User login
Early and Significant Reduction in C-Reactive Protein Levels After Corticosteroid Therapy Is Associated With Reduced Mortality in Patients With COVID-19
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.
After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
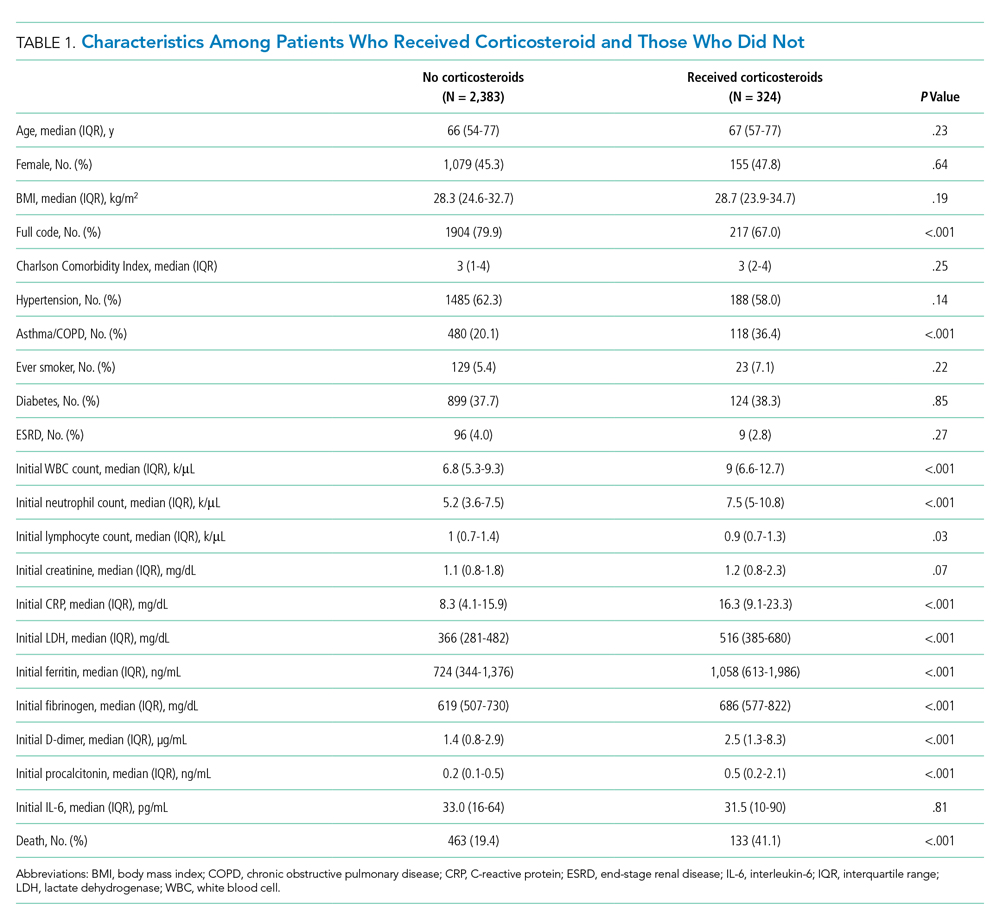
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
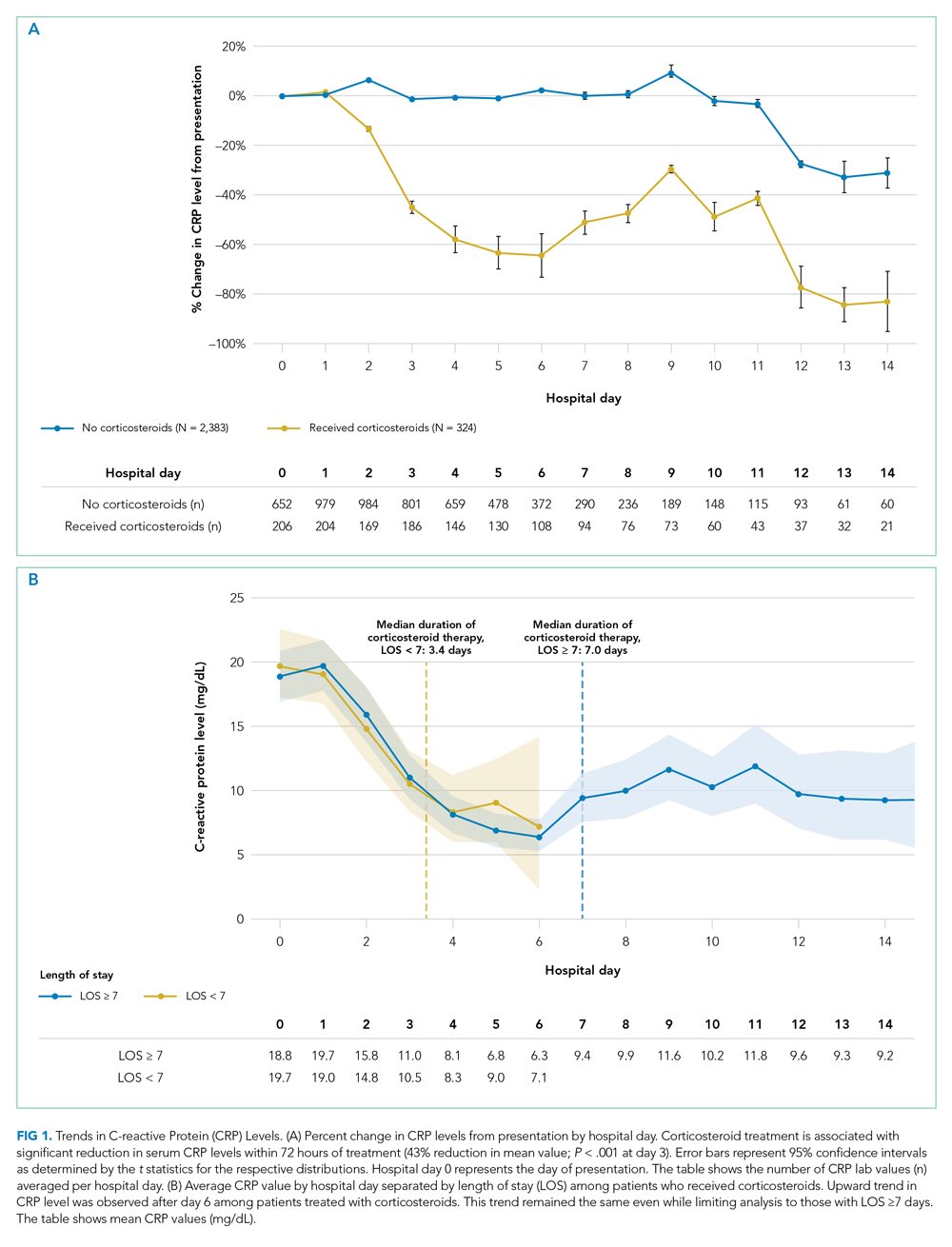
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.
CRP Responders vs Nonresponders
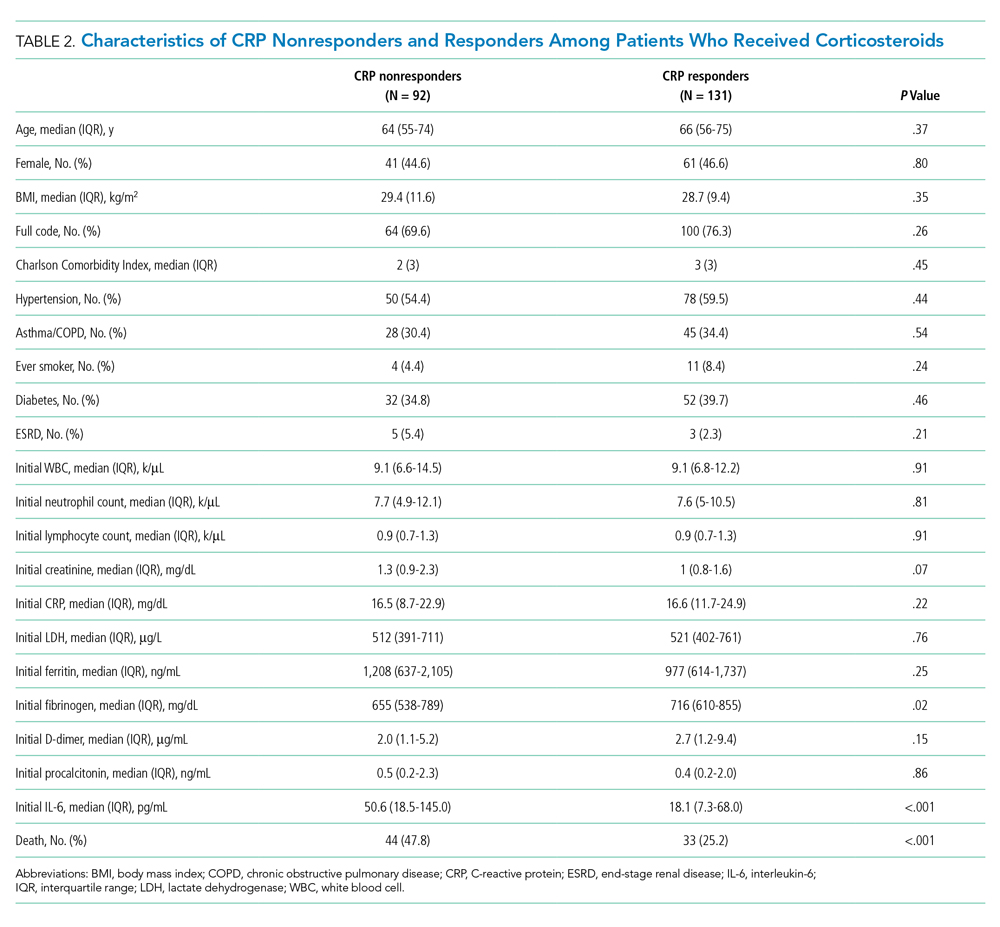
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.
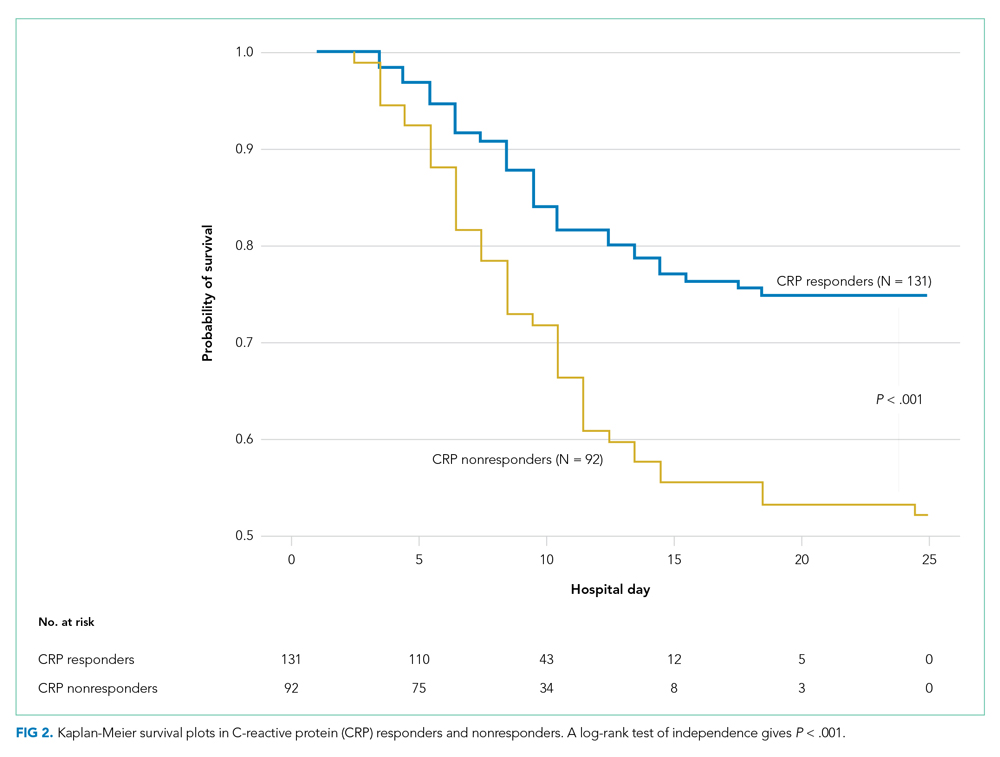
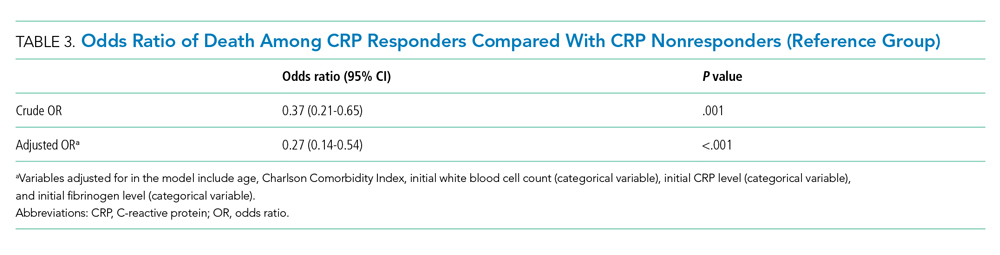
The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.
To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.
DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.

After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.

CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.

The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.

To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.

DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.

After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.

CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.

The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.

To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.

DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
© 2021 Society of Hospital Medicine
Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19
Coronavirus disease 2019 (COVID-19) is the most important public health emergency of the 21st century. The pandemic has devastated New York City, where over 17,000 confirmed deaths have occurred as of June 5, 2020.1 The most common cause of death in COVID-19 patients is respiratory failure from acute respiratory distress syndrome (ARDS). A recent study reported high mortality rates among COVID-19 patients who received mechanical ventilation (MV).2
Glucocorticoids are useful as adjunctive treatment for some infections with inflammatory responses, but their efficacy in COVID-19 is unclear. Prior experience with influenza and other coronaviruses may be relevant. A recent meta-analysis of influenza pneumonia showed increased mortality and a higher rate of secondary infections in patients who were administered glucocorticoids.3 For Middle East respiratory syndrome, severe acute respiratory syndrome, and influenza, some studies have demonstrated an association between glucocorticoid use and delayed viral clearance.4-7 However, a recent retrospective series of patients with COVID-19 and ARDS demonstrated a decrease in mortality with glucocorticoid use.8 Glucocorticoids are easily obtained and familiar to providers caring for COVID-19 patients. Hence their empiric use is widespread.8,9
The primary goal of this study was to determine whether early glucocorticoid treatment is associated with reduced mortality or need for MV in COVID-19 patients.
METHODS
Study Setting and Overview
Montefiore Medical Center comprises four hospitals totaling 1,536 beds in the Bronx borough of New York, New York. Based upon early experience, some clinicians began prescribing systemic glucocorticoids to patients with COVID-19 while others did not. We leveraged this variation in practice to examine the effectiveness of glucocorticoids in reducing mortality and the rate of MV in hospitalized COVID-19 patients.
Study Populations
There were 2,998 patients admitted with a positive COVID-19 test between March 11, 2020, and April 13, 2020. An a priori decision was made to include all hospitalized COVID-19 patients, including children. Because the outcomes of in-hospital mortality and in-hospital MV cannot be assessed in patients still hospitalized, we included only patients who either died or had been discharged from the hospital. Patients who died or were placed on MV within the first 48 hours of admission were excluded because outcome events occurred before having the opportunity for glucocorticoid treatment. To ensure treatment preceded outcome measurement, we included only patients treated with glucocorticoids within the first 48 hours of admission (treatment group) and compared them with patients never treated with glucocorticoids (control group).
Outcomes and Independent Variables
The primary outcome was a composite of in-hospital mortality or in-hospital MV. Secondary outcomes were the components of the primary. Timing of MV was determined using the first documentation of a ventilator respiratory rate or tidal volume. The independent variable of interest was treatment with glucocorticoids within the first 48 hours of admission. Formulations included are described in the Appendix.
To compare treatment and control groups and to perform adjusted analyses, we also examined the demographic and clinical characteristics, comorbidities, and laboratory values of each admission. For the comparison of study populations, missing values for each variable were ignored. In the primary (unstratified) multivariable analysis, continuous variables were categorized, with missing values assumed to be normal when used as an adjustment variable. All variables extracted, number of missing values, candidates for inclusion in the multivariable analysis, and those that fell out of the model are presented in the Appendix. Several subgroup analyses were predefined including age, diabetes, admission glucose, C-reactive protein (CRP), D-dimer, and troponin T levels.
Statistical Analysis
The treated and control groups were compared with respect to demographics, clinical characteristics, comorbidities, and laboratory values. Primary and secondary outcomes in the groups were compared in unadjusted and adjusted analyses using univariable and multivariable logistic regression models. All patient characteristics that were candidates for inclusion in the adjustment models are listed in the Appendix. Variables were included in the final model if they were associated with the primary outcome (Wald test P < .20) in univariable regression. A sensitivity analysis excluded all variables missing greater than 10% of data, including CRP. Interactions between treatment and six predefined subgroups were tested using logistic regression with interaction terms (eg, [steroids]*[age]). Stratified logistic regression was used to test the association between treatment and the primary outcome in each of the predefined subgroups. Patients who were missing CRP were excluded from the stratified analysis. Because a significant interaction between treatment and initial CRP level was discovered, we undertook a post hoc adjusted analysis within each of the 15 predefined subgroup variables. Because there were fewer outcome events in each subgroup, we constructed a parsimonious logistic regression model that included all variables independently associated with the exposure (P < .05). The same seven adjustment variables were used in each of the predefined subgroups. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. Stata 15.1 software (StataCorp) was used for data analysis.
RESULTS
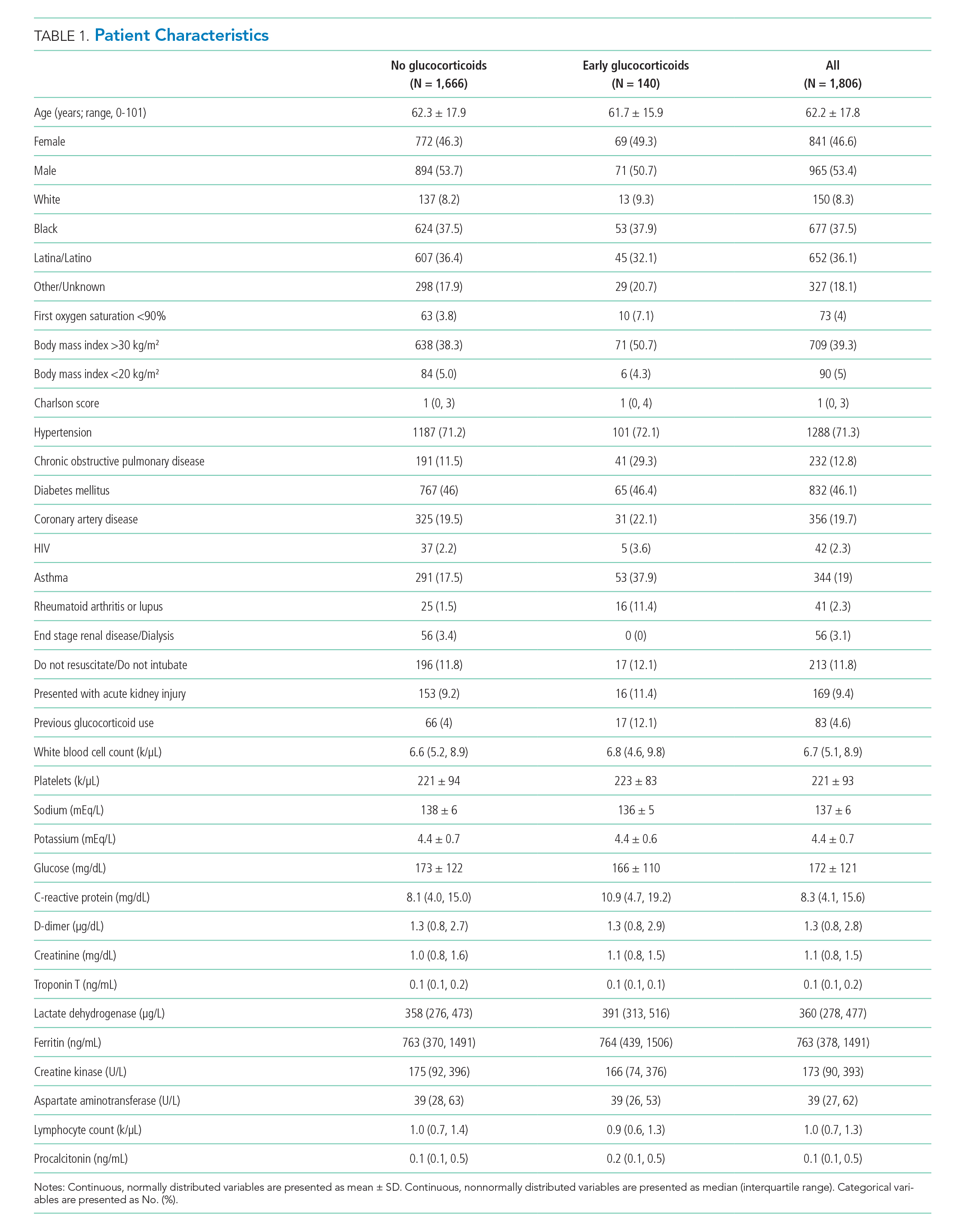
Of 2,998 patients examined, 1,806 met inclusion criteria and included 140 (7.7%) treated with glucocorticoids within 48 hours of admission and 1,666 who never received glucocorticoids. Reasons for exclusion of 1,192 patients are provided in the Appendix. Among patients who remained hospitalized and were excluded, 169 of 962 (17.6%) received glucocorticoids. Characteristics of the study population are presented in Table 1. Treatment and control groups were similar except that glucocorticoid-treated patients were more likely to have chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis or lupus, or to have received glucocorticoids in the year prior to admission.
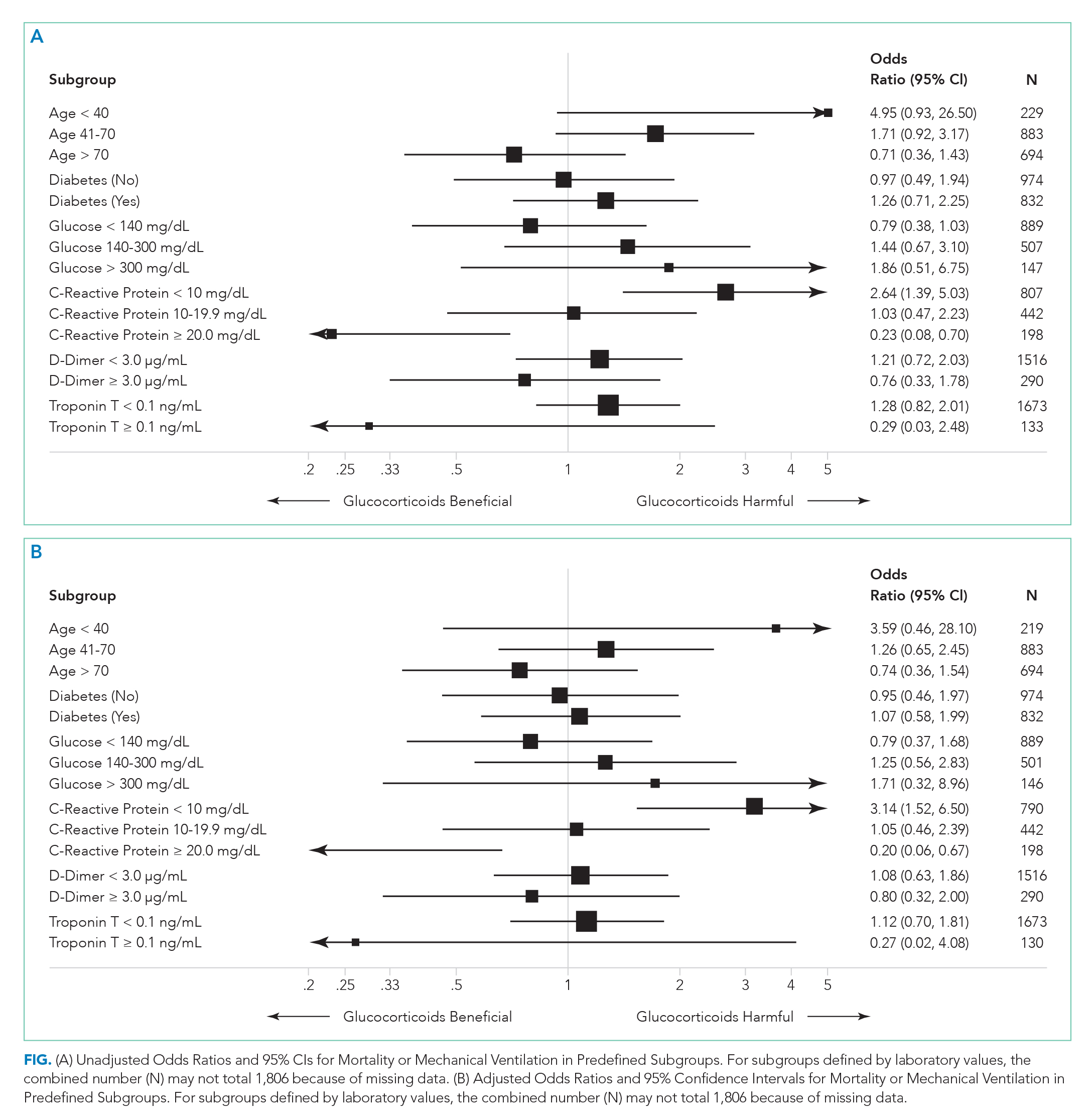
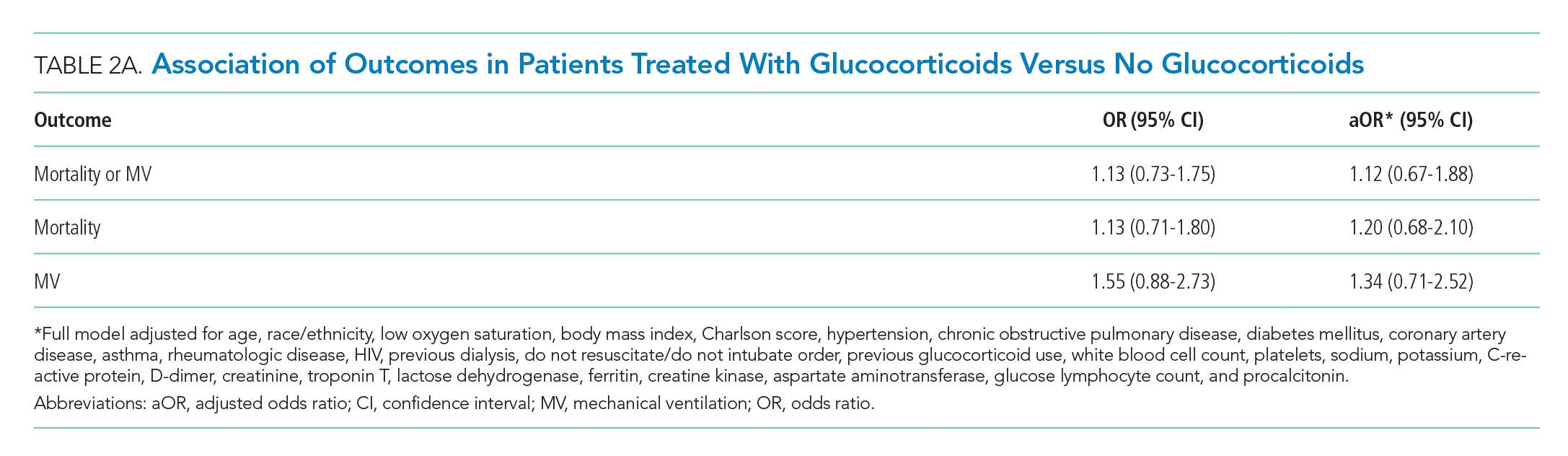
There were 318 who met the primary outcome of death or mechanical ventilation, 270 of whom died and 135 of whom required mechanical ventilation. Overall, early use of glucocorticoids was not associated with in-hospital mortality or MV as a composite outcome or as separate outcomes in both unadjusted and adjusted models (Table 2A). However, there was significant heterogeneity of treatment effect in the subgroups defined by CRP levels (P for interaction = .008; Figure). Early glucocorticoid use and an initial CRP of 20 mg/dL or higher was associated with a significantly reduced risk of mortality or MV in unadjusted (odds ratio, 0.23; 95% CI, 0.08-0.70) and adjusted (aOR, 0.20; 95% CI, 0.06-0.67) analyses (Table 2B). Conversely, glucocorticoid treatment in patients with CRP levels less than 10 mg/dL was associated with a significantly increased risk of mortality or MV in unadjusted (OR, 2.64; 95% CI, 1.39-5.03) and adjusted (aOR, 3.14; 95% CI, 1.52-6.50) analyses.
DISCUSSION
The results of this study indicate that early treatment with glucocorticoids is not associated with mortality or need for MV in unselected patients with COVID-19. Subgroup analyses suggest that glucocorticoid-treated patients with markedly elevated CRP may benefit from glucocorticoid treatment, whereas those patients with lower CRP may be harmed. Our findings were consistent after adjustment for clinical characteristics. The public health implications of these findings are hard to overestimate. Given the global growth of the pandemic and that glucocorticoids are widely available and inexpensive, glucocorticoid therapy may save many thousands of lives. Equally important because we have been able to identify a group that may be harmed, some patients may be saved because glucocorticoids will not be given.
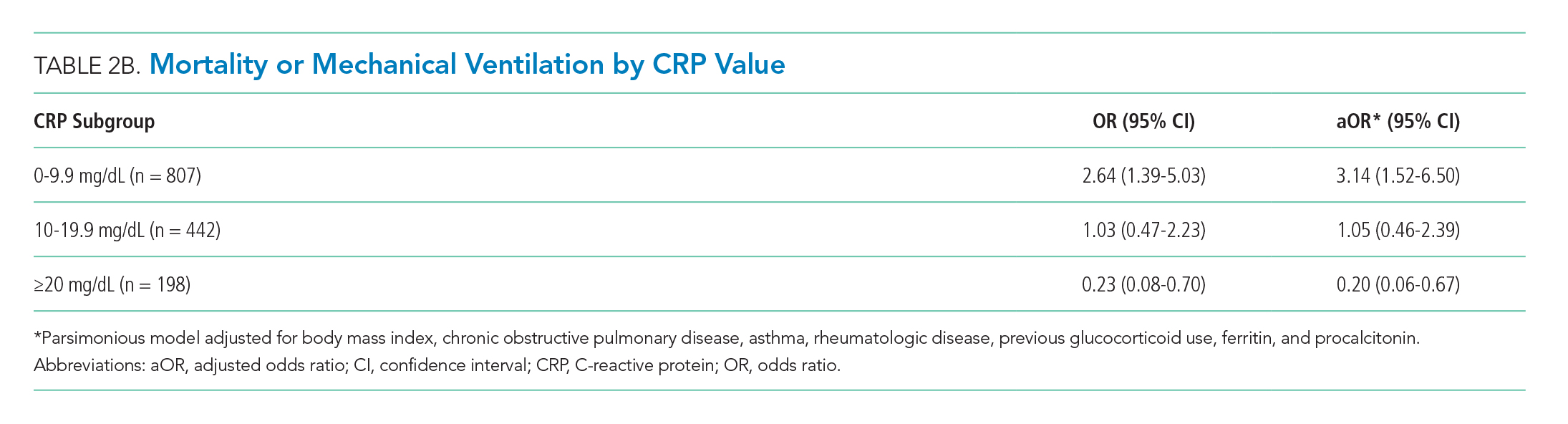
Our study reaffirms the finding of the as yet unpublished Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial that there is a subset of patients with COVID-19 who benefit from treatment with glucocorticoids.10 Our study extends the findings of the RECOVERY trial in two important ways. First, in addition to finding some patients who may benefit, we also have identified patient groups that may experience harm from treatment with glucocorticoids. This finding suggests choosing the right patients for glucocorticoid treatment is critical to maximize the likelihood of benefit and minimize the risk of harm. Second, we have identified patient groups who are likely to benefit (or be harmed) on the basis of a widely available lab test (CRP).
Our results are also consistent with previous studies of patients with SARS-CoV and MERS-CoV, in which no associations between glucocorticoid treatment and mortality were found.7 However, the results of studies examining the effect of glucocorticoids in patients with COVID-19 are less consistent.8,11,12
Few of the previous studies examined the effects of glucocorticoids in subgroups of patients. In our study, the improved outcomes associated with glucocorticoid use in patients with elevated CRPs is intriguing and may be clinically important. Proinflammatory cytokines, especially interleukin-6, acutely increase CRP levels. Cytokine storm syndrome (CSS) is a hyperinflammatory condition that occurs in a subset of COVID-19 patients, often resulting in multiorgan dysfunction.13 CRP is markedly elevated in CSS,14 and improved outcomes with glucocorticoid therapy in this subgroup may indicate benefit in this inflammatory phenotype. Patients with lower CRP are less likely to have CSS and may experience more harm than benefit associated with glucocorticoid treatment.
Several limitations are inherent to this study. Since it was done at a single center, the results may not be generalizable. As a retrospective analysis, it is subject to confounding and bias. In addition, because patients were included only if they had reached the outcome of death/MV or hospital discharge, the sample size was truncated. We believe glucocorticoid use in hospitalized patients excluded from the study reflects increased use with time because of a growing belief in their effectiveness.
Preliminary analysis from the RECOVERY study showed a reduced rate of mortality in patients randomized to dexamethasone, compared with those who received standard of care.10 These results led to the National Institutes for Health COVID-19 Treatment Guidelines Panel recommendation for dexamethasone treatment in patients with COVID-19 who require supplemental oxygen or MV.15 Our findings suggest a role for CRP to identify patients who may benefit from glucocorticoid therapy, as well as those in whom it may be harmful. Additional studies to further elucidate the role of CRP in guiding glucocorticoid therapy and to predict clinical response are needed.
1. COVID-19: Data. 2020. New York City Health. Accessed June 5, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
3. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. https://doi.org/10.1186/s13054-019-2395-8
4. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. https://doi.org/10.1186/s13063-017-2427-0
5. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. https://doi.org/10.1016/j.jcv.2004.07.006
6. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. https://doi.org/10.1086/600383
7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. https://doi.org/10.1016/s0140-6736(20)30317-2
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. https://doi.org/10.1001/jamainternmed.2020.0994
9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
10. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. Preprint posted June 22, 2020. https://doi.org/10.1101/2020.06.22.20137273
11. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. Published online April 2, 2020. https://doi.org/10.1093/cid/ciaa243
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. https://doi.org/10.1172/jci137244
14. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. https://doi.org/10.1016/j.autrev.2020.102537
15. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. National Institutes of Health. Updated June 25, 2020. Accessed June 25, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
Coronavirus disease 2019 (COVID-19) is the most important public health emergency of the 21st century. The pandemic has devastated New York City, where over 17,000 confirmed deaths have occurred as of June 5, 2020.1 The most common cause of death in COVID-19 patients is respiratory failure from acute respiratory distress syndrome (ARDS). A recent study reported high mortality rates among COVID-19 patients who received mechanical ventilation (MV).2
Glucocorticoids are useful as adjunctive treatment for some infections with inflammatory responses, but their efficacy in COVID-19 is unclear. Prior experience with influenza and other coronaviruses may be relevant. A recent meta-analysis of influenza pneumonia showed increased mortality and a higher rate of secondary infections in patients who were administered glucocorticoids.3 For Middle East respiratory syndrome, severe acute respiratory syndrome, and influenza, some studies have demonstrated an association between glucocorticoid use and delayed viral clearance.4-7 However, a recent retrospective series of patients with COVID-19 and ARDS demonstrated a decrease in mortality with glucocorticoid use.8 Glucocorticoids are easily obtained and familiar to providers caring for COVID-19 patients. Hence their empiric use is widespread.8,9
The primary goal of this study was to determine whether early glucocorticoid treatment is associated with reduced mortality or need for MV in COVID-19 patients.
METHODS
Study Setting and Overview
Montefiore Medical Center comprises four hospitals totaling 1,536 beds in the Bronx borough of New York, New York. Based upon early experience, some clinicians began prescribing systemic glucocorticoids to patients with COVID-19 while others did not. We leveraged this variation in practice to examine the effectiveness of glucocorticoids in reducing mortality and the rate of MV in hospitalized COVID-19 patients.
Study Populations
There were 2,998 patients admitted with a positive COVID-19 test between March 11, 2020, and April 13, 2020. An a priori decision was made to include all hospitalized COVID-19 patients, including children. Because the outcomes of in-hospital mortality and in-hospital MV cannot be assessed in patients still hospitalized, we included only patients who either died or had been discharged from the hospital. Patients who died or were placed on MV within the first 48 hours of admission were excluded because outcome events occurred before having the opportunity for glucocorticoid treatment. To ensure treatment preceded outcome measurement, we included only patients treated with glucocorticoids within the first 48 hours of admission (treatment group) and compared them with patients never treated with glucocorticoids (control group).
Outcomes and Independent Variables
The primary outcome was a composite of in-hospital mortality or in-hospital MV. Secondary outcomes were the components of the primary. Timing of MV was determined using the first documentation of a ventilator respiratory rate or tidal volume. The independent variable of interest was treatment with glucocorticoids within the first 48 hours of admission. Formulations included are described in the Appendix.
To compare treatment and control groups and to perform adjusted analyses, we also examined the demographic and clinical characteristics, comorbidities, and laboratory values of each admission. For the comparison of study populations, missing values for each variable were ignored. In the primary (unstratified) multivariable analysis, continuous variables were categorized, with missing values assumed to be normal when used as an adjustment variable. All variables extracted, number of missing values, candidates for inclusion in the multivariable analysis, and those that fell out of the model are presented in the Appendix. Several subgroup analyses were predefined including age, diabetes, admission glucose, C-reactive protein (CRP), D-dimer, and troponin T levels.
Statistical Analysis
The treated and control groups were compared with respect to demographics, clinical characteristics, comorbidities, and laboratory values. Primary and secondary outcomes in the groups were compared in unadjusted and adjusted analyses using univariable and multivariable logistic regression models. All patient characteristics that were candidates for inclusion in the adjustment models are listed in the Appendix. Variables were included in the final model if they were associated with the primary outcome (Wald test P < .20) in univariable regression. A sensitivity analysis excluded all variables missing greater than 10% of data, including CRP. Interactions between treatment and six predefined subgroups were tested using logistic regression with interaction terms (eg, [steroids]*[age]). Stratified logistic regression was used to test the association between treatment and the primary outcome in each of the predefined subgroups. Patients who were missing CRP were excluded from the stratified analysis. Because a significant interaction between treatment and initial CRP level was discovered, we undertook a post hoc adjusted analysis within each of the 15 predefined subgroup variables. Because there were fewer outcome events in each subgroup, we constructed a parsimonious logistic regression model that included all variables independently associated with the exposure (P < .05). The same seven adjustment variables were used in each of the predefined subgroups. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. Stata 15.1 software (StataCorp) was used for data analysis.

RESULTS
Of 2,998 patients examined, 1,806 met inclusion criteria and included 140 (7.7%) treated with glucocorticoids within 48 hours of admission and 1,666 who never received glucocorticoids. Reasons for exclusion of 1,192 patients are provided in the Appendix. Among patients who remained hospitalized and were excluded, 169 of 962 (17.6%) received glucocorticoids. Characteristics of the study population are presented in Table 1. Treatment and control groups were similar except that glucocorticoid-treated patients were more likely to have chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis or lupus, or to have received glucocorticoids in the year prior to admission.

There were 318 who met the primary outcome of death or mechanical ventilation, 270 of whom died and 135 of whom required mechanical ventilation. Overall, early use of glucocorticoids was not associated with in-hospital mortality or MV as a composite outcome or as separate outcomes in both unadjusted and adjusted models (Table 2A). However, there was significant heterogeneity of treatment effect in the subgroups defined by CRP levels (P for interaction = .008; Figure). Early glucocorticoid use and an initial CRP of 20 mg/dL or higher was associated with a significantly reduced risk of mortality or MV in unadjusted (odds ratio, 0.23; 95% CI, 0.08-0.70) and adjusted (aOR, 0.20; 95% CI, 0.06-0.67) analyses (Table 2B). Conversely, glucocorticoid treatment in patients with CRP levels less than 10 mg/dL was associated with a significantly increased risk of mortality or MV in unadjusted (OR, 2.64; 95% CI, 1.39-5.03) and adjusted (aOR, 3.14; 95% CI, 1.52-6.50) analyses.

DISCUSSION
The results of this study indicate that early treatment with glucocorticoids is not associated with mortality or need for MV in unselected patients with COVID-19. Subgroup analyses suggest that glucocorticoid-treated patients with markedly elevated CRP may benefit from glucocorticoid treatment, whereas those patients with lower CRP may be harmed. Our findings were consistent after adjustment for clinical characteristics. The public health implications of these findings are hard to overestimate. Given the global growth of the pandemic and that glucocorticoids are widely available and inexpensive, glucocorticoid therapy may save many thousands of lives. Equally important because we have been able to identify a group that may be harmed, some patients may be saved because glucocorticoids will not be given.

Our study reaffirms the finding of the as yet unpublished Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial that there is a subset of patients with COVID-19 who benefit from treatment with glucocorticoids.10 Our study extends the findings of the RECOVERY trial in two important ways. First, in addition to finding some patients who may benefit, we also have identified patient groups that may experience harm from treatment with glucocorticoids. This finding suggests choosing the right patients for glucocorticoid treatment is critical to maximize the likelihood of benefit and minimize the risk of harm. Second, we have identified patient groups who are likely to benefit (or be harmed) on the basis of a widely available lab test (CRP).
Our results are also consistent with previous studies of patients with SARS-CoV and MERS-CoV, in which no associations between glucocorticoid treatment and mortality were found.7 However, the results of studies examining the effect of glucocorticoids in patients with COVID-19 are less consistent.8,11,12
Few of the previous studies examined the effects of glucocorticoids in subgroups of patients. In our study, the improved outcomes associated with glucocorticoid use in patients with elevated CRPs is intriguing and may be clinically important. Proinflammatory cytokines, especially interleukin-6, acutely increase CRP levels. Cytokine storm syndrome (CSS) is a hyperinflammatory condition that occurs in a subset of COVID-19 patients, often resulting in multiorgan dysfunction.13 CRP is markedly elevated in CSS,14 and improved outcomes with glucocorticoid therapy in this subgroup may indicate benefit in this inflammatory phenotype. Patients with lower CRP are less likely to have CSS and may experience more harm than benefit associated with glucocorticoid treatment.
Several limitations are inherent to this study. Since it was done at a single center, the results may not be generalizable. As a retrospective analysis, it is subject to confounding and bias. In addition, because patients were included only if they had reached the outcome of death/MV or hospital discharge, the sample size was truncated. We believe glucocorticoid use in hospitalized patients excluded from the study reflects increased use with time because of a growing belief in their effectiveness.
Preliminary analysis from the RECOVERY study showed a reduced rate of mortality in patients randomized to dexamethasone, compared with those who received standard of care.10 These results led to the National Institutes for Health COVID-19 Treatment Guidelines Panel recommendation for dexamethasone treatment in patients with COVID-19 who require supplemental oxygen or MV.15 Our findings suggest a role for CRP to identify patients who may benefit from glucocorticoid therapy, as well as those in whom it may be harmful. Additional studies to further elucidate the role of CRP in guiding glucocorticoid therapy and to predict clinical response are needed.
Coronavirus disease 2019 (COVID-19) is the most important public health emergency of the 21st century. The pandemic has devastated New York City, where over 17,000 confirmed deaths have occurred as of June 5, 2020.1 The most common cause of death in COVID-19 patients is respiratory failure from acute respiratory distress syndrome (ARDS). A recent study reported high mortality rates among COVID-19 patients who received mechanical ventilation (MV).2
Glucocorticoids are useful as adjunctive treatment for some infections with inflammatory responses, but their efficacy in COVID-19 is unclear. Prior experience with influenza and other coronaviruses may be relevant. A recent meta-analysis of influenza pneumonia showed increased mortality and a higher rate of secondary infections in patients who were administered glucocorticoids.3 For Middle East respiratory syndrome, severe acute respiratory syndrome, and influenza, some studies have demonstrated an association between glucocorticoid use and delayed viral clearance.4-7 However, a recent retrospective series of patients with COVID-19 and ARDS demonstrated a decrease in mortality with glucocorticoid use.8 Glucocorticoids are easily obtained and familiar to providers caring for COVID-19 patients. Hence their empiric use is widespread.8,9
The primary goal of this study was to determine whether early glucocorticoid treatment is associated with reduced mortality or need for MV in COVID-19 patients.
METHODS
Study Setting and Overview
Montefiore Medical Center comprises four hospitals totaling 1,536 beds in the Bronx borough of New York, New York. Based upon early experience, some clinicians began prescribing systemic glucocorticoids to patients with COVID-19 while others did not. We leveraged this variation in practice to examine the effectiveness of glucocorticoids in reducing mortality and the rate of MV in hospitalized COVID-19 patients.
Study Populations
There were 2,998 patients admitted with a positive COVID-19 test between March 11, 2020, and April 13, 2020. An a priori decision was made to include all hospitalized COVID-19 patients, including children. Because the outcomes of in-hospital mortality and in-hospital MV cannot be assessed in patients still hospitalized, we included only patients who either died or had been discharged from the hospital. Patients who died or were placed on MV within the first 48 hours of admission were excluded because outcome events occurred before having the opportunity for glucocorticoid treatment. To ensure treatment preceded outcome measurement, we included only patients treated with glucocorticoids within the first 48 hours of admission (treatment group) and compared them with patients never treated with glucocorticoids (control group).
Outcomes and Independent Variables
The primary outcome was a composite of in-hospital mortality or in-hospital MV. Secondary outcomes were the components of the primary. Timing of MV was determined using the first documentation of a ventilator respiratory rate or tidal volume. The independent variable of interest was treatment with glucocorticoids within the first 48 hours of admission. Formulations included are described in the Appendix.
To compare treatment and control groups and to perform adjusted analyses, we also examined the demographic and clinical characteristics, comorbidities, and laboratory values of each admission. For the comparison of study populations, missing values for each variable were ignored. In the primary (unstratified) multivariable analysis, continuous variables were categorized, with missing values assumed to be normal when used as an adjustment variable. All variables extracted, number of missing values, candidates for inclusion in the multivariable analysis, and those that fell out of the model are presented in the Appendix. Several subgroup analyses were predefined including age, diabetes, admission glucose, C-reactive protein (CRP), D-dimer, and troponin T levels.
Statistical Analysis
The treated and control groups were compared with respect to demographics, clinical characteristics, comorbidities, and laboratory values. Primary and secondary outcomes in the groups were compared in unadjusted and adjusted analyses using univariable and multivariable logistic regression models. All patient characteristics that were candidates for inclusion in the adjustment models are listed in the Appendix. Variables were included in the final model if they were associated with the primary outcome (Wald test P < .20) in univariable regression. A sensitivity analysis excluded all variables missing greater than 10% of data, including CRP. Interactions between treatment and six predefined subgroups were tested using logistic regression with interaction terms (eg, [steroids]*[age]). Stratified logistic regression was used to test the association between treatment and the primary outcome in each of the predefined subgroups. Patients who were missing CRP were excluded from the stratified analysis. Because a significant interaction between treatment and initial CRP level was discovered, we undertook a post hoc adjusted analysis within each of the 15 predefined subgroup variables. Because there were fewer outcome events in each subgroup, we constructed a parsimonious logistic regression model that included all variables independently associated with the exposure (P < .05). The same seven adjustment variables were used in each of the predefined subgroups. The study was approved by the Albert Einstein College of Medicine Institutional Review Board. Stata 15.1 software (StataCorp) was used for data analysis.

RESULTS
Of 2,998 patients examined, 1,806 met inclusion criteria and included 140 (7.7%) treated with glucocorticoids within 48 hours of admission and 1,666 who never received glucocorticoids. Reasons for exclusion of 1,192 patients are provided in the Appendix. Among patients who remained hospitalized and were excluded, 169 of 962 (17.6%) received glucocorticoids. Characteristics of the study population are presented in Table 1. Treatment and control groups were similar except that glucocorticoid-treated patients were more likely to have chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis or lupus, or to have received glucocorticoids in the year prior to admission.

There were 318 who met the primary outcome of death or mechanical ventilation, 270 of whom died and 135 of whom required mechanical ventilation. Overall, early use of glucocorticoids was not associated with in-hospital mortality or MV as a composite outcome or as separate outcomes in both unadjusted and adjusted models (Table 2A). However, there was significant heterogeneity of treatment effect in the subgroups defined by CRP levels (P for interaction = .008; Figure). Early glucocorticoid use and an initial CRP of 20 mg/dL or higher was associated with a significantly reduced risk of mortality or MV in unadjusted (odds ratio, 0.23; 95% CI, 0.08-0.70) and adjusted (aOR, 0.20; 95% CI, 0.06-0.67) analyses (Table 2B). Conversely, glucocorticoid treatment in patients with CRP levels less than 10 mg/dL was associated with a significantly increased risk of mortality or MV in unadjusted (OR, 2.64; 95% CI, 1.39-5.03) and adjusted (aOR, 3.14; 95% CI, 1.52-6.50) analyses.

DISCUSSION
The results of this study indicate that early treatment with glucocorticoids is not associated with mortality or need for MV in unselected patients with COVID-19. Subgroup analyses suggest that glucocorticoid-treated patients with markedly elevated CRP may benefit from glucocorticoid treatment, whereas those patients with lower CRP may be harmed. Our findings were consistent after adjustment for clinical characteristics. The public health implications of these findings are hard to overestimate. Given the global growth of the pandemic and that glucocorticoids are widely available and inexpensive, glucocorticoid therapy may save many thousands of lives. Equally important because we have been able to identify a group that may be harmed, some patients may be saved because glucocorticoids will not be given.

Our study reaffirms the finding of the as yet unpublished Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial that there is a subset of patients with COVID-19 who benefit from treatment with glucocorticoids.10 Our study extends the findings of the RECOVERY trial in two important ways. First, in addition to finding some patients who may benefit, we also have identified patient groups that may experience harm from treatment with glucocorticoids. This finding suggests choosing the right patients for glucocorticoid treatment is critical to maximize the likelihood of benefit and minimize the risk of harm. Second, we have identified patient groups who are likely to benefit (or be harmed) on the basis of a widely available lab test (CRP).
Our results are also consistent with previous studies of patients with SARS-CoV and MERS-CoV, in which no associations between glucocorticoid treatment and mortality were found.7 However, the results of studies examining the effect of glucocorticoids in patients with COVID-19 are less consistent.8,11,12
Few of the previous studies examined the effects of glucocorticoids in subgroups of patients. In our study, the improved outcomes associated with glucocorticoid use in patients with elevated CRPs is intriguing and may be clinically important. Proinflammatory cytokines, especially interleukin-6, acutely increase CRP levels. Cytokine storm syndrome (CSS) is a hyperinflammatory condition that occurs in a subset of COVID-19 patients, often resulting in multiorgan dysfunction.13 CRP is markedly elevated in CSS,14 and improved outcomes with glucocorticoid therapy in this subgroup may indicate benefit in this inflammatory phenotype. Patients with lower CRP are less likely to have CSS and may experience more harm than benefit associated with glucocorticoid treatment.
Several limitations are inherent to this study. Since it was done at a single center, the results may not be generalizable. As a retrospective analysis, it is subject to confounding and bias. In addition, because patients were included only if they had reached the outcome of death/MV or hospital discharge, the sample size was truncated. We believe glucocorticoid use in hospitalized patients excluded from the study reflects increased use with time because of a growing belief in their effectiveness.
Preliminary analysis from the RECOVERY study showed a reduced rate of mortality in patients randomized to dexamethasone, compared with those who received standard of care.10 These results led to the National Institutes for Health COVID-19 Treatment Guidelines Panel recommendation for dexamethasone treatment in patients with COVID-19 who require supplemental oxygen or MV.15 Our findings suggest a role for CRP to identify patients who may benefit from glucocorticoid therapy, as well as those in whom it may be harmful. Additional studies to further elucidate the role of CRP in guiding glucocorticoid therapy and to predict clinical response are needed.
1. COVID-19: Data. 2020. New York City Health. Accessed June 5, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
3. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. https://doi.org/10.1186/s13054-019-2395-8
4. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. https://doi.org/10.1186/s13063-017-2427-0
5. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. https://doi.org/10.1016/j.jcv.2004.07.006
6. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. https://doi.org/10.1086/600383
7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. https://doi.org/10.1016/s0140-6736(20)30317-2
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. https://doi.org/10.1001/jamainternmed.2020.0994
9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
10. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. Preprint posted June 22, 2020. https://doi.org/10.1101/2020.06.22.20137273
11. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. Published online April 2, 2020. https://doi.org/10.1093/cid/ciaa243
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. https://doi.org/10.1172/jci137244
14. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. https://doi.org/10.1016/j.autrev.2020.102537
15. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. National Institutes of Health. Updated June 25, 2020. Accessed June 25, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
1. COVID-19: Data. 2020. New York City Health. Accessed June 5, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
2. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
3. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Crit Care. 2019;23(1):99. https://doi.org/10.1186/s13054-019-2395-8
4. Arabi YM, Alothman A, Balkhy HH, et al. Treatment of Middle East Respiratory Syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81. https://doi.org/10.1186/s13063-017-2427-0
5. Lee N, Allen Chan KC, Hui DS, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31(4):304-309. https://doi.org/10.1016/j.jcv.2004.07.006
6. Lee N, Chan PK, Hui DS, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492-500. https://doi.org/10.1086/600383
7. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473-475. https://doi.org/10.1016/s0140-6736(20)30317-2
8. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. Published online March 13, 2020. https://doi.org/10.1001/jamainternmed.2020.0994
9. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-1720. https://doi.org/10.1056/nejmoa2002032
10. Horby P, Lim WS, Emberson J, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. Preprint posted June 22, 2020. https://doi.org/10.1101/2020.06.22.20137273
11. Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. Published online April 2, 2020. https://doi.org/10.1093/cid/ciaa243
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. https://doi.org/10.1172/jci137244
14. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. https://doi.org/10.1016/j.autrev.2020.102537
15. The National Institutes of Health COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. National Institutes of Health. Updated June 25, 2020. Accessed June 25, 2020. https://www.covid19treatmentguidelines.nih.gov/dexamethasone/
© 2020 Society of Hospital Medicine