User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Sodium vs Potassium for Lowering Blood Pressure?
A pair of dueling editorials in the journal Hypertension debate whether our focus should be on sodium or its often neglected partner, potassium.
A meta-analysis of 85 trials showed a consistent and linear. It may also depend on where you live and whether your concern is treating individuals or implementing effective food policy.
The Case for Sodium Restriction
Stephen Juraschek, MD, PhD, of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, co-author of one editorial, told me in a zoom interview that he believes his side of the debate clearly has the stronger argument. Of the two cations in question, there has been infinitely more ink spilled about sodium.
Studies such as INTERSALT, the DASH diet, and TOHP may be the most well-known, but there are many, many intervention studies of sodium restriction’s effect on blood pressure. A meta-analysis of 85 trials of showed a consistent and linear relationship between sodium reduction and blood pressure. In contrast, the evidence base for potassium is more limited and less consistent. There are half as many trials with potassium, and its ability to lower blood pressure may depend on how much sodium is present in the diet.
An outlier in the sodium restriction evidence base is the PURE study, which suggested that extreme sodium restriction could increase cardiovascular mortality, but the trial suffered from two potential issues. First, it used a single spot urine specimen to measure sodium rather than the generally accepted more accurate 24-hour urine collection. A reanalysis of the TOHP study using a spot urine rather than a 24-hour urine collection changed the relationship between sodium intake and mortality and possibly explained the U-shaped association observed in PURE. Second, PURE was an observational cohort and was prone to confounding, or in this case, reverse causation. Why did people who consumed very little salt have an increased risk for cardiovascular disease? It is very possible that people with a high risk for cardiovascular disease were told to consume less salt to begin with. Hence B led to A rather than A leading to B.
The debate on sodium restriction has been bitter at times. Opposing camps formed, and people took sides in the “salt wars.” A group of researchers, termed the Jackson 6, met and decided to end the controversy by running a randomized trial in US prisons (having discounted the options of long-term care homes and military bases). They detailed their plan in an editorial in Hypertension. The study never came to fruition for two reasons: the obvious ethical problems of experimenting on prisoners and the revelation of undisclosed salt industry funding.
More recent studies have mercifully been more conventional. The SSaSS study, a randomized controlled trial of a salt substitute, provided the cardiovascular outcomes data that many were waiting for. And CARDIA-SSBP, a cross-over randomized trial recently presented at the American Heart Association meeting, showed that reducing dietary sodium was on par with medication when it came to lowering blood pressure.
For Dr. Juraschek, the evidence is clear: “If you were going to choose one, I would say the weight of the evidence is still really heavily on the sodium side.”
The Case for Potassium Supplementation
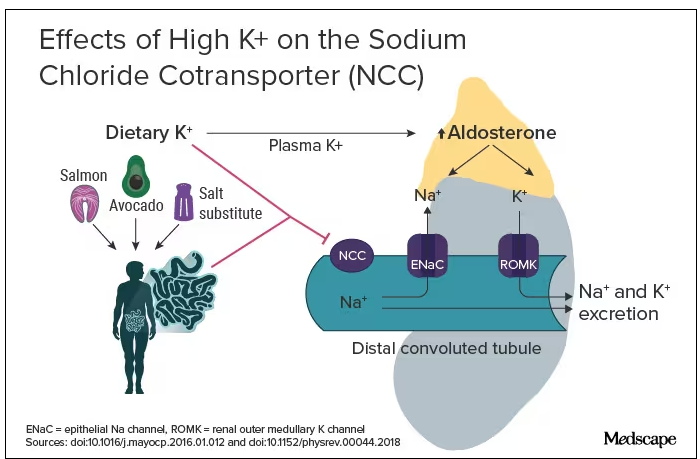
The evidence for salt restriction notwithstanding, Swapnil Hiremath, MD, MPH, from the University of Ottawa, Ontario, Canada, argued in his editorial that potassium supplementation has gotten short shrift. Though he admits the studies for potassium supplementation have been smaller and sometimes rely on observational evidence, the evidence is there. In the distal convoluted tubule, the sodium chloride cotransporter (NCC), aka the potassium switch, is turned on by low potassium levels and leads to sodium reabsorption by the kidney even in settings of high sodium intake (Figure). To nonnephrologists, renal physiology may be a black box. But if you quickly brush up on the mechanism of action of thiazide diuretics, the preceding descriptor will make more sense.
Dr. Hiremath points out that the DASH diet study also got patients to increase their potassium intake by eating more fruits and vegetables. Furthermore, the SSaSS study tested a salt substitute that was 25% potassium (and 75% sodium).
How much blood pressure lowering is due to sodium restriction vs potassium supplementation is a complex question because lowering sodium intake will invariably lead to more potassium intake. “It’s very hard to untangle the relationship,” Dr. Hiremath said in an interview. “It’s sort of synergistic but it’s not completely additive. It’s not as if you add four and four and get eight.” But he maintains there is more evidence regarding the benefit of potassium supplementation than many realize.
Realistic Diets and Taste Issues
“We know that increasing potassium, decreasing sodium is useful. The question is how do we do that?” says Dr. Hiremath. Should we encourage fruit and vegetable consumption in a healthy diet, give potassium supplements, or encourage the use of low-sodium salt substitutes?
Recommending a healthier diet with more fruits and vegetables is a no-brainer. But getting people to do it is hard. In a world where fruit is more expensive than junk food is, economic realities may drive food choice regardless of our best efforts. The 4700 mg of potassium in the DASH eating plan is the equivalent of eleven bananas daily; although not impossible, it would require a substantive shift in eating patterns for most people.
Given that we prescribe iron, vitamin B12, calcium, and vitamin D to patients who need them, why not potassium tablets to help with blood pressure? Granted, there are concerns about inducing hyperkalemia. Also, why not just prescribe a proven anti-hypertensive, such as ramipril, which has the added benefit of helping with renal protection or cardiac remodeling? Dr. Hiremath points out that patients are far less reluctant to take dietary supplements. Medication is something you take when sick. A supplement is seen as “natural” and “healthy” and might be more attractive to people resistant to prescription meds.
Another drawback of oral potassium supplementation is taste. In a Consumer Reports taste test, potassium chloride fared poorly. It was bitter and had a metallic aftertaste. At least one tester wouldn’t ever consume it again. Potassium citrate is slightly more palpable.
Salt substitutes, like the 75:25 ratio of sodium to potassium used in SSaSS, may be as high as you can go for potassium in any low-sodium salt alternative. If you go any higher than that, the taste will just turn people off, suggests Dr. Hiremath.
But SsaSS, which was done in China, may not be relevant to North America. In China, most sodium is added during cooking at home, and the consumption of processed foods is low. For the typical North American, roughly three quarters of the sodium eaten is added to their food by someone else; only about 15% is added during cooking at home or at the dinner table. If you aren’t someone who cooks, buying a salt substitute is probably not going to have much impact.
Given that reality, Dr. Juraschek thinks we need to target the sodium in processed foods. “There’s just so much sodium in so many products,” he says. “When you think about public policy, it’s most expeditious for there to be more regulation about how much is added to our food supply vs trying to get people to consume eight to 12 servings of fruit.”
No Salt War Here
Despite their different editorial takes, Dr. Hiremath and Dr. Juraschek largely agree on the broad strokes of the problem. This isn’t X (or Twitter) after all. Potassium supplementation may be useful in some parts of the world but may not address the underlying problem in countries where processed foods are the source of most dietary sodium.
The CARDIA-SSBP trial showed that a very low–sodium diet had the same blood pressure–lowering effect as a first-line antihypertensive, but most people will not be able to limit themselves to 500 mg of dietary sodium per day. In CARDIA-SSBP, just as in DASH, participants were provided with meals from study kitchens. They were not just told to eat less salt, which would almost certainly have failed.
“We should aim for stuff that is practical and doable rather than aim for stuff that cannot be done,” according to Dr. Hiremath. Whether that should be salt substitutes or policy change may depend on which part of the planet you live on.
One recent positive change may herald the beginning of a policy change, at least in the United States. In March 2023, the US Food and Drug Administration proposed a rule change to allow salt substitutes to be labeled as salt. This would make it easier for food manufacturers to swap out sodium chloride for a low-sodium alternative and reduce the amount of sodium in the US diet without having a large impact on taste and consumer uptake. Both Dr. Hiremath and Dr. Juraschek agree that it may not be enough on its own but that it’s a start.
Christopher Labos is a cardiologist with a degree in epidemiology. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally, he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal, and is host of the award-winning podcast The Body of Evidence.
A version of this article appeared on Medscape.com.
A pair of dueling editorials in the journal Hypertension debate whether our focus should be on sodium or its often neglected partner, potassium.
A meta-analysis of 85 trials showed a consistent and linear. It may also depend on where you live and whether your concern is treating individuals or implementing effective food policy.
The Case for Sodium Restriction
Stephen Juraschek, MD, PhD, of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, co-author of one editorial, told me in a zoom interview that he believes his side of the debate clearly has the stronger argument. Of the two cations in question, there has been infinitely more ink spilled about sodium.
Studies such as INTERSALT, the DASH diet, and TOHP may be the most well-known, but there are many, many intervention studies of sodium restriction’s effect on blood pressure. A meta-analysis of 85 trials of showed a consistent and linear relationship between sodium reduction and blood pressure. In contrast, the evidence base for potassium is more limited and less consistent. There are half as many trials with potassium, and its ability to lower blood pressure may depend on how much sodium is present in the diet.
An outlier in the sodium restriction evidence base is the PURE study, which suggested that extreme sodium restriction could increase cardiovascular mortality, but the trial suffered from two potential issues. First, it used a single spot urine specimen to measure sodium rather than the generally accepted more accurate 24-hour urine collection. A reanalysis of the TOHP study using a spot urine rather than a 24-hour urine collection changed the relationship between sodium intake and mortality and possibly explained the U-shaped association observed in PURE. Second, PURE was an observational cohort and was prone to confounding, or in this case, reverse causation. Why did people who consumed very little salt have an increased risk for cardiovascular disease? It is very possible that people with a high risk for cardiovascular disease were told to consume less salt to begin with. Hence B led to A rather than A leading to B.
The debate on sodium restriction has been bitter at times. Opposing camps formed, and people took sides in the “salt wars.” A group of researchers, termed the Jackson 6, met and decided to end the controversy by running a randomized trial in US prisons (having discounted the options of long-term care homes and military bases). They detailed their plan in an editorial in Hypertension. The study never came to fruition for two reasons: the obvious ethical problems of experimenting on prisoners and the revelation of undisclosed salt industry funding.
More recent studies have mercifully been more conventional. The SSaSS study, a randomized controlled trial of a salt substitute, provided the cardiovascular outcomes data that many were waiting for. And CARDIA-SSBP, a cross-over randomized trial recently presented at the American Heart Association meeting, showed that reducing dietary sodium was on par with medication when it came to lowering blood pressure.
For Dr. Juraschek, the evidence is clear: “If you were going to choose one, I would say the weight of the evidence is still really heavily on the sodium side.”
The Case for Potassium Supplementation
The evidence for salt restriction notwithstanding, Swapnil Hiremath, MD, MPH, from the University of Ottawa, Ontario, Canada, argued in his editorial that potassium supplementation has gotten short shrift. Though he admits the studies for potassium supplementation have been smaller and sometimes rely on observational evidence, the evidence is there. In the distal convoluted tubule, the sodium chloride cotransporter (NCC), aka the potassium switch, is turned on by low potassium levels and leads to sodium reabsorption by the kidney even in settings of high sodium intake (Figure). To nonnephrologists, renal physiology may be a black box. But if you quickly brush up on the mechanism of action of thiazide diuretics, the preceding descriptor will make more sense.
Dr. Hiremath points out that the DASH diet study also got patients to increase their potassium intake by eating more fruits and vegetables. Furthermore, the SSaSS study tested a salt substitute that was 25% potassium (and 75% sodium).
How much blood pressure lowering is due to sodium restriction vs potassium supplementation is a complex question because lowering sodium intake will invariably lead to more potassium intake. “It’s very hard to untangle the relationship,” Dr. Hiremath said in an interview. “It’s sort of synergistic but it’s not completely additive. It’s not as if you add four and four and get eight.” But he maintains there is more evidence regarding the benefit of potassium supplementation than many realize.
Realistic Diets and Taste Issues
“We know that increasing potassium, decreasing sodium is useful. The question is how do we do that?” says Dr. Hiremath. Should we encourage fruit and vegetable consumption in a healthy diet, give potassium supplements, or encourage the use of low-sodium salt substitutes?
Recommending a healthier diet with more fruits and vegetables is a no-brainer. But getting people to do it is hard. In a world where fruit is more expensive than junk food is, economic realities may drive food choice regardless of our best efforts. The 4700 mg of potassium in the DASH eating plan is the equivalent of eleven bananas daily; although not impossible, it would require a substantive shift in eating patterns for most people.
Given that we prescribe iron, vitamin B12, calcium, and vitamin D to patients who need them, why not potassium tablets to help with blood pressure? Granted, there are concerns about inducing hyperkalemia. Also, why not just prescribe a proven anti-hypertensive, such as ramipril, which has the added benefit of helping with renal protection or cardiac remodeling? Dr. Hiremath points out that patients are far less reluctant to take dietary supplements. Medication is something you take when sick. A supplement is seen as “natural” and “healthy” and might be more attractive to people resistant to prescription meds.
Another drawback of oral potassium supplementation is taste. In a Consumer Reports taste test, potassium chloride fared poorly. It was bitter and had a metallic aftertaste. At least one tester wouldn’t ever consume it again. Potassium citrate is slightly more palpable.
Salt substitutes, like the 75:25 ratio of sodium to potassium used in SSaSS, may be as high as you can go for potassium in any low-sodium salt alternative. If you go any higher than that, the taste will just turn people off, suggests Dr. Hiremath.
But SsaSS, which was done in China, may not be relevant to North America. In China, most sodium is added during cooking at home, and the consumption of processed foods is low. For the typical North American, roughly three quarters of the sodium eaten is added to their food by someone else; only about 15% is added during cooking at home or at the dinner table. If you aren’t someone who cooks, buying a salt substitute is probably not going to have much impact.
Given that reality, Dr. Juraschek thinks we need to target the sodium in processed foods. “There’s just so much sodium in so many products,” he says. “When you think about public policy, it’s most expeditious for there to be more regulation about how much is added to our food supply vs trying to get people to consume eight to 12 servings of fruit.”
No Salt War Here
Despite their different editorial takes, Dr. Hiremath and Dr. Juraschek largely agree on the broad strokes of the problem. This isn’t X (or Twitter) after all. Potassium supplementation may be useful in some parts of the world but may not address the underlying problem in countries where processed foods are the source of most dietary sodium.
The CARDIA-SSBP trial showed that a very low–sodium diet had the same blood pressure–lowering effect as a first-line antihypertensive, but most people will not be able to limit themselves to 500 mg of dietary sodium per day. In CARDIA-SSBP, just as in DASH, participants were provided with meals from study kitchens. They were not just told to eat less salt, which would almost certainly have failed.
“We should aim for stuff that is practical and doable rather than aim for stuff that cannot be done,” according to Dr. Hiremath. Whether that should be salt substitutes or policy change may depend on which part of the planet you live on.
One recent positive change may herald the beginning of a policy change, at least in the United States. In March 2023, the US Food and Drug Administration proposed a rule change to allow salt substitutes to be labeled as salt. This would make it easier for food manufacturers to swap out sodium chloride for a low-sodium alternative and reduce the amount of sodium in the US diet without having a large impact on taste and consumer uptake. Both Dr. Hiremath and Dr. Juraschek agree that it may not be enough on its own but that it’s a start.
Christopher Labos is a cardiologist with a degree in epidemiology. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally, he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal, and is host of the award-winning podcast The Body of Evidence.
A version of this article appeared on Medscape.com.
A pair of dueling editorials in the journal Hypertension debate whether our focus should be on sodium or its often neglected partner, potassium.
A meta-analysis of 85 trials showed a consistent and linear. It may also depend on where you live and whether your concern is treating individuals or implementing effective food policy.
The Case for Sodium Restriction
Stephen Juraschek, MD, PhD, of the Beth Israel Deaconess Medical Center, Boston, Massachusetts, co-author of one editorial, told me in a zoom interview that he believes his side of the debate clearly has the stronger argument. Of the two cations in question, there has been infinitely more ink spilled about sodium.
Studies such as INTERSALT, the DASH diet, and TOHP may be the most well-known, but there are many, many intervention studies of sodium restriction’s effect on blood pressure. A meta-analysis of 85 trials of showed a consistent and linear relationship between sodium reduction and blood pressure. In contrast, the evidence base for potassium is more limited and less consistent. There are half as many trials with potassium, and its ability to lower blood pressure may depend on how much sodium is present in the diet.
An outlier in the sodium restriction evidence base is the PURE study, which suggested that extreme sodium restriction could increase cardiovascular mortality, but the trial suffered from two potential issues. First, it used a single spot urine specimen to measure sodium rather than the generally accepted more accurate 24-hour urine collection. A reanalysis of the TOHP study using a spot urine rather than a 24-hour urine collection changed the relationship between sodium intake and mortality and possibly explained the U-shaped association observed in PURE. Second, PURE was an observational cohort and was prone to confounding, or in this case, reverse causation. Why did people who consumed very little salt have an increased risk for cardiovascular disease? It is very possible that people with a high risk for cardiovascular disease were told to consume less salt to begin with. Hence B led to A rather than A leading to B.
The debate on sodium restriction has been bitter at times. Opposing camps formed, and people took sides in the “salt wars.” A group of researchers, termed the Jackson 6, met and decided to end the controversy by running a randomized trial in US prisons (having discounted the options of long-term care homes and military bases). They detailed their plan in an editorial in Hypertension. The study never came to fruition for two reasons: the obvious ethical problems of experimenting on prisoners and the revelation of undisclosed salt industry funding.
More recent studies have mercifully been more conventional. The SSaSS study, a randomized controlled trial of a salt substitute, provided the cardiovascular outcomes data that many were waiting for. And CARDIA-SSBP, a cross-over randomized trial recently presented at the American Heart Association meeting, showed that reducing dietary sodium was on par with medication when it came to lowering blood pressure.
For Dr. Juraschek, the evidence is clear: “If you were going to choose one, I would say the weight of the evidence is still really heavily on the sodium side.”
The Case for Potassium Supplementation
The evidence for salt restriction notwithstanding, Swapnil Hiremath, MD, MPH, from the University of Ottawa, Ontario, Canada, argued in his editorial that potassium supplementation has gotten short shrift. Though he admits the studies for potassium supplementation have been smaller and sometimes rely on observational evidence, the evidence is there. In the distal convoluted tubule, the sodium chloride cotransporter (NCC), aka the potassium switch, is turned on by low potassium levels and leads to sodium reabsorption by the kidney even in settings of high sodium intake (Figure). To nonnephrologists, renal physiology may be a black box. But if you quickly brush up on the mechanism of action of thiazide diuretics, the preceding descriptor will make more sense.
Dr. Hiremath points out that the DASH diet study also got patients to increase their potassium intake by eating more fruits and vegetables. Furthermore, the SSaSS study tested a salt substitute that was 25% potassium (and 75% sodium).
How much blood pressure lowering is due to sodium restriction vs potassium supplementation is a complex question because lowering sodium intake will invariably lead to more potassium intake. “It’s very hard to untangle the relationship,” Dr. Hiremath said in an interview. “It’s sort of synergistic but it’s not completely additive. It’s not as if you add four and four and get eight.” But he maintains there is more evidence regarding the benefit of potassium supplementation than many realize.
Realistic Diets and Taste Issues
“We know that increasing potassium, decreasing sodium is useful. The question is how do we do that?” says Dr. Hiremath. Should we encourage fruit and vegetable consumption in a healthy diet, give potassium supplements, or encourage the use of low-sodium salt substitutes?
Recommending a healthier diet with more fruits and vegetables is a no-brainer. But getting people to do it is hard. In a world where fruit is more expensive than junk food is, economic realities may drive food choice regardless of our best efforts. The 4700 mg of potassium in the DASH eating plan is the equivalent of eleven bananas daily; although not impossible, it would require a substantive shift in eating patterns for most people.
Given that we prescribe iron, vitamin B12, calcium, and vitamin D to patients who need them, why not potassium tablets to help with blood pressure? Granted, there are concerns about inducing hyperkalemia. Also, why not just prescribe a proven anti-hypertensive, such as ramipril, which has the added benefit of helping with renal protection or cardiac remodeling? Dr. Hiremath points out that patients are far less reluctant to take dietary supplements. Medication is something you take when sick. A supplement is seen as “natural” and “healthy” and might be more attractive to people resistant to prescription meds.
Another drawback of oral potassium supplementation is taste. In a Consumer Reports taste test, potassium chloride fared poorly. It was bitter and had a metallic aftertaste. At least one tester wouldn’t ever consume it again. Potassium citrate is slightly more palpable.
Salt substitutes, like the 75:25 ratio of sodium to potassium used in SSaSS, may be as high as you can go for potassium in any low-sodium salt alternative. If you go any higher than that, the taste will just turn people off, suggests Dr. Hiremath.
But SsaSS, which was done in China, may not be relevant to North America. In China, most sodium is added during cooking at home, and the consumption of processed foods is low. For the typical North American, roughly three quarters of the sodium eaten is added to their food by someone else; only about 15% is added during cooking at home or at the dinner table. If you aren’t someone who cooks, buying a salt substitute is probably not going to have much impact.
Given that reality, Dr. Juraschek thinks we need to target the sodium in processed foods. “There’s just so much sodium in so many products,” he says. “When you think about public policy, it’s most expeditious for there to be more regulation about how much is added to our food supply vs trying to get people to consume eight to 12 servings of fruit.”
No Salt War Here
Despite their different editorial takes, Dr. Hiremath and Dr. Juraschek largely agree on the broad strokes of the problem. This isn’t X (or Twitter) after all. Potassium supplementation may be useful in some parts of the world but may not address the underlying problem in countries where processed foods are the source of most dietary sodium.
The CARDIA-SSBP trial showed that a very low–sodium diet had the same blood pressure–lowering effect as a first-line antihypertensive, but most people will not be able to limit themselves to 500 mg of dietary sodium per day. In CARDIA-SSBP, just as in DASH, participants were provided with meals from study kitchens. They were not just told to eat less salt, which would almost certainly have failed.
“We should aim for stuff that is practical and doable rather than aim for stuff that cannot be done,” according to Dr. Hiremath. Whether that should be salt substitutes or policy change may depend on which part of the planet you live on.
One recent positive change may herald the beginning of a policy change, at least in the United States. In March 2023, the US Food and Drug Administration proposed a rule change to allow salt substitutes to be labeled as salt. This would make it easier for food manufacturers to swap out sodium chloride for a low-sodium alternative and reduce the amount of sodium in the US diet without having a large impact on taste and consumer uptake. Both Dr. Hiremath and Dr. Juraschek agree that it may not be enough on its own but that it’s a start.
Christopher Labos is a cardiologist with a degree in epidemiology. He spends most of his time doing things that he doesn’t get paid for, like research, teaching, and podcasting. Occasionally, he finds time to practice cardiology to pay the rent. He realizes that half of his research findings will be disproved in 5 years; he just doesn’t know which half. He is a regular contributor to the Montreal Gazette, CJAD radio, and CTV television in Montreal, and is host of the award-winning podcast The Body of Evidence.
A version of this article appeared on Medscape.com.
Do Your Patients Hate Exercise? Suggest They Do This Instead
Have patients who want to lose weight? Tell them to put on their dancing shoes.
Dancing can be an effective fat-loss tool for people who are overweight or have obesity, according to a recent meta-analysis in PLOS One.
Participants who danced three times a week for at least 3 months reaped maximum benefits. And the more they let loose, the better — more creative dance forms led to more pronounced improvements in body composition.
The study builds on previous research that suggests dance can be beneficial for weight loss and overall health. A 2017 meta-analysis found that dance significantly improved body composition, blood biomarkers, and musculoskeletal function. Other research has linked dance with improvements in cognitive function, mental health, and quality of life.
What makes dance special? It’s a full-body workout that might be easier to stick with than other exercises. “Enjoyment” is key for sustainability, the researchers wrote: “As a form of physical activity that integrates exercise, entertainment, and sociality, dance possesses innate advantages in fostering motivation for exercise.”
“The best exercise is the one you’ll do every day, and something that you like to do,” said Nicholas Pennings, DO, chair and associate professor of family medicine at Campbell University, Buies Creek, NC. (Dr. Pennings was not involved in the study.) For patients who enjoy dancing, dance could be that thing — or at least one workout to add to the mix.
Help your patients get started with these tips.
Frame it as a hobby, not exercise. Ask what hobbies they used to enjoy in high school, suggests Deirdre Mattina, MD, a cardiologist at the Cleveland Clinic and a former professional dancer. “ This can sometimes evoke happy memories of younger years and perhaps hobbies that they’d given up because they thought they were too old,” she said. If they used to play sports or dance, that’s your in. “I usually talk about hot yoga as a transition to get back their flexibility and then something like a dance aerobics or Zumba class to start.”
Recommend a group class. “Any intervention promoting social relationships is expected to increase adherence,” said Giulio Marchesini Reggiani, MD, a recently retired professor of internal medicine and dietetics at the University of Bologna in Italy. “You are motivated by the group, and you create a relationship among participants, and this means that you are no longer alone.” Try local gyms, health clubs, or even dance studios (yes, where kids go — they offer adult classes, too).
Help patients find their unique groove. Dr. Mattina has some patients who take cardio dance classes, some who line dance, and others who pole dance or heels dance. “Those are the things that keep it fun,” she said. “It doesn’t seem like exercise. It seems more like going out and hanging out.”
Encourage those who “don’t know how to dance.” You don’t need fancy choreography or the grace of a prima ballerina.”Simply move aided by the music,” said Dr. Reggiani. “As long as you start engaging in physical activity, you improve your health, and you improve your movement.” Suggest patients start with beginner Zumba or a step class to get the hang of moving to a beat. Or try a home dance video, like Barre Blend by BODi (which offers a 14-day free trial). “You can try taking a couple classes in the privacy of your own home first, so you feel comfortable getting out there and doing it with a group,” said Dr. Mattina.
Modify as needed. If a patient has mobility limitations or lower-body pain, they can still dance — just do the upper-body portion of the moves. “Dance involves both upper and lower body movement, and so many dance activities could easily be performed in a chair,” said Dr. Pennings. A good joint-friendly option: Some health clubs offer dance classes that take place in a swimming pool.
Involve the whole family. Support from a partner can help patients stick with exercise, said Dr. Reggiani, and dance can also help a couple strengthen their bond. Invite kids and grandparents to join, too. “Dancing is something that can be done at any age,” said Dr. Reggiani. “For kids, it is important to make it fun,” said Dr. Pennings. “Start when they are young with music they are familiar with and enjoy.” For skeptical partners? “Keep it simple and nonjudgmental,” he said.
Remind patients to warm up. We lose flexibility with age, so ease into it, said Dr. Mattina. Many classes include warmups, but if you’re at home, do a few minutes of light, low-impact cardio — jumping jacks, mountain climbers, jogging, or brisk walking — before stretching. Or just put on a slow song and start lightly bouncing to the beat or stepping your feet to one side, together, then to the other side and together.
Tell them to take dance breaks. No time to join a class? Break up the workday with a few 10-minute dance parties. (That’s about three songs.) “Short bursts of exercise throughout the day, like if you do 10 minutes of exercise six times a day, actually has a greater health benefit than doing 60 minutes of continuous exercise,” said Dr. Pennings. It helps counter the negative effects of prolonged sitting “by increasing blood flow and increasing utilization of your muscles.”
Manage expectations about weight loss. Patients often have outsized expectations about how much weight they’ll lose when starting a new exercise regimen, Dr. Pennings said. Dancing burns about 300 calories per hour, so it takes roughly 12 hours to lose one pound. Consistency over time is the key. “My goal is to both emphasize the health benefits of exercise while maintaining realistic expectations about weight loss,” said Dr. Pennings. Focus less on the weight part and highlight other benefits: Dancing builds strength, balance, and coordination, said Dr. Pennings. It can help improve blood pressure and other heart health markers and boost cognition in older adults. And it’s fun.
A version of this article appeared on Medscape.com.
Have patients who want to lose weight? Tell them to put on their dancing shoes.
Dancing can be an effective fat-loss tool for people who are overweight or have obesity, according to a recent meta-analysis in PLOS One.
Participants who danced three times a week for at least 3 months reaped maximum benefits. And the more they let loose, the better — more creative dance forms led to more pronounced improvements in body composition.
The study builds on previous research that suggests dance can be beneficial for weight loss and overall health. A 2017 meta-analysis found that dance significantly improved body composition, blood biomarkers, and musculoskeletal function. Other research has linked dance with improvements in cognitive function, mental health, and quality of life.
What makes dance special? It’s a full-body workout that might be easier to stick with than other exercises. “Enjoyment” is key for sustainability, the researchers wrote: “As a form of physical activity that integrates exercise, entertainment, and sociality, dance possesses innate advantages in fostering motivation for exercise.”
“The best exercise is the one you’ll do every day, and something that you like to do,” said Nicholas Pennings, DO, chair and associate professor of family medicine at Campbell University, Buies Creek, NC. (Dr. Pennings was not involved in the study.) For patients who enjoy dancing, dance could be that thing — or at least one workout to add to the mix.
Help your patients get started with these tips.
Frame it as a hobby, not exercise. Ask what hobbies they used to enjoy in high school, suggests Deirdre Mattina, MD, a cardiologist at the Cleveland Clinic and a former professional dancer. “ This can sometimes evoke happy memories of younger years and perhaps hobbies that they’d given up because they thought they were too old,” she said. If they used to play sports or dance, that’s your in. “I usually talk about hot yoga as a transition to get back their flexibility and then something like a dance aerobics or Zumba class to start.”
Recommend a group class. “Any intervention promoting social relationships is expected to increase adherence,” said Giulio Marchesini Reggiani, MD, a recently retired professor of internal medicine and dietetics at the University of Bologna in Italy. “You are motivated by the group, and you create a relationship among participants, and this means that you are no longer alone.” Try local gyms, health clubs, or even dance studios (yes, where kids go — they offer adult classes, too).
Help patients find their unique groove. Dr. Mattina has some patients who take cardio dance classes, some who line dance, and others who pole dance or heels dance. “Those are the things that keep it fun,” she said. “It doesn’t seem like exercise. It seems more like going out and hanging out.”
Encourage those who “don’t know how to dance.” You don’t need fancy choreography or the grace of a prima ballerina.”Simply move aided by the music,” said Dr. Reggiani. “As long as you start engaging in physical activity, you improve your health, and you improve your movement.” Suggest patients start with beginner Zumba or a step class to get the hang of moving to a beat. Or try a home dance video, like Barre Blend by BODi (which offers a 14-day free trial). “You can try taking a couple classes in the privacy of your own home first, so you feel comfortable getting out there and doing it with a group,” said Dr. Mattina.
Modify as needed. If a patient has mobility limitations or lower-body pain, they can still dance — just do the upper-body portion of the moves. “Dance involves both upper and lower body movement, and so many dance activities could easily be performed in a chair,” said Dr. Pennings. A good joint-friendly option: Some health clubs offer dance classes that take place in a swimming pool.
Involve the whole family. Support from a partner can help patients stick with exercise, said Dr. Reggiani, and dance can also help a couple strengthen their bond. Invite kids and grandparents to join, too. “Dancing is something that can be done at any age,” said Dr. Reggiani. “For kids, it is important to make it fun,” said Dr. Pennings. “Start when they are young with music they are familiar with and enjoy.” For skeptical partners? “Keep it simple and nonjudgmental,” he said.
Remind patients to warm up. We lose flexibility with age, so ease into it, said Dr. Mattina. Many classes include warmups, but if you’re at home, do a few minutes of light, low-impact cardio — jumping jacks, mountain climbers, jogging, or brisk walking — before stretching. Or just put on a slow song and start lightly bouncing to the beat or stepping your feet to one side, together, then to the other side and together.
Tell them to take dance breaks. No time to join a class? Break up the workday with a few 10-minute dance parties. (That’s about three songs.) “Short bursts of exercise throughout the day, like if you do 10 minutes of exercise six times a day, actually has a greater health benefit than doing 60 minutes of continuous exercise,” said Dr. Pennings. It helps counter the negative effects of prolonged sitting “by increasing blood flow and increasing utilization of your muscles.”
Manage expectations about weight loss. Patients often have outsized expectations about how much weight they’ll lose when starting a new exercise regimen, Dr. Pennings said. Dancing burns about 300 calories per hour, so it takes roughly 12 hours to lose one pound. Consistency over time is the key. “My goal is to both emphasize the health benefits of exercise while maintaining realistic expectations about weight loss,” said Dr. Pennings. Focus less on the weight part and highlight other benefits: Dancing builds strength, balance, and coordination, said Dr. Pennings. It can help improve blood pressure and other heart health markers and boost cognition in older adults. And it’s fun.
A version of this article appeared on Medscape.com.
Have patients who want to lose weight? Tell them to put on their dancing shoes.
Dancing can be an effective fat-loss tool for people who are overweight or have obesity, according to a recent meta-analysis in PLOS One.
Participants who danced three times a week for at least 3 months reaped maximum benefits. And the more they let loose, the better — more creative dance forms led to more pronounced improvements in body composition.
The study builds on previous research that suggests dance can be beneficial for weight loss and overall health. A 2017 meta-analysis found that dance significantly improved body composition, blood biomarkers, and musculoskeletal function. Other research has linked dance with improvements in cognitive function, mental health, and quality of life.
What makes dance special? It’s a full-body workout that might be easier to stick with than other exercises. “Enjoyment” is key for sustainability, the researchers wrote: “As a form of physical activity that integrates exercise, entertainment, and sociality, dance possesses innate advantages in fostering motivation for exercise.”
“The best exercise is the one you’ll do every day, and something that you like to do,” said Nicholas Pennings, DO, chair and associate professor of family medicine at Campbell University, Buies Creek, NC. (Dr. Pennings was not involved in the study.) For patients who enjoy dancing, dance could be that thing — or at least one workout to add to the mix.
Help your patients get started with these tips.
Frame it as a hobby, not exercise. Ask what hobbies they used to enjoy in high school, suggests Deirdre Mattina, MD, a cardiologist at the Cleveland Clinic and a former professional dancer. “ This can sometimes evoke happy memories of younger years and perhaps hobbies that they’d given up because they thought they were too old,” she said. If they used to play sports or dance, that’s your in. “I usually talk about hot yoga as a transition to get back their flexibility and then something like a dance aerobics or Zumba class to start.”
Recommend a group class. “Any intervention promoting social relationships is expected to increase adherence,” said Giulio Marchesini Reggiani, MD, a recently retired professor of internal medicine and dietetics at the University of Bologna in Italy. “You are motivated by the group, and you create a relationship among participants, and this means that you are no longer alone.” Try local gyms, health clubs, or even dance studios (yes, where kids go — they offer adult classes, too).
Help patients find their unique groove. Dr. Mattina has some patients who take cardio dance classes, some who line dance, and others who pole dance or heels dance. “Those are the things that keep it fun,” she said. “It doesn’t seem like exercise. It seems more like going out and hanging out.”
Encourage those who “don’t know how to dance.” You don’t need fancy choreography or the grace of a prima ballerina.”Simply move aided by the music,” said Dr. Reggiani. “As long as you start engaging in physical activity, you improve your health, and you improve your movement.” Suggest patients start with beginner Zumba or a step class to get the hang of moving to a beat. Or try a home dance video, like Barre Blend by BODi (which offers a 14-day free trial). “You can try taking a couple classes in the privacy of your own home first, so you feel comfortable getting out there and doing it with a group,” said Dr. Mattina.
Modify as needed. If a patient has mobility limitations or lower-body pain, they can still dance — just do the upper-body portion of the moves. “Dance involves both upper and lower body movement, and so many dance activities could easily be performed in a chair,” said Dr. Pennings. A good joint-friendly option: Some health clubs offer dance classes that take place in a swimming pool.
Involve the whole family. Support from a partner can help patients stick with exercise, said Dr. Reggiani, and dance can also help a couple strengthen their bond. Invite kids and grandparents to join, too. “Dancing is something that can be done at any age,” said Dr. Reggiani. “For kids, it is important to make it fun,” said Dr. Pennings. “Start when they are young with music they are familiar with and enjoy.” For skeptical partners? “Keep it simple and nonjudgmental,” he said.
Remind patients to warm up. We lose flexibility with age, so ease into it, said Dr. Mattina. Many classes include warmups, but if you’re at home, do a few minutes of light, low-impact cardio — jumping jacks, mountain climbers, jogging, or brisk walking — before stretching. Or just put on a slow song and start lightly bouncing to the beat or stepping your feet to one side, together, then to the other side and together.
Tell them to take dance breaks. No time to join a class? Break up the workday with a few 10-minute dance parties. (That’s about three songs.) “Short bursts of exercise throughout the day, like if you do 10 minutes of exercise six times a day, actually has a greater health benefit than doing 60 minutes of continuous exercise,” said Dr. Pennings. It helps counter the negative effects of prolonged sitting “by increasing blood flow and increasing utilization of your muscles.”
Manage expectations about weight loss. Patients often have outsized expectations about how much weight they’ll lose when starting a new exercise regimen, Dr. Pennings said. Dancing burns about 300 calories per hour, so it takes roughly 12 hours to lose one pound. Consistency over time is the key. “My goal is to both emphasize the health benefits of exercise while maintaining realistic expectations about weight loss,” said Dr. Pennings. Focus less on the weight part and highlight other benefits: Dancing builds strength, balance, and coordination, said Dr. Pennings. It can help improve blood pressure and other heart health markers and boost cognition in older adults. And it’s fun.
A version of this article appeared on Medscape.com.
Colchicine May Benefit Patients With Diabetes and Recent MI
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
TOPLINE:
A daily low dose of colchicine significantly reduces ischemic cardiovascular events in patients with type 2 diabetes (T2D) and a recent myocardial infarction (MI).
METHODOLOGY:
- After an MI, patients with vs without T2D have a higher risk for another cardiovascular event.
- The Colchicine Cardiovascular Outcomes Trial (COLCOT), a randomized, double-blinded trial, found a lower risk for ischemic cardiovascular events with 0.5 mg colchicine taken daily vs placebo, initiated within 30 days of an MI.
- Researchers conducted a prespecified subgroup analysis of 959 adult patients with T2D (mean age, 62.4 years; 22.2% women) in COLCOT (462 patients in colchicine and 497 patients in placebo groups).
- The primary efficacy endpoint was a composite of cardiovascular death, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina requiring coronary revascularization within a median 23 months.
- The patients were taking a variety of appropriate medications, including aspirin and another antiplatelet agent and a statin (98%-99%) and metformin (75%-76%).
TAKEAWAY:
- The risk for the primary endpoint was reduced by 35% in patients with T2D who received colchicine than in those who received placebo (hazard ratio, 0.65; P = .03).
- The primary endpoint event rate per 100 patient-months was significantly lower in the colchicine group than in the placebo group (rate ratio, 0.53; P = .01).
- The frequencies of adverse events were similar in both the treatment and placebo groups (14.6% and 12.8%, respectively; P = .41), with gastrointestinal adverse events being the most common.
- In COLCOT, patients with T2D had a 1.86-fold higher risk for a primary endpoint cardiovascular event, but there was no significant difference in the primary endpoint between those with and without T2D on colchicine.
IN PRACTICE:
“Patients with both T2D and a recent MI derive a large benefit from inflammation-reducing therapy with colchicine,” the authors noted.
SOURCE:
This study, led by François Roubille, University Hospital of Montpellier, France, was published online on January 5, 2024, in Diabetes Care.
LIMITATIONS:
Patients were not stratified at inclusion for the presence of diabetes. Also, the study did not evaluate the role of glycated hemoglobin and low-density lipoprotein cholesterol, as well as the effects of different glucose-lowering medications or possible hypoglycemic episodes.
DISCLOSURES:
The COLCOT study was funded by the Government of Quebec, the Canadian Institutes of Health Research, and philanthropic foundations. Coauthors Jean-Claude Tardif and Wolfgang Koenig declared receiving research grants, honoraria, advisory board fees, and lecture fees from pharmaceutical companies, as well as having other ties with various sources.
A version of this article appeared on Medscape.com.
HPV Vaccine Shown to Be Highly Effective in Girls Years Later
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Cervical cancer is the fourth most common cancer among women worldwide.
- Programs to provide Cervarix, a bivalent vaccine, began in the United Kingdom in 2007.
- After the initiation of the programs, administering the vaccine became part of routine care for girls starting at age 12 years.
- Researchers collected data in 2020 from 447,845 women born between 1988 and 1996 from the Scottish cervical cancer screening system to assess the efficacy of Cervarix in lowering rates of cervical cancer.
- They correlated the rate of cervical cancer per 100,000 person-years with data on women regarding vaccination status, age when vaccinated, and deprivation in areas like income, housing, and health.
TAKEAWAY:
- No cases of cervical cancer were found among women who were immunized at ages 12 or 13 years, no matter how many doses they received.
- Women who were immunized between ages 14 and 18 years and received three doses had fewer instances of cervical cancer compared with unvaccinated women regardless of deprivation status (3.2 cases per 100,00 women vs 8.4 cases per 100,000).
IN PRACTICE:
“Continued participation in screening and monitoring of outcomes is required, however, to assess the effects of changes in vaccines used and dosage schedules since the start of vaccination in Scotland in 2008 and the longevity of protection the vaccines offer.”
SOURCE:
The study was led by Timothy J. Palmer, PhD, Scottish Clinical Lead for Cervical Screening at Public Health Scotland.
LIMITATIONS:
Only 14,645 women had received just one or two doses, which may have affected the statistical analysis.
DISCLOSURES:
The study was funded by Public Health Scotland. A coauthor reports attending an advisory board meeting for HOLOGIC and Vaccitech. Her institution received research funding or gratis support funding from Cepheid, Euroimmun, GeneFirst, SelfScreen, Hiantis, Seegene, Roche, Hologic, and Vaccitech in the past 3 years.
A version of this article appeared on Medscape.com.
Bone Mineral Density Higher in Children Living Near Green Areas
A recently published prospective study in JAMA Network Open identified a significant association between children’s bone health and their proximity to green areas.
The literature emphasized the benefits of childhood exposure to green spaces for neurocognitive, social, behavioral, and mental development, as well as well-being. In addition, such exposure is linked to lower body mass index, increased physical activity, and reduced risks for overweight, obesity, and hypertension. However, specific data on bone mineral density implications are limited.
To address this gap, Hanne Sleurs, PhD, a researcher at the Universiteit Hasselt in Belgium, and colleagues followed the bone health of 327 participants from birth to 4-6 years and examined correlations with individuals’ exposure to green areas. Data collection occurred from October 2014 to July 2021.
Green spaces were categorized as high (vegetation height > 3 m), low (vegetation height ≤ 3 m), and mixed (combination of both). The distances of green spaces from participants’ residences ranged from a radius of 100 m to 3 km. Radial bone mineral density assessment was conducted using quantitative ultrasound during follow-up consultations.
The scientists found that participants frequently exposed to high and mixed vegetation areas within a 500-m radius of their homes had significantly higher bone mineral density than those at other distances or those frequenting spaces with different vegetation. In addition, access to larger green spaces with mixed and high vegetation within a 1-km radius was significantly associated with a lower likelihood of low bone density in children.
“These findings illustrate the positive impact on bone health of early childhood exposure to green areas near their homes during critical growth and development periods, with long-term implications,” wrote the researchers.
The results aligned with those of a prior study in which authors noted factors contributing to families’ frequent park visits, including shorter distances, safety, and park organization, as well as the natural diversity and activities offered.
One hypothesis explaining improved bone density in children visiting green areas was increased physical activity practiced in these locations. The mechanical load from exercise can activate signaling pathways favoring bone development. Literature also gathered data on the influence of green areas on young populations engaging in physical activities, showing positive outcomes.
According to the study authors, the findings are crucial for public health because they emphasize the need for urban investments in accessible green spaces as a strategy for fracture and osteoporosis prevention. In the long term, such initiatives translate to reduced public health expenses, along with physical and emotional gains in communities adopting environmental strategies, they concluded.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
A recently published prospective study in JAMA Network Open identified a significant association between children’s bone health and their proximity to green areas.
The literature emphasized the benefits of childhood exposure to green spaces for neurocognitive, social, behavioral, and mental development, as well as well-being. In addition, such exposure is linked to lower body mass index, increased physical activity, and reduced risks for overweight, obesity, and hypertension. However, specific data on bone mineral density implications are limited.
To address this gap, Hanne Sleurs, PhD, a researcher at the Universiteit Hasselt in Belgium, and colleagues followed the bone health of 327 participants from birth to 4-6 years and examined correlations with individuals’ exposure to green areas. Data collection occurred from October 2014 to July 2021.
Green spaces were categorized as high (vegetation height > 3 m), low (vegetation height ≤ 3 m), and mixed (combination of both). The distances of green spaces from participants’ residences ranged from a radius of 100 m to 3 km. Radial bone mineral density assessment was conducted using quantitative ultrasound during follow-up consultations.
The scientists found that participants frequently exposed to high and mixed vegetation areas within a 500-m radius of their homes had significantly higher bone mineral density than those at other distances or those frequenting spaces with different vegetation. In addition, access to larger green spaces with mixed and high vegetation within a 1-km radius was significantly associated with a lower likelihood of low bone density in children.
“These findings illustrate the positive impact on bone health of early childhood exposure to green areas near their homes during critical growth and development periods, with long-term implications,” wrote the researchers.
The results aligned with those of a prior study in which authors noted factors contributing to families’ frequent park visits, including shorter distances, safety, and park organization, as well as the natural diversity and activities offered.
One hypothesis explaining improved bone density in children visiting green areas was increased physical activity practiced in these locations. The mechanical load from exercise can activate signaling pathways favoring bone development. Literature also gathered data on the influence of green areas on young populations engaging in physical activities, showing positive outcomes.
According to the study authors, the findings are crucial for public health because they emphasize the need for urban investments in accessible green spaces as a strategy for fracture and osteoporosis prevention. In the long term, such initiatives translate to reduced public health expenses, along with physical and emotional gains in communities adopting environmental strategies, they concluded.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
A recently published prospective study in JAMA Network Open identified a significant association between children’s bone health and their proximity to green areas.
The literature emphasized the benefits of childhood exposure to green spaces for neurocognitive, social, behavioral, and mental development, as well as well-being. In addition, such exposure is linked to lower body mass index, increased physical activity, and reduced risks for overweight, obesity, and hypertension. However, specific data on bone mineral density implications are limited.
To address this gap, Hanne Sleurs, PhD, a researcher at the Universiteit Hasselt in Belgium, and colleagues followed the bone health of 327 participants from birth to 4-6 years and examined correlations with individuals’ exposure to green areas. Data collection occurred from October 2014 to July 2021.
Green spaces were categorized as high (vegetation height > 3 m), low (vegetation height ≤ 3 m), and mixed (combination of both). The distances of green spaces from participants’ residences ranged from a radius of 100 m to 3 km. Radial bone mineral density assessment was conducted using quantitative ultrasound during follow-up consultations.
The scientists found that participants frequently exposed to high and mixed vegetation areas within a 500-m radius of their homes had significantly higher bone mineral density than those at other distances or those frequenting spaces with different vegetation. In addition, access to larger green spaces with mixed and high vegetation within a 1-km radius was significantly associated with a lower likelihood of low bone density in children.
“These findings illustrate the positive impact on bone health of early childhood exposure to green areas near their homes during critical growth and development periods, with long-term implications,” wrote the researchers.
The results aligned with those of a prior study in which authors noted factors contributing to families’ frequent park visits, including shorter distances, safety, and park organization, as well as the natural diversity and activities offered.
One hypothesis explaining improved bone density in children visiting green areas was increased physical activity practiced in these locations. The mechanical load from exercise can activate signaling pathways favoring bone development. Literature also gathered data on the influence of green areas on young populations engaging in physical activities, showing positive outcomes.
According to the study authors, the findings are crucial for public health because they emphasize the need for urban investments in accessible green spaces as a strategy for fracture and osteoporosis prevention. In the long term, such initiatives translate to reduced public health expenses, along with physical and emotional gains in communities adopting environmental strategies, they concluded.
This article was translated from the Medscape Portuguese edition. A version of this article appeared on Medscape.com.
Wearable Device Tracks IBD from Sweat
LAS VEGAS —
The device, in development by EnLiSense, can rapidly detect calprotectin, C-reactive protein (CRP), and interleukin-6 (IL-6), using miniaturized versions of biochemical lab tests.
Patient monitoring relies on identifying trends, whether biomarker levels are increasing or decreasing, according to Shalini Prasad, PhD, who presented the study during a poster session at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “In a blood test you don’t get that unless you’re willing to sample every month. That’s the benefit [of the device],” said Dr. Prasad, professor of bioengineering at University of Texas at Dallas and a cofounder of EnLiSense.
The project grew out of the involvement of EnLiSense with the Biomedical Advanced Research Development Authority (BARDA). “We were tracking infections, and we were looking at inflammatory markers associated with infections: Cytokines and chemokines. We thought it was a natural pivot for us because the disease of inflammation is IBD,” said Dr. Prasad.
The device need only be worn when the physician determines the disease is in a variable state. The patient “will wear it for the duration of time as determined by the clinician,” said Dr. Prasad.
The watch face–sized device, typically worn on the forearm, absorbs sweat and performs automated biochemical analysis independently, then beams its findings to the cloud. “What you get back is concentration [of inflammatory biomarkers]. It is essentially trend line reporting of how the concentration is fluctuating over time for markers,” said Dr. Prasad.
The Crohn’s and Colitis Foundation is supporting the company through its IBD Ventures program. EnLiSense is currently conducting a study tracking patients over 4 weeks to correlate biomarker concentrations in sweat with concentrations in stool.
A key remaining question is how long the device should be worn and during what clinical periods. The technology has the potential to provide too much information. “Just figuring the balance. We’re trying to find the right spot where it makes sense for both the clinician and the patient. This is something that is a work in progress. We don’t want this to be just like any other consumer wearable which gives you something but you’re not sure what it means,” said Dr. Prasad.
The study included 33 patients with IBD who were monitored between 40 and 130 minutes. The device measured levels of CRP, IL-6, and calprotectin. Serum samples were also measured the same day.
The researchers found higher levels of calprotectin among patients with active disease in perspiration (P = .0260), serum (P = .022), and in fecal samples (P = .0411). There were no significant differences between patients who are active and those in remission with respect to CRP levels in perspiration or serum, or IL-6 in perspiration. Serum Il-6 levels were higher in those with active disease.
There was no significant difference between serum and sweat calprotectin levels among patients who were active or in remission, but the median expression of IL-6 in perspiration was higher in the active group (P = .0016). In the active group, calprotectin was elevated in sweat, serum, and stool.
Levels of calprotectin measured in perspiration correlated with levels in the serum (R2 = 0.7195), as did CRP (R2 = 0.615) and IL-6 (R2 = 0.5411).
Treating to Target
The poster caught the interest of Jeremiah Faith, PhD, who attended the session and was asked to comment. “I think patients want to know what’s happening [with their disease], and we could probably give better care if we know day to day the status of someone, especially because every time we test them we get a point in time, but the reality is probably that people are kind of wavy, and knowing the wave is much better,” he said.
He noted that there was not a strong separation between mean perspiration calprotectin values, but he said the ability to take frequent measurements could overcome that weakness. “The difference between active and remission is not as drastic as what you’d see from blood, for example. But it’s the same thing with your watch. Your watch is a really poor sensor of what your heartbeat is doing, but if you measure it every few seconds, and you average over a long period of time, it can actually more be more [accurate]. So there’s a lot of potential for this,” said Dr. Faith, associate professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai in New York.
If perfected, the device could help efforts at treating to target, in which therapies are adjusted to achieve minimal disease. Currently, physicians are forced to adjust doses or change therapies based on infrequent testing. “If this is accurate ... maybe at some point we will have the tools to be smarter about it,” said Dr. Faith.
Dr. Prasad is a cofounder of EnLiSense. Dr. Faith has no relevant financial disclosures.
LAS VEGAS —
The device, in development by EnLiSense, can rapidly detect calprotectin, C-reactive protein (CRP), and interleukin-6 (IL-6), using miniaturized versions of biochemical lab tests.
Patient monitoring relies on identifying trends, whether biomarker levels are increasing or decreasing, according to Shalini Prasad, PhD, who presented the study during a poster session at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “In a blood test you don’t get that unless you’re willing to sample every month. That’s the benefit [of the device],” said Dr. Prasad, professor of bioengineering at University of Texas at Dallas and a cofounder of EnLiSense.
The project grew out of the involvement of EnLiSense with the Biomedical Advanced Research Development Authority (BARDA). “We were tracking infections, and we were looking at inflammatory markers associated with infections: Cytokines and chemokines. We thought it was a natural pivot for us because the disease of inflammation is IBD,” said Dr. Prasad.
The device need only be worn when the physician determines the disease is in a variable state. The patient “will wear it for the duration of time as determined by the clinician,” said Dr. Prasad.
The watch face–sized device, typically worn on the forearm, absorbs sweat and performs automated biochemical analysis independently, then beams its findings to the cloud. “What you get back is concentration [of inflammatory biomarkers]. It is essentially trend line reporting of how the concentration is fluctuating over time for markers,” said Dr. Prasad.
The Crohn’s and Colitis Foundation is supporting the company through its IBD Ventures program. EnLiSense is currently conducting a study tracking patients over 4 weeks to correlate biomarker concentrations in sweat with concentrations in stool.
A key remaining question is how long the device should be worn and during what clinical periods. The technology has the potential to provide too much information. “Just figuring the balance. We’re trying to find the right spot where it makes sense for both the clinician and the patient. This is something that is a work in progress. We don’t want this to be just like any other consumer wearable which gives you something but you’re not sure what it means,” said Dr. Prasad.
The study included 33 patients with IBD who were monitored between 40 and 130 minutes. The device measured levels of CRP, IL-6, and calprotectin. Serum samples were also measured the same day.
The researchers found higher levels of calprotectin among patients with active disease in perspiration (P = .0260), serum (P = .022), and in fecal samples (P = .0411). There were no significant differences between patients who are active and those in remission with respect to CRP levels in perspiration or serum, or IL-6 in perspiration. Serum Il-6 levels were higher in those with active disease.
There was no significant difference between serum and sweat calprotectin levels among patients who were active or in remission, but the median expression of IL-6 in perspiration was higher in the active group (P = .0016). In the active group, calprotectin was elevated in sweat, serum, and stool.
Levels of calprotectin measured in perspiration correlated with levels in the serum (R2 = 0.7195), as did CRP (R2 = 0.615) and IL-6 (R2 = 0.5411).
Treating to Target
The poster caught the interest of Jeremiah Faith, PhD, who attended the session and was asked to comment. “I think patients want to know what’s happening [with their disease], and we could probably give better care if we know day to day the status of someone, especially because every time we test them we get a point in time, but the reality is probably that people are kind of wavy, and knowing the wave is much better,” he said.
He noted that there was not a strong separation between mean perspiration calprotectin values, but he said the ability to take frequent measurements could overcome that weakness. “The difference between active and remission is not as drastic as what you’d see from blood, for example. But it’s the same thing with your watch. Your watch is a really poor sensor of what your heartbeat is doing, but if you measure it every few seconds, and you average over a long period of time, it can actually more be more [accurate]. So there’s a lot of potential for this,” said Dr. Faith, associate professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai in New York.
If perfected, the device could help efforts at treating to target, in which therapies are adjusted to achieve minimal disease. Currently, physicians are forced to adjust doses or change therapies based on infrequent testing. “If this is accurate ... maybe at some point we will have the tools to be smarter about it,” said Dr. Faith.
Dr. Prasad is a cofounder of EnLiSense. Dr. Faith has no relevant financial disclosures.
LAS VEGAS —
The device, in development by EnLiSense, can rapidly detect calprotectin, C-reactive protein (CRP), and interleukin-6 (IL-6), using miniaturized versions of biochemical lab tests.
Patient monitoring relies on identifying trends, whether biomarker levels are increasing or decreasing, according to Shalini Prasad, PhD, who presented the study during a poster session at the annual Crohn’s & Colitis Congress®, a partnership of the Crohn’s & Colitis Foundation and the American Gastroenterological Association. “In a blood test you don’t get that unless you’re willing to sample every month. That’s the benefit [of the device],” said Dr. Prasad, professor of bioengineering at University of Texas at Dallas and a cofounder of EnLiSense.
The project grew out of the involvement of EnLiSense with the Biomedical Advanced Research Development Authority (BARDA). “We were tracking infections, and we were looking at inflammatory markers associated with infections: Cytokines and chemokines. We thought it was a natural pivot for us because the disease of inflammation is IBD,” said Dr. Prasad.
The device need only be worn when the physician determines the disease is in a variable state. The patient “will wear it for the duration of time as determined by the clinician,” said Dr. Prasad.
The watch face–sized device, typically worn on the forearm, absorbs sweat and performs automated biochemical analysis independently, then beams its findings to the cloud. “What you get back is concentration [of inflammatory biomarkers]. It is essentially trend line reporting of how the concentration is fluctuating over time for markers,” said Dr. Prasad.
The Crohn’s and Colitis Foundation is supporting the company through its IBD Ventures program. EnLiSense is currently conducting a study tracking patients over 4 weeks to correlate biomarker concentrations in sweat with concentrations in stool.
A key remaining question is how long the device should be worn and during what clinical periods. The technology has the potential to provide too much information. “Just figuring the balance. We’re trying to find the right spot where it makes sense for both the clinician and the patient. This is something that is a work in progress. We don’t want this to be just like any other consumer wearable which gives you something but you’re not sure what it means,” said Dr. Prasad.
The study included 33 patients with IBD who were monitored between 40 and 130 minutes. The device measured levels of CRP, IL-6, and calprotectin. Serum samples were also measured the same day.
The researchers found higher levels of calprotectin among patients with active disease in perspiration (P = .0260), serum (P = .022), and in fecal samples (P = .0411). There were no significant differences between patients who are active and those in remission with respect to CRP levels in perspiration or serum, or IL-6 in perspiration. Serum Il-6 levels were higher in those with active disease.
There was no significant difference between serum and sweat calprotectin levels among patients who were active or in remission, but the median expression of IL-6 in perspiration was higher in the active group (P = .0016). In the active group, calprotectin was elevated in sweat, serum, and stool.
Levels of calprotectin measured in perspiration correlated with levels in the serum (R2 = 0.7195), as did CRP (R2 = 0.615) and IL-6 (R2 = 0.5411).
Treating to Target
The poster caught the interest of Jeremiah Faith, PhD, who attended the session and was asked to comment. “I think patients want to know what’s happening [with their disease], and we could probably give better care if we know day to day the status of someone, especially because every time we test them we get a point in time, but the reality is probably that people are kind of wavy, and knowing the wave is much better,” he said.
He noted that there was not a strong separation between mean perspiration calprotectin values, but he said the ability to take frequent measurements could overcome that weakness. “The difference between active and remission is not as drastic as what you’d see from blood, for example. But it’s the same thing with your watch. Your watch is a really poor sensor of what your heartbeat is doing, but if you measure it every few seconds, and you average over a long period of time, it can actually more be more [accurate]. So there’s a lot of potential for this,” said Dr. Faith, associate professor of genetics and genomic sciences at the Icahn School of Medicine at Mount Sinai in New York.
If perfected, the device could help efforts at treating to target, in which therapies are adjusted to achieve minimal disease. Currently, physicians are forced to adjust doses or change therapies based on infrequent testing. “If this is accurate ... maybe at some point we will have the tools to be smarter about it,” said Dr. Faith.
Dr. Prasad is a cofounder of EnLiSense. Dr. Faith has no relevant financial disclosures.
FROM CROHN’S & COLITIS CONGRESS
Robitussin Cough Syrup Recalled Nationwide Due to Fungus Concerns
The company that makes Robitussin syrups did not specify which microorganisms may be in the products. The recall announcement from the global consumer health products company Haleon stated that the contamination could lead to fungal infections or the presence of fungi or yeasts in a person’s blood. So far, the company has not received any reports of people being sickened by the recalled products.
The recall applies to bottles of Robitussin Honey CF Max Day and Robitussin Honey CF Max Nighttime. Both varieties are for adults. Affected products were sold nationwide and have specific lot numbers printed at the bottom of the back of the bottles. Consumers can view the lot numbers on the FDA’s recall webpage.
People with weakened immune systems have a higher risk of life-threatening health problems due to the cough syrup, the company warned.
“In non-immunocompromised consumers, the population most likely to use the product, life-threatening infections are not likely to occur,” the recall notice from Haleon stated. “However, the occurrence of an infection that may necessitate medical intervention cannot be completely ruled out.”
People who have affected products should stop using them immediately. The company asked that anyone with the products email Haleon at mystory.us@haleon.com, or call the company at 800-245-1040 Monday through Friday from 8 a.m. to 6 p.m. Eastern time.
A version of this article appeared on WebMD.com.
The company that makes Robitussin syrups did not specify which microorganisms may be in the products. The recall announcement from the global consumer health products company Haleon stated that the contamination could lead to fungal infections or the presence of fungi or yeasts in a person’s blood. So far, the company has not received any reports of people being sickened by the recalled products.
The recall applies to bottles of Robitussin Honey CF Max Day and Robitussin Honey CF Max Nighttime. Both varieties are for adults. Affected products were sold nationwide and have specific lot numbers printed at the bottom of the back of the bottles. Consumers can view the lot numbers on the FDA’s recall webpage.
People with weakened immune systems have a higher risk of life-threatening health problems due to the cough syrup, the company warned.
“In non-immunocompromised consumers, the population most likely to use the product, life-threatening infections are not likely to occur,” the recall notice from Haleon stated. “However, the occurrence of an infection that may necessitate medical intervention cannot be completely ruled out.”
People who have affected products should stop using them immediately. The company asked that anyone with the products email Haleon at mystory.us@haleon.com, or call the company at 800-245-1040 Monday through Friday from 8 a.m. to 6 p.m. Eastern time.
A version of this article appeared on WebMD.com.
The company that makes Robitussin syrups did not specify which microorganisms may be in the products. The recall announcement from the global consumer health products company Haleon stated that the contamination could lead to fungal infections or the presence of fungi or yeasts in a person’s blood. So far, the company has not received any reports of people being sickened by the recalled products.
The recall applies to bottles of Robitussin Honey CF Max Day and Robitussin Honey CF Max Nighttime. Both varieties are for adults. Affected products were sold nationwide and have specific lot numbers printed at the bottom of the back of the bottles. Consumers can view the lot numbers on the FDA’s recall webpage.
People with weakened immune systems have a higher risk of life-threatening health problems due to the cough syrup, the company warned.
“In non-immunocompromised consumers, the population most likely to use the product, life-threatening infections are not likely to occur,” the recall notice from Haleon stated. “However, the occurrence of an infection that may necessitate medical intervention cannot be completely ruled out.”
People who have affected products should stop using them immediately. The company asked that anyone with the products email Haleon at mystory.us@haleon.com, or call the company at 800-245-1040 Monday through Friday from 8 a.m. to 6 p.m. Eastern time.
A version of this article appeared on WebMD.com.
Lp(a) Packs a More Powerful Atherogenic Punch Than LDL
TOPLINE:
While low-density lipoprotein (LDL) particles are much more abundant than lipoprotein(a) [Lp(a)] particles and carry the greatest overall risk for coronary heart disease (CHD), .
METHODOLOGY:
- To compare the atherogenicity of Lp(a) relative to LDL on a per-particle basis, researchers used a genetic analysis because Lp(a) and LDL both contain one apolipoprotein B (apoB) per particle.
- In a genome-wide association study of 502,413 UK Biobank participants, they identified genetic variants uniquely affecting plasma levels of either Lp(a) or LDL particles.
- For these two genetic clusters, they related the change in apoB to the respective change in CHD risk, which allowed them to directly compare the atherogenicity of LDL and Lp(a), particle to particle.
TAKEAWAY:
- The odds ratio for CHD for a 50 nmol/L higher Lp(a)-apoB was 1.28 (95% CI, 1.24-1.33) compared with 1.04 (95% CI, 1.03-1.05) for the same increment in LDL-apoB.
- Additional supporting evidence was provided by using polygenic scores to rank participants according to the difference in Lp(a)-apoB vs LDL-apoB, which revealed a greater risk for CHD per 50 nmol/L apoB for the Lp(a) cluster (hazard ratio [HR], 1.47; 95% CI, 1.36-1.58) than the LDL cluster (HR, 1.04; 95% CI, 1.02-1.05).
- Based on the data, the researchers estimate that the atherogenicity of Lp(a) is roughly sixfold greater (point estimate of 6.6; 95% CI, 5.1-8.8) than that of LDL on a per-particle basis.
IN PRACTICE:
“There are two clinical implications. First, to completely characterize atherosclerotic cardiovascular disease risk, it is imperative to measure Lp(a) in all adult patients at least once. Second, these studies provide a rationale that targeting Lp(a) with potent and specific drugs may lead to clinically meaningful benefit,” wrote the authors of an accompanying commentary on the study.
SOURCE:
The study, with first author Elias Björnson, PhD, University of Gothenburg, Gothenburg, Sweden, and an editorial by Sotirios Tsimikas, MD, University of California, San Diego, and Vera Bittner, MD, University of Alabama at Birmingham, was published in the Journal of the American College of Cardiology.
LIMITATIONS:
The UK Biobank consists primarily of a Caucasian population, and confirmatory studies in more diverse samples are needed. The working range for the Lp(a) assay used in the study did not cover the full range of Lp(a) values seen in the population. Variations in Lp(a)-apoB and LDL-apoB were estimated from genetic analysis and not measured specifically in biochemical assays.
DISCLOSURES:
The study had no commercial funding. Some authors received honoraria from the pharmaceutical industry. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
While low-density lipoprotein (LDL) particles are much more abundant than lipoprotein(a) [Lp(a)] particles and carry the greatest overall risk for coronary heart disease (CHD), .
METHODOLOGY:
- To compare the atherogenicity of Lp(a) relative to LDL on a per-particle basis, researchers used a genetic analysis because Lp(a) and LDL both contain one apolipoprotein B (apoB) per particle.
- In a genome-wide association study of 502,413 UK Biobank participants, they identified genetic variants uniquely affecting plasma levels of either Lp(a) or LDL particles.
- For these two genetic clusters, they related the change in apoB to the respective change in CHD risk, which allowed them to directly compare the atherogenicity of LDL and Lp(a), particle to particle.
TAKEAWAY:
- The odds ratio for CHD for a 50 nmol/L higher Lp(a)-apoB was 1.28 (95% CI, 1.24-1.33) compared with 1.04 (95% CI, 1.03-1.05) for the same increment in LDL-apoB.
- Additional supporting evidence was provided by using polygenic scores to rank participants according to the difference in Lp(a)-apoB vs LDL-apoB, which revealed a greater risk for CHD per 50 nmol/L apoB for the Lp(a) cluster (hazard ratio [HR], 1.47; 95% CI, 1.36-1.58) than the LDL cluster (HR, 1.04; 95% CI, 1.02-1.05).
- Based on the data, the researchers estimate that the atherogenicity of Lp(a) is roughly sixfold greater (point estimate of 6.6; 95% CI, 5.1-8.8) than that of LDL on a per-particle basis.
IN PRACTICE:
“There are two clinical implications. First, to completely characterize atherosclerotic cardiovascular disease risk, it is imperative to measure Lp(a) in all adult patients at least once. Second, these studies provide a rationale that targeting Lp(a) with potent and specific drugs may lead to clinically meaningful benefit,” wrote the authors of an accompanying commentary on the study.
SOURCE:
The study, with first author Elias Björnson, PhD, University of Gothenburg, Gothenburg, Sweden, and an editorial by Sotirios Tsimikas, MD, University of California, San Diego, and Vera Bittner, MD, University of Alabama at Birmingham, was published in the Journal of the American College of Cardiology.
LIMITATIONS:
The UK Biobank consists primarily of a Caucasian population, and confirmatory studies in more diverse samples are needed. The working range for the Lp(a) assay used in the study did not cover the full range of Lp(a) values seen in the population. Variations in Lp(a)-apoB and LDL-apoB were estimated from genetic analysis and not measured specifically in biochemical assays.
DISCLOSURES:
The study had no commercial funding. Some authors received honoraria from the pharmaceutical industry. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
TOPLINE:
While low-density lipoprotein (LDL) particles are much more abundant than lipoprotein(a) [Lp(a)] particles and carry the greatest overall risk for coronary heart disease (CHD), .
METHODOLOGY:
- To compare the atherogenicity of Lp(a) relative to LDL on a per-particle basis, researchers used a genetic analysis because Lp(a) and LDL both contain one apolipoprotein B (apoB) per particle.
- In a genome-wide association study of 502,413 UK Biobank participants, they identified genetic variants uniquely affecting plasma levels of either Lp(a) or LDL particles.
- For these two genetic clusters, they related the change in apoB to the respective change in CHD risk, which allowed them to directly compare the atherogenicity of LDL and Lp(a), particle to particle.
TAKEAWAY:
- The odds ratio for CHD for a 50 nmol/L higher Lp(a)-apoB was 1.28 (95% CI, 1.24-1.33) compared with 1.04 (95% CI, 1.03-1.05) for the same increment in LDL-apoB.
- Additional supporting evidence was provided by using polygenic scores to rank participants according to the difference in Lp(a)-apoB vs LDL-apoB, which revealed a greater risk for CHD per 50 nmol/L apoB for the Lp(a) cluster (hazard ratio [HR], 1.47; 95% CI, 1.36-1.58) than the LDL cluster (HR, 1.04; 95% CI, 1.02-1.05).
- Based on the data, the researchers estimate that the atherogenicity of Lp(a) is roughly sixfold greater (point estimate of 6.6; 95% CI, 5.1-8.8) than that of LDL on a per-particle basis.
IN PRACTICE:
“There are two clinical implications. First, to completely characterize atherosclerotic cardiovascular disease risk, it is imperative to measure Lp(a) in all adult patients at least once. Second, these studies provide a rationale that targeting Lp(a) with potent and specific drugs may lead to clinically meaningful benefit,” wrote the authors of an accompanying commentary on the study.
SOURCE:
The study, with first author Elias Björnson, PhD, University of Gothenburg, Gothenburg, Sweden, and an editorial by Sotirios Tsimikas, MD, University of California, San Diego, and Vera Bittner, MD, University of Alabama at Birmingham, was published in the Journal of the American College of Cardiology.
LIMITATIONS:
The UK Biobank consists primarily of a Caucasian population, and confirmatory studies in more diverse samples are needed. The working range for the Lp(a) assay used in the study did not cover the full range of Lp(a) values seen in the population. Variations in Lp(a)-apoB and LDL-apoB were estimated from genetic analysis and not measured specifically in biochemical assays.
DISCLOSURES:
The study had no commercial funding. Some authors received honoraria from the pharmaceutical industry. A complete list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Insulin Resistance Doesn’t Affect Finerenone’s Efficacy
TOPLINE:
In patients with chronic kidney disease (CKD) and type 2 diabetes, baseline insulin resistance was associated with increased cardiovascular (CV) but not kidney risk and did not affect the efficacy of finerenone.
METHODOLOGY:
- Insulin resistance is implicated in CV disease in patients with CKD, but its role in CKD progression is less clear.
- This post hoc analysis of FIDELITY, a pooled analysis of the and trials, randomly assigned patients with type 2 diabetes and CKD (who received optimized renin-angiotensin system blockade) to receive finerenone (10 mg or 20 mg) once daily or placebo and followed them for a median of 3 years.
- An estimated glucose disposal rate (eGDR), a measure of insulin resistance, was calculated for 12,964 patients (median age, 65 years), using waist circumference, hypertension status, and glycated hemoglobin.
- Outcomes included a CV composite (time to CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and a kidney composite (time to renal failure, a sustained decrease ≥ 57% in the initial estimated glomerular filtration rate, or renal death).
TAKEAWAY:
- The median eGDR was 4.1 mg/kg/min. The 50% of patients with a lower eGDR were considered insulin resistant, whereas the remaining half with a higher eGDR were considered insulin sensitive.
- The incidence rate of CV outcomes was higher among patients with insulin resistance in both the finerenone group (incidence rate per 100 patient-years, 5.18 vs 3.47 among insulin-sensitive patients) and the placebo group (6.34 vs 3.76), but eGDR showed no association with kidney outcomes.
- The efficacy of finerenone vs placebo on CV (Wald test P = .063) and kidney outcomes (Wald test P = .51) did not change significantly across the range of baseline eGDR values.
- The incidences of treatment-emergent adverse events and severe adverse events with finerenone were similar between the insulin-resistant and insulin-sensitive subgroups.
IN PRACTICE:
“The efficacy and safety of finerenone were not modified by baseline insulin resistance. A higher risk of CV — but not kidney outcomes was observed in patients with CKD and T2D with greater insulin resistance,” the authors wrote.
SOURCE:
This study was led by Thomas Ebert of the Medical Department III — Endocrinology, Nephrology, Rheumatology, University of Leipzig Medical Center, Leipzig, Germany, and published online in Diabetes Care.
LIMITATIONS:
This study was not adequately powered to evaluate the statistical significance of the association of eGDR with CV and kidney outcomes and was hypothesis-generating. Further studies are needed to examine whether the effects of insulin resistance differ between individuals with diabetes vs those with advanced CKD with or without diabetes.
DISCLOSURES:
The FIDELIO-DKD and FIGARO-DKD trials were conducted and sponsored by Bayer AG. Three authors declared being full-time employees of Bayer. Several authors declared receiving personal fees, consulting fees, grants, or research support from; holding patents with; or having ownership interests in various pharmaceutical companies, including Bayer.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with chronic kidney disease (CKD) and type 2 diabetes, baseline insulin resistance was associated with increased cardiovascular (CV) but not kidney risk and did not affect the efficacy of finerenone.
METHODOLOGY:
- Insulin resistance is implicated in CV disease in patients with CKD, but its role in CKD progression is less clear.
- This post hoc analysis of FIDELITY, a pooled analysis of the and trials, randomly assigned patients with type 2 diabetes and CKD (who received optimized renin-angiotensin system blockade) to receive finerenone (10 mg or 20 mg) once daily or placebo and followed them for a median of 3 years.
- An estimated glucose disposal rate (eGDR), a measure of insulin resistance, was calculated for 12,964 patients (median age, 65 years), using waist circumference, hypertension status, and glycated hemoglobin.
- Outcomes included a CV composite (time to CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and a kidney composite (time to renal failure, a sustained decrease ≥ 57% in the initial estimated glomerular filtration rate, or renal death).
TAKEAWAY:
- The median eGDR was 4.1 mg/kg/min. The 50% of patients with a lower eGDR were considered insulin resistant, whereas the remaining half with a higher eGDR were considered insulin sensitive.
- The incidence rate of CV outcomes was higher among patients with insulin resistance in both the finerenone group (incidence rate per 100 patient-years, 5.18 vs 3.47 among insulin-sensitive patients) and the placebo group (6.34 vs 3.76), but eGDR showed no association with kidney outcomes.
- The efficacy of finerenone vs placebo on CV (Wald test P = .063) and kidney outcomes (Wald test P = .51) did not change significantly across the range of baseline eGDR values.
- The incidences of treatment-emergent adverse events and severe adverse events with finerenone were similar between the insulin-resistant and insulin-sensitive subgroups.
IN PRACTICE:
“The efficacy and safety of finerenone were not modified by baseline insulin resistance. A higher risk of CV — but not kidney outcomes was observed in patients with CKD and T2D with greater insulin resistance,” the authors wrote.
SOURCE:
This study was led by Thomas Ebert of the Medical Department III — Endocrinology, Nephrology, Rheumatology, University of Leipzig Medical Center, Leipzig, Germany, and published online in Diabetes Care.
LIMITATIONS:
This study was not adequately powered to evaluate the statistical significance of the association of eGDR with CV and kidney outcomes and was hypothesis-generating. Further studies are needed to examine whether the effects of insulin resistance differ between individuals with diabetes vs those with advanced CKD with or without diabetes.
DISCLOSURES:
The FIDELIO-DKD and FIGARO-DKD trials were conducted and sponsored by Bayer AG. Three authors declared being full-time employees of Bayer. Several authors declared receiving personal fees, consulting fees, grants, or research support from; holding patents with; or having ownership interests in various pharmaceutical companies, including Bayer.
A version of this article appeared on Medscape.com.
TOPLINE:
In patients with chronic kidney disease (CKD) and type 2 diabetes, baseline insulin resistance was associated with increased cardiovascular (CV) but not kidney risk and did not affect the efficacy of finerenone.
METHODOLOGY:
- Insulin resistance is implicated in CV disease in patients with CKD, but its role in CKD progression is less clear.
- This post hoc analysis of FIDELITY, a pooled analysis of the and trials, randomly assigned patients with type 2 diabetes and CKD (who received optimized renin-angiotensin system blockade) to receive finerenone (10 mg or 20 mg) once daily or placebo and followed them for a median of 3 years.
- An estimated glucose disposal rate (eGDR), a measure of insulin resistance, was calculated for 12,964 patients (median age, 65 years), using waist circumference, hypertension status, and glycated hemoglobin.
- Outcomes included a CV composite (time to CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure) and a kidney composite (time to renal failure, a sustained decrease ≥ 57% in the initial estimated glomerular filtration rate, or renal death).
TAKEAWAY:
- The median eGDR was 4.1 mg/kg/min. The 50% of patients with a lower eGDR were considered insulin resistant, whereas the remaining half with a higher eGDR were considered insulin sensitive.
- The incidence rate of CV outcomes was higher among patients with insulin resistance in both the finerenone group (incidence rate per 100 patient-years, 5.18 vs 3.47 among insulin-sensitive patients) and the placebo group (6.34 vs 3.76), but eGDR showed no association with kidney outcomes.
- The efficacy of finerenone vs placebo on CV (Wald test P = .063) and kidney outcomes (Wald test P = .51) did not change significantly across the range of baseline eGDR values.
- The incidences of treatment-emergent adverse events and severe adverse events with finerenone were similar between the insulin-resistant and insulin-sensitive subgroups.
IN PRACTICE:
“The efficacy and safety of finerenone were not modified by baseline insulin resistance. A higher risk of CV — but not kidney outcomes was observed in patients with CKD and T2D with greater insulin resistance,” the authors wrote.
SOURCE:
This study was led by Thomas Ebert of the Medical Department III — Endocrinology, Nephrology, Rheumatology, University of Leipzig Medical Center, Leipzig, Germany, and published online in Diabetes Care.
LIMITATIONS:
This study was not adequately powered to evaluate the statistical significance of the association of eGDR with CV and kidney outcomes and was hypothesis-generating. Further studies are needed to examine whether the effects of insulin resistance differ between individuals with diabetes vs those with advanced CKD with or without diabetes.
DISCLOSURES:
The FIDELIO-DKD and FIGARO-DKD trials were conducted and sponsored by Bayer AG. Three authors declared being full-time employees of Bayer. Several authors declared receiving personal fees, consulting fees, grants, or research support from; holding patents with; or having ownership interests in various pharmaceutical companies, including Bayer.
A version of this article appeared on Medscape.com.
The Breakthrough Drug Whose Full Promise Remains Unrealized
Celebrating a Decade of Sofosbuvir for Hepatitis C
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
Celebrating a Decade of Sofosbuvir for Hepatitis C
Celebrating a Decade of Sofosbuvir for Hepatitis C
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.
Prior to 2013, the backbone of hepatitis C virus (HCV) therapy was pegylated interferon (PEG) in combination with ribavirin (RBV). This year-long therapy was associated with significant side effects and abysmal cure rates. Although efficacy improved with the addition of first-generation protease inhibitors, cure rates remained suboptimal and treatment side effects continued to be significant.
Clinicians and patients needed better options and looked to the drug pipeline with hope. However, even among the most optimistic, the idea that HCV therapy could evolve into an all-oral option seemed a relative pipe dream.
The Sofosbuvir Revolution Begins
The Liver Meeting held in 2013 changed everything.
Several presentations featured compelling data with sofosbuvir, a new polymerase inhibitor that, when combined with RBV, offered an all-oral option to patients with genotypes 2 and 3, as well as improved efficacy for patients with genotypes 1, 4, 5, and 6 when it was combined with 12 weeks of PEG/RBV.
However, the glass ceiling of HCV care was truly shattered with the randomized COSMOS trial, a late-breaker abstract that revealed 12-week functional cure rates in patients receiving sofosbuvir in combination with the protease inhibitor simeprevir.
This phase 2a trial in treatment-naive and -experienced genotype 1 patients with and without cirrhosis showed that an all-oral option was not only viable for the most common strain of HCV but was also safe and efficacious, even in difficult-to-treat populations.
On December 6, 2013, the US Food and Drug Administration (FDA) approved sofosbuvir for the treatment of HCV, ushering in a new era of therapy.
Guidelines quickly changed to advocate for both expansive HCV screening and generous treatment. Yet, as this more permissive approach was being recommended, the high price tag and large anticipated volume of those seeking prescriptions were setting off alarms. The drug cost triggered extensive restrictions based on degree of fibrosis, sobriety, and provider type in an effort to prevent immediate healthcare expenditures.
Given its high cost, rules restricting a patient to only one course of sofosbuvir-based therapy also surfaced. Although treatment with first-generation protease inhibitors carried a hefty price of $161,813.49 per sustained virologic response (SVR), compared with $66,000-$100,000 for 12 weeks of all-oral therapy, its uptake was low and limited by side effects and comorbid conditions. All-oral treatment appeared to have few medical barriers, leading payers to find ways to slow utilization. These restrictions are now gradually being eliminated.
Because of high SVR rates and few contraindications to therapy, most patients who gained access to treatment achieved cure. This included patients who had previously not responded to treatment and prioritized those with more advanced disease.
This quickly led to a significant shift in the population in need of treatment. Prior to 2013, many patients with HCV had advanced disease and did not respond to prior treatment options. After uptake of all-oral therapy, individuals in need were typically treatment naive without advanced disease.
This shift also added new psychosocial dimensions, as many of the newly infected individuals were struggling with active substance abuse. HCV treatment providers needed to change, with increasing recruitment of advanced practice providers, primary care physicians, and addiction medication specialists.
Progress, but Far From Reaching Targets
Fast-forward to 2023.
Ten years after FDA approval, 13.2 million individuals infected with HCV have been treated globally, 82% with sofosbuvir-based regimens and most in lower-middle-income countries. This is absolutely cause for celebration, but not complacency.
In 2016, the World Health Assembly adopted a resolution of elimination of viral hepatitis by 2030. The World Health Organization (WHO) defined elimination of HCV as 90% reduction in new cases of infection, 90% diagnosis of those infected, 80% of eligible individuals treated, and 65% reduction of deaths by 2030.
Despite all the success thus far, the CDA Foundation estimates that the WHO elimination targets will not be achieved until after the year 2050. They also note that in 2020, over 50 million individuals were infected with HCV, of which only 20% were diagnosed and 1% annually treated.
The HCV care cascade, by which the patient journeys from screening to cure, is complicated, and a one-size-fits-all solution is not possible. Reflex testing (an automatic transition to HCV polymerase chain reaction [PCR] testing in the lab for those who are HCV antibody positive) has significantly improved diagnosis. However, communicating these results and linking a patient to curative therapy remain significant obstacles.
Models and real-life experience show that multiple strategies can be successful. They include leveraging the electronic medical record, simplified treatment algorithms, test-and-treat strategies (screening high-risk populations with a point-of-care test that allows treatment initiation at the same visit), and co-localizing HCV screening and treatment with addiction services and relinkage programs (finding those who are already diagnosed and linking them to treatment).
In addition, focusing on populations at high risk for HCV infection — such as people who inject drugs, men who have sex with men, and incarcerated individuals — allows for better resource utilization.
Though daunting, HCV elimination is not impossible. There are several examples of success, including in the countries of Georgia and Iceland. Although, comparatively, the United States remains behind the curve, the White House has asked Congress for $11 billion to fund HCV elimination domestically.
As we await action at the national level, clinicians are reminded that there are several things we can do in caring for patients with HCV:
- A one-time HCV screening is recommended in all individuals aged 18 or older, including pregnant people with each pregnancy.
- HCV antibody testing with reflex to PCR should be used as the screening test.
- Pan-genotypic all-oral therapy is recommended for patients with HCV. Cure rates are greater than 95%, and there are few contraindications to treatment.
- Most people are eligible for simplified treatment algorithms that allow minimal on-treatment monitoring.
Without increased screening and linkage to curative therapy, we will not meet the WHO goals for HCV elimination.
Dr. Reau is chief of the hepatology section at Rush University Medical Center in Chicago and a regular contributor to this news organization. She serves as editor of Clinical Liver Disease, a multimedia review journal, and recently as a member of HCVGuidelines.org, a web-based resource from the American Association for the Study of Liver Diseases (AASLD) and the Infectious Diseases Society of America, as well as educational chair of the AASLD hepatitis C special interest group. She continues to have an active role in the hepatology interest group of the World Gastroenterology Organisation and the American Liver Foundation at the regional and national levels. She disclosed ties with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article appeared on Medscape.com.