Atopic dermatitis (AD) is most often treated with mid-potency topical corticosteroids.1,2 Although this option is effective, not all patients respond to treatment, and those who do may lose efficacy over time, a phenomenon known as tachyphylaxis. The pathophysiology of tachyphylaxis to topical corticosteroids has been ascribed to loss of corticosteroid receptor function,3 but the evidence is weak.3,4 Patients with severe treatment-resistant AD improve when treated with mid-potency topical steroids in an inpatient setting; therefore, treatment resistance to topical corticosteroids may be largely due to poor adherence.5
Patients with treatment-resistant AD generally improve when treated with topical corticosteroids under conditions designed to promote treatment adherence, but this improvement often is reported for study groups, not individual patients. Focusing on group data may not give a clear picture of what is happening at the individual level. In this study, we evaluated changes at an individual level to determine how frequently AD patients who were previously treated with topical corticosteroids unsuccessfully would respond to desoximetasone spray 0.25% under conditions designed to promote good adherence over a 7-day period.
Methods
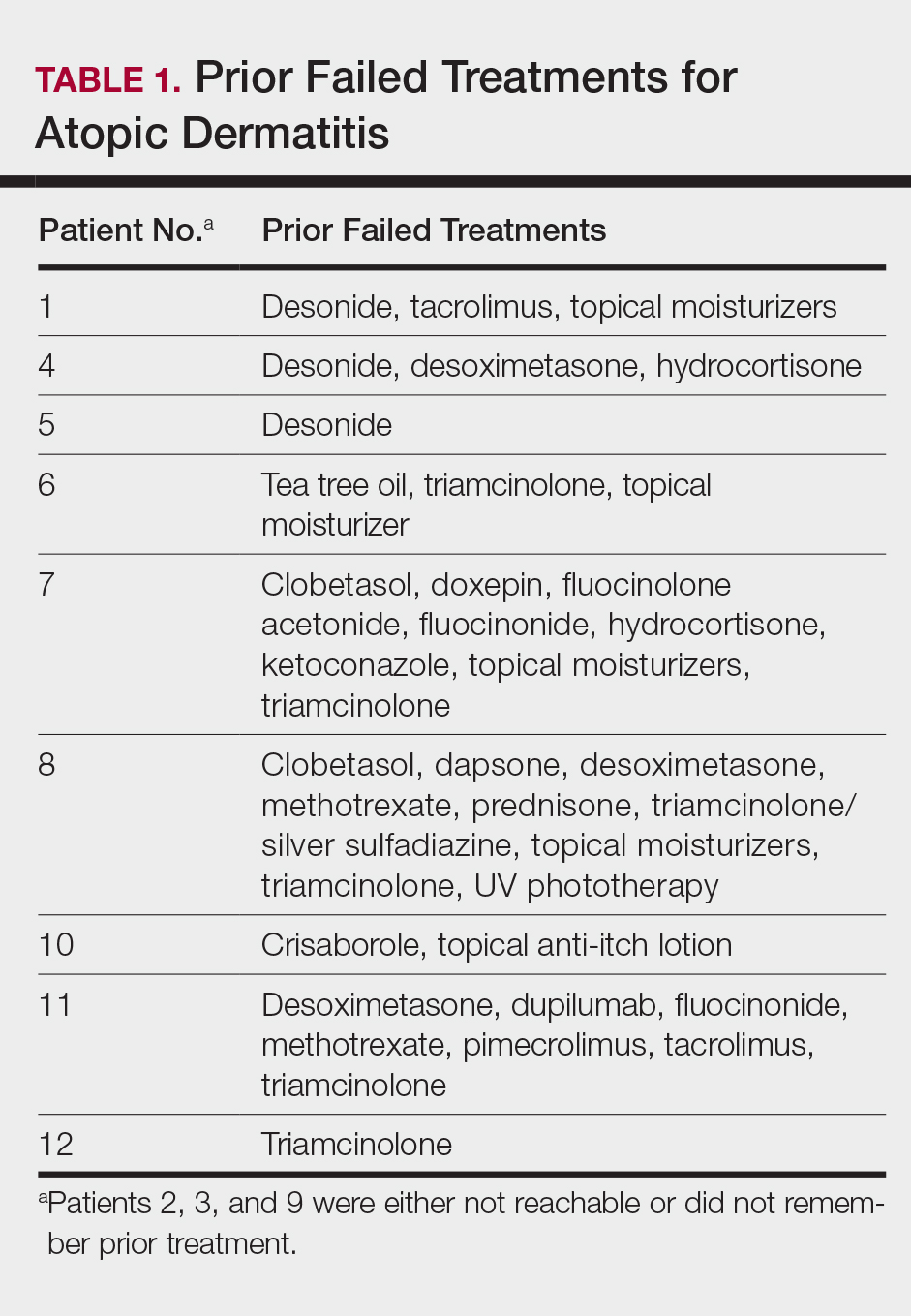
This open-label, randomized, single-center clinical study included 12 patients with AD who were previously unsuccessfully treated with topical corticosteroids in the Department of Dermatology at Wake Forest Baptist Medical Center (Winston-Salem, North Carolina)(Table 1). The study was approved by the local institutional review board.
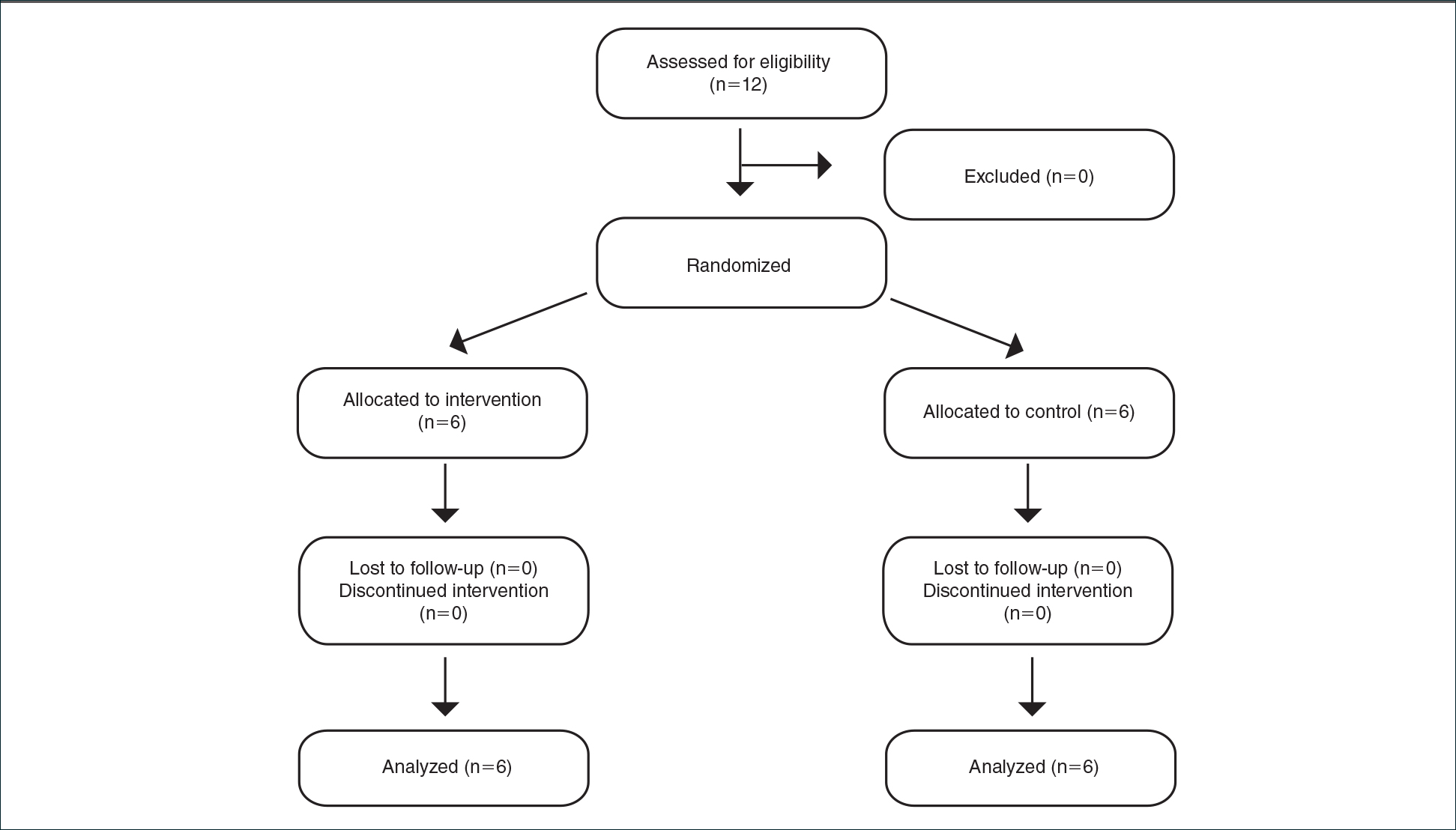
Inclusion criteria included men and women 18 years or older at baseline who had AD that was considered amenable to therapy with topical corticosteroids by the clinician and were able to comply with the study protocol (Figure). Written informed consent also was obtained from each patient. Women who were pregnant, breastfeeding, or unwilling to practice birth control during participation in the study were excluded. Other exclusion criteria included presence of a condition that in the opinion of the investigator would compromise the safety of the patient or quality of data as well as patients with no access to a telephone throughout the day. Patients diagnosed with conditions affecting adherence to treatment (eg, dementia, Alzheimer disease), those with a history of allergy or sensitivity to corticosteroids, and those with a history of drug hypersensitivity were excluded from the study.
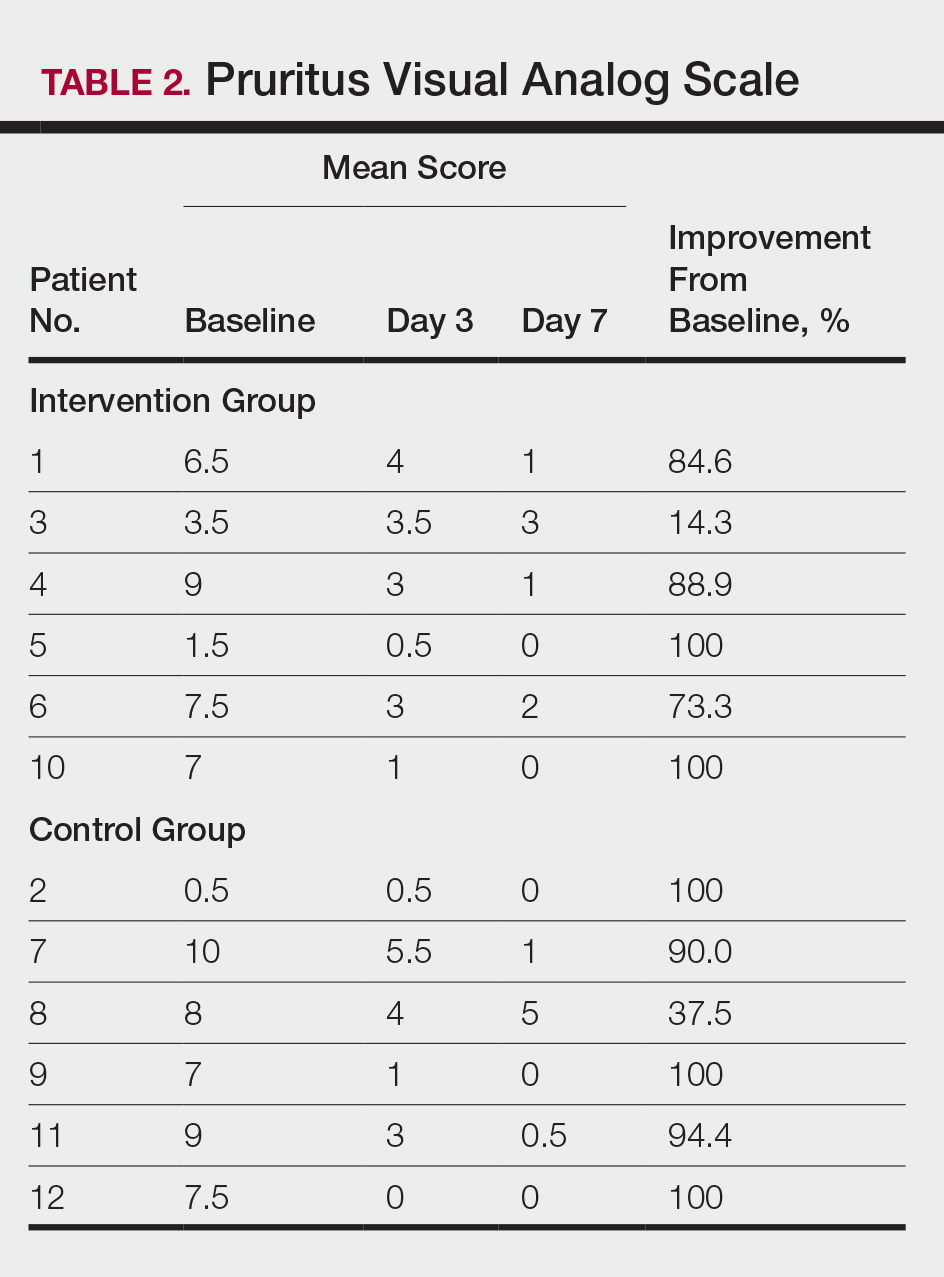
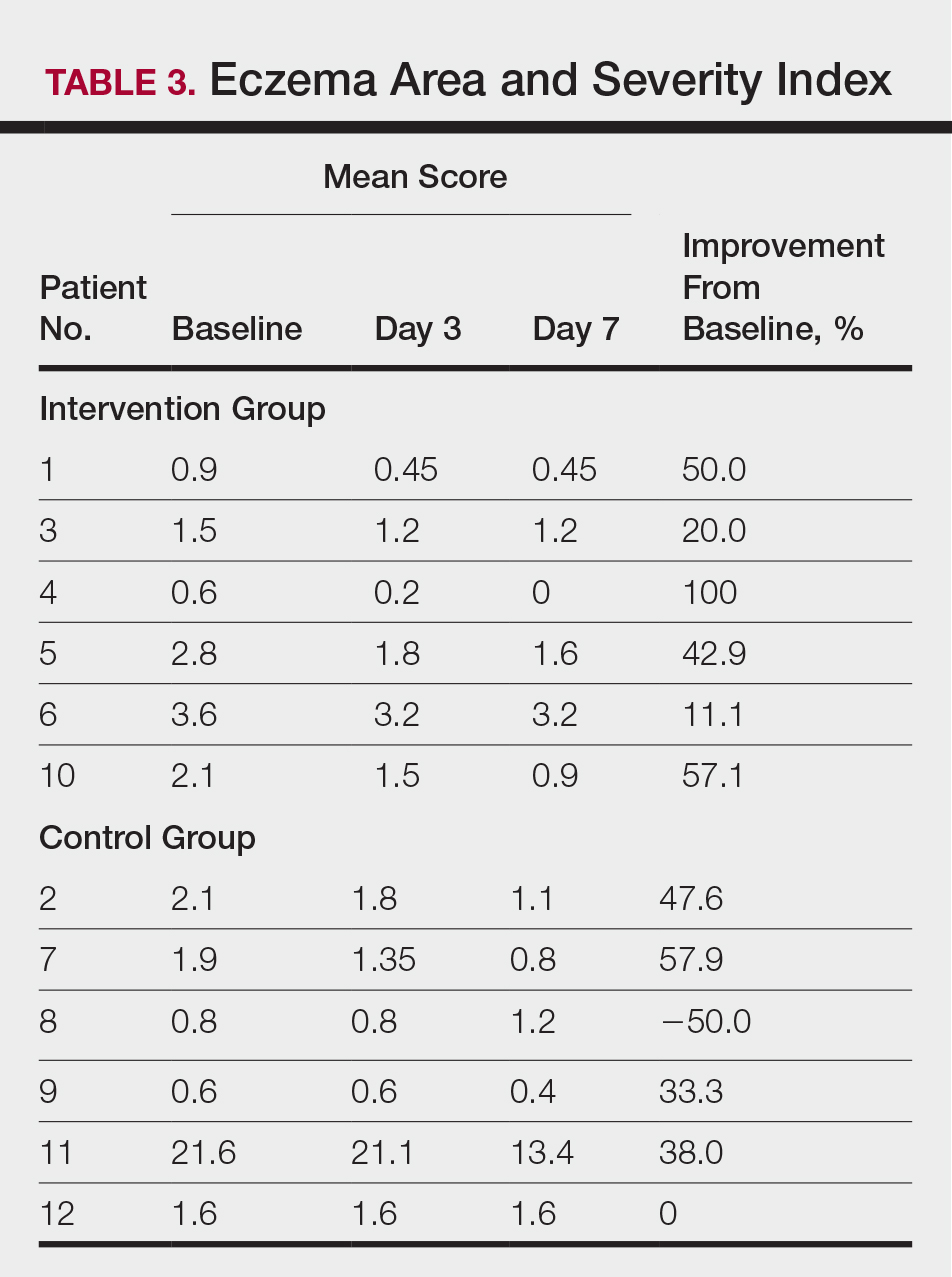
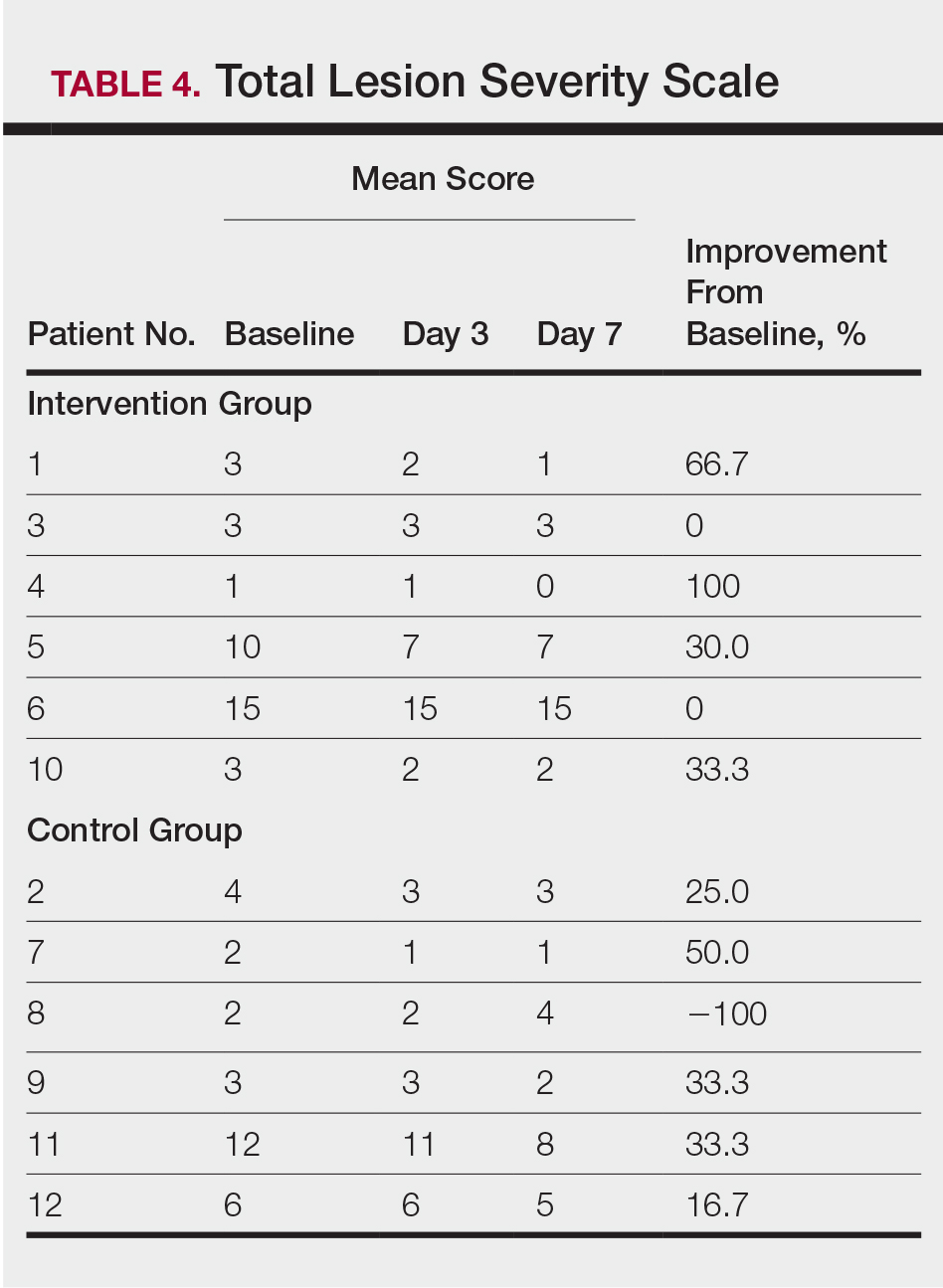
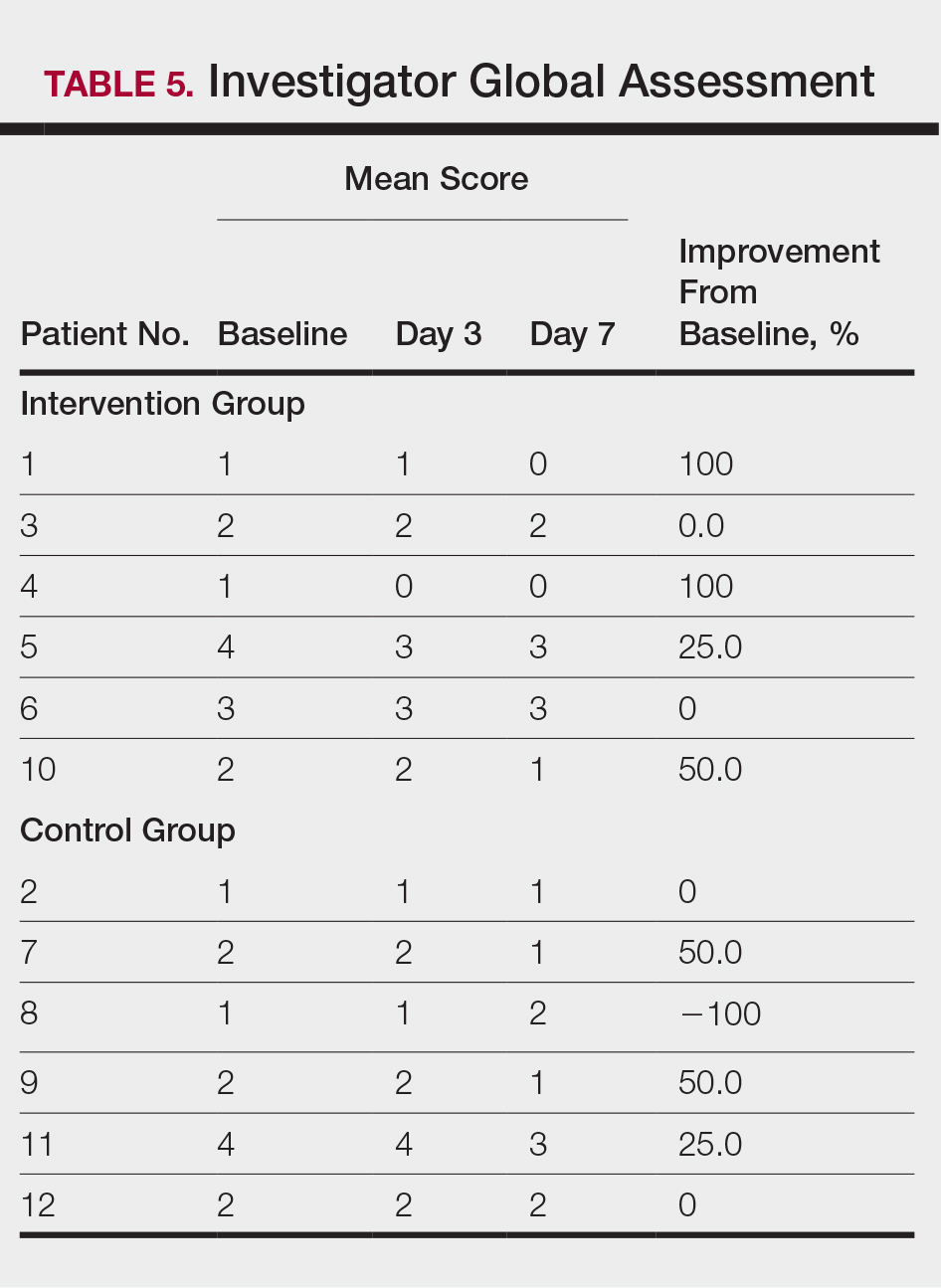
All 12 patients were treated with desoximetasone spray 0.25% for 7 days. Patients were instructed not to use other AD medications during the study period. At baseline, patients were randomized to receive either twice-daily telephone calls to discuss treatment adherence (intervention group) or no telephone calls (control) during the study period. Patients in both the intervention and control groups returned for evaluation on days 3 and 7. During these visits, disease severity was evaluated using the pruritus visual analog scale, Eczema Area and Severity Index (EASI), total lesion severity scale (TLSS), and investigator global assessment (IGA). Descriptive statistics were used to report the outcomes for each patient.
Results
Twelve AD patients who were previously unsuccessfully treated with topical corticosteroids were recruited for the study. Six patients were randomized to the intervention group and 6 were randomized to the control group. Fifty percent of patients were black, 50% were women, and the average age was 50.4 years. All 12 patients completed the study.
At the end of the study, most patients showed improvement in all evaluation parameters (eFigure). All 12 patients showed improvement in pruritus visual analog scores; 83.3% (10/12) showed improved EASI scores, 75.0% (9/12) showed improved TLSS scores, and 58.3% (7/12) showed improved IGA scores (Tables 2–5). Patients who received telephone calls in the intervention group showed greater improvement compared to those in the control group, except for pruritus; the mean reduction in pruritus was 76.9% in the intervention group versus 87.0% in the control group. The mean improvement in EASI score was 46.9% in the intervention group versus 21.1% in the control group. The mean improvement in TLSS score was 38.3% in the intervention group versus 9.7% in the control group. The mean improvement in IGA score was 45.8% in the intervention group versus 4.2% in the control group. Only one patient in the control group (patient 8) showed lower EASI, TLSS, and IGA scores at baseline.