be expected to be similar in both groups. Furthermore, confounding variables such as patient comorbidities are extracted from administrative data sets whose personnel might not be as familiar with the medical aspects of patient care. We used linear and logistic regression analyses to account for known differences in baseline characteristics despite the sample sizes being proportionally larger in the SOS group. Although we attribute the shortened length of stay in the SOS group to the interdisciplinary team approach, we were unable to determine to what extent this was a result of nursing staff or discharge planning. By using administrative databases, we were unable to abstract the consensus time and date of discharge, when all hospital staff deemed the patient ready for discharge, and hence relied on the actual time of discharge, which can be heavily reliant on availability at skilled nursing facilities. In addition, it was unknown whether patients discharged from SOS units were, by matter of protocol, discharged earlier in the day. Nevertheless, this small difference in length of stay can improve patient flow by opening up postoperative patient beds. Furthermore, such data sets are unable to provide information on patient satisfaction or quality‐of‐life measures, both of which are important determinants in specialized care pathways.42 The patient population served by our institution is generally ethnically homogeneous, thereby limiting potential generalizations to tertiary‐care centers or geographical areas with a population similar to ours. Our study also was not intended as a formal cost‐effectiveness analysis; hence, the impact of possible startup costs to begin a similar nursing unit was not explored. Although differences in practice management can be considered a limitation of not only operative but also perioperative care, we neither expected nor encountered any significant or drastic alterations during the study period, and year of surgery was adjusted for in our analysis. However, prospective randomized controlled studies testing specific clinical pathways and practice‐related innovations are needed to better examine these outcomes.
CONCLUSIONS
In conclusion, postoperative patients after elective knee arthroplasty cared for on specialized orthopedic surgery units have shorter length of stays and cost hospitals less than patients admitted to nonspecialized orthopedic nursing units. In an era in which quality indicators and external reviews are forcing practitioners and health care organizations to become increasingly responsible for their own practices, more research is required to better address specific questions pertaining to different processes of care. Our study is meant to increase the attention paid to patient flow and postoperative logistics in the elective TKA population. SOS units, as a unique model of care, may become an additional step toward ensuring quality care and improved resource utilization.
Acknowledgements
The authors thank Donna K. Lawson, LPN, for her assistance in data collection and management.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,,.The economic benefit for family/general medicine practices employing physician assistants.Am J Manag Care.2002;8:613–620.
- ,,, et al.Economic evaluation of Australian stroke services: a prospective, multicenter study comparing dedicated stroke units with other care modalities.Stroke.2006;37:2790–2795.
- ,,,.Geriatric evaluation and management units in the care of the frail elderly cancer patient.J Gerontol A Biol Sci Med Sci.2005;60:798–803.
- ,,,,,.A randomized, controlled trial of a geriatric assessment unit in a community rehabilitation hospital.N Engl J Med.1990;322:1572–1578.
- ,.The effect of longevity on spending for acute and long‐term care.N Engl J Med.2000;342:1409–1415.
- ,,, et al.The Bone and Joint Decade 2000‐2010.Acta Orthop Scand.1998;69:219–220.
- .The Bone and Joint Decade 2000‐2010— for prevention and treatment of musculoskeletal disease.Osteoarthr Cartil.1998;7:1–4.
- Prevalence of self‐reported arthritis or chronic joint symptoms among adults—United States, 2001.MMWR Morb Mortal Wkly Rep.2002;51:948–950.
- ,,, et al.Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States.Arthritis Rheum.1998;41:778–799.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, 2002. Available at: http://www.ahrq.gov. Accessed January 25,2005.
- ,,,,,.Costs and cost‐effectiveness in hip and knee replacements. A prospective study.Int J Technol Assess Health Care.1997;13:575–588.
- ,,,,.Trends in total knee replacement surgeries and implications for public health, 1990‐2000.Public Health Rep.2005;120:278–282.
- ,,, et al.Demographic variation in the rate of knee replacement: a multi‐year analysis.Health Serv Res.1996;31:125–140.
- ,,,,.Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990‐2000.Arthritis Rheum.2006;55:591–597.
- ,.2001 National Hospital Discharge Survey.Adv Data.2003;332.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004:1–29.
- ,,.Costs of health care administration in the United States and Canada.N Engl J Med2003;349:768–775.
- ,,.Maintaining a hip registry for 25 years. Mayo Clinic experience.Clin Orthop Relat Res.1997:61–68.
- ,,,.Validation of a combined comorbidity index.J Clin Epidemiol.1994;47:1245–1251.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,, et al.A prospective randomized comparison of laparoscopic appendectomy with open appendectomy: Clinical and economic analyses.Surgery.2001;129:390–400.
- ,,, et al.Incremental costs of enrolling cancer patients in clinical trials: a population‐based study.J Natl Cancer Inst.1999;91:847–853.
- ,.Understanding the complexity of redesigning care around the clinical microsystem.Qual Saf Health Care.2006;15(Suppl 1):i10–i16.
- ,,, et al.Length of stay after primary total hip and knee arthroplasty in Denmark, 2001‐2003.Ugeskr Laeger.2006;168:276–279.
- ,,,.Opportunities for control of hospital costs for total joint arthroplasty after initial cost containment.J Arthroplasty.1998;13:504–507.
- ,,,,.Effectiveness of clinical pathways for total knee and total hip arthroplasty: literature review.J Arthroplasty.2003;18:69–74.
- ,,, et al.The effect of a perioperative clinical pathway for knee replacement surgery on hospital costs.Anesth Analg.1998;86:978–984.
- The cost effectiveness of streamlined care pathways and product standardization in total knee arthroplasty.J Arthroplasty.1999;14:182–186.
- ,,,,.Impact of cost reduction programs on short‐term patient outcome and hospital cost of total knee arthroplasty.J Bone Joint Surg Am.2002;84‐A:348–353.
- ,,,.Success of clinical pathways for total joint arthroplasty in a community hospital.Clin Orthop Relat Res.2007;457:133–137.
- ,,,.Optimal timeframe for reporting short‐term complication rates after total knee arthroplasty.J Arthroplasty.2006;21:705–711.
- ,,.Associations with reduced length of stay and costs on an academic hospitalist service.Am J Manag Care.2004;10:561–568.
- ,,.Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs.J Hosp Med.2006;1:29–35.
- .Care of hospitalized older patients: opportunities for hospital‐based physicians.J Hosp Med.2006;1:42–47.
- ,,, et al.Effects of a hospitalist care model on mortality of elderly patients with hip fractures.J Hosp Med.2007;2:219–225.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,,,.Effect of feedback on resource use and morbidity in hip and knee arthroplasty in an integrated group practice setting.Mayo Clin Proc.1996;71:127–133.
- ,,,.Integrated care pathways.BMJ.1998;316:133–137.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,,.The economic benefit for family/general medicine practices employing physician assistants.Am J Manag Care.2002;8:613–620.
- ,,, et al.Economic evaluation of Australian stroke services: a prospective, multicenter study comparing dedicated stroke units with other care modalities.Stroke.2006;37:2790–2795.
- ,,,.Geriatric evaluation and management units in the care of the frail elderly cancer patient.J Gerontol A Biol Sci Med Sci.2005;60:798–803.
- ,,,,,.A randomized, controlled trial of a geriatric assessment unit in a community rehabilitation hospital.N Engl J Med.1990;322:1572–1578.
- ,.The effect of longevity on spending for acute and long‐term care.N Engl J Med.2000;342:1409–1415.
- ,,, et al.The Bone and Joint Decade 2000‐2010.Acta Orthop Scand.1998;69:219–220.
- .The Bone and Joint Decade 2000‐2010— for prevention and treatment of musculoskeletal disease.Osteoarthr Cartil.1998;7:1–4.
- Prevalence of self‐reported arthritis or chronic joint symptoms among adults—United States, 2001.MMWR Morb Mortal Wkly Rep.2002;51:948–950.
- ,,, et al.Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States.Arthritis Rheum.1998;41:778–799.
- HCUPnet, Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality, 2002. Available at: http://www.ahrq.gov. Accessed January 25,2005.
- ,,,,,.Costs and cost‐effectiveness in hip and knee replacements. A prospective study.Int J Technol Assess Health Care.1997;13:575–588.
- ,,,,.Trends in total knee replacement surgeries and implications for public health, 1990‐2000.Public Health Rep.2005;120:278–282.
- ,,, et al.Demographic variation in the rate of knee replacement: a multi‐year analysis.Health Serv Res.1996;31:125–140.
- ,,,,.Trends in the epidemiology of total shoulder arthroplasty in the United States from 1990‐2000.Arthritis Rheum.2006;55:591–597.
- ,.2001 National Hospital Discharge Survey.Adv Data.2003;332.
- ,.2002 National Hospital Discharge Survey.Adv Data.2004:1–29.
- ,,.Costs of health care administration in the United States and Canada.N Engl J Med2003;349:768–775.
- ,,.Maintaining a hip registry for 25 years. Mayo Clinic experience.Clin Orthop Relat Res.1997:61–68.
- ,,,.Validation of a combined comorbidity index.J Clin Epidemiol.1994;47:1245–1251.
- ,,,.A new method of classifying prognostic comorbidity in longitudinal studies: development and validation.J Chronic Dis.1987;40:373–383.
- ,,, et al.A prospective randomized comparison of laparoscopic appendectomy with open appendectomy: Clinical and economic analyses.Surgery.2001;129:390–400.
- ,,, et al.Incremental costs of enrolling cancer patients in clinical trials: a population‐based study.J Natl Cancer Inst.1999;91:847–853.
- ,.Understanding the complexity of redesigning care around the clinical microsystem.Qual Saf Health Care.2006;15(Suppl 1):i10–i16.
- ,,, et al.Length of stay after primary total hip and knee arthroplasty in Denmark, 2001‐2003.Ugeskr Laeger.2006;168:276–279.
- ,,,.Opportunities for control of hospital costs for total joint arthroplasty after initial cost containment.J Arthroplasty.1998;13:504–507.
- ,,,,.Effectiveness of clinical pathways for total knee and total hip arthroplasty: literature review.J Arthroplasty.2003;18:69–74.
- ,,, et al.The effect of a perioperative clinical pathway for knee replacement surgery on hospital costs.Anesth Analg.1998;86:978–984.
- The cost effectiveness of streamlined care pathways and product standardization in total knee arthroplasty.J Arthroplasty.1999;14:182–186.
- ,,,,.Impact of cost reduction programs on short‐term patient outcome and hospital cost of total knee arthroplasty.J Bone Joint Surg Am.2002;84‐A:348–353.
- ,,,.Success of clinical pathways for total joint arthroplasty in a community hospital.Clin Orthop Relat Res.2007;457:133–137.
- ,,,.Optimal timeframe for reporting short‐term complication rates after total knee arthroplasty.J Arthroplasty.2006;21:705–711.
- ,,.Associations with reduced length of stay and costs on an academic hospitalist service.Am J Manag Care.2004;10:561–568.
- ,,.Is there a geriatrician in the house? Geriatric care approaches in hospitalist programs.J Hosp Med.2006;1:29–35.
- .Care of hospitalized older patients: opportunities for hospital‐based physicians.J Hosp Med.2006;1:42–47.
- ,,, et al.Effects of a hospitalist care model on mortality of elderly patients with hip fractures.J Hosp Med.2007;2:219–225.
- ,,,,.Core competencies in hospital medicine: development and methodology.J Hosp Med.2006;1:48–56.
- ,,,,,.Effect of feedback on resource use and morbidity in hip and knee arthroplasty in an integrated group practice setting.Mayo Clin Proc.1996;71:127–133.
- ,,,.Integrated care pathways.BMJ.1998;316:133–137.
Copyright © 2008 Society of Hospital Medicine
Hospitalists and Hip Fractures
Because the incidence of hip fracture increases dramatically with age and the elderly are the fastest‐growing portion of the United States population, the number of hip fractures is expected to triple by 2040.1 With the associated increase in postoperative morbidity and mortality, the costs will likely exceed $16‐$20 billion annually.15 Already by 2002, the number of patients with hip fractures exceeded 340,000 in this country, resulting in $8.6 billion in health care expenditures from in‐hospital and posthospital costs.68 This makes hip fracture a serious public health concern and triggers a need to devise an efficient means of caring for these patients. We previously reported that a hospitalist service can decrease time to surgery and shorten length of stay without affecting the number of inpatient deaths or 30‐day readmissions of patients undergoing hip fracture surgery.9 However, one concern with reducing length of stay and time to surgery in the high‐risk hip fracture patient population is the effect on long‐term mortality because the death rate following hip fracture repair may be as high as 43% after 1 year.10 To evaluate this important issue, we assessed mortality over a 1‐year period in the same cohort of patients previously described.9 We also identified predictors associated with mortality. We hypothesized that the expedited surgical treatment and decreased length of stay of a hospitalist‐managed group would not have an adverse effect on 1‐year mortality.
METHODS
Patient Selection
Following approval by the Mayo Clinic Institutional Review Board, we used the Mayo Clinic Surgical Index to identify patients admitted between July 1, 2000, and June 30, 2002, who matched International Classification of Diseases (9th Edition) hip fracture codes.11 These patients were cross‐referenced with those having a primary surgical indication of hip fracture. Patients transferred to our facility more than 72 hours after fracture were excluded from our study. Study patients provided authorization to use their medical records for the purposes of research.
A cohort of 466 patients was identified. For purposes of comparison, patients admitted between July 1, 2000, and June 30, 2001, were deemed to belong to the standard care service, and patients admitted between July 1, 2001, and June 30, 2002, were deemed part of the hospitalist service.
Intervention
Prior to July 2001, Mayo Clinic patients aged 65 and older having surgical repair of a hip fracture were triaged directly to a surgical orthopedic or general medical teaching service. Patients with multiple medical diagnoses were managed initially on a medical teaching service prior to transfer to the operating room. The primary team (medical or surgical) was responsible for the postoperative care of the patient and any orders or consultations required.
After July 1, 2001, these patients were admitted by the orthopedic surgery service and medically comanaged by a hospitalist service, which consisted of a hospitalist physician and 2 allied‐health practitioners. Twelve hospitalists and 12 allied health care professionals cared for patients during the study period. All preoperative and postoperative evaluations, inpatient management decisions, and coordination of outpatient care were performed by the hospitalists. This model of care is similar to one previously studied and published elsewhere.12 A census cap of 20 patients limited the number of patients managed by the hospitalist service. Any overflow of hip fracture patients was triaged directly to a non‐hospitalist‐based primary medical or surgical service as before. Thus, 23 hip fracture patients (10%) admitted after July 1, 2001, were not managed by the hospitalists but are included in this group for an intent‐to‐treat analysis.
Data Collection
Study nurses abstracted all data including admitting diagnoses, demographic features, type and mechanism of hip fracture, admission date and time, American Society of Anesthesia (ASA) class, comorbid medical conditions, medications, all clinical data, and readmission rates. Date of last follow‐up was confirmed using the Mayo Clinic medical record, whereas date and cause of death were obtained from death certificates obtained from state and national sources. Length of stay was defined as the number of days between admission and discharge. Time to surgery was defined in hours as the time from hospital admission to the start of the surgery. Finally, time from surgery to dismissal was defined as the number of days from the initiation of the surgical procedure to the time of dismissal. Thirty‐day readmission was defined as readmission to our hospital within 30 days of discharge date.
Statistical Considerations
Power
The power analysis was based on the end point of survival following surgical repair of hip fracture and primary comparison of patients in the standard care group with those in the hospitalist group. With 236 patients in the standard care group, 230 in the hospitalist group, and 274 observed deaths during the follow‐up period, there was 80% power to detect a hazard ratio of 1.4 or greater as being statistically significant (alpha = 0.05, beta = 0.2).
Analysis
The analysis focused on the end point of survival following surgical repair of hip fracture. In addition to the hospitalist versus standard care service, demographic, baseline clinical, and in‐hospital data were evaluated as potential predictors of survival. Survival rates were estimated using the method of Kaplan and Meier, and relative differences in survival were evaluated using the Cox proportional hazards regression models.13, 14 Potential predictors were analyzed both univariately and in a multivariable model. For the multivariable model, initial variable selection was accomplished using stepwise selection, backward elimination, and recursive partitioning.15 Each method yielded similar results. Bootstrap resampling was then used to confirm the variables selected for each model.16, 17 The threshold of statistical significance was set at P = .05 for all tests. All analyses were conducted in SAS version 8.2 (SAS Institute Inc., Cary, NC) and Splus version 6.2.1 (Insightful Corporation, Seattle, WA).
RESULTS
There were 236 patients with hip fractures (50.6%) admitted to the standard care service, and 230 patients (49.4%) admitted to the hospitalist service. As shown in Table 1, the baseline characteristics of the patients admitted to the 2 services did not differ significantly except that a greater proportion of patients with hypoxia were admitted to the hospitalist service (11.3% vs. 5.5%; P = .02). However, time to surgery, postsurgery stay, and overall length of hospitalization of the hospitalist‐treated patients were all significantly shorter.
| Patient characteristic | Standard care n = 236 | Hospitalist care n = 230 | P value | ||
|---|---|---|---|---|---|
| |||||
| Age (years) | 82 | 83 | .34 | ||
| Female sex | 171 | 72.5% | 163 | 70.9% | .70 |
| Comorbidity | |||||
| Coronary artery disease | 69 | 29.2% | 77 | 33.5% | .32 |
| Congestive heart failure | 41 | 17.4% | 49 | 21.3% | .28 |
| Chronic obstructive pulmonary disease | 36 | 15.3% | 38 | 16.5% | .71 |
| Cerebral vascular accident or transient ischemic attack | 36 | 15.3% | 50 | 21.7% | .07 |
| Dementia | 54 | 22.9% | 62 | 27.0% | .31 |
| Diabetes | 45 | 19.1% | 46 | 20.0% | .80 |
| Renal insufficiency | 17 | 7.2% | 17 | 7.4% | .94 |
| Residence at time of admission | .07 | ||||
| Home | 149 | 63.1% | 138 | 60.0% | |
| Assisted living | 32 | 13.6% | 42 | 18.3% | |
| Nursing home | 55 | 23.3% | 50 | 21.7% | |
| Ambulatory status at time of admission | .14 | ||||
| Independent | 114 | 48.3% | 89 | 38.7% | |
| Assistive device | 99 | 41.9% | 115 | 50.0% | |
| Personal help | 9 | 3.8% | 16 | 7.0% | |
| Transfer to bed or chair | 9 | 3.8% | 7 | 3.0% | |
| Nonambulatory | 5 | 2.1% | 3 | 1.3% | |
| Signs at time of admission | |||||
| Hypotension | 4 | 1.7% | 3 | 1.3% | > .99 |
| Hypoxia | 13 | 5.5% | 26 | 11.3% | .02 |
| Pulmonary edema | 37 | 15.7% | 29 | 12.6% | .34 |
| Tachycardia | 19 | 8.1% | 25 | 10.9% | .3 |
| Fracture type | .78 | ||||
| Femoral neck | 118 | 50.0% | 118 | 51.3% | |
| Intertrochanteric | 118 | 50.0% | 112 | 48.7% | |
| Mechanism of fracture | .82 | ||||
| Fall | 219 | 92.8% | 212 | 92.2% | |
| Trauma | 1 | 0.4% | 3 | 1.3% | |
| Pathologic | 7 | 3.0% | 6 | 2.6% | |
| Unknown | 9 | 3.8% | 7 | 3.0% | |
| ASA* class | .38 | ||||
| I or II | 33 | 14.0% | 23 | 10.0% | |
| III | 166 | 70.3% | 166 | 72.2% | |
| IV | 37 | 15.7% | 41 | 17.8% | |
| Location discharged to | .07 | ||||
| Home or assisted living | 24 | 10.5% | 13 | 5.9% | |
| Nursing home | 196 | 86.0% | 192 | 87.3% | |
| Another hospital or hospice | 8 | 3.5% | 15 | 6.8% | |
| Time to surgery (hours) | 38 | 25 | .001 | ||
| Time from surgery to discharge (days) | 9 | 7 | .04 | ||
| Length of stay | 10.6 | 8.4 | < .00 | ||
| Readmission rate | 25 | 10.6% | 20 | 8.7% | .49 |
Patients were followed for a median of 4.0 years (range 5 days to 5.6 years), and 192 patients were still alive at the end of follow‐up (April 2006). As illustrated in Figure 1, survival did not differ between the 2 treatment groups (P = .36). Overall survival at 1 year was 70.6% (95% confidence interval [CI]: 66.5%, 74.9%). Survival at 1 year in the standard care group was 70.6% (95% CI: 64.9%, 76.8%), whereas in the hospitalist group, it was 70.5% (95% CI: 64.8%, 76.7%). As delineated in Table 2, cardiovascular causes accounted for 34 deaths (25.6%), with 14 of these in the standard care group and 20 in the hospitalist group; 29 deaths (21.8%) had respiratory causes, 20 in the standard care group and 9 in the hospitalist group; and 17 (12.8%) were due to cancer, with 7 and 10 in the standard care and hospitalist groups, respectively. Unknown causes accounted for 21 cases, or 15.8% of total deaths.
| Standard care | Hospitalist care | Total No. of deaths | % | |
|---|---|---|---|---|
| Cancer | 7 | 10 | 17 | 12.8% |
| Cardiovascular | 14 | 20 | 34 | 25.6% |
| Infectious | 5 | 4 | 9 | 6.8% |
| Neurological | 5 | 10 | 15 | 11.3% |
| Other | 0 | 2 | 2 | 1.5% |
| Renal | 4 | 2 | 6 | 4.5% |
| Respiratory | 20 | 9 | 29 | 21.8% |
| Unknown | 11 | 10 | 21 | 15.8% |
| Total | 66 | 67 | 133 | 100.0% |
In the univariate analysis, we found 29 variables that were significant predictors of survival (Table 3). A hospitalist model of care was not significantly associated with patient survival, despite the shorter length of stay (8.4 days vs. 10.6 days; P < .001) or expedited time to surgery (25 vs. 38 hours; P < .001), when compared with the standard care group, as previously reported by Phy et al.9 In the multivariable analysis (Table 4), however, the independent predictors of mortality were ASA class III or IV versus class II (hazard ratio [HR] 4.20; 95% CI: 2.21, 7.99), admission from a nursing home versus from home or assisted living (HR 2.24; 95% CI: 1.73, 2.90), and inpatient complications, which included patients requiring admission to the intensive care unit (ICU) and those who had a myocardial infarction or acute renal failure as an inpatient (HR 1.85; 95% CI: 1.45, 2.35). Even after adjusting for these factors, survival following hip fracture did not differ significantly between the hospitalist care patients and the standard care patients (HR 1.16; 95% CI: 0.91, 1.48).
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.41 (1.20, 1.65) | < .001 |
| ASA* II | 1.0 (referent) | |
| ASA* III | 5.27 (2.79, 9.96) | < .001 |
| ASA* IV | 11.7 (5.97, 22.9) | < .001 |
| History of chronic obstructive pulmonary disease | 1.82 (1.35, 2.43) | < .001 |
| History of renal insufficiency | 2.40 (1.62,3.55) | < .001 |
| History of stroke/transient ischemic attack | 1.46 (1.10, 1.95) | .01 |
| History of diabetes | 1.70 (1.29,2.25) | < .001 |
| History of congestive heart failure | 2.26 (1.73, 2.96) | < .001 |
| History of coronary artery disease | 1.53 (1.20, 1.97) | < .001 |
| History of dementia | 2.02 (1.57, 2.59) | < .001 |
| Admission from home | 1.0 (referent) | |
| Admission from assisted living | 1.47 (1.06, 2.04) | .02 |
| Admission from nursing home | 3.04 (2.33, 3.98) | < .001 |
| Independent | 1.0 (referent) | |
| Use of assistive device | 1.81 (1.39, 2.36) | < .001 |
| Personal help | 3.49 (2.16, 5.64) | < .001 |
| Nonambulatory | 3.96 (2.47, 6.35) | < .001 |
| Crackles on admission | 2.03 (1.50, 2.74) | < .001 |
| Hypoxia on admission | 1.56 (1.04, 2.32) | .03 |
| Hypotension on admission | 6.21 (2.72, 14.2) | < .001 |
| Tachycardia on admission | 1.66 (1.15, 2.41) | .007 |
| Coumadin on admission | 1.57 (1.13, 2.18) | .007 |
| Confusion/unconsciousness on admission | 2.23 (1.74, 2.87) | < .001 |
| Fever on admission | 1.98 (1.16, 3.40) | .01 |
| Tachypnea on admission | 1.95 (1.39, 2.72) | < .001 |
| Inpatient myocardial Infarction | 3.59 (2.35, 5.48) | < .001 |
| Inpatient atrial fibrillation | 2.00 (1.37, 2.92) | < .001 |
| Inpatient congestive heart failure | 2.62 (1.79, 3.84) | < .0001 |
| Inpatient delirium | 1.46 (1.13, 1.90) | < .005 |
| Inpatient lung infection | 2.52 (1.85, 3.42) | < .001 |
| Inpatient respiratory failure | 2.76 (1.64, 4.66) | < .001 |
| Inpatient mechanical ventilation | 2.56 (1.43, 4.57) | .002 |
| Inpatient renal failure | 3.60 (1.97, 6.61) | < .001 |
| Days from admission to surgery | 1.06 (1.005, 1.12) | .03 |
| Intensive care unit stay | 1.93 (1.51, 2.47) | < .001 |
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.17 (0.99, 1.38) | .07 |
| ASA* class III or IV | 4.20 (2.21, 7.99) | < .001 |
| ASA* class II | 1.0 (referent) | |
| Admission from nursing home | 2.24 (1.73, 2.90) | < .001 |
| Admission from home or assisted living | 1.0 (referent) | |
| Inpatient myocardial infarction, inpatient acute renal failure, or intensive care unit stay | 1.85 (1.45, 2.35) | < .001 |
| No inpatient myocardial infarction, no inpatient acute renal failure, and no intensive care unit stay | 1.0 (referent) | |
DISCUSSION
In our previous study, length of stay and time to surgery were significantly lower in a hospitalist care model.9 The present study shows that neither the reduced length of stay nor the shortened time to surgery of patients managed by the hospitalist group was associated with a difference in mortality compared with a standard care group, despite significantly improved efficiency and processes of care. Thus, our results refute initial concerns of increased mortality in a hospitalist model of care.
Delivery of perioperative medical care to hip fracture patients by hospitalists is associated with significant decreases in time to surgery and length of stay compared with standard care, with no differences in short‐term mortality.9, 18 Although there have been conflicting reports on the impact of length of stay and time to surgery on long‐term outcomes, our findings support previous results that decreased time to surgery was not associated with an observable effect on mortality.1923 A recent study by Orosz et al. that evaluated 1178 patients showed that earlier hip fracture surgery (performed less than 24 hours after admission) was not associated with reduced mortality, although it was associated with shorter length of stay.19 Our study also corroborates the results of an examination of 8383 hip fracture patients by Grimes et al., who found that time to surgery between 24 and 48 hours after admission had no effect on either 30‐day or long‐term mortality compared with that of those who underwent surgery between 48 and 72 hours, between 72 and 96 hours, or more than 96 hours after admission.20 However, both these results and our own are contrary to those of Gdalevich, whose study of 651 patients found that 1‐year mortality was 1.6‐fold higher for those whose hip fracture repair was postponed more than 48 hours.21 However, time to surgery in both the standard care and hospitalist model in our study was well below the 48‐hour cutoff, suggesting that operating anywhere within the normally accepted 48‐hour time frame may not influence long‐term mortality.
Because of the small number of events in both groups, we were unable to specifically compare whether a hospitalist model of care has any specific impact on long‐term cause of death. Although causes of death of patients with hip fracture were consistent with those of previous studies,10, 24 our death rate at 1 year, 29.4%, was higher than that seen among similar population groups at tertiary referral centers.19, 20, 2429 This is most likely a result of the cohort having a high proportion of nursing home patients (22%)19, 24, 26 transferred for evaluation to St. Mary's Hospital, which serves most of Olmsted County, Minnesota. This hospital also has some characteristics of a community‐based hospital, as it is where greater than 95% of all county patients receive care for surgical repair of hip fracture. Mortality rates are often higher at these types of hospitals.30 Previous studies using patients from Olmsted County indicate results can also be extrapolated to a large part of the U.S. population.31 In Pitto et al.'s study, the risk of death was 31% lower in those admitted from home than for those admitted from a nursing home.32 The latter patients normally have a higher number of comorbid conditions and tend to be less ambulatory than those in a community home‐dwelling setting. Our study also demonstrated that admission from a nursing home was a strong predictor of mortality for up to 1 year in the geriatric population. This may reflect the inherent decreased survival in this patient group, which is in agreement with the findings of other studies that showed inactivity and decreased ambulation prior to fracture were associated with increased mortality.3335
Multiple comorbidities, commonly seen in a geriatric population, translate into a higher ASA class and an increased risk of significant in‐hospital complications. Our study confirmed the findings of previous studies that a higher ASA class is a strong predictor of mortality,21, 26, 30, 3537 independent of decreased time to surgery.38 We also noted that significant in‐hospital complications, including renal failure, respiratory failure, and myocardial infarction, are documented predictors of mortality after hip fracture.27 Although mortality may vary depending on fracture type (femoral neck vs. intertrochanteric),3941 these differences were not observed in our study, in line with the results of previous published studies.37, 42 Controlling for age and comorbidities may be why an association was not found between fracture type and mortality. Finally, in a model containing comorbidity, ASA class, and nursing home residence prior to fracture, age was not a significant predictor of mortality.
Our study had a number of limitations. First, this was a retrospective cohort study based on chart review, so some data may have been subject to recording bias, and this might have differed between the serial models. Because of the retrospective nature of the study and referral of some of the patients from outside the community, our 1‐year follow‐up was not complete, but approached a respectable 93%. Other studies have described the benefits derived by a hospitalist practice only following the first year of its implementation, likely because of the hospitalist learning curve.43, 44 This may be why there was no difference in mortality between the standard care and hospitalist groups, as the latter was only in its first year of existence. Additional longitudinal study is required to find out if mortality differences emerge between the treatment groups. Furthermore, although in‐hospital care may influence short‐term outcomes, its effect on long‐term mortality has been unclear. Our data demonstrate that even though a hospitalist service can shorten length of stay and time to surgery, there were no appreciable intermediate differences in mortality at 1 year. Further prospective studies are needed to determine whether this medical‐surgical partnership in caring for these patients provides more favorable outcomes of reducing mortality and intercurrent complications.
Acknowledgements
We thank Donna K. Lawson for her assistance in data collection and management.
- ,,.The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen.Clin Orthop Relat Res1990 (252):163–166.
- ,,.Hip fractures in the elderly: a world‐wide projection.Osteoporos Int.1992;2:285–289.
- ,,,.The economic cost of hip fractures among elderly women. A one‐year, prospective, observational cohort study with matched‐pair analysis.Belgian Hip Fracture Study Group.J Bone Joint Surg Am.2001;83‐A:493–500.
- ,,.Estimating hip fracture morbidity, mortality and costs.J Am Geriatr Soc.2003;51:364–370.
- ,.The aging of America. Impact on health care costs.JAMA.1990;263:2335–2340.
- ,,,.Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation.J Bone Miner Res.1997;12(1):24–35.
- US Department of Health and Human Services.Surveillance for selected public health indicators affecting older adults —United States.MMWR Morb Mortal Wkly Rep1999;48:33–34.
- ,.Epidemiology and outcomes of osteoporotic fractures.Lancet.2002;359:1761–1767.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,.Hip fractures in Finland and Great Britain—a comparison of patient characteristics and outcomes.Int Orthop.2001;25:349–354.
- WHO.International Classification of Disease, Ninth Revision (ICD‐9).Geneva, Switzerland:World Health Organization;1977.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- .Regression models and life‐tables (with discussion).J R Stat Soc Ser B.1972;34:187–220.
- ,.Nonparametric estimation from incomplete observations.J Am Statistical Assoc.1958;53:457–481.
- ,.An Introduction to Recursive Partitioning using the RPART Routines: Section of Biostatistics, Mayo Clinic;1997.
- .Computer Intensive Statistical Methods, Validation, Model Selection, and Bootstrap.London:Chapman and Hall;1994.
- ,.A bootstrap resampling procedure for model building: application to the Cox regression model.Stat Med.1992;11:2093–2109.
- ,,.Associations between the hospitalist model of care and quality‐of‐care‐related outcomes in patients undergoing hip fracture surgery.Mayo Clin Proc.2006;81(1):28–31.
- ,,, et al.Association of timing of surgery for hip fracture and patient outcomes.JAMA.2004;291:1738–1743.
- ,,,,.The effects of time‐to‐surgery on mortality and morbidity in patients following hip fracture.Am J Med.2002;112:702–709.
- ,,,.Morbidity and mortality after hip fracture: the impact of operative delay.Arch Orthop Trauma Surg.2004;124:334–340.
- ,,.Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur.J Bone Joint Surg Br.2005;87:1123–1126.
- ,.The timing of surgery for proximal femoral fractures.J Bone Joint Surg Br.1992;74(2):203–205.
- ,,, et al.Hospital readmissions after hospital discharge for hip fracture: surgical and nonsurgical causes and effect on outcomes.J Am Geriatr Soc.2003;51:399–403.
- ,.Mortality after hip fractures.Acta Orthop Scand1979;50(2):161–167.
- ,,,,.Medical complications and outcomes after hip fracture repair.Arch Intern Med.2002;162:2053–2057.
- ,,, et al.Development and initial validation of a risk score for predicting in‐hospital and 1‐year mortality in patients with hip fractures.J Bone Miner Res.2005;20:494–500.
- ,,,,.Outcome after hip fracture in individuals ninety years of age and older.J Orthop Trauma.2001;15(1):34–39.
- ,,,.Hip fractures in the elderly: predictors of one year mortality.J Orthop Trauma.1997;11(3):162–165.
- ,,,.The effect of hospital type and surgical delay on mortality after surgery for hip fracture.J Bone Joint Surg Br.2005;87:361–366.
- .History of the Rochester Epidemiology Project.Mayo Clin Proc.1996;71:266–274.
- .The mortality and social prognosis of hip fractures. A prospective multifactorial study.Int Orthop.1994;18(2):109–113.
- ,.Functional outcome after hip fracture. A 1‐year prospective outcome study of 275 patients.Injury.2003;34:529–532.
- ,,.Rate of mortality for elderly patients after fracture of the hip in the 1980's.J Bone Joint Surg Am.1987;69:1335–1340.
- ,,,.Hip fractures in the elderly. Mortality, functional results and social readaptation.Int Surg.1989;74(3):191–194.
- ,,.Blood transfusion requirements in femoral neck fracture.Injury.2000;31(1):7–10.
- ,,.Mortality and causes of death after hip fractures in The Netherlands.Neth J Med.1992;41(1–2):4–10.
- ,,.Influence of preoperative medical status and delay to surgery on death following a hip fracture.ANZ J Surg.2002;72:405–407.
- ,,,Predictors of mortality and institutionalization after hip fracture: the New Haven EPESE cohort. Established Populations for Epidemiologic Studies of the Elderly.Am J Public Health.1994;84:1807–1812.
- ,,,.Mortality risk after hip fracture.J Orthop Trauma.2003;17(1):53–56.
- ,,.Thirty‐day mortality following hip arthroplasty for acute fracture.J Bone Joint Surg Am.2004;86‐A:1983–1988.
- ,,,,.Functional outcomes and mortality vary among different types of hip fractures: a function of patient characteristics.Clin Orthop Relat Res.2004:64–71.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
Because the incidence of hip fracture increases dramatically with age and the elderly are the fastest‐growing portion of the United States population, the number of hip fractures is expected to triple by 2040.1 With the associated increase in postoperative morbidity and mortality, the costs will likely exceed $16‐$20 billion annually.15 Already by 2002, the number of patients with hip fractures exceeded 340,000 in this country, resulting in $8.6 billion in health care expenditures from in‐hospital and posthospital costs.68 This makes hip fracture a serious public health concern and triggers a need to devise an efficient means of caring for these patients. We previously reported that a hospitalist service can decrease time to surgery and shorten length of stay without affecting the number of inpatient deaths or 30‐day readmissions of patients undergoing hip fracture surgery.9 However, one concern with reducing length of stay and time to surgery in the high‐risk hip fracture patient population is the effect on long‐term mortality because the death rate following hip fracture repair may be as high as 43% after 1 year.10 To evaluate this important issue, we assessed mortality over a 1‐year period in the same cohort of patients previously described.9 We also identified predictors associated with mortality. We hypothesized that the expedited surgical treatment and decreased length of stay of a hospitalist‐managed group would not have an adverse effect on 1‐year mortality.
METHODS
Patient Selection
Following approval by the Mayo Clinic Institutional Review Board, we used the Mayo Clinic Surgical Index to identify patients admitted between July 1, 2000, and June 30, 2002, who matched International Classification of Diseases (9th Edition) hip fracture codes.11 These patients were cross‐referenced with those having a primary surgical indication of hip fracture. Patients transferred to our facility more than 72 hours after fracture were excluded from our study. Study patients provided authorization to use their medical records for the purposes of research.
A cohort of 466 patients was identified. For purposes of comparison, patients admitted between July 1, 2000, and June 30, 2001, were deemed to belong to the standard care service, and patients admitted between July 1, 2001, and June 30, 2002, were deemed part of the hospitalist service.
Intervention
Prior to July 2001, Mayo Clinic patients aged 65 and older having surgical repair of a hip fracture were triaged directly to a surgical orthopedic or general medical teaching service. Patients with multiple medical diagnoses were managed initially on a medical teaching service prior to transfer to the operating room. The primary team (medical or surgical) was responsible for the postoperative care of the patient and any orders or consultations required.
After July 1, 2001, these patients were admitted by the orthopedic surgery service and medically comanaged by a hospitalist service, which consisted of a hospitalist physician and 2 allied‐health practitioners. Twelve hospitalists and 12 allied health care professionals cared for patients during the study period. All preoperative and postoperative evaluations, inpatient management decisions, and coordination of outpatient care were performed by the hospitalists. This model of care is similar to one previously studied and published elsewhere.12 A census cap of 20 patients limited the number of patients managed by the hospitalist service. Any overflow of hip fracture patients was triaged directly to a non‐hospitalist‐based primary medical or surgical service as before. Thus, 23 hip fracture patients (10%) admitted after July 1, 2001, were not managed by the hospitalists but are included in this group for an intent‐to‐treat analysis.
Data Collection
Study nurses abstracted all data including admitting diagnoses, demographic features, type and mechanism of hip fracture, admission date and time, American Society of Anesthesia (ASA) class, comorbid medical conditions, medications, all clinical data, and readmission rates. Date of last follow‐up was confirmed using the Mayo Clinic medical record, whereas date and cause of death were obtained from death certificates obtained from state and national sources. Length of stay was defined as the number of days between admission and discharge. Time to surgery was defined in hours as the time from hospital admission to the start of the surgery. Finally, time from surgery to dismissal was defined as the number of days from the initiation of the surgical procedure to the time of dismissal. Thirty‐day readmission was defined as readmission to our hospital within 30 days of discharge date.
Statistical Considerations
Power
The power analysis was based on the end point of survival following surgical repair of hip fracture and primary comparison of patients in the standard care group with those in the hospitalist group. With 236 patients in the standard care group, 230 in the hospitalist group, and 274 observed deaths during the follow‐up period, there was 80% power to detect a hazard ratio of 1.4 or greater as being statistically significant (alpha = 0.05, beta = 0.2).
Analysis
The analysis focused on the end point of survival following surgical repair of hip fracture. In addition to the hospitalist versus standard care service, demographic, baseline clinical, and in‐hospital data were evaluated as potential predictors of survival. Survival rates were estimated using the method of Kaplan and Meier, and relative differences in survival were evaluated using the Cox proportional hazards regression models.13, 14 Potential predictors were analyzed both univariately and in a multivariable model. For the multivariable model, initial variable selection was accomplished using stepwise selection, backward elimination, and recursive partitioning.15 Each method yielded similar results. Bootstrap resampling was then used to confirm the variables selected for each model.16, 17 The threshold of statistical significance was set at P = .05 for all tests. All analyses were conducted in SAS version 8.2 (SAS Institute Inc., Cary, NC) and Splus version 6.2.1 (Insightful Corporation, Seattle, WA).
RESULTS
There were 236 patients with hip fractures (50.6%) admitted to the standard care service, and 230 patients (49.4%) admitted to the hospitalist service. As shown in Table 1, the baseline characteristics of the patients admitted to the 2 services did not differ significantly except that a greater proportion of patients with hypoxia were admitted to the hospitalist service (11.3% vs. 5.5%; P = .02). However, time to surgery, postsurgery stay, and overall length of hospitalization of the hospitalist‐treated patients were all significantly shorter.
| Patient characteristic | Standard care n = 236 | Hospitalist care n = 230 | P value | ||
|---|---|---|---|---|---|
| |||||
| Age (years) | 82 | 83 | .34 | ||
| Female sex | 171 | 72.5% | 163 | 70.9% | .70 |
| Comorbidity | |||||
| Coronary artery disease | 69 | 29.2% | 77 | 33.5% | .32 |
| Congestive heart failure | 41 | 17.4% | 49 | 21.3% | .28 |
| Chronic obstructive pulmonary disease | 36 | 15.3% | 38 | 16.5% | .71 |
| Cerebral vascular accident or transient ischemic attack | 36 | 15.3% | 50 | 21.7% | .07 |
| Dementia | 54 | 22.9% | 62 | 27.0% | .31 |
| Diabetes | 45 | 19.1% | 46 | 20.0% | .80 |
| Renal insufficiency | 17 | 7.2% | 17 | 7.4% | .94 |
| Residence at time of admission | .07 | ||||
| Home | 149 | 63.1% | 138 | 60.0% | |
| Assisted living | 32 | 13.6% | 42 | 18.3% | |
| Nursing home | 55 | 23.3% | 50 | 21.7% | |
| Ambulatory status at time of admission | .14 | ||||
| Independent | 114 | 48.3% | 89 | 38.7% | |
| Assistive device | 99 | 41.9% | 115 | 50.0% | |
| Personal help | 9 | 3.8% | 16 | 7.0% | |
| Transfer to bed or chair | 9 | 3.8% | 7 | 3.0% | |
| Nonambulatory | 5 | 2.1% | 3 | 1.3% | |
| Signs at time of admission | |||||
| Hypotension | 4 | 1.7% | 3 | 1.3% | > .99 |
| Hypoxia | 13 | 5.5% | 26 | 11.3% | .02 |
| Pulmonary edema | 37 | 15.7% | 29 | 12.6% | .34 |
| Tachycardia | 19 | 8.1% | 25 | 10.9% | .3 |
| Fracture type | .78 | ||||
| Femoral neck | 118 | 50.0% | 118 | 51.3% | |
| Intertrochanteric | 118 | 50.0% | 112 | 48.7% | |
| Mechanism of fracture | .82 | ||||
| Fall | 219 | 92.8% | 212 | 92.2% | |
| Trauma | 1 | 0.4% | 3 | 1.3% | |
| Pathologic | 7 | 3.0% | 6 | 2.6% | |
| Unknown | 9 | 3.8% | 7 | 3.0% | |
| ASA* class | .38 | ||||
| I or II | 33 | 14.0% | 23 | 10.0% | |
| III | 166 | 70.3% | 166 | 72.2% | |
| IV | 37 | 15.7% | 41 | 17.8% | |
| Location discharged to | .07 | ||||
| Home or assisted living | 24 | 10.5% | 13 | 5.9% | |
| Nursing home | 196 | 86.0% | 192 | 87.3% | |
| Another hospital or hospice | 8 | 3.5% | 15 | 6.8% | |
| Time to surgery (hours) | 38 | 25 | .001 | ||
| Time from surgery to discharge (days) | 9 | 7 | .04 | ||
| Length of stay | 10.6 | 8.4 | < .00 | ||
| Readmission rate | 25 | 10.6% | 20 | 8.7% | .49 |
Patients were followed for a median of 4.0 years (range 5 days to 5.6 years), and 192 patients were still alive at the end of follow‐up (April 2006). As illustrated in Figure 1, survival did not differ between the 2 treatment groups (P = .36). Overall survival at 1 year was 70.6% (95% confidence interval [CI]: 66.5%, 74.9%). Survival at 1 year in the standard care group was 70.6% (95% CI: 64.9%, 76.8%), whereas in the hospitalist group, it was 70.5% (95% CI: 64.8%, 76.7%). As delineated in Table 2, cardiovascular causes accounted for 34 deaths (25.6%), with 14 of these in the standard care group and 20 in the hospitalist group; 29 deaths (21.8%) had respiratory causes, 20 in the standard care group and 9 in the hospitalist group; and 17 (12.8%) were due to cancer, with 7 and 10 in the standard care and hospitalist groups, respectively. Unknown causes accounted for 21 cases, or 15.8% of total deaths.

| Standard care | Hospitalist care | Total No. of deaths | % | |
|---|---|---|---|---|
| Cancer | 7 | 10 | 17 | 12.8% |
| Cardiovascular | 14 | 20 | 34 | 25.6% |
| Infectious | 5 | 4 | 9 | 6.8% |
| Neurological | 5 | 10 | 15 | 11.3% |
| Other | 0 | 2 | 2 | 1.5% |
| Renal | 4 | 2 | 6 | 4.5% |
| Respiratory | 20 | 9 | 29 | 21.8% |
| Unknown | 11 | 10 | 21 | 15.8% |
| Total | 66 | 67 | 133 | 100.0% |
In the univariate analysis, we found 29 variables that were significant predictors of survival (Table 3). A hospitalist model of care was not significantly associated with patient survival, despite the shorter length of stay (8.4 days vs. 10.6 days; P < .001) or expedited time to surgery (25 vs. 38 hours; P < .001), when compared with the standard care group, as previously reported by Phy et al.9 In the multivariable analysis (Table 4), however, the independent predictors of mortality were ASA class III or IV versus class II (hazard ratio [HR] 4.20; 95% CI: 2.21, 7.99), admission from a nursing home versus from home or assisted living (HR 2.24; 95% CI: 1.73, 2.90), and inpatient complications, which included patients requiring admission to the intensive care unit (ICU) and those who had a myocardial infarction or acute renal failure as an inpatient (HR 1.85; 95% CI: 1.45, 2.35). Even after adjusting for these factors, survival following hip fracture did not differ significantly between the hospitalist care patients and the standard care patients (HR 1.16; 95% CI: 0.91, 1.48).
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.41 (1.20, 1.65) | < .001 |
| ASA* II | 1.0 (referent) | |
| ASA* III | 5.27 (2.79, 9.96) | < .001 |
| ASA* IV | 11.7 (5.97, 22.9) | < .001 |
| History of chronic obstructive pulmonary disease | 1.82 (1.35, 2.43) | < .001 |
| History of renal insufficiency | 2.40 (1.62,3.55) | < .001 |
| History of stroke/transient ischemic attack | 1.46 (1.10, 1.95) | .01 |
| History of diabetes | 1.70 (1.29,2.25) | < .001 |
| History of congestive heart failure | 2.26 (1.73, 2.96) | < .001 |
| History of coronary artery disease | 1.53 (1.20, 1.97) | < .001 |
| History of dementia | 2.02 (1.57, 2.59) | < .001 |
| Admission from home | 1.0 (referent) | |
| Admission from assisted living | 1.47 (1.06, 2.04) | .02 |
| Admission from nursing home | 3.04 (2.33, 3.98) | < .001 |
| Independent | 1.0 (referent) | |
| Use of assistive device | 1.81 (1.39, 2.36) | < .001 |
| Personal help | 3.49 (2.16, 5.64) | < .001 |
| Nonambulatory | 3.96 (2.47, 6.35) | < .001 |
| Crackles on admission | 2.03 (1.50, 2.74) | < .001 |
| Hypoxia on admission | 1.56 (1.04, 2.32) | .03 |
| Hypotension on admission | 6.21 (2.72, 14.2) | < .001 |
| Tachycardia on admission | 1.66 (1.15, 2.41) | .007 |
| Coumadin on admission | 1.57 (1.13, 2.18) | .007 |
| Confusion/unconsciousness on admission | 2.23 (1.74, 2.87) | < .001 |
| Fever on admission | 1.98 (1.16, 3.40) | .01 |
| Tachypnea on admission | 1.95 (1.39, 2.72) | < .001 |
| Inpatient myocardial Infarction | 3.59 (2.35, 5.48) | < .001 |
| Inpatient atrial fibrillation | 2.00 (1.37, 2.92) | < .001 |
| Inpatient congestive heart failure | 2.62 (1.79, 3.84) | < .0001 |
| Inpatient delirium | 1.46 (1.13, 1.90) | < .005 |
| Inpatient lung infection | 2.52 (1.85, 3.42) | < .001 |
| Inpatient respiratory failure | 2.76 (1.64, 4.66) | < .001 |
| Inpatient mechanical ventilation | 2.56 (1.43, 4.57) | .002 |
| Inpatient renal failure | 3.60 (1.97, 6.61) | < .001 |
| Days from admission to surgery | 1.06 (1.005, 1.12) | .03 |
| Intensive care unit stay | 1.93 (1.51, 2.47) | < .001 |
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.17 (0.99, 1.38) | .07 |
| ASA* class III or IV | 4.20 (2.21, 7.99) | < .001 |
| ASA* class II | 1.0 (referent) | |
| Admission from nursing home | 2.24 (1.73, 2.90) | < .001 |
| Admission from home or assisted living | 1.0 (referent) | |
| Inpatient myocardial infarction, inpatient acute renal failure, or intensive care unit stay | 1.85 (1.45, 2.35) | < .001 |
| No inpatient myocardial infarction, no inpatient acute renal failure, and no intensive care unit stay | 1.0 (referent) | |
DISCUSSION
In our previous study, length of stay and time to surgery were significantly lower in a hospitalist care model.9 The present study shows that neither the reduced length of stay nor the shortened time to surgery of patients managed by the hospitalist group was associated with a difference in mortality compared with a standard care group, despite significantly improved efficiency and processes of care. Thus, our results refute initial concerns of increased mortality in a hospitalist model of care.
Delivery of perioperative medical care to hip fracture patients by hospitalists is associated with significant decreases in time to surgery and length of stay compared with standard care, with no differences in short‐term mortality.9, 18 Although there have been conflicting reports on the impact of length of stay and time to surgery on long‐term outcomes, our findings support previous results that decreased time to surgery was not associated with an observable effect on mortality.1923 A recent study by Orosz et al. that evaluated 1178 patients showed that earlier hip fracture surgery (performed less than 24 hours after admission) was not associated with reduced mortality, although it was associated with shorter length of stay.19 Our study also corroborates the results of an examination of 8383 hip fracture patients by Grimes et al., who found that time to surgery between 24 and 48 hours after admission had no effect on either 30‐day or long‐term mortality compared with that of those who underwent surgery between 48 and 72 hours, between 72 and 96 hours, or more than 96 hours after admission.20 However, both these results and our own are contrary to those of Gdalevich, whose study of 651 patients found that 1‐year mortality was 1.6‐fold higher for those whose hip fracture repair was postponed more than 48 hours.21 However, time to surgery in both the standard care and hospitalist model in our study was well below the 48‐hour cutoff, suggesting that operating anywhere within the normally accepted 48‐hour time frame may not influence long‐term mortality.
Because of the small number of events in both groups, we were unable to specifically compare whether a hospitalist model of care has any specific impact on long‐term cause of death. Although causes of death of patients with hip fracture were consistent with those of previous studies,10, 24 our death rate at 1 year, 29.4%, was higher than that seen among similar population groups at tertiary referral centers.19, 20, 2429 This is most likely a result of the cohort having a high proportion of nursing home patients (22%)19, 24, 26 transferred for evaluation to St. Mary's Hospital, which serves most of Olmsted County, Minnesota. This hospital also has some characteristics of a community‐based hospital, as it is where greater than 95% of all county patients receive care for surgical repair of hip fracture. Mortality rates are often higher at these types of hospitals.30 Previous studies using patients from Olmsted County indicate results can also be extrapolated to a large part of the U.S. population.31 In Pitto et al.'s study, the risk of death was 31% lower in those admitted from home than for those admitted from a nursing home.32 The latter patients normally have a higher number of comorbid conditions and tend to be less ambulatory than those in a community home‐dwelling setting. Our study also demonstrated that admission from a nursing home was a strong predictor of mortality for up to 1 year in the geriatric population. This may reflect the inherent decreased survival in this patient group, which is in agreement with the findings of other studies that showed inactivity and decreased ambulation prior to fracture were associated with increased mortality.3335
Multiple comorbidities, commonly seen in a geriatric population, translate into a higher ASA class and an increased risk of significant in‐hospital complications. Our study confirmed the findings of previous studies that a higher ASA class is a strong predictor of mortality,21, 26, 30, 3537 independent of decreased time to surgery.38 We also noted that significant in‐hospital complications, including renal failure, respiratory failure, and myocardial infarction, are documented predictors of mortality after hip fracture.27 Although mortality may vary depending on fracture type (femoral neck vs. intertrochanteric),3941 these differences were not observed in our study, in line with the results of previous published studies.37, 42 Controlling for age and comorbidities may be why an association was not found between fracture type and mortality. Finally, in a model containing comorbidity, ASA class, and nursing home residence prior to fracture, age was not a significant predictor of mortality.
Our study had a number of limitations. First, this was a retrospective cohort study based on chart review, so some data may have been subject to recording bias, and this might have differed between the serial models. Because of the retrospective nature of the study and referral of some of the patients from outside the community, our 1‐year follow‐up was not complete, but approached a respectable 93%. Other studies have described the benefits derived by a hospitalist practice only following the first year of its implementation, likely because of the hospitalist learning curve.43, 44 This may be why there was no difference in mortality between the standard care and hospitalist groups, as the latter was only in its first year of existence. Additional longitudinal study is required to find out if mortality differences emerge between the treatment groups. Furthermore, although in‐hospital care may influence short‐term outcomes, its effect on long‐term mortality has been unclear. Our data demonstrate that even though a hospitalist service can shorten length of stay and time to surgery, there were no appreciable intermediate differences in mortality at 1 year. Further prospective studies are needed to determine whether this medical‐surgical partnership in caring for these patients provides more favorable outcomes of reducing mortality and intercurrent complications.
Acknowledgements
We thank Donna K. Lawson for her assistance in data collection and management.
Because the incidence of hip fracture increases dramatically with age and the elderly are the fastest‐growing portion of the United States population, the number of hip fractures is expected to triple by 2040.1 With the associated increase in postoperative morbidity and mortality, the costs will likely exceed $16‐$20 billion annually.15 Already by 2002, the number of patients with hip fractures exceeded 340,000 in this country, resulting in $8.6 billion in health care expenditures from in‐hospital and posthospital costs.68 This makes hip fracture a serious public health concern and triggers a need to devise an efficient means of caring for these patients. We previously reported that a hospitalist service can decrease time to surgery and shorten length of stay without affecting the number of inpatient deaths or 30‐day readmissions of patients undergoing hip fracture surgery.9 However, one concern with reducing length of stay and time to surgery in the high‐risk hip fracture patient population is the effect on long‐term mortality because the death rate following hip fracture repair may be as high as 43% after 1 year.10 To evaluate this important issue, we assessed mortality over a 1‐year period in the same cohort of patients previously described.9 We also identified predictors associated with mortality. We hypothesized that the expedited surgical treatment and decreased length of stay of a hospitalist‐managed group would not have an adverse effect on 1‐year mortality.
METHODS
Patient Selection
Following approval by the Mayo Clinic Institutional Review Board, we used the Mayo Clinic Surgical Index to identify patients admitted between July 1, 2000, and June 30, 2002, who matched International Classification of Diseases (9th Edition) hip fracture codes.11 These patients were cross‐referenced with those having a primary surgical indication of hip fracture. Patients transferred to our facility more than 72 hours after fracture were excluded from our study. Study patients provided authorization to use their medical records for the purposes of research.
A cohort of 466 patients was identified. For purposes of comparison, patients admitted between July 1, 2000, and June 30, 2001, were deemed to belong to the standard care service, and patients admitted between July 1, 2001, and June 30, 2002, were deemed part of the hospitalist service.
Intervention
Prior to July 2001, Mayo Clinic patients aged 65 and older having surgical repair of a hip fracture were triaged directly to a surgical orthopedic or general medical teaching service. Patients with multiple medical diagnoses were managed initially on a medical teaching service prior to transfer to the operating room. The primary team (medical or surgical) was responsible for the postoperative care of the patient and any orders or consultations required.
After July 1, 2001, these patients were admitted by the orthopedic surgery service and medically comanaged by a hospitalist service, which consisted of a hospitalist physician and 2 allied‐health practitioners. Twelve hospitalists and 12 allied health care professionals cared for patients during the study period. All preoperative and postoperative evaluations, inpatient management decisions, and coordination of outpatient care were performed by the hospitalists. This model of care is similar to one previously studied and published elsewhere.12 A census cap of 20 patients limited the number of patients managed by the hospitalist service. Any overflow of hip fracture patients was triaged directly to a non‐hospitalist‐based primary medical or surgical service as before. Thus, 23 hip fracture patients (10%) admitted after July 1, 2001, were not managed by the hospitalists but are included in this group for an intent‐to‐treat analysis.
Data Collection
Study nurses abstracted all data including admitting diagnoses, demographic features, type and mechanism of hip fracture, admission date and time, American Society of Anesthesia (ASA) class, comorbid medical conditions, medications, all clinical data, and readmission rates. Date of last follow‐up was confirmed using the Mayo Clinic medical record, whereas date and cause of death were obtained from death certificates obtained from state and national sources. Length of stay was defined as the number of days between admission and discharge. Time to surgery was defined in hours as the time from hospital admission to the start of the surgery. Finally, time from surgery to dismissal was defined as the number of days from the initiation of the surgical procedure to the time of dismissal. Thirty‐day readmission was defined as readmission to our hospital within 30 days of discharge date.
Statistical Considerations
Power
The power analysis was based on the end point of survival following surgical repair of hip fracture and primary comparison of patients in the standard care group with those in the hospitalist group. With 236 patients in the standard care group, 230 in the hospitalist group, and 274 observed deaths during the follow‐up period, there was 80% power to detect a hazard ratio of 1.4 or greater as being statistically significant (alpha = 0.05, beta = 0.2).
Analysis
The analysis focused on the end point of survival following surgical repair of hip fracture. In addition to the hospitalist versus standard care service, demographic, baseline clinical, and in‐hospital data were evaluated as potential predictors of survival. Survival rates were estimated using the method of Kaplan and Meier, and relative differences in survival were evaluated using the Cox proportional hazards regression models.13, 14 Potential predictors were analyzed both univariately and in a multivariable model. For the multivariable model, initial variable selection was accomplished using stepwise selection, backward elimination, and recursive partitioning.15 Each method yielded similar results. Bootstrap resampling was then used to confirm the variables selected for each model.16, 17 The threshold of statistical significance was set at P = .05 for all tests. All analyses were conducted in SAS version 8.2 (SAS Institute Inc., Cary, NC) and Splus version 6.2.1 (Insightful Corporation, Seattle, WA).
RESULTS
There were 236 patients with hip fractures (50.6%) admitted to the standard care service, and 230 patients (49.4%) admitted to the hospitalist service. As shown in Table 1, the baseline characteristics of the patients admitted to the 2 services did not differ significantly except that a greater proportion of patients with hypoxia were admitted to the hospitalist service (11.3% vs. 5.5%; P = .02). However, time to surgery, postsurgery stay, and overall length of hospitalization of the hospitalist‐treated patients were all significantly shorter.
| Patient characteristic | Standard care n = 236 | Hospitalist care n = 230 | P value | ||
|---|---|---|---|---|---|
| |||||
| Age (years) | 82 | 83 | .34 | ||
| Female sex | 171 | 72.5% | 163 | 70.9% | .70 |
| Comorbidity | |||||
| Coronary artery disease | 69 | 29.2% | 77 | 33.5% | .32 |
| Congestive heart failure | 41 | 17.4% | 49 | 21.3% | .28 |
| Chronic obstructive pulmonary disease | 36 | 15.3% | 38 | 16.5% | .71 |
| Cerebral vascular accident or transient ischemic attack | 36 | 15.3% | 50 | 21.7% | .07 |
| Dementia | 54 | 22.9% | 62 | 27.0% | .31 |
| Diabetes | 45 | 19.1% | 46 | 20.0% | .80 |
| Renal insufficiency | 17 | 7.2% | 17 | 7.4% | .94 |
| Residence at time of admission | .07 | ||||
| Home | 149 | 63.1% | 138 | 60.0% | |
| Assisted living | 32 | 13.6% | 42 | 18.3% | |
| Nursing home | 55 | 23.3% | 50 | 21.7% | |
| Ambulatory status at time of admission | .14 | ||||
| Independent | 114 | 48.3% | 89 | 38.7% | |
| Assistive device | 99 | 41.9% | 115 | 50.0% | |
| Personal help | 9 | 3.8% | 16 | 7.0% | |
| Transfer to bed or chair | 9 | 3.8% | 7 | 3.0% | |
| Nonambulatory | 5 | 2.1% | 3 | 1.3% | |
| Signs at time of admission | |||||
| Hypotension | 4 | 1.7% | 3 | 1.3% | > .99 |
| Hypoxia | 13 | 5.5% | 26 | 11.3% | .02 |
| Pulmonary edema | 37 | 15.7% | 29 | 12.6% | .34 |
| Tachycardia | 19 | 8.1% | 25 | 10.9% | .3 |
| Fracture type | .78 | ||||
| Femoral neck | 118 | 50.0% | 118 | 51.3% | |
| Intertrochanteric | 118 | 50.0% | 112 | 48.7% | |
| Mechanism of fracture | .82 | ||||
| Fall | 219 | 92.8% | 212 | 92.2% | |
| Trauma | 1 | 0.4% | 3 | 1.3% | |
| Pathologic | 7 | 3.0% | 6 | 2.6% | |
| Unknown | 9 | 3.8% | 7 | 3.0% | |
| ASA* class | .38 | ||||
| I or II | 33 | 14.0% | 23 | 10.0% | |
| III | 166 | 70.3% | 166 | 72.2% | |
| IV | 37 | 15.7% | 41 | 17.8% | |
| Location discharged to | .07 | ||||
| Home or assisted living | 24 | 10.5% | 13 | 5.9% | |
| Nursing home | 196 | 86.0% | 192 | 87.3% | |
| Another hospital or hospice | 8 | 3.5% | 15 | 6.8% | |
| Time to surgery (hours) | 38 | 25 | .001 | ||
| Time from surgery to discharge (days) | 9 | 7 | .04 | ||
| Length of stay | 10.6 | 8.4 | < .00 | ||
| Readmission rate | 25 | 10.6% | 20 | 8.7% | .49 |
Patients were followed for a median of 4.0 years (range 5 days to 5.6 years), and 192 patients were still alive at the end of follow‐up (April 2006). As illustrated in Figure 1, survival did not differ between the 2 treatment groups (P = .36). Overall survival at 1 year was 70.6% (95% confidence interval [CI]: 66.5%, 74.9%). Survival at 1 year in the standard care group was 70.6% (95% CI: 64.9%, 76.8%), whereas in the hospitalist group, it was 70.5% (95% CI: 64.8%, 76.7%). As delineated in Table 2, cardiovascular causes accounted for 34 deaths (25.6%), with 14 of these in the standard care group and 20 in the hospitalist group; 29 deaths (21.8%) had respiratory causes, 20 in the standard care group and 9 in the hospitalist group; and 17 (12.8%) were due to cancer, with 7 and 10 in the standard care and hospitalist groups, respectively. Unknown causes accounted for 21 cases, or 15.8% of total deaths.

| Standard care | Hospitalist care | Total No. of deaths | % | |
|---|---|---|---|---|
| Cancer | 7 | 10 | 17 | 12.8% |
| Cardiovascular | 14 | 20 | 34 | 25.6% |
| Infectious | 5 | 4 | 9 | 6.8% |
| Neurological | 5 | 10 | 15 | 11.3% |
| Other | 0 | 2 | 2 | 1.5% |
| Renal | 4 | 2 | 6 | 4.5% |
| Respiratory | 20 | 9 | 29 | 21.8% |
| Unknown | 11 | 10 | 21 | 15.8% |
| Total | 66 | 67 | 133 | 100.0% |
In the univariate analysis, we found 29 variables that were significant predictors of survival (Table 3). A hospitalist model of care was not significantly associated with patient survival, despite the shorter length of stay (8.4 days vs. 10.6 days; P < .001) or expedited time to surgery (25 vs. 38 hours; P < .001), when compared with the standard care group, as previously reported by Phy et al.9 In the multivariable analysis (Table 4), however, the independent predictors of mortality were ASA class III or IV versus class II (hazard ratio [HR] 4.20; 95% CI: 2.21, 7.99), admission from a nursing home versus from home or assisted living (HR 2.24; 95% CI: 1.73, 2.90), and inpatient complications, which included patients requiring admission to the intensive care unit (ICU) and those who had a myocardial infarction or acute renal failure as an inpatient (HR 1.85; 95% CI: 1.45, 2.35). Even after adjusting for these factors, survival following hip fracture did not differ significantly between the hospitalist care patients and the standard care patients (HR 1.16; 95% CI: 0.91, 1.48).
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.41 (1.20, 1.65) | < .001 |
| ASA* II | 1.0 (referent) | |
| ASA* III | 5.27 (2.79, 9.96) | < .001 |
| ASA* IV | 11.7 (5.97, 22.9) | < .001 |
| History of chronic obstructive pulmonary disease | 1.82 (1.35, 2.43) | < .001 |
| History of renal insufficiency | 2.40 (1.62,3.55) | < .001 |
| History of stroke/transient ischemic attack | 1.46 (1.10, 1.95) | .01 |
| History of diabetes | 1.70 (1.29,2.25) | < .001 |
| History of congestive heart failure | 2.26 (1.73, 2.96) | < .001 |
| History of coronary artery disease | 1.53 (1.20, 1.97) | < .001 |
| History of dementia | 2.02 (1.57, 2.59) | < .001 |
| Admission from home | 1.0 (referent) | |
| Admission from assisted living | 1.47 (1.06, 2.04) | .02 |
| Admission from nursing home | 3.04 (2.33, 3.98) | < .001 |
| Independent | 1.0 (referent) | |
| Use of assistive device | 1.81 (1.39, 2.36) | < .001 |
| Personal help | 3.49 (2.16, 5.64) | < .001 |
| Nonambulatory | 3.96 (2.47, 6.35) | < .001 |
| Crackles on admission | 2.03 (1.50, 2.74) | < .001 |
| Hypoxia on admission | 1.56 (1.04, 2.32) | .03 |
| Hypotension on admission | 6.21 (2.72, 14.2) | < .001 |
| Tachycardia on admission | 1.66 (1.15, 2.41) | .007 |
| Coumadin on admission | 1.57 (1.13, 2.18) | .007 |
| Confusion/unconsciousness on admission | 2.23 (1.74, 2.87) | < .001 |
| Fever on admission | 1.98 (1.16, 3.40) | .01 |
| Tachypnea on admission | 1.95 (1.39, 2.72) | < .001 |
| Inpatient myocardial Infarction | 3.59 (2.35, 5.48) | < .001 |
| Inpatient atrial fibrillation | 2.00 (1.37, 2.92) | < .001 |
| Inpatient congestive heart failure | 2.62 (1.79, 3.84) | < .0001 |
| Inpatient delirium | 1.46 (1.13, 1.90) | < .005 |
| Inpatient lung infection | 2.52 (1.85, 3.42) | < .001 |
| Inpatient respiratory failure | 2.76 (1.64, 4.66) | < .001 |
| Inpatient mechanical ventilation | 2.56 (1.43, 4.57) | .002 |
| Inpatient renal failure | 3.60 (1.97, 6.61) | < .001 |
| Days from admission to surgery | 1.06 (1.005, 1.12) | .03 |
| Intensive care unit stay | 1.93 (1.51, 2.47) | < .001 |
| Variable | Hazard ratio (95% CI) | P value |
|---|---|---|
| ||
| Age on admission per 10 years | 1.17 (0.99, 1.38) | .07 |
| ASA* class III or IV | 4.20 (2.21, 7.99) | < .001 |
| ASA* class II | 1.0 (referent) | |
| Admission from nursing home | 2.24 (1.73, 2.90) | < .001 |
| Admission from home or assisted living | 1.0 (referent) | |
| Inpatient myocardial infarction, inpatient acute renal failure, or intensive care unit stay | 1.85 (1.45, 2.35) | < .001 |
| No inpatient myocardial infarction, no inpatient acute renal failure, and no intensive care unit stay | 1.0 (referent) | |
DISCUSSION
In our previous study, length of stay and time to surgery were significantly lower in a hospitalist care model.9 The present study shows that neither the reduced length of stay nor the shortened time to surgery of patients managed by the hospitalist group was associated with a difference in mortality compared with a standard care group, despite significantly improved efficiency and processes of care. Thus, our results refute initial concerns of increased mortality in a hospitalist model of care.
Delivery of perioperative medical care to hip fracture patients by hospitalists is associated with significant decreases in time to surgery and length of stay compared with standard care, with no differences in short‐term mortality.9, 18 Although there have been conflicting reports on the impact of length of stay and time to surgery on long‐term outcomes, our findings support previous results that decreased time to surgery was not associated with an observable effect on mortality.1923 A recent study by Orosz et al. that evaluated 1178 patients showed that earlier hip fracture surgery (performed less than 24 hours after admission) was not associated with reduced mortality, although it was associated with shorter length of stay.19 Our study also corroborates the results of an examination of 8383 hip fracture patients by Grimes et al., who found that time to surgery between 24 and 48 hours after admission had no effect on either 30‐day or long‐term mortality compared with that of those who underwent surgery between 48 and 72 hours, between 72 and 96 hours, or more than 96 hours after admission.20 However, both these results and our own are contrary to those of Gdalevich, whose study of 651 patients found that 1‐year mortality was 1.6‐fold higher for those whose hip fracture repair was postponed more than 48 hours.21 However, time to surgery in both the standard care and hospitalist model in our study was well below the 48‐hour cutoff, suggesting that operating anywhere within the normally accepted 48‐hour time frame may not influence long‐term mortality.
Because of the small number of events in both groups, we were unable to specifically compare whether a hospitalist model of care has any specific impact on long‐term cause of death. Although causes of death of patients with hip fracture were consistent with those of previous studies,10, 24 our death rate at 1 year, 29.4%, was higher than that seen among similar population groups at tertiary referral centers.19, 20, 2429 This is most likely a result of the cohort having a high proportion of nursing home patients (22%)19, 24, 26 transferred for evaluation to St. Mary's Hospital, which serves most of Olmsted County, Minnesota. This hospital also has some characteristics of a community‐based hospital, as it is where greater than 95% of all county patients receive care for surgical repair of hip fracture. Mortality rates are often higher at these types of hospitals.30 Previous studies using patients from Olmsted County indicate results can also be extrapolated to a large part of the U.S. population.31 In Pitto et al.'s study, the risk of death was 31% lower in those admitted from home than for those admitted from a nursing home.32 The latter patients normally have a higher number of comorbid conditions and tend to be less ambulatory than those in a community home‐dwelling setting. Our study also demonstrated that admission from a nursing home was a strong predictor of mortality for up to 1 year in the geriatric population. This may reflect the inherent decreased survival in this patient group, which is in agreement with the findings of other studies that showed inactivity and decreased ambulation prior to fracture were associated with increased mortality.3335
Multiple comorbidities, commonly seen in a geriatric population, translate into a higher ASA class and an increased risk of significant in‐hospital complications. Our study confirmed the findings of previous studies that a higher ASA class is a strong predictor of mortality,21, 26, 30, 3537 independent of decreased time to surgery.38 We also noted that significant in‐hospital complications, including renal failure, respiratory failure, and myocardial infarction, are documented predictors of mortality after hip fracture.27 Although mortality may vary depending on fracture type (femoral neck vs. intertrochanteric),3941 these differences were not observed in our study, in line with the results of previous published studies.37, 42 Controlling for age and comorbidities may be why an association was not found between fracture type and mortality. Finally, in a model containing comorbidity, ASA class, and nursing home residence prior to fracture, age was not a significant predictor of mortality.
Our study had a number of limitations. First, this was a retrospective cohort study based on chart review, so some data may have been subject to recording bias, and this might have differed between the serial models. Because of the retrospective nature of the study and referral of some of the patients from outside the community, our 1‐year follow‐up was not complete, but approached a respectable 93%. Other studies have described the benefits derived by a hospitalist practice only following the first year of its implementation, likely because of the hospitalist learning curve.43, 44 This may be why there was no difference in mortality between the standard care and hospitalist groups, as the latter was only in its first year of existence. Additional longitudinal study is required to find out if mortality differences emerge between the treatment groups. Furthermore, although in‐hospital care may influence short‐term outcomes, its effect on long‐term mortality has been unclear. Our data demonstrate that even though a hospitalist service can shorten length of stay and time to surgery, there were no appreciable intermediate differences in mortality at 1 year. Further prospective studies are needed to determine whether this medical‐surgical partnership in caring for these patients provides more favorable outcomes of reducing mortality and intercurrent complications.
Acknowledgements
We thank Donna K. Lawson for her assistance in data collection and management.
- ,,.The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen.Clin Orthop Relat Res1990 (252):163–166.
- ,,.Hip fractures in the elderly: a world‐wide projection.Osteoporos Int.1992;2:285–289.
- ,,,.The economic cost of hip fractures among elderly women. A one‐year, prospective, observational cohort study with matched‐pair analysis.Belgian Hip Fracture Study Group.J Bone Joint Surg Am.2001;83‐A:493–500.
- ,,.Estimating hip fracture morbidity, mortality and costs.J Am Geriatr Soc.2003;51:364–370.
- ,.The aging of America. Impact on health care costs.JAMA.1990;263:2335–2340.
- ,,,.Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation.J Bone Miner Res.1997;12(1):24–35.
- US Department of Health and Human Services.Surveillance for selected public health indicators affecting older adults —United States.MMWR Morb Mortal Wkly Rep1999;48:33–34.
- ,.Epidemiology and outcomes of osteoporotic fractures.Lancet.2002;359:1761–1767.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,.Hip fractures in Finland and Great Britain—a comparison of patient characteristics and outcomes.Int Orthop.2001;25:349–354.
- WHO.International Classification of Disease, Ninth Revision (ICD‐9).Geneva, Switzerland:World Health Organization;1977.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- .Regression models and life‐tables (with discussion).J R Stat Soc Ser B.1972;34:187–220.
- ,.Nonparametric estimation from incomplete observations.J Am Statistical Assoc.1958;53:457–481.
- ,.An Introduction to Recursive Partitioning using the RPART Routines: Section of Biostatistics, Mayo Clinic;1997.
- .Computer Intensive Statistical Methods, Validation, Model Selection, and Bootstrap.London:Chapman and Hall;1994.
- ,.A bootstrap resampling procedure for model building: application to the Cox regression model.Stat Med.1992;11:2093–2109.
- ,,.Associations between the hospitalist model of care and quality‐of‐care‐related outcomes in patients undergoing hip fracture surgery.Mayo Clin Proc.2006;81(1):28–31.
- ,,, et al.Association of timing of surgery for hip fracture and patient outcomes.JAMA.2004;291:1738–1743.
- ,,,,.The effects of time‐to‐surgery on mortality and morbidity in patients following hip fracture.Am J Med.2002;112:702–709.
- ,,,.Morbidity and mortality after hip fracture: the impact of operative delay.Arch Orthop Trauma Surg.2004;124:334–340.
- ,,.Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur.J Bone Joint Surg Br.2005;87:1123–1126.
- ,.The timing of surgery for proximal femoral fractures.J Bone Joint Surg Br.1992;74(2):203–205.
- ,,, et al.Hospital readmissions after hospital discharge for hip fracture: surgical and nonsurgical causes and effect on outcomes.J Am Geriatr Soc.2003;51:399–403.
- ,.Mortality after hip fractures.Acta Orthop Scand1979;50(2):161–167.
- ,,,,.Medical complications and outcomes after hip fracture repair.Arch Intern Med.2002;162:2053–2057.
- ,,, et al.Development and initial validation of a risk score for predicting in‐hospital and 1‐year mortality in patients with hip fractures.J Bone Miner Res.2005;20:494–500.
- ,,,,.Outcome after hip fracture in individuals ninety years of age and older.J Orthop Trauma.2001;15(1):34–39.
- ,,,.Hip fractures in the elderly: predictors of one year mortality.J Orthop Trauma.1997;11(3):162–165.
- ,,,.The effect of hospital type and surgical delay on mortality after surgery for hip fracture.J Bone Joint Surg Br.2005;87:361–366.
- .History of the Rochester Epidemiology Project.Mayo Clin Proc.1996;71:266–274.
- .The mortality and social prognosis of hip fractures. A prospective multifactorial study.Int Orthop.1994;18(2):109–113.
- ,.Functional outcome after hip fracture. A 1‐year prospective outcome study of 275 patients.Injury.2003;34:529–532.
- ,,.Rate of mortality for elderly patients after fracture of the hip in the 1980's.J Bone Joint Surg Am.1987;69:1335–1340.
- ,,,.Hip fractures in the elderly. Mortality, functional results and social readaptation.Int Surg.1989;74(3):191–194.
- ,,.Blood transfusion requirements in femoral neck fracture.Injury.2000;31(1):7–10.
- ,,.Mortality and causes of death after hip fractures in The Netherlands.Neth J Med.1992;41(1–2):4–10.
- ,,.Influence of preoperative medical status and delay to surgery on death following a hip fracture.ANZ J Surg.2002;72:405–407.
- ,,,Predictors of mortality and institutionalization after hip fracture: the New Haven EPESE cohort. Established Populations for Epidemiologic Studies of the Elderly.Am J Public Health.1994;84:1807–1812.
- ,,,.Mortality risk after hip fracture.J Orthop Trauma.2003;17(1):53–56.
- ,,.Thirty‐day mortality following hip arthroplasty for acute fracture.J Bone Joint Surg Am.2004;86‐A:1983–1988.
- ,,,,.Functional outcomes and mortality vary among different types of hip fractures: a function of patient characteristics.Clin Orthop Relat Res.2004:64–71.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
- ,,.The future of hip fractures in the United States. Numbers, costs, and potential effects of postmenopausal estrogen.Clin Orthop Relat Res1990 (252):163–166.
- ,,.Hip fractures in the elderly: a world‐wide projection.Osteoporos Int.1992;2:285–289.
- ,,,.The economic cost of hip fractures among elderly women. A one‐year, prospective, observational cohort study with matched‐pair analysis.Belgian Hip Fracture Study Group.J Bone Joint Surg Am.2001;83‐A:493–500.
- ,,.Estimating hip fracture morbidity, mortality and costs.J Am Geriatr Soc.2003;51:364–370.
- ,.The aging of America. Impact on health care costs.JAMA.1990;263:2335–2340.
- ,,,.Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation.J Bone Miner Res.1997;12(1):24–35.
- US Department of Health and Human Services.Surveillance for selected public health indicators affecting older adults —United States.MMWR Morb Mortal Wkly Rep1999;48:33–34.
- ,.Epidemiology and outcomes of osteoporotic fractures.Lancet.2002;359:1761–1767.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,.Hip fractures in Finland and Great Britain—a comparison of patient characteristics and outcomes.Int Orthop.2001;25:349–354.
- WHO.International Classification of Disease, Ninth Revision (ICD‐9).Geneva, Switzerland:World Health Organization;1977.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- .Regression models and life‐tables (with discussion).J R Stat Soc Ser B.1972;34:187–220.
- ,.Nonparametric estimation from incomplete observations.J Am Statistical Assoc.1958;53:457–481.
- ,.An Introduction to Recursive Partitioning using the RPART Routines: Section of Biostatistics, Mayo Clinic;1997.
- .Computer Intensive Statistical Methods, Validation, Model Selection, and Bootstrap.London:Chapman and Hall;1994.
- ,.A bootstrap resampling procedure for model building: application to the Cox regression model.Stat Med.1992;11:2093–2109.
- ,,.Associations between the hospitalist model of care and quality‐of‐care‐related outcomes in patients undergoing hip fracture surgery.Mayo Clin Proc.2006;81(1):28–31.
- ,,, et al.Association of timing of surgery for hip fracture and patient outcomes.JAMA.2004;291:1738–1743.
- ,,,,.The effects of time‐to‐surgery on mortality and morbidity in patients following hip fracture.Am J Med.2002;112:702–709.
- ,,,.Morbidity and mortality after hip fracture: the impact of operative delay.Arch Orthop Trauma Surg.2004;124:334–340.
- ,,.Delay to surgery prolongs hospital stay in patients with fractures of the proximal femur.J Bone Joint Surg Br.2005;87:1123–1126.
- ,.The timing of surgery for proximal femoral fractures.J Bone Joint Surg Br.1992;74(2):203–205.
- ,,, et al.Hospital readmissions after hospital discharge for hip fracture: surgical and nonsurgical causes and effect on outcomes.J Am Geriatr Soc.2003;51:399–403.
- ,.Mortality after hip fractures.Acta Orthop Scand1979;50(2):161–167.
- ,,,,.Medical complications and outcomes after hip fracture repair.Arch Intern Med.2002;162:2053–2057.
- ,,, et al.Development and initial validation of a risk score for predicting in‐hospital and 1‐year mortality in patients with hip fractures.J Bone Miner Res.2005;20:494–500.
- ,,,,.Outcome after hip fracture in individuals ninety years of age and older.J Orthop Trauma.2001;15(1):34–39.
- ,,,.Hip fractures in the elderly: predictors of one year mortality.J Orthop Trauma.1997;11(3):162–165.
- ,,,.The effect of hospital type and surgical delay on mortality after surgery for hip fracture.J Bone Joint Surg Br.2005;87:361–366.
- .History of the Rochester Epidemiology Project.Mayo Clin Proc.1996;71:266–274.
- .The mortality and social prognosis of hip fractures. A prospective multifactorial study.Int Orthop.1994;18(2):109–113.
- ,.Functional outcome after hip fracture. A 1‐year prospective outcome study of 275 patients.Injury.2003;34:529–532.
- ,,.Rate of mortality for elderly patients after fracture of the hip in the 1980's.J Bone Joint Surg Am.1987;69:1335–1340.
- ,,,.Hip fractures in the elderly. Mortality, functional results and social readaptation.Int Surg.1989;74(3):191–194.
- ,,.Blood transfusion requirements in femoral neck fracture.Injury.2000;31(1):7–10.
- ,,.Mortality and causes of death after hip fractures in The Netherlands.Neth J Med.1992;41(1–2):4–10.
- ,,.Influence of preoperative medical status and delay to surgery on death following a hip fracture.ANZ J Surg.2002;72:405–407.
- ,,,Predictors of mortality and institutionalization after hip fracture: the New Haven EPESE cohort. Established Populations for Epidemiologic Studies of the Elderly.Am J Public Health.1994;84:1807–1812.
- ,,,.Mortality risk after hip fracture.J Orthop Trauma.2003;17(1):53–56.
- ,,.Thirty‐day mortality following hip arthroplasty for acute fracture.J Bone Joint Surg Am.2004;86‐A:1983–1988.
- ,,,,.Functional outcomes and mortality vary among different types of hip fractures: a function of patient characteristics.Clin Orthop Relat Res.2004:64–71.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Intern Med.2002;137:866–874.
Copyright © 2007 Society of Hospital Medicine