User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Adding bortezomib does not improve MCL outcomes
Bortezomib added to an alternating chemoimmunotherapy regimen did not improve time to treatment failure in patients with newly diagnosed mantle cell lymphoma (MCL), results of a phase 2 study have suggested.
Response rates and time to treatment failure were similar to what has been seen historically without the addition of bortezomib, according to study investigator Jorge E. Romaguera, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The phase 2 study included 95 patients with newly diagnosed MCL treated with alternating cycles of bortezomib added to rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (BzR-hyperCVAD) and bortezomib added to rituximab plus high-dose methotrexate and high-dose cytarabine (BzR-MA).
Of 87 patients evaluable for response, alternating BzR-hyperCVAD/BzR-MA resulted in an overall response rate of 100% and a complete response rate of 82%, Dr. Romaguera and his colleagues reported in the journal Cancer. At a median follow-up of 44 months, median time to treatment failure was 55 months, and median overall survival had not yet been reached, according to the report.
Dr. Romaguera and his coauthors compared these results with those from a previous study of alternating R-hyperCVAD/R-MA, in which the median time to treatment failure was 56.4 months. “This suggests that the addition of bortezomib does not improve the outcome,” they wrote in the current report.
Although more follow-up is needed, the landscape of MCL treatment is changing quickly, they added. In particular, lenalidomide and ibrutinib, already approved for relapsed/refractory MCL, are now being evaluated as part of first-line MCL regimens. “These drugs will offer strategies of either consolidation or maintenance after induction and will hopefully help continue to improve the duration of the initial response and the overall outcome,” the researchers wrote.
In the current phase 2 study, the fact that 100% of patients achieved complete response suggested that relapses come from minimal residual disease, which “has clearly become a clinical factor for the outcomes of patients with MCL and will likely become the next endpoint,” they wrote.
The researchers reported having no financial disclosures related to the study, which was supported by Takeda Oncology.
SOURCE: Romaguera JE et al. Cancer. 2018 May 3. doi: 10.1002/cncr.31361.
Bortezomib added to an alternating chemoimmunotherapy regimen did not improve time to treatment failure in patients with newly diagnosed mantle cell lymphoma (MCL), results of a phase 2 study have suggested.
Response rates and time to treatment failure were similar to what has been seen historically without the addition of bortezomib, according to study investigator Jorge E. Romaguera, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The phase 2 study included 95 patients with newly diagnosed MCL treated with alternating cycles of bortezomib added to rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (BzR-hyperCVAD) and bortezomib added to rituximab plus high-dose methotrexate and high-dose cytarabine (BzR-MA).
Of 87 patients evaluable for response, alternating BzR-hyperCVAD/BzR-MA resulted in an overall response rate of 100% and a complete response rate of 82%, Dr. Romaguera and his colleagues reported in the journal Cancer. At a median follow-up of 44 months, median time to treatment failure was 55 months, and median overall survival had not yet been reached, according to the report.
Dr. Romaguera and his coauthors compared these results with those from a previous study of alternating R-hyperCVAD/R-MA, in which the median time to treatment failure was 56.4 months. “This suggests that the addition of bortezomib does not improve the outcome,” they wrote in the current report.
Although more follow-up is needed, the landscape of MCL treatment is changing quickly, they added. In particular, lenalidomide and ibrutinib, already approved for relapsed/refractory MCL, are now being evaluated as part of first-line MCL regimens. “These drugs will offer strategies of either consolidation or maintenance after induction and will hopefully help continue to improve the duration of the initial response and the overall outcome,” the researchers wrote.
In the current phase 2 study, the fact that 100% of patients achieved complete response suggested that relapses come from minimal residual disease, which “has clearly become a clinical factor for the outcomes of patients with MCL and will likely become the next endpoint,” they wrote.
The researchers reported having no financial disclosures related to the study, which was supported by Takeda Oncology.
SOURCE: Romaguera JE et al. Cancer. 2018 May 3. doi: 10.1002/cncr.31361.
Bortezomib added to an alternating chemoimmunotherapy regimen did not improve time to treatment failure in patients with newly diagnosed mantle cell lymphoma (MCL), results of a phase 2 study have suggested.
Response rates and time to treatment failure were similar to what has been seen historically without the addition of bortezomib, according to study investigator Jorge E. Romaguera, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues.
The phase 2 study included 95 patients with newly diagnosed MCL treated with alternating cycles of bortezomib added to rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (BzR-hyperCVAD) and bortezomib added to rituximab plus high-dose methotrexate and high-dose cytarabine (BzR-MA).
Of 87 patients evaluable for response, alternating BzR-hyperCVAD/BzR-MA resulted in an overall response rate of 100% and a complete response rate of 82%, Dr. Romaguera and his colleagues reported in the journal Cancer. At a median follow-up of 44 months, median time to treatment failure was 55 months, and median overall survival had not yet been reached, according to the report.
Dr. Romaguera and his coauthors compared these results with those from a previous study of alternating R-hyperCVAD/R-MA, in which the median time to treatment failure was 56.4 months. “This suggests that the addition of bortezomib does not improve the outcome,” they wrote in the current report.
Although more follow-up is needed, the landscape of MCL treatment is changing quickly, they added. In particular, lenalidomide and ibrutinib, already approved for relapsed/refractory MCL, are now being evaluated as part of first-line MCL regimens. “These drugs will offer strategies of either consolidation or maintenance after induction and will hopefully help continue to improve the duration of the initial response and the overall outcome,” the researchers wrote.
In the current phase 2 study, the fact that 100% of patients achieved complete response suggested that relapses come from minimal residual disease, which “has clearly become a clinical factor for the outcomes of patients with MCL and will likely become the next endpoint,” they wrote.
The researchers reported having no financial disclosures related to the study, which was supported by Takeda Oncology.
SOURCE: Romaguera JE et al. Cancer. 2018 May 3. doi: 10.1002/cncr.31361.
FROM CANCER
Key clinical point:
Major finding: Rates of overall and complete response were 100% and 82%, respectively, while time to treatment failure was 55 months.
Study details: A phase 2 trial that included 95 patients treated with alternating cycles of bortezomib added to rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (BzR-hyperCVAD) and bortezomib added to rituximab plus high-dose methotrexate and high-dose cytarabine (BzR-MA).
Disclosures: The study was supported by Takeda Oncology. The researchers reported having no financial disclosures related to the study.
Source: Romaguera JE et al. Cancer. 2018 May 3. doi: 10.1002/cncr.31361
Study: No link between non-Hodgkin lymphoma and Q fever
Sonja E. van Roeden, MD, and colleagues from Utrecht University, the Netherlands, performed a retrospective, population-based analysis of the entire general population of the Netherlands from 2002 to 2013, encompassing the 3-year period of a large Q fever epidemic in the country.
In total, there were 48,760 cases of NHL diagnosed between Jan. 1, 2002 and Dec. 31, 2013, with the annual incidence ranging from 21.4 cases per 100,000 population in 2002 to 26.7 in 2010. While researchers found a significant association between NHL incidence and areas of high endemicity of Q fever in 2009 (relative risk 1.16; P = .029), there were no other associations.
Among the 439 people with chronic Q fever, 5 went on to develop NHL, resulting in a relative risk of 4.99 (P = .0003), compared with the general population of the Netherlands.
“The absence of an exposure-response relation between the intensity of exposure and risk of non-Hodgkin lymphoma, based on reported incidence of acute Q fever, does not imply a causal relation,” the researchers wrote. “However, one could consider exposure to C. burnetii in people with chronic Q fever as more intense than in acute Q fever because of the ongoing character and duration of exposure, and assume an exposure-response relation on the basis of the higher risk for non-Hodgkin lymphoma in patients with chronic Q fever.”
The researchers reported having no financial disclosures.
SOURCE: van Roeden SE et al. Lancet Haematol. 2018 May;5:e211-9.
Sonja E. van Roeden, MD, and colleagues from Utrecht University, the Netherlands, performed a retrospective, population-based analysis of the entire general population of the Netherlands from 2002 to 2013, encompassing the 3-year period of a large Q fever epidemic in the country.
In total, there were 48,760 cases of NHL diagnosed between Jan. 1, 2002 and Dec. 31, 2013, with the annual incidence ranging from 21.4 cases per 100,000 population in 2002 to 26.7 in 2010. While researchers found a significant association between NHL incidence and areas of high endemicity of Q fever in 2009 (relative risk 1.16; P = .029), there were no other associations.
Among the 439 people with chronic Q fever, 5 went on to develop NHL, resulting in a relative risk of 4.99 (P = .0003), compared with the general population of the Netherlands.
“The absence of an exposure-response relation between the intensity of exposure and risk of non-Hodgkin lymphoma, based on reported incidence of acute Q fever, does not imply a causal relation,” the researchers wrote. “However, one could consider exposure to C. burnetii in people with chronic Q fever as more intense than in acute Q fever because of the ongoing character and duration of exposure, and assume an exposure-response relation on the basis of the higher risk for non-Hodgkin lymphoma in patients with chronic Q fever.”
The researchers reported having no financial disclosures.
SOURCE: van Roeden SE et al. Lancet Haematol. 2018 May;5:e211-9.
Sonja E. van Roeden, MD, and colleagues from Utrecht University, the Netherlands, performed a retrospective, population-based analysis of the entire general population of the Netherlands from 2002 to 2013, encompassing the 3-year period of a large Q fever epidemic in the country.
In total, there were 48,760 cases of NHL diagnosed between Jan. 1, 2002 and Dec. 31, 2013, with the annual incidence ranging from 21.4 cases per 100,000 population in 2002 to 26.7 in 2010. While researchers found a significant association between NHL incidence and areas of high endemicity of Q fever in 2009 (relative risk 1.16; P = .029), there were no other associations.
Among the 439 people with chronic Q fever, 5 went on to develop NHL, resulting in a relative risk of 4.99 (P = .0003), compared with the general population of the Netherlands.
“The absence of an exposure-response relation between the intensity of exposure and risk of non-Hodgkin lymphoma, based on reported incidence of acute Q fever, does not imply a causal relation,” the researchers wrote. “However, one could consider exposure to C. burnetii in people with chronic Q fever as more intense than in acute Q fever because of the ongoing character and duration of exposure, and assume an exposure-response relation on the basis of the higher risk for non-Hodgkin lymphoma in patients with chronic Q fever.”
The researchers reported having no financial disclosures.
SOURCE: van Roeden SE et al. Lancet Haematol. 2018 May;5:e211-9.
FROM LANCET HAEMATOLOGY
5-year data show deepening response with ibrutinib in CLL
Single-agent ibrutinib has had sustained efficacy and a rate of complete response that has increased over time, according to a 5-year follow-up report including 132 patients with chronic lymphocytic leukemia (CLL).
Efficacy has been maintained in both treatment-naive and relapsed/refractory CLL, despite the presence of high-risk genomic features in many patients, investigators reported in Blood.
Treatment has been well tolerated, and the occurrence of severe adverse events has diminished over time, according to Susan M. O’Brien, MD, of the Chao Family Comprehensive Cancer Center, University of California, Irvine, and her colleagues.
“The safety profile of ibrutinib over time remains acceptable and manageable, allowing almost one-half of the patients (48%) to be treated for more than 4 years and thus maximize response,” the investigators wrote.
The report was based on 5-year follow-up of patients with CLL who had been enrolled in a phase 1b/2 study (PCYC-1102) and an extension study (PCYC-1103). A total of 132 patients were evaluated, including 101 with relapsed/refractory disease and 31 who were treatment naive.
The overall response rate remained high, at 89% in this 5-year follow-up. Complete response rates increased over time, reaching 29% in treatment-naive patients and 10% in relapsed/refractory patients. In a previous report on 3-year follow-up for these patients, investigators reported complete response rates of 23% in the previously untreated group and 7% in the relapsed/refractory group. The new findings demonstrate “deepening of responses” with continued ibrutinib therapy, Dr. O’Brien and her coauthors wrote.
The 5-year rate of progression-free survival was 44% for relapsed/refractory patients and 92% for treatment-naive patients in this study. Median progression-free survival was 51 months for the relapsed/refractory cohort. “[Progression-free survival] with single-agent ibrutinib in [treatment-naive] patients appears particularly favorable, because the median has not been reached,” investigators wrote.
Adverse events that limited treatment were more frequent during the first year of treatment than in subsequent years, data show, while new onset of neutropenia and thrombocytopenia decreased over time.
The overall response rate was high even in patients with high-risk genetic features. Rates of overall response ranged from 79% to 97%, depending on genomic subgroup. That held true for relapsed/refractory CLL patients with del(17p), a significant negative prognostic factor for survival. In that group, the overall response rate was 79% and the duration of response was 31 months, representing “promising single-agent efficacy” in that group, investigators said.
Pharmacyclics, an AbbVie Company, provided funding for the study and writing support. The study was also supported by grants from the National Institutes of Health and a private foundation. Dr. O’Brien reported ties to AbbVie, Janssen, and Pharmacyclics. Other investigators reported financial relationships with various pharmaceutical companies.
SOURCE: O’Brien S et al. Blood. 2018;131(17):1910-9.
This report on the 5-year experience with single-agent ibrutinib in chronic lymphocytic leukemia (CLL) is a step forward in knowledge of the drug’s natural history, according to Jennifer R. Brown, MD, PhD.
“Ibrutinib data are starting to mature, but much opportunity for growth remains,” Dr. Brown said in an editorial.
One notable update in the report is that a median, progression-free survival has been reached in patients with relapsed/refractory CLL. The reported 51-month median progression-free survival is “strikingly good” in this cohort of patients with high-risk disease, and compares favorably to older regimens in similar patient populations, she said.
Regarding previously untreated CLL patients, it is notable that 45% of this cohort discontinued treatment, many apparently after year 4, Dr. Brown said. The finding that median duration on therapy was just about 5.5 years in the cohort could be “potentially quite useful” in counseling patients, if confirmed in a larger cohort, she added.
Given the discontinuation data, one unanswered question is the durability of remission after stopping ibrutinib in a “deep but probably not complete remission” versus continuing therapy.
“Further follow-up of patients who discontinue without disease progression, as well as systematic investigation of time-limited therapy, including novel likely combination approaches, is clearly warranted,” Dr. Brown said in her editorial.
Dr. Jennifer R. Brown is with the Dana-Farber Cancer Institute in Boston. These comments are adapted an accompanying editorial ( Blood. 2018;131:1880-2 ). Dr. Brown reported ties to Janssen, Pharmacyclics, AstraZeneca, Sun, Redx, Sunesis, Loxo, Gilead, TG Therapeutics, Verastem, and AbbVie.
This report on the 5-year experience with single-agent ibrutinib in chronic lymphocytic leukemia (CLL) is a step forward in knowledge of the drug’s natural history, according to Jennifer R. Brown, MD, PhD.
“Ibrutinib data are starting to mature, but much opportunity for growth remains,” Dr. Brown said in an editorial.
One notable update in the report is that a median, progression-free survival has been reached in patients with relapsed/refractory CLL. The reported 51-month median progression-free survival is “strikingly good” in this cohort of patients with high-risk disease, and compares favorably to older regimens in similar patient populations, she said.
Regarding previously untreated CLL patients, it is notable that 45% of this cohort discontinued treatment, many apparently after year 4, Dr. Brown said. The finding that median duration on therapy was just about 5.5 years in the cohort could be “potentially quite useful” in counseling patients, if confirmed in a larger cohort, she added.
Given the discontinuation data, one unanswered question is the durability of remission after stopping ibrutinib in a “deep but probably not complete remission” versus continuing therapy.
“Further follow-up of patients who discontinue without disease progression, as well as systematic investigation of time-limited therapy, including novel likely combination approaches, is clearly warranted,” Dr. Brown said in her editorial.
Dr. Jennifer R. Brown is with the Dana-Farber Cancer Institute in Boston. These comments are adapted an accompanying editorial ( Blood. 2018;131:1880-2 ). Dr. Brown reported ties to Janssen, Pharmacyclics, AstraZeneca, Sun, Redx, Sunesis, Loxo, Gilead, TG Therapeutics, Verastem, and AbbVie.
This report on the 5-year experience with single-agent ibrutinib in chronic lymphocytic leukemia (CLL) is a step forward in knowledge of the drug’s natural history, according to Jennifer R. Brown, MD, PhD.
“Ibrutinib data are starting to mature, but much opportunity for growth remains,” Dr. Brown said in an editorial.
One notable update in the report is that a median, progression-free survival has been reached in patients with relapsed/refractory CLL. The reported 51-month median progression-free survival is “strikingly good” in this cohort of patients with high-risk disease, and compares favorably to older regimens in similar patient populations, she said.
Regarding previously untreated CLL patients, it is notable that 45% of this cohort discontinued treatment, many apparently after year 4, Dr. Brown said. The finding that median duration on therapy was just about 5.5 years in the cohort could be “potentially quite useful” in counseling patients, if confirmed in a larger cohort, she added.
Given the discontinuation data, one unanswered question is the durability of remission after stopping ibrutinib in a “deep but probably not complete remission” versus continuing therapy.
“Further follow-up of patients who discontinue without disease progression, as well as systematic investigation of time-limited therapy, including novel likely combination approaches, is clearly warranted,” Dr. Brown said in her editorial.
Dr. Jennifer R. Brown is with the Dana-Farber Cancer Institute in Boston. These comments are adapted an accompanying editorial ( Blood. 2018;131:1880-2 ). Dr. Brown reported ties to Janssen, Pharmacyclics, AstraZeneca, Sun, Redx, Sunesis, Loxo, Gilead, TG Therapeutics, Verastem, and AbbVie.
Single-agent ibrutinib has had sustained efficacy and a rate of complete response that has increased over time, according to a 5-year follow-up report including 132 patients with chronic lymphocytic leukemia (CLL).
Efficacy has been maintained in both treatment-naive and relapsed/refractory CLL, despite the presence of high-risk genomic features in many patients, investigators reported in Blood.
Treatment has been well tolerated, and the occurrence of severe adverse events has diminished over time, according to Susan M. O’Brien, MD, of the Chao Family Comprehensive Cancer Center, University of California, Irvine, and her colleagues.
“The safety profile of ibrutinib over time remains acceptable and manageable, allowing almost one-half of the patients (48%) to be treated for more than 4 years and thus maximize response,” the investigators wrote.
The report was based on 5-year follow-up of patients with CLL who had been enrolled in a phase 1b/2 study (PCYC-1102) and an extension study (PCYC-1103). A total of 132 patients were evaluated, including 101 with relapsed/refractory disease and 31 who were treatment naive.
The overall response rate remained high, at 89% in this 5-year follow-up. Complete response rates increased over time, reaching 29% in treatment-naive patients and 10% in relapsed/refractory patients. In a previous report on 3-year follow-up for these patients, investigators reported complete response rates of 23% in the previously untreated group and 7% in the relapsed/refractory group. The new findings demonstrate “deepening of responses” with continued ibrutinib therapy, Dr. O’Brien and her coauthors wrote.
The 5-year rate of progression-free survival was 44% for relapsed/refractory patients and 92% for treatment-naive patients in this study. Median progression-free survival was 51 months for the relapsed/refractory cohort. “[Progression-free survival] with single-agent ibrutinib in [treatment-naive] patients appears particularly favorable, because the median has not been reached,” investigators wrote.
Adverse events that limited treatment were more frequent during the first year of treatment than in subsequent years, data show, while new onset of neutropenia and thrombocytopenia decreased over time.
The overall response rate was high even in patients with high-risk genetic features. Rates of overall response ranged from 79% to 97%, depending on genomic subgroup. That held true for relapsed/refractory CLL patients with del(17p), a significant negative prognostic factor for survival. In that group, the overall response rate was 79% and the duration of response was 31 months, representing “promising single-agent efficacy” in that group, investigators said.
Pharmacyclics, an AbbVie Company, provided funding for the study and writing support. The study was also supported by grants from the National Institutes of Health and a private foundation. Dr. O’Brien reported ties to AbbVie, Janssen, and Pharmacyclics. Other investigators reported financial relationships with various pharmaceutical companies.
SOURCE: O’Brien S et al. Blood. 2018;131(17):1910-9.
Single-agent ibrutinib has had sustained efficacy and a rate of complete response that has increased over time, according to a 5-year follow-up report including 132 patients with chronic lymphocytic leukemia (CLL).
Efficacy has been maintained in both treatment-naive and relapsed/refractory CLL, despite the presence of high-risk genomic features in many patients, investigators reported in Blood.
Treatment has been well tolerated, and the occurrence of severe adverse events has diminished over time, according to Susan M. O’Brien, MD, of the Chao Family Comprehensive Cancer Center, University of California, Irvine, and her colleagues.
“The safety profile of ibrutinib over time remains acceptable and manageable, allowing almost one-half of the patients (48%) to be treated for more than 4 years and thus maximize response,” the investigators wrote.
The report was based on 5-year follow-up of patients with CLL who had been enrolled in a phase 1b/2 study (PCYC-1102) and an extension study (PCYC-1103). A total of 132 patients were evaluated, including 101 with relapsed/refractory disease and 31 who were treatment naive.
The overall response rate remained high, at 89% in this 5-year follow-up. Complete response rates increased over time, reaching 29% in treatment-naive patients and 10% in relapsed/refractory patients. In a previous report on 3-year follow-up for these patients, investigators reported complete response rates of 23% in the previously untreated group and 7% in the relapsed/refractory group. The new findings demonstrate “deepening of responses” with continued ibrutinib therapy, Dr. O’Brien and her coauthors wrote.
The 5-year rate of progression-free survival was 44% for relapsed/refractory patients and 92% for treatment-naive patients in this study. Median progression-free survival was 51 months for the relapsed/refractory cohort. “[Progression-free survival] with single-agent ibrutinib in [treatment-naive] patients appears particularly favorable, because the median has not been reached,” investigators wrote.
Adverse events that limited treatment were more frequent during the first year of treatment than in subsequent years, data show, while new onset of neutropenia and thrombocytopenia decreased over time.
The overall response rate was high even in patients with high-risk genetic features. Rates of overall response ranged from 79% to 97%, depending on genomic subgroup. That held true for relapsed/refractory CLL patients with del(17p), a significant negative prognostic factor for survival. In that group, the overall response rate was 79% and the duration of response was 31 months, representing “promising single-agent efficacy” in that group, investigators said.
Pharmacyclics, an AbbVie Company, provided funding for the study and writing support. The study was also supported by grants from the National Institutes of Health and a private foundation. Dr. O’Brien reported ties to AbbVie, Janssen, and Pharmacyclics. Other investigators reported financial relationships with various pharmaceutical companies.
SOURCE: O’Brien S et al. Blood. 2018;131(17):1910-9.
FROM BLOOD
Key clinical point:
Major finding: The 5-year rate of progression-free survival was 44% for relapsed/refractory patients and 92% for treatment-naive patients.
Study details: Report on 5-year follow-up of 132 patients with CLL enrolled in a phase 1b/2 study (PCYC-1102) and an extension study (PCYC-1103).
Disclosures: Pharmacyclics, an AbbVie Company, provided funding for the study and writing support. The study was also supported by grants from the National Institutes of Health and a private foundation. The authors reported ties to Pharmacyclics and other companies.
Source: O’Brien S et al. Blood. 2018;131(17):1910-9.
In young MCL patients, optimal treatment may vary
, according to a recent review published in Best Practice & Research Clinical Haematology.
Use of high-dose cytarabine plus rituximab as frontline treatment is well established, with median overall survival now exceeding 10 years, said Rory McCulloch, MD, and Simon Rule, MD, of the department of Haematology, Derriford Hospital, Plymouth, England. However, there is no proven benefit to conventional therapy in patients with asymptomatic, non-bulky disease, making a watch-and-wait strategy appropriate for these patients, the authors said.
On the opposite end of the spectrum there is a subgroup of patients characterized by TP53 mutations and poor prognostic index scores that have poor outcomes in spite of conventional therapy.
These patients might have improved outcomes either with early allogeneic haematopoietic cell transplantation (allo-HCT), or, especially, clinical trials of novel agents in the upfront setting, the authors noted.
“There are a host of exciting novel agents, most prominently the BTK inhibitors, that are game changing with respect to their activity,” wrote Dr. McCulloch and Dr. Rule. “Based on the long-term results seen with conventional therapy, it is premature to be considering such new drugs in the frontline setting outside the context of a clinical trial, but it is hard to believe they will not become incorporated into treatment protocols in the future.”
Watch-and-wait treatment strategies for lower-risk patients are supported by the results of two single-center, retrospective studies published in 2009 that suggest the practice has no adverse impact on overall survival. More recent registry studies, published in 2016 and 2017, have shown that a significant proportion of patients can be managed according to the watch-and-wait strategy.
Although it’s been challenging to precisely define the group of patients for whom watch-and-wait is appropriate, enrollment criteria for studies have generally specified that patients be asymptomatic with non-bulky disease and non-blastoid morphology, they said.
For the minority of patients presenting with high-risk disease, allo-HCT may improve outcomes, according to Dr. McCulloch and Dr. Rule. One prospective study evaluating allogeneic transplants in frontline therapy showed favorable outcomes in younger patients, although few high-risk patients were enrolled.
However, a second prospective study of allo-HCT, involving 25 patients with untreated MCL in the United Kingdom, demonstrated a 2-year overall survival of 80%. “Although immature, the results are encouraging and provide data to support frontline allogeneic transplant for some patients,” Dr. McCulloch and Dr. Rule said in a comment on that study.
Novel agent studies have produced mixed results in treatment settings relevant to younger, high-risk MCL patients, though key trials are ongoing that could change practice.
One phase 2 study is evaluating obinituzumab, the fully humanized anti-CD20, as part of MCL induction and maintenance. Results of that study could challenge the role of rituximab in maintenance, the review authors noted. Likewise, the immune modulator lenalidomide has been evaluated as maintenance in an Italian phase 3 trial that recently closed to recruitment.
BTK inhibitors represent a “step change” in the management of MCL, according to the authors of this review.
“It has become clear that earlier use of ibrutinib leads to an improved outcome [in MCL] and it is logical to extend this into frontline treatment,” they wrote.
A randomized phase 3, multinational trial known as TRIANGLE, now open to recruitment, is designed to evaluate use of ibrutinib in both induction and maintenance. Investigators plan to enroll 870 patients into the three-arm study, which will also evaluate the use of ibrutinib as part of induction, but with no autologous stem cell transplant.
“The trial is the first to randomize to a non-ASCT arm since the introduction of rituximab and cytarabine to the induction regimen and the results have the potential to significantly reduce chemotherapy intensity and toxicity,” the authors said.
Dr. Rule reported consulting for Pharmacyclics, Napp, Sunesis, Acerta Pharma, Kite, AstraZeneca, Roche, Janssen, and Celgene, and research funding from Janssen, Celgene, and GSK. Dr. McCulloch reported having no financial disclosures.
SOURCE: McCulloch R et al. Best Pract Res Clin Haematol. 2018 Mar;31(1):90-8.
, according to a recent review published in Best Practice & Research Clinical Haematology.
Use of high-dose cytarabine plus rituximab as frontline treatment is well established, with median overall survival now exceeding 10 years, said Rory McCulloch, MD, and Simon Rule, MD, of the department of Haematology, Derriford Hospital, Plymouth, England. However, there is no proven benefit to conventional therapy in patients with asymptomatic, non-bulky disease, making a watch-and-wait strategy appropriate for these patients, the authors said.
On the opposite end of the spectrum there is a subgroup of patients characterized by TP53 mutations and poor prognostic index scores that have poor outcomes in spite of conventional therapy.
These patients might have improved outcomes either with early allogeneic haematopoietic cell transplantation (allo-HCT), or, especially, clinical trials of novel agents in the upfront setting, the authors noted.
“There are a host of exciting novel agents, most prominently the BTK inhibitors, that are game changing with respect to their activity,” wrote Dr. McCulloch and Dr. Rule. “Based on the long-term results seen with conventional therapy, it is premature to be considering such new drugs in the frontline setting outside the context of a clinical trial, but it is hard to believe they will not become incorporated into treatment protocols in the future.”
Watch-and-wait treatment strategies for lower-risk patients are supported by the results of two single-center, retrospective studies published in 2009 that suggest the practice has no adverse impact on overall survival. More recent registry studies, published in 2016 and 2017, have shown that a significant proportion of patients can be managed according to the watch-and-wait strategy.
Although it’s been challenging to precisely define the group of patients for whom watch-and-wait is appropriate, enrollment criteria for studies have generally specified that patients be asymptomatic with non-bulky disease and non-blastoid morphology, they said.
For the minority of patients presenting with high-risk disease, allo-HCT may improve outcomes, according to Dr. McCulloch and Dr. Rule. One prospective study evaluating allogeneic transplants in frontline therapy showed favorable outcomes in younger patients, although few high-risk patients were enrolled.
However, a second prospective study of allo-HCT, involving 25 patients with untreated MCL in the United Kingdom, demonstrated a 2-year overall survival of 80%. “Although immature, the results are encouraging and provide data to support frontline allogeneic transplant for some patients,” Dr. McCulloch and Dr. Rule said in a comment on that study.
Novel agent studies have produced mixed results in treatment settings relevant to younger, high-risk MCL patients, though key trials are ongoing that could change practice.
One phase 2 study is evaluating obinituzumab, the fully humanized anti-CD20, as part of MCL induction and maintenance. Results of that study could challenge the role of rituximab in maintenance, the review authors noted. Likewise, the immune modulator lenalidomide has been evaluated as maintenance in an Italian phase 3 trial that recently closed to recruitment.
BTK inhibitors represent a “step change” in the management of MCL, according to the authors of this review.
“It has become clear that earlier use of ibrutinib leads to an improved outcome [in MCL] and it is logical to extend this into frontline treatment,” they wrote.
A randomized phase 3, multinational trial known as TRIANGLE, now open to recruitment, is designed to evaluate use of ibrutinib in both induction and maintenance. Investigators plan to enroll 870 patients into the three-arm study, which will also evaluate the use of ibrutinib as part of induction, but with no autologous stem cell transplant.
“The trial is the first to randomize to a non-ASCT arm since the introduction of rituximab and cytarabine to the induction regimen and the results have the potential to significantly reduce chemotherapy intensity and toxicity,” the authors said.
Dr. Rule reported consulting for Pharmacyclics, Napp, Sunesis, Acerta Pharma, Kite, AstraZeneca, Roche, Janssen, and Celgene, and research funding from Janssen, Celgene, and GSK. Dr. McCulloch reported having no financial disclosures.
SOURCE: McCulloch R et al. Best Pract Res Clin Haematol. 2018 Mar;31(1):90-8.
, according to a recent review published in Best Practice & Research Clinical Haematology.
Use of high-dose cytarabine plus rituximab as frontline treatment is well established, with median overall survival now exceeding 10 years, said Rory McCulloch, MD, and Simon Rule, MD, of the department of Haematology, Derriford Hospital, Plymouth, England. However, there is no proven benefit to conventional therapy in patients with asymptomatic, non-bulky disease, making a watch-and-wait strategy appropriate for these patients, the authors said.
On the opposite end of the spectrum there is a subgroup of patients characterized by TP53 mutations and poor prognostic index scores that have poor outcomes in spite of conventional therapy.
These patients might have improved outcomes either with early allogeneic haematopoietic cell transplantation (allo-HCT), or, especially, clinical trials of novel agents in the upfront setting, the authors noted.
“There are a host of exciting novel agents, most prominently the BTK inhibitors, that are game changing with respect to their activity,” wrote Dr. McCulloch and Dr. Rule. “Based on the long-term results seen with conventional therapy, it is premature to be considering such new drugs in the frontline setting outside the context of a clinical trial, but it is hard to believe they will not become incorporated into treatment protocols in the future.”
Watch-and-wait treatment strategies for lower-risk patients are supported by the results of two single-center, retrospective studies published in 2009 that suggest the practice has no adverse impact on overall survival. More recent registry studies, published in 2016 and 2017, have shown that a significant proportion of patients can be managed according to the watch-and-wait strategy.
Although it’s been challenging to precisely define the group of patients for whom watch-and-wait is appropriate, enrollment criteria for studies have generally specified that patients be asymptomatic with non-bulky disease and non-blastoid morphology, they said.
For the minority of patients presenting with high-risk disease, allo-HCT may improve outcomes, according to Dr. McCulloch and Dr. Rule. One prospective study evaluating allogeneic transplants in frontline therapy showed favorable outcomes in younger patients, although few high-risk patients were enrolled.
However, a second prospective study of allo-HCT, involving 25 patients with untreated MCL in the United Kingdom, demonstrated a 2-year overall survival of 80%. “Although immature, the results are encouraging and provide data to support frontline allogeneic transplant for some patients,” Dr. McCulloch and Dr. Rule said in a comment on that study.
Novel agent studies have produced mixed results in treatment settings relevant to younger, high-risk MCL patients, though key trials are ongoing that could change practice.
One phase 2 study is evaluating obinituzumab, the fully humanized anti-CD20, as part of MCL induction and maintenance. Results of that study could challenge the role of rituximab in maintenance, the review authors noted. Likewise, the immune modulator lenalidomide has been evaluated as maintenance in an Italian phase 3 trial that recently closed to recruitment.
BTK inhibitors represent a “step change” in the management of MCL, according to the authors of this review.
“It has become clear that earlier use of ibrutinib leads to an improved outcome [in MCL] and it is logical to extend this into frontline treatment,” they wrote.
A randomized phase 3, multinational trial known as TRIANGLE, now open to recruitment, is designed to evaluate use of ibrutinib in both induction and maintenance. Investigators plan to enroll 870 patients into the three-arm study, which will also evaluate the use of ibrutinib as part of induction, but with no autologous stem cell transplant.
“The trial is the first to randomize to a non-ASCT arm since the introduction of rituximab and cytarabine to the induction regimen and the results have the potential to significantly reduce chemotherapy intensity and toxicity,” the authors said.
Dr. Rule reported consulting for Pharmacyclics, Napp, Sunesis, Acerta Pharma, Kite, AstraZeneca, Roche, Janssen, and Celgene, and research funding from Janssen, Celgene, and GSK. Dr. McCulloch reported having no financial disclosures.
SOURCE: McCulloch R et al. Best Pract Res Clin Haematol. 2018 Mar;31(1):90-8.
FROM BEST PRACTICE & RESEARCH CLINICAL HAEMATOLOGY
Early results favor combo IL-15/anti-CD20 in indolent NHL
CHICAGO – A combination of an immunostimulatory IL-15-based agent, ALT-803, with a therapeutic monoclonal antibody (mAb) against CD20, was well tolerated and had clinical activity in patients with indolent non-Hodgkin lymphoma (iNHL), according to preliminary findings from a phase 1 study.
“The cancer immunotherapy breakthrough that happened several years ago continues year after year, with a plethora of different modalities of immunotherapy at our disposal,” Todd A. Fehniger, MD, PhD, said at the annual meeting of the American Association for Cancer Research.
Immunotherapy with anti-CD20 mAbs, alone or in combination with chemotherapy, is a standard therapy for iNHL patients. Since iNHL cells express CD20, targeting it with mAbs triggers antitumor responses via cell surface receptors resulting in a potent antibody-dependent cellular toxicity. However, response in patients is highly heterogeneous, with relapse within a few months in a subset of patients. In addition, chemotherapeutic combinations can be toxic and result in serious and long-term complications.
“Relapsed or refractory iNHL is not curable and treatment strategies without long-term complications are needed,” said Dr. Fehniger, associate professor of medicine at Washington University, St. Louis.
In an attempt to address this, Dr. Fehniger and his colleagues combined rituximab, an anti-CD20 antibody, with a relatively new IL-15 agonist immunostimulatory agent called ALT-803.
In the phase 1 trial, the researchers enrolled patients with indolent non-Hodgkin lymphoma who had relapsed after at least 1 prior to CD20 antibody containing therapy. The study was a standard 3+3 dose escalation design with rituximab administered by intravenous infusion, 375 mg/m2 in four weekly doses, followed by a rest and four consolidation doses every 8 weeks for four cycles.
ALT-803 was administered concurrently at dose levels of 1 mcg/kg, 3 mcg/kg, and 6 mcg/kg IV followed by 6 mcg/kg, 10 mcg/kg, 15 mcg/kg, and 20 mcg/kg subcutaneously.
In total, 21 patients were treated: 16 patients had follicular lymphoma, four patients had marginal zone lymphoma, and one patient had small lymphocytic lymphoma. The median prior therapies received was two (range: 1-18) and five patients were treated who were refractory to prior anti-CD20 MAb therapy.
ALT-803 was well tolerated with no dose limiting toxicities or grade 4 or 5 adverse events. No patients discontinued ALT-803 and the recommended phase 2 dose was 20 mcg/kg subcutaneously. Grade 3 adverse events, regardless of attribution to ALT-803, included transient hypertension (14%), anemia (5%), nausea (5%), chills (5%), fever (5%), neutropenia (5%), and hyperglycemia (5%).
“Patients who received [subcutaneous] ALT-803 developed a unique injection site rash reaction that peaked 7-10 days later but resolved typically within 14 days. It was self-limited and resolved on its own,” Dr. Fehniger said.
At the time of the presentation, the best overall response rate was achieved in 11 of 21 patients (52%), with 9 complete responders (43%), and 2 partial responders (10%).
Of the 12 patients treated with ALT-803 subcutaneously, 11 patients had either stable disease, or partial or complete responses. All 11 patients remained on study and were in consolidation or follow-up and have not relapsed, Dr. Fehniger reported.
Among the five rituximab-refractory patients, the researchers observed one complete response, two patients with stable disease (45% and 36% tumor volume decrease), and two patients with partial disease. The durability of the responses can only be understood with longer follow-up, Dr. Fehniger said.
The peripheral blood of the patients was analyzed via flow cytometry and mass cytometry. Over the duration of four weekly doses, there was an increase in percentage (sixfold, P less than .001) and absolute number (10-fold, P less than .001) of natural killer cells at the 15-mcg/kg and 20-mcg/kg subcutaneous dose levels of ALT-803.
These results suggest that further studies of ALT-803 with other therapeutic targeting mAbs, or other immunotherapy modalities, are warranted, the researchers concluded.
Dr. Fehniger reported research funding from Altor BioScience.
SOURCE: Fehniger TA et al. AACR Annual Meeting, Abstract CT146.
CHICAGO – A combination of an immunostimulatory IL-15-based agent, ALT-803, with a therapeutic monoclonal antibody (mAb) against CD20, was well tolerated and had clinical activity in patients with indolent non-Hodgkin lymphoma (iNHL), according to preliminary findings from a phase 1 study.
“The cancer immunotherapy breakthrough that happened several years ago continues year after year, with a plethora of different modalities of immunotherapy at our disposal,” Todd A. Fehniger, MD, PhD, said at the annual meeting of the American Association for Cancer Research.
Immunotherapy with anti-CD20 mAbs, alone or in combination with chemotherapy, is a standard therapy for iNHL patients. Since iNHL cells express CD20, targeting it with mAbs triggers antitumor responses via cell surface receptors resulting in a potent antibody-dependent cellular toxicity. However, response in patients is highly heterogeneous, with relapse within a few months in a subset of patients. In addition, chemotherapeutic combinations can be toxic and result in serious and long-term complications.
“Relapsed or refractory iNHL is not curable and treatment strategies without long-term complications are needed,” said Dr. Fehniger, associate professor of medicine at Washington University, St. Louis.
In an attempt to address this, Dr. Fehniger and his colleagues combined rituximab, an anti-CD20 antibody, with a relatively new IL-15 agonist immunostimulatory agent called ALT-803.
In the phase 1 trial, the researchers enrolled patients with indolent non-Hodgkin lymphoma who had relapsed after at least 1 prior to CD20 antibody containing therapy. The study was a standard 3+3 dose escalation design with rituximab administered by intravenous infusion, 375 mg/m2 in four weekly doses, followed by a rest and four consolidation doses every 8 weeks for four cycles.
ALT-803 was administered concurrently at dose levels of 1 mcg/kg, 3 mcg/kg, and 6 mcg/kg IV followed by 6 mcg/kg, 10 mcg/kg, 15 mcg/kg, and 20 mcg/kg subcutaneously.
In total, 21 patients were treated: 16 patients had follicular lymphoma, four patients had marginal zone lymphoma, and one patient had small lymphocytic lymphoma. The median prior therapies received was two (range: 1-18) and five patients were treated who were refractory to prior anti-CD20 MAb therapy.
ALT-803 was well tolerated with no dose limiting toxicities or grade 4 or 5 adverse events. No patients discontinued ALT-803 and the recommended phase 2 dose was 20 mcg/kg subcutaneously. Grade 3 adverse events, regardless of attribution to ALT-803, included transient hypertension (14%), anemia (5%), nausea (5%), chills (5%), fever (5%), neutropenia (5%), and hyperglycemia (5%).
“Patients who received [subcutaneous] ALT-803 developed a unique injection site rash reaction that peaked 7-10 days later but resolved typically within 14 days. It was self-limited and resolved on its own,” Dr. Fehniger said.
At the time of the presentation, the best overall response rate was achieved in 11 of 21 patients (52%), with 9 complete responders (43%), and 2 partial responders (10%).
Of the 12 patients treated with ALT-803 subcutaneously, 11 patients had either stable disease, or partial or complete responses. All 11 patients remained on study and were in consolidation or follow-up and have not relapsed, Dr. Fehniger reported.
Among the five rituximab-refractory patients, the researchers observed one complete response, two patients with stable disease (45% and 36% tumor volume decrease), and two patients with partial disease. The durability of the responses can only be understood with longer follow-up, Dr. Fehniger said.
The peripheral blood of the patients was analyzed via flow cytometry and mass cytometry. Over the duration of four weekly doses, there was an increase in percentage (sixfold, P less than .001) and absolute number (10-fold, P less than .001) of natural killer cells at the 15-mcg/kg and 20-mcg/kg subcutaneous dose levels of ALT-803.
These results suggest that further studies of ALT-803 with other therapeutic targeting mAbs, or other immunotherapy modalities, are warranted, the researchers concluded.
Dr. Fehniger reported research funding from Altor BioScience.
SOURCE: Fehniger TA et al. AACR Annual Meeting, Abstract CT146.
CHICAGO – A combination of an immunostimulatory IL-15-based agent, ALT-803, with a therapeutic monoclonal antibody (mAb) against CD20, was well tolerated and had clinical activity in patients with indolent non-Hodgkin lymphoma (iNHL), according to preliminary findings from a phase 1 study.
“The cancer immunotherapy breakthrough that happened several years ago continues year after year, with a plethora of different modalities of immunotherapy at our disposal,” Todd A. Fehniger, MD, PhD, said at the annual meeting of the American Association for Cancer Research.
Immunotherapy with anti-CD20 mAbs, alone or in combination with chemotherapy, is a standard therapy for iNHL patients. Since iNHL cells express CD20, targeting it with mAbs triggers antitumor responses via cell surface receptors resulting in a potent antibody-dependent cellular toxicity. However, response in patients is highly heterogeneous, with relapse within a few months in a subset of patients. In addition, chemotherapeutic combinations can be toxic and result in serious and long-term complications.
“Relapsed or refractory iNHL is not curable and treatment strategies without long-term complications are needed,” said Dr. Fehniger, associate professor of medicine at Washington University, St. Louis.
In an attempt to address this, Dr. Fehniger and his colleagues combined rituximab, an anti-CD20 antibody, with a relatively new IL-15 agonist immunostimulatory agent called ALT-803.
In the phase 1 trial, the researchers enrolled patients with indolent non-Hodgkin lymphoma who had relapsed after at least 1 prior to CD20 antibody containing therapy. The study was a standard 3+3 dose escalation design with rituximab administered by intravenous infusion, 375 mg/m2 in four weekly doses, followed by a rest and four consolidation doses every 8 weeks for four cycles.
ALT-803 was administered concurrently at dose levels of 1 mcg/kg, 3 mcg/kg, and 6 mcg/kg IV followed by 6 mcg/kg, 10 mcg/kg, 15 mcg/kg, and 20 mcg/kg subcutaneously.
In total, 21 patients were treated: 16 patients had follicular lymphoma, four patients had marginal zone lymphoma, and one patient had small lymphocytic lymphoma. The median prior therapies received was two (range: 1-18) and five patients were treated who were refractory to prior anti-CD20 MAb therapy.
ALT-803 was well tolerated with no dose limiting toxicities or grade 4 or 5 adverse events. No patients discontinued ALT-803 and the recommended phase 2 dose was 20 mcg/kg subcutaneously. Grade 3 adverse events, regardless of attribution to ALT-803, included transient hypertension (14%), anemia (5%), nausea (5%), chills (5%), fever (5%), neutropenia (5%), and hyperglycemia (5%).
“Patients who received [subcutaneous] ALT-803 developed a unique injection site rash reaction that peaked 7-10 days later but resolved typically within 14 days. It was self-limited and resolved on its own,” Dr. Fehniger said.
At the time of the presentation, the best overall response rate was achieved in 11 of 21 patients (52%), with 9 complete responders (43%), and 2 partial responders (10%).
Of the 12 patients treated with ALT-803 subcutaneously, 11 patients had either stable disease, or partial or complete responses. All 11 patients remained on study and were in consolidation or follow-up and have not relapsed, Dr. Fehniger reported.
Among the five rituximab-refractory patients, the researchers observed one complete response, two patients with stable disease (45% and 36% tumor volume decrease), and two patients with partial disease. The durability of the responses can only be understood with longer follow-up, Dr. Fehniger said.
The peripheral blood of the patients was analyzed via flow cytometry and mass cytometry. Over the duration of four weekly doses, there was an increase in percentage (sixfold, P less than .001) and absolute number (10-fold, P less than .001) of natural killer cells at the 15-mcg/kg and 20-mcg/kg subcutaneous dose levels of ALT-803.
These results suggest that further studies of ALT-803 with other therapeutic targeting mAbs, or other immunotherapy modalities, are warranted, the researchers concluded.
Dr. Fehniger reported research funding from Altor BioScience.
SOURCE: Fehniger TA et al. AACR Annual Meeting, Abstract CT146.
REPORTING FROM THE AACR ANNUAL MEETING
Key clinical point:
Major finding: The ALT-803 plus rituximab combination achieved an overall response rate in 52% of patients, a complete response in 43%, and partial response in 10%.
Study details: A phase 1 study of 21 patients with indolent non-Hodgkin lymphoma.
Disclosures: Dr. Fehniger reported research funding from Altor BioScience LLC.
Source: Fehniger TA et al. AACR Annual Meeting, Abstract CT146.
Novartis CAR T-cell therapy adds a lymphoma indication
Novartis’s after failure of two or more lines of systemic therapy.
The Food and Drug Administration approved the expanded indication on May 1. The chimeric antigen receptor (CAR) T-cell therapy was initially approved in Aug. 2017 for refractory or relapsed B-cell precursor acute lymphoblastic leukemia (ALL) in patients up to 25 years old. The new approval brings tisagenlecleucel into direct competition with Gilead Science’s CAR T-cell therapy axicabtagene ciloleucel (Yescarta), which was approved in Oct. 2017 for B-cell lymphoma.
Besides matching the competition, she said the lower price is because tisagenlecleucel takes longer to work for lymphoma, and the response isn’t as potent as for childhood ALL. Novartis is looking into chronic lymphocytic leukemia, multiple myeloma, and solid tumor indications for tisagenlecleucel and other CAR T-cell agents, she added.
The Centers for Medicare & Medicaid Services recently committed to covering outpatient administration of both agents for their initial indications; Novartis is working with CMS for coverage of the new lymphoma indication.
With both products, T cells are collected then shipped off to a company facility where a CAR gene is spliced into their DNA, essentially programming the T cells to attack the targeted cancer. The cells are then infused back into the patient.
In the phase 2 JULIET trial, tisagenlecleucel showed an overall response rate of 50% among 68 B-cell lymphoma patients, with 32% achieving complete response (CR) and 18% achieving partial response (PR). The median duration of response was not reached.
Axicabtagene ciloleucel’s label reports an objective response rate of 72% among 101 patients, with CR in 51% and PR in 21%. Median duration of response was 9.2 months but was also not reached among complete responders.
“Different trials. Different CARTs. Different levels of disease. Our drug is cryopreserved and theirs is not. No way to compare them,” the Novartis spokeswoman said when asked about the response differences.
T-cell reprogramming isn’t clean at this point in medical history; both agents carry black box warnings of potentially fatal cytokine release syndrome and neurologic toxicity, and both are subject to Risk Evaluation and Mitigation Strategy programs.
The B-cell lymphoma indication for both therapies includes diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. The Gilead product carries an additional indication for primary mediastinal large B-cell lymphoma. Neither agent is indicated for primary central nervous system lymphoma. Both labels say that patients should not donate blood, organs, or tissues after treatment. Tisagenlecleucel labeling also notes that some commercial HIV nucleic acid tests may yield false positives after treatment.
Novartis said in a press release that T cells are treated at the company’s Morris Plains, N.J., facility with a turnaround time of about 22 days. Cryopreservation of the harvested cells gives providers some flexibility in treatment timing.
Novartis’s after failure of two or more lines of systemic therapy.
The Food and Drug Administration approved the expanded indication on May 1. The chimeric antigen receptor (CAR) T-cell therapy was initially approved in Aug. 2017 for refractory or relapsed B-cell precursor acute lymphoblastic leukemia (ALL) in patients up to 25 years old. The new approval brings tisagenlecleucel into direct competition with Gilead Science’s CAR T-cell therapy axicabtagene ciloleucel (Yescarta), which was approved in Oct. 2017 for B-cell lymphoma.
Besides matching the competition, she said the lower price is because tisagenlecleucel takes longer to work for lymphoma, and the response isn’t as potent as for childhood ALL. Novartis is looking into chronic lymphocytic leukemia, multiple myeloma, and solid tumor indications for tisagenlecleucel and other CAR T-cell agents, she added.
The Centers for Medicare & Medicaid Services recently committed to covering outpatient administration of both agents for their initial indications; Novartis is working with CMS for coverage of the new lymphoma indication.
With both products, T cells are collected then shipped off to a company facility where a CAR gene is spliced into their DNA, essentially programming the T cells to attack the targeted cancer. The cells are then infused back into the patient.
In the phase 2 JULIET trial, tisagenlecleucel showed an overall response rate of 50% among 68 B-cell lymphoma patients, with 32% achieving complete response (CR) and 18% achieving partial response (PR). The median duration of response was not reached.
Axicabtagene ciloleucel’s label reports an objective response rate of 72% among 101 patients, with CR in 51% and PR in 21%. Median duration of response was 9.2 months but was also not reached among complete responders.
“Different trials. Different CARTs. Different levels of disease. Our drug is cryopreserved and theirs is not. No way to compare them,” the Novartis spokeswoman said when asked about the response differences.
T-cell reprogramming isn’t clean at this point in medical history; both agents carry black box warnings of potentially fatal cytokine release syndrome and neurologic toxicity, and both are subject to Risk Evaluation and Mitigation Strategy programs.
The B-cell lymphoma indication for both therapies includes diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. The Gilead product carries an additional indication for primary mediastinal large B-cell lymphoma. Neither agent is indicated for primary central nervous system lymphoma. Both labels say that patients should not donate blood, organs, or tissues after treatment. Tisagenlecleucel labeling also notes that some commercial HIV nucleic acid tests may yield false positives after treatment.
Novartis said in a press release that T cells are treated at the company’s Morris Plains, N.J., facility with a turnaround time of about 22 days. Cryopreservation of the harvested cells gives providers some flexibility in treatment timing.
Novartis’s after failure of two or more lines of systemic therapy.
The Food and Drug Administration approved the expanded indication on May 1. The chimeric antigen receptor (CAR) T-cell therapy was initially approved in Aug. 2017 for refractory or relapsed B-cell precursor acute lymphoblastic leukemia (ALL) in patients up to 25 years old. The new approval brings tisagenlecleucel into direct competition with Gilead Science’s CAR T-cell therapy axicabtagene ciloleucel (Yescarta), which was approved in Oct. 2017 for B-cell lymphoma.
Besides matching the competition, she said the lower price is because tisagenlecleucel takes longer to work for lymphoma, and the response isn’t as potent as for childhood ALL. Novartis is looking into chronic lymphocytic leukemia, multiple myeloma, and solid tumor indications for tisagenlecleucel and other CAR T-cell agents, she added.
The Centers for Medicare & Medicaid Services recently committed to covering outpatient administration of both agents for their initial indications; Novartis is working with CMS for coverage of the new lymphoma indication.
With both products, T cells are collected then shipped off to a company facility where a CAR gene is spliced into their DNA, essentially programming the T cells to attack the targeted cancer. The cells are then infused back into the patient.
In the phase 2 JULIET trial, tisagenlecleucel showed an overall response rate of 50% among 68 B-cell lymphoma patients, with 32% achieving complete response (CR) and 18% achieving partial response (PR). The median duration of response was not reached.
Axicabtagene ciloleucel’s label reports an objective response rate of 72% among 101 patients, with CR in 51% and PR in 21%. Median duration of response was 9.2 months but was also not reached among complete responders.
“Different trials. Different CARTs. Different levels of disease. Our drug is cryopreserved and theirs is not. No way to compare them,” the Novartis spokeswoman said when asked about the response differences.
T-cell reprogramming isn’t clean at this point in medical history; both agents carry black box warnings of potentially fatal cytokine release syndrome and neurologic toxicity, and both are subject to Risk Evaluation and Mitigation Strategy programs.
The B-cell lymphoma indication for both therapies includes diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma, and DLBCL arising from follicular lymphoma. The Gilead product carries an additional indication for primary mediastinal large B-cell lymphoma. Neither agent is indicated for primary central nervous system lymphoma. Both labels say that patients should not donate blood, organs, or tissues after treatment. Tisagenlecleucel labeling also notes that some commercial HIV nucleic acid tests may yield false positives after treatment.
Novartis said in a press release that T cells are treated at the company’s Morris Plains, N.J., facility with a turnaround time of about 22 days. Cryopreservation of the harvested cells gives providers some flexibility in treatment timing.
Five-year survival for non-Hodgkin lymphoma tops 71%
The overall 5-year survival rate for non-Hodgkin lymphoma (NHL) is 71.4%, according to the National Cancer Institute.
That number falls neatly into the middle of the range for survival by stage at diagnosis, with stage I (81.8%) and stage II (75.3%) disease on the high side and stage III (69.1%) and stage IV (61.7%) on the low side, the most recent data from the Surveillance, Epidemiology, and End Results (SEER) Program show. Five-year survival for NHL of unknown stage at diagnosis is 76.4%.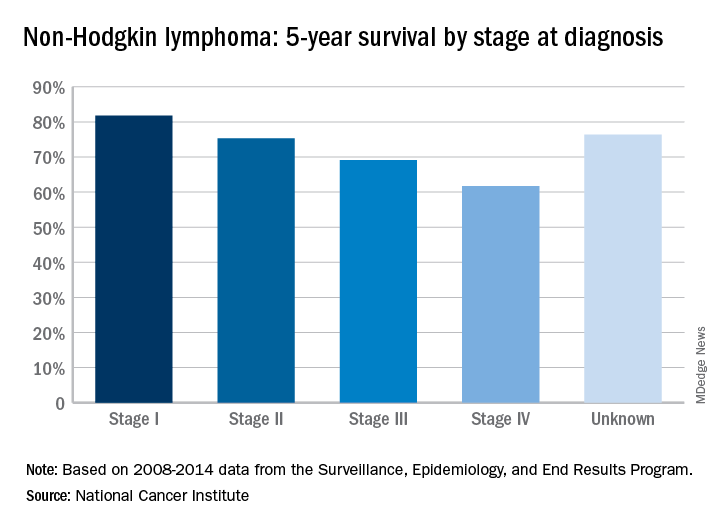
The overall 5-year survival rate for non-Hodgkin lymphoma (NHL) is 71.4%, according to the National Cancer Institute.
That number falls neatly into the middle of the range for survival by stage at diagnosis, with stage I (81.8%) and stage II (75.3%) disease on the high side and stage III (69.1%) and stage IV (61.7%) on the low side, the most recent data from the Surveillance, Epidemiology, and End Results (SEER) Program show. Five-year survival for NHL of unknown stage at diagnosis is 76.4%.
The overall 5-year survival rate for non-Hodgkin lymphoma (NHL) is 71.4%, according to the National Cancer Institute.
That number falls neatly into the middle of the range for survival by stage at diagnosis, with stage I (81.8%) and stage II (75.3%) disease on the high side and stage III (69.1%) and stage IV (61.7%) on the low side, the most recent data from the Surveillance, Epidemiology, and End Results (SEER) Program show. Five-year survival for NHL of unknown stage at diagnosis is 76.4%.
FDA places partial hold on trials after secondary lymphoma
The drugmaker after a pediatric patient developed a secondary T-cell lymphoma.
The Food and Drug Administration had issued a partial clinical hold in April on new enrollment of any patients with genetically defined solid tumors and hematologic malignancies. Patients already enrolled who have not had disease progression can continue to receive tazemetostat.
Tazemetostat is a first-in-class EZH2 inhibitor being studied as monotherapy in phase 1 and 2 trials for certain molecularly defined solid tumors, follicular lymphoma and diffuse large B-cell lymphoma, mesothelioma, and in combination studies of DLBCL and non–small cell lung cancer.
Epizyme is currently working to update informed consent, the investigator’s brochure, and study protocols, the company said in a statement.
The drugmaker after a pediatric patient developed a secondary T-cell lymphoma.
The Food and Drug Administration had issued a partial clinical hold in April on new enrollment of any patients with genetically defined solid tumors and hematologic malignancies. Patients already enrolled who have not had disease progression can continue to receive tazemetostat.
Tazemetostat is a first-in-class EZH2 inhibitor being studied as monotherapy in phase 1 and 2 trials for certain molecularly defined solid tumors, follicular lymphoma and diffuse large B-cell lymphoma, mesothelioma, and in combination studies of DLBCL and non–small cell lung cancer.
Epizyme is currently working to update informed consent, the investigator’s brochure, and study protocols, the company said in a statement.
The drugmaker after a pediatric patient developed a secondary T-cell lymphoma.
The Food and Drug Administration had issued a partial clinical hold in April on new enrollment of any patients with genetically defined solid tumors and hematologic malignancies. Patients already enrolled who have not had disease progression can continue to receive tazemetostat.
Tazemetostat is a first-in-class EZH2 inhibitor being studied as monotherapy in phase 1 and 2 trials for certain molecularly defined solid tumors, follicular lymphoma and diffuse large B-cell lymphoma, mesothelioma, and in combination studies of DLBCL and non–small cell lung cancer.
Epizyme is currently working to update informed consent, the investigator’s brochure, and study protocols, the company said in a statement.
Venetoclax shows muscle against CLL relapsed after idelalisib
For patients with relapsed or refractory chronic lymphocytic leukemia despite therapy with idelalisib (Zydelig), venetoclax (Venclexta) was associated with relatively high overall response and progression-free survival rates, results of a phase 2 study show.
Among 36 patients with relapsed/refractory CLL who had received idelalisib as their most recent B-cell receptor pathway inhibitor (BCRi), the overall response rate (ORR) was 67%, and median progression-free and overall survival (PFS and OS) had not been reached after 14 months of follow-up, reported Steven Coutre, MD, of Stanford (Calif.) University, and his colleagues.
“[V]enetoclax monotherapy is active and well-tolerated in patients with CLL progression after therapy with idelalisib, including a significant number of patients who also received prior therapy with ibrutinib [Imbruvica]. These results from the first prospective trial in this high-risk population provide evidence that venetoclax should be considered as a treatment option for such patients,” the investigators wrote. The report was published in Blood.
In clinical trials with idelalisib, approximately one-third of patients with CLL experienced disease progression on therapy, and other patients had to discontinue the drug, an inhibitor of the delta isoform of phosphoinositide 3-kinase (PI3K), because of toxicities, the investigators noted.
“The optimal treatment of patients with CLL progressing after idelalisib has not been well characterized,” they wrote. “Outcomes in patients who discontinued idelalisib treatment early are poor, with one retrospective analysis reporting a median overall survival (OS) after idelalisib discontinuation of approximately 2 months (range, 0-10 months).”
Venetoclax, an inhibitor of the apoptotic BCL-2 protein, has been shown to have activity against CLL, including in patients with high-risk features such as the chromosome 17p deletion (del17p), prompting the investigators to evaluate it as a follow-on in patients with relapsed/refractory CLL treated with a B-cell receptor pathway inhibitor.
They reported on the idelalisib cohort in a phase 2 trial in which patients with CLL that progressed on either idelalisib or ibrutinib were subsequently treated with venetoclax. The patients in this analysis included those treated with idelalisib in the main study cohort or an expansion cohort.
Patients were started on venetoclax 20 mg daily, followed by weekly dose escalations to a target of 400 mg daily by week 5, or to a maximum of 600 mg for patients who did not have a response by the week 12 assessment.
The overall response rate – the primary efficacy endpoint – was 67%. There were two complete remissions (CR) and one CR with incomplete bone marrow recovery. The remaining 21 patients with responses had partial responses.
At a median of 14 months of follow-up, neither median PFS, duration of response, or OS had been reached.
The investigator-estimated 12-month PFS rate was 79%.
The most common grade 3 or 4 adverse events were neutropenia in 50% of patients, thrombocytopenia in 25%, and anemia in 17%. There were no cases of clinical tumor lysis syndrome, which has been known to occur when venetoclax is initiated at full dose without a ramp-up.
The most common adverse events of any grade included neutropenia, diarrhea, upper respiratory tract infection, thrombocytopenia, nausea, fatigue, cough, rash, and anemia.
“The low number of CRs reported at the time of analysis may be a result of the follow-up time, particularly for patients in the expansion cohort, as other clinical studies with venetoclax report CR occurring after 1 year on therapy. Patients with prior ibrutinib exposure who had progressed on idelalisib as their most recent therapy before study entry had similar efficacy results,” the investigators wrote.
Genentech and AbbVie funded the study. Dr. Coutre is an advisory board member for both companies and others, and receives institutional funding from AbbVie and others. Multiple coauthors disclosed financial relationships with AbbVie, Genentech, or both, as well as other companies.
SOURCE: Coutre S et al. Blood. 2018;131(15):1704-11.
For patients with relapsed or refractory chronic lymphocytic leukemia despite therapy with idelalisib (Zydelig), venetoclax (Venclexta) was associated with relatively high overall response and progression-free survival rates, results of a phase 2 study show.
Among 36 patients with relapsed/refractory CLL who had received idelalisib as their most recent B-cell receptor pathway inhibitor (BCRi), the overall response rate (ORR) was 67%, and median progression-free and overall survival (PFS and OS) had not been reached after 14 months of follow-up, reported Steven Coutre, MD, of Stanford (Calif.) University, and his colleagues.
“[V]enetoclax monotherapy is active and well-tolerated in patients with CLL progression after therapy with idelalisib, including a significant number of patients who also received prior therapy with ibrutinib [Imbruvica]. These results from the first prospective trial in this high-risk population provide evidence that venetoclax should be considered as a treatment option for such patients,” the investigators wrote. The report was published in Blood.
In clinical trials with idelalisib, approximately one-third of patients with CLL experienced disease progression on therapy, and other patients had to discontinue the drug, an inhibitor of the delta isoform of phosphoinositide 3-kinase (PI3K), because of toxicities, the investigators noted.
“The optimal treatment of patients with CLL progressing after idelalisib has not been well characterized,” they wrote. “Outcomes in patients who discontinued idelalisib treatment early are poor, with one retrospective analysis reporting a median overall survival (OS) after idelalisib discontinuation of approximately 2 months (range, 0-10 months).”
Venetoclax, an inhibitor of the apoptotic BCL-2 protein, has been shown to have activity against CLL, including in patients with high-risk features such as the chromosome 17p deletion (del17p), prompting the investigators to evaluate it as a follow-on in patients with relapsed/refractory CLL treated with a B-cell receptor pathway inhibitor.
They reported on the idelalisib cohort in a phase 2 trial in which patients with CLL that progressed on either idelalisib or ibrutinib were subsequently treated with venetoclax. The patients in this analysis included those treated with idelalisib in the main study cohort or an expansion cohort.
Patients were started on venetoclax 20 mg daily, followed by weekly dose escalations to a target of 400 mg daily by week 5, or to a maximum of 600 mg for patients who did not have a response by the week 12 assessment.
The overall response rate – the primary efficacy endpoint – was 67%. There were two complete remissions (CR) and one CR with incomplete bone marrow recovery. The remaining 21 patients with responses had partial responses.
At a median of 14 months of follow-up, neither median PFS, duration of response, or OS had been reached.
The investigator-estimated 12-month PFS rate was 79%.
The most common grade 3 or 4 adverse events were neutropenia in 50% of patients, thrombocytopenia in 25%, and anemia in 17%. There were no cases of clinical tumor lysis syndrome, which has been known to occur when venetoclax is initiated at full dose without a ramp-up.
The most common adverse events of any grade included neutropenia, diarrhea, upper respiratory tract infection, thrombocytopenia, nausea, fatigue, cough, rash, and anemia.
“The low number of CRs reported at the time of analysis may be a result of the follow-up time, particularly for patients in the expansion cohort, as other clinical studies with venetoclax report CR occurring after 1 year on therapy. Patients with prior ibrutinib exposure who had progressed on idelalisib as their most recent therapy before study entry had similar efficacy results,” the investigators wrote.
Genentech and AbbVie funded the study. Dr. Coutre is an advisory board member for both companies and others, and receives institutional funding from AbbVie and others. Multiple coauthors disclosed financial relationships with AbbVie, Genentech, or both, as well as other companies.
SOURCE: Coutre S et al. Blood. 2018;131(15):1704-11.
For patients with relapsed or refractory chronic lymphocytic leukemia despite therapy with idelalisib (Zydelig), venetoclax (Venclexta) was associated with relatively high overall response and progression-free survival rates, results of a phase 2 study show.
Among 36 patients with relapsed/refractory CLL who had received idelalisib as their most recent B-cell receptor pathway inhibitor (BCRi), the overall response rate (ORR) was 67%, and median progression-free and overall survival (PFS and OS) had not been reached after 14 months of follow-up, reported Steven Coutre, MD, of Stanford (Calif.) University, and his colleagues.
“[V]enetoclax monotherapy is active and well-tolerated in patients with CLL progression after therapy with idelalisib, including a significant number of patients who also received prior therapy with ibrutinib [Imbruvica]. These results from the first prospective trial in this high-risk population provide evidence that venetoclax should be considered as a treatment option for such patients,” the investigators wrote. The report was published in Blood.
In clinical trials with idelalisib, approximately one-third of patients with CLL experienced disease progression on therapy, and other patients had to discontinue the drug, an inhibitor of the delta isoform of phosphoinositide 3-kinase (PI3K), because of toxicities, the investigators noted.
“The optimal treatment of patients with CLL progressing after idelalisib has not been well characterized,” they wrote. “Outcomes in patients who discontinued idelalisib treatment early are poor, with one retrospective analysis reporting a median overall survival (OS) after idelalisib discontinuation of approximately 2 months (range, 0-10 months).”
Venetoclax, an inhibitor of the apoptotic BCL-2 protein, has been shown to have activity against CLL, including in patients with high-risk features such as the chromosome 17p deletion (del17p), prompting the investigators to evaluate it as a follow-on in patients with relapsed/refractory CLL treated with a B-cell receptor pathway inhibitor.
They reported on the idelalisib cohort in a phase 2 trial in which patients with CLL that progressed on either idelalisib or ibrutinib were subsequently treated with venetoclax. The patients in this analysis included those treated with idelalisib in the main study cohort or an expansion cohort.
Patients were started on venetoclax 20 mg daily, followed by weekly dose escalations to a target of 400 mg daily by week 5, or to a maximum of 600 mg for patients who did not have a response by the week 12 assessment.
The overall response rate – the primary efficacy endpoint – was 67%. There were two complete remissions (CR) and one CR with incomplete bone marrow recovery. The remaining 21 patients with responses had partial responses.
At a median of 14 months of follow-up, neither median PFS, duration of response, or OS had been reached.
The investigator-estimated 12-month PFS rate was 79%.
The most common grade 3 or 4 adverse events were neutropenia in 50% of patients, thrombocytopenia in 25%, and anemia in 17%. There were no cases of clinical tumor lysis syndrome, which has been known to occur when venetoclax is initiated at full dose without a ramp-up.
The most common adverse events of any grade included neutropenia, diarrhea, upper respiratory tract infection, thrombocytopenia, nausea, fatigue, cough, rash, and anemia.
“The low number of CRs reported at the time of analysis may be a result of the follow-up time, particularly for patients in the expansion cohort, as other clinical studies with venetoclax report CR occurring after 1 year on therapy. Patients with prior ibrutinib exposure who had progressed on idelalisib as their most recent therapy before study entry had similar efficacy results,” the investigators wrote.
Genentech and AbbVie funded the study. Dr. Coutre is an advisory board member for both companies and others, and receives institutional funding from AbbVie and others. Multiple coauthors disclosed financial relationships with AbbVie, Genentech, or both, as well as other companies.
SOURCE: Coutre S et al. Blood. 2018;131(15):1704-11.
FROM BLOOD
Key clinical point:
Major finding: The overall response rate was 67%, including two complete responses (CRs) and one CR with incomplete bone marrow recovery.
Study details: Cohort of 36 patients with relapsed/refractory CLL previously treated with idelalisib.
Disclosures: Genentech and AbbVie funded the study. Dr. Coutre is an advisory board member for both companies and others, and receives institutional funding from AbbVie and others. Multiple coauthors disclosed financial relationships with AbbVie, Genentech, or both, as well as other companies.
Source: Coutre S et al. Blood. 2018;131(15):1704-11.
PDPK1 could be novel target in MCL
Researchers may have found a new therapeutic approach for treating mantle cell lymphoma (MCL) by targeting 3-phosphoinositide-dependent protein kinase 1 (PDPK1).
Saori Maegawa and colleagues at Kyoto Prefectural University of Medicine in Japan, evaluated PDPK1 activity in patient-derived primary B-cell lymphoma cells by immunohistochemical staining of p-PDPK1Ser241 (p-PDPK1) in tissue specimens from seven patients with MCL, six patients with diffuse large B-cell lymphoma, and five patients with follicular lymphoma. All specimens were biopsied at initial diagnosis, before starting treatment.
“Our study showed that PDPK1 inhibition caused inactivation of RSK2-NTKD, as well as the decrease of total RSK2 protein, but not of AKT, in MCL-derived cells,” the researchers wrote in Experimental Hematology. “This implies that RSK2 activity is mainly regulated by PDPK1 at both the transcriptional expression and post-translational levels, but AKT activity is regulated by a signaling pathway that does not interact with a PDPK1-mediated pathway in MCL.”
If a PDPK1 inhibitor is pursued as clinical target, the researchers said careful monitoring for hyperglycemia may be required since impaired glucose metabolism is commonly seen with AKT inhibitors. Future research in MCL could also be directed toward the targeting of RSK2-NTKD, the researchers wrote.
SOURCE: Maegawa S et al. Exp Hematol. 2018 Mar;59:72-81.e2.
Researchers may have found a new therapeutic approach for treating mantle cell lymphoma (MCL) by targeting 3-phosphoinositide-dependent protein kinase 1 (PDPK1).
Saori Maegawa and colleagues at Kyoto Prefectural University of Medicine in Japan, evaluated PDPK1 activity in patient-derived primary B-cell lymphoma cells by immunohistochemical staining of p-PDPK1Ser241 (p-PDPK1) in tissue specimens from seven patients with MCL, six patients with diffuse large B-cell lymphoma, and five patients with follicular lymphoma. All specimens were biopsied at initial diagnosis, before starting treatment.
“Our study showed that PDPK1 inhibition caused inactivation of RSK2-NTKD, as well as the decrease of total RSK2 protein, but not of AKT, in MCL-derived cells,” the researchers wrote in Experimental Hematology. “This implies that RSK2 activity is mainly regulated by PDPK1 at both the transcriptional expression and post-translational levels, but AKT activity is regulated by a signaling pathway that does not interact with a PDPK1-mediated pathway in MCL.”
If a PDPK1 inhibitor is pursued as clinical target, the researchers said careful monitoring for hyperglycemia may be required since impaired glucose metabolism is commonly seen with AKT inhibitors. Future research in MCL could also be directed toward the targeting of RSK2-NTKD, the researchers wrote.
SOURCE: Maegawa S et al. Exp Hematol. 2018 Mar;59:72-81.e2.
Researchers may have found a new therapeutic approach for treating mantle cell lymphoma (MCL) by targeting 3-phosphoinositide-dependent protein kinase 1 (PDPK1).
Saori Maegawa and colleagues at Kyoto Prefectural University of Medicine in Japan, evaluated PDPK1 activity in patient-derived primary B-cell lymphoma cells by immunohistochemical staining of p-PDPK1Ser241 (p-PDPK1) in tissue specimens from seven patients with MCL, six patients with diffuse large B-cell lymphoma, and five patients with follicular lymphoma. All specimens were biopsied at initial diagnosis, before starting treatment.
“Our study showed that PDPK1 inhibition caused inactivation of RSK2-NTKD, as well as the decrease of total RSK2 protein, but not of AKT, in MCL-derived cells,” the researchers wrote in Experimental Hematology. “This implies that RSK2 activity is mainly regulated by PDPK1 at both the transcriptional expression and post-translational levels, but AKT activity is regulated by a signaling pathway that does not interact with a PDPK1-mediated pathway in MCL.”
If a PDPK1 inhibitor is pursued as clinical target, the researchers said careful monitoring for hyperglycemia may be required since impaired glucose metabolism is commonly seen with AKT inhibitors. Future research in MCL could also be directed toward the targeting of RSK2-NTKD, the researchers wrote.
SOURCE: Maegawa S et al. Exp Hematol. 2018 Mar;59:72-81.e2.
FROM EXPERIMENTAL HEMATOLOGY