User login
Early and Significant Reduction in C-Reactive Protein Levels After Corticosteroid Therapy Is Associated With Reduced Mortality in Patients With COVID-19
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.
After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
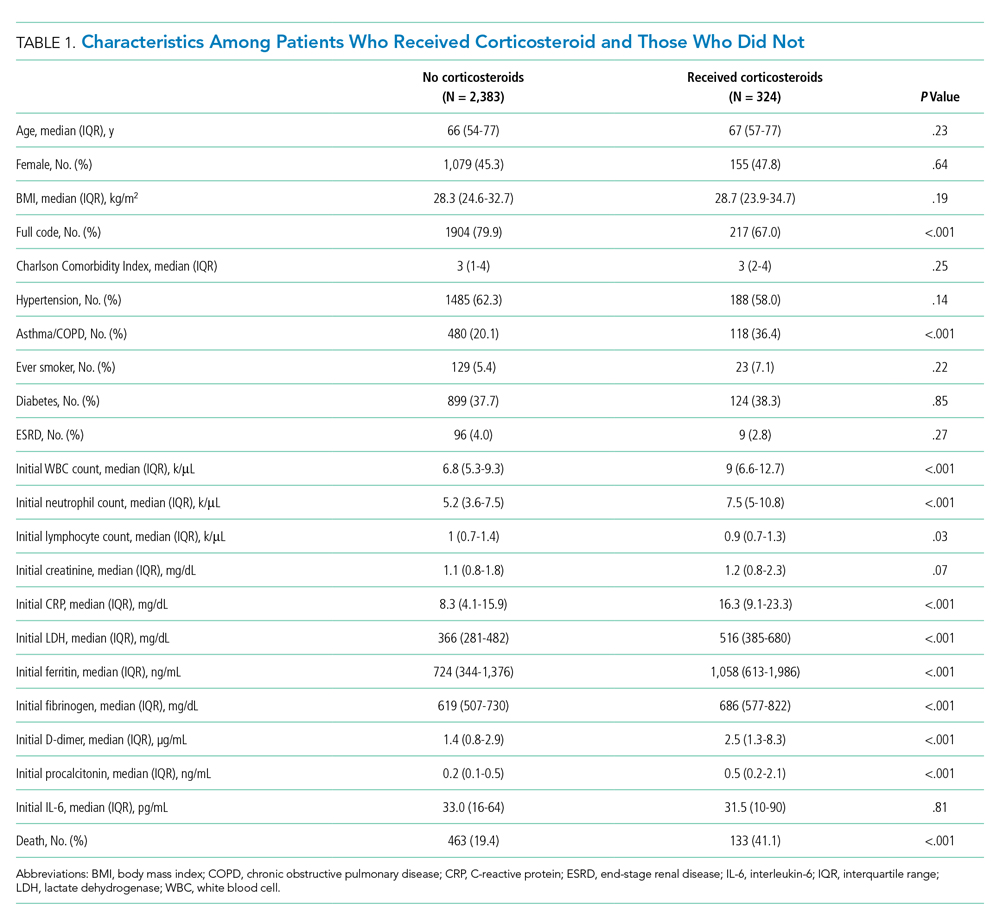
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
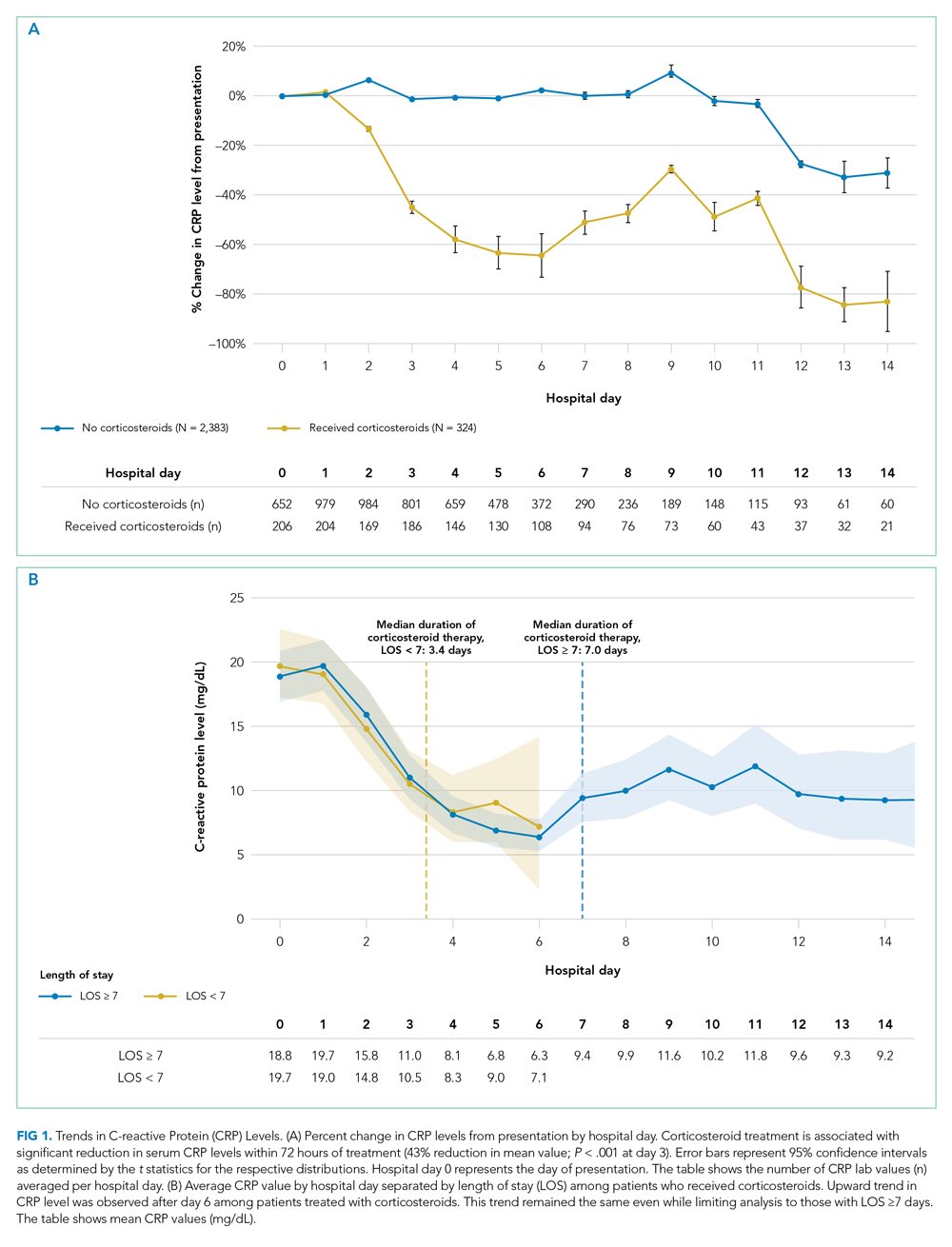
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.
CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.
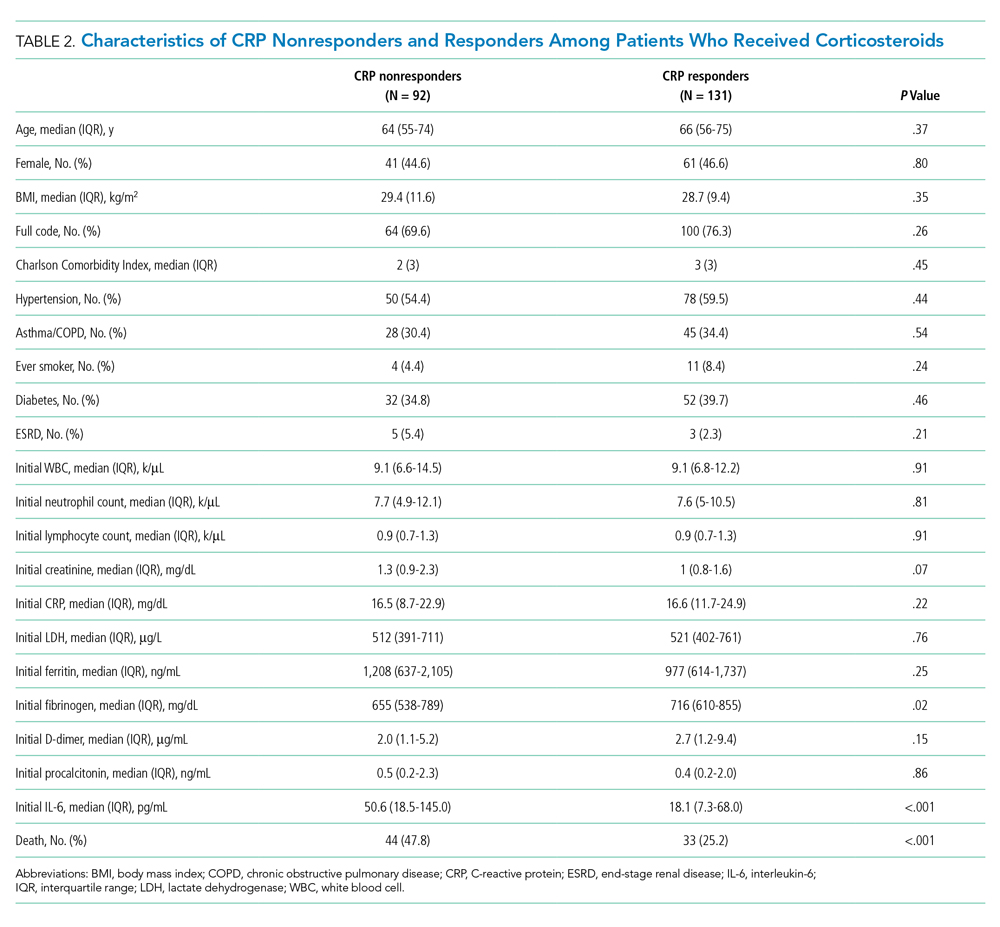
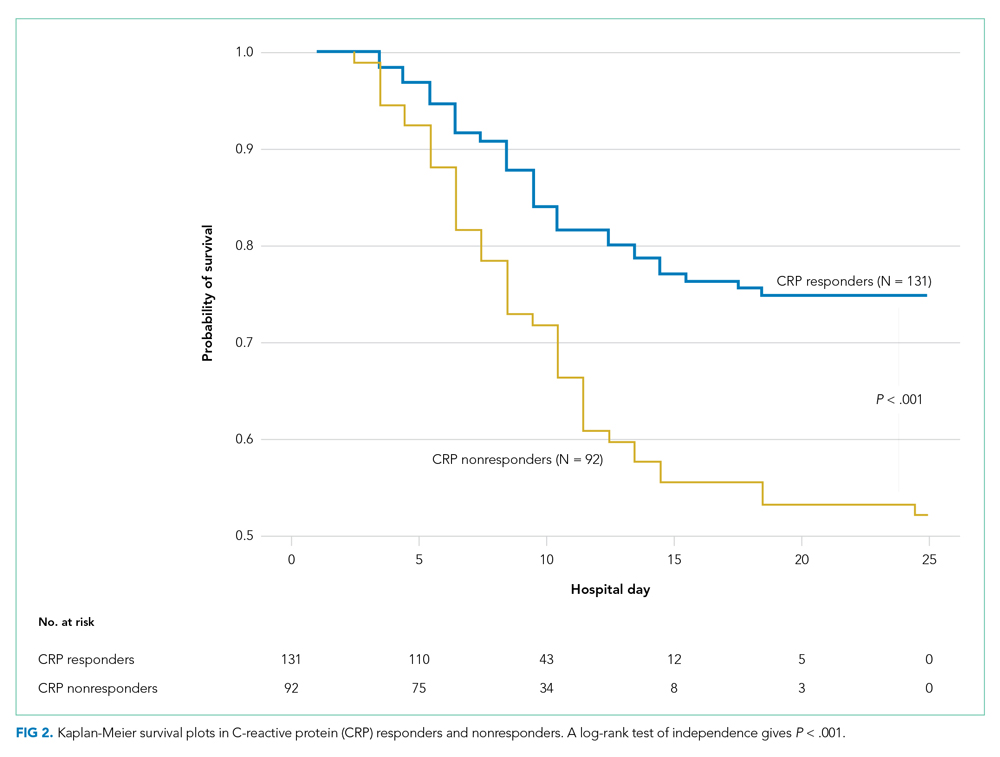
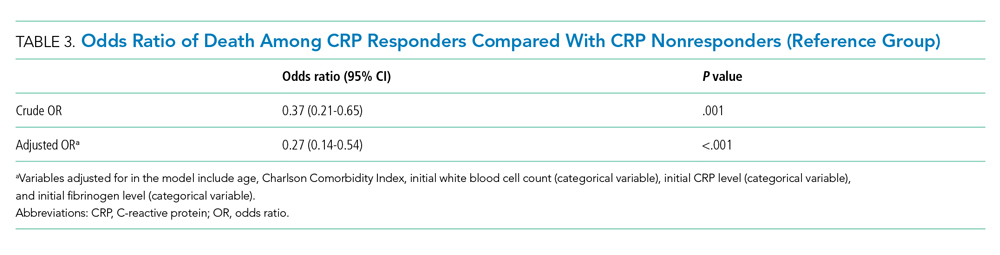
The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.
To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.
DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.

After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.

CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.

The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.

To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.

DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
Confirmed cases of coronavirus disease 2019 (COVID-19) exceed 111 million, and the disease is responsible for approximately 2.4 million deaths worldwide.1 In the United States, 28 million cases of COVID-19 have been reported, and the disease has caused more than 497,000 deaths.2 The clinical presentation of COVID-19 varies widely, with the most severe presentation characterized by acute respiratory distress syndrome and a marked systemic inflammatory response. Corticosteroids have emerged as a potential therapeutic option in a subset of patients. Results from the recently published RECOVERY trial suggest a substantial mortality benefit of dexamethasone in patients who require mechanical ventilation, with a risk reduction of approximately 33%.3 In addition, a recent large retrospective study demonstrated a reduction in the risk of mechanical ventilation or mortality with corticosteroids in a prespecified subset of patients with C-reactive protein (CRP) ≥20 mg/dL, which indicates a high burden of inflammation.4
Some patients with severe COVID-19 experience a positive feedback cascade of proinflammatory cytokines, called the cytokine storm, which can worsen lung injury and, in some cases, progress to vasodilatory shock and multiorgan failure.5 This complication’s cytokine cascade includes interleukin (IL) 6, IL-1β, and CC chemokine ligand 3 (CCL3), which are released by airway macrophages and all of which are heavily implicated in the maladaptive forms of immune response to COVID-19.6,7 The cytokine IL-6 is the primary signal for the production of CRP, and corticosteroids have been shown, both in vitro and in vivo, to reduce the production of IL-6 and other cytokines by airway macrophages.6 Levels of CRP have been shown to correlate with outcomes in COVID-19 and bacterial pneumonias.7,8 Reduction in CRP levels following the institution of therapy, known as CRP response, has been shown to predict outcomes in other inflammatory conditions, such as osteomyelitis, hidradenitis suppurativa, and some cases of bacterial pneumonia.8-10 Similar CRP response in hemophagocytic lymphohistiocytosis, an entity which closely resembles cytokine storm syndrome, has been shown to correlate with disease activity in patients following treatment with an IL-1 antagonist.11 Whether the CRP response as a response to therapeutics in COVID-19 is associated with improved outcomes remains unknown.
Laboratory measurement of CRP levels offers several advantages over the measurement of interleukins. Notably, the half-life of CRP is approximately 19 hours, which is comparable across different age groups and inflammatory conditions because its concentration depends primarily on synthesis in the liver, and a decreased level suggests decreased stimulus for synthesis.8 This makes CRP a useful biomarker to assess response to therapy, in contrast to interleukins, which have short half-lives, are variable in heterogeneous populations, and can be difficult to measure. In addition, CRP measurement is rapid and relatively inexpensive.
We hypothesized that reduction in CRP levels by 50% or more within 72 hours after the initiation of corticosteroids in patients with COVID-19 is associated with reduced inpatient mortality and may be an early indicator of therapeutic response.
METHODS
Study Participants
In this retrospective cohort study, we reviewed all adult patients admitted to Montefiore Medical Center (Bronx, New York) for COVID-19 between March 10, 2020, and May 2, 2020. Patients must have been discharged (alive or deceased) by the administrative censor date (May 2, 2020) to be included. Patients who died within the first 48 hours of admission were excluded to allow sufficient time for corticosteroid treatment to take effect. For inclusion in the corticosteroid group, patients needed to have received at least 2 consecutive days of corticosteroid treatment beginning within the first 48 hours of admission with a total daily dose of 0.5 mg/kg prednisone equivalent or greater. Patients who received treatment-dose corticosteroids later in the hospital course were excluded (Appendix Figure).
Comparison Group and Outcome
We examined trends in CRP levels for patients who received corticosteroids vs trends among patients who did not receive corticosteroids. In addition, among patients who were treated with corticosteroids, we compared the inpatient mortality of those who did have a reduction in CRP level after treatment with inpatient mortality of those who did not have a reduction in CRP level after treatment. First, CRP level trends over time were examined in all patients, and compared between those who received corticosteroid treatment and those who did not. Then, patients who received corticosteroids were categorized based on changes in CRP levels after beginning corticosteroids. The first CRP level obtained during the first 48 hours of admission was used as the initial CRP level. For each patient, the last CRP level within the 72 hours after initiation of treatment was used to calculate the change in CRP level from admission. A patient was considered to be a “CRP responder” if their CRP level decreased by 50% or more within 72 hours after treatment and a “CRP nonresponder” if their CRP level did not drop by at least 50% within 72 hours of treatment. Patients who did not have a CRP level within the initial 48 hours of admission or a subsequent CRP measured in the 72 hours after treatment were considered to have an “undetermined CRP response” and excluded from the mortality analysis.
We observed a rise in CRP starting around day 6 among patients treated with corticosteroids and performed a post hoc analysis to determine if this was due to a selection effect whereby patients staying in the hospital longer had higher CRP levels or represented actual rise. In order to address this, we performed a stratified analysis comparing the trends in CRP levels among patients with a length of stay (LOS) of 7 or more days with trends among those with an LOS less than 7 days.
Statistical Analysis
To characterize differences in patients who received corticosteroids and those who did not, we examined their demographic, clinical characteristics, and admission laboratory values, using chi-square test for categorical variables and Kruskal-Wallis test for continuous variables (Table 1). The change in CRP levels from day 0 (presentation to the hospital) in both groups was plotted in a time-series analysis. For each day in the time series, the 95% CIs for the changes in CRP were computed using the t statistic for the corresponding distribution. The Kruskal-Wallis test was used to assess the significance of differences between groups at 72 hours after initiation of treatment.

After categorizing patients by CRP response, we compared demographic, clinical, and laboratory characteristics of patients who were CRP responsive with those of patients who were not, using the same tests of statistical inference mentioned above. To compare time to inpatient mortality differences between CRP response groups, Kaplan-Meier survival curves were generated and statistical significance determined via log-rank test. Univariable logistic regression was used to estimate the odds ratio of inpatient mortality between comparison groups in an unadjusted analysis. Last, to examine the independent association between CRP response and mortality, we constructed a multivariate model that included variables that were significantly associated with mortality in univariable analysis and considered to be important potential confounders by the authors. Details on variable selection for the model are listed in Appendix Table 1.
Data Collection
Data were directly extracted from our center’s electronic health record system. Data processing and recoding was performed using the Python programming language (version 2.7.17) and data analysis was done using Stata 12 (StataCorp LLC; 2011). This study was approved by the institutional review board of the Albert Einstein College of Medicine.
RESULTS
Corticosteroids vs No Corticosteroids
Between March 10, 2020, and May 2, 2020, a total of 3,382 adult patients were admitted for COVID-19 at Montefiore Medical Center. Of these, 2,707 patients met the study inclusion criteria, and 324 of those received corticosteroid treatment. Their demographic characteristics, comorbidities, and admission lab values are shown in Table 1. Patients who received corticosteroids were older, had higher comorbidity scores, were more likely to have asthma or chronic obstructive pulmonary disease, and were less likely to be full code status, compared with patients who did not receive corticosteroids. Patients who received corticosteroids also had higher initial white blood cell (WBC) and neutrophil counts but lower lymphocyte count. The two groups were comparable in initial creatinine level. Additional patient characteristics and addmission lab values are shown in Appendix Table 2.
Average change in CRP levels by hospital day for those who received corticosteroids and those who did not are shown in Figure 1A. Among patients who received corticosteroid treatment, there was a significant decrease in CRP level at 72 hours of treatment (P < .001). In the post hoc analysis of trends in CRP levels, we found that CRP levels among those treated with corticosteroids started to rise around day 6 after the initial drop. This trend was observed even after removing patients with shorter LOS (<7 days) (Figure 1B). The median durations of corticosteroid therapy were 3 days among patients whose LOS was less than 7 days and 6 days among those whose LOS was 7 days or greater. The rise in CRP level was seen at day 5 and day 7 within each group, respectively. Crude death rate was 41.7% among patients with LOS of less than 7 days and 40.6% in those with LOS of 7 days or greater.

CRP Responders vs Nonresponders
Among the 324 patients who received corticosteroids, 131 (40.4%) were classified as responders, 92 (28.4%) were classified as nonresponders, and 101 (31.2%) were undetermined. Characteristics of CRP responders and CRP nonresponders are shown in Table 2 and Appendix Table 3. CRP responders were more likely to have dementia, higher median admission platelet count, and fibrinogen level compared with CRP nonresponders. Patients whose CRP response was undetermined were excluded from the analysis. Their characteristics are shown in Appendix Table 4.

The observed inpatient mortality rate was 25.2% among CRP responders and 47.8% among CRP nonresponders. This was also demonstrated in the Kaplan-Meier survival curve (Figure 2). The odds of inpatient mortality among CRP responders was strongly and significantly reduced compared with those among nonresponders in an unadjusted analysis (odds ratio [OR], 0.37; 95% CI, 0.21-0.65; P = .001) and after adjustment for demographic and clinical characteristics including age, Charlson Comorbidity Index, initial WBC count, initial CRP level, and initial fibrinogen level (OR, 0.27; 95% CI, 0.14-0.54; P < .001). Details on how variables were operationalized and information on missing data are included in Appendix Table 1.

To explore whether this observed effect differed depending on severity of the respiratory illness, we examined the association between CRP response and mortality in subgroups stratified by intubation status. Within our cohort of 223 patients (92 CRP responders and 131 CRP nonresponders), 166 patients were never intubated, 50 patients were intubated in the first 48 hours, and 7 patients were intubated later on during the admission. The odds ratios for death among CRP responders vs nonresponders were 0.50 (P = .07) among patients never intubated and 0.46 (P = .2) among patients intubated within the initial 48 hours of admission.

DISCUSSION
In this retrospective study, we found that, on average, patients treated with corticosteroids had a swift and marked reduction in serum CRP. In addition, among patients treated with corticosteroids, those whose CRP was reduced by 50% or more within 72 hours after treatment had a dramatically reduced risk of inpatient mortality compared with the risk among nonresponders. This study contributes to a growing body of evidence that suggests that corticosteroids may be an efficacious treatment to reduce adverse events in patients with COVID-19 who have evidence of high levels of inflammation as measured by CRP level.3,4,12,13
It remains unclear whether CRP is simply a biomarker of disease activity or if it plays a role in mediating inflammation. While CRP is commonly understood to be an acute phase reactant, it has been suggested that, after undergoing proteolysis, it functions as a chemoattractant for monocytes.14 In addition, it is now known that the inflammatory CD14+/CD16+ monocytes that express high levels of IL-6 are key drivers of the cytokine storm in COVID-19.15 Therefore, it may be possible that the high levels of circulating CRP in patients with cytokine storm recruits monocytes to the lungs, which leads to further lung injury.
Other mechanisms of immune dysregulation that may contribute to lung injury and respiratory failure in COVID-19, such as cytokine-induced T-cell suppression, have been proposed.7,16 The related markers, such as levels of T-cells or specific cytokines, may therefore represent different but related underlying immune mechanisms affecting the clinical course of COVID-19 that may respond to different therapeutic modalities such as direct IL-6 blockade or chemokine receptor blockade, among others that are currently under investigation.17,18
Regardless of the underlying mechanism of immune regulation, our study shows that serial measurement of CRP may serve as an early indicator of response to corticosteroids that correlates with decreased mortality. The association between CRP response and reduced risk of mortality was present in both subgroups, those requiring mechanical ventilation and those who did not. The risk reduction was similar in magnitude to the overall effect but was not statistically significant in either group. Interestingly, our time series analysis demonstrated a rise in CRP around day 6 among patients treated with corticosteroids (notably, most patients were treated for 5 to 7 days). Our post hoc analysis suggests that this may represent a “rebound” in inflammation after discontinuation of corticosteroids. However, the clinical significance of this rebound and whether a longer course of steroids would improve outcomes is not known. Because corticosteroid therapy may be associated with adverse effects in some patients,4 it is possible that CRP nonresponders represent a subset of patients in whom corticosteroids are not effective and for whom alternative therapies should be considered. In one study looking at the usefulness of IL-1 inhibition for severe COVID-19 infection, patients who received IL-1 inhibitor therapy had improved mortality and a significant decrease in CRP concentration as compared with the historical group.19 Finally, it is worth noting that, in one large retrospective study, there was harm associated with corticosteroid therapy in patients with low levels of CRP, and in the RECOVERY trial there was a trend toward harm for patients with no oxygen requirement.3,4 Serial measurement of CRP may further identify the subset of patients in whom corticosteroid therapy might be harmful.
This study has several limitations. First, the retrospective nature of this study is inherently prone to selection bias, and despite the large number of clinical variables accounted for, unmeasured confounders may still exist. This study was also conducted at a single clinical center operating under emergency circumstances at a time during which healthcare resources were limited. Overall in-hospital mortality was high but similar to mortality rates reported at other hospitals in the New York City area during the same months.20 The strengths of this study include a large cohort of COVID-19 patients from New York City, an epicenter of COVID-19, who received corticosteroids.
CONCLUSION
We found that therapy with corticosteroids in patients with COVID-19 is associated with a substantial reduction in CRP levels within 72 hours of therapy, and for those patients in whom CRP levels decrease by 50% or more, there is a significantly lower risk of inpatient mortality. Future studies are needed to validate these findings in other cohorts and to determine if markers other than CRP levels may be predictors of a therapeutic response or if CRP nonresponders would benefit from other targeted therapies.
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
1. WHO coronavirus disease (COVID-19) dashboard. World Health Organization. Updated February 22, 2021. Accessed February 22, 2021. https://covid19.who.int/
2. COVID Data Tracker: United States COVID-19 Cases and Deaths by State. Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed February 22, 2021. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
3. Horby P, Lim WS, Emberson JR, et al; RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. Published online July 17, 2020. https://doi.org/10.1056/NEJMoa2021436
4. Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8);489-493. https://doi.org/10.12788/jhm.3497
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363-374. https://doi.org/10.1038/s41577-020-0311-8
6. Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122(3):550-559.e3. https://doi.org/10.1016/j.jaci.2008.07.007
7. Giamarellos-Bourboulis EJ, Netea MG, Rovina N. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27(6):992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009
8. Luna CM. C-reactive protein in pneumonia: let me try again. Chest. 2004;125(4):1192-1195. https://doi.org/10.1378/chest.125.4.1192
9. Montaudié H, Seitz-Polski B, Cornille A, Benzaken S, Lacour JP, Passeron T. Interleukin 6 and high-sensitivity C-reactive protein are potential predictive markers of response to infliximab in hidradenitis suppurativa. J Am Acad Dermatol. 2017;76(1):156-158. https://doi.org/10.1016/j.jaad.2016.08.036
10. Menéndez R, Martínez R, Reyes S, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64(7):587-591. https://doi.org/10.1136/thx.2008.105312
11. Rajasekaran S, Kruse K, Kovey K, et al. Therapeutic role of anakinra, an interleukin-1 receptor antagonist, in the management of secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction/macrophage activating syndrome in critically ill children. Pediatr Crit Care Med. 2014;15(5):401-408. https://doi.org/10.1097/pcc.0000000000000078
12. Wang Y, Jiang W, He Q, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5(1):57. https://doi.org/10.1038/s41392-020-0158-2
13. Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. Published online May 19, 2020. https://doi.org/10.1093/cid/ciaa601
14. Robey FA, Ohura K, Futaki S, et al. Proteolysis of human c-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987;262(15):7053-7057.
15. Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. Published online March 13, 2020. https://doi.org/10.1093/nsr/nwaa041
16. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583(7816):437-440. https://doi/10.1038/s41586-020-2355-0(2020).
17. Tocilizumab in COVID-19 Pneumonia (TOCIVID-19). ClinicalTrials.gov identifier: NCT04317092. Updated October 22, 2020. Accessed October 22, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092
18. Study to Evaluate the Efficacy and Safety of Leronlimab for Patients With Severe or Critical Coronavirus Disease 2019 (COVID-19). ClinicalTrials.gov identifier: NCT04347239. Updated October 19, 2020. Accessed November 16, 2020.https://www.clinicaltrials.gov/ct2/show/NCT04347239
19. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393-e400. https://doi.org/10.1016/s2665-9913(20)30164-8
20. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. https://doi.org/10.1001/jama.2020.6775
© 2021 Society of Hospital Medicine
When Is Testing for Thrombophilia Indicated?
The Case
A healthy 42-year-old woman presents to the hospital with acute-onset pleuritic chest pain and shortness of breath. She has not had any recent surgeries, takes no medications, and is very active. A lung ventilation-perfusion scan reveals a high probability of pulmonary embolism (PE). The patient’s history is notable for two second-trimester pregnancy losses. The patient is started on low-molecular heparin and warfarin (LMHW).
Should this patient be tested for thrombophilia?
Background
Thrombophilia can now be identified in more than half of all patients presenting with VTE, and testing for underlying causes of thrombophilia has become widespread.1 Physicians believe that thrombophilia testing frequently changes management of patients with VTE.2
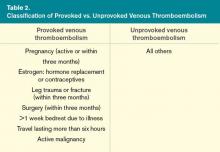
Thrombophilias can be classified into three major categories: deficiency of natural inhibitors of coagulation, abnormal function or elevated level of coagulation factors, and acquired thrombophilias (see Table 1).
The prevalence of specific thrombophilias varies widely. For example, the prevalence of activated protein C resistance (the factor V Leiden mutation) is 3% to 7%. In comparison, the prevalence of antithrombin deficiency is estimated at 0.02%. Each thrombophilia is associated with an increased VTE risk, but the level of risk associated with a given thrombophilia varies greatly.1
Before testing for thrombophilia in acute VTE, assess the risk of recurrent VTE by determining if the thrombosis was provoked or unprovoked. A VTE event is considered provoked if it occurs in the setting of pregnancy within the previous three months; estrogen therapy; immobility from acute illness for more than one week; travel lasting for more than six hours; leg trauma, fracture, or surgery within the previous three months; or active malignancy (see Table 2,).3 Unprovoked VTE has a recurrence rate of 7.4% per patient year, compared with 3.3% per patient year for a provoked VTE; the risk is even lower (0.7% per patient year) if the risk factor for the provoked VTE was surgical.4
Testing for thrombophilia is indicated if the results would add significant prognostic information beyond the clinical history, or if it would change patient management—in particular, the intensity or the duration of anticoagulation.
Review of the Data
Does presence of thrombophilia alter the intensity of anticoagulation for VTE?
If thrombophilia increases the risk of VTE recurrence while on anticoagulation, then a more intense level of anticoagulation might prevent future VTE. There are no studies investigating higher intensity of anticoagulation, but if standard anticoagulation were insufficient for patients with identifiable thrombophilia, one might expect to observe increased recurrence rates among patients with thrombophilia treated with standard warfarin therapy.
In a substudy of the Extended Low-Intensity Anticoagulation for Unprovoked Venous ThromboEmbolism (ELATE) trial, the risk of recurrence of VTE among treated subjects was very low overall, and the presence of thrombophilic abnormalities was not associated with significantly higher risk.5 Observational studies have found VTE recurrence rates are low in patients treated with warfarin, with or without thrombophilia.6-8
The impact of the initial level of anticoagulation on recurrence after the completion of the treatment period has been evaluated. Although one study suggested that patients with substandard levels of anticoagulation were at an increased risk of subsequent VTE, this was not confirmed in the Leiden Thrombophilia Study (LETS). 9,10
In sum, the majority of data do not suggest a significantly increased risk of recurrent VTE in patients with thrombophilia treated with standard anticoagulation. Therefore, treatment with warfarin to a goal INR of 2 to 3 is sufficient.
Does presence of thrombophilia alter duration of VTE treatment?
A major decision clinicians face when caring for VTE patients is the duration of anticoagulation treatment. The current ACCP recommendation for treatment of a provoked VTE is three months, with treatment for an unprovoked VTE three months or lifelong.11 If the presence of thrombophilia increases the risk of recurrence after cessation of anticoagulation treatment, longer duration of treatment might be indicated. One of the goals of thrombophilia testing should be to identify those patients.
Overall, the recurrence rate after first VTE is high, with a cumulative incidence of 25% at five years, 30% at eight years, and 56% at 20 years.12,13
Deficiency of natural inhibitors of coagulation.
Deficiency of a natural inhibitor of coagulation has been associated with a risk of recurrence of VTE of as much as 10% per year, according to some studies.6,14 However, the estimates are based on studies that include individuals from thrombosis-prone families, and selection bias might have contributed to the high recurrence rates.1 In the unselected population represented in the LETS study, only a modest elevation was seen in the estimated risk of recurrence for patients with inhibitor deficiencies.15
Testing for deficiency of inhibitors offers little prognostic information beyond that obtained when determining whether a VTE event is provoked or unprovoked. In studies that have separately examined subjects with provoked vs. unprovoked VTE, deficiency of an inhibitor is not associated with increased risk of recurrence.15,16
Abnormal function or level of anticoagulation factors.
Factor V Leiden (FVL) is the most common cause of inherited thrombophilia and is associated with as much as a sixfold increase in VTE risk, while the prothrombin gene mutation is associated with a twofold increase.17,18
In contrast, the evidence associating these mutations with recurrent VTE risk is not as consistent. Although a study conducted at a referral center in Italy found an increased risk of recurrence with either Factor V Leiden or prothrombin gene mutation, a large meta-analysis of 23 studies found increased risk only with Factor V Leiden.19,20 Another meta-analysis demonstrated only a modest increased risk of recurrence in subjects with Factor V Leiden or prothrombin gene mutation, and a prospective study from Austria found no increased risk of recurrence with Factor V Leiden two years after discontinuation of anticoagulation.18,21 Additionally, when using patients with unprovoked VTE as reference, there was no increased risk of recurrence among patients homozygous for Factor V Leiden or the prothrombin gene mutation.22
In summary, although Factor V Leiden and prothrombin gene defects are associated with increased risk of recurrent VTE, the magnitude of the risk increase is modest and, therefore, should not alter duration of therapy.
Acquired thrombophilia.
It appears that the only thrombophilic state that might have a significant impact on the risk of recurrence is the antiphospholipid syndrome. The cessation of warfarin therapy in patients with thrombosis associated with antiphospholipid antibodies carries a 69% risk of recurrent thrombosis within a year.23 Some studies have suggested that the presence of specific antibodies (i.e. anticardiolipin antibodies) is associated with increased risk in patients with antiphospholipid syndrome.24
However, at present, all patients with VTE and antiphospholipid syndrome should be candidates for lifelong anticoagulation. Antiphospholipid antibody testing should be performed in patients with a suggestive history, including those with recurrent fetal loss or a single fetal loss after 10 weeks, or known collagen vascular disease.25
The role of provoked vs. unprovoked VTE.
Identifying whether a VTE is provoked or unprovoked has been shown to be an important predictor of recurrence. For example, one prospective, cohort study found two-year recurrence rates of zero in patients with a surgery or pregnancy-related VTE, 9% with other provoked VTE, and 19% with unprovoked VTE.26 In the same study, thrombophilia testing failed to reliably predict recurrence risk. Patients with unprovoked VTE who were tested and found to not have a defect were at equally high risk of recurrent VTE as those found to have a thrombophilia.27
The most significant predictor for VTE recurrence is whether the first event was provoked, and thrombophilia testing offers little additional prognostic information.28
VTE as a multifactorial disorder.
It is becoming increasingly clear that VTE is multifactorial disorder, caused by the interactions of genotypic, phenotypic, and environmental factors. In the case of an unprovoked VTE, the patient already carries a significantly elevated risk for recurrence, and further testing for known causes of thrombophilia appears to add very little additional information. The optimal duration of anticoagulation for unprovoked VTE is unclear, but current guidelines suggest at least three months—and clinicians should consider lifelong treatment.
In the vast majority of cases, testing for thrombophilia has no impact on the management of VTE and is not warranted. In patients with antiphospholipid-antibody syndrome, given the high risk of recurrence, long-term anticoagulation after a first VTE might be indicated. In select patients with a clinical picture suggestive of antiphospholipid-antibody syndrome, or a strong family history, testing should be considered.
Back to the Case
Our patient appears to have an unprovoked VTE. She should receive regular anticoagulation with warfarin, with a goal INR of 2 to 3, for at least three months. Lifelong anticoagulation therapy should be considered. Testing for heritable thrombophilia will not change the current management or treatment duration and, hence, is not indicated. However, the patient’s history is suggestive of antiphospholipid-antibody syndrome, so she should be tested. If the diagnosis of antiphospholipid syndrome is made, lifelong anticoagulation should be considered.
Bottom Line
Unprovoked VTE provides the strongest predictor for recurrence. Thrombophilia testing adds little in predicting recurrence and rarely is indicated.
Dr. Stehlikova is a clinical hospitalist in the division of hospital medicine, department of medicine, at Albert Einstein College of Medicine and Montefiore Medical Center in Bronx, N.Y. Dr. Martin is director of the Einstein Hospitalist Service. Dr. Janakiram is a fellow in the department of hematology at Einstein, and Dr. Korcak is an instructor at Einstein in the department of medicine and director of the Weiler Medical Service. Dr. Galhotra is associate director for inpatient quality in the department of medicine at Einstein; Dr. Averbukh is an academic hospitalist; and Dr. Southern is chief of the division of hospital medicine at Einstein.
References
- Middeldorp S, van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. 2008;143:321-335.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5:1979-1981.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199-205.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170:1710-1716.
- Kearon C, Julian JA, Kovacs MJ, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112:4432-4436.
- Vossen CY, Walker ID, Svensson P, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol. 2005;25:1992-1997.
- Brown K, Luddington R, Williamson D, Baker P, Baglin T. Risk of venous thromboembolism associated with a G to A transition at position 20210 in the 3'-untranslated region of the prothrombin gene. Br J Haematol. 1997;98:907-909.
- Schulman S, Tengborn L. Treatment of venous thromboembolism in patients with congenital deficiency of antithrombin III. Thromb Haemost. 1992;68:634-636.
- Palareti G, Legnani C, Cosmi B, Guazzaloca G, Cini M, Mattarozzi S. Poor anticoagulation quality in the first 3 months after unprovoked venous thromboembolism is a risk factor for long-term recurrence. J Thromb Haemost. 2005;3:955-961.
- Gadisseur AP, Christiansen SC, van der Meer FJ, Rosendaal FR. The quality of oral anticoagulant therapy and recurrent venous thrombotic events in the Leiden Thrombophilia Study. J Thromb Haemost. 2007;5:931-936.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:454S-545S.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
- Laczkovics C, Grafenhofer H, Kaider A, et al. Risk of recurrence after a first venous thromboembolic event in young women. Haematologica. 2007;92:1201-1207.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93-99.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352-2361.
- De Stefano V, Simioni P, Rossi E, et al. The risk of recurrent venous thromboembolism in patients with inherited deficiency of natural anticoagulants antithrombin, protein C and protein S. Haematologica. 2006;91:695-698.
- Price DT, Ridker PM. Factor V Leiden mutation and the risks for thromboembolic disease: a clinical perspective. Ann Intern Med. 1997;127:895-903.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166:729-736.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood. 2000;96:3329-3333.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301:2472-2485.
- Eichinger S, Pabinger I, Stumpflen A, et al. The risk of recurrent venous thromboembolism in patients with and without factor V Leiden. Thromb Haemost. 1997;77:624-628.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation. 2010;121(15):1706-1712.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104:332-338.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737-1740.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362:523-526.
- Rosendaal FR. Once and only once. Circulation. 2010;121:1688-1690.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121:458-463.
The Case
A healthy 42-year-old woman presents to the hospital with acute-onset pleuritic chest pain and shortness of breath. She has not had any recent surgeries, takes no medications, and is very active. A lung ventilation-perfusion scan reveals a high probability of pulmonary embolism (PE). The patient’s history is notable for two second-trimester pregnancy losses. The patient is started on low-molecular heparin and warfarin (LMHW).
Should this patient be tested for thrombophilia?
Background
Thrombophilia can now be identified in more than half of all patients presenting with VTE, and testing for underlying causes of thrombophilia has become widespread.1 Physicians believe that thrombophilia testing frequently changes management of patients with VTE.2
Thrombophilias can be classified into three major categories: deficiency of natural inhibitors of coagulation, abnormal function or elevated level of coagulation factors, and acquired thrombophilias (see Table 1).
The prevalence of specific thrombophilias varies widely. For example, the prevalence of activated protein C resistance (the factor V Leiden mutation) is 3% to 7%. In comparison, the prevalence of antithrombin deficiency is estimated at 0.02%. Each thrombophilia is associated with an increased VTE risk, but the level of risk associated with a given thrombophilia varies greatly.1
Before testing for thrombophilia in acute VTE, assess the risk of recurrent VTE by determining if the thrombosis was provoked or unprovoked. A VTE event is considered provoked if it occurs in the setting of pregnancy within the previous three months; estrogen therapy; immobility from acute illness for more than one week; travel lasting for more than six hours; leg trauma, fracture, or surgery within the previous three months; or active malignancy (see Table 2,).3 Unprovoked VTE has a recurrence rate of 7.4% per patient year, compared with 3.3% per patient year for a provoked VTE; the risk is even lower (0.7% per patient year) if the risk factor for the provoked VTE was surgical.4
Testing for thrombophilia is indicated if the results would add significant prognostic information beyond the clinical history, or if it would change patient management—in particular, the intensity or the duration of anticoagulation.
Review of the Data
Does presence of thrombophilia alter the intensity of anticoagulation for VTE?
If thrombophilia increases the risk of VTE recurrence while on anticoagulation, then a more intense level of anticoagulation might prevent future VTE. There are no studies investigating higher intensity of anticoagulation, but if standard anticoagulation were insufficient for patients with identifiable thrombophilia, one might expect to observe increased recurrence rates among patients with thrombophilia treated with standard warfarin therapy.
In a substudy of the Extended Low-Intensity Anticoagulation for Unprovoked Venous ThromboEmbolism (ELATE) trial, the risk of recurrence of VTE among treated subjects was very low overall, and the presence of thrombophilic abnormalities was not associated with significantly higher risk.5 Observational studies have found VTE recurrence rates are low in patients treated with warfarin, with or without thrombophilia.6-8
The impact of the initial level of anticoagulation on recurrence after the completion of the treatment period has been evaluated. Although one study suggested that patients with substandard levels of anticoagulation were at an increased risk of subsequent VTE, this was not confirmed in the Leiden Thrombophilia Study (LETS). 9,10
In sum, the majority of data do not suggest a significantly increased risk of recurrent VTE in patients with thrombophilia treated with standard anticoagulation. Therefore, treatment with warfarin to a goal INR of 2 to 3 is sufficient.
Does presence of thrombophilia alter duration of VTE treatment?
A major decision clinicians face when caring for VTE patients is the duration of anticoagulation treatment. The current ACCP recommendation for treatment of a provoked VTE is three months, with treatment for an unprovoked VTE three months or lifelong.11 If the presence of thrombophilia increases the risk of recurrence after cessation of anticoagulation treatment, longer duration of treatment might be indicated. One of the goals of thrombophilia testing should be to identify those patients.
Overall, the recurrence rate after first VTE is high, with a cumulative incidence of 25% at five years, 30% at eight years, and 56% at 20 years.12,13
Deficiency of natural inhibitors of coagulation.
Deficiency of a natural inhibitor of coagulation has been associated with a risk of recurrence of VTE of as much as 10% per year, according to some studies.6,14 However, the estimates are based on studies that include individuals from thrombosis-prone families, and selection bias might have contributed to the high recurrence rates.1 In the unselected population represented in the LETS study, only a modest elevation was seen in the estimated risk of recurrence for patients with inhibitor deficiencies.15
Testing for deficiency of inhibitors offers little prognostic information beyond that obtained when determining whether a VTE event is provoked or unprovoked. In studies that have separately examined subjects with provoked vs. unprovoked VTE, deficiency of an inhibitor is not associated with increased risk of recurrence.15,16
Abnormal function or level of anticoagulation factors.
Factor V Leiden (FVL) is the most common cause of inherited thrombophilia and is associated with as much as a sixfold increase in VTE risk, while the prothrombin gene mutation is associated with a twofold increase.17,18
In contrast, the evidence associating these mutations with recurrent VTE risk is not as consistent. Although a study conducted at a referral center in Italy found an increased risk of recurrence with either Factor V Leiden or prothrombin gene mutation, a large meta-analysis of 23 studies found increased risk only with Factor V Leiden.19,20 Another meta-analysis demonstrated only a modest increased risk of recurrence in subjects with Factor V Leiden or prothrombin gene mutation, and a prospective study from Austria found no increased risk of recurrence with Factor V Leiden two years after discontinuation of anticoagulation.18,21 Additionally, when using patients with unprovoked VTE as reference, there was no increased risk of recurrence among patients homozygous for Factor V Leiden or the prothrombin gene mutation.22
In summary, although Factor V Leiden and prothrombin gene defects are associated with increased risk of recurrent VTE, the magnitude of the risk increase is modest and, therefore, should not alter duration of therapy.
Acquired thrombophilia.
It appears that the only thrombophilic state that might have a significant impact on the risk of recurrence is the antiphospholipid syndrome. The cessation of warfarin therapy in patients with thrombosis associated with antiphospholipid antibodies carries a 69% risk of recurrent thrombosis within a year.23 Some studies have suggested that the presence of specific antibodies (i.e. anticardiolipin antibodies) is associated with increased risk in patients with antiphospholipid syndrome.24
However, at present, all patients with VTE and antiphospholipid syndrome should be candidates for lifelong anticoagulation. Antiphospholipid antibody testing should be performed in patients with a suggestive history, including those with recurrent fetal loss or a single fetal loss after 10 weeks, or known collagen vascular disease.25
The role of provoked vs. unprovoked VTE.
Identifying whether a VTE is provoked or unprovoked has been shown to be an important predictor of recurrence. For example, one prospective, cohort study found two-year recurrence rates of zero in patients with a surgery or pregnancy-related VTE, 9% with other provoked VTE, and 19% with unprovoked VTE.26 In the same study, thrombophilia testing failed to reliably predict recurrence risk. Patients with unprovoked VTE who were tested and found to not have a defect were at equally high risk of recurrent VTE as those found to have a thrombophilia.27
The most significant predictor for VTE recurrence is whether the first event was provoked, and thrombophilia testing offers little additional prognostic information.28
VTE as a multifactorial disorder.
It is becoming increasingly clear that VTE is multifactorial disorder, caused by the interactions of genotypic, phenotypic, and environmental factors. In the case of an unprovoked VTE, the patient already carries a significantly elevated risk for recurrence, and further testing for known causes of thrombophilia appears to add very little additional information. The optimal duration of anticoagulation for unprovoked VTE is unclear, but current guidelines suggest at least three months—and clinicians should consider lifelong treatment.
In the vast majority of cases, testing for thrombophilia has no impact on the management of VTE and is not warranted. In patients with antiphospholipid-antibody syndrome, given the high risk of recurrence, long-term anticoagulation after a first VTE might be indicated. In select patients with a clinical picture suggestive of antiphospholipid-antibody syndrome, or a strong family history, testing should be considered.
Back to the Case
Our patient appears to have an unprovoked VTE. She should receive regular anticoagulation with warfarin, with a goal INR of 2 to 3, for at least three months. Lifelong anticoagulation therapy should be considered. Testing for heritable thrombophilia will not change the current management or treatment duration and, hence, is not indicated. However, the patient’s history is suggestive of antiphospholipid-antibody syndrome, so she should be tested. If the diagnosis of antiphospholipid syndrome is made, lifelong anticoagulation should be considered.
Bottom Line
Unprovoked VTE provides the strongest predictor for recurrence. Thrombophilia testing adds little in predicting recurrence and rarely is indicated.
Dr. Stehlikova is a clinical hospitalist in the division of hospital medicine, department of medicine, at Albert Einstein College of Medicine and Montefiore Medical Center in Bronx, N.Y. Dr. Martin is director of the Einstein Hospitalist Service. Dr. Janakiram is a fellow in the department of hematology at Einstein, and Dr. Korcak is an instructor at Einstein in the department of medicine and director of the Weiler Medical Service. Dr. Galhotra is associate director for inpatient quality in the department of medicine at Einstein; Dr. Averbukh is an academic hospitalist; and Dr. Southern is chief of the division of hospital medicine at Einstein.
References
- Middeldorp S, van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. 2008;143:321-335.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5:1979-1981.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199-205.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170:1710-1716.
- Kearon C, Julian JA, Kovacs MJ, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112:4432-4436.
- Vossen CY, Walker ID, Svensson P, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol. 2005;25:1992-1997.
- Brown K, Luddington R, Williamson D, Baker P, Baglin T. Risk of venous thromboembolism associated with a G to A transition at position 20210 in the 3'-untranslated region of the prothrombin gene. Br J Haematol. 1997;98:907-909.
- Schulman S, Tengborn L. Treatment of venous thromboembolism in patients with congenital deficiency of antithrombin III. Thromb Haemost. 1992;68:634-636.
- Palareti G, Legnani C, Cosmi B, Guazzaloca G, Cini M, Mattarozzi S. Poor anticoagulation quality in the first 3 months after unprovoked venous thromboembolism is a risk factor for long-term recurrence. J Thromb Haemost. 2005;3:955-961.
- Gadisseur AP, Christiansen SC, van der Meer FJ, Rosendaal FR. The quality of oral anticoagulant therapy and recurrent venous thrombotic events in the Leiden Thrombophilia Study. J Thromb Haemost. 2007;5:931-936.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:454S-545S.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
- Laczkovics C, Grafenhofer H, Kaider A, et al. Risk of recurrence after a first venous thromboembolic event in young women. Haematologica. 2007;92:1201-1207.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93-99.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352-2361.
- De Stefano V, Simioni P, Rossi E, et al. The risk of recurrent venous thromboembolism in patients with inherited deficiency of natural anticoagulants antithrombin, protein C and protein S. Haematologica. 2006;91:695-698.
- Price DT, Ridker PM. Factor V Leiden mutation and the risks for thromboembolic disease: a clinical perspective. Ann Intern Med. 1997;127:895-903.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166:729-736.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood. 2000;96:3329-3333.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301:2472-2485.
- Eichinger S, Pabinger I, Stumpflen A, et al. The risk of recurrent venous thromboembolism in patients with and without factor V Leiden. Thromb Haemost. 1997;77:624-628.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation. 2010;121(15):1706-1712.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104:332-338.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737-1740.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362:523-526.
- Rosendaal FR. Once and only once. Circulation. 2010;121:1688-1690.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121:458-463.
The Case
A healthy 42-year-old woman presents to the hospital with acute-onset pleuritic chest pain and shortness of breath. She has not had any recent surgeries, takes no medications, and is very active. A lung ventilation-perfusion scan reveals a high probability of pulmonary embolism (PE). The patient’s history is notable for two second-trimester pregnancy losses. The patient is started on low-molecular heparin and warfarin (LMHW).
Should this patient be tested for thrombophilia?
Background
Thrombophilia can now be identified in more than half of all patients presenting with VTE, and testing for underlying causes of thrombophilia has become widespread.1 Physicians believe that thrombophilia testing frequently changes management of patients with VTE.2
Thrombophilias can be classified into three major categories: deficiency of natural inhibitors of coagulation, abnormal function or elevated level of coagulation factors, and acquired thrombophilias (see Table 1).
The prevalence of specific thrombophilias varies widely. For example, the prevalence of activated protein C resistance (the factor V Leiden mutation) is 3% to 7%. In comparison, the prevalence of antithrombin deficiency is estimated at 0.02%. Each thrombophilia is associated with an increased VTE risk, but the level of risk associated with a given thrombophilia varies greatly.1
Before testing for thrombophilia in acute VTE, assess the risk of recurrent VTE by determining if the thrombosis was provoked or unprovoked. A VTE event is considered provoked if it occurs in the setting of pregnancy within the previous three months; estrogen therapy; immobility from acute illness for more than one week; travel lasting for more than six hours; leg trauma, fracture, or surgery within the previous three months; or active malignancy (see Table 2,).3 Unprovoked VTE has a recurrence rate of 7.4% per patient year, compared with 3.3% per patient year for a provoked VTE; the risk is even lower (0.7% per patient year) if the risk factor for the provoked VTE was surgical.4
Testing for thrombophilia is indicated if the results would add significant prognostic information beyond the clinical history, or if it would change patient management—in particular, the intensity or the duration of anticoagulation.
Review of the Data
Does presence of thrombophilia alter the intensity of anticoagulation for VTE?
If thrombophilia increases the risk of VTE recurrence while on anticoagulation, then a more intense level of anticoagulation might prevent future VTE. There are no studies investigating higher intensity of anticoagulation, but if standard anticoagulation were insufficient for patients with identifiable thrombophilia, one might expect to observe increased recurrence rates among patients with thrombophilia treated with standard warfarin therapy.
In a substudy of the Extended Low-Intensity Anticoagulation for Unprovoked Venous ThromboEmbolism (ELATE) trial, the risk of recurrence of VTE among treated subjects was very low overall, and the presence of thrombophilic abnormalities was not associated with significantly higher risk.5 Observational studies have found VTE recurrence rates are low in patients treated with warfarin, with or without thrombophilia.6-8
The impact of the initial level of anticoagulation on recurrence after the completion of the treatment period has been evaluated. Although one study suggested that patients with substandard levels of anticoagulation were at an increased risk of subsequent VTE, this was not confirmed in the Leiden Thrombophilia Study (LETS). 9,10
In sum, the majority of data do not suggest a significantly increased risk of recurrent VTE in patients with thrombophilia treated with standard anticoagulation. Therefore, treatment with warfarin to a goal INR of 2 to 3 is sufficient.
Does presence of thrombophilia alter duration of VTE treatment?
A major decision clinicians face when caring for VTE patients is the duration of anticoagulation treatment. The current ACCP recommendation for treatment of a provoked VTE is three months, with treatment for an unprovoked VTE three months or lifelong.11 If the presence of thrombophilia increases the risk of recurrence after cessation of anticoagulation treatment, longer duration of treatment might be indicated. One of the goals of thrombophilia testing should be to identify those patients.
Overall, the recurrence rate after first VTE is high, with a cumulative incidence of 25% at five years, 30% at eight years, and 56% at 20 years.12,13
Deficiency of natural inhibitors of coagulation.
Deficiency of a natural inhibitor of coagulation has been associated with a risk of recurrence of VTE of as much as 10% per year, according to some studies.6,14 However, the estimates are based on studies that include individuals from thrombosis-prone families, and selection bias might have contributed to the high recurrence rates.1 In the unselected population represented in the LETS study, only a modest elevation was seen in the estimated risk of recurrence for patients with inhibitor deficiencies.15
Testing for deficiency of inhibitors offers little prognostic information beyond that obtained when determining whether a VTE event is provoked or unprovoked. In studies that have separately examined subjects with provoked vs. unprovoked VTE, deficiency of an inhibitor is not associated with increased risk of recurrence.15,16
Abnormal function or level of anticoagulation factors.
Factor V Leiden (FVL) is the most common cause of inherited thrombophilia and is associated with as much as a sixfold increase in VTE risk, while the prothrombin gene mutation is associated with a twofold increase.17,18
In contrast, the evidence associating these mutations with recurrent VTE risk is not as consistent. Although a study conducted at a referral center in Italy found an increased risk of recurrence with either Factor V Leiden or prothrombin gene mutation, a large meta-analysis of 23 studies found increased risk only with Factor V Leiden.19,20 Another meta-analysis demonstrated only a modest increased risk of recurrence in subjects with Factor V Leiden or prothrombin gene mutation, and a prospective study from Austria found no increased risk of recurrence with Factor V Leiden two years after discontinuation of anticoagulation.18,21 Additionally, when using patients with unprovoked VTE as reference, there was no increased risk of recurrence among patients homozygous for Factor V Leiden or the prothrombin gene mutation.22
In summary, although Factor V Leiden and prothrombin gene defects are associated with increased risk of recurrent VTE, the magnitude of the risk increase is modest and, therefore, should not alter duration of therapy.
Acquired thrombophilia.
It appears that the only thrombophilic state that might have a significant impact on the risk of recurrence is the antiphospholipid syndrome. The cessation of warfarin therapy in patients with thrombosis associated with antiphospholipid antibodies carries a 69% risk of recurrent thrombosis within a year.23 Some studies have suggested that the presence of specific antibodies (i.e. anticardiolipin antibodies) is associated with increased risk in patients with antiphospholipid syndrome.24
However, at present, all patients with VTE and antiphospholipid syndrome should be candidates for lifelong anticoagulation. Antiphospholipid antibody testing should be performed in patients with a suggestive history, including those with recurrent fetal loss or a single fetal loss after 10 weeks, or known collagen vascular disease.25
The role of provoked vs. unprovoked VTE.
Identifying whether a VTE is provoked or unprovoked has been shown to be an important predictor of recurrence. For example, one prospective, cohort study found two-year recurrence rates of zero in patients with a surgery or pregnancy-related VTE, 9% with other provoked VTE, and 19% with unprovoked VTE.26 In the same study, thrombophilia testing failed to reliably predict recurrence risk. Patients with unprovoked VTE who were tested and found to not have a defect were at equally high risk of recurrent VTE as those found to have a thrombophilia.27
The most significant predictor for VTE recurrence is whether the first event was provoked, and thrombophilia testing offers little additional prognostic information.28
VTE as a multifactorial disorder.
It is becoming increasingly clear that VTE is multifactorial disorder, caused by the interactions of genotypic, phenotypic, and environmental factors. In the case of an unprovoked VTE, the patient already carries a significantly elevated risk for recurrence, and further testing for known causes of thrombophilia appears to add very little additional information. The optimal duration of anticoagulation for unprovoked VTE is unclear, but current guidelines suggest at least three months—and clinicians should consider lifelong treatment.
In the vast majority of cases, testing for thrombophilia has no impact on the management of VTE and is not warranted. In patients with antiphospholipid-antibody syndrome, given the high risk of recurrence, long-term anticoagulation after a first VTE might be indicated. In select patients with a clinical picture suggestive of antiphospholipid-antibody syndrome, or a strong family history, testing should be considered.
Back to the Case
Our patient appears to have an unprovoked VTE. She should receive regular anticoagulation with warfarin, with a goal INR of 2 to 3, for at least three months. Lifelong anticoagulation therapy should be considered. Testing for heritable thrombophilia will not change the current management or treatment duration and, hence, is not indicated. However, the patient’s history is suggestive of antiphospholipid-antibody syndrome, so she should be tested. If the diagnosis of antiphospholipid syndrome is made, lifelong anticoagulation should be considered.
Bottom Line
Unprovoked VTE provides the strongest predictor for recurrence. Thrombophilia testing adds little in predicting recurrence and rarely is indicated.
Dr. Stehlikova is a clinical hospitalist in the division of hospital medicine, department of medicine, at Albert Einstein College of Medicine and Montefiore Medical Center in Bronx, N.Y. Dr. Martin is director of the Einstein Hospitalist Service. Dr. Janakiram is a fellow in the department of hematology at Einstein, and Dr. Korcak is an instructor at Einstein in the department of medicine and director of the Weiler Medical Service. Dr. Galhotra is associate director for inpatient quality in the department of medicine at Einstein; Dr. Averbukh is an academic hospitalist; and Dr. Southern is chief of the division of hospital medicine at Einstein.
References
- Middeldorp S, van Hylckama Vlieg A. Does thrombophilia testing help in the clinical management of patients? Br J Haematol. 2008;143:321-335.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5:1979-1981.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92:199-205.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170:1710-1716.
- Kearon C, Julian JA, Kovacs MJ, et al. Influence of thrombophilia on risk of recurrent venous thromboembolism while on warfarin: results from a randomized trial. Blood. 2008;112:4432-4436.
- Vossen CY, Walker ID, Svensson P, et al. Recurrence rate after a first venous thrombosis in patients with familial thrombophilia. Arterioscler Thromb Vasc Biol. 2005;25:1992-1997.
- Brown K, Luddington R, Williamson D, Baker P, Baglin T. Risk of venous thromboembolism associated with a G to A transition at position 20210 in the 3'-untranslated region of the prothrombin gene. Br J Haematol. 1997;98:907-909.
- Schulman S, Tengborn L. Treatment of venous thromboembolism in patients with congenital deficiency of antithrombin III. Thromb Haemost. 1992;68:634-636.
- Palareti G, Legnani C, Cosmi B, Guazzaloca G, Cini M, Mattarozzi S. Poor anticoagulation quality in the first 3 months after unprovoked venous thromboembolism is a risk factor for long-term recurrence. J Thromb Haemost. 2005;3:955-961.
- Gadisseur AP, Christiansen SC, van der Meer FJ, Rosendaal FR. The quality of oral anticoagulant therapy and recurrent venous thrombotic events in the Leiden Thrombophilia Study. J Thromb Haemost. 2007;5:931-936.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:454S-545S.
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1-7.
- Laczkovics C, Grafenhofer H, Kaider A, et al. Risk of recurrence after a first venous thromboembolic event in young women. Haematologica. 2007;92:1201-1207.
- Brouwer JL, Lijfering WM, Ten Kate MK, Kluin-Nelemans HC, Veeger NJ, van der Meer J. High long-term absolute risk of recurrent venous thromboembolism in patients with hereditary deficiencies of protein S, protein C or antithrombin. Thromb Haemost. 2009;101:93-99.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352-2361.
- De Stefano V, Simioni P, Rossi E, et al. The risk of recurrent venous thromboembolism in patients with inherited deficiency of natural anticoagulants antithrombin, protein C and protein S. Haematologica. 2006;91:695-698.
- Price DT, Ridker PM. Factor V Leiden mutation and the risks for thromboembolic disease: a clinical perspective. Ann Intern Med. 1997;127:895-903.
- Ho WK, Hankey GJ, Quinlan DJ, Eikelboom JW. Risk of recurrent venous thromboembolism in patients with common thrombophilia: a systematic review. Arch Intern Med. 2006;166:729-736.
- Simioni P, Prandoni P, Lensing AW, et al. Risk for subsequent venous thromboembolic complications in carriers of the prothrombin or the factor V gene mutation with a first episode of deep-vein thrombosis. Blood. 2000;96:3329-3333.
- Segal JB, Brotman DJ, Necochea AJ, et al. Predictive value of factor V Leiden and prothrombin G20210A in adults with venous thromboembolism and in family members of those with a mutation: a systematic review. JAMA. 2009;301:2472-2485.
- Eichinger S, Pabinger I, Stumpflen A, et al. The risk of recurrent venous thromboembolism in patients with and without factor V Leiden. Thromb Haemost. 1997;77:624-628.
- Lijfering WM, Middeldorp S, Veeger NJ, et al. Risk of recurrent venous thrombosis in homozygous carriers and double heterozygous carriers of factor V Leiden and prothrombin G20210A. Circulation. 2010;121(15):1706-1712.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence of thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104:332-338.
- Pengo V, Tripodi A, Reber G, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737-1740.
- Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362:523-526.
- Rosendaal FR. Once and only once. Circulation. 2010;121:1688-1690.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121:458-463.