User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Multivitamins and dementia: Untangling the COSMOS study web
I have written before about the COSMOS study and its finding that multivitamins (and chocolate) did not improve brain or cardiovascular health. So I was surprised to read that a “new” study found that vitamins can forestall dementia and age-related cognitive decline.
Upon closer look, the new data are neither new nor convincing, at least to me.
Chocolate and multivitamins for CVD and cancer prevention
The large randomized COSMOS trial was supposed to be the definitive study on chocolate that would establish its heart-health benefits without a doubt. Or, rather, the benefits of a cocoa bean extract in pill form given to healthy, older volunteers. The COSMOS study was negative. Chocolate, or the cocoa bean extract they used, did not reduce cardiovascular events.
And yet for all the prepublication importance attached to COSMOS, it is scarcely mentioned. Had it been positive, rest assured that Mars, the candy bar company that cofunded the research, and other interested parties would have been shouting it from the rooftops. As it is, they’re already spinning it.
Which brings us to the multivitamin component. COSMOS actually had a 2 × 2 design. In other words, there were four groups in this study: chocolate plus multivitamin, chocolate plus placebo, placebo plus multivitamin, and placebo plus placebo. This type of study design allows you to study two different interventions simultaneously, provided that they are independent and do not interact with each other. In addition to the primary cardiovascular endpoint, they also studied a cancer endpoint.
The multivitamin supplement didn’t reduce cardiovascular events either. Nor did it affect cancer outcomes. The main COSMOS study was negative and reinforced what countless other studies have proven: Taking a daily multivitamin does not reduce your risk of having a heart attack or developing cancer.
But wait, there’s more: COSMOS-Mind
But no researcher worth his salt studies just one or two endpoints in a study. The participants also underwent neurologic and memory testing. These results were reported separately in the COSMOS-Mind study.
COSMOS-Mind is often described as a separate (or “new”) study. In reality, it included the same participants from the original COSMOS trial and measured yet another primary outcome of cognitive performance on a series of tests administered by telephone. Although there is nothing inherently wrong with studying multiple outcomes in your patient population (after all, that salami isn’t going to slice itself), they cannot all be primary outcomes. Some, by necessity, must be secondary hypothesis–generating outcomes. If you test enough endpoints, multiple hypothesis testing dictates that eventually you will get a positive result simply by chance.
There was a time when the neurocognitive outcomes of COSMOS would have been reported in the same paper as the cardiovascular outcomes, but that time seems to have passed us by. Researchers live or die by the number of their publications, and there is an inherent advantage to squeezing as many publications as possible from the same dataset. Though, to be fair, the journal would probably have asked them to split up the paper as well.
In brief, the cocoa extract again fell short in COSMOS-Mind, but the multivitamin arm did better on the composite cognitive outcome. It was a fairly small difference – a 0.07-point improvement on the z-score at the 3-year mark (the z-score is the mean divided by the standard deviation). Much was also made of the fact that the improvement seemed to vary by prior history of cardiovascular disease (CVD). Those with a history of CVD had a 0.11-point improvement, whereas those without had a 0.06-point improvement. The authors couldn’t offer a definitive explanation for these findings. Any argument that multivitamins improve cardiovascular health and therefore prevent vascular dementia has to contend with the fact that the main COSMOS study didn’t show a cardiovascular benefit for vitamins. Speculation that you are treating nutritional deficiencies is exactly that: speculation.
A more salient question is: What does a 0.07-point improvement on the z-score mean clinically? This study didn’t assess whether a multivitamin supplement prevented dementia or allowed people to live independently for longer. In fairness, that would have been exceptionally difficult to do and would have required a much longer study.
Their one attempt to quantify the cognitive benefit clinically was a calculation about normal age-related decline. Test scores were 0.045 points lower for every 1-year increase in age among participants (their mean age was 73 years). So the authors contend that a 0.07-point increase, or the 0.083-point increase that they found at year 3, corresponds to 1.8 years of age-related decline forestalled. Whether this is an appropriate assumption, I leave for the reader to decide.
COSMOS-Web and replication
The results of COSMOS-Mind were seemingly bolstered by the recent publication of COSMOS-Web. Although I’ve seen this study described as having replicated the results of COSMOS-Mind, that description is a bit misleading. This was yet another ancillary COSMOS study; more than half of the 2,262 participants in COSMOS-Mind were also included in COSMOS-Web. Replicating results in the same people isn’t true replication.
The main difference between COSMOS-Mind and COSMOS-Web is that the former used a telephone interview to administer the cognitive tests and the latter used the Internet. They also had different endpoints, with COSMOS-Web looking at immediate recall rather than a global test composite.
COSMOS-Web was a positive study in that patients getting the multivitamin supplement did better on the test for immediate memory recall (remembering a list of 20 words), though they didn’t improve on tests of memory retention, executive function, or novel object recognition (basically a test where subjects have to identify matching geometric patterns and then recall them later). They were able to remember an additional 0.71 word on average, compared with 0.44 word in the placebo group. (For the record, it found no benefit for the cocoa extract).
Everybody does better on memory tests the second time around because practice makes perfect, hence the improvement in the placebo group. This benefit at 1 year did not survive to the end of follow-up at 3 years, in contrast to COSMOS-Mind, where the benefit was not apparent at 1 year and seen only at year 3. A history of cardiovascular disease didn’t seem to affect the results in COSMOS-Web as it did in COSMOS-Mind. As far as replications go, COSMOS-Web has some very non-negligible differences, compared with COSMOS-Mind. This incongruity, especially given the overlap in the patient populations is hard to reconcile. If COSMOS-Web was supposed to assuage any doubts that persisted after COSMOS-Mind, it hasn’t for me.
One of these studies is not like the others
Finally, although the COSMOS trial and all its ancillary study analyses suggest a neurocognitive benefit to multivitamin supplementation, it’s not the first study to test the matter. The Age-Related Eye Disease Study looked at vitamin C, vitamin E, beta-carotene, zinc, and copper. There was no benefit on any of the six cognitive tests administered to patients. The Women’s Health Study, the Women’s Antioxidant Cardiovascular Study and PREADViSE have all failed to show any benefit to the various vitamins and minerals they studied. A meta-analysis of 11 trials found no benefit to B vitamins in slowing cognitive aging.
The claim that COSMOS is the “first” study to test the hypothesis hinges on some careful wordplay. Prior studies tested specific vitamins, not a multivitamin. In the discussion of the paper, these other studies are critiqued for being short term. But the Physicians’ Health Study II did in fact study a multivitamin and assessed cognitive performance on average 2.5 years after randomization. It found no benefit. The authors of COSMOS-Web critiqued the 2.5-year wait to perform cognitive testing, saying it would have missed any short-term benefits. Although, given that they simultaneously praised their 3 years of follow-up, the criticism is hard to fully accept or even understand.
Whether follow-up is short or long, uses individual vitamins or a multivitamin, the results excluding COSMOS are uniformly negative.
Do enough tests in the same population, and something will rise above the noise just by chance. When you get a positive result in your research, it’s always exciting. But when a slew of studies that came before you are negative, you aren’t groundbreaking. You’re an outlier.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
I have written before about the COSMOS study and its finding that multivitamins (and chocolate) did not improve brain or cardiovascular health. So I was surprised to read that a “new” study found that vitamins can forestall dementia and age-related cognitive decline.
Upon closer look, the new data are neither new nor convincing, at least to me.
Chocolate and multivitamins for CVD and cancer prevention
The large randomized COSMOS trial was supposed to be the definitive study on chocolate that would establish its heart-health benefits without a doubt. Or, rather, the benefits of a cocoa bean extract in pill form given to healthy, older volunteers. The COSMOS study was negative. Chocolate, or the cocoa bean extract they used, did not reduce cardiovascular events.
And yet for all the prepublication importance attached to COSMOS, it is scarcely mentioned. Had it been positive, rest assured that Mars, the candy bar company that cofunded the research, and other interested parties would have been shouting it from the rooftops. As it is, they’re already spinning it.
Which brings us to the multivitamin component. COSMOS actually had a 2 × 2 design. In other words, there were four groups in this study: chocolate plus multivitamin, chocolate plus placebo, placebo plus multivitamin, and placebo plus placebo. This type of study design allows you to study two different interventions simultaneously, provided that they are independent and do not interact with each other. In addition to the primary cardiovascular endpoint, they also studied a cancer endpoint.
The multivitamin supplement didn’t reduce cardiovascular events either. Nor did it affect cancer outcomes. The main COSMOS study was negative and reinforced what countless other studies have proven: Taking a daily multivitamin does not reduce your risk of having a heart attack or developing cancer.
But wait, there’s more: COSMOS-Mind
But no researcher worth his salt studies just one or two endpoints in a study. The participants also underwent neurologic and memory testing. These results were reported separately in the COSMOS-Mind study.
COSMOS-Mind is often described as a separate (or “new”) study. In reality, it included the same participants from the original COSMOS trial and measured yet another primary outcome of cognitive performance on a series of tests administered by telephone. Although there is nothing inherently wrong with studying multiple outcomes in your patient population (after all, that salami isn’t going to slice itself), they cannot all be primary outcomes. Some, by necessity, must be secondary hypothesis–generating outcomes. If you test enough endpoints, multiple hypothesis testing dictates that eventually you will get a positive result simply by chance.
There was a time when the neurocognitive outcomes of COSMOS would have been reported in the same paper as the cardiovascular outcomes, but that time seems to have passed us by. Researchers live or die by the number of their publications, and there is an inherent advantage to squeezing as many publications as possible from the same dataset. Though, to be fair, the journal would probably have asked them to split up the paper as well.
In brief, the cocoa extract again fell short in COSMOS-Mind, but the multivitamin arm did better on the composite cognitive outcome. It was a fairly small difference – a 0.07-point improvement on the z-score at the 3-year mark (the z-score is the mean divided by the standard deviation). Much was also made of the fact that the improvement seemed to vary by prior history of cardiovascular disease (CVD). Those with a history of CVD had a 0.11-point improvement, whereas those without had a 0.06-point improvement. The authors couldn’t offer a definitive explanation for these findings. Any argument that multivitamins improve cardiovascular health and therefore prevent vascular dementia has to contend with the fact that the main COSMOS study didn’t show a cardiovascular benefit for vitamins. Speculation that you are treating nutritional deficiencies is exactly that: speculation.
A more salient question is: What does a 0.07-point improvement on the z-score mean clinically? This study didn’t assess whether a multivitamin supplement prevented dementia or allowed people to live independently for longer. In fairness, that would have been exceptionally difficult to do and would have required a much longer study.
Their one attempt to quantify the cognitive benefit clinically was a calculation about normal age-related decline. Test scores were 0.045 points lower for every 1-year increase in age among participants (their mean age was 73 years). So the authors contend that a 0.07-point increase, or the 0.083-point increase that they found at year 3, corresponds to 1.8 years of age-related decline forestalled. Whether this is an appropriate assumption, I leave for the reader to decide.
COSMOS-Web and replication
The results of COSMOS-Mind were seemingly bolstered by the recent publication of COSMOS-Web. Although I’ve seen this study described as having replicated the results of COSMOS-Mind, that description is a bit misleading. This was yet another ancillary COSMOS study; more than half of the 2,262 participants in COSMOS-Mind were also included in COSMOS-Web. Replicating results in the same people isn’t true replication.
The main difference between COSMOS-Mind and COSMOS-Web is that the former used a telephone interview to administer the cognitive tests and the latter used the Internet. They also had different endpoints, with COSMOS-Web looking at immediate recall rather than a global test composite.
COSMOS-Web was a positive study in that patients getting the multivitamin supplement did better on the test for immediate memory recall (remembering a list of 20 words), though they didn’t improve on tests of memory retention, executive function, or novel object recognition (basically a test where subjects have to identify matching geometric patterns and then recall them later). They were able to remember an additional 0.71 word on average, compared with 0.44 word in the placebo group. (For the record, it found no benefit for the cocoa extract).
Everybody does better on memory tests the second time around because practice makes perfect, hence the improvement in the placebo group. This benefit at 1 year did not survive to the end of follow-up at 3 years, in contrast to COSMOS-Mind, where the benefit was not apparent at 1 year and seen only at year 3. A history of cardiovascular disease didn’t seem to affect the results in COSMOS-Web as it did in COSMOS-Mind. As far as replications go, COSMOS-Web has some very non-negligible differences, compared with COSMOS-Mind. This incongruity, especially given the overlap in the patient populations is hard to reconcile. If COSMOS-Web was supposed to assuage any doubts that persisted after COSMOS-Mind, it hasn’t for me.
One of these studies is not like the others
Finally, although the COSMOS trial and all its ancillary study analyses suggest a neurocognitive benefit to multivitamin supplementation, it’s not the first study to test the matter. The Age-Related Eye Disease Study looked at vitamin C, vitamin E, beta-carotene, zinc, and copper. There was no benefit on any of the six cognitive tests administered to patients. The Women’s Health Study, the Women’s Antioxidant Cardiovascular Study and PREADViSE have all failed to show any benefit to the various vitamins and minerals they studied. A meta-analysis of 11 trials found no benefit to B vitamins in slowing cognitive aging.
The claim that COSMOS is the “first” study to test the hypothesis hinges on some careful wordplay. Prior studies tested specific vitamins, not a multivitamin. In the discussion of the paper, these other studies are critiqued for being short term. But the Physicians’ Health Study II did in fact study a multivitamin and assessed cognitive performance on average 2.5 years after randomization. It found no benefit. The authors of COSMOS-Web critiqued the 2.5-year wait to perform cognitive testing, saying it would have missed any short-term benefits. Although, given that they simultaneously praised their 3 years of follow-up, the criticism is hard to fully accept or even understand.
Whether follow-up is short or long, uses individual vitamins or a multivitamin, the results excluding COSMOS are uniformly negative.
Do enough tests in the same population, and something will rise above the noise just by chance. When you get a positive result in your research, it’s always exciting. But when a slew of studies that came before you are negative, you aren’t groundbreaking. You’re an outlier.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
I have written before about the COSMOS study and its finding that multivitamins (and chocolate) did not improve brain or cardiovascular health. So I was surprised to read that a “new” study found that vitamins can forestall dementia and age-related cognitive decline.
Upon closer look, the new data are neither new nor convincing, at least to me.
Chocolate and multivitamins for CVD and cancer prevention
The large randomized COSMOS trial was supposed to be the definitive study on chocolate that would establish its heart-health benefits without a doubt. Or, rather, the benefits of a cocoa bean extract in pill form given to healthy, older volunteers. The COSMOS study was negative. Chocolate, or the cocoa bean extract they used, did not reduce cardiovascular events.
And yet for all the prepublication importance attached to COSMOS, it is scarcely mentioned. Had it been positive, rest assured that Mars, the candy bar company that cofunded the research, and other interested parties would have been shouting it from the rooftops. As it is, they’re already spinning it.
Which brings us to the multivitamin component. COSMOS actually had a 2 × 2 design. In other words, there were four groups in this study: chocolate plus multivitamin, chocolate plus placebo, placebo plus multivitamin, and placebo plus placebo. This type of study design allows you to study two different interventions simultaneously, provided that they are independent and do not interact with each other. In addition to the primary cardiovascular endpoint, they also studied a cancer endpoint.
The multivitamin supplement didn’t reduce cardiovascular events either. Nor did it affect cancer outcomes. The main COSMOS study was negative and reinforced what countless other studies have proven: Taking a daily multivitamin does not reduce your risk of having a heart attack or developing cancer.
But wait, there’s more: COSMOS-Mind
But no researcher worth his salt studies just one or two endpoints in a study. The participants also underwent neurologic and memory testing. These results were reported separately in the COSMOS-Mind study.
COSMOS-Mind is often described as a separate (or “new”) study. In reality, it included the same participants from the original COSMOS trial and measured yet another primary outcome of cognitive performance on a series of tests administered by telephone. Although there is nothing inherently wrong with studying multiple outcomes in your patient population (after all, that salami isn’t going to slice itself), they cannot all be primary outcomes. Some, by necessity, must be secondary hypothesis–generating outcomes. If you test enough endpoints, multiple hypothesis testing dictates that eventually you will get a positive result simply by chance.
There was a time when the neurocognitive outcomes of COSMOS would have been reported in the same paper as the cardiovascular outcomes, but that time seems to have passed us by. Researchers live or die by the number of their publications, and there is an inherent advantage to squeezing as many publications as possible from the same dataset. Though, to be fair, the journal would probably have asked them to split up the paper as well.
In brief, the cocoa extract again fell short in COSMOS-Mind, but the multivitamin arm did better on the composite cognitive outcome. It was a fairly small difference – a 0.07-point improvement on the z-score at the 3-year mark (the z-score is the mean divided by the standard deviation). Much was also made of the fact that the improvement seemed to vary by prior history of cardiovascular disease (CVD). Those with a history of CVD had a 0.11-point improvement, whereas those without had a 0.06-point improvement. The authors couldn’t offer a definitive explanation for these findings. Any argument that multivitamins improve cardiovascular health and therefore prevent vascular dementia has to contend with the fact that the main COSMOS study didn’t show a cardiovascular benefit for vitamins. Speculation that you are treating nutritional deficiencies is exactly that: speculation.
A more salient question is: What does a 0.07-point improvement on the z-score mean clinically? This study didn’t assess whether a multivitamin supplement prevented dementia or allowed people to live independently for longer. In fairness, that would have been exceptionally difficult to do and would have required a much longer study.
Their one attempt to quantify the cognitive benefit clinically was a calculation about normal age-related decline. Test scores were 0.045 points lower for every 1-year increase in age among participants (their mean age was 73 years). So the authors contend that a 0.07-point increase, or the 0.083-point increase that they found at year 3, corresponds to 1.8 years of age-related decline forestalled. Whether this is an appropriate assumption, I leave for the reader to decide.
COSMOS-Web and replication
The results of COSMOS-Mind were seemingly bolstered by the recent publication of COSMOS-Web. Although I’ve seen this study described as having replicated the results of COSMOS-Mind, that description is a bit misleading. This was yet another ancillary COSMOS study; more than half of the 2,262 participants in COSMOS-Mind were also included in COSMOS-Web. Replicating results in the same people isn’t true replication.
The main difference between COSMOS-Mind and COSMOS-Web is that the former used a telephone interview to administer the cognitive tests and the latter used the Internet. They also had different endpoints, with COSMOS-Web looking at immediate recall rather than a global test composite.
COSMOS-Web was a positive study in that patients getting the multivitamin supplement did better on the test for immediate memory recall (remembering a list of 20 words), though they didn’t improve on tests of memory retention, executive function, or novel object recognition (basically a test where subjects have to identify matching geometric patterns and then recall them later). They were able to remember an additional 0.71 word on average, compared with 0.44 word in the placebo group. (For the record, it found no benefit for the cocoa extract).
Everybody does better on memory tests the second time around because practice makes perfect, hence the improvement in the placebo group. This benefit at 1 year did not survive to the end of follow-up at 3 years, in contrast to COSMOS-Mind, where the benefit was not apparent at 1 year and seen only at year 3. A history of cardiovascular disease didn’t seem to affect the results in COSMOS-Web as it did in COSMOS-Mind. As far as replications go, COSMOS-Web has some very non-negligible differences, compared with COSMOS-Mind. This incongruity, especially given the overlap in the patient populations is hard to reconcile. If COSMOS-Web was supposed to assuage any doubts that persisted after COSMOS-Mind, it hasn’t for me.
One of these studies is not like the others
Finally, although the COSMOS trial and all its ancillary study analyses suggest a neurocognitive benefit to multivitamin supplementation, it’s not the first study to test the matter. The Age-Related Eye Disease Study looked at vitamin C, vitamin E, beta-carotene, zinc, and copper. There was no benefit on any of the six cognitive tests administered to patients. The Women’s Health Study, the Women’s Antioxidant Cardiovascular Study and PREADViSE have all failed to show any benefit to the various vitamins and minerals they studied. A meta-analysis of 11 trials found no benefit to B vitamins in slowing cognitive aging.
The claim that COSMOS is the “first” study to test the hypothesis hinges on some careful wordplay. Prior studies tested specific vitamins, not a multivitamin. In the discussion of the paper, these other studies are critiqued for being short term. But the Physicians’ Health Study II did in fact study a multivitamin and assessed cognitive performance on average 2.5 years after randomization. It found no benefit. The authors of COSMOS-Web critiqued the 2.5-year wait to perform cognitive testing, saying it would have missed any short-term benefits. Although, given that they simultaneously praised their 3 years of follow-up, the criticism is hard to fully accept or even understand.
Whether follow-up is short or long, uses individual vitamins or a multivitamin, the results excluding COSMOS are uniformly negative.
Do enough tests in the same population, and something will rise above the noise just by chance. When you get a positive result in your research, it’s always exciting. But when a slew of studies that came before you are negative, you aren’t groundbreaking. You’re an outlier.
Dr. Labos is a cardiologist at Hôpital Notre-Dame, Montreal. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
AHA updates CPR guidelines on cardiac arrest after poisoning
The update reflects treatment advances and new knowledge, including the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) for patients whose condition is refractory to poison antidotes and other therapies.
The new guidelines are designed primarily for North American health care professionals who treat adults and children who are critically ill because of poisoning, including intentional and unintentional drug overdose, chemical exposure, and drug-drug interactions, the authors note.
Published online in Circulation, the update was endorsed by the American Academy of Pediatrics.
‘Nearly miraculous’
“It’s been 13 years since the poisoning treatment guidelines had a comprehensive update,” lead author Eric J. Lavonas, MD, professor of emergency medicine at Denver Health and the Rocky Mountain Poison and Drug Center, Colo., told this news organization. “In that time, we’ve learned a lot about how to best use antidotes and other treatments to save the most critically poisoned patients.”
Highlighting a few key points from the update, he said, “For those rare situations when antidotes aren’t enough, the new guidelines include the use of heart-lung machines (VA-ECMO) for patients with beta-blocker, calcium channel blocker, or sodium channel blocker poisoning causing cardiogenic shock.”
Furthermore, he said, “High-dose insulin treatment for patients with beta-blocker and calcium channel blocker poisoning [also recommended in the update] has really become mainstream. The doses are up to 10 times higher than the amount used to treat diabetic emergencies.
“Some excellent science has shown that giving IV lipid emulsion can save the life of someone with an accidental overdose of local anesthetic medications, particularly bupivacaine,” he added. “The result is sometimes nearly miraculous.
“But when this treatment is extended to poisoning from other medications, it often doesn’t work as well, and in some situations may make things worse,” he said. “The issue may be that giving lipids increases absorption of drug from the stomach and intestines, which can be dangerous when the patient took an overdose of pills.”
Low level of evidence
The guidelines were compiled by the Critical Poisoning Writing Group, which includes experts from emergency medicine, pediatrics, medical toxicology, pharmacology, critical care, emergency medical services, education, research, and nursing. Group members were appointed by the AHA Emergency Cardiovascular Care Science Subcommittee and were approved by the AHA Manuscript Oversight Committee.
First and foremost, the group recommends timely consultation with a medical toxicologist, a clinical toxicologist, or a regional poison center to facilitate rapid, effective therapy, because treatment of cardiac arrest and toxicity from poisoning often requires treatments that most clinicians don’t use frequently.
Other key points include the following:
- Naloxone administration may reverse respiratory arrest due to opioid overdose, preventing progression to cardiac arrest.
- Give high-dose insulin therapy early in the treatment of patients with beta-blocker and calcium channel blocker poisoning, Dr. Lavonas noted.
- Standard advanced life support plus sodium bicarbonate is appropriate for life-threatening dysrhythmias caused by cocaine or other sodium channel blockers.
- If cyanide poisoning is suspected, clinicians should not wait for confirmatory testing; treatment should begin immediately with hydroxocobalamin (preferred) or sodium nitrite plus sodium thiosulfate.
- Digoxin-specific immune antibody fragments can reverse life-threatening dysrhythmias from digoxin poisoning.
- Use of 20% intravenous lipid emulsion can be efficacious in the resuscitation of life-threatening local anesthetic toxicity, especially from bupivacaine, Dr. Lavonas indicated.
- Sedation is recommended for patients with severe agitation from sympathomimetic poisoning to manage hyperthermia and acidosis, prevent rhabdomyolysis and injury, and allow evaluation for other life-threatening conditions.
- Although flumazenil reverses central nervous system and respiratory depression from benzodiazepine poisoning, risks and contraindications, provided in the guidelines, limit its use.
- VA-ECMO can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatments.
“Unfortunately, despite improvements in the design and funding support for resuscitation research, the overall certainty of the evidence base for resuscitation science and management of critical poisoning is low,” the group acknowledges.
Of the 73 guideline recommendations, only 2 are supported by level A evidence; 3 are supported by level B-randomized evidence, 12 by level B-nonrandomized evidence, and the rest by level C evidence.
“Accordingly, the strength of recommendations is weaker than optimal,” they write. “Clinical trials in resuscitation and the management of critical poisoning are sorely needed.”
‘Don’t go it alone!’
“Most critical poisonings are pretty uncommon, and each patient is different,” Dr. Lavonas said. “Even in the emergency department or ICU, most physicians will treat a patient who is critically ill with any given poison less than once a year. The antidotes and medication doses needed to effectively treat these patients are often very different than everyday medical practice.
“Don’t try to go it alone!” he urges. “Poisoning cases are complex, and the treatments work best when they are implemented quickly and assertively. A toxicologist can help sort through complex situations and get effective treatment started without delay.”
Every certified poison center has a medical toxicologist or clinical toxicologist on call 24/7 to give advice to physicians and hospitals about patients who are critically ill after being poisoned, he added. “Everyone in the U.S. has access to a poison center by calling one number: 1-800-222-1222.”
Dr. Lavonas has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The update reflects treatment advances and new knowledge, including the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) for patients whose condition is refractory to poison antidotes and other therapies.
The new guidelines are designed primarily for North American health care professionals who treat adults and children who are critically ill because of poisoning, including intentional and unintentional drug overdose, chemical exposure, and drug-drug interactions, the authors note.
Published online in Circulation, the update was endorsed by the American Academy of Pediatrics.
‘Nearly miraculous’
“It’s been 13 years since the poisoning treatment guidelines had a comprehensive update,” lead author Eric J. Lavonas, MD, professor of emergency medicine at Denver Health and the Rocky Mountain Poison and Drug Center, Colo., told this news organization. “In that time, we’ve learned a lot about how to best use antidotes and other treatments to save the most critically poisoned patients.”
Highlighting a few key points from the update, he said, “For those rare situations when antidotes aren’t enough, the new guidelines include the use of heart-lung machines (VA-ECMO) for patients with beta-blocker, calcium channel blocker, or sodium channel blocker poisoning causing cardiogenic shock.”
Furthermore, he said, “High-dose insulin treatment for patients with beta-blocker and calcium channel blocker poisoning [also recommended in the update] has really become mainstream. The doses are up to 10 times higher than the amount used to treat diabetic emergencies.
“Some excellent science has shown that giving IV lipid emulsion can save the life of someone with an accidental overdose of local anesthetic medications, particularly bupivacaine,” he added. “The result is sometimes nearly miraculous.
“But when this treatment is extended to poisoning from other medications, it often doesn’t work as well, and in some situations may make things worse,” he said. “The issue may be that giving lipids increases absorption of drug from the stomach and intestines, which can be dangerous when the patient took an overdose of pills.”
Low level of evidence
The guidelines were compiled by the Critical Poisoning Writing Group, which includes experts from emergency medicine, pediatrics, medical toxicology, pharmacology, critical care, emergency medical services, education, research, and nursing. Group members were appointed by the AHA Emergency Cardiovascular Care Science Subcommittee and were approved by the AHA Manuscript Oversight Committee.
First and foremost, the group recommends timely consultation with a medical toxicologist, a clinical toxicologist, or a regional poison center to facilitate rapid, effective therapy, because treatment of cardiac arrest and toxicity from poisoning often requires treatments that most clinicians don’t use frequently.
Other key points include the following:
- Naloxone administration may reverse respiratory arrest due to opioid overdose, preventing progression to cardiac arrest.
- Give high-dose insulin therapy early in the treatment of patients with beta-blocker and calcium channel blocker poisoning, Dr. Lavonas noted.
- Standard advanced life support plus sodium bicarbonate is appropriate for life-threatening dysrhythmias caused by cocaine or other sodium channel blockers.
- If cyanide poisoning is suspected, clinicians should not wait for confirmatory testing; treatment should begin immediately with hydroxocobalamin (preferred) or sodium nitrite plus sodium thiosulfate.
- Digoxin-specific immune antibody fragments can reverse life-threatening dysrhythmias from digoxin poisoning.
- Use of 20% intravenous lipid emulsion can be efficacious in the resuscitation of life-threatening local anesthetic toxicity, especially from bupivacaine, Dr. Lavonas indicated.
- Sedation is recommended for patients with severe agitation from sympathomimetic poisoning to manage hyperthermia and acidosis, prevent rhabdomyolysis and injury, and allow evaluation for other life-threatening conditions.
- Although flumazenil reverses central nervous system and respiratory depression from benzodiazepine poisoning, risks and contraindications, provided in the guidelines, limit its use.
- VA-ECMO can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatments.
“Unfortunately, despite improvements in the design and funding support for resuscitation research, the overall certainty of the evidence base for resuscitation science and management of critical poisoning is low,” the group acknowledges.
Of the 73 guideline recommendations, only 2 are supported by level A evidence; 3 are supported by level B-randomized evidence, 12 by level B-nonrandomized evidence, and the rest by level C evidence.
“Accordingly, the strength of recommendations is weaker than optimal,” they write. “Clinical trials in resuscitation and the management of critical poisoning are sorely needed.”
‘Don’t go it alone!’
“Most critical poisonings are pretty uncommon, and each patient is different,” Dr. Lavonas said. “Even in the emergency department or ICU, most physicians will treat a patient who is critically ill with any given poison less than once a year. The antidotes and medication doses needed to effectively treat these patients are often very different than everyday medical practice.
“Don’t try to go it alone!” he urges. “Poisoning cases are complex, and the treatments work best when they are implemented quickly and assertively. A toxicologist can help sort through complex situations and get effective treatment started without delay.”
Every certified poison center has a medical toxicologist or clinical toxicologist on call 24/7 to give advice to physicians and hospitals about patients who are critically ill after being poisoned, he added. “Everyone in the U.S. has access to a poison center by calling one number: 1-800-222-1222.”
Dr. Lavonas has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
The update reflects treatment advances and new knowledge, including the use of venoarterial extracorporeal membrane oxygenation (VA-ECMO) for patients whose condition is refractory to poison antidotes and other therapies.
The new guidelines are designed primarily for North American health care professionals who treat adults and children who are critically ill because of poisoning, including intentional and unintentional drug overdose, chemical exposure, and drug-drug interactions, the authors note.
Published online in Circulation, the update was endorsed by the American Academy of Pediatrics.
‘Nearly miraculous’
“It’s been 13 years since the poisoning treatment guidelines had a comprehensive update,” lead author Eric J. Lavonas, MD, professor of emergency medicine at Denver Health and the Rocky Mountain Poison and Drug Center, Colo., told this news organization. “In that time, we’ve learned a lot about how to best use antidotes and other treatments to save the most critically poisoned patients.”
Highlighting a few key points from the update, he said, “For those rare situations when antidotes aren’t enough, the new guidelines include the use of heart-lung machines (VA-ECMO) for patients with beta-blocker, calcium channel blocker, or sodium channel blocker poisoning causing cardiogenic shock.”
Furthermore, he said, “High-dose insulin treatment for patients with beta-blocker and calcium channel blocker poisoning [also recommended in the update] has really become mainstream. The doses are up to 10 times higher than the amount used to treat diabetic emergencies.
“Some excellent science has shown that giving IV lipid emulsion can save the life of someone with an accidental overdose of local anesthetic medications, particularly bupivacaine,” he added. “The result is sometimes nearly miraculous.
“But when this treatment is extended to poisoning from other medications, it often doesn’t work as well, and in some situations may make things worse,” he said. “The issue may be that giving lipids increases absorption of drug from the stomach and intestines, which can be dangerous when the patient took an overdose of pills.”
Low level of evidence
The guidelines were compiled by the Critical Poisoning Writing Group, which includes experts from emergency medicine, pediatrics, medical toxicology, pharmacology, critical care, emergency medical services, education, research, and nursing. Group members were appointed by the AHA Emergency Cardiovascular Care Science Subcommittee and were approved by the AHA Manuscript Oversight Committee.
First and foremost, the group recommends timely consultation with a medical toxicologist, a clinical toxicologist, or a regional poison center to facilitate rapid, effective therapy, because treatment of cardiac arrest and toxicity from poisoning often requires treatments that most clinicians don’t use frequently.
Other key points include the following:
- Naloxone administration may reverse respiratory arrest due to opioid overdose, preventing progression to cardiac arrest.
- Give high-dose insulin therapy early in the treatment of patients with beta-blocker and calcium channel blocker poisoning, Dr. Lavonas noted.
- Standard advanced life support plus sodium bicarbonate is appropriate for life-threatening dysrhythmias caused by cocaine or other sodium channel blockers.
- If cyanide poisoning is suspected, clinicians should not wait for confirmatory testing; treatment should begin immediately with hydroxocobalamin (preferred) or sodium nitrite plus sodium thiosulfate.
- Digoxin-specific immune antibody fragments can reverse life-threatening dysrhythmias from digoxin poisoning.
- Use of 20% intravenous lipid emulsion can be efficacious in the resuscitation of life-threatening local anesthetic toxicity, especially from bupivacaine, Dr. Lavonas indicated.
- Sedation is recommended for patients with severe agitation from sympathomimetic poisoning to manage hyperthermia and acidosis, prevent rhabdomyolysis and injury, and allow evaluation for other life-threatening conditions.
- Although flumazenil reverses central nervous system and respiratory depression from benzodiazepine poisoning, risks and contraindications, provided in the guidelines, limit its use.
- VA-ECMO can be lifesaving for patients with cardiogenic shock or dysrhythmias that are refractory to other treatments.
“Unfortunately, despite improvements in the design and funding support for resuscitation research, the overall certainty of the evidence base for resuscitation science and management of critical poisoning is low,” the group acknowledges.
Of the 73 guideline recommendations, only 2 are supported by level A evidence; 3 are supported by level B-randomized evidence, 12 by level B-nonrandomized evidence, and the rest by level C evidence.
“Accordingly, the strength of recommendations is weaker than optimal,” they write. “Clinical trials in resuscitation and the management of critical poisoning are sorely needed.”
‘Don’t go it alone!’
“Most critical poisonings are pretty uncommon, and each patient is different,” Dr. Lavonas said. “Even in the emergency department or ICU, most physicians will treat a patient who is critically ill with any given poison less than once a year. The antidotes and medication doses needed to effectively treat these patients are often very different than everyday medical practice.
“Don’t try to go it alone!” he urges. “Poisoning cases are complex, and the treatments work best when they are implemented quickly and assertively. A toxicologist can help sort through complex situations and get effective treatment started without delay.”
Every certified poison center has a medical toxicologist or clinical toxicologist on call 24/7 to give advice to physicians and hospitals about patients who are critically ill after being poisoned, he added. “Everyone in the U.S. has access to a poison center by calling one number: 1-800-222-1222.”
Dr. Lavonas has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
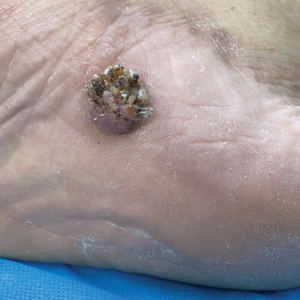
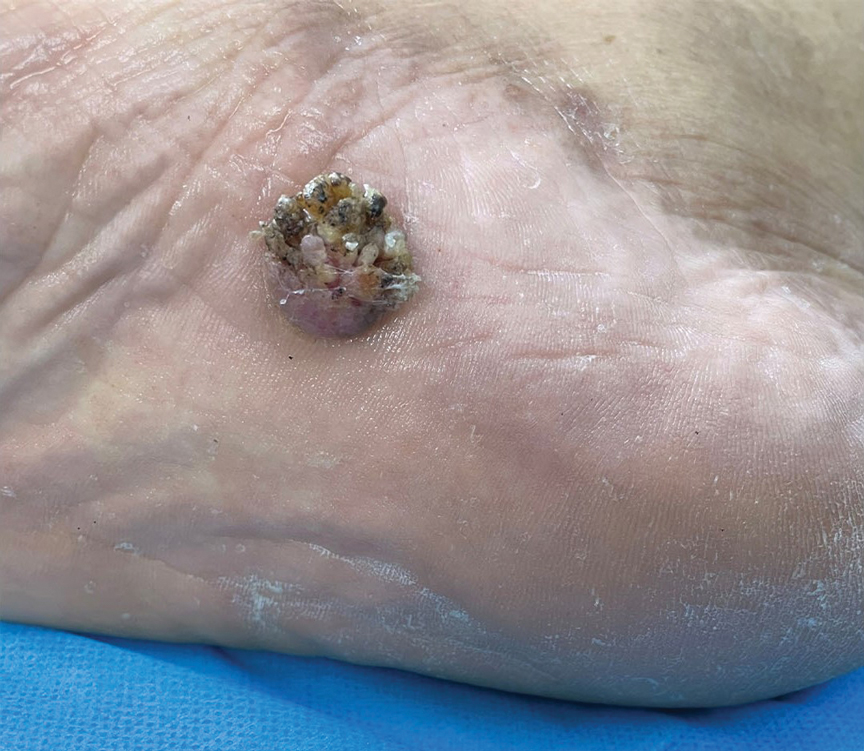
Verrucous Plaque on the Foot
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.
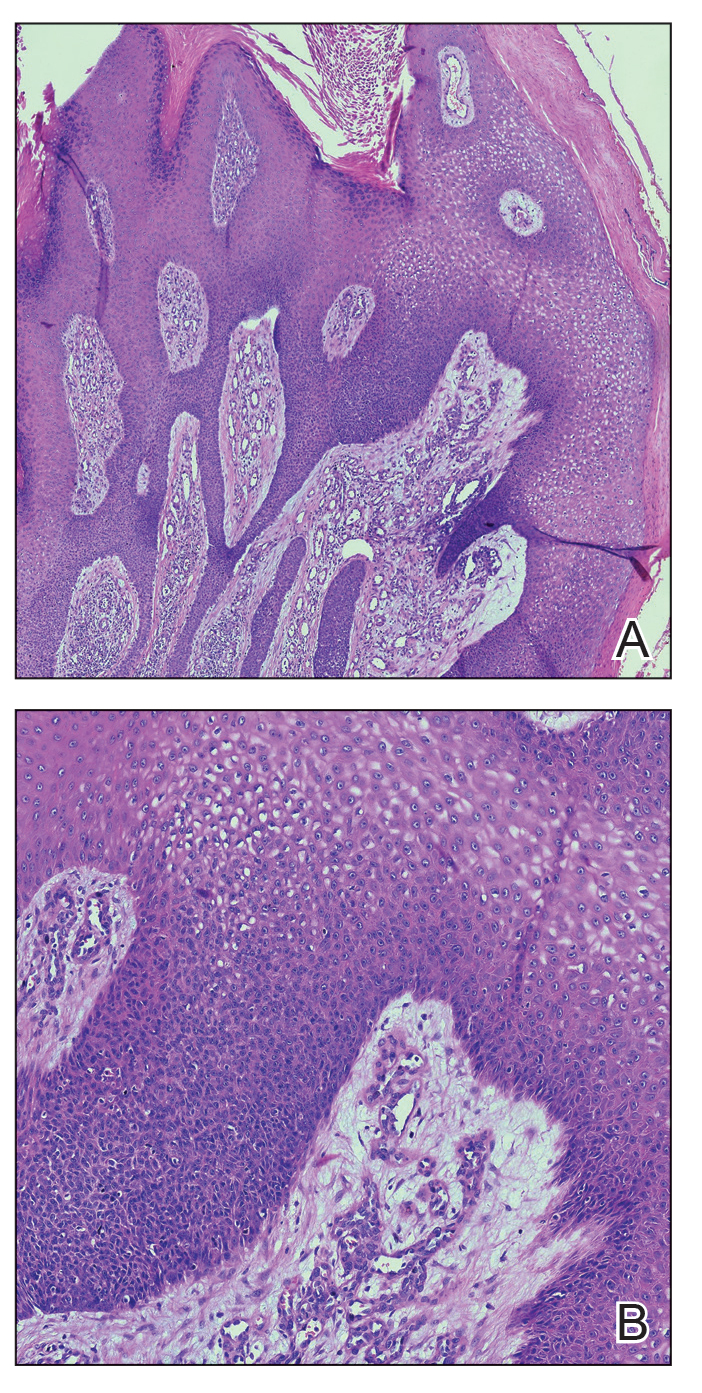
Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.
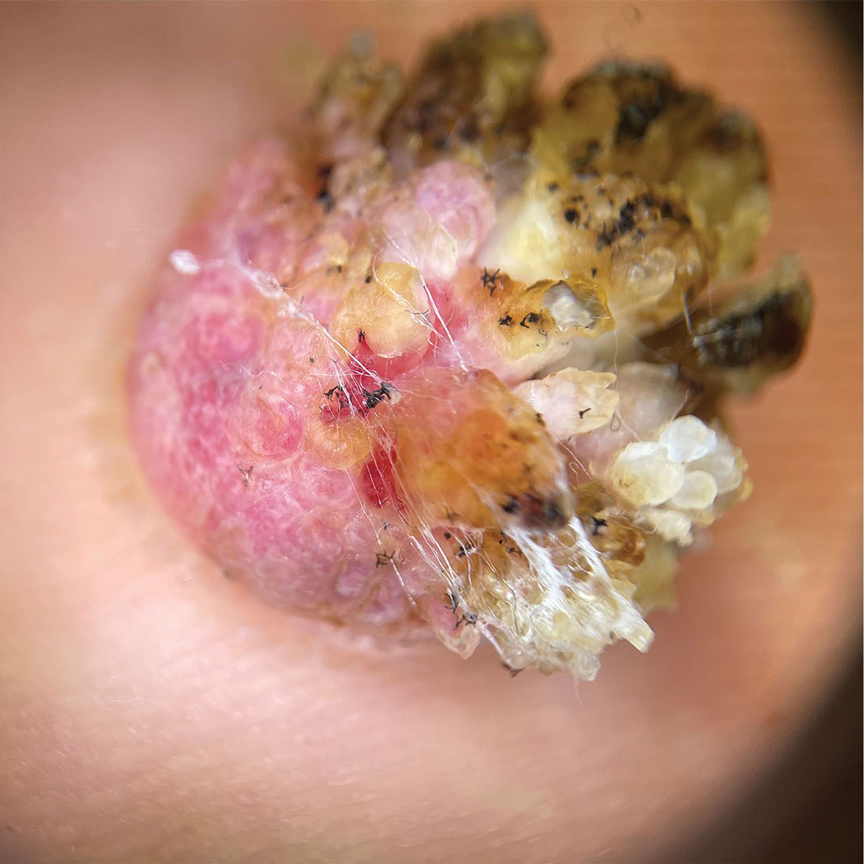
Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9
There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
A 62-year-old man presented with an enlarging plaque on the foot of 3 years’ duration. He experienced minor pain while walking but reported no other symptoms. His family history was negative for similar anomalies, and his medical history was negative for the presence of malignant tumors. Physical examination revealed a 2-mm erythematous plaque on the plantar aspect of the right foot with prominent overlying verrucous changes and no ulceration or regional lymphadenopathy. Dermoscopy and reflectance confocal microscopy of the lesion were performed along with a histopathologic examination after complete surgical excision.
Menstruation linked to underdiagnosis of type 2 diabetes?
The analysis estimates that an additional 17% of undiagnosed women younger than 50 years could be reclassified as having T2D, and that women under 50 had an A1c distribution that was markedly lower than that of men under 50, by a mean of 1.6 mmol/mol.
In a study that will be presented at this year’s annual meeting of the European Association for the Study of Diabetes (EASD), the researchers wanted to investigate whether a contributing factor to late diagnosis of T2D in women under 50 may be the difference in A1c levels due to hemoglobin replacement linked to menstrual blood loss.
The study was published online in Diabetes Therapy. “If the threshold for diagnosis of diabetes ... was lowered by 2 mmol/mol in women under the age of 50, an additional 17% of these women (approximately equivalent to 35,000 women in England and Wales) would be diagnosed with diabetes ... which may contribute to up to 64% of the difference in mortality rates between men/women with diabetes mellitus aged 16-50 years,” the researchers noted.
They added that A1c levels in women under 50 years were found to be consistently lower than those in men, and with A1c levels in women reaching the equivalent of those in men up to 10 years later, this “may result in delayed diagnosis of diabetes mellitus in premenopausal women.”
Noting that the study was observational, senior author Adrian Heald, MD, consultant endocrinologist, Salford (England) Royal NHS Foundation Trust, said that it “may be the case that prediabetes and type 2 diabetes in women are not being spotted because the set point needs to be slightly lower, but a systematic study sampling from the population of at-risk individuals is needed further to our findings.
“We also need to refer back to use of the glucose tolerance test, because A1c has been used for the past 15 years but it is not the gold standard,” added Dr. Heald. “Clinicians have often wondered if patients might be missed with A1c measurement, or even overdiagnosed.”
Lucy Chambers, PhD, from Diabetes UK, acknowledged that the research was valuable but added: “More research on sex differences in thresholds for a type 2 diagnosis is needed to inform any changes to clinical practice. In the meantime, we encourage clinicians to follow the current guidance of not ruling out type 2 diabetes based on a one-off A1c below the diagnostic threshold.”
But in support of greater understanding around the sex differences in A1c diagnostic thresholds, Dr. Chambers added: “Receiving an accurate and timely diagnosis ensures that women get the treatment and support needed to manage their type 2 diabetes and avoid long-term complications, including heart disease, where sex-based inequalities in care already contribute to poorer outcomes for women.”
Effect of A1c reference range on T2D diagnosis and associated CVD
Compared with men, women with T2D have poorer glycemic control; a higher risk for cardiovascular (CV) complications; reduced life expectancy (5.3 years shorter vs. 4.5 years shorter); and a higher risk factor burden, such as obesity and hypertension at diagnosis.
In addition, T2D is a stronger risk factor for CV disease (CVD) in women than in men, and those aged 35-59 years who receive a diagnosis have the highest relative CV death risk across all age and sex groups.
The researchers pointed out that previous studies have observed differences in A1c relative to menopause, and they too found that “A1c levels rose after the age of 50 in women.”
However, they noted that the implication of differing A1c reference ranges on delayed diabetes diagnosis with worsening CV risk profile had not been previously recognized and that their study “[h]ighlights for the first time that, while 1.6 mmol/mol may appear only a small difference in terms of laboratory measurement, at population level this has implications for significant number of premenopausal women.”
The researchers initially observed the trend in local data in Salford, in the northwest of England. “These ... data highlighted that women seemed to be diagnosed with type 2 diabetes at an older age, so we wanted to examine what the source of that might be,” study author Mike Stedman, BSc, director, Res Consortium, Andover, England, said in an interview.
Dr. Stedman and his colleagues assessed the sex and age differences of A1c in individuals who had not been diagnosed with diabetes (A1c ≤ 48 mmol/mol [≤ 6.5%]). “We looked at data from other labs [in addition to those in Salford, totaling 938,678 people] to see if this was a local phenomenon. They could only provide more recent data, but these also showed a similar pattern,” he added.
Finally, Dr. Stedman, Dr. Heald, and their colleagues estimated the possible national impact by extrapolating findings based on population data from the UK Office of National Statistics and on National Diabetes Audit data for type 2 diabetes prevalence and related excess mortality. This brought them to the conclusion that T2D would be diagnosed in an additional 17% of women if the threshold were lowered by 2 mmol/mol, to 46 mmol/mol, in women under 50 years.
Lower A1c in women under 50 may delay T2D diagnosis by up to 10 years
The analysis found that the median A1c increased with age, with values in women younger than 50 years consistently being 1 mmol/mol lower than values in men. In contrast, A1c values in women over 50 years were equivalent to those in men.
However, at age 50 years, compared with men, A1c in women was found to lag by approximately 5 years. Women under 50 had an A1c distribution that was lower than that of men by an average of 1.6 mmol/mol (4.7% of mean; P < .0001), whereas this difference in individuals aged 50 years or older was less pronounced (P < .0001).
The authors wrote that “an undermeasurement of approximately 1.6 mmol/mol A1c in women may delay their diabetes ... diagnosis by up to 10 years.”
Further analysis showed that, at an A1c of 48 mmol/mol, 50% fewer women than men under the age of 50 could be diagnosed with T2D, whereas only 20% fewer women than men aged 50 years or older could be diagnosed with T2D.
Lowering the A1c threshold for diagnosis of T2D from 48 mmol/mol to 46 mmol/mol in women under 50 led to an estimate that an additional 35,345 undiagnosed women in England could be reclassified as having a T2D diagnosis.
The authors pointed out that “gender difference in adverse cardiovascular risk factors are known to be present prior to the development of [type 2] diabetes” and that “once diagnosed, atherosclerotic CVD prevalence is twice as high in patients with diabetes ... compared to those without a diagnosis.”
Dr. Heald added that there is always the possibility that other factors might be at play and that the work posed questions rather than presented answers.
Taking a pragmatic view, the researchers suggested that “one alternative approach may be to offer further assessment using fasting plasma glucose or oral glucose tolerance testing in those with A1c values of 46 or 47 mmol/mol.”
“In anyone with an early diagnosis of type 2 diabetes, in addition to dietary modification and especially if there is cardiovascular risk, then one might start them on metformin due to the cardiovascular benefits as well as the sugar-lowering effects,” said Dr. Heald, adding that “we certainly don’t want women missing out on metformin that could have huge benefits in the longer term.”
Dr. Stedman and Dr. Heald declared no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Chambers has declared no conflicts.
A version of this article appeared on Medscape.com.
The analysis estimates that an additional 17% of undiagnosed women younger than 50 years could be reclassified as having T2D, and that women under 50 had an A1c distribution that was markedly lower than that of men under 50, by a mean of 1.6 mmol/mol.
In a study that will be presented at this year’s annual meeting of the European Association for the Study of Diabetes (EASD), the researchers wanted to investigate whether a contributing factor to late diagnosis of T2D in women under 50 may be the difference in A1c levels due to hemoglobin replacement linked to menstrual blood loss.
The study was published online in Diabetes Therapy. “If the threshold for diagnosis of diabetes ... was lowered by 2 mmol/mol in women under the age of 50, an additional 17% of these women (approximately equivalent to 35,000 women in England and Wales) would be diagnosed with diabetes ... which may contribute to up to 64% of the difference in mortality rates between men/women with diabetes mellitus aged 16-50 years,” the researchers noted.
They added that A1c levels in women under 50 years were found to be consistently lower than those in men, and with A1c levels in women reaching the equivalent of those in men up to 10 years later, this “may result in delayed diagnosis of diabetes mellitus in premenopausal women.”
Noting that the study was observational, senior author Adrian Heald, MD, consultant endocrinologist, Salford (England) Royal NHS Foundation Trust, said that it “may be the case that prediabetes and type 2 diabetes in women are not being spotted because the set point needs to be slightly lower, but a systematic study sampling from the population of at-risk individuals is needed further to our findings.
“We also need to refer back to use of the glucose tolerance test, because A1c has been used for the past 15 years but it is not the gold standard,” added Dr. Heald. “Clinicians have often wondered if patients might be missed with A1c measurement, or even overdiagnosed.”
Lucy Chambers, PhD, from Diabetes UK, acknowledged that the research was valuable but added: “More research on sex differences in thresholds for a type 2 diagnosis is needed to inform any changes to clinical practice. In the meantime, we encourage clinicians to follow the current guidance of not ruling out type 2 diabetes based on a one-off A1c below the diagnostic threshold.”
But in support of greater understanding around the sex differences in A1c diagnostic thresholds, Dr. Chambers added: “Receiving an accurate and timely diagnosis ensures that women get the treatment and support needed to manage their type 2 diabetes and avoid long-term complications, including heart disease, where sex-based inequalities in care already contribute to poorer outcomes for women.”
Effect of A1c reference range on T2D diagnosis and associated CVD
Compared with men, women with T2D have poorer glycemic control; a higher risk for cardiovascular (CV) complications; reduced life expectancy (5.3 years shorter vs. 4.5 years shorter); and a higher risk factor burden, such as obesity and hypertension at diagnosis.
In addition, T2D is a stronger risk factor for CV disease (CVD) in women than in men, and those aged 35-59 years who receive a diagnosis have the highest relative CV death risk across all age and sex groups.
The researchers pointed out that previous studies have observed differences in A1c relative to menopause, and they too found that “A1c levels rose after the age of 50 in women.”
However, they noted that the implication of differing A1c reference ranges on delayed diabetes diagnosis with worsening CV risk profile had not been previously recognized and that their study “[h]ighlights for the first time that, while 1.6 mmol/mol may appear only a small difference in terms of laboratory measurement, at population level this has implications for significant number of premenopausal women.”
The researchers initially observed the trend in local data in Salford, in the northwest of England. “These ... data highlighted that women seemed to be diagnosed with type 2 diabetes at an older age, so we wanted to examine what the source of that might be,” study author Mike Stedman, BSc, director, Res Consortium, Andover, England, said in an interview.
Dr. Stedman and his colleagues assessed the sex and age differences of A1c in individuals who had not been diagnosed with diabetes (A1c ≤ 48 mmol/mol [≤ 6.5%]). “We looked at data from other labs [in addition to those in Salford, totaling 938,678 people] to see if this was a local phenomenon. They could only provide more recent data, but these also showed a similar pattern,” he added.
Finally, Dr. Stedman, Dr. Heald, and their colleagues estimated the possible national impact by extrapolating findings based on population data from the UK Office of National Statistics and on National Diabetes Audit data for type 2 diabetes prevalence and related excess mortality. This brought them to the conclusion that T2D would be diagnosed in an additional 17% of women if the threshold were lowered by 2 mmol/mol, to 46 mmol/mol, in women under 50 years.
Lower A1c in women under 50 may delay T2D diagnosis by up to 10 years
The analysis found that the median A1c increased with age, with values in women younger than 50 years consistently being 1 mmol/mol lower than values in men. In contrast, A1c values in women over 50 years were equivalent to those in men.
However, at age 50 years, compared with men, A1c in women was found to lag by approximately 5 years. Women under 50 had an A1c distribution that was lower than that of men by an average of 1.6 mmol/mol (4.7% of mean; P < .0001), whereas this difference in individuals aged 50 years or older was less pronounced (P < .0001).
The authors wrote that “an undermeasurement of approximately 1.6 mmol/mol A1c in women may delay their diabetes ... diagnosis by up to 10 years.”
Further analysis showed that, at an A1c of 48 mmol/mol, 50% fewer women than men under the age of 50 could be diagnosed with T2D, whereas only 20% fewer women than men aged 50 years or older could be diagnosed with T2D.
Lowering the A1c threshold for diagnosis of T2D from 48 mmol/mol to 46 mmol/mol in women under 50 led to an estimate that an additional 35,345 undiagnosed women in England could be reclassified as having a T2D diagnosis.
The authors pointed out that “gender difference in adverse cardiovascular risk factors are known to be present prior to the development of [type 2] diabetes” and that “once diagnosed, atherosclerotic CVD prevalence is twice as high in patients with diabetes ... compared to those without a diagnosis.”
Dr. Heald added that there is always the possibility that other factors might be at play and that the work posed questions rather than presented answers.
Taking a pragmatic view, the researchers suggested that “one alternative approach may be to offer further assessment using fasting plasma glucose or oral glucose tolerance testing in those with A1c values of 46 or 47 mmol/mol.”
“In anyone with an early diagnosis of type 2 diabetes, in addition to dietary modification and especially if there is cardiovascular risk, then one might start them on metformin due to the cardiovascular benefits as well as the sugar-lowering effects,” said Dr. Heald, adding that “we certainly don’t want women missing out on metformin that could have huge benefits in the longer term.”
Dr. Stedman and Dr. Heald declared no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Chambers has declared no conflicts.
A version of this article appeared on Medscape.com.
The analysis estimates that an additional 17% of undiagnosed women younger than 50 years could be reclassified as having T2D, and that women under 50 had an A1c distribution that was markedly lower than that of men under 50, by a mean of 1.6 mmol/mol.
In a study that will be presented at this year’s annual meeting of the European Association for the Study of Diabetes (EASD), the researchers wanted to investigate whether a contributing factor to late diagnosis of T2D in women under 50 may be the difference in A1c levels due to hemoglobin replacement linked to menstrual blood loss.
The study was published online in Diabetes Therapy. “If the threshold for diagnosis of diabetes ... was lowered by 2 mmol/mol in women under the age of 50, an additional 17% of these women (approximately equivalent to 35,000 women in England and Wales) would be diagnosed with diabetes ... which may contribute to up to 64% of the difference in mortality rates between men/women with diabetes mellitus aged 16-50 years,” the researchers noted.
They added that A1c levels in women under 50 years were found to be consistently lower than those in men, and with A1c levels in women reaching the equivalent of those in men up to 10 years later, this “may result in delayed diagnosis of diabetes mellitus in premenopausal women.”
Noting that the study was observational, senior author Adrian Heald, MD, consultant endocrinologist, Salford (England) Royal NHS Foundation Trust, said that it “may be the case that prediabetes and type 2 diabetes in women are not being spotted because the set point needs to be slightly lower, but a systematic study sampling from the population of at-risk individuals is needed further to our findings.
“We also need to refer back to use of the glucose tolerance test, because A1c has been used for the past 15 years but it is not the gold standard,” added Dr. Heald. “Clinicians have often wondered if patients might be missed with A1c measurement, or even overdiagnosed.”
Lucy Chambers, PhD, from Diabetes UK, acknowledged that the research was valuable but added: “More research on sex differences in thresholds for a type 2 diagnosis is needed to inform any changes to clinical practice. In the meantime, we encourage clinicians to follow the current guidance of not ruling out type 2 diabetes based on a one-off A1c below the diagnostic threshold.”
But in support of greater understanding around the sex differences in A1c diagnostic thresholds, Dr. Chambers added: “Receiving an accurate and timely diagnosis ensures that women get the treatment and support needed to manage their type 2 diabetes and avoid long-term complications, including heart disease, where sex-based inequalities in care already contribute to poorer outcomes for women.”
Effect of A1c reference range on T2D diagnosis and associated CVD
Compared with men, women with T2D have poorer glycemic control; a higher risk for cardiovascular (CV) complications; reduced life expectancy (5.3 years shorter vs. 4.5 years shorter); and a higher risk factor burden, such as obesity and hypertension at diagnosis.
In addition, T2D is a stronger risk factor for CV disease (CVD) in women than in men, and those aged 35-59 years who receive a diagnosis have the highest relative CV death risk across all age and sex groups.
The researchers pointed out that previous studies have observed differences in A1c relative to menopause, and they too found that “A1c levels rose after the age of 50 in women.”
However, they noted that the implication of differing A1c reference ranges on delayed diabetes diagnosis with worsening CV risk profile had not been previously recognized and that their study “[h]ighlights for the first time that, while 1.6 mmol/mol may appear only a small difference in terms of laboratory measurement, at population level this has implications for significant number of premenopausal women.”
The researchers initially observed the trend in local data in Salford, in the northwest of England. “These ... data highlighted that women seemed to be diagnosed with type 2 diabetes at an older age, so we wanted to examine what the source of that might be,” study author Mike Stedman, BSc, director, Res Consortium, Andover, England, said in an interview.
Dr. Stedman and his colleagues assessed the sex and age differences of A1c in individuals who had not been diagnosed with diabetes (A1c ≤ 48 mmol/mol [≤ 6.5%]). “We looked at data from other labs [in addition to those in Salford, totaling 938,678 people] to see if this was a local phenomenon. They could only provide more recent data, but these also showed a similar pattern,” he added.
Finally, Dr. Stedman, Dr. Heald, and their colleagues estimated the possible national impact by extrapolating findings based on population data from the UK Office of National Statistics and on National Diabetes Audit data for type 2 diabetes prevalence and related excess mortality. This brought them to the conclusion that T2D would be diagnosed in an additional 17% of women if the threshold were lowered by 2 mmol/mol, to 46 mmol/mol, in women under 50 years.
Lower A1c in women under 50 may delay T2D diagnosis by up to 10 years
The analysis found that the median A1c increased with age, with values in women younger than 50 years consistently being 1 mmol/mol lower than values in men. In contrast, A1c values in women over 50 years were equivalent to those in men.
However, at age 50 years, compared with men, A1c in women was found to lag by approximately 5 years. Women under 50 had an A1c distribution that was lower than that of men by an average of 1.6 mmol/mol (4.7% of mean; P < .0001), whereas this difference in individuals aged 50 years or older was less pronounced (P < .0001).
The authors wrote that “an undermeasurement of approximately 1.6 mmol/mol A1c in women may delay their diabetes ... diagnosis by up to 10 years.”
Further analysis showed that, at an A1c of 48 mmol/mol, 50% fewer women than men under the age of 50 could be diagnosed with T2D, whereas only 20% fewer women than men aged 50 years or older could be diagnosed with T2D.
Lowering the A1c threshold for diagnosis of T2D from 48 mmol/mol to 46 mmol/mol in women under 50 led to an estimate that an additional 35,345 undiagnosed women in England could be reclassified as having a T2D diagnosis.
The authors pointed out that “gender difference in adverse cardiovascular risk factors are known to be present prior to the development of [type 2] diabetes” and that “once diagnosed, atherosclerotic CVD prevalence is twice as high in patients with diabetes ... compared to those without a diagnosis.”
Dr. Heald added that there is always the possibility that other factors might be at play and that the work posed questions rather than presented answers.
Taking a pragmatic view, the researchers suggested that “one alternative approach may be to offer further assessment using fasting plasma glucose or oral glucose tolerance testing in those with A1c values of 46 or 47 mmol/mol.”
“In anyone with an early diagnosis of type 2 diabetes, in addition to dietary modification and especially if there is cardiovascular risk, then one might start them on metformin due to the cardiovascular benefits as well as the sugar-lowering effects,” said Dr. Heald, adding that “we certainly don’t want women missing out on metformin that could have huge benefits in the longer term.”
Dr. Stedman and Dr. Heald declared no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; and no other relationships or activities that could appear to have influenced the submitted work. Dr. Chambers has declared no conflicts.
A version of this article appeared on Medscape.com.
FROM EASD 2023
Can zoo poo help manage diabetic foot ulcers?
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
In a striking convergence of veterinary biology and medical science, researchers from the University of Sheffield (England) have unveiled findings that could potentially advance the treatment of diabetic foot ulcers, a condition affecting an estimated 18.6 million people worldwide. The unexpected ingredient in this potentially transformative therapy? Feces from endangered species, sourced from Yorkshire Wildlife Park, Doncaster, England.
The scourge of antibiotic resistance
Diabetic foot ulcers are a significant challenge in health care, not only because of their prevalence but also because of the alarming rise of antibiotic-resistant bacterial infections. Current antibiotic treatments frequently fail, leading to life-altering consequences like amputations and significant health care costs – estimated at one-third of the total direct costs of diabetes care. The critical need for alternative therapies has propelled scientists into a pressing search for novel antimicrobial agents.
A pioneering approach: zoo poo as bioactive goldmine
Led by Professor Graham Stafford, chair of molecular microbiology at the University of Sheffield, the research team began to explore a rather unorthodox resource: the fecal matter of endangered animals like Guinea baboons, lemurs, and Visayan pigs. While such a source might seem surprising at first glance, the rationale becomes clear when considering the nature of bacteriophages.
What are bacteriophages?
Bacteriophages, commonly known as phages, are viruses that selectively target and kill bacteria. Despite being the most prevalent biological entities on Earth, their therapeutic potential has remained largely untapped. What makes bacteriophages particularly interesting is their ability to kill antibiotic-resistant bacteria – a feature making them prime candidates for treating otherwise unmanageable diabetic foot ulcers. (Armstrong DG, et al; Fish R, et al).
Findings and future directions
Professor Stafford and his team discovered that the feces of several endangered animals harbored bacteriophages capable of killing bacterial strains resistant to antibiotics. The findings not only hold promise for a groundbreaking treatment but also provide another compelling reason to conserve endangered species: Their inherent biodiversity might contain cures for a range of infectious diseases.
While research is ongoing and clinical trials have not yet begun, the preliminary results are overwhelmingly promising.
We often look to complex technologies and synthetic materials for medical science breakthroughs, yet sometimes the most innovative solutions can be found in the most overlooked places. In this case, the feces of endangered species could turn out to be a vital asset in battling antibiotic resistance, thus affecting diabetic foot care in ways we never imagined possible.
The research conducted at the University of Sheffield also serves as a powerful argument for a One Health approach – a multidisciplinary field focusing on the interconnectedness of human, animal, and environmental health.
This intriguing work reaffirms the need for an interdisciplinary approach in tackling the world’s pressing health care challenges. The collaborative efforts between the University of Sheffield and Yorkshire Wildlife Park exemplify how academic research and conservation can come together to yield solutions for some of the most devastating and costly health conditions, while also underscoring the invaluable role that biodiversity plays in our collective well-being. Here’s to teaming up to act against amputation worldwide.
Dr. Armstrong is professor of surgery and director of limb preservation at University of Southern California, Los Angeles. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
FDA issues letter regarding lebrikizumab review for atopic dermatitis
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
The Food and Drug Administration has issued a complete response letter regarding lebrikizumab, an investigational biologic for the treatment of adult and adolescent patients with moderate to severe atopic dermatitis, describing concerns about findings made during an inspection of a third-party contract manufacturer that included the “monoclonal antibody drug substance” for lebrikizumab, Eli Lilly announced in an Oct. 2 press release.
Lebrikizumab is under FDA review for treating atopic dermatitis; a complete response letter indicates that the review has been completed, and highlights issues that need to be addressed before a final decision on approval is made.
The press release noted that the agency did not raise any concerns about the clinical data package, safety, or label for lebrikizumab, an investigational, monoclonal antibody that binds to the cytokine interleukin (IL)-13, and is designed to be administered once per month.
In the press release, the company said it would work with the third-party manufacturer and the FDA to address the feedback “in order to make lebrikizumab available to patients.”
Hyaluronic acid suppository improves menopause symptoms
TOPLINE:
Among women with genitourinary syndrome of menopause, 12 weeks of treatment with vaginal suppositories containing hyaluronic acid (HLA) reduces vulvovaginal symptoms, according to trial results presented at the annual Menopause Meeting. HLA may be a promising nonhormonal therapy for this condition, the researchers said.
METHODOLOGY:
- Investigators randomly assigned 49 women to receive treatment with a vaginal suppository containing 5 mg of HLA or standard-of-care treatment with vaginal estrogen cream (0.01%).
- The trial was conducted between September 2021 and August 2022.
TAKEAWAY:
- Patients in both treatment arms experienced improvements on the Vulvovaginal Symptom Questionnaire (VSQ), the study’s primary outcome.
- The VSQ assesses vulvovaginal symptoms associated with menopause such as itching, burning, and dryness, as well as the emotional toll of symptoms and their effect on sexual activity.
- Change in VSQ score did not significantly differ between the treatment groups. The measure improved from 5.2 to 1.7 in the group that received estrogen, and from 5.8 to 2.5 in those who received HLA (P = .81).
- No treatment-related severe adverse events were reported.
IN PRACTICE:
“Women often need to decide between different therapies for genitourinary syndrome of menopause,” study author Benjamin Brucker, MD, of New York University said in an interview. “Now we can help counsel them about this formulation of HLA.”
SOURCE:
Poster P-1 was presented at the 2023 meeting of the Menopause Society, held Sept. 27-30 in Philadelphia.
DISCLOSURES:
The study was funded by Bonafide Health, a company that sells supplements to treat menopause symptoms, including vaginal suppositories containing HLA.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with genitourinary syndrome of menopause, 12 weeks of treatment with vaginal suppositories containing hyaluronic acid (HLA) reduces vulvovaginal symptoms, according to trial results presented at the annual Menopause Meeting. HLA may be a promising nonhormonal therapy for this condition, the researchers said.
METHODOLOGY:
- Investigators randomly assigned 49 women to receive treatment with a vaginal suppository containing 5 mg of HLA or standard-of-care treatment with vaginal estrogen cream (0.01%).
- The trial was conducted between September 2021 and August 2022.
TAKEAWAY:
- Patients in both treatment arms experienced improvements on the Vulvovaginal Symptom Questionnaire (VSQ), the study’s primary outcome.
- The VSQ assesses vulvovaginal symptoms associated with menopause such as itching, burning, and dryness, as well as the emotional toll of symptoms and their effect on sexual activity.
- Change in VSQ score did not significantly differ between the treatment groups. The measure improved from 5.2 to 1.7 in the group that received estrogen, and from 5.8 to 2.5 in those who received HLA (P = .81).
- No treatment-related severe adverse events were reported.
IN PRACTICE:
“Women often need to decide between different therapies for genitourinary syndrome of menopause,” study author Benjamin Brucker, MD, of New York University said in an interview. “Now we can help counsel them about this formulation of HLA.”
SOURCE:
Poster P-1 was presented at the 2023 meeting of the Menopause Society, held Sept. 27-30 in Philadelphia.
DISCLOSURES:
The study was funded by Bonafide Health, a company that sells supplements to treat menopause symptoms, including vaginal suppositories containing HLA.
A version of this article appeared on Medscape.com.
TOPLINE:
Among women with genitourinary syndrome of menopause, 12 weeks of treatment with vaginal suppositories containing hyaluronic acid (HLA) reduces vulvovaginal symptoms, according to trial results presented at the annual Menopause Meeting. HLA may be a promising nonhormonal therapy for this condition, the researchers said.
METHODOLOGY:
- Investigators randomly assigned 49 women to receive treatment with a vaginal suppository containing 5 mg of HLA or standard-of-care treatment with vaginal estrogen cream (0.01%).
- The trial was conducted between September 2021 and August 2022.
TAKEAWAY:
- Patients in both treatment arms experienced improvements on the Vulvovaginal Symptom Questionnaire (VSQ), the study’s primary outcome.
- The VSQ assesses vulvovaginal symptoms associated with menopause such as itching, burning, and dryness, as well as the emotional toll of symptoms and their effect on sexual activity.
- Change in VSQ score did not significantly differ between the treatment groups. The measure improved from 5.2 to 1.7 in the group that received estrogen, and from 5.8 to 2.5 in those who received HLA (P = .81).
- No treatment-related severe adverse events were reported.
IN PRACTICE:
“Women often need to decide between different therapies for genitourinary syndrome of menopause,” study author Benjamin Brucker, MD, of New York University said in an interview. “Now we can help counsel them about this formulation of HLA.”
SOURCE:
Poster P-1 was presented at the 2023 meeting of the Menopause Society, held Sept. 27-30 in Philadelphia.
DISCLOSURES:
The study was funded by Bonafide Health, a company that sells supplements to treat menopause symptoms, including vaginal suppositories containing HLA.
A version of this article appeared on Medscape.com.
Jury out on how tea drinking influences colorectal cancer risk
TOPLINE:
A meta-analysis finds that tea drinking may reduce the risk for colorectal cancer (CRC) by 24%, but the estimate is “uncertain,” and the actual effect on CRC risk can range from a reduction of 51% to an increase of 18%, researchers say.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis of 15 studies (11 cohort, three case-control, and one randomized controlled trial) with nearly 2.7 million participants.
- The studies were conducted in Asia, North America, Europe, and Oceania between 1986 and 2015 and included black and green tea.
- Tea consumption was dichotomized as < 1 cup vs. ≥ 1 cups daily. A random effects model was used for data analysis.
TAKEAWAY:
- No statistically significant association was found between tea consumption and CRC risk (relative risk, 0.76).
- By geographic region, results of an American subgroup analysis suggested tea drinking might be protective against CRC (RR, 0.33), while data from the United Kingdom (RR, 1.45) and Italian (RR, 1.15) subgroups had opposite results.
- In subgroups by tea type, green tea was associated with a lower CRC risk (RR, 0.05).
- Sensitivity analysis revealed that the effect on CRC risk can range from a reduction of 51% (RR, 0.49) to an increase of 18% (RR, 1.18).
IN PRACTICE:
“Taken together, this meta-analysis suggests that tea consumption may not be linked to the development of CRC. These relationships still need to be confirmed by additional well-designed large prospective studies and randomized clinical trials,” the authors write.
SOURCE:
The study, with co–first authors Yu Huang and Qiang Chen, with the Third Hospital of Hebei Medical University, Shijiazhuang, China, was published online in BMC Gastroenterology.
LIMITATIONS:
There was a high level of heterogeneity in the original studies, as well as variations in the quantity and types of tea consumed and in the design and quality of the studies. Some studies did not account for potentially important variables, such as alcohol use and diet.
DISCLOSURES:
The study was supported by grants from the Hebei Provincial Natural Science Foundation and the Hebei Provincial Department of Science and Technology. The authors have disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A meta-analysis finds that tea drinking may reduce the risk for colorectal cancer (CRC) by 24%, but the estimate is “uncertain,” and the actual effect on CRC risk can range from a reduction of 51% to an increase of 18%, researchers say.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis of 15 studies (11 cohort, three case-control, and one randomized controlled trial) with nearly 2.7 million participants.
- The studies were conducted in Asia, North America, Europe, and Oceania between 1986 and 2015 and included black and green tea.
- Tea consumption was dichotomized as < 1 cup vs. ≥ 1 cups daily. A random effects model was used for data analysis.
TAKEAWAY:
- No statistically significant association was found between tea consumption and CRC risk (relative risk, 0.76).
- By geographic region, results of an American subgroup analysis suggested tea drinking might be protective against CRC (RR, 0.33), while data from the United Kingdom (RR, 1.45) and Italian (RR, 1.15) subgroups had opposite results.
- In subgroups by tea type, green tea was associated with a lower CRC risk (RR, 0.05).
- Sensitivity analysis revealed that the effect on CRC risk can range from a reduction of 51% (RR, 0.49) to an increase of 18% (RR, 1.18).
IN PRACTICE:
“Taken together, this meta-analysis suggests that tea consumption may not be linked to the development of CRC. These relationships still need to be confirmed by additional well-designed large prospective studies and randomized clinical trials,” the authors write.
SOURCE:
The study, with co–first authors Yu Huang and Qiang Chen, with the Third Hospital of Hebei Medical University, Shijiazhuang, China, was published online in BMC Gastroenterology.
LIMITATIONS:
There was a high level of heterogeneity in the original studies, as well as variations in the quantity and types of tea consumed and in the design and quality of the studies. Some studies did not account for potentially important variables, such as alcohol use and diet.
DISCLOSURES:
The study was supported by grants from the Hebei Provincial Natural Science Foundation and the Hebei Provincial Department of Science and Technology. The authors have disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
A meta-analysis finds that tea drinking may reduce the risk for colorectal cancer (CRC) by 24%, but the estimate is “uncertain,” and the actual effect on CRC risk can range from a reduction of 51% to an increase of 18%, researchers say.
METHODOLOGY:
- Researchers conducted a systematic review and meta-analysis of 15 studies (11 cohort, three case-control, and one randomized controlled trial) with nearly 2.7 million participants.
- The studies were conducted in Asia, North America, Europe, and Oceania between 1986 and 2015 and included black and green tea.
- Tea consumption was dichotomized as < 1 cup vs. ≥ 1 cups daily. A random effects model was used for data analysis.
TAKEAWAY:
- No statistically significant association was found between tea consumption and CRC risk (relative risk, 0.76).
- By geographic region, results of an American subgroup analysis suggested tea drinking might be protective against CRC (RR, 0.33), while data from the United Kingdom (RR, 1.45) and Italian (RR, 1.15) subgroups had opposite results.
- In subgroups by tea type, green tea was associated with a lower CRC risk (RR, 0.05).
- Sensitivity analysis revealed that the effect on CRC risk can range from a reduction of 51% (RR, 0.49) to an increase of 18% (RR, 1.18).
IN PRACTICE:
“Taken together, this meta-analysis suggests that tea consumption may not be linked to the development of CRC. These relationships still need to be confirmed by additional well-designed large prospective studies and randomized clinical trials,” the authors write.
SOURCE:
The study, with co–first authors Yu Huang and Qiang Chen, with the Third Hospital of Hebei Medical University, Shijiazhuang, China, was published online in BMC Gastroenterology.
LIMITATIONS:
There was a high level of heterogeneity in the original studies, as well as variations in the quantity and types of tea consumed and in the design and quality of the studies. Some studies did not account for potentially important variables, such as alcohol use and diet.
DISCLOSURES:
The study was supported by grants from the Hebei Provincial Natural Science Foundation and the Hebei Provincial Department of Science and Technology. The authors have disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
Vegetarian diets tied to lower risk for some GI cancers
TOPLINE:
METHODOLOGY:
- Researchers did a systematic review and meta-analysis of seven original studies (six cohorts and one case-control) involving 686,691 people.
- Pooled relative risk for gastric, colorectal, and upper gastrointestinal cancers were assessed with confidence intervals in multivariate analysis accounting for potential confounders.
TAKEAWAY:
- Compared with nonvegetarian diets, vegetarian diets were inversely associated with the risk for GI tumor development (relative risk, 0.77).
- In a subgroup analysis, vegetarian diets were negatively correlated with the risk for gastric cancer (RR, 0.41) and colorectal cancer (RR, 0.85) but not with upper GI cancer (excluding stomach; RR, 0.93).
- Vegetarian diets were negatively correlated with the risk for GI cancer in men (RR, 0.57) but not women (RR, 0.89).
- Vegetarian diets were negatively correlated with the risk for GI cancer in North American (RR, 0.76) and Asian populations (RR, 0.43) but not in European populations (RR, 0.83).
IN PRACTICE:
“The results of this systematic review indicate that adherence to vegetarian diets can reduce the risk of gastrointestinal cancers, compared with non-vegetarian diets. This study provides a reference for primary prevention strategies for gastrointestinal cancers,” the authors write.
SOURCE:
The study, with first author Tongtong Bai, of Nanjing University of Chinese Medicine, was published online on in the European Journal of Gastroenterology & Hepatology.
LIMITATIONS:
The effects of vegetarian diets on GI tumorigenesis may be influenced by gender and geographical region. The heterogeneity of effects of vegetarian diets on different GI cancers could be due to the small number of studies included and could represent chance variation. The results need to be confirmed by studies of populations in other regions. There was evidence of publication bias.
DISCLOSURES:
The study had no specific funding. The authors have disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers did a systematic review and meta-analysis of seven original studies (six cohorts and one case-control) involving 686,691 people.
- Pooled relative risk for gastric, colorectal, and upper gastrointestinal cancers were assessed with confidence intervals in multivariate analysis accounting for potential confounders.
TAKEAWAY:
- Compared with nonvegetarian diets, vegetarian diets were inversely associated with the risk for GI tumor development (relative risk, 0.77).
- In a subgroup analysis, vegetarian diets were negatively correlated with the risk for gastric cancer (RR, 0.41) and colorectal cancer (RR, 0.85) but not with upper GI cancer (excluding stomach; RR, 0.93).
- Vegetarian diets were negatively correlated with the risk for GI cancer in men (RR, 0.57) but not women (RR, 0.89).
- Vegetarian diets were negatively correlated with the risk for GI cancer in North American (RR, 0.76) and Asian populations (RR, 0.43) but not in European populations (RR, 0.83).
IN PRACTICE:
“The results of this systematic review indicate that adherence to vegetarian diets can reduce the risk of gastrointestinal cancers, compared with non-vegetarian diets. This study provides a reference for primary prevention strategies for gastrointestinal cancers,” the authors write.
SOURCE:
The study, with first author Tongtong Bai, of Nanjing University of Chinese Medicine, was published online on in the European Journal of Gastroenterology & Hepatology.
LIMITATIONS:
The effects of vegetarian diets on GI tumorigenesis may be influenced by gender and geographical region. The heterogeneity of effects of vegetarian diets on different GI cancers could be due to the small number of studies included and could represent chance variation. The results need to be confirmed by studies of populations in other regions. There was evidence of publication bias.
DISCLOSURES:
The study had no specific funding. The authors have disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers did a systematic review and meta-analysis of seven original studies (six cohorts and one case-control) involving 686,691 people.
- Pooled relative risk for gastric, colorectal, and upper gastrointestinal cancers were assessed with confidence intervals in multivariate analysis accounting for potential confounders.
TAKEAWAY:
- Compared with nonvegetarian diets, vegetarian diets were inversely associated with the risk for GI tumor development (relative risk, 0.77).
- In a subgroup analysis, vegetarian diets were negatively correlated with the risk for gastric cancer (RR, 0.41) and colorectal cancer (RR, 0.85) but not with upper GI cancer (excluding stomach; RR, 0.93).
- Vegetarian diets were negatively correlated with the risk for GI cancer in men (RR, 0.57) but not women (RR, 0.89).
- Vegetarian diets were negatively correlated with the risk for GI cancer in North American (RR, 0.76) and Asian populations (RR, 0.43) but not in European populations (RR, 0.83).
IN PRACTICE:
“The results of this systematic review indicate that adherence to vegetarian diets can reduce the risk of gastrointestinal cancers, compared with non-vegetarian diets. This study provides a reference for primary prevention strategies for gastrointestinal cancers,” the authors write.
SOURCE:
The study, with first author Tongtong Bai, of Nanjing University of Chinese Medicine, was published online on in the European Journal of Gastroenterology & Hepatology.
LIMITATIONS:
The effects of vegetarian diets on GI tumorigenesis may be influenced by gender and geographical region. The heterogeneity of effects of vegetarian diets on different GI cancers could be due to the small number of studies included and could represent chance variation. The results need to be confirmed by studies of populations in other regions. There was evidence of publication bias.
DISCLOSURES:
The study had no specific funding. The authors have disclosed no conflicts of interest.
A version of this article appeared on Medscape.com.
COVID-19 virus infects coronary vasculature
TOPLINE:
, which could help explain why people with COVID-19 have an increased risk for ischemic cardiovascular complications up to 1 year after infection.
METHODOLOGY:
- Researchers obtained 27 coronary autopsy specimens from eight patients who died from COVID-19, mean age 70 years and 75% male. All had coronary artery disease and most had cardiovascular risk factors such as hypertension, were overweight or obese, and had hyperlipidemia and type 2 diabetes.
- All but one patient, who was pronounced dead before hospital admission, were hospitalized for an average of 17.6 days.
- To identify SARS-CoV-2 viral RNA (vRNA) in the autoptic coronary vasculature, researchers performed RNA fluorescence in situ hybridization (RNA-FISH) analysis for the vRNA encoding the spike (S) protein; they also probed the antisense strand of the S gene (S antisense), which is only produced during viral replication.
TAKEAWAY:
- The study found evidence of SARS-CoV-2 replication in all analyzed human autopsy coronaries regardless of their pathological classification, although viral replication was highest in early-stage lesions that progress to more advanced atherosclerotic plaques.
- Findings indicated that more than 79% of macrophages (white blood cells that help remove lipids) and more than 90% of foam cells (lipid-laden macrophages that are a hallmark of atherosclerosis at all stages of the disease) are S+, and more than 40% of both cell types are S antisense+, indicating SARS-CoV-2 can infect macrophages at a high rate.
- SARS-CoV-2 induced a strong inflammatory response as evidenced by release of cytokines (including interleukin-1 beta and interluekin-6 that are linked to myocardial infarction) in both macrophages and foam cells, which may contribute to the ischemic cardiovascular complications in patients with COVID-19.
IN PRACTICE:
“Our data conclusively demonstrate that SARS-CoV-2 is capable of infecting and replicating in macrophages within the coronary vasculature of patients with COVID-19,” write the authors, adding that SARS-CoV-2 preferentially replicates in foam cells, compared with other macrophages, suggesting these cells “might act as a reservoir of SARS-CoV-2 viral debris in the atherosclerotic plaque.”
SOURCE:
The study was led by Natalia Eberhardt, PhD, postdoctoral fellow, department of medicine, division of cardiology, New York University, and colleagues. It was published online in Nature Cardiovascular Research.
LIMITATIONS:
Findings are relevant only to the original strains of SARS-CoV-2 that circulated in New York between May 2020 and May 2021, and are not generalizable to patients younger and healthier than those from whom samples were obtained for the study.
DISCLOSURES:
The study received support from the National Institutes of Health. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, which could help explain why people with COVID-19 have an increased risk for ischemic cardiovascular complications up to 1 year after infection.
METHODOLOGY:
- Researchers obtained 27 coronary autopsy specimens from eight patients who died from COVID-19, mean age 70 years and 75% male. All had coronary artery disease and most had cardiovascular risk factors such as hypertension, were overweight or obese, and had hyperlipidemia and type 2 diabetes.
- All but one patient, who was pronounced dead before hospital admission, were hospitalized for an average of 17.6 days.
- To identify SARS-CoV-2 viral RNA (vRNA) in the autoptic coronary vasculature, researchers performed RNA fluorescence in situ hybridization (RNA-FISH) analysis for the vRNA encoding the spike (S) protein; they also probed the antisense strand of the S gene (S antisense), which is only produced during viral replication.
TAKEAWAY:
- The study found evidence of SARS-CoV-2 replication in all analyzed human autopsy coronaries regardless of their pathological classification, although viral replication was highest in early-stage lesions that progress to more advanced atherosclerotic plaques.
- Findings indicated that more than 79% of macrophages (white blood cells that help remove lipids) and more than 90% of foam cells (lipid-laden macrophages that are a hallmark of atherosclerosis at all stages of the disease) are S+, and more than 40% of both cell types are S antisense+, indicating SARS-CoV-2 can infect macrophages at a high rate.
- SARS-CoV-2 induced a strong inflammatory response as evidenced by release of cytokines (including interleukin-1 beta and interluekin-6 that are linked to myocardial infarction) in both macrophages and foam cells, which may contribute to the ischemic cardiovascular complications in patients with COVID-19.
IN PRACTICE:
“Our data conclusively demonstrate that SARS-CoV-2 is capable of infecting and replicating in macrophages within the coronary vasculature of patients with COVID-19,” write the authors, adding that SARS-CoV-2 preferentially replicates in foam cells, compared with other macrophages, suggesting these cells “might act as a reservoir of SARS-CoV-2 viral debris in the atherosclerotic plaque.”
SOURCE:
The study was led by Natalia Eberhardt, PhD, postdoctoral fellow, department of medicine, division of cardiology, New York University, and colleagues. It was published online in Nature Cardiovascular Research.
LIMITATIONS:
Findings are relevant only to the original strains of SARS-CoV-2 that circulated in New York between May 2020 and May 2021, and are not generalizable to patients younger and healthier than those from whom samples were obtained for the study.
DISCLOSURES:
The study received support from the National Institutes of Health. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.
TOPLINE:
, which could help explain why people with COVID-19 have an increased risk for ischemic cardiovascular complications up to 1 year after infection.
METHODOLOGY:
- Researchers obtained 27 coronary autopsy specimens from eight patients who died from COVID-19, mean age 70 years and 75% male. All had coronary artery disease and most had cardiovascular risk factors such as hypertension, were overweight or obese, and had hyperlipidemia and type 2 diabetes.
- All but one patient, who was pronounced dead before hospital admission, were hospitalized for an average of 17.6 days.
- To identify SARS-CoV-2 viral RNA (vRNA) in the autoptic coronary vasculature, researchers performed RNA fluorescence in situ hybridization (RNA-FISH) analysis for the vRNA encoding the spike (S) protein; they also probed the antisense strand of the S gene (S antisense), which is only produced during viral replication.
TAKEAWAY:
- The study found evidence of SARS-CoV-2 replication in all analyzed human autopsy coronaries regardless of their pathological classification, although viral replication was highest in early-stage lesions that progress to more advanced atherosclerotic plaques.
- Findings indicated that more than 79% of macrophages (white blood cells that help remove lipids) and more than 90% of foam cells (lipid-laden macrophages that are a hallmark of atherosclerosis at all stages of the disease) are S+, and more than 40% of both cell types are S antisense+, indicating SARS-CoV-2 can infect macrophages at a high rate.
- SARS-CoV-2 induced a strong inflammatory response as evidenced by release of cytokines (including interleukin-1 beta and interluekin-6 that are linked to myocardial infarction) in both macrophages and foam cells, which may contribute to the ischemic cardiovascular complications in patients with COVID-19.
IN PRACTICE:
“Our data conclusively demonstrate that SARS-CoV-2 is capable of infecting and replicating in macrophages within the coronary vasculature of patients with COVID-19,” write the authors, adding that SARS-CoV-2 preferentially replicates in foam cells, compared with other macrophages, suggesting these cells “might act as a reservoir of SARS-CoV-2 viral debris in the atherosclerotic plaque.”
SOURCE:
The study was led by Natalia Eberhardt, PhD, postdoctoral fellow, department of medicine, division of cardiology, New York University, and colleagues. It was published online in Nature Cardiovascular Research.
LIMITATIONS:
Findings are relevant only to the original strains of SARS-CoV-2 that circulated in New York between May 2020 and May 2021, and are not generalizable to patients younger and healthier than those from whom samples were obtained for the study.
DISCLOSURES:
The study received support from the National Institutes of Health. The authors report no relevant financial relationships.
A version of this article appeared on Medscape.com.