User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Social media makes kids with type 1 diabetes feel less alone
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
After being diagnosed with type 1 diabetes in 2021, British teenager Johnny Bailey felt isolated. That’s when he turned to social media, where he found others living with type 1 diabetes. He began to share his experience and now has more than 329,000 followers on his TikTok account, where he regularly posts videos.
These include short clips of him demonstrating how he changes his FreeStyle Libre sensor for his flash glucose monitor. In the videos, Johnny appropriately places his sensor on the back of his arm with background music, makes facial expressions, and transforms a dreaded diabetes-related task into an experience that appears fun and entertaining. In the limited videos I was able to review, he follows all the appropriate steps for sensor placement.
Many youths living with type 1 diabetes struggle with living with a chronic medical condition. Because type 1 diabetes is a rare condition, affecting about 1 in 500 children in the United States, many youth may not meet anyone else their age with type 1 diabetes through school, social events, or extracurricular activities.
For adolescents with intensively managed conditions like type 1 diabetes, this can present numerous psychosocial challenges – specifically, many youth experience shame or stigma associated with managing type 1 diabetes.
Diabetes-specific tasks may include wearing an insulin pump, monitoring blood glucose with finger pricks or a continuous glucose monitor (CGM), giving injections of insulin before meals and snacks, adjusting times for meals and snacks based on metabolic needs, waking up in the middle of the night to treat high or low blood glucose – the list goes on and on.
One study estimated that the average time it takes a child with type 1 diabetes to perform diabetes-specific tasks is over 5 hours per day.
Although much of this diabetes management time is spent by parents, as children get older and become teenagers, they are gradually transitioning to taking on more of this responsibility themselves. Wearing diabetes technology (insulin pumps and CGMs) can draw unwanted attention, leading to diabetes-specific body image concerns. Kids may also have to excuse themselves from an activity to treat a low or high blood glucose, creating uncomfortable situations when others inquire about why the activity was interrupted. As a result, many youths will avoid managing their diabetes properly to avoid drawing unwanted attention, consequently put their health at risk.
Those who are afraid of placing their glucose sensor owing to fear of pain may be reassured by seeing Johnny placing his sensor with a smile on his face. Some of his content also highlights other stigmatizing situations that teens may face, for example someone with a judgmental look questioning why he needed to give an insulin injection here.
This highlights an important concept – that people with type 1 diabetes may face criticism when dosing insulin in public, but it doesn’t mean they should feel forced to manage diabetes in private unless they choose to. Johnny is an inspirational individual who has bravely taken his type 1 diabetes experiences and used his creative skills to make these seemingly boring health-related tasks fun, interesting, and accessible.
Social media has become an outlet for people with type 1 diabetes to connect with others who can relate to their experiences.
However, there’s another side to consider. Although social media may provide a great source of support for youth, it may also adversely affect mental health. Just as quickly as social media outlets have grown, so has concern over excessive social media use and its impact on adolescents’ mental health. There’s a growing body of literature that describes the negative mental health aspects related to social media use.
Some adolescents struggling to manage type 1 diabetes may feel worse when seeing others thrive on social media, which has the potential to worsen stigma and shame. Youth may wonder how someone else is able to manage their type 1 diabetes so well when they are facing so many challenges.
Short videos on social media provide an incomplete picture of living with type 1 diabetes – just a glimpse into others’ lives, and only the parts that they want others to see. Managing a chronic condition can’t be fully represented in 10-second videos. And if youths choose to post their type 1 diabetes experiences on social media, they also risk receiving backlash or criticism, which can negatively their impact mental health in return.
Furthermore, the content being posted may not always be accurate or educational, leading to the potential for some youth to misunderstand type 1 diabetes.
Although I wouldn’t discourage youth with type 1 diabetes from engaging on social media and viewing diabetes-related content, they need to know that social media is flooded with misinformation. Creating an open space for youth to ask their clinicians questions about type 1 diabetes–related topics they view on social media is vital to ensuring they are viewing accurate information, so they are able to continue to manage their diabetes safely.
As a pediatric endocrinologist, I sometimes share resources on social media with patients if I believe it will help them cope with their type 1 diabetes diagnosis and management. I have had numerous patients – many of whom have struggled to accept their diagnosis – mention with joy and excitement that they were following an organization addressing type 1 diabetes on social media.
When making suggestions, I may refer them to The Diabetes Link, an organization with resources for young adults with type 1 diabetes that creates a space to connect with other young adults with type 1 diabetes. diaTribe is another organization created and led by people with diabetes that has a plethora of resources and provides evidence-based education for patients. I have also shared Project 50-in-50, which highlights two individuals with type 1 diabetes hiking the highest peak in each state in less than 50 days. Being able to see type 1 diabetes in a positive light is a huge step toward a more positive outlook on diabetes management.
Dr. Nally is an assistant professor, department of pediatrics, and a pediatric endocrinologist, division of pediatric endocrinology, at Yale University, New Haven, Conn. She reported conflicts of interest with Medtronic and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
The new word in liver disease: The story behind NAFLD’s rebranding as MASLD
A noteworthy shift recently occurred in the field of hepatology, but it didn’t stem from a clinical trial or medical finding. Instead, the change arose from a matter of semantics.
In a special article published online in the journal Hepatology, a diverse international consensus group introduced new terminology for one of the world’s most rapidly growing diseases.
The term nonalcoholic fatty liver disease (NAFLD) was to be officially retired, replaced with a more precise and descriptive term – metabolic dysfunction–associated steatotic liver disease (MASLD).
In addition, steatotic liver disease (SLD) would be used as an umbrella term encompassing both MASLD and a new subcategory, MetALD, for individuals with MASLD whose alcohol consumption ranges from 140 to 350 g/wk for women and from 210 to 420 g/wk for men. Nonalcoholic steatohepatitis (NASH) would be known as metabolic dysfunction-associated steatohepatitis (MASH).
said the NAFLD nomenclature consensus group’s co-lead, Mary E. Rinella, MD, professor of medicine at University of Chicago and director of the metabolic and fatty liver program at University of Chicago Hospitals.
“The only really new thing we did is identify a group of people who meet criteria for MASLD and also drink more than the allowable limit,” she said. “There are tons of these patients who were not being considered before. Now they’re in a category by themselves, where they are going to be able to be studied and better understood.”
Why make a change?
The unveiling of the new nomenclature marked the culmination of 3 years of dedicated work that was built upon decades of growing understanding about the pathophysiologic underpinnings of these disease states.
The terms NAFLD and NASH emerged in 1980 to describe patients with chronic liver disease who denied excessive alcohol consumption. However, in the past 2 decades, it became increasingly evident that the existing terminology was inadequate, the consensus group’s co-lead, Philip Newsome, PhD, said in an interview.
“There was a strong desire for a name that describes what the condition is, rather than what it isn’t; avoiding use of stigmatizing terms, such as fatty and alcoholic; and finally, a nomenclature that could recognize the coexistence of conditions,” said Dr. Newsome, former secretary general of the European Association for the Study of the Liver (EASL), and director of the Centre for Liver and Gastrointestinal Research at the University of Birmingham, England.
These forces, combined with the recognition that NAFLD and alcohol-related liver disease shared biological processes, created momentum for change.
The idea gained traction with a 2020 article that proposed “MAFLD” as a more suitable term because it would link the disease with its known cardiometabolic risks, Dr. Rinella explained.
“We thought that paper was going to be the beginning of a conversation, but what happened instead is it became a full-court press,” Dr. Rinella said.
Dr. Rinella and Dr. Newsome then spearheaded a study to determine whether content experts and patients supported change. The process was led by three prominent international liver societies: EASL, the American Association for the Study of Liver Diseases (AASLD), and the Asociación Latinoamericana para el Estudio del Hígado. The organizations received input from 236 panelists from 56 countries, reflecting the diverse voices essential for addressing a disease with an expanding global prevalence rate.
In this globalized world, you cannot make a decision from on high and then expect everybody to just adopt it, Dr. Rinella noted.
The panel utilized a modified Delphi consensus approach, necessitating a supermajority of respondents (67%) to vote in favor of the changes. Seventy-four percent felt that the current nomenclature was sufficiently flawed to consider a name change, and 89% preferred terminology that describes the underlying cause of the disease. A supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%).
The participants settled on the new terminology, and the study resulted in a conclusion: “The new nomenclature and diagnostic criteria are widely supported, nonstigmatizing, and can improve awareness and patient identification.”
It was by no means a simple or straightforward task, according to Dr. Rinella. “Anytime you have a contentious issue and you engage a broad range of stakeholders, many of which you know are in disagreement, you’re going to have a difficult time reaching consensus,” she said.
Reassuring reluctant adopters
The backing of international liver societies will be crucial to ensuring the smooth and relatively swift adoption of the new nomenclature. The AASLD announced in July that it would begin this process by holding conversations with key stakeholders, including the Food and Drug Administration, patient organizations, and pharmaceutical industry representatives.
“By engaging external groups, we have gained valuable insights into potential roadblocks or barriers that may impede the full implementation of the new MASLD nomenclature,” AASLD President Norah Terrault, MD, MPH, FAASLD, told this news organization. “Knowing the types of issues they face will allow us to build an implementation plan that will help guide the field through adoption.”
Even with buy-in from key stakeholders, implementing the changes will be no small feat. It’s a “vast undertaking” that may result in short-term frustrations for some groups, Dr. Terrault said.
“For instance, researchers whose work commenced under the old nomenclature may not be able to alter their research papers and will need to publish under the old nomenclature, which may impact which journals their research could be published in,” she said. “Some patient advocacy groups may have the old nomenclature in their names, resulting in a need to rebrand and revise their educational resources. Patient materials need to be updated. Primary care professionals need to be educated. The list goes on.”
These changes demand both patience and time, Dr. Terrault said. This applies to those tasked with persuading colleagues and patients, as well as clinicians, many of whom have already expressed some resistance to the updated terminology.
The panel anticipated pushback from clinicians who still advocate for NAFLD. However, Dr. Rinella countered that a diagnosis of MASLD requires only one cardiometabolic risk factor and has 99% overlap in most populations. In contrast, the MAFLD diagnostic criteria put forward in 2020 proposed even more restrictive cardiometabolic criteria and greater tolerance for alcohol consumption and would alter the disease natural history, she said.
Concerns have also been raised that replacing NAFLD with MASLD might complicate the value of prior research efforts. However, this should not be a cause for concern, as extensive examination across multiple populations has demonstrated near complete overlap between the two definitions, Dr. Rinella said. Biomarker development, natural history studies, and drug development research will remain unaffected, she said.
Some detractors argue that the term “fatty” is sufficiently descriptive and not stigmatizing. However, Dr. Newsome contends that the panel’s research unequivocally disproves this notion.
“Our Delphi process demonstrated very clearly that over 50% felt it was stigmatizing, and in particular, there were clear supportive views for this change from many patient groups,” he noted. “The new nomenclature empowers patients to explain what the condition means without the use of emotional language.”
An opportunity to improve care
One compelling way to persuade reluctant adopters of the new nomenclature’s value is to highlight the opportunities it presents.
The updated terminology opens avenues for research and clinical improvements for patients who meet MASLD criteria and consume alcohol at higher levels (MetALD), Dr. Newsome said.
“There are questions about the relative contribution of these two factors to liver injury, and I see this as an opportunity to explore this area further,” he said.
Hepatologists should embrace this change as a means of increasing awareness regarding the metabolic origins of the disease, Dr. Rinella said. This, in turn, will help identify more patients who require treatment but who are currently overlooked by the existing system, she noted.
“Right now, only around 1% of people with advanced disease are being identified by primary care physicians,” she said. “Hopefully, by elevating the role of metabolic disease, primary care physicians, endocrinologists, and gastroenterologists will be able to identify more patients and bring them to care before they develop cirrhosis.”
Such an outcome would signify much more than a mere semantic shift; it would represent a major advancement in the diagnosis and management of the disease.
A version of this article appeared on Medscape.com.
A noteworthy shift recently occurred in the field of hepatology, but it didn’t stem from a clinical trial or medical finding. Instead, the change arose from a matter of semantics.
In a special article published online in the journal Hepatology, a diverse international consensus group introduced new terminology for one of the world’s most rapidly growing diseases.
The term nonalcoholic fatty liver disease (NAFLD) was to be officially retired, replaced with a more precise and descriptive term – metabolic dysfunction–associated steatotic liver disease (MASLD).
In addition, steatotic liver disease (SLD) would be used as an umbrella term encompassing both MASLD and a new subcategory, MetALD, for individuals with MASLD whose alcohol consumption ranges from 140 to 350 g/wk for women and from 210 to 420 g/wk for men. Nonalcoholic steatohepatitis (NASH) would be known as metabolic dysfunction-associated steatohepatitis (MASH).
said the NAFLD nomenclature consensus group’s co-lead, Mary E. Rinella, MD, professor of medicine at University of Chicago and director of the metabolic and fatty liver program at University of Chicago Hospitals.
“The only really new thing we did is identify a group of people who meet criteria for MASLD and also drink more than the allowable limit,” she said. “There are tons of these patients who were not being considered before. Now they’re in a category by themselves, where they are going to be able to be studied and better understood.”
Why make a change?
The unveiling of the new nomenclature marked the culmination of 3 years of dedicated work that was built upon decades of growing understanding about the pathophysiologic underpinnings of these disease states.
The terms NAFLD and NASH emerged in 1980 to describe patients with chronic liver disease who denied excessive alcohol consumption. However, in the past 2 decades, it became increasingly evident that the existing terminology was inadequate, the consensus group’s co-lead, Philip Newsome, PhD, said in an interview.
“There was a strong desire for a name that describes what the condition is, rather than what it isn’t; avoiding use of stigmatizing terms, such as fatty and alcoholic; and finally, a nomenclature that could recognize the coexistence of conditions,” said Dr. Newsome, former secretary general of the European Association for the Study of the Liver (EASL), and director of the Centre for Liver and Gastrointestinal Research at the University of Birmingham, England.
These forces, combined with the recognition that NAFLD and alcohol-related liver disease shared biological processes, created momentum for change.
The idea gained traction with a 2020 article that proposed “MAFLD” as a more suitable term because it would link the disease with its known cardiometabolic risks, Dr. Rinella explained.
“We thought that paper was going to be the beginning of a conversation, but what happened instead is it became a full-court press,” Dr. Rinella said.
Dr. Rinella and Dr. Newsome then spearheaded a study to determine whether content experts and patients supported change. The process was led by three prominent international liver societies: EASL, the American Association for the Study of Liver Diseases (AASLD), and the Asociación Latinoamericana para el Estudio del Hígado. The organizations received input from 236 panelists from 56 countries, reflecting the diverse voices essential for addressing a disease with an expanding global prevalence rate.
In this globalized world, you cannot make a decision from on high and then expect everybody to just adopt it, Dr. Rinella noted.
The panel utilized a modified Delphi consensus approach, necessitating a supermajority of respondents (67%) to vote in favor of the changes. Seventy-four percent felt that the current nomenclature was sufficiently flawed to consider a name change, and 89% preferred terminology that describes the underlying cause of the disease. A supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%).
The participants settled on the new terminology, and the study resulted in a conclusion: “The new nomenclature and diagnostic criteria are widely supported, nonstigmatizing, and can improve awareness and patient identification.”
It was by no means a simple or straightforward task, according to Dr. Rinella. “Anytime you have a contentious issue and you engage a broad range of stakeholders, many of which you know are in disagreement, you’re going to have a difficult time reaching consensus,” she said.
Reassuring reluctant adopters
The backing of international liver societies will be crucial to ensuring the smooth and relatively swift adoption of the new nomenclature. The AASLD announced in July that it would begin this process by holding conversations with key stakeholders, including the Food and Drug Administration, patient organizations, and pharmaceutical industry representatives.
“By engaging external groups, we have gained valuable insights into potential roadblocks or barriers that may impede the full implementation of the new MASLD nomenclature,” AASLD President Norah Terrault, MD, MPH, FAASLD, told this news organization. “Knowing the types of issues they face will allow us to build an implementation plan that will help guide the field through adoption.”
Even with buy-in from key stakeholders, implementing the changes will be no small feat. It’s a “vast undertaking” that may result in short-term frustrations for some groups, Dr. Terrault said.
“For instance, researchers whose work commenced under the old nomenclature may not be able to alter their research papers and will need to publish under the old nomenclature, which may impact which journals their research could be published in,” she said. “Some patient advocacy groups may have the old nomenclature in their names, resulting in a need to rebrand and revise their educational resources. Patient materials need to be updated. Primary care professionals need to be educated. The list goes on.”
These changes demand both patience and time, Dr. Terrault said. This applies to those tasked with persuading colleagues and patients, as well as clinicians, many of whom have already expressed some resistance to the updated terminology.
The panel anticipated pushback from clinicians who still advocate for NAFLD. However, Dr. Rinella countered that a diagnosis of MASLD requires only one cardiometabolic risk factor and has 99% overlap in most populations. In contrast, the MAFLD diagnostic criteria put forward in 2020 proposed even more restrictive cardiometabolic criteria and greater tolerance for alcohol consumption and would alter the disease natural history, she said.
Concerns have also been raised that replacing NAFLD with MASLD might complicate the value of prior research efforts. However, this should not be a cause for concern, as extensive examination across multiple populations has demonstrated near complete overlap between the two definitions, Dr. Rinella said. Biomarker development, natural history studies, and drug development research will remain unaffected, she said.
Some detractors argue that the term “fatty” is sufficiently descriptive and not stigmatizing. However, Dr. Newsome contends that the panel’s research unequivocally disproves this notion.
“Our Delphi process demonstrated very clearly that over 50% felt it was stigmatizing, and in particular, there were clear supportive views for this change from many patient groups,” he noted. “The new nomenclature empowers patients to explain what the condition means without the use of emotional language.”
An opportunity to improve care
One compelling way to persuade reluctant adopters of the new nomenclature’s value is to highlight the opportunities it presents.
The updated terminology opens avenues for research and clinical improvements for patients who meet MASLD criteria and consume alcohol at higher levels (MetALD), Dr. Newsome said.
“There are questions about the relative contribution of these two factors to liver injury, and I see this as an opportunity to explore this area further,” he said.
Hepatologists should embrace this change as a means of increasing awareness regarding the metabolic origins of the disease, Dr. Rinella said. This, in turn, will help identify more patients who require treatment but who are currently overlooked by the existing system, she noted.
“Right now, only around 1% of people with advanced disease are being identified by primary care physicians,” she said. “Hopefully, by elevating the role of metabolic disease, primary care physicians, endocrinologists, and gastroenterologists will be able to identify more patients and bring them to care before they develop cirrhosis.”
Such an outcome would signify much more than a mere semantic shift; it would represent a major advancement in the diagnosis and management of the disease.
A version of this article appeared on Medscape.com.
A noteworthy shift recently occurred in the field of hepatology, but it didn’t stem from a clinical trial or medical finding. Instead, the change arose from a matter of semantics.
In a special article published online in the journal Hepatology, a diverse international consensus group introduced new terminology for one of the world’s most rapidly growing diseases.
The term nonalcoholic fatty liver disease (NAFLD) was to be officially retired, replaced with a more precise and descriptive term – metabolic dysfunction–associated steatotic liver disease (MASLD).
In addition, steatotic liver disease (SLD) would be used as an umbrella term encompassing both MASLD and a new subcategory, MetALD, for individuals with MASLD whose alcohol consumption ranges from 140 to 350 g/wk for women and from 210 to 420 g/wk for men. Nonalcoholic steatohepatitis (NASH) would be known as metabolic dysfunction-associated steatohepatitis (MASH).
said the NAFLD nomenclature consensus group’s co-lead, Mary E. Rinella, MD, professor of medicine at University of Chicago and director of the metabolic and fatty liver program at University of Chicago Hospitals.
“The only really new thing we did is identify a group of people who meet criteria for MASLD and also drink more than the allowable limit,” she said. “There are tons of these patients who were not being considered before. Now they’re in a category by themselves, where they are going to be able to be studied and better understood.”
Why make a change?
The unveiling of the new nomenclature marked the culmination of 3 years of dedicated work that was built upon decades of growing understanding about the pathophysiologic underpinnings of these disease states.
The terms NAFLD and NASH emerged in 1980 to describe patients with chronic liver disease who denied excessive alcohol consumption. However, in the past 2 decades, it became increasingly evident that the existing terminology was inadequate, the consensus group’s co-lead, Philip Newsome, PhD, said in an interview.
“There was a strong desire for a name that describes what the condition is, rather than what it isn’t; avoiding use of stigmatizing terms, such as fatty and alcoholic; and finally, a nomenclature that could recognize the coexistence of conditions,” said Dr. Newsome, former secretary general of the European Association for the Study of the Liver (EASL), and director of the Centre for Liver and Gastrointestinal Research at the University of Birmingham, England.
These forces, combined with the recognition that NAFLD and alcohol-related liver disease shared biological processes, created momentum for change.
The idea gained traction with a 2020 article that proposed “MAFLD” as a more suitable term because it would link the disease with its known cardiometabolic risks, Dr. Rinella explained.
“We thought that paper was going to be the beginning of a conversation, but what happened instead is it became a full-court press,” Dr. Rinella said.
Dr. Rinella and Dr. Newsome then spearheaded a study to determine whether content experts and patients supported change. The process was led by three prominent international liver societies: EASL, the American Association for the Study of Liver Diseases (AASLD), and the Asociación Latinoamericana para el Estudio del Hígado. The organizations received input from 236 panelists from 56 countries, reflecting the diverse voices essential for addressing a disease with an expanding global prevalence rate.
In this globalized world, you cannot make a decision from on high and then expect everybody to just adopt it, Dr. Rinella noted.
The panel utilized a modified Delphi consensus approach, necessitating a supermajority of respondents (67%) to vote in favor of the changes. Seventy-four percent felt that the current nomenclature was sufficiently flawed to consider a name change, and 89% preferred terminology that describes the underlying cause of the disease. A supermajority felt that having “metabolic disease or dysfunction” in the name would help patients better understand their disease (72%) and help health care professionals better explain or understand the disease (80%).
The participants settled on the new terminology, and the study resulted in a conclusion: “The new nomenclature and diagnostic criteria are widely supported, nonstigmatizing, and can improve awareness and patient identification.”
It was by no means a simple or straightforward task, according to Dr. Rinella. “Anytime you have a contentious issue and you engage a broad range of stakeholders, many of which you know are in disagreement, you’re going to have a difficult time reaching consensus,” she said.
Reassuring reluctant adopters
The backing of international liver societies will be crucial to ensuring the smooth and relatively swift adoption of the new nomenclature. The AASLD announced in July that it would begin this process by holding conversations with key stakeholders, including the Food and Drug Administration, patient organizations, and pharmaceutical industry representatives.
“By engaging external groups, we have gained valuable insights into potential roadblocks or barriers that may impede the full implementation of the new MASLD nomenclature,” AASLD President Norah Terrault, MD, MPH, FAASLD, told this news organization. “Knowing the types of issues they face will allow us to build an implementation plan that will help guide the field through adoption.”
Even with buy-in from key stakeholders, implementing the changes will be no small feat. It’s a “vast undertaking” that may result in short-term frustrations for some groups, Dr. Terrault said.
“For instance, researchers whose work commenced under the old nomenclature may not be able to alter their research papers and will need to publish under the old nomenclature, which may impact which journals their research could be published in,” she said. “Some patient advocacy groups may have the old nomenclature in their names, resulting in a need to rebrand and revise their educational resources. Patient materials need to be updated. Primary care professionals need to be educated. The list goes on.”
These changes demand both patience and time, Dr. Terrault said. This applies to those tasked with persuading colleagues and patients, as well as clinicians, many of whom have already expressed some resistance to the updated terminology.
The panel anticipated pushback from clinicians who still advocate for NAFLD. However, Dr. Rinella countered that a diagnosis of MASLD requires only one cardiometabolic risk factor and has 99% overlap in most populations. In contrast, the MAFLD diagnostic criteria put forward in 2020 proposed even more restrictive cardiometabolic criteria and greater tolerance for alcohol consumption and would alter the disease natural history, she said.
Concerns have also been raised that replacing NAFLD with MASLD might complicate the value of prior research efforts. However, this should not be a cause for concern, as extensive examination across multiple populations has demonstrated near complete overlap between the two definitions, Dr. Rinella said. Biomarker development, natural history studies, and drug development research will remain unaffected, she said.
Some detractors argue that the term “fatty” is sufficiently descriptive and not stigmatizing. However, Dr. Newsome contends that the panel’s research unequivocally disproves this notion.
“Our Delphi process demonstrated very clearly that over 50% felt it was stigmatizing, and in particular, there were clear supportive views for this change from many patient groups,” he noted. “The new nomenclature empowers patients to explain what the condition means without the use of emotional language.”
An opportunity to improve care
One compelling way to persuade reluctant adopters of the new nomenclature’s value is to highlight the opportunities it presents.
The updated terminology opens avenues for research and clinical improvements for patients who meet MASLD criteria and consume alcohol at higher levels (MetALD), Dr. Newsome said.
“There are questions about the relative contribution of these two factors to liver injury, and I see this as an opportunity to explore this area further,” he said.
Hepatologists should embrace this change as a means of increasing awareness regarding the metabolic origins of the disease, Dr. Rinella said. This, in turn, will help identify more patients who require treatment but who are currently overlooked by the existing system, she noted.
“Right now, only around 1% of people with advanced disease are being identified by primary care physicians,” she said. “Hopefully, by elevating the role of metabolic disease, primary care physicians, endocrinologists, and gastroenterologists will be able to identify more patients and bring them to care before they develop cirrhosis.”
Such an outcome would signify much more than a mere semantic shift; it would represent a major advancement in the diagnosis and management of the disease.
A version of this article appeared on Medscape.com.
CPAP adherence curbs severe cardiovascular disease outcomes
, based on data from more than 4,000 individuals.
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular diseases, but the association between management of OSA with a continuous positive-airway pressure device (CPAP) and major adverse cardiac or cerebrovascular events (MACCEs) remains unclear, wrote Manuel Sánchez-de-la-Torre, PhD, of the University of Lleida, Spain, and colleagues.
In a meta-analysis published in JAMA, the researchers reviewed data from 4,186 individuals with a mean age of 61.2 years; 82.1% were men. The study population included 2,097 patients who used CPAP and 2,089 who did not. The mean apnea-hypopnea index (AHI) was 31.2 events per hour, and OSA was defined as an oxygen desaturation index of 12 events or more per hour or an AHI of 15 events or more per hour. The composite primary outcome included the first MACCE, or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Each of these components was a secondary endpoint.
Overall, the primary outcome of MACCE was similar for CPAP and non-CPAP using patients (hazard ratio, 1.01) with a total of 349 MACCE events in the CPAP group and 342 in the non-CPAP group. The mean adherence to CPAP was 3.1 hours per day. A total of 38.5% of patients in the CPAP group met the criteria for good adherence, defined as a mean of 4 or more hours per day.
However, as defined, good adherence to CPAP significantly reduced the risk of MACCE, compared with no CPAP use (HR, 0.69), and a sensitivity analysis showed a significant risk reduction, compared with patients who did not meet the criteria for good adherence (HR, 0.55; P = .005).
“Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features,” the researchers wrote in their discussion.
The findings were limited by several factors including the evaluation only of CPAP as a treatment for OSA, and the inability to assess separate components of the composite endpoint, the researchers noted. Other limitations included the relatively small number of female patients, reliance mainly on at-home sleep apnea tests, and the potential for selection bias, they said.
However, the results suggest that CPAP adherence is important to prevention of secondary cardiovascular outcomes in OSA patients, and that implementation of specific and personalized strategies to improve adherence to treatment should be a clinical priority, they concluded.
The study was funded by the Instituto de Salud Carlos III, the European Union and FEDER, IRBLleida–Fundació Dr Pifarré, SEPAR, ResMed Ltd. (Australia), Associació Lleidatana de Respiratori, and CIBERES. Dr Sánchez-de-la-Torre also disclosed financial support from a Ramón y Cajal grant.
, based on data from more than 4,000 individuals.
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular diseases, but the association between management of OSA with a continuous positive-airway pressure device (CPAP) and major adverse cardiac or cerebrovascular events (MACCEs) remains unclear, wrote Manuel Sánchez-de-la-Torre, PhD, of the University of Lleida, Spain, and colleagues.
In a meta-analysis published in JAMA, the researchers reviewed data from 4,186 individuals with a mean age of 61.2 years; 82.1% were men. The study population included 2,097 patients who used CPAP and 2,089 who did not. The mean apnea-hypopnea index (AHI) was 31.2 events per hour, and OSA was defined as an oxygen desaturation index of 12 events or more per hour or an AHI of 15 events or more per hour. The composite primary outcome included the first MACCE, or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Each of these components was a secondary endpoint.
Overall, the primary outcome of MACCE was similar for CPAP and non-CPAP using patients (hazard ratio, 1.01) with a total of 349 MACCE events in the CPAP group and 342 in the non-CPAP group. The mean adherence to CPAP was 3.1 hours per day. A total of 38.5% of patients in the CPAP group met the criteria for good adherence, defined as a mean of 4 or more hours per day.
However, as defined, good adherence to CPAP significantly reduced the risk of MACCE, compared with no CPAP use (HR, 0.69), and a sensitivity analysis showed a significant risk reduction, compared with patients who did not meet the criteria for good adherence (HR, 0.55; P = .005).
“Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features,” the researchers wrote in their discussion.
The findings were limited by several factors including the evaluation only of CPAP as a treatment for OSA, and the inability to assess separate components of the composite endpoint, the researchers noted. Other limitations included the relatively small number of female patients, reliance mainly on at-home sleep apnea tests, and the potential for selection bias, they said.
However, the results suggest that CPAP adherence is important to prevention of secondary cardiovascular outcomes in OSA patients, and that implementation of specific and personalized strategies to improve adherence to treatment should be a clinical priority, they concluded.
The study was funded by the Instituto de Salud Carlos III, the European Union and FEDER, IRBLleida–Fundació Dr Pifarré, SEPAR, ResMed Ltd. (Australia), Associació Lleidatana de Respiratori, and CIBERES. Dr Sánchez-de-la-Torre also disclosed financial support from a Ramón y Cajal grant.
, based on data from more than 4,000 individuals.
Obstructive sleep apnea (OSA) is associated with an increased risk of cardiovascular diseases, but the association between management of OSA with a continuous positive-airway pressure device (CPAP) and major adverse cardiac or cerebrovascular events (MACCEs) remains unclear, wrote Manuel Sánchez-de-la-Torre, PhD, of the University of Lleida, Spain, and colleagues.
In a meta-analysis published in JAMA, the researchers reviewed data from 4,186 individuals with a mean age of 61.2 years; 82.1% were men. The study population included 2,097 patients who used CPAP and 2,089 who did not. The mean apnea-hypopnea index (AHI) was 31.2 events per hour, and OSA was defined as an oxygen desaturation index of 12 events or more per hour or an AHI of 15 events or more per hour. The composite primary outcome included the first MACCE, or death from cardiovascular causes, myocardial infarction, stroke, revascularization procedure, hospital admission for heart failure, hospital admission for unstable angina, or hospital admission for transient ischemic attack. Each of these components was a secondary endpoint.
Overall, the primary outcome of MACCE was similar for CPAP and non-CPAP using patients (hazard ratio, 1.01) with a total of 349 MACCE events in the CPAP group and 342 in the non-CPAP group. The mean adherence to CPAP was 3.1 hours per day. A total of 38.5% of patients in the CPAP group met the criteria for good adherence, defined as a mean of 4 or more hours per day.
However, as defined, good adherence to CPAP significantly reduced the risk of MACCE, compared with no CPAP use (HR, 0.69), and a sensitivity analysis showed a significant risk reduction, compared with patients who did not meet the criteria for good adherence (HR, 0.55; P = .005).
“Adherence to treatment is complex to determine and there are other potential factors that could affect patient adherence, such as health education, motivation, attitude, self-efficacy, psychosocial factors, and other health care system–related features,” the researchers wrote in their discussion.
The findings were limited by several factors including the evaluation only of CPAP as a treatment for OSA, and the inability to assess separate components of the composite endpoint, the researchers noted. Other limitations included the relatively small number of female patients, reliance mainly on at-home sleep apnea tests, and the potential for selection bias, they said.
However, the results suggest that CPAP adherence is important to prevention of secondary cardiovascular outcomes in OSA patients, and that implementation of specific and personalized strategies to improve adherence to treatment should be a clinical priority, they concluded.
The study was funded by the Instituto de Salud Carlos III, the European Union and FEDER, IRBLleida–Fundació Dr Pifarré, SEPAR, ResMed Ltd. (Australia), Associació Lleidatana de Respiratori, and CIBERES. Dr Sánchez-de-la-Torre also disclosed financial support from a Ramón y Cajal grant.
FROM JAMA
Hormone therapy less effective in menopausal women with obesity
PHILADELPHIA – , according to a small, retrospective study presented at the annual meeting of the Menopause Society (formerly the North American Menopause Society).
More than 40% of women over age 40 in the United States have obesity, presenter Anita Pershad, MD, an ob.gyn. medical resident at Eastern Virginia Medical School, Norfolk, told attendees. Yet most of the large-scale studies investigating perimenopausal and postmenopausal hormone therapy included participants without major medical comorbidities, so little data exist on how effectively HT works in women with these comorbidities, she said
“The main takeaway of our study is that obesity may worsen a woman’s menopausal symptoms and limit the amount of relief she gets from hormone therapy,” Dr. Pershad said in an interview. “It remains unclear if hormone therapy is less effective in women with obesity overall, or if the expected efficacy can be achieved with alternative design and administration routes. A potential mechanism of action for the observed decreased effect could be due to adipose tissue acting as a heat insulator, promoting the effects of vasomotor symptoms.”
Dr. Pershad and her colleagues conducted a retrospective review of the medical records of 119 patients who presented to a menopause clinic at a Midsouth urban academic medical center between July 2018 and December 2022. Obesity was defined as having a body mass index (BMI) of 30 kg/m2 or greater.
The patients with and without obesity were similar in terms of age, duration of menopause, use of hormone therapy, and therapy acceptance, but patients with obesity were more likely to identify themselves as Black (71% vs. 40%). Women with obesity were also significantly more likely than women without obesity to report vasomotor symptoms (74% vs. 45%, P = .002), genitourinary/vulvovaginal symptoms (60% vs. 21%, P < .001), mood disturbances (11% vs. 0%, P = .18), and decreased libido (29% vs. 11%, P = .017).
There were no significant differences in comorbidities between women with and without obesity, and among women who received systemic or localized HT, the same standard dosing was used for both groups.
Women with obesity were much less likely to see a satisfying reduction in their menopausal symptoms than women without obesity (odds ratio 0.07, 95% confidence interval, 0.01-0.64; P = .006), though the subgroups for each category of HT were small. Among the 20 women receiving systemic hormone therapy, only 1 of the 12 with obesity (8.3%) reported improvement in symptoms, compared with 7 of the 8 women without obesity (88%; P = .0004). Among 33 women using localized hormone therapy, 46% of the 24 women with obesity vs. 89% of the 9 women without obesity experienced symptom improvement (P = .026).
The proportions of women reporting relief from only lifestyle modifications or from nonhormonal medications, such as SSRIs/SNRIs, trazodone, and clonidine, were not statistically different. There were 33 women who relied only on lifestyle modifications, with 31% of the 16 women with obesity and 59% of the 17 women without obesity reporting improvement in their symptoms (P = .112). Similarly, among the 33 women using nonhormonal medications, 75% of the 20 women with obesity and 77% of the 13 women without obesity experienced relief (P = .9).
Women with obesity are undertreated
Dr. Pershad emphasized the need to improve care and counseling for diverse patients seeking treatment for menopausal symptoms.
“More research is needed to examine how women with medical comorbidities are uniquely impacted by menopause and respond to therapies,” Dr. Pershad said in an interview. “This can be achieved by actively including more diverse patient populations in women’s health studies, burdened by the social determinants of health and medical comorbidities such as obesity.”
Stephanie S. Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health, Rochester, Minn., and medical director for The Menopause Society, was not surprised by the findings, particularly given that women with obesity tend to have more hot flashes and night sweats as a result of their extra weight. However, dosage data was not adjusted for BMI in the study and data on hormone levels was unavailable, she said, so it’s difficult to determine from the data whether HT was less effective for women with obesity or whether they were underdosed.
“I think women with obesity are undertreated,” Dr. Faubion said in an interview. “My guess is people are afraid. Women with obesity also may have other comorbidities,” such as hypertension and diabetes, she said, and “the greater the number of cardiovascular risk factors, the higher risk hormone therapy is.” Providers may therefore be leery of prescribing HT or prescribing it at an appropriately high enough dose to treat menopausal symptoms.
Common practice is to start patients at the lowest dose and titrate up according to symptoms, but “if people are afraid of it, they’re going to start the lowest dose” and may not increase it, Dr. Faubion said. She noted that other nonhormonal options are available, though providers should be conscientious about selecting ones whose adverse events do not include weight gain.
Although the study focused on an understudied population within hormone therapy research, the study was limited by its small size, low overall use of hormone therapy, recall bias, and the researchers’ inability to control for other medications the participants may have been taking.
Dr. Pershad said she is continuing research to try to identify the mechanisms underlying the reduced efficacy in women with obesity.
The research did not use any external funding. Dr. Pershad had no industry disclosures, but her colleagues reported honoraria from or speaking for TherapeuticsMD, Astella Pharma, Scynexis, Pharmavite, and Pfizer. Dr. Faubion had no disclosures.
PHILADELPHIA – , according to a small, retrospective study presented at the annual meeting of the Menopause Society (formerly the North American Menopause Society).
More than 40% of women over age 40 in the United States have obesity, presenter Anita Pershad, MD, an ob.gyn. medical resident at Eastern Virginia Medical School, Norfolk, told attendees. Yet most of the large-scale studies investigating perimenopausal and postmenopausal hormone therapy included participants without major medical comorbidities, so little data exist on how effectively HT works in women with these comorbidities, she said
“The main takeaway of our study is that obesity may worsen a woman’s menopausal symptoms and limit the amount of relief she gets from hormone therapy,” Dr. Pershad said in an interview. “It remains unclear if hormone therapy is less effective in women with obesity overall, or if the expected efficacy can be achieved with alternative design and administration routes. A potential mechanism of action for the observed decreased effect could be due to adipose tissue acting as a heat insulator, promoting the effects of vasomotor symptoms.”
Dr. Pershad and her colleagues conducted a retrospective review of the medical records of 119 patients who presented to a menopause clinic at a Midsouth urban academic medical center between July 2018 and December 2022. Obesity was defined as having a body mass index (BMI) of 30 kg/m2 or greater.
The patients with and without obesity were similar in terms of age, duration of menopause, use of hormone therapy, and therapy acceptance, but patients with obesity were more likely to identify themselves as Black (71% vs. 40%). Women with obesity were also significantly more likely than women without obesity to report vasomotor symptoms (74% vs. 45%, P = .002), genitourinary/vulvovaginal symptoms (60% vs. 21%, P < .001), mood disturbances (11% vs. 0%, P = .18), and decreased libido (29% vs. 11%, P = .017).
There were no significant differences in comorbidities between women with and without obesity, and among women who received systemic or localized HT, the same standard dosing was used for both groups.
Women with obesity were much less likely to see a satisfying reduction in their menopausal symptoms than women without obesity (odds ratio 0.07, 95% confidence interval, 0.01-0.64; P = .006), though the subgroups for each category of HT were small. Among the 20 women receiving systemic hormone therapy, only 1 of the 12 with obesity (8.3%) reported improvement in symptoms, compared with 7 of the 8 women without obesity (88%; P = .0004). Among 33 women using localized hormone therapy, 46% of the 24 women with obesity vs. 89% of the 9 women without obesity experienced symptom improvement (P = .026).
The proportions of women reporting relief from only lifestyle modifications or from nonhormonal medications, such as SSRIs/SNRIs, trazodone, and clonidine, were not statistically different. There were 33 women who relied only on lifestyle modifications, with 31% of the 16 women with obesity and 59% of the 17 women without obesity reporting improvement in their symptoms (P = .112). Similarly, among the 33 women using nonhormonal medications, 75% of the 20 women with obesity and 77% of the 13 women without obesity experienced relief (P = .9).
Women with obesity are undertreated
Dr. Pershad emphasized the need to improve care and counseling for diverse patients seeking treatment for menopausal symptoms.
“More research is needed to examine how women with medical comorbidities are uniquely impacted by menopause and respond to therapies,” Dr. Pershad said in an interview. “This can be achieved by actively including more diverse patient populations in women’s health studies, burdened by the social determinants of health and medical comorbidities such as obesity.”
Stephanie S. Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health, Rochester, Minn., and medical director for The Menopause Society, was not surprised by the findings, particularly given that women with obesity tend to have more hot flashes and night sweats as a result of their extra weight. However, dosage data was not adjusted for BMI in the study and data on hormone levels was unavailable, she said, so it’s difficult to determine from the data whether HT was less effective for women with obesity or whether they were underdosed.
“I think women with obesity are undertreated,” Dr. Faubion said in an interview. “My guess is people are afraid. Women with obesity also may have other comorbidities,” such as hypertension and diabetes, she said, and “the greater the number of cardiovascular risk factors, the higher risk hormone therapy is.” Providers may therefore be leery of prescribing HT or prescribing it at an appropriately high enough dose to treat menopausal symptoms.
Common practice is to start patients at the lowest dose and titrate up according to symptoms, but “if people are afraid of it, they’re going to start the lowest dose” and may not increase it, Dr. Faubion said. She noted that other nonhormonal options are available, though providers should be conscientious about selecting ones whose adverse events do not include weight gain.
Although the study focused on an understudied population within hormone therapy research, the study was limited by its small size, low overall use of hormone therapy, recall bias, and the researchers’ inability to control for other medications the participants may have been taking.
Dr. Pershad said she is continuing research to try to identify the mechanisms underlying the reduced efficacy in women with obesity.
The research did not use any external funding. Dr. Pershad had no industry disclosures, but her colleagues reported honoraria from or speaking for TherapeuticsMD, Astella Pharma, Scynexis, Pharmavite, and Pfizer. Dr. Faubion had no disclosures.
PHILADELPHIA – , according to a small, retrospective study presented at the annual meeting of the Menopause Society (formerly the North American Menopause Society).
More than 40% of women over age 40 in the United States have obesity, presenter Anita Pershad, MD, an ob.gyn. medical resident at Eastern Virginia Medical School, Norfolk, told attendees. Yet most of the large-scale studies investigating perimenopausal and postmenopausal hormone therapy included participants without major medical comorbidities, so little data exist on how effectively HT works in women with these comorbidities, she said
“The main takeaway of our study is that obesity may worsen a woman’s menopausal symptoms and limit the amount of relief she gets from hormone therapy,” Dr. Pershad said in an interview. “It remains unclear if hormone therapy is less effective in women with obesity overall, or if the expected efficacy can be achieved with alternative design and administration routes. A potential mechanism of action for the observed decreased effect could be due to adipose tissue acting as a heat insulator, promoting the effects of vasomotor symptoms.”
Dr. Pershad and her colleagues conducted a retrospective review of the medical records of 119 patients who presented to a menopause clinic at a Midsouth urban academic medical center between July 2018 and December 2022. Obesity was defined as having a body mass index (BMI) of 30 kg/m2 or greater.
The patients with and without obesity were similar in terms of age, duration of menopause, use of hormone therapy, and therapy acceptance, but patients with obesity were more likely to identify themselves as Black (71% vs. 40%). Women with obesity were also significantly more likely than women without obesity to report vasomotor symptoms (74% vs. 45%, P = .002), genitourinary/vulvovaginal symptoms (60% vs. 21%, P < .001), mood disturbances (11% vs. 0%, P = .18), and decreased libido (29% vs. 11%, P = .017).
There were no significant differences in comorbidities between women with and without obesity, and among women who received systemic or localized HT, the same standard dosing was used for both groups.
Women with obesity were much less likely to see a satisfying reduction in their menopausal symptoms than women without obesity (odds ratio 0.07, 95% confidence interval, 0.01-0.64; P = .006), though the subgroups for each category of HT were small. Among the 20 women receiving systemic hormone therapy, only 1 of the 12 with obesity (8.3%) reported improvement in symptoms, compared with 7 of the 8 women without obesity (88%; P = .0004). Among 33 women using localized hormone therapy, 46% of the 24 women with obesity vs. 89% of the 9 women without obesity experienced symptom improvement (P = .026).
The proportions of women reporting relief from only lifestyle modifications or from nonhormonal medications, such as SSRIs/SNRIs, trazodone, and clonidine, were not statistically different. There were 33 women who relied only on lifestyle modifications, with 31% of the 16 women with obesity and 59% of the 17 women without obesity reporting improvement in their symptoms (P = .112). Similarly, among the 33 women using nonhormonal medications, 75% of the 20 women with obesity and 77% of the 13 women without obesity experienced relief (P = .9).
Women with obesity are undertreated
Dr. Pershad emphasized the need to improve care and counseling for diverse patients seeking treatment for menopausal symptoms.
“More research is needed to examine how women with medical comorbidities are uniquely impacted by menopause and respond to therapies,” Dr. Pershad said in an interview. “This can be achieved by actively including more diverse patient populations in women’s health studies, burdened by the social determinants of health and medical comorbidities such as obesity.”
Stephanie S. Faubion, MD, MBA, director for Mayo Clinic’s Center for Women’s Health, Rochester, Minn., and medical director for The Menopause Society, was not surprised by the findings, particularly given that women with obesity tend to have more hot flashes and night sweats as a result of their extra weight. However, dosage data was not adjusted for BMI in the study and data on hormone levels was unavailable, she said, so it’s difficult to determine from the data whether HT was less effective for women with obesity or whether they were underdosed.
“I think women with obesity are undertreated,” Dr. Faubion said in an interview. “My guess is people are afraid. Women with obesity also may have other comorbidities,” such as hypertension and diabetes, she said, and “the greater the number of cardiovascular risk factors, the higher risk hormone therapy is.” Providers may therefore be leery of prescribing HT or prescribing it at an appropriately high enough dose to treat menopausal symptoms.
Common practice is to start patients at the lowest dose and titrate up according to symptoms, but “if people are afraid of it, they’re going to start the lowest dose” and may not increase it, Dr. Faubion said. She noted that other nonhormonal options are available, though providers should be conscientious about selecting ones whose adverse events do not include weight gain.
Although the study focused on an understudied population within hormone therapy research, the study was limited by its small size, low overall use of hormone therapy, recall bias, and the researchers’ inability to control for other medications the participants may have been taking.
Dr. Pershad said she is continuing research to try to identify the mechanisms underlying the reduced efficacy in women with obesity.
The research did not use any external funding. Dr. Pershad had no industry disclosures, but her colleagues reported honoraria from or speaking for TherapeuticsMD, Astella Pharma, Scynexis, Pharmavite, and Pfizer. Dr. Faubion had no disclosures.
AT THE MENOPAUSE SOCIETY ANNUAL MEETING
Enlarging pink patches after traveling
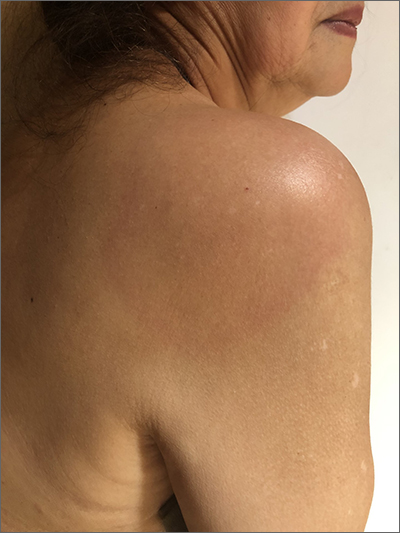
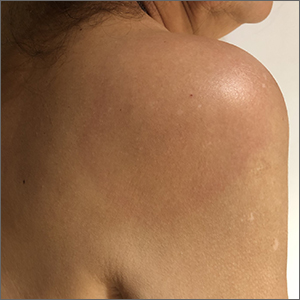
The patient’s multiple pink, subtly annular patches after recent travel to Lyme-endemic areas of the United States demonstrated a classic manifestation of disseminated Lyme disease. An enzyme-linked immunosorbent assay was positive for Borrelia burgdorferi IgM and IgG antibodies, confirming an acute infection.
While not usually necessary, skin biopsy shows a nonspecific perivascular cellular infiltrate that may be comprised of histiocytes, lymphocytes, and plasma cells. Spirochetes are not typically seen, but they may be identified with antibody-labeled or silver stains.
Lyme disease initially manifests as localized disease with erythema migrans, a targetoid lesion on the skin that appears at the site of the tick bite. This initial stage develops within the first few weeks of the bite and may be accompanied by fatigue and a low-grade fever.
If left untreated, the infection may progress to early disseminated disease, which occurs weeks to months after the initial bite. This second stage of Lyme disease manifests with multiple erythema migrans lesions on additional parts of the body, indicating spirochete dissemination through the bloodstream and lymphatic system. Early disseminated disease may also include borrelial lymphocytoma, Lyme neuroborreliosis, and cardiac conduction abnormalities such as AV block.
The third stage of Lyme disease, late Lyme disease, occurs months to years after an initial infection that has gone untreated. The key feature of this stage is arthritis, which tends to affect the knees and may be migratory in nature. Neurological symptoms such as encephalopathy and polyneuropathies may also develop. A minority of patients with late Lyme disease may develop acrodermatitis chronica atrophicans, a rash that typically occurs on the dorsal hands and feet as blue-red plaques that turn the affected skin atrophic.1
This patient was treated with a 3-week course of oral doxycycline 100 mg twice daily and was referred to an infectious disease specialist for further work-up of systemic symptoms, given the risk for cardiac pathology in disseminated Lyme disease.
Photo courtesy of Le Wen Chiu, MD. Text courtesy of Le Wen Chiu, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo-Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1

The patient’s multiple pink, subtly annular patches after recent travel to Lyme-endemic areas of the United States demonstrated a classic manifestation of disseminated Lyme disease. An enzyme-linked immunosorbent assay was positive for Borrelia burgdorferi IgM and IgG antibodies, confirming an acute infection.
While not usually necessary, skin biopsy shows a nonspecific perivascular cellular infiltrate that may be comprised of histiocytes, lymphocytes, and plasma cells. Spirochetes are not typically seen, but they may be identified with antibody-labeled or silver stains.
Lyme disease initially manifests as localized disease with erythema migrans, a targetoid lesion on the skin that appears at the site of the tick bite. This initial stage develops within the first few weeks of the bite and may be accompanied by fatigue and a low-grade fever.
If left untreated, the infection may progress to early disseminated disease, which occurs weeks to months after the initial bite. This second stage of Lyme disease manifests with multiple erythema migrans lesions on additional parts of the body, indicating spirochete dissemination through the bloodstream and lymphatic system. Early disseminated disease may also include borrelial lymphocytoma, Lyme neuroborreliosis, and cardiac conduction abnormalities such as AV block.
The third stage of Lyme disease, late Lyme disease, occurs months to years after an initial infection that has gone untreated. The key feature of this stage is arthritis, which tends to affect the knees and may be migratory in nature. Neurological symptoms such as encephalopathy and polyneuropathies may also develop. A minority of patients with late Lyme disease may develop acrodermatitis chronica atrophicans, a rash that typically occurs on the dorsal hands and feet as blue-red plaques that turn the affected skin atrophic.1
This patient was treated with a 3-week course of oral doxycycline 100 mg twice daily and was referred to an infectious disease specialist for further work-up of systemic symptoms, given the risk for cardiac pathology in disseminated Lyme disease.
Photo courtesy of Le Wen Chiu, MD. Text courtesy of Le Wen Chiu, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.

The patient’s multiple pink, subtly annular patches after recent travel to Lyme-endemic areas of the United States demonstrated a classic manifestation of disseminated Lyme disease. An enzyme-linked immunosorbent assay was positive for Borrelia burgdorferi IgM and IgG antibodies, confirming an acute infection.
While not usually necessary, skin biopsy shows a nonspecific perivascular cellular infiltrate that may be comprised of histiocytes, lymphocytes, and plasma cells. Spirochetes are not typically seen, but they may be identified with antibody-labeled or silver stains.
Lyme disease initially manifests as localized disease with erythema migrans, a targetoid lesion on the skin that appears at the site of the tick bite. This initial stage develops within the first few weeks of the bite and may be accompanied by fatigue and a low-grade fever.
If left untreated, the infection may progress to early disseminated disease, which occurs weeks to months after the initial bite. This second stage of Lyme disease manifests with multiple erythema migrans lesions on additional parts of the body, indicating spirochete dissemination through the bloodstream and lymphatic system. Early disseminated disease may also include borrelial lymphocytoma, Lyme neuroborreliosis, and cardiac conduction abnormalities such as AV block.
The third stage of Lyme disease, late Lyme disease, occurs months to years after an initial infection that has gone untreated. The key feature of this stage is arthritis, which tends to affect the knees and may be migratory in nature. Neurological symptoms such as encephalopathy and polyneuropathies may also develop. A minority of patients with late Lyme disease may develop acrodermatitis chronica atrophicans, a rash that typically occurs on the dorsal hands and feet as blue-red plaques that turn the affected skin atrophic.1
This patient was treated with a 3-week course of oral doxycycline 100 mg twice daily and was referred to an infectious disease specialist for further work-up of systemic symptoms, given the risk for cardiac pathology in disseminated Lyme disease.
Photo courtesy of Le Wen Chiu, MD. Text courtesy of Le Wen Chiu, MD, Department of Dermatology, University of New Mexico School of Medicine, Albuquerque, and Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker, MD School of Medicine, Kalamazoo.
1. Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo-Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
1. Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo-Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
Weight loss with semaglutide maintained for up to 3 years
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
Once weekly glucagon-like peptide 1 receptor agonist (GLP-1 RA) semaglutide (Ozempic, Novo Nordisk) significantly improved hemoglobin A1c level and body weight for up to 3 years in a large cohort of adults with type 2 diabetes, show real-world data from Israel.
and, in particular, in those patients with higher adherence to the therapy.
Avraham Karasik, MD, from the Institute of Research and Innovation at Maccabi Health Services, Tel Aviv, led the study and presented the work as a poster at this year’s annual meeting of the European Association for the Study of Diabetes.
“We found a clinically relevant improvement in blood sugar control and weight loss after 6 months of treatment, comparable with that seen in randomized trials,” said Dr. Karasik during an interview. “Importantly, these effects were sustained for up to 3 years, supporting the use of once weekly semaglutide for the long-term management of type 2 diabetes.”
Esther Walden, RN, deputy head of care at Diabetes UK, appreciated that the real-world findings reflected those seen in the randomized controlled trials. “This study suggests that improvements in blood sugars and weight loss can potentially be sustained in the longer term for adults with type 2 diabetes taking semaglutide as prescribed.”
Large scale, long term, and real world
Dr. Karasik explained that in Israel, there are many early adopters of once weekly semaglutide, and as such, it made for a large sample size, with a significant use duration for the retrospective study. “It’s a popular drug and there are lots of questions about durability of effect,” he pointed out.
Though evidence from randomized controlled trials support the effectiveness of once weekly semaglutide to treat type 2 diabetes, these studies are mostly of relatively short follow-up, explained Dr. Karasik, pointing out that long-term, large-scale, real-world data are needed. “In real life, people are acting differently to the trial setting and some adhere while others don’t, so it was interesting to see the durability as well as what happens when people discontinue treatment or adhere less.”
“Unsurprisingly, people who had a higher proportion of days covered ([PDC]; the total days of semaglutide use as a proportion of the total number of days followed up) had a higher effect,” explained Dr. Karasik, adding that, “if you don’t take it, it doesn’t work.”
A total of 23,442 patients were included in the study, with 6,049 followed up for 2 years or more. Mean baseline A1c was 7.6%-7.9%; body mass index (BMI) was 33.7-33.8 kg/m2; metformin was taken by 84%-88% of participants; insulin was taken by 30%; and 31% were treated with another GLP-1 RA prior to receiving semaglutide.
For study inclusion, participants were required to have had redeemed at least one prescription for subcutaneous semaglutide (0.25, 0.5, or 1 mg), and had at least one A1c measurement 12 months before and around 6 months after the start of semaglutide.
The primary outcome was change in A1c from baseline to the end of the follow-up at 6, 12, 18, 24, 30, and 36 months. Key secondary outcomes included change in body weight from baseline to the end of the follow-up (36 months); change in A1c and body weight in subgroups of patients who were persistently on therapy (at 12, 24, 36 months); and change in A1c and body weight in subgroups stratified by baseline characteristics. There was also an exploratory outcome, which was change in A1c and weight after treatment discontinuation. Dr. Karasik presented some of these results in his poster.
Median follow-up was 17.6 months in the total population and was 29.9 months in those who persisted with therapy for 2 years or more. “We have over 23,000 participants so it’s a large group, and these are not selected patients so the generalizability is better.”
Three-year sustained effect
Results from the total population showed that A1c lowered by a mean of 0.77% (from 7.6% to 6.8%) and body weight reduced by 4.7 kg (from 94.1 kg to 89.7 kg) after 6 months of treatment. These reductions were maintained during 3 years of follow-up in around 1,000 patients.
A significant 75% of participants adhered to once weekly semaglutide (PDC of more than 60%) within the first 6 months. In patients who used semaglutide for at least 2 years, those with high adherence (PDC of at least 80%) showed an A1c reduction of 0.76% after 24 months and of 0.43% after 36 months. Body weight was reduced by 6.0 kg after 24 months and 5.8 kg after 36 months.
Reductions in both A1c and weight were lower in patients with PDC of below 60%, compared with those with PDC of 60%-79% or 80% or over (statistically significant difference of P < .05 for between-groups differences for both outcomes across maximum follow-up time).
As expected, among patients who were GLP-1 RA–naive, reductions in A1c level and body weight were more pronounced, compared with GLP-1 RA–experienced patients (A1c reduction, –0.87% vs. –0.54%; weight loss, –5.5 kg vs. –3.0 kg, respectively; P < .001 for between-groups difference for both outcomes).
Dr. Karasik reported that some patients who stopped taking semaglutide did not regain weight immediately and that this potential residual effect after treatment discontinuation merits additional investigation. “This is not like in the randomized controlled trials. I don’t know how to interpret it, but that’s the observation. A1c did increase a little when they stopped therapy, compared to those with PDC [of 60%-79% or 80% or over] (P < .05 for between-groups difference for both outcomes in most follow-up time).”
He also highlighted that in regard to the long-term outcomes, “unlike many drugs where the effect fades out with time, here we don’t see that happening. This is another encouraging point.”
Dr. Karasik declares speaker fees and grants from Novo Nordisk, Boehringer Ingelheim, and AstraZeneca. The study was supported by Novo Nordisk.
A version of this article appeared on Medscape.com.
FROM EASD 2023
Tirzepatide with insulin glargine improves type 2 diabetes
HAMBURG, GERMANY – Once-weekly tirzepatide (Mounjaro, Lilly) added to insulin glargine resulted in greater reductions in hemoglobin A1c along with more weight loss and less hypoglycemia, compared with prandial insulin lispro (Humalog, Sanofi), for patients with inadequately controlled type 2 diabetes, show data from the SURPASS-6 randomized clinical trial.
It also resulted in a higher percentage of participants meeting an A1c target of less than 7.0%, wrote the researchers, whose study was presented at the annual meeting of the European Association for the Study of Diabetes and was published simultaneously in JAMA.
Also, daily insulin glargine use was substantially lower among participants who received tirzepatide, compared with insulin lispro. Insulin glargine was administered at a dosage 13 IU/day; insulin lispro was administered at a dosage of 62 IU/day. “At the highest dose, some patients stopped their insulin [glargine] in the tirzepatide arm,” said Juan Pablo Frias, MD, medical director and principal investigator of Velocity Clinical Research, Los Angeles, who presented the findings. “We demonstrated clinically meaningful and superior glycemic and body weight control with tirzepatide compared with insulin lispro, while tirzepatide was also associated with less clinically significant hypoglycemia.”
Weight improved for participants who received tirzepatide compared with those who received insulin lispro, at –10 kg and +4 kg respectively. The rate of clinically significant hypoglycemia (blood glucose < 54 mg/dL) or severe hypoglycemia was tenfold lower with tirzepatide, compared with insulin lispro.
The session dedicated to tirzepatide was comoderated by Apostolos Tsapas, MD, professor of medicine and diabetes, Aristotle University, Thessaloniki, Greece, and Konstantinos Toulis, MD, consultant in endocrinology and diabetes, General Military Hospital, Thessaloniki, Greece. Dr. Toulis remarked that, in the chronic disease setting, management and treatment intensification are challenging to integrate, and there are barriers to adoption in routine practice. “This is particularly true when it adds complexity, as in the case of multiple prandial insulin injections on top of basal insulin in suboptimally treated individuals with type 2 diabetes.
“Demonstrating superiority over insulin lispro in terms of the so-called trio of A1c, weight loss, and hypoglycemic events, tirzepatide offers both a simpler to adhere to and a more efficacious treatment intensification option.” He noted that, while long-term safety data are awaited, “this seems to be a definite step forward from any viewpoint, with the possible exception of the taxpayer’s perspective.”
Dr. Tsapas added: “These data further support the very high dual glucose and weight efficacy of tirzepatide and the primary role of incretin-related therapies amongst the injectables for the treatment of type 2 diabetes.”
Tirzepatide 5, 10, 15 mg vs. insulin lispro in addition to insulin glargine
The researchers aimed to assess the efficacy and safety of adding once-weekly tirzepatide, compared with thrice-daily prandial insulin lispro, as an adjunctive therapy to insulin glargine for patients with type 2 diabetes that was inadequately controlled with basal insulin.
Tirzepatide activates the body’s receptors for glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1). The study authors noted that “recent guidelines support adding an injectable incretin-related therapy such as GLP-1 receptor agonist for glycemic control, rather than basal insulin, when oral medications are inadequate.”
The open-label, phase 3b clinical trial drew data from 135 sites across 15 countries and included 1,428 adults with type 2 diabetes who were taking basal insulin. Participants were randomly assigned in a 1:1:1:3 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg [n = 243], 10 mg [n = 238], or 15 mg [n = 236]) or prandial thrice-daily insulin lispro (n = 708).
Both arms were well matched. The average age was 60 years, and 60% of participants were women. The average amount of time patients had type 2 diabetes was 14 years; 85% of participants continued taking metformin. The average A1c level was 8.8% at baseline. Patients were categorized as having obesity (average body mass index, 33 kg/m2). The average insulin glargine dose was 46 units, or 0.5 units/kg.
Outcomes included noninferiority of tirzepatide (pooled cohort) compared with insulin lispro, both in addition to insulin glargine; and A1c change from baseline to week 52 (noninferiority margin, 0.3%). Key secondary endpoints included change in body weight and percentage of participants who achieved an A1c target of less than 7.0%.
About 90% of participants who received the study drug completed the study, said Dr. Frias. “Only 0.5% of tirzepatide patients needed rescue therapy, while only 2% of the insulin lispro did.”
Prior to optimization, the average insulin glargine dose was 42 IU/kg; during optimization, it rose to an average of 46 IU/kg. “At 52 weeks, those on basal-bolus insulin found their insulin glargine dose stayed flat while insulin lispro was 62 units,” reported Dr. Frias. “The three tirzepatide doses show a reduction in insulin glargine, such that the pooled dose reached an average of 11 units, while 20% actually came off their basal insulin altogether [pooled tirzepatide].”
Tirzepatide (pooled) led to the recommended A1c target of less than 7.0% for 68% of patients versus 36% of patients in the insulin lispro group.
About 68% of the patients who received tirzepatide (pooled) achieved the recommended A1c target of less than 7.0% versus 36% of patients in the insulin lispro group.
“Individual tirzepatide doses and pooled doses showed significant reduction in A1c and up to a 2.5% reduction,” Dr. Frias added. “Normoglycemia was obtained by a greater proportion of patients on tirzepatide doses versus basal-bolus insulin – one-third in the 15-mg tirzepatide dose.”
Body weight reduction of 10% or more with tirzepatide
Further, at week 52, weight loss of 5% or more was achieved by 75.4% of participants in the pooled tirzepatide group, compared with 6.3% in the prandial lispro group. The weight loss was accompanied by clinically relevant improvements in cardiometabolic parameters.
In an exploratory analysis, weight loss of 10% or more was achieved by a mean of 48.9% of pooled tirzepatide-treated participants at week 52, compared with 2% of those taking insulin lispro, said Dr. Frias.
“It is possible that the body weight loss induced by tirzepatide therapy and its reported effect in reducing liver fat content may have led to an improvement in insulin sensitivity and decreased insulin requirements,” wrote the researchers in their article.
Hypoglycemia risk and the weight gain observed with complex insulin regimens that include prandial insulin have been main limitations to optimally up-titrate insulin therapy in clinical practice, wrote the authors.
Dr. Frias noted that, in this study, 48% of patients who received insulin lispro experienced clinically significant hypoglycemia, while only 10% of patients in the tirzepatide arms did. “This was 0.4 episodes per patient-year versus 4.4 in tirzepatide and insulin lispro respectively.”
There were more reports of adverse events among the tirzepatide groups than the insulin lispro group. “Typically, with tirzepatide, the commonest adverse events were GI in origin and were mild to moderate.” Rates were 14%-26% for nausea, 11%-15% for diarrhea, and 5%-13% for vomiting.
The study was sponsored by Eli Lilly. Dr. Frias has received grants from Eli Lilly paid to his institution during the conduct of the study and grants, personal fees, or nonfinancial support from Boehringer Ingelheim, Pfizer, Merck, Altimmune, 89BIO, Akero, Carmot Therapeutics, Intercept, Janssen, Madrigal, Novartis, Eli Lilly, Sanofi, and Novo Nordisk outside the submitted work. Dr. Toulis and Dr. Tsapas declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – Once-weekly tirzepatide (Mounjaro, Lilly) added to insulin glargine resulted in greater reductions in hemoglobin A1c along with more weight loss and less hypoglycemia, compared with prandial insulin lispro (Humalog, Sanofi), for patients with inadequately controlled type 2 diabetes, show data from the SURPASS-6 randomized clinical trial.
It also resulted in a higher percentage of participants meeting an A1c target of less than 7.0%, wrote the researchers, whose study was presented at the annual meeting of the European Association for the Study of Diabetes and was published simultaneously in JAMA.
Also, daily insulin glargine use was substantially lower among participants who received tirzepatide, compared with insulin lispro. Insulin glargine was administered at a dosage 13 IU/day; insulin lispro was administered at a dosage of 62 IU/day. “At the highest dose, some patients stopped their insulin [glargine] in the tirzepatide arm,” said Juan Pablo Frias, MD, medical director and principal investigator of Velocity Clinical Research, Los Angeles, who presented the findings. “We demonstrated clinically meaningful and superior glycemic and body weight control with tirzepatide compared with insulin lispro, while tirzepatide was also associated with less clinically significant hypoglycemia.”
Weight improved for participants who received tirzepatide compared with those who received insulin lispro, at –10 kg and +4 kg respectively. The rate of clinically significant hypoglycemia (blood glucose < 54 mg/dL) or severe hypoglycemia was tenfold lower with tirzepatide, compared with insulin lispro.
The session dedicated to tirzepatide was comoderated by Apostolos Tsapas, MD, professor of medicine and diabetes, Aristotle University, Thessaloniki, Greece, and Konstantinos Toulis, MD, consultant in endocrinology and diabetes, General Military Hospital, Thessaloniki, Greece. Dr. Toulis remarked that, in the chronic disease setting, management and treatment intensification are challenging to integrate, and there are barriers to adoption in routine practice. “This is particularly true when it adds complexity, as in the case of multiple prandial insulin injections on top of basal insulin in suboptimally treated individuals with type 2 diabetes.
“Demonstrating superiority over insulin lispro in terms of the so-called trio of A1c, weight loss, and hypoglycemic events, tirzepatide offers both a simpler to adhere to and a more efficacious treatment intensification option.” He noted that, while long-term safety data are awaited, “this seems to be a definite step forward from any viewpoint, with the possible exception of the taxpayer’s perspective.”
Dr. Tsapas added: “These data further support the very high dual glucose and weight efficacy of tirzepatide and the primary role of incretin-related therapies amongst the injectables for the treatment of type 2 diabetes.”
Tirzepatide 5, 10, 15 mg vs. insulin lispro in addition to insulin glargine
The researchers aimed to assess the efficacy and safety of adding once-weekly tirzepatide, compared with thrice-daily prandial insulin lispro, as an adjunctive therapy to insulin glargine for patients with type 2 diabetes that was inadequately controlled with basal insulin.
Tirzepatide activates the body’s receptors for glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1). The study authors noted that “recent guidelines support adding an injectable incretin-related therapy such as GLP-1 receptor agonist for glycemic control, rather than basal insulin, when oral medications are inadequate.”
The open-label, phase 3b clinical trial drew data from 135 sites across 15 countries and included 1,428 adults with type 2 diabetes who were taking basal insulin. Participants were randomly assigned in a 1:1:1:3 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg [n = 243], 10 mg [n = 238], or 15 mg [n = 236]) or prandial thrice-daily insulin lispro (n = 708).
Both arms were well matched. The average age was 60 years, and 60% of participants were women. The average amount of time patients had type 2 diabetes was 14 years; 85% of participants continued taking metformin. The average A1c level was 8.8% at baseline. Patients were categorized as having obesity (average body mass index, 33 kg/m2). The average insulin glargine dose was 46 units, or 0.5 units/kg.
Outcomes included noninferiority of tirzepatide (pooled cohort) compared with insulin lispro, both in addition to insulin glargine; and A1c change from baseline to week 52 (noninferiority margin, 0.3%). Key secondary endpoints included change in body weight and percentage of participants who achieved an A1c target of less than 7.0%.
About 90% of participants who received the study drug completed the study, said Dr. Frias. “Only 0.5% of tirzepatide patients needed rescue therapy, while only 2% of the insulin lispro did.”
Prior to optimization, the average insulin glargine dose was 42 IU/kg; during optimization, it rose to an average of 46 IU/kg. “At 52 weeks, those on basal-bolus insulin found their insulin glargine dose stayed flat while insulin lispro was 62 units,” reported Dr. Frias. “The three tirzepatide doses show a reduction in insulin glargine, such that the pooled dose reached an average of 11 units, while 20% actually came off their basal insulin altogether [pooled tirzepatide].”
Tirzepatide (pooled) led to the recommended A1c target of less than 7.0% for 68% of patients versus 36% of patients in the insulin lispro group.
About 68% of the patients who received tirzepatide (pooled) achieved the recommended A1c target of less than 7.0% versus 36% of patients in the insulin lispro group.
“Individual tirzepatide doses and pooled doses showed significant reduction in A1c and up to a 2.5% reduction,” Dr. Frias added. “Normoglycemia was obtained by a greater proportion of patients on tirzepatide doses versus basal-bolus insulin – one-third in the 15-mg tirzepatide dose.”
Body weight reduction of 10% or more with tirzepatide
Further, at week 52, weight loss of 5% or more was achieved by 75.4% of participants in the pooled tirzepatide group, compared with 6.3% in the prandial lispro group. The weight loss was accompanied by clinically relevant improvements in cardiometabolic parameters.
In an exploratory analysis, weight loss of 10% or more was achieved by a mean of 48.9% of pooled tirzepatide-treated participants at week 52, compared with 2% of those taking insulin lispro, said Dr. Frias.
“It is possible that the body weight loss induced by tirzepatide therapy and its reported effect in reducing liver fat content may have led to an improvement in insulin sensitivity and decreased insulin requirements,” wrote the researchers in their article.
Hypoglycemia risk and the weight gain observed with complex insulin regimens that include prandial insulin have been main limitations to optimally up-titrate insulin therapy in clinical practice, wrote the authors.
Dr. Frias noted that, in this study, 48% of patients who received insulin lispro experienced clinically significant hypoglycemia, while only 10% of patients in the tirzepatide arms did. “This was 0.4 episodes per patient-year versus 4.4 in tirzepatide and insulin lispro respectively.”
There were more reports of adverse events among the tirzepatide groups than the insulin lispro group. “Typically, with tirzepatide, the commonest adverse events were GI in origin and were mild to moderate.” Rates were 14%-26% for nausea, 11%-15% for diarrhea, and 5%-13% for vomiting.
The study was sponsored by Eli Lilly. Dr. Frias has received grants from Eli Lilly paid to his institution during the conduct of the study and grants, personal fees, or nonfinancial support from Boehringer Ingelheim, Pfizer, Merck, Altimmune, 89BIO, Akero, Carmot Therapeutics, Intercept, Janssen, Madrigal, Novartis, Eli Lilly, Sanofi, and Novo Nordisk outside the submitted work. Dr. Toulis and Dr. Tsapas declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
HAMBURG, GERMANY – Once-weekly tirzepatide (Mounjaro, Lilly) added to insulin glargine resulted in greater reductions in hemoglobin A1c along with more weight loss and less hypoglycemia, compared with prandial insulin lispro (Humalog, Sanofi), for patients with inadequately controlled type 2 diabetes, show data from the SURPASS-6 randomized clinical trial.
It also resulted in a higher percentage of participants meeting an A1c target of less than 7.0%, wrote the researchers, whose study was presented at the annual meeting of the European Association for the Study of Diabetes and was published simultaneously in JAMA.
Also, daily insulin glargine use was substantially lower among participants who received tirzepatide, compared with insulin lispro. Insulin glargine was administered at a dosage 13 IU/day; insulin lispro was administered at a dosage of 62 IU/day. “At the highest dose, some patients stopped their insulin [glargine] in the tirzepatide arm,” said Juan Pablo Frias, MD, medical director and principal investigator of Velocity Clinical Research, Los Angeles, who presented the findings. “We demonstrated clinically meaningful and superior glycemic and body weight control with tirzepatide compared with insulin lispro, while tirzepatide was also associated with less clinically significant hypoglycemia.”
Weight improved for participants who received tirzepatide compared with those who received insulin lispro, at –10 kg and +4 kg respectively. The rate of clinically significant hypoglycemia (blood glucose < 54 mg/dL) or severe hypoglycemia was tenfold lower with tirzepatide, compared with insulin lispro.
The session dedicated to tirzepatide was comoderated by Apostolos Tsapas, MD, professor of medicine and diabetes, Aristotle University, Thessaloniki, Greece, and Konstantinos Toulis, MD, consultant in endocrinology and diabetes, General Military Hospital, Thessaloniki, Greece. Dr. Toulis remarked that, in the chronic disease setting, management and treatment intensification are challenging to integrate, and there are barriers to adoption in routine practice. “This is particularly true when it adds complexity, as in the case of multiple prandial insulin injections on top of basal insulin in suboptimally treated individuals with type 2 diabetes.
“Demonstrating superiority over insulin lispro in terms of the so-called trio of A1c, weight loss, and hypoglycemic events, tirzepatide offers both a simpler to adhere to and a more efficacious treatment intensification option.” He noted that, while long-term safety data are awaited, “this seems to be a definite step forward from any viewpoint, with the possible exception of the taxpayer’s perspective.”
Dr. Tsapas added: “These data further support the very high dual glucose and weight efficacy of tirzepatide and the primary role of incretin-related therapies amongst the injectables for the treatment of type 2 diabetes.”
Tirzepatide 5, 10, 15 mg vs. insulin lispro in addition to insulin glargine
The researchers aimed to assess the efficacy and safety of adding once-weekly tirzepatide, compared with thrice-daily prandial insulin lispro, as an adjunctive therapy to insulin glargine for patients with type 2 diabetes that was inadequately controlled with basal insulin.
Tirzepatide activates the body’s receptors for glucose-dependent insulinotropic polypeptide and glucagonlike peptide–1 (GLP-1). The study authors noted that “recent guidelines support adding an injectable incretin-related therapy such as GLP-1 receptor agonist for glycemic control, rather than basal insulin, when oral medications are inadequate.”
The open-label, phase 3b clinical trial drew data from 135 sites across 15 countries and included 1,428 adults with type 2 diabetes who were taking basal insulin. Participants were randomly assigned in a 1:1:1:3 ratio to receive once-weekly subcutaneous injections of tirzepatide (5 mg [n = 243], 10 mg [n = 238], or 15 mg [n = 236]) or prandial thrice-daily insulin lispro (n = 708).
Both arms were well matched. The average age was 60 years, and 60% of participants were women. The average amount of time patients had type 2 diabetes was 14 years; 85% of participants continued taking metformin. The average A1c level was 8.8% at baseline. Patients were categorized as having obesity (average body mass index, 33 kg/m2). The average insulin glargine dose was 46 units, or 0.5 units/kg.
Outcomes included noninferiority of tirzepatide (pooled cohort) compared with insulin lispro, both in addition to insulin glargine; and A1c change from baseline to week 52 (noninferiority margin, 0.3%). Key secondary endpoints included change in body weight and percentage of participants who achieved an A1c target of less than 7.0%.
About 90% of participants who received the study drug completed the study, said Dr. Frias. “Only 0.5% of tirzepatide patients needed rescue therapy, while only 2% of the insulin lispro did.”
Prior to optimization, the average insulin glargine dose was 42 IU/kg; during optimization, it rose to an average of 46 IU/kg. “At 52 weeks, those on basal-bolus insulin found their insulin glargine dose stayed flat while insulin lispro was 62 units,” reported Dr. Frias. “The three tirzepatide doses show a reduction in insulin glargine, such that the pooled dose reached an average of 11 units, while 20% actually came off their basal insulin altogether [pooled tirzepatide].”
Tirzepatide (pooled) led to the recommended A1c target of less than 7.0% for 68% of patients versus 36% of patients in the insulin lispro group.
About 68% of the patients who received tirzepatide (pooled) achieved the recommended A1c target of less than 7.0% versus 36% of patients in the insulin lispro group.
“Individual tirzepatide doses and pooled doses showed significant reduction in A1c and up to a 2.5% reduction,” Dr. Frias added. “Normoglycemia was obtained by a greater proportion of patients on tirzepatide doses versus basal-bolus insulin – one-third in the 15-mg tirzepatide dose.”
Body weight reduction of 10% or more with tirzepatide
Further, at week 52, weight loss of 5% or more was achieved by 75.4% of participants in the pooled tirzepatide group, compared with 6.3% in the prandial lispro group. The weight loss was accompanied by clinically relevant improvements in cardiometabolic parameters.
In an exploratory analysis, weight loss of 10% or more was achieved by a mean of 48.9% of pooled tirzepatide-treated participants at week 52, compared with 2% of those taking insulin lispro, said Dr. Frias.
“It is possible that the body weight loss induced by tirzepatide therapy and its reported effect in reducing liver fat content may have led to an improvement in insulin sensitivity and decreased insulin requirements,” wrote the researchers in their article.
Hypoglycemia risk and the weight gain observed with complex insulin regimens that include prandial insulin have been main limitations to optimally up-titrate insulin therapy in clinical practice, wrote the authors.
Dr. Frias noted that, in this study, 48% of patients who received insulin lispro experienced clinically significant hypoglycemia, while only 10% of patients in the tirzepatide arms did. “This was 0.4 episodes per patient-year versus 4.4 in tirzepatide and insulin lispro respectively.”
There were more reports of adverse events among the tirzepatide groups than the insulin lispro group. “Typically, with tirzepatide, the commonest adverse events were GI in origin and were mild to moderate.” Rates were 14%-26% for nausea, 11%-15% for diarrhea, and 5%-13% for vomiting.
The study was sponsored by Eli Lilly. Dr. Frias has received grants from Eli Lilly paid to his institution during the conduct of the study and grants, personal fees, or nonfinancial support from Boehringer Ingelheim, Pfizer, Merck, Altimmune, 89BIO, Akero, Carmot Therapeutics, Intercept, Janssen, Madrigal, Novartis, Eli Lilly, Sanofi, and Novo Nordisk outside the submitted work. Dr. Toulis and Dr. Tsapas declared no relevant disclosures.
A version of this article first appeared on Medscape.com.
AT EASD 2023
Decoding AFib recurrence: PCPs’ role in personalized care
One in three patients who experience their first bout of atrial fibrillation (AFib) during hospitalization can expect to experience a recurrence of the arrhythmia within the year, new research shows.
The findings, reported in Annals of Internal Medicine, suggest these patients may be good candidates for oral anticoagulants to reduce their risk for stroke.
“Atrial fibrillation is very common in patients for the very first time in their life when they’re sick and in the hospital,” said William F. McIntyre, MD, PhD, a cardiologist at McMaster University, Hamilton, Ont., who led the study. These new insights into AFib management suggest there is a need for primary care physicians to be on the lookout for potential recurrence.
AFib is strongly linked to stroke, and patients at greater risk for stroke may be prescribed oral anticoagulants. Although the arrhythmia can be reversed before the patient is discharged from the hospital, risk for recurrence was unclear, Dr. McIntyre said.
“We wanted to know if the patient was in atrial fibrillation because of the physiologic stress that they were under, or if they just have the disease called atrial fibrillation, which should usually be followed lifelong by a specialist,” Dr. McIntyre said.
Dr. McIntyre and colleagues followed 139 patients (mean age, 71 years) at three medical centers in Ontario who experienced new-onset AFib during their hospital stay, along with an equal number of patients who had no history of AFib and who served as controls. The research team used a Holter monitor to record study participants’ heart rhythm for 14 days to detect incident AFib at 1 and 6 months after discharge. They also followed up with periodic phone calls for up to 12 months. Among the study participants, half were admitted for noncardiac surgeries, and the other half were admitted for medical illnesses, including infections and pneumonia. Participants with a prior history of AFib were excluded from the analysis.
The primary outcome of the study was an episode of AFib that lasted at least 30 seconds on the monitor or one detected during routine care at the 12-month mark.
Patients who experienced AFib for the first time in the hospital had roughly a 33% risk for recurrence within a year, nearly sevenfold higher than their age- and sex-matched counterparts who had not had an arrhythmia during their hospital stay (3%; confidence interval, 0%-6.4%).
“This study has important implications for management of patients who have a first presentation of AFib that is concurrent with a reversible physiologic stressor,” the authors wrote. “An AFib recurrence risk of 33.1% at 1 year is neither low enough to conclude that transient new-onset AFib in the setting of another illness is benign nor high enough that all such transient new-onset AFib can be assumed to be paroxysmal AFib. Instead, these results call for risk stratification and follow-up in these patients.”
The researchers reported that among people with recurrent AFib in the study, the median total time in arrhythmia was 9 hours. “This far exceeds the cutoff of 6 minutes that was established as being associated with stroke using simulated AFib screening in patients with implanted continuous monitors,” they wrote. “These results suggest that the patients in our study who had AFib detected in follow-up are similar to contemporary patients with AFib for whom evidence-based therapies, including oral anticoagulation, are warranted.”
Dr. McIntyre and colleagues were able to track outcomes and treatments for the patients in the study. In the group with recurrent AFib, 1 had a stroke, 2 experienced systemic embolism, 3 had a heart failure event, 6 experienced bleeding, and 11 died. In the other group, there was one case of stroke, one of heart failure, four cases involving bleeding, and seven deaths. “The proportion of participants with new-onset AFib during their initial hospitalization who were taking oral anticoagulants was 47.1% at 6 months and 49.2% at 12 months. This included 73% of participants with AFib detected during follow-up and 39% who did not have AFib detected during follow-up,” they wrote.
The uncertain nature of AFib recurrence complicates predictions about patients’ posthospitalization experiences within the following year. “We cannot just say: ‘Hey, this is just a reversible illness, and now we can forget about it,’ ” Dr. McIntyre said. “Nor is the risk of recurrence so strong in the other direction that you can give patients a lifelong diagnosis of atrial fibrillation.”
Role for primary care
Without that certainty, physicians cannot refer everyone who experiences new-onset AFib to a cardiologist for long-term care. The variability in recurrence rates necessitates a more nuanced and personalized approach. Here, primary care physicians step in, offering tailored care based on their established, long-term patient relationships, Dr. McIntyre said.
The study participants already have chronic health conditions that bring them into regular contact with their family physician. This gives primary care physicians a golden opportunity to be on lookout and to recommend care from a cardiologist at the appropriate time if it becomes necessary, he said.
“I have certainly seen cases of recurrent atrial fibrillation in patients who had an episode while hospitalized, and consistent with this study, this is a common clinical occurrence,” said Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart, New York. Primary care physicians must remain vigilant and avoid the temptation to attribute AFib solely to illness or surgery
“Ideally, we would have randomized clinical trial data to guide the decision about whether to use prophylactic anticoagulation,” said Dr. Bhatt, who added that a cardiology consultation may also be appropriate.
Dr. McIntyre reported no relevant financial relationships. Dr. Bhatt reported numerous relationships with industry.
A version of this article appeared on Medscape.com.
One in three patients who experience their first bout of atrial fibrillation (AFib) during hospitalization can expect to experience a recurrence of the arrhythmia within the year, new research shows.
The findings, reported in Annals of Internal Medicine, suggest these patients may be good candidates for oral anticoagulants to reduce their risk for stroke.
“Atrial fibrillation is very common in patients for the very first time in their life when they’re sick and in the hospital,” said William F. McIntyre, MD, PhD, a cardiologist at McMaster University, Hamilton, Ont., who led the study. These new insights into AFib management suggest there is a need for primary care physicians to be on the lookout for potential recurrence.
AFib is strongly linked to stroke, and patients at greater risk for stroke may be prescribed oral anticoagulants. Although the arrhythmia can be reversed before the patient is discharged from the hospital, risk for recurrence was unclear, Dr. McIntyre said.
“We wanted to know if the patient was in atrial fibrillation because of the physiologic stress that they were under, or if they just have the disease called atrial fibrillation, which should usually be followed lifelong by a specialist,” Dr. McIntyre said.
Dr. McIntyre and colleagues followed 139 patients (mean age, 71 years) at three medical centers in Ontario who experienced new-onset AFib during their hospital stay, along with an equal number of patients who had no history of AFib and who served as controls. The research team used a Holter monitor to record study participants’ heart rhythm for 14 days to detect incident AFib at 1 and 6 months after discharge. They also followed up with periodic phone calls for up to 12 months. Among the study participants, half were admitted for noncardiac surgeries, and the other half were admitted for medical illnesses, including infections and pneumonia. Participants with a prior history of AFib were excluded from the analysis.
The primary outcome of the study was an episode of AFib that lasted at least 30 seconds on the monitor or one detected during routine care at the 12-month mark.
Patients who experienced AFib for the first time in the hospital had roughly a 33% risk for recurrence within a year, nearly sevenfold higher than their age- and sex-matched counterparts who had not had an arrhythmia during their hospital stay (3%; confidence interval, 0%-6.4%).
“This study has important implications for management of patients who have a first presentation of AFib that is concurrent with a reversible physiologic stressor,” the authors wrote. “An AFib recurrence risk of 33.1% at 1 year is neither low enough to conclude that transient new-onset AFib in the setting of another illness is benign nor high enough that all such transient new-onset AFib can be assumed to be paroxysmal AFib. Instead, these results call for risk stratification and follow-up in these patients.”
The researchers reported that among people with recurrent AFib in the study, the median total time in arrhythmia was 9 hours. “This far exceeds the cutoff of 6 minutes that was established as being associated with stroke using simulated AFib screening in patients with implanted continuous monitors,” they wrote. “These results suggest that the patients in our study who had AFib detected in follow-up are similar to contemporary patients with AFib for whom evidence-based therapies, including oral anticoagulation, are warranted.”
Dr. McIntyre and colleagues were able to track outcomes and treatments for the patients in the study. In the group with recurrent AFib, 1 had a stroke, 2 experienced systemic embolism, 3 had a heart failure event, 6 experienced bleeding, and 11 died. In the other group, there was one case of stroke, one of heart failure, four cases involving bleeding, and seven deaths. “The proportion of participants with new-onset AFib during their initial hospitalization who were taking oral anticoagulants was 47.1% at 6 months and 49.2% at 12 months. This included 73% of participants with AFib detected during follow-up and 39% who did not have AFib detected during follow-up,” they wrote.
The uncertain nature of AFib recurrence complicates predictions about patients’ posthospitalization experiences within the following year. “We cannot just say: ‘Hey, this is just a reversible illness, and now we can forget about it,’ ” Dr. McIntyre said. “Nor is the risk of recurrence so strong in the other direction that you can give patients a lifelong diagnosis of atrial fibrillation.”
Role for primary care
Without that certainty, physicians cannot refer everyone who experiences new-onset AFib to a cardiologist for long-term care. The variability in recurrence rates necessitates a more nuanced and personalized approach. Here, primary care physicians step in, offering tailored care based on their established, long-term patient relationships, Dr. McIntyre said.
The study participants already have chronic health conditions that bring them into regular contact with their family physician. This gives primary care physicians a golden opportunity to be on lookout and to recommend care from a cardiologist at the appropriate time if it becomes necessary, he said.
“I have certainly seen cases of recurrent atrial fibrillation in patients who had an episode while hospitalized, and consistent with this study, this is a common clinical occurrence,” said Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart, New York. Primary care physicians must remain vigilant and avoid the temptation to attribute AFib solely to illness or surgery
“Ideally, we would have randomized clinical trial data to guide the decision about whether to use prophylactic anticoagulation,” said Dr. Bhatt, who added that a cardiology consultation may also be appropriate.
Dr. McIntyre reported no relevant financial relationships. Dr. Bhatt reported numerous relationships with industry.
A version of this article appeared on Medscape.com.
One in three patients who experience their first bout of atrial fibrillation (AFib) during hospitalization can expect to experience a recurrence of the arrhythmia within the year, new research shows.
The findings, reported in Annals of Internal Medicine, suggest these patients may be good candidates for oral anticoagulants to reduce their risk for stroke.
“Atrial fibrillation is very common in patients for the very first time in their life when they’re sick and in the hospital,” said William F. McIntyre, MD, PhD, a cardiologist at McMaster University, Hamilton, Ont., who led the study. These new insights into AFib management suggest there is a need for primary care physicians to be on the lookout for potential recurrence.
AFib is strongly linked to stroke, and patients at greater risk for stroke may be prescribed oral anticoagulants. Although the arrhythmia can be reversed before the patient is discharged from the hospital, risk for recurrence was unclear, Dr. McIntyre said.
“We wanted to know if the patient was in atrial fibrillation because of the physiologic stress that they were under, or if they just have the disease called atrial fibrillation, which should usually be followed lifelong by a specialist,” Dr. McIntyre said.
Dr. McIntyre and colleagues followed 139 patients (mean age, 71 years) at three medical centers in Ontario who experienced new-onset AFib during their hospital stay, along with an equal number of patients who had no history of AFib and who served as controls. The research team used a Holter monitor to record study participants’ heart rhythm for 14 days to detect incident AFib at 1 and 6 months after discharge. They also followed up with periodic phone calls for up to 12 months. Among the study participants, half were admitted for noncardiac surgeries, and the other half were admitted for medical illnesses, including infections and pneumonia. Participants with a prior history of AFib were excluded from the analysis.
The primary outcome of the study was an episode of AFib that lasted at least 30 seconds on the monitor or one detected during routine care at the 12-month mark.
Patients who experienced AFib for the first time in the hospital had roughly a 33% risk for recurrence within a year, nearly sevenfold higher than their age- and sex-matched counterparts who had not had an arrhythmia during their hospital stay (3%; confidence interval, 0%-6.4%).
“This study has important implications for management of patients who have a first presentation of AFib that is concurrent with a reversible physiologic stressor,” the authors wrote. “An AFib recurrence risk of 33.1% at 1 year is neither low enough to conclude that transient new-onset AFib in the setting of another illness is benign nor high enough that all such transient new-onset AFib can be assumed to be paroxysmal AFib. Instead, these results call for risk stratification and follow-up in these patients.”
The researchers reported that among people with recurrent AFib in the study, the median total time in arrhythmia was 9 hours. “This far exceeds the cutoff of 6 minutes that was established as being associated with stroke using simulated AFib screening in patients with implanted continuous monitors,” they wrote. “These results suggest that the patients in our study who had AFib detected in follow-up are similar to contemporary patients with AFib for whom evidence-based therapies, including oral anticoagulation, are warranted.”
Dr. McIntyre and colleagues were able to track outcomes and treatments for the patients in the study. In the group with recurrent AFib, 1 had a stroke, 2 experienced systemic embolism, 3 had a heart failure event, 6 experienced bleeding, and 11 died. In the other group, there was one case of stroke, one of heart failure, four cases involving bleeding, and seven deaths. “The proportion of participants with new-onset AFib during their initial hospitalization who were taking oral anticoagulants was 47.1% at 6 months and 49.2% at 12 months. This included 73% of participants with AFib detected during follow-up and 39% who did not have AFib detected during follow-up,” they wrote.
The uncertain nature of AFib recurrence complicates predictions about patients’ posthospitalization experiences within the following year. “We cannot just say: ‘Hey, this is just a reversible illness, and now we can forget about it,’ ” Dr. McIntyre said. “Nor is the risk of recurrence so strong in the other direction that you can give patients a lifelong diagnosis of atrial fibrillation.”
Role for primary care
Without that certainty, physicians cannot refer everyone who experiences new-onset AFib to a cardiologist for long-term care. The variability in recurrence rates necessitates a more nuanced and personalized approach. Here, primary care physicians step in, offering tailored care based on their established, long-term patient relationships, Dr. McIntyre said.
The study participants already have chronic health conditions that bring them into regular contact with their family physician. This gives primary care physicians a golden opportunity to be on lookout and to recommend care from a cardiologist at the appropriate time if it becomes necessary, he said.
“I have certainly seen cases of recurrent atrial fibrillation in patients who had an episode while hospitalized, and consistent with this study, this is a common clinical occurrence,” said Deepak L. Bhatt, MD, MPH, director of Mount Sinai Heart, New York. Primary care physicians must remain vigilant and avoid the temptation to attribute AFib solely to illness or surgery
“Ideally, we would have randomized clinical trial data to guide the decision about whether to use prophylactic anticoagulation,” said Dr. Bhatt, who added that a cardiology consultation may also be appropriate.
Dr. McIntyre reported no relevant financial relationships. Dr. Bhatt reported numerous relationships with industry.
A version of this article appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
Spironolactone safe, effective option for women with hidradenitis suppurativa
CARLSBAD, CALIF. –
Those are the key findings from a single-center retrospective study that Jennifer L. Hsiao, MD, and colleagues presented during a poster session at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
In an interview after the meeting, Dr. Hsiao, a dermatologist who directs the hidradenitis suppurativa clinic at the University of Southern California, Los Angeles, said that hormones are thought to play a role in HS pathogenesis given the typical HS symptom onset around puberty and fluctuations in disease activity with menses (typically premenstrual flares) and pregnancy. “Spironolactone, an anti-androgenic agent, is used to treat HS in women; however, there is a paucity of data on the efficacy of spironolactone for HS and whether certain patient characteristics may influence treatment response,” she told this news organization. “This study is unique in that we contribute to existing literature regarding spironolactone efficacy in HS and we also investigate whether the presence of menstrual HS flares or polycystic ovarian syndrome influences the likelihood of response to spironolactone.”
For the analysis, Dr. Hsiao and colleagues retrospectively reviewed the medical records of 53 adult women with HS who were prescribed spironolactone and who received care at USC’s HS clinic between January 2015 and December 2021. They collected data on demographics, comorbidities, HS medications, treatment response at 3 and 6 months, as well as adverse events. They also evaluated physician-assessed response to treatment when available.
The mean age of patients was 31 years, 37% were White, 30.4% were Black, 21.7% were Hispanic, 6.5% were Asian, and the remainder were biracial. The mean age at HS diagnosis was 25.1 years and the three most common comorbidities were acne (50.9%), obesity (45.3%), and anemia (37.7%). As for menstrual history, 56.6% had perimenstrual HS flares and 37.7% had irregular menstrual cycles. The top three classes of concomitant medications were antibiotics (58.5%), oral contraceptives (50.9%), and other birth control methods (18.9%).
The mean spironolactone dose was 104 mg/day; 84.1% of the women experienced improvement of HS 3 months after starting the drug, while 81.8% had improvement of their HS 6 months after starting the drug. The researchers also found that 56.6% of women had documented perimenstrual HS flares and 7.5% had PCOS.
“Spironolactone is often thought of as a helpful medication to consider if a patient reports having HS flares around menses or features of PCOS,” Dr. Hsiao said. However, she added, “our study found that there was no statistically significant difference in the response to spironolactone based on the presence of premenstrual flares or concomitant PCOS.” She said that spironolactone may be used as an adjunct therapeutic option in patients with more severe disease in addition to other medical and surgical therapies for HS. “Combining different treatment options that target different pathophysiologic factors is usually required to achieve adequate disease control in HS,” she said.
Dr. Hsiao acknowledged certain limitations of the study, including its single-center design and small sample size. “A confounding variable is that some patients were on other medications in addition to spironolactone, which may have influenced treatment outcomes,” she noted. “Larger prospective studies are needed to identify optimal dosing for spironolactone therapy in HS as well as predictors of treatment response.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that with only one FDA-approved systemic medication for the management of HS (adalimumab), “we off-label bandits must be creative to curtail the incredibly painful impact this chronic, destructive inflammatory disease can have on our patients.”
“The evidence supporting our approaches, whether it be antibiotics, immunomodulators, or in this case, antihormonal therapies, is limited, so more data is always welcome,” said Dr. Friedman, who was not involved with the study. “One very interesting point raised by the authors, one I share with my trainees frequently from my own experience, is that regardless of menstrual cycle abnormalities, spironolactone can be impactful. This is important to remember, in that overt signs of hormonal influences is not a requisite for the use or effectiveness of antihormonal therapy.”
Dr. Hsiao disclosed that she is a member of board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Novartis, UCB, as a speaker for AbbVie, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte. Dr. Friedman reported having no relevant financial disclosures.
CARLSBAD, CALIF. –
Those are the key findings from a single-center retrospective study that Jennifer L. Hsiao, MD, and colleagues presented during a poster session at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
In an interview after the meeting, Dr. Hsiao, a dermatologist who directs the hidradenitis suppurativa clinic at the University of Southern California, Los Angeles, said that hormones are thought to play a role in HS pathogenesis given the typical HS symptom onset around puberty and fluctuations in disease activity with menses (typically premenstrual flares) and pregnancy. “Spironolactone, an anti-androgenic agent, is used to treat HS in women; however, there is a paucity of data on the efficacy of spironolactone for HS and whether certain patient characteristics may influence treatment response,” she told this news organization. “This study is unique in that we contribute to existing literature regarding spironolactone efficacy in HS and we also investigate whether the presence of menstrual HS flares or polycystic ovarian syndrome influences the likelihood of response to spironolactone.”
For the analysis, Dr. Hsiao and colleagues retrospectively reviewed the medical records of 53 adult women with HS who were prescribed spironolactone and who received care at USC’s HS clinic between January 2015 and December 2021. They collected data on demographics, comorbidities, HS medications, treatment response at 3 and 6 months, as well as adverse events. They also evaluated physician-assessed response to treatment when available.
The mean age of patients was 31 years, 37% were White, 30.4% were Black, 21.7% were Hispanic, 6.5% were Asian, and the remainder were biracial. The mean age at HS diagnosis was 25.1 years and the three most common comorbidities were acne (50.9%), obesity (45.3%), and anemia (37.7%). As for menstrual history, 56.6% had perimenstrual HS flares and 37.7% had irregular menstrual cycles. The top three classes of concomitant medications were antibiotics (58.5%), oral contraceptives (50.9%), and other birth control methods (18.9%).
The mean spironolactone dose was 104 mg/day; 84.1% of the women experienced improvement of HS 3 months after starting the drug, while 81.8% had improvement of their HS 6 months after starting the drug. The researchers also found that 56.6% of women had documented perimenstrual HS flares and 7.5% had PCOS.
“Spironolactone is often thought of as a helpful medication to consider if a patient reports having HS flares around menses or features of PCOS,” Dr. Hsiao said. However, she added, “our study found that there was no statistically significant difference in the response to spironolactone based on the presence of premenstrual flares or concomitant PCOS.” She said that spironolactone may be used as an adjunct therapeutic option in patients with more severe disease in addition to other medical and surgical therapies for HS. “Combining different treatment options that target different pathophysiologic factors is usually required to achieve adequate disease control in HS,” she said.
Dr. Hsiao acknowledged certain limitations of the study, including its single-center design and small sample size. “A confounding variable is that some patients were on other medications in addition to spironolactone, which may have influenced treatment outcomes,” she noted. “Larger prospective studies are needed to identify optimal dosing for spironolactone therapy in HS as well as predictors of treatment response.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that with only one FDA-approved systemic medication for the management of HS (adalimumab), “we off-label bandits must be creative to curtail the incredibly painful impact this chronic, destructive inflammatory disease can have on our patients.”
“The evidence supporting our approaches, whether it be antibiotics, immunomodulators, or in this case, antihormonal therapies, is limited, so more data is always welcome,” said Dr. Friedman, who was not involved with the study. “One very interesting point raised by the authors, one I share with my trainees frequently from my own experience, is that regardless of menstrual cycle abnormalities, spironolactone can be impactful. This is important to remember, in that overt signs of hormonal influences is not a requisite for the use or effectiveness of antihormonal therapy.”
Dr. Hsiao disclosed that she is a member of board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Novartis, UCB, as a speaker for AbbVie, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte. Dr. Friedman reported having no relevant financial disclosures.
CARLSBAD, CALIF. –
Those are the key findings from a single-center retrospective study that Jennifer L. Hsiao, MD, and colleagues presented during a poster session at the annual symposium of the California Society of Dermatology & Dermatologic Surgery.
In an interview after the meeting, Dr. Hsiao, a dermatologist who directs the hidradenitis suppurativa clinic at the University of Southern California, Los Angeles, said that hormones are thought to play a role in HS pathogenesis given the typical HS symptom onset around puberty and fluctuations in disease activity with menses (typically premenstrual flares) and pregnancy. “Spironolactone, an anti-androgenic agent, is used to treat HS in women; however, there is a paucity of data on the efficacy of spironolactone for HS and whether certain patient characteristics may influence treatment response,” she told this news organization. “This study is unique in that we contribute to existing literature regarding spironolactone efficacy in HS and we also investigate whether the presence of menstrual HS flares or polycystic ovarian syndrome influences the likelihood of response to spironolactone.”
For the analysis, Dr. Hsiao and colleagues retrospectively reviewed the medical records of 53 adult women with HS who were prescribed spironolactone and who received care at USC’s HS clinic between January 2015 and December 2021. They collected data on demographics, comorbidities, HS medications, treatment response at 3 and 6 months, as well as adverse events. They also evaluated physician-assessed response to treatment when available.
The mean age of patients was 31 years, 37% were White, 30.4% were Black, 21.7% were Hispanic, 6.5% were Asian, and the remainder were biracial. The mean age at HS diagnosis was 25.1 years and the three most common comorbidities were acne (50.9%), obesity (45.3%), and anemia (37.7%). As for menstrual history, 56.6% had perimenstrual HS flares and 37.7% had irregular menstrual cycles. The top three classes of concomitant medications were antibiotics (58.5%), oral contraceptives (50.9%), and other birth control methods (18.9%).
The mean spironolactone dose was 104 mg/day; 84.1% of the women experienced improvement of HS 3 months after starting the drug, while 81.8% had improvement of their HS 6 months after starting the drug. The researchers also found that 56.6% of women had documented perimenstrual HS flares and 7.5% had PCOS.
“Spironolactone is often thought of as a helpful medication to consider if a patient reports having HS flares around menses or features of PCOS,” Dr. Hsiao said. However, she added, “our study found that there was no statistically significant difference in the response to spironolactone based on the presence of premenstrual flares or concomitant PCOS.” She said that spironolactone may be used as an adjunct therapeutic option in patients with more severe disease in addition to other medical and surgical therapies for HS. “Combining different treatment options that target different pathophysiologic factors is usually required to achieve adequate disease control in HS,” she said.
Dr. Hsiao acknowledged certain limitations of the study, including its single-center design and small sample size. “A confounding variable is that some patients were on other medications in addition to spironolactone, which may have influenced treatment outcomes,” she noted. “Larger prospective studies are needed to identify optimal dosing for spironolactone therapy in HS as well as predictors of treatment response.”
Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, who was asked to comment on the study, said that with only one FDA-approved systemic medication for the management of HS (adalimumab), “we off-label bandits must be creative to curtail the incredibly painful impact this chronic, destructive inflammatory disease can have on our patients.”
“The evidence supporting our approaches, whether it be antibiotics, immunomodulators, or in this case, antihormonal therapies, is limited, so more data is always welcome,” said Dr. Friedman, who was not involved with the study. “One very interesting point raised by the authors, one I share with my trainees frequently from my own experience, is that regardless of menstrual cycle abnormalities, spironolactone can be impactful. This is important to remember, in that overt signs of hormonal influences is not a requisite for the use or effectiveness of antihormonal therapy.”
Dr. Hsiao disclosed that she is a member of board of directors for the Hidradenitis Suppurativa Foundation. She has also served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Novartis, UCB, as a speaker for AbbVie, and as an investigator for Amgen, Boehringer Ingelheim, and Incyte. Dr. Friedman reported having no relevant financial disclosures.
AT CALDERM 2023
Preparing for the viral trifecta: RSV, influenza, and COVID-19
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
New armamentaria available to fight an old disease.
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.