User login
Pulmonary infarction due to pulmonary embolism
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
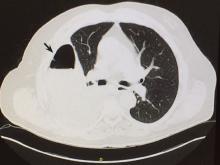
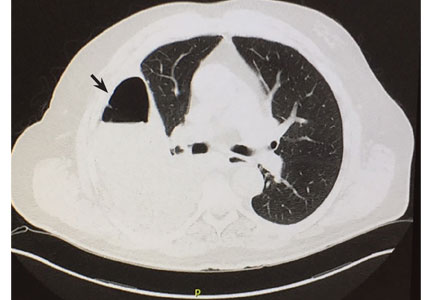
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
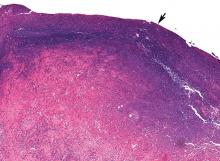
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
A 76-year-old man whose history included abdominal aortic aneurysm repair, bilateral femoral artery bypass for popliteal artery aneurysm, hypertension, and peptic ulcer disease was admitted to a community hospital with pleuritic chest pain and shortness of breath. Two days earlier, he had undergone repair of a ventral hernia.
At the time of that admission, he reported no fever, chills, night sweats, cough, or history of heart or lung disease. His vital signs were normal, and physical examination had revealed no apparent respiratory distress, no jugular venous distention, normal heart sounds, and no pedal edema; however, decreased air entry was noted in the right lung base. Initial serum levels of troponin and N-terminal pro-B-type natriuretic peptide were normal.
At that time, computed tomographic angiography of the chest showed segmental pulmonary emboli in the left upper and right lower lobes of the lungs and right pleural effusion. Transthoracic echocardiography showed normal atrial and ventricular sizes with no right or left ventricular systolic dysfunction and a left ventricular ejection fraction of 59%.
Treatment with intravenous heparin was started, and the patient was transferred to our hospital.
PLEURAL EFFUSION AND PULMONARY EMBOLISM
1. Which of the following is true about pleural effusion?
- It is rarely, if ever, associated with pulmonary embolism
- Most patients with pleural effusion due to pulmonary embolism do not have pleuritic chest pain
- Pulmonary embolism should be excluded in all cases of pleural effusion without a clear cause
Pulmonary embolism should be excluded in all cases of pleural effusion that do not have a clear cause. As for the other answer choices:
- Pulmonary embolism is the fourth leading cause of pleural effusion in the United States, after heart failure, pneumonia, and malignancy.1
- About 75% of patients who develop pleural effusion in the setting of pulmonary embolism complain of pleuritic chest pain on the side of the effusion.2 Most effusions are unilateral, small, and usually exudative.3
EVALUATION BEGINS: RESULTS OF THORACENTESIS
Our patient continued to receive intravenous heparin.
He underwent thoracentesis on hospital day 3, and 1,000 mL of turbid sanguineous pleural fluid was removed. Analysis of the fluid showed pH 7.27, white blood cell count 3.797 × 109/L with 80% neutrophils, and lactate dehydrogenase (LDH) concentration 736 U/L (a ratio of pleural fluid LDH to a concurrent serum LDH > 0.6 is suggestive of an exudate); the fluid was also sent for culture and cytology. Thoracentesis was terminated early due to cough, and follow-up chest radiography showed a moderate-sized pneumothorax.
Computed tomography (CT) of the chest at this time showed a small wedge-shaped area of lung consolidation in the right lower lobe (also seen on CT done 1 day before admission to our hospital), with an intrinsic air-fluid level suggesting a focal infarct or lung abscess, now obscured by adjacent consolidation and atelectasis. In the interval since the previous CT, the multiloculated right pleural effusion had increased in size (Figure 1).
THE NEXT STEP
2. What is the most appropriate next step for this patient?
- Consult an interventional radiologist for chest tube placement
- Start empiric antibiotic therapy and ask an interventional radiologist to place a chest tube
- Start empiric antibiotic therapy, withhold anticoagulation, and consult a thoracic surgeon
- Start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation
The most appropriate next step is to start empiric antibiotic therapy and consult a thoracic surgeon while continuing anticoagulation.
In this patient, it is appropriate to initiate antibiotics empirically on the basis of his significant pleural loculations, a wedge-shaped consolidation, and 80% neutrophils in the pleural fluid, all of which suggest infection. The unmasking of a wedge-shaped consolidation after thoracentesis, with a previously noted air-fluid level and an interval increase in multiloculated pleural fluid, raises suspicion of a necrotic infection that may have ruptured into the pleural space, a possible lung infarct, or a malignancy. Hence, simply placing a chest tube may not be enough.
Blood in the pleural fluid does not necessitate withholding anticoagulation unless the bleeding is heavy. A pleural fluid hematocrit greater than 50% of the peripheral blood hematocrit suggests hemothorax and is an indication to withhold anticoagulation.1 Our patient’s pleural fluid was qualitatively sanguineous but not frankly bloody, and therefore we judged that it was not necessary to stop his heparin.
HOW DOES PULMONARY INFARCTION PRESENT CLINICALLY?
3. Which of the following statements about pulmonary infarction is incorrect?
- Cavitation and infarction are more common with larger emboli
- Cavitation occurs in fewer than 10% of pulmonary infarctions
- Lung abscess develops in more than 50% of pulmonary infarctions
- Pulmonary thromboembolism is the most common cause of pulmonary infarction
Lung abscess develops in far fewer than 50% of cases of pulmonary infarction. The rest of the statements are correct.
Cavitation complicates about 4% to 7% of infarctions and is more common when the infarction is 4 cm or greater in diameter.4 These cavities are usually single and predominantly on the right side in the apical or posterior segment of the upper lobe or the apical segment of the right lower lobe, as in our patient.5–8 CT demonstrating scalloped inner margins and cross-cavity band shadows suggests a cavitary pulmonary infarction.9,10
Infection and abscess in pulmonary infarction are poorly understood but have been linked to larger infarctions, coexistent congestion or atelectasis, and dental or oropharyngeal infection. In an early series of 550 cases of pulmonary infarction, 23 patients (4.2%) developed lung abscess and 6 (1.1%) developed empyema.11 The mean time to cavitation for an infected pulmonary infarction has been reported to be 18 days.12
A reversed halo sign, generally described as a focal, rounded area of ground-glass opacity surrounded by a nearly complete ring of consolidation, has been reported to be more frequent with pulmonary infarction than with other diseases, especially when in the lower lobes.13
CASE CONTINUED: THORACOSCOPY
A cardiothoracic surgeon was consulted, intravenous heparin was discontinued, an inferior vena cava filter was placed, and the patient underwent video-assisted thoracoscopy.
Purulent fluid was noted on the lateral aspect of right lower lobe; this appeared to be the ruptured cavitary lesion functioning like an uncontrolled bronchopleural fistula. Two chest tubes, sizes 32F and 28F, were placed after decortication, resection of the lung abscess, and closure of the bronchopleural fistula. No significant air leak was noted after resection of this segment of lung.
Pathologic study showed acute organizing pneumonia with abscess formation; no malignant cells or granulomas were seen (Figure 2). Pleural fluid cultures grew Streptococcus intermedius, while the tissue culture was negative for any growth, including acid-fast bacilli and fungi.
On 3 different occasions, both chest tubes were shortened, backed out 2 cm, and resecured with sutures and pins, and Heimlich valves were applied before the patient was discharged.
Intravenous piperacillin-tazobactam was started on the fifth hospital day. On discharge, the patient was advised to continue this treatment for 3 weeks at home.
The patient was receiving enoxaparin subcutaneously in prophylactic doses; 72 hours after the thorascopic procedure this was increased to therapeutic doses, continuing after discharge. Bridging to warfarin was not advised in view of his chest tubes.
Our patient appeared to have developed a right lower lobe infarction that cavitated and ruptured into the pleural space, causing a bronchopleural fistula with empyema after a recent pulmonary embolism. Other reported causes of pulmonary infarction in pulmonary embolism are malignancy and heavy clot burden,6 but these have not been confirmed in subsequent studies.5 Malignancy was ruled out by biopsy of the resected portion of the lung, and our patient did not have a history of heart failure. A clear cavity was not noted (because it ruptured into the pleura), but an air-fluid level was described in a wedge-shaped consolidation, suggesting infarction.
How common is pulmonary infarction after pulmonary embolism?
Pulmonary infarction occurs in few patients with pulmonary embolism.13 Since the lungs receive oxygen from the airways and have a dual blood supply from the pulmonary and bronchial arteries, they are not particularly vulnerable to ischemia. However, the reported incidence of pulmonary infarction in patients with pulmonary embolism has ranged from 10% to higher than 30%.5,14,15
The reasons behind pulmonary infarction with complications after pulmonary embolism have varied in different case series in different eras. CT, biopsy, or autopsy studies reveal pulmonary infarction after pulmonary embolism to be more common than suspected by clinical symptoms.
In a Mayo Clinic series of 43 cases of pulmonary infarction diagnosed over a 6-year period by surgical lung biopsy, 18 (42%) of the patients had underlying pulmonary thromboembolism, which was the most common cause.16
RISK FACTORS FOR PULMONARY INFARCTION
4. Which statement about risk factors for pulmonary infarction in pulmonary embolism is incorrect?
- Heart failure may be a risk factor for pulmonary infarction
- Pulmonary hemorrhage is a risk factor for pulmonary infarction
- Pulmonary infarction is more common with more proximal sites of pulmonary embolism
- Collateral circulation may protect against pulmonary infarction
Infarction is more common with emboli that are distal rather than proximal.
Dalen et al15 suggested that after pulmonary embolism, pulmonary hemorrhage is an important contributor to the development of pulmonary infarction independent of the presence or absence of associated cardiac or pulmonary disease, but that the effect depends on the site of obstruction.
This idea was first proposed in 1913, when Karsner and Ghoreyeb17 showed that when pulmonary arteries are completely obstructed, the bronchial arteries take over, except when the embolism is present in a small branch of the pulmonary artery. This is because the physiologic anastomosis between the pulmonary artery and the bronchial arteries is located at the precapillary level of the pulmonary artery, and the bronchial circulation does not take over until the pulmonary arterial pressure in the area of the embolism drops to zero.
Using CT data, Kirchner et al5 confirmed that the risk of pulmonary infarction is higher if the obstruction is peripheral, ie, distal.
Using autopsy data, Tsao et al18 reported a higher risk of pulmonary infarction in embolic occlusion of pulmonary vessels less than 3 mm in diameter.
Collateral circulation has been shown to protect against pulmonary infarction. For example, Miniati et al14 showed that healthy young patients with pulmonary embolism were more prone to develop pulmonary infarction, probably because they had less efficient collateral systems in the peripheral lung fields. In lung transplant recipients, it has been shown that the risk of infarction decreased with development of collateral circulation.19
Dalen et al,15 however, attributed delayed resolution of pulmonary hemorrhage (as measured by resolution of infiltrate on chest radiography) to higher underlying pulmonary venous pressure in patients with heart failure and consequent pulmonary infarction. In comparison, healthy patients without cardiac or pulmonary disease have faster resolution of pulmonary hemorrhage when present, and less likelihood of pulmonary infarction (and death in submassive pulmonary embolism).
Data on the management of infected pulmonary infarction are limited. Mortality rates have been as high as 41% with noninfected and 73% with infected cavitary infarctions.4 Some authors have advocated early surgical resection in view of high rates of failure of medical treatment due to lack of blood supply within the cavity and continued risk of infection.
KEY POINTS
In patients with a recently diagnosed pulmonary embolism and concurrent symptoms of bacterial pneumonia, a diagnosis of cavitary pulmonary infarction should be considered.
Consolidations that are pleural-based with sharp, rounded margins and with focal areas of central hyperlucencies representing hemorrhage on the mediastinal windows on CT are more likely to represent a pulmonary infarct.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
- Light RW. Pleural Diseases. 4th ed. Baltimore, MD: Lippincott, Williams & Wilkins; 2001.
- Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest 1991; 100(3):598–603. pmid:1909617
- Light RW. Pleural effusion due to pulmonary emboli. Curr Opin Pulm Med 2001; 7(4):198–201. pmid:11470974
- Libby LS, King TE, LaForce FM, Schwarz MI. Pulmonary cavitation following pulmonary infarction. Medicine (Baltimore) 1985; 64(5):342–348. pmid:4033411
- Kirchner J, Obermann A, Stuckradt S, et al. Lung infarction following pulmonary embolism: a comparative study on clinical conditions and CT findings to identify predisposing factors. Rofo 2015; 187(6):440–444. doi:10.1055/s-0034-1399006
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging 2006; 21(1):1–7. doi:10.1097/01.rti.0000187433.06762.fb
- Scharf J, Nahir AM, Munk J, Lichtig C. Aseptic cavitation in pulmonary infarction. Chest 1971; 59(4):456–458. pmid:5551596
- Wilson AG, Joseph AE, Butland RJ. The radiology of aseptic cavitation in pulmonary infarction. Clin Radiol 1986; 37(4):327–333. pmid:3731699
- Butler MD, Biscardi FH, Schain DC, Humphries JE, Blow O, Spotnitz WD. Pulmonary resection for treatment of cavitary pulmonary infarction. Ann Thorac Surg 1997; 63(3):849–850. pmid:9066420
- Koroscil MT, Hauser TR. Acute pulmonary embolism leading to cavitation and large pulmonary abscess: a rare complication of pulmonary infarction. Respir Med Case Rep 2016; 20:72–74. doi:10.1016/j.rmcr.2016.12.001
- Levin L, Kernohan JW, Moersch HJ. Pulmonary abscess secondary to bland pulmonary infarction. Dis Chest 1948; 14(2):218–232. pmid:18904835
- Marchiori E, Menna Barreto M, Pereira Freitas HM, et al. Morphological characteristics of the reversed halo sign that may strongly suggest pulmonary infarction. Clin Radiol 2018; 73(5):503.e7–503.e13. doi:10.1016/j.crad.2017.11.022
- Smith GT, Dexter L, Dammin GJ. Postmortem quantitative studies in pulmonary embolism. In: Sasahara AA, Stein M, eds. Pulmonary Embolic Disease. New York, NY: Grune & Stratton, Inc; 1965:120–126.
- Miniati M, Bottai M, Ciccotosto C, Roberto L, Monti S. Predictors of pulmonary infarction. Medicine (Baltimore) 2015; 94(41):e1488. doi:10.1097/MD.0000000000001488
- Dalen JE, Haffajee CI, Alpert JS, Howe JP, Ockene IS, Paraskos JA. Pulmonary embolism, pulmonary hemorrhage and pulmonary infarction. N Engl J Med 1977; 296(25):1431–1435. doi:10.1056/NEJM197706232962503
- Parambil JG, Savci CD, Tazelaar HD, Ryu JH. Causes and presenting features of pulmonary infarctions in 43 cases identified by surgical lung biopsy. Chest 2005; 127(4):1178–1183. doi:10.1378/chest.127.4.1178
- Karsner HT, Ghoreyeb AA. Studies in infarction: III. The circulation in experimental pulmonary embolism. J Exp Med 1913; 18(5):507–511. pmid:19867725
- Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med 1982; 72(4):599–606. pmid:6462058
- Burns KE, Iacono AT. Incidence of clinically unsuspected pulmonary embolism in mechanically ventilated lung transplant recipients. Transplantation 2003; 76(6):964–968. doi:10.1097/01.TP.0000084523.58610.BA
- Yousem SA. The surgical pathology of pulmonary infarcts: diagnostic confusion with granulomatous disease, vasculitis, and neoplasia. Mod Pathol 2009; 22(5):679–685. doi:10.1038/modpathol.2009.20
Do HCAHPS doctor communication scores reflect the communication skills of the attending on record? A cautionary tale from a tertiary-care medical service
Communication is the foundation of medical care.1 Effective communication can improve health outcomes, safety, adherence, satisfaction, trust, and enable genuine informed consent and decision-making.2-9 Furthermore, high-quality communication increases provider engagement and workplace satisfaction, while reducing stress and malpractice risk.10-15
Direct measurement of communication in the healthcare setting can be challenging. The “Four Habits Model,” which is derived from a synthesis of empiric studies8,16-20 and theoretical models21-24 of communication, offers 1 framework for assessing healthcare communication. The conceptual model underlying the 4 habits has been validated in studies of physician and patient satisfaction.1,4,25-27 The 4 habits are: investing in the beginning, eliciting the patient’s perspective, demonstrating empathy, and investing in the end. Each habit is divided into several identifiable tasks or skill sets, which can be reliably measured using validated tools and checklists.28 One such instrument, the Four Habits Coding Scheme (4HCS), has been evaluated against other tools and demonstrated overall satisfactory inter-rater reliability and validity.29,30
The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, developed under the direction of the Centers for Medicare and Medicaid Services (CMS) and the Agency for Healthcare Research and Quality, is an established national standard for measuring patient perceptions of care. HCAHPS retrospectively measures global perceptions of communication, support and empathy from physicians and staff, processes of care, and the overall patient experience. HCAHPS scores were first collected nationally in 2006 and have been publicly reported since 2008.31 With the introduction of value-based purchasing in 2012, health system revenues are now tied to HCAHPS survey performance.32 As a result, hospitals are financially motivated to improve HCAHPS scores but lack evidence-based methods for doing so. Some healthcare organizations have invested in communication training programs based on the available literature and best practices.2,33-35 However, it is not known how, if at all, HCAHPS scores relate to physicians’ real-time observed communication skills.
To examine the relationship between physician communication, as reported by global HCAHPS scores, and the quality of physician communication skills in specific encounters, we observed hospitalist physicians during inpatient bedside rounds and measured their communication skills using the 4HCS.
METHODS
Study Design
The study utilized a cross sectional design; physicians who consented were observed on rounds during 3 separate encounters, and we compared hospitalists’ 4HCS scores to their HCAHPS scores to assess the correlation. The study was approved by the Institutional Review Board of the Cleveland Clinic.
Population
The study was conducted at the main campus of the Cleveland Clinic. All physicians specializing in hospital medicine who had received 10 or more completed HCAHPS survey responses while rounding on a medicine service in the past year were invited to participate in the study. Participation was voluntary; night hospitalists were excluded. A research nurse was trained in the Four Habits Model28 and in the use of the 4HCS coding scheme by the principal investigator. The nurse observed each physician and ascertained the presence of communication behaviors using the 4HCS tool. Physicians were observed between August 2013 and August 2014. Multiple observations per physician could occur on the same day, but only 1 observation per patient was used for analysis. Observations consisted of a physician’s first encounter with a hospitalized patient, with the patient’s consent. Observations were conducted during encounters with English-speaking and cognitively intact patients only. Resident physicians were permitted to stay and conduct rounds per their normal routine. Patient information was not collected as part of the study.
Measures
HCAHPS. For each physician, we extracted all HCAHPS scores that were collected from our hospital’s Press Ganey database. The HCAHPS survey contains 22 core questions divided into 7 themes or domains, 1 of which is doctor communication. The survey uses frequency-based questions with possible answers fixed on a 4-point scale (4=always, 3=usually, 2=sometimes, 1=never). Our primary outcome was the doctor communication domain, which comprises 3 questions: 1) During this hospital stay, how often did the doctors treat you with respect? 2) During this hospital stay, how often did the doctors listen to you? and 3) During this hospital stay, how often did the doctors explain things in a language you can understand? Because CMS counts only the percentage of responses that are graded “always,” so-called “top box” scoring, we used the same measure.
The HCAHPS scores are always attributed to the physician at the time of discharge even if he may not have been responsible for the care of the patient during the entire hospital course. To mitigate contamination from patients seen by multiple providers, we cross-matched length of stay (LOS) data with billing data to determine the proportion of days a patient was seen by a single provider during the entire length of stay. We stratified patients seen by the attending providers to less than 50%, 50% to less than 100%, and at 100% of the LOS. However, we were unable to identify which patients were seen by other consultants or by residents due to limitations in data gathering and the nature of the database.
The Four Habits. The Four Habits are: invest in the beginning, elicit the patient’s perspective, demonstrate empathy, and invest in the end (Figure 1). Specific behaviors for Habits 1 to 4 are outlined in the Appendix, but we will briefly describe the themes as follows. Habit 1, invest in the beginning, describes the ability of the physician to set a welcoming environment for the patient, establish rapport, and collaborate on an agenda for the visit. Habit 2, elicit the patient’s perspective, describes the ability of the physician to explore the patients’ worries, ideas, expectations, and the impact of the illness on their lifestyle. Habit 3, demonstrate empathy, describes the physician’s openness to the patient’s emotions as well as the ability to explore, validate; express curiosity, and openly accept these feelings. Habit 4, invest in the end, is a measure of the physician’s ability to counsel patients in a language built around their original concerns or worries, as well as the ability to check the patients’ understanding of the plan.2,29-30
4HCS. The 4HCS tool (Appendix) measures discreet behaviors and phrases based on each of the Four Habits (Figure 1). With a scoring range from a low of 4 to a high of 20, the rater at bedside assigns a range of points on a scale of 1 to 5 for each habit. It is an instrument based on a teaching model used widely throughout Kaiser Permanente to improve clinicians’ communication skills. The 4HCS was first tested for interrater reliability and validity against the Roter Interaction Analysis System using 100 videotaped primary care physician encounters.29 It was further evaluated in a randomized control trial. Videotapes from 497 hospital encounters involving 71 doctors from a variety of clinical specialties were rated by 4 trained raters using the coding scheme. The total score Pearson’s R and intraclass correlation coefficient (ICC) exceeded 0.70 for all pairs of raters, and the interrater reliability was satisfactory for the 4HCS as applied to heterogeneous material.30
STATISTICAL ANALYSIS
Physician characteristics were summarized with standard descriptive statistics. Pearson correlation coefficients were computed between HCAHPS and 4HCS scores. All analyses were performed with RStudio (Boston, MA). The Pearson correlation between the averaged HCAHPS and 4HCS scores was also computed. A correlation with a P value less than 0.05 was considered statistically significant. With 28 physicians, the study had a power of 88% to detect a moderate correlation (greater than 0.50) with a 2-sided alpha of 0.05. We also computed the correlations based on the subgroups of data with patients seen by providers for less than 50%, 50% to less than 100%, and 100% of LOS. All analyses were conducted in SAS 9.2 (SAS Institute Inc., Cary, NC).36
RESULTS
There were 31 physicians who met our inclusion criteria. Of 29 volunteers, 28 were observed during 3 separate inpatient encounters and made up the final sample. A total of 1003 HCAHPS survey responses were available for these physicians. Participants were predominantly female (60.7%), with an average age of 39 years. They were in practice for an average of 4 years (12 were in practice more than 5 years), and 9 were observed on a teaching rotation.
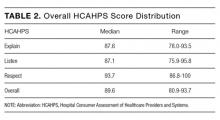
The means of the overall 4HCS scores per observation were 17.39 ± 2.33 for the first, 17.00 ± 2.37 for the second, and 17.43 ± 2.36 for third bedside observation. The mean 4HCS scores per observation, broken down by habit, appear in Table 1. The ICC among the repeated scores within the same physician was 0.81. The median number of HCAHPS survey returns was 32 (range = [8, 85], with mean = 35.8, interquartile range = [16, 54]). The median overall HCAHPS doctor communication score was 89.6 (range = 80.9-93.7). Participants scored the highest in the respect subdomain and the lowest in the explain subdomain. Median HCAHPS scores and ranges appear in Table 2.
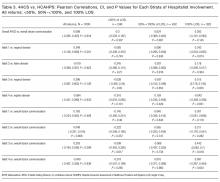
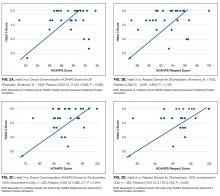
Because there were no significant associations between 4HCS scores or HCAHPS scores and physician age, sex, years in practice, or teaching site, correlations were not adjusted. Figure 2A and 2B show the association between mean 4HCS scores and HCAHPS scores by physician. There was no significant correlation between overall 4HCS and HCAHPS doctor communication scores (Pearson correlation coefficient 0.098; 95% confidence interval [CI], -0.285, 0.455). The individual habits also were not correlated with overall HCAHPS scores or with their corresponding HCAHPS domain (Table 3).
For 325 patients, 1 hospitalist was present for the entire LOS. In sensitivity analysis limiting observations to these patients (Figure 2C, Figure 2D, Table 3), we found a moderate correlation between habit 3 and the HCAHPS respect score (Pearson correlation coefficient 0.515; 95% CI, 0.176, 0.745; P = 0.005), and a weaker correlation between habit 3 and the HCAHPS overall doctor communication score (0.442; 95% CI, 0.082, 0.7; P = 0.019). There were no other significant correlations between specific habits and HCAHPS scores.
DISCUSSION
In this observational study of hospitalist physicians at a large tertiary care center, we found that communication skills, as measured by the 4HCS, varied substantially among physicians but were highly correlated within patients of the same physician. However, there was virtually no correlation between the attending physician of record’s 4HCS scores and their HCAHPS communication scores. When we limited our analysis to patients who saw only 1 hospitalist throughout their stay, there were moderate correlations between demonstration of empathy and both the HCAHPS respect score and overall doctor communication score. There were no trends across the strata of hospitalist involvement. It is important to note that the addition of even 1 different hospitalist to the LOS removes any association. Habits 1 and 2 are close to significance in the 100% subgroup, with a weak correlation. Interestingly, Habit 4, which focuses on creating a plan with the patient, showed no correlation at all with patients reporting that doctors explained things in language they could understand.
Development and testing of the HCAHPS survey began in 2002, commissioned by CMS and the Agency for Healthcare Research and Quality for the purpose of measuring patient experience in the hospital. The HCAHPS survey was endorsed by the National Quality Forum in 2005, with final approval of the national implementation granted by the Office of Management and Budget later that year. The CMS began implementation of the HCAHPS survey in 2006, with the first required public reporting of all hospitals taking place in March 2008.37-41 Based on CMS’ value-based purchasing initiative, hospitals with low HCAHPS scores have faced substantial penalties since 2012. Under these circumstances, it is important that the HCAHPS measures what it purports to measure. Because HCAHPS was designed to compare hospitals, testing was limited to assessment of internal reliability, hospital-level reliability, and construct validity. External validation with known measures of physician communication was not performed.41 Our study appears to be the first to compare HCAHPS scores to directly observed measures of physician communication skills. The lack of association between the 2 should sound a cautionary note to hospitals who seek to tie individual compensation to HCAHPS scores to improve them. In particular, the survey asks for a rating for all the patient’s doctors, not just the primary hospitalist. We found that, for hospital stays with just 1 hospitalist, the HCAHPS score reflected observed expression of empathy, although the correlation was only moderate, and HCAHPS were not correlated with other communication skills. Of all communication skills, empathy may be most important. Almost the entire body of research on physician communication cites empathy as a central skill. Empathy improves patient outcomes1-9,13-14,16-18,42 such as adherence to treatment, loyalty, and perception of care; and provider outcomes10-12,15 such as reduced burnout and a decreased likelihood of malpractice litigation.
It is less clear why other communication skills did not correlate with HCAHPS, but several differences in the measures themselves and how they were obtained might be responsible. It is possible that HCAHPS measures something broader than physician communication. In addition, the 4HCS was developed and normed on outpatient encounters as is true for virtually all doctor-patient coding schemes.43 Little is known about inpatient communication best practices. The timing of HCAHPS may also degrade the relationship between observed and reported communication. The HCAHPS questionnaires, collected after discharge, are retrospective reconstructions that are subject to recall bias and recency effects.44,45 In contrast, our observations took place in real time and were specific to the face-to-face interactions that take place when physicians engage patients at the bedside. Third, the response rate for HCAHPS surveys is only 30%, leading to potential sample bias.46 Respondents represent discharged patients who are willing and able to answer surveys, and may not be representative of all hospitalized patients. Finally, as with all global questions, the meaning any individual patient assigns to terms like “respect” may vary.
Our study has several limitations. The HCAHPS and 4HCS scores were not obtained from the same sample of patients. It is possible that the patients who were observed were not representative of the patients who completed the HCAHPS surveys. In addition, the only type of encounter observed was the initial visit between the hospitalist and the patient, and did not include communication during follow-up visits or on the day of discharge. However, there was a strong ICC among the 4HCS scores, implying that the 4HCS measures an inherent physician skill, which should be consistent across patients and encounters. Coding bias of the habits by a single observer could not be excluded. High intra-class correlation could be due in part to observer preferences for particular communication styles. Our sample included only 28 physicians. Although our study was powered to rule out a moderate correlation between 4HCS scores and HCAHPS scores (Pearson correlation coefficient greater than 0.5), we cannot exclude weaker correlations. Most correlations that we observed were so small that they would not be clinically meaningful, even in a much larger sample.
CONCLUSIONS
Our findings that HCAHPS scores did not correlate with the communication skills of the attending of record have some important implications. In an environment of value-based purchasing, most hospital systems are interested in identifying modifiable provider behaviors that optimize efficiency and payment structures. This study shows that directly measured communication skills do not correlate with HCAHPS scores as generally reported, indicating that HCAHPS may be measuring a broader domain than only physician communication skills. Better attribution based on the proportion of care provided by an individual physician could make the scores more useful for individual comparisons, but most institutions do not report their data in this way. Given this limitation, hospitals should refrain from comparing and incentivizing individual physicians based on their HCAHPS scores, because this measure was not designed for this purpose and does not appear to reflect an individual’s skills. This is important in the current environment in which hospitals face substantial penalties for underperformance but lack specific tools to improve their scores. Furthermore, there is concern that this type of measurement creates perverse incentives that may adversely alter clinical practice with the aim of improving scores.46
Training clinicians in communication and teaming skills is one potential means of increasing overall scores.15 Improving doctor-patient and team relationships is also the right thing to do. It is increasingly being demanded by patients and has always been a deep source of satisfaction for physicians.15,47 Moreover, there is an increasingly robust literature that relates face-to-face communication to biomedical and psychosocial outcomes of care.48 Identifying individual physicians who need help with communication skills is a worthwhile goal. Unfortunately, the HCAHPS survey does not appear to be the appropriate tool for this purpose.
Disclosure
The Cleveland Clinic Foundation, Division of Clinical Research, Research Programs Committees provided funding support. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors have no conflicts of interest for this study.
1. Glass RM. The patient-physician relationship. JAMA focuses on the center of medicine. JAMA. 1996;275(2):147-148. PubMed
2. Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns. 2005;58(1):4-12. PubMed
3. Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796-804. PubMed
4. Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213-220. PubMed
5. Like R, Zyzanski SJ. Patient satisfaction with the clinical encounter: social psychological determinants. Soc Sci Med. 1987;24(4):351-357. PubMed
6. Williams S, Weinman J, Dale J. Doctor-patient communication and patient satisfaction: a review. Fam Pract. 1998;15(5):480-492. PubMed
7. Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med. 2006;63(12):3067-3079. PubMed
8. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
9. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. PubMed
10. Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553-559. PubMed
11. Ambady N, Laplante D, Nguyen T, Rosenthal R, Chaumeton N, Levinson W. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9. PubMed
12. Weng HC, Hung CM, Liu YT, et al. Associations between emotional intelligence and doctor burnout, job satisfaction and patient satisfaction. Med Educ. 2011;45(8):835-842. PubMed
13. Mauksch LB, Dugdale DC, Dodson S, Epstein R. Relationship, communication, and efficiency in the medical encounter: creating a clinical model from a literature review. Arch Intern Med. 2008;168(13):1387-1395. PubMed
14. Suchman AL, Roter D, Green M, Lipkin M Jr. Physician satisfaction with primary care office visits. Collaborative Study Group of the American Academy on Physician and Patient. Med Care. 1993;31(12):1083-1092. PubMed
15. Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31(7):755-761. PubMed
16. Brody DS, Miller SM, Lerman CE, Smith DG, Lazaro CG, Blum MJ. The relationship between patients’ satisfaction with their physicians and perceptions about interventions they desired and received. Med Care. 1989;27(11):1027-1035. PubMed
17. Wasserman RC, Inui TS, Barriatua RD, Carter WB, Lippincott P. Pediatric clinicians’ support for parents makes a difference: an outcome-based analysis of clinician-parent interaction. Pediatrics. 1984;74(6):1047-1053. PubMed
18. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528. PubMed
19. Inui TS, Carter WB. Problems and prospects for health services research on provider-patient communication. Med Care. 1985;23(5):521-538. PubMed
20. Beckman H, Frankel R, Kihm J, Kulesza G, Geheb M. Measurement and improvement of humanistic skills in first-year trainees. J Gen Intern Med. 1990;5(1):42-45. PubMed
21. Keller S, O’Malley AJ, Hays RD, et al. Methods used to streamline the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2057-2077. PubMed
22. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535-544. PubMed
23. Lazare A, Eisenthal S, Wasserman L. The customer approach to patienthood. Attending to patient requests in a walk-in clinic. Arch Gen Psychiatry. 1975;32(5):553-558. PubMed
24. Eisenthal S, Lazare A. Evaluation of the initial interview in a walk-in clinic. The clinician’s perspective on a “negotiated approach”. J Nerv Ment Dis. 1977;164(1):30-35. PubMed
25. Kravitz RL, Callahan EJ, Paterniti D, Antonius D, Dunham M, Lewis CE. Prevalence and sources of patients’ unmet expectations for care. Ann Intern Med. 1996;125(9):730-737. PubMed
26. Froehlich GW, Welch HG. Meeting walk-in patients’ expectations for testing. Effects on satisfaction. J Gen Intern Med. 1996;11(8):470-474. PubMed
27. DiMatteo MR, Taranta A, Friedman HS, Prince LM. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387. PubMed
28. Frankel RM, Stein T. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16(4):184-191. PubMed
29. Krupat E, Frankel R, Stein T, Irish J. The Four Habits Coding Scheme: validation of an instrument to assess clinicians’ communication behavior. Patient Educ Couns. 2006;62(1):38-45. PubMed
30. Fossli Jensen B, Gulbrandsen P, Benth JS, Dahl FA, Krupat E, Finset A. Interrater reliability for the Four Habits Coding Scheme as part of a randomized controlled trial. Patient Educ Couns. 2010;80(3):405-409. PubMed
31. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. PubMed
32. Anonymous. CMS continues to shift emphasis to quality of care. Hosp Case Manag. 2012;20(10):150-151. PubMed
33. The R.E.D.E. Model 2015. Cleveland Clinic Center for Excellence in Healthcare
Communication. http://healthcarecommunication.info/. Accessed April 3 2016.
34. Empathetics, Inc. A Boston-based empathy training firm raises $1.5 million in
Series A Financing 2015. Empathetics Inc. http://www.prnewswire.com/news-releases/
empathetics-inc----a-boston-based-empathy-training-firm-raises-15-million-
in-series-a-financing-300072696.html). Accessed April 3, 2016.
35. Intensive Communication Skills 2016. Institute for Healthcare Communication.
http://healthcarecomm.org/. Accessed April 3, 2016.
36. Hu B, Palta M, Shao J. Variability explained by covariates in linear mixed-effect
models for longitudinal data. Canadian Journal of Statistics. 2010;38:352-368.
37. O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case-mix adjustment
of the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2162-2181. PubMed
38. O’Malley AJ, Zaslavsky AM, Hays RD, Hepner KA, Keller S, Cleary PD. Exploratory
factor analyses of the CAHPS Hospital Pilot Survey responses across
and within medical, surgical, and obstetric services. Health Serv Res. 2005;40(6 pt
2):2078-2095. PubMed
39. Goldstein E, Farquhar M, Crofton C, Darby C, Garfinkel S. Measuring hospital
care from the patients’ perspective: an overview of the CAHPS Hospital Survey
development process. Health Serv Res. 2005;40(6 pt 2):1977-1995. PubMed
40. Darby C, Hays RD, Kletke P. Development and evaluation of the CAHPS hospital
survey. Health Serv Res. 2005;40(6 pt 2):1973-1976. PubMed
41. Keller VF, Carroll JG. A new model for physician-patient communication. Patient
Educ Couns. 1994;23(2):131-140. PubMed
42. Quirk M, Mazor K, Haley HL, et al. How patients perceive a doctor’s caring attitude.
Patient Educ Couns. 2008;72(3):359-366. PubMed
43. Frankel RM, Levinson W. Back to the future: Can conversation analysis be used
to judge physicians’ malpractice history? Commun Med. 2014;11(1):27-39. PubMed
44. Furnham A. Response bias, social desirability and dissimulation. Personality and
individual differences 1986;7(3):385-400.
45. Shteingart H, Neiman T, Loewenstein Y. The role of first impression in operant
learning. J Exp Psychol Gen. 2013;142(2):476-488. PubMed
46. Tefera L, Lehrman WG, Conway P. Measurement of the patient experience: clarifying
facts, myths, and approaches. JAMA. 2016;315(2):2167-2168. PubMed
47. Horowitz CR, Suchman AL, Branch WT Jr, Frankel RM. What do doctors find
meaningful about their work? Ann Intern Med. 2003;138(9):772-775. PubMed
48. Rao JK, Anderson LA, Inui TS, Frankel RM. Communication interventions make
a difference in conversations between physicians and patients: a systematic review
of the evidence. Med Care. 2007;45(4):340-349. PubMed
Communication is the foundation of medical care.1 Effective communication can improve health outcomes, safety, adherence, satisfaction, trust, and enable genuine informed consent and decision-making.2-9 Furthermore, high-quality communication increases provider engagement and workplace satisfaction, while reducing stress and malpractice risk.10-15
Direct measurement of communication in the healthcare setting can be challenging. The “Four Habits Model,” which is derived from a synthesis of empiric studies8,16-20 and theoretical models21-24 of communication, offers 1 framework for assessing healthcare communication. The conceptual model underlying the 4 habits has been validated in studies of physician and patient satisfaction.1,4,25-27 The 4 habits are: investing in the beginning, eliciting the patient’s perspective, demonstrating empathy, and investing in the end. Each habit is divided into several identifiable tasks or skill sets, which can be reliably measured using validated tools and checklists.28 One such instrument, the Four Habits Coding Scheme (4HCS), has been evaluated against other tools and demonstrated overall satisfactory inter-rater reliability and validity.29,30
The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, developed under the direction of the Centers for Medicare and Medicaid Services (CMS) and the Agency for Healthcare Research and Quality, is an established national standard for measuring patient perceptions of care. HCAHPS retrospectively measures global perceptions of communication, support and empathy from physicians and staff, processes of care, and the overall patient experience. HCAHPS scores were first collected nationally in 2006 and have been publicly reported since 2008.31 With the introduction of value-based purchasing in 2012, health system revenues are now tied to HCAHPS survey performance.32 As a result, hospitals are financially motivated to improve HCAHPS scores but lack evidence-based methods for doing so. Some healthcare organizations have invested in communication training programs based on the available literature and best practices.2,33-35 However, it is not known how, if at all, HCAHPS scores relate to physicians’ real-time observed communication skills.
To examine the relationship between physician communication, as reported by global HCAHPS scores, and the quality of physician communication skills in specific encounters, we observed hospitalist physicians during inpatient bedside rounds and measured their communication skills using the 4HCS.
METHODS
Study Design
The study utilized a cross sectional design; physicians who consented were observed on rounds during 3 separate encounters, and we compared hospitalists’ 4HCS scores to their HCAHPS scores to assess the correlation. The study was approved by the Institutional Review Board of the Cleveland Clinic.
Population
The study was conducted at the main campus of the Cleveland Clinic. All physicians specializing in hospital medicine who had received 10 or more completed HCAHPS survey responses while rounding on a medicine service in the past year were invited to participate in the study. Participation was voluntary; night hospitalists were excluded. A research nurse was trained in the Four Habits Model28 and in the use of the 4HCS coding scheme by the principal investigator. The nurse observed each physician and ascertained the presence of communication behaviors using the 4HCS tool. Physicians were observed between August 2013 and August 2014. Multiple observations per physician could occur on the same day, but only 1 observation per patient was used for analysis. Observations consisted of a physician’s first encounter with a hospitalized patient, with the patient’s consent. Observations were conducted during encounters with English-speaking and cognitively intact patients only. Resident physicians were permitted to stay and conduct rounds per their normal routine. Patient information was not collected as part of the study.
Measures
HCAHPS. For each physician, we extracted all HCAHPS scores that were collected from our hospital’s Press Ganey database. The HCAHPS survey contains 22 core questions divided into 7 themes or domains, 1 of which is doctor communication. The survey uses frequency-based questions with possible answers fixed on a 4-point scale (4=always, 3=usually, 2=sometimes, 1=never). Our primary outcome was the doctor communication domain, which comprises 3 questions: 1) During this hospital stay, how often did the doctors treat you with respect? 2) During this hospital stay, how often did the doctors listen to you? and 3) During this hospital stay, how often did the doctors explain things in a language you can understand? Because CMS counts only the percentage of responses that are graded “always,” so-called “top box” scoring, we used the same measure.
The HCAHPS scores are always attributed to the physician at the time of discharge even if he may not have been responsible for the care of the patient during the entire hospital course. To mitigate contamination from patients seen by multiple providers, we cross-matched length of stay (LOS) data with billing data to determine the proportion of days a patient was seen by a single provider during the entire length of stay. We stratified patients seen by the attending providers to less than 50%, 50% to less than 100%, and at 100% of the LOS. However, we were unable to identify which patients were seen by other consultants or by residents due to limitations in data gathering and the nature of the database.
The Four Habits. The Four Habits are: invest in the beginning, elicit the patient’s perspective, demonstrate empathy, and invest in the end (Figure 1). Specific behaviors for Habits 1 to 4 are outlined in the Appendix, but we will briefly describe the themes as follows. Habit 1, invest in the beginning, describes the ability of the physician to set a welcoming environment for the patient, establish rapport, and collaborate on an agenda for the visit. Habit 2, elicit the patient’s perspective, describes the ability of the physician to explore the patients’ worries, ideas, expectations, and the impact of the illness on their lifestyle. Habit 3, demonstrate empathy, describes the physician’s openness to the patient’s emotions as well as the ability to explore, validate; express curiosity, and openly accept these feelings. Habit 4, invest in the end, is a measure of the physician’s ability to counsel patients in a language built around their original concerns or worries, as well as the ability to check the patients’ understanding of the plan.2,29-30
4HCS. The 4HCS tool (Appendix) measures discreet behaviors and phrases based on each of the Four Habits (Figure 1). With a scoring range from a low of 4 to a high of 20, the rater at bedside assigns a range of points on a scale of 1 to 5 for each habit. It is an instrument based on a teaching model used widely throughout Kaiser Permanente to improve clinicians’ communication skills. The 4HCS was first tested for interrater reliability and validity against the Roter Interaction Analysis System using 100 videotaped primary care physician encounters.29 It was further evaluated in a randomized control trial. Videotapes from 497 hospital encounters involving 71 doctors from a variety of clinical specialties were rated by 4 trained raters using the coding scheme. The total score Pearson’s R and intraclass correlation coefficient (ICC) exceeded 0.70 for all pairs of raters, and the interrater reliability was satisfactory for the 4HCS as applied to heterogeneous material.30
STATISTICAL ANALYSIS
Physician characteristics were summarized with standard descriptive statistics. Pearson correlation coefficients were computed between HCAHPS and 4HCS scores. All analyses were performed with RStudio (Boston, MA). The Pearson correlation between the averaged HCAHPS and 4HCS scores was also computed. A correlation with a P value less than 0.05 was considered statistically significant. With 28 physicians, the study had a power of 88% to detect a moderate correlation (greater than 0.50) with a 2-sided alpha of 0.05. We also computed the correlations based on the subgroups of data with patients seen by providers for less than 50%, 50% to less than 100%, and 100% of LOS. All analyses were conducted in SAS 9.2 (SAS Institute Inc., Cary, NC).36
RESULTS
There were 31 physicians who met our inclusion criteria. Of 29 volunteers, 28 were observed during 3 separate inpatient encounters and made up the final sample. A total of 1003 HCAHPS survey responses were available for these physicians. Participants were predominantly female (60.7%), with an average age of 39 years. They were in practice for an average of 4 years (12 were in practice more than 5 years), and 9 were observed on a teaching rotation.
The means of the overall 4HCS scores per observation were 17.39 ± 2.33 for the first, 17.00 ± 2.37 for the second, and 17.43 ± 2.36 for third bedside observation. The mean 4HCS scores per observation, broken down by habit, appear in Table 1. The ICC among the repeated scores within the same physician was 0.81. The median number of HCAHPS survey returns was 32 (range = [8, 85], with mean = 35.8, interquartile range = [16, 54]). The median overall HCAHPS doctor communication score was 89.6 (range = 80.9-93.7). Participants scored the highest in the respect subdomain and the lowest in the explain subdomain. Median HCAHPS scores and ranges appear in Table 2.
Because there were no significant associations between 4HCS scores or HCAHPS scores and physician age, sex, years in practice, or teaching site, correlations were not adjusted. Figure 2A and 2B show the association between mean 4HCS scores and HCAHPS scores by physician. There was no significant correlation between overall 4HCS and HCAHPS doctor communication scores (Pearson correlation coefficient 0.098; 95% confidence interval [CI], -0.285, 0.455). The individual habits also were not correlated with overall HCAHPS scores or with their corresponding HCAHPS domain (Table 3).
For 325 patients, 1 hospitalist was present for the entire LOS. In sensitivity analysis limiting observations to these patients (Figure 2C, Figure 2D, Table 3), we found a moderate correlation between habit 3 and the HCAHPS respect score (Pearson correlation coefficient 0.515; 95% CI, 0.176, 0.745; P = 0.005), and a weaker correlation between habit 3 and the HCAHPS overall doctor communication score (0.442; 95% CI, 0.082, 0.7; P = 0.019). There were no other significant correlations between specific habits and HCAHPS scores.
DISCUSSION
In this observational study of hospitalist physicians at a large tertiary care center, we found that communication skills, as measured by the 4HCS, varied substantially among physicians but were highly correlated within patients of the same physician. However, there was virtually no correlation between the attending physician of record’s 4HCS scores and their HCAHPS communication scores. When we limited our analysis to patients who saw only 1 hospitalist throughout their stay, there were moderate correlations between demonstration of empathy and both the HCAHPS respect score and overall doctor communication score. There were no trends across the strata of hospitalist involvement. It is important to note that the addition of even 1 different hospitalist to the LOS removes any association. Habits 1 and 2 are close to significance in the 100% subgroup, with a weak correlation. Interestingly, Habit 4, which focuses on creating a plan with the patient, showed no correlation at all with patients reporting that doctors explained things in language they could understand.
Development and testing of the HCAHPS survey began in 2002, commissioned by CMS and the Agency for Healthcare Research and Quality for the purpose of measuring patient experience in the hospital. The HCAHPS survey was endorsed by the National Quality Forum in 2005, with final approval of the national implementation granted by the Office of Management and Budget later that year. The CMS began implementation of the HCAHPS survey in 2006, with the first required public reporting of all hospitals taking place in March 2008.37-41 Based on CMS’ value-based purchasing initiative, hospitals with low HCAHPS scores have faced substantial penalties since 2012. Under these circumstances, it is important that the HCAHPS measures what it purports to measure. Because HCAHPS was designed to compare hospitals, testing was limited to assessment of internal reliability, hospital-level reliability, and construct validity. External validation with known measures of physician communication was not performed.41 Our study appears to be the first to compare HCAHPS scores to directly observed measures of physician communication skills. The lack of association between the 2 should sound a cautionary note to hospitals who seek to tie individual compensation to HCAHPS scores to improve them. In particular, the survey asks for a rating for all the patient’s doctors, not just the primary hospitalist. We found that, for hospital stays with just 1 hospitalist, the HCAHPS score reflected observed expression of empathy, although the correlation was only moderate, and HCAHPS were not correlated with other communication skills. Of all communication skills, empathy may be most important. Almost the entire body of research on physician communication cites empathy as a central skill. Empathy improves patient outcomes1-9,13-14,16-18,42 such as adherence to treatment, loyalty, and perception of care; and provider outcomes10-12,15 such as reduced burnout and a decreased likelihood of malpractice litigation.
It is less clear why other communication skills did not correlate with HCAHPS, but several differences in the measures themselves and how they were obtained might be responsible. It is possible that HCAHPS measures something broader than physician communication. In addition, the 4HCS was developed and normed on outpatient encounters as is true for virtually all doctor-patient coding schemes.43 Little is known about inpatient communication best practices. The timing of HCAHPS may also degrade the relationship between observed and reported communication. The HCAHPS questionnaires, collected after discharge, are retrospective reconstructions that are subject to recall bias and recency effects.44,45 In contrast, our observations took place in real time and were specific to the face-to-face interactions that take place when physicians engage patients at the bedside. Third, the response rate for HCAHPS surveys is only 30%, leading to potential sample bias.46 Respondents represent discharged patients who are willing and able to answer surveys, and may not be representative of all hospitalized patients. Finally, as with all global questions, the meaning any individual patient assigns to terms like “respect” may vary.
Our study has several limitations. The HCAHPS and 4HCS scores were not obtained from the same sample of patients. It is possible that the patients who were observed were not representative of the patients who completed the HCAHPS surveys. In addition, the only type of encounter observed was the initial visit between the hospitalist and the patient, and did not include communication during follow-up visits or on the day of discharge. However, there was a strong ICC among the 4HCS scores, implying that the 4HCS measures an inherent physician skill, which should be consistent across patients and encounters. Coding bias of the habits by a single observer could not be excluded. High intra-class correlation could be due in part to observer preferences for particular communication styles. Our sample included only 28 physicians. Although our study was powered to rule out a moderate correlation between 4HCS scores and HCAHPS scores (Pearson correlation coefficient greater than 0.5), we cannot exclude weaker correlations. Most correlations that we observed were so small that they would not be clinically meaningful, even in a much larger sample.
CONCLUSIONS
Our findings that HCAHPS scores did not correlate with the communication skills of the attending of record have some important implications. In an environment of value-based purchasing, most hospital systems are interested in identifying modifiable provider behaviors that optimize efficiency and payment structures. This study shows that directly measured communication skills do not correlate with HCAHPS scores as generally reported, indicating that HCAHPS may be measuring a broader domain than only physician communication skills. Better attribution based on the proportion of care provided by an individual physician could make the scores more useful for individual comparisons, but most institutions do not report their data in this way. Given this limitation, hospitals should refrain from comparing and incentivizing individual physicians based on their HCAHPS scores, because this measure was not designed for this purpose and does not appear to reflect an individual’s skills. This is important in the current environment in which hospitals face substantial penalties for underperformance but lack specific tools to improve their scores. Furthermore, there is concern that this type of measurement creates perverse incentives that may adversely alter clinical practice with the aim of improving scores.46
Training clinicians in communication and teaming skills is one potential means of increasing overall scores.15 Improving doctor-patient and team relationships is also the right thing to do. It is increasingly being demanded by patients and has always been a deep source of satisfaction for physicians.15,47 Moreover, there is an increasingly robust literature that relates face-to-face communication to biomedical and psychosocial outcomes of care.48 Identifying individual physicians who need help with communication skills is a worthwhile goal. Unfortunately, the HCAHPS survey does not appear to be the appropriate tool for this purpose.
Disclosure
The Cleveland Clinic Foundation, Division of Clinical Research, Research Programs Committees provided funding support. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors have no conflicts of interest for this study.
Communication is the foundation of medical care.1 Effective communication can improve health outcomes, safety, adherence, satisfaction, trust, and enable genuine informed consent and decision-making.2-9 Furthermore, high-quality communication increases provider engagement and workplace satisfaction, while reducing stress and malpractice risk.10-15
Direct measurement of communication in the healthcare setting can be challenging. The “Four Habits Model,” which is derived from a synthesis of empiric studies8,16-20 and theoretical models21-24 of communication, offers 1 framework for assessing healthcare communication. The conceptual model underlying the 4 habits has been validated in studies of physician and patient satisfaction.1,4,25-27 The 4 habits are: investing in the beginning, eliciting the patient’s perspective, demonstrating empathy, and investing in the end. Each habit is divided into several identifiable tasks or skill sets, which can be reliably measured using validated tools and checklists.28 One such instrument, the Four Habits Coding Scheme (4HCS), has been evaluated against other tools and demonstrated overall satisfactory inter-rater reliability and validity.29,30
The Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey, developed under the direction of the Centers for Medicare and Medicaid Services (CMS) and the Agency for Healthcare Research and Quality, is an established national standard for measuring patient perceptions of care. HCAHPS retrospectively measures global perceptions of communication, support and empathy from physicians and staff, processes of care, and the overall patient experience. HCAHPS scores were first collected nationally in 2006 and have been publicly reported since 2008.31 With the introduction of value-based purchasing in 2012, health system revenues are now tied to HCAHPS survey performance.32 As a result, hospitals are financially motivated to improve HCAHPS scores but lack evidence-based methods for doing so. Some healthcare organizations have invested in communication training programs based on the available literature and best practices.2,33-35 However, it is not known how, if at all, HCAHPS scores relate to physicians’ real-time observed communication skills.
To examine the relationship between physician communication, as reported by global HCAHPS scores, and the quality of physician communication skills in specific encounters, we observed hospitalist physicians during inpatient bedside rounds and measured their communication skills using the 4HCS.
METHODS
Study Design
The study utilized a cross sectional design; physicians who consented were observed on rounds during 3 separate encounters, and we compared hospitalists’ 4HCS scores to their HCAHPS scores to assess the correlation. The study was approved by the Institutional Review Board of the Cleveland Clinic.
Population
The study was conducted at the main campus of the Cleveland Clinic. All physicians specializing in hospital medicine who had received 10 or more completed HCAHPS survey responses while rounding on a medicine service in the past year were invited to participate in the study. Participation was voluntary; night hospitalists were excluded. A research nurse was trained in the Four Habits Model28 and in the use of the 4HCS coding scheme by the principal investigator. The nurse observed each physician and ascertained the presence of communication behaviors using the 4HCS tool. Physicians were observed between August 2013 and August 2014. Multiple observations per physician could occur on the same day, but only 1 observation per patient was used for analysis. Observations consisted of a physician’s first encounter with a hospitalized patient, with the patient’s consent. Observations were conducted during encounters with English-speaking and cognitively intact patients only. Resident physicians were permitted to stay and conduct rounds per their normal routine. Patient information was not collected as part of the study.
Measures
HCAHPS. For each physician, we extracted all HCAHPS scores that were collected from our hospital’s Press Ganey database. The HCAHPS survey contains 22 core questions divided into 7 themes or domains, 1 of which is doctor communication. The survey uses frequency-based questions with possible answers fixed on a 4-point scale (4=always, 3=usually, 2=sometimes, 1=never). Our primary outcome was the doctor communication domain, which comprises 3 questions: 1) During this hospital stay, how often did the doctors treat you with respect? 2) During this hospital stay, how often did the doctors listen to you? and 3) During this hospital stay, how often did the doctors explain things in a language you can understand? Because CMS counts only the percentage of responses that are graded “always,” so-called “top box” scoring, we used the same measure.
The HCAHPS scores are always attributed to the physician at the time of discharge even if he may not have been responsible for the care of the patient during the entire hospital course. To mitigate contamination from patients seen by multiple providers, we cross-matched length of stay (LOS) data with billing data to determine the proportion of days a patient was seen by a single provider during the entire length of stay. We stratified patients seen by the attending providers to less than 50%, 50% to less than 100%, and at 100% of the LOS. However, we were unable to identify which patients were seen by other consultants or by residents due to limitations in data gathering and the nature of the database.
The Four Habits. The Four Habits are: invest in the beginning, elicit the patient’s perspective, demonstrate empathy, and invest in the end (Figure 1). Specific behaviors for Habits 1 to 4 are outlined in the Appendix, but we will briefly describe the themes as follows. Habit 1, invest in the beginning, describes the ability of the physician to set a welcoming environment for the patient, establish rapport, and collaborate on an agenda for the visit. Habit 2, elicit the patient’s perspective, describes the ability of the physician to explore the patients’ worries, ideas, expectations, and the impact of the illness on their lifestyle. Habit 3, demonstrate empathy, describes the physician’s openness to the patient’s emotions as well as the ability to explore, validate; express curiosity, and openly accept these feelings. Habit 4, invest in the end, is a measure of the physician’s ability to counsel patients in a language built around their original concerns or worries, as well as the ability to check the patients’ understanding of the plan.2,29-30
4HCS. The 4HCS tool (Appendix) measures discreet behaviors and phrases based on each of the Four Habits (Figure 1). With a scoring range from a low of 4 to a high of 20, the rater at bedside assigns a range of points on a scale of 1 to 5 for each habit. It is an instrument based on a teaching model used widely throughout Kaiser Permanente to improve clinicians’ communication skills. The 4HCS was first tested for interrater reliability and validity against the Roter Interaction Analysis System using 100 videotaped primary care physician encounters.29 It was further evaluated in a randomized control trial. Videotapes from 497 hospital encounters involving 71 doctors from a variety of clinical specialties were rated by 4 trained raters using the coding scheme. The total score Pearson’s R and intraclass correlation coefficient (ICC) exceeded 0.70 for all pairs of raters, and the interrater reliability was satisfactory for the 4HCS as applied to heterogeneous material.30
STATISTICAL ANALYSIS
Physician characteristics were summarized with standard descriptive statistics. Pearson correlation coefficients were computed between HCAHPS and 4HCS scores. All analyses were performed with RStudio (Boston, MA). The Pearson correlation between the averaged HCAHPS and 4HCS scores was also computed. A correlation with a P value less than 0.05 was considered statistically significant. With 28 physicians, the study had a power of 88% to detect a moderate correlation (greater than 0.50) with a 2-sided alpha of 0.05. We also computed the correlations based on the subgroups of data with patients seen by providers for less than 50%, 50% to less than 100%, and 100% of LOS. All analyses were conducted in SAS 9.2 (SAS Institute Inc., Cary, NC).36
RESULTS
There were 31 physicians who met our inclusion criteria. Of 29 volunteers, 28 were observed during 3 separate inpatient encounters and made up the final sample. A total of 1003 HCAHPS survey responses were available for these physicians. Participants were predominantly female (60.7%), with an average age of 39 years. They were in practice for an average of 4 years (12 were in practice more than 5 years), and 9 were observed on a teaching rotation.
The means of the overall 4HCS scores per observation were 17.39 ± 2.33 for the first, 17.00 ± 2.37 for the second, and 17.43 ± 2.36 for third bedside observation. The mean 4HCS scores per observation, broken down by habit, appear in Table 1. The ICC among the repeated scores within the same physician was 0.81. The median number of HCAHPS survey returns was 32 (range = [8, 85], with mean = 35.8, interquartile range = [16, 54]). The median overall HCAHPS doctor communication score was 89.6 (range = 80.9-93.7). Participants scored the highest in the respect subdomain and the lowest in the explain subdomain. Median HCAHPS scores and ranges appear in Table 2.
Because there were no significant associations between 4HCS scores or HCAHPS scores and physician age, sex, years in practice, or teaching site, correlations were not adjusted. Figure 2A and 2B show the association between mean 4HCS scores and HCAHPS scores by physician. There was no significant correlation between overall 4HCS and HCAHPS doctor communication scores (Pearson correlation coefficient 0.098; 95% confidence interval [CI], -0.285, 0.455). The individual habits also were not correlated with overall HCAHPS scores or with their corresponding HCAHPS domain (Table 3).
For 325 patients, 1 hospitalist was present for the entire LOS. In sensitivity analysis limiting observations to these patients (Figure 2C, Figure 2D, Table 3), we found a moderate correlation between habit 3 and the HCAHPS respect score (Pearson correlation coefficient 0.515; 95% CI, 0.176, 0.745; P = 0.005), and a weaker correlation between habit 3 and the HCAHPS overall doctor communication score (0.442; 95% CI, 0.082, 0.7; P = 0.019). There were no other significant correlations between specific habits and HCAHPS scores.
DISCUSSION
In this observational study of hospitalist physicians at a large tertiary care center, we found that communication skills, as measured by the 4HCS, varied substantially among physicians but were highly correlated within patients of the same physician. However, there was virtually no correlation between the attending physician of record’s 4HCS scores and their HCAHPS communication scores. When we limited our analysis to patients who saw only 1 hospitalist throughout their stay, there were moderate correlations between demonstration of empathy and both the HCAHPS respect score and overall doctor communication score. There were no trends across the strata of hospitalist involvement. It is important to note that the addition of even 1 different hospitalist to the LOS removes any association. Habits 1 and 2 are close to significance in the 100% subgroup, with a weak correlation. Interestingly, Habit 4, which focuses on creating a plan with the patient, showed no correlation at all with patients reporting that doctors explained things in language they could understand.
Development and testing of the HCAHPS survey began in 2002, commissioned by CMS and the Agency for Healthcare Research and Quality for the purpose of measuring patient experience in the hospital. The HCAHPS survey was endorsed by the National Quality Forum in 2005, with final approval of the national implementation granted by the Office of Management and Budget later that year. The CMS began implementation of the HCAHPS survey in 2006, with the first required public reporting of all hospitals taking place in March 2008.37-41 Based on CMS’ value-based purchasing initiative, hospitals with low HCAHPS scores have faced substantial penalties since 2012. Under these circumstances, it is important that the HCAHPS measures what it purports to measure. Because HCAHPS was designed to compare hospitals, testing was limited to assessment of internal reliability, hospital-level reliability, and construct validity. External validation with known measures of physician communication was not performed.41 Our study appears to be the first to compare HCAHPS scores to directly observed measures of physician communication skills. The lack of association between the 2 should sound a cautionary note to hospitals who seek to tie individual compensation to HCAHPS scores to improve them. In particular, the survey asks for a rating for all the patient’s doctors, not just the primary hospitalist. We found that, for hospital stays with just 1 hospitalist, the HCAHPS score reflected observed expression of empathy, although the correlation was only moderate, and HCAHPS were not correlated with other communication skills. Of all communication skills, empathy may be most important. Almost the entire body of research on physician communication cites empathy as a central skill. Empathy improves patient outcomes1-9,13-14,16-18,42 such as adherence to treatment, loyalty, and perception of care; and provider outcomes10-12,15 such as reduced burnout and a decreased likelihood of malpractice litigation.
It is less clear why other communication skills did not correlate with HCAHPS, but several differences in the measures themselves and how they were obtained might be responsible. It is possible that HCAHPS measures something broader than physician communication. In addition, the 4HCS was developed and normed on outpatient encounters as is true for virtually all doctor-patient coding schemes.43 Little is known about inpatient communication best practices. The timing of HCAHPS may also degrade the relationship between observed and reported communication. The HCAHPS questionnaires, collected after discharge, are retrospective reconstructions that are subject to recall bias and recency effects.44,45 In contrast, our observations took place in real time and were specific to the face-to-face interactions that take place when physicians engage patients at the bedside. Third, the response rate for HCAHPS surveys is only 30%, leading to potential sample bias.46 Respondents represent discharged patients who are willing and able to answer surveys, and may not be representative of all hospitalized patients. Finally, as with all global questions, the meaning any individual patient assigns to terms like “respect” may vary.
Our study has several limitations. The HCAHPS and 4HCS scores were not obtained from the same sample of patients. It is possible that the patients who were observed were not representative of the patients who completed the HCAHPS surveys. In addition, the only type of encounter observed was the initial visit between the hospitalist and the patient, and did not include communication during follow-up visits or on the day of discharge. However, there was a strong ICC among the 4HCS scores, implying that the 4HCS measures an inherent physician skill, which should be consistent across patients and encounters. Coding bias of the habits by a single observer could not be excluded. High intra-class correlation could be due in part to observer preferences for particular communication styles. Our sample included only 28 physicians. Although our study was powered to rule out a moderate correlation between 4HCS scores and HCAHPS scores (Pearson correlation coefficient greater than 0.5), we cannot exclude weaker correlations. Most correlations that we observed were so small that they would not be clinically meaningful, even in a much larger sample.
CONCLUSIONS
Our findings that HCAHPS scores did not correlate with the communication skills of the attending of record have some important implications. In an environment of value-based purchasing, most hospital systems are interested in identifying modifiable provider behaviors that optimize efficiency and payment structures. This study shows that directly measured communication skills do not correlate with HCAHPS scores as generally reported, indicating that HCAHPS may be measuring a broader domain than only physician communication skills. Better attribution based on the proportion of care provided by an individual physician could make the scores more useful for individual comparisons, but most institutions do not report their data in this way. Given this limitation, hospitals should refrain from comparing and incentivizing individual physicians based on their HCAHPS scores, because this measure was not designed for this purpose and does not appear to reflect an individual’s skills. This is important in the current environment in which hospitals face substantial penalties for underperformance but lack specific tools to improve their scores. Furthermore, there is concern that this type of measurement creates perverse incentives that may adversely alter clinical practice with the aim of improving scores.46
Training clinicians in communication and teaming skills is one potential means of increasing overall scores.15 Improving doctor-patient and team relationships is also the right thing to do. It is increasingly being demanded by patients and has always been a deep source of satisfaction for physicians.15,47 Moreover, there is an increasingly robust literature that relates face-to-face communication to biomedical and psychosocial outcomes of care.48 Identifying individual physicians who need help with communication skills is a worthwhile goal. Unfortunately, the HCAHPS survey does not appear to be the appropriate tool for this purpose.
Disclosure
The Cleveland Clinic Foundation, Division of Clinical Research, Research Programs Committees provided funding support. No funding source had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The authors have no conflicts of interest for this study.
1. Glass RM. The patient-physician relationship. JAMA focuses on the center of medicine. JAMA. 1996;275(2):147-148. PubMed
2. Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns. 2005;58(1):4-12. PubMed
3. Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796-804. PubMed
4. Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213-220. PubMed
5. Like R, Zyzanski SJ. Patient satisfaction with the clinical encounter: social psychological determinants. Soc Sci Med. 1987;24(4):351-357. PubMed
6. Williams S, Weinman J, Dale J. Doctor-patient communication and patient satisfaction: a review. Fam Pract. 1998;15(5):480-492. PubMed
7. Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med. 2006;63(12):3067-3079. PubMed
8. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
9. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. PubMed
10. Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553-559. PubMed
11. Ambady N, Laplante D, Nguyen T, Rosenthal R, Chaumeton N, Levinson W. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9. PubMed
12. Weng HC, Hung CM, Liu YT, et al. Associations between emotional intelligence and doctor burnout, job satisfaction and patient satisfaction. Med Educ. 2011;45(8):835-842. PubMed
13. Mauksch LB, Dugdale DC, Dodson S, Epstein R. Relationship, communication, and efficiency in the medical encounter: creating a clinical model from a literature review. Arch Intern Med. 2008;168(13):1387-1395. PubMed
14. Suchman AL, Roter D, Green M, Lipkin M Jr. Physician satisfaction with primary care office visits. Collaborative Study Group of the American Academy on Physician and Patient. Med Care. 1993;31(12):1083-1092. PubMed
15. Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31(7):755-761. PubMed
16. Brody DS, Miller SM, Lerman CE, Smith DG, Lazaro CG, Blum MJ. The relationship between patients’ satisfaction with their physicians and perceptions about interventions they desired and received. Med Care. 1989;27(11):1027-1035. PubMed
17. Wasserman RC, Inui TS, Barriatua RD, Carter WB, Lippincott P. Pediatric clinicians’ support for parents makes a difference: an outcome-based analysis of clinician-parent interaction. Pediatrics. 1984;74(6):1047-1053. PubMed
18. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528. PubMed
19. Inui TS, Carter WB. Problems and prospects for health services research on provider-patient communication. Med Care. 1985;23(5):521-538. PubMed
20. Beckman H, Frankel R, Kihm J, Kulesza G, Geheb M. Measurement and improvement of humanistic skills in first-year trainees. J Gen Intern Med. 1990;5(1):42-45. PubMed
21. Keller S, O’Malley AJ, Hays RD, et al. Methods used to streamline the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2057-2077. PubMed
22. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535-544. PubMed
23. Lazare A, Eisenthal S, Wasserman L. The customer approach to patienthood. Attending to patient requests in a walk-in clinic. Arch Gen Psychiatry. 1975;32(5):553-558. PubMed
24. Eisenthal S, Lazare A. Evaluation of the initial interview in a walk-in clinic. The clinician’s perspective on a “negotiated approach”. J Nerv Ment Dis. 1977;164(1):30-35. PubMed
25. Kravitz RL, Callahan EJ, Paterniti D, Antonius D, Dunham M, Lewis CE. Prevalence and sources of patients’ unmet expectations for care. Ann Intern Med. 1996;125(9):730-737. PubMed
26. Froehlich GW, Welch HG. Meeting walk-in patients’ expectations for testing. Effects on satisfaction. J Gen Intern Med. 1996;11(8):470-474. PubMed
27. DiMatteo MR, Taranta A, Friedman HS, Prince LM. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387. PubMed
28. Frankel RM, Stein T. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16(4):184-191. PubMed
29. Krupat E, Frankel R, Stein T, Irish J. The Four Habits Coding Scheme: validation of an instrument to assess clinicians’ communication behavior. Patient Educ Couns. 2006;62(1):38-45. PubMed
30. Fossli Jensen B, Gulbrandsen P, Benth JS, Dahl FA, Krupat E, Finset A. Interrater reliability for the Four Habits Coding Scheme as part of a randomized controlled trial. Patient Educ Couns. 2010;80(3):405-409. PubMed
31. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. PubMed
32. Anonymous. CMS continues to shift emphasis to quality of care. Hosp Case Manag. 2012;20(10):150-151. PubMed
33. The R.E.D.E. Model 2015. Cleveland Clinic Center for Excellence in Healthcare
Communication. http://healthcarecommunication.info/. Accessed April 3 2016.
34. Empathetics, Inc. A Boston-based empathy training firm raises $1.5 million in
Series A Financing 2015. Empathetics Inc. http://www.prnewswire.com/news-releases/
empathetics-inc----a-boston-based-empathy-training-firm-raises-15-million-
in-series-a-financing-300072696.html). Accessed April 3, 2016.
35. Intensive Communication Skills 2016. Institute for Healthcare Communication.
http://healthcarecomm.org/. Accessed April 3, 2016.
36. Hu B, Palta M, Shao J. Variability explained by covariates in linear mixed-effect
models for longitudinal data. Canadian Journal of Statistics. 2010;38:352-368.
37. O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case-mix adjustment
of the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2162-2181. PubMed
38. O’Malley AJ, Zaslavsky AM, Hays RD, Hepner KA, Keller S, Cleary PD. Exploratory
factor analyses of the CAHPS Hospital Pilot Survey responses across
and within medical, surgical, and obstetric services. Health Serv Res. 2005;40(6 pt
2):2078-2095. PubMed
39. Goldstein E, Farquhar M, Crofton C, Darby C, Garfinkel S. Measuring hospital
care from the patients’ perspective: an overview of the CAHPS Hospital Survey
development process. Health Serv Res. 2005;40(6 pt 2):1977-1995. PubMed
40. Darby C, Hays RD, Kletke P. Development and evaluation of the CAHPS hospital
survey. Health Serv Res. 2005;40(6 pt 2):1973-1976. PubMed
41. Keller VF, Carroll JG. A new model for physician-patient communication. Patient
Educ Couns. 1994;23(2):131-140. PubMed
42. Quirk M, Mazor K, Haley HL, et al. How patients perceive a doctor’s caring attitude.
Patient Educ Couns. 2008;72(3):359-366. PubMed
43. Frankel RM, Levinson W. Back to the future: Can conversation analysis be used
to judge physicians’ malpractice history? Commun Med. 2014;11(1):27-39. PubMed
44. Furnham A. Response bias, social desirability and dissimulation. Personality and
individual differences 1986;7(3):385-400.
45. Shteingart H, Neiman T, Loewenstein Y. The role of first impression in operant
learning. J Exp Psychol Gen. 2013;142(2):476-488. PubMed
46. Tefera L, Lehrman WG, Conway P. Measurement of the patient experience: clarifying
facts, myths, and approaches. JAMA. 2016;315(2):2167-2168. PubMed
47. Horowitz CR, Suchman AL, Branch WT Jr, Frankel RM. What do doctors find
meaningful about their work? Ann Intern Med. 2003;138(9):772-775. PubMed
48. Rao JK, Anderson LA, Inui TS, Frankel RM. Communication interventions make
a difference in conversations between physicians and patients: a systematic review
of the evidence. Med Care. 2007;45(4):340-349. PubMed
1. Glass RM. The patient-physician relationship. JAMA focuses on the center of medicine. JAMA. 1996;275(2):147-148. PubMed
2. Stein T, Frankel RM, Krupat E. Enhancing clinician communication skills in a large healthcare organization: a longitudinal case study. Patient Educ Couns. 2005;58(1):4-12. PubMed
3. Stewart M, Brown JB, Donner A, et al. The impact of patient-centered care on outcomes. J Fam Pract. 2000;49(9):796-804. PubMed
4. Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR. Linking primary care performance to outcomes of care. J Fam Pract. 1998;47(3):213-220. PubMed
5. Like R, Zyzanski SJ. Patient satisfaction with the clinical encounter: social psychological determinants. Soc Sci Med. 1987;24(4):351-357. PubMed
6. Williams S, Weinman J, Dale J. Doctor-patient communication and patient satisfaction: a review. Fam Pract. 1998;15(5):480-492. PubMed
7. Ciechanowski P, Katon WJ. The interpersonal experience of health care through the eyes of patients with diabetes. Soc Sci Med. 2006;63(12):3067-3079. PubMed
8. Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995;152(9):1423-1433. PubMed
9. Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med. 2011;86(3):359-364. PubMed
10. Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553-559. PubMed
11. Ambady N, Laplante D, Nguyen T, Rosenthal R, Chaumeton N, Levinson W. Surgeons’ tone of voice: a clue to malpractice history. Surgery. 2002;132(1):5-9. PubMed
12. Weng HC, Hung CM, Liu YT, et al. Associations between emotional intelligence and doctor burnout, job satisfaction and patient satisfaction. Med Educ. 2011;45(8):835-842. PubMed
13. Mauksch LB, Dugdale DC, Dodson S, Epstein R. Relationship, communication, and efficiency in the medical encounter: creating a clinical model from a literature review. Arch Intern Med. 2008;168(13):1387-1395. PubMed
14. Suchman AL, Roter D, Green M, Lipkin M Jr. Physician satisfaction with primary care office visits. Collaborative Study Group of the American Academy on Physician and Patient. Med Care. 1993;31(12):1083-1092. PubMed
15. Boissy A, Windover AK, Bokar D, et al. Communication skills training for physicians improves patient satisfaction. J Gen Intern Med. 2016;31(7):755-761. PubMed
16. Brody DS, Miller SM, Lerman CE, Smith DG, Lazaro CG, Blum MJ. The relationship between patients’ satisfaction with their physicians and perceptions about interventions they desired and received. Med Care. 1989;27(11):1027-1035. PubMed
17. Wasserman RC, Inui TS, Barriatua RD, Carter WB, Lippincott P. Pediatric clinicians’ support for parents makes a difference: an outcome-based analysis of clinician-parent interaction. Pediatrics. 1984;74(6):1047-1053. PubMed
18. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528. PubMed
19. Inui TS, Carter WB. Problems and prospects for health services research on provider-patient communication. Med Care. 1985;23(5):521-538. PubMed
20. Beckman H, Frankel R, Kihm J, Kulesza G, Geheb M. Measurement and improvement of humanistic skills in first-year trainees. J Gen Intern Med. 1990;5(1):42-45. PubMed
21. Keller S, O’Malley AJ, Hays RD, et al. Methods used to streamline the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2057-2077. PubMed
22. Engel GL. The clinical application of the biopsychosocial model. Am J Psychiatry. 1980;137(5):535-544. PubMed
23. Lazare A, Eisenthal S, Wasserman L. The customer approach to patienthood. Attending to patient requests in a walk-in clinic. Arch Gen Psychiatry. 1975;32(5):553-558. PubMed
24. Eisenthal S, Lazare A. Evaluation of the initial interview in a walk-in clinic. The clinician’s perspective on a “negotiated approach”. J Nerv Ment Dis. 1977;164(1):30-35. PubMed
25. Kravitz RL, Callahan EJ, Paterniti D, Antonius D, Dunham M, Lewis CE. Prevalence and sources of patients’ unmet expectations for care. Ann Intern Med. 1996;125(9):730-737. PubMed
26. Froehlich GW, Welch HG. Meeting walk-in patients’ expectations for testing. Effects on satisfaction. J Gen Intern Med. 1996;11(8):470-474. PubMed
27. DiMatteo MR, Taranta A, Friedman HS, Prince LM. Predicting patient satisfaction from physicians’ nonverbal communication skills. Med Care. 1980;18(4):376-387. PubMed
28. Frankel RM, Stein T. Getting the most out of the clinical encounter: the four habits model. J Med Pract Manage. 2001;16(4):184-191. PubMed
29. Krupat E, Frankel R, Stein T, Irish J. The Four Habits Coding Scheme: validation of an instrument to assess clinicians’ communication behavior. Patient Educ Couns. 2006;62(1):38-45. PubMed
30. Fossli Jensen B, Gulbrandsen P, Benth JS, Dahl FA, Krupat E, Finset A. Interrater reliability for the Four Habits Coding Scheme as part of a randomized controlled trial. Patient Educ Couns. 2010;80(3):405-409. PubMed
31. Giordano LA, Elliott MN, Goldstein E, Lehrman WG, Spencer PA. Development, implementation, and public reporting of the HCAHPS survey. Med Care Res Rev. 2010;67(1):27-37. PubMed
32. Anonymous. CMS continues to shift emphasis to quality of care. Hosp Case Manag. 2012;20(10):150-151. PubMed
33. The R.E.D.E. Model 2015. Cleveland Clinic Center for Excellence in Healthcare
Communication. http://healthcarecommunication.info/. Accessed April 3 2016.
34. Empathetics, Inc. A Boston-based empathy training firm raises $1.5 million in
Series A Financing 2015. Empathetics Inc. http://www.prnewswire.com/news-releases/
empathetics-inc----a-boston-based-empathy-training-firm-raises-15-million-
in-series-a-financing-300072696.html). Accessed April 3, 2016.
35. Intensive Communication Skills 2016. Institute for Healthcare Communication.
http://healthcarecomm.org/. Accessed April 3, 2016.
36. Hu B, Palta M, Shao J. Variability explained by covariates in linear mixed-effect
models for longitudinal data. Canadian Journal of Statistics. 2010;38:352-368.
37. O’Malley AJ, Zaslavsky AM, Elliott MN, Zaborski L, Cleary PD. Case-mix adjustment
of the CAHPS Hospital Survey. Health Serv Res. 2005;40(6 pt 2):2162-2181. PubMed
38. O’Malley AJ, Zaslavsky AM, Hays RD, Hepner KA, Keller S, Cleary PD. Exploratory
factor analyses of the CAHPS Hospital Pilot Survey responses across
and within medical, surgical, and obstetric services. Health Serv Res. 2005;40(6 pt
2):2078-2095. PubMed
39. Goldstein E, Farquhar M, Crofton C, Darby C, Garfinkel S. Measuring hospital
care from the patients’ perspective: an overview of the CAHPS Hospital Survey
development process. Health Serv Res. 2005;40(6 pt 2):1977-1995. PubMed
40. Darby C, Hays RD, Kletke P. Development and evaluation of the CAHPS hospital
survey. Health Serv Res. 2005;40(6 pt 2):1973-1976. PubMed
41. Keller VF, Carroll JG. A new model for physician-patient communication. Patient
Educ Couns. 1994;23(2):131-140. PubMed
42. Quirk M, Mazor K, Haley HL, et al. How patients perceive a doctor’s caring attitude.
Patient Educ Couns. 2008;72(3):359-366. PubMed
43. Frankel RM, Levinson W. Back to the future: Can conversation analysis be used
to judge physicians’ malpractice history? Commun Med. 2014;11(1):27-39. PubMed
44. Furnham A. Response bias, social desirability and dissimulation. Personality and
individual differences 1986;7(3):385-400.
45. Shteingart H, Neiman T, Loewenstein Y. The role of first impression in operant
learning. J Exp Psychol Gen. 2013;142(2):476-488. PubMed
46. Tefera L, Lehrman WG, Conway P. Measurement of the patient experience: clarifying
facts, myths, and approaches. JAMA. 2016;315(2):2167-2168. PubMed
47. Horowitz CR, Suchman AL, Branch WT Jr, Frankel RM. What do doctors find
meaningful about their work? Ann Intern Med. 2003;138(9):772-775. PubMed
48. Rao JK, Anderson LA, Inui TS, Frankel RM. Communication interventions make
a difference in conversations between physicians and patients: a systematic review
of the evidence. Med Care. 2007;45(4):340-349. PubMed
© 2017 Society of Hospital Medicine
Incidence and nature of postoperative complications in patients with obstructive sleep apnea undergoing noncardiac surgery
Should we routinely screen for hypercapnia in sleep apnea patients before elective noncardiac surgery?
Yes. Obesity hypoventilation syndrome (OHS) is often undiagnosed and greatly increases perioperative risk. Therefore, we recommend trying to detect OHS in a timely manner. Treatment should begin without delay to avoid adverse perioperative outcomes, which can include acute-on-chronic respiratory failure requiring intensive-care monitoring and invasive mechanical ventilation, or death.
ALSO CALLED PICKWICKIAN SYNDROME
OHS is also known as Pickwickian syndrome, named for a character—a “fat boy” who is constantly falling asleep—in The Posthumous Papers of the Pickwick Club by Charles Dickens.
Salient features of OHS are:
- Obesity (body mass index ≥ 30 kg/m2)
- Sleep-disordered breathing (most patients with OHS are morbidly obese and have severe obstructive sleep apnea1)
- Chronic daytime alveolar hypoventilation: ie, Paco2 ≥ 45 mm Hg (normal range 35–45 mm Hg) and Pao2 < 70 mm Hg1 (normal range 85–95 mm Hg)
- No other identifiable cause of hypoventilation such as pulmonary disease (severe obstructive or restrictive), chest wall deformities, severe hypothyroidism, or neuromuscular disease.
WHY SCREEN FOR OHS?
Both obstructive sleep apnea and OHS worsen quality of life and increase the risk of serious disease and death.2–3 Patients with severe sleep apnea, particularly those with hypercapnia (ie, OHS) are at higher risk of cardiopulmonary complications in the perioperative period.
Compared with eucapnic patients with obstructive sleep apnea, patients with OHS have higher health care expenses, are at higher risk of developing serious cardiovascular diseases such as pulmonary hypertension and congestive heart failure, and are more likely to die sooner.4,5
Nowbar et al5 prospectively followed a group of severely obese patients after hospital discharge. At 18 months, 23% of those with OHS had died, compared with 9% of those without OHS. The groups were well matched for body mass index, age, and a number of comorbid conditions. Most of the deaths occurred in the first 3 months after hospital discharge. During the hospital stay, more patients with OHS were admitted to the intensive care unit and needed endotracheal intubation and mechanical ventilation, and more were discharged to a long-term facility.
A high level of suspicion can lead to early recognition and treatment, which may reduce the rate of adverse outcomes associated with undiagnosed and untreated OHS. Routine screening for hypercapnia in patients with sleep apnea might help to identify patients with OHS and allow for modifications in surgical approach, anesthetic technique, and postoperative monitoring, increasing patient safety.
HOW PREVALENT IS OHS?
Obstructive sleep apnea affects up to 20% of US adults and is undiagnosed and untreated in up to 90% of cases.6 Simple screening questionnaires have been shown to reliably identify patients at risk.7,8
To date, no population-based prevalence studies of OHS have been done.
The overall prevalence of OHS in patients with obstructive sleep apnea is better studied: multiple prospective and retrospective studies across various geographic regions with a variety of racial or ethnic populations have shown it to be between 10% and 20%.1,9 This range is very consistent among studies performed in Europe, the United States, and Japan, whether retrospective or prospective, and whether large or small.
The prevalence of OHS in the general adult population in the United States can, however, be estimated. If approximately 5% of the general US population has severe obesity (body mass index ≤ 40 kg/m2), if half of patients with severe obesity have obstructive sleep apnea,10 and if 15% of severely obese patients with sleep apnea have OHS, then a conservative estimated prevalence of OHS in the general adult US population is 0.37% (1 in 270 adults).
WHAT CAN BE DONE BEFORE ELECTIVE SURGERY?
Patients with OHS have an elevated serum bicarbonate level due to metabolic compensation for chronic respiratory acidosis. Moreover, they may have mild hypoxemia during wakefulness as measured by finger pulse oximetry.
The serum venous bicarbonate level is an easy and reasonable test to screen for hypercapnia in obese patients with obstructive sleep apnea because it is readily available, physiologically sensible, and less invasive than arterial puncture to measure blood gases.9
Arterial blood gas measurements, however, should be obtained to confirm the presence and severity of daytime hypercapnia in obese patients with hypoxemia during wakefulness or an elevated serum bicarbonate level.
Pulmonary function testing and chest imaging can exclude other causes of hypercapnia if hypercapnia is confirmed.
An overnight, attended polysomnographic study in a sleep laboratory is ultimately needed to establish the diagnosis and severity of obstructive sleep apnea and to titrate continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) therapy. Since most patients with OHS have severe obstructive sleep apnea, in-laboratory attended polysomnography allows the clinician to both diagnose and intervene with PAP therapy (a “split-night” study). Home titration with an auto-CPAP device is not recommended because it does not have the ability to titrate PAP pressures in response to hypoxemia or hypoventilation. Patients with OHS require attended, laboratory-based PAP titration with or without supplemental oxygen.
CPAP or BPAP therapy should be started during the few days or weeks before surgery, and adherence should be emphasized. Anesthesiologists might reconsider the choice of anesthetic technique in favor of regional anesthesia and modify postoperative pain management to reduce opioid requirements. Reinstituting CPAP or BPAP therapy upon extubation or arrival in the postoperative recovery unit can further reduce the risk of respiratory complications. Additional monitoring such as continuous pulse oximetry when the patient is on the general ward should be considered.
- Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc 2008; 5:218–225.
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005; 293:1861–1867.
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008; 31:1071–1078.
- Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001; 120:377–383.
- Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116:1–7.
- Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 2009; 10:753–758.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med 2008; 2:349–364.
Yes. Obesity hypoventilation syndrome (OHS) is often undiagnosed and greatly increases perioperative risk. Therefore, we recommend trying to detect OHS in a timely manner. Treatment should begin without delay to avoid adverse perioperative outcomes, which can include acute-on-chronic respiratory failure requiring intensive-care monitoring and invasive mechanical ventilation, or death.
ALSO CALLED PICKWICKIAN SYNDROME
OHS is also known as Pickwickian syndrome, named for a character—a “fat boy” who is constantly falling asleep—in The Posthumous Papers of the Pickwick Club by Charles Dickens.
Salient features of OHS are:
- Obesity (body mass index ≥ 30 kg/m2)
- Sleep-disordered breathing (most patients with OHS are morbidly obese and have severe obstructive sleep apnea1)
- Chronic daytime alveolar hypoventilation: ie, Paco2 ≥ 45 mm Hg (normal range 35–45 mm Hg) and Pao2 < 70 mm Hg1 (normal range 85–95 mm Hg)
- No other identifiable cause of hypoventilation such as pulmonary disease (severe obstructive or restrictive), chest wall deformities, severe hypothyroidism, or neuromuscular disease.
WHY SCREEN FOR OHS?
Both obstructive sleep apnea and OHS worsen quality of life and increase the risk of serious disease and death.2–3 Patients with severe sleep apnea, particularly those with hypercapnia (ie, OHS) are at higher risk of cardiopulmonary complications in the perioperative period.
Compared with eucapnic patients with obstructive sleep apnea, patients with OHS have higher health care expenses, are at higher risk of developing serious cardiovascular diseases such as pulmonary hypertension and congestive heart failure, and are more likely to die sooner.4,5
Nowbar et al5 prospectively followed a group of severely obese patients after hospital discharge. At 18 months, 23% of those with OHS had died, compared with 9% of those without OHS. The groups were well matched for body mass index, age, and a number of comorbid conditions. Most of the deaths occurred in the first 3 months after hospital discharge. During the hospital stay, more patients with OHS were admitted to the intensive care unit and needed endotracheal intubation and mechanical ventilation, and more were discharged to a long-term facility.
A high level of suspicion can lead to early recognition and treatment, which may reduce the rate of adverse outcomes associated with undiagnosed and untreated OHS. Routine screening for hypercapnia in patients with sleep apnea might help to identify patients with OHS and allow for modifications in surgical approach, anesthetic technique, and postoperative monitoring, increasing patient safety.
HOW PREVALENT IS OHS?
Obstructive sleep apnea affects up to 20% of US adults and is undiagnosed and untreated in up to 90% of cases.6 Simple screening questionnaires have been shown to reliably identify patients at risk.7,8
To date, no population-based prevalence studies of OHS have been done.
The overall prevalence of OHS in patients with obstructive sleep apnea is better studied: multiple prospective and retrospective studies across various geographic regions with a variety of racial or ethnic populations have shown it to be between 10% and 20%.1,9 This range is very consistent among studies performed in Europe, the United States, and Japan, whether retrospective or prospective, and whether large or small.
The prevalence of OHS in the general adult population in the United States can, however, be estimated. If approximately 5% of the general US population has severe obesity (body mass index ≤ 40 kg/m2), if half of patients with severe obesity have obstructive sleep apnea,10 and if 15% of severely obese patients with sleep apnea have OHS, then a conservative estimated prevalence of OHS in the general adult US population is 0.37% (1 in 270 adults).
WHAT CAN BE DONE BEFORE ELECTIVE SURGERY?
Patients with OHS have an elevated serum bicarbonate level due to metabolic compensation for chronic respiratory acidosis. Moreover, they may have mild hypoxemia during wakefulness as measured by finger pulse oximetry.
The serum venous bicarbonate level is an easy and reasonable test to screen for hypercapnia in obese patients with obstructive sleep apnea because it is readily available, physiologically sensible, and less invasive than arterial puncture to measure blood gases.9
Arterial blood gas measurements, however, should be obtained to confirm the presence and severity of daytime hypercapnia in obese patients with hypoxemia during wakefulness or an elevated serum bicarbonate level.
Pulmonary function testing and chest imaging can exclude other causes of hypercapnia if hypercapnia is confirmed.
An overnight, attended polysomnographic study in a sleep laboratory is ultimately needed to establish the diagnosis and severity of obstructive sleep apnea and to titrate continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) therapy. Since most patients with OHS have severe obstructive sleep apnea, in-laboratory attended polysomnography allows the clinician to both diagnose and intervene with PAP therapy (a “split-night” study). Home titration with an auto-CPAP device is not recommended because it does not have the ability to titrate PAP pressures in response to hypoxemia or hypoventilation. Patients with OHS require attended, laboratory-based PAP titration with or without supplemental oxygen.
CPAP or BPAP therapy should be started during the few days or weeks before surgery, and adherence should be emphasized. Anesthesiologists might reconsider the choice of anesthetic technique in favor of regional anesthesia and modify postoperative pain management to reduce opioid requirements. Reinstituting CPAP or BPAP therapy upon extubation or arrival in the postoperative recovery unit can further reduce the risk of respiratory complications. Additional monitoring such as continuous pulse oximetry when the patient is on the general ward should be considered.
Yes. Obesity hypoventilation syndrome (OHS) is often undiagnosed and greatly increases perioperative risk. Therefore, we recommend trying to detect OHS in a timely manner. Treatment should begin without delay to avoid adverse perioperative outcomes, which can include acute-on-chronic respiratory failure requiring intensive-care monitoring and invasive mechanical ventilation, or death.
ALSO CALLED PICKWICKIAN SYNDROME
OHS is also known as Pickwickian syndrome, named for a character—a “fat boy” who is constantly falling asleep—in The Posthumous Papers of the Pickwick Club by Charles Dickens.
Salient features of OHS are:
- Obesity (body mass index ≥ 30 kg/m2)
- Sleep-disordered breathing (most patients with OHS are morbidly obese and have severe obstructive sleep apnea1)
- Chronic daytime alveolar hypoventilation: ie, Paco2 ≥ 45 mm Hg (normal range 35–45 mm Hg) and Pao2 < 70 mm Hg1 (normal range 85–95 mm Hg)
- No other identifiable cause of hypoventilation such as pulmonary disease (severe obstructive or restrictive), chest wall deformities, severe hypothyroidism, or neuromuscular disease.
WHY SCREEN FOR OHS?
Both obstructive sleep apnea and OHS worsen quality of life and increase the risk of serious disease and death.2–3 Patients with severe sleep apnea, particularly those with hypercapnia (ie, OHS) are at higher risk of cardiopulmonary complications in the perioperative period.
Compared with eucapnic patients with obstructive sleep apnea, patients with OHS have higher health care expenses, are at higher risk of developing serious cardiovascular diseases such as pulmonary hypertension and congestive heart failure, and are more likely to die sooner.4,5
Nowbar et al5 prospectively followed a group of severely obese patients after hospital discharge. At 18 months, 23% of those with OHS had died, compared with 9% of those without OHS. The groups were well matched for body mass index, age, and a number of comorbid conditions. Most of the deaths occurred in the first 3 months after hospital discharge. During the hospital stay, more patients with OHS were admitted to the intensive care unit and needed endotracheal intubation and mechanical ventilation, and more were discharged to a long-term facility.
A high level of suspicion can lead to early recognition and treatment, which may reduce the rate of adverse outcomes associated with undiagnosed and untreated OHS. Routine screening for hypercapnia in patients with sleep apnea might help to identify patients with OHS and allow for modifications in surgical approach, anesthetic technique, and postoperative monitoring, increasing patient safety.
HOW PREVALENT IS OHS?
Obstructive sleep apnea affects up to 20% of US adults and is undiagnosed and untreated in up to 90% of cases.6 Simple screening questionnaires have been shown to reliably identify patients at risk.7,8
To date, no population-based prevalence studies of OHS have been done.
The overall prevalence of OHS in patients with obstructive sleep apnea is better studied: multiple prospective and retrospective studies across various geographic regions with a variety of racial or ethnic populations have shown it to be between 10% and 20%.1,9 This range is very consistent among studies performed in Europe, the United States, and Japan, whether retrospective or prospective, and whether large or small.
The prevalence of OHS in the general adult population in the United States can, however, be estimated. If approximately 5% of the general US population has severe obesity (body mass index ≤ 40 kg/m2), if half of patients with severe obesity have obstructive sleep apnea,10 and if 15% of severely obese patients with sleep apnea have OHS, then a conservative estimated prevalence of OHS in the general adult US population is 0.37% (1 in 270 adults).
WHAT CAN BE DONE BEFORE ELECTIVE SURGERY?
Patients with OHS have an elevated serum bicarbonate level due to metabolic compensation for chronic respiratory acidosis. Moreover, they may have mild hypoxemia during wakefulness as measured by finger pulse oximetry.
The serum venous bicarbonate level is an easy and reasonable test to screen for hypercapnia in obese patients with obstructive sleep apnea because it is readily available, physiologically sensible, and less invasive than arterial puncture to measure blood gases.9
Arterial blood gas measurements, however, should be obtained to confirm the presence and severity of daytime hypercapnia in obese patients with hypoxemia during wakefulness or an elevated serum bicarbonate level.
Pulmonary function testing and chest imaging can exclude other causes of hypercapnia if hypercapnia is confirmed.
An overnight, attended polysomnographic study in a sleep laboratory is ultimately needed to establish the diagnosis and severity of obstructive sleep apnea and to titrate continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) therapy. Since most patients with OHS have severe obstructive sleep apnea, in-laboratory attended polysomnography allows the clinician to both diagnose and intervene with PAP therapy (a “split-night” study). Home titration with an auto-CPAP device is not recommended because it does not have the ability to titrate PAP pressures in response to hypoxemia or hypoventilation. Patients with OHS require attended, laboratory-based PAP titration with or without supplemental oxygen.
CPAP or BPAP therapy should be started during the few days or weeks before surgery, and adherence should be emphasized. Anesthesiologists might reconsider the choice of anesthetic technique in favor of regional anesthesia and modify postoperative pain management to reduce opioid requirements. Reinstituting CPAP or BPAP therapy upon extubation or arrival in the postoperative recovery unit can further reduce the risk of respiratory complications. Additional monitoring such as continuous pulse oximetry when the patient is on the general ward should be considered.
- Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc 2008; 5:218–225.
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005; 293:1861–1867.
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008; 31:1071–1078.
- Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001; 120:377–383.
- Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116:1–7.
- Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 2009; 10:753–758.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med 2008; 2:349–364.
- Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc 2008; 5:218–225.
- Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA 2005; 293:1861–1867.
- Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008; 31:1071–1078.
- Berg G, Delaive K, Manfreda J, Walld R, Kryger MH. The use of health-care resources in obesity-hypoventilation syndrome. Chest 2001; 120:377–383.
- Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med 2004; 116:1–7.
- Kapur V, Strohl KP, Redline S, Iber C, O'Connor G, Nieto J. Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep Breath 2002; 6:49–54.
- Finkel KJ, Searleman AC, Tymkew H, et al. Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med 2009; 10:753–758.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108:812–821.
- Mokhlesi B, Tulaimat A, Faibussowitsch I, Wang Y, Evans AT. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath 2007; 11:117–124.
- Lee W, Nagubadi S, Kryger MH, Mokhlesi B. Epidemiology of obstructive sleep apnea: a population-based perspective. Expert Rev Respir Med 2008; 2:349–364.