User login
Duration of Adalimumab Therapy in Hidradenitis Suppurativa With and Without Oral Immunosuppressants
To the Editor:
The tumor necrosis factor α inhibitor adalimumab is the only US Food and Drug Administration–approved treatment of hidradenitis suppurativa (HS). Although 50.6% of patients fulfilled Hidradenitis Suppurativa Clinical Response criteria with adalimumab at 12 weeks, many responders were not satisfied with their disease control, and secondary loss of Hidradenitis Suppurativa Clinical Response fulfillment occurred in 15.9% of patients within approximately 3 years.1 Without other US Food and Drug Administration–approved HS treatments, some dermatologists have combined adalimumab with methotrexate (MTX) and/or mycophenolate mofetil (MMF) to attempt to increase the duration of satisfactory disease control while on adalimumab. Combining tumor necrosis factor α inhibitors with oral immunosuppressants is a well-established approach in psoriasis, psoriatic arthritis, and inflammatory bowel disease; however, to the best of our knowledge, this approach has not been studied for HS.2,3
To assess whether there is a role for combining adalimumab with MTX and/or MMF in the treatment of HS, we performed a single-institution retrospective chart review at the University of Connecticut Department of Dermatology to determine whether patients receiving combination therapy stayed on adalimumab longer than those who received adalimumab monotherapy. All patients receiving adalimumab for the treatment of HS with at least 1 follow-up visit 3 or more months after treatment initiation were included. Duration of treatment with adalimumab was defined as the length of time between initiation and termination of adalimumab, regardless of flares, adverse events, or addition of adjuvant therapy that occurred during this time span. Because standardized rating scales measuring the severity of HS at this time are not recorded routinely at our institution, treatment duration with adalimumab was used as a surrogate for measuring therapeutic success. Additionally, treatment duration is a meaningful end point, as patients with HS may require indefinite treatment. Patients were eligible for inclusion if they were receiving adalimumab for the treatment of HS. Patients were excluded if they were lost to follow-up or had received adalimumab for less than 6 months, as data suggest that biologics do not reach peak effect for up to 6 months in HS.4
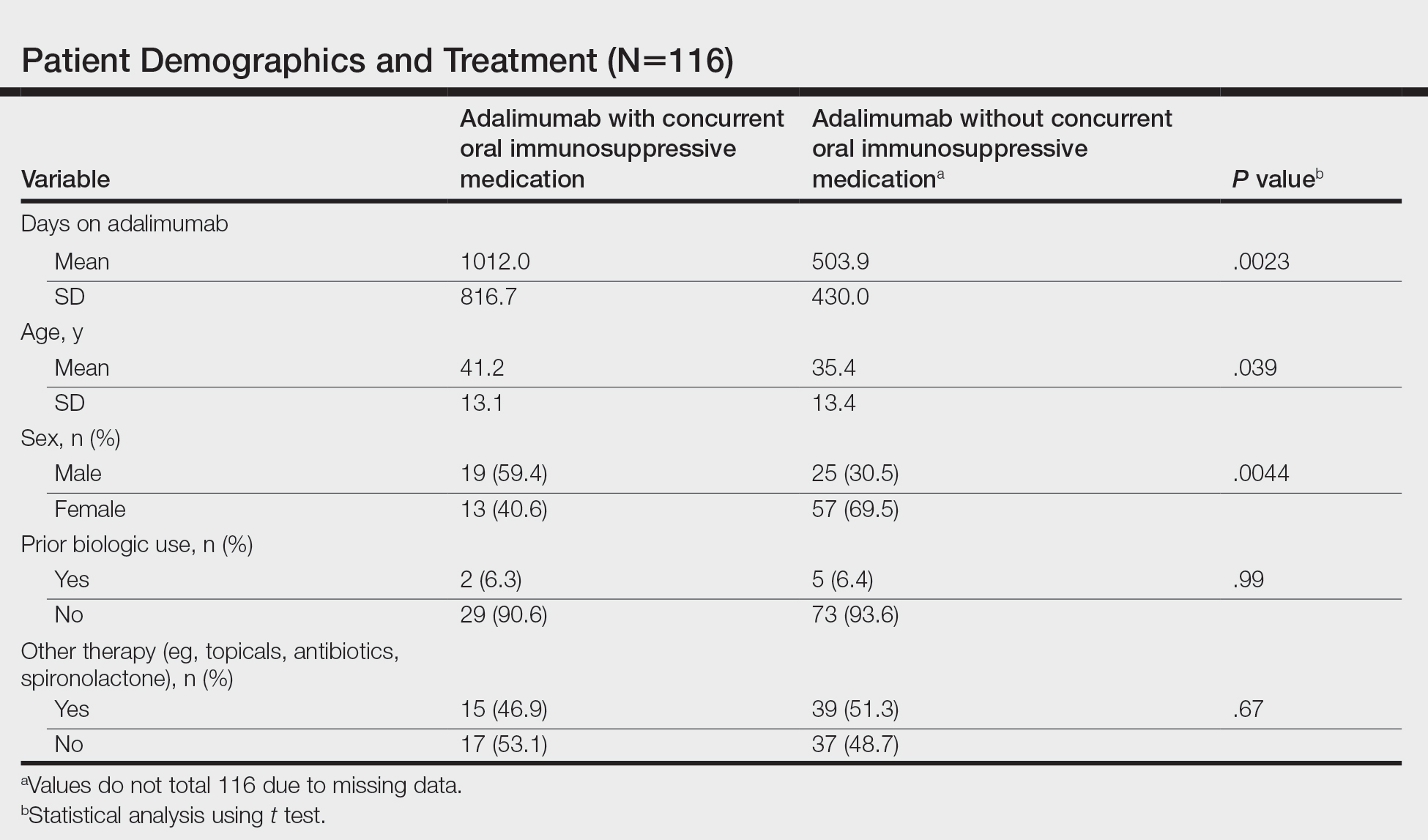
We identified 116 eligible patients with HS, 32 of whom received combination therapy. Five patients received 40 mg of adalimumab every other week, and 111 patients received 40 mg of adalimumab each week. Patients receiving oral immunosuppressants were more likely to be male and as likely to be biologic naïve compared to patients on monotherapy (Table). The average weekly dose of MTX was 14.63 mg, and the average daily dose of MMF was 1000 mg. The average number of days between starting adalimumab and starting an oral immunosuppressant was 114.5 (SD, 217; median, 0) days. Reasons for discontinuation of adalimumab included insufficient response, noncompliance, dislike of injections, adverse events, fear of adverse events, other medical issues unrelated to HS, and insurance coverage issues. Patients who ended treatment with adalimumab owing to insurance coverage issues were still included in our study because insurance coverage remains a major determinant of treatment choice in HS and is relevant to the dynamics of medical decision-making.
Statistical analysis was conducted on all patients inclusive of any reason for discontinuation to avoid bias in the calculation of treatment duration. Cox regression analysis was conducted for all independent variables and was noncontributory. Kaplan-Meier methodology was used to assess the duration of treatment of adalimumab with and without concomitant oral immunosuppressants, and quartile survival times were calculated. Quartile survival time is the time point after adalimumab initiation at which 25% of patients have discontinued adalimumab. We chose quartile survival time instead of average treatment duration to adequately power this study, given our small patient pool.
Although patients receiving adalimumab with oral immunosuppressants had a longer quartile treatment duration (450 days; 95% CI, 185-1800) than the group without oral immunosuppressants (360 days; 95% CI, 200-700), neither MTX nor MMF was shown to significantly prolong duration of therapy with adalimumab (log-rank test: P=.12). Additionally, patients receiving combination therapy were just as likely to discontinue adalimumab as those on monotherapy (χ2 test: P=.93). Patients who took both MTX and MMF at different times did show a statistically significant increase in adalimumab quartile treatment duration (1710 days; 95% CI, 1620 [upper limit not calculable]), but this is likely because these patients were kept on adalimumab while trialing adjunctive medications.
The results of our study indicate that MTX and MMF do not prolong duration of adalimumab therapy, which suggests that adalimumab combination therapy with MTX and MMF may not improve HS more than adalimumab alone, and/or partial responders to adalimumab monotherapy are unlikely to be converted to satisfactory responders with the addition of oral immunosuppressants. Limitations of our study include that it was retrospective, used treatment duration as a surrogate for objective efficacy measures, and relied on a single-institution data source. Additionally, owing to our small sample size, we were unable to account for certain potential confounders, including patient weight and insurance status. Future controlled prospective studies using objective end points are needed to further elucidate whether oral immunosuppressants have a role as an adjunct in the treatment of HS.
- Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi:10.1016/j.jaad.2018.05.040
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Sultan KS, Berkowitz JC, Khan S. Combination therapy for inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2017;8:103-113. doi:10.4292/wjgpt.v8.i2.103
- Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181:609-611.
To the Editor:
The tumor necrosis factor α inhibitor adalimumab is the only US Food and Drug Administration–approved treatment of hidradenitis suppurativa (HS). Although 50.6% of patients fulfilled Hidradenitis Suppurativa Clinical Response criteria with adalimumab at 12 weeks, many responders were not satisfied with their disease control, and secondary loss of Hidradenitis Suppurativa Clinical Response fulfillment occurred in 15.9% of patients within approximately 3 years.1 Without other US Food and Drug Administration–approved HS treatments, some dermatologists have combined adalimumab with methotrexate (MTX) and/or mycophenolate mofetil (MMF) to attempt to increase the duration of satisfactory disease control while on adalimumab. Combining tumor necrosis factor α inhibitors with oral immunosuppressants is a well-established approach in psoriasis, psoriatic arthritis, and inflammatory bowel disease; however, to the best of our knowledge, this approach has not been studied for HS.2,3
To assess whether there is a role for combining adalimumab with MTX and/or MMF in the treatment of HS, we performed a single-institution retrospective chart review at the University of Connecticut Department of Dermatology to determine whether patients receiving combination therapy stayed on adalimumab longer than those who received adalimumab monotherapy. All patients receiving adalimumab for the treatment of HS with at least 1 follow-up visit 3 or more months after treatment initiation were included. Duration of treatment with adalimumab was defined as the length of time between initiation and termination of adalimumab, regardless of flares, adverse events, or addition of adjuvant therapy that occurred during this time span. Because standardized rating scales measuring the severity of HS at this time are not recorded routinely at our institution, treatment duration with adalimumab was used as a surrogate for measuring therapeutic success. Additionally, treatment duration is a meaningful end point, as patients with HS may require indefinite treatment. Patients were eligible for inclusion if they were receiving adalimumab for the treatment of HS. Patients were excluded if they were lost to follow-up or had received adalimumab for less than 6 months, as data suggest that biologics do not reach peak effect for up to 6 months in HS.4
We identified 116 eligible patients with HS, 32 of whom received combination therapy. Five patients received 40 mg of adalimumab every other week, and 111 patients received 40 mg of adalimumab each week. Patients receiving oral immunosuppressants were more likely to be male and as likely to be biologic naïve compared to patients on monotherapy (Table). The average weekly dose of MTX was 14.63 mg, and the average daily dose of MMF was 1000 mg. The average number of days between starting adalimumab and starting an oral immunosuppressant was 114.5 (SD, 217; median, 0) days. Reasons for discontinuation of adalimumab included insufficient response, noncompliance, dislike of injections, adverse events, fear of adverse events, other medical issues unrelated to HS, and insurance coverage issues. Patients who ended treatment with adalimumab owing to insurance coverage issues were still included in our study because insurance coverage remains a major determinant of treatment choice in HS and is relevant to the dynamics of medical decision-making.
Statistical analysis was conducted on all patients inclusive of any reason for discontinuation to avoid bias in the calculation of treatment duration. Cox regression analysis was conducted for all independent variables and was noncontributory. Kaplan-Meier methodology was used to assess the duration of treatment of adalimumab with and without concomitant oral immunosuppressants, and quartile survival times were calculated. Quartile survival time is the time point after adalimumab initiation at which 25% of patients have discontinued adalimumab. We chose quartile survival time instead of average treatment duration to adequately power this study, given our small patient pool.
Although patients receiving adalimumab with oral immunosuppressants had a longer quartile treatment duration (450 days; 95% CI, 185-1800) than the group without oral immunosuppressants (360 days; 95% CI, 200-700), neither MTX nor MMF was shown to significantly prolong duration of therapy with adalimumab (log-rank test: P=.12). Additionally, patients receiving combination therapy were just as likely to discontinue adalimumab as those on monotherapy (χ2 test: P=.93). Patients who took both MTX and MMF at different times did show a statistically significant increase in adalimumab quartile treatment duration (1710 days; 95% CI, 1620 [upper limit not calculable]), but this is likely because these patients were kept on adalimumab while trialing adjunctive medications.
The results of our study indicate that MTX and MMF do not prolong duration of adalimumab therapy, which suggests that adalimumab combination therapy with MTX and MMF may not improve HS more than adalimumab alone, and/or partial responders to adalimumab monotherapy are unlikely to be converted to satisfactory responders with the addition of oral immunosuppressants. Limitations of our study include that it was retrospective, used treatment duration as a surrogate for objective efficacy measures, and relied on a single-institution data source. Additionally, owing to our small sample size, we were unable to account for certain potential confounders, including patient weight and insurance status. Future controlled prospective studies using objective end points are needed to further elucidate whether oral immunosuppressants have a role as an adjunct in the treatment of HS.
To the Editor:
The tumor necrosis factor α inhibitor adalimumab is the only US Food and Drug Administration–approved treatment of hidradenitis suppurativa (HS). Although 50.6% of patients fulfilled Hidradenitis Suppurativa Clinical Response criteria with adalimumab at 12 weeks, many responders were not satisfied with their disease control, and secondary loss of Hidradenitis Suppurativa Clinical Response fulfillment occurred in 15.9% of patients within approximately 3 years.1 Without other US Food and Drug Administration–approved HS treatments, some dermatologists have combined adalimumab with methotrexate (MTX) and/or mycophenolate mofetil (MMF) to attempt to increase the duration of satisfactory disease control while on adalimumab. Combining tumor necrosis factor α inhibitors with oral immunosuppressants is a well-established approach in psoriasis, psoriatic arthritis, and inflammatory bowel disease; however, to the best of our knowledge, this approach has not been studied for HS.2,3
To assess whether there is a role for combining adalimumab with MTX and/or MMF in the treatment of HS, we performed a single-institution retrospective chart review at the University of Connecticut Department of Dermatology to determine whether patients receiving combination therapy stayed on adalimumab longer than those who received adalimumab monotherapy. All patients receiving adalimumab for the treatment of HS with at least 1 follow-up visit 3 or more months after treatment initiation were included. Duration of treatment with adalimumab was defined as the length of time between initiation and termination of adalimumab, regardless of flares, adverse events, or addition of adjuvant therapy that occurred during this time span. Because standardized rating scales measuring the severity of HS at this time are not recorded routinely at our institution, treatment duration with adalimumab was used as a surrogate for measuring therapeutic success. Additionally, treatment duration is a meaningful end point, as patients with HS may require indefinite treatment. Patients were eligible for inclusion if they were receiving adalimumab for the treatment of HS. Patients were excluded if they were lost to follow-up or had received adalimumab for less than 6 months, as data suggest that biologics do not reach peak effect for up to 6 months in HS.4
We identified 116 eligible patients with HS, 32 of whom received combination therapy. Five patients received 40 mg of adalimumab every other week, and 111 patients received 40 mg of adalimumab each week. Patients receiving oral immunosuppressants were more likely to be male and as likely to be biologic naïve compared to patients on monotherapy (Table). The average weekly dose of MTX was 14.63 mg, and the average daily dose of MMF was 1000 mg. The average number of days between starting adalimumab and starting an oral immunosuppressant was 114.5 (SD, 217; median, 0) days. Reasons for discontinuation of adalimumab included insufficient response, noncompliance, dislike of injections, adverse events, fear of adverse events, other medical issues unrelated to HS, and insurance coverage issues. Patients who ended treatment with adalimumab owing to insurance coverage issues were still included in our study because insurance coverage remains a major determinant of treatment choice in HS and is relevant to the dynamics of medical decision-making.
Statistical analysis was conducted on all patients inclusive of any reason for discontinuation to avoid bias in the calculation of treatment duration. Cox regression analysis was conducted for all independent variables and was noncontributory. Kaplan-Meier methodology was used to assess the duration of treatment of adalimumab with and without concomitant oral immunosuppressants, and quartile survival times were calculated. Quartile survival time is the time point after adalimumab initiation at which 25% of patients have discontinued adalimumab. We chose quartile survival time instead of average treatment duration to adequately power this study, given our small patient pool.
Although patients receiving adalimumab with oral immunosuppressants had a longer quartile treatment duration (450 days; 95% CI, 185-1800) than the group without oral immunosuppressants (360 days; 95% CI, 200-700), neither MTX nor MMF was shown to significantly prolong duration of therapy with adalimumab (log-rank test: P=.12). Additionally, patients receiving combination therapy were just as likely to discontinue adalimumab as those on monotherapy (χ2 test: P=.93). Patients who took both MTX and MMF at different times did show a statistically significant increase in adalimumab quartile treatment duration (1710 days; 95% CI, 1620 [upper limit not calculable]), but this is likely because these patients were kept on adalimumab while trialing adjunctive medications.
The results of our study indicate that MTX and MMF do not prolong duration of adalimumab therapy, which suggests that adalimumab combination therapy with MTX and MMF may not improve HS more than adalimumab alone, and/or partial responders to adalimumab monotherapy are unlikely to be converted to satisfactory responders with the addition of oral immunosuppressants. Limitations of our study include that it was retrospective, used treatment duration as a surrogate for objective efficacy measures, and relied on a single-institution data source. Additionally, owing to our small sample size, we were unable to account for certain potential confounders, including patient weight and insurance status. Future controlled prospective studies using objective end points are needed to further elucidate whether oral immunosuppressants have a role as an adjunct in the treatment of HS.
- Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi:10.1016/j.jaad.2018.05.040
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Sultan KS, Berkowitz JC, Khan S. Combination therapy for inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2017;8:103-113. doi:10.4292/wjgpt.v8.i2.103
- Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181:609-611.
- Zouboulis CC, Okun MM, Prens EP, et al. Long-term adalimumab efficacy in patients with moderate-to-severe hidradenitis suppurativa/acne inversa: 3-year results of a phase 3 open-label extension study. J Am Acad Dermatol. 2019;80:60-69.e2. doi:10.1016/j.jaad.2018.05.040
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Sultan KS, Berkowitz JC, Khan S. Combination therapy for inflammatory bowel disease. World J Gastrointest Pharmacol Ther. 2017;8:103-113. doi:10.4292/wjgpt.v8.i2.103
- Prussick L, Rothstein B, Joshipura D, et al. Open-label, investigator-initiated, single-site exploratory trial evaluating secukinumab, an anti-interleukin-17A monoclonal antibody, for patients with moderate-to-severe hidradenitis suppurativa. Br J Dermatol. 2019;181:609-611.
Practice Points
- Adalimumab is the only medication approved by the US Food and Drug Administration for treatment of hidradenitis suppurativa (HS), yet many patients on adalimumab do not achieve satisfactory results. New treatment options are in demand for patients affected by HS.
- Although combining tumor necrosis factor α inhibitors with oral immunosuppressants such as methotrexate and mycophenolate mofetil appears to be beneficial in treating other conditions such as psoriasis, these treatments may not have as great a benefit for patients with HS.
Reflectance Confocal Microscopy to Facilitate Knifeless Skin Cancer Management
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
Practice Gap
Management of nonmelanoma skin cancer (NMSC) in elderly patients can cause morbidity because these patients frequently struggle to care for their biopsy sites and experience biopsy- and surgery-related complications. To minimize this treatment-related morbidity, we designed a knifeless treatment approach that employs reflectance confocal microscopy (RCM) in lieu of skin biopsy to establish the diagnosis of NMSC, then uses either intralesional or topical chemotherapy or immunotherapy (as appropriate, depending on depth of invasion) to cure the NMSC. With this approach, the patient is spared both biopsy- and surgery-related difficulties, though both intralesional and topical chemotherapy are accompanied by their own risks for adverse effects.
The Technique
Elderly patients, diabetic patients, and patients with lesions suspicious for NMSC on areas prone to poor wound healing or to notable treatment-related morbidity (eg, lower legs, genitals, the face of younger patients) are offered skin biopsy or RCM; the latter is performed during the appointment by an RC
When resolution is uncertain, RCM is repeated to assess for tumor clearance. Repeat RCM is performed at least 4 weeks after termination of treatment to avoid misinterpretation caused by treatment-related tissue inflammation. Patients who are not cured using this management approach are offered appropriate surgical management.
Practice Implications
Reflectance confocal microscopy has emerged as an effective modality for confirming the diagnosis of NMSC with high sensitivity and specificity.1,2 Emergence of this technology presents an opportunity for improving the way the NMSC is managed because RCM allows dermatologists to confirm the diagnosis of BCC and SCC by interpretation of RCM mosaics rather than by histopathologic examination of biopsied tissue. Our knifeless approach to skin cancer management is especially beneficial when biopsy and dermatologic surgery are likely to confer notable morbidity, such as managing NMSC on the face of a young adult, in the frail elderly population, or in diabetic patients, and when treating sites on the lower extremity prone to poor wound healing.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
- Song E, Grant-Kels JM, Swede H, et al. Paired comparison of the sensitivity and specificity of multispectral digital skin lesion analysis and reflectance confocal microscopy in the detection of melanoma in vivo: a cross-sectional study. J Am Acad Dermatol. 2016;75:1187-1192.
- Ferrari B, Salgarelli AC, Mandel VD, et al. Non-melanoma skin cancer of the head and neck: the aid of reflectance confocal microscopy for the accurate diagnosis and management. G Ital Dermatol Venereol. 2017;152:169-177.
Clinical Pearl: Topical Timolol for Refractory Hypergranulation
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
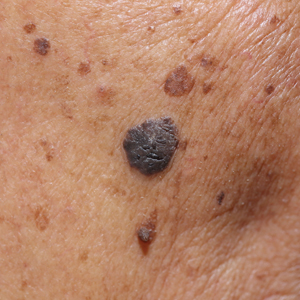
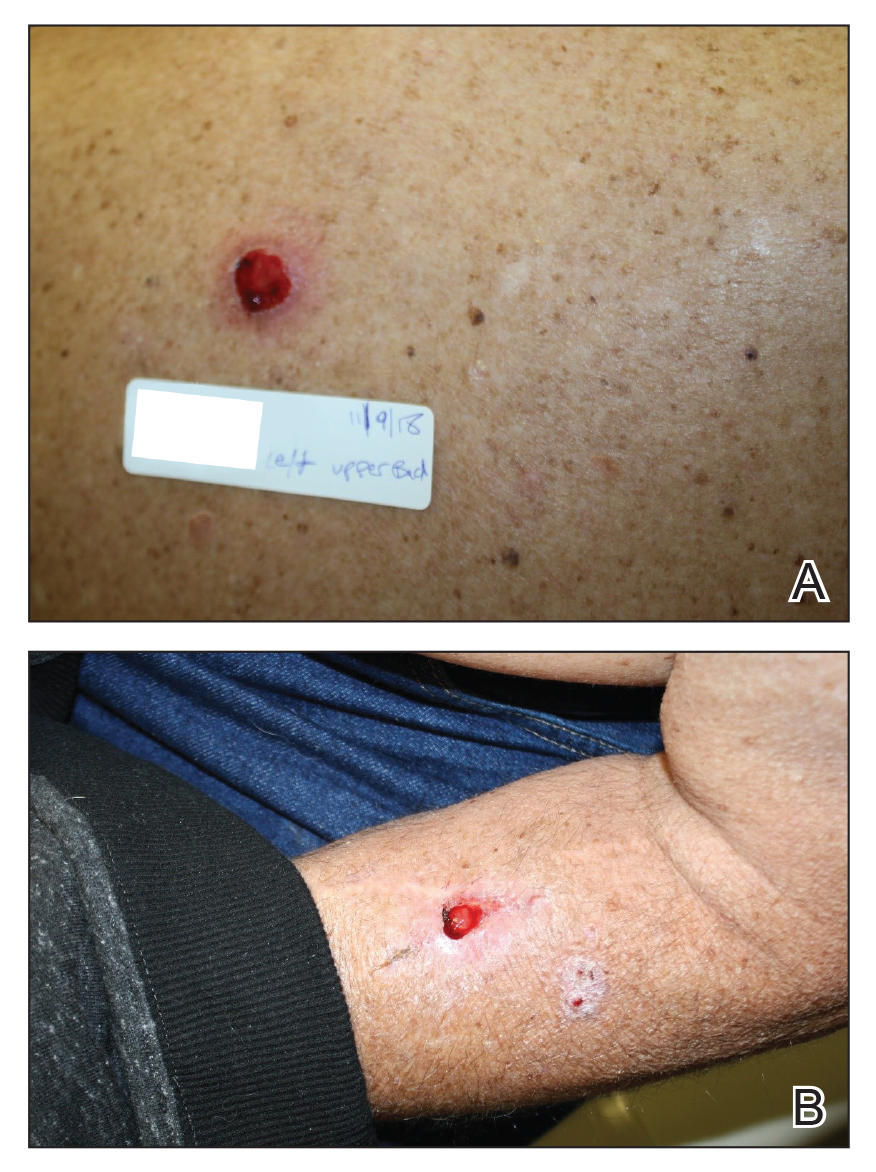
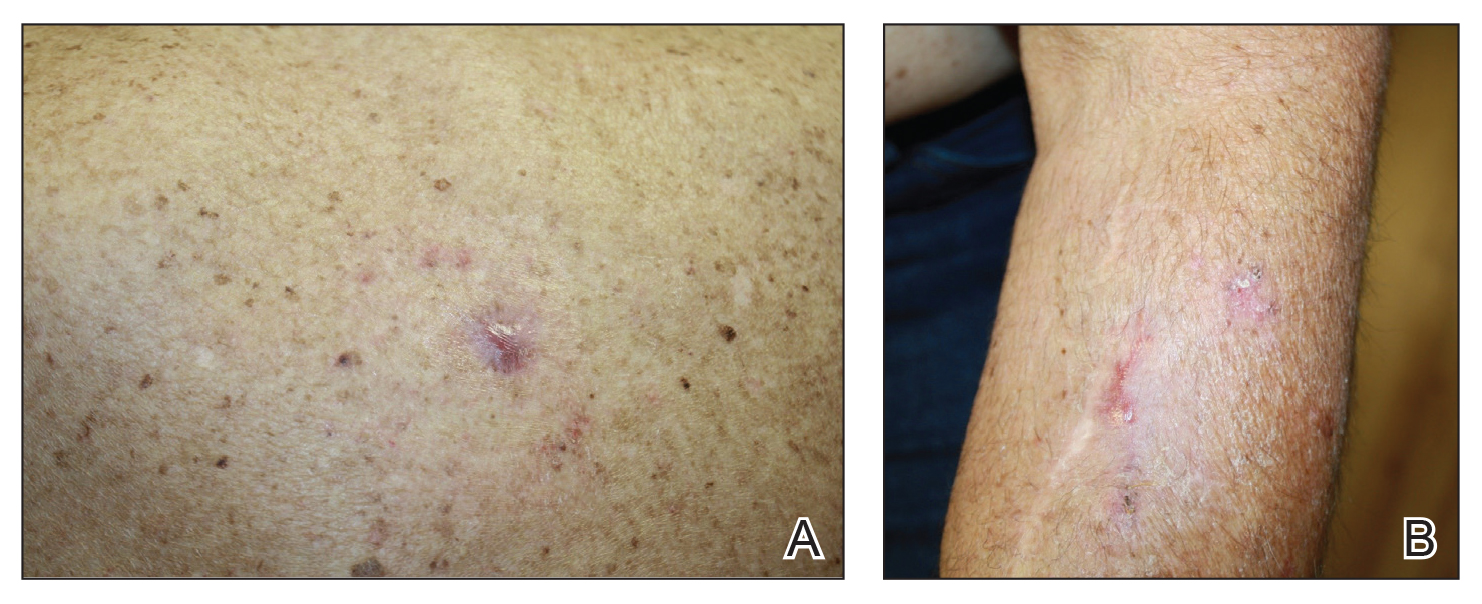
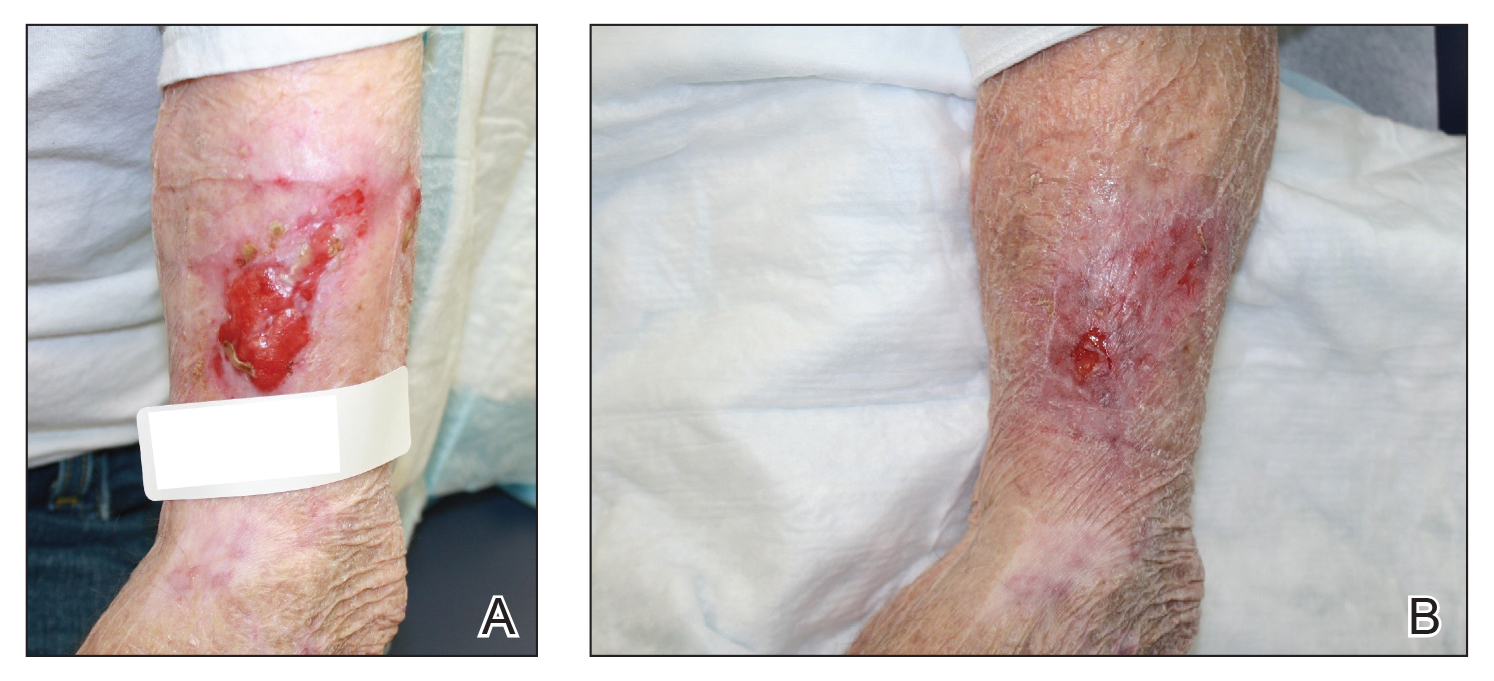
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.