User login
LOOP trial undercuts value of long-term continuous ECG screening for AFib
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
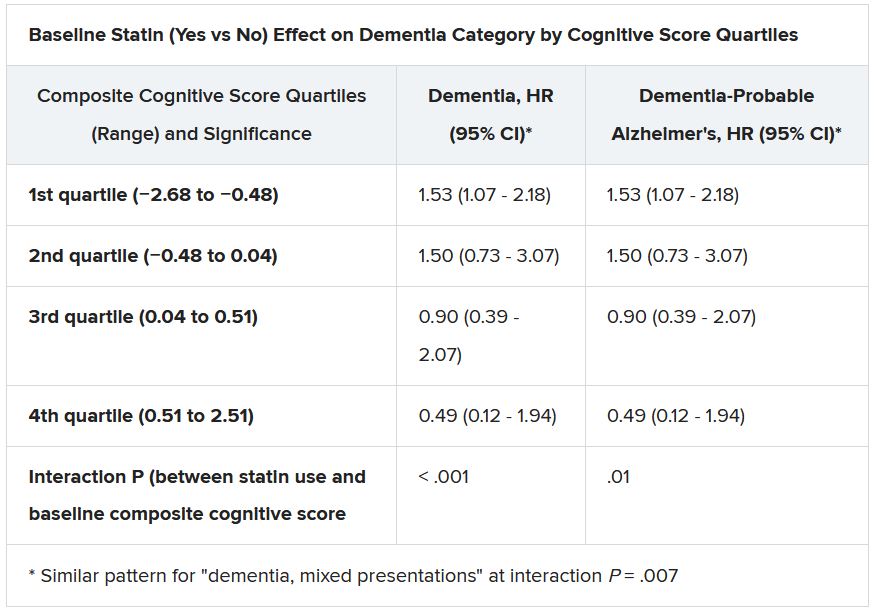
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
Perhaps short, asymptomatic bouts of atrial fibrillation (AFib) that show up on long-term, continuous monitoring aren’t worth hunting for just so oral anticoagulation (OAC) can be started, even in elderly people with other stroke risk factors.
That’s a potential message from a randomized trial that tested an AFib screening strategy relying on an implantable loop recorder (ILR) in older adults without AFib but with other stroke risk factors who were invited to participate. OAC was recommended to any participant found with even a short bout of the arrhythmia (that is, any lasting 6 minutes or longer).
More than three times as many in the monitoring group compared to a standard-care cohort were found to have AFib, and nearly all were put on OAC. In fact, monitored participants were almost three times as likely to be put on OAC (P < .0001) compared with controls.
But it didn’t make any apparent difference to outcomes. The risk for stroke or systemic embolism did not significantly differ between the two groups over more than 5 years in the trial of about 6,000 participants, called LOOP.
“This result was seen despite a high proportion of atrial fibrillation detection, and a high acceptance of anticoagulation therapy, and might imply that not all atrial fibrillation is worth screening for, and not all screen-detected atrial fibrillation merits anticoagulation,” contend the authors of the LOOP report, simultaneously published in The Lancet and presented Aug. 29 at the virtual European Society of Cardiology (ESC) Congress 2021.
“The rates of bleeding were modest, despite the low threshold for anticoagulation,” and was not significantly different between the two groups, Jesper H. Svendsen, MD, DMSc, Copenhagen University Hospital, Denmark, said at a media briefing before his presentation of the trial at the congress. He is lead author on the Lancet report.
At least 6 minutes of AFib was identified in more than 30% of the ILR-monitored patients, and about 90% of those were started on OAC, Dr. Svendsen observed.
But one take-home message from LOOP, he said in an interview, is that “short-lasting episodes” of AFib do not necessarily pose an untoward risk for stroke compared with AFib revealed by intermittent monitoring, which “primarily identifies longer-lasting atrial fibrillation episodes. So short-lasting episodes are probably not as serious as long-lasting.”
The LOOP trial “teaches us that perhaps short-lasting asymptomatic episodes may not benefit from being screened or found,” said Stefan James, MD, PhD, Uppsala University, Sweden. However, that may not be the case when the monitored individual is symptomatic or has longer-lasting AFib episodes, he said in an interview. “But certainly, this study teaches us that we need to understand much better the relationship between short episodes versus symptoms versus medical outcomes.”
In LOOP, 6,004 people aged 70-90 years without AFib but with at least one other stroke risk factor, which could include hypertension, diabetes, a history of stroke, or heart failure, were implanted with an ILR, the Reveal LINQ (Medtronic).
They were randomly assigned at four centers in Denmark to a monitoring group or a usual care group in a 1:3 ratio. Overwhelmingly, most had hypertension. Almost half the population were women.
OAC was recommended for all persons in the monitoring group who showed an episode of AFib lasting at least 6 minutes.
Atrial fibrillation was diagnosed in 31.8% of the 1,501 participants in the monitored group and 12.2% of the 4,503 assigned to usual care, for a hazard ratio (HR) of 3.17 (95% confidence interval, 2.81-3.59; P < .0001).
OAC was started in 29.7% of monitored participants and 13.1% of the control cohort, for an HR of 2.72 (95% CI, 2.41-3.08; P < .0001).
There were 315 strokes and three systemic arterial embolisms observed in the entire trial, for primary endpoint rates of 4.5% in the ILR monitoring group and 5.6% in the control group (HR, 0.80; 95% CI, 0.61-1.05; P = .11). Adding transient ischemic attack (TIA) or cardiovascular death to the endpoint did not make for a significant difference. The rates of major bleeding were 4.3% and 3.5%, respectively (P = .11).
“In general, the findings were consistent across subgroups,” including by age, sex, diabetes and heart failure status, stroke history, antiplatelet therapy, renal function, and even CHA2DS2–VASc score, Dr. Svendsen noted.
But, he said, participants in the highest tertile for baseline systolic blood pressure (BP), at least 157 mm Hg, “seemed to benefit from being screened,” with a 49% reduction in risk for the primary endpoint (P = .0066). The interaction between systolic BP and outcome was significant (P = .007).
Only 9.3% of participants in LOOP did not have a baseline diagnosis of hypertension and so had to have another risk factor to enroll, the published report notes. However, the significant interaction with systolic BP “suggests that patients with dysregulated hypertension could benefit from this type of screening and concomitant anticoagulation.”
“There is a tight association between our primary endpoint and hypertension,” Dr. Svendsen said in an interview. “But I think it’s very important to say that this subgroup analysis is only hypothesis-generating.”
An editorial accompanying the LOOP publication suggests, in line with Dr. Svendsen’s proposal, that “shorter atrial fibrillation episodes found by long-term ILRs might not have the same stroke risk as atrial fibrillation detected through single-timepoint or less intense monitoring.”
If much of the paroxysmal AFib observed in LOOP and other studies with similar monitoring methods “is not the actual cause of stroke and is instead predominantly a risk marker, further research is warranted to establish whether a different screening focus and treatment paradigm are required to prevent stroke and other vascular brain injury related to atrial fibrillation,” wrote editorialists Ben Freedman, MBBS, PhD, and Nicole Lowres, BPhty, PhD, University of Sydney, Australia.
LOOP was partially supported by Medtronic. Dr. Svendsen is a member of Medtronic advisory boards and has received speaker honoraria and research grants from Medtronic in relation to this work and outside the submitted work. Disclosures for the other authors are in the report. Dr. Freedman reports grants to the Heart Research Institute, speakers fees and nonfinancial support from the Bristol-Myers Squibb–Pfizer Alliance, speakers fees and nonfinancial support from Daiichi Sankyo, nonfinancial support from AliveCor, and speakers fees and nonfinancial support from Omron unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation. Dr. Lowres reports grants to the Heart Research Institute from the Bristol-Myers Squibb–Pfizer Alliance unrelated to the topic of the editorial but related to atrial fibrillation and screening for atrial fibrillation.
A version of this article first appeared on Medscape.com.
APAF-CRT: ‘Ablate and pace’ cuts mortality in narrow-QRS HF, permanent AFib
When a patient has permanent atrial fibrillation (AFib) and advanced heart failure (HF), rate control therapy is an option but an “ablate-and-pace” strategy may be better at improving symptoms. The ablate-and-pace approach, compared to pharmacologic rate control, may even prolong survival in a subset of such patients when the accompanying pacemaker provides cardiac resynchronization therapy (CRT), suggests a new randomized trial.
In the APAF-CRT trial, mortality fell more than 70% over 4 years for such patients with HF and narrow QRS intervals who were assigned to ablate-and-pace – that is, CRT after creation of heart block by atrioventricular (AV) junction ablation – compared to those managed medically.
The benefit was seen regardless of left ventricular ejection fraction (LVEF) at the start of the trial and probably stemmed from “the combination of strict rate control and rate regulation achieved by AV-junction ablation together with biventricular pacing,” said Michele Brignole, MD, Istituto Auxologico Italiano, Ospedale San Luca, Milan. The CRT substitution for a standard pacemaker, he explained, is thought to “counteract” the adverse remodeling effects of apical right ventricular (RV) pacing.
Dr. Brignole delivered the remarks at a media presentation before his presentation of the APAF-CRT during the virtual annual congress of the European Society of Cardiology.
The results “support ablation-CRT as a first-line therapy in patients with permanent AFib and narrow QRS who were hospitalized for heart failure,” regardless of ejection fraction, said Dr. Brignole, lead author on the study’s same-day publication in the European Heart Journal.
“The results are not surprising. They are in line with prior studies with shorter follow-up, and they justify a relatively common practice today, to implant CRT in these patients. It has previously been shown to improve heart failure and quality of life, and is now proven to improve survival because of the longer follow-up,” Michael Glikson, MD, Shaare Zedek Medical Center, Jerusalem, said at the media briefing.
“The APAF-CRT mortality trial makes an important contribution to establishment of AV-nodal ablation with CRT as first-line therapy of resistant atrial fibrillation with heart failure, mostly in patients with reduced ejection fraction,” said Dr. Glikson, who was not part of the trial.
However, he added, “the advantage of CRT over RV pacing is still somewhat unclear in patients with normal or preserved ejection fraction,” who were relatively few in APAF-CRT and in whom RV apical pacing after AV nodal ablation has not been shown to make a big difference to ventricular function.
The new analysis covered the trial’s second phase, which featured a mortality primary endpoint, in contrast to the previously reported initial stage that followed the first 102 patients over 2 years for death, worsening HF, or HF hospitalization.
The first phase had halted enrollment before reaching its planned target of 280 patients when an interim analysis showed a significant benefit for ablate and pace. The mortality trial continued to recruit at 11 centers in Europe, reaching 133 patients, who were followed for up to 4 years, the report notes. But its enrollment had also been suspended after an interim analysis saw superiority in the ablate-and-pace arm.
APAF-CRT entered patients with severely symptomatic permanent AFib for longer than 6 months, with a QRS interval no greater than 110 ms, who had at least one HF hospitalization in the last year and were considered poor candidates for AFib ablation. Their mean age was 73 years, and almost half, 47%, were women.
They were randomly assigned to ablate-and-pace with CRT or pharmacologic rate control therapy, 63 and 70 patients, respectively. Patients in either group could be given an implantable defibrillator at physician discretion.
Patients had been followed a median of 29 months when the trial was stopped for efficacy. The hazard ratio (HR) for death from any cause, ablate-and-pace vs. rate control, was 0.26 (95% confidence interval, 0.10-0.65; P = .004), with a number needed to treat to prevent an event of 3.7. The HR was 0.40 (95% CI, 0.22-0.73; P = .002) for the secondary endpoint of death or HF hospitalization.
The new ESC guidelines on cardiac pacing and cardiac resynchronization therapy recommend “that if the ejection fraction is subnormal, they should receive a CRT as the first choice,” Dr. Glikson said. “However, for patients who are undergoing AV nodal ablation and have normal ejection fractions, we thought that RV apical pacing should be okay,” so that was the main recommendation, he said.
“I think that the APAF-CRT study does not really change this approach” because the study was small and there were few data on such patients.
APAF-CRT was an investigator-initiated independent clinical trial, sponsored by a nonprofit organization, Centro Prevenzione Malattie Cardiorespiratorie ‘Nuccia e Vittore Corbella’, Rapallo, Italy, which received an unrestricted research grant from the Boston Scientific Investigator Sponsored Research (ISR) Committee. Dr. Brignole declared no conflicts. Disclosures for the other authors are in the report. Dr. Glikson had no disclosures.
A version of this article first appeared on Medscape.com.
When a patient has permanent atrial fibrillation (AFib) and advanced heart failure (HF), rate control therapy is an option but an “ablate-and-pace” strategy may be better at improving symptoms. The ablate-and-pace approach, compared to pharmacologic rate control, may even prolong survival in a subset of such patients when the accompanying pacemaker provides cardiac resynchronization therapy (CRT), suggests a new randomized trial.
In the APAF-CRT trial, mortality fell more than 70% over 4 years for such patients with HF and narrow QRS intervals who were assigned to ablate-and-pace – that is, CRT after creation of heart block by atrioventricular (AV) junction ablation – compared to those managed medically.
The benefit was seen regardless of left ventricular ejection fraction (LVEF) at the start of the trial and probably stemmed from “the combination of strict rate control and rate regulation achieved by AV-junction ablation together with biventricular pacing,” said Michele Brignole, MD, Istituto Auxologico Italiano, Ospedale San Luca, Milan. The CRT substitution for a standard pacemaker, he explained, is thought to “counteract” the adverse remodeling effects of apical right ventricular (RV) pacing.
Dr. Brignole delivered the remarks at a media presentation before his presentation of the APAF-CRT during the virtual annual congress of the European Society of Cardiology.
The results “support ablation-CRT as a first-line therapy in patients with permanent AFib and narrow QRS who were hospitalized for heart failure,” regardless of ejection fraction, said Dr. Brignole, lead author on the study’s same-day publication in the European Heart Journal.
“The results are not surprising. They are in line with prior studies with shorter follow-up, and they justify a relatively common practice today, to implant CRT in these patients. It has previously been shown to improve heart failure and quality of life, and is now proven to improve survival because of the longer follow-up,” Michael Glikson, MD, Shaare Zedek Medical Center, Jerusalem, said at the media briefing.
“The APAF-CRT mortality trial makes an important contribution to establishment of AV-nodal ablation with CRT as first-line therapy of resistant atrial fibrillation with heart failure, mostly in patients with reduced ejection fraction,” said Dr. Glikson, who was not part of the trial.
However, he added, “the advantage of CRT over RV pacing is still somewhat unclear in patients with normal or preserved ejection fraction,” who were relatively few in APAF-CRT and in whom RV apical pacing after AV nodal ablation has not been shown to make a big difference to ventricular function.
The new analysis covered the trial’s second phase, which featured a mortality primary endpoint, in contrast to the previously reported initial stage that followed the first 102 patients over 2 years for death, worsening HF, or HF hospitalization.
The first phase had halted enrollment before reaching its planned target of 280 patients when an interim analysis showed a significant benefit for ablate and pace. The mortality trial continued to recruit at 11 centers in Europe, reaching 133 patients, who were followed for up to 4 years, the report notes. But its enrollment had also been suspended after an interim analysis saw superiority in the ablate-and-pace arm.
APAF-CRT entered patients with severely symptomatic permanent AFib for longer than 6 months, with a QRS interval no greater than 110 ms, who had at least one HF hospitalization in the last year and were considered poor candidates for AFib ablation. Their mean age was 73 years, and almost half, 47%, were women.
They were randomly assigned to ablate-and-pace with CRT or pharmacologic rate control therapy, 63 and 70 patients, respectively. Patients in either group could be given an implantable defibrillator at physician discretion.
Patients had been followed a median of 29 months when the trial was stopped for efficacy. The hazard ratio (HR) for death from any cause, ablate-and-pace vs. rate control, was 0.26 (95% confidence interval, 0.10-0.65; P = .004), with a number needed to treat to prevent an event of 3.7. The HR was 0.40 (95% CI, 0.22-0.73; P = .002) for the secondary endpoint of death or HF hospitalization.
The new ESC guidelines on cardiac pacing and cardiac resynchronization therapy recommend “that if the ejection fraction is subnormal, they should receive a CRT as the first choice,” Dr. Glikson said. “However, for patients who are undergoing AV nodal ablation and have normal ejection fractions, we thought that RV apical pacing should be okay,” so that was the main recommendation, he said.
“I think that the APAF-CRT study does not really change this approach” because the study was small and there were few data on such patients.
APAF-CRT was an investigator-initiated independent clinical trial, sponsored by a nonprofit organization, Centro Prevenzione Malattie Cardiorespiratorie ‘Nuccia e Vittore Corbella’, Rapallo, Italy, which received an unrestricted research grant from the Boston Scientific Investigator Sponsored Research (ISR) Committee. Dr. Brignole declared no conflicts. Disclosures for the other authors are in the report. Dr. Glikson had no disclosures.
A version of this article first appeared on Medscape.com.
When a patient has permanent atrial fibrillation (AFib) and advanced heart failure (HF), rate control therapy is an option but an “ablate-and-pace” strategy may be better at improving symptoms. The ablate-and-pace approach, compared to pharmacologic rate control, may even prolong survival in a subset of such patients when the accompanying pacemaker provides cardiac resynchronization therapy (CRT), suggests a new randomized trial.
In the APAF-CRT trial, mortality fell more than 70% over 4 years for such patients with HF and narrow QRS intervals who were assigned to ablate-and-pace – that is, CRT after creation of heart block by atrioventricular (AV) junction ablation – compared to those managed medically.
The benefit was seen regardless of left ventricular ejection fraction (LVEF) at the start of the trial and probably stemmed from “the combination of strict rate control and rate regulation achieved by AV-junction ablation together with biventricular pacing,” said Michele Brignole, MD, Istituto Auxologico Italiano, Ospedale San Luca, Milan. The CRT substitution for a standard pacemaker, he explained, is thought to “counteract” the adverse remodeling effects of apical right ventricular (RV) pacing.
Dr. Brignole delivered the remarks at a media presentation before his presentation of the APAF-CRT during the virtual annual congress of the European Society of Cardiology.
The results “support ablation-CRT as a first-line therapy in patients with permanent AFib and narrow QRS who were hospitalized for heart failure,” regardless of ejection fraction, said Dr. Brignole, lead author on the study’s same-day publication in the European Heart Journal.
“The results are not surprising. They are in line with prior studies with shorter follow-up, and they justify a relatively common practice today, to implant CRT in these patients. It has previously been shown to improve heart failure and quality of life, and is now proven to improve survival because of the longer follow-up,” Michael Glikson, MD, Shaare Zedek Medical Center, Jerusalem, said at the media briefing.
“The APAF-CRT mortality trial makes an important contribution to establishment of AV-nodal ablation with CRT as first-line therapy of resistant atrial fibrillation with heart failure, mostly in patients with reduced ejection fraction,” said Dr. Glikson, who was not part of the trial.
However, he added, “the advantage of CRT over RV pacing is still somewhat unclear in patients with normal or preserved ejection fraction,” who were relatively few in APAF-CRT and in whom RV apical pacing after AV nodal ablation has not been shown to make a big difference to ventricular function.
The new analysis covered the trial’s second phase, which featured a mortality primary endpoint, in contrast to the previously reported initial stage that followed the first 102 patients over 2 years for death, worsening HF, or HF hospitalization.
The first phase had halted enrollment before reaching its planned target of 280 patients when an interim analysis showed a significant benefit for ablate and pace. The mortality trial continued to recruit at 11 centers in Europe, reaching 133 patients, who were followed for up to 4 years, the report notes. But its enrollment had also been suspended after an interim analysis saw superiority in the ablate-and-pace arm.
APAF-CRT entered patients with severely symptomatic permanent AFib for longer than 6 months, with a QRS interval no greater than 110 ms, who had at least one HF hospitalization in the last year and were considered poor candidates for AFib ablation. Their mean age was 73 years, and almost half, 47%, were women.
They were randomly assigned to ablate-and-pace with CRT or pharmacologic rate control therapy, 63 and 70 patients, respectively. Patients in either group could be given an implantable defibrillator at physician discretion.
Patients had been followed a median of 29 months when the trial was stopped for efficacy. The hazard ratio (HR) for death from any cause, ablate-and-pace vs. rate control, was 0.26 (95% confidence interval, 0.10-0.65; P = .004), with a number needed to treat to prevent an event of 3.7. The HR was 0.40 (95% CI, 0.22-0.73; P = .002) for the secondary endpoint of death or HF hospitalization.
The new ESC guidelines on cardiac pacing and cardiac resynchronization therapy recommend “that if the ejection fraction is subnormal, they should receive a CRT as the first choice,” Dr. Glikson said. “However, for patients who are undergoing AV nodal ablation and have normal ejection fractions, we thought that RV apical pacing should be okay,” so that was the main recommendation, he said.
“I think that the APAF-CRT study does not really change this approach” because the study was small and there were few data on such patients.
APAF-CRT was an investigator-initiated independent clinical trial, sponsored by a nonprofit organization, Centro Prevenzione Malattie Cardiorespiratorie ‘Nuccia e Vittore Corbella’, Rapallo, Italy, which received an unrestricted research grant from the Boston Scientific Investigator Sponsored Research (ISR) Committee. Dr. Brignole declared no conflicts. Disclosures for the other authors are in the report. Dr. Glikson had no disclosures.
A version of this article first appeared on Medscape.com.
GUIDE-HF: CardioMEMS-guided meds fall short in mild to moderate heart failure
Medical therapy for heart failure guided by an implanted pulmonary artery pressure (PAP) sensor didn’t improve survival or risk for HF events like hospitalization over a year in a major randomized trial that entered a broad range of patients with mild to moderate disease.
But medical therapy adjustments based on PAP readings from the miniature CardioMEMS (Abbott) implant might well have surpassed conventional HF management for outcomes had the world not been turned upside down by SARS-CoV-2 and the pandemic lockdowns, assert researchers from the GUIDE-HF trial.
Something about the crisis, they concluded – although not without some pushback – led to better outcomes in the standard-care control group, apparently muddling any potential differences from those on PAP-guided management.
Working with regulators, the team conducted a “pre–COVID-19 impact analysis” that compared outcomes before the March 2020 national COVID-19 emergency declaration that forced much of the United States with shelter in place.
By that time, all of the trial’s patients had been followed for at least 3 months, and about three-fourths of its endpoints had already been counted, JoAnn Lindenfeld, MD, Vanderbilt University Medical Center, Nashville, Tenn., said at a media briefing prior to unveiling GUIDE-HF at the all-virtual European Society of Cardiology Congress 2021.
The pre–COVID-19 analysis, approved several months before the end of the trial – while the data were still blinded – had been “suggested by both regulatory agencies and professional societies in Europe and in the United States,” Dr. Lindenfeld said.
It pointed to a possible benefit for the CardioMEMS-guided strategy, a barely significant 19% drop in risk (P = .049) for the primary endpoint of death, HF hospitalization, or urgent HF hospital visit. The effect was driven by a 24% decline in HF events (P = .014), with no significant contribution from mortality.
“The benefits of hemodynamic monitoring and management in reducing heart failure hospitalizations extended to patients with less severe heart failure”; that is, those in New York Heart Association class 2 and any in NYHA class 3 with “elevated natriuretic peptides but no previous hospitalization,” said Dr. Lindenfeld, who is also lead author on the GUIDE-HF report published in the Lancet.
Such benefits would suggest that CardioMEMS-guided management can improve outcomes in an HF population much broader than the device’s current indication.
But as it happens, the trial’s prospectively defined 12-month primary outcomes were less impressive. A 12% decline in risk for the composite endpoint among patients managed by CardioMEMS failed to reach significance compared with standard management (P = .16).
“Several factors could explain the considerable loss of benefit of hemodynamic-guided management during the COVID-19 pandemic,” the Lancet report explained. They include “improved patient compliance with medical and dietary regimens, reduced respiratory infections, altered health-care provider behavior, changes in disease progression due to COVID-19, or other as yet unknown effects of a major pandemic.”
Expanded population
Importantly, GUIDE-HF had entered 1,000 patients in NYHA class 2-4 and either an HF hospitalization in the previous year or elevated natriuretic peptide levels. About 44% of the entrants in NYHA class 3 did not have a 1-year history of HF hospitalization.
That’s a more heterogeneous and potentially lower-risk cohort than patients in the randomized CHAMPION study of 11 years ago, which led to the implant’s approval on both sides of the Atlantic.
In that trial, CardioMEMS-guided management was followed by 30% drop in risk for HF hospitalization over 6 months (P < .001). But CHAMPION was limited to patients in NYHA class 3 with a history of HF hospitalization, the device’s current indication in both the United States and Europe.
The GUIDE-HF findings “reinforce that patients with class 3 heart failure and prior heart failure hospitalization are those in whom there is the clearest benefit, based on the prior CHAMPION trial. These are the patients where this monitoring strategy may be best targeted,” Gregg C. Fonarow, MD, University of California Los Angeles Medical Center, said in an interview.
Although GUIDE-HF didn’t show a significant benefit for NYHA class 2 patients with elevated biomarkers, who aren’t covered by the device’s current labeling, that group showed “some suggestions of potential benefit,” noted Dr. Fonarow, who isn’t a coauthor on the Lancet report. So, “there may be select patients with class 2 heart failure where monitoring could be considered on a case-by-case basis.”
In an interview, Larry A. Allen, MD, MHS, said that, “while the technology is pretty amazing, the real question is whether it tells us something that we didn’t already know that leads to improved care. Unfortunately, as tested here, it doesn’t, or at least not enough to make a big difference.”
The pre–COVID-19 impact analysis “should be interpreted with caution, and not as the primary finding,” Dr. Allen, from the University of Colorado at Denver, Aurora, who is not a GUIDE-HF coauthor, said in an interview.
One might hypothesize, he said, “that, in the setting of limited in-person visits with loss of physical examination, perhaps CardioMEMS would be more – not less – helpful during the pandemic. And yet the opposite was seen.”
The pandemic has “markedly altered all kinds of aspects of patient care and trial conduct, but that doesn’t make the data derived during that period uninformative,” Dr. Allen said. “And as we are increasingly reminded, the future will be a new normal, not a prepandemic normal.”
A third group
The GUIDE-HF trial includes, in addition to the 1,000 randomized patients, a single-group observational cohort of 2,600 patients, whose outcomes will be reported at another time, noted the published report.
But in the randomized comparison, conducted at 118 centers in North America, all patients were implanted with the CardioMEMS device and blinded as to their assigned strategy. Enrollment took place between March 2018 and Dec. 20, 2019.
Of the 1,000 successfully implanted patients, 497 were assigned to the pressure-guided strategy, in which “titration of diuretics was recommended if pulmonary artery pressure provided evidence of excess intravascular volume, and titration of vasodilators was recommended if elevated vascular resistance was evident,” the report stated.
The remaining 503 patients assigned to standard care served as control subjects, for whom “investigators were aware of treatment assignment but did not have access to PAP data.”
The hazard ratio for the primary endpoint in the pressure-guided group, compared with the control group, was 0.88 (95% confidence interval, 0.74-1.05; P = .16) over a median follow-up of 11.7 months.
But in the sensitivity analysis comparing outcomes before and after the COVID-19 lockdowns, using established methodology, the report stated, the primary-endpoint HR was 0.81 (95% CI, 0.66-1.00; P = .049).
The difference is owed to improved outcomes in the control group under pandemic conditions, the researchers concluded. Patients assigned to conventional management –whatever that meant during shelter-in-place – experienced 21% fewer primary-endpoint events than their own rate before the pandemic. After the COVID-19 emergency was declared, there was no significant difference in event rates between the two randomization groups.
In the primary 12-month analysis, the HR for HF events in the guided-therapy was not significant reduced, at 0.85 (95% CI, 0.70-1.03; P = .096). But in the pre-COVID-19 analysis, that risk fell significantly with CardioMEMS-guided management, for an HR of 0.76 (95% CI, 0.61-0.95; P = .014).
An editorial accompanying the GUIDE-HF publication (Lancet. 2021 Aug 27. doi: 10.1016/S0140-6736[21]01914-0) asserts that the trial “did not enroll an ideal group of patients for showing the efficacy of pulmonary artery pressure monitoring, since many had baseline pressures in the target range with little possibility of short-term gain.”
Also, wrote John G. F. Cleland, MD, PhD, University of Glasgow, and Pierpaolo Pellicori, MD, Imperial College London, “follow-up was too short, and interventions did not substantially change pulmonary artery pressure.”
They continue: “Monitoring alone cannot improve outcome, but consequent actions might. The GUIDE-HF results are encouraging but inconclusive, and should inform further research, possibly a large, simple, open-label trial to investigate a system of care rather than a single technology.”
GUIDE-HF was funded by Abbott. Dr. Lindenfeld disclosed receiving research grants from AstraZeneca, Sensible Medical, and Volumetrix; and consulting for Abbott, Alleviant Medical, AstraZeneca, Boehringer Ingelheim, Boston Scientific, CVRx, Edwards, Impulse Dynamics, and VWave. Dr. Fonarow reported consulting for Abbott and that his institution has participated in the GUIDE-HF trial; he has elsewhere disclosed consulting for Amgen, AstraZeneca, CHF Solutions Lifesciences, Janssen, Medtronic, and Novartis. Dr. Allen had elsewhere reported consulting for Abbott, Amgen, Boston Scientific, and Novartis. Dr. Cleland disclosed receiving personal fees from Abbott for serving on an advisory board for the MitraClip device, unrelated to the CardioMEMS device. Dr. Pellicori reported no relevant conflicts.
A version of this article first appeared on Medscape.com.
Medical therapy for heart failure guided by an implanted pulmonary artery pressure (PAP) sensor didn’t improve survival or risk for HF events like hospitalization over a year in a major randomized trial that entered a broad range of patients with mild to moderate disease.
But medical therapy adjustments based on PAP readings from the miniature CardioMEMS (Abbott) implant might well have surpassed conventional HF management for outcomes had the world not been turned upside down by SARS-CoV-2 and the pandemic lockdowns, assert researchers from the GUIDE-HF trial.
Something about the crisis, they concluded – although not without some pushback – led to better outcomes in the standard-care control group, apparently muddling any potential differences from those on PAP-guided management.
Working with regulators, the team conducted a “pre–COVID-19 impact analysis” that compared outcomes before the March 2020 national COVID-19 emergency declaration that forced much of the United States with shelter in place.
By that time, all of the trial’s patients had been followed for at least 3 months, and about three-fourths of its endpoints had already been counted, JoAnn Lindenfeld, MD, Vanderbilt University Medical Center, Nashville, Tenn., said at a media briefing prior to unveiling GUIDE-HF at the all-virtual European Society of Cardiology Congress 2021.
The pre–COVID-19 analysis, approved several months before the end of the trial – while the data were still blinded – had been “suggested by both regulatory agencies and professional societies in Europe and in the United States,” Dr. Lindenfeld said.
It pointed to a possible benefit for the CardioMEMS-guided strategy, a barely significant 19% drop in risk (P = .049) for the primary endpoint of death, HF hospitalization, or urgent HF hospital visit. The effect was driven by a 24% decline in HF events (P = .014), with no significant contribution from mortality.
“The benefits of hemodynamic monitoring and management in reducing heart failure hospitalizations extended to patients with less severe heart failure”; that is, those in New York Heart Association class 2 and any in NYHA class 3 with “elevated natriuretic peptides but no previous hospitalization,” said Dr. Lindenfeld, who is also lead author on the GUIDE-HF report published in the Lancet.
Such benefits would suggest that CardioMEMS-guided management can improve outcomes in an HF population much broader than the device’s current indication.
But as it happens, the trial’s prospectively defined 12-month primary outcomes were less impressive. A 12% decline in risk for the composite endpoint among patients managed by CardioMEMS failed to reach significance compared with standard management (P = .16).
“Several factors could explain the considerable loss of benefit of hemodynamic-guided management during the COVID-19 pandemic,” the Lancet report explained. They include “improved patient compliance with medical and dietary regimens, reduced respiratory infections, altered health-care provider behavior, changes in disease progression due to COVID-19, or other as yet unknown effects of a major pandemic.”
Expanded population
Importantly, GUIDE-HF had entered 1,000 patients in NYHA class 2-4 and either an HF hospitalization in the previous year or elevated natriuretic peptide levels. About 44% of the entrants in NYHA class 3 did not have a 1-year history of HF hospitalization.
That’s a more heterogeneous and potentially lower-risk cohort than patients in the randomized CHAMPION study of 11 years ago, which led to the implant’s approval on both sides of the Atlantic.
In that trial, CardioMEMS-guided management was followed by 30% drop in risk for HF hospitalization over 6 months (P < .001). But CHAMPION was limited to patients in NYHA class 3 with a history of HF hospitalization, the device’s current indication in both the United States and Europe.
The GUIDE-HF findings “reinforce that patients with class 3 heart failure and prior heart failure hospitalization are those in whom there is the clearest benefit, based on the prior CHAMPION trial. These are the patients where this monitoring strategy may be best targeted,” Gregg C. Fonarow, MD, University of California Los Angeles Medical Center, said in an interview.
Although GUIDE-HF didn’t show a significant benefit for NYHA class 2 patients with elevated biomarkers, who aren’t covered by the device’s current labeling, that group showed “some suggestions of potential benefit,” noted Dr. Fonarow, who isn’t a coauthor on the Lancet report. So, “there may be select patients with class 2 heart failure where monitoring could be considered on a case-by-case basis.”
In an interview, Larry A. Allen, MD, MHS, said that, “while the technology is pretty amazing, the real question is whether it tells us something that we didn’t already know that leads to improved care. Unfortunately, as tested here, it doesn’t, or at least not enough to make a big difference.”
The pre–COVID-19 impact analysis “should be interpreted with caution, and not as the primary finding,” Dr. Allen, from the University of Colorado at Denver, Aurora, who is not a GUIDE-HF coauthor, said in an interview.
One might hypothesize, he said, “that, in the setting of limited in-person visits with loss of physical examination, perhaps CardioMEMS would be more – not less – helpful during the pandemic. And yet the opposite was seen.”
The pandemic has “markedly altered all kinds of aspects of patient care and trial conduct, but that doesn’t make the data derived during that period uninformative,” Dr. Allen said. “And as we are increasingly reminded, the future will be a new normal, not a prepandemic normal.”
A third group
The GUIDE-HF trial includes, in addition to the 1,000 randomized patients, a single-group observational cohort of 2,600 patients, whose outcomes will be reported at another time, noted the published report.
But in the randomized comparison, conducted at 118 centers in North America, all patients were implanted with the CardioMEMS device and blinded as to their assigned strategy. Enrollment took place between March 2018 and Dec. 20, 2019.
Of the 1,000 successfully implanted patients, 497 were assigned to the pressure-guided strategy, in which “titration of diuretics was recommended if pulmonary artery pressure provided evidence of excess intravascular volume, and titration of vasodilators was recommended if elevated vascular resistance was evident,” the report stated.
The remaining 503 patients assigned to standard care served as control subjects, for whom “investigators were aware of treatment assignment but did not have access to PAP data.”
The hazard ratio for the primary endpoint in the pressure-guided group, compared with the control group, was 0.88 (95% confidence interval, 0.74-1.05; P = .16) over a median follow-up of 11.7 months.
But in the sensitivity analysis comparing outcomes before and after the COVID-19 lockdowns, using established methodology, the report stated, the primary-endpoint HR was 0.81 (95% CI, 0.66-1.00; P = .049).
The difference is owed to improved outcomes in the control group under pandemic conditions, the researchers concluded. Patients assigned to conventional management –whatever that meant during shelter-in-place – experienced 21% fewer primary-endpoint events than their own rate before the pandemic. After the COVID-19 emergency was declared, there was no significant difference in event rates between the two randomization groups.
In the primary 12-month analysis, the HR for HF events in the guided-therapy was not significant reduced, at 0.85 (95% CI, 0.70-1.03; P = .096). But in the pre-COVID-19 analysis, that risk fell significantly with CardioMEMS-guided management, for an HR of 0.76 (95% CI, 0.61-0.95; P = .014).
An editorial accompanying the GUIDE-HF publication (Lancet. 2021 Aug 27. doi: 10.1016/S0140-6736[21]01914-0) asserts that the trial “did not enroll an ideal group of patients for showing the efficacy of pulmonary artery pressure monitoring, since many had baseline pressures in the target range with little possibility of short-term gain.”
Also, wrote John G. F. Cleland, MD, PhD, University of Glasgow, and Pierpaolo Pellicori, MD, Imperial College London, “follow-up was too short, and interventions did not substantially change pulmonary artery pressure.”
They continue: “Monitoring alone cannot improve outcome, but consequent actions might. The GUIDE-HF results are encouraging but inconclusive, and should inform further research, possibly a large, simple, open-label trial to investigate a system of care rather than a single technology.”
GUIDE-HF was funded by Abbott. Dr. Lindenfeld disclosed receiving research grants from AstraZeneca, Sensible Medical, and Volumetrix; and consulting for Abbott, Alleviant Medical, AstraZeneca, Boehringer Ingelheim, Boston Scientific, CVRx, Edwards, Impulse Dynamics, and VWave. Dr. Fonarow reported consulting for Abbott and that his institution has participated in the GUIDE-HF trial; he has elsewhere disclosed consulting for Amgen, AstraZeneca, CHF Solutions Lifesciences, Janssen, Medtronic, and Novartis. Dr. Allen had elsewhere reported consulting for Abbott, Amgen, Boston Scientific, and Novartis. Dr. Cleland disclosed receiving personal fees from Abbott for serving on an advisory board for the MitraClip device, unrelated to the CardioMEMS device. Dr. Pellicori reported no relevant conflicts.
A version of this article first appeared on Medscape.com.
Medical therapy for heart failure guided by an implanted pulmonary artery pressure (PAP) sensor didn’t improve survival or risk for HF events like hospitalization over a year in a major randomized trial that entered a broad range of patients with mild to moderate disease.
But medical therapy adjustments based on PAP readings from the miniature CardioMEMS (Abbott) implant might well have surpassed conventional HF management for outcomes had the world not been turned upside down by SARS-CoV-2 and the pandemic lockdowns, assert researchers from the GUIDE-HF trial.
Something about the crisis, they concluded – although not without some pushback – led to better outcomes in the standard-care control group, apparently muddling any potential differences from those on PAP-guided management.
Working with regulators, the team conducted a “pre–COVID-19 impact analysis” that compared outcomes before the March 2020 national COVID-19 emergency declaration that forced much of the United States with shelter in place.
By that time, all of the trial’s patients had been followed for at least 3 months, and about three-fourths of its endpoints had already been counted, JoAnn Lindenfeld, MD, Vanderbilt University Medical Center, Nashville, Tenn., said at a media briefing prior to unveiling GUIDE-HF at the all-virtual European Society of Cardiology Congress 2021.
The pre–COVID-19 analysis, approved several months before the end of the trial – while the data were still blinded – had been “suggested by both regulatory agencies and professional societies in Europe and in the United States,” Dr. Lindenfeld said.
It pointed to a possible benefit for the CardioMEMS-guided strategy, a barely significant 19% drop in risk (P = .049) for the primary endpoint of death, HF hospitalization, or urgent HF hospital visit. The effect was driven by a 24% decline in HF events (P = .014), with no significant contribution from mortality.
“The benefits of hemodynamic monitoring and management in reducing heart failure hospitalizations extended to patients with less severe heart failure”; that is, those in New York Heart Association class 2 and any in NYHA class 3 with “elevated natriuretic peptides but no previous hospitalization,” said Dr. Lindenfeld, who is also lead author on the GUIDE-HF report published in the Lancet.
Such benefits would suggest that CardioMEMS-guided management can improve outcomes in an HF population much broader than the device’s current indication.
But as it happens, the trial’s prospectively defined 12-month primary outcomes were less impressive. A 12% decline in risk for the composite endpoint among patients managed by CardioMEMS failed to reach significance compared with standard management (P = .16).
“Several factors could explain the considerable loss of benefit of hemodynamic-guided management during the COVID-19 pandemic,” the Lancet report explained. They include “improved patient compliance with medical and dietary regimens, reduced respiratory infections, altered health-care provider behavior, changes in disease progression due to COVID-19, or other as yet unknown effects of a major pandemic.”
Expanded population
Importantly, GUIDE-HF had entered 1,000 patients in NYHA class 2-4 and either an HF hospitalization in the previous year or elevated natriuretic peptide levels. About 44% of the entrants in NYHA class 3 did not have a 1-year history of HF hospitalization.
That’s a more heterogeneous and potentially lower-risk cohort than patients in the randomized CHAMPION study of 11 years ago, which led to the implant’s approval on both sides of the Atlantic.
In that trial, CardioMEMS-guided management was followed by 30% drop in risk for HF hospitalization over 6 months (P < .001). But CHAMPION was limited to patients in NYHA class 3 with a history of HF hospitalization, the device’s current indication in both the United States and Europe.
The GUIDE-HF findings “reinforce that patients with class 3 heart failure and prior heart failure hospitalization are those in whom there is the clearest benefit, based on the prior CHAMPION trial. These are the patients where this monitoring strategy may be best targeted,” Gregg C. Fonarow, MD, University of California Los Angeles Medical Center, said in an interview.
Although GUIDE-HF didn’t show a significant benefit for NYHA class 2 patients with elevated biomarkers, who aren’t covered by the device’s current labeling, that group showed “some suggestions of potential benefit,” noted Dr. Fonarow, who isn’t a coauthor on the Lancet report. So, “there may be select patients with class 2 heart failure where monitoring could be considered on a case-by-case basis.”
In an interview, Larry A. Allen, MD, MHS, said that, “while the technology is pretty amazing, the real question is whether it tells us something that we didn’t already know that leads to improved care. Unfortunately, as tested here, it doesn’t, or at least not enough to make a big difference.”
The pre–COVID-19 impact analysis “should be interpreted with caution, and not as the primary finding,” Dr. Allen, from the University of Colorado at Denver, Aurora, who is not a GUIDE-HF coauthor, said in an interview.
One might hypothesize, he said, “that, in the setting of limited in-person visits with loss of physical examination, perhaps CardioMEMS would be more – not less – helpful during the pandemic. And yet the opposite was seen.”
The pandemic has “markedly altered all kinds of aspects of patient care and trial conduct, but that doesn’t make the data derived during that period uninformative,” Dr. Allen said. “And as we are increasingly reminded, the future will be a new normal, not a prepandemic normal.”
A third group
The GUIDE-HF trial includes, in addition to the 1,000 randomized patients, a single-group observational cohort of 2,600 patients, whose outcomes will be reported at another time, noted the published report.
But in the randomized comparison, conducted at 118 centers in North America, all patients were implanted with the CardioMEMS device and blinded as to their assigned strategy. Enrollment took place between March 2018 and Dec. 20, 2019.
Of the 1,000 successfully implanted patients, 497 were assigned to the pressure-guided strategy, in which “titration of diuretics was recommended if pulmonary artery pressure provided evidence of excess intravascular volume, and titration of vasodilators was recommended if elevated vascular resistance was evident,” the report stated.
The remaining 503 patients assigned to standard care served as control subjects, for whom “investigators were aware of treatment assignment but did not have access to PAP data.”
The hazard ratio for the primary endpoint in the pressure-guided group, compared with the control group, was 0.88 (95% confidence interval, 0.74-1.05; P = .16) over a median follow-up of 11.7 months.
But in the sensitivity analysis comparing outcomes before and after the COVID-19 lockdowns, using established methodology, the report stated, the primary-endpoint HR was 0.81 (95% CI, 0.66-1.00; P = .049).
The difference is owed to improved outcomes in the control group under pandemic conditions, the researchers concluded. Patients assigned to conventional management –whatever that meant during shelter-in-place – experienced 21% fewer primary-endpoint events than their own rate before the pandemic. After the COVID-19 emergency was declared, there was no significant difference in event rates between the two randomization groups.
In the primary 12-month analysis, the HR for HF events in the guided-therapy was not significant reduced, at 0.85 (95% CI, 0.70-1.03; P = .096). But in the pre-COVID-19 analysis, that risk fell significantly with CardioMEMS-guided management, for an HR of 0.76 (95% CI, 0.61-0.95; P = .014).
An editorial accompanying the GUIDE-HF publication (Lancet. 2021 Aug 27. doi: 10.1016/S0140-6736[21]01914-0) asserts that the trial “did not enroll an ideal group of patients for showing the efficacy of pulmonary artery pressure monitoring, since many had baseline pressures in the target range with little possibility of short-term gain.”
Also, wrote John G. F. Cleland, MD, PhD, University of Glasgow, and Pierpaolo Pellicori, MD, Imperial College London, “follow-up was too short, and interventions did not substantially change pulmonary artery pressure.”
They continue: “Monitoring alone cannot improve outcome, but consequent actions might. The GUIDE-HF results are encouraging but inconclusive, and should inform further research, possibly a large, simple, open-label trial to investigate a system of care rather than a single technology.”
GUIDE-HF was funded by Abbott. Dr. Lindenfeld disclosed receiving research grants from AstraZeneca, Sensible Medical, and Volumetrix; and consulting for Abbott, Alleviant Medical, AstraZeneca, Boehringer Ingelheim, Boston Scientific, CVRx, Edwards, Impulse Dynamics, and VWave. Dr. Fonarow reported consulting for Abbott and that his institution has participated in the GUIDE-HF trial; he has elsewhere disclosed consulting for Amgen, AstraZeneca, CHF Solutions Lifesciences, Janssen, Medtronic, and Novartis. Dr. Allen had elsewhere reported consulting for Abbott, Amgen, Boston Scientific, and Novartis. Dr. Cleland disclosed receiving personal fees from Abbott for serving on an advisory board for the MitraClip device, unrelated to the CardioMEMS device. Dr. Pellicori reported no relevant conflicts.
A version of this article first appeared on Medscape.com.
Eyes on ESC ‘21: Hope for EMPEROR-Preserved, guidelines remade
There will be so much more to the annual congress of the European Society of Cardiology, which begins Aug. 27 with an all-virtual format, than detailed primary results of EMPEROR-Preserved, a trial that could mark a turning point for heart failure (HF) medical therapy.
Also among the featured Hot Line and Late-Breaking Science sessions are – along with many other studies – explorations of arrhythmia management (ablation or guided by loop recorder); secondary prevention, including by vaccination; oral anticoagulation, notably after transcatheter valve procedures; and colchicine or thrombosis prophylaxis in hospitalized patients with COVID-19.
There will even be a head-to-head comparison of two long-familiar left atrial appendage (LAA) occluders, and a population-based, randomized trial of sodium restriction through wide-scale use of a potassium-based salt substitute.
The congress will also introduce four guideline documents at sessions throughout the Congress, one on each day. They cover new and modified recommendations for heart failure; pacing, including cardiac resynchronization therapy (CRT); cardiovascular (CV) disease prevention; and, with cosponsorship from the European Association for Cardio-Thoracic Surgery, valvular heart disease.
The virtues of virtual
That next year’s Congress is slated for Aug. 27-30 in Barcelona should be welcome news for anyone whose “what if” curiosity about all-virtual conferences has already been satisfied. But with experience comes wisdom, as the medical societies have learned that online scientific meetings have some winning qualities that may be worth keeping, as least for a while.
“I think there is no doubt that the digital format will continue, for several reasons. One is that this pandemic is not over,” ESC Congress program committee chair Stephan Windecker, MD, Bern (Switzerland) University Hospital, , told this news organization. “As long as it is not over, the digital format is here to stay.”
But it also appears that people who haven’t been able to attend the congress in person are keen to log in and engage online, Dr. Windecker said. The 2020 all-virtual conference drew a much younger pool of registrants, on average, than did the live conferences before the pandemic.
“I think that’s an indication of people that may be in training, in early stages of their career, or they don’t have the support from departments or from their practice, or other financial means.” But they are able to participate via computer, tablet, or smartphone, he said.
“Another advantage is that the recorded content can be replayed at the convenience of whoever wants to consume it at a later point in time,” he added. “Those are just some examples why the digital format is likely to stay,” on its own or in a new age of hybrid meetings.
New and updated guidelines
Leading off the guideline series is the document on diagnosis and treatment of acute and chronic HF, which leveraged the past few busy years of HF clinical trials to arrive at a number of new recommendations and strengthened level-of-evidence ratings. It covers both drug and device therapy of HF with reduced ejection fraction (HFrEF) and acute decompensated HF, and tweaks and further enshrines the concept of HF with mildly reduced ejection fraction (HFmrEF).
Several updated recommendations for both long-used and novel medications, notably the sodium-glucose cotransporter 2 inhibitors, will be included because of the recently appreciated evidence-based impact in HFrEF, Dr. Windecker noted.
“I think it will be particularly interesting to look for the SGLT2 inhibitors as not a completely new class of drugs, but certainly one where there has been a lot of new evidence, to look at how those drugs will be integrated in the overall care pathway.”
A top-line preview of the new HF guideline limited to drug therapy, presented at July’s Heart Failure Association of the European Society of Cardiology (ESC-HFA), provided a simple answer to a common question in the new, bountiful age of HFrEF medications: Which meds, initiated in what order?
As it happens, the new recommendation for first-line HFrEF drug therapy is not a silver bullet, but a shotgun – prompt initiation of at least four meds, one from each of four drug classes: renin-angiotensin system inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and SGLT2 inhibitors. Each class, as described in the document, is to be started as soon as safely feasible, in a sequence deemed appropriate for each individual patient.
Spotlight on EMPEROR-Preserved
The world already knows that the trial, which tested the SGLT2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) on top of standard therapy, “met” its primary endpoint in almost 6,000 patients with HF with preserved ejection fraction (HFpEF), who included some with HFmrEF by more contemporary definitions.
That means patients in EMPEROR-Preserved assigned to take empagliflozin showed significantly fewer events that made up the study’s primary endpoint, a composite of CV death or HF hospitalization. It appears to be the first clearly significant overall medical therapy benefit for a clinical primary endpoint in a major randomized HFpEF drug trial.
And that, pending fuller presentation of trial results at the Congress on Aug. 27, could be a huge deal for the half of HF patients with left ventricular ejection fractions (LVEF) higher than the HFrEF range.
Those early top-line results weren’t a decisive bombshell for a field now filled with hope for a practice-changing empagliflozin outcome in EMPEROR-Preserved, which isn’t a certainty. They were more like the “boom” of a mortar launching a rocket of fireworks that may explode into a chrysanthemum or green comet or, sometimes, turn out to be no more than a dud. The promise of the early cursory results critically depends on further details.
“Provided there is a compelling benefit, this is what everyone has been waiting for in this condition for decades,” Mikhail N. Kosiborod, MD, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo., said.
“Already knowing that the trial met the primary endpoint is obviously very intriguing and encouraging,” he added. “But there are things we don’t know, such as: What is the magnitude of benefit? And whether that benefit, whatever the magnitude, is driven by reductions in both heart failure hospitalizations and cardiovascular death, or only one of the two.”
For example: “If we see an impressive benefit for reduction of hospitalizations, but not a significant reduction in death, that would still be a huge advance. That’s because, to date, we don’t have any drug for HFpEF that has convincingly demonstrated a compelling reduction in heart failure hospitalization or improvement in symptoms, function, or quality of life,” observed Dr. Kosiborod, who wasn’t part of EMPEROR-Preserved.
There have been “suggestions” from HFrEF trials that empagliflozin and dapagliflozin (Farxiga, AstraZeneca) “have very comparable effects on at least the endpoint of cardiovascular death or hospitalization for heart failure,” he said. “So, my expectation would be that whatever is observed in EMPEROR-Preserved is likely a class effect, as well.”
Following EMPEROR-Preserved on the agenda is EMPEROR-Pooled, a patient-level combined analysis of the EMPEROR series of trials that spans the range of HF, regardless of ejection fraction or diabetes status, primarily exploring the effects of empagliflozin on renal function.
Other offerings, Friday, Aug. 27
Scheduled immediately after EMPEROR-Preserved is a presentation on the SMART-MI trial, which should clarify whether management guided by continuous ambulatory monitoring is effective in patients considered at especially high arrhythmic risk. Entry called for recent myocardial infarction and an LVEF of 36%-50% with evidence of cardiac autonomic dysfunction.
The trial randomly assigned 400 such patients to be or not be implanted with a Reveal LINQ (Medtronic) loop recorder and followed them for up to 18 months, primarily for detection of potentially serious arrhythmic events. Endpoints that involved mortality, hospitalization or other clinical events were secondary.
In a time slot preceding both SMART-MI and EMPEROR-Preserved, the GUIDE-HF trial is following a projected 3,600 patients with HF implanted with a CardioMEMS HF System (Abbott) pulmonary artery (PA) pressure sensor to explore the its value for guiding management.
The trial’s three cohorts, followed for at least 12 months, include randomized sensor-monitored and control groups of patients with New York Heart Association class 2-4 symptoms, as well as a third observational set of patients in NYHA class 3. That’s the indication for which the CardioMEMS monitor gained approval in the United States in 2014 based on the 2011 CHAMPION trial, and which fared just as well in the 2017 CHAMPION Post-Approval Study.
The Friday Hot Lines also include Dal-GenE, which has entered about 6,000 patients with recent MI to test the once-abandoned cholesterol ester transfer protein (CETP) inhibitor dalcetrapib (DalCor) for any secondary-prevention benefits when used selectively. The trial’s hook: All its patients are confirmed to have the AA genotype of the rs1967309 variant in the ADCY9 gene, which has been associated with a pronounced clinical response to CETP inhibition.
Saturday, Aug. 28
The direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists in patients with nonvalvular atrial fibrillation (AFib). But whether DOACs are similarly preferable in the growing world population of people who have undergone transcatheter aortic valve replacement (TAVR or TAVI), an issue explored with variable results in the ATLANTIS and GALILEO trials, is far from settled.
The ENVISAGE-TAVI AF trial explored the question for the factor X inhibitor edoxaban (Savaysa, Lixiana, Daiichi-Sankyo) in 1,400 patients with AFib and a transfemoral TAVR in the previous 5 days, who were randomly assigned to the DOAC or standard management along with discretionary antiplatelet therapy. They’ve been followed for up to 3 years for a composite endpoint of clinical events – including death, MI, and stroke – and for major bleeding.
The day will also feature MASTER DAPT, a comparison of two dual-antiplatelet therapy (DAPT) regimens in an estimated 4,300 patients considered to be high-risk for bleeding who had received the sirolimus-eluting Ultimaster (Terumo) coronary stent, which has a bioresorbable polymer coating.
Investigators have randomly assigned patients to receive either very-short-duration DAPT, for about a month after stenting, followed by a P2Y12 inhibitor alone for up to a year after the procedure; or a more conventional regimen of a P2Y12 inhibitor for 6-12 months with aspirin maintained for a total of 12 months.
Later that day, investigators from the FIGARO-DKD trial will present their results based on 7,437 patients with type 2 diabetes and chronic kidney disease (CKD), a much fuller version than the top-line findings announced by sponsor Bayer 3 months ago.
Those top-line results suggested that patients assigned to receive the nonsteroidal nonselective mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) on top of standard care benefited with a drop in risk for the primary endpoint of CV death or nonfatal CV events.
Finerenone was recently approved in the United States for treating patients with both type 2 diabetes and CKD based on the published FIDELIO-DKD trial, which had seen less CKD progression and fewer CV events in such patients who took the novel MRA.
Although similar in design to FIGARO-DKD, FIDELIO-DKD had entered fewer patients with early-stage diabetic kidney disease (DKD). That led researchers to pool the two trials’ populations to create a cohort that spans the spectrum of DKD severity. An analysis of the pooled cohort, dubbed FIDELITY, is on the schedule after FIGARO-DKD.
After FIDELITY is the prospective APAF-CRT trial that is following a projected 1,830 patients with permanent, symptomatic AFib and a recent hospitalization for AFib or HF and who were not good candidates for standard ablation. They were assigned to receive either atrioventricular junctional ablation followed by CRT, with or without a defibrillation, on top of optimal meds – a so-called “ablate-and-pace” strategy – or an implantable cardioverter defibrillator with rate-control drug therapy.
The new analysis represents the trial’s second phase in which mortality was followed for 4 years as the primary endpoint, in contrast to the previously reported initial phase that followed the first 102 patients for 2 years for the composite primary endpoint of death, worsening HF, and HF hospitalization. The first phase had halted enrollment before reaching its planned target of 280 patients after an interim analysis showed a significant benefit for ablate and pace.
Next up: DECAAF 2, a randomized assessment of whether catheter ablation for AFib guided by delayed gadolinium enhancement on MRI, a proxy for scar tissue, can be more effective than standard AFib ablation by pulmonary vein isolation alone. An estimated 900 patients with persistent AFib who had never before undergone ablation for the arrhythmia were randomly assigned to one strategy or the other and followed for AFib recurrence over 18 months.
Sunday, Aug. 29
The TOMAHAWK trial aimed to clarify the optimal timing of invasive coronary angiography for resuscitated patients with non–ST-segment elevation out-of-hospital cardiac arrest, a broad population in a setting for which there is little randomized-trial guidance. Investigators randomly assigned 558 such patients to undergo immediate invasive angiography or to direct intensive care unit admission for initial standard care with discretionary delayed angiography. Patients were followed for all-cause mortality, with other clinical events and neurologic outcomes as secondary endpoints.
Next on the schedule, the RIPCORD-2 trial randomly assigned 1,100 patients with stable known or suspected coronary artery disease (CAD) to undergo conventional angiography alone or with added direct pressure-wire measurement of fractional flow reserve to guide management decisions. Primary outcomes include health care costs and patient-reported quality of life at 1 year.
Slated for later that day, the Asymptomatic Carotid Surgery Trial-2 (ACST-2) has entered an estimated 3600 patients with a substantial carotid artery narrowing not associated with symptoms but for which either carotid endarterectomy (CEA) or carotid artery stenting (CAS) was considered anatomically feasible. There also must have been “substantial uncertainty” regarding the optimal procedure choice.
The trial, conducted in 40 countries primarily in Europe and North America and launched in 2008, randomly assigned the patients to undergo either CEA or CAS, in both cases with appropriate medical therapy, and followed them for periprocedural events and up to 10 years for strokes and stroke-related events.
The LOOP study, which is to directly follow ACST-2, has explored whether screening for AFib using the Medtronic Reveal LINQ monitor in older patients with non-AFib stroke risk factors – with oral anticoagulation prescribed for those who test positive – can lower their risk for stroke or systemic embolism. It randomly assigned 6,000 such patients to care guided by the loop recorder or to standard care.
On a somewhat larger scale, the Salt Substitute and Stroke Study (SSaSS) randomly assigned a total of 20,996 people in about 600 villages across northern China and Tibet to sodium-restriction intervention and control groups by village. All participants had a history of stroke or were aged at least 60 years with uncontrolled hypertension.
As described by the trial’s online portal, participants in villages assigned to the intervention group were given a supply of a low-sodium, potassium-supplementing salt substitute to replace their own salt supplies, along with education on the health benefits of sodium restriction. Participants in control villages continued their normal diets and, at the trial’s beginning, received “advice to reduce their salt intake.” All were required to own a telephone.
Clinical events, including strokes and hospitalizations throughout a 5-year follow-up, were tracked by phone calls made to all participants every 6 months and were documented at follow-up home visits.
Sunday is also to feature a Late-Breaking Trials session with a focus on COVID-19, which leads off with COLCOVID, a test of colchicine in patients hospitalized for suspected SARS-CoV-2 infection and in acute respiratory distress.
The 1,279 participants in Argentina were randomly assigned to receive or not receive the potent anti-inflammatory agent on top of antivirals and other standard management and followed for death or new need for mechanical ventilation. A successful outcome would contrast with the RECOVERY trial, which terminated a colchicine group of patients hospitalized with COVID-19 because of a lack of efficacy earlier this year.
COLCOVID is to be followed by the MICHELLE trial of rivaroxaban (Xarelto, Bayer/Janssen) prophylaxis, compared with no preventive oral anticoagulant, in 320 patients who, when hospitalized with COVID-19, had been on parenteral anticoagulants because of an elevated risk for venous thromboembolism. The trial, conducted in Brazil, called for postdischarge rivaroxaban at a once-daily dosage of 10 mg for about 1 month.
The session also includes a presentation called “Insights into the Effects of the COVID-19 Pandemic: Comprehensive Analysis from the GUIDE-HF Trial,” the primary outcomes of which will be reported on the first day of the Congress.
Following is a presentation on the PREPARE-IT study of icosapent ethyl (Vascepa, Amarin), given at high dosages intended to be anti-inflammatory, compared with placebo, in an estimated 4,000 adults. The trial has two groups: A prevention group of adults living and circulating in the community; and a treatment group of patients aged at least 40 years with confirmed symptomatic SARS-CoV-2 infection for whom the need for hospitalization isn’t clear.
Monday, Aug. 30
The final day of the Congress features a trial called Influenza Vaccination after Myocardial Infarction (IAMI), which has tested the secondary preventive effect of influenza vaccination by randomly assigning 2,571 patients to receive a standard vaccine or a saline placebo injection on one occasion.
Entry to the international trial called for a diagnosis of MI with or without ST-segment elevation, or stable CAD and age at least 75 years with other risk factors. The patients were followed for death, MI, stent thrombosis, and a slew of secondary endpoints over 12 months.
Monday offerings continue later in a time block leading off with the STEP trial, which has randomly assigned an estimated 8,000 patients at 40 centers in China who are 60 to 80 years of age with a systolic blood pressure of 140 to <190 mm Hg to be on standard guideline-based therapy or an intensive drug-management strategy.
The systolic BP goals are 130 to <150 mm Hg for standard care and 110 to <130 mm Hg for the intensive regimen. The composite primary endpoint includes death and clinical events related to acute coronary syndromes, HF, revascularization, and stroke.
Following on heels of STEP, the Amulet IDE trial – the first major randomized comparison of two transcatheter LAA closure devices – entered 1,878 patients with nonvalvular AFib who were considered high-risk for bleeding and stroke or systemic embolism.
They were randomly assigned in the noninferiority trial to receive either the AMPLATZER Amulet (Abbott Medical Devices) or the WATCHMAN (Boston Scientific) closure devices and were followed for safety and efficacy for up to 5 years.
Both LAA closure devices, intended to make patients with AFib less reliant on oral anticoagulation, are now available on both sides of the Atlantic – as well as many other countries – after the Amulet’s United States market approval on Aug. 16, based largely on the Amulet IDE trial.
Rounding out the final Hot Line set is one of the latest efforts to show the efficacy and safety of a very short DAPT period after coronary stenting in patients with acute coronary syndromes, the STOPDAPT-2 ACS trial.
The study assigned 3,008 patients in Japan to receive aspirin and clopidogrel for either 1 month or 1 year after implantation with an everolimus-eluting cobalt-chromium stent and followed them for up to 5 years for a composite of MI, CV death, stent thrombosis, stroke, and bleeding.
The trial follows the published STOPDAPT-2 trial that showed superiority for the 1-month DAPT regimen in a predominantly stable-CAD population treated with the same kind of stent.
Program structure and format
A total of 15 online channels are to be available in the morning, European time, their schedules running in parallel. Presentations often are prerecorded, but also include live sessions at 8:00 a.m. Central time and 12 p.m. CET (2:00 a.m. and 6:00 a.m. Eastern time) to liven up the channel offerings, Dr. Windecker observed, and to make them more immediate and potentially interactive.
Many of the parallel channels are devoted throughout the Congress to particular silos of cardiology; for example, arrhythmias and device therapy is on channel 3; CAD and acute care is on 5; HF is on 6; and preventive cardiology is on 9.
Other channels swing across different topics from day to day, such as channel 1, which covers COVID-19 topics on the first and third day of the meeting, “advances in science” on day 2, and “digital health, public health, health economics” on day 4.
The focus each day, starting at 2:00 p.m. CET (8:00 a.m. ET) and continuing into the evening in Europe, shifts over to the Prime Time live program, which features the Hot Line and guideline presentations and many of the live abstract presentations.
Dr. Kosiborod, not a researcher with the EMPEROR trials, is chair of the Dapagliflozin in Preserved Ejection Fraction Heart Failure ( PRESERVED-HF ) trial, which is scheduled for presentation at the September 2021 Heart Failure Society of American meeting.
A version of this article first appeared on Medscape.com.
There will be so much more to the annual congress of the European Society of Cardiology, which begins Aug. 27 with an all-virtual format, than detailed primary results of EMPEROR-Preserved, a trial that could mark a turning point for heart failure (HF) medical therapy.
Also among the featured Hot Line and Late-Breaking Science sessions are – along with many other studies – explorations of arrhythmia management (ablation or guided by loop recorder); secondary prevention, including by vaccination; oral anticoagulation, notably after transcatheter valve procedures; and colchicine or thrombosis prophylaxis in hospitalized patients with COVID-19.
There will even be a head-to-head comparison of two long-familiar left atrial appendage (LAA) occluders, and a population-based, randomized trial of sodium restriction through wide-scale use of a potassium-based salt substitute.
The congress will also introduce four guideline documents at sessions throughout the Congress, one on each day. They cover new and modified recommendations for heart failure; pacing, including cardiac resynchronization therapy (CRT); cardiovascular (CV) disease prevention; and, with cosponsorship from the European Association for Cardio-Thoracic Surgery, valvular heart disease.
The virtues of virtual
That next year’s Congress is slated for Aug. 27-30 in Barcelona should be welcome news for anyone whose “what if” curiosity about all-virtual conferences has already been satisfied. But with experience comes wisdom, as the medical societies have learned that online scientific meetings have some winning qualities that may be worth keeping, as least for a while.
“I think there is no doubt that the digital format will continue, for several reasons. One is that this pandemic is not over,” ESC Congress program committee chair Stephan Windecker, MD, Bern (Switzerland) University Hospital, , told this news organization. “As long as it is not over, the digital format is here to stay.”
But it also appears that people who haven’t been able to attend the congress in person are keen to log in and engage online, Dr. Windecker said. The 2020 all-virtual conference drew a much younger pool of registrants, on average, than did the live conferences before the pandemic.
“I think that’s an indication of people that may be in training, in early stages of their career, or they don’t have the support from departments or from their practice, or other financial means.” But they are able to participate via computer, tablet, or smartphone, he said.
“Another advantage is that the recorded content can be replayed at the convenience of whoever wants to consume it at a later point in time,” he added. “Those are just some examples why the digital format is likely to stay,” on its own or in a new age of hybrid meetings.
New and updated guidelines
Leading off the guideline series is the document on diagnosis and treatment of acute and chronic HF, which leveraged the past few busy years of HF clinical trials to arrive at a number of new recommendations and strengthened level-of-evidence ratings. It covers both drug and device therapy of HF with reduced ejection fraction (HFrEF) and acute decompensated HF, and tweaks and further enshrines the concept of HF with mildly reduced ejection fraction (HFmrEF).
Several updated recommendations for both long-used and novel medications, notably the sodium-glucose cotransporter 2 inhibitors, will be included because of the recently appreciated evidence-based impact in HFrEF, Dr. Windecker noted.
“I think it will be particularly interesting to look for the SGLT2 inhibitors as not a completely new class of drugs, but certainly one where there has been a lot of new evidence, to look at how those drugs will be integrated in the overall care pathway.”
A top-line preview of the new HF guideline limited to drug therapy, presented at July’s Heart Failure Association of the European Society of Cardiology (ESC-HFA), provided a simple answer to a common question in the new, bountiful age of HFrEF medications: Which meds, initiated in what order?
As it happens, the new recommendation for first-line HFrEF drug therapy is not a silver bullet, but a shotgun – prompt initiation of at least four meds, one from each of four drug classes: renin-angiotensin system inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and SGLT2 inhibitors. Each class, as described in the document, is to be started as soon as safely feasible, in a sequence deemed appropriate for each individual patient.
Spotlight on EMPEROR-Preserved
The world already knows that the trial, which tested the SGLT2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) on top of standard therapy, “met” its primary endpoint in almost 6,000 patients with HF with preserved ejection fraction (HFpEF), who included some with HFmrEF by more contemporary definitions.
That means patients in EMPEROR-Preserved assigned to take empagliflozin showed significantly fewer events that made up the study’s primary endpoint, a composite of CV death or HF hospitalization. It appears to be the first clearly significant overall medical therapy benefit for a clinical primary endpoint in a major randomized HFpEF drug trial.
And that, pending fuller presentation of trial results at the Congress on Aug. 27, could be a huge deal for the half of HF patients with left ventricular ejection fractions (LVEF) higher than the HFrEF range.
Those early top-line results weren’t a decisive bombshell for a field now filled with hope for a practice-changing empagliflozin outcome in EMPEROR-Preserved, which isn’t a certainty. They were more like the “boom” of a mortar launching a rocket of fireworks that may explode into a chrysanthemum or green comet or, sometimes, turn out to be no more than a dud. The promise of the early cursory results critically depends on further details.
“Provided there is a compelling benefit, this is what everyone has been waiting for in this condition for decades,” Mikhail N. Kosiborod, MD, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo., said.
“Already knowing that the trial met the primary endpoint is obviously very intriguing and encouraging,” he added. “But there are things we don’t know, such as: What is the magnitude of benefit? And whether that benefit, whatever the magnitude, is driven by reductions in both heart failure hospitalizations and cardiovascular death, or only one of the two.”
For example: “If we see an impressive benefit for reduction of hospitalizations, but not a significant reduction in death, that would still be a huge advance. That’s because, to date, we don’t have any drug for HFpEF that has convincingly demonstrated a compelling reduction in heart failure hospitalization or improvement in symptoms, function, or quality of life,” observed Dr. Kosiborod, who wasn’t part of EMPEROR-Preserved.
There have been “suggestions” from HFrEF trials that empagliflozin and dapagliflozin (Farxiga, AstraZeneca) “have very comparable effects on at least the endpoint of cardiovascular death or hospitalization for heart failure,” he said. “So, my expectation would be that whatever is observed in EMPEROR-Preserved is likely a class effect, as well.”
Following EMPEROR-Preserved on the agenda is EMPEROR-Pooled, a patient-level combined analysis of the EMPEROR series of trials that spans the range of HF, regardless of ejection fraction or diabetes status, primarily exploring the effects of empagliflozin on renal function.
Other offerings, Friday, Aug. 27
Scheduled immediately after EMPEROR-Preserved is a presentation on the SMART-MI trial, which should clarify whether management guided by continuous ambulatory monitoring is effective in patients considered at especially high arrhythmic risk. Entry called for recent myocardial infarction and an LVEF of 36%-50% with evidence of cardiac autonomic dysfunction.
The trial randomly assigned 400 such patients to be or not be implanted with a Reveal LINQ (Medtronic) loop recorder and followed them for up to 18 months, primarily for detection of potentially serious arrhythmic events. Endpoints that involved mortality, hospitalization or other clinical events were secondary.
In a time slot preceding both SMART-MI and EMPEROR-Preserved, the GUIDE-HF trial is following a projected 3,600 patients with HF implanted with a CardioMEMS HF System (Abbott) pulmonary artery (PA) pressure sensor to explore the its value for guiding management.
The trial’s three cohorts, followed for at least 12 months, include randomized sensor-monitored and control groups of patients with New York Heart Association class 2-4 symptoms, as well as a third observational set of patients in NYHA class 3. That’s the indication for which the CardioMEMS monitor gained approval in the United States in 2014 based on the 2011 CHAMPION trial, and which fared just as well in the 2017 CHAMPION Post-Approval Study.
The Friday Hot Lines also include Dal-GenE, which has entered about 6,000 patients with recent MI to test the once-abandoned cholesterol ester transfer protein (CETP) inhibitor dalcetrapib (DalCor) for any secondary-prevention benefits when used selectively. The trial’s hook: All its patients are confirmed to have the AA genotype of the rs1967309 variant in the ADCY9 gene, which has been associated with a pronounced clinical response to CETP inhibition.
Saturday, Aug. 28
The direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists in patients with nonvalvular atrial fibrillation (AFib). But whether DOACs are similarly preferable in the growing world population of people who have undergone transcatheter aortic valve replacement (TAVR or TAVI), an issue explored with variable results in the ATLANTIS and GALILEO trials, is far from settled.
The ENVISAGE-TAVI AF trial explored the question for the factor X inhibitor edoxaban (Savaysa, Lixiana, Daiichi-Sankyo) in 1,400 patients with AFib and a transfemoral TAVR in the previous 5 days, who were randomly assigned to the DOAC or standard management along with discretionary antiplatelet therapy. They’ve been followed for up to 3 years for a composite endpoint of clinical events – including death, MI, and stroke – and for major bleeding.
The day will also feature MASTER DAPT, a comparison of two dual-antiplatelet therapy (DAPT) regimens in an estimated 4,300 patients considered to be high-risk for bleeding who had received the sirolimus-eluting Ultimaster (Terumo) coronary stent, which has a bioresorbable polymer coating.
Investigators have randomly assigned patients to receive either very-short-duration DAPT, for about a month after stenting, followed by a P2Y12 inhibitor alone for up to a year after the procedure; or a more conventional regimen of a P2Y12 inhibitor for 6-12 months with aspirin maintained for a total of 12 months.
Later that day, investigators from the FIGARO-DKD trial will present their results based on 7,437 patients with type 2 diabetes and chronic kidney disease (CKD), a much fuller version than the top-line findings announced by sponsor Bayer 3 months ago.
Those top-line results suggested that patients assigned to receive the nonsteroidal nonselective mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) on top of standard care benefited with a drop in risk for the primary endpoint of CV death or nonfatal CV events.
Finerenone was recently approved in the United States for treating patients with both type 2 diabetes and CKD based on the published FIDELIO-DKD trial, which had seen less CKD progression and fewer CV events in such patients who took the novel MRA.
Although similar in design to FIGARO-DKD, FIDELIO-DKD had entered fewer patients with early-stage diabetic kidney disease (DKD). That led researchers to pool the two trials’ populations to create a cohort that spans the spectrum of DKD severity. An analysis of the pooled cohort, dubbed FIDELITY, is on the schedule after FIGARO-DKD.
After FIDELITY is the prospective APAF-CRT trial that is following a projected 1,830 patients with permanent, symptomatic AFib and a recent hospitalization for AFib or HF and who were not good candidates for standard ablation. They were assigned to receive either atrioventricular junctional ablation followed by CRT, with or without a defibrillation, on top of optimal meds – a so-called “ablate-and-pace” strategy – or an implantable cardioverter defibrillator with rate-control drug therapy.
The new analysis represents the trial’s second phase in which mortality was followed for 4 years as the primary endpoint, in contrast to the previously reported initial phase that followed the first 102 patients for 2 years for the composite primary endpoint of death, worsening HF, and HF hospitalization. The first phase had halted enrollment before reaching its planned target of 280 patients after an interim analysis showed a significant benefit for ablate and pace.
Next up: DECAAF 2, a randomized assessment of whether catheter ablation for AFib guided by delayed gadolinium enhancement on MRI, a proxy for scar tissue, can be more effective than standard AFib ablation by pulmonary vein isolation alone. An estimated 900 patients with persistent AFib who had never before undergone ablation for the arrhythmia were randomly assigned to one strategy or the other and followed for AFib recurrence over 18 months.
Sunday, Aug. 29
The TOMAHAWK trial aimed to clarify the optimal timing of invasive coronary angiography for resuscitated patients with non–ST-segment elevation out-of-hospital cardiac arrest, a broad population in a setting for which there is little randomized-trial guidance. Investigators randomly assigned 558 such patients to undergo immediate invasive angiography or to direct intensive care unit admission for initial standard care with discretionary delayed angiography. Patients were followed for all-cause mortality, with other clinical events and neurologic outcomes as secondary endpoints.
Next on the schedule, the RIPCORD-2 trial randomly assigned 1,100 patients with stable known or suspected coronary artery disease (CAD) to undergo conventional angiography alone or with added direct pressure-wire measurement of fractional flow reserve to guide management decisions. Primary outcomes include health care costs and patient-reported quality of life at 1 year.
Slated for later that day, the Asymptomatic Carotid Surgery Trial-2 (ACST-2) has entered an estimated 3600 patients with a substantial carotid artery narrowing not associated with symptoms but for which either carotid endarterectomy (CEA) or carotid artery stenting (CAS) was considered anatomically feasible. There also must have been “substantial uncertainty” regarding the optimal procedure choice.
The trial, conducted in 40 countries primarily in Europe and North America and launched in 2008, randomly assigned the patients to undergo either CEA or CAS, in both cases with appropriate medical therapy, and followed them for periprocedural events and up to 10 years for strokes and stroke-related events.
The LOOP study, which is to directly follow ACST-2, has explored whether screening for AFib using the Medtronic Reveal LINQ monitor in older patients with non-AFib stroke risk factors – with oral anticoagulation prescribed for those who test positive – can lower their risk for stroke or systemic embolism. It randomly assigned 6,000 such patients to care guided by the loop recorder or to standard care.
On a somewhat larger scale, the Salt Substitute and Stroke Study (SSaSS) randomly assigned a total of 20,996 people in about 600 villages across northern China and Tibet to sodium-restriction intervention and control groups by village. All participants had a history of stroke or were aged at least 60 years with uncontrolled hypertension.
As described by the trial’s online portal, participants in villages assigned to the intervention group were given a supply of a low-sodium, potassium-supplementing salt substitute to replace their own salt supplies, along with education on the health benefits of sodium restriction. Participants in control villages continued their normal diets and, at the trial’s beginning, received “advice to reduce their salt intake.” All were required to own a telephone.
Clinical events, including strokes and hospitalizations throughout a 5-year follow-up, were tracked by phone calls made to all participants every 6 months and were documented at follow-up home visits.
Sunday is also to feature a Late-Breaking Trials session with a focus on COVID-19, which leads off with COLCOVID, a test of colchicine in patients hospitalized for suspected SARS-CoV-2 infection and in acute respiratory distress.
The 1,279 participants in Argentina were randomly assigned to receive or not receive the potent anti-inflammatory agent on top of antivirals and other standard management and followed for death or new need for mechanical ventilation. A successful outcome would contrast with the RECOVERY trial, which terminated a colchicine group of patients hospitalized with COVID-19 because of a lack of efficacy earlier this year.
COLCOVID is to be followed by the MICHELLE trial of rivaroxaban (Xarelto, Bayer/Janssen) prophylaxis, compared with no preventive oral anticoagulant, in 320 patients who, when hospitalized with COVID-19, had been on parenteral anticoagulants because of an elevated risk for venous thromboembolism. The trial, conducted in Brazil, called for postdischarge rivaroxaban at a once-daily dosage of 10 mg for about 1 month.
The session also includes a presentation called “Insights into the Effects of the COVID-19 Pandemic: Comprehensive Analysis from the GUIDE-HF Trial,” the primary outcomes of which will be reported on the first day of the Congress.
Following is a presentation on the PREPARE-IT study of icosapent ethyl (Vascepa, Amarin), given at high dosages intended to be anti-inflammatory, compared with placebo, in an estimated 4,000 adults. The trial has two groups: A prevention group of adults living and circulating in the community; and a treatment group of patients aged at least 40 years with confirmed symptomatic SARS-CoV-2 infection for whom the need for hospitalization isn’t clear.
Monday, Aug. 30
The final day of the Congress features a trial called Influenza Vaccination after Myocardial Infarction (IAMI), which has tested the secondary preventive effect of influenza vaccination by randomly assigning 2,571 patients to receive a standard vaccine or a saline placebo injection on one occasion.
Entry to the international trial called for a diagnosis of MI with or without ST-segment elevation, or stable CAD and age at least 75 years with other risk factors. The patients were followed for death, MI, stent thrombosis, and a slew of secondary endpoints over 12 months.
Monday offerings continue later in a time block leading off with the STEP trial, which has randomly assigned an estimated 8,000 patients at 40 centers in China who are 60 to 80 years of age with a systolic blood pressure of 140 to <190 mm Hg to be on standard guideline-based therapy or an intensive drug-management strategy.
The systolic BP goals are 130 to <150 mm Hg for standard care and 110 to <130 mm Hg for the intensive regimen. The composite primary endpoint includes death and clinical events related to acute coronary syndromes, HF, revascularization, and stroke.
Following on heels of STEP, the Amulet IDE trial – the first major randomized comparison of two transcatheter LAA closure devices – entered 1,878 patients with nonvalvular AFib who were considered high-risk for bleeding and stroke or systemic embolism.
They were randomly assigned in the noninferiority trial to receive either the AMPLATZER Amulet (Abbott Medical Devices) or the WATCHMAN (Boston Scientific) closure devices and were followed for safety and efficacy for up to 5 years.
Both LAA closure devices, intended to make patients with AFib less reliant on oral anticoagulation, are now available on both sides of the Atlantic – as well as many other countries – after the Amulet’s United States market approval on Aug. 16, based largely on the Amulet IDE trial.
Rounding out the final Hot Line set is one of the latest efforts to show the efficacy and safety of a very short DAPT period after coronary stenting in patients with acute coronary syndromes, the STOPDAPT-2 ACS trial.
The study assigned 3,008 patients in Japan to receive aspirin and clopidogrel for either 1 month or 1 year after implantation with an everolimus-eluting cobalt-chromium stent and followed them for up to 5 years for a composite of MI, CV death, stent thrombosis, stroke, and bleeding.
The trial follows the published STOPDAPT-2 trial that showed superiority for the 1-month DAPT regimen in a predominantly stable-CAD population treated with the same kind of stent.
Program structure and format
A total of 15 online channels are to be available in the morning, European time, their schedules running in parallel. Presentations often are prerecorded, but also include live sessions at 8:00 a.m. Central time and 12 p.m. CET (2:00 a.m. and 6:00 a.m. Eastern time) to liven up the channel offerings, Dr. Windecker observed, and to make them more immediate and potentially interactive.
Many of the parallel channels are devoted throughout the Congress to particular silos of cardiology; for example, arrhythmias and device therapy is on channel 3; CAD and acute care is on 5; HF is on 6; and preventive cardiology is on 9.
Other channels swing across different topics from day to day, such as channel 1, which covers COVID-19 topics on the first and third day of the meeting, “advances in science” on day 2, and “digital health, public health, health economics” on day 4.
The focus each day, starting at 2:00 p.m. CET (8:00 a.m. ET) and continuing into the evening in Europe, shifts over to the Prime Time live program, which features the Hot Line and guideline presentations and many of the live abstract presentations.
Dr. Kosiborod, not a researcher with the EMPEROR trials, is chair of the Dapagliflozin in Preserved Ejection Fraction Heart Failure ( PRESERVED-HF ) trial, which is scheduled for presentation at the September 2021 Heart Failure Society of American meeting.
A version of this article first appeared on Medscape.com.
There will be so much more to the annual congress of the European Society of Cardiology, which begins Aug. 27 with an all-virtual format, than detailed primary results of EMPEROR-Preserved, a trial that could mark a turning point for heart failure (HF) medical therapy.
Also among the featured Hot Line and Late-Breaking Science sessions are – along with many other studies – explorations of arrhythmia management (ablation or guided by loop recorder); secondary prevention, including by vaccination; oral anticoagulation, notably after transcatheter valve procedures; and colchicine or thrombosis prophylaxis in hospitalized patients with COVID-19.
There will even be a head-to-head comparison of two long-familiar left atrial appendage (LAA) occluders, and a population-based, randomized trial of sodium restriction through wide-scale use of a potassium-based salt substitute.
The congress will also introduce four guideline documents at sessions throughout the Congress, one on each day. They cover new and modified recommendations for heart failure; pacing, including cardiac resynchronization therapy (CRT); cardiovascular (CV) disease prevention; and, with cosponsorship from the European Association for Cardio-Thoracic Surgery, valvular heart disease.
The virtues of virtual
That next year’s Congress is slated for Aug. 27-30 in Barcelona should be welcome news for anyone whose “what if” curiosity about all-virtual conferences has already been satisfied. But with experience comes wisdom, as the medical societies have learned that online scientific meetings have some winning qualities that may be worth keeping, as least for a while.
“I think there is no doubt that the digital format will continue, for several reasons. One is that this pandemic is not over,” ESC Congress program committee chair Stephan Windecker, MD, Bern (Switzerland) University Hospital, , told this news organization. “As long as it is not over, the digital format is here to stay.”
But it also appears that people who haven’t been able to attend the congress in person are keen to log in and engage online, Dr. Windecker said. The 2020 all-virtual conference drew a much younger pool of registrants, on average, than did the live conferences before the pandemic.
“I think that’s an indication of people that may be in training, in early stages of their career, or they don’t have the support from departments or from their practice, or other financial means.” But they are able to participate via computer, tablet, or smartphone, he said.
“Another advantage is that the recorded content can be replayed at the convenience of whoever wants to consume it at a later point in time,” he added. “Those are just some examples why the digital format is likely to stay,” on its own or in a new age of hybrid meetings.
New and updated guidelines
Leading off the guideline series is the document on diagnosis and treatment of acute and chronic HF, which leveraged the past few busy years of HF clinical trials to arrive at a number of new recommendations and strengthened level-of-evidence ratings. It covers both drug and device therapy of HF with reduced ejection fraction (HFrEF) and acute decompensated HF, and tweaks and further enshrines the concept of HF with mildly reduced ejection fraction (HFmrEF).
Several updated recommendations for both long-used and novel medications, notably the sodium-glucose cotransporter 2 inhibitors, will be included because of the recently appreciated evidence-based impact in HFrEF, Dr. Windecker noted.
“I think it will be particularly interesting to look for the SGLT2 inhibitors as not a completely new class of drugs, but certainly one where there has been a lot of new evidence, to look at how those drugs will be integrated in the overall care pathway.”
A top-line preview of the new HF guideline limited to drug therapy, presented at July’s Heart Failure Association of the European Society of Cardiology (ESC-HFA), provided a simple answer to a common question in the new, bountiful age of HFrEF medications: Which meds, initiated in what order?
As it happens, the new recommendation for first-line HFrEF drug therapy is not a silver bullet, but a shotgun – prompt initiation of at least four meds, one from each of four drug classes: renin-angiotensin system inhibitors, beta-blockers, mineralocorticoid receptor antagonists (MRA), and SGLT2 inhibitors. Each class, as described in the document, is to be started as soon as safely feasible, in a sequence deemed appropriate for each individual patient.
Spotlight on EMPEROR-Preserved
The world already knows that the trial, which tested the SGLT2 inhibitor empagliflozin (Jardiance, Boehringer Ingelheim/Eli Lilly) on top of standard therapy, “met” its primary endpoint in almost 6,000 patients with HF with preserved ejection fraction (HFpEF), who included some with HFmrEF by more contemporary definitions.
That means patients in EMPEROR-Preserved assigned to take empagliflozin showed significantly fewer events that made up the study’s primary endpoint, a composite of CV death or HF hospitalization. It appears to be the first clearly significant overall medical therapy benefit for a clinical primary endpoint in a major randomized HFpEF drug trial.
And that, pending fuller presentation of trial results at the Congress on Aug. 27, could be a huge deal for the half of HF patients with left ventricular ejection fractions (LVEF) higher than the HFrEF range.
Those early top-line results weren’t a decisive bombshell for a field now filled with hope for a practice-changing empagliflozin outcome in EMPEROR-Preserved, which isn’t a certainty. They were more like the “boom” of a mortar launching a rocket of fireworks that may explode into a chrysanthemum or green comet or, sometimes, turn out to be no more than a dud. The promise of the early cursory results critically depends on further details.
“Provided there is a compelling benefit, this is what everyone has been waiting for in this condition for decades,” Mikhail N. Kosiborod, MD, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo., said.
“Already knowing that the trial met the primary endpoint is obviously very intriguing and encouraging,” he added. “But there are things we don’t know, such as: What is the magnitude of benefit? And whether that benefit, whatever the magnitude, is driven by reductions in both heart failure hospitalizations and cardiovascular death, or only one of the two.”
For example: “If we see an impressive benefit for reduction of hospitalizations, but not a significant reduction in death, that would still be a huge advance. That’s because, to date, we don’t have any drug for HFpEF that has convincingly demonstrated a compelling reduction in heart failure hospitalization or improvement in symptoms, function, or quality of life,” observed Dr. Kosiborod, who wasn’t part of EMPEROR-Preserved.
There have been “suggestions” from HFrEF trials that empagliflozin and dapagliflozin (Farxiga, AstraZeneca) “have very comparable effects on at least the endpoint of cardiovascular death or hospitalization for heart failure,” he said. “So, my expectation would be that whatever is observed in EMPEROR-Preserved is likely a class effect, as well.”
Following EMPEROR-Preserved on the agenda is EMPEROR-Pooled, a patient-level combined analysis of the EMPEROR series of trials that spans the range of HF, regardless of ejection fraction or diabetes status, primarily exploring the effects of empagliflozin on renal function.
Other offerings, Friday, Aug. 27
Scheduled immediately after EMPEROR-Preserved is a presentation on the SMART-MI trial, which should clarify whether management guided by continuous ambulatory monitoring is effective in patients considered at especially high arrhythmic risk. Entry called for recent myocardial infarction and an LVEF of 36%-50% with evidence of cardiac autonomic dysfunction.
The trial randomly assigned 400 such patients to be or not be implanted with a Reveal LINQ (Medtronic) loop recorder and followed them for up to 18 months, primarily for detection of potentially serious arrhythmic events. Endpoints that involved mortality, hospitalization or other clinical events were secondary.
In a time slot preceding both SMART-MI and EMPEROR-Preserved, the GUIDE-HF trial is following a projected 3,600 patients with HF implanted with a CardioMEMS HF System (Abbott) pulmonary artery (PA) pressure sensor to explore the its value for guiding management.
The trial’s three cohorts, followed for at least 12 months, include randomized sensor-monitored and control groups of patients with New York Heart Association class 2-4 symptoms, as well as a third observational set of patients in NYHA class 3. That’s the indication for which the CardioMEMS monitor gained approval in the United States in 2014 based on the 2011 CHAMPION trial, and which fared just as well in the 2017 CHAMPION Post-Approval Study.
The Friday Hot Lines also include Dal-GenE, which has entered about 6,000 patients with recent MI to test the once-abandoned cholesterol ester transfer protein (CETP) inhibitor dalcetrapib (DalCor) for any secondary-prevention benefits when used selectively. The trial’s hook: All its patients are confirmed to have the AA genotype of the rs1967309 variant in the ADCY9 gene, which has been associated with a pronounced clinical response to CETP inhibition.
Saturday, Aug. 28
The direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists in patients with nonvalvular atrial fibrillation (AFib). But whether DOACs are similarly preferable in the growing world population of people who have undergone transcatheter aortic valve replacement (TAVR or TAVI), an issue explored with variable results in the ATLANTIS and GALILEO trials, is far from settled.
The ENVISAGE-TAVI AF trial explored the question for the factor X inhibitor edoxaban (Savaysa, Lixiana, Daiichi-Sankyo) in 1,400 patients with AFib and a transfemoral TAVR in the previous 5 days, who were randomly assigned to the DOAC or standard management along with discretionary antiplatelet therapy. They’ve been followed for up to 3 years for a composite endpoint of clinical events – including death, MI, and stroke – and for major bleeding.
The day will also feature MASTER DAPT, a comparison of two dual-antiplatelet therapy (DAPT) regimens in an estimated 4,300 patients considered to be high-risk for bleeding who had received the sirolimus-eluting Ultimaster (Terumo) coronary stent, which has a bioresorbable polymer coating.
Investigators have randomly assigned patients to receive either very-short-duration DAPT, for about a month after stenting, followed by a P2Y12 inhibitor alone for up to a year after the procedure; or a more conventional regimen of a P2Y12 inhibitor for 6-12 months with aspirin maintained for a total of 12 months.
Later that day, investigators from the FIGARO-DKD trial will present their results based on 7,437 patients with type 2 diabetes and chronic kidney disease (CKD), a much fuller version than the top-line findings announced by sponsor Bayer 3 months ago.
Those top-line results suggested that patients assigned to receive the nonsteroidal nonselective mineralocorticoid receptor antagonist (MRA) finerenone (Kerendia) on top of standard care benefited with a drop in risk for the primary endpoint of CV death or nonfatal CV events.
Finerenone was recently approved in the United States for treating patients with both type 2 diabetes and CKD based on the published FIDELIO-DKD trial, which had seen less CKD progression and fewer CV events in such patients who took the novel MRA.
Although similar in design to FIGARO-DKD, FIDELIO-DKD had entered fewer patients with early-stage diabetic kidney disease (DKD). That led researchers to pool the two trials’ populations to create a cohort that spans the spectrum of DKD severity. An analysis of the pooled cohort, dubbed FIDELITY, is on the schedule after FIGARO-DKD.
After FIDELITY is the prospective APAF-CRT trial that is following a projected 1,830 patients with permanent, symptomatic AFib and a recent hospitalization for AFib or HF and who were not good candidates for standard ablation. They were assigned to receive either atrioventricular junctional ablation followed by CRT, with or without a defibrillation, on top of optimal meds – a so-called “ablate-and-pace” strategy – or an implantable cardioverter defibrillator with rate-control drug therapy.
The new analysis represents the trial’s second phase in which mortality was followed for 4 years as the primary endpoint, in contrast to the previously reported initial phase that followed the first 102 patients for 2 years for the composite primary endpoint of death, worsening HF, and HF hospitalization. The first phase had halted enrollment before reaching its planned target of 280 patients after an interim analysis showed a significant benefit for ablate and pace.
Next up: DECAAF 2, a randomized assessment of whether catheter ablation for AFib guided by delayed gadolinium enhancement on MRI, a proxy for scar tissue, can be more effective than standard AFib ablation by pulmonary vein isolation alone. An estimated 900 patients with persistent AFib who had never before undergone ablation for the arrhythmia were randomly assigned to one strategy or the other and followed for AFib recurrence over 18 months.
Sunday, Aug. 29
The TOMAHAWK trial aimed to clarify the optimal timing of invasive coronary angiography for resuscitated patients with non–ST-segment elevation out-of-hospital cardiac arrest, a broad population in a setting for which there is little randomized-trial guidance. Investigators randomly assigned 558 such patients to undergo immediate invasive angiography or to direct intensive care unit admission for initial standard care with discretionary delayed angiography. Patients were followed for all-cause mortality, with other clinical events and neurologic outcomes as secondary endpoints.
Next on the schedule, the RIPCORD-2 trial randomly assigned 1,100 patients with stable known or suspected coronary artery disease (CAD) to undergo conventional angiography alone or with added direct pressure-wire measurement of fractional flow reserve to guide management decisions. Primary outcomes include health care costs and patient-reported quality of life at 1 year.
Slated for later that day, the Asymptomatic Carotid Surgery Trial-2 (ACST-2) has entered an estimated 3600 patients with a substantial carotid artery narrowing not associated with symptoms but for which either carotid endarterectomy (CEA) or carotid artery stenting (CAS) was considered anatomically feasible. There also must have been “substantial uncertainty” regarding the optimal procedure choice.
The trial, conducted in 40 countries primarily in Europe and North America and launched in 2008, randomly assigned the patients to undergo either CEA or CAS, in both cases with appropriate medical therapy, and followed them for periprocedural events and up to 10 years for strokes and stroke-related events.
The LOOP study, which is to directly follow ACST-2, has explored whether screening for AFib using the Medtronic Reveal LINQ monitor in older patients with non-AFib stroke risk factors – with oral anticoagulation prescribed for those who test positive – can lower their risk for stroke or systemic embolism. It randomly assigned 6,000 such patients to care guided by the loop recorder or to standard care.
On a somewhat larger scale, the Salt Substitute and Stroke Study (SSaSS) randomly assigned a total of 20,996 people in about 600 villages across northern China and Tibet to sodium-restriction intervention and control groups by village. All participants had a history of stroke or were aged at least 60 years with uncontrolled hypertension.
As described by the trial’s online portal, participants in villages assigned to the intervention group were given a supply of a low-sodium, potassium-supplementing salt substitute to replace their own salt supplies, along with education on the health benefits of sodium restriction. Participants in control villages continued their normal diets and, at the trial’s beginning, received “advice to reduce their salt intake.” All were required to own a telephone.
Clinical events, including strokes and hospitalizations throughout a 5-year follow-up, were tracked by phone calls made to all participants every 6 months and were documented at follow-up home visits.
Sunday is also to feature a Late-Breaking Trials session with a focus on COVID-19, which leads off with COLCOVID, a test of colchicine in patients hospitalized for suspected SARS-CoV-2 infection and in acute respiratory distress.
The 1,279 participants in Argentina were randomly assigned to receive or not receive the potent anti-inflammatory agent on top of antivirals and other standard management and followed for death or new need for mechanical ventilation. A successful outcome would contrast with the RECOVERY trial, which terminated a colchicine group of patients hospitalized with COVID-19 because of a lack of efficacy earlier this year.
COLCOVID is to be followed by the MICHELLE trial of rivaroxaban (Xarelto, Bayer/Janssen) prophylaxis, compared with no preventive oral anticoagulant, in 320 patients who, when hospitalized with COVID-19, had been on parenteral anticoagulants because of an elevated risk for venous thromboembolism. The trial, conducted in Brazil, called for postdischarge rivaroxaban at a once-daily dosage of 10 mg for about 1 month.
The session also includes a presentation called “Insights into the Effects of the COVID-19 Pandemic: Comprehensive Analysis from the GUIDE-HF Trial,” the primary outcomes of which will be reported on the first day of the Congress.
Following is a presentation on the PREPARE-IT study of icosapent ethyl (Vascepa, Amarin), given at high dosages intended to be anti-inflammatory, compared with placebo, in an estimated 4,000 adults. The trial has two groups: A prevention group of adults living and circulating in the community; and a treatment group of patients aged at least 40 years with confirmed symptomatic SARS-CoV-2 infection for whom the need for hospitalization isn’t clear.
Monday, Aug. 30
The final day of the Congress features a trial called Influenza Vaccination after Myocardial Infarction (IAMI), which has tested the secondary preventive effect of influenza vaccination by randomly assigning 2,571 patients to receive a standard vaccine or a saline placebo injection on one occasion.
Entry to the international trial called for a diagnosis of MI with or without ST-segment elevation, or stable CAD and age at least 75 years with other risk factors. The patients were followed for death, MI, stent thrombosis, and a slew of secondary endpoints over 12 months.
Monday offerings continue later in a time block leading off with the STEP trial, which has randomly assigned an estimated 8,000 patients at 40 centers in China who are 60 to 80 years of age with a systolic blood pressure of 140 to <190 mm Hg to be on standard guideline-based therapy or an intensive drug-management strategy.
The systolic BP goals are 130 to <150 mm Hg for standard care and 110 to <130 mm Hg for the intensive regimen. The composite primary endpoint includes death and clinical events related to acute coronary syndromes, HF, revascularization, and stroke.
Following on heels of STEP, the Amulet IDE trial – the first major randomized comparison of two transcatheter LAA closure devices – entered 1,878 patients with nonvalvular AFib who were considered high-risk for bleeding and stroke or systemic embolism.
They were randomly assigned in the noninferiority trial to receive either the AMPLATZER Amulet (Abbott Medical Devices) or the WATCHMAN (Boston Scientific) closure devices and were followed for safety and efficacy for up to 5 years.
Both LAA closure devices, intended to make patients with AFib less reliant on oral anticoagulation, are now available on both sides of the Atlantic – as well as many other countries – after the Amulet’s United States market approval on Aug. 16, based largely on the Amulet IDE trial.
Rounding out the final Hot Line set is one of the latest efforts to show the efficacy and safety of a very short DAPT period after coronary stenting in patients with acute coronary syndromes, the STOPDAPT-2 ACS trial.
The study assigned 3,008 patients in Japan to receive aspirin and clopidogrel for either 1 month or 1 year after implantation with an everolimus-eluting cobalt-chromium stent and followed them for up to 5 years for a composite of MI, CV death, stent thrombosis, stroke, and bleeding.
The trial follows the published STOPDAPT-2 trial that showed superiority for the 1-month DAPT regimen in a predominantly stable-CAD population treated with the same kind of stent.
Program structure and format
A total of 15 online channels are to be available in the morning, European time, their schedules running in parallel. Presentations often are prerecorded, but also include live sessions at 8:00 a.m. Central time and 12 p.m. CET (2:00 a.m. and 6:00 a.m. Eastern time) to liven up the channel offerings, Dr. Windecker observed, and to make them more immediate and potentially interactive.
Many of the parallel channels are devoted throughout the Congress to particular silos of cardiology; for example, arrhythmias and device therapy is on channel 3; CAD and acute care is on 5; HF is on 6; and preventive cardiology is on 9.
Other channels swing across different topics from day to day, such as channel 1, which covers COVID-19 topics on the first and third day of the meeting, “advances in science” on day 2, and “digital health, public health, health economics” on day 4.
The focus each day, starting at 2:00 p.m. CET (8:00 a.m. ET) and continuing into the evening in Europe, shifts over to the Prime Time live program, which features the Hot Line and guideline presentations and many of the live abstract presentations.
Dr. Kosiborod, not a researcher with the EMPEROR trials, is chair of the Dapagliflozin in Preserved Ejection Fraction Heart Failure ( PRESERVED-HF ) trial, which is scheduled for presentation at the September 2021 Heart Failure Society of American meeting.
A version of this article first appeared on Medscape.com.
New-AFib risk may not rise with light drinking, may fall with wine
Alcoholic drinks are in the news again, served with a twist. A large cohort study saw a familiar J-shaped curve detailing risk for new atrial fibrillation (AFib) in which the risk rose steadily with greater number of drinks per week, except at the lowest levels of alcohol intake.
There, the curve turned the other way. Light drinkers overall showed no higher AFib risk than nondrinkers, and the risk was lowest at any degree of alcohol intake up to 56 g per week.
On closer analysis of risk patterns, the type of alcoholic beverage mattered.
Alcohol content per drink was defined by standards in the United Kingdom, where the cohort was based.
The risk of AFib also didn’t climb at low intake levels of white wine or with “very low” use of liquor or spirits. But it went up consistently at any level of beer or cider consumption, and to be sure, “high intake of any beverage was associated with greater AF[ib] risk,” notes a report on the study published July 27, 2021, in JACC: Clinical Electrophysiology.
The results, based on more than 400,000 adults in the community, “raise the possibility that, for current consumers, drinking red or white wine could potentially be a safer alternative to other types of alcoholic beverages with respect to AF[ib] risk,” the report proposes.
The J-shaped risk curve for new AFib by degree of alcohol consumption follows the pattern sometimes seen for cardiovascular risk in general. But the intake level at which AFib risk is flat or reduced “is at a far lower dose of alcohol than what we’ve seen for cardiovascular disease,” lead author Samuel J. Tu, BHlthMedSc, said in an interview.
“That being said, even with the threshold sitting quite low, it still tells us that cutting down on alcohol is a good thing and perhaps one of the best things for our heart,” said Mr. Tu, University of Adelaide and Royal Adelaide Hospital, who also presented the findings at the Heart Rhythm Society 2021 Scientific Sessions, held in Boston and virtually.
How much alcohol is in a drink?
In a caution for anyone looking to beer, wine, or liquor to protect against AFib, or at least not cause it, the weekly number of drinks associated with the lowest AFib risk may be fewer than expected. That bottom of 56 g per week works out to one drink a day or less for British and only four or fewer per week for Americans, according to the study’s internationally varying definitions for the alcohol content of one drink.
For example, a drink was considered to have 8 g of alcohol in the United Kingdom, 14 g in the United States and some other countries, and up to 20 g in Austria. Those numbers came from definitions used by the respective national health agencies, such as the National Health Service in the United Kingdom and Centers for Disease Control and Prevention in the United States, Mr. Tu explained.
“They all defined standard drinks slightly differently. But wherever we looked, the threshold we found was far lower than what our governments recommend” based on what is known about alcohol and overall cardiovascular risk, he said.
First to show a hint of protection
The current study “is especially noteworthy because it’s the really the first to demonstrate any hint that there could be a protective effect from any particular amount of alcohol in regard to atrial fibrillation,” Gregory M. Marcus, MD, MAS, University of California, San Francisco, said in an interview. “The J-shaped association fits with what’s been observed with myocardial infarction and overall mortality, and hasn’t previously been seen in the setting of atrial fibrillation.”
Quite interestingly, “it appeared to be the wine drinkers, rather than those who consumed other types of alcohol, that enjoyed this benefit,” said Dr. Marcus, who was not involved in the research but co-authored an accompanying editorial with UCSF colleague Thomas A. Dewland, MD.
“It’s important to recognize the overwhelming evidence that alcohol in general increases the risk for atrial fibrillation,” he said. But “perhaps there’s something in wine that is anti-inflammatory that has some beneficial effect that maybe overwhelms the proarrhythmic aspect.”
The current study “opens the door to the question as to whether there is a small amount of alcohol, perhaps in the form of wine, where there are some benefits that outweigh the risks of atrial fibrillation.”
Still, the findings are observational and “clearly prone to confounding,” Dr. Marcus said. “We need to be very cautious in inferring causality.”
For example, it’s possible that “there is something about individuals that are able to drink alcohol on a regular basis and in small amounts that is the actual causal factor in reducing atrial fibrillation episodes.”
The analysis was based on 403,281 participants in the UK Biobank registry, a prospective cohort study in the United Kingdom, who were aged 40-69 when recruited from 2006 to 2010; it excluded anyone with a history of AFib or who was a former drinker. About 52% were women, the report noted.
Their median alcohol consumption was eight U.K. drinks per week, with 5.5% reporting they had never consumed alcohol. About 21,300 incident cases of AFib or atrial flutter were documented over almost 4.5 million person-years, or a median follow-up of 11.4 years.
The hazard ratio for incident AFib among those with a weekly alcohol consumption corresponding to 1-7 U.K. drinks, compared with intake of less than 1 U.K. drink per week, was 0.95 (95% confidence interval, 0.91-1.00). Within that range of 1-7 drinks, the absolute lowest AFib risk on the J curve was at 5 per week.
No increased risk of new AFib was seen in association with weekly U.K. drink levels of 10 for red wine, 8 for white wine, and 3 for spirits.
Compared with weekly intake of less than 1 U.K. drink per week, red wine intake at 1-7 per week showed an HR for AFib of 0.94 (95% CI, 0.91-0.97). Indeed, at no observed consumption level was red wine associated with a significant increase in AFib risk. White wine until the highest observed level of intake, above 28 U.K. drinks per week, at which point the HR for AFib was 1.48 (98% CI 1.19-1.86). The curve for spirit intake followed a similar but steeper curve, its HR risk reaching 1.61 (95% CI, 1.34-1.93) at intake levels beyond 28 U.K. drinks per week.
Consumption of beer or cider showed a linear association with AFib risk, which was elevated at all recorded intake levels, including 8-14 U.K. drinks per week (HR, 1.11; 95% CI 1.06-1.17) and up to 28 or more per week (HR, 1.35; 95% CI, 1.26-1.45).
The analysis is hypothesis generating at best, Dr. Marcus emphasized. “Ultimately, a randomized trial would be the only way to be fairly certain if there is indeed a causal protective relationship between red wine, in low amounts, and atrial fib.”
The message for patients, proposed Dr. Dewland and Dr. Marcus, is that alcohol abstinence is best for secondary AFib prevention, “especially if alcohol is a personal trigger for acute AF[ib] episodes,” and that for primary AFib prevention, “continued consumption of some alcohol may be reasonable, but the exact threshold is unclear and is likely a very low amount.”
Mr. Tu has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Marcus disclosed receiving research funding from Baylis Medical; consulting for Johnson & Johnson and InCarda; and holding equity interest in InCarda. Dr. Dewland reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alcoholic drinks are in the news again, served with a twist. A large cohort study saw a familiar J-shaped curve detailing risk for new atrial fibrillation (AFib) in which the risk rose steadily with greater number of drinks per week, except at the lowest levels of alcohol intake.
There, the curve turned the other way. Light drinkers overall showed no higher AFib risk than nondrinkers, and the risk was lowest at any degree of alcohol intake up to 56 g per week.
On closer analysis of risk patterns, the type of alcoholic beverage mattered.
Alcohol content per drink was defined by standards in the United Kingdom, where the cohort was based.
The risk of AFib also didn’t climb at low intake levels of white wine or with “very low” use of liquor or spirits. But it went up consistently at any level of beer or cider consumption, and to be sure, “high intake of any beverage was associated with greater AF[ib] risk,” notes a report on the study published July 27, 2021, in JACC: Clinical Electrophysiology.
The results, based on more than 400,000 adults in the community, “raise the possibility that, for current consumers, drinking red or white wine could potentially be a safer alternative to other types of alcoholic beverages with respect to AF[ib] risk,” the report proposes.
The J-shaped risk curve for new AFib by degree of alcohol consumption follows the pattern sometimes seen for cardiovascular risk in general. But the intake level at which AFib risk is flat or reduced “is at a far lower dose of alcohol than what we’ve seen for cardiovascular disease,” lead author Samuel J. Tu, BHlthMedSc, said in an interview.
“That being said, even with the threshold sitting quite low, it still tells us that cutting down on alcohol is a good thing and perhaps one of the best things for our heart,” said Mr. Tu, University of Adelaide and Royal Adelaide Hospital, who also presented the findings at the Heart Rhythm Society 2021 Scientific Sessions, held in Boston and virtually.
How much alcohol is in a drink?
In a caution for anyone looking to beer, wine, or liquor to protect against AFib, or at least not cause it, the weekly number of drinks associated with the lowest AFib risk may be fewer than expected. That bottom of 56 g per week works out to one drink a day or less for British and only four or fewer per week for Americans, according to the study’s internationally varying definitions for the alcohol content of one drink.
For example, a drink was considered to have 8 g of alcohol in the United Kingdom, 14 g in the United States and some other countries, and up to 20 g in Austria. Those numbers came from definitions used by the respective national health agencies, such as the National Health Service in the United Kingdom and Centers for Disease Control and Prevention in the United States, Mr. Tu explained.
“They all defined standard drinks slightly differently. But wherever we looked, the threshold we found was far lower than what our governments recommend” based on what is known about alcohol and overall cardiovascular risk, he said.
First to show a hint of protection
The current study “is especially noteworthy because it’s the really the first to demonstrate any hint that there could be a protective effect from any particular amount of alcohol in regard to atrial fibrillation,” Gregory M. Marcus, MD, MAS, University of California, San Francisco, said in an interview. “The J-shaped association fits with what’s been observed with myocardial infarction and overall mortality, and hasn’t previously been seen in the setting of atrial fibrillation.”
Quite interestingly, “it appeared to be the wine drinkers, rather than those who consumed other types of alcohol, that enjoyed this benefit,” said Dr. Marcus, who was not involved in the research but co-authored an accompanying editorial with UCSF colleague Thomas A. Dewland, MD.
“It’s important to recognize the overwhelming evidence that alcohol in general increases the risk for atrial fibrillation,” he said. But “perhaps there’s something in wine that is anti-inflammatory that has some beneficial effect that maybe overwhelms the proarrhythmic aspect.”
The current study “opens the door to the question as to whether there is a small amount of alcohol, perhaps in the form of wine, where there are some benefits that outweigh the risks of atrial fibrillation.”
Still, the findings are observational and “clearly prone to confounding,” Dr. Marcus said. “We need to be very cautious in inferring causality.”
For example, it’s possible that “there is something about individuals that are able to drink alcohol on a regular basis and in small amounts that is the actual causal factor in reducing atrial fibrillation episodes.”
The analysis was based on 403,281 participants in the UK Biobank registry, a prospective cohort study in the United Kingdom, who were aged 40-69 when recruited from 2006 to 2010; it excluded anyone with a history of AFib or who was a former drinker. About 52% were women, the report noted.
Their median alcohol consumption was eight U.K. drinks per week, with 5.5% reporting they had never consumed alcohol. About 21,300 incident cases of AFib or atrial flutter were documented over almost 4.5 million person-years, or a median follow-up of 11.4 years.
The hazard ratio for incident AFib among those with a weekly alcohol consumption corresponding to 1-7 U.K. drinks, compared with intake of less than 1 U.K. drink per week, was 0.95 (95% confidence interval, 0.91-1.00). Within that range of 1-7 drinks, the absolute lowest AFib risk on the J curve was at 5 per week.
No increased risk of new AFib was seen in association with weekly U.K. drink levels of 10 for red wine, 8 for white wine, and 3 for spirits.
Compared with weekly intake of less than 1 U.K. drink per week, red wine intake at 1-7 per week showed an HR for AFib of 0.94 (95% CI, 0.91-0.97). Indeed, at no observed consumption level was red wine associated with a significant increase in AFib risk. White wine until the highest observed level of intake, above 28 U.K. drinks per week, at which point the HR for AFib was 1.48 (98% CI 1.19-1.86). The curve for spirit intake followed a similar but steeper curve, its HR risk reaching 1.61 (95% CI, 1.34-1.93) at intake levels beyond 28 U.K. drinks per week.
Consumption of beer or cider showed a linear association with AFib risk, which was elevated at all recorded intake levels, including 8-14 U.K. drinks per week (HR, 1.11; 95% CI 1.06-1.17) and up to 28 or more per week (HR, 1.35; 95% CI, 1.26-1.45).
The analysis is hypothesis generating at best, Dr. Marcus emphasized. “Ultimately, a randomized trial would be the only way to be fairly certain if there is indeed a causal protective relationship between red wine, in low amounts, and atrial fib.”
The message for patients, proposed Dr. Dewland and Dr. Marcus, is that alcohol abstinence is best for secondary AFib prevention, “especially if alcohol is a personal trigger for acute AF[ib] episodes,” and that for primary AFib prevention, “continued consumption of some alcohol may be reasonable, but the exact threshold is unclear and is likely a very low amount.”
Mr. Tu has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Marcus disclosed receiving research funding from Baylis Medical; consulting for Johnson & Johnson and InCarda; and holding equity interest in InCarda. Dr. Dewland reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alcoholic drinks are in the news again, served with a twist. A large cohort study saw a familiar J-shaped curve detailing risk for new atrial fibrillation (AFib) in which the risk rose steadily with greater number of drinks per week, except at the lowest levels of alcohol intake.
There, the curve turned the other way. Light drinkers overall showed no higher AFib risk than nondrinkers, and the risk was lowest at any degree of alcohol intake up to 56 g per week.
On closer analysis of risk patterns, the type of alcoholic beverage mattered.
Alcohol content per drink was defined by standards in the United Kingdom, where the cohort was based.
The risk of AFib also didn’t climb at low intake levels of white wine or with “very low” use of liquor or spirits. But it went up consistently at any level of beer or cider consumption, and to be sure, “high intake of any beverage was associated with greater AF[ib] risk,” notes a report on the study published July 27, 2021, in JACC: Clinical Electrophysiology.
The results, based on more than 400,000 adults in the community, “raise the possibility that, for current consumers, drinking red or white wine could potentially be a safer alternative to other types of alcoholic beverages with respect to AF[ib] risk,” the report proposes.
The J-shaped risk curve for new AFib by degree of alcohol consumption follows the pattern sometimes seen for cardiovascular risk in general. But the intake level at which AFib risk is flat or reduced “is at a far lower dose of alcohol than what we’ve seen for cardiovascular disease,” lead author Samuel J. Tu, BHlthMedSc, said in an interview.
“That being said, even with the threshold sitting quite low, it still tells us that cutting down on alcohol is a good thing and perhaps one of the best things for our heart,” said Mr. Tu, University of Adelaide and Royal Adelaide Hospital, who also presented the findings at the Heart Rhythm Society 2021 Scientific Sessions, held in Boston and virtually.
How much alcohol is in a drink?
In a caution for anyone looking to beer, wine, or liquor to protect against AFib, or at least not cause it, the weekly number of drinks associated with the lowest AFib risk may be fewer than expected. That bottom of 56 g per week works out to one drink a day or less for British and only four or fewer per week for Americans, according to the study’s internationally varying definitions for the alcohol content of one drink.
For example, a drink was considered to have 8 g of alcohol in the United Kingdom, 14 g in the United States and some other countries, and up to 20 g in Austria. Those numbers came from definitions used by the respective national health agencies, such as the National Health Service in the United Kingdom and Centers for Disease Control and Prevention in the United States, Mr. Tu explained.
“They all defined standard drinks slightly differently. But wherever we looked, the threshold we found was far lower than what our governments recommend” based on what is known about alcohol and overall cardiovascular risk, he said.
First to show a hint of protection
The current study “is especially noteworthy because it’s the really the first to demonstrate any hint that there could be a protective effect from any particular amount of alcohol in regard to atrial fibrillation,” Gregory M. Marcus, MD, MAS, University of California, San Francisco, said in an interview. “The J-shaped association fits with what’s been observed with myocardial infarction and overall mortality, and hasn’t previously been seen in the setting of atrial fibrillation.”
Quite interestingly, “it appeared to be the wine drinkers, rather than those who consumed other types of alcohol, that enjoyed this benefit,” said Dr. Marcus, who was not involved in the research but co-authored an accompanying editorial with UCSF colleague Thomas A. Dewland, MD.
“It’s important to recognize the overwhelming evidence that alcohol in general increases the risk for atrial fibrillation,” he said. But “perhaps there’s something in wine that is anti-inflammatory that has some beneficial effect that maybe overwhelms the proarrhythmic aspect.”
The current study “opens the door to the question as to whether there is a small amount of alcohol, perhaps in the form of wine, where there are some benefits that outweigh the risks of atrial fibrillation.”
Still, the findings are observational and “clearly prone to confounding,” Dr. Marcus said. “We need to be very cautious in inferring causality.”
For example, it’s possible that “there is something about individuals that are able to drink alcohol on a regular basis and in small amounts that is the actual causal factor in reducing atrial fibrillation episodes.”
The analysis was based on 403,281 participants in the UK Biobank registry, a prospective cohort study in the United Kingdom, who were aged 40-69 when recruited from 2006 to 2010; it excluded anyone with a history of AFib or who was a former drinker. About 52% were women, the report noted.
Their median alcohol consumption was eight U.K. drinks per week, with 5.5% reporting they had never consumed alcohol. About 21,300 incident cases of AFib or atrial flutter were documented over almost 4.5 million person-years, or a median follow-up of 11.4 years.
The hazard ratio for incident AFib among those with a weekly alcohol consumption corresponding to 1-7 U.K. drinks, compared with intake of less than 1 U.K. drink per week, was 0.95 (95% confidence interval, 0.91-1.00). Within that range of 1-7 drinks, the absolute lowest AFib risk on the J curve was at 5 per week.
No increased risk of new AFib was seen in association with weekly U.K. drink levels of 10 for red wine, 8 for white wine, and 3 for spirits.
Compared with weekly intake of less than 1 U.K. drink per week, red wine intake at 1-7 per week showed an HR for AFib of 0.94 (95% CI, 0.91-0.97). Indeed, at no observed consumption level was red wine associated with a significant increase in AFib risk. White wine until the highest observed level of intake, above 28 U.K. drinks per week, at which point the HR for AFib was 1.48 (98% CI 1.19-1.86). The curve for spirit intake followed a similar but steeper curve, its HR risk reaching 1.61 (95% CI, 1.34-1.93) at intake levels beyond 28 U.K. drinks per week.
Consumption of beer or cider showed a linear association with AFib risk, which was elevated at all recorded intake levels, including 8-14 U.K. drinks per week (HR, 1.11; 95% CI 1.06-1.17) and up to 28 or more per week (HR, 1.35; 95% CI, 1.26-1.45).
The analysis is hypothesis generating at best, Dr. Marcus emphasized. “Ultimately, a randomized trial would be the only way to be fairly certain if there is indeed a causal protective relationship between red wine, in low amounts, and atrial fib.”
The message for patients, proposed Dr. Dewland and Dr. Marcus, is that alcohol abstinence is best for secondary AFib prevention, “especially if alcohol is a personal trigger for acute AF[ib] episodes,” and that for primary AFib prevention, “continued consumption of some alcohol may be reasonable, but the exact threshold is unclear and is likely a very low amount.”
Mr. Tu has disclosed no relevant financial relationships. Disclosures for the other authors are in the report. Dr. Marcus disclosed receiving research funding from Baylis Medical; consulting for Johnson & Johnson and InCarda; and holding equity interest in InCarda. Dr. Dewland reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DOACs best aspirin after ventricular ablation: STROKE-VT
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
ESC heart failure guideline to integrate bounty of new meds
Today there are so many evidence-based drug therapies for heart failure with reduced ejection fraction (HFrEF) that physicians treating HF patients almost don’t know what to do them.
It’s an exciting new age that way, but to many vexingly unclear how best to merge the shiny new options with mainstay regimens based on time-honored renin-angiotensin system (RAS) inhibitors and beta-blockers.
To impart some clarity, the authors of a new HF guideline document recently took center stage at the Heart Failure Association of the European Society of Cardiology (ESC-HFA) annual meeting to preview their updated recommendations, with novel twists based on recent major trials, for the new age of HF pharmacotherapeutics.
The guideline committee considered the evidence base that existed “up until the end of March of this year,” Theresa A. McDonagh, MD, King’s College London, said during the presentation. The document “is now finalized, it’s with the publishers, and it will be presented in full with simultaneous publication at the ESC meeting” that starts August 27.
It describes a game plan, already followed by some clinicians in practice without official guidance, for initiating drugs from each of four classes in virtually all patients with HFrEF.
New indicated drugs, new perspective for HFrEF
Three of the drug categories are old acquaintances. Among them are the RAS inhibitors, which include angiotensin-receptor/neprilysin inhibitors, beta-blockers, and the mineralocorticoid receptor antagonists. The latter drugs are gaining new respect after having been underplayed in HF prescribing despite longstanding evidence of efficacy.
Completing the quartet of first-line HFrEF drug classes is a recent arrival to the HF arena, the sodium-glucose cotransporter 2 inhibitors.
“We now have new data and a simplified treatment algorithm for heart failure with reduced ejection fraction based on the early administration of the four major classes of drugs,” said Marco Metra, MD, University of Brescia (Italy), previewing the medical-therapy portions of the new guideline at the ESC-HFA sessions, which launched virtually and live in Florence, Italy, on July 29.
The new game plan offers a simple answer to a once-common but complex question: How and in what order are the different drug classes initiated in patients with HFrEF? In the new document, the stated goal is to get them all on board expeditiously and safely, by any means possible.
The guideline writers did not specify a sequence, preferring to leave that decision to physicians, said Dr. Metra, who stated only two guiding principles. The first is to consider the patient’s unique circumstances. The order in which the drugs are introduced might vary, depending on, for example, whether the patient has low or high blood pressure or renal dysfunction.
Second, “it is very important that we try to give all four classes of drugs to the patient in the shortest time possible, because this saves lives,” he said.
That there is no recommendation on sequencing the drugs has led some to the wrong interpretation that all should be started at once, observed coauthor Javed Butler, MD, MPH, University of Mississippi, Jackson, as a panelist during the presentation. Far from it, he said. “The doctor with the patient in front of you can make the best decision. The idea here is to get all the therapies on as soon as possible, as safely as possible.”
“The order in which they are introduced is not really important,” agreed Vijay Chopra, MD, Max Super Specialty Hospital Saket, New Delhi, another coauthor on the panel. “The important thing is that at least some dose of all the four drugs needs to be introduced in the first 4-6 weeks, and then up-titrated.”
Other medical therapy can be more tailored, Dr. Metra noted, such as loop diuretics for patients with congestion, iron for those with iron deficiency, and other drugs depending on whether there is, for example, atrial fibrillation or coronary disease.
Adoption of emerging definitions
The document adopts the emerging characterization of HFrEF by a left ventricular ejection fraction (LVEF) up to 40%.
And it will leverage an expanding evidence base for medication in a segment of patients once said to have HF with preserved ejection fraction (HFpEF), who had therefore lacked specific, guideline-directed medical therapies. Now, patients with an LVEF of 41%-49% will be said to have HF with mildly reduced ejection fraction (HFmrEF), a tweak to the recently introduced HF with “mid-range” LVEF that is designed to assert its nature as something to treat. The new document’s HFmrEF recommendations come with various class and level-of-evidence ratings.
That leaves HFpEF to be characterized by an LVEF of 50% in combination with structural or functional abnormalities associated with LV diastolic dysfunction or raised LV filling pressures, including raised natriuretic peptide levels.
The definitions are consistent with those proposed internationally by the ESC-HFA, the Heart Failure Society of America, and other groups in a statement published in March.
Expanded HFrEF med landscape
Since the 2016 ESC guideline on HF therapy, Dr. McDonagh said, “there’s been no substantial change in the evidence for many of the classical drugs that we use in heart failure. However, we had a lot of new and exciting evidence to consider,” especially in support of the SGLT2 inhibitors as one of the core medications in HFrEF.
The new data came from two controlled trials in particular. In DAPA-HF, patients with HFrEF who were initially without diabetes and who went on dapagliflozin (Farxiga, AstraZeneca) showed a 27% drop in cardiovascular (CV) death or worsening-HF events over a median of 18 months.
“That was followed up with very concordant results with empagliflozin [Jardiance, Boehringer Ingelheim/Eli Lilly] in HFrEF in the EMPEROR-Reduced trial,” Dr. McDonagh said. In that trial, comparable patients who took empagliflozin showed a 25% drop in a primary endpoint similar to that in DAPA-HF over the median 16-month follow-up.
Other HFrEF recommendations are for selected patients. They include ivabradine, already in the guidelines, for patients in sinus rhythm with an elevated resting heart rate who can’t take beta-blockers for whatever reason. But, Dr. McDonagh noted, “we had some new classes of drugs to consider as well.”
In particular, the oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo) emerged about a year ago from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization. In the trial with more than 5,000 patients, treatment with vericiguat atop standard drug and device therapy was followed by a significant 10% drop in risk for CV death or HF hospitalization.
Available now or likely to be available in the United States, the European Union, Japan, and other countries, vericiguat is recommended in the new guideline for VICTORIA-like patients who don’t adequately respond to other indicated medications.
Little for HFpEF as newly defined
“Almost nothing is new” in the guidelines for HFpEF, Dr. Metra said. The document recommends screening for and treatment of any underlying disorder and comorbidities, plus diuretics for any congestion. “That’s what we have to date.”
But that evidence base might soon change. The new HFpEF recommendations could possibly be up-staged at the ESC sessions by the August 27 scheduled presentation of EMPEROR-Preserved, a randomized test of empagliflozin in HFpEF and – it could be said – HFmrEF. The trial entered patients with chronic HF and an LVEF greater than 40%.
Eli Lilly and Boehringer Ingelheim offered the world a peek at the results, which suggest the SGLT2 inhibitor had a positive impact on the primary endpoint of CV death or HF hospitalization. They announced the cursory top-line outcomes in early July as part of its regulatory obligations, noting that the trial had “met” its primary endpoint.
But many unknowns remain, including the degree of benefit and whether it varied among subgroups, and especially whether outcomes were different for HFmrEF than for HFpEF.
Upgrades for familiar agents
Still, HFmrEF gets noteworthy attention in the document. “For the first time, we have recommendations for these patients,” Dr. Metra said. “We already knew that diuretics are indicated for the treatment of congestion. But now, ACE inhibitors, ARBs, beta-blockers, mineralocorticoid antagonists, as well as sacubitril/valsartan, may be considered to improve outcomes in these patients.” Their upgrades in the new guidelines were based on review of trials in the CHARM program and of TOPCAT and PARAGON-HF, among others, he said.

The new document also includes “treatment algorithms based on phenotypes”; that is, comorbidities and less common HF precipitants. For example, “assessment of iron status is now mandated in all patients with heart failure,” Dr. Metra said.
AFFIRM-HF is the key trial in this arena, with its more than 1,100 iron-deficient patients with LVEF less than 50% who had been recently hospitalized for HF. A year of treatment with ferric carboxymaltose (Ferinject/Injectafer, Vifor) led to a 26% drop in risk for HF hospitalization, but without affecting mortality.
For those who are iron deficient, Dr. Metra said, “ferric carboxymaltose intravenously should be considered not only in patients with low ejection fraction and outpatients, but also in patients recently hospitalized for acute heart failure.”
The SGLT2 inhibitors are recommended in HFrEF patients with type 2 diabetes. And treatment with tafamidis (Vyndaqel, Pfizer) in patients with genetic or wild-type transthyretin cardiac amyloidosis gets a class I recommendation based on survival gains seen in the ATTR-ACT trial.
Also recommended is a full CV assessment for patients with cancer who are on cardiotoxic agents or otherwise might be at risk for chemotherapy cardiotoxicity. “Beta-blockers and ACE inhibitors should be considered in those who develop left ventricular systolic dysfunction after anticancer therapy,” Dr. Metra said.
The ongoing pandemic made its mark on the document’s genesis, as it has with most everything else. “For better or worse, we were a ‘COVID guideline,’ ” Dr. McDonagh said. The writing committee consisted of “a large task force of 31 individuals, including two patients,” and there were “only two face-to-face meetings prior to the first wave of COVID hitting Europe.”
The committee voted on each of the recommendations, “and we had to have agreement of more than 75% of the task force to assign a class of recommendation or level of evidence,” she said. “I think we did the best we could in the circumstances. We had the benefit of many discussions over Zoom, and I think at the end of the day we have achieved a consensus.”
With such a large body of participants and the 75% threshold for agreement, “you end up with perhaps a conservative guideline. But that’s not a bad thing for clinical practice, for guidelines to be conservative,” Dr. McDonagh said. “They’re mainly concerned with looking at evidence and safety.”
A version of this article first appeared on Medscape.com.
Today there are so many evidence-based drug therapies for heart failure with reduced ejection fraction (HFrEF) that physicians treating HF patients almost don’t know what to do them.
It’s an exciting new age that way, but to many vexingly unclear how best to merge the shiny new options with mainstay regimens based on time-honored renin-angiotensin system (RAS) inhibitors and beta-blockers.
To impart some clarity, the authors of a new HF guideline document recently took center stage at the Heart Failure Association of the European Society of Cardiology (ESC-HFA) annual meeting to preview their updated recommendations, with novel twists based on recent major trials, for the new age of HF pharmacotherapeutics.
The guideline committee considered the evidence base that existed “up until the end of March of this year,” Theresa A. McDonagh, MD, King’s College London, said during the presentation. The document “is now finalized, it’s with the publishers, and it will be presented in full with simultaneous publication at the ESC meeting” that starts August 27.
It describes a game plan, already followed by some clinicians in practice without official guidance, for initiating drugs from each of four classes in virtually all patients with HFrEF.
New indicated drugs, new perspective for HFrEF
Three of the drug categories are old acquaintances. Among them are the RAS inhibitors, which include angiotensin-receptor/neprilysin inhibitors, beta-blockers, and the mineralocorticoid receptor antagonists. The latter drugs are gaining new respect after having been underplayed in HF prescribing despite longstanding evidence of efficacy.
Completing the quartet of first-line HFrEF drug classes is a recent arrival to the HF arena, the sodium-glucose cotransporter 2 inhibitors.
“We now have new data and a simplified treatment algorithm for heart failure with reduced ejection fraction based on the early administration of the four major classes of drugs,” said Marco Metra, MD, University of Brescia (Italy), previewing the medical-therapy portions of the new guideline at the ESC-HFA sessions, which launched virtually and live in Florence, Italy, on July 29.
The new game plan offers a simple answer to a once-common but complex question: How and in what order are the different drug classes initiated in patients with HFrEF? In the new document, the stated goal is to get them all on board expeditiously and safely, by any means possible.
The guideline writers did not specify a sequence, preferring to leave that decision to physicians, said Dr. Metra, who stated only two guiding principles. The first is to consider the patient’s unique circumstances. The order in which the drugs are introduced might vary, depending on, for example, whether the patient has low or high blood pressure or renal dysfunction.
Second, “it is very important that we try to give all four classes of drugs to the patient in the shortest time possible, because this saves lives,” he said.
That there is no recommendation on sequencing the drugs has led some to the wrong interpretation that all should be started at once, observed coauthor Javed Butler, MD, MPH, University of Mississippi, Jackson, as a panelist during the presentation. Far from it, he said. “The doctor with the patient in front of you can make the best decision. The idea here is to get all the therapies on as soon as possible, as safely as possible.”
“The order in which they are introduced is not really important,” agreed Vijay Chopra, MD, Max Super Specialty Hospital Saket, New Delhi, another coauthor on the panel. “The important thing is that at least some dose of all the four drugs needs to be introduced in the first 4-6 weeks, and then up-titrated.”
Other medical therapy can be more tailored, Dr. Metra noted, such as loop diuretics for patients with congestion, iron for those with iron deficiency, and other drugs depending on whether there is, for example, atrial fibrillation or coronary disease.
Adoption of emerging definitions
The document adopts the emerging characterization of HFrEF by a left ventricular ejection fraction (LVEF) up to 40%.
And it will leverage an expanding evidence base for medication in a segment of patients once said to have HF with preserved ejection fraction (HFpEF), who had therefore lacked specific, guideline-directed medical therapies. Now, patients with an LVEF of 41%-49% will be said to have HF with mildly reduced ejection fraction (HFmrEF), a tweak to the recently introduced HF with “mid-range” LVEF that is designed to assert its nature as something to treat. The new document’s HFmrEF recommendations come with various class and level-of-evidence ratings.
That leaves HFpEF to be characterized by an LVEF of 50% in combination with structural or functional abnormalities associated with LV diastolic dysfunction or raised LV filling pressures, including raised natriuretic peptide levels.
The definitions are consistent with those proposed internationally by the ESC-HFA, the Heart Failure Society of America, and other groups in a statement published in March.
Expanded HFrEF med landscape
Since the 2016 ESC guideline on HF therapy, Dr. McDonagh said, “there’s been no substantial change in the evidence for many of the classical drugs that we use in heart failure. However, we had a lot of new and exciting evidence to consider,” especially in support of the SGLT2 inhibitors as one of the core medications in HFrEF.
The new data came from two controlled trials in particular. In DAPA-HF, patients with HFrEF who were initially without diabetes and who went on dapagliflozin (Farxiga, AstraZeneca) showed a 27% drop in cardiovascular (CV) death or worsening-HF events over a median of 18 months.
“That was followed up with very concordant results with empagliflozin [Jardiance, Boehringer Ingelheim/Eli Lilly] in HFrEF in the EMPEROR-Reduced trial,” Dr. McDonagh said. In that trial, comparable patients who took empagliflozin showed a 25% drop in a primary endpoint similar to that in DAPA-HF over the median 16-month follow-up.
Other HFrEF recommendations are for selected patients. They include ivabradine, already in the guidelines, for patients in sinus rhythm with an elevated resting heart rate who can’t take beta-blockers for whatever reason. But, Dr. McDonagh noted, “we had some new classes of drugs to consider as well.”
In particular, the oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo) emerged about a year ago from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization. In the trial with more than 5,000 patients, treatment with vericiguat atop standard drug and device therapy was followed by a significant 10% drop in risk for CV death or HF hospitalization.
Available now or likely to be available in the United States, the European Union, Japan, and other countries, vericiguat is recommended in the new guideline for VICTORIA-like patients who don’t adequately respond to other indicated medications.
Little for HFpEF as newly defined
“Almost nothing is new” in the guidelines for HFpEF, Dr. Metra said. The document recommends screening for and treatment of any underlying disorder and comorbidities, plus diuretics for any congestion. “That’s what we have to date.”
But that evidence base might soon change. The new HFpEF recommendations could possibly be up-staged at the ESC sessions by the August 27 scheduled presentation of EMPEROR-Preserved, a randomized test of empagliflozin in HFpEF and – it could be said – HFmrEF. The trial entered patients with chronic HF and an LVEF greater than 40%.
Eli Lilly and Boehringer Ingelheim offered the world a peek at the results, which suggest the SGLT2 inhibitor had a positive impact on the primary endpoint of CV death or HF hospitalization. They announced the cursory top-line outcomes in early July as part of its regulatory obligations, noting that the trial had “met” its primary endpoint.
But many unknowns remain, including the degree of benefit and whether it varied among subgroups, and especially whether outcomes were different for HFmrEF than for HFpEF.
Upgrades for familiar agents
Still, HFmrEF gets noteworthy attention in the document. “For the first time, we have recommendations for these patients,” Dr. Metra said. “We already knew that diuretics are indicated for the treatment of congestion. But now, ACE inhibitors, ARBs, beta-blockers, mineralocorticoid antagonists, as well as sacubitril/valsartan, may be considered to improve outcomes in these patients.” Their upgrades in the new guidelines were based on review of trials in the CHARM program and of TOPCAT and PARAGON-HF, among others, he said.

The new document also includes “treatment algorithms based on phenotypes”; that is, comorbidities and less common HF precipitants. For example, “assessment of iron status is now mandated in all patients with heart failure,” Dr. Metra said.
AFFIRM-HF is the key trial in this arena, with its more than 1,100 iron-deficient patients with LVEF less than 50% who had been recently hospitalized for HF. A year of treatment with ferric carboxymaltose (Ferinject/Injectafer, Vifor) led to a 26% drop in risk for HF hospitalization, but without affecting mortality.
For those who are iron deficient, Dr. Metra said, “ferric carboxymaltose intravenously should be considered not only in patients with low ejection fraction and outpatients, but also in patients recently hospitalized for acute heart failure.”
The SGLT2 inhibitors are recommended in HFrEF patients with type 2 diabetes. And treatment with tafamidis (Vyndaqel, Pfizer) in patients with genetic or wild-type transthyretin cardiac amyloidosis gets a class I recommendation based on survival gains seen in the ATTR-ACT trial.
Also recommended is a full CV assessment for patients with cancer who are on cardiotoxic agents or otherwise might be at risk for chemotherapy cardiotoxicity. “Beta-blockers and ACE inhibitors should be considered in those who develop left ventricular systolic dysfunction after anticancer therapy,” Dr. Metra said.
The ongoing pandemic made its mark on the document’s genesis, as it has with most everything else. “For better or worse, we were a ‘COVID guideline,’ ” Dr. McDonagh said. The writing committee consisted of “a large task force of 31 individuals, including two patients,” and there were “only two face-to-face meetings prior to the first wave of COVID hitting Europe.”
The committee voted on each of the recommendations, “and we had to have agreement of more than 75% of the task force to assign a class of recommendation or level of evidence,” she said. “I think we did the best we could in the circumstances. We had the benefit of many discussions over Zoom, and I think at the end of the day we have achieved a consensus.”
With such a large body of participants and the 75% threshold for agreement, “you end up with perhaps a conservative guideline. But that’s not a bad thing for clinical practice, for guidelines to be conservative,” Dr. McDonagh said. “They’re mainly concerned with looking at evidence and safety.”
A version of this article first appeared on Medscape.com.
Today there are so many evidence-based drug therapies for heart failure with reduced ejection fraction (HFrEF) that physicians treating HF patients almost don’t know what to do them.
It’s an exciting new age that way, but to many vexingly unclear how best to merge the shiny new options with mainstay regimens based on time-honored renin-angiotensin system (RAS) inhibitors and beta-blockers.
To impart some clarity, the authors of a new HF guideline document recently took center stage at the Heart Failure Association of the European Society of Cardiology (ESC-HFA) annual meeting to preview their updated recommendations, with novel twists based on recent major trials, for the new age of HF pharmacotherapeutics.
The guideline committee considered the evidence base that existed “up until the end of March of this year,” Theresa A. McDonagh, MD, King’s College London, said during the presentation. The document “is now finalized, it’s with the publishers, and it will be presented in full with simultaneous publication at the ESC meeting” that starts August 27.
It describes a game plan, already followed by some clinicians in practice without official guidance, for initiating drugs from each of four classes in virtually all patients with HFrEF.
New indicated drugs, new perspective for HFrEF
Three of the drug categories are old acquaintances. Among them are the RAS inhibitors, which include angiotensin-receptor/neprilysin inhibitors, beta-blockers, and the mineralocorticoid receptor antagonists. The latter drugs are gaining new respect after having been underplayed in HF prescribing despite longstanding evidence of efficacy.
Completing the quartet of first-line HFrEF drug classes is a recent arrival to the HF arena, the sodium-glucose cotransporter 2 inhibitors.
“We now have new data and a simplified treatment algorithm for heart failure with reduced ejection fraction based on the early administration of the four major classes of drugs,” said Marco Metra, MD, University of Brescia (Italy), previewing the medical-therapy portions of the new guideline at the ESC-HFA sessions, which launched virtually and live in Florence, Italy, on July 29.
The new game plan offers a simple answer to a once-common but complex question: How and in what order are the different drug classes initiated in patients with HFrEF? In the new document, the stated goal is to get them all on board expeditiously and safely, by any means possible.
The guideline writers did not specify a sequence, preferring to leave that decision to physicians, said Dr. Metra, who stated only two guiding principles. The first is to consider the patient’s unique circumstances. The order in which the drugs are introduced might vary, depending on, for example, whether the patient has low or high blood pressure or renal dysfunction.
Second, “it is very important that we try to give all four classes of drugs to the patient in the shortest time possible, because this saves lives,” he said.
That there is no recommendation on sequencing the drugs has led some to the wrong interpretation that all should be started at once, observed coauthor Javed Butler, MD, MPH, University of Mississippi, Jackson, as a panelist during the presentation. Far from it, he said. “The doctor with the patient in front of you can make the best decision. The idea here is to get all the therapies on as soon as possible, as safely as possible.”
“The order in which they are introduced is not really important,” agreed Vijay Chopra, MD, Max Super Specialty Hospital Saket, New Delhi, another coauthor on the panel. “The important thing is that at least some dose of all the four drugs needs to be introduced in the first 4-6 weeks, and then up-titrated.”
Other medical therapy can be more tailored, Dr. Metra noted, such as loop diuretics for patients with congestion, iron for those with iron deficiency, and other drugs depending on whether there is, for example, atrial fibrillation or coronary disease.
Adoption of emerging definitions
The document adopts the emerging characterization of HFrEF by a left ventricular ejection fraction (LVEF) up to 40%.
And it will leverage an expanding evidence base for medication in a segment of patients once said to have HF with preserved ejection fraction (HFpEF), who had therefore lacked specific, guideline-directed medical therapies. Now, patients with an LVEF of 41%-49% will be said to have HF with mildly reduced ejection fraction (HFmrEF), a tweak to the recently introduced HF with “mid-range” LVEF that is designed to assert its nature as something to treat. The new document’s HFmrEF recommendations come with various class and level-of-evidence ratings.
That leaves HFpEF to be characterized by an LVEF of 50% in combination with structural or functional abnormalities associated with LV diastolic dysfunction or raised LV filling pressures, including raised natriuretic peptide levels.
The definitions are consistent with those proposed internationally by the ESC-HFA, the Heart Failure Society of America, and other groups in a statement published in March.
Expanded HFrEF med landscape
Since the 2016 ESC guideline on HF therapy, Dr. McDonagh said, “there’s been no substantial change in the evidence for many of the classical drugs that we use in heart failure. However, we had a lot of new and exciting evidence to consider,” especially in support of the SGLT2 inhibitors as one of the core medications in HFrEF.
The new data came from two controlled trials in particular. In DAPA-HF, patients with HFrEF who were initially without diabetes and who went on dapagliflozin (Farxiga, AstraZeneca) showed a 27% drop in cardiovascular (CV) death or worsening-HF events over a median of 18 months.
“That was followed up with very concordant results with empagliflozin [Jardiance, Boehringer Ingelheim/Eli Lilly] in HFrEF in the EMPEROR-Reduced trial,” Dr. McDonagh said. In that trial, comparable patients who took empagliflozin showed a 25% drop in a primary endpoint similar to that in DAPA-HF over the median 16-month follow-up.
Other HFrEF recommendations are for selected patients. They include ivabradine, already in the guidelines, for patients in sinus rhythm with an elevated resting heart rate who can’t take beta-blockers for whatever reason. But, Dr. McDonagh noted, “we had some new classes of drugs to consider as well.”
In particular, the oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo) emerged about a year ago from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization. In the trial with more than 5,000 patients, treatment with vericiguat atop standard drug and device therapy was followed by a significant 10% drop in risk for CV death or HF hospitalization.
Available now or likely to be available in the United States, the European Union, Japan, and other countries, vericiguat is recommended in the new guideline for VICTORIA-like patients who don’t adequately respond to other indicated medications.
Little for HFpEF as newly defined
“Almost nothing is new” in the guidelines for HFpEF, Dr. Metra said. The document recommends screening for and treatment of any underlying disorder and comorbidities, plus diuretics for any congestion. “That’s what we have to date.”
But that evidence base might soon change. The new HFpEF recommendations could possibly be up-staged at the ESC sessions by the August 27 scheduled presentation of EMPEROR-Preserved, a randomized test of empagliflozin in HFpEF and – it could be said – HFmrEF. The trial entered patients with chronic HF and an LVEF greater than 40%.
Eli Lilly and Boehringer Ingelheim offered the world a peek at the results, which suggest the SGLT2 inhibitor had a positive impact on the primary endpoint of CV death or HF hospitalization. They announced the cursory top-line outcomes in early July as part of its regulatory obligations, noting that the trial had “met” its primary endpoint.
But many unknowns remain, including the degree of benefit and whether it varied among subgroups, and especially whether outcomes were different for HFmrEF than for HFpEF.
Upgrades for familiar agents
Still, HFmrEF gets noteworthy attention in the document. “For the first time, we have recommendations for these patients,” Dr. Metra said. “We already knew that diuretics are indicated for the treatment of congestion. But now, ACE inhibitors, ARBs, beta-blockers, mineralocorticoid antagonists, as well as sacubitril/valsartan, may be considered to improve outcomes in these patients.” Their upgrades in the new guidelines were based on review of trials in the CHARM program and of TOPCAT and PARAGON-HF, among others, he said.

The new document also includes “treatment algorithms based on phenotypes”; that is, comorbidities and less common HF precipitants. For example, “assessment of iron status is now mandated in all patients with heart failure,” Dr. Metra said.
AFFIRM-HF is the key trial in this arena, with its more than 1,100 iron-deficient patients with LVEF less than 50% who had been recently hospitalized for HF. A year of treatment with ferric carboxymaltose (Ferinject/Injectafer, Vifor) led to a 26% drop in risk for HF hospitalization, but without affecting mortality.
For those who are iron deficient, Dr. Metra said, “ferric carboxymaltose intravenously should be considered not only in patients with low ejection fraction and outpatients, but also in patients recently hospitalized for acute heart failure.”
The SGLT2 inhibitors are recommended in HFrEF patients with type 2 diabetes. And treatment with tafamidis (Vyndaqel, Pfizer) in patients with genetic or wild-type transthyretin cardiac amyloidosis gets a class I recommendation based on survival gains seen in the ATTR-ACT trial.
Also recommended is a full CV assessment for patients with cancer who are on cardiotoxic agents or otherwise might be at risk for chemotherapy cardiotoxicity. “Beta-blockers and ACE inhibitors should be considered in those who develop left ventricular systolic dysfunction after anticancer therapy,” Dr. Metra said.
The ongoing pandemic made its mark on the document’s genesis, as it has with most everything else. “For better or worse, we were a ‘COVID guideline,’ ” Dr. McDonagh said. The writing committee consisted of “a large task force of 31 individuals, including two patients,” and there were “only two face-to-face meetings prior to the first wave of COVID hitting Europe.”
The committee voted on each of the recommendations, “and we had to have agreement of more than 75% of the task force to assign a class of recommendation or level of evidence,” she said. “I think we did the best we could in the circumstances. We had the benefit of many discussions over Zoom, and I think at the end of the day we have achieved a consensus.”
With such a large body of participants and the 75% threshold for agreement, “you end up with perhaps a conservative guideline. But that’s not a bad thing for clinical practice, for guidelines to be conservative,” Dr. McDonagh said. “They’re mainly concerned with looking at evidence and safety.”
A version of this article first appeared on Medscape.com.
FDA to revise statin pregnancy contraindication
The U.S. Food and Drug Administration (FDA) aims to update the labeling on all statins to remove the drugs’ blanket contraindication in all pregnant patients, the agency has announced. The change should reinforce for both physicians and patients that statin use in women with unrecognized pregnancy is unlikely to be harmful, it said.
“Because the benefits of statins may include prevention of serious or potentially fatal events in a small group of very high-risk pregnant patients, contraindicating these drugs in all pregnant women is not appropriate.”
The revision should emphasize for clinicians “that statins are safe to prescribe in patients who can become pregnant and help them reassure patients with unintended statin exposure in early pregnancy,” the FDA explained.
Removal of the broadly worded contraindication should “enable health care professionals and patients to make individual decisions about benefit and risk, especially for those at very high risk of heart attack or stroke." That includes women with homozygous familial hypercholesterolemia and those who are prescribed statins for secondary prevention, the agency said.
Clinicians “should discontinue statin therapy in most pregnant patients, or they can consider the ongoing therapeutic needs of the individual patient, particularly those at very high risk for cardiovascular events during pregnancy. Because of the chronic nature of cardiovascular disease, treatment of hyperlipidemia is not generally necessary during pregnancy.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) aims to update the labeling on all statins to remove the drugs’ blanket contraindication in all pregnant patients, the agency has announced. The change should reinforce for both physicians and patients that statin use in women with unrecognized pregnancy is unlikely to be harmful, it said.
“Because the benefits of statins may include prevention of serious or potentially fatal events in a small group of very high-risk pregnant patients, contraindicating these drugs in all pregnant women is not appropriate.”
The revision should emphasize for clinicians “that statins are safe to prescribe in patients who can become pregnant and help them reassure patients with unintended statin exposure in early pregnancy,” the FDA explained.
Removal of the broadly worded contraindication should “enable health care professionals and patients to make individual decisions about benefit and risk, especially for those at very high risk of heart attack or stroke." That includes women with homozygous familial hypercholesterolemia and those who are prescribed statins for secondary prevention, the agency said.
Clinicians “should discontinue statin therapy in most pregnant patients, or they can consider the ongoing therapeutic needs of the individual patient, particularly those at very high risk for cardiovascular events during pregnancy. Because of the chronic nature of cardiovascular disease, treatment of hyperlipidemia is not generally necessary during pregnancy.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration (FDA) aims to update the labeling on all statins to remove the drugs’ blanket contraindication in all pregnant patients, the agency has announced. The change should reinforce for both physicians and patients that statin use in women with unrecognized pregnancy is unlikely to be harmful, it said.
“Because the benefits of statins may include prevention of serious or potentially fatal events in a small group of very high-risk pregnant patients, contraindicating these drugs in all pregnant women is not appropriate.”
The revision should emphasize for clinicians “that statins are safe to prescribe in patients who can become pregnant and help them reassure patients with unintended statin exposure in early pregnancy,” the FDA explained.
Removal of the broadly worded contraindication should “enable health care professionals and patients to make individual decisions about benefit and risk, especially for those at very high risk of heart attack or stroke." That includes women with homozygous familial hypercholesterolemia and those who are prescribed statins for secondary prevention, the agency said.
Clinicians “should discontinue statin therapy in most pregnant patients, or they can consider the ongoing therapeutic needs of the individual patient, particularly those at very high risk for cardiovascular events during pregnancy. Because of the chronic nature of cardiovascular disease, treatment of hyperlipidemia is not generally necessary during pregnancy.”
A version of this article first appeared on Medscape.com.
Heart failure med undertreatment because of older age common, flouts evidence
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
, suggests a large cohort study.
About 80% of patients aged 80 years or older were prescribed renin-angiotensin-system inhibitors (RASi) in a multivariate-adjusted analysis of more than 27,000 patients in the Swedish Heart Failure Registry (SwedeHF). In contrast, such drugs – which included angiotensin receptor-neprilysin inhibitors (ARNi), angiotensin receptor blockers, and ACE inhibitors – were prescribed to 95% of patients younger than 70 years.
Similarly, fewer of the oldest patients were offered meds from the two other drug classes core to HF management at the time: Beta blockers and mineralocorticoid receptor antagonists (MRA).
And among those in the 80-and-older age group who were prescribed RASi or beta blockers, their uptitration more often fell short of even half the target dosage, compared with the youngest patients in the analysis.
Physicians may hold back on full guideline-directed medical therapy in their very elderly patients with HFrEF for many reasons, including a perceived likelihood of drug intolerance due to frailty or multiple comorbidities, including renal dysfunction, Davide Stolfo, MD, Karolinska Institutet, Stockholm, and University of Trieste, Italy, told this news organization.
But the current analysis was adjusted for about 80 variables “that in our interpretation may be main reasons for not introducing drugs and using them in the older patients,” he said. They included care setting (that is, inpatient or outpatient), HF severity by several measures, a range of comorbidities, renal dysfunction, and history of serious illness such as cancer.
Even then, age emerged as a significant, independent predictor of medical therapy underuse in the oldest patients. Some physicians apparently see advanced age, by itself, as an “intrinsic reason” not to abide by HFrEF medical therapy recommendations, said Dr. Stolfo, who presented the analysis at HFA 2021, the annual meeting of the Heart Failure Association of the European Society of Cardiology (ESC-HFA), conducted both virtually and live in Florence, Italy.
Most major HF-drug trials have excluded or admitted few patients aged 80 years or older, but “the guidelines recommend treatment regardless of age, and in the trials there has been no influence from age on the effectiveness of drugs,” Dr. Stolfo observed.
Moreover, in a prior SwedeHF analysis with propensity matching, patients with HFrEF aged 80 or older showed steeper reductions in risk for death or HF hospitalization from treatment with RASi than those in younger age groups.
One of the few randomized trials to focus on the very elderly, called SENIORS, enrolled patients aged 70 years and older – the average age was 76 – and saw a significantly reduced risk of death or cardiovascular hospitalization for those assigned to the beta blocker nebivolol. The benefits in the trial, which was conducted 15 years ago, were independent of left ventricular function.
So in the oldest patients, “we could question the need to achieve full dose of an evidence-based drug, but we shouldn’t question the use of these drugs.”
The findings are consistent with a need to individualize medical therapy in senior patients with HFrEF, especially those of more advanced age, some of whom may be robust enough to be managed similarly to younger patients while others who may be less suitable for full guideline-directed medical therapy, Dr. Stolfo said.
Even for those who are more frail or have major comorbidities, drug therapy of HFrEF continues to be important for symptom control even if competing causes of death make it harder to prolong survival, Dr. Stolfo said.
“We should provide to all patients the best strategy they can tolerate,” he said. “If we cannot greatly impact on the long-term survival for these patients, treatment can be aimed to improve the quality of life and keep the patient out of the hospital.”
The analysis was supported by Boehringer Ingelheim. Dr. Stolfo disclosed personal fees from Novartis, Merck, GlaxoSmithKline, and Acceleron.
A version of this article first appeared on Medscape.com.
No overall statin effect seen on dementia, cognition in ASPREE analysis
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.