User login
Nailing the Nail Biopsy: Surgical Instruments and Their Function in Nail Biopsy Procedures
Practice Gap
The term nail biopsy (NB) may refer to a punch, excisional, shave, or longitudinal biopsy of the nail matrix and/or nail bed.1 Nail surgeries, including NBs, are performed relatively infrequently. In a study using data from the Medicare Provider Utilization and Payment Database 2012-2017, only 1.01% of Mohs surgeons and 0.28% of general dermatologists in the United States performed NBs. Thirty-one states had no dermatologist-performed NBs, while 3 states had no nail biopsies performed by any physician, podiatrist, nurse practitioner, or physician assistant, indicating that there is a shortage of dermatology clinicians performing nail surgeries.2
Dermatologists may not be performing NBs due to unfamiliarity with nail unit anatomy and lack of formal NB training during residency.3 In a survey of 240 dermatology residents in the United States, 58% reported performing fewer than 10 nail procedures during residency, with 25% observing only.4 Of those surveyed, 1% had no exposure to nail procedures during 3 years of residency. Furthermore, when asked to assess their competency in nail surgery on a scale of not competent, competent, and very competent, approximately 30% responded that they were not competent.4 Without sufficient education on procedures involving the nail unit, residents may be reluctant to incorporate nail surgery into their clinical practice.
Due to their complexity, NBs require the use of several specialized surgical instruments that are not used for other dermatologic procedures, and residents and attending physicians who have limited nail training may be unfamiliar with these tools. To address this educational gap, we sought to create a guide that details the surgical instruments used for the nail matrix tangential excision (shave) biopsy technique—the most common technique used in our nail specialty clinic. This guide is intended for educational use by dermatologists who wish to incorporate NB as part of their practice.
Tools and Technique
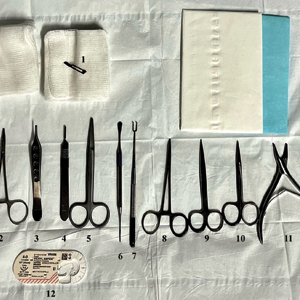
As a major referral center, our New York City–based nail specialty clinic performs a large volume of NBs, many of them performed for clinically concerning longitudinal melanonychias for which a nail matrix shave biopsy most often is performed. We utilize a standardized tray consisting of 12 surgical instruments that are needed to successfully perform a NB from start to finish (Figure). In addition to standard surgical tray items, such as sutures and tissue scissors, additional specialized instruments are necessary for NB procedures, including a nail elevator, an English nail splitter, and skin hook.
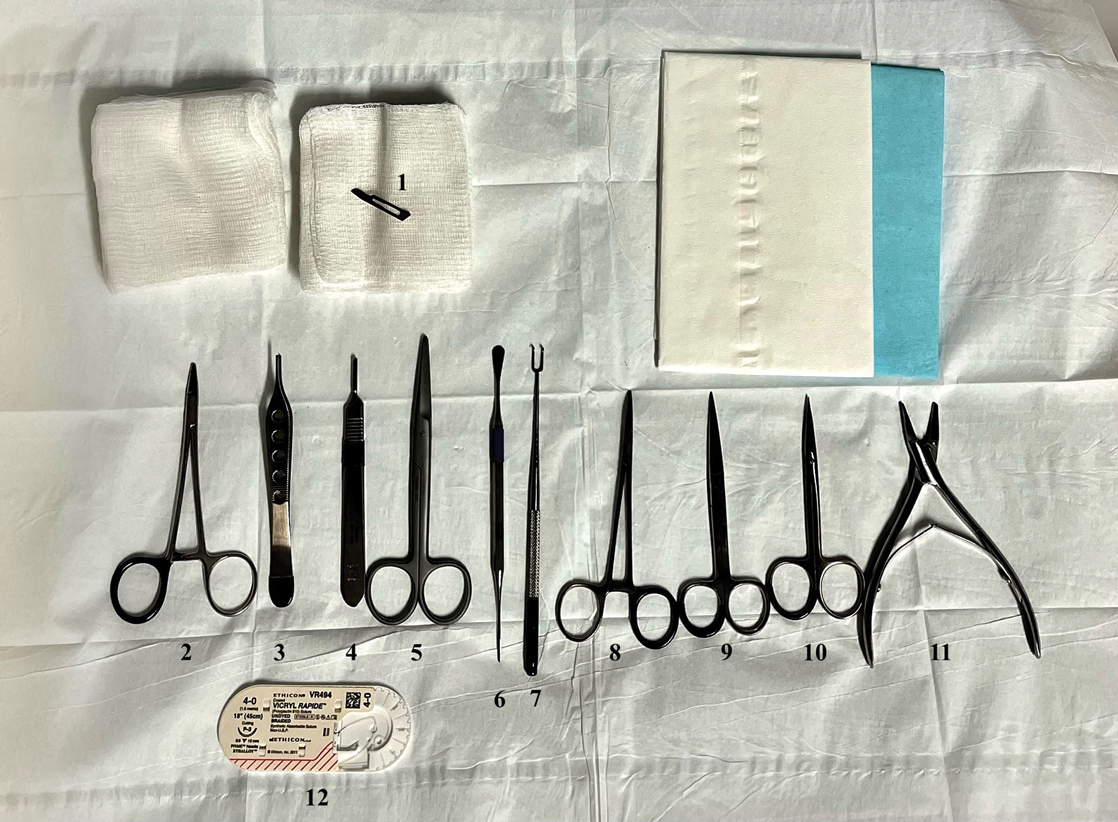
After the initial incisions are made at 45° angles to the proximal nail fold surrounding the longitudinal band, the nail elevator is used to separate the proximal nail plate from the underlying nail bed. The English nail splitter is used to create a transverse split separating the proximal from the distal nail plate, and the proximal nail plate then is retracted using a clamp. The skin hook is used to retract the proximal nail fold to expose the pigment in the nail matrix, which is biopsied using the #15 blade and sent for histopathology. The proximal nail fold and retracted nail plate then are put back in place, and absorbable sutures are used to repair the defect. In certain cases, a 3-mm punch biopsy may be used to sample the nail plate and/or the surrounding soft tissue.
Practice Implications
A guide to surgical tools used during NB procedures, including less commonly encountered tools such as a nail elevator and English nail splitter, helps to close the educational gap of NB procedures among dermatology trainees and attending physicians. In conjunction with practical training with cadavers and models, a guide to surgical tools can be reviewed by trainees before hands-on exposure to nail surgery in a clinical setting. By increasing awareness of the tools needed to complete the procedure from start to finish, dermatologists may feel more prepared and confident in their ability to perform NBs, ultimately allowing for more rapid diagnosis of nail malignancies.
- Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9:3-15. doi:10.4103/idoj.IDOJ_268_17
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:e14928. doi:10.1111/dth.14928
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273. doi:10.1016/j.det.2016.02.002
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.e4835. doi:10.1016/j.jaad.2010.05.044
Practice Gap
The term nail biopsy (NB) may refer to a punch, excisional, shave, or longitudinal biopsy of the nail matrix and/or nail bed.1 Nail surgeries, including NBs, are performed relatively infrequently. In a study using data from the Medicare Provider Utilization and Payment Database 2012-2017, only 1.01% of Mohs surgeons and 0.28% of general dermatologists in the United States performed NBs. Thirty-one states had no dermatologist-performed NBs, while 3 states had no nail biopsies performed by any physician, podiatrist, nurse practitioner, or physician assistant, indicating that there is a shortage of dermatology clinicians performing nail surgeries.2
Dermatologists may not be performing NBs due to unfamiliarity with nail unit anatomy and lack of formal NB training during residency.3 In a survey of 240 dermatology residents in the United States, 58% reported performing fewer than 10 nail procedures during residency, with 25% observing only.4 Of those surveyed, 1% had no exposure to nail procedures during 3 years of residency. Furthermore, when asked to assess their competency in nail surgery on a scale of not competent, competent, and very competent, approximately 30% responded that they were not competent.4 Without sufficient education on procedures involving the nail unit, residents may be reluctant to incorporate nail surgery into their clinical practice.
Due to their complexity, NBs require the use of several specialized surgical instruments that are not used for other dermatologic procedures, and residents and attending physicians who have limited nail training may be unfamiliar with these tools. To address this educational gap, we sought to create a guide that details the surgical instruments used for the nail matrix tangential excision (shave) biopsy technique—the most common technique used in our nail specialty clinic. This guide is intended for educational use by dermatologists who wish to incorporate NB as part of their practice.
Tools and Technique
As a major referral center, our New York City–based nail specialty clinic performs a large volume of NBs, many of them performed for clinically concerning longitudinal melanonychias for which a nail matrix shave biopsy most often is performed. We utilize a standardized tray consisting of 12 surgical instruments that are needed to successfully perform a NB from start to finish (Figure). In addition to standard surgical tray items, such as sutures and tissue scissors, additional specialized instruments are necessary for NB procedures, including a nail elevator, an English nail splitter, and skin hook.

After the initial incisions are made at 45° angles to the proximal nail fold surrounding the longitudinal band, the nail elevator is used to separate the proximal nail plate from the underlying nail bed. The English nail splitter is used to create a transverse split separating the proximal from the distal nail plate, and the proximal nail plate then is retracted using a clamp. The skin hook is used to retract the proximal nail fold to expose the pigment in the nail matrix, which is biopsied using the #15 blade and sent for histopathology. The proximal nail fold and retracted nail plate then are put back in place, and absorbable sutures are used to repair the defect. In certain cases, a 3-mm punch biopsy may be used to sample the nail plate and/or the surrounding soft tissue.
Practice Implications
A guide to surgical tools used during NB procedures, including less commonly encountered tools such as a nail elevator and English nail splitter, helps to close the educational gap of NB procedures among dermatology trainees and attending physicians. In conjunction with practical training with cadavers and models, a guide to surgical tools can be reviewed by trainees before hands-on exposure to nail surgery in a clinical setting. By increasing awareness of the tools needed to complete the procedure from start to finish, dermatologists may feel more prepared and confident in their ability to perform NBs, ultimately allowing for more rapid diagnosis of nail malignancies.
Practice Gap
The term nail biopsy (NB) may refer to a punch, excisional, shave, or longitudinal biopsy of the nail matrix and/or nail bed.1 Nail surgeries, including NBs, are performed relatively infrequently. In a study using data from the Medicare Provider Utilization and Payment Database 2012-2017, only 1.01% of Mohs surgeons and 0.28% of general dermatologists in the United States performed NBs. Thirty-one states had no dermatologist-performed NBs, while 3 states had no nail biopsies performed by any physician, podiatrist, nurse practitioner, or physician assistant, indicating that there is a shortage of dermatology clinicians performing nail surgeries.2
Dermatologists may not be performing NBs due to unfamiliarity with nail unit anatomy and lack of formal NB training during residency.3 In a survey of 240 dermatology residents in the United States, 58% reported performing fewer than 10 nail procedures during residency, with 25% observing only.4 Of those surveyed, 1% had no exposure to nail procedures during 3 years of residency. Furthermore, when asked to assess their competency in nail surgery on a scale of not competent, competent, and very competent, approximately 30% responded that they were not competent.4 Without sufficient education on procedures involving the nail unit, residents may be reluctant to incorporate nail surgery into their clinical practice.
Due to their complexity, NBs require the use of several specialized surgical instruments that are not used for other dermatologic procedures, and residents and attending physicians who have limited nail training may be unfamiliar with these tools. To address this educational gap, we sought to create a guide that details the surgical instruments used for the nail matrix tangential excision (shave) biopsy technique—the most common technique used in our nail specialty clinic. This guide is intended for educational use by dermatologists who wish to incorporate NB as part of their practice.
Tools and Technique
As a major referral center, our New York City–based nail specialty clinic performs a large volume of NBs, many of them performed for clinically concerning longitudinal melanonychias for which a nail matrix shave biopsy most often is performed. We utilize a standardized tray consisting of 12 surgical instruments that are needed to successfully perform a NB from start to finish (Figure). In addition to standard surgical tray items, such as sutures and tissue scissors, additional specialized instruments are necessary for NB procedures, including a nail elevator, an English nail splitter, and skin hook.

After the initial incisions are made at 45° angles to the proximal nail fold surrounding the longitudinal band, the nail elevator is used to separate the proximal nail plate from the underlying nail bed. The English nail splitter is used to create a transverse split separating the proximal from the distal nail plate, and the proximal nail plate then is retracted using a clamp. The skin hook is used to retract the proximal nail fold to expose the pigment in the nail matrix, which is biopsied using the #15 blade and sent for histopathology. The proximal nail fold and retracted nail plate then are put back in place, and absorbable sutures are used to repair the defect. In certain cases, a 3-mm punch biopsy may be used to sample the nail plate and/or the surrounding soft tissue.
Practice Implications
A guide to surgical tools used during NB procedures, including less commonly encountered tools such as a nail elevator and English nail splitter, helps to close the educational gap of NB procedures among dermatology trainees and attending physicians. In conjunction with practical training with cadavers and models, a guide to surgical tools can be reviewed by trainees before hands-on exposure to nail surgery in a clinical setting. By increasing awareness of the tools needed to complete the procedure from start to finish, dermatologists may feel more prepared and confident in their ability to perform NBs, ultimately allowing for more rapid diagnosis of nail malignancies.
- Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9:3-15. doi:10.4103/idoj.IDOJ_268_17
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:e14928. doi:10.1111/dth.14928
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273. doi:10.1016/j.det.2016.02.002
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.e4835. doi:10.1016/j.jaad.2010.05.044
- Grover C, Bansal S. Nail biopsy: a user’s manual. Indian Dermatol Online J. 2018;9:3-15. doi:10.4103/idoj.IDOJ_268_17
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare Provider Utilization and Payment Database 2012 to 2017. Dermatol Ther. 2021;34:e14928. doi:10.1111/dth.14928
- Hare AQ, Rich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273. doi:10.1016/j.det.2016.02.002
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.e4835. doi:10.1016/j.jaad.2010.05.044
Melasma Risk Factors: A Matched Cohort Study Using Data From the All of Us Research Program
To the Editor:
Melasma (also known as chloasma) is characterized by symmetric hyperpigmented patches affecting sun-exposed areas. Women commonly develop this condition during pregnancy, suggesting a connection between melasma and increased female sex hormone levels.1 Other hypothesized risk factors include sun exposure, genetic susceptibility, estrogen and/or progesterone therapy, and thyroid abnormalities but have not been corroborated.2 Treatment options are limited because the pathogenesis is poorly understood; thus, we aimed to analyze melasma risk factors using a national database with a nested case-control approach.
We conducted a matched case-control study using the Registered Tier dataset (version 7) from the National Institute of Health’s All of Us Research Program (https://allofus.nih.gov/), which is available to authorized users through the program’s Researcher Workbench and includes more than 413,000 total participants enrolled from May 1, 2018, through July 1, 2022. Cases included patients 18 years and older with a diagnosis of melasma (International Classification of Diseases, Tenth Revision, Clinical Modification code L81.1 [Chloasma]; concept ID 4264234 [Chloasma]; and Systematized Nomenclature of Medicine [SNOMED] code 36209000 [Chloasma]), and controls without a diagnosis of melasma were matched in a 1:10 ratio based on age, sex, and self-reported race. Concept IDs and SNOMED codes were used to identify individuals in each cohort with a diagnosis of alcohol dependence (concept IDs 433753, 435243, 4218106; SNOMED codes 15167005, 66590003, 7200002), depression (concept ID 440383; SNOMED code 35489007), hypothyroidism (concept ID 140673; SNOMED code 40930008), hyperthyroidism (concept ID 4142479; SNOMED code 34486009), anxiety (concept IDs 441542, 442077, 434613; SNOMED codes 48694002, 197480006, 21897009), tobacco dependence (concept IDs 37109023, 437264, 4099811; SNOMED codes 16077091000119107, 89765005, 191887008), or obesity (concept IDs 433736 and 434005; SNOMED codes 414916001 and 238136002), or with a history of radiation therapy (concept IDs 4085340, 4311117, 4061844, 4029715; SNOMED codes 24803000, 85983004, 200861004, 108290001) or hormonal medications containing estrogen and/or progesterone, including oral medications and implants (concept IDs 21602445, 40254009, 21602514, 21603814, 19049228, 21602529, 1549080, 1551673, 1549254, 21602472, 21602446, 21602450, 21602515, 21602566, 21602473, 21602567, 21602488, 21602585, 1596779, 1586808, 21602524). In our case cohort, diagnoses and exposures to treatments were only considered for analysis if they occurred prior to melasma diagnosis.
Multivariate logistic regression was performed to calculate odds ratios and P values between melasma and each comorbidity or exposure to the treatments specified. Statistical significance was set at P<.05.
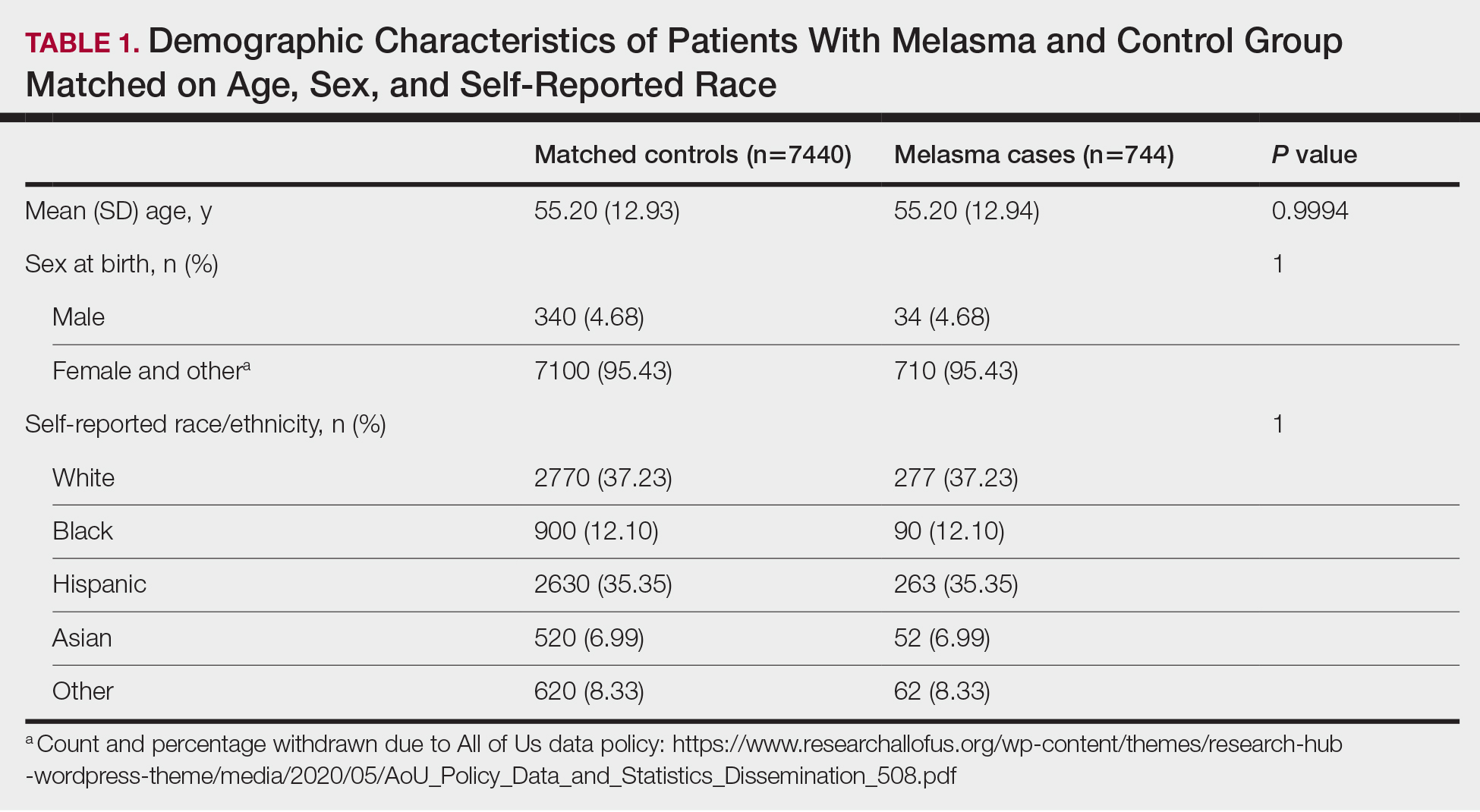
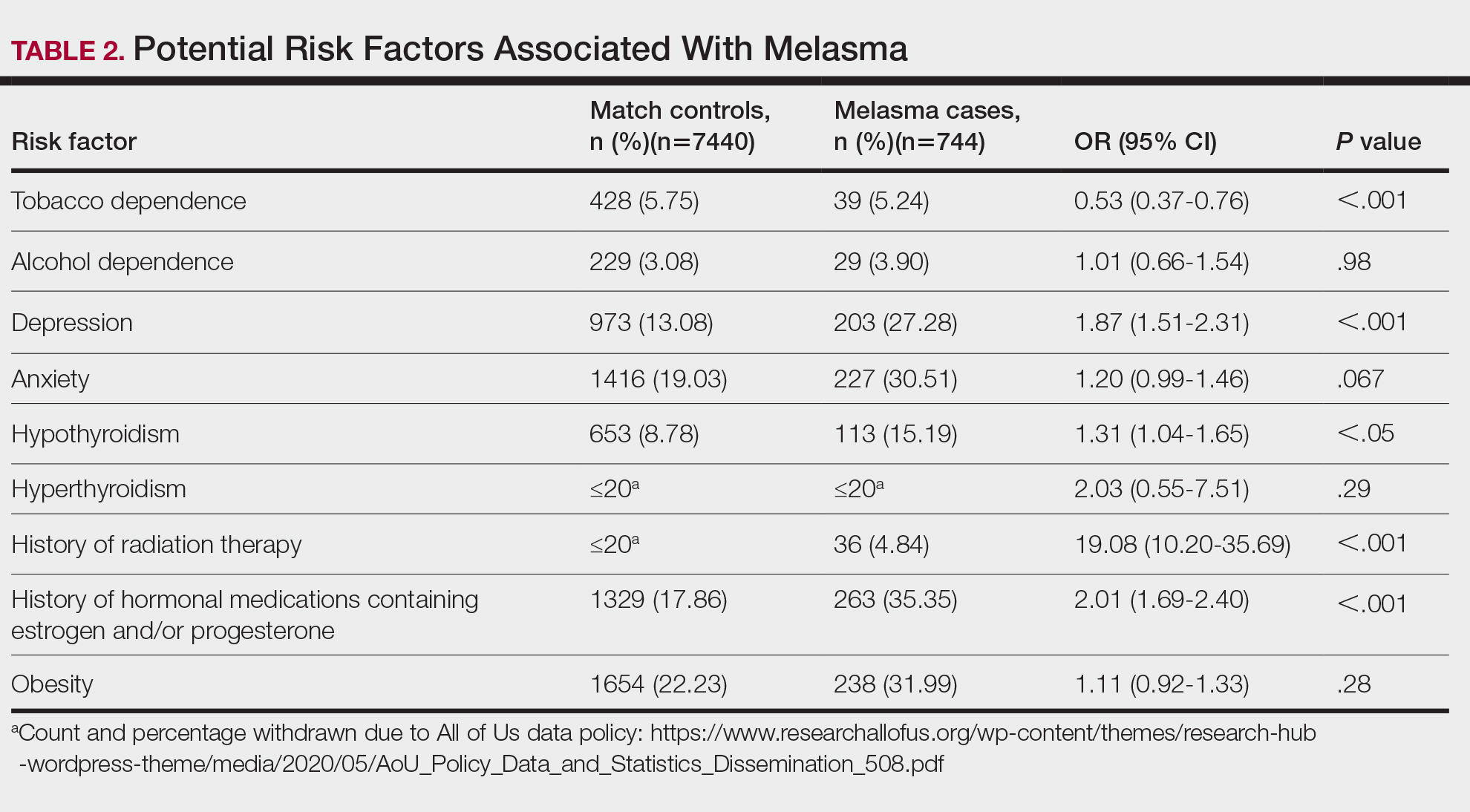
We identified 744 melasma cases (mean age, 55.20 years; 95.43% female; 12.10% Black) and 7440 controls with similar demographics (ie, age, sex, race/ethnicity) between groups (all P>.05 [Table 1]). Patients with a melasma diagnosis were more likely to have a pre-existing diagnosis of depression (OR, 1.87; 95% CI, 1.51-2.31 [P<.001]) or hypothyroidism (OR, 1.31; 95% CI, 1.04-1.65 [P<.05]), or a history of radiation therapy (OR, 19.08; 95% CI, 10.20-35.69 [P<.001]) and/or estrogen and/or progesterone therapy (OR, 2.01; 95% CI, 1.69-2.40 [P<.001]) prior to melasma diagnosis. A diagnosis of anxiety prior to melasma diagnosis trended toward an association with melasma (P=.067). Pre-existing alcohol dependence, obesity, and hyperthyroidism were not associated with melasma (P=.98, P=.28, and P=.29, respectively). A diagnosis of tobacco dependence was associated with a decreased melasma risk (OR, 0.53, 95% CI, 0.37-0.76)[P<.001])(Table 2).
Our study results suggest that pre-existing depression was a risk factor for subsequent melasma diagnosis. Depression may exacerbate stress, leading to increased activation of the hypothalamic-pituitary-adrenal axis as well as increased levels of cortisol and adrenocorticotropic hormone, which subsequently act on melanocytes to increase melanogenesis.3 A retrospective study of 254 participants, including 127 with melasma, showed that increased melasma severity was associated with higher rates of depression (P=.002)2; however, the risk for melasma following a depression diagnosis has not been reported.
Our results also showed that hypothyroidism was associated with an increased risk for melasma. On a cellular level, hypothyroidism can cause systemic inflammation, potentailly leading to increased stress and melanogenesis via activation of the hypothalamic-pituitary-adrenal axis.4 These findings are similar to a systematic review and meta-analysis reporting increased thyroid-stimulating hormone, anti–thyroid peroxidase, and antithyroglobulin antibody levels associated with increased melasma risk (mean difference between cases and controls, 0.33 [95% CI, 0.18-0.47]; pooled association, P=.020; mean difference between cases and controls, 0.28 [95% CI, 0.01-0.55], respectively).5
Patients in our cohort who had a history of radiation therapy were 19 times more likely to develop melasma, similar to findings of a survey-based study of 421 breast cancer survivors in which 336 (79.81%) reported hyperpigmentation in irradiated areas.6 Patients in our cohort who had a history of estrogen and/or progesterone therapy were 2 times more likely to develop melasma, similar to a case-control study of 207 patients with melasma and 207 controls that showed combined oral contraceptives increased risk for melasma (OR, 1.23 [95% CI, 1.08-1.41; P<.01).3
Tobacco use is not a well-known protective factor against melasma. Prior studies have indicated that tobacco smoking activates melanocytes via the Wnt/β-Catenin pathway, leading to hyperpigmentation.7 Although exposure to cigarette smoke decreases angiogenesis and would more likely lead to hyperpigmentation, nicotine exposure has been shown to increase angiogenesis, which could lead to increased blood flow and partially explain the protection against melasma demonstrated in our cohort.8 Future studies are needed to explore this relationship.
Limitations of our study include lack of information about melasma severity and information about prior melasma treatment in our cohort as well as possible misdiagnosis reported in the dataset.
Our results demonstrated that pre-existing depression and hypothyroidism as well as a history of radiation or estrogen and/or progesterone therapies are potential risk factors for melasma. Therefore, we recommend that patients with melasma be screened for depression and thyroid dysfunction, and patients undergoing radiation therapy or starting estrogen and/or progesterone therapy should be counseled on their increased risk for melasma. Future studies are needed to determine whether treatment of comorbidities such as hypothyroidism and depression improve melasma severity. The decreased risk for melasma associated with tobacco use also requires further investigation.
Acknowledgments—The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
In addition, the All of Us Research Program would not be possible without the partnership of its participants, who we gratefully acknowledge for their contributions and without whom this research would not have been possible. We also thank the All of Us Research Program for making the participant data examined in this study available to us.
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Platsidaki E, Efstathiou V, Markantoni V, et al. Self-esteem, depression, anxiety and quality of life in patients with melasma living in a sunny mediterranean area: results from a prospective cross-sectional study. Dermatol Ther (Heidelb). 2023;13:1127-1136. doi:10.1007/s13555-023-00915-1
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio (published January 22, 2023). Diseases. 2023;11:15. doi:10.3390/diseases11010015
- Kheradmand M, Afshari M, Damiani G, et al. Melasma and thyroid disorders: a systematic review and meta-analysis. Int J Dermatol. 2019;58:1231-1238. doi:10.1111/ijd.14497
- Chu CN, Hu KC, Wu RS, et al. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy (published March 31, 2021). BMC Cancer. 2021;21:330. doi:10.1186/s12885-021-08047-5
- Nakamura M, Ueda Y, Hayashi M, et al. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013;22:556-558. doi:10.1111/exd.12170
- Ejaz S, Lim CW. Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol. 2005;20:335-344. doi:10.1016/j.etap.2005.03.011
To the Editor:
Melasma (also known as chloasma) is characterized by symmetric hyperpigmented patches affecting sun-exposed areas. Women commonly develop this condition during pregnancy, suggesting a connection between melasma and increased female sex hormone levels.1 Other hypothesized risk factors include sun exposure, genetic susceptibility, estrogen and/or progesterone therapy, and thyroid abnormalities but have not been corroborated.2 Treatment options are limited because the pathogenesis is poorly understood; thus, we aimed to analyze melasma risk factors using a national database with a nested case-control approach.
We conducted a matched case-control study using the Registered Tier dataset (version 7) from the National Institute of Health’s All of Us Research Program (https://allofus.nih.gov/), which is available to authorized users through the program’s Researcher Workbench and includes more than 413,000 total participants enrolled from May 1, 2018, through July 1, 2022. Cases included patients 18 years and older with a diagnosis of melasma (International Classification of Diseases, Tenth Revision, Clinical Modification code L81.1 [Chloasma]; concept ID 4264234 [Chloasma]; and Systematized Nomenclature of Medicine [SNOMED] code 36209000 [Chloasma]), and controls without a diagnosis of melasma were matched in a 1:10 ratio based on age, sex, and self-reported race. Concept IDs and SNOMED codes were used to identify individuals in each cohort with a diagnosis of alcohol dependence (concept IDs 433753, 435243, 4218106; SNOMED codes 15167005, 66590003, 7200002), depression (concept ID 440383; SNOMED code 35489007), hypothyroidism (concept ID 140673; SNOMED code 40930008), hyperthyroidism (concept ID 4142479; SNOMED code 34486009), anxiety (concept IDs 441542, 442077, 434613; SNOMED codes 48694002, 197480006, 21897009), tobacco dependence (concept IDs 37109023, 437264, 4099811; SNOMED codes 16077091000119107, 89765005, 191887008), or obesity (concept IDs 433736 and 434005; SNOMED codes 414916001 and 238136002), or with a history of radiation therapy (concept IDs 4085340, 4311117, 4061844, 4029715; SNOMED codes 24803000, 85983004, 200861004, 108290001) or hormonal medications containing estrogen and/or progesterone, including oral medications and implants (concept IDs 21602445, 40254009, 21602514, 21603814, 19049228, 21602529, 1549080, 1551673, 1549254, 21602472, 21602446, 21602450, 21602515, 21602566, 21602473, 21602567, 21602488, 21602585, 1596779, 1586808, 21602524). In our case cohort, diagnoses and exposures to treatments were only considered for analysis if they occurred prior to melasma diagnosis.
Multivariate logistic regression was performed to calculate odds ratios and P values between melasma and each comorbidity or exposure to the treatments specified. Statistical significance was set at P<.05.
We identified 744 melasma cases (mean age, 55.20 years; 95.43% female; 12.10% Black) and 7440 controls with similar demographics (ie, age, sex, race/ethnicity) between groups (all P>.05 [Table 1]). Patients with a melasma diagnosis were more likely to have a pre-existing diagnosis of depression (OR, 1.87; 95% CI, 1.51-2.31 [P<.001]) or hypothyroidism (OR, 1.31; 95% CI, 1.04-1.65 [P<.05]), or a history of radiation therapy (OR, 19.08; 95% CI, 10.20-35.69 [P<.001]) and/or estrogen and/or progesterone therapy (OR, 2.01; 95% CI, 1.69-2.40 [P<.001]) prior to melasma diagnosis. A diagnosis of anxiety prior to melasma diagnosis trended toward an association with melasma (P=.067). Pre-existing alcohol dependence, obesity, and hyperthyroidism were not associated with melasma (P=.98, P=.28, and P=.29, respectively). A diagnosis of tobacco dependence was associated with a decreased melasma risk (OR, 0.53, 95% CI, 0.37-0.76)[P<.001])(Table 2).
Our study results suggest that pre-existing depression was a risk factor for subsequent melasma diagnosis. Depression may exacerbate stress, leading to increased activation of the hypothalamic-pituitary-adrenal axis as well as increased levels of cortisol and adrenocorticotropic hormone, which subsequently act on melanocytes to increase melanogenesis.3 A retrospective study of 254 participants, including 127 with melasma, showed that increased melasma severity was associated with higher rates of depression (P=.002)2; however, the risk for melasma following a depression diagnosis has not been reported.
Our results also showed that hypothyroidism was associated with an increased risk for melasma. On a cellular level, hypothyroidism can cause systemic inflammation, potentailly leading to increased stress and melanogenesis via activation of the hypothalamic-pituitary-adrenal axis.4 These findings are similar to a systematic review and meta-analysis reporting increased thyroid-stimulating hormone, anti–thyroid peroxidase, and antithyroglobulin antibody levels associated with increased melasma risk (mean difference between cases and controls, 0.33 [95% CI, 0.18-0.47]; pooled association, P=.020; mean difference between cases and controls, 0.28 [95% CI, 0.01-0.55], respectively).5
Patients in our cohort who had a history of radiation therapy were 19 times more likely to develop melasma, similar to findings of a survey-based study of 421 breast cancer survivors in which 336 (79.81%) reported hyperpigmentation in irradiated areas.6 Patients in our cohort who had a history of estrogen and/or progesterone therapy were 2 times more likely to develop melasma, similar to a case-control study of 207 patients with melasma and 207 controls that showed combined oral contraceptives increased risk for melasma (OR, 1.23 [95% CI, 1.08-1.41; P<.01).3
Tobacco use is not a well-known protective factor against melasma. Prior studies have indicated that tobacco smoking activates melanocytes via the Wnt/β-Catenin pathway, leading to hyperpigmentation.7 Although exposure to cigarette smoke decreases angiogenesis and would more likely lead to hyperpigmentation, nicotine exposure has been shown to increase angiogenesis, which could lead to increased blood flow and partially explain the protection against melasma demonstrated in our cohort.8 Future studies are needed to explore this relationship.


Limitations of our study include lack of information about melasma severity and information about prior melasma treatment in our cohort as well as possible misdiagnosis reported in the dataset.
Our results demonstrated that pre-existing depression and hypothyroidism as well as a history of radiation or estrogen and/or progesterone therapies are potential risk factors for melasma. Therefore, we recommend that patients with melasma be screened for depression and thyroid dysfunction, and patients undergoing radiation therapy or starting estrogen and/or progesterone therapy should be counseled on their increased risk for melasma. Future studies are needed to determine whether treatment of comorbidities such as hypothyroidism and depression improve melasma severity. The decreased risk for melasma associated with tobacco use also requires further investigation.
Acknowledgments—The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
In addition, the All of Us Research Program would not be possible without the partnership of its participants, who we gratefully acknowledge for their contributions and without whom this research would not have been possible. We also thank the All of Us Research Program for making the participant data examined in this study available to us.
To the Editor:
Melasma (also known as chloasma) is characterized by symmetric hyperpigmented patches affecting sun-exposed areas. Women commonly develop this condition during pregnancy, suggesting a connection between melasma and increased female sex hormone levels.1 Other hypothesized risk factors include sun exposure, genetic susceptibility, estrogen and/or progesterone therapy, and thyroid abnormalities but have not been corroborated.2 Treatment options are limited because the pathogenesis is poorly understood; thus, we aimed to analyze melasma risk factors using a national database with a nested case-control approach.
We conducted a matched case-control study using the Registered Tier dataset (version 7) from the National Institute of Health’s All of Us Research Program (https://allofus.nih.gov/), which is available to authorized users through the program’s Researcher Workbench and includes more than 413,000 total participants enrolled from May 1, 2018, through July 1, 2022. Cases included patients 18 years and older with a diagnosis of melasma (International Classification of Diseases, Tenth Revision, Clinical Modification code L81.1 [Chloasma]; concept ID 4264234 [Chloasma]; and Systematized Nomenclature of Medicine [SNOMED] code 36209000 [Chloasma]), and controls without a diagnosis of melasma were matched in a 1:10 ratio based on age, sex, and self-reported race. Concept IDs and SNOMED codes were used to identify individuals in each cohort with a diagnosis of alcohol dependence (concept IDs 433753, 435243, 4218106; SNOMED codes 15167005, 66590003, 7200002), depression (concept ID 440383; SNOMED code 35489007), hypothyroidism (concept ID 140673; SNOMED code 40930008), hyperthyroidism (concept ID 4142479; SNOMED code 34486009), anxiety (concept IDs 441542, 442077, 434613; SNOMED codes 48694002, 197480006, 21897009), tobacco dependence (concept IDs 37109023, 437264, 4099811; SNOMED codes 16077091000119107, 89765005, 191887008), or obesity (concept IDs 433736 and 434005; SNOMED codes 414916001 and 238136002), or with a history of radiation therapy (concept IDs 4085340, 4311117, 4061844, 4029715; SNOMED codes 24803000, 85983004, 200861004, 108290001) or hormonal medications containing estrogen and/or progesterone, including oral medications and implants (concept IDs 21602445, 40254009, 21602514, 21603814, 19049228, 21602529, 1549080, 1551673, 1549254, 21602472, 21602446, 21602450, 21602515, 21602566, 21602473, 21602567, 21602488, 21602585, 1596779, 1586808, 21602524). In our case cohort, diagnoses and exposures to treatments were only considered for analysis if they occurred prior to melasma diagnosis.
Multivariate logistic regression was performed to calculate odds ratios and P values between melasma and each comorbidity or exposure to the treatments specified. Statistical significance was set at P<.05.
We identified 744 melasma cases (mean age, 55.20 years; 95.43% female; 12.10% Black) and 7440 controls with similar demographics (ie, age, sex, race/ethnicity) between groups (all P>.05 [Table 1]). Patients with a melasma diagnosis were more likely to have a pre-existing diagnosis of depression (OR, 1.87; 95% CI, 1.51-2.31 [P<.001]) or hypothyroidism (OR, 1.31; 95% CI, 1.04-1.65 [P<.05]), or a history of radiation therapy (OR, 19.08; 95% CI, 10.20-35.69 [P<.001]) and/or estrogen and/or progesterone therapy (OR, 2.01; 95% CI, 1.69-2.40 [P<.001]) prior to melasma diagnosis. A diagnosis of anxiety prior to melasma diagnosis trended toward an association with melasma (P=.067). Pre-existing alcohol dependence, obesity, and hyperthyroidism were not associated with melasma (P=.98, P=.28, and P=.29, respectively). A diagnosis of tobacco dependence was associated with a decreased melasma risk (OR, 0.53, 95% CI, 0.37-0.76)[P<.001])(Table 2).
Our study results suggest that pre-existing depression was a risk factor for subsequent melasma diagnosis. Depression may exacerbate stress, leading to increased activation of the hypothalamic-pituitary-adrenal axis as well as increased levels of cortisol and adrenocorticotropic hormone, which subsequently act on melanocytes to increase melanogenesis.3 A retrospective study of 254 participants, including 127 with melasma, showed that increased melasma severity was associated with higher rates of depression (P=.002)2; however, the risk for melasma following a depression diagnosis has not been reported.
Our results also showed that hypothyroidism was associated with an increased risk for melasma. On a cellular level, hypothyroidism can cause systemic inflammation, potentailly leading to increased stress and melanogenesis via activation of the hypothalamic-pituitary-adrenal axis.4 These findings are similar to a systematic review and meta-analysis reporting increased thyroid-stimulating hormone, anti–thyroid peroxidase, and antithyroglobulin antibody levels associated with increased melasma risk (mean difference between cases and controls, 0.33 [95% CI, 0.18-0.47]; pooled association, P=.020; mean difference between cases and controls, 0.28 [95% CI, 0.01-0.55], respectively).5
Patients in our cohort who had a history of radiation therapy were 19 times more likely to develop melasma, similar to findings of a survey-based study of 421 breast cancer survivors in which 336 (79.81%) reported hyperpigmentation in irradiated areas.6 Patients in our cohort who had a history of estrogen and/or progesterone therapy were 2 times more likely to develop melasma, similar to a case-control study of 207 patients with melasma and 207 controls that showed combined oral contraceptives increased risk for melasma (OR, 1.23 [95% CI, 1.08-1.41; P<.01).3
Tobacco use is not a well-known protective factor against melasma. Prior studies have indicated that tobacco smoking activates melanocytes via the Wnt/β-Catenin pathway, leading to hyperpigmentation.7 Although exposure to cigarette smoke decreases angiogenesis and would more likely lead to hyperpigmentation, nicotine exposure has been shown to increase angiogenesis, which could lead to increased blood flow and partially explain the protection against melasma demonstrated in our cohort.8 Future studies are needed to explore this relationship.


Limitations of our study include lack of information about melasma severity and information about prior melasma treatment in our cohort as well as possible misdiagnosis reported in the dataset.
Our results demonstrated that pre-existing depression and hypothyroidism as well as a history of radiation or estrogen and/or progesterone therapies are potential risk factors for melasma. Therefore, we recommend that patients with melasma be screened for depression and thyroid dysfunction, and patients undergoing radiation therapy or starting estrogen and/or progesterone therapy should be counseled on their increased risk for melasma. Future studies are needed to determine whether treatment of comorbidities such as hypothyroidism and depression improve melasma severity. The decreased risk for melasma associated with tobacco use also requires further investigation.
Acknowledgments—The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
In addition, the All of Us Research Program would not be possible without the partnership of its participants, who we gratefully acknowledge for their contributions and without whom this research would not have been possible. We also thank the All of Us Research Program for making the participant data examined in this study available to us.
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Platsidaki E, Efstathiou V, Markantoni V, et al. Self-esteem, depression, anxiety and quality of life in patients with melasma living in a sunny mediterranean area: results from a prospective cross-sectional study. Dermatol Ther (Heidelb). 2023;13:1127-1136. doi:10.1007/s13555-023-00915-1
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio (published January 22, 2023). Diseases. 2023;11:15. doi:10.3390/diseases11010015
- Kheradmand M, Afshari M, Damiani G, et al. Melasma and thyroid disorders: a systematic review and meta-analysis. Int J Dermatol. 2019;58:1231-1238. doi:10.1111/ijd.14497
- Chu CN, Hu KC, Wu RS, et al. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy (published March 31, 2021). BMC Cancer. 2021;21:330. doi:10.1186/s12885-021-08047-5
- Nakamura M, Ueda Y, Hayashi M, et al. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013;22:556-558. doi:10.1111/exd.12170
- Ejaz S, Lim CW. Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol. 2005;20:335-344. doi:10.1016/j.etap.2005.03.011
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Platsidaki E, Efstathiou V, Markantoni V, et al. Self-esteem, depression, anxiety and quality of life in patients with melasma living in a sunny mediterranean area: results from a prospective cross-sectional study. Dermatol Ther (Heidelb). 2023;13:1127-1136. doi:10.1007/s13555-023-00915-1
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio (published January 22, 2023). Diseases. 2023;11:15. doi:10.3390/diseases11010015
- Kheradmand M, Afshari M, Damiani G, et al. Melasma and thyroid disorders: a systematic review and meta-analysis. Int J Dermatol. 2019;58:1231-1238. doi:10.1111/ijd.14497
- Chu CN, Hu KC, Wu RS, et al. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy (published March 31, 2021). BMC Cancer. 2021;21:330. doi:10.1186/s12885-021-08047-5
- Nakamura M, Ueda Y, Hayashi M, et al. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013;22:556-558. doi:10.1111/exd.12170
- Ejaz S, Lim CW. Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol. 2005;20:335-344. doi:10.1016/j.etap.2005.03.011
Practice Points
- Treatment options for melasma are limited due to its poorly understood pathogenesis.
- Depression and hypothyroidism and/or history of exposure to radiation and hormonal therapies may increase melasma risk.
- We recommend that patients with melasma be screened for depression and thyroid dysfunction. Patients undergoing radiation therapy or starting estrogen and/ or progesterone therapy should be counseled on the increased risk for melasma.
Analysis of Nail Excision Practice Patterns in the Medicare Provider Utilization and Payment Database 2012-2017
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.
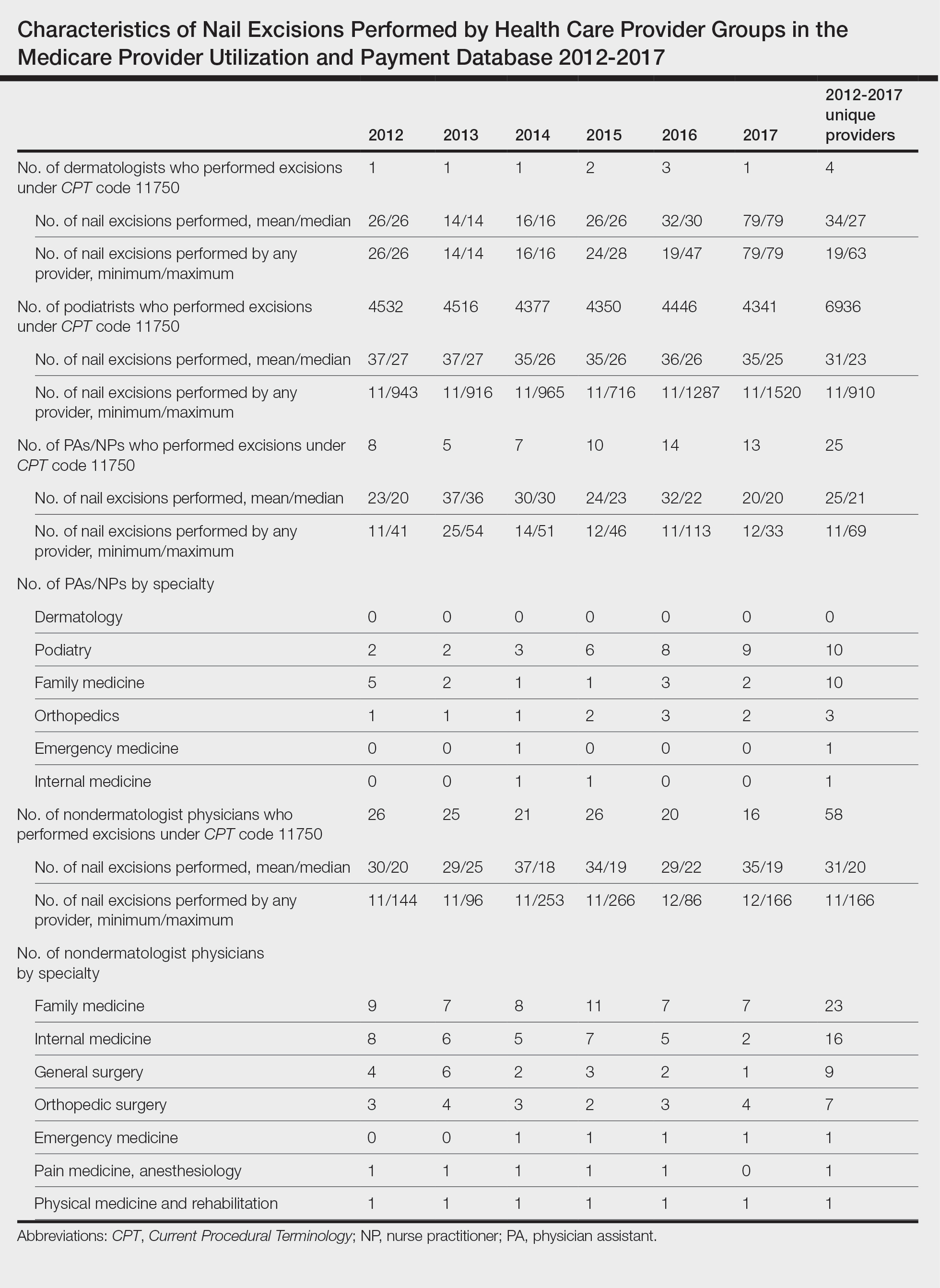
A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).
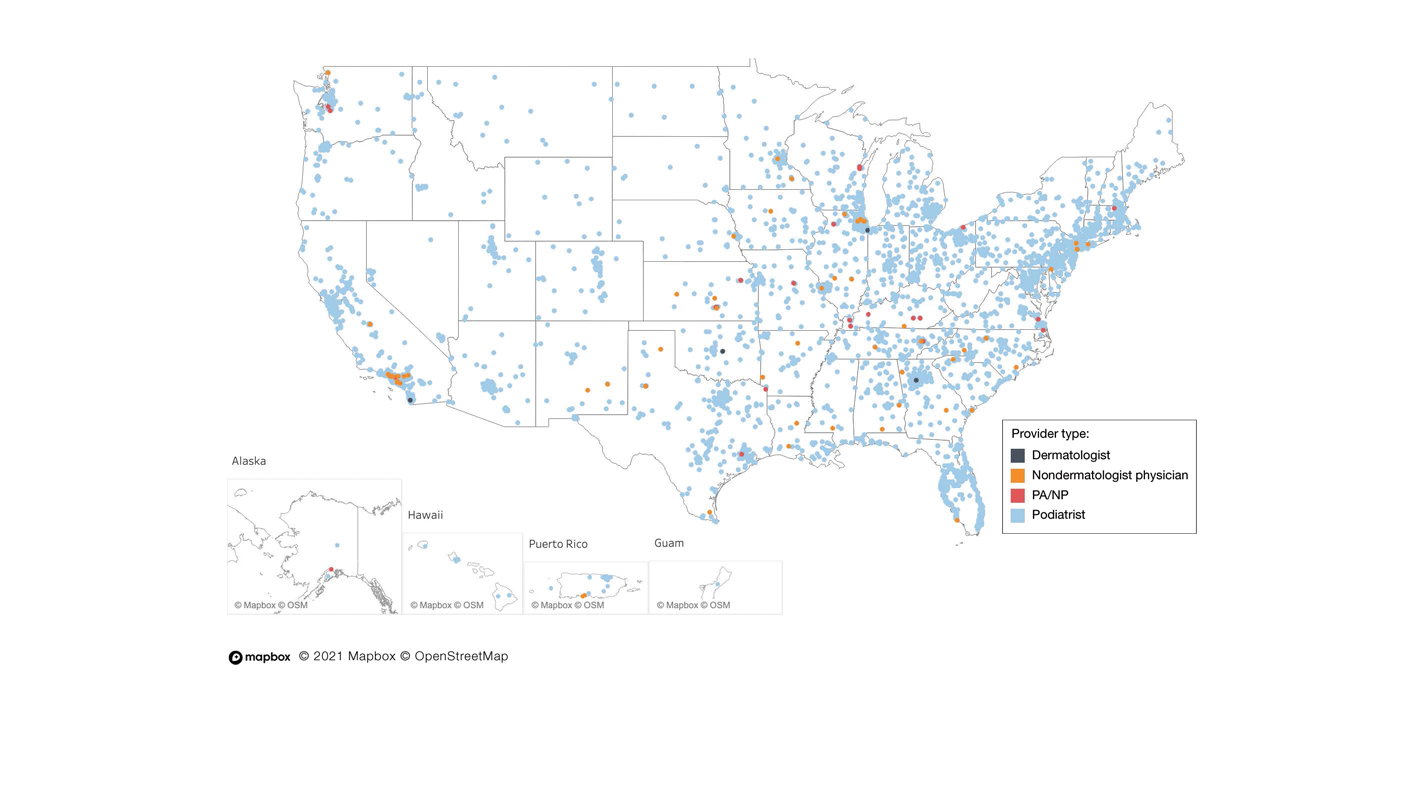
Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
To the Editor:
Partial or total nail plate excisions commonly are used for the treatment of onychocryptosis and nail spicules. Procedures involving the nail unit require advanced technical skills to achieve optimal functional and aesthetic outcomes, avoid complications, and minimize health care costs. Data on the frequency of nail plate excisions performed by dermatologists and their relative frequency compared to other medical providers are limited. The objective of our study was to analyze trends in nail excision practice patterns among medical providers in the United States.
A retrospective analysis on nail excisions using the Current Procedural Terminology (CPT) code 11750 (excision of nail and nail matrix, partial or complete [eg, ingrown or deformed nail] for permanent removal), which is distinct from code 11755 (biopsy of nail unit [eg, plate, bed, matrix, hyponychium, proximal and lateral nail folds][separate procedure]), was performed using data from the Medicare Provider Utilization and Payment Database 2012-2017.1,2 This file also is used by Peck et al3 in an article submitted to the Journal of the American Podiatric Medical Association and currently under consideration for publication. Procedures were recorded by year and provider type—dermatologist, podiatrist, physician assistant (PA)/nurse practitioner (NP), nondermatologist physician—and subcategorized by provider specialty (Table). Practice locations subcategorized by provider type were mapped using Tableau Software (Salesforce)(Figure). Descriptive statistics including number of providers, mean and median excisions per provider, and minimum/maximum nail excisions were calculated (Table). Practice types of PAs/NPs and specialization of nondermatologist physicians were determined using provider name, identification number, and practice address. This study did not require institutional review board review, as only publicly available data were utilized in our analysis.

A total of 6936 podiatrists, 58 nondermatologist physicians, 25 PAs/NPs, and 4 dermatologists performed 10 or more nail excisions annually under CPT code 11750 from January 2012 to December 2017 with annual means of 31, 31, 25, and 34, respectively (Table). No PAs/NPs included in the dataset worked in dermatology practices during the study period. Physician assistants and NPs most often practiced in podiatry and family medicine (FM) settings (both 40% [10/25]). Nondermatologist physicians most often specialized in FM (40% [23/58])(Table). The greatest number of providers practiced in 3 of the 4 most-populous states: California, Texas, and Florida; the fewest number practiced in 3 of the 10 least-populous states: Alaska, Hawaii, and Vermont. Vermont, Wyoming, and North Dakota—3 of the 5 least-populous states—had the fewest practitioners among the contiguous United States (Figure).

Our study showed that from January 2012 to December 2017, fewer dermatologists performed nail excisions than any other provider type (0.06%, 4 dermatologists of 7023 total providers), and dermatologists performed 1734-fold fewer nail excisions than podiatrists (99%, 6936 podiatrists of 7023 total providers). Only dermatologists practicing in California, Georgia, Indiana, and Oklahoma performed nail excisions. Conversely, podiatrists were more geographically distributed across the United States and other territories, with representation in all 50 states as well as the District of Columbia, Puerto Rico, and Guam.
Reasons for these large discrepancies in practice between dermatologists and other providers likely are multifactorial, encompassing a lack of emphasis on nail procedures in dermatology training, patient perception of the scope of dermatologic practice, and nail excision reimbursement patterns. Most dermatologists likely lack experience in performing nail procedures. The Accreditation Council for Graduate Medical Education requirements mandate that dermatology residents observe or perform 3 nail procedures over 3 years of residency, including 1 that may be performed on a human cadaver.4 In contrast, podiatry trainees must gain competency in toenail avulsion (both partial and complete), participate in anesthesia workshops, and become proficient in administering lower extremity blocks by the end of their training.5 Therefore, incorporating aspects of podiatric surgical training into dermatology residency requirements may increase the competency and comfort of dermatologists in performing nail excisions and practicing as nail experts as attending physicians.
It is likely that US patients do not perceive dermatologists as nail specialists and instead primarily consult podiatrists or FM and/or internal medicine physicians for treatment; for example, nail disease was one of the least common reasons for consulting a dermatologist (5%) in a German nationwide survey-based study (N=1015).6 Therefore, increased efforts are needed to educate the general public about the expertise of dermatologists in the diagnosis and management of nail conditions.
Reimbursement also may be a barrier to dermatologists performing nail procedures as part of their scope of practice; for example, in a retrospective study of nail biopsies using the Medicare Provider Utilization and Payment Database, there was no statistically significant difference in reimbursements for nail biopsies vs skin biopsies from 2012 to 2017 (P=0.69).7 Similar to nail biopsies, nail excisions typically are much more time consuming and technically demanding than skin biopsies, which may discourage dermatologists from routinely performing nail excision procedures.
Our study is subject to a number of limitations. The data reflected only US-based practice patterns and may not be applicable to nail procedures globally. There also is the potential for miscoding of procedures in the Medicare database. The data included only Part B Medicare fee-for-service and excludes non-Medicare insured, uninsured, and self-pay patients, as well as aggregated records from 10 or fewer Medicare beneficiaries.
Dermatologists rarely perform nail excisions and perform fewer nail excisions than any other provider type in the United States. There currently is an unmet need for comprehensive nail surgery education in US-based dermatology residency programs. We hope that our study fosters interdisciplinary collegiality and training between podiatrists and dermatologists and promotes expanded access to care across the United States to serve patients with nail disorders.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
- Centers for Medicare & Medicaid Services. Medicare Fee-For-Service Provider Utilization & Payment Data Physician and Other Supplier Public Use File: A Methodological Overview . Updated September 22, 2020. Accessed January 5, 2024. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/medicare-provider-charge-data/downloads/medicare-physician-and-other-supplier-puf-methodology.pdf
- Centers for Medicare and Medicaid Services. Billing and Coding: Surgical Treatment of Nails. Updated November 9, 2023. Accessed January 8, 2024. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleID=52998#:~:text=The%20description%20of%20CPT%20codes,date%20of%20service%20(DOS).
- Peck GM, Vlahovic TC, Hill R, et al. Senior podiatrists in solo practice are high performers of nail excisions. JAPMA. In press.
- Accreditation Council for Graduate Medical Education. Case log minimums. review committee for dermatology. Published May 2019. Accessed January 5, 2024. https://www.acgme.org/Portals/0/PFAssets/ProgramResources/CaseLogMinimums.pdf?ver=2018-04-03-102751-650
- Council on Podiatric Medical Education. Standards and Requirements for Approval of Podiatric Medicine and Surgery Residencies. Published July 2023. Accessed January 17, 2024. https://www.cpme.org/files/320%20Council%20Approved%20October%202022%20-%20April%202023%20edits.pdf
- Augustin M, Eissing L, Elsner P, et al. Perception and image of dermatology in the German general population 2002-2014. J Eur Acad Dermatol Venereol. 2017;31:2124-2130.
- Wang Y, Lipner SR. Retrospective analysis of nail biopsies performed using the Medicare provider utilization and payment database 2012 to 2017. Dermatol Ther. 2021;34:E14928.
Practice Points
- Dermatologists are considered nail experts but perform nail excisions less frequently than their podiatric counterparts and physicians in other specialties.
- Aspects of podiatric surgical training should be incorporated into dermatology residency to increase competency and comfort of dermatologists in nail excision procedures.
- Dermatologists may not be perceived as nail experts by the public, indicating a need for increased community education on the role of dermatologists in treating nail disease.